
SerpinPC in persons with severe hemophilia (PwH): long-term treatment from a multi- center, multi-part, first-in-human study T Baglin*, A Koch, I Mocanu+, L Makhaldiani$, J Huntington* *Centessa Pharmaceuticals plc, 1 Ashley Road, Altrincham, Cheshire, United Kingdom, WA14 2DT, Simbec-Orion Clinical Pharmacology, Merthyr Tydfil, CF48 4DR, United Kingdom, +Arensia Exploratory Medicine, Testemitanu Str. 30, Chisinau, Republic of Moldova, $Arensia Exploratory Medicine, 13a Tevdore Mgvdeli Str. 0112, Tbilisi, Georgia

This presentation has been prepared by Centessa Pharmaceuticals plc (the “Company”) for informational purposes only and not for any other purpose. This presentation does not contain all the information that is or may be material to investors or potential investors and should not be considered as advice or a recommendation to investors or potential investors in respect of the holding, purchasing or selling of securities or other financial instruments and does not take into account any investor’s particular objectives, financial situation or needs. The communication of this presentation may be restricted by law; it is not intended for distribution to, or use by any person in, any jurisdiction where such distribution or use would be contrary to local law or regulation. This presentation is not directed to or intended for distribution, or transfer, either directly or indirectly to, or use by, any person or entity that is a citizen or resident or located in any locality, state, country or other jurisdiction