As filed with the U.S. Securities and Exchange Commission on August 6, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
VSEE HEALTH, INC.
(Exact name of registrant as specified in its charter)
Delaware |
| 001-41015 |
| 86-2970927 |
(State or other jurisdiction of incorporation or organization) | | (Commission File Number) | | (I.R.S. Employer |
980 N Federal Hwy #304,
Boca Raton, Florida 33432
(561) 672-7068
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Imoigele Aisiku
Milton Chen
Co-Chief Executive Officers
980 N Federal Hwy #304
Boca Raton, FL 33432
Tel: (561) 672-7068
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With Copies to:
Thomas J. Poletti, Esq.
Veronica Lah, Esq.
Manatt, Phelps & Phillips, LLP
2049 Century Park East, Suite 1700
Los Angeles, CA 90067
(310) 312-4000
Approximate date of commencement of proposed sale to the public: As soon as possible after the effective date hereof.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ |
| Accelerated filer ☐ |
Non-accelerated filer ☒ | | Smaller reporting company ☒ |
| | Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 6, 2024
PRELIMINARY PROSPECTUS

VSEE HEALTH, INC.
Up to 11,500,000 Shares of Common Stock Issuable Upon the Exercise of Public Warrants
Up to 25,050,000 Shares of Common Stock
This prospectus relates to the issuance by us of up to 11,500,000 shares of our common stock, $0.0001 par value per share (the “Common Stock”) that are issuable upon the exercise of warrants (the “Public Warrants”) originally issued in the initial public offering of Digital Health Acquisition Corp. (“DHAC” or “Digital Health”).
This prospectus also relates to the resale, from time to time, of up to 25,050,000 shares of our Common Stock by the selling stockholder, Dominion Capital LLC (“Dominion” or the “Selling Stockholder”). The Common Stock to which this prospectus relates includes (1) up to 25,000,000 shares of Common Stock that have been or may be issued to Dominion pursuant to a purchase agreement between DHAC and Dominion dated as of November 21, 2023 (the “Equity Purchase Agreement”) and (2) 50,000 shares of Common Stock upon conversion of a senior unsecured note in a principal amount of $500,000 that is payable only in shares of our Common Stock at an initial price of $10 per share (the “Equity Purchase Note”) that we issued to Dominion on July 2, 2024 as a commitment fee for this equity purchase transaction. We may receive gross proceeds of up to $50,000,000 from the sale of Common Stock to Dominion under the Equity Purchase Agreement, from time to time, in our discretion after the date of the registration statement of which this prospectus is a part is declared effective and after satisfaction of other conditions in the Equity Purchase Agreement. See the section titled “Description of Equity Financing Transaction” for a description of the Purchase Agreement and the section titled “Selling Stockholder” for additional information regarding Dominion.
The shares of our Common Stock included in this prospectus are being registered for resale pursuant to the terms of the terms of the Public Warrants described herein under the section titled “Description of Our Securities — IPO Warrants” and the Equity Purchase Agreement described herein under the section titled “The Committed Equity Financing Transaction.” We will bear all fees and expenses incident to our obligation to register the shares of Common Stock.
We will not receive any proceeds from the sale of shares of Common Stock by Dominion. However, we may receive up to $50,000,000 in aggregate gross proceeds from sales of our Common Stock to Dominion that we may, in our discretion, elect to make, from time to time after the date of this prospectus, pursuant to the Equity Purchase Agreement. We will receive proceeds from any exercise of the Public Warrants for cash. Any proceeds received by us from the sale of shares of Common Stock to Dominion pursuant to the Equity Purchase Agreement or from the exercise of the Public Warrants will be used for general corporate purposes. See section titled “Use of Proceeds” for more information. We believe the likelihood that public warrant holders will exercise their Public Warrants and therefore the amount of cash proceeds that we would receive, is dependent upon the trading price of our shares of Common Stock. If the trading price for our shares of Common Stock continues to be over $11.50 per share, we believe holders of Public Warrants will likely exercise these warrants. In addition, to the extent the Public Warrants are exercised on a “cashless basis,” the amount of cash we would receive from the exercise of the Public Warrants will decrease. The Public Warrants may be exercised for cash or on a “cashless basis” on certain conditions. See “Description of Our Securities — IPO Warrants” for further discussion.
In addition, Dominion may sell or otherwise dispose of the shares of Common Stock included in this prospectus in a number of different ways and at varying prices. The price that Dominion will pay for the shares of Common Stock to be resold pursuant to this prospectus will depend upon the timing of sales and will fluctuate based on the trading price of our Common Stock and will be set as at the price per share pursuant to the purchase notice under the Equity Purchase Agreement that we deliver to Dominion (the “Advance Notice”) by multiplying 92% of the lowest average daily volume weighted average price (“VWAP”) during the five days prior to our submission of Advance Notice. If the VWAP pursuant to an Advance Notice is less than the floor price of $2.00, which will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, share split, or other similar transaction and effective upon the consummation of any such transactions (the “Floor Price”), then Dominion has the right but not the obligation to purchase shares of our Common Stock at the Floor Price. Although Dominion is obligated to purchase shares of our Common Stock under the terms and subject to the conditions and limitations of the Equity Purchase Agreement to the extent we choose to sell such shares of our Common Stock to it (subject to certain conditions), the timing and amount of any sales of Common Stock by Dominion are within the sole discretion of Dominion. There can be no assurances that we will choose to sell any shares of our Common Stock to Dominion, or that Dominion will sell any or all of the shares of our Common Stock, if any, purchased under the Equity Purchase Agreement pursuant to this prospectus. See the section titled “Plan of Distribution” for more information about how the Selling Stockholder may sell or otherwise dispose of the Common Stock being offered in this prospectus. While the Equity Purchase Agreement contains certain limitations regarding the number of shares of Common Stock that we can sell to Dominion under the Equity Purchase Agreement, the number of shares of Common Stock that we can sell to Dominion under the Equity Purchase Agreement could constitute a considerable percentage of our public float at the time of such sales. As a result, our stockholders may experience significant dilution as a result of the resale by Dominion of shares of Common Stock pursuant to this prospectus. See section titled “Risk Factors — Risks Related to This Offering” for more information.
We will pay the expenses of registering the shares of Common Stock offered by this prospectus, but all selling and other expenses incurred by Dominion will be paid by itself. Dominion acknowledges that it is disclosed as an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
You should read this prospectus, any applicable prospectus supplement and any related free writing prospectus carefully before you invest.
Our Common Stock and Public Warrants are listed on The Nasdaq Capital Market under the symbol “VSEE” and “VSEEW,” respectively. On August 5, 2024, the last reported sale price of our Common Stock on The Nasdaq Capital Market was $2.67 per share and the last reported sale price of our Public Warrant on The Nasdaq Capital Market was $0.097 per Public Warrant.
We are a “smaller reporting company” as defined under the federal securities laws and, as such, are eligible for reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary - Implications of Being a Smaller Reporting Company”.
Investing in our securities involves a high degree of risk. Before making any investment in our securities, you should read and carefully consider the risks described in this prospectus under the heading “Risk Factors” beginning on page 12 of this prospectus and in our filings with the Securities and Exchange Commission.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2024
TABLE OF CONTENTS
i
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-1 that we filed with the U.S. Securities and Exchange Commission (the “SEC”). Under this registration process, the Selling Stockholder may, from time to time, sell the securities offered by them described in this prospectus. We will not receive any proceeds from the sale by the Selling Stockholders of the securities offered by them described in this prospectus. We will receive proceeds from any exercise of the Public Warrants for cash.
We may also file a prospectus supplement or post-effective amendment to the registration statement of which this prospectus forms a part that may contain material information relating to this offering. The prospectus supplement or post-effective amendment may also add, update or change information contained in this prospectus. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement or post-effective amendment, you should rely on the prospectus supplement or post-effective amendment, as applicable. Before purchasing any securities, you should carefully read this prospectus, any post-effective amendment, and any applicable prospectus supplement, together with the additional information described under the headings “Where You Can Find More Information.”
Neither we nor the Selling Stockholders have authorized anyone to provide you with any information or to make any representations other than those contained, or incorporated by reference, in this prospectus, any post-effective amendment, or any applicable prospectus supplement prepared by or on behalf of us or to which we have referred you. We and the Selling Stockholders take no responsibility for and can provide no assurance as to the reliability of any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should not assume that the information contained in this prospectus or any applicable prospectus supplement is accurate on any date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus or any applicable prospectus supplement is delivered, or securities are sold, on a later date.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
1
SELECTED DEFINITIONS
“Additional Bridge Notes” refers to the 10% original issue discount senior secured promissory notes, as amended, in the principal amount of $111,111.33 purchased at signing of the Bridge Letter Agreement on November 21, 2023, which will mature on May 21, 2025 and (2) $55,555.67 purchased on January 25, 2024, which will mature on July 25, 2025, bearing guaranteed interest at a rate of 8.00% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at an initial fixed conversion price of $10.00 per share.
“Amended Charter” refers to the Second Amended & Restated Certificate of Incorporation of VSee Health, Inc., which took effect on June 24, 2024.
“A&R Loan Conversion SPA” refers to certain securities purchase agreement entered by and among DHAC, VSee Lab/iDoc (as applicable) and the Bridge Investor on November 21, 2023 and as amended and restated on February 13, 2024.
“Board” refers to the board of directors of VSee Health, Inc.
“Bridge Amendment” refers to the letter agreement, dated November 21, 2023, among DHAC, VSee, iDoc and the Bridge Investor amending the Original Bridge SPA.
“Bridge RRA” refers to the Registration Rights Agreement, dated October 5, 2022, between DHAC and the Bridge Investor and as amended further on January 22, 2024.
“Bridge SPA” or “Bridge Securities Purchase Agreement” refers to the Original Bridge SPA, as amended by the Bridge Amendment.
“Bridge Warrants” refers to 173,913 five-year warrants, each representing the right to purchase one share of Common Stock at an initial exercise price of $11.50, subject to certain adjustments, issued to the Bridge Investor on October 5, 2022.
“Bridge Investor” refers to Dominion Capital LLC.
“Business Combination” refers to the VSee Merger, the iDoc Merger and the other transactions contemplated by the Business Combination Agreement.
“Business Combination Agreement” refers to the Third Amended and Restated Business Combination Agreement, dated as of November 21, 2023, as amended by the First Amendment dated February 13, 2024 and the Second Amendment dated April 17, 2024, by and among DHAC, Merger Sub I, Merger Sub II, VSee and iDoc, as the same may be amended, modified, supplemented or waived from time to time in accordance with its terms.
“Bridge Notes” refers to the 10% original issue discount senior secured promissory notes issued to the Bridge Investor on October 5, 2022 in the principal amount of $888,888.80 (the “DHAC Bridge Notes”), $666,666.60 (the “VSee Bridge Notes”) and $666,666.60 (the “iDoc Bridge Notes”), respectively in an aggregate principal amount of approximately $2,222,222.
“Bylaws” refers to the bylaws of VSee Health, Inc.
“Closing” refers to the closing of the transactions contemplated by the Business Combination Agreement on June 24, 2024.
“Company,” “our,” “we” “us” or “VSee Health`” refers, prior to VSee Health, Inc. (or DHAC, as the context suggests, prior to the Business Combination).
“Common Stock” refers to the common stock, par value $0.0001 per share, of the Company.
“Continental” refers to Continental Stock Transfer & Trust Company.
2
“Conversion Shares” refers to the 600,000 shares of Common Stock granted to the Bridge Investor on June 24, 2024 under the A&R Loan Conversion SPAs.
“DGCL” refers to the General Corporation Law of the State of Delaware, as amended.
“DHAC” refers to Digital Health Acquisition Corp., a Delaware company and the predecessor of VSee Health, Inc. prior to the Business Combination.
“DHAC Bridge Notes” refers to the 10% original issue discount senior secured promissory notes in the principal amount of $888,888.80 issued by DHAC to the Bridge Investor on October 5, 2022.
“Dominion” (or “Selling Stockholder” as applicable) refers to Dominion Capital LLC.
“Equity Purchase Agreement” refers to the equity purchase agreement dated November 21, 2023 with the Bridge Investor pursuant to which the Company may sell and issue to the Bridge Investor, and the Bridge Investor is obligated to purchase from the Company, up to $50,000,000 of its newly issued shares of the Company’s Common Stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the Closing.
“Equity Purchase Note” refers to the senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of the Company’s common stock at a fixed conversion price of $10 per share.
“Exchange Act” refers to the Securities Exchange Act of 1934, as amended.
“Exchange Agreement” refers to the exchange agreement, dated November 21, 2023, among DHAC, VSee Lab, iDoc, and the Selling Stockholder pursuant to which the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for the Exchange Note.
“Exchange Note” refers to the senior secured convertible promissory note issued by the Company to the Selling Stockholder with an aggregate principle value of $2,523,744.29, which will be guaranteed by each of the Company, VSee and iDoc, bearing guaranteed interest at a rate of 8.00% per annum and will be convertible into shares of Company Common Stock at an initial fixed conversion price of $10 per share.
“Exchange RRA” refers to the registration rights agreement entered into between the Selling Stockholder and the Company on June 2024, 2024 pursuant to the terms of the Exchange Agreement.
“HSR Act” refers to the Hart-Scott Rodino Antitrust Improvements Act of 1976.
“iDoc” refers to iDoc Virtual Telehealth Solutions, Inc., a Texas corporation.
“iDoc Bridge Notes” refers to the 10% original issue discount senior secured promissory notes in the principal amount of $666,666.60 issued by iDoc to the Bridge Investor on October 5, 2022.
“Initial Public Offering” or “IPO” refers to DHAC’s initial public offering of its Public Shares pursuant to the IPO registration statement became effective on November 3, 2021 and closed on November 8, 2021.
“Investment Company Act” refers to the Investment Company Act of 1940, as amended, and the rules and regulations promulgated thereunder.
“Merger Sub I” refers to DHAC Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of DHAC.
“Merger Sub II” refers to DHAC Merger Sub II, Inc., a Texas corporation and wholly owned subsidiary of DHAC.
“Nasdaq” refers to The Nasdaq Stock Market.
3
“October 2022 Commitment Shares” refers to the 30,000 shares of Common Stock issued to the Selling Stockholder on October 5, 2022.
“Original Bridge SPA” refers to the securities purchase agreement entered by and among DHAC, VSee Lab, iDoc and the Bridge Investor on October 5, 2022.
“Quantum Investor” refers to the investor who executed the Quantum Purchase Agreement.
“Quantum Note” refers to a 7% original issue discount convertible promissory note in the aggregate principal amount of $3,000,000, bearing interest at rate of 12% per annum and are convertible into shares of common stock of DHAC at (1) a fixed conversion price of $10 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination, which conversion price is subject to reset.
“Quantum Purchase Agreement” refers to a convertible note purchase agreement DHAC entered into with the Quantum Investor on November 21, 2023, pursuant to which the Quantum Investor subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, the Quantum Note.
“SEC” refers to the U.S. Securities and Exchange Commission.
“Series A Preferred Stock” refers to the Series A Convertible Preferred Stock, par value $0.0001 per share, of the Company.
“Public Warrants” refers to the public warrants of VSee Health, Inc. listed on the Nasdaq Capital Market under the trading symbol “VSEEW.”
“VSee Lab” refers to VSee Lab, Inc., a Delaware corporation.
“VSee Bridge Notes” refers to the 10% original issue discount senior secured promissory notes in the principal amount of $666,666.60 issued by VSee Lab to the Bridge Investor on October 5, 2022.
4
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of VSee Health. These statements are based on the beliefs and assumptions of the management of VSee Health. Although VSee Health believes that its plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, VSee Health cannot assure you that it will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes”, “estimates”, “expects”, “projects”, “forecasts”, “may”, “will”, “should”, “seeks”, “plans”, “scheduled”, “anticipates” or “intends” or similar expressions. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| ● | the anticipated benefits of the Business Combination; |
| ● | the occurrence of any event, change or other circumstances, including the outcome of any legal proceedings that may be instituted against the Company; |
| ● | the ability to maintain the listing of the Common Stock on the Nasdaq, as applicable; |
| ● | the projected financial information, anticipated growth rate, market opportunity and financial performance of the Company; |
| ● | the risk of disruption to the Company’s current plans and operations; |
| ● | the ability to recognize the anticipated benefits of the Company’s business, which may be affected by, among other things, competition and the ability to grow and manage growth profitably and retain its key employees; |
| ● | the Company’s success in retaining or recruiting, or changes required in, its officers, key employees or directors following the Business Combination; |
| ● | the ability of the Company to raise financing in the future; |
| ● | the success, cost and timing of the Company’s product development activities; |
| ● | the commercialization and adoption of VSee Lab’s and iDoc’s existing products and the success of the Company’s future product offerings; |
| ● | the potential attributes and benefits of VSee Lab’s, iDoc’s and the Company’s products and services; |
| ● | the Company’s ability to obtain and maintain regulatory approval for its products, and any related restrictions and limitations of any approved product; |
| ● | the effect of legal, tax and regulatory changes; |
| ● | the Company’s ability to identify, in-license or acquire additional technology; |
| ● | the Company’s ability to maintain existing license, manufacturing and supply agreements; |
| ● | the size and growth potential of the markets for the Company’s products, and the ability of each to serve those markets, either alone or in partnership with others; |
| ● | the pricing of the Company’s products and services and reimbursement for medical procedures conducted using the Company’s products and services; |
5
| ● | the Company’s estimates regarding expenses, revenue, capital requirements and needs for additional financing; |
| ● | the costs related to the Company’s business; |
| ● | the ability of the Company to raise financing in the future; |
| ● | the Company’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| ● | the Company’s ability to compete with other companies currently marketing or engaged in the development of products and services that serve customers engaged in proteomic analysis, many of which have greater financial and marketing resources than the Company; |
| ● | factors relating to the business, operations and financial performance of the Company, including market conditions and global and economic factors beyond the Company’s control; |
| ● | other factors disclosed under the section entitled “Risk Factors” in the this prospectus beginning on page 12 thereof, which is incorporated herein by reference. |
6
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere in this prospectus and in documents incorporated by reference. This summary is not complete and may not contain all the information you should consider before investing in our securities. You should read this entire prospectus and the documents incorporated by reference in this prospectus carefully, especially the risks of investing in our securities discussed under the heading “Risk Factors,” and our financial statements and related notes incorporated by reference in this prospectus before making an investment decision. Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus and the documents incorporated by reference in this prospectus to, the “Company”, “we”, “us”, “our”, “VSee Health” and “VSEE” refer to VSee Health, Inc., a Delaware corporation, and its wholly-owned consolidated subsidiaries VSee Lab, Inc. (“VSee Lab”) and iDoc Virtual Telehealth Solutions, Inc. (“iDoc”).
This prospectus includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements.”
This prospectus includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this prospectus are the property of their respective owners.
Overview
Through our wholly-owned subsidiary VSee Lab, we offer a comprehensive telehealth platform for U.S. hospitals and enterprises. Through VSee Lab, we offer a set of telehealth software building blocks, data connectors, and workflow templates that can be rapidly configured into the client’s workflows. Our offerings allow clinicians without programming experience to configure our building blocks into their existing workflow without requiring programmers — i.e. — no code. In addition, our building blocks allow programmers to increase their productivity with simple coding to piece together our building blocks — i.e. — low code.
At the core of our platform is a comprehensive set of software building blocks for telehealth that include on-demand visits, scheduling appointments, in-take forms, signature for consent and compliance, team coordination, unified communication, remote exam and remote patient monitoring, payments including insurance processing, clinical notes, and administrative control panels and analytics. These set of building blocks can connect to electronic medical record systems such as EPIC and Cerner via HL7, FHIR, and sFTP. Lastly we provide a set of templates to make creating telehealth workflow fast and easy. The entire telehealth platform sits on a scalable server architecture and is HIPAA compliant and SOC2 externally audited. VSee Lab is also GDPR compliant and supports single-sign-on (SSO) and multi-factor-authentication (MFA).
We put telehealth software tools in the hands of clinicians to enable them to make changes without programming so that they can achieve the best patient outcomes. We provide our clients with capabilities specifically built to enable them to collaborate with their clinical and non-clinical colleagues, securely coordinate patient care, conduct virtual patient visits including remote physical exam and remote patient monitoring, and an analytical dashboard to manage their entire telehealth operations from patient satisfaction score to patient wait time to staffing allocations. We empower clinicians to create the workflow they want without waiting for IT; where today, most clinicians feel helpless given that IT departments often cannot give clinicians what they want.
To complement our offerings through VSee Lab, we also provide high acuity patient care solutions through our wholly-owned subsidiary, iDoc. Through iDoc we offer. specialty intensive care unit services by providing physician services in the neurology intensive care unit (neurointensivists), cardiac intensive care unit (surgical and anesthesia intensivists), and medical intensive care units (pulmonary and critical care intensivists).
We strive to be the solutions provider of access to the shortage of intensivists across the care continuum utilizing sophisticated telehealth solutions to bridge the care gap. In a post Covid, physician burnout health care system, we aim to provide a solution to physician burnout and to a lack of patient access to quality intensive care. By using the sophisticated leading telehealth software and hardware devices, we provide access to highly skilled physicians in the highest acuity in patient setting, the ICU. We provide elite physician services in the Intensive care units of major hospital systems and other customers. Our core service delivers general critical care, neurology, EEG reading, and neuro critical care through a custom internal virtual health care technology platform. We also serves a diverse range of customers from large hospital systems to small/micro hospitals, to long-term acute care (LTAC) facilities to the federal prison system and others. We connect critically ill patients to high quality Neurointensivists, general and cardiac intensivists and specialty specific e-consultations and helps to improve outcomes for patients as well as improved productivity and
7
physician burnout while reduced costs for health systems. We have developed a unique quality control program in collaboration with each hospital by development of a hospital specific reporting dashboard to monitor and achieve high quality critical care quality. In addition, current workflows and protocols are evaluated to adjust to incorporate critical care. Continuous process improvement and readjustment of target metrics with the ICU team to maximize patient safety and improve outcomes.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company,” meaning that the market value of our Common Stock held by non-affiliates is less than $700 million and our annual revenue is less than $100 million during the most recently completed fiscal year. We may continue to be a smaller reporting company after this offering if either (i) the market value of our stock held by non-affiliates is less than $250 million as of the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Summary of Risk Factors
Investing in our securities involves risks. You should carefully consider the risks described in “Risk Factors” beginning on page 12 before making a decision to invest in our securities. If any of these risks actually occurs, our business, financial condition and results of operations would likely be materially adversely affected. Some of the risks related our business and industry are summarized below.
| ● | We operate in a competitive industry, and if either is not able to compete effectively, its business, financial condition, and results of operations will be harmed. |
| ● | The level of demand for and market utilization of our software and solutions are subject to a high degree of uncertainty. |
| ● | We may incur losses in the future, and thereafter may never achieve or sustain profitability again. |
| ● | The developing and rapidly evolving nature of our business and the markets in which we operate may make it difficult to evaluate our business. |
| ● | Our business, results of operations, and financial condition may fluctuate on a quarterly and annual basis, which may result in a decline in our stock price if such fluctuations result in a failure to meet any projections that we may provide or the expectations of securities analysts or investors. |
| ● | The business, financial condition and results of our operations have been and may continue to be adversely impacted by the COVID-19 pandemic or similar epidemics in the future or other adverse public health developments, including government responses to such events. |
| ● | Our sales cycles can be long and unpredictable and requires considerable time and expense. As a result, our sales, revenues, and cash flows are difficult to predict and may vary substantially from period to period, which may cause our results of operations to fluctuate significantly. |
| ● | Developments affecting spending by the healthcare industry could adversely affect our revenues. |
| ● | Our practices relies on physician and physician extender’s abilities and therefore there are potential medical malpractice risks that may adversely affect our business. |
| ● | Economic uncertainties or prolonged downturns in the general economy, or political changes, could disproportionately affect the demand for our solutions and harm our business. |
8
| ● | Our existing clients do not continue or renew their contracts with us, renew at lower fee levels or decline to purchase additional services from them, our business may be harmed. |
| ● | Our telemedicine business and growth strategy depends on our ability to maintain and expand our network of established hospital system and telemedicine user bases, board-certified physicians and other provider specialists. If we are unable to maintain and expand our network, our future growth would be limited and ours business would be harmed. |
| ● | Our telemedicine business is dependent on our relationships with affiliated professional entities, which we do not own, to provide medical services, and our business would be harmed if those relationships were disrupted. |
| ● | If we are not able to develop and release new solutions, or successful enhancements, new features and modifications to our existing solutions, our business could be harmed. |
| ● | Any failure to offer high-quality technical support services may harm our relationships with our clients and our financial results. |
| ● | Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to support our continued growth. |
| ● | We depend on our senior management team, and the loss of one or more of these employees or an inability to attract and retain qualified key personnel could harm our business. |
| ● | Our management team has broad discretion in making strategic decisions to execute our growth plans, and there can be no assurance that management’s decisions will result in successful achievement of our business objectives or will not have unintended consequences that negatively impact our growth prospects. |
| ● | We may acquire other companies or technologies, which could divert our management’s attention, result in dilution to our stockholders, and otherwise disrupt our operations, and we may have difficulty integrating any such acquisitions successfully or realizing the anticipated benefits therefrom, any of which could harm our business. |
| ● | If we are unable to grow, or if we fail to manage future growth effectively, our revenues may not increase and we may be unable to implement our business strategy. |
| ● | If the estimates and assumptions we use to determine the size of our total addressable market are inaccurate, our future growth rate may be affected and our business would be harmed. |
| ● | We may not grow at the rates we historically have achieved or at all, even if our key metrics may indicate growth, which may adversely affect the market price of our common stock. |
| ● | We may in the future become subject to litigation, which could be costly and time-consuming to defend. |
| ● | We may become subject to medical liability claims, which could cause us to incur significant expenses, may require us to pay significant damages if not covered by insurance, and could harm our business. |
| ● | Taxing authorities may successfully assert that we should have collected or in the future should collect sales and use, value-added, or similar taxes, and we could be subject to liability with respect to past or future sales, which could adversely affect our results of operations. |
| ● | We will likely require additional capital from equity or debt financings to support business growth, and this capital might not be available on acceptable terms, if at all. |
| ● | The price of our Common Stock and Public Warrants may be volatile, which could result in substantial losses for investors. |
9
| ● | Although we consummated the Business Combination, there is no guarantee that the Public Warrants will ever be in the money, and they may expire worthless and the terms of our Public Warrants may be amended. |
Corporate Information
We were incorporated under the name “Digital Health Acquisition Corp.” on March 30, 2021 under the laws of the State of Delaware for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination, involving one or more other businesses. On June 24, 2024, we completed the Business Combination pursuant to the Business Combination Agreement dated as of November 21, 2023, as amended by the first amendment dated February 13, 2024 and the second amendment dated April 17, 2024 (as amended, the “Business Combination Agreement”) that we entered into with VSee Lab and iDoc. Upon the completion of the Business Combination, we changed our name to “VSee Health, Inc.” and the business of VSee Lab and iDoc became our business.
Our principal executive offices are located at 980 N Federal Hwy #304, Boca Raton, FL 33432, and our telephone number is (561) 672-7068. Our website can be found at https://vseehealth.com/.
10
THE OFFERING
Issuer | VSee Health, Inc. |
| |
Securities being offered | Up to 36,550,000 shares of the Company’s Common Stock consisting of: (i) up to 11,500,000 shares of Common Stock that are issuable upon the exercise of the Public Warrants; and (ii) up to 25,050,000 shares of our Common Stock including (1) up to 25,000,000 shares of Common Stock that we may sell to Dominion, from time to time over the 36 months following July 2, 2024 (which is the sixth trading day following the Closing of the Business Combination), pursuant to the Equity Purchase Agreement and (2) 50,000 shares of Common Stock upon conversion of the Equity Purchase Note that we issued to Dominion on July 2, 2024 as a commitment fee for this equity purchase transaction. |
| |
Shares of Common Stock outstanding prior to this offering | 14,821,019 shares |
| |
Exercise price of Public Warrants | $11.50 per share, subject to adjustments as described herein |
| |
Use of Proceeds | We will not receive any of the proceeds from the sale or other disposition of shares of Common Stock. by Dominion. We may receive up to $50,000,000 in gross proceeds from the sale of shares of Common Stock to Dominion pursuant to the Equity Purchase Agreement from time to time after the date that the registration statement of which this prospectus is a part is declared effective. We will receive proceeds from any exercise of the Public Warrants for cash. Any proceeds we receive, we intend to use for general corporate purposes. See the section of this prospectus titled “Use of Proceeds” for additional information. |
| |
Market for Common Stock and Ticker Symbol | Our Common Stock and Public Warrants are listed on the Nasdaq Capital Market under the symbol “VSEE” and “VSEEW,” respectively. On August 5, 2024, the last reported sale price of our Common Stock and Public Warrants on The Nasdaq Capital Market was $2.67 per share and $0.097 per warrant. |
| |
Risk Factors | Investment in our securities involves a high degree of risk and could result in a loss of your entire investment. See “Risk Factors” beginning on page 12, and the other information included and incorporated by reference in this prospectus for a discussion of the factors you should consider carefully before deciding to invest in our securities. |
The number of shares of Common Stock is based on shares of Common Stock outstanding as of August 5, 2024 and excludes, as of that date, the following: ● 6,158 shares of Series A Preferred Stock convertible into maximum 3,079,000 shares of Common Stock; and ● 2,544,021 shares of Common Stock available for future issuance pursuant to the Company’s 2024 Equity Incentive Plan. | |
11
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below before making an investment decision. Our business, prospects, financial condition, or operating results could be harmed by any of these risks, as well as other risks not known to us or that we consider immaterial as of the date of this prospectus. The trading price of our securities could decline due to any of these risks, and, as a result, you may lose all or part of your investment.
Risks Related to this Offering
The sale or issuance of our Common Stock to Dominion may cause dilution and the sale of the shares of Common Stock acquired by Dominion, or the perception that such sales may occur, could cause the price of our Common Stock to fall.
The purchase price for the shares that we may sell to Dominion under the Equity Purchase Agreement will fluctuate based on the price of our Common Stock. Depending on market liquidity at the time, sales of such shares may cause the trading price of our Common Stock to fall.
Subject to the terms of the Equity Purchase Agreement, we generally have the right to control the timing and amount of any future sales of our shares to Dominion. The extent to which we rely on Dominion as a source of funding will depend on a number of factors, including the prevailing market price of our Common Stock and the extent to which we are able to secure working capital from other sources and other factors to be determined by us. We may ultimately decide to sell to Dominion all, some, or none of the shares of our Common Stock that may be available for us to sell pursuant to the Equity Purchase Agreement. When we sell shares to Dominion and when Dominion converts the Equity Purchase Note into shares of our Common Stock, after Dominion has acquired the shares, Dominion may resell all or some of those shares at any time or from time to time in its discretion. Therefore, sales to Dominion by us could result in substantial dilution to the interests of other holders of our Common Stock. Additionally, the sale of a substantial number of shares of our Common Stock to Dominion, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
Dominion will pay less than the then-prevailing market price for our Common Stock, which could cause the price of our Common Stock to decline.
The purchase price of our Common Stock to be sold to Dominion under the Equity Purchase Agreements is derived from the market price of our Common Stock on Nasdaq. Shares to be sold to Dominion pursuant to the Equity Purchase Agreement will be purchased at a discounted price. We may effect sales to Dominion at a purchase price per share equal to the price per share pursuant to the purchase notice under the Equity Purchase Agreement that we deliver to Dominion (the “Advance Notice”) by multiplying (a) the lowest average daily volume weighted average price (“VWAP”) during the five days prior to our submission of Advance Notice by (2) 92%. See section entitled “The Committed Equity Financing Transaction” for more information.
As a result of this pricing structure, Dominion may sell the shares they receive immediately after receipt of such shares, which could cause the price of our Common Stock to decrease.
We may require additional financing to sustain our operations, without which we may not be able to continue operations, and the terms of subsequent financings may adversely impact our stockholders.
The extent we rely on Dominion as a source of funding will depend on a number of factors, including the prevailing market price of our Common Stock and the extent to which we are able to secure working and other capital from other sources. If obtaining sufficient funding from Dominion were to prove unavailable or prohibitively dilutive, we will need to secure another source of funding in order to satisfy our working and other capital needs. Even if we were to sell to Dominion all of the shares of Common Stock available for sale to Dominion under the Equity Purchase Agreement, we may still need additional capital to fully implement our business, operating and development plans. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences may be a material adverse effect on our business, operating results, financial condition and prospects. Depending on the type and the terms of any financing we pursue, stockholders’ rights and the value of their investment in our Common Stock could be reduced. A financing could involve one or more types of securities including Common Stock, convertible debt or warrants to acquire common stock. These securities could be issued at or below the then prevailing market price for our Common Stock. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our business, operating results, financial condition and prospects.
12
Our management will have broad discretion over the use of the net proceeds from our sale of shares of Common Stock to the Investor, you may not agree with how we use the proceeds and the proceeds may not be invested successfully.
Our management will have broad discretion as to the use of the net proceeds from our sale of shares of Common Stock to Dominion, and we could use them for purposes other than those contemplated at the time of commencement of this offering. Accordingly, you will be relying on the judgment of our management with regard to the use of those net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending their use, we may invest those net proceeds in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flows.
It is not possible to predict the actual number of shares we will sell under the Equity Purchase Agreement to Dominion, or the actual gross proceeds resulting from those sales.
Because the purchase price per share to be paid by Dominion for the shares of Common Stock that we may elect to sell to Dominion under the Equity Purchase Agreement, if any, will fluctuate based on the market prices of our common stock during the applicable period for each purchase made pursuant to the Equity Purchase Agreement, if any, it is not possible for us to predict, as of the date of this prospectus and prior to any such sales, the number of shares of Common Stock that we will sell to Dominion under the Equity Purchase Agreement, the purchase price per share that Dominion will pay for shares purchased from us under the Equity Purchase Agreement, or the aggregate gross proceeds that we will receive from those purchases by Dominion under the Equity Purchase Agreement, if any.
Investors who buy shares at different times will likely pay different prices.
Pursuant to the Equity Purchase Agreement, we will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold to Dominion. If and when we do elect to sell shares of our common stock to Dominion pursuant to the Equity Purchase Agreement, after it has acquired such shares, Dominion may resell all, some or none of such shares at any time or from time to time in its discretion and at different prices. As a result, the other investors who purchase shares from Dominion in this offering at different times will likely pay different prices for those shares, and so may experience different levels of dilution and in some cases substantial dilution and different outcomes in their investment results.
Our commitment to issue shares of Common Stock pursuant to the terms of the Equity Purchase Agreement could encourage short sales by third parties, which could contribute to the future decline of our stock price.
Our commitment to issue shares of Common Stock pursuant to the terms of the Equity Purchase Agreement and the Equity Purchase Note has the potential to cause significant downward pressure on the price of our Common Stock. In such an environment, short sellers may contribute exacerbate any decline of our stock price. If there are significant short sales of our Common Stock, the share price of our Common Stock may decline more than it would in an environment without such activity. This may cause other holders of our Common Stock to sell their shares. If there are many more shares of our Common Stock on the market for sale than the market will absorb, the price of our Common Stock will likely decline.
Although pursuant to the Equity Purchase Agreement and during the term thereof, Dominion shall not participate in short sales of our Common Stock or engage in hedging transactions, other third party investors may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of Common Stock in the course of hedging in positions they assume. Such third-party investors may also loan or pledge shares of our Common Stock to broker-dealers that in turn may sell such shares. Such activity could cause a decline in the market price of the shares of our Common Stock.
We may amend the terms of the warrants in a manner that may be adverse to holders of public warrants with the approval by the holders of at least 50% of the then outstanding public warrants. As a result, the exercise price of the warrants could be increased, the exercise period could be shortened and the number of shares of common stock purchasable upon exercise of a warrant could be decreased, all without approval of each warrant affected.
Our warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as Warrant Agent, and us. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants.
13
Accordingly, we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash, shorten the exercise period or decrease the number of shares of common stock, as applicable, purchasable upon exercise of a warrant.
Although we consummated the Business Combination, there is no guarantee that the Public Warrants will ever be in the money, and they may expire worthless and the terms of our Public Warrants may be amended.
The exercise price for the Public Warrants is $11.50 per share of common stock. There is no guarantee that the Public Warrants will ever be in the money prior to their expiration, and as such, the Public Warrants may expire worthless.
Risks Related to Our Operation of Business
We operate in a competitive industry, and if either is not able to compete effectively, its business, financial condition, and results of operations will be harmed.
The telemedicine market is rapidly evolving and highly competitive. We expect competition to intensify in the future as existing competitors and new entrants introduce new telemedicine services and software platforms or other technology to U.S. healthcare providers, particularly hospitals and healthcare systems. We currently face competition from a range of companies, including other incumbent providers of telemedicine consultation services and specialized software providers, that are continuing to grow and enhance their service offerings and develop more sophisticated and effective transaction and service platforms. In addition, large, well-financed healthcare providers have in some cases developed their own telemedicine services and technologies utilizing their own and third-party platforms and may provide these solutions to their patients. Electronic medical record vendors could build telemedicine functionality directly into their existing systems for healthcare providers instead of utilizing our solution. Competition from specialized telemedicine services and software providers, healthcare providers and other parties will result in continued pricing pressures, which is likely to lead to price declines in certain of our services, which could negatively impact our sales, profitability and market share.
Some of our competitors may have greater name recognition, longer operating histories and significantly greater resources than we do. Further, our current or potential competitors may be acquired by third parties with greater available resources. As a result, our competitors may be able to respond more quickly and effectively than either can to new or changing opportunities, technologies, standards or client requirements and may have the ability to initiate or withstand substantial price competition. In addition, current and potential competitors have established, and may in the future establish, cooperative relationships with vendors of complementary products, technologies or services to increase the availability of their solutions in the marketplace. Accordingly, new competitors or alliances may emerge that have greater market share, a larger client base, more widely adopted proprietary technologies, greater marketing expertise, greater financial resources and larger sales forces than we have, which could put eus at a competitive disadvantage. Our competitors could also be better positioned to serve certain segments of the telemedicine market, which could create additional price pressure. In light of these factors, even if the solutions we offer are more effective than those of our competitors, current or potential clients may accept competitive solutions in lieu of purchasing our solutions. If we are unable to compete successfully in the telemedicine industry, our business, financial condition and results of operations will be harmed.
Moreover, we expect that competition will continue to increase as a result of consolidation in the healthcare industry. Many healthcare industry participants are consolidating to create integrated healthcare delivery systems with greater market power. As provider networks and managed care organizations consolidate, thus decreasing the number of market participants, competition to provide services like ours will become more intense, and the importance of establishing and maintaining relationships with key industry participants will become greater. These industry participants may try to use their market power to negotiate price reductions for our telemedicine consultation and platform services. If we are forced to reduce our prices and are unable to achieve a corresponding reduction in our expenses, our revenues would decrease, which could harm our business.
The level of demand for and market utilization of our software and solutions are subject to a high degree of uncertainty.
The market for telemedicine services and related technology is characterized by rapid change. As telemedicine specialty consultation workflows and related business drivers continue to evolve, the level of demand for and market utilization of our telemedicine services and platform remain subject to a high degree of uncertainty. Our success will depend to a substantial extent on the willingness of healthcare organizations to use, and to increase the frequency and extent of their utilization of, our solutions and our
14
ability to demonstrate the value of telemedicine to healthcare providers. If healthcare organizations do not recognize or acknowledge the benefits of these telemedicine services platform or if either is unable to reduce healthcare costs or generate positive health outcomes, then the market for our solutions might not develop at all, or it might develop more slowly than we expect. Similarly, negative publicity regarding patient confidentiality and privacy in the context of technology-enabled healthcare or concerns about our solutions or the telemedicine market as whole could limit market acceptance of our solutions. If clients do not perceive the benefits of our solutions, then the market may not develop at all, or it may develop more slowly than we expect. Achieving and maintaining market acceptance of our solutions could be negatively affected by many factors, including:
| ● | the quality, popularity, pricing and timing of telemedicine consultation services utilized by us and our competitors; |
| ● | general economic conditions, particularly economic conditions adversely affecting discretionary and reimbursable healthcare spending; |
| ● | federal and state policy initiatives impacting the need for, fraud and abuse concerns regarding, and pricing of telemedicine services; |
| ● | changes in client needs and preferences; |
| ● | the development of specialty care practice standards or industry norms applicable to telemedicine consultation services; |
| ● | the availability of other forms of medical and telemedicine assistance; |
| ● | lack of additional evidence or peer-reviewed publication of clinical evidence supporting the safety, ease-of-use, cost-savings or other perceived benefits of our solutions over competitive products or other currently available methodologies; |
| ● | perceived risks associated with the use of our solutions or similar products or technologies generally; and |
| ● | critical reviews and public tastes and preferences, all of which change rapidly and cannot be predicted. |
In addition, our solutions may be perceived by clients or potential clients to be more complicated or less effective than traditional approaches, and may be unwilling to change their current healthcare practices. Healthcare providers are often slow to change their medical treatment practices for a variety of reasons, including perceived liability risks arising from the use of new products and services and the uncertainty of third-party reimbursement. Accordingly, healthcare providers may not recommend our solutions until there is sufficient evidence to convince them to alter their current approach. Any of these factors could adversely affect the demand for and market utilization of our solutions, which would harm its business.
We may incur losses in the future, and thereafter may never achieve or sustain profitability again.
We expect our costs will increase in the foreseeable future and we may incur losses. We also expect to invest significant additional funds towards enhancing our services and platform, growing our business and operating as a public company and as we continue to invest in increasing our hospital and healthcare system client base, expanding our operations, hiring additional employees, and developing future offerings. These efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues sufficiently to offset these higher expenses. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. As we expand, we may not generate positive cash flow from operations in any given period. If we are not able to achieve or maintain positive cash flow in the long term, we will require additional financing, which may not be available on favorable terms or at all or which would be dilutive to our stockholders. If we are unable to address these risks and challenges successfully as we encounter them, our business may be harmed. Our failure to achieve or maintain profitability or positive cash flow could negatively affect the value of our common stock.
The developing and rapidly evolving nature of our business and the markets in which we operate may make it difficult to evaluate our business.
We have been creating offerings for the developing and rapidly evolving market for telemedicine services. Each of VSee Lab and iDoc and telemedicine overall has limited operating history with their current solutions and business model makes it difficult to evaluate their business and prospects. It is difficult to evaluate trends that may affect our business and whether our expansion will be
15
profitable. You should consider our business and prospects in light of the risks and difficulties either VSee Lab and/or iDoc encounter or may encounter. These risks and difficulties include those frequently experienced by growing companies in rapidly changing industries, such as determining appropriate investments of our limited resources, market adoption of our existing and future solutions, competition from other companies, acquiring and retaining clients, hiring, integrating, training and retaining skilled personnel, developing new solutions, determining prices for our solutions, unforeseen expenses, and challenges in forecasting accuracy. If we have difficulty launching new solutions, our reputation and business may be harmed. Additional risks include our ability to effectively manage growth and to store, protect and use personal data in compliance with governmental regulation, contractual obligations and other legal obligations related to privacy and security. If our assumptions regarding these and other similar risks and uncertainties, which we use to plan our business, are incorrect or change as we gain more experience operating our businesses or due to changes in our industry, or if we do not address these challenges successfully, our business, financial condition and results of operations could differ materially from our expectations and our business could suffer.
Our business, results of operations, and financial condition may fluctuate on a quarterly and annual basis, which may result in a decline in our stock price if such fluctuations result in a failure to meet any projections that we may provide or the expectations of securities analysts or investors.
Our operating results have in the past and could in the future vary significantly from quarter-to-quarter and year-to-year and may fail to match our past performance, our projections or the expectations of securities analysts because of a variety of factors, many of which are outside of our control. As a result, we may not be able to accurately forecast our operating results and growth rate. Any of these events could cause the market price of our common stock to fluctuate. Factors that may contribute to the variability of our operating results include:
| ● | the addition or loss of large hospital and healthcare system clients, including through acquisitions or consolidations of such clients; |
| ● | seasonal and other variations in the timing of our sales and implementation cycles, especially in the case of our large clients; |
| ● | the timing of recognition of revenue, including possible delays in the recognition of revenue due to sometimes unpredictable implementation timelines; |
| ● | the amount and timing of operating expenses related to the maintenance and expansion of our business, operations and infrastructure; |
| ● | the timing and success of introductions of new products and services by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, hospital and healthcare system clients or strategic partners; |
| ● | hospital and healthcare system client renewal rates and the timing and terms of such renewals; |
| ● | the mix of services sold and utilization volume of our services during a period; |
| ● | the timing of expenses related to the development or acquisition of technologies or businesses and potential future charges for impairment of goodwill from acquired companies; |
| ● | technical difficulties or interruptions in our services; |
| ● | breaches of information security or privacy; |
| ● | our ability to hire and retain qualified personnel, including cross-licensing and privileging each of our physician networks; |
| ● | changes in the structure of healthcare provider and payment systems; |
| ● | changes in the legislative or regulatory environment, including with respect to healthcare, privacy, or data protection, or enforcement by government regulators, including fines, orders, or consent decrees; |
16
| ● | the cost and potential outcomes of ongoing or future regulatory investigations or examinations, or of future litigation; |
| ● | political, economic and social instability, including terrorist activities and health epidemics (including the COVID-19 pandemic), and any disruption these events may cause to the global economy; and |
| ● | changes in business or macroeconomic conditions. |
The impact of one or more of the foregoing and other factors may cause our operating results to vary significantly. As such, we believe that quarter-to-quarter and year-to-year comparisons of our operating results may not be meaningful and should not be relied upon as an indication of future performance.
The business, financial condition and results of our operations have been and may continue to be adversely impacted by the COVID-19 pandemic or similar epidemics in the future or other adverse public health developments, including government responses to such events.
Since December 2019, SARS-CoV-2, and the resulting disease, COVID-19, has spread to almost every country in the world and all 50 states within the United States. Global health concerns relating to the outbreak of COVID-19 have impacted the macroeconomic environment, and the outbreak has significantly increased economic uncertainty. In addition, any industry connected to the delivery of healthcare services has been significantly disrupted in the attempt to respond to the needs created by the outbreak. The duration and severity of this pandemic is unknown, and the extent of the business disruption and financial impact depend on factors beyond our knowledge and control.
COVID-19 pandemic-related market changes that have caused an increased demand in telehealth solutions and other increases in health systems spending may cause us to invest in additional solutions to meet these needs and may also cause an increase in competitive offerings. If we are not able to make a return on those investments, meet the market demands, or effectively compete in the marketplace, its business results may suffer. Also, the financial impact of COVID-19 or another pandemic, epidemic, or outbreak of an infectious disease may lead to an overall decrease in healthcare spending due to a potential economic downturn and overall uncertainty causing healthcare expenditures to be concentrated in emergency care, which may cause a material impact to our business.
While the potential economic impact brought by and the duration of any pandemic, epidemic, or outbreak of an infectious disease, including COVID-19, may be difficult to assess or predict, the widespread COVID-19 pandemic has resulted in, and may continue to result in, significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. The impact of any pandemic, epidemic, or outbreak of an infectious disease, including COVID-19, on the needs, expectations, and spending levels of our customers could impact our ability to maintain or grow our business and as a result our operating and financial results could be adversely affected.
The full extent to which the outbreak of COVID-19 will impact our business, results of operations, and financial condition is still unknown and will depend on future developments, which are highly uncertain and cannot be predicted, including, but not limited to, the duration and spread of the outbreak, its severity, the actions to contain the virus or treat its impact, and how quickly and to what extent normal economic and operating conditions can resume. Even after the outbreak of COVID-19 has subsided, we may experience materially adverse impacts to our business as a result of its global economic impact, including any recession that has occurred or may occur in the future.
Our sales cycles can be long and unpredictable and requires considerable time and expense. As a result, our sales, revenues, and cash flows are difficult to predict and may vary substantially from period to period, which may cause our results of operations to fluctuate significantly.
The sales cycle for our solutions from initial contact with a potential lead to contract execution and implementation varies widely by client. Some of our clients undertake a significant and prolonged evaluation process, including to determine whether our solutions meet their unique telemedicine service needs, which frequently involves evaluation of not only our solutions but also an evaluation of those of our competitors, which has in the past resulted in extended sales cycles. Our sales efforts involve educating our clients about the use, technical capabilities and potential benefits of our solutions. Moreover, our large hospital and healthcare system clients often begin to deploy our solutions on a limited basis, but nevertheless demand extensive configuration, integration services and pricing concessions, which increases our upfront investment in the sales effort with no guarantee that these clients will deploy our solution widely enough across their organization to justify our substantial upfront investment. It is possible that in the future that we may experience even longer sales cycles, more complex client needs, higher upfront sales costs and less predictability in completing some
17
of our sales, including as a result of the COVID-19 pandemic, we continue to expand our direct sales force, expand into new territories and market additional solutions and services. If our sales cycles lengthen or our substantial upfront sales and implementation investments do not result in sufficient sales to justify our investments, our business could be harmed.
Developments affecting spending by the healthcare industry could adversely affect our revenues.
The U.S. healthcare industry has changed significantly in recent years, and we expect that significant changes will continue to occur. General reductions in expenditures by healthcare industry participants could result from, among other things:
| ● | government regulations or private initiatives that affect the manner in which healthcare providers interact with patients, payors or other healthcare industry participants, including changes in pricing or means of delivery of healthcare products and services; |
| ● | consolidation of healthcare industry participants; |
| ● | reductions in government funding for healthcare, in particular telehealth; and |
| ● | adverse changes in business or economic conditions affecting healthcare payors or providers or other healthcare industry participants. |
Any of these changes in healthcare spending could adversely affect our revenues. Even if general expenditures by industry participants remain the same or increase, developments in the healthcare industry may result in reduced spending in some or all of the specific market segments that we serve now or in the future. However, the timing and impact of developments in the healthcare industry are difficult to predict. We cannot assure you that the demand for our solutions and services will continue to exist at current levels or that we will have adequate technical, financial, and marketing resources to react to changes in the healthcare industry.
Our practices relies on physician and physician extender’s abilities and therefore there are potential medical malpractice risks that may adversely affect our business.
Our business may expose them to potential medical malpractice, professional negligence, or other related actions or claims that are inherent in the provision of healthcare services. These claims, with or without merit, could cause them to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our core business, harm our reputation and adversely affect our ability to attract and retain clients, any of which could have a material adverse effect on our business, financial condition and results of operations.
Economic uncertainties or prolonged downturns in the general economy, or political changes, could disproportionately affect the demand for our solutions and harm our business.
Current or future economic uncertainties or prolonged downturns could harm our business. Negative conditions in the general economy in the United States, including conditions resulting from changes in gross domestic product growth, financial and credit market fluctuations, political deadlock, natural catastrophes, pandemics, social unrest, warfare and terrorist attacks, could cause a decrease in funds available to our clients and potential clients and negatively affect the growth rate of our business.
These economic conditions may make it difficult for us and our clients to forecast and plan future budgetary decisions or business activities accurately, and they could cause our clients to reevaluate their decisions to purchase our solutions, which could delay and lengthen our sales cycles or result in cancellations of planned purchases. Furthermore, during challenging economic times or as a result of political changes, our clients may tighten their budgets and face constraints in gaining timely access to sufficient funding or other credit, which could result in an impairment of their ability to make timely payments to us. In turn, we may be required to increase our allowance for doubtful accounts, which would adversely affect our financial results.
To the extent our solutions are perceived by clients and potential clients to be discretionary, our revenues may be disproportionately affected by delays or reductions in general information technology and telemedicine spending. Competitors may respond to market conditions by lowering prices and attempting to lure away our clients. In addition, the increased pace of consolidation in the healthcare industry may result in reduced overall spending on our solutions.
18
We cannot predict the timing, strength or duration of any economic slowdown, instability or recovery, generally or within the healthcare industry, or the effect of political changes. If the economic conditions of the general economy or the healthcare industry do not improve, or worsen from present levels, our business could be harmed.
Our existing clients do not continue or renew their contracts with us, renew at lower fee levels or decline to purchase additional services from them, our business may be harmed.
We expect to derive a significant portion of our revenues from renewal of existing client contracts and sales of additional services to existing clients. Factors that may affect our ability to sell additional solutions and services include, but are not limited to, the following:
| ● | the price, performance and functionality of our software solutions; |
| ● | the availability, price, performance and functionality of competing solutions; |
| ● | our ability to develop and sell complementary solutions and services; |
| ● | changes in healthcare laws, regulations or trends; and |
| ● | the business environment and strategic priorities of our clients. |
Most of our clients have no obligation to renew their subscriptions for our solutions after the initial term expires. In addition, our clients may negotiate terms less advantageous to them upon renewal, which may reduce our revenues from these clients. If our clients fail to renew their contracts, renew our contracts upon less favorable terms or at lower fee levels or fail to purchase new solutions and services from them, our revenues may decline, or our future revenue growth may be constrained.
Our telemedicine business and growth strategy depends on our ability to maintain and expand our network of established hospital system and telemedicine user bases, board-certified physicians and other provider specialists. If we are unable to maintain and expand our network, our future growth would be limited and ours business would be harmed.
Our success is dependent upon our continued ability to maintain a network of established health care systems providers and established, board-certified physicians and other provider specialists. Our ability to develop and maintain satisfactory relationships with these providers also may be negatively impacted by other factors not associated with either of them, such as changes in Medicare and/or Medicaid reimbursement levels and other pressures on healthcare providers and consolidation activity among hospitals, physician groups and healthcare providers. The failure to maintain or to secure new cost-effective provider contracts may result in a loss of or inability to grow our client base, higher costs, healthcare provider network disruptions, less attractive service for our clients and/or difficulty in meeting regulatory requirements, any of which could harm our business.
Our telemedicine business is dependent on our relationships with affiliated professional entities, which we do not own, to provide medical services, and our business would be harmed if those relationships were disrupted.
There is a risk that U.S. state authorities in some jurisdictions may find that our contractual relationships with our physicians and physician extenders who provide telehealth services violate state’s prohibition against the corporate practice of medicine and related professions. The corporate practice of medicine prohibition exists in some form, by statute, regulation, board of medicine or attorney general guidance, or case law, in most states, though there is broad variation between state application and enforcement of the doctrine makes an exact count difficult. These laws generally prohibit the practice of medicine or related professions by lay persons or entities and are intended to prevent unlicensed persons or entities from interfering with or inappropriately influencing a physician or physician extenders’ professional judgment. The extent to which each state considers particular actions or contractual relationships between us and our providers to constitute improper influence of professional judgment varies across the states and is subject to change and to evolving interpretations by state boards of medicine and state attorneys general, among others. As such, we must monitor our compliance with laws in every jurisdiction in which we operate on an ongoing basis, but we cannot guarantee that subsequent interpretation of the corporate practice of medicine or related professions laws will not circumscribe our business operations. State corporate practice of medicine doctrines also often impose penalties on the licensed providers themselves for aiding the corporate practice of medicine, which could discourage physicians from participating in our network of providers.
19
A material change in corporate practice of medicine interpretation could impact our operations and could impair our ability to provide services to our clients and harm our business.
If we are not able to develop and release new solutions, or successful enhancements, new features and modifications to our existing solutions, our business could be harmed.
To date, we derived a substantial majority of our revenues from sales of solutions from our telemedicine software platforms, and our longer-term results of operations and continued growth will depend on our ability successfully to develop and market new solutions and add in additional modules and feature in a timely manner. In addition, we have invested, and will continue to invest, significant resources in research and development to enhance our existing solutions. If existing clients are not willing to make additional payments for such new solutions, or if new clients do not value such new solutions or enhancements, it could harm our business. If we are unable to predict client and user preferences or other industry changes, or if we are unable to enhance or modify our solutions on a timely basis, we may lose clients. In addition, our results of operations would suffer if our innovations are not responsive to the needs of our clients or if such innovation are not appropriately timed with market opportunity or effectively brought to market. Delays in launching new solutions may open windows of opportunity for new and existing competitors to erode our market share and may negatively impact our revenues and profitability.
Any failure to offer high-quality technical support services may harm our relationships with our clients and our financial results.
Our clients depend on our support organization to resolve any technical issues relating to our services. In addition, our sales process is highly dependent on the quality of our solutions, our business reputation and on strong recommendations from our existing clients. Any failure to maintain high-quality and highly-responsive technical support, or a market perception that we do not maintain high-quality and highly-responsive support, could harm our reputation, adversely affect our ability to sell our solutions to existing and prospective clients, and harm our business.
We offer technical support services with our solutions and may be unable to respond quickly enough to accommodate short-term increases in demand for support services, particularly as we increase the size of our client base. We may also be unable to modify the format of our support services to compete with changes in support services provided by competitors. It is difficult to predict demand for technical support services and if demand increases significantly, we may be unable to provide satisfactory support services to our clients. Additionally, increased demand for these services, without corresponding revenue, could increase costs and adversely affect our results of operations.
Because competition for qualified personnel is intense, we may not be able to attract and retain the highly skilled employees we need to support our continued growth.
To continue to execute on our growth plan, we must attract and retain highly qualified personnel. The pool of qualified personnel with experience working in the healthcare market is limited overall and the competition to hire them is intense. As such, we may not be successful in continuing to attract and retain qualified personnel. We have from time to time in the past experienced, and expect to continue to experience in the future, difficulty in hiring and retaining highly skilled employees with appropriate qualifications. In addition, our search for replacements for departed employees may cause uncertainty regarding the future of our business, impact employee hiring and retention, and adversely impact our revenue, financial condition and results of operations. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects could be harmed.
We depend on our senior management team, and the loss of one or more of these employees or an inability to attract and retain qualified key personnel could harm our business.
Our success depends largely upon the continued services of our key executive officers. These executive officers are “at-will” employees and therefore may terminate employment with us at any time with no advance notice. We also rely on our leadership team in the areas of research and development, marketing, services and general and administrative functions. From time to time, there may be changes in our executive management team resulting from the hiring or departure of executives, which could disrupt our business. The replacement of one or more of our executive officers or other key employees would likely involve significant time and costs and may significantly delay or prevent the achievement of our business objectives. In addition, volatility or lack of performance in our stock price may affect our ability to attract and retain replacements should key personnel depart. If we are not able to retain any of our key personnel, our business could be harmed.
20
Our management team has broad discretion in making strategic decisions to execute our growth plans, and there can be no assurance that management’s decisions will result in successful achievement of our business objectives or will not have unintended consequences that negatively impact our growth prospects.
Our management has broad discretion in making strategic decisions to execute our growth plans and may devote time and company resources to new or expanded solution offerings, potential acquisitions, prospective customers or other initiatives that do not necessarily improve our operating results or contribute to our growth. Management’s failure to make strategic decisions that are ultimately accretive to our growth may result in unfavorable returns and uncertainty about its prospects, each of which could cause the price of our Common Stock to decline.
We may acquire other companies or technologies, which could divert our management’s attention, result in dilution to our stockholders, and otherwise disrupt our operations, and we may have difficulty integrating any such acquisitions successfully or realizing the anticipated benefits therefrom, any of which could harm our business.
We intend to acquire or invest in additional businesses, applications and services or technologies that we believe could complement or expand our solutions, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated.
In addition, if we acquire additional businesses, we may not be able to integrate the acquired personnel, operations and technologies successfully, or effectively manage the combined business following the acquisition. We also may not achieve the anticipated benefits from the acquired business due to a number of factors, including, but not limited to:
| ● | inability to integrate or benefit from acquired technologies or services in a profitable manner; |
| ● | unanticipated costs or liabilities, including legal liabilities, associated with the acquisition; |
| ● | difficulty integrating the accounting systems, operations and personnel of the acquired business; |
| ● | difficulties and additional expenses associated with supporting legacy products and hosting infrastructure of the acquired business; |
| ● | difficulty converting the clients of the acquired business onto our platform and contract terms, including disparities in the revenue, licensing, support or professional services model of the acquired company; |
| ● | diversion of management’s attention from other business concerns; |
| ● | adverse effects to our existing business relationships with business partners and clients as a result of acquisitions; |
| ● | the potential loss of key employees or contractors; |
| ● | use of resources that are needed in other parts of our business; and |
| ● | use of substantial portions of our available cash to consummate the acquisition. |
In addition, a significant portion of the purchase price of businesses we acquire may be allocated to acquired goodwill and other intangible assets, which must be assessed for impairment at least annually. In the future, if our acquisitions do not yield expected returns, we may be required to take charges to our results of operations based on this impairment assessment process, which could adversely affect our results of operations.
Acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our results of operations or cause the market price of our common stock to decline. In addition, if an acquired business fails to meet our expectations, our business may be harmed.
21
If we are unable to grow, or if we fail to manage future growth effectively, our revenues may not increase and we may be unable to implement our business strategy.
Our future success will depend upon our ability to grow, and if we are unable to manage our growth effectively, we may incur unexpected expenses and be unable to meet our clients’ requirements, all of which could harm our business. A key aspect to managing our growth is our ability to scale each of VSee Lab’s and iDoc’s capabilities, including in response to unexpected shifts in demand for telemedicine. To manage our current and anticipated future growth effectively, we must continue to maintain and enhance our IT infrastructure, financial and accounting systems and controls. We must also attract, train and retain a significant number of board-certified physicians, sales and marketing personnel, client support personnel, professional services personnel, software engineers, technical personnel and management personnel, and the availability of such personnel, in particular physicians and software engineers, may be constrained.
Our growth will depend on the acceptance of our solutions as a suitable supplement to traditional healthcare delivery systems and on our ability to overcome operational challenges. Our business model and solutions could lose our viability as a supplement to traditional healthcare delivery systems due to client dissatisfaction or new alternative solutions. If we are unable to address the needs of our clients, or our clients are dissatisfied with the quality of our solutions, our clients may not renew our contracts, seek to cancel or terminate their relationship with us or renew on less favorable terms, any of which could cause our annual net dollar retention rate to decrease.
As we continue to grow, including from the integration of employees and businesses acquired in connection with previous or future acquisitions, we may find it difficult to maintain important aspects of our corporate culture, which could negatively affect our profitability and our ability to retain and recruit qualified personnel who are essential for our future success. If we do not effectively manage our growth, we may not be able to execute on our business plan, respond to competitive pressures, take advantage of market opportunities, satisfy client requirements or maintain high-quality solutions. Additionally, we may not be able to expand and upgrade our systems and infrastructure to accommodate future growth.
Failure to effectively manage our growth could also lead us to over-invest or under-invest in development and operations, result in weaknesses in our infrastructure, systems or controls, give rise to operational mistakes, financial losses, loss of productivity or business opportunities and result in loss of employees and reduced productivity of remaining employees. Our growth is expected to require significant capital expenditures and may divert financial resources from other projects such as the development of new solutions and services. If we are unable to effectively manage our growth our expenses may increase more than expected, our revenues may not increase or may grow more slowly than expected and we may be unable to implement our business strategy. The quality of our services may also suffer, which could negatively affect our reputation and harm our ability to attract and retain clients.
If the estimates and assumptions we use to determine the size of our total addressable market are inaccurate, our future growth rate may be affected and our business would be harmed.
Market opportunity estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may prove to be inaccurate. Even if the markets in which we compete meet our size estimates and forecasted growth, our business could fail to grow at similar rates, if at all. The principal assumptions relating to our market opportunity include all hospitals in the United States adopting outsourced clinical resources via telemedicine and that we can successfully add specialties to tour solutions beyond those currently offered today. Our market opportunity is also based on the assumption that our existing and future offerings will be more attractive to our clients and potential clients than competing solutions. If these assumptions prove inaccurate, our business could be harmed.
We may not grow at the rates we historically have achieved or at all, even if our key metrics may indicate growth, which may adversely affect the market price of our common stock.
We have experienced significant growth in recent years. Future revenues may not grow at these same rates or may decline. Our future growth will depend, in part, on our ability to grow our revenues from existing clients, to complete sales to potential future clients, to expand our client base, and to develop new solutions and services. We can provide no assurances that we will be successful in executing on these growth strategies or that, even if our key metrics would indicate future growth, we will continue to grow our revenues or to generate net income. Our ability to execute on our existing sales pipeline, create additional sales pipelines and expand our client base depends on, among other things, the attractiveness of our services relative to those offered by our competitors, our ability to demonstrate the value of our existing and future services and our ability to attract and retain a sufficient number of qualified
22
sales and marketing leadership and support personnel. In addition, our existing clients may be slower to adopt our services than we currently anticipate, which could harm our business and growth prospects and adversely affect the market price of our common stock.
We may in the future become subject to litigation, which could be costly and time-consuming to defend.
We may become subject to legal proceedings and claims that arise in the ordinary course of business, such as claims brought by our clients in connection with commercial disputes or employment claims made by our current or former associates. Litigation may result in substantial costs and may divert management’s attention and resources, which may substantially harm our business, financial condition and results of operations. Insurance may not cover such claims, may not provide sufficient payments to cover all of the costs to resolve one or more such claims and may not continue to be available on terms acceptable to us. Resolution of some of these types of matters against us may result in our having to pay significant fines, judgments, or settlements, which, if uninsured, or if the fines, judgments, and settlements exceed insured levels, could adversely affect our results of operations and cash flows, thereby harming our business and stock price. For example, fines or assessments could be levied against us under domestic or foreign data privacy laws (such as the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), the General Data Protection Regulation (“GDPR”), or the California Consumer Privacy Act of 2018 (“CCPA”)) or under authority of privacy enforcing governmental entities (such as the Federal Trade Commission (“FTC”), or the U.S. Department of Health and Human Services (“HHS”)) or as a result of private actions, such as class actions based on data breaches or based on private rights of action (such as that contained in the CCPA). Certain litigation or the resolution of certain litigation may affect the availability or cost of some of our insurance coverage, which could adversely affect our results of operations and cash flows, expose us to increased risks that would be uninsured and adversely affect our ability to attract directors and officers. In addition, such litigation could result in increased scrutiny by government authorities having authority over our business, such as the FTC, the HHS, Office for Civil Rights (“OCR”), and state attorneys general.
We may become subject to medical liability claims, which could cause us to incur significant expenses, may require us to pay significant damages if not covered by insurance, and could harm our business.
Because our business delivers telehealth services to patients, each faces the risk of medical liability claims against us and our affiliated professional entities. We and our affiliated professional entities have in the past and may in the future be subject to medical liability claims and, if these claims are successful, substantial damage awards. Although we maintain insurance covering medical malpractice claims in amounts that we believe is appropriate in light of the risks attendant to our business, we cannot predict the outcomes of medical malpractice cases, the effect that any claims of this nature, regardless of their ultimate outcome, could have on our business or reputation or on our ability to attract and retain clients. Professional liability insurance is expensive and insurance premiums may increase significantly in the future, particularly as we expand our services. As a result, adequate professional liability insurance may not be available to our providers in the future at acceptable costs or at all.
Any claims made against us that are not fully covered by insurance could be costly to defend against, result in substantial damage awards against us and divert the attention of our management and our providers from our operations, which could harm our business. In addition, any claims may harm our business or reputation.
Taxing authorities may successfully assert that we should have collected or in the future should collect sales and use, value-added, or similar taxes, and we could be subject to liability with respect to past or future sales, which could adversely affect our results of operations.
We do not collect sales and use and similar taxes in any states for telemedicine services based on our belief that our services are not subject to such taxes in any state. Sales and use and similar tax laws and rates vary greatly from state to state. Certain states in which we do not collect such taxes may assert that such taxes are applicable, which could result in tax assessments, penalties and interest with respect to past services, and we may be required to collect such taxes for services in the future. Such tax assessments, penalties and interest or future requirements may adversely affect our results of operations.
We will likely require additional capital from equity or debt financings to support business growth, and this capital might not be available on acceptable terms, if at all.
We intend to make investments to support our anticipated business growth and will likely require additional funds to respond to business challenges, including the need to develop new solutions or enhance our existing solutions, enhance our operating infrastructure and acquire complementary businesses and technologies. In order to achieve these objectives, we may make future commitments of capital resources, including incurring additional indebtedness under our credit facility. Accordingly, we may need to
23
engage in equity or debt financings to secure additional funds. If we raise additional funds through further issuances of equity or debt securities, our existing stockholders could suffer significant dilution, and any new equity securities we issue could have rights, preferences and privileges superior to those of holders of our common stock. Any debt financing secured by us in the future could involve restrictive covenants relating to our capital raising activities and other financial and operational matters. In addition, we may not be able to obtain additional financing on terms favorable to us, if at all. If we are unable to obtain adequate financing or financing on terms satisfactory to us, when we require it, our ability to continue to support our business growth and to respond to business challenges could be significantly limited.
Risks Related to Governmental Regulation
In the U.S., we conduct business in a heavily regulated environment and if we fail to comply with health care laws and regulations, we could incur fines and other penalties, be prohibited from participating in certain reimbursement programs or be required to make significant changes to our operations or experience adverse publicity, which could have a material adverse effect on our business, financial condition, and results of operations.
The U.S. healthcare industry is heavily regulated and closely scrutinized by federal, state and local governments. Comprehensive statutes and regulations govern the manner in which we provide and bill for services and collect reimbursement from governmental programs and private payors, our contractual relationships with our providers, vendors and customers, our marketing activities and other aspects of our operations. Of particular importance are:
| ● | the federal physician self-referral law, commonly referred to as the Stark Law, that, subject to specific exceptions, prohibits physicians from referring Medicare or Medicaid patients to an entity for the provision of certain “designated health services” if the physician or a member of such physician’s immediate family has a direct or indirect financial relationship (including an ownership interest or a compensation arrangement) with the entity, and prohibit the entity from billing Medicare or Medicaid for such designated health services. Many states have adopted similar laws; |
| ● | the federal Anti-Kickback Statute that prohibits the knowing and willful offer, payment, solicitation or receipt of any bribe, kickback, rebate or other remuneration for referring an individual, in return for ordering, leasing, purchasing or recommending or arranging for or to induce the referral of an individual or the ordering, purchasing or leasing of items or services covered, in whole or in part, by any federal healthcare program, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
| ● | the federal False Claims Act that prohibits, among other things, presenting, or causing the presentment, of a false claim for payment or approval; making, using, or causing others to make or use, a false record or statement that is material to a false or fraudulent claim, Making, using, or causing to be made or used, a false record or statement material to an obligation to pay money to the government; or conceals, avoids, or decreases an obligation to pay money to the government. Many states have adopted state false claims act laws. |
We do not always have the benefit of significant regulatory or judicial interpretation of these laws and regulations to guide our operations. In the future, different interpretations or enforcement of these laws and regulations could subject our current or past practices to allegations of impropriety or illegality or could require them to make changes in our operations or structure. A determination that they have violated these laws, or the public announcement that we are being investigated for possible violations of these laws, could have a material adverse effect on our business, financial condition, results of operations and cash flows, and our business reputation could suffer significantly. In addition, other similar legislation or regulations at the federal or state level may be adopted that could have a material adverse effect on our business, financial condition, results of operations and cash flows.
To enforce compliance with the federal laws, the U.S. Department of Justice and the U.S. Department of Health and Human Services Office of Inspector General, or OIG, have recently increased their scrutiny of healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Dealing with investigations can be time- and resource-consuming and can divert management’s attention from the business. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business. In addition, because of the potential for large monetary exposure under the federal False Claims Act, which provides for treble damages and penalties of $12,537 to $25,076 per false claim or statement, healthcare providers often resolve allegations without admissions of liability for significant and material amounts to avoid the uncertainty of treble damages that may be awarded in litigation proceedings. Such settlements often contain additional compliance and
24
reporting requirements as part of a consent decree, settlement agreement or corporate integrity agreement. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ compliance with the healthcare reimbursement rules and fraud and abuse laws.
The laws, regulations and standards governing the provision of healthcare services may change significantly in the future. We cannot assure you that any new or changed healthcare laws, regulations or standards will not materially adversely affect our business. We cannot assure you that a review of our business by judicial, law enforcement, regulatory or accreditation authorities will not result in a determination that could adversely affect our operations.
State legislative and regulatory changes specific to the area of telehealth law may present the third party medical groups and independent physicians on our platform with additional requirements and state compliance costs, which may create additional operational complexity and increase costs.
Our affiliated professional entities and independent physicians’ and physician extenders’ ability to provide telehealth services to, and receive reimbursement for the services provided to patients in a particular state are dependent upon the laws and regulations of the state where the patient resides. Laws and regulations governing the provision of telehealth services are evolving at a rapid pace and are subject to changing political, regulatory, and other influences. Some states’ regulatory agencies or medical boards may have established rules or interpreted existing rules in a manner that limits or restricts providers’ ability to provide telehealth services or for physicians to supervise nurse practitioners and physician assistants remotely. Additionally, there may be limitations placed on the modality through which telehealth services may be provided or requirements related to the provision of telehealth services, such as having a prior in person visit or receipt of certain informed consents. For example, some states specifically require synchronous (or “live”) communications and restrict or exclude the use of asynchronous telehealth modalities, which is also known as “store-and-forward” telehealth. Because this is a developing area of law and regulation, we continually monitor our compliance in every jurisdiction in which we operate. However, we cannot be assured that our affiliated professional entities or independent providers’ activities and arrangements, if challenged, will be found to be in compliance with the state requirements or that a new or existing law or regulation will not be adopted, enforced, or changed in manner that is unfavorable to our business model. We cannot predict the regulatory landscape for those jurisdictions in which we operate and any significant changes in law, policies, or standards, or the interpretation or enforcement thereof, could occur with little or no notice. The majority of the consultations provided through our platforms are synchronous consultations for patients located in jurisdictions that permit the use of asynchronous telehealth. If there is a change in laws or regulations related to our business, or the interpretation or enforcement thereof, that adversely affects our structure or operations, including greater restrictions on the use of asynchronous telehealth or remote supervision of nurse practitioners or physician assistants, it could have a material adverse effect on our business, financial condition, and results of operations.
Evolving government regulations and enforcement activities may require increased costs or adversely affect our results of operations.
In a regulatory climate that is uncertain, our operations may be subject to direct and indirect adoption, expansion or reinterpretation of various laws and regulations. This risk is especially acute in the healthcare industry given the level of government spending and oversight of the industry as a whole.
In the ordinary course of business, we may be subject to inquiries and audits by federal and state agencies that oversee applicable healthcare program participation, licensure and payment regulations. We may also be subject to routine and targeted government audits and investigations. We believe that the regulatory environment surrounding most segments of the healthcare industry remains intense. Responding to audits and inquiries may require us to incur significant expense. If the results of any audit or investigation reveal material non-compliance, we may have to incur additional expense in defending our business and making modifications to our operations.
In the states in which we operate, we believe we are in material compliance with all applicable material regulations, but, due to the uncertain regulatory environment, certain states may determine that we are in violation of their laws and regulations. If we must remedy such violations, we may be required to modify our business and services in such states in a manner that undermines our respective platform’s attractiveness to customers, we may become subject to fines or other penalties or, if we determine that the requirements to operate in compliance in such states are overly burdensome, we may elect to terminate our operations in such states. In each case, our revenue may decline and our business, financial condition, and results of operations could be adversely affected.
25
If we fail to comply with extensive healthcare laws and government regulations, we could suffer penalties or be required to make significant changes to our operations.
The healthcare industry is required to comply with extensive and complex laws and regulations at the federal, and state government levels relating to, among other things:
| ● | licensure of health providers, and enrollment with government reimbursement programs; |
| ● | necessity and adequacy of telehealth services; |
| ● | relationships with physicians and other referral sources and referral recipients; |
| ● | billing and coding for services; |
| ● | properly handling any overpayments; |
| ● | quality of medical equipment, devices and services we make available; |
| ● | qualifications of medical professionals and support personnel; |
| ● | confidentiality, maintenance, data breach, identity theft and security issues associated with health-related and personal information and medical records; and |
| ● | communications with patients and consumers. |
Among these laws are the federal Stark Law, the federal Anti-Kickback Statute, the False Claims Act, and similar state laws. If we fail to comply with applicable laws and regulations, we could suffer civil sanctions and criminal penalties, including the loss of our ability to participate in the Medicare, Medicaid and other federal and state healthcare programs. While we endeavor to ensure that our financial relationships with referral sources such as hospitals and physicians comply with the applicable laws (including applicable safe harbors and exceptions), evolving interpretations or enforcement of these laws and regulations could subject our current practices to allegations of impropriety or illegality or could require them to make changes in our operations. A determination that we have violated these or other laws, or the public announcement that we are being investigated for possible violations of these or other laws, could harm our business, and our business reputation could suffer significantly. In addition, other legislation or regulations at the federal or state level may be adopted that could harm our business.
Our collection, use and disclosure of personally identifiable information, including health information, is subject to federal and state privacy and security regulations, and our failure to comply with those regulations or to adequately secure the information we hold could result in significant liability or reputational harm to us and, in turn, harm ou rr client base and our business.
There are a number of federal and state laws, rules and regulations, as well as contractual obligations, relating to the protection, collection, storage, use, retention, security, disclosure, transfer and other processing of confidential, sensitive and personal information, including certain patient protected health information (PHI), such as patient records. Existing laws and regulations are constantly evolving, and new laws and regulations that apply to our business are being introduced at every level of government in the United States. In many cases, these laws and regulations regarding transfer or disclosure of personal information apply not only to transfer or disclosure to third-parties, but also to transfers of information between or among VSee Lab and iDoc, our affiliates and other parties with whom we conduct business. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that may have a material adverse effect on our business. We monitor legal developments in data privacy and security regulations at the local, state and federal level, however, the regulatory framework for data privacy and security worldwide is continuously evolving and developing and, as a result, interpretation and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future.
The management of PHI is subject to HIPAA. HIPAA is the primary federal law that protects patients’ health care data and records. HIPAA consists of the HIPAA privacy rule (“Privacy Rule”) and the HIPAA security rule (“Security Rule”). The HIPAA Privacy Rule protects medical records and other personal health information by limiting our use and disclosure, giving individuals the right to access, amend, and seek accounting of our own health information, and limiting most uses and disclosures of health
26
information to the minimum amount reasonably necessary to accomplish the intended purpose. The HIPAA Security Rule protects individuals’ electronic personal health information that is created, received, used, or maintained, and requires appropriate administrative, physical and technical safeguards to ensure the confidentiality, integrity, and security of electronic protected health information. The HITECH Act strengthened HIPAA enforcement provisions, requires OCR to periodically audit covered entities and our business associates, and authorized State Attorneys General to bring civil actions for HIPAA violations. It permits the HHS to conduct audits of HIPAA compliance and impose significant civil monetary penalties even if we did not know or reasonably could not have known about the violation.
HIPAA requires healthcare providers and its business associates to develop and maintain policies and procedures with respect to PHI that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information. HIPAA also implemented the use of standard transaction code sets and standard identifiers that covered entities must use when submitting or receiving certain electronic healthcare transactions, including activities associated with the billing and collection of healthcare claims.
HIPAA imposes mandatory penalties for certain violations. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts are able to award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
In addition, HIPAA mandates that the Secretary of HHS conduct periodic compliance audits of HIPAA covered entities or business associates for compliance with the HIPAA Privacy and Security Standards. It also tasks HHS with establishing a methodology whereby harmed individuals who were the victims of breaches of unsecured PHI may receive a percentage of the Civil Monetary Penalty fine paid by the violator.
HIPAA further requires that patients be notified of any unauthorized acquisition, access, use or disclosure of our unsecured PHI that compromises the privacy or security of such information, with certain exceptions related to unintentional or inadvertent use or disclosure by employees or authorized individuals. HIPAA specifies that such notifications must be made “without unreasonable delay and in no case later than 60 calendar days after discovery of the breach.” If a breach affects 500 patients or more, it must be reported to HHS without unreasonable delay, and HHS will post the name of the breaching entity on its public web site. Breaches affecting 500 patients or more in the same state or jurisdiction must also be reported to the local media. If a breach involves fewer than 500 people, the covered entity must record it in a log and notify HHS at least annually. This reporting obligation is in addition to any state notification requirements.
There are proposed changes to the HIPAA regulations, which if enacted, may require us to make significant changes to our HIPAA compliance program and our patient access request procedures and may have other financial, and operational impacts.
There are other federal and state laws protect the confidentiality, privacy, availability, integrity and security of personally identifiable information (PII), including PHI. At the state and local level, there is increased focus on regulating the collection, store, use, retention, security, disclosure, transfer and other processing of confidential, sensitive and personal information. These laws in many cases are more restrictive than, and may not be preempted by, the HIPAA rules and may be subject to varying interpretations by courts and government agencies, creating complex compliance issues for us and our clients and potentially exposing us to additional expense, adverse publicity and liability.
In addition, all 50 U.S. states and the District of Columbia have enacted breach notification laws that may require us to notify patients, employees or regulators in the event of unauthorized access to, disclosure of, or acquisition of personal or confidential information experienced by us or our service providers. These laws are not consistent, and compliance in the event of a widespread data breach is difficult and may be costly. Moreover, states have been frequently amending existing laws, requiring attention to changing regulatory requirements. We also may be contractually required to notify patients or other counterparties of a security breach. Although we may have contractual protections with our service providers, any actual or perceived security breach could harm our reputation and brand, expose us to potential liability or require us to expend significant resources on data security and in responding to any such actual or perceived breach. Any contractual protections we may have from our service providers may not be sufficient to adequately protect us from any such liabilities and losses, and we may be unable to enforce any such contractual protections. In addition to government regulation, privacy advocates and industry groups have and may in the future propose self-regulatory standards from time to time. These and other industry standards may legally or contractually apply to us, or we may elect to comply with such standards.
27
New health and personal information security standards, whether implemented pursuant to HIPAA, congressional action or otherwise, could have a significant effect on the manner in which VSee and iDoc must handle healthcare related data, and the cost of complying with standards could be significant. If we do not comply with existing or new laws and regulations related to PHI, we could be subject to criminal or civil sanctions.
Because of the sensitivity of the PII we store and transmit, the security features of our technology platforms are very important. If our security measures are breached or fail, unauthorized persons may be able to obtain access to sensitive client and patient data, including HIPAA-regulated PHI. As a result, our reputation could be severely damaged, adversely affecting client or investor confidence. Clients may curtail their use of or stop using our services or our client base could decrease, which would cause our business to suffer. In addition, we could face litigation, damages for contract breach, penalties and regulatory actions for violation of HIPAA and other applicable laws or regulations and significant costs for remediation, notification to individuals and for measures to prevent future occurrences. Any potential security breach could also result in increased costs associated with liability for stolen assets or information, repairing system damage that may have been caused by such breaches, incentives offered to client or other business partners in an effort to maintain our business relationships after a breach and implementing measures to prevent future occurrences, including organizational changes, deploying additional personnel and protection technologies, training employees and engaging third-party experts and consultants. While we maintain insurance covering certain security and privacy damages and claim expenses, we may not carry insurance or maintain coverage sufficient to compensate for all liability and, in any event, insurance coverage would not address the reputational damage that could result from a security incident.
We also publish statements to our clients that describe how we handle and protect personal information. If federal or state regulatory authorities or private litigants consider any portion of these statements to be untrue, we may be subject to claims of deceptive practices, which could lead to significant liabilities and consequences, including, without limitation, costs of responding to investigations, defending against litigation, settling claims and complying with regulatory or court orders.
In March 2020, the Office of the National Coordinator for Health Information Technology (“ONC”) released a final rule implementing the information blocking prohibition of the 21st Century Cures Act, which went into effect on April 5, 2021. The rule, which applies to almost all health care providers, is designed to create a more interoperable health care system that supports seamless data exchange, improves care coordination, and removes barriers to the use and exchange of PHI between providers and plans and as directed by patients. “Information blocking” refers to activities that unreasonably limit the availability and use of electronic health information (“EHI”). The rule prohibits information blocking of EHI unless it is required by law or meets one of eight narrowly applied exceptions. Like most providers, we had to create new policies and procedures, trainings, and governance structures, and invest in new technology to comply with the rule. ONC has delegated oversight and compliance monitoring to the Office of Inspector General, and a provider may be subject to significant financial penalties if it fails to comply with these new rules. The exact penalties for providers will be determined through future rulemaking. Any individual can submit a complaint alleging that a provider has engaged in information blocking through an online portal made available by ONC.
If we fail to comply with federal and state laws and policies governing claim submissions to government healthcare programs or commercial insurance programs, we or our clients may be subject to civil and criminal penalties or loss of eligibility to participate in government healthcare programs and contractual claims by commercial insurers.
We offer revenue cycle management services to our clients that include the preparation and submission of claims for professional service and billing agent collection processing with payers on behalf of our clients. Certain of these reimbursement claims are governed by federal and state laws with potential civil and criminal penalties for non-compliance. The HIPAA security, privacy and transaction standards also have a potentially significant effect on our claims preparation, transmission and submission services, because such services must be structured and provided in a way that supports our clients’ HIPAA compliance obligations. Errors by us or our systems with respect to entry, formatting, preparation or transmission of claim information may be determined or alleged to be in violation of these laws and regulations. If our revenue cycle management services fail to comply with these laws and regulations, we may be subjected to federal or state government investigations and possible penalties may be imposed upon us, false claims actions may have to be defended, private payers may file claims against them, and we may be excluded from Medicare, Medicaid or other government-funded healthcare programs. Further, our clients may seek contractual remedies and indemnification. Any investigation or proceeding related to these topics, even if unwarranted or without merit, could adversely affect demand for our services, could force us to expend significant capital, research and development and other resources to address the failure, and may harm our business.
Private pay sources such as third-party insurance and managed care entities also often reserve the right to, and do actually conduct audits of our billing processes, and have from time to time conducted such reviews. Our costs to respond to and defend any such reviews, audits and investigations are significant and are likely to increase in the current enforcement environment. These audits and
28
investigations may require us to refund or retroactively adjust amounts that have been paid to us by the relevant government program or private pay source.
If our revenue cycle management services fail to comply with these laws and regulations, we may be subjected to federal or state government investigations and possible penalties may be imposed upon us, false claims actions may have to be defended, private payers may file claims against us, and we may be excluded from Medicare, Medicaid or other government-funded healthcare programs. Further, our clients may seek contractual remedies and indemnification. Any investigation or proceeding related to these topics, even if unwarranted or without merit, could adversely affect demand for our services, could force us to expend significant capital, research and development and other resources to address the failure, and may harm our business.
Physician licensing and credentialing, a cost of providing professional services, can negatively impact our margins as it may incur increased expenses to utilize appropriately licensed and credentialed physicians for consult demands, especially when expanding to new jurisdictions and new hospital clients.
A physician’s (or a physician extender’s) ability to perform telemedicine consults is dictated by where the physician is licensed to practice and with whom the physician is privileged to provide services. State licensure and physician credentialing requirements take time to procure, often necessitating months of lead-time before a physician is able to begin providing consults for a particular hospital facility. Our ability to manage and anticipate physician need and prioritize licensing and credentialing could impact profit margins and expense management. As consult demands increase in areas where only a limited number of physicians hold necessary licenses and credentials, those physicians with appropriate licensing and credentialing to meet client demands may assume additional overtime shifts or otherwise demand increased fees, thereby increasing its costs. Further, obtaining a license to practice medicine in a particular jurisdiction is at the discretion of the local state medical board, and, as such, timing to achieve licensure in certain jurisdictions may be outside our ability to accomplish within expected time frames.
Certain software products related to telemedicine platforms may be subject to FDA regulatory review and oversight. It is critical to identify applicable FDA requirements and ensure compliance with such requirements.
Certain software products often used in telemedicine platforms and offerings could fall under the broad category of digital health products that may, in certain circumstances, require FDA regulatory review prior to marketing. The FDA generally maintains regulatory oversight over products that meet the Agency’s statutory definition of a “medical device.” In certain circumstances, software applications and their corresponding platforms are considered medical devices when they are intended to be used for one or more medical purposes and are consequently regulated by the FDA. Determining whether a product meets the definition of a medical device requires assessment of both design and intended use. Intended use of a product is determined by the intent of the manufacturer as evidenced by the design of the product and the product labeling. Labeling is a broad term that includes marketing and advertising claims. The FDA’s regulatory approach toward digital health technologies is set forth in both regulations and guidance documents. This requires analyzing (1) whether a product meets the FDA’s definition of a medical device and, if it does, (2) whether it is carved out from active regulation by one of the FDA’s digital health “enforcement discretion” policies. In general, the FDA’s overarching approach is to apply its regulatory oversight in a risk-based manner to only software functions deemed to meet the definition of medical devices (i.e., those intended for the diagnosis of disease or other conditions, or the cure, mitigation, treatment, or prevention of disease) and whose functionality could create patient safety risks in the event of a malfunction.
Risks Related to the Use of Our Technology
Failure to keep pace with advances in technology could cause our solutions to become obsolete, which could harm our business, financial condition and results of operations.
The telemedicine industry is characterized by rapid technological change, changing consumer requirements, short product lifecycles and evolving industry standards. The successful implementation of our business model depends on our ability to anticipate and adapt to evolving technologies and industry standards and introduce new solutions accordingly. For example, we deployed our software platform to hospital organizations as a stand-alone software-as-a-service solution independent of its clinical services to enable these providers to optimize and scale its platform across all of our care sites. These new solutions carry risks, such as cost overruns, delays in delivery, performance problems, and lack of acceptance by our clients. If we cannot anticipate or adapt to rapidly evolving industry standards, technology, and increasingly sophisticated clients and our employees, our existing technology could become undesirable, obsolete, or harm our reputation. Moreover, we may not be successful in developing, using, marketing, selling or maintaining new technologies effectively or adapting our solutions to evolving client requirements or emerging industry standards, and, as a result, our business could be harmed. In addition, we have limited insight into trends that might develop and affect our
29
business, which could lead to errors in our predicting and reacting to relevant business, legal, and regulatory trends and healthcare reform. Further, there can be no assurance that technological advances by one or more of our competitors or future competitors will not result in our present or future solutions and services becoming uncompetitive or obsolete. If any of these events occur, it could harm our business.
If the systems that we use to provide our services experience security breaches, we may incur significant liabilities, and our reputation and business may be harmed.
Our services involve the storage and transmission of our clients’ proprietary information, sensitive or confidential data, including valuable personal information of patients, clients and others, as well as the PHI of our clients. Because of the sensitivity of the information we store and transmit, the security features of our computer, network and communications systems infrastructure are critical to the success of our business. A breach or failure of our security measures could result from a variety of circumstances and events, including third-party action, employee negligence or error, malfeasance, computer viruses, cyber-attacks by computer hackers, failures during the process of upgrading or replacing software and databases, power outages, hardware failures, telecommunication failures, user errors or catastrophic events. Information security risks have generally increased in recent years because of the proliferation of new technologies and the increased sophistication and activities of perpetrators of cyber-attacks. As cyber threats continue to evolve, We may be required to expend additional resources to further enhance our information security measures and/or to investigate and remediate any information security vulnerabilities. If our security measures fail or are breached, it could result in unauthorized persons accessing sensitive client or patient data (including PHI), a loss of or damage to our data, an inability to access data sources, or process data or provide our services to our clients. Such failures or breaches of our security measures, or our inability to effectively resolve such failures or breaches in a timely manner, could severely damage our reputation, adversely affect client or investor confidence in us and reduce the demand for our services from existing and potential clients. In addition, we could face litigation, damages for contract breach, monetary penalties, or regulatory actions for violation of applicable laws or regulations including HIPAA, and incur significant costs for remedial measures to prevent future occurrences and mitigate past violations. Although we maintain insurance covering certain security and privacy damages and claim expenses, we may not carry insurance or maintain coverage sufficient to compensate for all liability and, in any event, insurance coverage would not address the reputational damage that could result from a security incident.
We may experience cyber-security and other breach incidents that remain undetected for an extended period. Because techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are not recognized until launched, we may be unable to anticipate these techniques or to implement adequate preventive measures. If an actual or perceived breach of our security occurs, or if we are unable to effectively resolve such breaches in a timely manner, the market perception of the effectiveness of our security measures could be harmed and we could lose sales and clients, which could harm our business.
We rely on telecommunications and internet service providers for providing solutions to our clients, and any interruption or failure in the services provided by these third parties could harm our business.
Our business is highly dependent on telecommunications and internet service providers. Our services are designed to operate 24-hours-a-day, seven-days-a-week, without interruption. However, we may experience interruptions and delays in services and availability from time to time. We may not maintain redundant systems or facilities for some of these services. While we control and have access to our servers, we do not control the operation of internet providers.
Additionally, if our vendors or internet providers are unable to keep up with our growing needs, this could harm our business. Interruptions in our services may reduce our revenue, cause us to issue refunds to clients for prepaid and unused subscriptions, subject us to potential liability or adversely affect client renewal rates.
In the event of a catastrophic event with respect to one or more of these systems or facilities, we may experience an extended period of system unavailability, which could negatively impact our relationships with clients. To operate without interruption, we and our service providers must guard against:
| ● | damage from fire, power loss, natural disasters and other force majeure events outside our control; |
| ● | communications failures; |
| ● | software and hardware errors, failures and crashes; |
30
| ● | security breaches, computer viruses, hacking, denial-of-service attacks and similar disruptive problems; and |
| ● | other potential interruptions. |
Moreover, system failures may result in loss of data, including patient data, which is critical to the provision of our services. Any errors, failures, interruptions or delays experienced in connection with our or our third parties’ systems could negatively impact our relationships with clients, adversely affect our brand and expose us to liabilities to third parties, all of which could harm our business.
Failure to protect or enforce our intellectual property rights could impair our ability to protect our internally developed technology and our brand and the costs involved in such enforcement could harm our business.
Our intellectual property includes our internally developed processes, methodologies, algorithms, applications, technology platform, software code, website content, user interfaces, graphics, trade dress, databases and domain names. We rely on a combination of trademark, trade secret and copyright laws and confidentiality procedures and contractual provisions to protect our intellectual property rights in our internally developed technology and content. We believe that our intellectual property is an essential asset of our business. If we do not adequately protect our intellectual property, our brand and reputation could be harmed and competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business, negatively affect our position in the marketplace, limit our ability to commercialize our technology, and delay or render impossible our achievement of profitability. A failure to protect our intellectual property in a cost-effective and meaningful manner could adversely affect our ability to compete. We regard the protection of our trade secrets, copyrights, trademarks, trade dress, databases and domain names as critical to our success.
We strive to protect our intellectual property rights by relying on federal, state, and common law rights and other rights provided under foreign laws. However, the steps we take to protect our intellectual property rights may be inadequate. For example, other parties, including our competitors, may independently develop similar technology, duplicate our services, or design around our intellectual property and, in such cases, we may not be able to assert our intellectual property rights against such parties. Further, our contractual arrangements may not effectively prevent disclosure of our confidential information or provide an adequate remedy in the event of unauthorized disclosure of our confidential information, and we may be unable to detect the unauthorized use of, or take appropriate steps to enforce, our intellectual property rights.
We make business decisions about when to seek patent protection for a particular technology and when to rely upon trade secret protection, and the approach we select may ultimately prove to be inadequate. In particular, we do not currently hold a patent or other registered or applied for intellectual property protection for our software platform. Even in cases where we seek patent protection, there is no assurance that the resulting patents will effectively protect every significant feature of our solutions, technology or proprietary information, or provide us with any competitive advantages, since intellectual property law, including statutory and case law, particularly in the United States, is constantly developing, and any changes in the law could make it harder for us to enforce our rights.
In order to protect our intellectual property rights, we may be required to spend significant resources to monitor and protect these rights. Litigation brought to protect and enforce our intellectual property rights could be costly, time-consuming and distracting to management and could result in the impairment or loss of portions of our intellectual property. Furthermore, our efforts to enforce our intellectual property rights may be met with defenses, counterclaims and countersuits attacking the validity and enforceability of our intellectual property rights. An adverse determination of any litigation proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly and could put any related pending patent applications at risk of not issuing. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential or sensitive information could be compromised by disclosure in the event of litigation. In addition, during the course of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Negative publicity related to a decision by us to initiate such enforcement actions against a client or former client, regardless of its accuracy, may adversely impact our other client relationships or prospective client relationships, harm our brand and business, and could cause the market price of our common stock to decline. Our failure to secure, protect, and enforce our intellectual property rights could harm our brand and our business.
31
We could incur substantial costs as a result of any claim of infringement of another party’s intellectual property rights.
There is considerable patent and other intellectual property development activity in our industry. Our future success depends in part on not infringing upon the intellectual property rights of others. From time to time, third parties may claim that we are infringing upon our intellectual property rights or that we have misappropriated our intellectual property. As competition in our market grows, the possibility of patent infringement, trademark infringement and other intellectual property claims against us increases. In a patent infringement claim against us, we may assert, as a defense, that we do not infringe the relevant patent claims, that the patent is invalid or both. The strength of our defenses will depend on the patents asserted, the interpretation of these patents, and our ability to invalidate the asserted patents. However, we could be unsuccessful in advancing non-infringement and/or invalidity arguments in our defense. In the United States, issued patents enjoy a presumption of validity, and the party challenging the validity of a patent claim must present clear and convincing evidence of invalidity, which is a high burden of proof. Conversely, the patent owner need only prove infringement by a preponderance of the evidence, which is a lower burden of proof. We may be unaware of the intellectual property rights that others may claim cover some or all of our technology or services. Because patent applications can take years to issue and are often afforded confidentiality for some period of time, there may currently be pending applications, unknown to us, that later result in issued patents that could cover one or more aspects of our technology and services. Any claims or litigation could cause us to incur significant expenses and, whether or not successfully asserted against us, could require that we pay substantial damages, ongoing royalty or license payments or settlement fees, prevent us from offering our solutions or using certain technologies, require us to re-engineer all or a portion of our platforms, or require that we comply with other unfavorable terms. We may also be obligated to indemnify our clients or business partners or pay substantial settlement costs, including royalty payments, in connection with any such claim or litigation and to obtain licenses, modify applications or refund fees, which could be costly. Even if we were to prevail in such a dispute, any litigation regarding our intellectual property could be costly and time-consuming and divert the attention of our management and key personnel from our business operations.
Our software platforms may not perform properly due to errors or similar problems, which could damage our reputation, give rise to claims against us, or divert application of our resources from other purposes, any of which could harm our business.
