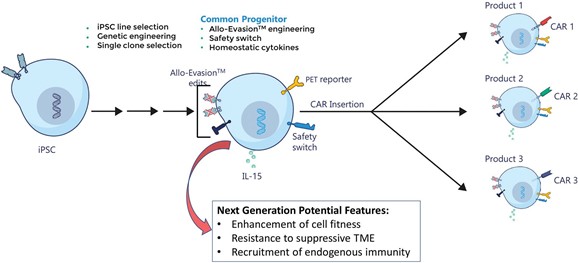
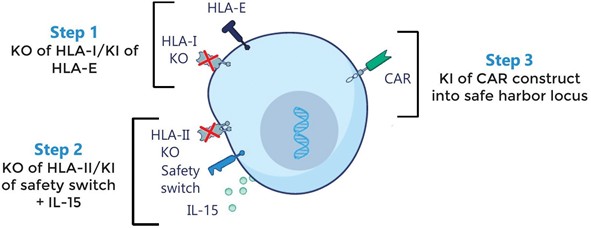
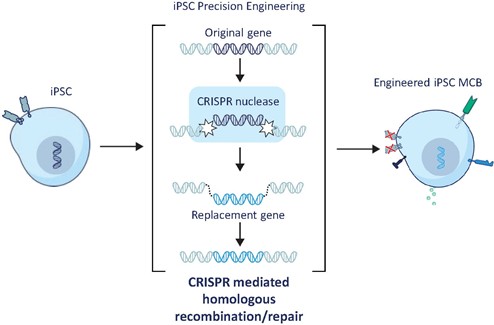
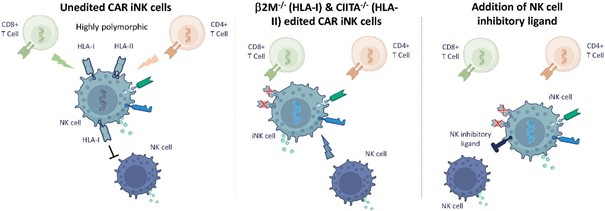
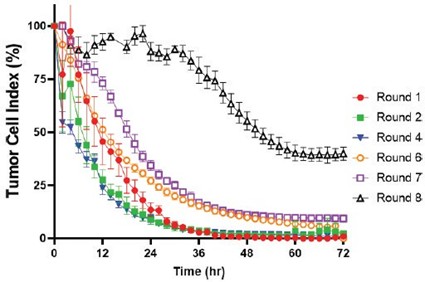
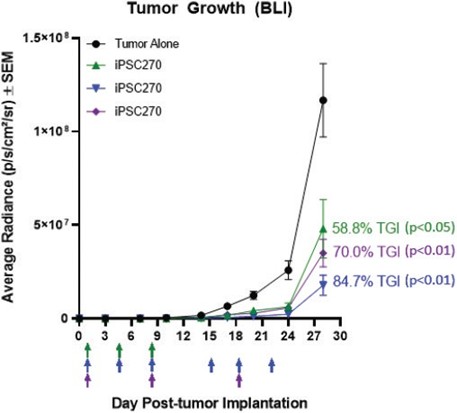
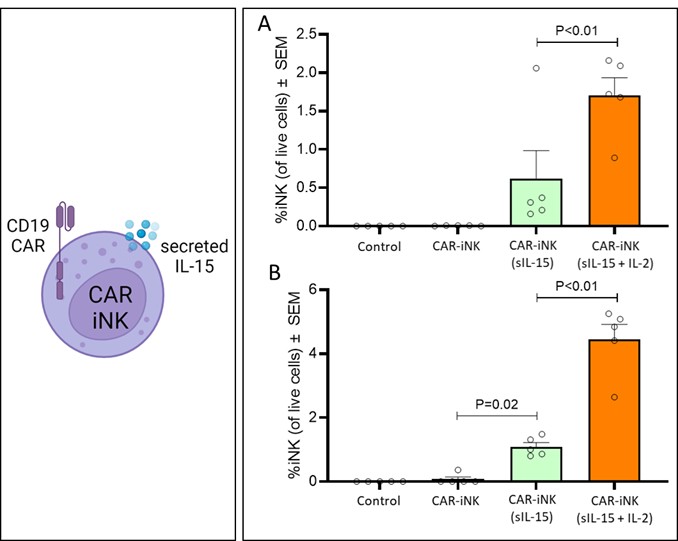
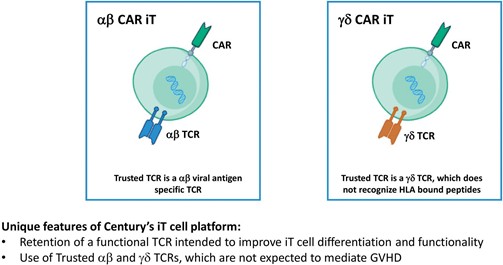
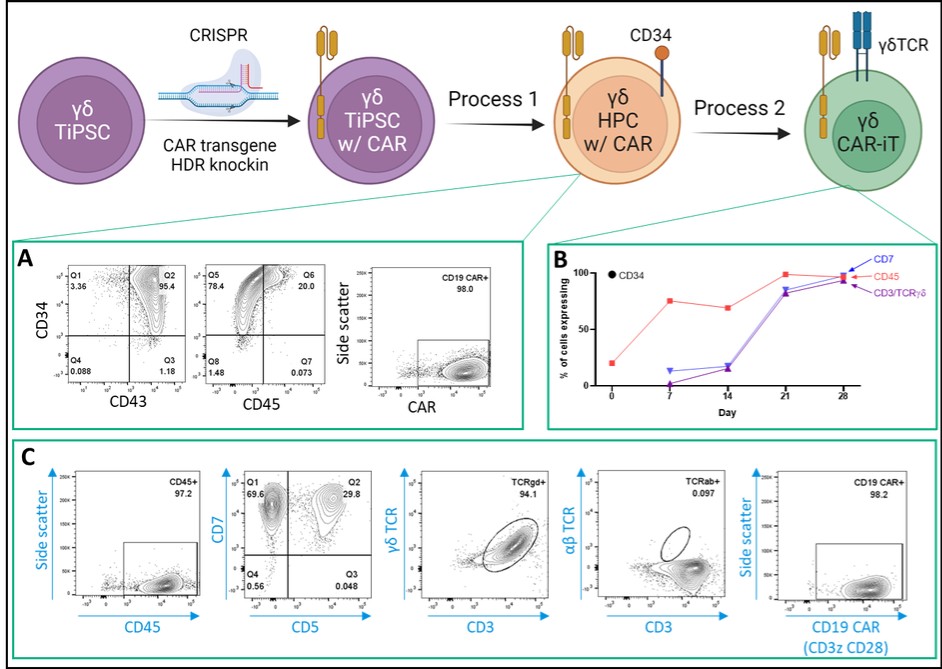
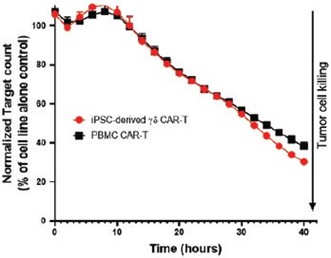
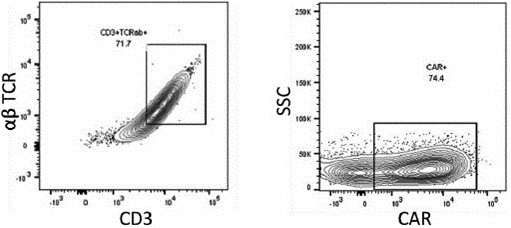
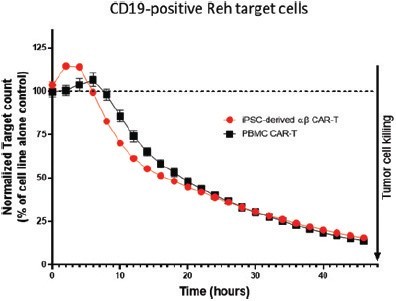
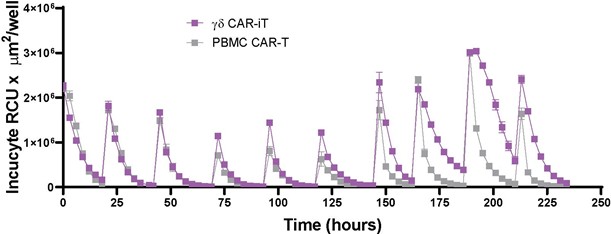
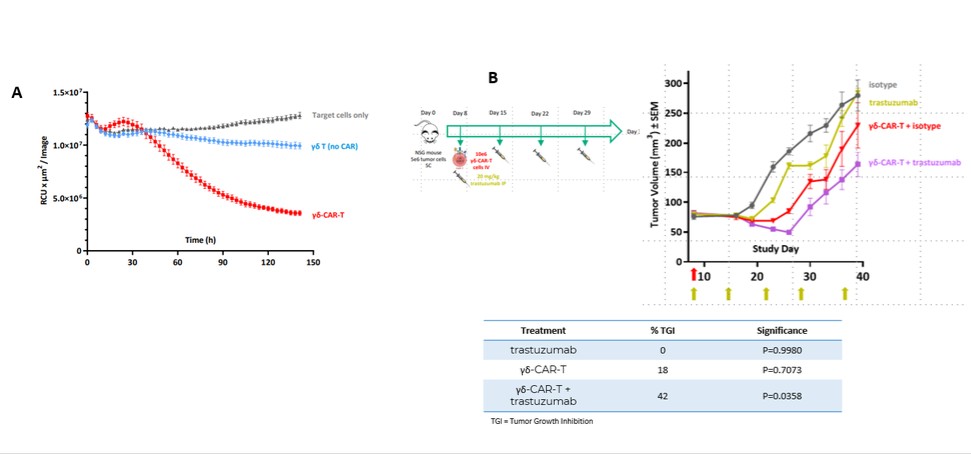
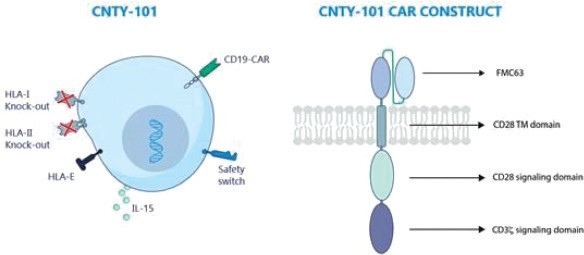
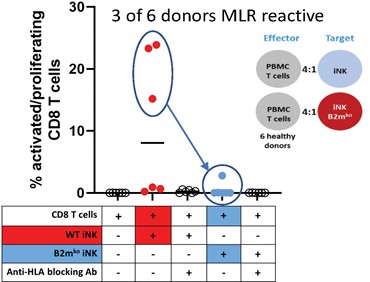
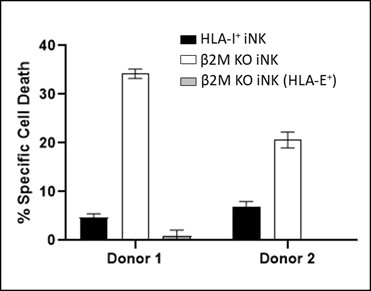
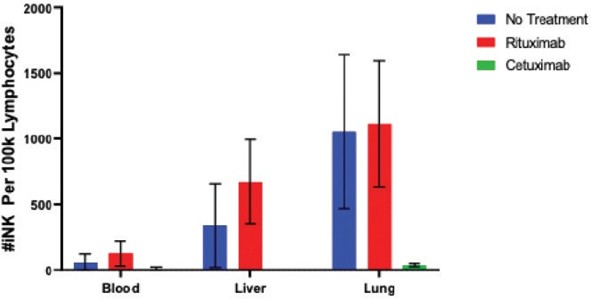
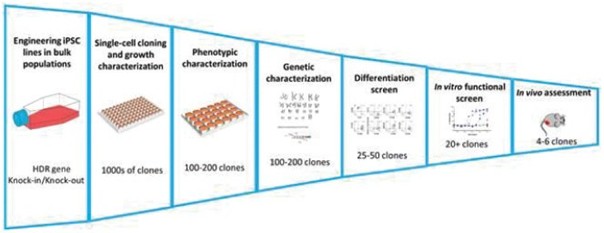
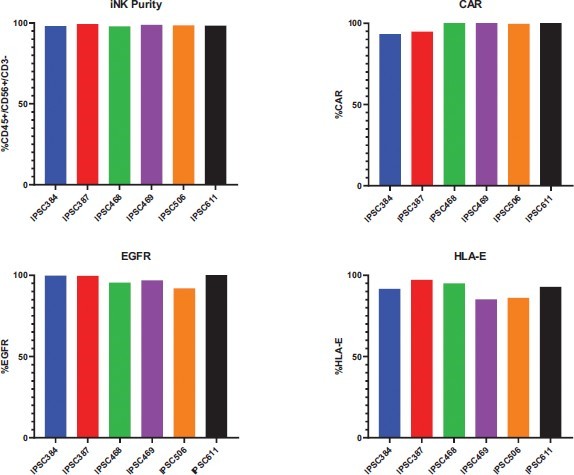
We have incurred indebtedness, and we may incur additional indebtedness, which could adversely affect our business.
