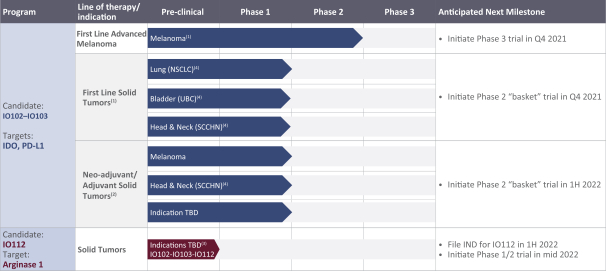
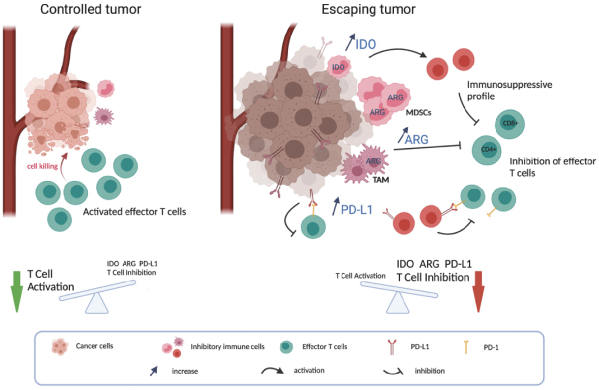
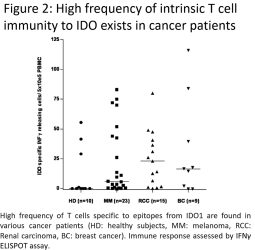
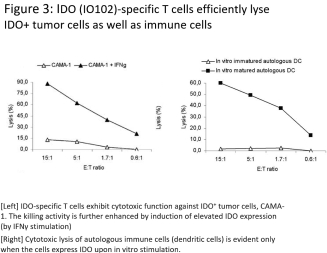
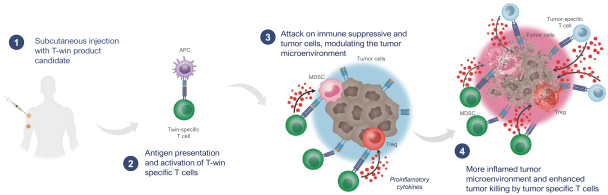
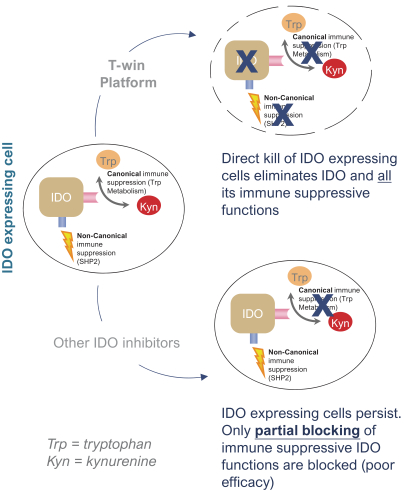
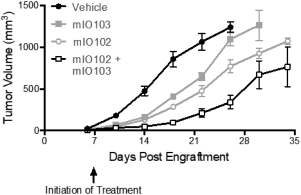
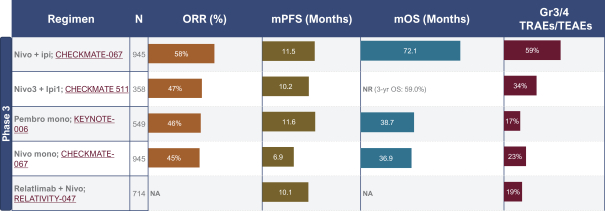
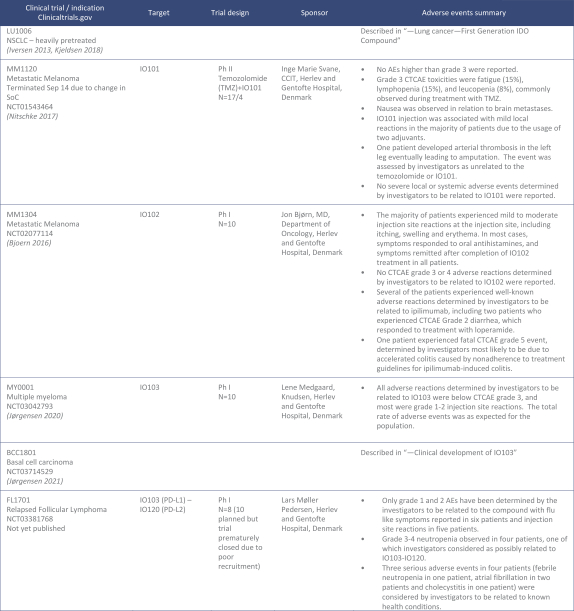
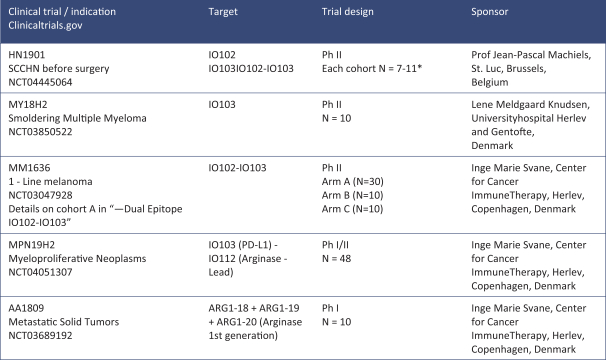
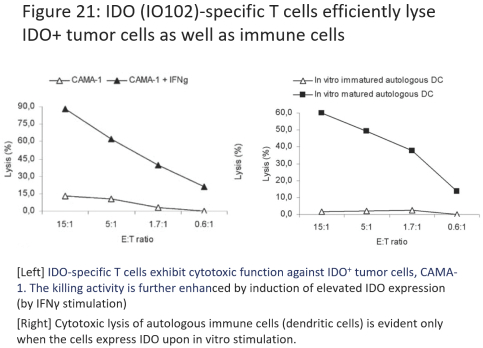
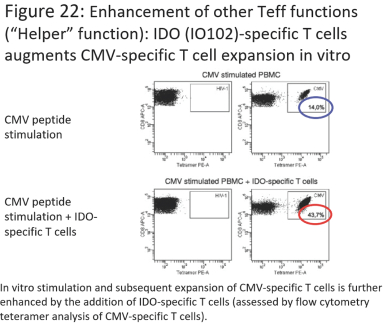
for data subjects to claim material and non-material damages resulting from infringements of the EU GDPR. Given the breadth and depth of changes in data protection obligations, maintaining compliance with the EU GDPR, will require significant time, resources and expense, and we may be required to put in place additional mechanisms ensuring compliance with the evolving data protection rules. This may be onerous and adversely affect our business, financial condition, results of operations and prospects. These requirements and risks are set out in further detail in the section of this prospectus titled “Risk Factors—Risks Related to Our Industry and Business Operations—We are subject to a variety of privacy and data security laws, and our failure to comply with them could harm our business.”
Rest of the World Regulation
For other countries outside of the EU (or in some cases, EEA) and the United States, such as the United Kingdom, countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. Additionally, the clinical trials must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Employees and Human Capital Resources
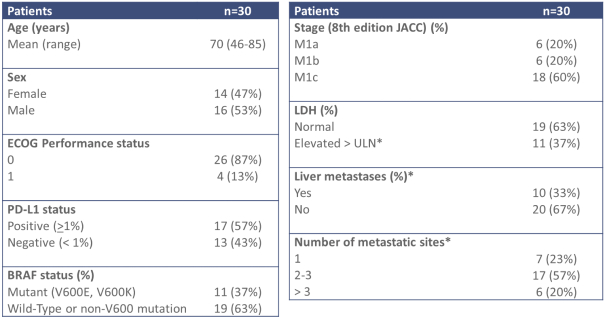
