Exhibit 99.1

Copyright © 2022 Allarity Therapeutics. All rights reserved. N a sdaq: A L LR Personalized Cancer Care. Realized. Steve R. Carchedi, CEO H1 2022

Legal Statement 1 /1 4 /2 0 2 2 Allarity Therapeutics 2 This presentation is provided for informational purposes only and is subject to change. The information contained herein does not purport to be all - inclusive. The data contained herein is derived from various internal and external sources, and may rely upon assumptions, stated or otherwise, and forward - looking statements discussed below. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any forward - looking statements, modeling or any other information contained herein. Allarity Therapeutics, Inc. (collectively “Allarity Therapeutics,” “Allarity,” or the “Company”) assume no obligation to update the information in this presentation. This material is not for the benefit of, and does not convey any rights or remedies for the benefit of, any holder of securities or any other person. This material is not intended to provide the basis for evaluation of any transaction and does not purport to contain all information that may be required and should not be considered a recommendation or opinion of any kind with respect to any transaction. No Offer or Solicitation. This material shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Forward - Looking Statements. This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward - looking statements provide Allarity’s current expectations or forecasts of future events. The words “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean that a statement is not forward - looking These forward - looking statements include, but are not limited to, statements relating to the submitted NDA for dovitinib and MMA for the drug - specific DRP ® companion diagnostic for dovitinib, and ongoing clinical trials for stenoparib and IXEMPRA ® . Any forward - looking statements in this press release are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward - looking statements. These risks and uncertainties include, but are not limited to, the risk that results of a clinical study do not necessarily predict final results and that one or more of the clinical outcomes may materially change following more comprehensive reviews of the data, and as more patient data become available, the risk that results of a clinical study are subject to interpretation and additional analyses may be needed and/or may contradict such results, the receipt of regulatory approval for dovitinib, the drug - specific DRP ® companion diagnostic for dovitinib, or any of our other therapeutic candidates or, if approved, the successful commercialization of such products, the risk of cessation or delay of any of the ongoing or planned clinical trials and/or our development of our product candidates, the risk that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our therapeutic candidates, and the risk that the current COVID - 19 pandemic will impact the Company’s current and future clinical trials and the timing of the Company’s preclinical studies and other operations. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward - looking statements, see the section entitled “Risk Factors” in our Form S - 1 registration statement on file with the Securities and Exchange Commission, available at the Securities and Exchange Commission’s website at www.sec.gov , and as well as discussions of potential risks, uncertainties and other important factors in the Company’s subsequent filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the presentation , and the Company undertakes no duty to update this information unless required by law. Any financial projections in this presentation are forward - looking statements that are based on assumptions that are inherently subject to significant uncertainties and contingencies, many of which are beyond Allarity’s control. While all projections are necessarily speculative, Allarity believes that the preparation of prospective financial information involves increasingly higher levels of uncertainty the further out the projection extends from the date of preparation. The assumptions and estimates underlying the projected results are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those contained in the projections. The inclusion of projections in this communication should not be regarded as an indication that Allarity or their representatives, considered or consider the projections to be a reliable prediction of future events.
