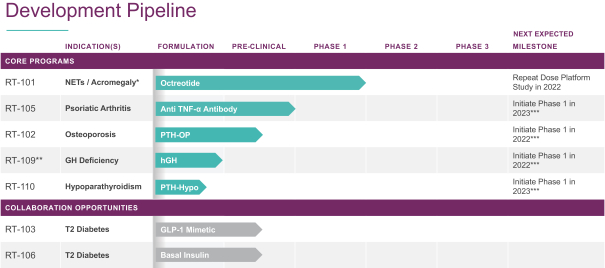
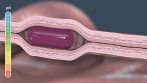
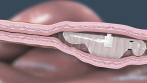
Device aspects of the RaniPill capsule and methods of using the RaniPill capsule are claimed in U.S. Patents 8,721,620, 8,759,284, and 8,562,589, and in PCT/US2010/062070 and PCT/US2010/062073, and in their respective progeny. Devices containing specific biologics and methods of delivering them are claimed in U.S. Patents 8,764,733, 8,809,269, 8,809,271, 8,846,040, 8,969,293, 8,980,822, 9,259,386, 9,283,179, 9,284,367, 9,402,806, 9,402,807, 9,415,004, 9,629,799, 9,861,683, and in PCT/US2012/044441 and PCT/US2014/024385, and in their respective progeny.
Microtablet Patent Family
Our microtablet patent family includes claims covering the microtablets delivered by the RaniPill capsule. This patent family has a priority date in 2014, includes several dozen patents and patent applications and is expected to have patent terms extending into at least 2035 if all fees are paid. As of June 1, 2021, this patent family included 7 patents issued in the United States and 1 patent issued in Australia, with applications pending in the United States, Australia, Canada, China, Europe, India, and Japan.
Microtablets having various characteristics and containing various biologics are claimed in U.S. Patents 10,098,931, 10,058,595, 10,039,810, and 10,220,076, and in PCT/US2016/030480, PCT/US2016/031548, and PCT/US2016/050832, and in their respective progeny.
Additional Patent Families
We own numerous additional patents and patent applications outside of the foundational and microtablet patent families, with claims to additional biologics, pharmacologic properties of various biologics and various next generation devices, with applications pending in the United States, Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Mexico, and South Korea. Patents in these families are expected to expire between the late 2030s and early 2040s if all fees are paid.
Trademarks
We have several trademark registrations and applications in 10 jurisdictions.
Trade Secrets and Other Proprietary Information
We rely in part on keeping our trade secrets and other proprietary information confidential. We protect proprietary information by executing confidentiality agreements and intellectual property assignment agreements prior to commencement of our relationship with our employees, consultants, scientific advisors, sponsored researchers, contractors and other collaborators. Confidentiality agreements limit use and disclosure of our confidential information during and after the relationship. Intellectual property assignment agreements require that all inventions resulting from work performed for us or relating to our business and conceived during the period of the relationship are our exclusive property. We take other appropriate precautions, such as physical and technological security measures, to guard against misappropriation of our proprietary information by third parties.
For information regarding the risks related to our intellectual property, see the section titled “Risk Factors— Risks Related to Our Intellectual Property.”
Government Regulation
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of biologics such as those we are developing.
169