As filed with the U.S. Securities and Exchange Commission on March 27, 2023
Registration No. 333-268456
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 4 to
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CytoMed Therapeutics Limited
(Exact Name of Registrant as Specified in its Charter)
Not Applicable
(Translation of Registrant’s name into English)
Singapore | 2834 | Not Applicable | ||
(State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
1 Commonwealth Lane
#08-22
Singapore 149544
+65 6250 7738
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Puglisi & Associates
850 Library Ave, Suite 204
Newark, DE 19711
(302)-738-6680
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
Richard I. Anslow, Esq. Lijia Sanchez, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, NY 10105 Tel: (212) 370-1300 Fax: (212) 370-7889 | Richard A. Friedman, Esq. Stephen A. Cohen, Esq. Sean F. Reid, Esq. Sheppard, Mullin, Richter & Hampton LLP 30 Rockefeller Plaza New York, NY 10112 Tel: (212) 653-8700 Fax: (212) 653-8701 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933:
Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this Prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This Prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion dated March 27, 2023

PRELIMINARY PROSPECTUS
2,412,369 ORDINARY SHARES
CYTOMED THERAPEUTICS LIMITED
We are offering ordinary shares. This is the initial public offering of ordinary shares of CytoMed Therapeutics Limited. We are offering, on a firm commitment basis, 2,412,369 ordinary shares. The offering price of our ordinary shares in this offering is expected to be between U.S.$4.00 and U.S.$5.00 per share. Prior to this offering, there has been no public market for our ordinary shares.
We have applied to list our ordinary shares on the Nasdaq Capital Market (“Nasdaq”) under the symbol “GDTC”. There is no assurance that our application will be approved, and if our application is not approved, this offering will not be completed.
We are an “emerging growth company” as defined in the Jumpstart Our Business Act of 2012, as amended, and, as such, will be subject to reduced public company reporting requirements.
Investing in our ordinary shares is highly speculative and involves a high degree of risk. Before buying any shares, you should carefully read the discussion of material risks of investing in our ordinary shares in “Risk Factors” beginning on page 26 of this Prospectus.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this Prospectus. Any representation to the contrary is a criminal offense.
| PER SHARE | TOTAL | |||||||
| Initial public offering Price | U.S.$ | U.S.$ | ||||||
| Underwriting discounts and commissions(1) | U.S.$ | U.S.$ | ||||||
| Proceeds, before expenses, to us | U.S.$ | U.S.$ |
(1) We have agreed to issue, on the closing date of this offering, warrants, or the representative’s warrants, to the representative of the underwriters, The Benchmark Company, LLC, in an amount equal to 5% of the aggregate number of ordinary shares sold by us in this offering, excluding any shares issued pursuant to exercise of the underwriter’s over-allotment option. For a description of other terms of the representative’s warrants and a description of the other compensation to be received by the underwriters, see “Underwriting” beginning on page 181.
We expect our total cash expenses for this offering (including cash expenses payable to our underwriters for their out-of-pocket expenses) to be approximately U.S.$1.31 million, exclusive of the above discounts. In addition, we will pay additional items of value in connection with this offering that are viewed by the Financial Industry Regulatory Authority, or FINRA, as underwriting compensation. These payments will further reduce proceeds available to us before expenses. See “Underwriting.”
This offering is being conducted on a firm commitment basis. The underwriters are obligated to take and pay for all of the shares if any such shares are taken. We have granted the underwriters an option for a period of 45 days after the closing of this offering to purchase up to 15% of the total number of our ordinary shares to be offered by us pursuant to this offering (excluding shares subject to this option), solely for the purpose of covering over-allotments, at the initial public offering price less the underwriting discounts. If the underwriters exercise the option in full, the total underwriting discounts payable will be U.S.$873,880.56 based on an assumed initial public offering price of U.S.$4.50 per ordinary share (the midpoint of the price range set forth on the cover page of this Prospectus), and the total gross proceeds to us, before underwriting discounts and expenses, will be U.S.$12,484,008.00.
The underwriters expect to deliver the ordinary shares against payment as set forth under the section titled “Underwriting” on or about , 2023.
| The Benchmark Company | Axiom Capital Management, Inc. |
The date of this Prospectus is , 2023.
| 2 |
Explanatory Note
As of the date of this Prospectus, the registrant is a public company limited by shares known as CytoMed Therapeutics Limited. On January 19, 2023, the registrant converted from a private company limited by shares incorporated in Singapore, known as CytoMed Therapeutics Pte. Ltd. to a public company limited by shares pursuant to the provisions of the Singapore Companies Act.
Effective on January 17, 2023, the registrant implemented a 1-for-380.83 reverse split of its ordinary shares pursuant to which the shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date.
References to “the date of this Prospectus” in this draft Prospectus shall refer to the date of confidential submission of this draft Prospectus.
| 3 |
TABLE OF CONTENTS
| 4 |
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this Prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This Prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this Prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our ordinary shares. Our business, financial condition, results of operations and prospects may have changed since that date.
You should rely only on the information contained in this Prospectus or in any related free-writing prospectus. We have not authorized anyone to provide you with information different from that contained in this Prospectus. We are offering to sell, and seeking offers to buy, ordinary shares in our Company only in jurisdictions where such offers and sales are permitted. The information contained in this Prospectus is current only as of the date of this Prospectus, regardless of the time of delivery of this Prospectus or of any sale of the ordinary shares.
For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this Prospectus in any jurisdiction, other than the United States, where action for that purpose is required. Persons outside the United States who come into possession of this Prospectus must inform themselves about, and observe any restrictions relating to, the offering of the ordinary shares and the distribution of this Prospectus outside the United States.
We are incorporated under the laws of Singapore as a company with limited liability, and as of the date of this Prospectus a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the U.S. Securities and Exchange Commission, or the SEC, we currently qualify for treatment as a “foreign private issuer.” As a foreign private issuer, we will not be required to file periodic reports and financial statements with the Securities and Exchange Commission, or the SEC, as frequently or as promptly as domestic registrants whose securities are registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Until , 2023 (25 days after the date of this Prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
| 5 |
“CytoMed”, “CytoMed Therapeutics”, the “Company”, “we”, “our”, “ours”, “us”, “our Group” and similar terms refer to CytoMed Therapeutics Limited and/or its subsidiaries (where applicable).