Company Snapshot: New Therapeutics for Unmet Cancer Needs We are building an oncology - focused pharmaceutical company using our world - class, highly validated companion diagnostics platform to achieve true, personalized cancer care. Robust pipeline of 5 clinical - stage cancer therapeutics that address significant oncology markets U.S. NDA submitted for lead candidate, Dovitinib, on December 21, 2022 Patented Drug Response Predictor (DRP ® ) platform creates drug - specific, companion diagnostics to select and treat likely responder patients Developed, validated and perfected over past 15 years U.S. Nasdaq listed (December 21, 2021) Anchored by a $20M institutional investment (U.S.) De - listed from Stockholm, SE stock market 1 /1 4 /2 0 2 2 Allarity Therapeutics 3
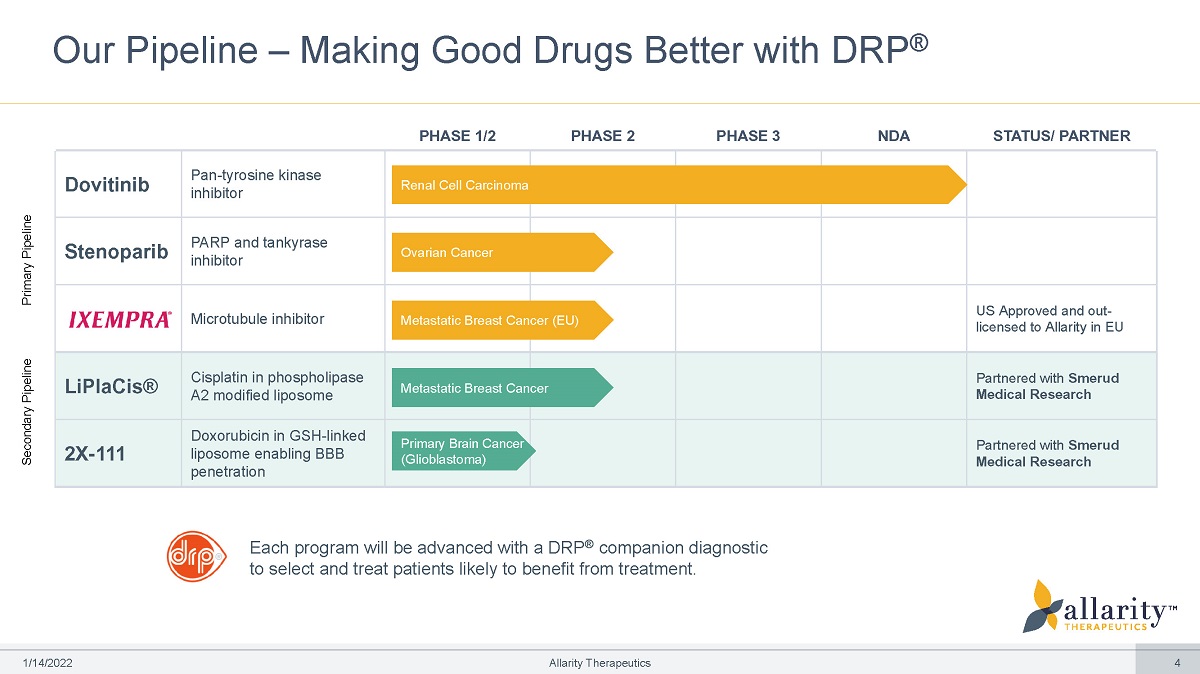
Our Pipeline – Making Good Drugs Better with DRP ® PHASE 1/2 PHASE 2 PHASE 3 NDA STATUS/ PARTNER Dovitinib Pan - tyrosine kinase inhibitor Renal Cell Carcinoma Stenoparib PARP and tankyrase inhibitor Ovarian Cancer Microtubule inhibitor Metastatic Breast Cancer (EU) US Approved and out - licensed to Allarity in EU LiPlaCis® Cisplatin in phospholipase A2 modified liposome Metastatic Breast Cancer Partnered with Smerud Medical Research 2X - 111 Doxorubicin in GSH - linked liposome enabling BBB penetration Primary Brain Cancer (Glioblastoma) Partnered with Smerud Medical Research Each program will be advanced with a DRP ® companion diagnostic to select and treat patients likely to benefit from treatment. Primary Pipeline 1 /1 4 /2 0 2 2 Allarity Therapeutics 4 Secondary Pipeline
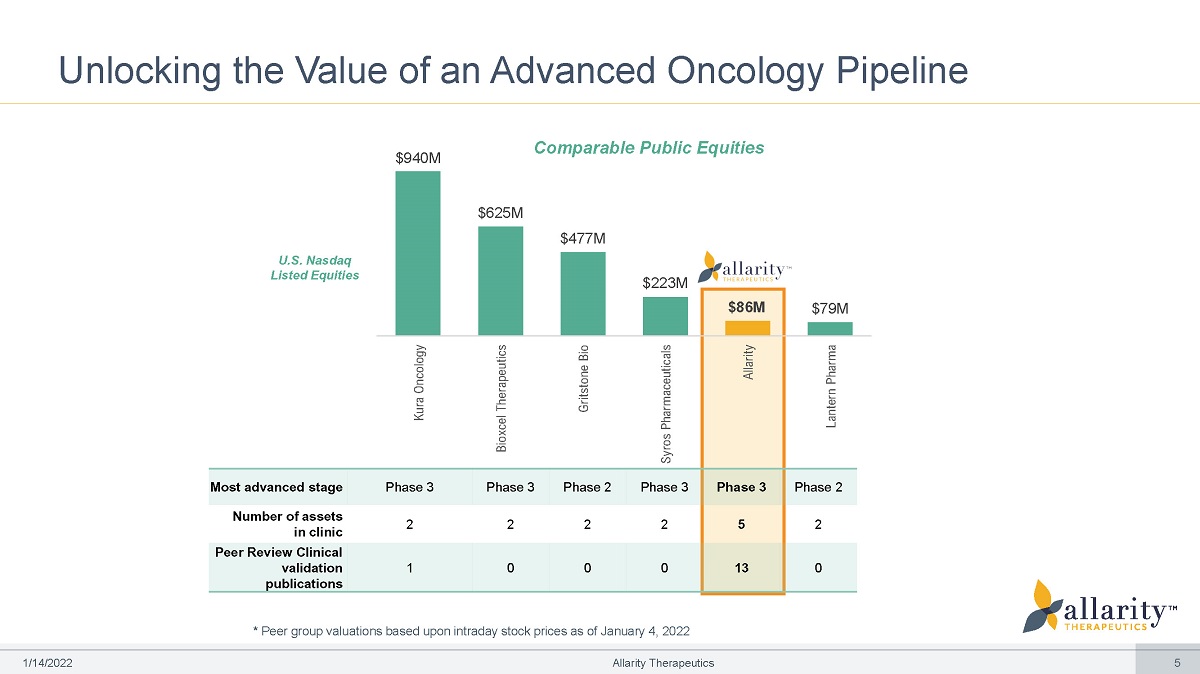
5 Most advanced stage Phase 3 Phase 3 Phase 2 Phase 3 Phase 3 Phase 2 Number of assets in clini c 2 2 2 2 5 2 Peer Review Clinical v a lidation publ ic a tio ns 1 0 0 0 13 0 $940M $625M $477M $223M $86M $79M Kura Oncology Bioxcel Therapeutics Gritstone Bio Syros Pharmaceuticals Allarity Lantern Pharma Unlocking the Value of an Advanced Oncology Pipeline Allarity Therapeutics * Peer group valuations based upon intraday stock prices as of January 4, 2022 Comparable Public Equities U.S. Nasdaq Listed Equ i ties 1 /1 4 /2 0 2 2

Advancing a Phase 2/3 Oncology Rx Pipeline Through Superior Companion Diagnostics
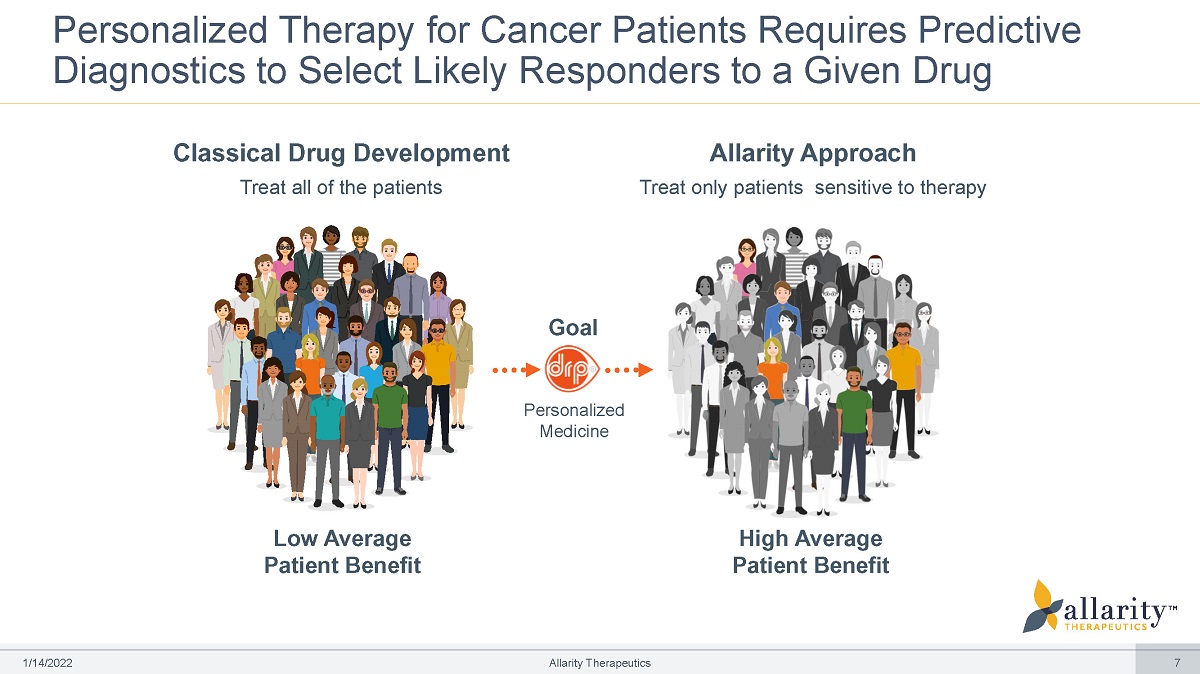
Personalized Therapy for Cancer Patients Requires Predictive Diagnostics to Select Likely Responders to a Given Drug Goal Personalized Medicine Low Average Patient Benefit High Average Patient Benefit Classical Drug Development Treat all of the patients 1 /1 4 /2 0 2 2 Allarity Therapeutics 7 Allarity Approach Treat only patients sensitive to therapy
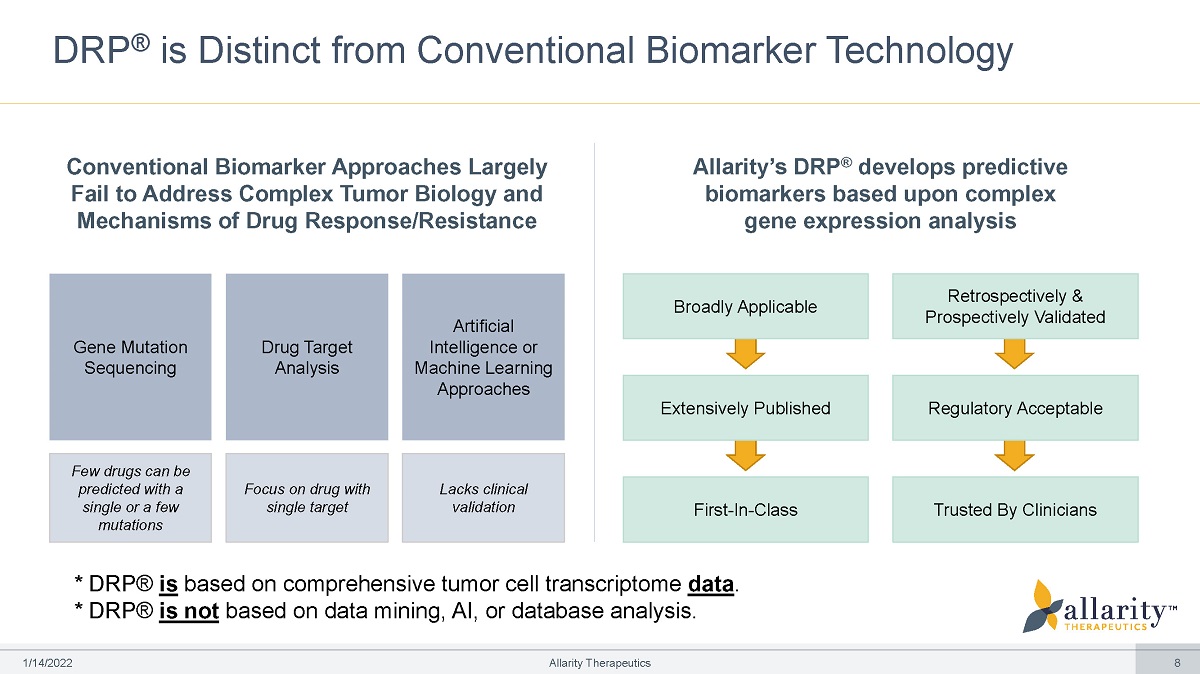
DRP ® is Distinct from Conventional Biomarker Technology Conventional Biomarker Approaches Largely Fail to Address Complex Tumor Biology and Mechanisms of Drug Response/Resistance Gene Mutation Sequencing Few drugs can be predicted with a single or a few mutations Drug T arget Analysis Focus on drug with single target Artificial Intelligence or Machine Learning Approaches Lacks clinical validation Allarity’s DRP ® develops predictive biomarkers based upon complex gene expression analysis Broadly Applicable Extensively Published First - In - Class Retrospectively & Prospectively Validated Reg u lato r y Accept a ble Trusted By Clinicians * DRP® is based on comprehensive tumor cell transcriptome data . * DRP® is not based on data mining, AI, or database analysis. 1 /1 4 /2 0 2 2 Allarity Therapeutics 8
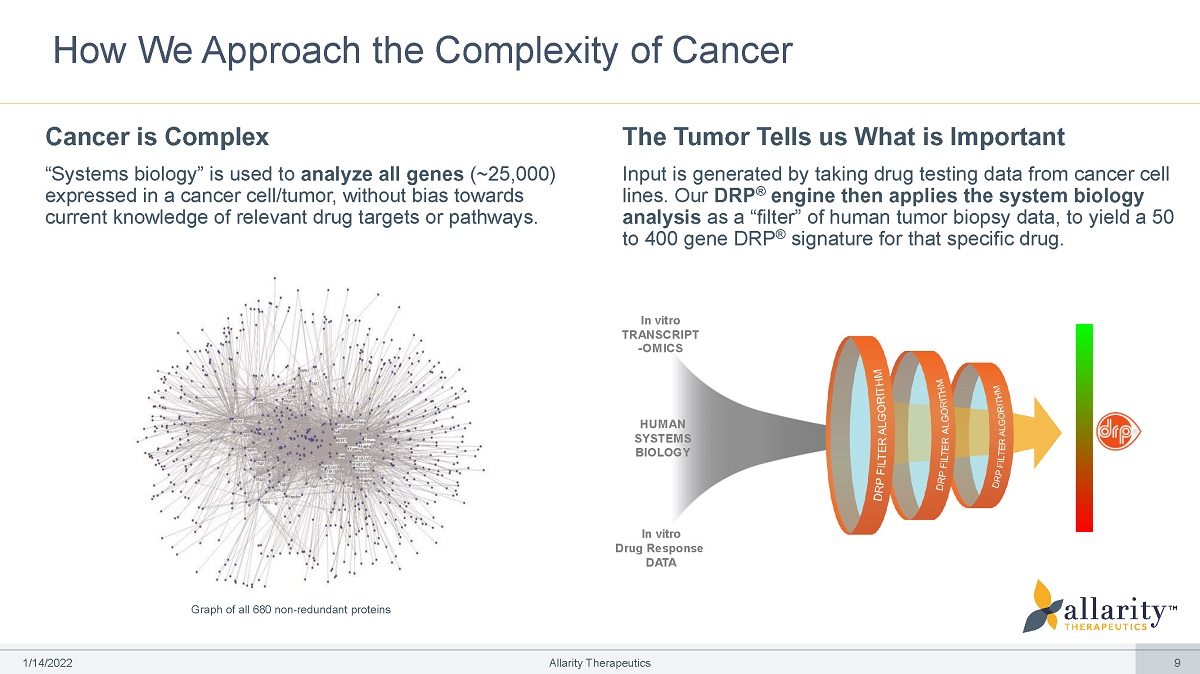
