
Exhibit 99.2 Corporate Overview September 10, 2024

2 DISCLAIMER AND FORWARD LOOKING STATEMENTS This presentation has been prepared by Centessa Pharmaceuticals plc (the “Company”) for planned clinical trials; our ability to obtain adequate financing, including through our financing facility informational purposes only and not for any other purpose. This presentation does not contain all the with Oberland, to fund our planned clinical trials and other expenses; trends in the industry; the legal information that is or may be material to investors or potential investors and should not be considered and regulatory framework for the industry, including the receipt and maintenance of clearances to as advice or a recommendation to investors or potential investors in respect of the holding, purchasing conduct or continue clinical testing; the risk that any one or more of our product candidates will not be or selling of securities or other financial instruments and does not take into account any investor’s successfully developed and commercialized; the risk that the results of preclinical studies or clinical particular objectives, financial situation or needs. The communication of this presentation may be studies will not be predictive of future results in connection with future studies; and geo-political risks restricted by law; it is not intended for distribution to, or use by any person in, any jurisdiction where such as the Russia-Ukraine war and the conflicts in the Middle East and other risk factors contained in such distribution or use would be contrary to local law or regulation. This presentation is not directed to our filings with the U.S. Securities and Exchange Commission. In light of these risks and uncertainties, or intended for distribution, or transfer, either directly or indirectly to, or use by, any person or entity the events or circumstances referred to in the forward-looking statements may not occur. The actual that is a citizen or resident or located in any locality, state, country or other jurisdiction where such results may vary from the anticipated results and the variations may be material. These forward- distribution, transfer, publication, availability or use would be contrary to law or regulation or which looking statements should not be taken as forecasts or promises nor should they be taken as implying would require any registration or licensing within such jurisdiction. any indication, assurance or guarantee that the assumptions on which such forward looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in This presentation may contain forward-looking statements made pursuant to the safe harbor this presentation. You are cautioned not to place undue reliance on these forward-looking statements, provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that which speak only as of the date this presentation is given. All projections, valuations and statistical are not statements of historical fact are forward-looking statements, including, without limitation, analyses are provided for information purposes only. We expressly disclaim any obligation or statements related to the Company’s ability to deliver impactful medicines to patients; the ability of undertaking to release publicly any updates or revisions to any forward looking statements our key executives to drive execution of the Company’s portfolio of programs; our asset-centric contained herein to reflect any change in our expectations or any changes in events, conditions business model and the intended advantages and benefits thereof; research and clinical development or circumstances on which any such statement is based, except as may be required by law. plans; the scope, progress, results and costs of developing our product candidates or any other future They may be based on subjective assessments and assumptions and may use one among alternative product candidates; the development and therapeutic potential of our product candidates, including methodologies that produce different results and to the extent they are based on historical information, SerpinPC, ORX750, ORX142 and, LB101; strategy; regulatory matters, including the timing and they should not be relied upon as an accurate prediction of future performance. likelihood of success of obtaining approvals to initiate or continue clinical trials or market any products; the Company’s ability to successfully conduct its clinical development of ORX750 below the This presentation discusses product candidates that are under clinical study, and which have not yet maximum exposure limit set by the U.S. Food and Drug Administration (“FDA”) or, in the event the been approved for marketing by the FDA or any other regulatory agency. No representation or warranty, Company plans to exceed the maximum exposure limit, the Company’s ability to successfully have the express or implied, is made as to the safety or effectiveness of these product candidates for the use for maximum exposure limit removed; enroll subjects in clinical trials; market size and opportunity for our which such product candidates are being studied. The trademarks included herein are the property of product candidates; and our anticipated cash runway. Words such as “may,” “might,” “will,” “could,” the owners thereof and are used for reference purposes only. Such use should not be construed as an “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” endorsement of such products. Certain information contained in this presentation relates to or is “predict,” “potential,” “continue,” “ongoing,” “aim,” “seek,” and variations of these words or similar based on studies, publications, surveys and other data obtained from third party sources and the expressions are intended to identify forward-looking statements, though not all forward-looking Company’s own internal estimates and research. While we believe these third-party sources to be statements necessarily contain these identifying words. These forward-looking statements are based reliable as of the date of this presentation, we have not independently verified, and make no on the beliefs of the Company's management as well as assumptions made by and information representation or warranty, express or implied, as to the adequacy, fairness, accuracy or currently available to the Company. Such statements reflect the current views of the Company with completeness of, any information obtained from third party sources. Finally, while we believe our own respect to future events and are subject to known and unknown risks, including, without limitation, internal research is reliable, such research has not been verified by any independent source. risks related to our ability to protect and maintain our intellectual property position; business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company; risks inherent in developing products and technologies; future results from our ongoing and 2

OUR MISSION Discovering and developing medicines that are transformational for patients Multiple potential blockbuster assets Ongoing momentum in 2024 with clinical milestones anticipated across our most advanced programs Strong balance sheet Centessa reported $294.8 million in cash, cash equivalents and short-term investments as of June 30, 2024. Cash runway into mid-2026. 3
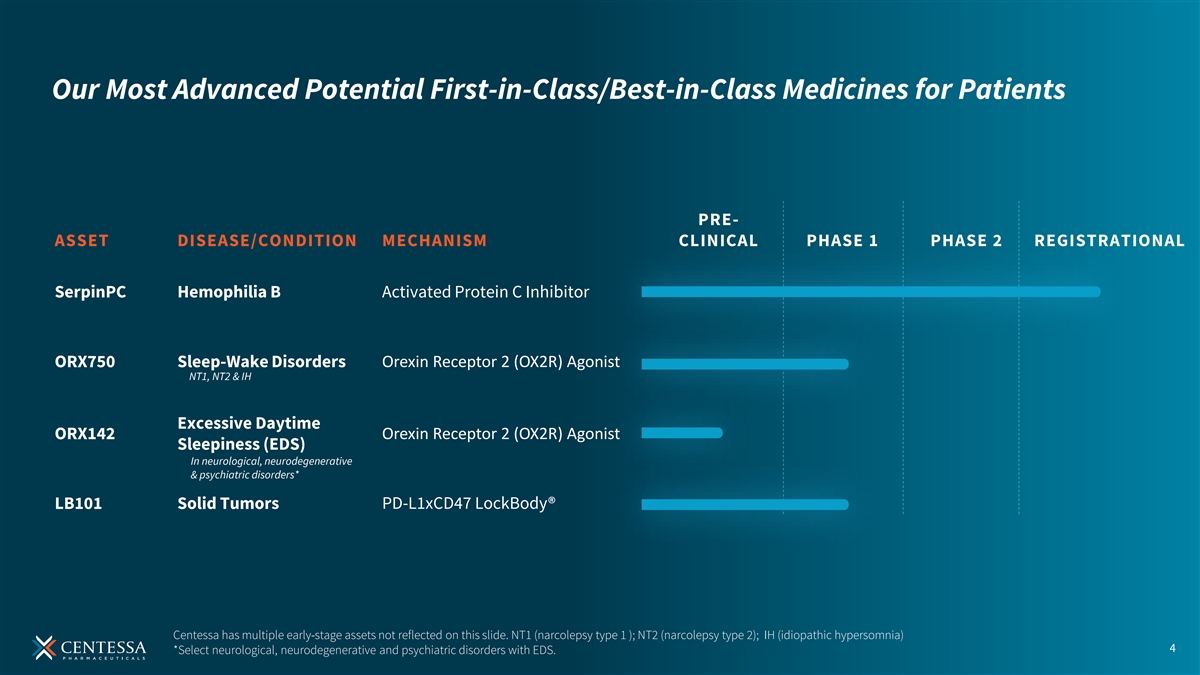
Our Most Advanced Potential First-in-Class/Best-in-Class Medicines for Patients PRE- ASSET DISEASE/CONDITION MECHANISM CLINICAL PHASE 1 PHASE 2 REGISTRATIONAL SerpinPC Hemophilia B Activated Protein C Inhibitor ORX750 Sleep-Wake Disorders Orexin Receptor 2 (OX2R) Agonist NT1, NT2 & IH Excessive Daytime Orexin Receptor 2 (OX2R) Agonist ORX142 Sleepiness (EDS) In neurological, neurodegenerative & psychiatric disorders* LB101 Solid Tumors PD-L1xCD47 LockBody® Centessa has multiple early-stage assets not reflected on this slide. NT1 (narcolepsy type 1 ); NT2 (narcolepsy type 2); IH (idiopathic hypersomnia) 4 *Select neurological, neurodegenerative and psychiatric disorders with EDS.

