| 
726 Exchange Street, Suite 800
Buffalo, New York 14210 Phone 716.845.6000 Fax 716.845.6474 www.kavinokycook.com |
By Electronic Mail Only
October 5, 2021
United States Securities and Exchange Commission
Division of Corporation Finance
Office of Technology
| | Re: | Predictmedix Inc. |
| | | Amendment No. 2 to Registration Statement on Form 20FR12G |
| | | Filed August 9, 2021 |
| | | File No. 000-56295 |
| | | |
| | Attn: | Anna Abramson |
Dear Securities and Exchange Commission:
We are responding to your letter dated August 20, 2021 (the "Comment Letter") concerning Amendment No. 1 to the Registration Statement on Form 20-F (the "Registration Statement") filed by Predictmedix Inc. (the "Company," "we," "us" or "our"). We have reproduced your comments and the Company's responses below. In addition, we have included financial information for the three months ended April 30, 2021 and the six months July 31, 2021.
Before addressing the comments individually, we have added the following preliminary statement on page 6 of the Registration Statement (included with this letter in PDF form). We believe that including this disclosure at the beginning of the Registration Statement will help readers better contextualize and understand the business of the Company and the Risk Factors that follow. Also, we realized from the thrust of your comments that we had combined discussion of our current primary product (the IDSS or "Safe Entry System") with the products in development such that they all appeared to have the same or similar primacy. Also, we realized that the role of Juiceworks (and the business model that was implemented with Juiceworks) with respect to the IDSS product was not adequately emphasized. Finally, we tried to bring greater clarity to the role of the screening systems as preliminary indicators for where more definitive testing and diagnosis is desirable. None of the IDSS, the Impairment Detection station or the Mental Illness detection stations provide a definitive diagnosis. Rather, they are intended to rapidly screen people in various settings where an early indication of factors that can be discerned using sensors and artificial intelligence can generate meaningful recommendations for next stage testing. The technology can be in facilities where there is movement of a large number of people for which screening has to be rapid and ongoing. The technology can also be deployed in settings such as hospitals, healthcare facilities, airports, malls, stadiums, government and office buildings, casinos, spiritual and cultural events, performances, festivals, and conferences. The IDSS is appropriate for any location where large numbers of people gather or where transmission security is paramount. The system can be used as a first step triage where individuals can be screened for the presence of symptoms and the ones who are symptomatic can undergo further secondary testing.
PRELIMINARY STATEMENT
The Company's business is focused on developing artificial intelligence ("AI") powered technologies for public settings, general workplace health and safety, and for the health care industry. In particular, the Company has developed A technology for the identification and detection of infectious diseases (including COVID-19), sometimes referred to in this Registration Statement as our Infectious Disease Symptom Screening Solutions ("IDSS") and marketed in North America under the name "Safe Entry System." In addition, the Company is developing AI powered products that address (1) detection of alcohol and/or cannabis impairment in individuals; (2) mental illness screening; and (3) remote patient monitoring and treatment plans, sometimes referred to herein as the Mobile Wellbeing product. The following is a description of our primary product, the IDSS, and the products under development.
Disease Symptom
Screening Solutions
("IDSS") | Our primary product that is being marketed and sold currently. All Canadian Display Company Ltd, an Ontario corporation doing business as "Juiceworks Exhibits" ("Juiceworks") is the constructor and selling agent for the Company's Safe Entry Systems in North America. An initial license fee is received by the Company from customers when a Safe Entry System is sold or leased by Juiceworks. The Company then will receive a recurring monthly licensing fee from customers. A formal agreement with Juiceworks covering exclusive fabrication rights and non-exclusive selling rights is under discussion but has not been completed as of the date of this registration statement. Outside of North America, the Company intends to market and sell the Safe Entry System directly, however, the Company may enter into arrangements with third parties similar to the arrangement with Juiceworks to cover other territories. As a screening system, our IDSS does not render conclusive diagnoses, nor can it identify asymptomatic cases. Rather, it can (and should) be used to identify persons who are more likely to test positive for certain infectious diseases and refer such persons to undergo a more definitive test, such as a rapid COVID test. Our IDSS marketing targets potential customers who may have to rapidly screen large numbers of people where it is not practical to diagnostically test all of them. Such a situation arises in many public events. In a place where large numbers of people are to be admitted to a common area, the IDSS could be used to identify individuals who are more likely to test positive and yield a smaller pool of individuals who could then undergo a rapid diagnostic test prior to admission. Our IDSS screening stations will be networked and will collect data on an entirely anonymous basis that will enhance the performance of our IDSS screening stations everywhere. Our AI technology incorporates machine learning techniques that enables the system to improve accuracy as additional data is available. |
Products Under Development |