In vitro T R A N S C RI PT - OMICS HU M AN S Y S T E M S BIOLOGY In vitro Drug Response DATA How We Approach the Complexity of Cancer Cancer is Complex “Systems biology” is used to analyze all genes (~25,000) expressed in a cancer cell/tumor, without bias towards current knowledge of relevant drug targets or pathways. The Tumor Tells us What is Important Input is generated by taking drug testing data from cancer cell lines. Our DRP ® engine then applies the system biology analysis as a “filter” of human tumor biopsy data, to yield a 50 to 400 gene DRP ® signature for that specific drug. Graph of all 680 non - redundant proteins 1 /1 4 /2 0 2 2 Allarity Therapeutics 9
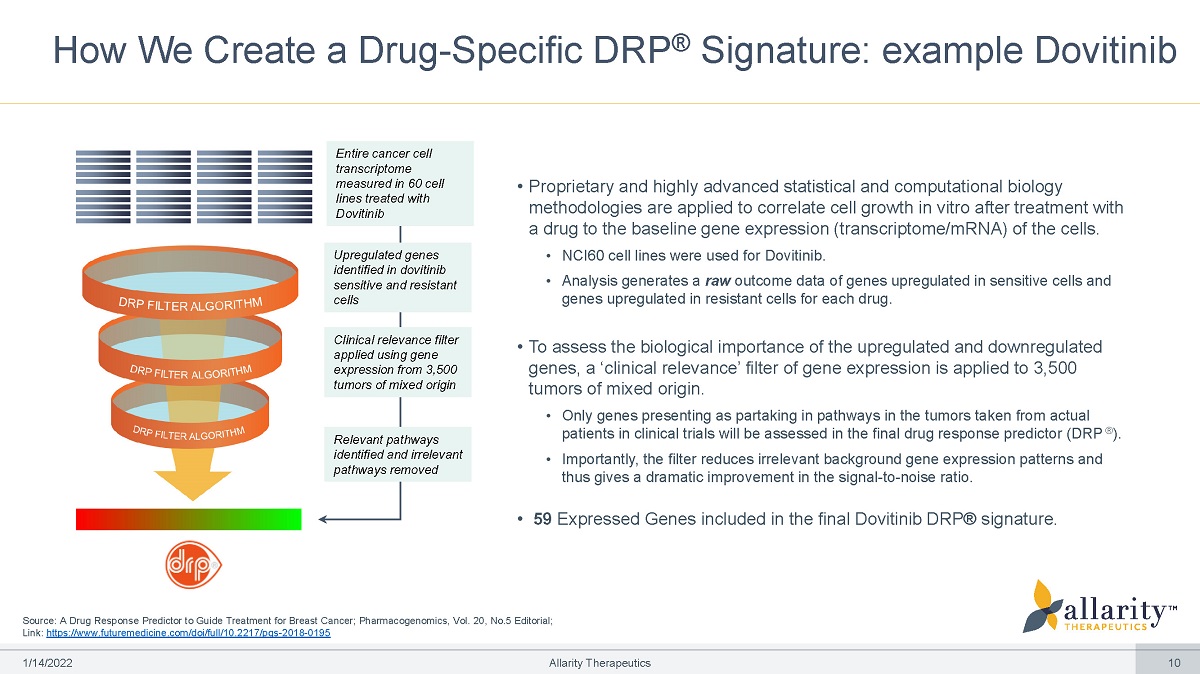
How We Create a Drug - Specific DRP ® Signature: example Dovitinib Source: A Drug Response Predictor to Guide Treatment for Breast Cancer; Pharmacogenomics, Vol. 20, No.5 Editorial; Link: https://www.futuremedicine.com/doi/full/10.2217/pgs - 2018 - 0195 • Proprietary and highly advanced statistical and computational biology methodologies are applied to correlate cell growth in vitro after treatment with a drug to the baseline gene expression (transcriptome/mRNA) of the cells. • NCI60 cell lines were used for Dovitinib. • Analysis generates a raw outcome data of genes upregulated in sensitive cells and genes upregulated in resistant cells for each drug. • To assess the biological importance of the upregulated and downregulated genes, a ‘clinical relevance’ filter of gene expression is applied to 3,500 tumors of mixed origin. • Only genes presenting as partaking in pathways in the tumors taken from actual patients in clinical trials will be assessed in the final drug response predictor (DRP ® ). • Importantly, the filter reduces irrelevant background gene expression patterns and thus gives a dramatic improvement in the signal - to - noise ratio. • 59 Expressed Genes included in the final Dovitinib DRP® signature. Entire cancer cell transcriptome measured in 60 cell lines treated with Dovitinib Upregulated genes identified in dovitinib sensitive and resistant cells Clinical relevance filter applied using gene expression from 3,500 tumors of mixed origin Relevant pathways identified and irrelevant pathways removed 1 /1 4 /2 0 2 2 Allarity Therapeutics 10
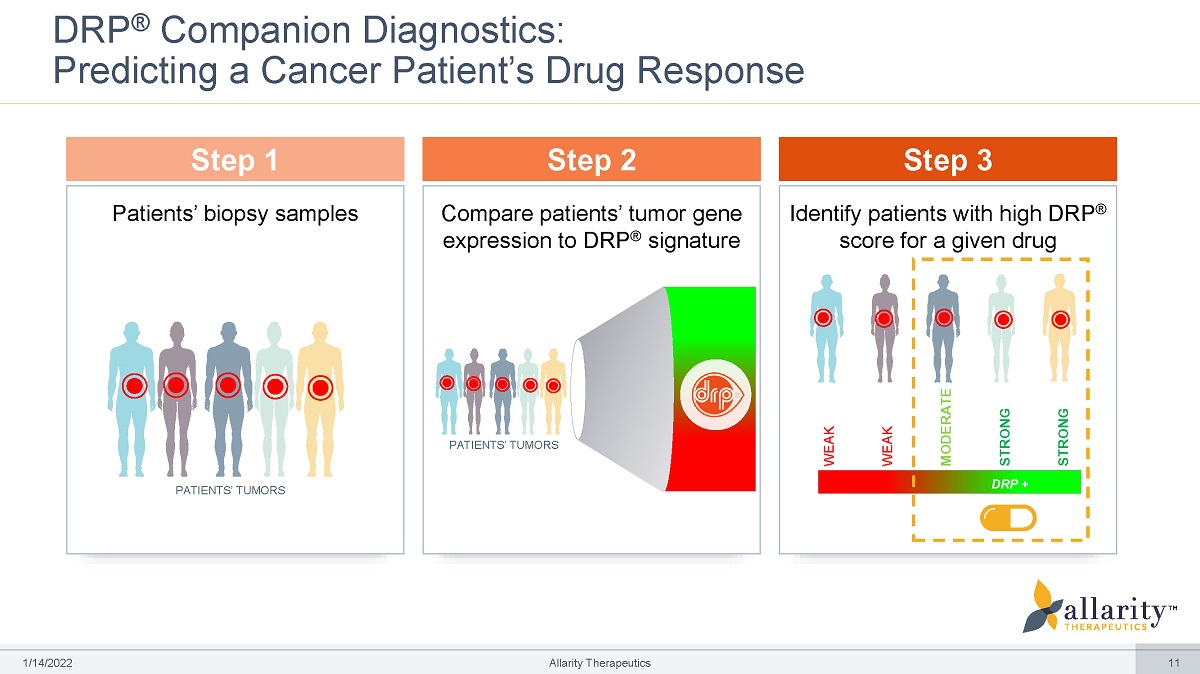
Compare patients’ tumor gene expression to DRP ® signature PATIENTS’ TUMORS DRP ® Companion Diagnostics: Predicting a Cancer Patient’s Drug Response STRONG WEAK Patients’ biopsy samples PATIENTS’ TUMORS Step 1 Step 2 Step 3 WEAK M O DERATE STRONG Identify patients with high DRP ® score for a given drug DRP + 1 /1 4 /2 0 2 2 Allarity Therapeutics 11

DRP ® Sample Collection Kits 1 /1 4 /2 0 2 2 Allarity Therapeutics 12
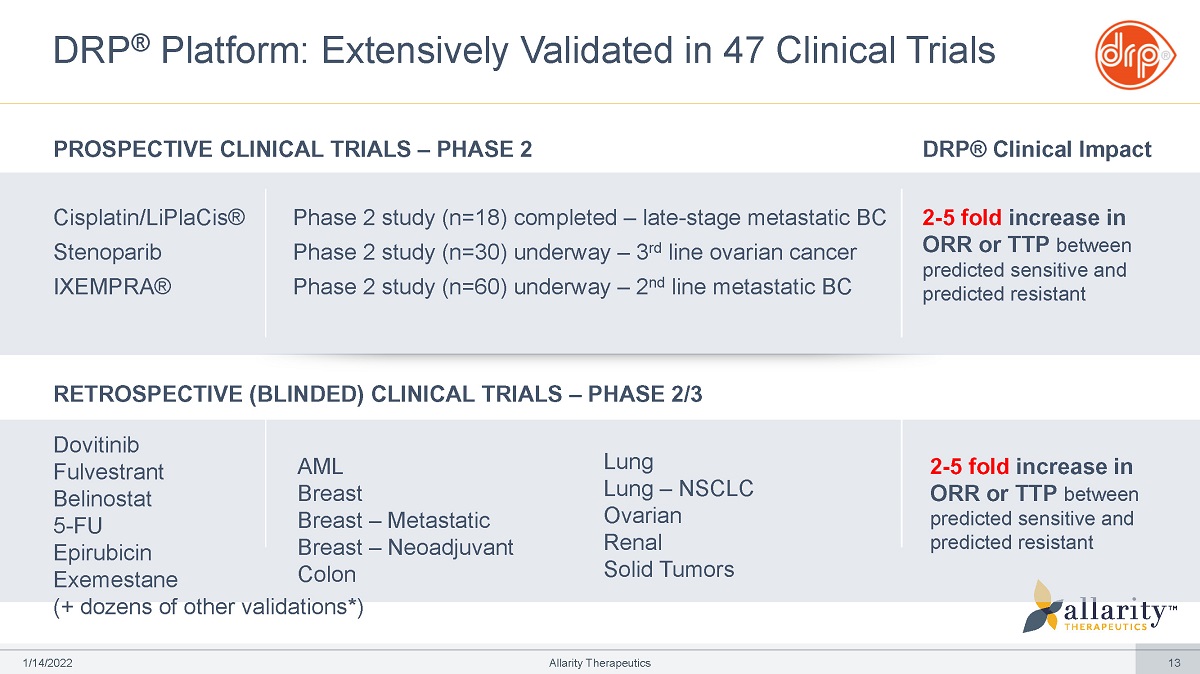
DRP ® Platform: Extensively Validated in 47 Clinical Trials Cisplatin/LiPlaCis® Stenoparib IXEMPRA® (+ dozens of other validations*) PROSPECTIVE CLINICAL TRIALS – PHASE 2 DRP® Clinical Impact 2 - 5 fold increase in ORR or TTP between predicted sensitive and predicted resistant RETROSPECTIVE (BLINDED) CLINICAL TRIALS – PHASE 2/3 1 /1 4 /2 0 2 2 Allarity Therapeutics 13 Dovitinib AML Breast Breast – Metastatic Breast – Neoadjuvant Lung Lung – NSCLC Ovarian Renal 2 - 5 fold increase in ORR or TTP between predicted sensitive and predicted resistant Fulvestrant Belinostat 5 - FU Epirubicin Exemestane Colon Solid Tumors Phase 2 study (n=18) completed – late - stage metastatic BC Phase 2 study (n=30) underway – 3 rd line ovarian cancer Phase 2 study (n=60) underway – 2 nd line metastatic BC
U.S. FDA Will Accept Retrospective, Blinded cDx Validation 1 /1 4 /2 0 2 2 Allarity Therapeutics 14 ”While prospective samples are preferred, well - characterized samples from banks can be used in your clinical validation study, provided that there is no collection or selection bias, and patient history and appropriate outcome information are available. We recommend you consult with FDA prior to performing pivotal studies using banked samples.” (FDA Guidance, May 2007) • Allarity consulted with FDA in 2020 on the use of banked samples from phase III trial of Dovitinib used to retrospectively validate the Dovitinib - DRP® companion diagnostic. • Pre - NDA meeting held in 2021. • PMA for DOVITINIB - DRP® is currently under evaluation by FDA medical device division. • IDEs have previously been granted by FDA medical device division for: • LiPlaCis® - DRP® companion diagnostic • Stenoparib - DRP® companion diagnostic • Examples of companion diagnostics approved by FDA and on oncology drug labels based on banked samples (retrospective validations): VECTIBIX® (KRAS cDx) and TARCEVA® (EGFR cDx)