For investors outside of the United States: We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the ordinary shares and the distribution of this prospectus outside of the United States.
For investors in Singapore: This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, our ordinary shares were not offered or sold or caused to be made the subject of an invitation for subscription or purchase and will not be offered or sold or caused to be made the subject of an invitation for subscription or purchase, and this prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of our ordinary shares, has not been circulated or distributed, nor will it be circulated or distributed, whether directly or indirectly, to any person in Singapore other than (i) to an institutional investor (as defined in Section 4A of the Securities and Futures Act 2001 of Singapore, as modified or amended from time to time (“SFA”)) pursuant to Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA and (where applicable) Regulation 3 of the Securities and Futures (Classes of Investors) Regulations 2018, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where our ordinary shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
(a) a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
(b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, securities or securities-based derivatives contracts (each term as defined in Section 2(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ordinary shares pursuant to an offer made under Section 275 of the SFA, except:
| ● | to an institutional investor or to a relevant person, or to any person arising from an offer referred to in Section 275(1A) of the SFA or Section 276(4)(c)(ii) of the SFA; | |
| ● | where no consideration is or will be given for the transfer; | |
| ● | where the transfer is by operation of law; | |
| ● | as specified in Section 276(7) of the SFA; or | |
| ● | as specified in Regulation 37A of the Securities and Futures (Offers of Investments) (Securities and Securities-based Derivatives Contracts) Regulations 2018. |
Any reference to the SFA is a reference to the Securities and Futures Act 2001 of Singapore and a reference to any term as defined in the SFA or any provision in the SFA is a reference to that term as modified or amended from time to time including by such of its subsidiary legislation as may be applicable at the relevant time.
Notification under Section 309B(1)(c) of the SFA: The Company has determined, and hereby notifies all persons (including relevant persons (as defined in Section 309A(1) of the SFA)) that the ordinary shares are prescribed capital markets products (as defined in the Securities and Futures (Capital Markets Products) Regulations 2018) and Excluded Investment Products (as defined in MAS Notice SFA 04-N12: Notice on the Sale of Investment Products and MAS Notice FAA-N16: Notice on Recommendations on Investment Products).
By accepting this prospectus, the recipient hereof and thereof represents and warrants that such recipient is entitled to receive it in accordance with the restrictions set forth above and agrees to be bound by the limitations contained herein. Any failure to comply with these limitations may constitute a violation of law.
| 6 |
PRESENTATION OF FINANCIAL INFORMATION
Basis of Presentation
Unless otherwise indicated, all financial information contained in this Prospectus is prepared and presented in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”). Certain differences exist between IFRS and generally accepted accounting principles in the United States (“U.S. GAAP”) which might be material to the financial information herein. We have not prepared a reconciliation of our consolidated financial statements and related footnote disclosures between IFRS and U.S. GAAP. Potential investors should consult their own professional advisers for an understanding of the differences between IFRS and U.S. GAAP and how these differences might affect the financial information herein.
All references in this Prospectus to “U.S. dollars”, “U.S.$” and “USD” refer to the currency of the United States of America, all references to “S$” or “Singapore dollars” or “SGD” refer to the currency of Singapore and all references to “RM” or “Malaysian Ringgit” or “Ringgit” refer to the currency of Malaysia.
Our financial year ends on December 31 of each year. References in this Prospectus to a financial year, such as “financial year 2020”, relate to our financial year ended on December 31 of that calendar year.
We have made rounding adjustments to some of the figures included in this Prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that precede them.
| 7 |
All translations from Singapore dollars to U.S. dollars and from U.S. dollars to Singapore dollars in this Prospectus are made at a rate of S$1.3903 to U.S.$1.00, the exchange rate in effect as of June 30, 2022 as set forth in the H.10 statistical release of the U.S. Board of Governors of the Federal Reserve System. Further, all translations from Malaysian Ringgit to U.S. dollars and from U.S. dollars to Malaysian Ringgit in this Prospectus are made at a rate of MYR 4.4075 to U.S.$1.00, the exchange rate in effect as of June 30, 2022 as set forth in the H.10 statistical release of the U.S. Board of Governors of the Federal Reserve System. We make no representation that any Singapore dollars amounts or U.S. dollars amounts could have been, or could be, converted into U.S. dollars or Singapore dollars, as the case may be, at any particular rate, or at all.
| 8 |
Certain market data and forecasts used throughout this Prospectus were obtained from internal company surveys, market research, consultant surveys, reports of governmental and international agencies and industry publications and surveys. Industry publications and third-party research, surveys and reports generally indicate that their information has been obtained from sources believed to be reliable. This information involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the section titled “Risk Factors” in this Prospectus. The industry in which we operate is also subject to a high degree of uncertainty and risks due to a variety of factors, including those described under the section titled “Risk Factors” in this Prospectus. These factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us in this Prospectus.
| 9 |
“CYTOMED THERAPEUTICS”, the CytoMed Therapeutics logo, and other trademarks, trade names or service marks of CytoMed Therapeutics Limited appearing in this Prospectus are the property of CytoMed Therapeutics Limited. All other trademarks, trade names and service marks appearing in this Prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Prospectus may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert their rights thereto.
| 10 |
PROSPECTUS SUMMARY
This summary only highlights the more detailed information appearing elsewhere in this Prospectus. As this is a summary, it does not contain all of the information that you should consider in making an investment decision. You should read this entire Prospectus carefully, including the information under the section titled “Risk Factors” and our financial statements and the related notes in this Prospectus, before investing.
