UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2023
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________ to _____________
Commission File No. 001-41290
| SMART FOR LIFE, INC. |
| (Exact name of registrant as specified in its charter) |
| Nevada | | 81-5360128 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| | | |
| 990 Biscayne Blvd, Suite 505, Miami, FL | | 33132 |
| (Address of principal executive offices) | | (Zip Code) |
| (786) 749-1221 |
| (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | | SMFL | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | Large accelerated filer | ☐ | Accelerated filer | ☐ |
| | Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2023 (the last business day of the registrant’s most recently completed second fiscal quarter), the aggregate market value of the registrant’s common stock held by non-affiliates (based upon the closing price of such shares as reported on the Nasdaq Capital Market) was approximately $6.37 million. Shares held by each executive officer and director and by each person who owns 10% or more of the outstanding common shares have been excluded from the calculation in that such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of September 20, 2024, there were a total of 7,090,728 shares of the registrant’s common stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Smart for Life, Inc.
Annual Report on Form 10-K
Year Ended December 31, 2023
TABLE OF CONTENTS
INTRODUCTORY NOTES
Use of Terms
Except as otherwise indicated by the context and for the purposes of this report only, references in this report to “we,” “us,” “our” and “our company” are to Smart for Life, Inc., a Nevada corporation, and its consolidated subsidiaries.
Special Note Regarding Forward-Looking Statements
This report contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to us. All statements other than statements of historical facts are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| ● | our goals and strategies; |
| ● | our future business development, financial condition and results of operations; |
| ● | expected changes in our revenue, costs or expenditures; |
| ● | growth of and competition trends in our industry; |
| ● | our expectations regarding demand for, and market acceptance of, our products; |
| ● | our expectations regarding our relationships with investors, institutional funding partners and other parties with which we collaborate; |
| ● | fluctuations in general economic and business conditions in the markets in which we operate; and |
| ● | relevant government policies and regulations relating to our industry. |
In some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under Item 1A “Risk Factors” and elsewhere in this report. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
The forward-looking statements made in this report relate only to events or information as of the date on which the statements are made in this report. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
PART I
ITEM 1. BUSINESS.
Overview
We are engaged in the development, marketing, manufacturing, acquisition, operation and sale of a broad spectrum of nutritional and related products with an emphasis on health and wellness. Structured as a global holding company, we are executing a buy-and-build strategy with serial accretive acquisitions creating a vertically integrated company. To drive growth and earnings, we are developing proprietary products as well as acquiring other profitable companies, encompassing brands, manufacturing, and distribution channels.
We also operate a network platform in the affiliate marketing space. Affiliate marketing is an advertising model in which a product vendor compensates third-party digital marketers to generate traffic or leads for the product vendor’s products and services. The third-party digital marketers are referred to as affiliates, and the commission fee incentivizes them to find ways to promote the products being sold by the product vendor.
Business Model
We are engaged in a comprehensive program to develop a robust pipeline of prospective acquisitions in addition to the companies currently operated by us. Our management has significant experience in locating and evaluating prospective target operating companies. We have also entered into buy-side agreements with certain advisers and consultants to assist management in identifying and evaluating prospective target operating companies. The nutritional products industry is highly fragmented, and we believe that there is a large pool of companies generating less than $20 million in revenues representing significant opportunity for industry consolidation.
We plan to acquire target companies utilizing a combination of cash, promissory notes, earnouts and public company stock, generally at 4x to 6x trailing adjusted EBITDA (earnings before interest, taxes, depreciation, and amortization). Aside from our first acquisition described below, we intend on paying no more than 60% cash on any acquisition that we execute with a target of 50%. The remainder is allocated between stock and a note and/or earnout with a heavier weighting toward the former. Although the acquisition consideration is structured, we believe that our acquisitions will provide three distinct benefits to the principals of an acquisition - first, a significant liquidity event; second, the creation of a significant equity position in an emerging growth public company; and third, ongoing employment at customary industry compensation.
We plan to acquire multiple companies creating a vertically integrated company. We do not currently have sufficient capital to complete these acquisitions. We intend to raise capital for additional acquisitions primarily through debt financing at our operating company level, additional equity offerings by our company, or by undertaking a combination of any of the above. The sale of additional equity securities could result in dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if at all.
There is no guarantee that we will be able to acquire additional businesses under the terms outlined above or that we will be able to find additional acquisition candidates should we terminate our plans for any of our current acquisition targets.
Corporate History and Structure
Our company was incorporated in the State of Delaware on February 2, 2017 under the name Bonne Santé Group, Inc. On August 4, 2021, we changed our name to Smart for Life, Inc. in connection with the acquisition of DSO described below. On April 10, 2023, we redomiciled as a Nevada company.
On March 8, 2018, we acquired 51% of Millenium Natural Manufacturing Corp. and Millenium Natural Health Products Inc. for a purchase price of $2,140,272. On October 8, 2019, we entered into an agreement to acquire the remaining 49% of these companies, which was completed on October 8, 2019. On September 30, 2020, we changed the name of Millenium Natural Manufacturing Corp. to Bonne Sante Natural Manufacturing, Inc., or BSNM, and on November 24, 2020, we merged Millenium Natural Health Products Inc. into BSNM to better reflect our vertical integration. On March 6, 2024, we entered into a sale and leaseback agreement relating to BSNM as more fully described under Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Recent Developments—Sale and Leaseback Agreement.” As a result of this transaction, BSNM is no longer a subsidiary of our company.
On February 11, 2020, we entered into securities purchase agreement, which was amended on July 7, 2020 and June 4, 2021, to acquire all of the issued and outstanding equity interests of Doctors Scientific Organica, LLC d/b/a Smart for Life, Oyster Management Services, Ltd., Lawee Enterprises, L.L.C. and U.S. Medical Care Holdings, L.L.C. On July 1, 2021, the acquisition was completed. On August 27, 2021, we transferred all of the equity interests of Oyster Management Services, Ltd., Lawee Enterprises, L.L.C. and U.S. Medical Care Holdings, L.L.C. to Doctors Scientific Organica, LLC. On December 13, 2022, we converted Oyster Management Services, Ltd. to a limited liability company known as Oyster Management Services, L.L.C. On May 19, 2022, we acquired Lavi Enterprises, LLC. On the same date, we transferred all of the equity interests of Lavi Enterprises, LLC to Doctors Scientific Organica, LLC. As a result, these entities are now wholly owned subsidiaries of Doctors Scientific Organica, LLC. In this report, we collectively refer to Doctors Scientific Organica, LLC and its consolidated subsidiaries as DSO.
On August 24, 2021, we established Smart for Life Canada Inc. as a wholly owned subsidiary of Doctors Scientific Organica, LLC in Canada. This subsidiary sells retail products through a retail store location in Montreal Canada and the same location also acts as distribution center for our international direct to consumer and big box customers. We maintain inventory and employees at this location.
On July 21, 2021, we entered into a securities purchase agreement, which was amended on November 8, 2021, to acquire all of the issued and outstanding capital stock of Nexus Offers, Inc., or Nexus. On November 8, 2021, the acquisition was completed.
On November 29, 2021, we entered into a contribution and exchange agreement to acquire all of the issued and outstanding capital stock of GSP Nutrition Inc., or GSP. On December 6, 2021, the acquisition was completed.
On March 14, 2022, we entered into securities purchase agreement, which was amended on July 29, 2022, to acquire all of the issued and outstanding equity interests of Ceautamed Worldwide LLC and its wholly-owned subsidiaries Wellness Watchers Global, LLC and Greens First Female LLC, which we collectively refer to in this report as Ceautamed. On July 29, 2022, the acquisition was completed. On January 29, 2024, we contributed nearly all of the assets of Ceautamed into a limited liability company, First Health FL LLC, or First Health, of which we own 49%. Pursuant to the limited liability company operating agreement, we do not exert operational control of First Health. The aggregate value of the contributed assets was $3,486,223.
On August 15, 2022, we organized Smart Acquisition Group, LLC in the State of Florida. This subsidiary is 50% owned by our company and 50% owned by Stuart Benson, the former owner and principal of Ceautamed. This special purpose subsidiary is dedicated exclusively to the identification, negotiation, financing, and acquisition of companies synergistic to our buy-and-build business model. On September 22, 2023, we dissolved this subsidiary.
Corporate Structure
The following charts depict our organization structure.

Our Industry
The markets in which we operate are characterized by rapid technological changes, frequent new product introductions, established and emerging competition, extensive intellectual property disputes and litigation, price competition, aggressive marketing practices, evolving industry standards and changing customer preferences. Accordingly, our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies operating in rapidly changing and competitive markets.
Nutraceutical Industry
The nutraceutical industry focuses on nutritional supplements intended to improve longevity, sports fitness and provide health benefits in addition to the basic nutritional value present in food. Most people are familiar with various nutraceutical products—and have likely used them—even if they are unfamiliar with the industry name. Nutraceuticals comprise such commonly used items as herbal products, specific diet products, vitamins, processed foods and beverages, functional foods, isolated nutrients and other dietary products. Functional foods are foods that have a potentially positive effect on health beyond basic nutrition. A familiar example of a functional food is oatmeal because it contains soluble fiber that can help lower cholesterol levels. Some foods are also modified to have health benefits. An example is orange juice that has been fortified with calcium for bone health.
The following table prepared by the Council for Responsible Nutrition (www.crnusa.org), or CRN, depicts the types of supplements taken by the population in the different indicated categories beginning in 2018 and estimated through 2027. We sell products across all of these product categories, and we believe that our market share in each of these categories is currently less than 1%.
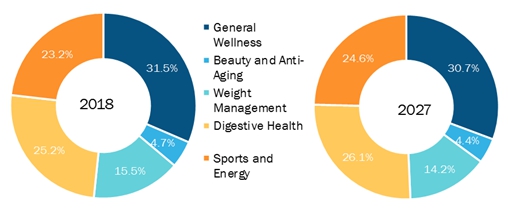
SOURCE: Council for Responsible Nutrition
Nutraceuticals are garnering immense attention in recent years due to various trends including changing lifestyles, burgeoning middle-class segment across emerging economies, transforming dietary habits, aging population, and increased life expectancy. In addition, the focus of R&D based pharmaceutical sector on expensive specialty drugs is increasing the burden on the healthcare system as well as resulting in higher out-of-pocket costs for drugs driving the focus on prevention than intervention. The self-care trend across the world is driving strong demand for nutraceuticals including superfoods, food and dietary supplements, sports nutrition, and functional foods and beverages. Given the hectic lifestyles and the lack of time for consumption of the required nutrients through regular diet, the need for replenishing such essential nutrients is increasing. In this context, nutraceuticals are emerging to be the solution for meeting this requirement. Nutraceuticals are considered to be the vital link between health and food.
The market is also experiencing strong demand for personalized approaches to wellness that is driving product innovation in the areas of weight management, sports nutrition, and healthy snacking. Other noteworthy trends benefiting market prospects in the near term include emergence of clean labeling as a new norm owing to increasing focus of consumers on ingredient list on the product; innovative delivery technologies such as microencapsulation, which protects the product from adverse conditions such as light and air.
As the overall population continues to turn to healthier living in hopes of offsetting rising healthcare expenditures and preventing general subpar health conditions, we believe that the demand for nutraceutical industry products will resemble a similar trend.
Digital Marketing
As a result of our acquisition of Nexus, we have entered the digital marketing industry as a way to promote the products and brands that we sell. Digital marketing is a component of marketing that uses internet and online based digital technologies such as desktop computers, mobile phones and other digital media and platforms to promote products and services.
The COVID-19 pandemic resulted in people staying at home and/or working remotely from home, resulting in huge increase in online traffic. Clicks and display ads are among the most prominent forms of digital marketing initiatives. Clicks are expensive compared to display ads, as clicks ensure the customer is directed to the advertiser’s website. However, clicks provide a better return on investment.
Our Operating Subsidiaries
DSO
DSO manufactures, sells and owns the Smart for Life brand of natural health and wellness meal replacement products. The brand includes proprietary hunger suppressing functional foods that are designed to work with the body’s natural ability to lose weight. The program uses an exact protein-to-sugar ratio, a low glycemic index and glycemic load as well as multiple small meals throughout the day to deliver specific amounts of protein, super fibers and complex carbs to suppress hunger, keep sugar and insulin low and trigger the body’s release of the fat releasing hormone glucagon.
Our Smart for Life products deliver:
| ● | Hunger controlling protein mix |
| ● | No toxins or preservatives |
| ● | The right amount of protein per calorie ratio |
| ● | No insulin spike, lets glucagon do its job |
| ● | A small amount of essential good fats |
| ● | Right amount of complex carbs |
DSO also develops premium supplements and commodities that will promote optimal health and wellness. This natural product line uses simple quality ingredients to help create a more sustainable lifestyle. DSO has over 15 years of experience providing high-quality products to premium retail locations and companies. DSO branded vitamins and supplements are also being sold through Amazon, and this sales channel is becoming a major contributor to the growth of the brand online. All products are packaged in eco-friendly and bio-degradable packaging.
GSP
GSP is a sports nutrition company. It offers nutritional supplements for athletes and active lifestyle consumers through a variety of wellness solutions and delivery methods, including powders, tablets and soft gels that are formulated to support energy and performance; nutrition and wellness; and focus and clarity.
In conjunction with the sale of BSNM, we entered into a lease agreement with the buyer to operate the facility owned by the buyer. Following the surrendering of the facility in Doral, Florida, GSP moved the equipment and operation to the manufacturing facility in Riviera Beach, Florida. This subsidiary primarily focuses on the contract manufacturing of vitamins and supplements, with a particular emphasis on the production of tablets, capsules and powders, along with turn-key solutions for packaging these health and wellness products in a wide variety of bottles, jars, sachets and stick packs. GSP’s initial line of nutritional products was marketed under the Sports Illustrated Nutrition brand. The product line consisted of whey protein isolate powder, tablet supplements for joint health, nitric oxide, post workout blends, Omega-3 supplements, and pre-workout supplements, among others. Due to the high cost of the license associated with Sports Illustrated Nutrition, the license agreement was surrendered, and future products will be marketed under the GSP brand.
First Health
First Health is primarily engaged in the development and distribution of a wide variety of nutritional products, including antioxidant rich supplements, plant-based protein, alkalizing nutrients and products designed for weight management.
First Health shares the Greens First line of branded products which have been specifically marketed to the healthcare provider sector. These vitamins and supplements have been sold on a business-to-business basis, direct-to-consumer, as well as sold utilizing an international medical distribution company.
The mission is to create a healthier world with nutritious wellness products made from the highest quality ingredients for superior benefits.
Nexus
Nexus operates a cost per action/cost per acquisition network. This network consists of hundreds of digital marketers who stand ready to market products introduced to the Nexus network. The cost per action/cost per acquisition model is where digital marketers are paid for an action (e.g., a product sale or lead generation) that is taken as a direct result of their marketing efforts. Through the digital marketer’s method of marketing, the digital marketer sends traffic to one of the product vendor’s offers listed on the network.
Nexus has relationships with both product vendors and digital marketers. A product vendor is a Nexus customer that has products, whether digital or physical, for sale and is looking for increased sales through digital marketing avenues from digital marketers. Digital marketers are Nexus contractors that engage in digital marketing. An example of a digital marketer is someone who has a strong Facebook following, or a strong knowledge of Facebook ad marketing. Other examples include google ad marketing or email marketers who send marketing messages to an opted in list of subscribers. Historically, Nexus’ customers consisted exclusively of owners of digital products that were also delivered digitally. Following our acquisition of Nexus, BSNM, DSO, GSP and Ceautamed, as well as any additional nutraceutical companies that we acquire in the future, will also become customers of Nexus. Nexus will use its online marketplace to market our nutraceutical products through its network of digital marketers. Our nutraceutical product companies will then sell and physically deliver the nutraceutical products to the end users identified through the efforts of the digital marketers. Nexus has the ability to “plug and play” with any of the products sold by companies that we may acquire in the future as we can take the consumer facing products being sold by those companies and seamlessly add them to the Nexus network to generate sales.
Product vendors come to Nexus to increase sales of their products and digital marketers come to Nexus to receive a commission in exchange for their marketing efforts, which are designed to generate sales for the product vendors. When a digital marketer’s marketing efforts results in a sale of a product by a product vendor, the digital marketer is then credited with a commission. The product vendor is billed weekly for the sales that the product vendor makes during the week as the result of such digital marketers’ marketing efforts. The product vendor pays Nexus and Nexus pays the digital marketer. This is an anonymous transaction as digital marketers and product vendors are only defined inside the marketplace by an offer name (product vendor) and an affiliate number (digital marketer).
Manufacturing, Distribution and Quality Control
DSO operates a 30,000 square foot manufacturing facility in Riviera Beach, Florida. This facility is primarily focused on the production of natural health and wellness meal replacement products, including nutrition bars, cookies, soups and shakes, as well as some vitamin and supplement capabilities such as powders. In addition to our own products, DSO manufactures products on behalf of third parties
GSP operated an 18,000 square foot manufacturing facility in Doral, Florida. This facility primarily focused on the contract manufacturing of vitamins and supplements, with a particular emphasis on the production of tablets, capsules and powders, along with turn-key solutions for packaging these health and wellness products in a wide variety of bottles, jars, sachets and stick packs. During 2024, the equipment and inventory located at the Doral facility was transferred to the facility in Riviera Beach, Florida.
First Health relies on third-party contract manufacturers to manufacture its products.
All our manufacturing operations are subject to good manufacturing practices, or GMPs, promulgated by the U.S. Food and Drug Administration, or the FDA, and other applicable regulatory standards. We believe our manufacturing processes comply with the GMPs for dietary supplements or foods, and our manufacturing and distribution facilities generally have sufficient capacity to meet our current business requirements and our currently anticipated sales. We place special emphasis on quality control. We assign lot numbers to all raw materials and initially hold them in quarantine while our quality department evaluates them for compliance with established specifications. Once released, we retain samples and process the material according to approved formulas by blending, mixing and technically processing as necessary. We manufacture products in final delivery form as a capsule, tablet, powder, or nutrition bar. After a product is manufactured, our laboratory analysts test its weight, purity, potency, disintegration and dissolution, if applicable, utilizing both internal equipment and third-party labs. We hold the product in quarantine until we complete the quality evaluation and determine that the product meets all applicable specifications before packaging. When the manufactured product meets all specifications, our automated packaging equipment packages the product with at least one tamper-evident safety seal and affixes a label, an indelible lot number and, in most cases, the expiration or “best by” date.
Our manufacturing operations are designed to allow low-cost production of a wide variety of products of different quantities, physical sizes and packaging formats, while maintaining a high level of customer service and quality. Flexible production line changeover capabilities and reduced cycle times allow us to respond quickly to changes in manufacturing schedules and customer demands.
We have inventory control systems at our facilities that track each manufacturing and packaging component as we receive it from our supply sources through manufacturing and shipment of each product to customers. To facilitate this tracking, most products we sell are bar coded. We believe our distribution capabilities increase our flexibility in responding to our customers’ delivery requirements.
Raw Materials and Suppliers
In fiscal 2023 and 2022, we spent approximately $1,988,000 and $9,335,000, respectively, on raw materials, excluding shipping packaging and similar product materials. The principal raw materials required in our operations are food ingredients, vitamins, minerals, herbs, gelatin, labels, and bottles. We believe that there are adequate sources of supply for all our principal raw materials, and in general we maintain two to three suppliers for many of our raw materials. From time to time, weather or unpredictable fluctuations in the supply and demand may affect price, quantity, availability, or selection of raw materials. We believe that our strong relationships with our suppliers yield high quality, competitive pricing, and overall good service to our customers. Although we cannot be sure that our sources of supply for our principal raw materials will be adequate in all circumstances, we believe that we can develop alternate sources in a timely and cost-effective manner if our current sources become inadequate. During fiscal 2023, we had one significant raw material supplier accounted for 22% of our raw material purchases. Due to availability of numerous alternative raw material suppliers, we do not believe that the loss of any single raw material supplier would have a material adverse effect on our consolidated financial condition or results of operations. See Item 1A “Risk Factors—Risks Related to Our Business and Industry—An increase in the price and shortage of supply of key raw materials could adversely affect our business.”
Sales and Marketing
We employ many different techniques and strategies within our marketing initiatives. These include direct to consumer outreach, use of influencers through social media, Facebook targeting, focused e-mail campaigns, and traditional media. Our marketing goal is always to increase visibility and relevance of our brands in the minds of our customers and potential customers. We hope to expand our programs to include experimental marketing techniques in the future.
We recently shifted the marketing focus of Nexus to target the nutraceutical industry away from all other efforts, which we believe will become a value-added component of our marketing strategies.
Customers
DSO, GSP and First Health primarily sell products to customers under individual purchase orders placed by them under their standard terms and conditions of sale. These terms and conditions generally include insurance requirements, representations by us with respect to the quality of our products and our manufacturing process, our obligations to comply with law, and indemnifications by us if we breach our representations or obligations. There is no commitment from any customer to purchase from us, or from us to sell to them, any minimum amount of product. During fiscal 2023, Amazon and Boxout, accounted for 31% and 31%, respectively, of our total revenues.
First Health has distribution agreements with Boxout whereby it acts as an exclusive distributor for certain of First Health’s products to professional health and wellness providers and service locations, along with distributors selling to such providers and locations, within the U.S. and Canada, as well as through e-commerce websites Amazon, Ebay and Walmart.
As described above, Nexus’ customers are product vendors. Although the number of customers that Nexus has fluctuates from year to year, it has established long-term relationships with its significant product vendors, but it does not have long-term contracts with any of its customers. The relationship with customers can be terminated at any time by either party; however, as a result of Nexus’ extensive network of digital marketers, which drive sales for product vendors, the average length of Nexus’ relationships with its significant customers is 3 years. Most of Nexus’ customers are acquired through existing customer referrals. Nexus also attends Internet marketing conferences to promote is service.
The loss of any major customer would have a material adverse effect on us if we were unable to replace that customer. See Item 1A “Risk Factors—Risks Related to Our Business and Industry—Our major customers account for a significant portion of our consolidated net sales and the loss of any major customer could have a material adverse effect on our results of operations.”
Competition
The nutraceutical industry is highly competitive. Our competitors include a number of large, nationally known brands such as Nature Made (Pharmavite), Nature’s Bounty, GNC, Spectrum (Hain Celestial), Country Life, Garden of Life and Jarrow Formulas, and many smaller brands, manufacturers and distributors. The sales of products through online marketplace platforms such as Amazon and firms’ websites continue to expand. Private label products also provide competition to our products. Whole Foods Market, Walmart, CVS, Walgreens and many health stores also sell a portion of their nutritional supplement offerings under their own private labels. Private label products are often sold at a discount to branded products. We also compete with distributors that sell products to health stores as well as mass market retailers such as United Natural Foods and KeHE Distributors. In addition, several major pharmaceutical companies continue to offer nutritional supplement lines in the mass market, including Centrum (Pfizer and GSK) and One-A-Day (Bayer). Pharmaceutical companies also offer prescription and over-the-counter products that are or may be competitive with nutritional supplements, particularly with regard to certain categories of products. Finally, as the nutraceutical market generally has low barriers to entry, additional competitors enter the market regularly.
Nexus’ competitors would be any digital marketing agency in the cost per acquisition space looking to acquire exclusive advertiser offers and high end publishers who can send high amounts of traffic through digital marketing media. Examples include Ca$hNetwork, OfferBlueprint and MaxBounty.
Competitive Strengths
Based on management’s belief and experience in the industry, we believe that the following competitive strengths enable us to compete effectively.
| ● | Proprietary manufacturing facilities. GSP and DSO own and operate proprietary manufacturing facilities, which allow for a high level of managerial control over all aspects of production, including sourcing, logistics and maintaining the highest levels of quality during the manufacturing process. Through direct ownership, we are able to optimize our sales and marketing practices and provide a completely integrated approach, all solidified by a single manufacturing platform for capsules, tablets, powders and various other delivery methods for all vitamins and supplements. In addition, as a private label contract manufacturer for third parties, we can provide a turnkey solution for brands and retailers who want to minimize their supply chain disruption and maximize their control over product flow to end customers. In addition, as a middle market-sized contract manufacturer, we are not encumbered by the often overly complex processes that our larger competitors may have. We can be nimble and highly adaptable, “flexing” with our customers’ needs as they change over time, which allows us to better service our ever-expanding international client base. We are able to maintain a competitive advantage due to our vertically integrated operational control. This vertical integration also allows us to minimize intellectual property and data security risks, while also eliminating costs, improving focus, optimizing quality and launching with a faster time-to-market for new products. We retain control over every step of the manufacturing processes, allowing us to establish our own institutional advantages and maximize efficiencies. |
| ● | Established and trusted brands. Smart for Life, Doctors Scientific Organica, and Greens First are well-established brands in the in the health and wellness industry. In particular, Smart for Life products are currently sold in many of the largest big-box retailers in the United States and Canada, including Costco, Walmart, Sam’s Club, BJ’s and Publix, as well as through online channels such as Amazon. DSO has established a dedicated following of consumers that are strong believers in the high-quality vitamins and supplements it sells to its customers, along with the eco-friendly and bio-degradable packaging, with Amazon sales numbers continuing to increase as a result. |
| ● | Client focused innovative research and development. We believe that our research and development team adds significant value to our company and our customers and is a differentiating factor for our company. We strive to be technology driven leveraging technology, science, and innovation in our research and development efforts. We work closely with our clients to create and develop new and exciting products. We frequently work directly with our customers in our research and development labs to create innovative solutions that create value for our customers in a timely manner. Our team works closely with physicians to create novel wholesome products that add nutritional and functional value. |
| ● | Ability to market through captive marketing subsidiary. We believe that our subsidiary, Nexus, allows us access to a broad spectrum of marketing tools to be utilized across the entire spectrum of our products. We believe that having an experienced management team and existing customer base accessible to all of our other brands in our portfolio will allow us to drive sales and revenue of existing products as well as test new product offerings generated through our research and development. |
| ● | Referral only network based on long term relationships. Nexus operates a referral only network, meaning that all of its digital marketers are referred. There is no way to get a Nexus account other than being directly referred by a known good account holder. This allows Nexus to stem any fraudulent traffic, which we believe is a substantial competitive advantage for product vendors. We believe that these factors set Nexus apart from its competition. |
Growth Strategies
We will strive to grow our business by pursuing the following growth strategies.
| ● | Acquisition of additional businesses. The nutritional products industry is highly fragmented, and we believe that there is a large pool of companies generating less than $20 million in revenues representing significant opportunity for industry consolidation. We plan to acquire multiple companies creating a vertically integrated company. As noted above, we do not currently have sufficient capital to complete these acquisitions. We intend to raise capital for additional acquisitions primarily through debt financing at our operating company level, additional equity offerings by our company, or by undertaking a combination of any of the above. The sale of additional equity securities could result in dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if at all. There is no guarantee that we will be able to acquire additional businesses under the terms outlined above or that we will be able to find additional acquisition candidates should we terminate our plans for any of our current acquisition targets. |
| ● | Increase sales from existing and new customers. We expect to continue to drive growth for our consumer products branded business through our increased focus on our top brands and continued expansion in various health and wellness categories, which we expect to result in incremental shelf space with existing customers and new customer additions. We expect that our focus on delivering tangible benefits to consumers through product innovation will not only benefit us but also benefit our customers. Our ability to supply both branded and private label products broadens and deepens our partnerships with key retail customers, providing us more opportunities for category leadership and growth. We view the private label business as an important and valuable service that we provide to key accounts. |
| ● | Further penetrate international markets. Our products are currently marketed and sold in approximately two countries. In fiscal 2023, approximately 4% of our sales were to customers outside the United States. We plan to capitalize on our marketing and distribution capabilities to drive incremental international sales of our consumer product brands in emerging markets, which are characterized by a rising middle class and a strong demand for high quality nutritional and wellness products from U.S.-based manufacturers. |
| ● | Drive productivity through operational efficiencies. We expect to continue to focus on improving efficiency across our operations to allow us to reduce costs in our manufacturing facilities as well as across our overhead cost areas. Our acquisition of DSO significantly increased our production capacity. In addition, we have launched an initiative to optimize our product portfolio, which we expect will enable further efficiencies across our manufacturing network. We are also introducing new initiatives that leverage automation, standardization and simplification and are expected to increase productivity across our operations. |
Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized brand names under which we market our products. We own or have rights to material trademarks or trade names that we use in conjunction with the sale of our products, including the Smart for Life, Doctors Scientific Organica, and Greens First, and brand names. We also own website domain names and have proprietary methodologies that we use in our manufacturing businesses. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
We protect our intellectual property rights through a variety of methods, including trademark, patent and trade secret laws, as well as confidentiality agreements and proprietary information agreements with vendors, employees, consultants and others who have access to our proprietary information. Protection of our intellectual property often affords us the opportunity to enhance our position in the marketplace by precluding our competitors from using or otherwise exploiting our technology and brands. We are also a party to several intellectual property license agreements relating to certain of our products. The duration of our trademark registrations is generally 10, 15 or 20 years, depending on the country in which the marks are registered, and we can renew the registrations. The scope and duration of our intellectual property protection varies throughout the world by jurisdiction and by individual product. Our global trademark portfolio, with the aforementioned registration durations, consists of our core marks for our business and our proprietary product brands which drive significant brand awareness for all of our businesses. Our proprietary product formulas and recipes, maintained as trade secrets, are significant to our growth and success as they form the foundation for our production and sales of effective, high quality products.
Employees
As of December 31, 2023, we had approximately 12 employees with approximately 7 of such employees being engaged in our manufacturing operations and the balance being engaged in management or middle management. None of our employees are represented by labor unions, and we believe that we have an excellent relationship with our employees.
Regulation
Our business is subject to varying degrees of regulation by a number of government authorities in the United States, including the FDA, the Federal Trade Commission, or the FTC, the Consumer Product Safety Commission, or the CPSC, the U.S. Department of Agriculture, or the USDA, and U.S. Environmental Protection Agency, or the EPA. Various agencies of the state and localities in which we operate and in which our products are sold also regulate our business.
The areas of our business that these and other authorities regulate include, among others:
| ● | product claims and advertising; |
| ● | product ingredients; and |
| ● | how we manufacture, package, distribute, import, export, sell and store our products. |
In addition, our products sold in foreign countries are also subject to regulation under various national, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements and over-the-counter drugs.
As a result of the acquisition of Nexus, we are also subject to laws and regulations generally applicable to providers of digital marketing services, including federal and state laws and regulations governing data security and privacy, unfair and deceptive acts and practices, advertising and content regulation.
We are also subject to a variety of other regulations in the United States, including those relating to taxes, employment, import and export, and intellectual property.
Food and Drug Administration
The Dietary Supplement Health and Education Act of 1994, or DSHEA, amended the Federal Food, Drug, and Cosmetic Act, or the FDC Act, to establish a new framework governing the composition, safety, labeling, manufacturing and marketing of dietary supplements. Generally, under the FDC Act, dietary ingredients (i.e., vitamins; minerals; herb or other botanical; amino acids; or dietary substances for use by humans to supplement diet by increasing total dietary intake; or any concentrate, metabolite, constituent, extract or combination of any of the above) that were marketed in the United States prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. New dietary ingredients (i.e., dietary ingredients that were not marketed in the United States before October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without being “chemically altered.” A new dietary ingredient notification must provide the FDA evidence of a “history of use or other evidence of safety” establishing that use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. The FDA may determine that a new dietary ingredient notification does not provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe. Such a determination could prevent the marketing of such dietary ingredient.
In 2011 and 2016, the FDA issued draft guidance setting forth recommendations for complying with the new dietary ingredient notification requirement. Although FDA guidance is non-binding and does not establish legally enforceable responsibilities, and companies are free to use an alternative approach if the approach satisfies the requirements of applicable laws and regulations, FDA guidance is a strong indication of the FDA’s current thinking on the topic discussed in the guidance, including its position on enforcement. At this time, it is difficult to determine whether the 2016 draft guidance (which replaced the 2011 draft guidance), if finalized, would have a material impact on our operations. However, if the FDA were to enforce the applicable statutes and regulations in accordance with the draft guidance as written, such enforcement could require us to incur additional expenses, which could be significant, and negatively impact our business in several ways, including, but not limited to, enjoining the manufacturing of our products until the FDA determines that we are in compliance and can resume manufacturing, increasing our liability and reducing our growth prospects.
The FDA or other agencies could take actions against products or product ingredients that, in their determination, present an unreasonable health risk to consumers that would make it illegal for us to sell such products. In addition, the FDA could issue consumer warnings with respect to the products or ingredients in such products that are sold in our stores. Such actions or warnings could be based on information received through FDC Act-mandated reporting of serious adverse events.
We take a number of actions to ensure the products we sell comply with the FDC Act. Some of these actions include maintaining and continuously updating a list of restricted ingredients that will be prohibited from inclusion in any products that we sell. In addition, we have developed and maintain a list of ingredients that we believe comply with the applicable provisions of the FDC Act. As is common in our industry, we rely on some third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of such products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations. A removal or recall could also result in negative publicity and damage to our reputation that could reduce future demand for our products. In the past, we have attempted to offset any losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall.
The FDC Act permits structure/function claims to be included in labels and labeling for dietary supplements without FDA pre-market approval. However, companies must have substantiation that the claims are “truthful and not misleading”, and must submit a notification with the text of the claims to the FDA no later than 30 days after marketing the dietary supplement with the claims. Permissible structure/function claims may describe how a particular nutrient or dietary ingredient affects the structure, function or general well-being of the body, or characterize the documented mechanism of action by which a nutrient or dietary ingredient acts to maintain such structure or function. The label or labeling of a product marketed as a dietary supplement may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat or prevent a disease (i.e. a disease claim). If the FDA determines that a particular structure/function claim is an unacceptable disease claim that causes the product to be regulated as a drug, a conventional food claim or an unauthorized version of a “health claim,” or, if the FDA determines that a particular claim is not adequately supported by existing scientific data or is false or misleading in any particular, we would be prevented from using the claim and would have to update our product labels and labeling accordingly.
In addition, DSHEA provides that so-called “third-party literature,” e.g., “a publication, including an article, a chapter in a book, or an official abstract of a peer-reviewed scientific publication that appears in an article and was prepared by the author or the editors of the publication” supplements, when reprinted in its entirety, may be used “in connection with the sale of a dietary supplement to consumers” without the literature being subject to regulation as labeling. Such literature: (1) must not be false or misleading; (2) may not “promote” a particular manufacturer or brand of dietary supplement; (3) must present a balanced view or is displayed or presented with other such items on the same subject matter so as to present a balanced view of the available scientific information; (4) if displayed in an establishment, must be physically separate from the dietary supplements; and (5) should not have appended to it any information by sticker or any other method. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any continued dissemination could subject our product to regulatory action as an illegal drug.
In June 2007, pursuant to the authority granted by the FDC Act as amended by DSHEA, the FDA published detailed GMP regulations that govern the manufacturing, packaging, labeling and holding operations of dietary supplement manufacturers. The GMP regulations, among other things, impose significant recordkeeping requirements on manufacturers. The GMP requirements are in effect for all dietary supplement manufacturers, and the FDA conducts inspections of dietary supplement manufacturers pursuant to these requirements. There remains considerable uncertainty with respect to the FDA’s interpretation of the regulations and their actual implementation in manufacturing facilities.
In addition, the FDA’s interpretation of the regulations governing dietary supplements will likely change over time as the agency becomes more familiar with the industry and the regulations. The failure of a manufacturing facility to comply with the GMP regulations renders products manufactured in such facility “adulterated,” and subjects such products and the manufacturer to a variety of potential FDA enforcement actions. In addition, under the Food Safety Modernization Act, or FSMA, which was enacted in January 2011, the manufacturing of dietary ingredients contained in dietary supplements will be subject to similar or even more burdensome manufacturing requirements, which will likely increase the costs of dietary ingredients and will subject suppliers of such ingredients to more rigorous inspections and enforcement. The FSMA will also require importers of food, including dietary supplements and dietary ingredients, to conduct verification activities to ensure that the food they might import meets applicable domestic requirements.
The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements, including powers to issue a public warning or notice of violation letter to a company, publicize information about illegal products, detain products intended for import, require the reporting of serious adverse events, require a recall of illegal or unsafe products from the market, and request the Department of Justice to initiate a seizure action, an injunction action or a criminal prosecution in the United States courts.
The FSMA expands the reach and regulatory powers of the FDA with respect to the production and importation of food, including dietary supplements. The expanded reach and regulatory powers include the FDA’s ability to order mandatory recalls, administratively detain domestic products, and require certification of compliance with domestic requirements for imported foods associated with safety issues. FMSA also gave FDA the authority to administratively revoke manufacturing facility registrations, effectively enjoining manufacturing of dietary ingredients and dietary supplements without judicial process. The regulation of dietary supplements may increase or become more restrictive in the future.
Federal Trade Commission
The FTC exercises jurisdiction over the advertising of dietary supplements and other health-related products and requires that all advertising to consumers be truthful and non-misleading. The FTC actively monitors the dietary supplement space and has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. FTC enforcement actions may result in consent decrees, cease and desist orders, judicial injunctions and the payment of fines with respect to advertising claims that are found to be unsubstantiated.
Environmental Regulation
Our facilities and operations, in common with those of similar industries making similar products, are subject to many federal, state, provincial and local requirements, rules and regulations relating to the protection of the environment and of human health and safety, including those regulating the discharge of materials into the environment. We continually examine ways to reduce our emissions, minimize waste and limit our exposure to any liabilities, as well as decrease costs related to environmental compliance. Costs to comply with current and anticipated environmental requirements, rules and regulations and any estimated capital expenditures for environmental control facilities are not anticipated to be material when compared with overall costs and capital expenditures. Accordingly, we do not anticipate that such costs will have a material effect on our financial position, results of operations, cash flows or competitive position.
New Legislation or Regulation
Legislation may be introduced which, if passed, would impose substantial new regulatory requirements on dietary supplements and other health products. We cannot determine what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
ITEM 1A. RISK FACTORS.
An investment in our securities involves a high degree of risk. You should carefully read and consider all of the risks described below, together with all of the other information contained or referred to in this report, before making an investment decision with respect to our securities. If any of the following events occur, our financial condition, business and results of operations (including cash flows) may be materially adversely affected. In that event, the market price of our stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business and Industry
We are an early-stage company with a limited operating history.
We were organized as a Delaware corporation in February 2017, and in April 2023 redomiciled in Nevada. We have a limited history upon which you can evaluate our business and prospects. Our prospects must be considered in light of the risks encountered by companies in the early stages of development in highly competitive markets, particularly the markets for nutraceuticals and related products. You should consider the frequency with which early-stage businesses encounter unforeseen expenses, difficulties, complications, delays and other adverse factors. These risks are described in more detail below.
We have incurred losses since our inception, and we may not be able to manage our businesses on a profitable basis.
We have generated losses since inception and have relied on cash on hand, sales of securities, external bank lines of credit, and issuance of third-party and related party debt to support our operations. For the year ended December 31, 2023, we generated an operating loss of $17,155,992 and a net loss of $22,675,741. We cannot assure you that we will achieve profitably or that we will have adequate working capital to meet our obligations as they become due. Management believes that our success will depend on our ability to successfully complete additional acquisitions of profitable nutraceutical companies and related products as well as develop our own brands. We cannot guarantee that we will be successful in completing acquisitions or any other companies or products, that we will successfully integrate acquired companies, or that we will be able to successfully develop our own brands. We cannot assure you that even if we are successful in completing the acquisitions or in developing our own branded products, we will be successful in profitably managing such companies, acquired assets and brands. We cannot assure you that we will maintain profitability for any period of time or that investors will not lose their entire investment.
Our auditors have issued a going concern opinion on our audited financial statements.
The report of our independent registered public accounting firm that accompanies our financial statements for the year ended December 31, 2023 contains a going concern qualification in which such firm expressed substantial doubt about our ability to continue as a going concern, based on the financial statements at that time. We have suffered recurring losses from operations and have a working capital deficiency of approximately $19.7 million and an accumulated deficit of $67.7 million as of December 31, 2023. Additionally, we had a net loss of $22.7 million, and cash used in operations of $5.8 million for the year ended December 31, 2023. These conditions raise substantial doubt about our ability to continue as a going concern.
Management believes that current available resources will not be sufficient to fund our planned expenditures over the next 12 months. Management has implemented restructuring activities, including staff reductions, a subsequent event sale of BSNM, and a subsequent event sale of Ceautamed’s assets. Accordingly, we will be dependent upon the raising of additional capital through placement of common stock and/or debt financing in order to implement our business plan. If we raise additional capital through the issuance of equity securities or securities convertible into equity, stockholders will experience dilution, and such securities may have rights, preferences, or privileges senior to those of the holders of common stock or convertible senior notes. If we raise additional funds by issuing debt, we may be subject to limitation on its operations, through debt covenants or other restrictions. There is no assurance that we will be successful with future financing ventures, and the inability to secure such financing may have a material adverse effect on our financial condition. The accompanying consolidated financial statements do not include any adjustments to the amounts and classifications of assets and liabilities that might be necessary should we be unable to continue as a going concern. If we cannot continue as a going concern, our stockholders would likely lose most or all of their investment in us.
If we fail to implement our business plan and complete acquisitions as planned, our mission will fail and our business will suffer accordingly.
Our mission is the creation of a world-class nutraceutical company engaged in the development, manufacture and sales of quality nutraceutical and related health and lifestyle products for distribution to an expanding global marketplace. We expect that our holding company strategy through which we plan to acquire profitable but undervalued target companies and products will enable us to accelerate the development and expansion of our product portfolio, manufacturing capacity and distribution channels. If we are unable to execute our strategy of completing acquisitions as planned, we will not be able to fulfill our mission or grow our business.
Our acquisitions may result in significant transaction expenses, integration and consolidation risks, and we may be unable to profitably operate our consolidated company.
We are structured as a holding company and we have executed a buy and hold strategy. We are engaged in the business of acquisition, operation and management of nutraceutical and related products. Our acquisitions may result in significant transaction expenses and present new risks associated with entering additional markets or offering new products and services and integrating the acquired companies. We may not have sufficient management, financial and other resources to integrate the companies we acquire or to successfully operate new businesses and we may be unable to profitably operate our expanded company. Moreover, any new businesses that we may acquire, once integrated with our existing operations, may not produce expected or intended results.
We may not be able to manage future growth effectively.
We expect to continue to experience significant growth. Should we keep growing rapidly, our financial, management and operating resources may not expand sufficiently to adequately manage our growth. If we are unable to manage our growth, our costs may increase disproportionately, our future revenues may not grow or may decline, and we may face dissatisfied customers. Our failure to manage our growth may adversely impact our business and the value of your investment.
Our ability to obtain continued financing is critical to the growth of our business. We will need additional financing to fund operations, which additional financing may not be available on reasonable terms or at all.
Our future growth, including the potential for future market expansion will require additional capital. We will consider raising additional funds through various financing sources, including the procurement of commercial debt financing. However, there can be no assurance that such funds will be available on commercially reasonable terms, if at all. If such financing is not available on satisfactory terms, we may be unable to execute our growth strategy, and operating results may be adversely affected. Any additional debt financing will increase expenses and must be repaid regardless of operating results and may involve restrictions limiting our operating flexibility.