Primary Pipeline Dovitinib Stenoparib IXEMPR A ®
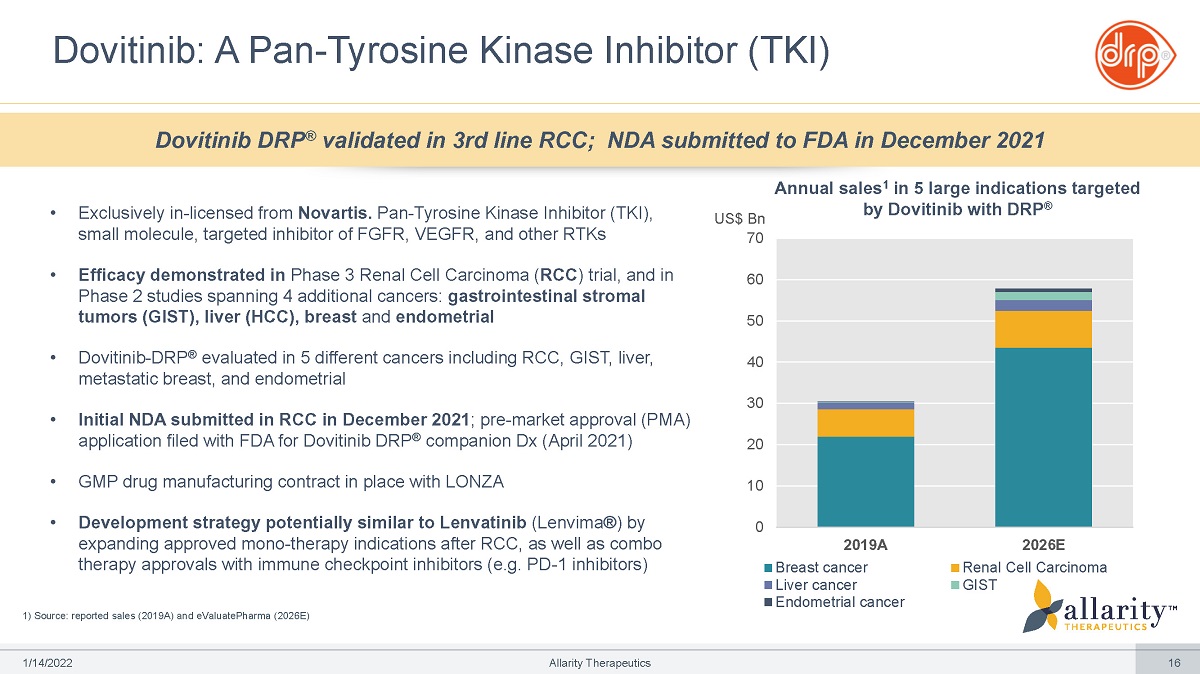
• Exclusively in - licensed from Novartis. Pan - Tyrosine Kinase Inhibitor (TKI), small molecule, targeted inhibitor of FGFR, VEGFR, and other RTKs • Efficacy demonstrated in Phase 3 Renal Cell Carcinoma ( RCC ) trial, and in Phase 2 studies spanning 4 additional cancers: gastrointestinal stromal tumors (GIST), liver (HCC), breast and endometrial • Dovitinib - DRP ® evaluated in 5 different cancers including RCC, GIST, liver, metastatic breast, and endometrial • Initial NDA submitted in RCC in December 2021 ; pre - market approval (PMA) application filed with FDA for Dovitinib DRP ® companion Dx (April 2021) • GMP drug manufacturing contract in place with LONZA • Development strategy potentially similar to Lenvatinib (Lenvima®) by expanding approved mono - therapy indications after RCC, as well as combo therapy approvals with immune checkpoint inhibitors (e.g. PD - 1 inhibitors) 1) Source: reported sales (2019A) and eValuatePharma (2026E) Dovitinib: A Pan - Tyrosine Kinase Inhibitor (TKI) 0 10 20 30 40 50 60 U S $ Bn 70 2019A Breast cancer Liver cancer Endometrial cancer 2026E Renal Cell Carcinoma GIST Annual sales 1 in 5 large indications targeted by Dovitinib with DRP ® Dovitinib DRP ® validated in 3rd line RCC; NDA submitted to FDA in December 2021 1 /1 4 /2 0 2 2 Allarity Therapeutics 16
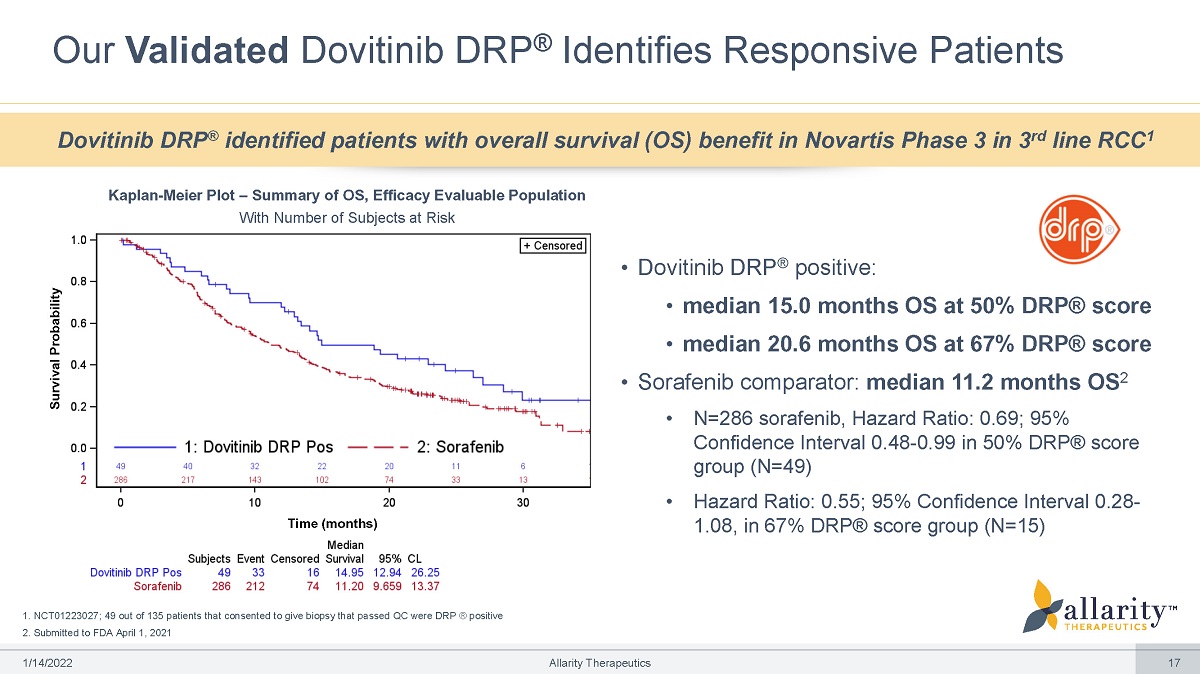
1. NCT01223027; 49 out of 135 patients that consented to give biopsy that passed QC were DRP ® positive 2. Submitted to FDA April 1, 2021 Our Validated Dovitinib DRP ® Identifies Responsive Patients • N=286 sorafenib, Hazard Ratio: 0.69; 95% Confidence Interval 0.48 - 0.99 in 50% DRP® score group (N=49) • Hazard Ratio: 0.55; 95% Confidence Interval 0.28 - 1.08, in 67% DRP® score group (N=15) Dovitinib DRP ® identified patients with overall survival (OS) benefit in Novartis Phase 3 in 3 rd line RCC 1 Kaplan - Meier Plot – Summary of OS, Efficacy Evaluable Population With Number of Subjects at Risk • Dovitinib DRP ® positive: • median 15.0 months OS at 50% DRP® score • median 20.6 months OS at 67% DRP® score • Sorafenib comparator: median 11.2 months OS 2 Tim e (months) Survival Probability 1 /1 4 /2 0 2 2 Allarity Therapeutics 17
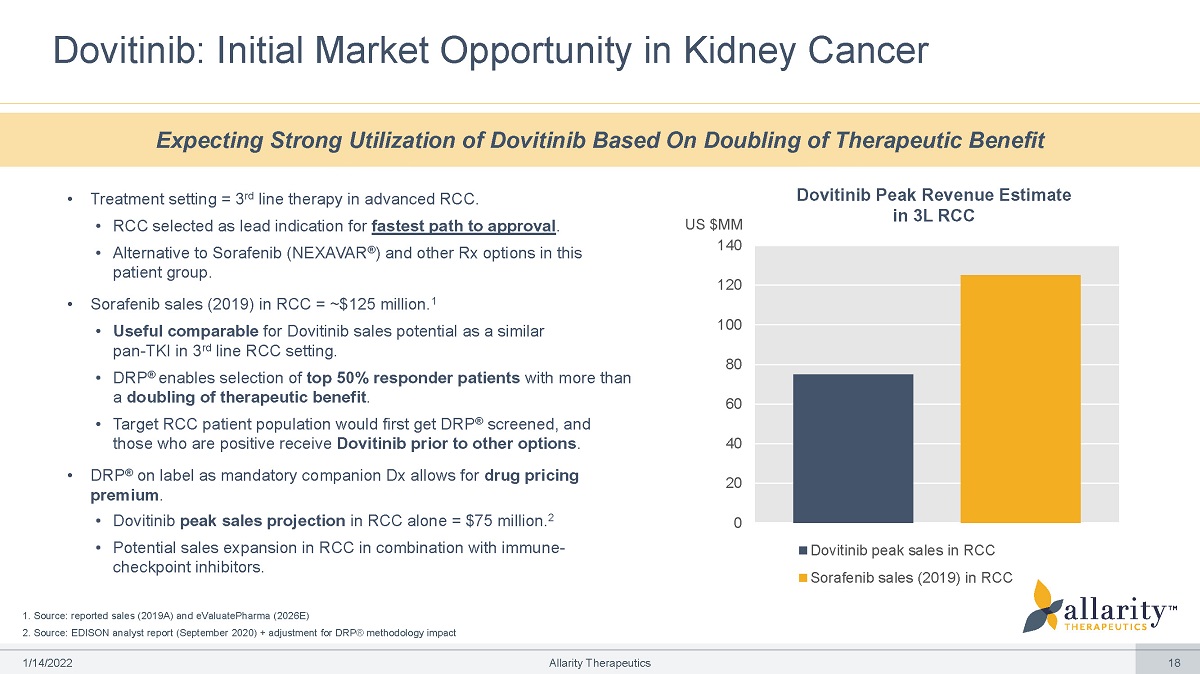
1. Source: reported sales (2019A) and eValuatePharma (2026E) 2. Source: EDISON analyst report (September 2020) + adjustment for DRP® methodology impact Dovitinib: Initial Market Opportunity in Kidney Cancer 0 20 40 60 80 100 120 US $M M 140 Dovitinib peak sales in RCC Sorafenib sales (2019) in RCC Expecting Strong Utilization of Dovitinib Based On Doubling of Therapeutic Benefit 1 /1 4 /2 0 2 2 Allarity Therapeutics 18 Dovitinib Peak Revenue Estimate in 3L RCC • Treatment setting = 3 rd line therapy in advanced RCC. • RCC selected as lead indication for fastest path to approval . • Alternative to Sorafenib (NEXAVAR ® ) and other Rx options in this patient group. • Sorafenib sales (2019) in RCC = ~$125 million. 1 • Useful comparable for Dovitinib sales potential as a similar pan - TKI in 3 rd line RCC setting. • DRP ® enables selection of top 50% responder patients with more than a doubling of therapeutic benefit . • Target RCC patient population would first get DRP ® screened, and those who are positive receive Dovitinib prior to other options . • DRP ® on label as mandatory companion Dx allows for drug pricing premium . • Dovitinib peak sales projection in RCC alone = $75 million. 2 • Potential sales expansion in RCC in combination with immune - checkpoint inhibitors.