Our software platforms provide our clients and providers with the ability to, among other things, complete, view and edit medical history; request a consult (either scheduled or on demand); conduct a consult (via video or phone); and initiate an expert medical service. Software development is time-consuming, expensive and complex, and may involve unforeseen difficulties. We may encounter technical obstacles, and it is possible that they may discover additional problems that prevent our software platforms from operating properly. If our solutions do not function reliably or fail to achieve client expectations in terms of performance, clients could assert liability claims against us or attempt to cancel their contracts with us. This could damage tour reputation and impair our ability to attract or maintain clients.
Moreover, complex software, such as ours, often contains defects and errors, some of which may remain undetected for a period of time. Material performance problems, defects or errors in our existing or new software and services may arise in the future and may result from interface of our solution with systems and data that we did not develop and the function of which is outside of our control or undetected in our testing. Such errors may be found after the introduction of new software or enhancements to existing software. If we detect any errors before we introduce a solution, we may have to delay deployment for an extended period of time while we address the problem. Any defects and errors, and any failure by us to identify and address them, could result in loss of revenue or market share, diversion of development resources, harm to our reputation and increased service and maintenance costs. Defects or errors may discourage existing or potential clients from purchasing our solutions from us. Correction of defects or errors could prove to be impossible or impracticable. The costs incurred in correcting any defects or errors may be substantial and could harm our business.
Risks Related to Us as Public Company
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our securities less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. We will remain an “emerging growth company” for up to five years. However, if our non-convertible debt issued within a three-year period exceeds $1.0 billion, or revenues exceeds $1.07 billion, or the market value of our shares of common stock that are held by non-affiliates exceeds $700 million on the last day of the second fiscal quarter of any given fiscal year, we would cease to be an emerging growth company as of the following fiscal year. As an emerging growth company, we are not being required to comply with the auditor attestation requirements of section 404 of the Sarbanes-Oxley Act, we have reduced disclosure obligations regarding executive compensation in our periodic reports and proxy
32
statements, and we are exempt from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. Additionally, as an emerging growth company, we have elected to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As such, our financial statements may not be comparable to companies that comply with public company effective dates. We cannot predict if investors will find our shares less attractive because we may rely on these provisions. If some investors find our shares less attractive as a result, there may be a less active trading market for our shares and our share price may be more volatile.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, will not adopt the new or revised standard until the time private companies are required to adopt the new or revised standard. This may make comparison of our financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accountant standards used.
We have no substantial combined operating history, and any failure to successfully integrate the business of VSee Lab and iDoc could adversely affect the results of our operations.
Until June 24, 2024, each of VSee Lab and iDoc operated independently since their inception. There can be no assurance that we will be able to integrate the operations of VSee Lab and iDoc successfully or to institute the necessary systems and procedures, including accounting and financial reporting systems, to manage the combined enterprise on a profitable basis and to report the results of operations of the combined entities on a timely basis. In addition, there can be no assurance that the management teams of each of VSee Lab and iDoc will be able to successfully manage the combined entity and effectively implement their operating or growth strategies. The financial results of VSee Lab and iDoc cover periods during which they were not under common control or management and, therefore, may not be indicative of their future financial or operating results. Our success will depend on management’s ability to integrate VSee Lab and iDoc into one organization. Our inability to successfully integrate these companies and to coordinate and integrate certain operational, administrative, financial and information technology systems would have a material adverse effect on our financial condition and results of operations.
In addition, although iDoc has been profitable historically, we expect our costs will increase in the foreseeable future and we may incur losses. We also expect to invest significant additional funds towards enhancing our services and platform, growing our business and operating as a public company and as we continue to invest in increasing our hospital and healthcare system client base, expanding our operations, hiring additional employees, and developing future offerings. These efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues sufficiently to offset these higher expenses.
Our current management team have no experience managing a public company.
Our current management have no experience managing a publicly-traded company, interacting with public company investors and research analysts, and complying with the increasingly complex laws and requirements pertaining to public companies, including those related to timely public disclosures, financial reporting, internal controls and enterprise risk management. As a result, we may not successfully or efficiently manage our new and additional roles and responsibilities. A public company is subject to significant regulatory oversight, reporting obligations under U.S. securities laws and the continuous scrutiny of securities analysts and investors. These new obligations and constituents will require significant attention of our senior management and could divert our attention away from the day-to-day management of our business. Failure to adequately comply with the requirements of being a public company, including deficiencies in financial reporting or ineffective disclosure controls and procedures and internal control over financial reporting, could cause investors to lose confidence in the our reported financial and other information and materially adversely affect our business, financial condition and results of operation, as well as severely negatively affect our stock price.
33
If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes- Oxley Act and the rules and regulations of the applicable listing standards of Nasdaq. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly and place significant strain on our personnel, systems and resources. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, we anticipate that we will continue to expend, significant resources, including accounting-related costs and significant management oversight. If any of these new or improved controls and systems do not perform as expected, we may experience material weaknesses in our controls. Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business.
Any failure to develop or maintain effective controls or any difficulties encountered in our implementation or improvement could harm our results of operations or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will eventually be required to include in our periodic reports that are filed with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on Nasdaq. We are required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act and are required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. We will be required to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our annual report on Form 10-K for the year ended December 31, 2024.
If the our business’ benefits do not meet the expectations of financial or industry analysts, the market price of our securities may decline.
The market price of our securities may decline if:
| ● | We do not achieve the perceived benefits of the acquisition as rapidly as, or to the extent anticipated by, financial or industry analysts; or |
| ● | The effect of the Business Combination on the financial statements is not consistent with the expectations of financial or industry analysts. |
Accordingly, investors may experience a loss as a result of decreasing stock prices.
We are be required to meet the continuing listing requirements of the Nasdaq Stock Market. However, we may be unable to maintain the listing of our securities in the future.
If we fail to meet the continued listing requirements and Nasdaq delists our securities, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; |
| ● | a limited amount of news and analyst coverage for the Company; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
34
VSee Lab’s independent registered public accounting firm’s report contained an explanatory paragraph that expresses substantial doubt about its ability to continue as a “going concern.”
VSee Lab’s recurring losses from operations and financial condition raise substantial doubt about its ability to continue as a going concern. In VSee Lab’s financial statements for the year ended December 31, 2023, VSee concluded that its recurring losses from operations and need for additional financing to fund future operations raise substantial doubt about VSee Lab’s ability to continue as a going concern. Similarly, VSee Lab’s independent registered public accounting firm included an explanatory paragraph in its report on VSee Lab’s financial statements for the year ended December 31, 2023 with respect to this uncertainty. VSee Lab’s ability to continue as a going concern will require it to obtain additional funding. If VSee Lab is unable to obtain sufficient funding, its business, prospects, financial condition and results of operations will be materially and adversely affected, and VSee Lab may be unable to continue as a going concern. In such an event, it would be forced to delay, limit, reduce or terminate its product development or commercialization efforts, or may be forced to reduce or terminate its operations. If VSee Lab is unable to continue as a going concern, VSee Lab may have to liquidate its assets and may receive less than the value at which those assets are carried on its audited financial statements, and it is likely that investors will lose all or part of their investment.
iDoc’s independent registered public accounting firm’s report contained an explanatory paragraph that expresses substantial doubt about its ability to continue as a “going concern.”
iDoc’s recurring losses from operations and financial condition raise substantial doubt about its ability to continue as a going concern. In iDoc’s financial statements for the year ended December 31, 2023, iDoc concluded that its recurring losses from operations and need for additional financing to fund future operations raise substantial doubt about iDoc’s ability to continue as a going concern. Similarly, iDoc’s independent registered public accounting firm included an explanatory paragraph in its report on iDoc’s financial statements for the year ended December 31, 2023 with respect to this uncertainty. iDoc’s ability to continue as a going concern will require it to obtain additional funding. If iDoc is unable to obtain sufficient funding, its business, prospects, financial condition and results of operations will be materially and adversely affected, and iDoc may be unable to continue as a going concern. In such an event, it would be forced to limit, reduce or terminate its operations. If iDoc is unable to continue as a going concern, iDoc may have to liquidate its assets and may receive less than the value at which those assets are carried on its audited financial statements, and it is likely that investors will lose all or part of their investment.
35
USE OF PROCEEDS
We will not receive any proceeds from the sale of Common Stock by Dominion in this offering. We may receive up to $50 million in gross proceeds from the shares of Common Stock that we may sell to Dominion pursuant to the Equity Purchase Agreement from time to time after the date that the registration statement of which this prospectus is a part is declared effective. However, we are unable to estimate the actual amount of proceeds that we may receive, as it will depend on the number of shares of Common Stock that we choose to sell, our ability to meet the conditions set forth in the Equity Purchase Agreement, market conditions and the price of shares of our Common Stock, among other factors. See section titled “Plan of Distribution” in this prospectus for more information.
Assuming the exercise of all the Public Warrants for cash, we will receive an aggregate of approximately $115 million, but will not receive any proceeds from the sale of the shares of Common Stock issuable upon such exercise. There is no assurance that the holders of the Public Warrants will elect to exercise for cash any or all of such warrants. To the extent that any Public Warrants are exercised on a “cashless basis,” the amount of cash we would receive from the exercise of the warrants will decrease.
We expect to use the net proceeds that we receive from the sales of our Common Stock to Dominion under the Equity Purchase Agreement and the net proceeds from the exercise of the Public Warrants, if any, for general corporate purposes. We may find it necessary or advisable to use the net proceeds for other purposes. We will have broad discretion over the use of any proceeds from the sales of our Common Stock to Dominion and the exercise of the Public Warrants. The amounts and timing of our actual expenditures will depend on numerous factors, including the factors described under “Risk Factors” in this prospectus and in any accompanying prospectus supplements, as well as the amount of cash used in our operations.
36
MARKET PRICE OF OUR COMMON STOCK
Our Common Stock and Public Warrants are currently listed on the Nasdaq Capital Market of The Nasdaq Stock Market LLC (“Nasdaq”) under the symbol “VSEE” and “VSEEW” respectively. Prior to the consummation of the Business Combination, DHAC’s Units, common stock and Public Warrants were listed on the Nasdaq Capital Market under the symbols “DHACU,” “DHAC” and “DHACW,” respectively.
On August 5, 2024, the closing sale price of our Common Stock was $2.67 per share and the closing sale price of our Public Warrants was $0.097 per warrant.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends for the foreseeable future. We expect to retain future earnings, if any, to fund the development and growth of our business. Any future determination to pay dividends on our Common Stock will be at the discretion of our board of directors and will depend upon, among other factors, our financial condition, operating results, current and anticipated cash needs, plans for expansion and other factors that our board of directors may deem relevant.
37
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Vsee Health, Inc ((formerly known as Digital Healthcare Acquisition Corp.) (“DHAC”)) (“VSee”, the “Company”) is providing the following unaudited pro forma condensed combined financial information to aid you in your analysis of the financial aspects of the Business Combination and related transactions. The following unaudited pro forma condensed combined financial information presents the combination of the financial information of DHAC and VSee and iDoc adjusted to give effect to the Business Combination and related transactions. The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.”
The historical financial information of DHAC was derived from the audited financial statements of DHAC as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022, and the unaudited financial statements as of March 31, 2024 and 2023 included elsewhere in this Form 8-K. The historical financial information of VSee was derived from the audited financial statements of VSee as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022 and the unaudited financial statements as of March 31, 2024 and 2023, included elsewhere in this Form 8-K. The historical financial information of iDoc was derived from the audited financial statements of iDoc as of December 31, 2023 and 2022 and for the years ended December 31, 2023 and 2022 and the unaudited financial statements as of March 31, 2024 and 2023, included elsewhere in this Form 8-K.
This information should be read together with DHAC’s and VSee and iDoc’s audited financial statements and related notes, the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of DHAC,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations of VSee,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations of iDoc” and other financial information included elsewhere in this proxy statement/prospectus/consent solicitation.
Notwithstanding the legal form of the business combination pursuant to the Business Combination Agreement, the Business Combination will be accounted for as a reverse recapitalization with VSee as the accounting acquirer and DHAC and iDoc as the accounting acquirees. Accordingly for accounting purposes, the Business combination will be treated as the equivalent of VSee issuing stock for the net assets of DHAC, accompanied by a recapitalization. The net assets of DHAC will be stated as historical cost with no goodwill or other intangible assets recorded. For accounting purposes, the acquirer is the entity that has obtained control of another entity and, thus, consummated a business combination.
As VSee is determined to be the accounting acquirer in the Business Combination, the acquisition of iDoc will be treated as a business combination under Accounting Standards Codification (“ASC”) Topic 805, Business Combinations (“ASC 805”), and will be accounted for using the acquisition method of accounting. The consideration transferred to acquire iDoc will be allocated to the assets acquired and liabilities assumed based on the estimated acquisition-date fair values. The excess of consideration transferred to effect the acquisition over the fair values of assets acquired and liabilities assumed will be recorded as goodwill. Transaction costs will be expensed as if the Business Combination had occurred on January 1, 2023.
The determination of whether control has been obtained begins with the evaluation of whether control should be evaluated based on the variable interest or voting interest model pursuant to ASC Topic 810, Consolidation (“ASC 810”). In all redemption scenarios, VSee has been determined to be the accounting acquirer based on evaluation of the following factors:
| ● | VSee’s stockholders will have the most significant voting interest post business combination under both the No Redemption and Maximum Redemption scenarios. |
| ● | VSee’s Chief Executive Officer will remain as Chief Executive officer post the business combination. |
| ● | VSee’s telehealth software platform has more notoriety. As such the post business combination name will be VSee Health, Inc. |
| ● | VSee will obtain the benefit of the merger post business combination, as its growth potential will be expanded with the addition of iDoc and its access to capital will be expanded with the addition of DHAC. |
| ● | VSee’s telehealth software platform along with its historical revenue stream management determined at the time of entering into the initial business combination agreement that VSee was the more significant entity under the relative size consideration. |
38
| ● | Although iDoc has a greater asset holding as of December 31, 2022 compared to VSee and DHAC, this is as a result of current year growth and the addition of Encompass Medical Billing LLC acquisition which occurred in early 2022. |
The unaudited pro forma condensed combined balance sheet as of March 31, 2024 assumes that the Business Combination and related transactions occurred on March 31, 2024. The unaudited pro forma condensed combined statement of operations for the three months ended March 31, 2024 and for the years ended December 31, 2023 gives pro forma effect to the Business Combination and related transactions as if they had occurred on January 1, 2023. DHAC and VSee and iDoc have not had any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
These unaudited pro forma condensed combined and financial statements are for informational purposes only. They do not purport to indicate the results that would have been obtained had the Business Combination and related transactions actually been completed on the assumed date or for the periods presented, or which may be realized in the future. The pro forma adjustments are based on the information currently available and the assumptions and estimates underlying the pro forma adjustments are described in the accompanying notes. Actual results may differ materially from the assumptions within the accompanying unaudited pro forma condensed combined financial information.
Description of the Business Combination
On June 15, 2022, DHAC executed the Original Business Combination Agreement with Merger Sub I, Merger Sub II, VSee and iDoc with respect to the Business Combination. On August 9, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the First Amended and Restated Business Combination Agreement to provide for the concurrent execution of financing documents for a PIPE consisting of convertible notes and warrants and delivery of Cassel Salpeter’s opinion to the Board. On October 6, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the Second Amended and Restated Business Combination Agreement to make the consideration payable to VSee and iDoc stockholders 100% Common Stock and to provide for the concurrent execution of revised PIPE financing documents, which provided for the purchase of shares of preferred stock and warrants in connection with the closing of the Business Combination. On November 3, 2022 the parties entered into a First Amendment to the Second Amended and Restated Business Combination Agreement to remove a closing condition that DHAC have at least $10 million in cash proceeds from the transactions at closing. On July 11, 2023, each of the PIPE investors provided notice to the Company that since certain closing condition was not met, PIPE financing was terminated. On November 21, 2023, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the Third Amended and Restated Business Combination Agreement to, among other things, provide for the removal of the PIPE financing and the concurrent execution of the Additional Bridge Financing, the Exchange Financing, the Quantum Financing, the Equity Financing and the Loan Conversions. On February 13, 2024, the parties entered into a First Amendment to the Third Amended and Restated Business Combination Agreement to provide that the Assumed Notes would be assumed by DHAC and converted into DHAC Common Stock following the closing instead of being converted into class B common stock of VSee and iDoc prior to the closing. On April 17, 2024, the parties entered into a Second Amendment to the Third Amended and Restated Business Combination Agreement to extend the termination date therein to June 30, 2024. On June 24, 2024 the parties closed on the proposed Business Combination.
The pro forma adjustments giving effect to the Business Combination and related transactions are summarized below, and are discussed further in the footnotes to these unaudited pro forma condensed combined and consolidated financial statements:
| ● | the consummation of the Business Combination and reclassification of cash held in DHAC’s Trust Account to cash and cash equivalents, net of redemptions (see below); and |
| ● | the accounting for certain offering costs and transaction costs incurred by both DHAC and VSee and iDoc. |
| ● | the accounting for certain financing arrangements which were entered into and agreed upon on November 21, 2023 as further disclosed below. |
The unaudited pro forma condensed combined and consolidated financial information has been prepared using actual redemptions and the result of the closing which occurred on June 24, 2024.
Included in the shares outstanding and weighted average shares outstanding as presented in the pro forma combined financial statements are an aggregate of 5,246,354 combined company shares to be issued to VSee shareholders, 4,950,000 combined company shares to be issued to iDoc shareholders, 437,000 combined company shares issued to settle the DHAC Deferred Underwriting Fee
39
issued to Underwriters, 600,000 shares issued to Bridge Investor for the balance of the VSee and iDoc Bridge Note, 35,000 combined company shares issued to DHAC sponsor to settle certain indebtedness, 436,300 combined company shares issued to Sponsor affiliates, VSee and iDoc lenders to settled certain indebtedness.
Assuming a $10.00 conversion price of each of the Series A Preferred Stock and the Convertible Notes:
| | | | | |
Sponsor(1)(2)(4) |
| 2,665,250 |
| 16.7 | % |
Sponsor affiliates(2)(3) |
| 936,300 |
| 5.9 | % |
Current Management, Board and Advisors |
| 301,750 |
| 1.9 | % |
Public Stockholders whose shares are subject to redemption(6) |
| 114,966 |
| 0.7 | % |
VSee |
| 5,246,354 |
| 32.9 | % |
iDoc |
| 4,950,000 |
| 31.1 | % |
AGP(5) |
| 437,000 |
| 2.7 | % |
Bridge Investor(4)(5)(7) |
| 950,375 |
| 6.0 | % |
Quantum Investor(3)(4) |
| 300,000 |
| 1.9 | % |
Other Stockholders(8) |
| 27,000 |
| 0.2 | % |
Assuming a $5.00 conversion price of each of the Series A Preferred Stock and the Convertible Notes (except for the Equity Purchase Note, which converts at a fixed $10.00 conversion price):
| | | | | |
Sponsor(1)(2)(4) |
| 2,700,250 |
| 15.8 | % |
Sponsor affiliates(2)(3) |
| 1,080,100 |
| 6.3 | % |
Current Management, Board and Advisors |
| 301,750 |
| 1.8 | % |
Public Stockholders whose shares are subject to redemption(6) |
| 114,966 |
| 0.7 | % |
VSee |
| 5,246,354 |
| 30.7 | % |
iDoc |
| 4,950,000 |
| 28.9 | % |
AGP(5) |
| 874,000 |
| 5.1 | % |
Bridge Investor(4)(5)(7) |
| 1,220,749 |
| 7.1 | % |
Quantum Investor(3)(4) |
| 600,000 |
| 3.5 | % |
Other Stockholders(8) |
| 27,000 |
| 0.2 | % |
Assuming a $2.00 conversion price of each of the Series A Preferred Stock and the Convertible Notes (except for the Equity Purchase Note, which converts at a fixed $10.00 conversion price):
| | | | | |
Sponsor(1)(2)(4) |
| 2,805,250 |
| 13.6 | % |
Sponsor affiliates(2)(3) |
| 1,511,500 |
| 7.3 | % |
Current Management, Board and Advisors |
| 301,750 |
| 1.5 | % |
Public Stockholders whose shares are subject to redemption(6) |
| 114,966 |
| 0.6 | % |
VSee |
| 5,246,354 |
| 25.4 | % |
iDoc |
| 4,950,000 |
| 23.9 | % |
AGP(5) |
| 2,185,000 |
| 10.6 | % |
Bridge Investor(4)(5)(7) |
| 2,031,872 |
| 9.8 | % |
Quantum Investor(3)(4) |
| 1,500,000 |
| 7.3 | % |
Other Stockholders(8) |
| 27,000 |
| 0.1 | % |
| (1) | Excludes 557,000 Private Warrants exercisable at the exercise price of $11.50. |
| (2) | Marc Munro, through his ownership of Tidewater and Whacky beneficially owns 66.35% of the Sponsor and will beneficially own 344,500 shares of Common Stock held by Sponsor Affiliates, consisting of 292,500 shares of Common Stock and 520 shares of Series A Preferred Stock to be received upon conversion of notes held by such entities controlled by him. |
| (3) | Lawrence Sands, through his ownership of SCS and SCS Capital Partners beneficially owns 500,000 founder shares and 33% of the Quantum Investor, and will beneficially own 591,800 shares of Common Stock held by Sponsor Affiliates, consisting of 500,000 founder shares and 918 shares of Series A Preferred Stock to be received upon conversion of notes held by such entities controlled by him. |
40
| (4) | The Bridge Investor owns 4.91% of the Sponsor and 33% of the Quantum Investor. |
| (5) | Includes 600,000 shares of the Company common stock issuable to the Bridge Investor upon conversion of certain of the Assumed Notes following the closing at a fixed conversion price of $2.00 per share. The conversion price of the Series A Shares and the Convertible Notes (other than the Equity Purchase Note) is subject to reset and adjustment under certain circumstances. See “Risk Factors — Risks Related to DHAC’s Business and the Business Combination — The DHAC Public Stockholders will experience dilution as a consequence of, among other transactions, the issuance of Common Stock as consideration in the Business Combination” for additional information related to the risk of dilution to our public stockholders. |
| (6) | Excludes 11,500,000 Public Warrants. |
| (7) | Excludes 173,913 warrants held by the Bridge Investor exercisable at the initial exercise price of $11.50. |
| (8) | Excludes 26,086 warrants held by an Other Stockholder exercisable at the initial exercise price of $11.50. |
The foregoing scenarios are for illustrative purposes only given the current trading price of DHAC’s common stock, the Company cannot reasonably clarify the rate at which it is expected that these investors will convert the Series A Shares or the Convertible Notes. Such rate will largely be determined by the trading price of DHAC’s common stock following the closing of the Business Combination — to the extent that such trading price is at or below $10.00 per share at the time of conversion following the closing of the Business Combination, the Preferred A shareholders and the Convertible Note holders will convert to common at price less than $10.00 and could convert at a price as low as $5.00 as described above. The $2.00 conversion price referenced above assumes the floor price.
The following presents the calculation of basic and diluted weighted average shares outstanding. The computation of diluted income (loss) per share excludes the effect of DHAC warrants to purchase 12,057,000 shares, Bridge Warrants to purchase 173,913 shares, Extension Note warrants of 26,086 and options to purchase 803,646 shares, the Convertible notes held by Quantum for 300,000, the Exchange Convertible Note and Additional Bridget Note to Bridge Investor for 270,375 and ELOC commitment fee Convertible Note 50,000 to be issued because the inclusion of any of these securities would be anti-dilutive.
| | |
|
| Closing Date |
| | Shareholding |
Weighted average shares calculation, basic and diluted |
|
|
DHAC public shares |
| 114,966 |
DHAC Sponsor affiliate |
| 936,300 |
DHAC Sponsor and director shares |
| 2,967,000 |
Bridge Investors |
| 630,000 |
A.G.P. Underwriter |
| 437,000 |
Other current stockholder |
| 27,000 |
VSee company shares issued in Business Combination |
| 5,246,354 |
iDoc company shares issued in Business Combination |
| 4,950,000 |
Weighted average shares outstanding |
| 15,308,620 |
41
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF MARCH 31, 2024
| | | | | | | | | | | | | | | | |
|
| | |
| | |
| | |
| Transaction |
|
|
| | |
| | | | | | | | | | | Accounting | | | | Pro Forma | |
| | (A) | | (A) | | (B) | | Adjustments | | | | Combined | ||||
| | VSEE | | IDOC | | DHAC | | (Actual | | | | (Actual | ||||
| | (Historical) | | (Historical) | | (Historical) | | Redemptions) | | | | Redemptions) | ||||
Assets |
| |
|
| |
|
| |
|
|
|
|
|
| |
|
Current assets: |
| |
|
| |
|
| |
|
|
|
|
|
| |
|
Cash and cash equivalents | | $ | 689,280 |
| | 74,184 | | $ | 724 |
| 1,400,355 |
| A | | $ | 1,488,488 |
| | | | | | | | | |
| (2,590,305) |
| C | | | |
| | | | | | | | | |
| 2,700,000 |
| D | | | |
| | | | | | | | | |
| (170,000) |
| G | | | |
| | | | | | | | | |
| (365,750) |
| I | | | |
| | | | | | | | | |
| (250,000) |
| M | | | |
Accounts receivable | |
| 627,395 |
| | 2,964,616 | |
| — |
| — |
|
| |
| 3,592,011 |
Due from related party | |
| — |
| | 1,047,771 | |
| — |
| (210,508) |
| L | |
| 837,263 |
Prepaids and other current assets | |
| 102,325 |
| | 140,665 | |
| — |
| 210,000 |
| C | |
| 452,990 |
Total Current Assets | |
| 1,419,000 |
| | 4,227,236 | |
| 724 |
| 723,792 |
|
| |
| 6,370,752 |
Customer list | |
| — |
| | — | |
| — |
| 5,302,497 |
| F | |
| 5,302,497 |
Trade name | |
| — |
| | — | |
| — |
| 1,376,002 |
| F | |
| 1,376,002 |
Right-of-use asset | |
| — |
| | 1,316,153 | |
| — |
| — |
|
| |
| 1,316,153 |
Fixed assets | |
| 11,779 |
| | 110,296 | |
| — |
| — |
|
| |
| 122,075 |
Note receivable | |
| — |
| | 245,500 | |
| — |
| — |
|
| |
| 245,500 |
Goodwill | |
| — |
| | — | |
| — |
| 44,877,668 |
| F | |
| 44,877,668 |
Deferred tax assets | |
| — |
| | 563,094 | |
| — |
| — |
|
| |
| 563,094 |
Deposit | |
| — |
| | 20,720 | |
| — |
| — |
|
| |
| 20,720 |
Cash and marketable securities held in Trust Account | |
| — |
| | — | |
| 1,386,490 |
| (1,400,355) |
| A | |
| — |
| | | | | | | | | |
| 13,865 |
| A | | | |
Total Assets | | $ | 1,430,779 | | $ | 6,482,999 | | $ | 1,387,214 |
| 50,893,469 |
|
| | $ | 60,194,461 |
Liabilities and Stockholders’ Equity | |
|
| |
|
| |
|
|
|
|
|
| |
|
|
Current Liabilities | |
|
| |
|
| |
|
|
|
|
|
| |
|
|
Accounts payable and accrued liabilities | | $ | 1,819,512 | |
| 1,804,892 | | $ | 3,642,780 |
| (519,829) |
| C | | $ | 6,656,805 |
| | | | | | | | | |
| (90,550) |
| G | | | |
Deferred revenue | |
| — | |
| 20,000 | |
| — |
| — |
|
| |
| 20,000 |
Excise tax payable | |
| — | |
| — | |
| 72,396 |
| — |
|
| |
| 72,396 |
Income tax payable | |
| — | |
| — | |
| 187,225 |
| — |
|
| |
| 187,225 |
Right-of-use liability | |
| — | |
| 698,480 | |
| — |
| — |
|
| |
| 698,480 |
Line of credit | |
| — | |
| 456,097 | |
| — |
| — |
|
| |
| 456,097 |
Deferred revenue | |
| 1,371,527 | |
| — | |
| — |
| — |
|
| |
| 1,371,527 |
Factoring payable | |
| — | |
| 491,974 | |
| — |
| (250,000) |
| M | |
| 241,974 |
Note payable | |
| 550,000 | |
| 1,581,183 | |
| 926,500 |
| (926,500) |
| G | |
| 1,026,183 |
| | | | | | | | | |
| (1,105,000) |
| H | | | |
Due on share purchase | |
| 135,000 | |
| — | |
| — |
| (135,000) |
| E | |
| — |
Bridge Note | |
| 600,000 | |
| 600,000 | |
| — |
| (1,200,000) |
| K | |
| — |
Accrued interest on Exchange Note | |
| — | |
| — | |
| 78,061 |
| — |
|
| |
| 78,061 |
Additional Bridge Note | |
| — | |
| — | |
| 156,564 |
| — |
|
| |
| 156,566 |
Exchange Note | |
| — | |
| — | |
| 2,814,359 |
| — |
|
| |
| 2,814,359 |
Extension note, net of discount | |
| — | |
| — | |
| 285,614 |
| (285,614) |
| I | |
| — |
42
| | | | | | | | | | | | | | | | |
|
| | |
| | |
| | |
| Transaction |
|
|
| | |
| | | | | | | | | | | Accounting | | | | | |
| | | | | | | | | | | Adjustments | | | | Pro Forma | |
| | (A) | | (A) | | (B) | | (Assuming No | | | | Combined | ||||
| | VSEE | | IDOC | | DHAC | | Additional | | | | (Assuming No | ||||
| | (Historical) | | (Historical) | | (Historical) | | Redemptions) | | | | Redemptions) | ||||
Extension Note- Bifurcated Derivative |
| | — |
| | — |
| | 22,868 |
| (22,868) |
| I |
| | — |
ELOC – Commitment Fee convertible note payable |
| | — |
| | — |
| | 189,764 |
| 500,000 |
| J |
| | 689,764 |
Quantum Convertible Note, net of discount |
| | — |
| | — |
| | — |
| 3,000,000 |
| D |
| | 3,000,000 |
Advances from related parties |
| | 338,506 |
| | 500,000 |
| | 592,800 |
| (241,101) |
| G |
| | 979,940 |
| | | | | | | | | |
| (210,508) |
| L | | | |
Total current liabilities |
| | 4,814,257 |
| | 6,152,626 |
| | 8,968,931 |
| (1,486,970) |
|
|
| | 18,448,844 |
Deferred underwriting fee payable |
| | — |
| | — |
| | 4,370,000 |
| (4,370,000) |
| C |
| | — |
Note payable |
| | — |
| | 1,500,600 |
| | — |
| — |
|
|
| | 1,500,600 |
Right-of-use liability, less current portion |
| | — |
| | 885,940 |
| | — |
| — |
|
|
| | 885,940 |
Total Liabilities |
| | 4,814,257 |
| | 8,539,166 |
| | 13,338,931 |
| (5,856,970) |
|
|
| | 20,835,384 |
Common stock subject to possible redemption |
| | — |
| | — |
| | 1,281,957 |
| (1,295,822) |
| B |
| | — |
| | | | | | | | | |
| 13,865 |
| A | | | |
|
| | — |
| | — |
| | 1,281,957 |
| (1,281,957) | | |
| | — |
Stockholders’ Equity |
| |
|
| |
|
| |
|
|
|
|
|
| |
|
Serries A Preferred |
| | 37 |
| | — |
| | — |
| (37) |
| E |
| | — |
Series A-1 Preferred |
| | 123 |
| | — |
| | — |
| (123) |
| E |
| | — |
Common stock |
| | 1,000 |
| | 4,978 |
| | 350 |
| (1,000) |
| E |
| | 1,470 |
| | | | | | | | | |
| 11 |
| B | | | |
| | | | | | | | | |
| (4,483) |
| F | | | |
| | | | | | | | | |
| 525 |
| E | | | |
| | | | | | | | | |
| 60 |
| K | | | |
| | | | | | | | | |
| 29 |
| H | | | |
Additional paid-in capital |
| | 6,026,457 |
| | 209,521 |
| | 550,246 |
| (13,865) |
| A |
| | 51,359,131 |
| | | | | | | | | |
| 1,295,811 |
| B | | | |
| | | | | | | | | |
| (13,941,934) |
| E | | | |
| | | | | | | | | |
| 1,268,000 |
| G | | | |
| | | | | | | | | |
| 49,289,984 |
| F | | | |
| | | | | | | | | |
| 4,370,000 |
| C | | | |
| | | | | | | | | |
| 1,104,971 |
| H | | | |
| | | | | | | | | |
| 1,199,940 |
| K | | | |
Accumulated deficit |
| | (9,117,796) |
| | (2,270,666) |
| | (13,784,270) |
| 13,865 |
| A |
| | (12,001,524) |
| | | | | | | | | |
| (1,860,476) |
| C | | | |
| | | | | | | | | |
| (300,000) |
| D | | | |
| | | | | | | | | |
| 2,270,666 |
| F | | | |
| | | | | | | | | |
| 13,784,270 |
| E | | | |
| | | | | | | | | |
| (57,268) |
| I | | | |
| | | | | | | | | |
| (500,000) |
| J | | | |
Non-controlling interest |
| | (293,299) |
| | — |
| | — |
| 293,299 |
| E |
| | — |
Total Stockholders’ Equity |
| | (3,383,478) |
| | (2,056,167) |
| | (13,233,674) |
| 58,032,396 |
|
|
| | 39,359,077 |
Total Liabilities and Stockholders’ Equity | | $ | 1,430,779 | | $ | 6,482,999 | | $ | 1,387,214 |
| 50,893,469 |
|
| | $ | 60,194,461 |
43
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE THREE MONTHS ENDED MARCH 31, 2024
| | | | | | | | | | | | | | | | | |
|
| | |
| | |
| | |
| Transaction |
|
|
| | | |
| | | | | | | | | | | Accounting | | | | Pro Forma | ||
| | | | | | | | | | | Adjustments | | | | Combined | ||
| | VSEE | | IDOC | | DHAC | | (Actual | | | | (Actual | |||||
| | (Historical) | | (Historical) | | (Historical) | | Redemptions) | | | | Redemptions) | |||||
VSee revenue | | $ | 1,495,995 | | $ | — | | $ | — | | $ | — |
|
| | $ | 1,495,995 |
Patient fees | |
| — | |
| 1,121,355 | |
| — | |
| — |
|
| |
| 1,121,355 |
Telehealth fee | |
| — | |
| 512,710 | |
| — | |
| — |
|
| |
| 512,710 |
Institution fees | |
| — | |
| 5,700 | |
| — | |
| — |
|
| |
| 5,700 |
Total revenue | |
| 1,495,995 | |
| 1,639,765 | |
| — | |
| — |
|
| |
| 3,135,760 |
Cost of revenue | |
| 386,253 | |
| 400,563 | |
| — | |
| — |
|
| |
| 786,816 |
Gross profit / (loss) | |
| 1,109,742 | |
| 1,239,202 | |
| — | |
| — |
|
| |
| 2,348,944 |
Costs and expenses: | |
|
| |
|
| |
|
| |
|
|
|
| |
|
|
Compensation and related benefits | |
| 893,577 | |
| 402,333 | |
| — | |
| — |
|
| |
| 1,295,910 |
General and administrative | |
| 151,348 | |
| 288,684 | |
| 674,262 | |
| — |
|
| |
| 1,114,294 |
Profossional fees | |
| — | |
| 110,182 | |
| — | |
| — |
|
| |
| 110,182 |
Amortization of client list | |
| — | |
| — | |
| — | |
| 132,562 |
| C | |
| 132,562 |
Operating expenses | |
| 26,338 | |
| 92,000 | |
| — | |
| — |
|
| |
| 118,338 |
Total costs and expenses | |
| 1,071,263 | |
| 893,199 | |
| 674,262 | |
| 132,562 |
|
| |
| 2,771,286 |
Operating loss | |
| 38,479 | |
| 346,003 | |
| (674,262) | |
| (132,562) |
|
| |
| (422,342) |
Interest expense | |
| (9,310) | |
| (78,714) | |
| (113,989) | |
| (717,268) |
| D | |
| (919,281) |
Default interest | |
| — | |
| — | |
| (20,296) | |
| — |
|
| |
| (20,296) |
Initial fair value of Additional Bridge Note | |
| — | |
| — | |
| 3,851 | |
| — |
|
| |
| 3,851 |
Change in fair value of Additional Bridge Note | |
| — | |
| — | |
| (2,133) | |
| — |
|
| |
| (2,133) |
Change in fair value of Exchange Note | |
| — | |
| — | |
| (192,801) | |
| — |
|
| |
| (192,801) |
Change in fair value of ELOC | |
| — | |
| — | |
| 13,956 | |
| — |
|
| |
| 13,956 |
Change in fair value of Extension Note embedded derivative | |
| — | |
| — | |
| 4 | |
| — |
|
| |
| 4 |
Other income (expense) | |
| — | |
| (18,200) | |
| — | |
| — |
|
| |
| (18,200) |
Interest earned on investments held in Trust Account | |
| — | |
| — | |
| 17,853 | |
| (17,853) |
| A | |
| — |
Income (loss) before taxes | |
| 29,169 | |
| 249,089 | |
| (967,817) | |
| (867,683) |
|
| |
| (1,557,242) |
Provision for taxes | |
| — | |
| (55,603) | |
| — | |
| 55,603 |
| F | |
| — |
Net loss | | $ | 29,169 | | $ | 193,486 | | $ | (967,817) | | $ | (812,080) |
|
| | $ | (1,557,242) |
Weighted average shares outstanding, basic and diluted | |
| 9,998,446 | |
| 4,978 | |
| 4,096,353 | |
| 10,264,555 |
|
| |
| 14,360,908 |
Basic and diluted net loss per share | | $ | (0.00) | | $ | 38.87 | | $ | (0.27) | |
|
|
|
| | $ | (0.10) |
44
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2023
| | | | | | | | | | | | | | | | | |
|
| | |
| | |
| | |
| Transaction |
|
|
| Pro Forma | ||
| | | | | | | | | | | Accounting | | | | Combined | ||
| | | | | | | | | | | Adjustments | | | | (Assuming No | ||
| | VSEE | | IDOC | | DHAC | | (Assuming No | | | | Additional | |||||
| | (Historical) | | (Historical) | | (Historical) | | Redemptions) | | | | Redemptions) | |||||
VSee revenue | | $ | 5,840,889 | | $ | — | | $ | — | | $ | — | | | | $ | 5,840,889 |
Patient fees | |
| — | |
| 3,475,666 | |
| — | |
| — | | | |
| 3,475,666 |
Telehealth fee | |
| — | |
| 2,434,210 | |
| — | |
| — | | | |
| 2,434,210 |
Institution fees | |
| — | |
| 716,314 | |
| — | |
| — | | | |
| 716,314 |
Total revenue | |
| 5,840,889 | |
| 6,626,190 | |
| — | |
| — | | | |
| 12,467,079 |
Cost of revenue | |
| 1,933,195 | |
| 2,451,633 | |
| — | |
| — | | | |
| 4,384,828 |
Gross profit / (loss) | |
| 3,907,694 | |
| 4,174,557 | |
| — | |
| — | | | |
| 8,082,251 |
Costs and expenses: | | | | | | | | | | | | | | | | | |
Compensation and related benefits | |
| 4,417,028 | |
| 2,044,822 | |
| — | |
| 804,774 |
| E | |
| 7,266,624 |
General and administrative | |
| 962,616 | |
| 6,052,031 | |
| 2,593,765 | |
| — | | | |
| 9,608,412 |
Profossional fees | |
| — | |
| 87,886 | |
| — | |
| — | | | |
| 87,886 |
Amortization of client list | |
| — | |
| — | |
| — | |
| 530,250 |
| C | |
| 530,250 |
Operating expenses | |
| 86,799 | |
| 358,471 | |
| — | |
| 1,860,476 |
| B | |
| 907,737 |
Total costs and expenses | |
| 5,466,443 | |
| 8,543,210 | |
| 2,593,765 | |
| 3,195,500 | | | |
| 19,798,918 |
Operating loss | |
| (1,558,749) | |
| (4,368,653) | |
| (2,593,765) | |
| (3,195,500) | | | |
| (11,716,667) |
Interest expense | |
| (191,323) | |
| (317,048) | |
| (598,355) | |
| (717,268) |
| D | |
| (1,823,994) |
Default interest | |
| — | |
| — | |
| (1,579,927) | |
| — | | | |
| (1,579,927) |
Gain on forgiveness of debt | |
| 107,862 | |
| 107,862 | |
| — | |
| — | | | |
| 215,724 |
Initial fair value of Additional Bridge Note | |
| — | |
| — | |
| 11,111 | |
| — | | | |
| 11,111 |
Initial fair value of ELOC | |
| — | |
| — | |
| (204,039) | |
| — | | | |
| (204,039) |
Change in fair value of Additional Bridge Note | |
| — | |
| — | |
| (2,726) | |
| — | | | |
| (2,726) |
Change in fair value of Exchange Note | |
| — | |
| — | |
| (97,814) | |
| — | | | |
| (97,814) |
Change in fair value of ELOC | |
| — | |
| — | |
| 319 | |
| — | | | |
| 319 |
Change in fair value of Bridge Note embedded derivative | |
| 90,200 | |
| 90,200 | |
| 120,267 | |
| — | | | |
| 300,667 |
Change in fair value of Extension Note embedded derivative | |
| — | |
| — | |
| 1,630 | |
| — | | | |
| 1,630 |
Change in fair value of PIPE Forward Contract derivative | |
| — | |
| — | |
| 170,666 | |
| — | | | |
| 170,666 |
Other income (expense) | |
| (20,114) | |
| (338,813) | |
| — | |
| — | | | |
| (358,927) |
Impairment charges | |
| — | |
| (104,076) | |
| — | | | | | | |
| (104,076) |
Interest earned on investments held in Trust Account | |
| — | |
| — | |
| 358,767 | |
| (358,767) |
| A | |
| — |
Loss before taxes | |
| (1,572,124) | |
| (4,930,528) | |
| (4,413,866) | |
| (4,271,535) | | | | | (15,188,053) |
Provision for taxes | |
| (1,838,490) | |
| 1,070,410 | |
| — | |
| 768,080 |
| F | |
| — |
Net loss | | $ | (3,410,614) | | $ | (3,860,118) | | $ | (4,413,866) | | $ | (3,503,455) | | | | $ | (15,188,053) |
Weighted average shares outstanding, basic and diluted | |
| 9,998,446 | |
| 4,978 | |
| 4,096,353 | |
| 10,264,555 | | | |
| 14,360,908 |
Basic and diluted net loss per share | | $ | (0.34) | | $ | (775.44) | | $ | (1.08) | | | | | | | $ | (0.99) |
45
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Note 1. Basis of Presentation
The Business Combination between VSee and DHAC will be accounted for as a reverse recapitalization, with no goodwill or other intangible assets recorded, in accordance with GAAP. Under this method of accounting, DHAC will be treated as the “accounting acquiree” and VSee as the “accounting acquirer” for financial reporting purposes. Accordingly, for accounting purposes, the Business Combination will be treated as the equivalent of VSee and iDoc issuing shares for the net assets of DHAC, followed by a recapitalization. The net assets of DHAC will be stated at historical cost. Operations prior to the Business Combination will be those of VSee.
The acquisition of iDoc will be treated as a business combination for which VSee is the accounting acquirer under Accounting Standards Codification (“ASC”) Topic 805, Business Combinations (“ASC 805”) because iDoc meets the definition of a business and VSee indirectly obtain control of iDoc through its acquisition of DHAC under ASC 810. As a result, the acquisition of iDoc will be accounted for using the acquisition method whereby VSee will record the fair value of assets and liabilities acquired from iDoc. The excess of consideration transferred over the fair values of assets acquired and liabilities assumed will be recorded as goodwill.
The unaudited pro forma condensed combined balance sheet as of March 31, 2024 gives effect to the Business Combination and related transactions as if they occurred on March 31, 2024. The unaudited pro forma condensed combined statements of operations for the three months period ended March 31, 2024 and for the years ended December 31, 2023 give pro forma effect to the Business Combination as if it had been completed on January 1, 2023. These periods are presented on the basis that VSee is the acquirer for accounting purposes.
The pro forma adjustments reflecting the consummation of the Business Combination and the related transaction are based on certain currently available information and certain assumptions and methodologies that DHAC management believes are reasonable under the circumstances. The unaudited condensed combined pro forma adjustments, which are described in the accompanying notes, may be revised as additional information becomes available and is evaluated. Therefore, it is likely that the actual adjustments will differ from the pro forma adjustments, and it is possible that the difference may be material. DHAC management believes that its assumptions and methodologies provide a reasonable basis for presenting all of the significant effects of the Business Combination and the related transactions based on information available to management at this time and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information does not give effect to any anticipated synergies, operating efficiencies, tax savings, or cost savings that may be associated with the Business Combination. The unaudited pro forma condensed combined financial information is not necessarily indicative of what the actual results of operations and financial position would have been had the Business Combination and related transactions taken place on the dates indicated, nor are they indicative of the future consolidated results of operations or financial position of the post-combination company. They should be read in conjunction with the historical financial statements and notes thereto of DHAC and VSee and iDoc.
Note 2. Accounting Policies and Reclassifications
Upon consummation of the Business Combination, management will perform a comprehensive review of the three entities’ accounting policies. As a result of the review, management may identify differences between the accounting policies of the three entities which, when conformed, could have a material impact on the financial statements of the post-combination company. Based on its initial analysis, management did not identify any differences that would have a material impact on the unaudited pro forma condensed combined financial information. As a result, the unaudited pro forma condensed combined financial information does not assume any differences in accounting policies.
As part of the preparation of these unaudited pro forma condensed combined financial statements, certain reclassifications were made to align DHAC’s financial statement presentation with that of VSee and iDoc.
Note 3. Adjustments to Unaudited Pro Forma Condensed Combined Financial Information
The unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the Business Combination and related transactions and has been prepared for informational purposes only.
46
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” Release No. 33-10786 replaces the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”) and present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). DHAC has elected not to present Management’s Adjustments and will only be presenting Transaction Accounting Adjustments in the unaudited pro forma condensed combined financial information. DHAC and VSee and iDoc have not had any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
The pro forma basic and diluted earnings per share amounts presented in the unaudited pro forma condensed combined statement of operations are based upon the number of VSee and iDoc’s ordinary shares outstanding, assuming the Business Combination and related transactions occurred on January 1, 2023.
Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet
The adjustments included in the unaudited pro forma condensed combined balance sheet as of March 31, 2024 are as follows:
| A. | Reflects the reclassification of $1.40 million held in the Trust Account to cash and cash equivalents that becomes available at closing of the Business Combination and the incremental interest earned in trust through June 24, 2024. |
| B. | Reflect the reclassification of the common stock subject to redemption for cash amounting to $1.30 million is transferred to permanent equity. |
| C. | Represents the payment of $2.59 million estimated transaction costs, including banking, printing, legal and accounting services. $1.86 million was expensed as part of the Business Combination that are deemed to be direct and incremental cost of the Business Combination and recorded in accumulated deficit, $0.52 million was net impact of transactions cost included in accounts payable and agreed vendor deferral of fees. Additionally, approximately $4.37 million of deferred underwriting fees payable by DHAC have been satisfied via the issuance of preferred stock to the underwriter, representing 4,370 Preferred Shares to with par value of $0.0001. The par value is minimal as such $4.37 million was applied to additional paid in capital. |
| D. | Reflects the proceeds from the convertible note purchase agreement (the “Quantum Purchase Agreement”), pursuant to which an institutional and accredited investor (the “Quantum Investor”) subscribed for and will purchase, and DHAC has issue to the Quantum Investor, at the Closing, a 7% ($0.21million) original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000 for cash proceeds of $2,700,000 at closing. The originally issued discount of $0.30 million is included net of the Convertible Note payable balance and the debt discount will be expensed at issuance. The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) a fixed conversion price of $10 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30th-day anniversary of the Quantum Note issuance date (the “Average Price”) is less than $10, to a price equal to the Average Price but in no event less than $2.00. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10. If an event of default occurs, the Quantum Note would bear interest at a rate of 18.00% per annum. The Convertible Promissory Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings. |
| E. | Represents recapitalization of VSee outstanding equity as a result of the reverse recapitalization. Upon closing all of the outstanding shares held by VSee Equity Holders will be exchanged for 5,246,354 Common Stock of VSee Health Inc. (which includes 338,387 Common Shares allocated to the TAD shareholders in connection with the TAD Exchange representing approximately $3.38 million estimated fair value at $10 per share) and 803,646 fully vested stock options will be granted to VSee employees to purchase common stock of VSee Health Inc., at $10 per share and as exchange for the reverse recapitalization of VSee and the removal of the non-controlling interest in TAD. The entry also reflects and the elimination of historical accumulated deficit of DHAC. The shares and options granted to VSee shareholders were determined based on the |
47
| estimated value attributed to VSee of $60.50 million as determined by the Board in its negotiations with VSee management. The options are fully vested at the closing of the business combination and in accordance with ASC 805 are deemed to be part of the consideration granted in the business combination exchange as such no compensation expense is recognized. There are a total of 174,302 options issued to employees whom will vest between 40% and 60% over a one year service period subsequent to the business combination which will be valued at the effective grant date to be recognized as compensation over the vesting period post business combination. As such, no adjustment was included on the balance sheet as no expense would be incurred for these options. Refer to Note E below on adjustment to the statement of operations for further discussion. |
| F. | Reflects the allocation of the purchase price for the acquisition of iDoc. Upon closing all of the outstanding shares of iDoc will be exchanged for 4,950,000 Common Stock of VSee Health Inc. The number of shares was based on the estimated value attributed to iDoc of $49.50 million. iDoc provider of tele-intensive acute care, and tele-neurocritical care in high value hospital environments. iDoc leverages its extensive telehealth platform, and neuro and general critical expertise to treat and monitor acutely ill patients with diseases of the brain, spinal cord, heart, and lungs that often have complicated medical problems. The iDoc is a virtual health services management company that is responding to the need for rapid, effective treatment of emergency patients and the shortage of critical care experts. The Telehealth market today is one characterized by rapid transformation, with major customers and hospital systems looking to build or add capabilities and major legacy competitors looking to shore up historical limitations. The rapid transformation of the telehealth market indicates strong future growth of the market, and its current offerings provide an attractive value proposition to health systems, medical groups, and individual medical practitioners, driving higher market share. The goodwill of $44.88 million is from the plan to continue to harness its scale to further grow the value proposition of the platform for all stakeholders. |
Below is the summary and allocation against the preliminary estimated of the purchase price to the assets acquired and liabilities assumed (in millions) as follows:
| | | |
Customer list |
| $ | 5.30 |
Trade name | | | 1.38 |
Net liabilities |
| | (2.06) |
Total identified assets and liabilities |
| | 4.62 |
Purchase consideration |
| | (49.50) |
Goodwill | | $ | 44.88 |
Customer list — To determine the estimated value of the customer list for iDoc the Multiple Period Excess Earnings Method “MPEEM” discounted cash flow approach was used to measure the fair value of an intangible asset. Under the MPEEM, the free cash flows for a group of assets is determined, and then adjusted for a contributory charge for the use of other identifiable tangible and intangible assets. The present value of the resulting excess cash flows is adjusted for any tax benefits and the resulting amount represents the fair value of the intangible asset. Since the intellectual property is the core asset of iDoc, the primary intellectual property consisting of iDoc’s designs, trademarks and processes, were evaluated using the MPEEM. Based on industry information and perceived risk of each individual contributory asset, it was determined that a reasonable return for fixed assets was 12.0% and the reasonable rate of return for working capital was 6.5% based on iDoc’s current line of credit. Management has assigned estimated lives of 10 years for the iDoc customer list.
Trade name — The Relief from Royalty method considers what a purchaser could afford, or would be willing to pay, for a license of similar intellectual property rights. The royalty stream is then capitalized reflecting the risk and return relationship of investing in the asset. The Relief from Royalty technique has been used for many years in the valuation of intangible assets and intellectual property. It is based on the assumption that the owner of intangible property is “relieved” from paying a royalty to obtain its use. This method requires that the intellectual property have been developed to the point where it can be expected that products encompassing the technologies could be produced within a reasonable period of time. Second, there was sufficient data to develop reasonable estimates of the royalty base (i.e., projected revenues). Third, there was adequate data to support the determination of a reasonable royalty rate. Finally, the Relief from Royalty Approach accounts for market conditions, the economic life of the Intellectual Property and the risk associated with receiving future economic benefits. A Royalty rate of 1% was used to determine the estimated value of the trade name. The trade name is a non-depreciable asset that will be analyzed for impairment annually or when indicator are present that would indicate impairment.
| G. | Reflect the issuance of shares of certain indebtedness owed by DHAC will be converted into Series A Preferred Stock at the Closing of the Business Combination, resulting in the issuance of 126,800 common share equivalents for the settlement of |
48
| $0.93 million in promissory notes held at March 31, 2024 by DHAC, $0.25 million in related party advances held as March 31, 2024, $0.01 million of accrued administrative fees and accrued expenses held at March 31, 2024. As a result 1,268 Preferred Shares to be issued with par value of $0.0001. The par value is minimal as such $1.27 was applied to additional paid in capital. |
| H. | Reflect the issuance of shares of certain indebtedness owed by VSee and iDoc will be converted into Series A Preferred Stock at the Closing of the Business Combination, resulting in the issuance of 300 Preferred Shares for the settlement of $0.30 million in Promissory notes held at March 31, 2024 by iDoc, and 220 Preferred Shares for settlement of $0.22 million in notes payable by VSee held as March 31, 2024. The par value is minimal for the Preferred Shares as such $0.52 was applied to additional paid in capital. In additional $0.59 million of Notes payable held at March 31, 2024 by iDoc were settled via the issuance of 292,500 shares of common stock. |
| I. | Reflect the pay off of the investor note payable held by DHAC in cash at the closing for $0.37 million, resulting in an additional $0.57 million in related amortization and interest that would become due as part of the mandatory early repayment. |
| J. | Reflect the convertible note issued as a commitment fee in connection with the Equity Purchase Agreement with an institutional and accredited investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s Common Stock, from time to time over a 36-month period (the “Equity Purchase Commitment Period”) beginning from the sixth (6th) trading day following the closing of the Business Combination transaction (the “Equity Purchase Effective Day”), provided that certain conditions are met. The Company also agreed to file a resale registration statement to register shares of Common Stock to be purchased under the Equity Purchase Agreement with the SEC within 45 days following the Equity Purchase Effective Day, and shall use commercially reasonable efforts to have such registration statement declared effective by the SEC within 30 days of such filing. During the Equity Purchase Commitment Period, DHAC may suspend the use of the resale registration statement to (i) delay the disclosure of material nonpublic information concerning the Company in good faith or (ii) amend the registration statement concerning material information, by providing written notice to the investor. Such suspension cannot be longer than 90 consecutive days (or 120 days in any calendar year). The investor has agreed not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of the Company’s Common Stock. On the Equity Purchase Effective Day, the Company will issue to the investor, as a commitment fee for this equity purchase transaction, a senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of the Company’s Common Stock at a fix conversion price of $10 per share (the “Equity Purchase Note”). |
| K. | Reflect the issuance of shares of certain indebtedness owed by iDoc and Vsee of $0.60 million each for a total of $1.2 million, settled with the issuance of 300,000 common stock for a total of 600,000 shares. |
| L. | Reflects the elimination of due to due from between iDoc and Vsee of $0.21 million. |
| M. | Reflects the payment of $250,000 at closing for the factoring arrangement held by iDoc. |
Adjustments to Unaudited Pro Forma Condensed Combined Statements of Operations
The pro forma adjustments included in the unaudited pro forma condensed combined and consolidated statements of operations for the three months period ended March 31, 2024 and for the years ended December 31, 2023 is as follows:
| A. | Reflects elimination of investment income on the Trust Account. |
| B. | Reflects the estimated transaction costs of approximate $1.86 million as if incurred on January 1, 2023, the date the Business Combination occurred for the purposes of the unaudited pro forma condensed combined statement of operations. This is a non-recurring item. See Note C of the unaudited proforma balance sheet adjustment. |
| C. | To reflect incremental amortization expense as a result of the fair value adjustment to intangible assets. Amortization expense related to customer list valued at $5.30 million over 10 years. Amortization expense of $0.53 million for year ended December 31, 2023. Amortization expense related to the customer list as general and administrative expense. The trade name is a non-depreciable asset that will be analyzed for impairment annually or when indicator are present that would indicate impairment. See Note F of the unaudited proforma balance sheet adjustment. |
49
| D. | To reflect an adjustment to record interest expense of $0.63 million for the following financing arrangements: To adjust for interest expense of 12.00% reflecting interest since January 1, 2023 of $0.36 million and the 7% original issue discount of $0.30 million on under the Quantum Note. See Note D of the unaudited proforma balance sheet adjustment. In addition, the adjustment reflects interest expense of $0.06 million in related amortization and interest that would become due as part of the mandatory early repayment. See Note I of the unaudited proforma balance sheet adjustment. |
| E. | To reflect the stock based compensation for the 174,302 options granted to the employees which vest over a one-year period. The value of the options was $0.80 million using Black Scholes valuation model. Resulting in $0.80 million for the year ended December 31, 2023. The inputs were as follows: |
| | | | |
Risk-free interest rate |
| | 5.10 | % |
Expected terms (years) |
| | 1.0 | |
Expected volatility |
| | 95.0 | % |
Exercise price | | $ | 12.11 | |
Stock price | | $ | 12.11 | |
| F. | Although the blended statutory rate for the entity post business combination would be 21%, the combined pro forma information under both scenarios results in a net loss for tax purposes. As such, a full valuation allowance has been applied resulting in no anticipated tax provision. |
Note 4. Net Income (Loss) per Share
Net income (loss) per share was calculated using the historical weighted average shares outstanding, and the issuance of additional shares in connection with the Business Combination and the related transactions, assuming the shares were outstanding since January 1, 2023. As the Business Combination and the related transactions are being reflected as if they had occurred at the beginning of the period presented, the calculation of weighted average shares outstanding for basic and diluted net income (loss) per share assumes that the shares issuable relating to the Business Combination and related have been outstanding for the entirety of all periods presented.
The unaudited pro forma condensed combined financial information has been prepared to present two alternative scenarios with respect to redemption of Common Stock by DHAC Public Stockholders at the time of the Business Combination for the years ended December 31, 2023 (in thousands, except shares and per share numbers):
| | | | | | |
|
| Three months Ended |
| Year Ended | ||
| | March 31, 2024(1) | | December 31, 2023(1) | ||
Pro forma net loss | | $ | (1,557) | | $ | (15,188) |
Weighted average shares outstanding – basic and diluted | | | 15,308,620 | | | 15,308,620 |
Net loss per share – basic and diluted | | $ | (0.10) | | $ | (0.99) |
Excluded securities:(2) | |
|
| |
|
|
Public Warrants | |
| 11,500,000 | |
| 11,500,000 |
Private Warrants | |
| 557,000 | |
| 557,000 |
Bridge Warrants | |
| 173,913 | |
| 173,913 |
Extension Warrants | |
| 26,086 | |
| 26,086 |
Quantum Convertible Note | |
| 300,000 | |
| 300,000 |
Bridge Convertible Notes. | |
| 320,375 | |
| 320,375 |
Tidewater assumed by DHAC Note | |
| 292,500 | |
| 292,500 |
Bridge assumed by DHAC Notes | |
| 600,000 | |
| 600,000 |
Stock options to be issued at effective time under stock option plan to be adopted at effective time | |
| 803,646 | |
| 803,646 |
| (1) | Pro forma income (loss) per share includes the related pro forma adjustments as referred to within the section “Unaudited Pro Forma Condensed Combined Financial Information.” |
| (2) | The potentially dilutive outstanding securities were excluded from the computation of pro forma net loss per share, basic and diluted, because their effect would have been anti-dilutive, issuance or vesting of such shares is contingent upon the satisfaction of certain conditions which were not satisfied by the end of the periods presented. |
50
DESCRIPTION OF BUSINESS
Overview
Through our wholly-owned subsidiary VSee Lab, we offer a comprehensive telehealth platform for U.S. hospitals and enterprises. Through VSee Lab, we offer a set of telehealth software building blocks, data connectors, and workflow templates that can be rapidly configured into the client’s workflows. Our offerings allow clinicians without programming experience to configure our building blocks into their existing workflow without requiring programmers - i.e. - no code. In addition, our building blocks allow programmers to increase their productivity with simple coding to piece together our building blocks - i.e. - low code.
At the core of our platform is a comprehensive set of software building blocks for telehealth that include on-demand visits, scheduling appointments, in-take forms, signature for consent and compliance, team coordination, unified communication, remote exam and remote patient monitoring, payments including insurance processing, clinical notes, and administrative control panels and analytics. These set of building blocks can connect to electronic medical record systems such as EPIC and Cerner via HL7, FHIR, and sFTP. Lastly we provide a set of templates to make creating telehealth workflow fast and easy. The entire telehealth platform sits on a scalable server architecture and is HIPAA compliant and SOC2 externally audited. VSee Lab is also GDPR compliant and supports single-sign-on (SSO) and multi-factor-authentication (MFA).
We put telehealth software tools in the hands of clinicians to enable them to make changes without programming so that they can achieve the best patient outcomes. We provide our clients with capabilities specifically built to enable them to collaborate with their clinical and non-clinical colleagues, securely coordinate patient care, conduct virtual patient visits including remote physical exam and remote patient monitoring, and an analytical dashboard to manage their entire telehealth operations from patient satisfaction score to patient wait time to staffing allocations. We empower clinicians to create the workflow they want without waiting for IT; where today, most clinicians feel helpless given that IT departments often cannot give clinicians what they want.
To complement our offerings through VSee Lab, we also provide high acuity patient care solutions through our wholly-owned subsidiary — iDoc. Through iDoc, we offer. specialty intensive care unit services by providing physician services in the neurology intensive care unit (neurointensivists), cardiac intensive care unit (surgical and anesthesia intensivists), and medical intensive care units (pulmonary and critical care intensivists).
We strive to be the solutions provider of access to the shortage of intensivists across the care continuum utilizing sophisticated telehealth solutions to bridge the care gap. In a post Covid, physician burnout health care system, we aim to provide a solution to physician burnout and to a lack of patient access to quality intensive care. By using the sophisticated leading telehealth software and hardware devices, we provide access to highly skilled physicians in the highest acuity in patient setting, the ICU. We provide elite physician services in the Intensive care units of major hospital systems and other customers. Our core service delivers general critical care, neurology, EEG reading, and neuro critical care through a custom internal virtual health care technology platform. We also serves a diverse range of customers from large hospital systems to small/micro hospitals, to long-term acute care (LTAC) facilities to the federal prison system and others. We connect critically ill patients to high quality Neurointensivists, general and cardiac intensivists and specialty specific e-consultations and helps to improve outcomes for patients as well as improved productivity and physician burnout while reduced costs for health systems. We have developed a unique quality control program in collaboration with each hospital by development of a hospital specific reporting dashboard to monitor and achieve high quality critical care quality. In addition, current workflows and protocols are evaluated to adjust to incorporate critical care. Continuous process improvement and readjustment of target metrics with the ICU team to maximize patient safety and improve outcomes.
VSee Lab Product Offerings
The telehealth platform we have created provides a set of powerful building blocks to solve clients’ urgent and growing needs.
Our “Patient Engagement Solutions” enable our health system customers to create a modern, warm, and productive experience, from scheduling an in-person appointment to conducting a virtual visit to reviewing the instructions from the physicians. Patients often need to use a legacy telephone system to engage their healthcare team or use the patient portal from the electronic medical record (EMR) companies where the user experience is often poor. Our patient engagement solutions deliver high engagement and high value and help our customers embrace the shift to digital patient experience.
Our “Clinician Staffing Solutions” enable our health system customers to create a shared coverage model of staffing. Due to a shortage of clinicians, often times coverage is required to shift to external medical teams where the medical records and notes are
51
passed back to the hospital clinicians. To address this, we offer a solution where an on-demand, i.e., walk-in, patient can be matched to a small set of physicians. If one of these physicians takes this patient, the other physicians are notified and they can accept the next patient in the waiting queue. If all these physicians are busy, our solution will use a hunt group protocol to notify another set of physicians, until this patient is seen by a physician. This routing system allows a patient to be efficiently routed among the clinicians directly employed by the hospital as well as routing to external medical groups for additional coverage. Without innovative digital solutions, hospitals would pay often pay locum tenens nurses and physicians at two times or more the rate of an employed clinician.
Our “Remote Physical Exam and Remote Patient Monitoring Solutions” allow the streaming of medical devices such as otoscope and stethoscope to conduct a remote physical exam live. This contrasts with video-only telehealth that does not allow remote exams. In addition, our solution allows remote patient monitoring where our customers can pull data from blood pressure cuffs, digital scales, etc., to allow clinicians to monitor a patient remotely. Many existing telehealth tools are limited to only seeing someone via video while our solution augments the video experience with medical devices. Such capability allows our clients to seamlessly go from text only, audio only, video only, to full medical device streaming and monitoring.
Our “AI for telesitter and telenursing Solutions” enable healthcare systems to use AI and remote nurses to augment the staffing of bedside nurses, thereby minimizing the impact of nursing shortages. Our offering includes remote admission and discharge, asynchronous and on-demand nursing mentoring, and AI that monitors events in the patient’s room - such as creating virtual fencing for fall prevention, detecting if a patient is under stress, etc. VSee Lab’s AI is able to convert events in the patient’s room into events in VSee Lab’s task queues, where VSee Lab can route the events to first line telenurses. If the first line nurses do not respond the events within a threshold, the events are routed to nurses in a command center - where this 2 layer nursing coverage minimizes the chance that an adverse event is not processed.
Our solutions and technologies have the following features and advantages:
Our Simple Patient Experience. Before the virtual visit, our telehealth platform allows healthcare providers to:
| ● | Invite patients to your branded waiting room by email, SMS or website embedded button; |
| ● | Allow on-demand walk-ins and/or scheduled visits; |
| ● | Customize intake forms with logic such as calculating GAD7, etc.; |
| ● | Automate compliance with consent and provider state-matching, i.e., patient will only see providers with medical license in the patient’s state; |
| ● | Verify insurance eligibility; |
| ● | Collect online credit card payments; |
| ● | Lower wait times with wait queue tagging of specific clinician and hold my spot; |
| ● | Show educational videos or articles as patients wait; and |
| ● | Live chat with the front desk. |
During the virtual visit, we allow healthcare providers to:
| ● | Add in remote family members, interpreters, and other care team members scheduled or on-demand; |
| ● | Share and annotate images, documents and websites as though you were face-to-face; |
| ● | Send files; |
52
| ● | Push additional forms for patients to fill out or sign; |
| ● | Live stream digital peripherals such as otoscopes and stethoscopes for remote physical examination; and |
| ● | Far end pan, tilt, zoom camera control. |
After the virtual visit, we allow healthcare providers to:
| ● | Pass patients back to front desk staff or scheduler to schedule the next appointment; |
| ● | Have patients self-schedule follow-up visits via VSee patient portal; |
| ● | Send auto-confirmations and reminders via email & SMS; |
| ● | Let patients immediately review their visit notes & attachments via VSee patient portal; |
| ● | Get instant feedback with a post-visit survey; |
| ● | Have patients pick up ePrescribe medication (including EPCS) from self-selected pharmacy; and |
| ● | Receive SuperBill for billing insurance. |
In addition to virtual visits, we also allow patients to engage their everyday wellness:
| ● | Set personal health and wellness goals with their care team; |
| ● | Track their own progress with wellness device data from Fitbit, wireless scales, blood pressure cuffs, etc.; |
| ● | Share their food diary, mood chart, or other wellness charts; and |
| ● | Securely message questions to their provider or just share vacation photos. |
Our Productive Clinician Experience. Setting up telehealth is often a complex experience, but our system allows fast setup and go live by which healthcare providers can:
| ● | Set up a tailored online practice in as a fast as a few hours; |
| ● | Add logo, room description, provider profiles, legal documents; |
| ● | Create or remove new providers, patients, and waiting rooms; |
| ● | Turn on/off options for 250+ points of configuration without doing programming (no code); |
| ● | asynchronous (eConsult) workflow; |
| ● | walk-ins, appointments, group appointments; |
| ● | online payments, eligibility, claims submissions; |
| ● | flexible intake forms with logic and attachments; |
| ● | scheduled appointment interpreter service dispatch; and |
| ● | Integrate with electronic medical records (EMRs) such as EPIC, Cerner, etc. |
53
VSee supports efficient team coordination by allowing healthcare providers to:
| ● | Set sound alerts and mobile notifications for when a patient is ready to be seen; |
| ● | Manage all patients from different waiting rooms in a single dashboard; |
| ● | Track patients throughout their visit — know exactly where they are at any point In the patient journey; and |
| ● | Coordinate among the Medical Administrative Specialists, nurses, physicians, schedulers with internal chats and customizable visit tags. |
Our Powerful Administrative Features. In addition to providing a simple patient and productive clinician user experience, VSee also provides powerful administrative features to enable enterprise control of their telehealth operations by allowing healthcare providers to:
| ● | Enforce HIPAA with user access roles and intrusion logging; |
| ● | Manage clinician staffing and patient scheduling with rich master calendar; |
| ● | Set visit payment amounts, generate invoices and superbills, and setup insurance claims engines; |
| ● | Build an actionable data dashboard with drag-and-drop widgets to monitor patient satisfaction, wait time, etc.; |
| ● | Create admin dashboard reports and export to third party visualization engines; and |
| ● | Manage call recordings policies and archives. |
In addition to the features above, our platform supports numerous enterprise scaling features, such as ensuring compliance with state medical license rules across thousands of providers, using routing to balance patient load across all the clinicians in a healthcare system, and using patient tagging to efficiently move a patient within a department. VSee also supports powerful security models such as strong encryption, single-sign-on (SSO), multi-factor-authentication (MFA), and VSee has passed the SOC2 audit.
iDoc’s Offering of Services and Technologies
As a solutions company focused on inpatient specific disease areas with intentional purpose driven growth, and areas to provide access to general and specialty care to vulnerable patient populations, iDoc has a patient e-consultation service that is currently tailored to provide outpatient care to correctional facilities predominantly at the Federal level. The unique security challenges for digital healthcare as well as in person care positions iDoc’s platform to meet the rigorous requirements of the Federal Bureau of Prisons (FBOP). Through our own network of physicians, we provide e-consults in over 14 specialties to the FBOP including areas such as mental health, cardiology, oncology, rheumatology, neurology, nephrology, and more. We connect the patient to the specialty physicians to minimize care delays and transport safety concerns.
We are focused on providing the highest level of clinical and operational quality. We have developed a comprehensive quality management program that supports evidence-based practices, tracks customer satisfaction levels and encourages continuous improvement of telemedicine services. Our clinical leaders regularly review industry accepted standards and, when appropriate, make changes to our protocols. As new practice standards are introduced, our network of board-certified physicians and other provider specialists review these standards and adapt them for national telemedicine practice. Our network physicians and other specialists are continuously trained and evaluated to appropriately integrate and utilize these updated practice standards.
We have developed a care delivery model that intentionally collaborates with hospital systems, healthcare providers, and health care administrators to deliver a customizable solution and experience for the patient. iDoc uses its internally developed telehealth software to deliver the right care at the right time bridging specialty critical care physicians with critically ill patients.
The iDoc technology platform consists of video conferencing, electronic health records, and billing technology working seamlessly to provide telemedicine consults for clinical practice. The virtual healthcare platform can be used individually or in
54
conjunction with native applications/resources or as a whole package. All conference connections are HIPAA compliant and secured through HTTPS/ TLS 1.2+ level encryption. Recorded video EEG data (no other video or audio recordings are made/stored) is transmitted using industry standard HTTPS/TLS 1.2+ and stored in the iDoc AWS cloud. The data stays encrypted at rest No ePHI is stored by this module.
The iDoc clinical dashboard gives providers an intuitive and multi-level view of patients in both aggregate and individual modes. This system interfaces with the client’s vital signs monitors (and other data when available) to facilitate the patient monitoring process. Support for handoffs includes sticky notes and messaging. Customizable alert levels ensure alarms are valid and help prevent “alert fatigue”. This system interfaces with the client’s vital signs monitors (and other data when available) to facilitate the patient monitoring process.
To ensure quality, iDoc has developed a comprehensive quality management program that supports evidence-based practices, tracks customer satisfaction levels and encourages continuous improvement of telemedicine services. We regularly review industry accepted standards and, when appropriate, make changes to our protocols. As new practice standards are introduced, our network of board-certified physicians and other provider specialists review these standards and adapt them for national telemedicine practice. Our network physicians and other specialists are continuously trained and evaluated to appropriately integrate and utilize these updated practice standards.
Market Opportunity
We believe there are two significant trends and challenges facing healthcare in the United States: first, we believe that hospitals need a better method to engage with their patients, to make the patient’s experience dealing with healthcare easier; second, we believe that hospitals need better clinician staffing options since there is an ever growing shortage of nurses and physicians across America.
In addition, intensive care units have become increasingly complex environments, the dramatic increase in surgical therapeutic options for stroke, and the proliferation of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and Det Norske Veritas (DNV) stroke centers with the advent of multi-modal monitoring in the neurointensive care unit, the need and use of electroencephalogram (EEG), the demand for intensivists with the knowledge to care for these patients has led to the growth of neurointensivists (critical care physicians with specialty training in neurosciences). The increasing utilization of extracoporal membrane oxygenators (ECMO) and left ventricular assist devices (LVAD) has led to increase demand for cardiac intensivists (critical care physicians with specialty training in cardiac disorders). The growth and evolution of cloud services and technology focused on delivering health care has led to the ability and need to provide greater and more specialty tele-intensive care and telemedicine solutions to be developed and adopted.
Our business model is designed to both empower the clinicians while satisfying the IT security requirements, where we become the digital transformation tool that allows healthcare enterprises to tackle the twin mega trends and challenges of patient engagement the clinician shortage. Our revenue-generating customers, primarily healthcare enterprises, have access to a suite of telehealth building blocks to support their use cases. Our focus on clinician-centric product design and productivity is what led us to create the no code approach to telehealth configuration, where instead of having clinicians at the mercy of the IT, the clinicians can configure their own telehealth software using a graphical user interface and simple setup instructions.
We believe that the health system is not generally capable of training the necessary critical care physicians to meet the increasing demand from densely populated to rural hospitals or from large academic medical centers to micro hospitals (hospitals with typically less than 25 beds). For hospitals which are lacking physician staffing, iDoc physicians develop and staff ICU facilities through telemedicine. We collaborate in tracking the signs of early disease recognition and recovery in a patient’s condition. We specialize in providing state of the art equipment for audiovisual monitoring of the patient in a real time synchronous interface with hospital EHR systems.
Sales and Marketing
We employ a direct sales organization composed of highly trained team members. The sales organization is segmented primarily by customer type. For example, there is one enterprise-focused team concentrating on mental health and another concentrated on health systems. Our direct sales organization also reaches customers through indirect channels, such as third-party resellers and premiers. We also have a team of experienced sales executives who are primarily responsible for selling our solutions and services directly to hospitals and health systems. In addition, we have developed channel customers who incorporate our platform as part of a model that combines on-site staffing solutions with telemedicine
55
The direct sales organization is supported by marketing and customer success specialists. Our marketing program supports our growth and lead generation though content development, brand awareness, search engine optimization, field marketing events, integrated campaigns, industry relations and public media. We generate customer leads, accelerate sales opportunities, and build brand awareness through our marketing programs, both digitally and via strong word of mouth and client references. These programs target decision makers to provide information about our company and solutions through digital channels such as LinkedIn, our annual conference, online webinars, and tradeshows. Our customer success team supports customer retention by working directly with customers to produce higher engagement with our solutions, which in turn expands their use of the platform in the future.
Competition
We view as competitors those companies that currently or in the future will develop and market virtual care technology (devices, software, and systems) or provide virtual care services, such as the delivery of on-demand access to healthcare and specialty disease state and care management and services. Competition focuses on, among other factors, software as a service (SAAS), experience in operations, customer service, quality of technology and know-how, and reputation. Competitors in the telehealth and specialty medical services market include MDLive, Inc. (now owned by Cigna), American Well Corporation, Included Health, and Accolade, Inc., among other smaller industry participants. Neuro and/or ICU specialty competitors include NeuroCall, Ceribell, and Specialist on Call. Technology solution competitors include American Well Corporation, MDLive, Teladoc, as well as smaller technology providers. Each of VSee and iDoc also faces competition from large, well-financed health plans that in some cases have developed their own virtual care, expert medical service or in-house software platforms, as well as large technology and retail companies, such as Google, Microsoft, Amazon and Walmart, which have or may in the future develop or acquire their own virtual care solutions.
Many of our competitors are well financed, have been in business for substantially longer, have substantial financial resources and long standing contracts and relationships with major customers. Many of our competitors have public financial structures which enable them access to significant amounts of capital at a relatively low cost of capital. We have experienced, and expect to continue to experience, intense competition from a number of companies, and we expect such competition to increase as our industry evolves.
Our competitors may announce new products, services, or enhancements that better address changing industry standards or the needs of customers. Any such increased competition could cause pricing pressure, loss of market share or decreased client engagement, any of which could adversely affect our business and operating results. Internet search engines could also change their methodologies in ways that adversely affect our ability to optimize our page rankings within their search results. If this occurs, our ability to successfully market our services to customers may be harmed and our business results may suffer.
Particularly, VSee Lab faces competition across three categories: 1) EMR’s built in telehealth tool, 2) TelaDoc, AmWell, Zoom, Microsoft Teams, and 3) home-grown custom-built solutions.
| ● | Competing with EMR’s build in telehealth: Almost all electronic medical record (EMR) systems now have built in telehealth tools. These tools are mainly video conference; such as adding a button to make a Zoom or Microsoft Teams or Twilio video call. VSee is designed to integrate with the EMR, thus while we compete with the EMR’s built-in telehealth tool, we also add value to the EMRs. While the software offered by such competitors are more rigid and more time consuming with respect to requested changes to the workflow, VSee allows changing workflow in minutes via our no code option and in days and weeks via our low code option. |
| ● | Competing with Telehealth and Video Conference Software: In many healthcare enterprises, Zoom and Microsoft Teams have approached telehealth with video conferencing. In contrast, VSee provides productivity and patient engagement features that go beyond video only. We also face competition from TelaDoc, specifically their InTouch offering, and AmWell, both of whichare mature and established companies. |
| ● | Competing with home grown or custom-built software: Given that some existing telehealth vendors such do not satisfy many client requirements, many clients decide to build their telehealth from scratch. Such projects require dozens of engineers and often take many months or even years. |
iDoc’s primary competitors include Hicuity Health, INTELEICU, and enVision teleICU (segment of INOVA), among others. While there are several competitors in this industry, many began from a hardware-centric focus, aiming to extend and integrate their devices into hospitals. We approached the development of our services and software platform differently by focusing on optimizing an extensive network of board-certified physicians and other provider specialists across numerous complex workflows. As a result, configurability, modularity ,and optimization became imperative, and we subsequently made these capabilities available on a low-
56
code development platform to address the configurability needs of our customers. We believe we compete favorably based on the following key competitive factors for our industry:
| ● | access to a broad network of established, board-certified physicians and other provider specialists; |
| ● | purpose-built acute care platform with highly configurable workflows and easy integration; |
| ● | demonstrated scalability; |
| ● | clinical and service quality; |
| ● | customer satisfaction; |
| ● | value; |
| ● | reporting, analytics and benchmarking; |
| ● | experience; and |
| ● | flexibility. |
Research and Development
The Telemedicine and Tele-intensive care market is a rapidly evolving industry. Our ability to continue differentiating and enhancing our platform and services depends on our capacity to introduce new services, technologies, and functionality. Due to capital constraints, we currently do not have active research and development spending. Strategically, to maintain and grow our market viability and strength, we plan to focus our future research and development on delivering new products and further enhancing our solutions’ functionality, performance, and flexibility.
U.S. Law and Regulations
Our operations are subject to comprehensive United States federal, state and local regulation in the jurisdictions in which we do business. The laws and rules governing our business and interpretations of those laws and rules continue to expand and become more restrictive each year and are subject to frequent change, especially health regulatory requirements. Our ability to operate profitably will depend in part upon our ability, and that of our affiliated provider network, to operate in compliance with applicable laws and rules. Those laws and rules continue to evolve, and we therefore devote significant resources to monitoring developments in healthcare regulation. As the applicable laws and rules change, we are likely to make conforming modifications in our business processes from time to time. No assurance can be made that a review of our business by courts or regulatory authorities will not result in determinations that could adversely affect our operations or that the healthcare regulatory environment will not change in a way that restricts our operations.
Data Protection, Security, and Regulatory Compliance
The data we collect and process is an integral part of our tools and solutions. In addition, our business is subject to extensive, complex, and rapidly changing federal and state laws and regulations governing data collection, healthcare regulation, financial services laws, regulations and rules, such as the Payment Card Industry Data Security Standards, and related matters. Our respect for laws and regulations regarding the collection and processing of personal data underlies our strategy to improve our security model and implementation. While we believe we comply in all material respects with applicable laws and regulations, these regulations can vary significantly from jurisdiction to jurisdiction, and interpretation and enforcement of existing laws and regulations may change periodically. Federal and state legislatures also may enact various legislative proposals that could materially impact certain aspects of our business. For additional information, see “Risk Factors - Risks Related to Our Business - We are subject to stringent and changing laws, regulations, self-regulatory schemes, contractual obligations, and standards related to privacy, data protection, and information security. The actual or perceived failure by us, our customers, partners, or vendors to comply with such obligations could harm our reputation, subject us to significant fines and liability, or otherwise adversely affect our business.”
57
Data Collection and Protection
We collect and use personal information for the purpose of clinical care on the behalf of our healthcare clients. In some instances, we may use third-party service providers to assist us in the collection efforts.
All data is encrypted in transit and at rest using TLS 1.2, and personal health information is encrypted at rest using AES-256 encryption. Along with a dedicated in-house security team and contracted security researchers, we are SOC2 audited by an external team.
U.S. State and Federal Health Information Privacy and Security Laws
There are numerous U.S. federal and state laws and regulations related to the privacy and security of personally identifiable information, including health information. In particular, HIPAA established privacy and security standards that limit the use and disclosure of protected health information, referred to as PHI, and require the implementation of administrative, physical, and technical safeguards to ensure the confidentiality, integrity, and availability of individually identifiable health information in electronic form. Our members as well as certain of our enterprise customers are regulated as covered entities under HIPAA. As a service provider who creates, receives, maintains, or transmits PHI on behalf of these covered entities for certain of our services, we are a “business associate” as defined under HIPAA.
Violations of HIPAA may result in civil and criminal penalties and a single breach incident can result in violations of multiple standards. In the event of a breach, we must also comply with HIPAA’s breach notification rule and our covered entity enterprise customers may require we provide assistance in the breach notification process and may seek indemnification and other contractual remedies. State attorneys general also have the right to prosecute HIPAA violations committed against residents of their states, and individuals have used HIPAA standards as the basis for the duty of care in state civil suits, such as those for negligence or recklessness in misusing personal information. In addition, HIPAA mandates that HHS conduct periodic compliance audits of HIPAA covered entities and their business associates for compliance.
Further, many states in which we operate and in which our members and customers as well as their patients reside also have laws that protect the privacy and security of sensitive and personal information, including health information, information regarding mental health and substance use treatment, and other information related to the provision of healthcare services. Some of these laws also prohibit unfair privacy and security practices and deceptive statements about privacy and security place specific requirements on certain types of activities, such as data security and texting. These laws may be similar to or even more protective than HIPAA and other federal privacy laws. For example, the laws of the State of California, in which we operate, are more restrictive than HIPAA, including the provisions of the California Consumer Privacy Act, or CCPA, which went into effect January 1, 2020. While any information we maintain in our role as a business associate may be exempt from the CCPA, other records and information we maintain on our members may be subject to the CCPA. Where state laws are more protective than HIPAA or require us to take action such as breach notification, we must comply with the state laws we are subject to, in addition to HIPAA. In certain cases, it may be necessary to modify our planned operations and procedures to comply with these more stringent state laws. Not only may some of these state laws impose fines and penalties upon violators, but also some, unlike HIPAA, may afford private rights of action to individuals who believe their personal information has been misused. In addition, state laws are changing rapidly, and there is discussion of a new federal privacy law or federal breach notification law, to which we may be subject. For additional information, see “Risk Factors - Risks Related to the Healthcare Industry.”
In addition to HIPAA, state health information privacy and state health information privacy laws, we may be subject to other state and federal privacy laws, including laws that prohibit unfair privacy and security practices and deceptive statements about privacy and security and laws that place specific requirements on certain types of activities, such as data security and texting.
In recent years, there have been a number of well-publicized data breaches involving the improper use and disclosure of PII and PHI. Many states have responded to these incidents by enacting laws requiring holders of personal information to maintain safeguards and to take certain actions in response to a data breach, such as providing prompt notification of the breach to affected individuals and state officials. In addition, under HIPAA and pursuant to the related contracts that we enter into with our business associates, we must report breaches of unsecured PHI to our contractual partners following discovery of the breach. Notification must also be made in certain circumstances to affected individuals, federal authorities and others.
58
Federal and State Telecommunications Laws
There are a number of federal and state laws and regulations potentially applicable to communications by phone, text message, or facsimile, including the TCPA, and those laws and regulations are continuously evolving. Our services that allow members and other platform users to leverage such telephonic communications may be subject to these laws and regulations.
Other Healthcare Laws and Regulations and Health Reform
There are many laws that govern the activities of healthcare professionals, some of which may be applied to us because of our relationships with them. Some of these requirements may apply to us even if we do not have a physical presence in the state, based solely on our agreements with providers licensed in the state. Many states limit the scope of business relationships between business entities and medical professionals. For example, while many states’ fee-splitting laws only prohibit a physician from sharing medical fees with a referral source, some states have interpreted certain management agreements between business entities and physicians as unlawful fee-splitting. These laws generally prohibit us from exercising control over the medical judgments or decisions of physicians and non-physician healthcare providers and from engaging in certain financial arrangements, such as splitting professional fees with healthcare providers. In addition, certain federal and state anti-kickback and false claims laws may apply to us indirectly through our arrangements with healthcare professionals and entities. Statutes and regulations relating to the practice of medicine, anti-kickback, fraud, fee-splitting, and similar issues vary widely from state to state. Because these laws are often vague, their application is frequently dependent on court rulings and attorney general opinions.
In addition, there have been several legislative and regulatory changes and proposed reforms of the healthcare system to contain costs, improve quality, and expand access to care. It is also possible that additional governmental action is taken in response to the COVID-19 pandemic. Failure to comply with any of these laws or regulations could lead to adverse judicial or administrative action against us and/or our provider customers, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, the need to make changes to the terms of engagement of our provider customers that interfere with our business, and other materially adverse consequences.
U.S. Federal and State Fraud and Abuse Laws
Successfully commercializing a telehealth technology will depend on broad health insurance or third party payor coverage. Government and private payors institute coverage criteria to ensure the appropriate utilization of products and services and to control costs. Limited third party payor coverage for a technology or procedure may limit adoption and commercial viability, while broader coverage supports optimal market uptake. Favorable coverage decisions by government payors like Medicare or Medicaid is critical because private payors typically follow the government’s lead regarding reimbursement. However, manufacturers whose technology is reimbursed by the government payors are subject to various U.S. federal and state laws pertaining to healthcare fraud and abuse. These laws can be implicated by inappropriate sales and marketing arrangements with healthcare providers. Many commonly accepted commercial practices are illegal in the healthcare industry and violations of these laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in U.S. federal and state healthcare programs, including Medicare and Medicaid.
Federal Stark Law. Our affiliated provider network may be subject to the federal self-referral prohibitions, commonly known as the Stark Law. Where applicable, this law prohibits a physician from referring beneficiaries of certain government programs to an entity providing “designated health services” if the physician or a member of such physician’s immediate family has a “financial relationship” with the entity, unless an exception applies. The penalties for violating the Stark Law include the denial of payment for services ordered in violation of the statute, mandatory refunds of any sums paid for such services, civil penalties for each violation, and possible exclusion from future participation in the federally funded healthcare programs. A person who engages in a scheme to circumvent the Stark Law’s prohibitions may be fined for each applicable arrangement or scheme. The Stark Law is a strict liability statute, which means proof of specific intent to violate the law is not required. In addition, the government and some courts have taken the position that claims presented in violation of the various statutes, including the Stark Law can be considered a violation of the federal False Claims Act (described below) based on the contention that a provider impliedly certifies compliance with all applicable laws, regulations and other rules when submitting claims for reimbursement. A determination of liability under the Stark Law could harm our business.
Federal Anti-Kickback Statute. We are also subject to the federal Anti-Kickback Statute. The Anti-Kickback Statute is broadly worded and prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for, or to induce, (i) the referral of a person covered by Medicare, Medicaid or other governmental programs, (ii) the furnishing or arranging for
59
the furnishing of items or services reimbursable under Medicare, Medicaid or other governmental programs or (iii) the purchasing, leasing or ordering or arranging or recommending purchasing, leasing or ordering of any item or service reimbursable under Medicare, Medicaid or other governmental programs. Certain federal courts have held that the Anti-Kickback Statute can be violated if “one purpose” of a payment is to induce referrals. In addition, a person or entity does not need to have actual knowledge of this statute or specific intent to violate it to have committed a violation, making it easier for the government to prove that a defendant had the requisite state of mind or “scienter” required for a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act, as discussed below. Violations of the Anti-Kickback Statute can result in exclusion from Medicare, Medicaid or other governmental programs as well as civil and criminal penalties, including fines per violation and damages of up to three times the amount of the unlawful remuneration, and imprisonment of up to ten years. Imposition of any of these remedies could harm our business. In addition to a few statutory exceptions, the U.S. Department of Health and Human Services Office of Inspector General, or OIG, has published safe harbor regulations that outline categories of activities deemed protected from prosecution under the Anti-Kickback Statute provided all applicable criteria are met. The failure of a financial relationship to meet all of the applicable safe harbor criteria does not necessarily mean that the particular arrangement violates the Anti-Kickback Statute. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG.
False Claims Act. Both federal and state government agencies have continued civil and criminal enforcement efforts as part of numerous ongoing investigations of healthcare companies and their executives and managers. Although there are a number of civil and criminal statutes that can be applied to healthcare providers, a significant number of these investigations involve the federal False Claims Act. These investigations can be initiated not only by the government but also by a private party asserting direct knowledge of fraud. These “qui tam” whistleblower lawsuits may be initiated against any person or entity alleging such person or entity has knowingly or recklessly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or has made a false statement or used a false record to get a claim approved. In addition, the improper retention of an overpayment for 60 days or more is also a basis for a False Claim Act action. Penalties for False Claims Act violations include fines for each false claim, plus up to three times the amount of damages sustained by the federal government. A False Claims Act violation may provide the basis for exclusion from the federally funded healthcare programs. In addition, some states have adopted similar fraud, whistleblower and false claims provisions.
Federal Physician Self-Referral Law. The Federal Physician Self-Referral Law, also referred to as the Stark Law, prohibits a physician (or an immediate family member of a physician) who has a financial relationship with an entity from referring patients to that entity for certain designated health services, including durable medical equipment and supplies, payable by Medicare, unless an exception applies. The Stark Law also prohibits such an entity from presenting or causing to be presented a claim to the Medicare program for such designated health services provided pursuant to a prohibited referral, and provides that certain collections related to any such claims must be refunded in a timely manner. Exceptions to the Stark Law include, among other things, exceptions for certain financial relationships, including both ownership and compensation arrangements. The Stark Law is a strict liability statute: to the extent that the statute is implicated and an exception does not apply, the statute is violated. In addition to the Stark Law, many states have implemented similar physician self-referral prohibitions that may extend to Medicaid, third party payors, and self-pay patients. Violations of the Stark Law must be reported and unauthorized claims must be refunded to Medicare in order to avoid potential liability under the federal False Claims Act for avoiding a known obligation to return identified overpayments. Violations of the Stark Law, the Anti-Kickback Statute, the Civil Monetary Penalties Law and/or the federal False Claims Act can also form the basis for exclusion from participation in federal and state healthcare programs.
Civil Monetary Penalties Law. The Civil Monetary Penalties Law (“CMPL”) authorizes the imposition of substantial civil money penalties against an entity that engages in certain prohibited activities including but not limited to violations of the Stark Law or Anti-Kickback Statute, knowing submission of a false or fraudulent claim, employment of an excluded individual, and the provision or offer of anything of value to a Medicare or Medicaid beneficiary that the transferring party knows or should know is likely to influence beneficiary selection of a particular provider for which payment may be made in whole or part by a federal healthcare program, commonly known as the Beneficiary Inducement CMP. Remuneration is defined under the CMPL as any transfer of items or services for free or for less than fair market value. There are certain exceptions to the definition of remuneration for offerings that meet the Financial Need, Preventative Care, or Promoting Access to Care exceptions (as defined in the CMPL). Sanctions for violations of the CMPL include civil monetary penalties and administrative penalties up to and including exclusion from participation in federal healthcare programs.
FCPA and Other Anti-Bribery and Anti-Corruption Laws. The U.S. Foreign Corrupt Practices Act (“FCPA”) prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official,
60
government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA would include interactions with certain healthcare professionals or organizations in many countries. Our present and future businesses have been and will continue to be subject to various other U.S. and foreign laws, rules and/or regulations.
State Fraud and Abuse Laws. Most states in which we operate have also adopted similar fraud and abuse laws as described above. The scope of these laws and the interpretations of them vary from state to state and are enforced by state courts and regulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any payor, including patients and commercial insurers, not just those reimbursed by a federally funded healthcare program. A determination of liability under such state fraud and abuse laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
Reimbursement Related Regulation
Medicare. The Medicare program offers beneficiaries different ways to obtain medical benefits: (i) Medicare Part A, which covers, among other things, in-patient hospital, SNFs, home healthcare, and certain other types of healthcare services; (ii) Medicare Part B, which covers physicians’ services, outpatient services, durable medical equipment, and certain other types of items and healthcare services; (iii) Medicare Part C, also known as Medicare Advantage, which is a managed care option for beneficiaries who are entitled to Medicare Part A and enrolled in Medicare Part B; and (iv) Medicare Part D, which provides coverage for prescription drugs that are not otherwise covered under Medicare Part A or Part B for those beneficiaries that enroll.
Our affiliated provider network is reimbursed by the Part B and Part C programs for certain of the telemedicine services it provides to Medicare beneficiaries. Medicare coverage for telemedicine services is treated distinctly from other types of professional medical services and is limited by federal statute and subject to specific conditions of participation and payment pursuant to Medicare regulations, policies and guidelines, including the location of the patient, the type of service, and the modality for delivering the telemedicine service, among others.
Medicaid. Medicaid programs are funded jointly by the federal government and the states and are administered by states (or the state’s designated managed care or other similar organizations) under approved plans. Our affiliated provider network is reimbursed by certain state Medicaid programs for certain of the telemedicine services it provides to Medicaid beneficiaries. Medicaid coverage for telemedicine services varies by state and is subject to specific conditions of participation and payment.
Participation in Medicare/Medicaid Programs. Participation in the Medicare, including Medicare Advantage, and Medicaid programs is heavily regulated by federal and state (in the case of Medicaid) statute, regulation, policy, and guidance protocols. If a provider fails to comply substantially with the requirements for participating in the programs, the provider’s participation may be terminated and/or civil or criminal penalties may be imposed. Our affiliated network providers are enrolled with Medicare and certain Medicaid programs, and they also participate in arrangements administered by commercial payers under the Medicare Advantage program. In the ordinary course of business, we may from time to time be subject to inquiries, investigations and audits by federal and state agencies that oversee applicable government program participation. In addition to auditing compliance with program requirements, these audits can trigger, particularly when issues are identified, investigations, repayments, and requirements under certain of the U.S. Federal and State Fraud and Abuse Laws described above.
Commercial Insurance Providers. iDoc participates with commercial and/or private insurance carriers for its patient reimbursement fees. The fee schedule basis for payment by the commercial insurance providers is determined with Medicare reimbursement fee structure guidelines and if the Company is in network or out of network with the insurance carriers which varies based on state and insurer requirements.
COVID-19 Waivers and Limited Statutory Changes. As a result of the COVID-19 pandemic, federal and state governments have enacted legislation, promulgated regulations, and taken other administrative actions intended to assist healthcare providers seeking to utilize telemedicine methods in providing care to patients during the public health emergency. These measures include temporary relief from certain Medicare conditions of participation requirements for healthcare providers, temporary relaxation of licensure requirements for healthcare professionals by some states, temporary relaxation of privacy restrictions for telemedicine remote communications, and temporarily expanding the scope of services for which Medicare and Medicaid reimbursement is available during the emergency period. These changes have temporarily increased reimbursement available to our affiliated provider network for telemedicine services provided. We acknowledge the Public Health Emergency (PHE) expired on May 11, 2023. We also acknowledge that a significant number of limited statutory changes related to telehealth have been extended through December 2024 and in some states made permanent. As a result of the PHE expiration date of May 11, 2023, we have seen differing impacts to
61
telehealth at the federal and state level but overall leaning towards increased adoption of telehealth services compared to pre-COVID-19 era.
FDA Regulation of Medical Devices
Certain software products often used in telemedicine platforms and offerings could fall under the broad category of digital health products that may, in certain circumstances, require the U.S. Food and Drug Administration (the “FDA”) regulatory review prior to marketing. The FDA generally maintains regulatory oversight over products that meet the Agency’s statutory definition of a “medical device.” In certain circumstances, software applications and their corresponding platforms are considered medical devices when they are intended to be used for one or more medical purposes and are consequently regulated by the FDA. Determining whether a product meets the definition of a medical device requires assessment of both design and intended use. Intended use of a product is determined by the intent of the manufacturer as evidenced by the design of the product and the product labeling. Labeling is a broad term that includes marketing and advertising claims. The FDA’s regulatory approach toward digital health technologies is set forth in both regulations and guidance documents. This requires analyzing (1) whether a product meets the FDA’s definition of a medical device and, if it does, (2) whether it is carved out from active regulation by one of the FDA’s digital health “enforcement discretion” policies. In general, the FDA’s overarching approach is to apply its regulatory oversight in a risk-based manner to only software functions deemed to meet the definition of medical devices (i.e., those intended for the diagnosis of disease or other conditions, or the cure, mitigation, treatment, or prevention of disease) and whose functionality could create patient safety risks in the event of a malfunction.