where such distribution, transfer, publication, availability or use would be contrary to law or regulation or which would require any registration or licensing within such jurisdiction. This presentation may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements, including, without limitation, statements related to the Company’s ability to deliver impactful medicines to patients; the ability of our key executives to drive execution of the Company’s portfolio of programs; our asset-centric business model and the intended advantages and benefits thereof; research and clinical development plans; the scope, progress, results and costs of developing our product candidates or any other future product candidates; the development and therapeutic potential of our product candidates, including SerpinPC; strategy; regulatory matters, including the timing and likelihood of success of obtaining approvals to initiate or continue clinical trials or market any products; market size and opportunity for our product candidates; and our anticipated cash runway. Words such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” “aim,” “seek,” and variations of these words or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the beliefs of the Company's management as well as assumptions made by and information currently available to the Company. Such statements reflect the current views of the Company with respect to future events and are subject to known and unknown risks, including, without limitation, risks related to our ability to protect and maintain our intellectual property position; business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about the Company; risks inherent in developing products and technologies; future results from our ongoing and planned clinical trials; our ability to obtain adequate financing, including through our financing facility with Oberland, to fund our planned clinical trials and other expenses; trends in the industry; the legal and regulatory framework for the industry, including the receipt and maintenance of clearances to conduct or continue clinical testing; future expenditures risks related to our asset-centric corporate model; the risk that any one or more of our product candidates will not be successfully developed and commercialized; the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies; and risks related to the COVID-19 pandemic including the effects of the Delta, Omicron and any other variants, geo-political risks such as the Russia-Ukraine conflict and other risk factors contained in our filings with the U.S. Securities and Exchange Commission. In light of these risks and uncertainties, the events or circumstances referred to in the forward-looking statements may not occur. The actual results may vary from the anticipated results and the variations may be material. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that the assumptions on which such forward looking statements have been made are correct or exhaustive or, in the case of the assumptions, fully stated in this presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. All projections, valuations and statistical analyses are provided for information purposes only. They may be based on subjective assessments and assumptions and may use one among alternative methodologies that produce different results and to the extent they are based on historical information, they should not be relied upon as an accurate prediction of future performance. This presentation discusses product candidates that are under clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration or any other regulatory agency. No representation or warranty, express or implied, is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation or warranty, express or implied, as to the adequacy, fairness, accuracy or completeness of, any information obtained from third party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. Disclaimer 2

SerpinPC: a subcutaneously administered biologic inhibitor of APC 3 • Unprecedented biology with novel pharmacology • Intended for subcutaneous prophylaxis across hemophilia subtypes • Modified a1 anti-trypsin with 3 substitution mutations to confer selective inhibition of activated protein C (APC) • Prevents bleeds by inhibiting APC to prolong prothrombinase activity and allow sufficient thrombin generation in the absence of intrinsic tenase 3D-model of SerpinPC* * SerpinPC is an investigational agent that has not been approved by the FDA or any other regulatory authority

SerpinPC and thrombin generation 4 Extrinsic Tenase Intrinsic Tenase Prothrombinase Prothrombin Thrombin SerpinPC APC Protein C
