- AKLI Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
S-1 Filing
Akili (AKLI) S-1IPO registration
Filed: 23 Aug 22, 9:23pm
Delaware | 3841 | 98-1586159 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
Daniel J. Espinoza, Esq. Arthur R. McGivern, Esq. Sarah Ashfaq, Esq. Goodwin Procter LLP 100 Northern Avenue Boston, Massachusetts (617) 570-1000 | Jacqueline Studer, Esq. Chief Legal Officer Akili, Inc. 125 Broad Street, Fifth Floor Boston, Massachusetts 02110 (617) 313-8853 |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☒ | |||||
Page | ||||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 5 | ||||
| 7 | ||||
| 13 | ||||
| 15 | ||||
| 64 | ||||
| 65 | ||||
| 66 | ||||
| 74 | ||||
| 80 | ||||
| 135 | ||||
| 137 | ||||
| 155 | ||||
| 162 | ||||
| 173 | ||||
| 176 | ||||
| 180 | ||||
| 186 | ||||
| 190 | ||||
| 191 | ||||
| 195 | ||||
| 195 | ||||
| 195 | ||||
F-1 | ||||
II-1 | ||||
| • | “2011 Plan” are to Akili Interactive Labs, Inc. Amended and Restated 2011 Stock Incentive Plan, as amended from time to time; |
| • | “2022 Incentive Plan” or “2022 Plan” are to the 2022 Stock Option and Incentive Plan for Akili, Inc.; |
| • | “2022 ESPP” are to the 2022 Employee Stock Purchase Plan for Akili Interactive Labs, Inc.; |
| • | “Akili common stock” are to common stock, par value $0.0001 per share, of Akili; |
| • | “Akili Options” are to options to purchase shares of Akili common stock; |
| • | “Akili, Inc.” are to SCS after the Domestication and its name change from Social Capital Suvretta Corp. I; |
| • | “Akili, Inc. common stock” are to common stock, par value $0.0001 per share, of Akili, Inc.; |
| • | “Akili Stockholders” are to the stockholders of Akili, holders of Akili Options and holders of warrants to acquire Akili common stock, in each case prior to the Business Combination; |
| • | “ASC” are to Accounting Standards Codification; |
| • | “Business Combination” are to the Domestication together with the Merger; |
| • | “Bylaws” are to the bylaws of Akili, Inc. |
| • | “Cayman Constitutional Documents” are to SCS’ amended and restated memorandum and articles of association, as amended from time to time prior to the Domestication; |
| • | “Certificate of Incorporation” are to the certificate of incorporation of Akili, Inc.; |
| • | “Closing” are to the closing of the Business Combination; |
| • | “Company,” “we,” “us” and “our” are to SCS prior to its domestication as a corporation in the State of Delaware and to Akili, Inc. after its domestication as a corporation incorporated in the State of Delaware, including after its change of name to Akili, Inc.; |
| • | “Continental” are to Continental Stock Transfer & Trust Company |
| • | “COVID-19” are to the novel coronavirus pandemic; |
| • | “DGCL” are to the General Corporation Law of the State of Delaware; |
| • | “Domestication” are to the domestication of Social Capital Suvretta Holdings Corp. I as a corporation incorporated in the State of Delaware; |
| • | “Exchange Act” are to the Securities Exchange Act of 1934, as amended; |
| • | “founder shares” are to the SCS Class B ordinary shares purchased by the Sponsor in a private placement prior to the initial public offering, and the SCS Class A ordinary shares that will be issued upon the conversion thereof; |
| • | “initial public offering” are to SCS’ initial public offering that was consummated on July 2, 2021; |
| • | “IPO registration statement” are to the Registration Statements on Form S-1 (333-256723 and333-257543) filed by SCS in connection with its initial public offering, which became effective on June 29, 2021 and June 30, 2021, respectively; |
| • | “IRS” are to the U.S. Internal Revenue Service; |
| • | “Merger” are to the merger of Merger Sub with and into Akili, with Akili surviving the merger as a wholly owned subsidiary of Akili, Inc.; |
| • | “Merger Sub” are to a Delaware corporation and subsidiary of SCS; |
| • | “Nasdaq” are to the Nasdaq Capital Market; |
| • | “ordinary shares” are to the SCS Class A ordinary shares and the SCS Class B ordinary shares, collectively; |
| • | “Person” are to any individual, firm, corporation, partnership, limited liability company, incorporated or unincorporated association, joint venture, joint stock company, governmental authority or instrumentality or other entity of any kind; |
| • | “PIPE Investment” are to the purchase of shares of Akili, Inc. common stock pursuant to the Subscription Agreements; |
| • | “PIPE Investors” are to those certain investors participating in the PIPE Investment pursuant to the Subscription Agreements; |
| • | “private placement shares” are to the Class A ordinary shares issued to Sponsor in a private placement that was consummated concurrently with the closing of the initial public offering and the shares of common stock of Akili, Inc. issued as a matter of law upon the conversion thereof at the time of the Domestication; |
| • | “pro forma” are to giving pro forma effect to the Business Combination; |
| • | “public shareholders” are to holders of public shares, whether acquired in SCS’ initial public offering or acquired in the secondary market; |
| • | “public shares” are to the SCS Class A ordinary shares that were offered and sold by SCS in its initial public offering and registered pursuant to the IPO registration statement or the shares of Akili, Inc. common stock issued as a matter of law upon the conversion thereof at the time of the Domestication, as the context requires; |
| • | “redemption” are to each redemption of public shares for cash pursuant to the Cayman Constitutional Documents; |
| • | “Registration Rights Agreement” are to the Amended and Restated Registration Rights Agreement dated as of August 19, 2022, by and among Akili, Inc. (following the Domestication), the Sponsor, certain affiliates of the Sponsor, certain directors and advisors of SCS and certain former stockholders of Akili; |
| • | “Sarbanes-Oxley Act” are to the Sarbanes-Oxley Act of 2002; |
| • | “SCS” are to Social Capital Suvretta Holdings Corp. I, prior to its domestication as a corporation in the State of Delaware; |
| • | “SCS Class A ordinary shares” are to SCS’ Class A ordinary shares, par value $0.0001 per share; |
| • | “SCS Class B ordinary shares” are to SCS’ Class B ordinary shares, par value $0.0001 per share; |
| • | “SEC” are to the U.S. Securities and Exchange Commission; |
| • | “Securities Act” are to the Securities Act of 1933, as amended; |
| • | “SPE” are to special-purpose entity; |
| • | “Sponsor” are to SCS Sponsor I LLC, a Cayman Islands limited liability company, the sponsor of SCS; |
| • | “Sponsor Related PIPE Investor” are to a PIPE Investor that is an affiliate of the Sponsor (together with its permitted transferees); |
| • | “Subscription Agreements” are to the subscription agreements pursuant to which the PIPE Investment was consummated; |
| • | “Third-Party PIPE Investor” are to any PIPE Investor who is not a Sponsor Related PIPE Investor; and |
| • | “trust account” are to the trust account established at the consummation of SCS’ initial public offering and maintained by Continental, acting as trustee. |
| • | the effect of uncertainties related to the ongoing COVID-19 pandemic; |
| • | our ability to achieve and maintain profitability in the future; |
| • | our financial performance and ability to respond to general economic conditions; |
| • | our ability to manage our growth effectively and our expectations regarding the development and expansion of our business; |
| • | our ability to access sources of capital, including debt financing and other sources of capital to finance operations and growth; |
| • | our ability to achieve and maintain market acceptance and adoption of EndeavorRx and other prescription digital therapeutics by patients and physicians; |
| • | our ability to obtain or maintain adequate insurance coverage and reimbursement for EndeavorRx and its other products; |
| • | our ability to accurately forecast demand for EndeavorRx and its other products; |
| • | our ability to maintain access for EndeavorRx and our other products via the Apple Store and the Google Play Store; |
| • | our ability to achieve or maintain profitability; |
| • | our ability to maintain or obtain patent protection and/or the patent rights relating to EndeavorRx and its other product candidates and our ability to prevent third parties from competing against us; |
| • | our ability to successfully commercialize EndeavorRx and its other products; |
| • | our ability to obtain and maintain regulatory approval for EndeavorRx and our other product candidates, in the U.S. and in foreign markets, and any related restrictions or limitations of an approved product candidate; |
| • | our ability to obtain funding for our operations, including funding necessary to complete further development of EndeavorRx and further development, approval and, if approved, commercialization of our other product candidates; |
| • | our ability to identify, in-license or acquire additional technology or product candidates; |
| • | our ability to successfully protect against security breaches and other disruptions to our information technology structure; |
| • | the impact of applicable laws and regulations, whether in the U.S. or foreign jurisdictions, and any changes thereof; |
| • | our ability to successfully compete against other companies developing similar products to our current and future product offerings; |
| • | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| • | our ability to establish and maintain an effective system of internal controls over financial reporting; |
| • | our ability to maintain the listing of our securities on Nasdaq; |
| • | the risk that the Business Combination disrupts our plans and operations; |
| • | our inability to realize the anticipated benefits of the Business Combination; |
| • | the outcome of any legal or governmental proceedings that may be instituted against us; and |
| • | other factors detailed under the section titled “ Risk Factors |
| • | Targeted treatments that are personalized to patients’ needs |
| • | Clinically validated therapeutics like drugs and medical devices. |
| • | Therapeutics that are experienced as entertainment |
| • | Patient focused and adaptive |

| (1) | Timeframes are estimates and are subject to change—see Disclaimer and Risk Factors. |
| (2) | AKL-T01 is marketed as EndeavorRx in the U.S. for children ages8-12 old with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue. |
| (3) | Shionogi is responsible for the clinical development and commercialization of SDT-001 (a version ofAKL-T01 localized for Japanese language and culture), as well as any future development and commercialization ofAKL-T02, another version of our SSME engine that has been used for our ASD program, each in Japan and Taiwan. |
| (4) | AKL-M01 is designed to use SSME algorithms to monitor and assess certain aspects of cognition, as opposed to providing cognitive therapy. |
| (5) | To the extent we are unable to access additional sources of funding following the completion of the Business Combination, our current estimated timeframe for initiating a pivotal study in this development program could be delayed. |
| * | Estimated timeframes in figure above correspond to applicable milestone start times, and are subject to change. Please refer to the section entitled “ Risk Factors Risks Related to Our Business and Industry—Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside of our control. If we experience delays or difficulties in the enrollment or retention of patients in clinical trials, our ability to obtain necessary marketing authorizations for our product candidates could be delayed or preventedRisks Related to Our Products—Our current product candidates are in various stages of development. Our product candidates may fail in development or suffer delays that adversely affect their commercial viability. If we fail to maintain clearance, de novo classification or approval to market our product candidates, including AKL-T01 for expanded indications, or if we are delayed in obtaining such marketing authorizations, our business, prospects, results of operations and financial condition could be materially and adversely affected |
| • | Establishing a commercial foothold in pediatric ADHD. |
| • | Leveraging our initial success to expand into other ADHD populations. |
| • | Applying our clinically-validated technology to other mental health and neurology conditions. |
| • | Simultaneously pursuing new technologies designed to address other cognitive impairments. |
| • | Further evolving the treatment paradigm by creating new methods of cognitive assessment. |
| • | We have a history of significant losses, anticipate losses increasing expenses in the future, and may not be able to achieve or maintain profitability. |
| • | The failure of our prescription digital therapeutics to achieve and maintain market acceptance and adoption by patients and physicians could have a material adverse effect on our business, prospects, results of operation and financial condition. |
| • | The market for prescription digital therapeutics is new, rapidly evolving, and increasingly competitive, as the healthcare industry in the U.S. is undergoing significant structural change, which makes it difficult to forecast demand for our products. As a result, all prospective financial information included herein are subject to change. |
| • | Our development programs represent novel and innovative potential therapeutic areas, and negative perception of any product or product candidate that we develop could adversely affect our ability to conduct our business, obtain marketing authorizations or identify alternate regulatory pathways to market for such product candidate. |
| • | We face competition, and new products may emerge that provide different or better alternatives for treatment of the conditions that EndeavorRx or our future products, if granted marketing authorization, are authorized to treat. |
| • | If we fail to achieve and maintain clearance, de novo classification or approval to market our product candidates, including AKL-T01 for expanded indications, or if we are delayed in obtaining such marketing authorizations, our business, prospects, results of operations and financial condition could be materially and adversely affected. |
| • | Clinical trials of any of our products or product candidates may fail to produce results necessary to support marketing authorization. |
| • | EndeavorRx is made available via the Apple App Store® and on Google PlayTM, and supported by third-party infrastructure. If our ability to access these markets or access necessary third-party infrastructure was stopped or otherwise restricted or limited, it could have a material adverse effect on our business, prospects, results of operations and financial condition. |
| • | If we are not able to develop and release new products, or successful enhancements, new features and modifications to EndeavorRx or any future products, our business, prospects, results of operations and financial condition could be materially and adversely affected. |
| • | We rely on a single third party digital pharmacy for the fulfillment of prescriptions. This reliance on a single third party increases the risk that we could have disruption in the fulfillment of prescriptions, which could have a material and adverse effect on our reputation, business, results of operations and financial condition. |
| • | If we are unable to adequately protect and enforce our intellectual property and proprietary technology, obtain and maintain patent protection for our technology and products where appropriate or if the scope of the patent protection obtained is not sufficiently broad, or if we are unable to protect the confidentiality of our trade secrets and know-how, our competitors could develop and commercialize technology and products similar or identical to our products, and our ability to successfully commercialize our technology and products may be impaired. |
| • | If we fail to comply with obligations in the agreements under which we collaborate with or license intellectual property rights from third parties, or otherwise experience disruptions to our business relationships with collaborators or licensors, we could lose rights that are important to its business. |
| • | We will need substantial additional funding, and if it is unable to raise capital when needed or on terms favorable to us, our business, financial condition and results of operation could be materially and adversely affected. |
| • | The amount of our future losses is uncertain and our quarterly and annual operating results may fluctuate significantly or fall below the expectations of investors or securities analysists, each of which may cause our stock price to fluctuate or decline. |
| • | exemption from the requirement to have our registered independent public accounting firm attest to management’s assessment of our internal control over financial reporting; |
| • | exemption from compliance with the requirement of the Public Company Accounting Oversight Board, or PCAOB, regarding the communication of critical audit matters in the auditor’s report on the financial statements; |
| • | reduced disclosure about our executive compensation arrangements; and |
| • | exemption from the requirement to hold non-binding advisory votes on executive compensation or golden parachute arrangements. |
Issuer | Akili, Inc. |
Shares of common stock to be issued by us | 2,096,106 shares of common stock reserved for issuance upon the exercise of stock options to purchase common stock. |
Shares of common stock outstanding prior to the exercise of stock options and settlement of restricted stock units | 85,395,207 shares |
Use of proceeds | We will receive up to an aggregate of approximately $6,440,833 from the exercise of stock options to which this registration statement relates (assuming the exercise in full of all of the outstanding stock options for cash). There is no assurance that stock options will be in the money prior to their expiration or that the holders of such stock options will elect to exercise any or all of such stock options. We believe the likelihood that these holders will exercise such stock options, and therefore any cash proceeds that we may receive in relation to the exercise of such stock options being offered for sale in this prospectus, will be dependent on the trading price of our common stock. If the market price for our common stock is less than the exercise price of stock options, we believe the holders of such stock options will be unlikely to exercise such securities. We expect to use any net proceeds from the exercise of stock options for general corporate purposes. See “ Use of Proceeds |
Shares of common stock offered by the Selling Securityholders | 43,414,721 shares consisting of: |
| • | 16,200,000 shares of common stock issued in the PIPE Investment by certain of the selling securityholders; |
| • | 6,250,000 shares of common stock issued upon consummation of the Business Combination, in exchange for Class B ordinary shares of SCS originally issued to the Sponsor; |
| • | 640,000 shares of common stock issued upon consummation of the Business Combination, in exchange for Class A ordinary shares of SCS originally issued to the Sponsor; |
| • | 2,494,549 shares of common stock held by certain of our current and former directors; |
| • | 15,684,066 shares of common stock issued to certain former equity holders of Akili pursuant to the Business Combination; |
| • | 2,096,106 shares of common stock reserved for issuance upon the exercise of options to purchase common stock held by persons who previously ended their relationship with Akili prior to or concurrently with the Closing to the Business Combination; and |
| • | 50,000 shares of common stock reserved for issuance upon the settlement of restricted stock units. |
Terms of the offering | The Selling Securityholders will determine when and how they will dispose of the securities registered under this prospectus for resale. See “Plan of Distribution”. |
Use of proceeds | We will not receive any proceeds from the sale of the securities registered under this prospectus by the Selling Securityholders. |
Risk factors | See “Risk Factors” and the other information included in this prospectus for a discussion of factors you should consider before investing in our securities. |
Nasdaq symbols | Our common stock is listed on Nasdaq under the symbol “AKLI”. |
| • | the failure of EndeavorRx to achieve wide acceptance among patients, self-insured employers, commercial and government payers, health plans, physicians and other government entities, and key opinion leaders in the treatment community; |
| • | lack of additional evidence of peer-reviewed publication of clinical or real world evidence supporting the effectiveness, safety, cost-savings or other advantages of our products over competitive products or other currently available methodologies; |
| • | perceived risks associated with the use of our product or similar products or technologies generally; |
| • | our ability to maintain U.S. Food and Drug Administration, or FDA, marketing authorization and other marketing authorizations for EndeavorRx; |
| • | our ability to secure and maintain other regulatory clearance, authorization or approval for AKL-T01 for expanded indications and our other product candidates; |
| • | the introduction of competitive products and the rate of acceptance of those products as compared to our products; and |
| • | results of clinical, real world and health economics and outcomes research studies relating to chronic condition products or similar competitive products. |
| • | lack of availability of adequate third-party payer coverage or reimbursement; |
| • | lack of experience with our products; |
| • | our inability to convince key opinion leaders to recommend our products; |
| • | perceived inadequacy of evidence supporting clinical benefits, safety or cost-effectiveness of our products; |
| • | liability risks generally associated with the use of new products; and |
| • | the training required to use new products. |
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | clinical trial holds; |
| • | fines, warning letters or other regulatory enforcement action; |
| • | refusal by the FDA or comparable foreign regulatory authorities to clear or approve pending submissions filed by us; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
| • | We may not be able to demonstrate to the FDA’s satisfaction that our product candidates meet the applicable regulatory standards for clearance, de novo classification, or approval, as applicable; |
| • | The FDA may disagree that our clinical data supports the label and use that we are seeking; and |
| • | The FDA may disagree that the data from our preclinical or pilot studies and clinical trials is sufficient to support marketing authorization. |
| • | the possible breach of the manufacturing agreement by the third party; and |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
| • | we may lose marketing authorization of such product; |
| • | regulatory authorities may require additional warnings on the product’s label; |
| • | we may be required to issue safety communications to patients or healthcare providers that outline the risks of such side effects; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
| • | multiple, conflicting and changing laws and regulations such as tax laws, privacy and data protection laws and regulations, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
