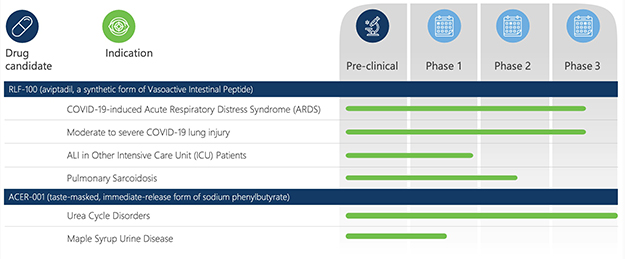
On November 5, 2021, NRx announced that the FDA had declined NeuroRx’s application for EUA of IV aviptadil for the treatment of acute respiratory failure due to critical COVID-19. In its press release, NRx stated that in the letter from the FDA denying EUA, the FDA noted that it has only reviewed safety data on 131 patients treated with aviptadil. NRx further announced in its press release that it will attempt to coordinate a review by the FDA of 150 or more additional patients treated with aviptadil through other trials. Additionally, NRx stated in its press release that the study’s Data Safety and Monitoring Board reviewing the trial found no safety issues. Further, on November 24, 2021, NRx reported that it was denied breakthrough therapy designation for the product. On June 10, 2022, NRx reported that its second application for Breakthrough Therapy Designation was also denied.
On January 6, 2022, NRx reported that NeuroRx had submitted an additional application to the FDA seeking EUA for the use of aviptadil to treat patients with critical COVID-19 who are at immediate risk for death from respiratory failure despite treatment with approved therapy, including Remdesivir. Additionally, on January 26, 2022, NRx issued a press release reporting NeuroRx’s receipt of a first safety report from a southwestern hospital where physicians have administered aviptadil to patients with COVID-19 respiratory failure. According to NRx’s press release, the patients were treated under the United States’ Right to Try Act, which gives access to investigational medicines for patients who have been diagnosed with life-threatening diseases or conditions, who have tried all approved treatment options, and who are unable to participate in a clinical trial to access certain unapproved treatments. The press release stated that of the first 19 patients treated by December 31, 2021, three had died and sixteen (84%) were reported to be alive as of January 22, 2022. Further, NRx’s press release reported that 14 of these 16 patients had been discharged to a rehabilitation facility or to home. By way of comparison, according to “Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the ISARIC WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study”, published in the journal ERJ Open Research in January 2021, the overall 28-day fatality rate for COVID-19 patients admitted to the ICU was approximately 30.7%. The press release also indicated that this use of aviptadil had occurred during the then-current COVID-19 surge caused by the omicron variant, although patients were not necessarily tested for the specific COVID variant that caused their ICU admission. Finally, NRx stated that no serious adverse events were reported. There can be no assurance that NeuroRx’s reapplication seeking EUA for aviptadil for the treatment of acute lung disease caused by COVID-19 will be successful.
On November 29, 2021, NRx issued a press release announcing the results of a subsequent statistical analysis it commissioned from Dr. David Schoenfeld, a statistician with expertise in life-threatening diseases of the lung. According to the press release, Dr. Schoenfeld analyzed the subgroup of patients in the Phase 2b/3 trial that remained in respiratory failure despite treatment with remdesivir and stated that the analysis identified a statistically significant (p=0.03) 2.5-fold increased odds of a patient having survived and being free of respiratory failure at 60 days (the primary endpoint) and a statistically significant (p=0.006) four-fold higher odds of 60-day survival among patients treated with ZYESAMI compared to those treated with placebo.
On March 3, 2022, two U.S. Senators and two members of the House of Representatives sent a letter to Dr. Robert Califf, Commissioner of the FDA, and Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Disease regarding the results of the right-to-try administration of ZYESAMI. The letter discusses the results and seeks comment on the FDA review of the ZYESAMI EUA application and the FDA’s stance that the EUA will not be reviewed until the completion of clinical trials later this year. There can be no assurance that the letter will have any effect on the review and approval of the current EUA application.
While we have received a phase 2b/3 Study Report summary from NeuroRx and are reviewing the contents of the report to decide on the best path forward for the development of RLF-100 IV in Europe and other territories, NeuroRx has refused to share the full clinical trial data with us, which has prevented us from moving forward to seek approval for the product in its territories. They have also reported publicly their intent to file their own applications in Europe and the U.K. We believe that all of these actions, along with many others, constitute breaches of the Collaboration Agreement.