In the United States, medical devices are subject to extensive regulation at the federal level by the FDA under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. The laws and regulations govern, among other things, medical device design and development, pre-clinical and clinical testing, pre-market clearance, authorization or approval, establishment registration and product listing, product manufacturing, product packaging and labeling, product storage, advertising and promotion, product distribution, recalls and field actions, servicing and post-market clinical surveillance. A number of U.S. states also impose licensing and compliance regimes on companies that manufacture or distribute prescription devices into or within the state.
If our products are marketed for clinical monitoring or therapeutic uses, they could be regulated by the FDA as medical devices. It is presently unclear what level of risk the agency would assign to such products, what special controls may be imposed on such products (if any), and what regulatory requirements would be applicable to such products.
Regulation Under the FTC
The Federal Trade Commission (“FTC”) also oversees the advertising and promotion of our products pursuant to broad authority to police deceptive advertising for goods or services within the United States. Under the Federal Trade Commission Act, the FTC is empowered, among other things, to
Further, medical device systems that include wireless radio frequency transmitters and/or receivers are subject to equipment authorization requirements in the United States. The Federal Communications Commission (“FCC”) requires advance clearance of all radio frequency devices before they can be sold or marketed in the United States. These clearances ensure that the proposed products comply with FCC radio frequency emission and power level standards and will not cause interference.
62
Legal Proceedings
We may in the future be involved in, legal proceedings, claims, and government investigations in the ordinary course of business. These include proceedings, claims, and investigations relating to, among other things, regulatory matters, commercial matters, intellectual property, competition, tax, employment, pricing, discrimination, consumer rights, personal injury, and property rights.
Depending on the nature of the proceeding, claim, or investigation, we may be subject to settlement awards, monetary damage awards, fines, penalties, or injunctive orders. Furthermore, the outcome of these matters could materially adversely affect each of their respective business, results of operations, and financial condition. The outcomes of legal proceedings, claims, and government investigations are inherently unpredictable and subject to significant judgment to determine the likelihood and amount of loss related to such matters.
As of the filing of the registration statement, we were not a party to any material legal proceedings.
Properties
Our principal executive offices are located at 980 N Federal Hwy #304, Boca Raton, FL 33432, and our telephone number is (561) 672-7068. Our website can be found at https://vseehealth.com/.
Furthermore, iDoc has physical operations in Boston, Massachusetts and Houston, Texas. Such office locations for personnel are contracted via short-term leases.
Employees
We currently have approximately 154 full-time equivalent employees and contractors, of which approximately 24 are board certified practicing physicians. Particularly, iDoc has an experienced team of board-certified neurointensivists, cardiac specialty trained intensivists, and medical intensivists that treat and coordinate care for acutely ill patients 24/7 in the neurointensive Care Unit (NICU), cardiac intensive care unit, and medical intensive care unit. None of our employees are represented by a labor union.
63
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF VSEE HEALTH
References in this section to “we,” “us”, “DHAC” or the “Company” refer to Digital Health Acquisition Corp. which was renamed VSee Health, Inc. in connection with the closing of the Business combination. The information set forth herein does not include the results of operations of either of VSee Lab or iDoc as the Business Combination occurred after the date of the information set forth herein. References to the “Sponsor” refer to Digital Health Sponsor LLC. Certain information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
Overview
DHAC was incorporated as a blank check company formed under the laws of the State of Delaware on March 30, 2021. DHAC was formed for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities. DHAC’s Sponsor is Digital Health Sponsor LLC, a Delaware limited liability company (“Sponsor”).
As previously disclosed in DHAC’s Current Report on Form 8-K filed with the SEC on June 12, 2024, DHAC held a special stockholder meeting (the “Special Meeting”) on June 7, 2024, at which the DHAC stockholders considered and adopted, among other matters, a proposal to approve the transactions contemplated by the Business Combination Agreement. On June 24, 2024, the parties consummated the business combination by and among DHAC, DHAC Merger Sub I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of DHAC (“Merger Sub I”), DHAC Merger Sub II, Inc., a Texas corporation and a direct, wholly owned subsidiary of DHAC (“Merger Sub II”), VSee Lab, Inc., a Delaware corporation (“VSee Lab”), and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”) (the “Business Combination”). In connection with the Business Combination, DHAC changed its name from Digital Health Acquisition Corp. to VSee Health, Inc. (“VSee Health”)
At the closing of the Business Combination, (1) each share of DHAC common stock was re-designated as a share of VSee Health’s common stock, par value $0.0001 and each outstanding warrant of DHAC was re-designated as a warrant of VSee Health and each whole warrant exercisable for one share of the VSee Health’s Common Stock at an exercise price of $11.50; (2) each issued and outstanding share of Class A common stock of VSee Lab (including all securities that are converted or exchanged into shares of VSee Lab Class A common stock) immediately prior to the Business Combination was automatically cancelled and extinguished and converted into the right to receive approximately 0.40 shares of VSee Health Common Stock; and (3) each issued and outstanding share of Class A common stock of iDoc immediately prior to the Business Combination was automatically cancelled and extinguished and converted into the right to receive approximately 994.38 shares of VSee Health Common Stock.
Furthermore, with the closing of the Business Combination, (1) pursuant to certain securities purchase agreements entered into on November 21, 2023, (the “Loan Conversion SPAs”), by and among DHAC, VSee Lab and/or iDoc with certain lenders of each of DHAC, Vsee Lab and iDoc, certain indebtedness of each of DHAC, VSee Lab and iDoc was converted into shares of series A preferred stock of VSee Health, par value $0.0001 per share (the “Series A Preferred Stock”) upon Closing and VSee Health issued 1,788 Series A Preferred Stock to such lenders; (2) pursuant to certain securities purchase agreements entered into on November 21, 2023 and as further amended and restated on February 13, 2024 (the “A&R Loan Conversion SPAs”), by and among DHAC, VSee Lab and/or iDoc and certain lenders, following assumption and conversion of the underlying loans, VSee Health issued 892,500 shares of Common Stock to such lenders after the Closing; and (3) in connection with services performed by A.G.P./Alliance Global Partners (“A.G.P.”) during DHAC’s initial public offering and pursuant to a securities purchase agreement entered into on November 3, 2022 and as further amended on November 21, 2023 (the “A.G.P. Securities Purchase Agreement”), VSee Health issued 4,370 shares of Series A Preferred Stock to A.G.P. upon Closing. For additional descriptions of the Loan Conversion SPAs dated November 21, 2023, the A&R Loan Conversion SPAs dated February 13, 2024 and the A.G.P. Securities Purchase Agreement, please refer to VSee Health’s Current Reports on Form 8-K filed with the SEC on November 22, 2023 and on February 13, 2024.
As previously disclosed in DHAC’s Current Report on Form 8-K filed with the SEC on November 22, 2023, in connection with the Closing, pursuant to the exchange agreement (the “Exchange Agreement”) entered by and among DHAC, VSee Lab and iDoc on November 21, 2023, VSee Health consummated the exchange of a senior convertible promissory note with an aggregate principle value of $2,523,744.29 (the “Exchange Note”) and issued the Exchange Note to the bridge investor (the “Bridge Investor”) on the Closing Date. The Exchange Note is guaranteed by each of VSee Health, VSee Lab and iDoc and is fully secured by collateral of VSee Health and its subsidiaries including, without limitation, the intellectual property, trademark, and patent rights. The parties entered into an Amended and Restated Security Agreement (the “Security Agreement”) and certain intellectual property security agreements on the Closing Date granting such security interest in favor of the Bridge Investor.
64
On the Closing Date, VSee Health also entered into a registration rights agreement pursuant to the Exchange Agreement (the “Exchange Registration Rights Agreement”). In addition, each of VSee Health’s directors and officers entered into a lock-up agreement contemplated by the Exchange Agreement (the “Exchange Lock-Up Agreement”).
As previously disclosed in DHAC’s Current Report on Form 8-K filed with the SEC on November 22, 2023, in connection with the Closing and pursuant to the convertible note purchase agreement (the “Quantum Purchase Agreement”) entered by and between DHAC and an institutional and accredited investor (the “Quantum Investor”) on November 21, 2023, VSee Health agreed to issue and sell to the Quantum Investor a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000. Such Quantum Note was issued on June 25, 2024 and certain terms were amended on July 3, 2024 as reported in VSee Health’s Current Report on Form 8-K filed with the SEC on July 9, 2024. Concurrent with the issuance of the Quantum Note, VSee Health entered into a Registration Rights Agreement, pursuant to which it agreed to register the shares of Common Stock underlying the Quantum Note (the “Quantum Registration Rights Agreement”).
Results of Operations
DHAC’s entire activity from inception to March 31, 2024, was in preparation for its formation, its initial public offering, and since the closing of its initial public offering, a search for business combination candidates. DHAC did not generate any operating revenues until the closing and completion of its initial business combination. DHAC generates non-operating income in the form of interest income on investments held in trust account. We expect to incur increased expenses as a result of being a public company (for legal, financial reporting, accounting and auditing compliance), as well as for due diligence expenses.
For the three months ended March 31, 2024, we had a net loss of $967,817 which consisted of $674,262 in general and administrative expenses, default interest expense related to M2B Note of $20,296, interest expense related to Additional Bridge Notes of $8,617, interest expense related to the M2B Note of $2,496, interest expense relate to the Exchange Note of $51,036 interest expense related to the Extension Note of $51,840, initial fair value of Additional Bridge of $3,851, change in fair value of Additional Bridge Notes of $2,133, change in fair value of Exchange Note of $192,801, partially offset by change in fair value of the Extension Note embedded derivative of $4, income from our investments held in the trust account of $17,853, and change in fair value of ELOC of $13,956.
For the three months ended March 31, 2023, we had a net loss of $1,894,642 which consisted of $707,592 in general and administrative expenses, change in fair value of PIPE forward contract derivative $1,163,950 and interest expense related to Bridge promissory note of $133,138, partially offset by income from our investments held in the trust account of $75,280 and change in fair value of Bridge note embedded derivative of $34,758.
Liquidity and Capital Resources
As of March 31, 2024, DHAC had $724 in cash and no cash equivalents.
DHAC’s liquidity needs up to the Initial Public Offering were satisfied through receipt of a $25,000 capital contribution from its Sponsor and certain of its executive officers, directors, and advisors in exchange for the issuance of the founder shares, and loans from its Sponsor for an aggregate amount of $602,720 to cover organizational expenses and expenses related to the Initial Public Offering pursuant to promissory notes (the “Notes”).
On November 8, 2021, DHAC consummated the Initial Public Offering of 11,500,000 Units, including the full exercise of the underwriters’ over-allotment option, at a price of $10.00 per Unit, generating gross proceeds of $115 million. Simultaneously with the closing of the Initial Public Offering, DHAC completed the private sale of 557,000 Units (the “Private Placement Units”) at a purchase price of $10.00 per Private Placement Unit (the “Private Placement”), to the Sponsor, generating gross proceeds of $5,570,000. As of November 8, 2021, we received $3,680,000 from the proceeds of the Private Placement and recorded $1,890,000 in subscription receivable. The Sponsor paid the subscription in full on November 12, 2021.
Following the Initial Public Offering and the Private Placement, a total of $116,725,000 was placed in the Trust Account and we had $9,478 of cash held outside of the Trust Account, after payment of costs related to the Initial Public Offering, and available for working capital purposes. We incurred $6,877,164 in transaction costs, consisting of $1,955,000 of underwriting fees, $4,370,000 of deferred underwriting fees and $552,164 of other offering costs. On October 20, 2022, in connection with our stockholders meeting to approve the extension, 10,805,877 shares of our common stock were redeemed leaving 694,123 shares subject to redemption. In connection with the redemption we withdrew $110,472,254 from the Trust Account. On November 6, 2023, in connection with the
65
stockholders meeting to approve the extension, 579,157 shares of DHAC’s common stock were redeemed leaving 114,966 shares subject to redemption.
On October 6, 2022, in connection with the execution of the Second Amended and Restated Business Combination Agreement, DHAC, VSee and iDoc entered into the Bridge SPA, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222. $888,889 of the Bridge Note was allocated to DHAC. The Bridge Notes will be assumed by DHAC in connection with the closing of the Business Combination. The Bridge Notes bear guaranteed interest at a rate of 10% per annum. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments and (ii) 30,000 shares of DHAC common stock as additional consideration for the purchase of the Bridge Notes and Bridge Warrants. DHAC as a result received $738,200 in proceeds for working capital purposes. On October 4, 2023 DHAC defaulted on the Bridge Note and accordingly the default provision were allocated and applied resulting in the triggering of 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24% and the Company assumed the penalties and interest which were due and payable under the VSee and iDoc portion of the note, resulting in total amount due of $2,523,744. As a result of the default, the Company entered into an Exchange Agreement on November 21, 2023 with the holder and recognized $1,579,927 in default interest.
On October 26, 2022, the Company issued an unsecured promissory note in the aggregate principal amount of $350,000 to Digital Health Sponsor LLC, the Company’s “Sponsor.” The Company deposited to the trust account all of the loan amount and extended the amount of time it has available to complete a business combination from November 8, 2022 to February 8, 2023. The promissory note does not bear interest and will be repaid only upon closing of a business combination by the Company.
In February 2023, SCS Capital Partners LLC issued an unsecured promissory note to the Company, pursuant to which the Company may borrow up to an aggregate principal amount of $250,000. The promissory note was non-interest bearing and shall be used to pay for general operating expenses. On August 17, 2023, the promissory note was amended and restated to allow for an additional $315,000, in the aggregate of $565,000.
On May 5, 2023, the Company issued a promissory note to SCS Capital Partners LLC in the aggregate principal amount of $200,000 (the “SCS Note”). The SCS Note bears interest at a rate of 10% per annum and is due and payable on May 5, 2024. If the Company’s PIPE financing closes in connection with the closing of its business combination, 100% of all unpaid principal under the SCS Note and any accrued but unpaid interest are due and payable at the closing of the PIPE financing. In connection with the termination of the PIPE financing on July 11, 2023, this note was terminated, and advances were allocated to the amended note for $565,000 on August 17, 2023, as discussed above.
On May 5, 2023 and as amended on April 17, 2024, the Company entered into a securities purchase agreement (the “May 2023 SPA”) with an institutional investor (the “Holder”). Pursuant to the May 2023 SPA, the Company issued the Holder a 16.67% original issue discount promissory note, in favor of the Holder, in the aggregate principal amount of $300,000 (the “Extension Note”). The Extension Note bears guaranteed interest at a rate of 10% per annum and is due and payable on June 30, 2024.
On October 4, 2023, the Company issued a promissory note in the aggregate principal amount of $165,000 to M2B Funding Corp., an affiliate of the Sponsor for a purchase price of $150,000 and included $5,000 in legal fees. The note original issued discount of $15,000 plus $5,000 of offering cost were recorded as a debt discount and amortized over the term of the note. The note had a maturity date of January 5, 2024. The Company defaulted on the note and amended the note on February 8,2024. As a result of the amendment the Company was to repay the remaining amount due by February 8, 2024. On January 31, 2024 the note was paid in full for a total of $190,750.
On November 21, 2023, DHAC, VSee and iDoc entered into an amendment to the Original Bridge SPA, pursuant to which the Bridge Investor agreed to purchase the Additional Bridge Notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,556 note. The Additional Bridge Notes bear guaranteed interest at a rate of 8% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at a fixed conversion price of $10 per share. The conversion price of the Additional Bridge Notes is subject to reset if DHAC’s common stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the DHAC common stock in the 10 trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Additional Bridge Notes requires the payment of 110% of the outstanding obligations, including the guaranteed minimum interest. If an event of default occurs, the Additional Bridge Notes would bear interest
66
at a rate of 24% per annum and require the payment of 125% of the outstanding obligations, including the guaranteed minimum interest. As of March 31, 2024, $150,000 has been funded.
On November 21, 2023, DHAC entered into the Quantum Purchase Agreement, pursuant to which the Quantum Investor purchased a 7% original issue discount Quantum Note in the aggregate principal amount of $3,000,000 on June 25, 2024, which was further amended on July 3, 2024 as reported in VSee Health’s Current Report on Form 8-K filed with the SEC on July 9, 2024. The Quantum Note bears interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) a fixed conversion price of $10.00 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30th-day anniversary of the Quantum Note issuance date is less than $10.00, to a price equal to the Average Price but in no event less than $2.00. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10.00. If an event of default occurs, the Quantum Note would bear interest at a rate of 18% per annum and as amended on July 3, 2024, eighteen months of such interest shall be guaranteed regardless of early pay or redemption. The Quantum Note will mature on June 30, 2026. Concurrently with the consummation of the transactions contemplated by the Quantum Purchase Agreement, the Company will enter into a registration rights agreement pursuant to which it will agree to register the shares of DHAC common stock underlying the Quantum Note.
On November 21, 2023, DHAC entered into an equity line of purchase agreement (the “Equity Purchase Agreement”) with an affiliate of the Bridge Investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s common stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the closing of the Business Combination, provided that certain conditions are met.
For the three months ended March 31, 2024, cash used in operating activities was $335,318. Net loss of $967,817 was primarily comprised of initial loss on Additional Bridge Note of $3,851, change in fair value of Exchange note of $192,801, change in fair value of ELOC note $13,956, change in fair value of Additional Bridge Note of $2,133, change in fair value of Extension Note bifurcated derivative of $4, and interest earned on investments held in Trust Account of $17,853. Changes in operating assets and liabilities provided $473,299 of cash for operating activities.
For the three months ended March 31, 2023, cash used in operating activities was $340,667. Net loss of $1,894,642 was primarily comprised of change in fair value of bride note bifurcated derivative of $34,758, change in fair value of PIPE forward contract derivative of $1,163,950, and interest earned on investments held in Trust Account of $75,280. Changes in operating assets and liabilities provided $500,063 of cash for operating activities.
Critical Accounting Estimates
Management’s discussion and analysis of our results of operations and liquidity and capital resources are based on our financial information. We describe our significant accounting policies in Note 2 — Summary of Significant Accounting Policies, of the Notes to Condensed consolidated Financial Statements included in this report. Our condensed consolidated financial statements have been prepared in accordance with U.S. GAAP. Certain of our accounting policies require that management apply significant judgments in defining the appropriate assumptions integral to financial estimates. On an ongoing basis, management reviews the accounting policies, assumptions, estimates and judgments to ensure that our condensed consolidated financial statements are presented fairly and in accordance with U.S. GAAP. Judgments are based on historical experience, terms of existing contracts, industry trends and information available from outside sources, as appropriate. The most significant accounting estimates were the assumptions used to fair value the PIPE Forward Contract (as defined in financial statement Note 6 — Commitments), the Extension Note Bifurcated Derivative, the Bridge Note Bifurcated Derivative (as defined in financial statement Note 2), the Additional Bridge Note and the Exchange Note. However, by their nature, judgments are subject to an inherent degree of uncertainty, and, therefore, actual results could differ from our estimates.
Warrant Instruments
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”), and ASC 815, “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the warrants are freestanding
67
financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. The Company has analyzed the Public Warrants, Private Warrants, Bridge Note Warrants and the Extension Warrants and determined they are considered to be freestanding instruments and do not exhibit any of the characteristics in ASC 480 and therefore are not classified as liabilities under ASC 480. The warrants meet all of the requirements for equity classification under ASC 815 and therefore are classified in equity.
Financial Instruments
The Company evaluates its financial instruments to determine if such instruments should be accounted for as a liability under ASC 480 or if they are derivatives or contain features that qualify as bifurcated derivatives in accordance with ASC 815.
Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the statements of operations. Derivative assets and liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date. The Company has determined the PIPE financing agreement is a derivative instrument, the Bridge Note and the Extension Note’s early redemption provisions are embedded feature that are required to be bifurcated as a derivative. FASB ASC 470-20, “Debt with Conversion and Other Options,” addresses the allocation of proceeds from the issuance of debt into its debt and bifurcated derivative components. The Company applies this guidance to allocate the Bridge Note and the Extension Note proceeds between the Bridge Note and the Extension Note, respectively and the respective Bifurcated Derivative, using the residual method by allocating the principal first to fair value of the bifurcated derivative and then to the debt.
The Senior Secured Convertible Promissory Notes (the “Exchange Note” and the “Additional Bridge Note” represent share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Senior Secured Convertible Promissory Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
Recent Accounting Standards
We do not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material impact on our condensed consolidated financial statements.
68
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF VSEE LAB
The following discussion and analysis provide information that VSee Lab’s management believes is relevant to an assessment and understanding of the results of operations and financial condition of VSee Lab, Inc. (“VSee Lab” and for purposes of this section only, referred to as the “Company”, “we,” “us” and “our”). The discussion and analysis should be read together with VSee Lab’s consolidated financial statements as of and for the three months ended March 31, 2024 and 2023, and the related respective notes thereto. This discussion may contain forward-looking statements based upon VSee Lab’s current expectations, estimates and projections that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking statements due to, among other considerations, the matters discussed under “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.”
Overview
VSee Lab is a telehealth software platform. VSee Lab’s proprietary technology platform and modular software solution empower users to plug and play telehealth services with end-to-end encrypted video streaming integrated with medical device data, electronic medical records, and other sensitive data, with multiple other interactive functionalities that enable teamwork that VSee Lab believes are not available from any other system worldwide. Our company’s core platform is a highly scalable, integrated, application program interface (“API”) driven technology platform, for virtual healthcare delivery, with multiple real-time integrations spanning the healthcare ecosystem. Our platform’s APIs power external connectivity and deep integration with a wide range of payors, electronic medical records, third party applications, and other interfaces with employers, hospital systems, and health systems, which we believe uniquely positions us as a long-term partner meeting the unique needs of the rapidly changing, healthcare industry. Our company will also be able to white label our solutions so they fit into the plans and strategies of our clients, all on a platform that is high-performance and highly scalable.
Telehealth Platform Solutions
VSee Lab offers Telehealth Solutions to health systems, medical groups, and individual medical practitioners. Our customers purchase access to a suite of pre-built telehealth modules to fully customize their virtual care model, including API driven technology platform for virtual healthcare delivery, and multiple real-time integrations of smart connected devices with sophisticated data science to deliver personalized health insight for use by medical professionals. Our customers purchase a subscription for access to our Telehealth Solutions. Pricing is based on the customer size ranging from a simple solo practice room to a custom multi-clinic solution for hundreds of providers. VSee Lab offers Telehealth Solutions on a per- user subscription basis as basic or enterprise levels, and a reduced-functionality version to our single physician users for free.
Material Trends, Events and Uncertainties
Impact of the COVID-19 Pandemic
The extent to which the coronavirus (“COVID-19”) outbreak and measures taken in response thereto impact our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted. Global health concerns relating to the COVID-19 outbreak have been weighing on the macroeconomic environment, and the outbreak has significantly increased economic uncertainty. Risks related to consumers and businesses lowering or changing spending, which impact domestic and international spend. The outbreak has resulted in authorities implementing numerous measures to try to contain the virus, such as travel bans and restrictions, quarantines, shelter in place orders, and business shutdowns. These measures have not only negatively impacted consumer spending and business spending habits, but they have also adversely impacted and may further impact our workforce and operations and the operations of its customers, suppliers, and business partners. These measures may remain in place for a significant period of time, and they are likely to continue to adversely affect our business, results of operations and financial condition. The spread of COVID-19 has caused us to modify our company’s business practices (including employee travel, employee work locations, and cancellation of physical participation in meetings, events, and conferences), and we may take further actions as may be required by government authorities or that we determine are in the best interests of our employees, customers, and business partners. There is no certainty that such measures will be sufficient to mitigate the risks posed by the virus or otherwise be satisfactory to government authorities.
The extent to which the COVID-19 outbreak impacts our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted, including, but not limited to, the duration and spread of the
69
outbreak, its severity, the actions to contain the virus or treat its impact, and how quickly and to what extent normal economic and operating conditions can resume. Even after the COVID-19 outbreak has subsided, we may continue to experience materially adverse impacts to our business because of its global economic impact, including any recession that has occurred or may occur in the future.
There are no comparable recent events which may provide guidance as to the effect of the spread of COVID-19 and a global pandemic, and, as a result, the ultimate impact of the COVID-19 outbreak or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of the impacts on our business, our operations, or the global economy. However, the effects could have a material impact on our results of operations, and we will continue to monitor the COVID-19 situation closely.
Performance Factors
We believe that our future performance will depend on many factors, including the following:
The Rapid Transformation of the Telehealth Market
The Telehealth market today is one characterized by rapid transformation, with major customers and hospital systems looking to build or add capabilities and major legacy competitors looking to shore up historical limitations. We believe that the rapid transformation of the telehealth market indicates strong future growth of the market, and our current offerings provide an attractive value proposition to health systems, medical groups, and individual medical practitioners, driving higher market share. We plan to continue to harness our scale to further grow the value proposition of our platform for all stakeholders.
Ability to Expand Within the Market and Attract New Customers
Telehealth is still in its total infancy stages in terms of utilization, scope, and services. Most of the growth is expected within hospital systems, definition, and segmentation structure, and we believe our software platform and services have significant potential. We plan to leverage our industry relationships with government, hospital systems and insurance providers to increase our customer base.
Innovation and New Product Offerings
Despite the rapid advancements in technology, growth in virtual healthcare delivery, and improvement in decision support algorithms and machine learning tools, Telehealth Technology Solutions have not fully penetrated medicine and hospital systems to become the standard methodology of care and represent less than 1% of total healthcare spending according to Grandview Research. Major reasons for Telehealth solutions not capturing its full potential include:
| ● | Many of the existing video and hardware and software used in telehealth are repurposed businesses that are not healthcare specific. |
| ● | Remote monitoring/diagnostic devices do not readily integrate into telehealth systems limiting doctors real time metrics to enable diagnostics and assessment. |
| ● | Backend software coordination is not optimized for telehealth use and connectivity, resulting in significant greater complexity and costs for implementation. |
| ● | The software and code foundations of the early telemedicine companies have major functionality limitations and arduous implementation and incremental coding/connectivity requirements adding significant cost and reducing functionality. |
We believe our technology solutions meet the performance and compliance standards in healthcare, increase the sharing of patient history, files and scheduling are integrated into the video view for doctors, create sophisticated video engagement between patients, staff and doctors and seamlessly integrate patients’ records to provide more comprehensive telehealth care. We believe our ability to invest in new technology and develop new features, modules, and solutions will be critical to our long-term success.
70
Critical Accounting Policies and Estimates
Basis of Presentation and Consolidation
The accompanying consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
Use of Estimates
The preparation of the VSee Lab’s consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, allowance for doubtful accounts, and income taxes.
VSee Lab bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
VSee Lab recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits as a component of general and administrative expenses. VSee Lab’s federal tax return and any state tax returns are not currently under examination.
VSee Lab has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
VSee Lab recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services.
VSee Lab determines revenue recognition in accordance with ASC 606, through the following five steps:
1)Identify the contract with a customer
The Company considers the terms and conditions of its contracts and the Company’s customary business practices in identifying its contracts under ASC 606. The Company determines it has a contract with a customer when the contract has been approved by both parties, it can identify each party’s rights regarding the services to be transferred and the payment terms for the services, it has determined the customer to have the ability and intent to pay, and the contract has commercial substance. The
71
Company applies judgment in determining the customer’s ability and intent to pay, which is based on a variety of factors, including the customer’s payment history or, in the case of a new customer, credit and financial information pertaining to the customer.
Contractual terms for subscription services are typically 12 months. Contracts are cancellable with a 30-day notice period and customers are billed in annual, quarterly, or monthly installments in advance of the service period of the subscription. The Company is not required to refund any prorated prepayment fees invoiced to cover services that were provided.
2)Identify the performance obligations in the contract
Performance obligations promised in a contract are identified based on the services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the service either on its own or together with other resources that are readily available, and are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. The Company’s contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that they have any material outstanding commitments for future revenues beyond one year from the end of a reporting period.
The Company treats each subscription to a specific module as a distinct performance obligation because each module is capable of being distinct as the customer can benefit from the subscription to each module on its own and each subscription can be sold standalone.
Furthermore, the subscriptions to individual modules are distinct in the context of the contract as (1) the Company is not integrating the services with other services promised in the contract into a bundle of services that represent a combined output, (2) the subscriptions to specific modules do not significantly modify or customize the subscription to another module, and (3) the specific modules are not highly interdependent or highly interrelated. The subscription to each module is treated as a series of distinct performance obligations because it is distinct and substantially the same, satisfied over time, and has the same measure of progress.
3)Determine the transaction price
The transaction price is determined based on the consideration the Company expects to be entitled to in exchange for transferring services to the customer. Under the contracts, the clients pay a fixed rate per user per subscription service. Prior to the start of a contract, clients generally make upfront nonrefundable payments to the Company when contracting for implementation services.
The contract pricing is fixed and stated in the arrangements based on the work to be performed by the Company and represents the amount the Company is entitled to for delivering such goods and services. The quoted fees per promise and performance obligation are based on the most likely amount method for what the Company expects to collect.
4)Allocate the transaction price to performance obligations in the contract
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative stand-alone selling price (“SSP”). The determination of a SSP for each distinct performance obligation requires judgment. The Company believes the quoted transaction prices in the customer contracts represent the stand alone selling prices for each of the separate performance obligations that are distinct and priced separately within the contract.
5)Recognize revenue when or as the Company satisfies a performance obligation
Revenue is recognized when or as control of the promised goods or service is transferred to the customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. Subscriptions represent a series of distinct goods or services because the performance obligations are satisfied over time as customers simultaneously receive and consume the benefits related to the services as the Company performs. In the case of module specific subscriptions, a consistent level of service is provided during each monthly period of subscription to the Company’s platform. The Company commences revenue recognition when the customer is provided with platform subscription for the initial monthly period and
72
revenue is recognized over time as a consistent level of subscription service during the subsequent period is delivered. The Company’s obligation for its integrated subscriptions is to stand-ready throughout the subscription period; therefore, the Company considers an output method of time to measure progress towards satisfaction of its obligations with revenue commencing upon the beginning of the subscription period.
Upfront nonrefundable fees do not result in the transfer of a promised good or service to the client, therefore, the Company defers this revenue and recognizes it over the subscription period of the customer contract. Deferred revenue consists of the unamortized balance of nonrefundable upfront fees which are classified as current and non-current based on the timing of when the Company expects to recognize revenue.
Cost of Revenue
Cost of revenue consists primarily of expenses related to cloud hosting, personnel-related expenses for the Company’s customer success team, costs for third-party software services and contractors, and other services used in connection with delivery and support of VSee Lab’s platform subscription services.
Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses since inception and continues to have negative operating cash flows. These conditions raise substantial doubt about VSee Lab’s ability to continue as a going concern. The continuation of VSee Lab’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should VSee Lab be unable to continue as a going concern.
VSee Lab’s ability to continue as a going concern will require it to obtain additional funding. If VSee Lab is unable to obtain sufficient funding, its business, prospects, financial condition and results of operations will be materially and adversely affected, and VSee Lab may be unable to continue as a going concern. In such an event, it would be forced to delay, limit, reduce or terminate its product development or commercialization efforts, or may be forced to reduce or terminate its operations. If VSee Lab is unable to continue as a going concern, the Company may have to liquidate its assets and may receive less than the value at which those assets are carried on its audited financial statements, and it is likely that investors will lose all or part of their investment.
Net Income (Loss) Per Common Share
VSee Lab computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants. No potential dilutive common shares are included in the computation of any diluted per share amount when a loss is reported.
Cash and Cash Equivalents
VSee Lab considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates their fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
VSee Lab carries its accounts receivable at net realizable value. VSee Lab maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses - Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on
73
certain types of financial instruments, including trade receivables. No credit losses were recognized for the years ended December 31, 2023 and 2022.
The allowance for doubtful accounts is calculated based on a general reserve for at-risk balances considering the Company’s ability to collect. The allowance for doubtful accounts was $32,457 and $0 as of December 31, 2023 and 2022, respectively.
Prepaid Assets
Prepaid expenses are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the consolidated statements of operations.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3: Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable and accrued liabilities as reflected in the consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the consolidated financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. Derivative assets and liabilities are classified in the consolidated balance sheets as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the consolidated balance sheet date. The Company has determined the early mandatory redemption provision in the Bridge Note as described in Note 9 is an embedded derivative instrument. FASB ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components. The Company applies this guidance to allocate the Bridge Note proceeds between the Bridge Note and the Embedded Early Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt.
Fixed Assets
Fixed Assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets. During the year ended December 31, 2023, the Company purchased office and medical equipment, which is being depreciated over a three-year useful life.
74
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Credit Losses - Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. This may result in the earlier recognition of allowances for losses. ASU 2016-13 is effective for the Company beginning January 1, 2023, and the adoption did not have a material impact on the consolidated financial statements.
In August 2021, the FASB issued ASU No. 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”). ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP.
Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective January 1, 2024, for the Company. Early adoption is permitted. Management is currently evaluating the effect of the adoption of ASU 2020-06 on the consolidated financial statements, but currently does not believe ASU 2020-06 will have a significant impact on the Company’s consolidated financial statements.
Financial Statement Components
Revenue
VSee Lab generates revenue from subscription services to its software platform. Subscriptions represent a series of distinct goods or services because the performance obligations are satisfied over time as customers simultaneously receive and consume the benefits related to the services as VSee Lab performs.
Cost of Revenue
VSee Lab’s cost of revenue consists primarily of expenses related to cloud hosting, personnel-related expenses for VSee’s customer success team, costs for third-party software services and contractors, and other services.
Operating expenses
VSee Lab’s operating expenses include all operating costs not included in cost of revenue. These costs consist of general and administrative expenses composed primarily of all payroll and payroll-related expenses, professional fees, and other costs related to the administration of its business.
75
VSee Lab, Inc.
Three Months Ended March 31, 2024 and 2023 Results of Operations
The following table presents VSee Lab’s results of operations for the three months ended March 31, 2024 and 2023:
| | | | | | | | | | | | |
|
| For the Three Months Ended March 31, |
| |||||||||
| | 2024 |
| 2023 |
| Change |
| % |
| |||
Revenue | | $ | 1,495,995 | | $ | 1,596,268 | | $ | (100,273) |
| (6) | % |
Cost of revenue | |
| 386,253 | |
| 575,322 | |
| (189,069) |
| (33) | % |
Gross profit | |
| 1,109,742 | |
| 1,020,946 | |
| 88,796 |
| 9 | % |
Operating expenses | |
| 1,071,263 | |
| 1,658,091 | |
| (586,828) |
| (35) | % |
Other (expenses)/income | |
| (9,310) | |
| (1,717) | |
| (7,593) |
| 442 | % |
Net income (loss) before taxes | |
| 29,169 | |
| (638,862) | |
| 668,031 |
| 105 | % |
Income tax (expense) benefit | |
| — | |
| 182,843 | |
| (182,843) |
| (100) | % |
Net income (loss) | | $ | 29,169 | | $ | (456,019) | | $ | 485,188 |
| 106 | % |
Revenue
Revenue was $1,495,995 for the three months ended March 31, 2024, compared to $1,596,268 for the three months ended March 31, 2023, a decrease of ($100,273) or (6%). Revenue decline was driven by lower subscription and technical engineering fees and slightly offset by higher professional services and other fees. Subscription fees declined ($155,989) or (13%) due to lower client customer utilization and customer termination. Technical engineering fees declined ($12,737) or (7%) from less engineering work on existing and new accounts during the first quarter of 2024 compared to last year. The declines in revenue were offset by $68,453 or 26% of higher professional services and other fees, primarily from $78,750 of project management and consulting fees.
Cost of Revenue
Cost of revenue for the three months ended March 31, 2024, decreased ($189,069) or (33)% over the same period last year. The decrease was primarily driven by ($99,242) of lower compensation expenses from time spent and expense realignment across the business and a ($85,613) decrease in hosting expenses from lower usage and new vendor price reductions.
Operating Expenses
Operating expenses for the three months ended March 31, 2024 decreased ($586,828), or (35%), over the same period last year. The decrease was driven by ($441,975) or (33%) of lower compensation and related benefits, primarily from lower utilization of independent contractors and employee headcount reduction. General and Administrative expenses decreased by ($129,905) or (46%) primarily from lower software cost and business service expenses driven by a higher percentage of cost allocations to the cost of revenue compared to the same period last year and lower reseller services fees. Transaction expenses decreased by ($14,948) or (36%), driven by lower professional fees related to the Business Combination transactions procured during the current year compared to last year.
Other (Expense) Income
Other (expense) income during the three months ended March 31, 2024, increased ($7,593), primarily driven by ($26,069) of the fair value gain on the embedded derivative last year compared to none in the current period and ($19,616) of income tax refund, bank rewards, and interest income received last year compared to none during the current period, driving a ($45,685) reduction in other income. These were slightly offset by $38,092 of lower interest expenses.
Net Income (loss)
Net income was $29,169 for the three months ended March 31, 2024, compared to a net (loss) of ($456,019) for the three months ended March 31, 2023, resulting in an improvement in net income of $485,188 or 106%, primarily driven by $586,828 of lower operating expenses and offset by $100,273 of lower revenue.
76
Cash Flows
The following table presents selected captions from VSee Lab’s consolidated statements of cash flows for the three months ended March 31, 2024 and 2023:
| | | | | | |
|
| For the Three Months Ended March 31, | ||||
| | 2024 |
| 2023 | ||
Net cash from operating activities | | $ | 579,286 | | $ | (353,316) |
Net cash (used in) from investing activities | | $ | (8,740) | | $ | (1,690) |
Net cash provided by financing activities | | $ | — | | $ | 320,000 |
Change in cash | | $ | 570,546 | | $ | (35,006) |
VSee Lab’s principal sources of liquidity are cash and cash equivalents, totaling $689,280 and $195,658 as of March 31, 2024 and 2023, respectively.
VSee Lab’s future capital requirements will depend on many factors, including our growth rate, contract renewal activity, number of subscription renewals, the continuing market acceptance of telehealth, and debt funding.
Cash Provided by (Used in) Operating Activities
Cash used in operating activities was $579,286 for three months ended March 31, 2024. This consisted of a net income of $29,169, adjusted for non-cash items of $16,819, and an increase in net changes in operating assets and liabilities of $533,298. The net changes in operating assets and liabilities were primarily driven by the increase in deferred revenue.
Cash used in operating activities was ($353,316) for the three months ended March 31, 2023. This consisted of net (loss) of ($456,019), adjusted for non-cash items of $17,672, and an increase in net changes in operating assets and liabilities of $85,031. The net changes in operating assets and liabilities were primarily driven by increases in accounts payable and accrued liabilities and partially offset by an increase in deferred tax assets and a decrease in deferred revenue.
Cash Provided by Investing Activities
Cash used for investing activities for the three months ended March 31, 2024, was ($8,740) and was used to purchase fixed assets. Cash used for investing activities for the three months ended March 31, 2023 was ($1,690) used to purchase fixed assets.
Cash Provided by Financing Activities
The Company had no cash from financing activities during the three months ended March 31, 2024. Cash provided by financing activities for the three months ended March 31, 2023, was $320,000, consisting of $200,000 and $120,000 proceeds from note payable and related party loan payable, respectively.
77
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF IDOC
The following discussion and analysis provide information that iDoc’s management believes is relevant to an assessment and understanding of the results of operations and financial condition of iDoc Virtual Telehealth Solutions, Inc. (“iDoc”) (for purposes of this section, collectively referred to as the “Company”, “we,” “us” and “our”). The discussion and analysis should be read together with iDoc’s consolidated financial statements as of and for the three months ended March 31, 2024 and 2023, and the related respective notes thereto. This discussion may contain forward-looking statements based upon iDoc’s current expectations, estimates and projections that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking statements due to, among other considerations, the matters discussed under “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.”
Overview
iDoc is a high acuity patient care solution providing elite physician services in intensive care units of our major hospital systems and other customers. iDoc delivers neuro-critical care through a proprietary technology platform. iDoc serves a diverse range of customers from large hospital systems to small/micro hospitals, long-term acute care (LTAC) facilities, correctional facilities and others. In addition to the specialization of neuro critical care, iDoc provides general tele-critical care services, and specialty e-consults to large organizations such as correctional facilities. To provide these specialized services, iDoc pays meticulous attention to all government requirements that are required to deliver these services at a state and national level. Physician licensure is governed at the state level with its private clients and governed by Federal regulations with its Federal Bureau of Prison clients. iDoc complies with all state and federal level requirements for physician licensure. iDoc Virtual Telehealth Solutions currently operates in three states with private clients. These states are Texas, Georgia, and New Hampshire. iDoc’s wholly owned subsidiary billing company, Encompass, does not provide physician services and therefore there are no physician requirements and it operates out of Colorado. We operate out of five states with our Federal Bureau of Prisons clients. These states are South Carolina, Pennsylvania, Washington, Texas, and Alabama. All iDoc physicians practicing in each state require a state license and iDoc as the corporate entity manages and verifies this process directly with its physicians. The licensures are required for credentials to practice at any hospital and iDoc as the corporate entity also manages and verifies the credentialing process for all states and sites it operates in. The federal government currently does not require a separate state licensure for practicing at a federal site. However, the federal government does requires credentialing, which iDoc manages that with each of its federal practice locations. From the corporate level of medicine perspective, the government regulations apply to patient specific revenue that requires billing to Medicare, Medicaid, or third party insurers, which is governed at the state level. iDoc creates a subsidiary company in each state it operates in and obtains a company specific National Provider Identification (“NPI”) number for the corporate entity in each state to comply with the state mandates. iDoc, as a corporate entity complies with these requirements. Physicians are independently contracted or employed under the parent company — iDoc Virtual Telehealth Solutions and utilize the company NPI in each state and therefore physicians do not need separate NPI numbers just state specific licensure and maintain one NPI number. iDoc manages and verifies each physician is properly licensed and credentialed in each state under each corporate entity to which they provide services.
iDoc has an experienced team of board-certified intensivists, neurointensivists, neurologists, and advanced practice providers that treat and coordinate care for acutely ill patients 24/7 in the Neurointensive Care Unit (“NICU”) and Intensive Care Unit (“ICU”) for stroke, brain trauma, spinal cord, and all other neurological conditions. Our Neurocritical care experts will also help develop multidisciplinary plans of care to optimally treat neurological conditions in relation to their overall medical needs. Our Neuro Critical care service delivery will focus on physicians and provider services in Teleneurocritical care, epileptology, and teleneurology. In addition to standard interventions, our Neurocritical care experts will offer specific care including monitoring Intracranial pressure, cerebral hemodynamics, advanced multimodal neuro monitoring (brain oximetry, cerebral microdialysis and continuous electroencephalography).
iDoc aspires to be a leading tele-neuro critical care provider and have the potential to become a market leader in the specialty provider market. iDoc generates revenue from our patient fees related to insurance billings for Tele-Neuro critical and ICU patients at hospitals and hospital systems. iDoc also generates revenue from telehealth and institutional fees by providing Tele-Neuro critical and ICU management and administration at hospitals and hospital systems, telehealth consulting and monitoring services, including EEG reads and other ancillary medical services.
78
Patient Fees
iDoc collaborates with partner hospitals’ critical care team to provide health care for patients suffering from neurological ailments such as stroke, traumatic brain injury, neuromuscular disorders, spine injury and more. Our fees are paid by national insurance companies covering the cost of inpatient healthcare services.
Telehealth and Institutional Fees
iDoc provides hospitals and hospital systems with consulting, management, and administrative services to appropriately create or ensure efficient operation of Neurocritical Care Telemedicine or ICU Platforms. These services also include telemedicine platform software, telemedicine hardware, EEG on-call services, EEG interpretation fees and medical billing fees.
Material Trends, Events and Uncertainties
Impact of the COVID-19 Pandemic
The extent to which the coronavirus (“COVID-19”) outbreak and measures taken in response thereto impact our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted.
Global health concerns relating to the COVID-19 outbreak have been weighing on the macroeconomic environment, and the outbreak has significantly increased economic uncertainty. Risks related to consumers and businesses lowering or changing spending, which impact domestic and international spend. The outbreak has resulted in authorities implementing numerous measures to try to contain the virus, such as travel bans and restrictions, quarantines, shelter in place orders, and business shutdowns. These measures have not only negatively impacted consumer spending and business spending habits, but they have also adversely impacted and may further impact our workforce and operations and the operations of its customers, suppliers, and business partners. These measures may remain in place for a significant period of time, and they are likely to continue to adversely affect our business, results of operations and financial condition. The spread of COVID-19 has caused us to modify our company’s business practices (including employee travel, employee work locations, and cancellation of physical participation in meetings, events, and conferences), and we may take further actions as may be required by government authorities or that we determine are in the best interests of our employees, customers, and business partners. There is no certainty that such measures will be sufficient to mitigate the risks posed by the virus or otherwise be satisfactory to government authorities.
The extent to which the COVID-19 outbreak impacts our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted, including, but not limited to, the duration and spread of the outbreak, its severity, the actions to contain the virus or treat its impact, and how quickly and to what extent normal economic and operating conditions can resume.
Even after the COVID-19 outbreak has subsided, we may continue to experience materially adverse impacts to our business because of its global economic impact, including any recession that has occurred or may occur in the future.
There are no comparable recent events which may provide guidance as to the effect of the spread of COVID-19 and a global pandemic, and, as a result, the ultimate impact of the COVID-19 outbreak or a similar health epidemic is highly uncertain and subject to change. We do not yet know the full extent of the impacts on our business, our operations, or the global economy. However, the effects could have a material impact on our results of operations, and we will continue to monitor the COVID-19 situation closely.
Performance Factors
We believe that our future performance will depend on many factors, including the following:
The Rapid Transformation of the Telehealth Market
The Telehealth market today is one characterized by rapid transformation, with major customers and hospital systems looking to build or add capabilities and major legacy competitors looking to shore up historical limitations. We believe that the rapid transformation of the telehealth market indicates strong future growth of the market, and our current offerings provide an attractive value proposition to health systems, medical groups, and individual medical practitioners, driving higher market share. We plan to continue to harness our scale to further grow the value proposition of our platform for all stakeholders.
79
Ability to Expand Within the Market and Attract New Customers
Telehealth is still in its total infancy stages in terms of utilization, scope, and services. Most of the growth is expected within hospital systems, definition, and segmentation structure, and we believe our software platform and services have significant potential. We plan to leverage our industry relationships with government, hospital systems and insurance providers to increase our customer base.
Critical Accounting Policies and Estimates
Basis of Presentation and Consolidation
The accompanying consolidated financial statements of iDoc have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
Use of Estimates
The preparation of iDoc’s consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, the valuation of iDoc’s common stock, allowance for doubtful accounts, and income taxes.
iDoc bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss, and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits as a component of general and administrative expenses. The Company’s federal tax return and any state tax returns are not currently under examination.
The Company has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
iDoc recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects
80
the consideration the entity expects to be entitled in exchange for those goods or services. The Company recognizes revenue using a five-step model:
| 1. | Identify the contract(s) with a customer; |
| 2. | Identify the performance obligation(s) in the contract; |
| 3. | Determine the transaction price; |
| 4. | Allocate the transaction price to the performance obligations in the contract; and |
| 5. | Recognize revenue when (or as) it satisfies a performance obligation. |
The Company derives revenue from business services associated with direct tele-physician provider patient fee services, telehealth services, and institutional services provided to our clients.
Patient Fees Services and Performance Obligation
All of iDoc’s telemedicine contracts for patient reimbursement fees are directly billed through the Encompass healthcare billing services subsidiary. iDoc earns patient fees by providing high acuity patient care solutions. For patient fees, performance obligations are met when the Company’s physicians provide professional medical services to patients at the client site as this is deemed as transfer of goods and services to respective patients. The patient benefits from the professional services when care is rendered by the Company’s medical professionals. The revenue is determined based on the telemedicine billing code(s) associated with the respective professional service rendered to patients. iDoc earns primarily from reimbursement from the following third-party payors:
Medicare
The Medicare program offers beneficiaries different ways to obtain medical benefits: (i) Medicare Part A, which covers, among other things, in-patient hospital, SNFs, home healthcare, and certain other types of healthcare services; (ii) Medicare Part B, which covers physicians’ services, outpatient services, durable medical equipment, and certain other types of items and healthcare services; (iii) Medicare Part C, also known as Medicare Advantage, which is a managed care option for beneficiaries who are entitled to Medicare Part A and enrolled in Medicare Part B; and (iv) Medicare Part D, which provides coverage for prescription drugs that are not otherwise covered under Medicare Part A or Part B for those beneficiaries that enroll.
iDoc’s affiliated provider network is reimbursed by the Part B and Part C programs for certain of the telemedicine services it provides to Medicare beneficiaries. Medicare coverage for telemedicine services is treated distinctly from other types of professional medical services and is limited by federal statute and subject to specific conditions of participation and payment pursuant to Medicare regulations, policies and guidelines, including the location of the patient, the type of service, and the modality for delivering the telemedicine service, among others.
Medicaid
Medicaid programs are funded jointly by the federal government and the states and are administered by states (or the state’s designated managed care or other similar organizations) under approved plans. Our affiliated provider network is reimbursed by certain state Medicaid programs for certain of the telemedicine services it provides to Medicaid beneficiaries. Medicaid coverage for telemedicine services varies by state and is subject to specific conditions of participation and payment.
Commercial Insurance Providers
iDoc is reimbursed by commercial insurance carriers. The basis for payment to the commercial insurance providers is consistent with Medicare reimbursement fee structure guidelines and the Company is in-network or out-of-network with the commercial insurance carriers based on state and insurer requirements.
81
Telehealth Fees Service Contracts and Performance Obligation
iDoc enters into service contracts mainly in the following categories with hospitals or hospital systems, physician practice groups, and other users. iDoc’s customer contracts typically range in length from two to three years, with an automatic renewal process. The Company either invoices its customers for the monthly fixed fee in advance or at the end of the month, depending on the terms of the contract. The contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that it has any material outstanding commitment for future revenues beyond one year from the end of a reporting period. Under the contracts, the customers pay a fixed monthly fee for the services described below.
Contract For Telemedicine Care Services
Performance obligations in the contract for telemedicine care are based on services provided via the use of hardware and software integration that includes multi-participant video conferencing, and electronic communication for 24 hours per day, seven days per week for the duration of the contract. iDoc provides administrative support for the tele-physician services and coordinates the services of its clinicians network through administrative support, hardware support, and software support and provider coverage availability. The Company provides coverage availability of its physician services ranging from 12-24h per day. Performance obligations in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from patient services and institutional services obligations. Performance obligations are met when the Company provides administrative, business and medical records and reports related to their professional services rendered pursuant to the agreement in such format and upon such interval as hospitals may require. Revenue from telemedicine care services is included in telehealth fees in the consolidated financial statements. The Company commences revenue recognition when the Company satisfies its performance obligation to provide the contractual tele-physician hours services monthly. Prior to the commencement of services, customers generally make initial start-up nonrefundable payments to the Company when contracting for Company training, hardware and software installation and integration, which includes a one-time setup of software security, API interfaces, and compatibility between hospital existing equipment and hardware and software. The Company recognizes revenue upon completion of the implementation when the performance obligation of equipment setup and initial training is completed. The start-up fees do not significantly modify or customize the other goods in the contract. As the start-up service primarily covers initial administrative services for which the Company’s clients can cancel future services upon completion, management considers it to be separable from the ongoing business services, and the Company records start-up fees as one-time revenue when the start-up service is complete.
Institutional Fees Service Contracts and Performance Obligation
Contract For Electroencephalogram (“EEG”) Professional Interpretation Services
Performance obligations in the contract for EEG professional interpretation services are based on the number of professional services EEG interpretation provides monthly. The performance obligation in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. To facilitate the delivery of the EEG professional interpretation services, the Company’s physicians use EEG telemedicine equipment provided by the Company. The performance obligation is satisfied based on the number of EEG professional interpretations performed by the Company’s physicians. The number of professional interpretations is traced monthly by both parties and used to determine the revenue earned based on established contractual rates and are included in institutional fees in the consolidated financial statements. The Company commences revenue recognition on EEG professional interpretation services when the Company satisfies its performance obligation to provide professional interpretation monthly.
Encompass Healthcare Client Billing Services
iDoc enters into contracts with hospitals, physician practice groups, and other users for billing services. Medical billing service fees include amounts charged for ongoing billing, clinical-related, and other related services and are generally billed to the customer as a percentage of total collections. The Company does not recognize revenue for business service fees until these collections are made, as the service fees are not fixed and determinable until such time. Medical billing service fees also include amounts charged to customers for generating and mailing patient statements and are recognized as the related services are performed. The Company’s clients typically purchase one-year contracts that renew automatically upon completion. In most cases, the clients may terminate their agreements with 90 days notice without cause. The Company typically retains the right to terminate client agreements in a similar timeframe. The Company’s clients are billed monthly, in arrears, based either upon a percentage of collections, minimum fees, flat fees, or per-claim fees where applicable. Invoices are generated within the first two weeks of the subsequent month and delivered to clients primarily by email.
82
Determination of Pricing for Services
iDoc believes the quoted transaction prices in the customer contracts represent the stand- alone selling prices for each of the separate performance obligations which are distinct and priced separately within the contract. The transaction price for each service provided is independent and established in the contract and based on the duration of service provided or for a rate for service provided. Fees are established based on the service transferred to the client.
Telehealth and Institutional Services Contracts
Under most of iDoc’s contracts, including contracts with its two top customers, the customer pays fixed monthly fees for telemedicine consultation services, EEG professional interpretation services, platform software services, and hardware fees. The fixed monthly fee provides for a predetermined number of daily, monthly, or annual physician hours of coverage and agreed upon rates for interpretation and software services. To facilitate the delivery of the consultation services, the facilities use telemedicine equipment and the Company’s virtual health care platform, which is provided and installed by the Company. The Company also provides the hospitals with user training, maintenance and support services for the telemedicine equipment used to perform the consultation services. The Company recognizes revenue for variable consideration when it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. iDoc estimates the amount of revenue to be recognized on variable consideration, using the expected value or the most likely amount method, whichever is expected to better predict the amount. iDoc’s estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on assessments of legal enforceability, performance, and all information that is reasonably available to the Company. The determination of the amount of revenue the Company can recognize each accounting period requires management to make estimates and judgments on the estimated expected customer life or expected performance period, of at least 3 years.
Patient Fee Contracts Involving Third-Party Payors
iDoc receives payments from patients, third-party payers and others for patient fee services. Third-party payers pay the Company based on contracted rates or the entities’ billed charges. Payments received from third-party payers are generally less than billed charges. The Company receives less than its total established charges for its services. The Company determines the transaction price on patient fees based on standard charges for services provided, reduced by adjustments provided to third-party payors, and implicit price concessions provided to uninsured patients. The Company monitors its revenue and receivables from third-party payers and records an estimated contractual allowance to properly account for the differences between billed and reimbursed amounts.
In the post PHE environment, iDoc recognized the need to have a more timely recognition of when claims were denied compared to pre PHE. As part of a long-term solution, the Company switched insurance clearinghouses This new clearinghouse allowed for automation and direct visualization of the process as compared to the prior system, which was paper mail notifications. The change in clearinghouse was intended to ultimately allow the Company to address this and any future changes from any of the Company’s commercial or Medicare payors in a more nimble and direct electronic fashion versus relying on a mail delivery system. Setting up the automation, testing and validation was not completed until February 2024. The new process will positively allow the Company to better estimate for contractual allowances and receivables in a timelier manner as the Company will be able to identify any issues regarding denials and respond significantly quicker, thereby improving its revenue cycle management process with its insurance payors. The Company believes this will improve its collections and decrease our bad debt write off in a step wise manner moving forward.
Revenue from third-party payers is presented net of an estimated provision for contractual adjustments. Patient revenues are net of service credits and service adjustments, and allowance for doubtful accounts receivable. These adjustments and implicit price concessions represent the difference between the amount billed and the estimated consideration the Company expects to receive, based on historical collection experience, market conditions and other factors. Although the Company believes that its approach to estimates and judgments as described herein is reasonable, actual results could differ and the Company may be exposed to increases or decreases in revenue that could be material.
Cost of Revenue
Cost of revenue consists primarily of expenses related to compensation-related expenses for the Company’s telehealth service providers, costs for third-party software and hardware services and independent medical providers, and other services used in connection with the delivery and support of the Company’s telehealth platform.
83
Going Concern
The accompanying consolidated financial statements have been prepared assuming iDoc will continue as a going concern. iDoc has incurred losses since inception and continues to have negative operating cash flows. These conditions raise substantial doubt about iDoc’s ability to continue as a going concern. The continuation of iDoc’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should the Company be unable to continue as a going concern.
The Company’s ability to continue as a going concern will require it to obtain additional funding. If the Company is unable to obtain sufficient funding, its business, prospects, financial condition and results of operations will be materially and adversely affected, and the Company may be unable to continue as a going concern. In such an event, it would be forced to delay, limit, reduce or terminate its product development or commercialization efforts, or may be forced to reduce or terminate its operations. If the Company is unable to continue as a going concern, the Company may have to liquidate its assets and may receive less than the value at which those assets are carried on its audited financial statements, and it is likely that investors will lose all or part of their investment.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC-insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates its fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
The Company carries its accounts receivable at net realizable value. The Company maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses - Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. No credit losses were recognized for the years ended December 31, 2023 and 2022.
The allowance for doubtful accounts is calculated based on a general reserve for at-risk balances considering the Company’s ability to collect as well as the current credit conditions of third-party payers. The allowance for doubtful accounts was $1,576,415 and $1,038,956 as of December 31, 2023 and 2022, respectively.
Prepaid Expenses
Prepaid expenses are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the consolidated statements of operations.
Leases
iDoc accounts for leases under ASU 2016-02, “Leases” (Topic 842). Based on this standard, the Company determines if an agreement is a lease at inception. Operating and finance leases are included in right-of-use asset, current portion of right-of-use liability, and right-of-use liability less current portion in the Company’s consolidated balance sheets. Operating and finance lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date. As permitted under ASU 2016-02, the Company has made an accounting policy election not to apply the recognition provisions of ASU 2016-02 to short-term leases (leases with a lease term of 12 months or less that do not include an option to purchase the underlying asset that the lessee is reasonably certain to exercise); instead, the Company will recognize the lease payments for short term leases on a straight-line basis over the lease term.
84
Net Income (Loss) Per Common Share
iDoc computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants. No potential dilutive common shares are included in the computation of any diluted per share amount when a loss is reported.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3: Inputs are unobservable inputs that reflect the reporting entity’s assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable, and accrued liabilities as reflected in the consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities, and equity instruments of the Company are either recognized or disclosed in the consolidated financial statements together with other information relevant to making a reasonable assessment of future cash flows, interest rate risk, and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. Derivative assets and liabilities are classified in the consolidated balance sheets as current or non-current based on whether net-cash settlement or conversion of the instrument could be required within 12 months of the consolidated balance sheet date. The Company has determined the early mandatory redemption provision in the Bridge Note as described in Note 8 is an embedded derivative instrument. ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components. The Company applies this guidance to allocate the Bridge Note proceeds between the Bridge Note and the Embedded Early Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt.
Fixed Assets
Fixed assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets, which is three to ten years.
Intangible Assets
Intangible assets are presented at fair value, net of amortization. The fair value is determined based on the appraised value of the asset. Amortization is calculated on the straight-line method over the five-year estimated useful lives of the respective assets.
85
Intangible assets comprise of goodwill and a customer list. As of December 31, 2023 and 2022, the fair value of goodwill is $0 and $95,076, respectively, as described in Note 3, Business Acquisition. During the year ended December 31, 2022, the Company acquired a customer list related to the acquisition valued at $15,000. The balance of the customer list is $0 and $12,000 as of December 31, 2023 and 2022, respectively. The Company recognized $3,000 of amortization expenses during the years ended December 31, 2023 and 2022, respectively.
Impairment of Long-lived and Intangible Assets
In accordance with ASC 360-10, the Company, on a regular basis, reviews the carrying amount of long-lived assets for the existence of facts or circumstances, both internally and externally, that suggest impairment. The Company determines if the carrying amount of a long-lived asset is impaired based on anticipated undiscounted cash flows, before interest, from the use of the asset. In the event of impairment, a loss is recognized based on the amount by which the carrying amount exceeds the fair value of the asset. Fair value is determined based on the appraised value of the assets or the anticipated cash flows from the use of the asset, discounted at a rate commensurate with the risk involved. The Company recorded $104,076 and $0 of impairment charges during the years ended December 31, 2023 and 2022, respectively, on its goodwill and customer list intangible assets.
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Credit Losses - Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivable. This may result in the earlier recognition of allowances for losses. ASU 2016-13 is effective for the Company beginning January 1, 2023, and the adoption did not have a material impact on the consolidated financial statements.
In August 2021, the FASB issued ASU No. 2021-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2021-06”). ASU 2021-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP.
Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2021-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2021-06 is effective for the Company beginning January 1, 2024.
Early adoption is permitted, but no earlier than January 1, 2022. Management is currently evaluating the effect of the adoption of ASU 2021-06 on the consolidated financial statements but currently does not believe ASU 2021-06 will have a significant impact on the Company’s consolidated financial statements.
Financial Statement Components
Revenue
iDoc establishes management and administrative services contracts with hospitals or hospital systems to provide telehealth physician services to acute patients of the hospitals or hospital systems. iDoc also generate revenue by directly billing the insurance companies for care provided at hospitals or hospital systems.
iDoc’s contracts typically range in length from two to three years, with an automatic renewal process.
86
Cost of Revenue
iDoc’s cost of revenue is primarily comprised of personnel-related expenses for our employee and consulting physicians and other medical providers, and the costs for third-party software services and hardware used in connection with delivery of high acuity patient care solution when providing elite physician services in the Intensive care units of our major hospital systems and other customers.
Operating expenses
iDoc’s operating expenses include all operating costs not included in cost of revenue. These costs consist of compensation, general and administrative expenses composed primarily of all payroll and payroll- related expenses, professional fees, insurance, software costs, occupancy expenses related to iDoc’s operations, including utilities, depreciation and amortization, and other costs related to the administration of its business.
iDoc Virtual Telehealth Solutions, Inc.
Three Months Ended March 31, 2024 and 2023 Results of Operations
The following table presents iDoc ‘s results of operations for the three months ended March 31, 2024 and 2023:
| | | | | | | | | | | | |
|
| For the Three Months Ended March 31, |
| |||||||||
| | 2024 |
| 2023 |
| Change |
| % |
| |||
Revenues | | $ | 1,639,765 | | $ | 1,948,691 | | $ | (308,926) |
| (16) | % |
Cost of revenue | |
| 400,563 | |
| 790,133 | |
| (389,570) |
| (49) | % |
Gross profit | |
| 1,239,202 | |
| 1,158,558 | |
| 80,644 |
| 7 | % |
Operating expenses | |
| 893,199 | |
| 1,380,942 | |
| (487,743) |
| (35) | % |
Other (expenses)/income | |
| (96,914) | |
| (99,253) | |
| 2,339 |
| (2) | % |
Net income (loss) before taxes | |
| 249,089 | |
| (321,637) | |
| 570,726 |
| 177 | % |
Income tax (expense) benefit | |
| (55,603) | |
| 72,270 | |
| (127,873) |
| 177 | % |
Net income (loss) before taxes | | $ | 193,486 | | $ | (249,367) | | $ | 442,853 |
| 178 | % |
Revenue
Revenue during the three months ended March 31, 2024, decreased ($308,926) or (16%) compared to last year. The revenue decrease was driven by the ($269,776) or (98%) reduction in institutional fees primarily from the termination of the Encompass Busines and lower EEG revenue. The revenue decrease was also driven by ($160,627) or (24%) reduction in telehealth fees due to client terminations in the fourth quarter last year. The decrease in revenue was offset by $121,477, or 12% of higher patient fees from higher patient volume.
Cost of Revenue
Cost of revenue for the three months ended March 31, 2024, decreased ($389,570), or (49%), compared to the three months ending March 31, 2023. The decrease was driven by ($369,033) lower compensation to the Company’s full-time employees and consulting physicians due to reduced employee salary levels and lower consulting physicians’ service hours. The decrease was also driven by ($20,537) of lower non-compensation-related expenses.
Operating Expenses
Operating expenses for the three months ended March 31, 2024, decreased by ($487,743,) or (35)%, compared to the three months ended March 31, 2023. The decrease was primarily driven by ($286,295) or (50)% of lower general and administrative expenses, primarily from ($189,713) of lower bad debt expenses and ($44,405) expense reduction from employee recruiting and professional fees. The decrease was also due to ($215,461) or (35%) of lower compensation primarily due to cost savings from no Encompass-related compensation due to the termination of the Encompass business and ($48,769) or (35%) of lower one-time transaction expenses related to this proxy statement/prospectus for legal, business consulting, valuation, auditing, and taxation services. These decreases were slightly offset by $62,782, or 132% of higher professional fees, from higher medical billing fees.
87
Other Income (Expense)
Other (expenses) decreased by $2,339, during the three months ended March 31, 2024, compared to March 31, 2023. The decrease was driven by $42,673 of lower interest expenses and partially offset by ($26,069) of the fair value gain on the embedded derivative last year compared to none in the current period and ($14,265) of higher other expenses, primarily from losses on factoring payable.
Net Income (loss)
Net income was $193,486 for the three months ended March 31, 2024, compared to a net (loss) of ($249,367) for the three months ended March 31, 2023, resulting in a higher net income of $442,853. The improvement was primarily due to the $487,743 of lower operating expenses.
Cash Flows
The following table presents selected captions from iDoc’s consolidated statements of cash flows for the three months ended March 31, 2024 and 2023:
| | | | | | |
|
| For the Three Months Ended March 31, | ||||
| | 2024 |
| 2023 | ||
Net cash (used in) operating activities | | $ | 185,120 | | $ | (685,769) |
Net cash (used in) investing activities | | $ | — | | $ | 90,500 |
Net cash provided by financing activities | | $ | (173,973) | | $ | 483,239 |
Change in cash | | $ | 11,147 | | $ | (112,030) |
iDoc’s principal sources of liquidity are cash and cash equivalents, totaling $74,184 and $35,655 as of March 31, 2024 and 2023, respectively.
iDoc’s future capital requirements will depend on many factors, including our growth rate, contract renewal activity, number of visits, the continuing market acceptance of telehealth, and debt funding.
Cash Provided by (Used in) Operating Activities
iDoc’s uses of cash from operating activities primarily pay cash compensation to its employees and independent contractors, as well as costs for third-party hardware and professional services fees.
Cash used in operating activities was $185,120 for the three months ended March 31, 2024, consisting of net income of $193,486, adjustments for non-cash items of $164,760, and a decrease in net changes in operating assets and liabilities of ($173,126). The net changes in operating assets and liabilities were primarily driven by the increase in accounts receivable and offset by the increase in accounts payable and accrued liabilities.
Cash used in operating activities was ($685,769) for the three months ended March 31, 2023. This consisted of net loss of ($249,367) adjusted for non-cash items of $352,075 and a decrease in net changes in operating assets and liabilities of ($788,477). The net changes in operating assets and liabilities were primarily driven by increases in accounts receivable.
Cash Provided by (Used in) Investing Activities
There were no investing activities for the three months ended March 31, 2024. For the three months ended March 31, 2023, cash from investing activities was $90,500, consisting of $90,500 in proceeds from the note receivable.
Cash Provided by (Used in) Financing Activities
Cash provided by financing activities for the three months ended March 31, 2024, was ($173,973), consisting of ($221,673) payments on factoring payable and were offset by $31,500 and $16,200 proceeds from factoring payable and notes payable, respectively.
Cash provided by financing activities for the three months ended March 31, 2023, was $483,239, primarily consisting of $585,000 proceeds from notes payable and offset by ($53,816) of payments on notes payable and ($47,945) in lease repayments.
88
MANAGEMENT
Executive Officers and Directors
The following table sets forth certain information concerning our executive officers and directors:
Name |
| Position |
| Age |
Milton Chen | | Co-Chief Executive Officer and Director | | 51 |
Imoigele Aisiku | | Co-Chief Executive Officer, Chairman, Director | | 52 |
Jerry Leonard | | Chief Financial Officer and Secretary | | 56 |
Kevin Lowdermilk | | Director | | 60 |
Colin O’Sullivan | | Director | | 49 |
Scott Metzger | | Director | | 56 |
Cydonii V. Fairfax | | Director | | 51 |
David L. Wickersham | | Director | | 54 |
Milton Chen is the co-CEO of the Company. Mr. Chen is the co-founder and current CEO of VSee Lab Mr. Chen founded VSee Lab in January 2008 and has been the CEO of VSee Lab since then. In December 2016, Mr. Chen co-founded another company called “This American Doc” - a tele-staffing company for medical professionals. Mr. Chen has served as the CEO of This American Doc from 2016 to the present. Milton has donated his time, efforts and technologies to support refugees and the homeless in Ukraine, Iraq, Nigeria, Gabon and other countries around the world. While finishing his PhD at Stanford University, Mr. Chen researched human factors and design of video collaboration. Mr. Chen received a Bachelor of Science degree in Computer Science from University of UC Berkley and PhD from Standard University.
Imoigele Aisiku is the Co-CEO and chairman of the board of directors to the Company. Dr. Aisiku founded iDoc. in February 2014 and has been the CEO of iDoc since then. Dr. Aisiku is also the Division Chief of Emergency Critical Care of Brigham and Women’s Hospital in the Department of Emergency Medicine since January 2016. Dr. Aisiku has been practicing in the field of telemedicine for over 15 years and has consulted on telemedicine development nationally and internationally. Dr. Aisiku is board certified in Emergency Medicine, Internal Medicine Critical Care, and Neurocritical care. He did his medical school training at the University of Massachusetts and his emergency medicine and critical care training at Emory University. His neurocritical care training was at Washington University in St. Louis. He received his MBA from Emory University. Dr. Aisiku is currently an Associate Professor at Harvard Medical School Faculty and is also the Vice-Chair for Diversity Equity and Chief of Division of Emergency Critical Care Medicine in the Department of Emergency Medicine since 2020.
Jerry Leonard is the Chief Financial Officer of the Company. He has served as the CFO of iDoc since March 2021. Prior to his position with iDoc Mr. Leonard was the Vice President of Finance from January 2010 to June 2021 within the Asset Management business of Voya Financial, Inc. (NYSE: Voya). Preceding his role at Voya, he held various finance leadership positions at IBM and Colgate Palmolive (NYSE: IBM, CL). He started his career in Public Accounting at Arthur Andersen and PricewaterhouseCoopers. Mr. Leonard is a Certified Public Accountant (CPA). Mr. Leonard received his MBA from Emory University and a BBA in Accounting from Baruch College in New York City.
Kevin Lowdermilk is a member of the board of directors to the Company as an Independent Director. Mr. Lowdermilk has over 30 years of executive leadership experience. Currently, he is the CFO of Vaya Space, a hybrid rocket propulsion and small satellite launch company and has served on that position since August 2022. Prior to Vaya Space, between March 2016 and July 2022, he was the CFO of CFO Strategic Partners, a company that provides outsourced CFO services to small and medium-sized business and nonprofit entities. Mr. Lowdermilk’s past executive leadership experience also includes serving as the CEO of ISO Group, Inc. - a defense and aerospace supply chain company, serving as the CFO and then CEO of Exostar - a SaaS company with a focus on the aerospace and defense sector, and serving as the Vice President of Finance for a multi-national aerospace division of Rolls-Royce Holdings PLC in North America. He has also held board positions for a number of private companies across a variety of industries. Between 2009 and 2015, he was a board member of Global Healthcare Exchange, LLC (“GHX”) and chaired the board’s compensation committee through the sale of GHX to Thoma Bravo, LP. He earned his undergraduate degree in Economics from Western Kentucky University and his MBA from Ball State University.
Colin O’Sullivan is a member of the board of directors to the Company as an Independent Director. Colin O’Sullivan has nearly 25 years of executive healthcare leadership experience. He is currently the Executive Vice President of Cornerstone Healthcare Group and has served in that capacity since June 2014. Cornerstone owns and operates fifteen specialty acute hospitals, nine senior living
89
facilities, behavioral health hospital and rehabilitation division across seven states. Prior to Cornerstone, Mr. O’Sullivan was a senior executive in multiple healthcare companies including Lifecare Management Services, Regency Hospital Company, Coastal Carolinas Healthcare Alliance and others. He began his career in the US Air Force and was an Officer Candidate School Graduate from the U.S. Air Force Academy of Military Science. He earned his Doctor of Healthcare Administration from Central Michigan University, his Master of Healthcare Management from Marshall University, and his BS in Business Administration from Concord University in West Virginia.
Scott Metzger has served as a member of board of directors for Digital Health Acquisition Corp. since May of 2021 and is serving as a member of the board of directors to the Company as an Independent Director. Dr. Metzger has been a Medical Director with Optum, Inc. since September 2018. Between June 2000 to August 2018, Dr. Metzger worked as a physician for Premier Pain Centers and Specialty Anesthesia Associates. Dr. Metzger is the founder and former partner Premier Pain Centers and Specialty Anesthesia Associates, some of the most comprehensive centers for treatment of acute and chronic pain. Dr. Metzger has been active as a medical society leader and executive with experience ranging from starting the state branch of national pain society to serving as president of the state medical board. Dr. Metzger received his B.A. and M.D. from Boston University School of Medicine after completion of a combined 6-year program. He has also completed his residency and specialty training at Johns Hopkins Medicine through the Department of Anesthesiology and Critical Care Medicine.
Cydonii V. Fairfax is a member of the board of directors to the Company as an Independent Director. Ms. Fairfax is a legal and compliance executive with significant experience advising on corporate governance, finance, litigation and regulatory compliance matters in highly regulated industries. Her broad legal experience draws upon over 25 years of practice at leading institutions in both public and private sectors. Ms. Fairfax currently serves and has served since 2017 as Executive Vice President of the Metropolitan Transit Authority of Harris County, Texas (“Metro”), where she works extensively with senior management, board members and other stakeholders to structure and implement innovative transit solutions and capital projects. Additionally, Ms. Fairfax oversees the development of strategies and systems to manage risk and ensure adherence to legal and regulatory requirements. She has also served as Metro’s general counsel, directing and managing all legal affairs. Prior to joining Metro, Ms. Fairfax was Senior Vice President and Deputy General Counsel of American Capital, Ltd. (NASDAQ: ACAS), a publicly-traded private equity firm and global asset manager where she oversaw corporate governance for the fund group and advised on corporate transactions. Ms. Fairfax also practiced corporate and securities law at Arnold & Porter in Washington, D.C. Ms. Fairfax holds a Bachelor of Science degree in Finance from the University of Maryland and a Juris Doctor from Harvard Law School.
David L. Wickersham is a member of the board of directors to the Company as an Independent Director. Mr. Wickersham is the Chief Executive Officer and founder of Progressive Pipeline Management, which was started in 2002. Mr. Wickersham has over twenty-five years of experience in emergency response management and utility infrastructure rehabilitation. Mr. Wickersham holds a Bachelor of Science degree in Marine Transportation from the U.S. Merchant Marine Academy and served as an U.S. Veteran of the first Gulf War in Operation Desert Shield/Storm.
Director Independence
Nasdaq listing rules require that a majority of the board of directors of a company listed on Nasdaq be composed of “independent directors,” which is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship, which, in the opinion of the company’s board of directors, would interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director. We have determined that each of Kevin Lowdermilk, Colin O’Sullivan, Scott Metzger, Cydonii V. Fairfax and David L. Wickersham will be an independent director under the Nasdaq listing rules and Rule 10A-3 of the Exchange Act. In making these determinations, we considered the current and prior relationships that each non-employee director has with the Company and its subsidiaries, and all other facts and circumstances that we deemed relevant in determining independence, including the beneficial ownership of our common stock by each non-employee director, and the transactions involving them described in the section entitled “Certain Relationships and Related Transactions.”
Committees of the Board of Directors
The standing committees of the Board will consist of an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. The composition of each committee is set forth below.
90
Audit Committee
Our Audit Committee has been established in accordance with Section 3(a)(58)(A) of the Exchange Act and following the Business Combination will consist of Kevin Lowdermilk, Colin O’Sullivan Scott Metzger and Cydonii V. Fairfax, each of whom are independent directors and are “financially literate” as defined under the Nasdaq listing standards. Kevin Lowdermilk will serve as chairman of the Audit Committee. We have determined that Kevin Lowdermilk qualifies as an “audit committee financial expert,” as defined under rules and regulations of the SEC.
The audit committee’s duties are specified in our Audit Committee Charter.
Compensation Committee
Our Compensation Committee consists of Kevin Lowdermilk, Scott Metzger and David L. Wickersham. The chair of our compensation committee is David L. Wickersham. We have determined that each of Kevin Lowdermilk, Scott Metzger and David L. Wickersham is independent under Nasdaq listing standards, a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act. The compensation committee’s duties are specified in our Compensation Committee Charter.
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee will consist of Kevin Lowdermilk, Colin O’Sullivan and Cydonii V. Fairfax. The chair of our nominating and corporate governance committee will be Cydonii V. Fairfax. We have determined that each of Kevin Lowdermilk, Colin O’Sullivan and Cydonii V. Fairfax is independent under Nasdaq listing standards. The nominating and corporate governance committee’s duties are specified in our Nominating and Corporate Governance Committee Charter.
Code of Business Conduct and Ethics
We adopted a new Code of Business Conduct and Ethics for our directors, officers, employees and certain affiliates following the Business Combination in accordance with applicable federal securities laws, a copy of which will be available on the Company’s website at www.vseehealth.com. The Company will make a printed copy of the Code of Business Conduct and Ethics available to any stockholder who so requests. Requests for a printed copy may be directed to: VSee Health Inc., 980 N Federal Hwy #304 Boca Raton, FL 33432, Attention: Corporate Secretary.
If we amend or grant a waiver of one or more of the provisions of our Code of Business Conduct and Ethics, we intend to satisfy the requirements under Item 5.05 of Form 8-K regarding the disclosure of amendments to or waivers from provisions of our Code of Business Conduct and Ethics that apply to our principal executive officer, principal financial officer and principal accounting officer by posting the required information on the Company’s website at www.veseehealth.com. The information on this website is not part of this prospectus.
91
EXECUTIVE COMPENSATION
Compensation of Named Executive Officers
The following table shows information concerning the annual compensation received by our named executive officers (“NEOs”) for the year ended December 31, 2023 and 2022.
| | | | | | | | | | | | |
|
| |
| |
| |
| Option |
| All Other |
| |
| | | | Salary | | Bonus | | Awards | | Compensation | | Total |
Name and Position | | Year | | ($) | | ($) | | ($)(1) | | ($)(2) | | ($) |
Imoigele P. Aisiku |
| 2023 |
| 180,000 |
| — |
| — |
| — |
| 180,000 |
Co- Chief Executive Officer and Chairman |
| 2022 |
| 305,667 |
| — |
| — |
| — |
| 305,667 |
Milton Chen |
| 2023 |
| 41,666 |
| — |
| — |
| — |
| 41,666 |
Co- Chief Executive Officer and Director |
| 2022 |
| 137,500 |
| — |
| — |
| — |
| 137,500 |
Jerry Leonard |
| 2023 |
| 143,500 |
| — |
| — |
| — |
| 143,500 |
Senior Vice President of Business Development |
| 2022 |
| 183,333.37 |
| — |
| — |
| — |
| 183,333.37 |
Narrative Disclosure to Summary Compensation Table
Employment Agreements
Existing Employment Agreements
Imoigele P. Aisiku
Effective January 1, 2022, iDoc Telehealth Solutions, LLC (“iDoc LLC”), the wholly-owned subsidiary of iDoc entered into a contract of employment with Imoigele P. Aisiku, pursuant to which Mr. Aisiku will serve as our Chief Executive Officer and Medical Director for a period of two (2) years, which term will be extended for successive two (2) year periods upon the parties’ mutual agreement. Under this employment contract, Mr. Aisiku is entitled to an annual base salary of three hundred fifty thousand dollars ($350,000) for Mr. Aisiku’s service as Chief Executive Officer of iDoc and twenty five thousand ($25,000) for Mr. Aisiku’s service as the medical director for iDoc.
Milton Chen
Effective Jan 1, 2008, VSee Lab, entered an at will employment agreement with Mr. Chen with a base salary of $100,000.
Jerry Leonard
On October 1, 2021, iDoc LLC entered into a consulting agreement with Magnus Analytix, Inc., an entity wholly owned and controlled by Jerry Leonard, pursuant to which Mr. Leonard would serve as iDoc’s Chief Financial Officer. Pursuant to Mr. Leonard’s consulting agreement, he is entitled to monthly compensation of ten thousand four hundred seventeen dollars ($10,417).
Effective October 1, 2021, iDoc LLC also entered into a contract of employment with Mr. Leonard, pursuant to which Mr. Leonard would serve as iDoc’s Chief Financial Officer for a period of two (2) years, which term will be extended for successive two (2) year periods upon the parties’ mutual agreement. Pursuant to his contract of employment, Mr. Leonard is entitled to an annual base salary of thirty six thousand dollars ($36,000) and will be eligible for annual salary increases based on iDoc’s performance and Mr. Leonard’s performance as iDoc’s Chief Financial Officer.
92
Outstanding Equity Awards at 2023 Fiscal Year-End
None of our NEOs have received any equity awards in the year of 2023.
| | | | | | | | | | |
|
| Option Awards | ||||||||
| | |
| |
| Equity Incentive |
| |
| |
| | Number of | | Number of | | Plan Awards: | | | | |
| | Securities | | Securities | | Number of | | | | |
| | Underlying | | Underlying | | Securities | | | | |
| | Unexercised | | Unexercised | | Underlying | | Option | | |
| | Options | | Options | | Unexercised | | Exercise | | Option |
| | Exercisable | | Unexercisable | | Unearned Options | | Price | | Expiration |
Name | | (#) | | (#)(1) | | (#) | | ($) | | Date |
Imoigele P. Aisiku |
| — |
| — |
| — |
| — |
| — |
Milton Chen |
| — |
| — |
| — |
| — |
| — |
Jerry Leonard |
| — |
| — |
| — |
| — |
| — |
Director Compensation
None of the Company’s non-employee directors have received any compensation for services rendered to the Company for the year of 2023.
93
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
DHAC Related Person Transactions
Founder Shares
On June 7, 2021, Digital Health Sponsor, LLC, our sponsor (“Sponsor”), and certain of our directors, officers and advisors set forth below (the “Initial Stockholders”) purchased 4,312,500 of our common shares for an aggregate purchase price of $25,000. On October 26, 2021, our Sponsor and certain of the Initial Stockholders set for the below forfeited an aggregate of 1,437,500 shares of common stock; up to 375,000 Founder Shares were subject to forfeiture by the subscribers in case the underwriters did not fully exercise their over-allotment option.
| | | | | | |
|
| Initial |
| |
| Current |
Initial Stockholders | | Founder Shares | | Forfeited Shares | | Founder Shares |
Digital Health Sponsor LLC(1)(2)(3) |
| 3,044,500 |
| 971,250 |
| 2,073,250 |
Scott Wolf |
| 230,000 |
| 55,000 |
| 175,000 |
Daniel Sullivan |
| 86,250 |
| 11,250 |
| 75,000 |
SCS Capital Partners, LLC(2) |
| 900,000 |
| 400,000 |
| 500,000 |
Brent Willis |
| 8,625 |
| — |
| 8,625 |
Frank Ciufo |
| 8,625 |
| — |
| 8,625 |
George McNellage |
| 8,625 |
| — |
| 8,625 |
Scott Metzger |
| 8,625 |
| — |
| 8,625 |
Andrew Singer |
| 5,750 |
| — |
| 5,750 |
Lane Ostrow |
| 5,750 |
| — |
| 5,750 |
Basil Harris |
| 5,750 |
| — |
| 5,750 |
Marc Munro, through his ownership of Tidewater and Whacky beneficially owns 66.35% of the Sponsor and will beneficially own 344,500 shares of Common Stock held by Sponsor Affiliates, consisting of 292,500 shares of Common Stock and 520 shares of Series A Preferred Stock to be received upon conversion of notes held by such entities controlled by him.
Lawrence Sands, through his ownership of SCS and SCS Capital Partners beneficially owns 500,000 founder shares and 33% of the Quantum Investor, and will beneficially own 591,800 shares of Common Stock held by Sponsor Affiliates, consisting of 500,000 founder shares and 918 shares of Series A Preferred Stock to be received upon conversion of notes held by such entities controlled by him.
The Selling Stockholder owns 4.91% of the Sponsor and 33% of the Quantum Investor.
Prior to the initial investment in DHAC of $25,000 by our Initial Stockholders, we had no assets, tangible or intangible. Simultaneously with the consummation of the IPO, DHAC sold 557,000 Private Placement Shares to the Sponsor at $10.00 per unit for a total purchase price of $5,570,000 on a private placement basis simultaneously with the consummation of the IPO. Since the underwriters exercised the overallotment option in full, none of the Founder Shares are subject to forfeiture any longer.
The Initial Stockholders have agreed, subject to limited exceptions, not to transfer, assign or sell any of their respective Founder Shares until the earlier to occur of: (A) 180 days after the completion of our initial business combination and (B) subsequent to our initial business combination, if the reported last sale price of our common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 90 days after our initial business combination.
In connection with the execution of the Original Business Combination Agreement, our Sponsor and each of our Initial Stockholders (collectively, the “DHAC Supporting Stockholders”), DHAC, VSee and iDoc entered into a support agreement, dated as of June 15, 2022 (the “Sponsor Support Agreement”), pursuant to which the Sponsor and each other DHAC Supporting Stockholder has agreed to, among other things (a) vote in favor of the Business Combination Agreement and the transactions contemplated hereby (including the Mergers), (b) not effect any sale or distribution of any equity securities of DHAC held by such stockholders subject to the terms described therein and (c) not to redeem any of the equity securities of DHAC such stockholder owns, in each case, on the terms and subject to the conditions set forth in the Sponsor Support Agreement.
94
Working Capital Loans
As described in the prospectus covering our initial public offering, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or any of DHAC’s officers and directors may, but are not obligated to, loan us funds as may be required (“Working Capital Loans”). If we complete a Business Combination, we would repay the Working Capital Loans out of the proceeds of the Trust Account released to DHAC. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, we may use a portion of the proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. Except as described as follows, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans.
On June 7, 2021, our Sponsor agreed to loan DHAC up to $625,000 to be used for a portion of the expenses of the initial public offering. These notes were non-interest bearing and any outstanding balance on these notes were due immediately following our proposed public offering. Such notes were repaid in full on November 12, 2021.
On October 24, 2022, the Sponsor agreed to loan DHAC $350,000 to be used for payment of the fee to extend the termination date of DHAC from November 8, 2022 to February 8, 2023, which loan is non-interest bearing. On November 21, 2023, DHAC entered into a Conversion SPA with the Sponsor, pursuant to which the loans in aggregate amount of $350,000 will be converted into Series A Shares at the Closing.
In February 2023, SCS Capital Partners LLC issued a $250,000 interest-free loan to DHAC for Nasdaq fee payment and litigation expense, and on August 17, 2023, such loan was amended and restated to include an additional $315,000 interest-free loan to DHAC for operating expenses, making the aggregate principal amount to be $565,000. On May 5, 2023, SCS Capital Partners, LLC issued a $200,000 loan to DHAC for payment of the term extension fee. The related note bears interest of 10%, matures on May 5, 2024. The proceeds of the note were used to extend the termination date of DHAC from May 8, 2023 to August 8, 2023. On November 21, 2023, DHAC entered into a Conversion SPA with SCS Capital Partners LLC, pursuant to which the loans in aggregate amount of $765,000 will be converted into Series A Shares at the Closing.
Between April 2023 to October 2023, DHAC became indebted to SCS, LLC, the administer of DHAC, in the aggregate amount of approximately $153,000 for office lease, business operation expenses and secretarial services. On November 21, 2023, DHAC entered into a Conversion SPA with SCS, LLC, pursuant to which the loans in aggregate amount of $153,000 will be converted into Series A Shares at the Closing.
On October 4, 2023 and as amended on January 22, 2024, DHAC issued an unsecured promissory note in the aggregate principal amount of $165,000 to M2B, an affiliate of the Sponsor and which is owned by Daniel Kordash, and such note was satisfied and paid off on January 31, 2024.
On November 21, 2023, DHAC and VSee entered into a Conversion SPA with Whacky - a Sponsor Affiliate, pursuant to which certain loans incurred by VSee to Whacky in the aggregate amount of $220,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and VSee, entered into a Conversion SPA with the Bridge Investor who is also an investor in our Sponsor, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by VSee to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Munro Trust - a Sponsor Affiliate, pursuant to which certain loans incurred by iDoc to Munro Trust in the aggregate amount of $300,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Tidewater - a Sponsor Affiliate, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by iDoc to Tidewater in the aggregate amount of $585,000 will be convertible into the Company’s Common Stock following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with the Bridge Investor who is also an investor in our Sponsor, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by iDoc to
95
the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On November 21, 2023, DHAC entered into the Quantum Purchase Agreement, pursuant to which the Quantum Investor subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note in the aggregate principal amount of $3,000,000. See further description under the section titled “Quantum Financing” below.
On November 21, 2023, DHAC entered into the Equity Purchase Agreement with an affiliate of the Bridge Investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s common stock, from time to time over a 36- month period beginning from the sixth (6th) trading day following the Closing. See further description under the section titled “Equity Financing” below.
Registration Rights
The Initial Stockholders, as holders of our founder shares, as well as our Sponsor, as the holder of our Private Placement Units (and all underlying securities), are entitled to registration rights pursuant to a registration rights agreement signed on the effective date of the IPO. The holders of a majority of these securities are entitled to make up to two demands that we register such securities. The holders of the majority of these securities can elect to exercise these registration rights at any time after we consummate a business combination. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to our consummation of a business combination. We will bear the expenses incurred in connection with the filing of any such registration statements.
In connection with the Original Bridge SPA, DHAC entered into a registration rights agreement dated October 5, 2022 and as amended further on January 22, 2024 with the Bridge Investor who is also an investor in our Sponsor (the “Bridge RRA”), which provides that DHAC will file a registration statement to register the shares of Common Stock underlying the (i) Bridge Warrants,(ii) the bridge commitment shares,(iii) the shares of Common Stock issuable pursuant to the Bridge Notes and the Additional Bridge Notes and any anti-dilution or any remedies provisions of the Bridge Note and the Additional Bridge Notes; and (iv) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event no later than 30 calendar days after the Closing of Business Combination.
In connection with the Exchange Agreement, the Company and the Bridge Investor who is also an investor in our Sponsor will enter into a registration rights agreement, which provides that the Company will file a registration statement to register (i) the shares of Common Stock underlying the Exchange Note; (ii) the shares of Common Stock issuable as interest or principal on the Exchange Note; (iii) the shares of Common Stock issuable pursuant to any anti-dilution or any remedies provisions of the Exchange Note; and (iv) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event no later than 30 calendar days after the Closing of Business Combination.
Pursuant to the Equity Purchase Agreement entered between DHAC and the Bridge Investor who is also an investor in our Sponsor, DHAC agreed to file a resale registration statement to register shares of Common Stock to be purchased under the Equity Purchase Agreement with the SEC within 45 days following the sixth (6th) trading day following the Closing of the Business Combination, and shall use commercially reasonable efforts to have such registration statement declared effective by the SEC within 30 days of such filing.
Pursuant to the amended and restated Conversion SPAs executed on February 13, 2024, DHAC agreed to provide registration rights to the Bridge Investor and Tidewater with respect to the shares of Common Stock issuable upon conversion of the Assumed Notes following the Closing of the Business Combination.
Quantum Financing
On November 21, 2023, DHAC entered into a convertible note purchase agreement (the “Quantum Purchase Agreement”), pursuant to which an institutional and accredited investor (the “Quantum Investor”) subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000 (the “Quantum Financing”). The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) a fixed conversion price of $10 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date
96
of conversion or other date of determination. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30th-day anniversary of the Quantum Note issuance date (the “Average Price”) is less than $10.00, to a price equal to the Average Price but in no event less than $2.00. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10. If an event of default occurs, the Quantum Note would bear interest at a rate of 18.00% per annum. Concurrently with the consummation of the transactions contemplated by the Quantum Purchase Agreement, the Company will enter into a registration rights agreement pursuant to which it will agree to register the shares of DHAC common stock underlying the Quantum Note.
The Quantum Investor is a Delaware LLC that is owned 33% by SCS Capital Partners, an entity owned by Lawrence Sands who is a beneficial owner of founder shares and the manager of our Sponsor, 33% by the Bridge Investor and 33% by M2B Funding Corp.
Equity Financing
On November 21, 2023, DHAC entered into an equity line of purchase agreement (the “Equity Purchase Agreement”) with an affiliate of the Bridge Investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s common stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the Closing, provided that certain conditions are met. DHAC also agreed to file a resale registration statement to register shares of common stock to be purchased under the Equity Purchase Agreement with the SEC within 45 days following the Equity Purchase Effective Day, and shall use commercially reasonable efforts to have such registration statement declared effective by the SEC within 30 days of such filing. On the Equity Purchase Effective Day, the Company will issue to the investor, as a commitment fee, a senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of the Company’s common stock at a fix conversion price of $10 per share.
Bridge Financing and Exchange.
In connection with the execution of the Second Business Combination Agreement, DHAC, along with VSee and iDoc, the target companies in our Business Combination, entered into a securities purchase agreement with the Bridge Investor, who is also an investor in our Sponsor, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). The Bridge Notes bear guaranteed interest at a rate of 10.00% per annum and are convertible into shares of DHAC common stock under certain conditions described below. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments (the “Bridge Warrants”) and (ii) 30,000 shares of DHAC common stock.
On November 21, 2023, DHAC, VSee and iDoc entered into a letter agreement, pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111.33 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,555.67 note purchased on January 25, 2024, which will mature on July 25, 2025 (as amended, the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8.00% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at a fixed conversion price of $10 per share. The conversion price of the Additional Bridge Notes is subject to reset if DHAC’s common stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the DHAC common stock in the 10 trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Additional Bridge Notes requires the payment of 110% of the outstanding obligations, including the guaranteed minimum interest. If an event of default occurs, the Additional Bridge Notes would bear interest at a rate of 24.00% per annum and require the payment of 125% of the outstanding obligations, including the guaranteed minimum interest. In addition, on April 17, 2024, DHAC, VSee, iDoc and the Bridge Investor entered a letter agreement, which amended the business combination timelines in the Additional Bridge Notes.
On November 21, 2023, DHAC, VSee and iDoc entered into an exchange agreement (the “Exchange Agreement”) with the Bridge Investor, pursuant to which the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for a senior secured convertible promissory note issued by DHAC with an aggregate principle value of $2,523,744.29 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc. The Exchange Note will bear interest at a rate of 8.00% per annum and will be convertible into shares of common stock of the Company at a fixed
97
conversion price of $10 per share. The conversion price of the Exchange Note is subject to reset if DHAC’s common stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of DHAC’s common stock in the 10th trading dates prior to the measurement date and (y) $2.00.
Administrative Services Agreement
We agreed, commencing on November 3, 2021, to pay an affiliate of the Sponsor a total of $10,000 per month for office space and secretarial, administrative, and other services. The monthly fees will cease upon completion of an initial business combination or liquidation. For the year ended December 31, 2023, we incurred $120,000, of which $55,000 is included in accrued expenses in the accompanying consolidated balance sheets at December 2023. For the year ended December 31, 2022, we incurred $120,000, of which $10,550 is included in accrued expenses in the accompanying consolidated balance sheets at December 2022.
VSee Lab Related Person Transactions
On January 1, 2008, Milton Chen, the CEO of VSee Lab, Inc., received 7,186,237 shares of VSee Lab, Inc common stock and entered an employment agreement with VSee Lab, Inc, with annual compensation of $100,000.
On December 23, 2010, Salesforce, Inc., an investor in VSee Lab, Inc., received 1,195,019 shares of VSee Lab, Inc preferred stock for its investment of $3,570,000 in VSee Lab, Inc. No other person or entity has more than 5% ownership in VSee Lab, Inc.
During the year ended December 31, 2022, $127,710 of cash was provided by Milton Chen and will be used for operating expenses in the future. During the year, VSee received $18,612 from iDoc for cost-sharing expenses. The balance in the due to related party payable as of December 31, 2022 and 2021, was $146,322 and $0, respectively.
During the year ended December 31, 2022, VSee received a loan of $110,000 from Milton Chen, VSee’s Chief Executive Officer, for advanced cash and paid operating expenses on behalf of VSee. No repayments were made during the year ended December 31, 2022. The loan balance as of December 31, 2022 and 2021, was $110,000 and $0, respectively.
On October 5, 2022, DHAC, along with VSee and iDoc, the target companies in our Business Combination, entered into a securities purchase agreement with the Bridge Investor, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222. Such note was exchanged on November 21, 2023 pursuant to the Exchange Agreement, which provides that the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for a senior secured convertible promissory note issued by DHAC with an aggregate principle value of $2,523,744.29 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc.
On November 21, 2023, DHAC and VSee entered into a Conversion SPA with Whacky - a Sponsor Affiliate, pursuant to which certain loans incurred by VSee to Whacky in the aggregate amount of $220,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and VSee, entered into a Conversion SPA with the Bridge Investor who is also an investor in our Sponsor, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by VSee to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On November 21, 2023, DHAC, VSee and iDoc entered into a letter agreement to the October 2022 securities purchase agreement, pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111.33 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,555.67 note purchased on January 25, 2024, which will mature on July 25, 2025 (as amended, the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8.00% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at a fixed conversion price of $10 per share. In addition, on April 17, 2024, DHAC, VSee, iDoc and the Bridge Investor entered a letter agreement, which amended the business combination timelines in the Additional Bridge Notes.