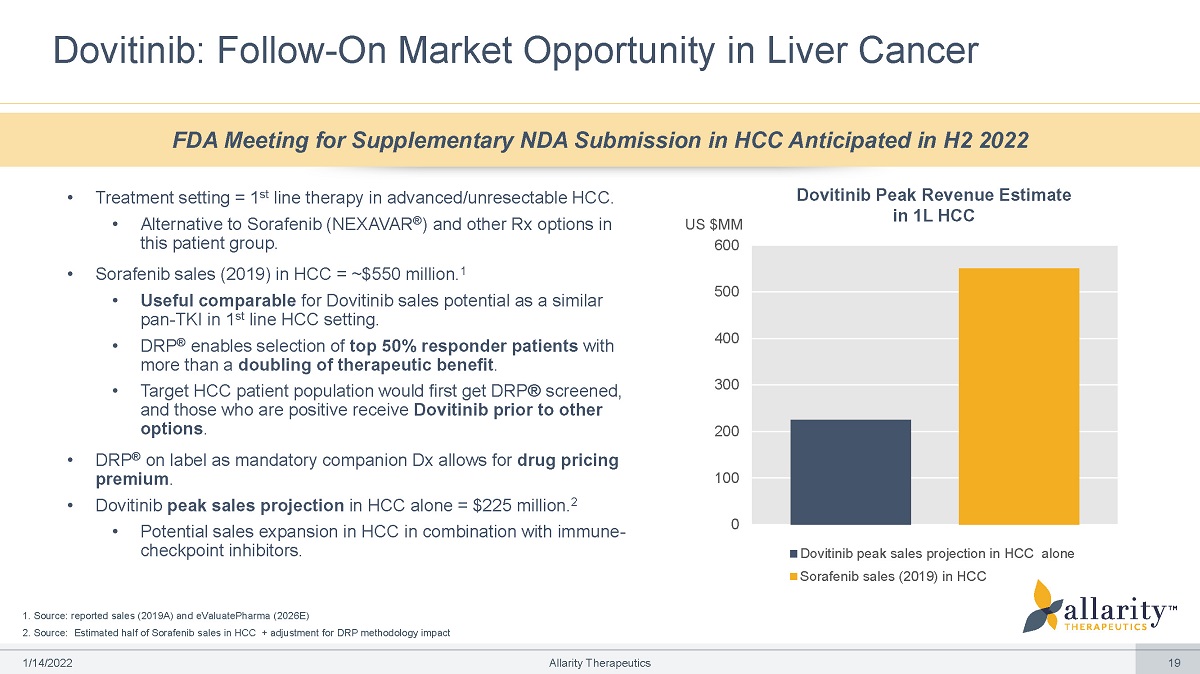
0 100 200 300 400 500 US $MM 600 Dovitinib peak sales projection in HCC alone Sorafenib sales (2019) in HCC Dovitinib Peak Revenue Estimate in 1L HCC 1. Source: reported sales (2019A) and eValuatePharma (2026E) 2. Source: Estimated half of Sorafenib sales in HCC + adjustment for DRP methodology impact Dovitinib: Follow - On Market Opportunity in Liver Cancer FDA Meeting for Supplementary NDA Submission in HCC Anticipated in H2 2022 1 /1 4 /2 0 2 2 Allarity Therapeutics 19 • Treatment setting = 1 st line therapy in advanced/unresectable HCC. • Alternative to Sorafenib (NEXAVAR ® ) and other Rx options in this patient group. • Sorafenib sales (2019) in HCC = ~$550 million. 1 • Useful comparable for Dovitinib sales potential as a similar pan - TKI in 1 st line HCC setting. • DRP ® enables selection of top 50% responder patients with more than a doubling of therapeutic benefit . • Target HCC patient population would first get DRP® screened, and those who are positive receive Dovitinib prior to other options . • DRP ® on label as mandatory companion Dx allows for drug pricing premium . • Dovitinib peak sales projection in HCC alone = $225 million. 2 • Potential sales expansion in HCC in combination with immune - checkpoint inhibitors.
Dovitinib: Evaluating Multiple Commercial Opportunities Potential Regional Oncology Commercialization Partners Have Been Identified • Dovitinib commercialization strategy: enter into marketing partnerships with leading regional oncology therapeutics companies to achieve optimal market penetration in key regions. • Existing discussions on - going with prominent regional partners in: • MENA region • LATM region • China region • EU region • U.S. • Regional partnership deals will include: • Approval milestone payments to Allarity. • Tiered royalties on Dovitinib sales in each region. 1 /1 4 /2 0 2 2 Allarity Therapeutics 20
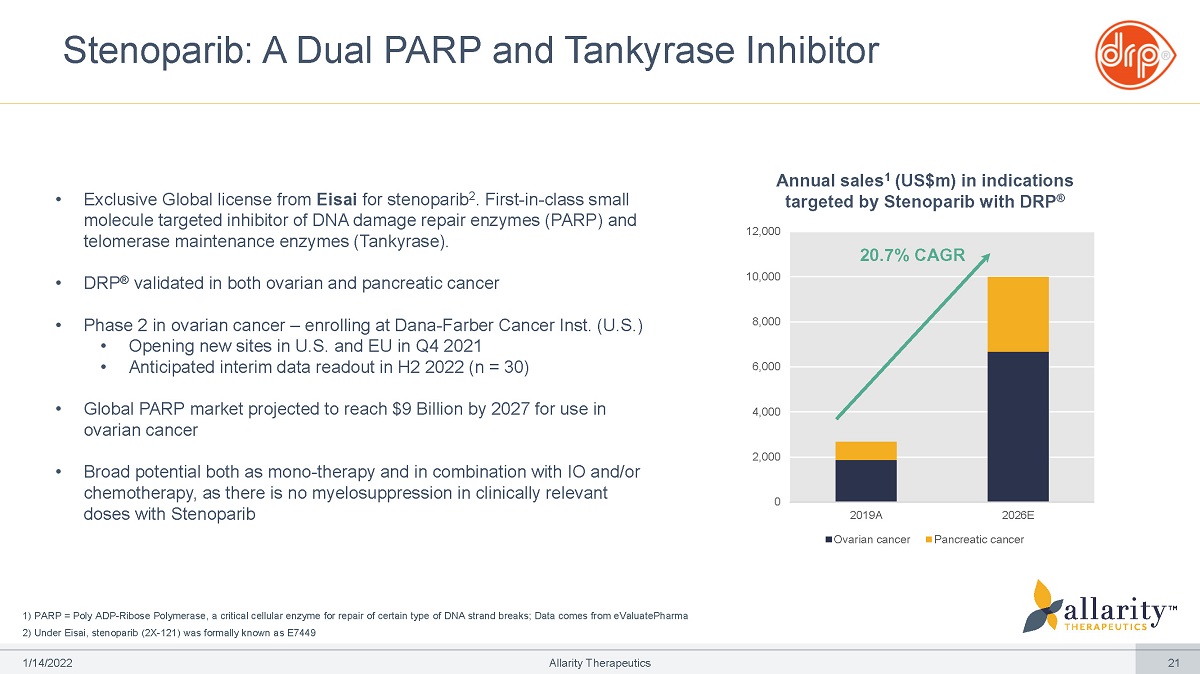
1) PARP = Poly ADP - Ribose Polymerase, a critical cellular enzyme for repair of certain type of DNA strand breaks; Data comes from eValuatePharma 2) Under Eisai, stenoparib (2X - 121) was formally known as E7449 Stenoparib: A Dual PARP and Tankyrase Inhibitor Annual sales 1 (US$m) in indications targeted by Stenoparib with DRP ® 0 2,0 00 4,0 00 6,0 00 8,0 00 10, 0 0 0 12, 0 0 0 2019A 2026E Ovarian cancer Pancreatic cancer • Exclusive Global license from Eisai for stenoparib 2 . First - in - class small molecule targeted inhibitor of DNA damage repair enzymes (PARP) and telomerase maintenance enzymes (Tankyrase). • DRP ® validated in both ovarian and pancreatic cancer • Phase 2 in ovarian cancer – enrolling at Dana - Farber Cancer Inst. (U.S.) • Opening new sites in U.S. and EU in Q4 2021 • Anticipated interim data readout in H2 2022 (n = 30) • Global PARP market projected to reach $9 Billion by 2027 for use in ovarian cancer • Broad potential both as mono - therapy and in combination with IO and/or chemotherapy, as there is no myelosuppression in clinically relevant doses with Stenoparib 20.7% CAGR 1 /1 4 /2 0 2 2 Allarity Therapeutics 21
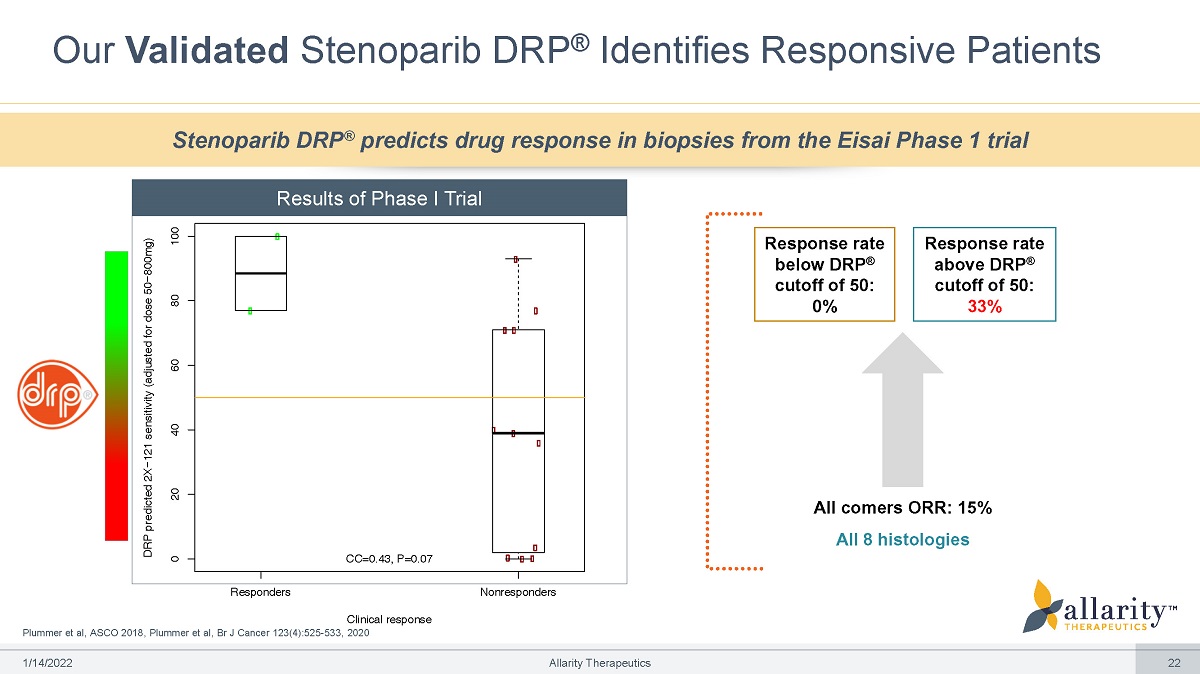
Clinical response Plummer et al, ASCO 2018, Plummer et al, Br J Cancer 123(4):525 - 533, 2020 Our Validated Stenoparib DRP ® Identifies Responsive Patients Response rate below DRP ® cutoff of 50: 0% Response rate above DRP ® cutoff of 50: 33% All comers ORR: 15% All 8 histologies DRP predicted 2X−121 sensitivity (adjusted for dose 50−800mg) Responders Nonresponders 0 20 40 60 80 1 00 CC=0.43, P=0.07 Stenoparib DRP ® predicts drug response in biopsies from the Eisai Phase 1 trial Results of Phase I Trial 1 /1 4 /2 0 2 2 Allarity Therapeutics 22

1) Lars M. Wagner, Division of Pediatric Hematology/Oncology, University of Kentucky, Lexington, KY, USA, in “Profile of veliparib and its potential in the treatment of solid tumors.” Onco Targets Ther. 2015; 8: 1931 – 1939. https ://www .nc bi.nlm.nih.gov/pmc/articles/PMC4524591 Stenoparib: Well Positioned in PARP Competitive Landscape “… the identification of reliable biomarkers will be critical for the success of this targeted agent” 1 Allarity PARP inhibitor (Stenoparib) with exclusive DRP ® : a novel, multi - targeted drug (Tankyrase and PARP inhibition) with improved efficacy, no myelotoxicity, and less drug resistance resulting in improved patient outcomes Product Owner Stage R e sponse bio m ar k er Export Resistance (PgP mediated) Absence of M y elotoxicity (Myelotox) Multi - targeted (PARP/ Tankyrase) PARP trapping BBB penetration Strong mainten a nce opportunity Olaparib Approved BRCA غ غ غ ض غ ض Niraparib Approved BRCA/HRD غ غ غ ض غ ض Veliparib Phase 3 BRCA غ غ غ غ غ غ Rucaparib Approved BRCA غ غ ض ض غ ض Talazoparib Approved BRCA ض غ غ ض غ ض Stenoparib Phase 2 DRP ® ض ض ض ض ض ض 1 /1 4 /2 0 2 2 Allarity Therapeutics 23 Value Proposition
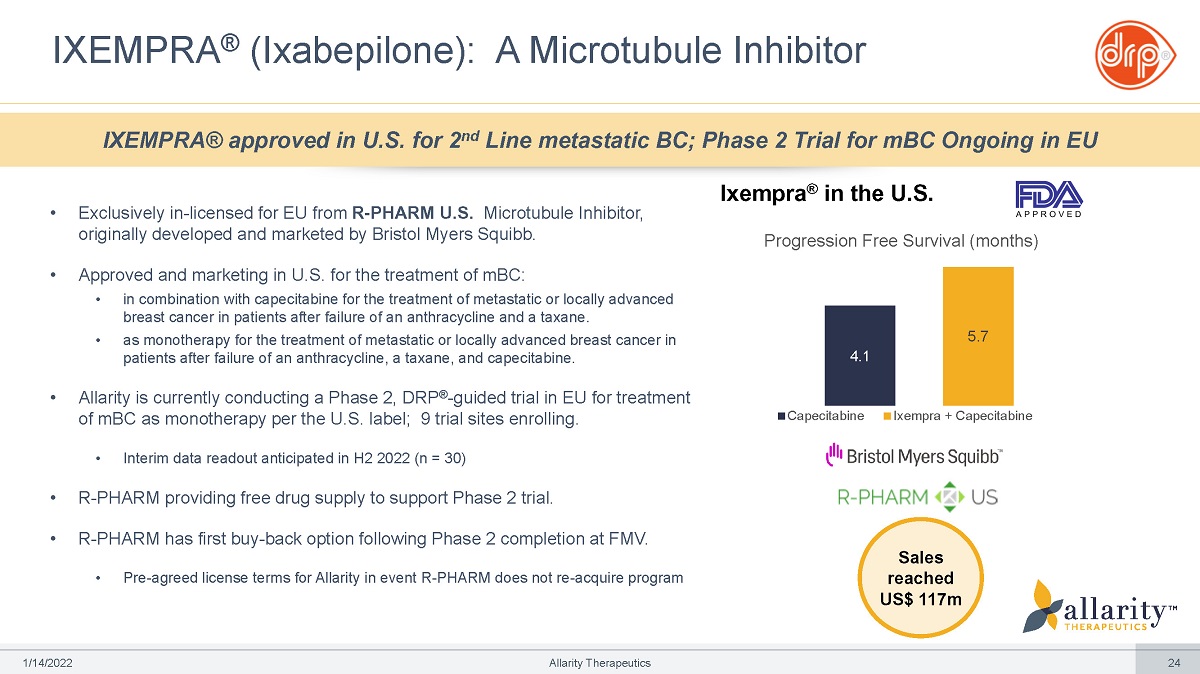
