
Exhibit 99.2 CORPORATE PRESENTATION January 2025

Forward Looking Statements Ventyx Biosciences, Inc. (“Ventyx” or the “Company”) cautions you that statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. These statements are based on the Company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding: the expected year-end 2024 cash balance based on preliminary, unaudited information for the year ended December 31, 2024; the potential of Ventyx’s product candidates, including the potential of meaningful value creation through clinical trial results, potential of VTX3232 to demonstrate best in class profile, treat various neuroinflammatory diseases, including Parkinson’s disease modification, or to achieve certain drug concentrations in the CSF, the potential of VTX2735 to demonstrate best in class profile or treat various systemic diseases, and the potential of VTX002 in UC and class-leading safety and efficacy profile; the design of clinical studies to be conducted by the Company; the total addressable market for a Parkinson’s disease modifying therapy; the timing of clinical updates for all three Phase 2 studies of VTX3232 and VTX2735, including the publication of any clinical data from these studies in 2025; the regulatory pathway for VTX2735 and any expedited pathways that may be available; management’s plans with respect to a potential pivotal Phase 3 trial for tamuzimod (VTX002) in UC, supported by a partner or other source of non-dilutive financing; the need for a single pivotal study for VTX002; and the expected timeframe for funding Ventyx’s operating plan with current cash, cash equivalents and marketable securities. We are in the process of finalizing our financial statements for the year ended December 31, 2024, and the preliminary, unaudited information presented in this press release for the year ended December 31, 2024 is based on management’s initial review of the information presented and its current expectations and is subject to adjustment as a result of, among other things, the completion of Ventyx’s end-of-period reporting processes and related activities, including the audit by Ventyx’s independent registered public accounting firm of Ventyx’s financial statements. As such, any financial information contained in this press release may differ materially from the information reflected in Ventyx’s financial statements as of and for the year ended December 31, 2024. You should carefully review our audited, consolidated financial statements for the year ended December 31, 2024 when they become available. The inclusion of forward-looking statements should not be regarded as a representation by Ventyx that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in Ventyx’s business, including, without limitation: potential delays in the commencement, enrollment and completion of clinical trials; Ventyx’s dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; disruptions in the supply chain, including raw materials needed for manufacturing and animals used in research, delays in site activations and enrollment of clinical trials; the results of preclinical studies and early clinical trials not necessarily being predictive of future results; interim results not necessarily being predictive of final results; the potential of one or more outcomes to materially change as a trial continues and more patient data become available and following more comprehensive audit and verification procedures; regulatory developments in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of Ventyx’s product candidates that may limit their development, regulatory approval and/or commercialization, or may result in recalls or product liability claims; Ventyx’s ability to obtain and maintain intellectual property protection for its product candidates; the use of capital resources by Ventyx sooner than expected; disruption to Ventyx’s operations from the ongoing military conflicts in Ukraine and the Middle East, including clinical trial delays; and other risks described in Ventyx’s press releases and Ventyx’s filings with the Securities and Exchange Commission (SEC), including in in Part II, Item 1A (Risk Factors) of Ventyx’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, filed on or about November 7, 2024, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Ventyx undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward- looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our products include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reliable, such assumptions have not been verified by any third party. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors that could cause results to differ materially from those expressed in the estimates made by third parties and by us. Trademarks in this presentation are the property of their respective owners and used for informational and education purposes only. 2