OREXIN AGONIST PROGRAM ORX750 Plan to rapidly advance into Phase 2 studies in patients with NT1, NT2, and IH beginning in Q4 of 2024 ORX142 Preclinical data to be presented at Sleep Europe 2024 2024 Driving HEMOPHILIA PROGRAM Momentum SerpinPC ANTICIPATED MILESTONES PRESent-2 Part 1 interim analysis planned in 2024; Part 1 data planned for late 2024/early 2025 LOCKBODY TECHNOLOGY PLATFORM LB101 Phase 1/2 study ongoing 5

LockBody Orexin Agonist Hemophilia Technology Program Program Platform 6

Orexin agonists have the potential to transform the standard of care for individuals with sleep-wake disorders and excessive daytime sleepiness (EDS) across select disorders 7
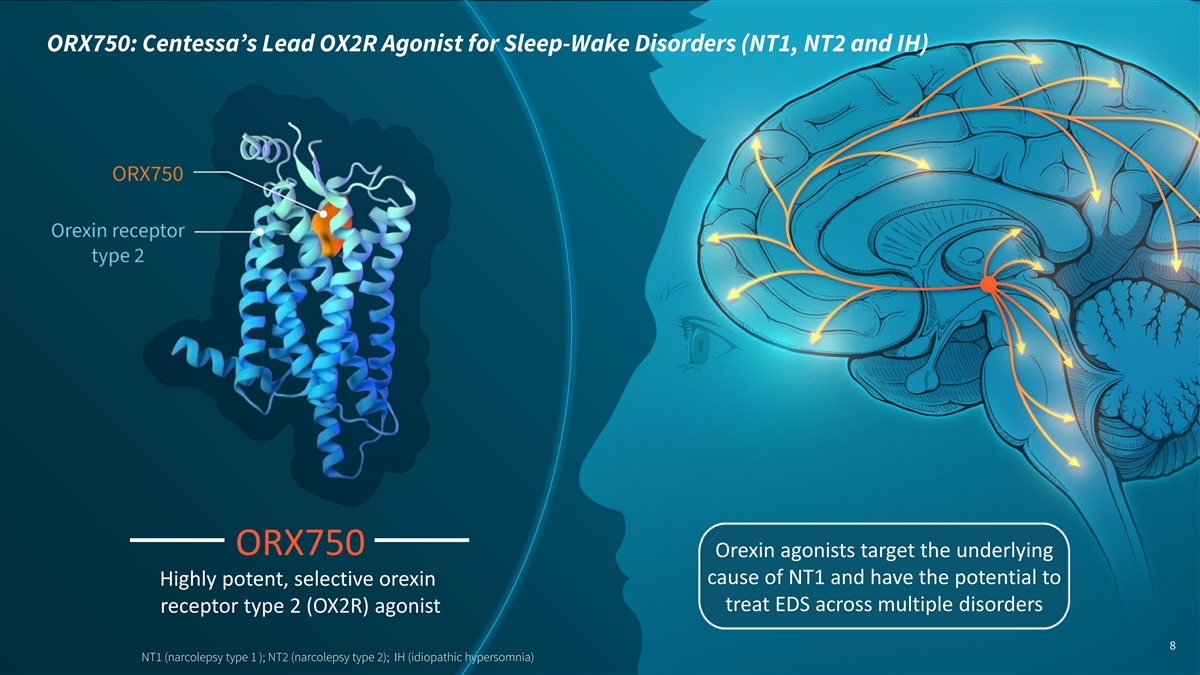
ORX750: Centessa’s Lead OX2R Agonist for Sleep-Wake Disorders (NT1, NT2 and IH) ORX750 Orexin agonists target the underlying cause of NT1 and have the potential to Highly potent, selective orexin treat EDS across multiple disorders receptor type 2 (OX2R) agonist 8 NT1 (narcolepsy type 1 ); NT2 (narcolepsy type 2); IH (idiopathic hypersomnia)
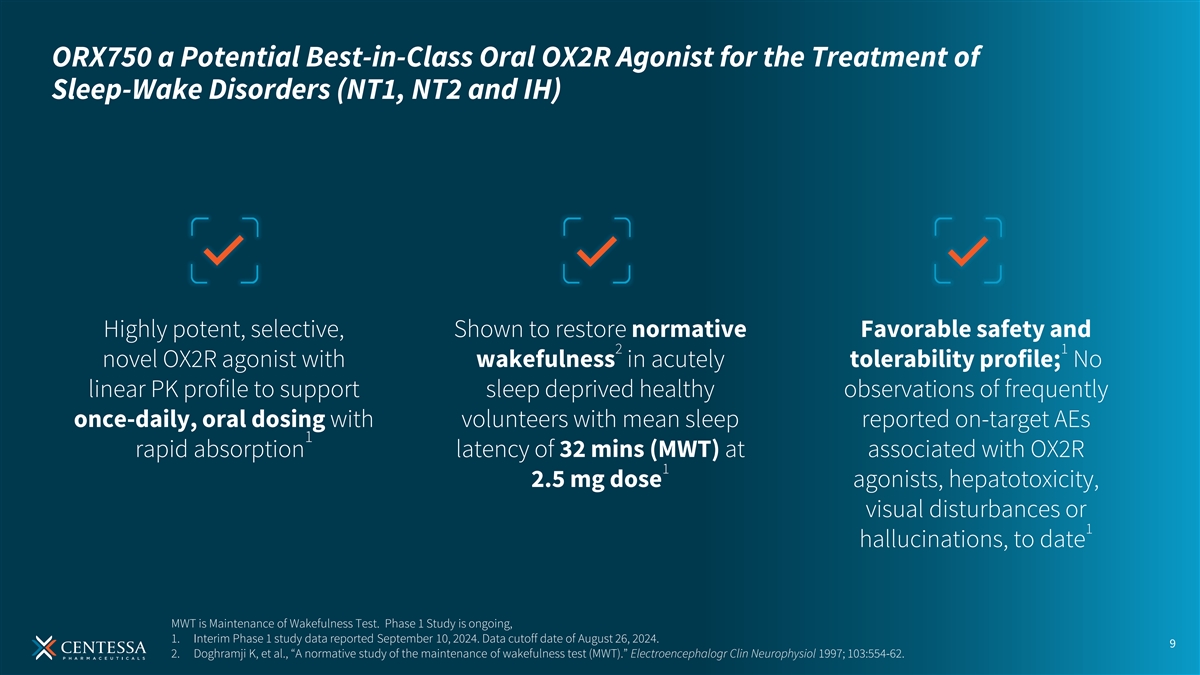
ORX750 a Potential Best-in-Class Oral OX2R Agonist for the Treatment of Sleep-Wake Disorders (NT1, NT2 and IH) Highly potent, selective, Shown to restore normative Favorable safety and 2 1 novel OX2R agonist with wakefulness in acutely tolerability profile; No linear PK profile to support sleep deprived healthy observations of frequently once-daily, oral dosing with volunteers with mean sleep reported on-target AEs 1 rapid absorption latency of 32 mins (MWT) at associated with OX2R 1 2.5 mg dose agonists, hepatotoxicity, visual disturbances or 1 hallucinations, to date MWT is Maintenance of Wakefulness Test. Phase 1 Study is ongoing, 1. Interim Phase 1 study data reported September 10, 2024. Data cutoff date of August 26, 2024. 9 2. Doghramji K, et al., “A normative study of the maintenance of wakefulness test (MWT).” Electroencephalogr Clin Neurophysiol 1997; 103:554-62.

ORX750 is a Highly Potent and Selective OX2R Agonist 100 EC 0.11 nM for hOX2R 50 ORX750 75 9,800-fold selectivity vs. hOX1R at hOX2R Activation 50 (% total) OXA at Activation pattern was indistinguishable from OXA hOX2R 1 with lack of biased agonism 25 ORX750 No significant differences in OX2R potency were at hOX1R 2 observed across species 0 No significant pharmacological activity observed in 3 GPCR selectivity and in vitro safety panels -12 -10 -8 -6 Log concentration (M) Fluorescent imaging plate reader (FLIPR) assay with Chinese hamster ovary (CHO) cells stably expressing recombinant human OX1R or OX2R; OXA EC50 at hOX2R = 0.035 nM; ORX750 EC50 at hOX1R = 1100 nM. 1 Pathhunter β-arrestin recruitment assay with CHO cells co-expressing ProLink (PK)-tagged OX2R and Enzyme Acceptor (EA)-tagged β-arrestin. 10 2 HumSafetyan, mouse, rat, dog, monkey recombinant receptors in vitro. 3 Safety 47 and GPCRMax168 from >60 receptor families.