Our ability to obtain financing may be impaired by such factors as the capital markets, both generally and specifically in our industry, which could impact the availability or cost of future financings. If the amount of capital we are able to raise from financing activities, together with our revenues from operations, are not sufficient to satisfy our capital needs, we may be required to decrease the pace of, or eliminate, our future product offerings and market expansion opportunities and potentially curtail operations.
Unfavorable publicity or consumer perception of our products and any similar products distributed by other companies could have a material adverse effect on our business.
We believe the nutritional supplement market is highly dependent upon consumer perception regarding the safety, efficacy and quality of nutritional supplements generally, as well as of products distributed specifically by us. Consumer perception of our products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, national media attention and other publicity regarding the consumption of nutritional supplements. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the nutritional supplement market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for our products and our business, results of operations, financial condition and cash flows. Our dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on us, the demand for our products, and our business, results of operations, financial condition and cash flows. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of nutritional supplements in general, or our products specifically, or associating the consumption of nutritional supplements with illness, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed.
Our success is linked to the size and growth rate of the vitamin, mineral and supplement market and an adverse change in the size or growth rate of that market could have a material adverse effect on us.
An adverse change in size or growth rate of the vitamin, mineral and supplement market could have a material adverse effect on us. Underlying market conditions are subject to change based on economic conditions, consumer preferences, and other factors that are beyond our control, including media attention and scientific research, which may be positive or negative.
General economic conditions, including a prolonged macroeconomic downturn, may negatively affect consumer purchases, which could adversely affect our sales, as well as our ability to access credit on terms previously obtained.
Our results are dependent on a number of factors impacting consumer spending, including general economic and business conditions; consumer confidence; wages and employment levels; the housing market; consumer debt levels; availability of consumer credit; credit and interest rates; fuel and energy costs; energy shortages; taxes; and general political conditions, both domestic and abroad. Consumer product purchases, including purchases of our products, may decline during recessionary periods. A prolonged downturn or an uncertain outlook in the economy may materially adversely affect our business, revenues and profits and the market price of our common stock, and we cannot be certain that funding for our capital needs will be available from our existing financial institutions and the credit markets if needed, and if available, to the extent required and on acceptable terms. If we cannot obtain funding when needed, in each case on acceptable terms, we may be unable to adequately fund our operating expenses and fund required capital expenditures, which may have an adverse effect on our revenues and results of operations.
We operate in highly competitive and fast-evolving industries, and our failure to compete effectively could affect our market share, financial condition, and growth prospects adversely.
The markets in which we operate are characterized by rapid technological changes, frequent new product introductions, established and emerging competition, extensive intellectual property disputes and litigation, price competition, aggressive marketing practices, evolving industry standards and changing customer preferences. Accordingly, our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies operating in rapidly changing and competitive markets.
The nutritional supplement industry is a large and growing industry and is highly fragmented in terms of both geographical market coverage and product categories. The market for nutritional supplements is highly competitive in all our channels of distribution. We compete with companies that may have broader product lines or larger sales volumes, or both, than we do, and our products compete with nationally advertised brand name products. These national brand companies have resources greater than ours. Numerous companies compete with us in the development, manufacture, and marketing of nutritional supplements worldwide. The market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. We also may face competition from low-cost entrants to the industry, including from international markets. Increased competition from companies that distribute through the wholesale channel, especially the private label market, could have a material adverse effect on our business, results of operations, financial condition and cash flows as these competitors may have greater financial and other resources available to them and possess extensive manufacturing, distribution, and marketing capabilities far greater than ours. We are also subject to competition in the attraction and retention of employees. Many of our competitors have greater financial resources and can offer employees compensation packages with which it is difficult for us to compete.
There has been an increase in the access and popularity of weight management medications which may negatively impact the consumer demand for nutritional supplements and weight management foods. As individuals migrate towards such prescription medications, and health insurance companies make such medications more affordable, there may be a decline in the demand for our products and services.
With our acquisition of Nexus in 2021, we entered the digital marketing industry as a way to promote the products and brands that we sell. We compete with other advertising service providers that may reach our target audience by means that are more effective than our services. Further, if such other providers of advertising have a long operating history, large product and service suites, more capital resources and broad international or local recognition, our operating results may be adversely affected if we cannot successfully compete.
The digital advertising market is rapidly developing. Accordingly, the development of the markets in which we operate makes it difficult to evaluate the viability and sustainability of our business and its acceptance by advertisers and clients. We cannot assure you that we will be profitable every year. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in operating losses.
We may not be able to compete effectively in some or all our markets, and our attempt to do so may require us to reduce our prices, which may result in lower margins. Failure to compete effectively could have a material adverse effect on our market share, business, results of operations, financial condition, cash flows and growth prospects.
Our major customers account for a significant portion of our consolidated net sales and the loss of any major customer could have a material adverse effect on our results of operations.
During fiscal 2023, Amazon and Boxout accounted for 31% and 31%, respectively, of our total revenues. We do not have a long-term contract with these major customers, and the loss of any major customer could have a material adverse effect on our results of operations. In addition, our results of operations and ability to service our debt obligations would be impacted negatively to the extent that any major customer is unable to make payments to us or does not make timely payments on outstanding accounts receivables.
Failure to develop new products and production technologies or to implement productivity and cost reduction initiatives successfully may harm our competitive position.
Our business depends significantly on the development of commercially viable new products as well as process technologies. If we are unsuccessful in developing new products and production processes in the future, our competitive position and results of operations may be negatively affected. However, as we invest in new technology, we face the risk of unanticipated operational or commercialization difficulties, including an inability to obtain necessary permits or governmental approvals, the development of competing technologies, failure of facilities or processes to operate in accordance with specifications or expectations, construction delays, cost over-runs, the unavailability of financing, required materials or equipment and various other factors. Likewise, our initiatives to improve productivity and performance and to generate cost savings may not be completed or beneficial or the estimated cost savings from such activities may not be realized.
Resources devoted to product innovation may not yield new products that achieve commercial success.
The development of new and innovative products requires significant investment in research and development and testing of new ingredients, formulas, and possibly new production processes. The research and development process can be expensive and prolonged and entails considerable uncertainty. Products may appear promising in development but fail to reach market within the expected time frame, or at all. We may face significant challenges with regard to a key product launch. Further, products also may fail to achieve commercial viability due to pricing competitiveness with other retailers, failure to timely bring the product to market, failure to differentiate the product with our competitors and other reasons. Finally, there is no guarantee that our development teams will be able to successfully respond to competitive products that could render some of our offerings obsolete. Development of a new product, from discovery through testing to the store shelf, typically takes between four to seven months, but may require an even longer timeline if clinical trials are involved. Each of these time periods can vary considerably from product to product and therefore the costs and risks of producing a commercially viable product can increase significantly as time passes.
Our failure to appropriately respond to changing consumer preferences and demand for new products and services could harm our customer relationships and product sales significantly.
The nutritional supplement industry is characterized by rapid and frequent changes in demand for products and new product introductions. Our failure to accurately predict these trends could negatively impact consumer opinion of us as a source for the latest products, which, in turn, could harm our customer relationships and cause decreases in our net sales. The success of our new product offerings depends upon a number of factors, including our ability to:
| ● | accurately anticipate customer needs; |
| ● | innovate and develop new products; |
| ● | successfully commercialize new products in a timely manner; |
| ● | price our products competitively; |
| ● | manufacture and deliver our products in sufficient volumes and in a timely manner; and |
| ● | differentiate our product offerings from those of our competitors. |
If any new products fail to gain market acceptance, are restricted by regulatory requirements or have quality problems, this would harm our results of operations. If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could be rendered obsolete, which could have a material adverse effect on our business, results of operations, financial condition and cash flows.
If we experience product recalls, we may incur significant and unexpected costs, and our business reputation could be adversely affected.
We may be exposed to product recalls and adverse public relations if our products are mislabeled or alleged to cause injury or illness, or if we are alleged to have violated governmental regulations. A product recall could result in substantial and unexpected expenditures, which would reduce operating profit and cash flow. In addition, a product recall may require significant management attention. Product recalls may hurt the value of our brands and lead to decreased demand for our products. Product recalls also may lead to increased scrutiny by federal, state or international regulatory agencies of our operations and increased litigation and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may incur material product liability claims, which could increase our costs and adversely affect our reputation, revenues, and operating income.
As a manufacturer and distributor of products designed for human consumption, we are subject to product liability claims if the use of our products is alleged to have resulted in injury. Our products consist of vitamins, minerals, dietary supplements, and other ingredients that are classified as foods and dietary supplements, and, in most cases, are not necessarily subject to pre-market regulatory approval in the United States. Some of our products contain innovative ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur. In addition, some of the products we sell are produced by third-party manufacturers. As a marketer of products manufactured by third parties, we also may be liable for various product liability claims for products we do not manufacture. We have been in the past, and may be in the future, subject to various product liability claims, including, among others, that our products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. A product liability claim against us could result in increased costs and could adversely affect our reputation with our customers, which, in turn, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Insurance coverage, even where available, may not be sufficient to cover losses we may incur.
Our business exposes us to the risk of liabilities arising from our operations. For example, we may be liable for claims brought by users of our products or by employees, customers or other third parties for personal injury or property damage occurring in the course of our operations. We seek to minimize these risks through various insurance contracts from third-party insurance carriers. However, our insurance coverage is subject to large individual claim deductibles, individual claim and aggregate policy limits, and other terms and conditions. We retain an insurance risk for the deductible portion of each claim and for any gaps in insurance coverage. We do not view insurance, by itself, as a material mitigant to these business risks.
We cannot assure that our insurance will be sufficient to cover our losses. Any losses that insurance does not substantially cover could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
We rely on our manufacturing operations to produce the vast majority of the nutritional supplements that we sell, and disruptions in our manufacturing system or losses of manufacturing certifications could affect our results of operations adversely.
We currently operate a manufacturing facility in Riviera Beach, Florida. All our domestic and foreign operations manufacturing products for sale to the United States are subject to GMPs promulgated by the FDA and other applicable regulatory standards, including in the areas of environmental protection and worker health and safety. Any significant disruption in our operations at the facility, including any disruption due to any regulatory requirement, could affect our ability to respond quickly to changes in consumer demand and could have a material adverse effect on our business, results of operations, financial condition, and cash flows. Additionally, we may be exposed to risks relating to the opening of new facilities or closing existing facilities that may cause a disruption in our operations. Although we have implemented GMPs in our facility, there can be no assurance that products manufactured in our plant will not be contaminated or otherwise fail to meet our quality standards. Any such contamination or other quality failures could result in costly recalls, litigation, regulatory actions, or damage to our reputation, which could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
We are also dependent on certain third-party contract manufacturers and suppliers.
Some of our own brands of vitamins and supplements, are produced by third party contract manufacturers. We also purchase certain important ingredients and raw materials from third-party suppliers. The principal raw materials required in our operations are vitamins, minerals, herbs, gelatin, and packaging components. Real or perceived quality control problems with products manufactured by contract manufacturers or raw materials outsourced from certain suppliers could negatively impact consumer confidence in our products or expose us to liability. In addition, disruption in the operations of any such manufacturer or supplier or material increases in the price of raw materials, for any reason, such as changes in economic and political conditions, tariffs, trade disputes, regulatory requirements, import restrictions, loss of certifications, power interruptions, fires, hurricanes, drought or other climate-related events, war or other events, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
Natural disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts and global political events could cause permanent or temporary facility closures, impair our ability to purchase, receive or replenish raw materials or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreak, terrorist acts or disruptive global political events, such as civil unrest in locations where our facilities, contract manufacturers or suppliers are located, or similar disruptions could adversely affect our operations and financial performance. To the extent these events result in the closure of one or more of our manufacturing facilities or our corporate headquarters, or impact one or more of our contract manufacturers or key suppliers, our operations and financial performance could be materially adversely affected through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from some local and overseas suppliers, the temporary disruption in the transport of goods from overseas, delay in the delivery of goods to our customers, the temporary reduction in the availability of our products, expiration of inventory, future long-lived asset impairment charges and disruption to our information systems. These events also could have indirect consequences, such as increases in the cost of insurance, if they were to result in significant loss of property or other insurable damage.
An increase in the price and shortage of supply of key raw materials could adversely affect our business.
Our products are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, the costs to manufacture our products or to purchase products from our contract manufacturers could increase significantly and we may not be able to pass on such increases to our customers. Additionally, in the event any of our, or our contract manufacturer’s, third-party suppliers or vendors become unable or unwilling to continue to provide raw materials in the required volumes and quality levels or in a timely manner, we, or our contract manufacturers, would be required to identify and obtain acceptable replacement supply sources. If we, or they, are unable to identify and obtain alternative supply sources in a timely manner or at all, our business could be adversely affected. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our results of operations and financial condition. Events such as COVID-19, the threat of political or social unrest, or the perceived threat thereof, may also have a significant impact on raw material prices and transportation costs for our products. In addition, the interruption in supply of certain key raw materials essential to the manufacturing of our products may have an adverse impact on us and our suppliers’ ability to provide us with the necessary products needed to maintain our customer relationships and an adequate level of sales.
General trade tensions between the U.S. and China have been escalating since 2018, with multiple rounds of U.S. tariffs on Chinese goods taking effect, with some subsequently being de-escalated. Furthermore, China or other countries may institute retaliatory trade measures in response to existing or future tariffs imposed by the U.S. that could have a negative impact on our business. If any of these events continue as described, we may need to seek alternative suppliers or vendors, raise prices, or make changes to our operations, any of which could have a material adverse effect on our sales and profitability, results of operations and financial condition.
In addition, the continuing conflicts in Ukraine and the Middle East may have impacts on our ability to obtain certain raw materials or raise prices on certain raw materials. These regions are significant worldwide suppliers for certain ingredients used for our production. If the availability of the ingredients becomes negatively effected, it may be more difficult or expensive for us to obtain these ingredients.
Our expansion into new business lines and services may result in unseen risks, challenges and uncertainties.
As a result of our acquisition of Nexus in November 2021, we have entered the digital marketing business as a way to promote the products and brands that we sell. Such acquisition may result in unseen risks, challenges and uncertainties. We may incur additional capital expenditure to support the expansion of our business and there is no guarantee that we may increase our revenues generated from such new business. Also, our failure to manage costs and expenses and evaluate consumer demands with respect to such new business could materially and adversely affect the prospects of us achieving overall profitability of and recouping our investments in this new business line. Moreover, this new business line may require significant managerial, financial, operational and other resources, as well as the smooth cooperation with our company. We may also face higher regulatory, legal and counterparty risks from entering this business. If we fail to manage the development of this new business line successfully, our growth potential, business and results of operations may be materially and adversely affected.
Declines in foot traffic, rising real estate prices and other costs and risks relating to operating a brick and mortar retail store could affect our results.
On August 24, 2021, we established Smart for Life Canada Inc. as a wholly owned subsidiary of Doctors Scientific Organica, LLC in Canada. This subsidiary sells retail products through a retail store location in Montreal Canada and the same location also acts as distribution center for our international direct to consumer and big box customers.
The success of our retail store is affected by (1) the location of the store; (2) surrounding tenants or vacancies; (3) increased competition in the area where the store is located; (4) the amount spent on advertising and promotion to attract consumers to the store; and (5) a shift towards online shopping resulting in a decrease in retail store traffic. Declines in consumer traffic could have a negative impact on our net sales and could materially adversely affect our financial condition and results of operations. Furthermore, declines in traffic could result in store impairment charges if expected future cash flows of the related asset group do not exceed the carrying value.
We rent this store under a three-year lease agreement ending in September 2024. If we fail to negotiate appropriate terms for new leases or lease renewals, we may incur lease costs that are excessive and cause operating margins to be below acceptable levels. We may also make term commitments that are too long or too short, without the option to exit early or extend. Factors such as the condition of local property markets, availability of lease financing, taxes, zoning and environmental issues, and competitive actions may impact the availability of, and our ability to successfully negotiate, leases. Furthermore, the success of the store depends on a number of factors, including the success of the shopping center where our store is located, consumer demographics and consumer shopping patterns. These factors cannot be predicted with complete accuracy. If we fail to profitably operate this new store, our financial performance could be adversely affected.
Our success is dependent on the accuracy, reliability, and proper use of sophisticated and dependable information processing systems and management information technology and any interruption in these systems could have a material adverse effect on our business, financial condition, and results of operations.
Our success is dependent on the accuracy, reliability, and proper use of sophisticated and dependable information processing systems and management information technology. Our information technology systems are designed and selected to facilitate order entry and customer billing, maintain customer records, accurately track purchases, manage accounting, finance, and manufacturing operations, generate reports, and provide customer service and technical support. Any interruption in these systems or any interruption associated with the transition of these systems to a new information technology platform could have a material adverse effect on our business, financial condition, and results of operations.
System interruptions or security breaches may affect sales.
Customer access to, and ability to use, our websites affect our sales. If we are unable to maintain and continually enhance the efficiency of our systems, we could experience system interruptions or delays that could affect our operating results negatively. In addition, we could be liable for breaches of security on our websites, loss or misuse of our customers’ personal information or payment data. Although we have developed systems and processes that are designed to protect consumer information and prevent fraudulent credit card transactions and other security breaches, failure to prevent or mitigate such fraud or breaches may negatively affect our operating results.
We must successfully maintain and/or upgrade our information technology systems, and our failure to do so could have a material adverse effect on our business, financial condition, or results of operations.
We rely on various information technology systems to manage our operations. Recently, we have implemented, and we continue to implement, modifications and upgrades to such systems and acquired new systems with new functionality. These types of activities subject us to inherent costs and risks associated with replacing and changing these systems, including impairment of our ability to fulfill customer orders, potential disruption of our internal control structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate the new systems, demands on management time and other risks and costs of delays or difficulties in transitioning to or integrating new systems into our current systems. These implementations, modifications and upgrades may not result in productivity improvements at a level that outweighs the costs of implementation, or at all. In addition, the difficulties with implementing new technology systems may cause disruptions in our business operations and have a material adverse effect on our business, financial condition or results of operations.
Privacy protection is increasingly demanding, and we may be exposed to risks and costs associated with security breaches, data loss, credit card fraud and identity theft that could cause us to incur unexpected expenses and loss of revenue, suffer reputational harm with our customers, as well as other risks.
The protection of customer, employee, vendor and other business data is critical to us. We receive confidential customer data, including payment cards and personally identifiable information, in the normal course of customer transactions. In order for our sales channels to function, we and other parties involved in processing customer transactions must be able to transmit confidential information, including credit card information, securely over public networks. While we have taken significant steps to protect customer and confidential information, the intentional or negligent actions of employees, business associates or third parties may undermine our security measures and result in unauthorized parties obtaining access to our data systems and misappropriating confidential data. There can be no assurance that advances in computer capabilities, new discoveries in the field of cryptography or other developments will prevent a compromise of our customer transaction processing capabilities and personal data. Because the techniques used to obtain unauthorized access to, disable, degrade, or sabotage systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. Any compromise of our data security could result in a violation of applicable privacy and other laws or standards, significant legal and financial exposure beyond the scope or limits of our insurance coverage, interruption of our operations, increased operating costs associated with remediation, equipment acquisitions or disposal, added personnel, and a loss of confidence in our security measures, which could harm our business or investor confidence. Any security breach involving the misappropriation, loss or other unauthorized disclosure of sensitive or confidential information could attract a substantial amount of media attention, damage our reputation, expose us to risk of litigation and material liability, disrupt our operations and harm our business.
Federal, state, provincial and international laws and regulations govern the collection, retention, sharing and security of data that we receive from and about our employees, customers, and vendors. The regulatory environment surrounding information security and privacy has been increasingly demanding in recent years, including the recent implementation of the California Consumer Privacy Act. In Canada, we are subject to Canada’s Personal Information and Protection of Electronic Documents Act, which provides Canadian residents with privacy protections and sets out rules for how companies may collect, use and disclose personal information in the course of commercial activities. The costs of compliance with, and other burdens imposed by, these and other international data privacy and security laws may limit our business and services and could have a materially adverse impact on our business.
We believe that we are in material compliance with all laws, regulations and self-regulatory regimes that are applicable to us. However, the laws, regulations, and self-regulatory regimes may be modified, and new laws may be enacted in the future that may apply to us and affect our business. Further, data protection authorities may interpret existing laws in new ways. We may deploy new services from time to time, which may also require us to change our compliance practices. Any such developments (or developments stemming from enactment or modification of other laws) or the failure to anticipate accurately the application or interpretation of these laws could create liability for us, result in adverse publicity, increase our future compliance costs, make our products and services less attractive to our customers, or cause us to change or limit our business practices, and materially affect our business and operating results. Further, any failure or perceived failure by us or third-party service providers to comply with international data privacy and security laws may lead to regulatory enforcement actions, fines, private lawsuits or reputational damage.
We may not be able to protect our intellectual property rights.
We regard our trademarks, service marks, copyrights, patents, trade secrets, proprietary technologies, domain names and similar intellectual property as important to our success. We rely on trademark, copyright and patent law, trade secret protection and confidentiality agreements with our future employees, consultants, vendors, customers and others to protect our proprietary rights. Many of the trademarks that we use contain words or terms having a somewhat common usage and, as a result, we may have difficulty registering them in certain jurisdictions. We have not yet obtained registrations for our most important marks. If other companies have registered or have been using in commerce similar trademarks for products similar to ours, we may have difficulty in registering, or enforcing an exclusive right to use, our marks.
There can be no assurance that our efforts to protect our proprietary rights will be sufficient or effective, that any pending or future patent and trademark applications will lead to issued patents and registered trademarks in all instances, that others will not develop or patent similar or superior technologies, products, or that our patents, trademarks, and other intellectual property will not be challenged, invalidated, misappropriated, or infringed by others. Additionally, the intellectual property laws and enforcement practices of other countries in which our product is or may in the future be offered may not protect our products and intellectual property rights to the same extent as the laws of the United States. If we are unable to protect our intellectual property from unauthorized use, our brand image may be harmed, and our business and results of operations may suffer.
Assertions by third parties of infringement, misappropriation, or other violation by us of their intellectual property rights could result in significant costs and substantially harm our business and operating results.
In recent years, there has been significant litigation involving intellectual property rights in many technology-based industries. Any infringement, misappropriation, or related claims, whether or not meritorious, is time-consuming, diverts technical and management personnel and is costly to resolve. As a result of any such dispute, we may have to develop non-infringing technology, pay damages, enter into royalty or licensing agreements, cease providing our product or take other actions to resolve the claims. These actions, if required, may be costly or unavailable on terms acceptable to us. Any of these events could result in increases in operating expenses, limit our product offerings or result in a loss of business.
We may be required to indemnify our vendors and/or customers, the payment of which could have a material adverse effect on our business, financial condition, and operating results.
We provide certain rights of indemnification to our vendors and/or customers in certain circumstances. If any plaintiff is successful in certifying a class and thereafter prevailing on the merits of their complaint, such an adverse result could have a material adverse effect on us. In addition, due to the nature and scope of the indemnity and defense we will likely need to provide, the legal fees associated with such indemnification could be significant enough to have a material adverse effect on our cash flows until such matters are fully and finally resolved.
Compliance with new and existing laws and governmental regulations could increase our costs significantly and adversely affect our results of operations.
The processing, formulation, safety, manufacturing, packaging, labeling, advertising, and distribution of our products are subject to federal laws and regulation by one or more federal agencies, including the FDA, the FTC, the CPSC, the USDA and the EPA. These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products are sold. Government regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost revenues and increased costs to us. For instance, the FDA regulates, among other things, the composition, safety, manufacture, labeling and marketing of dietary ingredients and dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for human use). Dietary supplements and dietary ingredients that do not comply with FDA’s regulations and/or the Dietary Supplement Health and Education Act of 1994 will be deemed adulterated or misbranded. Manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded, and the FDA may take enforcement action against any adulterated or misbranded dietary supplement on the market. The FDA has broad enforcement powers. If we violate applicable regulatory requirements, the FDA may bring enforcement actions against us, which could have a material adverse effect on our business, prospects, financial condition, and results of operations. The FDA may not accept the evidence of safety for any new ingredient that we may wish to market, may determine that a particular supplement or ingredient presents an unacceptable health risk based on the required submission of serious adverse events or other information, and may determine that a particular claim or statement of nutritional value that we use to support the marketing of a supplement is an impermissible drug claim, is not substantiated, or is an unauthorized version of a “health claim.” See Item 1 “Business—Regulation—Food and Drug Administration” for additional information. Any of these actions could prevent us from marketing particular nutritional supplement products or making certain claims or statements with respect to those products. The FDA could also require us to remove a particular product from the market. Any future recall or removal would result in additional costs to us, including lost revenues from any products that we are required to remove from the market, any of which could be material. Any product recalls or removals could also lead to an increased risk of litigation and liability, substantial costs, and reduced growth prospects.
Additional or more stringent laws and regulations of dietary supplements and other products have been considered from time to time. These developments could require reformulation of some products to meet new standards, recalls or discontinuance of some products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of some products, additional or different labeling, additional scientific substantiation, or other new requirements. Any of these developments could increase our costs significantly. In addition, regulators’ evolving interpretation of existing laws could have similar effects.
Our failure to comply with FTC regulations could result in substantial monetary penalties and could adversely affect our operating results.
The FTC exercises jurisdiction over the advertising of dietary supplements and requires that all advertising to consumers be truthful and non-misleading. The FTC actively monitors the dietary supplement space and has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. Failure to comply with applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
Our operations are subject to environmental and health and safety laws and regulations that may increase our cost of operations or expose us to environmental liabilities.
We are subject, directly or indirectly, to numerous federal, state, local and foreign environmental and health and safety laws and regulations governing our operations, including the handling, transportation and disposal of our non-hazardous and hazardous substances and wastes, as well as emissions and discharges from our operations into the environment, including discharges to air, surface water and groundwater. Failure to comply with such laws and regulations could result in costs for remedial actions, penalties, or the imposition of other liabilities. New laws, changes in existing laws or the interpretation thereof, or the development of new facts or changes in their processes could also cause us to incur additional capital and operating expenditures to maintain compliance with environmental laws and regulations and environmental permits. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of our operations, including operations at our manufacturing facility. We also could incur monetary fines, civil or criminal sanctions, third-party claims or cleanup or other costs as a result of violations of or liabilities under such requirements.
We also are subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties, or for properties to which substances or wastes that were sent in connection with current or former operations at our facilities. The presence of contamination from such substances or wastes could also adversely affect our ability to sell or lease our properties, or to use them as collateral for financing.
Failure to comply with federal, state, and international privacy, data protection, marketing and consumer protection laws, regulations and industry standards, or the expansion of current or the enactment or adoption of new privacy, data protection, marketing and consumer protection laws, regulations or industry standards, could adversely affect our business.
We are subject to a variety of federal, state, and foreign laws, regulations and industry standards regarding privacy, data protection, data security, marketing and consumer protection, which address the collection, storing, sharing, using, processing, disclosure and protection of data relating to individuals, as well as the tracking of consumer behavior and other consumer data. We are also subject to laws, regulations and industry standards relating to endorsements and influencer marketing. Many of these laws, regulations and industry standards are changing and may be subject to differing interpretations, are costly to comply with or inconsistent among jurisdictions. For example, the FTC expects companies like ours to comply with guidelines issued under the Federal Trade Commission Act that govern the collection, use, disclosure, and storage of consumer information, and establish principles relating to notice, consent, access and data integrity and security. The laws and regulations in many foreign countries relating to privacy, data protection, data security, marketing and consumer protection often are more restrictive than in the United States and may in some cases be interpreted to have a greater scope. Additionally, the laws, regulations, and industry standards, both foreign and domestic, relating to privacy, data protection, data security, marketing and consumer protection are dynamic and may be expanded or replaced by new laws, regulations or industry standards.
We strive to comply with applicable laws, policies, contractual and other legal obligations, and certain applicable industry standards of conduct relating to privacy, data security, data protection, marketing and consumer protection. However, these obligations and standards of conduct often are complex, vague, and difficult to comply with fully, and it is possible that these obligations and standards of conduct may be interpreted and applied in new ways and/or in a manner that is inconsistent with each other or that new laws, regulations, or other obligations may be enacted. It is possible that our practices may be argued or held to conflict with applicable laws, policies, contractual or other legal obligations, or applicable industry standards of conduct relating to privacy, data security, data protection, marketing or consumer protection. Any failure, or perceived failure, by us to comply with our posted privacy policies or with any data-related consent orders, the FTC, other regulatory requirements or orders or other federal, state or, as we continue to expand internationally, international privacy, data security, data protection, marketing or consumer protection-related laws, regulations, contractual obligations or self-regulatory principles or other industry standards could result in claims, proceedings or actions against us by governmental entities or others or other liabilities or could result in a loss of consumers. Any of these circumstances could adversely affect our business.
We expect that there will continue to be new proposed laws, regulations and industry standards concerning privacy, data protection and information security in the United States and other jurisdictions, and we cannot yet determine the impact such future laws, regulations and standards may have on our business. For instance, with the increased focus on the use of data for advertising, the anticipation and expectation of future laws, regulations, standards, and other obligations could impact us. In addition, as we expand our data analytics and other data-related product offerings there may be increased scrutiny on our use of data and we may be subject to new and unexpected regulations. Future laws, regulations, standards, and other obligations could, for example, impair our ability to collect or use information that we utilize to provide targeted digital promotions and media to consumers, thereby impairing our ability to maintain and grow our total customers and increase revenues. Future restrictions on the collection, use, sharing or disclosure of our users’ data or additional requirements for express or implied consent of users for the use and disclosure of such information could require us to modify our solutions, possibly in a material manner, and could limit our ability to develop or outright prohibit new solutions and features. Any such new laws, regulations, other legal obligations or industry standards, or any changed interpretation of existing laws, regulations or other standards may require us to incur additional costs and restrict our business operations. If our measures fail to comply with current or future laws, regulations, policies, legal obligations, or industry standards relating to privacy, data protection, data security, marketing or consumer protection, we may be subject to litigation, regulatory investigations, fines or other liabilities, as well as negative publicity and a potential loss of business. Moreover, if future laws, regulations, other legal obligations or industry standards, or any changed interpretations of the foregoing limit our ability to store, process and share personally identifiable information or other data, demand for our products could decrease, our costs could increase, our revenue growth could slow, and our business, financial condition and operating results could be harmed.
We are exposed to potential liability for information on our customers’ websites and for products and services sold through their websites and we may incur significant costs and damage to our reputation as a result of defending against such potential liability.
We are exposed to potential liability for information on our customers’ websites. We could be exposed to liability with respect to such third-party information such as their products, links to third-party websites, advertisements and content provided by customers. Among other things, we may face assertions that, by directly or indirectly providing such third-party content or links to other websites, we should be liable for defamation, negligence, copyright or trademark infringement, or other actions by parties providing such content or operating those websites. We may also face assertions that content on our publishers and advertisers’ websites, including statistics or other data we compile internally, or information contained in websites linked to our websites contains false information, errors or omissions, and users and our customers could seek damages for losses incurred as a result of their reliance upon or otherwise relating to incorrect information. We may also be subject to fines and other sanctions by the government for such incorrect information. In addition, our services could be used as a platform for fraudulent transactions and third party products and services sold through us may be defective. The measures we take to guard against liability for third-party content, information, products and services may not be adequate to exonerate us from relevant civil and other liabilities.
Any such claims, with or without merit, could be time-consuming to defend and result in litigation and significant diversion of management’s attention and resources. Even if these claims do not result in liability to us, we could incur significant costs in investigating and defending against these claims and suffer damage to our reputation.
If the use of third-party cookies or other tracking technology is rejected by Internet users, restricted by third parties outside of our control, or otherwise subject to unfavorable regulation, our performance could decline and we could lose customers and revenue.
We use a number of technologies to collect information about our customers. For instance, we use small text files (referred to as “cookies”), placed through an Internet browser on an Internet user’s machine which corresponds to a data set that we keep on our servers, to gather important data. Our cookies collect anonymous information, such as when an Internet user views an advertisement, clicks on an advertisement, or visits one of our advertisers’ websites. In some countries, including countries in the European Economic Area, this information may be considered personal information under applicable data protection laws. On mobile devices, we may also obtain location-based information about the user’s device through our cookies or other tracking technologies. We use these technologies to achieve our campaign goals, to ensure that the same Internet user does not unintentionally see the same media too frequently, to report aggregate information regarding the performance of our digital promotions and marketing campaigns, and to detect and prevent fraudulent activity throughout our network.
Cookies may easily be deleted or blocked by Internet users. All of the most commonly used Internet browsers (including Chrome, Firefox, Internet Explorer, and Safari) allow Internet users to prevent cookies from being accepted by their browsers. Internet users can also delete cookies from their computers at any time. Some Internet users also download “ad blocking” software that prevents cookies from being stored on a user’s computer. If more Internet users adopt these settings or delete their cookies more frequently than they currently do, our business could be harmed. In addition, the Safari and Firefox browsers blocks third-party cookies by default, and other browsers may do so in the future. Unless such default settings in browsers were altered by Internet users to permit the placement of third-party cookies, we would be able to set fewer of our cookies in users’ browsers, which could adversely affect our business. In addition, companies such as Google have publicly disclosed their intention to move away from cookies to another form of persistent unique identifier, or ID, to identify individual Internet users or Internet-connected devices in the bidding process on advertising exchanges. If companies do not use shared IDs across the entire ecosystem, this could have a negative impact on our ability to find the same anonymous user across different web properties, and reduce the effectiveness of our marketing efforts.
In addition, in the European Union, or EU, Directive 2009/136/EC, commonly referred to as the “Cookie Directive,” directs EU member states to ensure that collecting information on an Internet user’s computer, such as through a cookie, is allowed only if the Internet user has appropriately given his or her prior freely given, specific, informed and unambiguous consent. Similarly, this Directive, which also contains specific rules for the sending of marketing communications, limits the use of marketing texts messages and e-mails. Additionally, an e-Privacy Regulation, which will replace the Cookie Directive with requirements that could be stricter in certain respects, apply directly to activities within the EU without the need to be transposed in each member state’s law, and could impose stricter requirements regarding the use of cookies and marketing e-mails and text messages and additional penalties for noncompliance, has been proposed, although at this time it is unclear whether it will be approved as it is currently drafted or when its requirements will be effective. We may experience challenges in obtaining appropriate consent to our use of cookies from consumers or to send marketing communications to consumers within the EU, which may affect our ability to run promotions and our operating results and business in European markets, and we may not be able to develop or implement additional tools that compensate for the lack of data associated with cookies. Moreover, even if we are able to do so, such additional tools may be subject to further regulation, time consuming to develop or costly to obtain, and less effective than our current use of cookies.
Economic, political and other risks associated with our international operations could adversely affect our revenues and international growth prospects.
On August 24, 2021, we established Smart for Life Canada Inc. as a wholly owned subsidiary of DSO in Canada. This subsidiary sells retail products through a retail store location in Montreal Canada and the same location also acts as distribution center for our international direct to consumer and big box customers. We maintain inventory and employees at this location. We have sales outside of the United States. For fiscal 2023 and 2022, international sales represented approximately 4% and 10%, respectively, of our total revenues.
We intend to expand our international presence as part of our business strategy. Our international operations are subject to a number of risks inherent to operating in foreign countries, and any expansion of our international operations will amplify the effects of these risks, which include, among others:
| ● | differences in culture, economic and labor conditions and practices; |
| ● | the policies of the U.S. and foreign governments; |
| ● | disruptions in trade relations and economic instability; |
| ● | differences in enforcement of contract and intellectual property rights; |
| ● | social and political unrest; |
| ● | natural disasters, terrorist attacks, pandemics or other catastrophic events; |
| ● | complex, varying and changing government regulations and legal standards and requirements, particularly with respect to tax regulations, price protection, competition practices, export control regulations and restrictions, customs and tax requirements, immigration, anti-boycott regulations, data privacy, intellectual property, anti-corruption and environmental compliance, including the Foreign Corrupt Practices Act; |
| ● | greater difficulty enforcing intellectual property rights and weaker laws protecting such rights; and |
| ● | greater difficulty in accounts receivable collections and longer collection periods. |
We are also affected by domestic and international laws and regulations applicable to companies doing business abroad or importing and exporting goods and materials. These include tax laws, laws regulating competition, anti-bribery/anti-corruption and other business practices, and trade regulations, including duties and tariffs. Compliance with these laws is costly, and future changes to these laws may require significant management attention and disrupt our operations. Additionally, while it is difficult to assess what changes may occur and the relative effect on our international tax structure, significant changes in how U.S. and foreign jurisdictions tax cross-border transactions could materially and adversely affect our results of operations and financial position.
Our results of operations and financial position are also impacted by changes in currency exchange rates. Unfavorable currency exchange rates between the US Dollar and foreign currencies, particularly the Canadian dollar, could adversely affect us in the future. Fluctuations in currency exchange rates may present challenges in comparing operating performance from period to period.
There are other risks that are inherent in our Canadian and other international operations, including the potential for changes in socio-economic conditions, laws and regulations, including, among others, competition, import, export, labor and environmental, health and safety laws and regulations, and monetary and fiscal policies, protectionist measures that may prohibit acquisitions or joint ventures, or impact trade volumes, unsettled political conditions; government-imposed plant or other operational shutdowns, backlash from foreign labor organizations related to our restructuring actions, corruption; natural and man-made disasters, hazards and losses, violence, civil and labor unrest, and possible terrorist attacks.
Additionally, if the opportunity arises, we may expand our operations into new and high-growth international markets. However, there is no assurance that we will expand our operations in such markets in our desired time frame. To expand our operations into new international markets, we may enter into business combination transactions, make acquisitions or enter into strategic partnerships, joint ventures or alliances, any of which may be material. We may enter into these transactions to acquire other businesses or products to expand our products or take advantage of new developments and potential changes in the industry. Our lack of experience operating in new international markets and our lack of familiarity with local economic, political and regulatory systems could prevent us from achieving the results that we expect on our anticipated time frame or at all. If we are unsuccessful in expanding into new or high-growth international markets, it could adversely affect our operating results and financial condition.
Our international operations require us to comply with anti-corruption laws and regulations of the U.S. government and various international jurisdictions in which we do business.
Doing business on a worldwide basis requires us to comply with the laws and regulations of the U.S. government and various international jurisdictions, and our failure to successfully comply with these rules and regulations may expose us to liabilities. These laws and regulations apply to companies, individual directors, officers, employees, and agents, and may restrict our operations, trade practices, investment decisions and partnering activities. In particular, our international operations are subject to U.S. and foreign anti-corruption laws and regulations, such as the Foreign Corrupt Practices Act, or the FCPA. The FCPA prohibits us from providing anything of value to foreign officials for the purposes of influencing official decisions or obtaining or retaining business or otherwise obtaining favorable treatment, and requires us to maintain adequate record- keeping and internal accounting practices to accurately reflect our transactions. As part of our business, we may deal with state-owned business enterprises, the employees and representatives of which may be considered foreign officials for purposes of the FCPA. In addition, some of the international locations in which we operate lack a developed legal system and have elevated levels of corruption. As a result of the above activities, we are exposed to the risk of violating anti-corruption laws. Violations of these legal requirements are punishable by criminal fines and imprisonment, civil penalties, disgorgement of profits, injunctions, debarment from government contracts as well as other remedial measures. We have established policies and procedures designed to assist us and our personnel in complying with applicable U.S. and international laws and regulations. However, there can be no assurance that our policies and procedures will effectively prevent us from violating these regulations in every transaction in which we may engage, and such a violation could adversely affect our reputation, business, financial condition and results of operations.
Our success depends on the experience and skill of our board of directors, executive officers and key personnel, whom we may not be able to retain and we may not be able to hire enough additional personnel to meet our needs.
We are dependent on Alfonso J. Cervantes, Jr. (Executive Chairman), Darren C. Minton (Chief Executive Officer and President), and Alan B. Bergman (Chief Financial Officer). There can be no assurance that they will continue to be employed by us for a particular period of time. The loss of any member of the board of directors or executive officer or advisors could harm our business, financial condition, cash flow and results of operations.
The success of our strategy will depend on a well-defined management structure and the availability of a management team with proven competencies in the identification, acquisition and integration of complementary companies and assets. To implement our business plan, we will need to keep the personnel that we currently have and, if our business is to grow as planned, we will need additional personnel. We cannot assure you that we will be successful in retaining our present team or in attracting and retaining additional personnel. If we are unable to attract and retain key personnel or are unable to do so in a cost-effective manner, our business may be materially and adversely affected.
Although dependent on certain key personnel, we do not have any key man life insurance policies on any such people.
We are dependent on our management team to conduct our operations and execute our business plan, however, we have not purchased any insurance policies with respect to the management in the event of the death or disability of any of our key managers. Therefore, if any of the members of our management team dies or becomes disabled, we will not receive any compensation to assist with his absence.
We may be a party to lawsuits that arise in the ordinary course of business.
We may be a party to lawsuits in the future (including product liability, false advertising, and intellectual property claims) that arise in the ordinary course of business. The possibility of such litigation, and its timing, is in large part outside our control. It is possible that future litigation could arise that could have material adverse effects on us.
We have identified material weaknesses in our internal control over financial reporting. If we fail to develop or maintain an effective system of internal controls, we may not be able to accurately report our financial results and prevent fraud. As a result, current and potential stockholders could lose confidence in our financial statements, which would harm the trading price of our common stock.
Companies that file reports with the Securities and Exchange Commission, or the SEC, including us, are subject to the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or SOX 404. SOX 404 requires management to establish and maintain a system of internal control over financial reporting and annual reports on Form 10-K filed under the Securities Exchange Act of 1934, as amended, or the Exchange Act, to contain a report from management assessing the effectiveness of a company’s internal control over financial reporting. Separately, under SOX 404, as amended by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, public companies that are large accelerated filers or accelerated filers must include in their annual reports on Form 10-K an attestation report of their regular auditors attesting to and reporting on management’s assessment of internal control over financial reporting. Non-accelerated filers and smaller reporting companies, like us, are not required to include an attestation report of their auditors in annual reports.
A report of our management is included under Item 9A. “Controls and Procedures.” We are a smaller reporting company and, consequently, are not required to include an attestation report of our auditor in our annual report. However, if and when we become subject to the auditor attestation requirements under SOX 404, we can provide no assurance that we will receive a positive attestation from our independent auditors.
During its evaluation of the effectiveness of internal control over financial reporting as of December 31, 2023, management identified material weaknesses as described under Item 9A. “Controls and Procedures.” We are undertaking remedial measures, which measures will take time to implement and test, to address these material weaknesses. There can be no assurance that such measures will be sufficient to remedy the material weaknesses identified or that additional material weaknesses or other control or significant deficiencies will not be identified in the future. If we continue to experience material weaknesses in our internal controls or fail to maintain or implement required new or improved controls, such circumstances could cause us to fail to meet our periodic reporting obligations or result in material misstatements in our financial statements, or adversely affect the results of periodic management evaluations and, if required, annual auditor attestation reports. Each of the foregoing results could cause investors to lose confidence in our reported financial information and lead to a decline in our stock price.
Risks Related to Ownership of Our Common Stock
We may not be able to maintain a listing of our common stock on Nasdaq.