Company Highlights NLRP3 inhibition represents a paradigm shift in the treatment of autoimmune, inflammatory and neurodegenerative disorders via upstream regulation of key cytokines (e.g., IL-1, IL-18, IL-6) VTX2735 and VTX3232 demonstrate potential best-in-class profiles: low nanomolar potency; proven target engagement; clinical proof-of-concept in CAPS; favorable safety profile Clinical catalysts represent three potential paths to meaningful value creation in 2025: § VTX2735 Phase 2 recurrent pericarditis trial – data expected H2 2025 § VTX3232 Phase 2 cardiometabolic trial in obese participants – data expected H2 2025 § VTX3232 Phase 2 early Parkinson’s disease study – data expected H1 2025 Late-stage Inflammatory Bowel Disease portfolio provides potential additional source of value: § Tamuzimod is a Phase 3-ready, potential best-in-class S1P1R modulator for Ulcerative Colitis § VTX958 (TYK2 inhibitor) demonstrated robust endoscopic response in a Phase 2 Crohn’s disease study * Strong balance sheet: $252.9M in cash to fund operations into at least H2 2026 CAPS: Cryopyrin-associated autoinflammatory syndromes *Preliminary cash, cash equivalents and marketable securities balance as of December 31, 2024 (unaudited), subject to adjustments resulting from, among other things, the completion of our end-of-period reporting processes and related activities, including the audit by our independent registered public accounting firm of our financial statements. 3

Experienced Leadership Team Executive Team Raju Mohan, PhD Mark Forman, MD, PhD Matthew Moore John Nuss, PhD CHIEF EXECUTIVE OFFICER, CHIEF MEDICAL OFFICER CHIEF OPERATING OFFICER CHIEF SCIENTIFIC OFFICER FOUNDER Board Of Directors Sheila Gujrathi, PhD Raju Mohan, PhD Onaiza Cadoret-Manier EXECUTIVE CHAIR, VENTYX CHIEF EXECUTIVE OFFICER, VENTYX CHIEF EXECUTIVE OFFICER, STEALTH BIOTECH Allison Hulme, PhD Somu Subramaniam William White CHIEF EXECUTIVE OFFICER, AEOVIAN MANAGING PARTNER, NEW SCIENCE VENTURES CHIEF FINANCIAL OFFICER, AKERO THERAPEUTICS PHARMACEUTICALS 4
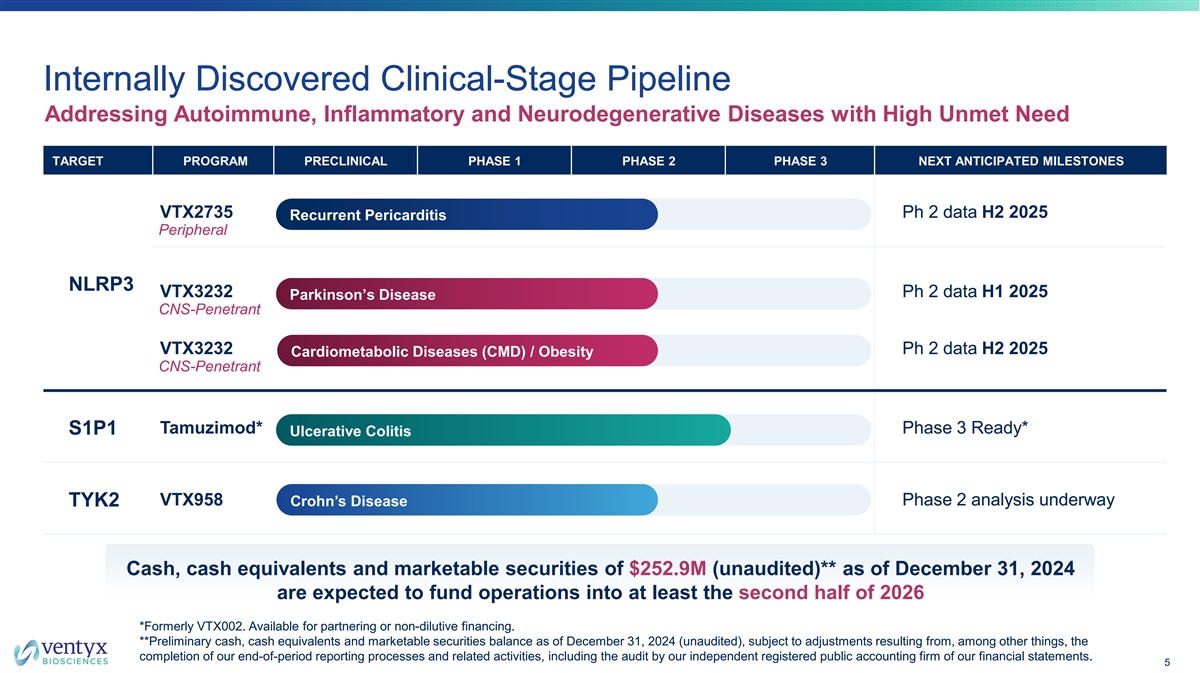
Internally Discovered Clinical-Stage Pipeline Addressing Autoimmune, Inflammatory and Neurodegenerative Diseases with High Unmet Need TARGET PROGRAM PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NEXT ANTICIPATED MILESTONES VTX2735 Ph 2 data H2 2025 Recurrent Pericarditis Peripheral NLRP3 VTX3232 Ph 2 data H1 2025 Parkinson’s Disease CNS-Penetrant VTX3232 Ph 2 data H2 2025 Cardiometabolic Diseases (CMD) / Obesity CNS-Penetrant Tamuzimod* Phase 3 Ready* S1P1 Ulcerative Colitis VTX958 Phase 2 analysis underway TYK2 Crohn’s Disease Cash, cash equivalents and marketable securities of $252.9M (unaudited)** as of December 31, 2024 are expected to fund operations into at least the second half of 2026 *Formerly VTX002. Available for partnering or non-dilutive financing. **Preliminary cash, cash equivalents and marketable securities balance as of December 31, 2024 (unaudited), subject to adjustments resulting from, among other things, the completion of our end-of-period reporting processes and related activities, including the audit by our independent registered public accounting firm of our financial statements. 5

NLRP3 Inhibition Broad potential in inflammatory diseases
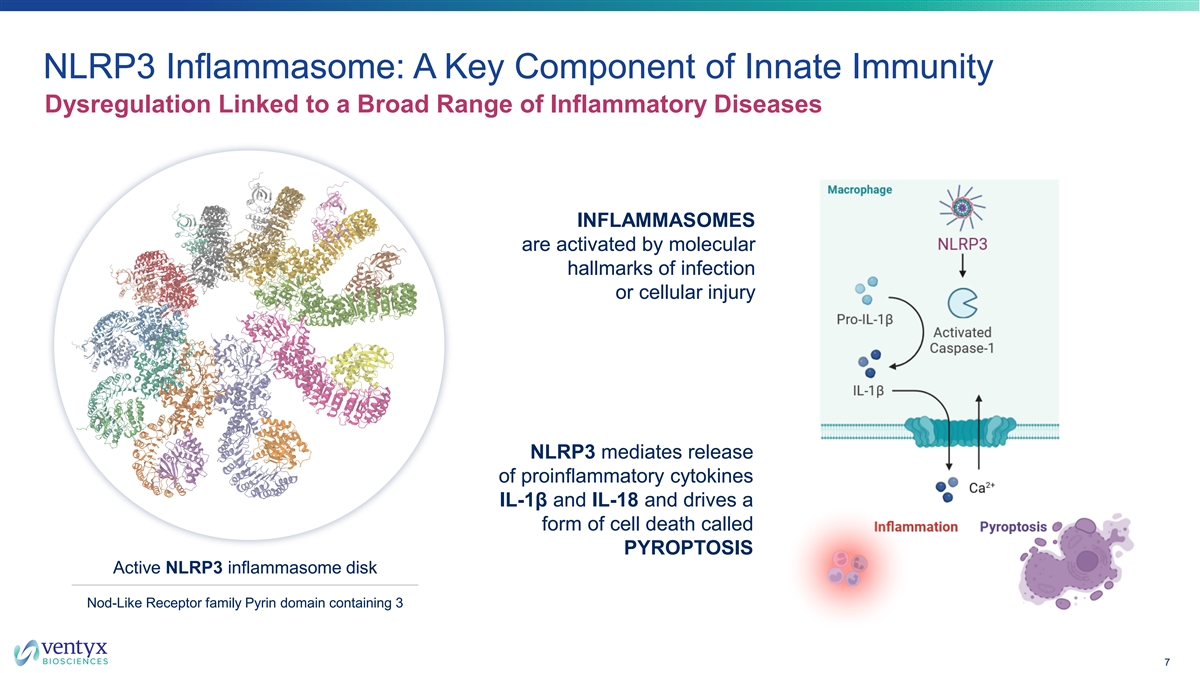
NLRP3 Inflammasome: A Key Component of Innate Immunity Dysregulation Linked to a Broad Range of Inflammatory Diseases INFLAMMASOMES are activated by molecular hallmarks of infection or cellular injury NLRP3 mediates release of proinflammatory cytokines IL-1β and IL-18 and drives a form of cell death called PYROPTOSIS Active NLRP3 inflammasome disk Nod-Like Receptor family Pyrin domain containing 3 7
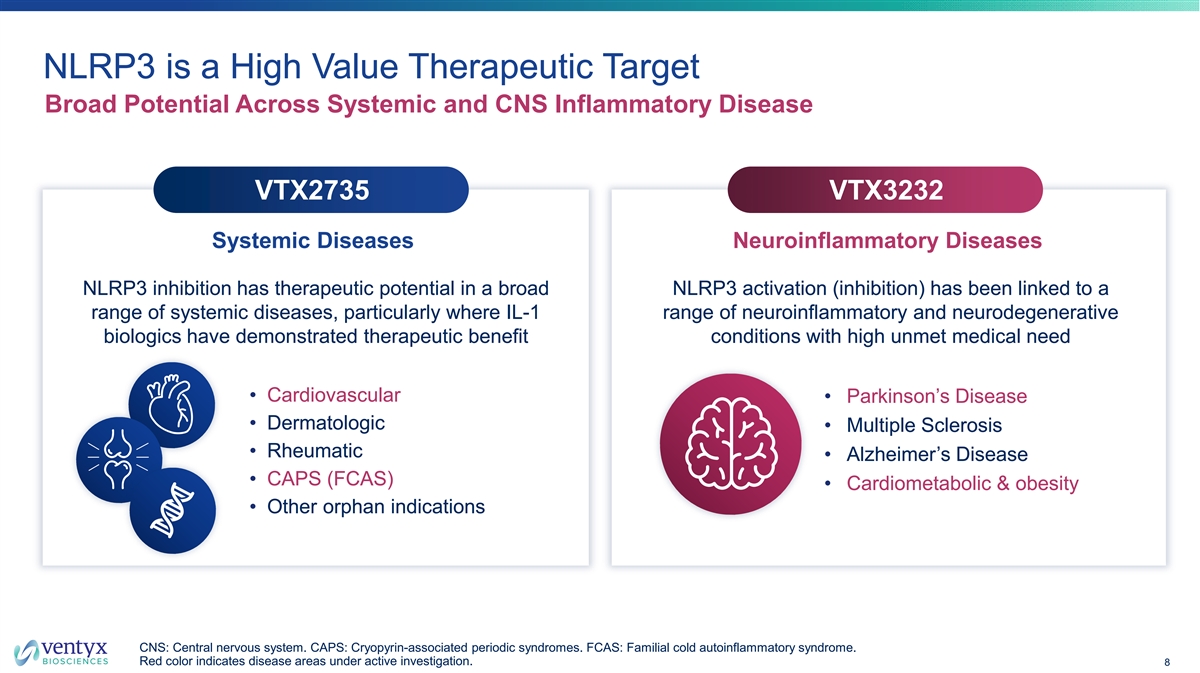
NLRP3 is a High Value Therapeutic Target Broad Potential Across Systemic and CNS Inflammatory Disease VTX2735 VTX3232 Systemic Diseases Neuroinflammatory Diseases NLRP3 inhibition has therapeutic potential in a broad NLRP3 activation (inhibition) has been linked to a range of systemic diseases, particularly where IL-1 range of neuroinflammatory and neurodegenerative biologics have demonstrated therapeutic benefit conditions with high unmet medical need • Cardiovascular • Parkinson’s Disease • Dermatologic • Multiple Sclerosis • Rheumatic • Alzheimer’s Disease • CAPS (FCAS) • Cardiometabolic & obesity • Other orphan indications CNS: Central nervous system. CAPS: Cryopyrin-associated periodic syndromes. FCAS: Familial cold autoinflammatory syndrome. Red color indicates disease areas under active investigation. 8