98
It is the policy of VSee that all transactions with related parties be at arm’s length and on terms generally available to an unaffiliated third party under the same or similar circumstances.
iDoc Related Person Transactions
On November 29, 2021, Dr. Imoigele P. Aisiku, iDoc’s Executive Chairman, entered into a Commercial Guaranty with Frost Bank, pursuant to which Dr. Aisiku agreed to guaranty iDoc’s term loan indebtedness owed to Frost Bank in an aggregate principal amount of six hundred fifty four thousand forty four dollars ($654,044). Pursuant to a Business Loan Agreement, dated November 29, 2021, by and between iDoc and Frost Bank, Dr. Aisiku also agreed to guarantee iDoc’s line of credit with Frost Bank in an aggregate principal amount of five hundred thousand dollars ($500,000).
On December 5, 2021, iDoc entered into a Business Loan Agreement with Dr. Aisiku, pursuant to which iDoc provided a loan to Dr. Aisiku in an aggregate principal amount of one hundred twenty thousand dollars ($120,000) (the “Loan”), accruing not interest and maturing on December 31, 2022. The Loan has since been repaid in full.
During the year ended December 31, 2022, iDoc advanced $158,964 in cash to Dr. Aisiku through a company controlled by him. During the year ended December 31, 2022, iDoc advanced $18,612 to VSee Lab for cost-sharing expenses. During the year ended December 31, 2021, $56,110 was repaid and used for operating expenses. The balance due at December 31, 2022 and 2021 was $678,936 and $501,360, respectively.
On February 10, 2022, iDoc entered into a lease with Dr. Aisiku pursuant to which iDoc leases from Mr. Aisiku the property located at 2311 W. Main St., Houston, Texas 77098 for a term beginning on February 1, 2022 and ending on January 31, 2027, which term automatically renews on a month-to-month basis unless terminated by iDoc or Dr. Aisiku. Pursuant to the lease, iDoc pays Dr. Aisiku rent at a rate of ten thousand dollars ($10,000) per month. iDoc paid $59,100 and $69,386 on auto leases on behalf of Dr. Aisiku for the years ended December 31, 2022 and 2021, respectively. iDoc made office space lease payments of $162,000 and $120,000 to Dr. Aisiku during the years ended December 31, 2022 and 2021, respectively.
On October 5, 2022, DHAC, along with VSee and iDoc, the target companies in our Business Combination, entered into a securities purchase agreement with the Bridge Investor, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222. Such note was exchanged on November 21, 2023 pursuant to the Exchange Agreement, which provides that the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for a senior secured convertible promissory note issued by DHAC with an aggregate principle value of $2,523,744.29 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Munro Trust - a Sponsor Affiliate, pursuant to which certain loans incurred by iDoc to Munro Trust in the aggregate amount of $300,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Tidewater - a Sponsor Affiliate, which Conversion SPA was amended on February 13, 2024, pursuant to which certain loans incurred by iDoc to Tidewater in the aggregate amount of $585,000 will be converted into the Company’s Common Stock following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA, with the Bridge Investor who is also an investor in our Sponsor, which Conversion SPA was amended on February 13, 2024, pursuant to which certain loans incurred by iDoc to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On November 21, 2023, DHAC, VSee and iDoc entered into a letter agreement to the October 2022 securities purchase agreement, pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111.33 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,555.67 note purchased on January 25, 2024, which will mature on July 25, 2025 (as amended, the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8.00% per annum and are convertible into shares of DHAC common stock, par value
99
$0.0001 at a fixed conversion price of $10 per share. In addition, on April 17, 2024, DHAC, VSee, iDoc and the Bridge Investor entered a letter agreement, which amended the business combination timelines in the Additional Bridge Notes.
On March 28, 2024, iDoc issued and sold a secured convertible promissory note in the principal amount of two hundred twenty four thousand ($224,000) (the “Note”) to Mr. David L. Wickersham who became a member of the Company’s board of directors on July 17, 2024. The Note was fully satisfied and paid off by the issuance of 114,000 shares of the Company common stock to Mr. Wickerman on the maturity date.
It is the policy of iDoc that all transactions with related parties be at arm’s length and on terms generally available to an unaffiliated third party under the same or similar circumstances.
Policies and Procedures for Related Party Transactions
We have adopt a related person transaction policy that sets forth its procedures for the identification, review, consideration and approval or ratification of related person transactions.
For purposes of the Company’s policy only, a related person transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which the Company and any related person are, were or will be participants in which the amount involved exceeds $120,000. Transactions involving compensation for services provided to the Company as an employee or director are not covered by this policy. A related person is any executive officer, director or beneficial owner of more than 5% of any class of the Company’s voting securities and any of their respective immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, the Company’s management must present information regarding the related person transaction to the Company’s audit committee, or, if audit committee approval would be inappropriate, to another independent body of the Company’s Board of Directors, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to the Company of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, the Company will collect information that the Company deems reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable the Company to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under the Company’s Code of Conduct that the Company expects to adopt prior to the closing of this Business Combination, the Company’s employees and directors will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, the Company’s audit committee, or other independent body of the Company’s Board of Directors, will take into account the relevant available facts and circumstances including, but not limited to:
| ● | the risks, costs and benefits to the Company; |
| ● | the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated; |
| ● | the availability of other sources for comparable services or products; and |
| ● | the terms available to or from, as the case may be, unrelated third parties or to or from employees generally. |
The policy requires that, in determining whether to approve, ratify or reject a related person transaction, the Company’s audit committee, or other independent body of the Company’s Board of Directors, must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, the Company’s best interests and those of the Company’s stockholders, as the Company’s audit committee, or other independent body of the Company’s Board of Directors, determines in the good faith exercise of its discretion.
100
BENEFICIAL OWNERSHIP OF SECURITIES
The following table sets forth information known to the Company regarding the beneficial ownership of the Company’s Common Stock as of August 5, 2024 by:
| ● | each person known to the Company to be the beneficial owner of more than 5% of outstanding Company common stock; |
| ● | each of the Company’s executive officers and directors; and |
| ● | all executive officers and directors of the Company as a group. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days and restricted stock units that vest within 60 days. Common stock issuable upon exercise of options and warrants currently exercisable within 60 days and restricted stock units that vest within 60 days are deemed outstanding solely for purposes of calculating the percentage of total ownership and total voting power of the beneficial owner thereof.
The beneficial ownership of Company Common Stock is based on 14,821,019 shares of the Company’s Common Stock issued and outstanding as of August 5, 2024.
Unless otherwise indicated, the Company believes that each person named in the table below has sole voting and investment power with respect to all shares of the Company’s Common Stock beneficially owned by them. Unless otherwise indicated, the business address of each of the following entities or individuals is c/o VSee Health, Inc., 980 N. Federal Highway #304 Boca Raton, Florida 33432.
Company Common Stock
| | | | | |
| | Number of Shares | | |
|
Name and Address of Beneficial Owner |
| Beneficially Owned |
| % of Class (1) | |
Five Percent Holders of the Company | | | | | |
Digital Health Sponsor LLC (our sponsor) (2) |
| 3,222,250 | (3) | 20.91 | % |
Directors and Executive Officers of the Company (4) | | | | | |
Milton Chen |
| 2,870,069 |
| 19.36 | % |
Imoigele Aisiku |
| 3,536,990 |
| 23.86 | % |
Jerry Leonard |
| — |
| — | |
Kevin Lowdermilk |
| — |
| — | |
Colin O’Sullivan |
| — |
| — | |
Scott Metzger | | 8,625 |
| * | |
Cydonii V. Fairfax | | — |
| — | |
David L. Wickersham |
| 114,000 | | * | |
All Directors and Executive Officers of the Company as a group (6 individuals) | | 6,529,684 | | 44.06 | % |
* | Less than 1%. |
| (1) | Shares and percentages are based on (a) 14,821,019 shares of the Company’s Common Stock, (b) 6,158 shares of Series A Convertible Preferred Stock at $10 per share, (c) 620,375 shares of the Company’s Common Stock issuable upon conversion of the Convertible Notes at $10 per share, and (d) 12,256,999 shares of the Company’s Common Stock issuable upon exercise of all warrants, in each case, outstanding as of June 24, 2024 (the “Closing Date”), which securities are convertible or exercisable within 60 days of the Closing Date and deemed outstanding for purposes of computing the percentage ownership of the person holding such convertible securities, but are not deemed outstanding for purposes of computing the percentage ownership of any other person. |
| (2) | Our sponsor is the record holder of the shares of common stock reported herein. Our affiliate, Mr. Lawrence Sands, is the manager of our sponsor and as such may be deemed to have sole voting and investment discretion with respect to the common |
101
| stock held by our sponsor. Mr. Sands disclaims any beneficial ownership of the securities held by Digital Health Sponsor LLC other than to the extent of any pecuniary interest he may have therein, directly or indirectly. The business address of the Sponsor is c/o VSee Health, Inc., 980 N Federal Hwy #304, Boca Raton, FL 33432. |
| (3) | Consists of 2,073,250 founder shares, 557,000 shares of Company Common Stock underlying the Private Placement Units from the IPO, 557,000 warrants for Company Common Stock underlying the Private Placement Units from the IPO at an exercise price of $11.50, and 35,000 shares of the Company Common Stock issuable upon conversion of 350 shares of Series A Convertible Preferred Stock at a $10 conversion price. |
| (4) | Unless otherwise indicated, the business address of each of the individuals is c/o VSee Health, Inc., 980 N Federal Hwy #304, Boca Raton, FL 33432. |
102
THE COMMITTED EQUITY FINANCING TRANSACTION
General
On November 21, 2023, DHAC entered into a common stock purchase agreement (the “Equity Purchase Agreement”) with Dominion Capital LLC (“Dominion”) pursuant to which Dominion has agreed to purchase from the Company, at the Company’s direction from time to time, in its sole discretion, from and after the sixth (6th) trading day following the Closing of the Business Combination (the “Effective Date”), and until the earlier of (i) the 36-month anniversary of the Effective Date or (ii) the termination of the Equity Purchase Agreement in accordance with the terms thereof (the “Commitment Period”), shares of the Company’s Common Stock, having a total maximum aggregate purchase price of $50,000,000 (the “Purchase Shares”), upon the terms and subject to the conditions and limitations set forth therein.
As consideration for its commitment to purchase the Company’s Common Stock under the Equity Purchase Agreement, on July 2, 2024, the Company issued a senior unsecured note in a principal amount of $500,000 that is payable only in shares of our Common Stock at an initial price of $10 per share (the “Equity Purchase Note”) to Dominion. The Equity Purchase Note is convertible into 50,000 shares of Common Stock and such shares are registered under this prospectus.
Purchase of Shares of Common Stock Under the Equity Purchase Agreement
During the Commitment Period, the Company may, from time to time and at its sole discretion, direct Dominion to purchase such number of shares of Common Stock (the “Advance Notice”) that does not exceed the lesser of: (i) an amount equal to fifteen percent (15%) of the aggregate daily traded volume of the Company’s Common Stock on Nasdaq for the ten (10) trading days immediately preceding an Advance Notice and (ii) $500,000 (which amount can be increased at the sole option of Dominion per each Advance Notice) at a purchase price per share equal to 92% of the of the lowest average daily volume weighted average price (“VWAP”) during the five days prior to the Company’s submission of Advance Notice (the “Purchase Price”). If the VWAP pursuant to an Advance Notice is less than the floor price of $2.00, which will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, share split, or other similar transaction and effective upon the consummation of any such transactions (the “Floor Price”), then Dominion has the right but not the obligation to purchase shares of our Common Stock at the Floor Price. In addition, Advance Notices cannot be submitted more than once per any given calendar week, unless otherwise mutually agreed upon by Dominion and the Company in writing. The Company also agreed to make a payment adjustment (a “Payment Adjustment”) to Dominion for any difference in the Purchase Price and 92% of the lowest average daily VWAP in the next five (5) trading days, but only if such price is lower than the Purchase Price and which shall be paid to Dominion by the Company in Common Stock pursuant to forms of the settlement document in the Equity Purchase Agreement.
The Company will control the timing and amount of any sales of its Common Stock to Dominion, and Dominion has no right to require the Company to sell any shares to it under the Equity Purchase Agreement. Actual sales of shares of Common Stock to Dominion under the Equity Purchase Agreement will depend on a variety of factors to be determined by the Company from time to time, including (among others) market conditions, the trading price of its Common Stock and determinations by the Company as to available and appropriate sources of funding for the Company and its operations. Dominion may not assign its rights and obligations under the Equity Purchase Agreement.
The Equity Purchase Agreement prohibits the Company from directing Dominion to purchase any shares of Common Stock if those shares, when aggregated with all other shares of Common Stock then beneficially owned by Dominion and its affiliates (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, and Rule 13d-3 thereunder), would result in Dominion and its affiliates beneficially owning more than 4.99% of the then total outstanding shares of the Company’s Common Stock.
If the Company fails to issue and deliver the shares purchased pursuant to an Advance Notice to Dominion within two trading days of the issuance of an Advance Notice or fails to have any restrictive legends removed from any shares purchased pursuant to an Advance Notice, the Company will be considered breaching its obligations under the Equity Purchase Agreement.
The Equity Purchase Agreement contains customary representations, warranties, covenants, closing conditions and indemnification and termination provisions. Sales under the Equity Purchase Agreement may commence only after certain conditions have been satisfied, including effectiveness of the registration statement to which this prospectus relates.
103
Termination of the Equity Purchase Agreement
Unless earlier terminated as provided in the Equity Purchase Agreement, the Equity Purchase Agreement will terminate automatically on the earliest to occur of: (i) the first day of the month next following the 36 month anniversary of the Effective Date, or (ii) the date on which Dominion shall have purchased shares of our Common Stock under the Equity Purchase Agreement for an aggregate gross purchase price equal to $50 million.
The Company has the right to terminate the Equity Purchase Agreement at any time for any reason or for no reason, without any liability whatsoever, upon five trading days’ prior written notice to Dominion, provided that (i) there are no outstanding Advance Notices, the Common Stock under which have yet to be issued, and (ii) the Company has paid all amounts owed to Dominion pursuant to the Equity Purchase Agreement.
The Company and Dominion also have the option to terminate the Equity Purchase Agreement by mutual written consent, which shall be effective as of the date of such mutual written consent unless otherwise provided in such written consent.
No Short-Selling or Hedging by Dominion
Dominion has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of our common stock during any time prior to the termination of the Equity Purchase Agreement.
Registration Rights Granted to Dominion
As provided under the Equity Purchase Agreement, the Company agreed to file a registration statement with the SEC covering the resale of the shares of the Company’s Common Stock issued to Dominion pursuant to the Equity Purchase Agreement. The Company shall also use commercially reasonable efforts to continuously maintain the effectiveness of such registration statement until all of the Purchase Shares have been sold or may be sold without restriction pursuant to Rule 144 of the Securities Act of 1933, as amended.
104
THE SELLING STOCKHOLDER
This prospectus relates to the possible resale from time to time by the Selling Stockholder of any or all of the shares of common stock that may be issued by us to the Selling Stockholder under the Equity Purchase Agreement. We are registering the shares of common stock pursuant to the registration rights granted to the Selling Stockholder as provided under the Equity Purchase Agreement we entered into with the Selling Stockholder as described under the section titled “The Committed Equity Financing Transaction” in order to permit the selling stockholder to offer the shares for resale from time to time. Except for the transactions entered with the Selling Stockholder and the Equity Purchase Agreement and other financings that are disclosed in our public filings, the Selling Stockholder has not had any material relationship with us within the past three years. As used in this prospectus, the term “Selling Stockholder” (or “Dominion” as applicable) means Dominion Capital LLC.
The table below presents information regarding the selling stockholder and the shares of common stock that it may offer from time to time under this prospectus. This table is prepared based on information supplied to us by the selling stockholder, and reflects holdings as of August 5, 2024. The number of shares in the column “Maximum Number of Shares of Common Stock to be Offered Pursuant to this Prospectus” represents all of the shares of common stock that the selling stockholder may offer under this prospectus. The selling stockholder may sell some, all or none of its shares in this offering. We do not know how long the selling stockholder will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the selling stockholder regarding the sale of any of the shares.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act, and includes shares of common stock with respect to which the selling stockholder has voting and investment power. The percentage of shares of common stock beneficially owned by the selling stockholder prior to the offering shown in the table below is based on an aggregate of 14,821,019 shares of our common stock outstanding on August 5, 2024. The fourth column assumes the sale of all of the shares offered by the selling stockholder pursuant to this prospectus.
| | | | | | | | | | | | |
| | Number of Shares of | | Maximum Number of | | |
| |||||
| | Common Stock | | Shares of Common | | Number of Shares of |
| |||||
| | Beneficially Owned | | Stock to be Offered | | Common Stock Owned |
| |||||
| | Prior to Offering |
| Pursuant to this | | After Offering |
| |||||
Name of Selling Stockholder(1) |
| Number(2) |
| Percent(3) | | Prospectus |
| Number(2)(4) |
| Percent(5) |
| |
Dominion Capital LLC (1) |
| 489,714 | | 3.27 | % | 25,050,000 | | 439,714 | | 1.10 | % | |
| (1) | Dominion Capital GP LLC is the manager of Dominion Capital LLC. Dominion Capital Holdings LLC is the manager of Dominion Capital GP LLC. Mikhail and Gennadiy Gurevich are managing members of Dominion Capital Holdings LLC and as such have voting and dispositive power over the securities held by Dominion Capital LLC. The business address for Dominion Capital LLC is 256 West 38th Street, 15th Floor, New York, NY 10018. We have been advised that none of Messrs. Mikhail and Gennadiy Gurevich or Dominion is a member of the Financial Industry Regulatory Authority, or FINRA, or an independent broker-dealer, or an affiliate or associated person of a FINRA member or independent broker-dealer. The foregoing should not be construed in and of itself as an admission by Messrs. Mikhail Gurevich and Gennadiy Gurevich as to beneficial ownership of the securities beneficially owned directly by Dominion. |
| (2) | Includes up to 173,913 shares of Common Stock issuable upon full exercise of the Bridge Warrants directly held by Dominion, and an aggregate of 315,801 shares of Common Stock upon full conversion of the Additional Bridge Notes, the Exchange Note and the Equity Purchase Note of the Company directly held by Dominion (which number assume the conversion of such notes at a conversion price of $10 and the current interest amounts due thereon being paid in cash) which notes are in each case, subject to a 4.99% beneficial ownership blocker. |
| (3) | Applicable percentage ownership is based on 14,821,019 shares of our common stock outstanding as of August 5, 2024 and 489,714 shares of Common Stock issuable upon full exercise of the Bridge Warrants and full conversion of the Additional Bridge Notes, the Exchange Note and the Equity Purchase Note directly held by Dominion. |
| (4) | Assumes the sale of all shares being offered pursuant to this prospectus. |
| (5) | Applicable percentage ownership is based on 14,821,019 shares of our common stock outstanding as of August 5, 2024 and 439,714 shares of Common Stock issuable upon full exercise of the Bridge Warrants and full conversion of the Additional Bridge Notes and the Exchange Note directly held by Dominion. |
105
DESCRIPTION OF OUR SECURITIES
The following summary of the material terms of securities of VSee Health, Inc. (formerly Digital Health Acquisition Corp.) is not intended to be a complete summary of the rights and preferences of such securities and is qualified by reference to our certificate of incorporation, as amended (“Amended Charter”), and our Bylaws (“Bylaws”), each of which are incorporated by reference as an exhibit to the registration statement of which this prospectus is a part, and certain provisions of Delaware law. We urge you to read each of our Amended Charter and Bylaws described herein in their entirety for a complete description of the rights and preferences of our securities.
Authorized and Outstanding Capital Stock
We are authorized to issue 110,000,000 shares. The total number of shares of Common Stock that we are authorized to issue is 100,000,000, having a par value of $0.0001 per share, and the total number of shares of Preferred Stock that we are authorized to issue is 10,000,000, having a par value of $0.0001 per share. The Series A Preferred Certificate of Designation authorizes the issuance of 6,500 shares of Series A Preferred Stock. As of the date of this prospectus, our issued and outstanding capital stock consists of approximately 14,821,019 shares of Common Stock and approximately 6,158 shares of Series A Preferred Stock.
Common Stock
Voting Rights
Except as otherwise provided herein or expressly required by law, each holder of Common Stock, as such, shall be entitled to vote on each matter submitted to a vote of stockholders and shall be entitled to one (1) vote for each share of Common Stock of the Company held of record by such holder as of the record date for determining stockholders entitled to vote on such matter. Except as otherwise required by law, holders of Common Stock of the Company, as such, shall not be entitled to vote on any amendment to this Amended Charter (including any Certificate of Designation) that relates solely to the rights, powers, preferences (or the qualifications, limitations or restrictions thereof) or other terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together with the holders of one or more other such series, to vote thereon pursuant to this Amended Charter (including any Certificate of Designation) or pursuant to the DGCL.
Subject to the rights of any holders of any outstanding series of Preferred Stock of the Company, the number of authorized shares of Common Stock of the Company may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the stock of the Company entitled to vote, irrespective of the provisions of Section 242(b)(2) of the DGCL.
Dividend Rights
Subject to applicable law and the rights and preferences of any holders of any outstanding series of Preferred Stock of the Company, the holders of Common Stock of the Company, as such, shall be entitled to the payment of dividends on the Common Stock of the Company when, as and if declared by the Company’s board of directors in accordance with applicable law.
Liquidation, Dissolution and Winding Up
Subject to the rights and preferences of any holders of any shares of any outstanding series of Preferred Stock of the Company, in the event of any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, the funds and assets of the Company that may be legally distributed to the Company’s stockholders shall be distributed among the holders of the then outstanding Common Stock of the Company pro rata in accordance with the number of shares of Common Stock of the Company held by each such holder.
Preferred Stock
The Amended Charter provides that shares of preferred stock of the Company may be issued from time to time in one or more series, each of such series to have such terms as stated or expressed herein and in the resolution or resolutions providing for the creation and issuance of such series adopted by the board of directors of the Company as hereinafter provided.
106
The board of directors of the Company has authority from time to time to issue the preferred stock in one or more series, and in connection with the creation of any such series, by adopting a resolution or resolutions providing for the issuance of the shares thereof and by filing a certificate of designation relating thereto in accordance with the DGCL (a “Certificate of Designation”), to determine and fix the number of shares of such series and such voting powers, full or limited, or no voting powers, and such designations, preferences and relative participating, optional or other special rights, and qualifications, limitations or restrictions thereof, including without limitation thereof, dividend rights, conversion rights, redemption privileges and liquidation preferences, and to increase or decrease (but not below the number of shares of such series then outstanding) the number of shares of any series as shall be stated and expressed in such resolutions, all to the fullest extent now or hereafter permitted by the DGCL. Without limiting the generality of the foregoing, the resolution or resolutions providing for the creation and issuance of any series of preferred stock of the Company may provide that such series shall be superior or rank equally or be junior to any other series of preferred stock of the Company to the extent permitted by law and this Amended Charter (including any Certificate of Designation). Except as otherwise required by law, holders of any series of preferred stock of the Company shall be entitled only to such voting rights, if any, as shall expressly be granted thereto by the Amended Charter (including any Certificate of Designation).
The number of authorized shares of preferred stock of the Company may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the stock of the Company entitled to vote, irrespective of the provisions of Section 242(b)(2) of the DGCL.
Series A Preferred Stock
As of the date of this prospectus/proxy statement/consent solicitation statement, no shares of Series A Preferred Stock are outstanding. After giving effect of the Business Combination, 6,158 shares of Series A Preferred Stock will be outstanding
Authorized Shares, Par Value
Pursuant to the Series A Preferred Certificate of Designation, we authorized 6,500 Series A Convertible Preferred Shares, par value $0.0001.
Ranking
The Series A Preferred Stock will rank senior to the Common Stock with respect to rights on dividends, distribution of assets on any voluntary or involuntary liquidation, and dissolution or winding up of the affairs of the Company.
Dividends
Holders of the Series A Preferred Stock participate on dividends and any other distributions of the Company’s assets as if such holder had held the number of shares of Common Stock acquirable upon complete conversion of the Series A Preferred Stock immediately prior to the date on which a record is taken for such dividend or distribution, subject to certain limitations on beneficial ownership.
Conversion Rights
The number of shares of Common Stock into which the Series A Preferred Stock are convertible (the “Conversion Rate”) is equal to the Conversion Amount divided by the initial conversion price of $10.00 (the “Conversion Price”), subject to adjustment. “Conversion Amount” means, with respect to each share of Series A Preferred Stock, the stated value.
The Series A Preferred Stock is convertible upon the earlier of (i) twelve (12) months after the issuance of the Series A Preferred Stock or (ii) the date on which no shares of Series A Preferred Stock remain outstanding, into Common Stock at the Conversion Rate (subject to equitable adjustment in the event of a stock split, stock consolidation, subdivision or certain other events of a similar nature that increase or decrease the number of shares of Series A Preferred Stock outstanding).
The Conversion Price of the Series A Preferred Stock is subject to reset in the event the holders convert all or any part of the Series A Preferred Stock at the Alternate Conversion Price. “Alternate Conversion Price” means the lowest of (i) the applicable conversion price as in effect on the applicable conversion date of the applicable alternate conversion, and (ii) the greater of (x) the Floor Price (as defined below) and (y) 90% of the price computed as the quotient of (I) the sum of the VWAP of the Common Stock for each of the three (3) trading days with the lowest VWAP of the Common Stock during the ten (10) consecutive trading day
107
period ending and including the trading day immediately preceding the delivery or deemed delivery of the applicable conversion notice, divided by (II) three (3), as appropriately adjusted for any stock dividend, stock split, stock combination, reclassification or similar transaction.
“Floor Price” means $10.00 (as adjusted for stock splits, stock dividends, stock combinations, recapitalizations and similar events); provided, that after the Resale Adjustment Date (as defined below), the Floor Price shall be lowered to $5.00 (as adjusted for stock splits, stock dividends, stock combinations, recapitalizations and similar events); provided further, upon any Price Adjustment Reset, the Floor Price shall be lowered to $2.00 (as adjusted for stock splits, stock dividends, stock combinations, recapitalizations and similar events).
Any conversion will be settled only in shares of Common Stock; provided, that the Company shall not effect any conversion to the extent that after giving effect to such conversion, the converting holder would beneficially own in excess of 4.99% of the Common Stock outstanding immediately after giving effect to the conversion.
Purchase Rights
If at any time the Company grants, issues or sells any options, convertible securities, or rights to purchase stock, warrants, securities or other property pro rata to all or substantially all of the record holders of any class of Common Stock (the “Purchase Rights”), then each holder of Series A Preferred Stock will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which such holder could have acquired if such holder had held the number of shares of Common Stock acquirable upon complete conversion of all the Series A Preferred Shares held by such holder immediately prior to the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights; subject to certain limitations on beneficial ownership.
Other Corporate Events
Prior to the consummation of any “Fundamental Transaction,” defined to include a merger, change or control, transfer of all or substantially all equity of the company, or sale of 50% or more of the Company’s outstanding shares of Common Stock, pursuant to which holders of shares of Common Stock are entitled to receive securities or other assets with respect to or in exchange for shares of Common Stock (a “Corporate Event”), the Company shall make appropriate provision to ensure that each holder of Series A Preferred Stock will thereafter have the right, at such holder’s option, to receive upon a conversion of all the Series A Preferred Stock held by such holder (i) in addition to the shares of Common Stock receivable upon such conversion, such securities or other assets (the “Corporate Event Consideration”) to which such holder would have been entitled with respect to such shares of Common Stock had such shares of Common Stock been held by such holder upon the consummation of such Corporate Event (without taking into account any limitations or restrictions on the convertibility of the Series A Preferred Stock) or (ii) in lieu of the shares of Common Stock otherwise receivable upon such conversion, such securities or other assets received by the holders of shares of Common Stock in connection with the consummation of such Corporate Event in such amounts as such holder of Series A Preferred Stock would have been entitled to receive had the Series A Preferred Stock held by such holder initially been issued with conversion rights for the form of such consideration (as opposed to shares of Common Stock) at a conversion rate for such consideration commensurate with the Conversion Rate.
Rights Upon Issuance of Other Securities; Adjustment of Conversion Price upon Subdivision or Combination of Common Stock
If the Company at any time subdivides (or combines) one or more classes of its outstanding shares of Common Stock into a greater (or lesser) number of shares, the Conversion Price will be proportionately reduced (or increased).
Adjustment of Conversion Price
The Company may, with the prior written consent of the holders of Series A Preferred Stock, reduce the Conversion Price to any amount and for any period of time deemed appropriate by the Board.
On the later (such later date, the “Resale Adjustment Date”) of (A) the 90th calendar day after the initial issuance of the Series A Preferred Stock and (B) the earlier of (x) the initial date the shares of Common Stock issuable upon conversion of the Series A Preferred Stock are eligible to be resold by the holders pursuant to Rule 144 or (y) the date a registration statement registering the resale by the holders of all shares of Common Stock issuable upon conversion of the Series A Preferred Stock is declared effective by
108
the SEC, as applicable, if the Conversion Price then in effect is greater than the Resale Adjustment Price (as defined below), on the Resale Adjustment Date, the Conversion Price shall automatically adjust downward to the Resale Adjustment Price.
On the earlier (such earlier date, the “Price Adjustment Date”) to occur after the Resale Adjustment Date of (A) such date the VWAP of the Common Stock for each trading day during a period of ten (10) consecutive trading days is less than $5.00 (as adjusted for stock splits, stock dividends, stock combinations, recapitalizations and similar events) or (B) the first anniversary of the issuance of the Series A Preferred Stock, as applicable, if the Conversion Price then in effect is greater than $2.00 (as adjusted for stock splits, stock dividends, stock combinations, recapitalizations and similar events) on the Price Adjustment Date, the Conversion Price shall automatically adjust downward to the Resale Adjustment Price (each, a “Price Adjustment Reset”).
“Resale Adjustment Price” means, with respect to any Resale Adjustment Date that price which shall be the lowest of (i) the applicable Conversion Price as in effect on the applicable Resale Adjustment Date, and (ii) the greater of (x) the Floor Price and (y) 90% of the price computed as the quotient of (I) the sum of the VWAP of the Common Stock for each of the three (3) Trading Days with the lowest VWAP of the Common Stock during the ten (10) consecutive Trading Day period ending and including the Trading Day immediately preceding the applicable Resale Adjustment Date, divided by (II) three (3) (such period, the “Resale Adjustment Measuring Period”). All such determinations to be appropriately adjusted for any stock dividend, stock split, stock combination, reclassification or similar transaction that proportionately decreases or increases the Common Stock during such Resale Adjustment Measuring Period.
Rights Upon Fundamental Transactions
Pursuant to the Series A Preferred Certificate of Designation, the Company shall not be party to a Fundamental Transaction, defined to include a merger, change or control, transfer of all or substantially all equity of the company, or sale of 50% or more of the Company’s outstanding shares of Common Stock, unless the successor entity assumes in writing all obligations of the Company under the Series A Preferred Certificate of Designation and the Series A Securities Purchase Agreement Transaction Documents, and such successor entity shall issue upon conversion or redemption of the Series A Preferred Stock shares of the publicly traded common stock (or their equivalent) of the successor entity had all the Series A Preferred Stock of the holder been converted immediately prior to the Fundamental Transaction.
Voting Rights
The holders of Series A Preferred Stock are entitled to vote with shareholders of Common Stock, together as a single class, with a number of votes per share equal to the number of shares of Common Stock into which such holders’ Series A Preferred Stock is then convertible. Series A Preferred Stock will be entitled to certain consent rights on matters related to the authorization of any adverse change to the powers, preferences, or special rights of the Series A Preferred Stock set forth in the Amended Charter or Bylaws, and shall have voting rights as required by law.
Additionally, without first obtaining the affirmative vote at a meeting duly called for such purpose or the written consent without a meeting of the holders of the Series A Preferred Stock, the Company shall not amend or repeal any provision of, or add any provision to, its Certificate of Incorporation or bylaws, or file any certificate of designations or articles of amendment of any series of shares of preferred stock, if such action would adversely alter or change in any respect the preferences, rights, privileges or powers, or restrictions provided for the benefit of the Series A Preferred Stock, regardless of whether any such action shall be by means of amendment to the Certificate of Incorporation or by merger, consolidation or otherwise.
Purchase Rights
If at any time the Company grants, issues or sells any options, convertible securities, or rights to purchase stock, warrants, securities or other property pro rata to all or substantially all of the record holders of any class of Common Stock (the “Purchase Rights”), then each holder of Series A Preferred Stock will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which such holder could have acquired if such holder had held the number of shares of Common Stock acquirable upon complete conversion of all the Series A Preferred Shares held by such holder immediately prior to the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights; subject to certain limitations on beneficial ownership.
109
Redemption
At any time, the Company may optionally redeem the Series A Preferred Stock in cash at a price equal to the 100% of the Conversion Amount.
Reservation Requirements
So long as any Series A Preferred Stock remains outstanding, the Company shall at all times reserve at least 200% of the number of shares of Common Stock as shall from time to time be necessary to effect the conversion of all Series A Preferred Stock then outstanding.
Liquidation
In the event of the liquidation of the Company, the holders of Series A Preferred Stock shall be entitled to receive in cash out of the assets of the Company, whether from capital or from earnings available for distribution to its stockholders, before any amount shall be paid to the holders of any of shares of junior and shall receive an amount per share of Series A Preferred Stock equal to the amount per share such holder would receive if such holder converted its Series A Preferred Stock into Common Stock immediately prior to the date of such payment.
Amendments
The Series A Preferred Certificate of Designation may be amended by the affirmative vote of the holders of Series A Preferred Stock voting as a separate class, and the stockholder approval as required pursuant to the DGCL and the Company’s Certificate of Incorporation.
Warrants
IPO Warrants
As of the date of this prospectus, 11,500,000 Public Warrants and 557,000 private placement warrants are outstanding (collectively, the “IPO Warrants”). Each whole IPO Warrant entitles the registered holder to purchase one share of common stock at a price of $11.50 per share, subject to adjustment as discussed below, at any time commencing 30 days after the completion of the Business Combination. However, no IPO Warrants will be exercisable for cash unless we have an effective and current registration statement covering the shares of common stock issuable upon exercise of the IPO Warrants and a current prospectus relating to such shares of common stock. Notwithstanding the foregoing, if a registration statement covering the shares of common stock issuable upon exercise of the Public Warrants is not effective within 90 days following the consummation of the Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when we shall have failed to maintain an effective registration statement, exercise IPO Warrants on a cashless basis pursuant to the exemption provided by Section 3(a)(9) of the Securities Act, provided that such exemption is available. If that exemption, or another exemption, is not available, holders will not be able to exercise their IPO Warrants on a cashless basis. In such event, each holder would pay the exercise price by surrendering the IPO Warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the IPO Warrants, multiplied by the difference between the exercise price of the IPO Warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose will mean the average reported last sale price of the shares of common stock for the five (5) trading days ending on the trading day prior to the date of exercise. The Warrants will expire on the fifth anniversary of our completion of an initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
The private placement warrants are identical to the Public Warrants.
We may call the IPO Warrants for redemption, in whole and not in part, at a price of $0.01 per warrant,
| ● | at any time after the IPO Warrants become exercisable; |
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; |
110
| ● | if, and only if, the reported last sale price of the shares of common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations and recapitalizations), for any 20 trading days within a 30 trading day period commencing after the IPO Warrants become exercisable and ending on the third business day prior to the notice of redemption to warrant holders; and |
| ● | if, and only if, there is a current registration statement in effect with respect to the shares of common stock underlying such IPO Warrants. |
The right to exercise will be forfeited unless the IPO Warrants are exercised prior to the date specified in the notice of redemption. On and after the redemption date, a record holder of a IPO Warrants will have no further rights except to receive the redemption price for such holder’s IPO Warrant upon surrender of such warrant.
The redemption criteria for our IPO Warrants have been established at a price which is intended to provide warrant holders a reasonable premium to the initial exercise price and provide a sufficient differential between the then-prevailing share price and the warrant exercise price so that if the share price declines as a result of our redemption call, the redemption will not cause the share price to drop below the exercise price of the IPO Warrants.
If we call the IPO Warrants for redemption as described above, our management will have the option to require all holders that wish to exercise IPO Warrants to do so on a “cashless basis.” In such event, each holder would pay the exercise price by surrendering the IPO Warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the IPO Warrants, multiplied by the difference between the exercise price of the IPO Warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the average reported last sale price of the shares of common stock for the five (5) trading days ending on the third (3rd) trading day prior to the date on which the notice of redemption is sent to the holders of IPO Warrants.
The IPO Warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. The warrant agreement provides that the terms of the IPO Warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval, by written consent or vote, of the holders of at least 50% of the then outstanding Public Warrants, in order to make any change that adversely affects the interests of the registered holders.
The exercise price and number of shares of common stock issuable on exercise of the IPO Warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or our recapitalization, reorganization, merger or consolidation. However, except as described below, the IPO Warrants will not be adjusted for issuances of shares of common stock at a price below their respective exercise prices.
In addition, if (x) we issue additional shares of common stock or equity-linked securities for capital raising purposes in connection with the Closing of the Business Combination at an issue price or effective issue price of less than $9.20 per share (with such issue price or effective issue price to be determined in good faith by the Board, and in the case of any such issuance to our sponsor, initial stockholders or their affiliates, without taking into account any founder shares held by them prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of our initial business combination on the date of the consummation of our initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, the exercise price of the IPO Warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of (i) the Market Value or (ii) the price at which we issue the additional shares of common stock or equity-linked securities, and the $18.00 redemption trigger price will be adjusted to 180% of this amount.
The IPO Warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by certified or official bank check payable to us, for the number of IPO Warrants being exercised. The warrant holders do not have the rights or privileges of holders of shares of common stock and any voting rights until they exercise their IPO Warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the IPO Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Warrant holders may elect to be subject to a restriction on the exercise of their IPO Warrants such that an electing warrant holder would not be able to exercise their IPO Warrants to the extent that, after giving effect to such exercise, such holder would beneficially own in excess of 9.8% of the shares of common stock outstanding.
111
No fractional shares will be issued upon exercise of the IPO Warrants. If, upon exercise of the IPO Warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round up to the nearest whole number the number of shares of common stock to be issued to the warrant holder.
Subject to applicable law, any action, proceeding or claim against us arising out of or relating in any way to the warrant agreement will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and we irrevocably submit to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim. This provision applies to claims under the Securities Act but does not apply to claims under the Exchange Act or any claim for which the federal district courts of the United States of America are the sole and exclusive forum.
Bridge Warrants
DHAC executed the Bridge Securities Purchase Agreement with the Bridge Investor pursuant to which, among other things, DHAC issued to the Bridge Investor warrants exercisable for 173,913 shares of Common Stock of the Company (collectively, the “Bridge Warrants”).
Exercise
The Bridge Warrants are exercisable for shares of Common Stock of the Company at a price of $11.50 per share of Common Stock (the “Exercise Price”), and expire at 5:30 p.m. Pacific Time five years after the date of issuance (the “Expiration Date”).
Cashless Exercise
If at any time after the date of issuance of the Bridge Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, then in lieu of exercising the Bridge Warrant by payment of cash or check, the holder may elect to receive the number of shares of Common Stock equal to the value of the Bridge Warrant, or the portion thereof being exercised, by surrender of the Bridge Warrant to the Company or its transfer agent, after which the holder will receive shares of Common Stock in accordance with the following formula:
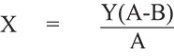
| | | |
Where, | X | = | The number of shares of Common Stock to be issued to the holder; |
| Y | = | The number of shares of Common Stock for which the Bridge Warrant is being exercised; |
| A | = | The fair market value of one share of Common Stock; and |
| B | = | The Exercise Price. |
No Fractional Shares
In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the Exercise Price multiplied by such fraction
No Rights of Shareholders
Except as provided in the Bridge Warrant, the Bridge Warrant does not entitle its holder to any rights of a shareholder of the Company.
Reservation of Common Stock
During the term the Bridge Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the Bridge Warrant and, from time to time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock issuable upon exercise of the Bridge Warrants.
112
Taxes
All shares that may be issued upon the exercise of rights represented by the Bridge Warrants and payment of the Exercise Price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Bridge Warrants).
Adjustments
Prior to the Expiration Date, the Exercise Price and the number of shares of Common Stock purchasable upon the exercise of the Bridge Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
113
Governing Law
The Bridge Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Bridge Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
Extension Warrants
DHAC executed the Extension Securities Purchase Agreement with the lender pursuant to which, among other things, DHAC issued to the lender warrants exercisable for 26,086 shares of Common Stock of the Company (collectively, the “Extension Warrants”).
Exercise
The Bridge Warrants are exercisable for shares of Common Stock of the Company at a price of $11.50 per share of Common Stock (the “Exercise Price”), and expire at 5:30 p.m. Pacific Time five years after the date of issuance (the “Expiration Date”).
Cashless Exercise
If at any time after the date of issuance of the Bridge Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, then in lieu of exercising the Bridge Warrant by payment of cash or check, the holder may elect to receive the number of shares of Common Stock equal to the value of the Bridge Warrant, or the portion thereof being exercised, by surrender of the Bridge Warrant to the Company or its transfer agent, after which the holder will receive shares of Common Stock in accordance with the following formula:
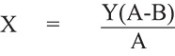
| | | |
Where, | X | = | The number of shares of Common Stock to be issued to the holder; |
| Y | = | The number of shares of Common Stock for which the Bridge Warrant is being exercised; |
| A | = | The fair market value of one share of Common Stock; and |
| B | = | The Exercise Price. |
No Fractional Shares
In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the Exercise Price multiplied by such fraction
No Rights of Shareholders
Except as provided in the Bridge Warrant, the Bridge Warrant does not entitle its holder to any rights of a shareholder of the Company.
114
Reservation of Common Stock
During the term the Bridge Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the Bridge Warrant and, from time to time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock issuable upon exercise of the Bridge Warrants.
Taxes
All shares that may be issued upon the exercise of rights represented by the Bridge Warrants and payment of the Exercise Price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Bridge Warrants).
Adjustments
Prior to the Expiration Date, the Exercise Price and the number of shares of Common Stock purchasable upon the exercise of the Bridge Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
115
Governing Law
The Extension Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Extension Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
Outstanding Notes
Bridge Notes
Pursuant to the Bridge SPA, DHAC, VSee, and iDoc issued the Bridge Investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the principal amount of $888,888.80, $666,666.60 and $666,666.60, respectively (the “Bridge Notes” and individually, the “DHAC Bridge Notes,” “VSee Bridge Notes” and “iDoc Bridge Notes” when referring to Bridge Notes issued to the Company, VSee, and iDoc, respectively) in an aggregate principal amount of approximately $2,222,222. On November 21, 2023 and as further amended and restated on February 13, 2024 (the “A&R Loan Conversion SPAs”), VSee, and iDoc entered into certain of the A&R Loan Conversion SPAs with the Bridge Investor, pursuant to which (i) the $600,000 balance of the VSee Bridge Note not included in the Exchange Note (as defined below) was assumed by the Company and was converted into Common Stock of the Company following the Closing, and (ii) the $600,000 balance of the iDoc Bridge Note not included in the Exchange Note and certain indebtedness owed by iDoc to Tidewater were assumed by the Company and were converted into Common Stock of the Company following the Closing.
Additional Bridge Notes
On November 21, 2023 and as further amended on April 17, 2024, DHAC entered into the Bridge Letter Agreement (the “Bridge Letter Agreement”), pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount convertible promissory notes in the aggregate principal amount of $166,667 (with a subscription amount of $150,000) from the Company with (1) a $111,111.33 note purchased at signing of the Bridge Letter Agreement, which will mature on May 21, 2025 and (2) a $55,555.67 note purchased on January 25, 2024, which will mature on July 25, 2025 (as amended, the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8.00% per annum and are convertible into shares of the Company’s Common Stock at a fixed conversion price of $10 per share. The note will be convertible into fully paid and non-assessable shares of Common Stock at any time after the original issue date. The conversion price of the Additional Bridge Notes is subject to reset if the Company’s Common Stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the Company’s Common Stock in the 10 trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Additional Bridge Notes requires the payment of 110% of the outstanding obligations, including the guaranteed minimum interest. If an event of
116
default occurs, the Additional Bridge Notes would bear interest at a rate of 24.00% per annum and require the payment of 125% of the outstanding obligations, including the guaranteed minimum interest.
Exchange Note
Pursuant to the Exchange Agreement with the Bridge Investor, the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, were exchanged for a senior secured convertible promissory note issued by DHAC the Exchange Note with an aggregate principle value of $2,523,744.29 (the “Exchange Note”), which was secured by the assets of the Company and guaranteed by each of the Company, VSee Lab and iDoc. The Exchange Note will bear interest at a rate of 8.00% per annum and will be convertible into shares of Common Stock at an initial fixed conversion price of $10 per share. The Exchange Note will be convertible into fully paid and non-assessable shares of Common Stock at any time after the original issue date. The conversion price of the Exchange Note is subject to reset if the Company’s Common Stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the Company’s Common Stock in the 10th trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Exchange Note requires the payment of 110% of the outstanding obligations. If an event of default occurs, the Exchange Note would bear interest at a rate of 24.00% per annum and require the payment of 125% of the outstanding obligations.
Quantum Note
Pursuant to the Quantum Purchase Agreement, the Company issued and sold to the Quantum Investor on June 25, 2024 and as further amended on July 3, 2024, a 7% original issue discount convertible promissory note in the aggregate principal amount of $3,000,000, which will mature on June 30, 2026. The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) an initial fixed conversion price of $10 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination. The Quantum Note will be convertible into fully paid and non-assessable shares of Common Stock at any time after the issuance date. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30th-day anniversary of the Quantum Note issuance date (the “Average Price”) is less than $10, to a price equal to the Average Price but in no event less than $2.00. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10. If an event of default occurs, the Quantum Note would bear interest at a rate of 18.00% per annum regardless of early pay or redemption.
Equity Purchase Note
Pursuant to the Equity Purchase Agreement, on July 2, 2024, the Company issued to the investor, as a commitment fee for the equity purchase transaction, a senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of Common Stock at an initial fixed conversion price of $10 per share. The Equity Purchase Note will be convertible into fully paid and non-assessable shares of Common Stock at any time after the original issue date. Commencing at any time after 90 calendar days from the closing of the Business Combination, the holder of the Note may, upon five days prior written notice, require the Company to pay off and satisfy its full obligations only in shares of the Company’s Common Stock at an initial price of $10 per share under the note.
Stock Options
In June 2024, the DHAC board of directors and stockholders approved the VSee Health, Inc. 2024 Equity Incentive Plan. There are currently 2,544,021 shares reserved for issuance under the 2024 Equity Incentive Plan. At the Closing of the Business Combination on June 24, 2024, the Company granted stock options with an exercise price equal to $10.00 pursuant to the 2024 Equity Incentive Plan to the individuals, in the amounts, and on the terms set forth in the Business Combination Agreement.
Dividends
Declaration and payment of any dividend will be subject to the discretion of the board of directors of the Company. The time and amount of dividends will be dependent upon, among other things, the Company’s business prospects, results of operations, financial condition, cash requirements and availability, debt repayment obligations, capital expenditure needs, contractual restrictions, covenants in the agreements governing current and future indebtedness, industry trends, the provisions of Delaware law affecting the
117
payment of dividends and distributions to stockholders and any other factors or considerations the board of directors of the Company may regard as relevant.
The Company currently intends to retain all available funds and any future earnings to fund the development and growth of the business, and therefore does not anticipate declaring or paying any cash dividends on Common Stock in the foreseeable future.
Exclusive Forum
The Amended Charter provides that, to the fullest extent permitted by law, unless the Company otherwise consents in writing, the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for (1) any derivative action or proceeding brought on behalf of the Company, (2) any action asserting a claim of breach of a fiduciary duty owed by, or any other wrongdoing by, any current or former director, officer, other employee or stockholder of the Company, (3) any action asserting a claim against the Company arising pursuant to any provision of the DGCL, the Amended Charter or the Amended Bylaws, or as to which the DGCL confers jurisdiction on the Court of Chancery, (4) any action to interpret, apply, enforce or determine the validity of any provisions of the Amended Charter or the Amended Bylaws, or (5) any other action asserting a claim governed by the internal affairs doctrine. Notwithstanding the foregoing, the federal district courts of the United States shall be the exclusive forum for the resolution of any action, suit or proceeding asserting a cause of action arising under the Securities Act and the provisions of the Amended Charter described above will not apply to claims arising under the Exchange Act or other federal securities laws for which there is exclusive federal jurisdiction.
Anti-Takeover Effects of Provisions of the Amended Charter, the Amended Bylaw and Applicable Law
Certain provisions of the Amended Charter, Amended Bylaws, and laws of the State of Delaware, where the Company is incorporated, may discourage or make more difficult a takeover attempt that a stockholder might consider in his or her best interest. These provisions may also adversely affect prevailing market prices for the common stock of the Company. We believe that the benefits of increased protection give the Company the potential ability to negotiate with the proponent of an unsolicited proposal to acquire or restructure Company and outweigh the disadvantage of discouraging those proposals because negotiation of the proposals could result in an improvement of their terms.
Limitations on Stockholder Action by Written Consent
The Amended Charter provides that, subject to the terms of any series of the Company preferred stock, any action required or permitted to be taken by the stockholders of the Company must be effected at an annual or special meeting of the stockholders and may not be effected by written consent in lieu of a meeting.
Amendment of the Amended Charter and Amended Bylaws
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate of incorporation requires a greater percentage.
The Amended Charter provides that it may be amended by the Company in the manners provided therein or prescribed by statute. In addition to any vote required by applicable law, the following provisions in the Amended Charter may be amended, altered, repealed or rescinded, in whole or in part, or any provision inconsistent therewith or herewith may be adopted, only by the affirmative vote of the holders of at least two-thirds (66 and 2/3%) of the total voting power of all the then outstanding shares of stock of the Corporation entitled to vote thereon, voting together as a single class: Part B of Article IV, Article V, Article VI, Article VII, Article VIII, Article IX and this Article X.
The Amended Charter also provides that the Company Board will have the power to adopt, amend, alter, or repeal the Amended Bylaws. Amendment or repeal of the Bylaws of the Corporation by the stockholders of the Corporation shall require the affirmative vote of the holders of at least two-thirds (66 and 2/3%) of the voting power of all of the then outstanding shares of voting stock of the Corporation entitled to vote generally in an election of directors.
118
Business Combinations
Under Section 203 of the DGCL, a corporation will not be permitted to engage in a business combination with any interested stockholder for a period of three years following the time that such interested stockholder became an interested stockholder, unless:
| (1) | prior to such time the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| (2) | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; |
| (3) | at or subsequent to such time the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662∕3% of the outstanding voting stock which is not owned by the interested stockholder. |
| (4) | Generally, a “business combination” includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of the Company’s outstanding voting stock. For purposes of this section only, “voting stock” has the meaning given to it in Section 203 of the DGCL. |
Since the Company has not opted out of Section 203 of the DGCL, it will apply to the Company. As a result, this provision will make it more difficult for a person who would be an “interested stockholder” to effect various business combinations with the Company for a three-year period. This provision may encourage companies interested in acquiring the Company to negotiate in advance with the Company Board because the stockholder approval requirement would be avoided if the Company Board approves either the business combination or the transaction which results in the stockholder becoming an interested stockholder. These provisions also may have the effect of preventing changes in the Company Board and may make it more difficult to accomplish transactions, which stockholders may otherwise deem to be in their best interests.
Cumulative Voting
Under Delaware law, the right to vote cumulatively does not exist unless the charter specifically authorizes cumulative voting. The Amended Charter does not authorize cumulative voting.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors or officers of corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. The Amended Charter includes a provision that eliminates the personal liability of directors or officers for damages for any breach of fiduciary duty as a director or officer where, in civil proceedings, the person acted in good faith and in a manner that person reasonably believed to be in or not opposed to the best interests of the Company or, in criminal proceedings, where the person had no reasonable cause to believe that his or her conduct was unlawful.
The Amended Bylaws provide that the Company must indemnify and advance expenses to the Company’s directors and officers to the fullest extent authorized by the DGCL. the Company also is expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for the Company directors, officers, and certain employees for some liabilities. the Company believes that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, advancement and indemnification provisions in the Amended Charter and Amended Bylaws may discourage stockholders from bringing lawsuits against directors for any alleged breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if
119
successful, might otherwise benefit the Company and its stockholders. In addition, your investment may be adversely affected to the extent the Company pays the costs of settlement and damage awards against directors and officer pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of the Company’s directors, officers, or employees for which indemnification is sought.
Corporate Opportunities
The Amended Charter provides for the renouncement by the Company of any interest or expectancy of the Company in, or being offered an opportunity to participate in any matter, transaction, or interest that is presented to, or acquired, created, or developed by, or which otherwise comes into possession of, any director of the Company who is not an employee of the Company or any of its subsidiaries, unless such matter, transaction, or interest is presenting to, or acquired, created, or developed by, or otherwise comes into the possession of a director of the Company expressly and solely in that director’s capacity as a director of the Company.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, the Company’s stockholders will have appraisal rights in connection with a merger or consolidation of the Company. Pursuant to the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of the Company’s stockholders may bring an action in the Company’s name to procure a judgment in the Company’s favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of the Company’s shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
Our Transfer Agent and Warrant Agent
The transfer agent and warrant agent for the Company’s Common Stock and Public Warrants is Continental Stock Transfer & Trust Company, 1 State Street, New York, New York 10004.
Listing of our Securities
Our Common Stock and Public Warrants are trading on the Nasdaq Capital Market of The Nasdaq Stock Market LLC (“Nasdaq”) under the symbols “VSEE” and “VSEEW” respectively.
120
SECURITIES ACT RESTRICTIONS ON RESALE OF OUR SECURITIES
Pursuant to Rule 144 under the Securities Act (“Rule 144”), a person who has beneficially owned restricted our common stock or our warrants for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been our affiliate at the time of, or at any time during the three months preceding, a sale and (ii) we are subject to the Exchange Act periodic reporting requirements for at least three months before the sale and have filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months (or such shorter period as the Company was required to file reports) preceding the sale.
Persons who have beneficially owned restricted our common stock shares or our warrants for at least six months but who are our affiliates at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of:
| ● | 1% of the total number of our common stock then outstanding; or |
| ● | the average weekly reported trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by our affiliates under Rule 144 are also limited by manner of sale provisions and notice requirements and to the availability of current public information about us.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies (other than business combination related shell companies) or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| ● | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| ● | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| ● | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Current Reports on Form 8-K; and |
| ● | at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company. |
As a result, the Sponsor will be able to sell their founder shares and private placement warrants, as applicable, pursuant to Rule 144 without registration one year after the Company has completed its initial business combination.
Following the recent consummation of the Business Combination, the Company is no longer be a shell company, and, once the conditions set forth in the exceptions listed above are satisfied, Rule 144 will become available for the resale of the above noted restricted securities.
Lock-up Agreements
Pursuant to certain lock-up restrictions agreed to into in connection with the Business Combination Agreement, subject to certain exceptions, the Sponsor, certain of the Company’s key stockholders and the executive officers and directors of the Company will be contractually restricted from selling or transferring any of its or their shares of our common stock. Such restrictions generally began upon the closing of the Business Combination and end 180 days after the closing of the Business Combination or until certain notes issues to the Selling Stockholder are no longer outstanding.
121
PLAN OF DISTRIBUTION
The shares of Common Stock offered by this prospectus are being offered by the Selling Stockholder, Dominion Capital LLC. The shares may be sold or distributed from time to time by the Selling Stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the shares of our Common Stock offered by this prospectus could be effected in one or more of the following methods:
| ● | ordinary brokers’ transactions; |
| ● | transactions involving cross or block trades; |
| ● | through brokers, dealers, or underwriters who may act solely as agents; |
| ● | “at the market” into an existing market for our Common Stock; |
| ● | in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents; |
| ● | in privately negotiated transactions; or any combination of the foregoing. |
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
Dominion Capital LLC is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
The Selling Stockholder has informed us that it intends to use one or more registered broker-dealers to effectuate all sales, if any, of our Common Stock that it has acquired and may in the future acquire from us pursuant to the Equity Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price. Each such registered broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. The Selling Stockholder has informed us that each such broker-dealer will receive commissions from the Selling Stockholder that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters or agents participating in the distribution of the shares of our Common Stock offered by this prospectus may receive compensation in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as agent, of the shares sold by the Selling Stockholder through this prospectus. The compensation paid to any such particular broker-dealer by any such purchasers of shares of our Common Stock sold by the Selling Stockholder may be less than or in excess of customary commissions. Neither we nor the Selling Stockholder can presently estimate the amount of compensation that any agent will receive from any purchasers of shares of our Common Stock sold by the Selling Stockholder.
Other than as set forth below, we know of no existing arrangements between the Selling Stockholder or any other stockholder, broker, dealer, underwriter or agent relating to the sale or distribution of the shares of our Common Stock offered by this prospectus. Capitalized terms used herein are defined in this prospectus. Please refer to the Company’s Current Report on Form 8-Ks filed with the SEC and the exhibits filed thereunder on October 7, 2022, November 22, 2023, February 13, 2024, April 18, 2024, June 28, 2024 and July 9, 2024 for further descriptions of the transactions set forth below. Also see section entitled “The Selling Stockholder” in this prospectus for further information about the securities beneficially owned by the Selling Stockholder.
| ● | On October 5, 2022, DHAC, VSee Lab and iDoc entered into the Original Bridge SPA with the Selling Stockholder, pursuant to which DHAC, VSee Lab and iDoc each issued and sold to the Selling Stockholder 10% original issue discount senior secured promissory notes due October 5, 2023 in the respective principal amount of $888,888.80, $666,666.60 and $666,666.60 for an aggregate principal amount of $2,222,222. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 Bridge Warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments and (ii) 30,000 shares of DHAC common stock, whereby the respective warrants and shares of DHAC common stock were redesignated as warrants and shares of Common Stock of the Company at the Closing of the Business Combination. |
122
| ● | On November 21, 2023 and as further amended on April 17, 2024, DHAC, VSee Lab and iDoc entered into a letter agreement amending the Original Bridge SPA, pursuant to which the Selling Stockholder agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111.33 note purchased on November 21, 2023, which will mature on May 21, 2025 and (2) a $55,555.67 note purchased on January 25, 2024, which will mature on July 25, 2025. |
| ● | On November 21, 2023 and as further amended and restated on February 13, 2024, DHAC, VSee Lab, and/or iDoc, as applicable, entered into securities purchase agreements pursuant to which the $600,000 balance of the VSee Lab Bridge Note and the $600,000 balance of the iDoc Bridge Note, which are not included in the Exchange Note would be assumed by the Company and converted into 600,000 shares of Common Stock at a conversion price of $2.00 per share following the Closing. In connection with the Closing of the Business Combination on June 24, 2024, the Company issued 600,000 shares of Common Stock to the Selling Stockholder. |
| ● | On July 2, 2024, pursuant to the Equity Purchase Agreement, the Company issued a senior unsecured note in a principal amount of $500,000 that is payable only in shares of the Company’s Common Stock at an initial price of $10 per share to the Selling Stockholder. |
We may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and when required under the Securities Act, to disclose certain information relating to a particular sale of shares offered by this prospectus by the Selling Stockholder, including the names of any brokers, dealers, underwriters or agents participating in the distribution of such shares by the Selling Stockholder, any compensation paid by the Selling Stockholder to any such brokers, dealers, underwriters or agents, and any other required information.
We will pay the expenses incident to the registration under the Securities Act of the offer and sale of the shares of our Common Stock covered by this prospectus by the Selling Stockholder. As consideration for the Selling Stockholder’s commitment to purchase the Company’s Common Stock under the Equity Purchase Agreement, on July 2, 2024, we issued a senior unsecured note in a principal amount of $500,000 that is payable only in shares of our Common Stock at an initial price of $10 per share (the “Equity Purchase Note”) to the Selling Stockholder. The Equity Purchase Note is convertible into 50,000 shares of Common Stock.
We also have agreed to indemnify the Selling Stockholder and certain other persons against certain liabilities in connection with the offering of shares of our Common Stock offered hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
The Selling Stockholder has represented to us that at no time prior to the date of the Equity Purchase Agreement has the Selling Stockholder or its agents, representatives or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our common stock or any hedging transaction, which establishes a net short position with respect to our Common Stock. The Selling Stockholder has agreed that during the term of the Equity Purchase Agreement, neither the Selling Stockholder, nor any of its agents, representatives or affiliates will enter into or effect, directly or indirectly, any of the foregoing transactions.
We have advised the Selling Stockholder that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the Selling Stockholder, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the securities offered by this prospectus.
Our Common Stock is currently listed on The Nasdaq Capital Market under the symbol “VSEE”.
123
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by Manatt, Phelps & Phillips, LLP, Costa Mesa, California.
EXPERTS
The consolidated financial statements of VSee Health, Inc. (f/k/a Digital Health Acquisition Corp.) as of December 31, 2023, and 2022, and the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for the year ended December 31, 2023 and 2022, appearing in this prospectus have been audited by Withum Smith + Brown, PC, independent registered public accounting firm, as set forth in their report thereon (which report expresses an unqualified opinion and includes an explanatory paragraph relating to a going concern), appearing elsewhere in this proxy statement/registration statement, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of VSee Lab, Inc. as of December 31, 2023 and 2022 and for the years then ended included in this prospectus have been so included in reliance on the report of Accell Audit & Compliance, PA, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The financial statements of iDoc Virtual Telehealth Solutions, Inc. as of December 31, 2023 and 2022 and for the years then ended included in this prospectus have been so included in reliance on the report of Accell Audit & Compliance, PA, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a website, at http://www.sec.gov, that contains registration statements, reports, proxy statements and other information regarding registrants that file electronically with the SEC, including us. Our website address is http://www.vseehealth.com.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities being offered by this prospectus. This prospectus is part of that registration statement. This prospectus does not contain all of the information set forth in the registration statement or the exhibits to the registration statement. For further information with respect to us and the securities we are offering pursuant to this prospectus, you should refer to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete, and you should refer to the copy of that contract or other documents filed as an exhibit to the registration statement. You may read or obtain a copy of the registration statement at the SEC’s website referred to above.
124
INDEX TO FINANCIAL STATEMENTS
VSEE LAB, INC. FINANCIAL STATEMENTS
Audited Financial Statements of VSee Lab, Inc. | |
F-5 | |
F-6 | |
Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 | F-7 |
F-8 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 | F-9 |
F-10 | |
| |
Unaudited Condensed Financial Statements of VSee Lab, Inc. | |
Condensed Consolidated Balance Sheets at March 31, 2024 (Unaudited) and December 31, 2023 | F-25 |
F-26 | |
F-27 | |
F-29 | |
F-30 |
IDOC VIRTUAL TELEHEALTH SOLUTIONS, LLC FINANCIAL STATEMENTS
Audited Financial Statements of iDoc Virtual Telehealth Solutions, LLC | |
F-43 | |
F-44 | |
Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 | F-45 |
F-46 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 | F-47 |
F-48 |
Unaudited Condensed Financial Statements of iDoc Virtual Telehealth Solutions, LLC | |
Condensed Consolidated Balance Sheets at March 31, 2024 (Unaudited) and December 31, 2023 | F-70 |
F-71 | |
F-72 | |
F-74 | |
F-75 |
F-1
DIGITAL HEALTH ACQUISITION CORP. FINANCIAL STATEMENTS
Audited Financial Statements of Digital Health Acquisition Corp | |
F-91 | |
Financial Statements: | |
Consolidated Balance Sheets as of December 31, 2023 and 2022 | F-92 |
Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 | F-93 |
F-94 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 | F-95 |
F-96 | |
| |
Unaudited Financial Statements of Digital Health Acquisition Corp. | |
Condensed Consolidated Balance Sheets as of March 31, 2024 (Unaudited) and December 31, 2023 | F-130 |
F-131 | |
F-132 | |
F-133 | |
Notes to Unaudited Condensed Consolidated Financial Statements | F-134 |
F-2
F-3
VSEE LAB, INC.
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| |
Consolidated Financial Statements | |
F-5 | |
F-6 | |
Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 | F-7 |
F-8 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 | F-9 |
F-10 |
F-4

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of VSee Lab, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of VSee Lab, Inc. (the Company) as of December 31, 2023 and 2022, and the related consolidated statements of operations, changes in stockholders’ (deficit) equity, and cash flows for each of the years in the two-year period ended December 31, 2023, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company has incurred net losses and negative cash flow from operations since inception. These factors, and the need for additional financing in order for the Company to meet its business plans raises substantial doubt about the Company’s ability to continue as a going concern. Our opinion is not modified with respect to that matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.

We have served as the Company’s auditor since 2022.
PCAOB Firm ID# 3289
Tampa, Florida
April 22, 2024

F-5
VSEE LAB, INC.
CONSOLIDATED BALANCE SHEETS
| | | | | | |
|
| December 31, |
| December 31, | ||
|
| 2023 |
| 2022 | ||
ASSETS |
| |
|
| |
|
Current assets |
| |
|
| |
|
Cash and cash equivalents | | $ | 118,734 | | $ | 230,664 |
Accounts receivable, net | |
| 628,480 | |
| 389,453 |
Prepaids and other current assets | |
| 79,920 | |
| 139,661 |
Total current assets | |
| 827,134 | |
| 759,778 |
Fixed assets, net | |
| 3,657 | |
| — |
Deferred tax asset | |
| — | |
| 1,852,826 |
Total assets | | $ | 830,791 | | $ | 2,612,604 |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
|
| |
|
|
Current liabilities | | | | | | |
Accounts payable and accrued liabilities | | $ | 1,824,408 | | $ | 721,089 |
Deferred revenue | |
| 802,524 | |
| 956,561 |
Due to related party | |
| 338,506 | |
| 146,322 |
Due on share purchase | |
| 135,000 | |
| — |
Embedded derivative | |
| — | |
| 273,534 |
Contingent liability | |
| 600,000 | |
| — |
Loan payable, related party, net of discount | |
| 323,000 | |
| 110,000 |
Note payable, net of discount | |
| 220,000 | |
| 407,131 |
Total liabilities | |
| 4,243,438 | |
| 2,614,637 |
Commitments and contingencies (Note 4) | |
|
| |
|
|
Stockholders’ deficit | |
|
| |
|
|
Preferred stock, $0.0001 par value, 1,701,715 shares authorized; Series A: 371,715 shares authorized, issued and outstanding | |
| 37 | |
| 37 |
Series A-1: 1,330,000 shares authorized, and 1,228,492 issued and outstanding | |
| 123 | |
| 123 |
Common stock, $0.0001 par value; 18,000,000 shares authorized 9,998,446 shares issued and outstanding | |
| 1,000 | |
| 1,000 |
Additional paid in capital | |
| 6,026,457 | |
| 6,026,457 |
Accumulated deficit | |
| (9,114,985) | |
| (5,666,895) |
Non-controlling interest | |
| (325,279) | |
| (362,755) |
Total stockholders’ deficit | |
| (3,412,647) | |
| (2,033) |
Total liabilities and stockholders’ deficit | | $ | 830,791 | | $ | 2,612,604 |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
VSEE LAB, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | |
|
| For the years ended December 31, | ||||
|
| 2023 |
| 2022 | ||
Revenues | | $ | 5,840,889 | | $ | 6,377,760 |
Cost of goods sold | |
| 1,933,195 | |
| 1,542,657 |
Gross margin | |
| 3,907,694 | |
| 4,835,103 |
Operating expenses | |
|
| |
|
|
Compensation and related benefits | |
| 4,417,028 | |
| 5,015,940 |
General and administrative | |
| 962,616 | |
| 1,283,172 |
Transaction expenses | |
| 86,799 | |
| 216,025 |
Total operating expenses | |
| 5,466,443 | |
| 6,515,137 |
Net operating loss | |
| (1,558,749) | |
| (1,680,034) |
Other income (expenses): | |
|
| |
|
|
Interest expense | |
| (191,323) | |
| (31,868) |
Other (expense) income | |
| (20,114) | |
| 156,516 |
Change in fair value on embedded derivative | |
| 90,200 | |
| (64,731) |
Gain on forgiveness of debt | |
| 107,862 | |
| — |
Total other (expenses) income | |
| (13,375) | |
| 59,917 |
Loss before income taxes | |
| (1,572,124) | |
| (1,620,117) |
Income tax (expense) benefit | |
| (1,838,490) | |
| 694,363 |
Net loss | |
| (3,410,614) | |
| (925,754) |
Net income (loss) attributable to non-controlling interest | | $ | 37,476 | | $ | (89,549) |
Net loss attributable to VSee Lab, Inc. | | $ | (3,448,090) | | $ | (836,205) |
Basic and diluted loss per share | | $ | (0.34) | | $ | (0.08) |
Weighted average number of shares outstanding, basic and diluted income | |
| 9,998,446 | |
| 9,998,446 |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
VSEE LAB, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A | | Series A-1 | |
| | |
| | Additional | | |
| | |
| | |
| |||||||
|
| Preferred Stock |
| Preferred Stock |
| Common Stock |
| Paid-In |
| | Accumulated |
| Non: controlling | | | | |||||||||||
|
| Shares |
| Amount |
| Shares |
| Amount |
| Shares |
| Amount |
| Capital |
| Deficit |
| Interest |
| Total | |||||||
Balance, December 31, 2021 |
| 371,715 | | $ | 37 |
| 1,228,492 | | $ | 123 |
| 9,998,446 | | $ | 1,000 | | $ | 6,026,457 | | $ | (4,830,690) | | $ | (273,206) | | $ | 923,721 |
Net loss |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| (836,205) | |
| — | |
| (836,205) |
Non-controlling interest |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| — | |
| (89,549) | |
| (89,549) |
Balance, December 31, 2022 |
| 371,715 | |
| 37 |
| 1,228,492 | |
| 123 |
| 9,998,446 | |
| 1,000 | |
| 6,026,457 | |
| (5,666,895) | |
| (362,755) | |
| (2,033) |
Net loss |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| (3,448,090) | |
| — | |
| (3,448,090) |
Non-controlling interest |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| — | |
| 37,476 | |
| 37,476 |
Balance, December 31, 2023 |
| 371,715 | | $ | 37 |
| 1,228,492 | | $ | 123 |
| 9,998,446 | | $ | 1,000 | | $ | 6,026,457 | | $ | (9,114,985) | | $ | (325,279) | | $ | (3,412,647) |
The accompanying notes are an integral part of these consolidated financial statements.
F-8
VSEE LAB, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | |
|
| For the Years Ended December 31, | ||||
|
| 2023 |
| 2022 | ||
Net loss | | $ | (3,410,614) | | $ | (925,754) |
Adjustments to reconcile net loss to net cash used by operating activities: | |
|
| |
|
|
Amortization of discount on note payable | |
| 93,733 | |
| 15,934 |
Change in fair value on embedded derivative | |
| (90,200) | |
| 64,731 |
Gain on forgiveness of debt | |
| (107,862) | |
| — |
Provision for bad debt | |
| 32,457 | |
| 15,131 |
Depreciation expense | |
| 678 | |
| — |
Changes in working capital requirements: | |
|
| |
|
|
Accounts receivable | |
| (271,484) | |
| (118,490) |
Prepaids and other current assets | |
| 59,741 | |
| 5,559 |
Deferred tax asset | |
| 1,852,826 | |
| (694,363) |
Accounts payable and accrued liabilities | |
| 1,169,983 | |
| 518,639 |
Deferred revenue | |
| (154,037) | |
| 146,515 |
Due to related party | |
| 192,184 | |
| 146,322 |
Net cash from operating activities | |
| (632,595) | |
| (825,776) |
CASH FLOWS FROM INVESTING ACTIVITIES: | |
|
| |
|
|
Purchase of fixed assets | |
| (4,335) | |
| — |
Net cash from financing activities | |
| (4,335) | |
| — |
CASH FLOWS FROM FINANCING ACTIVITIES: | |
|
| |
|
|
Proceeds from note payable | |
| 200,000 | |
| 600,000 |
Proceeds from loan payable, related party | |
| 190,000 | |
| 110,000 |
Proceeds from share purchase liability | |
| 135,000 | |
| — |
Net cash from financing activities | |
| 525,000 | |
| 710,000 |
NET CHANGE IN CASH AND CASH EQUIVALENTS | |
| (111,930) | |
| (115,776) |
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | |
| 230,664 | |
| 346,440 |
CASH AND CASH EQUIVALENTS, END OF PERIOD | | $ | 118,734 | | $ | 230,664 |
Supplemental disclosure of cash flow information | |
|
| |
|
|
Cash paid for interest expense | | $ | — | | $ | — |
Cash paid for income taxes | | $ | — | | $ | 16,000 |
The accompanying notes are an integral part of these consolidated financial statements.
F-9
VSEE LAB, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Note 1 Organization and Description of Business
VSee Lab, Inc. was incorporated on December 23, 2010 under the laws of the State of Delaware. ThisAmericanDoc, Inc. (“TAD”), a majority owned subsidiary, was incorporated on December 27, 2016, in the State of Delaware. VSee Lab, Inc. and TAD (collectively, the “Company”, “VSee”) are one of the leading providers of virtual healthcare platform services with a focus on high quality, lower costs, and improved outcomes around the world with its integrated platform. The Company is committed to creating a telemedicine experience that’s as simple and accessible as shopping online by providing an advanced, no code, low code telemedicine platform to integrate seamlessly across channels, other existing platforms, and healthcare devices for any virtual care delivery model. The Company is a health services technology company that is responding to the need for rapid, effective system integration within healthcare. The Company operates as a single operating and reportable segment.
Note 2 Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
The consolidated financial statements include the accounts of VSee Lab, Inc. and its 53.8% partially owned subsidiary, TAD. The accompanying consolidated financial statements reflect adjustments (including normal, recurring adjustments) necessary to present fairly the financial position of the Company as of December 31, 2023 and 2022, its results of operations, changes in stockholders’ deficit, and statements of cash flows for the years ended December 31, 2023 and 2022, in conformity with U.S. GAAP.
Use of Estimates
The preparation of the Company’s consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, allowance for doubtful accounts, and income taxes.
The Company bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits as a component of general and administrative expenses. The Company’s federal tax return and any state tax returns are not currently under examination.
The Company has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or
F-10
deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services.
The Company determines revenue recognition in accordance with ASC 606, through the following five steps:
1) Identify the contract with a customer
The Company considers the terms and conditions of its contracts and the Company’s customary business practices in identifying its contracts under ASC 606. The Company determines it has a contract with a customer when the contract has been approved by both parties, it can identify each party’s rights regarding the services to be transferred and the payment terms for the services, it has determined the customer to have the ability and intent to pay, and the contract has commercial substance. The Company applies judgment in determining the customer’s ability and intent to pay, which is based on a variety of factors, including the customer’s payment history or, in the case of a new customer, credit and financial information pertaining to the customer.
Contractual terms for subscription services are typically 12 months. Contracts are cancellable with a 30-day notice period and customers are billed in annual, quarterly, or monthly installments in advance of the service period of the subscription. The Company is not required to refund any prorated prepayment fees invoiced to cover services that were provided.
2) Identify the performance obligations in the contract
Performance obligations promised in a contract are identified based on the services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the service either on its own or together with other resources that are readily available, and are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. The Company’s contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that they have any material outstanding commitments for future revenues beyond one year from the end of a reporting period.
The Company treats each subscription to a specific module as a distinct performance obligation because each module is capable of being distinct as the customer can benefit from the subscription to each module on its own and each subscription can be sold standalone.
Furthermore, the subscriptions to individual modules are distinct in the context of the contract as (1) the Company is not integrating the services with other services promised in the contract into a bundle of services that represent a combined output, (2) the subscriptions to specific modules do not significantly modify or customize the subscription to another module, and (3) the specific modules are not highly interdependent or highly interrelated. The subscription to each module is treated as a series of distinct performance obligations because it is distinct and substantially the same, satisfied over time, and has the same measure of progress.
3) Determine the transaction price
The transaction price is determined based on the consideration the Company expects to be entitled to in exchange for transferring services to the customer. Under the contracts, the clients pay a fixed rate per user per subscription service. Prior to the start of a contract, clients generally make upfront nonrefundable payments to the Company when contracting for implementation services.
The contract pricing is fixed and stated in the arrangements based on the work to be performed by the Company and represents the amount the Company is entitled to for delivering such goods and services. The quoted fees per promise and performance obligation are based on the most likely amount method for what the Company expects to collect.
F-11
4) Allocate the transaction price to performance obligations in the contract
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative stand-alone selling price (“SSP”). The determination of a SSP for each distinct performance obligation requires judgment. The Company believes the quoted transaction prices in the customer contracts represent the stand alone selling prices for each of the separate performance obligations that are distinct and priced separately within the contract.
5) Recognize revenue when or as the Company satisfies a performance obligation
Revenue is recognized when or as control of the promised goods or service is transferred to the customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. Subscriptions represent a series of distinct goods or services because the performance obligations are satisfied over time as customers simultaneously receive and consume the benefits related to the services as the Company performs. In the case of module specific subscriptions, a consistent level of service is provided during each monthly period of subscription to the Company’s platform. The Company commences revenue recognition when the customer is provided with platform subscription for the initial monthly period and revenue is recognized over time as a consistent level of subscription service during the subsequent period is delivered. The Company’s obligation for its integrated subscriptions is to stand-ready throughout the subscription period; therefore, the Company considers an output method of time to measure progress towards satisfaction of its obligations with revenue commencing upon the beginning of the subscription period.
Upfront nonrefundable fees do not result in the transfer of a promised good or service to the client, therefore, the Company defers this revenue and recognizes it over the subscription period of the customer contract. Deferred revenue consists of the unamortized balance of nonrefundable upfront fees which are classified as current and non-current based on the timing of when the Company expects to recognize revenue.
Cost of Revenue
Cost of revenue consists primarily of expenses related to cloud hosting, personnel-related expenses for the Company’s customer success team, costs for third-party software services and contractors, and other services used in connection with delivery and support of the Company’s platform subscription services.
Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses since inception and continues to have negative operating cash flows. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The continuation of the Company’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Transaction Expenses
On June 15, 2022, the Company entered into a business combination agreement with Digital Health Acquisition Company (“DHAC”), a Delaware blank check company established for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business transaction with one or more businesses or entities, referred to as “targets.” On October 6, 2022, the business combination agreement was amended, and the Special Purchase Acquisition Company public offering transaction is ongoing. During the years ended December 31, 2023 and 2022, the Company incurred one-time transaction expenses related to the business combination of $86,799 and $216,025, respectively, for professional fees, including legal, taxation, business consulting, and audit services.
Net Income (Loss) Per Common Share
The Company computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net
F-12
income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants. No potential dilutive common shares are included in the computation of any diluted per share amount when a loss is reported.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates their fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
The Company carries its accounts receivable at net realizable value. The Company maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. No credit losses were recognized for the years ended December 31, 2023 and 2022.
The allowance for doubtful accounts is calculated based on a general reserve for at-risk balances considering the Company’s ability to collect. The allowance for doubtful accounts was $32,457 and $0 as of December 31, 2023 and 2022, respectively.
Prepaid Assets
Prepaid expenses are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the consolidated statements of operations.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3: Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable and accrued liabilities as reflected in the consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the consolidated financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
F-13
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. Derivative assets and liabilities are classified in the consolidated balance sheets as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the consolidated balance sheet date. The Company has determined the early mandatory redemption provision in the Bridge Note as described in Note 9 is an embedded derivative instrument. FASB ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components. The Company applies this guidance to allocate the Bridge Note proceeds between the Bridge Note and the Embedded Early Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt.
Fixed Assets
Fixed Assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets. During the year ended December 31, 2023, the Company purchased office and medical equipment, which is being depreciated over a three-year useful life.
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. This may result in the earlier recognition of allowances for losses. ASU 2016-13 is effective for the Company beginning January 1, 2023, and the adoption did not have a material impact on the consolidated financial statements.
In August 2021, the FASB issued ASU No. 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”). ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP.
Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective January 1, 2024, for the Company. Early adoption is permitted. Management is currently evaluating the effect of the adoption of ASU 2020-06 on the consolidated financial statements, but currently does not believe ASU 2020-06 will have a significant impact on the Company’s consolidated financial statements.
Note 3 Equity
Preferred Stock
The Company has two outstanding series of redeemable preferred stock. The Company has 1,701,715 shares of preferred stock authorized with a par value of $0.0001. The Company has allocated 371,715 shares for Series A preferred, and 1,330,000 shares for Series A-1 preferred.
F-14
Series A Preferred Stock
The Series A Preferred has the following rights and privileges:
Voting — Series A preferred stockholders are permitted to vote with the same voting rights as common stockholders in any actions to be taken by the stockholders of the Corporation, including any action with respect to the election of directors to the Board of Directors of the Corporation.
Dividend — Series A preferred stockholders shall be entitled to receive non-cumulative dividends when and if declared at the annual rate of $0.0484 per share for Series A preferred stock, payable quarterly. Dividends are prior and in preference to any declaration or payment of any dividend to the common stockholders of the Company.
Liquidation — In the event of any liquidation, dissolution or winding-up of the Company, the Series A preferred stockholders are entitled to distributions equal to their initial purchase price per share plus accrued and unpaid dividends (all amounts are prior and in preference to any distribution of any assets to the holders of the other series of preferred stock and common stock).
Conversion — Series A preferred stock is convertible into common stock at the option of the holder, except for mandatory conversion upon the earlier of an initial public offering or the date specified by written consent or agreement with majority stockholders. Upon conversion, all shares of Series A preferred stock would be converted automatically into a number of shares of common stock as determined by multiplying the number of shares of Series A preferred stock to be so converted by the Series A Original Issue Price and dividing the result by the applicable conversion price per share.
Series A-1 Preferred Stock
The Series A-1 Preferred has the following rights and privileges:
Voting — Series A-1 preferred stockholders are permitted to vote with the same voting rights as common stockholders in any actions to be taken by the stockholders of the Corporation, including any action with respect to the election of directors to the Board of Directors of the Corporation.
Dividend — Series A preferred stockholders shall be entitled to receive non-cumulative dividends if and when declared at the annual rate of $0.239 per share for Series A-1 preferred stock, payable quarterly.
Dividends are prior and in preference to any declaration or payment of any dividend to the common stockholders of the Company.
Liquidation — In the event of any liquidation, dissolution or winding-up of the Company, the Series A-1 preferred stockholders are entitled to distributions equal to their initial purchase price per share plus accrued and unpaid dividends (all amounts are prior and in preference to any distribution of any assets to the holders of the other series of preferred stock and common stock).
Conversion — Series A-1 preferred stock is convertible into common stock at the option of the holder, except for mandatory conversion upon the earlier of an initial public offering or the date specified by written consent or agreement of the majority stockholders. Upon conversion, all shares of Series A-1 preferred stock would be converted automatically into a number of shares of common stock as determined by multiplying the number of shares of Series A-1 preferred Stock to be so converted by the Series A-1 Original Issue Price and dividing the result by the applicable conversion price per share.
Note 4 Commitments Contingencies and Concentration Risk
Contingencies
During the normal course of business, the Company may be exposed to litigation. When the Company becomes aware of potential litigation, it evaluates the merits of the case in accordance with ASC 450, Contingencies. Litigation and contingency accruals are based on the Company’s assessment, including advice of legal counsel, regarding the expected outcome of litigation or other dispute resolution proceedings.
F-15
If the Company determines that an unfavorable outcome is probable and can be reasonably assessed, it establishes the necessary accruals. As of December 31, 2023 and 2022, the Company has $0 and $90,000 in contingent liabilities reflected in the consolidated financial statements for a legal settlement related to compensation disputes by a former employee.
The Company has a reseller agreement with a vendor to generate revenue opportunities in the international market. As of December 31, 2023 and 2022, the Company has an unpaid commitment of $410,233 and $714,555, respectively, on this contract. The commitment is not reflected in the consolidated financial statements as the commitment is due and payable once revenues are generated under the reseller agreement. The Company entered into the reseller agreement to generate market share in the international market, and payments are based on revenues generated by the reseller.
On November 21, 2023, the Company entered into a Securities Purchase Agreement (“SPA”) with an accredited investor to purchase 300,000 shares of common stock from the Company at $2 per share in exchange for the principal amount (excluding the original issue discount of $66,667) of $600,000 of the Company’s Bridge Note, effective immediately prior to the consummation of the Business Combination. The SPA was entered into concurrently with an Exchange Agreement on November 21, 2023. Refer to Note 9.
Indemnities
The Company generally indemnifies its customers for the services it provides under its contracts, as well as other specified liabilities, which may subject the Company to indemnity claims, liabilities, and related litigation. As of December 31, 2023 and 2022, the Company was not aware of any material asserted or unasserted claims in connection with these indemnity obligations.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to credit risk concentrations consist of cash and cash equivalents and accounts receivables. The Company maintains all of its cash and cash equivalents in commercial depository accounts, insured by the Federal Deposit Insurance Corporation (“FDIC”). At times, cash deposits may exceed federally insured limits.
Major Customer Concentration
The Company has five customers whose accounts receivable represented 86% of the Company’s total accounts receivable as of December 31, 2023. The Company has four customers whose accounts receivable represented 66% of the Company’s total accounts receivable as of December 31, 2022.
The Company has two customers whose revenue accounted for approximately 24% of the Company’s total revenue for the year ended December 31, 2023. The Company has one customer whose revenue accounted for approximately 11% of the Company’s total revenue for the year ended December 31, 2022.
Note 5 Fixed Assets
The components of fixed assets are summarized below:
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
Office equipment | | $ | 3,335 | | $ | — |
Medical equipment | |
| 1,000 | |
| — |
| |
| 4,335 | |
| — |
Less accumulated depreciation | |
| (678) | |
| — |
Fixed Assets, net | | $ | 3,657 | | $ | — |
During the year ended December 31, 2023, the Company purchased medical equipment with a useful life of 3 years. Depreciation expense totaling $678 and $0 was recorded during the years ended December 31, 2023 and 2022, respectively.
F-16
Note 6 Related Party
During the year ended December 31, 2022, a related party provided $127,710 of cash, which will be used for future operating expenses. During the years ended December 31, 2023 and 2022, a related party paid o$192,184 and $18,612 of the Company’s operating expenses, respectively. The balance due to the related party as of December 31, 2023 and 2022 was $338,506 and $146,322, respectively.
During the year ended December 31, 2022, the Company received a loan of $110,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. On March 29, 2023, the Company revised the terms of the loan to a 10.00% original issue discount promissory note with a principal balance of $121,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on June 27, 2023. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of December 31, 2023, the related party promissory note net of unamortized debt discount was $121,000. The Company recognized $11,000 of amortized debt discount and $17,930 in accrued interest, including $14,300 of default interest, for a total interest expense of $28,930 for the year ended December 31, 2023. The Company had $17,930 and $0 in accrued interest as of December 31, 2023 and 2022, respectively, which is included within accounts payable and accrued liabilities on the consolidated balance sheets.
On March 29, 2023, the Company received a 10.00% original issue discount promissory note with a principal balance of $132,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and amortized as interest expense over the life of the underlying note payable. The promissory note matured on June 27, 2023. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of December 31, 2023, the related party promissory note net of unamortized debt discount was $132,000. The Company recognized $12,000 of amortized debt discount and $21,120 in accrued interest, including $17,100 of default interest, for a total interest expense of $33,120 for the year ended December 31, 2023. The Company had $21,120 in accrued interest as of December 31, 2023, which is included within accounts payable and accrued liabilities on the consolidated balance sheets.
On December 26, 2023, the Company received a 10.00% original issue discount promissory note with a principal balance of $77,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and amortized as interest expense over the life of the underlying note payable. The promissory note matures on March 28, 2024. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of December 31, 2023, the related party promissory note net of unamortized debt discount was $70,000. Amortized debt discount and interest were $0 for the year ending December 31, 2023.
Note 7 Simple Agreement for Future Equity
On August 1, 2023, the Company entered into a Simple Agreement for Future Equity (“SAFE”) with a purchase price of $135,000. The SAFE is considered a mandatorily redeemable financial instrument under ASC 480-10-15-8. Per section 1 (a) of the SAFE “If there is an Equity Financing before the termination of this Safe, on the initial closing of such Equity Financing, this Safe will automatically convert into the greater of: (1) the number of shares of Standard Preferred Stock equal to the Purchase Amount divided by the lowest price per share of the Standard Preferred Stock; or (2) the number of shares of Safe Preferred Stock equal to the Purchase Amount divided by the Safe Price”. The fixed monetary amount known at inception (i.e., “Purchase Amount” of $135,000) embodies an obligation that the issuer must or may settle by issuing a variable number of shares, based on the safe price which is defined as “Safe Price” means the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization.” Since the capitalization can change through the termination events, the shares to be issued can vary. The SAFE may require the issuer to redeem the instrument for cash upon a change of control. The SAFE is classified and recorded as a liability under ASC 480-10-25-8 because a change of control is an event that is considered not under the sole control of the issuer.
F-17
Note 8 Income Taxes
The major components of income tax (expense) benefit for the years ended December 31, 2023 and 2022:
| | | | | | |
Consolidated income statement |
| December 31, 2023 |
| December 31, 2022 | ||
Current income Tax: | | $ | — | | $ | — |
Current tax on profits | |
| 14,334 | |
| — |
Tax regarding prior years | |
| | |
| — |
Deferred tax: | |
|
| |
|
|
Deferred taxation – current year | |
| (1,994,609) | |
| 694,363 |
Deferred taxation – prior years | |
| 141,785 | |
| — |
Income tax (expense) benefit reported in the income statement | | $ | (1,838,490) | | $ | 694,363 |
A reconciliation follows between tax expense and the product of accounting profit multiplied by the United States domestic tax rate for the years ended December 31, 2023 and 2022:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Accounting (loss) profit before tax from continuing operations | | $ | (1,572,124) | | $ | (1,620,117) |
Accounting (loss) profit before income tax | |
| (1,572,124) | |
| (1,620,117) |
Federal income tax benefit at federal statutory rate of 21% | |
| 330,146 | |
| 340,198 |
State income tax benefit, net of federal benefit | |
| 121,463 | |
| 93,644 |
Permanent differences, net | |
| 17,377 | |
| 17,892 |
Other | |
| 156,123 | |
| 242,629 |
Valuation allowance charges affecting the income tax provision | |
| (2,463,599) | |
| — |
Total | | $ | (1,838,490) | | $ | 694,363 |
Deferred Tax
Deferred tax is comprised of the following as of December 31, 2023 and 2022:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Non-Current |
| |
|
| |
|
Deferred revenue | | $ | 121,052 | | $ | 9,402 |
Loan Loss Reserve | |
| 9,083 | |
| — |
Fixed assets | |
| 16 | |
| — |
NOL carryforward | |
| 2,333,448 | |
| 1,843,424 |
Valuation allowance | |
| (2,463,599) | |
| — |
Net deferred tax assets | |
| — | |
| 1,852,826 |
Reflected in the Balance Sheets: | |
|
| |
|
|
position as follows: | |
|
| |
|
|
Deferred tax assets | |
| — | |
| 1,852,826 |
Deferred tax liabilities | |
| — | |
| — |
Deferred tax assets net | | $ | — | | $ | 1,852,826 |
Reconciliation of deferred tax assets, net
| | | | | | |
|
| 2023 |
| 2022 | ||
Opening balance as of January 1, | | $ | 1,852,826 | | $ | 1,158,463 |
Tax (expense)/benefit during the period recognized in profit or loss | |
| (1,852,826) | |
| 694,363 |
Closing balance as of December 31, | | $ | — | | $ | 1,852,826 |
The Company offsets tax assets and liabilities only if it has a legally enforceable right to set off current tax assets and current tax liabilities and the deferred tax assets and deferred tax liabilities relate to income taxes levied by the same tax authority.
F-18
The Company has US federal and State of California tax losses totaling $8.8 million and $7.1 million, respectively which have an unlimited carryover period for federal and 20 years for state. State of California losses begin to expire in 2037. As of December 31, 2023 and 2022, the Company had no provision for uncertain tax positions and no provisions for penalties or interest. In addition, the Company does not believe that there are any uncertain tax benefits that could be recognized in the near future that would impact the Company’s effective tax rate. The company recorded a valuation allowance of $2,463,599 on their deferred tax assets as there not enough positive evidence to support their utilization.
Note 9 Note Payable
The following is a summary of notes payable as of December 31, 2023 and 2022:
| | | | | | |
Notes Payable |
| December 31, 2023 |
| December 31, 2022 | ||
Note payable issued October 6, 2022 (Face Value: $666,667) | | $ | — | | $ | 666,667 |
Note payable issued January 12, 2023 (Face Value: $220,000) | |
| 220,000 | |
| — |
Total notes payable and line of credit | |
| 220,000 | |
| 666,667 |
Less: unamortized debt discount, net | |
| — | |
| (259,536) |
Total notes payable at carrying value | | $ | 220,000 | | $ | 407,131 |
As of December 31, 2023, the Company had no required principal payments on its notes payable.
Notes Payable
On October 6, 2022, in connection with the execution of a Business Combination Agreement, the Company entered into a securities purchase agreement (“The Agreement”) with an accredited investor (“Holder”), issued and sold to such investor a 10.00% original issue discount senior secured promissory notes due October 5, 2023, in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). $666,667 of the Bridge Note was allocated to the Company. The Company received cash proceeds of $600,000 from the note.
Concurrently with the execution of the Business Combination Agreement, on October 6, 2022, Digital Health Acquisition Company (‘DHAC”) entered into an Amended and Restated Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with certain investors (the “PIPE Investors”). If the PIPE Financing closes in connection with the closing of the Business Combination, 110% if paid after 90 days of the original issue date, 100% if before 90 days under the Bridge Notes and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Bridge Note has a mandatory default payment of 125% of the sum of the outstanding principal and all accrued interest unpaid and all other amounts, costs, fees (including Late Fees), expenses, indemnification and liquidated and other damages and other obligations due to the Holder. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The Agreement includes a mandatory prepayment that upon the closing of the Business Combination under the Business Combination Agreement, the Company shall repay the note in its entirety to the Holder in an amount equal to the mandatory prepayment Amount.
The Company reviewed the contingent early repayment option granted in the Bridge Note under ASC 815 and concluded that as a result of the significant discount granted in the note, the contingent mandatory repayment provision is therefore considered an embedded derivative. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Note proceeds between the Bridge Note and the Embedded Early Mandatory Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $208,803 and the residual value of $457,864 was allocated to the principal balance of the note. (see Note 10. Fair Value Measurements for additional disclosure on the derivative).
On October 5, 2023 the Company defaulted on the Bridge Note and accordingly the default provisions were allocated and applied resulting in the triggering of a 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24% resulting in default interest expense of $383,790.
An Exchange Agreement (this “Agreement”) was dated as of November 21, 2023, between Digital Health Acquisition Corp., a Delaware corporation (“DHAC”), VSee Lab, Inc., a Delaware corporation (“VSee”) and iDoc Virtual Telehealth Solutions, Inc., a
F-19
Texas corporation (“iDoc”, and together with DHAC and VSee, each a “Company” and collectively, the “Companies”) and the Holder.
The Holder beneficially owns and holds (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744, including interest.
The Holder has agreed to purchase from VSee and iDoc, their respective shares of common stock, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the VSee Note and the principal amount (excluding the original issue discount of $66,667) of $600,000 of the iDoc Note, effective immediately prior to the consummation of the Business Combination. As of December 31, 2023, the Company has $600,000 recorded as a contingent liability on the consolidated balance sheets.
The Holder, severally and not jointly, desire to, upon consummation of the Business Combination, exchange all amounts currently due and owing (the “Original Notes Amount”) under (i) the DHAC Note, (ii) the VSee Note other than the principal amount of $600,000 thereof, and (iii) the iDoc Note other than the principal amount of $600,000 thereof (the “Exchange”) for senior secured convertible promissory notes with an aggregate principle value of $2,523,744 (such notes, the “Notes” or the “Securities”), in the form of the Exchange Note was assumed by DHAC.
As a result of the Exchange Agreement, DHAC assumed The Company’s default interest of $383,789. The carrying balance of the Bridge note and the bifurcated derivative was offset by the principal amount of $600,000, resulting in a gain on forgiveness of debt of $107,862.
As of December 31, 2023 and 2022, the Bridge note net of unamortized debt discount was $0 and $407,131, respectively. The Company recognized $50,734 of amortized debt discount and $50,731 in interest for a total Bridge note interest expense of $101,465 for the year ended December 31, 2023. The Company recognized $15,934 of amortized debt discount for the year ended December 31, 2022. The Company had $0 and $15,934 in accrued interest as of December 31, 2023 and December 31, 2022, respectively.
On January 12, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $220,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023. On November 21, 2023, the Company entered into various securities purchase agreements (the “Conversion SPAs”) to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. As of December 31, 2023, the promissory note net of unamortized debt discount was $220,000. The Company recognized $20,000 of amortized debt discount and $12,980 in accrued interest for a total interest expense of $32,980 for the year ended December 31, 2023.
F-20
Note 10 Fair Value Measurements
The following table presents fair value information as of December 31, 2023 and 2022 of the Company’s financial liabilities that were accounted for at fair value on a recurring basis and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | | | | | | | | | | | |
| | December 31, 2023 | ||||||||||
| | Carrying | | | | | | | | | | |
|
| value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: | | | | | | | | | | | | |
Bridge Note – Embedded Derivative | | $ | — | | $ | — | | $ | — | | $ | — |
Total | | $ | — | | $ | — | | $ | — | | $ | — |
| | | | | | | | | | | | |
| | December 31, 2022 | ||||||||||
| | Carrying | | | | | | | | | | |
|
| value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: |
| |
|
| |
|
| |
|
| |
|
Bridge Note – Embedded Derivative | | $ | 273,534 | | $ | — | | $ | — | | $ | 273,534 |
Total | | $ | 273,534 | | $ | — | | $ | — | | $ | 273,534 |
Measurement
Bridge Note Embedded Derivative
The Company established the initial fair value for the Bridge Note Embedded Derivative as of October 5, 2022, which was the date the Bridge Note was executed. As a result of the exchange agreement on November 21, 2023, the carrying balance of the Bridge note and related default interest, as well as the carrying balance of the bifurcated derivative, was offset with the fair value of the stock payable. The fair value of the Embedded Derivative was remeasured as of November 21, 2023 and December 31, 2022. As such, the Company used a Probability Weighted Expected Return Method (“PWERM”) that fair values the early termination/repayment features of the debt. The PWERM is a multi-step process in which value is estimated based on the probability-weighted present value of various future outcomes. The PWERM was used to value the Bridge Note Embedded Derivative for the initial periods and subsequent measurement periods.
