approximately 35% still have uncontrolled blood pressure. Although the evidence for the benefits of reducing blood pressure is overwhelming and has been consistently supported by the medical community’s recommendations to drive blood pressure to lower levels, the current standard-of-care has not meaningfully changed in more than a decade, with no new classes of antihypertensive agents approved during that period.
There are multiple standard-of-care antihypertensive agents currently available, including angiotensin converting enzyme, or ACE, inhibitors and angiotensin receptor blockers, or ARBs, which are designed to reduce angiotensin activity but can also secondarily produce a lowering of aldosterone levels. Mineralocorticoid receptor antagonists, or MRAs, which block the effects of aldosterone at the mineralocorticoid receptor, typically cause aldosterone levels to rise, though they block the genomic effects triggered by aldosterone. Despite the widespread availability and use of these antihypertensive agents, many of which are available as generic products, each class of drugs currently available is associated with certain limitations, including limited efficacy, limited duration of aldosterone lowering, or significant side effects. Given both the importance of reducing aldosterone and the limitations of currently available therapies, we believe that baxdrostat has the potential to have a significant impact on the treatment paradigm for hypertension and other cardio-renal diseases.
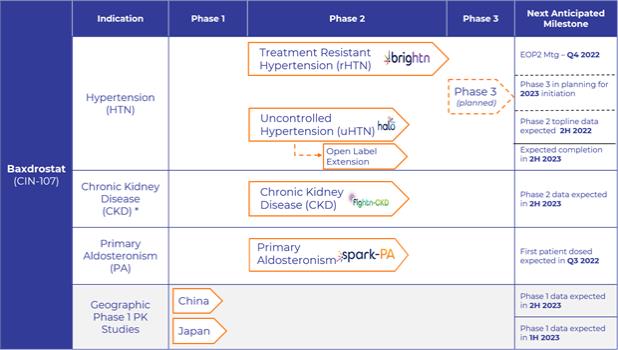
RECENT DEVELOPMENTS
Topline Results of BrigHtn Trial
Overview
Our BrigHtn trial was a Phase 2, randomized, double-blind, multicenter, placebo-controlled, dose-ranging clinical trial to evaluate the efficacy and safety of baxdrostat in patients with rHTN. Patients with rHTN were defined as being on a stable regimen of at least three antihypertensive agents at their maximally tolerated doses, one of which must be a diuretic, with a mean seated blood pressure, or BP, equal to or greater than 130/80 mm Hg. The primary endpoint was the change from baseline in mean seated systolic blood pressure, or SBP, after 12 weeks of treatment. The secondary objectives were to evaluate the change from baseline in mean seated DBP with each of the selected dose strengths of baxdrostat compared to placebo and to evaluate the percentage of patients achieving a seated BP response of less than 130/80 mm Hg with each of the dose strengths of baxdrostat compared to placebo after 12 weeks of treatment. A data monitoring committee, or DMC, determined that the trial met pre-specified statistical criteria of overwhelming efficacy at the highest dose, allowing completion of the trial with 275 patients randomized.
Summary of Results
BrigHtn was initiated in July 2020 in the United States. In the trial, 275 subjects were randomly assigned to one of the four treatment cohorts (0.5mg, 1 mg, 2 mg of baxdrostat or placebo once daily) and 248 patients completed the trial. Fifteen of the 27 discontinued patients withdrew due to consent withdrawal (n=7) or were lost to follow-up (n=8) or, with most occurring in late 2020 and early 2021 when we believe the Coronavirus Disease 2019 (COVID-19) pandemic situation may have interfered with patient retention.
TESAEs were reported in 10 patients and deemed by investigators to be unrelated to baxdrostat. These TESAEs included hyponatremia, hyperkalemia, cellulitis, urinary tract infection, dehydration, hyperglycemia, arthralgia, dizziness, syncope, acute kidney injury, nephrolithiasis, acute respiratory failure, and respiratory failure, with one patient discontinuing trial participation due to adverse events of intervertebral disc degeneration (n=1) and one patient experiencing a total of 6 SAEs, with 3 of these 6 SAEs — hyperglycemia, hyponatremia and hyperkalemia — leading to trial withdrawal. Both patient discontinuations were deemed by the investigator to be unrelated to study drug.
The demographics and baseline characteristics of patients in the four study arms were well balanced. Most of the participants were over age 60, overweight, or obese and had normal or mildly impaired renal functions. All but one patient was taking at least three antihypertensive medications at their maximally tolerated doses including a diuretic. In addition, over 90% of patients took an antihypertensive agent in the RAAS class.
2