Impairment Detection Screening | Our Impairment Detection Screening scanner is under development and is not being marketed or sold currently. The product incorporates the Company's proprietary AI technology and is being developed to detect cannabis and/or alcohol drug impairment in individuals. The product will be developed as a screening station that is capable of making a recommendation as to whether individuals passing through the screening station should undergo additional definitive testing. |
Mental Health Screening | Our Mental Health Screening station is under development and is not being marketed or sold currently. The product will incorporate the Company's proprietary AI technology to detect behavioral and physiological indicators of mental illness such as depression, autism, ADHD and dementia. As a step in the screening process, persons would be asked to read a script while undergoing the scan that detects vocal qualities, vocal cadence and physiological factors such as blood flow in the face. The intended use for this product will be to screen for indicators of mental disorders and make recommendations as to whether individuals passing through the screening station should undergo additional evaluation. In this way, the system could be used as a triage tool at mental health centers, police stations or any setting where a preliminary mental health assessment is required, and mental health professionals are not readily available to perform an assessment or are over-taxed by an inflow of possible cases. This technology represents an ambitious application of the Company's AI technology and is in a developmental stage. A prototype is not expected prior to the second half of 2022 and may not be ready for public demonstration prior to 2023. |
Remote patient monitoring and treatment plans application, sometimes referred to herein as the Mobile Wellbeing product (the "Mobile Wellbeing App" | This product was substantially developed by Mobile Wellbeing, a company that we acquired in July of 2020. The product is a telemedicine remote patient monitoring platform. We are currently adding modules to this platform that will enable us to use a patient's history and real time medical data, such as blood pressure or blood glucose level, to provide patients and their medical professionals with treatment plans for chronic disease management and in some cases lifesaving advance notice of when a patient should go to a hospital. This product also is under development and is not being marketed or sold by the Company currently. |
Item 3. Key Information
D. Risk Factors, page 9
1) In your response to prior comment 2, you included a risk factor titled "We are exposed to cybersecurity incidents resulting from deliberate attacks or unintentional events." Please include this risk factor in your filing.
Response 1)
We have included the risk factor on pages 11 of the included Amendment No. 2. Thank you. We have also added additional risk factors.
While we believe our infectious disease screening technology is unique…. Page 11
2) Please clarify whether you currently have competitors for your infectious disease screening technology. In that regard, we note your disclosure that "one or more potential competitors may emerge," indicating you do not have competitors, but also that "[c]ompetition continues to intensify," indicating that you do have competitors.
Response 2)
To the Company's knowledge, it does not have direct competitors for its IDSS product. Breathalyzer devices may be considered competitive with the Company's Impairment Detection product (under development). The Company has revised the following risk factors in Item 3(D) on page 13 of the included marked draft, reproduced below.
Impairment Screening Business
We are aware of two potential competitors for our Impairment Screening station (which is in development). They are Cannabix Technologies Inc. and Hound Labs, Inc., both of which are developing, or have developed, breathalyzer devices that detect THC in the breath. The Company does not believe that these products compete directly with the Company's Impairment Screening stations that can be used to rapidly screen large numbers of people. However, the Company may experience additional competition from competitors who may develop and introduce new products, services or enhancements that better meet the needs of end-users or changing industry standards, or achieve greater market acceptance due to pricing, sales channels, or other factors.
We may face competition with respect to all of our products.
Our artificial intelligence ("AI") based technologies may face competition from other companies that are developing their own AI based products and that may have superior technology, a larger base of data that can be used to "train" their AI technology or may have greater funding for research and development. As a result of competition, our product and service offerings may not be successful, we may fail to gain or may lose business, and we may be required to increase our spending or lower prices, any of which could materially negatively affect our potential sales and profits.
Our technology cannot screen for asymptomatic COVID-19.
Persons who have been infected with COVID but manifest no symptoms are sometimes referred to as "asymptomatic." Asymptomatic screening for COVID-19 is beyond the scope of our technology. Our IDSS is unlikely to identify a COVID infection in an asymptomatic person. This aspect may adversely affect our ability to market and sell our IDDSS product.
Also, on page 12 we added the following risk factor.
Our Development Stage Products are Substantially Untested
Without substantial testing in the field by third parties operating our products, we cannot speak definitively as to how well our products work or if they work at all. Our IDSS technology has been tested in the field. Our development-stage products have not been well tested. There is no assurance that our products will be viewed by the market as being sufficiently for their intended purpose.