• Exclusively in - licensed for EU from R - PHARM U.S. Microtubule Inhibitor, originally developed and marketed by Bristol Myers Squibb. • Approved and marketing in U.S. for the treatment of mBC: • in combination with capecitabine for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline and a taxane. • as monotherapy for the treatment of metastatic or locally advanced breast cancer in patients after failure of an anthracycline, a taxane, and capecitabine. • Allarity is currently conducting a Phase 2, DRP ® - guided trial in EU for treatment of mBC as monotherapy per the U.S. label; 9 trial sites enrolling. • Interim data readout anticipated in H2 2022 (n = 30) • R - PHARM providing free drug supply to support Phase 2 trial. • R - PHARM has first buy - back option following Phase 2 completion at FMV. • Pre - agreed license terms for Allarity in event R - PHARM does not re - acquire program IXEMPRA ® (Ixabepilone): A Microtubule Inhibitor IXEMPRA® approved in U.S. for 2 nd Line metastatic BC; Phase 2 Trial for mBC Ongoing in EU Ixempra ® in the U.S. 4.1 5.7 Capecitabine Ixempra + Capecitabine Sales reached US$ 117m Progression Free Survival (months) 1 /1 4 /2 0 2 2 Allarity Therapeutics 24
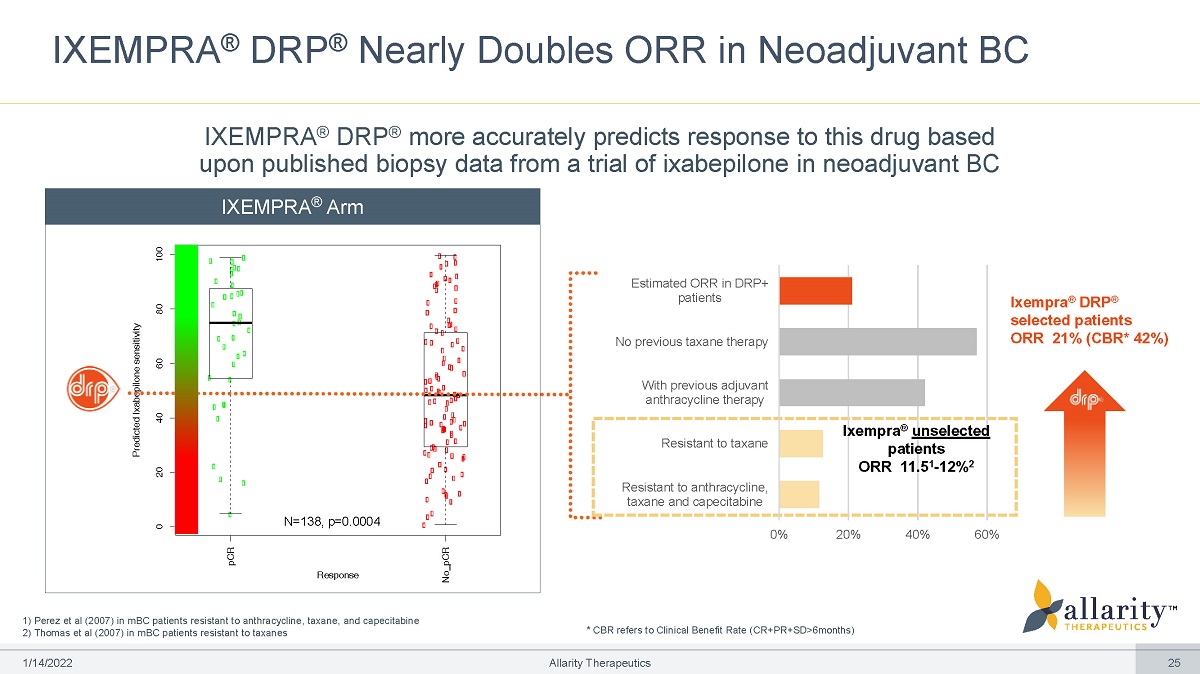
1) Perez et al (2007) in mBC patients resistant to anthracycline, taxane, and capecitabine 2) Thomas et al (2007) in mBC patients resistant to taxanes IXEMPRA ® DRP ® Nearly Doubles ORR in Neoadjuvant BC R e spons e Predicted Ixabepilone sensitivity pCR No_ pCR 0 20 4 0 60 80 1 00 Ixempra ® DRP ® selected patients ORR 21% (CBR* 42%) 0% 2 0 % 4 0 % 6 0 % Resistant to anthracycline, taxane and capecitabine Resistant to taxane No previous taxane therapy Estimated ORR in DRP+ patients With previous adjuvant anthracycline therapy Ixempra ® unselected patients ORR 11.5 1 - 12% 2 N=13 8 , p=0. 0 00 4 IXEMPRA ® DRP ® more accurately predicts response to this drug based upon published biopsy data from a trial of ixabepilone in neoadjuvant BC IXEMPRA ® Arm * CBR refers to Clinical Benefit Rate (CR+PR+SD>6months) 1 /1 4 /2 0 2 2 Allarity Therapeutics 25

• We expect to monetize our secondary pipeline programs through partnerships • LiPlaCis ® and 2X - 111 out - licensed to Smerud Medical Research International in June 2020 for further clinical and commercial development in connection with each program’s DRP ® companion Dx. • LiPlaCis ® in late - stage metastatic breast cancer • 2X - 111 in glioblastoma multiforme • Upon achievement of regulatory and commercial milestones, Allarity eligible to receive: • Fees of up to US $30M • Double - digits royalties on future sales for each drug • Irofulven reacquired by Lantern Pharma in July 2021 for further clinical and commercial development in bladder and prostate cancers, optionally in connection with DRP® companion Dx. • Upon achievement of regulatory and commercial milestones, Allarity eligible to receive: • Fees of up to US $18M • Single - digit tiered royalties on future sales of drug 1 /1 4 /2 0 2 2 Allarity Therapeutics 26 Secondary Pipeline Programs – Strategy & Opportunities

Leadership, I.P., & Strategic Milestones
James G. Cullem, JD Chief Business Officer Our Senior Leadership Team Marie Foegh, MD Chief Medical Officer Steen Knudsen, PhD Chief Scientific Officer, Co - Founder Steve R. Carchedi Chief Executive Officer (Joined September 2019) Jens E. Knudsen Chief Financial Officer (Joined November 2020) Thomas Jensen Senior Vice President, Information Technologies Co - Founder 1 /1 4 /2 0 2 2 Allarity Therapeutics 28
Advisory Board: World - Class Key Opinion Leaders DAN VON HOFF, MD Physician in Chief, Distinguished Professor at the Translational Genomics Research Institute (TGen) in Phoenix, Arizona. Virginia G. Piper Distinguished Chair for Innovative Cancer Research at HonorHealth. Chief Scientific Officer for US Oncology Research UR S ULA A. M A TU L ONIS, MD Chief and Director of the Division of Gynecologic Oncology at DFCI. Professor of Medicine at Harvard Medical School. First recipient of Brock - Wilson Family Chair at DFCI. JOYCE A. O’SHAUGHNESSY, MD Co - Chair of Breast Cancer Research and Chair of Breast Cancer Prevention Research at Baylor - Sammons Cancer Center and for The US Oncology Network MANSOOR RAZA MIRZA, MD Chief Oncologist at the Dept. of Oncology, Rigshospitalet, Copenhagen University Hospital, Denmark and Medical Director of the Nordic Society of Gynecologic Oncology - Clinical Trial Unit (NSGO - CTU). He is also Vice - Chairman of the Danish Society of Gynecologic Oncology (DGCG). 1 /1 4 /2 0 2 2 Allarity Therapeutics 29
Our Board of Directors GAIL J. MADERIS Director Gail is currently CEO of Antiva Biosciences, Inc., and a Board member at Valitor, Inc., the BIO Emerging Companies governing group, and the U.C. Berkeley Foundation Board of Trustees. Earlier in her career, Gail was CEO at Five Prime Therapeutics, Inc., President of Genzyme Molecular Oncology, and a Manager at Bain & Company. DUNCAN MOORE Chairman Duncan is a partner in East West Capital Partners and currently serves as Chairman of Lamellar Biomedical, a Director at Braidlock Limited, and Chairman the Advisory Board of the Scottish Life Sciences Association. Earlier in his career, Duncan was Global Head of Healthcare Research at Morgan Stanley. STEVE R. CARCHEDI Director, CEO Steve was previously CEO at Apexian Pharmaceuticals and Raphael Pharmaceuticals (formerly Cornerstone Pharmaceuticals). Earlier in his career, Steve held senior leadership positions at Mallinckrodt, General Electric, Johnson & Johnson, Eli Lilly & Company and Bristol Myers Squibb. SØREN GADE Director Søren is currently Member of the European Parliament and was former Minister of Defense in Denmark. He current serves on the Board of TecLeaf ApS, CSR Invest ApS, and the Fulton Foundation, and also serves as Protektor for the Danish Colon Cancer Association. He holds an MSc degree in Economics. 1 /1 4 /2 0 2 2 Allarity Therapeutics 30
