Exhibit 99.1

19/09/2024 1 Review of Ongoing Phase 2b Treatment-Resistant Depression Clinical Trial (GH001-TRD-201) Wiesław J. Cubała Medical University of Gdańsk

Author Disclosure 19/09/2024 2 Conflict of interest statement regarding my presentation in the ‘Industry session financially supported by GH Research during the 37th ECNP congress.’ Review of Ongoing Phase 2b TRD Clinical Trial (GH001-TRD-201) on 22 September 2024. I have an interest in relation to one or more organisations that could be perceived as a possible conflict of interest in the context of the subject of this presentation. The relationships are summarised belowa: Interests Name of Organisation Grants Acadia, Angelini, Beckley Psytech, GH Research, HMNC Brain Health, IntraCellular Therapies, Janssen, MSD, Novartis, Otsuka, Recognify Life Sciences Honoraria Angelini, Janssen, Novartis Shares - Paid positions Professor of Psychiatry (full-time), Medical University of Gdańsk, Poland Advisory boards Douglas Pharmaceuticals, GH Research, Janssen, MSD, Novartis Other involvement - a Relationships reported within the last three years.

19/09/2024 3 A synthetic formulation of the serotonergic psychedelic mebufotenina,b administered via pulmonary inhalation Rapid onset (within seconds) and short duration (<30mins) of psychoactive effects Single-dose administration OR an individualized dosing regimen (IDR) with up to three escalating doses administered on a single day; escalation guided by peak experiencec No additional structured psychological support visits before or after dosing in clinical trial protocol GH Research is also developing an intravenous formulation of mebufotenin: GH002 Abbreviations: PES = Peak experience scale. a Mebufotenin is not currently authorized as a treatment for any therapeutic indications. b Mebufotenin is more commonly known as 5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine). c Peak experience is defined as achieving a score of ≥75 on the proprietary PES. 1. Reckweg JT, et al. Eur Psychiatry. 