PHASE 1 STUDY ORX750 First-in-Human Healthy Volunteer (HV) Study Phase 1 clinical study of ORX750: Evaluate the safety, tolerability and pharmacokinetics (PK) of single-ascending and multiple-ascending doses in healthy adult subjects In parallel Efficacy assessments are being performed using the Maintenance of Wakefulness Test (MWT)* and Karolinska Sleepiness Scale (KSS) in acutely sleep-deprived healthy adult subjects *MWT is an established registrational and objective endpoint in EDS in sleep-wake disorders. 11 The Phase 1 study has a maximum exposure limit specified by the FDA which the company believes significantly exceeds the predicted efficacious doses of ORX750 in indications associated with or without orexin loss.
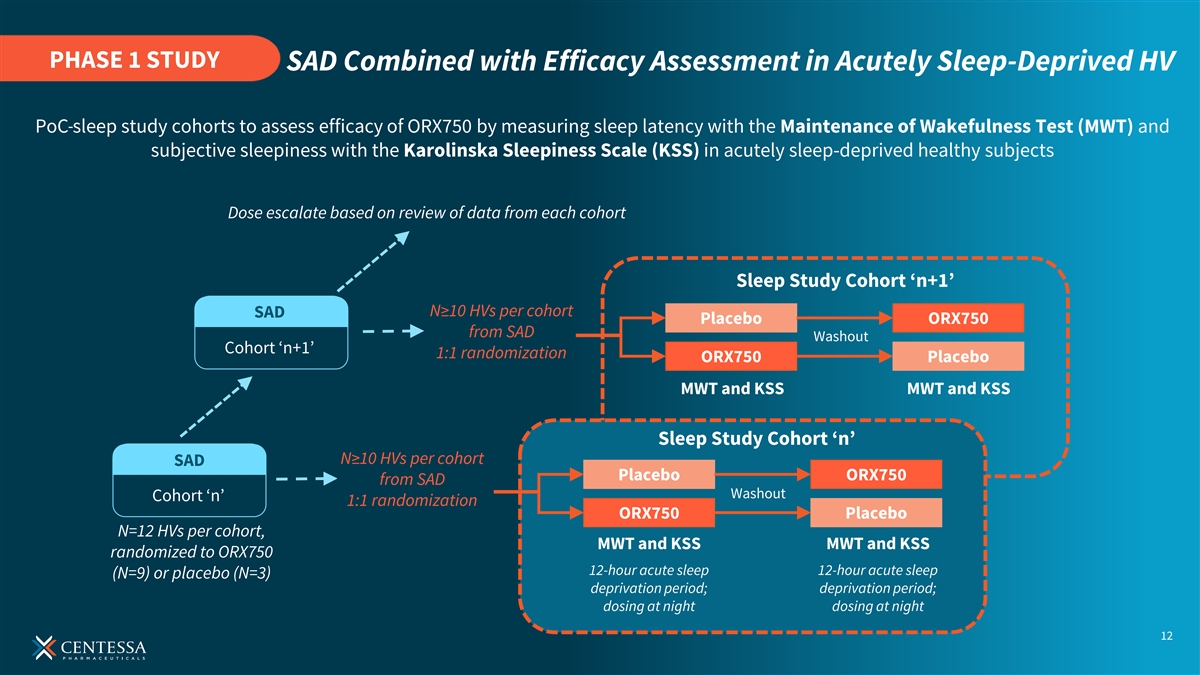
PHASE 1 STUDY SAD Combined with Efficacy Assessment in Acutely Sleep-Deprived HV PoC sleep study cohorts to assess efficacy of ORX750 by measuring sleep latency with the Maintenance of Wakefulness Test (MWT) and subjective sleepiness with the Karolinska Sleepiness Scale (KSS) in acutely sleep-deprived healthy subjects Dose escalate based on review of data from each cohort Sleep Study Cohort ‘n+1’ N≥10 HVs per cohort SAD Placebo ORX750 from SAD Washout Cohort ‘n+1’ 1:1 randomization ORX750 Placebo MWT and KSS MWT and KSS Sleep Study Cohort ‘n’ N≥10 HVs per cohort SAD Placebo ORX750 from SAD Washout Cohort ‘n’ 1:1 randomization ORX750 Placebo N=12 HVs per cohort, MWT and KSS MWT and KSS randomized to ORX750 12-hour acute sleep 12-hour acute sleep (N=9) or placebo (N=3) deprivation period; deprivation period; dosing at night dosing at night 12

INTERIM PHASE 1 ORX750 Significantly Improved Mean Sleep Onset Latency (measured by MWT) at First Two Doses Compared to Placebo LS Mean Difference Compared Post Dose LS Mean (95% CI) To Placebo In Mean Sleep Onset Sleep Onset Latency (Minutes) Latency (95% CI) Estimate (95% CI) P-value ORX750 1.0 mg (n= 8) 17.6 (12.1, 23.2) 8.1 (0.3, 15.9) 0.04 ORX750 2.5 mg (n=8) 32.0 (22.2, 41.8) 15.2 (4.7, 25.8) 0.01 1 The 2.5 mg dose was shown to restore normative wakefulness in acutely sleep-deprived healthy volunteers with mean sleep onset latency of 32 minutes (MWT) Acutely sleep-deprived healthy volunteers who received a 2.5 mg dose of ORX750 showed a significant 1.6 point improvement versus placebo in mean KSS score compared to baseline (p-value = 0.03) Phase 1 study is ongoing. Interim data cutoff date of August 26, 2024. Least squares (LS) mean. Consistent with the Phase 1 study design, a sleep study cohort (MWT) is optional at each SAD level, and as of the data cut off date, has been conducted only for 1 mg and 2.5 mg doses. 13 Mean sleep onset latency on the MWT (time to sleep onset over the four sessions performed at ~2, 4, 6, and 8 h after dosing at 11 p.m.; maximum 40 min per session). 1. Doghramji K, et al., “A normative study of the maintenance of wakefulness test (MWT).” Electroencephalogr Clin Neurophysiol 1997; 103:554-62.
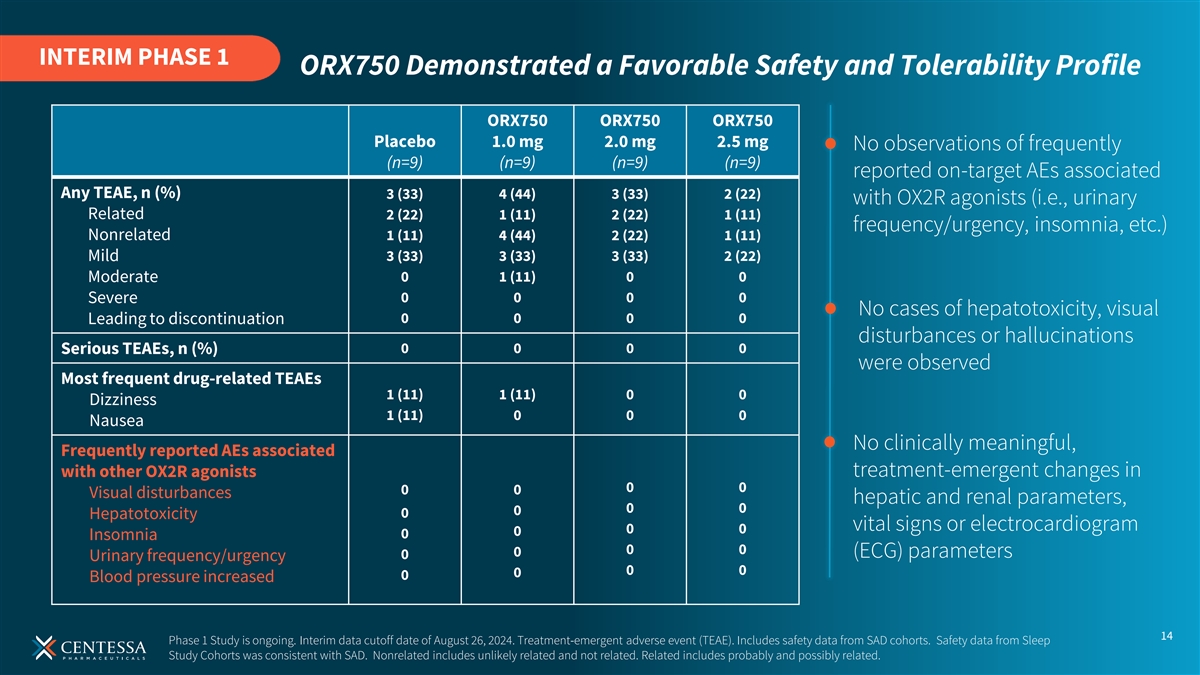
INTERIM PHASE 1 ORX750 Demonstrated a Favorable Safety and Tolerability Profile ORX750 ORX750 ORX750 Placebo 1.0 mg 2.0 mg 2.5 mg No observations of frequently (n=9) (n=9) (n=9) (n=9) reported on-target AEs associated Any TEAE, n (%) 3 (33) 4 (44) 3 (33) 2 (22) with OX2R agonists (i.e., urinary Related 2 (22) 1 (11) 2 (22) 1 (11) frequency/urgency, insomnia, etc.) Nonrelated 1 (11) 4 (44) 2 (22) 1 (11) Mild 3 (33) 3 (33) 3 (33) 2 (22) 0 1 (11) 0 0 Moderate 0 0 0 0 Severe No cases of hepatotoxicity, visual Leading to discontinuation 0 0 0 0 disturbances or hallucinations Serious TEAEs, n (%) 0 0 0 0 were observed Most frequent drug-related TEAEs 1 (11) 1 (11) 0 0 Dizziness 1 (11) 0 0 0 Nausea No clinically meaningful, Frequently reported AEs associated with other OX2R agonists treatment-emergent changes in 0 0 0 0 Visual disturbances hepatic and renal parameters, 0 0 0 0 Hepatotoxicity vital signs or electrocardiogram 0 0 0 0 Insomnia 0 0 0 (ECG) parameters 0 Urinary frequency/urgency 0 0 0 0 Blood pressure increased 14 Phase 1 Study is ongoing. Interim data cutoff date of August 26, 2024. Treatment-emergent adverse event (TEAE). Includes safety data from SAD cohorts. Safety data from Sleep Study Cohorts was consistent with SAD. Nonrelated includes unlikely related and not related. Related includes probably and possibly related.
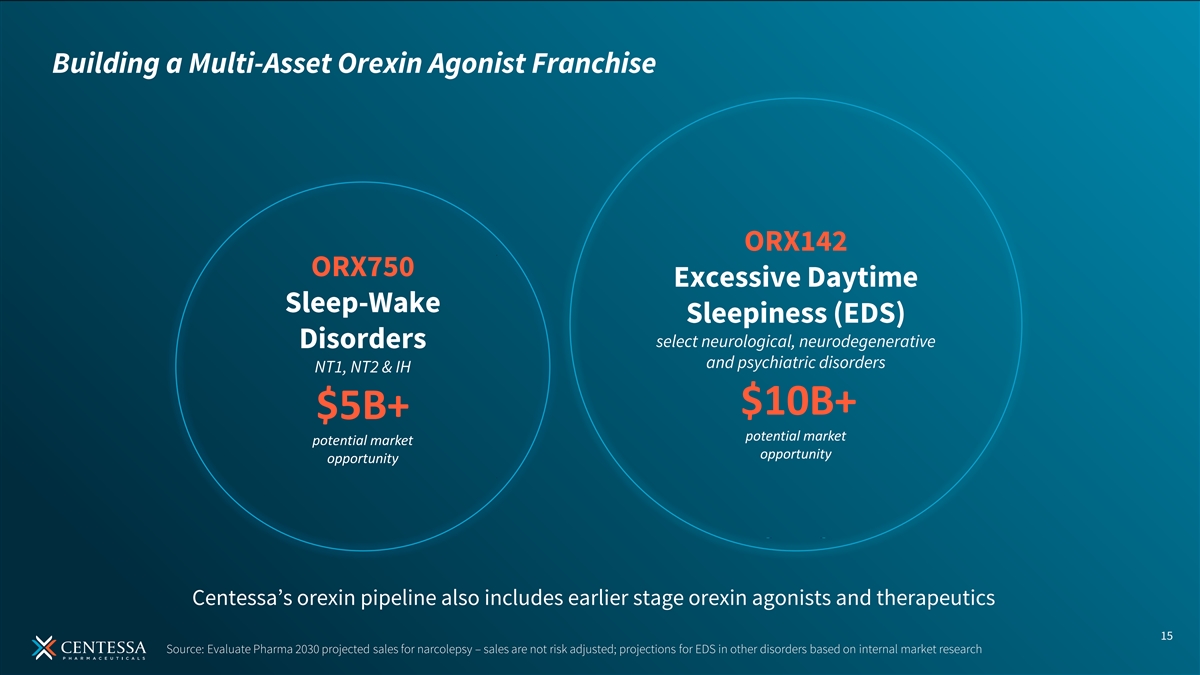
Building a Multi-Asset Orexin Agonist Franchise ORX142 ORX750 Excessive Daytime Sleep-Wake Sleepiness (EDS) select neurological, neurodegenerative Disorders and psychiatric disorders NT1, NT2 & IH $10B+ $5B+ potential market potential market opportunity opportunity Centessa’s orexin pipeline also includes earlier stage orexin agonists and therapeutics 15 Source: Evaluate Pharma 2030 projected sales for narcolepsy – sales are not risk adjusted; projections for EDS in other disorders based on internal market research

