Intellectual Property
Our ability to obtain and maintain intellectual property protection for our product candidates and core technologies is fundamental to the long-term success of our business. We rely on a combination of intellectual property protection strategies, including patents, trademarks, trade secrets, license agreements, confidentiality policies and procedures, nondisclosure agreements, invention assignment agreements and technical measures designed to protect the intellectual property and commercially valuable confidential information and data used in our business.
In summary, our patent estate includes issued patents and patent applications which claims cover our Vaxxine Platform and each of our product candidates. As of August 31, 2021, our patent estate includes ten U.S. issued patents, twelve U.S. patent applications, three U.S. provisional patent applications, four pending Patent Cooperation Treaty (“PCT”) patent applications, 98 issued non-U.S. patents and 194 pending non-U.S. patent applications.
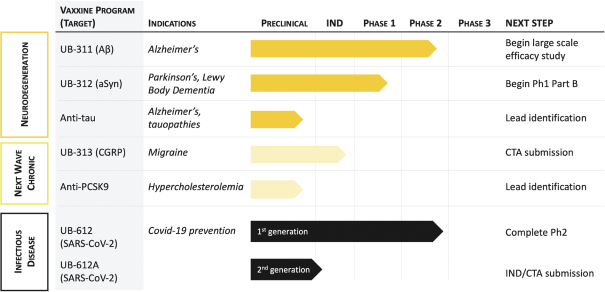
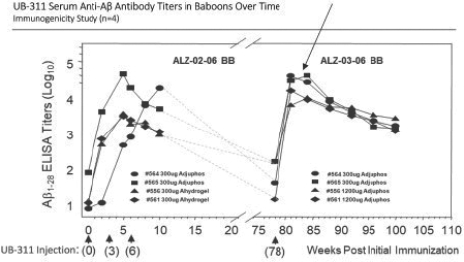
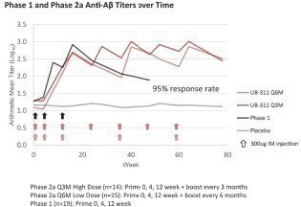
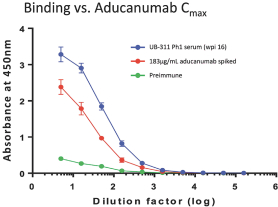
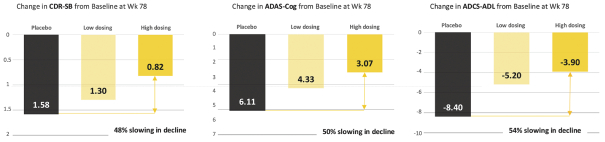
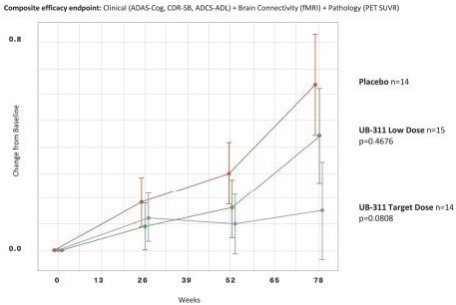
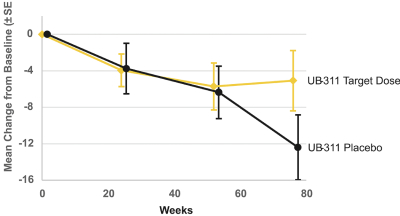
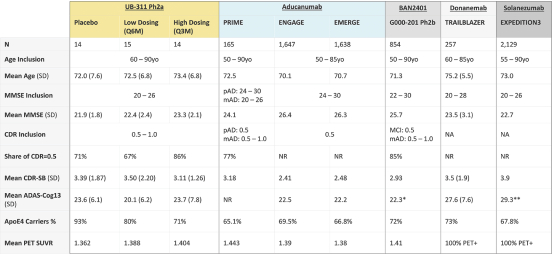
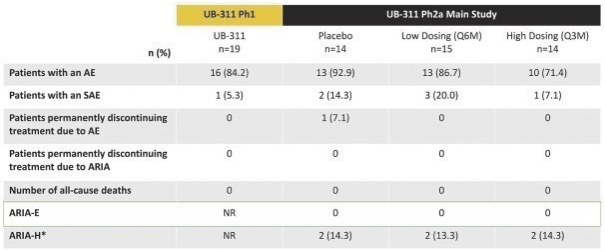
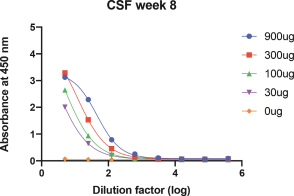
For our product candidates targeting the prevention and treatment of neurodegenerative disease, including claims covering UB-311, UB-312, patent rights are provided by patents and patent applications, the majority of which are being prosecuted in the United States, Australia, Brazil, Canada, China, the EPO, Hong Kong, Indonesia, India, Israel, Japan, the Republic of Korea, Mexico, Russia, Singapore, South Africa, Taiwan and the United Arab Emirates directed to peptide vaccines for the prevention and treatment of neurodegenerative diseases. These issued patents and patent applications, if issued, are expected to expire between 2022 and 2039, excluding any patent term adjustments or patent term extensions.