Item 4. Information on the Company
B. Business Overview, page 14
3) Please refer to prior comment 4 and provide disclosure regarding the current status for your Cannabis and/or Alcohol Impairment and Mental Health Screening technologies. Specifically address any current revenue-generating activities, customers and their geographic location, the status of development efforts for, anticipated timeframe for completion of, material costs associated with your planned products and services, and a description of the sources and availability of the materials used to create the hardware for your screening products
Response 3)
We have reproduced the table included as the "Preliminary Statement" in the first few pages of the 20-F again in the Business Overview section. While repetitive, we believe this table provides a coherent overview of the Company's products and makes clear that the only product currently offered for sale is the IDSS screening station. Also, as background, for the Company's disclosure as to its capital requirements going forward we believe it is important for the reader to understand the current arrangement that the Company has with Juiceworks for the construction, marketing and sale of the IDSS screening stations. This arrangement would be the model for the alcohol/cannabis Impairment Screening station and the Mental Health screening station when they are ready to be introduced to the market. Under the current arrangement, Juiceworks markets and sells the IDSS screening stations in North America, building each screening station as orders are received in its own plant in Canada according to the Company's specifications. When an IDSS scanning station is sold and installed at a customer's site, the Company installs its software for the scanning station remotely, over the internet. Significantly, the Company does not have to make a capital investment in a manufacturing facility, a manufacturing workforce or absorb the cost and overhead of building and maintaining a sales and marketing department. The Company receives an ongoing licensing fee from each customer for the use of its AI software. Juiceworks receives the entire profit on the sale and physical installation of the screening station hardware.
The Company believes this business model will enable it to rapidly scale up its sales and establish a steady stream of income while enabling the Company to focus on developing its technology and commercializing its development stage products. In addition, the Company has been able to control its product development costs by contracting with accomplished engineers in India. By keeping its capital investments low the Company believes it can carry forward the development and eventual marketing of the Impairment Detection scanner and the Mental Health screening scanner and the Mobile Wellbeing application (which is a different type of product) using its existing resources and, eventually, revenue from the licensing of its software in these scanning systems. The principal risk, which has now been prominently disclosed in Risk Factors and also in the description of the Company's business, is that Juiceworks (or other companies fulfilling the same role as Juiceworks) does not, or cannot, perform as expected or fails to add fabrication, marketing and sales capacity if demand increases.
We added the following risk factor in Item 4(D) on page 12.
Risks Associated with Juiceworks and the Business Model Being implemented by the Company
Our primary product, the IDSS, is fabricated, marketed and sold in North America by All Canadian Display Company Ltd, an Ontario corporation doing business as "Juiceworks Exhibits" ("Juiceworks"). With each sale or lease to a customer, an initial license fee is received by the Company. The Company then will receive a recurring monthly licensing fee from customers. Juiceworks receives the revenue from the sale or lease of the Safe Entry System. A formal agreement with Juiceworks covering exclusive fabrication rights and non-exclusive selling rights is under discussion but has not been entered into. Outside of North America, the Company intends to market and sell the Safe Entry System directly, however, the Company may enter into arrangements with third parties similar to the arrangement with Juiceworks to cover other territories. The current arrangement that the Company has with Juiceworks will be the model for the alcohol/cannabis Impairment Screening stations and the Mental Health screening stations when they are ready to be introduced to the market. The Company believes this model enables the Company to avoid substantial capital investment in a fabrication plant and the operating overhead associated with having an in-house manufacturing, sales and marketing department. However, this arrangement also exposes the Company to certain risks that could be material, including:
- Juiceworks or other companies fulfilling the same role as Juiceworks do not, or cannot, perform as expected;
- Juiceworks or other companies fulfilling the same role as Juiceworks fail to add fabrication, marketing and sales capacity as demand increases, thereby stymieing growth and frustrating customers;
- Juiceworks or other companies fulfilling the same role as Juiceworks breach their agreements which, currently, are not formal and would be difficult to enforce; and
- Juiceworks or other companies fulfilling the same role as Juiceworks compete with the Company using customer lists and contacts developed while marketing and selling the Company's products.
In the event that any of the forgoing breaches or setbacks occur, the Company's business could be materially adversely affected.
The hardware components of the IDSS screening stations are substantially the same standard components as will be used in the Impairment Detection screening station and the Mental Health screening station. These components of the IDSS are described in Item 4(B) on page 21 of Amendment No. 2 (the version attached with this letter as a PDF document). These components are readily available and to the Company's knowledge, are not adversely affected by supply chain shortages.