| 11 |
Unless otherwise stated in this Prospectus or the context otherwise requires, references to:
| ● | “γδ TCR” are to gamma delta T-cell receptor; | |
| ● | “A*STAR” are to the Agency for Science, Technology and Research of Singapore; | |
| ● | “ACCA” are to the Association of Chartered Certified Accountants; | |
| ● | “ACRA” are to the Accounting and Corporate Regulatory Authority of Singapore; | |
| ● | “ACRA Code” are to the Code of Professional Conduct and Ethics for Public Accountants and Accounting Entities; | |
| ● | “ACTRIS” are to the Advanced Cell Therapy and Research Institute, Singapore; | |
| ● | “Advertisement Regulations” are to the Health Products (Advertisement of Specified Health Products) Regulations 2016 enacted under the HPA (as defined below); | |
| ● | “ANGELICA Trial” are to the Phase I trial to evaluate allogeneic NKG2DL-targeting chimeric antigen receptor-grafted gamma delta T cells (CTM-N2D) in subjects with advanced solid tumours or haematological malignancies; | |
| ● | “ASX” are to the Australian Securities Exchange Ltd; | |
| ● | “ATPL” are to Accelerate Technologies Pte. Ltd. (formerly known as ETPL); | |
| ● | “BAS” are to building automated system; | |
| ● | “BCMA” are to B-cell maturation antigen; | |
| ● | “BMR” are to batch manufacturing records; | |
| ● | “BNM” are to the Central Bank of Malaysia or Bank Negara Malaysia; | |
| ● | “Board” or “Board of Directors” are to the board of directors of the Company; | |
| ● | “CAR” are to chimeric antigen receptor; | |
| ● | “CAR-T” are to chimeric antigen receptor-modified T cells; | |
| ● | “CDCR” are to the Control of Drugs and Cosmetics Regulations 1984 of Malaysia, as amended; | |
| ● | “cGMP” are to current good manufacturing practice, a system for ensuring that products are consistently produced and controlled according to quality standards; |
| 12 |
| ● | “cGMP Facility” are to the Company’s current good manufacturing practice and processing facility located at 12 Jalan Permas 9/16, Bandar Baru Permas Jaya, 81750 Johor, Malaysia; | |
| ● | “CGTP” are to Cell and Gene Therapy Products; | |
| ● | “Clean Air Regulations” are to the Environmental Quality (Clean Air) Regulations 2014 of Malaysia; | |
| ● | “Clinical Study Agreement” are to the Investigator-Initiated Clinical Study Agreement entered into between the Company and National University Hospital Singapore on March 10, 2023. | |
| ● | “Clinical Trials Regulations” are to the Health Products (Clinical Trials) Regulations of 2016 enacted under the HPA (as defined below), which governs clinical trials of therapeutic products and applicable CTGTP (as defined below) that are not observational trials within Singapore; | |
| ● | “Code” are to the Internal Revenue Code of 1986 of the United States of America; | |
| ● | “Comptroller” are to the comptroller of income tax in Singapore; | |
| ● | “Contract Manufacturing Organization” or “CMO” are to companies that provide drug development and drug manufacturing services to the companies in the pharmaceutical industry on a contract basis; | |
| ● | “Contract Research Organization” or “CRO” are to companies that provide research support to the companies in pharmaceutical and biotechnology industries on a contract basis; | |
| ● | “COVID-19” are to the worldwide novel coronavirus disease pandemic; | |
| ● | “CRIS” are to the Consortium for Clinical Research and Innovation Singapore, a wholly-owned subsidiary of the Ministry of Health Singapore; | |
| ● | “CRM” are to clinical research materials, which refer to any registered or unregistered therapeutic product, medicinal product, medicinal device, applicable CTGTP or placebo, that is manufactured, imported or supplied for the purpose of being used in clinical research, by way of administration to a trial participant in accordance with the research protocol or for a clinical purpose; | |
| ● | “CRM Regulations” are to the Health Products (Clinical Research Materials) Regulations of 2016 enacted under the HPA (as defined below), which govern the import, manufacture and supply of clinical research materials for use in clinical research studies within Singapore; | |
| ● | “CRS” are to cytokine release syndrome; | |
| ● | “CTA” are to Clinical Trial Authorization, which is required prior to the initiation of a clinical trial of a therapeutic product; | |
| ● | “CTGT” are to cell, tissue and gene therapy; | |
| ● | “CTGTP” are to cell, tissue and gene therapy products; | |
| ● | “CTGTP Regulations” are to the Health Products (Cell, Tissue and Gene Therapy Products) Regulations of 2021 enacted under the HPA (as defined below), which provide for regulating the manufacture, import, supply, presentation, registration, duties, and obligations of manufacturers, importers and other persons carrying out such activities as related to CTGTP in Singapore; | |
| ● | “CTIL” are to Clinical Trial Import License in Malaysia; | |
| ● | “CTM-N2D” are to CAR-gamma delta T cell proprietary product developed by our Group; | |
| ● | “CTM-GDT” are to allogeneic gamma delta T cell proprietary product developed by our Group; | |
| ● | “CTX” are to Clinical Trial Exemption in Malaysia; |
| 13 |
| ● | “CytoMed Malaysia” are to CytoMed Therapeutics (Malaysia) Sdn Bhd, a company incorporated on December 18, 2013 in Malaysia (Company No. 201301044786 (1074609-M)) with its registered address at Room 503, 5th Floor, Merlin Tower, Jalan Meldrum, 80000 Johor Bahru, Johor, Malaysia, the manufacturing arm of the Group where a PIC/S GMP laboratory is located and which manufacturing capacity includes stem cells and cancer living medicine; | |
| ● | “CytoMed Therapeutics”, “CytoMed” or “Company” are to CytoMed Therapeutics Limited, a company incorporated on March 9, 2018 in Singapore (Company Registration Number: 201808327H) with its registered address at 1 Commonwealth Lane, #08-22, Singapore 149544; | |
| ● | “Director” are to the directors of the Company; | |
| ● | “Director General” are to the Director General of Environmental Quality as referred to in the EQA; | |
| ● | “DNA” are to deoxyribonucleic acid, a large complex macromolecule containing the genetic code and which is typically found in the nucleus of a human cell; | |
| ● | “DTC” are to Depository Trust Company; | |
| ● | “ECEG 2016” are to the Ethical Code and Ethical Guidelines (2016 edition) published by the SMC; | |
| ● | “EIS” are to the Employment Insurance System in Malaysia; | |
| ● | “EISA” are to the Employment Insurance System Act 2017 of Malaysia; | |
| ● | “EPFA” are to the Employee Provident Fund Act 1991 of Malaysia; | |