VTX2735 Peripheral NLRP3 Inhibitor
VTX2735: Potent and Selective Peripheral NLRP3 Inhibitor Phase 2 Ready for Systemic Inflammatory Diseases POTENT & SELECTIVE NONCLINICAL & PHASE 1 PACKAGE • hu WB IC (IL-1β) = 80 nM • Demonstrated pharmacodynamic effects and in 50 vivo efficacy in rodent models • No inhibition of other • High exposures and target coverage achieved inflammasomes in Phase 1 • Safety profile established in nonclinical and clinical studies § Well-tolerated in healthy adults § No safety signals in nonclinical in vitro and in vivo studies § Chronic tox studies completed PATENT GRANTED (US Pat. No. 11,603,375) • Potent inhibitor of NLRP3 in PBMC from CAPS patients (FCAS mutations) Phase 2 proof-of-concept study in CAPS patients completed Source: Ventyx internal data. CAPS: Cryopyrin-associated periodic syndromes. FCAS: Familial cold autoinflammatory syndrome. WB: whole blood. PMBC: peripheral blood mononuclear cells. 10
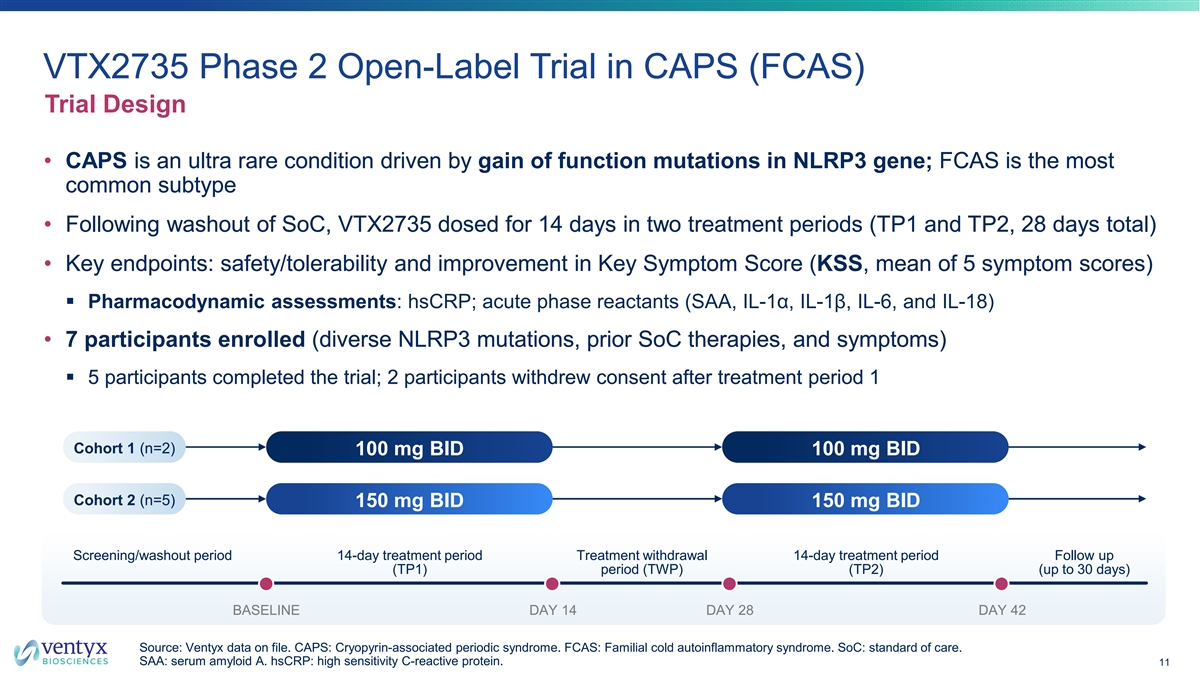
VTX2735 Phase 2 Open-Label Trial in CAPS (FCAS) Trial Design • CAPS is an ultra rare condition driven by gain of function mutations in NLRP3 gene; FCAS is the most common subtype • Following washout of SoC, VTX2735 dosed for 14 days in two treatment periods (TP1 and TP2, 28 days total) • Key endpoints: safety/tolerability and improvement in Key Symptom Score (KSS, mean of 5 symptom scores) § Pharmacodynamic assessments: hsCRP; acute phase reactants (SAA, IL-1α, IL-1β, IL-6, and IL-18) • 7 participants enrolled (diverse NLRP3 mutations, prior SoC therapies, and symptoms) § 5 participants completed the trial; 2 participants withdrew consent after treatment period 1 Cohort 1 (n=2) 100 mg BID 100 mg BID Cohort 2 (n=5) 150 mg BID 150 mg BID Screening/washout period 14-day treatment period Treatment withdrawal 14-day treatment period Follow up (TP1) period (TWP) (TP2) (up to 30 days) BASELINE DAY 14 DAY 28 DAY 42 Source: Ventyx data on file. CAPS: Cryopyrin-associated periodic syndrome. FCAS: Familial cold autoinflammatory syndrome. SoC: standard of care. SAA: serum amyloid A. hsCRP: high sensitivity C-reactive protein. 11

Treatment with VTX2735 Drives Reductions in Disease Activity Disease Activity as Assessed by Key Symptom Score (KSS) and General Well-Being KEY SYMPTOM SCORE (0-10)* GENERAL WELL-BEING (0-10)* Daily mean of five symptom scores “Considering all the ways FCAS affects you, please rate how you are doing” Treatment Treatment withdrawal withdrawal Mean 85% reduction during Mean 68% reduction during Treatment Period 1 Treatment Period 1 Source: Ventyx data on file (07 March 2024). *Note: Number next to each line represents individual participant number. One participant did not complete Treatment Period 1; the final measurement prior to study discontinuation is reported. 12
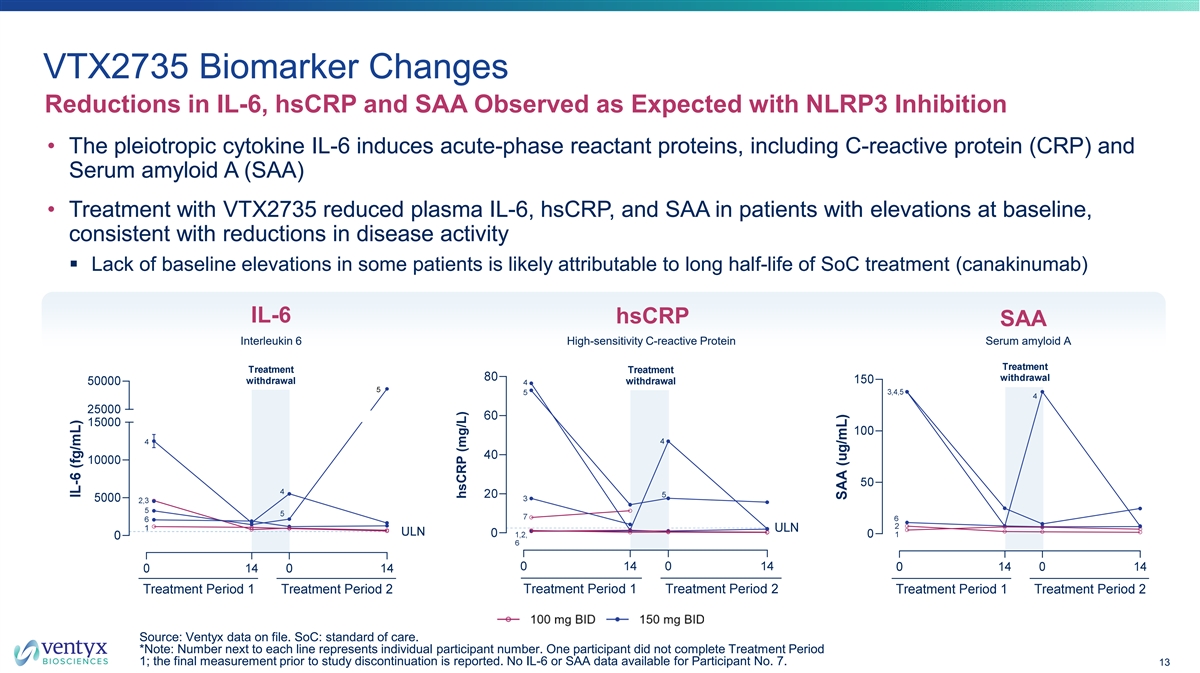
VTX2735 Biomarker Changes Reductions in IL-6, hsCRP and SAA Observed as Expected with NLRP3 Inhibition • The pleiotropic cytokine IL-6 induces acute-phase reactant proteins, including C-reactive protein (CRP) and Serum amyloid A (SAA) • Treatment with VTX2735 reduced plasma IL-6, hsCRP, and SAA in patients with elevations at baseline, consistent with reductions in disease activity § Lack of baseline elevations in some patients is likely attributable to long half-life of SoC treatment (canakinumab) IL-6 hsCRP SAA Interleukin 6 High-sensitivity C-reactive Protein Serum amyloid A Source: Ventyx data on file. SoC: standard of care. *Note: Number next to each line represents individual participant number. One participant did not complete Treatment Period 1; the final measurement prior to study discontinuation is reported. No IL-6 or SAA data available for Participant No. 7. 13 hsCRP (mg/L)
Conclusions from the Phase 2 Trial of VTX2735 in FCAS Patients Clinical Proof of Concept Achieved in CAPS Patients VTX2735 was well-tolerated; all adverse events VTX2735 showed categorized as mild or moderate and resolved without clinically-meaningful treatment interruption effects on disease activity and relevant Clinical outcomes and biomarker changes represent a biomarkers major milestone for VTX2735 and for NLRP3 inhibition • Dr. Hal Hoffman (UCSD): “Results similar to what we have seen with IL-1 targeted biologics; particularly impressive in a treatment- experienced population.” CAPS: Cryopyrin-associated periodic syndrome. FCAS: Familial cold autoinflammatory syndrome. 14