• 15 DRP ® patents granted covering 70 different oncology drugs • Additional DRP ® patent applications pending focused on primary pipeline • Recent USPTO allowance on 3 pending applications including our Dovitinib DRP ® • Patent strategy allows us to file new patent applications as we develop DRP®’s for additional oncology drugs • Additional protection from composition - of - matter, formulation, and method - of - use patents for priority programs Strong & Expanding Intellectual Property Portfolio Global IP Protection for Foundational DRP® Technology 1 /1 4 /2 0 2 2 Allarity Therapeutics 31
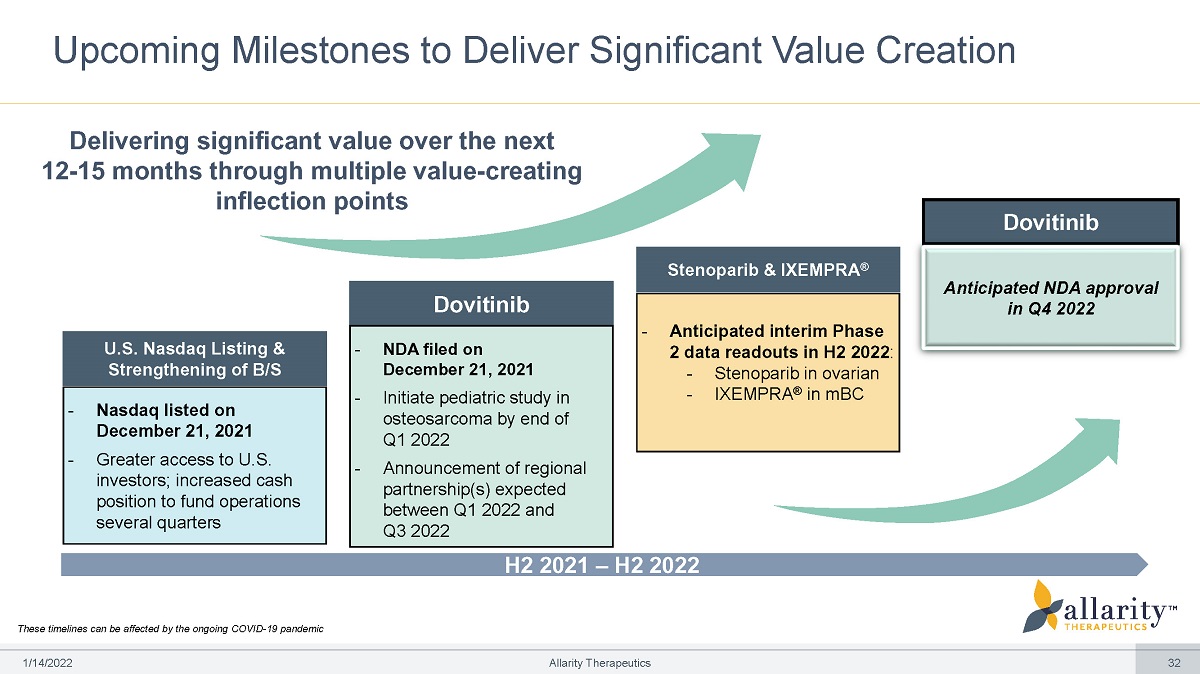
H2 2021 – H2 2022 Upcoming Milestones to Deliver Significant Value Creation Delivering significant value over the next 12 - 15 months through multiple value - creating inflection points These timelines can be affected by the ongoing COVID - 19 pandemic U.S. Nasdaq Listing & Strengthening of B/S - Nasdaq listed on December 21, 2021 - Greater access to U.S. investors; increased cash position to fund operations several quarters Dovitinib - NDA filed on December 21 , 2021 - Initiate pediatric study in osteosarcoma by end of Q 1 2022 - Announcement of regional partnership(s) expected between Q1 2022 and Q3 2022 Stenoparib & IXEMPRA ® - Anticipated interim Phase 2 data readouts in H2 2022 : - Stenoparib in ovarian - IXEMPRA ® in mBC Dovitinib Anticipated NDA approval in Q4 2022 1 /1 4 /2 0 2 2 Allarity Therapeutics 32

Advanced oncology pipeline with multiple mid to late clinical - stage drug candidates and opportunities for external partnering. Key value inflection points in 2022. Industry leading DRP ® companion Dx technology platform validated across more clinical trials than any other competitor. NDA for lead drug candidate, Dovitinib, filed on December 21, 2021; supported by PMA application for use of Dovitinib DRP ® companion Dx. NASDAQ listed on December 21, 2021. Strong balance sheet (~$20M) and no debt. Key Investment Highlights 1 /1 4 /2 0 2 2 Allarity Therapeutics 33

James G. Cullem Chief Business Officer +1 (978) 500 - 0863 jcullem@allarity.com Steve R. Carchedi Chief Executive Officer +1 (908) 720 - 1786 scarchedi@allarity.com allari t y .com
FAQs About Our DRP ® Platform Technology Q: What are the different filter rings in the graphic of how DRP® works? A: They represent (i) our proprietary correlation analysis, (ii) our proprietary clinical biopsy filter, and (iii) our final statistical refinement step. Q: Why is a 50% cut - off optimal for DRP ® companion Dx patient scoring? A: Because our 35+ prior clinical validations for DRP® have proven that selecting the top 50% of patients, by DRP® score, reliably captures most responders while excluding most non - responders. ROC analysis after a trial confirm the cutoff selected before a trial. Q: Why does a DRP ® companion Dx typically range between 50 - 400 key expressed genes? A: Because that is the number of expressed genes our algorithm identifies as relevant to response or resistance to a given drug, incorporating necessary biomarkers of both response and resistance. 1 /1 4 /2 0 2 2 Allarity Therapeutics 35

FAQs About Our DRP® Platform Technology Q: Are DRP ® companion Dx validations based upon retrospective or prospective trial analysis? A: Both. We have completed 35+ retrospective validations (including blinded retrospective validations) and 1 prospective validation to date. Q: Is there any special or proprietary way that individual patient biopsies are collected for DRP® analysis? A: No. We typically receive standard formalin - fixed, paraffin - embedded (FFPE) biopsy material, from which we extract all expressed mRNA, which we then analyze by industry standard AffyChip Œ array technology. Q: Why is the DRP® Platform distinct and superior to other companion Dx approaches? A: Most importantly, because it is more highly, clinically validated than any other oncology companion Dx platform, and more extensively peer - review published and patented. 1 /1 4 /2 0 2 2 Allarity Therapeutics 36
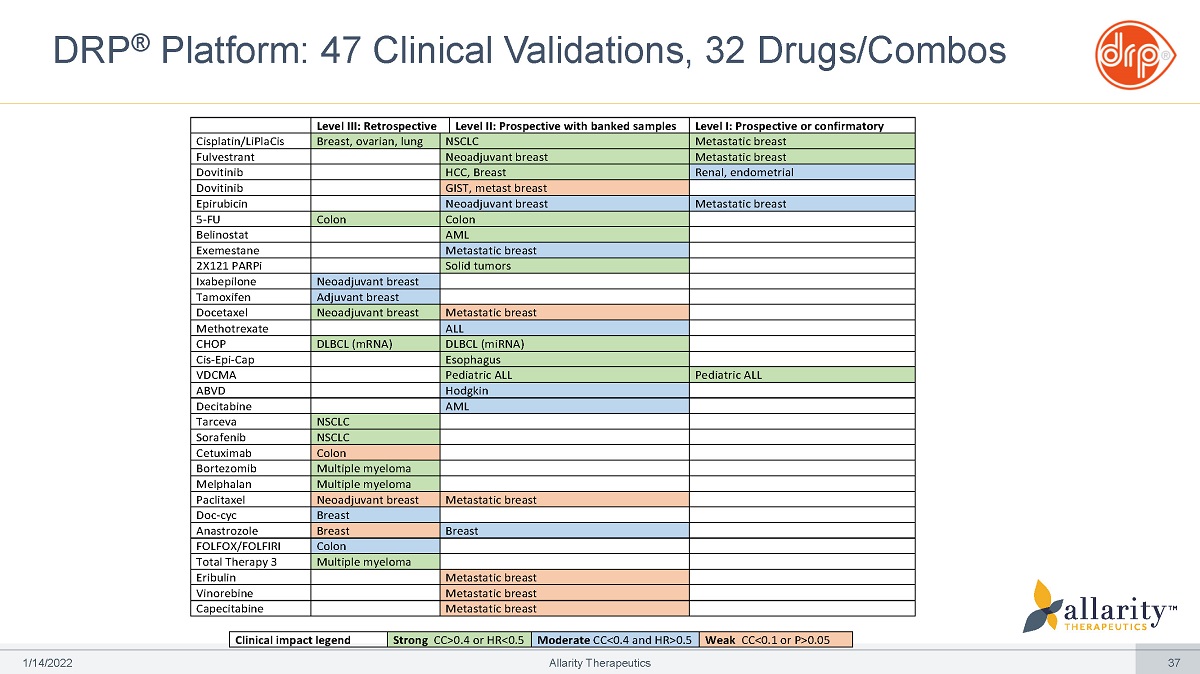
DRP ® Platform: 47 Clinical Validations, 32 Drugs/Combos Level III: Retrospective Level II: Prospective with banked samples Level I: Prospective or confirmatory Cisplatin/LiPlaCis Breast, ovarian, lung NSCLC Metastatic breast Fulvestrant Neoadjuvant breast Metastatic breast Dovitinib HCC, Breast Renal, endometrial Dovitinib GIST, metast breast Epirubicin Neoadjuvant breast Metastatic breast 5 - FU Colon Colon Belinostat AML Exemestane Metastatic breast 2X121 PARPi Solid tumors Ixabepilone Neoadjuvant breast Tamoxifen Adjuvant breast Docetaxel Neoadjuvant breast Metastatic breast Methotrexate ALL CHOP DLBCL (mRNA) DLBCL (miRNA) Cis - Epi - Cap Esophagus VDCMA Pediatric ALL Pediatric ALL ABVD Hodgkin Decitabine AML Tarceva NSCLC Sorafenib NSCLC Cetuximab Colon Bortezomib Multiple myeloma Melphalan Multiple myeloma Paclitaxel Neoadjuvant breast Metastatic breast Doc - cyc Breast Anastrozole Breast Breast FOLFOX/FOLFIRI Colon Total Therapy 3 Multiple myeloma Eribulin Metastatic breast Vinorebine Metastatic breast Capecitabine Metastatic breast 1 /1 4 /2 0 2 2 Allarity Therapeutics 37 Clinical impact legend Strong CC>0.4 or HR<0.5 Moderate CC<0.4 and HR>0.5 Weak CC<0.1 or P>0.05