Our common stock is currently listed on the Nasdaq Capital Market. We must meet certain financial and liquidity criteria to maintain the listing of our common stock on The Nasdaq Stock Market LLC, or Nasdaq. If we fail to meet any of Nasdaq’s continued listing standards or we violate Nasdaq listing requirements, our common stock may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing.
On December 5, 2023, we received a notification letter from Nasdaq notifying us that we were not in compliance with the Nasdaq stockholders’ equity requirement of $2,500,000 or the alternative criteria for continued listing on the Nasdaq Capital Market as set forth in Listing Rule 5550(b)(1), or the Equity Rule, given that our Form 10-Q for the period ended September 30, 2023 evidenced stockholders’ equity of $951,836, and that the staff of Nasdaq had determined to delist our securities from Nasdaq unless we requested an appeal of the determination. Based on the foregoing, we timely requested a hearing before a Nasdaq hearings panel. The hearing request stayed the delisting pending the conclusion of the hearings process.
On January 5, 2024, we received an additional notification letter from Nasdaq notifying us that we were not in compliance with the requirement to hold an annual meeting of shareholders since we did not hold an annual meeting in 2023. The letter stated that the hearings panel will consider this matter in rendering a determination regarding our continued listing on Nasdaq.
At the hearing held on March 12, 2024, we presented our plan for regaining compliance with the Equity Rule and presented our views with respect to the additional deficiency relating to the annual meeting, and requested a further extension so that we may complete the execution of our plan. Although we believe our plan will be sufficient to enable us to regain compliance, no assurance can be provided that Nasdaq will ultimately accept our plan or that we will ultimately regain compliance with the Equity Rule. As of the date of this report, we have not received a determination from the hearings panel. Notably, on March 7, 2024, we filed a Form 8-K disclosing that as a result of our restructuring plan, including recapitalization with equity and debt financings, the sale of certain non-performing assets and the liquidation of our senior debt facility, we had stockholder’s equity of over $2.5 million. In addition, through a series of debt to equity conversions and other activities, our stockholders’ equity as of September 20, 2024 was approximately $9,459,834. Key actions taken to achieve this include:
| Stockholders’ deficit as of December 31, 2023 | | $ | (6,387,454 | ) |
| | | | | |
| Conversion of debt and interest to stock (common and preferred) | | $ | 15,318,716 | |
| Stock issued for professional services | | | 91,250 | |
| Gain on sale of BSNM | | | 1,376,000 | |
| Warrant exercises | | | 817,322 | |
| Total restructurings | | $ | 17,603,288 | |
| | | | | |
| Net loss as of September 20, 2024 (estimated) | | | (1,756,000 | ) |
| Stockholders’ equity as of September 20, 2024 (estimated) | | $ | 9,459,834 | |
A delisting of our common stock from Nasdaq may materially impair our stockholders’ ability to buy and sell our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock. The delisting of our common stock could significantly impair our ability to raise capital and the value of your investment.
The market price of our stock may be highly volatile, and you could lose all or part of your investment.
The market for our common stock may be characterized by significant price volatility when compared to the shares of larger, more established companies that have large public floats, and our stock price will likely be more volatile than the shares of such larger, more established companies for the indefinite future. The stock market in general has recently been highly volatile. Furthermore, there have been recent instances of extreme stock price run-ups followed by rapid price declines and stock price volatility following a number of recent initial public offerings, particularly among companies with relatively smaller public floats. We also experienced such volatility following our initial public offering in February 2022 and may continue to experience such volatility, which may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock.
The market price of our common stock is likely to be volatile due to a number of factors. First, as noted above, our common stock is likely to be more sporadically and thinly traded compared to the shares of such larger, more established companies. The price for our common stock could, for example, decline precipitously in the event that a large number of shares is sold on the market without commensurate demand. Secondly, we are a speculative or “risky” investment due to our lack of profits to date. As a consequence of this enhanced risk, more risk-adverse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a larger, more established company that has a large public float. Many of these factors are beyond our control and may decrease the market price of our common stock regardless of our operating performance. The market price of our common stock could also be subject to wide fluctuations in response to a broad and diverse range of factors, including the following:
| ● | actual or anticipated variations in our periodic operating results; |
| ● | increases in market interest rates that lead investors of our common stock to demand a higher investment return; |
| ● | changes in earnings estimates; |
| ● | changes in market valuations of similar companies; |
| ● | actions or announcements by our competitors; |
| ● | adverse market reaction to any increased indebtedness we may incur in the future; |
| ● | additions or departures of key personnel; |
| ● | actions by stockholders; |
| ● | speculation in the media, online forums, or investment community; and |
| ● | our ability to maintain the listing of our common stock on Nasdaq. |
Volatility in the market price of our common stock may prevent investors from being able to sell their common stock at or above the price at which they purchased our common stock. As a result, you may suffer a loss on your investment.
We have not paid in the past and do not expect to declare or pay dividends in the foreseeable future.
We have not paid in the past and do not expect to declare or pay dividends in the foreseeable future, as we anticipate that we will invest future earnings in the development and growth of our business. Therefore, holders of our common stock will not receive any return on their investment unless they sell their securities, and holders may be unable to sell their securities on favorable terms or at all.
Future issuances of our common stock or securities convertible into, or exercisable or exchangeable for, our common stock, could cause the market price of our common stock to decline and would result in the dilution of your holdings.
Future issuances of our common stock or securities convertible into, or exercisable or exchangeable for, our common stock, could cause the market price of our common stock to decline. We cannot predict the effect, if any, of future issuances of our securities on the price of our common stock. In all events, future issuances of our common stock would result in the dilution of your holdings. In addition, the perception that new issuances of our securities could occur could adversely affect the market price of our common stock.
Future issuances of debt securities, which would rank senior to our common stock upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our common stock for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our common stock.
In the future, we may attempt to increase our capital resources by offering debt securities. Upon bankruptcy or liquidation, holders of our debt securities, and lenders with respect to other borrowings we may make, would receive distributions of our available assets prior to any distributions being made to holders of our common stock. Moreover, if we issue preferred stock, the holders of such preferred stock could be entitled to preferences over holders of common stock in respect of the payment of dividends and the payment of liquidating distributions. Because our decision to issue debt or preferred stock in any future offering, or borrow money from lenders, will depend in part on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any such future offerings or borrowings. Holders of our common stock must bear the risk that any future offerings we conduct or borrowings we make may adversely affect the level of return, if any, they may be able to achieve from an investment in our common stock.
If securities industry analysts do not publish research reports on us, or publish unfavorable reports on us, then the market price and market trading volume of our common stock could be negatively affected.
Any trading market for our common stock may be influenced in part by any research reports that securities industry analysts publish about us. We do not currently have and may never obtain research coverage by securities industry analysts. If no securities industry analysts commence coverage of us, the market price and market trading volume of our common stock could be negatively affected. In the event we are covered by analysts, and one or more of such analysts downgrade our securities, or otherwise reports on us unfavorably, or discontinues coverage of us, the market price and market trading volume of our common stock could be negatively affected.
If our shares of common stock become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on Nasdaq or another national securities exchange and if the price of our common stock is less than $5.00, our common stock could be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
We are subject to ongoing public reporting requirements that are less rigorous than rules for companies that are not emerging growth companies and our stockholders could receive less information than they might expect to receive from more mature public companies.
We are required to publicly report on an ongoing basis as an “emerging growth company” under the reporting rules set forth under the Exchange Act. For so long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not emerging growth companies. For so long as we are an emerging growth company, we will not be required to:
| ● | have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
| ● | submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay” and “say-on-frequency;” and |
| ● | disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. |
In addition, Section 107 of the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year following the fifth anniversary of our initial public offering, (ii) the last day of the first fiscal year in which our total annual gross revenues are $1.235 billion or more, (iii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iv) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
Because we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not emerging growth companies, our stockholders could receive less information than they might expect to receive from more mature public companies. We cannot predict if investors will find our common stock less attractive if we elect to rely on these exemptions, or if taking advantage of these exemptions would result in less active trading or more volatility in the price of our common stock.
Anti-takeover provisions in our charter documents and under Nevada law could make an acquisition of our company more difficult, and limit attempts by our stockholders to replace or remove our current management.
Provisions in our articles of incorporation and bylaws may have the effect of delaying or preventing a change of control or changes in our management. Our articles of incorporation and bylaws include provisions that:
| ● | permit the board of directors to establish the number of directors and fill any vacancies and newly created directorships; |
| ● | provide that directors may only be removed by the majority of the shares of voting stock then outstanding; and |
| ● | establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
Not applicable.
ITEM 1C. CYBERSECURITY.
Risk Management and Strategy
We recognize the critical importance of developing, implementing, and maintaining robust cybersecurity measures to safeguard our information systems and protect the confidentiality, integrity, and availability of our data. We have developed the following processes as part of our strategy for assessing, identifying, and managing material risks from cybersecurity threats.
Managing Material Risks & Integrated Overall Risk Management
We have integrated cybersecurity risk management into our risk management processes. This integration is intended to ensure that cybersecurity considerations are part of our decision-making processes. We continuously evaluate and address cybersecurity risks in alignment with our business objectives and operational needs.
Engaging Third-parties on Risk Management
Recognizing the complexity and evolving nature of cybersecurity threats, we plan to engage external experts, including consultants and auditors, in evaluating and testing our risk management systems. These services will enable us to leverage specialized knowledge and insights, ensuring our cybersecurity strategies and processes remain at the forefront of industry best practices. Our collaboration with these third-parties is expected to include annual audits, ongoing threat assessments, and regular consultations on security enhancements.
Overseeing Third-Party Risk
Because we are aware of the risks associated with third-party service providers, we implement processes to oversee and manage these risks. We conduct thorough security assessments of all third-party providers before engagement and maintain ongoing monitoring to ensure compliance with our cybersecurity standards. This approach is designed to mitigate risks related to data breaches or other security incidents originating from third parties.
Risks from Cybersecurity Threats
We have not encountered cybersecurity challenges that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition.
Governance
Board of Directors Oversight
Our board of directors oversees the management of risks associated with cybersecurity threats.
Management’s Role Managing Risk
Management is primarily responsible for assessing, monitoring and managing our cybersecurity risks. Management must ensure that all industry standard cybersecurity measures are functioning as required to prevent or detect cybersecurity threats and related risks. Management oversees and tests our compliance with standards, remediates known risks, and leads our employee training program.
Monitoring Cybersecurity Incidents
Management is continually informed about the latest developments in cybersecurity, including potential threats and innovative risk management techniques. Management implements and oversees processes for the regular monitoring of our information systems. This includes the deployment of industry-standard security measures and regular system audits to identify potential vulnerabilities. In the event of a cybersecurity incident, management will implement an incident response plan. This plan includes immediate actions to mitigate the impact and long-term strategies for remediation and prevention of future incidents.
Reporting to Board of Directors
Significant cybersecurity matters, and strategic risk management decisions, will be escalated to the board of directors.
ITEM 2. PROPERTIES.
Our corporate offices are located at 990 Biscayne Blvd, Suite 505, Miami, Florida 33132.
DSO’s manufacturing and corporate offices are located at 1210 W 13th St, Riviera Beach, Florida 33404. It operates a 30,000 square foot manufacturing facility at this address. The building housing this manufacturing facility is under a five-year lease ending in August 2028 at a rental rate of $28,845 per month with a 3% annual increase. DSO has an option to renew this lease for an additional three years with a 3% annual increase in the rental amount.
We believe that all our properties have been adequately maintained, are generally in good condition, and are suitable and adequate for our business.
ITEM 3. LEGAL PROCEEDINGS.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock is listed on the Nasdaq Capital Market under the symbol “SMFL.”
Number of Holders of our Common Shares
As of September 20, 2024, there were approximately 112 stockholders of record of our common stock. In computing the number of holders of record of our common stock, each broker-dealer and clearing corporation holding shares on behalf of its customers is counted as a single stockholder.
Dividend Policy
We have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends on our common stock in the near future. We may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash dividends on our common stock. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, contractual restrictions, general business conditions and other factors that our board of directors may deem relevant. See also Item 1A “Risk Factors—Risks Related Ownership of Our Common Stock—We have not paid in the past and do not expect to declare or pay dividends in the foreseeable future.”
Securities Authorized for Issuance under Equity Compensation Plans
See Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Recent Sales of Unregistered Securities
We have not sold any equity securities during the 2023 fiscal year that were not previously disclosed in a quarterly report on Form 10-Q or a current report on Form 8-K that was filed during the 2023 fiscal year.
Purchases of Equity Securities
No repurchases of our common stock were made during the fourth quarter of 2023.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis summarizes the significant factors affecting our operating results, financial condition, liquidity and cash flows as of and for the periods presented below. The following discussion and analysis should be read in conjunction with our financial statements and the related notes thereto included elsewhere in this report. The discussion contains forward-looking statements that are based on the beliefs of management, as well as assumptions made by, and information currently available to, management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this report, particularly in the sections titled “Risk Factors” and “Special Note Regarding Forward-Looking Statements.”
Overview
We are engaged in the development, marketing, manufacturing, acquisition, operation and sale of a broad spectrum of nutritional and related products with an emphasis on health and wellness. Structured as a global holding company, we are executing a buy-and-build strategy with serial accretive acquisitions creating a vertically integrated company. To drive growth and earnings, we are developing proprietary products as well as acquiring other profitable companies, encompassing brands, manufacturing and distribution channels.
We also operate a network platform in the affiliate marketing space. Affiliate marketing is an advertising model in which a product vendor compensates third-party digital marketers to generate traffic or leads for the product vendor’s products and services. The third-party digital marketers are referred to as affiliates, and the commission fee incentivizes them to find ways to promote the products being sold by the product vendor.
Our wholly-owned subsidiary DSO manufactures, sells and owns the Smart for Life brand of natural health and wellness meal replacement products. The brand includes proprietary hunger suppressing functional foods that are designed to work with the body’s natural ability to lose weight. It also develops premium supplements and commodities that will promote optimal health and wellness. DSO has over 15 years of experience providing high-quality products to premium retail locations and companies. Its branded vitamins and supplements are also being sold through Amazon, and this sales channel is becoming a major contributor to the growth of the brand online.
Our wholly-owned subsidiary GSP is a sports nutrition company. It offers nutritional supplements for athletes and active lifestyle consumers through a variety of wellness solutions and delivery methods, including powders, tablets and soft gels that are formulated to support energy and performance; nutrition and wellness; and focus and clarity. GSP operates within the 36,000 square foot manufacturing facility in Riviera Beach, Florida that is also occupied by DSO. This section of the facility primarily focuses on the contract manufacturing of vitamins and supplements, with a particular emphasis on the production of tablets, capsules and powders, along with turn-key solutions for packaging these health and wellness products in a wide variety of bottles, jars, sachets and stick packs.
We also own 49% of First Health, which is primarily engaged in the development and distribution of a wide variety of nutritional products, including antioxidant rich supplements, plant-based protein, alkalizing nutrients and products designed for weight management. First Health owns the Greens First line of branded products which have been specifically marketed to the healthcare provider sector. These vitamins and supplements have been sold on a business-to-business basis, direct-to-consumer, as well as sold utilizing an international medical distribution company.
Our wholly-owned subsidiary Nexus operates a cost per action/cost per acquisition network. This network consists of hundreds of digital marketers who stand ready to market products introduced to the Nexus network. The cost per action/cost per acquisition model is where digital marketers are paid for an action (e.g., a product sale or lead generation) that is taken as a direct result of their marketing efforts. Through the digital marketer’s method of marketing, the digital marketer sends traffic to one of the product vendor’s offers listed on the network.
Recent Developments
Ceautamed Transaction and Related Transactions
On January 29, 2024, we entered into an asset purchase agreement with First Health and Ceautamed, along with its wholly owned subsidiaries, Wellness Watchers Global, LLC and Greens First Female LLC, pursuant to which we agreed to sell 51% of the assets of Ceautamed and its subsidiaries to First Health for an aggregate price of $3,486,233, consisting of (i) $210,993.50 paid to our creditors for outstanding debt owed and (ii) $3,275,239 in the form of the assumption of certain assumed liabilities (as defined in the asset purchase agreement), including the assumption of certain debt and the release of Ceautamed and its subsidiaries from such liabilities.
In connection with the asset purchase agreement, we also entered into a limited liability company agreement, or the LLC Agreement, pursuant to which First Health was organized. Pursuant to the LLC Agreement, the voting members of First Health are Joseph X. Xiras, Stuart Benson, and Ryan Benson, each with a 17% voting interest in First Health. We will also maintain a 49% non-voting ownership interest in First Health. The voting members of First Health have the option to purchase such 49% non-voting ownership interest for $1 upon notice to us pursuant to the LLC Agreement.
Loan Agreement
On March 5, 2024, our company and DSO, as borrowers, entered into a loan agreement with Abbsi, LLC, or Abbsi, for a loan of up to $500,000 and issued a promissory note to Abbsi in the principal amount of $250,000. Pursuant to the loan agreement and note, Abbsi is providing funds directly to various vendors and service providers of DSO. During the term of the loan, Abbsi agreed to pay DSO’s suppliers directly, or reimburse us if DSO’s suppliers have been paid directly by us, for all costs associated with the production and sale of our branded products by Abbsi, as well as payroll costs attributable to the sale of such products. All such costs shall be reimbursed directly by us or applied as a reduction to the principal amount due under the loan agreement and note. The note bears interest at the rate of 12% per annum and has a term of 60-months, if not prepaid earlier, and shall otherwise be due and payable as provided in the note. The loan may be prepaid at any time without penalty and is secured by a security interest in all of DSO’s assets. As of the date hereof, Abbsi has advanced $250,000 under the loan.
The loan agreement also provides that, for so long as any amounts due under the note remain outstanding and subject to a cap equal to the principal amount of the loan, Abbsi shall be entitled to receive 20% of the annual positive cash flow generated by DSO, which shall be calculated on an annualized basis. Calculation of such payment shall be made contemporaneously with the end of each fiscal quarter and shall be paid concurrently with our quarterly or annual filings with the SEC.
Sale and Leaseback Agreement
On March 6, 2024, we entered into a sale and leaseback agreement with Efraim Elias, or the Purchaser, related to BSNM’s Doral, Florida production facility, or the Facility. Under the sale and leaseback agreement, we sold BSNM to the Purchaser for a purchase price of $1.00 and agreed to lease the Facility from the Purchaser. The lease shall expire on March 6, 2074 and either party may extend the lease for up to ten additional one-year periods.
In addition, we agreed to enter into a management services agreement, pursuant to which GSP shall (i) be obligated to make certain future payments to the Purchaser; (ii) assume and pay all existing and future liabilities of the Facility; (iii) manage the operations and affairs of the Facility; and (iv) be entitled to all revenues of the Facility and transactions in respect of all assets of the Facility, in its sole discretion. As of the date of this report, the management services agreement has not yet been signed.
The sale and leaseback agreement includes a right of first offer, pursuant to which we have a right to repurchase the Facility in the event that one or both parties determine that the Purchaser cannot maintain title ownership to the Facility and/or fulfill all of its obligations under the sale and leaseback agreement or the management services agreement. The terms of any such repurchase shall be in our sole discretion. The Purchaser may not transfer the Facility to any other party.
Securities Purchase Agreement
On April 3, 2024, we entered into a securities purchase agreement with Purely Optimal Nutrition Inc., or Purely Optimal, and Tan Enterprises, Inc., Availiant Holdings Corporation, Dannel Tan, Jason Kwan and Timur Kim, or the Sellers, pursuant to which we agreed to acquire all of the issued and outstanding membership interests of Purely Optimal, a health supplement brand, from the Sellers for an aggregate purchase price of $11,965,966.10, or the Purchase Price, consisting of (i) $7,859,579.66 in cash and (ii) $4,106,386.44 payable as 18,414 newly issued shares of series D convertible preferred stock, a new series of preferred stock, subject to certain adjustments described below.
The Purchase Price is based upon a six (6) times multiple of an estimated EBITDA of $1,467,073.35 for the twelve-month period ending on November 30, 2023, or the Reconstructed EBITDA. The Purchase Price will be adjusted upwards or downwards based upon the difference between (i) six (6) times the Reconstructed EBITDA and the Purchase Price and (ii) the Estimated Inventory Payment (as defined in the securities purchase agreement) and the actual amount of Inventory (as defined in the securities purchase agreement) on the closing date. In addition, the cash portion of the Purchase Price will be decreased by the amount of any outstanding indebtedness of Purely Optimal for borrowed money existing as of the closing date and any unpaid transaction expenses.
The securities purchase agreement contains customary representations, warranties and covenants, including a covenant that the Sellers will not compete with the business of Purely Optimal for a period of two (2) years following closing. The securities purchase agreement also contains mutual indemnification for breaches of representations or warranties and failure to perform covenants or obligations contained in the securities purchase agreement. In the case of the indemnification provided by the Sellers with respect to breaches of certain non-fundamental representations and warranties, the Sellers will only become liable for indemnified losses if the amount exceeds ten percent (10%) of the Purchase Price, whereupon the Sellers will be liable for all losses relating back to the first dollar, provided that the liability of the Sellers for breaches of certain non-fundamental representations and warranties shall not exceed thirty five (35%) of the Purchase Price and each Seller’s aggregate liability for the breach of fundamental representations shall be limited to the Purchase Price.
The closing of the securities purchase agreement is subject to customary closing conditions, including, without limitation, the completion of accounting and legal due diligence investigations; the receipt of all authorizations, consents and approvals of all governmental authorities and third parties; the release of any liens against any of the assets of Purely Optimal; our obtaining the requisite acquisition financing; and delivery of all documents required for the transfer of the equity interests of Purely Optimal to us.
Warrant Solicitation
On May 30, 2024, we entered into warrant solicitation inducement letters with the holders of warrants for the purchase of an aggregate of 183,370 shares of common stock at an exercise price of $10.64 issued on December 4, 2023, or the Existing Warrants, pursuant to which the holders agreed to exercise the Existing Warrants for cash at a reduced exercise price of $4.25 per share, or for gross proceeds of $779,322.50 in the aggregate. In consideration for the immediate exercise of the Existing Warrants for cash, we agreed to issue to the holders new warrants for the purchase of an aggregate of 550,110 shares of common stock, or the New Warrants.
On June 3, 2024, the closing of this transaction was completed, and we issued the New Warrants. The New Warrants are exercisable for a period of eighteen months at an initial exercise price of $4.25 per share and may be exercised on a cashless basis if at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available for the resale of, the shares issuable upon exercise of the New Warrants. The exercise price will be subject to customary adjustments in the event of stock splits, stock dividends, stock combinations and similar recapitalization transactions. The New Warrants also contain a beneficial ownership limitation which provides that we shall not effect any exercise, and a holder shall not have the right to exercise, any portion of a New Warrant to the extent that, after giving effect to the exercise, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the exercise. This limitation may be waived (up to a maximum of 9.99%) by a holder in its sole discretion upon not less than sixty-one (61) days’ prior notice to us.
H.C. Wainwright & Co., LLC acted as warrant inducement agent and financial advisor in connection with the transaction and received a cash fee equal to 7.5% of the gross proceeds, a management fee equal to 1% of the gross proceeds and reimbursement of certain expenses. After these fees, the Company received net proceeds of approximately $458,473. In addition, the Company issued to certain designees of H.C. Wainwright & Co., LLC warrants to purchase 13,753 shares of common stock at an exercise price of $5.3125 per share, which such warrants will have the same terms of the New Warrants (other than the exercise price).
Debt Conversions and Creation of Series C Preferred Stock
Subsequent to December 31, 2023, we entered into conversion agreements with certain lenders, pursuant to which such lenders converted an aggregate of $15,318,716 of debt and interest owed by us to such lenders in exchange for an aggregate of 6,141,229 shares of common stock and 36,506.81 shares of series C preferred stock. The conversions were completed in multiple transactions at a price per underlying share of common stock that was at least equal to the lower of (i) the official closing price as reflected on Nasdaq.com immediately preceding signing of the conversion agreement or (ii) the average official closing price as reflected on Nasdaq.com for the five trading days immediately preceding signing of the conversion agreement.
In connection with these debt conversions, we filed a certificate of designation with the Nevada Secretary of State on March 1, 2024 to create a new series of preferred stock designated as series C preferred stock. Pursuant to the certificate of designation, we designated 200,000 shares of our preferred stock as series C preferred stock. Following is a summary of the material terms of the series C preferred stock:
Dividend Rights. Holders of series C preferred stock are entitled to receive dividends in the same form as dividends paid on shares of the common stock only when and if such dividends are paid on shares of common stock. No other dividends shall be paid on shares of series C preferred stock.
Liquidation Rights. Upon any liquidation, dissolution or winding-up of our company, whether voluntary or involuntary, the holders of series C preferred stock shall be entitled to receive out of the assets of our company the same amount that a holder of common stock would receive if the series C preferred stock were fully converted (disregarding for such purposes any conversion limitations) to common stock, which amounts shall be paid prior to all holders of common stock and pari passu with all holders of our series B preferred stock.
Voting Rights. The series C preferred stock shall vote together with the common stock on an as-converted basis.
Conversion Rights. Each share of series C preferred stock is convertible, at any time and from time to time at the option of the holder thereof, into that number of shares of common stock determined by dividing the stated value of such share of series C preferred stock ($100) by the conversion price. The conversion price is $7.00 (subject to adjustments for stock dividends, stock splits, recapitalizations and certain fundamental transactions). Notwithstanding the foregoing, we shall not effect any conversion, and a holder shall not have the right to convert, any portion of the series C preferred stock to the extent that, after giving effect to the conversion, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the conversion. This limitation may be waived (up to a maximum of 9.99%) by the holder and in its sole discretion, upon not less than sixty-one (61) days’ prior notice to us.
No Redemption. The series C preferred stock is not redeemable.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by the following factors:
| ● | our ability to acquire new customers or retain existing customers; |
| ● | our ability to offer competitive product pricing; |
| ● | our ability to broaden product offerings; |
| ● | industry demand and competition; and |
| ● | market conditions and our market position. |
Emerging Growth Company
We qualify as an “emerging growth company” under the JOBS Act. As a result, we will be permitted to, and intend to, rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, we will not be required to:
| ● | have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
| ● | submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay” and “say-on-frequency;” and |
| ● | disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. |
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year following the fifth anniversary of our initial public offering, (ii) the last day of the first fiscal year in which our total annual gross revenues are $1.235 billion or more, (iii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iv) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
Discontinued Operations
As noted above, on March 6, 2024, we entered into a sale and leaseback agreement relating to BSNM. As a result, the financial results and balances have been classified as discontinued operations within this report and the accompanying consolidated financial statements.
BSNM is a nutraceutical contract manufacturer that specializes in a wide variety of products to fill its client’s needs, from the private labelling of vitamins, dietary supplements, nutraceuticals, sport nutrition and broad-spectrum nutritional supplements.
Results of Operations
Comparison of Years Ended December 31, 2023 and 2022
The following table sets forth key components of our results of operations during the years ended December 31, 2023 and 2022, both in dollars and as a percentage of our revenues.
| | | December 31, 2023 | | | December 31, 2022 | |
| | | Amount | | | % of
Revenues | | | Amount | | | % of
Revenues | |
| Revenues | | | | | | | | | | | | |
| Products | | $ | 7,863,977 | | | | 95.60 | % | | $ | 12,467,650 | | | | 78.40 | % |
| Advertising | | | 361,815 | | | | 4.40 | % | | | 3,434,879 | | | | 21.60 | % |
| Total revenues | | | 8,225,792 | | | | 100.00 | % | | | 15,902,529 | | | | 100.00 | % |
| Cost of revenues | | | | | | | | | | | | | | | | |
| Products | | | 4,854,554 | | | | 59.02 | % | | | 8,250,733 | | | | 51.88 | % |
| Advertising | | | 281,131 | | | | 3.42 | % | | | 2,561,276 | | | | 16.11 | % |
| Total cost of revenues | | | 5,135,685 | | | | 62.43 | % | | | 10,812,009 | | | | 67.99 | % |
| Gross profit | | | 3,090,107 | | | | 37.57 | % | | | 5,090,520 | | | | 32.01 | % |
| Operating expenses | | | | | | | | | | | | | | | | |
| General and administrative | | | 4,258,202 | | | | 51.77 | % | | | 5,337,925 | | | | 33.57 | % |
| Compensation | | | 5,561,017 | | | | 67.60 | % | | | 6,167,637 | | | | 38.78 | % |
| Professional services | | | 2,263,405 | | | | 27.52 | % | | | 2,165,198 | | | | 13.62 | % |
| Consulting fee - related party | | | 46,686 | | | | 0.57 | % | | | 1,471,199 | | | | 9.25 | % |
| Impairment of intangible assets | | | 5,843,501 | | | | 71.04 | % | | | — | | | | — | |
| Depreciation and amortization expense | | | 2,273,288 | | | | 27.64 | % | | | 1,899,542 | | | | 11.94 | % |
| Total operating expenses | | | 20,246,099 | | | | 246.13 | % | | | 17,041,501 | | | | 107.16 | % |
| Operating loss from continuing operations | | | (17,155,992 | ) | | | (208.56 | )% | | | (11,950,981 | ) | | | (75.15 | )% |
| Other income (expense) | | | | | | | | | | | | | | | | |
| Other income (expense) | | | 400,397 | | | | 4.87 | % | | | (474 | ) | | | (0.00 | )% |
| IPO expenses | | | — | | | | — | | | | (702,420 | ) | | | (4.42 | )% |
| Gain on extinguishment of debt | | | 269,828 | | | | 3.28 | % | | | 161,039 | | | | 1.01 | % |
| Interest expense | | | (4,634,839 | ) | | | (56.35 | )% | | | (15,689,565 | ) | | | (98.66 | )% |
| Total other expense | | | (3,964,614 | ) | | | (48.20 | )% | | | (16,231,420 | ) | | | (102.07 | )% |
| Net loss from continuing operations | | | (21,120,606 | ) | | | (256.76 | )% | | | (28,182,401 | ) | | | (177.22 | )% |
| Net loss from discontinued operations | | | (1,555,135 | ) | | | (18.91 | )% | | | (1,795,415 | ) | | | (11.29 | )% |
| Net loss | | $ | (22,675,741 | ) | | | (275.67 | )% | | $ | (29,977,816 | ) | | | (188.51 | )% |
Revenues. Our total revenues decreased by $7,676,737, or 48.27%, to $8,225,792 for the year ended December 31, 2023 from $15,902,529 for the year ended December 31, 2022. Such decrease was primarily due a lack of cash to fund the purchase of ingredients leading to our inability to meet production needs. The lack of ingredients inventory impacted our ability to meet the production needs of contract manufacturing clients as well as the sales of our own products. The lack of adequate cash also caused us to significantly reduce our advertising and marketing efforts to promote our products with online retailers.
Our nutraceutical business generates revenue from the sales of nutritional and related products. Revenues from our nutraceutical business (products) decreased by $4,603,673, or 36.92%, to $7,863,977 for the year ended December 31, 2023 from $12,467,650 for the year ended December 31, 2022. This decrease was primarily due to our cash constraints and our inability to pay for raw materials used in the production of both branded and contract manufacturing products. The decreased revenues were the result of a decrease in the volume of products sold and not due to pricing changes.
Our digital marketing business generates revenues when sales of listed products are sold by product vendors through our network as a result of the marketing efforts of digital marketers. Revenues from our digital marketing business (advertising), which is operated by Nexus, decreased by $3,073,064, or 89.47%, to $361,815 for the year ended December 31, 2023 from $3,434,879 for the year ended December 31, 2022. The decrease in revenue is attributable to our cash constraints and the impact it had with our affiliate advertisers.
Cost of revenues. Our total cost of revenues decreased by $5,676,324, or 52.50%, to $5,135,685 for the year ended December 31, 2023 from $10,812,009 for the year ended December 31, 2022. Such decrease is directly related to the decrease in revenues.
Cost of revenues for our nutraceutical business consist of ingredients, packaging materials, freight, and labor associated with the production of various products. Cost of revenues for our nutraceutical business (products) decreased by $3,396,179, or 41.16%, to $4,854,554 for the year ended December 31, 2023 from $8,250,733 for the year ended December 31, 2022. Such decrease was primarily due to the cash constraints described above. As a percentage of product revenues, cost of revenues for product sales decreased from 66.18% in 2022 to 61.73% in 2023 due to rising prices for raw materials and the utilization of existing inventory items.
Cost of revenues for our digital marketing business consist of commissions and bonuses paid to digital marketers. Cost of revenues from our digital marketing business (advertising) decreased by $2,280,145, or 89.02%, to $281,131 for the year ended December 31, 2023 from $2,561,276 for the year ended December 31, 2022. As a percentage of advertising revenues, cost of revenues for advertising sales was 77.70% for the year ended December 31, 2023 and 74.57% for the year ended December 31, 2022.
Gross profit. As a result of the foregoing, our gross profit decreased by $2,000,413 or 39.30%, to $3,090,107 for the year ended December 31, 2023 from $5,090,520 for the year ended December 31, 2022. As a percentage of revenues, our gross profit increased from 32.01% in 2022 to 37.57% in 2023.
General and administrative expenses. Our general and administrative expenses consist primarily of advertising expenses, bad debts, rent expense, insurance and other expenses incurred in connection with general operations. Our general and administrative expenses decreased by $1,079,723, or 20.23% to $4,258,202 for the year ended December 31, 2023 from $5,337,925 for the year ended December 31, 2022. Such decrease was primarily due to decreased rates for insurance, a significant reduction in advertising spending as discussed above. As a percentage of revenues, general and administrative expenses increased from 33.57% in 2022 to 51.77% in 2023.
Compensation. Our compensation expenses include both cash and non-cash items, including salaries plus related payroll taxes. Our compensation expenses decreased by $606,620, or 9.84% to $5,561,017 for the year ended December 31, 2023 from $6,167,637 for the year ended December 31, 2022. Such decrease was primarily due to the temporary closure of the production facilities during 2023 due to the cash constraints discussed above, offset by the increased stock based compensation recognized in 2023 of $797,535. As a percentage of revenues, compensation expenses increased from 38.78% in 2022 to 67.60% in 2023.
Professional services. Our professional services expenses consist primarily of investor relations, consulting, advisory, legal and audit expenses incurred in connection with general operations. Our professional services expenses increased by $98,207, or 4.54% to $2,263,405 for the year ended December 31, 2023 from $2,165,198 for the year ended December 31, 2022. Such increase was primarily due to advisor fees related to our compliance with Nasdaq. As a percentage of revenues, professional services expenses increased from 13.62% in 2022 to 27.52% in 2023.
Consulting fee related party. Consulting fees to related parties decreased by $1,424,513, or 96.83%, to $46,686 for the year ended December 31, 2023, from $1,471,199 for the year ended December 31, 2022. The decrease is associated with the decreased financing arrangements provided by the related party during 2023. As a percentage of revenues, consulting fees to related parties decreased from 9.25% in 2022 to 0.57% in 2023.
Impairment of intangible assets. We recognized an impairment of intangible assets of $5,843,501, or 71.04% of revenues, during 2023 related to the intangible assets associated with Nexus of $2,897,737, an impairment of the license agreement associated with GSP of $342,531, an impairment of the intangible assets of Ceautamed of $2,603,233. We did not recognize such an impairment during the year ended December 31, 2022.
Depreciation and amortization. Depreciation and amortization was $2,273,288, or 27.64% of revenues, for the year ended December 31, 2023, as compared to $1,899,542, or 11.94% of revenues, for the year ended December 31, 2022. The increase in amortization is associated with the amortization of the intangible assets acquired in the acquisition of Ceautamed.
Total other income (expense). We had $3,964,614 in total other expense, net, for the year ended December 31, 2023, as compared to total other expense, net, of $16,231,420 for the year ended December 31, 2022. Total other expense, net, for the year ended December 31, 2023 consisted of interest expense of $4,634,839, offset by other income of $400,397 and gain on debt extinguishment of $269,828. The other expense, net, for the year ended December 31, 2022 consisted of interest expense of $15,689,565, initial public offering expenses of $702,420, and other expense of $474, offset by a gain on debt extinguishment of $161,039.
Loss from discontinued operations. We incurred a loss on discontinued operations of $1,555,135 and $1,795,415 for the years ended December 31, 2023 and 2022, respectively. The decrease is attributable to the reduction of workforce and related compensation as production slowed.
Net loss. As a result of the cumulative effect of the factors described above, we had a net loss of $22,675,741 for the year ended December 31, 2023, as compared to $29,977,816 for the year ended December 31, 2022, a decrease of $7,302,075, or 24.36%.
Liquidity and Capital Resources
As of December 31, 2023, we had cash of $188,596. To date, we have financed our operations primarily through revenue generated from operations, bank borrowings and sales of our securities. Since our inception in 2017, we have experienced losses and as a result have continued to use cash in our operations. We have been dependent upon financing activities as we implement our acquisition strategy.
Our consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. We have suffered recurring losses from operations and have a working capital deficiency of approximately $19.7 million and an accumulated deficit of $67.7 million. Additionally, we had a net loss of $22.7 million, and cash used in operations of $5.8 million for the year ended December 31, 2023. These conditions raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Management believes that current available resources will not be sufficient to fund our planned expenditures over the next 12 months. Management has implemented restructuring activities, including staff reductions, a subsequent event sale of BSNM, and a subsequent event sale of Ceautamed’s assets. Accordingly, we will be dependent upon the raising of additional capital through placement of common stock and/or debt financing in order to implement our business plan. If we raise additional capital through the issuance of equity securities or securities convertible into equity, stockholders will experience dilution, and such securities may have rights, preferences, or privileges senior to those of the holders of common stock or convertible senior notes. If we raise additional funds by issuing debt, we may be subject to limitation on its operations, through debt covenants or other restrictions. There is no assurance that we will be successful with future financing ventures, and the inability to secure such financing may have a material adverse effect on our financial condition. The accompanying consolidated financial statements do not include any adjustments to the amounts and classifications of assets and liabilities that might be necessary should we be unable to continue as a going concern.
Summary of Cash Flow
The following table provides detailed information about our net cash flow for the years ended December 31, 2023 and 2022.
| | | Year Ended December 31, | |
| | | 2023 | | | 2022 | |
| Net cash used in operating activities | | $ | (5,848,553 | ) | | $ | (9,685,871 | ) |
| Net cash used in investing activities | | | (3,450 | ) | | | (3,029,720 | ) |
| Net cash provided by financing activities | | | 5,969,779 | | | | 12,588,241 | |
| Net change in cash | | | 117,776 | | | | (127,350 | ) |
| Cash and cash equivalents at beginning of year | | | 70,820 | | | | 198,170 | |
| Cash and cash equivalents at end of year | | $ | 188,596 | | | $ | 70,820 | |
Our net cash used in operating activities was $5,848,553 for the year ended December 31, 2023, as compared to $9,685,871 for the year ended December 31, 2022. For the year ended December 31, 2023, our net loss of $22,675,741, offset by impairment of intangibles of $5,843,501, depreciation and amortization of $2,273,345, an increase in accrued expenses of $2,556,332, amortization of debt discount and debt issuance cost of $1,266,191, a decrease in inventory of $939,574, stock based compensation of $797,535, along with a decrease in the value of discontinued operation of $980,192, were the primary drivers for cash used in operations. For the year ended December 31, 2022, our net loss of $29,977,816, offset by interest expense associated with future equity agreements of $10,844,743, debt issuance cost of $2,698,080, depreciation and amortization of $2,111,346 and an increase in accounts payable of $1,973,391, were the primary drivers for cash used in operations.
Our net cash used in investing activities was $3,450 for the year ended December 31, 2023, as compared to $3,029,720 for the year ended December 31, 2022. Net cash used in investing activities for the year ended December 31, 2023 consisted of cash paid for equipment used at BSNM, while the net cash used in investing activities for the year ended December 31, 2022 consisted of cash paid for the acquisition of Ceautamed of $3,000,000, and purchases of property and equipment of $29,720.
Our net cash provided by financing activities was $5,969,779 for the year ended December 31, 2023, as compared to $12,588,241 for the year ended December 31, 2022. Net cash provided by financing activities for the year ended December 31, 2023 consisted of proceeds from warrant exercises of $7,269,531, proceeds from the issuance of notes payable of $2,885,527, proceeds from the issuance of common stock in a private placement of $2,151,310 and receipts from related parties of $888,130, offset by repayments of notes payable of $5,873,881, repayments to related parties of $1,220,274, and repayments on notes payable of discontinued operations of $130,564. Net cash provided by financing activities for the year ended December 31, 2022 consisted of proceeds from our initial public offering of $12,812,008, proceeds from convertible notes and notes payable of $9,797,275 and proceeds from a private placement of common stock of $909,873, offset by repayments on convertible notes and notes payable of $10,621,431, and repayments on notes payable of discontinued operation of $309,484.
Initial Public Offering
On February 16, 2022, we entered into an underwriting agreement with Dawson James Securities, Inc., as representative of the several underwriters named on Schedule I thereto, relating to our initial public offering of units, each unit consisting of one share of common stock, a series A warrant to purchase one share of common stock and a series B warrant to purchase one share of common stock. Pursuant to the underwriting agreement, we agreed to sell 457 units to the underwriters, at a purchase price per unit of $28,665.00 (the offering price to the public of $31,500.00 per unit minus the underwriters’ discount), and also agreed to grant to the underwriters a 45-day option to purchase up to 69 additional shares of common stock, up to 69 additional series A warrants, and/or up to 69 additional series B warrants, in any combination thereof, at a purchase price to the public of $31,437.00 per share and $31.50 per warrant, less underwriting discounts and commissions, solely to cover over-allotments, if any.
On February 18, 2022, the closing of our initial public offering was completed. At the closing, the underwriters partially exercised the option and purchased 66 series A warrants and 66 series B warrants. Therefore, we sold 457 shares of common stock, 523 series A warrants and 523 series B warrants for total gross proceeds of $14,404,128. After deducting the underwriting commission and expenses, we received net proceeds of approximately $12,684,739. We used the proceeds of the offering to pay off certain debt and used the remaining net proceeds for working capital and general corporate purposes.
The series A warrants are exercisable until the fifth anniversary of the issuance date at an exercise price equal to $22,050.00 per share and may be exercised on a cashless basis if the issuance of common stock upon exercise of the warrants is not covered by an effective registration statement.
The series B warrants are exercisable until the fifth anniversary of the issuance date at an exercise price equal to $31,500.00 per share and may be exercised on a cashless basis, whereby the holder will receive one share of common stock for each series B warrant exercised. As of December 31, 2023, 457 of the series B warrants were exercised on a cashless basis and we issued 457 shares of common stock upon such exercise.
Private Placement of Common Stock and Prefunded Warrants
On December 8, 2022, we entered into a securities purchase agreement with certain accredited investors, pursuant to which we issued to the investors an aggregate of 407 shares of common stock and prefunded warrants to purchase an aggregate of 500 shares of common stock for an aggregate purchase price of $1,000,000, or $1,102.50 per underlying share.
The investors were also previously issued warrants for the purchase of an aggregate of 3,908 shares of common stock at an exercise price of $19,687.50, which contain a full ratchet anti-dilution adjustment provision. As a result of the issuance of shares at $1,102.50 per share, the exercise price of these warrants was reduced to $1,102.50 per share and the number of shares underlying the warrants was increased to 68,024 shares in accordance with the terms of the warrants. Pursuant to the securities purchase agreement, the investors then agreed to amend and restate the terms of the warrants to, among other things, remove full ratchet anti-dilution adjustment provision.