| • | requirements to maintain data and the processing of that data on servers located within the United States or in such countries; |
| • | protecting and enforcing our intellectual property rights; |
| • | converting our products as well as the accompanying instructional and marketing materials to conform to the language and customs of different countries; |
| • | complexities associated with managing multiple payer reimbursement regimes, and government payers; |
| • | competition from companies with significant market share in our market and with a better understanding of user preferences; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the effect of local and regional financial pressures on demand and payment for our products and services and exposure to foreign currency exchange rate fluctuations; |
| • | natural disasters, political and economic instability, including wars, terrorism, political unrest, outbreak of disease (including the recent coronavirus outbreak), boycotts, curtailment of trade, and other market restrictions; and |
| • | regulatory and compliance risks that relate to maintaining accurate information and control over activities subject to regulation under the U.S. Foreign Corrupt Practices Act (the “FCPA”), and comparable laws and regulations in other countries. |
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | supported by robust clinical data from well-controlled clinical research; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our technology and processing infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under our collaborative development relationships; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | amount of royalty payments under the license agreement; |
| • | whether and to what extent our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | our right to sublicense patent and other rights to collaborators and other third parties; |
| • | our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our products, and what activities satisfy those diligence obligations; and |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our collaborators. |
| • | the scope, progress, results and costs of researching and developing our current product candidates, as well as other additional product candidates we may develop and pursue in the future; |
| • | the timing of, and the costs involved in, obtaining marketing authorization for our product candidates and any other additional product candidates we may develop and pursue in the future; |
| • | the number of future product candidates that we may pursue and their development requirements; |
| • | the costs of commercialization activities for our product candidates, including the costs and timing of establishing product sales, marketing, and distribution capabilities; |
| • | revenue received from commercial sales of our current products and, subject to receipt of authorization, revenue, if any, received from commercial sales of our product candidates; |
| • | the extent to which we in-license or acquires rights to other products, product candidates or technologies; |
| • | our investment in our human capital required to grow the business and the associated costs as we expand our research and development and establishes a commercial infrastructure; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights, including enforcing and defending intellectual property-related claims; and |
| • | the cost of operating a public company. |
| • | the timing and success or failure of clinical trials for our product candidates or competing product candidates, or any other change in the competitive landscape of our industry, including consolidation among our competitors or partners or as a result of COVID-19; |
| • | our ability to successfully recruit and retain subjects for clinical trials, and any delays caused by difficulties in such efforts, including as a result of COVID-19; |
| • | our ability to obtain marketing authorization for our product candidates and the timing and scope of any such marketing authorizations we may receive; |
| • | the timing and cost of, and level of investment in, research and development activities relating to our product candidates, which may change from time to time; |
| • | our ability to attract, hire and retain qualified personnel; |
| • | expenditures that we will or may incur to develop additional product candidates; |
| • | the level of demand for EndeavorRx and our other product candidates should such product candidates receive marketing authorizations, which may vary significantly; |
| • | the risk/benefit profile, cost and reimbursement policies with respect to EndeavorRx and our other product candidates, if granted marketing authorization, and existing and potential future therapeutics that compete with our product candidates; |
| • | the changing and volatile U.S. and global economic environments including global inflationary pressures; and |
| • | future accounting pronouncements or changes in our accounting policies. |
| • | changes in the industries in which we and our customers operate; |
| • | developments involving our competitors; |
| • | changes in laws and regulations affecting our business; |
| • | variations in our operating performance and the performance of our competitors in general; |
| • | actual or anticipated fluctuations in our quarterly or annual operating results; |
| • | publication of research reports by securities analysts about our or our competitors or our industry; |
| • | the public’s reaction to our press releases, our other public announcements and our filings with the SEC; |
| • | actions by stockholders, including the sale by the Third-Party PIPE Investors of any of their shares of our common stock; |
| • | additions and departures of key personnel; |
| • | commencement of, or involvement in, litigation involving us; |
| • | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| • | the volume of shares of our common stock available for public sale; and |
| • | general economic and political conditions, such as the effects of the ongoing COVID-19 pandemic, recessions, interest rates, local and national elections, fuel prices, international currency fluctuations, corruption, political instability and acts of war or terrorism. |
| • | the accompanying notes to the unaudited pro forma condensed combined financial statements; |
| • | the historical unaudited condensed financial statements of SCS as of June 30, 2022 and for the six months ended June 30, 2022 and the related notes included in the prospectus; |
| • | the historical audited financial statements of SCS as of December 31, 2021 and for the period from February 25, 2021 (inception) through December 31, 2021 and the related notes included in this prospectus; |
| • | the historical unaudited condensed consolidated financial statements of Akili as of June 30, 2022 and for the six months ended June 30, 2022 and the related notes included elsewhere in this prospectus; |
| • | the historical audited consolidated financial statements of Akili as of December 31, 2021 and for the year ended December 31, 2021 and the related notes included elsewhere in this prospectus; and |
| • | the section entitled “ SCS’s Management’s Discussion and Analysis of Financial Condition and Results of Operations Akili’s Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Akili Interactive Labs, Inc. Stock Outstanding at June 30, 2022 | Additional Akili Interactive Labs, Inc. Stock Issued and Options Exercised/Expired After June 30, 2022 (1) | Conversion of Akili Interactive Labs, Inc. Preferred Stock and automatically exercised Warrants into Akili Interactive Labs, Inc. Common Stock | Akili Interactive Labs, Inc. Stock Prior to Closing | Akili, Inc. Stock Held by Akili Stockholders Post Closing (2) | ||||||||||||||||
COMMON STOCK | ||||||||||||||||||||
Common Stock (3) | 1,482,520 | 22,750 | 45,872,861 | (5) | 47,378,131 | 54,541,224 | ||||||||||||||
PREFERRED STOCK | ||||||||||||||||||||
Series A-1 Convertible Preferred Stock | 4,000,000 | — | (4,000,000 | ) | — | — | ||||||||||||||
Series A-2 Convertible Preferred Stock | 4,427,072 | — | (4,427,072 | ) | — | — | ||||||||||||||
Series B Convertible Preferred Stock | 7,341,485 | — | (7,341,485 | ) | — | — | ||||||||||||||
Series C Convertible Preferred Stock | 8,016,645 | — | (8,016,645 | ) | — | — | ||||||||||||||
Series D Convertible Preferred Stock | 13,843,858 | 8,236,127 | (22,079,985 | ) | — | — | ||||||||||||||
Total Common and Preferred Stock (3) | 39,111,580 | 8,258,877 | 7,674 | 47,378,131 | 54,541,224 | |||||||||||||||
Akili Interactive Labs, Inc. Warrants (4) | 134,729 | — | (7,674 | ) | 127,055 | 137,628 | ||||||||||||||
Akili Interactive Labs, Inc. Options (4) | 4,124,718 | (56,389 | ) | — | 4,068,330 | 5,321,115 | ||||||||||||||
Total Stock, Warrants and Options | 43,371,027 | 8,202,488 | — | 51,573,516 | 59,999,967 | |||||||||||||||
| (1) | Reflects the capitalization activity of Akili subsequent to the latest balance sheet date through August 19, 2022, in connection with the Merger Agreement. The amount includes cumulative dividends that accrue on each share of Series D at an annual rate of 10%, which are issued in additional shares. Upon conversion, Series D shares are multiplied by 150% when calculating the number of shares of common stock. |
| (2) | Per the terms of the Merger Agreement, no fractional Akili, Inc. common stock were issued. Each holder of Akili stock entitled to a fraction of a Akili, Inc. common stock had its fractional share rounded down to the nearest whole share. Each holder of an Akili option and warrant underlying a fraction of a share of Akili, Inc. common stock had its fractional option rounded down to the nearest whole share. |
| (3) | Amount excludes the issuance of 7,536,461 Earnout Shares, to certain eligible Akili equity holders as a result of Akili, Inc. satisfying certain performance conditions described below with the Earnout Period. |
| (4) | Stock options and warrants to purchase common stock were converted using the treasury stock method at each date. |
| (5) | Includes 7,674 shares representing the cashless exercise of certain outstanding Akili Interactive Labs, Inc. warrants prior to Closing. |
| Shares | % | |||||||
SCS Public Stockholders (a) | 227,522 | 0.3 | % | |||||
SCS Sponsor and Independent Director | 6,890,000 | 8.8 | % | |||||
Total SCS | 7,117,522 | 9.1 | % | |||||
Akili Stockholders (b) | 54,541,224 | 70.1 | % | |||||
PIPE Investors | 16,200,000 | 20.8 | % | |||||
Total Shares at Closing (excluding certain Akili shares) | 77,858,746 | 100 | % | |||||
Akili—Remaining Consideration Shares (b)(c) | 5,458,743 | |||||||
Total Shares at Closing (including certain Akili shares) | 83,317,489 | |||||||
| (a) | Reflects redemptions of 24,772,478 public shares of SCS Class A common stock in connection with the Transactions at a redemption price of $10.03 per share based on funds held in the trust account as of August 17, 2022, two business days prior to closing. |
| (b) | Total consideration issued to Akili is $600.0 million or 60,000,000 shares ($10 per share price with no fractional shares). The total shares issued includes those in respect of Akili common and preferred stock and stock options and warrants to purchase common stock, converted using the treasury stock method. The Akili—Remaining Consideration Shares reflect a Conversion Ratio of approximately 1.15. |
| (c) | Amount excludes the issuance of 7,536,461 Earnout Shares, to certain eligible Akili equity holders as a result of Akili, Inc. satisfying certain performance conditions described above with the Earnout Period. |
Historical | ||||||||||||||||||||
SCS | Akili | Transaction Accounting Adjustments | Pro Forma Balance Sheet | |||||||||||||||||
ASSETS | ||||||||||||||||||||
Current assets: | ||||||||||||||||||||
Cash and cash equivalents | $ | 145 | $ | 40,638 | $ | 250,814 | 3 | (a) | $ | 175,684 | ||||||||||
| (2,226 | ) | 3 | (d) | |||||||||||||||||
| (9,045 | ) | 3 | (f) | |||||||||||||||||
| 162,000 | 3 | (i) | ||||||||||||||||||
| (2,700 | ) | 3 | (k) | |||||||||||||||||
| (7,752 | ) | 3 | (l) | |||||||||||||||||
| (5,520 | ) | 3 | (e) | |||||||||||||||||
| (248,531 | ) | 3 | (j) | |||||||||||||||||
| (2,139 | ) | 3 | (n) | |||||||||||||||||
Restricted cash | — | 305 | — | 305 | ||||||||||||||||
Short-term investments | — | 4,987 | — | 4,987 | ||||||||||||||||
Account receivable, net | — | 17 | — | 17 | ||||||||||||||||
Prepaid expenses and other current assets | 524 | 5,328 | (2,338 | ) | 3 | (f) | 3,514 | |||||||||||||
Total current assets | 669 | 51,275 | 132,563 | 184,507 | ||||||||||||||||
Property and equipment, net | — | 1,054 | — | 1,054 | ||||||||||||||||
Operating lease right-of-use | — | 2,686 | — | 2,686 | ||||||||||||||||
Marketable securities held in Trust Account | 250,371 | — | (250,371 | ) | 3 | (a) | — | |||||||||||||
Total assets | $ | 251,040 | $ | 55,015 | $ | (117,808 | ) | $ | 188,247 | |||||||||||
LIABILITIES, REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT | ||||||||||||||||||||
Current liabilities: | ||||||||||||||||||||
Accounts payable | $ | 17 | $ | 4,309 | $ | (17 | ) | 3 | (l) | $ | 4,309 | |||||||||
Accrued expenses and other current liabilities | 7,405 | 5,044 | (7,405 | ) | 3 | (l) | 5,044 | |||||||||||||
Promissory note—related party | 250 | — | (250 | ) | 3 | (l) | — | |||||||||||||
Due to related party | 80 | — | (80 | ) | 3 | (l) | — | |||||||||||||
Deferred revenue | — | 109 | — | 109 | ||||||||||||||||
Deferred rent, short term | — | 9 | — | 9 | ||||||||||||||||
Operating lease liability | — | 625 | — | 625 | ||||||||||||||||
Note payable, short term | — | 625 | — | 625 | ||||||||||||||||
Total current liabilities | 7,752 | 10,721 | (7,752 | ) | 10,721 | |||||||||||||||
Deferred underwriting fee payable | 7,700 | — | (7,700 | ) | 3 | (e) | — | |||||||||||||
Note payable, long term | — | 14,213 | — | 14,213 | ||||||||||||||||
Operating lease liability, net of current portion | — | 2,832 | — | 2,832 | ||||||||||||||||
Corporate bond, net of bond discount | — | 1,735 | — | 1,735 | ||||||||||||||||
Earnout liability | — | — | 57,197 | 3 | (m) | 57,197 | ||||||||||||||
Total liabilities | $ | 15,452 | $ | 29,501 | $ | 41,745 | $ | 86,698 | ||||||||||||
Historical | ||||||||||||||||||||
SCS | Akili | Transaction Accounting Adjustments | Pro Forma Balance Sheet | |||||||||||||||||
Commitments and contingencies | ||||||||||||||||||||
Class A ordinary shares | 250,371 | — | (250,371 | ) | 3 | (b) | — | |||||||||||||
Class A common stock | — | — | 250,371 | 3 | (b) | — | ||||||||||||||
| (250,371 | ) | 3 | (j) | |||||||||||||||||
REDEEMABLE CONVERTIBLE PREFERRED STOCK: | ||||||||||||||||||||
Akili Series A-1 redeemable convertible preferred stock | — | — | — | — | ||||||||||||||||
Akili Series A-2 redeemable convertible preferred stock | — | 7,128 | (7,128 | ) | 3 | (g) | — | |||||||||||||
Akili Series B redeemable convertible preferred stock | — | 41,854 | (41,854 | ) | 3 | (g) | — | |||||||||||||
Akili Series C redeemable convertible preferred stock | — | 67,904 | (67,904 | ) | 3 | (g) | — | |||||||||||||
Akili Series D redeemable convertible preferred stock | — | 183,668 | (183,668 | ) | 3 | (g) | — | |||||||||||||
Stockholders equity (deficit): | ||||||||||||||||||||
SCS Preference shares | — | — | — | — | ||||||||||||||||
Class A ordinary shares | — | — | — | 3 | (b) | — | ||||||||||||||
Class A common stock | — | — | — | 3 | (b) | 11 | ||||||||||||||
| 1 | 3 | (c) | ||||||||||||||||||
| 3 | 3 | (j) | ||||||||||||||||||
| 5 | 3 | (h) | ||||||||||||||||||
| 2 | 3 | (i) | ||||||||||||||||||
Class B ordinary shares | 1 | — | (1 | ) | 3 | (b) | — | |||||||||||||
Class B common stock | — | — | 1 | 3 | (b) | — | ||||||||||||||
| (1 | ) | 3 | (c) | |||||||||||||||||
Akili Common stock | — | — | 5 | 3 | (g) | — | ||||||||||||||
| (5 | ) | 3 | (h) | |||||||||||||||||
Additional paid-in capital | — | — | 2,280 | 3 | (j) | 383,783 | ||||||||||||||
| 161,998 | 3 | (i) | ||||||||||||||||||
| (2,700 | ) | 3 | (k) | |||||||||||||||||
| (14,784 | ) | 3 | (h) | |||||||||||||||||
| 300,549 | 3 | (g) | ||||||||||||||||||
| (2,226 | ) | 3 | (d) | |||||||||||||||||
| (11,383 | ) | 3 | (f) | |||||||||||||||||
| (57,197 | ) | 3 | (m) | |||||||||||||||||
| 2,180 | 3 | (e) | ||||||||||||||||||
| 5,066 | 3 | (o) | ||||||||||||||||||
Accumulated deficit | (14,784 | ) | (275,033 | ) | 14,784 | 3 | (h) | (282,238 | ) | |||||||||||
| (2,139 | ) | 3 | (n) | |||||||||||||||||
| (5,066 | ) | 3 | (o) | |||||||||||||||||
| 443 | 3 | (a) | ||||||||||||||||||
| (443 | ) | 3 | (j) | |||||||||||||||||
Accumulated other comprehensive loss | — | (7 | ) | — | (7 | ) | ||||||||||||||
Total stockholders’ equity (deficit) | $ | (14,783 | ) | $ | (275,040 | ) | $ | 391,372 | $ | 101,549 | ||||||||||
Total liabilities, redeemable convertible preferred stock and stockholders’ deficit | $ | 251,040 | $ | 55,015 | $ | (117,808 | ) | $ | 188,247 | |||||||||||
Historical | ||||||||||||||||||||
SCS | Akili Interactive Labs, Inc. | Transaction Accounting Adjustments | Pro Forma Statement of Operations | |||||||||||||||||
Revenue | $ | — | $ | 130 | $ | — | $ | 130 | ||||||||||||
Operating expenses: | ||||||||||||||||||||
Cost of revenue | — | 193 | — | 193 | ||||||||||||||||
Research and development | — | 13,662 | 49 | 4 | (c) | 13,711 | ||||||||||||||
Selling, general and administrative | — | 30,339 | 59 | 4 | (c) | 36,671 | ||||||||||||||
| 6,273 | 4 | (b) | ||||||||||||||||||
Operating and formation costs | 6,273 | — | (6,273 | ) | 4 | (b) | — | |||||||||||||
Total operating expenses | 6,273 | 44,194 | 108 | 50,575 | ||||||||||||||||
Loss from operations | (6,273 | ) | (44,064 | ) | (108 | ) | (50,445 | ) | ||||||||||||
Interest earned on marketable securities held in Trust Account | 363 | — | (363 | ) | 4 | (a) | — | |||||||||||||
Loss on extinguishment of debt | — | — | — | — | ||||||||||||||||
Interest expense | — | (370 | ) | — | (370 | ) | ||||||||||||||
Other income | — | 49 | — | 49 | ||||||||||||||||
Loss before provision for income taxes | (5,910 | ) | (44,385 | ) | (471 | ) | (50,766 | ) | ||||||||||||
Provision for income taxes | — | — | — | — | ||||||||||||||||
Net loss | $ | (5,910 | ) | $ | (44,385 | ) | $ | (471 | ) | $ | (50,766 | ) | ||||||||
Unrealized loss on short-term investments | $ | — | $ | (7 | ) | $ | — | $ | — | |||||||||||
Comprehensive loss | $ | (5,910 | ) | $ | (44,392 | ) | $ | (471 | ) | $ | (50,766 | ) | ||||||||
Net loss | $ | (5,910 | ) | $ | (44,385 | ) | $ | (471 | ) | $ | (50,766 | ) | ||||||||
Dividends on Series D convertible preferred stock | — | (5,785 | ) | — | (5,785 | ) | ||||||||||||||
Accretion of Series D convertible preferred stock | — | (2,893 | ) | — | (2,893 | ) | ||||||||||||||
Net loss attributable to common stockholders, basic and diluted | $ | (5,910 | ) | $ | (53,063 | ) | $ | (471 | ) | $ | (59,444 | ) | ||||||||
Net loss per share, basic and diluted | $ | (0.19 | ) | $ | (36.18 | ) | $ | (0.76 | ) | |||||||||||
Weighted-average common shares outstanding: Basic and diluted | 31,890,000 | 1,466,462 | 77,858,746 | |||||||||||||||||
Historical | ||||||||||||||||||||
SCS | Akili Interactive Labs, Inc. | Transaction Accounting Adjustments | Pro Forma Statement of Operations | |||||||||||||||||
Revenue | $ | — | $ | 538 | $ | — | $ | 538 | ||||||||||||
Operating expenses: | ||||||||||||||||||||
Cost of revenue | — | 355 | — | 355 | ||||||||||||||||
Research and development | — | 18,234 | 2,733 | 5 | (c) | 20,967 | ||||||||||||||
Selling, general and administrative | — | 42,668 | 5,879 | 5 | (c) | 53,133 | ||||||||||||||
| 2,447 | 5 | (b) | ||||||||||||||||||
| 2,139 | 5 | (d) | ||||||||||||||||||
Operating and formation costs | 2,447 | — | (2,447 | ) | 5 | (b) | — | |||||||||||||
Total operating expenses | 2,447 | 61,257 | 10,751 | 74,455 | ||||||||||||||||
Loss from operations | (2,447 | ) | (60,719 | ) | (10,751 | ) | (73,917 | ) | ||||||||||||
Interest earned on marketable securities held in Trust Account | 8 | — | (8 | ) | 5 | (a) | — | |||||||||||||
Loss on extinguishment of debt | — | (181 | ) | — | (181 | ) | ||||||||||||||
Interest expense | — | (465 | ) | — | (465 | ) | ||||||||||||||
Other income | — | 17 | — | 17 | ||||||||||||||||
Loss before provision for income taxes | (2,439 | ) | (61,348 | ) | (10,759 | ) | (74,546 | ) | ||||||||||||
Provision for income taxes | — | — | — | — | ||||||||||||||||
Net loss | (2,439 | ) | (61,348 | ) | (10,759 | ) | (74,546 | ) | ||||||||||||
Dividends on Series D convertible preferred stock | — | (6,660 | ) | — | (6,660 | ) | ||||||||||||||
Accretion of Series D convertible preferred stock | — | (58,649 | ) | — | (58,649 | ) | ||||||||||||||
Net loss attributable to common stockholders, basic and diluted | $ | (2,439 | ) | $ | (126,657 | ) | $ | (10,759 | ) | $ | (139,855 | ) | ||||||||
Net loss per share, basic and diluted | $ | (0.12 | ) | $ | (105.77 | ) | $ | (1.80 | ) | |||||||||||
Weighted-average common shares outstanding: Basic and diluted | 20,954,628 | 1,197,489 | 77,858,746 | |||||||||||||||||
| (1) | For the period from February 25, 2021 (date of inception) to December 31, 2021. |
| • | Triggering Event I is $15.00 |
| • | Triggering Event II is $20.00 |
| • | Triggering Event III is $30.00 |
Combined Pro Forma | ||||||||
Year Ended December 31, 2021 | Six Months Ended June 30, 2022 | |||||||
Pro forma net loss | $ | (139,855 | ) | $ | (59,444 | ) | ||
Pro forma weighted average shares outstanding—basic and diluted | 77,858,746 | 77,858,746 | ||||||
Pro forma net loss per share-basic and diluted | $ | (1.80 | ) | $ | (0.76 | ) | ||
Pro Forma weighted average shares calculation—basic and diluted | ||||||||
SCS Sponsors | 6,890,000 | 6,890,000 | ||||||
SCS common stock subject to redemption | 227,522 | 227,522 | ||||||
Total SCS | 7,117,522 | 7,117,522 | ||||||
Issuance of SCS common stock in connection with closing of the PIPE Transaction | 16,200,000 | 16,200,000 | ||||||
Issuance of SCS common stock to Akili shareholders in connection with Business Combination (a)(b) | 54,541,224 | 54,541,224 | ||||||
Pro forma weighted average shares outstanding—basic and diluted (c) | 77,858,746 | 77,858,746 | ||||||
| a. | Excludes 5,458,743 Akili consideration shares that will be issued upon the occurrence of future events (i.e., exercise of stock options and warrants). Total consideration to be issued to Akili is $600.0 million or 60,000,000 shares ($10 per share price with no fractional shares issued). The total shares to be issued includes those in respect of all issued and outstanding Akili common and preferred stock and shares underlying stock options and warrants. Accordingly, the weighted average pro forma shares outstanding at Closing has been adjusted to exclude the portion of consideration shares that were unissued at Closing. |
| b. | Amount excludes the issuance of approximately 7,536,461 Earnout Shares to certain eligible Akili equity holders as a result of Akili, Inc. satisfying certain conditions described above within the Earnout Period. |
| c. | For the purposes of applying the if converted method for calculating diluted earnings per share, it was assumed that all Akili common stock options are exchanged for common stock. However, since this results in anti-dilution, the effect of such exchange was not included in the calculation of diluted loss per share. Shares underlying these instruments include 5,458,743 Akili consideration shares for unexercised stock options and warrants. |