The Bridge Note Embedded Derivative was classified within Level 3 of the fair value hierarchy at the initial measurement date and as of November 21, 2023 and December 31, 2022 due to the use of unobservable inputs. The key inputs into the simulation model for the Bridge Note Embedded Derivative were as follows at November 21, 2023, December 31, 2022 and October 5, 2022:
| | | | | | | |
|
| November 21, |
| December 31, |
| October 5, |
|
| | 2023 | | 2022 | | 2022 |
|
CCC bond rates |
| — |
| 15.09 | % | 14.09 | % |
Probability of early termination/repayment – BC not completed |
| — |
| 5 | % | 10 | % |
Probability of early termination/repayment – BC completed or PIPE completed |
| — |
| 95 | % | 90 | % |
Probability of completing a business combination by March 31, 2023 |
| — |
| 50 | % | 50 | % |
Probability of completing a business combination by June 30, 2023 |
| — |
| 50 | % | 50 | % |
Implied volatility |
| 0.1 | % | 6 | % | 12 | % |
Risk free rate |
| 5.38 | % | 4.76 | % | 4.01 | % |
The change in the fair value of the Level 3 financial liabilities for the years ended December 31, 2023 and 2022 are summarized as follows:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Bridge Note Embedded Derivative, Beginning Fair Value | | $ | 273,534 | | $ | — |
Fair value at October 5, 2022 (Initial measurement) | |
| — | |
| 208,803 |
Change in fair value | |
| (92,449) | |
| 64,731 |
Derivative adjustment from the Exchange Agreement (Note 9) | |
| (181,085) | |
| — |
Bridge Note Embedded Derivative, Ending Fair Value | | $ | — | | $ | 273,534 |
F-21
Transfers to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs. There were no transfers to or from the various Levels during the years ended December 31, 2023 and 2022.
Note 11 Subsequent Events
In accordance with ASC 855-10, the Company has analyzed its operations subsequent to December 31, 2023, through the date when the consolidated financial statements were issued and has determined that it does not have any material subsequent events to disclose in these consolidated financial statements.
On February 13, 2024, the Company amended the November 21, 2023, “SPA” with the accredited investor. Under the amended SPA, in connection with the Business Combination, VSee Health will assume the contingent liability. Under the amended SPA, the accredited investor will purchase from VSee Health 300,000 shares of the common stock at $2 per share in exchange for the contingent liability of $600,000.
F-22
VSee Lab, Inc.
UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three Months Ended
March 31, 2024 and 2023
F-23
VSEE LAB, INC.
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023
INDEX TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Condensed Financial Statements | |
Condensed Consolidated Balance Sheets at March 31, 2024 (Unaudited) and December 31, 2023 | F-25 |
F-26 | |
F-27 | |
F-29 | |
Notes to the Unaudited Condensed Consolidated Financial Statements | F-30 |
F-24
VSEE LAB, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
| | (Unaudited) | | | | |
ASSETS |
| | |
| |
|
Current assets |
| |
|
| |
|
Cash and cash equivalents | | $ | 689,280 | | $ | 118,734 |
Accounts receivable, net | |
| 627,395 | |
| 628,480 |
Prepaids and other current assets | |
| 102,325 | |
| 79,920 |
Total current assets | |
| 1,419,000 | |
| 827,134 |
Fixed assets, net | |
| 11,779 | |
| 3,657 |
Total assets | | $ | 1,430,779 | | $ | 830,791 |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
|
| |
|
|
Current liabilities | |
|
| |
|
|
Accounts payable and accrued liabilities | | $ | 1,819,512 | | $ | 1,824,408 |
Deferred revenue | |
| 1,371,527 | |
| 802,524 |
Due to related party | |
| 338,218 | |
| 338,506 |
Due on share purchase | |
| 135,000 | |
| 135,000 |
Contingent liability | |
| 600,000 | |
| 600,000 |
Loan payable, related party, net of discount | |
| 330,000 | |
| 323,000 |
Note payable, net of discount | |
| 220,000 | |
| 220,000 |
Total liabilities | |
| 4,814,257 | |
| 4,243,438 |
Commitments and contingencies (Note 4) | |
|
| |
|
|
Stockholders’ deficit | |
|
| |
|
|
Preferred stock, $0.0001 par value, 1,701,715 shares authorized; Series A: 371,715 shares authorized, issued and outstanding | |
| 37 | |
| 37 |
Series A-1: 1,330,000 shares authorized, and 1,228,492 issued and outstanding | |
| 123 | |
| 123 |
Common stock, $0.0001 par value; 18,000,000 shares authorized 9,998,446 shares issued and outstanding | |
| 1,000 | |
| 1,000 |
Additional paid in capital | |
| 6,026,457 | |
| 6,026,457 |
Accumulated deficit | |
| (9,117,796) | |
| (9,114,985) |
Non-controlling interest | |
| (293,299) | |
| (325,279) |
Total stockholders’ deficit | |
| (3,383,478) | |
| (3,412,647) |
Total liabilities and stockholders’ deficit | | $ | 1,430,779 | | $ | 830,791 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-25
VSEE LAB, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023 (UNAUDITED)
| | | | | | |
| | For the Three Months Ended March 31, | ||||
|
| 2024 |
| 2023 | ||
Revenues | | | | | |
|
Subscription fees | | $ | 1,005,202 | | $ | 1,161,191 |
Professional services and other fees | |
| 327,843 | |
| 259,390 |
Technical engineering Fees | |
| 162,950 | |
| 175,687 |
Total Revenue | |
| 1,495,995 | |
| 1,596,268 |
Cost of goods sold | |
| 386,253 | |
| 575,322 |
Gross margin | |
| 1,109,742 | |
| 1,020,946 |
Operating expenses | |
|
| |
|
|
Compensation and related benefits | |
| 893,577 | |
| 1,335,552 |
General and administrative | |
| 151,348 | |
| 281,253 |
Transaction expenses | |
| 26,338 | |
| 41,286 |
Total operating expenses | |
| 1,071,263 | |
| 1,658,091 |
Net operating profit (loss) | |
| 38,479 | |
| (637,145) |
Other income (expenses): | |
|
| |
|
|
Interest expense | |
| (9,310) | |
| (47,402) |
Other income | |
| — | |
| 19,616 |
Change in fair value on embedded derivative | |
| — | |
| 26,069 |
Total other expenses | |
| (9,310) | |
| (1,717) |
Income (loss) before income taxes | |
| 29,169 | |
| (638,862) |
Income tax benefit | |
| — | |
| 182,843 |
Net income (loss) | |
| 29,169 | |
| (456,019) |
Net income (loss) attributable to non-controlling interest | | $ | 31,980 | | $ | (4,767) |
Net loss attributable to stockholders | | $ | (2,811) | | $ | (451,252) |
Basic and diluted loss per share | | $ | (0.00) | | $ | (0.05) |
Weighted average number of shares outstanding, basic and diluted income | |
| 9,998,446 | |
| 9,998,446 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-26
VSEE LAB, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT (UNUADITED)
FOR THE THREE MONTHS ENDED MARCH 31, 2024
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A Preferred Stock | | Series A-1 Preferred Stock | | Common Stock | | Additional Paid-In | | Accumulated | | Non: controlling | | | | ||||||||||||
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | Capital | | Deficit | | Interest | | Total | |||||||
Balance, December 31, 2023 |
| 371,715 |
| $ | 37 |
| 1,228,492 |
| $ | 123 |
| 9,998,446 |
| $ | 1,000 |
| $ | 6,026,457 |
| $ | (9,114,985) |
| $ | (325,279) |
| $ | (3,412,647) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| (2,811) | |
| — | |
| (2,811) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Non-controlling interest |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| — | |
| 31,980 | |
| 31,980 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, March 31, 2024 |
| 371,715 | | $ | 37 |
| 1,228,492 | | $ | 123 |
| 9,998,446 | | $ | 1,000 | | $ | 6,026,457 | | $ | (9,117,796) | | $ | (293,299) | | $ | (3,383,478) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-27
VSEE LAB, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT (UNUADITED)
FOR THE THREE MONTHS ENDED MARCH 31, 2023
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Series A Preferred Stock | | Series A-1 Preferred Stock | | Common Stock | | Additional Paid-In | | Accumulated | | Non-controlling | | | | ||||||||||||
| | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | Capital | | Deficit | | Interest | | Total | |||||||
Balance, December 31, 2022 |
| 371,715 |
| $ | 37 |
| 1,228,492 |
| $ | 123 |
| 9,998,446 |
| $ | 1,000 |
| $ | 6,026,457 |
| $ | (5,666,895) |
| $ | (362,755) |
| $ | (2,033) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| (451,252) | |
| — | |
| (451,252) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Non-controlling interest |
| — | |
| — |
| — | |
| — |
| — | |
| — | |
| — | |
| — | |
| (4,767) | |
| (4,767) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, March 31, 2023 |
| 371,715 | | $ | 37 |
| 1,228,492 | | $ | 123 |
| 9,998,446 | | $ | 1,000 | | $ | 6,026,457 | | $ | (6,118,147) | | $ | (367,522) | | $ | (458,052) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-28
VSEE LAB, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023 (UNAUDITED)
| | | | | | |
| | For the Three Months Ended March 31, | ||||
| | 2024 | | 2023 | ||
Net income (loss) | | $ | 29,169 | | $ | (456,019) |
Adjustments to reconcile net income (loss) to net cash used by operating activities: |
|
|
|
|
|
|
Amortization of discount on note payable | |
| 7,000 | |
| 25,386 |
Change in fair value on embedded derivative | |
| — | |
| (26,069) |
Provision for bad debt | |
| 9,201 | |
| 18,308 |
Depreciation expense | |
| 618 | |
| 47 |
Changes in working capital requirements: | |
|
| |
|
|
Accounts receivable | |
| (8,116) | |
| (92,933) |
Prepaids and other current assets | |
| (22,405) | |
| 16,150 |
Deferred tax asset | |
| — | |
| (182,841) |
Accounts payable and accrued liabilities | |
| (4,896) | |
| 408,549 |
Deferred revenue | |
| 569,003 | |
| (129,418) |
Due to related party | |
| (288) | |
| 65,524 |
Net cash from operating activities | |
| 579,286 | |
| (353,316) |
| | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | |
|
| |
|
|
Purchase of fixed assets | |
| (8,740) | |
| (1,690) |
Net cash from financing activities | |
| (8,740) | |
| (1,690) |
| | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | |
|
| |
|
|
Proceeds from note payable | |
| — | |
| 200,000 |
Proceeds from loan payable, related party | |
| — | |
| 120,000 |
Net cash from financing activities | |
| — | |
| 320,000 |
| | | | | | |
NET CHANGE IN CASH AND CASH EQUIVALENTS | |
| 570,546 | |
| (35,006) |
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | |
| 118,734 | |
| 230,664 |
CASH AND CASH EQUIVALENTS, END OF PERIOD | | $ | 689,280 | | $ | 195,658 |
| | | | | | |
Supplemental disclosure of cash flow information | |
|
| |
|
|
Cash paid for interest expense | | $ | — | | $ | — |
Cash paid for income taxes | | $ | — | | $ | — |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-29
VSEE LAB, INC.
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Note 1Organization and Description of Business
VSee Lab, Inc. was incorporated on December 23, 2010 under the laws of the State of Delaware. ThisAmericanDoc, Inc. (“TAD”), a majority owned subsidiary, was incorporated on December 27, 2016, in the State of Delaware. VSee Lab, Inc. and TAD (collectively, the “Company”, “VSee”) are one of the leading providers of virtual healthcare platform services with a focus on high quality, lower costs, and improved outcomes around the world with its integrated platform. The Company is committed to creating a telemedicine experience that’s as simple and accessible as shopping online by providing an advanced, no code, low code telemedicine platform to integrate seamlessly across channels, other existing platforms, and healthcare devices for any virtual care delivery model. The Company is a health services technology company that is responding to the need for rapid, effective system integration within healthcare. The Company operates as a single operating and reportable segment.
Note 2Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying condensed consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Certain information or footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted, pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial reporting. Accordingly, they do not include all the information and footnotes necessary for a complete presentation of financial position, results of operations, or cash flows. In the opinion of management, the accompanying unaudited condensed consolidated financial statements include all adjustments, consisting of a normal recurring nature, which are necessary for a fair presentation of the financial position, operating results and cash flows for the periods presented.
The condensed consolidated financial statements include the accounts of VSee Lab, Inc. and its 53.8% partially owned subsidiary, TAD. The accompanying condensed consolidated financial statements reflect adjustments (including normal, recurring adjustments) necessary to present fairly the financial position of the Company as of March 31, 2024, its results of operations, changes in stockholders’ deficit, and statements of cash flows for the three months ended March 31, 2024 and 2023, in conformity with U.S. GAAP.
Use of Estimates
The preparation of the Company’s condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the condensed consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, allowance for doubtful accounts, and income taxes.
The Company bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
F-30
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits as a component of general and administrative expenses. The Company’s federal tax return and any state tax returns are not currently under examination.
The Company has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services.
The Company determines revenue recognition in accordance with ASC 606, through the following five steps:
1) Identify the contract with a customer
The Company considers the terms and conditions of its contracts and the Company’s customary business practices in identifying its contracts under ASC 606. The Company determines it has a contract with a customer when the contract has been approved by both parties, it can identify each party’s rights regarding the services to be transferred and the payment terms for the services, it has determined the customer to have the ability and intent to pay, and the contract has commercial substance. The Company applies judgment in determining the customer’s ability and intent to pay, which is based on a variety of factors, including the customer’s payment history or, in the case of a new customer, credit and financial information pertaining to the customer.
Contractual terms for subscription services are typically 12 months. Contracts are cancellable with a 30-day notice period and customers are billed in annual, quarterly, or monthly installments in advance of the service period of the subscription. The Company is not required to refund any prorated prepayment fees invoiced to cover services that were provided.
2) Identify the performance obligations in the contract
Performance obligations promised in a contract are identified based on the services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the service either on its own or together with other resources that are readily available, and are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. The Company’s contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that they have any material outstanding commitments for future revenues beyond one year from the end of a reporting period.
The Company treats each subscription to a specific module as a distinct performance obligation because each module is capable of being distinct as the customer can benefit from the subscription to each module on its own and each subscription can be sold standalone.
Furthermore, the subscriptions to individual modules are distinct in the context of the contract as (1) the Company is not integrating the services with other services promised in the contract into a bundle of services that represent a combined output, (2) the subscriptions to specific modules do not significantly modify or customize the subscription to another module, and (3) the specific modules are not highly interdependent or highly interrelated. The subscription to each module is treated as a series of distinct performance obligations because it is distinct and substantially the same, satisfied over time, and has the same measure of progress.
F-31
3) Determine the transaction price
The transaction price is determined based on the consideration the Company expects to be entitled to in exchange for transferring services to the customer. Under the contracts, the clients pay a fixed rate per user per subscription service. Prior to the start of a contract, clients generally make upfront nonrefundable payments to the Company when contracting for implementation services.
The contract pricing is fixed and stated in the arrangements based on the work to be performed by the Company and represents the amount the Company is entitled to for delivering such goods and services. The quoted fees per promise and performance obligation are based on the most likely amount method for what the Company expects to collect.
4) Allocate the transaction price to performance obligations in the contract
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative stand-alone selling price (“SSP”). The determination of a SSP for each distinct performance obligation requires judgment. The Company believes the quoted transaction prices in the customer contracts represent the stand alone selling prices for each of the separate performance obligations that are distinct and priced separately within the contract.
5) Recognize revenue when or as the Company satisfies a performance obligation
Revenue is recognized when or as control of the promised goods or service is transferred to the customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. Subscriptions represent a series of distinct goods or services because the performance obligations are satisfied over time as customers simultaneously receive and consume the benefits related to the services as the Company performs. In the case of module specific subscriptions, a consistent level of service is provided during each monthly period of subscription to the Company’s platform. The Company commences revenue recognition when the customer is provided with platform subscription for the initial monthly period and revenue is recognized over time as a consistent level of subscription service during the subsequent period is delivered. The Company’s obligation for its integrated subscriptions is to stand-ready throughout the subscription period; therefore, the Company considers an output method of time to measure progress towards satisfaction of its obligations with revenue commencing upon the beginning of the subscription period.
Upfront nonrefundable fees do not result in the transfer of a promised good or service to the client, therefore, the Company defers this revenue and recognizes it over the subscription period of the customer contract. Deferred revenue consists of the unamortized balance of nonrefundable upfront fees which are classified as current and non-current based on the timing of when the Company expects to recognize revenue.
Cost of Revenue
Cost of revenue consists primarily of expenses related to cloud hosting, personnel-related expenses for the Company’s customer success team, costs for third-party software services and contractors, and other services used in connection with delivery and support of the Company’s platform subscription services.
Going Concern
The accompanying condensed consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses since inception and historically has had negative operating cash flows. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The continuation of the Company’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These condensed consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should the Company be unable to continue as a going concern.
F-32
Transaction Expenses
On June 15, 2022, the Company entered into a business combination agreement with Digital Health Acquisition Company (“DHAC”), a Delaware blank check company established for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business transaction with one or more businesses or entities, referred to as “targets.” On October 6, 2022, the business combination agreement was amended, and the Special Purchase Acquisition Company public offering transaction is ongoing. During the three months ended March 31, 2024 and 2023, the Company incurred transaction expenses related to the business combination of $26,338 and $41,286, respectively, for professional fees, including legal, taxation, business consulting, and audit services.
Net Income (Loss) Per Common Share
The Company computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants. No potential dilutive common shares are included in the computation of any diluted per share amount when a loss is reported. The Company’s potential dilutive shares, which include preferred shares convertible into common stock have not been included in the computation of diluted net loss per share for the quarters ended March 31, 2024 and 2023 as the result would be anti-dilutive. The total shares excluded from the calculation total 3,894,996.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates their fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
The Company carries its accounts receivable at net realizable value. The Company maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. As of March 31, 2024 and December 31, 2023, respectively, the allowance for doubtful accounts was $32,457. As of March 31, 2024 and 2023, the Company recognized $9,201 and $18,308 of bad debt expenses.
Prepaid Assets
Prepaid assets are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the condensed consolidated statements of operations.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
F-33
Level 3: Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable and accrued liabilities as reflected in the condensed consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the condensed consolidated financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the condensed consolidated statements of operations. Derivative assets and liabilities are classified in the condensed consolidated balance sheets as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the condensed consolidated balance sheet date. FASB ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components.
Fixed Assets
Fixed Assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets. During the three months ended March 31, 2024, the Company purchased office and medical equipment, which is being depreciated over a three-year useful life.
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures” (“ASU 2023-09”), which will require the Company to disclose specified additional information in its income tax rate reconciliation and provide additional information for reconciling items that meet a quantitative threshold. ASU 2023-09 will also require the Company to disaggregate its income taxes paid disclosure by federal, state and foreign taxes, with further disaggregation required for significant individual jurisdictions. ASU 2023-09 will become effective for annual periods beginning after December 15, 2024. The Company is still reviewing the impact of ASU 2023-09. Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s condensed consolidated financial statements.
Note 3Equity
Preferred Stock
The Company has two outstanding series of redeemable preferred stock. The Company has 1,701,715 shares of preferred stock authorized with a par value of $0.0001. The Company has allocated 371,715 shares for Series A preferred, and 1,330,000 shares for Series A-1 preferred.
F-34
Series A Preferred Stock
The Series A Preferred has the following rights and privileges:
Voting — Series A preferred stockholders are permitted to vote with the same voting rights as common stockholders in any actions to be taken by the stockholders of the Corporation, including any action with respect to the election of directors to the Board of Directors of the Corporation.
Dividend — Series A preferred stockholders shall be entitled to receive non-cumulative dividends when and if declared at the annual rate of $0.0484 per share for Series A preferred stock, payable quarterly. Dividends are prior and in preference to any declaration or payment of any dividend to the common stockholders of the Company.
Liquidation — In the event of any liquidation, dissolution or winding-up of the Company, the Series A preferred stockholders are entitled to distributions equal to their initial purchase price per share plus accrued and unpaid dividends (all amounts are prior and in preference to any distribution of any assets to the holders of the other series of preferred stock and common stock).
Conversion — Series A preferred stock is convertible into common stock at the option of the holder, at the rate of $0.6053 per share, except for mandatory conversion upon the earlier of an initial public offering or the date specified by written consent or agreement with majority stockholders. Upon conversion, all shares of Series A preferred stock would be converted automatically into a number of shares of common stock as determined by multiplying the number of shares of Series A preferred stock to be so converted by the Series A Original Issue Price and dividing the result by the applicable conversion price of $0.6053 per share.
Series A-1 Preferred Stock
The Series A-1 Preferred has the following rights and privileges:
Voting — Series A-1 preferred stockholders are permitted to vote with the same voting rights as common stockholders in any actions to be taken by the stockholders of the Corporation, including any action with respect to the election of directors to the Board of Directors of the Corporation.
Dividend — Series A preferred stockholders shall be entitled to receive non-cumulative dividends if and when declared at the annual rate of $0.239 per share for Series A-1 preferred stock, payable quarterly. Dividends are prior and in preference to any declaration or payment of any dividend to the common stockholders of the Company.
Liquidation — In the event of any liquidation, dissolution or winding-up of the Company, the Series A-1 preferred stockholders are entitled to distributions equal to their initial purchase price per share plus accrued and unpaid dividends (all amounts are prior and in preference to any distribution of any assets to the holders of the other series of preferred stock and common stock).
Conversion — Series A-1 preferred stock is convertible into common stock at the option of the holder, at the rate of $2.9874 per share, except for mandatory conversion upon the earlier of an initial public offering or the date specified by written consent or agreement of the majority stockholders. Upon conversion, all shares of Series A-1 preferred stock would be converted automatically into a number of shares of common stock as determined by multiplying the number of shares of Series A-1 preferred Stock to be so converted by the Series A-1 Original Issue Price and dividing the result by the applicable conversion price of $2.9874 per share.
Note 4Commitments Contingencies and Concentration Risk
Contingencies
During the normal course of business, the Company may be exposed to litigation. When the Company becomes aware of potential litigation, it evaluates the merits of the case in accordance with ASC 450, Contingencies. Litigation and contingency accruals are based on the Company’s assessment, including advice of legal counsel, regarding the expected outcome of litigation or other dispute resolution proceedings. If the Company determines that an unfavorable outcome is probable and can be reasonably assessed, it establishes the necessary accruals.
F-35
The Company has a reseller agreement with a vendor to generate revenue opportunities in the international market. As of March 31, 2024 and December 31, 2023, the Company has an unpaid commitment of $382,765 and $410,233, respectively, on this contract. The commitment is not reflected in the condensed consolidated financial statements as the commitment is due and payable once revenues are generated under the reseller agreement. The Company entered into the reseller agreement to generate market share in the international market, and payments are based on revenues generated by the reseller.
On November 21, 2023, the Company entered into a Securities Purchase Agreement (“SPA”) with an accredited investor to purchase 300,000 shares of common stock from the Company at $2 per share in exchange for the principal amount (excluding the original issue discount of $66,667) of $600,000 of the Company’s Bridge Note, effective immediately prior to the consummation of the Business Combination. The SPA was entered into concurrently with an Exchange Agreement on November 21, 2023. Refer to Note 9.
On February 13, 2024, the Company amended the November 21, 2023, “SPA” with the accredited investor. Under the amended SPA, in connection with the Business Combination, VSee Health will assume the contingent liability. Under the amended SPA, the accredited investor will purchase from VSee Health 300,000 shares of the common stock at $2 per share in exchange for the contingent liability of $600,000.
Indemnities
The Company generally indemnifies its customers for the services it provides under its contracts, as well as other specified liabilities, which may subject the Company to indemnity claims, liabilities, and related litigation. As of March 31, 2024 and December 31, 2023, the Company was not aware of any material asserted or unasserted claims in connection with these indemnity obligations.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to credit risk concentrations consist of cash and cash equivalents and accounts receivables. The Company maintains all of its cash and cash equivalents in commercial depository accounts, insured by the Federal Deposit Insurance Corporation (“FDIC”). At times, cash deposits may exceed federally insured limits.
Major Customer Concentration
The Company has five customers whose accounts receivable represented 81% and 76% of the Company’s total accounts receivable as of March 31, 2024 and December 31, 2023, respectively.
The Company has one customer whose revenue accounted for approximately 13% of the Company’s total revenue for the three months ended March 31, 2024. The Company has two customers whose revenue accounted for approximately 21% of the Company’s total revenue for the three months ended March 31, 2023.
Note 5Fixed Assets
The components of fixed assets are summarized below:
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Office equipment | | $ | 5,932 | | $ | 3,335 |
Medical equipment | |
| 7,143 | |
| 1,000 |
| |
| 13,075 | |
| 4,335 |
Less accumulated depreciation | |
| (1,296) | |
| (678) |
Fixed Assets, net | | $ | 11,779 | | $ | 3,657 |
During the three months ended March 31, 2024, the Company purchased office and medical equipment with a useful life of 3 years. Depreciation expense totaling $618 and $47 was recorded during the three months ended March 31, 2024 and 2023, respectively.
F-36
Note 6Related Party
During the year ended December 31, 2022, a related party provided $127,710 of cash, which will be used for future operating expenses. During the three months ended March 31, 2024 and the year ended December 31, 2023, a related party paid $0 and $192,184 of the Company’s operating expenses, respectively. The balance due to the related party as of March 31, 2024 and December 31, 2023 was $338,218 and $338,506, respectively. The above amounts and transactions are not necessarily what third parties would agree to.
During the year ended December 31, 2022, the Company received a loan of $110,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. On March 29, 2023, the Company revised the terms of the loan to a 10.00% original issue discount promissory note with a principal balance of $121,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on June 27, 2023. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of March 31, 2024 and December 31, 2023, the related party promissory note net of unamortized debt discount was $121,000. The Company recognized $0 of amortized debt discount and $0 in accrued interest for the three months ended March 31, 2024. The Company recognized $367 of amortized debt discount and $121 in accrued interest for a total interest expense of $488 for the three months ended March 31, 2023. The Company had $17,930 in accrued interest as of March 31, 2024 and December 31, 2023, respectively, which is included within accounts payable and accrued liabilities on the condensed consolidated balance sheets. The above amounts and transactions are not necessarily what third parties would agree to.
On March 29, 2023, the Company received a 10.00% original issue discount promissory note with a principal balance of $132,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and amortized as interest expense over the life of the underlying note payable. The promissory note matured on June 27, 2023. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of March 31, 2024 and December 31, 2023, the related party promissory note net of unamortized debt discount was $132,000. The Company recognized $0 of amortized debt discount and $0 in accrued interest for the three months ended March 31, 2024. The Company recognized $400 of amortized debt discount and $132 in accrued interest for a total interest expense of $532 for the three months ended March 31, 2023. The Company had $21,120 in accrued interest as of March 31, 2024 and December 31, 2023, which is included within accounts payable and accrued liabilities on the condensed consolidated balance sheets. The above amounts and transactions are not necessarily what third parties would agree to.
On December 26, 2023, the Company received a 10.00% original issue discount promissory note with a principal balance of $77,000 from the CEO for advanced cash and paid operating expenses on behalf of the Company. Notes payable issued with a face value higher than the proceeds received are recognized as a debt discount and amortized as interest expense over the life of the underlying note payable. The promissory note matures on March 28, 2024. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. The note has a default interest rate of 26% from the default date. As of March 31, 2024 and December 31, 2023, the related party promissory note net of unamortized debt discount was $77,000 and 70,000, respectively. The Company recognized $7,000 of amortized debt discount and $2,310 in accrued interest for a total interest expense of $9,310 for the three months ended March 31, 2024. The Company had $9,310 and $0 in accrued interest as of March 31, 2024 and December 31, 2023, which is included within accounts payable and accrued liabilities on the condensed consolidated balance sheets. The above amounts and transactions are not necessarily what third parties would agree to.
Note 7Simple Agreement for Future Equity
On August 1, 2023, the Company entered into a Simple Agreement for Future Equity (“SAFE”) with a purchase price of $135,000. The SAFE is considered a mandatorily redeemable financial instrument under ASC 480-10-15-8. Per section 1 (a) of the SAFE “If there is an Equity Financing before the termination of this Safe, on the initial closing of such Equity Financing, this Safe will automatically convert into the greater of: (1) the number of shares of Standard Preferred Stock equal to the Purchase Amount divided by the lowest price per share of the Standard Preferred Stock; or (2) the number of shares of Safe Preferred Stock equal to the Purchase Amount divided by the Safe Price”. The fixed monetary amount known at inception (i.e., “Purchase Amount” of $135,000) embodies an obligation that the issuer must or may settle by issuing a variable number of shares, based on the safe price which is defined as “Safe Price” means the price per share equal to the Post-Money Valuation Cap divided by the Company Capitalization.” Since the capitalization can change through the termination events, the shares to be issued can vary. The SAFE may require the issuer
F-37
to redeem the instrument for cash upon a change of control. The SAFE is classified and recorded as a liability under ASC 480-10-25-8 because a change of control is an event that is considered not under the sole control of the issuer.
Note 8Income Taxes
The components of income tax expense for the three months ended March 31 were as follows:
| | | | | | |
|
| March 31, |
| March 31, | ||
| | 2024 | | 2023 | ||
Income (loss) before taxes | | $ | 29,169 | | $ | (456,019) |
Expected United States income tax (expense) benefit at a statutory rate of 21% |
| $ | (6,125.49) | | $ | 137,171 |
Expected State income tax (expense) benefit at a statutory rate of 0% and 8.84% at March 31, 2024 and 2023, respectively | |
| — | |
| 45,672 |
Valuation allowance | |
| 6,125.49 | |
| — |
Total income tax benefit | | $ | — | | $ | 182,843 |
For the three months ended March 31, 2024, the Company recorded income tax benefit of $0 for continuing operations. The effective tax rate of 0% for the three months ended March 31, 2024 varied from the statutory United States federal income tax rate of 21.0% primarily due to the effect of state income taxes, net of the federal benefit, and adjustments for meals and entertainment and changes in valuation allowance. The Company recognizes income tax benefits from uncertain tax positions where the realization of the ultimate benefit is uncertain. As of March 31, 2024, the Company has no unrecognized income tax benefits.
Note 9Note Payable
The following is a summary of notes payable as of March 31, 2024 and December 31, 2023:
| | | | | | |
|
| March 31, |
| December 31, | ||
Notes Payable | | 2024 | | 2023 | ||
Note payable issued January 12, 2023 (Face Value: $220,000) | | $ | 220,000 | | $ | 220,000 |
Total note payable and line of credit | | $ | 220,000 | | $ | 220,000 |
As of March 31, 2024, the Company had no required principal payments on its notes payable.
Notes Payable
On October 6, 2022, in connection with the execution of a Business Combination Agreement, the Company entered into a securities purchase agreement (“The Agreement”) with an accredited investor (“Holder”), issued and sold to such investor a 10.00% original issue discount senior secured promissory notes due October 5, 2023, in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). $666,667 of the Bridge Note was allocated to the Company. The Company received cash proceeds of $600,000 from the note.
Concurrently with the execution of the Business Combination Agreement, on October 6, 2022, Digital Health Acquisition Company (‘DHAC”) entered into an Amended and Restated Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with certain investors (the “PIPE Investors”). If the PIPE Financing closes in connection with the closing of the Business Combination, 110% if paid after 90 days of the original issue date, 100% if before 90 days under the Bridge Notes and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Bridge Note has a mandatory default payment of 125% of the sum of the outstanding principal and all accrued interest unpaid and all other amounts, costs, fees (including Late Fees), expenses, indemnification and liquidated and other damages and other obligations due to the Holder. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The Agreement includes a mandatory prepayment that upon the closing of the Business Combination under the Business Combination Agreement, the Company shall repay the note in its entirety to the Holder in an amount equal to the mandatory prepayment Amount.
The Company reviewed the contingent early repayment option granted in the Bridge Note under ASC 815 and concluded that as a result of the significant discount granted in the note, the contingent mandatory repayment provision is therefore considered an
F-38
embedded derivative. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Note proceeds between the Bridge Note and the Embedded Early Mandatory Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $208,803 and the residual value of $457,864 was allocated to the principal balance of the note. (see Note 10. Fair Value Measurements for additional disclosure on the derivative).
On October 5, 2023 the Company defaulted on the Bridge Note and accordingly the default provisions were allocated and applied resulting in the triggering of a 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24% resulting in default interest expense of $383,790 during the year ended December 31, 2023.
An Exchange Agreement (this “Agreement”) was dated as of November 21, 2023, between Digital Health Acquisition Corp., a Delaware corporation (“DHAC”), VSee Lab, Inc., a Delaware corporation (“VSee”) and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”, and together with DHAC and VSee, each a “Company” and collectively, the “Companies”) and the Holder.
The Holder beneficially owns and holds (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744, including interest.
The Holder has agreed to purchase from VSee and iDoc, their respective shares of common stock, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the VSee Note and the principal amount (excluding the original issue discount of $66,667) of $600,000 of the iDoc Note, effective immediately prior to the consummation of the Business Combination. As of March 31, 2024 and December 31, 2023, the Company has $600,000 recorded as a contingent liability on the condensed consolidated balance sheets.
The Holder, severally and not jointly, desire to, upon consummation of the Business Combination, exchange all amounts currently due and owing (the “Original Notes Amount”) under (i) the DHAC Note, (ii) the VSee Note other than the principal amount of $600,000 thereof, and (iii) the iDoc Note other than the principal amount of $600,000 thereof (the “Exchange”) for senior secured convertible promissory notes with an aggregate principle value of $2,523,744 (such notes, the “Notes” or the “Securities”), in the form of the Exchange Note was assumed by DHAC.
As a result of the Exchange Agreement, DHAC assumed The Company’s default interest of $383,789. The carrying balance of the Bridge note and the bifurcated derivative was offset by the principal amount of $600,000, resulting in a gain on forgiveness of debt of $107,862 during the year ended December 31, 2023.
As of March 31, 2024 and December 31, 2023, the Bridge note net of unamortized debt discount was $0. No amortized debt discount and interest were recognized during the three months ended March 31, 2024. The Company recognized $16,484 of amortized debt discount and $16,484 in accrued interest for a total Bridge note interest expense of $32,968 for the three months ended March 31, 2023. The Company had $0 in accrued interest as of March 31, 2024 and December 31, 2023.
On January 12, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $220,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023. On November 21, 2023, the Company entered into various securities purchase agreements (the “Conversion SPAs”) to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $220,000. No amortized debt discount and interest were recognized on the loan during the three months ended March 31, 2024. The Company recognized $8,136 of amortized debt discount and $5,280 in accrued interest for a total Bridge note interest expense of $13,416 for the three months ended March 31, 2023. As of March 31, 2024 and December 31, 2023, the Company had $12,980 in accrued interest, which is included within accounts payable and accrued liabilities on the condensed consolidated balance sheets.
F-39
Note 10Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the condensed consolidated balance sheet date up to the date that the unaudited condensed consolidated financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the unaudited condensed consolidated financial statements.
On April 17, 2024, the Company, DHAC and iDoc entered into a letter agreement with the Bridge Investor which amended the date with respect to the termination or closing of the business combination referenced in the Additional Bridge Notes from March 31, 2024 to June 30, 2024.
On April 17, 2024, the parties to the Third Amended and Restated Business Combination Agreement executed a Second Amendment to the Third Amended and Restated Business Combination Agreement to extend the termination date therein to June 30, 2024.
On April 17, 2024, the parties to the Extension Financing documents executed a letter agreement, which extended the maturity date of the Extension Note to June 30, 2024 On May 1, 2024, the term of DHAC was extended from May 8, 2024 to August 8, 2024.
On June 7, 2024, the DHAC board agreed to the Third Amended and Restated Business Combination agreement to close the business combination transaction on or before June 30, 2024.
F-40
iDoc Virtual Telehealth Solutions, INC.
CONSOLIDATED FINANCIAL STATEMENTS
For the Years Ended December 31, 2023 and 2022
F-41
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Consolidated Financial Statements |
| |
| F-43 | |
| F-44 | |
Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 | | F-45 |
| F-46 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 | | F-47 |
| F-48 |
F-42

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of iDoc Virtual Telehealth Solutions, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of iDoc Virtual Telehealth Solutions, Inc. (the Company) as of December 31, 2023 and 2022, and the related consolidated statements of operations, changes in stockholders’ (deficit) equity, and cash flows for each of the years in the two-year period ended December 31, 2023, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2, the Company has incurred net losses and negative cash flow from operations since inception. These factors, and the need for additional financing in order for the Company to meet its business plans raises substantial doubt about the Company’s ability to continue as a going concern. Our opinion is not modified with respect to that matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.

We have served as the Company’s auditor since 2022.
PCAOB Firm ID# 3289
Tampa, Florida
April 22, 2024

F-43
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONSOLIDATED BALANCE SHEETS
DECEMBER 31, 2023 AND 2022
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
ASSETS |
| |
|
| |
|
Current assets |
| |
|
| |
|
Cash and cash equivalents | | $ | 63,037 | | $ | 147,685 |
Accounts receivable, net | |
| 2,266,302 | |
| 5,107,835 |
Due from related party | |
| 1,008,101 | |
| 678,936 |
Prepaids and other current assets | |
| 123,205 | |
| 100,000 |
Total current assets | |
| 3,460,645 | |
| 6,034,456 |
Note receivable, related party | |
| 245,500 | |
| 336,000 |
Right-of-use asset, net | |
| 1,422,017 | |
| 1,542,249 |
Intangible assets, net | |
| — | |
| 107,076 |
Deferred tax asset | |
| 598,585 | |
| — |
Deposit | |
| 20,720 | |
| — |
Fixed assets, net | |
| 114,044 | |
| 38,706 |
Total assets | | $ | 5,861,511 | | $ | 8,058,487 |
LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | |
|
| |
|
|
Current liabilities | |
|
| |
|
|
Accounts payable | | $ | 391,923 | | $ | 111,630 |
Accrued liabilities | |
| 841,514 | |
| 650,677 |
Deferred revenue | |
| 20,000 | |
| — |
Income taxes payable | |
| — | |
| 22,281 |
Right-of-use liability | |
| 608,695 | |
| 350,962 |
Line of credit | |
| 456,097 | |
| 495,000 |
Factoring payable | |
| 660,578 | |
| — |
Notes payable, net of discount | |
| 1,540,983 | |
| 829,505 |
Due on acquisition purchase | |
| 300,000 | |
| 300,000 |
Contingent liability | |
| 600,000 | |
| — |
Embedded derivative | |
| — | |
| 273,534 |
Loan payable, related party | |
| 200,000 | |
| — |
Total current liabilities | |
| 5,619,790 | |
| 3,033,589 |
Notes payable, less current portion, net of discount | |
| 1,500,600 | |
| 1,808,925 |
Right-of-use liability, less current portion | |
| 990,774 | |
| 1,202,260 |
Deferred tax liability | |
| — | |
| 403,248 |
Total liabilities | |
| 8,111,164 | |
| 6,448,022 |
Commitments and contingencies (Note 11) | |
|
| |
|
|
Stockholders’ (deficit) equity | |
|
| |
|
|
Common stock, $1.00 par value; 5,000 shares authorized 4,978 issued and outstanding | |
| 4,978 | |
| 4,978 |
Additional paid in capital | |
| 209,521 | |
| 209,521 |
Accumulated (deficit) retained earnings | |
| (2,464,152) | |
| 1,395,966 |
Total stockholders’ (deficit) equity | |
| (2,249,653) | |
| 1,610,465 |
Total liabilities and stockholders’ (deficit) equity | | $ | 5,861,511 | | $ | 8,058,487 |
The accompanying notes are an integral part of these audited consolidated financial statements.
F-44
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | |
| | For the Years Ended December 31, | ||||
|
| 2023 |
| 2022 | ||
Revenues |
| |
|
| |
|
Patient Fees | | $ | 3,475,666 | | $ | 5,398,566 |
Telehealth Fees | |
| 2,434,210 | |
| 2,053,497 |
Institutional Fees | |
| 716,314 | |
| 1,057,174 |
Total Revenue | |
| 6,626,190 | |
| 8,509,237 |
Cost of Goods Sold | |
| 2,451,633 | |
| 3,229,891 |
Gross Margin | |
| 4,174,557 | |
| 5,279,346 |
Operating expenses | |
|
| |
|
|
General and administrative | |
| 6,052,031 | |
| 1,824,460 |
Compensation and related benefits | |
| 2,044,822 | |
| 2,688,844 |
Transaction expenses | |
| 358,471 | |
| 587,852 |
Professional fees | |
| 87,886 | |
| 105,996 |
Total operating expenses | |
| 8,543,210 | |
| 5,207,152 |
Net operating (loss) profit | |
| (4,368,653) | |
| 72,194 |
Other income (expenses): | |
|
| |
|
|
Interest expense | |
| (317,048) | |
| (152,626) |
Change in fair value on derivative | |
| 90,200 | |
| (64,731) |
Gain on forgiveness of debt | |
| 107,862 | |
| — |
Impairment charges | |
| (104,076) | |
| — |
Other (expense) income | |
| (338,813) | |
| 135,570 |
Total other expense | |
| (561,875) | |
| (81,787) |
Loss before income tax | |
| (4,930,528) | |
| (9,593) |
Income tax benefit (expense) | |
| 1,070,410 | |
| (8,531) |
Net loss | | $ | (3,860,118) | | $ | (18,124) |
Net loss income per share attributable to common shareholders: | |
|
| |
|
|
Basic | | $ | (775.4) | | $ | (3.6) |
Diluted | | $ | (775.4) | | $ | (3.6) |
Weighted average number of shares outstanding, basic, and diluted | |
| 4,978 | |
| 4,978 |
The accompanying notes are an integral part of these audited consolidated financial statements.
F-45
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | | | | | | | | | | | | |
| | | | | | | Additional | | | | | | | |
| | Common Stock | | Paid In | | (Accumulated Deficit) | | | | |||||
|
| Shares |
| Amount |
| Capital |
| Retained Earnings |
| Total | ||||
Balance December 31, 2021 |
| 4,000 | | $ | 4,000 | | $ | 499 | | $ | 1,414,090 | | $ | 1,418,589 |
Reallocation of existing shares |
| 957 | |
| 957 | |
| (957) | |
| — | |
| — |
Sale of common stock |
| 21 | |
| 21 | |
| 209,979 | |
| — | |
| 210,000 |
Net loss |
| — | |
| — | |
| — | |
| (18,124) | |
| (18,124) |
Balance December 31, 2022 |
| 4,978 | | $ | 4,978 | | $ | 209,521 | | $ | 1,395,966 | | $ | 1,610,465 |
Net loss |
| — | |
| — | |
| — | |
| (3,860,118) | |
| (3,860,118) |
Balance December 31, 2023 |
| 4,978 | | $ | 4,978 | | $ | 209,521 | | $ | (2,464,152) | | $ | (2,249,653) |
The accompanying notes are an integral part of these audited consolidated financial statements.
F-46
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | | | | |
|
| 2023 |
| 2022 | ||
Net loss | | $ | (3,860,118) | | $ | (18,124) |
Adjustments to reconcile net loss to net cash used by operating activities: | |
|
| |
|
|
Amortization of discount on note payable | |
| 51,816 | |
| 19,712 |
Amortization of right of use asset | |
| 212,415 | |
| 93,541 |
Impairment expense | |
| 104,078 | |
| 3,000 |
Depreciation and amortization | |
| 16,396 | |
| 3,776 |
Provision for doubtful accounts | |
| 534,460 | |
| 784,519 |
Change in fair value on embedded derivative | |
| (90,200) | |
| 64,731 |
Gain on forgiveness of debt | |
| (107,862) | |
| — |
Loss on factoring payable | |
| 339,611 | |
| — |
Changes in working capital requirements: | |
|
| |
|
|
Accounts receivable | |
| 2,307,073 | |
| (3,114,354) |
Due from related party | |
| (329,165) | |
| (177,576) |
Prepaid and other current assets | |
| (23,205) | |
| (72,854) |
Deferred tax asset | |
| (598,585) | |
| — |
Accounts payable | |
| 280,293 | |
| 29,180 |
Accrued liabilities | |
| 329,310 | |
| 258,279 |
Deferred revenue | |
| 20,000 | |
| — |
Income taxes payable | |
| (22,281) | |
| (394,719) |
Deferred tax liability | |
| (403,248) | |
| 403,248 |
Net cash from operating activities | |
| (1,239,212) | |
| (2,117,641) |
CASH FLOWS FROM INVESTING ACTIVITIES: | |
|
| |
|
|
Acquisition of business, net cash received | |
| — | |
| 39,313 |
Proceeds on note receivable, related party | |
| 90,500 | |
| 120,000 |
Issuance of note receivable, related party | |
| — | |
| (336,000) |
Deposits | |
| (20,720) | |
| — |
Purchase of fixed assets | |
| (88,734) | |
| (42,483) |
Net cash from investing activities | |
| (18,954) | |
| (219,170) |
CASH FLOWS FROM FINANCING ACTIVITIES: | |
|
| |
|
|
Proceeds from revolving line of credit | |
| — | |
| 70,000 |
Proceeds from factoring payable | |
| 608,916 | |
| — |
Proceeds from notes payable | |
| 894,000 | |
| 2,400,600 |
Proceeds from loan payable, related party | |
| 200,000 | |
| — |
Payments on notes payable | |
| (128,842) | |
| (227,122) |
Repayment on factoring payable | |
| (324,547) | |
| — |
Repayment on leased equipment | |
| (76,009) | |
| (82,568) |
Sale of common stock | |
| — | |
| 210,000 |
Net cash from financing activities | |
| 1,173,518 | |
| 2,370,910 |
CHANGE IN CASH AND CASH EQUIVALENTS | |
| (84,648) | |
| 34,099 |
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | |
| 147,685 | |
| 113,586 |
CASH AND CASH EQUIVALENTS, END OF PERIOD | | $ | 63,037 | | $ | 147,685 |
Supplemental disclosure of cash flow information | |
|
| |
|
|
Cash paid for interest expense | | $ | 86,529 | | $ | 56,365 |
Cash paid for income taxes | | $ | — | | $ | — |
Non-cash investing and financing activities: | |
|
| |
|
|
Right of use asset and liability | | $ | 555,562 | | $ | 1,824,981 |
Stock issued for acquisition | | $ | — | | $ | 300,000 |
The accompanying notes are an integral part of these audited consolidated financial statements.
F-47
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Note 1Organization and Description of Business
iDoc Telehealth Solutions, Inc. was incorporated in the state of Virginia on February 26, 2014. The Company subsequently changed its name to iDoc Virtual Telehealth Solutions, Inc. on September 10, 2018, and incorporated in the state of Texas. Encompass Healthcare Billing, LLC (“Encompass”), a wholly owned subsidiary, was incorporated on December 17, 2014, in the state of Colorado and was acquired by the Company on January 1, 2022 (iDoc Virtual Telehealth Solutions, Inc., and Subsidiary collectively referred to as the “Company,” or “iDoc”). The Company is headquartered in Houston, Texas and is one of the leading providers of tele-intensive acute care and tele-neurocritical care in high-value hospital environments. The Company leverages its extensive telehealth platform and neuro and general critical expertise to treat and monitor acutely ill patients with diseases of the brain, spinal cord, heart, and lungs that often have complicated medical problems. The Company is a virtual health services management company responding to the need for rapid, effective treatment of emergency patients and the shortage of critical care experts. The Company operates as a single operating and reportable segment. Encompass is a nationwide full service medical billing service provider, specializing in intraoperative neuromonitoring services medical billing.
Note 2Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
The consolidated financial statements include the accounts of iDoc Virtual Telehealth Solutions, Inc., and its subsidiary, Encompass Healthcare Billing, LLC, a 100% wholly owned subsidiary of the Company.
The accompanying consolidated financial statements reflect adjustments (including normal, recurring adjustments) necessary to present fairly the financial position of the Company as of December 31, 2023 and 2022, its results of operations, changes in stockholders’ (deficit) equity, and statements of cash flows for the years ended Decembers 31, 2023 and 2022, in conformity with U.S. GAAP.
Use of Estimates
The preparation of the Company’s consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, the valuation of the Company’s common stock, allowance for doubtful accounts, and income taxes.
The Company bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss, and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and
F-48
penalties related to unrecognized tax benefits as a component of general and administrative expenses. The Company’s federal tax return and any state tax returns are not currently under examination.
The Company has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services. The Company recognizes revenue using a five-step model:
1)Identify the contract(s) with a customer;
2)Identify the performance obligation(s) in the contract;
3)Determine the transaction price;
4)Allocate the transaction price to the performance obligations in the contract; and
5)Recognize revenue when (or as) it satisfies a performance obligation.
The Company derives revenue from business services associated with direct tele-physician provider patient fee services, telehealth services, and institutional services provided to our clients.
Patient Fees Services and Performance Obligation
All of the Company’s telemedicine contracts for patient reimbursement fees are directly billed through the Encompass healthcare billing services subsidiary. The Company earns patient fees by providing high acuity patient care solutions. For patient fees, performance obligations are met when the Company’s physicians provide professional medical services to patients at the client site as this is deemed as transfer of goods and services to respective patients. The patient benefits from the professional services when care is rendered by the Company’s medical professionals. The revenue is determined based on the telemedicine billing code(s) associated with the respective professional service rendered to patients. The Company earns primarily from reimbursement from the following third-party payors:
Medicare
The Medicare program offers beneficiaries different ways to obtain medical benefits: (i) Medicare Part A, which covers, among other things, in-patient hospital, SNFs, home healthcare, and certain other types of healthcare services; (ii) Medicare Part B, which covers physicians’ services, outpatient services, durable medical equipment, and certain other types of items and healthcare services; (iii) Medicare Part C, also known as Medicare Advantage, which is a managed care option for beneficiaries who are entitled to Medicare Part A and enrolled in Medicare Part B; and (iv) Medicare Part D, which provides coverage for prescription drugs that are not otherwise covered under Medicare Part A or Part B for those beneficiaries that enroll.
The Company’s affiliated provider network is reimbursed by the Part B and Part C programs for certain of the telemedicine services it provides to Medicare beneficiaries. Medicare coverage for telemedicine services is treated distinctly from other types of professional medical services and is limited by federal statute and subject to specific conditions of participation and payment pursuant to Medicare regulations, policies and guidelines, including the location of the patient, the type of service, and the modality for delivering the telemedicine service, among others.
F-49
Medicaid
Medicaid programs are funded jointly by the federal government and the states and are administered by states (or the state’s designated managed care or other similar organizations) under approved plans. Our affiliated provider network is reimbursed by certain State Medicaid programs for certain of the telemedicine services it provides to Medicaid beneficiaries. Medicaid coverage for telemedicine services varies by state and is subject to specific conditions of participation and payment.
Commercial Insurance Providers
The Company is reimbursed by commercial insurance carriers. The basis for payment to the commercial insurance providers is consistent with Medicare reimbursement fee structure guidelines and the Company is in- network or out-of-network with the commercial insurance carriers based on state and insurer requirements.
Telehealth Fees Service Contracts and Performance Obligation
The Company enters into service contracts mainly in the following categories with hospitals or hospital systems, physician practice groups, and other users. The Company’s customer contracts typically range in length from two to three years, with an automatic renewal process. The Company either invoices its customers for the monthly fixed fee in advance or at the end of the month, depending on the terms of the contract. The contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that it has any material outstanding commitment for future revenues beyond one year from the end of a reporting period. Under the contracts, the customers pay a fixed monthly fee for the services described below.
Contract For Telemedicine Care Services
Performance obligations in the contract for telemedicine care are based on services provided via the use of hardware and software integration that includes multi-participant video conferencing, and electronic communication for 24 hours per day, seven days per week for the duration of the contract. The Company provides administrative support for the tele-physician services and coordinates the services of its clinicians network through administrative support, hardware support, and software support and provider coverage availability. The Company provides coverage availability of its physician services ranging from 12-24h per day. Performance obligations in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from patient services and institutional services obligations. Performance obligations are met when the Company provides administrative, business and medical records and reports related to their professional services rendered pursuant to the agreement in such format and upon such interval as hospitals may require. Revenue from telemedicine care services is included in telehealth fees in the consolidated financial statements. The Company commences revenue recognition when the Company satisfies its performance obligation to provide the contractual tele-physician hours services monthly. Prior to the commencement of services, customers generally make initial start-up nonrefundable payments to the Company when contracting for Company training, hardware and software installation and integration, which includes a one-time setup of software security, API interfaces, and compatibility between hospital existing equipment and hardware and software. The Company recognizes revenue upon completion of the implementation when the performance obligation of equipment setup and initial training is completed. The start-up fees do not significantly modify or customize the other goods in the contract. As the start-up service primarily covers initial administrative services for which the Company’s clients can cancel future services upon completion, management considers it to be separable from the ongoing business services, and the Company records start-up fees as one-time revenue when the start-up service is complete.
Institutional Fees Service Contracts and Performance Obligation
Contract For Electroencephalogram (“EEG”) Professional Interpretation Services
Performance obligations in the contract for EEG professional interpretation services are based on the number of professional services EEG interpretation provides monthly. The performance obligation in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. To facilitate the delivery of the EEG professional interpretation services, the Company’s physicians use EEG telemedicine equipment provided by the Company. The performance obligation is satisfied based on the number of EEG professional interpretations performed by the Company’s physicians. The number of professional interpretations is traced monthly by both parties and used to determine the revenue earned based on established contractual rates and are included in institutional fees in the consolidated financial statements. The Company commences revenue recognition on EEG professional interpretation services when the Company satisfies its performance obligation to provide professional interpretation monthly.
F-50
Encompass Healthcare Client Billing Services
The Company enters into contracts with hospitals, physician practice groups, and other users for billing services. Medical billing service fees include amounts charged for ongoing billing, clinical-related, and other related services and are generally billed to the customer as a percentage of total collections. The Company does not recognize revenue for business service fees until these collections are made, as the service fees are not fixed and determinable until such time. Medical billing service fees also include amounts charged to customers for generating and mailing patient statements and are recognized as the related services are performed. The Company’s clients typically purchase one-year contracts that renew automatically upon completion. In most cases, the clients may terminate their agreements with 90 days notice without cause. The Company typically retains the right to terminate client agreements in a similar timeframe. The Company’s clients are billed monthly, in arrears, based either upon a percentage of collections, minimum fees, flat fees, or per-claim fees where applicable. Invoices are generated within the first two weeks of the subsequent month and delivered to clients primarily by email.
Determination of Pricing for Services
The Company believes the quoted transaction prices in the customer contracts represent the stand- alone selling prices for each of the separate performance obligations which are distinct and priced separately within the contract. The transaction price for each service provided is independent and established in the contract and based on the duration of service provided or for a rate for service provided. Fees are established based on the service transferred to the client.
Telehealth and Institutional Services Contracts
Under most of the Company’s contracts, including contracts with its two top customers, the customer pays fixed monthly fees for telemedicine consultation services, EEG professional interpretation services, platform software services, and hardware fees. The fixed monthly fee provides for a predetermined number of daily, monthly, or annual physician hours of coverage and agreed upon rates for interpretation and software services. To facilitate the delivery of the consultation services, the facilities use telemedicine equipment and the Company’s virtual health care platform, which is provided and installed by the Company. The Company also provides the hospitals with user training, maintenance and support services for the telemedicine equipment used to perform the consultation services. The Company recognizes revenue for variable consideration when it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. The Company estimates the amount of revenue to be recognized on variable consideration, using the expected value or the most likely amount method, whichever is expected to better predict the amount. The Company’s estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on assessments of legal enforceability, performance, and all information that is reasonably available to the Company. The determination of the amount of revenue the Company can recognize each accounting period requires management to make estimates and judgments on the estimated expected customer life or expected performance period, of at least 3 years.
Patient Fee Contracts Involving Third-Party Payors
The Company receives payments from patients, third-party payers and others for patient fee services. Third-party payers pay the Company based on contracted rates or the entities’ billed charges. Payments received from third-party payers are generally less than billed charges. The Company receives less than its total established charges for its services. The Company determines the transaction price on patient fees based on standard charges for services provided, reduced by adjustments provided to third-party payors, and implicit price concessions provided to uninsured patients. The Company monitors its revenue and receivables from third-party payers and records an estimated contractual allowance to properly account for the differences between billed and reimbursed amounts.
Revenue from third-party payers is presented net of an estimated provision for contractual adjustments. Patient revenues are net of service credits and service adjustments, and allowance for doubtful accounts receivable. These adjustments and implicit price concessions represent the difference between the amount billed and the estimated consideration the Company expects to receive, based on historical collection experience, market conditions and other factors. Although the Company believes that its approach to estimates and judgments as described herein is reasonable, actual results could differ and the Company may be exposed to increases or decreases in revenue that could be material.
F-51
Cost of Revenue
Cost of revenue consists primarily of expenses related to compensation-related expenses for the Company’s telehealth service providers, costs for third-party software and hardware services and independent medical providers, and other services used in connection with the delivery and support of the Company’s telehealth platform.
Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses since inception and continues to have negative operating cash flows. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The continuation of the Company’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Transaction Expenses
On June 15, 2022, the Company entered into a business combination agreement with Digital Health Acquisition Company (“DHAC”), a Delaware blank check company established for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business transaction with one or more businesses or entities, referred to as “targets.” On October 6, 2022, the business combination agreement was amended, and the Special Purchase Acquisition Company public offering transaction is ongoing. During the years ended December 31, 2023 and 2022, the Company incurred one-time transaction expenses related to the business combination of $358,471 and $587,852, respectively, for professional fees, including legal, taxation, business consulting, and auditing services.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC-insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates its fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
The Company carries its accounts receivable at net realizable value. The Company maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. No credit losses were recognized for the years ended December 31, 2023 and 2022.
The allowance for doubtful accounts is calculated based on a general reserve for at-risk balances considering the Company’s ability to collect as well as the current credit conditions of third-party payers. The allowance for doubtful accounts was $1,576,415 and $1,038,956 as of December 31, 2023 and 2022, respectively.
Prepaid Expenses
Prepaid expenses are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the consolidated statements of operations.
F-52
Leases
The Company accounts for leases under ASU 2016-02, “Leases” (Topic 842). Based on this standard, the Company determines if an agreement is a lease at inception. Operating and finance leases are included in right-of-use asset, current portion of right-of-use liability, and right-of-use liability less current portion in the Company’s consolidated balance sheets. Operating and finance lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date.
As permitted under ASU 2016-02, the Company has made an accounting policy election not to apply the recognition provisions of ASU 2016-02 to short-term leases (leases with a lease term of 12 months or less that do not include an option to purchase the underlying asset that the lessee is reasonably certain to exercise); instead, the Company will recognize the lease payments for short term leases on a straight-line basis over the lease term.
Net Income (Loss) Per Common Share
The Company computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants. No potential dilutive common shares are included in the computation of any diluted per share amount when a loss is reported.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3: Inputs are unobservable inputs that reflect the reporting entity’s assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable, and accrued liabilities as reflected in the consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities, and equity instruments of the Company are either recognized or disclosed in the consolidated financial statements together with other information relevant to making a reasonable assessment of future cash flows, interest rate risk, and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. Derivative assets and liabilities are classified in the consolidated balance sheets as current or non-current based on whether net- cash settlement or conversion of the instrument could be required within 12 months of the consolidated balance sheet date. The Company has determined the early mandatory redemption provision in the Bridge Note as described in Note 8 is an embedded derivative instrument. ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components. The Company applies this guidance to allocate the Bridge Note proceeds
F-53
between the Bridge Note and the Embedded Early Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt.
Fixed Assets
Fixed assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets, which is three to ten years.
Intangible Assets
Intangible assets are presented at fair value, net of amortization. The fair value is determined based on the appraised value of the asset. Amortization is calculated on the straight-line method over the five-year estimated useful lives of the respective assets. Intangible assets comprise of goodwill and a customer list. As of December 31, 2023 and 2022, the fair value of goodwill is $0 and $95,076, respectively, as described in Note 3, Business Acquisition. During the year ended December 31, 2022, the Company acquired a customer list related to the acquisition valued at $15,000. The balance of the customer list is $0 and $12,000 as of December 31, 2023 and 2022, respectively. The Company recognized $3,000 of amortization expenses during the years ended December 31, 2023 and 2022, respectively.
Impairment of Long-lived and Intangible Assets
In accordance with ASC 360-10, the Company, on a regular basis, reviews the carrying amount of long- lived assets for the existence of facts or circumstances, both internally and externally, that suggest impairment. The Company determines if the carrying amount of a long-lived asset is impaired based on anticipated undiscounted cash flows, before interest, from the use of the asset. In the event of impairment, a loss is recognized based on the amount by which the carrying amount exceeds the fair value of the asset. Fair value is determined based on the appraised value of the assets or the anticipated cash flows from the use of the asset, discounted at a rate commensurate with the risk involved. The Company recorded $104,076 and $0 of impairment charges during the years ended December 31, 2023 and 2022, respectively, on its goodwill and customer list intangible assets.
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. This may result in the earlier recognition of allowances for losses. ASU 2016-13 is effective for the Company beginning January 1, 2023, and the adoption did not have a material impact on the consolidated financial statements.
In August 2021, the FASB issued ASU No. 2021-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2021-06”).
ASU 2021-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models will result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP.
Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2021-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2021-06 is effective for the Company beginning January 1, 2024.
F-54
Early adoption is permitted, but no earlier than January 1, 2022. Management is currently evaluating the effect of the adoption of ASU 2021-06 on the consolidated financial statements but currently does not believe ASU 2021-06 will have a significant impact on the Company’s consolidated financial statements.
Note 3Business Acquisition
Encompass Healthcare Billing, LLC
On January 1, 2022, the Company completed the acquisition of 100% of Encompass Healthcare Billing, LLC. (“Encompass”) with a stock purchase agreement to acquire the equity interests of Encompass, pursuant to the acquisition agreement (“Acquisition Agreement”). In accordance with the Acquisition Agreement, the Company acquired all the outstanding shares of Encompass in exchange for 22 shares or $300,000 of the Company’s issued and outstanding shares of common stock.
The Acquisition agreement was amended during the year ended December 31, 2022 to a cash payment of $300,000 in lieu of the Company’s common stock. As of December 31, 2023, the cash payment of $300,000 was due on the Acquisition Agreement.
Encompass is a nationwide full service medical billing service provider, specializing in intraoperative neuromonitoring services (“IONM”) medical billing. IONM medical billing is very complex compared to any other specialty billing. Encompass takes steps proactively to provide the payers with the information required to collect the proper reimbursement and is very successful in appealing the claims and getting the reimbursement on originally denied claims. The Company acquired Encompass to drive operational efficiencies and capture market growth in the IONM medical billing segment.
| | | |
Consideration |
|
| |
Cash payment due | | $ | 300,000 |
Total consideration | | $ | 300,000 |
Fair values of identifiable net assets and liabilities: | |
|
|
Assets | |
|
|
Cash | | $ | 39,313 |
Accounts receivable | |
| 157,954 |
Customer list | |
| 15,000 |
Right-of -use asset | |
| 78,464 |
Total assets | |
| 290,731 |
Liabilities Accounts payable | |
| 7,343 |
Right-of -use liability | |
| 78,464 |
Total liabilities | |
| 85,807 |
Total fair value of identifiable net assets and liabilities | | $ | 204,924 |
Goodwill (consideration given minus fair value of identifiable net assets and liabilities) | | $ | 95,076 |
The Company analyzed the acquisition under applicable guidance and determined that the acquisition should be accounted for as a business combination in accordance with ASC 805, Business Combinations. The acquisition resulted in $95,076 in goodwill which is recorded on the reporting unit’s books (see Note 5). The values assigned to the balance sheet items are based on the independent valuation of the Company’s consideration in the transaction and the total fair value of Encompass’s identifiable net assets and liabilities.
F-55
Note 4Fixed Assets
The components of fixed assets are summarized below:
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
Office equipment | | $ | 28,506 | | $ | 28,506 |
Medical equipment | |
| 89,246 | |
| 13,976 |
Furniture | |
| 6,153 | |
| — |
Leasehold improvements | |
| 7,311 | |
| — |
| |
| 131,216 | |
| 42,482 |
Less accumulated. Depreciation | |
| (17,172) | |
| (3,776) |
Fixed Assets, net | | $ | 114,044 | | $ | 38,706 |
The Company recorded $13,396 and $3,776 in depreciation expense during the years ended December 31, 2023 and 2022, respectively.
Note 5Intangible Assets
At December 31, 2023 and 2022, intangible assets consist of the following:
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
Goodwill (Business Combination – Note 3) | | $ | — | | $ | 95,076 |
Customer list, net (Business Combination – Note 3) | |
| — | |
| 12,000 |
Ending balance | | $ | — | | $ | 107,076 |
During the year ending December 31, 2023, the Company determined these assets were impaired due to the operating losses of the acquired company, Encompass Healthcare Billing, LLC. The Company recognized $95,076 of goodwill impairment expenses during the year ended December 31, 2023. No impairment was identified for the years ended December 31, 2022. The customer list is presented at fair value, net of amortization. Amortization is calculated using the straight-line method over the five-year estimated useful life. During the year ended December 31, 2023, the Company recognized $3,000 of amortization expenses, and $9,000 of impairment expenses. The Company recognized $3,000 of amortization expenses during the year ended December 31, 2022.
Note 6Leases
Operating Leases
The Company leases office space in Boston, Massachusetts (“Massachusetts Lease”), Houston, Texas (“Texas Lease”), Atlanta, Georgia (“Georgia Lease”) and Lakewood, Colorado (“Colorado Lease”). The Company commenced a new Massachusetts lease on September 1, 2023, ending on August 31, 2028. The Texas Lease was renewed on February 1, 2022 and ends on January 31, 2027. The Georgia Lease commenced on May 25, 2021, and ended on June 24, 2022. The Company commenced a new Georgia lease on June 1, 2022, ending on May 31, 2027. The new Georgia leas was terminated on November 30, 2023. The Colorado Lease commenced on April 1, 2020, and ended on March 31, 2023. The monthly lease payments for the Massachusetts Lease are $9,380 between September 1, 2023 and August 31, 2024, $9,630 between September 1, 2024 and August 31, 2025, $9,870 between September 1, 2025 and August 31, 2026, $10,120 between September 1, 2026 and August 31, 2027, and $10,360 between September 1, 2027 and August 31, 2028. The monthly lease payments for the Texas Lease are $10,000, and for the Georgia Lease are $6,000 for the lease commenced on June 1, 2022. The monthly lease payments for the Georgia lease terminated on June 24, 2022 were $4,097. The monthly lease payments for the Colorado Lease are $4,678 between April 1, 2020 and March 31, 2021, $4,851 between April 1, 2021 and March 31, 2022 and $5,024 between April 1, 2022 and March 31, 2023. The Colorado lease was terminated on March 31, 2023.
Operating lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date. The interest rate used to determine the present value is the Company’s incremental borrowing rate, estimated to be 5.00%, as the interest rate implicit in most of its leases is not readily determinable. Operating lease expense is recognized on a straight-line basis over the lease term.
F-56
During the years ended December 31, 2023 and 2022, the Company recorded $243,525 and $256,029 as operating lease expense which is included in general and administrative expenses on the consolidated statements of operations, respectively.
Operating right-of-use assets are summarized below.
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Office Lease | | $ | 1,216,055 | | $ | 1,130,642 |
Less accumulated amortization | |
| (337,743) | |
| (344,514) |
Right-of-use, net | | $ | 878,312 | | $ | 786,128 |
Operating lease liabilities are summarized below:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Office Lease | | $ | 886,602 | | $ | 786,128 |
Less: current portion | |
| (222,325) | |
| (194,834) |
Long term portion | | $ | 664,277 | | $ | 591,294 |
Future minimum rent payments under the operating lease are as follows:
| | | |
|
| Total | |
Year ending December 31, 2024 | | $ | 241,850 |
Year ending December 31, 2025 | |
| 236,520 |
Year ending December 31, 2026 | |
| 239,440 |
Year ending December 31, 2027 | |
| 132,400 |
Year ending December 31, 2028 | |
| 82,880 |
Total future minimum lease payments | |
| 933,090 |
Less imputed interest | |
| (46,488) |
PV of Payments | | $ | 886,602 |
Finance Leases
Commencing during the year ended December 31, 2022, the Company leases office equipment under three finance leases with combined monthly payments of $20,313. The leases mature on June 2026 and August 2026. On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024 (Note 11). Equipment lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date.
Finance right-of-use assets are summarized below:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Equipment Lease | | $ | 849,662 | | $ | 849,662 |
Less accumulated amortization | |
| (305,957) | |
| (93,541) |
Right-of-use, net | | $ | 543,705 | | $ | 756,121 |
Finance lease liabilities are summarized below:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Equipment Lease | | $ | 712,867 | | $ | 767,094 |
Less: current portion | |
| (386,370) | |
| (156,128) |
Long term portion | | $ | 326,497 | | $ | 610,966 |
F-57
Future minimum rent payments under the finance lease are as follows:
| | | |
|
| Total | |
Year ending December 31, 2024 | | $ | 386,370 |
Year ending December 31, 2025 | |
| 243,758 |
Year ending December 31, 2026 | |
| 136,484 |
Total future minimum lease payments | |
| 766,612 |
Less imputed interest | |
| (53,745) |
PV of Payments | | $ | 712,867 |
Expenses incurred with respect to the Company’s finance leases during the years ended December 31, 2023 and 2022 which are included in general and administrative expenses on the consolidated statements of operations are set forth below.
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
Finance lease amortization | | $ | 212,416 | | $ | 93,540 |
Finance lease interest | |
| 47,990 | |
| 24,706 |
Total finance lease expense | | $ | 260,406 | | $ | 118,246 |
The weighted average remaining lease term and the weighted average discount rate on the finance leases at December 31, 2023 and 2022 are set forth below.
| | | | | |
|
| December 31, |
| December 31, |
|
| | 2023 | | 2022 | |
Weighted average remaining lease term | | 2.6 | years | 3.6 | years |
Weighted average discount rate |
| 6.92 | % | 6.92 | % |
Note 7Factoring Payable
On June 21, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $299,000 for a net purchase price of $207,639. Under the agreement, the Company authorized the Purchaser to collect $7,475 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $91,361 and administrative fees associated with processing the transaction of $7,267 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $130,977 on December 31, 2023.
On July 28, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $140,000 for a net purchase price of $100,000. Under the agreement, the Company authorized the Purchaser to collect $5,000 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $40,000 and administrative fees associated with processing the transaction of $1,295 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $52,189 on December 31, 2023.
On October 13, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $186,250 for a net purchase price of $125,000. Under the agreement, the Company authorized the Purchaser to collect $7,760 weekly. The agreement was not collateralized by a general security agreement over all the Company’s accounts, including, without limitation, all deposit accounts, accounts receivable, and other receivables, chattel paper, documents, equipment, general intangibles, instruments, and inventory. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts
F-58
generated by the Company. The Company recognized a loss on the sale of future receipts of $61,250 and administrative fees associated with processing the transaction of $7,546 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $150,866 on December 31, 2023.
On October 13, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $108,000 for a net purchase price of $75,000. Under the agreement, the Company authorized the Purchaser to collect $3,484 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $33,000 and administrative fees associated with processing the transaction of $1,740 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $97,548 on December 31, 2023.
On November 8, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $75,000 for a net purchase price of $111,000. Under the agreement, the Company authorized the Purchaser to collect $6,937 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $36,000 and an administrative fee associated with processing the transaction of $3,750 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $92,125 on December 31, 2023.
On December 20, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $228,000 for a net purchase price of $150,000. Under the agreement, the Company authorized the Purchaser to collect $10,364 weekly. The agreement is collateralized with a security interest in all accounts, including, without limitation, all deposit accounts, accounts receivable, and other receivables of the Company. No interest rate is associated with this transaction, and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $78,000 and an administrative fee and underwriting fees associated with processing the transaction of $15,000 for the year ended December 31, 2023, in operating expenses on the consolidated statements of operations. The factoring payable was $136,873 on December 31, 2023.
F-59
Note 8Line of Credit and Notes Payable
The following is a summary of the notes payable and line of credit as of December 31, 2023 and 2022:
| | | | | | |
| | December 31, | | December 31, | ||
Notes Payable & Line of Credit |
| 2023 |
| 2022 | ||
Note payable issued November 29, 2021 (Face Value: $654,044) | | $ | 336,983 | | $ | 426,922 |
Line of credit issued November 29, 2021 (Face Value: $500,000) | |
| 456,097 | |
| 495,000 |
Note payable issued December 1, 2021 (Face Value: $1,500,700) | |
| 1,500,600 | |
| 1,500,600 |
Note payable issued October 6, 2022 (Face Value: $666,667) | |
| — | |
| 666,667 |
Note payable issued November 15, 2022 (Face Value: $200,000) | |
| 200,000 | |
| 100,000 |
Note payable issued January 25, 2023 (Face Value: $100,000) | |
| 100,000 | |
| — |
Note payable issued February 14, 2023 and December 15, 2022 (Face Value: $585,500, $200,000) | |
| 585,000 | |
| 220,000 |
Note payable issued August 3, 2023 (Face Value: $33,000) | |
| 33,000 | |
| — |
Note payable issued August 18, 2023 (Face Value: $64,000) | |
| 64,000 | |
| — |
Note payable issued November 13, 2023 (Face Value: $22,000) | |
| 22,000 | |
| — |
Note payable issued November 30, 2023 (Face Value: $200,000) | |
| 200,000 | |
| — |
Total notes payable and line of credit | |
| 3,497,680 | |
| 3,409,189 |
Less: unamortized discount on notes payable | |
| — | |
| (275,759) |
Less: current portion | |
| (1,997,080) | |
| (1,324,505) |
Total notes payable and line of credit | | $ | 1,500,600 | | $ | 1,808,925 |
Required principal payments under the company’s notes payable and line of credit are as follows:
| | | |
Year Ending December 31, 2024 |
| $ | 1,997,080 |
Year Ending December 31, 2025 | |
| 4,567 |
Year Ending December 31, 2026 | |
| 26,534 |
Year Ending December 31, 2027 | |
| 37,720 |
Year Ending December 31, 2028 | |
| 39,008 |
Thereafter | |
| 1,392,771 |
Total | | $ | 3,497,680 |
Notes Payable
On November 29, 2021, the Company received a $654,044 promissory note from a bank, collateralized by all the assets of the Company. Interest was payable monthly at the annual fixed rate of 4.284%. On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024, and increased the effective interest rate to 3% above the Wall Street Journal prime rate (8.5% at December 31, 2023) (Note 11). The Company is required to pay the loan in 36 payments of $19,409. As of December 31, 2023 and 2022, the Company had an outstanding balance of $336,983 and $426,922, respectively, on the promissory note. For the years ended December 31, 2023 and 2022, the Company paid and recorded $18,742 and $25,198 in interest, respectively. The Company accrued interest of $7,509 and $105 at December 31, 2023 and 2022, respectively.
On December 1, 2021, the Company received a promissory note from a bank in the amount of $500,000. On February 25, 2022, the Company received an extension of $1,000,700 on the promissory note. The promissory note is collateralized by all the assets of the Company and the private property of the Company’s CEO. Interest is accrued monthly at the annual fixed rate of 3.75%. The promissory note matures on December 19, 2051. As of December 31, 2023 and 2022, the Company had an outstanding balance of $1,500,600 on the promissory note. Commencing on January 1, 2024, the Company is required to make monthly installment payments, including principal and interest, of $7,682. The Company recorded $56,272 and $33,269 in interest related to the promissory note for the years ended December 31, 2023 and 2022, respectively. The accrued interest balance, which is included within accrued liabilities on the consolidated balance sheets, as of December 31, 2023 and 2022 are $89,541 and $33,269, respectively.
F-60
On October 6, 2022, in connection with the execution of a Business Combination Agreement, the Company entered into a securities purchase agreement (“The Agreement”) with an accredited investor (“Holder”), issued and sold to such investor a 10.00% original issue discount senior secured promissory notes due October 5, 2023, in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). $666,667 of the Bridge Note was allocated to the Company. The Company received cash proceeds of $600,000 from the note.
Concurrently with the execution of the Business Combination Agreement, on October 6, 2022, Digital Health Acquisition Company (‘DHAC”) entered into an Amended and Restated Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with certain investors (the “PIPE Investors”). If the PIPE Financing closes in connection with the closing of the Business Combination, 110% if paid after 90 days of the original issue date, 100% if before 90 days under the Bridge Notes, and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Bridge Note has a mandatory default payment of 125% of the sum of the outstanding principal and all accrued interest unpaid and all other amounts, costs, fees (including Late Fees), expenses, indemnification and liquidated and other damages and other obligations due to the Holder. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The Agreement includes a mandatory prepayment that upon the closing of the Business Combination under the Business Combination Agreement, the Company shall repay the note in its entirety to the Holder in an amount equal to the mandatory prepayment Amount.
The Company reviewed the contingent early repayment option granted in the Bridge Note under ASC 815 and concluded that as a result of the significant discount granted in the note, the contingent mandatory repayment provision is therefore considered an embedded derivative. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Note proceeds between the Bridge Note and the Embedded Early Mandatory Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $208,803 and the residual value of $457,864 was allocated to the principal balance of the note. (See Note 9. Fair Value Measurements for additional disclosure on the derivative).
On October 5, 2023 the Company defaulted on the Bridge Note and accordingly the default provision were allocated and applied resulting in the triggering of 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24% resulting in default interest expense of $383,789.
An Exchange Agreement (this “Agreement”) was dated as of November 21, 2023, between Digital Health Acquisition Corp., a Delaware corporation (“DHAC”), VSee Lab, Inc., a Delaware corporation (“VSee”) and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”, and together with DHAC and VSee, each a “Company” and collectively, the “Companies”) and the holders.
The Holder beneficially own and hold (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, each as further detailed on Schedule I hereto, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744, including interest.
The Holder has agreed to purchase from VSee and iDoc, their respective shares of common stock, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the VSee Note and the principal amount (excluding the original issue discount of $66,667) of $600,000 of the iDoc Note, effective immediately prior to the consummation of the Business Combination. As of December 31, 2023, the Company has $600,000 recorded as a contingent liability on the consolidated balance sheets.
The Holder, severally and not jointly, desire to, upon consummation of the Business Combination, exchange all amounts currently due and owing (the “Original Notes Amount”) under (i) the DHAC Note, (ii)the VSee Note other than the principal amount of $600,000 thereof, and (iii) the iDoc Note other than the principal amount of $600,000 thereof (the “Exchange”) for senior secured convertible promissory notes with an aggregate principle value of $2,523,744 (such notes, the “Notes” or the “Securities”), in the form of the Exchange Note was assumed by DHAC.
F-61
As a result of the Exchange Agreement, DHAC assumed The Company’s default interest of $383,789. The carrying balance of the Bridge note and the bifurcated derivative was offset by the principal amount of $600,000, resulting in a gain on forgiveness of debt of $107,862.
As of December 31, 2023 and 2022, the Bridge note net of unamortized debt discount was $0 and $407,131, respectively. The Company recognized $50,916 of amortized debt discount and $50,733 in interest for a total Bridge Note interest expense of $101,649 for the year ended December 31, 2023. The Company had $0 and $15,934 in accrued interest as of December 31, 2023 and 2022, respectively.
On November 15, 2022, the Company received a $200,000 promissory note from an accredited investor. The promissory note matures on December 31, 2024, and is collateralized by all the assets of the Company. $100,000 of the promissory note was funded on November 15, 2022 and the remaining $100,000 was funded on January 12, 2023. Interest is accrued monthly at the annual fixed rate of 10.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into various securities purchase agreements (the “Conversion SPAs”) to convert the promissory note into Series A Preferred Stock at the Closing of the Business combination. As of December 31, 2023 and 2022, the Company had an outstanding balance of $200,000 and $100,000, respectively, and an accrued interest balance of $0 and $2,583, respectively.
On December 15, 2022, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $220,000. On February 14, 2023, the Company received an extension of $423,500 on the promissory note. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into the “Conversion SPAs” to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. As of December 31, 2023 and 2022, the promissory note net of unamortized debt discount was $585,000 and $203,778. No amortized debt discount and interest expense were recognized for the year ended December 31, 2023. The Company had $0 and $1,454 in accrued interest as of December 31, 2023 and 2022, respectively.
On January 25, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $110,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into the Conversion SPAs to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. As of December 31, 2023, the promissory note net of unamortized debt discount was $100,000. No amortized debt discount and interest expense were recognized for the year ended December 31, 2023. The Company had no accrued interest as of December 31, 2023.
On August 3, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $33,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matures on November 1, 2023, and is collateralized by all the assets of the Company. The Company entered into the “Conversion SPAs” to convert the promissory note into Common Stock at the Closing of the Business combination. Interest is accrued monthly at the annual fixed rate of 8.00%, with principal and interest due upon maturity. As of December 31, 2023, the promissory note net of unamortized debt discount was $33,000. The Company recognized $3,000 of amortized debt discount and $660 in accrued interest for a total interest expense of $3,660 for the year ended December 31, 2023. The Company had $660 in accrued interest as of December 31, 2023.
On August 18, 2023, the Company received a 8.5% original issue discount promissory note from an accredited investor with a principal balance of $64,000. Notes payable issued with a face value higher than the proceeds it receives are recognized as a debt discount and are amortized as interest expense over the life of the underlying note payable. The promissory note matures on November 16, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 8.00%, with principal and interest due upon maturity. Upon an Event of Default, the interest rate on the note shall increase to the
F-62
greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of December 31, 2023, the promissory note net of unamortized debt discount was $64,000. The Company recognized $5,000 of amortized debt discount and $1,280 in accrued interest for a total interest expense of $6,280 for the year ended December 31, 2023. The Company had $1,280 in accrued interest as of December 31, 2023.
On November 13, 2023, the Company received a 10% original issue discount promissory note from an accredited investor with a principal balance of $22,000. Notes payable issued with a face value higher than the proceeds it receives are recognized as a debt discount and are amortized as interest expense over the life of the underlying note payable. The promissory note matures on December 13, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of December 31, 2023, the promissory note net of unamortized debt discount was $22,000. The Company recognized $2,000 of amortized debt discount and $220 in accrued interest for a total interest expense of $2,220 for the year ended December 31, 2023. The Company had $220 in accrued interest as of December 31, 2023.
On November 30, 2023, the Company received a note payable from a lender with a purchase price of $200,000. The note payable has a 24% interest rate over the 3-month loan term. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of December 31, 2023, the Company had an outstanding balance of $200,000 on the loan. No interest expense was recognized on the loan for the year ended December 31, 2023.
Line of credit amendment
On November 29, 2021, the Company received a revolving line of credit from the same bank as the $500,000 promissory note. The line of credit is collateralized by the Company’s assets. Interest was payable monthly at 1.25% above the Wall Street Journal prime rate (8.5% at December 31, 2023). On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024, and increased the effective interest rate to 3% above the Wall Street Journal prime rate (8.5% at December 31, 2023) (Note 11). As of December 31, 2023 and 2022, the Company had an outstanding balance of $456,097 and $495,000, respectively, on the line of credit. The Company paid and recorded $47,239 and $30,863 in interest related to the line of credit for the years ended December 31, 2023 and 2022, respectively. The accrued interest balance, which is included within accrued liabilities on the consolidated balance sheets, as of December 31, 2023 and 2022, are $4,201 and $1,004, respectively.
Note 9Fair Value Measurements
The following table presents fair value information as of December 31, 2023 and 2022 of the Company’s financial liabilities that were accounted for at fair value on a recurring basis and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value.
| | | | | | | | | | | | |
| | December 31, 2023 | ||||||||||
|
| Carrying value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: |
| |
|
| |
|
| |
|
| |
|
Bridge Note – Embedded Derivative | | $ | — | | $ | — | | $ | — | | $ | — |
Total | | $ | — | | $ | — | | $ | — | | $ | — |
| | | | | | | | | | | | |
| | December 31, 2022 | ||||||||||
|
| Carrying value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: |
| |
|
| |
|
| |
|
| |
|
Bridge Note – Embedded Derivative | | $ | 273,534 | | $ | — | | $ | — | | $ | 273,534 |
Total | | $ | 273,534 | | $ | — | | $ | — | | $ | 273,534 |
Measurement
Bridge Note Embedded Derivative
The Company established the initial fair value for the Bridge Note Embedded Derivative as of October 5, 2022, which was the date the Bridge Note was executed. As a result of the exchange agreement on November 21, 2023, the carrying balance of the Bridge
F-63
note and related default interest, as well as the carrying balance of the bifurcated derivative, was offset with the fair value of the stock payable. The fair value of the Embedded Derivative was remeasured as of November 21, 2023 and December 31, 2022. As such, the Company used a Probability Weighted Expected Return Method (“PWERM”) that fair values the early termination/repayment features of the debt. The PWERM is a multi-step process in which value is estimated based on the probability-weighted present value of various future outcomes. The PWERM was used to value the Bridge Note Embedded Derivative for the initial periods and subsequent measurement periods.
The Bridge Note Embedded Derivative was classified within Level 3 of the fair value hierarchy at the initial measurement date and as of November 21, 2023 and December 31, 2022 due to the use of unobservable inputs. The key inputs into the simulation model for the Bridge Note Embedded Derivative were as follows at November 21, 2023, December 31, 2022 and October 5, 2022:
| | | | | | | |
| | November 21, | | December 31, | | October 5, |
|
|
| 2023 |
| 2022 |
| 2022 |
|
CCC bond rates |
| — |
| 15.09 | % | 14.09 | % |
Probability of early termination/repayment – BC not completed |
| — |
| 5 | % | 10 | % |
Probability of early termination/repayment – BC completed or PIPE completed |
| — |
| 95 | % | 90 | % |
Probability of completing a business combination by March 31, 2023 |
| — |
| 50 | % | 50 | % |
Probability of completing a business combination by June 30, 2023 |
| — |
| 50 | % | 50 | % |
Implied volatility |
| 0.1 | % | 6 | % | 12 | % |
Risk free rate |
| 5.38 | % | 4.76 | % | 4.01 | % |
The change in the fair value of the Level 3 financial liabilities for the years ended December 31, 2023 and 2022 are summarized as follows:
| | | | | | |
| | December 31, | | December 31, | ||
|
| 2023 |
| 2022 | ||
Bridge Note Embedded Derivative, Beginning Fair Value | | $ | 273,534 | | $ | — |
Fair value at October 5, 2022 (Initial measurement) | |
| — | |
| 208,803 |
Change in fair value | |
| (92,449) | |
| 64,731 |
Derivative adjustment from the Exchange Agreement (Note 8) | |
| (181,085) | |
| — |
Bridge Note Embedded Derivative, Ending Fair Value | | $ | — | | $ | 273,534 |
Transfers to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs. There were no transfers to or from the various Levels during the years ended December 31, 2023 and 2022.
Note 10Related Party
During the years ended December 31, 2023 and 2022, the Company advanced $136,981 and $146,684 in cash from the CEO through a company controlled by him. During the years ended December 31, 2023 and 2022, the Company advanced $192,184 and $18,612 to related parties for cost-sharing expenses. The balance due from the related party on December 31, 2023 and 2022, was $1,008,101 and $678,936, respectively.
The Company paid and incurred $32,450 and $59,100 on auto leases on behalf of the CEO for the years ended December 31, 2023 and 2022, respectively. The Company made office space lease payments of $186,000 and $162,000 to the CEO during the years ended December 31, 2023 and 2022, respectively.
On May 15, 2023, the Company received a promissory note with a principal balance of $200,000 from an accredited investor (“Holder”). The note bears no interest and matures on May 15, 2026. The Company shall use the funds solely for the purchase of telepresence robots. The Holder has security rights to eight (8) telepresence robots deployed. The Company is required to make payments to the Holder based on eighty percent (80%) of the monthly revenue generated on eight telepresence robots from the twelfth through the twentieth deployment of the telepresence robots. At December 31, 2023, the related party promissory note was $200,000, and is included in loan payable, related party, on the consolidated balance sheets. No interest is recognized for the year ending December 31, 2023.
F-64
Note 11Commitments, Contingencies, and Concentration Risk
Contingencies
During the normal course of business, the Company may be exposed to litigation. When the Company becomes aware of potential litigation, it evaluates the merits of the case in accordance with ASC 450, Contingencies. Litigation and contingency accruals are based on our assessment, including advice of legal counsel, regarding the expected outcome of litigation or other dispute resolution proceedings. If the Company determines that an unfavorable outcome is probable and can be reasonably assessed, it establishes the necessary accruals. As of December 31, 2023 and 2022, the Company has $0 and $90,000 in contingent liabilities in the consolidated financial statements for a legal settlement related to compensation disputes by a former employee.
The Company entered into a purchase agreement with a vendor to purchase twenty (20) Telepresence Robots, receive maintenance services, and access user-related Ava Telepresence applications and the Ava Cloud Service for a total purchase commitment of $711,900. As of December 31, 2023, the company had an unpaid commitment of $531,900 on this agreement. The commitment is not reflected in the consolidated financial statements as it is due and payable upon invoicing from the vendor for delivery and servicing installation of the Telepresence Robots and software applications.
The Company has a promissory note with an accredited investor to make payments on the promissory notes (Note 9). The accredited investor is entitled to payments for the lifetime use (to include initial lease term and any extension terms) of the first 125 telepresence robots deployed. The note payments will be used to initially pay down the principal. Once the principal is paid, the Company will continue to make payments for the lifetime of the first 125 telepresence robots deployed.
On November 1, 2023, the Company entered a forbearance agreement related to the promissory note and line of credit issued November 29, 2021, and the Company’s finance leases. Per the agreement, effective November 1, 2023, interest is payable monthly at 3% above the Wall Street Journal prime rate (8.5% at December 31, 2023) on the promissory note and the line of credit. In consideration of the Bank forbearing on its right to collect the amount due and owing until January 10, 2024, the Company agreed to make payments on November 13, 2023, and November 30, 2023, payments of $20,000 and $80,000, respectively.
On November 21, 2023, the Company entered into a Securities Purchase Agreement (“SPA”) with an accredited investor to purchase from the Company 300,000 shares of common stock at $2 per share, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the Company’s Bridge Note, effective immediately prior to the consummation of the Business Combination. The SPA was entered into concurrently with an Exchange Agreement on November 21, 2023. Refer to Note 8.
Indemnities
The Company generally indemnifies its customers for the services it provides under its contracts and other specified liabilities, which may subject the Company to indemnity claims, liabilities, and related litigation. As of December 31, 2023 and 2022, the Company was unaware of any material asserted or unasserted claims concerning these indemnity obligations.
Concentrations of Credit Risk
Financial instruments potentially subject the Company to credit risk concentrations consisting of cash and cash equivalents and trade accounts receivables. The Company maintains all its cash and cash equivalents in commercial depository accounts, insured by the Federal Deposit Insurance Corporation (“FDIC”). At times, cash deposits may exceed federally insured limits.
Major Customer Concentration
The Company has two customers whose revenue accounted for approximately 33% and 25% of the Company’s total revenue for the years ended December 31, 2023 and 2022, respectively. The Company has no customers whose accounts receivable represented 10% or more of the Company’s total accounts receivable.
F-65
Note 12Income Taxes
The components of income tax expense for the years ended December 31 were as follows:
| | | | | | |
Consolidated income statement |
| December 31, 2023 |
| December 31, 2022 | ||
Current income Tax: |
| |
|
| |
|
Current tax on profits | | $ | (12,489) | | $ | (22,281) |
Tax regarding prior years | |
| 81,066 | |
| — |
Deferred tax: | |
|
| |
|
|
Deferred taxation – current year | |
| 1,071,219 | |
| (52,665) |
Deferred taxation – prior years | |
| (69,386) | |
| 66,415 |
Income tax benefit (expense) reported in the income statement | | $ | 1,070,410 | | $ | (8,531) |
A reconciliation follows between tax expense and the product of accounting profit multiplied by the United States domestic tax rate for the years ended December 31, 2023 and 2022:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Accounting loss income before tax from continuing operations | | $ | (4,930,528) | | $ | (9,593) |
Accounting loss before income tax | |
| (4,930,528) | |
| (9,593) |
Federal income tax benefit (expense) at statutory income tax rate of 21% | |
| 1,035,411 | |
| 2,015 |
State income tax benefit (expense), net of federal benefit | |
| 61,164 | |
| 10,295 |
Remeasurement of deferred taxes due to US tax legislative changes | |
| — | |
| — |
Permanent differences, net | |
| (46,979) | |
| (87,256) |
Deferred tax true-up | |
| (69,386) | |
| 66,415 |
Other | |
| 90,200 | |
| — |
Total | | $ | 1,070,410 | | $ | (8,531) |
Deferred tax
Deferred tax is comprised of the following:
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Non-current |
| |
|
| |
|
Cash to accrual | | $ | (285,668) | | $ | (952,237) |
Right of use assets | |
| (317,376) | |
| (323,872) |
Right of use liabilities | |
| 356,981 | |
| 326,176 |
NOL carryforward | |
| 839,597 | |
| 546,861 |
Interest expense disallowance | |
| — | |
| — |
Fixed assets | |
| (2,157) | |
| (176) |
Deferred revenue | |
| 4,463 | |
| — |
Charitable contribution carryover | |
| 2,745 | |
| — |
Net deferred tax assets (liabilities) | | $ | 598,585 | | $ | (403,248) |
Reconciliation of deferred tax assets (liabilities), net
| | | | | | |
|
| December 31, 2023 |
| December 31, 2022 | ||
Opening balance | | $ | (403,248) | | $ | — |
Tax benefit/(expense) during the period recognized in profit or loss | |
| 1,071,219 | |
| (52,665) |
Reclass from current taxes payable | |
| — | |
| (416,998) |
Deferred tax true up | |
| (69,386) | |
| 66,415 |
Closing balance | | $ | 598,585 | | $ | (403,248) |
F-66
The Company offsets tax assets and liabilities only if it has a legally enforceable right to set off current tax assets and current tax liabilities, and the deferred tax assets and deferred tax liabilities relate to income taxes levied by the same tax authority. The Company has US federal and State of Georgia and Colorado tax losses totaling $3.6 million and $0.7 million, respectively, which have an unlimited carryover period. Additionally, the Company has State of Colorado tax losses totaling $1.0 million, which begin to expire in 2042.
As of December 31, 2023 and 2022, the Company had no provision for uncertain tax positions and no provisions for penalties or interest. In addition, the Company does not believe that there are any uncertain tax benefits that could be recognized in the near future that would impact the Company’s effective tax rate.
Note 13Subsequent Events
In accordance with ASC 855-10, the Company has analyzed its operations subsequent to December 31, 2023, through the date when the consolidated financial statements were issued and has determined that it does not have any material subsequent events to disclose in these consolidated financial statements.
On February 13, 2024, the Company amended the November 21, 2023, “SPA” with the accredited investor. Under the amended SPA, in connection with the Business Combination, VSee Health will assume the contingent liability. Under the amended SPA, the accredited investor will purchase from VSee Health 300,000 shares of the common stock at $2 per share in exchange for the contingent liability of $600,000.
F-67
iDoc Virtual Telehealth Solutions, INC.
UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
For the Three Months Ended
March 31, 2024 and 2023
F-68
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023
INDEX TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Condensed Consolidated Financial Statements | |
Condensed Consolidated Balance Sheets at March 31, 2024 (Unaudited) and December 31, 2023 | F-70 |
F-71 | |
F-72 | |
F-74 | |
Notes to the Unaudited Condensed Consolidated Financial Statements | F-75 |
F-69
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
MARCH 31, 2024 (UNAUDITED) AND DECEMBER 31, 2023
| | | | | | |
|
| March 31, 2024 |
| December 31, 2023 | ||
| | (Unaudited) | | | | |
ASSETS |
| | |
| |
|
Current assets |
| |
|
| |
|
Cash and cash equivalents | | $ | 74,184 | | $ | 63,037 |
Accounts receivable, net | |
| 2,964,616 | |
| 2,266,302 |
Due from related party | |
| 1,047,771 | |
| 1,008,101 |
Prepaids and other current assets | |
| 140,665 | |
| 123,205 |
Total current assets | |
| 4,227,236 | |
| 3,460,645 |
| | | | | | |
Note receivable, related party | |
| 245,500 | |
| 245,500 |
Right-of-use asset, net | |
| 1,316,153 | |
| 1,422,017 |
Deferred tax asset | |
| 563,094 | |
| 598,585 |
Deposit | |
| 20,720 | |
| 20,720 |
Fixed assets, net | |
| 110,296 | |
| 114,044 |
Total assets | | $ | 6,482,999 | | $ | 5,861,511 |
| | | | | | |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
|
| |
|
|
Current liabilities | |
|
| |
|
|
Accounts payable | | $ | 609,838 | | $ | 391,923 |
Accrued liabilities | |
| 1,195,054 | |
| 841,514 |
Deferred revenue | |
| 20,000 | |
| 20,000 |
Right-of-use liability | |
| 698,480 | |
| 608,695 |
Line of credit | |
| 456,097 | |
| 456,097 |
Factoring payable | |
| 491,974 | |
| 660,578 |
Notes payable, net of discount | |
| 1,581,183 | |
| 1,540,983 |
Due on acquisition purchase | |
| 300,000 | |
| 300,000 |
Contingent liability | |
| 600,000 | |
| 600,000 |
Loan payable, related party | |
| 200,000 | |
| 200,000 |
Total current liabilities | |
| 6,152,626 | |
| 5,619,790 |
| | | | | | |
Notes payable, less current portion, net of discount | |
| 1,500,600 | |
| 1,500,600 |
Right-of-use liability, less current portion | |
| 885,940 | |
| 990,774 |
Total liabilities | |
| 8,539,166 | |
| 8,111,164 |
| | | | | | |
Commitments and contingencies (Note 8) | |
|
| |
|
|
| | | | | | |
Stockholders’ deficit | |
|
| |
|
|
Common stock, $1.00 par value; 5,000 shares authorized 4,978 issued and outstanding | |
| 4,978 | |
| 4,978 |
Additional paid in capital | |
| 209,521 | |
| 209,521 |
Accumulated deficit | |
| (2,270,666) | |
| (2,464,152) |
Total stockholders’ deficit | |
| (2,056,167) | |
| (2,249,653) |
Total liabilities and stockholders’ deficit | | $ | 6,482,999 | | $ | 5,861,511 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-70
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023 (UNAUDITED)
| | | | | | |
| | For the three months ended March 31, | ||||
|
| 2024 |
| 2023 | ||
Revenues |
| |
|
| |
|
Patient Fees | | $ | 1,121,355 | | $ | 999,878 |
Telehealth Fees | |
| 512,710 | |
| 673,337 |
Institutional Fees | |
| 5,700 | |
| 275,476 |
Total Revenue | |
| 1,639,765 | |
| 1,948,691 |
Cost of Goods Sold | |
| 400,563 | |
| 790,133 |
Gross Margin | |
| 1,239,202 | |
| 1,158,558 |
| | | | | | |
Operating expenses | |
|
| |
|
|
General and administrative | |
| 288,684 | |
| 574,979 |
Compensation and related benefits | |
| 402,333 | |
| 617,794 |
Professional fees | |
| 110,182 | |
| 47,400 |
Transaction expenses | |
| 92,000 | |
| 140,769 |
Total operating expenses | |
| 893,199 | |
| 1,380,942 |
| | | | | | |
Net operating profit (loss) | |
| 346,003 | |
| (222,384) |
| | | | | | |
Other income (expenses): | |
|
| |
|
|
Interest expense | |
| (78,714) | |
| (121,387) |
Change in fair value on derivative | |
| — | |
| 26,069 |
Other expense | |
| (18,200) | |
| (3,935) |
Total other expense | |
| (96,914) | |
| (99,253) |
| | | | | | |
Income (loss) before income tax | |
| 249,089 | |
| (321,637) |
| | | | | | |
Income tax (expense) benefit | |
| (55,603) | |
| 72,270 |
| | | | | | |
Net income (loss) | | $ | 193,486 | | $ | (249,367) |
| | | | | | |
Net income (loss) per share attributable to common shareholders: | |
|
| |
|
|
Basic | | $ | 38.9 | | $ | (50.1) |
Diluted | | $ | 38.9 | | $ | (50.1) |
Weighted average number of shares outstanding, basic, and diluted | |
| 4,978 | |
| 4,978 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-71
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
FOR THE THREE MONTHS ENDED MARCH 31, 2024 (UNAUDITED)
| | | | | | | | | | | | | | |
| | | | | | | Additional | | | | | | | |
| | Common Stock | | Paid In | | Accumulated | | | | |||||
| | Shares | | Amount | | Capital | | Deficit | | Total | ||||
Balance December 31, 2023 |
| 4,978 |
| $ | 4,978 |
| $ | 209,521 |
| $ | (2,464,152) |
| $ | (2,249,653) |
| | | | | | | | | | | | | | |
Net income |
| — | |
| — | |
| — | |
| 193,486 | |
| 193,486 |
| | | | | | | | | | | | | | |
Balance March 31, 2024 |
| 4,978 | | $ | 4,978 | | $ | 209,521 | | $ | (2,270,666) | | $ | (2,056,167) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-72
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2023 (UNAUDITED)
| | | | | | | | | | | | | | |
| | | | | | | Additional | | | | |
| | |
| | Common Stock | | Paid In | | Retained | |
| | |||||
| | Shares | | Amount | | Capital | | Earnings | | Total | ||||
Balance December 31, 2022 |
| 4,978 |
| $ | 4,978 |
| $ | 209,521 |
| $ | 1,395,966 |
| $ | 1,610,465 |
| | | | | | | | | | | | | | |
Net loss |
| — | |
| — | |
| — | |
| (249,367) | |
| (249,367) |
| | | | | | | | | | | | | | |
Balance March 31, 2023 |
| 4,978 | | $ | 4,978 | | $ | 209,521 | | $ | 1,146,599 | | $ | 1,361,098 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-73
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE THREE MONTHS ENDED MARCH 31, 2024 AND 2023 (UNAUDITED)
| | | | | | |
| | For the three months ended March 31, | ||||
| | 2024 | | 2023 | ||
Net income (loss) |
| $ | 193,486 |
| $ | (249,367) |
Adjustments to reconcile net income (loss) to net | |
|
| |
|
|
cash used by operating activities: | |
|
| |
|
|
Amortization of discount on note payable | |
| — | |
| 41,795 |
Amortization of right of use asset | |
| 53,104 | |
| 53,104 |
Depreciation and amortization | |
| 3,748 | |
| 3,824 |
Provision for doubtful accounts | |
| 89,708 | |
| 279,421 |
Change in fair value on embedded derivative | |
| — | |
| (26,069) |
Loss on factoring payable | |
| 18,200 | |
| — |
Changes in working capital requirements: | |
|
| |
|
|
Accounts receivable | |
| (788,023) | |
| (708,299) |
Due from related party | |
| (39,670) | |
| (115,193) |
Prepaid and other current assets | |
| (17,460) | |
| (6,651) |
Deferred tax asset | |
| 35,491 | |
| — |
Accounts payable | |
| 217,914 | |
| 155,574 |
Accrued liabilities | |
| 418,622 | |
| (41,638) |
Deferred tax liability | |
| — | |
| (72,270) |
Net cash from operating activities | |
| 185,120 | |
| (685,769) |
| | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES: | |
|
| |
|
|
Proceeds on note receivable, related party | |
| — | |
| 90,500 |
Net cash from investing activities | |
| — | |
| 90,500 |
| | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES: | |
|
| |
|
|
Proceeds from factoring payable | |
| 31,500 | |
| — |
Proceeds from notes payable | |
| 16,200 | |
| 585,000 |
Payments on notes payable | |
| — | |
| (53,816) |
Repayment on factoring payable | |
| (221,673) | |
| — |
Repayment on leased equipment | |
| — | |
| (47,945) |
Net cash from financing activities | |
| (173,973) | |
| 483,239 |
| | | | | | |
NET CHANGE IN CASH AND CASH EQUIVALENTS | |
| 11,147 | |
| (112,030) |
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD | |
| 63,037 | |
| 147,685 |
CASH AND CASH EQUIVALENTS, END OF PERIOD | | $ | 74,184 | | $ | 35,655 |
| | | | | | |
Supplemental disclosure of cash flow information | |
|
| |
|
|
Cash paid for interest expense | | $ | 1,351 | | $ | 15,696 |
Cash paid for income taxes | | $ | — | | $ | — |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-74
IDOC VIRTUAL TELEHEALTH SOLUTIONS, INC.
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Note 1Organization and Description of Business
iDoc Telehealth Solutions, Inc. was incorporated in the state of Virginia on February 26, 2014. The Company subsequently changed its name to iDoc Virtual Telehealth Solutions, Inc. on September 10, 2018, and incorporated in the state of Texas. Encompass Healthcare Billing, LLC (“Encompass”), a wholly owned subsidiary, was incorporated on December 17, 2014, in the state of Colorado and was acquired by the Company on January 1, 2022 (iDoc Virtual Telehealth Solutions, Inc., and Subsidiary collectively referred to as the “Company,” or “iDoc”). The Company is headquartered in Houston, Texas and is one of the leading providers of tele-intensive acute care and tele-neurocritical care in high-value hospital environments. The Company leverages its extensive telehealth platform and neuro and general critical expertise to treat and monitor acutely ill patients with diseases of the brain, spinal cord, heart, and lungs that often have complicated medical problems. The Company is a virtual health services management company responding to the need for rapid, effective treatment of emergency patients and the shortage of critical care experts. The Company operates as a single operating and reportable segment. Encompass is a nationwide full service medical billing service provider, specializing in intraoperative neuromonitoring services medical billing.
Note 2 | Summary of Significant Accounting Policies |
Basis of Presentation and Consolidation
The accompanying condensed consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Certain information or footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted, pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial reporting. Accordingly, they do not include all the information and footnotes necessary for a complete presentation of financial position, results of operations, or cash flows. In the opinion of management, the accompanying unaudited condensed consolidated financial statements include all adjustments, consisting of a normal recurring nature, which are necessary for a fair presentation of the financial position, operating results and cash flows for the periods presented.
The condensed consolidated financial statements include the accounts of IDoc Virtual Telehealth Solutions, Inc., and its subsidiary, Encompass Healthcare Billing, LLC, a 100% wholly owned subsidiary of the Company. All intercompany amounts are eliminated upon consolidation.
The accompanying condensed consolidated financial statements reflect adjustments (including normal, recurring adjustments) necessary to present fairly the financial position of the Company as of March 31, 2024, its results of operations, changes in stockholders’ deficit, and statements of cash flows for the three months ended March 31, 2024 and 2023, in conformity with U.S. GAAP. Interim results are not necessarily indicative of full year performance.
Use of Estimates
The preparation of the Company’s condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts stated in the condensed consolidated financial statements and accompanying notes. These judgments, estimates, and assumptions are used for, but not limited to, the determination of revenue recognition, the valuation of the Company’s common stock, allowance for doubtful accounts, and income taxes.
The Company bases its estimates and judgments on historical experience and on various other assumptions that it believes are reasonable under the circumstances. However, future events are subject to change and best estimates and judgments routinely require adjustment. Actual results could differ from those estimates.
F-75
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis and operating loss, capital loss, and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits as a component of general and administrative expenses. The Company’s federal tax return and any state tax returns are not currently under examination.
The Company has adopted Accounting Standards Codification (“ASC”) 740-10, Accounting for Income Taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually from differences between the financial statement and tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 establishes a principle for recognizing revenue upon the transfer of promised goods or services to customers, in an amount that reflects the expected consideration received in exchange for those goods or services. The core principle of ASC 606 is to recognize revenue to depict the transfer of promised goods or services to clients in an amount that reflects the consideration the entity expects to be entitled in exchange for those goods or services. The Company recognizes revenue using a five-step model:
| 1) | Identify the contract(s) with a customer; |
| 2) | Identify the performance obligation(s) in the contract; |
| 3) | Determine the transaction price; |
| 4) | Allocate the transaction price to the performance obligations in the contract; and |
| 5) | Recognize revenue when (or as) it satisfies a performance obligation. |
The Company derives revenue from business services associated with direct tele-physician provider patient fee services, telehealth services, and institutional services provided to our clients.
Patient Fees Services and Performance Obligation
All of the Company’s telemedicine contracts for patient reimbursement fees are directly billed to the payers by the Company. The Company earns patient fees by providing high acuity patient care solutions. For patient fees, performance obligations are met when the Company’s physicians provide professional medical services to patients at the client site as this is deemed as transfer of goods and services to respective patients. The patient benefits from the professional services when care is rendered by the Company’s medical professionals. The revenue is determined based on the telemedicine billing code(s) associated with the respective professional service rendered to patients. The Company earns primarily from reimbursement from the following third-party payors:
F-76
Medicare
The Medicare program offers beneficiaries different ways to obtain medical benefits: (i) Medicare Part A, which covers, among other things, in-patient hospital, SNFs, home healthcare, and certain other types of healthcare services; (ii) Medicare Part B, which covers physicians’ services, outpatient services, durable medical equipment, and certain other types of items and healthcare services; (iii) Medicare Part C, also known as Medicare Advantage, which is a managed care option for beneficiaries who are entitled to Medicare Part A and enrolled in Medicare Part B; and (iv) Medicare Part D, which provides coverage for prescription drugs that are not otherwise covered under Medicare Part A or Part B for those beneficiaries that enroll.
The Company’s affiliated provider network is reimbursed by the Part B and Part C programs for certain of the telemedicine services it provides to Medicare beneficiaries. Medicare coverage for telemedicine services is treated distinctly from other types of professional medical services and is limited by federal statute and subject to specific conditions of participation and payment pursuant to Medicare regulations, policies and guidelines, including the location of the patient, the type of service, and the modality for delivering the telemedicine service, among others.
Medicaid
Medicaid programs are funded jointly by the federal government and the states and are administered by states (or the state’s designated managed care or other similar organizations) under approved plans. Our affiliated provider network is reimbursed by certain State Medicaid programs for certain of the telemedicine services it provides to Medicaid beneficiaries. Medicaid coverage for telemedicine services varies by state and is subject to specific conditions of participation and payment.
Commercial Insurance Providers
The Company is reimbursed by commercial insurance carriers. The basis for payment to the commercial insurance providers is consistent with Medicare reimbursement fee structure guidelines and the Company is in-network or out-of-network with the commercial insurance carriers based on state and insurer requirements.
Telehealth Fees Service Contracts and Performance Obligation
The Company enters into service contracts mainly in the following categories with hospitals or hospital systems, physician practice groups, and other users. The Company’s customer contracts typically range in length from two to three years, with an automatic renewal process. The Company either invoices its customers for the monthly fixed fee in advance or at the end of the month, depending on the terms of the contract. The contracts typically contain cancellation clauses with advance notice; therefore, the Company does not believe that it has any material outstanding commitment for future revenues beyond one year from the end of a reporting period. Under the contracts, the customers pay a fixed monthly fee for the services described below.
Contract For Telemedicine Care Services
Performance obligations in the contract for telemedicine care are based on services provided via the use of hardware and software integration that includes multi-participant video conferencing, and electronic communication for 24 hours per day, seven days per week for the duration of the contract. The Company provides administrative support for the tele-physician services and coordinates the services of its clinicians network through administrative support, hardware support, and software support and provider coverage availability. The Company provides coverage availability of its physician services ranging from 12-24h per day. Performance obligations in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from patient services and institutional services obligations. Performance obligations are met when the Company provides administrative, business and medical records and reports related to their professional services rendered pursuant to the agreement in such format and upon such interval as hospitals may require. Revenue from telemedicine care services is included in telehealth fees in the condensed consolidated financial statements. The Company commences revenue recognition when the Company satisfies its performance obligation to provide the contractual tele-physician hours services monthly. Prior to the commencement of services, customers generally make initial start-up nonrefundable payments to the Company when contracting for Company training, hardware and software installation and integration, which includes a one-time setup of software security, API interfaces, and compatibility between hospital existing equipment and hardware and software. The Company recognizes revenue upon completion of the implementation when the performance obligation of equipment setup and initial training is completed. The start-up fees do not significantly modify or customize the other goods in the contract. As the start-up service primarily covers initial administrative services for which the Company’s clients can cancel future services upon completion, management considers it to be separable from the ongoing business services, and the Company records start-up fees as one-time revenue when the start-up service is complete.
F-77
Institutional Fees Service Contracts and Performance Obligation
Contract For Electroencephalogram (“EEG”) Professional Interpretation Services
Performance obligations in the contract for EEG professional interpretation services are based on the number of professional services EEG interpretation provides monthly. The performance obligation in the contract for these services transferred to the customer are distinct in the context of the contract, whereby the transfer of the services is separately identifiable from other promises in the contract. To facilitate the delivery of the EEG professional interpretation services, the Company’s physicians use EEG telemedicine equipment provided by the Company. The performance obligation is satisfied based on the number of EEG professional interpretations performed by the Company’s physicians. The number of professional interpretations is traced monthly by both parties and used to determine the revenue earned based on established contractual rates and are included in institutional fees in the condensed consolidated financial statements. The Company commences revenue recognition on EEG professional interpretation services when the Company satisfies its performance obligation to provide professional interpretation monthly.
Determination of Pricing for Services
The Company believes the quoted transaction prices in the customer contracts represent the stand- alone selling prices for each of the separate performance obligations which are distinct and priced separately within the contract. The transaction price for each service provided is independent and established in the contract and based on the duration of service provided or for a rate for service provided. Fees are established based on the service transferred to the client.
Telehealth and Institutional Services Contracts
Under most of the Company’s contracts, including contracts with its two top customers, the customer pays fixed monthly fees for telemedicine consultation services, EEG professional interpretation services, platform software services, and hardware fees. The fixed monthly fee provides for a predetermined number of daily, monthly, or annual physician hours of coverage and agreed upon rates for interpretation and software services. To facilitate the delivery of the consultation services, the facilities use telemedicine equipment and the Company’s virtual health care platform, which is provided and installed by the Company. The Company also provides the hospitals with user training, maintenance and support services for the telemedicine equipment used to perform the consultation services.
The Company recognizes revenue for variable consideration when it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. The Company estimates the amount of revenue to be recognized on variable consideration, using the expected value or the most likely amount method, whichever is expected to better predict the amount. The Company’s estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on assessments of legal enforceability, performance, and all information that is reasonably available to the Company. The determination of the amount of revenue the Company can recognize each accounting period requires management to make estimates and judgments on the estimated expected customer life or expected performance period, of at least 3 years.
Patient Fee Contracts Involving Third-Party Payors
The Company receives payments from patients, third-party payers and others for patient fee services. Third-party payers pay the Company based on contracted rates or the entities’ billed charges. Payments received from third-party payers are generally less than billed charges. The Company receives less than its total established charges for its services. The Company determines the transaction price on patient fees based on standard charges for services provided, reduced by adjustments provided to third-party payors, and implicit price concessions provided to uninsured patients. The Company monitors its revenue and receivables from third-party payers and records an estimated contractual allowance to properly account for the differences between billed and reimbursed amounts.
Revenue from third-party payers is presented net of an estimated provision for contractual adjustments. Patient revenues are net of service credits and service adjustments, and allowance for doubtful accounts receivable. These adjustments and implicit price concessions represent the difference between the amount billed and the estimated consideration the Company expects to receive, based on historical collection experience, market conditions and other factors. Although the Company believes that its approach to estimates and judgments as described herein is reasonable, actual results could differ and the Company may be exposed to increases or decreases in revenue that could be material.
F-78
Cost of Revenue
Cost of revenue consists primarily of expenses related to compensation-related expenses for the Company’s telehealth service providers, costs for third-party software and hardware services and independent medical providers, and other services used in connection with the delivery and support of the Company’s telehealth platform.
Going Concern
The accompanying condensed consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses during 2022 and 2023 and historically has negative operating cash flows. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The continuation of the Company’s business is dependent upon its ability to achieve profitability and positive cash flows and, pending such achievement, future issuances of equity or other financings to fund ongoing operations. These condensed consolidated financial statements do not include any adjustments relating to the recovery of the recorded assets or the classifications of the liabilities that might be necessary should the Company be unable to continue as a going concern.
Transaction Expenses
On June 15, 2022, the Company entered into a business combination agreement with Digital Health Acquisition Company (“DHAC”), a Delaware blank check company established for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business transaction with one or more businesses or entities, referred to as “targets.” On October 6, 2022, the business combination agreement was amended, and the Special Purchase Acquisition Company public offering transaction is ongoing. During the three months ended March 31, 2024 and 2023, the Company incurred transaction expenses related to the business combination of $92,000 and $140,769 respectively, for professional fees, including legal, taxation, business consulting, and auditing services.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of acquisition to be cash equivalents. The Company maintains its cash balances with commercial banks in non-interest-bearing accounts, which do not exceed the FDIC-insured limits. Cash and cash equivalents include cash held in checking and savings accounts. The carrying amount of its cash equivalents approximates its fair value due to the short maturities of these instruments.
Accounts Receivable and Credit losses
The Company carries its accounts receivable at net realizable value. The Company maintains an allowance for doubtful accounts for the estimated losses resulting from the inability of the Company’s clients to pay their invoices. Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-13, Credit Losses — Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires entities to use a forward-looking approach based on current expected credit losses (“CECL”) to estimate credit losses on certain types of financial instruments, including trade receivables. Credit losses were recognized for the three months ended March 31, 2024 and 2023. The Company recognized $89,708 and $279,421 of bad debt expenses for the three months ended March 31, 2024 and 2023, respectively. As of March 31, 2024 and December 31, 2023, respectively, the allowance for doubtful accounts was $1,666,139 and $1,576,415.
Prepaid Expenses
Prepaid expenses are costs that have been paid but are not yet used up or have not yet expired. As the amount expires, the current asset is reduced, and the amount of the reduction is reported as an expense on the condensed consolidated statements of operations.
Leases
The Company accounts for leases under ASU 2016-02, “Leases” (Topic 842). Based on this standard, the Company determines if an agreement is a lease at inception. Operating and finance leases are included in right-of-use asset, current portion of right-of-use liability, and right-of-use liability less current portion in the Company’s condensed consolidated balance sheets. Operating and finance lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date.
F-79
As permitted under ASU 2016-02, the Company has made an accounting policy election not to apply the recognition provisions of ASU 2016-02 to short-term leases (leases with a lease term of 12 months or less that do not include an option to purchase the underlying asset that the lessee is reasonably certain to exercise); instead, the Company will recognize the lease payments for short term leases on a straight-line basis over the lease term.
Net Income (Loss) Per Common Share
The Company computes income (loss) per common share, in accordance with ASC Topic 260, Earnings Per Share, which requires dual presentation of basic and diluted earnings per share. Basic income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, that could result from the exercise of outstanding stock options and warrants.
Fair Value Measurements
ASC Topic 820, Fair Value Measurements, clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1: Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2: Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3: Inputs are unobservable inputs that reflect the reporting entity’s assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
Fair Value of Financial Instruments
ASC subtopic 825-10, Financial Instruments (“ASC 825-10”) requires disclosure of the fair value of certain financial instruments. The carrying value of cash and cash equivalents, accounts payable, and accrued liabilities as reflected in the condensed consolidated balance sheets, approximate fair value because of the short-term maturity of these instruments. All other significant financial assets, financial liabilities, and equity instruments of the Company are either recognized or disclosed in the condensed consolidated financial statements together with other information relevant to making a reasonable assessment of future cash flows, interest rate risk, and credit risk. Where practicable the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the condensed consolidated statements of operations. Derivative assets and liabilities are classified in the condensed consolidated balance sheets as current or non-current based on whether net-cash settlement or conversion of the instrument could be required within 12 months of the condensed consolidated balance sheet date. FASB ASC 470-20, “Debt with Conversion and Other Options” addresses the allocation of proceeds from the issuance of debt into its debt and embedded derivative components.
Fixed Assets
Fixed assets are recorded at historical cost. Depreciation is calculated on the straight-line method over the estimated useful lives of the respective assets, which is three to ten years.
F-80
Impairment of Long-lived and Intangible Assets
In accordance with ASC 360-10, the Company, on a regular basis, reviews the carrying amount of long-lived assets for the existence of facts or circumstances, both internally and externally, that suggest impairment. The Company determines if the carrying amount of a long-lived asset is impaired based on anticipated undiscounted cash flows, before interest, from the use of the asset. In the event of impairment, a loss is recognized based on the amount by which the carrying amount exceeds the fair value of the asset. Fair value is determined based on the appraised value of the assets or the anticipated cash flows from the use of the asset, discounted at a rate commensurate with the risk involved. The Company recorded $0 of impairment charges during the three months ended March 31, 2024 and 2023.
Original issue discount on Debt
When the Company issues notes payable with a face value higher than the proceeds it receives, it records the difference as a debt discount and amortizes the discount as interest expense over the life of the underlying note payable.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures” (“ASU 2023-09”), which will require the Company to disclose specified additional information in its income tax rate reconciliation and provide additional information for reconciling items that meet a quantitative threshold. ASU 2023-09 will also require the Company to disaggregate its income taxes paid disclosure by federal, state and foreign taxes, with further disaggregation required for significant individual jurisdictions. ASU 2023-09 will become effective for annual periods beginning after December 15, 2024. The Company is still reviewing the impact of ASU 2023-09. Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s condensed consolidated financial statements.
Note 3 | Fixed Assets |
The components of fixed assets are summarized below:
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Office equipment | | $ | 28,506 | | $ | 28,506 |
Medical equipment | |
| 89,246 | |
| 89,246 |
Furniture | |
| 6,153 | |
| 6,153 |
Leasehold improvements | |
| 7,311 | |
| 7,311 |
| |
| 131,216 | |
| 131,216 |
Less accumulated. Depreciation | |
| (20,920) | |
| (17,172) |
Fixed Assets, net | | $ | 110,296 | | $ | 114,044 |
The Company recorded $3,748 and $3,074 in depreciation expenses during the three months ended March 31, 2024 and 2023, respectively.
F-81
Note 4 | Leases |
Operating Leases
The Company leases office space in Boston, Massachusetts (“Massachusetts Lease”), Houston, Texas (“Texas Lease”), Atlanta, Georgia (“Georgia Lease”) and Lakewood, Colorado (“Colorado Lease”). The Company commenced a new Massachusetts lease on September 1, 2023, ending on August 31, 2028. The Texas Lease was renewed on February 1, 2022 and ends on January 31, 2027. The Company commenced a new Georgia lease on June 1, 2022, ending on May 31, 2027. The new Georgia lease was terminated on November 30, 2023. The Colorado Lease commenced on April 1, 2020, and ended on March 31, 2023. The monthly lease payments for the Massachusetts Lease are $9,380 between September 1, 2023 and August 31, 2024, $9,630 between September 1, 2024 and August 31, 2025, $9,870 between September 1, 2025 and August 31, 2026, $10,120 between September 1, 2026 and August 31, 2027, and $10,360 between September 1, 2027 and August 31, 2028. The monthly lease payments for the Texas Lease are $10,000, and for the Georgia Lease are $6,000 for the lease commenced on June 1, 2022. The monthly lease payments for the Colorado Lease are $4,678 between April 1, 2020 and March 31, 2021, $4,851 between April 1, 2021 and March 31, 2022 and $5,024 between April 1, 2022 and March 31, 2023. The Colorado lease was terminated on March 31, 2023.
Operating lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date. The interest rate used to determine the present value is the Company’s incremental borrowing rate, estimated to be 5.00%, as the interest rate implicit in most of its leases is not readily determinable. Operating lease expense is recognized on a straight-line basis over the lease term.
During the three months ended March 31, 2024 and 2023, the Company recorded $58,140 and $63,072 as operating lease expense which is included in general and administrative expenses on the condensed consolidated statements of operations, respectively.
Operating right-of-use assets are summarized below.
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Office Lease | | $ | 1,119,026 | | $ | 1,216,055 |
Less accumulated amortization | |
| (293,475) | |
| (337,743) |
Right-of-use, net | | $ | 825,551 | | $ | 878,312 |
Operating lease liabilities are summarized below:
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Office Lease | | $ | 861,981 | | $ | 886,602 |
Less: current portion | |
| (251,169) | |
| (222,325) |
Long term portion | | $ | 610,812 | | $ | 664,277 |
Future minimum rent payments under the operating lease are as follows:
| | | |
|
| Total | |
Year ending December 31, 2024 | | $ | 270,740 |
Year ending December 31, 2025 | |
| 237,240 |
Year ending December 31, 2026 | |
| 220,190 |
Year ending December 31, 2027 | |
| 123,120 |
Year ending December 31, 2028 | |
| 51,800 |
Total future minimum lease payments | |
| 903,090 |
Less imputed interest | |
| (41,109) |
PV of Payments | | $ | 861,981 |
F-82
Finance Leases
Commencing during the year ended December 31, 2022, the Company leases office equipment under three finance leases with combined monthly payments of $20,313. The leases mature on June 2026 and August 2026. On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024 (Note 11). Equipment lease right-of-use assets and liabilities are recognized at the present value of the future lease payments at the lease commencement date.
Finance right-of-use assets are summarized below:
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Equipment Lease | | $ | 849,662 | | $ | 849,662 |
Less accumulated amortization | |
| (359,060) | |
| (305,957) |
Right-of-use, net | | $ | 490,602 | | $ | 543,705 |
Finance lease liabilities are summarized below:
| | | | | | |
|
| March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
Equipment Lease | | $ | 722,439 | | $ | 712,867 |
Less: current portion | |
| (447,311) | |
| (386,370) |
Long term portion | | $ | 275,128 | | $ | 326,497 |
Future minimum rent payments under the finance lease are as follows:
| | | |
|
| Total | |
Year ending December 31, 2024 | | $ | 447,309 |
Year ending December 31, 2025 | |
| 243,758 |
Year ending December 31, 2026 | |
| 75,545 |
Total future minimum lease payments | |
| 766,612 |
Less imputed interest | |
| (44,173) |
PV of Payments | | $ | 722,439 |
Expenses incurred with respect to the Company’s finance leases during the three months ended March 31, 2024 and 2023 which are included in general and administrative expenses on the condensed consolidated statements of operations are set forth below.
| | | | | | |
|
| March 31, |
| March 31, | ||
| | 2024 | | 2023 | ||
Finance lease amortization | | $ | 53,104 | | $ | 53,104 |
Finance lease interest | |
| 9,573 | |
| 12,995 |
Total finance lease expense | | $ | 62,677 | | $ | 66,099 |
The weighted average remaining lease term and the weighted average discount rate on the finance leases at March 31, 2024 and December 31, 2023 are set forth below.
| | | | | |
|
| March 31, |
| December 31, |
|
| | 2024 | | 2023 |
|
Weighted average remaining lease term |
| 2.3 | years | 2.6 | years |
Weighted average discount rate |
| 6.92 | % | 6.92 | % |
F-83
Note 5Factoring Payable
On June 21, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $299,000 for a net purchase price of $207,639. Under the agreement, the Company authorized the Purchaser to collect $7,475 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The factoring payable was $110,477 and $130,977 on March 31, 2024 and December 31, 2023, respectively.
On July 28, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $140,000 for a net purchase price of $100,000. Under the agreement, the Company authorized the Purchaser to collect $5,000 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The factoring payable was $40,273 and $52,189 on March 31, 2024 and December 31, 2023, respectively.
On October 13, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $186,250 for a net purchase price of $125,000. Under the agreement, the Company authorized the Purchaser to collect $7,760 weekly. The agreement was not collateralized by a general security agreement over all the Company’s accounts, including, without limitation, all deposit accounts, accounts receivable, and other receivables, chattel paper, documents, equipment, general intangibles, instruments, and inventory. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The factoring payable was $129,660 and $150,866 on March 31, 2024 and December 31, 2023, respectively.
On October 13, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $108,000 for a net purchase price of $75,000. Under the agreement, the Company authorized the Purchaser to collect $3,484 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The factoring payable was $57,548 and $97,548 on March 31, 2024 and December 31, 2023, respectively.
On November 8, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $75,000 for a net purchase price of $111,000. Under the agreement, the Company authorized the Purchaser to collect $6,937 weekly. The agreement was not collateralized by a general security agreement over the Company’s personal property and interests. No interest rate is associated with this transaction and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on the future receipts generated by the Company. The factoring payable was $0 and 92,125 on March 31, 2024 and December 31, 2023, respectively.
On December 20, 2023, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $228,000 for a net purchase price of $150,000. Under the agreement, the Company authorized the Purchaser to collect $10,364 weekly. The agreement is collateralized with a security interest in all accounts, including, without limitation, all deposit accounts, accounts receivable, and other receivables of the Company. No interest rate is associated with this transaction, and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The factoring payable was $114,941 and $136,873 on March 31, 2024 and December 31, 2023, respectively.
On January 11, 2024, the Company entered into a Future Receipts Sale Agreement (“Agreement”) with a Purchasing Company (“Purchaser”) under which it sells future receipts without recourse. Under the terms of the agreement, the total dollar amount of future receipts being sold by the Company was $53,200 for a net purchase price of 31,500. Under the agreement, the Company authorized
F-84
the Purchaser to collect $2,500 weekly for twelve weeks and a $23,200 balloon collection on April 30, 2024. The agreement is collateralized with a security interest in all accounts, including, without limitation, all deposit accounts, accounts receivable, and other receivables of the Company. No interest rate is associated with this transaction, and no time during which the amount sold must be collected because the weekly amount is subject to adjustment based on future receipts generated by the Company. The Company recognized a loss on the sale of future receipts of $18,200 and an administrative fee and underwriting fees associated with processing the transaction of $3,500 for the three months ended March 31, 2024, in operating expenses on the condensed consolidated statements of operations. The factoring payable was $39,200 on March 31, 2024.
Note 6Line of Credit and Notes Payable
The following is a summary of the notes payable and line of credit as of March 31, 2024 and December 31, 2023:
| | | | | | |
|
| March 31, |
| December 31, | ||
Notes Payable & Line of Credit | | 2024 | | 2023 | ||
Note payable issued November 29, 2021 (Face Value: $654,044) | | $ | 336,983 | | $ | 336,983 |
Line of credit issued November 29, 2021 (Face Value: $500,000) | |
| 456,097 | |
| 456,097 |
Note payable issued December 1, 2021 (Face Value: $1,500,700) | |
| 1,500,600 | |
| 1,500,600 |
Note payable issued November 15, 2022 (Face Value: $200,000) | |
| 200,000 | |
| 200,000 |
Note payable issued January 25, 2023 (Face Value: $100,000) | |
| 100,000 | |
| 100,000 |
Note payable issued February 14, 2023 and December 15, 2022 (Face Value: $585,500) | |
| 585,000 | |
| 585,000 |
Note payable issued August 3, 2023 (Face Value: $33,000) | |
| 33,000 | |
| 33,000 |
Note payable issued August 18, 2023 (Face Value: $64,000) | |
| 64,000 | |
| 64,000 |
Note payable issued November 13, 2023 (Face Value: $22,000) | |
| 22,000 | |
| 22,000 |
Note payable issued November 30, 2023 (Face Value: $200,000) | |
| 224,000 | |
| 200,000 |
Note payable issued January 14, 2024 (Face Value: $16,200) | |
| 16,200 | |
| — |
Total notes payable and line of credit | |
| 3,537,880 | |
| 3,497,680 |
Less: current portion | |
| (2,037,280) | |
| (1,997,080) |
Total notes payable and line of credit | | $ | 1,500,600 | | $ | 1,500,600 |
Required principal payments under the company’s notes payable and line of credit are as follows:
| | | |
Year Ending December 31, 2024 |
| $ | 2,037,280 |
Year Ending December 31, 2025 | |
| 4,567 |
Year Ending December 31, 2026 | |
| 26,534 |
Year Ending December 31, 2027 | |
| 37,720 |
Year Ending December 31, 2028 | |
| 39,008 |
Thereafter | |
| 1,392,771 |
Total | | $ | 3,537,880 |
Notes Payable
On November 29, 2021, the Company received a $654,044 promissory note from a bank, collateralized by all the assets of the Company. Interest was payable monthly at the annual fixed rate of 4.284%. On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024, and increased the effective interest rate to 3% above the Wall Street Journal prime rate (8.5% at March 31, 2024) (Note 11). The Company is required to pay the loan in 36 payments of $19,409. As of March 31, 2024 and December 31, 2023, the Company had an outstanding balance of $336,983 on the promissory note. For the three months ended March 31, 2024 and 2023, the Company paid and recorded $9,794 and $4,411 in interest, respectively. The Company accrued interest of $17,304 and $7,509 at March 31, 2024 and December 31, 2023, respectively.
F-85
On December 1, 2021, the Company received a promissory note from a bank in the amount of $500,000. On February 25, 2022, the Company received an extension of $1,000,700 on the promissory note. The promissory note is collateralized by all the assets of the Company and the private property of the Company’s CEO. Interest is accrued monthly at the annual fixed rate of 3.75%. The promissory note matures on December 19, 2051. As of March 31, 2024 and December 31, 2023, the Company had an outstanding balance of $1,500,600 on the promissory note. Commencing on January 1, 2024, the Company is required to make monthly installment payments, including principal and interest, of $7,682. The Company recorded $14,029 and $13,875 in interest related to the promissory note for the three months ended March 31, 2024 and 2023, respectively. The accrued interest balance, which is included within accrued liabilities on the condensed consolidated balance sheets, as of March 31, 2024 and December 31, 2023 are $103,571 and $89,541, respectively.
On October 6, 2022, in connection with the execution of a Business Combination Agreement, the Company entered into a securities purchase agreement (“The Agreement”) with an accredited investor (“Holder”), issued and sold to such investor a 10.00% original issue discount senior secured promissory notes due October 5, 2023, in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). $666,667 of the Bridge Note was allocated to the Company. The Company received cash proceeds of $600,000 from the note.
Concurrently with the execution of the Business Combination Agreement, on October 6, 2022, Digital Health Acquisition Company (‘DHAC”) entered into an Amended and Restated Securities Purchase Agreement (the “PIPE Securities Purchase Agreement”) with certain investors (the “PIPE Investors”). If the PIPE Financing closes in connection with the closing of the Business Combination, 110% if paid after 90 days of the original issue date, 100% if before 90 days under the Bridge Notes, and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Bridge Note has a mandatory default payment of 125% of the sum of the outstanding principal and all accrued interest unpaid and all other amounts, costs, fees (including Late Fees), expenses, indemnification and liquidated and other damages and other obligations due to the Holder. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The Agreement includes a mandatory prepayment that upon the closing of the Business Combination under the Business Combination Agreement, the Company shall repay the note in its entirety to the Holder in an amount equal to the mandatory prepayment Amount.
The Company reviewed the contingent early repayment option granted in the Bridge Note under ASC 815 and concluded that as a result of the significant discount granted in the note, the contingent mandatory repayment provision is therefore considered an embedded derivative. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Note proceeds between the Bridge Note and the Embedded Early Mandatory Repayment option, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $208,803 and the residual value of $457,864 was allocated to the principal balance of the note. (See Note 9. Fair Value Measurements for additional disclosure on the derivative).
On October 5, 2023 the Company defaulted on the Bridge Note and accordingly the default provision were allocated and applied resulting in the triggering of 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24% resulting in default interest expense of $383,789 during the year ended December 31, 2023.
An Exchange Agreement (this “Agreement”) was dated as of November 21, 2023, between Digital Health Acquisition Corp., a Delaware corporation (“DHAC”), VSee Lab, Inc., a Delaware corporation (“VSee”) and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”, and together with DHAC and VSee, each a “Company” and collectively, the “Companies”) and the holders.
The Holder beneficially own and hold (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, each as further detailed on Schedule I hereto, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744, including interest.
The Holder has agreed to purchase from VSee and iDoc, their respective shares of common stock, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the VSee Note and the principal amount (excluding the original issue discount of $66,667) of $600,000 of the iDoc Note, effective immediately prior to the consummation of the Business
F-86
Combination. As of March 31, 2024 and December 31, 2023, the Company has $600,000 recorded as a contingent liability on the condensed consolidated balance sheets.
The Holder, severally and not jointly, desire to, upon consummation of the Business Combination, exchange all amounts currently due and owing (the “Original Notes Amount”) under (i) the DHAC Note, (ii) the VSee Note other than the principal amount of $600,000 thereof, and (iii) the iDoc Note other than the principal amount of $600,000 thereof (the “Exchange”) for senior secured convertible promissory notes with an aggregate principle value of $2,523,744 (such notes, the “Notes” or the “Securities”), in the form of the Exchange Note was assumed by DHAC.
As a result of the Exchange Agreement, DHAC assumed The Company’s default interest of $383,789. The carrying balance of the Bridge note and the bifurcated derivative was offset by the principal amount of $600,000, resulting in a gain on forgiveness of debt of $107,862 during the year ended December 31, 2023.
As of March 31, 2024 and December 31, 2023, the Bridge note net of unamortized debt discount was $0. The Company recognized $0 of amortized debt discount and $0 in interest for the three months ended March 31, 2024. The Company recognized $16,484 of amortized debt discount and $16,484 in interest for a total Bridge Note interest expense of $32,968 for the three months ended March 31, 2023. The Company had $0 in accrued interest as of March 31, 2024 and December 31, 2023.
On November 15, 2022, the Company received a $200,000 promissory note from an accredited investor. The promissory note matures on December 31, 2024, and is collateralized by all the assets of the Company. $100,000 of the promissory note was funded on November 15, 2022 and the remaining $100,000 was funded on January 12, 2023. Interest is accrued monthly at the annual fixed rate of 10.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into various securities purchase agreements (the “Conversion SPAs”) to convert the promissory note into Series A Preferred Stock at the Closing of the Business combination. For the three months ended March 31, 2024 and 2023, the Company incurred $0 and $5,000 in interest, respectively. As of March 31, 2024 and December 31, 2023, the Company had an outstanding balance of $200,000, and an accrued interest balance of $0.
On December 15, 2022, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $220,000. On February 14, 2023, the Company received an extension of $423,500 on the promissory note. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into the “Conversion SPAs” to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $585,000. No amortized debt discount and interest expense were recognized for the three months ended March 31, 2024. The Company had $0 in accrued interest as of March 31, 2024 and December 31, 2023.
On January 25, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $110,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on July 15, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. On June 30, 2023, the accredited investor forgave the interest expense on the loan from the date of origination. On November 21, 2023, concurrently with the execution of the Business Combination Agreement, the Company entered into the Conversion SPAs to convert the promissory note into Series A Preferred Stock at the Closing of the Business Combination. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $100,000. No amortized debt discount and interest expense were recognized for the three months ended March 31, 2024. The Company had $0 accrued interest as of March 31, 2024 and December 31, 2023.
On August 3, 2023, the Company received a 10.00% original issue discount promissory note from an accredited investor with a principal balance of $33,000. Notes payable issued with a face value higher than the proceeds it receives is recognized as a debt discount and is amortized as interest expense over the life of the underlying note payable. The promissory note matured on November 1, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 8.00%, with principal and interest due upon maturity. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $33,000. The Company recognized $0 of amortized
F-87
debt discount and $2,145 in default interest for a total interest expense of $2,145 for the three months ended March 31, 2024. The Company had $2,805 and $660 in accrued interest as of March 31, 2024 and December 31, 2023, respectively.
On August 18, 2023, the Company received a 8.5% original issue discount promissory note from an accredited investor with a principal balance of $64,000. Notes payable issued with a face value higher than the proceeds it receives are recognized as a debt discount and are amortized as interest expense over the life of the underlying note payable. The promissory note matures on November 16, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 8.00%, with principal and interest due upon maturity. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $64,000. The Company recognized $0 of amortized debt discount and $4,160 in default interest for a total interest expense of $4,160 for the three months ended March 31, 2024. The Company had $5,440 and $1,280 in accrued interest as of March 31, 2024 and December 31, 2023, respectively.
On November 13, 2023, the Company received a 10% original issue discount promissory note from an accredited investor with a principal balance of $22,000. Notes payable issued with a face value higher than the proceeds it receives are recognized as a debt discount and are amortized as interest expense over the life of the underlying note payable. The promissory note matures on December 13, 2023, and is collateralized by all the assets of the Company. Interest is accrued monthly at the annual fixed rate of 12.00%, with principal and interest due upon maturity. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. As of March 31, 2024 and December 31, 2023, the promissory note net of unamortized debt discount was $22,000. The Company recognized $0 of amortized debt discount and $1,430 in default interest for a total interest expense of $1,430 for the three months ended March 31, 2024. The Company had $1,650 and $220 in accrued interest as of March 31, 2024 and December 31, 2023, respectively.
On November 30, 2023, the Company received a note payable from a lender with a purchase price of $200,000. The note payable has a 24% interest rate over the 3-month loan term. Upon an Event of Default, the interest rate on the note shall increase to the greater of 24% per annum or the maximum rate allowed by the laws governing this agreement. On March 28, 2023, the Company modified the loan agreement to a loan principal of $224,000. The Company recorded $24,000 of interest expense on the loan for the three months ended March 31, 2024. As of March 31, 2024 and December 31, 2023, the Company had an outstanding balance of $224,000 and $200,000 on the loan. The Company had $0 in accrued interest as of March 31, 2024 and December 31, 2023.
On January 14, 2024, the Company received a note payable from a lender for $16,200. The note payable has an 8% interest rate over the 180-day loan term. The Company recorded $324 of interest expense on the loan for the three months ended March 31, 2024. As of March 31, 2024, the Company had an outstanding balance of $16,200 on the loan. The Company had $324 in accrued interest as of March 31, 2024.
Line of credit amendment
On November 29, 2021, the Company received a revolving line of credit from the same bank as the $500,000 promissory note. The line of credit is collateralized by the Company’s assets. Interest was payable monthly at 1.25% above the Wall Street Journal prime rate (8.5% at December 31, 2023). On November 1, 2023, the Company entered into a forbearance agreement with a maturity date of January 10, 2024, and increased the effective interest rate to 3% above the Wall Street Journal prime rate (8.5% at December 31, 2023) (Note 11). As of March 31, 2024 and December 31, 2023, the Company had an outstanding balance of $456,097 on the line of credit. The Company recorded $13,259 and $11,184 in interest related to the line of credit for the three months ended March 31, 2024 and 2023, respectively. The accrued interest balance, which is included within accrued liabilities on the condensed consolidated balance sheets, as of March 31, 2024 and December 31, 2023, are $16,109 and $4,201, respectively.
Note 7 | Related Party |
During the three months ended March 31, 2024 and the year ended December 31, 2023, the Company advanced $39,670 and $136,981 in cash to the CEO through a company controlled by him. During the three months ended March 31, 2024 and the year ended December 31, 2023, the Company advanced $0 and $192,184 to related parties for cost-sharing expenses. The balance due from the related party on March 31, 2024 and December 31, 2023, was $1,047,771 and $1,008,101, respectively. The above transactions and amounts are unsecured and non-interest-bearing and are not necessarily what third parties would agree to.
The Company incurred $7,800 and $2,600 on auto leases on behalf of the CEO for the three months ended March 31, 2024 and 2023, respectively. The Company made office space lease payments of $30,000 and $48,000 to the CEO during the three months
F-88
ended March 31, 2024 and 2023, respectively. The above transactions and amounts are not necessarily what third parties would agree to.
On September 1, 2022, the Company issued the CEO a note receivable with a principal balance of $336,000. The note bears no interest and matures on January 31, 2023. As of March 31, 2024, the related party note receivable was $245,000, and is included in note receivable, related party, on the condensed consolidated balance sheets. No interest income is recognized for the three months ending March 31, 2024 and the note is in default. The above transactions and amounts are not necessarily what third parties would agree to.
On May 15, 2023, the Company received a promissory note with a principal balance of $200,000 from an accredited investor (“Holder”). The note bears no interest and matures on May 15, 2026. The Company shall use the funds solely for the purchase of telepresence robots. The Holder has security rights to eight (8) telepresence robots deployed. The Company is required to make payments to the Holder based on eighty percent (80%) of the monthly revenue generated on eight telepresence robots from the twelfth through the twentieth deployment of the telepresence robots. At March 31, 2024, the related party promissory note was $200,000, and is included in loan payable, related party, on the condensed consolidated balance sheets. No interest is recognized for the three months ending March 31, 2024. The above transactions and amounts are not necessarily what third parties would agree to.
Note 8 | Commitments, Contingencies, and Concentration Risk |
Contingencies
During the normal course of business, the Company may be exposed to litigation. When the Company becomes aware of potential litigation, it evaluates the merits of the case in accordance with ASC 450, Contingencies. Litigation and contingency accruals are based on our assessment, including advice of legal counsel, regarding the expected outcome of litigation or other dispute resolution proceedings. If the Company determines that an unfavorable outcome is probable and can be reasonably assessed, it establishes the necessary accruals. As of March 31, 2024 and December 31, 2023, the Company has $0 in contingent liabilities in the condensed consolidated financial statements for a legal settlement related to compensation disputes by a former employee.
The Company entered into a purchase agreement with a vendor to purchase twenty (20) Telepresence Robots, receive maintenance services, and access user-related Ava Telepresence applications and the Ava Cloud Service for a total purchase commitment of $711,900. As of March 31, 2024 and December 31, 2023, the Company had an unpaid commitment of $530,300 and $531,900, respectively on this agreement. The commitment is not reflected in the condensed consolidated financial statements as it is due and payable upon invoicing from the vendor for delivery and servicing installation of the Telepresence Robots and software applications.
The Company has a promissory note with an accredited investor to make payments on the promissory notes (Note 6). The accredited investor is entitled to payments for the lifetime use (to include initial lease term and any extension terms) of the first 125 telepresence robots deployed. The note payments will be used to initially pay down the principal. Once the principal is paid, the Company will continue to make payments for the lifetime of the first 125 telepresence robots deployed.
On November 1, 2023, the Company entered a forbearance agreement related to the promissory note and line of credit issued November 29, 2021, and the Company’s finance leases. Per the agreement, effective November 1, 2023, interest is payable monthly at 3% above the Wall Street Journal prime rate (8.5% at March 31, 2024) on the promissory note and the line of credit. In consideration of the Bank forbearing on its right to collect the amount due and owing until January 10, 2024, the Company agreed to make payments on November 13, 2023 and November 30, 2023, of $20,000 and $80,000, respectively.
On November 21, 2023, the Company entered into a Securities Purchase Agreement (“SPA”) with an accredited investor to purchase from the Company 300,000 shares of common stock at $2 per share, in exchange of the principal amount (excluding the original issue discount of $66,667) of $600,000 of the Company’s Bridge Note, effective immediately prior to the consummation of the Business Combination. The SPA was entered into concurrently with an Exchange Agreement on November 21, 2023. Refer to Note 8.
On February 13, 2024, the Company amended the November 21, 2023, “SPA” with the accredited investor. Under the amended SPA, in connection with the Business Combination, VSee Health will assume the contingent liability. Under the amended SPA, the accredited investor will purchase from VSee Health 300,000 shares of the common stock at $2 per share in exchange for the contingent liability of $600,000.
F-89
Indemnities
The Company generally indemnifies its customers for the services it provides under its contracts and other specified liabilities, which may subject the Company to indemnity claims, liabilities, and related litigation. As of March 31, 2024 and December 31, 2023, the Company was unaware of any material asserted or unasserted claims concerning these indemnity obligations.
Concentrations of Credit Risk
Financial instruments potentially subject the Company to credit risk concentrations consisting of cash and cash equivalents and trade accounts receivables. The Company maintains all its cash and cash equivalents in commercial depository accounts, insured by the Federal Deposit Insurance Corporation (“FDIC”). At times, cash deposits may exceed federally insured limits.
Major Customer Concentration
The Company has two customers whose revenue accounted for approximately 28% and 29% of the Company’s total revenue for the three months ended March 31, 2024 and 2023. The Company has no customers whose accounts receivable represented 10% or more of the Company’s total accounts receivable.
Note 9 | Income Taxes |
The components of income tax expense for the three months ended March 31 were as follows:
| | | | | | |
|
| 2024 |
| 2023 | ||
Income (loss) before taxes | | $ | 249,089 | | $ | (321,637) |
Expected United States income tax (expense) benefit at statutory rate of 21% | | $ | (52,318) | | $ | 68,000 |
Expected State income tax (expense) benefit at statutory rate of 1.32% at March 31, 2024 and 2023, respectively | |
| (3,285) | |
| 4,270 |
Total income tax (expense) benefit | | $ | (55,603) | | $ | 72,270 |
For the three months ended March 31, 2024, the Company recorded income tax expense of $55,603 for continuing operations. The effective tax rate of 22.32% applied to income for the three months ended March 31, 2024 varied from the statutory United States federal income tax rate of 21.0% primarily due to the effect of state income taxes, net of the federal benefit, and adjustments for meals, entertainment and penalties. The Company recognizes income tax benefits from uncertain tax positions where the realization of the ultimate benefit is uncertain. As of March 31, 2024 and December 31, 2023, the Company has no unrecognized income tax benefits.
Note 10 | Subsequent Events |
The Company evaluated subsequent events and transactions that occurred after the condensed consolidated balance sheet date up to the date that the unaudited condensed consolidated financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the unaudited condensed consolidated financial statements.
On April 17, 2024, the Company, DHAC and VSee entered into a letter agreement with the Bridge Investor which amended the date with respect to the termination or closing of the business combination referenced in the Additional Bridge Notes from March 31, 2024 to June 30, 2024.
On April 17, 2024, the parties to the Third Amended and Restated Business Combination Agreement executed a Second Amendment to the Third Amended and Restated Business Combination Agreement to extend the termination date therein to June 30, 2024.
On April 17, 2024, the parties to the Extension Financing documents executed a letter agreement, which extended the maturity date of the Extension Note to June 30, 2024 On May 1, 2024, the term of DHAC was extended from May 8, 2024 to August 8, 2024.
On June 7, 2024, the DHAC board agreed to the Third Amended and Restated Business Combination agreement to close the business combination transaction on or before June 30, 2024.
F-90
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
Digital Health Acquisition Corp.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Digital Health Acquisition Corp. (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations, changes in stockholders’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, if the Company is unable to raise additional funds to alleviate liquidity needs as well as complete a Business Combination by the close of business on November 8, 2024, then the Company will cease all operations except for the purpose of liquidating. This date for mandatory liquidation, subsequent dissolution and liquidity condition raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company’s auditor since 2021.
New York, New York
April 12, 2024
PCAOB ID Number 100
F-91
DIGITAL HEALTH ACQUISITION CORP.
CONSOLIDATED BALANCE SHEETS
|
| December 31, |
| December 31, | ||
| | 2023 | | 2022 | ||
ASSETS | | | | | | |
Current assets: | | | | | |
|
Cash | | $ | 1,863 | | $ | 106,998 |
Total Current Assets | |
| 1,863 | |
| 106,998 |
Investments held in Trust Account | |
| 1,368,637 | |
| 7,527,369 |
Total Assets | | $ | 1,370,500 | | $ | 7,634,367 |
| | | | | | |
LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| | |
| |
Current liabilities: | |
| | |
| |
Accounts payable and accrued expenses | | $ | 3,303,836 | | $ | 1,886,312 |
Excise tax payable | | | 72,396 | | | — |
Income taxes payable | | | 187,225 | | | 187,225 |
Advances from related parties | | | 117,871 | | | 43,900 |
Bridge Note, net of discount | | | — | | | 292,800 |
Accrued interest on Exchange Note | | | 23,964 | | | — |
Additional Bridge Promissory note, net of discount | | | 102,726 | | | — |
Promissory note – M2B | | | 167,958 | | | — |
Exchange Note | | | 2,621,558 | | | — |
ELOC | | | 203,720 | | | — |
Promissory note – related party | | | 926,500 | | | 350,000 |
Extension Note, net of discount | | | 233,774 | | | — |
Bridge Note – Bifurcated Derivative | | | — | | | 364,711 |
Extension Note – Bifurcated Derivative | | | 22,872 | | | — |
PIPE Forward Contract Derivative | | | — | | | 170,666 |
Total Current Liabilities | |
| 7,984,400 | |
| 3,295,614 |
| | | | | | |
Deferred underwriting fee payable | |
| 4,370,000 | |
| 4,370,000 |
Total Liabilities | |
| 12,354,400 | |
| 7,665,614 |
| | | | | | |
Commitments | |
| | |
| |
Common stock, $0.0001 par value, subject to possible redemption; 114,966 and 694,123 shares issued and outstanding at redemption value of $11.15 and $10.65 per share as of December 31, 2023 and 2022, respectively | |
| 1,281,957 | |
| 7,395,349 |
Stockholders’ Deficit | | | | | | |
Common stock, $0.0001 par value; 50,000,000 shares authorized; 3,489,000 and 3,462,000 shares issued and outstanding (excluding 114,966 and 694,123 shares subject to redemption) as of December 31, 2023 and 2022, respectively | |
| 350 | |
| 347 |
Additional paid-in capital | |
| 550,246 | |
| 292,973 |
Accumulated deficit | |
| (12,816,453) | |
| (7,719,916) |
Total Stockholders’ Deficit | |
| (12,265,857) | |
| (7,426,596) |
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 1,370,500 | | $ | 7,634,367 |
The accompanying notes are an integral part of the consolidated financial statements.
F-92
DIGITAL HEALTH ACQUISITION CORP.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | |
|
| For the Years Ended December 31, | ||||
| | 2023 |
| 2022 | ||
General and administrative expenses | | $ | 2,593,765 | | $ | 3,594,967 |
Loss from operations | | | (2,593,765) | |
| (3,594,967) |
| | | | | | |
Other (expense) income: | | | | |
| |
Default interest expense – Bridge Note | | | (1,579,927) | | | — |
Interest expense – Bridge Note | | | (429,007) | | | (125,980) |
Interest expense – Additional Bridge | | | (12,642) | | | — |
Interest expense – M2B | | | (22,958) | | | — |
Interest expense – Extension Note | | | (133,748) | | | — |
Initial fair value of Additional Bridge Note | | | 11,111 | | | — |
Initial fair value of ELOC | | | (204,039) | | | — |
Change in fair value of Additional Bridge Note | | | (2,726) | | | — |
Change in fair value of Exchange Note | | | (97,814) | | | — |
Change in fair value of ELOC | | | 319 | | | — |
Change in fair value of Bridge Note – Bifurcated Derivative | | | 120,267 | | | (86,307) |
Change in fair value of Extension Note – Bifurcated Derivative | | | 1,630 | | | — |
Change in fair value of PIPE Forward Contract Derivative | | | 170,666 | | | (170,666) |
Interest earned on investments held in Trust Account | | | 358,767 | | | 922,644 |
Total other (expense) income | | | (1,820,101) | | | 539,691 |
| | | | | | |
Loss before provision for income taxes | | | (4,413,866) | | | (3,055,276) |
Provision for income taxes | | | — | | | (187,225) |
Net loss | | $ | (4,413,866) | | $ | (3,242,501) |
| | | | | | |
Basic and diluted weighted average common shares outstanding | | | 4,096,353 | |
| 12,741,219 |
Basic and diluted net loss per common share | | $ | (1.08) | | $ | (0.25) |
The accompanying notes are an integral part of the consolidated financial statements.
F-93
DIGITAL HEALTH ACQUISITION CORP.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| | | | | | | | | | | | | | |
| | | | | | | Additional | | | | | Total | ||
| | Common Stock | | Paid-in | | Accumulated | | Stockholders’ | ||||||
|
| Shares |
| Amount |
| Capital |
| Deficit |
| Deficit | ||||
Balance – December 31, 2021 | | 3,432,000 | | $ | 344 | | $ | — | | $ | (3,334,812) | | $ | (3,334,468) |
Accretion of common stock subject to redemption value | | — | | | — | | | — | | | (1,142,603) | | | (1,142,603) |
Issuance of 30,000 shares issued with Bridge Note, net of offering cost | | 30,000 | | | 3 | | | 284,421 | | | — | | | 284,424 |
Issuance of 173,913 warrants issued with Bridge Note, net of offering cost | | — | | | — | | | 8,552 | | | — | | | 8,552 |
Net loss | | — | | | — | | | — | | | (3,242,501) | | | (3,242,501) |
Balance – December 31, 2022 |
| 3,462,000 | | | 347 | | | 292,973 | | | (7,719,916) | | | (7,426,596) |
Issuance of 20,000 shares issued to settle legal claim |
| 20,000 | |
| 2 | |
| 214,198 | |
| — | |
| 214,200 |
Issuance of 7,000 shares and warrants issued with Extension Note, net of offering costs |
| 7,000 | |
| 1 | |
| 115,471 | |
| — | |
| 115,472 |
Excise tax payable attributable to redemption of common stock |
| — | |
| — | |
| (72,396) | |
| — | |
| (72,396) |
Accretion of common stock subject to redemption value |
| — | |
| — | |
| — | |
| (682,671) | |
| (682,671) |
Net loss |
| — | |
| — | |
| — | |
| (4,413,866) | | | (4,413,866) |
Balance – December 31, 2023 |
| 3,489,000 | | $ | 350 | | $ | 550,246 | | $ | (12,816,453) | | $ | (12,265,857) |
The accompanying notes are an integral part of the consolidated financial statements.
F-94
DIGITAL HEALTH ACQUISITION CORP.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | |
| | For the Years Ended | ||||
| | December 31, | ||||
|
| 2023 |
| 2022 | ||
Cash Flows from Operating Activities: | | | | | | |
Net loss | | $ | (4,413,866) | | $ | (3,242,501) |
Adjustments to reconcile net loss to net cash used in operating activities: | |
| | |
| |
Interest earned on investments held in Trust Account | |
| (358,767) | |
| (922,644) |
Initial loss on and change in fair value of ELOC | | | 203,720 | | | — |
Initial gain on fair value of Additional Bridge Note | | | (11,111) | | | — |
Change in fair value of Exchange Note | | | 97,814 | | | — |
Change in fair value of Additional Bridge Note | | | 2,726 | | | — |
Change in fair value of Bridge Note – Bifurcated Derivative | | | (120,267) | | | 86,307 |
Change in fair value of Extension Note – Bifurcated Derivative | | | (1,630) | | | — |
Change in fair value of PIPE Forward Contract Derivative | | | (170,666) | | | 170,666 |
Changes in operating assets and liabilities: | | | | | | |
Prepaid and other current assets | |
| — | |
| 457,605 |
Accounts payable and accrued expenses | |
| 1,631,724 | |
| 1,746,149 |
Default interest on Bridge Note | |
| 1,579,927 | |
| — |
Accrued interest – Bridge Note | | | 429,006 | | | 125,980 |
Accrued interest – Additional Bridge Note | | | 12,642 | | | — |
Accrued interest – M2B Note | | | 22,958 | | | — |
Accrued interest – Extension Note | | | 133,748 | | | — |
Income taxes payable | | | — | | | 187,225 |
Net cash used in operating activities | |
| (962,042) | |
| (1,391,213) |
| | | | | | |
Cash Flows from Investing Activities: | |
| | |
| |
Investment of cash into Trust Account | |
| (350,000) | |
| (350,000) |
Cash withdrawn from Trust Account to pay franchise and income taxes | | | 71,436 | | | 110,472,253 |
Cash withdrawn from Trust Account in connection with redemption | | | 6,796,063 | | | — |
Net cash provided by investing activities | |
| 6,517,499 | |
| 110,122,253 |
| | | | | | |
Cash Flows from Financing Activities: | |
| | |
| |
Advances from related party | |
| 95,037 | |
| — |
Repayment of advances from related party | | | (21,066) | | | — |
Proceeds from Bridge Note | | | 100,000 | | | 800,000 |
Proceeds from M2B Note | | | 145,000 | | | — |
Payment of Financing Cost in Bridge Note | | | — | | | (61,800) |
Proceeds from promissory note – related party | |
| 576,500 | |
| 350,000 |
Proceeds from promissory note | |
| 240,000 | |
| — |
Redemption of common stock | | | (6,796,063) | | | (110,472,254) |
Net cash used in financing activities | |
| (5,660,592) | |
| (109,384,054) |
| | | | | | |
Net Change in Cash | |
| (105,135) | |
| (653,014) |
Cash – Beginning of year | |
| 106,998 | |
| 760,012 |
Cash – End of year | | $ | 1,863 | | $ | 106,998 |
| | | | | | |
Non-cash investing and financing activities: | |
| | |
| |
Common stock issued for legal settlement | | $ | 214,200 | | $ | 284,424 |
Financing costs included in Extension Note | | $ | 60,000 | | $ | — |
Warrants issued as financing cost in Extension Note | | $ | 40,130 | | $ | 8,552 |
Bridge Promissory note, net of discount – settled with Exchange Note | | $ | 2,279,300 | | $ | — |
Bridge Note – Embedded Derivative – settled with Exchange Note | | $ | 244,444 | | $ | — |
Excise tax attributable to redemption of common stock | | $ | 72,396 | | $ | — |
Common stock issued as financing cost in Extension Note | | $ | 78,349 | | $ | — |
The accompanying notes are an integral part of the consolidated financial statements.
F-95
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
NOTE 1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
Digital Health Acquisition Corp. (the “Company” or “DHAC”) is a blank check company incorporated as a Delaware corporation on March 30, 2021. The Company was incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar Business Combination with one or more businesses (the “Business Combination”).
On June 9, 2022, DHAC Merger Sub I, Inc. (“Merger Sub I”), a Delaware corporation and a wholly owned subsidiary of the Company, was formed. On June 9, 2022, DHAC Merger Sub II, Inc. (“Merger Sub II”), a Texas corporation and a wholly owned subsidiary of the Company, was formed.
As of December 31, 2023, the Company had not commenced any significant operations. All activity for the period from inception, the date which operations commenced, through December 31, 2023 relates to the Company’s formation, the Company’s Initial Public Offering (as defined below), and identifying a target company for a Business Combination. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering (as defined below).
The registration statement for the Company’s Initial Public Offering was declared effective on November 3, 2021. On November 8, 2021, the Company consummated the Initial Public Offering of 11,500,000 units (the “Units” and, with respect to the shares of common stock included in the Units sold, the “Public Shares”), which includes the full exercise by the underwriter of its over-allotment option in the amount of 1,500,000 Units, at $10.00 per Unit, generating gross proceeds of $115,000,000, which is described in Note 3. On October 20, 2022, in connection with the stockholders meeting to approve the extension, 10,805,877 shares of DHAC’s common stock were redeemed leaving 694,123 shares of common stock subject to redemption. On November 6, 2023, in connection with the stockholders meeting to approve the extension, 579,157 shares of DHAC’s common stock were redeemed leaving 114,966 shares of common stock subject to redemption.
Simultaneously with the closing of the Initial Public Offering, the Company consummated the sale of 557,000 units (each, a “Private Placement Unit” and, collectively, the “Private Placement Units”) at a price of $10.00 per Private Placement Unit in a private placement to Digital Health Sponsor LLC (the “Sponsor”), generating gross proceeds of $5,570,000, which is described in Note 4. As of November 8, 2021, the Company received $3,680,000 from the proceeds of the Private Placement and recorded $1,890,000 in subscription receivable. The Sponsor paid the subscription in full on November 12, 2021.
Transaction costs amounted to $6,877,164, consisting of $1,955,000 of underwriting fees, $4,370,000 of deferred underwriting fees and $552,164 of other offering costs. In addition, cash of $9,478 was held outside of the Trust Account (as defined below) and is available for the payment of offering costs and for working capital purposes.
Following the closing of the Initial Public Offering on November 8, 2021, an amount of $116,725,000 ($10.15 per Unit) from the net proceeds of the sale of the Units in the Initial Public Offering and the sale of the Private Placement Units was placed in a trust account (the “Trust Account”), invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or in any open-ended investment company that holds itself out as a money market fund meeting the conditions of Rule 2a-7 of the Investment Company Act of 1940, as amended (the “Investment Company Act”). The Trust Account is intended as a holding place for funds pending the earliest to occur of either (i) the completion of the initial Business Combination; (ii) the redemption of any public shares properly submitted in connection with a stockholder vote to amend the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares if the Company does not complete the initial Business Combination within 27 months from the closing of the Initial Public Offering (as extended as of December 31, 2023) or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; or (iii) absent an initial Business Combination within 27 months from the closing of the Initial Public Offering (as extended as of December 31, 2023), the Company’s return of the funds held in the Trust Account to the Company’s public stockholders as part of the Company’s redemption of the public shares.
On October 20, 2022, stockholders of DHAC approved a proposal to amend DHAC’s amended and restated certificate of incorporation to (a) extend the date by which DHAC has to consummate a Business Combination (the “Extension”) for an additional three (3) months, from November 8, 2022 to February 8, 2023, (b) provide DHAC’s board of directors the ability to further extend the date by which DHAC has to consummate a Business Combination up to three (3) additional times for three (3) months each time, for a
F-96
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
maximum of nine (9) additional months if the Sponsor pays an amount equal to $350,000 for each three-month extension (the “Extension Fee”), which amount shall be deposited in the trust account of DHAC; provided, that if as of the time of an extension DHAC has filed a Form S-4 registration statement in connection with its initial Business Combination, then no Extension Fee would be required in connection with such extension; provided further that for each three — month extension (if any) following such extension where no deposit into the Trust Account or other payment has been made, an Extension Fee is required, and (c) allow for DHAC to provide redemption rights to DHAC’s public stockholders in accordance with the requirements of the amended and restated certificate of incorporation without complying with the tender offer rules. In connection with such stockholder vote, an aggregate of 10,805,877 shares of DHAC’s common stock were redeemed leaving 4,156,123 shares issued and outstanding and entitled to vote as of October 20, 2022. The Company subsequently extended the date by which the Company has to consummate a Business Combination pursuant to the three additional three — month extensions to November 8, 2023, and deposited an aggregate of $700,000 into the Trust Account as extension fees.
On September 8, 2023, DHAC held a Special Meeting and the stockholders approved an amendment of the Company’s amended and restated certificate of incorporation (as amended from time to time, the “Charter”) to expand the methods that the Company may employ to not become subject to the “penny stock” rules of the U.S. Securities and Exchange Commission (“SEC”). On September 8, 2023, DHAC filed such amendment, which provided that DHAC would be able to consummate the Business Combination even if as a result of the transactions the combined company does not have net tangible assets of at least $5,000,001 upon consummation of such business combination.
On November 6, 2023, DHAC held its 2023 annual stockholders meeting (“2023 Annual Meeting”). At the 2023 Annual Meeting, the stockholders of DHAC approved amendments to DHAC’s Charter to extend the date by which the Company must consummate a Business Combination (as defined in the Charter) up to four (4) times, each by an additional three (3) months, for an aggregate of twelve (12) additional months (i.e., from November 8, 2023 up to November 8, 2024) or such earlier date as determined by the Company’s board of directors. In connection with the amended Charter, on November 6, 2023, DHAC extended the period of time that it has to consummate its business combination by three months from November 8, 2023 to February 8, 2024. In addition, on February 2, 2024, DHAC further extended the period of time that it has to consummate its business combination by another three months from February 8, 2024 to May 8, 2024.
Furthermore, at the 2023 Annual Meeting, the stockholders of DHAC also approved an amendment to DHAC’s investment management trust agreement (the “Trust Agreement”), dated as of November 3, 2021 and as amended on October 26, 2022, by and between the Company and Continental Stock Transfer & Trust Company, which allows the Company to extend the business combination period from November 8, 2023 to up to four (4) times, each by an additional three (3) months, for an aggregate of twelve (12) additional months to November 8, 2024 (the “Combination Period”).
In connection with the 2023 Annual Meeting and amendments to DHAC’s Charter and Trust Agreement, 579,157 shares of Common Stock were redeemed.
The Company’s Business Combination must be with one or more target businesses that together have a fair market value equal to at least 80% of the net balance in the Trust Account (as defined below) (excluding the amount of deferred underwriting discounts held and taxes payable on the income earned on the Trust Account) at the time of the signing an agreement to enter into a Business Combination. However, the Company will only complete a Business Combination if the post-Business Combination company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act. There is no assurance that the Company will be able to successfully effect a Business Combination.
The Company will provide the Company’s public stockholders with the opportunity to redeem all or a portion of their common shares in connection with the initial Business Combination either (i) in connection with a general meeting called to approve the Business Combination or (ii) without a stockholder vote by means of a tender offer. The decision as to whether the Company will seek stockholder approval of a proposed Business Combination or conduct a tender offer will be made by the Company, solely in its discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would require the Company to seek stockholder approval under applicable law or stock exchange listing requirement. The public stockholders will be entitled to redeem their shares at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account calculated as of two business days prior to the consummation of the initial Business Combination, including
F-97
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
interest earned on the funds held in the Trust Account and not previously released to the Company to pay its taxes, divided by the number of then outstanding public shares, subject to the limitations.
If the Company is unable to complete its initial Business Combination within the Combination Period, the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account (less taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any) and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholders and the Company’s board of directors, liquidate and dissolve, subject, in each case, to the Company’s obligations under Delaware law to provide for claims of creditors and in all cases subject to the other requirements of applicable law. The shares of common stock subject to redemption are recorded at a redemption value and classified as temporary equity in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 480, “Distinguishing Liabilities from Equity”.
The Sponsor, along with certain advisors, officers and directors, has entered into a letter agreement with the Company, pursuant to which they have agreed to (i) waive their redemption rights with respect to their founder shares (as defined in Note 5) and public shares in connection with the completion of the initial Business Combination; (ii) waive their redemption rights with respect to their founder shares and public shares in connection with a stockholder vote to approve an amendment to the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares. If the Company have not consummated an initial Business Combination within the Combination Period or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; (iii) waive their rights to liquidating distributions from the Trust Account with respect to their founder shares if the Company fails to complete the initial Business Combination within the Combination Period, although they will be entitled to liquidating distributions from the Trust Account with respect to any public shares they hold if the Company fails to complete the initial Business Combination within the prescribed time frame; and (iv) vote any founder shares held by them and any public shares purchased during or after the Initial Public Offering (including in open market and privately negotiated transactions) in favor of the initial Business Combination.
The Company’s Sponsor has agreed that it will be liable to the Company if and to the extent any claims by a third party (other than the Company’s independent registered public accounting firm) for services rendered or products sold to the Company, or a prospective target business with which the Company have entered into a written letter of intent, confidentiality or other similar agreement or Business Combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.15 per public share and (ii) the actual amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.15 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). However, the Company has not asked the Sponsor to reserve for such indemnification obligations, nor have the Company independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations and the Company believes that the Company’s Sponsor’s only assets are securities of the Company. Therefore, the Company cannot assure that the Sponsor would be able to satisfy those obligations.
On June 15, 2022, DHAC entered into the original Business Combination Agreement, by and among DHAC, DHAC Merger Sub I, Inc. (“Merger Sub I”), DHAC Merger Sub II, Inc. (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”), VSee Lab, Inc., a Delaware corporation (“VSee”), and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”). On August 9, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the First Amended and Restated Business Combination Agreement to provide for the concurrent execution of financing documents for a PIPE financing consisting of convertible notes and warrants and delivery of the Cassel Salpeter’s opinion to the Board. On October 6, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into a Second Amended and Restated Business Combination Agreement to make the consideration payable to VSee and iDoc stockholders 100% DHAC common stock and to provide for the concurrent execution of amended PIPE financing documents providing for the issuance of the shares and warrants to the PIPE investors. On November 3, 2022, the parties entered into a First Amendment to the Second Amended and Restated Business Combination Agreement to remove a closing condition that DHAC have at least $10 million in cash proceeds from the transactions at closing. On July 11, 2023, each of the PIPE Investors provided notice to
F-98
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
the Company that since a closing condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing. Accordingly, the PIPE financing was terminated. On November 21, 2023, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the Third Amended and Restated Business Combination Agreement (as amended and restated, the “Business Combination Agreement” and the transactions contemplated thereby, the “Business Combination”) to, among other things, provide for the removal of the PIPE financing and the concurrent execution of the Additional Bridge Financing, the Exchange Financing, the Quantum Financing, the Equity Financing and the Loan Conversions, which are described in Note 6 — Commitments. The DHAC Board has (i) approved and declared advisable the Business Combination Agreement, the Business Combination and the other transactions contemplated thereby and (ii) resolved to recommend approval of the Business Combination Agreement and related matters by the stockholders of DHAC.
Pursuant to the Business Combination Agreement and subject to the terms and conditions set forth therein, Merger Sub I will merge with and into VSee (the “VSee Merger”), with VSee surviving the VSee Merger as a wholly owned subsidiary of DHAC, and Merger Sub II will merge with and into iDoc (the “iDoc Merger” and, together with the VSee Merger, the “Mergers”), with iDoc surviving the iDoc Merger as a wholly owned subsidiary of DHAC. At the effective time of the Mergers (the “Effective Time”), DHAC will change its name to VSee Health, Inc.
NASDAQ Trading Status
On March 31, 2023, DHAC received a letter from the staff (the “Staff”) at The Nasdaq Global Market (“Nasdaq Global”) notifying DHAC that for the 30 consecutive trading days prior to the date of the Letter, DHAC’s securities listed on the Nasdaq Global (including the Common Stock, Units and Warrants) (the “Securities”) had traded at a value below the minimum $50,000,000 “Market Value of Listed Securities (“MVLS”) requirement set forth in Nasdaq Listing Rule 5450(b)(2)(A), which is required for continued listing of DHAC’s Securities on Nasdaq Global. In accordance with Nasdaq listing rule 5810I(3)I, DHAC had 180 calendar days, or until September 27, 2023, to regain compliance.
On May 23, 2023, DHAC received a second letter from the Staff notifying DHAC that for the prior 30 consecutive business days, DHAC’s market value of publicly held shares (“MVPHS”) was below the $15 million required for continued listing on the Nasdaq Global and therefore, DHAC no longer met Nasdaq Listing Rule 5450(b)(3)(C) (the “MVPHS Requirement”). In accordance with Nasdaq Listing Rule 5810I(3)(D), DHAC had 180 calendar days, or until November 20, 2023, to regain compliance.
On September 28, 2023, DHAC received a third letter from the Staff notifying DHAC that the Staff had determined to delist DHAC’s Securities because it had not regained compliance with the MVLS standard. Pursuant to the third letter, on October 4, 2023, DHAC requested a hearing (the “Hearing”) to appeal this determination and also applied to transfer the listing of its Securities from Nasdaq Global to the Nasdaq Capital Market (“NasdaqCM”).
On October 9, 2023, DHAC received a fourth letter from the Staff notifying DHAC that its not meeting the 400 total shareholders requirement under the Nasdaq Listing Rule 5450(a)(2) served as an additional basis for delisting DHAC’s Securities from Nasdaq Global.
On October 26, 2023, the Nasdaq Listing Qualifications Department of the Nasdaq Stock Market notified DHAC in writing (the “Notice”) that its application to transfer the listing of its Securities to NasdaqCM had been approved. DHAC’s Securities were transferred to the NasdaqCM at the opening of business on October 30, 2023. On November 1, 2023, DHAC received a letter from the Nasdaq Global Hearing panel that due to DHAC’s transfer of its listed Securities to NasdaqCM, the Hearing on November 30, 2023 regarding non-compliance with the Nasdaq Global listing standards had been cancelled.
As of October 30, 2023, DHAC’s Securities are listed and traded on The Nasdaq Stock Market on NasdaqCM and will continue to be listed and traded on NasdaqCM.
F-99
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements are presented in U.S. dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the accounting and disclosure rules and regulations of the SEC.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Liquidity and Going Concern
The Company may raise additional capital through loans or additional investments from the Sponsor or its stockholders, officers, directors, or third parties. The Company’s officers and directors and the Sponsor may but are not obligated to loan the Company funds, from time to time, in whatever amount they deem reasonable in their sole discretion, to meet the Company’s working capital needs. Based on the foregoing, the Company believes it will have sufficient working capital and borrowing capacity from the Sponsor or an affiliate of the Sponsor, or certain of the Company directors to meet its needs through the earlier of the consummation of a Business Combination or at least one year from the date that the consolidated financial statements were issued.
As of December 31, 2023, the Company had a cash balance of $1,863 and a working capital deficit of $7,982,537. In addition, in connection with the Company’s assessment of going concern considerations in accordance with ASC 205-40, “Presentation of Financial Statements — Going Concern,” management has determined that the liquidity condition, mandatory liquidation and subsequent dissolution on November 8, 2024 raise substantial doubt about the Company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities of the Company as of December 31, 2023. The Company intends to complete a Business Combination before the mandatory liquidation date or file for an extension.
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
F-100
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Offering Costs
Offering costs consisted of legal, accounting, and other expenses incurred through the Initial Public Offering that were directly related to the Initial Public Offering. Offering costs were allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs allocated to warrants were allocated to equity. Offering costs allocated to the common stock issued were initially charged to temporary equity.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the consolidated financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. The most significant accounting estimates were the assumptions used to fair value the PIPE Forward Contract, the Extension Note Bifurcated Derivative, the Bridge Note Bifurcated Derivative, the Additional Bridge Note and the Exchange Note (each term as defined below). Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company did not have any cash equivalents as of December 31, 2023 and 2022.
Investments Held in Trust Account
At December 31, 2023 and 2022, the assets held in the Trust Account were held in money market funds, which are invested primarily in U.S. Treasury securities.
Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC Topic 480, “Distinguishing Liabilities from Equity.” Common stock subject to mandatory redemption is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified in temporary equity. At all other times, common stock is classified as stockholders’ deficit. The Company’s common stock sold in the Initial Public Offering features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence of uncertain future events. Accordingly, at December 31, 2023 and 2022, common stock subject to possible redemption is presented at redemption value as temporary equity, outside of the stockholders’ deficit section of the Company’s consolidated balance sheets.
The Company recognizes changes in redemption value immediately as they occur and adjusts the carrying value of redeemable common stock to equal the redemption value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. Immediately upon the closing of the Initial Public Offering, increases or decreases in the carrying amount of redeemable common stock are affected by charges against additional paid-in capital and accumulated deficit.
F-101
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
At December 31, 2023 and 2022, the common stock subject to possible redemption reflected in the consolidated balance sheets is reconciled in the following table:
| | | |
Gross proceeds |
| $ | 115,000,000 |
Less: | |
|
|
Proceeds allocated to public warrants | |
| (12,483,555) |
Common stock issuance costs | |
| (6,923,767) |
Plus: | |
|
|
Accretion of carrying value to redemption value | |
| 21,132,322 |
Common stock subject to possible redemption, December 31, 2021 | | | 116,725,000 |
Plus: | | | |
Accretion of carrying value to redemption value | | | 1,142,603 |
Less: | | | |
Redemptions | | | (110,472,254) |
Common stock subject to possible redemption, December 31, 2022 | | | 7,395,349 |
Plus: | | | |
Accretion of carrying value to redemption value | | | 682,671 |
Less: | | | |
Redemptions | | | (6,796,063) |
Common stock subject to possible redemption, December 31, 2023 | | $ | 1,281,957 |
Income Taxes
The Company accounts for income taxes under ASC 740, “Income Taxes.” ASC 740 requires the recognition of deferred tax assets and liabilities for both the expected impact of differences between the unaudited condensed consolidated financial statements and tax basis of assets and liabilities and for the expected future tax benefit to be derived from tax loss and tax credit carry forwards. ASC 740 additionally requires a valuation allowance to be established when it is more likely than not that all or a portion of deferred tax assets will not be realized. ASC 740-270-25-2 requires that an annual effective tax rate be determined, and such annual effective rate applied to year to date income in interim periods under ASC 740-270-30-5. As of December 31, 2023 and 2022, the Company’s deferred tax asset had a full valuation allowance recorded against it.
ASC 740-270-25-2 requires that an annual effective tax rate be determined and such annual effective rate applied to year to date income in interim periods under ASC 740-270-30-5. The Company’s effective tax rate was 0.0% and 6.1% for the years ended December 31, 2023 and 2022, respectively. The effective tax rate differs from the statutory tax rate of 21.0% for the years ended December 31, 2023 and 2022 due to the valuation allowance on the deferred tax assets.
ASC 740 also clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. ASC 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim period, disclosure and transition.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2023 and 2022. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
The Company has identified the United States as its only “major” tax jurisdiction. The Company has been subject to income taxation by major taxing authorities since inception. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
F-102
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Net Loss per Common Stock
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” Net loss per common stock is computed by dividing net loss by the weighted average number of common stocks outstanding for the period. Accretion associated with the redeemable shares of common stock is excluded from net loss per common stock as the redemption value approximates fair value.
The calculation of diluted loss per share does not consider the effect of the warrants issued in connection with the (i) Initial Public Offering, and (ii) the private placement (iii) the Bridge Notes and the Extension Note because the exercise of the warrants is contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive. The warrants are exercisable to purchase 12,256,999 shares of common stock in the aggregate. As of December 31, 2023 and 2022, the Company did not have any dilutive securities or other contracts that could, potentially, be exercised or converted into common stock and then share in the earnings of the Company. As a result, diluted net loss per common stock is the same as basic net loss per common stock for the periods presented.
The following table reflects the calculation of basic and diluted net loss per common stock (in dollars, except per share amounts):
| | | | | | |
| | For the years ended December 31, | ||||
|
| 2023 |
| 2022 | ||
|
| Common Stock |
| Common Stock | ||
Basic and diluted net loss per of common stock |
| |
|
| |
|
Numerator: |
| |
|
| |
|
Allocation of net loss | | $ | (4,413,866) | | $ | (3,242,501) |
Denominator: | |
|
| |
|
|
Basic and diluted weighted average common shares outstanding | |
| 4,096,353 | |
| 12,741,219 |
Basic and diluted net loss per common share | | $ | (1.08) | | $ | (0.25) |
Concentration of Credit Risk
The Company has significant cash balances at a financial institutions which throughout the year regularly exceeded the federally insured limited of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial condition, results of operations, and cash flows.
Warrant Instruments
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”), and ASC 815, “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. The Company has analyzed the Public Warrants, Private Warrants, Bridge Warrants and the Extension Warrants and determined they are considered to be freestanding instruments and do not exhibit any of the characteristics in ASC 480 and therefore are not classified as liabilities under ASC 480. The warrants meet all of the requirements for equity classification under ASC 815 and therefore are classified in equity.
F-103
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Financial Instruments
The Company evaluates its financial instruments to determine if such instruments should be accounted for as a liability under ASC 480 or if they are derivatives or contain features that qualify as bifurcated derivatives in accordance with ASC 815.
Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. Derivative assets and liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date. The Company has determined the PIPE financing agreement is a derivative instrument, the Bridge Notes and the Extension Note’s early redemption provisions are embedded feature that are required to be bifurcated as a derivative. FASB ASC 470-20, “Debt with Conversion and Other Options,” addresses the allocation of proceeds from the issuance of debt into its debt and bifurcated derivative components. The Company applies this guidance to allocate the Bridge Notes and the Extension Note proceeds between the Bridge Notes and the Extension Note, respectively, and the respective bifurcated derivative, using the residual method by allocating the principal first to fair value of the bifurcated derivative and then to the debt.
The Exchange Note and the Additional Bridge Note represent share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Exchange Note and the Additional Bridge Note are required to be accounted for as a liability under ASC 480. As required under ASC 480, the liabilities will be re-measured at fair value at each reporting period with the changes in the fair value of the liabilities recognized in earnings.
Fair Value Measurement
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement. |
Recent Accounting Pronouncements
In June 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”). This update requires financial assets measured at amortized cost basis to be presented at the net amount expected to be collected. The measurement of expected credit losses is based on relevant information about past events, including historical experience, current conditions, and reasonable and supportable forecasts that affect the collectability of the reported amount. Since June 2016, the FASB issued clarifying updates to the new standard including changing the effective date for smaller reporting companies. The guidance is effective for fiscal years beginning after December 15, 2022, and interim periods within those fiscal years, with early adoption permitted. The Company adopted ASU 2016-13 on January 1, 2023. The adoption of ASU 2016-13 did not have a material impact on its consolidated financial statements.
F-104
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
In December 2023, the FASB issued ASU No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures” (“ASU 2023-09”), which will require the Company to disclose specified additional information in its income tax rate reconciliation and provide additional information for reconciling items that meet a quantitative threshold. ASU 2023-09 will also require the Company to disaggregate its income taxes paid disclosure by federal, state and foreign taxes, with further disaggregation required for significant individual jurisdictions. ASU 2023-09 will become effective for annual periods beginning after December 15, 2024. The Company is still reviewing the impact of ASU 2023-09.
Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s consolidated financial statements.
Inflation Reduction Act of 2022
On August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for, among other things, a new U.S. federal 1% excise tax on certain repurchases of stock by publicly traded U.S. domestic corporations and certain U.S. domestic subsidiaries of publicly traded foreign corporations occurring on or after January 1, 2023. The excise tax is imposed on the repurchasing corporation itself, not its stockholders from which shares are repurchased. The amount of the excise tax is generally 1% of the fair market value of the shares repurchased at the time of the repurchase. However, for purposes of calculating the excise tax, repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market value of stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department of the Treasury (the “Treasury”) has been given authority to provide regulations and other guidance to carry out and prevent the abuse or avoidance of the excise tax.
Any redemption or other repurchase that occurs after December 31, 2022, in connection with a Business Combination, extension vote or otherwise, may be subject to the excise tax. Whether and to what extent the Company would be subject to the excise tax in connection with a Business Combination, extension vote or otherwise would depend on a number of factors, including (i) the fair market value of the redemptions and repurchases in connection with the Business Combination, extension or otherwise, (ii) the structure of a Business Combination, (iii) the nature and amount of any “PIPE” or other equity issuances in connection with a Business Combination (or otherwise issued not in connection with a Business Combination but issued within the same taxable year of a Business Combination) and (iv) the content of regulations and other guidance from the Treasury. In addition, because the excise tax would be payable by the Company and not by the redeeming holder, the mechanics of any required payment of the excise tax have not been determined. The foregoing could cause a reduction in the cash available on hand to complete a Business Combination and in the Company’s ability to complete a Business Combination.
The Company held a meeting on November 6, 2023 to vote on a proposal to amend the Charter to extend the date by which the Company must consummate a Business Combination or, if it fails to do so, cease its operations and redeem or repurchase 100% of the shares of the Company’s common stock issued in the Company’s initial public offering, from November 8, 2023 to February 8, 2024, with additional extensions up to November 8, 2024. In connection with the meeting, 579,157 shares of the Company’s common stock were redeemed with a total redemption payment of $6,462,504. As a result, the Company booked a liability of $72,396 for the excise tax based on 1% of shares redeemed during the reporting period. For interim periods, an entity is not required to estimate future stock repurchases and stock issuances to measure its excise tax obligation. Rather, an entity can generally record the obligation on an as-incurred basis. In other words, the excise tax obligation recognized at the end of a quarterly financial reporting period is calculated as if the end of the quarterly period was the end of the annual period for which the excise tax obligation is payable.
NOTE 3. INITIAL PUBLIC OFFERING
In the “Initial Public Offering,” the Company sold 11,500,000 units, which included a full exercise by the underwriters of their over-allotment option in the amount of 1,500,000 Units, at a purchase price of $10.00 per unit. Each unit consists of one common share and one warrant. Each warrant will entitle the holder to purchase one (1) share of common stock at a price of $11.50 per whole share, subject to adjustment (see Note 7). Each warrant will become exercisable 30 days after the completion of the initial Business Combination or 12 months from the closing of the Initial Public Offering and will expire five years after the completion of the initial Business Combination, or earlier upon redemption or liquidation.
F-105
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
NOTE 4. PRIVATE PLACEMENT
Simultaneously with the closing of the Initial Public Offering, the Sponsor purchased 557,000 units, at $10.00 per unit for a total purchase price of $5,570,000 in a private placement. As of November 8, 2021, the Company received $3,680,000 from the proceeds of the Private Placement and recorded $1,890,000 in subscription receivable. The Sponsor paid the subscription in full on November 12, 2021. The private placement units are identical to the units sold in the Initial Public Offering but are not redeemable. There will be no underwriting fees or commissions with respect to the private placement units. The proceeds from the private placement were added to the proceeds of Initial Public Offering and placed in a Trust Account in the United States maintained by Continental Stock Transfer & Trust Company, as trustee. If the Company does not complete its initial business combination within 27 months (as extended as of December 31, 2023), the Sponsor will waive any and all rights and claims to any proceeds and interest thereon in respect to the private placement units and the proceeds from the sale of the private placement units will be included in the liquidating distribution to the holders of the Company’s public shares.
The Sponsor, advisors, officers and directors have entered into a letter agreement with the Company, pursuant to which they have agreed to (i) waive their redemption rights with respect to their founder shares and public shares in connection with the completion of the initial Business Combination; (ii) waive their redemption rights with respect to their founder shares and public shares in connection with a stockholder vote to approve an amendment to the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares if the Company has not consummated an initial Business Combination within the Combination Period or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; (iii) waive their rights to liquidating distributions from the Trust Account with respect to their founder shares if the Company fails to complete the initial Business Combination within the Combination Period, although they will be entitled to liquidating distributions from the Trust Account with respect to any public shares they hold if the Company fails to complete the initial Business Combination within the prescribed time frame; and (iv) vote any founder shares held by them and any public shares purchased during or after the Initial Public Offering (including in open market and privately negotiated transactions) in favor of the initial Business Combination.
NOTE 5. RELATED PARTY TRANSACTIONS
Founder Shares
On June 7, 2021, the Sponsor, along with certain of the Company’s directors, officers and advisors purchased 4,312,500 shares for an aggregate purchase price of $25,000. In October 2021, the Sponsor, officers and certain advisors forfeited an aggregate of 1,437,500 shares of common stock, resulting in 2,875,000 founder shares outstanding. Such shares are referred to herein as “founder shares” or “insider shares”.
Sponsor Note Payable
On June 7, 2021, the Sponsor agreed to loan the Company up to $625,000 to be used for a portion of the expenses of the Initial Public Offering. These notes were non-interest bearing and any outstanding balance on the notes was due immediately following the Company’s Initial Public Offering. There was an amount of $602,720 borrowed under the Notes. The Notes were repaid on November 12, 2021 Borrowings under this note are no longer available.
Advances from Related Party
As of November 8, 2021, the Sponsor paid for $402,936 on expenses on behalf of the Company. The advance was repaid on November 12, 2021.
The Company owes the Sponsor $117,871 and $43,900 as of December 31, 2023 and 2022, respectively.
Working Capital Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working
F-106
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Capital Loans”). If the Company completes a Business Combination, the Company will repay the Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company may use a portion of the proceeds held outside the Trust Account to repay the Working Capital Loans, but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would be repaid upon consummation of a Business Combination, without interest. As of December 31, 2023 and 2022, the Company had no borrowings under the Working Capital Loans.
Promissory Note Related Party
On October 24, 2022, the Company issued an unsecured promissory note in the aggregate principal amount of $350,000 to the Sponsor. The Company deposited to the trust account all of the loan amount and extended the amount of time it has available to complete a business combination from November 8, 2022 to February 8, 2023. On November 21, 2023, DHAC entered into a Conversion Securities Purchase Agreement (“Conversion SPA”) with the Sponsor, pursuant to which the loans in aggregate amount of $350,000 will be converted into Series A Preferred Shares at the Closing.
On February 2, 2023, SCS Capital Partners LLC, a Sponsor affiliate and a stockholder who currently holds more than 5% shares in the Company, issued a $250,000 interest-free loan to DHAC for Nasdaq fee payment and litigation expense, and on August 17, 2023, such loan was amended and restated to include an additional $315,000 interest-free loan to DHAC for operating expenses, making the aggregate principal amount to be $565,000. On May 5, 2023, SCS Capital Partners, LLC issued a $200,000 loan to DHAC for payment of the term extension fee. The related note bears interest of 10%, matures on May 5, 2024. The proceeds of the note were used to extend the liquidation date of DHAC from May 8, 2023 to August 8, 2023. On November 21, 2023, DHAC entered into a Conversion SPA with SCS Capital Partners LLC, pursuant to which the loans in aggregate amount of $765,000 will be converted into Series A Preferred Shares at the Closing.
Promissory Note — M2B
On October 4, 2023, the Company issued a promissory note in the aggregate principal amount of $165,000 to M2B Funding Corp., an affiliate of the Sponsor, for a purchase price of $150,000 and included $5,000 in legal fees (the “M2B Note”). The original issued discount of $15,000 plus $5,000 of offering cost were recorded as a debt discount and amortized over the term of the note. The note had a 10% interest and a maturity date of January 5, 2024. The Company defaulted on the note and amended the note on January 22, 2024. As a result of the amendment, the Company was to repay the remaining amount due by February 8, 2024. On January 31, 2024, the note was paid in full for a total of $190,750.
As of December 31, 2023, the M2B Note net of unamortized debt discount was $167,958. The Company recognized interest expense of $22,958 for the year ended December 31, 2023.
Post-Business Combination Financing Transactions
Bridge Financing
In connection with the execution of the Second Business Combination Agreement, DHAC, along with VSee and iDoc, the target companies in the Business Combination, entered into a securities purchase agreement with the Bridge Investor, who is also an investor in the Sponsor, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments (the “Bridge Warrants”) and (ii) 30,000 shares of DHAC common stock.
As a result of the default, on November 21, 2023, DHAC, VSee and iDoc entered into an exchange agreement (the “Exchange Agreement”) with the Bridge Investor, pursuant to which the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for a senior secured convertible promissory note issued by DHAC with an
F-107
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
aggregate principle value of $2,523,744 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc. The Exchange Note will bear interest at a rate of 8% per annum and will be convertible into shares of common stock of the Company at a fixed conversion price of $10.00 per share (see Note 6 — Commitments — Bridge Financing and Bifurcated Derivative for further information).
On November 21, 2023, DHAC, VSee and iDoc entered into a letter agreement, pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,556 note purchased at a later date mutually agreed upon by DHAC and the Bridge Investor, which is currently expected to be upon the filing of an amendment to DHAC’s Registration Statement on Form S-4 in connection with the Business Combination (the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at a fixed conversion price of $10.00 per share (see Note 6 — Commitments — Additional Bridge Financing for further information).
Loan Conversions
On November 21, 2023, DHAC, VSee, and/or iDoc, as applicable, entered into Securities Purchase Agreements (the “Conversion SPAs”) with various lenders of each of DHAC, VSee and iDoc, pursuant to which certain indebtedness owed by DHAC, VSee and iDoc will be converted into Series A Preferred Stock of DHAC at the closing of the Business Combination.
On November 21, 2023, DHAC and VSee entered into a Conversion SPA with Whacky — a Sponsor Affiliate, pursuant to which certain loans incurred by VSee to Whacky in the aggregate amount of $220,000 will be converted into Series A Preferred Shares at the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Mark E. Munro Charitable Remainder Unitrust (“Munro Trust”) — a Sponsor Affiliate, pursuant to which certain loans incurred by iDoc to Munro Trust in the aggregate amount of $300,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and VSee, entered into a Conversion SPA with the Bridge Investor who is also an investor in the Sponsor, which Conversion SPA was amended and restated on February 13, 2024 (as further described under Note 11 — Subsequent Events), pursuant to which certain loans incurred by VSee to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Tidewater — a Sponsor Affiliate, which Conversion SPA was amended and restated on February 13, 2024 (as further described under Note 11 — Subsequent Events), pursuant to which certain loans incurred by iDoc to Tidewater in the aggregate amount of $585,000 will be convertible into the Company’s Common Stock following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with the Bridge Investor who is also an investor in the Sponsor, which Conversion SPA was amended and restated on February 13, 2024 (as further described under Note 11 — Subsequent Events), pursuant to which certain loans incurred by iDoc to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
Quantum Financing Securities Purchase Agreement
On November 21, 2023, DHAC entered into the Quantum Purchase Agreement, pursuant to which the Quantum Investor subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note in the aggregate principal amount of $3,000,000 (see Note 6 — Commitments — Quantum Financing Securities Purchase Agreement for further information).
F-108
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Equity Financing
On November 21, 2023, DHAC entered into the Equity Purchase Agreement with an affiliate of the Bridge Investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s common stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the Closing (see Note 6 — Commitments — Equity Financing for further information).