To that end, on October 7, 2021, we filed a lawsuit against NeuroRx and its then - CEO, Dr. Jonathan Javitt, for multiple breaches of the Collaboration Agreement between Relief and NeuroRx relating to the development and commercialization of RLF-100. The complaint was filed in the Supreme Court of the State of New York in Manhattan. The complaint alleges that the defendants are in breach of numerous provisions of the Collaboration Agreement. The complaint, among other remedies, seeks damages, an order compelling defendants to comply with multiple provisions of the Collaboration Agreement, and a declaration directing NeuroRx to deliver the entire data set from the Phase 2b/3 clinical trial of intravenously-administering aviptadil to Relief. On January 10, 2022, NeuroRx filed a complaint against us alleging that we are in breach of the Collaboration Agreement and have thus repudiated and cancelled the Collaboration Agreement. Additionally, NeuroRx claims that we, through our press releases and statements to investors, have defamed NeuroRx and Dr. Javitt. We believe that such claims are without merit. There can be no assurance as to the result of this litigation.
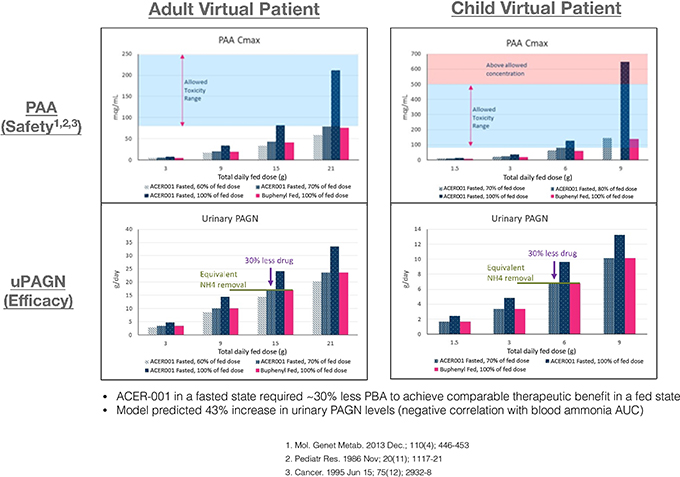
In March 2021, we signed a Collaboration and License Agreement with Acer Therapeutics, Inc. (“Acer”) for the worldwide development and commercialization of ACER-001 for the treatment of Urea Cycle Disorders (“UCDs”) and Maple Syrup Urine Disease (“MSUD”). ACER-001 is a proprietary powder formulation of sodium phenylbutyrate (NaPB) designed to be both taste-masked and immediate release.
In August 2021, Acer submitted an NDA for ACER-001 to the FDA for use as a treatment of UCD, which submission was accepted for filing in November 2021 with a PDUFA decision date of June 5, 2022. On June 7, 2022, Acer announced that it has not yet received a decision from the FDA on its NDA. Further, in accordance with our collaboration agreement with ACER, we are planning to submit an application for marketing authorization for this product to European and U.K. regulatory authorities, assuming ACER-001 is approved by the FDA.
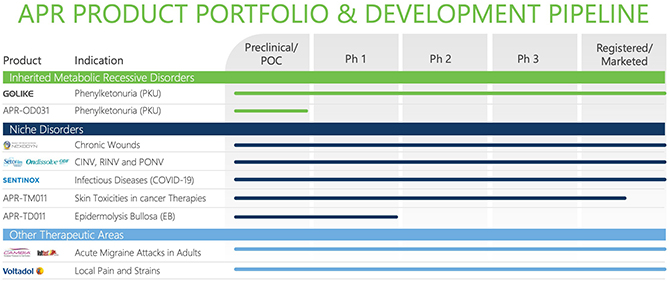
On June 29, 2021, we announced that we had signed and closed a definitive agreement to acquire all outstanding shares of APR Applied Pharma Research SA (“APR”), a privately held Swiss pharmaceutical company with over 25 years’ experience in identifying, developing and commercializing known molecules engineered with drug delivery systems in niche and rare diseases on a global basis.
APR is applying advanced patented pharma technologies, as well as proprietary delivery systems and novel dosage forms, to optimize the therapeutic potential of pharmaceuticals and improve patient outcomes. Its products are commercialized in about 50 countries worldwide. APR’s pipeline and portfolio include products for the treatment of rare or debilitating diseases. APR is, for example, commercializing Golike to improve metabolic control in patients suffering from phenylketonuria, a rare genetic metabolic disorder. A direct sales and marketing team is in place in selected European countries to support Golike, as well as established distribution partnerships for other countries in Europe and beyond. APR also has a strong pipeline of programs in development, including two orphan drug designations. Additionally, Sentinox, an intranasal spray to help block the transmission of the COVID-19 virus, recently received clearance as a Class III medical device in the EU.
99