Dawson James Securities, Inc. acted as placement agent in connection with the private placement described above and received a cash commission of $90,000 and warrants for the purchase of 73 shares of common at an exercise price of $1,102.50.
Registered Direct Offering and Related Private Placement
On May 2, 2023, we entered into a securities purchase agreement with an institutional investor, pursuant to which we agreed to issue and sell, in a registered direct offering, 1,502 shares of common stock and a pre-funded warrant to purchase up to 2,952 shares of common stock at an exercise price of $0.0063, at an offering price per share and pre-funded warrant of $201.915 and $201.9087, respectively. In addition, under the securities purchase agreement, we agreed to issue to the investor a warrant to purchase up to 4,454 shares of common stock at an exercise price of $194.04 per share in a concurrent private placement.
On May 5, 2023, the offering was completed. At closing, the investor exercised the pre-funded warrant in full. Accordingly, we issued to the investor 4,454 shares of common stock and a warrant to purchase up to 4,454 shares of common stock at an exercise price of $194.04 per share for total gross proceeds of $899,326 and net proceeds of $776,933.
H.C. Wainwright & Co., LLC, or the Placement Agent, acted as the exclusive placement agent in connection with this offering and received a cash fee equal to 7.5% of the gross proceeds of the offering and a management fee equal to 1% of the gross proceeds of the offering. We also agreed to reimburse the Placement Agent $30,000 for fees and expenses of its legal counsel and other out-of-pocket expenses and $15,950 for certain closing costs. In addition to the cash fees, we issued to the Placement Agent warrants to purchase up to 334 shares of common stock at an exercise price of $252.39375 per share (or 125% of the offering price).
Second Registered Direct Offering and Related Private Placement
On May 17, 2023, we entered into a securities purchase agreement with an institutional investor, pursuant to which we agreed to issue and sell, in a registered direct offering 3,270 shares of common stock and a pre-funded warrant to purchase up to 6,014 shares of common stock at an exercise price of $0.0063, at an offering price per share and pre-funded warrant of $170.73 and $170.7237, respectively. In addition, under the securities purchase agreement, we agreed to issue to the investor a warrant to purchase up to 9,284 shares of common stock at an exercise price of $163.17 per share in a concurrent private placement.
On May 19, 2023, the offering was completed. At closing, the investor exercised the pre-funded warrant in full. Accordingly, we issued to the investor 9,284 shares of common stock and a warrant to purchase up to 9,284 shares of common stock at an exercise price of $163.17 per share for total gross proceeds of $1,585,057 and net proceeds of $1,374,367.
The Placement Agent acted as the exclusive placement agent in connection with this offering and received a cash fee equal to 7.5% of the gross proceeds of the offering and a management fee equal to 1% of the gross proceeds of the offering. We also agreed to reimburse the Placement Agent $60,000 for fees and expenses of its legal counsel and other out-of-pocket expenses and $15,950 for certain closing costs. In addition to the cash fees, we issued to the Placement Agent warrants to purchase up to 697 shares of common stock at an exercise price of $213.4125 per share (or 125% of the offering price).
Outstanding Debt
We have historically funded our acquisitions and operations through debt agreements. We have outstanding debt of $12,364,800 at December 31, 2023, with $10,616,114 of principal payments due within the next 12 months. We have not generated cash flow from operations and the repayment of debt is dependent on raising new equity transactions and new debt financings. Between January 1, 2024 and September 20, 2024, we have converted $12,901,227 of the debt into equity instruments. There is no assurance that we will be successful with future financings, and the inability to secure financings may have a material adverse effect on our financial condition.
The following is a roll-forward of the debt balances for the year ended December 31, 2023:
| Balance at December 31, 2022 | | $ | 19,835,728 | |
| Proceeds from new debt issued in 2023 | | | 2,885,527 | |
| Repayment of principal during 2023 | | | (5,873,881 | ) |
| Amortization of debt discounts | | | 1,266,191 | |
| Non-cash debt issued | | | 295,462 | |
| Stock issued for conversion of principal of debt | | | (6,264,000 | ) |
| Net cash advance activity | | | 154,689 | |
| Gain on extinguishment of debt in 2023 | | | (243,395 | ) |
| Conversion of interest to debt | | | 334,950 | |
| Conversion of principal to accrued expenses | | | (26,471 | ) |
| Balance at December 31, 2023 | | $ | 12,364,800 | |
Please see Note 10 to our audited consolidated financial statements beginning on page F-1 of this annual report for a complete description of the terms of our outstanding debt.
Contractual Obligations
Our principal commitments consist mostly of obligations under the loans described above, the operating leases described under Item 2 “Properties” and pricing/margin structures for products established with our clients. We do not have any purchase obligations with any suppliers.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
The following discussion relates to critical accounting policies for our company. The preparation of financial statements in conformity with United States generally accepted accounting principles, or GAAP, requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Revenue Recognition. We evaluate and recognize revenue by: identifying the contract(s) with the customer; identifying the performance obligations in the contract; determining the transaction price; allocating the transaction price to performance obligations in the contract; and recognizing revenue as each performance obligation is satisfied through the transfer of a promised good or service to a customer (i.e., “transfer of control”).
Products (DSO, GSP and Ceautamed)
We primarily generate product revenues by manufacturing and packaging nutraceutical products as a contract manufacturer for our customers. The majority of our revenue is recognized when we satisfy a single performance obligation by transferring control of products to a customer. Control is generally transferred when our products are either shipped or delivered based on the terms contained within the underlying contracts or agreements. Our general payment terms are short-term in duration. We do not have significant financing components or payment terms. We did not have any material unsatisfied performance obligations at December 31, 2023 or 2022.
Distribution expenses to transport our products, where applicable, and warehousing expense after manufacture are accounted for within operating expenses.
Advertising/Marketing (Nexus)
Nexus generates advertising revenues when sales of listed products are sold by product vendors through its network as a result of the marketing efforts of digital marketers. The products on the network come from several different customers, which pay Nexus a specific amount per sale, the amount of which is dictated by the customer. The revenue is recognized upon the sale of a product by the customer, net of fraudulent traffic or disputed transactions. A portion of the specific amount received by Nexus for that sale is paid out to the digital marketer as a commission, which is recorded in cost of sales. To illustrate the revenue process, a digital marketer logs onto the platform and selects an offer to promote for the day. The platform generates a unique link which the digital marketer distributes either via email or a banner ad. As the link is distributed to the consumer via the marketing efforts of the digital marketer, the consumer visits that link to make a purchase from the customer’s website, and when such purchase is complete, revenue is recognized by Nexus and the sale is credited to the digital marketer’s Nexus account. The benefit to the digital marketer operating on Nexus’ network is that the digital marketer receives a commission without the possibility of a claw back or refund. The customer benefits through increased sales of its products as a result of the marketing efforts of the digital marketers. Nexus’ platform acts as the transaction ledger, keeping track of clicks, sales and commissions.
Nexus’ general payment terms are short-term in duration. Insertion orders are utilized between Nexus and the customer for each campaign related to a particular product being marketed. The insertion order remains in effect until the customer or Nexus terminates the order, and either party may terminate the order at any time upon 14 days’ written notice. The customer is billed weekly for the sales digital marketers have generated for the week. Nexus does not have significant financing components or payment terms. Nexus did not have any material unsatisfied performance obligations at December 31, 2023 or 2022.
Inventory, net. Inventory consists of raw materials, packaging materials, and finished goods and is valued at the lower of cost (first-in, first-out) or replacement cost or net realizable value. An allowance for inventory obsolescence is provided for slow moving or obsolete inventory to write down historical cost to net realizable value. We primarily perform manufacturing for functional foods and nutraceuticals in the form of bars, cookies, powders, tablets and capsules. The allowance for obsolescence is an estimate established through charges to cost of goods sold. Management’s judgment in determining the adequacy of the allowance is based upon several factors which include, but are not limited to, analysis of slow-moving inventory, analysis of the selling price of inventory, the predetermined shelf life of the product, and management’s judgment with respect to current economic conditions. Given the nature of the inventory, it is reasonably possible our estimate of the allowance for obsolescence will change in the near term.
Property and Equipment. Property and equipment are recorded at cost. Expenditures for major betterments and additions are charged to the asset accounts, while replacements, maintenance and repairs which do not improve or extend the lives of the respective assets are charged to expense as incurred. We provide for depreciation and amortization over the estimated useful lives of various assets using the straight-line method ranging from 3-15 years.
Intangible Assets. In accordance with ASC Topic 360, Intangibles and Other, we review intangible assets for impairment. In connection with any such review, if the recorded value of intangible assets is greater than its fair value, they are written down and charged to results of operations. Intangible assets (excluding goodwill) are assessed at each reporting date for indications that an asset may be impaired. If any such indication exists, we make an estimate of the asset’s recoverable amount. The asset’s recoverable amount is the higher of an asset’s or cash-generating unit’s fair value less costs of disposal and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets. Where the carrying amount of an asset or a group of assets exceeds its recoverable amount, the asset is considered impaired and is written down to its recoverable amount. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset or the group of assets. Intangible assets consist of customer relationships, tradename, developed technology, patents, software platform, supplier contracts, non-compete agreements, license agreements, and intellectual property acquired in the acquisitions of DSO, Nexus, GSP and Ceautamed. We amortize intangible assets with finite lives on a straight-line basis over their estimated useful lives which ranges from 3 to 15 years.
Goodwill. We allocate goodwill to reporting units based on the reporting unit expected to benefit from the business combination. We evaluate our reporting units on an annual basis and, if necessary, reassign goodwill using a relative fair value allocation approach. Goodwill is tested for impairment at the reporting unit level (operating segment or one level below an operating segment) on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value. These events or circumstances could include a significant change in the business climate, legal factors, operating performance indicators, competition, or sale or disposition of a significant portion of a reporting unit. In accordance with ASC Topic 350, Intangibles—Goodwill and Other, we review intangible assets at least annually for impairment. In connection with any such review, if the recorded value of intangible assets is greater than its fair value, they are written down and charged to results of operations. Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units, assignment of goodwill to reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated primarily through the use of a discounted cash flow methodology. This analysis requires significant judgments, including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth for our business, estimation of the useful life over which cash flows will occur, and determination of our weighted average cost of capital. The estimates used to calculate the fair value of a reporting unit change from year to year based on operating results, market conditions, and other factors. Changes in these estimates and assumptions could materially affect the determination of fair value and goodwill impairment for each reporting unit. In connection with the acquisition of DSO on July 1, 2021, we recognized goodwill which had a balance of $1,342,000 as of December 31, 2023. In connection with the acquisition of Nexus on November 8, 2021, we recognized goodwill which had a balance of $1,703,000 as of December 31, 2023. In connection with the acquisition of Ceautamed on July 29, 2022, we recognized goodwill which had a balance of $0 as of December 31, 2023
Long-Lived Assets. We assess potential impairments to long-lived assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. An impairment loss is recognized when the undiscounted cash flows expected to be generated by an asset (or group of assets) is less than its carrying amount. Any required impairment loss is measured as the amount by which the asset’s carrying value exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to operating results. We recognized an impairment of the intangible assets associated with Nexus during the year ended December 31, 2023 of $2,897,737. We recognized an impairment of the license agreement associated with GSP during the year ended December 31, 2023 of $342,531. We recognized an impairment of the intangible assets of Ceautamed during the year ended December 31, 2023 of $2,603,233. There were no impairments recognized during the year ended December 31, 2022.
Lease Right-of-Use Asset. We record a right-of-use asset and lease liability on the balance sheet for all leases with terms longer than 12 months. Leases are classified either as finance or operating with the classification affecting the pattern of expense recognition. Lease liabilities are recognized based on the present value of the remaining lease payments and are discounted using the most reasonable incremental borrowing rate. We use the implicit rate when it is readily determinable. Since our lease does not provide an implicit rate, to determine the present value of lease payments, management uses our incremental borrowing rate based on the information available at lease commencement. Leases with a term of 12 months or less at inception are not recorded on our balance sheet and are expensed on a straight- line basis over the lease term.
Stock-based Compensation. We recognize expense for stock options and warrants granted over the vesting period based on the fair value of the award at the grant date, are valued using a Black-Scholes option pricing model to determine the fair market value of the stock options. We calculate the amount of tax benefit available by tracking each stock option award on an employee-by-employee basis and on a grant-by-grant basis. We then compare the recorded expense to the tax deduction received for each stock option grant. Our policy is to recognize forfeitures as they occur.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
The full text of our audited consolidated financial statements begins on page F-1 of this annual report.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
As required by Rule 13a-15(e) of the Exchange Act, our management has carried out an evaluation, with the participation and under the supervision of our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2023. Based upon, and as of the date of this evaluation, our chief executive officer and chief financial officer determined that our disclosure controls and procedures were not effective due to the material weakness in internal control over financial reporting described below. Notwithstanding the identified material weakness, management, including our chief executive officer and chief financial officer, believes the consolidated financial statements included in this report fairly represent, in all material respects, our financial condition, results of operations and cash flows as of and for the periods presented in accordance with GAAP.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Internal control over financial reporting refers to the process designed by, or under the supervision of, our principal executive officer and principal financial and accounting officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with GAAP, and includes those policies and procedures that:
| (1) | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| (2) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with the authorization of our management and directors; and |
| (3) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Our management evaluated the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this evaluation, management used the framework established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. The COSO framework summarizes each of the components of a company’s internal control system, including (i) the control environment, (ii) risk assessment, (iii) control activities, (iv) information and communication, and (v) monitoring.
We identified a material weakness in our internal controls over financial reporting. A material weakness is defined as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness identified related to the timely review and detection of potential accounting misstatements, which in the aggregate, constituted a material weakness. Notwithstanding the material weakness, we believe that our financial statements contained in this report fairly present our financial position, results of operations and cash flows for the periods covered by this report in all material respects.
As part of our plan to remediate this material weakness, we are performing a full review of our internal control procedures. We have implemented, and plan to continue to implement, new controls and new processes. We have hired and plan to continue to hire additional qualified personnel and establish more robust processes to support our internal control over financial reporting, including clearly defined roles and responsibilities. We anticipate time being required to complete the implementation and to assess and ensure the sustainability of these controls. The material weakness will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Controls over Financial Reporting
We regularly review our system of internal control over financial reporting and make changes to our processes and systems to improve controls and increase efficiency, while ensuring that we maintain an effective internal control environment. Changes may include such activities as implementing new, more efficient systems, consolidating activities, and migrating processes.
Except in connection with the remediation plan described above, there have been no changes in our internal control over financial reporting during the fourth quarter of fiscal year 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Executive Officers
Set forth below is information regarding our directors and executive officers as of the date of this report.
| Name | | Age | | Position |
| Alfonso J. Cervantes, Jr. | | 74 | | Executive Chairman of the Board |
| Darren C. Minton | | 41 | | Chief Executive Officer, President and Director |
| Alan B. Bergman | | 55 | | Chief Financial Officer |
| Loren Brown | | 61 | | Director |
| Heather Granato | | 54 | | Director |
| Arthur S. Reynolds | | 80 | | Director |
Alfonso J. Cervantes, Jr. Mr. Cervantes is the founder of our company and has served as our Executive Chairman since our inception. Mr. Cervantes is also Executive Chairman of Trilogy Capital Group, LLC, or Trilogy, a private equity firm and a principal stockholder, and served as Chairman and Chief Executive Officer of its predecessor, Trilogy Capital Partners, Inc. since 2002. Through more than 35 years as an executive in diversified businesses, Mr. Cervantes has accumulated extensive experience in the public markets with experience in corporate finance and emerging growth companies. His significant corporate finance experience includes mergers and acquisitions, initial public offerings, private placements as well as the reorganization of middle-market companies. Prior to joining us, Mr. Cervantes was the founder and Vice Chairman of Staffing 360 Solutions, Inc., from 2012 to 2015, where he facilitated, in association with Mr. Minton, multiple acquisitions and drove the company from pure startup to over $100 million in revenues in approximately two years. Mr. Cervantes is a graduate of Webster University with a degree in Communications. We believe Mr. Cervantes is qualified to serve on our board of directors due to his extensive corporate finance experience and knowledge of our company.
Darren C. Minton. Mr. Minton has served as our Chief Executive Officer since April 2022, President since September 2017 and as a member of our board of directors since November 2018. Mr. Minton has more than 15 years of capital markets experience in both small and large organizations. Over the years, his capacities have ranged from various executive positions, as well as president and chief executive officer positions with entrepreneurial ventures to established roles reporting to public company boards, with significant leadership and team building skills. Prior to joining us, Mr. Minton was a co-founder and Executive Vice President at Staffing 360 Solutions, Inc., from 2012 to 2017, where he facilitated the company’s alternative public offering and listing on Nasdaq. He previously served as President of Trilogy Capital Partners, Inc. and as an Analyst at Mesa West Capital, a privately held portfolio lender with a multi-billion dollar offering capability headquartered in Los Angeles, as well as First Republic Bank in Palo Alto. Mr. Minton is a graduate of Stanford University with a degree in Economics. We believe Mr. Minton is qualified to serve on our board of directors due to his extensive management and capital markets experience.
Alan B. Bergman. Mr. Bergman has served as our Chief Financial Officer since January 2021. Mr. Bergman’s expertise includes corporate financial management, mergers and acquisitions, corporate reorganizations, cost reduction and avoidance, financial analysis and reporting, IPO management, contract negotiations, ISO 9000 Quality Systems and SEC reporting and compliance. Prior to joining us, he served as Chief Financial Officer, Vice President Finance at Bright Mountain Media, Inc. from June 2019 to December 2020. Prior to that, he served as Vice President Finance at Greenlane Holdings, Inc., from December 2018 to May 2019. He previously served as Controller for Woodfield Distribution from October 2013 to February 2018 and as Vice President Finance at Latitude Solutions from May 2011 to March 2013. Mr. Bergman commenced his career in 2000 with Deloitte as a Senior Auditor and subsequently as Audit Manager at Mallah Furman, P.A. and as Senior Auditor at Weinberg & Company, P.A. In addition, Mr. Bergman has been an Adjunct Professor of Accounting at Florida Atlantic University and Millennia Atlantic University. Mr. Bergman received his Master’s in Accounting from University of Miami.
Loren Brown. Mr. Brown has been a member of our board of directors since March 2024. Mr. Brown is a seasoned executive with over 30 years of experience serving in executive positions with various nutraceutical and health and wellness businesses. He has served as the Director of Business Development for Human and Animal Services at SGS/Nutrasource Diagnostics, a global contract research organization with a global team of 96,000 employees that operates in 2,600 offices and labs worldwide. Mr. Brown specializes in regulatory compliance, product development, and testing solutions for dietary supplements, supporting clients in commercializing health and wellness products in the human and animal markets. His prior experience encompasses similar roles at a global contract research organization, as well as serving as the Associate Director of Business Development at Burdock Group, a comprehensive Food Safety and Regulatory Compliance Consulting Firm. We believe Mr. is qualified to serve on our board of directors due to his extensive experience in our industry.
Heather Granato. Ms. Granato has been a member of our board of directors since April 2024. Ms. Granato is a 30-year veteran of the natural products industry. In February 2024, she founded, and currently serves as President of, Nutrachievement, which provides consultancy services to companies in the nutraceutical industry. Prior to that, she served as Vice President of Partnerships & Sustainability for Informa PLC, an international events, digital services and academic knowledge group, from June 2022 to December 2023, and served as Vice President of Content for Informa PLC from December 2012 to June 2022. Ms. Granato has been a presenter at events including SupplySide, Vitafoods, Natural Products Expo, the Natural Gourmet Show and the Folio: Show. Her publishing experience includes Natural Products Insider, Food Product Design, Country Living’s Healthy Living, Natural Foods Merchandiser, Delicious Magazine and WomenOf.com. She was named to the FOLIO: 100 list of top media professionals in 2018, received the 2014 Visionary Award and the 2018 Journalistic Excellence Award from the American Herbal Products Association, and was recognized by the United Natural Products Alliance in 2023 with its Ignition…Liftoff Award. Ms. Granato graduated magna cum laude from the University of Richmond, Virginia, in 1992 with a bachelor’s degree in journalism. The board believes that Ms. Granato is qualified to serve on the board due to her extensive experience in the nutraceutical industry, including in the areas of journalistic outreach, content creation and marketing initiatives.
Arthur S. Reynolds. Mr. Reynolds has been a member of our board of directors since October 2022. Mr. Reynolds is an accomplished international financier bringing more than 35 years of capital markets and financial experience providing cross-border financial consulting services in Europe for clients principally located in the United States. He is the founder of Rexon Limited of London and New York where, since 1999, he has served as managing director. Mr. Reynolds was founder and, from 1997 to 1999, managing partner of London-based Value Management & Research (UK) Limited. Mr. Reynolds was the founder and, from 1982 to 1997, served as managing director of Ferghana Financial Services Limited. Prior thereto, Mr. Reynolds held executive positions at Merrill Lynch International Bank Limited, Banque de la Société Financière Européene, J.P. Morgan & Company and Mobil Corporation. Between 2006 and 2016, Mr. Reynolds served on the Board of Directors of ThermoEnergy Corporation, first as Chairman of the Audit Committee, subsequently as Chief Financial Officer, and finally as Chairman. Mr. Reynolds is a member of the Board of the International Festival Society and serves as Chairman of the Elgar Society’s North America Branch. Mr. Reynolds holds an B.A. from Columbia University, a M.A. from Cambridge University, and an M.B.A. in Finance from New York University. Mr. Reynolds brings to the board extensive financial and executive experience across multiple sectors, with special strength in the international arena. We believe that Mr. Reynolds possesses the appropriate skills to act as the audit committee chair.
Our directors currently have terms which will end at our next annual meeting of the stockholders or until their successors are elected and qualify, subject to their prior death, resignation or removal. Officers serve at the discretion of the board of directors. There is no arrangement or understanding between any director or executive officer and any other person pursuant to which he was or is to be selected as a director, nominee or officer.
Family Relationships
There are no family relationships among any of our officers or directors.
Involvement in Certain Legal Proceedings
To the best of our knowledge, except as described below, none of our directors or executive officers has, during the past ten years:
| ● | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| ● | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| ● | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| ● | been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| ● | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Corporate Governance
Governance Structure
We chose to appoint a separate Chairman of the Board who is not our Chief Executive Officer. Our board of directors has made this decision based on their belief that a separate Chairman of the Board can act as a balance to the Chief Executive Officer, who also serves as a non-independent director.
The Board’s Role in Risk Oversight
The board of directors oversees that the assets of our company are properly safeguarded, that the appropriate financial and other controls are maintained, and that our business is conducted wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the board’s oversight of the various risks facing our company. In this regard, our board seeks to understand and oversee critical business risks. Our board does not view risk in isolation. Risks are considered in virtually every business decision and as part of our business strategy. Our board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is essential for our company to be competitive on a global basis and to achieve its objectives.
While the board oversees risk management, company management is charged with managing risk. Management communicates routinely with the board and individual directors on the significant risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management.
Our board administers its risk oversight function as a whole by making risk oversight a matter of collective consideration; however, much of the work is delegated to committees, which will meet regularly and report back to the full board. The audit committee oversees risks related to our financial statements, the financial reporting process, accounting and legal matters, the compensation committee evaluates the risks and rewards associated with our compensation philosophy and programs, and that the nominating and corporate governance committee evaluates risk associated with management decisions and strategic direction.
Independent Directors
Our board of directors has determined that all of our directors, other than Messrs. Cervantes and Minton, qualify as “independent” directors in accordance with the rules and regulations of Nasdaq. Messrs. Cervantes and Minton are not considered independent because they are employees of our company and/or its subsidiaries. In making its independence determinations, the board considered, among other things, relevant transactions between our company and entities associated with the independent directors, as described under the heading Item 13 “Certain Relationships and Related Party Transactions, and Director Independence,” and determined that none have any relationship with our company or other relationships that would impair the directors’ independence.
Committees of the Board of Directors
Our board has established an audit committee, a compensation committee and a nominating and corporate governance committee, each with its own charter approved by the board. Each committee’s charter available on our website at www.smartforlifecorp.com. In addition, our board of directors may, from time to time, designate one or more additional committees, which shall have the duties and powers granted to it by our board of directors.
Audit Committee
Loren Brown, Heather Granato and Arthur S. Reynolds have been appointed to serve on our audit committee, with Mr. Reynolds serving as the chair. Our board has determined that each member of the audit committee is an independent director under Nasdaq’s rules and under Rule 10A-3 under the Exchange Act. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq. Our board has determined that Mr. Reynolds is an “audit committee financial expert” as defined by applicable SEC rules and has the requisite financial sophistication as defined under the applicable Nasdaq rules and regulations.
The audit committee oversees our accounting and financial reporting processes and the audits of our consolidated financial statements. The audit committee is responsible for, among other things: (i) retaining and overseeing our independent accountants; (ii) assisting the board in its oversight of the integrity of our financial statements, the qualifications, independence and performance of our independent auditors and our compliance with legal and regulatory requirements; (iii) reviewing and approving the plan and scope of the internal and external audit; (iv) pre-approving any audit and non-audit services provided by our independent auditors; (v) approving the fees to be paid to our independent auditors; (vi) reviewing with our chief executive officer and chief financial officer and independent auditors the adequacy and effectiveness of our internal controls; (vii) reviewing and approving related party transactions; (viii) reviewing hedging transactions; and (ix) reviewing and assessing annually the audit committee’s performance and the adequacy of its charter.
Compensation Committee
Loren Brown, Heather Granato and Arthur S. Reynolds and have been appointed to serve on our compensation committee, with Ms. Granato serving as the chair. Our board has determined that each member of the compensation committee is independent under the applicable rules and regulations of Nasdaq and is a “non-employee director” as defined under Rule 16b-3 of the Exchange Act.
The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. The compensation committee is responsible for, among other things: (i) reviewing and approving the compensation of our executive officers; (ii) evaluating and making recommendations to the board regarding the compensation of our independent directors; (iii) evaluating and making recommendations to the board regarding equity-based and incentive compensation plans, policies and programs; and (iv) administering all such equity-based and incentive compensation plans, policies and programs.
Nominating and Corporate Governance Committee
Loren Brown, Heather Granato and Arthur S. Reynolds and have been appointed to serve on our nominating and corporate governance committee, with Mr. Brown serving as the chair. Our board has determined that each member of the nominating and corporate governance committee is an independent director under the applicable rules and regulations of Nasdaq.
The nominating and corporate governance committee assists the board in selecting individuals qualified to become directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things: (i) identifying and evaluating individuals qualified to become members of the board by reviewing nominees for election to the board submitted by stockholders and recommending to the board director nominees for each annual meeting of stockholders and for election to fill any vacancies on the board, (ii) advising the board with respect to board organization, desired qualifications of board members, the membership, function, operation, structure and composition of committees (including any committee authority to delegate to subcommittees), and self-evaluation and policies, (iii) advising on matters relating to corporate governance and monitoring developments in the law and practice of corporate governance, and (iv) overseeing compliance with our code of business conduct and ethics and conduct of our officers and directors.
The nominating and corporate governance committee’s methods for identifying candidates for election to the board (other than those proposed by our stockholders, as discussed below) will include the solicitation of ideas for possible candidates from a number of sources - members of our board, our executives, individuals personally known to the members of our board, and other research. The nominating and corporate governance committee may also, from time-to-time, retain one or more third-party search firms to identify suitable candidates.
In making director recommendations, the nominating and corporate governance committee may consider some or all of the following factors: (i) the candidate’s judgment, skill, experience with other organizations of comparable purpose, complexity and size, and subject to similar legal restrictions and oversight; (ii) the interplay of the candidate’s experience with the experience of other board members; (iii) the extent to which the candidate would be a desirable addition to the board and any committee thereof; (iv) whether or not the person has any relationships that might impair his or her independence; and (v) the candidate’s ability to contribute to the effective management of our company, taking into account the needs of our company and such factors as the individual’s experience, perspective, skills and knowledge of the industry in which we operate.
A stockholder may nominate one or more persons for election as a director at an annual meeting of stockholders if the stockholder complies with the notice and information provisions contained in our bylaws. Such notice must be in writing not less than 90 days and not more than 120 days prior to the anniversary date of the preceding year’s annual meeting of stockholders or as otherwise required by requirements of the Exchange Act. In addition, stockholders furnishing such notice must be a holder of record on both (i) the date of delivering such notice and (ii) the record date for the determination of stockholders entitled to vote at such meeting.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. Such code of ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, and reporting of violations of the code.
We are required to disclose any amendment to, or waiver from, a provision of our code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar functions. We intend to use our website as a method of disseminating this disclosure, as permitted by applicable SEC rules. Any such disclosure will be posted to our website within four (4) business days following the date of any such amendment to, or waiver from, a provision of our code of ethics.
Insider Trading Policy
We have adopted an insider trading policy which prohibits our directors, officers and employees from engaging in transactions in our common stock while in the possession of material non-public information; engaging in transactions in the stock of other companies while in possession of material non-public information that they become aware of in performing their duties; and disclosing material non-public information to unauthorized persons outside our company.
Our insider trading policy restricts trading by directors, officers and certain key employees during blackout periods, which generally begin 15 calendar days before the end of each fiscal quarter and end two business days after the issuance of our earnings release for the quarter. Additional blackout periods may be imposed with or without notice, as the circumstances require.
Our insider trading policy also prohibits our directors, officers and employees from purchasing financial instruments (such as prepaid variable forward contracts, equity swaps, collars and exchange funds) designed to hedge or offset any decrease in the market value of our common stock they hold, directly or indirectly. In addition, directors, officers and employees are expressly prohibited from pledging our common stock to secure personal loans or other obligations, including by holding their common stock in a margin account, unless such arrangement is specifically approved in advance by the administrator of our insider trading policy, or making short-sale transactions in our common stock.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than 10% of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the company. Officers, directors and greater than 10% stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. We believe, based solely on a review of the copies of such reports furnished to us and representations of these persons, that all reports were timely filed for the year ended December 31, 2023.
ITEM 11. EXECUTIVE COMPENSATION.
Summary Compensation Table - Years Ended December 31, 2023 and 2022
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the noted periods. No other executive officers received total annual salary and bonus compensation in excess of $100,000.
| Name and Principal Position | | Year | | Salary
($) | | | Bonus
($) | | | Stock
Awards
($)(1) | | | Option
Awards
($)(1) | | | Total
($) | |
| Alfonso J. Cervantes, Jr., | | 2023 | | | 300,000 | | | | - | | | | - | | | | 385,323 | | | | 685,323 | |
| Executive Chairman | | 2022 | | | 300,000 | | | | - | | | | 234,500 | | | | 154,200 | | | | 688,700 | |
| Darren C. Minton, | | 2023 | | | 250,000 | | | | - | | | | - | | | | 71,356 | | | | 321,356 | |
| Chief Executive Officer | | 2022 | | | 200,000 | | | | - | | | | 93,800 | | | | 51,900 | | | | 345,700 | |
| Alan Bergman, | | 2023 | | | 250,000 | | | | 25,000 | | | | - | | | | 28,452 | | | | 303,452 | |
| Chief Financial Officer | | 2022 | | | 200,000 | | | | 25,000 | | | | 32,830 | | | | 51,900 | | | | 309,730 | |
| (1) | The amount is equal to the aggregate grant-date fair value with respect to the awards, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. |
Employment Agreements
On July 1, 2020, we entered into an employment agreement with Mr. Cervantes, our Executive Chairman. Pursuant to the employment agreement, Mr. Cervantes was entitled to an annual base salary of $200,000, which was increased to $250,000 on July 1, 2021 and was increased to $300,000 on November 8, 2021. In addition, Mr. Cervantes is eligible to receive a bonus of $100,000 for each bona fide acquisition that we complete. He will also be entitled to an annual bonus of 20% of his base salary based on meeting company objectives and the remainder will be based on meeting mutually agreed employee objections or as otherwise determined by the board. Mr. Cervantes is eligible to participate in all equity incentive plans and other employee benefit plans, including health insurance, commensurate with his position. The term of Mr. Cervantes’ agreement is five years, commencing July 1, 2020 and terminating June 30, 2025. His employment agreement is terminable on 30 days’ notice; however, we may terminate Mr. Cervantes’ employment without notice for cause (as defined in the employment agreement). If we terminate Mr. Cervantes’ employment without cause or due to a disability, he is entitled to twelve (12) months of severance pay equal to the base salary of the current year, which will be paid on a bi-weekly schedule. The employment agreement contains standard confidentiality provisions and restrictive covenants prohibiting Mr. Cervantes from owning or operating a business that competes with our company during the term of his employment.
On July 1, 2020, we entered into an employment agreement with Mr. Minton, our Chief Executive Officer and President. Pursuant to the employment agreement, Mr. Minton was entitled to an annual base salary of $200,000, which was increased to $250,000 on July 1, 2021. He will also be entitled to receive an annual bonus of up to 20% of his base salary based on meeting mutually agreed objectives or as otherwise determined by the board. Mr. Minton is eligible to participate in all equity incentive plans and other employee benefit plans, including health insurance, commensurate with his position. The term of Mr. Minton’s agreement is three years, commencing July 1, 2020 and terminating June 30, 2023. Mr. Minton’s contract was extended for 12 months at an annual base salary of $300,000 and continuation of all other benefits. His employment agreement is terminable on 30 days’ notice; however, we may terminate Mr. Minton’s employment without notice for cause (as defined in the employment agreement). If we terminate Mr. Minton’s employment without cause or due to disability, he is entitled to six (6) months of severance pay equal to the base salary of the current year, which will be paid on a bi-weekly schedule. The employment agreement contains standard confidentiality provisions and restrictive covenants prohibiting Mr. Minton from owning or operating a business that competes with our company during the term of his employment.
On December 12, 2020, we entered into an employment agreement with Mr. Bergman, our Chief Financial Officer. Pursuant to the employment agreement, Mr. Bergman was entitled to an annual base salary of $175,000, which was increased to $200,000 on January 1, 2022 and to $250,000 on January 1, 2023. He will also be entitled to receive an annual bonus between 10% to 20% of his base salary based on meeting mutually agreed objectives or as otherwise determined by the board. Mr. Bergman is eligible to participate in all equity incentive plans and other employee benefit plans, including health insurance, commensurate with his position. The term of Mr. Bergman’s agreement commenced on January 1, 2021 and will continue until terminated. His employment agreement is terminable on 90 days’ notice; however, we may terminate Mr. Bergman’s employment without notice for cause (as defined in the employment agreement). If we terminate Mr. Bergman’s employment without cause or due to a disability, he is entitled to one (1) month of severance pay equal to the base salary of the current year, which will be paid on a bi-weekly schedule. The employment agreement contains standard confidentiality provisions and restrictive covenants prohibiting Mr. Bergman from owning or operating a business that competes with our company during the term of his employment.
Retirement Benefits
We have not maintained, and do not currently maintain, a defined benefit pension plan or nonqualified deferred compensation plan. We currently make available a retirement plan intended to provide benefits under Section 401(k) of the Internal Revenue Code of 1986, as amended, or the Code, pursuant to which employees, including the executive officers named above, can make voluntary pre-tax contributions.
Potential Payments Upon Termination or Change in Control
As described under “—Employment Agreements” above, Messrs. Cervantes, Minton and Bergman are entitled severance if their employment is terminated without cause.
Outstanding Equity Awards at Fiscal Year-End
The following table includes certain information with respect to the value of all unexercised options and unvested shares of restricted stock previously awarded to the executive officers named above at the fiscal year ended December 31, 2023.
| | | Option Awards |
| Name | | Number of Securities Underlying Unexercised
Options (#)
Exercisable | | | Number of Securities Underlying Unexercised
Options (#)
Unexercisable | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned
Options (#) | | | Option Exercise
Price ($) | | | Option Expiration
Date |
| Alfonso J. Cervantes, Jr. | | | 318 | | | | - | | | | - | | | $ | 31.500 | | | 09/14/2030 |
| Alfonso J. Cervantes, Jr. | | | 16 | | | | 80 | | | | - | | | $ | 2,182.95 | | | 08/12/2027 |
| Alfonso J. Cervantes, Jr. | | | - | | | | 12,858 | | | | - | | | $ | 23.31 | | | 09/05/2033 |
| Alfonso J. Cervantes, Jr. | | | 12,858 | | | | - | | | | - | | | $ | 20.7921 | | | 09/06/2033 |
| Darren C. Minton | | | 80 | | | | - | | | | - | | | $ | 31.500 | | | 09/14/2030 |
| Darren C. Minton | | | 5 | | | | 27 | | | | - | | | $ | 1,984.50 | | | 08/12/2032 |
| Darren C. Minton | | | - | | | | 2,381 | | | | - | | | $ | 23.31 | | | 09/05/2033 |
| Darren C. Minton | | | 2,381 | | | | - | | | | - | | | $ | 20.7921 | | | 09/06/2033 |
| Alan B. Bergman | | | 5 | | | | 27 | | | | - | | | $ | 1,984.50 | | | 08/12/2032 |
| Alan B. Bergman | | | - | | | | 953 | | | | - | | | $ | 23.31 | | | 09/05/2033 |
| Alan B. Bergman | | | 953 | | | | - | | | | - | | | $ | 20.7921 | | | 09/06/2033 |
Director Compensation
The table below sets forth the compensation paid to our independent directors during the fiscal year ended December 31, 2023.
| Name | | Fees Earned
or Paid in
Cash
($) | | | Option
Awards
($)(1) | | | Total
($) | |
| Robert S. Rein | | | 18,000 | | | | 78,492 | | | | 96,492 | |
| Roger Conley Wood | | | 18,000 | | | | 21,407 | | | | 39,407 | |
| Arthur S. Reynolds | | | 43,000 | | | | 21,407 | | | | 64,407 | |
| (1) | The amount is equal to the aggregate grant-date fair value with respect to the awards, computed in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718. |
Commencing in March 2022, our independent directors received an annual fee of $10,000, payable monthly, with each committee chair receiving an additional annual fee of $4,000 and each other committee member receiving an additional annual fee of $2,000; provided that Mr. Reynolds receives an annual fee of $35,000. Each independent director also receives $2,000 for each in person board meeting and may be reimbursed for pre-approved reasonable business-related expenses incurred in good faith in connection with his or her duties to our company.
March 8, 2024, we modified the compensation paid to our independent directors to (i) a cash fee of $10,000 per quarter and (ii) an annual stock option grant for the purchase of 5,715 shares of common stock, which will vest quarterly over a one-year period.
2020 Stock Incentive Plan
On September 14, 2020, our board adopted the Bonne Santé Group, Inc. 2020 Stock Incentive Plan, or the 2020 Plan, which was approved by our stockholders on September 14, 2020. The following is a summary of certain significant features of the 2020 Plan.
Purposes: The purpose of the 2020 Plan is to offer selected employees, consultants, advisors and outside directors the opportunity to acquire equity in our company.
Types of Awards: Awards that may be granted include incentive stock options as described in section 422(b) of the Code, non-qualified stock options (i.e., options that are not incentive stock options) and awards of restricted stock. These awards offer our employees, consultants, advisors and outside directors the possibility of future value, depending on the long-term price appreciation of our common stock and the award holder’s continuing service with our company or one or more of its subsidiaries.
Eligible Recipients: Persons eligible to receive awards under the 2020 Plan will be those employees, consultants, advisors and outside directors of our company and its subsidiaries who are selected by the Administrator.
Administration: The 2020 Plan is administered by our compensation committee. Among other things, the administrator has the authority to select persons who will receive awards, determine the types of awards and the number of shares to be covered by awards, and to establish the terms, conditions, restrictions and other provisions of awards.
Shares Available: The maximum number of shares of common stock that may be delivered to participants under the 2020 Plan is 635, subject to adjustment for certain corporate changes affecting the shares, such as stock splits. Shares subject to an award under the 2020 Plan for which the award is canceled, forfeited or expires again become available for grants under the 2020 Plan. Shares subject to an award that is settled in cash will not again be made available for grants under the 2020 Plan. As of the date of this report, no shares remain available for issuance under the 2020 Plan.
Stock Options:
General. Subject to the provisions of the 2020 Plan, the administrator has the authority to determine all grants of stock options. That determination will include: (i) the number of shares subject to any option; (ii) the exercise price per share; (iii) the expiration date of the option; (iv) the manner, time and date of permitted exercise; (v) other restrictions, if any, on the option or the shares underlying the option; and (vi) any other terms and conditions as the administrator may determine.
Option Price. The exercise price for stock options will be determined at the time of grant. Normally, the exercise price will not be less than the fair market value on the date of grant, as determined in good faith by the administrator. As a matter of tax law, the exercise price for any incentive stock option awarded may not be less than the fair market value of the shares on the date of grant. However, incentive stock option grants to any person owning more than 10% of our voting stock must have an exercise price of not less than 110% of the fair market value on the grant date.
Exercise of Options. An option may be exercised only in accordance with the terms and conditions for the option agreement as established by the administrator at the time of the grant. The option must be exercised by notice to us, accompanied by payment of the exercise price. Payments may be made in cash or, at the option of the administrator, by actual or constructive delivery of shares of common stock to the holder of the option based upon the fair market value of the shares on the date of exercise.
Expiration or Termination. Options, if not previously exercised, will expire on the expiration date established by the administrator at the time of grant; provided that such term cannot exceed ten years and that such term of an incentive stock option granted to a holder of more than 10% of our voting stock cannot exceed five years. Options will terminate before their expiration date if the holder’s service with us terminates before the expiration date. The option may remain exercisable for specified periods after certain terminations of service, including terminations as a result of death, disability or retirement, with the precise period during which the option may be exercised to be established by the administrator and reflected in the grant evidencing the award.
Stock Awards: Stock awards can also be granted under the 2020 Plan. A stock award is a grant of shares of common stock. These awards will be subject to such conditions, restrictions and contingencies as the administrator shall determine at the date of grant. Those may include requirements for continuous service and/or the achievement of specified performance goals.
Other Material Provisions: Awards will be evidenced by a written agreement, in such form as may be approved by the administrator. In the event of various changes to our capitalization, such as stock splits, stock dividends and similar re-capitalizations, an appropriate adjustment will be made by the administrator to the number of shares covered by outstanding awards or to the exercise price of such awards. The administrator is also permitted to include in the written agreement provisions that provide for certain changes in the award in the event of a change of control of our company, including acceleration of vesting. Except as otherwise determined by the administrator at the date of grant, awards will not be transferable, other than by will or the laws of descent and distribution. Prior to any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any employee withholding tax requirements. The board also has the authority, at any time, to discontinue the granting of awards. The board also has the authority to alter or amend the 2020 Plan or any outstanding award or may terminate the 2020 Plan as to further grants, provided that no amendment will, without the approval of our stockholders, increase the number of shares available under the 2020 Plan or change the persons eligible for awards under the 2020 Plan. No amendment that would adversely affect any outstanding award made under the 2020 Plan can be made without the consent of the holder of such award.
2022 Equity Incentive Plan
On January 13, 2022, our board of directors adopted the Smart for Life, Inc. 2022 Equity Incentive Plan, or the 2022 Plan, which was approved by our stockholders on January 13, 2022. On January 12, 2023, our board approved an amendment to the 2022 Plan to increase the number of shares reserved for issuance under the 2022 Plan from 635 shares to 22,222 shares, which was approved by our stockholders on March 15, 2023. The following is a summary of certain significant features of the 2022 Plan.