VTX2735 is a Phase 2 Ready Peripheral NLRP3 Inhibitor BIOLOGIC-LIKE ACTIVITY IN CAPS TRIAL HIGHLY POTENT & SELECTIVE • Structurally unique, selective inhibitor of NLRP3 • Concentration dependent suppression of IL-1β ex vivo • Potent inhibitor of NLRP3 with IC = 80 nM 50 in human whole blood assay • Reduction in hsCRP and other inflammation biomarkers (IL-6, SAA, neutrophils) • Highly potent vs. CAPS mutation variants • Clinically-meaningful benefits observed in CAPS patients PROMISING SAFETY PROFILE PHASE 2 READY • Well-tolerated in healthy adults and CAPS • IP position secure; patent issued patients (US Pat. No. 11,603,375) • No toxicological signals of concern • Multi-kilo API production completed • No CYP, hERG or transporter interactions • Once-daily dosing form in development Source: Ventyx data on file. CAPS: Cryopyrin-associated periodic syndrome. nM: nanomolar. hsCRP: high sensitivity C-reactive protein. SAA: serum amyloid A. CYP: Cytochrome P450. hERG: human ether-à-gogo gene. API: active pharmaceutical ingredient. 15
Attractive Opportunity for NLRP3 in Recurrent Pericarditis De-risked Mechanism and Efficient Path to Market 1 Recurrent pericarditis is a ~40,000 patient U.S. prevalent population with recurrent pericarditis debilitating autoinflammatory Autoinflammatory process characterized by IL-1 release condition (downstream of NLRP3) TREATMENT PARADIGM • 2021 approval of Arcalyst (rilonacept) validates IL-1 approach (de-risking for NLRP3) Refractory § Arcalyst generated $233M in 2023 sales in 2nd full year of commercial 2 availability; consensus sales >$1B in 2030 Arcalyst azathioprine Corticosteroids • Regulatory precedent for efficient path to market methotrexate § Open-label Phase 2 trial followed by a single Phase 3 trial VTX2735 NSAIDs ± • Topline Phase 2 data for VTX2735 expected in H2 2025 Colchicine Potential as steroid-sparing therapy Source: 1. Klein et al., J Am Heart Assoc. 2021 Aug 3;10(15):e018950. doi: 10.1161/JAHA.120.018950; 2. Historical sales from Kiniksa investor presentation; consensus sales from Evaluate Pharma. 16
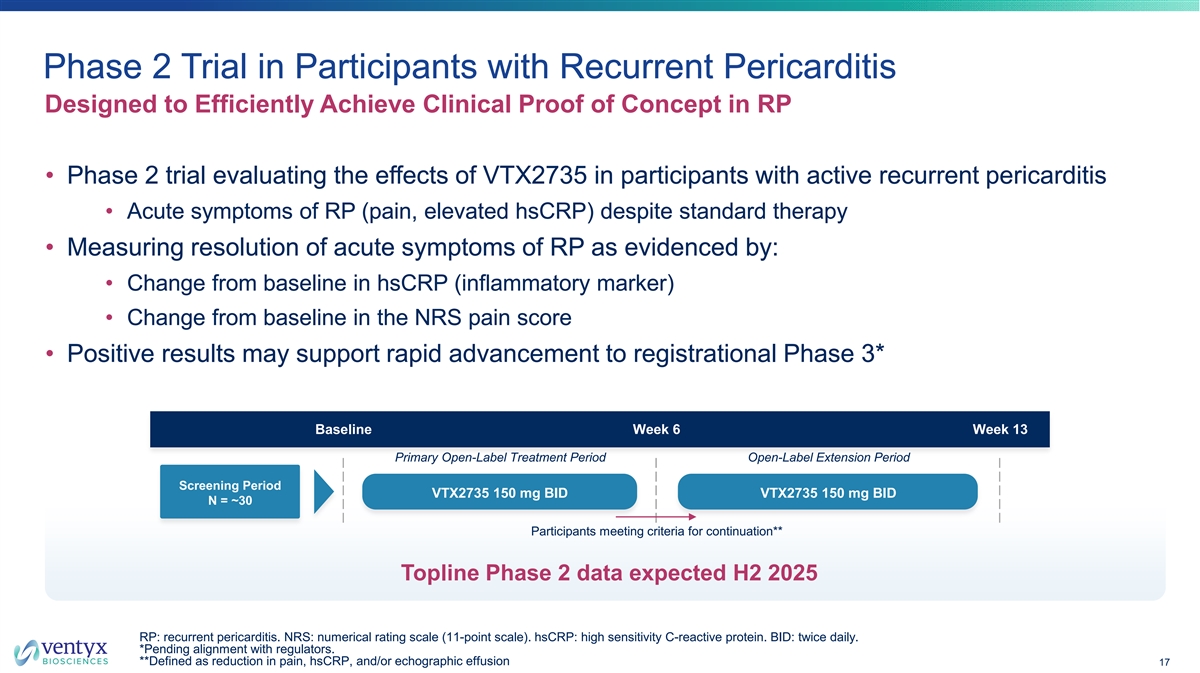
Phase 2 Trial in Participants with Recurrent Pericarditis Designed to Efficiently Achieve Clinical Proof of Concept in RP • Phase 2 trial evaluating the effects of VTX2735 in participants with active recurrent pericarditis • Acute symptoms of RP (pain, elevated hsCRP) despite standard therapy • Measuring resolution of acute symptoms of RP as evidenced by: • Change from baseline in hsCRP (inflammatory marker) • Change from baseline in the NRS pain score • Positive results may support rapid advancement to registrational Phase 3* Baseline Week 6 Week 13 Primary Open-Label Treatment Period Open-Label Extension Period Screening Period VTX2735 150 mg BID VTX2735 150 mg BID N = ~30 Participants meeting criteria for continuation** Topline Phase 2 data expected H2 2025 RP: recurrent pericarditis. NRS: numerical rating scale (11-point scale). hsCRP: high sensitivity C-reactive protein. BID: twice daily. *Pending alignment with regulators. **Defined as reduction in pain, hsCRP, and/or echographic effusion 17

VTX3232 CNS-Penetrant NLRP3 Inhibitor
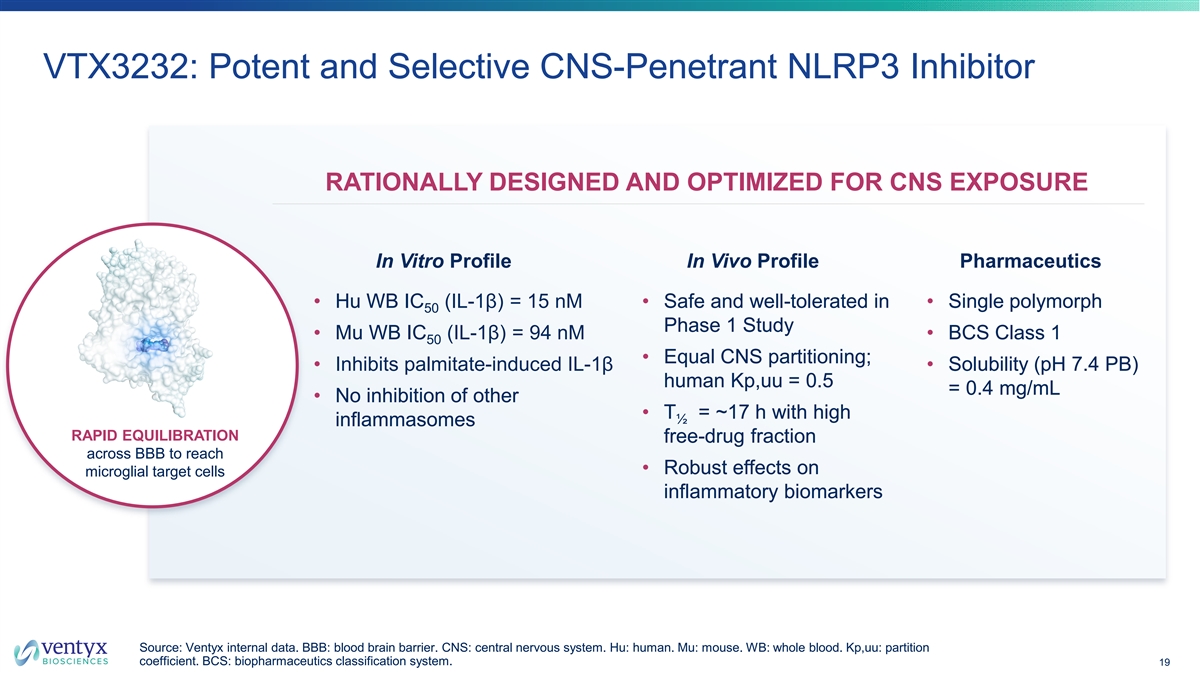
VTX3232: Potent and Selective CNS-Penetrant NLRP3 Inhibitor RATIONALLY DESIGNED AND OPTIMIZED FOR CNS EXPOSURE In Vitro Profile In Vivo Profile Pharmaceutics • Hu WB IC (IL-1β) = 15 nM • Safe and well-tolerated in • Single polymorph 50 Phase 1 Study • Mu WB IC (IL-1β) = 94 nM • BCS Class 1 50 • Equal CNS partitioning; • Inhibits palmitate-induced IL-1β • Solubility (pH 7.4 PB) human Kp,uu = 0.5 = 0.4 mg/mL • No inhibition of other • T = ~17 h with high ½ inflammasomes RAPID EQUILIBRATION free-drug fraction across BBB to reach • Robust effects on microglial target cells inflammatory biomarkers Source: Ventyx internal data. BBB: blood brain barrier. CNS: central nervous system. Hu: human. Mu: mouse. WB: whole blood. Kp,uu: partition coefficient. BCS: biopharmaceutics classification system. 19
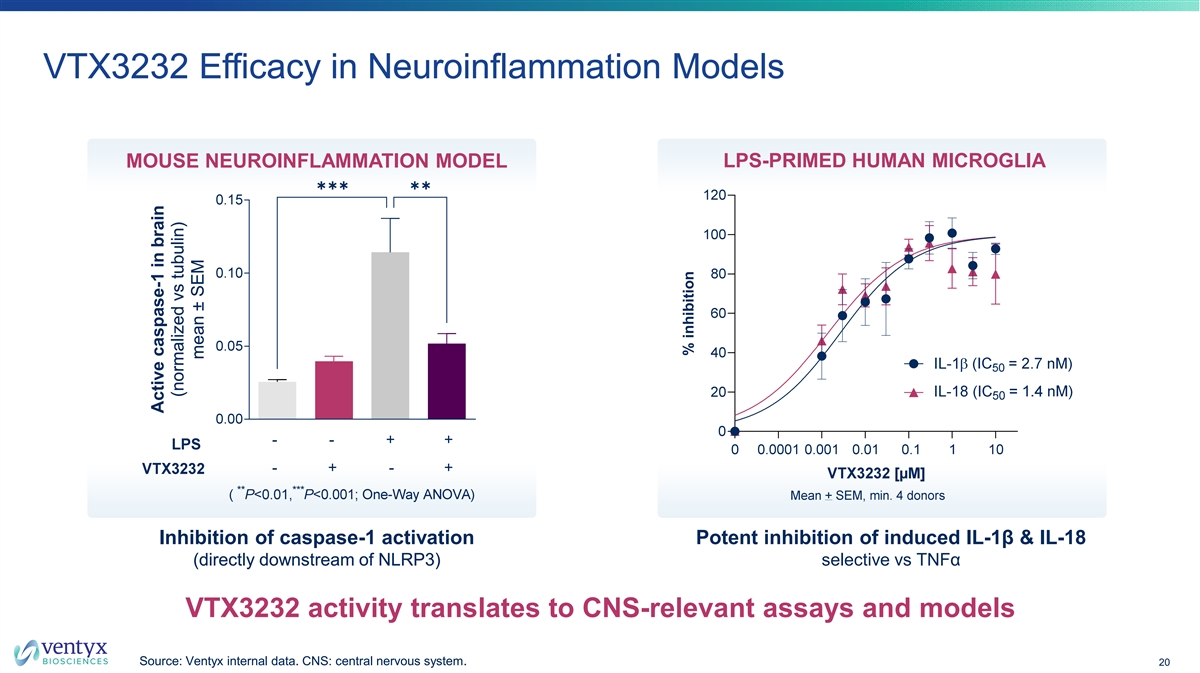
VTX3232 Efficacy in Neuroinflammation Models MOUSE NEUROINFLAMMATION MODEL LPS-PRIMED HUMAN MICROGLIA ✱✱✱✱✱ Inhibition of caspase-1 activation Potent inhibition of induced IL-1β & IL-18 (directly downstream of NLRP3) selective vs TNFα VTX3232 activity translates to CNS-relevant assays and models Source: Ventyx internal data. CNS: central nervous system. 20 Active caspase-1 in brain (normalized vs tubulin) mean ± SEM % inhibition
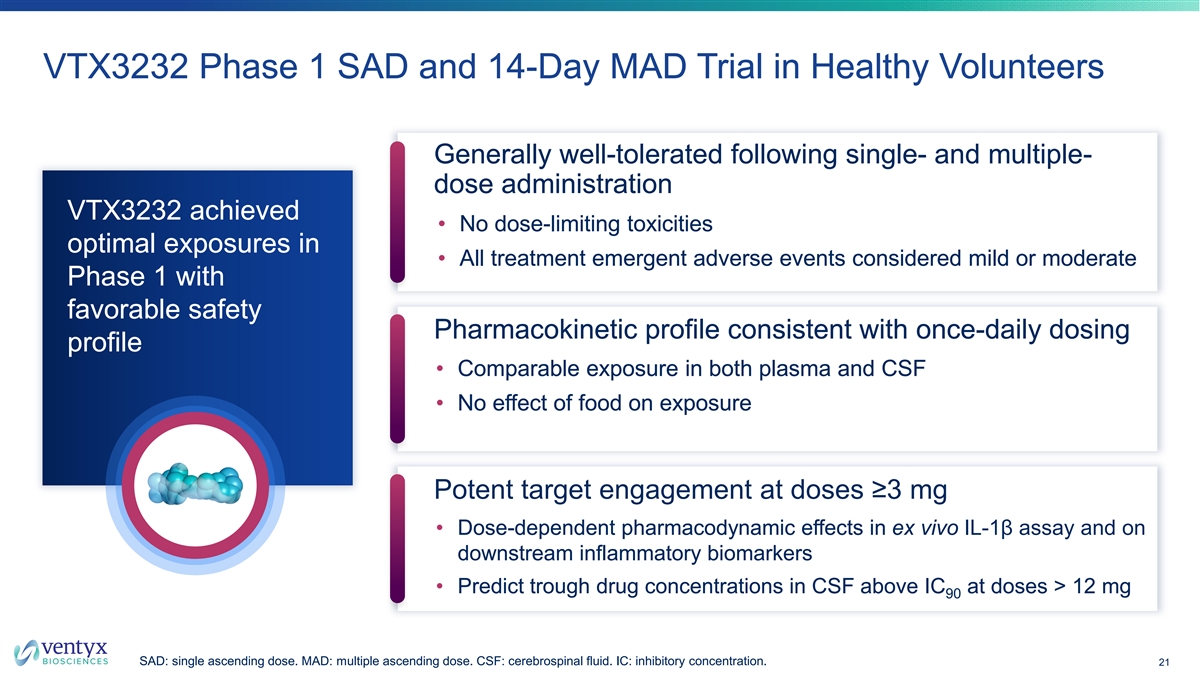