Plan to rapidly advance ORX750 into Phase 2 studies in patients with NT1, NT2 and IH beginning in Q4 of 2024 ORX142 preclinical data to be presented at Sleep Europe 2024 16

LockBody Orexin Agonist Hemophilia Technology Program Program Platform 17

Hemophilia B: Large Growing Market with Unmet Need A safe, subcutaneous and effective treatment has the potential to transform care for hemophilia B No subcutaneous treatment option currently ~$2.6B+ 2 available for hemophilia B in the US Hemophilia B 1 Market Limited options for hemophilia B with 2 inhibitors 1. Evaluate Pharma Analyst Consensus for 2023. 2. In Canada, a subcutaneous option is available for hemophilia B with inhibitors. 18

Novel mechanism of action SerpinPC has the potential to be a first-in-class 1 Showed significant reduction in bleeding subcutaneous therapy with a differentiated safety Shown to have a favorable safety and well profile for people with 1 tolerated profile to date; No thrombosis hemophilia B 1 observed to date SerpinPC is an investigational serine protease inhibitor (SERPIN) engineered to specifically inhibit activated protein C (APC), that has not been approved by the FDA or any other regulatory authority. ABR is annualized bleed rate. 1. Ongoing Phase 2a Study being conducted in Georgia and Moldova to evaluate safety, tolerability, pharmacokinetics and efficacy of SerpinPC in a population of severe hemophilia A and B subjects not on previous prophylaxis and with a history of frequent bleeding. Part 5: Blood (2023) 142 (Supplement 1): 2619. https://doi.org/10.1182/blood-2023-179969. 19 Part 3-4: Blood (2022) 140 (Supplement 1): 460–461. https://doi.org/10.1182/blood-2022-159631. Additional information on the trial can be accessed at www.clinicaltrials.gov (NCT04073498).
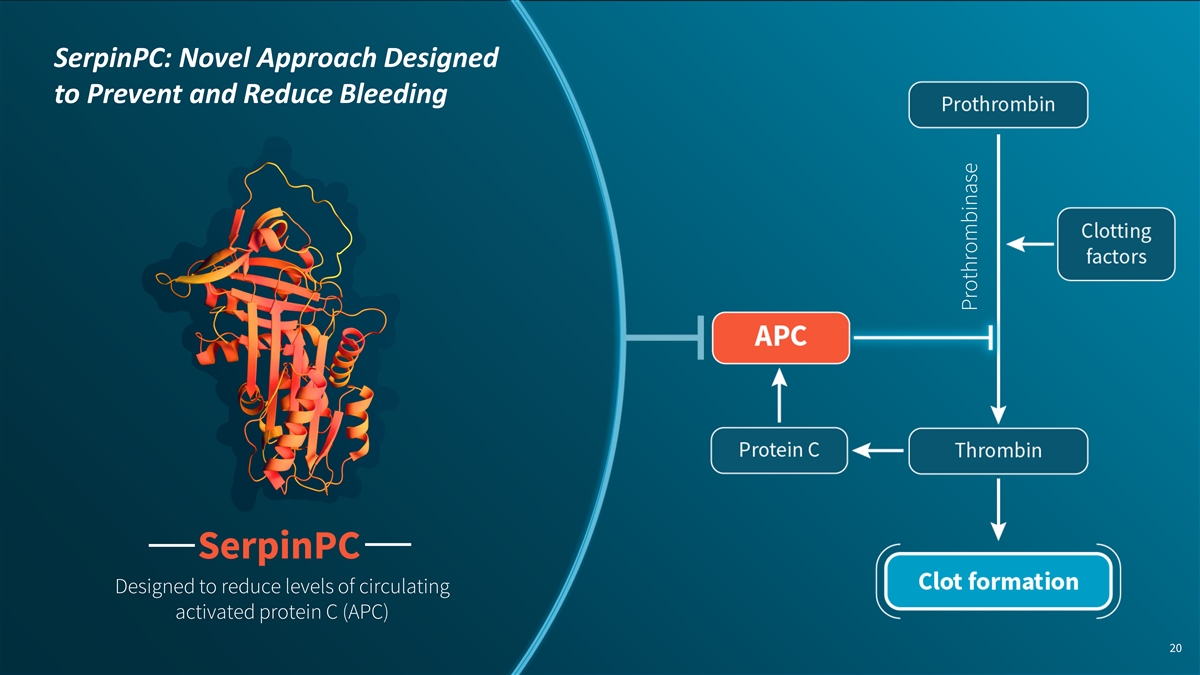
SerpinPC: Novel Approach Designed to Prevent and Reduce Bleeding SerpinPC Designed to reduce levels of circulating activated protein C (APC) 20 Prothrombinase
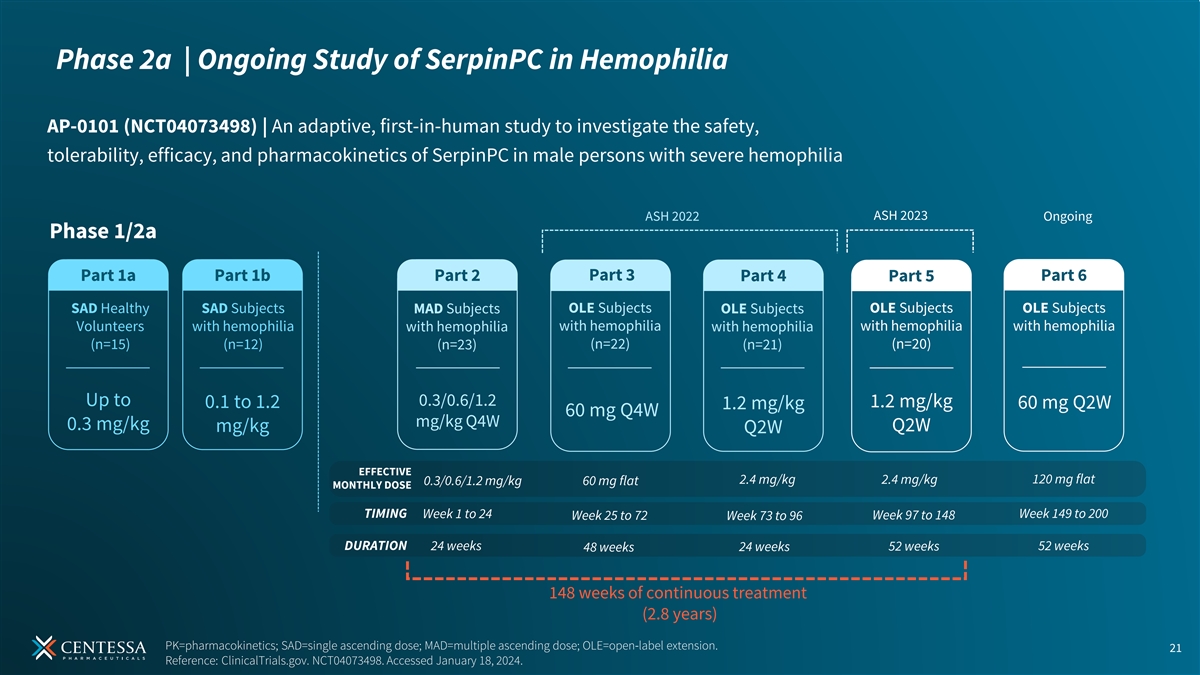