| ● | “ESSA” are to the Employees’ Social Security Act 1969 of Malaysia; | |
| ● | “ETPL” are to Exploit Technologies Pte Ltd (now known as ATPL); | |
| ● | “EQA” are to the Environmental Quality Act 1974 of Malaysia; | |
| ● | “Exchange Act” are to the Securities Exchange Act of 1934 in the United States of America, as amended; | |
| ● | “FDA” are to the United States Food and Drug Administration; | |
| ● | “Federal Reserve System” are to the central bank of the United States of America; | |
| ● | “Financial Institution” is defined in the FX Notices as a person carrying out a financial business regulated under the laws administered by BNM and any person carrying out any other financial business as may be specified by BNM; | |
| ● | “Financial Instrument” is defined in the FX Notices and FSA to include any agreement, including an option, a swap, futures or forward contract, whose market price, value, delivery or payment obligations is derived from, referenced to or based on, but not limited to, securities, commodities, assets, rates (including interest rates or exchange rates) or indices; | |
| ● | “FMA” are to the Factories and Machinery Act 1967 of Malaysia; | |
| ● | “Foreign Exchange Notices” or “FX Notices” are to the notices issued by the BNM under the FSA and IFSA, which local and foreign investors are subject to; |
| 14 |
| ● | “FRS” or “SFRS(I) 9” are to the Singapore Financial Reporting Standard or the Singapore Financial Reporting Standard (International) 9, respectively; | |
| ● | “FSA” are to the Financial Services Act 2013 of Malaysia; | |
| ● | “gamma delta T-iPSCs” are to gamma delta T cells converted into iPSCs; | |
| ● | “GCP” or “Good Clinical Practice” are to good clinical practice; | |
| ● | “GMP” are to good manufacturing practice; | |
| ● | “Group”, “We”, “Us” and “Our” are to the Company and its subsidiaries; | |
| ● | “GST” means goods and services tax imposed pursuant to the Goods and Services Tax Act 1993 of Singapore; | |
| ● | “Guidelines on CGTPs” are to the Guidelines For Registration of Cell and Gene Therapy Products of Malaysia, issued under regulation 29 of the CDCR and effective on January 1, 2021; | |
| ● | “GvHD” are to graft versus host disease; | |
| ● | “HCS Bill” are to the Healthcare Services Bill, which was passed in the Singapore Parliament on January 6, 2020; | |
| ● | “HCSA” are to the upcoming Healthcare Services Act of Singapore, which will be implemented in phases. As of the date of this Prospectus, the phased implementation of the HCSA commenced from January 3, 2022, with all phases targeted to be implemented in Singapore by end- 2023; | |
| ● | “Health Products Act” or “HPA” are to the Health Products Act 2007 of Singapore that regulates the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the manufacture of health products within Singapore; | |
| ● | “Health Sciences Authority” or “HSA” are to the statutory board under the Ministry of Health Singapore; | |
| ● | “hESCs” are to human embryonic stem cells; | |
| ● | “HME 2016” are to the Handbook on Medical Ethics (2016 edition) issued by the SMC; | |
| ● | “HPA Order 2021” are to the Health Products Act (Amendment of First Schedule) Order 2021 of Singapore enacted under the HPA, an order in which a new category of health products, namely cell, tissue or gene therapy product, is included in the First Schedule of the HPA; | |
| ● | “hPSCs” are to human pluripotent stem cells; | |
| ● | “HSA Act” are to Health Sciences Authority Act 2001 of Singapore; | |
| ● | “Human Biomedical Research Act” or “HBRA” are to the Human Biomedical Research Act 2015 of Singapore that regulates the conduct of human biomedical research, further regulates certain restricted human biomedical research, and prohibits certain types of human biomedical research; |
| 15 |
| ● | “IASB” are to International Accounting Standards Board; | |
| ● | “ICA” are to the Industrial Co-ordination Act 1975 of Malaysia, as amended; | |
| ● | “IFRS” are to the international financial reporting standards as adopted by the International Accounting Standards Board; | |
| ● | “IFSA” are to the Islamic Financial Services Act 2013 of Malaysia, as amended; | |
| ● | “IL-2” are to interleukin-2; | |
| ● | “IMCB” are to the Institute of Molecular and Cell Biology, a research institute of A*STAR launched on January 23, 1985 to develop and support the biomedical research and development capabilities in Singapore; | |
| ● | “Incentive Plan” are to the 2023 Equity Incentive Plan of the Company, adopted on January 18, 2023 and to be effective upon the consummation of this offering; | |
| ● | “Income Tax Act” are to the Income Tax Act 1947 of Singapore; | |
| ● | “iPSC” are to induced pluripotent stem cells; | |
| ● | “iPSC-gdNKT” are to iPSC-derived gamma delta natural killer T cell product developed by our Group; | |
| ● | “IRAS” are to the Inland Revenue Authority of Singapore; | |
| ● | “ISAs” are to the International Standards on Auditing; | |
| ● | “ISCA” are to the Institute of Singapore Chartered Accountants; | |
| ● | “JOBS Act” are to the Jumpstart Our Business Startups Act of 2012 of the United States, as amended; | |
| ● | “Johor Bahru By-Laws” are to the Trade, License, Business and Industrial (MBJB) By-Laws 2016 of Malaysia; | |
| ● | “JSE” are to the Johannesburg Stock Exchange; | |
| ● | “K562 cells” are to the human myelogenous leukemia cell line; | |
| ● | “K562 Cell License for NK cell expansion” are to the license granted pursuant to the license agreement entered into between the Company and ATPL in December 2020; | |
| ● | “Know-How License” are to the license granted pursuant to the license agreement entered into between Puricell and ATPL in October 2020; | |
| ● | “LMC” are to the Landmark Medical Centre Sdn Bhd, a licensed private hospital located in Johor Bahru, Malaysia; | |
| ● | “Lower-tier PFIC” are to an entity in which a PFIC owns equity interests in; | |
| ● | “MA” are to the Medicines Act 1975 of Singapore; | |
| ● | “Malaysian Ringgit” or “RM” or “Ringgit” are to the currency of Malaysia; | |
| ● | “Management” or our “Management Team” are to our executive officers and Directors; | |
| ● | “Marketing Authorization Application” or “MAA” are to an application submitted by a drug manufacturer seeking marketing authorization, that is permission from a medical regulatory authority to bring a medicinal product to the market; | |
| ● | “Medicare” are to the national health insurance program in the United States of America; | |
