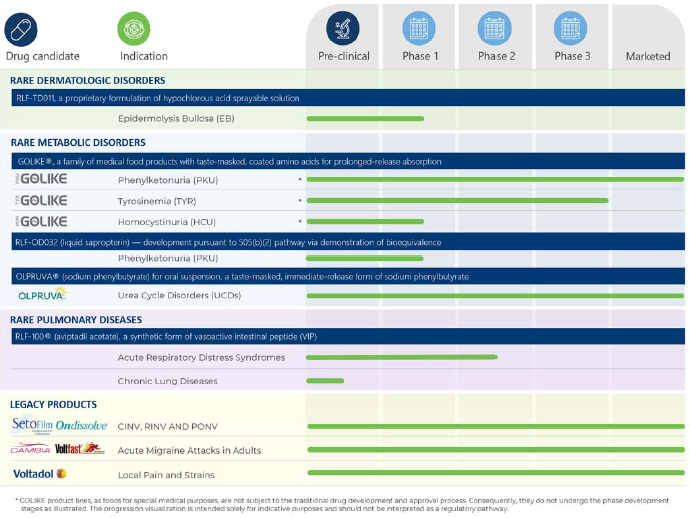
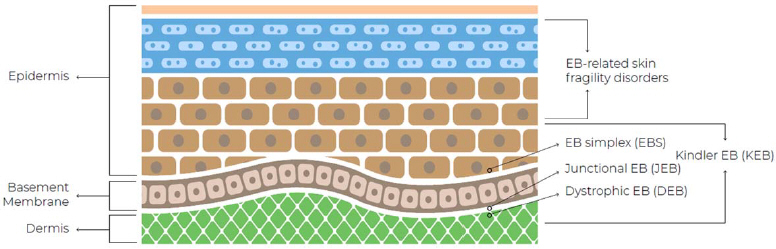
Considerable genetic heterogeneity and complex genotype-phenotype correlations are observed across the EB subtype and are attributed to several factors. These include the type of the mutation (homozygosity versus heterozygosity), the number of genes involved (monogenic, digenic inheritance), the location of mutations within each gene, and the range of resulting alterations in protein expression. Beyond the primary structural-functional defects, secondary epigenetic factors (e.g. the differentially regulated expression of a myriad of other genes involved in the maintenance and function of this microenvironment, as well as induction of inflammatory cascades) and environmental factors further contribute to the highly variable phenotype of EB (Laimer et al. 2015, Küttner et al. 2013).
In most cases, symptoms of EB are apparent from birth or shortly thereafter. A physician may suspect EB from the appearance of the affected skin. To confirm the diagnosis, a limited number of laboratory tests are available, which may include a skin biopsy for immunofluorescent mapping or a genetic testing. The signs and symptoms of this disorder vary greatly among the different EB subtypes and individuals affected. In milder cases, blistering mainly affects the hands and feet, whereas severe forms of EB are characterized by generalized skin fragility and devastating blistering from minor trauma, severely impairing the quality of life of affected patients. Some EB types may also affect the eyes, tongue, and esophagus, leading to mutilating scarring and disabling musculoskeletal deformities. Complications in EB may include infections, fusion of fingers and joint changes, nutritional problems, dental and oral issues, skin cancer, and premature death.
VYJUVEK®, was approved by the FDA on May 19, 2023, for the treatment of DEB, and the company Krystal Biotech, Inc. (NASDAQ: KRYS) subsequently initiated the U.S. commercial launch. VYJUVEK is the first medicine approved by the FDA for the treatment of DEB. VYJUVEK® is a re-dosable, off-the-shelf gene therapy designed to deliver two copies of the COL7A1 gene when applied topically, directly onto an open wound. Unlike the previous standard of care, VYJUVEK treats DEB at the molecular level by providing patient’s skin cells the template to produce normal COL7 protein, thereby addressing the fundamental disease-causing mechanism.
FILSUVEZ® was approved by the FDA on December 19, 2023. It is a topical gel indicated for the treatment of partial thickness wounds in patients six months and older with JEB and DEB. FILSUVEZ contains a dry extract from two species of birch bark consisting of naturally occurring substances known as triterpenes, including betulin, betulinic acid, erythrodiol, lupeol and oleanolic acid. The topical gel is applied on the wound and covered by a wound dressing.
The current standard of care for EB patients includes wound management to prevent infection, pain management to reduce discomfort, and nutritional support to promote healing. This involves careful wound cleaning and disinfection to minimize the risk of infection. Gentle cleansing of the affected areas with mild, non-irritating solutions helps remove bacteria and other pathogens from the wound surface. Antibiotics may be used to prevent and treat infections, while analgesics are prescribed for pain relief.
Management of bioburden in EB patients involves antiseptics and often requires the use of antibiotics. However, topical antibiotics should be used sparingly in EB due to the risk of promoting antibiotic-resistant bacteria strains and potential wound sensitization. The emergence of antibiotic-resistant strains of bacteria, such as methicillin-resistant Staphylococcus aureus and increasingly ciprofloxacin-resistant Pseudomonas, is a significant challenge, potentially compromising the efficacy of current treatments. These resistant strains are frequently isolated from EB wounds (Singer et al. 2018). While both infection and inflammation can impair wound healing, no specific product has been developed for EB wounds that can simultaneously control infection and bioburden while reducing inflammation.
Wound care management for EB patients and their care givers is a complex and time-consuming process. Nurses and families often find their lives overwhelmed by the continuous routine of wound management and medication administration. Care involves piercing, draining, and dressing blisters, with bathing and dressing changes alone requiring more than three hours. Pain medications and antibiotics must be administered regularly. Additionally, a significant amount is dedicated to frequent visits to doctors, clinics and support groups (Denyer et al. 2007).
The goal of developing RLF-TD011 is to efficiently control bioburden while reducing the need for antibiotics and alleviating inflammation and pain associated with EB. Additionally, the method of administration may reduce the complexity and time required to treat EB thereby consuming less healthcare and care giver resources.
RLF-TD011 for the Potential Treatment of Epidermolysis Bullosa
We are developing RLF-TD011 as a differentiated acid oxidizing solution of hypochlorous acid (HCIO) that combines strong antimicrobial action with anti-inflammatory properties, thereby allowing for infection control, reduction of wound colonization and improved wound healing. We believe RLF TD011, if approved, may be a fast, easy to use, and effective treatment for EB wound care management. Importantly, RLF-TD011 could also enhance the efficacy and usability of newly developed EB treatments given its unique properties.
Developed with our proprietary, patent-protected TEHCLO Nanotechnology, RLF-TD011 employs an exclusive combination of four physio-chemical properties—high-purity HCIO, hypotonic, low pH and high oxidation-reduction potential, which we believe can support a faster physiological healing of wounds by creating a favorable wound microenvironment. HCIO is well known as a broad-spectrum, fast acting antimicrobial agent, which reinforced by low pH and high ORP contributes to the prevention and treatment of skin infections.
RLF-TD011 is a self-administered, sprayable solution enabling targeted application while avoiding skin contact and cross-contamination. Wound care remains the cornerstone of treatment for patients with EB, potentially facilitating a rapid and natural wound healing, while minimizing or preventing infections (and thereby reducing the reliance on antibiotics), and avoiding or limiting the chronicization of wounds.
33