2022;65(supple 1):S716. 2. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414. Overview of GH001 Clinical Trial Aspects1,2
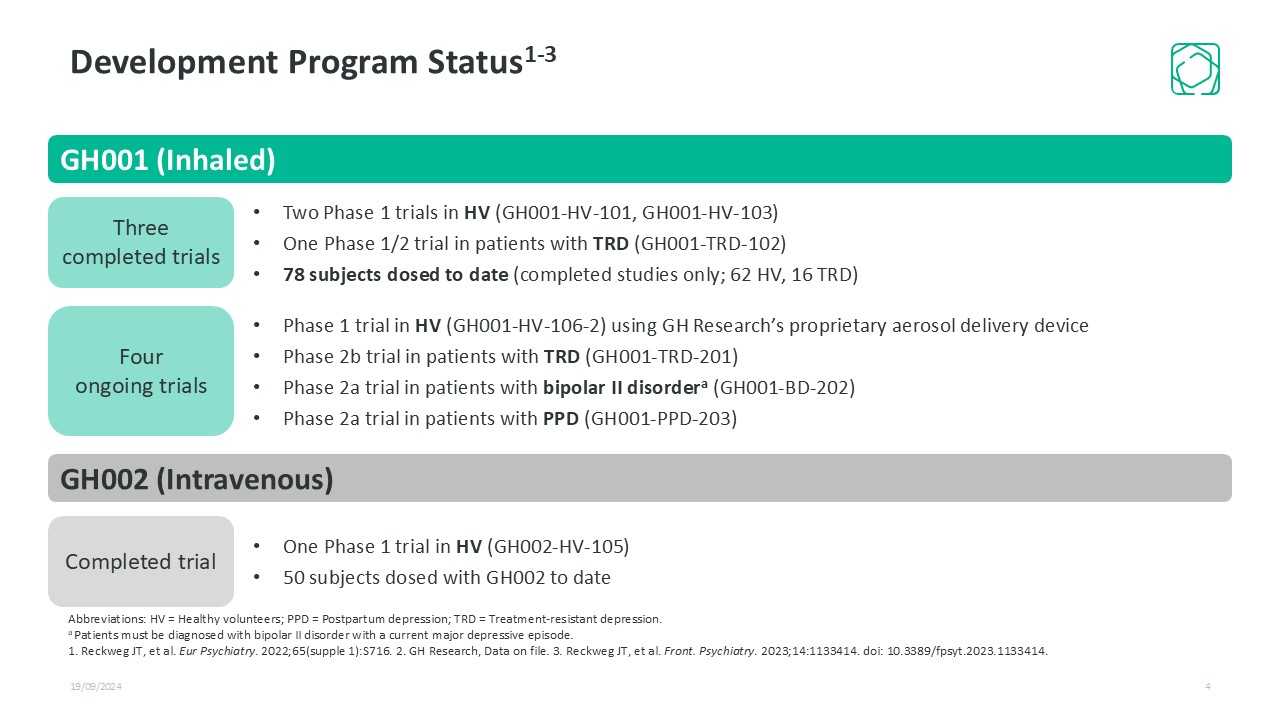
19/09/2024 4 Two Phase 1 trials in HV (GH001-HV-101, GH001-HV-103) One Phase 1/2 trial in patients with TRD (GH001-TRD-102) 78 subjects dosed to date (completed studies only; 62 HV, 16 TRD) Three completed trials Four ongoing trials Phase 1 trial in HV (GH001-HV-106-2) using GH Research’s proprietary aerosol delivery device Phase 2b trial in patients with TRD (GH001-TRD-201) Phase 2a trial in patients with bipolar II disordera (GH001-BD-202) Phase 2a trial in patients with PPD (GH001-PPD-203) GH002 (Intravenous) Completed trial One Phase 1 trial in HV (GH002-HV-105) 50 subjects dosed with GH002 to date Abbreviations: HV = Healthy volunteers; PPD = Postpartum depression; TRD = Treatment-resistant depression. a Patients must be diagnosed with bipolar II disorder with a current major depressive episode. 1. Reckweg JT, et al. Eur Psychiatry. 2022;65(supple 1):S716. 2. GH Research, Data on file. 3. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414. Development Program Status1-3 GH001 (Inhaled)
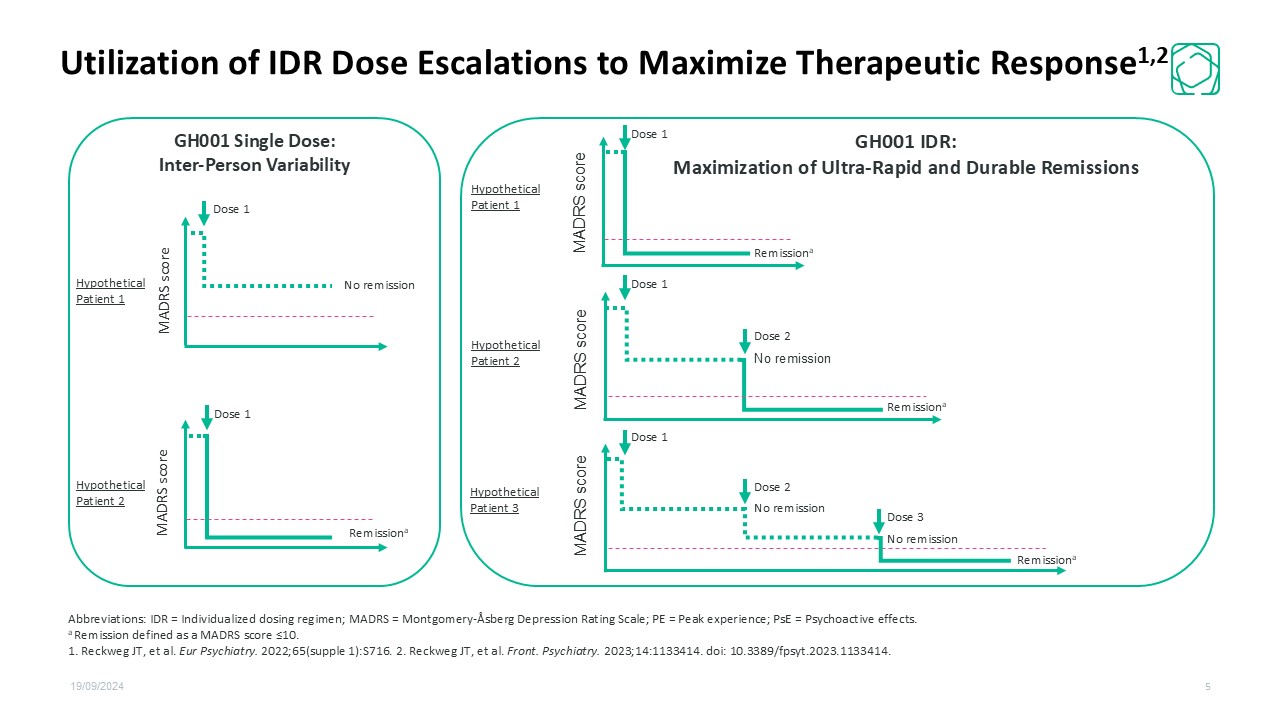
GH001 Single Dose: Inter-Person Variability GH001 IDR:Maximization of Ultra-Rapid and Durable Remissions MADRS score MADRS score MADRS score MADRS score Dose 1 Dose 1 Dose 2 Dose 3 Dose 2 Dose 1 No remission Remissiona Remissiona Remissiona Remissiona Hypothetical Patient 1 Hypothetical Patient 2 Hypothetical Patient 3 Hypothetical Patient 1 