VTX3232 Phase 1 SAD and 14-Day MAD Trial in Healthy Volunteers Generally well-tolerated following single- and multiple- dose administration VTX3232 achieved • No dose-limiting toxicities optimal exposures in • All treatment emergent adverse events considered mild or moderate Phase 1 with favorable safety Pharmacokinetic profile consistent with once-daily dosing profile • Comparable exposure in both plasma and CSF • No effect of food on exposure Potent target engagement at doses ≥3 mg • Dose-dependent pharmacodynamic effects in ex vivo IL-1β assay and on downstream inflammatory biomarkers • Predict trough drug concentrations in CSF above IC at doses > 12 mg 90 SAD: single ascending dose. MAD: multiple ascending dose. CSF: cerebrospinal fluid. IC: inhibitory concentration. 21
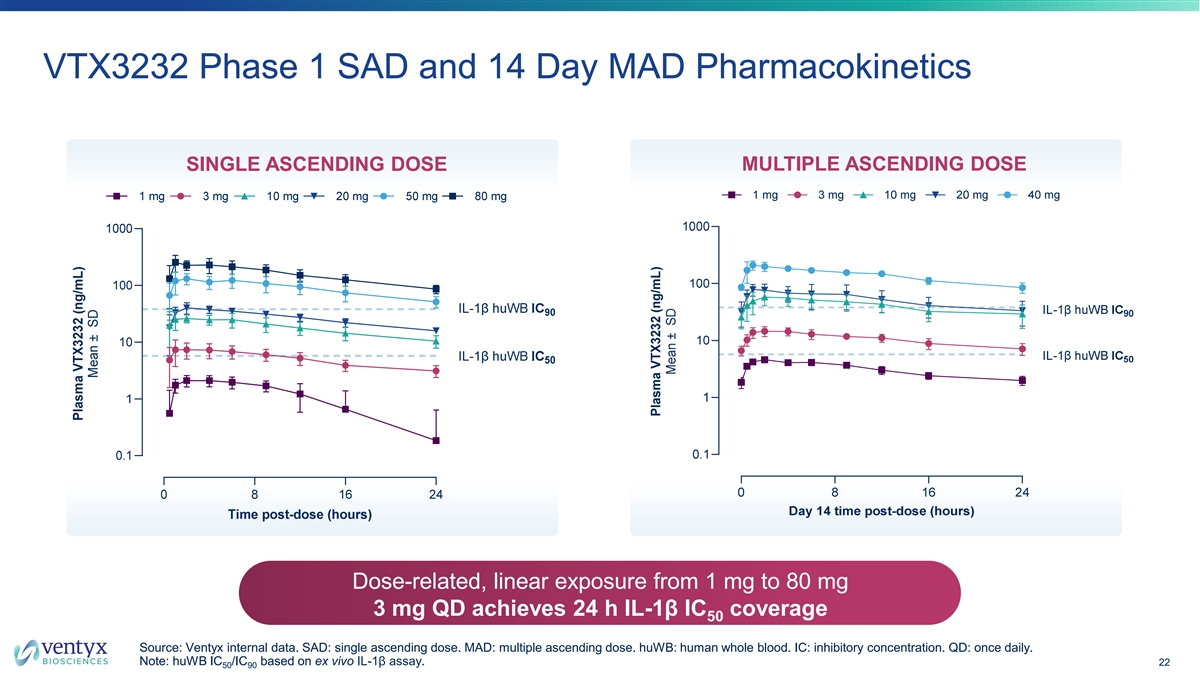
VTX3232 Phase 1 SAD and 14 Day MAD Pharmacokinetics SINGLE ASCENDING DOSE MULTIPLE ASCENDING DOSE Dose-related, linear exposure from 1 mg to 80 mg 3 mg QD achieves 24 h IL-1β IC coverage 50 Source: Ventyx internal data. SAD: single ascending dose. MAD: multiple ascending dose. huWB: human whole blood. IC: inhibitory concentration. QD: once daily. Note: huWB IC /IC based on ex vivo IL-1β assay. 22 50 90
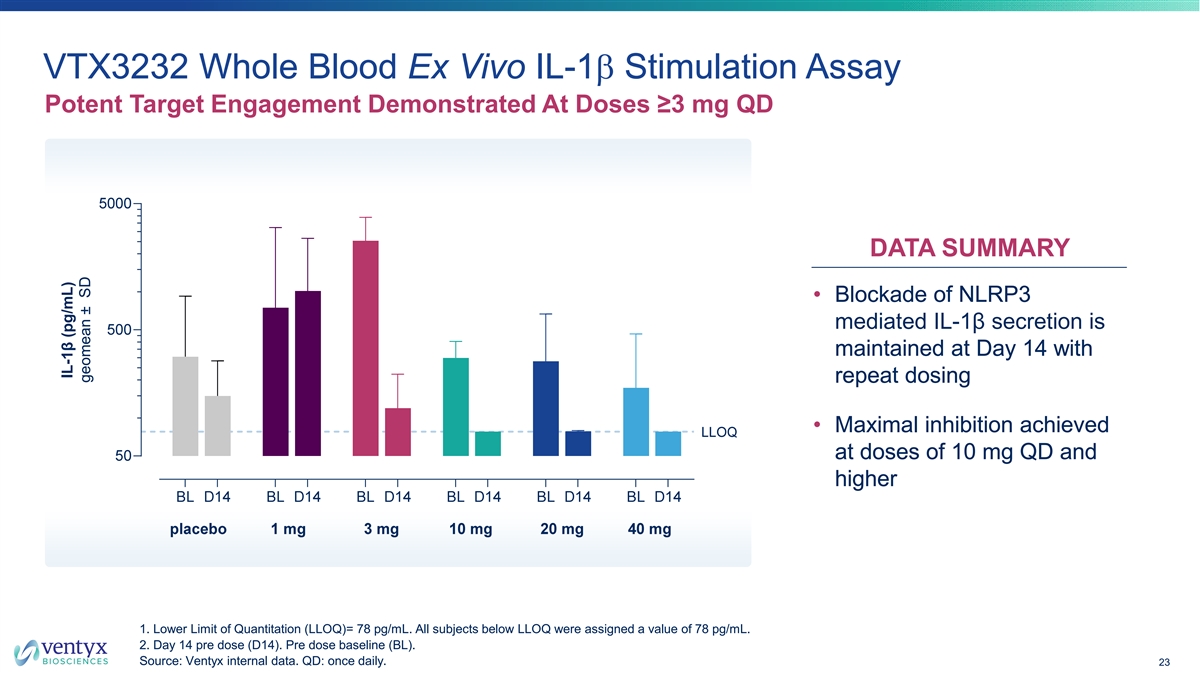
VTX3232 Whole Blood Ex Vivo IL-1b Stimulation Assay Potent Target Engagement Demonstrated At Doses ≥3 mg QD DATA SUMMARY • Blockade of NLRP3 mediated IL-1β secretion is maintained at Day 14 with repeat dosing • Maximal inhibition achieved at doses of 10 mg QD and higher 1. Lower Limit of Quantitation (LLOQ)= 78 pg/mL. All subjects below LLOQ were assigned a value of 78 pg/mL. 2. Day 14 pre dose (D14). Pre dose baseline (BL). Source: Ventyx internal data. QD: once daily. 23 IL-1β (pg/mL) geomean ± SD
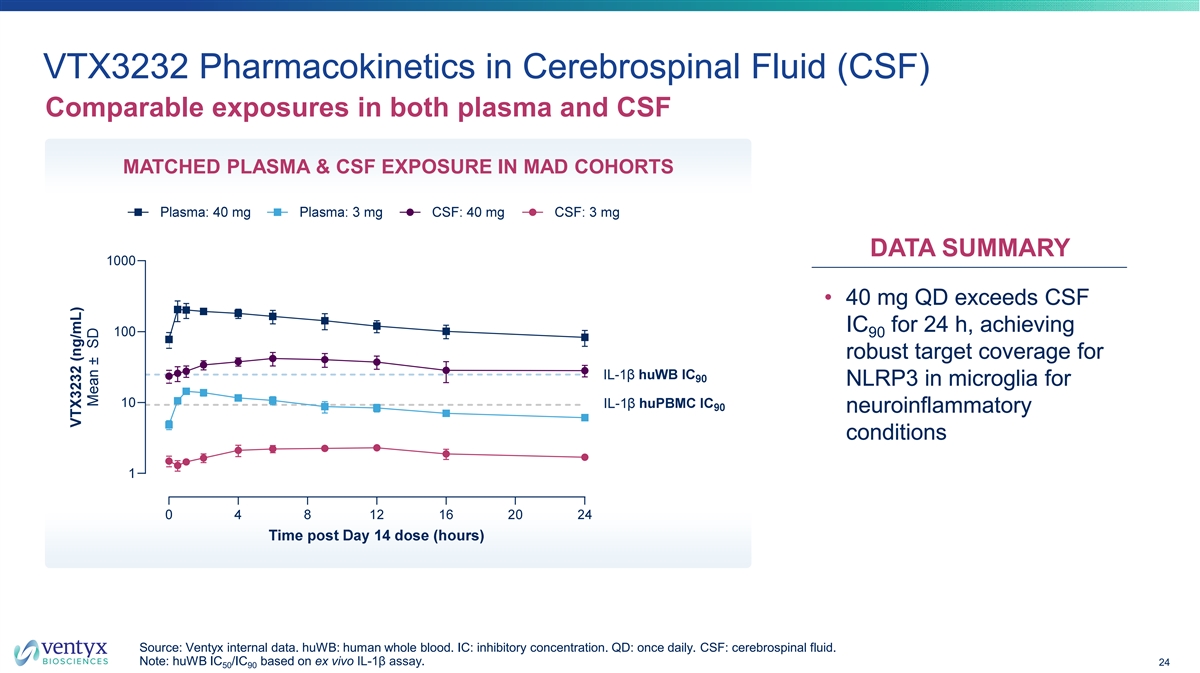
VTX3232 Pharmacokinetics in Cerebrospinal Fluid (CSF) Comparable exposures in both plasma and CSF MATCHED PLASMA & CSF EXPOSURE IN MAD COHORTS DATA SUMMARY • 40 mg QD exceeds CSF IC for 24 h, achieving 90 robust target coverage for NLRP3 in microglia for neuroinflammatory conditions Source: Ventyx internal data. huWB: human whole blood. IC: inhibitory concentration. QD: once daily. CSF: cerebrospinal fluid. Note: huWB IC /IC based on ex vivo IL-1β assay. 24 50 90
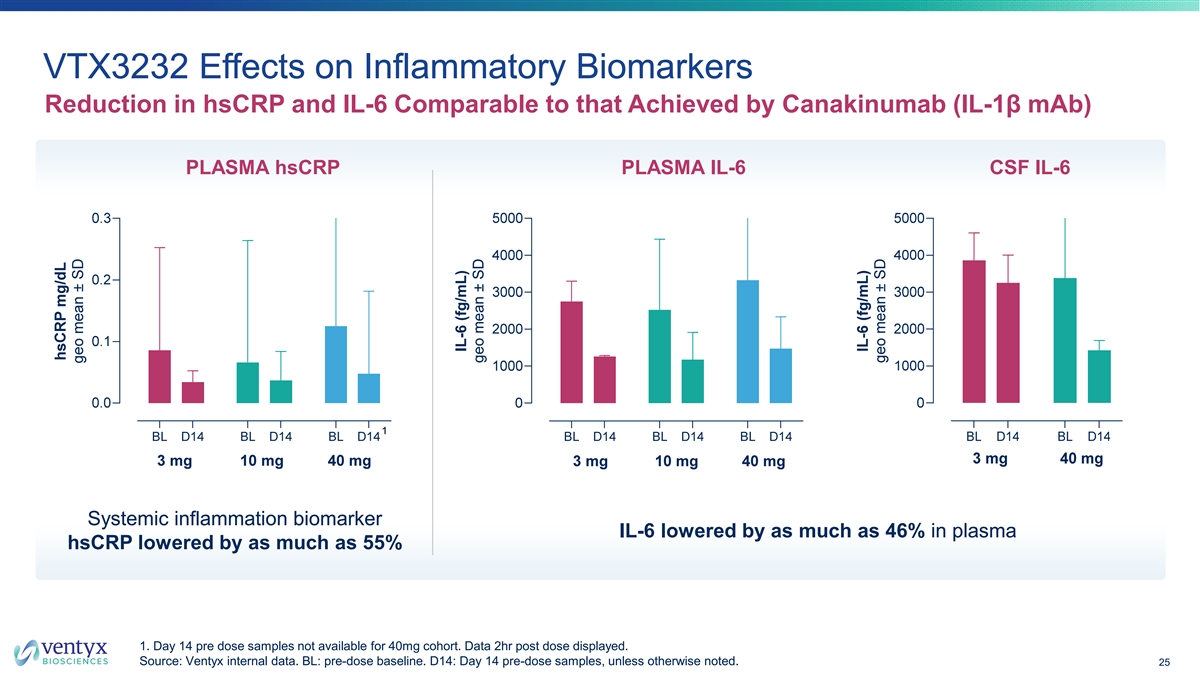
VTX3232 Effects on Inflammatory Biomarkers Reduction in hsCRP and IL-6 Comparable to that Achieved by Canakinumab (IL-1β mAb) PLASMA hsCRP PLASMA IL-6 CSF IL-6 Systemic inflammation biomarker IL-6 lowered by as much as 46% in plasma hsCRP lowered by as much as 55% 1. Day 14 pre dose samples not available for 40mg cohort. Data 2hr post dose displayed. Source: Ventyx internal data. BL: pre-dose baseline. D14: Day 14 pre-dose samples, unless otherwise noted. 25 IL-6 (fg/mL) geo mean ± SD
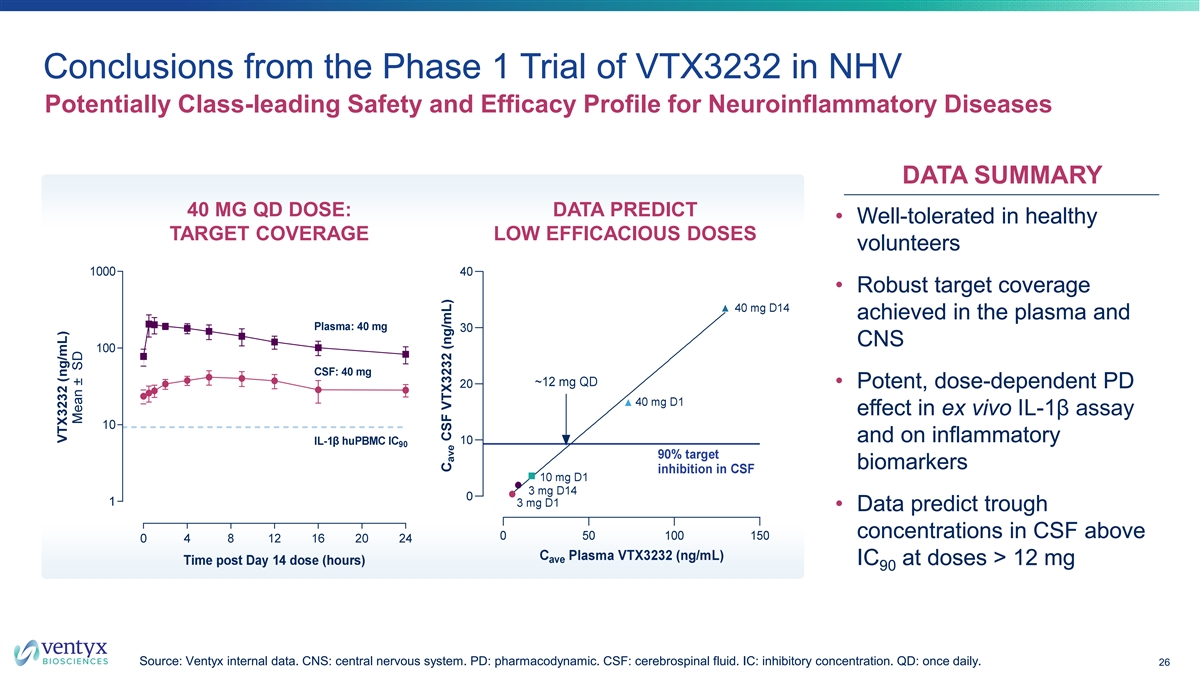
Conclusions from the Phase 1 Trial of VTX3232 in NHV Potentially Class-leading Safety and Efficacy Profile for Neuroinflammatory Diseases DATA SUMMARY 40 MG QD DOSE: DATA PREDICT • Well-tolerated in healthy TARGET COVERAGE LOW EFFICACIOUS DOSES volunteers • Robust target coverage achieved in the plasma and CNS • Potent, dose-dependent PD effect in ex vivo IL-1β assay and on inflammatory biomarkers • Data predict trough concentrations in CSF above IC at doses > 12 mg 90 Source: Ventyx internal data. CNS: central nervous system. PD: pharmacodynamic. CSF: cerebrospinal fluid. IC: inhibitory concentration. QD: once daily. 26
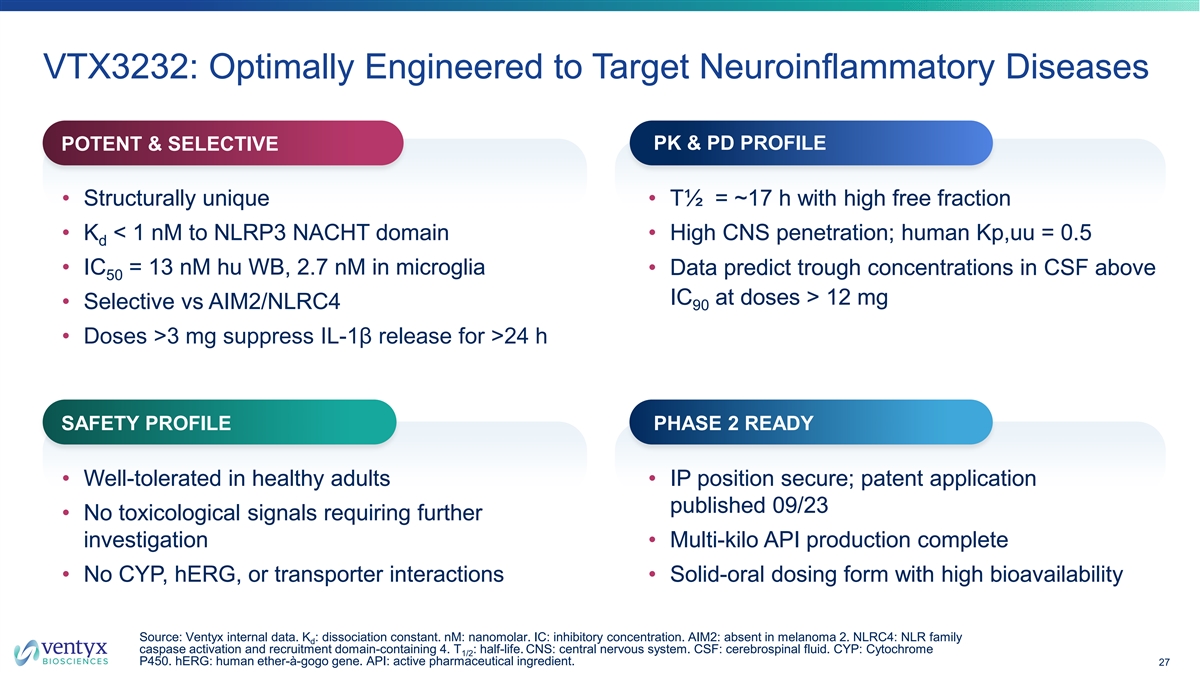
VTX3232: Optimally Engineered to Target Neuroinflammatory Diseases PK & PD PROFILE POTENT & SELECTIVE • Structurally unique • T½ = ~17 h with high free fraction • K < 1 nM to NLRP3 NACHT domain • High CNS penetration; human Kp,uu = 0.5 d • IC = 13 nM hu WB, 2.7 nM in microglia • Data predict trough concentrations in CSF above 50 IC at doses > 12 mg • Selective vs AIM2/NLRC4 90 • Doses >3 mg suppress IL-1β release for >24 h SAFETY PROFILE PHASE 2 READY • Well-tolerated in healthy adults • IP position secure; patent application published 09/23 • No toxicological signals requiring further investigation • Multi-kilo API production complete • No CYP, hERG, or transporter interactions • Solid-oral dosing form with high bioavailability Source: Ventyx internal data. K : dissociation constant. nM: nanomolar. IC: inhibitory concentration. AIM2: absent in melanoma 2. NLRC4: NLR family d caspase activation and recruitment domain-containing 4. T : half-life. CNS: central nervous system. CSF: cerebrospinal fluid. CYP: Cytochrome 1/2 P450. hERG: human ether-à-gogo gene. API: active pharmaceutical ingredient. 27
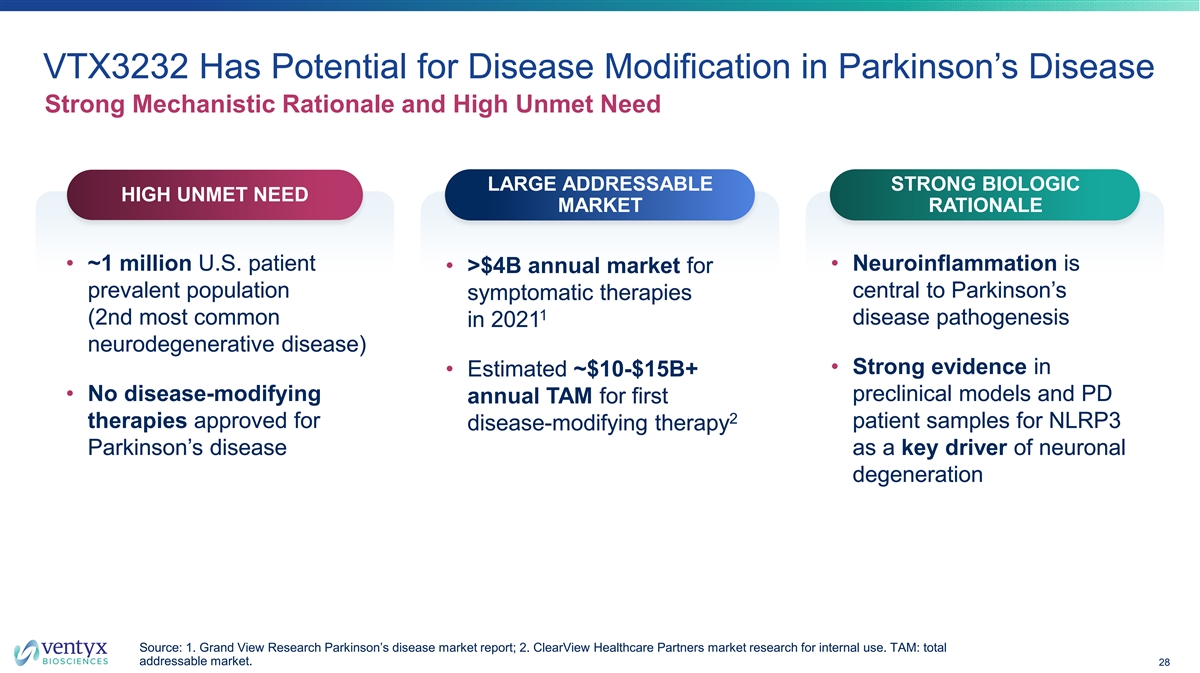
