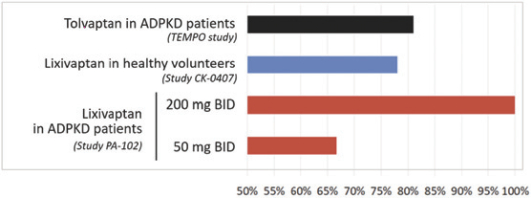
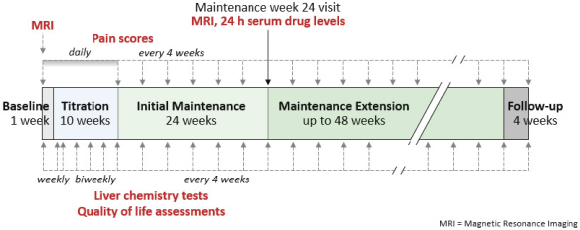
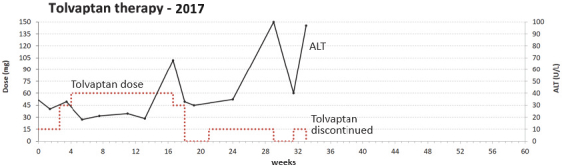
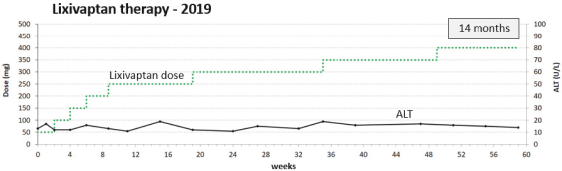
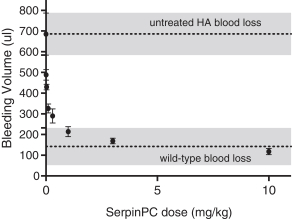
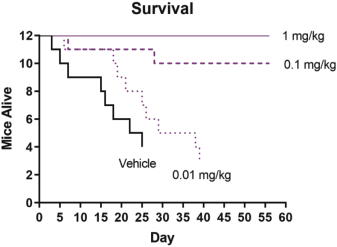
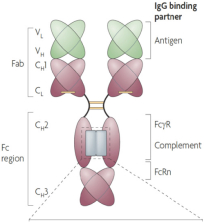
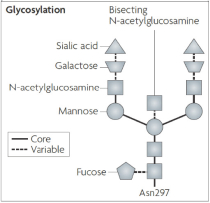
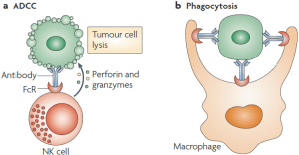
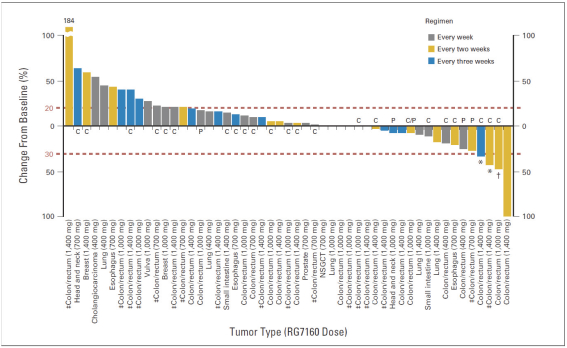
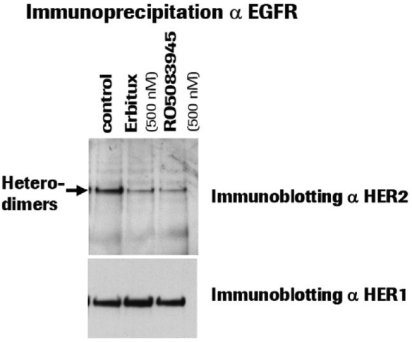
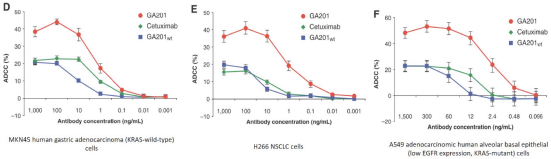
If Pega-One terminates without cause, breaches the agreement, or becomes insolvent, Roche may elect to continue development of the imgatuzumab product, and Pega-One must transfer to Roche (free of charge) all regulatory filings and approvals, clinical and non-clinical agreements, CMC agreements, and other related development contracts. Pega-One must also grant Roche a worldwide, exclusive, sublicensable, transferable license under its patent, know-how, and joint patent rights to research, develop, manufacture, have manufactured, use, offer to sell, sell, promote, export and import imgatuzumab and related products. If termination occurs after completion of a Phase 2 study of the first product, Roche will pay to Pega-One a royalty percentage rate in the low single digits based on net sales of the imgatuzumab product for ten years after the first commercial sale of the product on a country-by-country basis. If termination occurs after the first regulatory approval of the first product, Roche will pay to Pega-One a royalty percentage rate in the mid-single digits of net sales for ten years after the first commercial sale of the product on a country-by-country basis.
Pega-One may not assign its rights or obligations under this Agreement without prior written consent from Roche, except to an affiliate or in the context of a merger, acquisition, sale or other transaction involving all or substantially all of the assets of Pega-One.