Purposes: The purposes of the 2022 Plan are to attract and retain officers, employees, directors and consultants for our company and its subsidiaries; motivate them by means of appropriate incentives to achieve long-range goals; provide incentive compensation opportunities; and further align their interests with those of our stockholders through compensation that is based on our common stock.
Types of Awards: Awards that may be granted include: (a) incentive stock options, (b) non-qualified stock options, (c) stock appreciation rights, (d) restricted awards, (e) performance share awards, and (f) performance compensation awards. These awards offer our officers, employees, consultants and directors the possibility of future value, depending on the long-term price appreciation of our common stock and the award holder’s continuing service with our company.
Eligible Recipients: Persons eligible to receive awards under the 2022 Plan will be those officers, employees, directors and consultants of the Company and its subsidiaries who are selected by the administrator.
Administration: The 2022 Plan is administered by our compensation committee. Among other things, the administrator has the authority to select persons who will receive awards, determine the types of awards and the number of shares to be covered by awards, and to establish the terms, conditions, performance criteria, restrictions and other provisions of awards. The administrator has authority to establish, amend and rescind rules and regulations relating to the 2022 Plan.
Shares Available: The maximum number of shares of our common stock that may be delivered to participants under the 2022 Plan is 22,222, subject to adjustment for certain corporate changes affecting the shares, such as stock splits. Shares subject to an award under the 2022 Plan for which the award is canceled, forfeited or expires again become available for grants under the 2022 Plan. Shares subject to an award that is settled in cash will not again be made available for grants under the 2022 Plan. As of the date of this report, 4,404 shares remain available for issuance under the 2022 Plan.
Stock Options:
General. Stock options give the option holder the right to acquire from us a designated number of shares of common stock at a purchase price that is fixed upon the grant of the option. Stock options granted may be either tax-qualified stock options (so-called “incentive stock options”) or non-qualified stock options. Subject to the provisions of the 2022 Plan, the administrator has the authority to determine all grants of stock options. That determination will include: (i) the number of shares subject to any option; (ii) the exercise price per share; (iii) the expiration date of the option; (iv) the manner, time and date of permitted exercise; (v) other restrictions, if any, on the option or the shares underlying the option; and (vi) any other terms and conditions as the administrator may determine.
Option Price. The exercise price for stock options will be determined at the time of grant. Normally, the exercise price will not be less than the fair market value on the date of grant. As a matter of tax law, the exercise price for any incentive stock option awarded may not be less than the fair market value of the shares on the date of grant. However, incentive stock option grants to any person owning more than 10% of our voting stock must have an exercise price of not less than 110% of the fair market value on the grant date.
Exercise of Options. An option may be exercised only in accordance with the terms and conditions for the option agreement as established by the administrator at the time of the grant. The option must be exercised by notice to us, accompanied by payment of the exercise price. Payments may be made in cash or, at the option of the administrator, by actual or constructive delivery of shares of common stock to the holder of the option based upon the fair market value of the shares on the date of exercise.
Expiration or Termination. Options, if not previously exercised, will expire on the expiration date established by the administrator at the time of grant. In the case of incentive stock options, such term cannot exceed ten years provided that in the case of holders of more than 10% of our voting stock, such term cannot exceed five years. Options will terminate before their expiration date if the holder’s service with our company or a subsidiary terminates before the expiration date. The option may remain exercisable for specified periods after certain terminations of employment, including terminations as a result of death, disability or retirement, with the precise period during which the option may be exercised to be established by the administrator and reflected in the grant evidencing the award.
Incentive and Non-Qualified Options. As described elsewhere in this summary, an incentive stock option is an option that is intended to qualify under certain provisions of the Code for more favorable tax treatment than applies to non-qualified stock options. Any option that does not qualify as an incentive stock option will be a non-qualified stock option. Under the Code, certain restrictions apply to incentive stock options. For example, the exercise price for incentive stock options may not be less than the fair market value of the shares on the grant date and the term of the option may not exceed ten years. In addition, an incentive stock option may not be transferred, other than by will or the laws of descent and distribution, and is exercisable during the holder’s lifetime only by the holder. In addition, no incentive stock options may be granted to a holder that is first exercisable in a single year if that option, together with all incentive stock options previously granted to the holder that also first become exercisable in that year, relate to shares having an aggregate fair market value in excess of $100,000, measured at the grant date.
Stock Appreciation Rights: Stock appreciation rights, or SARs, which may be granted alone or in tandem with options, have an economic value similar to that of options. When an SAR for a particular number of shares is exercised, the holder receives a payment equal to the difference between the market price of the shares on the date of exercise and the exercise price of the shares under the SAR. Again, the exercise price for SARs normally is the market price of the shares on the date the SAR is granted. Under the 2022 Plan, holders of SARs may receive this payment - the appreciation value - either in cash or shares valued at the fair market value on the date of exercise. The form of payment will be determined by us.
Restricted Awards: Restricted awards are shares awarded to participants at no cost. Restricted awards can take the form of awards of restricted stock, which represent issued and outstanding shares subject to vesting criteria, or restricted stock units, which represent the right to receive shares subject to satisfaction of the vesting criteria. Restricted stock awards are forfeitable and non-transferable until the shares vest. The vesting date or dates and other conditions for vesting are established when the shares are awarded. These awards will be subject to such conditions, restrictions and contingencies as the administrator shall determine at the date of grant. Those may include requirements for continuous service and/or the achievement of specified performance goals.
Performance Awards: A performance award is an award that may be in the form of cash or shares or a combination, based on the attainment of pre-established performance goals and other conditions, restrictions and contingencies identified by the administrator.
Performance Criteria: Under the 2022 Plan, one or more performance criteria will be used by the administrator in establishing performance goals. Any one or more of the performance criteria may be used on an absolute or relative basis to measure the performance of our company, as the administrator may deem appropriate, or as compared to the performance of a group of comparable companies, or published or special index that the administrator deems appropriate. In determining the actual size of an individual performance compensation award, the administrator may reduce or eliminate the amount of the award through the use of negative discretion if, in its sole judgment, such reduction or elimination is appropriate. The administrator shall not have the discretion to (i) grant or provide payment in respect of performance compensation awards if the performance goals have not been attained or (ii) increase a performance compensation award above the maximum amount payable under the 2022 Plan.
Other Material Provisions: Awards will be evidenced by a written agreement, in such form as may be approved by the administrator. In the event of various changes to the capitalization of our company, such as stock splits, stock dividends and similar re-capitalizations, an appropriate adjustment will be made by the administrator to the number of shares covered by outstanding awards or to the exercise price of such awards. The administrator is also permitted to include in the written agreement provisions that provide for certain changes in the award in the event of a change of control of our company, including acceleration of vesting. Except as otherwise determined by the administrator at the date of grant, awards will not be transferable, other than by will or the laws of descent and distribution. Prior to any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any employee withholding tax requirements. Our board also has the authority, at any time, to discontinue the granting of awards. The board also has the authority to alter or amend the 2022 Plan or any outstanding award or may terminate the 2022 Plan as to further grants, provided that no amendment will, without the approval of our stockholders, to the extent that such approval is required by law or the rules of an applicable exchange, increase the number of shares available under the 2022 Plan, change the persons eligible for awards under the 2022 Plan, extend the time within which awards may be made, or amend the provisions of the 2022 Plan related to amendments. No amendment that would adversely affect any outstanding award made under the 2022 Plan can be made without the consent of the holder of such award.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of September 20, 2024 for (i) each of our named executive officers and directors; (ii) all of our named executive officers and directors as a group; and (iii) each other shareholder known by us to be the beneficial owner of more than 5% of our outstanding common stock. Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o our company, 990 Biscayne Blvd, Suite 505, Miami, Florida 33132.
| Name and Address of Beneficial Owner | | Title of Class | | Amount and Nature of Beneficial
Ownership(1) | | | Percent of
Class(2) | |
| Alfonso J. Cervantes, Jr., Executive Chairman(3) | | Common Stock | | | 21,301 | | | | * | |
| Darren C. Minton, Chief Executive Officer and Director(4) | | Common Stock | | | 5,401 | | | | * | |
| Alan B. Bergman, Chief Financial Officer(5) | | Common Stock | | | 977 | | | | * | |
| Loren Brown, Director | | Common Stock | | | 0 | | | | * | |
| Heather Granato, Director | | Common Stock | | | 0 | | | | * | |
| Arthur S. Reynolds, Director(6) | | Common Stock | | | 819 | | | | * | |
| All executive officers and directors as a group (6 persons above) | | Common Stock | | | 28,498 | | | | * | |
| (1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares that such person or any member of such group has the right to acquire within sixty (60) days. For purposes of computing the percentage of outstanding shares of our common shares held by each person or group of persons named above, any shares that such person or persons has the right to acquire within sixty (60) days of September 20, 2024 are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership by any person. |
| (2) | Based on 7,090,728 shares of common stock issued and outstanding as of September 20, 2024. |
| (3) | Includes 635 shares of common stock, 5,901 shares of common stock issuable upon the conversion of 3,711 shares of series B preferred stock, 13,200 shares of common stock which Mr. Cervantes has the right to acquire within 60 days through the exercise of vested options and 1,565 shares of common stock held by Trilogy. Mr. Cervantes is the Chairman of Trilogy and has voting and investment power over the shares held by it. Mr. Cervantes disclaims beneficial ownership of the shares held by Trilogy except to the extent of his pecuniary interest, if any, in such shares. |
| (4) | Includes 429 shares of common stock, 2,503 shares of common stock issuable upon the conversion of 1,574 shares of series B preferred stock and 2,469 shares of common stock which Mr. Minton has the right to acquire within 60 days through the exercise of vested options. |
| (5) | Includes 16 shares of common stock held directly and 961 shares of common stock which Mr. Bergman has the right to acquire within 60 days through the exercise of vested options. |
| (6) | Includes 32 shares of common stock, 72 shares of common stock issuable upon the conversion of 45 shares of series B preferred stock and 715 shares of common stock which Mr. Reynolds has the right to acquire within 60 days through the exercise of vested options. |
Changes in Control
We do not currently have any arrangements which if consummated may result in a change of control of our company.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth certain information about the securities authorized for issuance under our incentive plans as of December 31, 2023.
| Plan Category | | Number of
securities to
be issued upon
exercise of
outstanding
options, warrants
and rights
(a) | | | Weighted-
average exercise price of outstanding options, warrants and rights
(b) | | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |
| Equity compensation plans approved by security holders | | | 21,340 | | | $ | 42.41 | | | | 318 | |
| Equity compensation plans not approved by security holders | | | 21,919 | | | $ | 23.31 | | | | 4,271 | |
| Total | | | 43,259 | | | | | | | | 4,589 | |
On September 14, 2020, we established the 2020 Plan. As of December 31, 2023, the maximum number of shares of common stock that may be issued pursuant to awards granted under the 2020 Plan is 635 shares. On January 13, 2022, we established the 2022 Plan. On September 5, 2023, our board of directors approved an amendment to the 2022 Plan to (i) increase the number of shares of common stock reserved for issuance under the 2022 Plan to 47,620 shares and (ii) add a provision pursuant to which the number of shares available for issuance under the 2022 Plan shall be automatically increased on January 1 of each year by an amount equal to the lesser of (i) ten percent (10%) of the total number of shares of common stock issued and outstanding on December 31 of the immediately preceding calendar year or (ii) such number of shares of common stock as may be established by the board of directors. This amendment has not yet been approved by stockholders. As of December 31, 2023, the maximum number of shares of common stock that may be issued pursuant to awards granted under the 2022 Plan is 47,620 shares.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Transactions with Related Persons
The following includes a summary of transactions since the beginning of our 2022 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under Item 11 “Executive Compensation” above). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
We are party to a management services agreement with Trilogy, a company controlled by our Executive Chairman. For the year ended December 31, 2023 and 2022, we incurred expenses related to Trilogy of $46,686 and $0, respectively, and paid Trilogy $17,858 and $0, respectively, for services rendered under a consulting agreement. At December 31, 2023 and 2022, the related party receivable was $332,142 and $0, respectively. Subsequent to December 31, 2023, the receivable balance as of December 31, 2023 was collected. At December 31, 2023 and 2022, the accrued expenses related party was $264,141 and $947,951, respectively.
Director Independence
Our board of directors has determined that Loren Brown, Heather Granato and Arthur S. Reynolds are independent within the meaning of the rules of Nasdaq.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Independent Auditors’ Fees
The following is a summary of the fees billed to us for professional services rendered for the fiscal years ended December 31, 2023 and 2022. The 2023 services, which related to the review of the Form 10-Q for the quarter ended September 30, 2023 and audit of the financial statements for the year ended December 31, 2023, were provided by RBSM LLP, and the 2022 services were provided by Daszkal Bolton LLP.
| | | Years Ended December 31, | |
| | | 2023 | | | 2022 | |
| Audit Fees | | $ | 244,152 | | | $ | 106,750 | |
| Audit-Related Fees | | | — | | | | — | |
| Tax Fees | | | 22,080 | | | | 7,000 | |
| All Other Fees | | | — | | | | — | |
| TOTAL | | $ | 266,232 | | | $ | 113,750 | |
“Audit Fees” consisted of fees billed for professional services rendered by the principal accountant for the audit of our annual financial statements and review of the financial statements included in our registration statement or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements.
“Audit-Related Fees” consisted of fees billed for assurance and related services by the principal accountant that were reasonably related to the performance of the audit or review of our financial statements and are not reported under the paragraph captioned “Audit Fees” above.
“Tax Fees” consisted of fees billed for professional services rendered by the principal accountant for tax returns preparation.
“All Other Fees” consisted of fees billed for products and services provided by the principal accountant, other than the services reported above under other captions of this Item 14.
Pre-Approval Policies and Procedures
Under the Sarbanes-Oxley Act of 2002, all audit and non-audit services performed by our auditors must be approved in advance by our board of directors to assure that such services do not impair the auditors’ independence from us. In accordance with its policies and procedures, our board of directors pre-approved the audit service performed by RBSM LLP for our financial statements as of and for the year ended December 31, 2023.
PART IV
ITEM 15. EXHIBIT AND FINANCIAL STATEMENT SCHEDULES.
(a) List of Documents Filed as a Part of This Report:
(1) Index to Financial Statements:
(2) Index to Financial Statement Schedules:
All schedules have been omitted because the required information is included in the financial statements or the notes thereto, or because it is not required.
(3) Index to Exhibits:
See exhibits listed under Part (b) below.
(b) Exhibits:
| Exhibit No. | | Description |
| 2.1 | | Plan of Conversion, dated January 12, 2023 (incorporated by reference to Exhibit 2.1 to the Current Report on Form 8-K filed on April 13, 2023) |
| 2.2 | | Certificate of Conversion as filed with the Secretary of State of the State of Delaware on April 10, 2023 (incorporated by reference to Exhibit 2.2 to the Current Report on Form 8-K filed on April 13, 2023) |
| 2.3 | | Articles of Conversion as filed with the Secretary of State of the State of Nevada on April 10, 2023 (incorporated by reference to Exhibit 2.3 to the Current Report on Form 8-K filed on April 13, 2023) |
| 3.1 | | Articles of Incorporation of Smart for Life, Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on April 13, 2023) |
| 3.2 | | Certificate of Amendment to Articles of Incorporation of Smart for Life, Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on April 28, 2023) |
| 3.3 | | Certificate of Change to Articles of Incorporation of Smart for Life, Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on August 8, 2023) |
| 3.4 | | Certificate of Change to Articles of Incorporation of Smart for Life, Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on November 2, 2023) |
| 3.5* | | Certificate of Change to Articles of Incorporation of Smart for Life, Inc. |
| 3.6 | | Certificate of Designation of Series B Preferred Stock (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on May 30, 2023) |
| 3.7 | | Certificate of Designation of Series C Preferred Stock (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on March 7, 2024) |
| 3.8 | | Bylaws of Smart for Life, Inc. (incorporated by reference to Exhibit 3.3 to the Current Report on Form 8-K filed on April 13, 2023) |
| 4.1* | | Description of Securities of Smart for Life, Inc. |
| 4.2 | | Form of Common Stock Purchase Warrant issued on June 3, 2024 (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed on June 7, 2024) |
| 4.3 | | Form of Placement Agent Common Stock Purchase Warrant issued on June 3, 2024 (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed on June 7, 2024) |
| 4.4 | | Form of Placement Agent Common Stock Purchase Warrant issued on December 4, 2023 (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed on December 6, 2023) |
| 4.5 | | Placement Agent Common Stock Purchase Warrant issued by Smart for Life, Inc. to Charles Worthman on May 31, 2023 (incorporated by reference to Exhibit 4.6 to the Registration Statement on Form S-3 filed on June 5, 2023) |
| 4.6 | | Placement Agent Common Stock Purchase Warrant issued by Smart for Life, Inc. to Craig Schwabe on May 31, 2023 (incorporated by reference to Exhibit 4.7 to the Registration Statement on Form S-3 filed on June 5, 2023) |
| 4.7 | | Placement Agent Common Stock Purchase Warrant issued by Smart for Life, Inc. to Michael Vasinkevich on May 31, 2023 (incorporated by reference to Exhibit 4.8 to the Registration Statement on Form S-3 filed on June 5, 2023) |
| 4.8 | | Placement Agent Common Stock Purchase Warrant issued by Smart for Life, Inc. to Noam Rubinstein on May 31, 2023 (incorporated by reference to Exhibit 4.9 to the Registration Statement on Form S-3 filed on June 5, 2023) |
| 4.9 | | Form of Placement Agent Common Stock Purchase Warrant issued on May 19, 2023 (incorporated by reference to Exhibit 4.3 to the Current Report on Form 8-K filed on May 23, 2023) |
| 4.10 | | Placement Agent Common Stock Purchase Warrant issued by Smart for Life, Inc. to H.C. Wainwright & Co., LLC on May 5, 2023 (incorporated by reference to Exhibit 4.2 to the Registration Statement on Form S-3 filed on May 5, 2023) |
| 4.11 | | Common Stock Purchase Warrant issued by Smart for Life, Inc. to Dawson James Securities, Inc. on December 8, 2022 (incorporated by reference to Exhibit 4.21 to the Current Report on Form 8-K filed on December 9, 2022) |
| 4.12 | | Common Stock Purchase Warrant issued by Smart for Life, Inc. to Dawson James Securities, Inc. on December 8, 2022 (incorporated by reference to Exhibit 4.22 to the Current Report on Form 8-K filed on December 9, 2022) |
| 4.13 | | Common Stock Purchase Warrant issued by Smart for Life, Inc. to Robert D. Keyser, Jr. on December 8, 2022 (incorporated by reference to Exhibit 4.23 to the Current Report on Form 8-K filed on December 9, 2022) |
| 4.14 | | Common Stock Purchase Warrant issued by Smart for Life, Inc. to James Hopkins on December 8, 2022 (incorporated by reference to Exhibit 4.24 to the Current Report on Form 8-K filed on December 9, 2022) |
| 4.15 | | Warrant Agent Agreement, dated February 16, 2022, between Smart for Life, Inc. and VStock Transfer, LLC and Forms of Warrants (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed on February 23, 2022) |
| 4.16 | | Warrant issued by Smart for Life, Inc. to Joseph Xiras on January 13, 2022 (incorporated by reference to Exhibit 4.21 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.17 | | Warrant issued by Smart for Life, Inc. to Leonite Fund I, LP on January 13, 2022 (incorporated by reference to Exhibit 4.22 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.18 | | Warrant issued by Smart for Life, Inc. to Laurie Rosenthal on January 7, 2022 (incorporated by reference to Exhibit 4.20 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.19 | | Warrant issued by Smart for Life, Inc. to Robert Rein on January 3, 2022 (incorporated by reference to Exhibit 4.19 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.20 | | Warrant issued by Smart for Life, Inc. to Thomas L Calkins II and Diane M Calkins JTIC on December 27, 2021 (incorporated by reference to Exhibit 4.18 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.21 | | Warrant issued by Smart for Life, Inc. to Ryan Hazel on December 23, 2021 (incorporated by reference to Exhibit 4.17 to Amendment No. 2 to Registration Statement on Form S-1/A filed on January 21, 2022) |
| 4.22 | | Amended and Restated Warrant issued by Smart for Life, Inc. to Dawson James Securities, Inc. on February 1, 2022 (incorporated by reference to Exhibit 4.25 to Amendment No. 3 to Registration Statement on Form S-1/A filed on February 2, 2022) |
| 4.23 | | Warrant issued by Smart for Life, Inc. to Dawson James Securities, Inc. on July 1, 2021 (incorporated by reference to Exhibit 4.23 to Amendment No. 3 to Registration Statement on Form S-1/A filed on February 2, 2022) |
| 4.24 | | Warrant issued by Smart for Life, Inc. to Dawson James Securities, Inc. on July 1, 2021 (incorporated by reference to Exhibit 4.24 to Amendment No. 3 to Registration Statement on Form S-1/A filed on February 2, 2022) |
| 4.25 | | Common Stock Purchase Warrant issued by Smart for Life, Inc. to Peah Capital, LLC on December 18, 2020 (incorporated by reference to Exhibit 4.14 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 4.26 | | Amendment No 1 to Common Stock Purchase Warrant, dated June 30, 2021, between Smart for Life, Inc. and Peah Capital, LLC (incorporated by reference to Exhibit 4.15 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.1 | | Securities Purchase Agreement, dated April 3, 2024, among Smart for Life, Inc., Purely Optimal Nutrition Inc., Tan Enterprises, Inc., Avaliant Holdings Corporation, Dannel Tan, Jason Kwan, and Timur Kim (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on April 9, 2024) |
| 10.2 | | Asset Purchase Agreement, dated January 29, 2024, among Smart for Life, Inc., First Health FL LLC, Ceautamed Worldwide, LLC, Wellness Watchers Global, LLC and Greens First Female, LLC (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.3 | | Bill of Sale and Assignment and Assumption Agreement, dated January 29, 2024, among First Health FL LLC, Ceautamed Worldwide, LLC, Wellness Watchers Global, LLC and Greens First Female, LLC (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.4 | | Assignment of Intellectual Property, dated as of January 29, 2024, among First Health FL LLC, Ceautamed Worldwide LLC, Wellness Watchers, LLC and Greens First Female, LLC (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.5 | | Limited Liability Company Agreement, dated as of January 29, 2024, among Smart for Life, Inc., Joseph X. Xiras, Stuart Benson and Ryan Benson (incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.6 | | Securities Purchase Agreement, dated May 17, 2023, between Smart for Life, Inc. and the investor signatory thereto (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on May 23, 2023) |
| 10.7 | | Securities Purchase Agreement, dated May 2, 2023, between Smart for Life, Inc. and the investor signatory thereto (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on May 5, 2023) |
| 10.8 | | Form of Inducement Letter, dated May 30, 2024 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on May 31, 2024) |
| 10.9 | | Form of Inducement Letter, dated December 4, 2023 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on December 6, 2023) |
| 10.10 | | Form of Inducement Letter, dated May 29, 2023 (incorporated by reference to Exhibit 10.26 to the Current Report on Form 8-K filed on May 30, 2023) |
| 10.11* | | Form of Conversion Agreement for conversions of debt to Common Stock |
| 10.12* | | Form of Conversion Agreement for conversions of debt to Series C Preferred Stock |
| 10.13* | | Loan Agreement, dated March 5, 2024, among Smart for Life, Inc., Doctors Scientific Organica, LLC, Oyster Management Services Ltd., Lavi Enterprises, LLC and Abbsi LLC |
| 10.14* | | Secured Promissory Note issued by Smart for Life, Inc., Doctors Scientific Organica, LLC, Oyster Management Services Ltd. and Lavi Enterprises, LLC to Abbsi LLC on March 5, 2024 |
| 10.13 | | Original Issue Discount Secured Subordinated Note issued by Smart for Life, Inc. to Joseph X. Xiras on July 29, 2022 (incorporated by reference to Exhibit 10.14 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.14 | | Amendment No. 1 to Original Issue Discount Secured Subordinated Note issued by Smart for Life, Inc. to Joseph X. Xiras on May 24, 2023 (incorporated by reference to Exhibit 10.7 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.15 | | Amendment No. 2 to Original Issue Discount Secured Subordinated Note issued by Smart for Life, Inc. to Joseph X. Xiras on January 26, 2024 (incorporated by reference to Exhibit 10.8 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.16 | | Amended and Restated 6% Secured Subordinated Promissory Note issued by Smart for Life, Inc. to Sasson E. Moulavi on November 29, 2022 (incorporated by reference to Exhibit 10.10 to the Current Report on Form 8-K filed on December 2, 2022) |
| 10.17 | | Secured Subordinated Promissory Note issued by Smart for Life, Inc. to D&D Hayes, LLC on July 29, 2022 (incorporated by reference to Exhibit 10.8 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.18 | | Letter Agreement, dated August 9, 2023, between Smart for Life, Inc. and D&D Hayes, LLC (incorporated by reference to Exhibit 10.10 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.19 | | Agreement, dated January 29, 2024, among D&D Hayes, LLC, Ceautamed Worldwide, LLC, Wellness Watchers Global, LLC, Greens First Female, LLC, First Group Acquisition Company, LLC and First Health FL LLC (incorporated by reference to Exhibit 10.11 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.20 | | Agreement, dated January 29, 2024, among Smart for Life, Inc., D&D Hayes, LLC, Ceautamed Worldwide, LLC, Wellness Watchers Global, LLC, Greens First Female, LLC, First Group Acquisition Company, LLC and First Health FL LLC (incorporated by reference to Exhibit 10.12 to the Current Report on Form 8-K filed on February 2, 2024) |
| 10.21 | | Secured Subordinated Promissory Note issued by Smart for Life, Inc. to RMB Industries, Inc. on July 29, 2022 (incorporated by reference to Exhibit 10.9 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.22 | | Letter Agreement, dated November 28, 2022, between Smart for Life, Inc. and RMB Industries, Inc. (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed on December 2, 2022) |
| 10.23 | | Letter Agreement, dated April 1, 2023, between Smart for Life, Inc. and RMB Industries, Inc. (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed on April 6, 2023) |
| 10.24 | | Secured Subordinated Promissory Note issued by Smart for Life, Inc. to D&D Hayes, LLC on July 29, 2022 (incorporated by reference to Exhibit 10.10 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.25 | | Letter Agreement, dated November 28, 2022, between Smart for Life, Inc. and D&D Hayes, LLC (incorporated by reference to Exhibit 10.5 to the Current Report on Form 8-K filed on December 2, 2022) |
| 10.26 | | Letter Agreement, dated April 1, 2023, between Smart for Life, Inc. and D&D Hayes, LLC (incorporated by reference to Exhibit 10.6 to the Current Report on Form 8-K filed on April 6, 2023) |
| 10.27 | | Secured Subordinated Promissory Note issued by Smart for Life, Inc. to Bactolac Pharmaceuticals, Inc. on July 29, 2022 (incorporated by reference to Exhibit 10.11 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.28 | | Secured Subordinated Promissory Note issued by Smart for Life, Inc. to Stuart Benson on July 29, 2022 (incorporated by reference to Exhibit 10.12 to the Current Report on Form 8-K filed on August 4, 2022) |
| 10.29 | | Letter Agreement, dated November 28, 2022, between Smart for Life, Inc. and Stuart Benson (incorporated by reference to Exhibit 10.7 to the Current Report on Form 8-K filed on December 2, 2022) |
| 10.30 | | Letter Agreement, dated April 1, 2023, between Smart for Life, Inc. and Stuart Benson (incorporated by reference to Exhibit 10.9 to the Current Report on Form 8-K filed on April 6, 2023) |
| 10.31 | | Form of Debenture relating to 2022 private placement (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed on October 5, 2022) |
| 10.32 | | Loan Agreement, dated July 1, 2021, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.21 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.33 | | First Amendment to Loan Agreement, dated June 29, 2022, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.24 to the Annual Report on Form 10-K filed on March 31, 2023) |
| 10.34 | | Second Amendment to Loan Agreement, dated December 29, 2022, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on January 5, 2023) |
| 10.35 | | Third Amendment to Loan Agreement, dated April 20, 2023, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.29 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.36 | | Fourth Amendment to Loan Agreement, dated May 22, 2023, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.30 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.37 | | Term Loan Promissory Note issued by Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc. and Doctors Scientific Organica, LLC to Diamond Creek Capital, LLC on July 1, 2021 (incorporated by reference to Exhibit 10.22 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.38 | | Security Agreement, dated July 1, 2021, among Smart for Life, Inc., Bonne Sante Natural Manufacturing, Inc., Doctors Scientific Organica, LLC and Diamond Creek Capital, LLC (incorporated by reference to Exhibit 10.23 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.39 | | Lease Agreement, dated November 28, 2022, between 990 S Rogers Circle, LLC and Smart for Life, Inc. (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on December 2, 2022) |
| 10.40 | | Commercial Lease, dated September 1, 2018, between Scientific Real Estate Holdings LLC and Doctors Scientific Organica, LLC (incorporated by reference to Exhibit 10.35 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.41 | | First Amendment to Commercial Lease, dated August 16, 2021, between Scientific Real Estate Holdings LLC and Doctors Scientific Organica, LLC (incorporated by reference to Exhibit 10.36 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.42 | | Second Amendment to Commercial Lease, dated September 9, 2022, between Soflo 111 Properties, LLC and Doctors Scientific Organica, LLC (incorporated by reference to Exhibit 10.37 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.43 | | Third Amendment to Commercial Lease, dated May 24, 2023, between Soflo 111 Properties, LLC and Doctors Scientific Organica, LLC (incorporated by reference to Exhibit 10.38 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.44 | | Memorandum of Agreement of Lease, dated September 30, 2021, between The Linger Corporation and Smart for Life Canada Inc. (incorporated by reference to Exhibit 10.36 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.45† | | Employment Agreement, dated July 1, 2020, between Smart for Life, Inc. and Alfonso J. Cervantes (incorporated by reference to Exhibit 10.38 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.46† | | Employment Agreement, dated July 1, 2020, between Smart for Life, Inc. and Darren C. Minton (incorporated by reference to Exhibit 10.40 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.47†* | | Employment Agreement, dated December 12, 2020, between Smart for Life, Inc. and Alan B. Bergman |
| 10.48 | | Independent Director Agreement, dated October 17, 2022, between Smart for Life, Inc. and Arthur S. Reynolds (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on October 21, 2022) |
| 10.49 | | Independent Director Agreement, dated April 18, 2024, between Smart for Life, Inc. and Heather Granato (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on April 24, 2024) |
| 10.50 | | Independent Director Agreement, dated March 8, 2024, between Smart for Life, Inc. and Loren Brown (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed on March 14, 2024) |
| 10.51 | | Form of Indemnification Agreement between Smart for Life, Inc. and each independent director (incorporated by reference to Exhibit 10.42 to Amendment No. 1 to Registration Statement on Form S-1/A filed on January 14, 2022) |
| 10.52† | | 2020 Stock Incentive Plan (incorporated by reference to Exhibit 10.43 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.53† | | Form of Stock Option Agreement for 2020 Stock Incentive Plan (incorporated by reference to Exhibit 10.44 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.54† | | Form of Restricted Stock Award Agreement for 2020 Stock Incentive Plan (incorporated by reference to Exhibit 10.45 to the Registration Statement on Form S-1 filed on December 16, 2021) |
| 10.55† | | 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.46 to Amendment No. 1 to Registration Statement on Form S-1/A filed on January 14, 2022) |
| 10.56† | | Amendment No. 1 to 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.42 to the Annual Report on Form 10-K filed on March 31, 2023) |
| 10.57† | | Amendment No. 2 to 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.50 to the Registration Statement on Form S-1 filed on December 15, 2023) |
| 10.58† | | Form of Stock Option Agreement for 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.47 to Amendment No. 1 to Registration Statement on Form S-1/A filed on January 14, 2022) |
| 10.59† | | Form of Restricted Stock Award Agreement for 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.48 to Amendment No. 1 to Registration Statement on Form S-1/A filed on January 14, 2022) |
| 10.60† | | Form of Restricted Stock Unit Award Agreement for 2022 Equity Incentive Plan (incorporated by reference to Exhibit 10.49 to Amendment No. 1 to Registration Statement on Form S-1/A filed on January 14, 2022) |
| † | Executive compensation plan or arrangement |
ITEM 16. FORM 10-K SUMMARY.
None.
FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Smart For Life, Inc. and subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Smart For Life, Inc. and subsidiaries (the Company) as of December 31, 2023 and the related consolidated statement of operations, stockholders’ deficit, and cash flows for the year ended December 31, 2023, and the related notes and schedules (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and has a working capital deficiency of approximately $19.7 million and an accumulated deficit of $67.6 million at December 31, 2023. Additionally, the Company had a net loss of $22.7 million, and cash used in operations of $5.8 million for the year ended December 31, 2023. These factors raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 2. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Retrospective Adjustment for Reverse Stock Splits
We also have audited the retrospective adjustments to the 2022 consolidated financial statements to retrospectively apply the change due to reverse stock splits as described in Note 2. In our opinion, such retrospective adjustments are appropriate and have been properly applied. We were not engaged to audit, review, or apply any procedures to the 2022 consolidated financial statements of the Company other than with respect to these retrospective adjustments for the reverse stock splits and, accordingly, we do not express an opinion or any other form of assurance on the 2022 consolidated financial statements taken as a whole.
Retrospective Adjustment for Discontinued Operations
We also have audited the reclassification adjustments to the 2022 consolidated financial statements to retrospectively apply the change due to discontinued operations, as described in Note 2 and 5. In our opinion, such reclassification adjustments are appropriate and have been properly applied. We were not engaged to audit, review, or apply any procedures to the 2022 consolidated financial statements of the Company other than with respect to the reclassification adjustments and, accordingly, we do not express an opinion or any other form of assurance on the 2022 consolidated financial statements taken as a whole.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statement, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that are communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved are especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Goodwill and Other Intangible Assets Impairment Assessments
Critical Audit Matter Description
As described in Notes 2 and 8 to the consolidated financial statements, the Company has goodwill and other intangible assets of approximately $14.1 million at December 31, 2023. Management tests these assets annually for impairment or more frequently when potential impairment triggering events are present. Intangible assets are evaluated based on the asset group based on product as well as being evaluated between definite-lived and indefinite-lived intangible assets for the purpose of the impairment assessment. The Company assesses potential impairments whenever events or circumstances indicate that the asset may be impaired. For finite-lived intangibles assets the impairment is based on recoverability. Recoverability of an asset group is measured by a comparison of the carrying amount of an asset group to its forecasted cash flows expected to be generated by the asset group. If the carrying amount of the asset group exceeds its estimated forecasted cash flows, an impairment charge is recognized as the amount by which the carrying amount of the asset group exceeds the fair value of the asset group. An indefinite-lived intangible asset is considered impaired if the carrying amount exceeds the fair value of the asset group. The fair value of the asset group was determined using the income approach.
Goodwill is tested for impairment by comparing the estimated fair value of a reporting unit to its carrying value. Management uses an income approach to estimate the fair value of the reporting unit.
The principal considerations for our determination that performing procedures relating to the valuation of goodwill and intangible assets as a critical audit matter are (1) there was a high degree of auditor judgment and subjectivity in applying procedures relating to the fair value of intangible assets acquired due to the significant judgment by management when developing the estimates and (2) significant audit effort was required in evaluating the significant assumptions relating to the estimates, including the income projections and discount rates. In addition, the audit effort involved the use of professionals with specialized skill and knowledge to assist in performing these procedures and evaluating the audit evidence obtained.
How the Critical Audit Matter Was Addressed in the Audit
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included the following:
| ● | Inquiry of management regarding the development of the assumptions used in the valuation of the intangible assets. |
| ● | Testing management’s process included evaluating the appropriateness of the valuation models, testing the completeness, accuracy, and relevance of underlying data used in the models, and testing the reasonableness of significant assumptions, including the future levels of revenue, gross profit margin, EBITDA, capital expenditures, debt free cash free working capital and discount rates involved evaluating whether the assumptions were reasonable considering (i) current and past performance; (ii) the consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. |
| ● | Professionals with specialized skill and knowledge were used to assist in evaluating the (i) the appropriateness of the discounted cash flow approach; (ii) reasonableness of significant assumptions. |
| ● | Evaluated the experience, qualifications and objectivity of the Company’s specialist, a third-party valuation firm. |
| ● | Obtained an understanding of the nature of the work the Company’s specialist performed, including the objectives and scope of the specialist’s work; the methods or assumptions used; and a comparison of the methods or assumptions used with those used in the preceding period. Identified and evaluated assumptions developed by the specialist considering assumptions generally used in the specialist’s field; supporting evidence provided by the specialist; existing market data; historical or recent experience and changes in conditions and events affecting the Company. |
| ● | Tested the accuracy and completeness of company-produced data used by the specialist, and evaluated the relevance and reliability of externally obtained data. For assumptions provided to the specialist by the company, evaluated whether there is a reasonable basis for using each assumption considering whether other reasonably likely outcomes could materially affect the relevant financial statement assertions. Identified and evaluated significant assumptions used by the specialist for reasonableness. |
| ● | Evaluated the Company’s estimates of future revenue projections by completing a retrospective comparison to historical revenue projections. We tested the significant assumptions discussed above, as well as the completeness and accuracy of the underlying data used in the projected cash flows and valuations. |
| ● | We tested the reconciliation of the carrying value of the enterprise value to the fair value of the enterprise value of the Company as of the valuation date. |
| /s/ RBSM LLP | |
| | |
| We have served as the Company’s auditor since 2023. | |
| PCAOB ID 587 | |
| New York, NY | |
| September 20, 2024 | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Smart for Life, Inc.
Doral, Florida
Opinion on the Consolidated Financial Statements
We have audited, before the effects of the adjustments to retrospectively apply (1) the effects of the reverse stock splits described in Note 2 – Summary of Significant Accounting Policies – Basis of Presentation – Reverse Stock Splits (Reverse Stock Splits) and (2) the effects of the reclassifications for Discontinued operations described in Note 2 – Summary of Significant Accounting Policies – Discontinued Operations (Discontinued Operations), the accompanying consolidated balance sheet of Smart for Life, Inc. (the “Company”) at December 31, 2022, and the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for the year ended December 31, 2022, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, before the adjustments to retrospectively apply the reverse stock splits described in Note 2 – Summary of significant Accounting Policies, Reverse Stock Splits and the adjustments to reflect the reclassifications for Discontinued Operations described in Note 2 – Summary of Significant Accounting Policies, Discontinued Operations, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
We were not engaged to audit, review, or apply any procedures to the adjustments for the retrospective effect of the Reverse Stock Splits or Discontinued Operations described in Note 2 – Summary of Significant Accounting Policies – Basis of Presentation – Reverse Stock Splits and Note 2 – Summary of Significant Accounting Policies – Discontinued Operations and accordingly, we do not express an opinion or any other form of assurance about whether such adjustments and disclosures are appropriate and have been properly applied. Those adjustments and disclosures were audited by RBSM. (The 2022 consolidated financial statements before the effects of the adjustments discussed in Note 2 – Reverse Stock Splits, Note 2 – Discontinued Operations, Note 5 – Discontinued Operations, Note 6 – Inventory, Note 7 – Property and Equipment, Note 8 – Intangible assets, Note 9 – Lease Commitments, Note 10 – Debt and Note 13 – Stockholders’ Equity are not presented herein).
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2 to the consolidated financial statements, the Company has sustained recurring losses and has a deficiency in working capital of approximately $11.5 million at December 31, 2022, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the audit of the 2022 consolidated financial statements that are communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved are especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Valuation of Intangible Assets and Goodwill in Acquisitions
As described in Notes 3 to the consolidated financial statements, the Company completed an acquisition for consideration of $8.6 million and the transaction was accounted for as business combinations. The Company recorded acquired intangible assets at fair value on the date of acquisition. The methods used to estimate the fair value of acquired intangible assets involve significant assumptions. The significant assumptions applied by management in estimating the fair value of the acquired intangible assets included income projections and discount rates.
The principal considerations for our determination that performing procedures relating to the valuation of acquired intangible assets is a critical audit matter are (1) there was a high degree of auditor judgment and subjectivity in applying procedures relating to the fair value of intangible assets acquired due to the significant judgment by management when developing the estimates and (2) significant audit effort was required in evaluating the significant assumptions relating to the estimates, including the income projections and discount rates. In addition, the audit effort involved the use of professionals with specialized skill and knowledge to assist in performing these procedures and evaluating the audit evidence obtained.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others, reading the purchase agreements, and testing management’s process for estimating the fair value of intangible assets. Testing management’s process included evaluating the appropriateness of the valuation models, testing the completeness, accuracy, and relevance of underlying data used in the models, and testing the reasonableness of significant assumptions, including the income projections and discount rates. Evaluating the reasonableness of the income projections involved considering the current performance of the acquired businesses, the consistency with external market and industry data, and whether these assumptions were consistent with other evidence obtained in other areas of the audit.
Intangible Assets Impairment Assessments
As described in Notes 3 and 6 to the consolidated financial statements, the Company has goodwill and intangible assets of $24.8 million at December 31, 2022. In most cases, no directly observable market inputs are available to measure the fair value to determine if the asset is impaired. Therefore, an estimate is derived indirectly and is based on net present value techniques utilizing post-tax cash flows and discount rates. The estimates that management used in calculating the net present values depend on assumptions specific to the nature of the management service activities with regard to the amount and timing of projected future cash flows; long-term forecasts; actions of competitors (competing services), future tax and discount rates.
The principal considerations for our determination that performing procedures relating to the intangible assets impairment assessment is a critical audit matter are the significant judgment by management when developing the net present value of the intangible assets. This in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to the amount and timing of projected future cash flows and the discount rate.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing management’s process for developing the fair value estimate; evaluating the appropriateness of the net present value techniques; testing the completeness and accuracy of underlying data used in the model; and evaluating the significant assumptions used by management, including the amount and timing of projected future cash flows and the discount rate. Evaluating management’s assumptions related to the amount and timing of projected future cash flows and the discount rate involved evaluating whether the assumptions used by management were reasonable considering the current and past performance of the intangible assets and whether these assumptions were consistent with evidence obtained in other areas of the audit.
/s/ Daszkal Bolton LLP
Daszkal Bolton LLP
Fort Lauderdale, Florida
March 31, 2023
We served as the Company’s auditor from 2021 to March 2023
SMART FOR LIFE, INC.
