Exhibit 99.6

Providing accurate and clinically actionable urologic solutions to inform patient diagnosis and treatment Annual Report 2021 BelgiumOfficeCAP Business Center Rue d’Abhooz 314040 HertsalUnited States Office and Laboratory 15279 Alton ParkwaySuite 100Irvine, CA 92618EuropeLaboratory NovioTech Campus Transistorweg 56534 AT Nijmegen

AboutMDxHealth is a commercial-stage precision diagnostics company that provides actionable molecular diagnostic information to personalize the diagnosis and treatment of prostate cancer and other urologic diseases. The Company’s tests are based on proprietary genetic, epigenetic and other complex molecular technologies.MDxHealth provides highly accurate and clinically actionable urologic solutions to inform patient diagnosis and treatment while improving healthcare economics for payers and providers.The Company’s US headquarters and laboratory operations are in Irvine, California.The European headquarters are located in Herstal, Belgium, with laboratory operations in Nijmegen, The Netherlands. MDxHealth is listed on the NASDAQ and Euronext Brussels stock exchange under the ticker symbol MDXH.Visit mdxhealth.com and follow us on social media at: twitter.com/mdxhealth, facebook.com/ mdxhealth and linkedin.com/company/mdxhealth.For more information: info@mdxhealth.com

Part I: Strategy & Business Review Contents
Message from the CEO4Part I: Business Review5Key figures6Share facts72021 Business review8Part II: Corporate Governance20Board of directors24Executive management32Internal Control and risk management35Shareholder information39Statutory auditor58Remuneration report59Part III: Principle Risks & Uncertainties72Part IV: Financial Statements108Consolidated financial statements109Auditor’s opinion159Condensed non-consolidated financial statements167 Annual Report 2021 3

Letter to shareholders Message from the CEODear Shareholders,Message from the CEODear Shareholders,The MDxHealth team continued to advance our growth initiatives in 2021 amidst another challenging year of effect from the global pandemic. While prostate cancer screenings, patient flow, and sales rep access were limited, we continue to make progress toward building a growth company as defined by consistent and sustainable revenue growth coupled with a clear and consistent focus on our business fundamentals.It is important to note the following developments for MDxHealth in 2021, all of which underscore and reflect the commitment we made to focus and execution, and will serve as the basis for our success and growth going forward:• Our menu of SelectMDx and ConfirmMDx continued to be adopted by our urology customer base in 2021, and while our volumes were impacted by the pandemic, our customer base remains intact, and we are confident that return of patient flow into the system will reflect this engagement.• On the commercial side, our sales team remained highly engaged with our focus on driving sustainable adoption of our clinical diagnostic pathway that provides actionable results for the diagnosis and treatment of prostate cancer.• We secured support for our growth initiatives from our Initial Public Offering in the U.S. as MDxHealth was listed on the NASDAQ exchange under the ticker MDXH. This offering included support from high quality U.S. and European investors as well as our reference shareholders, and provides us with a strong balance sheet to execute on our growth objectives.• Our SelectMDx test was issued a draft coverage decision. This is a significant development for MDXHealth as it signals coverage by Medicare in 2022 and, coupled with our inclusion in the gold standard National Comprehensive Cancer Network (NCCN) Guidelines, provides a leading indicator for continued growth in our adoption as well as growth in both revenue and gross margin.• In 2021, we introduced an exciting channel opportunity with the market introduction of Urinary Tract Infection, or UTI, testing services for the rapid and clinically actionable diagnosis of infections of the urinary tract. We are confident that this is the first of potential growth opportunities we can explore that are consistent with our sales channel and focus, and will contribute to growth going forward.• In addition, as we communicated in 2020, we intend to focus on expansion of our menu into active surveillance testing for prostate cancer patients. We are confident and believe that our menu as conceived will allow MDxHealth to be the only company in the space to take a patient from positive screen all the way through the diagnostic and therapeutic pathway of prostate cancer.Each of these, and all of these collectively, point to a restructured and focused business with a fact-based and evidence-based approach to our focus, investment of resources, and commitment to excellence. As I emphasized last year, the one factor that has not changed is our people. They have all risen to the challenge and are committed to delivering on our promise and positive path forward.We remain confident in the potential of our unique menu to provide urologists with a clear clinical pathway to accurately identify high-grade prostate cancer and challenging complex infections while improving healthcare economics. We believe this clinical pathway, with SelectMDx guiding cancer detection in a pre-biopsy setting and ConfirmMDx in a post-biopsy setting, will continue to drive momentum and market share.I would like to close by thanking our shareholders and employees for your continued support and restating our unwavering commitment to operating discipline and delivering value to all our stakeholders including patients, customers, employees and shareholders.Respectfully,Belgium, 20 April, 2022Michael K. McGarrityChief Executive Officer Annual Report 2021 4

Business Review Annual Report 2021 5
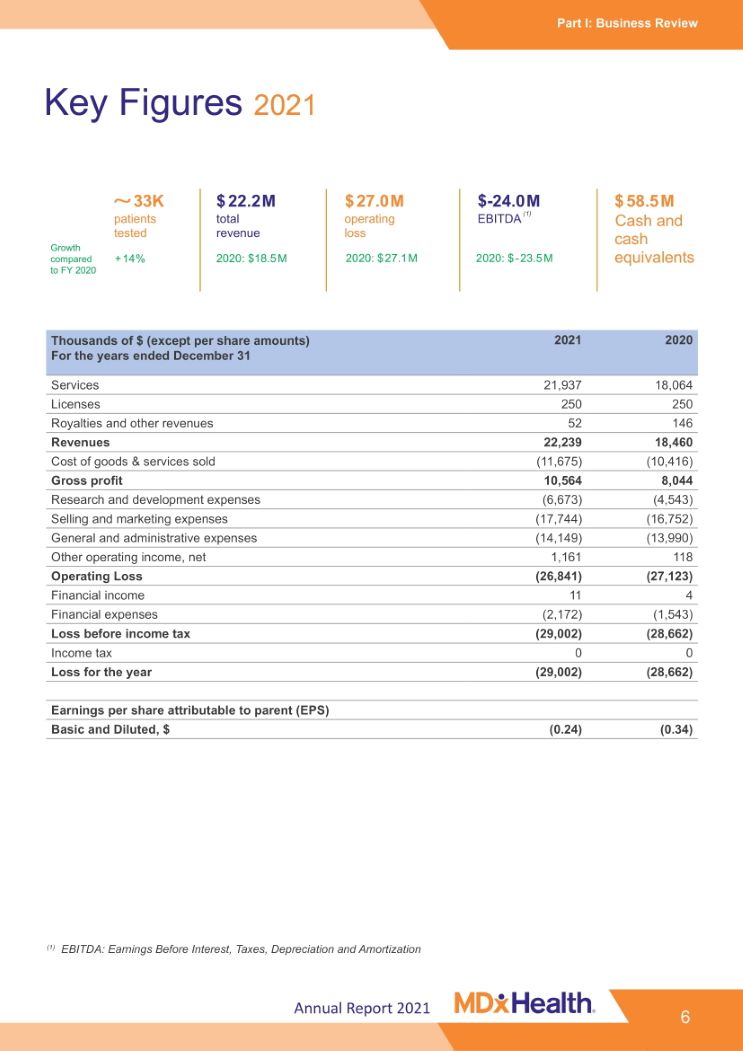
Part I: Business Review Key Figures 2021 33K $ 22.2 M $ 27.0 M $-24.0 M $ 58.5 M patients total operating EBITDA (1) Cash and tested + 14% revenue 2020: $18.5 M loss 2020: $ 27.1 M 2020: $ - 23.5 M cash equivalents Growth compared to FY 2020 Thousands of $ (except per share amounts) For the years ended December 31 2021 2020 Services 21,937 18,064 Licenses 250 250 Royalties and other revenues 52 146 Revenues 22,239 18,460 Cost of goods & services sold (11,675) (10,416) Gross profit 10,564 8,044 Research and development expenses (6,673) (4,543) Selling and marketing expenses (17,744) (16,752) General and administrative expenses (14,149) (13,990) Other operating income, net 1,161 118 Operating Loss (26,841) (27,123) Financial income 11 4 Financial expenses (2,172) (1,543) Loss before income tax (29,002) (28,662) Income tax 0 0 Loss for the year (29,002) (28,662) Earnings per share attributable to parent (EPS) Basic and Diluted, $ (0.24) (0.34) (1) EBITDA: Earnings Before Interest, Taxes, Depreciation and Amortization Annual Report 2021 6
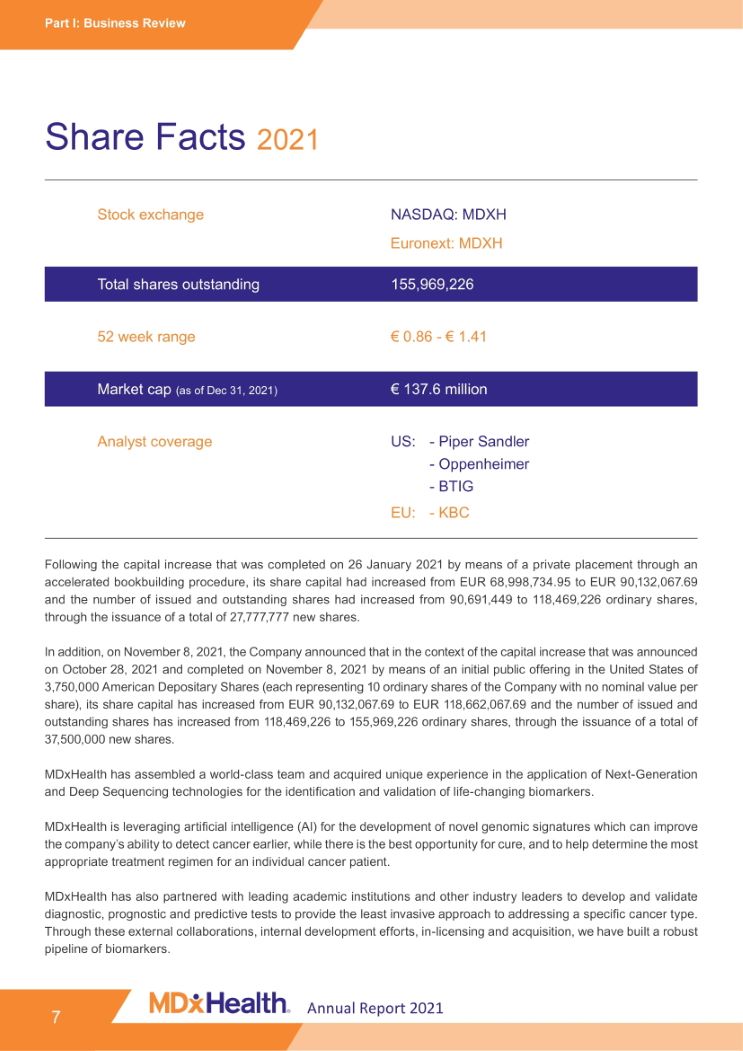
Part I: Business Review Share Facts 2021 Stock exchange NASDAQ: MDXH Euronext: MDXH Total shares outstanding 155,969,226 52 week range € 0.86 - € 1.41 Market cap (as of Dec 31, 2021) € 137.6 million Analyst coverage US: - Piper Sandler Oppenheimer BTIG EU: - KBC Following the capital increase that was completed on 26 January 2021 by means of a private placement through an accelerated bookbuilding procedure, its share capital had increased from EUR 68,998,734.95 to EUR 90,132,067.69 and the number of issued and outstanding shares had increased from 90,691,449 to 118,469,226 ordinary shares, through the issuance of a total of 27,777,777 new shares. In addition, on November 8, 2021, the Company announced that in the context of the capital increase that was announced on October 28, 2021 and completed on November 8, 2021 by means of an initial public offering in the United States of 3,750,000 American Depositary Shares (each representing 10 ordinary shares of the Company with no nominal value per share), its share capital has increased from EUR 90,132,067.69 to EUR 118,662,067.69 and the number of issued and outstanding shares has increased from 118,469,226 to 155,969,226 ordinary shares, through the issuance of a total of 37,500,000 new shares. MDxHealth has assembled a world-class team and acquired unique experience in the application of Next-Generation and Deep Sequencing technologies for the identification and validation of life-changing biomarkers. MDxHealth is leveraging artificial intelligence (AI) for the development of novel genomic signatures which can improve the company’s ability to detect cancer earlier, while there is the best opportunity for cure, and to help determine the most appropriate treatment regimen for an individual cancer patient. MDxHealth has also partnered with leading academic institutions and other industry leaders to develop and validate diagnostic, prognostic and predictive tests to provide the least invasive approach to addressing a specific cancer type. Through these external collaborations, internal development efforts, in-licensing and acquisition, we have built a robust pipeline of biomarkers. Annual Report 2021 7;

Part I: Business Review Annual Report 2021 Business Highlights 2021 Overview 2021 2021 Business Review While 2021 presented continued headwinds related to the pandemic and its continued effect on patient flow for our particular patient population in prostate cancer, we have made significant progress and we believe our results provide evidence that MDxHealth will emerge from this challenging period with strong and sustainable growth. We believe this progress is evidenced by the following: Publication of draft foundational Local Coverage Determination (LCD) for Biomarkers to Stratify Patients at Increased Risk for Prostate Cancer by Palmetto GBA under its MolDx program, which cites evidence of the clinical utility of SelectMDx® and, when finalized, is expected to support coverage for qualified Medicare patients throughout the United States; Improvement in cash collections and capital allocation, driven by continued focus on operating discipline; Advancement of development programs to expand our prostate cancer menu into Active Surveillance; and Introduction of novel Urinary Tract Infection (UTI) testing services into our urology channel which we are confident will contribute to our total revenue in 2022. Highlights for the year ended December 31, 2021 Revenues increased by 20% to $22.2 million versus $18.5 million for 2020 Billable test volume for ConfirmMDx® and SelectMDx increased by 3% to 15,324 and 13,615, for 2021, respectively, versus 14,945 and 13,201, respectively, for 2020 Successfully completed initial public offering (IPO) in the United States on the NASDAQ, raising gross proceeds of $45 million Cash balance as of December 31, 2021 of $58.5 million MDxHealth Business Overview We are a commercial-stage precision diagnostics company committed to providing non-invasive, clinically actionable and cost-effective urologic solutions to improve patient care. Our novel prostate cancer genomic testing solutions, SelectMDx and ConfirmMDx, provide physicians with a clear clinical pathway to accurately identify clinically significant prostate cancer while minimizing the use of invasive procedures that are prone to complications. Our unique approach combines advanced clinical modeling with genomic data to provide each patient with a personalized cancer risk profile, which provides more accurate and actionable information than standard risk factors (e.g., PSA, DRE, age) used by clinicians. Our lead products address men at risk for developing prostate cancer. In addition, we are actively developing testing solutions to help with the management of men diagnosed with prostate cancer, with the goal to provide our clients with a menu of tools spanning the continuum of prostate cancer diagnosis and care. Our team’s collective decades of experience in precision diagnostics and our portfolio of novel biomarkers for diagnostic, prognostic and predictive molecular assays supports our active pipeline of new testing solutions for prostate and other urologic diseases. 8

Part I: Strategy & Business Review Annual Report 2021
Prostate cancer is presently the most common, and second deadliest, form of cancer in men. The broad adoption of PSA testing in the 1980s created a paradigm shift in men’s health, reducing the incidence of metastatic prostate cancers by more than 50%. However, widespread PSA testing also significantly increased the pool of symptomatic men, resulting in overdiagnosis, overtreatment, serious complications, and potential anxiety — triggering a retreat from standardized PSA screening — culminating with the U.S. Preventative Services Task Force’s (“USPSTF’s”) decision to recommend against all PSA screening in 2012. Following recommendations from clinicians and patient advocates together with building evidence of an uptick in metastatic prostate cancer incidence, the USPSTF softened its position in 2017, upgrading PSA screening for middle aged men. However, the USPSTF’s reversal left unresolved the clinical dilemma posed by the estimated pool of over ten million men living with an elevated PSA in the United States. Approximately 25 million PSA tests are performed each year, and over 15% of these reveal heightened PSA levels — leading to an estimated pool of over three million undiagnosed men informed each year of their heightened risk for prostate cancer based on elevated PSA test results and/or negative biopsy results. Other than repeated invasive needle biopsy procedures, these symptomatic men and their clinicians have limited tools to manage their cancer risk. Our core testing solutions directly address this challenge. Since the commercial launch of ConfirmMDx in 2012 and SelectMDx in 2016, we have performed over 200,000 tests ordered by more than 1,000 practicing urologists in the United States. SelectMDx for Prostate Cancer (a liquid biopsy test for men being considered for their first prostate biopsy) and ConfirmMDx for Prostate Cancer (an epigenetic test for men post-prostate biopsy), are designed to (i) improve the early detection of clinically significant prostate cancer in at-risk men and (ii) reduce the unnecessary costs and patient anxiety associated with the diagnosis and treatment of the disease. Both tests have been included in the NCCN Guideline for the Early Prostate Cancer Detection. Both tests have also successfully completed formal technical assessment review for Medicare reimbursement and have received either a final or draft local coverage determination. Building from the foundation of our complementary marketed products, we are committed to sustained growth, with our core management principles defined by a commitment to focus, commercial execution and operating discipline throughout our organization. While MDxHealth is domiciled and listed as a public company in Belgium, our primary commercial focus is in the United States, where over 95% of our tests are performed and revenues are generated. Our leadership change in 2019 and coincident organizational and operational discipline implemented throughout the MDxHealth group of companies has further focused our commitment to U.S.-sourced growth, with our entire executive management team and over 90% of staff based in or reporting to our U.S. laboratory and headquarters in Irvine, California. We have established a systematic approach to commercializing our precision diagnostic solutions in our target markets in the United States, focusing on active engagement, education and market development directed toward health care professionals and their patients. Our commercial team is focused on prioritizing large and high-volume community urology centers, and on building long-standing relationships with key physicians and practice groups who have strong connections to the population of men who may be eligible for our solutions. Our ultimate goal is to support physicians using our tests through all aspects of the patient’s journey, starting from initial diagnosis through to advanced prostate cancer management. We also seek to build on our long-term partnerships with key opinion leaders (“KOLs”) and patient associations that are oriented towards the needs of our patients and customers. Our sales and marketing organization is focused on building physician awareness of the clinical and economic benefits provided by ConfirmMDx and SelectMDx through education of urologists and their clinical staff as well as pathology and laboratory staff, targeted KOL development and training, and development of tools for our customers to interact with patients and consumers (doctor-to-consumer education). Our Product Portfolio Our core commercial tests address a substantial unmet clinical need in the prostate cancer diagnostic and treatment pathway. According to the American Cancer Society, prostate cancer is the most common, and second deadliest, form of cancer in males in the United States. Prior to the emergence of precision diagnostic solutions, existing 9

Part I: Strategy & Business Review Annual Report 2021
diagnostic tests were critically flawed, with high false negatives and false positives, leading to costly and invasive diagnostic protocols and attendant complications. Approximately 25 million PSA tests are performed each year, and over 15% of those reveal heightened levels of PSA. An elevated PSA level can be caused by many different sources, the majority of which are not cancer. Current clinical guidelines suggest that men with an elevated PSA should be considered for a prostate biopsy, so that a pathologist can visually inspect the sampled tissue to identify any sign of malignancy. However, 60% of biopsies are negative, not revealing any cancer, and as many as a third of these negative biopsies are false negatives, providing limited comfort to patients and their physicians that cancer was not missed. The relatively modest sensitivity and specificity of these current standard-of-care tests and procedures has led to increased patient anxiety, potentially unnecessary, invasive and costly interventions, and increased complications and hospitalizations. Upon a determination that a patient’s PSA level is elevated or an abnormal digital rectal exam result, our SelectMDx test — which is a noninvasive urine test with 95% NPV — can be used to help physicians determine whether a costly, painful and complication-prone needle-core biopsy is advisable. For those men who proceed to a biopsy procedure, our ConfirmMDx test — which measures biomarker signals in the same biopsied tissue examined by the pathologist — provides additional information to physicians and increases the accuracy of the biopsy, with a 96% NPV. Improving the decision for initial prostate biopsy Improving the decision for repeat prostate biopsy Our Competitive Strengths Routine Cancer Screening Positive Biopsy / Imaging Negative No Cancer* We believe we have the following competitive strengths which underpin our commercial execution success and will position us for sustainable growth: • Targeted Menu Improving Prostate Cancer Diagnosis and Treatment. We offer a menu of tests that provide clinically actionable results for at-risk men who may or may not have prostate cancer. Collectively, SelectMDx and ConfirmMDx provide urologists with a clear clinical pathway to accurately identify clinically significant prostate cancer while minimizing the use of invasive procedures, improving health outcomes and significantly lowering costs to the healthcare system. Strong Commercial Focus and Presence. We aim to increase adoption of our two commercial tests by leveraging our direct sales force in the United States to continue to market and sell to our urology-focused network. We have significant experience in building effective commercial teams consisting of sales reps, strategic account managers, and clinical liaisons led by a management team with a track record of success. In addition, our payor and reimbursement, revenue cycle management and client services groups provide expert support for our field sales team as well as our patients and customer base. We believe we can leverage these groups to explore additional opportunities for growth based on this commercial channel. Outside the United States we will continue to evaluate distribution partners to drive adoption in markets where our menu is best suited. 10

Part I: Business Review Annual Report 2021• Commercial Channel Advantage. Building from the launch of our first commercial test in 2012, we have established MDxHealth as an industry leader in precision diagnostics for early prostate cancer detection. We intend to take advantage of our client relationships — urologists, pathologists, physicians assistants, nurses, office administrators — to support menu expansion and additional growth opportunities as appropriate and within our focus. • Compelling Reimbursement Strategy. Adoption of our ConfirmMDx test has been supported by its LCD issued via the Palmetto GBA-administered MolDX Program in 2014, its inclusion in the NCCN guidelines in 2016 and the European Association of Urology (EAU) guidelines in 2018, as well as consistent expansion of coverage by commercial payors. We expect our SelectMDx test to follow the same progress path of payor coverage by both Medicare and commercial payors, based on inclusion of the test in the NCCN guidelines in 2020. There is no guarantee that SelectMDx will receive a final LCD and there can be no assurance that Medicare coverage and reimbursement will be granted or, if granted, that it will be maintained. • Robust and Reliable Technology. We possess a proprietary intellectual property (“IP”) portfolio capable of advancing our diagnostic pathway in prostate cancer as well as high quality laboratory operations, including our CAP accredited, CLIA certified and New York State Department of Health (“NYSDOH”) approved molecular laboratory facility located at our U.S. headquarters in Irvine, California. We also have an extensive library of biomarkers which can be applied in additional urology and men’s health diagnostics. • Proven Leadership with Industry Expertise. Our management team members have proven track records of execution and value creation across medical devices, diagnostics and biotech. We believe we have built a culture of performance, responsibility and accountability — from research and development, to sales and marketing, and operations and management, we are committed to building value for all of our stakeholders, including patients, customers, employees and shareholders. Our Strategy Our ultimate goal with our core testing solutions is to take a prostate cancer patient from positive screen all the way through the diagnostic and therapeutic pathway of prostate cancer. As such, we are focused on continuing to drive adoption of our SelectMDx and ConfirmMDx tests and expand our product offerings. The key elements of our strategy include: • Physician and Patient Education. One important component of our efforts to successfully penetrate the urology market and promote clinical adoption of our SelectMDx and ConfirmMDx tests is to drive awareness of these tests. We educate physicians and patients through a variety of channels including by supporting clinical studies for the publication of peer reviewed journals and abstracts at key scientific conferences, forging relationships with the leading medical and scientific opinion leaders in urology, developing strategic partnerships with leading pathology laboratories with large urology client bases and via public relations and advertising campaigns. Expand Test Menu. We intend to build on our leadership in the prostate cancer diagnostic space by expanding our menu of tests beyond SelectMDx and ConfirmMDx. We are currently developing additional products for the prostate cancer diagnostic and treatment pathway. Not all men diagnosed with localized prostate cancer benefit from intervention as some tumors are slow and non-life threatening. Our AS-MDx product candidate is intended to risk-stratify patients who may benefit from immediate intervention versus active surveillance. Patients under active surveillance are currently monitored by invasive and costly prostate biopsies. Our MonitorMDx product candidate is intended to be a non-invasive alternative that risk stratifies patients for continued active surveillance versus intervention, which may also improve patient compliance with active surveillance protocols. 11

Part I: Business Review Annual Report 2021• Expand Reimbursement. An important component of our commercial strategy is to expand reimbursement for our SelectMDx and ConfirmMDx tests. Our ConfirmMDx test has been covered by a Medicare MoIDX LCD since 2014. Although our SelectMDx test is not currently covered by Medicare, in May 2021 a draft foundational LCD supporting the clinical utility of this test was issued by the MoIDX Program which, if finalized, is expected to support Medicare coverage of both SelectMDx and ConfirmMDx for qualified Medicare patients throughout the United States. There is no guarantee that SelectMDx will receive a final LCD and there can be no assurance that Medicare coverage and reimbursement will be granted or, if granted, that it will be maintained. Our managed care team continues to pursue adoption of positive coverage and reimbursement policies and contracts by other payors. We believe the clinical utility and actionability of our ConfirmMDx and SelectMDx tests, combined with our experience and knowledge of the complex coverage and reimbursement landscape in the United States, will enable us to expand coverage and reimbursement of ConfirmMDx and SelectMDx among the commercial payor market. We continue to build upon our successful strategy, supported by governmental and commercial coverage policies, as a foundation to secure additional contracts from major payors. Market Opportunity Among U.S. males, prostate cancer is the most diagnosed cancer and the second leading cause of cancer death. According to the American Cancer Society, in 2021, over 260,000 men are expected to be diagnosed with prostate cancer in the United States, with more than 34,000 dying from the disease. There are currently significant challenges with diagnosing prostate cancer in the United States. Approximately 25 million PSA tests are performed each year, and over 15% of those reveal heightened levels of PSA. Current clinical guidelines suggest that men with an elevated PSA should be considered for a prostate biopsy, so that a pathologist can visually inspect the sampled tissue to identify any sign of malignancy. However, 60% of biopsies are negative, not revealing any cancer, and as many as a third of these negative biopsies are false negatives, providing limited comfort to patients and their physicians that cancer was not missed. The relatively modest sensitivity and specificity of these current standard-of-care tests and procedures has led to increased patient anxiety, potentially unnecessary, invasive and costly interventions, and increased complications and hospitalizations. Our suite of commercial products addresses these issues, presenting a substantial market opportunity. Based on the estimated 3 million men annually that demonstrate an elevated PSA level, and assuming average revenue per test of $500, management estimates the addressable market in the United States for the SelectMDx test at approximately $1.5 billion. Based on the estimated 300,000 men annually that receive a negative biopsy result, and assuming average revenue per test of $1,600, management estimates the addressable market in the United States for the ConfirmMDx test at approximately $500 million. Prostate Cancer is the most common cancer in American men. Who needs a biopsy? Who needs a repeat biopsy? Who needs treatment? 25M PSA tests performed 3M Demonstrate elevated PSA 500K Prostate biopsies performed 200K New prostate cancer cases 33K Est. deaths >10% of tests are elevated Disposition of elevated PSA ~60% of biopsies are negative Active surveillance or treatment 3M men annually eligible for USD 1,5B market opportunity 300K men annually eligible for USD 500M market opportunity 12

Part I: Business Review Annual Report 2021 Commercial Products SelectMDx for Prostate Cancer liquid biopsy assay The current standard for prostate cancer screening is the PSA blood test. Unfortunately, the PSA is not specific to clinically significant prostate cancer — it is more of an indicator of prostate health. There are many factors such as benign prostatic hyperplasia (“BPH”), inflammation, prostatitis and a naturally occurring enlarged prostate that can cause an elevated PSA. In men with an elevated PSA level between 3-10 ng/mL, only 25-40% of biopsies reveal cancer — and the majority of these identified cancers are indolent. Also, following a prostate biopsy procedure, around 18% of men suffer complications (blood in urine) and around 3% are hospitalized for infection (sepsis). SelectMDx helps physicians determine if a patient is at higher or lower risk for prostate cancer and which men can safely avoid biopsy. SelectMDx is a non-invasive urine test that measures the expression of two mRNA cancer-related biomarkers (HOXC6 and DLX1). The test provides binary results that, when combined with the patient’s clinical risk factors, help the physician determine whether: • The patient may benefit from a biopsy and early prostate cancer detection; or • The patient can avoid a biopsy and return to routine screening. Men identified by the test as having a high likelihood of clinically significant cancer can, upon biopsy, be diagnosed and treated sooner, while men identified at very low risk may avoid biopsy. The following chart depicts the functioning of the SelectMDx test: Guidelines Inclusion SelectMDx has been included in the NCCN Prostate Cancer Early Detection guidelines since 2020. NCCN is a non- profit alliance of the 31 leading cancer centers in the United States. SelectMDx has also been included in the European Association of Urology (EAU) Prostate Cancer guidelines since 2018. Clinical Validation Studies The use of SelectMDx as a predictive test to identify men at low risk for aggressive prostate cancer has been well validated in both scientific and clinical studies. Results from the clinical validation study for SelectMDx confirmed its superior performance compared to other commonly used biomarker tests and risk calculators. The test’s NPV of 95% in the validation study means that if the test identifies a very low risk, the physician and patient can be 95% sure that a subsequent biopsy will not detect Gleason score ≥7 prostate cancer, information that may provide a level of confidence needed to avoid a biopsy. The test has a very high predictive accuracy (AUC 0.85) for high-grade prostate cancer, which is significantly better than the PCPT risk calculator version 2. 13

Part I: Business Review Annual Report 2021 There are twelve published studies assessing the SelectMDx test and which together demonstrate its analytical validity, clinical validity, clinical utility and positive health economic outcomes. These studies, all of which have been published in peer-reviewed publications, evaluated more than 4,500 patients in the aggregate. The following is a summary that highlights key findings from some of these studies. • Analytical validity. A study published in 2017 illustrated, in an independent laboratory, the performance characteristics and robustness of the SelectMDx mRNA assay, covering all aspects of analytical method validation including assay sensitivity, specificity, linearity, precision, repeatability and reproducibility using pre-specified acceptance criteria. • Clinical validity. In a study published in 2019, the SelectMDx test demonstrated an NPV of 95%. Urine samples were collected from 1,955 men from The Netherlands, France and Germany prior to an initial prostate biopsy. SelectMDx molecular biomarker results were combined with other risk factors in a clinical model optimized to detect International Society of Urological Pathology Grade Group 2 or greater prostate cancer in men. Results in the validation cohort were compared with the independent PCPT risk calculator version 2. The full validation cohort of 916 men including all prostate specific antigen levels yielded an AUC of 0.85 with 93% sensitivity, 47% specificity and 95% negative predictive value. The Prostate Cancer Prevention Trial Risk Calculator (“PCPTRC”) AUC was 0.76. In the 715-patient validation cohort, limited to subjects with PSA less than 10 ng/ml, the AUC was 0.82 with 89% sensitivity, 53% specificity and 95% negative predictive value. The PCPTRC AUC was 0.70. • Clinical utility. In a 2019 study, SelectMDx had a significant impact on initial prostate biopsy decision-making in a U.S. community urology setting. Biopsy rates in SelectMDx positive men were 5-fold higher than in SelectMDx negatives. • Health economic outcomes. A 2018 study demonstrated that routine use of the SelectMDx test to guide biopsy decision making improved health outcomes and significantly lowered costs in American men at risk for prostate cancer. Compared to the current standard of care, SelectMDx implementation would result in an average of 0.045 quality-adjusted life years (“QALYs”) gained at a cost savings of $1,694 per patient. Assuming approximately 300,000 men are biopsied each year, this translates to an incremental 14,000 QALYs gained at cost savings of $500,000 annually. : Robust Clinical Evidence 12 published studies on genes and technology (>3.500 patients) Analytical Validity Clinical validity Clinical Utility Health Economics Pivotal Clinical Studies Analytical validation Hessels et al. BMC Urology 2013 Clinically validated for a 95% NPV >$500M in savings to health care system Hease et al. Significantly impact prostate biopsy decision making Shore et al. Urology Practice 2019 Journal of Urology 2019 Govers et al. Journal of Urology 2018 14

Part I: Business Review Annual Report 2021 ConfirmMDx for Prostate Cancer epigenetic assay Approximately 30% of men with a cancer-negative prostate biopsy actually have cancer. Prostate cancer is difficult to diagnose because it is both heterogenous and multi-focal. The standard of care for diagnosing prostate cancer is a transrectal ultrasound guided biopsy. However, this procedure samples less than 1% of the entire gland, leaving men at risk for undetected prostate cancer. ConfirmMDx is a well-validated epigenetic test that guides the detection of occult prostate cancer on a patient’s previously biopsied negative tissue. The test can help urologists determine a man’s risk for harboring clinically significant prostate cancer despite having a cancer-negative biopsy result, and it has a number of unique features/advantages. For patients with an initial negative biopsy, few options are currently available to guide a urologist in determining whether or when an additional biopsy procedure is warranted. Fear of occult (hidden) prostate cancer leads to additional procedures, leading many men to receive multiple follow-up biopsy procedures to rule out the presence of cancer. The ConfirmMDx test addresses prostate biopsy sampling concerns, helping urologists to: • “Rule-out” men from undergoing potentially unnecessary repeat biopsies and screening procedures, helping to reduce complications, patient anxiety and excessive healthcare expenses associated with these procedures; and • “Rule-in” high-risk men with a previous negative biopsy result who may be harboring undetected cancer (false negative biopsy result) and therefore may benefit from a repeat biopsy and potentially treatment. For men with a negative biopsy, independently published clinical studies have shown that the ConfirmMDx test is the most significant, independent predictor of prostate biopsy outcomes relative to other available clinical factors such as age, PSA and DRE results. Incorporating ConfirmMDx into clinical practice can reduce the number of unnecessary repeat biopsies, yielding clinical and economic value for healthcare providers, patients and payors. ConfirmMDx can aid urologists with patient management decisions regarding the need for follow-up testing and procedures with the identification of low-risk patients testing negative for DNA hypermethylation. The use of ConfirmMDx for prostate cancer detection using methylation-specific PCR (MSP) and cancer-associated epigenetic biomarkers to improve upon histopathology has been well validated in both scientific and clinical studies. DNA methylation, the most common and useful measure of epigenetic abnormality testing, is responsible for the silencing of key tumor suppressor genes. DNA methylation biomarkers associated with prostate cancer have been extensively evaluated. GSTP1 is a widely studied and reported epigenetic biomarker associated with prostate cancer diagnosis, encoding the glutathione S-transferase Pi 1 (GSTP1) protein involved in detoxification, due to its high sensitivity and specificity. Complementing GSTP1, methylation of the APC and RASSF1 genes is frequently found in prostate cancer, and these markers have demonstrated a “field effect” aiding in the identification of biopsies with false-negative histopathological results. The epigenetic field effect is a molecular mechanism whereby cells adjacent to cancer foci can contain DNA methylation changes, which may be indistinguishable by histopathology, but detectable by MSP testing. The presence of epigenetic field effects associated with prostate cancer has been widely published and is the basis of activity for the ConfirmMDx assay to aid in the detection of occult prostate cancer on previously biopsied, histopathologically negative tissue. 15
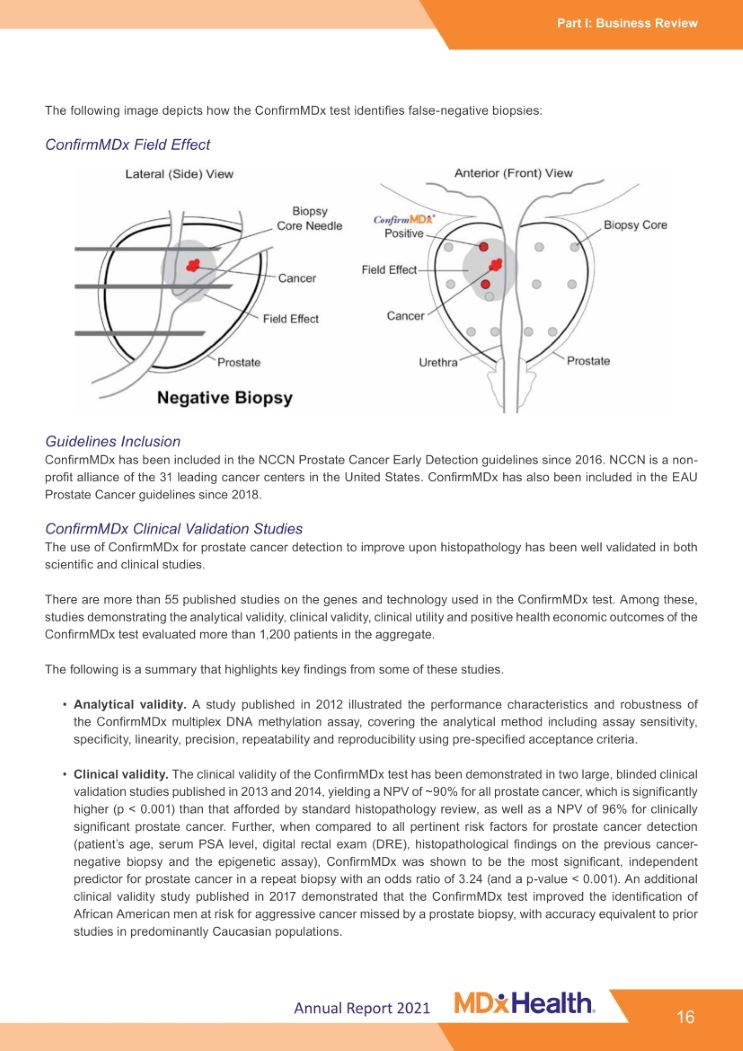
Part I: Business Review Annual Report 2021 The following image depicts how the ConfirmMDx test identifies false-negative biopsies: ConfirmMDx Field Effect Guidelines Inclusion ConfirmMDx has been included in the NCCN Prostate Cancer Early Detection guidelines since 2016. NCCN is a non- profit alliance of the 31 leading cancer centers in the United States. ConfirmMDx has also been included in the EAU Prostate Cancer guidelines since 2018. ConfirmMDx Clinical Validation Studies The use of ConfirmMDx for prostate cancer detection to improve upon histopathology has been well validated in both scientific and clinical studies. There are more than 55 published studies on the genes and technology used in the ConfirmMDx test. Among these, studies demonstrating the analytical validity, clinical validity, clinical utility and positive health economic outcomes of the ConfirmMDx test evaluated more than 1,200 patients in the aggregate. The following is a summary that highlights key findings from some of these studies. • Analytical validity. A study published in 2012 illustrated the performance characteristics and robustness of the ConfirmMDx multiplex DNA methylation assay, covering the analytical method including assay sensitivity, specificity, linearity, precision, repeatability and reproducibility using pre-specified acceptance criteria. • Clinical validity. The clinical validity of the ConfirmMDx test has been demonstrated in two large, blinded clinical validation studies published in 2013 and 2014, yielding a NPV of ~90% for all prostate cancer, which is significantly higher (p < 0.001) than that afforded by standard histopathology review, as well as a NPV of 96% for clinically significant prostate cancer. Further, when compared to all pertinent risk factors for prostate cancer detection (patient’s age, serum PSA level, digital rectal exam (DRE), histopathological findings on the previous cancer- negative biopsy and the epigenetic assay), ConfirmMDx was shown to be the most significant, independent predictor for prostate cancer in a repeat biopsy with an odds ratio of 3.24 (and a p-value < 0.001). An additional clinical validity study published in 2017 demonstrated that the ConfirmMDx test improved the identification of African American men at risk for aggressive cancer missed by a prostate biopsy, with accuracy equivalent to prior studies in predominantly Caucasian populations. 16

Part I: Business Review Annual Report 2021 • Clinical utility. A 2014 study reported on the real-world use of the ConfirmMDx assay, demonstrating that the test impacts physician behavior. A very low rate of repeat biopsies (4.4%) was observed in the ConfirmMDx negative men, as compared to the expected 43% rate of repeat biopsy reported in a large population-based randomized trial sponsored by the National Cancer Institute. • Health economic outcomes. In a study published in 2013, a budget impact model developed to evaluate the effect of the ConfirmMDx assay on healthcare spending demonstrated significant potential healthcare savings associated with the reduction of repeat biopsies and complications avoided. Under the study’s model, utilization of ConfirmMDx would bring approximately $500,000 in annual savings per 1 million covered patients. Addressing other urological unmet clinical needs Urinary tract infections (“UTIs”) account for over 10 million clinic and ER visits every year. Up to 30% of UTIs are polymicrobial, driven by biofilm-producing bacteria. Traditional culture-only testing leaves clinicians with no more than a “mixed flora” result, and ultimately empirical treatment. We seek to address this unmet clinical need with a non-invasive urine test that identifies and quantifies infectious bacteria and their antibiotics susceptibility to help ensure patients receive the correct diagnosis and treatment as quickly as possible. Our UTI solution is intended for prompt sample-to-answer results, combining molecular testing to identify and quantify each microbe with culture-based testing to look for in-vitro susceptibility. Our goal is to help pinpoint not only the offending organisms, regardless of how many are identified, but also the antibiotics capable of clearing the entire infection. We estimate the addressable market in the United States for UTI testing at approximately 2 million men annually, or $1 billion. Pipeline We intend to build on our leadership in the urologic diagnostic space by expanding our menu of tests beyond SelectMDx and ConfirmMDx. We are currently developing two additional products for the prostate cancer diagnostic and treatment pathway. Not all men diagnosed with localized prostate cancer benefit from intervention as some tumors are slow growing and non-life threatening. Our AS-MDx solution is intended to risk-stratify patients who may benefit Analytical validation Van Neste et al. BMC Urology 2013 Meta analysis validatinh High NPV Partin et al. Trans, of the Am. Clin. And Clim. Assoc 2016 Validated in African American men Waterhouse et al. Urology 2016 Savings to health care system Aubry et al. Armican Health Drug and Benefits 2013 : Robust Clinical Evidence Over 55 published studies on genes and technology Analytical Validity Clinical validity Clinical Utility Health Economics Pivotal Clinical Studies Validation of high NPV Partin et al. Journal of Urology 2014 Risk score development NPV 96% CS PCa Van Neste et al. The Prostate 2016 Validation of clinical utility/actionability Wojno., et al., 2014 17

Part I: Business Review Annual Report 2021 from immediate intervention versus active surveillance. Patients under active surveillance are currently monitored by invasive and costly prostate biopsies. Our Monitor-MDx solution is being developed as a non-invasive alternative that risk stratifies patients for continued active surveillance versus intervention, which may also improve patient compliance with active surveillance protocols. We estimate the addressable market in the United States for the AS-MDx indication at approximately 134,000 men annually, or $134 million, and for the Monitor-MDx indication at approximately 1.5 million men annually, or $1.5 billion. Active Surveillance (AS-MDx) for men with localized Prostate Cancer In the United States, prostate cancer is the most frequently diagnosed cancer among men, with an estimated 268,490 new cases and 34,500 deaths in 2021. Despite its high prevalence, the five-year survival rate for men diagnosed with localized prostate cancer is nearly 100%. Definitive treatment by surgery or radiation carries significant risk of co- morbidities, and Active Surveillance (“AS”) in lieu of treatment has been increasingly adopted as an alternate care plan for patients with low-risk prostate cancer. To aid in the identification of candidates for AS, the NCCN Guidelines defines a series of Risk Groups based on clinical factors and pathologic features. However, prostate cancer is a heterogeneous disease with varying potential to progress to lethal forms, and clinical information available at the time of diagnosis may not provide an accurate assessment of the extent of the disease and/or its aggressiveness for all patients. There is an unmet need for better patient risk stratification in order to optimize disease management. Our AS-MDx candidate is intended to improve the risk-stratification of men diagnosed with localized prostate cancer who are being considered for Active Surveillance in lieu of immediate treatment. AS-MDx is a nucleic acid amplification assay intended to provide clinically actionable information via a direct, cost-effective approach. MonitorMDx for Men being considered for a Surveillance Biopsy Men on Active Surveillance are monitored using PSA, MRI and periodic biopsies to determine if the prostate cancer has progressed and whether definitive treatment is appropriate. We are actively analyzing urine and blood biomarker panels with the goal of developing a non-invasive test for monitoring these patients. If MonitorMDx could allow physicians to forego or delay surveillance biopsy, the test would represent a significant business opportunity with little or no direct competition. If these projects are successful, MDxHealth would have a full offering of biomarker-based prostate cancer tests from early detection to treatment and management. SelectMDx and ConfirmMDx would help determine which patients should (or should not) undergo a prostate biopsy. In the post-biopsy setting, AS-MDx and MonitorMDx would provide methods to identify and monitor patients who could choose Active Surveillance as a treatment option. The figure below shows how AS-MDx and MonitorMDx would fit in the current standard of care pathways for management of men with localized prostate cancer. AS-MDx MonitorMDx Patient diagnosed with localized prostate cancer Does the patient have potentially lethal Pca, or is he a candidate for Active Surveillance? AS-MDx Interventional treatment (e.g. surgery, radiation) Active Surveillance Has the cancer progressed to high-risk disease? Would the patient benefit from intervention? MonitorMDx Interventional treatment (e.g. surgery, radiation) Continue Active Surveillance Current SOC: risk stratification - Bx pathology - Clinical risk factors - mpMRI - Biomarker tests - Other patient-specific info Current SOC: monitor for disease progression - Periodic repeat biopsy(ies) - Rising PSA, changes to other clinical risk factors - mpMRI - Biomarker tests 18

Part II: Corporate Governance Annual Report 2021 19

Part II: Corporate Governance This section summarizes the main rules and principles of MDxHealth’s Corporate Governance Charter. The complete Corporate Governance Charter is available on the MDxHealth website, at http://www.mdxhealth.com/shareholder-information Introduction This Corporate Governance Statement is included in the Company's report of the Board of Directors on the statutory accounts for the financial year ended on 31 December 2021 in accordance with article 3:6, §2 of the Belgian Companies and Associations Code of 23 March 2019 (as amended) (the "Belgian Companies and Associations Code"). On 14 April 2021, in accordance with the Belgian Royal Decree of 12 May 2019 designating the corporate governance code to be complied with by listed companies, the Company designated the new 2020 Belgian Corporate Governance Code (the "2020 Code") as reference code within the meaning of article 3:6, §2 of the Belgian Companies and Associations Code. At the same occasion, the Company's corporate governance charter was adopted in accordance with the recommendations set out in the 2020 Code, which replaces the previous 2009 Belgian Corporate Governance Code. For the financial year ended on 31 December 2021, the Company complied to a large extent with the provisions of the 2020 Code, except for the following deviations which the Company believed were justified in view of the Company’s specific situation. Notably, in line with the “comply-or-explain” principle of said 2020 Code, MDxHealth does not fully comply with the following provisions: • Given the size of the Company, no internal audit function is in place. In line with provision 4.14 of the 2020 Code, the need for an internal audit function will be reviewed annually. • Following the modification of the Directors' remuneration on 30 July 2020, effective as from 1 July 2020, the Non-Executive Directors that are not Independent Directors shall not be entitled to a remuneration in cash, but shall each year be entitled to receive share options for a maximum of 10,000 shares of the Company. This is contrary to provision 7.6 of the 2020 Code, which provides that no share options should be granted to Non-Executive Directors. The Company believes that this provision of the 2020 Code is not appropriate and adapted to take into account the realities of companies in the biotech and life sciences Annual Report 2021 20

Part II: Corporate Governance Annual Report 2021 industry. Notably, the ability to remunerate Non-Executive Directors with share options allowes the Company to limit the portion of remuneration in cash that the Company would otherwise need to pay to attract or retain renowned experts with the most relevant skills, knowledge and expertise. The Company is of the opinion that granting Non-Independent Non-Executive Directors the opportunity to be remunerated in part in share- based incentives rather than all in cash enables the Non-Independent Non-Executive Directors to link their effective remuneration to the performance of the Company and to strengthen the alignment of their interests with the interests of the Company’s shareholders. The Company believes that this is in the interest of the Company and its stakeholders. Furthermore, the Company believes that this is customary for Directors active in companies in the life sciences industry. • In accordance with provision 7.6 of the 2020 Code, Non-Executive Directors should receive a part of their remuneration in the form of shares of the Company. The Company has however no distributable reserves and therefore does not meet the legal requirements to proceed to a share buy-back. As a result, the Company does not own any treasury shares and is unable to grant existing shares to Non-Executive Directors as part of their remuneration. The interests of the Non-Independent Non-Executive Directors are currently considered to be sufficiently oriented to the creation of long-term value for the Company. Finally, the Board will propose to remunerate the Independent Directors in cash, but leaving it at the own initiative of the Independent Directors whether or not they wish to use such funds (in whole or in part) to acquire existing shares of the Company. • In accordance with provision 7.9 of the 2020 Code, the Board should set a minimum threshold of shares to be held by the executive management. A part of the remuneration of the executive management consists of options to subscribe for the Company’s shares, which should allow the executive management over time to acquire shares of the Company, in line with the objectives of the option plans. • Pursuant to article 7:91 of the Belgian Companies and Associations Code and provision 7.11 of the 2020 Code, shares should not vest and share options should not be exercisable within three years as of their granting. It has been expressly provided by the Company's general shareholders' meeting that the Board of Directors is explicitly authorized to deviate from the provisions of 7:91 of the Belgian Companies and Associations Code, for all persons who fall within the scope of these provisions (whether directly or pursuant to articles 7:108 and 7:121 of the Belgian Companies and Associations Code, or otherwise). The Company is of the opinion that this allows for more flexibility when structuring share-based awards. For example, it is customary for option plans to provide for a vesting in several instalments over a well-defined period of time, instead of vesting after three years only. This seems to be more in line with prevailing practice. • In accordance with provision 7.12 of the 2020 Code, the Board of Directors should include provisions that would enable the Company to recover variable remuneration paid, or withhold the payment of variable remuneration, and specify the circumstances in which it would be appropriate to do so, insofar as enforceable by law. The Company believes that this provision of the 2020 Code is not appropriate and adapted to take into account the realities of companies in the biotech and life sciences industry, including, notably, for management teams located in the United States. The share option plans set up by the Company do however contain bad leaver provisions that can result in the share options, whether vested or not, automatically and immediately becoming null and void. Notwithstanding the company's position that share options are not to be qualified as variable remuneration, the Board of Directors is of the opinion that such bad leaver provisions sufficiently protect the Company's interests and that it is therefore currently no necessary to provide for additional contractual provisions that give the company a contractual right to reclaim any (variable) remuneration from the members of the executive management. For that reason, there are no contractual provisions in place between the Company and the members of the executive management that give the Company a contractual right to reclaim from said executives any variable remuneration that would be awarded. 21

Part II: Corporate Governance Annual Report 2021 The performance and functioning of the Board of Directors, its committees, and the executive management team are summarized below. On November 8, 2021, following the Company's initial public offering in the United States of 3,750,000 American Depositary Shares (each, an "ADS", and each ADS representing 10 ordinary shares of the Company with no nominal value per share) and the listing of the ADSs on the Nasdaq Capital Market, the Board of Directors approved a revised version of the Company's corporate governance charter to reflect the fact that, under United States securities law, the Company is currently eligible for treatment as a “foreign private issuer” and "emerging growth company". As a foreign private issuer and emerging growth company, the Company may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to U.S. public companies. For further details on the qualification of the Company as “foreign private issuer” and "emerging growth company", reference is made to section 1.9 of the Company's corporate governance charter. The articles of association and the corporate governance charter are available on the Company's website (https://mdxhealth.com/) and can be obtained free of charge at the Company's registered office. The 2020 Code can be accessed on the following website: www.corporategovernancecommittee.be/ 22

Part II: Corporate Governance Annual Report 2021 23

Part II: Corporate Governance Annual Report 2021 Board of Directors The Company has opted for a "one tier" governance structure whereby the Board of Directors is the ultimate decision making body, with the overall responsibility for the management and control of the Company, and is authorized to carry out all actions that are considered necessary or useful to achieve the Company's object. The Board of Directors has all powers except for those reserved to the general shareholders' meeting by law or the Company's articles of association. The Board of Directors acts as a collegiate body. The Board of Directors’ role is to pursue sustainable value creation by the Company, by determining the Company’s strategy, putting in place effective, responsible and ethical leadership, and monitoring the Company’s performance. The Board of Directors acts as a collegiate body. Pursuant to the Belgian Companies and Associations Code and the articles of association of the Company, the Board of Directors should be composed of at least three Directors. In accordance with the 2020 Code, the Board of Directors should have a composition appropriate to the company’s purpose, its operations, phase of development, structure of ownership and other specifics. The Board of Directors shall be composed of at least three Independent Directors and a majority of the Board shall consist of Non-Executive Directors. Currently, the Board of Directors comprises 9 Directors, of which 5 are Independent Non-Executive Directors and 3 are Non-Independent Non-Executive Directors. The Directors of the Company are appointed by the general shareholders’ meeting. The Company’s Board of Directors strives to maintain a well-balanced general diversity at the Board of Directors. Currently, there are 3 female Directors among a total of 9 Board members (representing a ratio of 33.33% female Directors against 66.67% male Directors). The Belgian Companies and Associations Code requires that at least one third of the members of the Board of Directors should be of the opposite gender. In order to calculate the required number of directors of a different gender, fractions must be rounded to the nearest whole number, which means that the Company's Board in its current composition must include at least 3 female Directors. The Company has met the one- third gender diversity requirement since 1 January 2018 and continues to comply with such requirement at the date of this Annual Report. The Board of Directors is a collegial body, and deliberates and makes decisions as such. Excluding the Board committees meetings, the Board of Directors met 16 times throughout 2021. All Directors were present or represented at these 16 meetings, except that Hilde Windels BV, represented by its permanent representative, Ms. Hilde Windels, did not participate to three meetings during this period. In addition, in accordance with article 7:95 of the Belgian Companies and Associations Code and article 23 of the Company's articles of association, the Board of Directors passed resolutions with unanimous and written consent of all Directors at 6 occasions. Chair The chair of the Board of Directors is responsible for the leadership of the Board of Directors. The chair takes the necessary measures to develop a climate of trust within the Board of Directors, contributing to open discussion, constructive dissent and support for the decisions of the Board of Directors. The chair promotes effective interaction between the Board and the executive management. The chair establishes a close relationship with the CEO, providing support and advice, while fully respecting the executive responsibilities of the CEO. The Board of Directors appoints a chair amongst the Non-Executive Directors. Currently, Ahok BV, with Mr. Koen Hoffman as permanent representative, is the chair of the Board of Directors. Mr. Hoffman assumed the role of Board chair since 2020. 24