VTX3232 Has Potential for Disease Modification in Parkinson’s Disease Strong Mechanistic Rationale and High Unmet Need LARGE ADDRESSABLE STRONG BIOLOGIC HIGH UNMET NEED MARKET RATIONALE • ~1 million U.S. patient • Neuroinflammation is • >$4B annual market for prevalent population central to Parkinson’s symptomatic therapies 1 (2nd most common disease pathogenesis in 2021 neurodegenerative disease) • Strong evidence in • Estimated ~$10-$15B+ • No disease-modifying preclinical models and PD annual TAM for first 2 therapies approved for patient samples for NLRP3 disease-modifying therapy Parkinson’s disease as a key driver of neuronal degeneration Source: 1. Grand View Research Parkinson’s disease market report; 2. ClearView Healthcare Partners market research for internal use. TAM: total addressable market. 28
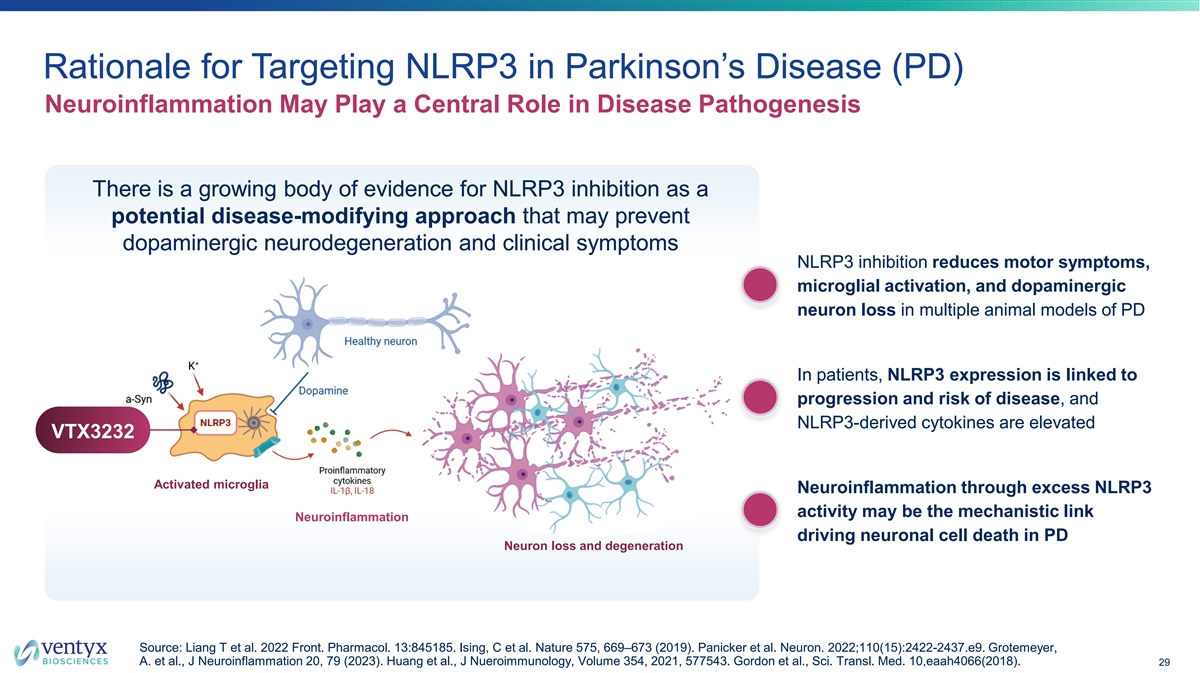
Rationale for Targeting NLRP3 in Parkinson’s Disease (PD) Neuroinflammation May Play a Central Role in Disease Pathogenesis There is a growing body of evidence for NLRP3 inhibition as a potential disease-modifying approach that may prevent dopaminergic neurodegeneration and clinical symptoms NLRP3 inhibition reduces motor symptoms, microglial activation, and dopaminergic neuron loss in multiple animal models of PD In patients, NLRP3 expression is linked to progression and risk of disease, and NLRP3-derived cytokines are elevated VTX3232 Activated microglia Neuroinflammation through excess NLRP3 activity may be the mechanistic link Neuroinflammation driving neuronal cell death in PD Neuron loss and degeneration Source: Liang T et al. 2022 Front. Pharmacol. 13:845185. Ising, C et al. Nature 575, 669–673 (2019). Panicker et al. Neuron. 2022;110(15):2422-2437.e9. Grotemeyer, A. et al., J Neuroinflammation 20, 79 (2023). Huang et al., J Nueroimmunology, Volume 354, 2021, 577543. Gordon et al., Sci. Transl. Med. 10,eaah4066(2018). 29
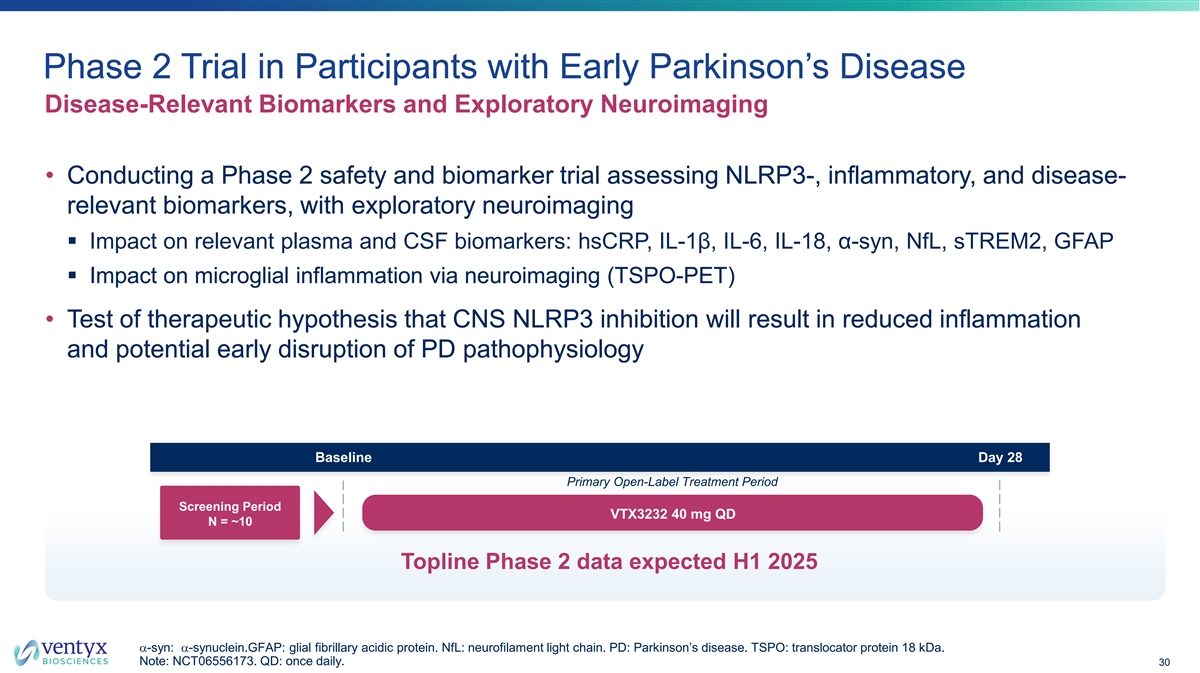
Phase 2 Trial in Participants with Early Parkinson’s Disease Disease-Relevant Biomarkers and Exploratory Neuroimaging • Conducting a Phase 2 safety and biomarker trial assessing NLRP3-, inflammatory, and disease- relevant biomarkers, with exploratory neuroimaging § Impact on relevant plasma and CSF biomarkers: hsCRP, IL-1β, IL-6, IL-18, α-syn, NfL, sTREM2, GFAP § Impact on microglial inflammation via neuroimaging (TSPO-PET) • Test of therapeutic hypothesis that CNS NLRP3 inhibition will result in reduced inflammation and potential early disruption of PD pathophysiology Baseline Day 28 Primary Open-Label Treatment Period Screening Period VTX3232 40 mg QD N = ~10 Topline Phase 2 data expected H1 2025 a-syn: a-synuclein.GFAP: glial fibrillary acidic protein. NfL: neurofilament light chain. PD: Parkinson’s disease. TSPO: translocator protein 18 kDa. Note: NCT06556173. QD: once daily. 30
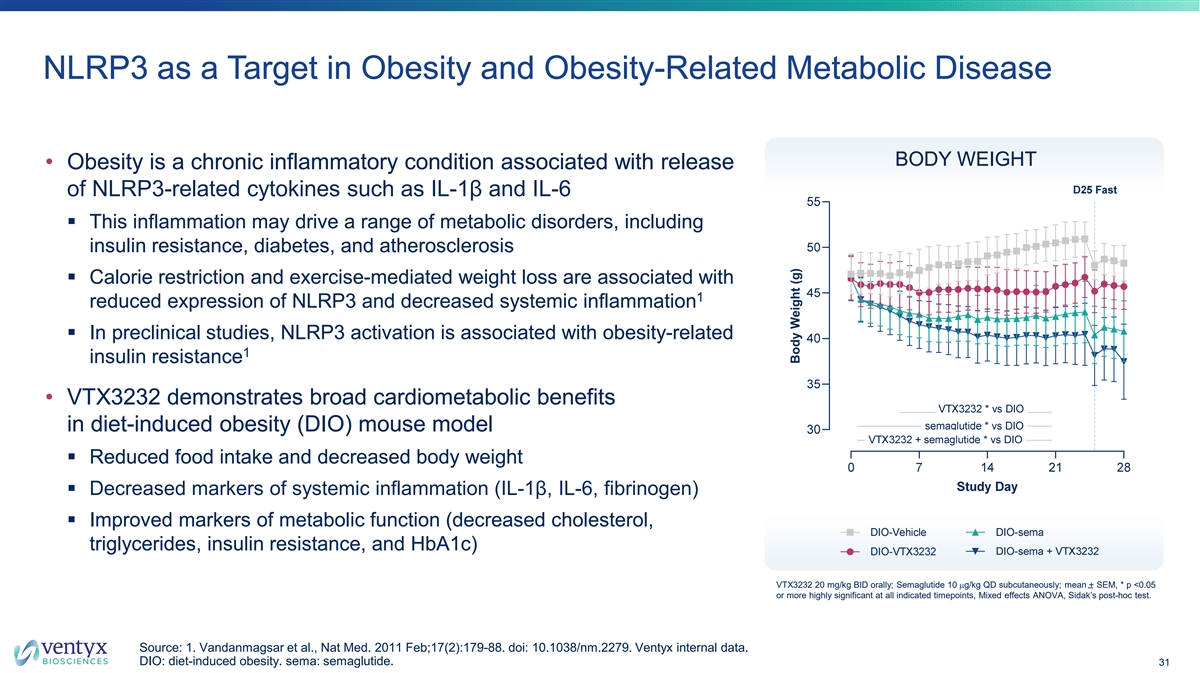
NLRP3 as a Target in Obesity and Obesity-Related Metabolic Disease BODY WEIGHT • Obesity is a chronic inflammatory condition associated with release of NLRP3-related cytokines such as IL-1β and IL-6 § This inflammation may drive a range of metabolic disorders, including insulin resistance, diabetes, and atherosclerosis § Calorie restriction and exercise-mediated weight loss are associated with 1 reduced expression of NLRP3 and decreased systemic inflammation § In preclinical studies, NLRP3 activation is associated with obesity-related 1 insulin resistance • VTX3232 demonstrates broad cardiometabolic benefits in diet-induced obesity (DIO) mouse model § Reduced food intake and decreased body weight § Decreased markers of systemic inflammation (IL-1β, IL-6, fibrinogen) § Improved markers of metabolic function (decreased cholesterol, triglycerides, insulin resistance, and HbA1c) VTX3232 20 mg/kg BID orally; Semaglutide 10 mg/kg QD subcutaneously; mean + SEM, * p <0.05 or more highly significant at all indicated timepoints, Mixed effects ANOVA, Sidak’s post-hoc test. Source: 1. Vandanmagsar et al., Nat Med. 2011 Feb;17(2):179-88. doi: 10.1038/nm.2279. Ventyx internal data. DIO: diet-induced obesity. sema: semaglutide. 31 Body Weight (g)
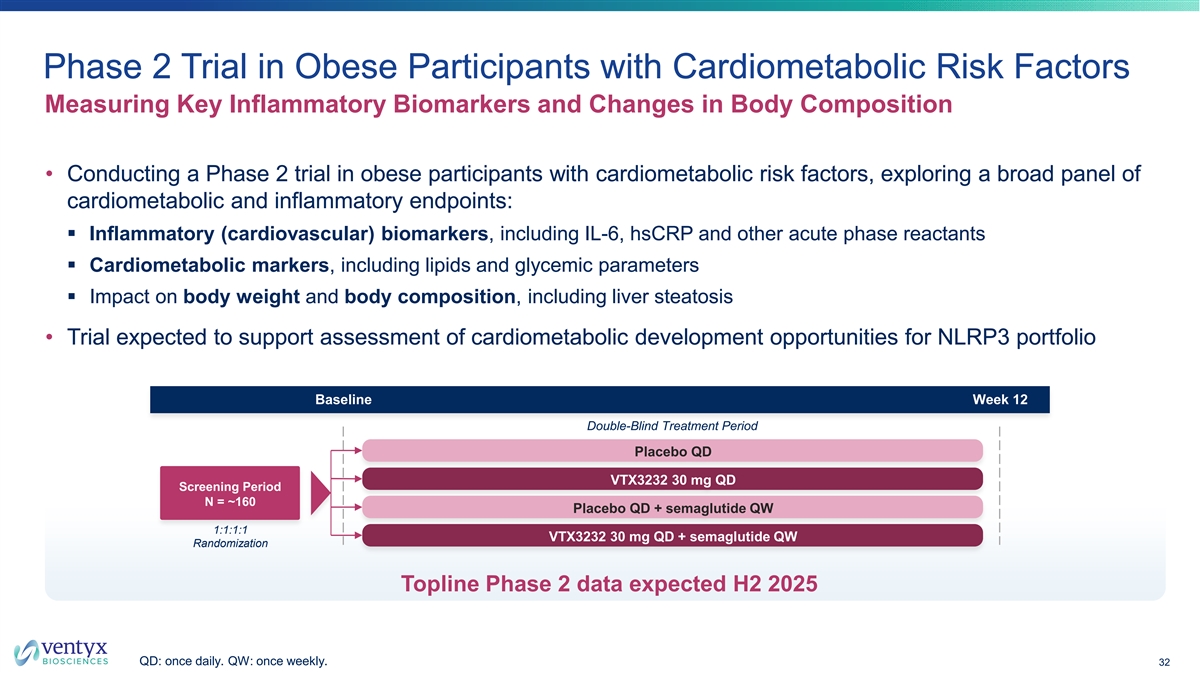
Phase 2 Trial in Obese Participants with Cardiometabolic Risk Factors Measuring Key Inflammatory Biomarkers and Changes in Body Composition • Conducting a Phase 2 trial in obese participants with cardiometabolic risk factors, exploring a broad panel of cardiometabolic and inflammatory endpoints: § Inflammatory (cardiovascular) biomarkers, including IL-6, hsCRP and other acute phase reactants § Cardiometabolic markers, including lipids and glycemic parameters § Impact on body weight and body composition, including liver steatosis • Trial expected to support assessment of cardiometabolic development opportunities for NLRP3 portfolio Baseline Week 12 Double-Blind Treatment Period Placebo QD VTX3232 30 mg QD Screening Period N = ~160 Placebo QD + semaglutide QW 1:1:1:1 VTX3232 30 mg QD + semaglutide QW Randomization Topline Phase 2 data expected H2 2025 QD: once daily. QW: once weekly. 32