| • | Targeted treatments that are personalized to patients’ needs de-identified, aggregate level view of each patient’s activity, informing our product development. The therapeutics’ mechanics, algorithms and designs are protected by patents, trade secrets and copyrights, combining protections typically seen in both the medicine and technology industries to create a robust intellectual property portfolio. |
| • | Clinically validated therapeutics like drugs and medical devices The American Journal of Psychiatry, The Lancet Digital Health and Nature: Digital Medicine |
| • | Therapeutics that are experienced as entertainment |
unique user experiences also allows us to adapt the experience to appeal to different patient populations by developing and testing product candidates in clinical trials. We believe we have the potential to offer the first treatments that both rival the experience of consumer entertainment products and can be utilized as part of a treatment plan. |
| • | Patient focused and adaptive |
| • | pivotal study in adolescents with ADHD |
| • | pivotal study in adults with ADHD |
| • | Weill Cornell Medicine/NewYork-Presbyterian Hospital in acute cognitive dysfunction in COVID-19 survivors |
| • | Vanderbilt University Medical Center in acute cognitive dysfunction in COVID-19 survivors |
| • | pivotal study in pediatric ADHD in Japan by Shionogi & Co., Ltd (“Shionogi”) |
| • | proof of concept study in early childhood (3-8 year olds) ADHD by TALi Digital Limited (“TALi”) |
| • | post-operative cognitive dysfunction by Vanderbilt University Medical Center |
| • | chemotherapy-related cognitive impairment by the University of California San Francisco (“UCSF”) |
| • | cognitive monitoring in a healthy aging population |
| • | proof of concept and/or pivotal study in inattention in ASD |
| • | pivotal study in cognitive dysfunction in MS |
| • | pivotal study in cognitive dysfunction in MDD |
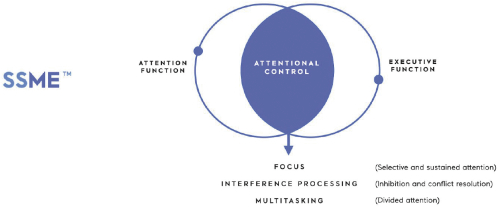

Core Mechanic | Description | Targeted Physiology | ||
SSME Stimulus Management Engine | Targets attentional control |  | ||
SNAV Navigation Engine | Targets spatial navigation and episodic memory |  | ||
BBT Trainer | Targets attentional control, goal management and working memory |  |

| • | Navigation: Steering over gates and/or avoiding obstacles |
| • | Targeting: Tap for targets and ignore non-targets |
| • | Multitasking: Simultaneous navigation and targeting |

Disease Area | Total U.S. Population with Disease Diagnosis | Initial Target Population Subset with applicable cognitive impairment, as noted | ||
Attention-deficit/hyperactivity disorder (“ADHD”), all ages | 10.8M (ADHD + inattention) | 8.1M | ||
Autism spectrum disorder (“ASD”) | 1.3M | 410k (ASD + inattention) | ||
Multiple sclerosis (“MS”) | 900K | 180K | ||
| (MS + cognitive dysfunction) | ||||
Major depressive disorder (“MDD”) | 19M | 2.1M | ||
| (MDD + cognitive dysfunction) | ||||
Acute cognitive dysfunction | 81M | 3.3M | ||
(COVID fog, ICU-related, TBI, cancer-related) |
| * | Figures in table above are based on our management’s good faith estimates based on various publications, public health data and national health statistics including from the NIH and CDC. |

| (1) | Timeframes are estimates and are subject to change—see Disclaimer and Risk Factors. |
| (2) | AKL-T01 is marketed as EndeavorRx in the U.S. for children ages8-12 old with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue. |
| (3) | Shionogi is responsible for the clinical development and commercialization of SDT-001 (a version ofAKL-T01 localized for Japanese language and culture), as well as any future development and commercialization ofAKL-T02, another version of our SSME engine that has been used for our ASD program, each in Japan and Taiwan. |
| (4) | AKL-M01 is designed to use SSME algorithms to monitor and assess certain aspects of cognition, as opposed to providing cognitive therapy. |
| (5) | To the extent we are unable to access additional sources of funding, our current estimated timeframe for initiating a pivotal study in this development program could be delayed. |
| * | Estimated timeframes in figure above correspond to applicable milestone start times, and are subject to change. Please refer to the section entitled “ Risk Factors Risks Related to Our Business and Industry—Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside of our control. If we experience delays or difficulties in the enrollment or retention of patients in clinical trials, our ability to obtain necessary marketing authorizations for our product candidates could be delayed or prevented Risks Related to Our Products—Our current product candidates are in various stages of development. Our product candidates may fail in development or suffer delays that adversely affect their commercial viability. If we fail to maintain clearance, de novo classification or approval to market our product candidates, including AKL-T01 for expanded indications, or if we are delayed in obtaining such marketing authorizations, our business, prospects, results of operations and financial condition could be materially and adversely affected |
| STARS-ADHD pivotal study | ||
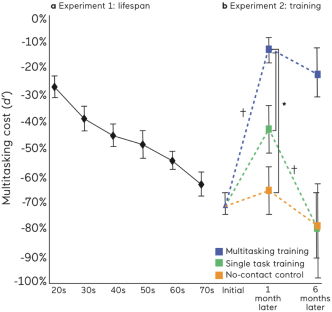
| The pivotal STARS-ADHD study was a 4-week multi-center, randomized, blinded, controlled trial conducted between July 2016 and November 2017 in 348 children aged8-12 years and diagnosed with ADHD. Children enrolled into the study were instructed to useAKL-T01 or an educational-style video game control for approximately 25 minutes a day for 28 days. |  | |
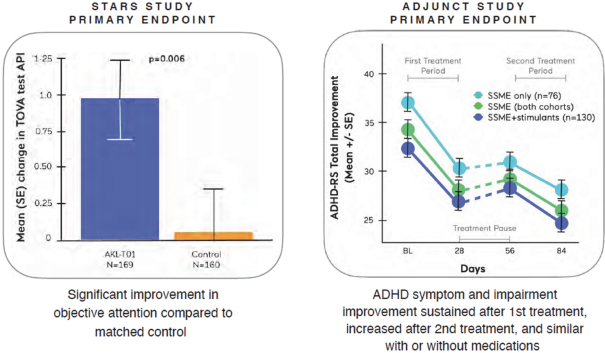
| The predefined primary endpoint of the study was the change from baseline in the TOVA Attention Performance Index (TOVA API), a measure of objective attention for which the study was statistically powered. TOVA is a computerized test cleared by the FDA to assess attention deficits and evaluate the effects of interventions in ADHD; the API is a composite measure of attention functioning. This objective attention endpoint was the primary endpoint for which the study was statistically powered. The control condition used in this study was specifically designed to enable the assessment of changes in the primary endpoint of objective attention. The control was in the form of an educational style word search digital game matched to AKL-T01 for expectation of benefit and time on task.AKL-T01 showed a statistically significant improvement on the TOVA API compared to the control (p=0.006). | ||
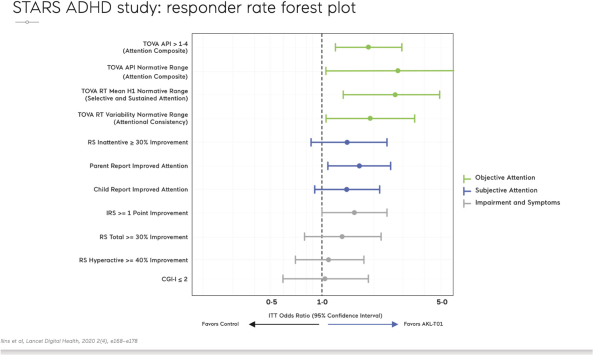
| The study demonstrated statistically significant improvement in the IRS from baseline after one month as well as to the end of the three- month trial in both the children on-stimulants andoff-stimulants (both cohorts: p<0.001). The second period ofAKL-T01 treatment resulted in further increases in efficacy on this primary outcome measure, beyond the effects already seen after the first period of treatment. The magnitude of improvement in IRS throughout the study was similar for children independent of their ADHD medication use. Responder rates for IRS (improvement of greater than 1 point or more on the IRS scale) were 41% and 56% at the end of the first period of treatment withAKL-T01 in theoff-stimulant andon-stimulant groups respectively (50% across both groups). This increased to 69% and 68% respectively by the end of the second period of treatment. Further, across both groups, responder rates forADHD-RS Total (% children with ≥30% improvement) after the first period of treatment was 27% and increased to 45% after the second period of treatment. Additionally, after the second period of treatment, 60% of parents said the intervention helped their child’s attention in real life, and 75% of children reported feeling an improvement in their attention when asked via an exit survey. |  |

| • | Australia, in which the technology does not require a medical prescription; and |
| • | India, in which the technology does not require a medical prescription. |
| • | U.S. commercial launch of EndeavorRx in 8-12 year-old patients with primarily inattentive or combined-type ADHD, who have a demonstrated attention issue: Expected H2 2022 |
| • | Shionogi pivotal trial data in Japan: Expected H2 2023 |
| • | Initiation of TALi technology study in 3-8 year-old children with ADHD: Expected H1 2023 |
| • | Pivotal trial data in adolescent ADHD patients: Expected H2 2023 |
| • | Pivotal trial data in adult ADHD patients: Expected H2 2023 |
The study was conducted at the Children’s Hospital of Philadelphia Center for Autism Research, which enrolled 19 children with autism, aged 9-13 years old and with an average age of 10 years old. Patients received either our investigational treatment(AKL-T02) or a control educational style video game based on a word challenge game. Patients were asked to play the game for 30 minutes a day, five days a week, for four weeks.This pilot study found that not only did the child participants like and engage with our investigational treatment, their attention on the TOVA test of attention improved similar to what was seen in our studies of children with only an ADHD diagnosis. The control video game did not demonstrate improvement in the mean TOVA score. There was one adverse event (decreased frustration tolerance) in the AKL-T02 group; no serious adverse events were reported. |  |
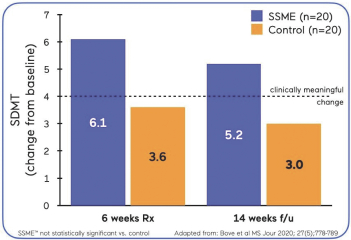
| Our proof of concept study in MDD was a multi-center, randomized, controlled trial of our SSME technology engine, utilizing the AKL-T03 variation of our treatment software, in 74 adult patients diagnosed withmild-to-moderate mild-to-moderate AKL-T03 or a control game. Both groups used the treatment/control at home, five days per week for 25 minutes per day, on a tablet device for six weeks. Following the treatment period, anin-clinic assessment was conducted to assess key outcomes. The primary outcome of the study assessed sustained attention as measured by TOVA, anFDA-cleared objective measure of attention. | 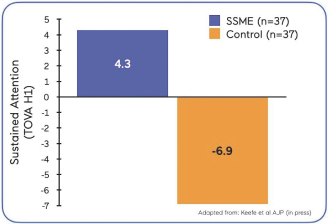 |
| • | SDMT: No difference between group, p=0.21. Both the AKL-T03 and control groups showed statistically significant improvements, p<0.001 and p=0.024, respectively. |
| • | At 8 weeks follow up, responders analysis (clinically meaningful +4 point increase in SDMT relative to baseline SDMT score) was statistically significant favoring AKL-T03, p=0.038. |
| • | PASAT: No difference between group, p=0.93. Both the AKL-T03 and control groups showed statistically significant improvements, p=0.002 and p=0.07 (marginally significant), respectively. |
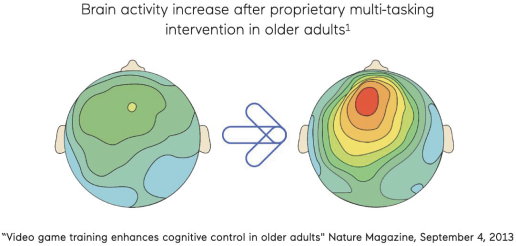
Clinical evidence in acute cognitive function Evaluating the ability of our SSME technology to improve impairments related to acute cognitive dysfunction, we conducted a pilot study between September 2015 and April 2019 of 84 patients with TBI, including 60– 85-year-old HVLT-R learning, delayed recall and recognition. | 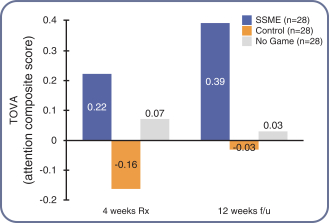 |
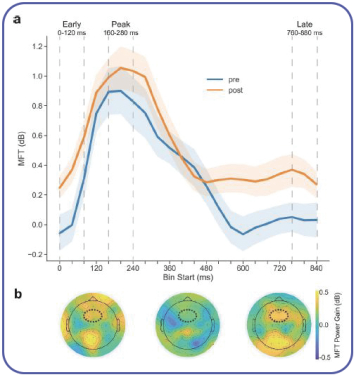
 We also conducted a pilot study in 54 healthy older adults in collaboration with Pfizer. The study showed the potential for SSME assessment to detect cognitive differences between amyloid positive and amyloid negative status, where amyloid is a protein biomarker associated with a higher risk of progression to dementia, in otherwise healthy individuals (graph on right). | 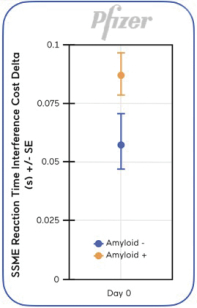 |
| • | Consumer-driven model: |
| • | Active participation by a physician: |
| • | Delivered as a comprehensive care program: |
| • | Coverage by formulary decision-makers: self-pay/reimbursement model to enable growth in the short term with potential to track toward expanded access via insurance coverage over time. |
| • | Power of data to inform and adapt: |
PHASE | PRE-LAUNCH | PRE-LAUNCH | PRE-LAUNCH | |||||||||||
METRIC | FY 2021 | Q 1’22 | GROWTH VS. Q1’21 | |||||||||||
DEMAND | Total Rx Written | 1,713 | 668 | 229 | % | |||||||||
| New Rx | 1,422 | 503 | 166 | % | ||||||||||
| Refill Rx | 291 | 165 | 1079 | % | ||||||||||
ENGAGEMENT | Total Prescribers | 877 | 437 | 191 | % | |||||||||
| New Prescribers | 832 | 239 | 96 | % | ||||||||||
| Conversion (Enrolled To Dispense) | 57 | % | 53 | % | (14 | %) | ||||||||
REIMBURSEMENT | % of TRx Self Paid | 86 | % | 89 | % | N/A | ||||||||
| % of TRx Reimbursed | 10 | % | 4.3 | % | N/A | |||||||||
| Total Covered Lives | 2.8 | M | 2.6 | M | N/A | |||||||||

| • | families with children not well-managed on ADHD drugs due to side effects or lack of efficacy, |
| • | families choosing to not put their children on ADHD drugs, and |
| • | families with children actively taking ADHD drugs but looking for additional options to add to their treatment. |

| * | Estimated timeframes in figure above correspond to applicable milestone start times, and are subject to change—please refer to the section entitled “ Risk Factors Risks Related to Our Business and Industry—Enrollment and retention of patients in clinical trials is an expensive and time- consuming process and could be made more difficult or rendered impossible by multiple factors outside of our control. If we experience delays or difficulties in the enrollment or retention of patients in clinical trials, our ability to obtain necessary marketing authorizations for our product candidates could be delayed or |
prevented Risks Related to Our Products—Our current product candidates are in various stages of development. Our product candidates may fail in development or suffer delays that adversely affect their commercial viability. If we fail to maintain clearance, de novo classification or approval to market our product candidates, including AKL-T01 for expanded indications, or if we are delayed in obtaining such marketing authorizations, our business, prospects, results of operations and financial condition could be materially and adversely affected |
| ** | Population data in table above are estimates based on our management’s good faith estimates based on various publications, public health data, national health statistics including from the NIH and CDC and partner research data. |
| • | An exclusive license from UCSF to a patent family directed to software and methods for enhancing cognition via a task performed in the presence of interferences (distractions and/or interrupters) (see “Agreements/Third Parties—UCSF Neuroracer Agreement” below for a description of this exclusive license agreement). Two U.S. patents, five Japanese patents and one Canadian patent have been allowed in this family, the patents expiring as early as 2031 (Japan and Canada) and 2032 (U.S.). Additional applications are pending in this family in Australia, Canada, and Europe. |
| • | An exclusive license from UCSF to a patent family directed to software and methods for enhancing cognition via a task with both a physical and cognitive component. One Japanese patent has been allowed in this family, expiring as early as 2035. Additional applications are pending in this family in the U.S., Australia, Canada, Europe, Hong Kong and Japan. |
| • | A patent family directed to a personalized cognitive training regimen through difficulty progression. One U.S. patent, one Japanese patent and one South Korean patent have been allowed in this family and will expire as early as 2035. Additional applications are pending in this family in Australia, Canada, Europe and Hong Kong. |
| • | A patent family directed to processor-implemented systems and methods for measuring cognitive abilities. One U.S. patent has been allowed in this family and will expire as early as 2036. Additional applications are pending in this family in Australia, Canada, Europe, Japan and South Korea. |
| • | A patent family directed to signal detection metrics in adaptive response-deadline procedures. One Chinese patent has been allowed in this family and will expire as early as 2037. Additional applications are pending in this family in the U.S., Australia, Canada, Europe, Japan and South Korea. |
| • | A patent family directed to audio-only interference training for cognitive disorder screening and treatment. One U.S. patent has been allowed in this family and will expire as early as 2039. Additional applications are pending in this family in China, Hong Kong, South Korea, Canada, Europe, Australia and Japan. |
| • | A patent family directed to facial expression detection for screening and treatment of affective disorders. One U.S. patent has been allowed in this family and will expire as early as 2039. Additional applications are pending in this family in China, Hong Kong, South Korea, Canada, Europe, Australia and Japan. |
| • | A patent family directed to a platform configured to render computerized emotional/affective elements for use as stimuli in computerized tasks. One South Korean patent and one Chinese patent have been allowed in this family and will expire as early as 2037. Additional applications are pending in this family in Australia, Canada, Europe, Japan, the U.S, and Hong Kong. |
| • | A patent family directed to a cognitive platform coupled with a physiological component. One U.S. patent, one Japanese patent, and one South Korean patent have been allowed in this family and will expire as early as 2037. Additional applications are pending in this family in Australia, Canada, China, Europe, and Hong Kong. |
| • | A pending patent family directed to a cognitive platform for deriving effort metric for optimizing cognitive treatment, with applications pending in the U.S., Canada, Europe, Australia, Japan, South Korea, China and Hong Kong. If any patents are allowed in this family, they could expire as early as 2039. |
| • | A pending patent family directed to a distributed network for the secured collection, analysis, and sharing of data across platforms, with applications pending in the U.S., Canada, Europe, Australia, Japan and China. If any patents are allowed in this family, they could expire as early as 2038. |
| • | A pending patent family directed to systems and methods for scientific evaluation of program code outputs, with applications pending in the U.S. and pursuant to the international Patent Cooperation Treaty. If any patents are allowed in this family, they could expire as early as 2040. |
| • | A pending patent family directed to systems and methods for software design control and quality assurance, with applications pending in the U.S., Taiwan, and pursuant to the international Patent Cooperation Treaty. If any patents are allowed in this family, they could expire as early as 2040. |
| • | A pending patent family directed to a system and method for adaptive configuration of computerized cognitive training programs, with an application pending in the U.S. If any patents are allowed in this family, they could expire as early as 2041. |
| • | A pending patent family directed to a method for algorithmic rendering of graphical user interface elements, with a provisional application pending in the U.S. If any patents are allowed in this family, they could expire as early as 2041. |
| • | A pending patent family directed to a method and system for determining equitable benefit in digital products and services, with a provisional application pending in the U.S. If any patents are allowed in this family, they could expire as early as 2042. |
| • | A pending patent family directed to a cognitive platform including computerized evocative elements in modes, with applications pending in the U.S., Australia, Canada, China, Europe, Hong Kong, Japan, and South Korea. If any patents are allowed in this family, they could expire as early as 2037. |
| • | A pending patent family directed to a cognitive platform including computerized elements, with applications pending in the U.S., Canada, Europe, Japan, Australia, China, and Hong Kong. If any patents are allowed in this family, they could expire as early as 2038. |
| • | A pending patent family directed to a cognitive platform for identification of biomarkers and other types of markers, with applications pending in the U.S., Europe, Canada, Australia, and Japan. If any patents are allowed in this family, they could expire as early as 2037. |
| • | A pending patent family directed to a platform for identification of biomarkers using navigation tasks and treatments using navigation tasks, with applications pending in the U.S., Australia, Canada, China, Europe, Japan, and South Korea. If any patents are allowed in this family, they could expire as early as 2037. |
| • | A pending patent family directed to cognitive screens, monitor and cognitive treatments targeting immune-mediated and neuro-degenerative disorders, with applications pending in the U.S., Taiwan, Canada, Europe, Australia, Japan, China, Hong Kong, and South Korea. If any patents are allowed in this family, they could expire as early as 2039. |
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or |
service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | federal civil and criminal false claims laws, including the False Claims Act (“FCA”), which can be enforced through civil “qui tam” or “whistleblower” actions and civil monetary penalty laws, impose criminal and civil penalties against individuals or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other federal health care programs that are false or fraudulent; knowingly making or causing a false statement material to a false or fraudulent claim or an obligation to pay money to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing such an obligation. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payers if they are deemed to “cause” the submission of false or fraudulent claims. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in the proceeds of any monetary recovery; |
| • | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payer (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating these statutes without actual knowledge of the statutes or specific intent to violate them in order to have committed a violation; |
| • | the federal Physician Payment Sunshine Act, created under the ACA and its implementing regulations, which require manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the US Department of Health and |
| • | Human Services (“HHS”) information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations will extend to include payments and transfers of value made to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives during the previous year; |
| • | federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs; |
| • | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and |
| • | analogous state and foreign laws and regulations, such as state and foreign anti-kickback, false claims, consumer protection and unfair competition laws which may apply to pharmaceutical business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving healthcare items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government that otherwise restricts payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to file reports with states regarding pricing and |
marketing information, such as the tracking and reporting of gifts, compensations and other remuneration and items of value provided to healthcare professionals and entities; and state and local laws requiring the registration of pharmaceutical sales representatives. |
| • | increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1% of the average manufacturer price; |
| • | required collection of rebates for drugs paid by Medicaid managed care organizations; |