Phase 2a | Ongoing Study of SerpinPC in Hemophilia AP-0101 (NCT04073498) | An adaptive, first-in-human study to investigate the safety, tolerability, efficacy, and pharmacokinetics of SerpinPC in male persons with severe hemophilia ASH 2023 ASH 2022 Ongoing Phase 1/2a Part 1a Part 1b Part 2 Part 3 Part 4 Part 5 Part 6 OLE Subjects OLE Subjects OLE Subjects SAD Healthy SAD Subjects MAD Subjects OLE Subjects Volunteers with hemophilia with hemophilia with hemophilia with hemophilia with hemophilia with hemophilia (n=15) (n=12) (n=22) (n=20) (n=23) (n=21) Up to 0.3/0.6/1.2 1.2 mg/kg 0.1 to 1.2 60 mg Q2W 1.2 mg/kg 60 mg Q4W mg/kg Q4W 0.3 mg/kg Q2W mg/kg Q2W EFFECTIVE 2.4 mg/kg 2.4 mg/kg 120 mg flat 0.3/0.6/1.2 mg/kg 60 mg flat MONTHLY DOSE TIMING Week 1 to 24 Week 149 to 200 Week 25 to 72 Week 73 to 96 Week 97 to 148 DURATION 24 weeks 52 weeks 48 weeks 24 weeks 52 weeks 148 weeks of continuous treatment (2.8 years) PK=pharmacokinetics; SAD=single ascending dose; MAD=multiple ascending dose; OLE=open-label extension. 21 Reference: ClinicalTrials.gov. NCT04073498. Accessed January 18, 2024.
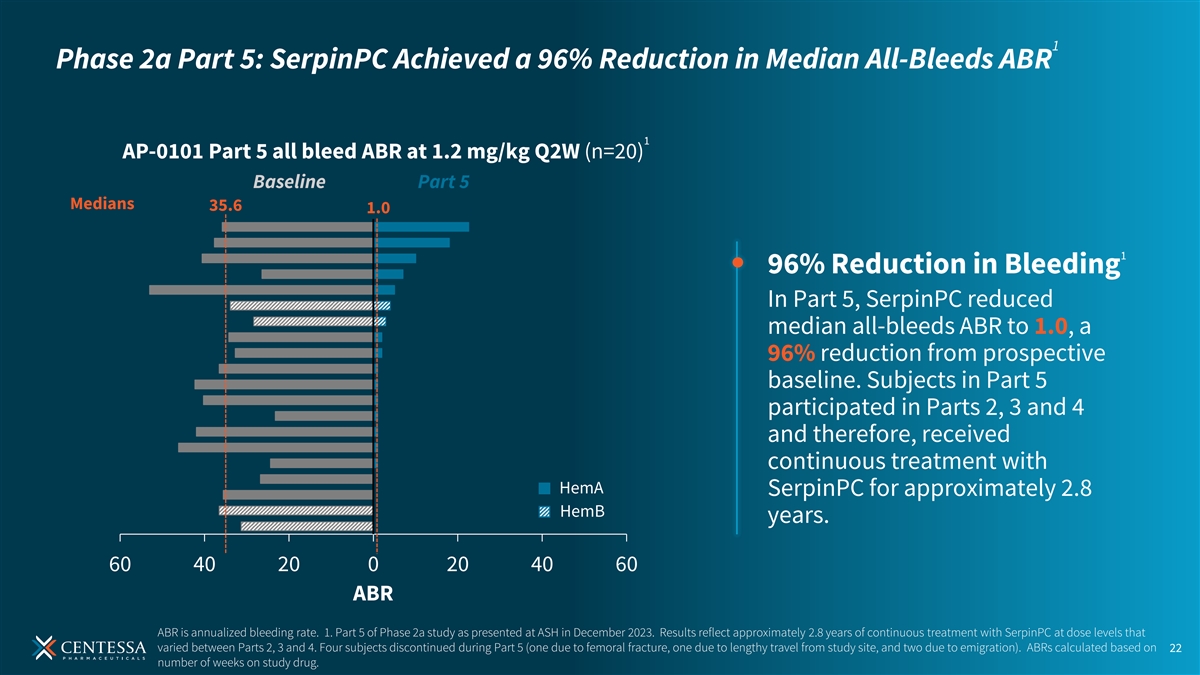
1 Phase 2a Part 5: SerpinPC Achieved a 96% Reduction in Median All-Bleeds ABR 1 AP-0101 Part 5 all bleed ABR at 1.2 mg/kg Q2W (n=20) Baseline Part 5 Medians 35.6 1.0 1 96% Reduction in Bleeding In Part 5, SerpinPC reduced median all-bleeds ABR to 1.0, a 96% reduction from prospective baseline. Subjects in Part 5 participated in Parts 2, 3 and 4 and therefore, received continuous treatment with HemA SerpinPC for approximately 2.8 HemB years. 60 40 20 0 20 40 60 ABR ABR is annualized bleeding rate. 1. Part 5 of Phase 2a study as presented at ASH in December 2023. Results reflect approximately 2.8 years of continuous treatment with SerpinPC at dose levels that varied between Parts 2, 3 and 4. Four subjects discontinued during Part 5 (one due to femoral fracture, one due to lengthy travel from study site, and two due to emigration). ABRs calculated based on 22 number of weeks on study drug.
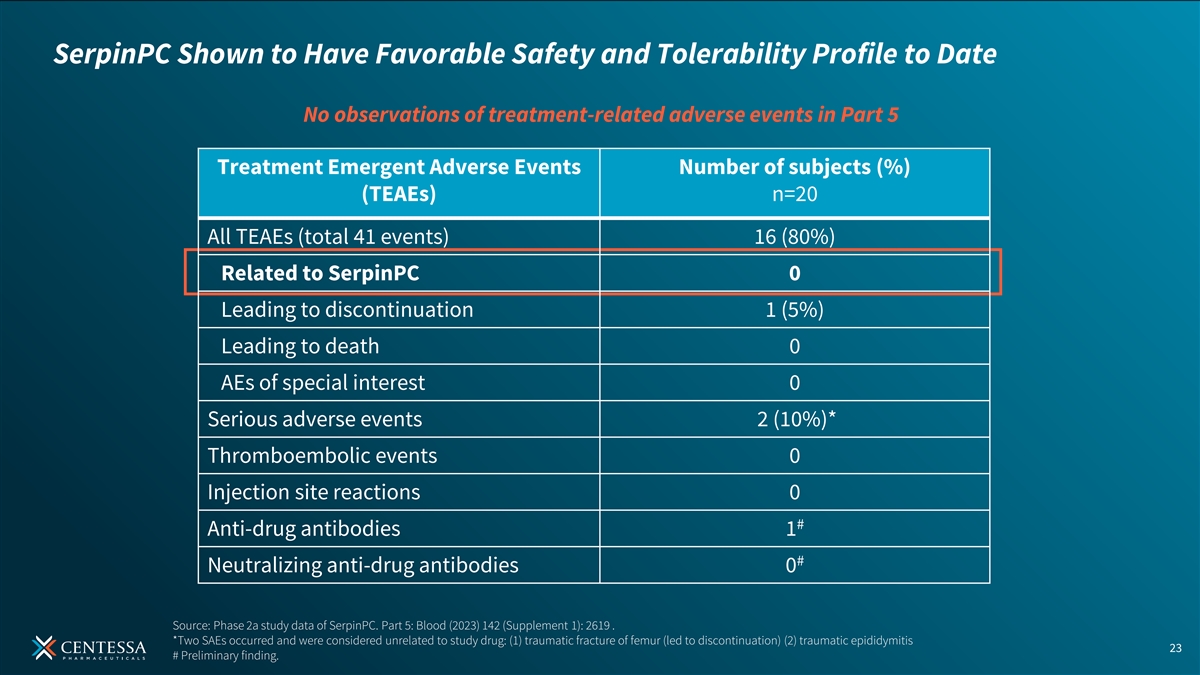
SerpinPC Shown to Have Favorable Safety and Tolerability Profile to Date No observations of treatment-related adverse events in Part 5 Treatment Emergent Adverse Events Number of subjects (%) (TEAEs) n=20 All TEAEs (total 41 events) 16 (80%) Related to SerpinPC 0 Leading to discontinuation 1 (5%) Leading to death 0 AEs of special interest 0 Serious adverse events 2 (10%)* Thromboembolic events 0 Injection site reactions 0 # Anti-drug antibodies 1 # Neutralizing anti-drug antibodies 0 Source: Phase 2a study data of SerpinPC. Part 5: Blood (2023) 142 (Supplement 1): 2619 . *Two SAEs occurred and were considered unrelated to study drug: (1) traumatic fracture of femur (led to discontinuation) (2) traumatic epididymitis 23 # Preliminary finding.