We have added the following disclosure commencing on page 21of Amendment No. 2 with respect to the Cannabis/Alcohol Impairment screening product.
Current Revenue Generating Activities and Customers
Our Impairment Detection screening station is currently in the development stage and is not available for sale. While we are exploring relationships with third parties for the construction, marketing and sale of this technology, no such relationships have been undertaken. The Company cannot project the size or acceptance of the potential market, nor is the Company able to project when this product can be brought to market. Among other factors, the Company may choose to extend the development period while it builds its base of data that would be available to the AI algorithms being used by the screening station.
Anticipated Timeframe & Development Costs
The Company believes that it will have a functioning prototype of the Impairment Detection screening station by the end of 2021 or early in 2022. While we are exploring relationships with third parties for the construction, marketing and sale of this technology, no such relationships have been undertaken. We view the advantages of this business model as allowing the Company to avoid having to incur substantial capital costs for plant and manufacturing and overhead costs for sales and marketing. We also believe that having a third party undertake these functions will enable the Company to scale up more rapidly than it could otherwise. However, this business model results in the Company having less control over the timing and implementation of bringing this product to market.
The remaining projected development costs for the Impairment Detection screening stations consist of: (a) a Full Stack Developer at CDN$2,200/month, (c) a Senior Data Scientist at CDN$2,500/month, (d) two Junior Data Scientists at CDN$1,200/month each, for a total projected cost of approximately $CDN 50,000 to CDN$60,000 (6-7 months of development and refinement). The foregoing persons are in India. The Company believes that it has adequate resources to fund this development without having to raise additional capital. The Company also will implement a testing period.
Sources and Materials
The hardware for our Impairment Detection Screening Technology will be substantially similar to the hardware used in our IDSS screeners. We anticipate that the following components will be needed for each unit: a multispectral camera, a specialized computer module, a power-over-ethernet switch, a hard drive, an LED monitor, relay switches, a router, a wireless keyboard and a mouse. We believe that all of these components are widely commercially available and can be purchased from multiple manufacturers. The Company is not aware of supply chain difficulties related to these components.
We have added the following disclosure commencing on page 22 of Amendment No. 2 with respect to the Mental Health screening product.
Mental Health Screening
Current Revenue Generating Activities and Customers
Our Mental Health Screening station is currently in the development stage and does not produce any revenue. We will seek to establish a relationship with a third party for the fabrication of individual units, as they are sold, and for sales and marketing of the product, similar to the relationship the Company has with Juiceworks for its IDSS product. While we are exploring relationships with third parties for the construction, marketing and sale of this technology, no such relationships have been undertaken. We view the advantages of this business model as allowing the Company to avoid having to incur substantial capital costs for plant and manufacturing and overhead costs for sales and marketing. We also believe that having a third party undertake these functions will enable the Company to scale up more rapidly than it could otherwise. However, this business model results in the Company having less control over the timing and implementation of bringing this product to market. Our target markets for this technology are hospitals, residential facilities, police stations and health care providers.
Anticipated Timeframe & Development Costs
The Company believes that it will have a functioning prototype of the Mental Health Screening stations by the end of 2022 or in the first half of 2023, however, the Company may choose to delay introduction of the product to the market in order to test the scanning stations in the field and further build the data underlying the AI engine.
The remaining projected development costs for the prototype software consist of: (a) a Full Stack Developer at CDN$2,200/month, (b) Senior Data Scientist - $2,500/month and (c) 2-3 Junior Data Scientists - $1,200/month for a total projected cost of CDN$140,000. The development process may take 12-16 months. The foregoing engineers are in India. The Company believes that it has adequate resources to fund this development without having to raise additional capital. Once a prototype is built, the Company also will implement a testing period, the duration of which is difficult to predict. There are no marketing and sales partners identified for the Impairment Screening Technology and there is no projected time for when the Mental Health screening product may generate revenue. The Company cannot forecast how the market will respond.
Like any true AI based technology, the system gets "trained" on data. In the early stages of development, the data available to the system will be considerably less than the data base that is expected once the system is in more widespread use. It may make sense for the Company to donate scanning stations to users in the field in order to build the Company's data base. If this is done, revenue generation would be delayed.
Sources and Materials
Our Mental Health Screening devices will be modeled upon our IDSS screeners. We anticipate that the following components will be needed: multispectral camera, specialized computer module, power-over-ethernet switch, hard drive, LED monitor, relay switches, router and wireless keyboard and mouse. We believe that all of these components are widely commercially available and can be purchased from multiple manufacturers.