| ● | “mDR Convertible Loan” are to the convertible loan provided by mDR Limited to the Company pursuant to the convertible loan agreement dated December 10, 2019, as amended by the supplemental agreements dated December 31, 2021, January 3, 2022 and January 3, 2023, entered into between the Company and mDR Limited; | |
| ● | “mGFP-γδ T” are to the gamma delta T cells subjected to electroporation using mRNA encoding mGFP; | |
| ● | “Ministry of Health Malaysia” or “MOH Malaysia” are to the Ministry of Health of the Malaysian Government; | |
| ● | “Ministry of Health Singapore” or “MOH Singapore” are to the Ministry of Health of the Singapore Government; | |
| ● | “MITI” are to the Ministry of International Trade and Industry of Malaysia; |
| 16 |
| ● | “mRNA” are to messenger RNA, a type of RNA that acts as a messenger that is read by ribosomes to build proteins; | |
| ● | “MSCs” are to mesenchymal stem cells; | |
| ● | “Nasdaq” are to The Nasdaq Stock Market LLC; | |
| ● | “Nasdaq Listing Rules” are to the listing requirements of Nasdaq, as amended, modified or supplemented from time to time; | |
| ● | “NDA” are to the new drug application to the HSA and/or other relevant health and regulatory authorities, through which formal proposal is made to the HSA and/or other relevant health and regulatory authorities for approval of a new drug; | |
| ● | “NEOs” are to the Company’s named executive officers, being CHOO Chee Kong, Dr. ZENG Jieming and Dr. TAN Wee Kiat as at the date of this Prospectus; | |
| ● | “NK cells” are to natural killer cells, a type of immune cell found in the human body; | |
| ● | “NKG2D” are to natural killer group 2D receptor, a type of receptor typically found on the NK cell; | |
| ● | “NKG2DL” are to natural killer group 2D ligands, a type of ligand typically expressed by tumor cells, enabling NK cells to activate and kill tumor cells; | |
| ● | “NKG2Dz-γδT” are to gamma delta T cells grafted with NKG2DL-targeting CAR; | |
| ● | “NKT” are to natural killer T cells; | |
| ● | “NOL” means net operating loss; | |
| ● | “NPCB” are to the National Pharmaceutical Control Bureau, Ministry of Health Malaysia; | |
| ● | “NPRA” are to the National Pharmaceutical Regulatory Authority, Ministry of Health Malaysia; | |
| ● | “Occupational Safety and Health Laws” are to the legislation, regulation and rules in respect of occupational safety and health in Malaysia; | |
| ● | “OECD” are to the Organization for Economic Co-operation and Development; | |
| ● | “off-the-shelf” are to the manufacturing of the stated cell therapies in quantities, which utilizes either donor blood cells or iPSCs as starting materials, but not the limited patient’s own blood cells and no matching is required between such donor and recipient of the product; | |
| ● | “one-tier system” are to the one-tier corporate tax system in Singapore; | |
| ● | “OSHA” are to Occupational Safety and Health Act 1994 of Malaysia; | |
| ● | “PA 1952” are to the Poisons Act 1952 of Malaysia; | |
| ● | “PAg” are to phosphoantigen, a type of small molecule that stimulates gamma delta T cells expressing Vγ9 and Vδ2 molecules; | |
| ● | “Patent License” are to the license granted pursuant to the license agreement entered into between the Company and ETPL in June 2018, as varied by an addendum entered into in December 2020, second addendum entered into in September 2021, and third addendum entered into effective as of October 18, 2022; | |
| ● | “PCID Regulations” are to the Prevention and Control of Infectious Diseases (Importation and Exportation of Human Remains, Human Tissues and Pathogenic Organisms or Substances) Regulations 2005 of Malaysia, as amended; | |
| ● | “PDPA Malaysia” are to the Personal Data Protection Act 2010 of Malaysia; | |
| ● | “PDPA Singapore” are to the Personal Data Protection Act 2012 of Singapore; | |
| ● | “PFIC” are to passive foreign investment company; | |
| ● | “PHFSA” are to the Private Healthcare Facilities and Services Act 1998 of Malaysia; | |
| ● | “PHMCA” are to the Private Hospitals and Medical Clinics Act 1980 of Singapore; |
| 17 |
| ● | “PIC/S” are to the pharmaceutical inspection co-operation scheme; | |
| ● | “Prospectus” are to this prospectus of the Company dated March 27, 2023, as may be amended; | |
| ● | “PTAB” are to the Patent Trial and Appeal Board; | |
| ● | “Puricell” are to Puricell Lab Pte Ltd, a 95% owned subsidiary of the Company; | |
| ● | “QA” are to quality assurance; | |
| ● | “QC” are to quality control; | |
| ● | “R&D” are to research and development; | |
| ● | “REJA” are to the Reciprocal Enforcement of Judgments Act 1958 of Malaysia; | |
| ● | “Resident” is defined in the FX Notices as, among others, a body corporate incorporated or established, or registered with or approved by any authority, in Malaysia; | |
| ● | “RNA” are to ribonucleic acid, a biological macromolecule that functions to convert the genetic information of DNA into proteins; | |
| ● | “Sarbanes-Oxley Act” are to the Sarbanes-Oxley Act of 2002, as amended; | |
| ● | “scFv” are to single-chain variable fragment, a technique used to produce a functional antigen-binding fragment on a cellular level; | |
| ● | “Scientific Advisory Board” or “SAB” are to the Company’s board of scientific advisors which provide the Company with on-going scientific advice; | |
| ● | “Singapore National Healthcare Group Domain Specific Review Boards” are to the independent committee constituted of medical, scientific and nonscientific members who are empowered by the National Healthcare Group Chief Executive Officer, to review research involving patients, staff, premises, or facilities of National Healthcare Group institutions and all other institutions under its oversight. | |
| ● | “SEC” are to the Securities and Exchange Commission of the United States of America; | |
| ● | “Securities Act” are to the Securities Act of 1933 of the United States of America, as amended; | |
| ● | “SFRS(I)” are to the Singapore Financial Reporting Standard (International); | |
| ● | “SGX-ST” are to the Singapore Exchange Securities Trading Limited; | |
| ● | “SIC” are to the Securities Industry Council of Singapore; | |
| ● | “SID” are to the Singapore Institute of Directors; | |
| ● | “Singapore Companies Act” are to the Companies Act 1967 of Singapore; | |
| ● | “Singapore Laboratory” are to the Company’s property located at 1 Commonwealth Lane, #08-22, Singapore 149544, with intended use as an office and research laboratory; | |
| ● | “Singapore Medical Council” or “SMC” are to the statutory board under the Ministry of Health Singapore which regulates the practice of medicine in Singapore; | |
| ● | “Singapore Take-over Code” are to the Singapore Code on Take-overs and Mergers; | |