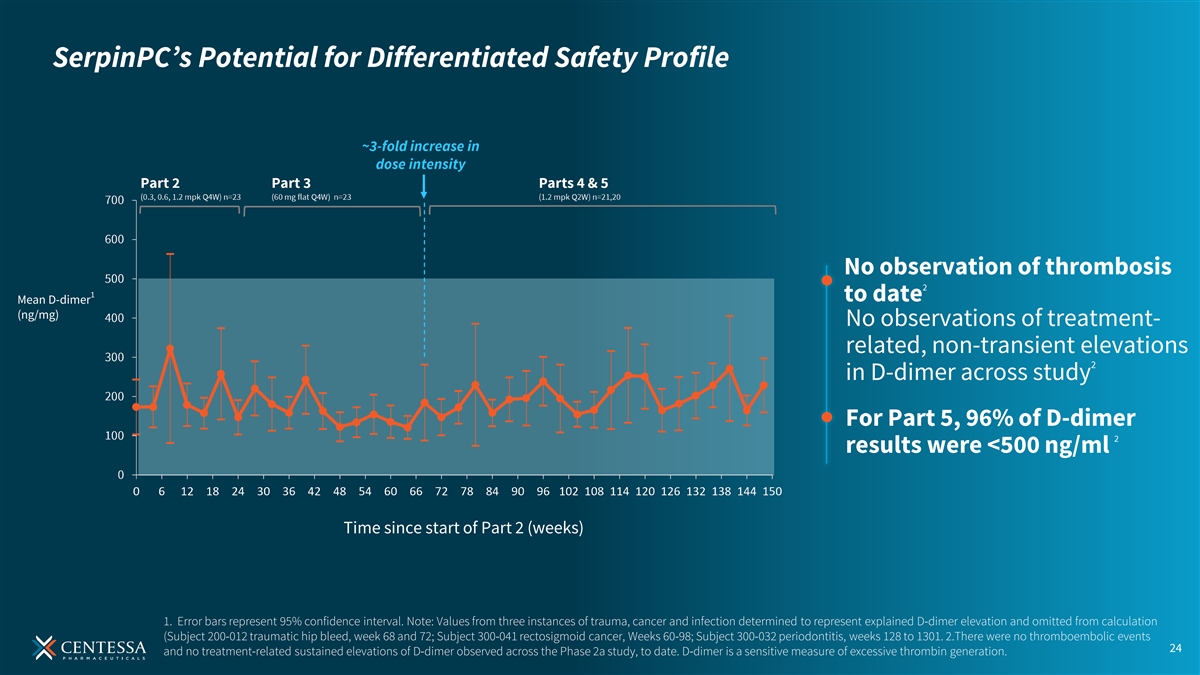
SerpinPC’s Potential for Differentiated Safety Profile No observation of thrombosis 2 1 to date Mean D-dimer (ng/mg) No observations of treatment- related, non-transient elevations 2 in D-dimer across study For Part 5, 96% of D-dimer 2 results were <500 ng/ml Time since start of Part 2 (weeks) 1. Error bars represent 95% confidence interval. Note: Values from three instances of trauma, cancer and infection determined to represent explained D-dimer elevation and omitted from calculation (Subject 200-012 traumatic hip bleed, week 68 and 72; Subject 300-041 rectosigmoid cancer, Weeks 60-98; Subject 300-032 periodontitis, weeks 128 to 1301. 2.There were no thromboembolic events 24 and no treatment-related sustained elevations of D-dimer observed across the Phase 2a study, to date. D-dimer is a sensitive measure of excessive thrombin generation.
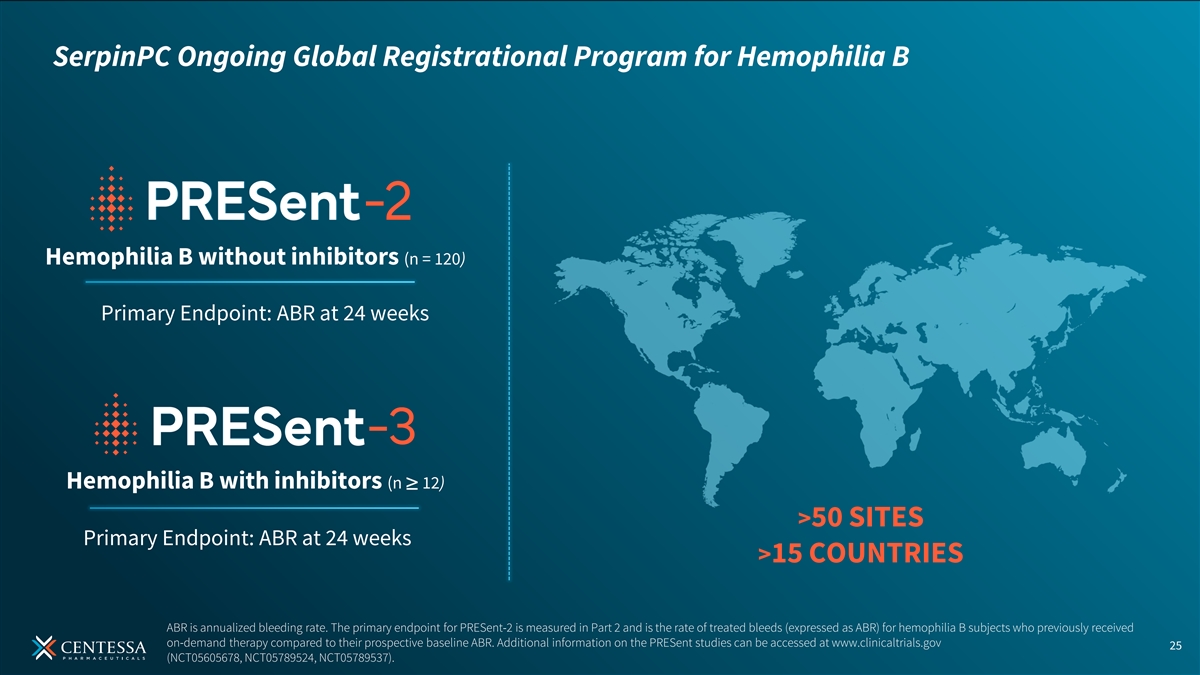
SerpinPC Ongoing Global Registrational Program for Hemophilia B Hemophilia B without inhibitors (n = 120) Primary Endpoint: ABR at 24 weeks Hemophilia B with inhibitors (n ≥ 12) >50 SITES Primary Endpoint: ABR at 24 weeks >15 COUNTRIES ABR is annualized bleeding rate. The primary endpoint for PRESent-2 is measured in Part 2 and is the rate of treated bleeds (expressed as ABR) for hemophilia B subjects who previously received on-demand therapy compared to their prospective baseline ABR. Additional information on the PRESent studies can be accessed at www.clinicaltrials.gov 25 (NCT05605678, NCT05789524, NCT05789537).
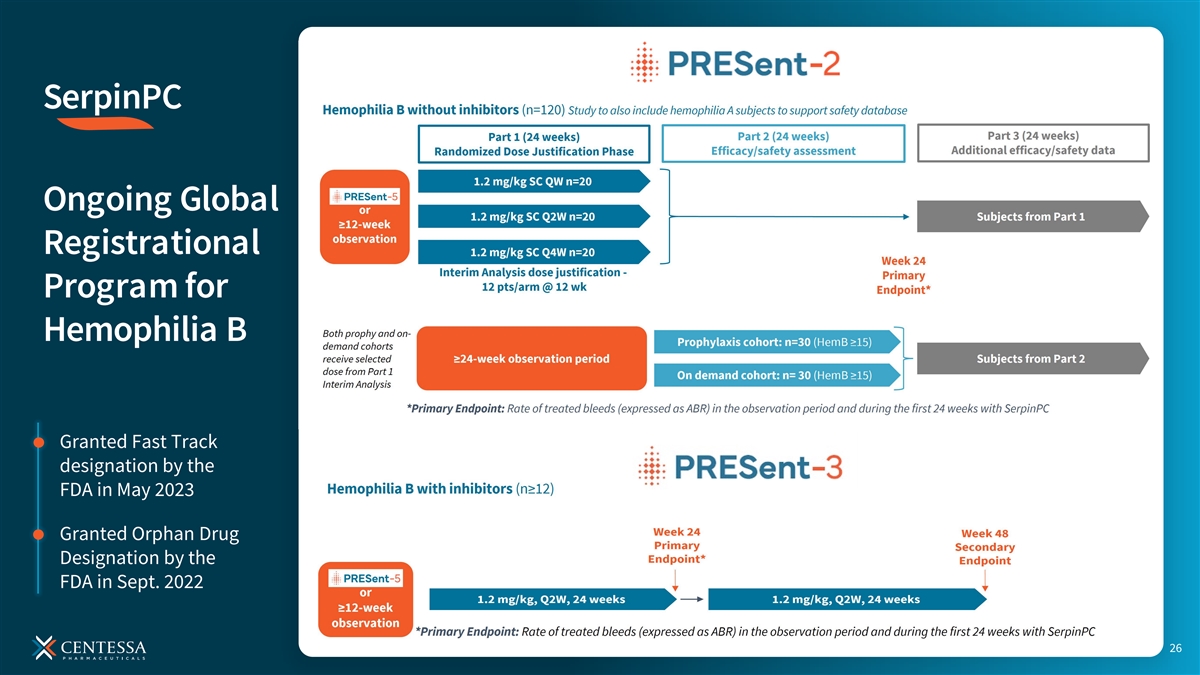
SerpinPC Ongoing Global Registrational Program for Hemophilia B Granted Fast Track designation by the FDA in May 2023 Granted Orphan Drug Designation by the FDA in Sept. 2022 26
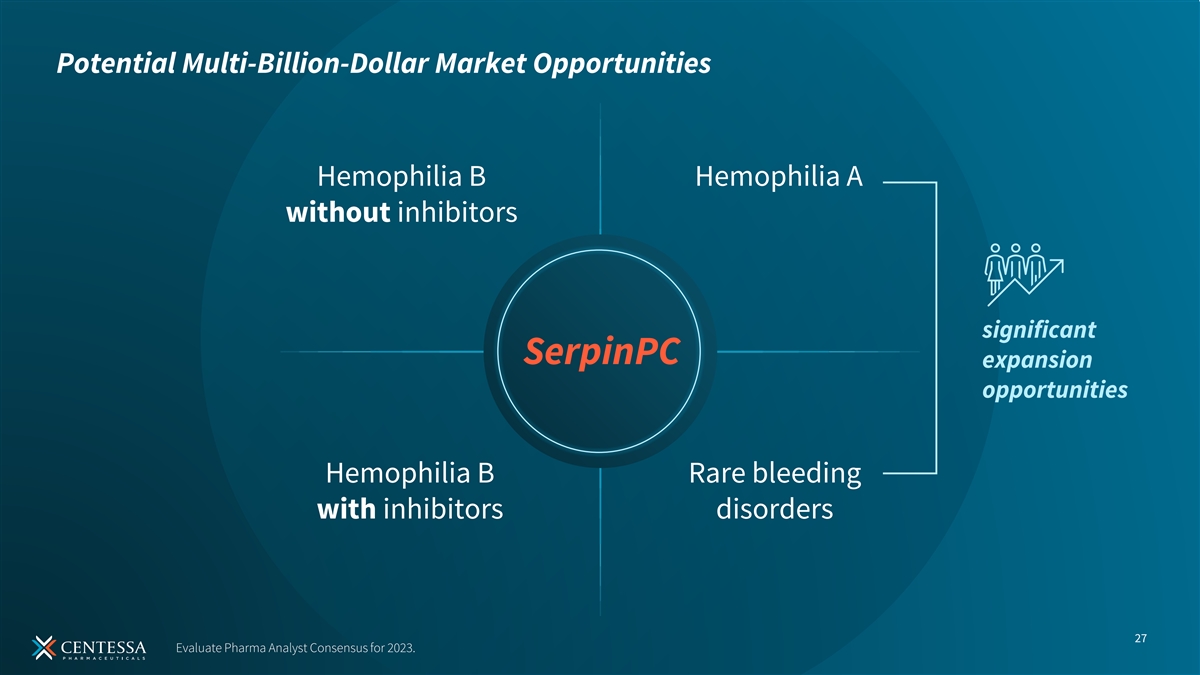
Potential Multi-Billion-Dollar Market Opportunities Hemophilia B Hemophilia A without inhibitors significant SerpinPC expansion opportunities Hemophilia B Rare bleeding with inhibitors disorders 27 Evaluate Pharma Analyst Consensus for 2023.
Orexin Agonist Hemophilia LockBody Program Program Technology Platform 28