Part II: Corporate Governance Annual Report 2021 Independent Directors The Company has currently five Independent (Non-Executive) Directors. A Director in a listed company is considered to be independent if he or she does not have a relationship with that company or with a major shareholder of the Company that compromises his or her independence. If the Director is a legal entity, his or her independence must be assessed on the basis of both the legal entity and his or her permanent representative. A Director will be presumed to qualify as an Independent Director if he or she meets at least the criteria set out in article 7:87 of the Belgian Companies and Associations Code and Clause 3.5 of the 2020 Code, which can be summarized as follows: 1. Not being an executive, or exercising a function as a person entrusted with the daily management of the company or an affiliated company or person, and not have been in such a position for the previous three years before their appointment. Alternatively, no longer enjoying share options of the company related to this position. 2. Not having served for a total term of more than twelve years as a Non-Executive Board member. 3. Not being an employee of the senior management (as defined in article 19,2° of the law of 20 September 1948 regarding the organization of the business industry) of the company or an affiliated company or person, and not have been in such a position for the previous three years before their appointment. Alternatively, no longer enjoying share options of the company related to this position. 4. Not receiving, or having received during their mandate or for a period of three years prior to their appointment, any significant remuneration or any other significant advantage of a patrimonial nature from the company or an affiliated company or person, apart from any fee they receive or have received as a Non-Executive Board member. 5. Not holding shares, either directly or indirectly, either alone or in concert, representing globally one tenth or more of the company’s capital or one tenth or more of the voting rights in the company at the moment of appointment. 6. Not having been nominated, in any circumstances, by a shareholder fulfilling the conditions covered under point 5. 7. Not having, nor having had in the past year before their appointment, a significant business relationship with the company or an affiliated company or person, either directly or as partner, shareholder, Board member, member of the senior management (as defined in article 19,2° of the law of 20 September 1948 regarding the organization of the business industry) of a company or person who maintains such a relationship. 8. Not being or having been within the last three years before their appointment, a partner or member of the audit team of the company or person who is, or has been within the last three years before their appointment, the external auditor of the company or an affiliated company or person. 9. Not being an executive of another company in which an executive of the company is a Non-Executive Board member, and not have other significant links with executive Board members of the company through involvement in other companies or bodies. 10. Not having, in the company or an affiliated company or person, a spouse, legal partner or close family member to the second degree, exercising a function as Board member or executive or person entrusted with the daily management or employee of the senior management (as defined in article 19,2° of the law of 20 September 1948 regarding the organization of the business industry), or falling in one of the other cases referred to in 1 to 9 above, and as far as point 2 is concerned, up to three years after the date on which the relevant relative has terminated their last term. If the Board of Directors submits the nomination of an Independent Director who does not meet the abovementioned criteria to the general meeting, it shall explain the reasons why it assumes that the candidate is in fact independent. The Company is of the view that the Independent Directors comply with each of the criteria of the Belgian Companies and Associations Code and the 2020 Code. 25
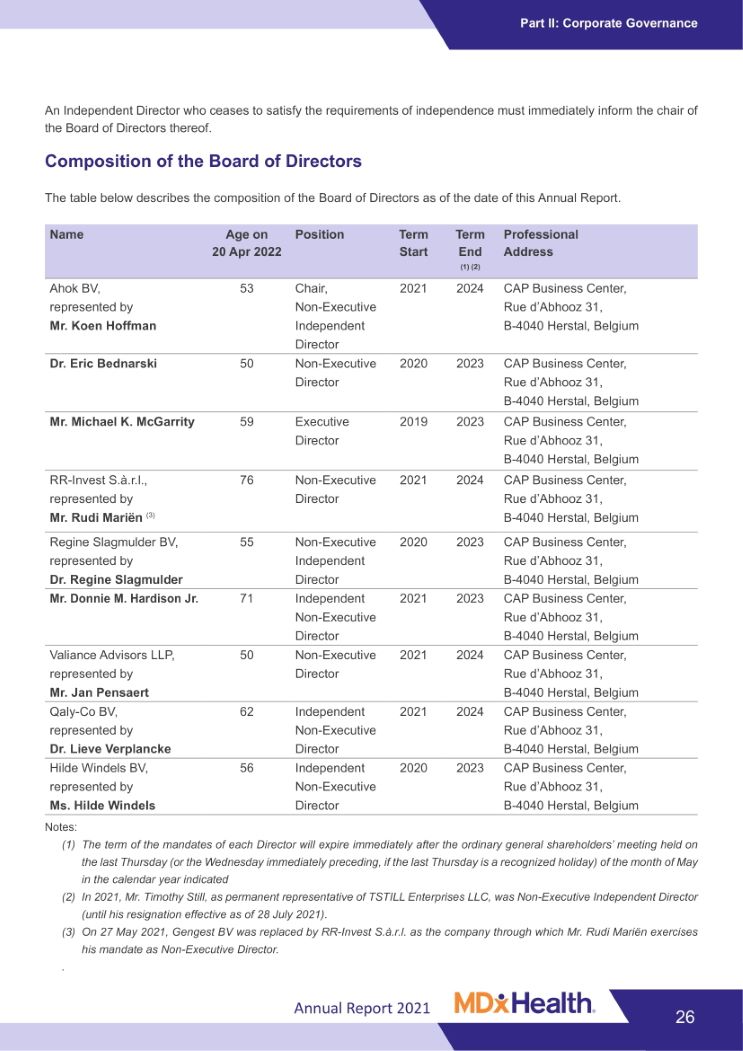
Part II: Corporate Governance Annual Report 2021 An Independent Director who ceases to satisfy the requirements of independence must immediately inform the chair of the Board of Directors thereof. Composition of the Board of Directors The table below describes the composition of the Board of Directors as of the date of this Annual Report. Name Age on Position Term Term Professional 20 Apr 2022 Start End (1) (2) Address Ahok BV, 53 Chair, 2021 2024 CAP Business Center, represented by Non-Executive Rue d’Abhooz 31, Mr. Koen Hoffman Independent B-4040 Herstal, Belgium Director Dr. Eric Bednarski 50 Non-Executive 2020 2023 CAP Business Center, Director Rue d’Abhooz 31, B-4040 Herstal, Belgium Mr. Michael K. McGarrity 59 Executive 2019 2023 CAP Business Center, Director Rue d’Abhooz 31, B-4040 Herstal, Belgium RR-Invest S.à.r.l., 76 Non-Executive 2021 2024 CAP Business Center, represented by Director Rue d’Abhooz 31, Mr. Rudi Mariën (3) B-4040 Herstal, Belgium Regine Slagmulder BV, 55 Non-Executive 2020 2023 CAP Business Center, represented by Independent Rue d’Abhooz 31, Dr. Regine Slagmulder Director B-4040 Herstal, Belgium Mr. Donnie M. Hardison Jr. 71 Independent 2021 2023 CAP Business Center, Non-Executive Rue d’Abhooz 31, Director B-4040 Herstal, Belgium Valiance Advisors LLP, 50 Non-Executive 2021 2024 CAP Business Center, represented by Director Rue d’Abhooz 31, Mr. Jan Pensaert B-4040 Herstal, Belgium Qaly-Co BV, 62 Independent 2021 2024 CAP Business Center, represented by Non-Executive Rue d’Abhooz 31, Dr. Lieve Verplancke Director B-4040 Herstal, Belgium Hilde Windels BV, 56 Independent 2020 2023 CAP Business Center, represented by Non-Executive Rue d’Abhooz 31, Ms. Hilde Windels Director B-4040 Herstal, Belgium Notes: (1) The term of the mandates of each Director will expire immediately after the ordinary general shareholders’ meeting held on the last Thursday (or the Wednesday immediately preceding, if the last Thursday is a recognized holiday) of the month of May in the calendar year indicated (2) In 2021, Mr. Timothy Still, as permanent representative of TSTILL Enterprises LLC, was Non-Executive Independent Director (until his resignation effective as of 28 July 2021). (3) On 27 May 2021, Gengest BV was replaced by RR-Invest S.à.r.l. as the company through which Mr. Rudi Mariën exercises his mandate as Non-Executive Director. 26

Part II: Corporate Governance Annual Report 2021 Mr. Koen Hoffman obtained a Master in Applied Economics and an MBA at Vlerick Business School. Between 1992 and July 2016, he was active at KBC Group in which he started his career in the corporate finance department and later became the CEO of KBC Securities as from October 2012. Since August 2016, he is the CEO of Value Square asset management. Mr Koen Hoffman serves also as board member at Fagron (Chair),Greenyard (chair), Mithra Pharmaceuticals and SnowWorld. Dr. Eric Bednarski currently serves as a Partner of MVM Partners LLP. Before joining MVM in 2008, he was a Partner at Advent Healthcare Ventures and a Principal at Advent International Corporation. Prior to Advent, he was a Director in the Corporate Finance Group of Silicon Valley Bank. Dr. Bednarski has a B.S. degree in Neural Science from Brown University and a Ph.D. in Biological Sciences from the University of California, Irvine. Mr. Donnie M. Hardison Jr. currently is the sole proprietor of DMH Consulting, a management consulting firm that he founded and previously operated from April 2016 to January 2017. He was most recently the President and Chief Executive Officer, and served on the board of directors, of Biotheranostics, Inc., a molecular diagnostic company focused on oncology, from February 2017 until it was acquired by Hologic, Inc. in February 2021. From April 2010 to March 2016, Mr. Hardison was the President and Chief Executive Officer of Good Start Genetics, a molecular genetic testing and information company. For more than 20 years prior to that, Mr. Hardison held various executive and senior management positions at companies including Laboratory Corporation of America (LabCorp) a clinical laboratory company, Exact Sciences Corporation, a molecular diagnostics company, OnTarget, Inc., a sales and marketing consulting company, Quest Diagnostics Inc., a clinical laboratory company, SmithKline Beecham Corporation, a pharmaceutical company, and others. He served on the board of directors of Exact Sciences Corporation (Nasdaq: EXAS) from May 2000, through its initial public offering in February 2001, until August 2007. Mr. Hardison received his Bachelor of Arts degree, in political science, from the University of North Carolina, Chapel Hill. Mr. Michael K. McGarrity has more than 25 years of experience in the healthcare industry with a unique combination of device, diagnostics and biotechnology experience. Michael was most recently the CEO of Sterilis Medical. Prior to Sterilis Michael was the CEO of Nanosphere (NASDAQ: NSPH), a nanotechnology-based molecular diagnostics company, where he engineered an operational and strategic turnaround that resulted in its successful sale to Luminex (NASDAQ: LMNX) in 2016. Prior to Nanosphere, McGarrity spent 13 years at Stryker Corporation (NYSE: SYK). Mr. Rudi Mariën is President and Managing Director of RR-Invest S.à.r.l. and Biovest NV. He was the Vice President of Cerba European Lab. Through his management company, Gengest BV, Mr. Mariën has Board mandates in different listed and private biotech companies. Mr. Mariën was co-founder, reference shareholder and Chair of Innogenetics, and has been the founder, shareholder and Managing Director of several clinical reference laboratories including the Barc Group, a leading international centralized clinical laboratory, exclusively dedicated to pharmaceutical studies. Mr. Mariën holds a degree in pharmaceutical sciences from the University of Gent and is specialized in clinical biology. 27

Part II: Corporate Governance Annual Report 2021 Dr. Regine Slagmulder is a partner and full professor in management accounting & control at Vlerick Business School. Previously, she worked as a strategy practice consultant at McKinsey & Company. She also previously worked as a professor of management accounting at INSEAD and at the University of Tilburg. She serves as an independent director and chair of the audit committee on the board of the investment company Quest for Growth (since 2011) and of Ekopak (since 2021), both listed on Euronext. Dr. Slagmulder graduated in civil electrotechnical engineering and industrial management from the University of Gent, after which she received a management doctorate at Vlerick Business School. As part of her research activities, she was a research fellow attached to INSEAD, Boston University (USA) and the P. Drucker Graduate Management Center at Claremont University (USA). Mr. Jan Pensaert is the Founding Managing Partner of Valiance. He brings over 20 years of experience in growth investing. He leads the Investment Committee for the Valiance Funds and is responsible for all aspects of the Funds’ investment processes. Jan currently serves on the Board of several Valiance entities funds and portfolio companies including MDxHealth, JenaValve, MyCartis and 4Tech. Prior to founding Valiance, Jan was CEO of La Fayette, where during his tenure the La Fayette Funds increased in AUM from USD 750 million to USD 5.5 billion. Before that, he was responsible for the Permal Group’s European-based investment management and research activities, and prior to that he worked at Lazard in Corporate Finance M&A. Jan holds a BA in Business Economics from Gent University in Belgium, and a Masters in Banking & Finance from the University of Aix-Marseille, France. Dr. Lieve Verplancke MD, a Belgian national, began her career in 1984 with The Beecham Group (now part of GlaxoSmithKline), and has since held key management positions with Merck & Co., as well as Bristol-Myers Squibb, where she served as Managing Director, leading their Belgian/GDL subsidiary, until 2012. Ms. Verplancke also serves as a Board Member for Brussels-based Europe Hospitals; the Imelda Hospital in Bonheiden; and the Euronext fund, Quest for Growth and Materialise. She is also the Founder and Managing Director of Qaly@Beersel, an elderly care center in Belgium. In addition to being a medical doctor (MD– KULeuven University), Ms. Verplancke holds a postgraduate degree in Economics and an MBA from the University of Antwerp. She has also completed courses at INSEAD, CEDEP, Columbia University and the Vlerick Business School, and is a certified Executive Coach (PCC). Ms. Hilde Windels is the CEO of immunodiagnostic company Antelope Dx BV and has 20 years of experience in the biotechnology sector with a track record of building and structuring organizations, fundraising, M&A, public capital markets and corporate strategies. At Biocartis, she was CEO ad interim and Deputy CEO from September 2015 until September 2017 and CFO from 2011 until September 2015. Previously, Mrs. Windels worked as independent CFO for several private biotech companies and from 1999 to 2008 she was CFO of Devgen. Currently, Mrs. Windels serves as a board member at Erytech and Celyad. In the past, she also served on the boards of Devgen, Biocartis, Ablynx, VIB and FlandersBio. Mrs. Windels holds a Masters in Economics (commercial engineer) from the University of Leuven, Belgium. 28

Part II: Corporate Governance Annual Report 2021 Committees of the Board of Directors The Board of Directors of MDxHealth has set up two permanent Board committees which are responsible for assisting the Board of Directors and making recommendations in specific fields: the audit committee (in accordance with article 7:99 of the Belgian Companies and Associations Code and provision 4.10 of the 2020 Code) and the nomination and remuneration committee (in accordance with article 7:100 of the Belgian Companies and Associations Code and provision 4.17 and 4.19 of the 2020 Code). The terms of reference of these Board committees are primarily set out in the corporate governance charter. Audit Committee MDxHealth has had an audit committee in place since the Company’s inception. According to article 7:99 §3 of the Belgian Companies and Associations Code, MDxHealth would meet the size criteria in order to operate without a separate audit committee, but the Company has chosen to continue operating with a separate audit committee. The audit committee of the Company consists of three Directors, all of whom are currently Independent Non-Executive Directors. According to the Belgian Companies and Associations Code, all members of the audit committee must be Non-Executive Directors, and at least one member must be independent within the meaning of article 7:87 of the Belgian Companies and Associations Code. Furthermore, each member of the committee must meet the criteria for independence set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended. The chairperson of the audit committee is to be appointed by the members of the audit committee. Notwithstanding anything to the contrary, in appointing members of the committee, the Board of Directors may rely on the applicable phase-in rules applicable to initial public offerings in accordance with Rule 5615(b)(1) of the Listing Rules of the Nasdaq Capital Market. The composition of the audit committee complies with the 2020 Code, which require that a majority of the members of the audit committee are independent. The members of the audit committee must have a collective competence in the business activities of the Company as well as in accounting, auditing and finance, and at least one member of the audit committee must have the necessary competence in accounting and auditing. According to the Board of Directors, the members of the audit committee satisfy this requirement, as evidenced by the different senior management and Director mandates that they have held in the past and currently hold. The role of the audit committee is to assist the Board of Directors in fulfilling its financial, legal and regulatory monitoring responsibilities. The committee reports regularly to the Board of Directors on the exercise of its duties, identifying any matters in respect of which it considers that action or improvement is needed, and making recommendations as to the steps to be taken. The audit review and the reporting on that review cover the Company and its subsidiaries as a whole. The specific tasks of the audit committee are outlined in the Company’s governance charter and include the following: • to inform the Board of Directors of the result of the audit of the financial statements and the manner in which the audit has contributed to the integrity of the financial reporting and the role that the audit committee has played in that process; • to monitor the financial reporting process, and to make recommendations or proposals to ensure the integrity of the process; • to monitor the effectiveness of the Company’s internal control and risk management systems, and the Company’s internal audit process and its effectiveness; • to monitor the audit of the annual statutory and consolidated financial statements, including the follow-up questions and recommendations by the statutory auditor and, as the case may be, the auditor responsible for the audit of the consolidated financial statements; • to assess and monitor the independence of the statutory auditor, in particular with respect to the appropriateness 29

Part II: Corporate Governance Annual Report 2021 of the provision of additional services to the Company. More specifically, the audit committee analyses, together with the statutory auditor, the threats for the statutory auditor's independence and the security measures taken to limit these threats, when the total amount of fees exceeds the criteria specified in article 4 §3 of Regulation (EU) No 537/2014; and • to make recommendations to the Board of Directors on the selection, appointment and remuneration of the Company’s statutory auditor in accordance with article 16 § 2 of Regulation (EU) No 537/2014. On the date of this report, the following Non-Executive Independent Directors are members of the audit committee: Regine Slagmulder BV, represented by its permanent representative, Dr. Regine Slagmulder (chair); Qaly-Co BV, represented by its permanent representative; Dr. Lieve Verplancke; and Hilde Windels BV, represented by its permanent representative, Ms. Hilde Windels (replacing Valiance Advisors LLP, represented by its permanent representative, Mr. Jan Pensaert, since August 2021). As required by Belgian law, the chair of the audit committee is competent in accounting and auditing, as is evidenced by her role as partner and full professor in management accounting and control at Vlerick Business School, as well as serving as chair of the audit committee of multiple publicly listed companies. The audit committee is a collegial body and deliberates and makes decisions as such. The audit committee met four times in 2021. All members of the audit committee were present or represented at all meetings. Nomination and Remuneration Committee According to article 7:100 §4 of the Belgian Companies and Associations Code, MDxHealth would meet the size criteria in order to operate without a separate remuneration committee, but the Company has chosen to continue operating with a separate remuneration committee. MDxHealth’s nomination and remuneration committee must be composed of at least three members and must be composed exclusively of Non-Executive Directors who have the necessary competence in terms of remuneration policy. A majority of its members must be Independent Directors. The nomination and remuneration committee is chaired by the chair of the Board of Directors or another Non-Executive Director appointed by the committee. The chair of the Board of Directors should not chair the committee when dealing with the designation of his successor. The CEO should participate in an advisory capacity in the meetings of the committee when it deals with the remuneration of other executive managers. The role of the nomination and remuneration committee is to make recommendations to the Board of Directors with regard to the appointment and remuneration of Directors and members of the executive management and, in particular, to: • identify, recommend and nominate, for the approval of the Board of Directors, candidates to fill vacancies in the Board of Directors and executive management positions as they arise. In this respect, the nomination and remuneration committee must consider and advise on proposals made by relevant parties, including management and shareholders; • advise the Board of Directors on any proposal for the appointment of the chief executive officer and on the chief executive officer’s proposals for the appointment of other members of the executive management; • draft appointment procedures for members of the Board of Directors and the chief executive officer; • ensure that the appointment and re-election process is organized objectively and professionally; • periodically assess the size and composition of the Board of Directors and make recommendations to the Board of Directors with regard to any changes; • consider issues related to succession planning; • make proposals to the Board of Directors on the remuneration policy for Directors and members of the executive management and the persons responsible for the day-to-day management of the Company, as well as, where appropriate, on the resulting proposals to be submitted by the Board of Directors to the shareholders’ meeting; 30

Part II: Corporate Governance Annual Report 2021 • make proposals to the Board of Directors on the individual remuneration of Directors and members of the executive management, and the persons responsible for the day-to-day management of the Company, including variable remuneration and long-term incentives, whether or not share-related, in the form of share options or other financial instruments, and arrangements on early termination, and where applicable, on the resulting proposals to be submitted by the Board of Directors to the shareholders’ meeting; • prepare a remuneration report to be included by the Board of Directors in the annual corporate governance statement; • present and provide explanations in relation to the remuneration report at the ordinary general shareholders’ meeting; and • report regularly to the Board of Directors on the exercise of its duties. On the date of this report, the following Non-Executive Directors are members of the nomination and remuneration committee: Mr. Donnie M. Hardison Jr. (chair), replacing TSTILL Enterprises LLC, represented by its permanent representative, Mr. Timothy Still, since September 2021; Dr. Eric Bednarski; Qaly-Co BV, represented by its permanent representative, Dr. Lieve Verplancke; Ahok BV, represented by its permanent representative, Mr. Koen Hoffman; and Valiance Advisors LLP, represented by its permanent representative, Mr. Jan Pensaert. The nomination and remuneration committee is a collegial body and deliberates and makes decisions as such. The nomination and remuneration committee met three times in 2021. All of the committee members with the exception of Mr. Donnie M. Hardison Jr. attended all of the committee meetings. Mr. Donnie M. Hardison Jr. did not attend the two initial meetings of the nomination and remuneration committee held in 2021, as they occurred prior to his becoming a member of the committee. Process for evaluating the Board, its committees, and its individual Directors The Board should assess at least every three years its own performance and its interaction with the executive management, as well as its size, composition, functioning and that of its committees. The evaluation should be carried out through a formal process, whether or not externally facilitated, in accordance with a methodology approved by the Board. At the end of each Board member’s term, the nomination and remuneration committee should evaluate this Board member’s presence at the Board or committee meetings, their commitment and their constructive involvement in discussions and decision-making in accordance with a pre-established and transparent procedure. The nomination and remuneration committee should also assess whether the contribution of each Board member is adapted to changing circumstances. The Board will act on the results of the performance evaluation. Where appropriate, this will involve proposing new Board members for appointment, proposing not to re-appoint existing Board members or taking any measure deemed appropriate for the effective operation of the Board. Click here to return to table of contents 31

Executive management Annual Report 2021 32
Part II: Corporate Governance Annual Report 2021 Executive management The Board of Directors has appointed the executive management of the Company. The terms of reference of the executive management have been determined by the Board of Directors in close consultation with the CEO. Chief Executive Officer The CEO is appointed, and can be removed, by the Board of Directors of the Company. The CEO is charged by the Board of Directors with the day-to-day management of the Company and is therefore also managing Director of the Company. In this function, the CEO has the following general responsibilities: • the implementation of the decisions of the Board of Directors, within the strategy, planning, values and budgets approved by the Board of Directors, • overseeing the different central departments and business units of the Company, and reporting to the Board of Directors on their activities, • the development of proposals for the Board of Directors relating to strategy, planning, finances, operations, human resources and budgets, and other matters that are to be dealt with at the level of the Board of Directors. The specific tasks of the CEO are further described in the Company's corporate governance charter. Other members of the executive management team The other members of the executive management team, being the heads of the main activities and central departments (and their divisions) of MDxHealth, are appointed and removed by the CEO in close consultation with the Board of Directors of the Company. The main tasks of the executive management are to organize their department in accordance with the guidelines determined by the CEO and to report to the CEO on the operation and activities of their department. Composition of the executive management team The composition of the Management Team is set out below and reflects the situation at the date of this Annual Report: Name Age on Dec 31, 2021 Position Permanent Address Mr. Michael K. McGarrity 59 Chief Executive Officer 15279 Alton Parkway Ste 100, Irvine, CA 92618 USA Mr. John Bellano 53 Chief Commercial Officer 15279 Alton Parkway Ste 100, Irvine, CA 92618 USA Mr. Ron Kalfus 47 Chief Financial Officer 15279 Alton Parkway Ste 100, Irvine, CA 92618 USA Mr. Joseph Sollee 57 Executive Vice President 15279 Alton Parkway of Corporate Ste 100, Irvine, Development & General CA 92618 Counsel USA 33

Part II: Corporate Governance Annual Report 2021 In 2021 the Management Team consisted of Mr. Michael McGarrity, as Chief Executive Officer, Mr. Ron Kalfus, as Chief Financial Officer, Mr. John Bellano, as Chief Commercial Officer, and Mr. Joseph Sollee, as Executive Vice President of Corporate Development and General Counsel. Following are biographies of the executive management team members (also referred to as executives) as of the date of this Annual Report: Mr. Michael K. McGarrity, Chief Executive Officer See “Board of Directors - Composition of the Board of Directors”. Mr. John Bellano, Chief Commercial Officer Mr. Bellano joined MDxHealth in June 2019. He has more than 25 years of experience in the healthcare Industry. Mr. Bellano started his career in pharmaceuticals and transitioned to molecular diagnostics where he has spent the past 20 years of his career, most recently as Chief Commercial Officer of Sterilis Solutions Prior to Sterilis Solutions he served as the commercial leader for pharmacogenomic companies Assurex Health and AltheaDx. While at Assurex Health (Myriad Genetics) revenue grew from USD 700 thousand to a run rate of USD 100 Million during his 5-year span with the organization. Mr. Ron Kalfus, Chief Financial Officer Mr. Kalfus joined MDxHealth in July 2019. He has over 20 years of leadership experience in both public and private companies within diagnostics/biotech and other sectors, and brings extensive knowledge in financial operations and management. Mr. Kalfus joined MDxHealth from Rosetta Genomics, where he helped lead efforts to reposition the company for commercial success with its oncology diagnostic products, and raised over USD 60 million in capital to fund these efforts. Prior to Rosetta, Mr. Kalfus served as the CFO and Treasurer of MabCure, a Belgium-based publicly- traded biotechnology startup in the field of early cancer detection using antibodies. Mr. Joseph Sollee, Executive Vice President, General Counsel & Chief Compliance Officer Mr. Sollee has provided legal counsel to MDxHealth since its inception in 2003, and in April 2008 joined our management team. Prior to joining the Company, Mr. Sollee served as Special Counsel with the law firm of Kennedy Covington (now K&L Gates), where he led the Life Sciences Practice Group. Mr. Sollee has more than 20 years of experience in the life sciences industry, and has held senior legal and management positions at Triangle Pharmaceuticals and TherapyEdge. In addition, he has practiced as a corporate attorney in the Washington D.C. legal firm Swidler & Berlin and in investment banking at Smith Barney in New York. Mr. Sollee received a Juris Doctorate in Law (JD) and a Master’s degree in International & Comparative Law (LLM) from Duke University, a BA degree from Harvard University, and has been certified into the legal bars of New York, Washington D.C. and North Carolina. Click here to return to table of contents 34

Part II: Corporate Governance Annual Report 2021 Internal control and risk management The rules and procedures that apply when Board members and executive managers deal in MDxHealth securities are defined in the Company’s Dealing Code. The code prohibits Board members and executive managers from dealing with MDxHealth securities during periods prohibited by applicable laws and regulation or during specific closed periods announced by the Company. The dealing code is available in its entirety on the Company’s website (www.mdxhealth.com). Introduction The Company operates a risk management and control framework in accordance with the Belgian Companies and Associations Code and the 2020 Code. MDxHealth is exposed to a wide variety of risks within the context of its business operations that can result in its objectives being affected or not achieved. Controlling those risks is a core task of the Board of Directors (including the audit committee), the executive management and all other employees with managerial responsibilities. The risk management and control system has been set up to reach the following goals: • achievement of the Company's objectives; • achieving operational excellence; • ensuring correct and timely financial reporting; and • compliance with all applicable laws and regulations. 35

Part II: Corporate Governance Annual Report 2021 Control Environment Three lines of defense The Company applies the 'three lines of defense model' to clarify roles, responsibilities and accountabilities, and to enhance communication within the area of risk and control. Within this model, the lines of defense to respond to risks are: • First line of defense: line management is responsible for assessing risks on a day-to-day basis and implementing controls in response to these risks. • Second line of defense: the oversight functions like Finance and Controlling and Quality and Regulatory oversee and challenge risk management as executed by the first line of defense. The second line of defense functions provide guidance and direction and develop a risk management framework. • Third line of defense: independent assurance providers such as external accounting and external audit challenge the risk management processes as executed by the first and second line of defense. Policies, procedures and processes The Company fosters an environment in which its business objectives and strategy are pursued in a controlled manner. This environment is created through the implementation of different Company-wide policies, procedures and processes such as the Company's values, the Quality Management System and the Delegation of Authorities rule set. The employees are regularly informed and trained on these subjects in order to develop sufficient risk management and control at all levels and in all areas of the organization. Risk management Sound risk management starts with identifying and assessing the risks associated with the Company's business and external factors. Once the relevant risks are identified, the Company strives to prudently manage and minimize such risks, acknowledging that certain calculated risks are necessary to ensure that the Company achieves its objectives and continues to create value for its stakeholders. All employees of the Company are accountable for the timely identification and qualitative assessment of the risks within their area of responsibility. Control activities Control measures are in place to minimize the effect of risks on the Company's ability to achieve its objectives. These control activities are embedded in the Company's key processes and systems to assure that the risk responses and the Company's overall objectives are carried out as designed. Control activities are conducted throughout the organization, at all levels and within all departments. 36

Part II: Corporate Governance Annual Report 2021 Information and communication The Company recognizes the importance of timely, complete and accurate communication and information both top down as well as bottom-up. The Company therefore put several measures in place to assure amongst others: • security of confidential information; • clear communication about roles and responsibilities; and • timely communication to all stakeholders about external and internal changes impacting their areas of responsibility. Monitoring of control mechanisms Monitoring helps to ensure that internal control systems operate effectively. The quality of the Company's risk management and control framework is assessed by the following functions: • Quality and Regulatory: All employees of the Company are instructed on the rules and policies of the Company via a booklet of work rules, the terms of their employment arrangements, standard operating procedures defined by task/area, and by numerous documents (such as the Code of Business Conduct and Ethics and the Dealing Code) that are distributed and explained to the personnel. • External Audit: In the Company's review of the annual accounts, the statutory auditor focuses on the design and effectiveness of internal controls and systems relevant for the preparation of the financial statements. The outcome of the audits, including work on internal controls, is reported to management and the audit committee. • Audit Committee: The Board of Directors and the audit committee have the ultimate responsibility with respect to internal control and risk management. In addition, the legal department of MDxHealth, under supervision of the CEO and together with the management team, has set up internal procedures in order to ensure that acts performed within or by the Company are in compliance with the existing laws and external regulations. The management is also responsible to comply with internal regulations and the Board of Directors is ensuring that the management is respecting the general policies and the corporate plans. The Board of Directors has established a Code of Business Conduct and Ethics to aid MDxHealth’s Directors, officers and employees in making ethical and legal decisions when conducting Company business and performing their day- to-day duties. The Code of Business Conduct and Ethics is available in its entirety on the Company’s website (https:// mdxhealth.com). In addition, the Board has appointed a Chief Compliance Officer to oversee ongoing compliance with the Code of Business Conduct and Ethics and existing laws and external regulations, and to report regularly to the Board of Directors and the Audit Committee on compliance matters. 37

Part II: Corporate Governance Annual Report 2021 Risk management and internal control with regard to the process of financial reporting The accurate and consistent application of accounting rules throughout the Company is assured by means of set of control procedures, including: • The audit committee reviews all financial information before it is released • The Board of Directors reviews internal monthly financial information • The financial auditors not only audit the year-end financial statements, but at the request of the Company they also perform a limited review of the Interim half-year financial statements • The Company managers and finance department personnel explain all material variances in historical figures and between the budget and actual figures • The Board of Directors, the Company managers and finance department personnel perform reviews and controls of the key financial figures at each reporting period, some of which are described below • At the Board of Directors level, there is a periodic review and approval of the following main topics: - Overall strategy and strategic options; - Multi-year business plan and company goals; - Ensuing year budget and targets; - Comparison of actual results and budgeted figures; - Hiring, motivation, and retention of key talent; - Remuneration and benefits; - Financial statements; and - Internal controls. Management of the Company is organized on the basis of plans, departments, projects, and corresponding budgets and targets. Progress on the core projects, budgets, and plans are reviewed on a periodic basis. The management has clearly aligned responsibilities as described in the job descriptions which are prepared for all employees of the Company. A set of measures has been taken to assure the quality of the financial and management information, amongst others: • The appointment of qualified personnel in key positions with all entities of the Company; • The definition of a set of standard procedures for key activities such as steps for the approval, purchasing and payment of services and goods; • The request for the external auditors to pay special attention to areas with specific company and industry risk; • The request for specialized consultants to assist in designing and/or reviewing key procedures, systems, or reports; • The audit committee or individual Directors periodically review and are consulted on key matters and procedures and when needed external specialist assistance is sought. The Board periodically reviews and provides instructions to the management team on how to manage credit risks, interest risks, exchange risks, and liquidity risks. As an example, the Board has given instructions on what type of financial instruments the Company can place its cash and on which it is not allowed to do so. The management also seeks external specialized advice on managing such risks. 38
Part II: Corporate Governance Annual Report 2021 Shareholder information Principal shareholders The Company has an international shareholder base with both large and smaller specialised shareholders focused on the healthcare and life sciences sectors, and a number of more local retail investors. Based on the number of shares on the date of this report, transparency notifications received by the Company until that date, and statements of acquisition of beneficial ownership filed with the SEC under U.S. securities law until that date, the shareholder base of the Company is as set out in the table below. It is possible that the information below in relation to a shareholder is not or no longer up-to-date. All notification and declaration are available on the Company's website (https://mdxhealth.com/). Date of notification On a non-diluted basis % of the voting rights attached to Shares (1) On a fully diluted basis % of the voting rights attached to shares (2) MVM Partners LLP (3) 27 December 2021 22.8% 21.47% Bleichroeder LP (4) 14 February 2022 15.25% 14.38% Valiance Asset Management Limited (5) 27 December 2021 12.2% 11.50% Biovest NV (6) 14 February 2022 7.1% 6.66% Notes: (1) The percentage of voting rights is calculated on the basis of the number of outstanding shares at the date of the notification. On the date of this report, the share capital of the Company amounts to EUR 118,662,067.69. It is divided into 155,969,226 shares of no nominal value, each representing the same fraction of the share capital. (2) The percentage of voting rights is calculated on the basis of a total of 165,405,601 shares, consisting of 155,969,226 shares outstanding on the date of this report and the issuance 9,436,375 additional shares, assuming that (i) 264,000 new shares were issued upon the exercise of 264,000 share options, issued under the form of subscription rights on 15 June 2012, (ii) 582,500 new shares were issued upon the exercise of 582,500 share options, issued under the form of subscription rights on 23 June 2014 (of which 66,500 share options remained available for grant but the Company decided no longer to grant these share options), (iii) 1,999,875 new shares were issued upon the exercise of 1,999,875 share options, issued under the form of subscription rights on 19 June 2017, (iv) 2,990,000 new shares were issued upon the exercise of 2,990,000 share options, issued under the form of subscription rights on 21 June 2019 (of which 69,500 share options have not yet been granted), (v) 3,600,000 new shares were issued upon the exercise of 3,600,000 share options, issued under the form of subscription rights on 27 May 2021 (of which 430,000 share options have not yet been granted),and (vi) 1,047,267 new shares were issued to the benefit of Kreos Capital (as defined below) upon the conversion of a drawdown fee, an amount of EUR 180,000 and an amount of EUR 202,500 into new shares pursuant to a loan agreement, as amended, entered into by the Company with Kreos Capital. (3) MVM Partners LLP, MVM V LP and MVM GP (No. 5) (collectively, the "MVM Entities") jointly filed with the SEC a statement on Schedule 13D according to which the aggregate number of shares beneficially owned by the MVM Entities represents 22.8% of the outstanding shares and voting rights of the Company at the time of statement on Schedule 13D. Notably, it follows from the statement on Schedule 13D that an aggregate of 35,504,584 ordinary shares are beneficially owned by MVM Partners LLP, which consists of (i) 25,805,845 ordinary shares and 898,147 ADSs representing 8,981,470 ordinary shares held by MVM V LP and (ii) 532,079 ordinary shares and 18,519 ADSs representing 185,190 ordinary shares held by MVM GP (No. 5). The statement on Schedule 13D also specifies that (i) MVM Partners LLP provides investment advisory services to the MVM V LP and MVM GP (No. 5), which directly hold the ordinary shares reflected as being beneficially owned by MVM V LP and MVM GP (No. 5) herein, and in such capacity MVM Partners LLP has voting and dispositive power over such shares; (ii) investment decisions for MVM V LP and MVM GP (No. 5) are made by an investment committee at MVM Partners LLP which consists of five individuals; (iii) no single 39

Part II: Corporate Governance Annual Report 2021 individual member of the investment committee, or any other individual at MVM Partners LLP, has the power to unilaterally make investment decisions for the MVM Entities or to direct the voting or disposition of the shares; (iv) Dr. Eric Bednarski, an investment manager and partner at MVM Partners LLP, is a member of the board of directors of the Company; and (v) the MVM Entities entered into a joint filing agreement, dated 27 December 2021, a copy of which is attached to the statement on Schedule 13D. (4) Bleichroeder LP and Bleichroeder Holdings LLC jointly (collectively, the "Bleichroeder Entities") filed with the SEC a statement on Schedule 13G according to which the aggregate number of shares beneficially owned by the Bleichroeder Entities represents 15.25% of the outstanding shares and voting rights of the Company at the time of statement on Schedule 13G. Notably, it follows from the statement on Schedule 13D that Bleichroeder LP, an investment adviser registered under Section 203 of the Investment Advisers Act of 1940, is deemed to be the beneficial owner of 23,783,330 shares, or 15.25%, of the common stock believed to be outstanding. The 23,783,330 shares include 4,200,000 shares of common stock and 19,583,330 shares of common stock underlying ADSs. 21 April Fund Ltd., a Cayman Islands company for which Bleichroeder LP acts as investment adviser, holds 15,042,162 of these 23,783,330 shares, which equates to 9.64% of common stock believed to be outstanding. Clients of Bleichroeder have the right to receive and the ultimate power to direct the receipt of dividends from, or the proceeds of the sale of, such securities. (5) Valiance Asset Management Limited ("Valiance Management"), TopMDx Ltd. ("TopMDx"), Valiance Life Sciences Growth Investments SICAV-SIF ("LSGI Fund") and Valiance Life Sciences Growth Investments GP S.à r.l. ("LSGI GP") (collectively, the "Valiance Entities") jointly filed with the SEC a statement on Schedule 13D according to which the aggregate number of shares beneficially owned by the Valiance Entities represents 12.2% of the outstanding shares and voting rights of the Company at the time of statement on Schedule 13D. Notably, it follows from the statement on Schedule 13D that an aggregate of 19,027,014 ordinary shares are beneficially owned by Valiance Management, which consist of (i) 8,834,387 ordinary shares, and 160,083 ADSs representing 1,600,830 ordinary shares held by TopMDx, an exempted closed-ended fund registered in British Virgin Islands of which Valiance Asset Management is the investment manager, and (ii) 8,591,797 ordinary shares held by LSGI Fund, a Luxembourg investment fund of which LSGI GP serves as investment manager. The statement on Schedule 13D also specifies that (i) Jan Pensaert, the Founding Managing Partner of Valiance Asset Management, which is affiliated with the Valiance Entities, serves as a member of the Company's board of directors and, in such capacity, may have influence over the corporate activities of the the Company; and (ii) Valiance Management serves as the investment manager of LSGI GP, which is the investment manager of LSGI Fund; however, no agreement exists between Valiance Management and LSGI GP for the purposes of acquiring, holding, voting, or disposing of the equity securities of the the Company and, accordingly, the Valiance Entities disclaim the existence of, or membership in, a “group” for purposes of the statement on Schedule 13D. The shareholding on a fully diluted basis takes into account the exercise of 80,000 share options for new shares of the Company, held by Valiance Advisors LLP, a Director of the Company and a related person to Valiance Asset Management Limited, TopMDx Limited and Valiance Life Sciences Growth Investments SICAV-SIF. (6) Biovest NV and RMM, S.A. (collectively, the "Biovest Entities") jointly filed with the SEC a statement on Schedule 13G according to which the aggregate number of shares beneficially owned by the Biovest Entities represents 7.1% of the outstanding shares and voting rights of the Company at the time of statement on Schedule 13G. Notably, it follows from the statement on Schedule 13G that 11,008,257 ordinary shares held by Biovest NV. The statement on Schedule 13G also specifies that (i) RMM, S.A. is the sole owner of Biovest NV and pursuant to an understanding with Biovest NV, decisions relating to the voting and dispositive power of the shares are shared between Biovest NV and the board of director of RMM, S.A. (the "Board"); and (ii) voting and investment power over the shares managed by the Board is exercised jointly by more than three natural persons and voting and disposition decisions require the approval of a majority of such persons; accordingly, no single natural person has a controlling decision and no individual director of RMM, S.A. should be deemed to be a beneficial owner of the shares. The shareholding on a fully diluted basis does not take into account the exercise of 92,000 share options for new shares of the Company, held by RR-Invest S.à.r.l., a Director of the Company and a company controlled by Mr. Rudi Mariën, who is a director of RMM, S.A. No other shareholders, acting alone or in concert with other shareholders, notified the Company of a participation or an agreement to act in concert in relation to 3% or more of the current total existing voting rights attached to the voting securities of the Company. Each shareholder of the Company is entitled to one vote per share. 40

Part II: Corporate Governance Annual Report 2021 Share capital and shares On the date of this report, the share capital of the Company amounts to EUR 118,662,067.69 and is fully paid-up. It is represented by 155,969,226 ordinary shares, each representing a fractional value of (rounded) EUR 0.7608 and representing one 155,969,226th of the share capital. The Company's shares do not have a nominal value. In addition to the outstanding shares, the Company has a number of outstanding options that are exercisable into ordinary shares, consisting of: • 264,000 outstanding share options issued under the form of subscription rights on 15 June 2012 ("May 2012 Share Options"); • 582,500 outstanding share options issued under the form of subscription rights on 23 June 2014 ("2014 Share Options") (of which 66,500 share options remained available for grant but the Company decided no longer to grant these share options); • 1,999,875 outstanding share options issued under the form of subscription rights on 19 June 2017 ("2017 Share Options"); • 2,990,000 outstanding share options issued under the form of subscription rights on 21 June 2019 ("2019 Share Options") (of which 69,500 share options have not yet been granted); and • 3,600,000 outstanding share options issued under the form of subscription rights on 27 May 2021 ("2021 Share Options") (of which 430,000 share options have not yet been granted). On 23 September 2019, the Company entered into loan agreements with Kreos Capital VI (UK) Limited ("Kreos Capital") with respect to a loan facility of up to EUR 9,000,000, which was fully drawn on 1 November 2019. The Company and Kreos Capital agreed that (i) a drawdown fee equal to 7% of the amounts drawn down under the loan agreements (being EUR 630,000 in aggregate) would remain outstanding as a payable (without accruing interest), and would be convertible into ordinary shares by means of a contribution in kind to the share capital of the Company at a price EUR 0.85 per share (the "DF Convertible Loan Payable"), (ii) according to an amendment to the loan agreements dated 19 October 2020, an amount of EUR 180,000 out of the EUR 9,000,000 loan facility would be convertible into ordinary shares by means of a contribution in kind to the share capital of the Company at a conversion price representing a 25% premium to the 30- day volume weighted average price immediately prior to signing the amendment (i.e., EUR 0.95) (rounded) (the "2020 Discretionary Convertible Loan Payable"), and (iii) according to an amendment to the loan agreements dated 19 April 2021, an additional amount of EUR 202,500 out of the EUR 9,000,000 loan facility would be convertible into ordinary shares by means of a contribution in kind to the share capital of the Company at a conversion price representing a 25% premium to the 30-day volume weighted average price ending 10 days prior to signing the amendment (i.e., EUR 1.41) (rounded) (the "2021 Discretionary Convertible Loan Payable", and together with the DF Convertible Loan and the 2020 Discretionary Convertible Loan Payable, the "Kreos Convertible Loan Payables"). Should the full amount of the Kreos Convertible Loan Payables be converted into new shares of the Company, by means of contributions in kind to the share capital of the Company at their respective conversion prices per share, 1,074,267 new shares would have to be issued by the Company to the benefit of Kreos Capital. 41
Part II: Corporate Governance Annual Report 2021 Part II: Corporate Governance Annual Report 2021 History of share capital At the end of 2021, the issued capital of MDxHealth amounted to EUR 118,662,067.69 represented by 155,969,226 common shares without nominal value. Transaction Number of shares issued Issue price per share (EUR) Issue price per share post stock- split (EUR) Capital increase (EUR) Share capital after transaction (EUR) Share Issu- ance Premium after transaction (EUR) Aggregate # of shares after capi- tal increase The table below provides an overview of the history of the Company’s share capital since its incorporation in 2003. The overview should be read together with the notes set out below the table. Date Incorporation Jan. 10, 2003 Incorporation 202,975 0.30 0.06 61,500.00 61,500.00 0 202,975 Phase I Financing Round December 20, 2002 (Pre Feb. 7, 2003 Capital increase 197,025 ferred A 20.00 Shares) 4.00 3,940,500.00 4,002,000.00 0 400,000 Jun. 30, 2003 Capital increase 33,333 20.00 4.00 666,660.00 4,668,660.00 0 433,333 Sep. 30, 2003 Capital increase 218,139 22.31 4.46 4,866,681.09 9,535,341.09 0 651,472 Jun. 20, 2004 Capital increase 195,504 23.87 4.77 4,666,680.48 14,202,021.57 0 846,976 Phase II Financing Round October 19, 2005 (Preferred B Sh Oct. 28, 2005 Capital increase 375,000 24.00 ares) 4.80 9,000,000.00 23,202,021.57 0 1,221,976 Mar. 31, 2006 Capital increase 193,548 31.00 6.20 5,999,988.00 29,202,009.57 0 1,415,524 Stock Split May 23, 2006 Stock split 5/1 / / / / / 0 7,077,620 Initial Public Off Jun. 30, 2006 ering and Exercise Capital increase in cash of Over-Allotm 2,933,334 ent Warrants 7.50 7.50 22,000,005.00 51,202,014.57 0 10,010,954 Jun. 30, 2006 Capital decrease / / / -10,217,809.00 40,984,205.57 0 10,010,954 Jun. 30, 2006 Capital increase through exercise 440,000 7.50 7.50 1,817,200.00 42,801,405.57 1,482,800.00 10,450,954 of warrants Exercise of Warrants in cash in cash in cash in cash in cash in cash Apr. 18, 2007 Capital increase through exercise of warrants 182,560 4.70 4.70 747,666.16 43,549,071.73 1,593,731.31 10,633,514 Private Placement Oct. 19, 2007 Capital increase in cash 1,063,351 10.00 10.00 4,354,954.02 47,904,025.75 7,872,287.29 11,696,865 Exercise of Warrants Oct. 25, 2007 Capital increase through exercise of warrants 50,837 4.73 4.73 208,202.93 48,112,228.68 7,904,487.77 11,747,702 42

Part II: Corporate Governance Annual Report 2021 Exercise of Warrants Apr. 24, 2008 Capital increase through exercise 61,120 of warrants 4.59 4.59 250,316.96 48,362,545.64 7,934,871.81 11,808,822 Nov.5 , 2008 Capital increase through exercise 19,375 4.73 4.73 79,350.31 48,441,895.95 7,947,140.25 11,828,197 of warrants Private Placement Dec. 18, 2008 Capital increase in cash 1,332,877 6.29 6.29 5,458,797.75 53,900,693.70 10,872,138.83 13,161,074 Exercise of Warrants Apr. 17, 2009 Capital increase through exercise of warrants 24,540 4.49 4.49 100,503.57 54,001,197.27 10,881,808.74 13,185,614 Reduction of Share Capital Jun. 21, 2010 Share Capital reduction / / / / 10,517,661.90 10,881,808.74 13,185,614 Private Placement Apr. 8, 2011 Capital increase in cash 5,436,713 1.50 1.50 4,336,865.96 14,854,527.86 14,700,012.24 18,622,327 Private Placement Jul. 4, 2012 Capital increase in cash 6,891,113 1,45 1,45 5,497,040.84 20,351,568.70 19,202,971.61 25,513,440 Private Placement Jun. 25, 2013 Capital increase in cash 8,737,863 2,05 2,05 6,970,193.32 27,321,762.02 30,232,776.07 34,251,303 Private Placement Nov. 7, 2014 Capital increase in cash 3,425,000 3,60 3,60 2,732,122.50 30,053,884.52 39,830,653.57 37,676,303 Exercise of Warrants Apr. 30, 2015 Capital increase through exercise of warrants 172,187 2.01 2.01 137,353.57 30,191,238.09 40,039,189.53 37,848,490 Private Placement Jun. 26, 2015 Capital increase in cash 6,150,000 4.50 4.50 4,905,855.00 35,097,093.09 62,808,334.53 43,998,490 Private Placement Sep. 18, 2015 Capital increase in cash 1,086,956 4.14 4.14 867,064.80 35,964,157.89 66,441,267.57 45,085,446 Exercise of Warrants Nov. 27, 2015 Capital increase through exercise of warrants 68,187 1.70 1.70 54,392.77 36,018,550.66 66,502,756.44 45,153,633 Exercise of Warrants May 9, 2016 Capital increase through exercise of warrants 116,000 1.70 1.70 92,533.20 36,111,083.86 66,607,143.24 45,269,633 Private Placement Nov. 7, 2016 Capital increase in cash 4,526,962 4.50 4.50 3,611,157.59 39,722,241.45 83,367,314.65 49,796,595 Exercise of Warrants Nov. 10,2016 Capital increase through exercise of warrants 49,000 1.69 1.69 39,087.30 39,761,328.75 83,410,887.35 49,845,595 43
Part II: Corporate Governance Annual Report 2021 Exercise of Warrants May 5, 2017 Capital increase through exercise 103,813 1.94 1.94 82,811.63 39,844,140.38 83,529,614.08 49,949,408 of warrants Private Placement Mar. 26, 2018 Capital increase in cash 9,989,881 3.60 3.60 7,968,928.07 47,813,068.45 111,524,257.61 59,939,289 Private Placement Oct. 1, 2019 Capital increase in cash 10,589,236 0.85 0.85 8,447,033.56 56,260,102.01 112,078,074.65 70,528,525 Private Placement May 15, 2020 Capital increase in cash 20,162,924 0.63 0.63 12,738,632.94 68,998,734.95 112,078,074.65 90,691,449 Private Placement Jan. 26, 2021 Capital increase in cash 27,777,777 0.90 0.90 21,133,332.74 90,132,067.69 115,944,741.21 118,469,226 Initial Public Offering Nasdaq Nov. 8, 2021 Capital increase 37,500,000* 1.04 1.04 28,530,000.00 118,662,067.69 126,480,632.34 155,969,226 Per statutory accounts 118,662,067.69 126,480,632.34 155,969,226 Per IFRS consolidated accounts 106,098,267.06 126,480,632.34 155,969,226 * represented by 3,750,000 American Depositary Shares 44

Part II: Corporate Governance Annual Report 2021 Authorized capital Description of the authorized capital By virtue of the resolution of the extraordinary general shareholders' meeting of the Company held on 27 May 2021, as published by excerpt in the Annexes to the Belgian Official Gazette of 1 June 2021 under number 21333389, the Board of Directors of the Company has been granted certain powers to increase the Company's share capital in the framework of the authorized capital. The powers under the authorized capital have been set out in Article 6 of the Company's articles of association. In the framework of this authorization granted by the extraordinary general shareholders' meeting, the Board of Directors is authorized to increase the share capital of the Company on one or several occasions by a maximum aggregate amount of EUR 90,132,067.69 (excluding issue premium), for a period of five years as from 27 May 2021. This authorization is valid for a period of five years as from 1 June 2021. The Board of Directors may increase the share capital by contributions in cash or in kind, by capitalization of reserves, whether available or unavailable for distribution, and capitalization of issue premiums, with or without the issuance of new shares, with or without voting rights, that will have the rights as will be determined by the Board of Directors. The Board of Directors is also authorized to use this authorization for the issuance of convertible bonds or subscription rights, bonds with subscription rights or other securities. In the event of a capital increase decided by the Board of Directors within the framework of the authorized capital, all issue premiums booked, if any, will be accounted for in accordance with the provisions of the Company's articles of association. The Board of Directors is authorized, when exercising its powers within the framework of the authorized capital, to restrict or cancel, in the interest of the Company, the preferential subscription rights of the shareholders. This restriction or cancellation of the preferential subscription rights can also be done in favor of members of the personnel of the Company or of its subsidiaries, or in favor of one or more persons other than members of the personnel of the Company or of its subsidiaries. The Board of Directors is authorized, with the right of substitution, to amend the articles of association, after each capital increase that has occurred within the framework of the authorized capital, in order to bring them in conformity with the new situation of the share capital and the shares. Available amount in the framework of the authorized capital So far, the Board of Directors has used its powers under the authorized capital on 8 November 2021, by issuing 37,500,000 new shares (3,750,000 American Depositary Shares) for an aggregate amount of EUR 28,530,000.00 (excluding issue premium). As a result, the Board of Directors still has the authority under the authorized capital to increase the share capital of the Company with an aggregate amount of EUR 61,602,067.69 (excluding issue premium). Form and transferability of the shares The shares of the Company can take the form of registered and dematerialized shares. All the Company's shares are fully paid-up and are freely transferable. On 21 January 2021, the Board of Directors decided to increase the share capital of the Company in the framework of the authorized capital by the issuance of a maximum number of shares which still had to be determined, with dis- 45

Part II: Corporate Governance Annual Report 2021 application of the preferential subscription right of the existing shareholders of the Company and, in so far as required, of the existing holders of subscription rights (share options) of the Company, subject to, amongst other things, the condition that the new shares would be offered to a broad group of unidentified Belgian and foreign institutional, qualified, professional and/or other investors, in and outside of Belgium, on the basis of applicable private placement exemptions, in the framework of a private placement through an accelerated bookbuilding procedure. On that basis, the Company decided to instruct investment banks to organize, launch and close the offering of new shares via a private placement through an accelerated bookbuilding procedure. The transaction was launched on 21 January 2021, and later that same day the Company announced that it successfully raised an amount of approximately EUR 25.0 million in gross proceeds by means of a private placement via an accelerated bookbuilding procedure of 27,777,777 new shares at an issue price of EUR 0.90 per share. The settlement and payment of the 27,777,777 new shares took place on 26 January 2021. Of these new shares, 18,138,288 shares were immediately admitted to listing and trading on the regulated market of Euronext Brussels upon their issuance, and 9,639,489 shares were not immediately admitted to listing and trading on the regulated market of Euronext Brussels upon their issuance. The Company prepared a listing prospectus to have the 9,639,489 unlisted shares admitted to listing and trading on the regulated market of Euronext Brussels. The 9,639,489 shares were admitted to listing and trading on the regulated market of Euronext Brussels on 23 April 2021. On 28 October 2021, the Board of Directors decided to increase the share capital of the Company within the framework of the authorised capital through the issuance of new shares, the maximum number and the issue price of which still had to be determined, with dis-application of the preferential subscription right of the existing shareholders of the Company and, in so far as required, of the existing holders of subscription rights (share options) of the Company, all or part of the new shares being represented by ADSs, which were to be registered under the United States Securities Act of 1933, as amended and were to be listed on the Nasdaq Capital Market (the number of new shares to be represented by one ADS was still to be determined). The new shares, represented by ADSs, were to be offered (i) via an initial public offering to retail and institutional investors in the United States, and potentially (ii) via private placements with qualified, professional, institutional and other investors, as the case may be, in countries and jurisdictions outside of the United States in accordance with applicable securities laws and regulations. On that basis, the Company decided to instruct investment banks to organize, launch and close the initial public offering of new shares represented by ADSs in the United States. The transaction was launched on 28 October 2021 and, on 4 November 2021, the Company announced the pricing of its initial public offering in the United States of 3,750,000 ADSs (representing 37,500,000 new shares) at a price to the public of USD 12.00 per ADS for total gross proceeds of USD 45.0 million before deducting underwriting discounts and commissions and estimated offering expenses. The settlement and payment of the 37,500,000 new shares (represented by 3,750,0000 ADSs) took place on 8 November 2021, at an issue price per share of EUR 1,04 (rounded), for a total issue price of EUR 39.065.891,13. The 37,500,000 new shares were not immediately admitted to listing and trading on the regulated market of Euronext Brussels upon their issuance, and the Company prepared a listing prospectus to have the 37,500,000 unlisted shares admitted to listing and trading on the regulated market of Euronext Brussels. The 37,500,000 shares were admitted to listing and trading on the regulated market of Euronext Brussels on 16 December 2021. All of the 155,969,226 existing shares have been admitted to listing and trading on the regulated market of Euronext Brussels. Currency The Company's shares do not have a nominal value, but each reflect the same fraction of the Company's share capital, which is denominated in euro. 46