| ● | “SOCSO” are to the Social Security Organization Fund of Malaysia; | |
| ● | “Stamp Duties Act” are to the Stamp Duties Act 1929 of Singapore; | |
| ● | “TCR” or “TCRs” are to T cell receptors; | |
| ● | “UK” are to the United Kingdom; | |
| ● | “United States” or “U.S.” are to the United States of America; | |
| ● | “VStock” are to V Stock Transfer, LLC, the transfer agent and registrar of the Company; | |
| ● | “U.S. Board of Governors” are to the governing body of the Federal Reserve System in the United States of America; | |
| ● | “U.S. GAAP” are to generally accepted accounting principles in the United States of America; and |
| 18 |
| ● | “U.S. Holder” are to a person that is, for U.S. federal income tax purposes, a beneficial owner of ordinary shares and a citizen or individual resident of the United States, a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia, or an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source. |
Our Business
We are a pre-clinical biopharmaceutical company focused on harnessing our licensed proprietary technologies to create novel cell-based immunotherapies for the treatment of human cancers. The development of our novel technologies has been inspired by the clinical success of existing CAR-T in treating hematological malignancies as well as the current clinical limitations and commercial challenges in extrapolating the CAR-T principle into treatment of solid tumors.
We believe that the current development of CD19-targeting CAR-T cells in treating B-cell malignancies signifies that cellular immunotherapy is becoming one of the pillars in cancer care. However, we believe that it remains challenging to apply the current CAR-T principle into treatments of other type of cancers, in particular solid tumors, due to a variety of reasons, including (i) the reliance on the limited cell quality and quantity of patients, (ii) the lack of suitable surface cancer antigens and their recognition system, and (iii) the ability of cancer to escape because of a single antigen-targeting strategy. To this end, we have established two novel patient blood cell-independent platform technologies to manufacture “off-the-shelf” cell-based cancer immunotherapies, meaning the manufacturing of the stated cell therapies in quantities, which utilizes either donor blood cells or iPSCs as starting materials, but not the limited patient’s own blood cells and no matching is required between such donor and recipient of the product. Our two novel platforms depend on healthy donor blood cells and induced pluripotent stem cells as starting material. Such platform technologies and the resulting product candidates exploit the multiple antigen recognition systems of NK cells and gamma delta T cells in the human body and so as to recognize and treat a broad range of cancers. Built on proprietary platform technologies, we are developing three product candidates: CTM-N2D, iPSC-gdNKT and CTM-GDT. CTM-N2D is our lead product candidate and it consists of expanded gamma delta T cells grafted with NKG2DL-targeting CAR to enhance anti-cancer cytotoxicity. We submitted a CTA application for Phase I clinical trial to HSA, Singapore in December 2021 and, in July 2022, were granted a CTA relating to the use of our CTM-N2D for the ANGELICA Trial to be conducted with the National University Hospital Singapore, subject to conditions specified by the HSA. On January 6, 2023, the HSA acknowledged that we had submitted the relevant documents to meet the approval conditions and no further action was required. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects. We expect to expand our pipeline further in Phase II trials of CTM-N2D therapy for specific cancer indications. Our second product candidate, iPSC-gdNKT, utilizes iPSC as a starting material to generate gdNKT, which is a synthetic hybrid of a gamma delta T cell and a NK cell. The hybrid cell express receptors of both cells which potentially allow the gdNKT cells to recognize and treat a broad range of cancers. This product is currently undergoing pre-clinical development. Our third product candidate, CTM-GDT, consists of expanded allogeneic gamma delta T cells and exploits the potential of these cells to recognize and treat a broad range of cancers. We are looking to submit a CTX application for Phase I clinical trial to NPRA, Malaysia after 2023. We have also appointed an agent in the United States to prepare a Drug Master File for a potential research collaboration in the United States.
As of the date of this Prospectus, we are a holding company incorporated in Singapore which oversees our operations in Malaysia. We conduct our business activities primarily through our direct wholly-owned subsidiary in Malaysia, CytoMed Malaysia, but may be commencing more research and clinical trial activities in Singapore through CytoMed moving forward. Other than CytoMed Malaysia, our other subsidiaries have minimal operations as of the date of this Prospectus.
We have an operating cGMP Facility in Johor, Malaysia (which is near Singapore) which has been built in accordance with the international PIC/S GMP standards for the manufacture of cell therapy products and clinical trials. In addition, we have a well-trained operations team who conducts all essential cGMP activities including manufacture, quality control, quality assurance and documentation. We manufacture our product candidates in our cGMP Facility instead of engaging and relying on an external CMO.
Industry Overview
Our products are focused in the global immunotherapy market. Immunotherapy is the treatment of disease by promoting or inhibiting the immune responses and is gaining recognition and acceptance as an alternative therapy to treatment of cancer, in particular, in cancer patients who do not respond well to conventional cancer treatment, such as surgery, chemotherapy or radiation.
| 19 |
Current CAR-T cell therapy to treat cancer
Currently, CAR-T cell therapy is a type of cancer treatment in which a patient’s T cells (a type of immune system cell) are modified in the laboratory so they may attack cancer cells. Typically, T cells are extracted from the patient’s blood. Subsequently, the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added to the T cells. Such special receptor is called a chimeric antigen receptor (CAR) and such modified T cells are called CAR-T cells. Large numbers of CAR-T cells are grown in the laboratory and given to the patient by infusion. CAR-T cells are being used to treat certain blood cancers, and are being studied in the treatment of other types of cancer.