Administrative Services Agreement
The Company agreed, commencing on November 3, 2021, to pay an affiliate of the Sponsor a total of $10,000 per month for office space and secretarial, administrative, and other services. The monthly fees will cease upon completion of an initial business combination or liquidation. For the year ended December 31, 2023, the Company incurred $120,000, of which $55,500 are included in accrued expenses in the accompanying consolidated balance sheets as of December 31, 2023. For the year ended December 31, 2022, the Company incurred $120,000, of which $10,550 is included in accrued expenses in the accompanying consolidated balance sheets as of December 31, 2022.
The Company will reimburse its officers and directors for any reasonable out-of-pocket business expenses incurred by them in connection with certain activities on the Company’s behalf such as identifying and investigating possible target businesses and business combinations. There is no limit on the amount of out-of-pocket expenses reimbursable by the Company; provided, however, that to the extent such expenses exceed the available proceeds not deposited in the Trust Account and the interest income earned on the amounts held in the Trust Account, such expenses would not be reimbursed by the Company unless the Company consummates an initial business combination. The audit committee will review and approve all reimbursements and payments made to any initial stockholder or member of the management team, or the Company’s or their respective affiliates, and any reimbursements and payments made to members of the audit committee will be reviewed and approved by the Board of Directors, with any interested director abstaining from such review and approval.
No compensation or fees of any kind, including finder’s fees, consulting fees or other similar compensation, will be paid to any of the initial stockholders, officers or directors who owned the shares of common stock prior to this offering, or to any of their respective affiliates, prior to or with respect to the Business Combination (regardless of the type of transaction that it is).
All ongoing and future transactions between the Company and any of its officers and directors or their respective affiliates will be on terms believed by the Company to be no less favorable to the Company than are available from unaffiliated third parties. Such transactions, including the payment of any compensation, will require prior approval by a majority of the Company’s uninterested “independent” directors (to the extent the Company has any) or the members of the board who do not have an interest in the transaction, in either case who had access, at the Company’s expense, to the Company’s attorneys or independent legal counsel. The Company will not enter into any such transaction unless the Company’s disinterested “independent” directors (or, if there are no “independent” directors, the Company’s disinterested directors) determine that the terms of such transaction are no less favorable to the Company than those that would be available to the Company with respect to such a transaction from unaffiliated third parties.
NOTE 6. COMMITMENTS
Initial Public Offering Registration and Stockholders’ Rights
Pursuant to a registration rights agreement entered into on November 3, 2021, the holders of the (i) founder shares, which were issued in a private placement prior to the closing of the Initial Public Offering and (ii) private placement units (including all underlying securities), issued in a private placement simultaneously with the closing of the Initial Public Offering have registration rights to require the Company to register a sale of any of its securities held by them pursuant to a registration rights agreement. These holders are entitled to make up to two demands that the Company registers such securities for sale under the Securities Act. In addition, these holders will have “piggyback” registration rights to include their securities in other registration statements filed by the Company.
F-109
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Underwriters’ Agreement
The Representative is entitled to a deferred underwriting commission of 3.8% of the gross proceeds of the Initial Public Offering held in the Trust Account upon the completion of the Company’s initial Business Combination subject to the terms of the underwriting agreement.
The Company executed a Securities Purchase Agreement (the “A.G.P. Securities Purchase Agreement”) dated November 3, 2022 with A.G.P., which was amended on November 21, 2023, whereby A.G.P. subscribed for and will purchase, and DHAC will issue and sell, at the closing of the Business Combination, 4,370 shares of Series A Preferred Stock (“Series A Shares”) convertible into shares of DHAC common stock. The purchase price for the Series A Shares will be paid by conversion of A.G.P.’s $4,370,000 deferred underwriting fee into such Series A Shares. The Certificate of Designation of the Series A Preferred Stock establishes the terms and conditions of the Series A Preferred Stock. The Company reviewed the Series A Preferred Stock under ASC 480 and ASC 815 and concluded that Series A Preferred Stock did not include any elements that would preclude them from equity treatment and therefore are not subject to the liability treatment under ASC 480 or derivative guidance under ASC 815.
The Business Combination Agreement
On June 15, 2022, Digital Health Acquisition Corp (“DHAC”) entered into the Business Combination Agreement, with Merger Sub I, Merger Sub II, VSee and iDoc. On August 9, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the First Amended and Restated Business Combination Agreement to provide for the concurrent execution of financing documents for a PIPE consisting of convertible notes and warrants and delivery of the Cassel Salpeter’s opinion to the Board. On October 6, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into a Second Amended and Restated Business Combination Agreement to make the consideration payable to VSee and iDoc stockholders 100% DHAC common stock and to provide for the concurrent execution of amended PIPE financing documents providing for the issuance of the shares and warrants to the PIPE investors. On November 3, 2022, the parties entered into a First Amendment to the Second Amended and Restated Business Combination Agreement to remove a closing condition that DHAC have at least $10 million in cash proceeds from the transactions at closing. On July 11, 2023, each of the PIPE Investors provided notice to the Company that since a closing condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing. Accordingly, the PIPE financing was terminated. On November 21, 2023, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the Third Amended and Restated Business Combination Agreement (as amended and restated, the “Business Combination Agreement”) to, among other things, provide for the removal of the PIPE financing and the concurrent execution of the Additional Bridge Financing, the Exchange Financing, the Quantum Financing, the Equity Financing and the Loan Conversions, which are described in Note 6 — Commitments.
Pursuant to the terms of the Business Combination Agreement, a business combination by and among DHAC, VSee and iDoc will be effected through the merger of Merger Sub I with and into VSee, with VSee surviving the Merger as a wholly owned subsidiary of DHAC and the merger of Merger Sub II with and into iDoc, with iDoc surviving the Merger as a wholly owned subsidiary of DHAC. The Board of Directors of DHAC (the “Board”) has (i) approved and declared advisable the Business Combination Agreement, the Business Combination and the other transactions contemplated thereby and (ii) resolved to recommend approval of the Business Combination Agreement and related matters by the stockholders of DHAC.
The Merger Consideration
The Business Combination combined equity value of VSee and iDoc is $110 million. At the Closing, each of VSee and iDoc will convert each share of VSee and iDoc capital stock (excluding shares of the holders who perfect rights of appraisal under Delaware or Texas law, as the case may be) into the right to receive the applicable merger consideration as further described below.
| ● | VSee Merger Consideration |
The aggregate merger consideration that the holders of VSee Class A Common Stock (including the holders of VSee Preferred Stock as converted and holders of VSee Class A Common Stock in connection with the TAD Exchange) as of the Effective Time are entitled to receive in the Business Combination, referred to as the “VSee Class A Consideration,” is an amount equal to (1) $60,500,000, minus (2) an amount equal to the Effective Time Option Grants multiplied by $10, minus (3) the aggregate amount of VSee’s transaction expenses. “Effective Time Option Grants” refer to the stock options with an exercise price of $10 per share pursuant to the VSee Incentive Plan to the individuals, in the amounts, and on the terms set forth on Exhibit E to the Business
F-110
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Combination Agreement. 100% of the VSee Closing Consideration will be paid in shares of Company Common Stock in accordance with the terms of the Business Combination Agreement and subject to deductions for the VSee Indemnity Escrow Amount. The “VSee Per Share Class A Consideration” refers to a number of shares of Common Stock equal to (a) (1) the VSee Class A Closing Consideration, divided by (2) the total number of VSee Class A Outstanding Shares, divided by (b) 10. “VSee Class A Outstanding Shares” refer to the total number of shares of VSee Class A Common Stock outstanding immediately prior to the Effective Time, expressed on a fully diluted and as-converted to VSee Class A Common Stock basis, and including, without limitation or duplication, the number of shares of VSee Class A Common Stock issuable upon conversion of the VSee Preferred Stock and upon closing of the TAD Exchange, which refers to a transaction where This American Doc, Inc. becomes a wholly owned subsidiary of VSee immediately prior to the consummation of the Business Combination.
| ● | iDoc Merger Consideration |
The aggregate merger consideration that the holders of iDoc Class A Common Stock as of the Effective Time are entitled to receive in the Business Combination, referred to as the “iDoc Class A Closing Consideration,” is an amount equal to (1) $49,500,000, minus (2) the aggregate amount of iDoc’s transaction expenses. 100% of the iDoc Closing Consideration will be paid in shares of Company Common Stock in accordance with the terms of the Business Combination Agreement and subject to deductions for the iDoc Indemnity Escrow Amount as described below. The “iDoc Per Share Class A Consideration” refers to a number of shares of Common Stock equal to (a) (1) the iDoc Class A Closing Consideration, divided by (2) the total number of iDoc Class A Outstanding Shares, divided by (b) 10. “iDoc Class A Outstanding Shares” refer to the total number of shares of iDoc Class A Common Stock outstanding immediately prior to the Effective Time, expressed on a fully diluted and as-converted to iDoc Class A Common Stock basis.
Conditions to Closing
The obligations of DHAC, VSee and iDoc to consummate the Business Combination are subject to certain closing conditions, including, but not limited to, (i) the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (ii) the approval of DHAC’s shareholders, (iii) the approval of VSee’s stockholders, (iv) the approval of iDoc’s stockholders, and (v) the delivery of applicable closing deliverables.
In addition, the obligations of VSee and iDoc to consummate the Business Combination are subject to the fulfillment of other closing conditions, including, but not limited to, (i) the approval by the Nasdaq Capital Market of DHAC’s listing application in connection with the Business Combination and (ii) the DHAC board of directors consisting of the number of directors, and comprising the individuals, as contemplated by the Business Combination Agreement.
Third Amended and Restated Transaction Support Agreement
On November 21, 2023, the parties to the Business Combination Agreement entered into the Third Amended and Restated Business Combination Agreement, pursuant to which the Second A&R Business Combination Agreement was amended and restated to provide for, among other things, the concurrent execution of the other agreements and transactions described as below. The transactions contemplated by the Business Combination Agreement are referred to as the “Business Combination” and the closing and closing date of the Business Combination are referred to as the “Closing” and the “Closing Date,” respectively.
In connection with the execution of the Business Combination Agreement, DHAC, Milton Chen, the Executive Vice Chairman of VSee, Dr. Imoigele Aisiku, the Executive Chairman of the Board of Directors of iDoc, and certain other stockholders of VSee and iDoc (collectively, the “Supporting Stockholders”) entered into a Third Amended and Restated Transaction Support Agreement, dated as of November 21, 2023 (the “Transaction Support Agreement”) which amended and restated the Second Amended and Restated Transaction Support Agreement executed on October 6, 2022, pursuant to which the Supporting Stockholders have agreed to, among other things, (i) support and vote in favor of the Business Combination Agreement and the Business Combination at DHAC’s stockholder meeting; (ii) not affect any sale or distribution of any shares of capital stock of DHAC, VSee, or iDoc; and (iii) take or cause to be done such further acts and things as may be reasonably necessary or advisable to cause the parties to fulfill their respective obligations under the Business Combination Agreement and consummate the Business Combination.
F-111
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
VSee Health, Inc. Incentive Plan
DHAC has agreed to approve and adopt the VSee Health, Inc. 2024 Equity Incentive Plan (the “Incentive Plan”) to be effective as of one day prior to the closing Business Combination and in a form mutually acceptable to DHAC, VSee and iDoc. The Incentive Plan shall provide for an initial aggregate share reserve equal to 15% of the number of shares of DHAC Common Stock outstanding following the closing after giving effect to the Business Combination, including without limitation, the PIPE Financing. Subject to approval of the Incentive Plan by DHAC’s Stockholders, DHAC has agreed to file a Form S-8 Registration Statement with the SEC following the Effective Time with respect to the shares of DHAC Common Stock issuable under the Incentive Plan.
PIPE Securities Purchase Agreement
In connection with the execution of the Business Combination Agreement, DHAC executed an Amended and Restated Securities Purchase Agreement (as amended, the “PIPE Securities Purchase Agreement” or “PIPE Forward Contract”) dated October 6, 2022 with certain PIPE Investors whereby the PIPE Investors subscribed for and will purchase, and DHAC will issue and sell, (i) 8,000 shares of Series A Preferred Stock (“Initial PIPE Shares”) convertible into shares of DHAC common stock and (ii) warrants (“Initial PIPE Warrants”) exercisable for 424,000 shares of DHAC Common Stock (such transactions, the “Initial PIPE Financing”) for aggregate proceeds of at least $8,000,000.
The PIPE Securities Purchase Agreement also provides that at any time after the date of the PIPE Securities Purchase Agreement and including (x) with respect to the PIPE Investors’ right to purchase Additional Offering Securities further to an Additional Offering (as each term is defined below) the earlier to occur of (I) the first anniversary of the date of the PIPE Securities Purchase Agreement and (II) the date of the consummation of one or more Subsequent Placements (as defined in the PIPE Securities Purchase Agreement) with the PIPE Investors on terms identical to the PIPE Securities Purchase Agreement and the other PIPE Financing documents in all material respects with an aggregate purchase price of at least $10 million (the “Additional Offering”, and the securities thereof, the “Additional Offering Securities”) and (y) with respect to Buyer’s right to participate in a Subsequent Placement other than an Additional Offering the earlier to occur of (I) the initial date after the Closing that no PIPE Shares remain outstanding, and (II) the date of the consummation of a Subsequent Placement by the Company with gross proceeds, paid in cash, of at least $5,000,000, in either case, neither the Company nor any of its subsidiaries shall, directly or indirectly, effect any Subsequent Placement unless the Company shall have first complied with the PIPE Investors’ participation right described herein and set forth in the PIPE Securities Purchase Agreement. With respect to (i) Additional Offerings, DHAC is required to offer 100% of the Additional Offering Securities to the PIPE Investors; and (ii) Subsequent Placements, DHAC is required to offer 25% of the Offered Securities to the PIPE Investors.
The Aggregate Closing PIPE Proceeds will be a part of the aggregate cash proceeds available for release to DHAC, Merger Sub I, and Merger Sub II in connection with the transactions contemplated by the Business Combination Agreement. The PIPE Warrants are exercisable into shares of DHAC Common Stock at a price of $12.50 per share and expire 5 years from the date of issuance. The PIPE Shares are convertible into shares of DHAC Common Stock at a price of $10.00 per share, subject to certain adjustments. The Certificate of Designation of the Series A Preferred Stock establishes the terms and conditions of the Series A Preferred Stock.
The Company reviewed the PIPE Securities Purchase Agreement’s underlying securities under ASC 480 and ASC 815 and concluded that Series Preferred A Stock includes a contingent redemption that would require temporary equity treatment at issuance and the warrants do not have any elements that would preclude them from equity treatment and therefore are not subject to the derivative guidance under ASC 815. However, under ASC 480-10-55-33, a forward contract that permits the holder to purchase redeemable shares (the Series A Preferred Stock) is a liability pursuant to ASC 480 because (1) the forward contract itself is indexed to an underlying share (i.e., the option’s value varies with the fair value of the share) that embodies the issuer’s obligation to repurchase the share and (2) the issuer has a conditional obligation to transfer assets if the shares are put back. Accordingly, the Company determined the fair value of the PIPE Forward Contract and noted the value at the October 6, 2022, the executed date of agreement was zero. As of December 31, 2023, the value of the PIPE Forward Contract was $0 (see Note 10 — Fair Value Measurements for additional disclosure on the PIPE Forward Contract).
On April 11, 2023 but effective March 31, 2023, the Company entered into an amendment to the PIPE Securities Purchase Agreement to, among other things, (a) amend and restate the form of Certificate of Designation of the Series A Preferred Stock to provide the aggregate number of shares of Series A Preferred Stock issuable thereunder shall not exceed 15,000, (b) amend and restate the form of PIPE Warrant to correct an error in the redemption provision of the PIPE Warrants, and (c) revise certain closing conditions for the PIPE Financing. As previously disclosed in its Current Report on Form 8-K filed on April 12, 2023, the Company
F-112
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
and each of the PIPE Investors entered into amendments to the PIPE SPA to, among other things, add a closing condition providing that the closing date of the business combination shall occur on or prior to July 10, 2023 (the “Outside Date Closing Condition”).
In connection with the closing of the transactions contemplated by the PIPE Securities Purchase Agreement, DHAC and the PIPE Investors will enter into the registration rights agreement (the “PIPE Registration Rights Agreement”). The PIPE Registration Rights Agreement provides the PIPE Investors with customary registration rights with respect to the shares of Common Stock underlying the PIPE Shares and PIPE Warrants issued to the PIPE Investors.
Pursuant to the PIPE Securities Purchase Agreement, certain of DHAC’s stockholders agreed to enter into a lock-up agreement (the “PIPE Lock-Up Agreement”) with DHAC. Under the PIPE Lock-Up Agreement, the PIPE Lock-Up Period means the period beginning on the date of the Lock-Up Agreement and ending on the earliest of (i) eight months after the Closing Date, or (ii) on the trading day after DHAC’s Common Stock exceeds $12.50 (as adjusted for any stock splits, stock dividends, stock combinations recapitalizations and similar events) for a period of twenty consecutive trading days after the Closing Date.
On July 11, 2023, each of the PIPE Investors provided notice to the Company that since the Outside Date Closing Condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing.
Backstop Agreement
On January 18, 2023, DHAC and the Sponsor entered into a Backstop Agreement (the “Backstop Agreement”) pursuant to which DHAC agreed to offer on or prior to the closing of the Business Combination the PIPE Investors the option to purchase up to an additional 2,000 shares of Series A Preferred Stock initially convertible into 234,260 shares of DHAC common stock (the “Additional PIPE Shares” and together with the Initial PIPE Shares, the “PIPE Shares”), together with additional warrants to purchase up to 106,000 shares of DHAC common stock (the “Additional PIPE Warrants” and together with the Initial PIPE Warrants, the “PIPE Warrants”; the Additional PIPE Shares and Additional PIPE Warrants are referred to as the “Additional PIPE Securities”) pursuant to a participation right granted to the PIPE Investors under the PIPE Securities Purchase Agreement, in each case, on the same terms and conditions set forth in the PIPE Securities Purchase Agreement for an aggregate purchase price of up to $2,000,000 (such proceeds together with the proceeds from the Initial PIPE Financing, as increased pursuant to the amendment to the Backstop Agreement described below, the “Aggregate Closing PIPE Proceeds”). Pursuant to the Backstop Agreement, if the PIPE Investors do not elect to purchase all of the Additional PIPE Securities, the Sponsor has agreed to purchase any such unsubscribed Additional PIPE Securities concurrent with the closing of the transactions contemplated by the PIPE Securities Purchase Agreement on the same terms and conditions set forth in the PIPE Securities Purchase Agreement.
On April 11, 2023 but effective March 31, 2023, the Sponsor and DHAC entered into an amendment to the Backstop Agreement to increase the Additional PIPE Shares that may be purchased pursuant to the Backstop Agreement from 2,000 shares of Series A Preferred Stock to 7,000 shares of Series A Preferred Stock, for an aggregate additional PIPE financing of up to $7,000,000, increasing the Aggregate Closing PIPE Proceeds to a total of $15,000,000.
Pursuant to the PIPE Securities Purchase Agreement and the Backstop Agreement, each as amended, any purchaser of Additional PIPE Securities will enter into a lock-up agreement with the Company.
On July 11, 2023, each of the PIPE Investors provided notice to the Company that since the Outside Date Closing Condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing, and, as such the Backstop Agreement is terminated as of July 11, 2023.
Bridge Financing and Bifurcated Derivative
On October 6, 2022, in connection with the execution of the Business Combination Agreement, DHAC, VSee and iDoc entered into a Securities Purchase Agreement (the “Original Bridge SPA”) with an accredited investor (the “Bridge Investor”) who is also an investor in the Sponsor, pursuant to which DHAC, VSee and iDoc each issued and sold to such Bridge Investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222 (the “Bridge Notes”).An amount of $888,889 of the Bridge Note was allocated to DHAC. The Bridge Notes bear guaranteed interest at a rate of 10% per annum. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain
F-113
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
adjustments (the “Bridge Warrants”) and (ii) 30,000 shares of DHAC common stock (the “Bridge Shares”) as additional consideration for the purchase of the Bridge Notes and Bridge Warrants. If the PIPE Financing closes in connection with the closing of the Business Combination, 110% of all unpaid principal under the Bridge Notes and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Company reviewed the warrants and common stock issued in connection with the securities purchase agreement under ASC 815 and concluded that the Bridge Warrants are not in scope of ASC 480 and are not subject to the Derivative guidance under ASC 815. The Bridge Warrants and the Bridge Shares should be recorded as equity. As such the principal value of the Bridge Notes was allocated using the relative fair value basis of all three instruments. As the Bridge Warrants were issued with various instruments the purchase price needs to be allocated using the relative fair value method (i.e., warrant at its fair value and the common stock at its fair value the promissory note at its principal value allocated using the relative fair value of the proceeds received an applied proportionally to the equity classified stock, warrants and promissory note).
The Company reviewed the contingent early repayment option granted in the Bridge Notes under ASC 815 and concluded that as a result of the significant discount granted in the note the contingent repayment provision is therefore considered an embedded derivative that should be bifurcated from the debt host. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Notes proceeds between the Bridge Notes and the Bifurcated Derivative, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $278,404 and the residual value of $610,485 was allocated to the principal balance of the note (see Note 10 — Fair Value Measurements for additional disclosure on the derivative).
DHAC as a result received cash proceeds of $738,200 net of $61,800 of direct cost attributable to the financing. The Bridge Warrants and Bridge Shares issued to Bridge Investor were analyzed under ASC 815 and noted there were no elements that would preclude equity treatment. As such the Company recorded the fair value of the Bridge Warrants of $8,552, net of $613 of offering cost allocated based on the relative value basis and Bridge Shares of $284,424, net of $20,376 of offering cost allocated based on the relative value basis. As a result, of the bifurcated derivative discussed above, the offering cost allocated to the debt, and the value of the share and warrants granted, the Company recorded amortizable debt discount of $443,665, consisting of $40,811 in financing cost allocated to the Bridge Note, $9,165 the issuance date fair value of the Bridge Warrants, $304,800 the fair value of the Bridge Shares and $88,889 originally issued discount.
The Company recognized a total Bridge Note interest expense of $429,007 for the year ended December 31, 2023. In connection with the financing, the Company entered into a Registration Rights Agreement with the Bridge Investor, dated October 5, 2022, which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Bridge Warrants and the Bridge Shares.
On October 4, 2023, the Company defaulted on the Bridge Notes, and accordingly, the default provision was allocated and applied resulting in the triggering of 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24%, and the Company assumed the penalties and interest which were due and payable under the VSee and iDoc portion of the note, resulting in total amount due of $2,523,744. As a result, the Company entered into an Exchange Agreement dated November 21, 2023 (the “Exchange Agreement”) with the Bridge Investor and recognized $1,579,927 in default interest.
The Bridge Investor, beneficially owns and holds (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, each as further detailed on Schedule I hereto, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744.
Exchange Note Exchange Financing
Pursuant to the Exchange Agreement, the Bridge Investor agreed to exchange all amounts currently due and owing under (i) the DHAC Note, (ii) the VSee Note other than the principal amount of $600,000.00 thereof, and (iii) the iDoc Note other than the principal amount of $600,000.00 thereof for a senior secured convertible promissory note with an aggregate principle value of $2,523,744 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc. The Exchange Note will bear interest
F-114
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
at a rate of 8% per annum and will be convertible into shares of common stock of the Company at a fixed conversion price of $10 per share. The conversion price of the Exchange Note is subject to reset if DHAC’s common stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of DHAC’s common stock in the 10th trading dates prior to the measurement date and (y) $2.0. Amounts repaid may not be reborrowed. The Bridge Investor may set off and deduct pursuant to and in accordance with the Exchange Agreement amounts due to the Bridge Investor. The transactions contemplated by the Exchange Agreement and the Exchange Note is hereby referred as the “Exchange Financing.”
The monetary amount of the obligation is a fixed monetary amount known at inception as represented by the Amortization of Principal Schedule 2(a) (each, an “Amortization Payment”). As a result of Section 2(a), the Exchange Note represents a debt instrument that the Company must or may settle by issuing a variable number of its equity shares as each Amortization Payment shall, at the option of the Company, be made in whole or in part, in immediately available Dollars equal to the sum of the Amortization Payment provided for in Schedule 2(a), or, subject to the Company complying with the Equity Conditions on the date of such Amortization Payment, in Common Stock issued at 95% of the lowest VWAP in the prior ten (10) trading days prior to such Amortization Payment (the “Amortization Conversion Price”) but in no event shall Common Stock be used to make such Amortization Payment if the Amortization Conversion Price is less than $2.00.
The Exchange Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Exchange Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
At November 21, 2023 the Exchange Note was recognized at fair value of $2,523,744 in accordance with ASC 480. As of December 31, 2023, the Exchange Note’s fair value was $2,621,558. The Company recognized a total Exchange Note interest expense of $22,433 for the year ended December 31, 2023 and the change in fair value of $97,814.
Additional Bridge Financing
On November 21, 2023, DHAC entered into an amendment to the Original Bridge SPA (the “Bridge Amendment”), pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount convertible promissory notes in the aggregate principal amount of $166,667 (with a subscription amount of $150,000) from the Company with (1) a $111,111 note purchased at signing of the Bridge Letter Agreement, which will mature on May 21, 2025 and (2) a $55,556 note purchased at a later date mutually agreed upon by the Company and the Bridge Investor (the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8% per annum and are convertible into shares of the Company’s common stock, par value $0.0001, at a fixed conversion price of $10.00 per share. The conversion price of the Additional Bridge Notes is subject to reset if the Company’s Common Stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the Company’s Common Stock in the 10 trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Additional Bridge Notes requires the payment of 110% of the outstanding obligations, including the guaranteed minimum interest. If an event of default occurs, the Additional Bridge Notes would bear interest at a rate of 24% per annum and require the payment of 125% of the outstanding obligations, including the guaranteed minimum interest. As of December 31, 2023, $100,000 has been funded. The transactions contemplated by the Bridge Amendment and the Additional Bridge Note is hereby referred as the “Additional Bridge Financing.”
The monetary amount of the obligation is a fixed monetary amount known at inception as represented by the Amortization of Principal Schedule 2(a) (each, an “Amortization Payment”). As a result of Section 2(a), the Additional Bridge Note represents a debt instrument that the Company must or may settle by issuing a variable number of its equity shares as each Amortization Payment shall, at the option of the Company, be made in whole or in part, in immediately available Dollars equal to the sum of the Amortization Payment provided for in Schedule 2(a), or, subject to the Company complying with the Equity Conditions on the date of such Amortization Payment, in Common Stock issued at 95% of the lowest VWAP in the prior ten (10) trading days prior to such Amortization Payment (the “Amortization Conversion Price”) but in no event shall Common Stock be used to make such Amortization Payment if the Amortization Conversion Price is less than $2.00.
F-115
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
The Additional Bridge Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Senior Secured Convertible Promissory Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
At November 21, 2023, $100,000 of proceeds were received under the Additional Bridge Note, as such the original issued discount was immediately expensed as interest of $11,111 as the Note was recognized at fair value of $100,000 in accordance with ASC 480. As of December 31, 2023, the Additional Bridge Note’s fail value was $102,726. The Company recognized a total Additional Bridge Note interest expense of $12,642 for the year ended December 31, 2023 and the change in fair value of $2,726.
Extension Note (Extension Financing) and Bifurcated Derivative
On May 5, 2023, the Company entered into a securities purchase agreement (the “Extension Purchase Agreement”) with an institutional investor (the “Holder”). Pursuant to the Extension Purchase Agreement, the Company issued the Holder a 16.67% original issue discount promissory note, in favor of the Holder, in the aggregate principal amount of $300,000 (the “Extension Note”). The Extension Note bears guaranteed interest at a rate of 10% per annum and is due and payable on May 5, 2024.
VSee Lab, Inc., a Delaware corporation (“VSee”), and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”), guaranteed the Company’s obligations under the Extension Purchase Agreement, the Extension Note and the other transaction documents (the “May 2023 Financing Documents”) pursuant to a Subsidiary Guaranty dated May 5, 2023. The Company’s, VSee’s and iDoc’s obligations to the Holder under the May 2023 Financing Documents are subordinated to the Company’s, VSee’s and iDoc’s obligations to its existing bridge lender.
In connection with the Extension Purchase Agreement, the Company issued to the Holder (i) warrants with an exercise period of five years to purchase up to 26,086 shares of the Company’s Common Stock at an exercise price of $11.50 per share (the “Extension Warrants”), and (ii) 7,000 shares of the Company’s Common Stock as commitment shares (the “Extension Shares”). The Company also entered into a Registration Rights Agreement with the Holder, dated May 5, 2023 (the “May 2023 RRA”), which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Extension Warrants and the Extension Shares, subject to the terms thereof.
The Company reviewed the Extension Warrants and Extension Shares issued in connection with the Extension Purchase Agreement under ASC 815 and concluded that the Extension Warrants are not in scope of ASC 480 and are not subject to the Derivative guidance under ASC 815. The Extension Warrants and the Extension Shares should be recorded as equity. As such the principal value of the Extension Note was allocated using the relative fair value basis of all three instruments. As the Extension Warrants were issued with various instruments the purchase price needs to be allocated using the relative fair value method (i.e., warrant at its fair value and the common stock at its fair value the promissory note at its principal value allocated using the relative fair value of the proceeds received an applied proportionally to the equity classified stock, warrants and promissory note).
The Company reviewed the contingent early repayment option granted in the Extension Note under ASC 815 and concluded that as a result of the significant discount granted in the note the contingent repayment provision is therefore considered an embedded derivative that should be bifurcated from the debt host. Accordingly, in accordance with ASC 470-20, the Company allocated the Extension Note proceeds between the Extension Note and the Bifurcated Derivative, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $71,755 and the residual value of $228,245 was allocated to the principal balance of the note (see Note 10 — Fair Value Measurements for additional disclosure on the derivative).
DHAC as a result received cash proceeds of $240,000 net of $10,000 of direct cost attributable to the financing. The Extension Warrants and Extension Shares were analyzed under ASC 815 and noted there were no elements that would preclude equity treatment. As such the Company recorded the fair value of the Extension Warrants of $2,461, net of $82 of offering cost allocated based on the relative value basis and Extension Shares of $76,102, net of $2,542 of offering cost allocated based on the relative value basis. As a result of the bifurcated derivative discussed above, the offering cost allocated to the debt, and the value of the shares and warrants granted, the Company recorded amortizable debt discount of $175,472, consisting of $56,993 in financing cost allocated to the
F-116
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Extension Note, $40,130 the issuance date fair value of the Investor Warrants, $78,349 the fair value of the Extension Shares and $50,000 originally issued discount.
As of December 31, 2023, the Extension Note net of unamortized debt discount was $233,774. The Company recognized $97,814 of amortized debt discount and $19,597 in accrued interest for a total Extension Note interest expense of $133,748 for the year ended December 31, 2023. In connection with the Extension Purchase Agreement, the Company entered into the May 2023 RRA with the Holder, dated May 5, 2023, which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Extension Warrants and the Extension Shares.
Quantum Financing Securities Purchase Agreement
On November 21, 2023, DHAC entered into a convertible note purchase agreement (the “Quantum Purchase Agreement”), pursuant to which an institutional and accredited investor (the “Quantum Investor”) subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000. The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) a fixed conversion price of $10.00 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30-day anniversary of the Quantum Note issuance date (the “Average Price”) is less than $10.00, to a price equal to the Average Price but in no event less than $2. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10.00. If an event of default occurs, the Quantum Note would bear interest at a rate of 18% per annum. The transactions contemplated by the Quantum Purchase Agreement and the Quantum Note is hereby referred as the “Quantum Financing.”
Concurrently with the consummation of the transactions contemplated by the Quantum Purchase Agreement, DHAC will enter into a Registration Rights Agreement in a form under the Quantum Purchase Agreement, pursuant to which it agreed to register the shares of Common Stock underlying the Quantum Note (the “Quantum Registration Rights Agreement”).
As of December 31, 2023, the Quantum Purchase Agreement has not yet been funded and is expected to be funded at the closing of the business combination. The Quantum Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Quantum Note will be required to be accounted for as a liability under ASC 480 upon funding of the note. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
Equity Financing
On November 21, 2023, DHAC entered into an equity purchase agreement (the “Equity Purchase Agreement”) with the Bridge Investor pursuant to which DHAC may sell and issue to the Bridge Investor, and the Bridge Investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s Common Stock, from time to time over a 36-month period (the “Equity Purchase Commitment Period”) beginning from the sixth (6th) trading day following the closing of the Business Combination transaction (the “Equity Purchase Effective Day”), provided that certain conditions are met. The Company also agreed to file a resale registration statement to register shares of Common Stock to be purchased under the Equity Purchase Agreement with the SEC within 45 days following the Equity Purchase Effective Day, and shall use commercially reasonable efforts to have such registration statement declared effective by the SEC within 30 days of such filing. During the Equity Purchase Commitment Period, DHAC may suspend the use of the resale registration statement to (i) delay the disclosure of material nonpublic information concerning the Company in good faith or (ii) amend the registration statement concerning material information, by providing written notice to the investor. Such suspension cannot be longer than 90 consecutive days (or 120 days in any calendar year). The investor has agreed not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of the Company’s Common Stock. On the Equity Purchase Effective Day, the Company will issue to the investor, as a commitment fee for this equity purchase transaction, a senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of the Company’s Common Stock at a fix conversion price of $10.00 per share (the “Equity Purchase Commitment Note”). The transaction contemplated by the Equity Purchase Agreement is hereby referred as “Equity Financing.”
F-117
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
The Company has analyzed the Equity Purchase Agreement and determined that the contract should be recorded as a liability under ASC 815 and measured at fair value. As a result of the ASC 815 liability classification, the Company is required to re-measure the liability at fair value at each reporting period until the liability is settled.
The Company has determined that the likelihood of exercising this contract is low as the contract provides no scenario in which the Company may exercise the contract at above market rates (i.e., sell shares at a price above which the shares are currently trading in the active market). Furthermore, the choice to exercise the contract is solely at the discretion of the Company (i.e., does not obligate the Company in any manner), which, as stated above, is unlikely. Additionally, the contract does not impose a fee or fine if the Company chooses not to exercise the contract, as such that the fair value of the equity contract is considered de minimis.
NOTE 7. STOCKHOLDERS’ DEFICIT
Common Stock
The Company is authorized to issue 50,000,000 of common shares with a par value of $0.0001 per share. On June 7, 2021, the Sponsor, along with certain of the Company’s directors, officers and advisors purchased 4,312,500 shares for an aggregate purchase price of $25,000. In October 2021, the Sponsor, officers and certain advisors forfeited an aggregate of 1,437,500 shares of common stock, resulting in 2,875,000 founder shares outstanding. At the closing of the Initial Public Offering, 557,000 shares were issued as part of the Private Placement sale. On October 6, 2022, in connection with the Original Bridge SPA, 30,000 shares were issued to the Bridge Investor. In February 2023, 20,000 shares were issued to an additional stockholder. On May 5, 2023 in connection with the Extension Purchase Agreement, 7,000 shares were issued to the investor. As of December 31, 2023 and 2022, there were 3,489,000 and 3,462,000 shares of common stock issued and outstanding, respectively, excluding 114,966 and 694,123 shares subject to redemption which were classified outside of permanent deficit on the consolidated balance sheets. The holders of record of the Company’s common stock are entitled to one vote for each share held on all matters to be voted on by stockholders. In connection with any vote held to approve the Company’s initial business combination, the initial stockholders, insiders, officers and directors, have agreed to vote their respective shares of common stock owned by them immediately prior to this offering, including both the insider shares and any shares acquired in this offering or following this offering in the open market, in favor of the proposed business combination.
Pursuant to the amended and restated certificate of incorporation, if the Company does not consummate its initial business combination within 27 months from the closing of this offering (as extended as of December 31, 2023), it will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. The Company’s insiders have agreed to waive their rights to share in any distribution with respect to their insider shares.
The stockholders have no conversion, preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to the shares of common stock, except that public stockholders have the right to sell their shares to the Company in any tender offer or have their shares of common stock converted to cash equal to their pro rata share of the Trust Account if they vote on the proposed business combination and the business combination is completed.
If the Company holds a stockholder vote to amend any provisions of the certificate of incorporation relating to stockholders’ rights or pre-business combination activity (including the substance or timing within which it has to complete a business combination), it will provide its public stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its franchise and income taxes, divided by the number of then outstanding public shares, in connection with any such vote. In either of such events, converting stockholders would be paid their pro rata portion of the Trust Account promptly following consummation of the business combination or the approval of the amendment to the certificate of incorporation. If the business combination is not consummated or the amendment is not approved, stockholders will not be paid such amounts.
F-118
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
NOTE 8. WARRANTS
Initial Public Offering Warrants
There are 12,057,000 warrants issued and outstanding as of December 31, 2023 and 2022 issued in connection with the Initial Public Offering. Each warrant entitles the registered holder to purchase one (1) share of common stock at a price of $11.50 per whole share, subject to adjustment as discussed below, at any time commencing on the later of 30 days after the completion of an initial business combination or 12 months from the closing of the Initial Public Offering.
However, no warrants will be exercisable for cash unless the Company has an effective and current registration statement covering the shares of common stock issuable upon exercise of the warrants and a current prospectus relating to such shares of common stock. Notwithstanding the foregoing, if a registration statement covering the shares of common stock issuable upon exercise of the public warrants is not effective within a specified period following the consummation of the initial business combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company shall have failed to maintain an effective registration statement, exercise warrants on a cashless basis pursuant to the exemption provided by Section 3(a)(9) of the Securities Act, provided that such exemption is available. If that exemption, or another exemption, is not available, holders will not be able to exercise their warrants on a cashless basis. In the event of such cashless exercise, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose will mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the trading day prior to the date of exercise. The warrants will expire on the fifth anniversary of the completion of an initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
The Private Placement Warrants is identical to the warrants underlying the units in the Initial Public Offering. The Company may call the warrants for redemption, in whole and not in part, at a price of $0.01 per warrant,
| ● | at any time after the warrants become exercisable; |
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; |
| ● | if, and only if, the reported last sale price of the shares of common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations and recapitalizations), for any 20 trading days within a 30 trading day period commencing at any time after the warrants become exercisable and ending on the third business day prior to the notice of redemption to warrant holders; and |
| ● | if, and only if, there is a current registration statement in effect with respect to the shares of common stock underlying such warrants. |
The right to exercise will be forfeited unless the warrants are exercised prior to the date specified in the notice of redemption. On and after the redemption date, a record holder of a warrant will have no further rights except to receive the redemption price for such holder’s warrant upon surrender of such warrant.
The redemption criteria for the warrants have been established at a price which is intended to provide warrant holders a reasonable premium to the initial exercise price and provide a sufficient differential between the then-prevailing share price and the warrant exercise price so that if the share price declines as a result of the redemption call, the redemption will not cause the share price to drop below the exercise price of the warrants.
If the Company calls the warrants for redemption as described above, the Company’s management will have the option to require all holders that wish to exercise warrants to do so on a “cashless basis.” In such event, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the
F-119
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
average reported last sale price of the shares of common stock for the 5 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
The warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and the Company. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision or to make any other change that does not adversely affect the interests of the registered holders. For any other change, the warrant agreement requires the approval by the holders of at least a majority of the then outstanding public warrants if such amendment is undertaken prior to or in connection with the consummation of a business combination or at least a majority of the then outstanding warrants if the amendment is undertaken after the consummation of a business combination.
The exercise price and number of shares of common stock issuable on exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described below, the warrants will not be adjusted for issuances of shares of common stock at a price below their respective exercise prices.
If (x) the Company issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of the initial business combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by the board of directors, and in the case of any such issuance to the Company’s Sponsor, initial stockholders or their affiliates, without taking into account any founders’ shares held by them prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial business combination on the date of the consummation of the initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of (i) the Market Value or (ii) the price at which the Company issue the additional shares of common stock or equity-linked securities and the $18.00 per share redemption trigger price described above will be adjusted (to the nearest cent) to be equal to 180% of the Market Value. The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by certified or official bank check payable to the Company, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of shares of common stock and any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Warrant holders may elect to be subject to a restriction on the exercise of their warrants such that an electing warrant holder would not be able to exercise their warrants to the extent that, after giving effect to such exercise, such holder would beneficially own in excess of 9.8% of the shares of common stock outstanding.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, the Company will, upon exercise, round up to the nearest whole number the number of shares of common stock to be issued to the warrant holder.
Bridge Warrants
On October 6, 2022, 173,913 warrants were issued pursuant to the Bridge Purchase Agreement. The purchase right represented by the Bridge Warrants shall terminate on or before 5:30 p.m., Pacific Time, on the date five years from the date of issuance (the “Expiration Date”). The exercise price at which the Bridge Warrants may be exercised shall be $11.50 per share of Common Stock. If at any time after the date of issuance of the Bridge Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, the Bridge Warrants may be exercised by cashless exercise. In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the Exercise Price multiplied by such fraction. Except as provided in the Bridge Warrant, the Bridge Warrant does not entitle its holder to any rights of a shareholder of the Company.
During the term the Bridge Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the Bridge Warrant and, from time to
F-120
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock issuable upon exercise of the Bridge Warrants. All shares that may be issued upon the exercise of rights represented by the Bridge Warrants and payment of the Exercise Price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Bridge Warrants). Prior to the Expiration Date, the Exercise Price and the number of shares of Common Stock purchasable upon the exercise of the Bridge Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
| (a) | In the event that the Company shall at any time after the date of issuance of the Bridge Warrants (i) declare a dividend on Common Stock in shares or other securities of the Company, (ii) split or subdivide the outstanding Common Stock, (iii) combine the outstanding Common Stock into a smaller number of shares, or (iv) issue by reclassification of its Common Stock any shares or other securities of the Company, then, in each such event, the Exercise Price in effect at the time shall be adjusted so that the holder shall be entitled to receive the kind and number of such shares or other securities of the Company which the holder would have owned or have been entitled to receive after the happening of any of the events described above had such Bridge Warrant been exercised immediately prior to the happening of such event (or any record date with respect thereto). |
| (b) | No adjustment in the number of shares of Common Stock receivable upon exercise of the Bridge Warrant shall be required unless such adjustment would require an increase or decrease of at least 0.1% in the aggregate number of shares of Common Stock purchasable upon exercise of all Bridge Warrants; provided that any adjustments which are not required to be made shall be carried forward and taken into account in any subsequent adjustment. |
| (c) | If at any time, as a result of an adjustment, the holder of any Bridge Warrant thereafter exercised shall become entitled to receive any shares of the Company other than shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Bridge Warrant shall be subject to adjustment from time to time in a manner and on terms as nearly equivalent as practicable to the provisions with respect to the Common Stock receivable upon execution of the Bridge Warrant. |
| (d) | Whenever the Exercise Price payable upon exercise of each Bridge Warrant is adjusted, the Warrant Shares shall be adjusted by multiplying the number of shares of Common Stock receivable upon execution of the Bridge Warrant immediately prior to such adjustment by a fraction, the numerator of which shall be the Exercise Price in effect immediately prior to such adjustment, and the denominator of which shall be the Exercise Price as adjusted. |
| (e) | In the event of any capital reorganization of the Company, or of any reclassification of the Common Stock, or in case of the consolidation of the Company with or the merger of the Company with or into any other corporation or of the sale of the properties and assets of the Company as, or substantially as, an entirety to any other corporation, each Bridge Warrant shall, after such capital reorganization, reclassification of Common Stock, consolidation, merger or sale, and in lieu of being exercisable for shares of Common Stock of the Company, be exercisable, upon the terms and conditions specified in the Bridge Warrant, for the number of shares of stock or other securities or assets to which holder of the number of shares of Common Stock purchasable upon exercisable of such Bridge Warrant immediately prior to such capital organization, reclassification of Common Stock, consolidation, merger or sale would have been entitled upon such capital organization, reclassification of Common Stock, consolidation, merger or sale. The Company shall not effect any such consolidation, merger or sale, unless prior to or simultaneously with the consummation thereof, the successor corporation (if other than the Company) resulting from such consolidation or merger or the corporation purchasing such assets or the appropriate corporation or entity shall assume, by written instrument, the obligation to deliver to holder of each Bridge Warrant the shares of stock, securities or assets to which, in accordance with the foregoing provisions, such holder may be entitled and all other obligations of the Company under the Bridge Warrant. |
| (f) | If the Company in any manner issues or sells or enters into any agreement to issue or sell, any Common Stock, options or convertible securities (any such securities, “Variable Price Securities”) after the issuance of the Bridge Warrants that are issuable pursuant to such agreement or convertible into or exchangeable or exercisable for shares of Common Stock at a price which varies or may vary with the market price of the shares of Common Stock, including by way of one or more reset(s) to a fixed price, but exclusive of such formulations reflecting customary anti-dilution provisions (such as share splits, share combinations, share dividends and similar transactions) (each of the formulations for such variable price being herein referred to as, the “Variable Price”), the Company shall provide notice thereof to the holder on the date of such agreement and the |
F-121
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
| issuance of such convertible securities or options. From and after the date the Company enters into such agreement or issues any such Variable Price Securities, the holder shall have the right, but not the obligation, in its sole discretion to substitute the Variable Price for the Exercise Price upon exercise of the Bridge Warrant by designating in the exercise form delivered upon any exercise of the Bridge Warrant that solely for purposes of such exercise the holder is relying on the Variable Price rather than the Exercise Price then in effect. |
| (g) | In case any event shall occur as to which the other provisions above are not strictly applicable or the failure to make any adjustment would result in an unfair enlargement or dilution of the purchase rights represented by the Bridge Warrants in accordance with the essential intent and principles hereof, then, in each such case, the independent auditors of the Company shall give an opinion as to the adjustment, if any, on a basis consistent with the essential intent and principles above, necessary to preserve, without enlargement or dilution, the purchase rights presented by the Bridge Warrants. Upon receipt of such opinion, the Company shall promptly make the adjustment described therein. |
The Bridge Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Bridge Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
Extension Warrants
On May 5, 2023, the Company issued 26,086 warrants pursuant to the Extension Purchase Agreement. The purchase right represented by the Extension Warrants shall terminate on the date five years from the date of issuance (the “Expiration Date”). The exercise price at which the Extension Warrants may be exercised shall be $11.50 per share of Common Stock. If at any time after the date of issuance of the Extension Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, the Extension Warrants may be exercised by cashless exercise. In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the exercise price multiplied by such fraction. Except as provided in the Extension Warrants, the Extension Warrant does not entitle its holder to any rights of a stockholder of the Company.
During the term, the May 2023 Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the May 2023 Warrant and, from time to time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock issuable upon exercise of the Extension Warrants. All shares that may be issued upon the exercise of rights represented by the Extension Warrants and payment of the exercise price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Extension Warrants). Prior to the Expiration Date, the exercise price and the number of shares of Common Stock purchasable upon the exercise of the Extension Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
| (a) | In the event that the Company shall at any time after the date of issuance of the Extension Warrants (i) declare a dividend on Common Stock in shares or other securities of the Company, (ii) split or subdivide the outstanding Common Stock, (iii) combine the outstanding Common Stock into a smaller number of shares, or (iv) issue by reclassification of its Common Stock any shares or other securities of the Company, then, in each such event, the exercise price in effect at the time shall be adjusted so that the holder shall be entitled to receive the kind and number of such shares or other securities of the Company which the holder would have owned or have been entitled to receive after the happening of any of the events described above had such Extension Note Warrant been exercised immediately prior to the happening of such event (or any record date with respect thereto). |
| (b) | No adjustment in the number of shares of Common Stock receivable upon exercise of the Extension Warrants shall be required unless such adjustment would require an increase or decrease of at least 0.1% in the aggregate number of shares of Common Stock purchasable upon exercise of all Extension Warrants; provided that any adjustments which are not required to be made shall be carried forward and taken into account in any subsequent adjustment. |
| (c) | If at any time, as a result of an adjustment, the holder of any Extension Note Warrant thereafter exercised shall become entitled to receive any shares of the Company other than shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Extension Note Warrant shall be subject to adjustment from time to time in a manner and |
F-122
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
| on terms as nearly equivalent as practicable to the provisions with respect to the Common Stock receivable upon execution of the Extension Warrant. |
| (d) | Whenever the exercise price payable upon exercise of each Extension Warrant is adjusted, the Extension Warrant shares shall be adjusted by multiplying the number of shares of Common Stock receivable upon execution of the Extension Warrant immediately prior to such adjustment by a fraction, the numerator of which shall be the exercise price in effect immediately prior to such adjustment, and the denominator of which shall be the exercise price as adjusted. |
| (e) | In the event of any capital reorganization of the Company, or of any reclassification of the Common Stock, or in case of the consolidation of the Company with or the merger of the Company with or into any other corporation or of the sale of the properties and assets of the Company as, or substantially as, an entirety to any other corporation, each Extension Warrant shall, after such capital reorganization, reclassification of Common Stock, consolidation, merger or sale, and in lieu of being exercisable for shares of Common Stock of the Company, be exercisable, upon the terms and conditions specified in the Extension Warrant, for the number of shares of stock or other securities or assets to which holder of the number of shares of Common Stock purchasable upon exercisable of such Extension Warrant immediately prior to such capital organization, reclassification of Common Stock, consolidation, merger or sale would have been entitled upon such capital organization, reclassification of Common Stock, consolidation, merger or sale. The Company shall not effect any such consolidation, merger or sale, unless prior to or simultaneously with the consummation thereof, the successor corporation (if other than the Company) resulting from such consolidation or merger or the corporation purchasing such assets or the appropriate corporation or entity shall assume, by written instrument, the obligation to deliver to holder of each Extension Warrant the shares of stock, securities or assets to which, in accordance with the foregoing provisions, such holder may be entitled and all other obligations of the Company under the Extension Warrant. |
| (f) | If the Company in any manner issues or sells or enters into any agreement to issue or sell, any Common Stock, options or convertible securities (any such securities, “Variable Price Securities”) after the issuance of the Extension Warrants that are issuable pursuant to such agreement or convertible into or exchangeable or exercisable for shares of Common Stock at a price which varies or may vary with the market price of the shares of Common Stock, including by way of one or more reset(s) to a fixed price, but exclusive of such formulations reflecting customary anti-dilution provisions (such as share splits, share combinations, share dividends and similar transactions) (each of the formulations for such variable price being herein referred to as, the “Variable Price”), the Company shall provide notice thereof to the holder on the date of such agreement and the issuance of such convertible securities or options. From and after the date the Company enters into such agreement or issues any such Variable Price Securities, the holder shall have the right, but not the obligation, in its sole discretion to substitute the Variable Price for the exercise price upon exercise of the Extension Warrant by designating in the exercise form delivered upon any exercise of the Extension Warrant that solely for purposes of such exercise the holder is relying on the Variable Price rather than the exercise price then in effect. |
| (g) | In case any event shall occur as to which the other provisions above are not strictly applicable or the failure to make any adjustment would result in an unfair enlargement or dilution of the purchase rights represented by the Extension Warrants in accordance with the essential intent and principles hereof, then, in each such case, the independent auditors of the Company shall give an opinion as to the adjustment, if any, on a basis consistent with the essential intent and principles above, necessary to preserve, without enlargement or dilution, the purchase rights presented by the Extension Warrants. Upon receipt of such opinion, the Company shall promptly make the adjustment described therein. |
The Extension Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Extension Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
NOTE 9. INCOME TAX
The Company’s net deferred tax assets were offset with a valuation allowance resulting in zero deferred tax assets, net of allowance as of December 31, 2023 and 2022.
F-123
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
The Company’s net deferred tax assets are as follows:
| | | | | | |
| | December 31, | ||||
|
| 2023 |
| 2022 | ||
Deferred tax assets |
| | | | |
|
Net operating loss carryforward | | $ | 461,882 | | $ | (379) |
Start-up/organization expenses | |
| 1,622,610 | |
| 962,297 |
Total deferred tax assets | | | 2,084,492 | |
| 961,918 |
Valuation allowance | | | (2,084,492) | |
| (961,918) |
Deferred tax assets, net of allowance | | $ | — | | $ | — |
The income tax provision consists of the following:
| | | | | | |
| | For the years ended December 31, | ||||
|
| 2023 |
| 2022 | ||
Federal |
| | | | |
|
Current | | $ | — | | $ | 187,225 |
Deferred | | | (926,728) | |
| (741,805) |
| | | | | | |
State | | | | |
|
|
Current | | | — | |
| — |
Deferred | | | (191,524) | |
| (153,306) |
Change in valuation allowance | | | 1,118,252 | |
| 895,111 |
Income tax provision | | $ | — | | $ | 187,225 |
As of December 31, 2023 and 2022, the Company has $1,822,738 and $0 of U.S. federal and state net operating loss carryovers available to offset future taxable income, respectively.
In assessing the realization of the deferred tax assets, management considers whether it is more likely than not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all of the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance. For the years ended December 31, 2023 and 2022, the change in the valuation allowance was $1,118,252 and $895,111, respectively.
A reconciliation of the federal income tax rate to the Company’s effective tax rate at December 31, 2023 and 2022 is as follows:
| | | | | |
| | December 31, |
| ||
|
| 2023 |
| 2022 |
|
Statutory federal income tax rate | | 21.0 | % | 21.0 | % |
State taxes, net of federal tax benefit | | 4.3 | % | 4.3 | % |
Change in fair value of Bridge Note – bifurcated derivative | | 0.7 | % | (0.7) | % |
Change in fair value of PIPE forward contract derivative | | 1.0 | % | (1.4) | % |
Initial fair value of ELOC | | (1.2) | % | — | |
Initial fair value of Additional Bridge | | 0.1 | % | — | |
Change in fair value of Exchange Note | | (0.6) | % | — | |
Change in fair value of ELOC | | 0.0 | % | — | |
Change in fair value of Additional Bridge Note | | 0.0 | % | — | |
Change in valuation allowance | | (25.3) | % | (29.3) | % |
Income tax provision | | 0.0 | % | (6.1) | % |
F-124
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
The Company files income tax returns in the U.S. federal jurisdiction in various state and local jurisdictions and is subject to examination by the various taxing authorities.
NOTE 10. FAIR VALUE MEASUREMENTS
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
On December 31, 2023, assets held in the Trust Account were comprised of $1,368,637 in money market funds primarily invested in U.S. Treasury securities. During the year ended December 31, 2023, the Company did withdrew an amount of $71,436 from the Trust Account to pay tax obligations and $6,796,063 in connection with redemptions.
On December 31, 2022, assets held in the Trust Account were comprised of $7,527,369 in money market funds primarily invested in U.S. Treasury securities. During the year ended December 31, 2022, the Company withdrew $110,472,254 as a result of an aggregate of 10,805,877 shares of common stock redeemed on October 20, 2022 and the Company did not withdraw any interest income from the Trust Account.
The following table presents information about the Company’s assets that are measured at fair value on a recurring basis on December 31, 2023 and 2022 and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value. The fair value of securities held in the Trust on December 31, 2023 and , 2022 are as follows:
| | | | | | | |
|
| Trading Securities |
| Level |
| Fair Value | |
December 31, 2023 | | Money Market Funds | | 1 | | $ | 1,368,637 |
| | | | | | | |
|
| Trading Securities |
| Level |
| Fair Value | |
December 31, 2022 |
| Money Market Funds |
| 1 | | $ | 7,527,369 |
The following table presents fair value information as of December 31, 2023 and 2022 of the Company’s financial liabilities that were accounted for at fair value on a recurring basis and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
| | | | | | | | | | | | |
December 31, 2023 |
| Fair Value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: | | | | | | | | | | | | |
Extension Note – Bifurcated Derivative | | $ | 22,872 | | $ | — | | $ | — | | $ | 22,872 |
ELOC | | $ | 203,720 | | $ | — | | $ | — | | $ | 203,720 |
Additional Bridge Note | | $ | 102,726 | | $ | — | | $ | — | | $ | 102,726 |
Exchange Note | | $ | 2,621,558 | | $ | — | | $ | — | | $ | 2,621,558 |
| | | | | | | | | | | | |
December 31, 2022 |
| Fair Value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: |
| |
|
| |
|
| |
|
| |
|
PIPE Forward Contract | | $ | 170,666 | | $ | — | | $ | — | | $ | 170,666 |
Bridge Note – Bifurcated Derivative | | $ | 364,711 | | $ | — | | $ | — | | $ | 364,711 |
Measurement
Bridge Note Bifurcated Derivative
The Company established the initial fair value for the Bridge Note Bifurcated Derivative as of October 5, 2022, which was the date the Bridge Note was executed. On December 31, 2022, the fair value was remeasured. As such, the Company used a Probability
F-125
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
Weighted Expected Return Method (“PWERM”) that fair values the early termination/repayment features of the debt. The PWERM is a multi-step process in which value is estimated based on the probability-weighted present value of various future outcomes. The PWERM was used to value the Bridge Note Bifurcated Derivative for the initial periods and subsequent measurement periods. As a result of the Exchange Agreement on November 21, 2023, the Bridge Note was extinguished and the Derivative no longer exists.
The Bridge Note Bifurcated Derivative was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of November 21, 2023 and December 31, 2022 due to the use of unobservable inputs. The key inputs into PWERM for the Bridge Note Bifurcated Derivative at the termination date of November 21, 2023 and as of December 31, 2022 were as follows:
| | | | | | |
|
| November 21, 2023 |
| December 31, 2022 |
| |
CCC bond rates | | | n/a | | 15.09 | % |
Risk-free interest rate | | | 5.38 | % | n/a | |
Stock price | | $ | 12.64 | | n/a | |
Volatility | | | 0.1 | % | n/a | |
Weighted term | | | 0.61 | | n/a | |
Probability of early termination/repayment – business combination not completed |
| | — | % | 5 | % |
Probability of early termination/repayment – business combination completed, or PIPE completed |
| | — | % | 95 | % |
Probability of completing a business combination by March 31, 2023 |
| | — | % | 50 | % |
Probability of completing a business combination by June 30, 2023 |
| | — | % | 50 | % |
Extension Note Bifurcated Derivative
The Company established the initial fair value for the Extension Note Bifurcated Derivative as of May 5, 2023, which was the date the Extension Note was executed. On December 31, 2023, the fair value was remeasured. As such, the Company used a Discounted Cash Flow model (“DCF”) that fair values the early termination/repayment features of the debt. The DCF was used to value the Extension Note Bifurcated Derivative for the initial periods and subsequent measurement periods.
The Extension Note Bifurcated Derivative was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of May 5, 2023 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the DCF model for the Extension Note Bifurcated Derivative were as follows at May 5, 2023, initial value, and at December 31, 2023:
| | | | | |
|
| December 31, 2023 |
| May 5, 2023 |
|
Risk-free interest rate | | — | % | 5.13 | % |
CCC bond rates | | 12.96 | % | 14.69 | % |
Expected term (years) |
| 0.25 |
| 0.38 | |
Probability of completing a business combination by August 30, 2023 |
| — | % | 25 | % |
Probability of completing a business combination by September 30, 2023 |
| — | % | 75 | % |
Probability of completing a business combination by December 31, 2023 |
| — | % | — | % |
Probability of completing a business combination by March 31, 2024 |
| 100 | % | — | % |
PIPE Forward Contract
The Company established the initial fair value for the PIPE Forward Contract as of October 6, 2022, which was the date of the PIPE Securities Purchase Agreement was executed. On December 31, 2022, the fair value was remeasured. As such, the Company utilizing a PWERM. The PWERM is a multistep process in which value is estimated based on the probability-weighted present value of various future outcomes to value the PIPE Forward Contract for the initial periods and subsequent measurement periods. On July 11, 2023, each of the PIPE Investors provided notice to the Company that since the Outside Date Closing Condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing. As a result, the forward contract is terminated and derecognized as of July 11, 2023.
The PIPE Forward Contract was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of December 31, 2022 due to the use of unobservable inputs. As a result of the termination of the PIPE Forward Contract on July 11,
F-126
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
2023, the PIPE Forward Contract was derecognized. The key inputs into the PWERM for the PIPE Forward Contract were as follows at June 30, 2023 (as there were no significant transactions or events related to the close of the business combination that would effect the valuation between June 30, 2023 and July 11, 2023, the date the contract was terminated) and December 31, 2022:
| | | | | | | |
|
| June 30, 2023 | | December 31, 2022 |
| October 6, 2022 |
|
Risk-free interest rate |
| 5.43 | % | 4.76 | % | 4.00 | % |
Expected term (years) |
| 0.23 | | 0.37 |
| 0.61 | |
Probability of completing a business combination |
| 75 | % | 95 | % | 90 | % |
Additional Bridge Note
The Company established the initial fair value for the Additional Bridge as of November 21, 2023, which was the date the initial Additional Bridge Note was executed. On December 31, 2023, the fair value was remeasured. As such, the Company used a Monte Carlo model (“MCM”) that fair values the early termination/repayment features of the debt. The MCM was used to value the Additional Bridge Note for the initial periods and subsequent measurement periods.
The Additional Bridge Note was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of November 21, 2023 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the Additional Bridge Note were as follows at November 21, 2023, initial value, and at December 31, 2023:
| | | | | | | |
|
| December 31, 2023 |
| November 21, 2023 |
| ||
Risk-free interest rate | | | 5.40 | % | | 5.48 | % |
Expected term (years) | | | 0.25 | | | 0.36 | |
Volatility | | | 95 | % | | 95 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Debt discount rate | | | 39.7 | % | | 41.5 | % |
Probability of early termination/repayment – business combination not completed | | | 20 | % | | 20 | % |
Probability of completing a business combination by March 31, 2024 |
| | 80 | % | | 80 | % |
Exchange Note
The Company established the initial fair value for the Exchange Note as of November 21, 2023, which was the date the Exchange Note was executed. On December 31, 2023, the fair value was remeasured. As such, the Company using the MCM that fair values the early termination/repayment features of the debt. The MCM was used to value the Exchange Note for the initial periods and subsequent measurement periods.
The Exchange Note was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of November 21, 2023 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the Exchange Note were as follows at November 21, 2023, initial value, and at December 31, 2023:
| | | | | | | |
|
| December 31, 2023 |
| November 21, 2023 |
| ||
Risk-free interest rate | | | 5.21 | % | | 5.48 | % |
Expected term (years) | | | 0.71 | | | 0.61 |
|
Volatility | | | 95 | % | | 96 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Debt discount rate | | | 47.54 | % | | 49.17 | % |
Probability of completing a business combination by March 31, 2024 |
| | 80 | % | | 80 | % |
ELOC/Equity Financing
The Company established the initial fair value for the ELOC as of November 21, 2023, which was the date the ELOC Equity Purchase Agreement was executed. On December 31, 2023, the fair value was remeasured. As such, the Company using the MCM
F-127
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
that fair values the early termination/repayment features of the debt. The MCM was used to value the ELOC for the initial periods and subsequent measurement periods.
The ELOC was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of November 21, 2023 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the ELOC were as follows at November 21, 2023, initial value, and at December 31, 2023:
| | | | | | | |
|
| December 31, 2023 |
| November 21, 2023 |
| ||
Risk-free interest rate |
| | 3.99 | % | | 4.57 | % |
Expected term (years) |
| | 3.25 |
| | 3.36 |
|
Volatility | | | 96.4 | % | | 96.4 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Probability of completing a business combination by March 31, 2024 |
| | 80 | % | | 80 | % |
Level 3 Changes in Fair Value
The change in the fair value of the Level 3 financial liabilities for the period from contract inception through December 31, 2023 and 2022 is summarized as follows:
Level 3 Changes in Fair Value of Derivatives for the year ended December 31, 2023:
| | | | | | | | | |
|
| | |
| Bridge Note - |
| Extension Note - | ||
| | Forward | | Bifurcated | | Bifurcated | |||
|
| Contract |
| Derivative |
| Derivative | |||
Fair value as of December 31, 2022 | | $ | 170,666 | | $ | 364,711 | | $ | — |
Initial value of Extension Note – Bifurcated Derivative May 5, 2023 | |
| — | |
| — | |
| 24,502 |
Change in valuation inputs or other assumptions | | | 529,840 | | | (120,267) | | | (1,630) |
Derecognized value at termination date | |
| (700,506) | |
| (244,444) | |
| — |
Fair value as of December 31, 2023 | | $ | — | | $ | — | | $ | 22,872 |
Level 3 Change in Fair Value of Notes for the year ended December 31, 2023:
| | | | | | | | | |
|
|
| | | | |
|
| |
| | Exchange | | Additional | | | |||
|
| Note |
| Bridge Note |
| ELOC | |||
Fair value as of January 1, 2023 | | $ | — | | $ | — | | $ | — |
Initial value of Extension Note, Additional Bridge Note and ELOC November 21, 2023 | |
| 2,523,744 | | | 100,000 | | | 204,039 |
Change in valuation inputs or other assumptions | |
| (97,814) | | | 2,726 | | | (318) |
Fair value as of December 31, 2023 | | $ | 2,621,558 | | $ | 102,726 | | $ | 203,720 |
Level 3 Changes in Fair Value of Derivatives for the year ended December 31, 2022:
| | | | | | | | | |
|
| | |
| Bridge Note - |
| Extension Note - | ||
| | Forward | | Bifurcated | | Bifurcated | |||
|
| Contract |
| Derivative |
| Derivative | |||
Fair value at October 5, 2022 (Initial measurement) | | $ | — | | $ | 278,404 | | $ | — |
Fair value at October 6, 2022 (Initial measurement) | |
| — | |
| — | |
| — |
Change in valuation inputs or other assumptions | |
| 170,666 | |
| 86,307 | |
| — |
Fair value as of December 31, 2022 | | $ | 170,666 | | $ | 364,711 | | $ | — |
Transfers to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs. There were no transfers to or from the various Levels for the years ended December 31, 2023 and 2022.
F-128
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2023
NOTE 11. SUBSEQUENT EVENTS
The Company evaluated subsequent events and transactions that occurred after the consolidated balance sheet date up to the date that the consolidated financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the consolidated financial statements.
On January 22, 2024, the Company amended an unsecured promissory note in the aggregate principal amount of $165,000 to M2B Funding Corp., an affiliate of the Sponsor. As a result of the amendment, the Company was to repay the remaining amount due by February 8, 2024. On January 31, 2024, the note was paid in full for a total of $190,750.
On January 22, 2024, the Company and the Bridge Investor entered into a side letter to the registration rights agreement with the Bridge Investor dated October 5, 2022 whereby the Company agreed to register the shares of common stock underlying the Bridge Notes and the Additional Bridge Notes.
On January 25, 2024, the Bridge Investor purchased the second Additional Bridge Note in the principal amount of $55,556 from DHAC as contemplated by the Bridge SPA.
On February 2, 2024, the Company extended the date by which the Company has to consummate a business combination from February 8, 2024 to May 8, 2024. The extension is the second of four additional three-month extensions permitted under the Company’s governing documents and provides the Company with additional time to complete the business combination.
On February 13, 2024, the parties entered into a First Amendment to the Third Amended and Restated Business Combination Agreement to provide that certain indebtedness of VSee and iDoc would be assumed by DHAC and converted into DHAC common stock following the closing instead of being converted into class B common stock of VSee and iDoc prior to the closing.
On February 13, 2024, the Company, VSee and/or iDoc, as applicable, amended and restated certain of the Conversion SPAs (the “Amended and Restated Conversion SPAs”) pursuant to which (1) a $600,000 balance of certain indebtedness of VSee will be assumed by the Company and converted into the Company’s common stock after the closing of the business combination; (2) a $600,000 balance certain indebtedness of iDoc will be assumed by the Company and will then be converted into the Company’s common stock subject to executing of certain registration rights agreement and filing of a registration statement thereunder after the closing of the business combination; and (3) certain indebtedness owned by iDoc will be assumed by the Company and will then be converted into the Company’s common stock subject to executing of certain registration rights agreement and filing of a registration statement thereunder after the closing of the business combination.
On April 17, 2024, the parties entered into a Second Amendment to the Third Amended and Restated Business Combination Agreement to extend certain termination date therein to June 30, 2024.
On April 17, 2024, DHAC, VSee, iDoc and the Bridge Investor entered a Letter Agreement, which amended certain business combination timeline in the Additional Bridge Notes.
On April 17, 2024, the parties to the Extension Purchase Agreement and Extension Note executed a letter agreement, which extended the maturity date of the Extension Note to June 30, 2024.
F-129
DIGITAL HEALTH ACQUISITION CORP.
CONDENSED CONSOLIDATED BALANCE SHEETS
| | | | | | |
| | March 31, |
| December 31, | ||
| | 2024 | | 2023 | ||
|
| (unaudited) |
| | ||
ASSETS |
| |
|
| |
|
Current assets: |
| |
|
| |
|
Cash | | $ | 724 | | $ | 1,863 |
Total current assets | |
| 724 | | | 1,863 |
Investments held in Trust Account | | | 1,386,490 | | | 1,368,637 |
Total Assets | | $ | 1,387,214 | | $ | 1,370,500 |
| | | | | | |
LIABILITIES, COMMON STOCK SUBJECT TO POSSIBLE REDEMPTION AND STOCKHOLDERS’ DEFICIT | |
|
| |
|
|
Current liabilities: | |
|
| |
|
|
Accounts payable and accrued expenses | | $ | 3,642,780 | | $ | 3,303,836 |
Excise tax payable | | | 72,396 | | | 72,396 |
Income taxes payable | | | 187,225 | | | 187,225 |
Advances from related parties | | | 592,800 | | | 117,871 |
Accrued interest on Exchange and Additional Bridge Notes | | | 78,061 | | | 23,964 |
Additional Bridge Notes | | | 156,564 | | | 102,726 |
Promissory Note – M2B | |
| — | |
| 167,958 |
Exchange Note | | | 2,814,359 | | | 2,621,558 |
ELOC | | | 189,764 | | | 203,720 |
Promissory Note – related party | | | 926,500 | | | 926,500 |
First Fire Note, net of discount | | | 285,614 | | | 233,774 |
First Fire Note – Bifurcated Derivative | | | 22,868 | | | 22,872 |
Total current liabilities | |
| 8,968,931 | | | 7,984,400 |
| | | | | | |
Deferred underwriting fee payable | |
| 4,370,000 | | | 4,370,000 |
Total Liabilities | |
| 13,338,931 | | | 12,354,400 |
| | | | | | |
Commitments | |
| | | | |
Common stock subject to possible redemption, $0.0001 par value; 114,966 shares issued and outstanding at redemption value of $11.15 per share as of March 31, 2024 and December 31, 2023 | |
| 1,281,957 | | | 1,281,957 |
| | | | | | |
Stockholders’ Deficit | |
| | | | |
Common stock, $0.0001 par value; 50,000,000 shares authorized; 3,489,000 shares issued and outstanding (excluding 114,966 shares subject to redemption) as of March 31, 2024 and December 31, 2023, respectively | |
| 350 | | | 350 |
Additional paid-in capital | |
| 550,246 | | | 550,246 |
Accumulated deficit | |
| (13,784,270) | | | (12,816,453) |
Total Stockholders’ Deficit | |
| (13,233,674) | | | (12,265,857) |
TOTAL LIABILITIES, COMMON STOCK SUBJECT TO POSSIBLE REDEMPTION AND STOCKHOLDERS’ DEFICIT | | $ | 1,387,214 | | $ | 1,370,500 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-130
DIGITAL HEALTH ACQUISITION CORP.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | | |
| | For the Three Months | ||||
| Ended March 31, | |||||
|
| 2024 |
| 2023 | ||
| | | | | | |
General and administrative costs | | $ | 674,262 | | $ | 707,592 |
Loss from operations | |
| (674,262) | | | (707,592) |
| | | | | | |
Other (expense) income: | |
| | | | |
Interest expense- Bridge/Exchange Note | | | (51,036) | | | (133,138) |
Interest expense – Additional Bridge Note | | | (8,617) | | | — |
Interest expense – M2B Note | | | (2,496) | | | — |
Interest expense – Extension Note | | | (51,840) | | | — |
Default interest expense- M2B Note | | | (20,296) | | | — |
Initial fair value of Additional Bridge Note – additional draw | | | 3,851 | | | — |
Change in fair value of Bridge Note- Bifurcated Derivative | | | — | | | 34,758 |
Change in fair value of Extension Note- Bifurcated Derivative | | | 4 | | | — |
Change in fair value of Additional Bridge Note | | | (2,133) | | | — |
Change in fair value of Exchange Note | | | (192,801) | | | — |
Change in fair value of ELOC Note | | | 13,956 | | | — |
Change in fair value of PIPE Forward Contract Derivative | | | — | | | (1,163,950) |
Interest earned on investments held in Trust Account | |
| 17,853 | | | 75,280 |
Total other expense | | | (293,555) | | | (1,187,050) |
| | | | | | |
Loss before provision for income taxes | | | (967,817) | | | (1,894,642) |
Provision for income taxes | | | — | | | — |
Net loss | | $ | (967,817) | | $ | (1,894,642) |
| | | | | | |
Basic and diluted weighted average shares outstanding | | | 3,603,966 | | | 4,168,567 |
Basic and diluted net loss per share | | $ | (0.27) | | $ | (0.45) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-131
DIGITAL HEALTH ACQUISITION CORP.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE THREE MONTHS ENDED MARCH 31, 2024
| | | | | | | | | | | | | | |
| | |
| |
| Additional |
| |
| Total | ||||
| | Common Stock | | Paid-in | | Accumulated | | Stockholders’ | ||||||
|
| Shares |
| Amount |
| Capital |
| Deficit |
| Deficit | ||||
Balance – December 31, 2023 (audited) |
| 3,489,000 | | $ | 350 | | $ | 550,246 | | $ | (12,816,453) | | $ | (12,265,857) |
| | | | | | | | | | | | | | |
Net loss |
| — | |
| — | |
| — | |
| (967,817) | |
| (967,817) |
| | | | | | | | | | | | | | |
Balance – March 31, 2024 (unaudited) |
| 3,489,000 | | $ | 350 | | $ | 550,246 | | $ | (13,784,270) | | $ | (13,233,674) |
FOR THE THREE MONTHS ENDED MARCH 31, 2023
| | | | | | | | | | | | | | |
| | | | | | | Additional | | | | | Total | ||
| | Common Stock | | Paid-in | | Accumulated | | Stockholders’ | ||||||
|
| Shares |
| Amount |
| Capital |
| Deficit |
| Deficit | ||||
Balance – December 31, 2022 (audited) | | 3,462,000 | | $ | 347 | | $ | 292,973 | | $ | (7,719,916) | | $ | (7,426,596) |
| | | | | | | | | | | | | | |
Issuance of 20,000 shares issued to settle legal claim | | 20,000 | | | 2 | | | 214,198 | | | — | | | 214,200 |
| | | | | | | | | | | | | | |
Net loss | | — | | | — | | | — | | | (1,894,642) | | | (1,894,642) |
|
| | |
| | |
| | |
| | |
| |
Balance – March 31, 2023 (unaudited) |
| 3,482,000 | | $ | 349 | | $ | 507,171 | | $ | (9,614,558) | | $ | (9,107,038) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-132
DIGITAL HEALTH ACQUISITION CORP.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | |
| | For the Three Months Ended | ||||
| | March 31, | ||||
|
| 2024 |
| 2023 | ||
Cash Flows from Operating Activities: | | | | | | |
Net loss | | $ | (967,817) | | $ | (1,894,642) |
Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | | |
Interest earned on investments held in Trust Account | |
| (17,853) | | | (75,280) |
Initial fair value of Additional Bridge Note – additional draw | | | (3,851) | | | — |
Change in fair value of Bridge Note – Bifurcated Derivative | | | — | | | (34,758) |
Change in fair value of Extension Note – Bifurcated Derivative | | | (4) | | | — |
Change in fair value of Exchange Note | |
| 192,801 | | | — |
Change in fair value of Additional Bridge Note | |
| 2,133 | | | — |
Change in fair value of ELOC | |
| (13,956) | | | — |
Change in fair value of PIPE Forward Contract Derivative | | | — | | | 1,163,950 |
Changes in operating assets and liabilities: | | | | | | |
Prepaid expenses and other current assets | | | — | | | (52,500) |
Accounts payable and accrued expenses | | | 338,944 | | | 419,424 |
Accrued interest expense – Exchange Note | | | 59,653 | | | 133,139 |
Accrued interest expense – M2B Note | | | 2,496 | | | — |
Accrued interest expense – Extension Note | | | 51,840 | | | — |
Default Interest M2B | | | 20,296 | | | — |
Net cash used in operating activities | |
| (335,318) | | | (340,667) |
| | | | | | |
Cash Flows from Financing Activities: | | | | | | |
Advances from related party | | | 474,929 | | | — |
Proceeds from Additional Bridge Note – related party | | | 50,000 | | | — |
Proceeds from promissory note | | | — | | | 250,000 |
Repayment of M2B Note | | | (190,750) | | | — |
Net cash provided by financing activities | | | 334,179 | | | 250,000 |
| | | | | | |
Net Change in Cash | |
| (1,139) | | | (90,667) |
Cash – Beginning of period | |
| 1,863 | | | 106,998 |
Cash – End of period | | $ | 724 | | $ | 16,331 |
| | | | | | |
Non-cash investing and financing activities: | | | | | | |
Common stock issued from legal settlement | | $ | — | | $ | 214,200 |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-133
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
NOTE 1. DESCRIPTION OF ORGANIZATION AND BUSINESS OPERATIONS
Digital Health Acquisition Corp. (the “Company” or “DHAC”) is a blank check company incorporated as a Delaware corporation on March 30, 2021. The Company was incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar Business Combination with one or more businesses (the “Business Combination”).
On June 9, 2022, DHAC Merger Sub I, Inc. (“Merger Sub I”), a Delaware corporation and a wholly owned subsidiary of the Company, was formed. On June 9, 2022, DHAC Merger Sub II, Inc. (“Merger Sub II”), a Texas corporation and a wholly owned subsidiary of the Company, was formed.
As of March 31, 2024, the Company had not commenced any significant operations. All activity for the period from inception, the date which operations commenced, through March 31, 2024 relates to the Company’s formation, the Company’s Initial Public Offering (as defined below), and identifying a target company for a Business Combination. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering (as defined below).
The registration statement for the Company’s Initial Public Offering was declared effective on November 3, 2021. On November 8, 2021, the Company consummated the Initial Public Offering of 11,500,000 units (the “Units” and, with respect to the shares of common stock included in the Units sold, the “Public Shares”), which includes the full exercise by the underwriter of its over-allotment option in the amount of 1,500,000 Units, at $10.00 per Unit, generating gross proceeds of $115,000,000, which is described in Note 3. On October 20, 2022, in connection with the stockholders meeting to approve the extension, 10,805,877 shares of DHAC’s common stock were redeemed leaving 694,123 shares of common stock subject to redemption. On November 6, 2023, in connection with the stockholders meeting to approve the extension, 579,157 shares of DHAC’s common stock were redeemed leaving 114,966 shares of common stock subject to redemption.
Simultaneously with the closing of the Initial Public Offering, the Company consummated the sale of 557,000 units (each, a “Private Placement Unit” and, collectively, the “Private Placement Units”) at a price of $10.00 per Private Placement Unit in a private placement to Digital Health Sponsor LLC (the “Sponsor”), generating gross proceeds of $5,570,000, which is described in Note 4. As of November 8, 2021, the Company received $3,680,000 from the proceeds of the Private Placement and recorded $1,890,000 in subscription receivable. The Sponsor paid the subscription in full on November 12, 2021.
Transaction costs amounted to $6,877,164, consisting of $1,955,000 of underwriting fees, $4,370,000 of deferred underwriting fees and $552,164 of other offering costs. In addition, cash of $9,478 was held outside of the Trust Account (as defined below) and is available for the payment of offering costs and for working capital purposes.
Following the closing of the Initial Public Offering on November 8, 2021, an amount of $116,725,000 ($10.15 per Unit) from the net proceeds of the sale of the Units in the Initial Public Offering and the sale of the Private Placement Units was placed in a trust account (the “Trust Account”), invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or in any open-ended investment company that holds itself out as a money market fund meeting the conditions of Rule 2a-7 of the Investment Company Act of 1940, as amended (the “Investment Company Act”). The Trust Account is intended as a holding place for funds pending the earliest to occur of either (i) the completion of the initial Business Combination; (ii) the redemption of any public shares properly submitted in connection with a stockholder vote to amend the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares if the Company does not complete the initial Business Combination within 27 months from the closing of the Initial Public Offering (as extended as of December 31, 2023) or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; or (iii) absent an initial Business Combination within 27 months from the closing of the Initial Public Offering (as extended as of December 31, 2023), the Company’s return of the funds held in the Trust Account to the Company’s public stockholders as part of the Company’s redemption of the public shares.
F-134
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
On October 20, 2022, stockholders of DHAC approved a proposal to amend DHAC’s amended and restated certificate of incorporation to (a) extend the date by which DHAC has to consummate a Business Combination (the “Extension”) for an additional three (3) months, from November 8, 2022 to February 8, 2023, (b) provide DHAC’s board of directors the ability to further extend the date by which DHAC has to consummate a Business Combination up to three (3) additional times for three (3) months each time, for a maximum of nine (9) additional months if the Sponsor pays an amount equal to $350,000 for each three-month extension (the “Extension Fee”), which amount shall be deposited in the trust account of DHAC; provided, that if as of the time of an extension DHAC has filed a Form S-4 registration statement in connection with its initial Business Combination, then no Extension Fee would be required in connection with such extension; provided further that for each three-month extension (if any) following such extension where no deposit into the Trust Account or other payment has been made, an Extension Fee is required, and (c) allow for DHAC to provide redemption rights to DHAC’s public stockholders in accordance with the requirements of the amended and restated certificate of incorporation without complying with the tender offer rules. In connection with such stockholder vote, an aggregate of 10,805,877 shares of DHAC’s common stock were redeemed leaving 4,156,123 shares issued and outstanding and entitled to vote as of October 20, 2022. The Company subsequently extended the date by which the Company has to consummate a Business Combination pursuant to the three additional three-month extensions to November 8, 2023, and deposited an aggregate of $700,000 into the Trust Account as extension fees.
On September 8, 2023, DHAC held a Special Meeting and the stockholders approved an amendment of the Company’s amended and restated certificate of incorporation (as amended from time to time, the “Charter”) to expand the methods that the Company may employ to not become subject to the “penny stock” rules of the U.S. Securities and Exchange Commission (“SEC”). On September 8, 2023, DHAC filed such amendment, which provided that DHAC would be able to consummate the Business Combination even if as a result of the transactions the combined company does not have net tangible assets of at least $5,000,001 upon consummation of such business combination.
On November 6, 2023, DHAC held its 2023 annual stockholders meeting (“2023 Annual Meeting”). At the 2023 Annual Meeting, the stockholders of DHAC approved amendments to DHAC’s Charter to extend the date by which the Company must consummate a Business Combination (as defined in the Charter) up to four (4) times, each by an additional three (3) months, for an aggregate of twelve (12) additional months (i.e., from November 8, 2023 up to November 8, 2024) or such earlier date as determined by the Company’s board of directors. In connection with the amended Charter, on November 6, 2023, DHAC extended the period of time that it has to consummate its business combination by three months from November 8, 2023 to February 8, 2024. In addition, on February 2, 2024, DHAC further extended the period of time that it has to consummate its business combination by another three months from February 8, 2024 to May 8, 2024 and on May 1, 2024, DHAC further extended the period of time that it has to consummate its business combination by another three months from May 8, 2024 to August 8, 2024. The extension is the third of four additional three-month extensions permitted under the Company’s governing documents and provides the Company with additional time to complete the business combination.
Furthermore, at the 2023 Annual Meeting, the stockholders of DHAC also approved an amendment to DHAC’s investment management trust agreement (the “Trust Agreement”), dated as of November 3, 2021 and as amended on October 26, 2022, by and between the Company and Continental Stock Transfer & Trust Company, which allows the Company to extend the business combination period from November 8, 2023 to up to four (4) times, each by an additional three (3) months, for an aggregate of twelve (12) additional months to November 8, 2024 (the “Combination Period”).
In connection with the 2023 Annual Meeting and amendments to DHAC’s Charter and Trust Agreement, 579,157 shares of Common Stock were redeemed.
The Company’s Business Combination must be with one or more target businesses that together have a fair market value equal to at least 80% of the net balance in the Trust Account (as defined below) (excluding the amount of deferred underwriting discounts held and taxes payable on the income earned on the Trust Account) at the time of the signing an agreement to enter into a Business Combination. However, the Company will only complete a Business Combination if the post-Business Combination company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act. There is no assurance that the Company will be able to successfully effect a Business Combination.
F-135
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
The Company will provide the Company’s public stockholders with the opportunity to redeem all or a portion of their common shares in connection with the initial Business Combination either (i) in connection with a general meeting called to approve the Business Combination or (ii) without a stockholder vote by means of a tender offer. The decision as to whether the Company will seek stockholder approval of a proposed Business Combination or conduct a tender offer will be made by the Company, solely in its discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would require the Company to seek stockholder approval under applicable law or stock exchange listing requirement. The public stockholders will be entitled to redeem their shares at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account calculated as of two business days prior to the consummation of the initial Business Combination, including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its taxes, divided by the number of then outstanding public shares, subject to the limitations.
If the Company is unable to complete its initial Business Combination within the Combination Period, the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account (less taxes payable and up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any) and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholders and the Company’s board of directors, liquidate and dissolve, subject, in each case, to the Company’s obligations under Delaware law to provide for claims of creditors and in all cases subject to the other requirements of applicable law. The shares of common stock subject to redemption are recorded at a redemption value and classified as temporary equity in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 480, “Distinguishing Liabilities from Equity”.
The Sponsor, along with certain advisors, officers and directors, has entered into a letter agreement with the Company, pursuant to which they have agreed to (i) waive their redemption rights with respect to their founder shares (as defined in Note 5) and public shares in connection with the completion of the initial Business Combination; (ii) waive their redemption rights with respect to their founder shares and public shares in connection with a stockholder vote to approve an amendment to the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares. If the Company have not consummated an initial Business Combination within the Combination Period or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; (iii) waive their rights to liquidating distributions from the Trust Account with respect to their founder shares if the Company fails to complete the initial Business Combination within the Combination Period, although they will be entitled to liquidating distributions from the Trust Account with respect to any public shares they hold if the Company fails to complete the initial Business Combination within the prescribed time frame; and (iv) vote any founder shares held by them and any public shares purchased during or after the Initial Public Offering (including in open market and privately negotiated transactions) in favor of the initial Business Combination.
The Company’s Sponsor has agreed that it will be liable to the Company if and to the extent any claims by a third party (other than the Company’s independent registered public accounting firm) for services rendered or products sold to the Company, or a prospective target business with which the Company have entered into a written letter of intent, confidentiality or other similar agreement or Business Combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.15 per public share and (ii) the actual amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.15 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). However, the Company has not asked the Sponsor to reserve for such indemnification obligations, nor have the Company independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations and the Company believes that the Company’s Sponsor’s only assets are securities of the Company. Therefore, the Company cannot assure that the Sponsor would be able to satisfy those obligations.
On June 15, 2022, DHAC entered into the original Business Combination Agreement, by and among DHAC, DHAC Merger Sub I, Inc. (“Merger Sub I”), DHAC Merger Sub II, Inc. (“Merger Sub II” and together with Merger Sub I, the “Merger Subs”), VSee Lab,
F-136
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Inc., a Delaware corporation (“VSee”), and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”). On August 9, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the First Amended and Restated Business Combination Agreement to provide for the concurrent execution of financing documents for a PIPE financing consisting of convertible notes and warrants and delivery of the Cassel Salpeter’s opinion to the Board. On October 6, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into a Second Amended and Restated Business Combination Agreement to make the consideration payable to VSee and iDoc stockholders 100% DHAC common stock and to provide for the concurrent execution of amended PIPE financing documents providing for the issuance of the shares and warrants to the PIPE investors. On November 3, 2022, the parties entered into a First Amendment to the Second Amended and Restated Business Combination Agreement to remove a closing condition that DHAC have at least $10 million in cash proceeds from the transactions at closing. On July 11, 2023, each of the PIPE Investors provided notice to the Company that since a closing condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing. Accordingly, the PIPE financing was terminated. On November 21, 2023, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the Third Amended and Restated Business Combination Agreement (as amended and restated, the “Business Combination Agreement” and the transactions contemplated thereby, the “Business Combination”) to, among other things, provide for the removal of the PIPE financing and the concurrent execution of the Additional Bridge Financing, the Exchange Financing, the Quantum Financing, the Equity Financing and the Loan Conversions, which are described in Note 6 — Commitments. The DHAC Board has (i) approved and declared advisable the Business Combination Agreement, the Business Combination and the other transactions contemplated thereby and (ii) resolved to recommend approval of the Business Combination Agreement and related matters by the stockholders of DHAC.
Pursuant to the Business Combination Agreement and subject to the terms and conditions set forth therein, Merger Sub I will merge with and into VSee (the “VSee Merger”), with VSee surviving the VSee Merger as a wholly owned subsidiary of DHAC, and Merger Sub II will merge with and into iDoc (the “iDoc Merger” and, together with the VSee Merger, the “Mergers”), with iDoc surviving the iDoc Merger as a wholly owned subsidiary of DHAC. At the effective time of the Mergers (the “Effective Time”), DHAC will change its name to VSee Health, Inc.
NASDAQ Trading Status
On March 31, 2023, DHAC received a letter from the staff (the “Staff”) at the Nasdaq Global Market (“Nasdaq Global”) notifying DHAC that for the 30 consecutive trading days prior to the date of the Letter, DHAC’s securities listed on the Nasdaq Global (including the Common Stock, Units and Warrants) (the “Securities”) had traded at a value below the minimum $50,000,000 “Market Value of Listed Securities (“MVLS”) requirement set forth in Nasdaq Listing Rule 5450(b)(2)(A), which is required for continued listing of DHAC’s Securities on Nasdaq Global. In accordance with Nasdaq listing rule 5810I(3)I, DHAC had 180 calendar days, or until September 27, 2023, to regain compliance.
On May 23, 2023, DHAC received a second letter from the Staff notifying DHAC that for the prior 30 consecutive business days, DHAC’s market value of publicly held shares (“MVPHS”) was below the $15 million required for continued listing on the Nasdaq Global and therefore, DHAC no longer met Nasdaq Listing Rule 5450(b)(3)(C) (the “MVPHS Requirement”). In accordance with Nasdaq Listing Rule 5810I(3)(D), DHAC had 180 calendar days, or until November 20, 2023, to regain compliance.
On September 28, 2023, DHAC received a third letter from the Staff notifying DHAC that the Staff had determined to delist DHAC’s Securities because it had not regained compliance with the MVLS standard. Pursuant to the third letter, on October 4, 2023, DHAC requested a hearing (the “Hearing”) to appeal this determination and also applied to transfer the listing of its Securities from Nasdaq Global to the Nasdaq Capital Market (“NasdaqCM”).
On October 9, 2023, DHAC received a fourth letter from the Staff notifying DHAC that it is not meeting the 400 total shareholders requirement under the Nasdaq Listing Rule 5450 (a)(2) served as an additional basis for delisting DHAC’s Securities from Nasdaq Global.
On October 26, 2023, the Nasdaq Listing Qualifications Department of the Nasdaq Stock Market notified DHAC in writing (the “Notice”) that its application to transfer the listing of its Securities to NasdaqCM had been approved. DHAC’s Securities were transferred to the NasdaqCM at the opening of business on October 30, 2023. On November 1, 2023, DHAC received a letter from the Nasdaq Global Hearing panel that due to DHAC’s transfer of its listed Securities to NasdaqCM, the Hearing on November 30, 2023 regarding non-compliance with the Nasdaq Global listing standards had been cancelled.
F-137
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
As of October 30, 2023, DHAC’s Securities are listed and traded on the Nasdaq Stock Market on NasdaqCM and will continue to be listed and traded on NasdaqCM.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information and in accordance with the instructions to Form 10-Q and Article 8 of Regulation S-X of the SEC. Certain information or footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted, pursuant to the rules and regulations of the SEC for interim financial reporting. Accordingly, they do not include all the information and footnotes necessary for a complete presentation of financial position, results of operations, or cash flows. In the opinion of management, the accompanying unaudited condensed consolidated financial statements include all adjustments, consisting of a normal recurring nature, which are necessary for a fair presentation of the financial position, operating results and cash flows for the periods presented.
The accompanying unaudited condensed consolidated financial statements should be read in conjunction with the Company’s Annual Report on Form 10-K for the period ended December 31, 2023, as filed with the SEC on April 12, 2024. The interim results for the three months ended March 31, 2024 are not necessarily indicative of the results to be expected for the year ending December 31, 2024 or for any future periods.
Principles of Consolidation
The accompanying condensed consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Liquidity and Going Concern
The Company may raise additional capital through loans or additional investments from the Sponsor or its stockholders, officers, directors, or third parties. The Company’s officers and directors and the Sponsor may but are not obligated to loan the Company funds, from time to time, in whatever amount they deem reasonable in their sole discretion, to meet the Company’s working capital needs. Based on the foregoing, the Company believes it will have sufficient working capital and borrowing capacity from the Sponsor or an affiliate of the Sponsor, or certain of the Company directors to meet its needs through the earlier of the consummation of a Business Combination or at least one year from the date that the condensed consolidated financial statements were issued.
As of March 31, 2024, the Company had a cash balance of $724 and a working capital deficit of $8,968,207. In addition, in connection with the Company’s assessment of going concern considerations in accordance with ASC 205-40, “Presentation of Financial Statements — Going Concern,” management has determined that the liquidity condition, mandatory liquidation and subsequent dissolution on November 8, 2024 raise substantial doubt about the Company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities of the Company as of March 31, 2024. The Company intends to complete a Business Combination before the mandatory liquidation date.
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
F-138
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Offering Costs
Offering costs consisted of legal, accounting, and other expenses incurred through the Initial Public Offering that were directly related to the Initial Public Offering. Offering costs were allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs allocated to warrants were allocated to equity. Offering costs allocated to the common stock issued were initially charged to temporary equity.
Use of Estimates
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the condensed consolidated financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. The most significant accounting estimates were the assumptions used to fair value the PIPE Forward Contract, the Extension Note Bifurcated Derivative, the Bridge Note Bifurcated Derivative, the Additional Bridge Note and the Exchange Note (each term as defined below). Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company did not have any cash equivalents as of March 31, 2024 and December 31, 2023.
Investments Held in Trust Account
At March 31, 2024 and December 31, 2023, the assets held in the Trust Account were held in money market funds, which are invested primarily in U.S. Treasury securities.
Common Stock Subject to Possible Redemption
The Company accounts for its common stock subject to possible redemption in accordance with the guidance in ASC Topic 480, “Distinguishing Liabilities from Equity.” Common stock subject to mandatory redemption is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that features redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified in temporary equity. At all other times, common stock is classified as stockholders’ equity (deficit). The Company’s common stock sold in the Initial Public Offering features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence of uncertain future events. Accordingly, at March 31, 2024 and December 31,
F-139
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
2023, common stock subject to possible redemption is presented at redemption value as temporary equity, outside of the stockholders’ deficit section of the Company’s condensed consolidated balance sheets.
The Company recognizes changes in redemption value immediately as they occur and adjusts the carrying value of redeemable common stock to equal the redemption value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. Immediately upon the closing of the Initial Public Offering, increases or decreases in the carrying amount of redeemable common stock are affected by charges against additional paid-in capital and accumulated deficit.
At March 31, 2024 and December 31, 2023, the common stock subject to possible redemption reflected in the condensed consolidated balance sheets is reconciled in the following table:
| | | |
Gross proceeds |
| $ | 115,000,000 |
Less: | |
| |
Proceeds allocated to public warrants | |
| (12,483,555) |
Common stock issuance costs | |
| (6,923,767) |
Plus: | |
| |
Accretion of carrying value to redemption value | |
| 21,132,322 |
Common stock subject to possible redemption, December 31, 2021 | | | 116,725,000 |
Plus: | | | |
Accretion of carrying value to redemption value | | | 1,142,603 |
Less: | | | |
Redemptions | | | (110,472,254) |
Common stock subject to possible redemption, December 31, 2022 | | | 7,395,349 |
Plus: | | | |
Accretion of carrying value to redemption value | | | 682,671 |
Less: | | | |
Redemptions | | | (6,796,063) |
Common stock subject to possible redemption, December 31, 2023 and March 31, 2024 | | $ | 1,281,957 |
Income Taxes
The Company accounts for income taxes under ASC 740, “Income Taxes.” ASC 740 requires the recognition of deferred tax assets and liabilities for both the expected impact of differences between the unaudited condensed consolidated financial statements and tax basis of assets and liabilities and for the expected future tax benefit to be derived from tax loss and tax credit carry forwards. ASC 740 additionally requires a valuation allowance to be established when it is more likely than not that all or a portion of deferred tax assets will not be realized. ASC 740-270-25-2 requires that an annual effective tax rate be determined, and such annual effective rate applied to year to date income in interim periods under ASC 740-270-30-5. As of March 31, 2024 and December 31, 2023, the Company’s deferred tax asset had a full valuation allowance recorded against it.
ASC 740-270-25-2 requires that an annual effective tax rate be determined and such annual effective rate applied to year to date income in interim periods under ASC 740-270-30-5. The Company’s effective tax rate was 0.0% and 0.0% for the three months ended March 31, 2024 and 2023, respectively. The effective tax rate differs from the statutory tax rate of 21.0% for the three months ended March 31, 2024 and 2023 due to the valuation allowance on the deferred tax assets.
ASC 740 also clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. ASC 740 also provides guidance on derecognition, classification, interest and penalties, accounting in interim period, disclosure and transition.
The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of March 31, 2024 and December 31, 2023. The
F-140
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
The Company has identified the United States as its only “major” tax jurisdiction. The Company has been subject to income taxation by major taxing authorities since inception. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
Net Loss per Common Stock
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” Net loss per common stock is computed by dividing net loss by the weighted average number of common stocks outstanding for the period. Accretion associated with the redeemable shares of common stock is excluded from net loss per common stock as the redemption value approximates fair value.