As of June 30, 2021, we had 19 full-time employees, seven of whom have Ph.D. or M.D. degrees. Of these full-time employees, 10 employees are engaged in research and development activities and one employee is engaged in finance, legal, human resources, facilities and general management. We have no collective bargaining agreements with our employees and we have not experienced any work stoppages. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our employees. We also place a high value on the diversity of our team –including gender, background and expertise – to foster our culture of innovation. The principal purposes of our equity incentive plans are to align the interests of our stockholders and those eligible for awards, to retain and incentivize officers, directors, employees, and other service providers, and to encourage them to act in our long-term best interests. We value our employees and regularly evaluate total compensation we provide, including pension contributions paid time off personal leave and other benefits, to ensure we remain competitive and attractive to potential new hires.
Facilities
We lease a facility containing approximately 302 square meters of office space for our main office, which is located at COBIS, Ole Maaloes Vej 3, 2200 Copenhagen N, Denmark. The lease expires on January 31, 2025. We also lease lab space at COBIS, Ole Maaloes Vej 3, 2200 Copenhagen N, Denmark. The lease expires on December 31, 2022.
In the United States, we lease facilities in New York, located at 430 East 29th Street, New York, New York, and in Maryland, located at 5640 Fishers Lane, Suite C, Rockville, Maryland. The New York lease expires on February 28, 2027 and the Maryland lease expires on March 31, 2027.
For our UK team, we lease an office facility located at 18 Maryport Street, Monmouthshire, UK. This lease can be terminated at our convenience.
175