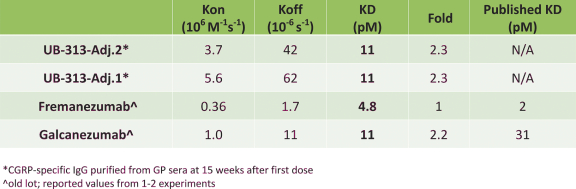
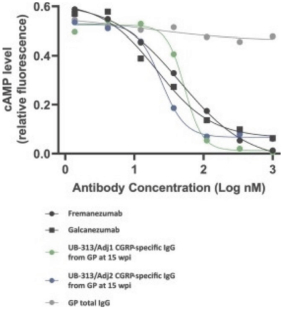
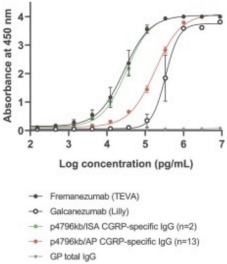
For our product candidates directed to peptide immunogens targeting CGRP and formulations thereof for the prevention and treatment of migraine, including UB-313, patent rights may be provided by a patent family being prosecuted in the United States, Australia, Brazil, Canada, China, India, Indonesia, Japan, Mexico, Russia, the Republic of Korea, Singapore, Taiwan and the United Arab Emirates. These patent applications, if issued, are expected to expire in 2039, excluding any patent term adjustments or patent term extensions.
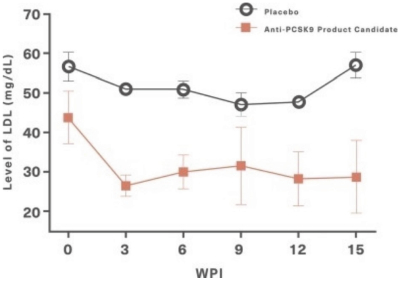
For our product candidates targeting cholesterol and cardiovascular disease, including our anti-PCSK9 product candidate targeting PCSK9 and formulations thereof for prevention and treatment of PCSK9-mediated disorders, we are in the process of acquiring a pending patent application in Taiwan and a pending PCT patent application. This Taiwanese patent application, if issued, and any U.S. or non-U.S. patent issuing from this PCT patent application, if such patent is issued, is expected to expire in 2041, excluding any patent term adjustment or patent term extension.