Part II: Corporate Governance Annual Report 2021 Rights attached to shares Dividend and dividend policy All of the shares of the Company entitle the holder thereof to an equal right to participate in dividends in respect of the financial year ending 31 December 2021 and future years. All of the shares participate equally in the Company's profits (if any). Pursuant to the Belgian Companies and Associations Code, the shareholders can in principle decide on the distribution of profits with a simple majority vote at the occasion of the ordinary general shareholders' meeting, based on the most recent statutory audited financial statements, prepared in accordance with Belgian GAAP and based on a (non-binding) proposal of the Company's Board of Directors. The Belgian Companies and Associations Code and the Company's articles of association also authorize the Board of Directors to declare interim dividends without shareholder approval. The right to pay such interim dividends is, however, subject to certain legal restrictions. The Company's ability to distribute dividends is subject to availability of sufficient distributable profits as defined under Belgian law on the basis of the Company's stand-alone statutory accounts prepared in accordance with Belgian GAAP. In particular, dividends can only be distributed if following the declaration and issuance of the dividends the amount of the Company's net assets on the date of the closing of the last financial year as follows from the statutory non- consolidated financial statements (i.e. summarized, the amount of the assets as shown in the balance sheet, decreased with provisions and liabilities, all in accordance with Belgian accounting rules), decreased with, except in exceptional cases, to be disclosed and justified in the notes to the annual accounts, the non-amortized costs of incorporation and extension and the non-amortized costs for research and development, does not fall below the amount of the paid-up capital (or, if higher, the issued capital), increased with the amount of non-distributable reserves. In addition, pursuant to Belgian law and the Company's articles of association, the Company must allocate an amount of 5% of its Belgian GAAP annual net profit (nettowinst/bénéfices nets) to a legal reserve in its stand-alone statutory accounts, until the legal reserve amounts to 10% of the Company's share capital. The Company's legal reserve currently does not meet this requirement. Accordingly, 5% of its Belgian GAAP annual net profit during future years will need to be allocated to the legal reserve, limiting the Company's ability to pay out dividends to its shareholders. Under the senior secured loan agreement entered into between with Kreos Capital and the Company on 1 November 2019 and amended on 19 October 2020 and 19 April 2021, no distributions can be declared or made without consent of the Kreos Capital. Finally, additional financial restrictions and other limitations may be contained in future credit agreements. American Depositary Shares On 8 November 2021, the Company closed a capital increase following an initial public offering in the United States of 37,500,000 new shares represented by 3,750,000 ADSs and the listing of those ADSs on the NASDAQ Capital Market under the symbol "MDXH" on 4 November 2021. Each ADS represents 10 New shares. The ADSs were offered by means of (i) an initial public offering to retail and institutional investors in the United States, and (ii) private placements with qualified, professional, institutional and other investors, as the case may be, in countries and jurisdictions outside of the United States. The ADSs have been registered under the United States Securities Act of 1933, as amended, by means of a registration statement on Form F-1 filed with the SEC and declared effective by the SEC on 3 November 2021. The Bank of New York Mellon, as depositary, registered and delivered the ADSs. Each ADS represents the right to receive 10 shares. ING Belgium SA/NV acts as custodian for the depositary in Belgium. The depositary’s principal office is located at 240 Greenwich Street, New York, New York 10286 . 47

Part II: Corporate Governance Annual Report 2021 An ADS holder is not be treated as one of the Company's shareholders and does not have any shareholder rights. The depositary will be the holder of the shares represented by the ADSs. A holder of ADSs will have ADS holder rights. A deposit agreement among the Company, the depositary and all persons directly and indirectly holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. The depositary has agreed to pay ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. An ADS holder may surrender its ADSs for the purpose of withdrawal of shares. Upon payment of the depository's fees and expenses and of any taxes or charges, such as stamp taxes or share transfer taxes or fees, the depositary will deliver the shares and any other deposited securities represented by the ADSs to the ADS holder or a person designated by it at the office of the custodian or through a book-entry delivery. The ADS holder may instruct the depositary to vote the number of whole deposited shares its ADSs represent. The depositary will notify the ADS holder of shareholders' meetings or other solicitations of consents and arrange to deliver its voting materials to ADS holders if the Company asks it to in a timely fashion. Those materials will describe the matters to be voted on and explain how the ADS holder may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, and subject to the laws of Belgium and the provisions of the Company's articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. Preferential Subscription Rights In the event of a capital increase in cash with the issue of new shares of the Company, or in the event of an issue of convertible bonds or subscription rights, the existing shareholders have a preferential right to subscribe, pro rata, to the new shares of the Company, convertible bonds or subscription rights. These preferential subscription rights are transferable during the subscription period. The general shareholders' meeting may decide to limit or cancel this preferential subscription right, subject to special reporting requirements. Such decision by the general shareholders' meeting needs to satisfy the same quorum and majority requirements as the decision to increase the Company's share capital. The shareholders may also decide to authorize the Board of Directors to limit or cancel the preferential subscription right within the framework of the authorized capital, subject to the terms and conditions set forth in the Belgian Companies and Associations Code. As mentioned above, the Board of Directors of the Company has been granted certain powers to increase the Company's share capital in the framework of the authorized capital and to cancel the statutory preferential subscription rights of the shareholders (within the meaning of articles 7:191 and 7:193 of the Belgian Companies and Associations Code). The powers under the authorized capital have been set out in article 6 of the Company's articles of association. Generally, unless expressly authorized in advance by the general shareholders' meeting, the authorization of the Board of Directors to increase the share capital of the Company through contributions in cash with cancellation or limitation of the preferential subscription right of the existing shareholders is suspended as of the notification to the Company by the FSMA of a public takeover bid on the financial instruments of the Company. The Company's general shareholders' meeting did not grant such express authorization to the Board of Directors. 48

Part II: Corporate Governance Annual Report 2021 Voting Rights Each shareholder of the Company is entitled to one vote per share. Shareholders may vote by proxy, subject to the rules described in the Company's articles of association. Voting rights can be mainly suspended in relation to shares: • which are not fully paid up, notwithstanding the request thereto of the Board of Directors of the Company; • to which more than one person is entitled or on which more than one person has rights in rem (droits réels) on, except in the event a single representative is appointed for the exercise of the voting right vis-à-vis the Company; • which entitle their holder to voting rights above the threshold of 3%, 5%, 10%, 15%, 20% and any further multiple of 5% of the total number of voting rights attached to the outstanding financial instruments of the Company on the date of the relevant general shareholders' meeting, in the event that the relevant shareholder has not notified the Company and the FSMA at least 20 calendar days prior to the date of the general shareholders' meeting in accordance with the applicable rules on disclosure of major shareholdings; and • of which the voting right was suspended by a competent court or the FSMA. Pursuant to the Belgian Companies and Associations Code, the voting rights attached to shares owned by the Company, or a person acting in its own name but on behalf of the Company, or acquired by a subsidiary of the Company, as the case may be, are suspended. Generally, the general shareholders' meeting has sole authority with respect to: • the approval of the annual financial statements of the Company; • the distribution of profits (except interim dividends); • the appointment (at the proposal of the Board of Directors and upon recommendation by the remuneration and nomination committee) and dismissal of Directors of the Company; • the appointment (at the proposal of the Board of Directors and upon recommendation by the audit committee) and dismissal of the statutory auditor of the Company; • the granting of release from liability to the Directors and the statutory auditor of the Company; • the determination of the remuneration of the Directors and of the statutory auditor for the exercise of their mandate; • the advisory vote on the remuneration report included in the annual report of the Board of Directors, the binding vote on the remuneration policy that the Company submitted for the first time to the general shareholders’ meeting held on 27 May 2021, and subsequently upon every material change to the remuneration policy and in any case at least every four years, and the determination of the following features of the remuneration or compensation of Directors, members of the executive management and certain other executives (as the case may be): (i) in relation to the remuneration of Executive and Non-Executive Directors, members of the executive management and other executives, an exemption from the rule that share based awards can only vest after a period of at least three years as of the grant of the awards, (ii) in relation to the remuneration of Executive Directors, members of the executive management and other executives, an exemption from the rule that (unless the variable remuneration is less than a quarter of the annual remuneration) at least one quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least two years and that at least another quarter of the variable remuneration must be based on performance criteria that have been determined in advance and that can be measured objectively over a period of at least three years, (iii) in relation to the remuneration of Non-Executive Directors, any variable part of the remuneration (provided, however that no variable remuneration can be granted to Independent Non-Executive Directors), and (iv) any service agreements to be entered into with Executive Directors, members of the executive management and other executives providing for severance payments exceeding twelve (12) months’ remuneration (or, subject to a motivated opinion by the remuneration and nomination committee, eighteen (18) months' remuneration); 49

Part II: Corporate Governance Annual Report 2021 • the filing of a claim for liability against Directors; • the decisions relating to the dissolution, merger and certain other reorganisations of the Company; and • the approval of amendments to the articles of association. Right to attend and vote at general shareholders' meetings Ordinary general shareholders’ meeting The ordinary general shareholders' meeting is held at the registered office of the Company or at the place determined in the notice convening the general shareholders' meeting. The meeting is held every year on the last Thursday of May at 10:00 a.m. If this day would be a Belgian public holiday, the ordinary general shareholders' meeting shall be held on the previous business day. At the ordinary general shareholders' meeting, the Board of Directors submits to the shareholders the audited non-consolidated and consolidated annual financial statements and the reports of the Board of Directors and of the statutory auditor with respect thereto. The ordinary general shareholders' meeting then decides on the approval of the statutory annual financial statements, the proposed allocation of the Company's profit or loss, the release from liability of the directors and the statutory auditor, the approval of the remuneration report included in the annual report of the Board of Directors (it being understood that the vote on the remuneration report is only an advisory vote and that the Company must explain in the remuneration report of the subsequent financial year how it took into account the advisory vote of the general shareholders' meeting of the previous financial year), of the remuneration policy (as the case may be), and, when applicable, the (re-)appointment or dismissal of the statutory auditor and/or of all or certain directors. In addition, as relevant, the ordinary general shareholders' meeting must also decide on the approval of the remuneration of the directors and statutory auditor for the exercise of their mandate, and on the approval of provisions of service agreements to be entered into with executive directors, members of the executive management and other executives providing (as the case may be) for severance payments exceeding twelve (12) months' remuneration (or, subject to a motivated opinion by the remuneration and nomination committee, eighteen (18) months' remuneration). Special and extraordinary general shareholders’ meetings The Board of Directors or the statutory auditor (or the liquidators, if appropriate) may, whenever the interest of the Company so requires, convene a special or extraordinary general shareholders' meeting. Such general shareholders' meeting must also be convened every time one or more shareholders holding, alone or together, at least 10% of the Company's share capital so request. Shareholders that do not hold at least 10% of the Company's share capital do not have the right to have the general shareholders' meeting convened. Right to put items on the agenda of the general meeting and to table draft resolutions Shareholders who hold alone or together with other shareholders at least 3% of the Company's share capital have the right to put additional items on the agenda of a general shareholders' meeting that has been convened and to table draft resolutions in relation to items that have been or are to be included in the agenda. This right does not apply to general shareholders' meetings that are being convened on the grounds that the quorum was not met at the first duly convened meeting. Shareholders wishing to exercise this right must prove on the date of their request that they own at least 3% of the outstanding share capital. The ownership must be based, for dematerialised shares, on a certificate issued by the applicable settlement institution for the shares concerned, or by a certified account holder, confirming the number of shares that have been registered in the name of the relevant shareholders and, for registered shares, on a certificate of registration of the relevant shares in the share register book of the Company. In addition, the shareholder concerned must register for the meeting concerned with at least 3% of the outstanding share capital. A request to put additional items on the agenda and/or to table draft resolutions must be submitted in writing, and must contain, in the event of an 50

Part II: Corporate Governance additional agenda item, the text of the agenda item concerned and, in the event of a new draft resolution, the text of the draft resolution. The request must reach the Company at the latest on the twenty-second calendar day preceding the date of the general shareholders' meeting concerned. If the Company receives a request, it will have to publish at the latest on the fifteenth calendar day preceding the general shareholders' meeting an update of the agenda of the meeting with the additional agenda items and draft resolutions. Notices convening the general meeting The notice convening the general shareholders' meeting must state the place, date and hour of the meeting and must include an agenda indicating the items to be discussed and the proposed resolutions. The notice must, as the case may be, include the proposal of the audit committee to nominate a statutory auditor responsible for auditing the consolidated financial statements. The notice also needs to contain a description of the formalities that security holders must fulfil in order to be admitted to the general shareholders' meeting and (as the case may be) exercise their voting right, information on the manner in which shareholders can put additional items on the agenda and table draft resolutions, information on the manner in which security holders can ask questions during the general shareholders' meeting and prior to the meeting via the Company's email address or a specific email address mentioned in this notice, information on the procedure to participate to the general shareholders' meeting by means of a proxy or to vote by means of a remote vote, and, as applicable, the registration date for the general shareholders' meeting. The notice must also mention where shareholders can obtain a copy of the documentation that will be submitted to the general shareholders' meeting, the agenda with the proposed resolutions or, if no resolutions are proposed, updates of the agenda if shareholders have put additional items or draft resolutions on the agenda, the forms to vote by proxy or by means of a remote vote, and the address of the webpage on which the documentation and information relating to the general shareholders' meeting will be made available. This documentation and information, together with the notice and the total number of outstanding voting rights, must also be made available on the Company's website at the same time as the publication of the notice convening the meeting, for a period of five years after the relevant general shareholders' meeting. The notice convening the general shareholders' meeting has to be published at least 30 calendar days prior to the general shareholders' meeting in the Belgian Official Gazette, in a newspaper that is published nation-wide in Belgium, in paper or electronically, in media that can be reasonably relied upon for the dissemination of information within the EEA in a manner ensuring fast access to such information on a non-discriminatory basis, and on the Company's website. A publication in a nation-wide newspaper is not needed for ordinary general shareholders' meetings taking place on the date, hour and place indicated in the articles of association of the Company if the agenda is limited to the treatment and approval of the financial statements, the annual report of the Board of Directors, the report of the statutory auditor, the remuneration report, the severance pay for executive directors, and the discharge from liability of the directors and statutory auditor. In addition to this publication, the notice has to be distributed at least 30 calendar days prior to the meeting via the normal publication means that the Company uses for the publication of press releases and regulated information. The term of 30 calendar days prior to the general shareholders' meeting for the publication and distribution of the convening notice can be reduced to 17 calendar days for a second meeting if, as the case may be, the applicable quorum for the meeting is not reached at the first meeting, the date of the second meeting was mentioned in the notice for the first meeting and no new item is put on the agenda of the second meeting. At the same time as its publication, the convening notice must also be sent to the holders of registered shares, holders of registered convertible bonds, holders of registered subscription rights, holders of registered certificates issued with the co-operation of the Company (if any), and, as the case may be, to the directors and statutory auditor of the Company. This communication needs to be made by e-mail unless the addressee has informed the Company that it wishes to receive the relevant documentation by another equivalent means of communication. If the relevant addressee does not have an e-mail address or if it did not inform the Company thereof, the relevant documentation will be sent by ordinary mail. 51 Annual Report 2021

Part II: Corporate Governance Formalities to attend the meeting All holders of shares, profit-sharing certificates, non-voting shares, convertible bonds, subscription rights or other securities issued by the Company, as the case may be, and all holders of certificates issued with the co-operation of the Company (if any) can attend the general shareholders' meetings insofar as the law or the articles of association entitles them to do so and, as the case may be, gives them the right to participate in voting. In order to be able to attend a general shareholders' meeting, a holder of securities issued by the Company must satisfy two criteria: being registered as holder of securities on the registration date for the meeting, and notify the Company: Firstly, the right to attend general shareholders' meetings applies only to persons who are registered as owning securities on the fourteenth calendar day prior to the general shareholders' meeting at midnight (Belgian time) via registration, in the applicable register book for the securities concerned (for registered securities) or in the accounts of a certified account holder or relevant settlement institution for the securities concerned (for dematerialized securities or securities in book-entry form). Secondly, in order to be admitted to the general shareholders' meeting, securities holders must notify the Company at the latest on the sixth calendar day prior to the general shareholders' meeting whether they intend to attend the meeting and indicate the number of shares in respect of which they intend to do so. For the holders of dematerialized securities or securities in book-entry form, the notice should include a certificate confirming the number of securities that have been registered in their name on the record date. The certificate can be obtained by the holder of the dematerialized securities or securities in book-entry form with the certified account holder or the applicable settlement institution for the securities concerned. The formalities for the registration of securities holders, and the notification of the Company must be further described in the notice convening the general shareholders' meeting. Electronic participation The Board of Directors has the possibility to organize the general shareholders' meeting by means of electronic communication which must (i) allow the Company to verify the capacity and identity of the shareholders using it; (ii) at least enable (a) the securities holders to directly, simultaneously and continuously follow the discussions during the meeting and (b) the shareholders to exercise their voting rights on all points on which the general shareholders' meeting is required to take a decision; and (iii) allow the securities holders to actively participate to the deliberations and to ask questions during the meeting. Voting by proxy or remote voting Each shareholder has, the right to attend a general shareholders' meeting and to vote at the general shareholders' meeting in person or through a proxy holder, who need not be a shareholder. A shareholder may designate, for a given meeting, only one person as proxy holder, except in circumstances where Belgian law allows the designation of multiple proxy holders. The appointment of a proxy holder may take place in paper form or electronically (in which case the form shall be signed by means of an electronic signature in accordance with applicable Belgian law), through a form which shall be made available by the Company. The signed original paper (handwritten) or electronic form must be received by the Company at the latest on the sixth calendar day preceding the meeting. The appointment of a proxy holder must be made in accordance with the applicable rules of Belgian law, including in relation to conflicts of interest and the keeping of a register. The notice convening the meeting may allow shareholders to vote remotely in relation to the general shareholders' meeting, by sending a paper form or, if specifically allowed in the notice convening the meeting, by sending a form Annual Report 2021 52

Part II: Corporate Governance electronically (in which case the form shall be signed by means of an electronic signature in accordance with applicable Belgian law). These forms shall be made available by the Company. The original signed paper form must be received by the Company at the latest on the sixth calendar day preceding the date of the meeting. Voting through the signed electronic form may occur until the last calendar day before the meeting. The Company may also organize a remote vote in relation to the general shareholders' meeting through other electronic communication methods, such as, among others, through one or several websites. The Company shall specify the practical terms of any such remote vote in the convening notice. When votes are cast electronically, an electronic confirmation of receipt of the votes is sent to the relevant shareholders that cast the vote. After the general shareholders' meeting, shareholders can obtain, at least upon request (which must be made no later than three months after the vote), the confirmation that their votes have been validly recorded and taken into account by the Company, unless that information is already available to them. If an intermediary receives such confirmation, it must transmit it without delay to the shareholder. Holders of securities who wish to be represented by proxy or vote remotely must, in any case comply with the formalities to attend the meeting. Holders of shares without voting rights, profit-sharing certificates without voting rights, convertible bonds, warrants or certificates issued with the cooperation of the Company may attend the general shareholders' meeting but only with an advisory vote. Quorum and majorities In general, there is no attendance quorum requirement for a general shareholders' meeting and decisions are generally passed with a simple majority of the votes of the shares present or represented. However, capital increases (other than those decided by the Board of Directors pursuant to the authorized capital), decisions with respect to the Company's dissolution, mergers, de-mergers and certain other reorganizations of the Company, amendments to the articles of association (other than an amendment of the corporate purpose), and certain other matters referred to in the Belgian Companies and Associations Code do not only require the presence or representation of at least 50% of the share capital of the Company but also a majority of at least 75% of the votes cast. An amendment of the Company's corporate purpose requires the approval of at least 80% of the votes cast at a general shareholders' meeting, which can only validly pass such resolution if at least 50% of the share capital of the Company and at least 50% of the profit certificates, if any, are present or represented. In the event where the required quorum is not present or represented at the first meeting, a second meeting needs to be convened through a new notice. The second general shareholders' meeting may validly deliberate and decide regardless of the number of shares present or represented. The special majority requirements, however, remain applicable. Right to ask questions Within the limits of article 7:139 of the Belgian Companies and Associations Code, security holders have a right to ask questions to the directors in connection with the report of the Board of Directors or the items on the agenda of such general shareholders' meeting. However, directors may, in the interest of the Company, refuse to answer questions when the communication of certain information or facts is likely to cause prejudice to the Company or is contrary to the obligations of confidentiality entered into by them or by the Company. Shareholders can also ask questions to the statutory auditor in connection with its report. Such questions can be submitted in writing prior to the meeting or can be asked at the meeting. Written questions to the statutory auditor must be submitted to the Company at the same time. The statutory auditor may, in the interest of the Company, refuse to answer questions when the communication of certain information or facts is likely to cause prejudice to the Company 53 Annual Report 2021

Part II: Corporate Governance or is contrary to its professional secrecy or to obligations of confidentiality entered into by the Company. The statutory auditor has the right to speak at the general meeting in connection with the performance of its duties. Written and oral questions will be answered during the meeting concerned in accordance with applicable law. In addition, in order for written questions to be considered, the shareholders who submitted the written questions concerned must comply with the formalities to attend the meeting. Information that has an impact in case of public takeover bids The Company provides the following information in accordance with article 34 of the Belgian Royal Decree dated 14 November 2007: The share capital of the Company amounts to EUR 118,662,067.69 and is fully paid-up. It is represented by 155,969,226 ordinary shares, each representing a fractional value of (rounded) EUR 0.7608 and representing one 155,969,226th of the share capital. The Company's shares do not have a nominal value. Other than the applicable legislations on the disclosure of significant shareholdings and the Company's articles of association, there are no restrictions on the transfer of shares. There are no holders of any shares with special control rights. There are no share option plans for members of the personnel other than the share option plans disclosed elsewhere in this report. These share option plans contain provisions on accelerated vesting in case of change of control. Each shareholder of the Company is entitled to one vote per share. Voting rights may be suspended as provided in the Company's articles of association and the applicable laws and articles. There are no agreements between shareholders which are known by the Company that may result in restrictions on the transfer of securities and/or the exercise of voting rights. The rules governing appointment and replacement of Board members and amendment to articles of association are set out in the Company's articles of association and the Company's Corporate Governance Charter. The powers of the Board of Directors, more specifically with regard to the power to issue or redeem shares are set out in the Company's articles of association. The Board of Directors was not granted the authorization to purchase its own shares "to avoid imminent and serious danger to the Company" (i.e., to defend against public takeover bids). The Company's articles of association do not provide for any other specific protective mechanisms against public takeover bids. At the date of this report, the Company is a party to the following significant agreements which, upon a change of control of the Company or following a takeover bid can enter into force or, subject to certain conditions, as the case may be, can be amended, be terminated by the other parties thereto or give the other parties thereto a right to an accelerated repayment of outstanding debt obligations of the Company under such agreements: The Company has borrowed an amount equal to EUR 9,000,000, as of 1 November 2019, under a senior secured loan agreement with Kreos Capital, which was amended on 19 October 2020 and on 19 April 2021. The main characteristics of the loan agreement are: Annual Report 2021 54

Part II: Corporate Governance Balance: As of 31 December 2021 the outstanding balance on the loan agreement was EUR 9.0 million (USD 10.5 million). In addition, in connection with the facility, a drawdown fee of EUR 630,000 (USD 714,000) was due to Kreos Capital which was not payable in cash but remained outstanding as a "convertible loan" (the "Initial Convertible Loan"); Term: The Company is required to make monthly interest-only payments on the loan through July 2022. As of August 2022 until maturity, MDxHealth is required to make monthly interest and principal payments. The loan matures in October 2023; Interest: The loan accrues interest at a rate of 9.5% per annum; End-of-loan payment: Upon final repayment of the loan, an end-of-loan payment equal to EUR 585,000 (USD 692,000) will be due to Kreos Capital; Initial Convertible Loan: The Initial Convertible Loan does not accrue interest and is not required to be repaid. The Company will not have the right to prepay or otherwise terminate the Initial Convertible Loan. The Initial Convertible Loan expires on the earlier of (i) the tenth anniversary of the drawdown of the loan (i.e., 1 November 2029) and (ii) the sale of the entire issued share capital of MDxHealth (the "Expiration Date"); Conversion of the Initial Convertible Loan: Upon the Expiration Date, the Initial Convertible Loan will convert automatically into ordinary shares. Prior to the Expiration Date, Kreos Capital may at any time convert the Initial Convertible Loan into new ordinary shares at its election. Upon conversion of the Initial Convertible Loan, the relevant shares of the Company will be valued at EUR 0.85 per share; Cancellation of the Initial Convertible Loan: In lieu of converting the Initial Convertible Loan, Kreos Capital may instead cancel the convertible loan at any time (but before the Expiration Date) after the earlier to occur of (i) a repayment or prepayment in full of the loan, and (ii) sale of the entire issued share capital of the Company. In such case, Kreos Capital will be paid an amount equal to 150% of the principal amount of the Initial Convertible Loan. Additional convertible amounts: In the framework of amendments to the loan after the initial signing date, it has been agreed that an additional EUR 180,000 (USD 204,000) of the loan will be convertible into shares of the Company at a 25% premium to the 30-day volume weighted average price immediately prior to signing the amendment of 19 October 2020 (i.e., EUR 0.95) (rounded) and EUR 202,500 (USD 229,000) of the loan will be convertible into shares of the Company at a 25% premium to the 30-day volume weighted average price ten days prior to signing the amendment of 19 April 2021 (i.e., EUR 1.41) (rounded). These amounts form part of the loan and are thus subject to the amortization schedule and the voluntary prepayment provisions of the loan agreement. If exercised, these amounts will be reduced from the principal amount due under the loan agreement. Board observer: Kreos Capital has a non-voting board observer; Change of control: The loan agreement contains a change of control clause, which was approved by the Company's shareholders at the ordinary general shareholders' meeting that was held on 28 May 2020; Collateral: Security has been granted over all assets owned by the Company and its subsidiaries, including IP rights (but excluding any shares in, and IP rights licensed to, the Company or its subsidiaries); Contractual restrictions: The loan agreement does not contain financial covenants, but it does contain other customary restrictions on the business of the Company and its subsidiaries (such as limitations on future disposals, financial indebtedness, security and acquisitions subject to certain carve-outs and limitations). In addition, the Company's share option plans provide for an accelerated vesting of the subscription rights in case of a change of control event. No takeover bid has been instigated by third parties in respect of the Company's equity during the current financial year. 55 Annual Report 2021

Part II: Corporate Governance Notification of significant shareholdings Pursuant to the Belgian Act of 2 May 2007 on the disclosure of significant shareholdings in issuers whose securities are admitted to trading on a regulated market and containing various provisions, as amended from time to time (the "Belgian Transparency Act"), a notification to the Company and to the FSMA is required by all natural persons and legal entities (i.e. legal person, enterprise without legal personality, or trust), in the following circumstances: an acquisition or disposal of voting securities, voting rights or financial instruments that are treated as voting securities; the reaching of a threshold by persons or legal entities acting in concert; the conclusion, modification or termination of an agreement to act in concert; the downward reaching of the lowest threshold; the passive reaching of a threshold; the holding of voting securities in the Company upon first admission thereof to trading on a regulated market; where a previous notification concerning the financial instruments treated as equivalent to voting securities is updated; the acquisition or disposal of the control of an entity that holds voting securities in the Company; and where the Company introduces additional notification thresholds in the articles of association, in each case where the percentage of voting rights attached to the securities held by such persons reaches, exceeds or falls below the legal threshold, set at 5% of the total voting rights, and 10%, 15%, 20% and so on in increments of 5% or, as the case may be, the additional thresholds provided in the articles of association. The Company has provided for an additional threshold of 3% in its articles of association. The notification must be made promptly and at the latest within four trading days following the moment on which the person who is subject to the notification obligation received knowledge or could be deemed to have received knowledge of the acquisition or disposal of the voting rights triggering the reaching of the threshold. Where the Company receives a notification of information regarding the reaching of a threshold, it has to publish such information within three trading days following receipt of the notification. Subject to certain exceptions, no shareholder may, pursuant to article 25/1 of the Belgian Transparency Act, cast a greater number of votes at a general shareholders' meeting of the Company than those attached to the rights and securities that it has notified in accordance with the aforementioned disclosure rules at least 20 calendar days prior to the date of the general shareholders' meeting. The forms on which such notifications must be made, as well as further explanations, can be found on the website of the FSMA (www.FSMA.be). Violation of the disclosure requirements may result in the suspension of voting rights, a court order to sell the securities to a third party and/or criminal liability. The FSMA may also impose administrative sanctions. The Company is required to publicly disclose any notifications received regarding increases or decreases in a shareholder's ownership of the Company's securities, and must mention these notifications in the notes to its financial statements. A list as well as a copy of such notifications will be accessible on the Company's website (www.mdxhealth.com). The obligation to disclose significant shareholdings as well as certain other provisions of Belgian law (e.g. merger control, authorized capital and the requirement to have certain change of control clauses approved by an extraordinary shareholders' meeting) that may apply to the Company, may make an unsolicited tender offer, merger, change in man- agement or other change in control, more difficult. Such provisions could discourage potential takeover attempts that third parties may consider and that other shareholders may consider to be in their best interest and could adversely affect the market price of the shares and ADSs. These provisions may also deprive shareholders of the opportunity to sell their shares and ADSs at a premium (which is typically offered in the context of a takeover bid). Annual Report 2021 56

Part II: Corporate Governance In accordance with U.S. federal securities laws, holders of shares and holders of ADSs will be required to comply with disclosure requirements relating to their ownership of the Company's securities. Any person that, after acquiring ben- eficial ownership of shares or ADSs, is the beneficial owners of more than 5% of shares or shares underlying ADSs must file with the SEC a Schedule 13D or Schedule 13G, as applicable, disclosing the information required by such schedules, including the number of shares or shares underlying ADSs that such person has acquired (whether alone or jointly with one or more other persons). In addition, if any material change occurs in the facts set forth in the report filed on Schedule 13D (including a more than 1% increase or decrease in the percentage of the total shares beneficially owned), the beneficial owner must promptly file an amendment disclosing such change. Click here to return to table of contents 57 Annual Report 2021

Part II: Corporate Governance Statutory auditor Services performed by the auditor and performance of exceptional activities or execution of special instructions (Article 3:65 Belgian Companies and Associations Code) BDO Réviseurs d’Entreprises. SRL, a limited liability company (société à responsabilité limitée/besloten vennootschap) organized and existing under the laws of Belgium, with registered office at Da Vincilaan 9, 1930 Zaventem, Belgium, was re-appointed on 27 May 2020 as the statutory auditor of the Company for a term of 3 years ending immediately after the closing of the ordinary general shareholders’ meeting to be held in 2023. As Mr. Gert Claes had been the permanent representative of the statutory auditor for a period of 6 years, since 29 May 2015, in accordance with Belgian law, Mr. Gert Claes has been replaced by Mr. Bert Kegels as permanent representative of the statutory auditor of the Company with effect as of the closing of the ordinary general shareholders' meeting held on 27 May 2021 and for the remaining term of the mandate of the statutory auditor of the Company. The statutory auditor and the auditor responsible for the audit of the consolidated financial statements, confirms annually in writing to the audit committee his or her independence from the Company, discloses annually to the audit committee any additional services provided to the Company, and discusses with the audit committee the threats to his or her independence and the safeguards applied to mitigate those threats as documented by him or her. During the past fiscal year, in addition to their usual activity, the statutory auditor performed additional activities on behalf of the Company mainly for the issuance of special reports related to warrant plans, grant report certification, for participation to the audit committees and for participation to special projects. The Company expensed $382,346 (€325,401) in fees to the auditor in 2021. The fees are broken down as follows: Audit fee for statutory and consolidated financials of $182,125 (€155,000) Audit related and other services $200,221 (€ 170,401) Click here to return to table of contents Annual Report 2021 58

Part II: Corporate Governance Remuneration report The following remuneration report has been prepared by the nomination and remuneration committee and approved by the Board of Directors of MDxHealth on 20 April 2022. This remuneration report is part of the Corporate Governance Statement, which is part of the Company's annual report of the Board of Directors on the statutory accounts for the financial year ended on 31 December 2021 in accordance to in article 3:6, §3 of the Belgian Companies and Associations Code (the “Remuneration Report”). The Company has reviewed the remuneration policy of its management, Executive and Non-Executive Directors in light of article 3:6 of the Belgian Companies and Associations Code, as supplemented by the relevant provisions of the 2020 Code, and has prepared this Remuneration Report in accordance with the requirements contained therein. Introduction In accordance with article 3:6, §3 of the Belgian Companies and Associations Code, the Company prepared this remuneration report in order to provide an overview of the remuneration, including all benefits granted or due during the financial year ended on 31 December 2021 to each of the Directors and members of the executive management team, including newly recruited officers and former officers, in accordance with the Company's remuneration policy. The remuneration for Non-Executive Directors was modified at a special general shareholders’ meeting of 30 July 2020. In addition, the ordinary general shareholders' meeting held on 27 May 2021 approved an increase of the additional maximum annual fixed remuneration of the chair of the Board of Directors from EUR 31,000.00 (ca. USD 36,673) to EUR 59,500.00 (ca. USD 70,388) (all amounts being exclusive of VAT and similar charges), effective as from 1 July 2021. In conformity with the applicable legislation, the nomination and remuneration committee of the Board of Directors, composed of Non-Executive members of the Board, has the tasks (i) to formulate proposals on the remuneration policy applicable to Directors, managers and other executives, as well as on the determination of their remuneration on an individual basis, and (ii) to prepare the remuneration report to be inserted in the corporate governance statement of the annual report. In accordance with article 7:89/1 of the Belgian Companies and Associations Code, listed companies must establish a remuneration policy with respect to Directors, other officers and delegates for day-to-day management. This article details the objectives of, as well as the information that needs to be included in, the remuneration policy. The remuneration policy must be approved by a binding vote of the general shareholders' meeting and must be submitted 59 Annual Report 2021

Part II: Corporate Governance to the general shareholders' meeting for approval whenever there is a material change and in any case at least every four years. In view hereof, in accordance with article 7:89/1 of the Belgian Companies and Associations Code, the shareholders approved a new remuneration policy that the Board of Directors submitted to the ordinary general shareholders' meeting held on 27 May 2021. No significant change to the remuneration policy is envisaged for 2022 or the following accounting years. However, the Company will continuously review the remuneration of Directors and executive managers against market practice. This remuneration report will be submitted to a vote by the ordinary general shareholders' meeting Procedure adopted in 2021 to determine the level of remuneration Directors Annually, the nomination and remuneration committee reviews the fee levels paid to Directors and compares them to fee levels paid at other comparable companies. Grants of subscription rights to Non-Independent Non-Executive Directors were recommended by the non-conflicted members of the nomination and remuneration committee, reviewed by the Board of Directors and submitted to the general shareholders’ meeting for approval. The number of subscription rights granted in the past to Non-Executive Directors (including Independent Directors) has remained low compared to the number of total outstanding security instruments. Non-Executive Directors (including Independent Directors) are not entitled to bonuses, fringe benefits or pension benefits. Non-Executive Board members who provide services to the Company outside of the formal Board meetings or Board committee meetings, must have their work and fees pre-approved by the non-conflicted members of the nomination and remuneration committee. These fees are then submitted for approval at the ensuing ordinary general shareholders’ meeting. For the executive Director position, the nomination and remuneration committee proposes remuneration changes and bonuses, if any to the Board of Directors for approval. CEO and executive managers The remuneration of the executive management is designed to attract, retain and motivate executive managers. The level and structure of the remuneration are subject to an annual review by the nomination and remuneration committee to take into account market practice. The annual review does not provide mechanisms for automatic adjustments, except for changes that are legally required. The fixed remuneration level, the variable bonus, and the objectives of the CEO are reviewed by the nomination and remuneration committee, compared to industry and market levels, and confirmed by the Board of Directors. The Board of Directors sets the Company objectives and the personal objectives of the CEO. The CEO sets the personal objectives of the other executive managers. He recommends grants of subscription rights, bonuses and changes, if any, in the fixed remuneration of executive managers to the nomination and remuneration committee. The nomination and remuneration committee reviews these recommendations and compares them to industry and market practices. It then proposes the subscription rights grants, bonuses and remuneration changes, if any, to the Board of Directors, and to the extent required by applicable law, to the general shareholders’ meeting, for approval. Annual Report 2021 60

Part II: Corporate Governance Directors' remuneration in 2021 A record of Board attendance is maintained by the secretary to the Board of Directors. This record is then reviewed by the Board of Directors and confirmed by the approval of the Board minutes. Regular attendance at scheduled meetings of the Board of Directors, including committee meetings, is expected. In the event that a Director fails to attend at least 75% of the scheduled meeting of the Board of Directors during a calendar year, the Board may reduce such Director’s applicable annual retainer fee by a pro rata amount to reflect actual attendance. The Directors' remuneration was last modified at the ordinary general shareholders’ meeting of 27 May 2021. Independent Non-Executive Directors Following the modification of the Directors' remuneration on 30 July 2020, effective as from July 1, 2020, the Independent Non-Executive Directors are remunerated on the basis of a pre-defined fixed annual retainer fee as follows: EUR 35,000.00 (USD 41,394.50)1 base fee for each Non-Executive Director; In addition to the base fee, the following fees apply: EUR 31,000.00 (USD 36,663.70) 1 for the chair of the Board of Directors; EUR 17,500.00 (USD 20,697.25) 1 for the chair of the audit committee; EUR 9,000.00 (USD 10,644.30) 1 for the members of the audit committee (other than the chair of the committee); EUR 17,500.00 (USD 20,697.25) 1 for the chair of the nomination and remuneration committee; and EUR 5,500.00 (USD 6,504.85) 1 for the members of the nomination and remuneration committee (other than the chair of the committee). The foregoing additional remuneration amounts are in addition to the base fee and can be combined, depending on whether the applicable eligibility criteria have been met. The remuneration can be reduced pro rata temporis depending on the duration of the mandate, chairpersonship or membership of a Director during a given year. Furthermore, at the occasion of the ordinary general shareholders' meeting held on 27 May 2021, without prejudice to the remuneration of the members of the Board of Directors approved by the special general shareholders' meeting held on 30 July 2020, which resolution shall continue to apply, effective as from 1 July 2021, the additional maximum annual fixed remuneration of the chair of the Board of Directors has been increased from EUR 31,000.00 (ca. USD 36,663.70) 1 to EUR 59,500.00 (ca. USD 70,370.65) 1 (all amounts being exclusive of VAT and similar charges). This fee structure was proposed by the nomination and remuneration committee on the basis of a bench-mark study that was carried out in 2020 and is in line with the existing market practices. The Company's Board of Directors considers that it contributes to the long-term performance of the company. Non-Independent Non-Executive Directors Following the modification of the Directors' remuneration on 30 July 2020, effective as from 1 July 2020, the Non- Executive Directors that are not Independent Directors shall not be entitled to a remuneration in cash, but shall each year be entitled to receive share options for a maximum of 10,000 shares of the Company. This is contrary to provision 7.6 of the 2020 Code, which provides that no share options should be granted to Non- Executive Directors. The Company believes that this provision of the 2020 Code is not appropriate and adapted to take into account the realities of companies in the biotech and life sciences industry. Notably, the ability to remunerate 1 exchange rate EUR 1 = USD 1.1827 (historical rate 2021) 61 Annual Report 2021

Part II: Corporate Governance Non-Executive Directors with share options allowes the Company to limit the portion of remuneration in cash that the Company would otherwise need to pay to attract or retain renowned experts with the most relevant skills, knowledge and expertise. The Company is of the opinion that granting Non-Independent Non-Executive Directors the opportunity to be remunerated in part in share-based incentives rather than all in cash enables the Non-Independent Non-Executive Directors to link their effective remuneration to the performance of the Company and to strengthen the alignment of their interests with the interests of the Company’s shareholders. The Company believes that this is in the interest of the Company and its stakeholders. Furthermore, the Company believes that this is customary for Directors active in companies in the life sciences industry. Furthermore, as the Company currently does not hold any of its own shares as treasury stock and does not have the ability to acquire its own shares, in 2021, Non-Executive Directors did not receive a part of their remuneration in the form of shares of the Company. Even though this deviates from provision 7.6 of the 2020 Code, the Company's Board of Directors considers that this remuneration contributes to aligning the interests of the Non-Independent Non-Executive Directors with those of MDxHealth, amongst other things, by involving them in the risks and prospects of its activities in a long-term perspective. Their remuneration contributes to MDxHealth's long-term performance. Non-Executive Directors Apart from the above remuneration, Non-Executive Directors are entitled to a reimbursement of out-of-pocket expenses actually incurred to participate to Board meetings. The mandate of Non-Executive Directors can be terminated at any time without any compensation. Non-Executive Directors do not receive any form of pension plan benefits from the Company. The Company has not made any loans to the members of the Board of Directors. Executive Directors Executive Directors do not receive any remuneration for their position as a Director. Executive Directors are only remunerated for their role as executive managers. These individuals receive a fixed remuneration plus a variable bonus that is linked to their personal achievements and the achievements of the Company. They do not receive any additional remuneration for the exercise of their Board mandate. The mandate of executive Directors may be terminated at any time without any form of compensation. Their remuneration package is approved by the general shareholders’ meeting. The CEO is the only executive Director of the Board of Directors of the Company and he does not earn any remuneration in respect of his executive Director position. All Directors Relative importance of the components of remuneration: The relative importance of the various components of remuneration of the Directors as referred to in article 3:6, §3, indent. 3, 1°, b) of the Belgian Companies and Associations Code, is provided below under the “Remuneration earned by the Directors for the reported year“ section of this remuneration report. No deviation from the remuneration, as decided by the general shareholders' meetings held on 30 July 2020 and 27 May 2021: During the course of 2021, the Company has not deviated from its remuneration for Directors. The total remuneration of the Board of Directors (excluding the Executive Director who is only remunerated for his role as CEO) in 2021 and 2020 was of EUR 730,000 (USD 863,000) and EUR 678,000 (USD 775,000) respectively (excluding VAT, share-based compensation and expenses reimbursement). Annual Report 2021 62

Part II: Corporate Governance Insurances: On 23 May 2006, the Board of Directors decided, with application of the old article 523 of the Belgian Company Code (article 7:96 of the Belgian Companies and Associations Code), that the Company would indemnify the Directors against any claim by a third party based on Directors’ liability, except in the event of gross negligence and willful misconduct. Therefore, the Company has taken out Directors’ liability insurance. The insurance policy was renewed in 2021. Additionally, the Company’s US subsidiary, MDxHealth, Inc., has entered into indemnification agreements directly with each of its Directors, as well as each Director of the Company, to indemnify each such person for liabilities to the extent that they may arise from, or claims therefor which are based on, US- associated activities of the US subsidiary or of the Company, including any claims based on a theory of derivative liability in the right of the US subsidiary. No possibility to recover variable remuneration: Once paid, the Company does not have the ability to recover the variable part of the remuneration of the Directors. 63 Annual Report 2021
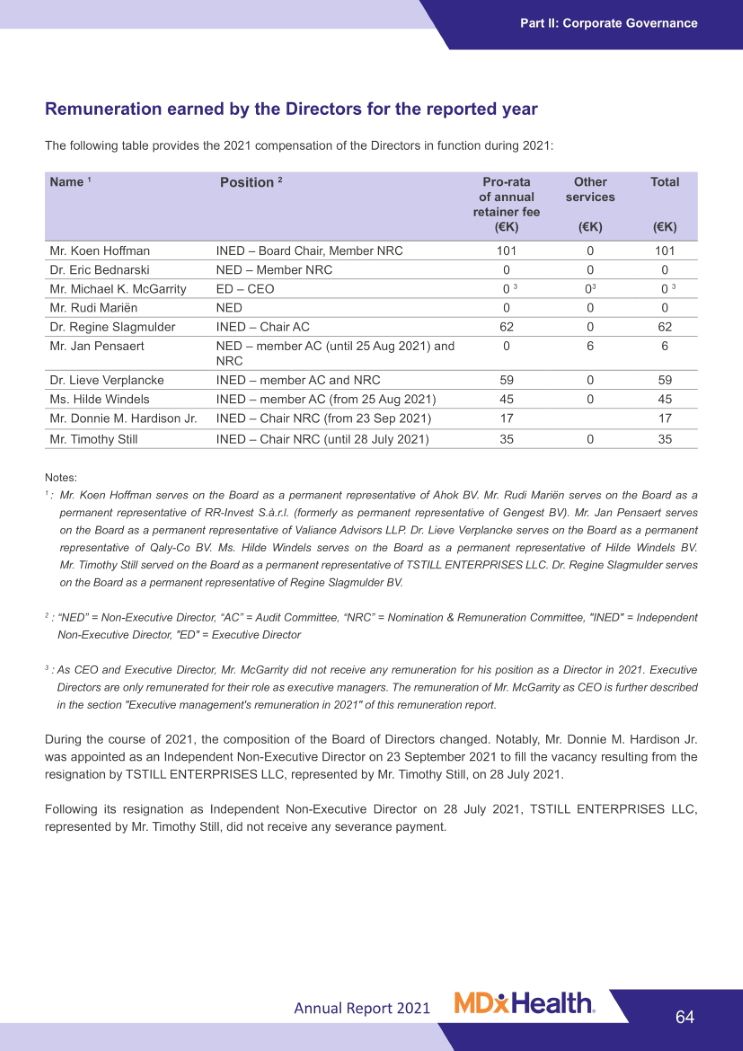
Part II: Corporate Governance Remuneration earned by the Directors for the reported year The following table provides the 2021 compensation of the Directors in function during 2021: Name 1 Position 2 Pro-rata of annual retainer fee Other services Total (€K) (€K) (€K) Mr. Koen Hoffman INED – Board Chair, Member NRC 101 0 101 Dr. Eric Bednarski NED – Member NRC 0 0 0 Mr. Michael K. McGarrity ED – CEO 0 3 03 0 3 Mr. Rudi Mariën NED 0 0 0 Dr. Regine Slagmulder INED – Chair AC 62 0 62 Mr. Jan Pensaert NED – member AC (until 25 Aug 2021) and NRC 0 6 6 Dr. Lieve Verplancke INED – member AC and NRC 59 0 59 Ms. Hilde Windels INED – member AC (from 25 Aug 2021) 45 0 45 Mr. Donnie M. Hardison Jr. INED – Chair NRC (from 23 Sep 2021) 17 17 Mr. Timothy Still INED – Chair NRC (until 28 July 2021) 35 0 35 Notes: 1 : Mr. Koen Hoffman serves on the Board as a permanent representative of Ahok BV. Mr. Rudi Mariën serves on the Board as a permanent representative of RR-Invest S.à.r.l. (formerly as permanent representative of Gengest BV). Mr. Jan Pensaert serves on the Board as a permanent representative of Valiance Advisors LLP. Dr. Lieve Verplancke serves on the Board as a permanent representative of Qaly-Co BV. Ms. Hilde Windels serves on the Board as a permanent representative of Hilde Windels BV. Mr. Timothy Still served on the Board as a permanent representative of TSTILL ENTERPRISES LLC. Dr. Regine Slagmulder serves on the Board as a permanent representative of Regine Slagmulder BV. 2 : “NED” = Non-Executive Director, “AC” = Audit Committee, “NRC” = Nomination & Remuneration Committee, "INED" = Independent Non-Executive Director, "ED" = Executive Director 3 : As CEO and Executive Director, Mr. McGarrity did not receive any remuneration for his position as a Director in 2021. Executive Directors are only remunerated for their role as executive managers. The remuneration of Mr. McGarrity as CEO is further described in the section "Executive management's remuneration in 2021" of this remuneration report. During the course of 2021, the composition of the Board of Directors changed. Notably, Mr. Donnie M. Hardison Jr. was appointed as an Independent Non-Executive Director on 23 September 2021 to fill the vacancy resulting from the resignation by TSTILL ENTERPRISES LLC, represented by Mr. Timothy Still, on 28 July 2021. Following its resignation as Independent Non-Executive Director on 28 July 2021, TSTILL ENTERPRISES LLC, represented by Mr. Timothy Still, did not receive any severance payment. Annual Report 2021 64

Part II: Corporate Governance Executive management's remuneration in 2021 Each member of the executive management is entitled to a basic fixed remuneration designed to fit responsibilities, relevant experience and competences, in line with market rates for equivalent positions. The majority of the annual remuneration is a fixed compensation amount. There is no minimum or maximum variable bonus. The CEO has a fixed remuneration, a fixed bonus and a variable bonus linked to the performance of the Company and to his capacity to manage remuneration costs. The management team members receive a fixed remuneration plus a variable bonus that is linked to their personal achievements (i.e. experience, know-how, education, skills, responsibilities, and performance) and the achievements of the Company. The remuneration is closely linked to performance. Bonuses, if any, are linked to identifiable objectives and to special projects and are set and measured on a calendar-year basis. Non-performers are not retained in the Company. The performance objectives of the management team members are primarily evaluated with regard to the following criteria: (i) respect of the Board-approved annual budget, and (ii) meeting measurable operational targets. The various objectives and their weighting may differ for the individual managers. The nomination and remuneration committee of the Board of Directors meets annually to review the performance of the managers, to compare the actual measurable results to the objectives that were pre-defined by the committee, and to establish the measurable objectives for the ensuing calendar year. In addition, members of the executive management team are also granted with subscription rights. This policy contributes to aligning the interests of the members of the executive management with those of MDxHealth, amongst other things, by involving them in the risks and prospects of its activities in a long-term perspective. Their remuneration contributes to the MDxHealth's long-term performance. Each member of the executive management who is a salaried employee may be entitled to a number of fringe benefits, which may include participating in a defined contribution pension or retirement scheme, disability insurance, a company car, a mobile telephone, internet access and/or a laptop computer according to general Company policy, and other collective benefits (such as hospitalization insurance and meal vouchers). In 2021, all the members of the executive management were engaged on the basis of an employment contract. The employment contracts are generally for an indefinite term, with a trial period. The employment contracts may be terminated at any time by the Company, subject to a severance notice or payment in line with market standards (see also above). The employment contracts include, where appropriate, non-competition undertakings, as well as confidentiality and IP transfer undertakings (that will try to seek maximum protection of the Company’s interests, under applicable laws and subject to the employee’s agreement). Executive members who are engaged on the basis of a services contract do not receive fringe benefits, except that they may be provided with a mobile phone and laptop computer according to General Company policy, and they qualify for reimbursement of expenses incurred while carrying out their professional responsibilities. Executive managers of the Company that are employed under employee contracts are entitled to enroll in defined- contribution type pension plans (such as 401K plans in the United States). The assets of these pension plans are held and managed by third-party organizations and the Company only makes contributions to these plans during the term of service of the employee. Executive managers of the Company that are engaged on the basis of a service agreement are not entitled to any pension plans or pension plan contributions from the Company. The relative importance of the various components of remuneration of the members of the executive management as referred to in article 3:6, §3, indent. 3, 1°, b) of the Belgian Companies and Associations Code, is provided below under the “Remuneration earned by the CEO for the reported year“, "Remuneration earned by other executive managers for the reported year" sections of this remuneration report. 65 Annual Report 2021
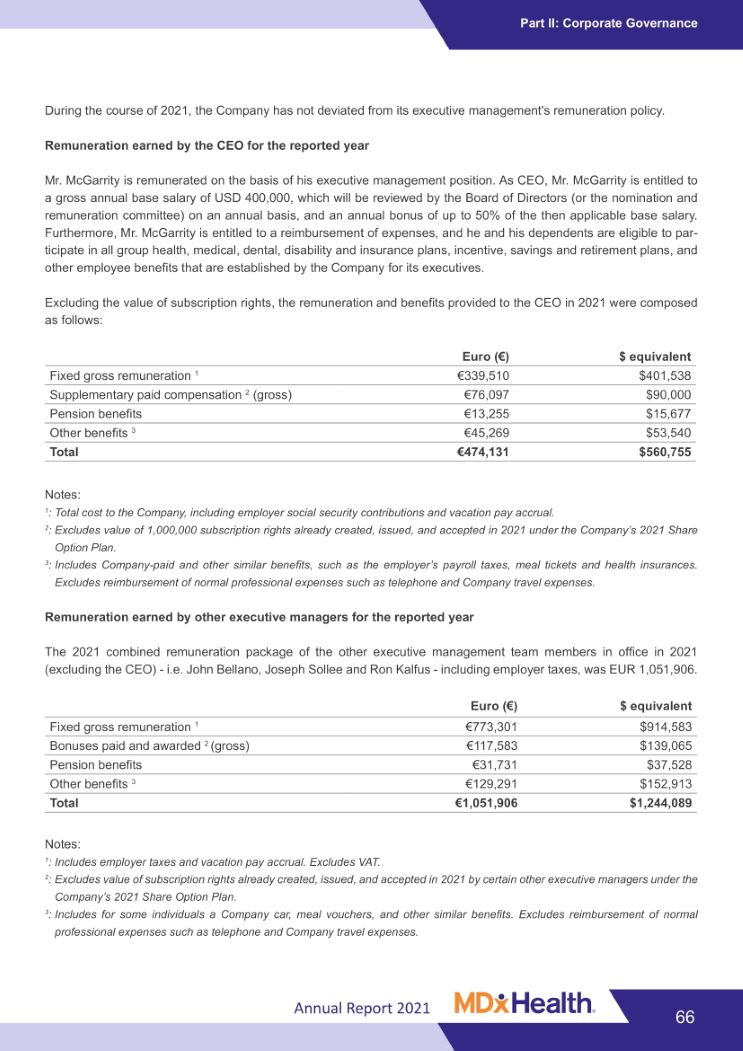
Part II: Corporate Governance During the course of 2021, the Company has not deviated from its executive management's remuneration policy. Remuneration earned by the CEO for the reported year Mr. McGarrity is remunerated on the basis of his executive management position. As CEO, Mr. McGarrity is entitled to a gross annual base salary of USD 400,000, which will be reviewed by the Board of Directors (or the nomination and remuneration committee) on an annual basis, and an annual bonus of up to 50% of the then applicable base salary. Furthermore, Mr. McGarrity is entitled to a reimbursement of expenses, and he and his dependents are eligible to par- ticipate in all group health, medical, dental, disability and insurance plans, incentive, savings and retirement plans, and other employee benefits that are established by the Company for its executives. Excluding the value of subscription rights, the remuneration and benefits provided to the CEO in 2021 were composed as follows: Euro (€) $ equivalent Fixed gross remuneration 1 €339,510 $401,538 Supplementary paid compensation 2 (gross) €76,097 $90,000 Pension benefits €13,255 $15,677 Other benefits 3 €45,269 $53,540 Total €474,131 $560,755 Notes: 1: Total cost to the Company, including employer social security contributions and vacation pay accrual. 2: Excludes value of 1,000,000 subscription rights already created, issued, and accepted in 2021 under the Company’s 2021 Share Option Plan. 3: Includes Company-paid and other similar benefits, such as the employer’s payroll taxes, meal tickets and health insurances. Excludes reimbursement of normal professional expenses such as telephone and Company travel expenses. Remuneration earned by other executive managers for the reported year The 2021 combined remuneration package of the other executive management team members in office in 2021 (excluding the CEO) - i.e. John Bellano, Joseph Sollee and Ron Kalfus - including employer taxes, was EUR 1,051,906. Euro (€) $ equivalent Fixed gross remuneration 1 €773,301 $914,583 Bonuses paid and awarded 2 (gross) €117,583 $139,065 Pension benefits €31,731 $37,528 Other benefits 3 €129,291 $152,913 Total €1,051,906 $1,244,089 Notes: 1: Includes employer taxes and vacation pay accrual. Excludes VAT. 2: Excludes value of subscription rights already created, issued, and accepted in 2021 by certain other executive managers under the Company’s 2021 Share Option Plan. 3: Includes for some individuals a Company car, meal vouchers, and other similar benefits. Excludes reimbursement of normal professional expenses such as telephone and Company travel expenses. Annual Report 2021 66