| • | required manufacturers to participate in a coverage gap discount program, under which they must agree to offer 70 percent point-of-sale |
| • | imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs. |
| • | On January 2, 2013, the U.S. American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers. |
| • | On April 13, 2017, CMS published a final rule that gives states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans sold through such marketplaces. |
| • | On May 30, 2018, the Right to Try Act, was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA permission under the FDA expanded access program. There is no obligation for a pharmaceutical manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act. |
| • | On May 23, 2019, CMS published a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. |
| • | On December 20, 2019, former President Trump signed into law the Further Consolidated Appropriations Act (H.R. 1865), which repealed the Cadillac tax, the health insurance provider tax and the medical device excise tax. It is impossible to determine whether similar taxes could be instated in the future. |
| • | establishment registration and device listing; |
| • | compliance with FDA’s QSR requirements; |
| • | labeling regulations; |
| • | medical device reporting regulations, which, for example, require that manufacturers report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
| • | voluntary and mandatory device recalls to address problems when a device is defective and/or could be a risk to health; and |
| • | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health. |
| • | warning letters or untitled letters that require corrective action; |
| • | fines, injunctions and civil penalties; |
| • | recall or seizure of products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | withdrawing PMA approvals already granted; and; |
| • | criminal prosecution. |
Three Months Ended June 30, | Six Months Ended June 30, | Years Ended December 31, | ||||||||||||||||||||||
| (dollars in thousands, except per share data) | 2022 | 2021 | 2022 | 2021 | 2021 | 2020 | ||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||
Total revenue | $ | 64 | $ | 105 | $ | 130 | $ | 222 | $ | 538 | $ | 3,939 | ||||||||||||
Total cost of revenues and operating expenses | 22,403 | 14,663 | 44,194 | 23,526 | 61,257 | 29,375 | ||||||||||||||||||
Loss from operations | (22,339 | ) | (14,558 | ) | (44,064 | ) | (23,304 | ) | (60,719 | ) | (25,436 | ) | ||||||||||||
Other income (expense): | ||||||||||||||||||||||||
Other income | 40 | 4 | 49 | 5 | 17 | 124 | ||||||||||||||||||
Interest expense | (194 | ) | (39 | ) | (370 | ) | (120 | ) | (465 | ) | (333 | ) | ||||||||||||
Loss on extinguishment of debt | — | (181 | ) | — | (181 | ) | (181 | ) | — | |||||||||||||||
Net loss before income taxes | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,600 | ) | $ | (61,348 | ) | $ | (25,645 | ) | ||||||
Income tax expense | $ | — | $ | — | $ | — | $ | 1 | $ | — | $ | 1 | ||||||||||||
Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,601 | ) | $ | (61,348 | ) | $ | (25,646 | ) | ||||||
Unrealized (loss) gain on short-term investments | $ | (1 | ) | $ | — | $ | (7 | ) | $ | — | $ | — | $ | — | ||||||||||
Comprehensive loss | $ | (22,494 | ) | $ | (14,774 | ) | $ | (44,392 | ) | $ | (23,601 | ) | $ | (61,348 | ) | $ | (25,646 | ) | ||||||
Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,601 | ) | $ | (61,348 | ) | $ | (25,646 | ) | ||||||
Dividends on Series D convertible preferred stock | (2,908 | ) | (1,115 | ) | (5,785 | ) | (1,115 | ) | (6,660 | ) | — | |||||||||||||
Redemption value of Series D convertible preferred stock | (1,455 | ) | (55,877 | ) | (2,893 | ) | (55,877 | ) | (58,649 | ) | — | |||||||||||||
Net loss attributable to common shareholders | $ | (26,856 | ) | $ | (71,766 | ) | $ | (53,063 | ) | $ | (80,593 | ) | $ | (126,657 | ) | $ | (25,646 | ) | ||||||
Net loss per share - basic and diluted | $ | (18.25 | ) | $ | (61.98 | ) | $ | (36.18 | ) | $ | (69.60 | ) | $ | (105.77 | ) | $ | (22.20 | ) | ||||||
June 30, 2022 | December 31, | |||||||||||
| (in thousands) | 2021 | 2020 | ||||||||||
Balance Sheet Data: | ||||||||||||
Cash and cash equivalents | $ | 40,638 | $ | 76,899 | $ | 18,528 | ||||||
Working capital, net (1) | 40,554 | 71,692 | 15,230 | |||||||||
Total assets | 55,015 | 80,937 | 20,181 | |||||||||
Total liabilities | 29,501 | 15,175 | 8,197 | |||||||||
Redeemable convertible preferred stock | 300,554 | 291,876 | 116,886 | |||||||||
Total stockholders' equity (deficit) | (275,040 | ) | (226,114 | ) | (104,902 | ) | ||||||
Statement of Cash Flows Data: | ||||||||||||
Net cash used in operating activities | $ | (41,379 | ) | $ | (53,982 | ) | $ | (24,551 | ) | |||
Net cash used in investing activities | (4,987 | ) | (492 | ) | (116 | ) | ||||||
Net cash provided by financing activities | 10,105 | 112,845 | 1,998 | |||||||||
| (1) | Working capital, net is defined as current assets less current liabilities. |
| • | expenses incurred in connection with the development of our pipeline of products; |
| • | personnel-related expenses, including salaries, bonuses, benefits and stock-based compensation for employees engaged in R&D functions; |
| • | cost of clinical trials; |
| • | cost of regulatory submissions, reviews, external consultants; |
| • | expenses incurred in connection with the discovery and development of our products, including under agreements with third parties, such as consultants; |
| • | expenses incurred under agreements with consultants who supplement our internal capabilities, including software development; and |
| • | facilities, depreciation and other expenses, which include direct and allocated expenses, such as rent and maintenance of facilities, insurance and other operating costs. |
| • | attention in ASD: pilot study completed and planning on an FDA Q-submission meeting in the second half of 2022 to discuss our next planned development phase; |
| • | cognitive dysfunction in MS: POC completed and planning on an FDA Q-submission meeting in the first half of 2023 to discuss our next planned development phase; and |
| • | cognitive dysfunction in MDD: POC completed and planning on an FDA Q-submission meeting in the second half of 2023 to discuss our next planned development phase. |
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||||||||||||
| (dollars in thousands, except percentages) | 2022 | 2021 | $ Change | % Change | 2022 | 2021 | $ Change | % Change | ||||||||||||||||||||||||
Revenues | $ | 64 | $ | 105 | $ | (41 | ) | -39 | % | $ | 130 | $ | 222 | $ | (92 | ) | -41 | % | ||||||||||||||
Cost of revenues | 97 | 98 | (1 | ) | -1 | % | 193 | 160 | 33 | 21 | % | |||||||||||||||||||||
Gross profit (loss) | (33 | ) | 7 | (40 | ) | -571 | % | (63 | ) | 62 | (125 | ) | -202 | % | ||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||||||
Research and development | 7,358 | 4,039 | 3,319 | 82 | % | 13,662 | 7,983 | 5,679 | 71 | % | ||||||||||||||||||||||
Selling, general and administrative | 14,948 | 10,526 | 4,422 | 42 | % | 30,339 | 15,383 | 14,956 | 97 | % | ||||||||||||||||||||||
Total operating expenses | 22,306 | 14,565 | 7,741 | 53 | % | 44,001 | 23,366 | 20,635 | 88 | % | ||||||||||||||||||||||
Operating loss | (22,339 | ) | (14,558 | ) | (7,781 | ) | 53 | % | (44,064 | ) | (23,304 | ) | (20,760 | ) | 89 | % | ||||||||||||||||
Other income (expense): | ||||||||||||||||||||||||||||||||
Other income | 40 | 4 | 36 | 900 | % | 49 | 5 | 44 | 880 | % | ||||||||||||||||||||||
Interest expense | (194 | ) | (39 | ) | (155 | ) | 397 | % | (370 | ) | (120 | ) | (250 | ) | 208 | % | ||||||||||||||||
Extinguishment of debt | — | (181 | ) | 181 | * | — | (181 | ) | 181 | -100 | % | |||||||||||||||||||||
Total other income (expense) | (154 | ) | (216 | ) | 62 | -29 | % | (321 | ) | (296 | ) | (25 | ) | 8 | % | |||||||||||||||||
Loss before income taxes | (22,493 | ) | (14,774 | ) | (7,719 | ) | 52 | % | (44,385 | ) | (23,600 | ) | (20,785 | ) | 88 | % | ||||||||||||||||
Expense from income taxes | — | — | — | — | — | 1 | (1 | ) | -100 | % | ||||||||||||||||||||||
Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (7,719 | ) | 52 | % | $ | (44,385 | ) | $ | (23,601 | ) | $ | (20,784 | ) | 88 | % | ||||||||||
Unrealized (loss) gain on short-term investments | $ | (1 | ) | $ | — | $ | (1 | ) | * | $ | (7 | ) | $ | — | $ | (7 | ) | * | ||||||||||||||
Comprehensive loss | $ | (22,494 | ) | $ | (14,774 | ) | $ | (7,720 | ) | 52 | % | $ | (44,392 | ) | $ | (23,601 | ) | $ | (20,791 | ) | 88 | % | ||||||||||
| * | Percentage change not meaningful |
| • | an increase of $1.9 million of personnel related expenses due to an increase in R&D personnel headcount, which increased the expense from $2.9 million in the three months ended June 30, 2021 to $4.8 million in the three months ended June 30, 2022; |
| • | an increase of $0.8 million of clinical studies and expenses incurred in discovery and development primarily due to age expansion studies for ADHD patients in the 13-17 year old and adult categories as well as collaborative studies to treat acute cognitive dysfunction inCOVID-19 survivors, which increased from $0.6 million in the three months ended June 30, 2021 to $1.4 million in the three months ended June 30, 2022; |
| • | an increase of $0.2 million of computer and software equipment expenses due to an increase in software subscriptions, which increased the expense from $0.2 million for the three months ended June 30, 2021 to $0.4 million for the three months ended June 30, 2022; and |
| • | an increase of $0.5 million related to various other expenses such as an increase in external consulting fees and travel expenses, which increased the expense from $0.3 million for the three months ended June 30, 2021 to $0.8 million for the three months ended June 30, 2022. |
| • | an increase of $3.1 million of personnel related expenses due to an increase in R&D personnel headcount, which increased the expense from $6.1 million in the six months ended June 30, 2021 to $9.2 million in the six months ended June 30, 2022; |
| • | an increase of $1.9 million of clinical studies and expenses incurred in discovery and development primarily due to age expansion studies for ADHD patients in the 13-17 year old and adult categories as well as collaborative studies to treat acute cognitive dysfunction inCOVID-19 survivors, which increased from $0.8 million in the six months ended June 30, 2021 to $2.7 million in the six months ended June 30, 2022; |
| • | an increase of $0.3 million of computer and software equipment expenses due to an increase in software subscriptions, which increased the expense from $0.3 million for the six months ended June 30, 2021 to $0.6 million for the six months ended June 30, 2022; and |
| • | an increase of $0.4 million related to various other expenses such as an increase in external consulting fees and travel expenses, which increased the expense from $0.8 million for the six months ended June 30, 2021 to $1.2 million for the six months ended June 30, 2022. |
| • | an increase of $2.9 million in personnel-related costs, primarily due to the build-out of our HR, marketing and sales departments; |
| • | an increase of $1.3 million in consulting, legal, accounting and other professional service costs; |
| • | an increase of $0.6 million related to various other expenses such as software subscriptions and travel expenses, and |
| • | a decrease of $0.4 million in marketing and advertising costs. |
| • | an increase of $4.7 million in marketing and advertising costs; |
| • | an increase of $5.6 million in personnel-related costs, primarily due to the build-out of our HR, marketing and sales departments; |
| • | an increase of $3.7 million in consulting, legal, accounting and other professional service costs; and |
| • | an increase of $0.9 million related to various other expenses such as software subscriptions and travel expenses. |
December 31, | ||||||||||||||||
| (dollars in thousands, except percentages) | 2021 | 2020 | $ Change | % Change | ||||||||||||
Revenues | $ | 538 | $ | 3,939 | $ | (3,401 | ) | -86 | % | |||||||
Cost of revenues | 355 | 416 | (61 | ) | -15 | % | ||||||||||
Net margin | 183 | 3,523 | (3,340 | ) | -95 | % | ||||||||||
Operating expenses: | ||||||||||||||||
Research and development | 18,234 | 15,418 | 2,816 | 18 | % | |||||||||||
Selling, general and administrative | 42,668 | 13,541 | 29,127 | 215 | % | |||||||||||
Total operating expenses | 60,902 | 28,959 | 31,943 | 110 | % | |||||||||||
Operating loss | (60,719 | ) | (25,436 | ) | (35,283 | ) | 139 | % | ||||||||
Other income (expense): | ||||||||||||||||
Other income | 17 | 124 | (107 | ) | -86 | % | ||||||||||
Interest expense | (465 | ) | (333 | ) | (132 | ) | 40 | % | ||||||||
Loss on extinguishment of debt | (181 | ) | — | (181 | ) | * | ||||||||||
Total other income (expense) | (629 | ) | (209 | ) | (420 | ) | 201 | % | ||||||||
Loss before income taxes | (61,348 | ) | (25,645 | ) | (35,703 | ) | 139 | % | ||||||||
Expense from income taxes | — | 1 | (1 | ) | -100 | % | ||||||||||
Net loss | $ | (61,348 | ) | $ | (25,646 | ) | $ | (35,702 | ) | 139 | % | |||||
| * | Percentage change not meaningful. |
| • | an increase of $1.6 million of personnel related expenses from $11.4 million in 2020 to $13.0 million in 2021 due to an increase in R&D personnel headcount; |
| • | a decrease of $0.9 million of external consulting fees from $1.6 million in 2020 to $0.7 million in 2021 due to an increase in R&D personnel headcount, which allowed more projects to be performed internally; |
| • | a decrease of $0.2 million of facilities and overhead allocation from $1.0 million in 2020 to $0.8 million in 2021 due to a lower allocation to R&D expenses as the SG&A headcount increased at a faster rate; |
| • | an increase of $2.3 million of clinical studies and expenses incurred in discovery and development from $0.6 million in 2020 to $2.9 million in 2021 primarily due to age expansion studies for ADHD patients in the 13-17 year old and adult categories as well as collaborative studies to treat acute cognitive dysfunction inCOVID-19 survivors; and |
| • | an increase of $0.2 million of computer and software equipment expense from $0.6 million in 2020 to $0.8 million in 2021. |
| • | an increase of $18.9 million in marketing and advertising costs; |
| • | an increase of $4.2 million in consulting, legal, accounting and other professional service costs; |
| • | an increase of $5.0 million in personnel-related costs, primarily due to the build-out of our HR, marketing and sales departments; and |
| • | an increase of $1.1 million related to various other expenses such as software subscriptions and rent expense. |
| • | our revenue growth; |
| • | the ability of our patients to obtain third-party payer reimbursement for our current product; |
| • | the amount and timing of sales and other revenues from our product candidates, if approved, including the sales price and the availability of coverage and adequate third-party payer reimbursement; |
| • | our sales and marketing activities; |
| • | our R&D efforts; |
| • | the emergence and effect of competing or complementary products; |
| • | the outcome, timing and cost of meeting regulatory requirements established by the FDA, or comparable foreign regulatory authorities; |
| • | the progress, timing, scope and costs of our preclinical studies, clinical trials, potential future clinical trials and other related activities; |
| • | the costs of commercialization activities for any of our product candidates that receive marketing authorization, including the costs and timing of establishing product sales, marketing and hosting capabilities, or entering into strategic collaborations with third parties to leverage or access these capabilities; |
| • | the cash requirements of any future discovery of product candidates; |
| • | our ability to retain our current employees and the need to hire additional management and sales, technical and medical personnel; |
| • | the extent to which we acquire or invest in business, products or technology; and |
| • | the impact of the COVID-19 pandemic. |
Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||
2022 | 2021 | 2021 | 2020 | |||||||||||||
(in thousands) | (in thousands) | |||||||||||||||
Net cash used in operating activities | $ | (41,379 | ) | $ | (21,229 | ) | $ | (53,982 | ) | $ | (24,551 | ) | ||||
Net cash used in investing activities | (4,987 | ) | (103 | ) | (492 | ) | (116 | ) | ||||||||
Net cash provided by financing activities | 10,105 | 112,581 | 112,845 | 1,998 | ||||||||||||
Net increase (decrease) in cash | $ | (36,261 | ) | $ | 91,249 | $ | 58,371 | $ | (22,669 | ) | ||||||
| • | the prices at which we sold our preferred stock to outside investors in arm’s-length transactions; |
| • | our results of operations, financial position, and capital resources; |
| • | contemporaneous third-party valuations common stock; |
| • | rights, preferences, and privileges of convertible preferred stock relative to common stock; |
| • | the lack of marketability of common stock; |
| • | stage and development of Akili’s business; |
| • | the history and nature of our business, industry trends and competitive environment; |
| • | general economic conditions; and |
| • | the likelihood of achieving a liquidity event, such as an initial public offering or sale of Akili, given prevailing market conditions. |
Name | Age | Title | ||
Executive Officers: | ||||
W. Edward Martucci, Ph.D. | 40 | Chief Executive Officer and Director | ||
Santosh Shanbhag | 45 | Chief Financial Officer | ||
Matthew Franklin | 49 | President and Chief Operating Officer | ||
Anil Jina, MB, BCh, BAO | 51 | Chief Medical Officer | ||
Jacqueline Studer, J.D. | 63 | Chief Legal Officer | ||
Jonathan David . | 44 | Chief Product Officer | ||
Non-Executive Directors: | ||||
Chamath Palihapitiya | 45 | Chairman and Director | ||
Bharatt Chowrira, J.D., Ph.D. | 57 | Director | ||
Kenneth Ehlert | 53 | Director | ||
Adam Gazzaley, M.D., Ph.D. | 53 | Director | ||
William “BJ” Jones, Jr. | 59 | Director | ||
Christine Lemke | 45 | Director |
| • | the Class I directors are Kenneth Ehlert and Bharatt Chowrira, J.D., Ph.D., and their terms will expire at the annual meeting of stockholders to be held in 2023; |
| • | the Class II directors are Christine Lemke and William “BJ” Jones, Jr., and their terms will expire at the annual meeting of stockholders to be held in 2024; and |
| • | the Class III directors are W. Edward Martucci, Ph.D., Chamath Palihapitiya and Adam Gazzaley, M.D., Ph.D., and their terms will expire at the annual meeting of stockholders to be held in 2025. |
| • | selecting a qualified firm to serve as the independent registered public accounting firm to audit the financial statements of the company; |
| • | helping to ensure the independence and performance of the independent registered public accounting firm; |
| • | discussing the scope and results of the audit with the independent registered public accounting firm, and reviewing, with management and the independent accountants, the annual audited financial statements and quarterly financial statements of the company; |
| • | developing procedures for employees to submit concerns anonymously about questionable accounting or audit matters; |
| • | reviewing policies on risk assessment and risk management; |
| • | reviewing related party transactions; |
| • | obtaining and reviewing a report by the independent registered public accounting firm at least annually that describes the internal quality-control procedures, any material issues with such procedures, and any steps taken to deal with such issues when required by applicable law; and |
| • | approving (or, as permitted, pre-approving) all audit and all permissiblenon-audit service to be performed by the independent registered public accounting firm. |
| • | recommending to the company board goals and objectives, non-equity compensation, and equity grants of all senior officers; |
| • | recommending to the company board goals and objectives, non-equity compensation, and equity grants for the Chief Executive Officer; |
| • | recommending to the board non-equity compensation and equity grants for the directors and the Chair; |
| • | selecting independent compensation consultants and assessing whether there are any conflicts of interest with any of the committee’s compensation advisors; |
| • | reviewing and approving employment agreements, severance arrangements and change-of-control |
| • | reviewing the policies relating to compensation and benefits of employees. |
| • | identify individuals qualified to become board members, consistent with criteria approved by the board of directors; |
| • | recommend that the board of directors select the nominees for election as directors at each annual meeting of stockholders; |
| • | develop and recommend to the board of directors corporate governance guidelines and periodically review those guidelines and recommend any changes; and |
| • | oversee an annual evaluation of the board of directors, its committees and management. |
Non-Equity | ||||||||||||||||||||||||||||
Option | Incentive Plan | All Other | ||||||||||||||||||||||||||
Salary | Bonus | Awards | Compensation | Compensation | Total | |||||||||||||||||||||||
Name and Principal Position | Year | ($) | ($) (1) | ($) (2) | ($) (3) | ($) (4) | ($) | |||||||||||||||||||||
W. Edward Martucci | 2021 | 400,000 | — | 2,221,997 | 162,000 | 9,800 | 2,793,797 | |||||||||||||||||||||
Chief Executive Officer | — | |||||||||||||||||||||||||||
Jacqueline Studer | 2021 | 367,800 | 25,000 | 394,380 | 99,306 | 9,800 | 896,286 | |||||||||||||||||||||
Chief Legal Officer | ||||||||||||||||||||||||||||
Santosh Shanbhag | 2021 | 343,419 | 25,000 | 379,024 | 92,723 | 9,800 | 849,966 | |||||||||||||||||||||
Chief Financial Officer | ||||||||||||||||||||||||||||
| (1) | The amounts reported represent discretionary bonuses earned by Ms. Studer and Mr. Shanbhag for performance during December 31, 2021. |
| (2) | The amounts reported represent the aggregate grant date fair value of stock option awards granted to our named executive officers during the fiscal year ended December 31, 2021, computed in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures related to service-based vesting. A discussion of the assumptions used in determining grant date fair value may be found in Note 9 to Akili’s financial statements for the year ended December 31, 2021, included elsewhere in this prospectus. These amounts also include the incremental fair value of certain modified stock options associated with a stock option repricing in 2021 (determined as of such repricing date in accordance with FASB ASC Topic 718). These amounts do not correspond to the actual value that may be recognized by the named executive officers upon exercise of the applicable award or sale of the underlying shares of stock. |
| (3) | The amounts in the “Non-Equity Incentive Plan Compensation” column reflects the actual payout of the 2021 cash bonus, as describe in more details below. |
| (4) | The amounts reported represent matching contributions contributed by Akili to each named executive’s account in Akili’s 401(k) plan. |
Name | Base Salary | |||
W. Edward Martucci | $ | 400,000 | ||
Jacqueline Studer (1) | $ | 380,000 | ||
Santosh Shanbhag (2) | $ | 350,000 | ||
| (1) | Ms. Studer’s base salary was increased from $328,000 to $380,000 effective as of March 26, 2021. |
| (2) | Mr. Shanbhag’s base salary was increased from $317,200 to $350,000 effective March 12, 2021. |