The calculation of diluted loss per share does not consider the effect of the warrants issued in connection with the (i) Initial Public Offering, and (ii) the private placement (iii) the Bridge Notes and the Extension Note because the exercise of the warrants is contingent upon the occurrence of future events and the inclusion of such warrants would be anti-dilutive. The warrants are exercisable to purchase 12,256,999 shares of common stock in the aggregate. For the three months ended March 31, 2024 and 2023, the Company did not have any dilutive securities or other contracts that could, potentially, be exercised or converted into common stock and then share in the earnings of the Company. As a result, diluted net loss per common stock is the same as basic net loss per common stock for the periods presented.
The following table reflects the calculation of basic and diluted net loss per common stock (in dollars, except per share amounts):
| | | | | | |
| | For the three months ended March 31, | ||||
|
| 2024 |
| 2023 | ||
| | Common Stock | | Common Stock | ||
Basic and diluted net loss per of common stock | | | | | | |
Numerator: | | | | | | |
Net loss | | $ | (967,817) | | $ | (1,894,642) |
Denominator: | | | | | | |
Basic and diluted weighted average common shares outstanding | | | 3,603,966 | | | 4,168,567 |
Basic and diluted net loss per common share | | $ | (0.27) | | $ | (0.45) |
Concentration of Credit Risk
The Company has significant cash balances at a financial institutions which throughout the year regularly exceeded the federally insured limited of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial condition, results of operations, and cash flows.
F-141
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Warrant Instruments
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC 480, “Distinguishing Liabilities from Equity” (“ASC 480”), and ASC 815, “Derivatives and Hedging” (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be recorded at their initial fair value on the date of issuance, and each balance sheet date thereafter. The Company has analyzed the Public Warrants, Private Warrants, Bridge Warrants and the Extension Warrants and determined they are considered to be freestanding instruments and do not exhibit any of the characteristics in ASC 480 and therefore are not classified as liabilities under ASC 480. The warrants meet all of the requirements for equity classification under ASC 815 and therefore are classified in equity.
Financial Instruments
The Company evaluates its financial instruments to determine if such instruments should be accounted for as a liability under ASC 480 or if they are derivatives or contain features that qualify as bifurcated derivatives in accordance with ASC 815.
Derivative instruments are recorded at fair value on the grant date and re-valued at each reporting date, with changes in the fair value reported in the condensed consolidated statements of operations. Derivative assets and liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date. The Company has determined the PIPE financing agreement is a derivative instrument, the Bridge Notes and the Extension Note’s early redemption provisions are embedded feature that are required to be bifurcated as a derivative. FASB ASC 470-20, “Debt with Conversion and Other Options,” addresses the allocation of proceeds from the issuance of debt into its debt and bifurcated derivative components. The Company applies this guidance to allocate the Bridge Notes and the Extension Note proceeds between the Bridge Notes and the Extension Note, respectively, and the respective bifurcated derivative, using the residual method by allocating the principal first to fair value of the bifurcated derivative and then to the debt.
The Exchange Note and the Additional Bridge Note represent share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Exchange Note and the Additional Bridge Note are required to be accounted for as a liability under ASC 480. As required under ASC 480, the liabilities will be re-measured at fair value at each reporting period with the changes in the fair value of the liabilities recognized in earnings.
Fair Value Measurement
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
F-142
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement. |
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures” (“ASU 2023-09”), which will require the Company to disclose specified additional information in its income tax rate reconciliation and provide additional information for reconciling items that meet a quantitative threshold. ASU 2023-09 will also require the Company to disaggregate its income taxes paid disclosure by federal, state and foreign taxes, with further disaggregation required for significant individual jurisdictions. ASU 2023-09 will become effective for annual periods beginning after December 15, 2024. The Company is still reviewing the impact of ASU 2023-09.
Management does not believe that any recently issued, but not effective, accounting standards, if currently adopted, would have a material effect on the Company’s condensed consolidated financial statements.
Inflation Reduction Act of 2022
On August 16, 2022, the Inflation Reduction Act of 2022 (the “IR Act”) was signed into federal law. The IR Act provides for, among other things, a new U.S. federal 1% excise tax on certain repurchases of stock by publicly traded U.S. domestic corporations and certain U.S. domestic subsidiaries of publicly traded foreign corporations occurring on or after January 1, 2023. The excise tax is imposed on the repurchasing corporation itself, not its stockholders from which shares are repurchased. The amount of the excise tax is generally 1% of the fair market value of the shares repurchased at the time of the repurchase. However, for purposes of calculating the excise tax, repurchasing corporations are permitted to net the fair market value of certain new stock issuances against the fair market value of stock repurchases during the same taxable year. In addition, certain exceptions apply to the excise tax. The U.S. Department of the Treasury (the “Treasury”) has been given authority to provide regulations and other guidance to carry out and prevent the abuse or avoidance of the excise tax.
Any redemption or other repurchase that occurs after December 31, 2022, in connection with a Business Combination, extension vote or otherwise, may be subject to the excise tax. Whether and to what extent the Company would be subject to the excise tax in connection with a Business Combination, extension vote or otherwise would depend on a number of factors, including (i) the fair market value of the redemptions and repurchases in connection with the Business Combination, extension or otherwise, (ii) the structure of a Business Combination, (iii) the nature and amount of any “PIPE” or other equity issuances in connection with a Business Combination (or otherwise issued not in connection with a Business Combination but issued within the same taxable year of a Business Combination) and (iv) the content of regulations and other guidance from the Treasury. In addition, because the excise tax would be payable by the Company and not by the redeeming holder, the mechanics of any required payment of the excise tax have not been determined. The foregoing could cause a reduction in the cash available on hand to complete a Business Combination and in the Company’s ability to complete a Business Combination.
The Company held a meeting on November 6, 2023 to vote on a proposal to amend the Charter to extend the date by which the Company must consummate a Business Combination or, if it fails to do so, cease its operations and redeem or repurchase 100% of the shares of the Company’s common stock issued in the Company’s initial public offering, from November 8, 2023 to February 8, 2024, with additional extensions up to November 8, 2024. In connection with the meeting, 579,157 shares of the Company’s common stock were redeemed with a total redemption payment of $6,462,504. As a result, the Company booked a liability of $72,396 for the excise tax based on 1% of shares redeemed during the reporting period. For interim periods, an entity is not required to estimate future stock repurchases and stock issuances to measure its excise tax obligation. Rather, an entity can generally record the obligation on an as-incurred basis. In other words, the excise tax obligation recognized at the end of a quarterly financial reporting period is calculated as if the end of the quarterly period was the end of the annual period for which the excise tax obligation is payable.
F-143
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
NOTE 3. INITIAL PUBLIC OFFERING
In the “Initial Public Offering,” the Company sold 11,500,000 units, which included a full exercise by the underwriters of their over-allotment option in the amount of 1,500,000 Units, at a purchase price of $10.00 per unit. Each unit consists of one common share and one warrant. Each warrant will entitle the holder to purchase one (1) share of common stock at a price of $11.50 per whole share, subject to adjustment (see Note 8). Each warrant will become exercisable 30 days after the completion of the initial Business Combination or 12 months from the closing of the Initial Public Offering and will expire five years after the completion of the initial Business Combination, or earlier upon redemption or liquidation.
NOTE 4. PRIVATE PLACEMENT
Simultaneously with the closing of the Initial Public Offering, the Sponsor purchased 557,000 units, at $10.00 per unit for a total purchase price of $5,570,000 in a private placement. As of November 8, 2021, the Company received $3,680,000 from the proceeds of the Private Placement and recorded $1,890,000 in subscription receivable. The Sponsor paid the subscription in full on November 12, 2021. The private placement units are identical to the units sold in the Initial Public Offering but are not redeemable. There will be no underwriting fees or commissions with respect to the private placement units. The proceeds from the private placement were added to the proceeds of Initial Public Offering and placed in a Trust Account in the United States maintained by Continental Stock Transfer & Trust Company, as trustee. If the Company does not complete its initial business combination within 33 months (as extended as of May 1, 2024), the Sponsor will waive any and all rights and claims to any proceeds and interest thereon in respect to the private placement units and the proceeds from the sale of the private placement units will be included in the liquidating distribution to the holders of the Company’s public shares.
The Sponsor, advisors, officers and directors have entered into a letter agreement with the Company, pursuant to which they have agreed to (i) waive their redemption rights with respect to their founder shares and public shares in connection with the completion of the initial Business Combination; (ii) waive their redemption rights with respect to their founder shares and public shares in connection with a stockholder vote to approve an amendment to the Company’s amended and restated certificate of incorporation (A) to modify the substance or timing of the Company’s obligation to allow redemption in connection with the initial Business Combination or to redeem 100% of the Company’s public shares if the Company has not consummated an initial Business Combination within the Combination Period or (B) with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity; (iii) waive their rights to liquidating distributions from the Trust Account with respect to their founder shares if the Company fails to complete the initial Business Combination within the Combination Period, although they will be entitled to liquidating distributions from the Trust Account with respect to any public shares they hold if the Company fails to complete the initial Business Combination within the prescribed time frame; and (iv) vote any founder shares held by them and any public shares purchased during or after the Initial Public Offering (including in open market and privately negotiated transactions) in favor of the initial Business Combination.
NOTE 5. RELATED PARTY TRANSACTIONS
Founder Shares
On June 7, 2021, the Sponsor, along with certain of the Company’s directors, officers and advisors purchased 4,312,500 shares for an aggregate purchase price of $25,000. In October 2021, the Sponsor, officers and certain advisors forfeited an aggregate of 1,437,500 shares of common stock, resulting in 2,875,000 founder shares outstanding. Such shares are referred to herein as “founder shares” or “insider shares”.
Advances from Related Party
As of November 8, 2021, the Sponsor paid for $402,936 of expenses on behalf of the Company. The advance was repaid on November 12, 2021.
The Company owes the Sponsor and Sponsor affiliates $592,800 and $117,871 as of March 31, 2024 and December 31, 2023, respectively.
F-144
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Working Capital Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company will repay the Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company may use a portion of the proceeds held outside the Trust Account to repay the Working Capital Loans, but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would be repaid upon consummation of a Business Combination, without interest. As of March 31, 2024 and December 31, 2023, the Company had no borrowings under the Working Capital Loans.
Promissory Note Related Party
On October 24, 2022, the Company issued an unsecured promissory note in the aggregate principal amount of $350,000 to the Sponsor. The Company deposited to the trust account all of the loan amount and extended the amount of time it has available to complete a business combination from November 8, 2022 to February 8, 2023. On November 21, 2023, DHAC entered into a Conversion Securities Purchase Agreement (“Conversion SPA”) with the Sponsor, pursuant to which the loans in aggregate amount of $350,000 will be converted into Series A Preferred Shares at the Closing.
On February 2, 2023, SCS Capital Partners LLC, a Sponsor affiliate and a stockholder who currently holds more than 5% shares in the Company, issued a $250,000 interest-free loan to DHAC for Nasdaq fee payment and litigation expense, and on August 17, 2023, such loan was amended and restated to include an additional $315,000 interest-free loan to DHAC for operating expenses, making the aggregate principal amount to be $565,000. On May 5, 2023, SCS Capital Partners, LLC issued a $200,000 loan to DHAC for payment of the term extension fee. The related note bears interest of 10%, matures on May 5, 2024. The proceeds of the note were used to extend the liquidation date of DHAC from May 8, 2023 to August 8, 2023. On November 21, 2023, DHAC entered into a Conversion SPA with SCS Capital Partners LLC, pursuant to which the loans in aggregate amount of $765,000 will be converted into Series A Preferred Shares at the Closing.
On January 22, 2024, the Company amended an unsecured promissory note in the aggregate principal amount of $165,000 to M2B Funding Corp., an affiliate of the Sponsor. As a result of the amendment, the Company was to repay the remaining amount due by February 8, 2024. On January 31, 2024, the note was paid in full for a total of $190,750.
Promissory Note — M2B
On October 4, 2023, the Company issued a promissory note in the aggregate principal amount of $165,000 to M2B Funding Corp., an affiliate of the Sponsor, for a purchase price of $150,000 and included $5,000 in legal fees (the “M2B Note”). The original issued discount of $15,000 plus $5,000 of offering cost were recorded as a debt discount and amortized over the term of the note. The note had a 10% interest and a maturity date of January 5, 2024. The Company defaulted on the note and amended the note on January 22, 2024. As a result of the amendment, the Company was to repay the remaining amount due by February 8, 2024. On January 31, 2024, the note was paid in full for a total of $190,750. For the three months ended March 31, 2024, the Company recognized interest expense of $22,792 (including $20,296 of default interest) for the three months ended March 31, 2024.
Post-Business Combination Financing Transactions
Bridge Financing
In connection with the execution of the Second Business Combination Agreement, DHAC, along with VSee and iDoc, the target companies in the Business Combination, entered into a securities purchase agreement with the Bridge Investor, who is also an investor in the Sponsor, pursuant to which DHAC, VSee and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one
F-145
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments (the “Bridge Warrants”) and (ii) 30,000 shares of DHAC common stock.
As a result of the default, on November 21, 2023, DHAC, VSee and iDoc entered into an exchange agreement (the “Exchange Agreement”) with the Bridge Investor, pursuant to which the amounts currently due and owing under (i) the DHAC Bridge Note, (ii) the VSee Bridge Note other than $600,000 of the principal amount thereof, and (iii) the iDoc Bridge Note other than $600,000 of the principal amount thereof, will be exchanged at the Closing for a senior secured convertible promissory note issued by DHAC with an aggregate principle value of $2,523,744 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc. The Exchange Note will bear interest at a rate of 8% per annum and will be convertible into shares of common stock of the Company at a fixed conversion price of $10.00 per share (see Note 6 — Commitments — Bridge Financing and Bifurcated Derivative for further information).
On November 21, 2023, DHAC, VSee and iDoc entered into a letter agreement, pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount senior secured convertible promissory notes in the aggregate principal amount of $166,667 (with an aggregate subscription amount of $150,000) from DHAC with (1) a $111,111 note purchased at signing of the Bridge Amendment, which will mature on May 21, 2025 and (2) a $55,556 note purchased at a later date mutually agreed upon by DHAC and the Bridge Investor, which is currently expected to be upon the filing of an amendment to DHAC’s Registration Statement on Form S-4 in connection with the Business Combination (the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8% per annum and are convertible into shares of DHAC common stock, par value $0.0001 at a fixed conversion price of $10.00 per share (see Note 6 — Commitments — Additional Bridge Financing for further information).
On January 22, 2024, the Company and the Bridge Investor entered into a side letter to the registration rights agreement with the Bridge Investor dated October 5, 2022 whereby the Company agreed to register the shares of common stock underlying the Bridge Notes and the Additional Bridge Notes.
On January 25, 2024, the Bridge Investor purchased the second Additional Bridge Note in the principal amount of $55,556 from DHAC as contemplated by the Bridge SPA.
On April 17, 2024, the Company, VSee, and iDoc entered into a letter agreement with the Bridge Investor (the “Bridge Letter Agreement”), which amended the date with respect to the termination or closing of the business combination referenced in the Additional Bridge Notes from March 31, 2024 to June 30, 2024.
Loan Conversions
On November 21, 2023, DHAC, VSee, and/or iDoc, as applicable, entered into Securities Purchase Agreements (the “Conversion SPAs”) with various lenders of each of DHAC, VSee and iDoc, pursuant to which certain indebtedness owed by DHAC, VSee and iDoc will be converted into Series A Preferred Stock of DHAC at the closing of the Business Combination.
On November 21, 2023, DHAC and VSee entered into a Conversion SPA with Whacky — a Sponsor Affiliate, pursuant to which certain loans incurred by VSee to Whacky in the aggregate amount of $220,000 will be converted into Series A Preferred Shares at the Closing.
On November 1, 2023, DHAC and iDoc, entered into a Conversion SPA with Mark E. Munro Charitable Remainder Unitrust (“Munro Trust”) — a Sponsor Affiliate, pursuant to which certain loans incurred by iDoc to Munro Trust in the aggregate amount of $300,000 will be converted into Series A Shares at the Closing.
On November 21, 2023, DHAC and VSee, entered into a Conversion SPA with the Bridge Investor who is also an investor in the Sponsor, which Conversion SPA was amended and restated on February 13, 2024 (as further described under Note 10 — Subsequent Events), pursuant to which certain loans incurred by VSee to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
F-146
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with Tidewater — a Sponsor Affiliate, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by iDoc to Tidewater in the aggregate amount of $585,000 will be convertible into the Company’s Common Stock following the Closing.
On November 21, 2023, DHAC and iDoc, entered into a Conversion SPA with the Bridge Investor who is also an investor in the Sponsor, which Conversion SPA was amended and restated on February 13, 2024, pursuant to which certain loans incurred by iDoc to the Bridge Investor in the aggregate amount of $600,000 will be converted into the Company’s Common Stock subject to executing of certain registration rights agreement and filing a registration statement thereunder following the Closing.
On February 13, 2024, the parties entered into a First Amendment to the Third Amended and Restated Business Combination Agreement to provide that certain indebtedness of VSee and iDoc would be assumed by DHAC and converted into DHAC common stock following the closing instead of being converted into class B common stock of VSee and iDoc prior to the closing.
On February 13, 2024, the Company, VSee and/or iDoc, as applicable, amended and restated certain of the Conversion SPAs (the “Amended and Restated Conversion SPAs”) pursuant to which (1) a $600,000 balance of certain indebtedness of VSee will be assumed by the Company and converted into the Company’s common stock after the closing of the business combination; (2) a $600,000 balance certain indebtedness of iDoc will be assumed by the Company and will then be converted into the Company’s common stock subject to executing of certain registration rights agreement and filing of a registration statement thereunder after the closing of the business combination; and (3) certain indebtedness owned by iDoc will be assumed by the Company and will then be converted into the Company’s common stock subject to executing of certain registration rights agreement and filing of a registration statement thereunder after the closing of the business combination.
Quantum Financing Securities Purchase Agreement
On November 21, 2023, DHAC entered into the Quantum Purchase Agreement, pursuant to which the Quantum Investor subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note in the aggregate principal amount of $3,000,000 (see Note 6 — Commitments — Quantum Financing Securities Purchase Agreement for further information).
Equity Financing
On November 21, 2023, DHAC entered into the Equity Purchase Agreement with an affiliate of the Bridge Investor pursuant to which DHAC may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s common stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the Closing (see Note 6 — Commitments — Equity Financing for further information).
Administrative Services Agreement
The Company agreed, commencing on November 3, 2021, to pay an affiliate of the Sponsor a total of $10,000 per month for office space and secretarial, administrative, and other services. The monthly fees will cease upon completion of an initial business combination or liquidation. For the three months ended March 31, 2024 and 2023, the Company incurred $30,000. At March 31, 2024 and December 31, 2023, $55,500 and $90,550 is included in accrued expenses in the accompanying unaudited condensed consolidated balance sheets, respectively.
The Company will reimburse its officers and directors for any reasonable out-of-pocket business expenses incurred by them in connection with certain activities on the Company’s behalf such as identifying and investigating possible target businesses and business combinations. There is no limit on the amount of out-of-pocket expenses reimbursable by the Company; provided, however, that to the extent such expenses exceed the available proceeds not deposited in the Trust Account and the interest income earned on the amounts held in the Trust Account, such expenses would not be reimbursed by the Company unless the Company consummates an initial business combination. The audit committee will review and approve all reimbursements and payments made to any initial stockholder or member of the management team, or the Company’s or their respective affiliates, and any reimbursements and payments made to members of the audit committee will be reviewed and approved by the Board of Directors, with any interested director abstaining from such review and approval.
F-147
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
No compensation or fees of any kind, including finder’s fees, consulting fees or other similar compensation, will be paid to any of the initial stockholders, officers or directors who owned the shares of common stock prior to this offering, or to any of their respective affiliates, prior to or with respect to the Business Combination (regardless of the type of transaction that it is).
All ongoing and future transactions between the Company and any of its officers and directors or their respective affiliates will be on terms believed by the Company to be no less favorable to the Company than are available from unaffiliated third parties. Such transactions, including the payment of any compensation, will require prior approval by a majority of the Company’s uninterested “independent” directors (to the extent the Company has any) or the members of the board who do not have an interest in the transaction, in either case who had access, at the Company’s expense, to the Company’s attorneys or independent legal counsel. The Company will not enter into any such transaction unless the Company’s disinterested “independent” directors (or, if there are no “independent” directors, the Company’s disinterested directors) determine that the terms of such transaction are no less favorable to the Company than those that would be available to the Company with respect to such a transaction from unaffiliated third parties.
NOTE 6. COMMITMENTS
Initial Public Offering Registration and Stockholders’ Rights
Pursuant to a registration rights agreement entered into on November 3, 2021, the holders of the (i) founder shares, which were issued in a private placement prior to the closing of the Initial Public Offering and (ii) private placement units (including all underlying securities), issued in a private placement simultaneously with the closing of the Initial Public Offering have registration rights to require the Company to register a sale of any of its securities held by them pursuant to a registration rights agreement. These holders are entitled to make up to two demands that the Company registers such securities for sale under the Securities Act. In addition, these holders will have “piggyback” registration rights to include their securities in other registration statements filed by the Company.
Underwriters’ Agreement
The Representative is entitled to a deferred underwriting commission of 3.8% of the gross proceeds of the Initial Public Offering held in the Trust Account upon the completion of the Company’s initial Business Combination subject to the terms of the underwriting agreement.
The Company executed a Securities Purchase Agreement (the “A.G.P. Securities Purchase Agreement”) dated November 3, 2022 with A.G.P., which was amended on November 21, 2023, whereby A.G.P. subscribed for and will purchase, and DHAC will issue and sell, at the closing of the Business Combination, 4,370 shares of Series A Preferred Stock (“Series A Shares”) convertible into shares of DHAC common stock. The purchase price for the Series A Shares will be paid by conversion of A.G.P.’s $4,370,000 deferred underwriting fee into such Series A Shares. The Certificate of Designation of the Series A Preferred Stock establishes the terms and conditions of the Series A Preferred Stock. The Company reviewed the Series A Preferred Stock under ASC 480 and ASC 815 and concluded that Series A Preferred Stock did not include any elements that would preclude them from equity treatment and therefore are not subject to the liability treatment under ASC 480 or derivative guidance under ASC 815.
The Business Combination Agreement
On June 15, 2022, Digital Health Acquisition Corp (“DHAC”) entered into the Business Combination Agreement, with Merger Sub I, Merger Sub II, VSee and iDoc. On August 9, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into the First Amended and Restated Business Combination Agreement to provide for the concurrent execution of financing documents for a PIPE consisting of convertible notes and warrants and delivery of the Cassel Salpeter’s opinion to the Board. On October 6, 2022, DHAC, Merger Sub I, Merger Sub II, VSee and iDoc entered into a Second Amended and Restated Business Combination Agreement to make the consideration payable to VSee and iDoc stockholders 100% DHAC common stock and to provide for the concurrent execution of amended PIPE financing documents providing for the issuance of the shares and warrants to the PIPE investors. On November 3, 2022, the parties entered into a First Amendment to the Second Amended and Restated Business Combination Agreement to remove a closing condition that DHAC have at least $10 million in cash proceeds from the transactions at closing. On July 11, 2023, each of the PIPE Investors provided notice to the Company that since a closing condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing. Accordingly, the PIPE financing was terminated. On November 21, 2023, DHAC, Merger Sub
F-148
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
I, Merger Sub II, VSee and iDoc entered into the Third Amended and Restated Business Combination Agreement (as amended and restated, the “Business Combination Agreement”) to, among other things, provide for the removal of the PIPE financing and the concurrent execution of the Additional Bridge Financing, the Exchange Financing, the Quantum Financing, the Equity Financing and the Loan Conversions, which are described in Note 6 — Commitments.
Pursuant to the terms of the Business Combination Agreement, a business combination by and among DHAC, VSee and iDoc will be effected through the merger of Merger Sub I with and into VSee, with VSee surviving the Merger as a wholly owned subsidiary of DHAC and the merger of Merger Sub II with and into iDoc, with iDoc surviving the Merger as a wholly owned subsidiary of DHAC. The Board of Directors of DHAC (the “Board”) has (i) approved and declared advisable the Business Combination Agreement, the Business Combination and the other transactions contemplated thereby and (ii) resolved to recommend approval of the Business Combination Agreement and related matters by the stockholders of DHAC.
On February 13, 2024, the parties to the Business Combination Agreement executed a First Amendment to the Business Combination Agreement to provide that the Assumed Notes (as defined below) would be assumed by DHAC and converted into DHAC Common Stock following the closing of the business combination instead of being converted into class B common stock of VSee and iDoc prior to the closing.
On April 17, 2024, the parties to the Business Combination Agreement entered into a Second Amendment (the “Second Amendment”) to the Business Combination Agreement, pursuant to which the termination date in the Business Combination Agreement was amended from March 31, 2024 to June 30, 2024.
The Merger Consideration
The Business Combination combined equity value of VSee and iDoc is $110 million. At the Closing, each of VSee and iDoc will convert each share of VSee and iDoc capital stock (excluding shares of the holders who perfect rights of appraisal under Delaware or Texas law, as the case may be) into the right to receive the applicable merger consideration as further described below.
The aggregate merger consideration that the holders of VSee Class A Common Stock (including the holders of VSee Preferred Stock as converted and holders of VSee Class A Common Stock in connection with the TAD Exchange) as of the Effective Time are entitled to receive in the Business Combination, referred to as the “VSee Class A Consideration,” is an amount equal to (1) $60,500,000, minus (2) an amount equal to the Effective Time Option Grants multiplied by $10, minus (3) the aggregate amount of VSee’s transaction expenses. “Effective Time Option Grants” refer to the stock options with an exercise price of $10 per share pursuant to the VSee Incentive Plan to the individuals, in the amounts, and on the terms set forth on Exhibit E to the Business Combination Agreement. 100% of the VSee Closing Consideration will be paid in shares of Company Common Stock in accordance with the terms of the Business Combination Agreement and subject to deductions for the VSee Indemnity Escrow Amount. The “VSee Per Share Class A Consideration” refers to a number of shares of Common Stock equal to (a) (1) the VSee Class A Closing Consideration, divided by (2) the total number of VSee Class A Outstanding Shares, divided by (b) 10. “VSee Class A Outstanding Shares” refer to the total number of shares of VSee Class A Common Stock outstanding immediately prior to the Effective Time, expressed on a fully diluted and as-converted to VSee Class A Common Stock basis, and including, without limitation or duplication, the number of shares of VSee Class A Common Stock issuable upon conversion of the VSee Preferred Stock and upon closing of the TAD Exchange, which refers to a transaction where This American Doc, Inc. becomes a wholly owned subsidiary of VSee immediately prior to the consummation of the Business Combination.
The aggregate merger consideration that the holders of iDoc Class A Common Stock as of the Effective Time are entitled to receive in the Business Combination, referred to as the “iDoc Class A Closing Consideration,” is an amount equal to (1) $49,500,000, minus (2) the aggregate amount of iDoc’s transaction expenses. 100% of the iDoc Closing Consideration will be paid in shares of Company Common Stock in accordance with the terms of the Business Combination Agreement and subject to deductions for the iDoc Indemnity Escrow Amount as described below. The “iDoc Per Share Class A Consideration” refers to a number of shares of Common Stock equal to (a) (1) the iDoc Class A Closing Consideration, divided by (2) the total number of iDoc Class A Outstanding
F-149
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Shares, divided by (b) 10. “iDoc Class A Outstanding Shares” refer to the total number of shares of iDoc Class A Common Stock outstanding immediately prior to the Effective Time, expressed on a fully diluted and as-converted to iDoc Class A Common Stock basis.
Conditions to Closing
The obligations of DHAC, VSee and iDoc to consummate the Business Combination are subject to certain closing conditions, including, but not limited to, (i) the expiration or termination of any applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (ii) the approval of DHAC’s shareholders, (iii) the approval of VSee’s stockholders, (iv) the approval of iDoc’s stockholders, and (v) the delivery of applicable closing deliverables.
In addition, the obligations of VSee and iDoc to consummate the Business Combination are subject to the fulfillment of other closing conditions, including, but not limited to, (i) the approval by the Nasdaq Capital Market of DHAC’s listing application in connection with the Business Combination and (ii) the DHAC board of directors consisting of the number of directors, and comprising the individuals, as contemplated by the Business Combination Agreement.
Third Amended and Restated Transaction Support Agreement
On November 21, 2023, the parties to the Business Combination Agreement entered into the Third Amended and Restated Business Combination Agreement, pursuant to which the Second A&R Business Combination Agreement was amended and restated to provide for, among other things, the concurrent execution of the other agreements and transactions described as below. The transactions contemplated by the Business Combination Agreement are referred to as the “Business Combination” and the closing and closing date of the Business Combination are referred to as the “Closing” and the “Closing Date,” respectively.
In connection with the execution of the Business Combination Agreement, DHAC, Milton Chen, the Executive Vice Chairman of VSee, Dr. Imoigele Aisiku, the Executive Chairman of the Board of Directors of iDoc, and certain other stockholders of VSee and iDoc (collectively, the “Supporting Stockholders”) entered into a Third Amended and Restated Transaction Support Agreement, dated as of November 21, 2023 (the “Transaction Support Agreement”) which amended and restated the Second Amended and Restated Transaction Support Agreement executed on October 6, 2022, pursuant to which the Supporting Stockholders have agreed to, among other things, (i) support and vote in favor of the Business Combination Agreement and the Business Combination at DHAC’s stockholder meeting; (ii) not affect any sale or distribution of any shares of capital stock of DHAC, VSee, or iDoc; and (iii) take or cause to be done such further acts and things as may be reasonably necessary or advisable to cause the parties to fulfill their respective obligations under the Business Combination Agreement and consummate the Business Combination.
VSee Health, Inc. Incentive Plan
DHAC has agreed to approve and adopt the VSee Health, Inc. 2024 Equity Incentive Plan (the “Incentive Plan”) to be effective as of one day prior to the closing Business Combination and in a form mutually acceptable to DHAC, VSee and iDoc. The Incentive Plan shall provide for an initial aggregate share reserve equal to 15% of the number of shares of DHAC Common Stock outstanding following the closing after giving effect to the Business Combination, including without limitation, the PIPE Financing. Subject to approval of the Incentive Plan by DHAC’s Stockholders, DHAC has agreed to file a Form S-8 Registration Statement with the SEC following the Effective Time with respect to the shares of DHAC Common Stock issuable under the Incentive Plan.
PIPE Securities Purchase Agreement
In connection with the execution of the Business Combination Agreement, DHAC executed an Amended and Restated Securities Purchase Agreement (as amended, the “PIPE Securities Purchase Agreement” or “PIPE Forward Contract”) dated October 6, 2022 with certain PIPE Investors whereby the PIPE Investors subscribed for and will purchase, and DHAC will issue and sell, (i) 8,000 shares of Series A Preferred Stock (“Initial PIPE Shares”) convertible into shares of DHAC common stock and (ii) warrants (“Initial PIPE Warrants”) exercisable for 424,000 shares of DHAC Common Stock (such transactions, the “Initial PIPE Financing”) for aggregate proceeds of at least $8,000,000.
F-150
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
The PIPE Securities Purchase Agreement also provides that at any time after the date of the PIPE Securities Purchase Agreement and including (x) with respect to the PIPE Investors’ right to purchase Additional Offering Securities further to an Additional Offering (as each term is defined below) the earlier to occur of (I) the first anniversary of the date of the PIPE Securities Purchase Agreement and (II) the date of the consummation of one or more Subsequent Placements (as defined in the PIPE Securities Purchase Agreement) with the PIPE Investors on terms identical to the PIPE Securities Purchase Agreement and the other PIPE Financing documents in all material respects with an aggregate purchase price of at least $10 million (the “Additional Offering”, and the securities thereof, the “Additional Offering Securities”) and (y) with respect to Buyer’s right to participate in a Subsequent Placement other than an Additional Offering the earlier to occur of (I) the initial date after the Closing that no PIPE Shares remain outstanding, and (II) the date of the consummation of a Subsequent Placement by the Company with gross proceeds, paid in cash, of at least $5,000,000, in either case, neither the Company nor any of its subsidiaries shall, directly or indirectly, effect any Subsequent Placement unless the Company shall have first complied with the PIPE Investors’ participation right described herein and set forth in the PIPE Securities Purchase Agreement. With respect to (i) Additional Offerings, DHAC is required to offer 100% of the Additional Offering Securities to the PIPE Investors; and (ii) Subsequent Placements, DHAC is required to offer 25% of the Offered Securities to the PIPE Investors.
The Aggregate Closing PIPE Proceeds will be a part of the aggregate cash proceeds available for release to DHAC, Merger Sub I, and Merger Sub II in connection with the transactions contemplated by the Business Combination Agreement. The PIPE Warrants are exercisable into shares of DHAC Common Stock at a price of $12.50 per share and expire 5 years from the date of issuance. The PIPE Shares are convertible into shares of DHAC Common Stock at a price of $10.00 per share, subject to certain adjustments. The Certificate of Designation of the Series A Preferred Stock establishes the terms and conditions of the Series A Preferred Stock.
The Company reviewed the PIPE Securities Purchase Agreement’s underlying securities under ASC 480 and ASC 815 and concluded that Series Preferred A Stock includes a contingent redemption that would require temporary equity treatment at issuance and the warrants do not have any elements that would preclude them from equity treatment and therefore are not subject to the derivative guidance under ASC 815. However, under ASC 480-10-55-33, a forward contract that permits the holder to purchase redeemable shares (the Series A Preferred Stock) is a liability pursuant to ASC 480 because (1) the forward contract itself is indexed to an underlying share (i.e., the option’s value varies with the fair value of the share) that embodies the issuer’s obligation to repurchase the share and (2) the issuer has a conditional obligation to transfer assets if the shares are put back. Accordingly, the Company determined the fair value of the PIPE Forward Contract and noted the value at the October 6, 2022, the executed date of agreement was zero. As of March 31, 2024, the value of the PIPE Forward Contract was $0 as the PIPE was terminated on July 11, 2023.
On April 11, 2023 but effective March 31, 2023, the Company entered into an amendment to the PIPE Securities Purchase Agreement to, among other things, (a) amend and restate the form of Certificate of Designation of the Series A Preferred Stock to provide the aggregate number of shares of Series A Preferred Stock issuable thereunder shall not exceed 15,000, (b) amend and restate the form of PIPE Warrant to correct an error in the redemption provision of the PIPE Warrants, and (c) revise certain closing conditions for the PIPE Financing. As previously disclosed in its Current Report on Form 8-K filed on April 12, 2023, the Company and each of the PIPE Investors entered into amendments to the PIPE SPA to, among other things, add a closing condition providing that the closing date of the business combination shall occur on or prior to July 10, 2023 (the “Outside Date Closing Condition”).
In connection with the closing of the transactions contemplated by the PIPE Securities Purchase Agreement, DHAC and the PIPE Investors will enter into the registration rights agreement (the “PIPE Registration Rights Agreement”). The PIPE Registration Rights Agreement provides the PIPE Investors with customary registration rights with respect to the shares of Common Stock underlying the PIPE Shares and PIPE Warrants issued to the PIPE Investors.
Pursuant to the PIPE Securities Purchase Agreement, certain of DHAC’s stockholders agreed to enter into a lock-up agreement (the “PIPE Lock-Up Agreement”) with DHAC. Under the PIPE Lock-Up Agreement, the PIPE Lock-Up Period means the period beginning on the date of the Lock-Up Agreement and ending on the earliest of (i) eight months after the Closing Date, or (ii) on the trading day after DHAC’s Common Stock exceeds $12.50 (as adjusted for any stock splits, stock dividends, stock combinations recapitalizations and similar events) for a period of twenty consecutive trading days after the Closing Date.
On July 11, 2023, each of the PIPE Investors provided notice to the Company that since the Outside Date Closing Condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing.
F-151
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Backstop Agreement
On January 18, 2023, DHAC and the Sponsor entered into a Backstop Agreement (the “Backstop Agreement”) pursuant to which DHAC agreed to offer on or prior to the closing of the Business Combination the PIPE Investors the option to purchase up to an additional 2,000 shares of Series A Preferred Stock initially convertible into 234,260 shares of DHAC common stock (the “Additional PIPE Shares” and together with the Initial PIPE Shares, the “PIPE Shares”), together with additional warrants to purchase up to 106,000 shares of DHAC common stock (the “Additional PIPE Warrants” and together with the Initial PIPE Warrants, the “PIPE Warrants”; the Additional PIPE Shares and Additional PIPE Warrants are referred to as the “Additional PIPE Securities”) pursuant to a participation right granted to the PIPE Investors under the PIPE Securities Purchase Agreement, in each case, on the same terms and conditions set forth in the PIPE Securities Purchase Agreement for an aggregate purchase price of up to $2,000,000 (such proceeds together with the proceeds from the Initial PIPE Financing, as increased pursuant to the amendment to the Backstop Agreement described below, the “Aggregate Closing PIPE Proceeds”). Pursuant to the Backstop Agreement, if the PIPE Investors do not elect to purchase all of the Additional PIPE Securities, the Sponsor has agreed to purchase any such unsubscribed Additional PIPE Securities concurrent with the closing of the transactions contemplated by the PIPE Securities Purchase Agreement on the same terms and conditions set forth in the PIPE Securities Purchase Agreement.
On April 11, 2023 but effective March 31, 2023, the Sponsor and DHAC entered into an amendment to the Backstop Agreement to increase the Additional PIPE Shares that may be purchased pursuant to the Backstop Agreement from 2,000 shares of Series A Preferred Stock to 7,000 shares of Series A Preferred Stock, for an aggregate additional PIPE financing of up to $7,000,000, increasing the Aggregate Closing PIPE Proceeds to a total of $15,000,000.
Pursuant to the PIPE Securities Purchase Agreement and the Backstop Agreement, each as amended, any purchaser of Additional PIPE Securities will enter into a lock-up agreement with the Company.
On July 11, 2023, each of the PIPE Investors provided notice to the Company that since the Outside Date Closing Condition was not met, the PIPE Investors were under no obligation to close the PIPE Financing, and, as such the Backstop Agreement is terminated as of July 11, 2023.
Bridge Financing and Bifurcated Derivative
On October 6, 2022, in connection with the execution of the Business Combination Agreement, DHAC, VSee and iDoc entered into a Securities Purchase Agreement (the “Original Bridge SPA”) with an accredited investor (the “Bridge Investor”) who is also an investor in the Sponsor, pursuant to which DHAC, VSee and iDoc each issued and sold to such Bridge Investor 10% original issue discount senior secured promissory notes due October 5, 2023 in the aggregate principal amount of $2,222,222 (the “Bridge Notes”). An amount of $888,889 of the Bridge Note was allocated to DHAC. The Bridge Notes bear guaranteed interest at a rate of 10% per annum. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments (the “Bridge Warrants”) and (ii) 30,000 shares of DHAC common stock (the “Bridge Shares”) as additional consideration for the purchase of the Bridge Notes and Bridge Warrants. If the PIPE Financing closes in connection with the closing of the Business Combination, 110% of all unpaid principal under the Bridge Notes and guaranteed interest of 10% are due and payable at the closing of the PIPE Financing.
The Company reviewed the warrants and common stock issued in connection with the securities purchase agreement under ASC 815 and concluded that the Bridge Warrants are not in scope of ASC 480 and are not subject to the Derivative guidance under ASC 815. The Bridge Warrants and the Bridge Shares should be recorded as equity. As such the principal value of the Bridge Notes was allocated using the relative fair value basis of all three instruments. As the Bridge Warrants were issued with various instruments the purchase price needs to be allocated using the relative fair value method (i.e., warrant at its fair value and the common stock at its fair value the promissory note at its principal value allocated using the relative fair value of the proceeds received an applied proportionally to the equity classified stock, warrants and promissory note).
The Company reviewed the contingent early repayment option granted in the Bridge Notes under ASC 815 and concluded that as a result of the significant discount granted in the note the contingent repayment provision is therefore considered an embedded derivative that should be bifurcated from the debt host. Accordingly, in accordance with ASC 470-20, the Company allocated the Bridge Notes proceeds between the Bridge Notes and the Bifurcated Derivative, using the residual method by allocating the principal
F-152
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $278,404 and the residual value of $610,485 was allocated to the principal balance of the note (see Note 9 — Summary of Significant Accounting Policies — Fair Value Measurements for additional disclosure on the derivative).
DHAC as a result received cash proceeds of $738,200 net of $61,800 of direct cost attributable to the financing. The Bridge Warrants and Bridge Shares issued to Bridge Investor were analyzed under ASC 815 and noted there were no elements that would preclude equity treatment. As such the Company recorded the fair value of the Bridge Warrants of $8,552, net of $613 of offering cost allocated based on the relative value basis and Bridge Shares of $284,424, net of $20,376 of offering cost allocated based on the relative value basis. As a result, of the bifurcated derivative discussed above, the offering cost allocated to the debt, and the value of the share and warrants granted, the Company recorded amortizable debt discount of $443,665, consisting of $40,811 in financing cost allocated to the Bridge Note, $9,165 the issuance date fair value of the Bridge Warrants, $304,800 the fair value of the Bridge Shares and $88,889 originally issued discount.
In connection with the financing, the Company entered into a Registration Rights Agreement with the Bridge Investor, dated October 5, 2022, which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Bridge Warrants and the Bridge Shares.
On October 4, 2023, the Company defaulted on the Bridge Notes, and accordingly, the default provision was allocated and applied resulting in the triggering of 125% mandatory default penalty, a 10% late fee and default interest from the date of default of 24%, and the Company assumed the penalties and interest which were due and payable under the VSee and iDoc portion of the note, resulting in total amount due of $2,523,744. As a result, the Company entered into an Exchange Agreement dated November 21, 2023 (the “Exchange Agreement”) with the Bridge Investor and recognized $1,579,927 in default interest.
The Bridge Investor, beneficially owns and holds (i) a promissory note of DHAC in the principal amount (including the original issue discount of $88,889) of $888,889 (the “DHAC Note”); (ii) a promissory note of VSee in the principal amount (including the original issue discount of $66,667) of $666,667 (the “VSee Note”); and (iii) a promissory note of iDoc in the principal amount (including the original issue discount of $66,667) of $666,667 (the “iDoc Note”, together with the DHAC Note and the VSee Note, each as further detailed on Schedule I hereto, collectively, the “Original Notes”) which are currently due and owing, and have an aggregate current value of $3,723,744.
Exchange Note Exchange Financing
Pursuant to the Exchange Agreement, the Bridge Investor agreed to exchange all amounts currently due and owing under (i) the DHAC Note, (ii) the VSee Note other than the principal amount of $600,000.00 thereof, and (iii) the iDoc Note other than the principal amount of $600,000.00 thereof for a senior secured convertible promissory note with an aggregate principle value of $2,523,744 (the “Exchange Note”), which will be guaranteed by each of DHAC, VSee and iDoc. The Exchange Note will bear interest at a rate of 8% per annum and will be convertible into shares of common stock of the Company at a fixed conversion price of $10 per share. The conversion price of the Exchange Note is subject to reset if DHAC’s common stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of DHAC’s common stock in the 10th trading dates prior to the measurement date and (y) $2.0. Amounts repaid may not be reborrowed. The Bridge Investor may set off and deduct pursuant to and in accordance with the Exchange Agreement amounts due to the Bridge Investor. The transactions contemplated by the Exchange Agreement and the Exchange Note is hereby referred as the “Exchange Financing.”
The monetary amount of the obligation is a fixed monetary amount known at inception as represented by the Amortization of Principal Schedule 2(a) (each, an “Amortization Payment”). As a result of Section 2(a), the Exchange Note represents a debt instrument that the Company must or may settle by issuing a variable number of its equity shares as each Amortization Payment shall, at the option of the Company, be made in whole or in part, in immediately available Dollars equal to the sum of the Amortization Payment provided for in Schedule 2(a), or, subject to the Company complying with the Equity Conditions on the date of such Amortization Payment, in Common Stock issued at 95% of the lowest VWAP in the prior ten (10) trading days prior to such Amortization Payment (the “Amortization Conversion Price”) but in no event shall Common Stock be used to make such Amortization Payment if the Amortization Conversion Price is less than $2.00.
F-153
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
The Exchange Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Exchange Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
At November 21, 2023, the Exchange Note was recognized at fair value of $2,523,744 in accordance with ASC 480. As of March 31, 2024, the Exchange Note’s fair value was $2,814,359. The Company recognized a total Exchange Note interest expense of $51,036 for the three months ended March 31, 2024 and a change in fair value of $192,801.
Additional Bridge Financing
On November 21, 2023, DHAC entered into an amendment to the Original Bridge SPA (the “Bridge Amendment”), pursuant to which the Bridge Investor agreed to purchase additional 10% original issue discount convertible promissory notes in the aggregate principal amount of $166,667 (with a subscription amount of $150,000) from the Company with (1) a $111,111 note purchased at signing of the Bridge Letter Agreement, which will mature on May 21, 2025 and (2) a $55,556 note purchased at a later date mutually agreed upon by the Company and the Bridge Investor (the “Additional Bridge Notes”). The Additional Bridge Notes bear guaranteed interest at a rate of 8% per annum and are convertible into shares of the Company’s common stock, par value $0.0001, at a fixed conversion price of $10.00 per share. The conversion price of the Additional Bridge Notes is subject to reset if the Company’s Common Stock trades below $10.00 on the 10th business day after the conversion shares are registered or may otherwise be freely resold, and every 90th day thereafter, to a price equal to the greater of (x) 95% of the average lowest VWAP of the Company’s Common Stock in the 10 trading dates prior to the measurement date and (y) $2.00. In addition, optional prepayment of the Additional Bridge Notes requires the payment of 110% of the outstanding obligations, including the guaranteed minimum interest. If an event of default occurs, the Additional Bridge Notes would bear interest at a rate of 24% per annum and require the payment of 125% of the outstanding obligations, including the guaranteed minimum interest. As of March 31, 2024, $150,000 has been funded. The transactions contemplated by the Bridge Amendment and the Additional Bridge Note are hereby referred as the “Additional Bridge Financing.”
The monetary amount of the obligation is a fixed monetary amount known at inception as represented by the Amortization of Principal Schedule 2(a) (each, an “Amortization Payment”). As a result of Section 2(a), the Additional Bridge Note represents a debt instrument that the Company must or may settle by issuing a variable number of its equity shares as each Amortization Payment shall, at the option of the Company, be made in whole or in part, in immediately available Dollars equal to the sum of the Amortization Payment provided for in Schedule 2(a), or, subject to the Company complying with the Equity Conditions on the date of such Amortization Payment, in Common Stock issued at 95% of the lowest VWAP in the prior ten (10) trading days prior to such Amortization Payment (the “Amortization Conversion Price”) but in no event shall Common Stock be used to make such Amortization Payment if the Amortization Conversion Price is less than $2.00.
The Additional Bridge Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Senior Secured Convertible Promissory Note is required to be accounted for as a liability under ASC 480. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
At November 21, 2023, $100,000 of proceeds were received under the Additional Bridge Note, as such the original issued discount was immediately expensed as interest of $11,111 as the note was recognized at fair value of $100,000 in accordance with ASC 480. At January 25, 2024, $50,000 of proceeds were received under the Additional Bridge Note, as such the original issued discount was immediately expensed as interest of $5,556 as the Note was recognized at fair value of $51,705 in accordance with ASC 480. As of March 31, 2024, the fair value of the Additional Bridge Notes was $156,564. The Company recognized a total Additional Bridge Note interest expense of $3,062 for the three months ended March 31, 2024 and a change in fair value of $2,133.
F-154
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Extension Note (Extension Financing) and Bifurcated Derivative
On May 5, 2023, the Company entered into a securities purchase agreement (the “Extension Purchase Agreement”) with an institutional investor (the “Holder”). Pursuant to the Extension Purchase Agreement, the Company issued the Holder a 16.67% original issue discount promissory note, in favor of the Holder, in the aggregate principal amount of $300,000 (the “Extension Note”). The Extension Note bears guaranteed interest at a rate of 10% per annum and is due and payable on May 5, 2024. On April 17, 2024, the Company and the investor entered into a letter agreement (the “Extension Letter Agreement”), which amended the maturity date of the Extension Note to June 30, 2024 and clarified certain definitions and transaction terms in both the Extension Purchase Agreement and the Extension Note.
VSee Lab, Inc., a Delaware corporation (“VSee”), and iDoc Virtual Telehealth Solutions, Inc., a Texas corporation (“iDoc”), guaranteed the Company’s obligations under the Extension Purchase Agreement, the Extension Note and the other transaction documents (the “May 2023 Financing Documents”) pursuant to a Subsidiary Guaranty dated May 5, 2023. The Company’s, VSee’s and iDoc’s obligations to the Holder under the May 2023 Financing Documents are subordinated to the Company’s, VSee’s and iDoc’s obligations to its existing bridge lender.
In connection with the Extension Purchase Agreement, the Company issued to the Holder (i) warrants with an exercise period of five years to purchase up to 26,086 shares of the Company’s Common Stock at an exercise price of $11.50 per share (the “Extension Warrants”), and (ii) 7,000 shares of the Company’s Common Stock as commitment shares (the “Extension Shares”). The Company also entered into a Registration Rights Agreement with the Holder, dated May 5, 2023 (the “May 2023 RRA”), which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Extension Warrants and the Extension Shares, subject to the terms thereof.
The Company reviewed the Extension Warrants and Extension Shares issued in connection with the Extension Purchase Agreement under ASC 815 and concluded that the Extension Warrants are not in scope of ASC 480 and are not subject to the Derivative guidance under ASC 815. The Extension Warrants and the Extension Shares should be recorded as equity. As such the principal value of the Extension Note was allocated using the relative fair value basis of all three instruments. As the Extension Warrants were issued with various instruments the purchase price needs to be allocated using the relative fair value method (i.e., warrant at its fair value and the common stock at its fair value the promissory note at its principal value allocated using the relative fair value of the proceeds received an applied proportionally to the equity classified stock, warrants and promissory note).
The Company reviewed the contingent early repayment option granted in the Extension Note under ASC 815 and concluded that as a result of the significant discount granted in the note the contingent repayment provision is therefore considered an embedded derivative that should be bifurcated from the debt host. Accordingly, in accordance with ASC 470-20, the Company allocated the Extension Note proceeds between the Extension Note and the Bifurcated Derivative, using the residual method by allocating the principal first to fair value of the embedded derivative and then to the debt. Accordingly, the fair value of the embedded derivative at issuance was $24,502 and the residual value of $275,498 was allocated to the principal balance of the note.
DHAC as a result received cash proceeds of $240,000 net of $10,000 of direct cost attributable to the financing. The Extension Warrants and Extension Shares were analyzed under ASC 815 and noted there were no elements that would preclude equity treatment. As such, the Company recorded the fair value of the Extension Warrants of $39,111, net of $1,019 of offering cost allocated based on the relative value basis and Extension Shares of $78,349, net of $1,989 of offering cost allocated based on the relative value basis. As a result of the bifurcated derivative discussed above, the offering cost allocated to the debt, and the value of the shares and warrants granted, the Company recorded amortizable debt discount of $175,472, consisting of $6,993 in financing cost allocated to the Extension Note, $40,130 the issuance date fair value of the Investor Warrants, $78,349 the fair value of the Extension Shares and $50,000 originally issued discount.
As of March 31, 2024, the Extension Note net of unamortized debt discount was $285,614. For the three months ended March 31, 2024, the Company recognized $44,340 of amortized debt discount and $7,500 in accrued interest for a total Extension Note interest expense of $51,840. As of December 31, 2023, the Extension Note net of unamortized debt discount was $233,774. For the year ended December 31, 2023, the Company recognized $114,151 of amortized debt discount and $7,500 in accrued interest for a total Extension Note interest expense of $133,748. In connection with the Extension Purchase Agreement, the Company entered into the May 2023
F-155
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
RRA with the Holder, dated May 5, 2023, which provides that the Company will file a registration statement to register the shares of Common Stock underlying the Extension Warrants and the Extension Shares.
Quantum Financing Securities Purchase Agreement
On November 21, 2023, DHAC entered into a convertible note purchase agreement (the “Quantum Purchase Agreement”), pursuant to which an institutional and accredited investor (the “Quantum Investor”) subscribed for and will purchase, and DHAC will issue and sell to the Quantum Investor, at the Closing, a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000. The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of Common Stock of DHAC at (1) a fixed conversion price of $10.00 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination. The conversion price of the Quantum Note is subject to reset if the average of the daily VWAPs for the three (3) trading days prior to the 30-day anniversary of the Quantum Note issuance date (the “Average Price”) is less than $10.00, to a price equal to the Average Price but in no event less than $2. In addition, the Company at its option can redeem early a portion or all amounts outstanding under the Quantum Note if the Company provides the Quantum Note holder a notice at least ten (10) trading days prior to such redemption and on the notice day the VWAP of the Company’s Common Stock is less than $10.00. If an event of default occurs, the Quantum Note would bear interest at a rate of 18% per annum. The transactions contemplated by the Quantum Purchase Agreement and the Quantum Note is hereby referred as the “Quantum Financing.”
Concurrently with the consummation of the transactions contemplated by the Quantum Purchase Agreement, DHAC will enter into a Registration Rights Agreement in a form under the Quantum Purchase Agreement, pursuant to which it agreed to register the shares of Common Stock underlying the Quantum Note (the “Quantum Registration Rights Agreement”).
As of March 31, 2024, the Quantum Purchase Agreement has not yet been funded and is expected to be funded at the closing of the business combination. The Quantum Note represents share-settled debt that requires or may require the Company to settle the debt instrument by delivering a variable number of shares with a then-current fair value equal to the principal amount of the note plus accrued and unpaid interest. As a result, the Quantum Note will be required to be accounted for as a liability under ASC 480 upon funding of the note. As required under ASC 480, the liability will be re-measured at fair value at each reporting period with the changes in the fair value of the liability recognized in earnings.
Equity Financing
On November 21, 2023, DHAC entered into an equity purchase agreement (the “Equity Purchase Agreement”) with the Bridge Investor pursuant to which DHAC may sell and issue to the Bridge Investor, and the Bridge Investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s Common Stock, from time to time over a 36-month period (the “Equity Purchase Commitment Period”) beginning from the sixth (6th) trading day following the closing of the Business Combination transaction (the “Equity Purchase Effective Day”), provided that certain conditions are met. The Company also agreed to file a resale registration statement to register shares of Common Stock to be purchased under the Equity Purchase Agreement with the SEC within 45 days following the Equity Purchase Effective Day, and shall use commercially reasonable efforts to have such registration statement declared effective by the SEC within 30 days of such filing. During the Equity Purchase Commitment Period, DHAC may suspend the use of the resale registration statement to (i) delay the disclosure of material nonpublic information concerning the Company in good faith or (ii) amend the registration statement concerning material information, by providing written notice to the investor. Such suspension cannot be longer than 90 consecutive days (or 120 days in any calendar year). The investor has agreed not to cause or engage in any manner whatsoever, any direct or indirect short selling or hedging of the Company’s Common Stock. On the Equity Purchase Effective Day, the Company will issue to the investor, as a commitment fee for this equity purchase transaction, a senior unsecured convertible note in a principal amount of $500,000 that is convertible into shares of the Company’s Common Stock at a fix conversion price of $10.00 per share (the “Equity Purchase Commitment Note”). The transaction contemplated by the Equity Purchase Agreement is hereby referred as “Equity Financing.”
The Company has analyzed the Equity Purchase Agreement and determined that the contract should be recorded as a liability under ASC 815 and measured at fair value. As a result of the ASC 815 liability classification, the Company is required to re-measure the liability at fair value at each reporting period until the liability is settled.
F-156
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
The Company has determined that the likelihood of exercising this contract is low as the contract provides no scenario in which the Company may exercise the contract at above market rates (i.e., sell shares at a price above which the shares are currently trading in the active market). Furthermore, the choice to exercise the contract is solely at the discretion of the Company (i.e., does not obligate the Company in any manner), which, as stated above, is unlikely. Additionally, the contract does not impose a fee or fine if the Company chooses not to exercise the contract, as such that the fair value of the equity contract was determined to be $203,720 as of December 31, 2023 and $189,764 as of March 31, 2024, resulting in a change in fair value of the ELOC of $13,956.
NOTE 7. STOCKHOLDERS’ DEFICIT
Common Stock
The Company is authorized to issue 50,000,000 of common shares with a par value of $0.0001 per share. On June 7, 2021, the Sponsor, along with certain of the Company’s directors, officers and advisors purchased 4,312,500 shares for an aggregate purchase price of $25,000. In October 2021, the Sponsor, officers and certain advisors forfeited an aggregate of 1,437,500 shares of common stock, resulting in 2,875,000 founder shares outstanding. At the closing of the Initial Public Offering, 557,000 shares were issued as part of the Private Placement sale. On October 6, 2022, in connection with the Original Bridge SPA, 30,000 shares were issued to the Bridge Investor. In February 2023, 20,000 shares were issued to an additional stockholder. On May 5, 2023 in connection with the Extension Purchase Agreement, 7,000 shares were issued to the investor. As of March 31, 2024 and December 31, 2023, there were 3,489,000 shares of common stock issued and outstanding, excluding 114,966 shares subject to redemption which were classified outside of permanent deficit on the condensed consolidated balance sheets. The holders of record of the Company’s common stock are entitled to one vote for each share held on all matters to be voted on by stockholders. In connection with any vote held to approve the Company’s initial business combination, the initial stockholders, insiders, officers and directors, have agreed to vote their respective shares of common stock owned by them immediately prior to this offering, including both the insider shares and any shares acquired in this offering or following this offering in the open market, in favor of the proposed business combination.
Pursuant to the amended and restated certificate of incorporation, if the Company does not consummate its initial business combination within 33 months from the closing of this offering (as extended as of May 1, 2024), it will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem 100% of the outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the board of directors, dissolve and liquidate, subject (in the case of (ii) and (iii) above) to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. The Company’s insiders have agreed to waive their rights to share in any distribution with respect to their insider shares.
The stockholders have no conversion, preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to the shares of common stock, except that public stockholders have the right to sell their shares to the Company in any tender offer or have their shares of common stock converted to cash equal to their pro rata share of the Trust Account if they vote on the proposed business combination and the business combination is completed.
If the Company holds a stockholder vote to amend any provisions of the certificate of incorporation relating to stockholders’ rights or pre-business combination activity (including the substance or timing within which it has to complete a business combination), it will provide its public stockholders with the opportunity to redeem their shares of common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its franchise and income taxes, divided by the number of then outstanding public shares, in connection with any such vote. In either of such events, converting stockholders would be paid their pro rata portion of the Trust Account promptly following consummation of the business combination or the approval of the amendment to the certificate of incorporation. If the business combination is not consummated or the amendment is not approved, stockholders will not be paid such amounts.
F-157
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
NOTE 8. WARRANTS
Initial Public Offering Warrants
There are 12,057,000 warrants issued and outstanding as of March 31, 2024 and December 31, 2023 issued in connection with the Initial Public Offering. Each warrant entitles the registered holder to purchase one (1) share of common stock at a price of $11.50 per whole share, subject to adjustment as discussed below, at any time commencing on the later of 30 days after the completion of an initial business combination or 12 months from the closing of the Initial Public Offering.
However, no warrants will be exercisable for cash unless the Company has an effective and current registration statement covering the shares of common stock issuable upon exercise of the warrants and a current prospectus relating to such shares of common stock. Notwithstanding the foregoing, if a registration statement covering the shares of common stock issuable upon exercise of the public warrants is not effective within a specified period following the consummation of the initial business combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company shall have failed to maintain an effective registration statement, exercise warrants on a cashless basis pursuant to the exemption provided by Section 3(a)(9) of the Securities Act, provided that such exemption is available. If that exemption, or another exemption, is not available, holders will not be able to exercise their warrants on a cashless basis. In the event of such cashless exercise, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose will mean the average reported last sale price of the shares of common stock for the 5 trading days ending on the trading day prior to the date of exercise. The warrants will expire on the fifth anniversary of the completion of an initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
The Private Placement Warrants is identical to the warrants underlying the units in the Initial Public Offering. The Company may call the warrants for redemption, in whole and not in part, at a price of $0.01 per warrant,
| ● | at any time after the warrants become exercisable; |
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; |
| ● | if, and only if, the reported last sale price of the shares of common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations and recapitalizations), for any 20 trading days within a 30 trading day period commencing at any time after the warrants become exercisable and ending on the third business day prior to the notice of redemption to warrant holders; and |
| ● | if, and only if, there is a current registration statement in effect with respect to the shares of common stock underlying such warrants. |
The right to exercise will be forfeited unless the warrants are exercised prior to the date specified in the notice of redemption. On and after the redemption date, a record holder of a warrant will have no further rights except to receive the redemption price for such holder’s warrant upon surrender of such warrant.
The redemption criteria for the warrants have been established at a price which is intended to provide warrant holders a reasonable premium to the initial exercise price and provide a sufficient differential between the then-prevailing share price and the warrant exercise price so that if the share price declines as a result of the redemption call, the redemption will not cause the share price to drop below the exercise price of the warrants.
If the Company calls the warrants for redemption as described above, the Company’s management will have the option to require all holders that wish to exercise warrants to do so on a “cashless basis.” In such event, each holder would pay the exercise price by surrendering the warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the
F-158
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
average reported last sale price of the shares of common stock for the 5 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
The warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and the Company. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision or to make any other change that does not adversely affect the interests of the registered holders. For any other change, the warrant agreement requires the approval by the holders of at least a majority of the then outstanding public warrants if such amendment is undertaken prior to or in connection with the consummation of a business combination or at least a majority of the then outstanding warrants if the amendment is undertaken after the consummation of a business combination.
The exercise price and number of shares of common stock issuable on exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described below, the warrants will not be adjusted for issuances of shares of common stock at a price below their respective exercise prices. If (x) the Company issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of the initial business combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by the board of directors, and in the case of any such issuance to the Company’s Sponsor, initial stockholders or their affiliates, without taking into account any founders’ shares held by them prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial business combination on the date of the consummation of the initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of (i) the Market Value or (ii) the price at which the Company issue the additional shares of common stock or equity-linked securities and the $18.00 per share redemption trigger price described above will be adjusted (to the nearest cent) to be equal to 180% of the Market Value. The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by certified or official bank check payable to the Company, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of shares of common stock and any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Warrant holders may elect to be subject to a restriction on the exercise of their warrants such that an electing warrant holder would not be able to exercise their warrants to the extent that, after giving effect to such exercise, such holder would beneficially own in excess of 9.8% of the shares of common stock outstanding.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, the Company will, upon exercise, round up to the nearest whole number the number of shares of common stock to be issued to the warrant holder.
Bridge Warrants
On October 6, 2022, 173,913 warrants were issued pursuant to the Bridge Purchase Agreement. The purchase right represented by the Bridge Warrants shall terminate on or before 5:30 p.m., Pacific Time, on the date five years from the date of issuance (the “Expiration Date”). The exercise price at which the Bridge Warrants may be exercised shall be $11.50 per share of Common Stock. If at any time after the date of issuance of the Bridge Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, the Bridge Warrants may be exercised by cashless exercise. In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the Exercise Price multiplied by such fraction. Except as provided in the Bridge Warrant, the Bridge Warrant does not entitle its holder to any rights of a shareholder of the Company.
During the term the Bridge Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the Bridge Warrant and, from time to time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock
F-159
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
issuable upon exercise of the Bridge Warrants. All shares that may be issued upon the exercise of rights represented by the Bridge Warrants and payment of the Exercise Price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Bridge Warrants). Prior to the Expiration Date, the Exercise Price and the number of shares of Common Stock purchasable upon the exercise of the Bridge Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
(a) | In the event that the Company shall at any time after the date of issuance of the Bridge Warrants (i) declare a dividend on Common Stock in shares or other securities of the Company, (ii) split or subdivide the outstanding Common Stock, (iii) combine the outstanding Common Stock into a smaller number of shares, or (iv) issue by reclassification of its Common Stock any shares or other securities of the Company, then, in each such event, the Exercise Price in effect at the time shall be adjusted so that the holder shall be entitled to receive the kind and number of such shares or other securities of the Company which the holder would have owned or have been entitled to receive after the happening of any of the events described above had such Bridge Warrant been exercised immediately prior to the happening of such event (or any record date with respect thereto). |
(b) | No adjustment in the number of shares of Common Stock receivable upon exercise of the Bridge Warrant shall be required unless such adjustment would require an increase or decrease of at least 0.1% in the aggregate number of shares of Common Stock purchasable upon exercise of all Bridge Warrants; provided that any adjustments which are not required to be made shall be carried forward and taken into account in any subsequent adjustment. |
(c) | If at any time, as a result of an adjustment, the holder of any Bridge Warrant thereafter exercised shall become entitled to receive any shares of the Company other than shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Bridge Warrant shall be subject to adjustment from time to time in a manner and on terms as nearly equivalent as practicable to the provisions with respect to the Common Stock receivable upon execution of the Bridge Warrant. |
(d) | Whenever the Exercise Price payable upon exercise of each Bridge Warrant is adjusted, the Warrant Shares shall be adjusted by multiplying the number of shares of Common Stock receivable upon execution of the Bridge Warrant immediately prior to such adjustment by a fraction, the numerator of which shall be the Exercise Price in effect immediately prior to such adjustment, and the denominator of which shall be the Exercise Price as adjusted. |
(e) | In the event of any capital reorganization of the Company, or of any reclassification of the Common Stock, or in case of the consolidation of the Company with or the merger of the Company with or into any other corporation or of the sale of the properties and assets of the Company as, or substantially as, an entirety to any other corporation, each Bridge Warrant shall, after such capital reorganization, reclassification of Common Stock, consolidation, merger or sale, and in lieu of being exercisable for shares of Common Stock of the Company, be exercisable, upon the terms and conditions specified in the Bridge Warrant, for the number of shares of stock or other securities or assets to which holder of the number of shares of Common Stock purchasable upon exercisable of such Bridge Warrant immediately prior to such capital organization, reclassification of Common Stock, consolidation, merger or sale would have been entitled upon such capital organization, reclassification of Common Stock, consolidation, merger or sale. The Company shall not effect any such consolidation, merger or sale, unless prior to or simultaneously with the consummation thereof, the successor corporation (if other than the Company) resulting from such consolidation or merger or the corporation purchasing such assets or the appropriate corporation or entity shall assume, by written instrument, the obligation to deliver to holder of each Bridge Warrant the shares of stock, securities or assets to which, in accordance with the foregoing provisions, such holder may be entitled and all other obligations of the Company under the Bridge Warrant. |
(f) | If the Company in any manner issues or sells or enters into any agreement to issue or sell, any Common Stock, options or convertible securities (any such securities, “Variable Price Securities”) after the issuance of the Bridge Warrants that are issuable pursuant to such agreement or convertible into or exchangeable or exercisable for shares of Common Stock at a price which varies or may vary with the market price of the shares of Common Stock, including by way of one or more reset(s) to a fixed price, but exclusive of such formulations reflecting customary anti-dilution provisions (such as share splits, share combinations, share dividends and similar transactions) (each of the formulations for such variable price being herein referred to as, the “Variable Price”), the Company shall provide notice thereof to the holder on the date of such agreement and the |
F-160
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
issuance of such convertible securities or options. From and after the date the Company enters into such agreement or issues any such Variable Price Securities, the holder shall have the right, but not the obligation, in its sole discretion to substitute the Variable Price for the Exercise Price upon exercise of the Bridge Warrant by designating in the exercise form delivered upon any exercise of the Bridge Warrant that solely for purposes of such exercise the holder is relying on the Variable Price rather than the Exercise Price then in effect.
(g) | In case any event shall occur as to which the other provisions above are not strictly applicable or the failure to make any adjustment would result in an unfair enlargement or dilution of the purchase rights represented by the Bridge Warrants in accordance with the essential intent and principles hereof, then, in each such case, the independent auditors of the Company shall give an opinion as to the adjustment, if any, on a basis consistent with the essential intent and principles above, necessary to preserve, without enlargement or dilution, the purchase rights presented by the Bridge Warrants. Upon receipt of such opinion, the Company shall promptly make the adjustment described therein. |
The Bridge Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Bridge Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
Extension Warrants
On May 5, 2023, the Company issued 26,086 warrants pursuant to the Extension Purchase Agreement. The purchase right represented by the Extension Warrants shall terminate on the date five years from the date of issuance (the “Expiration Date”). The exercise price at which the Extension Warrants may be exercised shall be $11.50 per share of Common Stock. If at any time after the date of issuance of the Extension Warrants there is no effective registration statement available for the resale of shares of Common Stock held by the holder, the Extension Warrants may be exercised by cashless exercise. In lieu of any fractional share to which the holder would otherwise be entitled, the Company shall make a cash payment equal to the exercise price multiplied by such fraction. Except as provided in the Extension Warrants, the Extension Warrant does not entitle its holder to any rights of a stockholder of the Company.
During the term, the May 2023 Warrants are exercisable, the Company will reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of Common Stock upon the exercise of the May 2023 Warrant and, from time to time, will take all steps necessary to amend its Certificate of Incorporation to provide sufficient reserves of shares of Common Stock issuable upon exercise of the Extension Warrants. All shares that may be issued upon the exercise of rights represented by the Extension Warrants and payment of the exercise price will be free from all taxes, liens and charges in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously or otherwise specified in the Extension Warrants). Prior to the Expiration Date, the exercise price and the number of shares of Common Stock purchasable upon the exercise of the Extension Warrants are subject to adjustment from time to time upon the occurrence of any of the following events:
| (a) | In the event that the Company shall at any time after the date of issuance of the Extension Warrants (i) declare a dividend on Common Stock in shares or other securities of the Company, (ii) split or subdivide the outstanding Common Stock, (iii) combine the outstanding Common Stock into a smaller number of shares, or (iv) issue by reclassification of its Common Stock any shares or other securities of the Company, then, in each such event, the exercise price in effect at the time shall be adjusted so that the holder shall be entitled to receive the kind and number of such shares or other securities of the Company which the holder would have owned or have been entitled to receive after the happening of any of the events described above had such Extension Note Warrant been exercised immediately prior to the happening of such event (or any record date with respect thereto). |
| (b) | No adjustment in the number of shares of Common Stock receivable upon exercise of the Extension Warrants shall be required unless such adjustment would require an increase or decrease of at least 0.1% in the aggregate number of shares of Common Stock purchasable upon exercise of all Extension Warrants; provided that any adjustments which are not required to be made shall be carried forward and taken into account in any subsequent adjustment. |
| (c) | If at any time, as a result of an adjustment, the holder of any Extension Note Warrant thereafter exercised shall become entitled to receive any shares of the Company other than shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Extension Note Warrant shall be subject to adjustment from time to time in a manner and |
F-161
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
| on terms as nearly equivalent as practicable to the provisions with respect to the Common Stock receivable upon execution of the Extension Warrant. |
| (d) | Whenever the exercise price payable upon exercise of each Extension Warrant is adjusted, the Extension Warrant shares shall be adjusted by multiplying the number of shares of Common Stock receivable upon execution of the Extension Warrant immediately prior to such adjustment by a fraction, the numerator of which shall be the exercise price in effect immediately prior to such adjustment, and the denominator of which shall be the exercise price as adjusted. |
| (e) | In the event of any capital reorganization of the Company, or of any reclassification of the Common Stock, or in case of the consolidation of the Company with or the merger of the Company with or into any other corporation or of the sale of the properties and assets of the Company as, or substantially as, an entirety to any other corporation, each Extension Warrant shall, after such capital reorganization, reclassification of Common Stock, consolidation, merger or sale, and in lieu of being exercisable for shares of Common Stock of the Company, be exercisable, upon the terms and conditions specified in the Extension Warrant, for the number of shares of stock or other securities or assets to which holder of the number of shares of Common Stock purchasable upon exercisable of such Extension Warrant immediately prior to such capital organization, reclassification of Common Stock, consolidation, merger or sale would have been entitled upon such capital organization, reclassification of Common Stock, consolidation, merger or sale. The Company shall not effect any such consolidation, merger or sale, unless prior to or simultaneously with the consummation thereof, the successor corporation (if other than the Company) resulting from such consolidation or merger or the corporation purchasing such assets or the appropriate corporation or entity shall assume, by written instrument, the obligation to deliver to holder of each Extension Warrant the shares of stock, securities or assets to which, in accordance with the foregoing provisions, such holder may be entitled and all other obligations of the Company under the Extension Warrant. |
| (f) | If the Company in any manner issues or sells or enters into any agreement to issue or sell, any Common Stock, options or convertible securities (any such securities, “Variable Price Securities”) after the issuance of the Extension Warrants that are issuable pursuant to such agreement or convertible into or exchangeable or exercisable for shares of Common Stock at a price which varies or may vary with the market price of the shares of Common Stock, including by way of one or more reset(s) to a fixed price, but exclusive of such formulations reflecting customary anti-dilution provisions (such as share splits, share combinations, share dividends and similar transactions) (each of the formulations for such variable price being herein referred to as, the “Variable Price”), the Company shall provide notice thereof to the holder on the date of such agreement and the issuance of such convertible securities or options. From and after the date the Company enters into such agreement or issues any such Variable Price Securities, the holder shall have the right, but not the obligation, in its sole discretion to substitute the Variable Price for the exercise price upon exercise of the Extension Warrant by designating in the exercise form delivered upon any exercise of the Extension Warrant that solely for purposes of such exercise the holder is relying on the Variable Price rather than the exercise price then in effect. |
| (g) | In case any event shall occur as to which the other provisions above are not strictly applicable or the failure to make any adjustment would result in an unfair enlargement or dilution of the purchase rights represented by the Extension Warrants in accordance with the essential intent and principles hereof, then, in each such case, the independent auditors of the Company shall give an opinion as to the adjustment, if any, on a basis consistent with the essential intent and principles above, necessary to preserve, without enlargement or dilution, the purchase rights presented by the Extension Warrants. Upon receipt of such opinion, the Company shall promptly make the adjustment described therein. |
The Extension Warrants are governed by, and construed in accordance with, the laws of the State of Delaware, without regard to principles of conflicts of law. The Company and the holders of the Extension Warrants consent to the exclusive jurisdiction of the federal courts of the United States sitting in Delaware.
NOTE 9. FAIR VALUE MEASUREMENTS
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to
F-162
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
As of March 31, 2024, assets held in the Trust Account were comprised of $1,386,490 in money market funds primarily invested in U.S. Treasury securities.
As of December 31, 2023, assets held in the Trust Account were comprised of $1,368,637 in money market funds primarily invested in U.S. Treasury securities. During the year ended December 31, 2023, the Company withdrew an amount of $71,436 from the Trust Account to pay tax obligations and $6,796,063 in connection with redemptions.
The following tables present information about the Company’s assets that are measured at fair value on a recurring basis as of March 31, 2024 and December 31, 2023 and indicate the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value. The fair value of securities held in the Trust as of March 31, 2024 and December 31, 2023 are as follows:
| | | | | | | |
| | |
| |
| | |
| | | | | | Fair | |
|
| Trading Securities | | Level | | Value | |
March 31, 2024 |
| Money Market Funds |
| 1 | | $ | 1,386,490 |
| | | | | | | |
| | |
| |
| | |
| | | | | | Fair | |
|
| Trading Securities | | Level | | Value | |
December 31, 2023 |
| Money Market Funds |
| 1 | | $ | 1,368,637 |
The following tables present fair value information as of March 31, 2024 and December 31, 2023 of the Company’s financial liabilities that were accounted for at fair value on a recurring basis and indicate the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
| | | | | | | | | | | | |
March 31, 2024 |
| Fair Value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: | | | | | | | | | | | | |
Extension Note – Bifurcated Derivative | | $ | 22,868 | | $ | — | | $ | — | | $ | 22,868 |
ELOC | | $ | 189,764 | | $ | — | | $ | — | | $ | 189,764 |
Additional Bridge Note | | $ | 156,564 | | $ | — | | $ | — | | $ | 156,564 |
Exchange Note | | $ | 2,814,359 | | $ | — | | $ | — | | $ | 2,814,359 |
| | | | | | | | | | | | |
December 31, 2023 |
| Fair Value |
| (Level 1) |
| (Level 2) |
| (Level 3) | ||||
Liabilities: |
| |
|
| |
|
| |
|
| |
|
Extension Note – Bifurcated Derivative | | $ | 22,872 | | $ | — | | $ | — | | $ | 22,872 |
ELOC | | $ | 203,720 | | $ | — | | $ | — | | $ | 203,720 |
Additional Bridge Note | | $ | 102,726 | | $ | — | | $ | — | | $ | 102,726 |
Exchange Note | | $ | 2,621,558 | | $ | — | | $ | — | | $ | 2,621,558 |
Measurement
Extension Note Bifurcated Derivative
The Company established the initial fair value for the Extension Note Bifurcated Derivative as of May 5, 2023, which was the date the Extension Note was executed. As of March 31, 2024 and December 31, 2023, the fair value was remeasured. As such, the
F-163
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
Company used a Discounted Cash Flow model (“DCF”) that fair values the early termination/repayment features of the debt. The DCF was used to value the Extension Note Bifurcated Derivative for the initial periods and subsequent measurement periods.
The Extension Note Bifurcated Derivative was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of March 31, 2024 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the DCF model for the Extension Note Bifurcated Derivative were as follows at March 31, 2024, and December 31, 2023:
| | | | | |
|
| March 31, 2024 |
| December 31, 2023 |
|
CCC bond rates |
| 13.07 | % | 12.96 | % |
Expected term (years) | | 0.25 | | 0.25 | |
Probability of completing a business combination by June 30, 2024 and March 31, 2024, respectively | | 80 | % | 80 | % |
Additional Bridge Note
The Company established the initial fair value for the Additional Bridge as of November 21, 2023, which was the date the initial Additional Bridge Note was executed. As of March 31, 2024 and December 31, 2023, the fair value was remeasured. As such, the Company used a Monte Carlo model (“MCM”) that fair values the early termination/repayment features of the debt. The MCM was used to value the Additional Bridge Note for the initial periods and subsequent measurement periods.
The Additional Bridge Note was classified within Level 3 of the fair value hierarchy at March 31, 2024 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the Additional Bridge Note were as follows at March 31, 2024, and December 31, 2023:
| | | | | | | |
|
| March 31, 2024 |
| December 31, 2023 |
| ||
Risk-free interest rate | |
| 5.46 | % | | 5.40 | % |
Expected term (years) | |
| 0.25 |
| | 0.25 | |
Volatility | | | 95 | % | | 95 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Debt discount rate | | | 39.83 | % | | 39.7 | % |
Probability of early termination/repayment – business combination not completed | | | 20 | % | | 20 | % |
Probability of completing a business combination by March 31, 2024 | | | — | % | | 80 | % |
Probability of completing a business combination by June 30, 2024 | |
| 80 | % | | — | % |
Exchange Note
The Company established the initial fair value for the Exchange Note as of November 21, 2023, which was the date the Exchange Note was executed. As of March 31, 2024 and December 31, 2023, the fair value was remeasured. As such, the Company using the MCM that fair values the early termination/repayment features of the debt. The MCM was used to value the Exchange Note for the initial periods and subsequent measurement periods.
The Exchange Note was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of March 31, 2024 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the Exchange Note were as follows at March 31, 2024 and December 31, 2023:
| | | | | | | |
|
| March 31, 2024 |
| December 31, 2023 |
| ||
Risk-free interest rate | |
| 5.46 | % | | 5.21 | % |
Expected term (years) | | | 0.71 | | | 0.71 | |
Volatility | | | 110.1 | % | | 95 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Debt discount rate | | | 47.50 | % | | 47.54 | % |
Probability of completing a business combination by March 31, 2024 | |
| — | % | | 80 | % |
Probability of completing a business combination by June 30, 2024 | |
| 80 | % | | — | % |
F-164
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
ELOC/Equity Financing
The Company established the initial fair value for the ELOC as of November 21, 2023, which was the date the ELOC Equity Purchase Agreement was executed. As of March 31, 2024 and December 31, 2023, the fair value was remeasured. As such, the Company used the MCM that fair values the early termination/repayment features of the debt. The MCM was used to value the ELOC for the initial periods and subsequent measurement periods.
The ELOC was classified within Level 3 of the fair value hierarchy at the initial measurement dates and as of March 31, 2024 and December 31, 2023 due to the use of unobservable inputs. The key inputs into the MCM model for the ELOC were as follows at March 31, 2024 and at December 31, 2023:
| | | | | | | |
|
| March 31, 2024 |
| December 31, 2023 |
| ||
Risk-free interest rate | |
| 4.45 | % | | 3.99 | % |
Expected term (years) | | | 3.00 | | | 3.25 | |
Volatility | | | 105.0 | % | | 96.4 | % |
Stock price | | $ | 2.00 | | $ | 2.00 | |
Probability of completing a business combination by March 31, 2024 | | | — | % | | 80 | % |
Probability of completing a business combination by June 30, 2024 | |
| 80 | % | | — | % |
Level 3 Changes in Fair Value
The change in the fair value of the Level 3 financial liabilities for the period from December 31, 2023 through March 31, 2024 is summarized as follows:
Level 3 Changes in Fair Value of Derivatives for the three months ended March 31, 2024:
| | | | | | | | | | | | |
|
| Extension Note |
| Exchange |
| Additional |
|
| | |||
| | Bifurcated Derivative |
| Note |
| Bridge Note |
| ELOC | ||||
Fair value as of December 31, 2023 | | $ | 22,872 | | $ | 2,621,558 | | $ | 102,726 | | $ | 203,720 |
Initial value of Additional Bridge on January 25, 2024 |
| | — |
| | — |
| | 51,705 |
| | — |
Change in valuation inputs or other assumptions |
| | (4) |
| | 192,801 |
| | 2,133 |
| | (13,956) |
Fair value as of March 31, 2024 | | $ | 22,868 | | $ | 2,814,359 | | $ | 156,564 | | $ | 189,764 |
Level 3 Changes in Fair Value of Derivatives for the three months ended March 31, 2023:
| | | | | | |
|
| PIPE |
| Bridge Note | ||
| | Forward | | Bifurcated | ||
| | Contract | | Derivative | ||
Fair value at December 31, 2022 | | $ | 170,666 | | $ | 364,711 |
Change in fair value | |
| 1,163,950 | | | (34,758) |
Fair value at March 31, 2023 (unaudited) | | $ | 1,334,616 | | $ | 329,953 |
Transfers to/from Levels 1, 2 and 3 are recognized at the end of the reporting period in which a change in valuation technique or methodology occurs. There were no transfers to or from the various levels for the three months ended March 31, 2024 and 2023.
F-165
DIGITAL HEALTH ACQUISITION CORP.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
MARCH 31, 2024
NOTE 10. SUBSEQUENT EVENTS
The Company evaluated subsequent events and transactions that occurred after the condensed consolidated balance sheet date up to the date that the unaudited condensed consolidated financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the unaudited condensed consolidated financial statements.
On April 17, 2024, the Company, VSee and iDoc entered into a letter agreement with the Bridge Investor which amended the date with respect to the termination or closing of the business combination referenced in the Additional Bridge Notes from March 31, 2024 to June 30, 2024.
On April 17, 2024, the parties to the Third Amended and Restated Business Combination Agreement executed a Second Amendment to the Third Amended and Restated Business Combination Agreement to extend the termination date therein to June 30, 2024.
On April 17, 2024, the parties to the Extension Financing documents executed a letter agreement, which extended the maturity date of the Extension Note to June 30, 2024
On May 1, 2024, the term of DHAC was extended from May 8, 2024 to August 8, 2024.
On June 24, 2024, the parties consummated the Business Combination pursuant to the terms of the Business Combination Agreement (and upon all other conditions pursuant to the Business Combination Agreement being satisfied or waived) whereby (i) Merger Sub I merged with and into VSee Lab, with VSee Lab as the surviving company and, after giving effect to such merger, VSee Lab became a wholly-owned subsidiary of the Company, (ii) Merger Sub II merged with and into iDoc, with iDoc as the surviving company and, after giving effect to such merger, iDoc became a wholly-owned subsidiary of the Company, and (iii) DHAC changed its name to VSee Health, Inc.
On June 24, 2024, in connection with the Closing of the Business Combination, the Company consummated the exchange of a senior convertible promissory note with an aggregate principle value of $2,523,744.29 (the “Exchange Note”) and issued the Exchange Note to the Bridge Investor.
On June 24, 2024, in connection with the Closing of the Business Combination, the Company issued and sold to the Quantum Investor a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000.
On July 3, 2024, the Company and the Quantum Investor entered into an amendment to the Quantum Note to change the maturity date from June 25, 2025 to June 30, 2026, and to provide that eighteen months of interest will be guaranteed regardless of early pay or redemption.
On July 2, 2024, pursuant to the Equity Purchase Agreement, the Company issued a senior unsecured note in a principal amount of $500,000 that is payable only in shares of the Company’s Common Stock at an initial price of $10 per share (the “Equity Purchase Commitment Note”) to the investor.
F-166
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The estimated expenses payable by us in connection with the offering described in this registration statement (other than the underwriting discount and commissions) will be as follows:
| | | | |
EXPENSE |
| AMOUNT | | |
SEC registration fee | | $ | 13,353 | |
Legal fees and expenses | | $ | 100,000 | * |
Accounting fees and expenses | | $ | 10,000 | * |
Printing and engraving expenses | | $ | 5,000 | * |
Miscellaneous expenses | | $ | 2,500 | * |
Total | | $ | 130,853 | * |
* | Estimated Expenses. |
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Section 102(b)(7) of the DGCL permits a corporation to provide in its charter that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for payments of unlawful dividends or unlawful stock purchases or redemptions or (4) for any transaction from which the director derived an improper personal benefit. The current certificate of incorporation, as amended, of the registrant provide for such limitation of liability.
We have entered into indemnification agreements with each of our directors and executive officers in which we have agreed to indemnify and hold harmless, and also advance expenses as incurred, to the fullest extent permitted under applicable law, against all expenses, losses and liabilities incurred by the indemnitee or on the indemnitee’s behalf arising from the fact that such person is or was a director, officer, employee or agent of our company or our subsidiaries.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, our certificate of incorporation, as amended, our bylaws, as amended, any agreement, any vote of stockholders or disinterested directors or otherwise.
II-1
Item 15. Recent Sales of Unregistered Securities.
The following sets forth information regarding all unregistered securities sold by the registrant in the three years preceding the date of this registration statement. This information has been retroactively adjusted to reflect the reverse stock splits for all periods presented. Unless otherwise indicated, all issuances of shares were made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), and no underwriting discounts or commissions were paid with respect to the issuance of the securities.
On June 7, 2021, the initial stockholders of DHAC purchased an aggregate of 4,312,500 founder shares in exchange for a capital contribution of $25,000. On October 26, 2021, the sponsor, officers and certain advisors of DHAC forfeited an aggregate of 1,437,500 founder shares and up to 375,000 founder shares were subject to forfeiture depending on the extent to which the underwriters’ over-allotment option was exercised. Simultaneously with the closing of DHAC’s IPO, the sponsor of DHAC purchased 557,000 private placement units. Such units were separated at the Closing of the Business Combination into one private warrant in the Company exercisable to purchase one share of Common Stock at $12.50 per share and one share of Common Stock.
On October 5, 2022, DHAC, VSee Lab, iDoc and the Bridge Investor entered into the Original Bridge SPA, pursuant to which DHAC, VSee Lab and iDoc each issued and sold to such investor 10% original issue discount senior secured promissory notes due October 5, 2023 (the Bridge Note) in the respective principal amount of $888,888.80 (the “DHAC Bridge Notes”), $666,666.60 (the “VSee Lab Bridge Notes”) and $666,666.60 (the “iDoc Bridge Notes”) for an aggregate principal amount of $2,222,222. In connection with the purchase of the Bridge Notes, DHAC issued the investor (i) 173,913 warrants, each representing the right to purchase one share of DHAC common stock at an initial exercise price of $11.50, subject to certain adjustments and (ii) 30,000 shares of DHAC common stock, whereby the respective warrants and shares of DHAC common stock were redesignated as warrants and shares of Common Stock of the Company at the Closing.
On October 26, 2022, DHAC issued an unsecured promissory note in the aggregate principal amount of $350,000 to Digital Health Sponsor LLC (the “Sponsor”). As further described below, on November 21, 2023, DHAC and certain creditors including the Sponsor entered into certain loan conversion securities purchase agreements whereby such note with the Sponsor was converted into the right to receive Series A Preferred Shares of the Company and the Company issued such Series A Preferred Shares at the Closing of the Business Combination.
On November 3, 2022, DHAC and A.G.P./Alliance Global Partners (the “AGP”) entered into a securities purchase agreement (the “Purchase Agreement”), pursuant to which the Company will issue 4,370 shares of its Series B Convertible Preferred Stock (the “Series B Preferred Stock”), at a per share price of $1,000 to AGP upon the closing of the transactions contemplated by the Business Combination Agreement in full satisfaction of AGP’s $4,370,000 deferred underwriting fee payable by the Company to AGP pursuant to the Underwriting Agreement, dated November 3, 2021, between the Company and AGP. On November 21, 2023, the parties entered into an amendment to the Purchase Agreement pursuant to which DHAC would issue Series A Preferred Stocks at a per share price of $1,000 to AGP at the Closing of the Business Combination. On June 24, 2024, upon the Closing of the Business Combination, the Company issued 4,370 shares of Series A Preferred Stocks to AGP.
In February 2023, DHAC issued an unsecured promissory note to SCS Capital Partners LLC, pursuant to which the Company may borrow up to an aggregate principal amount of $250,000. The promissory note was non-interest bearing and shall be used to pay for general operating expenses. On August 17, 2023, the promissory note was amended and restated to allow for an additional $315,000, in the aggregate of $565,000. In addition, on May 5, 2023, the Company issued a promissory note to SCS Capital Partners LLC in the aggregate principal amount of $200,000. The note bears interest at a rate of 10% per annum. As further described below, on November 21, 2023, DHAC and certain creditors including SCS Capital Partners LLC entered into certain loan conversion securities purchase agreements whereby such notes with SCS Capital Partners LLC were converted into the right to receive Series A Preferred Shares of the Company and the Company issued such Series A Preferred Shares to SCS Capital Partners LLC at the Closing of the Business Combination. Such Series A Preferred Stocks are convertible into shares of the Company Common Stock.
On May 5, 2023 and as amended on April 17, 2024, DHAC entered into a securities purchase agreement (the “Extension Purchase Agreement”) with an institutional investor (the “Holder”). Pursuant to the Extension Purchase Agreement, DHAC issued the Holder a 16.67% original issue discount promissory note, in favor of the Holder, in the aggregate principal amount of $300,000 (the “Extension Note”). The Extension Note was paid off at the Closing of the Business Combination. In connection with the purchase of the Extension Note, DHAC issued the investor (i) 26,086 Warrants, each representing the right to purchase one share of the Company’s Common Stock at an initial exercise price of $11.50, subject to certain adjustments and (ii) 7,000 shares of the Company Common Stock as additional consideration for the purchase of the Extension Note and the warrants.
II-2
Pursuant to certain securities purchase agreements entered into on November 21, 2023, (the “Loan Conversion SPAs”), by and among DHAC, VSee Lab and/or iDoc with certain lenders of each of DHAC, VSee Lab and iDoc, certain indebtedness of each of DHAC, VSee Lab and iDoc was converted into shares of Series A Preferred Stocks of VSee Health. On June 24, 2024, upon Closing of the Business Combination, the Company issued in aggregate 1,788 Series A Preferred Stock to such lenders. Such Series A Preferred Stocks are convertible into shares of the Company Common Stock.
Pursuant to certain securities purchase agreements entered into on November 21, 2023 and as further amended and restated on February 13, 2024 (the “A&R Loan Conversion SPAs”), by and among DHAC, VSee Lab and/or iDoc and certain lenders (including the Bridge Investor), following assumption and conversion of the underlying loans, on June 24, 2024, upon Closing of the Business Combination, the Company issued 892,500 shares of Common Stock to such lenders.
Pursuant to the exchange agreement entered by and among DHAC, VSee Lab and iDoc on November 21, 2023, the Company consummated the exchange of the Bridge Note (excluding $600,000 of the respective VSee Lab Bridge Note and the iDoc Bridge Note) with an aggregate principle value of $2,523,744.29 (the “Exchange Note”). On June 24, 2024, upon Closing of the Business Combination, the Company issued the Exchange Note to the Bridge Investor. The Exchange Note will bear interest at a rate of 8.00% per annum and will be convertible into shares of the Company Common Stock at a fixed conversion price of $10 per share.
Pursuant to the convertible note purchase agreement (the “Quantum Purchase Agreement”) entered by and between DHAC and an institutional and accredited investor (the “Quantum Investor”) on November 21, 2023, the Company issued and sold to the Quantum Investor a 7% original issue discount convertible promissory note (the “Quantum Note”) in the aggregate principal amount of $3,000,000 on June 25, 2024 and as further amended on July 3, 2024. The Quantum Note will bear interest at rate of 12% per annum and are convertible into shares of the Company Common Stock at (1) a fixed conversion price of $10 per share; or (2) 85% of the lowest daily VWAP (as defined in the Quantum Note) during the seven (7) consecutive trading days immediately preceding the date of conversion or other date of determination.
On November 21, 2023, DHAC entered into an equity purchase agreement (the “Equity Purchase Agreement”) with an institutional and accredited investor pursuant to which the Company may sell and issue to the investor, and the investor is obligated to purchase from DHAC, up to $50,000,000 of its newly issued shares of the Company’s Common Stock, from time to time over a 36-month period beginning from the sixth (6th) trading day following the Closing of the Business Combination transaction. In connection with the Equity Purchase Agreement, on July 2, 2024, the Company issued a senior unsecured note in a principal amount of $500,000 that is payable only in shares of the Company’s Common Stock at an initial price of $10 per share to the investor.
II-3
Item 16. Exhibits and Financial Statement Schedules
The financial statements filed as part of this registration statement are listed in the index to the financial statements immediately preceding such financial statements, which index to the financial statements is incorporated herein by reference.
Exhibit No. |
| Description |
|---|---|---|
2.1 | | |
2.2 | | |
2.3 | | |
3.1 | | |
3.2 | | |
3.3 | | |
4.1 | | |
5.1 | | |
10.1 | | |
10.2 | | |
10.3 | | |
10.4 | | |
10.5 | | |
10.6 | | |
10.7 | | |
10.8 | |
II-4
Exhibit No. |
| Description |
|---|---|---|
10.9 | | |
10.10 | | |
10.11 | | |
10.12 | | |
10.13 | | |
10.14 | | |
10.15 | | |
10.16 | | |
10.17 | | |
10.18 | | |
10.19 | | |
10.20 | | |
10.21 | | |
10.22 | | |
10.23 | | |
10.24 | | |
10.25 | | |
10.26 | |
II-5
Exhibit No. |
| Description |
|---|---|---|
10.27 | | |
10.28 | | |
10.29 | | |
10.30 | | |
10.31 | | |
10.32 | | |
10.33 | | |
10.34 | | |
10.35 | | |
10.36 | | |
10.37 | | |
10.38 | | |
10.39 | | |
10.40 | | |
10.41 | | |
10.42 | | |
10.43+ | | |
10.44+† | | |
16.1 | |
II-6
Exhibit No. |
| Description |
|---|---|---|
23.1 | | |
23.2 | | Consent of Accell Audit & Compliance, PA, independent registered accounting firm for VSee Lab, Inc. |
23.3 | | |
23.4 | | Consent of Manatt, Phelps, and Phillips LLP (included in Exhibit 5.1) |
24.1 | | Power of Attorney (included on signature page to the initial filing of the Registration Statement) |
101.INS | | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
101.SCH | | XBRL Taxonomy Extension Schema Document. |
101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document. |
101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document. |
101.LAB | | XBRL Taxonomy Extension Label Linkbase Document. |
101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document. |
104 | | Cover Page Interactive Data File - the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
107 | |
+ Indicates management contract or compensatory plan or arrangement.
† Schedules and exhibits to this Exhibit omitted pursuant to Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or 15(d) of the Exchange Act that are incorporated by reference in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-7
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered that remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§ 230.430A of Title 17 of the Code of Federal Regulations), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of Title 17 of the Code of Federal Regulations); |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (6) | The undersigned registrant hereby undertakes that: |
| (i) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance on Rule 430A and contained in a form of prospectus filed by the undersigned registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and |
| (ii) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (7) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the Boca Raton, Florida as of August 6, 2024.
| VSEE HEALTH, INC. | |
| | |
| By: | /s/ Imoigele Aisiku |
| | Imoigele Aisiku |
| | Co-Chief Executive Officer |
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints each of Imoigele Aisiku and Milton Chen acting alone or together with another attorney-in-fact, as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any or all further amendments (including post-effective amendments) to this registration statement (and any additional registration statement related hereto permitted by Rule 462(b) promulgated under the Securities Act (and all further amendments, including post-effective amendments, thereto)), and to file the same, with all exhibits thereto, and other documents in connection therewith, with the SEC, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | |
Signature |
| Title |
| Date |
| | | | |
/s/ Imoigele Aisiku | | Co-Chief Executive Officer, and Director | | August 6, 2024 |
Imoigele Aisiku | | (Principal Executive Officer) | | |
| | | | |
/s/ Milton Chen | | Co-Chief Executive Officer, and Director | | August 6, 2024 |
Milton Chen | | (Principal Executive Officer) | | |
| | | | |
/s/ Jerry Leonard | | Chief Financial Officer | | August 6, 2024 |
Jerry Leonard | | (Principal Financial Officer) | | |
| | | | |
/s/ Kevin Lowdermilk | | Director | | August 6, 2024 |
Kevin Lowdermilk | | | | |
| | | | |
/s/ Scott Metzger | | Director | | August 6, 2024 |
Scott Metzger | | | | |
| | | | |
/s/ Collin O’Sullivan | | Director | | August 6, 2024 |
Collin O’Sullivan | | | | |
| | | | |
/s/ Cydonii V. Fairfax | | Director | | August 6, 2024 |
Cydonii V. Fairfax | | | | |
| | | | |
/s/ David L. Wickersham | | Director | | August 6, 2024 |
David L. Wickersham | | | | |
| | | | |
II-9