As of December 31, 2022, we had an outstanding balance of $10.0 million under our Loan and Security Agreement with Hercules Capital, Inc., or the Loan Agreement. Our indebtedness could have important consequences to our stockholders. For example, it:
●increases our vulnerability to adverse general economic and industry conditions;
●limits our flexibility in planning for, or reacting to, changes in our business or the industries in which we operate by restricting our ability to make acquisitions, investments or divestments, or take other corporate actions quickly; and
●limits our ability to obtain additional financing or refinancing in the future for working capital, clinical trials, research and development, or other purposes.
Any of the above-listed factors could materially adversely affect our business, financial condition, results of operations, and cash flows. The Loan Agreement also contains certain financial and other covenants, including limitations on, among other things, additional indebtedness, out licensing, paying dividends in certain circumstances, and making certain acquisitions and investments. Any failure to comply with the terms, covenants and conditions of the Loan Agreement may limit our ability to draw upon additional tranches of term loans and may result in an event of default under such agreement, which could have a material adverse effect on our business, financial condition, and results of operations.
We are subject to various foreign, federal, and state healthcare and privacy laws and regulations, and our failure to comply with these laws and regulations could harm our results of operations and financial condition.
Our business operations and current and future arrangements with investigators, healthcare professionals, consultants, third-party payors, and customers expose us to broadly applicable foreign, federal and state fraud and abuse, and other healthcare and privacy laws and regulations. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell, and distribute any products for which we obtain marketing approval. Such laws include:
●the federal Anti-Kickback Statute, which prohibits, among other things, persons, or entities from knowingly and willfully soliciting, offering, receiving, or providing any remuneration (including any kickback, bribe, or certain rebates), directly or indirectly, overtly or covertly, in cash or in-kind, in return for, either the referral of an individual or the purchase, lease, or order, or arranging for or recommending the purchase, lease, or order of any good, facility, item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
●the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making or causing to be made a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government;