Name | Target Bonus Percentage | Actual Payout | ||||||
W. Edward Martucci | 45 | % | 162,000 | |||||
Jacqueline Studer | 30 | % | 99,306 | |||||
Santosh Shanbhag | 30 | % | 92,723 | |||||
Option Awards (1) | ||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Vesting Commencement Date (“VCD”) | Option Exercise Price ($) | Option Expiration Date | |||||||||||||||
W. Edward Martucci | 75,271 | (2) | — | 4/3/2015 | 2.38 | 8/26/2025 | ||||||||||||||
| 276,540 | (3) | — | 5/6/2016 | 2.46 | 5/20/2026 | |||||||||||||||
| 470,112 | (4) | 156,705 | (4) | 4/2/2019 | 4.40 | 10/2/2028 | ||||||||||||||
| 9,375 | (5) | 5,625 | (5) | 3/15/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 2,500 | (6) | 2,500 | (6) | 11/18/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 90,416 | (7) | 529,584 | (7) | 6/25/2021 | 4.40 | 9/29/2031 | ||||||||||||||
Jacqueline Studer | 125,000 | (8) | 75,000 | (8) | 3/26/2020 | 4.40 | 6/12/2029 | |||||||||||||
| 9,375 | (5) | 5,625 | (5) | 3/15/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 2,500 | (6) | 2,500 | (6) | 11/18/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 15,312 | (7) | 89,688 | (7) | 6/25/2021 | 4.40 | 9/29/2031 | ||||||||||||||
Santosh Shanbhag | 189,687 | (9) | 113,813 | (9) | 3/12/2020 | 4.40 | 6/12/2029 | |||||||||||||
| 9,375 | (5) | 5,625 | (5) | 3/15/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 2,500 | (6) | 2,500 | (6) | 11/18/2020 | 4.40 | 5/21/2030 | ||||||||||||||
| 13,854 | (7) | 81,146 | (7) | 6/25/2021 | 4.40 | 9/29/2031 | ||||||||||||||
| (1) | Each equity award was granted under Akili’s Amended and Restated 2011 Stock Incentive Plan (the “2011 Plan”). |
| (2) | Represents an option to purchase shares of common stock granted on April 3, 2015. The shares underlying this option vested as follows: 50% of the shares vested on the vesting commencement date (the “VCD”) and 16.67% vested on each 12 month anniversary of the VCD, subject to continued service through the applicable vesting date. |
| (3) | Represents an option to purchase shares of common stock granted on May 6, 2016. The shares underlying this option vest as follows: 28.6% of the shares vested on the VCD and 8.925% vests at the end of each 6 month period, subject to continued service through the applicable vesting date. |
| (4) | Represents an option to purchase shares of common stock granted on April 2, 2019. The shares underlying this option vest as follows: 12.5% of the shares vested on the VCD and 12.5% vested at the end of each 6 month period, subject to continued service through the applicable vesting date. |
| (5) | Represents an option to purchase shares of common stock granted on March 15, 2020. The shares underlying this option vest as follows: 25% of the shares vested on the VCD and 12.5% vests at the end of each 6 month period, subject to continued service through the applicable vesting date. |
| (6) | Represents an option to purchase shares of common stock granted on November 18, 2020. The shares underlying this option vest as follows: 16.67% of the shares vested on the VCD and 16.67% vests at the end of each 6 month period, subject to continued service through the applicable vesting date. |
| (7) | Represents an option to purchase shares of common stock granted on June 25, 2021. The shares underlying this option vest and become exercisable in 48 equal monthly installments over four years, subject to continued service through the applicable vesting date. |
| (8) | Represents an option to purchase shares of common stock granted on March 26, 2020. The shares underlying this option vest as follows: 25% of the shares vested on the VCD and 12.5% vests at the end of each 6 month period, subject to continued service through the applicable vesting date. |
| (9) | Represents an option to purchase shares of common stock granted on March 12, 2020. The shares underlying this option vest as follows: 25% of the shares vested on the VCD and 12.5% vested at the end of each 6 month period, subject to continued service through the applicable vesting date. |
Name | Fees Earned or Paid in Cash ($) | Option Awards ($) (1) | All Other Compensation ($) | Total ($) | ||||||||||||
Robert Perez* (2) | 100,000 | 589,841 | — | 689,841 | ||||||||||||
Adam Gazzaley (3) | — | 89,411 | 100,000 | 189,411 | ||||||||||||
Christine Lemke (4) | 13,125 | 237,623 | — | 250,748 | ||||||||||||
James Gates* (5) | — | — | — | — | ||||||||||||
John Spinale* (6) | — | — | — | — | ||||||||||||
Bharatt Chowrira* (7) | — | — | — | — | ||||||||||||
Ken Ehlert (8) | 3,646 | — | — | 3,646 | ||||||||||||
| * | Not serving as a director of Akili, Inc. |
| (1) | The amounts reported represent the aggregate grant date fair value of the stock option awards granted to Akili’s directors during 2021, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures related to service-based vesting. A discussion of the assumptions used in determining grant date fair value may be found in Note 9 to Akili’s financial statements for the year ended December 31, 2021, included elsewhere in this prospectus. These amounts also include the incremental fair value of certain modified stock options associated with a stock option repricing in 2021(determined as of such repricing date in accordance with FASB ASC Topic 718). The amounts reported in this column reflect the accounting cost for these stock option awards and do not correspond to the actual economic value that may be received by such directors upon the exercise of the stock option awards or any sale of the underlying shares. |
| (2) | Mr. Perez has entered into an agreement with Akili, pursuant to which he is entitled to receive a fee of $8,333.33 per month for certain services provided to the Company solely with respect to his role as Executive Chairman. For more information, see “Certain Relationships and Related Person Transactions.” As of December 31, 2021, Mr. Perez held options to purchase an aggregate of 974,925 shares of Akili’s common stock. |
| (3) | Dr. Gazzaly has entered into an advisor agreement with Akili, pursuant to which he is entitled to receive a consulting fee of $8,333.33 per month for certain services provided to the Company. For more information, see “Certain Relationships and Related Person Transactions.” As of December 31, 2021, Dr. Gazzaley held options to purchase an aggregate of 75,000 shares of Akili’s common stock. |
| (4) | As of December 31, 2021, Ms. Lemke held options to purchase an aggregate of 70,000 shares of Akili’s common stock. |
| (5) | As of December 31, 2021, Mr. Gates did not hold any outstanding equity awards. |
| (6) | As of December 31, 2021, Mr. Spinale held options to purchase an aggregate of 29,000 shares of Akili’s common stock. |
| (7) | As of December 31, 2021, Dr. Chowrira did not hold any outstanding equity awards. |
| (8) | As of December 31, 2021, Mr. Ehlert did not hold any outstanding equity awards. |
Annual Retainer for Board Membership |
$40,000 for general availability and participation in meetings and conference calls of our Board of Directors |
Additional Annual Retainer for Non-Executive Chair |
$40,000 |
Additional Annual Retainer for Committee Membership |
Audit Committee Chairperson: $20,000 |
Audit Committee member (other than Chairperson): $10,000 |
Compensation Committee Chairperson: $15,000 |
Compensation Committee member (other than Chairperson): $7,500 |
Nominating and Corporate Governance Committee Chairperson: $10,000 |
Nominating and Corporate Governance Committee member (other than Chairperson): $5,000 |
| • | each person who is known to be the beneficial owner of more than 5% of shares of Akili, Inc. common stock; |
| • | each of our named executive officers and directors; and |
| • | all executive officers and directors of Akili, Inc. as a group. |
Name and Address of Beneficial Owner (1) | Number of Shares | % of Ownership | ||||||
5% Holders | ||||||||
Aaron Cowen (2) | 6,627,000 | 7.8 | % | |||||
Chamath Palihapitiya (3) | 13,773,000 | 16.1 | % | |||||
PureTech Health, LLC (4) | 12,527,477 | 14.7 | % | |||||
TLS Beta Pte. Ltd. (5) | 10,336,425 | 12.1 | % | |||||
Entities affiliated with Neuberger Berman (6) | 5,776,884 | 6.8 | % | |||||
Directors and Named Executive Officers | ||||||||
Chamath Palihapitiya (3) | 13,773,000 | 16.1 | % | |||||
W. Edward Martucci, Ph.D. (7) | 1,390,346 | 1.6 | % | |||||
Santosh Shanbhag (8) | 369,551 | * | ||||||
Jacqueline Studer (9) | 269,135 | * | ||||||
Bharatt Chowrira, Ph.D. | — | — | ||||||
Kenneth Ehlert (10) | 16,787 | * | ||||||
Adam Gazzaley, M.D., Ph.D. (11) | 1,156,464 | 1.4 | % | |||||
William Jones, Jr. | — | — | ||||||
Christine Lemke (12) | 23,502 | * | ||||||
All directors and executive officers as a group (12 individuals) | 17,366,634 | 20.3 | % | |||||
| * | Less than one percent |
| (1) | Unless otherwise noted, the business address of each of those listed in the table above is 125 Broad Street, 5th Floor, Boston, MA 02110. |
| (2) | Consists of 6,627,000 shares of Akili, Inc. common stock. Averill Master Fund, Ltd. (“Averill Fund”) is the record holder of 3,540,000 shares of Akili, Inc. common stock reported herein. Mr. Cowen may be deemed to control Suvretta Capital Management, LLC, the investment manager of the Averill Fund, and therefore may be deemed to beneficially own the Akili, Inc. common stock held by the Averill Fund. SVAV Sponsor I, LLC (“SVAV”) is the record holder of 3,087,000 shares of Akili, Inc. common stock reported herein. Mr. Cowen may be deemed to control SVAV and therefore may be deemed to beneficially own the Akili, Inc. common stock held by SVAV. Mr. Cowen disclaims beneficial ownership of the Akili, Inc. common |
| stock reported herein except to the extent of any indirect pecuniary interest therein. The address of Mr. Cowen is c/o Suvretta Capital Management, LLC, 540 Madison Avenue, 7th Floor, New York, NY 10022. |
| (3) | Consists of 13,773,000 shares of Akili, Inc. common stock. SC PIPE Holdings LLC (“SC PIPE Holdings”) is the record holder of 8,100,000 of the shares of Akili, Inc. common stock reported herein. The shares held by SC PIPE Holdings may be pledged pursuant to a security and pledge agreement. SC PIPE Holdings is controlled by Mr. Palihapitiya, the current Chairman of the Board and the former Chief Executive Officer and Chairman of SCS’s Board of Directors SC Master Holdings is the sole member of SC PIPE Holdings. Mr. Palihapitiya and SC Master Holdings may be deemed to beneficially own shares of common stock held directly by SC PIPE Holdings by virtue of their indirect or direct interests in SC PIPE Holdings or their control over SC PIPE Holdings, as the case may be. SC Master Holdings, LLC (“SC Master Holdings”) is the record holder of 3,773,000 of the shares of Akili, Inc. common stock reported herein. SC Master Holdings is controlled by Mr. Palihapitiya. Mr. Palihapitiya may be deemed to beneficially own shares of Akili, Inc. common stock held directly by SC Master Holdings by virtue of his indirect interests in SC Master Holdings or his control over SC Master Holdings, as the case may be. A trust for the benefit of members of Mr. Palihapitiya’s immediate family (the “Family Trust”), is the record holder of 1,900,000 of the shares of Akili, Inc. common stock reported herein. Mr. Palihapitiya may be deemed to beneficially own shares of Akili, Inc. common stock held directly by the Family Trust. The address of each of Mr. Palihapitiya, SC Master Holdings and SC PIPE Holdings is c/o SC Master Holdings, LLC, 506 Santa Cruz Avenue, Suite 300, Menlo Park, California 94025. |
| (4) | Represents 12,027,477 shares of Akili and 500,000 PIPE Shares. Voting and investment power over the shares held by PureTech Health LLC is exercised by its parent entity, PureTech Health plc. The board of directors of PureTech Health plc consists of Ms. Sharon Barber-Lui, Dr. Bharatt Chowrira, Dr. Raju Kucherlapati, Dr. John LaMattina, Dr. Robert Langer, Ms. Kiran Mazumdar-Shaw, Dame Marjorie Scardino, Mr. Christopher Viehbacher and Ms. Daphne Zohar. None of the members of the board of directors of PureTech Health plc or PureTech Health LLC has individual voting or investment power with respect to such shares. The address for PureTech Health LLC and the individuals listed above is c/o PureTech Health LLC, 6 Tide Street, Boston, Massachusetts 02210. |
| (5) | Represents 9,836,425 shares of Akili and 500,000 PIPE Shares. TLS Beta Pte. Ltd. is a direct wholly-owned subsidiary of Temasek Life Sciences Private Limited, which in turn is a direct wholly-owned subsidiary of Fullerton Management Pte Ltd, which in turn is a direct wholly-owned subsidiary of Temasek Holdings (Private) Limited. The address of each of the foregoing entities is 60B Orchard Road, #06-18 Tower 2, The Atrium@Orchard, Singapore. |
| (6) | Consists of shares held directly by (i) Neuberger Berman Principal Strategies PRIMA Fund LP (“NB PRIMA Fund”), (ii) PRIMA MLP Fund LP (“PRIMA MLP”) and (iii) Neuberger Berman Principal Strategies PRIMA Co-Invest Fund VI LP (“NB PRIMA Co-Invest VI” and together with NB PRIMA Fund and PRIMA MLP, the “PRIMA Funds”). Neuberger Berman Investment Advisers LLC (“NBIA”) serves as the adviser to the PRIMA Funds. Neuberger Berman Principal Strategies PRIMA Associates LP (“PRIMA Associates”) serves as the general partner of NB PRIMA Fund and NB PRIMA Co-Invest VI (a separate third-party serves as general partner of PRIMA MLP). Neuberger Berman Group LLC and certain of its affiliates may be deemed to be the beneficial owners of the securities held directly by the PRIMA Funds because it or certain affiliated persons, including NBIA and PRIMA Associates, have shared power to retain, dispose of or vote the securities owned by the PRIMA Funds pursuant to the terms of investment advisory agreements with the PRIMA Funds. Neuberger Berman Group LLC or its affiliated persons do not, however, have any economic interest in the securities held by the PRIMA Funds. Investment and voting decisions with respect to the securities held by the PRIMA Funds made by Neuberger Berman are made by an investment committee consisting of Joseph Rotter, Gabriel Cahill and Sean Badcock (the “PRIMA Investment Committee”), each of whom is an employee of Neuberger Berman. All members of the PRIMA Investment Committee disclaim beneficial ownership of the securities held by the PRIMA Funds. The principal address for the above referenced entities is 1290 Avenue of the Americas, New York, New York 10104. |
| (7) | Includes 122,311 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| (8) | Includes 50,651 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| (9) | Includes 36,238 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| (10) | Includes 3,358 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| (11) | Includes (i) 1,089,314 shares of common stock and (ii) 1,199 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| (12) | Includes 3,357 shares of Akili, Inc. common stock issuable upon the exercise of options exercisable as of or within 60 days of August 19, 2022. |
| • | the resale of 16,200,000 shares of common stock issued in the PIPE Investment by certain of the Selling Securityholders; |
| • | the resale of 6,250,000 shares of common stock issued upon consummation of the Business Combination, in exchange for shares of Class B ordinary shares of SCS originally issued the Sponsor; |
| • | the resale of 640,000 shares of common stock issued upon consummation of the Business Combination, in exchange for Class A ordinary shares of SCS originally issued to the Sponsor; |
| • | the resale of 2,494,549 shares of common stock held by certain of our former and current directors; |
| • | the resale of 15,684,066 shares of common stock issued to former equity holders of Akili pursuant to the Business Combination; |
| • | the issuance by us and resale of 2,096,106 shares of common stock reserved for issuance upon the exercise of options to purchase common stock held by persons who previously ended their relationship with Akili prior to or concurrently with the Closing of the Business Combination; and |
| • | the issuance by us and resale of 50,000 shares of common stock reserved for issuance upon the settlement of restricted stock units. |
Securities Beneficially Owned Prior to this Offering | Securities to be Sold in this Offering | Securities Beneficially Owned After this Offering | ||||||||||||||
Name of Selling Securityholder (1) | Akili, Inc. Common Stock | Akili, Inc. Common Stock | Akili, Inc. Common Stock | Percent | ||||||||||||
Adam Gazzaley (2) | 1,156,464 | 1,089,314 | 67,150 | * | ||||||||||||
Alyeska Master Fund, L.P. (3) | 500,000 | 500,000 | — | — | ||||||||||||
Amgen Ventures LLC (4) | 1,982,322 | 300,000 | 1,682,322 | 2.0 | ||||||||||||
Apeiron Investment Group Ltd. (5) | 100,000 | 100,000 | — | — | ||||||||||||
Averill Master Fund, Ltd. (6) | 3,540,000 | 3,540,000 | — | — | ||||||||||||
Securities Beneficially Owned Prior to this Offering | Securities to be Sold in this Offering | Securities Beneficially Owned After this Offering | ||||||||||||||
Name of Selling Securityholder (1) | Akili, Inc. Common Stock | Akili, Inc. Common Stock | Akili, Inc. Common Stock | Percent | ||||||||||||
Chamath Palihapitiya (7) | 11,873,000 | 11,873,000 | — | — | ||||||||||||
CP WY Remainder Interest Trust U/A/D Dated December 22, 2021. (8) | 1,900,000 | 1,900,000 | — | — | ||||||||||||
David Spiegel, M.D., Ph.D. (9) | 10,000 | 10,000 | — | — | ||||||||||||
Frigga, Ltd. (10) | 1,757,256 | 100,000 | 1,657,256 | 1.9 | ||||||||||||
Gates Irrevocable Trust 2004 fbo the children of James R. Gates (11) | 79,806 | 79,806 | — | — | ||||||||||||
James R. Gates Separate Property Revocable Trust (12) | 1,325,429 | 1,325,429 | — | — | ||||||||||||
JAZZ Human Performance Opportunity Fund, L.P. (13) | 693,226 | 693,226 | — | — | ||||||||||||
JAZZ Human Performance Technology Fund, LP (14) | 3,063,364 | 3,063,364 | — | — | ||||||||||||
John Spinale (15) | 33,384 | 33,384 | — | — | ||||||||||||
Omidyar Technology Ventures, LLC (16) | 1,063,422 | 60,000 | 1,003,422 | 1.2 | ||||||||||||
Polaris Healthcare Technology Opportunities Fund, L.P. (17) | 827,685 | 250,000 | 577,685 | * | ||||||||||||
Polaris Partners IX, L.P. (18) | 827,687 | 250,000 | 577,687 | * | ||||||||||||
PureTech Health LLC (19) | 12,527,476 | 12,527,476 | — | — | ||||||||||||
Robert Perez (20) | 1,122,322 | 1,122,322 | — | — | ||||||||||||
Senthil Sundaram (21) | 30,000 | 30,000 | ||||||||||||||
Sukumar Nagendran (22) | 10,000 | 10,000 | ||||||||||||||
SVAV Sponsor I, LLC (24) | 3,087,000 | 3,087,000 | — | — | ||||||||||||
TLS Beta Pte. Ltd. (24) | 10,336,425 | 500,000 | 9,836,425 | 11.5 | ||||||||||||
Vladimir Coric (25) | 30,000 | 30,000 | — | — | ||||||||||||
| * | represents less than one percent of shares outstanding |
| (1) | Unless otherwise noted, the business address of each of those listed in the table above is 125 Broad Street, 5th Floor, Boston, MA 02110. |
| (2) | Consist of 1,089,314 shares of common stock held by Adam Gazzaley and 67,150 shares of common stock reserved for issuance upon the exercise of options held by Adam Gazzaley. Adam Gazzaley is on the board of directors of Akili, Inc. |
| (3) | Consist of 500,000 shares of common stock held by Alyeska Master Fund, L.P. issued in the PIPE Investment. Alyeska Investment Group, L.P., the investment manager of Alyeska Master Fund, L.P., has voting and investment control of the shares held by Alyeska Master Fund, L.P. Anand Parekh is the Chief Executive Officer of Alyeska Investment Group, L.P. and may be deemed to be the beneficial owner of such shares. Mr. Parekh, however, disclaims any beneficial ownership of the shares held by the Selling Securityholder. The registered address of Alyeska Master Fund, L.P. is at c/o Maples Corporate Services Limited, P.O. Box 309, Ugland House, South Church Street George Town, Grand Cayman, KY1-1104, Cayman Islands. Alyeska Investment Group, L.P. is located at 77 W. Wacker, Suite 700, Chicago IL 60601. |
| (4) | Consist of 1,682,322 shares of common stock held of record by Amgen Ventures LLC and 300,000 shares of common stock held by Amgen Ventures LLC issued in the PIPE Investment. Amgen Ventures LLC is a wholly owned subsidiary of Amgen Inc. The address for Amgen Ventures LLC and Amgen Inc. is One Amgen Center Drive, Thousand Oaks, CA 91320. |
| (5) | Consist of 100,000 shares of common stock held by Apeiron Investment Group Ltd. issued in the PIPE Investment. Julien Höfer, Jefim Gewiet are managing directors of Apeiron Investment Group Ltd. and therefore may be deemed to beneficially own the shares of common stock held by Apeiron Investment Group Ltd. The address for Apeiron Investment Group Ltd. is 66 & 67, Beatrice, Amery Street, SLM1707, Sliema, Malta. |
| (6) | Consist of 3,540,000 shares of common stock held by Averill Master Fund, Ltd. issued in the PIPE Investment. Suvretta Capital Management, LLC, the investment manager of Averill Master Fund, Ltd., may be deemed to beneficially own the shares of common stock held by Averill Master Fund, Ltd. Aaron Cowen may be deemed to control Suvretta Capital Management, LLC, and therefore may be deemed to beneficially own the shares held by Averill Master Fund, Ltd. The business address of Averill Master Fund, Ltd. is 540 Madison Avenue, 7th Floor, New York, NY 10022. |
| (7) | Consists of 11,873,000 shares of common stock, including 8,100,000 shares of common stock held of record by SC PIPE Holdings LLC (“SC PIPE Holdings”) and 3,773,000 shares of common stock held of record by SC Master Holdings, LLC (“SC Master Holdings”). SC PIPE Holdings is controlled by Mr. Palihapitiya, the current Chairman of the Board and the former Chief Executive Officer and Chairman of SCS’s Board of Directors. SC Master Holdings is the sole member of SC PIPE Holdings. Mr. Palihapitiya and SC Master Holdings may be deemed to beneficially own shares of common stock held directly by SC PIPE Holdings by virtue of their indirect or direct interests in SC PIPE Holdings or their control over SC PIPE Holdings, as the case may be. SC Master Holdings is controlled by |
| Mr. Palihapitiya. Mr. Palihapitiya may be deemed to beneficially own shares of common stock held directly by SC Master Holdings by virtue of his indirect interests in SC Master Holdings or his control over SC Master Holdings, as the case may be. The shares of common stock reported herein do not include 1,900,000 shares of common stock held directly by a trust for the benefit of members of Mr. Palihapitiya’s immediate family (the “Family Trust”), which are otherwise reported in this table. Mr. Palihapitiya may be deemed to beneficially own shares of common stock held directly by the Family Trust. The address of each of Mr. Palihapitiya, SC Master Holdings and SC PIPE Holdings is c/o SC Master Holdings, LLC, 506 Santa Cruz Avenue, Suite 300, Menlo Park, California 94025. |
| (8) | Consist of 1,900,000 shares of common stock held by the CP WY Remainder Interest Trust U/A/D Dated December 22, 2021. Mr. Palihapitiya, the current Chairman of the Board and the former Chief Executive Officer and Chairman of SCS’s Board of Directors, may be deemed to beneficially own shares of common stock held directly by CP WY Remainder Interest Trust U/A/D Dated December 22, 2021. The business address of the CP WY Remainder Interest Trust U/A/D Dated December 22, 2021 is 415 W 17th Street, Ste B2, Cheyenne, WY 82001. |
| (9) | Consists of 10,000 shares of common stock underlying the RSUs held by David Spiegel, M.D., Ph.D. that vested at Closing and are issuable on the date on which this prospectus is declared effective by the SEC. |