Mental Health Screening, page 16
4) We note your response to prior comment 5. Please further explain what early signs of mental illness your technology will detect.
Response 4)
Attached as Exhibit A to this letter are examples of the data that is collected by the IDSS screening stations. Similar data will be collected by the impairment and mental illness screening stations. While the data and features being studied are different for each of the screening products, the processes undertaken by each AI engine at a fundamental level are similar. The AI engine searches for features that correlate with the condition to be detected and generates a recommendation coupled with a confidence level. The mental illness screening process is different in that the person being screened is asked to read a prepared script while standing in front of the screening station. In addition to physiological data, the mental illness screener will collect information regarding the vocal inflection and cadence of the person reading the script. The Company has not included this example in the registration statement because it would not be meaningful to the average reader. However, we include it for your review as Exhibit A.
We have provided a better description of how the Mental Illness screening will work on page 22 and in the summary table under Preliminary Statement and in Item 4(B) - Business Overview.
Significant Business Developments During the Year ended January 31, 2021
Other Developments, page 18
5) We note your revised disclosure that you have not entered into any material contracts or agreements with the companies listed in this section. Given that you do not currently have any relationship with these companies, a discussion of the companies appears inappropriate. Please revise.
Response 5)
We have removed this discussion from Amendment No. 2.
A - Operating Results, page 21
6) We note the revised disclosure provided in response to comment 8. Please also discuss your plans to develop the IDSS technologies and product lines. Address and quantify the capital resource requirements you have determined are necessary to bring these technologies to completion. Identify and quantify those available capital resources you plan to rely upon to complete the development of these technologies. Explain how and when you expect to generate IDSS revenues. Also, address any known uncertainties related to the recovery of your investments in this product and service line.
Response 6)
We note that for the six months ended July 31, 2021, the Company had revenue of $49,640 from the licensing of its IDSS (page 32 of the included marked draft).
We have added the following disclosure in Item 5 of the Registration Statement.
Development of IDSS Technologies and Product Lines
The IDSS technology is complete and is currently being marketed.
The IDSS utilizes multispectral imaging cameras and other sensors (including audio) placed in the scanner station as data collection sources. These cameras and sensors provide the system with visual spectral imaging plus infrared thermography that falls outside of visual spectrum wavelengths. This data is processed using a Cloud/Edge configuration comparing, in real time, the complex thermographic patterns of persons being screened with learned data sets from healthy control groups and Covid-19 patients. The result is an evidence-based determination of whether symptoms of COVID or other infectious diseases are present. The potential presence of COVID-19 may be indicated by symptoms such as fatigue, headache, fever, pink eyes and other key determinant factors in combination. Built as an AI machine learning system, all of the IDSS screening stations are networked and contribute to the data base of the AI system. The system continuously evolves as more data is received through screenings and from testing partners and customers. In other words, as more screening stations are implemented in the field and data is fed into the Company's systems, the greater the accuracy and usefulness of the screening stations as the AI "learns." Among other benefits, screening stations operating in the Company's network will provide the most recent physiological patterns associated with symptoms of the COVID virus. The output shows up as a green or a red light on the module itself with a red light associated with the individual exhibiting symptoms.
The technology can be in facilities where there is large movement of people for which screening has to be rapid and ongoing. The technology can also be deployed in settings such as hospitals, healthcare facilities, airports, malls, stadiums, government and office buildings, casinos, spiritual and cultural events, performances, festivals, and conferences. Any location where large numbers of people gather or where transmission security is paramount. The system can be used as first step triage whereby individuals can be screened for the presence of symptoms and the ones who are symptomatic can undergo further secondary testing.
As noted above, with respect to the IDSS Scanning Station, which is the Company's primary product, the Company has a relationship with All Canadian Display Company Ltd, an Ontario corporation doing business as "Juiceworks Exhibits" ("Juiceworks"). Juiceworks is authorized to market and sell the IDSS Scanning Stations in the United States and Canada. Juiceworks receives the profit from the sale and installation of each unit. The Company receives a licensing fee for installation and ongoing use of its proprietary artificial intelligence software in each IDSS that is sold.
Capital Resource Requirements
The business model, which is based upon using one or more third parties for fabricating, marketing and selling the IDSS product, minimizes the Company's need to make capital investment in a physical plant and incurring the overhead cost of maintaining a manufacturing, marketing and sales department. This model also enables the Company to focus on developing its technology and creating new products. However, the Company's reliance on Juiceworks to perform these functions also results in less control over how they are carried out. Currently, the Company does not have a formal agreement with Juiceworks governing this relationship. If Juiceworks does not perform well or fails to invest adequate resources to satisfy customers and propel sales, the results will be disappointing and material revenue generation could be delayed. The Company is pursuing a more formal agreement with Juiceworks. See Risk Factors - Risks Associated with Juiceworks and the Business Model Being implemented by the Company.