Novel pharmacology combining tumor enrichment with activation of effector function LockBody Technology Platform aims to redefine Designed as single agent systemic immuno-oncology treatment treatment 1 Potential wide therapeutic index LB101 is an investigational agent that has not been approved by the FDA or any other regulatory authority. Information on the Phase 1/2a trial of LB101 can be accessed at www.clinicaltrials.gov 29 (NCT05821777). 1. LB101 in-vivo preclinical data: MC38 hPD-L1+ syngeneic model in mouse, and in non-human primates where LB101 was delivered IV at 5, 20, 50mg/kg (q7d x 4).
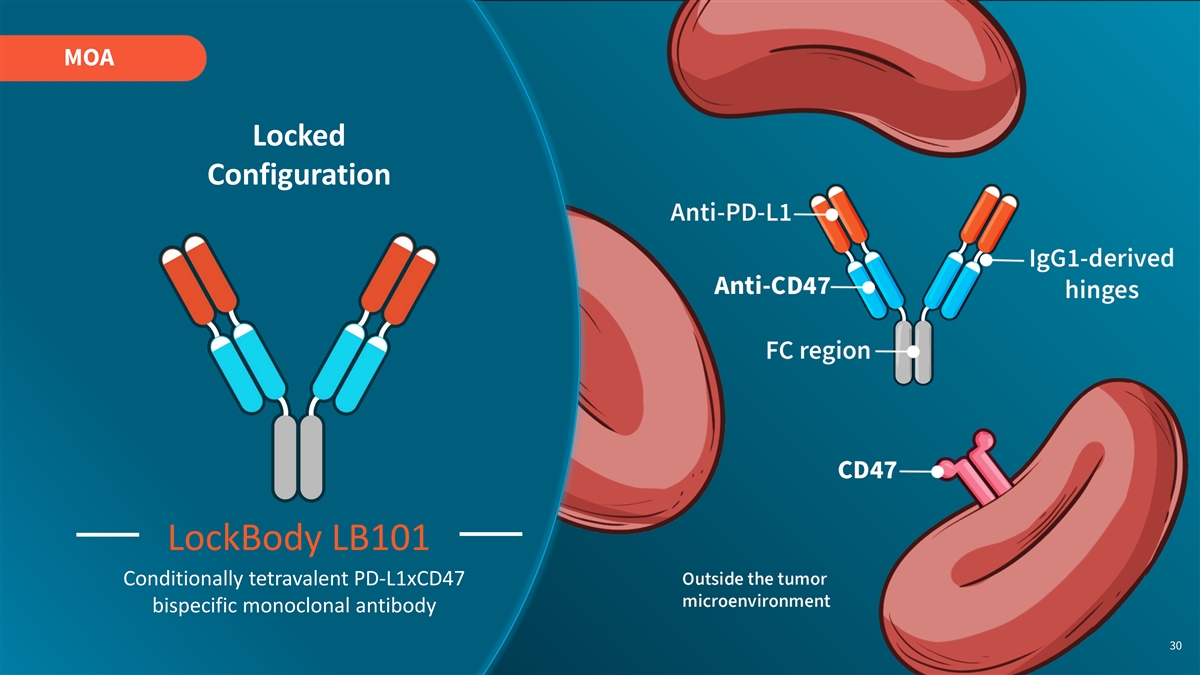
MOA Locked Configuration LockBody LB101 Conditionally tetravalent PD-L1xCD47 bispecific monoclonal antibody 30
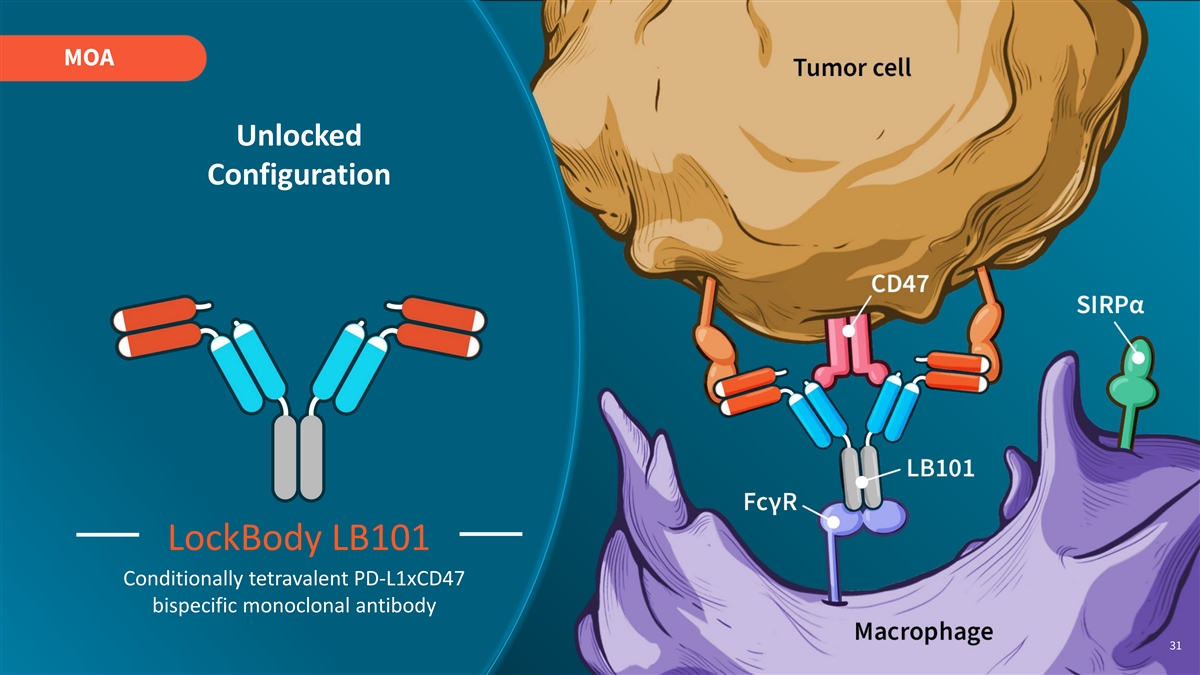
MOA Unlocked Configuration LockBody LB101 Conditionally tetravalent PD-L1xCD47 bispecific monoclonal antibody 31
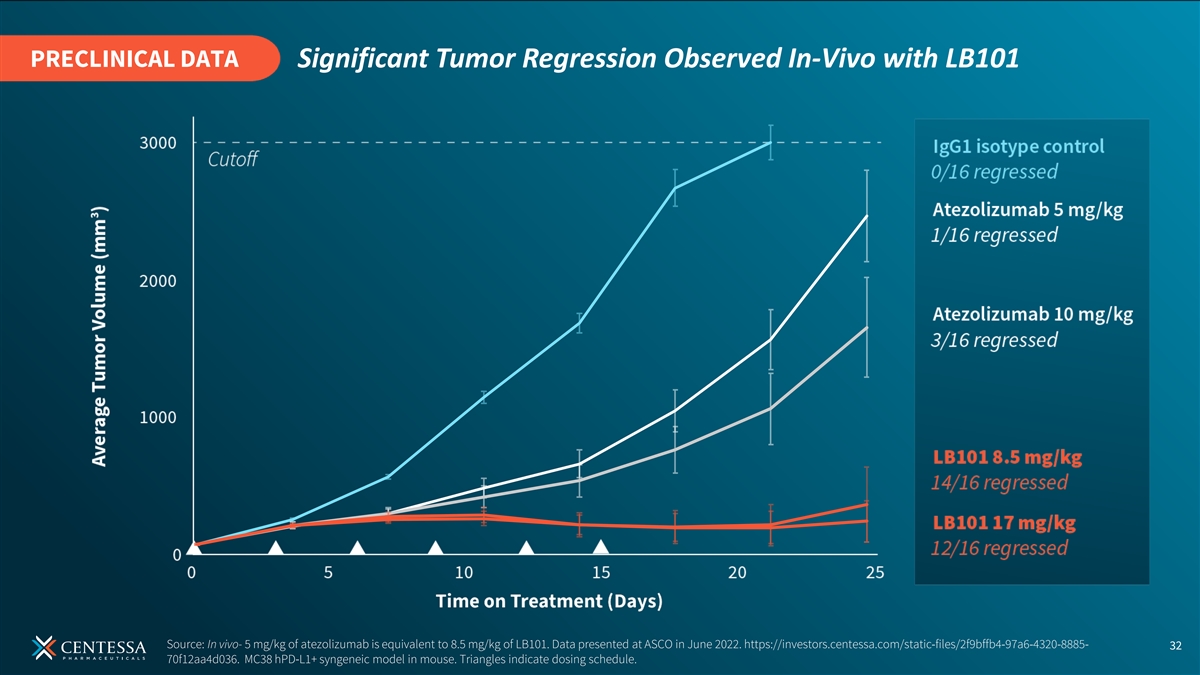
PRECLINICAL DATA Significant Tumor Regression Observed In-Vivo with LB101 Source: In vivo- 5 mg/kg of atezolizumab is equivalent to 8.5 mg/kg of LB101. Data presented at ASCO in June 2022. https://investors.centessa.com/static-files/2f9bffb4-97a6-4320-8885- 32 70f12aa4d036. MC38 hPD-L1+ syngeneic model in mouse. Triangles indicate dosing schedule.