CAR-T cell therapy market
Since the approval of the first CAR-T cell therapeutic treatment in 2017 by the FDA, there has been widespread research and an exponential increase in clinical trial activity in the field of immunotherapy. Between 2017 to 2022, there have been at least six (6) CAR-T products that have entered the market, including two (2) of the earliest CAR-T products - Kymriah® by Novartis International AG (NYSE:NVS) and Yescarta® by Gilead Sciences, Inc. (NASDAQ:GILD), which received the earliest approvals and have been commercially available since 2018, and have been infused into many patients worldwide.
However, all approved CAR-T cell therapies thus far have taken an autologous treatment approach. Autologous CAR-T products are expensive to produce because they are manufactured on a patient-by-patient basis, and can be hampered by a shortage of CAR-T cells or viral vectors. Hence, current CAR-T cell therapies have been usually recommended for end-stage cancer patients who have exhausted all conventional treatment options.
CAR-T cell therapy has been a game-changer in the cancer treatment industry, creating hope that it could usher in a new era of cancer treatment. However, the success stories have typically come from targeting CD19, which is now considered an antigen that holds the key to a limited range of blood cancers. CAR-T cell therapy treatment for other types of cancers, in particular, solid tumors, has been severely limited so far.
Cell Therapy Industry in Singapore and Malaysia
The cell therapy industry in Singapore and Malaysia is at a nascent stage, with only a handful of biopharmaceutical companies within the cellular immunotherapy space in the treatment of cancer. Singapore approved the first CAR-T therapy for cancer treatment in March 2021, Kymriah® by Novartis (Singapore) Pte Ltd. This is the first and only commercially approved CAR-T therapy in Singapore and is currently in use for certain specific forms of B-cell malignancies. As of the date of this Prospectus, Malaysia has not yet approved any CAR-T therapy for treatment of cancer for commercial use.
Singapore established ACTRIS in April 2020 to meet the potential demand of cell therapy process development and product manufacturing to enable clinical utility. ACTRIS is the national center for facilitating discovery, process development and manufacturing of cellular-based therapeutics in immunotherapy and regenerative medicine in Singapore. ACTRIS works with other government agencies such as A*STAR and other industry players, such as hospitals, universities, research organizations and clinicians. ACTRIS is a business unit under the CRIS.
We intend to capitalize on our close connections with A*STAR to tap on the latest developments and advances in cell-based therapeutics in the field of cancer treatment.
Business Strategies and Future Plans
We intend to focus on developing our two (2) “off-the-shelf, allogeneic” technology platforms centered on gamma delta T cells and iPSCs, respectively.
| 20 |
Our aim is to be amongst the pioneers of cellular immunotherapy treatment for human cancer to serve the emerging economies within South-east Asia. We aim to strategically position ourselves as a bridge between the cutting-edge of cellular therapy in the U.S. and our origin amongst emerging economies in South-east Asia, with our headquarter in Singapore. We believe that the U.S. has the depth and breadth of expertise and experience to accelerate our R&D efforts in cellular therapy. We aim to establish our profile with like-minded biopharmaceutical companies, researchers, scientists and sources of investment capital within the U.S. In addition, we anticipate that cellular science will advance and gain more mainstream acceptance in the future. We are considering conducting research in regenerative medicine, in particular, stem cell application in an aging population in Asia. We believe we are well-placed with the necessary expertise, experience and resources to take advantage of such prospects.
Close connections with scientific and medical community
We have appointed immunotherapy industry advisors to our Scientific Advisory Board and our key executive officers have the relevant specialized technical and medical expertise in cellular therapy.
Our core technologies are licensed from A*STAR, Singapore’s lead public sector R&D agency. By working closely with research organizations and government bodies, we are continually kept abreast of the latest developments in the field of cellular therapy. We believe that our strategic positioning, experience and expertise will give us an edge to take advantage of the growing and developing cellular therapy market.
In addition, we intend to seek collaborations and/or enter into joint-ventures and out-licensing arrangements to jointly develop our two (2) novel “off-the-shelf” technology platforms with industry players using our infrastructure in Singapore and Malaysia so as to speed up commercialization of our product candidates.
Lower business and operating costs in South-east Asia
The relatively lower business and operating costs in South-east Asia should position us well to achieve our vision to develop our donor blood cell-based and iPSC-based platform technologies into “off-the-shelf” cancer immunotherapies with the ultimate goal to offer lower cost cell therapies as an alternate form of cancer treatment to patients.
We have invested in and completed construction of our own cGMP Facility which is fully equipped with state-of-the-art equipment, and which we believe is capable of handling the manufacturing for Phase I and Phase II clinical trials for our clinically-ready CAR T therapy, trade-named “CTM-N2D”. Our cGMP Facility has been audited by the NPRA in Malaysia, and the compliance standard of our cGMP Facility has been found to be satisfactory. We believe our proprietary product candidate, CTM-N2D, is clinically ready to commence clinical trials. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s acknowledgement on January 6, 2023 that we had submitted the relevant documents to meet the approval conditions of the CTA initially granted in July 2022 relating to the use of our CTM-N2D for the aforementioned trial by HSA, and no further action was required. We have already recruited a competent team of scientists and technicians and given the necessary training to manufacture the product for clinical trial. We target to recruit our first patient in second half of 2023. Our iPSC-gdNKT platform has been in the pre-clinical process development stage since fourth quarter of 2022, and is targeting to commence pre-clinical studies after 2024.
