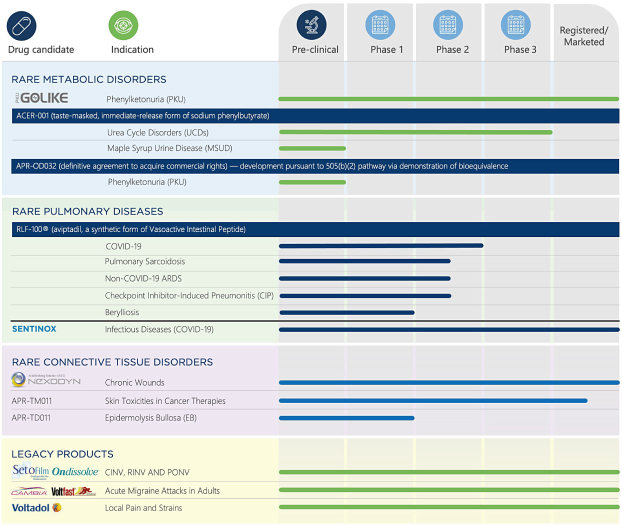
| Modified Release Orally Administered Amino Acid Formulations | | Granted: United States (Nos. 10,500,180 and 11,419,837), Armenia, Austria, Australia, Azerbaijan, Belgium, Bulgaria, Belarus, Switzerland, China, Colombia, Czechia, Germany, Denmark, Eurasian Patent Convention, European Patent Convention, Spain, Finland, France, United Kingdom, Greece, Croatia, Hungary, Indonesia, Ireland, Israel, Italy, Jordan, Kyrgyzstan, Kazakhstan, Lebanon, Lithuania, Malta, Mexico, Malaysia, Norway, Netherlands, Poland, Portugal, Romania, Russian Federation, Sweden, Slovenia, Slovakia, Tajikistan, Turkmenistan, Turkey, Taiwan Pending: United States (Application No. 17/660,999), Argentina, Australia, Brazil, Canada, Chile, China, Egypt, European Patent Convention, Gulf Cooperation Council, Hong Kong*, Israel, Iraq, Macao, Malaysia, Philippines, Pakistan, Saudi Arabia, Uruguay, Venezuela, Vietnam, South Africa | | September 25, 2036 (Jordan), September 28, 2036 (Taiwan), September 27, 2036 (all other jurisdictions). Applications, if granted, will expire no earlier than September 27, 2036. |