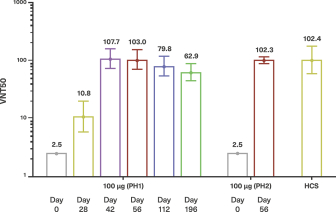
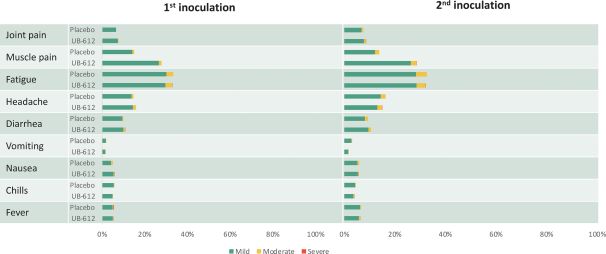
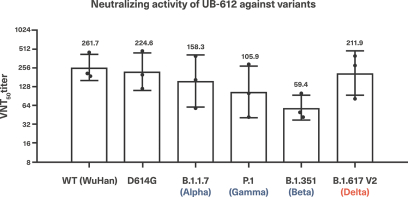
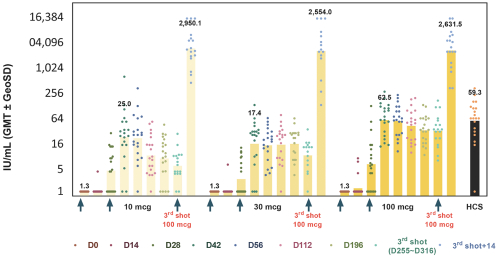
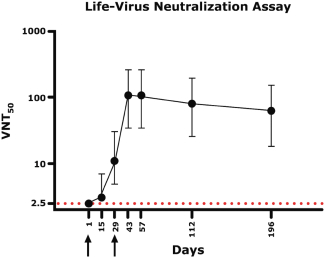
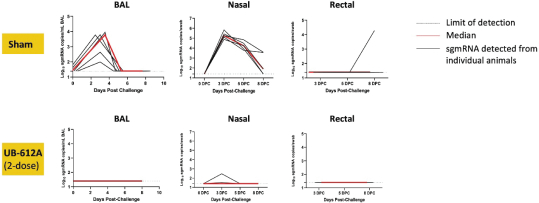
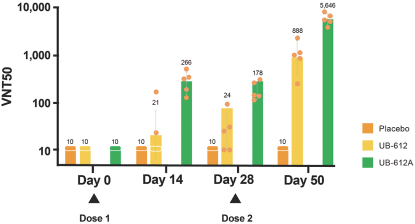
For our product candidates targeting SARS-CoV-2, including UB-612 and UB-612A for COVID-19, we have pending patent applications in Brazil, Pakistan and Taiwan, one pending PCT patent application and three provisional patent applications in the United States. These patent applications, if issued, and any U.S. or non-U.S. patent issuing from this PCT or provisional patent application, are expected to expire between 2041 and 2042, excluding any patent term adjustments or patent term extensions.
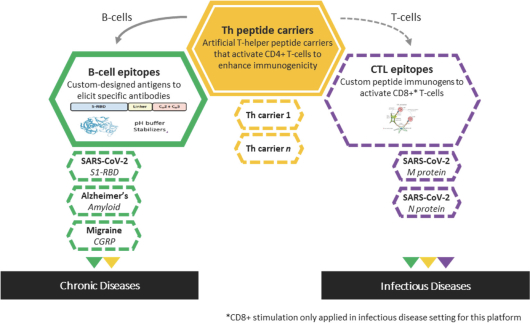
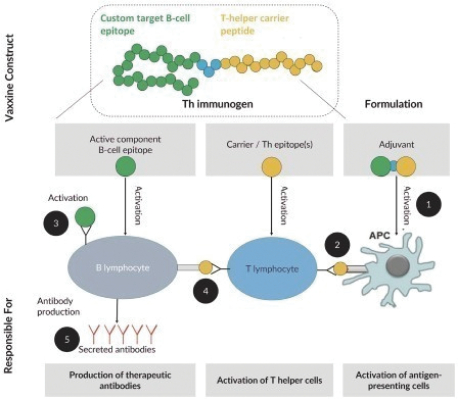
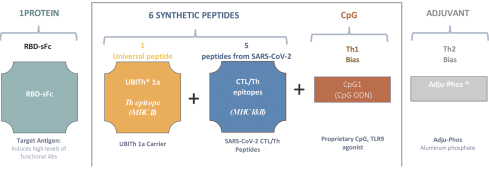
For each product candidate utilizing the Vaxxine platform, additional patent rights directed to artificial T helper cell epitopes and to a CpG delivery system are provided by patents and patent applications, the majority of which are being prosecuted in the United States, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Colombia, Denmark, the EPO, France, Germany, Hong Kong, Indonesia, India, Ireland, Israel, Italy, Japan, Mexico, the Netherlands, New Zealand, Peru, Philippines, the Republic of Korea, Russia, Singapore, South Africa, Spain, Sweden, Switzerland/Liechtenstein, Taiwan, Thailand, the United Arab Emirates, the United Kingdom and Vietnam. These issued patents and patent applications, if issued, are expected to expire between 2023 and 2039, excluding any patent term adjustments or patent term extensions.
146