Part II: Corporate Governance The total remuneration and benefits paid to the executive management team members (including the CEO) in 2021 and 2020 was EUR 1,526,037 (USD 1,804,845) and EUR 1,516,682 (USD 1,732,354) respectively (gross amount, excluding VAT and share based compensation). In the aforementioned figures, the service fees of the managers hired on the basis of a service agreement are included with the salaries of the other management team members. The primary performance objectives for the bonuses of the above management team members in 2021 were the following: respect of the Board-approved annual budget, with a focus on cash-flow management meeting measurable operational targets, such as the commercialization of its ConfirmMDx for Prostate and SelectMDx for Prostate tests and attainment of revenue targets Special provisions of the contractual relationship with the executive management Each of the executive managers has a contractual employment agreement. The Company hired Mr. Michael K. McGarrity, acting in the role of Chief Executive Officer, effective as of 18 February 2019. The executive employment agreement with Mr. McGarrity provides that if the Company terminates the employment agreement without cause or if Mr. McGarrity resigns for good reason, Mr. McGarrity shall be eligible to receive as severance an amount equal to twelve (12) months of base salary in effect at the time of the separation. Acting under the direction of the Board, the Company hired Mr. Ron Kalfus, acting in the role of Chief Financial Officer, effective as of 22 July 2019. The employment agreement with Mr. Kalfus provides that if the Company terminates the employment agreement without cause or if Mr. Kalfus resigns for good reason, Mr. Kalfus shall be eligible to receive as severance an amount equal to six (6) months of base salary in effect at the time of the separation, which amount was automatically increased to twelve (12) months of base salary after 22 July 2020. Acting under the direction of Board, the Company hired Mr. John Bellano, acting in the role of Chief Commercial Officer, effective as of 19 June 2019. The employment agreement with Mr. Bellano provides that if the Company terminates the employment agreement without cause or if Mr. Bellano resigns for good reason, Mr. Bellano shall be eligible to receive as severance an amount equal to six (6) months of base salary in effect at the time of the separation, which amount was automatically increased to twelve (12) months of base salary after 19 June 2020. The employment contract with Mr. Joe Sollee dates from before the entry into force of the law of 6 April 2010 on corporate governance in public and listed companies and is in conformity with common employment law. The contract with Mr. Sollee provides that if his employment is terminated for a reason other than serious misconduct or if Mr. Sollee resigns for good reason, he will be entitled to a severance pay of nine (9) months gross remuneration and benefits. The contracts with the executive managers and the Executive Director do not include any provision stating that the variable part of the remuneration based upon faulty financial information will be recovered by the Company. Subscription rights Share options granted by the Company generally take the form of subscription rights in the sense of article 7:67 and seq. of the Belgian Companies and Associations Code. Subscription rights can periodically be awarded to members of the personnel as defined under article 1:27 of the Belgian Companies and Associations code (with the exception of Non-Independent Directors), or even certain consultants, primarily as a retention and motivation tool. Subscription rights typically vest over time (subject to the beneficiary remaining with the Company) and can only be exercised after a specific period of time, except where the Company decides otherwise. During 2020, the Company modified its 67 Annual Report 2021

Part II: Corporate Governance remuneration policy to provide that the Company will no longer grant share options to Independent Directors. In the course of 2021, no subscription rights were exercised by Directors and executive managers. 2021 Share-based compensation of Directors and executive managers During the course of 2021, the following share-based compensation was awarded to Directors and executive managers of MDxHealth: On 3 July 2021, each Non-Independent Non-Executive Director serving on the Board as of 27 May 2021 (at the occasion of the ordinary general shareholders meeting), were granted 10,000 new subscription rights with the following characteristics: Exercise price of EUR 1.375 (one share option (subscription right) gives right to buy one share); Cliff vesting over 1 year for all beneficiaries; and Duration of options: 10 years. Mr. Eric Bednarski, a Non-Independent Non-Executive Director serving on the Board, declined to accept any of the new subscription rights upon his receipt of notice of the grant. On 3 July 2021, a total of 2,200,000 subscription rights were granted to members of the executive management team. Of these 2,200,000 granted subscription rights, 1,100,000 vest in accordance with a straight-line vesting schedule over three years for all beneficiaries, with the following additional characteristics: Exercise price of EUR 1.375 (one subscription rights gives right to buy one share); Exercise Period: the subscription rights are not exercisable until after the third anniversary the date of their grant; Duration of the subscription right: 10 years. The 1,100,000 subscription rights were granted as follows: Mr. McGarrity received 500,000 subscription rights; Mr. Bellano received 225,000 subscription rights; Mr. Kalfus received 200,000 subscription rights; Mr. Sollee received 175,000 subscription rights. Of these 2,200,000 granted subscription rights, 1,100,000 were granted with the following characteristics: Exercise price of EUR 1.375 (one subscription rights gives right to buy one share); Cliff vesting in the first calendar quarter of 2023, if the Company attains specified corporate goals for the full fiscal year 2022 approved by the Board of Directors; Exercise Period: the subscription rights are not exercisable until after the third anniversary the date of their grant; Duration of the subscription right: 10 years. The 1,100,000 subscription rights were granted as follows:: Mr. McGarrity received 500,000 subscription rights; Mr. Bellano received 225,000 subscription rights; Mr. Kalfus received 200,000 subscription rights; Mr. Sollee received 175,000 subscription rights. Annual Report 2021 68
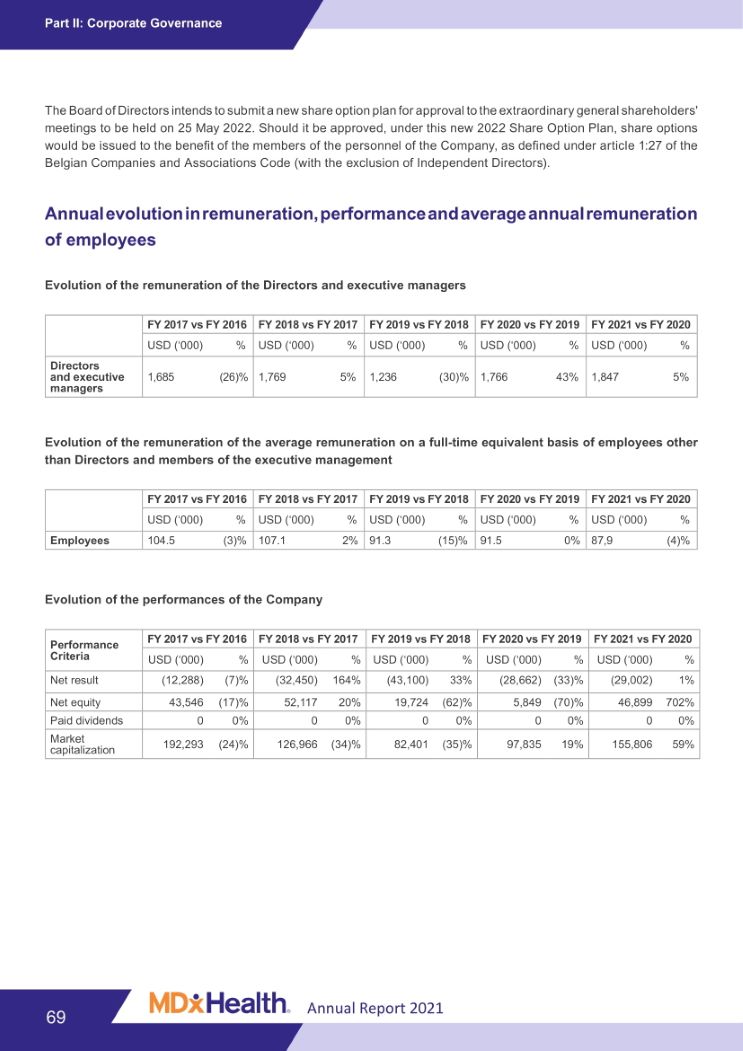
Part II: Corporate Governance The Board of Directors intends to submit a new share option plan for approval to the extraordinary general shareholders' meetings to be held on 25 May 2022. Should it be approved, under this new 2022 Share Option Plan, share options would be issued to the benefit of the members of the personnel of the Company, as defined under article 1:27 of the Belgian Companies and Associations Code (with the exclusion of Independent Directors). Annual evolution in remuneration, performance and average annual remuneration of employees Evolution of the remuneration of the Directors and executive managers FY 2017 vs FY 2016 FY 2018 vs FY 2017 FY 2019 vs FY 2018 FY 2020 vs FY 2019 FY 2021 vs FY 2020 USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % Directors and executive managers 1,685 (26)% 1,769 5% 1,236 (30)% 1,766 43% 1,847 5% Evolution of the remuneration of the average remuneration on a full-time equivalent basis of employees other than Directors and members of the executive management FY 2017 vs FY 2016 FY 2018 vs FY 2017 FY 2019 vs FY 2018 FY 2020 vs FY 2019 FY 2021 vs FY 2020 USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % Employees 104.5 (3)% 107.1 2% 91.3 (15)% 91.5 0% 87,9 (4)% Evolution of the performances of the Company Performance Criteria FY 2017 vs FY 2016 FY 2018 vs FY 2017 FY 2019 vs FY 2018 FY 2020 vs FY 2019 FY 2021 vs FY 2020 USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % USD (‘000) % Net result (12,288) (7)% (32,450) 164% (43,100) 33% (28,662) (33)% (29,002) 1% Net equity 43,546 (17)% 52,117 20% 19,724 (62)% 5,849 (70)% 46,899 702% Paid dividends 0 0% 0 0% 0 0% 0 0% 0 0% Market capitalization 192,293 (24)% 126,966 (34)% 82,401 (35)% 97,835 19% 155,806 59% 69 Annual Report 2021

Part II: Corporate Governance Ratio between the highest and the lowest remuneration For the financial year 2021, the ratio, by country, between the highest and the lowest remuneration, expressed on a full-time equivalent basis is: Country Ratio (Highest / Lowest) Belgium 4.19 The Netherlands 2.24 United States of America 16.00 * (Highest / Lowest) Done on 20 April 2022 On behalf of the Board of Directors Click here to return to table of contents Annual Report 2021 70

Part III: Principle Risks & Uncertainties 71 Annual Report 2021

Part III: Principle Risks & Uncertainties Part III: Principle Risks & Uncertainties Our business and our industry are subject to significant risks. You should carefully consider the risks and uncertainties described below, together with all of the other information in this annual report, including our audited consolidated financial statements and related notes. This annual report also includes forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements.” If any of the following risks are realized, our business, financial condition, operating results and prospects could be materially and adversely affected. Summary of Risk Factors The ongoing outbreak of the novel coronavirus (“COVID-19”) resulted in significant declines in sales of the Company’s ConfirmMDx and SelectMDx tests during 2020, adversely affected sales in 2021, and could continue to impact volumes in 2022, and the business may experience other adverse effects as a result of the pandemic. We have a history of losses, and expect to incur net losses in the future and may never achieve profitability. We might require substantial additional funding to continue our operations and to respond to business needs or take advantage of new business opportunities, which may not be available on acceptable terms, or at all. Our commercial success will depend on the market acceptance and adoption of our current and future tests. Our financial results are largely dependent on sales of one test, and we will need to generate sufficient revenues from this and other future solutions to grow our business. We face uncertainties over the reimbursement of our tests by third party payors. Billing and collections processing for our tests is complex and time-consuming, and any delay in transmitting and collecting claims could have an adverse effect on revenue. Annual Report 2021 72

Part III: Principle Risks & Uncertainties Our business and reputation will suffer if we are unable to establish and comply with, stringent quality standards to assure that the highest level of quality is observed in the performance of our tests. We expect to make significant investments to research and develop new tests, which may not be successful. Failure to comply with governmental payor regulations could result in us being excluded from participation in Medicare, Medicaid or other governmental payor programs, which would adversely affect our business. We conduct business in a heavily regulated industry, and changes in regulations or violations of regulations may, directly or indirectly, adversely affect our results of operations and financial condition and harm our business. If the FDA were to begin requiring approval or clearance of our tests, we could incur substantial costs and time delays associated with meeting requirements for premarket clearance or approval. The dual listing of our ordinary shares and ADSs following the U.S. offering may adversely affect the liquidity and value of the ADSs. Risks Related to Our Business and Industry The ongoing outbreak of the novel coronavirus (“COVID-19”) resulted in significant declines in sales of our ConfirmMDx and SelectMDx tests during 2020, adversely affected sales in 2021, and could continue to impact volumes in 2022, and our business may experience other adverse effects as a result of the pandemic. In March 2020, the World Health Organization declared COVID-19 as a global pandemic. COVID-19 variants, including the “delta” variant and the “omicron” variant have led to a resurgence of individuals affected by the virus and, as such, the pandemic continues to affect various industries, the financial markets globally, which may lead to a further economic downturn and increased market volatility, as well as renewed orders to shelter in place, travel restrictions, and mandated business closures. Economic and business prospects in the United States and other countries declined rapidly due to the COVID-19 pandemic and restrictions on individual and business activity to mitigate the pandemic. Because substantially all of our business operations and our workforce are concentrated in the United States, which has reported significant COVID-19 related cases and mortalities, our business, results of operations, and financial condition have been, and may continue to be, significantly adversely affected. In terms of the impact of the COVID-19 pandemic on our operations, representative contact with clinicians began to decline in March 2020 due to COVID-19. This affected both ConfirmMDx and SelectMDx volumes and had a negative effect on our revenues and cash flows. Overall, ConfirmMDx and SelectMDx billed volumes declined by 18% and 39% for the full year 2020, respectively, compared to 2019 pre-pandemic volumes. In 2021, compared to 2019 pre-pandemic volumes, ConfirmMDx and SelectMDx billed volumes were lower by 16% and 37%, respectively. Other impacts of COVID-19 on our business, financial condition, and results of operations have included, but are not limited to, the following: although our laboratory facilities remain operational, we temporarily implemented staggered laboratory shifts and work-from-home policies for non-essential personnel beginning in March 2020 which reduced the level of laboratory throughput capacity available to process testing services by around 20% compared to 2019. During 2021, we began to relax our pandemic-related workplace controls with the implementation of our COVID-19 Reopen Plan, but staggered laboratory shifts and work-from-home policies remain in place pending the continuing resolution of pandemic-related risks in the general population; 73 Annual Report 2021

Part III: Principle Risks & Uncertainties we have adjusted, and expect to continue to adjust, our precautionary measures at our various locations based on our perception of local recovery levels and applicable governmental regulations and our business could be negatively affected if these precautionary measures prove to be excessive, ineffective or inadequate; while we believe that our laboratories’ current throughput capacity, which was temporarily reduced due to staggered shift policies implemented following the onset of the COVID-19 pandemic, is sufficient to handle current customer demand, there can be no assurance that further resource limitations or interruptions or increases in expected demand will not result in service delays or extended turn-around times for our testing services; while our inventories were not materially impacted and we believe that we have and maintain adequate inventories of critical components necessary to process our ConfirmMDx and SelectMDx tests in amounts sufficient to avoid potential disruptions for the next several months, there can be no assurance that our outstanding and future orders needed to maintain appropriate inventories with our component manufacturers will not be delayed or cancelled due to the COVID-19 pandemic; pandemic-related supply chain disruptions (whether caused by restrictions, congestion, or slowdowns in shipping or logistics, increases in demand for certain goods used on our operations, or otherwise) may hinder, or even force us to suspend, operations at some or all of our clinical laboratories; and the healthcare industry and our customers have been negatively impacted by the pandemic, shifting resources toward coronavirus care and limiting non-essential contact with patients, which reduced orders for our testing solutions beginning in March 2020. This has had a negative impact on volumes of our ConfirmMDx and SelectMDx tests. In light of the still high level of cases in the United States and other countries globally, there may be further negative impacts arising from the pandemic. The extent to which COVID-19 affects our operations in 2022 and beyond will ultimately depend on future developments, which remain uncertain and cannot be predicted with confidence, including the progress in vaccinations, the impact of any emerging variants and any additional information that may emerge concerning the severity of COVID-19 and ongoing actions to contain COVID-19 or mitigate its impact. These and other factors arising from the COVID-19 pandemic could worsen in the United States or locally at the location of our offices or the offices of our collaborator companies, each of which could further adversely impact our business generally and could have a material adverse impact on our operations and financial condition and results. We have a history of losses and expect to incur net losses in the future and may never achieve profitability. We have incurred substantial net losses since our inception, and there can be no assurance that we will achieve profitability. As of December 31, 2021, we had an accumulated deficit of $244.3 million and for the year ended December 31, 2021, we had a net loss of $29.0 million and net cash used in operating activities of $22.5 million. We expect our losses to continue as a result of costs relating to ongoing research and development and for increased sales and marketing costs for existing and planned solutions. These losses have had, and will continue to have, an adverse effect on our working capital, total assets, and stockholders’ equity. Even if we achieve significant revenues, we may not become profitable, and even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Failure to become and remain consistently profitable could adversely affect the market price of our common stock and could significantly impair our ability to raise capital or expand our business in accordance with our growth strategy. Historically, we have been able to raise capital at regular occasions. If we are unable to continue to do this, our ability to operate as a going concern could be seriously compromised. We may require substantial additional funding to continue our operations and to respond to business needs or take advantage of new business opportunities, which may not be available on acceptable terms, or at all. Annual Report 2021 74

Part III: Principle Risks & Uncertainties Our capital outlays and operating expenditures are expected to increase over the next several years as commercial operations expand. We may require additional equity or debt funding from time to time in case of a shortfall in cash inflows from operations or to respond to business needs or take advantage of new business opportunities, which may not be available at acceptable terms, or at all. For more information about our cash and cash equivalent position or total liquidity position. If additional funds are raised through the sale of equity, convertible debt or other equity-linked securities, our securityholders’ ownership will be diluted. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of ordinary shares. If additional funds are raised by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of shareholders, and the terms of the debt securities issued could impose significant restrictions on our operations. If adequate funds are not available, we may have to scale back our operations or limit our research and development activities, which may cause us to grow at a slower pace, or not at all, and our business could be adversely affected. Our term loan contains restrictions that limit our flexibility in operating our business, and if we fail to comply with the covenants and other obligations under our loan agreement, the lenders may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations. In September 2019, we entered into a loan facility agreement with Kreos Capital VI (UK) Limited (“Kreos Capital”) which was amended in October 2020 and April 2021. As of December 31, 2021, the facility consisted of a total of €9.0 million ($10.2 million) in term loans (of which €382,500 ($433,000) is convertible into shares of the Company) and a €630,000 ($714,000) convertible loan. We are required to make monthly interest-only payments on the loan through July 2022. Beginning in August 2022 until maturity we are required to make monthly interest and principal payments. The loan matures in October 2023. The loan agreement is collateralized by substantially all of our assets, including intellectual property related to our ConfirmMDx and SelectMDx tests. The loan agreement also subjects us to certain affirmative and negative covenants, including limitations on our ability to transfer or dispose of assets, merge with or acquire other companies, make investments, pay dividends, incur additional indebtedness and liens and conduct transactions with affiliates. As a result of these covenants, we have certain limitations on the manner in which we can conduct our business, and we may be restricted from engaging in favorable business activities or financing future operations or capital needs until our current debt obligations are paid in full or we obtain the consent of Kreos Capital, which we may not be able to obtain. We cannot be certain that we will be able to generate sufficient cash flow or revenue to meet the financial covenants or pay the principal and accrued interest on the debt. In addition, upon the occurrence of an event of default, Kreos Capital, among other things, can declare all indebtedness due and payable immediately, which would adversely impact liquidity and reduce the availability of cash flows to fund working capital needs, capital expenditures and other general corporate purposes. An event of default includes, but is not limited to, our failure to pay any amount due and payable under the loan agreement, the breach of any representation or warranty in the loan agreement, the breach of any covenant in the loan agreement (subject to a cure period in some cases), a change in control as defined in the loan agreement, the default on any debt payments to a third party or any voluntary or involuntary insolvency proceeding. If an event of default occurs and we are unable to repay amounts due under the loan agreement, Kreos Capital could foreclose on substantially all of our assets, including secured intellectual property. We cannot be certain that future working capital, borrowings or equity financings will be available to repay or refinance our debt to Kreos Capital or any other debt we may incur in the future. Our acceptance of a Paycheck Protection Program loan subjects us to a variety of federal regulations and although we may apply for forgiveness of this loan it may not be forgiven. 75 Annual Report 2021

Part III: Principle Risks & Uncertainties In April 2020, we qualified for a $2.3 million loan through the Paycheck Protection Program (the “PPP”) of the U.S. Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”), under a loan agreement administered by the U.S. Small Business Administration. By participating in a federal loan program, we become subject to increased governmental oversight and federal regulatory compliance obligations, including potential civil and criminal liability for making false claims or statements under the U.S. False Claims Act, 31 USC. § 3729 et seq. (the “FCA”). Liability under the FCA and similar federal statutes can carry significant potential monetary penalties and potential jail time, and can arise from both “knowing” and “willful” misstatements. FCA violations will result in a civil penalty per false claim, of not less than $11,181 and not more than $22,363, plus treble the government’s actual damages. A person who violates § 3729 will also be held liable for the government’s costs for bringing a civil action to recover any penalty or damages. If, despite our good faith belief that we satisfied all eligibility requirements for the PPP loan, we are found to have been ineligible to receive the PPP loan or in violation of any of the laws or regulations that apply to us in connection with the PPP loan, we may be subject to penalties, including under the FCA, and could be required to repay the PPP loan. Additionally, if we choose to apply for forgiveness, a review or audit by the SBA or other government entity in connection with any future forgiveness application or claims under the False Claims Act could consume significant financial and management resources. Any of these events could harm our business, results of operations and financial condition. We may engage in acquisitions that could disrupt our business, cause dilution to our stockholders and reduce our financial resources. In addition to the acquisition of NovioGendix, a privately held company based in Nijmegen (The Netherlands), in September 2015, we may enter into other transactions in the future to acquire other businesses, products or technologies. We may be unable to realize the anticipated benefits of the acquisitions or do so within the anticipated timeframe. Any acquisitions may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by the indemnification we may obtain from the seller. In addition, we may not be able to successfully integrate the acquired personnel, technologies and operations into our existing business in an effective, timely and non- disruptive manner. If we are unable to do so, the disruption to our operations could result in additional costs or could distract management’s attention from other initiatives. The molecular diagnostics industry is highly competitive and characterized by rapid technological changes and we may be unable to keep pace with our competitors. The molecular diagnostics field is characterized by rapid technological changes, frequent new product introductions, changing customer preferences, emerging competition, evolving industry and regulatory compliance standards, reimbursement uncertainty and price competition. Moreover, the molecular diagnostics field is intensely competitive both in terms of service and price, and continues to undergo significant consolidation, permitting larger clinical laboratory service providers to increase cost efficiencies and service levels, resulting in more intense competition. The market for assessing men at risk for prostate cancer is large. As a result, this market has attracted competitors, some of which possess substantially greater financial, selling, logistical and laboratory resources, more experience in dealing with third-party payors, and greater market penetration, purchasing power and marketing budgets, as well as more experience in providing diagnostic services. Some companies and institutions are developing serum-based tests and diagnostic tests based on the detection of proteins, nucleic acids or the presence of fragments of mutated genes in the blood that are associated with prostate cancer. These competitors could have technological, financial, reputational, and market access advantages over us. Regarding our ConfirmMDx for Prostate Cancer tissue-based test, several directly competitive products are currently commercially available. In 2014, OPKO Health, Inc., a NYSE listed company, launched the 4Kscore test, a blood based 4-plex test which combines the results of the blood test with clinical information in an algorithm that calculates a patient’s Annual Report 2021 76

Part III: Principle Risks & Uncertainties percent risk for aggressive prostate cancer prior to a biopsy. OPKO is the third largest clinical laboratory in the United States, with a significantly larger sales and marketing team than we have. The 4Kscore test obtained FDA marketing approval in December 2021. Offered at a lower price point, the 4Kscore test offers a competitive price advantage over the ConfirmMDx test. The PCA-3 test from Hologic, a urine-based test, is on the U.S. market as an FDA approved test, which may be perceived as providing a competitive advantage since the ConfirmMDx for Prostate Cancer test is not FDA approved. The PCA-3 test is intended for the same patient population as ConfirmMDx for Prostate Cancer, but our performance has only been established in men who were already recommended by urologists for repeat biopsy. Regarding our SelectMDx for Prostate Cancer tissue-based test, several directly competitive products are currently commercially available. In 2016, ExosomeDx launched the ExoDx (Intelliscore), a urine-based test designed to assess whether a patient presenting for an initial biopsy is at greater risk for high-grade prostate cancer. The ExoDx test competes directly with SelectMDx. In 2018, Bio-Techne Corporation, a large U.S.-based, diversified life sciences company, acquired the ExoDx test. Bio-Techne has greater resources and a significantly larger sales and marketing team than we have. In addition, the ExoDx test may also provide a competitive advantage since, unlike the SelectMDx test, it does not require a prostate massage as part of its specimen collection procedures. In addition to ExoDx, the 4Kscore test offered by OPKO and the Prostate Health Index test, or the “phi score”, offered by Beckman Coulter, both compete directly with the SelectMDx test. Both OPKO and Beckman Coulter have greater resources and a significantly larger sales and marketing team than we do. As a result of these significantly greater resources, these competitors are able to make larger investments into the tests they produce and the sales and marketing of these tests, which may cause us to lose market share. In addition to competitive products, the ConfirmMDx and SelectMDx tests also face competition from multiparametric MRI (“mpMRI”), a clinical diagnostic imaging procedure available to and used by physicians for many years, which focuses on visual tissue analysis. The mpMRI procedure can visually reveal potential locations of abnormal and potentially cancerous prostate tissue characteristics that distinguish tumors from healthy tissue. The visual aspect of diagnostic imaging may feel more accessible and be considered preferable by some physicians over molecular analysis, and there likely is an economic incentive for some physicians to earn a professional fee from the performance of mpMRI procedures. It may be difficult to change the methods or behavior of physicians to incorporate our testing solutions into their practices in conjunction with, or instead of, mpMRI clinical diagnostic imaging procedures. In addition, companies developing or offering capital equipment or point-of-care kits to physicians represent another source of potential competition. These devices are used directly by the physicians or their institutions, which can facilitate adoption. If we are unable to compete effectively with the abovementioned competitors and with new technologies and procedures such as mpMRI, we may lose market share, which could in turn adversely affect our revenues. Our commercial success will depend on the market acceptance and adoption of our current and future tests. Healthcare providers typically take a long time to adopt new products, testing practices and clinical treatments, partly because of perceived liability risks and the uncertainty of third-party coverage and reimbursement. It is critical to the success of our sales efforts that we educate enough patients, clinicians and administrators about molecular diagnostics testing, in general, as well as about our ConfirmMDx and SelectMDx tests, and demonstrate their clinical benefits. It is likely that clinicians may not adopt, and third-party payors may not cover or adequately reimburse for, our tests unless they determine, based on published peer-reviewed journal articles and the experience of other clinicians, that they provide accurate, reliable and cost-effective information. As the healthcare reimbursement system in the United States evolves to place greater emphasis on comparative effectiveness and outcomes data, we cannot predict whether we will have sufficient data, or whether the data we have will be presented to the satisfaction of any payors seeking such data, in the process of determining and maintaining coverage for our diagnostic tests. The administration of clinical and economic utility studies is expensive and demands significant attention from the management team. Our largest ongoing study, a multicenter U.S. observational study of 77 Annual Report 2021

Part III: Principle Risks & Uncertainties ConfirmMDx and SelectMDx entitled a Prospective Validation of Prostate Biomarkers for Repeat Biopsy (“PRIORITY”), has encountered and is expected to continue to experience delays in enrolment and completion as a result of the COVID-19 pandemic. Additionally, we have several smaller post-marketing clinical studies ongoing or planned that are primarily intended to support expanded indications for our ConfirmMDx and SelectMDx tests. There can be no assurance that the PRIORITY study or our other clinical studies will be successfully initiated, enrolled or completed. Also, data collected from these studies may not be positive or consistent with our existing data or may not be statistically significant or compelling to the medical community. If the results obtained from ongoing or future studies are inconsistent with certain results obtained from previous studies, adoption of diagnostic services would suffer, and our business would be harmed. If our tests or the technology underlying our current or future tests do not receive sufficient favorable exposure in peer- reviewed publications, the rate of clinician adoption of our tests and positive reimbursement coverage decisions for our tests could be negatively affected. See “Risk Factors — We face uncertainties over the reimbursement of our tests by third party payors”. The publication of clinical data in peer-reviewed journals is a crucial step in commercializing and obtaining reimbursement for diagnostic tests, and our inability to control when, if ever, our results are published may delay or limit our ability to derive sufficient revenue from any product that is the subject of a study. Our financial results are largely dependent on sales of one test, and we will need to generate sufficient revenues from this and other future solutions to grow our business. Revenues in 2021 and 2020 were largely dependent on the sales of our ConfirmMDx test for Prostate Cancer. Revenues from sales of ConfirmMDx accounted for approximately 91% and 94% of total revenues in 2021 and 2020, respectively. We launched our second test, SelectMDx for Prostate Cancer, in 2016 and we anticipate that sales of SelectMDx will increase and complement sales of ConfirmMDx; however, sales of ConfirmMDx are expected to continue to account for a substantial portion of total revenues for at least the next several years. The commercial success of the ConfirmMDx and SelectMDx tests and our ability to generate sales will depend on several factors, including: acceptance by the medical community; the number of patients undergoing a prostate biopsy procedure; acceptance, endorsement and formal policy approval of favorable reimbursement for the test by Medicare and other third-party payors; our ability to successfully market the tests; the amount and nature of competition from other prostate cancer products and procedures; and our ability to establish and maintain commercial distribution, sales force and laboratory testing capabilities. Based on our expectation that reimbursement for SelectMDx will increase, we expect that sales of the ConfirmMDx test as a proportion of our total revenues will decrease over the next several years. However, there can be no assurance that SelectMDx will be successfully commercialized. If we are unable to increase sales and reimbursement of SelectMDx and ConfirmMDx or successfully develop and commercialize other solutions or enhancements, our revenues and our ability to achieve profitability would be impaired, and the market price of our shares could decline. We face uncertainties concerning the coverage and reimbursement of our tests by third-party payors. Successful commercialization of our tests depends, in large part, on the availability of coverage and adequate reimbursement from government and private payors. Favorable third-party payor coverage and reimbursement are essential to meeting our immediate objectives and long-term commercial goals. In the United States, for new diagnostic solutions, each private and government payor decides whether to cover the test, the amount it will reimburse clinical laboratories or other providers for a covered test, and any specific conditions for coverage and reimbursement. Providers may be unlikely to order a specific diagnostic test unless an applicable third-party payor offers meaningful Annual Report 2021 78

Part III: Principle Risks & Uncertainties reimbursement for the test. Therefore, adequate coverage and reimbursement is critical to the commercial success of a diagnostic product, and if we are unable to secure and maintain favorable coverage determinations and reimbursement, this will undermine our ability to earn revenue from our products. Medicare Reimbursement for diagnostic tests furnished to Medicare beneficiaries (typically patients aged 65 or older) is usually based on a fee schedule set by the U.S. Centers for Medicare & Medicaid Services (“CMS”), a division of the U.S. Department of Health and Human Services (“HHS”). As a Medicare-enrolled laboratory based in California, we bill Noridian Healthcare Solutions (“Noridian”), the Medicare Administrative Contractor (“MAC”), for California, and we are subject to Noridian’s local coverage and reimbursement policies. Noridian participates in the Molecular Diagnostic Services Program (“MolDX”), administered by Palmetto GBA, which handles technical assessments for U.S. laboratories that perform molecular diagnostic testing. In 2014, we obtained a positive LCD under the MolDX program, which provides coverage for ConfirmMDx testing of Medicare patients throughout the United States. However, Medicare does not currently provide coverage and reimbursement for the SelectMDx test. In early 2019, we submitted clinical and outcomes data on our SelectMDx test to the MolDX program as part of a technical assessment process seeking Medicare coverage. In August 2019, Palmetto GBA issued a favorable draft LCD recommending coverage for the SelectMDx test. However, we were subsequently requested to and submitted an update to our technical assessment under the MolDX program for Medicare coverage of SelectMDx. On May 21, 2021, the MolDX Program issued a draft foundational LCD supporting the clinical utility of SelectMDx. This draft foundational LCD that identifies evidence supporting the clinical utility of the SelectMDx test and, if/when finalized, is expected to would support coverage and reimbursement for SelectMDx testing for qualified Medicare patients throughout the United States. The final determination with respect to Medicare coverage and reimbursement of the SelectMDx test therefore remains pending, and there can be no assurance that such coverage and reimbursement will be granted or, if granted, that it will be maintained. Commercial payors Obtaining coverage and reimbursement by commercial payors is a time-consuming and costly process, without a guaranteed outcome, since each commercial payor makes its own decision with respect to whether to cover a particular test and, if so, at what rate to reimburse providers for that test. In addition, several payors and other entities conduct technology assessments of new medical tests and devices and provide the results of these assessments for informational purposes to other parties. These assessments may be used by third-party payors and healthcare providers as grounds to deny coverage for a particular test, or to refuse to use or order a particular test or procedure. The ConfirmMDx and SelectMDx tests have received initial negative technology assessments from several of these entities and are likely to receive more negative technology assessments. We continue to work with third-party payors to obtain coverage and reimbursement for our ConfirmMDx and SelectMDx tests and to appeal coverage denial decisions based on existing and ongoing studies, peer reviewed publications, and support from physician and patient groups. There are no assurances that commercial payors will continue to issue positive coverage and reimbursement policies and/or contracts and, if issued, that such policies and/or contracts will be maintained in the future. If our tests are considered on a policy-wide level by major third-party payors, whether at our request or on the payor’s own initiative, and the payor determines that such tests are ineligible for coverage and reimbursement, our revenue potential could be adversely impacted. Outside the United States Outside of the United States, various coverage, pricing and reimbursement approvals are required, including through coverage determinations made at the national level under public benefit programs. We expect that it will take several years to establish broad coverage and reimbursement for our tests with payors in countries outside of the United States 79 Annual Report 2021

Part III: Principle Risks & Uncertainties where we commercialize our solutions, and our efforts may not be successful. Even if public or private reimbursement is obtained, it may cover competing tests, the reimbursement may be conditioned upon local performance of the tests or other requirements we may encounter difficulties in satisfying. Reimbursement levels outside of the United States may vary considerably from the reimbursement amounts we receive in the United States. In addition, because we plan in many circumstances to rely on distributors to obtain reimbursement for our tests, to the extent the distributor does not have direct reimbursement arrangements with payors, we may not be able to retain reimbursement coverage in certain countries with a particular payor; further, if our agreement with a particular distributor is terminated or expires or a distributor fails to pay for other reasons, we could lose reimbursement coverage in that jurisdiction. Currently, we rely almost entirely on the sale of ConfirmMDx tests in the United States for our revenues, with these tests accounting for approximately 91% and 94% of total revenues in 2021 and 2020, respectively. As noted above, we have not yet obtained Medicare reimbursement for the SelectMDx test and hence the failure to receive a favorable Medicare reimbursement decision will mainly have an impact on our future prospects rather than resulting in an immediate decrease in revenues. If, however, reimbursement for the ConfirmMDx test were to be revoked either by CMS or any of the commercial payors, this could have an immediate impact on our revenues. While we do not believe that revocation of Medicare reimbursement for the ConfirmMDx test is likely, if this were to occur, the impact on us could be severe. Risks Related to Our Intellectual Property If we are unable to retain intellectual property protection in relation to our main test ConfirmMDx and our second test SelectMDx or if we are required to expend significant resources to protect our intellectual property position, our competitive position could be undercut. Our ability to protect our discoveries, know-how and technologies affects our ability to compete and to achieve profitability. We rely on a combination of U.S. and foreign patents and patent applications, copyrights, trademarks and trademark applications, confidentiality or non-disclosure agreements, material transfer agreements, licenses and consulting agreements to protect our intellectual property rights. We also maintain certain company know-how, algorithms, and technological innovations designed to provide us with a competitive advantage in the marketplace as trade secrets. As of December 31, 2021, we owned or had exclusive rights to more than 22 patent families related to our molecular technology and cancer-specific biomarkers. Specifically, there are 116 granted or pending patent applications in this group comprised of 16 issued or allowed U.S. patents, 12 pending U.S. provisional or non-provisional applications, 51 pending international patent applications filed under the Patent Cooperation Treaty (“PCT”) and 40 granted or allowed patents in jurisdictions outside the United States, including Japan, Canada, Israel and the major European countries. Our issued U.S. patents expire at various times between 2024 and 2036. Of these issued patents, two cover intellectual property used in our ConfirmMDx test, one of which expires in November 2022 and the other of which expires in 2024, and one covers intellectual property used in our SelectMDx test which expires in 2036. When these patents expire other companies will no longer be prohibited from incorporating the subject intellectual property into competing tests they may seek to develop. While we intend to pursue additional and future patent applications, it is possible that pending patent applications and any future applications may not result in issued patents. Even if patents are issued, third parties may independently develop similar or competing technology that avoids our patents. Third parties may also assert infringement or other intellectual property claims against us or against our licensors, licensees, suppliers or strategic partners. Any actions regarding patents could be costly and time-consuming and could divert the attention of management and key personnel from other areas of our business. Further, we cannot be certain that the steps we have taken will prevent the misappropriation of our trade secrets and other confidential information as well as the misuse of our patents and other intellectual property, particularly in foreign countries with no patent protection Annual Report 2021 80

Part III: Principle Risks & Uncertainties Although we have licensed and own issued patents in the United States and foreign countries, we cannot be certain the claims will continue to be considered patentable by the U.S. Patent and Trademark Office (the “USPTO”), U.S. courts patent offices and courts in other jurisdictions. The U.S. Supreme Court, other federal courts and/or the USPTO, may change the standards of patentability and any such changes could have a negative impact on our business. For instance, the Federal Circuit has recently ruled on several patent cases, such as Univ. of Utah Research Found. v. Ambry Genetics Corp., 774 F.3d 755 (Fed. Cir. 2014), Ariosa Diagnostics, Inc. v. Sequenom, Inc., 788 F.3d 1371 (Fed. Cir. 2015), Genetic Tech. Ltd. v. Merial LLC, 818 F.3d 1369 (Fed. Cir. 2016), and Cleveland Clinic Found. v. True Health Diagnostics, 859 F.3d 1352 (Fed. Cir. 2017), that some diagnostic method claims are not patent eligible. These decisions have narrowed the scope of patent protection available in certain circumstances or weakened the rights of patent owners in certain situations. Some aspects of our technology involve processes that may be subject to this evolving standard and we cannot guarantee that any of our issued or pending process claims will be patentable as a result of such evolving standards. In addition, this combination of decisions has created uncertainty as to the value of certain issued patents, in particular in the detection of prostate cancer and other cancers. We may be subject to substantial costs and liabilities, or be prevented from using technologies incorporated in our ConfirmMDx and SelectMDx tests, as a result of litigation or other proceedings relating to patent rights. Third parties may assert infringement or other intellectual property claims against us or our licensors, licensees, suppliers or strategic partners. We pursue a patent strategy that we believe provides us with a competitive advantage in the assessment of prostate cancer and is designed to maximize patent protection against third parties in the United States and, potentially, in certain foreign countries. In order to protect or enforce our patent rights, we may have to initiate actions against third parties. Any actions regarding patents could be costly and time-consuming and could divert the attention of management and key personnel from other areas of our business. Additionally, such actions could result in challenges to the validity or applicability of our patents. Because the USPTO maintains patent applications in secrecy until a patent application is published or the patent is issued, we have no way of knowing if others may have filed patent applications covering technologies used by us or our partners. Additionally, there may be third-party patents, patent applications and other intellectual property relevant our technologies that may block or compete with our technologies. Even if third-party claims are without merit, defending a lawsuit may result in substantial expense to us and may divert the attention of management and key personnel. In addition, we cannot provide assurance that we would prevail in any such suits or that the damages or other remedies, if any, awarded against us would not be substantial. Claims of intellectual property infringement may require us, or our strategic partners, to enter into royalty or license agreements with third parties that may not be available on acceptable terms, if at all. These claims may also result in injunctions which could prevent us from further developing and commercializing services or products containing our technologies, which could in turn adversely affect our ability to earn revenues from these services or products. Also, patents and patent applications owned by us may become the subject of post grant challenges or interference proceedings in the USPTO to determine validity and the priority of invention, which could result in substantial cost as well as a possible adverse decision as to the validity or priority of invention of the patent or patent application involved. An adverse decision in an interference proceeding may result in the loss of rights under a patent or patent application subject to such a proceeding. Ultimately, the potential weakening of our intellectual property position as a result of the evolution of case law or otherwise may make us more vulnerable to competition. While we are unable to quantify the impact of this risk given that our patents remain untested in the courts, the impact could be severe if our competitors are able to take advantage of any weakening of our intellectual property position. 81 Annual Report 2021

Part III: Principle Risks & Uncertainties We rely on strategic collaborative and license arrangements with third parties to develop critical intellectual property. We may not be able to successfully establish and maintain such intellectual property. The development and commercialization of our products and services rely, directly or indirectly, upon strategic collaborations and license agreements with third parties. We have a license agreement with an academic institution pursuant to which we have incorporated licensed technology into our ConfirmMDx test and may incorporate licensed technology into our pipeline products. Our dependence on license, collaboration and other similar agreements with third parties may subject us to a number of risks. There can be no assurance that any current contractual arrangements between us and third parties or between our strategic partners and other third parties will be continued on materially similar terms and will not be breached or terminated early. Any failure to obtain or retain the rights to necessary technologies on acceptable commercial terms could require us to re-configure our products and services, which could negatively impact their commercial sale or increase the associated costs, either of which could materially harm our business and adversely affect our future revenues and ability to achieve sustained profitability. We expect to continue and expand our reliance on collaboration and license arrangements. Establishing new strategic collaborations and license arrangements is difficult and time-consuming. Discussions with potential collaborators or licensors may not lead to the establishment of collaborations on favorable terms, if at all. To the extent we agree to work exclusively with one collaborator in a given area, our opportunities to collaborate with other entities could be limited. Potential collaborators or licensors may reject collaborations with us based upon their assessment of our financial, regulatory or intellectual property position or other factors. Even if we successfully establish new collaborations, these relationships may never result in the successful commercialization of any product or service. In addition, the success of the projects that require collaboration with third parties will be dependent on the continued success of such collaborators. There is no guarantee that our collaborators will continue to be successful and, as a result, we may expend considerable time and resources developing products or services that will not ultimately be commercialized. Risks Related to Our Operations Billing and collections processing for our tests is complex and time-consuming, and any delay in transmitting and collecting for claims could adversely impact revenue. Substantially all of our current revenue is derived from the use of our ConfirmMDx test, which is billed on a fee-for- service basis and paid, for example by hospitals and direct payments from individual patients, and may be reimbursed by third-party payors, including Medicare and other governmental payor programs, private insurance plans and managed care organizations. Billing for molecular diagnostics testing services is complex, time-consuming, and expensive. We are often obligated to bill services in the specific manner required by each particular third-party payor. Failure to comply with these complex billing requirements (including complex federal and state regulations related to billing government health care programs, e.g., Medicare and Medicaid) may significantly hinder our collection and retention efforts, including not only potential write-offs of doubtful accounts and long collection cycles for accounts receivable, but also the potential disgorgement of previously paid claims based on third-party payor program integrity investigations into billing discrepancies, fraud, waste and abuse. With CMS’ recent implementation of a comprehensive oversight regime that consolidated program integrity powers into a single Unified Program Integrity Contractor (“UPIC”), audit and investigatory activity into billing fraud, waste and abuse in the industry has in recent years significantly increased. Responding to requests from a UPIC, or other auditor, is often time-consuming and requires dedication of internal, and sometimes external, resources. UPICs also have the authority to implement Medicare payment suspensions during the pendency of an audit, which could significantly impact cash flows, even where no improper billing is ultimately found to have occurred. Commercial payors may also engage in audit activity, requiring timely production of medical documentation in support of billed claims.MDxHealth faces an inherent risk of product liability claims. Annual Report 2021 82

Part III: Principle Risks & Uncertainties Among the potential factors that can complicate third-party payor billing are: Differences between the list price for our tests and the reimbursement rates of payors; compliance with complex federal and state regulations related to billing government health care programs, (e.g., Medicare and Medicaid); disputes among payors as to which party is responsible for payment; differences in coverage among payors and the effect of patient co-payments or co-insurance; differences in information and billing requirements among payors; incorrect or missing billing information; and the resources required to manage the billing and claims appeals process. During the fourth quarter of 2019, and based on recent and historical collections data, we updated certain assumptions to our estimates, which affected our revenues. These included a revision to the period that a vast majority of collections would occur (from 24 months to 12 months); an updated lookback period for historical collection experience in order to use more recent and relevant collection data; and recognition on a cash basis if no historical payment experience is available. Updating these revenue recognition estimates negatively affected our revenues in 2019 in the amount of $10.1 million. We face an inherent risk of product liability claims. The marketing, sale and use of our tests could lead to product or professional liability claims against us if someone were to allege that our tests failed to perform as they were designed, or if someone were to misinterpret test results or improperly rely on them for clinical decisions. Although we maintain product and professional liability insurance which is deemed to be appropriate and adequate, it may not fully protect us from the financial impact of defending against product liability or professional liability claims or any judgments, fines or settlement costs arising out of any such claims. Furthermore, any product liability lawsuit, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could harm our reputation, which could impact our results of operations, or cause collaboration partners to terminate existing agreements and potential partners to seek alternate partners, any of which could negatively impact our results of operations. Failure to attract or retain key personnel or to secure the support of key scientific collaborators could materially adversely impact our business. Our success in implementing our business strategy depends largely on the skills, experience, and performance of key members of our executive management team and others in key management positions, including Michael McGarrity, our Chief Executive Officer. The collective efforts of our executive management team are critical to us as we continue to develop our technologies, tests, and R&D and sales programs. As a result of the difficulty in locating qualified new management, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more of these key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies and implementing our business strategy. Our executives have employment agreements; however, the existence of an employment agreement does not guarantee retention of members of our executive management team. We do not maintain “key person” life insurance on any of our employees. We have established relationships with leading key opinion leaders and scientists at important research and academic institutions that we believe are key to establishing tests using our technologies as a standard of care for cancer assessment and diagnosis. If our collaborators determine that cancer testing using our technologies are not appropriate options for prostate cancer diagnosis, or superior to available prostate cancer methods, or that alternative technologies 83 Annual Report 2021

Part III: Principle Risks & Uncertainties would be more effective in the early diagnosis of prostate cancer, we would encounter significant difficulty establishing tests using our technologies as a standard of care for prostate cancer diagnosis, which would limit our revenue growth and profitability. Our results of operations can be adversely affected by labor shortages, turnover and labor cost increases. Labor is a significant component of operating our business. A number of factors may adversely affect the labor force available to us or increase labor costs, including high employment levels, federal unemployment subsidies, including unemployment benefits offered in response to the COVID-19 pandemic, increased wages offered by other employers, vaccine mandates and other government regulations and our responses thereto. As more employers offer remote work, we may have more difficulty recruiting for jobs that require on-site attendance, such as certain clinical laboratory and sales roles. Although we have not experienced any material labor shortage to date, we have recently observed an overall tightening and increasingly competitive labor market. A sustained labor shortage or increased turnover rates within our employee base, caused by COVID-19 or as a result of general macroeconomic factors, could lead to increased costs, such as increased overtime or financial incentives to meet demand and increased wage rates to attract and retain employees, and could negatively affect our ability to efficiently operate our clinical laboratories and overall business. If we are unable to hire and retain employees capable of performing at a high-level, or if mitigation measures we may take to respond to a decrease in labor availability have unintended negative effects, our business could be adversely affected. Additionally, the operations of our vendors and partners could also suffer from labor shortages, turnover and labor cost increases which could result in supply change disruptions and increases in the costs of the products and services we purchase, each of which could adversely affect our operations. Our business and reputation will suffer if we are unable to establish and comply with, stringent quality standards to assure that the highest level of quality is observed in the performance of our tests. Inherent risks are involved in providing and marketing cancer tests and related services. Patients and healthcare providers rely on us to provide accurate clinical and diagnostic information that may be used to make critical healthcare decisions. As such, users of our tests may have a greater sensitivity to errors than users of some other types of products and services. Past or future performance or accuracy defects, incomplete or improper quality and process controls, excessively slow turnaround times, unanticipated uses of our tests or mishandling of samples or test results (whether by us, patients, healthcare providers, courier delivery services or others) can lead to adverse outcomes for patients and interruptions to our services. These events could lead to voluntary or legally mandated safety alerts relating to our tests or our laboratory facilities and could result in the removal of our products and services from the market or the suspension of our laboratories’ operations. Insufficient quality controls and any resulting negative outcomes could result in significant costs and litigation, as well as negative publicity that could reduce demand for our tests and payors’ willingness to cover our tests. Even if we maintain adequate controls and procedures, damaging and costly errors may occur. Our laboratory facilities may become inoperable due to natural or man-made disasters or regulatory sanctions. We currently perform all of our testing in our laboratory facilities located in Irvine, California and Nijmegen, The Netherlands. Our laboratory facilities could become inoperable due to circumstances that may be beyond our control, and such inoperability could adversely affect our business and operations. The facilities, equipment and other business process systems would be costly to replace and could require substantial time to repair or replace. Annual Report 2021 84

Part III: Principle Risks & Uncertainties The facilities may be damaged or destroyed by natural or man-made disasters, including earthquakes, wildfires, floods, outbreak of disease (such as the ongoing COVID-19 pandemic), acts of terrorism or other criminal activities and power outages, which may render it difficult or impossible for us to perform our tests for some period. The facilities may also be rendered inoperable because of regulatory sanction. In the United States, we are subject to federal and state laws and regulations regarding the operation of clinical laboratories. Our U.S. laboratory facilities in Irvine, California and Plano, Texas are certified under CLIA. CLIA and the laws of California and certain other states, impose certification requirements for clinical laboratories, and establish standards for quality assurance and quality control, among other things. Clinical laboratories are subject to inspection by regulators, and to sanctions for failing to comply with applicable requirements. Sanctions available under CLIA include prohibiting a laboratory from running tests, requiring a laboratory to implement a corrective action plan, and imposing civil monetary penalties. Our U.S. laboratory facility in Irvine, California holds a certificate of accreditation from CMS to perform high-complexity testing, which is managed by California Laboratory Field Services (“CA LFS”). To renew this certificate, the facility is subject to survey and inspection every two years. We also hold a certificate of accreditation from the College of American Pathologists (“CAP”), which sets standards that are higher than those contained in the CLIA regulations. CAP is an independent, non-governmental organization of board-certified pathologists that accredits laboratories nationwide on a voluntary basis. Because CAP has deemed status with CA LFS, biennial inspections will be performed by teams formed by CAP. Sanctions for failure to comply with CAP or CLIA requirements, including proficiency testing violations, may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as the imposition of significant fines or criminal penalties. In addition, our Irvine facility is subject to regulation under state laws and regulations governing laboratory licensure. Two states, one of which is New York, have enacted state licensure laws that are more stringent than CLIA. Failure to maintain CLIA certification, CAP accreditation, or required state licenses could have a material adverse effect on the sales of our tests and results of operations. The Irvine facility receives samples from all 50 U.S. states and certain provinces in Canada. Many states maintain independent licensure, registration, or certification procedures with which our facility must maintain compliance in order to receive and test samples from that location. Maintaining compliance with the myriad of governmental requirements is time and resource intensive, and failure to maintain compliance could result in sanctions. We rely on a limited number of third-party suppliers for services and items used in the production and operation of our testing solutions, and some of those services and items are supplied from a single source. Disruption of the supply chain, unavailability of third-party services required for the performance of the tests, modifications of certain items or failure to achieve economies of scale could have a material adverse effect on us. In connection with our role as a CLIA-certified provider of laboratory services, we assist healthcare providers with certain logistics related to the collection and return of samples for testing. To provide our ConfirmMDx and SelectMDx services, we are required to obtain customized components and services that are currently available from a limited number of sources. Most of these components and services are sourced externally from approximately 40 external suppliers. Many of the consumable supplies and reagents used as raw materials in our testing process are procured from a limited number of suppliers, some of which are single source. In addition, we rely on a limited number of suppliers, or in some cases a single supplier (for example, for the automation of our deparaffination steps for our ConfirmMDx test), for certain services and equipment with which we provide testing services. If we have to switch to a replacement supplier for any of these items that are sub-components or for certain services required for the performance of our tests, or if we have to commence our own manufacturing or testing to satisfy market demand, we may face additional delays. For example, in the past, a supplier has delivered critical non-conforming components that failed our acceptance testing, requiring us to audit the supplier and assist the supplier in improving our internal quality processes. In addition, third party suppliers may be subject to circumstances which impact their ability to supply, including enforcement action by regulatory authorities, natural disasters (e.g., hurricanes, earthquakes, disease and terrorism), epidemics (e.g., 85 Annual Report 2021

Part III: Principle Risks & Uncertainties the ongoing COVID-19 pandemic), industrial action (e.g., strikes), financial difficulties including insolvency, among a variety of other internal or external factors. Any such supply disruptions could in turn result in service disruptions for an extended period of time, which could delay completion of our clinical studies or commercialization activities and prevent us from achieving or maintaining profitability. While we were able to qualify alternative suppliers to address COVID-19 related disruptions, in the future alternative suppliers may be unavailable, may be unwilling to supply, may not have the necessary regulatory approvals, or may not have in place an adequate quality management systems. Furthermore, modifications to a service or items or inclusions of certain services or items made by a third-party supplier could require new approvals from the relevant regulatory authorities before the modified service or item may be used, for example any modifications to the assembly and packaging of items for our testing services supplied to healthcare providers. While we have not experienced any material supply chain disruptions to date, if we were to experience such disruptions, whether as a result of the COVID-19 pandemic or otherwise, this could have an immediate impact on revenues if it related to the ConfirmMDx test, and the impact could be material depending on the length of the supply disruption. Failures in our information technology, telecommunications or other systems could significantly disrupt our operations. We use information technology and telecommunications systems across virtually all aspects of our business, including laboratory testing, sales, billing, customer service, logistics and management of data, including patient information. Our information technology, telecommunications and other systems, are vulnerable to damage and failure, computer viruses, acts of God and physical or electronic break-ins. Despite the precautionary measures we have taken to prevent breakdowns in our information technology and telecommunications systems, sustained or repeated system failures that interrupt our ability to process test orders, deliver test results or perform tests in a timely manner or that cause us to lose patient information could adversely affect our business, results of operations and financial condition. Although we maintain cyber liability insurance which we believe to be appropriate and adequate, the levels and terms of coverage may not be adequate to compensate us for losses that may arise from any such disruption, failure or security breach. In addition, such insurance may not be available to us in the future on economically reasonable terms, or at all. Further, insurance may not cover all claims made against us and could have high deductibles in any event, and defending a suit, regardless of its merit, could be costly and divert management attention. Security breaches or loss of data may harm our reputation, expose us to liability and adversely affect our business. If we experience any security breaches or loss of data or if we fail to comply with data protection laws and regulations, we could be subject to government enforcement actions (which could include civil or criminal penalties), private litigation and/or adverse publicity, which could negatively affect our results of operations and business. We face four primary risks relative to protecting sensitive and critical personally identifiable information, intellectual property or other proprietary business information about our customers, payors, recipients and collaboration partners, including test results: (1) loss of access risk, (2) inappropriate disclosure or access risk, (3) inappropriate modification risk, and (4) the risk of being unable to identify and audit controls over the first three risks. While we devote significant resources to protecting such information, the measures we introduce may not be sufficient to guard against security breaches, the loss or misappropriation of data, privacy violations or the failure to implement satisfactory remedial measures, which could in turn disrupt operations and lead to reputational damage, regulatory penalties and other material financial losses. Furthermore, we are subject to privacy and data security laws and regulations at the state, federal and international level. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., section 5 of the Federal Annual Report 2021 86

