| Item 2.05 | Costs Associated with Exit or Disposal Activities. |
On January 11, 2023, the Board of Directors (the “Board”) of Akili, Inc. (the “Company”) approved an operating plan for 2023 that will result in a reduction of the Company’s operating expenses. As part of this plan, the Company’s workforce will be reduced by approximately 30% across different areas and functions in the Company. This workforce reduction was communicated to employees on January 12, 2023 and is expected to be completed by the end of the first quarter of 2023. Affected employees will be offered severance and other benefits, and the Company estimates that these severance and termination-related costs will be approximately $1.5 – $2.5 million and expects to record these charges in the first quarter of 2023. The Company also expects that payments of these costs will be made in the first quarter of 2023.
In addition, the operating plan includes a pipeline reprioritization of the Company’s preclinical and clinical development programs and further prioritization of the Company’s resources on its commercial organization and efforts.
| Item 7.01 | Regulation FD Disclosure. |
2023 Non-GAAP Total Operating Expense Guidance
The Company expects 2023 non-GAAP total operating expenses of between $55.0 – $60.0 million.
The information contained in this Item 7.01 is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
About Non-GAAP Financial Measures
This Current Report on Form 8-K includes the following non-GAAP financial measure: non-GAAP total operating expenses on a projected basis. The Company derives this non-GAAP financial measure by excluding certain expenses and other items from the GAAP financial measure that is most directly comparable to the non-GAAP financial measure. Specifically, the projected non-GAAP total operating expenses financial measure excludes stock-based compensation expense. The Company’s management believes that this non-GAAP financial measure is useful to both management and investors in analyzing its ongoing business and operating performance. Management does not intend the presentation of this non-GAAP financial measure to be considered in isolation or as a substitute for results prepared in accordance with GAAP, but as a complement to provide greater transparency. In addition, this non-GAAP financial measure may differ from similarly named measures used by other companies. A quantitative reconciliation of projected non-GAAP total operating expenses to projected GAAP operating expenses is not available, nor is the probable significance of such reconciling information, due to Akili’s inability to predict with reasonable certainty the amount of future stock-based compensation expense at this time.
| Item 8.01 | Other Information. |
On January 12, 2023, the Company’s chief executive officer sent an email to all Company employees with a business update, which included information regarding the approved workforce reduction as part of the Company’s 2023 operating plan. A copy of the email is attached as Exhibit 99.1 to this Current Report on Form 8-K.
In addition, the Company is providing the below business updates to its previously-disclosed pipeline programs and cash runway.
Pipeline and Business Updates
As shown in the updated pipeline chart included below, there are several updates to the Company’s pipeline as part of the Company’s recently approved 2023 operating plan, and these updates are briefly summarized below.
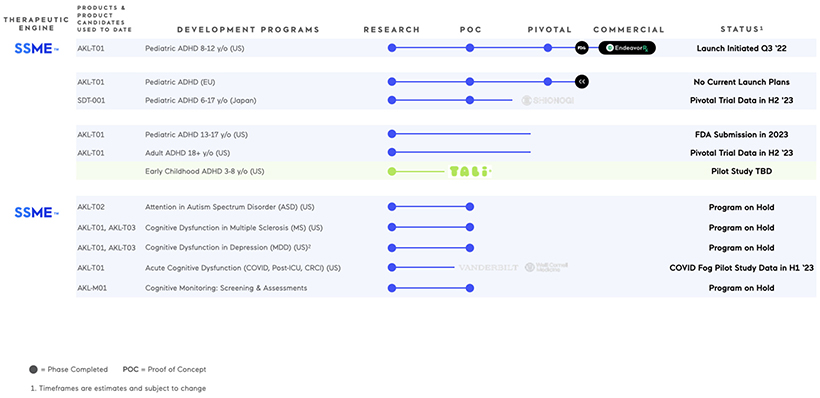
| | • | | Pivotal Trial of EndeavorRx (AKL-T01) in Adolescents Ages 13-17 with ADHD (listed above in the pipeline chart as “Pediatric ADHD 13-17 y/o (U.S.)”): As disclosed on January 5, 2023, the Company announced topline results of this STARS-ADHD-Adolescents label expansion trial evaluating the efficacy and safety of EndeavorRx (AKL-T01) in adolescents ages 13-17 with attention-deficit/hyperactivity disorder (ADHD), |