PRECLINICAL DATA Observed to be Well Tolerated in Non-Human Primates (NHPs) with LB101 Doses up to 50mg/kg No anemia/ No weight loss No change in red blood thrombocytopenia cell or hemoglobin 33 Source: In-vivo- LB101 delivered IV at 5, 20, 50mg/kg (q7d x 4) in non-human primates.
LB101 is in an ongoing Phase 1/2a first-in-human clinical trial 34

OREXIN AGONIST PROGRAM ORX750 Plan to rapidly advance into Phase 2 studies in patients with NT1, NT2, and IH beginning in Q4 of 2024 ORX142 Preclinical data to be presented at Sleep Europe 2024 2024 Driving HEMOPHILIA PROGRAM Momentum SerpinPC ANTICIPATED MILESTONES PRESent-2 Part 1 interim analysis planned in 2024; Part 1 data planned for late 2024/early 2025 LOCKBODY TECHNOLOGY PLATFORM LB101 Phase 1/2 study ongoing 35
OUR MISSION Discovering and developing medicines that are transformational for patients Multiple potential blockbuster assets Ongoing momentum in 2024 with clinical milestones anticipated across our most advanced programs Strong balance sheet Centessa reported $294.8 million in cash, cash equivalents and short-term investments as of June 30, 2024. Cash runway into mid-2026. 36

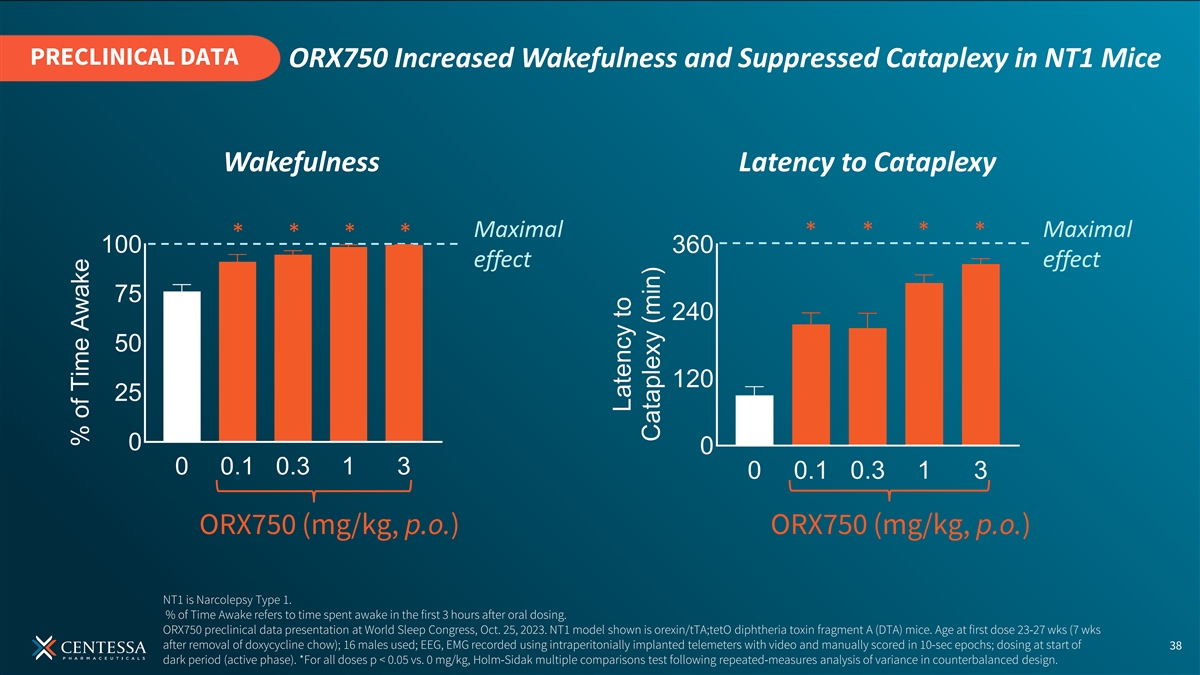
PRECLINICAL DATA ORX750 Increased Wakefulness and Suppressed Cataplexy in NT1 Mice Wakefulness Latency to Cataplexy Maximal Maximal effect effect ORX750 (mg/kg, p.o.) ORX750 (mg/kg, p.o.) NT1 is Narcolepsy Type 1. % of Time Awake refers to time spent awake in the first 3 hours after oral dosing. ORX750 preclinical data presentation at World Sleep Congress, Oct. 25, 2023. NT1 model shown is orexin/tTA;tetO diphtheria toxin fragment A (DTA) mice. Age at first dose 23-27 wks (7 wks after removal of doxycycline chow); 16 males used; EEG, EMG recorded using intraperitonially implanted telemeters with video and manually scored in 10-sec epochs; dosing at start of 38 dark period (active phase). *For all doses p < 0.05 vs. 0 mg/kg, Holm-Sidak multiple comparisons test following repeated-measures analysis of variance in counterbalanced design.
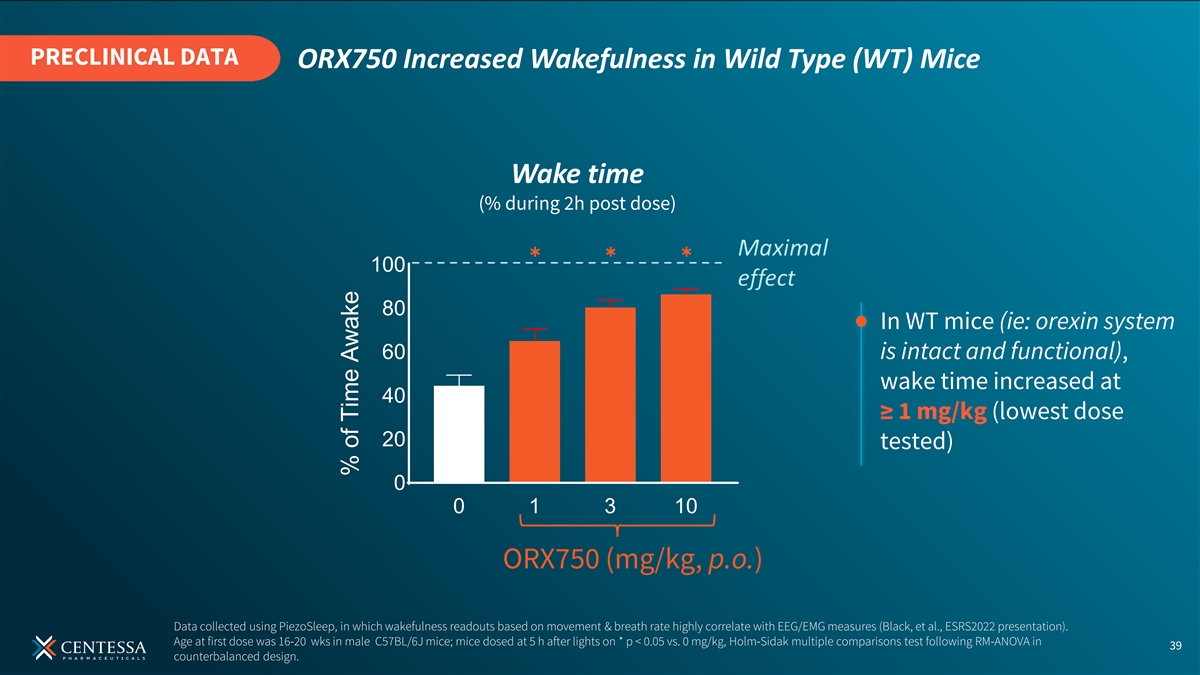
PRECLINICAL DATA ORX750 Increased Wakefulness in Wild Type (WT) Mice Wake time (% during 2h post dose) ✱✱✱ 100 80 In WT mice (ie: orexin system 60 is intact and functional), wake time increased at 40 ≥ 1 mg/kg (lowest dose 20 tested) 0 0 1 3 10 ORX750 (mg/kg, p.o.) Data collected using PiezoSleep, in which wakefulness readouts based on movement & breath rate highly correlate with EEG/EMG measures (Black, et al., ESRS2022 presentation). Age at first dose was 16-20 wks in male C57BL/6J mice; mice dosed at 5 h after lights on * p < 0.05 vs. 0 mg/kg, Holm-Sidak multiple comparisons test following RM-ANOVA in 39 counterbalanced design. % of Time Awake






