Part III: Principle Risks & Uncertainties Trade Commission Act), govern the collection, use, disclosure, and protection of health-related and other personal information. Failure to comply with data protection laws and regulations could result in (1) government enforcement actions and potential liability thereunder (potentially including civil and/or criminal penalties), (2) private litigation, and/or (3) adverse publicity that could negatively affect our operations and/or business. In addition, we obtain health information from third parties (e.g., healthcare providers) and are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”). These laws contain significant fines and other penalties for wrongful use or disclosure of protected data. For example, HIPAA violations can result in civil and criminal penalties. We expect to make significant investments to research and develop new tests, which may not be successful. We are seeking to improve the performance of our existing SelectMDx and ConfirmMDx commercial test offerings and to develop a pipeline for future products and services. Developing new or improved diagnostic tests is a speculative and risky endeavor. Candidate products and services that may initially show promise may fail to achieve the desired results in larger clinical validation studies or may not achieve acceptable levels of clinical accuracy. Results from early studies or trials are not necessarily predictive of future clinical validation or clinical trial results, and interim results of a validation study or trial are not necessarily indicative of final results. From time to time, we may publicly disclose then-available data from clinical validation studies before completion, and the results and related findings and conclusions may be subject to change following the final analysis of the data related to the particular study. As a result, such data should be viewed with caution until the final data are available. Additionally, such data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment and/or follow-up continues and more patient data become available. Significant differences between initial or interim data and final data from either our clinical validation studies or clinical trials could significantly alter our plans to proceed with additional studies or trials, and harm our reputation and business prospects. If we determine that any of our current or future development programs is unlikely to succeed, we may abandon it without any return on our investment into the program. We may need to raise additional capital to bring any new products or services to market, which may not be available on acceptable terms, if at all. Our research and development efforts will be hindered if we are not able to obtain samples, contract with third parties for access to samples or complete timely enrollment in future clinical trials. Access to human sample types, such as blood, tissue, stool, or urine is necessary for our research and product development. Acquiring samples from individuals with clinical diagnoses or associated clinical outcomes through purchase or clinical studies is necessary. Lack of available samples can delay development timelines and increase costs of development. Generally, the agreements under which we gain access to human samples are non-exclusive. Other companies may compete with us for access. Additionally, the process of negotiating access to samples can be lengthy and it may involve numerous parties and approval levels to resolve complex issues such as usage rights, institutional review board approval and patient informed consent, privacy rights, publication rights, intellectual property ownership and research parameters. If we are not able to negotiate access to clinical samples with research institutions, hospitals, clinical partners, pharmaceutical companies, or companies developing therapeutics on a timely basis, or at all, or if other laboratories or our competitors secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed. Finally, we may not be able to conduct or complete clinical trials on a timely basis if we are not able to enroll sufficient numbers of patients in such trials, and our failure to do so could have an adverse effect on our research and development and product commercialization efforts. 87 Annual Report 2021

Part III: Principle Risks & Uncertainties Risks Related to Regulation of Our Business Failure to comply with governmental payor regulations could result in us being excluded from participation in Medicare, Medicaid or other governmental payor programs, which would adversely affect our business. Failure to comply with applicable Medicare, Medicaid and other governmental payor rules could result in us being excluded from participation in one or more governmental payor programs, returning funds already paid, civil monetary penalties, criminal penalties and/or limitations on the operational function of our laboratories. Additionally, with the recent implementation by CMS of a comprehensive oversight regime that consolidates program integrity powers into a single UPIC, audit and investigatory activity into potential billing fraud, waste and abuse in the industry has increased. These changes have adversely affected and may in the future adversely affect coverage and reimbursement for laboratory services, including the molecular diagnostics testing services we provide. If we were unable to receive reimbursement under a governmental payor program, this would have a severe impact on our revenues, given the importance of reimbursement under these programs in our revenue base We conduct business in a heavily regulated industry, and changes in, or violations of, applicable regulations may, directly or indirectly, adversely affect our operational results and financial condition, which could harm our business. Our business operations and activities may be subject to a range of local, state, federal, and international healthcare laws and regulations, including investigatory and program integrity audits and other oversight federal and state health care programs. These laws and regulations currently include, among others: CLIA (which requires laboratories to obtain certification from the federal government) and state laboratory licensure laws; Federal Trade Commission standards regarding advertising and business practices; FDA laws and regulations; HIPAA (which imposes comprehensive federal standards with respect to the privacy and security of protected health information, and requirements for the use of certain standardized electronic transactions), and the amendments to HIPAA under HITECH (which strengthened and expanded HIPAA privacy and security compliance requirements, increased penalties for violators, extended enforcement authority to state attorneys general and imposed requirements for breach notification); state laws regulating genetic testing and the privacy protection of genetic test results, as well as state laws protecting the privacy and security of health information and personal data and mandating reporting of breaches to affected individuals and state regulators; the federal Anti-Kickback Statute (which prohibits knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program) and parallel state anti-kickback laws (which contain similar prohibitions on remuneration between referral sources, although these state laws are not always limited in application to items or services reimbursable by federal or state health care programs); Annual Report 2021 88

Part III: Principle Risks & Uncertainties the federal False Claims Act (which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government or the improper retention of identified overpayments or other financial obligations to the federal government) and parallel state false claims acts (which contain similar prohibition on presenting false or fraudulent claims, although these state may extend to items or services by any third-party payor, including commercial insurers); the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transferring of remuneration to a Medicare or state health care program (e.g., Medicaid) beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies; the federal physician self-referral law, commonly known as the “Stark Law,” which prohibits a physician from making a referral to an entity for certain “designated health services” (“DHS”) payable by Medicare if the physician, or an immediate family member of the physician, has a financial relationship with that entity, unless an exception applies. The Stark Law further prohibits the entity from billing the Medicare program for DHS furnished pursuant to a prohibited referral. In addition, the Stark Law, through the addition of section 1903(s) to the Social Security Act, prohibits the federal government from making federal financial participation payments to state Medicaid programs for DHS furnished as a result of a referral that would violate the Stark Law if Medicare “covered the service to the same extent and under the same conditions” as the state Medicaid Program. The U.S. Department of Justice (“DOJ”) and several state agencies have successfully argued that Section 1903(s) expands the Stark Law to Medicaid-covered claims, even absent a separate state self-referral law prohibiting the same conduct; other federal and state fraud and abuse laws, including (i) the state anti-kickback laws described above, (ii) the state physician self-referral laws, and (iii) the state false claims acts described above; Section 216 of the Protecting Access to Medicare Act of 2014, which requires applicable laboratories to report commercial payor data in a timely and accurate manner beginning in 2017 and every three years thereafter (and in some cases annually); Federal and state laws that impose reporting and other compliance-related requirements; and similar foreign laws and regulations that apply to us in the countries in which we operate. In addition, in October 2018, the Eliminating Kickbacks in Recovery Act of 2018 (“EKRA”), was enacted by the U.S. Congress as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act. EKRA is an all-payor anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory. Although it appears that EKRA was intended to reach patient brokering and similar arrangements to induce patronage of substance use recovery and treatment, the language in EKRA is broadly written. Further, certain of EKRA’s exceptions, such as the exception applicable to relationships with employees that effectively prohibits incentive compensation, are inconsistent with the federal anti-kickback statute and regulations, which permit payment of employee incentive compensation, a practice that is common in the industry. Significantly, EKRA permits the U.S. Department of Justice to issue regulations clarifying EKRA’s exceptions or adding additional exceptions, but such regulations have not yet been issued. Laboratory industry stakeholders are reportedly seeking clarification regarding EKRA’s scope and/or amendments to its language. Our business practices, in operating a U.S. clinical laboratory, may face heightened scrutiny from U.S. government enforcement agencies such as the DOJ, the HHS Office of Inspector General (“OIG”), and CMS. The OIG has issued 89 Annual Report 2021

Part III: Principle Risks & Uncertainties fraud alerts in recent years that identify certain arrangements between clinical laboratories and referring physicians as implicating the federal Anti-Kickback Statute. The OIG has stated that it is particularly concerned about these types of arrangements because the choice of laboratory, as well as the decision to order laboratory tests, typically are made or strongly influenced by the physician, with little or no input from the patient. Moreover, the provision of payments or other items of value by a clinical laboratory to a referring physician could be prohibited under the Stark Law, unless the arrangement meets all criteria of an applicable exception. The government has actively enforced these laws against clinical laboratories in recent years. These U.S. laws and regulations are complex and are subject to interpretation by the U.S. courts and government agencies. Our failure to comply with such laws and regulations could lead to significant civil or criminal penalties, exclusion from participation in state and federal health care programs, individual imprisonment, disgorgement of profits, contractual damages, reputational harm, diminished profits and future earnings, additional reporting or oversight obligations if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with the law, curtailment or restructuring of our operations, or prohibitions or restrictions on our laboratories’ ability to provide or receive payment for our services, any of which could adversely affect our ability to operate our business and pursue our strategy. Even where we are able to successfully defend against any such claims, any potential audit, enforcement action, or litigation would involve substantial internal and external resources, detract from our executives’ day to day responsibilities, and result in legal expenditures, all of which could materially adversely affect our results of operations. While we believe that we are in material compliance with all applicable laws and regulations, there remains a risk that one or more government agencies could take a contrary position, or that a private party could file suit under the qui tam provisions of the federal False Claims Act or a similar state law. Such occurrences, regardless of their outcome, could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations, and other private third-party payors. Our employees, independent contractors, consultants, commercial partners, and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. We are exposed to the risk of fraud, misconduct, or other illegal activity by our employees, independent contractors, consultants, commercial partners, and vendors. Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: comply with the rules and regulations of the CMS, FDA, and other federal and state government agencies as well as comparable foreign regulatory authorities; provide true, complete and accurate information to such regulatory authorities; comply with manufacturing and clinical laboratory standards; comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or report financial information or data accurately or to disclose unauthorized activities to us. In particular, research, sales, marketing, education, and other business arrangements in the healthcare industry are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing, and other abusive practices, as well as off-label product promotion. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, educating, marketing and promotion, sales and commission, certain customer incentive programs, and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of participant recruitment for clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of business conduct and ethics and provide compliance training to our workforce members upon onboarding and annually thereafter, but it is not always possible to identify and deter misconduct by employees and third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions. Even if it is later determined after an action is instituted against us that we were not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our actions, and have to divert significant management resources from other matters. Annual Report 2021 90

Part III: Principle Risks & Uncertainties Our expansion of our business beyond the United States has resulted in additional regulatory requirements with which we must comply. Our expansion of our business outside of the United States increases the potential of violating foreign laws similar to those described above under “ — We conduct business in a heavily regulated industry, and changes in regulations or violations of regulations may, directly or indirectly, adversely affect our results of operations and financial condition and harm our business”. In order to market our tests in other countries, we may be required to obtain regulatory approvals and comply with extensive safety and quality regulations in other countries. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ. The European Union/European Economic Area (the “EU/EEA”), requires a CE conformity mark in order to market medical devices. Many other countries accept CE or FDA clearance or approval, although others, require separate regulatory filings. Further, the advertising and promotion of our products in the EEA is subject to the laws of individual EEA Member States implementing the EU Medical Devices Directives including Directive 98/79/EC on Invitro Diagnostic Medical Devices, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EEA Member State laws governing the advertising and promotion of medical devices. Going forward, CE marking will be pursuant to Regulation 2017/745 (the “Medical Devices Regulation” or “MDR”) and Regulation 2017/746 (the “Invitro Diagnostic Medical Devices Regulation” or “IVDR”), which were passed by the European Parliament on April 5, 2017. The Medical Devices Regulation and the Invitro Diagnostic Medical Devices Regulation contain further obligations for medical devices and invitro diagnostic medical devices with which we will be required to comply as applicable. These new laws are generally stricter than the requirements previously in place and contain increased evidence requirements for CE marking. They may limit or restrict the advertising and promotion of our tests to the general public and may impose limitations on promotional activities with healthcare professionals. The risk of being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even in case of successful defense against it, could result in significant legal expenses and divert management’s attention from the operation of our business. While our business is primarily based in the United States, these laws or regulations would not have an immediate material impact on our revenues. However, in the longer term, our prospects could be seriously harmed. If the FDA were to take the position that our tests are not within the scope of its policy on enforcement discretion for laboratory-developed tests, or Congress or FDA were otherwise to begin requiring approval or clearance of our tests, responding to such a development could lead to a halt in the commercial provision of our tests until we meet the requirements for premarket approval or clearance, enforcement action from FDA, and we could incur substantial costs and time delays associated with meeting FDA requirements for premarket clearance or approval. Although we believe we are within the scope of the FDA’s policy on enforcement discretion for laboratory-developed tests, commercial availability of LDTs is subject to uncertainty given the FDA’s latitude in interpreting and applying its laws and policies. For example, although the FDA has historically exercised enforcement discretion over most LDTs, it does not consider tests to be subject to this enforcement discretion if they were or are designed or manufactured completely, or partly, outside of the laboratory that offers and uses them, or if they are offered “over-the-counter” (as opposed to being available to patients only when prescribed by a health care provider). Even for tests that appear to fall within FDA’s previously stated policy on enforcement discretion, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time. Furthermore, the laws and regulations governing the marketing of diagnostic products are evolving, extremely complex and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. Pursuant to its authority under the federal Food, Drug, and Cosmetic Act (the “FDCA”), the FDA has jurisdiction over Annual Report 2021 91

Part III: Principle Risks & Uncertainties medical devices, including in vitro diagnostics and, therefore, potentially our clinical laboratory tests. Among other things, pursuant to the FDCA and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. Although the FDA has asserted that it has authority to regulate the development and use of LDTs, such as our and many other laboratories’ tests, as medical devices, it has generally exercised enforcement discretion and is not otherwise regulating most tests developed and performed within a single high complexity CLIA-certified laboratory. Even though the ConfirmMDx and SelectMDx tests are commercialized in the United States as LDTs, they may in the future become subject to more onerous regulation by the FDA. For example, the FDA may disagree with the assessment that the tests fall within the definition of an LDT and seek to regulate them as medical devices. The FDA has, for over the past decade, been introducing proposals to end enforcement discretion and to bring LDTs clearly under existing FDA regulatory frameworks and the U.S. Congress has recently been working on legislation to create an LDT and in vitro diagnostic regulatory framework that would be separate and distinct from the existing medical device regulatory framework. If the FDA begins to enforce its medical device requirements for LDTs, or if the FDA disagrees with our assessment that our ConfirmMDx and SelectMDx tests are LDTs, our company and these tests could for the first time be subject to a variety of regulatory requirements, including registration and listing, medical device reporting, and adherence to good manufacturing practices under the quality system regulations, and we could be required to obtain premarket clearance or approval for these existing tests and any new tests we may develop, which may force us to cease or delay marketing our tests until the required clearance or approval are obtained. The premarket review process for diagnostic products can be lengthy, expensive, time-consuming, and unpredictable. Further, obtaining premarket clearance or approval may involve, among other things, successfully completing clinical trials. Clinical trials require significant time and cash resources and are subject to a high degree of risk, including risks of experiencing delays, failing to complete the trial or obtaining unexpected or negative results. If we are required to obtain premarket clearance or approval and/ or conduct premarket clinical trials, development costs could significantly increase, the introduction of any new tests under development may be delayed, and sales of ConfirmMDx and SelectMDx could be interrupted or stopped. Any of these outcomes could reduce revenues or increase costs and materially adversely affect our business, prospects, results of operations, or financial condition. Moreover, any cleared or approved labelling claims may not be consistent with current claims or be adequate to support continued adoption of and reimbursement for our tests. For instance, if FDA requires that ConfirmMDx or SelectMDx be labelled as investigational, or if the labelling claims the FDA allows are limited, order levels may decline and reimbursement may be adversely affected. If after commercialization under the LDT framework our tests are allowed to remain on the market but there is uncertainty about the regulatory status of our tests, including questions that may be raised if competitors object to our regulatory positioning as an LDT, we may encounter ongoing regulatory and legal challenges and related costs. Such challenges or related developments (for example if the labeling claims the FDA allows us to make are more limited than the claims we currently plan to make) may impact our commercialization efforts as orders or reimbursement may be less than anticipated. As a result, we could experience significantly increased development costs and a delay in generating additional revenue. Until the FDA finalizes its regulatory position regarding LDTs, or federal legislation is passed concerning regulation of LDTs, it is unknown how the FDA may regulate our tests in the future and what testing and data may be required to support any required clearance or approval. The requirement of premarket review could negatively affect our business until such review is completed and regulatory clearance or approval is obtained. The FDA could require that sales of ConfirmMDx and SelectMDx be halted pending premarket clearance or approval. In December 2018 the FDA Commissioner and the Director of the Center for Devices and Radiological Health (CDRH) expressed significant concerns regarding disparities between some LDTs and in vitro diagnostics that have been reviewed and cleared or approved by FDA. If the FDA were to determine that our tests are Annual Report 2021 92

Part III: Principle Risks & Uncertainties not within the policy for LDTs for any reason, including new rules, policies, or guidance, or due to changes in statute, our tests may become subject to FDA requirements, including premarket review. If required, the regulatory marketing authorization process may involve, among other things, successfully completing additional clinical trials and submitting a premarket clearance (510(k)) submission or filing a de novo or premarket approval application with the FDA. If premarket review and authorization is required by the FDA, we may need to incur additional expenses or require additional time to seek it, or we may be unable to satisfy FDA standards, and our tests may not be cleared or approved on a timely basis, if at all, and the labeling claims permitted by the FDA may not be consistent with our currently planned claims or adequate to support adoption of and reimbursement for our tests. If the FDA requires any form of premarket review, the ConfirmMDx and SelectMDx tests may not be cleared or approved on a timely basis, if at all. We may also decide voluntarily to pursue FDA premarket review and authorization of the ConfirmMDx and SelectMDx tests if it appears that doing so would be appropriate. In addition, we believe that the sample collection kits provided by us for collection and transport of specimens from a health care provider to our Irvine, California clinical laboratory are considered a Class I medical devices subject to the FDA’s general device controls but exempt from premarket review. However, the FDA could assert the specimen collection kits are non-exempt or Class II devices, which would subject them to premarket clearance or approval processes, which could be time-consuming and expensive. Failure to comply with any applicable FDA requirements could trigger a range of enforcement actions by the FDA, including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity. Our operating results could be materially adversely affected by unanticipated changes in tax laws and regulations, adjustments to our tax provisions, exposure to additional tax liabilities, or forfeiture of our tax assets. We are subject to laws and regulations on tax levies and other charges or contributions in different countries, including transfer pricing and tax regulations for the compensation of personnel and third parties. Our tax structure involves several transfers and transfer price determinations between our parent company and our subsidiaries or other affiliates. Our effective tax rates could be adversely affected by changes in tax laws, treaties and regulations, both internationally and domestically. An increase of the effective tax rates could have an adverse effect on our business, financial position, results of operations and cash flows. The net operating loss (“NOL”) carry forwards of our corporate subsidiaries may be unavailable to offset future taxable income because of restrictions under U.S. tax law. As of December 31, 2021, consolidated net tax loss carry forward amounted to $305.0 million. Our NOLs generated in tax years ending on or prior to December 31, 2017 are only permitted to be carried forward for 20 taxable years under applicable U.S. federal tax law, and therefore could expire unused. We consider that it is highly likely that we will be unable to use at least a portion of these NOLs, in light of our continued losses. Under tax legislation commonly referred to as the Tax Cuts and Jobs Act (“TCJA”), as modified by the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”), our federal NOLs generated in tax years ending after December 31, 2017 may be carried forward indefinitely and NOLs arising in taxable years beginning after December 31, 2017 and before January 1, 2021 may be carried back to each of the five taxable years preceding the tax year of such loss, but NOLs arising in taxable years beginning after December 31, 2020 may not be carried back. In addition, under the TCJA, as modified by the CARES Act, for taxable years beginning after December 31, 2020, the deductibility of federal NOLs generated in taxable years beginning after December 31, 2017 is limited to 80% of current year taxable income. It is uncertain if and to what extent various states will conform to the TCJA, as modified by the CARES Act. Annual Report 2021 93

Part III: Principle Risks & Uncertainties In addition, under sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change” (generally defined as a cumulative change in ownership by “5-percent shareholders” that exceeds 50 percentage points over a rolling three-year period), the corporation’s ability to use its pre-change NOLs and certain other pre-change tax attributes to offset post-change income and taxes may be limited. Similar rules may apply under state tax laws. Our existing NOLs and other certain tax attributes may be subject to limitations arising from previous ownership changes, and if we undergo an ownership change in connection with, or we undergo an ownership change following, this offering, our ability to utilize NOLs and such other tax attributes could be further limited by Sections 382 and 383 of the Code. In addition, future changes in our stock ownership, many of which are outside of our control, could result in an ownership change under Sections 382 and 383 of the Code. We have not conducted any studies to determine annual limitations, if any, that could result from such changes in the ownership. Our ability to utilize those NOLs and certain other tax attributes could be limited by an “ownership change” as described above and consequently, we may not be able to utilize a material portion of our NOLs and certain other tax attributes, which could have a material adverse effect on our cash flows and results of operations by effectively increasing our future tax obligations. Also under Belgian tax law, certain restrictions regarding the use of Belgian tax losses carried forward apply and these losses may also be forfeited upon certain changes of control over Belgian corporate taxpayers. As a Coronavirus measure, some limited tax loss carried back mechanism was introduced in Belgian tax law. Given that we have historically generated operating losses, any change in our ability to use NOLs could have a severe impact on us if and when we become profitable. As of December 31, 2021, we had an accumulated deficit of $244.3 million and for the year ended December 31, 2021, we had a net loss of $29.0 million. Risks Related to Ownership of the ADSs and Ordinary Shares. The trading price of our ordinary shares and ADSs may be volatile due to factors beyond our control, and purchasers of the ADSs could incur substantial losses. The market prices of the ADSs and ordinary shares may be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their ADSs or shares at or above the price originally paid for the security. The market price for the ADSs may be influenced by many factors, including: • actual or anticipated fluctuations in our financial condition and operating results; • the release of new data from our PRIORITY or other clinical trials; • actual or anticipated changes in our growth rate relative to our competitors; • competition from existing products or new products that may emerge; • announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; • failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; • issuance of new or updated research or reports by securities analysts; Annual Report 2021 94

Part III: Principle Risks & Uncertainties • fluctuations in the valuation of companies perceived by investors to be comparable to us; • currency fluctuations; • additions or departures of key management or scientific personnel; • disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; • changes to coverage policies or reimbursement levels by commercial third-party payors and government payors and any announcements relating to coverage policies or reimbursement levels; • announcement or expectation of additional debt or equity financing efforts; • uncertainty caused by and the unprecedented nature of the current COVID-19 pandemic; • issuances or sales of the ADSs by us, our insiders or our other holders; and • general economic and market conditions. These and other market and industry factors may cause the market price and demand for the ADSs to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ADSs and may otherwise negatively affect the liquidity of the trading market for ADSs. Certain of our significant shareholders may have different interests from us and may be able to control us, including the outcome of shareholder votes. As of December 31, 2021, (i) MVM Partners LLP beneficially owned approximately 22.23% of our ordinary shares and has one representative at the board level (Dr. Eric Bednarski), (ii) Bleichroeder LP owned approximately 15.25% of our ordinary shares, (iii) Biovest NV beneficially owned approximately 9.36% of our ordinary shares and has one representative at the board level (Rudi Mariën), and (iv) Valiance Asset Management beneficially owned approximately 12.30% of our ordinary shares and has one representative at the board level (Jan Pensaert). In addition, as long as two of MVM Partners LLP’s funds (MVM V LP and MVM GP (No.5) LP) hold in aggregate 5% of our company’s outstanding shares, they are entitled to have one observer at the board level. As a result, these shareholders will be able to exercise a significant level of control over all matters requiring stockholder approval, including the election of directors, amendment of our articles of association and approval of certain significant corporate transactions. This control could have the effect of delaying or preventing a change of control of the Company or changes in management, in each case, which other shareholders might find favorable, and will make the approval of certain transactions difficult or impossible without the support of these significant shareholders. If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the price of the ADSs and their trading volume could decline. The trading market for the ADSs depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or only limited securities or industry analysts cover our company, the trading price for the ADSs could be negatively impacted. If one or more of the analysts who covers us downgrades our equity securities or publishes inaccurate or unfavorable research about our business, the price of ADSs would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades our securities, demand for ADSs could decrease, which could cause the price of the ADSs or their trading volume to decline. Annual Report 2021 95

Part III: Principle Risks & Uncertainties We intend to retain all available funds and any future earnings and, consequently, ADS holders’ ability to achieve a return on their investment will depend on appreciation in the price of the ADSs. We have never declared or paid any cash dividends on our ordinary shares or ADSs, and we intend to retain all available funds and any future earnings to fund the development and expansion of our business. In addition, our loan agreement with Kreos Capital limits our ability to pay any such dividends. Therefore, ADS holders are not likely to receive any dividends on your ADSs for the foreseeable future and the success of an investment in ADSs will depend upon any future appreciation in their value. Consequently, investors may need to sell all or part of their holdings of ADSs after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that the ADSs will appreciate in value or even maintain the price at which our investors have purchased them. Investors seeking cash dividends should not purchase the ADSs. In addition, if we choose to pay dividends in the future, exchange rate fluctuations may affect the amount of Euros that we are able to distribute, and the amount in U.S. dollars that our shareholders receive upon the payment of cash dividends or other distributions we declare and pay in euros, if any. Any dividends will generally be subject to Belgian withholding tax. These factors could harm the value of the ADSs. Holders of ADSs should be aware that the rights provided to our ADS holders under Belgian corporate law and our articles of association differ in certain respects from the rights that you would typically enjoy as a shareholder of a U.S. company under applicable U.S. federal and state laws. We are, and will upon the consummation of the offering be, a Belgian public company with limited liability. Our corporate affairs are governed by our articles of association and by the laws governing companies incorporated in Belgium. The rights of shareholders and the responsibilities of members of our board of directors may be different from the rights and obligations of shareholders and boards of directors in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our board is required by Belgian law to consider the interests of our company, its shareholders, its employees, and other stakeholders. It is possible that some of these parties will have interests that are different from, or in addition to, the interests of our shareholders. See “Description of Share Capital and Articles of Association.” Concentration of ownership of our ordinary shares (including ordinary shares in the form of ADSs) among our existing executive officers, directors and principal shareholders may prevent ADS holders from influencing significant corporate decisions. Our executive officers, directors, greater than five percent shareholders and their affiliates beneficially owned approximately 39.7% of our outstanding ordinary shares as of December 31, 2021. Depending on the level of attendance at our general meetings of shareholders, these shareholders either alone or voting together as a group will be in a position to determine the outcome of decisions taken at any such general meeting. Any shareholder or group of shareholders controlling more than 50% of the share capital present and voting at our general meetings of shareholders may control any shareholder resolution requiring a simple majority, including the appointment of board members, as well as certain decisions relating to our capital structure, the approval of certain significant corporate transactions and amendments to our articles of association. Among other consequences, this concentration of ownership may prevent or discourage unsolicited acquisition proposals that you may believe are in your best interest as one of our shareholders. Some of these persons or entities may have interests different than yours. Future sales, or the perception of future sales, of a substantial number of our ordinary shares could adversely affect the price of the ADSs, and actual sales of our equity will dilute ADS holders. Future sales of a substantial number of our ADSs or ordinary shares, or the perception that such sales will occur, could cause a decline in the market price of the ADSs. 55,598,249 ordinary shares (including ordinary shares underlying Annual Report 2021 96

Part III: Principle Risks & Uncertainties ADSs) held by our directors, executive officers and certain shareholders are subject to the lock-up agreements with terms generally expiring in May 2022. If, after the period during which such lock-up agreements restrict sales of the our ordinary shares and ADSs or if the underwriters of our initial public offering of ADSs waive the restrictions set forth therein (which may occur at any time), one or more of these securityholders sell substantial amounts of ordinary shares or ADSs in the public market, or the market perceives that such sales may occur, the market price of the ADSs and our ability to raise capital through an issue of equity securities in the future could be adversely affected. If we issue ordinary shares in future financings, shareholders may experience dilution and, as a result, the price of the ADSs may decline. We may from time-to-time issue additional ordinary shares at a discount from the trading price of the ADSs. As a result, holders of the ADSs would experience immediate dilution upon the issuance of any of our ordinary shares at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preference shares or shares. If we issue ordinary shares or other equity or equity-linked securities, holders of ADSs would experience additional dilution and, as a result, the price or the ADSs may decline. It may be difficult for ADS holders outside Belgium to serve process on, or enforce foreign judgments against, us or our directors and senior management. We are a Belgian public limited liability company. Less than a majority of the members of our board of directors and members of our executive management team are residents of the United States. All or a substantial portion of the assets of such non-resident persons and most of our assets are located outside the United States. As a result, it may not be possible for ADS holders to effect service of process upon such persons or on us or to enforce against them or us a judgment obtained in U.S. courts. Original actions or actions for the enforcement of judgments of U.S. courts relating to the civil liability provisions of the federal or state securities laws of the United States are not directly enforceable in Belgium. The United States and Belgium do not currently have a multilateral or bilateral treaty providing for reciprocal recognition and enforcement of judgments, other than arbitral awards, in civil and commercial matters. In order for a final judgment for the payment of money rendered by U.S. courts based on civil liability to produce any effect on Belgian soil, it is accordingly required that this judgment be recognized or be declared enforceable by a Belgian court in accordance with Articles 22 to 25 of the 2004 Belgian Code of Private International Law. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium, unless (in addition to compliance with certain technical provisions) the Belgian courts are satisfied of the following: • the effect of the enforcement judgment is not manifestly incompatible with Belgian public policy; • the judgment did not violate the rights of the defendant; • the judgment was not rendered in a matter where the parties transferred rights subject to transfer restrictions with the sole purpose of avoiding the application of the law applicable according to Belgian international private law; • the judgment is not subject to further recourse under U.S. law; Annual Report 2021 97

Part III: Principle Risks & Uncertainties • the judgment is not incompatible with a judgment rendered in Belgium or with a subsequent judgment rendered abroad that might be recognized in Belgium; • the claim was not filed outside Belgium after the same claim was filed in Belgium, while the claim filed in Belgium is still pending; • the Belgian courts did not have exclusive jurisdiction to rule on the matter; • the U.S. court did not accept its jurisdiction solely on the basis of the presence of the plaintiff or the location of goods not direct linked to the dispute in the United States; • the judgment did not concern the deposit or validity of intellectual property rights when the deposit or registration of those intellectual property rights was requested, done or should have been done in Belgium pursuant to international treaties; • the judgment did not relate to the validity, operation, dissolution, or liquidation of a legal entity that has its main seat in Belgium at the time of the petition of the U.S. court; • if the judgment relates to the opening, progress or closure of insolvency proceedings, it is rendered on the basis of the European Insolvency Regulation (EC Regulation No. 1346/2000 of May 29, 2000) or, if not, that (a) a decision in the principal proceedings is taken by a judge in the state where the most important establishment of the debtor was located or (b) a decision in territorial proceedings was taken by a judge in the state where the debtor had another establishment than its most important establishment; and • the judgment submitted to the Belgian court is authentic under the laws of the state where the judgment was issued; in case of a default judgment, it can be shown that under locally applicable laws the invitation to appear in court was properly served on the defendant; a document can be produced showing that the judgment is, under the rules of the state where it was issued, enforceable and was properly served on the defendant. In addition to recognition or enforcement, a judgment by a federal or state court in the United States against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. The findings of a federal or state court in the United States will not, however, be taken into account to the extent they appear incompatible with Belgian public policy. Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against us or members of our board of directors or our executive management any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws. We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, the ADSs may be less attractive to investors. We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Annual Report 2021 98

Part III: Principle Risks & Uncertainties Section 404, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We may take advantage of these exemptions until we are no longer an emerging growth company. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the aggregate market value of our ordinary shares held by non-affiliates exceeds $700 million as of the end of our second fiscal quarter before that time, in which case we would no longer be an emerging growth company as of the following December 31st (the last day of our fiscal year). We cannot predict if investors will find the ADSs less attractive because we may rely on these exemptions. If some investors find the ADSs less attractive as a result, there may be a less active trading market for the ADSs and the price of the ADSs may be more volatile. As a foreign private issuer and as permitted by the listing requirements of Nasdaq, we rely on certain home country corporate governance practices rather than the corporate governance requirements of Nasdaq. We qualify as a foreign private issuer and our ADSs are listed on Nasdaq. In accordance with the listing requirements of Nasdaq, we rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of Nasdaq. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. In addition, our officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, while we currently publish annual and semi-annual reports on our website pursuant to the rules of Euronext Brussels and expect to file such financial reports with the SEC, we are not required to file periodic reports with the SEC as frequently or as promptly as U.S. public companies. Specifically, we are not required to file quarterly reports on Form 10-Q or current reports on Form 8-K that a domestic company would be required to file under the Exchange Act. Accordingly, there may be less publicly available information concerning our company than there would be if we were not a foreign private issuer. In addition, the Listing Rules of the Nasdaq Stock Market require a majority of the directors of a listed U.S. company to be independent, whereas in Belgium, only three directors need to be independent. The Listing Rules of the Nasdaq Stock Market further require that each of the nominating, compensation and audit committees of a listed U.S. company be comprised entirely of independent directors. However, the Belgian Corporate Governance Code recommends only that a majority of the directors on the nomination committee meet the technical requirements for independence under Belgian corporate law. At present, our audit committee is composed of three independent directors out of three members, whereas our nomination and remuneration committees are composed of two independent directors out of three members. Our board of directors has no plan to change the composition of our audit committee and nomination and remuneration committee, and we intend to follow home country practice to the maximum extent possible. Therefore, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers. We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses. As a foreign private issuer, we are not required to comply with all the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. The determination of foreign private issuer status is made annually on the last business day of our most recently completed second fiscal quarter. Accordingly, we will next make a determination with respect to our foreign private issuer status on June 30, 2022. There is a risk that we will lose our foreign private issuer status in the future. Annual Report 2021 99

Part III: Principle Risks & Uncertainties We would lose our foreign private issuer status if, for instance more than 50% of our ordinary shares are owned by U.S. residents or persons and more than 50% of our assets are located in the United States and we continue to fail to meet additional requirements necessary to maintain our foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly greater than the costs we incur as a foreign private issuer. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. GAAP and modify certain of our policies to comply with corporate governance practices associated with U.S. domestic issuers. Such conversion and modifications would involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers, which could also increase our costs. U.S. holders of ADSs may suffer adverse tax consequences if we are characterized as a passive foreign investment company, or PFIC. In general, a non-U.S. corporation is a PFIC for U.S. federal income tax purposes for any taxable year in which (i) 50% or more of value of its assets (based on an average of the quarterly values of the assets during such taxable year) consists of assets that produce, or are held for the production of, passive income, or (ii) 75% or more of its gross income consists of passive income. A separate determination must be made after the close of each fiscal year as to whether a non-U.S. corporation is a PFIC for that year. For purposes of the above calculations, a non-U.S. corporation that owns, directly or indirectly, at least 25% by value of the shares of another corporation is treated as if it held its proportionate share of the assets of the other corporation and received directly its proportionate share of the income of the other corporation. Passive income generally includes dividends, interest, investment gains and certain rents and royalties. Cash is generally a passive asset for these purposes. The value goodwill is generally treated as an active asset if it is associated with business activities that produce active income. If we are a PFIC for any taxable year during which a U.S. Holder holds ADSs, we will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which the U.S. Holder owns the ADSs regardless of whether we continue to meet the PFIC test described above, unless the U.S. Holder makes a specified election once we cease to be a PFIC. If we are classified as a PFIC for any taxable year during which a U.S. Holder holds ADSs, the U.S. Holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements. Based on the current estimates, and expected future composition, of our income and the value of our assets, including goodwill, we do not expect to be a PFIC for our current taxable year. However, our PFIC status for any taxable year is an annual determination that can be made only after the end of that year and will depend on the composition of our income and assets and the value of our assets from time to time. The determination of whether we are a PFIC is fact-intensive and the applicable law is subject to varying interpretation. There can be no assurance that the U.S. Internal Revenue Service, or IRS, will agree with our position or that the IRS will not successfully challenge our position including our classification of certain income and assets as non-passive or our valuation of our tangible and intangible assets. A U.S. Holder may in certain circumstances mitigate the adverse tax consequences of the PFIC rules by filing an election to treat the PFIC as a Qualified Electing Fund (“QEF”) or, if shares of the PFIC are “marketable stock” for purposes of the PFIC rules, by making a mark-to-market election with respect to the shares of the PFIC. However, we do not currently intend to provide the information necessary for U.S. Holders to make a QEF election if we were treated as a PFIC for any taxable year and prospective investors should assume that a QEF election will not be available. Furthermore, if a U.S. Annual Report 2021 100

Part III: Principle Risks & Uncertainties Holder were to make a mark-to-market election with respect to its ADSs, the U.S. Holder would be required to include annually in its U.S. federal taxable income (taxable at ordinary income rates) an amount reflecting any year end increase in the value of its ADSs. For further discussion of the PFIC rules and the adverse U.S. federal income tax consequences in the event we are classified as a PFIC. The United States federal income tax rules relating to PFICs are very complex. Current and prospective U.S. Holders are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of ADSs, the consequences to them of an investment in a PFIC, any elections available with respect to the ADSs and the IRS information reporting obligations with respect to the purchase, ownership and disposition of ADSs of a PFIC. If a U.S. Holder is treated as owning at least 10% of our ordinary share capital, such holder may be subject to adverse U.S. federal income tax consequences. If a U.S. Holder is treated as owning, directly, indirectly or constructively, at least 10% of the value or voting power of our share capital, such U.S. Holder may be treated as a “U.S. shareholder” with respect to each “controlled foreign corporation” in our group, if any. Because our group currently includes at least one U.S. subsidiary, under current law, any of our current non-U.S. subsidiaries and any future newly formed or acquired non-U.S. subsidiaries will be treated as controlled foreign corporations, regardless of whether we are treated as a controlled foreign corporation. A U.S. shareholder of a controlled foreign corporation may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, regardless of whether we make any distributions. An individual that is a U.S. shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a U.S. shareholder that is a U.S. corporation. Failure to comply with controlled foreign corporation reporting obligations may subject a U.S. shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any U.S. shareholder information that may be necessary to comply with the reporting and tax paying obligations applicable under the controlled foreign corporation rules of the Code. U.S. Holders should consult their tax advisors regarding the potential application of these rules to their investment in ADSs. We incur significant costs as a result of operating as a company that is publicly listed on both Nasdaq in the United States and Euronext Brussels in Belgium, and our management is required to devote substantial time to new compliance initiatives. As a U.S. public company listed on Nasdaq, we incur legal, accounting, and other expenses that we did not previously incur. We will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the Nasdaq listing requirements and other applicable securities rules and regulations. Compliance with these rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company” and/or a foreign private issuer. The Exchange Act requires that, as a public company, we file annual, semi-annual and current reports with respect to our business, financial condition and result of operations. However, as a foreign private issuer, we are not required to file quarterly and current reports with respect to our business and results. We currently make annual and semi-annual reporting with respect to our listing on Euronext Brussels. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our board of directors. Annual Report 2021 101

Part III: Principle Risks & Uncertainties However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Further, being a U.S. listed company and a Belgian public company with shares admitted to trading on Euronext Brussels impacts the disclosure of information and requires compliance with two sets of applicable rules. From time to time, this may result in uncertainty regarding compliance matters and result in higher costs necessitated by legal analysis of dual legal regimes, ongoing revisions to disclosure and adherence to heightened governance practices. As a result of the enhanced disclosure requirements of the U.S. securities laws, business and financial information that we report is broadly disseminated and highly visible to investors, which we believe may increase the likelihood of threatened or actual litigation, including by competitors and other third parties, which could, even if unsuccessful, divert financial resources and the attention of our management from our operations. As a result of being a U.S. public company, we are subject to regulatory compliance requirements, including Section 404, and if we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud. Pursuant to Section 404, our management is required to assess and attest to the effectiveness of our internal control over financial reporting in connection with issuing our consolidated financial statements as of and for each fiscal year. Section 404 also requires an attestation report on the effectiveness of internal control over financial reporting be provided by our independent registered public accounting firm beginning with our annual report following the date on which we are no longer an “emerging growth company”, which may be up to five fiscal years from the initial public offering of our ADSs. The cost of complying with Section 404 significantly increases and management’s attention may be diverted from other business concerns, which could adversely affect our results. We may need to hire more employees in the future or engage outside consultants to comply with these requirements, which will further increase expenses. If we fail to comply with the requirements of Section 404 in the required timeframe, we may be subject to sanctions or investigations by regulatory authorities, including the SEC and Nasdaq. Furthermore, if we are unable to attest to the effectiveness of our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, and the market price of the ADSs could decline. Failure to implement or maintain effective internal control over financial reporting could also restrict our future access to the capital markets and subject each of us, our directors and our officers to both significant monetary and criminal liability. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expense and a diversion of management’s time and attention from revenue generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business, financial position, results and prospects may be adversely affected. If we fail to implement and maintain effective internal controls over financial reporting, our ability to produce accurate financial statements on a timely basis could be impaired. Annual Report 2021 102

Part III: Principle Risks & Uncertainties We are subject to reporting obligations under U.S. securities laws and the Sarbanes-Oxley Act of 2002. Section 404 of the Sarbanes-Oxley Act requires that we include a report from management on the effectiveness of our internal control over financial reporting in our second annual report on Form 20-F after we become public. If we fail to implement and maintain adequate disclosure controls and procedures, our management may conclude that our internal control over financial reporting is not effective. This conclusion could adversely impact the market price of the ADSs due to a loss of investor confidence in the reliability of our reporting processes. We are required to perform system and process evaluations and testing of our internal controls over financial reporting, to allow our management and our independent public registered accounting firm to report on the effectiveness of our internal control over financial reporting. In addition, our compliance with Section 404 of the Sarbanes-Oxley Act will require that we incur substantial accounting expense, expend significant management effort and we may need to hire additional accounting and financial staff with the appropriate experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or any subsequent testing by our independent registered public accounting firm, may reveal additional deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. We cannot assure you that there will not be additional material weaknesses or significant deficiencies in our internal control over financial reporting in the future. If we are unable to conclude that our internal controls are effective or if we have material weaknesses, investors could lose confidence in the accuracy or completeness of our reported financial information, which could have a negative effect on the trading price of ADSs. For as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We could be an “emerging growth company” for up to five years following the initial public offering of our ADSs. At the time when we are no longer an emerging growth company, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Our remediation efforts may not enable us to avoid a material weakness in the future. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur remediation costs. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets. We may be subject to securities litigation, which is expensive and could divert management’s attention. The market price of the ADSs may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business. Investors resident in countries other than Belgium may suffer dilution if they are unable to participate in future preferential subscription rights offerings. Under Belgian law and our constitutional documents, shareholders have a waivable and cancellable preferential subscription right to subscribe pro rata to their existing shareholdings to the issuance, against a contribution in cash, Annual Report 2021 103

Part III: Principle Risks & Uncertainties of new shares or other securities entitling the holder thereof to new shares, unless such rights are limited or cancelled by resolution of our general shareholders’ meeting or, if so authorized by a resolution of such meeting, our board of directors. The exercise of preferential subscription rights by certain shareholders not residing in Belgium (including those in the United States, Australia, Israel, Canada or Japan as a result of the offering and taking into account the current shareholding and international network of our current board of directors) may be restricted by applicable law, practice or other considerations, and such shareholders may not be entitled to exercise such rights, unless the rights and shares are registered or qualified for sale under the relevant legislation or regulatory framework. In particular, we may not be able to establish an exemption from registration under the U.S. Securities Act, and we are under no obligation to file a registration statement with respect to any such preferential subscription rights or underlying securities or to endeavor to have a registration statement declared effective under the U.S. Securities Act. Shareholders in jurisdictions outside Belgium who are not able or not permitted to exercise their preferential subscription rights in the event of a future preferential subscription rights, equity or other offering may suffer dilution of their shareholdings. ADS holders may not be able to exercise their right to vote the ordinary shares underlying their ADSs. ADS holders do not have the same rights as our shareholders. For example, ADS holders may not attend shareholders’ meetings or directly exercise the voting rights attaching to the ordinary shares underlying their ADSs. ADS holders may vote only by instructing the depositary to vote on their behalf. If we request the depositary to solicit your voting instructions (and we are not required to do so), the depositary will notify you of a shareholders’ meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, to vote or to have its agents vote the deposited ordinary shares as instructed by ADS holders. If we do not request the depositary to solicit ADS holders’ voting instructions, ADS holders can still send voting instructions, and, in that case, the depositary may try to vote as the ADS holder instructs, but it is not required to do so. Except by instructing the depositary as described above, ADS holders won’t be able to exercise voting rights unless the ADS holder surrenders the ADS holder’s ADSs and withdraw the ordinary shares. However, ADS holders may not know about the meeting enough in advance to withdraw the ordinary shares. We cannot assure ADS holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ordinary shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may not be able to exercise voting rights and there may be nothing an ADS holder can do if the ADS holder’s ordinary shares are not voted as the ADS holder requested. In addition, ADS holders have no right to call a shareholders’ meeting. Holders of ADSs may be subject to limitations on the transfer of their ADSs and the withdrawal of the underlying ordinary shares. ADSs, which may be evidenced by American Depositary Receipts (“ADRs”), are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason subject to a holder of ADSs’ right to cancel his or her ADSs and withdraw the underlying ordinary shares as specified in the deposit agreement. Temporary delays in the cancellation of ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, in connection with voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, a holder of ADSs may not be able to cancel his or her ADSs and withdraw the underlying ordinary shares when he or she owes money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. Annual Report 2021 104

Part III: Principle Risks & Uncertainties ADS holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiffs in any such action. The deposit agreement governing the ADSs representing our ordinary shares provides that, to the fullest extent permitted by law, ADS holders, including holders who acquire ADSs in the secondary market, waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws. If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws has not been finally adjudicated by the U.S. Supreme Court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement, by a federal or state court in the City of New York, which has non-exclusive jurisdiction over matters arising under the deposit agreement. In determining whether to enforce a contractual pre- dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the deposit agreement and the ADSs. It is advisable that you consult legal counsel regarding the jury waiver provision before entering into the deposit agreement. If any owners or holders of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under federal securities laws, such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and the depositary. If a lawsuit is brought against either or both of us and the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have, including results that could be less favorable to the plaintiffs in any such action. Nevertheless, if this jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the deposit agreement with a jury trial. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any owner or holder of ADSs or by us or the depositary of compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder. Takeover provisions in the national law of Belgium may make a takeover difficult. Public takeover bids on our shares and other voting securities, such as warrants or convertible bonds, if any, are subject to the Belgian Act of April 1, 2007 on public takeover bids, as amended and implemented by the Belgian Royal Decree of April 27, 2007, or Royal Decree, and to the supervision by the Belgian Financial Services and Markets Authority, or FSMA. Public takeover bids must be made for all of our voting securities, as well as for all other securities that entitle the holders thereof to the subscription to, the acquisition of or the conversion into voting securities. Prior to making a bid, a bidder must issue and disseminate a prospectus, which must be approved by the FSMA. The bidder must also obtain approval of the relevant competition authorities, where such approval is legally required for the acquisition of our company. The Belgian Act of April 1, 2007 provides that a mandatory bid will be required to be launched for all of our outstanding shares and securities giving access to shares if a person, as a result of its own acquisition or the acquisition by persons acting in concert with it or by persons acting on their account, directly or indirectly holds more than 30% of the voting securities in a company that has its registered office in Belgium and of which at least part of the voting securities are traded on a regulated market or on a multilateral trading facility designated by the Royal Decree. The mere fact of exceeding the relevant threshold through the acquisition of one or more shares will give rise to a mandatory bid, irrespective of whether or not the price paid in the relevant transaction exceeds the current market price. Annual Report 2021 105
Part III: Principle Risks & Uncertainties There are several provisions of Belgian company law and certain other provisions of Belgian law, such as the obligation to disclose important shareholdings and merger control, that may apply to us and which may make an unfriendly tender offer, merger, change in management or other change in control, more difficult. These provisions could discourage potential takeover attempts that third parties may consider and thus deprive the shareholders of the opportunity to sell their shares at a premium (which is typically offered in the framework of a takeover bid). Click here to return to table of contents Annual Report 2021 106

Part IV: Financial Statements Annual Report 2021 107

Part IV: Financial Statements Part IV: Financial Statements Annual Report 2021 108

Part IV: Financial Statements Part IV: Financial Statements Consolidated financial statements MDxHealth’s consolidated financial statements have been prepared in accordance with the International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB) and as adopted by the EU (“EU-IFRS”) and collectively “IFRS”. The accounting policies and notes are an integral part of these consolidated financial statements. The following consolidated accounts differ from the non-consolidated statutory annual accounts of the Company, which have been prepared in accordance with Belgian GAAP. The financial statements in this section of the Annual Report have been approved and authorized for issue by the Board of Directors at its meeting of April 20, 2022. The financial statements have been signed by Mr. Michael McGarrity, Executive Director, on behalf of the Board of Directors. The financial statements will be submitted to the shareholders for their final approval at the annual general shareholders’ meeting of May 25, 2022.Annual Report 2021 109
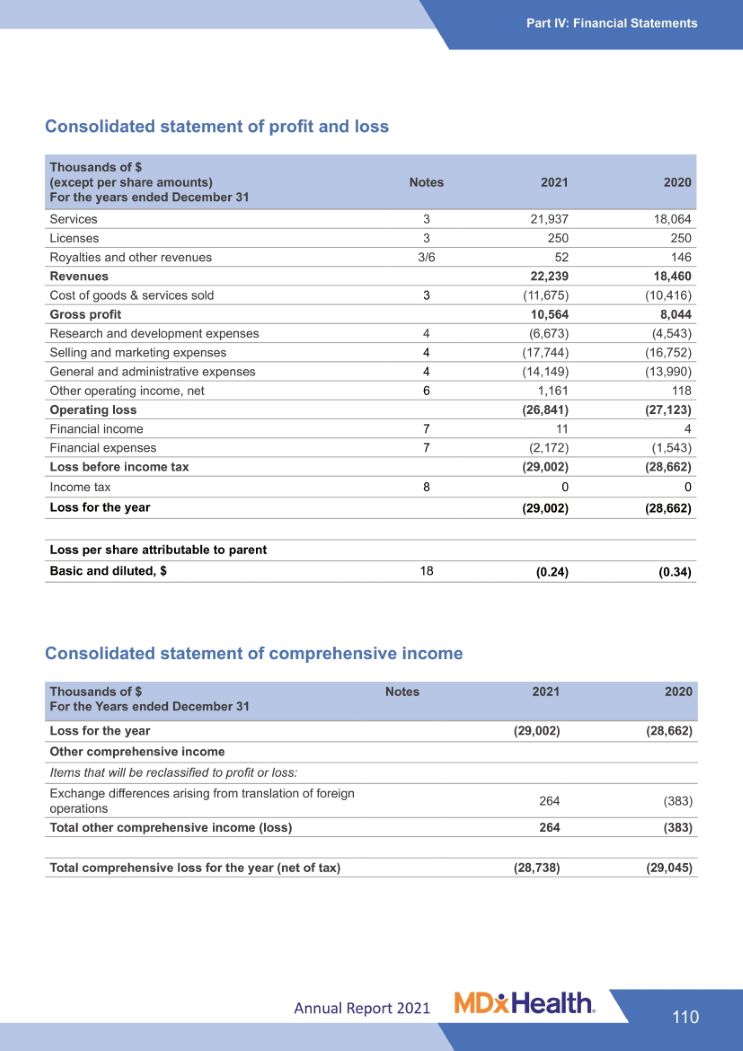
Part IV: Financial Statements Consolidated statement of profit and loss Thousands of $ (except per share amounts) Notes 2021 2020 For the years ended December 31 Services 3 21,937 18,064 Licenses 3 250 250 Royalties and other revenues 3/6 52 146 Revenues 22,239 18,460 Cost of goods & services sold 3 (11,675) (10,416) Gross profit 10,564 8,044 Research and development expenses 4 (6,673) (4,543) Selling and marketing expenses 4 (17,744) (16,752) General and administrative expenses 4 (14,149) (13,990) Other operating income, net 6 1,161 118 Operating loss (26,841) (27,123) Financial income 7 11 4 Financial expenses 7 (2,172) (1,543) Loss before income tax (29,002) (28,662) Income tax 8 0 0 Loss for the year (29,002) (28,662) Loss per share attributable to parent Basic and diluted, $ 18 (0.24) (0.34) Consolidated statement of comprehensive income Thousands of $ For the Years ended December 31 Notes 2021 2020 Loss for the year (29,002) (28,662) Other comprehensive income Items that will be reclassified to profit or loss: Exchange differences arising from translation of foreign 264 (383) operations Total other comprehensive income (loss) 264 (383) Total comprehensive loss for the year (net of tax) (28,738) (29,045) Annual Report 2021 110
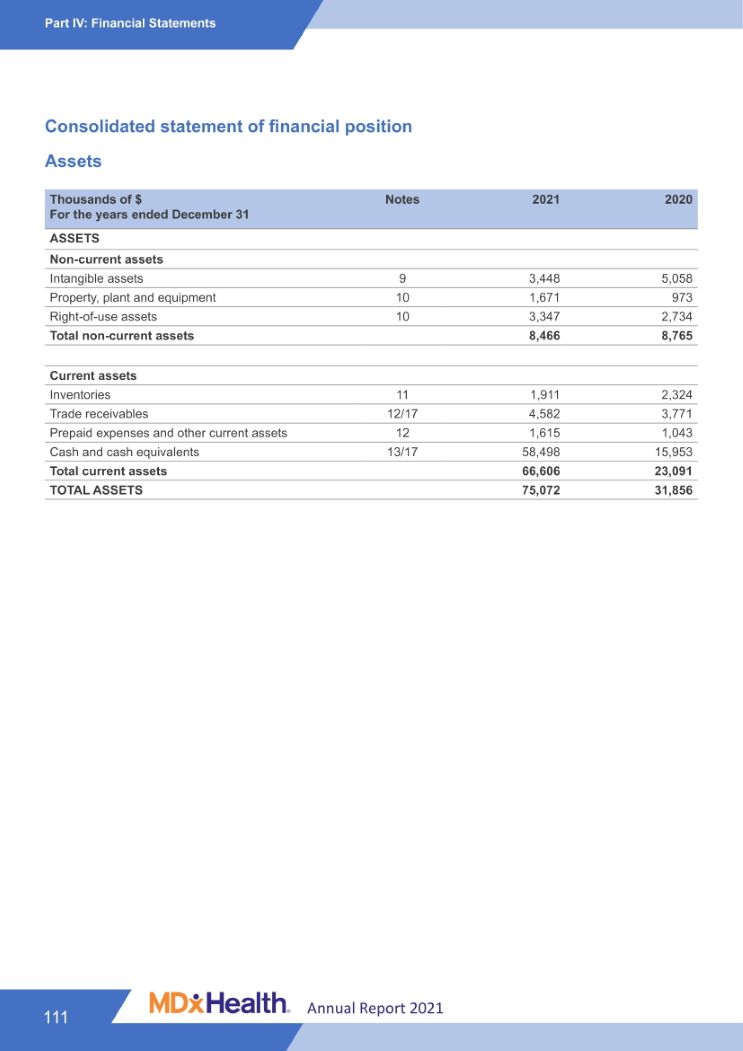
Part IV: Financial Statements Consolidated statement of financial position Assets Thousands of $ For the years ended December 31 Notes 2021 2020 ASSETS Non-current assets Intangible assets 9 3,448 5,058 Property, plant and equipment 10 1,671 973 Right-of-use assets 10 3,347 2,734 Total non-current assets 8,466 8,765 Current assets Inventories 11 1,911 2,324 Trade receivables 12/17 4,582 3,771 Prepaid expenses and other current assets 12 1,615 1,043 Cash and cash equivalents 13/17 58,498 15,953 Total current assets 66,606 23,091 TOTAL ASSETS 75,072 31,856 Annual Report 2021 111