| (10) | Consist of 1,657,256 shares of common stock held of record by Frigga Ltd. and 100,000 shares of common stock held by Frigga Ltd. issued in the PIPE Investment. Evidity Capital, LLC is the discretionary investment advisor of Canepa Advanced Healthcare Partners, Ltd., which is the general partner of Canepa Advanced Healthcare Fund, L.P., which is the sole shareholder of Frigga Ltd. Voting and dispositive determinations with respect to the securities held by Frigga Ltd. are made by the investment committee of Evidity Capital, LLC, which is comprised of Paul Enever, Alejandro Sanchez and Adam Lessler. Accordingly, each of the entities and individuals named herein may be deemed to share beneficial ownership of the securities held of record by Frigga Ltd. The address for Frigga Ltd. is Clarendon House, 2 Church Street. Hamilton HM 11. Bermuda. |
| (11) | Consists of 79,806 shares of common stock held by the Gates Irrevocable Trust 2004 fbo the children of James R. Gates. James R. Gates is the trustee and may be deemed to control the Gates Irrevocable Trust 2004 fbo the children of James R. Gates, and therefore may be deemed to beneficially own the shares held by the Gates Irrevocable Trust 2004 fbo the children of James R. Gates. James R. Gates was on the board of directors of Akili. The business address for the Gates Irrevocable Trust 2004 fbo the children of James R. Gates is 433 El Arroyo Road, Hillsborough, CA 94010. |
| (12) | Consist of 1,325,429 shares of common stock held by the James R. Gates Separate Property Revocable Trust. James R. Gates may be deemed to control the James R. Gates Separate Property Revocable Trust, and therefore may be deemed to beneficially own the shares held by the James R. Gates Separate Property Revocable Trust. James R. Gates was on the board of directors of Akili. The business address for the James R. Gates Separate Property Revocable Trust is 433 El Arroyo Road, Hillsborough, CA 94010. |
| (13) | Consist of 693,226 shares of common stock held by JAZZ Human Performance Opportunity Fund, LP. John Harris, John Spinale, Zack Lynch, Andrew Firlik are managing members of JAZZ Human Performance Opportunity GP, LLC, and therefore may be deemed to beneficially own the shares held by JAZZ Human Performance Opportunity Fund, LP. John Spinale was on the board of directors of Akili. The business address for JAZZ Human Performance Opportunity Fund, LP is 548 Market Street #27799, San Francisco, CA 94014. |
| (14) | Consist of 2,963,364 shares of common stock held of record by JAZZ Human Performance Technology Fund, LP and 100,000 shares of common stock held by JAZZ Human Performance Technology Fund, LP issued in the PIPE Investment. John Harris, John Spinale, Zack Lynch, Andrew Firlik are managing members of JAZZ Human Performance Technology GP, LLC, and therefore may be deemed to beneficially own the shares held by JAZZ Human Performance Technology Fund, LP. John Spinale was on the board of directors of Akili. The business address for JAZZ Human Performance Technology Fund, LP is 548 Market Street #27799, San Francisco, CA 94104. |
| (15) | Consist of 33,384 shares of common stock reserved for issuance upon the exercise of options held by John Spinale. John Spinale was on the board of directors of Akili. The address of John Spinale is 261 Jersey St, San Francisco, CA 94114. |
| (16) | Consist of 1,003,422 shares of common stock held of record by Omidyar Technology Ventures, LLC (“Omidyar”) and 60,000 shares of common stock held by Omidyar issued in the PIPE Investment. Jeffery R. Alvord is the director and president of Omidyar and therefore may be deemed to beneficially own the shares of common stock held by Omidyar. Each of Robinson C. Jacobs and Matthew K. Stepan is the director and vice president of Omidyar and therefore may be deemed to beneficially own the shares of common stock held by Omidyar. Matthew J. Deakin is the trustee of the Pierre M. Omidyar Trust, the ultimate beneficial owner of Omidyar, and therefore may be deemed to beneficially own the shares of common stock held by Omidyar. The address for Omidyar is 720 University Avenue, Suite 200, Los Gatos, California, 95032. |
| (17) | Consist of 577,685 shares of common stock held by record by Polaris Healthcare Technology Opportunities Fund, L.P. (“PHCT”) and 250,000 shares of common stock held by PHCT issued in the PIPE Investment. Polaris Healthcare Technology Opportunities Fund GP, L.L.C. (“PHCT GP”) is the general partner of PHCT and may be deemed to have sole voting and dispositive power with respect to the shares held by PHCT. David Barrett, Brian Chee, Amir Nashat and Amy Schulman (collectively, the “PHCT GP Managing Members”) are the managing members of PHCT GP. Each of the PHCT GP Managing Members, in their respective capacities with respect to PHCT GP, may be deemed to have shared voting and dispositive power with respect to the shares held by PHCT. The principal business address for all entities and individuals affiliated with Polaris Partners is c/o Polaris Partners, One Marina Park Drive, 10th Floor, Boston, Massachusetts 02210. |
| (18) | Consist of 577,687 shares of common stock held of record by Polaris Partners IX, L.P. (“PP IX”) and 250,000 shares of common stock held by PP IX issued in the PIPE Investment. Polaris Partners GP IX, L.L.C. (“PP GP IX”) is the general partner of PP IX and may be deemed to have sole voting and dispositive power with respect to the shares held by PP IX. David Barrett, Brian Chee, Amir Nashat and Amy Schulman (collectively, the “PP GP IX Managing Members”) are the managing members of PP GP IX. Each of the PP GP IX Managing Members, in their respective capacities with respect to PP GP IX, may be deemed to have shared voting and dispositive power |
| with respect to the shares held by PP IX. The principal business address for all entities and individuals affiliated with Polaris Partners is c/o Polaris Partners, One Marina Park Drive, 10th Floor, Boston, Massachusetts 02210. |
| (19) | Consist of 12,027,476 shares of common stock held of record by PureTech Health LLC and 500,000 shares of common stock held by PureTech Health LLC issued in the PIPE Investment. Voting and investment power over the shares held by PureTech Health LLC is exercised by its parent entity, PureTech Health plc. The board of directors of PureTech Health plc consists of Ms. Sharon Barber-Lui, Dr. Bharatt Chowrira, Dr. Raju Kucherlapati, Dr. John LaMattina, Dr. Robert Langer, Ms. Kiran Mazumdar-Shaw, Dame Marjorie Scardino, Mr. Christopher Viehbacher and Ms. Daphne Zohar. None of the members of the board of directors of PureTech Health plc or PureTech Health LLC has individual voting or investment power with respect to such shares. Dr. Bharatt Chowrira is a member of the board of directors of Akili, Inc. The address for PureTech Health LLC and the individuals listed above is c/o PureTech Health LLC, 6 Tide Street, Suite 400, Boston, Massachusetts 02210. |
| (20) | Consist of 1,122,322 shares of common stock reserved for issuance upon the exercise of options held by Robert Perez. Robert Perez was on the board of directors of Akili. The address of Robert Perez is 290 Beacon St, Apt 5, Boston MA 02116. |
| (21) | Consists of 30,000 shares of common stock underlying the RSUs held by Senthil Sundaram that vested at Closing and are issuable on the date on which this prospectus is declared effective by the SEC. |
| (22) | Consists of 10,000 shares of common stock underlying the RSUs held by Sukumar Nagendran that vested at Closing and are issuable on the date on which this prospectus is declared effective by the SEC. |
| (23) | Consist of 3,087,000 shares of common stock held by SVAV Sponsor I, LLC. Aaron Cowen and Kishan Mehta may be deemed to control SVAV Sponsor I, LLC, and therefore may be deemed to beneficially own the shares held by SVAV Sponsor I, LLC. Kishan Mehta was the president and on the board of directors of SCS. The business address of SVAV Sponsor I, LLC is 540 Madison Avenue, 7th Floor, New York, NY 10022. |
| (24) | Consist of 9,836,425 shares of common stock held of record by TLS Beta Pte. Ltd. and 500,000 shares of common stock held by TLD Beta Pte. Ltd issued in the PIPE Investment. TLS Beta Pte. Ltd., or TLS, is a direct wholly-owned subsidiary of Temasek Life Sciences Private Limited, or TLSPL, which in turn is a direct wholly-owned subsidiary of Fullerton Management Pte Ltd, or Fullerton, which in turn is a direct wholly-owned subsidiary of Temasek Holdings (Private) Limited, or Temasek. In such capacities, each of TLSPL, Fullerton and Temasek may be deemed to have voting and dispositive power over the shares held by TLS. The address for TLS, TLSPL, Fullerton and Temasek is 60B Orchard Road #06-18 Tower 2, The Atrium@Orchard, Singapore 238891. |
| (25) | Consist of 30,000 shares of common stock held by Vladimir Coric. |
Name | Akili Series D Preferred Shares | Total Purchase Price | ||||||
TLS Beta Pte. Ltd. (1) | 2,966,706 | $ | 24,999,998.32 | |||||
Neuberger Berman Principal Strategies | ||||||||
PRIMA Fund LP (2) | 1,201,813 | $ | 10,127,502.69 | |||||
PRIMA MLP Fund LP (2) | 1,186,682 | $ | 9,999,995.95 | |||||
Neuberger Berman Principal Strategies | ||||||||
PRIMA Co-Invest Fund VI LP(2) | 578,211 | $ | 4,872,499.68 | |||||
JAZZ Human Performance Technology Fund, L.P. (3) | 534,007 | $ | 4,499,999.02 | |||||
JAZZ Human Performance Opportunity Fund, L.P. (3) | 356,005 | $ | 3,000,002.16 | |||||
| (1) | TLS Beta Pte. Ltd. (“Temasek”) was a holder of more than 5% of Akili’s outstanding capital stock. |
| (2) | Neuberger Berman Principal Strategies PRIMA Fund LP and its affiliated entities, PRIMA MLP Fund LP and Neuberger Berman Principal Strategies PRIMA Co-Invest Fund VI LP (collectively, “Neuberger”), was a holder of more than 5% of Akili’s outstanding capital stock. |
| (3) | John Spinale is a member of the board of directors of Akili and an affiliate of JAZZ Human Performance Technology Fund, L.P. and JAZZ Human Performance Opportunity Fund, L.P. (collectively, “JAZZ”). JAZZ was a holder of more than 5% of Akili’s outstanding capital stock. |
Name | Akili Series C Preferred Shares | Total Purchase Price | ||||||
TLS Beta Pte. Ltd. (1) | 3,526,383 | $ | 29,999,998.10 | |||||
JAZZ Human Performance Technology Fund, L.P. (2) | 587,731 | $ | 5,000,003.94 | |||||
| (1) | Temasek was a holder of more than 5% of Akili’s outstanding capital stock. |
| (2) | John Spinale is a member of the board of directors of Akili and an affiliate of JAZZ. JAZZ was a holder of more than 5% of Akili’s outstanding capital stock. |
| • | The audit committee will review the material facts of all related person transactions. |
| • | In reviewing any related person transaction, the audit committee will take into account, among other factors that it deems appropriate, whether the related person transaction is on terms no less favorable to us than terms generally available in a transaction with an unaffiliated third-party under the same or similar circumstances and the extent of the related person’s interest in the transaction. |
| • | In connection with its review of any related person transaction, we will provide the audit committee with all material information regarding such related person transaction, the interest of the related person and any potential disclosure obligations of us in connection with such related person transaction. |
| • | If a related person transaction will be ongoing, the audit committee may establish guidelines for our management to follow in its ongoing dealings with the related person. |
| • | 1% of the total number of shares of Akili, Inc. common stock then outstanding; or |
| • | the average weekly reported trading volume of Akili, Inc. common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
| • | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| • | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| • | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Current Reports on Form 8-K; and |
| • | at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company. |
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | one or more underwritten offerings on a firm commitment or best efforts basis; |
| • | block trades in which the broker-dealer will attempt to sell the securities as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its accounts; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | distributions or transfers to their members, partners, employees or shareholders; |
| • | short sales effected after the date of the registration statement of which this prospectus is a part is declared effective by the SEC; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | in market transactions, including transactions on a national securities exchange or quotations service or over-the-counter |
| • | through trading plans entered into by a Selling Securityholder pursuant to Rule 10b5-1 under the Exchange Act that are in place at the time of an offering pursuant to this prospectus and any applicable prospectus supplement hereto that provide for periodic sales of their securities on the basis of parameters described in such trading plans; |
| • | directly to one or more purchasers, including through a specific bidding, auction or other process or in privately negotiated transactions; |
| • | in “at the market” offerings, as defined in Rule 415 under the Securities Act, at negotiated prices, at prices prevailing at the time of sale or at prices related to such prevailing market prices, including sales made directly on a national securities exchange or sales made through a market maker other than on an exchange or other similar offerings through sales agents; |
| • | through agents; |
| • | through broker-dealers who may agree with the Selling Securityholders to sell a specified number of such securities at a stipulated price per share or warrant; and |
| • | by entering into transactions with third parties who may (or may cause others to) issue securities convertible or exchangeable into, or the return of which is derived in whole or in part from the value of, our ordinary shares and a combination of any such methods of sale or any other method permitted pursuant to applicable law. |
Page | ||||
Condensed Consolidated Financial Statements for the Quarter Ended June 30, 2022 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
Audited Financial Statements as of December 31, 2021 and for the Period from February 25, 2021 (inception) to December 31, 2021 | ||||
| F-24 | ||||
| F-25 | ||||
| F-26 | ||||
| F-27 | ||||
| F-28 | ||||
| F-29 | ||||
PAGE | ||||
| F-42 | ||||
| F-43 | ||||
| F-44 | ||||
| F-46 | ||||
| F-47 | ||||
Financial Statements for the years ended December 31, 2021 and December 31, 2020 | ||||
PAGE | ||||
| F-58 | ||||
| F-59 | ||||
| F-60 | ||||
| F-61 | ||||
| F-62 | ||||
| F-63 | ||||
June 30, 2022 | December 31, 2021 | |||||||
| (Unaudited) | ||||||||
ASSETS | ||||||||
Current assets | ||||||||
Cash | $ | 145,044 | $ | 428,189 | ||||
Prepaid expenses | 524,750 | 504,034 | ||||||
Total Current Assets | 669,794 | 932,223 | ||||||
Non-current prepaid insurance | — | 247,500 | ||||||
Marketable securities held in Trust Account | 250,371,095 | 250,008,324 | ||||||
TOTAL ASSETS | $ | 251,040,889 | $ | 251,188,047 | ||||
LIABILITIES, TEMPORARY EQUITY AND PERMANENT DEFICIT | ||||||||
Current liabilities | ||||||||
Accounts payable | $ | 17,465 | $ | 5,000 | ||||
Accrued expenses | 7,405,342 | 1,974,837 | ||||||
Promissory note - related party | 250,000 | — | ||||||
Due to related party | 80,127 | 10,000 | ||||||
Total current liabilities | 7,752,934 | 1,989,837 | ||||||
Deferred underwriting fee payable | 7,700,000 | 7,700,000 | ||||||
Total Liabilities | 15,452,934 | 9,689,837 | ||||||
Commitments and Contingencies (Note 6) | ||||||||
Temporary Equity | ||||||||
Class A ordinary shares subject to possible redemption, 25,000,000 shares at redemption value as of June 30, 2022 and December 31, 2021 | 250,371,095 | 250,008,324 | ||||||
Permanent Deficit | ||||||||
Preference shares, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding as of June 30, 2022 and December 31, 2021 | — | — | ||||||
Class A ordinary shares, $0.0001 par value; 500,000,000 shares authorized; 640,000 shares issued and outstanding (excluding 25,000,000 shares subject to possible redemption) as of June 30, 2022 and December 31, 2021 | 64 | 64 | ||||||
Class B ordinary shares, $0.0001 par value; 50,000,000 shares authorized; 6,250,000 shares issued and outstanding as of June 30, 2022 and December 31, 2021 | 625 | 625 | ||||||
Additional paid-in capital | — | — | ||||||
Accumulated deficit | (14,783,829 | ) | (8,510,803 | ) | ||||
Total Permanent Deficit | (14,783,140 | ) | (8,510,114 | ) | ||||
TOTAL LIABILITIES, TEMPORARY EQUITY AND PERMANENT DEFICIT | $ | 251,040,889 | $ | 251,188,047 | ||||
For the Three Months Ended June 30, 2022 | For the Three Months Ended June 30, 2021 | For the Six Months Ended June 30, 2022 | For the Period from February 25, 2021 (Inception) Through June 30, 2021 | |||||||||||||
Operating and formation costs | $ | 2,071,642 | $ | 143 | $ | 6,273,026 | $ | 5,325 | ||||||||
Loss from operations | (2,071,642 | ) | (143 | ) | (6,273,026 | ) | (5,325 | ) | ||||||||
Other expense: | ||||||||||||||||
Interest earned on marketable securities held in Trust Account | 337,595 | — | 362,771 | — | ||||||||||||
Net loss | $ | (1,734,047 | ) | $ | (143 | ) | $ | (5,910,255 | ) | $ | (5,325 | ) | ||||
Weighted average shares outstanding, Class A ordinary shares | 25,640,000 | — | 25,640,000 | — | ||||||||||||
Basic and diluted net loss per share, Class A ordinary shares | $ | (0.05 | ) | $ | — | $ | (0.19 | ) | $ | — | ||||||
Weighted average shares outstanding, Class B ordinary shares | 6,250,000 | 5,500,000 | 6,250,000 | 5,500,000 | ||||||||||||
Basic and diluted net loss per share, Class B ordinary shares | $ | (0.05 | ) | $ | (0.00 | ) | $ | (0.19 | ) | $ | (0.00 | ) | ||||
Temporary Equity | Class A Ordinary Shares | Class B Ordinary Shares | Additional Paid-in Capital | Accumulated Deficit | Total Permanent Deficit | |||||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||
Balance – January 1, 2022 | 25,000,000 | $ | 250,008,324 | 640,000 | $ | 64 | 6,250,000 | $ | 625 | $ | — | $ | (8,510,803 | ) | $ | (8,510,114 | ) | |||||||||||||||||||
Remeasurement for Class A ordinary shares to redemption value | — | (8,324 | ) | — | — | — | — | — | 8,324 | 8,324 | ||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (4,176,208 | ) | (4,176,208 | ) | |||||||||||||||||||||||||
Balance – March 31, 2022 | 25,000,000 | $ | 250,000,000 | 640,000 | $ | 64 | 6,250,000 | $ | 625 | $ | — | $ | (12,678,687 | ) | $ | (12,677,998 | ) | |||||||||||||||||||
Remeasurement for Class A ordinary shares to redemption value | — | 371,095 | — | — | — | — | — | (371,095 | ) | (371,095 | ) | |||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (1,734,047 | ) | (1,734,047 | ) | |||||||||||||||||||||||||
Balance – June 30, 2022 | 25,000,000 | $ | 250,371,095 | 640,000 | $ | 64 | 6,250,000 | $ | 625 | $ | — | $ | (14,783,829 | ) | $ | (14,783,140 | ) | |||||||||||||||||||
Temporary Equity | Class A Ordinary Shares | Class B Ordinary Shares | Additional Paid-in Capital | Accumulated Deficit | Total Permanent Equity | |||||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||
Balance – February 25, 2021 (inception) | — | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||||||
Issuance of Class B ordinary shares to Sponsor | — | — | — | — | 6,325,000 | 633 | 24,367 | — | 25,000 | |||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (5,182 | ) | (5,182 | ) | |||||||||||||||||||||||||
Balance – March 31, 2021 | — | $ | — | — | $ | — | 6,325,000 | $ | 633 | $ | 24,367 | $ | (5,182 | ) | $ | 19,818 | ||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (143 | ) | (143 | ) | |||||||||||||||||||||||||
Balance – June 30, 2021 | — | $ | — | — | $ | — | 6,325,000 | $ | 633 | $ | 24,367 | $ | (5,325 | ) | $ | 19,675 | ||||||||||||||||||||
For the Six Months Ended June 30, 2022 | For the Period from February 25, 2021 (Inception) Through June 30, 2021 | |||||||
Cash Flows from Operating Activities: | ||||||||
Net loss | $ | (5,910,255 | ) | $ | (5,325 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Formation costs paid by Sponsor in exchange for issuance of Founder Shares | — | 5,000 | ||||||
Interest earned on marketable securities held in Trust Account | (362,771 | ) | — | |||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses | (20,716 | ) | (19,600 | ) | ||||
Non-current prepaid insurance | 247,500 | — | ||||||
Accounts payable and accrued expenses | 5,442,970 | 88 | ||||||
Advance from related party | 70,127 | — | ||||||
Net cash used in operating activities | (533,145 | ) | (19,837 | ) | ||||
Cash Flows from Financing Activities: | ||||||||
Advance from related party | — | 25,692 | ||||||
Proceeds from promissory note – related party | 250,000 | 300,000 | ||||||
Payment of offering costs | — | (82,317 | ) | |||||
Net cash provided by financing activities | 250,000 | 243,375 | ||||||
Net Change in Cash | (283,145 | ) | 223,538 | |||||
Cash – Beginning of period | 428,189 | — | ||||||
Cash – End of period | $ | 145,044 | $ | 223,538 | ||||
Non-Cash investing and financing Activities: | ||||||||
Offering costs included in accrued offering costs | $ | — | $ | 285,873 | ||||
Offering costs paid by Sponsor in exchange for issuance of Founder Shares | $ | — | $ | 20,000 | ||||
Remeasurement of Class A ordinary shares subject to possible redemption | $ | 362,771 | $ | — | ||||
Gross proceeds | $ | 250,000,000 | ||
Less: | ||||
Class A ordinary shares issuance costs | (12,488,190 | ) | ||
Plus: | ||||
Remeasurement of carrying value to redemption value | 12,496,514 | |||
Class A ordinary shares subject to possible redemption, December 31, 2021 | $ | 250,008,324 | ||
Plus: | ||||
Remeasurement of carrying value to redemption value | (8,324 | ) | ||
Class A ordinary shares subject to possible redemption, March 31, 2022 | $ | 250,000,000 | ||
Plus: | ||||
Remeasurement of carrying value to redemption value | 371,095 | |||
Class A ordinary shares subject to possible redemption, June 30, 2022 | $ | 250,371,095 | ||
Three Months Ended June 30, 2022 | Three Months Ended June 30, 2021 | Six Months Ended June 30, 2022 | For the Period from February 25, 2021 (Inception) Through June 30, 2021 | |||||||||||||||||||||||||||||
Class A | Class B | Class A | Class B | Class A | Class B | Class A | Class B | |||||||||||||||||||||||||
Basic and diluted net loss per ordinary share | ||||||||||||||||||||||||||||||||
Numerator: | ||||||||||||||||||||||||||||||||
Allocation of net loss, as adjusted | $ | (1,394,198 | ) | $ | (339,849 | ) | $ | — | (143 | ) | $ | (4,751,927 | ) | $ | (1,158,328 | ) | $ | — | $ | (5,325 | ) | |||||||||||
Denominator: | ||||||||||||||||||||||||||||||||
Basic and diluted weighted average shares outstanding | 25,640,000 | 6,250,000 | — | 5,500,000 | 25,640,000 | 6,250,000 | — | 5,500,000 | ||||||||||||||||||||||||
Basic and diluted net loss per ordinary share | $ | (0.05 | ) | $ | (0.05 | ) | $ | — | (0.00 | ) | $ | (0.19 | ) | $ | (0.19 | ) | $ | — | $ | (0.00 | ) | |||||||||||
| Level 1: | Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. | |
| Level 2: | Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. | |
| Level 3: | Unobservable inputs based on the Company’s assessment of the assumptions that market participants would use in pricing the asset or liability. | |