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2023 AND 2022
| | | December 31,
2023 | | | December 31,
2022 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 188,596 | | | $ | 70,820 | |
| Accounts receivable, net | | | 70,231 | | | | 540,072 | |
| Inventory, net | | | 1,242,777 | | | | 2,182,351 | |
| Related party receivable, net | | | 332,142 | | | | — | |
| Prepaid expenses and other current assets | | | 148,993 | | | | 301,037 | |
| Current assets of discontinued operations | | | 348,971 | | | | 509,696 | |
| Total current assets | | | 2,331,710 | | | | 3,603,976 | |
| | | | | | | | | |
| Property and equipment, net | | | 100,539 | | | | 219,191 | |
| Intangible assets, net | | | 11,046,767 | | | | 16,287,098 | |
| Goodwill | | | 3,045,000 | | | | 5,816,100 | |
| Deposits and other assets | | | 57,324 | | | | 72,441 | |
| Operating lease right-of-use assets, net | | | 2,015,299 | | | | 2,298,381 | |
| Assets of discontinued operations | | | 1,062,588 | | | | 849,768 | |
| Total other assets | | | 17,327,517 | | | | 25,542,979 | |
| Total assets | | $ | 19,659,227 | | | $ | 29,146,955 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 3,612,578 | | | $ | 3,255,322 | |
| Accrued expenses | | | 3,718,070 | | | | 1,647,865 | |
| Accrued expenses, related parties | | | 264,141 | | | | 947,951 | |
| Contract liabilities | | | 464,721 | | | | 675,426 | |
| Preferred stock dividends payable | | | 450,562 | | | | 600,750 | |
| Operating lease liability, current | | | 298,644 | | | | 250,646 | |
| Debt, current, net of unamortized debt discounts | | | 10,616,114 | | | | 6,383,557 | |
| Current liabilities of discontinued operations | | | 2,631,078 | | | | 1,382,001 | |
| Total current liabilities | | | 22,055,908 | | | | 15,143,518 | |
| | | | | | | | | |
| Long-term liabilities: | | | | | | | | |
| Operating lease liability, noncurrent | | | 1,800,158 | | | | 2,098,803 | |
| Debt, noncurrent, net of unamortized debt discounts | | | 1,748,686 | | | | 13,452,171 | |
| Liabilities of discontinued operations, noncurrent | | | 441,929 | | | | 814,445 | |
| Total long-term liabilities | | | 3,990,773 | | | | 16,365,419 | |
| Total liabilities | | | 26,046,681 | | | | 31,508,937 | |
| | | | | | | | | |
| Commitments and contingencies – Note 14 | | | | | | | | |
| | | | | | | | | |
| Stockholders’ Deficit | | | | | | | | |
| Series A Convertible Preferred Stock, $0.0001 par value, 1,000 shares authorized, 0 and 1,000 shares issued and outstanding as of December 31, 2023 and 2022, respectively | | | — | | | | — | |
| Series B Preferred Stock, $0.0001 par value, 5,000,000 shares authorized, 26,239 and 0 shares issued and outstanding as of December 31, 2023 and 2022, respectively | | | 3 | | | | — | |
| Common Stock, $.0001 par value, 7,936,508 shares authorized, 373,526 and 77,614 shares issued and outstanding as of December 31, 2023 and 2022, respectively | | | 37 | | | | 8 | |
| Additional paid in capital | | | 61,280,662 | | | | 42,630,425 | |
| Accumulated deficit | | | (67,668,156 | ) | | | (44,992,415 | ) |
| Total stockholders’ deficit | | | (6,387,454 | ) | | | (2,361,982 | ) |
| Total liabilities and stockholders’ deficit | | $ | 19,659,227 | | | $ | 29,146,955 | |
The accompanying notes are an integral part of these consolidated financial statements
SMART FOR LIFE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | December 31,
2023 | | | December 31,
2022 | |
| Revenues | | | | | | |
| Products | | $ | 7,863,977 | | | $ | 12,467,650 | |
| Advertising | | | 361,815 | | | | 3,434,879 | |
| Total revenues | | | 8,225,792 | | | | 15,902,529 | |
| Cost of revenues | | | | | | | | |
| Products | | | 4,854,554 | | | | 8,250,733 | |
| Advertising | | | 281,131 | | | | 2,561,276 | |
| Total cost of revenues | | | 5,135,685 | | | | 10,812,009 | |
| Gross profit | | | 3,090,107 | | | | 5,090,520 | |
| Operating expenses | | | | | | | | |
| General and administrative | | | 4,258,202 | | | | 5,337,925 | |
| Compensation | | | 5,561,017 | | | | 6,167,637 | |
| Professional services | | | 2,263,405 | | | | 2,165,198 | |
| Consulting fee – related party | | | 46,686 | | | | 1,471,199 | |
| Impairment of intangible asset | | | 5,843,501 | | | | — | |
| Depreciation and amortization expense | | | 2,273,288 | | | | 1,899,542 | |
| Total operating expenses | | | 20,246,099 | | | | 17,041,501 | |
| Operating loss from continuing operations | | | (17,155,992 | ) | | | (11,950,981 | ) |
| Other income (expense) | | | | | | | | |
| Other income (expense) | | | 400,397 | | | | (474 | ) |
| IPO expenses | | | — | | | | (702,420 | ) |
| Gain on debt extinguishment | | | 269,828 | | | | 161,039 | |
| Interest expense | | | (4,634,839 | ) | | | (15,689,565 | ) |
| Total other expense | | | (3,964,614 | ) | | | (16,231,420 | ) |
| Loss before income taxes | | | (21,120,606 | ) | | | (28,182,401 | ) |
| Income tax expense | | | — | | | | — | |
| Net loss from discontinued operations, net of tax | | | (1,555,135 | ) | | | (1,795,415 | ) |
| Net loss | | $ | (22,675,741 | ) | | $ | (29,977,816 | ) |
| Series A Preferred Stock dividends | | | — | | | | 600,750 | |
| Net loss attributable to common shareholders | | $ | (22,675,741 | ) | | $ | (30,578,565 | ) |
| Weighted average shares outstanding | | | 136,346 | | | | 9,766 | |
| Loss per share continuing operations | | $ | (154.90 | ) | | $ | (2,885.77 | ) |
| Loss per share discontinued operations | | $ | (11.41 | ) | | $ | (183.84 | ) |
| Net loss per share | | $ | (166.31 | ) | | $ | (3,069.61 | ) |
The accompanying notes are an integral part of these consolidated financial statements
SMART FOR LIFE, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | Preferred Stock | | | Common Stock | | | Additional Paid-In | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| Balance, December 31, 2021 | | | 8,000 | | | $ | 1 | | | | 70,577 | | | | 6 | | | $ | 8,924,148 | | | $ | (15,014,599 | ) | | $ | (6,090,444 | ) |
| Common stock issued for cash with initial public offering | | | — | | | | — | | | | 457 | | | | — | | | | 10,668,316 | | | | — | | | | 10,668,316 | |
| Series A warrants issued in connection with initial public offering | | | — | | | | — | | | | — | | | | — | | | | 1,910,743 | | | | — | | | | 1,910,743 | |
| Series B warrants in connection with initial public offering | | | — | | | | — | | | | — | | | | — | | | | 159,229 | | | | — | | | | 159,229 | |
| Warrants issued in connection with debt | | | — | | | | — | | | | — | | | | — | | | | 65,624 | | | | — | | | | 65,624 | |
| Common stock issued upon exercise of series B warrants | | | — | | | | — | | | | 457 | | | | — | | | | (144 | ) | | | — | | | | (144 | ) |
| Common stock issued upon conversion of convertible notes | | | — | | | | — | | | | 1,201 | | | | 1 | | | | 8,165,385 | | | | — | | | | 8,165,386 | |
| Common stock issued in connection with acquisition | | | — | | | | — | | | | 13 | | | | — | | | | (4 | ) | | | — | | | | (4 | ) |
| Common stock issued for conversion of accounts payable | | | — | | | | — | | | | 5 | | | | — | | | | 147,223 | | | | — | | | | 147,223 | |
| Common stock issued for services | | | — | | | | — | | | | 358 | | | | — | | | | 922,375 | | | | — | | | | 922,375 | |
| Common stock issued upon conversion of preferred stock | | | (7,000 | ) | | | (1 | ) | | | 3,333 | | | | 1 | | | | — | | | | — | | | | — | |
| Common stock issued under future equity agreements | | | — | | | | — | | | | 689 | | | | — | | | | 10,844,960 | | | | — | | | | 10,844,960 | |
| Common stock issued upon option exercise | | | — | | | | — | | | | 62 | | | | — | | | | — | | | | — | | | | — | |
| Common stock issued upon conversion of promissory note | | | — | | | | — | | | | 23 | | | | — | | | | 73,727 | | | | — | | | | 73,727 | |
| Stock issued for cash in private placement | | | — | | | | — | | | | 407 | | | | — | | | | 910,000 | | | | — | | | | 910,000 | |
| Stock issued for debt forbearance | | | — | | | | — | | | | 32 | | | | — | | | | 30,500 | | | | — | | | | 30,500 | |
| Preferred stock Series A dividend payable | | | — | | | | — | | | | — | | | | — | | | | (245,333 | ) | | | — | | | | (245,333 | ) |
| Stock based compensation | | | — | | | | — | | | | — | | | | — | | | | 53,676 | | | | — | | | | 53,676 | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | (29,977,816 | ) | | | (29,977,816 | ) |
| Balance, December 31, 2022 | | | 1,000 | | | $ | — | | | | 77,614 | | | $ | 8 | | | $ | 42,630,425 | | | $ | (44,992,415 | ) | | $ | (2,361,982 | ) |
| Common stock issued upon warrant exercise | | | — | | | | — | | | | 243,222 | | | | 24 | | | | 7,269,507 | | | | — | | | | 7,269,531 | |
| Stock based compensation | | | — | | | | — | | | | — | | | | — | | | | 797,535 | | | | — | | | | 797,535 | |
| Series B preferred stock issued for conversion of notes | | | 29,398 | | | | 3 | | | | — | | | | — | | | | 6,555,255 | | | | — | | | | 6,555,258 | |
| Conversion of Series A preferred stock to common stock | | | (1,000 | ) | | | — | | | | 477 | | | | — | | | | — | | | | — | | | | — | |
| Conversion of Series B preferred stock to common stock | | | (8,579 | ) | | | (1 | ) | | | 13,618 | | | | 1 | | | | 9 | | | | — | | | | 9 | |
| Common stock issued for services | | | — | | | | — | | | | 7,316 | | | | 1 | | | | 207,198 | | | | — | | | | 207,199 | |
| Conversion of accrued board fees | | | 135 | | | | — | | | | — | | | | — | | | | 30,000 | | | | — | | | | 30,000 | |
| Conversion of accrued compensation | | | 5,285 | | | | 1 | | | | — | | | | — | | | | 1,178,340 | | | | — | | | | 1,178,341 | |
| Stock issued for cash in private placement | | | — | | | | — | | | | 13,738 | | | | 1 | | | | 2,151,309 | | | | — | | | | 2,151,310 | |
| Conversion of accrued dividends | | | — | | | | — | | | | 17,541 | | | | 2 | | | | 186,632 | | | | — | | | | 186,634 | |
| Warrant placement fee | | | — | | | | — | | | | — | | | | — | | | | 274,452 | | | | — | | | | 274,452 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (22,675,741 | ) | | | (22,675,741 | ) |
| Balance, December 31, 2023 | | | 26,239 | | | $ | 3 | | | | 373,526 | | | $ | 37 | | | $ | 61,280,662 | | | $ | (67,668,156 | ) | | $ | (6,387,454 | ) |
The accompanying notes are an integral part of these consolidated financial statements
SMART FOR LIFE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | December 31, 2023 | | | December 31, 2022 | |
| Cash flows from operating activities: | | | | | | |
| Net loss | | $ | (22,675,741 | ) | | $ | (29,977,816 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Bad debt expense | | | 83,826 | | | | 46,763 | |
| Amortization of debt discount and debt issuance cost | | | 1,266,191 | | | | 2,698,080 | |
| Depreciation and amortization expense | | | 2,273,345 | | | | 2,111,346 | |
| Gain on extinguishment of debt | | | (269,828 | ) | | | (161,039 | ) |
| Stock-based compensation | | | 797,535 | | | | 54,285 | |
| Impairment of intangibles | | | 5,843,501 | | | | — | |
| Stock issued for services | | | 207,199 | | | | 922,262 | |
| Non-cash interest expense on conversion of notes payable | | | 92,569 | | | | — | |
| Non-cash finance fees | | | 753,933 | | | | — | |
| Non-cash finance expense – preferred stock dividend | | | 36,456 | | | | — | |
| Interest expense associated with warrants issued with debt | | | — | | | | 65,624 | |
| Interest expense associated with future equity agreements | | | — | | | | 10,844,743 | |
| Accretion related to right of use asset | | | 32,435 | | | | 25,177 | |
| Change in operating assets and liabilities: | | | | | | | | |
| Accounts receivable, net | | | 386,015 | | | | (226,594 | ) |
| Inventory | | | 939,574 | | | | 524,200 | |
| Prepaid expenses and other current assets | | | 152,044 | | | | 44,170 | |
| Deposits and other assets | | | 15,116 | | | | (47,771 | ) |
| Accounts payable | | | 396,928 | | | | 1,973,391 | |
| Accrued expenses | | | 2,556,332 | | | | 263,632 | |
| Accrued expenses, related parties | | | 494,530 | | | | 576,632 | |
| Contract liabilities | | | (210,705 | ) | | | 277,134 | |
| Discontinued operations | | | 980,192 | | | | 299,910 | |
| Net cash used in operating activities | | | (5,848,553 | ) | | | (9,685,871 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Cash paid for acquisition of Ceautamed | | | — | | | | (3,000,000 | ) |
| Additions to property and equipment | | | — | | | | (29,720 | ) |
| Discontinued operations | | | (3,450 | ) | | | — | |
| Net cash used in investing activities | | | (3,450 | ) | | | (3,029,720 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Receipts from related parties | | | 888,130 | | | | — | |
| Payments to related parties | | | (1,220,274 | ) | | | — | |
| Proceeds from initial public offering | | | — | | | | 12,812,008 | |
| Proceeds from issuance of common stock | | | 2,151,310 | | | | 909,873 | |
| Proceeds from exercise of warrants | | | 7,269,531 | | | | — | |
| Proceeds from convertible notes and notes payable | | | 2,885,527 | | | | 9,797,275 | |
| Repayments on convertible notes and notes payable | | | (5,873,881 | ) | | | (10,621,431 | ) |
| Repayments on notes payable of discontinued operations | | | (130,564 | ) | | | (309,484 | ) |
| Net cash provided by financing activities | | | 5,969,779 | | | | 12,588,241 | |
| | | | | | | | | |
| Net increase (decrease) in cash | | | 117,776 | | | | (127,350 | ) |
| Cash, beginning of year | | | 70,820 | | | | 198,170 | |
| Cash, end of year | | $ | 188,596 | | | $ | 70,820 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | | | | |
| Interest paid | | $ | 274,747 | | | $ | 4,112,081 | |
| Taxes paid | | $ | — | | | $ | — | |
| | | | | | | | | |
| Non-cash investing and financing activities: | | | | | | | | |
| Stock issued for conversion of accounts payable | | $ | — | | | $ | 147,223 | |
| Stock issued for conversion of convertible notes and interest | | $ | 6,555,258 | | | $ | 8,165,385 | |
| Stock issued for conversion of accrued dividends | | $ | 186,634 | | | $ | — | |
| Stock issued for conversion of series B preferred Stock to common stock | | $ | 9 | | | $ | — | |
| Non-cash equipment financing of discontinued operations | | $ | — | | | $ | 181,815 | |
| Debt issued for acquisition of Ceautamed | | $ | — | | | $ | 5,600,000 | |
| Equipment obtained with financing | | $ | — | | | $ | 8,463 | |
| Equipment transferred from discontinued operation | | $ | 57,659 | | | $ | — | |
| Stock issued for conversion of accrued compensation | | $ | 1,178,341 | | | $ | — | |
| Stock issued for conversion of accrued board fees | | $ | 30,000 | | | $ | — | |
| Conversion of notes payable to accrued expenses | | $ | 26,471 | | | $ | — | |
| Conversion of interest to notes payable | | $ | 334,950 | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Note 1 — Description of Business
Smart for Life, Inc., formerly Bonne Santé Group, Inc. (“SMFL”), is a Nevada corporation which was originally formed in the State of Delaware on February 2, 2017 and converted to a Nevada corporation on April 10, 2023. Structured as a global holding company, it is engaged in the development, marketing, manufacturing, acquisition, operation and sale of a broad spectrum of nutraceutical and related products with an emphasis on health and wellness.
On March 8, 2018, SMFL acquired 51% of Millenium Natural Manufacturing Corp. and Millenium Natural Health Products, Inc. On October 8, 2019, SMFL entered into an agreement to acquire the remaining 49% of these companies, subject to certain conditions which were subsequently met. On September 30, 2020, the name of Millenium Natural Manufacturing Corp. was changed to Bonne Sante Natural Manufacturing, Inc. (“BSNM”), and on November 24, 2020, Millenium Natural Health Products Inc. was merged into BSNM. Based in Doral, Florida, BSNM operates a 22,000 square-foot FDA-certified manufacturing facility. It manufactures nutritional products for a significant number of customers. On March 6, 2024, the Company entered into a sale and leaseback agreement relating to BSNM as more fully described under Note 5.
On July 1, 2021, SMFL acquired Doctors Scientific Organica, LLC d/b/a Smart for Life, Oyster Management Services, Ltd., Lawee Enterprises, L.L.C. and U.S. Medical Care Holdings, L.L.C. On August 27, 2021, SMFL transferred all of the equity interests of Oyster Management Services, Ltd., Lawee Enterprises, L.L.C. and U.S. Medical Care Holdings, L.L.C. to Doctors Scientific Organica, LLC. On May 19, 2022, SMFL acquired Lavi Enterprises, LLC. On the same date, SMFL transferred all of the equity interests of Lavi Enterprises, LLC to Doctors Scientific Organica, LLC. On December 13, 2022, Oyster Management Services, Ltd. was converted to a limited liability company known as Oyster Management Services, L.L.C. As a result of the foregoing, Oyster Management Services, L.L.C., Lawee Enterprises, L.L.C., U.S. Medical Care Holdings, L.L.C. and Lavi Enterprises, LLC are now wholly owned subsidiaries of Doctors Scientific Organica, LLC (collectively, “DSO”). Based in Riviera Beach, Florida, DSO operates a 30,000 square-foot FDA-certified manufacturing facility. DSO manufactures and sells weight management foods and related products. Additionally, DSO provides manufacturing services for other customers.
On August 24, 2021, Smart for Life Canada Inc. (“DSO Canada”) was established as a wholly owned subsidiary of Doctors Scientific Organica, LLC in Canada. SMFL Canada sells retail products through a retail store location in Montreal Canada and the same location also acts as distribution center for international direct to consumer and big box customers. It maintains inventory and employees at this location.
On November 8, 2021, SMFL acquired Nexus Offers, Inc. (“Nexus”). Nexus is a network platform in the affiliate marketing space. Affiliate marketing is an advertising model in which a product vendor compensates third-party digital marketers to generate traffic or leads for the product vendor’s products and services. The third-party digital marketers are referred to as affiliates, and the commission fee incentivizes them to find ways to promote the products being sold by the product vendor. Based in Miami, Florida, Nexus operates virtually.
On December 6, 2021, SMFL acquired GSP Nutrition Inc. (“GSP”). GSP is a sports nutrition company that offers nutritional supplements for athletes and active lifestyle consumers. Based in Miami, Florida, GSP operates virtually.
On July 29, 2022, SMFL acquired Ceautamed Worldwide, LLC and its wholly-owned subsidiaries Wellness Watchers Global, LLC and Greens First Female LLC (collectively, “Ceautamed”). Ceautamed is based in Boca Raton, Florida and owns the Greens First line of branded products which have been specifically marketed to the healthcare provider sector. On January 29, 2024, the Company entered into an asset purchase agreement pursuant to which the Company agreed to sell nearly all of the assets of Ceautamed and its subsidiaries for a 49% ownership interest in a new limited liability company. The agreement allows for the 51% owner to purchase the remaining 49% for $1 at an unspecified future date. See Note 16.
On August 15, 2022, SMFL entered into a joint venture with a seller of Ceautamed to form Smart Acquisition Group, LLC. This company was formed to expand M&A growth initiatives through the identification, negotiation, financing and acquisition of companies by SMFL.
Note 2 — Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements reflect the consolidated operations of SMFL and its wholly owned subsidiaries DSO, DSO Canada, Nexus, GSP and Ceautamed (collectively the “Company”) and are prepared in the United States Dollars in accordance with generally accepted accounting principles in the United States of America (“GAAP”). BSNM has been deemed to be a discontinued operation. Intercompany balances and transactions have been eliminated in consolidation.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Reclassifications
Certain prior period amounts have been reclassified to conform with the current year presentation. BSNM has been deemed to be a discontinued operation. These reclassifications had no impact on the reported results.
Basis of Presentation
The December 31, 2022 consolidated balance sheet reflected within the accompanying consolidated financial statements reflects a change for a correction of immaterial errors. An error pertained to the classification of assets acquired in the acquisitions of Nexus and Ceautamed. A reclassification of $4,464,100 from intangible assets to goodwill is reflected within these consolidated financial statements. Secondly, an error in the number of warrants granted and forfeited. See Note 3.
On April 24, 2023, the Company effected a 1-for-50 reverse stock split. The impact of this transaction is reflected within all common stock, options, and warrant information retrospectively in these consolidated financial statements.
On August 2, 2023, the Company effected a 1-for-3 reverse stock split. The impact of this transaction is reflected within all common stock, options, and warrant information retrospectively in these consolidated financial statements. As a result of the reverse stock split, the Company’s authorized common stock decreased to 166,666,667 shares.
On October 27, 2023, the Company effected a 1-for-3 reverse stock split. The impact of this transaction is reflected within all common stock, options, and warrant information retrospectively in these consolidated financial statements. As a result of the reverse stock split, the Company’s authorized common stock decreased to 55,555,556 shares.
On April 22, 2024, the Company effected a 1-for-7 reverse stock split. The impact of this transaction is reflected within all common stock, options, and warrant information retrospectively in these consolidated financial statements. As a result of the reverse stock split, the Company’s authorized common stock decreased to 7,936,508 shares.
Liquidity, Capital Resources and Going Concern
The Company’s consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has suffered recurring losses from operations and has a working capital deficiency of approximately $19.7 million and an accumulated deficit of $67.7 million. Additionally, the Company had a net loss of $22.7 million, and cash used in operations of $5.8 million for the year ended December 31, 2023. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Management believes that current available resources will not be sufficient to fund the Company’s planned expenditures over the next 12 months. Management has implemented restructuring activities of the Company including staff reductions, a subsequent event sale of BSNM, and a subsequent event sale of Ceautamed’s assets. Accordingly, the Company will be dependent upon the raising of additional capital through placement of common stock and/or debt financing in order to implement its business plan. If the Company raises additional capital through the issuance of equity securities or securities convertible into equity, stockholders will experience dilution, and such securities may have rights, preferences, or privileges senior to those of the holders of common stock or convertible senior notes. If the Company raises additional funds by issuing debt, the Company may be subject to limitation on its operations, through debt covenants or other restrictions. There is no assurance that the Company will be successful with future financing ventures, and the inability to secure such financing may have a material adverse effect on the Company’s financial condition. These consolidated financial statements do not include any adjustments to the amounts and classifications of assets and liabilities that might be necessary should the Company be unable to continue as a going concern.
Use of Estimates
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. These estimates include, among other items, assessing the collectability of receivables, the realization of deferred taxes, useful lives and recoverability of tangible and intangible assets, assumptions used in the valuation of options and warrants, incremental borrowing rate on lease liability, valuation of inventory, impairment of long lived assets, and accruals for commitments and contingencies. Some of these estimates can be subjective and complex and, consequently, actual results could differ materially from those estimates.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Fair Value Measurements
Under ASC Topic 820, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (i.e., an exit price). ASC Topic 820 establishes a hierarchy for inputs to valuation techniques used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that reflect assumptions market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. There are three levels to the hierarchy based on the reliability of inputs, as follows:
Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 - Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets and liabilities in markets that are not active.
Level 3 - Unobservable inputs for the asset or liability. The degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3.
The Company’s cash and cash equivalents are measured using Level 1 inputs and include cash on hand and deposits in banks. Due to their short-term nature, the carrying amounts reported in the consolidated balance sheets approximate the fair value of cash and cash equivalents.
The Company has certain assets that are measured at fair value on a non-recurring basis including those described in Note 8 and are adjusted to fair value only when the carrying values are more than the fair values. The categorization of the framework used to price the assets is considered a Level 3 measurement due to the subjective nature of the unobservable inputs used to determine the fair value.
Certain nonfinancial assets and liabilities are measured at fair value on a non-recurring basis and are subject to fair value adjustments in certain circumstances, such as when there is evidence of impairment. The estimated fair value of financial instruments is determined using the best available market information and appropriate valuation methodologies. Considerable judgment is necessary, however, in interpreting market data to develop the estimates of fair value. Accordingly, the estimates presented are not necessarily indicative of the amounts that the Company could realize in a current market exchange, or the value that ultimately will be realized upon maturity or disposition. In the evaluation of the estimated value of such assets, data for determining the value of the estimates are utilized based on the relevant facts and circumstances. The use of different market assumptions may have a material effect on the estimated fair value amounts.
Cash Equivalents
The Company considers all highly liquid investments purchased with an original maturity of three (3) months or less to be cash equivalents. At December 31, 2023 and 2022, there were no cash equivalents.
Accounts Receivable and Allowance for Doubtful Accounts
The Company’s allowance for doubtful accounts represents the Company’s estimate for uncollectible receivables based on a review of specific accounts and the Company’s historical collection experience. The Company writes off specific accounts based on an ongoing review of collectability, as well as management’s past experience with the customers. Accounts receivable are presented net of an allowance for doubtful accounts of $0 and $57,581 at December 31, 2023 and 2022, respectively.
Inventory, Net
Inventory consists of raw materials, packaging materials, and finished goods and is valued at the lower of cost (first-in, first-out) or replacement cost or net realizable value. An allowance for inventory obsolescence is provided for slow moving or obsolete inventory to write down historical cost to net realizable value. The Company primarily performs its manufacturing for functional foods and nutraceuticals in the form of bars, cookies, powders, tablets and capsules.
The allowance for obsolescence is an estimate established through charges to cost of goods sold. Management’s judgment in determining the adequacy of the allowance is based upon several factors which include, but are not limited to, analysis of slow-moving inventory, analysis of the selling price of inventory, the predetermined shelf life of the product, and management’s judgment with respect to current economic conditions. Given the nature of the inventory, it is reasonably possible the Company’s estimate of the allowance for obsolescence will change in the near term.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Property and Equipment
Property and equipment are recorded at cost. Expenditures for major betterments and additions are charged to the asset accounts, while replacements, maintenance and repairs which do not improve or extend the lives of the respective assets are charged to expense as incurred. The Company provides for depreciation and amortization over the estimated useful lives of various assets using the straight-line method ranging from 3-5 years.
Intangible Assets
In accordance with ASC Topic 360, Intangibles and Other, the Company reviews intangible assets for impairment. In connection with any such review, if the recorded value of intangible assets is greater than its fair value, they are written down and charged to results of operations. Intangible assets (excluding goodwill) are assessed at each reporting date for indications that an asset may be impaired. If any such indication exists, the Company makes an estimate of the asset’s recoverable amount. The asset’s recoverable amount is the higher of an asset’s or cash-generating unit’s fair value less costs of disposal and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets. Where the carrying amount of an asset or a group of assets exceeds its recoverable amount, the asset is considered impaired and is written down to its recoverable amount. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset or the group of assets.
Intangible assets consist of customer relationships, tradename, developed technology, patents, software platform, supplier contracts, non-compete agreements, license agreements, and intellectual property acquired in the acquisitions of DSO, Nexus, GSP and Ceautamed. The Company amortizes intangible assets with finite lives on a straight-line basis over their estimated useful lives which ranges from 3 to 15 years.
Goodwill
The Company allocates goodwill to reporting units based on the reporting unit expected to benefit from the business combination. The Company evaluates its reporting units on an annual basis and, if necessary, reassign goodwill using a relative fair value allocation approach. Goodwill is tested for impairment at the reporting unit level (operating segment or one level below an operating segment) on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value. These events or circumstances could include a significant change in the business climate, legal factors, operating performance indicators, competition, or sale or disposition of a significant portion of a reporting unit.
In accordance with ASC Topic 350, Intangibles—Goodwill and Other, the Company reviews intangible assets at least annually for impairment. In connection with any such review, if the recorded value of intangible assets is greater than its fair value, they are written down and charged to results of operations. Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units, assignment of goodwill to reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated primarily through the use of a discounted cash flow methodology. This analysis requires significant judgments, including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth for our business, estimation of the useful life over which cash flows will occur, and determination of our weighted average cost of capital.
The estimates used to calculate the fair value of a reporting unit change from year to year based on operating results, market conditions, and other factors. Changes in these estimates and assumptions could materially affect the determination of fair value and goodwill impairment for each reporting unit.
In connection with the acquisition of DSO on July 1, 2021, the Company recognized goodwill which had a balance of $1,342,000 as of December 31, 2023. In connection with the acquisition of Nexus on November 8, 2021, the Company recognized goodwill which had a balance of $1,703,000 as of December 31, 2023. In connection with the acquisition of Ceautamed on July 29, 2022, the Company recognized goodwill which had a balance of $0 as of December 31, 2023.
Long-Lived Assets
The Company assesses potential impairments to its long-lived assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. An impairment loss is recognized when the undiscounted cash flows expected to be generated by an asset (or group of assets) is less than its carrying amount. Any required impairment loss is measured as the amount by which the asset’s carrying value exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to operating results. The Company recognized an impairment of the intangible assets associated with Nexus during the year ended December 31, 2023 of $2,897,737. The Company recognized an impairment of the license agreement associated with GSP during the year ended December 31, 2023 of $342,531. The Company recognized an impairment of the intangible assets of Ceautamed during the year ended December 31, 2023 of $2,603,233.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Lease Right-of-Use Asset
The Company records a right-of-use (“ROU”) asset and lease liability on the balance sheet for all leases with terms longer than 12 months. Leases are classified either as finance or operating with the classification affecting the pattern of expense recognition.
Lease liabilities are recognized based on the present value of the remaining lease payments and are discounted using the most reasonable incremental borrowing rate. The Company uses the implicit rate when it is readily determinable. Since the Company’s lease does not provide an implicit rate, to determine the present value of lease payments, management uses the Company’s incremental borrowing rate based on the information available at lease commencement. Leases with a term of 12 months or less at inception are not recorded on the balance sheet and are expensed on a straight- line basis over the lease term.
Related Parties
The Company follows ASC 850, “Related Party Disclosures,” for the identification of related parties and disclosure of related party transactions (see Note 14).
Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses represent liabilities incurred in the normal course of business which may or may not have related invoices from third parties. Examples of accrued expenses include compensation, interest, and taxes. Included in accrued liabilities at December 31, 2023 are accrued interest of $1,898,384 and accrued payroll liabilities of $1,308,748.
Debt Issuance Cost
In accordance with ASC 835-30, Other Presentation Matters, the Company has reported debt issuance cost as a deduction from the carrying amount of debt and amortizes these costs using the effective interest method over the term of the debt as interest expense.
Revenue Recognition
The Company evaluates and recognizes revenue by:
| ● | identifying the contract(s) with the customer, |
| ● | identifying the performance obligations in the contract, |
| ● | determining the transaction price, |
| ● | allocating the transaction price to performance obligations in the contract; and |
| ● | recognizing revenue as each performance obligation is satisfied through the transfer of a promised good or service to a customer (i.e., “transfer of control”). |
Products (BSNM, DSO, GSP and Ceautamed)
The Company primarily generates revenues by manufacturing and packaging of nutraceutical products as a contract manufacturer for customers. The majority of the Company’s revenue is recognized when it satisfies a single performance obligation by transferring control of its products to a customer. Control is generally transferred when the Company’s products are either shipped or delivered based on the terms contained within the underlying contracts or agreements. The Company’s general payment terms are short-term in duration. The Company does not have significant financing components or payment terms. The Company did not have any material unsatisfied performance obligations at December 31, 2023 or 2022.
Distribution expenses to transport the Company’s products, where applicable, and warehousing expense after manufacture are accounted for within operating expenses.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Advertising/Marketing (Nexus)
Nexus generates revenues when sales of listed products are sold by product vendors through its network as a result of the marketing efforts of digital marketers. The products on the network come from several different customers, which pay Nexus a specific amount per sale, the amount of which is dictated by the customer. The revenue is recognized upon the sale of a product by the customer, net of fraudulent traffic or disputed transactions. A portion of the specific amount received by Nexus for that sale is paid out to the digital marketer as a commission, which is recorded in cost of sales. To illustrate the revenue process, a digital marketer logs onto the platform and selects an offer to promote for the day. The platform generates a unique link which the digital marketer distributes either via email or a banner ad. As the link is distributed to the consumer via the marketing efforts of the digital marketer, the consumer visits that link to make a purchase from the customer’s website, and when such purchase is complete, revenue is recognized by Nexus and the sale is credited to the digital marketer’s Nexus account. The benefit to the digital marketer operating on Nexus’ network is that the digital marketer receives a commission without the possibility of a claw back or refund. The customer benefits through increased sales of its products as a result of the marketing efforts of the digital marketers. Nexus’ platform acts as the transaction ledger, keeping track of clicks, sales and commissions.
Nexus’ general payment terms are short-term in duration. Insertion orders are utilized between Nexus and the customer for each campaign related to a particular product being marketed. The insertion order remains in effect until the customer or Nexus terminates the order, and either party may terminate the order at any time upon 14 days’ written notice. The customer is billed weekly for the sales digital marketers have generated for the week. Nexus does not have significant financing components or payment terms. Nexus did not have any material unsatisfied performance obligations at December 31, 2023 or 2022.
The table below represents the desegregation of revenue by type:
| | | December 31, 2023 | | | December 31, 2022 | |
| | | Amount | | | % of
Revenues | | | Amount | | | % of
Revenues | |
| Revenues | | | | | | | | | | | | |
| Retail | | $ | 6,880,713 | | | | 83.65 | % | | $ | 10,755,082 | | | | 67.63 | % |
| Contract manufacturing | | | 983,264 | | | | 11.95 | % | | | 1,712,568 | | | | 10.77 | % |
| Total Products | | | 7,863,977 | | | | 95.60 | % | | | 12,467,650 | | | | 78.40 | % |
| | | | | | | | | | | | | | | | | |
| Advertising | | | 361,815 | | | | 4.40 | % | | | 3,434,879 | | | | 21.60 | % |
| Total Revenues | | $ | 8,225,792 | | | | 100.00 | % | | $ | 15,902,529 | | | | 100.00 | % |
Contract Liabilities
The Company requires contract manufacturing clients to pay a deposit with the submission of purchase orders. These amounts are recorded as contract liabilities until such time as the revenue recognition criteria are fully met. The table below illustrates the activity during the year ended December 31, 2023.
| | | Amount | |
| Balance at December 31, 2022 | | $ | 675,426 | |
| Deposits received | | | 465,263 | |
| Recognized as revenue | | | (675,968 | ) |
| Balance at December 31, 2023 | | $ | 464,721 | |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Freight
For the years ended December 31, 2023 and 2022, freight costs amounted to $256,249 and $889,493, respectively, and have been recorded in cost of goods sold in the accompanying consolidated statement of operations.
Advertising
Advertising costs are expensed as incurred. Advertising costs for the years ended December 31, 2023 and 2022 were $1,580,248 and $2,173,594, respectively.
Paycheck Protection Program
The Company records Paycheck Protection Program (“PPP”) loan proceeds in accordance with ASC 470, Debt. Debt is extinguished when either the debtor pays the creditor or the debtor is legally released from being the primary obligor, either judicially or by the creditor.
Stock-based Compensation
The Company recognizes expense for stock options and warrants granted over the vesting period based on the fair value of the award at the grant date, valued using a Black-Scholes option pricing model to determine the fair market value of the stock options. The Company calculates the amount of tax benefit available by tracking each stock option award on an employee-by-employee basis and on a grant-by-grant basis. The Company then compares the recorded expense to the tax deduction received for each stock option grant. The Company’s policy is to recognize forfeitures as they occur.
Income Taxes
The Company accounts for income tax under the provisions of ASC 740, Income Taxes. The Company records a liability for uncertain tax positions when it is probable that a loss has been incurred and the amount can be reasonably estimated. At December 31, 2023 and 2022, the Company has no liabilities for uncertain tax positions. The Company continually evaluates expiring statutes of limitations, audits, proposed settlements, changes in tax law and new authoritative rulings. The Company’s tax years subject to examination by tax authorities generally remain open for three (3) years from the date of filing. Due to the continued losses, the Company has recorded a full valuation at the end of December 31, 2023 and 2022.
The provision for income taxes is computed using the asset and liability method, under which deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities, and for operating losses and tax credit carry forwards. Deferred tax assets and liabilities are measured using the currently enacted tax rates that apply to taxable income in effect for the years in which those tax assets are expected to be realized or settled. The Company records a valuation allowance to reduce deferred tax assets to the amount that is believed more likely than not to be realized.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Employee Retention Credits
In accordance with the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”), the Company filed for Employee Retention Credits for applicable periods in 2020 and 2021. As amounts to be refunded are subject to IRS calculations, and the timing for processing of the credits are unknown, the Company recognizes as other income amounts refunded upon the receipt of the payment. During the years ended December 31, 2023 and 2022, the Company received $586,556 and $0, respectively.
Discontinued Operations
A component of an entity that is disposed of by sale or abandonment is reported as discontinued operations if the transaction represents a strategic shift that will have a major effect on an entity's operations and financial results. The results of discontinued operations are aggregated and presented separately in the Consolidated Statement of Operations. Assets and liabilities of the discontinued operations are aggregated and reported separately as assets and liabilities of discontinued operations in the Consolidated Balance Sheet, including the comparative prior year period. Cash flows from such entities are reflected as cash flows from discontinued operations within the Company’s Consolidated Statements of Cash Flows for each period presented.
Amounts presented in discontinued operations have been derived from our consolidated financial statements and accounting records using the historical basis of assets, liabilities, and historical results. The discontinued operations exclude general corporate allocations.
Net Loss Per Share
Basic and diluted net loss per share of common stock for the years ended December 31, 2023, and 2022 was determined by dividing net loss by the weighted average shares of common stock outstanding during the period. The Company’s potentially dilutive shares, consisting of 217,318, and 71,513 warrants and 43,259 and 818 stock options, have not been included in the computation of dilutive net loss per share for the years ended December 31, 2023 and 2022, respectively, as the results would be antidilutive.
Recent Accounting Standards Issued Adopted
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments—Credit Losses (Topic 326)—Measurement of Credit Losses on Financial Instruments”, which has been subsequently amended (“ASU 2016-13”). This updated accounting guidance significantly changes the impairment model for most financial assets and certain other instruments. ASU 2016-13 will require immediate recognition of estimated credit losses expected to occur over the remaining life of many financial assets, which will generally result in earlier recognition of allowances for credit losses on trade receivables, loans and other financial instruments. This update is effective for the Company’s fiscal year beginning after December 15, 2022, including interim periods within those fiscal years. The Company adopted this new standard on January 1, 2023, and the adoption had no material impact on the Company’s consolidated financial statements.
Recent Accounting Standards Issued Not Yet Adopted
On August 5, 2020, the FASB issued ASU 2020-06, which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in GAAP. This ASU is effective for fiscal years beginning after December 31, 2023. The Company feels the adoption of this ASU will not have a material impact to the financial statements.
Note 3 — Correction of Prior Period Immaterial Errors
The Company has identified immaterial errors in the Company’s previously issued consolidated financial statements related to intangible assets and goodwill. The error pertains to the classification of assets acquired in the acquisition of Nexus, whereby intangible assets, and the value of such assets, were overstated and goodwill was understated.
The Company has identified immaterial errors in the Company’s previously issued consolidated financial statements related to the number of warrants issued, forfeited, and outstanding.
In evaluating whether the previously issued consolidated financial statements were materially misstated for the interim or annual periods prior to December 31, 2022, the Company applied the guidance of ASC 250, Accounting Changes and Error Corrections, SEC Staff Accounting Bulletin (“SAB”) Topic 1.M, Assessing Materiality and SAB Topic 1.N, Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements, and concluded that the effect of the errors on prior period annual financial statements was immaterial. The guidance states that prior-year misstatements which, if corrected in the current year would materially misstate the current year’s financial statements, must be corrected by adjusting prior year financial statements, even though such correction previously was and continues to be immaterial to the prior-year financial statements. Correcting prior-year financial statements for such immaterial misstatements does not require previously filed reports to be amended.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
The adjustments required to correct the misstatement in the financial statements related to the intangible assets and goodwill for the year ended December 31, 2022 are reflected in the consolidated balance sheet. The amount of the reclassification is $4,134,000.
As a result of the misstatement pertaining to the reclassification of the intangible assets, the Company noted an immaterial error associated with the recording of amortization expense. The Company recorded an error totaling $279,575 of amortization during the year ended December 31, 2022, and $67,075 for the three months ended March 31, 2023, respectively, collectively $346,768 that should not have been expensed. In accordance with ASC 250, Accounting Changes and Error Correction, the change in estimate for the fiscal year 2023 and beyond is reported on a prospective basis and the prior financial statements are not restated.
The Company also reclassified $340,100 from intangible assets to goodwill as the result of the obtained independent professional purchase price allocation related to the acquisition of Ceautamed.
The Company’s consolidated balance sheets have been revised from the amounts previously reported to correct the error and the impact of the purchase price allocation change as shown in the table below. See Note 4.
Consolidated Balance Sheet as of December 31, 2022
| | | As
Previously
Reported | | | PPA
Finalization | | | Corrections | | | As Adjusted | |
| Intangible assets, net | | $ | 20,936,258 | | | $ | (340,100 | ) | | $ | (4,134,000 | ) | | $ | 16,462,158 | |
| Goodwill | | | 1,342,000 | | | | 340,100 | | | | 4,134,000 | | | | 5,816,100 | |
The Company noted that it had not properly reported the number of warrants issued and forfeited during the year ended December 31, 2022 and the number of warrants outstanding as of December 31, 2022 was also misreported. The exclusion of the warrants in the previously issued financial statements did not have an impact on the Company’s consolidated balance sheet nor the consolidated statement of operations and loss per share calculations as of and for the year ended December 31, 2022. These amounts have been updated as presented below:
| | | As
Previously
Reported | | | Corrections | | | Corrected | | | Adjusted for Stock Splits | |
| Outstanding at December 31, 2021 | | | 14,802,006 | | | | — | | | | 14,802,006 | | | | 4,699 | |
| Granted | | | 11,021,560 | | | | 201,255,072 | | | | 212,276,632 | | | | 67,389 | |
| Exercised | | | 1,439,230 | | | | — | | | | 1,439,230 | | | | 457 | |
| Forfeited | | | 275,988 | | | | 100,000 | | | | 375,988 | | | | 118 | |
| Outstanding at December 31, 2022 | | | 24,108,348 | | | | 201,155,072 | | | | 225,263,420 | | | | 71,513 | |
Note 4 — Acquisitions
On March 14, 2022, the Company entered into securities purchase agreement, which was amended on July 29, 2022, to acquire Ceautamed. On July 29, 2022, the acquisition was completed.