Uncertainties Related to the Recovery of Our Investments in IDSS
We cannot guarantee that we will be able to recover our investment in the development of the IDSS product. However, the Company believes that once the product becomes established and has multiple customers in the field, there will be more than sufficient sales to cover our recovery of this investment. Moreover, the Company believes that such recovery is likely to be achieved during the current fiscal year ended January 31, 2022.
7) In regard to the disclosure concerning the Mobile Wellbeing (MWB) technologies provided in response to comment 8, please describe your plans to develop your MWB technologies and product lines in accordance with Item 5 of Form 20-F. Address and quantify the capital resource requirements you have determined are necessary to being these technologies to completion. Identify and quantify those available capital resources you plan to rely upon to complete the development of these technologies. Explain how and when you expect to generate MWB revenues. Also address any known uncertainties related to the recovery of your investment in this product and service line.
Response 7)
Capital Resource Requirements for the Development and Deployment of Mobile Wellbeing
When the Company acquired the Mobile Wellbeing assets in July of 2021, it acquired a beta version of the product. The development costs for the product were substantially incurred at the time the acquisition took place. Because the Mobile Wellbeing product is an application that is installed on cell phones or a computer making use of its web-based portal, there is no physical device that requires manufacturing investment. The development to be undertaken is entirely in the area of enhancing the existing software.
We have added the following disclosure in Section 4 on page 24 of Amendment No. 2.
As of July 2020, the date on which the Company acquired the Mobile Wellbeing product, there was a beta version of the application in existence. Prior to introducing the product to the market, the Company will modify the beta version of the product by adding certain modules consisting of artificial intelligence / machine learning layers which will allow the system to analyze in real time any discrepancies that may show up with patient monitoring which can inadvertently be associated with an adverse reaction: The human resources needed to add the modules include: (a) two Front End Developers, (b) three Back End Developers, (c) one Senior Data Scientist, (d) one Full Stack Developer and (e) some involvement by the Company's in-house Project Manager. The projected time for the product to be ready for the market is six months. The projected cost is: (a) Front End Developer - CDN$1,800/month, (b) Back End Developer - CDN$2,000/month, (c) Senior Data Scientist - CDN$2,500/month, (d) Full Stack Developer - CDN$2,200/month, for a total projected cost of CDN$85,800. The foregoing engineers are in India. The Company believes that it has adequate resources to fund this development without having to raise additional capital.
In July and August of 2021, an affiliate of the Company applied for the following patents related to the Mobile Wellbeing product.
Patent Application
No. 17384686 | System and method to automatically recommend and adapt a treatment regime for patients; Submitted July 23, 2021by Rajiv Muradia |
| | |
Patent Application
No. 17384773 | System and method to manage a rewards program for patient treatment protocols; Submitted July 23, 2021by Rajiv Muradia |
| | |
Patent Application
No. 17385889 | Utilizing healthcare providers network effect to increase compliance for better health outcomes; Submitted July 26, 2021by Rajiv Muradia |
| |
Provisional application that was converted to a non-provisional application: |
| | |
Patent Application
No. 63072392 | System and method to provide product recommendation and sponsored content to patients managed by computerized workflows for treatment protocols; Submitted August 15, 2021by Rajiv Muradia |
Uncertainty Related to Recovery Investment
Currently, the Company does not have a projected time for when the Mobile Wellbeing product will generate revenue. However, the Company estimates that the product will be available for download and sale by the end of 2021 or in the first half of 2022 and that it should generate revenue shortly after it is made available. The Company believes it will recover its investment in this product (currently shown as an intangible asset in our financial statements for the years ended January 31, 2020 and 2021) by the start of the 2023 fiscal year.
Item 6. Directors, Senior Management and Employees
A. Directors and senior management, page 27
8) Please refer to prior comment 10 and provide disclosure regarding the percentage of time or hours per week Mr. Kales and Mr. Kushwah anticipate spending on your business.
Response 8)
Mr. Kales and Dr. Kushwah divide their business time evenly between the Company and Deepspatial Inc. Their expectation is that the demand of each company will fluctuate from week to week, depending upon what they are doing. For example, if one of the companies is negotiating an important relationship or transaction, all of their attention will be applied to that endeavor. A conflict could arise if both companies are very active at the same time, but such a situation is unlikely (their time is expandable) and is expected to be brief. We have added further disclosure to this effect in Item 3(D) in Conflicts of Interest on page 13. The disclosure is as follows.
