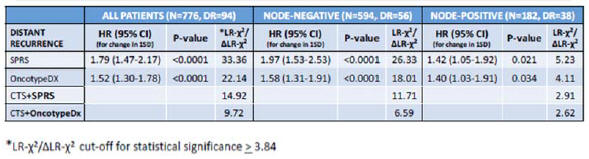
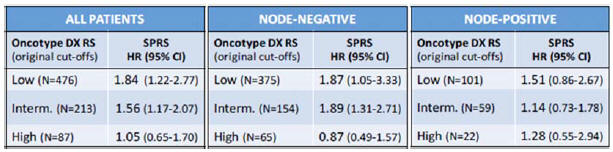
U.S. health regulatory overview
The following provides an overview of key aspects of laboratory service and medical device regulation within the U.S. It should be noted this overview does not address every facet of regulation at the federal and state level, but only those that would generally be most relevant to the activities described in this registration statement.
Federal and state clinical laboratory licensing requirements
The CLIA, governs the operations of all clinical laboratories operating in or returning results to individuals in the U.S. CLIA is administered by CMS, in partnership with state health departments. A clinical laboratory is defined as a laboratory that performs testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the assessment of health. Clinical laboratories must hold a certificate applicable to the type of laboratory examinations they perform and must demonstrate compliance with regulations addressing, among other things, personnel qualification and training, record keeping, quality control, and proficiency testing, all of which are intended to ensure the timeliness, reliability, and accuracy of clinical laboratory testing services. CLIA requires that laboratories demonstrate or verify the analytical validity of all tests they perform. Where a clinical laboratory analyses specimens based on a proprietary test method (i.e., an LDT), the laboratory must, among other things, document the accuracy, precision, specificity, sensitivity of, and establish a reference range for, such test.
CMS provides for exemption from CLIA for states that develop clinical laboratory standards that are at least as stringent as federal requirements. Both New York and Washington State are exempt from CLIA. The NYS Clinical Laboratory Evaluation Program requires all independent clinical laboratories operating in, or testing specimens from, NYS to obtain a laboratory permit prior to commencing operations, and all clinical laboratories performing LDTs to submit test validation documentation demonstrating the tests’ analytical and clinical validity.
Failure to comply with CLIA certification and state clinical laboratory licensure requirements may result in a range of enforcement actions, including certificate or license suspension, limitation, or revocation, directed plan of action, onsite monitoring, civil monetary penalties, criminal sanctions, and revocation of the laboratory’s approval to receive Medicare and Medicaid payment for its services, as well as significant adverse publicity.
FDA
The FDA regulates, among other medical products, “medical devices” which include certain articles intended for use in the diagnosis, prevention, cure, mitigation, or treatment of disease or intended to effect the structure or function of the body. Whether a product is intended for use as a medical device is generally determined, in the first instance, based on the manufacturer’s product labelling, which includes the label affixed to the product, materials distributed with the product, and promotional communications concerning the product.
Devices classified as Class I (low risk), generally may be marketed without FDA pre-market review, but are subject to “general controls”, including establishment registration, device listing, record keeping, medical device reporting, and quality system regulations, including design controls. Devices classified as Class II (moderate risk), may, in addition to general controls, also be subject to “special controls” (e.g., performance standards / manufacturing standards, post-market surveillance, patient registries, special labelling requirements, pre-market data requirements and guidelines), and also generally must obtain 510(k) premarket clearance or DeNovo authorization from FDA. Class III (high risk) devices must, in addition to general controls, obtain FDA pre-market approval through the submission of a pre- market approval application that contains evidence, including data from adequate and well- controlled clinical studies, demonstrating that the device is safe and effective for its intended use. In general, devices that require FDA pre-market clearance or DeNovo authorization may not be commercially distributed or promoted prior to obtaining such authorization, although they may be distributed and used for the purpose of developing the clinical data necessary to support FDA marketing applications, subject to certain limitations. Post-market changes to a cleared / authorized or approved device also may be subject to prior review by FDA, depending on the scope of the change and its potential impact on device safety and effectiveness.
It should also be emphasized that this pre-market review process is only one facet of FDA’s regulation. For example, FDA regulates product labelling, including promotional claims; the manufacturing of medical devices, including their design, under FDA quality system requirements; clinical trials with new or modified products; and post-market monitoring for, reporting of, and action related to, safety concerns. Failure to comply with applicable pre- and post-market device
35