Pursuant to the terms of the securities purchase agreement, as amended, the Company acquired Ceautamed for an aggregate purchase price of $8,600,000. The purchase price consists of (i) $3,000,000 in cash, of which $1,000,000 was previously paid by the Company and $2,000,000 was paid at closing, (ii) secured subordinated convertible promissory notes in the aggregate principal amount of $2,150,000; (iii) secured subordinated promissory notes in the aggregate principal amount of $2,150,000 and (iv) secured subordinated promissory notes in the aggregate principal amount of $1,300,000.
The table below summarizes the value of the total consideration given in the transaction.
| | | Amount | |
| Cash issued | | $ | 3,000,000 | |
| Debt issued | | | 5,600,000 | |
| Total consideration | | $ | 8,600,000 | |
Under the acquisition method of accounting outlined in ASC 805, the identifiable assets acquired and liabilities assumed in the acquisitions are recorded at their acquisition-date fair values and are included in the Company’s consolidated financial position.
The following table summarizes the preliminary purchase price allocation for the assets acquired and liabilities assumed in connection with the acquisition of Ceautamed.
| | | Amount | |
| Tangible assets acquired | | $ | 635,223 | |
| Liabilities assumed | | | (392,529 | ) |
| Intangible assets | | | 8,357,306 | |
| Net assets acquired | | $ | 8,600,000 | |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
The intangible assets acquired from Ceautamed were comprised of the following:
| | | Useful life
in years | | | Amount | |
| Non-compete agreements | | | 3 | | | $ | 800,000 | |
| Customer contracts | | | 10 | | | | 450,000 | |
| Trademark | | | 15 | | | | 2,160,000 | |
| Supplier contracts | | | 3 | | | | 1,800,000 | |
| Patent license | | | 10 | | | | 2,720,000 | |
| | | | | | | $ | 7,930,000 | |
The following unaudited supplemental proforma financial information reflects the combined results of operations had the Ceautamed acquisition had occurred at the beginning of 2022. The proforma information reflects certain adjustments related to the acquisition including adjusted amortization and depreciation expense based on the fair values of the assets acquired. The proforma combined results of operations are as follows:
| | | Year Ended
December 31,
2022 | |
| Net sales | | $ | 19,596,961 | |
| Net income (loss) | | $ | (29,742,915 | ) |
| | | | | |
| Earnings (loss) per share, basic and diluted | | $ | (3,045.56 | ) |
| Weighted average shares outstanding, basic and diluted | | | 9,766 | |
Note 5 — Discontinued Operations
ASC 360-10-45-9 requires that a long-lived asset (disposal group) to be sold shall be classified as held for sale in the period in which a set of criteria have been met, including criteria that the sale of the asset (disposal group) is probable, and actions required to complete the plan indicate that it is unlikely that significant changes to the plan will be made or that the plan will be withdrawn. On March 6, 2024, the Company entered into a sale and leaseback agreement with a third party related to BSNM as more fully described under Note 16.
For comparability purposes, certain prior period line items relating to the assets held for sale have been reclassified and presented as discontinued operations for all periods presented in the accompanying consolidated statements of operations, consolidated statements of cash flows, and the consolidated balance sheets.
In accordance with ASC 205-20-S99, “Allocation of Interest to Discontinued Operations,” the Company elected to not allocate consolidated interest expense to discontinued operations where the debt is not directly attributable to or related to discontinued operations.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
The following information presents the major classes of line item of assets and liabilities included as part of discontinued operations in the consolidated balance sheets as of December 31, 2023 and 2022:
| | | December 31,
2023 | | | December 31,
2022 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Accounts receivable, net | | $ | 76,761 | | | $ | 21,823 | |
| Inventory | | | 272,210 | | | | 483,150 | |
| Prepaid expenses and other current assets | | | — | | | | 4,723 | |
| Total current assets | | | 348,971 | | | | 509,696 | |
| | | | | | | | | |
| Property and equipment, net | | | 168,586 | | | | 263,028 | |
| Intangible assets, net | | | — | | | | 175,060 | |
| Deposits and other assets | | | 41,621 | | | | 37,196 | |
| Operating lease right-of-use assets | | | 852,381 | | | | 374,484 | |
| Total other assets | | | 1,062,588 | | | | 849,768 | |
| Total assets | | $ | 1,411,559 | | | $ | 1,359,464 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 987,786 | | | $ | 855,978 | |
| Accrued expenses | | | 484,972 | | | | 218,434 | |
| Due to related parties, net | | | 6,200 | | | | 21,700 | |
| Contract liabilities | | | 135,507 | | | | — | |
| Lease liability, current | | | 878,230 | | | | 53,173 | |
| Debt, current, net of debt discounts | | | 138,383 | | | | 232,716 | |
| Total current liabilities | | | 2,631,078 | | | | 1,382,001 | |
| | | | | | | | | |
| Long-term liabilities: | | | | | | | | |
| Lease liability, noncurrent | | | — | | | | 324,646 | |
| Debt, noncurrent | | | 441,929 | | | | 489,799 | |
| Total long-term liabilities | | | 441,929 | | | | 814,445 | |
| Total liabilities | | $ | 3,073,007 | | | $ | 2,196,446 | |
The following information presents the major classes of line items constituting the after-tax loss from discontinued operations in the consolidated statements of operations for the years ended December 31, 2023 and 2022:
| | | December 31,
2023 | | | December 31,
2022 | |
| Revenues | | | | | | |
| Products | | $ | 505,643 | | | $ | 1,863,832 | |
| Cost of revenues | | | | | | | | |
| Products | | | 834,489 | | | | 2,077,223 | |
| Gross loss | | | (328,846 | ) | | | (213,391 | ) |
| Operating expenses | | | | | | | | |
| General and administrative | | | 700,340 | | | | 732,369 | |
| Compensation | | | 296,893 | | | | 529,380 | |
| Professional services | | | 17,073 | | | | 4,869 | |
| Impairment | | | 119,776 | | | | — | |
| Depreciation and amortization expense | | | 153,175 | | | | 245,899 | |
| Total operating expenses | | | 1,287,257 | | | | 1,512,517 | |
| Operating loss | | | (1,616,103 | ) | | | (1,725,908 | ) |
| Other income (expense) | | | | | | | | |
| Other income (expense) | | | 194,721 | | | | (8,425 | ) |
| Interest expense | | | (136,983 | ) | | | (229,095 | ) |
| Gain on debt extinguishment | | | 3,230 | | | | 168,013 | |
| Total other income (expense) | | | 60,968 | | | | (69,507 | ) |
| Net loss from discontinued operations | | | (1,555,135 | ) | | | (1,795,415 | ) |
| Income tax expense | | | — | | | | — | |
| Net loss | | $ | (1,555,135 | ) | | $ | (1,795,415 | ) |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Note 6 — Inventory
Inventory consisted of the following at December 31 after the effects of the reclassification adjustments for discontinued operations:
| | | 2023 | | | 2022 | |
| Raw materials | | $ | 252,322 | | | $ | 361,623 | |
| Packaging materials | | | 617,388 | | | | 946,884 | |
| Finished goods | | | 373,067 | | | | 873,844 | |
| | | | 1,242,777 | | | | 2,182,351 | |
| Less: allowance for obsolescence | | | — | | | | — | |
| | | $ | 1,242,777 | | | $ | 2,182,351 | |
Note 7 — Property and Equipment
Property and equipment consisted of the following at December 31 after the effects of the reclassification adjustments for discontinued operations:
| | | Estimated
Useful Lives
(in Years) | | | 2023 | | | 2022 | |
| Furniture and fixtures | | | 7 | | | $ | 8,049 | | | $ | 8,049 | |
| Equipment – manufacturing | | | 5 | | | | 387,493 | | | | 316,535 | |
| Building and equipment | | | 5 | | | | 3,840 | | | | 3,840 | |
| Leasehold improvements | | | 2.5 | | | | 72,483 | | | | 72,483 | |
| | | | | | | | 471,865 | | | | 400,907 | |
| Less: accumulated depreciation | | | | | | | (371,326 | ) | | | (181,716 | ) |
| Property and equipment, net | | | | | | $ | 100,539 | | | $ | 219,191 | |
Depreciation and amortization expense for the years ended December 31, 2023 and 2022 totaled $118,595 and $112,878, respectively. During the year ended December 31, 2023, the Company transferred certain manufacturing equipment of $70,958 including the related accumulated depreciation of $70,958 from BSNM to DSO. As BSNM is presented as discontinued operations, the balances are not included within the presented December 31, 2022 amounts in the table above.
Note 8 — Intangible Assets
Intangible assets consisted of the following at December 31 after the effects of the reclassification adjustments for discontinued operations:
| | | Estimated
Useful Lives
(in Years) | | | 2023 | | | 2022 | |
| Goodwill | | | | | | $ | 3,045,000 | | | $ | 5,816,100 | |
| | | | | | | | | | | | | |
| Customer contracts | | | 7-10 | | | $ | 5,933,263 | | | $ | 6,400,000 | |
| Developed technology | | | 15 | | | | 1,660,000 | | | | 1,660,000 | |
| Non-compete agreements | | | 3 | | | | 840,000 | | | | 840,000 | |
| Patents | | | 5-10 | | | | 2,950,000 | | | | 2,950,000 | |
| Tradename | | | 10-15 | | | | 2,010,000 | | | | 4,170,000 | |
| Supplier contracts | | | 10 | | | | 646,933 | | | | 1,800,000 | |
| Licenses agreements | | | 5 | | | | — | | | | 584,220 | |
| Total intangible assets | | | | | | | 14,040,196 | | | | 18,404,220 | |
| Less: amortization | | | | | | | (2,993,429 | ) | | | (2,117,122 | ) |
| Intangibles, net | | | | | | $ | 11,046,767 | | | $ | 16,287,098 | |
The allocation of the Nexus purchase price in 2021 to intangibles assets, including customer contracts and non-compete agreements, was based on provisional valuations performed to estimate the fair value of the assets as of the acquisition date. During the three months ended June 30, 2023, the Company noted that an immaterial error existed in the recording of the allocation of the intangible assets and has reclassified $4,134,000 from intangible assets to goodwill.
The allocation of the Ceautamed purchase price in 2022 to intangibles assets, including customer contracts and non-compete agreements, was based on provisional valuations performed to estimate the fair value of the assets as of the acquisition date. The Company engaged an independent valuation firm to perform a purchase price allocation, noting differences in the intangible assets and values. The independent valuation resulted in reclassification of the assets which have been represented in the December 31, 2022 balances. Additionally, $340,100 was reclassified from intangible assets to goodwill.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
The table below identifies the changes to each of the intangible assets as a result of the purchase price adjustments obtained related to the Nexus and Ceautamed acquisitions.
| | | December 31,
2022
previously
reported | | | Purchase
Price
Adjustments | | | December 31,
2022
(As Adjusted) | |
| Customer contracts | | $ | 17,046,076 | | | $ | (10,203,814 | ) | | $ | 6,842,262 | |
| Developed technology | | | 1,570,000 | | | | 90,000 | | | | 1,660,000 | |
| Non-compete agreements | | | 1,595,530 | | | | (755,530 | ) | | | 840,000 | |
| Patents | | | 230,000 | | | | 2,720,000 | | | | 2,950,000 | |
| Tradename | | | 2,010,000 | | | | 2,160,000 | | | | 4,170,000 | |
| Intellectual property | | | 385,199 | | | | (385,199 | ) | | | — | |
| Supplier contracts | | | — | | | | 1,800,000 | | | | 1,800,000 | |
| Licenses agreements | | | 584,220 | | | | — | | | | 584,220 | |
| Total intangible assets | | $ | 23,421,025 | | | $ | (4,574,543 | ) | | $ | 18,846,482 | |
During 2023, identifiable intangible assets and goodwill decreased due to an impairment expense of $5,843,501.
Amortization (included in depreciation and amortization expense) for the years ended December 31, 2023 and 2022 was $2,154,693 and $1,841,948, respectively.
The future amortization is as follows:
| Years Ending December 31: | | | |
| 2024 | | $ | 4,750,590 | |
| 2025 | | | 836,332 | |
| 2026 | | | 836,238 | |
| 2027 | | | 836,238 | |
| 2028 | | | 813,261 | |
| Thereafter | | | 2,974,108 | |
| Total | | $ | 11,046,767 | |
Note 9 — Lease Commitments
The Company enters into lessee arrangements consisting of operating leases for premises. The Company had three and four operating leases for premises as of December 31, 2023 and 2022, respectively.
Leases Involving Real Estate
Leases of distribution and manufacturing facilities, customer support centers and the Company’s corporate headquarters have lease terms that generally range from 5 to 7 years.
Rental payments on these leases typically provide for fixed minimum payments that increase over the lease term at predetermined amounts. Certain leases of real estate provide for rental increases based on the consumer price index, which are included in the Company’s measurement of lease payments based on the rate or index in effect at lease commencement and are therefore included in the measurement of the lease liabilities.
Leases Involving Equipment
Equipment leases have lease terms that generally range from less than 4 years to 5 years. Rental payments on these leases typically provide for fixed minimum payments that increase over the lease term at predetermined amounts, are included in the measurement of lease payments, and are included in the measurement of lease liabilities. Certain leases involving equipment have purchase options. When those options are reasonably certain of being exercised, the Company reflects such purchase options when measuring the lease term and lease payments for those leases.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Financial Information
The following provides information about the Company’s right of use assets and lease liabilities for its operating and finance leases as of December 31, 2023 and 2022 after the effects of the reclassification adjustments for discontinued operations:
| | | Balance Sheet Classification | | December 31,
2023 | | | December 31,
2022 | |
| Right of use assets operating leases | | Operating leases right-of-use asset | | $ | 2,015,299 | | | $ | 2,298,381 | |
| | | | | | | | | | | |
| Lease liabilities | | | | | | | | | | |
| Current | | | | | | | | | | |
| Operating leases | | Operating lease liabilities, current portion | | | 298,644 | | | | 250,646 | |
| Noncurrent | | | | | | | | | | |
| Operating leases | | Operating lease liabilities, non-current portion | | | 1,800,158 | | | | 2,098,803 | |
| Total lease liabilities | | | | $ | 2,098,802 | | | $ | 2,349,449 | |
The components of the Company’s lease costs for the years ended December 31, 2023 and 2022 are as follows:
| | | Income Statement Classification | | December 31,
2023 | | | December 31,
2022 | |
| Rent expense | | General and administration | | $ | 533,036 | | | $ | 183,709 | |
Supplemental cash flow information related to Company’s leases for the year ended December 31, 2023:
| | | Operating
Leases | |
| Cash paid for amounts included in the measurement of lease liabilities | | $ | 246,140 | |
Weighted average remaining lease term and weighted average discount rate for the Company’s leases as of December 31, 2023:
| | | Operating
Leases | |
| Weighted average remaining term (in years) | | | 5.09 | |
| Weighted average discount rate | | | 12.00 | % |
Minimum lease payments under the operating lease are recognized on a straight-line basis over the term of the lease.
| For the Year Ended December 31: | | | |
| 2024 | | $ | 534,475 | |
| 2025 | | | 550,309 | |
| 2026 | | | 566,648 | |
| 2027 | | | 550,920 | |
| 2028 | | | 437,474 | |
| Thereafter | | | 199,302 | |
| Total payments | | | 2,839,128 | |
| Less: amount representing interest | | | (740,326 | ) |
| Lease obligation, net | | | 2,098,802 | |
| Less: current portion | | | (298,644 | ) |
| Lease obligation – long-term | | $ | 1,800,158 | |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Note 10 — Debt
Original Issue Discount Subordinated Debentures
In June 2022, the Company commenced offerings of original issue discount subordinated debentures. As of December 31, 2023, the Company has completed multiple closings and issued debentures in the aggregate principal amount of $5,264,099. The debentures contain an original issue discount of 15%, or an aggregate original issue discount of $788,830. As a result, the total purchase price was $4,475,269. The debentures bear interest at a rate of 17.5% per annum and are due from January through November 2024. The outstanding principal amount and all accrued interest is due and payable on the earlier of (i) the completion of the Company’s next equity financing in which it receives gross proceeds in excess of $20 million, (ii) twenty-four months after the date of issuance or (iii) within 30 days after election of repayment from the holder so long as the election is after the 6-month anniversary of the debenture. The Company may voluntarily prepay the debentures in whole or in part without premium or penalty. The debentures contain customary events of default for a loan of this type. The debentures are unsecured and are subordinated in right of payment to the prior payment in full of all senior indebtedness and are pari passu in right of payment to any other unsecured indebtedness incurred by the Company in favor of any third party. As of December 31, 2023, the outstanding principal balance of the debentures was $4,415,476, the unamortized debt issuance cost was $84,776 and accrued interest was $1,053,261. On May 30, 2023, two of the debenture agreements totaling $276,471 were converted into 1,186 shares of the Company’s series B preferred stock, at a conversion price of $223.01. During the year ended December 31, 2023, an aggregate of $1,347,060 was raised via these debentures.
Original Issue Discount Secured Subordinated Note
On July 29, 2022, the Company entered into a securities purchase agreement with an accredited investor, pursuant to which it sold an original issue discount secured subordinated note in the principal amount of $2,272,727 to such investor. The note contains an original issue discount of 12%, or $272,727. As a result, the total purchase price was $2,000,000, the proceeds of which were used to fund the acquisition of Ceautamed. The note shall bear interest at the rate of 16% per annum and matures on July 29, 2027. The outstanding principal and all accrued interest shall be amortized on a 60-month straight-line basis. The Company may prepay the principal and all accrued and unpaid interest on the note without penalty, in whole or in part. The note contains customary events of default for a loan of this type. The note is guaranteed by DSO, Nexus, GSP and Ceautamed and is secured by a security interest in all of the assets of the Company and such guarantors; provided that such security interest is subordinate to the rights of the lenders under any senior indebtedness (as defined in the note). Following payments made and payments not made on a timely basis, the note was modified on May 24, 2023 whereby the maturity date was extended to August 1, 2029. Additionally, the previously unpaid loan repayments identified in the original note would be paid by increasing the loan repayments until the “past due” payments are caught up by increasing the payments by approximately $22,500 per payment. In consideration of the modifications, the Company granted the investor 80 shares of common stock valued at $11,700 and agreed to make an additional payment of $45,141.05 by June 30, 2023. On August 29, 2023, a second modification occurred, whereby 2,155 shares valued at $50,000 were granted as a forbearance fee due to non-timely payments, and no adjustments to the loan amount or timing of maturity. The total value of the consideration was $106,841, or 4.7% of the original note principal. As of December 31, 2023 the outstanding principal balance of the note was $2,182,853, the unamortized debt issuance cost was $172,807 and accrued interest was $494,700. During the year ended December 31, 2023, the Company recognized $77,218 of interest expense associated with the amortization of the debt issuance cost.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Acquisition Notes
On July 1, 2021, the Company issued a 6% secured subordinated promissory note in the principal amount of $3,000,000 to a related party, Sasson E. Moulavi, in connection with the acquisition of DSO. This note accrues interest at 6% per annum and the outstanding principal and interest will be amortized on a straight-line basis and are payable quarterly in accordance with the amortization schedule, with all amounts due and payable on July 1, 2024. On November 29, 2022, the Company entered into a letter agreement with Dr. Moulavi to amend the terms of the note. Pursuant to the letter agreement, the parties agreed to amend and restate the note to amend the amortization schedule, with the first payment deferred until February 15, 2023 and all amounts due and payable on August 15, 2024. In exchange for the agreement of Dr. Moulavi to enter into the letter agreement, the Company agreed to (i) issue to Dr. Moulavi 32 shares of common stock under the Company’s 2022 Equity Incentive Plan and (ii) pay to Dr. Moulavi a fee of $50,000 in cash, which shall be paid upon completion of the Company’s anticipated debt financing and is included in debt balance. The Company may prepay all or any portion of this note any time prior to maturity without premium or penalty. This note contains customary covenants and events of default for a loan of this type, including if a default occurs under any senior secured indebtedness to banks and other financial institutions or private equity funds, and is secured by a security interest in all of the assets of DSO; provided that such security interest is subordinate to the rights of the lenders under any such senior secured indebtedness. During the year ended December 31, 2023, the Company made principal cash payments of $150,000 and $196,000 for the new note. On May 25, 2023 the note was modified whereby $1,500,000 in principal amount of the note was converted to 6,727 shares of the Company’s series B preferred stock. The remaining $1,400,000 of principal plus accrued interest of $334,950 was converted into a new note with the same interest rate and maturity date. The modification of the terms and debt was accounted for as a debt modification. As of December 31, 2023, the outstanding principal balance of this note was $1,388,950 and accrued interest was $37,697.
On November 8, 2021, the Company issued a 5% secured subordinated promissory note in the principal amount of $1,900,000 to related parties, Justin Francisco and Steven Rubert, in connection with the acquisition of Nexus. This note accrued interest at 5% per annum and the outstanding principal and interest was amortized on a straight-line basis and are payable quarterly in accordance with the amortization schedule attached to the note, with all amounts due and payable on November 8, 2024. On May 25, 2023, the note and accrued interest $12,966 was converted to 8,579 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
On July 29, 2022, the Company issued secured subordinated convertible promissory notes in the aggregate principal amount of $2,150,000 in connection with the acquisition of Ceautamed, which are partially with a related party. The notes bore interest at the rate of 5% per annum with all principal and accrued interest being due and payable in one lump sum on July 29, 2025; provided that upon an event of default (as defined in the notes), such interest rate shall increase to 10%. On May 25, 2023, the note and accrued interest of $182,153 was converted to 10,458 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
On July 29, 2022, the Company issued secured subordinated promissory notes in the aggregate principal amount of $2,150,000 in connection with the acquisition of Ceautamed, which are partially with a related party. The notes shall bear interest at the rate of 5% per annum and mature on July 29, 2025; provided that upon an event of default (as defined in the notes), such interest rate shall increase to 10%. The outstanding principal and all accrued interest shall be amortized on a five-year straight-line basis and payable quarterly in accordance with the amortization schedule to the notes. The Company may redeem all or any portion of the notes at any time without premium or penalty. The notes contain customary covenants and events of default for loans of this type, including upon any default under the senior indebtedness (as defined in the notes). The notes are guaranteed by Ceautamed and are secured by a security interest in all of the assets of such guarantors; provided that such security interest is subordinate to the rights of the lenders under any such senior indebtedness. As of December 31, 2023, the outstanding principal balance of these notes was $2,189,493, unamortized debt issuance cost was $0 and accrued interest was $237,430.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
On July 29, 2022, the Company issued secured subordinated promissory notes in the aggregate principal amount of $1,300,000 in connection with the acquisition of Ceautamed, which are partially with a related party. The notes bear interest at the rate of 5% per annum with all principal and accrued interest being due and payable in one lump sum ninety (90) days from the date of the note; provided that upon an event of default (as defined in the notes), such interest rate shall increase to 10%. On November 28, 2022, the Company entered into letter agreements with the holders of most of the notes to amend the terms of these notes. Pursuant to letter agreements, the parties agreed to extend the maturity date to June 1, 2023 and agreed to a seven month payment schedule, with the first payment due December 1, 2022. The parties also agreed to increase the default interest rate from 10% to 15%. The Company also agreed that if an event of default (as defined in the notes) has occurred and is continuing, then the Company shall not create any senior indebtedness (as defined in the notes) without the consent of the holders of a majority of the principal amount of the notes. In exchange for the agreement of the holders to enter into the letter agreements, the Company agreed to pay certain amendment fees as more particularly described in the letter agreements. On April 1, 2023, the Company entered into letter agreements with the holders of most of the notes, pursuant to which the Company agreed to pay the remaining balance of the notes in two payments on May 1, 2023 and June 1, 2023. One of the note holders agreed to settle the amount due to the holder for a lesser amount, resulting in a gain on extinguishment of debt of $60,764. In addition, the Company agreed to prepay a portion of the outstanding principal and accrued interest on the notes upon the occurrence of any of the following events: (i) in the event the Company receives any tax refunds claimed with respect to an “employee retention credit” pursuant to Section 2301 of the CARES Act (or any corresponding or similar provisions of state or local law) or receives any cash from factoring or assigning the right to receive such tax refunds, the Company must prepay $150,000 of the outstanding principal and accrued interest on the notes; (ii) in the event the Company or any its subsidiaries raise capital through the issuance of any convertible debentures, the Company must prepay the outstanding principal and accrued interest on the notes in an amount equal to one-third (1/3) of the net proceeds from such financing; and (iii) in the event the Company or any of its subsidiaries consummates an equity or equity-linked capital raise with the assistance of an investment bank, the Company must prepay the outstanding principal and accrued interest on the notes in an amount equal to one-third (1/3) of the proceeds raised by the Company or any of its subsidiaries, up to an aggregate amount of $350,000, plus any then overdue principal payments. The Company is in the process of negotiating a similar extension of one remaining note in the principal amount of $100,000. The Company may redeem all or any portion of the notes at any time without premium or penalty. The notes contain customary covenants and events of default for loans of this type, including upon any default under the senior indebtedness (as defined in the notes). The notes are guaranteed by Ceautamed and are secured by a security interest in all of the assets of such guarantors; provided that such security interest is subordinate to the rights of the lenders under any such senior indebtedness. As of December 31, 2023, the outstanding principal balance of these notes was $90,000 and they were in default. The unamortized debt issuance cost was $0 and accrued interest was $6,500.
Other Promissory Notes and Cash Advances
Promissory Notes
On July 1, 2021, the Company entered into a loan agreement with Diamond Creek Capital, LLC for a term loan in the principal amount of up to $3,000,000. The loan bears interest at a rate of 15.0% per annum, provided that upon an event of default, such rate shall increase by 5%. The loan was due and payable on the earlier of July 1, 2022 or upon completion of the Company’s initial public offering (the “IPO”). The Company repaid $1,325,000 of the principal balance and $27,604 of the interest from the proceeds of the IPO. In connection with such repayment, the lender agreed that the remaining loan was due and payable on July 1, 2023. On April 20, 2023, the Company entered into an amendment to the loan agreement, which provides that the Company shall make a payment towards the reduction of principal in the amount of $250,000 within two business days of the earlier to occur of the following events: (i) the Company receives any tax refunds claimed with respect to an “employee retention credit” pursuant to Section 2301 of the CARES Act (or any corresponding or similar provisions of state or local law) or receives any cash from factoring or assigning the right to receive such tax refunds, or (ii) the completion of a public offering of securities pursuant to a Registration Statement on Form S-3. In addition, within two business days following the completion of a public offering of securities pursuant to a Registration Statement on Form S-1, the Company agreed to pay in full all outstanding amounts owing under the loan agreement. The amendment also provides that following the payment of $250,000 described above, monthly payments of principal and interest pursuant to shall be an aggregate of $30,000 per month, until the amounts owing under the loan agreement are paid in full. The loan is secured by all of the Company’s assets and contains customary events of default. As of December 31, 2023, the outstanding principal balance of this note was $542,163 and accrued interest was $0. As of December 31, 2023, the Company was in default on this note. This note was fully paid as part of the transfer of Ceautamed assets into First Health FL LLC in January 2024.
In 2018, the Company entered into an amended note agreement and convertible promissory note for $200,000 with a third party. At December 31, 2023, the outstanding amount was $100,000 and accrued interest was $161,351. The amended loan agreement and convertible promissory note (convertible at fair value of shares) was in default at December 31, 2023. This note is related to BSNM and included within liabilities of discontinued operations, noncurrent.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
On October 12, 2022, the Company issued promissory notes in the principal amount of $258,000 to third parties. These promissory notes bear interest of 15% to 20% and are payable on December 2022 and January 2023. During the year ended December 31, 2023, the Company made various principal and interest payments on the notes. On May 26, 2023, only the remaining principal balance of $105,000 was converted into 471 shares of the Company’s series B preferred stock, the accrued interest was not converted. As of December 31, 2023, the outstanding principal balance was $0 and accrued interest was $14,329.
On November 2, 2022, the Company issued a promissory note to a board member in the principal amount of $50,000. This note bears interest at a rate of 12% and is due on demand. During the year ended December 31, 2023 the principal balance was paid in full, however the accrued interest was not paid. At December 31, 2023, the outstanding amount was $0 and accrued interest was $2,387.
On December 6, 2022, the Company issued a promissory note to a board member in the principal amount of $30,000. This note bore interest at a rate of 12% and was due on demand. The remaining principal balance of $30,000 as of May 26, 2023 was converted into 135 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
On December 21, 2022, the Company issued a promissory note to a board member in the principal amount of $100,000. This note bore interest at a rate of 12% and was due on demand. The remaining principal balance of $100,000 as of May 26, 2023 was converted into 449 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
On February 8, 2023, the Company issued a promissory note to a board member in the principal amount of $50,000. This note bore interest at a rate of 12% and was due on demand. The remaining principal balance as of May 26, 2023 was converted into 225 shares of the Company’s series B preferred stock which resulted in the note being fully paid At December 31, 2023.
On February 14, 2023, the Company issued a promissory note to a third party in the principal amount of $50,000. This note bore interest at a rate of 12% and was due on demand. During the year ended December 31, 2023, the Company made various principal and interest payments on the notes. The remaining principal balance of $20,000 as of May 26, 2023 was converted into 90 shares of the Company’s series B preferred stock. At December 31, 2023, the outstanding amount was $0 and accrued interest was $992.
On February 16, 2023, the Company issued a promissory note to a third party in the principal amount of $50,000. This note bore interest at a rate of 12% and was due on August 26, 2023. The remaining principal balance as of May 26, 2023 was converted into 225 shares of the Company’s series B preferred stock which resulted in the note being fully paid At December 31, 2023. At December 31, 2023, the outstanding amount was $0 and accrued interest was $2,153.
On March 7, 2023, the Company issued a promissory note to a board member in the principal amount of $137,000. This note bears interest at a rate of 12% and is due on demand. During the year ended December 31, 2023, the Company made various principal and interest payments on the notes. On May 26, 2023, $50,000 of principal and $1,137 of interest was converted 230 shares of into the Company’s series B preferred stock. At December 31, 2023, the outstanding amount was $51,294 and accrued interest was $5,312.
On April 13, 2023, the Company issued a promissory note to a third party in the principal amount of $59,000. This note bore interest at a rate of 12% and was due on demand. The remaining principal balance as of May 26, 2023, was converted into 265 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
On April 21, 2023, the Company issued a promissory note to a board member in the principal amount of $53,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $30,000 and accrued interest was $800.
On June 22, 2023, the Company issued an original issue discount promissory note to a board member in the principal amount of $118,000. This note bears interest at a rate of 17.5% and is due on demand. At December 31, 2023, the outstanding amount was $118,000, an unamortized discount of $8,400, and accrued interest of $10,862.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
On July 12, 2023, the Company issued a promissory note to a third party in the principal amount of $25,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $25,000 and accrued interest was $1,414.
On July 25, 2023, the Company issued an original issue discount note to a board member in the principal amount of $100,000. This note bears interest at a rate of 17.5% and is due on demand. At December 31, 2023, the outstanding amount was $117,647 and accrued interest was $8,969. At December 31, 2023, the unamortized debt discount on this note was $8,235.
On August 10, 2023, the Company issued a promissory note to a board member in the principal amount of $50,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $50,000 and accrued interest was $2,351.
On August 15, 2023, the Company issued a promissory note to a board member in the principal amount of $30,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $30,000 and accrued interest was $1,361.
On August 24, 2023, the Company issued a promissory note to a third party in the principal amount of $60,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $60,000 and accrued interest was $760.
On September 7, 2023, the Company issued a promissory note to a board member in the principal amount of $35,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $35,000 and accrued interest was $1,323.
On September 14, 2023, the Company issued a promissory note to a third party in the principal amount of $100,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $100,000 and accrued interest was $3,551.
On September 29, 2023, the Company issued a promissory note to a board member in the principal amount of $35,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $35,000 and accrued interest was $1,070.
On October 4, 2023, the Company issued a promissory note to a board member in the principal amount of $15,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $15,000 and accrued interest was $434.
On October 11, 2023, the Company issued a promissory note to a third party in the principal amount of $24,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $24,000 and accrued interest was $639.
On November 14, 2023, the Company issued a promissory note to a board member in the principal amount of $30,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $30,000 and accrued interest was $464.
On November 30, 2023, the Company issued a promissory note to a board member in the principal amount of $15,000. This note bears interest at a rate of 12% and is due on demand. At December 31, 2023, the outstanding amount was $15,000 and accrued interest was $153.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Cash Advances
In June 2022, the Company entered into a cash advance agreement for $341,150 with a required repayment amount of $490,000, which required weekly payments of approximately $19,738. The cash advance agreement was fully paid at December 31, 2023.
In July 2022, the Company entered into a cash advance agreement for $650,000 with a required repayment amount of $897,750, which requires weekly payments of approximately $40,806. In May 2023, the Company agreed to a settlement for a lesser amount resulting in a gain on extinguishment of $50,814. At December 31, 2023, the outstanding amount was $147,500.
In August 2022, the Company entered into a cash advance agreement for $100,000 with a required repayment amount of $146,260, which requires weekly payments of approximately $6,200. At December 31, 2023, the outstanding amount was $4,360.
In September 2022, the Company entered into a cash advance agreement for $243,750 with a required repayment amount of $372,500, which requires weekly payments of approximately $15,000. In May 2023, the Company agreed to a settlement for a lesser amount resulting in a gain on extinguishment of $37,500. At December 31, 2023, the outstanding amount was $12,584.
In November 2022, the Company entered into cash advance agreements for an aggregate of $592,236 with an aggregated required repayment amount of $994,460, which requires weekly payments of approximately $52,422. In June 2023, the Company agreed to a settlement with one of the agreements for a lesser amount resulting in a gain on extinguishment of $63,968. At December 31, 2023, the outstanding amount was $83,037.
In December 2022, the Company entered into cash advance agreements for $293,000 with a required repayment amount of $439,207, which requires weekly payments of approximately $39,905. At December 31, 2023, the outstanding amount was $8,271.
In January 2023, the Company entered into cash advance agreements for $387,000 with a required repayment amount of $593,604, which required weekly payments of approximately $39,980. The cash advance agreements were fully satisfied with payments at December 31, 2023.
In June 2023, the Company refinanced the January 2023 agreements with balances of $284,029 and entered into cash advance agreements and received an additional $107,071 with a required repayment amount of $652,065, which requires weekly payments of approximately $48,625. At December 31, 2023, the outstanding amount was $30,518 and unamortized debt issuance cost of $0.
In July 2023, the Company entered into cash advance agreements for $74,950 with a required repayment amount of $74,950, which requires weekly payments of approximately $7,495. At December 31, 2023, the outstanding amount was $30,974.
In August 2023, the Company refinanced an agreement from June 2023, as a result the Company received an additional $48,030. At December 31, 2023, the outstanding amount was $514,670 and unamortized debt issuance cost of $0.
In November 2023, the Company entered into cash advance agreements for $49,476 with a required repayment amount of $49,476. At December 31, 2023, the outstanding amount was $49,476.
Equipment Financing Loan
In May 2022, the Company entered into an equipment financing loan for $146,765 used for the purchase of equipment within BSNM’s operations. The loan bears interest at 10.18% and matures on April 1, 2027. At December 31, 2023, the outstanding amount was $108,249.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
In August 2022, the Company entered into an equipment financing loan for $35,050 used for the purchase of equipment within BSNM’s operations. The loan bears interest at 10.18% and matures on August 1, 2027. At December 31, 2023, the outstanding amount was $27,831.
In July 2022, the Company entered into an equipment financing loan for $8,463 used for the purchase of equipment within Ceautamed’s operations. At December 31, 2023, the outstanding amount was $4,360.
Revolving Lines of Credit
In 2021, DSO entered into two revolving lines of credit with a bank, which permit borrowings up to $958,000 and bear interest at 9.49% with no expiration date. As of December 31, 2023, the outstanding principal balance of these lines of credit was $0.
In August 2022, Ceautamed entered into a revolving line of credit with a bank, which permits borrowings up to $50,000 and bears interest at 45.09% with no expiration date. In 2023, the Company borrowed an additional $43,000. As of December 31, 2023, the outstanding principal balance of these lines of credit was $47,407.
In September 2022, DSO entered into a revolving line of credit with a bank, which permits borrowings up to $70,000 and bears interest at 9.49% with no expiration date. As of December 31, 2023, the outstanding principal balance of this line of credit was $16,741.
In September 2023, DSO entered into a merchant loan agreement for $113,000. Repayment amounts are 17% of the daily account credits processed through the provider until the loan amount is paid in full. As of December 31, 2023, the outstanding principal balance was $103,657.
EIDL Loan
In June 2020, pursuant to the economic injury disaster loan (“EIDL”) program under the under the provisions of the CARES Act, the Company entered into a promissory note with the U.S. Small Business Administration with a principal amount of $300,000. This loan matures in 30 years and bears interest at a rate of 3.75%. The loan is secured by all of the Company’s assets. As of December 31, 2023, the outstanding principal balance of this loan was $300,000 and accrued interest was $39,656.
PPP Loans
In February 2021, the Company received an additional $197,457 in PPP loans under the CARES Act. This loan bears interest at a rate of 1% per annum and matures in April 2025. As of December 31, 2023, the outstanding balance of this loan was $197,457 and accrued interest was $10,431.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Total Debt
Debt is comprised of the following components as of December 31, 2023:
| Original issue discount subordinated debentures | | $ | 4,415,476 | |
| Original issue discount secured subordinated note | | | 2,182,853 | |
| Acquisition notes | | | 3,668,443 | |
| Promissory notes and cash advances | | | 2,041,847 | |
| Revolving lines of credit | | | 167,805 | |
| Equipment financing loan | | | 4,360 | |
| EIDL loan | | | 150,000 | |
| | | | 12,630,784 | |
| Debt issuance costs | | | (265,984 | ) |
| Debt, net | | | 12,364,800 | |
| Debt, current | | | 10,757,040 | |
| Debt issuance costs, current | | | (140,926 | ) |
| Current portion of debt, net | | | 10,616,114 | |
| Debt, non-current | | | 1,873,744 | |
| Debt issuance costs, non-current | | | (125,058 | ) |
| Non-current portion of debt, net | | $ | 1,748,686 | |
The Company was not in compliance with all debt covenants as of December 31, 2023, but waivers of non-compliance were obtained for all such debt, except as disclosed.
The following is a roll-forward of the debt balances for the year ended December 31, 2023 after the effects of the reclassification adjustments for discontinued operations:
| Balance at December 31, 2022 | | $ | 19,835,728 | |
| Proceeds from new debt issued in 2023 | | | 2,885,527 | |
| Repayment of principal during 2023 | | | (5,873,881 | ) |
| Amortization of debt discounts | | | 1,266,191 | |
| Non-cash debt issued | | | 295,462 | |
| Stock issued for conversion of principal of debt | | | (6,264,000 | ) |
| Net cash advance activity | | | 154,689 | |
| Gain on extinguishment of debt in 2023 | | | (243,395 | ) |
| Conversion of interest to debt | | | 334,950 | |
| Conversion of principal to accrued expenses | | | (26,471 | ) |
| Balance at December 31, 2023 | | $ | 12,364,800 | |
The future contractual maturities of the debt are as follows:
| For the Year Ended December 31: | | | |
| 2024 | | $ | 10,616,114 | |
| 2025 | | | 336,627 | |
| 2026 | | | 460,397 | |
| 2027 | | | 460,397 | |
| 2028 | | | 385,126 | |
| Thereafter | | | 106,139 | |
| Total | | $ | 12,364,800 | |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Note 11 — Concentrations of Credit Risks
Credit Risks
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and accounts receivable. The Company maintains bank accounts with several financial institutions. Concentrations of credit risk with respect to accounts receivable are limited to the dispersion of customers across different industries and geographic regions.
Cash
The Company places its cash with high credit quality financial institutions. At December 31, 2023 and 2022, the Company had cash balances of $0 in excess of the Federal Deposit Insurance Corporation coverage of $250,000 per institution. The Company has not experienced any losses in such accounts.
Major Customers
For the year ended December 31, 2023, the Company had two significant customers representing an aggregate of 62% of revenues and 92% of the accounts receivable balance. The Company’s officers are closely monitoring the relationships with all customers.
Major Vendors
For the year ended December 31, 2023, the Company had one significant vendor representing 22% of total supplier purchases and 10% of the accounts payable balance. The Company’s officers are closely monitoring the relationships with all suppliers.
Note 12 — Income Taxes
The Company has evaluated the positive and negative evidence in assessing the realizability of its deferred tax assets. This assessment included the evaluation of scheduled reversals of deferred tax liabilities, estimates of projected future taxable income and tax planning strategies to determine which deferred tax assets are more likely than not to be realized in the future.
The Company records a liability for uncertain tax positions when it is probable that a loss has been incurred and the amount can be reasonably estimated. Interest and penalties related to income tax matters, if any, would be recognized as a component of income tax expense. At December 31, 2023 and 2022, the Company had no liabilities for uncertain tax positions. The Company continually evaluates expiring statutes of limitations, audits, proposed settlements, changes in tax law and new authoritative rulings. Currently, the tax years subsequent to 2018 are open and subject to examination by the taxing authorities.
At December 31, 2023, the Company had net operating loss carry forwards for federal income tax purposes of approximately $17.8 million, which will be available to offset future taxable income. Additionally, there is deferred tax asset associated with the impairment of intangible assets of approximately $1.2 million. Due to the uncertainty of the timing for the use of the carryforward, the Company has recognized a valuation allowance of approximately $19.0 million equaling the amount of the loss carryforward. The tax carryforwards have an indefinite life.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Deferred tax asset and valuation allowance computations are provided in the table below:
| Deferred tax asset at December 31, 2022 | | $ | 14,285,091 | |
| Valuation allowance at December 31, 2022 | | | (14,285,091 | ) |
| Net value at December 31, 2022 | | | — | |
| | | | | |
| Taxable net loss for the year ended December 31, 2023 | | | 16,832,240 | |
| Impairment of intangible asset | | | 5,843,501 | |
| Taxable benefit | | | 22,675,741 | |
| Tax rate | | | 21 | % |
| 2023 Tax benefit | | | 4,761,906 | |
| 2023 Valuation allowance | | | (4,761,906 | ) |
| Net value at December 31, 2023 | | | — | |
| | | | | |
| Deferred tax asset at December 31, 2023 | | | 19,046,997 | |
| Deferred tax asset valuation allowance at December 31, 2023 | | | (19,046,997 | ) |
| Net value at December 31, 2023 | | $ | — | |
The components of the net deferred tax assets for the years ended December 31, 2023 and 2022 are as follows:
| | | December 31,
2023 | | | December 31,
2022 | |
| Net operating loss tax asset | | $ | 17,819,862 | | | $ | 14,285,091 | |
| Impairment of intangible asset tax asset | | | 1,227,135 | | | | — | |
| Deferred tax asset | | | 19,046,997 | | | | 14,285,091 | |
| Less: Valuation allowance | | | (19,046,997 | ) | | | (14,285,091 | ) |
| Net deferred tax assets | | $ | — | | | $ | — | |
The Company has recorded a valuation allowance against the entire net deferred tax asset, as management believes it is more likely than not that the Company will not be able to benefit from the net deferred tax asset. The valuation allowance increased approximately $4,762,000 from December 31, 2022 to December 31, 2023.