Dr. Kushwah (Chief Operating Officer), Mr. Malhotra (Chief Financial Officer) and Mr. Kales (Chairman of the Board) are each officers of other companies, the principle one being Deepspatial Inc. (("Deepspatial"). Deepspatial is a development-stage company that is Geodemographic customer profiling tools for businesses looking to enter new foreign markets. Neither Dr. Kushwah not Mr. Kales will devote his full business time and energy to the Company. Rather, they expect to roughly split their time between the Company and Deepspatial. It is possible that conflicts for their time and attention will arise and that they may not be able to allocate sufficient time to the business of the Company during periods when Deepspatial is also very active. In addition. Mr. Malhotra is an officer of five other companies. He expects to allocate approximately twenty percent of his business time to the Company.
Any decisions made by Mr. Kushwah or Mr. Kales as directors of the Company will be made in accordance with the duties and obligations of directors to deal fairly and in good faith with the Company. Such directors may declare, and refrain from voting on, any matters in which such directors may have a conflict of interest. For more information regarding the outside activities of our officers, please see Item 6.A "Directors and Senior Management" below.
E. Share Ownership, page 37
9) We note your response to prior comment 11. Please include the number of options in each individual's total shares beneficially owned, use that total to calculate the percentage of outstanding shares beneficially owned by each individual, and include in a footnote the number of options held by each individual.
Response 9)
We have added the following table in Item 6 on page 47 of the attached draft.
Name | Number and percentage of Common Shares beneficially owned,
or controlled or directed, directly or indirectly* |
Sheldon Kales | 30,666,000 (28%) (1) |
Dr. Rahul Kushwah | 9,625,000 (9%) (2) |
Thomas Sipos | 650,000 (0.6%) (3) |
Ajit Kumar | 300,000 (0.3%) (4) |
Rakesh Malhotra | 275,000 (0.2%) (5) |
*Based on issued and outstanding common shares at January 31, 2021 plus vested options
(1) Includes 1,625,000 vested options to purchase common shares
(2) Includes 1,625,000 vested options to purchase common shares
(3) Includes 200,000 vested options to purchase common shares
(4) Includes 150,000 vested options to purchase common shares
(5) Includes 50,000 vested options to purchase common shares
Financial Statements
8. Intangible Assets, page F-23
10) Please tell us how you determined that your MWB and IDSS intangible assets are recoverable. Explain how and when you expect these technologies to generate sufficient revenues and margins to justify their current carrying values. Tell us how you plan to complete the development of these technologies. Describe and quantify for us the technical, manpower and financial resources you have determined will be required. Identify and quantify those available capital resources you plan to rely upon to complete development and marketing of those technologies. Finally, tell us why impairment charges are unnecessary.
Response 10)
We refer to our responses to Comments 3, 6 and 7 above for information on the cost and timing of development of the MWB and IDSS products. We added the following disclosure to Item 5.A (Operating Results) under "Research and Development" on page 33.
Intangible Assets
Total intangible assets were $435,419 as reflected in the Company's balance sheet as of July 31, 2021. Of the total amount shown as intangible assets, $138,561 is attributable to the Mobile Wellbeing product and $ 296,858 is attributable to the IDSS product. The IDSS product is currently being offered for sale/license. The Mobile Wellbeing product is in development (as discussed above), but the Company believes it will have a complete product by the end of this current fiscal year. The Company believes that it will recover the IDSS related asset in the next fiscal year and the Mobile Wellbeing asset in the following fiscal year or by the beginning of fiscal 2024.
Consolidated Statements of Loss and Comprehensive Loss, page F-41
11) We note your response to comment 15. We also note under Item 18, on pages F-40 through F-65, you provided financial statements for the comparative years January 31, 2020 and January 31, 2019 and a discussion of comparative results of operations on page 25. Please either remove these financial statements and related disclosures or revise all prior period financial statements to account for the disposition of Cultivar JA in accordance with paragraph 34 of IFRS 5.
Response 11)
In Amendment No. 2, the Company has removed the financial statements for the years ended January 31, 2020 and January 31, 2019. The Financial Statements for the years ended January 31, 2021 and 2020 are included along with quarterly reports for the three months ended April 30, 2021 and the six months ended July 31, 2021.
12) We note your response to comments 13 - 15. Please remove the report of Buckley Dodds LLP along with the financial statements for the years ended January 31, 2020 and 2019, or revise it as previously requested to include a statement that the audit was conducted "in accordance with the standards of the Public Company Accounting Oversight Board" and explicitly state that the financial statements are presented in accordance with International Financial Reporting Standards (IFRS) "as issued by the IASB".