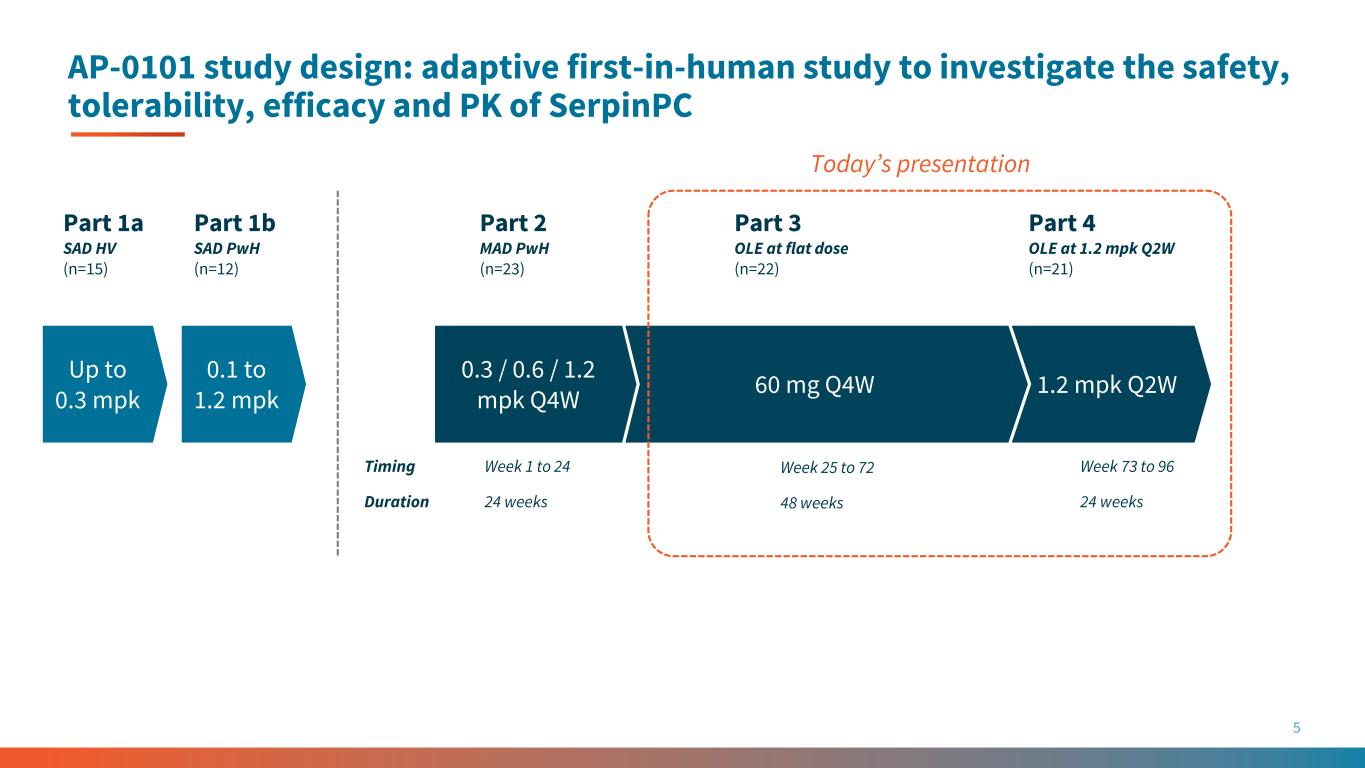
1.2 mpk Q2W AP-0101 study design: adaptive first-in-human study to investigate the safety, tolerability, efficacy and PK of SerpinPC 5 60 mg Q4W Part 2 MAD PwH (n=23) Part 3 OLE at flat dose (n=22) Part 4 OLE at 1.2 mpk Q2W (n=21) Part 1b SAD PwH (n=12) Week 1 to 24 Week 25 to 72 Week 73 to 96Timing 0.3 / 0.6 / 1.2 mpk Q4W 24 weeks 48 weeks 24 weeksDuration Today’s presentation Up to 0.3 mpk 0.1 to 1.2 mpk Part 1a SAD HV (n=15)

AP-0101 Parts 2-4: demographics, baseline characteristics and early terminations 6 * “Target joint” = joint with >3 bleeds in any 6-month period ** Determined by Safety Review Group Characteristic Value Age, median (min to max) 39 (21 to 56) Number of subjects 23 (including 12 from Part 1b SAD) Prospective baseline Annualized Bleed Rate (ABR), median (min to max) 34.1 (22.8 to 53.0) % subjects receiving previous prophylaxis 0% % subjects with target joints* 100% No. of target joints, median (min. to max.) 2.5 (1 to 4) Part Early termination Part 2 1 subject due to skin-rash – treatment-related** Part 3 1 subject due to emigration to another country Part 4 1 subject due to recto-sigmoid cancer – not related to treatment** Demographics and baseline characteristics Early terminations
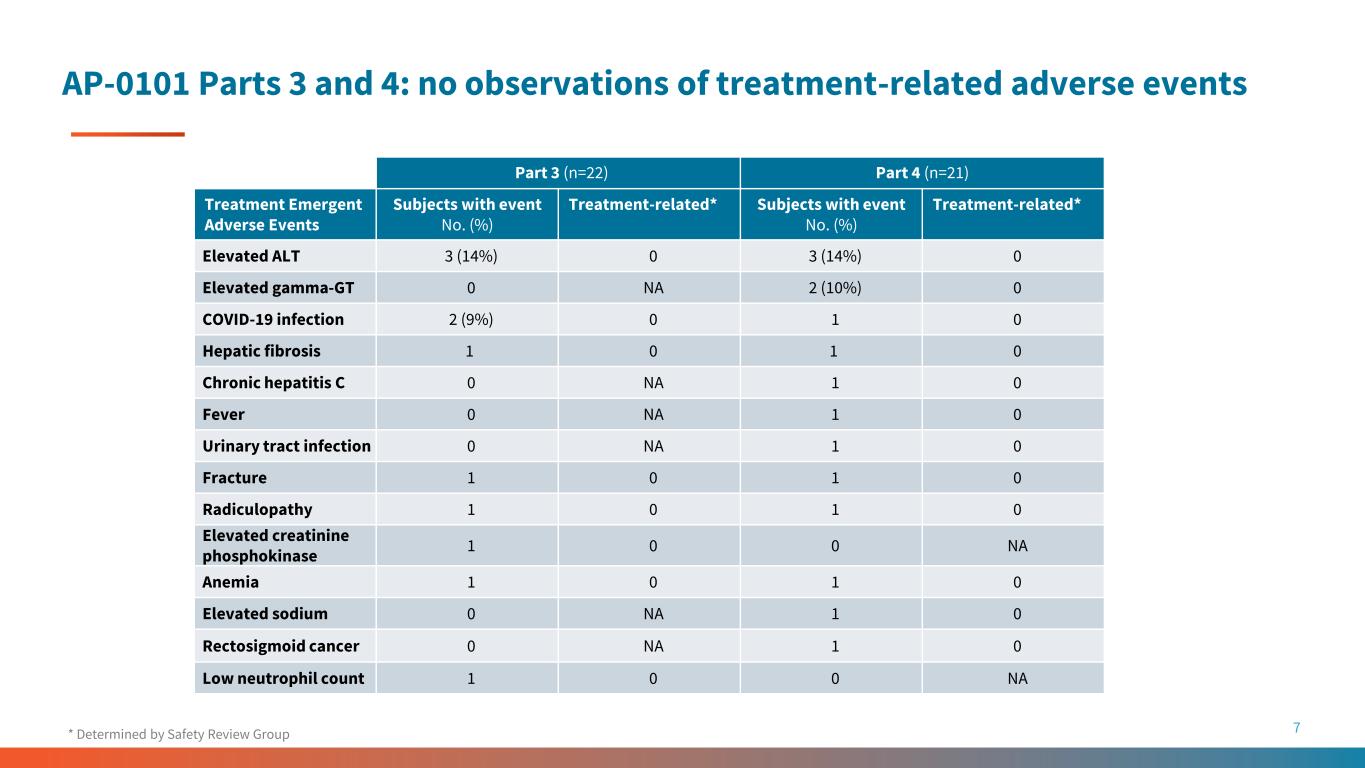