Publications of Clinical/Method Validation for Drug - Specific DRP®’s 1 /1 4 /2 0 2 2 Allarity Therapeutics 38 1. Chen J, et al. A 71 - gene signature of TRAIL sensitivity in cancer cells . (October 2011); Mol Cancer Ther , 10.1158/1535 - 7163. 2. Wang W, et al. Independent validation of a model using cell line chemosensitivity to predict response to therapy . J Natl Cancer Inst . (2013) Sep 4;105(17):1284 - 91 3. Knudsen S, et al. Development and validation of a gene expression score that predicts response to fulvestrant in breast cancer patients . PLoS One . 2014 Feb 5;9(2):e87415 4. Knudsen S, et al. Development and blind clinical validation of a microRNA based predictor of response to treatment with R - CHO(E)P in DLBCL . PLoS One. (2015) Feb 18;10(2):e0115538 5. Buhl IK, et al.. Cell Line Derived 5 - FU and Irinotecan Drug - Sensitivity Profiles Evaluated in Adjuvant Colon Cancer Trial Data . PLoS One. (2016); 11(5): e0155123. 6. Winther M, et al. Clinical Impact of a Novel MicroRNA Chemo - Sensitivity Predictor in Gastrooesophageal Cancer . PLoS One. (2016); 11(2): e0148070. 7. Rücker FG, et al. Molecular dissection of valproic acid effects in acute myeloid leukemia identifies predictive networks . Epigenetics. (2016) Jul 2;11(7):517 - 25. 8. Prahm KP, et al. Clinical validation of chemotherapy predictors developed on global microRNA expression in the NCI60 cell line panel tested in ovarian cancer . PLoS ONE (2017) 12(3): e0174300. 9. Bohl S, et al. Gene expression analysis of decitabine treated AML: high impact of tumor suppressor gene expression changes . Leukemia & Lymphoma (2017) Vol. 58, Iss. 9, 2264 - 2267 10. Vangsted A.J., et al. Drug response prediction in high - risk multiple myeloma . Gene (2018) 644 80 - 86 11. Buhl IK, et al. Molecular prediction of adjuvant cisplatin efficacy in Non - Small Cell Lung Cancer (NSCLC) — Validation in two independent cohorts . PLoS ONE (2018) 13(3): e0194609. 12. Buhl ASK, et al. Predicting efficacy of epirubicin by a multigene assay in advanced breast cancer within a Danish Breast Cancer Cooperative Group (DBCG) cohort: a retrospective - prospective blinded study . Breast Cancer Res Treat . (2018) Aug 11. 13. Christensen TD, et al. Prediction of fulvestrant efficacy in patients with advanced breast cancer: retrospective - prospective evaluation of the predictive potential of a multigene expression assay . Breast Cancer . (2019) Oct 25. doi: 10.1007/s12282 - 019 - 01017 - 7 14. Plummer R., et al. First - in - human study of the PARP/tankyrase inhibitor E7449 in patients with advanced solid tumours and evaluation of a novel drug - response predictor . Br J Cancer (2020) 123(4):525 - 533. https://doi.org/10.1038/s41416 - 020 - 0916 - 5

Dovitinib’s Place in the 3 rd Line RCC Treatment Landscape 1 /1 4 /2 0 2 2 Allarity Therapeutics 39 NCCN GUIDELINES V 2.2022 - SEPTEMBER 8, 2021 CLEAR CELL RCC THERAPY BEYOND FIRST LINE Preferred Regimen Other Recommended Regimens Useful in Certain Circumstances Cabozantinib Axitinib Everolimus Lenvatinib + Everolimus Axitinib + Pembrolizumab Bevacizumab Nivolumab Cabozantinib + Nivolumab High - dose IL - 2 Dovitinib DRP® Selected * Ipilimumab + Nivolumab Sorafenib Lenvatinib + Pembrolizumab Temsirolimus Pazopanib Sunitinib Tivozanib* Axitinib + Avelumab *For patients who received > 2 prior systemic therapies November 10, 2021

• We are opportunistically exploring the potential of Stenoparib as an anti - viral against COVID - 19 • Dual Mechanism - of - Action on: • Virus replication: PARP may be important for the SARS - CoV - 2 viral capsid maturation, which is ADP ribosylated • “Cytokine storm”: PARP inhibitors limit the expression of pro - inflammatory cytokines like IL - 6, IL - 1 and TNF - α • Stenoparib demonstrated activity against Coronavirus and variants in preclinical testing • Inhibitory activity demonstrated as a single agent and at lower dose in combination with remdesivir • Inhibitory activity also demonstrated against the SARS - CoV - 2 alpha variant B.1.1.7 (“British” variant), beta variant B.1351 (“South African” variant), and gamma variant P.1 (“Brazilian” variant) • Further pre - clinical testing against delta variant B.1.617.2 (“Indian” variant) is being initiated • Clear Clinical Development Pathway • Allarity will submit preclinical findings to the U.S. NIH/NIAID as part of the new U.S. federal Antiviral Program for Pandemics (APP) that has replaced the prior “Warp Speed” grant program, to support human clinical trials. • A multi - modal approach needed: • Remdesivir is the only approved antiviral therapeutic against COVID - 19, but has limited efficacy • Combination therapy is key to increased efficacy of remdesivir 1) PARP = Poly ADP - Ribose Polymerase, a critical cellular enzyme for repair of certain type of DNA strand breaks 2) BARDA = Biomedical Advanced Research and Development Authority 1 /1 4 /2 0 2 2 Allarity Therapeutics 40 Stenoparib: Anti - viral Activity vs. COVID - 19







