Hypothetical Patient 2 No remission No remission No remission Dose 1 MADRS score Dose 1 19/09/2024 5 Abbreviations: IDR = Individualized dosing regimen; MADRS = Montgomery-Åsberg Depression Rating Scale; PE = Peak experience; PsE = Psychoactive effects. a Remission defined as a MADRS score ≤10. 1. Reckweg JT, et al. Eur Psychiatry. 2022;65(supple 1):S716. 2. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414. Utilization of IDR Dose Escalations to Maximize Therapeutic Response1,2
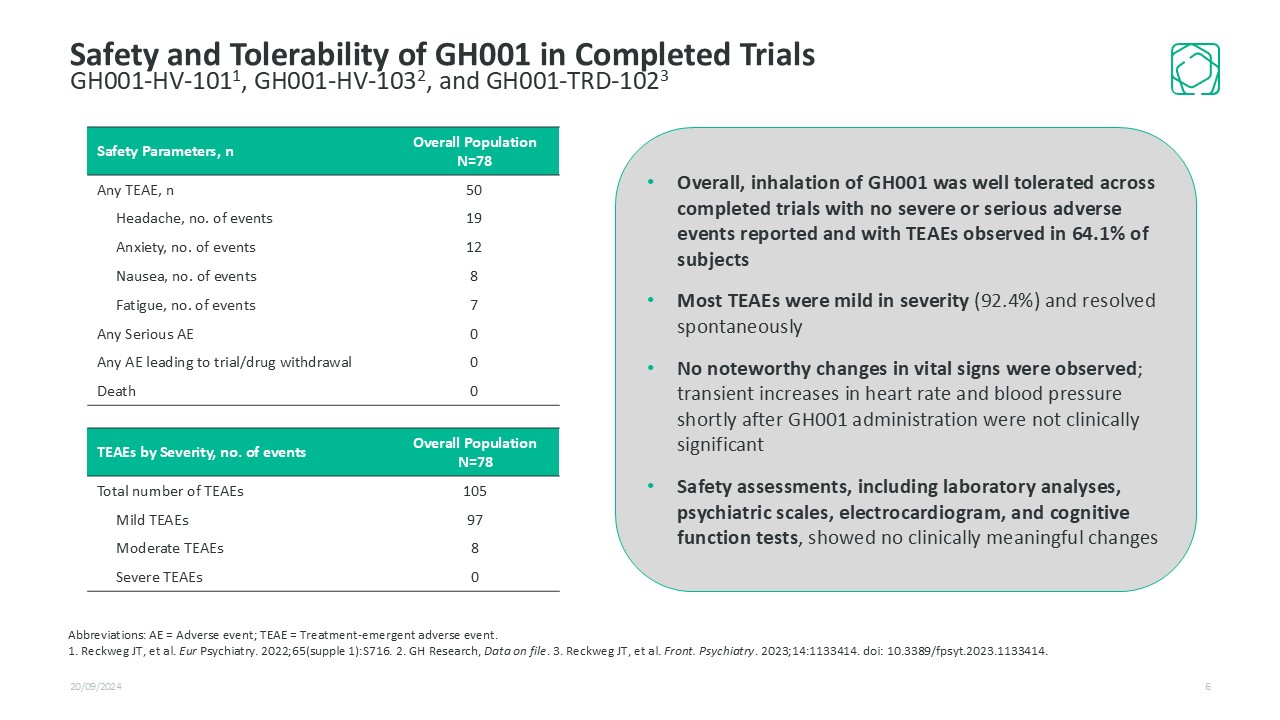
20/09/2024 6 Safety and Tolerability of GH001 in Completed TrialsGH001-HV-1011, GH001-HV-1032, and GH001-TRD-1023 Safety Parameters, n Overall Population N=78 Any TEAE, n 50 Headache, no. of events 19 Anxiety, no. of events 12 Nausea, no. of events 8 Fatigue, no. of events 7 Any Serious AE 0 Any AE leading to trial/drug withdrawal 0 Death 0 TEAEs by Severity, no. of events Overall Population N=78 Total number of TEAEs 105 Mild TEAEs 97 Moderate TEAEs 8 Severe TEAEs 0 Abbreviations: AE = Adverse event; TEAE = Treatment-emergent adverse event. 1. Reckweg JT, et al. Eur Psychiatry. 2022;65(supple 1):S716. 2. GH Research, Data on file. 3. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414. Overall, inhalation of GH001 was well tolerated across completed trials with no severe or serious adverse events reported and with TEAEs observed in 64.1% of subjects Most TEAEs were mild in severity (92.4%) and resolved spontaneously No noteworthy changes in vital signs were observed; transient increases in heart rate and blood pressure shortly after GH001 administration were not clinically significant Safety assessments, including laboratory analyses, psychiatric scales, electrocardiogram, and cognitive function tests, showed no clinically meaningful changes