Tamuzimod (VTX002) S1P1 Receptor Modulator for Ulcerative Colitis
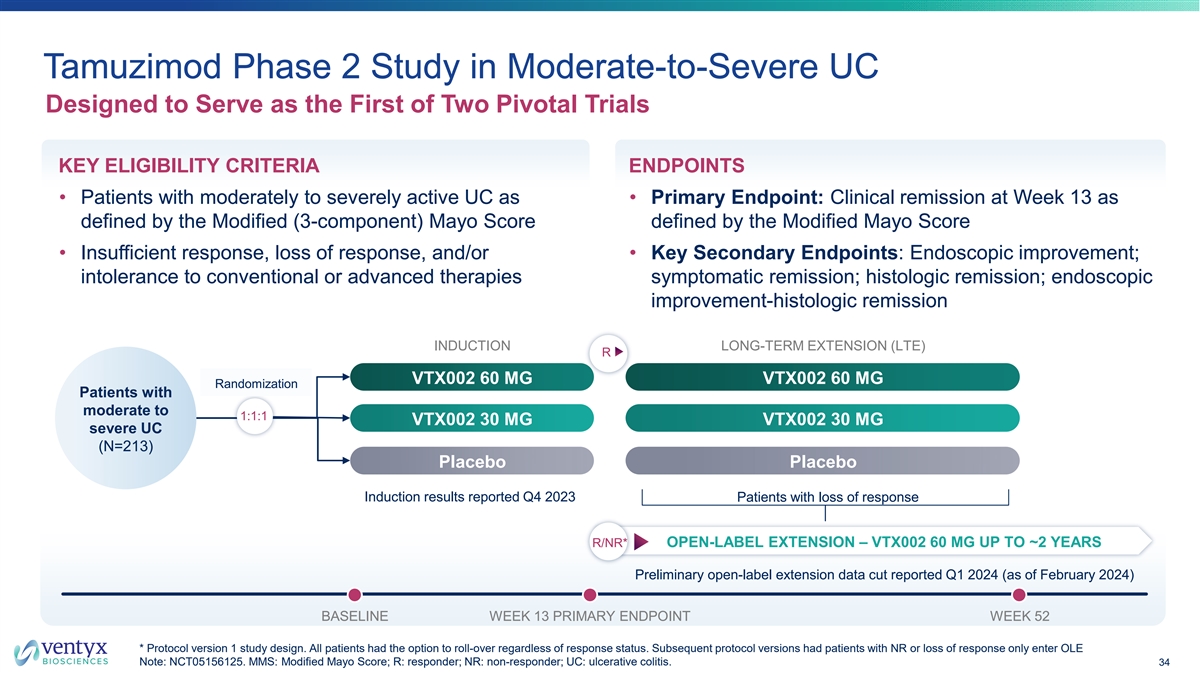
Tamuzimod Phase 2 Study in Moderate-to-Severe UC Designed to Serve as the First of Two Pivotal Trials KEY ELIGIBILITY CRITERIA ENDPOINTS • Patients with moderately to severely active UC as • Primary Endpoint: Clinical remission at Week 13 as defined by the Modified (3-component) Mayo Score defined by the Modified Mayo Score • Insufficient response, loss of response, and/or • Key Secondary Endpoints: Endoscopic improvement; intolerance to conventional or advanced therapies symptomatic remission; histologic remission; endoscopic improvement-histologic remission INDUCTION LONG-TERM EXTENSION (LTE) R VTX002 60 MG VTX002 60 MG Randomization Patients with moderate to 1:1:1 VTX002 30 MG VTX002 30 MG severe UC (N=213) Placebo Placebo Induction results reported Q4 2023 Patients with loss of response R/NR* OPEN-LABEL EXTENSION – VTX002 60 MG UP TO ~2 YEARS Preliminary open-label extension data cut reported Q1 2024 (as of February 2024) BASELINE WEEK 13 PRIMARY ENDPOINT WEEK 52 * Protocol version 1 study design. All patients had the option to roll-over regardless of response status. Subsequent protocol versions had patients with NR or loss of response only enter OLE Note: NCT05156125. MMS: Modified Mayo Score; R: responder; NR: non-responder; UC: ulcerative colitis. 34
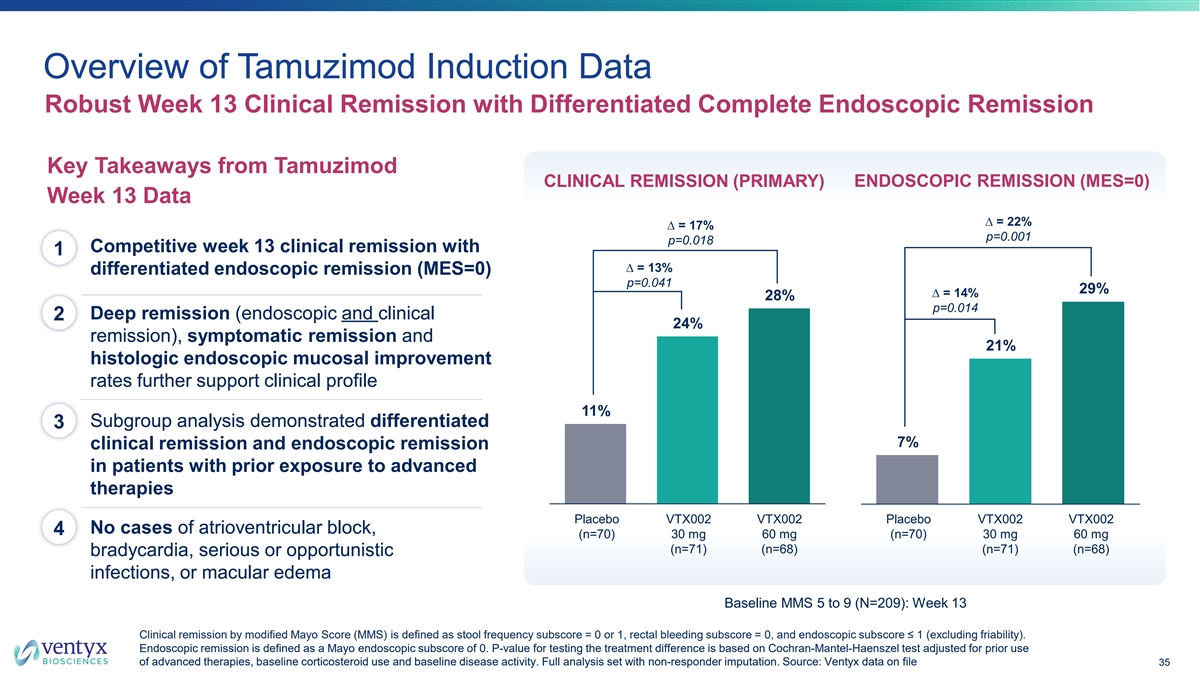
Overview of Tamuzimod Induction Data Robust Week 13 Clinical Remission with Differentiated Complete Endoscopic Remission Key Takeaways from Tamuzimod CLINICAL REMISSION (PRIMARY) ENDOSCOPIC REMISSION (MES=0) Week 13 Data ∆ = 22% ∆ = 17% p=0.001 p=0.018 Competitive week 13 clinical remission with 1 ∆ = 13% differentiated endoscopic remission (MES=0) p=0.041 29% ∆ = 14% 28% p=0.014 Deep remission (endoscopic and clinical 2 24% remission), symptomatic remission and 21% histologic endoscopic mucosal improvement rates further support clinical profile 11% Subgroup analysis demonstrated differentiated 3 7% clinical remission and endoscopic remission in patients with prior exposure to advanced therapies P Pllac ace eb bo o V VT TX X0 00 02 2 V VT TX X0 00 02 2 Placebo VTX002 VTX002 Placebo VTX002 VTX002 No cases of atrioventricular block, 4 (n=70) 3 30 0 m mg g 6 60 0 m mg g (n=70) 3 30 0 m mg g 66 0 0m mg g (n=71) (n=68) (n=71) (n=68) bradycardia, serious or opportunistic infections, or macular edema Baseline MMS 5 to 9 (N=209): Week 13 Clinical remission by modified Mayo Score (MMS) is defined as stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1 (excluding friability). Endoscopic remission is defined as a Mayo endoscopic subscore of 0. P-value for testing the treatment difference is based on Cochran-Mantel-Haenszel test adjusted for prior use of advanced therapies, baseline corticosteroid use and baseline disease activity. Full analysis set with non-responder imputation. So urce: Ventyx data on file 35
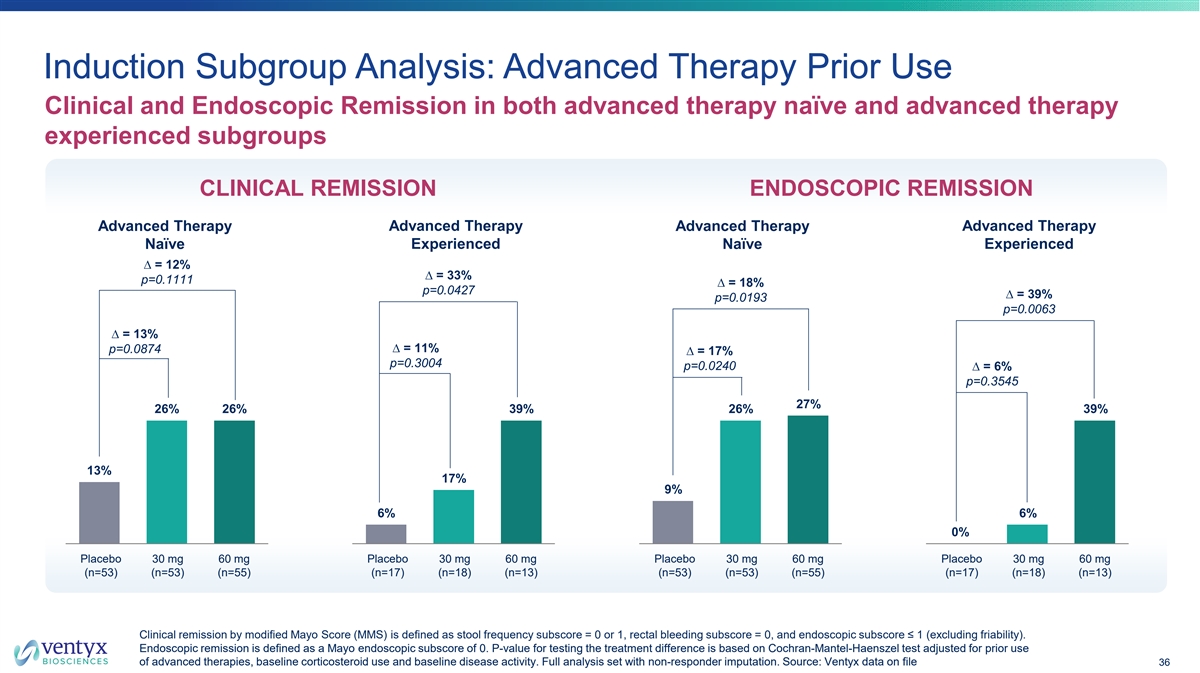
Induction Subgroup Analysis: Advanced Therapy Prior Use Clinical and Endoscopic Remission in both advanced therapy naïve and advanced therapy experienced subgroups CLINICAL REMISSION ENDOSCOPIC REMISSION Advanced Therapy Advanced Therapy Advanced Therapy Advanced Therapy Experienced Experienced Naïve Naïve ∆ = 12% ∆ = 33% p=0.1111 ∆ = 18% p=0.0427 ∆ = 39% p=0.0193 p=0.0063 ∆ = 13% ∆ = 11% p=0.0874 ∆ = 17% p=0.3004 p=0.0240 ∆ = 6% p=0.3545 27% 26% 26% 39% 26% 39% 13% 17% 9% 6% 6% 0% Placebo 30 mg 60 mg Placebo 30 mg 60 mg Placebo 30 mg 60 mg Placebo 30 mg 60 mg (n=53) (n=53) (n=55) (n=17) (n=18) (n=13) (n=53) (n=53) (n=55) (n=17) (n=18) (n=13) Clinical remission by modified Mayo Score (MMS) is defined as stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1 (excluding friability). Endoscopic remission is defined as a Mayo endoscopic subscore of 0. P-value for testing the treatment difference is based on Cochran-Mantel-Haenszel test adjusted for prior use of advanced therapies, baseline corticosteroid use and baseline disease activity. Full analysis set with non-responder imputation. So urce: Ventyx data on file 36