Response 12)
As noted above, the Company has removed the financial statements for the years ended January 31, 2020 and January 31, 2019. With this change, the report of Buckley Dodds LLP is no longer part of the registration statement.
We look forward to hearing from you. Thank you.
| | Very truly yours, |
| | |
| | KAVINOKY COOK LLP |
| | |
| | /s/ Jonathan H. Gardner |
| | Jonathan H. Gardner |
EXHIBIT A
The Company's IDSS technology, as well as the Impairment Detection screeners and the Mental Illness screeners under development, use multispectral imaging and audio analysis collected by screening stations that employ substantially similar hardware. The data collection is aimed at identifying features that are associated with disease, impairment or mental state for which the scanner is seeking a correlation. The AI algorithms used by the processor have been "trained" on data collected from individuals who have been given controlled amounts of alcohol and/or THC, or who are known to have COVID-19 or who are known to be suffering from a diagnosed mental state. Among other things, the systems are able to identify are multiple physiological features in the face which can be detected and that are associated with relevant disease, impairment or mental states. For example, several of these features relate to changes in blood flow which can be detected in the face following consumption of cannabis and/or alcohol. Audio features also have been identified that can be associated with the relevant disease, impairment or mental state.
The screening stations capture at least one multispectral image associated with each individual under examination and identify at least one indicative feature in each image. With respect to each such feature, the AI processing is used to: (a) generate an intensity representation for that feature, (b) apply at least one impairment analytical model to the intensity representation to determine a respective impairment likelihood and (c) determine a confidence level for each impairment likelihood based on characteristics associated with the applied impairment analytical model and that feature. The processor arrives at a recommendation based upon at least one impairment likelihood and the respective confidence level.
The following is an example of data collected from a multispectral imaging device screening for alcohol or cannabis impairment. This example is one of multiple features that are examined.
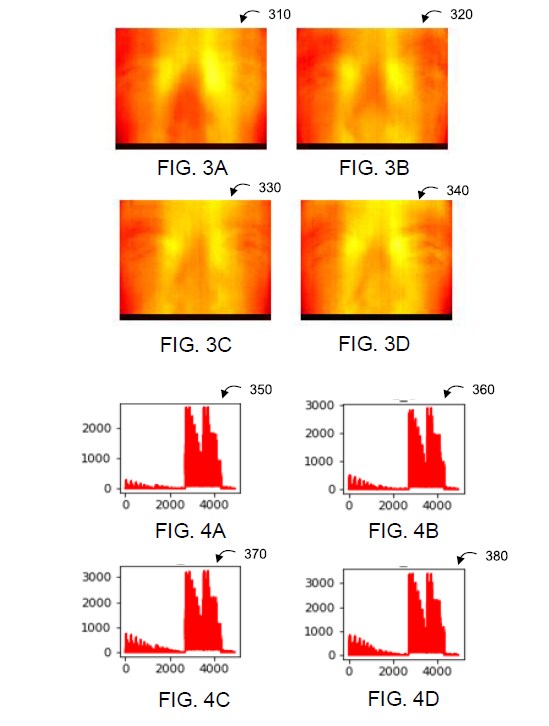
FIG. 3A is an infrared image of an individual's eyes and nose when the individual is under no impairment, in accordance with an example embodiment;
FIG. 3B shows the individual's eyes and nose (as in FIG. 3A) when the individual is under impairment, in accordance with an example embodiment;
FIG. 3C shows the individual's eyes and nose (as in FIGS. 3A and 3B) when the individual is at another impairment level, in accordance with an example embodiment;
FIG. 3D shows the individual's eyes and nose (as in FIGS. 3A to 3C) when the individual is at another impairment level, in accordance with an example embodiment;
FIG. 4A is an example intensity representation corresponding to the infrared image in FIG. 3A, in accordance with at example embodiment;
FIG. 4B is an example of intensity representation corresponding to the infrared image in FIG. 3B, in accordance with at example embodiment;
FIG. 4C is an example of intensity representation corresponding to the infrared image in FIG. 3C, in accordance with at example embodiment;
FIG. 4D is an example of intensity representation corresponding to the infrared image in FIG. 3D, in accordance with at example embodiment;
Similarly, multiple features have been identified within audio samples which have a strong correlation with impairment, following is one of the examples.

FIG. 13 is an example of an intensity representation that shows an audio property associated with different impairment levels, in accordance with an example embodiment