Part IV: Financial Statements Liabilities & Shareholders’ Equity Thousands of $ For the years ended December 31 Notes 2021 2020 EQUITY Share capital 20 128,454 76,716 Issuance premium 20 153,177 136,349 Accumulated deficit (244,302) (215,300) Share-based compensation 22 10,607 9,385 Translation reserve (1,037) (1,301) Total equity 46,899 5,849 LIABILITIES Non-current liabilities Loans and borrowings 14/17 7,651 10,279 Lease liabilities 14 2,624 2,017 Other non-current financial liabilities 14/17 1,466 690 Total non-current liabilities 11,741 12,986 Current liabilities Loans and borrowings 14/17 4,441 2,818 Lease liabilities 14 840 757 Trade payables 16/17 7,455 5,320 Other current liabilities 16 2,735 3,217 Other current financial liabilities 14/17 961 909 Total current liabilities 16,432 13,021 Total liabilities 28,173 26,007 TOTAL EQUITY AND LIABILITIES 75,072 31,856 Annual Report 2021 112
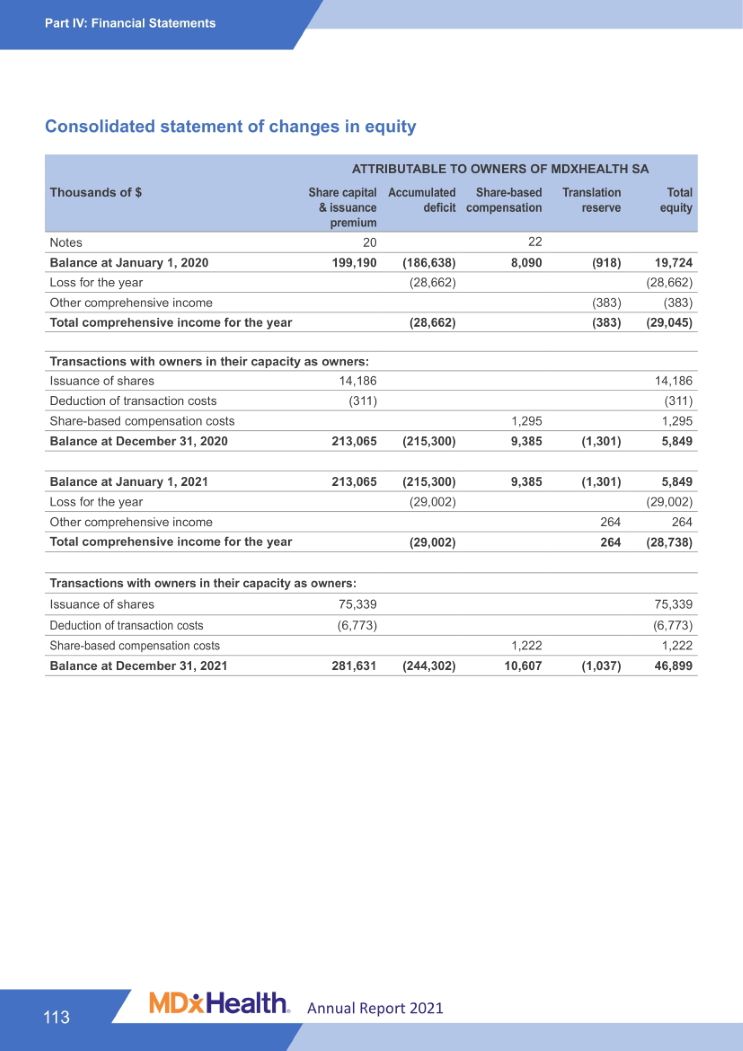
Part IV: Financial Statements Consolidated statement of changes in equity ATTRIBUTABLE TO OWNERS OF MDXHEALTH SA Thousands of $ Share capital & issuance premium Accumulated deficit Share-based compensation Translation reserve Total equity Notes 20 22 Balance at January 1, 2020 199,190 (186,638) 8,090 (918) 19,724 Loss for the year (28,662) (28,662) Other comprehensive income (383) (383) Total comprehensive income for the year (28,662) (383) (29,045) Transactions with owners in their capacity as owners: Issuance of shares 14,186 14,186 Deduction of transaction costs (311) (311) Share-based compensation costs 1,295 1,295 Balance at December 31, 2020 213,065 (215,300) 9,385 (1,301) 5,849 Balance at January 1, 2021 213,065 (215,300) 9,385 (1,301) 5,849 Loss for the year (29,002) (29,002) Other comprehensive income 264 264 Total comprehensive income for the year (29,002) 264 (28,738) Transactions with owners in their capacity as owners: Issuance of shares 75,339 75,339 Deduction of transaction costs (6,773) (6,773) Share-based compensation costs 1,222 1,222 Balance at December 31, 2021 281,631 (244,302) 10,607 (1,037) 46,899 Annual Report 2021 113
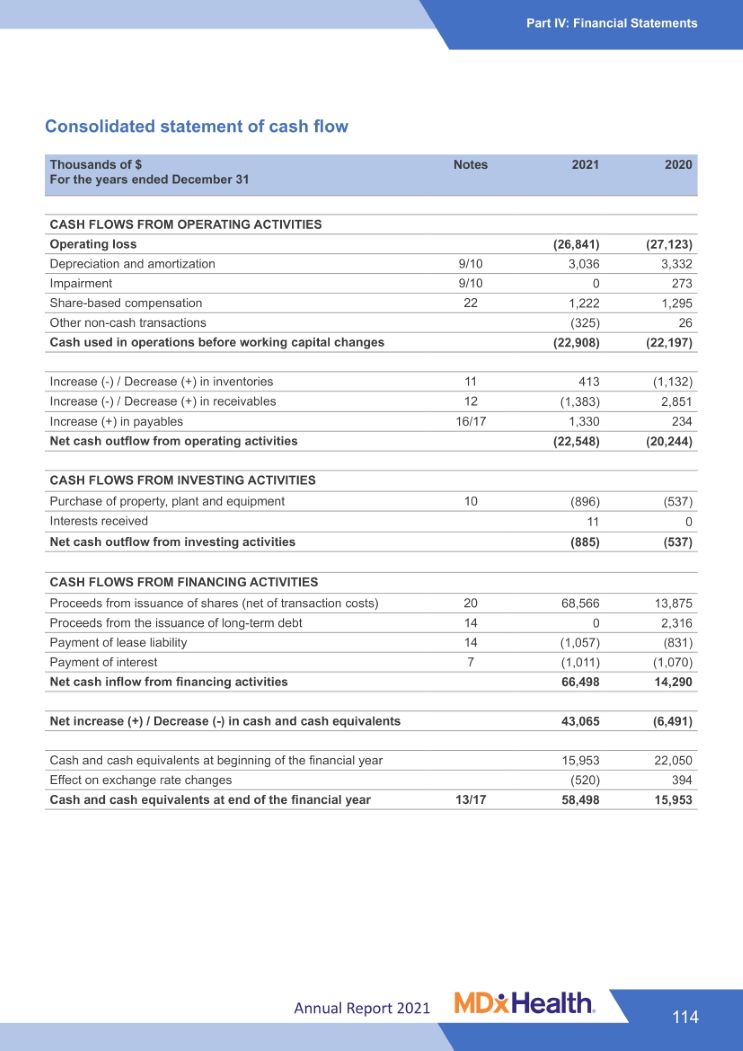
Part IV: Financial Statements Consolidated statement of cash flow Thousands of $ For the years ended December 31Notes20212020 CASH FLOWS FROM OPERATING ACTIVITIES Operating loss(26,841)(27,123) Depreciation and amortization9/103,0363,332 Impairment9/100273 Share-based compensation221,2221,295 Other non-cash transactions(325)26 Cash used in operations before working capital changes(22,908)(22,197) Increase (-) / Decrease (+) in inventories11413(1,132) Increase (-) / Decrease (+) in receivables12(1,383)2,851 Increase (+) in payables16/171,330234 Net cash outflow from operating activities(22,548)(20,244) CASH FLOWS FROM INVESTING ACTIVITIES Purchase of property, plant and equipment10(896)(537) Interests received110 Net cash outflow from investing activities(885)(537) CASH FLOWS FROM FINANCING ACTIVITIES Proceeds from issuance of shares (net of transaction costs)2068,56613,875 Proceeds from the issuance of long-term debt1402,316 Payment of lease liability14(1,057)(831) Payment of interest7(1,011)(1,070) Net cash inflow from financing activities66,49814,290 Net increase (+) / Decrease (-) in cash and cash equivalents43,065(6,491) Cash and cash equivalents at beginning of the financial year15,95322,050 Effect on exchange rate changes(520)394 Cash and cash equivalents at end of the financial year13/1758,49815,953 Annual Report 2021 114

Part IV: Financial Statements Annual Report 2021 115

Part IV: Financial Statements Notes Notes to consolidated financial statements NOTE 1 : Status and principal activity NOTE 2 : Summary of Significant Accounting policies NOTE 3 : Revenue and Cost of goods & services sold NOTE 4 : Nature of expenses NOTE 5 : Personnel costs NOTE 6 : Other operating income, net NOTE 7 : Finance income / (expenses) NOTE 8 : IncomeTax NOTE 9 : Intangible assets NOTE 10 : Property, plant and equipment and right of-use assets NOTE 11 : Inventories NOTE 12 : Trade and other receivables NOTE 13 : Cash and cash equivalents NOTE 14 : Loans, borrowings, lease obligations and other financial liabilities NOTE 15 : Contractual obligations NOTE 16 : Trade and other payables NOTE 17 : Financial instruments and fair value NOTE 18 : Loss per share NOTE 19 : Financial risk management NOTE 20 : Share capital and reserves NOTE 21 : Retirement benefit schemes NOTE 22 : Share-based payments NOTE 23 : Related parties NOTE 24 : Significant agreements, commitments and contingencies NOTE 25 : Subsequent events NOTE 26 : Subsidiaries NOTE 27: Principal audit fees and services NOTE 28 : Alternative performance measures (APMs) Annual Report 2021 116

Part IV: Financial Statements NOTE 1: Status and principal activity Back to Notes list When used in this report, all references to "MDxHealth", the "company", "we", "our" and "us" refer to MDxHealth, SA and its subsidiaries. MDxHealth is a limited liability company domiciled in Belgium, with offices and labs in Belgium, the United States and The Netherlands. MDxHealth is a molecular diagnostics company that develops and commercializes advanced epigenetic and other molecular tests for cancer assessment and the personalized treatment of patients. Applying its DNA methylation platform and proprietary biomarkers, the Company helps address a large and growing unmet medical need for better cancer diagnosis and treatment information. The Company develops and commercializes advanced molecular diagnostic products for personalized cancer treatment that provide physicians with tools to aid in the diagnosis and or prognosis of cancers, and aid in the physician’s ability to predict disease progression and response to therapy. MDxHealth’s products and pipeline cover primarily urologic cancers, but in addition, MDxHealth has numerous proprietary biomarkers for other solid cancer types ready for development. MDxHealth offers its laboratory solutions from our state-of-the-art, 13,448 sqft, College of American Pathologists (CAP)- accredited and Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) certified, molecular laboratory facility located at its U.S. headquarters in Irvine, California. MDxHealth also operates a molecular laboratory facility, MDxHealth B.V., located in Nijmegen, the Netherlands. This site is ISO 13485:2016 certified and operates a management quality system with the following scope: The design and development, manufacture, service laboratory activities and client services of in vitro diagnostic test kits, in vitro diagnostic reagents used for molecular diagnostic detection of oncological diseases. The Company is headquartered in Belgium. The parent company, MDxHealth SA, has its registered and corporate office in Cap Business Center, Rue d’Abhooz 31, 4040 Herstal, Belgium. MDxHealth, Inc., the Company’s U.S. subsidiary, is located at 15279 Alton Parkway, Suite 100, Irvine, CA 92618, United States. MDxHealth B.V., the Company’s Dutch subsidiary, is located at Transistorweg 5, 6534 Nijmegen, The Netherlands. The functional and presentation currency is the US Dollar. NOTE 2: Summary of Significant Accounting policies Back to Notes list 2.1. Basis of preparation and statement of compliance MDxHealth’s consolidated financial statements of have been prepared in accordance with the International Financial Reporting Standards (IFRS) and interpretations issued by the IFRS Interpretation Committee (IFRS-IC) applicable to companies reported under IFRS. The financial statements comply with IFRS as issued by the International Accounting Standards Board (IASB) and as adopted by the EU (“EU-IFRS”), collectively “IFRS”. The principal accounting policies applied in the preparation of the consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated. All amounts are presented in thousands of US Dollars ($) unless otherwise indicated, rounded to the nearest thousand. 2.2. Basis of consolidation The consolidated financial statements incorporate the financial statements of MDxHealth SA (Belgium) and its wholly- owned subsidiaries, including MDxHealth Inc. (United States), and MDxHealth BV (The Netherlands) for each fiscal year ending on December 31.Annual Report 2021 117

Part IV: Financial Statements Subsidiaries are all entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. The acquisition method of accounting is used to account for business combinations by the Company. All intercompany balances, profits and transactions are eliminated upon consolidation. 2.3. Going Concern The Company has experienced net losses and significant cash used in operating activities since its inception in 2003, and as of December 31, 2021, had an accumulated deficit of $244.3 million, a net loss of $29.0 million, and net cash used in operating activities of $22.5 million. Management expects the Company to continue to incur net losses and have significant cash outflows for at least the next twelve months. While these conditions, among others, could raise doubt about its ability to continue as a going concern, these consolidated financial statements have been prepared assuming that the Company will continue as a going concern. This basis of accounting contemplates the recovery of its assets and the satisfaction of liabilities in the normal course of business. A successful transition to attaining profitable operations is dependent upon achieving a level of positive cash flows adequate to support the Company’s cost structure. As of December 31, 2021, the Company had cash and cash equivalents of $58.5 million. Taking into account the above financial situation and on the basis of the most recent business plan, the Company believes that it has sufficient cash to be able to continue its operations for at least the next twelve months from the date of issuance of these financial statements, and accordingly has prepared the consolidated financial statements assuming that it will continue as a going concern. This assessment is based on forecasts and projections within management’s most recent business plan as well as the Company’s expected ability to realize cost reductions should these forecasts and projections not be met. 2.4. Use of estimates and judgments Management makes certain critical accounting estimates and management judgment when applying the Company’s accounting policies, which affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Estimates and judgments are continuously evaluated based on historical experience and other factors, including expectations of future events, which are believed to be reasonable under the circumstances. In the future, actual experience may differ from these estimates and assumptions. The areas where assumptions and estimation uncertainties in the financial statements have potentially the most significant effect in 2021, are listed below: Revenue recognition (see Note 3): The Company analyzes historical collection data on a quarterly basis and makes adjustments to its estimates. In accordance with IFRS 15, revenue is recognized where such variable consideration is included in the transaction price only to the extent that it is highly probable that the amount of revenue recognized will not be subject to significant future reversals as a result of subsequent re-estimation. Deferred income tax (see Note 8) Management estimates unused tax credits and tax losses to the extent that it is probable that taxable profit will be available against which the tax credits and tax losses can be utilized. On December 31, 2021, the Company had a consolidated net tax loss carried forward amounting to $305,022,000 (2020: $276,166,000), implying a potential deferred tax asset of $76,255,500 (2020: $69,041,000). No deferred tax assets have been recognized on December 31, 2021. Annual Report 2021 118

Part IV: Financial Statements Impairment Testing (see Note 9 and 10) Management periodically assess whether there are events or circumstances that indicate that the carrying amount of the intangible or tangible asset may not be recoverable. When such indications exist, management estimates the recoverable amount and records an impairment accordingly. Management estimates indications exist that intangible assets ConfirmMDx and SelectMDx could be impaired and has accordingly performed an impairment test which is described in Note 9. Key underlying estimates are considered to be cashflows and the weighted average cost of capital. In addition, as of December 31, 2021 and 2020, the Company had no outstanding goodwill remaining after the company fully impaired its goodwill in 2019. Share-Based Payments (see Note 22) Management estimates the fair value of the equity-settled share-based payment transactions by using the Black- Scholes option valuation model: • The dividend return is estimated by reference to the historical dividend payment of the Company. Currently, this is estimated to be zero as no dividends have been paid since inception. • The expected volatility was determined using the average volatility of the stock over the last two years at the date of grant. • Risk-free interest rate is based on the interest rate applicable for the 10Y Belgian government bond at the grant date Going Concern (note 2.3) Management needs to make significant judgements whether the Company will have sufficient liquidity to continue operations during the next twelve months. We refer to Note 2.3 for management assessment. Financial liabilities (note 14) Other financial liabilities are accounted for at fair value through P&L and include: • The contingent consideration related to the acquisition of NovioGendix in 2015. The fair value of this contingent consideration is reviewed on a regular basis and includes management’s estimates related to the risk-adjusted future cash flows of different scenarios discounted using appropriate interest rates. The structure of the possible scenario’s and the probability assigned to each one of them is reassessed by management at every reporting period and requires judgement from management about the outcome and probability of the different scenarios. • The derivative financial liability for the initial Kreos drawdown fee of €630,000 ($713,538) that initially is based upon the evolution of the share price of MDxHealth and also includes management’s estimates on probabilities that either payment or conversion will be requested by Kreos. 2.5. New Standards, Interpretations and Amendments 2.5.1. New Standards, Interpretations and Amendments adopted by the Company The accounting policies have been consistently applied by the Company and are consistent with those used in previous years. Annual Report 2021 119

Part IV: Financial Statements In the current financial year, the Company has applied the amendments to IFRS standards issued by the IASB for the annual period starting on 1 January 2021. This adoption has not had any material impact on the disclosures or on the amounts reported in these financial statements. 2.5.2. Standards and Interpretations issued but not yet effective in the current period Certain new accounting standards and amendments to standards have been published, but were not mandatory for 31 December 2021 reporting period. No amendments to standards that are issued but not yet effective are considered to affect the Company’s accounting policies or any of the disclosures when applied for the first time. 2.6. Foreign currency translation Functional and presentation currency Items included in the financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entity operates (the functional currency). The Company’s functional and presentation currency is the U.S. dollar based on the continuing development of the commercial activities in the U.S. market. Foreign currency transactions are translated into the functional currency using the exchange rates at the date of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of monetary assets and liabilities denominated in foreign currencies at year end exchange rates are generally recognized in profit or loss. The results and financial positions of foreign operations that have a functional currency different from the presentation currency are translated into the presentation currency as follows: • Assets and liabilities for each statement of financial position presented are translated at the closing rate at the date of that balance sheet • Income and expenses for each statement of profit or loss and statement of comprehensive income are translated at average exchange rates, and • All resulting exchange differences are recognized in other comprehensive income 2.7. Revenue recognition Performance obligations and timing of revenue recognition The majority of the Company’s revenue is derived from laboratory services with revenue recognized at a point in time when control of the services has transferred to the customer. This is generally when the test results are delivered to the customer. Minor other Company’s revenue is derived from license fees and royalties: • License fees are recognized when the Company has fulfilled all conditions and obligations. If the Company has continuing performance obligations towards the fees, the fee will be recognized on a straight-line basis over the contractual performance period. • Royalties are recognized as revenue once the amounts due can be reliably estimated based on the sale of the underlying products and services and when the collection of the royalties can be reasonably assured. Annual Report 2021 120

Part IV: Financial Statements License up-front (signature fees) and non-refundable fees for access to prior research results and databases are recognized when earned, if the Company has no continuing performance obligations and all conditions and obligations are fulfilled. If the Company has continuing performance obligations towards the fees, the fee will be recognized on a straight-line basis over the contractual performance period. Royalties are generated from the sales by third parties of products or services which incorporate the Company’s proprietary technology. Royalties are recognized as revenue once the amounts due can be reliably estimated based on the sale of the underlying products and services and when the collection of the royalties can be reasonably assured. Determining the transaction price A large portion of the Company’s revenues are derived from Medicare, which has set a fixed price (via a Local Coverage Determination or “LCD”) for the Company’s ConfirmMDx test. Therefore, the amount of revenue recognized from Medicare for ConfirmMDx is determined by reference to the fixed price in the LCD. For other commercial insurance companies for ConfirmMDx, SelectMDx, and Urinary Tract Infection (UTI) where there is no certainty of the amount that will be paid for services rendered, the Company uses historical collection data – on an individual payor basis – to estimate its future collection and corresponding revenues that should be recognized for each type of test. The Company analyzes historical collection data on a monthly basis and makes monthly adjustments to its estimates. In accordance with IFRS 15, revenue is recognized where such a variable consideration is included in the transaction price only to the extent that it is highly probable that the amount of revenue recognized will not be subject to significant future reversals as a result of subsequent re-estimation. When historical collection data is insufficient to estimate future collections, the Company defaults to cash basis, meaning that revenues will not be recognized until actual cash payment is received from the payor. Total revenue in any given year includes amounts related to tests performed in previous years as: • unrecognized amounts are collected; • recognized amounts are collected for different amounts than initially accrued for; and • balances outstanding for more than 12 months are reversed. Annual Report 2021 121

Part IV: Financial Statements 2.8. Segment information Information for the Company’s operating segments has been determined by reference to the information used by the chief operating decision maker (“CODM”) of the Company to review the performance of the Company and in making decisions on allocation of resources, the nature of the activities and the management structure and accountabilities. The Company’s CEO has been identified as the chief operating decision maker in accordance with his designated responsibility for the allocation of resources to operating segments and assessing their performance through periodic reporting. The CODM periodically reviews the Company’s performance based on information at a company level. The Company does not distinguish different business segments since most revenues are generated from clinical laboratory service testing, or the out-licensing of the Company’s patented DNA methylation platform and biomarkers. On an ancillary and opportunistic basis, the Company may engage in contracting out its R&D and scientific expertise to commercial and non-commercial entities. The Company is not organized, nor does it operate along business lines and all functions supported all the Company’s commercial endeavors. 2.9. Externally acquired intangible assets Intangible assets are recognized on business combinations if they are separable from the acquired entity or give rise to other contractual/legal rights. The amounts ascribed to such intangibles are determined using appropriate valuation techniques. Externally generated intangible assets are carried at their cost less any accumulated amortization and any accumulated impairment losses. Externally acquired patents and software licenses are initially recognized at cost and are subsequently amortized on a straight-line basis over their estimated useful lives on the following basis: • Patents: shorter of 5 years or the remaining patent life • Software: shorter of 5 years or the software license period • Developed technology: 10 years • In-Process Research and Development: indefinite until the completion or abandonment of the associated research and development effort. 2.10. Internally generated intangible assets (development costs) Development costs are capitalized if it can be demonstrated that: • It is technically feasible to develop the product for it to be sold; • Adequate resources are available to complete the development; • There is an intention to complete and sell the product; • The Company is able to sell the product; • Sale of the product will generate future economic benefits; and, • Expenditures on the project can be measured reliably. Internally generated intangible assets are carried at their cost less any accumulated amortization and any accumulated impairment losses. Amortization over the asset’s useful life shall begin when the asset is available for use.Annual Report 2021 122

Part IV: Financial Statements 2.11. Property, plant and equipment Property, plant and equipment are stated at historical cost less accumulated depreciation and impairment. Repair and maintenance costs are charged to the income statement as incurred. Gains and losses on the disposal of property, plant and equipment are included in other income or expenses. Depreciation is charged to write off the cost or valuation of assets over their useful lives, using the straight-line method, on the following basis: • Equipment: 5 years • IT hardware and software: 3 years • Furniture: 5 years • Vehicles: 5 years • Leasehold improvements: in line with the lease agreement period 2.12. Right-of-use assets and liabilities Right-of-use assets: The Company recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and impairment losses, and adjusted for any remeasurement of lease liabilities. The cost of right-of-use assets includes the amount of lease liabilities recognized, initial direct costs incurred, and lease payments made at or before the commencement date less any lease incentives received. Unless the Company is reasonably certain to obtain ownership of the leased asset at the end of the lease term, the recognized right-of-use assets are depreciated on a straight-line basis over the shorter of its estimated useful life (see 2.11) and the lease term. Right-of-use assets are subject to impairment. Lease liabilities: At the commencement date of the lease, the Company recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option reasonably certain to be exercised by the Company and payments of penalties for terminating a lease, if the lease term reflects the Company exercising the option to terminate. The variable lease payments that do not depend on an index or a rate are recognized as expense in the period on which the event or condition that triggers the payment occurs. In calculating the present value of lease payments, the Company uses the incremental borrowing rate at the lease commencement date if the interest rate implicit in the lease is not readily determinable. After the commencement date, the amount of lease liabilities is increased to reflect the accretion of interest and reduced for the lease payments made. In addition, the carrying amount of lease liabilities is remeasured if there is a modification, a change in the lease term, a change in the in-substance fixed lease payments or a change in the assessment to purchase the underlying asset. Short-term leases and leases of low-value assets: The Company applies the short-term lease recognition exemption to its short-term leases of machinery and equipment (i.e., those leases that have a lease term of 12 months or less from the commencement date and do not contain a purchase option). It also applies the lease of low-value assets recognition exemption to leases of office equipment that are considered of low value (i.e., below $5,000). Lease payments on short-term leases and low-value assets are recognized in the consolidated statement of profit or loss as incurred. Annual Report 2021 123

Part IV: Financial Statements 2.13. Impairment of assets Intangible assets that have an indefinite useful life are not subject to amortizations and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs of disposal and value in use. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash inflows which are largely independent of the cash inflows from other assets or groups of assets (cash generating units). Non- financial assets other than goodwill that suffered an impairment are reviewed for possible reversal of the impairment at the end of each reporting period. 2.14. Inventories Inventories are initially recognized at cost, and subsequently at the lower of cost and net realizable value. Cost comprises merely purchase costs, as the inventory consists solely of raw materials. Raw materials are not ordinarily interchangeable, and they are as such accounted for using the specific identification of their individual cost. The Company does not account for work in progress and finished products. 2.15. Government Grants A government grant is only recorded as a receivable when (i) the grant has been approved by the granting party, (ii) the amounts are measurable, and (iii) the Company believes it will meet the conditions necessary to be able to receive/use the grant. Government grants are recognized as other operating income over the life of the grant as the required or planned activities are performed and the related costs are incurred, and when there is reasonable assurance that the Company will comply with the conditions of the grant. 2.16. Cash and cash equivalents Cash and cash equivalents are carried on the balance sheet at nominal value. For the purposes of the cash flow statements, cash and cash equivalents comprise cash on hand, deposits held on call with banks, other short-term highly liquid investments and bank overdrafts. Bank overdrafts, if any, are included in borrowings included in current liabilities. 2.17. Taxation Current tax assets and liabilities for the current period are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted, at the reporting date. Deferred income tax is provided in full using the “balance sheet liability method”, on temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Deferred tax liabilities are recognized for all taxable differences. Deferred tax assets are recognized for all deductible temporary differences, carry forward of unused tax credits and unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences, and the carry forward of unused tax credits and unused tax losses can be utilized. Annual Report 2021 124

Part IV: Financial Statements The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred tax asset to be utilized. Unrecognized deferred tax assets are reassessed at each reporting date and are recognized to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered. Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the reporting date. Deferred tax assets and deferred tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred taxes relate to the same taxable entity and the same taxation authority. 2.18. Share capital Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds. 2.19. Financial Assets The financial assets consist mainly of trade receivables and other current assets (deposits). Classification and measurement on initial recognition The classification of financial assets at initial recognition depends on the financial asset’s contractual cash flow characteristics and the Company’s business model for managing them. With the exception of trade receivables that do not contain a significant financing component or for which the Company has applied the practical expedient, the Company initially measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss, transaction costs. Trade receivables that do not contain a significant financing component or for which the Company has applied the practical expedient are measured at the transaction price. Trade receivables do not carry any interest and are recognized initially at fair value and subsequently measured at amortized cost using the effective interest method, less provision for impairment based on expected credit losses, where applicable. Subsequent measurement After initial recognition, trade receivables and some other current assets are measured at amortized cost using the effective interest method, less provision for impairment based on expected credit losses. 2.20. Financial Liabilities The financial liabilities consist mainly of loans and borrowings, lease liabilities, trade and other payables and other financial liabilities that include derivative financial liabilities and contingent consideration related to business combinations. Measurement on initial recognition At initial recognition financial liabilities are measured at fair value minus transaction costs unless the financial liability is carried at fair value through profit or loss, in which case the transaction costs are immediately recognized in profit Annual Report 2021 125

Part IV: Financial Statements or loss. The best estimate of the fair value at initial recognition is usually the transaction price, represented by the fair value of the consideration given or received in exchange for the financial instrument. Any difference between the fair value estimated by the entity and the transaction price (“day one gain or loss”) is recognized: • in the income statement if the estimate is evidenced by a quoted price in an active market; and • deferred as an adjustment to the carrying amount of the financial instrument in all other cases. The fair value of the contingent consideration payable at the date of acquisition is computed as the sum of the probability weighted values of the fair values of the purchase prices associated with each of the potential product development routes. The fair value of each route is in turn computed as the sum of the survival probability discounted present values of the contingent payments in each such route including the milestone and commercialization payments. Any other financial liability included in the consideration payable for a business combination is recorded at fair value at the date of acquisition. The fair value of the financial derivative financial instrument related to the initial drawdown fee of the loan which is either convertible into shares or repayable at 150% is computed as the sum of the probability weighted values of the fair values associated with each of the possible outcomes further described in note 14. Subsequent measurement After initial recognition, loans & borrowings, lease liabilities, trade and other payables, are measured at amortized cost using the effective interest method. The contingent consideration and the derivative financial liability of the Kreos- drawdown fee are measured at fair value are reviewed on a regular basis, and at least at each reporting date, and any changes in fair value are recorded in the consolidated statement of profit and loss. 2.21. Retirement benefit schemes and employee savings schemes Payments to defined contribution employee savings schemes are charged as an expense as they fall due. The Company does not offer nor operate any material defined benefit schemes for its employees. With respect to Belgian pension plans and as explained in Note 21, the Company has considered the potential impact of the employer’s legal obligation to guarantee a minimum return on the Belgian pension plans and that this was assessed not to be significant. 2.22. Share-based compensation plans for personnel, directors and business associates The Company grants stock options in accordance with several share-based compensation plans in consideration for services performed by personnel, directors and business associates. The cost of the services rendered is measured at the fair value of the granted options and recognized as an expense in the statement of profit or loss. The corresponding credit is recorded directly into equity. The estimate of the number of options which will ultimately vest is revised at each reporting date. The change in estimate is recorded as an expense with a corresponding correction in equity. The received amount, less directly attributable transaction costs, will be recorded as share capital and share premium when the options are exercised. Annual Report 2021 126
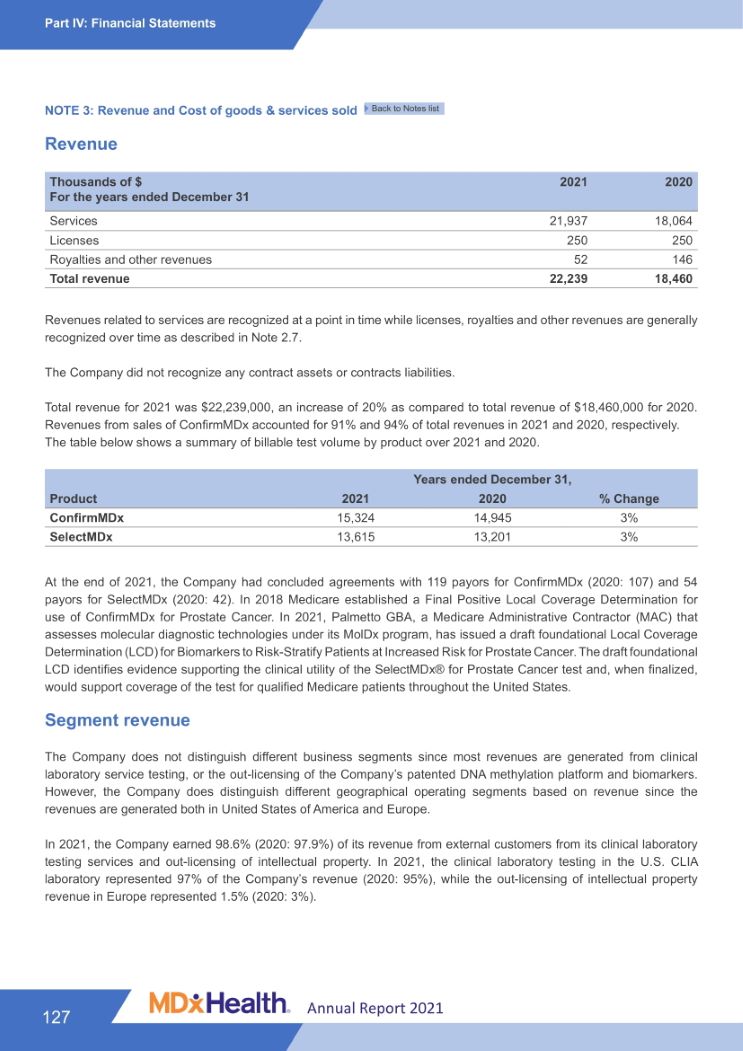
Part IV: Financial Statements NOTE 3: Revenue and Cost of goods & services sold Revenue Back to Notes list Thousands of $ For the years ended December 31 2021 2020 Services 21,937 18,064 Licenses 250 250 Royalties and other revenues 52 146 Total revenue 22,239 18,460 Revenues related to services are recognized at a point in time while licenses, royalties and other revenues are generally recognized over time as described in Note 2.7. The Company did not recognize any contract assets or contracts liabilities. Total revenue for 2021 was $22,239,000, an increase of 20% as compared to total revenue of $18,460,000 for 2020. Revenues from sales of ConfirmMDx accounted for 91% and 94% of total revenues in 2021 and 2020, respectively. The table below shows a summary of billable test volume by product over 2021 and 2020. Years ended December 31, Product 2021 2020 % Change ConfirmMDx 15,324 14,945 3% SelectMDx 13,615 13,201 3% At the end of 2021, the Company had concluded agreements with 119 payors for ConfirmMDx (2020: 107) and 54 payors for SelectMDx (2020: 42). In 2018 Medicare established a Final Positive Local Coverage Determination for use of ConfirmMDx for Prostate Cancer. In 2021, Palmetto GBA, a Medicare Administrative Contractor (MAC) that assesses molecular diagnostic technologies under its MolDx program, has issued a draft foundational Local Coverage Determination (LCD) for Biomarkers to Risk-Stratify Patients at Increased Risk for Prostate Cancer. The draft foundational LCD identifies evidence supporting the clinical utility of the SelectMDx® for Prostate Cancer test and, when finalized, would support coverage of the test for qualified Medicare patients throughout the United States. Segment revenue The Company does not distinguish different business segments since most revenues are generated from clinical laboratory service testing, or the out-licensing of the Company’s patented DNA methylation platform and biomarkers. However, the Company does distinguish different geographical operating segments based on revenue since the revenues are generated both in United States of America and Europe. In 2021, the Company earned 98.6% (2020: 97.9%) of its revenue from external customers from its clinical laboratory testing services and out-licensing of intellectual property. In 2021, the clinical laboratory testing in the U.S. CLIA laboratory represented 97% of the Company’s revenue (2020: 95%), while the out-licensing of intellectual property revenue in Europe represented 1.5% (2020: 3%). Annual Report 2021 127

Part IV: Financial Statements In 2021, Medicare represented the only payer generating over 38% of the Company’s revenues, for a total of $8,509,733 (2020 : $8,805,000). The amount of its revenue from external customers broken down by location is shown in the table below: Thousands of $ For the years ended December 31 2021 2020 United States of America 21,785 17,760 The Netherlands 195 352 Belgium 26 29 Spain 110 132 Poland 23 16 Italy 15 38 Rest of EU 72 112 Rest of the world 13 21 Total segment revenue 22,239 18,460 At the end of 2021, 38% of the non-current assets were located in the US (2020: 40%) and the remaining 62% in Europe (2020: 60%). Cost of goods & services sold Thousands of $ For the years ended December 31 2021 2020 Cost of goods & services sold 11,675 10,416 Total cost of goods & services sold 11,675 10,416 The costs of goods & services sold include the costs associated with providing testing services to third parties and include the cost of materials, labor (including salaries, bonuses, and benefits), transportation, collection kits, and allocated overhead costs associated with processing samples. Allocated overhead costs include depreciation of laboratory equipment, facility occupancy and information technology costs. Costs associated with processing samples are expensed when incurred, regardless of the timing of revenue recognition. Annual Report 2021 128
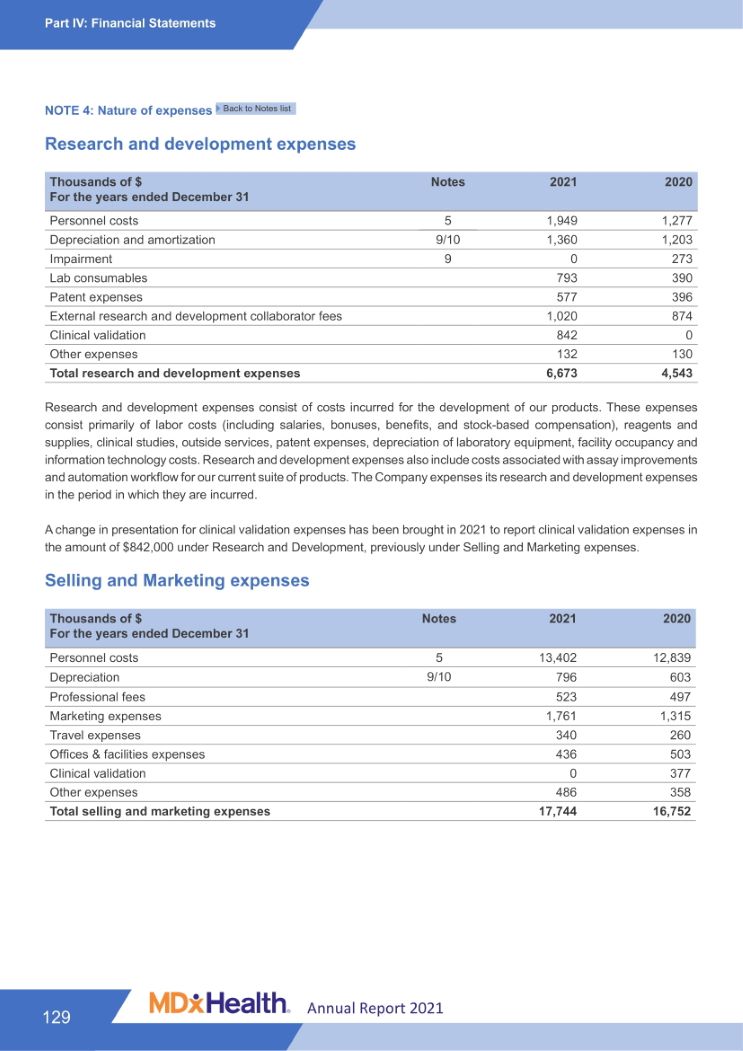
Part IV: Financial Statements NOTE 4: Nature of expenses Back to Notes list Research and development expenses Thousands of $ For the years ended December 31 Notes 2021 2020 Personnel costs 5 1,949 1,277 Depreciation and amortization 9/10 1,360 1,203 Impairment 9 0 273 Lab consumables 793 390 Patent expenses 577 396 External research and development collaborator fees 1,020 874 Clinical validation 842 0 Other expenses 132 130 Total research and development expenses 6,673 4,543 Research and development expenses consist of costs incurred for the development of our products. These expenses consist primarily of labor costs (including salaries, bonuses, benefits, and stock-based compensation), reagents and supplies, clinical studies, outside services, patent expenses, depreciation of laboratory equipment, facility occupancy and information technology costs. Research and development expenses also include costs associated with assay improvements and automation workflow for our current suite of products. The Company expenses its research and development expenses in the period in which they are incurred. A change in presentation for clinical validation expenses has been brought in 2021 to report clinical validation expenses in the amount of $842,000 under Research and Development, previously under Selling and Marketing expenses. Selling and Marketing expenses Thousands of $ For the years ended December 31 Notes 2021 2020 Personnel costs 5 13,402 12,839 Depreciation 9/10 796 603 Professional fees 523 497 Marketing expenses 1,761 1,315 Travel expenses 340 260 Offices & facilities expenses 436 503 Clinical validation 0 377 Other expenses 486 358 Total selling and marketing expenses 17,744 16,752 Annual Report 2021 129
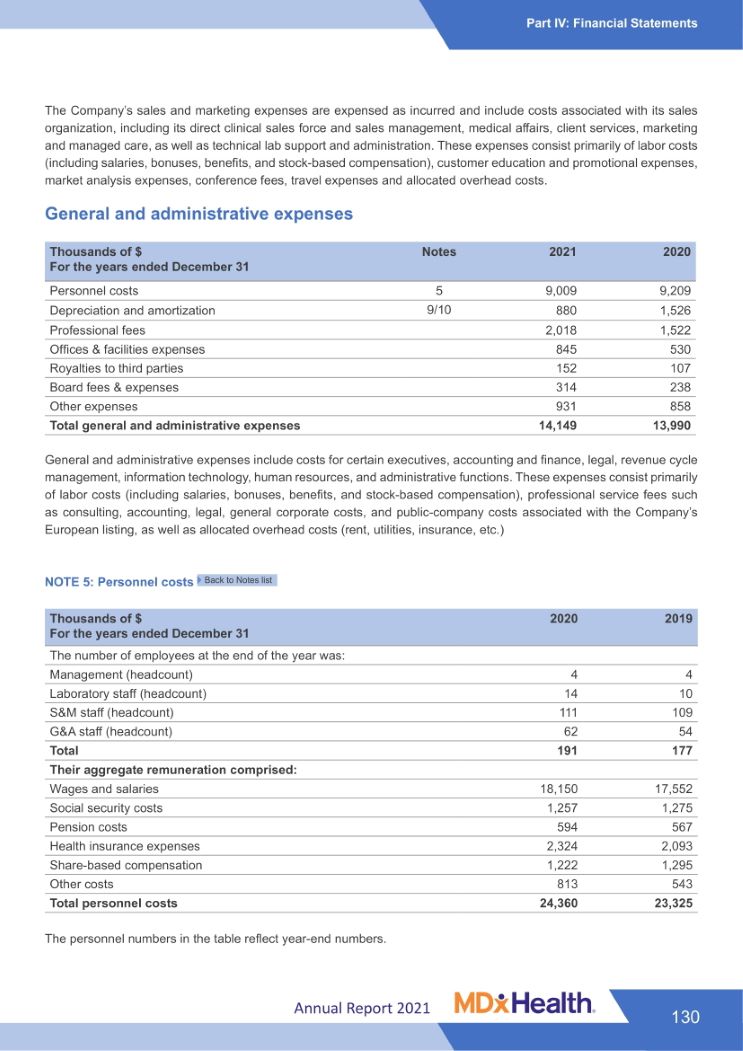
Part IV: Financial Statements The Company’s sales and marketing expenses are expensed as incurred and include costs associated with its sales organization, including its direct clinical sales force and sales management, medical affairs, client services, marketing and managed care, as well as technical lab support and administration. These expenses consist primarily of labor costs (including salaries, bonuses, benefits, and stock-based compensation), customer education and promotional expenses, market analysis expenses, conference fees, travel expenses and allocated overhead costs.General and administrative expensesThousands of $For the years ended December 31Notes20212020Personnel costs59,0099,209Depreciation and amortization9/108801,526Professional fees2,0181,522Offices & facilities expenses845530Royalties to third parties152107Board fees & expenses314238Other expenses931858Total general and administrative expenses14,14913,990 General and administrative expenses include costs for certain executives, accounting and finance, legal, revenue cycle management, information technology, human resources, and administrative functions. These expenses consist primarily of labor costs (including salaries, bonuses, benefits, and stock-based compensation), professional service fees such as consulting, accounting, legal, general corporate costs, and public-company costs associated with the Company’s European listing, as well as allocated overhead costs (rent, utilities, insurance, etc.) NOTE 5: Personnel costs Back to Notes list Thousands of $For the years ended December 3120202019The number of employees at the end of the year was:Management (headcount)44Laboratory staff (headcount)1410S&M staff (headcount)111109G&A staff (headcount)6254Total191177Their aggregate remuneration comprised:Wages and salaries18,15017,552Social security costs1,2571,275Pension costs594567Health insurance expenses2,3242,093Share-based compensation1,2221,295Other costs813543Total personnel costs24,36023,325The personnel numbers in the table reflect year-end numbers. Annual Report 2021 130
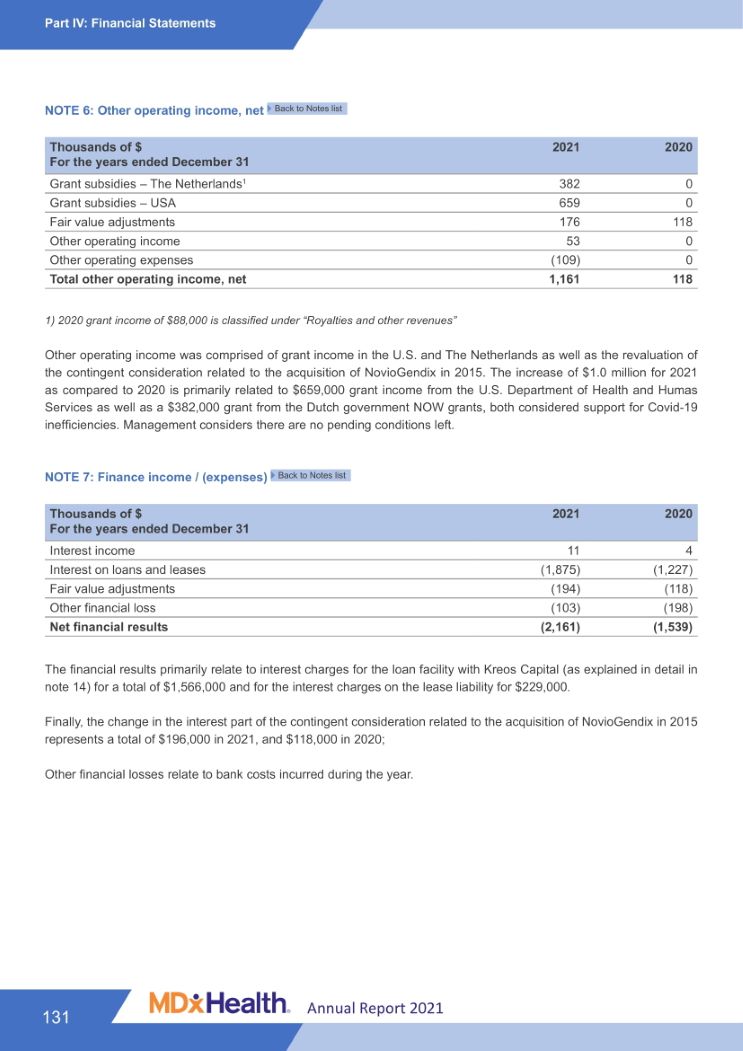
Part IV: Financial Statements NOTE 6: Other operating income, net Back to Notes list Thousands of $For the years ended December 3120212020Grant subsidies – The Netherlands13820Grant subsidies – USA6590Fair value adjustments176118Other operating income530Other operating expenses(109)0Total other operating income, net1,1611181) 2020 grant income of $88,000 is classified under “Royalties and other revenues”Other operating income was comprised of grant income in the U.S. and The Netherlands as well as the revaluation of the contingent consideration related to the acquisition of NovioGendix in 2015. The increase of $1.0 million for 2021 as compared to 2020 is primarily related to $659,000 grant income from the U.S. Department of Health and Humas Services as well as a $382,000 grant from the Dutch government NOW grants, both considered support for Covid-19 inefficiencies. Management considers there are no pending conditions left.NOTE 7: Finance income / (expenses) Back to Notes list Thousands of $For the years ended December 3120212020Interest income114Interest on loans and leases(1,875)(1,227)Fair value adjustments(194)(118)Other financial loss(103)(198)Net financial results(2,161)(1,539)The financial results primarily relate to interest charges for the loan facility with Kreos Capital (as explained in detail in note 14) for a total of $1,566,000 and for the interest charges on the lease liability for $229,000.Finally, the change in the interest part of the contingent consideration related to the acquisition of NovioGendix in 2015 represents a total of $196,000 in 2021, and $118,000 in 2020;Other financial losses relate to bank costs incurred during the year. Annual Report 2021 131
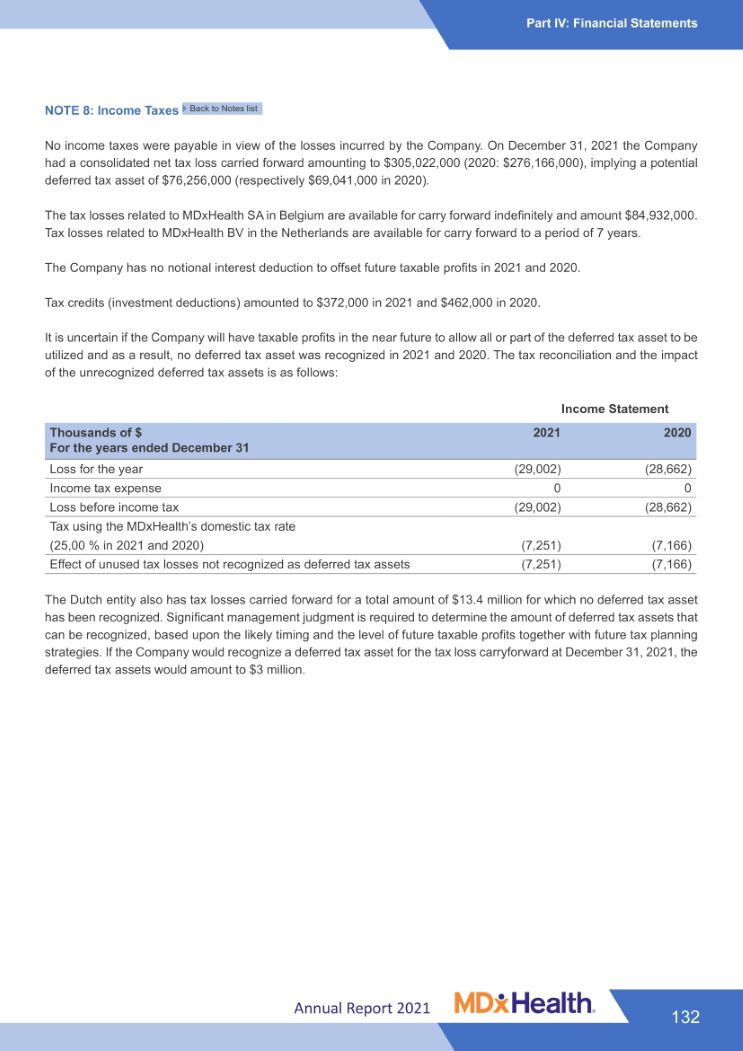
Part IV: Financial Statements. NOTE 8: Income Taxes Back to Notes list No income taxes were payable in view of the losses incurred by the Company. On December 31, 2021 the Company had a consolidated net tax loss carried forward amounting to $305,022,000 (2020: $276,166,000), implying a potential deferred tax asset of $76,256,000 (respectively $69,041,000 in 2020).The tax losses related to MDxHealth SA in Belgium are available for carry forward indefinitely and amount $84,932,000. Tax losses related to MDxHealth BV in the Netherlands are available for carry forward to a period of 7 years.The Company has no notional interest deduction to offset future taxable profits in 2021 and 2020. Tax credits (investment deductions) amounted to $372,000 in 2021 and $462,000 in 2020.It is uncertain if the Company will have taxable profits in the near future to allow all or part of the deferred tax asset to be utilized and as a result, no deferred tax asset was recognized in 2021 and 2020. The tax reconciliation and the impact of the unrecognized deferred tax assets is as follows:Income StatementThousands of $For the years ended December 3120212020Loss for the year(29,002)(28,662)Income tax expense00Loss before income tax(29,002)(28,662)Tax using the MDxHealth’s domestic tax rate(25,00 % in 2021 and 2020)(7,251)(7,166)Effect of unused tax losses not recognized as deferred tax assets(7,251)(7,166)The Dutch entity also has tax losses carried forward for a total amount of $13.4 million for which no deferred tax asset has been recognized. Significant management judgment is required to determine the amount of deferred tax assets that can be recognized, based upon the likely timing and the level of future taxable profits together with future tax planning strategies. If the Company would recognize a deferred tax asset for the tax loss carryforward at December 31, 2021, the deferred tax assets would amount to $3 million. Annual Report 2021 132
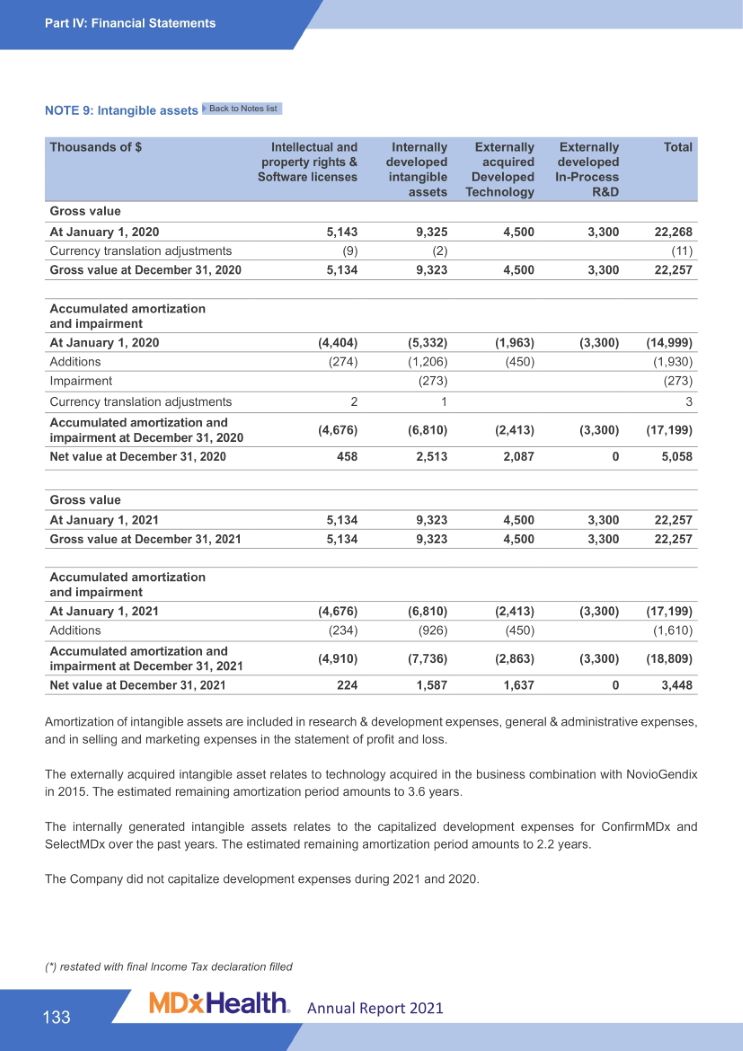
Part IV: Financial Statements. NOTE 9: Intangible assets Back to Notes listThousands of $Intellectual and property rights & Software licensesInternally developed intangibleassetsExternally acquired Developed TechnologyExternally developed In-ProcessR&DTotalGross valueAt January 1, 20205,1439,3254,5003,30022,268Currency translation adjustments(9)(2)(11)Gross value at December 31, 20205,1349,3234,5003,30022,257Accumulated amortization and impairmentAt January 1, 2020(4,404)(5,332)(1,963)(3,300)(14,999)Additions(274)(1,206)(450)(1,930)Impairment(273)(273)Currency translation adjustments213Accumulated amortization and(4,676)(6,810)(2,413)(3,300)(17,199)Net value at December 31, 20204582,5132,08705,058Gross valueAt January 1, 20215,1349,3234,5003,30022,257Gross value at December 31, 20215,1349,3234,5003,30022,257Accumulated amortization and impairmentAt January 1, 2021(4,676)(6,810)(2,413)(3,300)(17,199)Additions(234)(926)(450)(1,610)Accumulated amortization and(4,910)(7,736)(2,863)(3,300)(18,809)Net value at December 31, 20212241,5871,63703,448Amortization of intangible assets are included in research & development expenses, general & administrative expenses, and in selling and marketing expenses in the statement of profit and loss.The externally acquired intangible asset relates to technology acquired in the business combination with NovioGendix in 2015. The estimated remaining amortization period amounts to 3.6 years.The internally generated intangible assets relates to the capitalized development expenses for ConfirmMDx and SelectMDx over the past years. The estimated remaining amortization period amounts to 2.2 years.The Company did not capitalize development expenses during 2021 and 2020.(*) restated with final Income Tax declaration filled Annual Report 2021 133