Preliminary Open-Label Extension Data Further improvement in clinical and endoscopic remission rates at OLE week 39 % absolute endpoint rate (clinical or endoscopic remission) in induction dose arm at 13 weeks 1 2 1 % CLINICAL REMISSION AT OLE WEEK 39 % ENDOSCOPIC REMISSION AT OLE WEEK 39 BY INDUCTION DOSE ARM BY INDUCTION DOSE ARM NRI NRI 62% 62% (n/N = 8/13) (n/N = 8/13) Observed Observed 50% 50% 28% 42% (n/N = 5/18) (n/N = 8/16) 36% (n/N = 5/12) (n/N = 8/16) (n/N = 4/11) 27% 28% 42% 29% 28% (n/N = 3/11) 25% 24% (60mg) (60mg) (n/N = 5/18) 21% 19% (30mg) (n/N = 4/16) (n/N = 5/12) (30mg) (n/N = 3/16) 11% 7% (PBO) (PBO) 60mg 52 week 30mg 13 weeks 60mg 52 week Placebo Placebo 30mg 13 weeks treat-through + 60mg 39 weeks treat-through 13 weeks + 13 weeks + + 60mg 39 weeks 60mg 39 weeks 60mg 39 weeks At least half (NRI) of patients in 60mg treat-through group reach clinical remission or endoscopic remission at week 52 Note: NRI = non-responder imputation; participant discontinuations are assumed to be non-remitters 1 Irrespective of the clinical response at the end of the 13-week induction phase; VTX002 60mg / 60mg represents 52 weeks treat-through efficacy; other groups received 60mg for 39 weeks post- induction; 2 MES =0; Source: Ventyx data on file. 37
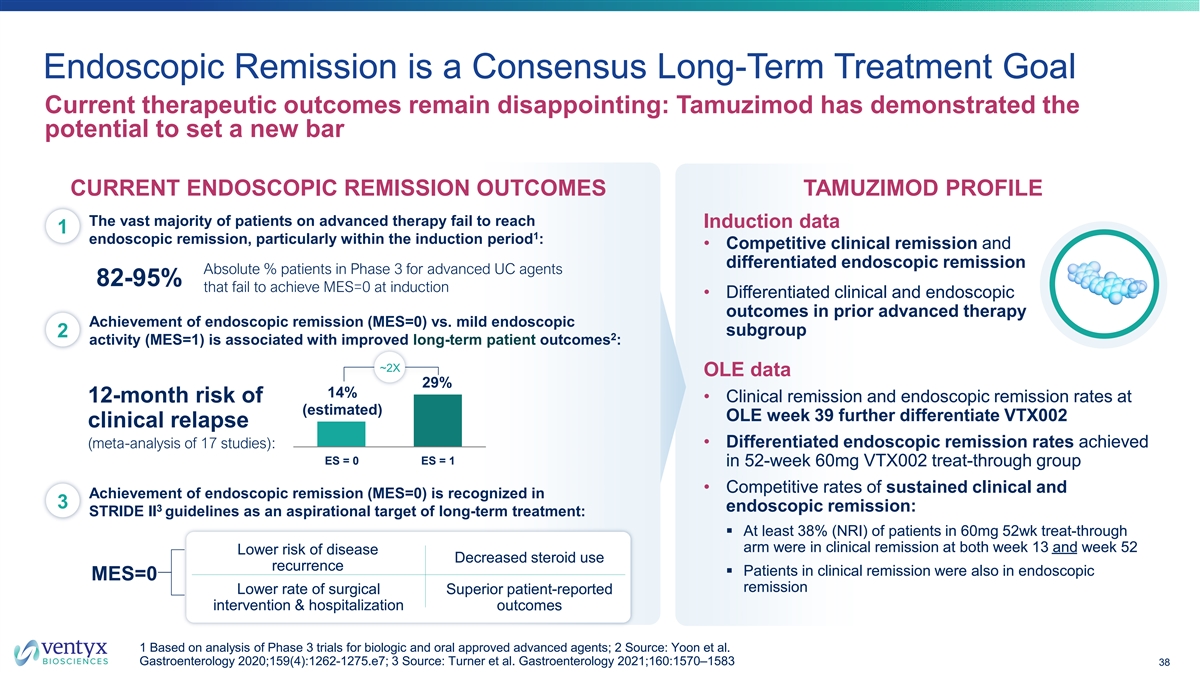
Endoscopic Remission is a Consensus Long-Term Treatment Goal Current therapeutic outcomes remain disappointing: Tamuzimod has demonstrated the potential to set a new bar CURRENT ENDOSCOPIC REMISSION OUTCOMES TAMUZIMOD PROFILE The vast majority of patients on advanced therapy fail to reach Induction data 1 1 endoscopic remission, particularly within the induction period : • Competitive clinical remission and differentiated endoscopic remission Absolute % patients in Phase 3 for advanced UC agents 82-95% that fail to achieve MES=0 at induction • Differentiated clinical and endoscopic outcomes in prior advanced therapy Achievement of endoscopic remission (MES=0) vs. mild endoscopic subgroup 2 2 activity (MES=1) is associated with improved long-term patient outcomes : ~2X OLE data 29% 14% • Clinical remission and endoscopic remission rates at 12-month risk of (estimated) OLE week 39 further differentiate VTX002 clinical relapse (meta-analysis of 17 studies): • Differentiated endoscopic remission rates achieved ES = 0 ES = 1 in 52-week 60mg VTX002 treat-through group • Competitive rates of sustained clinical and Achievement of endoscopic remission (MES=0) is recognized in 3 3 endoscopic remission: STRIDE II guidelines as an aspirational target of long-term treatment: § At least 38% (NRI) of patients in 60mg 52wk treat-through arm were in clinical remission at both week 13 and week 52 Lower risk of disease Decreased steroid use recurrence § Patients in clinical remission were also in endoscopic MES=0 remission Lower rate of surgical Superior patient-reported intervention & hospitalization outcomes 1 Based on analysis of Phase 3 trials for biologic and oral approved advanced agents; 2 Source: Yoon et al. Gastroenterology 2020;159(4):1262-1275.e7; 3 Source: Turner et al. Gastroenterology 2021;160:1570–1583 38
Tamuzimod (VTX002) Program Status OLE/LTE data continue to support the differentiated profile of tamuzimod in ulcerative colitis LTE phase completed mid 2024; data presented at UEGW in October 2024 Tamuzimod is Phase 3 ready (clinical, CMC, regulatory) • End of Phase 2 meeting with FDA completed; EMA Scientific Advice meeting completed • Phase 2 trial expected to serve as the first of two pivotal trials* Ventyx working to identify partner or other source of non-dilutive financing to support future trials of VTX002 in ulcerative colitis *Pending alignment with regulators. 39
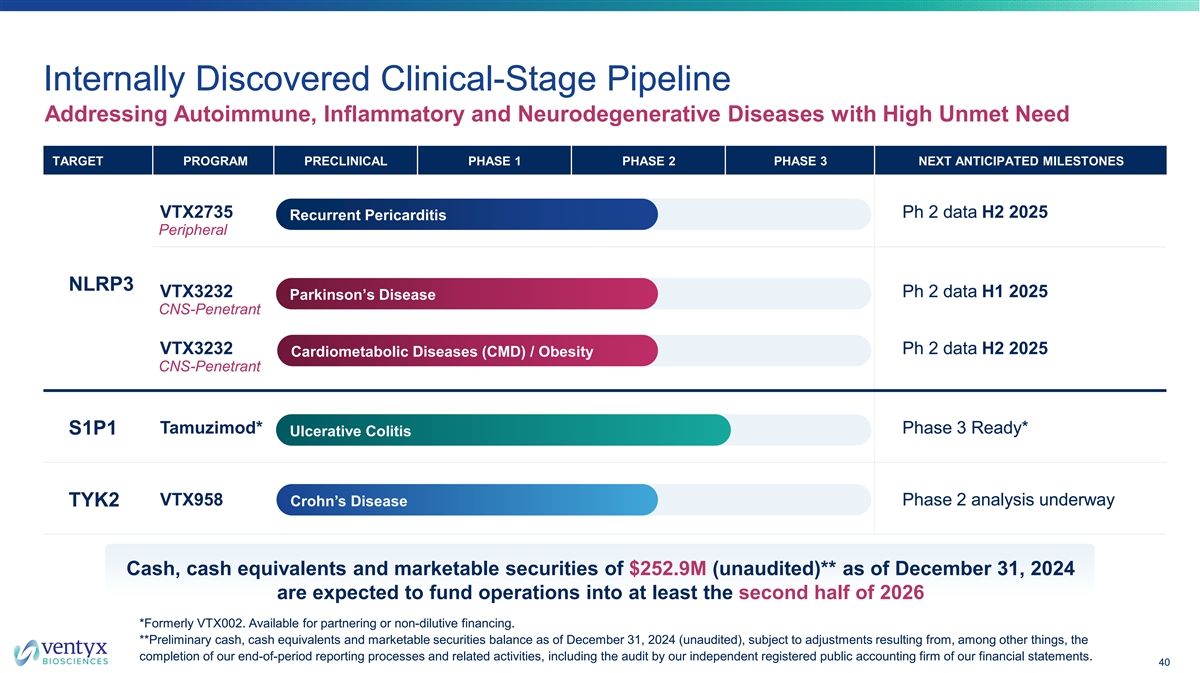
Internally Discovered Clinical-Stage Pipeline Addressing Autoimmune, Inflammatory and Neurodegenerative Diseases with High Unmet Need TARGET PROGRAM PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NEXT ANTICIPATED MILESTONES VTX2735 Ph 2 data H2 2025 Recurrent Pericarditis Peripheral NLRP3 VTX3232 Ph 2 data H1 2025 Parkinson’s Disease CNS-Penetrant VTX3232 Ph 2 data H2 2025 Cardiometabolic Diseases (CMD) / Obesity CNS-Penetrant Tamuzimod* Phase 3 Ready* S1P1 Ulcerative Colitis VTX958 Phase 2 analysis underway TYK2 Crohn’s Disease Cash, cash equivalents and marketable securities of $252.9M (unaudited)** as of December 31, 2024 are expected to fund operations into at least the second half of 2026 *Formerly VTX002. Available for partnering or non-dilutive financing. **Preliminary cash, cash equivalents and marketable securities balance as of December 31, 2024 (unaudited), subject to adjustments resulting from, among other things, the completion of our end-of-period reporting processes and related activities, including the audit by our independent registered public accounting firm of our financial statements. 40







