The table below summarizes the differences between the U.S. statutory federal rate and the Company’s estimated effective tax rate for the years ended December 31, 2023 and 2022:
| | | December 31,
2023 | | | December 31,
2022 | |
| U.S. Statutory Rate | | | (21 | )% | | | (21 | )% |
| Increase in valuation allowance | | | 21 | % | | | 21 | % |
| Total provision for income taxes | | | — | % | | | — | % |
Tax expense was $0 and $0 for 2023 and 2022, respectively.
The Company also has net operating loss carryforwards of approximately $61,825,000 and approximately $45,000,000 included in the deferred tax asset table above for 2023 and 2022, respectively. However, due to limitations of carryover attributes and separate return limitation year rules, it is unlikely the Company will benefit from the net operating loss carryforwards and thus management has determined that a 100% valuation reserve is required.
Note 13 — Stockholders’ Equity
Series A Preferred Stock
On June 29, 2021, the Company filed a certificate of designation with the Delaware Secretary of State, and on April 10, 2023, in connection with the conversion to Nevada, the Company filed a certificate of designation with the Nevada Secretary of State to establish its series A convertible preferred stock. The Company designated a total of 1,000 shares of its preferred stock as series A convertible preferred stock. The series A convertible preferred stock has the following voting powers, designations, preferences and relative rights, qualifications, limitations or restrictions:
Dividend Rights. Prior to February 14, 2022, holders of series A convertible preferred stock were entitled to receive cumulative dividends at a rate of 7.5% of the stated value per share ($1,000, subject to adjustment) per annum, which increased to 15% per annum after November 23, 2021 and 24% per annum after December 31, 2022. Holders of series A convertible preferred stock are no longer entitled to dividends. The dividend accrual was $450,562 as of December 31, 2023.
Liquidation Rights. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, or upon a change of control, the holders of series A convertible preferred stock shall be entitled to receive out of the assets of the Company the same amount that a holder of common stock would receive if the series A convertible preferred stock were fully converted (disregarding for such purposes any conversion limitations) to common stock which amounts shall be paid pari passu with all holders of common stock.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Voting Rights. The series A convertible preferred stock have no voting rights except as set forth below. As long as any shares of series A convertible preferred stock are outstanding, the Company shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the series A convertible preferred stock, (a) alter or change adversely the powers, preferences or rights given to the series A convertible preferred stock or alter or amend the certificate of designation, (b) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon a liquidation senior to, or otherwise pari passu with, the series A convertible preferred stock, (c) amend the certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of series A convertible preferred stock, or (d) enter into any agreement with respect to any of the foregoing.
Conversion Rights. Each share of series A convertible preferred stock is convertible, at any time and from time to time from at the option of the holder thereof, into that number of shares of common stock determined by dividing the stated value of such share of series A convertible preferred stock (plus any accrued but unpaid dividends thereon) by the conversion price. The conversion price is equal $2,100.105 (subject to adjustments). Notwithstanding the foregoing, the Company shall not effect any conversion, and a holder shall not have the right to convert, any portion of the series A convertible preferred stock to the extent that, after giving effect to the conversion, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the conversion. This limitation may be waived (up to a maximum of 9.99%) by the holder and in its sole discretion, upon not less than sixty-one (61) days’ prior notice to the Company.
During the first quarter of 2022, the holders converted an aggregate of 7,000 shares of series A convertible preferred stock into 3,333 shares of common stock.
During the year ended December 31, 2023, the remaining 1,000 shares of series A convertible preferred stock was converted into 477 shares of common stock.
As of December 31, 2023 and 2022, there were 0 and 1,000 shares of series A convertible preferred stock issued and outstanding.
On December 15, 2023, the Company filed a certificate of withdrawal with the Nevada Secretary of State to withdraw the designation and return the 1,000 shares designated to undesignated preferred stock.
Series B Preferred Stock
On May 25, 2023, the Company filed a certificate of designation with the Nevada Secretary of State to establish its series B preferred stock. The Company designated a total of 5,000,000 shares of its preferred stock as series B preferred stock. The series B preferred stock has the following voting powers, designations, preferences and relative rights, qualifications, limitations or restrictions:
Dividend Rights. Holders of series B preferred stock are entitled to receive dividends in the same form as dividends paid on shares of the common stock only when and if such dividends are paid on shares of the common stock. No other dividends shall be paid on shares of series B preferred stock.
Liquidation Rights. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, the holders of series B preferred stock shall be entitled to receive out of the assets of the Company the same amount that a holder of common stock would receive if the series B preferred stock were fully converted (disregarding for such purposes any conversion limitations) to common stock which amounts shall be paid prior to all holders of common stock.
Voting Rights. The series B preferred stock shall vote together with the common stock on an as-converted basis.
Conversion Rights. Each share of series B preferred stock is convertible, at any time and from time to time from at the option of the holder thereof, into that number of shares of common stock determined by dividing the stated value of such share of series B preferred stock ($223) by the conversion price. The conversion price is $140.49 (subject to adjustments). Notwithstanding the foregoing, the Company shall not effect any conversion, and a holder shall not have the right to convert, any portion of the series B preferred stock to the extent that, after giving effect to the conversion, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the conversion. This limitation may be waived (up to a maximum of 9.99%) by the holder and in its sole discretion, upon not less than sixty-one (61) days’ prior notice to the Company.
In May 2023, the Company issued 34,001 shares of series B preferred stock as settlement of debt, accrued interest, accrued compensation, and accrued board fees for a total value of $7,581,446.
In June 2023, the Company issued 817 shares of series B preferred stock as settlement of accrued interest totaling $182,153.
A note from 2018 with a principal balance of $55,000 plus accrued interest of $24,776 was converted into 358 shares of the Company’s series B preferred stock which resulted in the note being fully paid at December 31, 2023.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
In October 2023, an aggregate of 8,579 shares of series B preferred stock were converted into an aggregate of 13,618 shares of common stock.
As of December 31, 2023 and 2022, there were 26,239 and 0 shares of series B preferred stock issued and outstanding.
Common Stock
After the effects of the retrospective treatment of the equity splits in the reclassification adjustments for discontinued operations:
On February 16, 2022, the Company entered into an underwriting agreement with Dawson James Securities, Inc., as representative of the several underwriters named on Schedule I thereto, relating to its IPO of units, each unit consisting of one share of common stock, a series A warrant to purchase one share of common stock and a series B warrant to purchase one share of common stock. Pursuant to the underwriting agreement, the Company agreed to sell 458 units to the underwriters, at a purchase price per unit of $28,665.00 (the offering price to the public of $31,500.00 per unit minus the underwriters’ discount), and also agreed to grant to the underwriters a 45-day option to purchase up to 69 additional shares of common stock, up to 69 additional series A warrants, and/or up to 69 additional series B warrants, in any combination thereof, at a purchase price to the public of $31,437.00 per share and $31.50 per warrant, less underwriting discounts and commissions, solely to cover over-allotments, if any.
On February 18, 2022, the closing of the IPO was completed. At the closing, the underwriters partially exercised the option and purchased 66 series A warrants and 66 series B warrants. Therefore, the Company sold 457 shares of common stock, 523 series A warrants and 523 series B warrants for total gross proceeds of $14,404,128. After deducting the underwriting commission and expenses, the Company received net proceeds of $12,738,288.
On February 18, 2022, the Company issued 123 shares of common stock upon the conversion of the 5% secured subordinated convertible promissory note in the principal amount of $1,900,000 along with interest of $32,500 issued to Justin Francisco and Steven Rubert in connection with the acquisition of Nexus.
On February 18, 2022, the Company issued 198 shares of common stock upon the conversion of the 6% secured subordinated convertible promissory note in the principal amount of $3,000,000 along with interest of $116,000 issued to Sasson E. Moulavi in connection with the acquisition of DSO.
On February 18, 2022, the Company issued 73 shares of common stock upon the conversion of the convertible promissory note in the principal amount of $500,000 along with interest of $74,583 issued to East West Capital LLC.
On February 18, 2022, the Company issued 13 additional shares of common stock to the stockholders of GSP and 5 additional shares of common stock to certain vendors of GSP in accordance with the terms of the contribution and exchange agreement described above. Based on the IPO share allocation of the unit, it was determined that the Company would issue the additional 13 shares.
On February 18, 2022, the Company issued an aggregate of 688 shares of common stock to various lenders pursuant to future equity agreements which required the Company to issue shares of common stock upon closing of the IPO, which resulted in an interest expense of $10,844,960.
In February through March 2022, the Company issued 375 shares of common stock upon the conversion of the convertible promissory note in the principal amount of $2,250,000 along with interest of $292,500 issued to several debt holders.
On March 10, 2022, the Company granted restricted stock awards for an aggregate of 278 shares of common stock to certain directors, officers, and consultants. A total of 215 of these shares vested in full on the date of grant. The remaining 63 shares, which were granted to independent directors, vest monthly over a one-year period which were recorded as a prepaid of $46,900 at December 31, 2022. A total of 174 of these shares were granted under the 2020 Stock Incentive Plan described below. The remaining 105 were granted under the 2022 Equity Incentive Plan described below. The shares, valued at $822,626, were based on the closing trading price per share of $2,954.70 on the date of the grant.
On April 8, 2022, the Company issued 23 shares of common stock to Bevilacqua PLLC upon conversion of its convertible promissory note in the principal amount of $73,727.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
On June 9, 2022, the Company issued 62 shares of common stock to a director upon a cashless exercise of a stock option.
On December 8, 2022, the Company entered into a securities purchase agreement with certain accredited investors pursuant to which the Company issued to the investors an aggregate of 407 shares of common stock and prefunded warrants to purchase an aggregate of 500 shares of common stock for an aggregate purchase price of $1,000,000, or $1,102.50 per underlying share, with a $90,000 placement fee resulting in $910,000 in net proceeds.
On December 14, 2022, the Company issued 32 shares of common stock to a debt holder in exchange for a debt forbearance. The shares, valued at $30,500, were based on the closing trading price per share of $1,102.50 on the date of the grant.
During the year ended December 31, 2022, a total of 457 of the series B warrants were exercised on a cashless basis and the Company issued 457 shares of common stock upon such exercise.
During the year ended December 31, 2022, the Company issued an aggregate of 3,333 shares of common stock upon the conversion of 7,000 shares of series A convertible preferred stock.
On April 17, 2023, the Company issued 79 shares of common stock as compensation for amending the license agreement associated with Sports Illustrated Nutrition.
On May 4, 2023, the Company issued 477 shares of common stock upon the conversion of 1,000 shares of series A convertible preferred stock.
On May 2, 2023, the Company entered into a securities purchase agreement with an institutional investor, pursuant to which the Company agreed to issue and sell, in a registered direct offering, 1,502 shares of common stock and a pre-funded warrant to purchase up to 2,952 shares of common stock at an exercise price of $.0063, at an offering price per share and pre-funded warrant of $201.915 and $201.908, respectively. In addition, under the securities purchase agreement, the Company agreed to issue to the investor a warrant to purchase up to 4,454 shares of common stock at an exercise price of $194.04 per share in a concurrent private placement. At closing, the investor exercised the pre-funded warrant in full. Accordingly, the Company issued to the investor 4,454 shares of common stock and a warrant to purchase up to 4,454 shares of common stock at an exercise price of $194.04 per share for total gross proceeds of $899,326 and net proceeds of $776,933.
H.C. Wainwright & Co., LLC (the “Placement Agent”) acted as the exclusive placement agent in connection with this offering and received a cash fee equal to 7.5% of the gross proceeds of the offering and a management fee equal to 1% of the gross proceeds of the offering. The Company also agreed to reimburse the Placement Agent $30,000 for fees and expenses of its legal counsel and other out-of-pocket expenses and $15,950 for certain closing costs. In addition to the cash fees, the Company issued to the Placement Agent warrants to purchase up to 334 shares of common stock at an exercise price of $252.39375 per share (or 125% of the offering price).
On May 17, 2023, the Company entered into a securities purchase agreement with an institutional investor, pursuant to which the Company agreed to issue and sell, in a registered direct offering 3,270 shares of common stock and a pre-funded warrant to purchase up to 6,014 shares of common stock at an exercise price of $0.0063, at an offering price per share and pre-funded warrant of $170.73 and $170.7237, respectively. In addition, under the securities purchase agreement, the Company agreed to issue to the investor a warrant to purchase up to 9,284 shares of common stock at an exercise price of $163.17 per share in a concurrent private placement. At closing, the investor exercised the pre-funded warrant in full. Accordingly, the Company issued to the investor 9,284 shares of common stock and a warrant to purchase up to 9,284 shares of common stock at an exercise price of $163.17 per share for total gross proceeds of $1,585,057 and net proceeds of $1,374,367.
The Placement Agent acted as the exclusive placement agent in connection with this offering and received a cash fee equal to 7.5% of the gross proceeds of the offering and a management fee equal to 1% of the gross proceeds of the offering. The Company also agreed to reimburse the Placement Agent $60,000 for fees and expenses of its legal counsel and other out-of-pocket expenses and $15,950 for certain closing costs. In addition to the cash fees, the Company issued to the Placement Agent warrants to purchase up to 697 shares of common stock at an exercise price of $213.4125 per share (or 125% of the offering price).
On May 24, 2023, the Company issued 79 shares of common stock to a note holder as compensation for amending the note.
On August 29, 2023, the Company issued 2,155 shares of common stock to a note holder as compensation for amending the note.
In October 2023, the Company issued an aggregate of 13,618 shares of common stock upon the conversion of an aggregate of 8,579 shares of series B preferred stock.
On November 30, 2023, the Company issued an aggregate of 5,000 shares of common stock as compensation for advisory services.
In December 2023, the Company issued 17,541 shares of common stock upon the conversion of $186,634 of dividends payable associated with the series A convertible preferred stock.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
During the year ended December 31, 2023, the Company issued 243,222 shares of common stock upon exercise of warrants for cash of $7,269,531.
Stock Options and Warrants
In September 2020, the Company adopted its 2020 Incentive Plan (the “2020 Plan”) under which the Company is authorized to issue awards for up to 635 shares of common stock to directors, officers, employees, and consultants who provide services to the Company. Awards that may be granted include incentive stock options, non-qualified stock options and awards of restricted stock.
At December 31, 2023 and December 2022, there were 0 and 2 shares of common stock available for issuance under the 2020 Plan, respectively. The Company did not issue any stock options under the 2020 Plan During the years ended December 31, 2023 and 2022.
In January 2022, the Company adopted its 2022 Equity Inventive Plan (the “2022 Plan”) under which the Company is authorized to issue awards for up to 635 shares of common stock to directors, officers, employees, and consultants who provide services to the Company. Awards that may be granted include incentive stock options, non-qualified stock options, stock appreciation rights, restricted awards, performance share awards and performance compensation awards. At December 31, 2023, there were 629 shares of common stock available for issuance under the 2022 Plan.
On August 12, 2022, the Company issued stock options under the 2022 Plan for an aggregate of 422 shares of common stock. The stock options have an exercise price of $1,984.50 per share, will vest quarterly over a three-year period and expire ten (10) years after the date of issuance; provided that an option granted to Alfonso J. Cervantes, Jr., the Company’s Executive Chairman, for the purchase of 95 shares of common stock has an exercise price of $2,182.95 per share and expires five (5) years after the date of issuance. The Company did not issue any other stock options under the 2022 Plan during the year ended December 31, 2022.
On September 5, 2023, the Company issued options under the 2022 Plan for an aggregate of 22,024 shares of common stock. The stock options have an exercise price of $23.31 per share, will vest upon stockholder approval of Amendment No. 2 to the 2022 Plan and expire ten (10) years after the date of issuance.
On September 6, 2023, the Company issued options under the 2022 Plan for an aggregate of 20,714 shares of common stock. The stock options have an exercise price of $20.79 per share, vested in full on the date of grant and expire ten (10) years after the date of issuance.
The Company did not issue any other stock options under the 2022 Plan during the year ended December 31, 2023.
The Company recognized $797,535 and $53,676 of compensation expense related to the vesting of options based on the service period during the year ended December 31, 2023 and 2022, respectively.
The series A warrants sold in the IPO are exercisable until the fifth anniversary of the issuance date at an exercise price equal to $22,050.00 per share and may be exercised on a cashless basis if the issuance of common stock upon exercise of the warrants is not covered by an effective registration statement. The exercise price and number of shares of common stock issuable upon exercise of the series A warrants may be adjusted in certain circumstances, including in the event of a stock dividend, extraordinary dividend on or recapitalization, reorganization, merger, or consolidation.
The series B warrants sold in the IPO are exercisable until the fifth anniversary of the issuance date at an exercise price equal to $31,500.00 per share and may be exercised on a cashless basis, whereby the holder will receive one share of common stock for each series B warrant exercised. As of December 31, 2023, 457 of the series B warrants were exercised on a cashless basis and the Company issued 457 shares of common stock upon such exercise.
On May 29, 2023, the Company entered into warrant solicitation inducement letters with several holders of warrants for the purchase of an aggregate of 55,781 shares of common stock at an exercise price of $163.17 per share. Pursuant to the inducement letters, the holders agreed to exercise the warrants at a reduced exercise price of $81.90; provided that the inducement letters provided that in the event that any warrant exercise would otherwise cause a holder to exceed the beneficial ownership limitation set forth in the warrants, the Company shall only issue such number of shares to such holder that would not cause such holder to exceed the maximum number of shares permitted thereunder, with the balance to be held in abeyance for the benefit of such holder until notice from such holder that the balance (or portion thereof) may be issued in compliance with such limitation. Upon closing of the warrant solicitation on May 31, 2023, the Company issued 17,574 shares of common stock to the warrant holders, with the remaining 38,207 shares remaining unissued but held in abeyance until the Company received notice from the holders that the remaining shares may be issued in compliance with the beneficial ownership limitation. On June 30, 2023, in order to further clarify the status of the abeyance shares, the remaining shares held in abeyance, which totaled 25,767 shares as of such date, were substituted for second amended and restated warrants to purchase 25,767 shares of common stock. Since the exercise price of the second amended and restated warrants was paid in full upon closing of the warrant solicitation, the second amended and restated warrants had no exercise price and could be exercised at any time. In consideration for the immediate exercise of the warrants for cash, the Company issued new warrants to the holders for the purchase of an aggregate of 111,549 shares of common stock at an exercise price of $136.71 per share. The Placement Agent acted as warrant inducement agent and financial advisor in connection with the transaction and received a cash fee of 7.5% of the gross proceeds, a management fee of 1% of the gross proceeds and reimbursement of certain expenses. The Company also issued to certain designees of the Placement Agent warrants for the purchase of an aggregate of 4,185 shares of common stock at an exercise price of $102.375.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
On November 30, 2023, the Company entered into warrant solicitation inducement letters with several holders of (i) warrants for the purchase of an aggregate of 4,454 shares of common stock at an exercise price of $194.04, (ii) warrants for the purchase of an aggregate of 9,284 shares of common stock at an exercise price of $163.17 per share and (iii) warrants for the purchase of an aggregate of 111,552 shares of common stock at an exercise price of $136.71 per share. Pursuant to the inducement letters, the holders agreed to exercise the warrants for cash at a reduced exercise price of $12.39 per share, or for gross proceeds of $1,552,292 in the aggregate. In consideration for the immediate exercise of the warrants for cash, we agreed to issue to the holders new warrants for the purchase of an aggregate of 250,572 shares of common stock at an exercise price of $10.64 per share. The closing of this transaction was completed on December 4, 2023. The Placement Agent acted as warrant inducement agent and financial advisor in connection with the transaction and received a cash fee equal to 7.5% of the gross proceeds, a management fee equal to 1% of the gross proceeds and reimbursement of certain expenses. In addition, the Company issued to certain designees of the Placement Agent warrants to purchase an aggregate 9,398 shares of common stock at an exercise price of $15.4875 per share.
In the years ended December 31, 2023 and 2022, the Company granted 391,399 and 67,389 warrants, respectively. In the years ended December 31, 2023 and 2022, warrant holders exercised an aggregate of 243,222 and 457 shares, respectively.
The warrant terms include a fundamental transaction clause whereby there is a change in control of the ownership of the Company outside the control of the Company – such as an unsolicited takeover of the company by an outsider – the holder of the warrant can demand either the fair value of the warrants in cash (using a Black Scholes formula) or common stock in the successor entity.
The following is a summary of options granted, exercised, forfeited and outstanding reflecting on a retroactive basis the reverse stock splits during the year ended December 31, 2023:
| | | 2023-Stock Options | | | 2023-Warrants | |
| | | Number of
Options | | | Weighted
Average
Exercise
Price | | | Number of
Warrants | | | Weighted
Average
Exercise
Price | |
| Outstanding at beginning of year | | | 818 | | | $ | 1,039.50 | | | | 71,513 | | | $ | 2,438.58 | |
| Granted | | | 42,736 | | | | 22.01 | | | | 391,399 | | | | 12.84 | |
| Exercised | | | — | | | | — | | | | (243,222 | ) | | | (10.71 | ) |
| Forfeited | | | (296 | ) | | | (1,984.50 | ) | | | (2,377 | ) | | | (81.83 | ) |
| Outstanding at December 31, | | | 43,259 | | | $ | 32.73 | | | | 217,313 | | | $ | 812.73 | |
| Exercisable at December 31, | | | 21,205 | | | $ | 32.06 | | | | 217,313 | | | $ | 812.73 | |
As of December 31, 2023, based on the weighted average exercise price of the stock options exercisable of $32.06 and the Company’s stock price at December 31, 2023 of $11.55, the intrinsic value of the options is $0.00. The weighted average remaining life of the options as of December 31, 2023 is 9.64 years.
As of December 31, 2023, based on the weighted average exercise price of the warrants exercisable of $812.73 and the Company’s stock price at December 31, 2023 of $11.55, the intrinsic value of the warrants is $0.00. The weighted average remaining life of the warrants as of December 31, 2023 is 5.40 years.
During 2023, there were 42,736 stock options granted. At December 31, 2023, total future compensation costs related to non-vested stock options, less estimated forfeitures, are approximately $127,787 and will be recognized over the next twelve months.
The following is a summary of options granted, exercised, forfeited and outstanding during the year ended December 31, 2022:
| | | 2022-Stock Options | | | 2022-Warrants | |
| | | Number of
Options | | | Weighted
Average
Exercise
Price | | | Number of
Warrants | | | Weighted
Average
Exercise
Price | |
| Outstanding at beginning of year | | | 460 | | | $ | 31.50 | | | | 4,699 | | | $ | 16,317.00 | |
| Granted | | | 422 | | | | 1,984.50 | | | | 67,389 | | | | 1,481.94 | |
| Exercised | | | (63 | ) | | | (31.50 | ) | | | (457 | ) | | | (4,693.50 | ) |
| Forfeited | | | (1 | ) | | | (31.50 | ) | | | (118 | ) | | | (69.37 | ) |
| Outstanding at December 31, | | | 818 | | | $ | 1,039.50 | | | | 71,513 | | | $ | 2,438.58 | |
| Exercisable at December 31, | | | 397 | | | | | | | | 71,513 | | | | | |
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
During 2022, there were 422 stock options granted. At December 31, 2022, total future compensation costs related to non-vested stock options, less estimated forfeitures, are approximately $3,000 and will be recognized over the next three years.
Valuation Assumptions for Stock Options
The fair value of each option was estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions:
| | | 2023 | | | 2022 | |
| Risk-free interest rate | | | 4.27 – 4.30 | % | | | 2.90 | % |
| Expected volatility | | | 53 | % | | | 80 | % |
| Expected life (years) | | | 10 | | | | 5 | |
| Dividend yield | | | 0 | % | | | 0 | % |
The expected life represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and the Company’s historical exercise patterns. The risk-free rate is based on the U.S. Treasury yield constant maturity in effect at the time of grant for periods corresponding with the expected life of the option.
Valuation Assumptions for Warrants
The fair value of each warrant was estimated on the date of issuance using the Black-Scholes option-pricing model with the following assumptions:
| | | 2023 | | | 2022 | |
| Risk-free interest rate | | | 3.41 – 4.23 | % | | | 2.90 | % |
| Expected volatility | | | 56 | % | | | 80 | % |
| Expected life (years) | | | 5 – 5.5 | | | | 5 | |
| Dividend yield | | | 0 | % | | | 0 | % |
The expected life represents the weighted average period of time that warrants issued are expected to be outstanding giving consideration to the Company’s historical exercise patterns. The risk-free rate is based on the U.S. Treasury yield constant maturity in effect at the time of issuance for periods corresponding with the expected life of the warrant.
Note 14 — Commitments and Contingencies
Legal Proceedings
From time to time, the Company may become subject to threatened and/or asserted claims arising in the ordinary course of business. Management is not aware of any matters, either individually or in the aggregate, that are reasonably likely to have a material adverse effect on the Company’s financial condition, results of operations or liquidity.
Employment Agreements
In April 2022, the Board of Directors appointed a member of the Board of Directors as the new Chief Executive Officer of the Company. In connection with this transition of position there was no modification to his employment agreement or compensation.
Note 15 — Related Party Transactions
The Company entered into debt with related parties which are reflected in Note 10.
The Company is party to a management services agreement with Trilogy Capital Group, LLC (“Trilogy”), a company controlled by the Company’s Executive Chairman. For the year ended December 31, 2023 and 2022, the Company incurred expenses related to Trilogy of $46,686 and $0, respectively, and paid Trilogy $17,858 and $0, respectively, for services rendered under a consulting agreement which are reflected in the statements of operations as consulting fees – related parties. At December 31, 2023 and 2022, the related party receivable was $332,142 and $0, respectively. Subsequent to December 31, 2023, the receivable balance as of December 31, 2023 was collected. At December 31, 2023 and 2022, the accrued expenses related party was $264,141 and $947,951, respectively.
Note 16 — Subsequent Events
Ceautamed Disposition and Related Transactions
On January 29, 2024, the Company entered into an asset purchase agreement with First Health FL LLC (the “Buyer”) and Ceautamed, along with its wholly owned subsidiaries, Wellness Watchers Global, LLC and Greens First Female LLC, pursuant to which the Company agreed to sell nearly all of the assets of Ceautamed and its subsidiaries to the Buyer for an aggregate price of $3,486,233, consisting of (i) $210,993.50 paid to creditors for outstanding debt owed and (ii) $3,275,239 in the form of the assumption of certain assumed liabilities (as defined in the asset purchase agreement), including the assumption of certain debt and the release of Ceautamed and its subsidiaries from such liabilities.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
In connection with the asset purchase agreement, the Company also entered into a limited liability company agreement (the “LLC Agreement”), pursuant to which the Buyer was organized. Pursuant to the LLC Agreement, the voting members of the Buyer are Joseph X. Xiras, Stuart Benson, and Ryan Benson, each with a 17% voting interest in the Buyer. The Company will also maintain a 49% non-voting ownership interest in the Buyer. The voting members of the Buyer have the option to purchase such 49% non-voting ownership interest for nominal consideration at their discretion upon notice to the Company pursuant to the LLC Agreement.
Note Purchase Agreement Amendment
On August 4, 2022, the Company entered into a note purchase agreement with an accredited investor, pursuant to which the Company issued to such investor an original issue discount note in the principal amount of $2,272,727. The note, as amended, was further amended by a promissory note modification agreement on January 26, 2024, which amended the principal amount to $2,751,233.45 with an interest rate of 13%. Further, the Company agreed to pay an administration fee of $6,000 per month to the investor. In the event that any such payments are not made under the note within three days, then the Company must pay a late fee equal to five percent (5%) of the late principal and interest payments. The principal and interest payments will be payable on the first day of the month beginning on April 1, 2024, and amortized on a 15-year amortization schedule. Further, on the first day of each June and October prior to the maturity date, the Company will pay an additional principal reduction payment of $50,000. The maturity date of the note was also extended to January 26, 2026.
Pursuant to the modification agreement, in the event that the Company (i) receives payment from the exercise its outstanding warrants in an amount up to $2,200,000, the Company shall pay the investor 20% of the gross proceeds generated by such exercise, (ii) successfully completes a public offering with a specified investment bank, the Company shall pay to the investor 25% of the gross proceeds of such public offering, (iii) successfully completes a private debenture offering, the Company shall pay the investor 30% of the gross proceeds of such private placement, and (iv) successfully completes any other capital fund raising, the Company shall pay to the investor 25% of such gross proceeds generated by such capital fund raising.
In connection with the disposition of Ceautamed, the investor released its security interests relating to the Ceautamed and its subsidiaries, but not the Company, and the remaining amount of the note that is due on behalf of the Company will remain outstanding.
Amendment and Assignment of Amortized Note
In connection with the closing of the disposition of Ceautamed, the Company, Ceautamed and its subsidiaries, First Group Acquisition Company, LLC (“First Group”) and the Buyer entered into an agreement to amend that certain 5% Secured Subordinated Promissory Note, dated as of July 29, 2022, by and between the Company and D&D Hayes, LLC in the initial principal amount of $1,075,000. Pursuant to the amendment, (i) the Company shall be entitled to discharge the note for a cash payment to First Group in the amount of $300,000, plus interest of 10% per annum, in lieu of the existing outstanding principal balance on the note, (ii) in the event of an equity or debt financing consummated by the Company or any of its subsidiaries prior to the payments described in (i) have been satisfied, the Company must prepay to First Group an amount equal to $100,000 (or if less received, then the total proceeds from any such financing), (iii) until such time that the Company has completely repaid the amounts due and owing in (i), the encumbrances on the Company shall not be released. In the event of a default on the obligations, the Company shall be obligated to pay the full original balance of the note, plus interest of 10% per annum.
Forgiveness of Certain Outstanding Buyer Notes
On August 4, 2022, the Company entered into secured subordinated convertible promissory notes in connection with the acquisition of Ceautamed. In connection with the closing of the disposition of Ceautamed, certain note, specifically those to RMB Industries, Inc. in the initial principal amount of $967,500 and to RTB Childrens Trust in the principal amount of $107,500, were forgiven and discharged in their entirety and cancelled, pursuant to a letter agreement, dated January 29, 2024, among the parties.
On June 19, 2024, the parties entered into an agreement which implemented amendments to the original terms of the transactions described above. In particular, the Company and First Group agreed and established that the outstanding amount of the note was $351,293 and First Group elected to convert $300,000 in principal under the note into 92,593 shares of common stock. Additionally, the parties agreed to extend the initial date upon which the voting members of the Buyer may exercise the option to any time on or after October 1, 2024.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Loan Agreement
On March 5, 2024, the Company and DSO, as borrowers, entered into a loan agreement with Abbsi, LLC (“Abbsi”) for a loan of up to $500,000 and issued a promissory note to Abbsi in the principal amount of $250,000. Pursuant to the loan agreement and note, Abbsi is providing funds directly to various vendors and service providers of DSO. During the term of the loan, Abbsi agreed to pay DSO’s suppliers directly, or reimburse the Company if DSO’s suppliers have been paid directly by DSO or the Company, for all costs associated with the production and sale of Company branded products by Abbsi, as well as payroll costs attributable to the sale of such products. All such costs shall be reimbursed directly by the Company or applied as a reduction to the principal amount due under the loan agreement and note. The note bears interest at the rate of 12% per annum and has a term of 60-months, if not prepaid earlier, and shall otherwise be due and payable as provided in the note. The loan may be prepaid at any time without penalty and is secured by a security interest in all of DSO’s assets. As of the date hereof, Abbsi has advanced $250,000 under the loan.
The loan agreement also provides that, for so long as any amounts due under the note remain outstanding and subject to a cap equal to the principal amount of the loan, Abbsi shall be entitled to receive 20% of the annual positive cash flow generated by DSO, which shall be calculated on an annualized basis. Calculation of such payment shall be made contemporaneously with the end of each fiscal quarter and shall be paid concurrently with the Company’s quarterly or annual filings with the SEC.
Sale/Leaseback Agreement
On March 6, 2024, the Company entered into a sale and leaseback agreement with Efraim Elias (the “Purchaser”) related to BSNM’s production facility (the “Facility”). Under the sale and leaseback agreement, the Company sold BSNM to the Purchaser for a purchase price of $1.00 and agreed to lease the Facility from the Purchaser. The lease shall expire on March 6, 2074 and either party may extend the lease for up to ten additional one-year periods.
In addition, the Company agreed to enter into a management services agreement, pursuant to which GSP shall (i) be obligated to make certain future payments to the Purchaser; (ii) assume and pay all existing and future liabilities of the Facility; (iii) manage the operations and affairs of the Facility; and (iv) be entitled to all revenues of the Facility and transactions in respect of all assets of the Facility, in its sole discretion. The management services agreement has not yet been signed.
The sale and leaseback agreement includes a right of first offer, pursuant to which the Company has a right to repurchase the Facility in the event that one or both parties determine that the Purchaser cannot maintain title ownership to the Facility and/or fulfill all of its obligations under the sale and leaseback agreement or the management services agreement. The terms of any such repurchase shall be in the Company’s sole discretion. The Purchaser may not transfer the Facility to any other party.
SEC Order
On September 9, 2024, the SEC issued an order against the Company which found that the Company entered into two separation agreements with former employees that each contained language violating Exchange Act Rule 21F-17(a). The order imposes a civil money penalty against the Company for the two noted violations in the total amount of $19,500, to be paid in four installments over the course of 360 days from the date of the order and according to the schedule set forth in the order. The first three installments are each in the amount of $5,000. The last installment is in the principal amount of $4,500 and must also contain an amount for interest accrued. The [SEC] Division of Enforcement (‘Division) may, at any time following the entry of this Order, petition the Commission to: (1) reopen this matter to consider whether Smart for Life provided accurate and complete financial information at the time representations in its Form 10-Q for the quarter ended September 30, 2023 were made; and (2) seek an order directing payment of the maximum civil penalty allowable under the law.
Securities Purchase Agreement
On April 3, 2024, the Company entered into a securities purchase agreement with Purely Optimal Nutrition Inc. (“Purely Optimal”) and Tan Enterprises, Inc., Availiant Holdings Corporation, Dannel Tan, Jason Kwan and Timur Kim (the “Sellers”), pursuant to which the Company agreed to acquire all of the issued and outstanding membership interests of Purely Optimal, a health supplement brand, from the Sellers for an aggregate purchase price of $11,965,966.10 (the “Purchase Price”), consisting of (i) $7,859,579.66 in cash and (ii) $4,106,386.44 payable as 18,414 newly issued shares of series D convertible preferred stock, a new series of preferred stock, subject to certain adjustments described below.
The Purchase Price is based upon a six (6) times multiple of an estimated EBITDA of $1,467,073.35 for the twelve-month period ending on November 30, 2023 (the “Reconstructed EBITDA”). The Purchase Price will be adjusted upwards or downwards based upon the difference between (i) six (6) times the Reconstructed EBITDA and the Purchase Price and (ii) the Estimated Inventory Payment (as defined in the securities purchase agreement) and the actual amount of Inventory (as defined in the securities purchase agreement) on the closing date. In addition, the cash portion of the Purchase Price will be decreased by the amount of any outstanding indebtedness of Purely Optimal for borrowed money existing as of the closing date and any unpaid transaction expenses.
The securities purchase agreement contains customary representations, warranties and covenants, including a covenant that the Sellers will not compete with the business of Purely Optimal for a period of two (2) years following closing. The securities purchase agreement also contains mutual indemnification for breaches of representations or warranties and failure to perform covenants or obligations contained in the securities purchase agreement. In the case of the indemnification provided by the Sellers with respect to breaches of certain non-fundamental representations and warranties, the Sellers will only become liable for indemnified losses if the amount exceeds ten percent (10%) of the Purchase Price, whereupon the Sellers will be liable for all losses relating back to the first dollar, provided that the liability of the Sellers for breaches of certain non-fundamental representations and warranties shall not exceed thirty five (35%) of the Purchase Price and each Seller’s aggregate liability for the breach of fundamental representations shall be limited to the Purchase Price.
The closing of the securities purchase agreement is subject to customary closing conditions, including, without limitation, the completion of accounting and legal due diligence investigations; the receipt of all authorizations, consents and approvals of all governmental authorities and third parties; the release of any liens against any of the assets of Purely Optimal; the Company obtaining the requisite acquisition financing; and delivery of all documents required for the transfer of the equity interests of Purely Optimal to the Company.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Warrant Solicitation
On May 30, 2024, the Company entered into warrant solicitation inducement letters (the “Inducement Letters”) with the holders (the “Exercising Holders”) of warrants for the purchase of an aggregate of 183,370 shares of common stock at an exercise price of $10.64 issued on December 4, 2023 (the “Existing Warrants”), pursuant to which the Exercising Holders agreed to exercise the Existing Warrants for cash at a reduced exercise price of $4.25 per share, or for gross proceeds of $779,322.50 in the aggregate. In consideration for the immediate exercise of the Existing Warrants for cash, the Company agreed to issue to the Exercising Holders new warrants for the purchase of an aggregate of 550,110 shares of common stock (the “New Warrants”).
On June 3, 2024, the closing of this transaction was completed, and the Company issued the New Warrants. The New Warrants are exercisable for a period of eighteen months at an initial exercise price of $4.25 per share and may be exercised on a cashless basis if at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available for the resale of, the shares issuable upon exercise of the New Warrants. The exercise price will be subject to customary adjustments in the event of stock splits, stock dividends, stock combinations and similar recapitalization transactions. The New Warrants also contain a beneficial ownership limitation which provides that the Company shall not effect any exercise, and a holder shall not have the right to exercise, any portion of a New Warrant to the extent that, after giving effect to the exercise, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the exercise. This limitation may be waived (up to a maximum of 9.99%) by a holder in its sole discretion upon not less than sixty-one (61) days’ prior notice to the Company.
H.C. Wainwright & Co., LLC acted as warrant inducement agent and financial advisor in connection with the transaction and received a cash fee equal to 7.5% of the gross proceeds, a management fee equal to 1% of the gross proceeds and reimbursement of certain expenses. After these fees, the Company received net proceeds of approximately $458,473. In addition, the Company issued to certain designees of H.C. Wainwright & Co., LLC warrants to purchase 13,753 shares of common stock at an exercise price of $5.3125 per share, which such warrants will have the same terms of the New Warrants (other than the exercise price).
Debt Conversions and Creation of Series C Preferred Stock
Subsequent to December 31, 2023, the Company entered into conversion agreements with certain lenders, pursuant to which such lenders converted an aggregate of $15,318,716 of debt and interest owed by the Company to such lenders in exchange for an aggregate of 6,141,229 shares of common stock and 36,506.81 shares of series C preferred stock. The conversions were completed in multiple transactions at a price per underlying share of common stock that was at least equal to the lower of (i) the official closing price as reflected on Nasdaq.com immediately preceding signing of the conversion agreement or (ii) the average official closing price as reflected on Nasdaq.com for the five trading days immediately preceding signing of the conversion agreement.
In connection with these debt conversions, the Company filed a certificate of designation with the Nevada Secretary of State on March 1, 2024 to create a new series of preferred stock designated as series C preferred stock. Pursuant to the certificate of designation, the Company designated 200,000 shares of its preferred stock as series C preferred stock. Following is a summary of the material terms of the series C preferred stock:
Dividend Rights. Holders of series C preferred stock are entitled to receive dividends in the same form as dividends paid on shares of the common stock only when and if such dividends are paid on shares of common stock. No other dividends shall be paid on shares of series C preferred stock.
Liquidation Rights. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, the holders of series C preferred stock shall be entitled to receive out of the assets of the Company the same amount that a holder of common stock would receive if the series C preferred stock were fully converted (disregarding for such purposes any conversion limitations) to common stock, which amounts shall be paid prior to all holders of common stock and pari passu with all holders of series B preferred stock.
SMART FOR LIFE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2023 AND 2022
Voting Rights. The series C preferred stock shall vote together with the common stock on an as-converted basis.
Conversion Rights. Each share of series C preferred stock is convertible, at any time and from time to time at the option of the holder thereof, into that number of shares of common stock determined by dividing the stated value of such share of series C preferred stock ($100) by the conversion price. The conversion price is $7.00 (subject to adjustments for stock dividends, stock splits, recapitalizations and certain fundamental transactions). Notwithstanding the foregoing, the Company shall not effect any conversion, and a holder shall not have the right to convert, any portion of the series C preferred stock to the extent that, after giving effect to the conversion, such holder (together with such holder’s affiliates) would beneficially own in excess of 4.99% of the number of shares of common stock outstanding immediately after giving effect to the issuance of shares issuable upon the conversion. This limitation may be waived (up to a maximum of 9.99%) by the holder and in its sole discretion, upon not less than sixty-one (61) days’ prior notice to the Company.
No Redemption. The series C preferred stock is not redeemable.
Additional Equity Transactions
On January 8, 2024, the Company issued 3,572 shares of common stock upon the exercise of warrants for proceeds of $38,000.
Between January 10, 2024 and February 9, 2024, the Company issued an aggregate of 9,377 shares of common stock in exchange for professional services rendered with an aggregate value of $91,250.
On March 31, 2024, the Company issued an aggregate of 102,172 shares of common stock as payment of accrued dividends on the Company’s series A preferred stock in the amount of $450,564.
On June 3, 2024, the Company issued 44,322 shares of common stock upon the cashless exercise of warrants.
Subsequent to December 31, 2023, an aggregate of 6,727 shares of series B preferred stock were converted into an aggregate of 10,678 shares of common stock and an aggregate of 1,527 shares of series B preferred stock were converted into debt, which was immediately converted into shares of common stock (which debt and shares of common stock are included under “Debt Conversions and Creation of Series C Preferred Stock” above).
Subsequent to December 31, 2023, an aggregate of 26,775.83 shares of series C preferred stock that were issued upon the conversion of debt as described above were converted into an aggregate of 382,519 shares of common stock. However, subsequent to such issuances, holders returned an aggregate of 105,670 shares of common stock in exchange for the return of an aggregate of 7,396.57 shares of series C preferred stock. All but 977.98 of those shares of series C preferred stock were then converted into debt and then immediately converted back into common stock (which debt and shares of common stock included under “Debt Conversions and Creation of Series C Preferred Stock” above).
Subsequent to December 31, 2023, an aggregate of 54,367 shares of common stock were cancelled by the holders thereof.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: September 20, 2024 | SMART FOR LIFE, INC. |
| | |
| | /s/ Darren C. Minton |
| | Name: | Darren C. Minton |
| | Title: | Chief Executive Officer |
| | | (Principal Executive Officer) |
| | |
| | /s/ Alan B. Bergman |
| | Name: | Alan B. Bergman |
| | Title: | Chief Financial Officer |
| | | (Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| SIGNATURE | | TITLE | | DATE |
| | | | | |
| /s/ Darren C. Minton | | Chief Executive Officer and Director (principal executive officer) | | September 20, 2024 |
| Darren C. Minton | | | | |
| | | | | |
| /s/ Alan B. Bergman | | Chief Financial Officer (principal financial and accounting officer) | | September 20, 2024 |
| Alan B. Bergman | | | | |
| | | | | |
| /s/ Alfonso J. Cervantes, Jr. | | Executive Chairman | | September 20, 2024 |
| Alfonso J. Cervantes, Jr. | | | | |
| | | | | |
| /s/ Loren Brown | | Director | | September 20, 2024 |
| Loren Brown | | | | |
| | | | | |
| /s/ Heather Granato | | Director | | September 20, 2024 |
| Heather Granato | | | | |
| | | | | |
| /s/ Arthur S. Reynolds | | Director | | September 20, 2024 |
| Arthur S. Reynolds | | | | |
66
iso4217:USD xbrli:shares