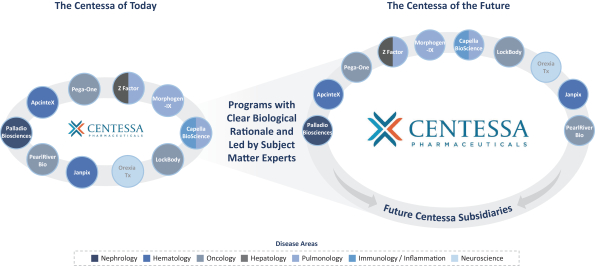
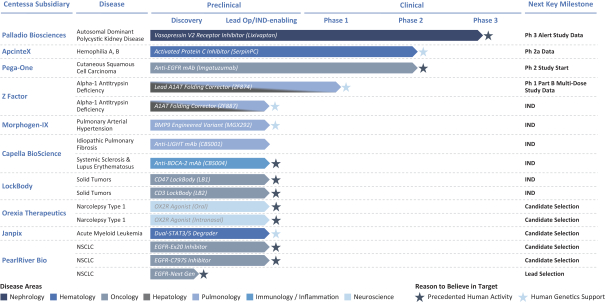
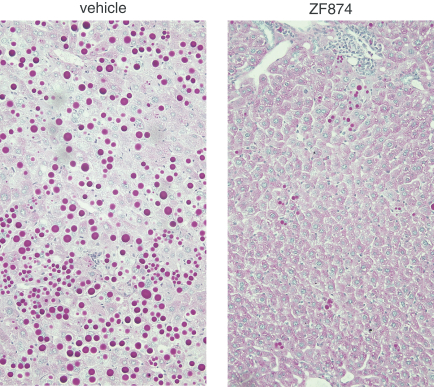
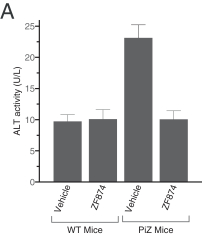
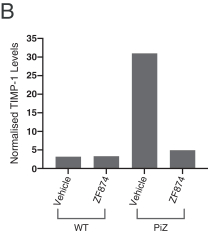
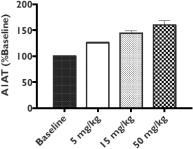
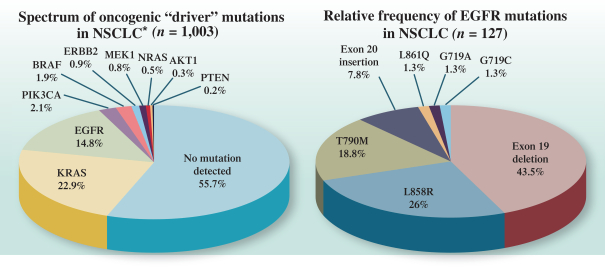
Z Factor
As of December 15, 2020, Z Factor, owned six pending foreign applications and six pending PCT applications. Z Factor’s patent portfolio includes composition of matter claims directed to ZF874, polymorphs thereof and variants thereof, method of treatment claims with ZF874, and method of manufacturing claims related to ZF874. The pending patent applications, once nationalized and if issued, are expected to expire between 2039 and 2041, without taking into account any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations of The Centessa Predecessor Group and Certain Other Acquired Entities — Licensing Arrangements — Z Factor License Agreement” for more information.
Morphogen-IX
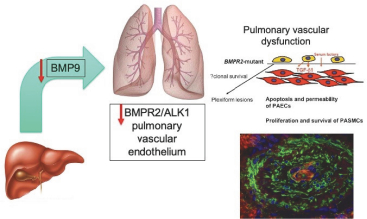
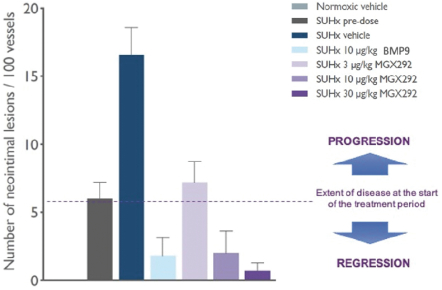
As of December 15, 2020, Morphogen-IX has a license to one issued U.S. patent, 41 issued foreign patents, e.g., France, Germany, UK, and China issued foreign patents, one U.S. pending patent application and nine pending foreign patent applications. Morphogen-IX’s licensed patent portfolio includes issued U.S. patents and issued foreign patents, which have composition of matter claims directed to MGX292 and BMP9 variants, and method of treatment claims with MGX292. The issued patents expire in 2035, and the pending patent applications, if issued, are expected to expire in 2035, without taking into account any possible patent term adjustments or extensions and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Morophogen-IX Licence Agreement
On October 30, 2015, our subsidiary, Morphogen-IX Limited, or Morphogen-IX, entered into a Patent and Know-How Licence Agreement, or License, with Cambridge Enterprise Limited (a company wholly owned by the University of Cambridge), or CE, relating to BMP 9 and 10. Pursuant to the agreement, Morphogen-IX obtained from CE an exclusive, worldwide, royalty bearing, sublicensable (through multiple tiers) license, or the Exclusive CE License, under certain patent rights, or BMP Patents, and certain technical information and materials relating to BMP 9 and 10, or BMP Know-How, for the treatment of all diseases, including prophylaxis, for human and animal health or any related research or development, or the Field. Morphogen-IX also obtained a non-exclusive, worldwide, royalty-bearing, sublicensable (through multiple tiers) license, or the CE Non-Exclusive License, to under certain, data, technical information and other know-how that is not specific to BMP 9 and 10, or the Non-Exclusive Know-How. Under the CE Exclusive License and the CE Non-Exclusive License, Morphogen-IX has the right to develop and commercialize any product, process, service or use that uses or incorporates any BMP Patents, the BMP Know-How or the Non-Exclusive Know-How, or any materials that are sold in conjunction with any such products or services, in each such case, a Licensed Product. CE has reserved a customary limited right to use the BMP Patents, BMP Know-How and Non-Exclusive Know-How for academic publication, teaching, and academic research.
247