AP-0101 Parts 3 and 4: no observations of treatment-related adverse events 7* Determined by Safety Review Group Part 3 (n=22) Part 4 (n=21) Treatment Emergent Adverse Events Subjects with event No. (%) Treatment-related* Subjects with event No. (%) Treatment-related* Elevated ALT 3 (14%) 0 3 (14%) 0 Elevated gamma-GT 0 NA 2 (10%) 0 COVID-19 infection 2 (9%) 0 1 0 Hepatic fibrosis 1 0 1 0 Chronic hepatitis C 0 NA 1 0 Fever 0 NA 1 0 Urinary tract infection 0 NA 1 0 Fracture 1 0 1 0 Radiculopathy 1 0 1 0 Elevated creatinine phosphokinase 1 0 0 NA Anemia 1 0 1 0 Elevated sodium 0 NA 1 0 Rectosigmoid cancer 0 NA 1 0 Low neutrophil count 1 0 0 NA
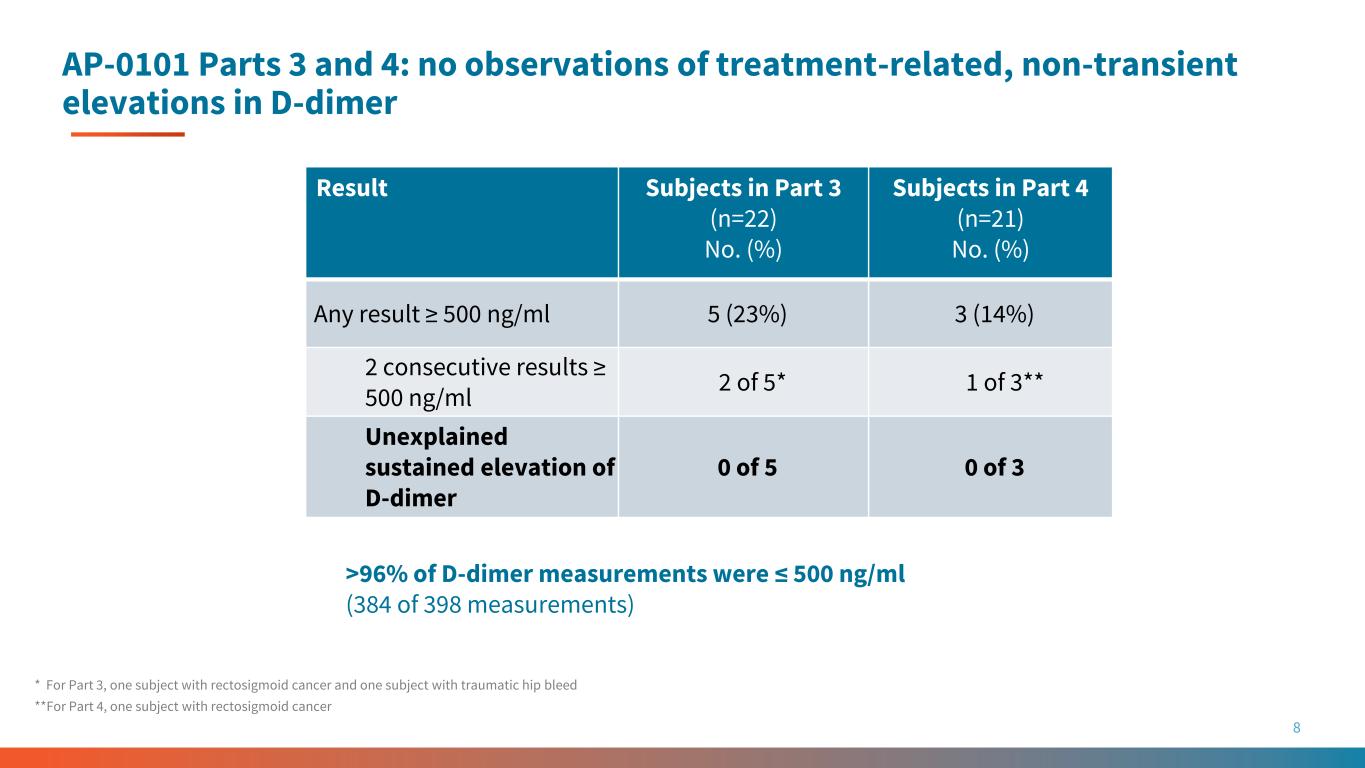
AP-0101 Parts 3 and 4: no observations of treatment-related, non-transient elevations in D-dimer 8 * For Part 3, one subject with rectosigmoid cancer and one subject with traumatic hip bleed **For Part 4, one subject with rectosigmoid cancer Result Subjects in Part 3 (n=22) No. (%) Subjects in Part 4 (n=21) No. (%) Any result ≥ 500 ng/ml 5 (23%) 3 (14%) 2 consecutive results ≥ 500 ng/ml 2 of 5* 1 of 3** Unexplained sustained elevation of D-dimer 0 of 5 0 of 3 >96% of D-dimer measurements were ≤ 500 ng/ml (384 of 398 measurements)
AP-0101: Anti-drug Antibodies (ADAs) and Pharmacokinetics (PK) 9 • Samples for ADA characterization including neutralizing capacity and cross-reactivity ongoing • PK analysis ongoing, including exposure-response modeling
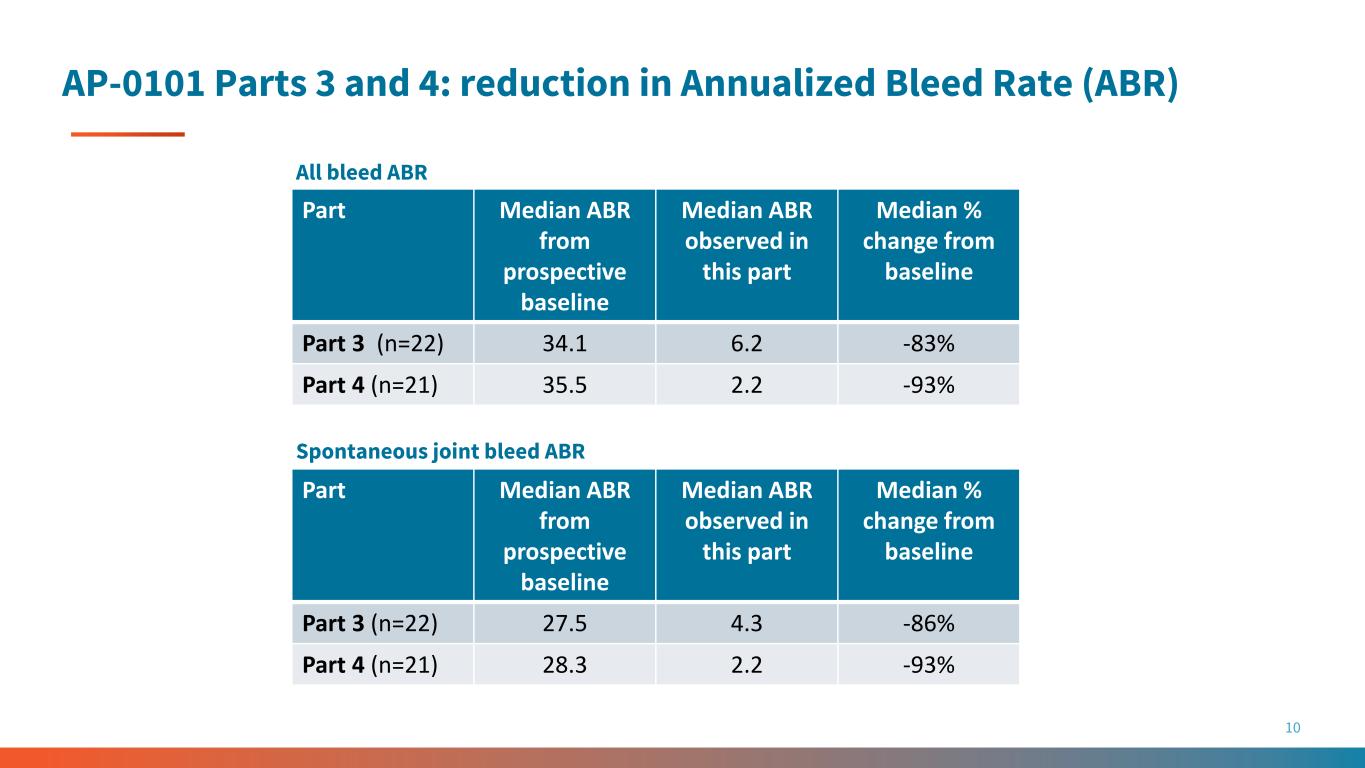
AP-0101 Parts 3 and 4: reduction in Annualized Bleed Rate (ABR) 10 Part Median ABR from prospective baseline Median ABR observed in this part Median % change from baseline Part 3 (n=22) 34.1 6.2 -83% Part 4 (n=21) 35.5 2.2 -93% Part Median ABR from prospective baseline Median ABR observed in this part Median % change from baseline Part 3 (n=22) 27.5 4.3 -86% Part 4 (n=21) 28.3 2.2 -93% All bleed ABR Spontaneous joint bleed ABR