19/09/2024 7 Single Dose (Day 7) Individualized Dosing Regimen (Day 7) 2/4 (50%) patients in the 12 mg group and 1/4 (25%) in the 18 mg group had a MADRS remission at Day 7 2/8 patients had a PEc and both had a MADRS remission at Day 7 7/8 (87.5%) patients had a MADRS remission at Day 7 7/8 patients had a PEc and 6 of those had a MADRS remission at Day 7 a b a b Abbreviations: MADRS = Montgomery–Åsberg Depression Rating Scale; PE = Peak experience; PES = Peak experience scale. a Remission is defined as a patient achieving a MADRS score of ≤10 after dosing. b Response is defined as a patient achieving ≥50% reduction from baseline in MADRS total score after dosing. c PE is defined as achieving a score of ≥75 on the proprietary PES. 1. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414. Efficacy of GH001 GH001-TRD-1021

Hour 2 Day 1 Day 7 GH001 p=0.0018 p<0.0001 p<0.0001 Baselinea 19/09/2024 8 Efficacy of GH001 (MADRS Change from Baseline) GH001-TRD-102: Individualized Dosing Regimen1 Abbreviations: MADRS = Montgomery–Åsberg Depression Rating Scale. a Baseline mean MADRS = 32. 1. Reckweg JT, et al. Front. Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414.

19/09/2024 9 Abbreviations: D = Day; H = Hour; IDR = Individualized dosing regimen; M = Month; MADRS = Montgomery–Åsberg Depression Rating Scale; OLE = Open-label extension; PRN = Pro re nata (as needed); TRD = Treatment-resistant depression. aThe double-blind phase was a fixed duration of 7 days (± 1 day) after an IDR with visits on D0, D1 and D7. After the double-blind phase there was a variable duration until a potential GH001 IDR in the OLE. bDuring the OLE, additional clinic visits can be scheduled if required for medical reasons. cThe GH001 IDR consists of up to 3 increasing doses (6, 12, 18 mg) and the placebo IDR consists of up to three placebo doses. As in previously completed trials, the GH001-TRD-201 trial will be conducted under the supervision of a healthcare provider, but without any planned psychotherapeutic interventions before, during, or after dosing. 1. NCT05800860. (2024). A Trial of GH001 in Patients With Treatment-Resistant Depression. ClinicalTrials.gov. Accessed August 23, 2024. https://clinicaltrials.gov/ct2/show/NCT05800860. GH001-TRD-201 Trial DesignPhase 2b trial in patients with TRD1 Baseline (H2) Double-Blind Phasea n=80 Randomization 1:1 GH001 IDRc Placebo IDRc Up to 5 GH001 IDRs may be administered during the OLE PRN, based on specific re-treatment criteria Baseline (H2) Scheduled Visit D14 M1 M2 M3 M4 M5 M6 MADRS assessment D0 D1 D7 D1 D0 Primary Endpoint ΔMADRS D7 Open-Label Extension Phaseb PRN

Thank you for your attention! 19/09/2024 10