Part IV: Financial Statements. The In-process R&D resulted from the allocation of the purchase price paid for the acquisition of MDxHealth BV in September 2015 and is related to the development of AssureMDx. Development costs for AssureMDx are included in development assets.Considering the uncertainties about the future commercialization of AssureMDx, during 2019, the Company impaired the entire In-Process R&D for $3,300,000, in addition to the previously capitalized development expenses for $1,847,000.The impairment test is based on the projected discounted cash flows resulting from ConfirmMDx and SelectMDx assays, considering a period of ten years. The main assumptions for impairment testing include a discount rate (based on WACC) of 12.16% and a perpetual growth rate of 3.0%. Other assumptions include the year-on-year growth rate of the revenue, gross margin and the operating costs which has been determined by management based on past experience. It was concluded that the value in use is higher than the carrying value of the cash generating unit of $3.4 million. There are no reasonably possible changes in assumptions that would reduce the value in use below its carrying value of the cash generating unit.NOTE 10: Property, plant and equipment and right of-useBack to Notes list In December 2019, the Company entered into a lease agreement (the “Alton Lease”) for approx. 11,000 square feet of office space in Irvine, California. In April 2020, the company amended the Alton Lease to add approx. 8,000 additional square feet of adjacent office space. The term of the Alton Lease is 6 years and commenced in September 2020. In March 2021, the Company amended the Alton Lease a second time for purposes of renewing its existing 13,000 square foot laboratory space for an additional 5 years with a commencement date of October 2021. Under the terms of the Alton Lease Agreement, the Company has an option to extend the Alton Lease for a period of 5 years. In October of 2021, the company entered into a 35-month sublease agreement (the “Second Lease Agreement”) for 6,000 square feet for an additional office space adjacent to the laboratory. Under the terms of the Alton Lease and Second Lease Agreement, rental payments escalate through the term of each agreement and the Company is subject to additional charges for common area maintenance and other costs. The new lease agreements from 2021 represent an additional right of use assets of a total value of $1,518,000.Thousands of $Laboratory equipmentFurnitureITequipmentLeasehold improve-mentsTOTALGross valueAt January 1, 20205,2562703335636,422Additions10116317898540Disposals(4)(4)Exchange rate difference arising21(3)55Gross value at December 31, 20205,3594345046666,963Accumulated depreciationAt January 1, 2020(4,398)(211)(272)(474)(5,355)Additions(467)(34)(77)(53)(631)Disposals145Exchange rate difference arising(10)12(2)(9)Accumulated depreciation at December 31, 2020(4,874)(244)(343)(529)(5,990)Net value at December 31, 2020485190161137973Annual Report 2021 134
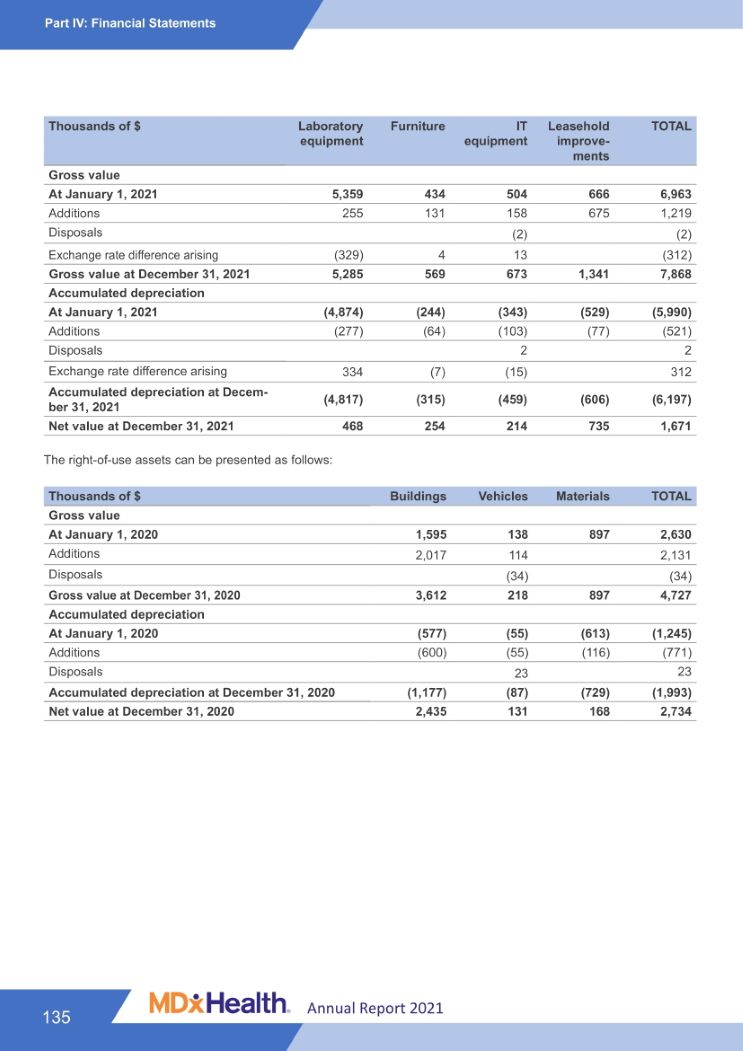
Part IV: Financial Statements. Thousands of $Laboratory equipmentFurnitureITequipmentLeasehold improve-mentsTOTALGross valueAt January 1, 20215,3594345046666,963Additions2551311586751,219Disposals(2)(2)Exchange rate difference arising(329)413(312)Gross value at December 31, 20215,2855696731,3417,868Accumulated depreciationAt January 1, 2021(4,874)(244)(343)(529)(5,990)Additions(277)(64)(103)(77)(521)Disposals22Exchange rate difference arising334(7)(15)312Accumulated depreciation at Decem- ber 31, 2021(4,817)(315)(459)(606)(6,197)Net value at December 31, 20214682542147351,671The right-of-use assets can be presented as follows:Thousands of $BuildingsVehiclesMaterialsTOTALGross valueAt January 1, 20201,5951388972,630Additions2,0171142,131Disposals(34)(34)Gross value at December 31, 20203,6122188974,727Accumulated depreciationAt January 1, 2020(577)(55)(613)(1,245)Additions(600)(55)(116)(771)Disposals2323Accumulated depreciation at December 31, 2020(1,177)(87)(729)(1,993)Net value at December 31, 20202,4351311682,734Annual Report 2021 135
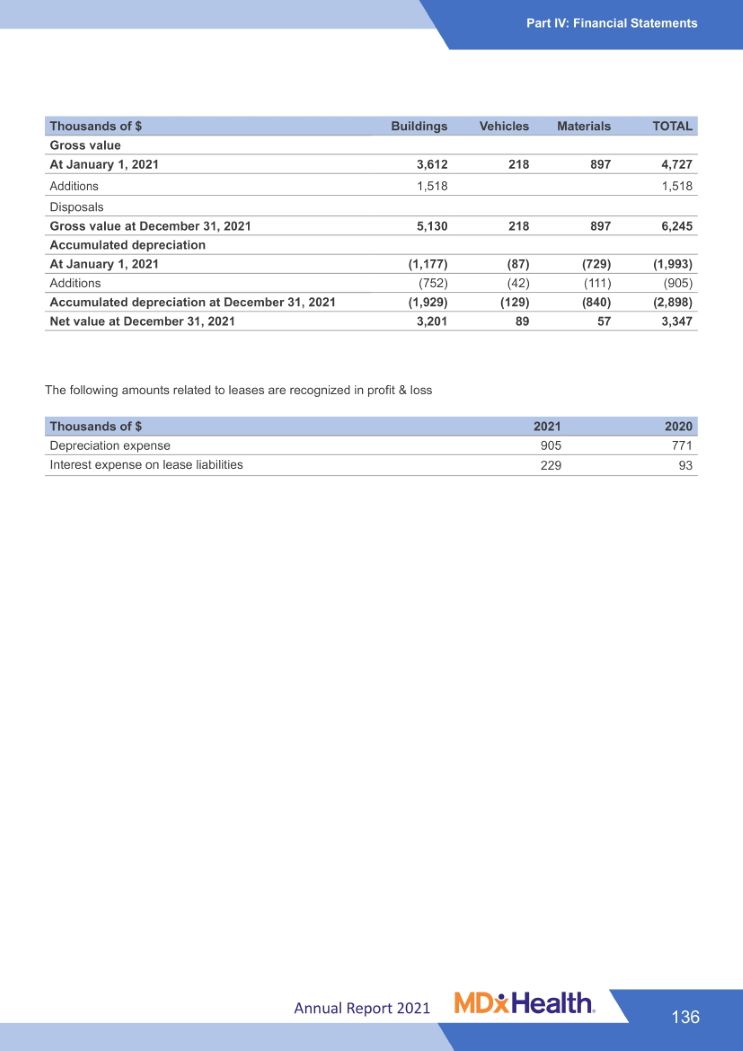
Part IV: Financial Statements. Thousands of $BuildingsVehiclesMaterialsTOTALGross valueAt January 1, 20213,6122188974,727Additions1,5181,518DisposalsGross value at December 31, 20215,1302188976,245Accumulated depreciationAt January 1, 2021(1,177)(87)(729)(1,993)Additions(752)(42)(111)(905)Accumulated depreciation at December 31, 2021(1,929)(129)(840)(2,898)Net value at December 31, 20213,20189573,347The following amounts related to leases are recognized in profit & lossThousands of $20212020Depreciation expense905771Interest expense on lease liabilities22993 Annual Report 2021 136
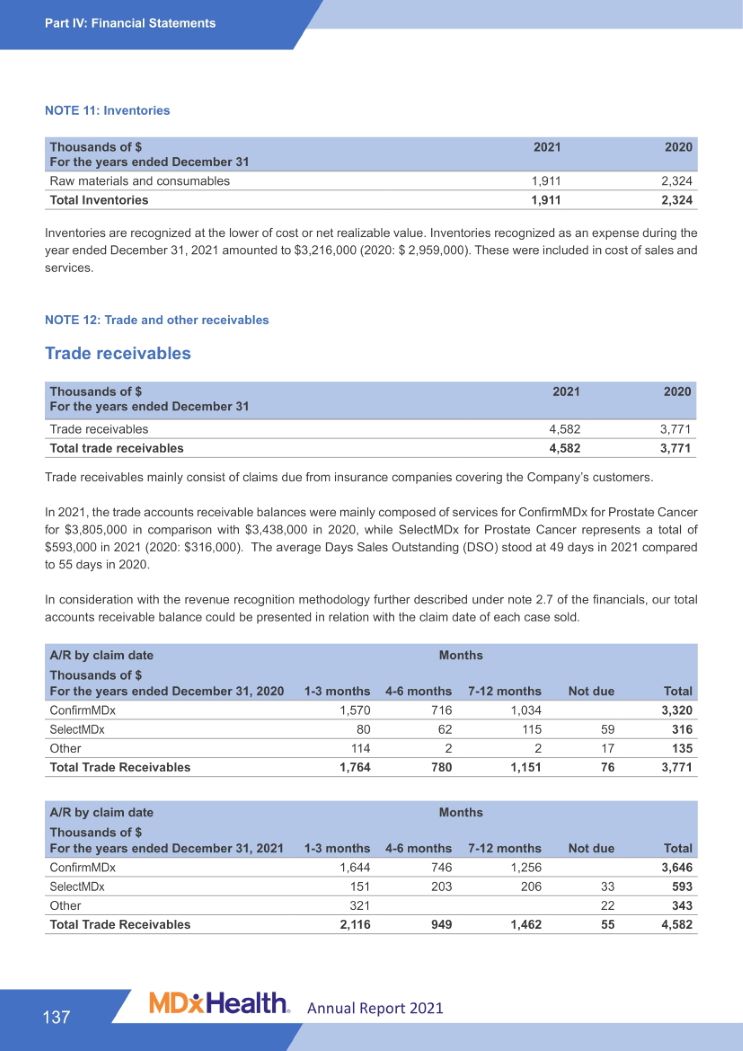
Part IV: Financial Statements. NOTE 11: InventoriesThousands of $For the years ended December 3120212020Raw materials and consumables1,9112,324Total Inventories1,9112,324Inventories are recognized at the lower of cost or net realizable value. Inventories recognized as an expense during the year ended December 31, 2021 amounted to $3,216,000 (2020: $ 2,959,000). These were included in cost of sales and services.NOTE 12: Trade and other receivablesTrade receivablesThousands of $For the years ended December 3120212020Trade receivables4,5823,771Total trade receivables4,5823,771Trade receivables mainly consist of claims due from insurance companies covering the Company’s customers.In 2021, the trade accounts receivable balances were mainly composed of services for ConfirmMDx for Prostate Cancer for $3,805,000 in comparison with $3,438,000 in 2020, while SelectMDx for Prostate Cancer represents a total of$593,000 in 2021 (2020: $316,000). The average Days Sales Outstanding (DSO) stood at 49 days in 2021 compared to 55 days in 2020.In consideration with the revenue recognition methodology further described under note 2.7 of the financials, our total accounts receivable balance could be presented in relation with the claim date of each case sold.A/R by claim dateMonthsThousands of $For the years ended December 31, 20201-3 months4-6 months7-12 monthsNot dueTotalConfirmMDx1,5707161,0343,320SelectMDx806211559316Other1142217135Total Trade Receivables1,7647801,151763,771A/R by claim dateMonthsThousands of $For the years ended December 31, 20211-3 months4-6 months7-12 monthsNot dueTotalConfirmMDx1,6447461,2563,646SelectMDx15120320633593Other32122343Total Trade Receivables2,1169491,462554,582Annual Report 2021 137
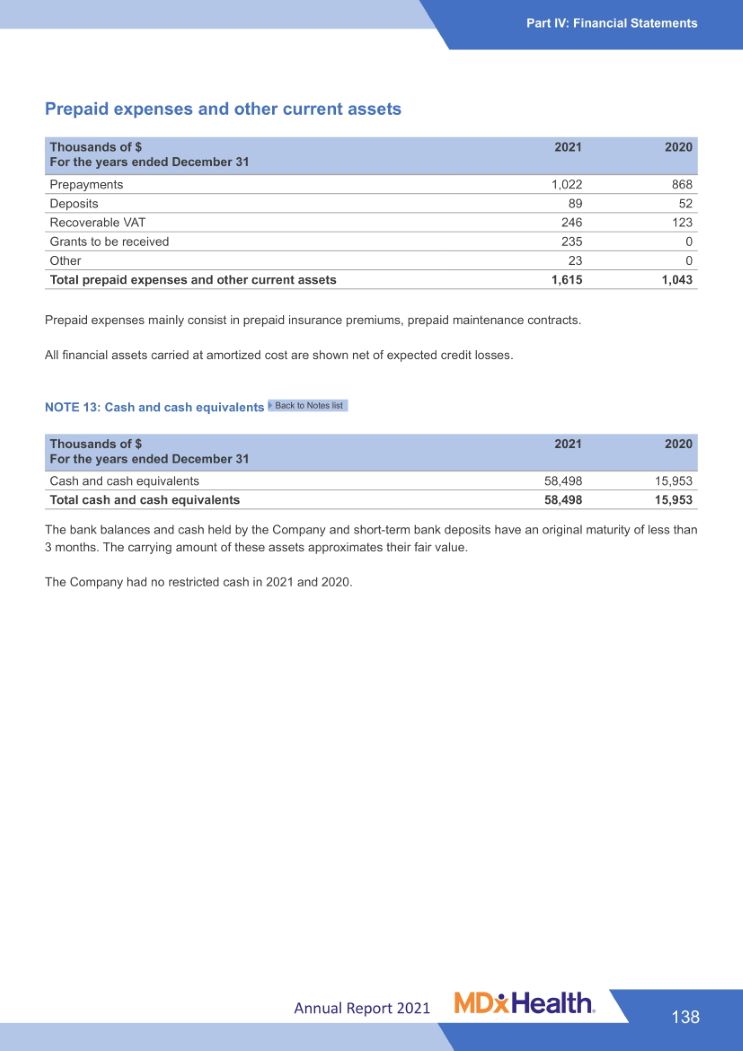
Part IV: Financial Statements. Prepaid expenses and other current assetsThousands of $For the years ended December 3120212020Prepayments1,022868Deposits8952Recoverable VAT246123Grants to be received2350Other230Total prepaid expenses and other current assets1,6151,043Prepaid expenses mainly consist in prepaid insurance premiums, prepaid maintenance contracts. All financial assets carried at amortized cost are shown net of expected credit losses.NOTE 13: Cash and cash equivalentsBack to Notes listThousands of $For the years ended December 3120212020Cash and cash equivalents58,49815,953Total cash and cash equivalents58,49815,953The bank balances and cash held by the Company and short-term bank deposits have an original maturity of less than 3 months. The carrying amount of these assets approximates their fair value.The Company had no restricted cash in 2021 and 2020.Annual Report 2021 138
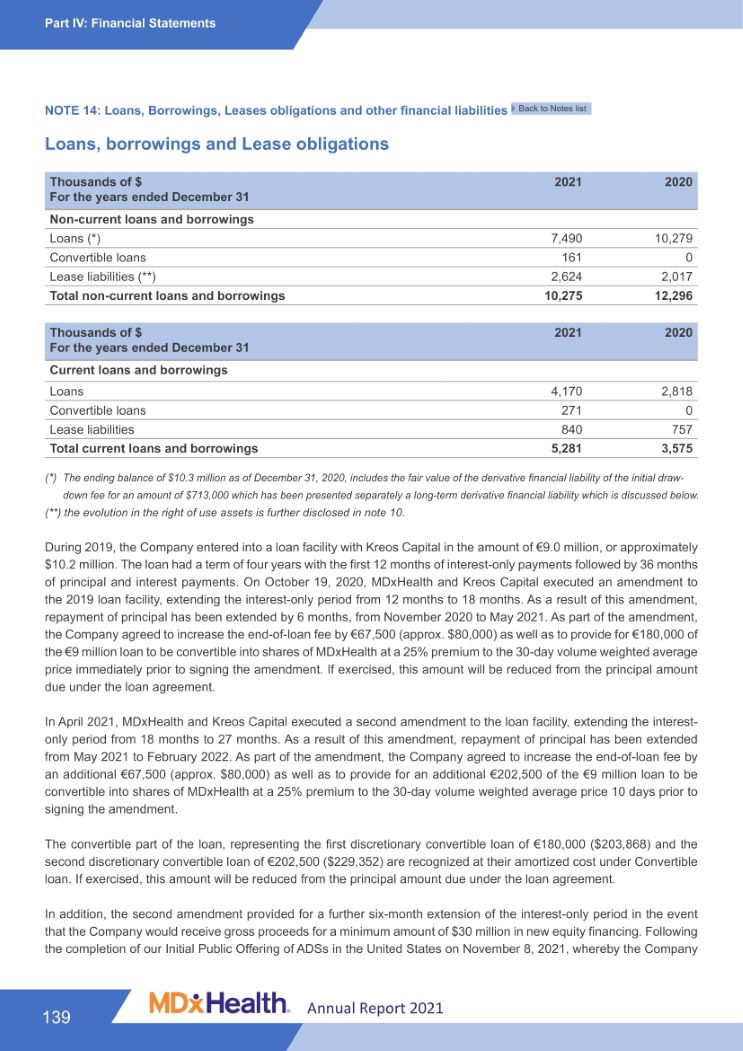
Part IV: Financial Statements. NOTE 14: Loans, Borrowings, Leases obligations and other financial liabilitiesLoans, borrowings and Lease obligationsBack to Notes listThousands of $For the years ended December 3120212020Non-current loans and borrowingsLoans (*)7,49010,279Convertible loans1610Lease liabilities (**)2,6242,017Total non-current loans and borrowings10,27512,296Thousands of $For the years ended December 3120212020Current loans and borrowingsLoans4,1702,818Convertible loans2710Lease liabilities840757Total current loans and borrowings5,2813,575(*) The ending balance of $10.3 million as of December 31, 2020, includes the fair value of the derivative financial liability of the initial draw- down fee for an amount of $713,000 which has been presented separately a long-term derivative financial liability which is discussed below.(**) the evolution in the right of use assets is further disclosed in note 10.During 2019, the Company entered into a loan facility with Kreos Capital in the amount of €9.0 million, or approximately$10.2 million. The loan had a term of four years with the first 12 months of interest-only payments followed by 36 months of principal and interest payments. On October 19, 2020, MDxHealth and Kreos Capital executed an amendment to the 2019 loan facility, extending the interest-only period from 12 months to 18 months. As a result of this amendment, repayment of principal has been extended by 6 months, from November 2020 to May 2021. As part of the amendment, the Company agreed to increase the end-of-loan fee by €67,500 (approx. $80,000) as well as to provide for €180,000 of the €9 million loan to be convertible into shares of MDxHealth at a 25% premium to the 30-day volume weighted average price immediately prior to signing the amendment. If exercised, this amount will be reduced from the principal amount due under the loan agreement.In April 2021, MDxHealth and Kreos Capital executed a second amendment to the loan facility, extending the interest- only period from 18 months to 27 months. As a result of this amendment, repayment of principal has been extended from May 2021 to February 2022. As part of the amendment, the Company agreed to increase the end-of-loan fee by an additional €67,500 (approx. $80,000) as well as to provide for an additional €202,500 of the €9 million loan to be convertible into shares of MDxHealth at a 25% premium to the 30-day volume weighted average price 10 days prior to signing the amendment.The convertible part of the loan, representing the first discretionary convertible loan of €180,000 ($203,868) and the second discretionary convertible loan of €202,500 ($229,352) are recognized at their amortized cost under Convertible loan. If exercised, this amount will be reduced from the principal amount due under the loan agreement.In addition, the second amendment provided for a further six-month extension of the interest-only period in the event that the Company would receive gross proceeds for a minimum amount of $30 million in new equity financing. Following the completion of our Initial Public Offering of ADSs in the United States on November 8, 2021, whereby the Company Annual Report 2021 139
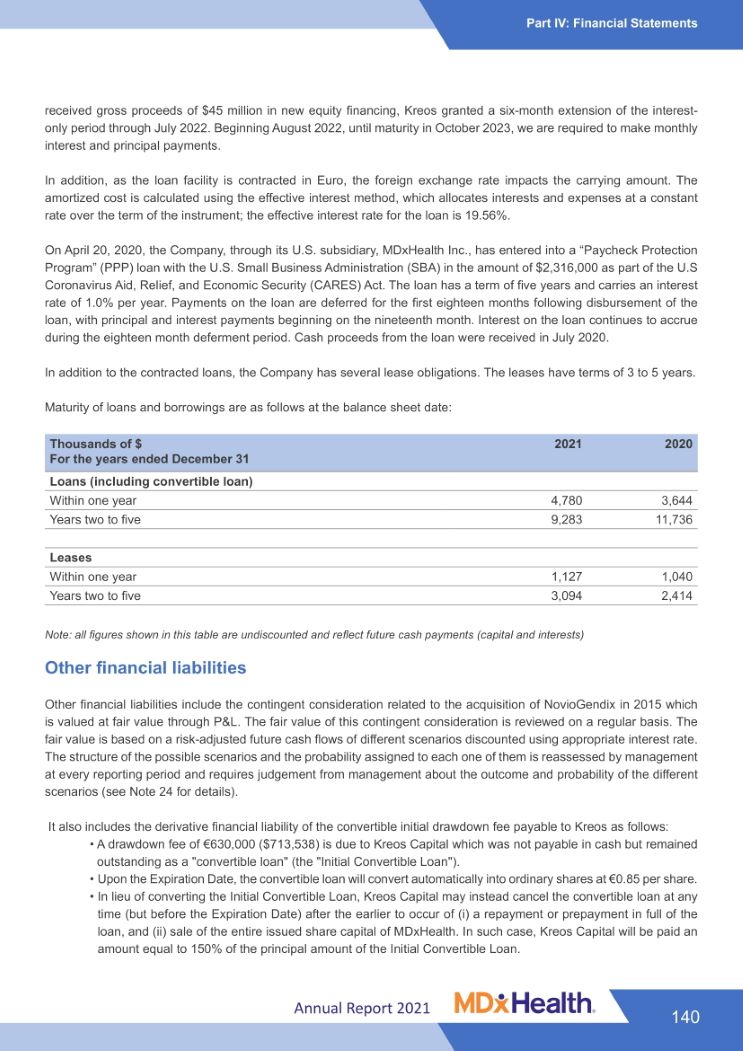
Part IV: Financial Statements received gross proceeds of $45 million in new equity financing, Kreos granted a six-month extension of the interest- only period through July 2022. Beginning August 2022, until maturity in October 2023, we are required to make monthly interest and principal payments.In addition, as the loan facility is contracted in Euro, the foreign exchange rate impacts the carrying amount. The amortized cost is calculated using the effective interest method, which allocates interests and expenses at a constant rate over the term of the instrument; the effective interest rate for the loan is 19.56%.On April 20, 2020, the Company, through its U.S. subsidiary, MDxHealth Inc., has entered into a “Paycheck Protection Program” (PPP) loan with the U.S. Small Business Administration (SBA) in the amount of $2,316,000 as part of the U.S Coronavirus Aid, Relief, and Economic Security (CARES) Act. The loan has a term of five years and carries an interest rate of 1.0% per year. Payments on the loan are deferred for the first eighteen months following disbursement of the loan, with principal and interest payments beginning on the nineteenth month. Interest on the loan continues to accrue during the eighteen month deferment period. Cash proceeds from the loan were received in July 2020.In addition to the contracted loans, the Company has several lease obligations. The leases have terms of 3 to 5 years.Maturity of loans and borrowings are as follows at the balance sheet date:Thousands of $For the years ended December 3120212020Loans (including convertible loan)Within one year4,7803,644Years two to five9,28311,736LeasesWithin one year1,1271,040Years two to five3,0942,414Note: all figures shown in this table are undiscounted and reflect future cash payments (capital and interests)Other financial liabilitiesOther financial liabilities include the contingent consideration related to the acquisition of NovioGendix in 2015 which is valued at fair value through P&L. The fair value of this contingent consideration is reviewed on a regular basis. The fair value is based on a risk-adjusted future cash flows of different scenarios discounted using appropriate interest rate. The structure of the possible scenarios and the probability assigned to each one of them is reassessed by management at every reporting period and requires judgement from management about the outcome and probability of the different scenarios (see Note 24 for details).It also includes the derivative financial liability of the convertible initial drawdown fee payable to Kreos as follows:A drawdown fee of €630,000 ($713,538) is due to Kreos Capital which was not payable in cash but remained outstanding as a "convertible loan" (the "Initial Convertible Loan").Upon the Expiration Date, the convertible loan will convert automatically into ordinary shares at €0.85 per share.In lieu of converting the Initial Convertible Loan, Kreos Capital may instead cancel the convertible loan at any time (but before the Expiration Date) after the earlier to occur of (i) a repayment or prepayment in full of the loan, and (ii) sale of the entire issued share capital of MDxHealth. In such case, Kreos Capital will be paid an amount equal to 150% of the principal amount of the Initial Convertible Loan.Annual Report 2021 140
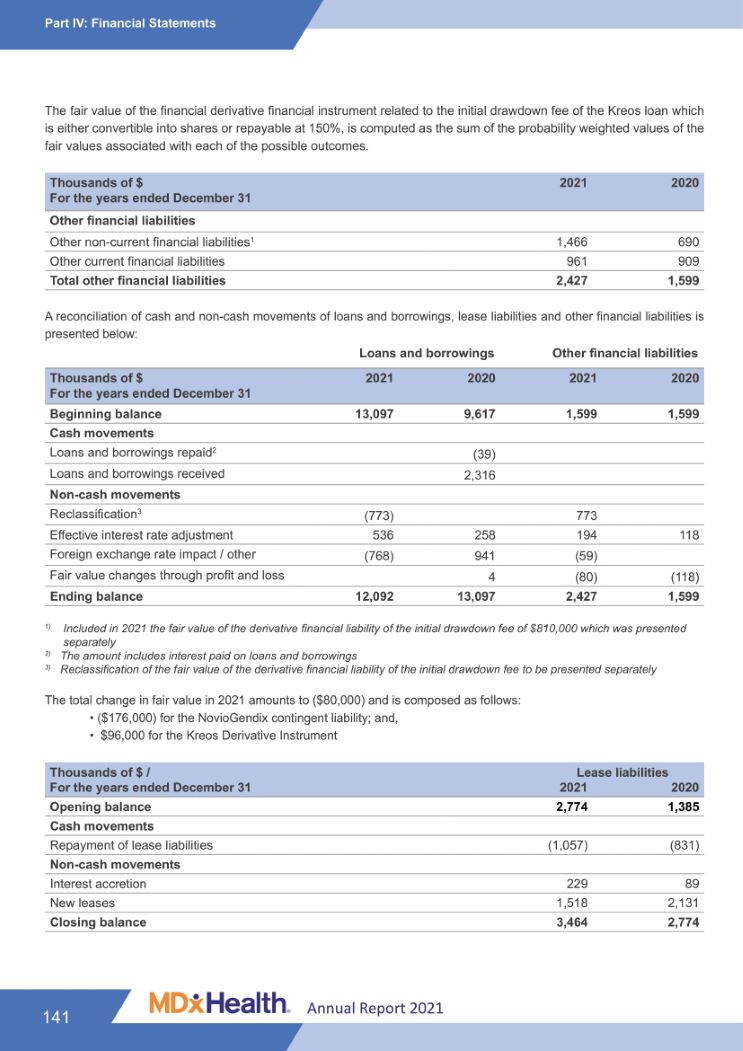
Part IV: Financial Statements The fair value of the financial derivative financial instrument related to the initial drawdown fee of the Kreos loan which is either convertible into shares or repayable at 150%, is computed as the sum of the probability weighted values of the fair values associated with each of the possible outcomes. Thousands of $ For the years ended December 31 2021 2020 Other financial liabilities Other non-current financial liabilities1 1,466 690 Other current financial liabilities 961 909 Total other financial liabilities 2,427 1,599 A reconciliation of cash and non-cash movements of loans and borrowings, lease liabilities and other financial liabilities is presented below: Loans and borrowings Other financial liabilities Thousands of $ 2021 2020 2021 2020 For the years ended December 31 Beginning balance 13,097 9,617 1,599 1,599 Cash movements Loans and borrowings repaid2 (39) Loans and borrowings received 2,316 Non-cash movements Reclassification3 (773) 773 Effective interest rate adjustment 536 258 194 118 Foreign exchange rate impact / other (768) 941 (59) Fair value changes through profit and loss 4 (80) (118) Ending balance 12,092 13,097 2,427 1,599 1) Included in 2021 the fair value of the derivative financial liability of the initial drawdown fee of $810,000 which was presented separately 2) The amount includes interest paid on loans and borrowings 3) Reclassification of the fair value of the derivative financial liability of the initial drawdown fee to be presented separately The total change in fair value in 2021 amounts to ($80,000) and is composed as follows: • ($176,000) for the NovioGendix contingent liability; and, • $96,000 for the Kreos Derivative Instrument Thousands of $ / For the years ended December 31 Lease liabilities 2021 2020 Opening balance 2,774 1,385 Cash movements Repayment of lease liabilities (1,057) (831) Non-cash movements Interest accretion 229 89 New leases 1,518 2,131 Closing balance 3,464 2,774 Annual Report 2021 141
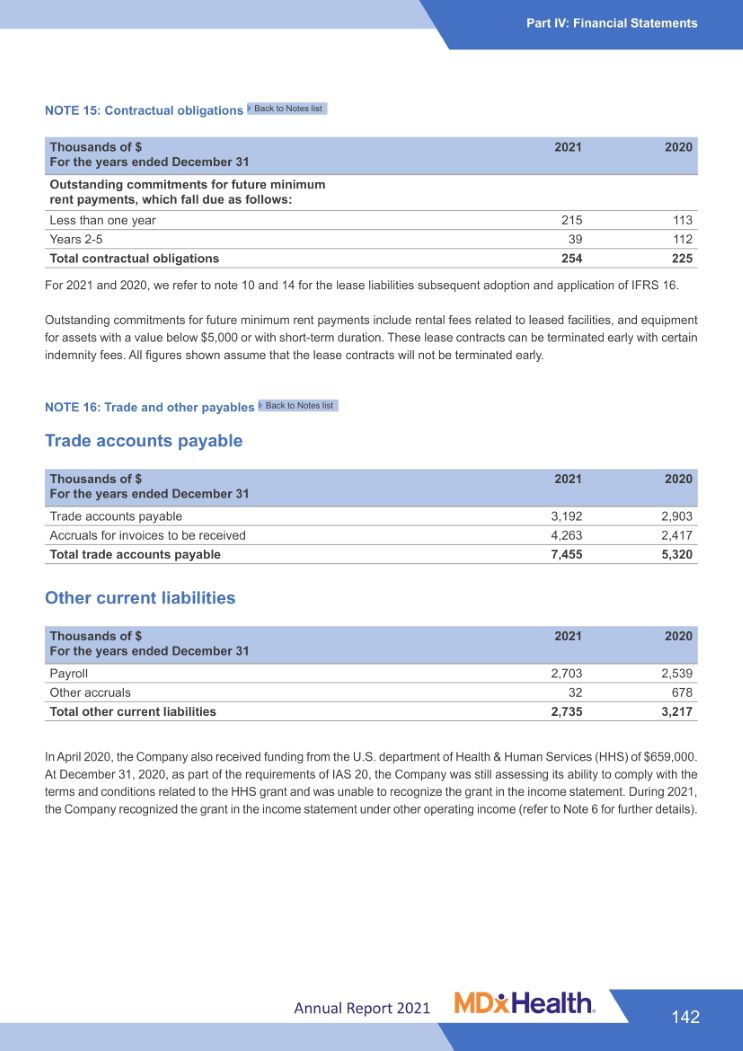
Part IV: Financial Statements NOTE 15: Contractual obligations Back to Notes list Thousands of $ For the years ended December 31 2021 2020 Outstanding commitments for future minimum rent payments, which fall due as follows: Less than one year 215 113 Years 2-5 39 112 Total contractual obligations 254 225 For 2021 and 2020, we refer to note 10 and 14 for the lease liabilities subsequent adoption and application of IFRS 16. Outstanding commitments for future minimum rent payments include rental fees related to leased facilities, and equipment for assets with a value below $5,000 or with short-term duration. These lease contracts can be terminated early with certain indemnity fees. All figures shown assume that the lease contracts will not be terminated early. NOTE 16: Trade and other payables Trade accounts payable Back to Notes list Thousands of $ For the years ended December 31 2021 2020 Trade accounts payable 3,192 2,903 Accruals for invoices to be received 4,263 2,417 Total trade accounts payable 7,455 5,320 Other current liabilities Thousands of $ For the years ended December 31 2021 2020 Payroll 2,703 2,539 Other accruals 32 678 Total other current liabilities 2,735 3,217 In April 2020, the Company also received funding from the U.S. department of Health & Human Services (HHS) of $659,000. At December 31, 2020, as part of the requirements of IAS 20, the Company was still assessing its ability to comply with the terms and conditions related to the HHS grant and was unable to recognize the grant in the income statement. During 2021, the Company recognized the grant in the income statement under other operating income (refer to Note 6 for further details). Annual Report 2021 142
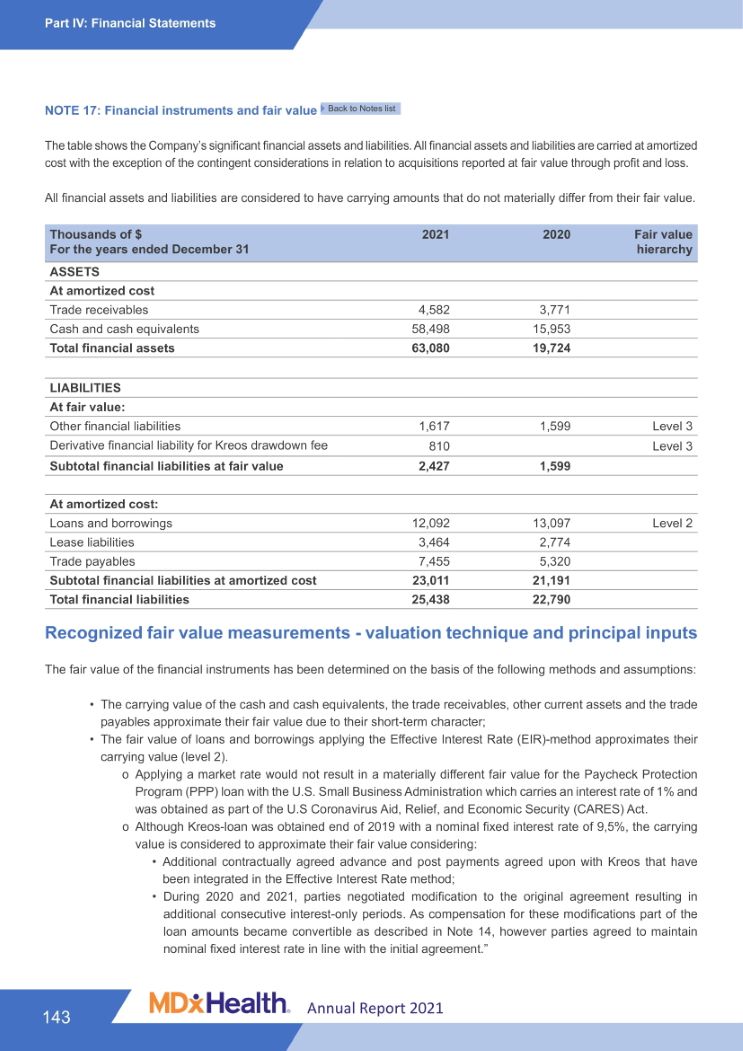
Part IV: Financial Statements NOTE 17: Financial instruments and fair value Back to Notes list The table shows the Company’s significant financial assets and liabilities. All financial assets and liabilities are carried at amortized cost with the exception of the contingent considerations in relation to acquisitions reported at fair value through profit and loss. All financial assets and liabilities are considered to have carrying amounts that do not materially differ from their fair value. Thousands of $ For the years ended December 31 2021 2020 Fair value hierarchy ASSETS At amortized cost Trade receivables 4,582 3,771 Cash and cash equivalents 58,498 15,953 Total financial assets 63,080 19,724 LIABILITIES At fair value: Other financial liabilities 1,617 1,599 Level 3 Derivative financial liability for Kreos drawdown fee 810 Level 3 Subtotal financial liabilities at fair value 2,427 1,599 At amortized cost: Loans and borrowings 12,092 13,097 Level 2 Lease liabilities 3,464 2,774 Trade payables 7,455 5,320 Subtotal financial liabilities at amortized cost 23,011 21,191 Total financial liabilities 25,438 22,790 Recognized fair value measurements - valuation technique and principal inputs The fair value of the financial instruments has been determined on the basis of the following methods and assumptions: • The carrying value of the cash and cash equivalents, the trade receivables, other current assets and the trade payables approximate their fair value due to their short-term character; • The fair value of loans and borrowings applying the Effective Interest Rate (EIR)-method approximates their carrying value (level 2). o Applying a market rate would not result in a materially different fair value for the Paycheck Protection Program (PPP) loan with the U.S. Small Business Administration which carries an interest rate of 1% and was obtained as part of the U.S Coronavirus Aid, Relief, and Economic Security (CARES) Act. o Although Kreos-loan was obtained end of 2019 with a nominal fixed interest rate of 9,5%, the carrying value is considered to approximate their fair value considering: • Additional contractually agreed advance and post payments agreed upon with Kreos that have been integrated in the Effective Interest Rate method; • During 2020 and 2021, parties negotiated modification to the original agreement resulting in additional consecutive interest-only periods. As compensation for these modifications part of the loan amounts became convertible as described in Note 14, however parties agreed to maintain nominal fixed interest rate in line with the initial agreement.” Annual Report 2021 143
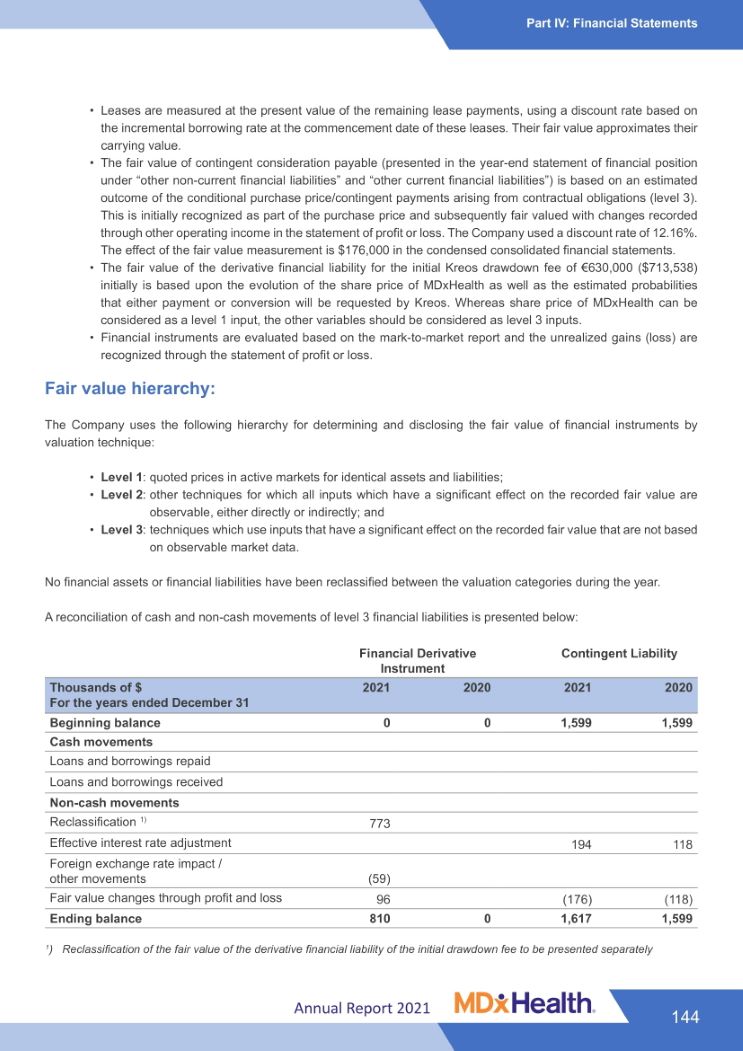
Part IV: Financial Statements • Leases are measured at the present value of the remaining lease payments, using a discount rate based on the incremental borrowing rate at the commencement date of these leases. Their fair value approximates their carrying value. • The fair value of contingent consideration payable (presented in the year-end statement of financial position under “other non-current financial liabilities” and “other current financial liabilities”) is based on an estimated outcome of the conditional purchase price/contingent payments arising from contractual obligations (level 3). This is initially recognized as part of the purchase price and subsequently fair valued with changes recorded through other operating income in the statement of profit or loss. The Company used a discount rate of 12.16%. The effect of the fair value measurement is $176,000 in the condensed consolidated financial statements. • The fair value of the derivative financial liability for the initial Kreos drawdown fee of €630,000 ($713,538) initially is based upon the evolution of the share price of MDxHealth as well as the estimated probabilities that either payment or conversion will be requested by Kreos. Whereas share price of MDxHealth can be considered as a level 1 input, the other variables should be considered as level 3 inputs. • Financial instruments are evaluated based on the mark-to-market report and the unrealized gains (loss) are recognized through the statement of profit or loss. Fair value hierarchy: The Company uses the following hierarchy for determining and disclosing the fair value of financial instruments by valuation technique: • Level 1: quoted prices in active markets for identical assets and liabilities; • Level 2: other techniques for which all inputs which have a significant effect on the recorded fair value are observable, either directly or indirectly; and • Level 3: techniques which use inputs that have a significant effect on the recorded fair value that are not based on observable market data.No financial assets or financial liabilities have been reclassified between the valuation categories during the year. A reconciliation of cash and non-cash movements of level 3 financial liabilities is presented below: Financial Derivative Instrument Contingent Liability Thousands of $ For the years ended December 31 2021 2020 2021 2020 Beginning balance 0 0 1,599 1,599 Cash movements Loans and borrowings repaid Loans and borrowings received Non-cash movements Reclassification 1) 773 Effective interest rate adjustment 194 118 Foreign exchange rate impact / other movements (59) Fair value changes through profit and loss 96 (176) (118) Ending balance 810 0 1,617 1,599 1) Reclassification of the fair value of the derivative financial liability of the initial drawdown fee to be presented separately Annual Report 2021 144
Part IV: Financial Statements NOTE 18: Loss per share Back to Notes list The basic loss per share is calculated by dividing the net result attributable to shareholders by the weighted average number of shares outstanding during the year. Years ended December 31 2021 2020 Loss for the year, in thousands of $ (29,002) (28,662) Basic and diluted loss per share, in $ (0.24) (0.34) Weighted average number of shares 2021 2020 Weighted average number of shares for basic and diluted loss per share 121,935,741 83,199,215 At December 31, 2021 and 2020, the Company had potential dilutive shares in the form of warrants. Diluted loss per share considers the impact of potentially dilutive securities except in periods in which there is a loss because the inclusion of the potential common shares would have an anti-dilutive effect. NOTE 19: Financial Risk Management Back to Notes list Capital management Capital is comprised of equity attributable to shareholders, borrowings, and cash and cash equivalents. The Company aims to maintain a strong capital base in order to maintain investor and creditor confidence and to sustain the future development of the business. The Company’s objectives when managing capital are to maintain sufficient liquidity to meet its working capital requirements, fund capital investment and purchases, and safeguard its ability to continue operating as a going concern. The Company monitors capital regularly to ensure that the statutory capital requirements are met and may propose capital increases to the shareholders’ meeting to ensure the necessary capital remains intact. Credit risk Credit risk arises from cash and cash equivalents, short-term bank deposits, as well as credit exposure to collaboration partners. Credit risk refers to the risks that counterparty will default on its contractual obligations resulting in financial loss to the Group. At the end of 2021, the Company operated with more than 1,000 different customers, systematically reducing credit risk compared to prior periods. In the US healthcare system, and particularly within the molecular diagnostic CLIA laboratory industry, where there are rapid technological advances in diagnostic services, companies provide services to healthcare professionals and their patients, while being reimbursed from commercial and governmental insurance systems. Often these services are provided out of network and without supplier contracts. As a result, there is reimbursement risk, separate from credit risk that is characterized by uncertainty in reimbursement value, delays in payment, and ultimately non-payment. This impacts the Company’s revenue recognition and cash collections. Annual Report 2021 145