11 HemA HemB AP-0101 Part 3: ABR at 60mg Q4W flat dose All bleeds ABR Spontaneous joint bleeds ABR Medians Part 3 48 weeks of treatment Baseline -60 -40 -20 0 20 40 60 ABR 4.327.5Medians Baseline Part 3 48 weeks of treatment -60 -40 -20 0 20 40 60 ABR 6.234.1
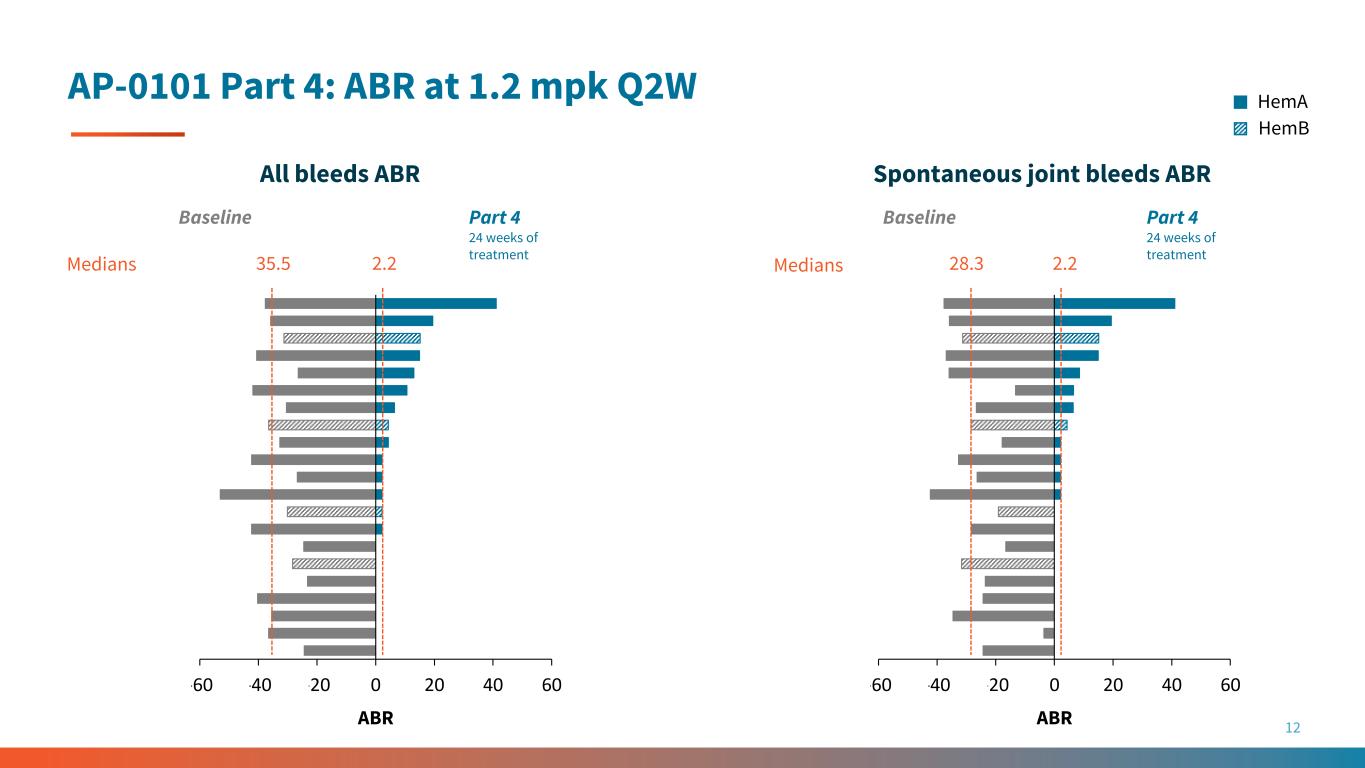
12 HemA HemB AP-0101 Part 4: ABR at 1.2 mpk Q2W -60 -40 -20 0 20 40 60 2.2 28.3 2.2 -60 -40 -20 0 20 40 60 ABR ABR All bleeds ABR Spontaneous joint bleeds ABR Baseline Part 4 24 weeks of treatment Part 4 24 weeks of treatment Baseline 35.5Medians Medians
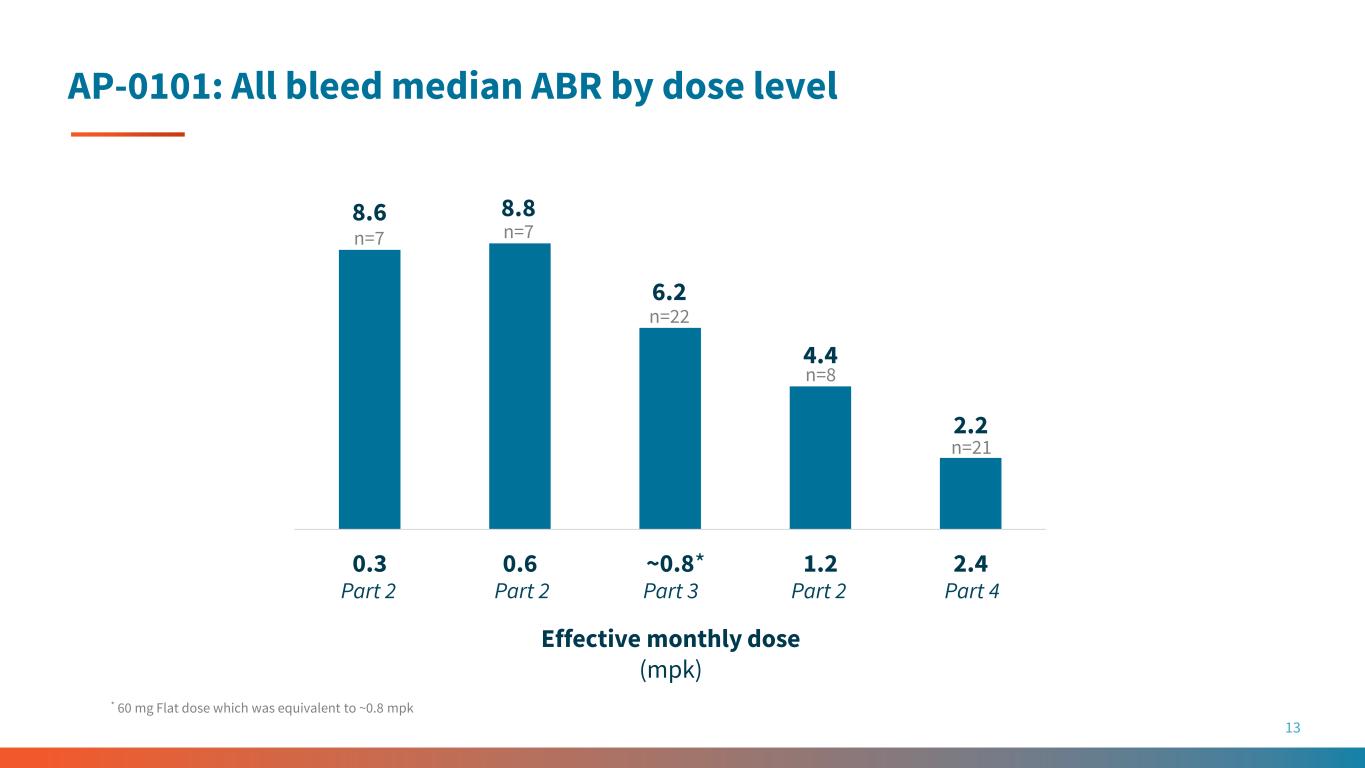
AP-0101: All bleed median ABR by dose level 13 8.6 8.8 6.2 4.4 2.2 0.3 0.6 ~0.8 1.2 2.4 Effective monthly dose (mpk) * 60 mg Flat dose which was equivalent to ~0.8 mpk * n=7n=7 n=8 n=22 n=21 Part 2 Part 2Part 2 Part 3 Part 4

Summary 14 • SerpinPC – Novel MoA: inhibition of APC to rebalance coagulation – Broad potential to treat all subtypes of hemophilia – Subcutaneous route of administration • Results of Phase 2, Parts 3 and 4 – No observations of treatment-related adverse events – No observations of treatment-related sustained elevations of D-dimer – All bleed median ABR of 2.2 (median percentage reduction from baseline of 93%) in Part 4

15 Thank you to all the persons who have and continue to participate in this study