Description | Level | June 30, 2022 | December 31, 2021 | |||||||||||||
Assets: | ||||||||||||||||
Marketable securities held in Trust Account | 1 | $ | 250,371,095 | $ | 250,008,324 | |||||||||||
ASSETS | ||||
Current Assets | ||||
Cash | $ | 428,189 | ||
Prepaid expenses | 504,034 | |||
Total Current Assets | 932,223 | |||
Non-current prepaid insurance | 247,500 | |||
Marketable securities held in Trust Account | 250,008,324 | |||
TOTAL ASSETS | $ | 251,188,047 | ||
LIABILITIES, TEMPORARY EQUITY AND PERMANENT DEFICIT | ||||
Current liabilities | ||||
Accounts payable | $ | 5,000 | ||
Accrued expense | 1,974,837 | |||
Advances from related party | 10,000 | |||
Total Current Liabilities | 1,989,837 | |||
Deferred underwriting fee payable | 7,700,000 | |||
TOTAL LIABILITIES | 9,689,837 | |||
Commitments and Contingencies (Note 6) | ||||
Temporary Equity | ||||
Class A ordinary shares subject to possible redemption, 25,000,000 shares at redemption value | 250,008,324 | |||
Permanent Deficit | ||||
Preference shares, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding | — | |||
Class A ordinary shares, $0.0001 par value; 500,000,000 shares authorized; 640,000 shares issued and outstanding (excluding 25,000,000 shares subject to possible redemption) | 64 | |||
Class B ordinary shares, $0.0001 par value; 50,000,000 shares authorized; 6,250,000 shares issued and outstanding | 625 | |||
Additional paid-in capital | — | |||
Accumulated deficit | (8,510,803 | ) | ||
Total Permanent Deficit | (8,510,114 | ) | ||
TOTAL LIABILITIES, TEMPORARY EQUITY AND PERMANENT DEFICIT | $ | 251,188,047 | ||
| Operating and formation costs | $ | 2,446,924 | ||
| Loss from operations | (2,446,924 | ) | ||
| Other income: | ||||
| Interest earned on marketable securities held in Trust Account | 8,324 | |||
| Net loss | $ | (2,438,600 | ) | |
| Basic and diluted weighted average shares outstanding, Class A ordinary shares | 15,101,877 | |||
| Basic and diluted net loss per share, Class A ordinary shares | $ | (0.12 | ) | |
| Basic and diluted weighted average shares outstanding, Class B ordinary shares | 5,852,751 | |||
| Basic and diluted net loss per share, Class B ordinary shares | $ | (0.12 | ) | |
Temporary Equity | Class A Ordinary Shares | Class B Ordinary Shares | Additional Paid-in | Accumulated | Total Permanent | |||||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||||||||
Balance – February 25, 2021 (inception) | — | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||||||
| Issuance of Class B ordinary shares to Sponsor | — | — | — | — | 6,325,000 | 633 | 24,367 | — | 25,000 | |||||||||||||||||||||||||||
| Sale of 25,000,000 Public Shares, net of underwriting discounts and offering expenses | 25,000,000 | 237,511,810 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Remeasurement of Class A ordinary shares to redemption value | — | 12,496,514 | — | — | — | — | (6,424,311 | ) | (6,072,203 | ) | (12,496,514 | ) | ||||||||||||||||||||||||
| Sale of 640,000 Private Placement Shares | — | — | 640,000 | 64 | — | — | 6,399,936 | — | 6,400,000 | |||||||||||||||||||||||||||
| Forfeiture of Founder Shares | — | — | — | — | (75,000 | ) | (8 | ) | 8 | — | — | |||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | (2,438,600 | ) | (2,438,600 | ) | |||||||||||||||||||||||||
Balance – December 31, 2021 | 25,000,000 | $ | 250,008,324 | 640,000 | $ | 64 | 6,250,000 | $ | 625 | $ | — | $ | (8,510,803 | ) | $ | (8,510,114 | ) | |||||||||||||||||||
Cash Flows from Operating Activities: | ||||
| Net loss | $ | (2,438,600 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Formation costs paid by Sponsor in exchange for issuance of Founder Shares | 5,000 | |||
| Interest earned on marketable securities held in Trust Account | (8,324 | ) | ||
| Changes in operating assets and liabilities: | ||||
| Prepaid expenses | (751,534 | ) | ||
| Accounts payable and accrued expenses | 1,979,837 | |||
Net cash used in operating activities | (1,213,621 | ) | ||
Cash Flows from Investing Activities: | ||||
| Investment of cash into Trust Account | (250,000,000 | ) | ||
Net cash used in investing activities | $ | (250,000,000 | ) | |
Cash Flows from Financing Activities: | ||||
| Proceeds from sale of Public Shares, net of underwriting discounts paid | 245,600,000 | |||
| Proceeds from sale of Private Placement Shares | 6,400,000 | |||
| Advances from related party | 97,640 | |||
| Repayment of advances from related party | (87,640 | ) | ||
| Proceeds from promissory note – related party | 300,000 | |||
| Repayment of promissory note – related party | (300,000 | ) | ||
| Payment of offering costs | (368,190 | ) | ||
Net cash provided by financing activities | $ | 251,641,810 | ||
Net Change in Cash | 428,189 | |||
| Cash – Beginning of period (inception) | — | |||
Cash – End of period | $ | 428,189 | ||
Non-Cash Investing and Financing Activities: | ||||
| Offering costs paid by Sponsor in exchange for issuance of Founder Shares | $ | 20,000 | ||
| Remeasurement of Class A ordinary share subject to possible redemption | $ | 12,496,514 | ||
| Deferred underwriting fee payable | $ | 7,700,000 | ||
| Forfeiture of Founder Shares | $ | (8 | ) | |
| Gross proceeds | $ | 250,000,000 | ||
| Less: | ||||
| Class A ordinary shares issuance costs | (12,488,190 | ) | ||
| Plus: | ||||
| Accretion of carrying value to redemption value | 12,496,514 | |||
Class A ordinary shares subject to possible redemption | $ | 250,008,324 | ||
For the Period from February 25, 2021 (Inception) Through December 31, 2021 | ||||||||
Class A | Class B | |||||||
Basic and diluted net loss per ordinary share | ||||||||
| Numerator: | ||||||||
| Allocation of net loss | $ | (1,757,485 | ) | $ | (681,115 | ) | ||
| Denominator: | ||||||||
| Basic and diluted weighted average shares outstanding | 15,101,877 | 5,852,751 | ||||||
| Basic and diluted net loss per ordinary share | $ | (0.12 | ) | $ | (0.12 | ) | ||
| Level 1: | Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. |
| Level 2: | Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. | |
| Level 3: | Unobservable inputs based on our assessment of the assumptions that market participants would use in pricing the asset or liability. | |
Description | Level | December 31, 2021 | ||||||
| Assets: | ||||||||
| Marketable securities held in Trust Account | 1 | $ | 250,008,324 | |||||
June 30, | December 31, | |||||||
2022 | 2021 | |||||||
Assets | ||||||||
Current assets: | ||||||||
| Cash and cash equivalents | $ | 40,638 | $ | 76,899 | ||||
| Restricted cash | 305 | 305 | ||||||
| Short-term investments | 4,987 | — | ||||||
| Accounts receivable | 17 | 29 | ||||||
| Prepaid expenses and other current assets | 5,328 | 2,500 | ||||||
Total current assets | 51,275 | 79,733 | ||||||
| Property and equipment, net | 1,054 | 1,193 | ||||||
Operating lease right-of-use | 2,686 | — | ||||||
| Prepaid expenses and other long-term assets | — | 11 | ||||||
Total assets | $ | 55,015 | $ | 80,937 | ||||
Liabilities, redeemable convertible preferred stock and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | 4,309 | 2,345 | ||||||
| Accrued expenses and other current liabilities | 5,044 | 5,477 | ||||||
| Deferred revenue | 109 | 96 | ||||||
| Deferred rent, short term | 9 | 123 | ||||||
| Operating lease liability | 625 | — | ||||||
| Note payable, short term | 625 | — | ||||||
Total current liabilities | 10,721 | 8,041 | ||||||
| Note payable, long term | 14,213 | 4,784 | ||||||
| Operating lease liability, net of current portion | 2,832 | — | ||||||
| Corporate bond, net of bond discount | 1,735 | 1,638 | ||||||
| Deferred rent, long term | — | 712 | ||||||
Total liabilities | 29,501 | 15,175 | ||||||
| Commitments and contingencies | 0 | 0 | ||||||
Redeemable convertible preferred stock, $0.0001 par value—authorized 41,785,202 shares: | ||||||||
Series A-1: designated, 4,000,000 shares; issued and outstanding shares, 4,000,000 at June 30, 2022 and December 31, 2021 (liquidation value of $4,000 at June 30, 2022 and December 31, 2021) | — | — | ||||||
Series A-2: designated 4,427,072 shares; issued and outstanding shares, 4,427,072 at June 30, 2022 and December 31, 2021 (liquidation value of $8,832 at June 30, 2022 and December 31, 2021) | 7,128 | 7,128 | ||||||
Series B: designated 7,341,485 shares; issued and outstanding shares, 7,341,485 at June 30, 2022 and December 31, 2021 (liquidation value of $42,360 at June 30, 2022 and December 31, 2021) | 41,854 | 41,854 | ||||||
Series C: designated 8,016,645 shares; issued and outstanding shares, 8,016,645 at June 30, 2022 and December 31, 2021 (liquidation value $68,200 at June 30, 2022 and December 31, 2021) | 67,904 | 67,904 | ||||||
Series D: designated 18,000,000 shares; issued and outstanding shares, 13,843,858 at June 30, 2022 and December 31, 2021 (liquidation value | 183,668 | 174,990 | ||||||
| Stockholders’ deficit: | ||||||||
Common stock, $0.0001 par value: 55,000,000 shares authorized at June 30, 2022 and December 31, 2021; 1,482,520 and 1,454,239 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively | — | — | ||||||
Additional paid-in capital | — | — | ||||||
| Accumulated deficit | (275,033 | ) | (226,114 | ) | ||||
| Accumulated other comprehensive loss | (7 | ) | — | |||||
Total stockholders’ deficit | (275,040 | ) | (226,114 | ) | ||||
Total liabilities, redeemable convertible preferred stock and stockholders’ deficit | $ | 55,015 | $ | 80,937 | ||||
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
| Revenues | $ | 64 | $ | 105 | $ | 130 | $ | 222 | ||||||||
| Cost of revenues | 97 | 98 | 193 | 160 | ||||||||||||
| Gross profit (loss) | (33 | ) | 7 | (63 | ) | 62 | ||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 7,358 | 4,039 | 13,662 | 7,983 | ||||||||||||
| Selling, general and administrative | 14,948 | 10,526 | 30,339 | 15,383 | ||||||||||||
| Total operating expenses | 22,306 | 14,565 | 44,001 | 23,366 | ||||||||||||
| Operating loss | (22,339 | ) | (14,558 | ) | (44,064 | ) | (23,304 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Other income | 40 | 4 | 49 | 5 | ||||||||||||
| Interest expense | (194 | ) | (39 | ) | (370 | ) | (120 | ) | ||||||||
| Extinguishment of debt | — | (181 | ) | — | (181 | ) | ||||||||||
| Total other income (expense) | (154 | ) | (216 | ) | (321 | ) | (296 | ) | ||||||||
| Loss before income taxes | (22,493 | ) | (14,774 | ) | (44,385 | ) | (23,600 | ) | ||||||||
| Income tax expense | — | — | — | 1 | ||||||||||||
| Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,601 | ) | ||||
| Unrealized loss on short-term investments | $ | (1 | ) | $ | — | $ | (7 | ) | $ | — | ||||||
| Comprehensive loss | $ | (22,494 | ) | $ | (14,774 | ) | $ | (44,392 | ) | $ | (23,601 | ) | ||||
| Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,601 | ) | ||||
| Dividends on Series D convertible preferred stock | (2,908 | ) | (1,115 | ) | (5,785 | ) | (1,115 | ) | ||||||||
| Redemption value of Series D convertible preferred stock | (1,455 | ) | (55,877 | ) | (2,893 | ) | (55,877 | ) | ||||||||
| Net loss attributable to common stockholders | $ | (26,856 | ) | $ | (71,766 | ) | $ | (53,063 | ) | $ | (80,593 | ) | ||||
| Net loss per share: | ||||||||||||||||
| Basic and diluted | $ | (18.25 | ) | $ | (61.98 | ) | $ | (36.18 | ) | $ | (69.60 | ) | ||||
| Weighted average common shares outstanding: | ||||||||||||||||
| Basic and diluted | 1,471,296 | 1,157,870 | 1,466,462 | 1,157,869 | ||||||||||||
Six Months Ended June 30, 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A-1 | Series A-2 | Series B | Series C | Series D | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Common Stock | Additional Paid-in | Accumulated | Accumulated other comprehensive | Total Stockholders’ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | Capital | Deficit | (loss) income | Deficit | |||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2021 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | 13,843,858 | $ | 174,990 | 1,454,239 | $ | — | $ | — | $ | (226,114 | ) | $ | — | $ | (226,114 | ) | ||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 2,070 | — | — | 2,070 | ||||||||||||||||||||||||||||||||||||||||||||||||
Exercise of stock options | — | — | — | — | — | — | — | — | — | — | 15,000 | — | 64 | — | — | 64 | ||||||||||||||||||||||||||||||||||||||||||||||||
Stock divdend | — | — | — | — | — | — | — | — | — | 2,877 | — | — | (2,134 | ) | (743 | ) | — | (2,877 | ) | |||||||||||||||||||||||||||||||||||||||||||||
Redemption value | — | — | — | — | — | — | — | — | — | 1,438 | — | — | — | (1,438 | ) | — | (1,438 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Other comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (6 | ) | (6 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (21,892 | ) | — | (21,892 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Balance at March 31, 2022 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | 13,843,858 | $ | 179,305 | 1,469,239 | $ | — | $ | — | $ | (250,187 | ) | $ | (6 | ) | $ | (250,193 | ) | |||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 1,969 | — | — | 1,969 | ||||||||||||||||||||||||||||||||||||||||||||||||
Exercise of stock options | — | — | — | — | — | — | — | — | — | — | 13,281 | — | 41 | — | — | 41 | ||||||||||||||||||||||||||||||||||||||||||||||||
Stock divdend | — | — | — | — | — | — | — | — | — | 2,908 | — | — | (2,010 | ) | (898 | ) | — | (2,908 | ) | |||||||||||||||||||||||||||||||||||||||||||||
Redemption value | — | — | — | — | — | — | — | — | — | 1,455 | — | — | — | (1,455 | ) | — | (1,455 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Other comprehensive loss | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (1 | ) | (1 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (22,493 | ) | — | (22,493 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Balance at June 30, 2022 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | 13,843,858 | $ | 183,668 | 1,482,520 | $ | — | $ | — | $ | (275,033 | ) | $ | (7 | ) | $ | (275,040 | ) | |||||||||||||||||||||||||||||||||||
Six Months Ended June 30, 2021 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A-1 | Series A-2 | Series B | Series C | Series D | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Redeemable Convertible Preferred Stock | Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | Capital | Deficit | Deficit | ||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2020 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | — | $ | — | 1,157,868 | $ | — | $ | 9,905 | $ | (114,807 | ) | $ | (104,902 | ) | ||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 842 | — | 842 | |||||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (8,827 | ) | (8,827 | ) | |||||||||||||||||||||||||||||||||||||||||||
Balance at March 31, 2021 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | — | $ | — | 1,157,868 | $ | — | $ | 10,747 | $ | (123,634 | ) | $ | (112,887 | ) | ||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 958 | — | 958 | |||||||||||||||||||||||||||||||||||||||||||||
Exercise of stock options | — | — | — | — | — | — | — | — | — | — | 5 | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Issuance of common stock warrants | — | — | — | — | — | — | — | — | — | — | — | — | 268 | — | 268 | |||||||||||||||||||||||||||||||||||||||||||||
Issuance of convertible preferred stock, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
net of issuance costs | — | — | — | — | — | — | — | — | 13,053,508 | 109,681 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Stock divdend | — | — | — | — | — | — | — | — | — | 1,115 | — | — | (1,115 | ) | — | (1,115 | ) | |||||||||||||||||||||||||||||||||||||||||||
Redemption value | — | — | — | — | — | — | — | — | — | 55,877 | — | — | (10,858 | ) | (45,019 | ) | (55,877 | ) | ||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (14,774 | ) | (14,774 | ) | |||||||||||||||||||||||||||||||||||||||||||
Balance at June 30, 2021 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | 13,053,508 | $ | 166,673 | 1,157,873 | $ | — | $ | — | $ | (183,427 | ) | $ | (183,427 | ) | ||||||||||||||||||||||||||||||||||
Six Months Ended June 30, | ||||||||
2022 | 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (44,385 | ) | $ | (23,601 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 154 | 138 | ||||||
| Reduction in the carrying amount of right-of-use assets | 239 | — | ||||||
| Stock-based compensation expense | 4,039 | 1,800 | ||||||
| Loss on extinguishment of debt | — | 181 | ||||||
| Amortization of premium on short-term investments | (20 | ) | — | |||||
| Non cash interest expense | 152 | 53 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | 12 | (19 | ) | |||||
| Prepaid expenses and other current assets | (2,828 | ) | (1,171 | ) | ||||
| Prepaid expenses and other long-term assets | 11 | (116 | ) | |||||
| Accounts payable | 1,961 | 1,328 | ||||||
| Accrued expenses and other current liabilities | (433 | ) | 343 | |||||
| Deferred rent | (14 | ) | (42 | ) | ||||
| Operating lease liabilities | (280 | ) | — | |||||
| Deferred revenue | 13 | (123 | ) | |||||
Net cash used in operating activities | (41,379 | ) | (21,229 | ) | ||||
Cash flows from investing activities: | ||||||||
| Acquisition of property and equipment | (13 | ) | (46 | ) | ||||
| Capitalized software development costs | — | (57 | ) | |||||
| Purchases of short-term investments | (14,974 | ) | — | |||||
| Proceeds from maturities of short-term investments | 10,000 | — | ||||||
Net cash used in investing activities | (4,987 | ) | (103 | ) | ||||
Cash flows from financing activities: | ||||||||
| Proceeds from exercise of stock options | 105 | — | ||||||
| Proceeds from note payable | 10,000 | 5,000 | ||||||
| Proceeds from issuance of preferred stock, net issuance costs | — | 109,681 | ||||||
| Payment of debt issuance costs | — | (74 | ) | |||||
| Payment of premium on note payable | — | (26 | ) | |||||
| Repayment of principal on note payable | — | (2,000 | ) | |||||
Net cash provided by financing activities | 10,105 | 112,581 | ||||||
Net increase (decrease) in cash, cash equivalents, and restricted cash | (36,261 | ) | 91,249 | |||||
| Cash, cash equivalents, and restricted cash at beginning of period | 77,204 | 18,833 | ||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 40,943 | $ | 110,082 | ||||
Supplementary Information: | ||||||||
| Cash paid for income taxes | $ | — | $ | — | ||||
| Cash paid for interest | 181 | 39 | ||||||
Noncash investing and financing activities: | ||||||||
| Purchase of property and equipment included in accounts payable and accrued expenses | 10 | 7 | ||||||
| Common stock warrants issued related to note payable | — | 268 | ||||||
| Redemption value of Series D preferred stock | 2,893 | 55,877 | ||||||
| Dividends accrued for Series D preferred stock | 5,785 | 1,115 | ||||||
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
| Product revenue | $ | 64 | $ | 37 | $ | 130 | $ | 66 | ||||||||
| Collaboration revenue | $ | — | $ | 68 | — | 156 | ||||||||||
| Total | $ | 64 | $ | 105 | $ | 130 | $ | 222 | ||||||||
Contract Liabilities | Product | |||
| Balance at December 31, 2021 | $ | 96 | ||
| Revenue recognized | (130 | ) | ||
| Revenue deferred | 143 | |||
| Balance at June 30, 2022 | $ | 109 | ||
June 30, 2022 | December 31, 2021 | |||||||
| Deferred issuance costs | $ | 2,999 | $ | 572 | ||||
| Prepaid clinical trials | 879 | 872 | ||||||
| Other current assets | 1,450 | 1,056 | ||||||
| Prepaid expenses and other current assets | $ | 5,328 | $ | 2,500 | ||||
June 30, 2022 | December 31, 2021 | |||||||
| Furniture and fixtures | $ | 184 | $ | 184 | ||||
| Computer equipment and software | 459 | 443 | ||||||
| Office equipment | 60 | 60 | ||||||
| Leasehold improvements | 975 | 975 | ||||||
| Capitalized internal-use software costs | 427 | 427 | ||||||
| Total property and equipment | 2,105 | 2,089 | ||||||
| Less: accumulated depreciation and amortization | (1,051 | ) | (896 | ) | ||||
| Property and equipment, net | $ | 1,054 | $ | 1,193 | ||||
Years Ending December 31, | Amounts | |||
| 2022 (remaining 6 months) | $ | 429 | ||
| 2023 | 878 | |||
| 2024 | 914 | |||
| 2025 | 950 | |||
| 2026 | 905 | |||
| Total undiscounted payments due under operating leases | 4,076 | |||
| Less imputed interest | (619 | ) | ||
| Total | $ | 3,457 | ||
| Current operating lease liability | $ | 625 | ||
| Non-current operating lease liability | 2,832 | |||
| Total | $ | 3,457 | ||
Years Ending December 31, | Amounts | |||
2022 | $ | 538 | ||
| 2023 | 878 | |||
| 2024 | 914 | |||
| 2025 | 950 | |||
| 2026 | 905 | |||
| Total | $ | 4,185 | ||
June 30, 2022 | December 31, 2021 | |||||||
| Accrued bonus | $ | 1,801 | $ | 2,516 | ||||
| Accrued royalties | 106 | 106 | ||||||
| Accrued wages and benefits | 669 | 421 | ||||||
| Accrued clinical study expenses | 418 | 363 | ||||||
| Accrued consulting service expenses | 1,489 | 766 | ||||||
| Other accrued expenses | 561 | 1,305 | ||||||
| Total | $ | 5,044 | $ | 5,477 | ||||
June 30, 2022 | December 31, 2021 | |||||||
| Corporate bond | 5,000 | $ | 5,000 | |||||
| Unamortized discount on corporate bond | (3,265 | ) | (3,362 | ) | ||||
| Corporate bond, net of discount | $ | 1,735 | $ | 1,638 | ||||
| Note payable, long term | $ | 14,375 | ||
| Final Payment | 750 | |||
| Unamortized debt issuance costs | (912 | ) | ||
| Note payable, long term (net of debt issuance costs) | $ | 14,213 | ||
Years Ending December 31, | ||||
| Remainder of 2022 | — | |||
| 2023 | 4,375 | |||
| 2024 | 7,500 | |||
| 2025 | 3,125 | |||
| Total | $ | 15,000 | ||
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
| Research and development | $ | 841 | $ | 265 | $ | 1,450 | $ | 512 | ||||||||
| Selling, general and administrative | 1,128 | 693 | 2,589 | 1,288 | ||||||||||||
| Total | $ | 1,969 | $ | 958 | $ | 4,039 | $ | 1,800 | ||||||||
| Number of Options | Weighted- Average Exercise Price Per Share | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Balance, December 31, 2021 | 7,529,063 | $ | 3.89 | 7.28 | $ | 4,020 | ||||||||||
| Granted | 1,334,379 | $ | 11.58 | |||||||||||||
| Cancelled | (458,986 | ) | $ | 4.55 | ||||||||||||
| Exercised | (28,281 | ) | $ | 3.73 | ||||||||||||
| Balance, June 30, 2022 | 8,376,175 | $ | 5.08 | 7.13 | $ | 54,482 | ||||||||||
| Exercisable, June 30, 2022 | 5,110,692 | $ | 3.77 | 5.99 | $ | 39,901 | ||||||||||
| Options vested and expected to vest, June 30, 2022 | 8,376,175 | $ | 5.08 | 7.13 | $ | 54,482 | ||||||||||
| Fair value of common stock | $ | 11.58 | ||
| Expected volatility | 96.21 | % | ||
| Expected term (in years) | 6.03 | |||
| Risk-free interest rate | 1.73 | % | ||
| Expected dividend yield | 0.00 | % |
| Fair Value Measurements as of June 30, 2022 | ||||||||||||||||
Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Assets | ||||||||||||||||
Cash equivalents: | ||||||||||||||||
| Money market funds | $ | 16,550 | $ | 0— | $ | 0— | $ | 16,550 | ||||||||
| Short-term investments: | ||||||||||||||||
| United States Treasuries | 4,987 | 0— | 0— | 4,987 | ||||||||||||
| Total assets | $ | 21,537 | $ | 0— | $ | 0— | $ | 21,537 | ||||||||
| Fair Value Measurements as of December 31, 2021 | ||||||||||||||||
Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Assets | ||||||||||||||||
Cash equivalents: | ||||||||||||||||
| Money market funds | $ | 61,510 | $ | 0— | $ | 0— | $ | 61,510 | ||||||||
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
2022 | 2021 | 2022 | 2021 | |||||||||||||
Numerator: | ||||||||||||||||
| Net loss | $ | (22,493 | ) | $ | (14,774 | ) | $ | (44,385 | ) | $ | (23,601 | ) | ||||