Part IV: Financial Statements In addition to reimbursement risk associated with commercial third-party payors, credit risk may also arise from amounts due directly from patients. In many cases, payors will cover the entire cost of testing. For example, for tests that fall under the Clinical Laboratory Fee Schedule, there is no co-payment, co-insurance or deductible for patients covered under traditional Medicare. However, patients covered by commercial insurance companies may be responsible for a co-payment, co-insurance, and/or deductible depending on the health insurance plan and individual patient benefit. Credit risk exists for those patients who cannot meet their co-payment or deductible portions. Customer’s compliance with agreed credit terms is regularly and closely monitored. Trade accounts receivable amounted to $4,582,000 at December 31, 2021 and no allowance for expected credit loss was recorded. The Company applies the simplified approach to providing for expected credit losses (ECL) prescribed by IFRS 9, which requires the use of the lifetime expected loss provision for all trade receivables. No ECL has been recorded for other financial assets carried at amortized cost as there is no related credit risk. The credit risk on cash and cash equivalents of $58,498,000 is limited given that the counterparties are banks with high credit scores attributed by international rating agencies. Interest risk In the course on 2019, the Company has entered into a 48-months loan agreement for a total amount of €9 million (approximately $10.2 million), and has been amended in October 2020 and in April 2021 (refer to Note 14 for further details). In application to IFRS 9 given the change in estimated cash-flows following the signed amendment, considering that the modification is non-substantial, the Company recognized in profit and loss the amount of the remeasurement. The amortized cost is calculated using the effective interest method, which allocates interests and expenses at a constant rate over the term of the instrument; the effective interest rate for the loan is 19.56%. In addition, on April 20, 2020, the Company, through its U.S. subsidiary, MDxHealth Inc., has entered into a “Paycheck Protection Program” (PPP) loan with the U.S. Small Business Administration (SBA) in the amount of $2,316,000 as part of the U.S Coronavirus Aid, Relief, and Economic Security (CARES) Act. The amortized cost is calculated using the effective interest method, which allocates interests and expenses at a constant rate over the term of the instrument; the effective interest rate for the loan is 1.00%. Considering the fixed interest rate, the Company is not exposed to interest risk, thus did not perform any sensitivity analysis. Currency risk The functional currency changed from the EURO to the US Dollar as of July 1, 2014. Consequently, the currency risk is concentrated on European operations. As of December 31, 2021, cash on hand held in EURO amounted to €7,195,000. The Company performed a sensitivity analysis of an increase/decrease of exchange rate on operations of 10%. The exposure of operations to the currency risk is limited to the net amount of €7,037,000 (€741,000 revenue and €7,778,000 costs), resulting in a potential gain of €816,000 in case of an increase of the USD/Euro exchange rate by 10%, and a potential loss of €668,000 in case of a decrease of the exchange rate by 10%. Annual Report 2021 146
Part IV: Financial Statements Liquidity risk The Company manages liquidity risk by maintaining adequate reserves and by continuously monitoring forecast and actual cash flows and matching the maturity profiles of financial assets and liabilities. At the date of this document, the Company has two loan agreements with banks and state institutions, and eleven leases (see notes 14 and 16). For the years ended December 31, 2021 Less than 1 year Between 1 and 2 years Between 3 and 5 years Total contractual cash flows Carrying amount Non derivatives Trade payables 7,455 7,455 7,455 Loans 4,780 8,200 1,083 14,063 12,092 Lease liabilities 1,127 915 2,179 4,221 3,464 Total 13,362 9,115 3,262 25,739 23,011 Note: Except for carrying amount, all figures shown in this table are undiscounted and reflect future cash payments The Company is also committed to a potential additional cash out of €945,000 ($1,070,307) if Kreos requests payment of the conversion loan (see note 14). For the years ended December 31, 2020 Less than 1 year Between 1 and 2 years Between 3 and 5 years Total contractual cash flows Carrying amount Non derivatives Trade payables 5,320 5,320 5,320 Loans (incl. convertible loan) 3,644 5,568 6,168 15,380 13,097 Lease liabilities 1,040 670 1,744 3,454 2,774 Total 10,004 6,238 7,912 24,154 21,191 Other risks The Company subscribes to certain insurance policies to cover matters such as (i) fire, theft, and other damage to its assets, (ii) product and liability insurance and clinical trial insurance, and (iii) D&O insurance. To date, no significant claims have been made under these insurance policies and there is no guarantee that the insurances will cover all damages if they should ever occur. To date, the Company has received several government grants for various R&D projects. Some of these grant amounts can be re-claimed if the Company does not fulfill all the conditions of the grant agreements. Annual Report 2021 147
Part IV: Financial Statements NOTE 20: Share capital and reserves Back to Notes list At December 31, 2021, the Company’s share capital was represented by the following number of shares (units). Only one class of shares (common shares) exists and they have no par value. For the Years ended December 31 2021 2020 Common shares 155,969,226 90,691,449 Total outstanding shares 155,969,226 90,691,449 On November 8, 2021, the Company announced that completion of a capital increase by means of an initial public offering in the United States of 3,750,000 American Depositary Shares, or “ADS” (each representing 10 ordinary shares of the Company with no nominal value per share) at an issue price of $12 per ADS, resulting in gross proceeds of $45.0 million. As a result of this capital increase, its share capital has increased from €90,132,067.69 to €118,662,067.69 and the number of issued and outstanding shares has increased from 118,469,226 to 155,969,226 ordinary shares, through the issuance of a total of 37,500,000 new shares. On January 21, 2021, the company announced the successful pricing of its capital increase with the offering of new ordinary shares. The Company raised €25.0 million ($30.4 million) in gross proceeds by means of a private placement of 27,777,777 new shares at an issue price of €0.90 per share through an accelerated bookbuild offering. As a result of the issuance of new shares, the Company’s share capital increased from €68,998,734.95 to €90,132,067.69 and its issued and outstanding shares increased from 90,691,449 to 118,469,226 ordinary shares. On May 15, 2020, the Company announced that MVM V LP and MVM GP (No.5) LP, funds managed by MVM Partners LLP (collectively “MVM“), completed their equity investment in the Company for an aggregate amount of €12.7 million ($13.7 Million). As a result of the investment, the Company’s share capital was increased from €56,260,102.01 to €68,998,734.95, through the issuance of 20,162,924 new ordinary shares of the Company at an issue price of (rounded) €0.632 per share. Thousands of $/ Thousands of €/ For the Years ended December 31 Share Issuance Share Issuance Capital Premium Capital Premium As of January 1, 2020 62,841 136,349 49,754 112,078 May 2020 – Issuance 13,875 0 12,460 0 As of December 31st, 2020 76,716 136,349 62,214 112,078 January 2021 – Issuance 23,632 4,693 19,473 3,867 November 2021 – Issuance 28,106 12,135 24,412 10,536 As of December 31st, 2021 128,454 153,177 106,099 126,481 of 20,162,924 shares (*) of 27,777,777 shares (*) of 37,500,000 shares (*) (*) net of expenses Annual Report 2021 148
Part IV: Financial Statements The capital stock and the issuance premium at December 31 amounted to the following: Thousands of $/ Thousands of €/ For the Years ended December 31 2021 2020 2021 2020 Share capital as per statutory accounts 143,419 84,903 118,662 68,999 Capital increase costs (14,965) (8,187) (12,564) (6,785) Share capital under IFRS 128,454 76,716 106,098 62,214 Issuance premium 153,177 136,349 126,481 112,078 Share capital and issuance premium 281,631 213,065 232,579 174,292 The history of the Share capital can be found in “General Information; Capital and Shares”. NOTE 21: Retirement benefit schemes Back to Notes list The Company operates defined contribution schemes for all its qualifying employees. The assets of these schemes are held separately from those of the Company in designated funds. A total cost of $594,000 in 2021 (2020: $567,000) represents contributions payable to these schemes by the Company at rates specified in the rules of the plans. The employees of the Company in Belgium are members of a state-managed retirement benefit scheme operated by the government (i.e., legal pension) and are members of a bank-operated private pension scheme. The Company is required to contribute a specified percentage of payroll costs to the retirement benefit scheme to fund the benefits. The obligation of the Company with respect to the retirement benefit scheme is to make the specified contributions. Because the Company must guarantee the statutory minimum return on these plans, not all actuarial and investment risks relating to these plans are transferred to the insurance company or pension fund managing the plans. The Company has considered the potential impact of the employer’s obligation to guarantee a minimum return and that this was assessed not to be significant. NOTE 22: Share-based payments Back to Notes list This section provides an overview of the outstanding warrants as of December 31, 2021. The warrants were created within the context of stock-based incentive plans for employees, directors and consultants of the Company. The Company has created several pools of warrants under stock option plans for grant to eligible employees, Directors, and consultants. On March 15, 2012 (195,000), June 15, 2012 (700,000), June 23, 2014 (1,500,000), June 19, 2017 (2,500,000), June 21, 2019 (3,000,000) and May 27, 2021 (3,600,000). In aggregate 12,310,800 warrants were issued, subject to warrants being granted to and accepted by the beneficiaries. 16,150 of these warrants never allocated have become null and void, resulting in a remaining and outstanding 12,294,650 warrants, (i) 2,250,052 warrants were terminated or lapsed, (ii) 577,123 warrants were exercised, (iii) 8,917,625 warrants were granted but not yet exercised, and (iv) 566,000 warrants were not yet granted by the Company. For the year 2021, 304,968 warrants (2020: 357,594) were terminated or lapsed, no warrants were exercised, and 795,250 warrants (2020: 859,999) were vested. As a result, as at December 31, 2021, there are 8,917,625 warrants outstanding, entitling their holders to subscribe to 8,917,625 shares of the Company. Annual Report 2021 149
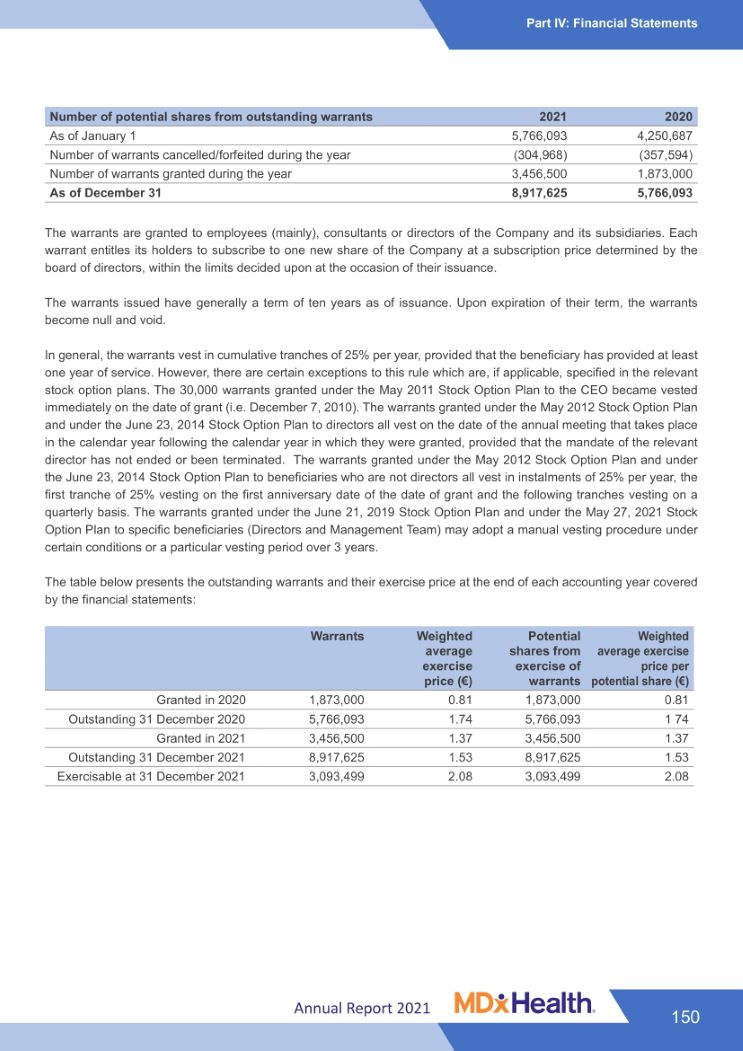
Part IV: Financial Statements Number of potential shares from outstanding warrants 2021 2020 As of January 1 5,766,093 4,250,687 Number of warrants cancelled/forfeited during the year (304,968) (357,594) Number of warrants granted during the year 3,456,500 1,873,000 As of December 31 8,917,625 5,766,093 The warrants are granted to employees (mainly), consultants or directors of the Company and its subsidiaries. Each warrant entitles its holders to subscribe to one new share of the Company at a subscription price determined by the board of directors, within the limits decided upon at the occasion of their issuance. The warrants issued have generally a term of ten years as of issuance. Upon expiration of their term, the warrants become null and void. In general, the warrants vest in cumulative tranches of 25% per year, provided that the beneficiary has provided at least one year of service. However, there are certain exceptions to this rule which are, if applicable, specified in the relevant stock option plans. The 30,000 warrants granted under the May 2011 Stock Option Plan to the CEO became vested immediately on the date of grant (i.e. December 7, 2010). The warrants granted under the May 2012 Stock Option Plan and under the June 23, 2014 Stock Option Plan to directors all vest on the date of the annual meeting that takes place in the calendar year following the calendar year in which they were granted, provided that the mandate of the relevant director has not ended or been terminated. The warrants granted under the May 2012 Stock Option Plan and under the June 23, 2014 Stock Option Plan to beneficiaries who are not directors all vest in instalments of 25% per year, the first tranche of 25% vesting on the first anniversary date of the date of grant and the following tranches vesting on a quarterly basis. The warrants granted under the June 21, 2019 Stock Option Plan and under the May 27, 2021 Stock Option Plan to specific beneficiaries (Directors and Management Team) may adopt a manual vesting procedure under certain conditions or a particular vesting period over 3 years. The table below presents the outstanding warrants and their exercise price at the end of each accounting year covered by the financial statements: Warrants Weighted average exercise price (€) Potential shares from exercise of warrants Weighted average exercise price per potential share (€) Granted in 2020 1,873,000 0.81 1,873,000 0.81 Outstanding 31 December 2020 5,766,093 1.74 5,766,093 1 74 Granted in 2021 3,456,500 1.37 3,456,500 1.37 Outstanding 31 December 2021 8,917,625 1.53 8,917,625 1.53 Exercisable at 31 December 2021 3,093,499 2.08 3,093,499 2.08 Annual Report 2021 150
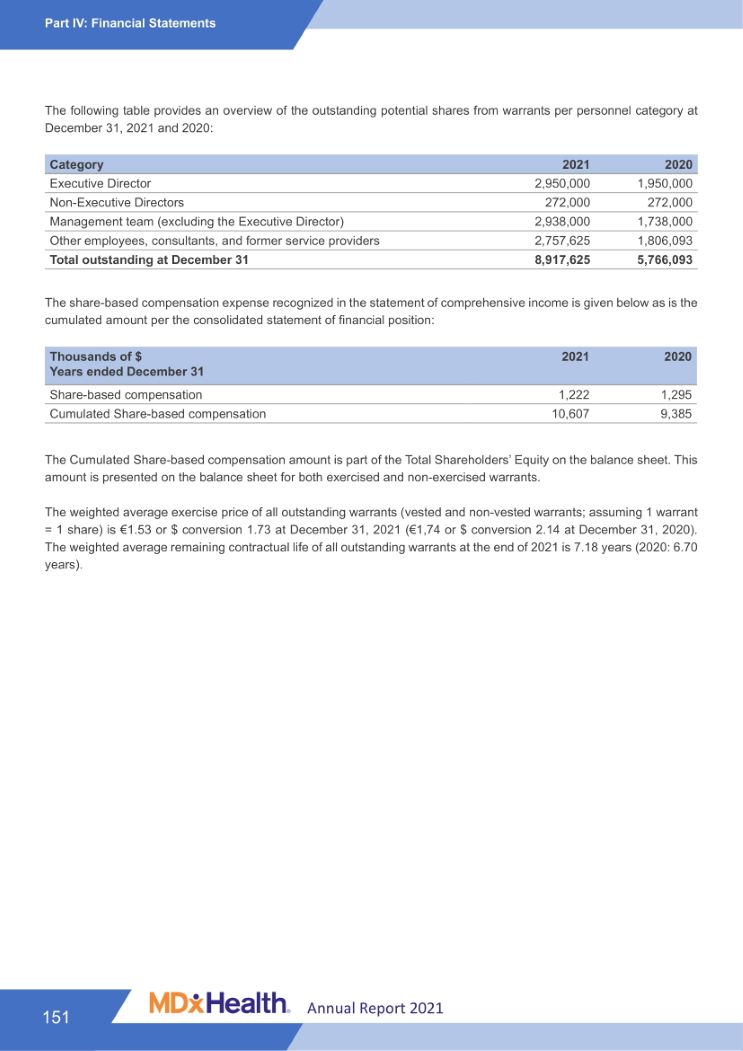
Part IV: Financial Statements The following table provides an overview of the outstanding potential shares from warrants per personnel category at December 31, 2021 and 2020: Category 2021 2020 Executive Director 2,950,000 1,950,000 Non-Executive Directors 272,000 272,000 Management team (excluding the Executive Director) 2,938,000 1,738,000 Other employees, consultants, and former service providers 2,757,625 1,806,093 Total outstanding at December 31 8,917,625 5,766,093 The share-based compensation expense recognized in the statement of comprehensive income is given below as is the cumulated amount per the consolidated statement of financial position: Thousands of $ Years ended December 31 2021 2020 Share-based compensation 1,222 1,295 Cumulated Share-based compensation 10,607 9,385 The Cumulated Share-based compensation amount is part of the Total Shareholders’ Equity on the balance sheet. This amount is presented on the balance sheet for both exercised and non-exercised warrants. The weighted average exercise price of all outstanding warrants (vested and non-vested warrants; assuming 1 warrant = 1 share) is €1.53 or $ conversion 1.73 at December 31, 2021 (€1,74 or $ conversion 2.14 at December 31, 2020). The weighted average remaining contractual life of all outstanding warrants at the end of 2021 is 7.18 years (2020: 6.70 years). Annual Report 2021 151
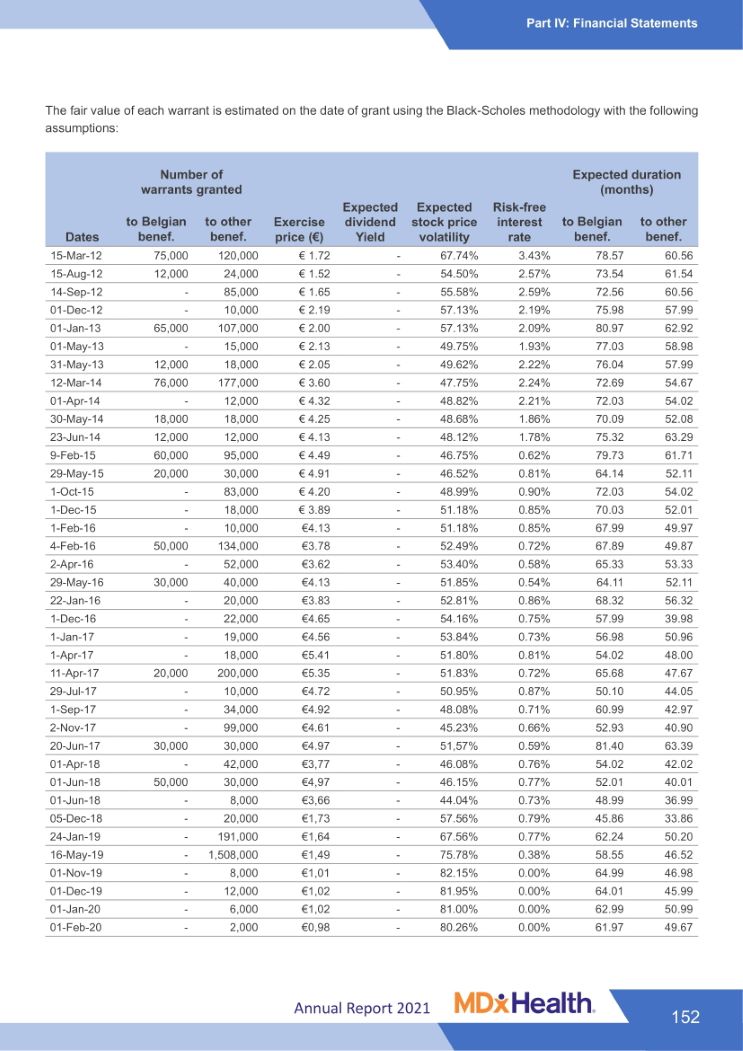
Part IV: Financial Statements The fair value of each warrant is estimated on the date of grant using the Black-Scholes methodology with the following assumptions: Number of Expected duration warrants granted (months) to Belgian to other Exercise Expected dividend Expected stock price Risk-free interest to Belgian to other Dates benef. benef. price (€) Yield volatility rate benef. benef. 15-Mar-12 75,000 120,000 € 1.72 - 67.74% 3.43% 78.57 60.56 15-Aug-12 12,000 24,000 € 1.52 - 54.50% 2.57% 73.54 61.54 14-Sep-12 - 85,000 € 1.65 - 55.58% 2.59% 72.56 60.56 01-Dec-12 - 10,000 € 2.19 - 57.13% 2.19% 75.98 57.99 01-Jan-13 65,000 107,000 € 2.00 - 57.13% 2.09% 80.97 62.92 01-May-13 - 15,000 € 2.13 - 49.75% 1.93% 77.03 58.98 31-May-13 12,000 18,000 € 2.05 - 49.62% 2.22% 76.04 57.99 12-Mar-14 76,000 177,000 € 3.60 - 47.75% 2.24% 72.69 54.67 01-Apr-14 - 12,000 € 4.32 - 48.82% 2.21% 72.03 54.02 30-May-14 18,000 18,000 € 4.25 - 48.68% 1.86% 70.09 52.08 23-Jun-14 12,000 12,000 € 4.13 - 48.12% 1.78% 75.32 63.29 9-Feb-15 60,000 95,000 € 4.49 - 46.75% 0.62% 79.73 61.71 29-May-15 20,000 30,000 € 4.91 - 46.52% 0.81% 64.14 52.11 1-Oct-15 - 83,000 € 4.20 - 48.99% 0.90% 72.03 54.02 1-Dec-15 - 18,000 € 3.89 - 51.18% 0.85% 70.03 52.01 1-Feb-16 - 10,000 €4.13 - 51.18% 0.85% 67.99 49.97 4-Feb-16 50,000 134,000 €3.78 - 52.49% 0.72% 67.89 49.87 2-Apr-16 - 52,000 €3.62 - 53.40% 0.58% 65.33 53.33 29-May-16 30,000 40,000 €4.13 - 51.85% 0.54% 64.11 52.11 22-Jan-16 - 20,000 €3.83 - 52.81% 0.86% 68.32 56.32 1-Dec-16 - 22,000 €4.65 - 54.16% 0.75% 57.99 39.98 1-Jan-17 - 19,000 €4.56 - 53.84% 0.73% 56.98 50.96 1-Apr-17 - 18,000 €5.41 - 51.80% 0.81% 54.02 48.00 11-Apr-17 20,000 200,000 €5.35 - 51.83% 0.72% 65.68 47.67 29-Jul-17 - 10,000 €4.72 - 50.95% 0.87% 50.10 44.05 1-Sep-17 - 34,000 €4.92 - 48.08% 0.71% 60.99 42.97 2-Nov-17 - 99,000 €4.61 - 45.23% 0.66% 52.93 40.90 20-Jun-17 30,000 30,000 €4.97 - 51,57% 0.59% 81.40 63.39 01-Apr-18 - 42,000 €3,77 - 46.08% 0.76% 54.02 42.02 01-Jun-18 50,000 30,000 €4,97 - 46.15% 0.77% 52.01 40.01 01-Jun-18 - 8,000 €3,66 - 44.04% 0.73% 48.99 36.99 05-Dec-18 - 20,000 €1,73 - 57.56% 0.79% 45.86 33.86 24-Jan-19 - 191,000 €1,64 - 67.56% 0.77% 62.24 50.20 16-May-19 - 1,508,000 €1,49 - 75.78% 0.38% 58.55 46.52 01-Nov-19 - 8,000 €1,01 - 82.15% 0.00% 64.99 46.98 01-Dec-19 - 12,000 €1,02 - 81.95% 0.00% 64.01 45.99 01-Jan-20 - 6,000 €1,02 - 81.00% 0.00% 62.99 50.99 01-Feb-20 - 2,000 €0,98 - 80.26% 0.00% 61.97 49.67 Annual Report 2021 152
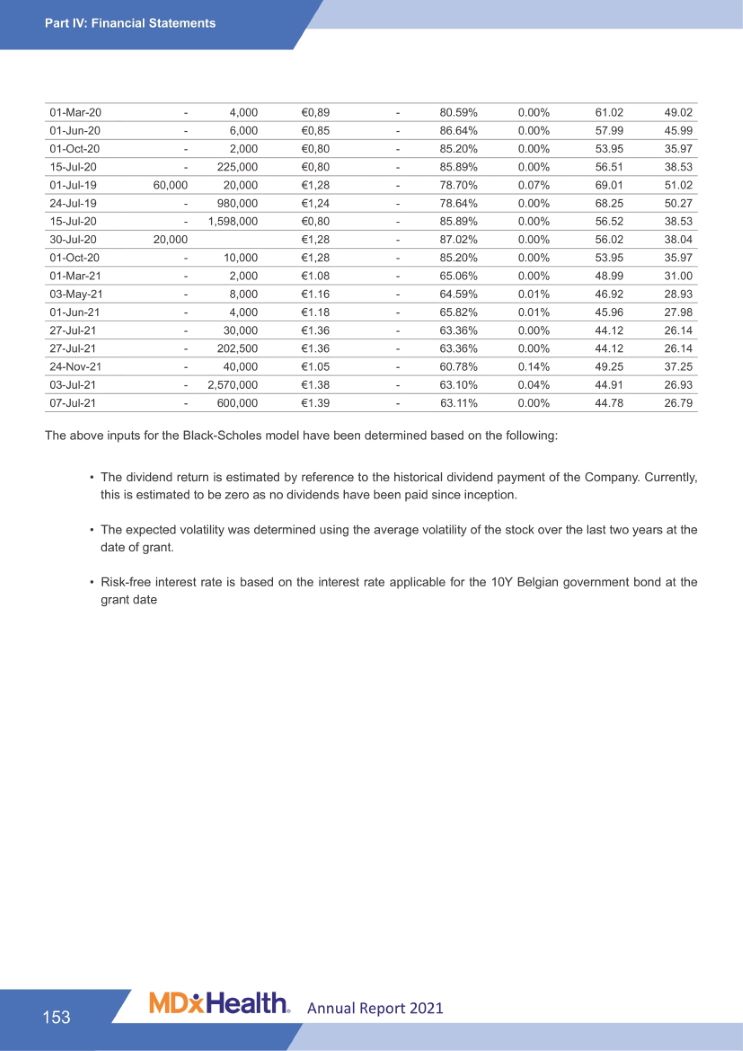
Part IV: Financial Statements 01-Mar-20 - 4,000 €0,89 - 80.59% 0.00% 61.02 49.02 01-Jun-20 - 6,000 €0,85 - 86.64% 0.00% 57.99 45.99 01-Oct-20 - 2,000 €0,80 - 85.20% 0.00% 53.95 35.97 15-Jul-20 - 225,000 €0,80 - 85.89% 0.00% 56.51 38.53 01-Jul-19 60,000 20,000 €1,28 - 78.70% 0.07% 69.01 51.02 24-Jul-19 - 980,000 €1,24 - 78.64% 0.00% 68.25 50.27 15-Jul-20 - 1,598,000 €0,80 - 85.89% 0.00% 56.52 38.53 30-Jul-20 20,000 €1,28 - 87.02% 0.00% 56.02 38.04 01-Oct-20 - 10,000 €1,28 - 85.20% 0.00% 53.95 35.97 01-Mar-21 - 2,000 €1.08 - 65.06% 0.00% 48.99 31.00 03-May-21 - 8,000 €1.16 - 64.59% 0.01% 46.92 28.93 01-Jun-21 - 4,000 €1.18 - 65.82% 0.01% 45.96 27.98 27-Jul-21 - 30,000 €1.36 - 63.36% 0.00% 44.12 26.14 27-Jul-21 - 202,500 €1.36 - 63.36% 0.00% 44.12 26.14 24-Nov-21 - 40,000 €1.05 - 60.78% 0.14% 49.25 37.25 03-Jul-21 - 2,570,000 €1.38 - 63.10% 0.04% 44.91 26.93 07-Jul-21 - 600,000 €1.39 - 63.11% 0.00% 44.78 26.79 The above inputs for the Black-Scholes model have been determined based on the following: • The dividend return is estimated by reference to the historical dividend payment of the Company. Currently, this is estimated to be zero as no dividends have been paid since inception. • The expected volatility was determined using the average volatility of the stock over the last two years at the date of grant. • Risk-free interest rate is based on the interest rate applicable for the 10Y Belgian government bond at the grant date Annual Report 2021 153
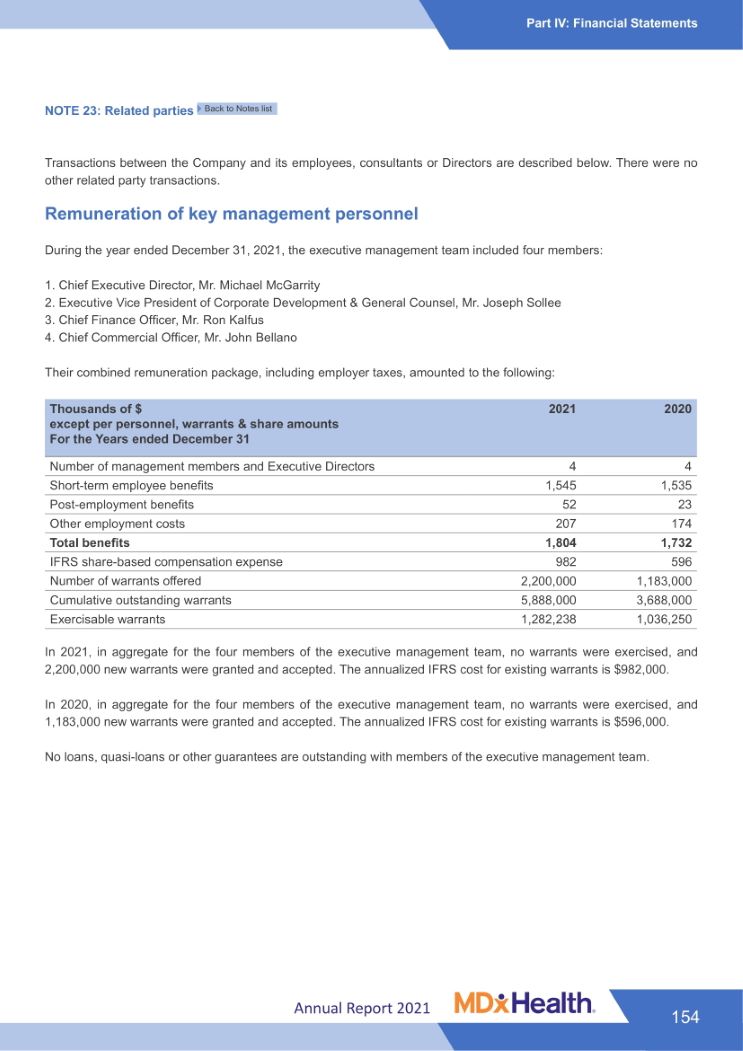
Part IV: Financial Statements NOTE 23: Related parties Back to Notes list Transactions between the Company and its employees, consultants or Directors are described below. There were no other related party transactions. Remuneration of key management personnel During the year ended December 31, 2021, the executive management team included four members: 1. Chief Executive Director, Mr. Michael McGarrity 2. Executive Vice President of Corporate Development & General Counsel, Mr. Joseph Sollee 3. Chief Finance Officer, Mr. Ron Kalfus 4. Chief Commercial Officer, Mr. John Bellano Their combined remuneration package, including employer taxes, amounted to the following: Thousands of $ except per personnel, warrants & share amounts For the Years ended December 31 2021 2020 Number of management members and Executive Directors 4 4 Short-term employee benefits 1,545 1,535 Post-employment benefits 52 23 Other employment costs 207 174 Total benefits 1,804 1,732 IFRS share-based compensation expense 982 596 Number of warrants offered 2,200,000 1,183,000 Cumulative outstanding warrants 5,888,000 3,688,000 Exercisable warrants 1,282,238 1,036,250 In 2021, in aggregate for the four members of the executive management team, no warrants were exercised, and 2,200,000 new warrants were granted and accepted. The annualized IFRS cost for existing warrants is $982,000. In 2020, in aggregate for the four members of the executive management team, no warrants were exercised, and 1,183,000 new warrants were granted and accepted. The annualized IFRS cost for existing warrants is $596,000. No loans, quasi-loans or other guarantees are outstanding with members of the executive management team. Annual Report 2021 154

Part IV: Financial Statements Remuneration of the Board The total remuneration of the Board of Directors (including the Executive Director) in 2021 and 2020 was $863,000, and $775,000 respectively (excluding VAT, stock-based compensation and reimbursement of expenses). No advances or credits have been granted to any member of the Board of Directors. None of the members of the Board of Directors have received any non-monetary remuneration other than warrants as disclosed above. Transactions with Non-Executive Directors Since 2012, the Non-Independent Directors do not receive a fee payment for attending and preparing for Board meetings or for assisting the Company with Board matters. They receive reimbursement for expenses directly related to the Board meetings, totaling less than $12,000 in 2021. The Independent Directors receive a fee for attending and preparing meetings of the Board of Directors and for assisting the Company with Board matters, and they receive reimbursement for expenses directly related to the Board meetings. In 2021 and 2020, respectively $302,000 and $231,000 were paid as fees and expense reimbursement to independent members of the Board of Directors. A total of 20,000 warrants were granted to Non-Executive Directors in 2021 and no warrants were exercised in 2021. NOTE 24: Significant agreements, commitments and contingencies Back to Notes list Fair value of Other financial liabilities On September 18, 2015, MDxHealth acquired MDxHealth BV (former NovioGendix), a Dutch molecular diagnostic research and service company with expertise in the urological oncology. The terms of the acquisition consisted of initial consideration paid in 1,086,956 shares of MDxHealth common stock, issued at €4.14 representing the average closing price of the Company’s shares on Euronext Brussels during a period of 30 days ending on September 17, 2015. In addition to this equity, additional cash consideration of €250,000 was paid. On top of the acquisition price, MDxHealth is committed to pay future milestone fees. The Company paid €1,000,000, being $1,105,000 regarding these milestone fees in 2017. The fair value of this contingent consideration as of December 31, 2021 is estimated at $1,617,000 over the period 2021-2022 (2020: $1,599,000). The Company is contractually required to pay at maturity to the holder of the obligation the amount of maximum $2,200,000. Collaborative research agreements and clinical research agreements The Company has entered into numerous agreements with universities, medical centers and external researchers for research and development work and for the validation of the Company’s technology and products. These agreements typically have durations of one to three years. The Company must pay fixed fees to the collaborators and in exchange typically receives access and rights to the results of the work. MDxHealth collaborates on research and clinical development with many of the world’s leading academic and government cancer research institutes. These important relationships provide the Company with additional resources and expertise for clinical marker validation as well as access to patient samples for testing. MDxHealth’s collaborators include such prestigious institutions as Johns Hopkins University Medical Institutions (US), Duke University Medical Center (US), Harvard Medical School (US), Cleveland Clinic (US), University of Colorado (US), University of California at Los Angeles (US), Radboud University (The Netherlands) and University of Gent (Belgium) among others. Annual Report 2021 155

Part IV: Financial Statements Intellectual property in-licensing agreements The Company has entered into numerous agreements with universities and companies for in-licensing intellectual property. These agreements typically require the Company to pay an up-front fee, annual maintenance fees and/or minimum annual royalty fees, legal fees related to the patents, and certain milestone and royalty fees if the patents are eventually used in a commercialized product. In addition, the Company must provide the licensor with periodic reports. Commercial and intellectual property sub-licensing agreements The Company has entered into numerous partnering and sub-licensing agreements. In regard to the Company’s developed tests, the Company has entered into a range of marketing and sales arrangements with commercial entities. These important relationships provide the Company with additional resources and infrastructure to expand the geographic reach and awareness of the Company’s solutions, primarily in relation to the ConfirmMDx and SelectMDx tests. MDxHealth’s marketing partners include Cerba Healthcare (Belgium), Ferrer Internacional (Spain), Teva Pharmaceuticals (Israel), and SouthGenetics (South and Central America), LifeLabs (Canada) and, in the US, LabCorp, Miraca Life Sciences, Bostwick Laboratories. In regard to intellectual property that MDxHealth has developed or improved, MDxHealth has sublicensed certain of its non-core epigenetic technologies to commercial partners, several of whom have launched products that generate royalties and other fees. These sublicenses include: • an exclusive sublicense to Laboratory Corporation of America (LabCorp) for the MGMT test (for the North American market only, of indefinite duration, and limited to service testing only). MDxHealth retained certain rights to develop and commercialize the MGMT test as a companion diagnostic on a worldwide basis. LabCorp began to commercialize the MGMT test in North America in 2008. • non-exclusive sublicense agreements for the Company’s patented methylation specific PCR (MSP) technology for diagnostic applications, in exchange for certain license fees and running royalties, to several partners including oncgnostics GmbH, Qiagen GmbH and Takara Bio. Litigation As of the date of this document and as far as MDxHealth is aware, the Company is not involved in any material legal proceedings. NOTE 25: Subsequent events Back to Notes list There is no subsequent event at the date of this report. Annual Report 2021 156
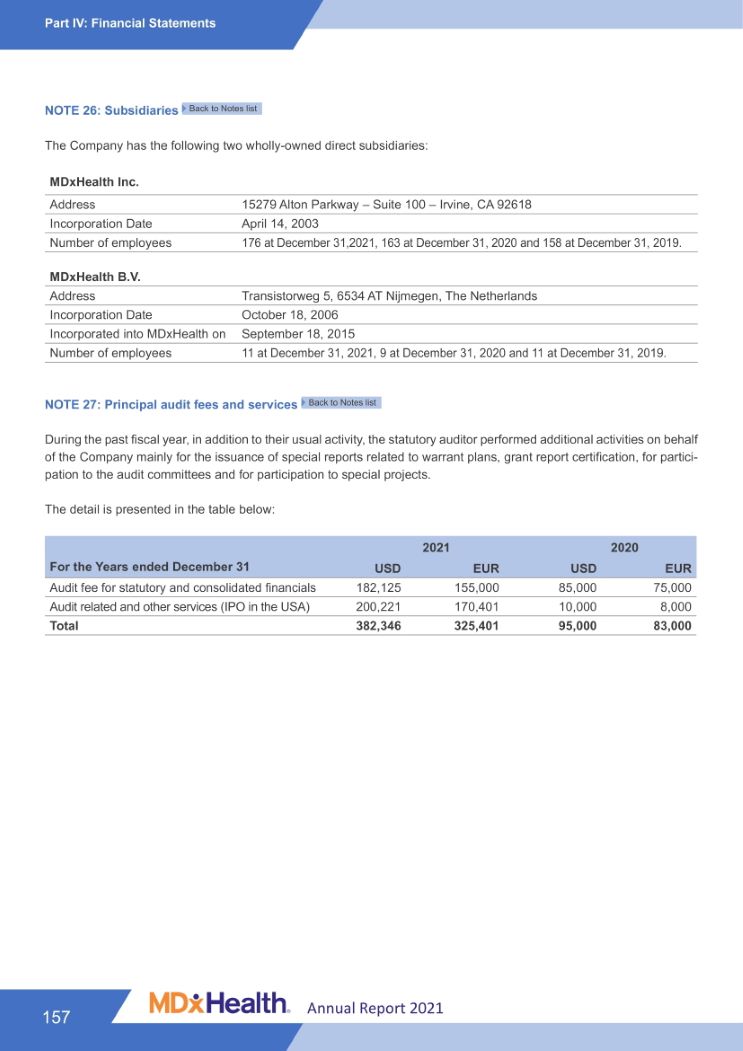
Part IV: Financial Statements NOTE 26: Subsidiaries Back to Notes list The Company has the following two wholly-owned direct subsidiaries: MDxHealth Inc. Address 15279 Alton Parkway – Suite 100 – Irvine, CA 92618 Incorporation Date April 14, 2003 Number of employees 176 at December 31,2021, 163 at December 31, 2020 and 158 at December 31, 2019. MDxHealth B.V. Address Transistorweg 5, 6534 AT Nijmegen, The Netherlands Incorporation Date October 18, 2006 Incorporated into MDxHealth on September 18, 2015 Number of employees 11 at December 31, 2021, 9 at December 31, 2020 and 11 at December 31, 2019. NOTE 27: Principal audit fees and services Back to Notes list During the past fiscal year, in addition to their usual activity, the statutory auditor performed additional activities on behalf of the Company mainly for the issuance of special reports related to warrant plans, grant report certification, for partici- pation to the audit committees and for participation to special projects. The detail is presented in the table below: For the Years ended December 31 USD 2021 EUR USD 2020 EUR Audit fee for statutory and consolidated financials 182,125 155,000 85,000 75,000 Audit related and other services (IPO in the USA) 200,221 170,401 10,000 8,000 Total 382,346 325,401 95,000 83,000 Annual Report 2021 157

Part IV: Financial Statements . NOTE 28: Alternative performance measures (APMs) Back to Notes list In its decision making, the Company uses some alternative performance measures (APMs) that are not defined in IFRS. They are used because they provide information useful to assess the Company’s development and performance. These measures should not be viewed in isolation or as an alternative to the measures presented in accordance with IFRS. These APMs may not be comparable to similar measures presented by other companies. The main alternative performance measures used by the Company are explained and reconciled as follows: Thousands of $ For the Years ended December 31 2021 2020 Operating loss (EBIT) (26,841) (27,123) Depreciation and amortization 3,036 3,332 Impairment 0 273 EBITDA (23,805) (23,518) APM Definition Reason for use EBITDA Earnings before interest, other financial income/(expense), tax, amortization, depreciation and impairment. This measure is used to show profit generation in the operating activities excluding non-cash-based depreciation, amortization and impairment. This measure gives an approximation of the cash generation potential before reinvestment in the business. Annual Report 2021 158

Part IV: Financial Statements . Annual Report 2021 159

Part IV: Financial Statements . AUDITOR’S OPINION In the context of the statutory audit of the consolidated financial statements of MDxHealth SA (‘the Company’) and its subsidiaries (together referred to as 'the Group'), we hereby present our statutory auditor’s report. It includes our report of the consolidated financial statements and the other legal and regulatory requirements. This report is an integrated whole and is indivisible. We have been appointed as statutory auditor by the general meeting of May 28, 2020, following the proposal formulated by the board of directors issued upon recommendation of the Audit Committee. Our statutory auditor’s mandate expires on the date of the General Meeting deliberating on the financial statements closed on December 31, 2022. We have performed the statutory audit of the consolidated financial statements of MDxHealth SA for sixteen consecutive years. REPORT ON THE CONSOLIDATED FINANCIAL STATEMENTS Unqualified opinion We have performed the statutory audit of the Group’s consolidated financial statements, which comprise the consolidated statement of financial position as at December 31, 2021, and the consolidated statement of profit or loss and other comprehensive income, the consolidated statement of changes in equity and the consolidated statement of cash flows for the year then ended, and notes to the consolidated financial statements, including a summary of significant accounting policies and other explanatory information, and which is characterized by a consolidated statement of financial position total of $ 75,072 (000) and for which the consolidated income statement and other comprehensive income shows a loss for the year of $ 29,002 (000). In our opinion, the consolidated financial statements give a true and fair view of the Group’s net equity and financial position as at December 31, 2021, as well as of its consolidated financial performance and its consolidated cash flows for the year then ended, in accordance with International Financial Reporting Standards (IFRS) as adopted by the European Union and with the legal and regulatory requirements applicable in Belgium. Basis for unqualified opinion We conducted our audit in accordance with International Standards on Auditing (ISA) as applicable in Belgium. Our responsibilities under those standards are further described in the 'Statutory auditor's responsibilities for the audit of the consolidated financial statements' section in this report. We have complied with all the ethical requirements that are relevant to the audit of consolidated financial statements in Belgium, including those concerning independence. We have obtained from the administrative body and company officials the explanations and information necessary for performing our audit. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion. Annual Report 2021 160

Part IV: Financial Statements . Key audit matters Key audit matters are those matters that, in our professional judgment, were of most significance in our audit of the consolidated financial statements of the current year. These matters were addressed in the context of our audit of the consolidated financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters. Revenue recognition Discussion of the matter As described in notes 2.7 and 3 of the financial statements, the majority of the Group’s revenue is derived from laboratory services with revenue recognized at a point in time when control of the services has transferred to the customer. This is generally when the test results are delivered to the customer. Other Group’s revenue is derived from license fees, royalties and other revenues. The group’s revenue recognition model includes critical accounting estimates based on management judgment. These estimates and underlying judgments are continuously revisited based on updated historical experience and the expected evolution of collections from third party payers. Revenue recognition was significant to our audit procedures, because of its financial impact on the consolidated annual accounts, and the significant level of management judgment required in making the accounting estimates. Procedures performed Our audit procedures included, amongst others: · We tested the Group’s internal control procedures on revenues and evaluated the Group’s assumptions and estimates used in assessing revenue recognition, in particular with respect to completeness, existence and accuracy. · We tested the existence of persuasive evidence of underlying agreements and contracts and we substantively tested and challenged the underlying calculations, key assumptions and estimates used in the revenue model. · We evaluated the reasonableness of the calculations of the ratio of claims collected in relation to claims billed, and of the trend of such ratio. · We considered the historical accuracy of accrued amounts of revenue and used the information obtained as evidence for evaluating the appropriateness of the assumptions made in the current year including how these compare to the experience in previous years. · We reviewed the adequacy of the Group’s disclosures in notes 2.7 and 3 in respect of the use of estimates and judgments in the revenue recognition model. Annual Report 2021 161

Part IV: Financial Statements . Responsibilities of the administrative body for the drafting of the consolidated financial statements The administrative body is responsible for the preparation of consolidated financial statements that give a true and fair view in accordance with the International Financial Reporting Standards (IFRS) as adopted by the European Union and with the legal and regulatory provisions applicable in Belgium, and for such internal control as the administrative body determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatements, whether due to fraud or error. In preparing the consolidated financial statements, the administrative body is responsible for assessing the Group’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the administrative body either intends to liquidate the Group or to cease operations, or has no realistic alternative but to do so. Statutory auditor’s responsibilities for the audit of the consolidated financial statements Our objectives are to obtain reasonable assurance about whether the consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue a statutory auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but it is not a guarantee that an audit conducted in accordance with ISAs will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consolidated financial statements. When executing our audit, we respect the legal, regulatory and normative framework applicable for the audit of the consolidated financial statements in Belgium. However, a statutory audit does not guarantee the future viability of the Group, neither the efficiency and effectiveness of the management of the Group by the administrative body. Our responsibilities regarding the continuity assumption applied by the administrative body are described below. As part of an audit in accordance with ISAs, we exercise professional judgment and maintain professional skepticism throughout the audit. We also: • Identify and assess the risks of material misstatement of the consolidated financial statements, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control; • Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control; • Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the administrative body; Annual Report 2021 162

Part IV: Financial Statements . • Conclude on the appropriateness of the administrative body’s use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Group’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our statutory auditor’s report to the related disclosures in the consolidated financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our statutory auditor’s report. However, future events or conditions may cause the Group to cease to continue as a going concern;• Evaluate the overall presentation, structure and content of the consolidated financial statements and whether the consolidated financial statements represent the underlying transactions and events in a manner that achieves fair presentation; • Obtain sufficient appropriate audit evidence regarding the financial information of the entities or business activities within the Group to express an opinion on the consolidated financial statements. We are responsible for the management, the supervision and the performance of the Group audit. We assume full responsibility for the auditor’s opinion. We communicate with the Audit Committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control identified during the audit. We also provide the Audit Committee with a statement that we respected the relevant ethical requirements relating to independence, and we communicate with them about all relationships and other issues which may influence our independence, and, if applicable, about the related measures to guarantee our independence. From the matters communicated with the Audit Committee, we determine those matters that were of most significance in the audit of the consolidated financial statements of the current year, and are therefore the key audit matters. We describe these matters in our statutory auditor’s report, unless law or regulation precludes public disclosure about the matter. OTHER LEGAL AND REGULATORY REQUIREMENTS Responsibilities of the administrative body The administrative body is responsible for the preparation and the contents of the director’s report on the consolidated financial statements and for the other information included in the annual report on the consolidated financial statements. Responsibilities of the statutory auditor In the context of our mission and in accordance with the Belgian standard (version revised 2020) which is complementary to the International Standards on Auditing (ISA) as applicable in Belgium, it is our responsibility to verify, in all material aspects, the director’s report on the consolidated financial statements and the other information included in the director’s report on the consolidated financial statements, as well as to report on these elements. Annual Report 2021 163

Part IV: Financial Statements Aspects relating to the management report on the consolidated financial statements and to the other information included in the annual report on the consolidated financial statements In our opinion, after having performed specific procedures in relation to the director’s report, this report is consistent with the consolidated financial statements for the same financial year, and it is prepared in accordance with article 3:32 of the Code of companies and associations. In the context of our audit of the consolidated financial statements, we are also responsible for considering, in particular based on the knowledge we have obtained during the audit, whether the director’s report on the consolidated financial statements and the other information included in the annual report on the consolidated financial statements, namely: Part I: Strategy & Business Review; Part II: Corporate Governance; Part III: Principle Risks & Uncertainties contain a material misstatement, i.e. information which is inadequately disclosed or otherwise misleading. Based on the procedures we have performed, there are no material misstatements we have to report to you. Statement concerning independence Our audit firm and our network did not provide services which are incompatible with the statutory audit of the consolidated financial statements and our audit firm remained independent of the Group during the terms of our mandate. The fees related to additional services which are compatible with the statutory audit as referred to in article 3:65 of the Code of companies and associations were duly itemised and valued in the notes to the consolidated financial statements.Annual Report 2021 164

Part IV: Financial Statements European Single Electronic Format (ESEF) In accordance with the standard on auditing the conformity of financial statements with the European Single Electronic Format (hereinafter “ESEF”), we also audited the conformity of the ESEF format with the regulatory technical standards established by Commission Delegated Regulation (EU) 2019/815 of 17 December 2018 (hereinafter: “Delegated Regulation”).The managing body is responsible for preparing, in accordance with ESEF requirements, the consolidated financial statements in the form of an electronic file in ESEF format (hereinafter “digital consolidated financial statements”) included in the annual financial report.It is our responsibility to obtain sufficient and appropriate supporting information to conclude that the format and mark- up language of the digital consolidated financial statements comply in all material aspects with the ESEF requirements under the Delegated Regulation.Based on our work, we believe that the format and the marking of information in the official French version of the digital consolidated financial statements included in the annual financial report of MDxHealth SA as at December 31, 2021 comply in all material aspects with the ESEF requirements under the Delegated Regulation.Other statements This report is in compliance with the contents of our additional report to the Audit Committee as referred to in article 11 of regulation (EU) No 537/2014. Zaventem, April 25, 2022 BDO Réviseurs d’Entreprises SRL Statutory auditor Represented by Bert Kegels Auditor Annual Report 2021 165

Part IV: Financial Statements Annual Report 2021 166

Part IV: Financial Statements Condensed non-consolidated financial statements The statutory financial statements to be filed with the Belgian National Bank are prepared in accordance with Belgian GAAP. An unqualified audit opinion will be issued by the statutory auditor.The information included in this section is an extract from the statutory accounts and does not include all information as required by articles 3:10 and 3:12 of the Belgian Companies and Associations Code. The full statutory accounts have not yet been filed with the Belgian National Bank as of the date of this document. Once filed with the Belgian National Bank, the full statutory accounts will also be made available in the investor section of MDxHealth’s website (www.mdxhealth.com) Annual Report 2021 167
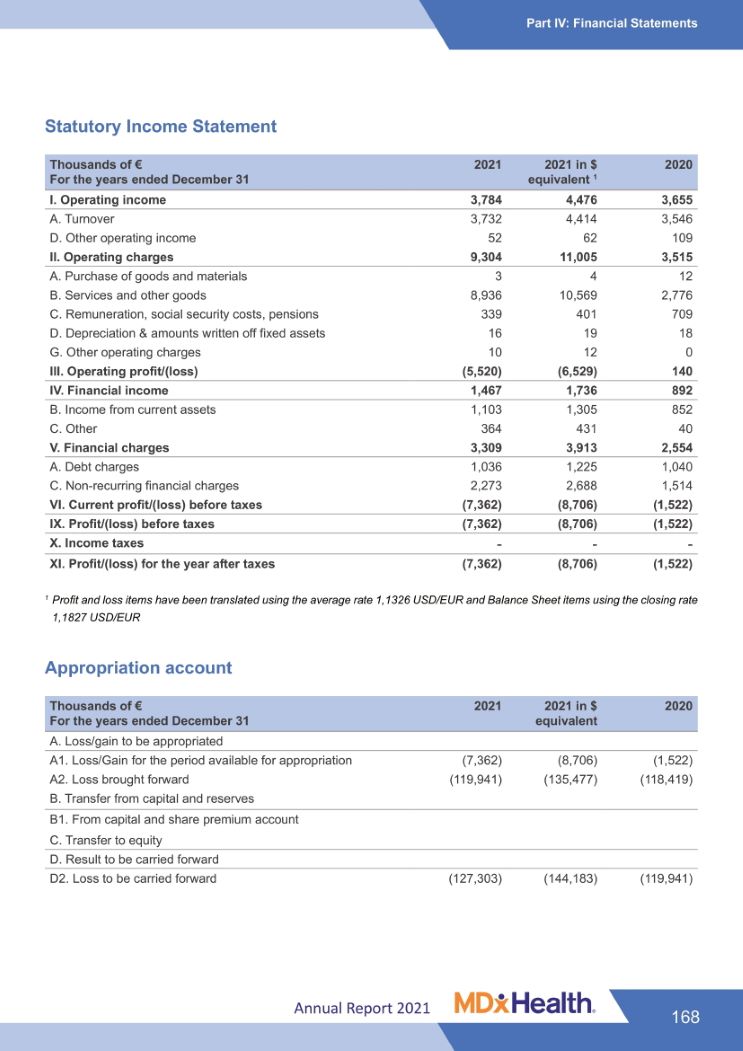
Part IV: Financial Statements Thousands of € For the years ended December 31 2021 2021 in $ equivalent 1 2020 I. Operating income 3,784 4,476 3,655 A. Turnover 3,732 4,414 3,546 D. Other operating income 52 62 109 II. Operating charges 9,304 11,005 3,515 A. Purchase of goods and materials 3 4 12 B. Services and other goods 8,936 10,569 2,776 C. Remuneration, social security costs, pensions 339 401 709 D. Depreciation & amounts written off fixed assets 16 19 18 G. Other operating charges 10 12 0 III. Operating profit/(loss) (5,520) (6,529) 140 IV. Financial income 1,467 1,736 892 B. Income from current assets 1,103 1,305 852 C. Other 364 431 40 V. Financial charges 3,309 3,913 2,554 A. Debt charges 1,036 1,225 1,040 C. Non-recurring financial charges 2,273 2,688 1,514 VI. Current profit/(loss) before taxes (7,362) (8,706) (1,522) IX. Profit/(loss) before taxes (7,362) (8,706) (1,522) X. Income taxes - - - XI. Profit/(loss) for the year after taxes (7,362) (8,706) (1,522) 1 Profit and loss items have been translated using the average rate 1,1326 USD/EUR and Balance Sheet items using the closing rate 1,1827 USD/EUR Appropriation account Thousands of € For the years ended December 31 2021 2021 in $ equivalent 2020 A. Loss/gain to be appropriated A1. Loss/Gain for the period available for appropriation (7,362) (8,706) (1,522) A2. Loss brought forward B. Transfer from capital and reserves (119,941) (135,477) (118,419) B1. From capital and share premium account C. Transfer to equity D. Result to be carried forward D2. Loss to be carried forward (127,303) (144,183) (119,941) Annual Report 2021 168
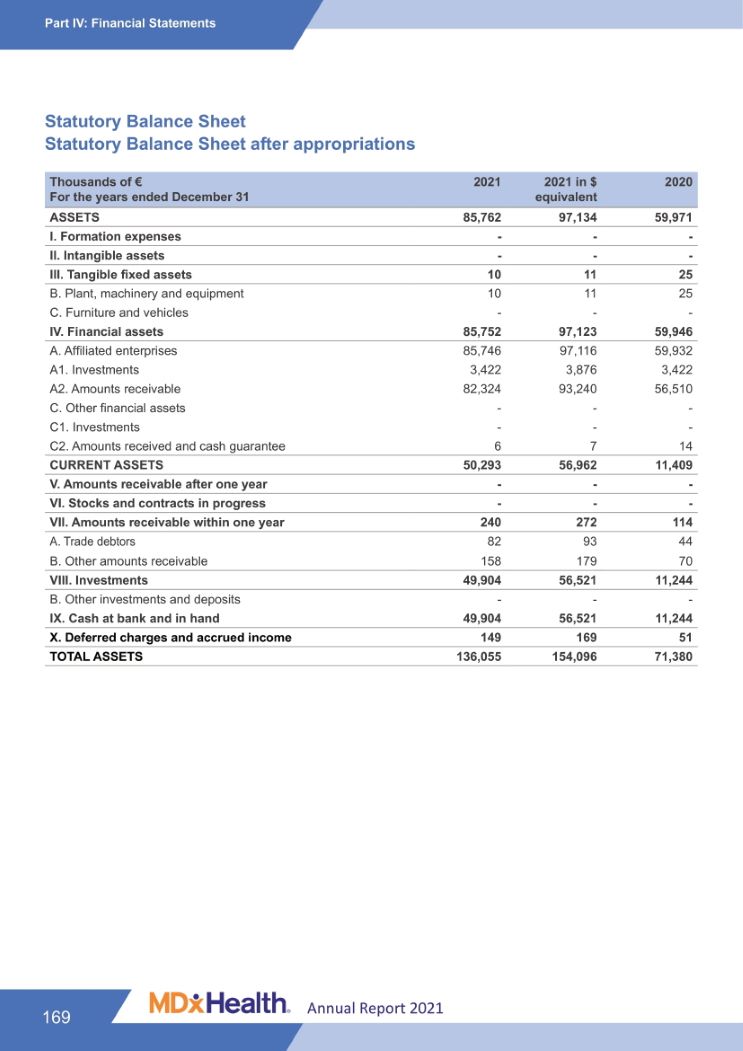
Part IV: Financial Statements Statutory Balance Sheet Statutory Balance Sheet after appropriations Thousands of € For the years ended December 31 2021 2021 in $ equivalent 2020 ASSETS 85,762 97,134 59,971 I. Formation expenses - - - II. Intangible assets - - - III. Tangible fixed assets 10 11 25 B. Plant, machinery and equipment 10 11 25 C. Furniture and vehicles - - - IV. Financial assets 85,752 97,123 59,946 A. Affiliated enterprises 85,746 97,116 59,932 A1. Investments 3,422 3,876 3,422 A2. Amounts receivable 82,324 93,240 56,510 C. Other financial assets - - - C1. Investments - - - C2. Amounts received and cash guarantee 6 7 14 CURRENT ASSETS 50,293 56,962 11,409 V. Amounts receivable after one year - - - VI. Stocks and contracts in progress - - - VII. Amounts receivable within one year 240 272 114 A. Trade debtors 82 93 44 B. Other amounts receivable 158 179 70 VIII. Investments 49,904 56,521 11,244 B. Other investments and deposits - - - IX. Cash at bank and in hand 49,904 56,521 11,244 X. Deferred charges and accrued income 149 169 51 TOTAL ASSETS 136,055 154,096 71,380 Annual Report 2021 169
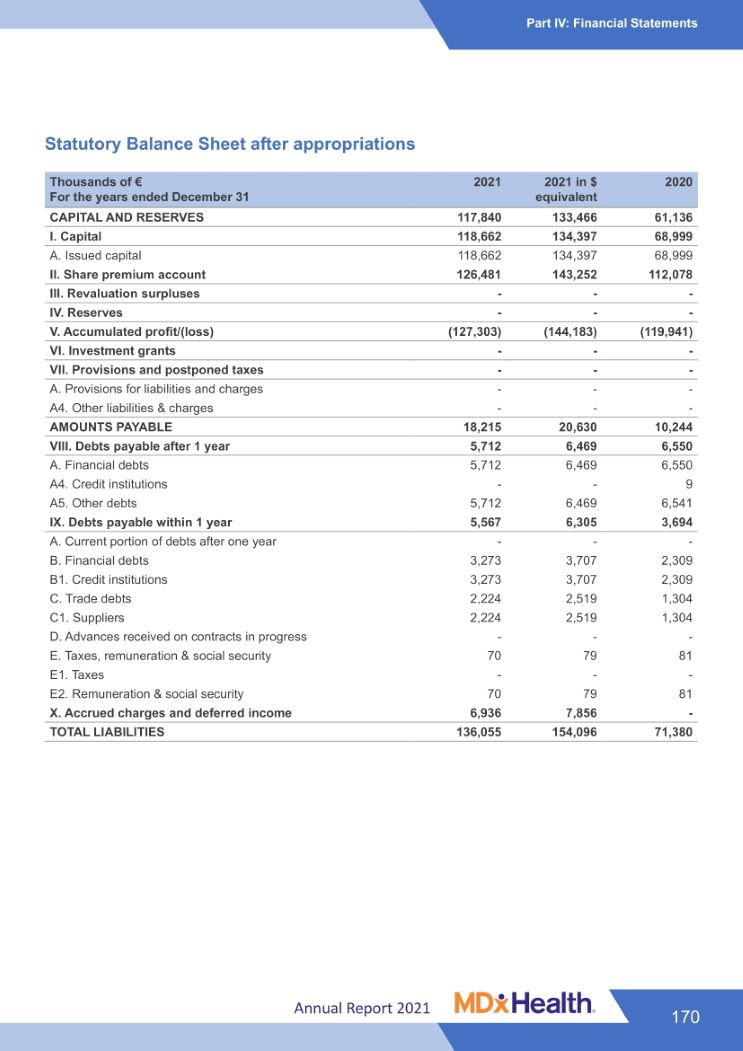
Part IV: Financial Statements Statutory Balance Sheet after appropriations Thousands of € For the years ended December 31 2021 2021 in $ equivalent 2020 CAPITAL AND RESERVES 117,840 133,466 61,136 I. Capital 118,662 134,397 68,999 A. Issued capital 118,662 134,397 68,999 II. Share premium account 126,481 143,252 112,078 III. Revaluation surpluses - - - IV. Reserves - - - V. Accumulated profit/(loss) (127,303) (144,183) (119,941) VI. Investment grants - - - VII. Provisions and postponed taxes - - - A. Provisions for liabilities and charges - - - A4. Other liabilities & charges - - - AMOUNTS PAYABLE 18,215 20,630 10,244 VIII. Debts payable after 1 year 5,712 6,469 6,550 A. Financial debts 5,712 6,469 6,550 A4. Credit institutions - - 9 A5. Other debts 5,712 6,469 6,541 IX. Debts payable within 1 year 5,567 6,305 3,694 A. Current portion of debts after one year - - - B. Financial debts 3,273 3,707 2,309 B1. Credit institutions 3,273 3,707 2,309 C. Trade debts 2,224 2,519 1,304 C1. Suppliers 2,224 2,519 1,304 D. Advances received on contracts in progress - - - E. Taxes, remuneration & social security 70 79 81 E1. Taxes - - - E2. Remuneration & social security 70 79 81 X. Accrued charges and deferred income 6,936 7,856 - TOTAL LIABILITIES 136,055 154,096 71,380 Annual Report 2021 170









































































































































