| Dividends on Series D convertible preferred stock | (2,908 | ) | (1,115 | ) | (5,785 | ) | (1,115 | ) | ||||||||
| Redemption value of Series D convertible preferred stock | (1,455 | ) | (55,877 | ) | (2,893 | ) | (55,877 | ) | ||||||||
Net loss attributable to common stockholders—basic and diluted | $ | (26,856 | ) | $ | (71,766 | ) | $ | (53,063 | ) | $ | (80,593 | ) | ||||
| Denominator: | ||||||||||||||||
| Weighted-average common stock outstanding | 1,471,296 | 1,157,870 | 1,466,462 | 1,157,869 | ||||||||||||
Net loss per share attributable to common stockholders—basic and diluted | $ | (18.25 | ) | $ | (61.98 | ) | $ | (36.18 | ) | $ | (69.60 | ) | ||||
As of June 30, | ||||||||
2022 | 2021 | |||||||
| Series A-1 convertible preferred stock (as converted to common stock) | 4,000,000 | 4,000,000 | ||||||
| Series A-2 convertible preferred stock (as converted to common stock) | 4,427,072 | 4,427,072 | ||||||
| Series B convertible preferred stock (as converted to common stock) | 7,341,485 | 7,341,485 | ||||||
| Series C convertible preferred stock (as converted to common stock) | 8,016,645 | 8,016,645 | ||||||
| Series D convertible preferred stock (as converted to common stock) | 21,795,525 | 19,778,730 | ||||||
| Warrants to purchase common stock | 226,196 | 226,196 | ||||||
| Stock options to purchase common stock | 8,376,175 | 5,912,116 | ||||||
| Total | 54,183,098 | 49,702,244 | ||||||
December 31, | ||||||||
Assets | 2021 | 2020 | ||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 76,899 | $ | 18,528 | ||||
| Restricted cash | 305 | 305 | ||||||
| Accounts receivable | 29 | 8 | ||||||
| Prepaid expenses and other current assets | 2,500 | 314 | ||||||
Total current assets | 79,733 | 19,155 | ||||||
| Property and equipment, net | 1,193 | 1,004 | ||||||
| Deposits | — | 22 | ||||||
| Prepaid expenses and other long-term assets | 11 | — | ||||||
Total assets | $ | 80,937 | $ | 20,181 | ||||
Liabilities, redeemable convertible preferred stock and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Note payable | $ | — | $ | 114 | ||||
| Accounts payable | 2,345 | 876 | ||||||
| Accrued expenses and other current liabilities | 5,477 | 2,445 | ||||||
| Deferred revenue | 96 | 369 | ||||||
| Deferred rent, short term | 123 | 121 | ||||||
Total current liabilities | 8,041 | 3,925 | ||||||
| Note payable, long term | 4,784 | 1,814 | ||||||
| Corporate bond, net of bond discount | 1,638 | 1,462 | ||||||
| Deferred rent, long term | 712 | 774 | ||||||
| Other long-term liabilities | — | 222 | ||||||
Total liabilities | 15,175 | 8,197 | ||||||
| Commitments and contingencies (Note 11) | 0 | 0 | ||||||
| Redeemable convertible preferred stock, $0.0001 par value—authorized 41,785,202 shares: | ||||||||
Series A-1: designated, 4,000,000 shares; issued and outstanding shares, 4,000,000 at December 31, 2021 and 2020 (liquidation value of $4,000 at December 31, 2021 and 2020) | — | — | ||||||
Series A-2: designated 4,427,072 shares; issued and outstanding shares, 4,427,072 at December 31, 2021 and 2020 (liquidation value of $8,832 at December 31, 2021 and 2020) | 7,128 | 7,128 | ||||||
| Series B: designated 7,341,485 shares; issued and outstanding shares, 7,341,485 at December 31, 2021 and 2020 (liquidation value of $42,360 at December 31, 2021 and 2020) | 41,854 | 41,854 | ||||||
| Series C: designated 8,016,645 shares; issued and outstanding shares, 8,016,645 at December 31, 2021 and 2020 (liquidation value of $68,200 at December 31, 2021 and 2020) | 67,904 | 67,904 | ||||||
| Series D: designated 18,000,000 shares; issued and outstanding shares, 13,843,858 at December 31, 2021 (liquidation value of $174,990 at December 31, 2021) | 174,990 | — | ||||||
| Stockholders’ deficit: | ||||||||
| Common shares, $0.0001 par value: 55,000,000 and 32,000,000 shares authorized at December 31, 2021 and 2020, respectively; 1,454,239 and 1,157,868 shares issued and outstanding at December 31, 2021 and 2020, respectively | — | — | ||||||
Additional paid-in capital | — | 9,905 | ||||||
| Accumulated deficit | (226,114 | ) | (114,807 | ) | ||||
Total stockholders’ deficit | (226,114 | ) | (104,902 | ) | ||||
Total liabilities, redeemable convertible preferred stock and stockholders’ deficit | $ | 80,937 | $ | 20,181 | ||||
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Revenues | $ | 538 | $ | 3,939 | ||||
| Cost of revenues | 355 | 416 | ||||||
| Gross profit | 183 | 3,523 | ||||||
| Operating expenses: | ||||||||
| Research and development | 18,234 | 15,418 | ||||||
| Selling, general and administrative | 42,668 | 13,541 | ||||||
| Total operating expenses | 60,902 | 28,959 | ||||||
| Operating loss | (60,719 | ) | (25,436 | ) | ||||
| Other income (expense): | ||||||||
| Other income | 17 | 124 | ||||||
| Interest expense | (465 | ) | (333 | ) | ||||
| Loss on extinguishment of debt | (181 | ) | — | |||||
| Total other income (expense) | (629 | ) | (209 | ) | ||||
| Loss before income taxes | (61,348 | ) | (25,645 | ) | ||||
| Income tax expense | — | 1 | ||||||
| Net loss and comprehensive loss | $ | (61,348 | ) | $ | (25,646 | ) | ||
| Dividends on Series D convertible preferred stock | $ | (6,660 | ) | — | ||||
| Redemption value of Series D convertible preferred stock | (58,649 | ) | — | |||||
| Net loss attributable to common stockholders | $ | (126,657 | ) | $ | (25,646 | ) | ||
| Net loss per share: | ||||||||
| Basic and diluted | $ | (105.77 | ) | $ | (22.20 | ) | ||
| Weighted average common shares outstanding: | ||||||||
| Basic and diluted | 1,197,489 | 1,155,319 | ||||||
Series A-1 Redeemable Convertible Preferred Stock | Series A-2 Redeemable Convertible Preferred Stock | Series B Redeemable Convertible Preferred Stock | Series C Redeemable Convertible Preferred Stock | Series D Redeemable Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders’ Deficit | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | Shares | Value | |||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2019 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | — | $ | — | 1,153,368 | $ | — | $ | 6,913 | $ | (89,161 | ) | $ | (82,248 | ) | ||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 2,898 | — | 2,898 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of stock options | — | — | — | — | — | — | — | — | — | — | 4,500 | — | 19 | — | 19 | |||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants | — | — | — | — | — | — | — | — | — | — | — | — | 75 | — | 75 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (25,646 | ) | (25,646 | ) | |||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2020 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | — | $ | — | 1,157,868 | $ | — | $ | 9,905 | $ | (114,807 | ) | $ | (104,902 | ) | ||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | — | — | 4,913 | — | 4,913 | |||||||||||||||||||||||||||||||||||||||||||||
| Exercise of stock options | — | — | — | — | — | — | — | — | — | — | 296,371 | — | 264 | — | 264 | |||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock warrants | — | — | — | — | — | — | — | — | — | — | — | — | 268 | — | 268 | |||||||||||||||||||||||||||||||||||||||||||||
| Issuance of convertible preferred stock, net of issuance costs | — | — | — | — | — | — | — | — | 13,053,508 | 109,681 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Stock divdend | — | — | — | — | — | — | — | — | 790,350 | 6,660 | — | — | (4,661 | ) | (1,999 | ) | (6,660 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Redemption value | — | — | — | — | — | — | — | — | — | 58,649 | — | — | (10,689 | ) | (47,960 | ) | (58,649 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | — | — | — | — | — | — | — | — | — | (61,348 | ) | (61,348 | ) | |||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2021 | 4,000,000 | $ | — | 4,427,072 | $ | 7,128 | 7,341,485 | $ | 41,854 | 8,016,645 | $ | 67,904 | 13,843,858 | $ | 174,990 | 1,454,239 | $ | — | $ | — | $ | (226,114 | ) | $ | (226,114 | ) | ||||||||||||||||||||||||||||||||||
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (61,348 | ) | $ | (25,646 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization of property and equipment | 279 | 300 | ||||||
| Stock-based compensation expense | 4,913 | 2,898 | ||||||
| Loss on disposal of fixed assets | 13 | 6 | ||||||
| Loss on extinguishment of debt | 181 | — | ||||||
| Non cash interest expense | 219 | 294 | ||||||
| Premium of end of term payment | — | 12 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (21 | ) | (8 | ) | ||||
| Prepaid expenses and other current assets | (2,186 | ) | 311 | |||||
| Deposits | 22 | — | ||||||
| Prepaid expenses and other long-term assets | (11 | ) | — | |||||
| Accounts payable | 1,480 | (38 | ) | |||||
| Accrued expenses and other current liabilities | 3,032 | 637 | ||||||
| Deferred rent, short term | 2 | — | ||||||
| Deferred rent and other long term liabilities | (284 | ) | 133 | |||||
| Deferred revenue | (273 | ) | (3,450 | ) | ||||
Net cash used in operating activities | (53,982 | ) | (24,551 | ) | ||||
Cash flows from investing activities: | ||||||||
| Acquisition of property and equipment | (65 | ) | (116 | ) | ||||
| Capitalized software development costs | (427 | ) | — | |||||
Net cash used in investing activities | (492 | ) | (116 | ) | ||||
Cash flows from financing activities: | ||||||||
| Proceeds from note payable | 5,000 | 2,000 | ||||||
| Proceeds from issuance of preferred stock, net issuance costs | 109,681 | — | ||||||
| Payment of debt issuance costs | (74 | ) | (21 | ) | ||||
| Payment of premium on note payable | (26 | ) | — | |||||
| Repayment of principal on note payable | (2,000 | ) | — | |||||
| Proceeds from exercise of stock options | 264 | 19 | ||||||
Net cash provided by financing activities | 112,845 | 1,998 | ||||||
Net increase (decrease) in cash, cash equivalents, and restricted cash | 58,371 | (22,669 | ) | |||||
| Cash, cash equivalents, and restricted cash at beginning of period | 18,833 | 41,502 | ||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 77,204 | $ | 18,833 | ||||
Supplementary Information: | ||||||||
| Cash paid for income taxes | $ | — | $ | 15 | ||||
| Cash paid for interest | 217 | 20 | ||||||
Noncash investing and financing activities: | ||||||||
| Purchase of property and equipment included in accounts payable and accrued expenses | 7 | 18 | ||||||
| Common stock warrants issued related to note payable | 268 | 75 | ||||||
| Redemption value of Series D preferred stock | 58,649 | — | ||||||
| Dividends accrued for Series D preferred stock | 6,660 | — | ||||||
1. | Nature of the Business and Basis of Presentation |
2. | Summary of Significant Accounting Policies |
Level 1: | Inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. |
Level 2: | Valuations based on quoted prices in markets that are not active or for which all significant inputs are observable, either directly or indirectly. |
Level 3: | Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. |
| Furniture and fixtures | 5-7 years | |
| Computer equipment and software | 3 years | |
| Office equipment | 3 years | |
| Leasehold improvements | 3-7 years (Or remaining term of the lease, if shorter) | |
Internal-use software | 2-5 years |
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Product revenue | $ | 186 | $ | 12 | ||||
| Collaboration revenue | 352 | 3,927 | ||||||
| Total | $ | 538 | $ | 3,939 | ||||
Contract Liabilities | Product | Collaboration | ||||||
| Balance at December 31, 2019 | $ | — | $ | 3,819 | ||||
| Revenue recognized | (12 | ) | (3,928 | ) | ||||
| Revenue deferred | 29 | 461 | ||||||
| Balance at December 31, 2020 | 17 | 352 | ||||||
| Revenue recognized | (186 | ) | (352 | ) | ||||
| Revenue deferred | 265 | — | ||||||
| Balance at December 31, 2021 | $ | 96 | $ | — | ||||
3. | Option and Collaboration Agreements |
| Payment associated with option period | $ | 10,000 | ||
| Payment to exercise agreement | 10,000 | |||
| Discount on issuance on corporate bond (see Note 6) | 3,805 | |||
| Contract modification | 387 | |||
| Total transaction price | 24,192 | |||
| Less: Revenue recognized | (24,192 | ) | ||
| Deferred revenue at December 31, 2021 | $ | — | ||
4. | Property and Equipment |
December 31, | ||||||||
2021 | 2020 | |||||||
| Furniture and fixtures | $ | 184 | $ | 184 | ||||
| Computer equipment and software | 443 | 390 | ||||||
| Office equipment | 60 | 60 | ||||||
| Leasehold improvements | 975 | 1,020 | ||||||
Capitalized internal-use software costs | 427 | — | ||||||
| Total property and equipment | 2,089 | 1,654 | ||||||
| Less: accumulated depreciation and amortization | (896 | ) | (650 | ) | ||||
| Property and equipment, net | $ | 1,193 | $ | 1,004 | ||||
5. | Accrued Expenses and Other Current Liabilities |
December 31, | ||||||||
2021 | 2020 | |||||||
| Accrued bonus | $ | 2,516 | $ | 1,715 | ||||
| Accrued royalties | 106 | 101 | ||||||
| Accrued wages and benefits | 421 | 287 | ||||||
| Accrued clinical study expenses | 363 | 9 | ||||||
| Accrued consulting service expenses | 766 | 100 | ||||||
| Other accrued expenses | 1,305 | 233 | ||||||
| Total | $ | 5,477 | $ | 2,445 | ||||
6. | Corporate Bond |
December 31, | ||||||||
2021 | 2020 | |||||||
| Corporate bond | 5,000 | $ | 5,000 | |||||
| Unamortized discount on corporate bond | (3,362 | ) | (3,538 | ) | ||||
| Corporate bond, net of discount | $ | 1,638 | $ | 1,462 | ||||
7. | Note Payable |
| • | Tranche 2—$5,000, is available following the achievement of a certain revenue milestone, the occurrence of which must be on or prior to December 31, 2022 or prior to an event of default. |
| • | Tranche 3—$10,000, is available based on the satisfaction of certain conditions, including a certain revenue milestone, and at sole discretion of the Lenders, the occurrence of which must be on or prior to December 31, 2022 or prior to an event of default. |
| Note payable | $ | 5,000 | ||
| Final Payment | 250 | |||
| Unamortized debt issuance costs | (466 | ) | ||
| Note payable, net of debt issuance costs | $ | 4,784 | ||
Years Ending December 31, | ||||
| 2022 | — | |||
| 2023 | $ | 1,250 | ||
| 2024 | 2,500 | |||
| 2025 | 1,250 | |||
| Total | $ | 5,000 | ||
8. | Redeemable Convertible Preferred Stock |
9. | Common Stock |
First Loan Modification Agreement Warrants | ||||
| Fair value of common stock | $ | 6.84 | ||
| Expected volatility | 67.70 | % | ||
| Expected term (in years) | 9.95 | |||
| Risk-free interest rate | 0.72 | % | ||
| Expected dividend yield | 0.00 | % | ||
Amended and Restated Loan and Security Agreement Warrants | ||||
| Fair value of common stock | $ | 4.40 | ||
| Expected volatility | 95.00 | % | ||
| Expected term (in years) | 10.00 | |||
| Risk-free interest rate | 1.56 | % | ||
| Expected dividend yield | 0.00 | % |
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Research and development | $ | 1,340 | $ | 988 | ||||
| Selling, general and administrative | 3,573 | 1,910 | ||||||
| Total | $ | 4,913 | $ | 2,898 | ||||
| Number of Options | Weighted- Average Exercise Price Per Share | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Balance, December 31, 2020 | 6,020,392 | $ | 4.53 | 7.25 | $ | 13,878 | ||||||||||
| Granted | 5,783,942 | $ | 4.40 | |||||||||||||
| Cancelled | (3,978,900 | ) | $ | 5.85 | ||||||||||||
| Exercised | (296,371 | ) | $ | 0.89 | ||||||||||||
| Balance, December 31, 2021 | 7,529,063 | $ | 3.89 | 7.28 | $ | 4,020 | ||||||||||
| Exercisable, December 31, 2021 | | 4,366,874 | | $ | 3.52 | 6.11 | $ | 4,020 | ||||||||
| Options vested and expected to vest, December 31, 2021 | 7,529,063 | $ | 3.89 | 7.28 | $ | 4,020 | ||||||||||
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Fair value of common stock | $ | 4.40 | $ | 6.29 | ||||
| Expected volatility | 98.73 | % | 93.50 | % | ||||
| Expected term (in years) | 4.88 | 5.94 | ||||||
| Risk-free interest rate | 0.81 | % | 0.46 | % | ||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
10. | Fair Value of Financial Assets and Liabilities |
| Fair Value Measurements as of December 31, 2021 | ||||||||||||||||
| Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Assets | ||||||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | 61,510 | $ | 0— | $ | 0— | $ | 61,510 | ||||||||
| Fair Value Measurements as of December 31, 2020 | ||||||||||||||||
| Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Assets | ||||||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | 17,508 | $ | 0— | $ | 0— | $ | 17,508 | ||||||||
11. | Commitments and Contingencies |
| 2022 | $ | 1,118 | ||
| 2023 | 878 | |||
| 2024 | 914 | |||
| 2025 | 950 | |||
| 2026 | 904 | |||
| Total | $ | 4,764 | ||
12. | Income Taxes |
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Current | ||||||||
| Federal | $ | — | $ | — | ||||
| State | — | 1 | ||||||
| Total current expense (benefit) | — | 1 | ||||||
| Deferred | ||||||||
| Federal | — | — | ||||||
| State | — | — | ||||||
| Total deferred expense (benefit) | — | — | ||||||
| Total tax recognized | $ | — | $ | 1 | ||||
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Benefit at federal statutory rate | 21.00 | % | 21.00 | % | ||||
| State taxes | 3.41 | % | 2.70 | % | ||||
| Credits | 1.20 | % | 2.61 | % | ||||
| Share-based payment measurement | (0.57 | %) | (1.01 | %) | ||||
| Other | (1.90 | %) | 0.18 | % | ||||
| Change in valuation allowance | (23.14 | %) | (25.49 | %) | ||||
| Effective tax rate | 0.00 | % | (0.01 | %) | ||||
2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Operating tax losses | $ | 38,084 | $ | 26,016 | ||||
| Research credits | 5,920 | 4,810 | ||||||
| Temporary differences | 981 | 773 | ||||||
| Share based payments | 1,902 | 1,099 | ||||||
| Gross deferred tax assets | 46,887 | 32,698 | ||||||
| Valuation Allowance | (46,287 | ) | (32,090 | ) | ||||
| Deferred tax assets, Less: valuation allowance | 600 | 608 | ||||||
| Deferred tax liabilities: | ||||||||
| Other temporary differences | (600 | ) | (608 | ) | ||||
| Deferred tax liabilities | (600 | ) | (608 | ) | ||||
| Total | $ | — | $ | — | ||||
13. | Net Loss Per Share |
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (61,348 | ) | $ | (25,646 | ) | ||
| Dividends on Series D convertible preferred stock | (6,660 | ) | — | |||||
| Redemption value of Series D convertible preferred stock | (58,649 | ) | — | |||||
| Net loss attributable to common stockholders—basic and diluted | $ | (126,657 | ) | $ | (25,646 | ) | ||
| Denominator: | ||||||||
| Weighted-average common stock outstanding | 1,197,489 | 1,155,319 | ||||||
| Net loss per share attributable to common stockholders—basic and diluted | $ | (105.77 | ) | $ | (22.20 | ) | ||
Years Ended December 31, | ||||||||
2021 | 2020 | |||||||
Series A-1 convertible preferred stock (as converted to common stock) | 4,000,000 | 4,000,000 | ||||||
Series A-2 convertible preferred stock (as converted to common stock) | 4,427,072 | 4,427,072 | ||||||
| Series B convertible preferred stock (as converted to common stock) | 7,341,485 | 7,341,485 | ||||||
| Series C convertible preferred stock (as converted to common stock) | 8,016,645 | 8,016,645 | ||||||
| Series D convertible preferred stock (as converted to common stock) | 20,765,787 | — | ||||||
| Warrants to purchase common stock | 226,196 | 77,672 | ||||||
| Stock options to purchase common stock | 7,529,063 | 6,020,392 | ||||||
| Total | 52,306,248 | 29,883,266 | ||||||
14. | Employee Benefit Plan |
15. | Subsequent Events |
Item 13. | Other Expenses of Issuance and Distribution |
SEC registration fee | $ | 38,395 | ||
Accounting fees and expenses | 55,810 | |||
Legal fees and expenses | 125,000 | |||
Financial printing and miscellaneous expenses | 125,000 | |||
Total | $ | 344,205 | ||
Item 14. | Indemnification of directors and officers. |
Item 15. | Recent Sales of Unregistered Securities. |
| • | Prior to SCS’s initial public offering, the Sponsor purchased 5,750,000 SCS Class B ordinary shares for an aggregate purchase price of $25,000. In June 2021, the Sponsor transferred 30,000 SCS Class B ordinary shares to Vladimir Coric (an independent director of SCS, and with the Sponsor, “SCS’s initial shareholders”). On June 29, 2021, SCS effected a share capitalization with respect to the SCS Class B ordinary shares of 575,000 shares thereof, resulting in SCS’s initial shareholders holding an aggregate of 6,325,000 founder shares (up to 825,000 of which were subject to forfeiture depending on the extent to which the underwriter’s over-allotment option in the initial public offering was exercised), resulting in an effective purchase price per SCS Class B ordinary share of approximately $0.004. As a result of the underwriters’ election to partially exercise their over-allotment option, a total of 750,000 SCS Class B ordinary shares are no longer subject to forfeiture and 75,000 SCS Class B ordinary shares were forfeited, resulting in an aggregate of 6,250,000 SCS Class B ordinary shares outstanding. |
| • | On July 2, 2021, SCS issued 640,000 SCS Class A ordinary shares, par value $0.0001 per share, to the Sponsor concurrently with the closing of SCS’s IPO for a total purchase price of $6,400,000; and |
| • | On August 19, 2022, we issued 16,200,000 shares of common stock to certain qualified institutional buyers and accredited investors that agreed to purchase such shares in connection with the Business Combination for aggregate consideration of $162,000,000. |
Item 16. | Exhibits and Financial Statements Schedules. |
| (a) | Exhibits. |
| * | Filed herewith. |
| ** | To be filed, if necessary, subsequent to the effectiveness of this registration statement by an amendment to this registration statement or incorporated by reference pursuant to a Current Report on Form 8-K |
| + | Schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. |
| † | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with Item 601(b)(10) of Regulation S-K. |
Item 17. | Undertakings. |
| (1) | to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: (i) to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”); (ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any |
| deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and (iii) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (i), (ii) and (iii) do not apply if the registration statement is on Form S-1 and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement; |
| (2) | that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
| (3) | to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; |
| (4) | that, for the purpose of determining liability under the Securities Act to any purchaser: |
| (5) | that, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
Akili, Inc. | ||
| By: | /s/ W. Edward Martucci, Ph.D. | |
| Name: | W. Edward Martucci, Ph.D. | |
| Title: | Chief Executive Officer | |
Signature | Title | Date | ||
| /s/ W. Edward Martucci, Ph.D. | Chief Executive Officer and Director | August 23, 2022 | ||
| W. Edward Martucci, Ph.D. | (Principal Executive Officer) | |||
| /s/ Santosh Shanbhag | Chief Financial Officer | August 23, 2022 | ||
| Santosh Shanbhag | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Chamath Palihapitiya | Chairman and Director | August 23, 2022 | ||
| Chamath Palihapitiya | ||||
| /s/ Bharatt Chowrira, Ph.D. | Director | August 23, 2022 | ||
| Bharatt Chowrira, Ph.D. | ||||
| /s/ Kenneth Ehlert | Director | August 23, 2022 | ||
| Kenneth Ehlert | ||||
| /s/ Adam Gazzaley, M.D., Ph.D. | Director | August 23, 2022 | ||
| Adam Gazzaley, M.D., Ph.D. | ||||
| /s/ William Jones, Jr. | Director | August 23, 2022 | ||
| William Jones, Jr. | ||||
| /s/ Christine Lemke | Director | August 23, 2022 | ||
| Christine Lemke | ||||