Summary of Risks Affecting Our Company
We are subject to numerous risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flow and prospects. You should carefully consider the following risks, those risks described in “Risk Factors” and the other information in this Prospectus before deciding whether to invest in our ordinary shares:
| 21 |
| ● | We are a global business subject to complex economic, legal, political, tax, foreign currency and other risks associated with international operations, which risks may be difficult to adequately address. | |
| ● | We do not have a long operating history and we do not currently have any product approved for commercial sale. | |
| ● | Our limited operating history may not provide an adequate basis to judge our future prospects and results of operation and may increase the risk of your investment. | |
| ● | We may not be able to access the capital markets in the future. | |
| ● | Our business depends upon the success of our CTM-N2D, iPSC-gdNKT and CTM-GDT product candidates. | |
| ● | Our business, our industry and the economy are subject to the impact of the ongoing COVID-19 pandemic. | |
| ● | We may not be able to obtain regulatory approval for our current or future product candidates. | |
| ● | Our pre-clinical procedures and trials may experience delays or may not progress to clinical trials. | |
| ● | The results of our clinical trials may not be successful. | |
| ● | We may not be able to maintain our key personnel or attract, train and retain other highly qualified personnel. | |
| ● | We rely on our cGMP Facility to produce our product candidates and it may not be sufficient to handle the large-scale manufacture and production of product candidates. | |
| ● | Save for a registered trademark in relation to our name we currently own no other intellectual property rights and rely entirely on intellectual property rights we licensed from others for our main operations. | |
| ● | We may encounter adverse developments or disputes concerning our licensed intellectual property rights or other proprietary rights. | |
| ● | We rely on third-party intellectual property licensors and the terms of such licenses. | |
| ● | We may not be able to successfully acquire or in-license additional product candidates on reasonable terms. | |
| ● | We may be subject to adverse developments relating to our competitors and our industry. | |
| ● | We may not realize market opportunities for our product candidates. | |
| ● | We may be adversely impacted by government laws and regulations and liabilities thereunder which could impede our progress. | |
| ● | There has been no public market for our ordinary shares prior to this offering and you may not be able to resell our ordinary shares at or above the price you paid (if at all). |
Foreign Private Issuer Status
We are a foreign private issuer within the meaning of the rules under the Exchange Act. As such, we are exempt from certain provisions applicable to United States domestic public companies. For example:
| ● | we are permitted to follow certain home country corporate governance practices in lieu of certain requirements under the Nasdaq listing standards. This may afford less protection to holders of our ordinary shares than U.S. regulations; | |
| ● | we are not subject to proxy rules and are not required to provide as many Exchange Act reports, or as frequently, as a domestic public company; | |
| ● | we may lose our private foreign issuer status, which would require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur additional legal, accounting and other expenses; | |
| ● | for interim reporting, we are permitted to comply solely with our home country requirements, which are less rigorous than the rules that apply to domestic public companies; | |
| ● | we are not required to provide the same level of disclosure on certain issues, such as executive compensation; | |
| ● | we are exempt from provisions of Regulation FD aimed at preventing issuers from making selective disclosures of material information; | |
| ● | we are not required to comply with the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; and | |
| ● | we are not required to comply with Section 16 of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and establishing insider liability for profits realized from any “short-swing” trading transaction. |
| 22 |
Implications of Being an Emerging Growth Company
As a company with revenue of less than U.S.$1.235 billion for the previous financial year, we qualify as an “emerging growth company” under the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act, or Section 404, in the assessment of the emerging growth company’s internal control over financial reporting. The JOBS Act also provides that an emerging growth company does not need to comply with any new or revised financial accounting standards until such date that a private company is otherwise required to comply with such new or revised accounting standards.
We will remain an “emerging growth company” until the earliest of (i) the last day of our financial year during which we have total annual gross revenues of at least U.S.$1.235 billion; (ii) the last day of our financial year following the fifth anniversary of the consummation of this offering; (iii) the date on which we have, during the previous three year period, issued more than U.S.$1 billion in non-convertible debt; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act, which would occur if the market value of our ordinary shares that are held by non-affiliates exceeds U.S.$700 million as of the last business day of our most recently completed second financial quarter and we have been publicly reporting for at least 12 months. Upon ceasing to be an “emerging growth company”, we will not be entitled to the exemptions provided in the JOBS Act as set out above.
Our Corporate Information
As of the date of this Prospectus, the registrant is a public company limited by shares known as CytoMed Therapeutics Limited. On January 19, 2023, the registrant converted from a private company limited by shares incorporated in Singapore, known as CytoMed Therapeutics Pte. Ltd. to a public company limited by shares pursuant to the provisions of the Singapore Companies Act.
Effective on January 17, 2023, the registrant’s shareholders and directors approved a 1-for-380.83 reverse split of its ordinary shares pursuant to which the shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date.
Our principal executive office in Singapore is located at 1 Commonwealth Lane, #08-22, Singapore 149544. Our telephone number is +65 6250 7738. Investors should submit any inquiries to the address and telephone number of our principal executive office. Our agent for service of process in the United States is Puglisi & Associates. Our website is located at https://www.cytomed.sg/. Information contained on, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into, this Prospectus.
| 23 |
Securities offered by us
| 2,412,369 ordinary shares (or 2,774,224 ordinary shares if the underwriters exercise in full their option to purchase additional shares).(1)
| |
| Underwriters’ over-allotment option | We have granted to the underwriters an option, exercisable for 45 days from the date of this Prospectus, to purchase up to 361,855 additional ordinary shares at the public offering price listed on the cover page of this Prospectus, less underwriting discounts and commissions. |
| Ordinary shares to be outstanding before and after this offering | As of the date of this Prospectus, our issued and outstanding share capital consists of 8,527,450 ordinary shares.
Immediately after the offering, we will have 10,939,819 ordinary shares outstanding (or 11,301,674 ordinary shares if the underwriters exercise in full their option to purchase additional shares).
| |
| Listing | We have applied to list our ordinary shares on the Nasdaq Capital Market, under the symbol “GDTC”. There is no assurance that such application will be approved, and if our application is not approved, this offering may not be completed.
| |
Use of Proceeds
| We estimate that the net proceeds to us from the offering will be approximately U.S.$9.31 million, based on the assumed initial public offering price of U.S.$4.50 per share after deducting estimated underwriting discounts and commissions and expenses of the offering that are payable by us. We intend to use the net proceeds from the offering for clinical trials, R&D, manufacturing expansion, working capital and general corporate purposes. Please refer to the section titled “Use of Proceeds”.
| |
| Lock-Up Agreements | We have agreed with the underwriters, subject to certain exceptions, not to offer, pledge, sell, or dispose of, directly or indirectly, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares during the 6-month period following the date of this Prospectus.
Members of our Board, our executive officers and all shareholders beneficially owning more than 5% of our ordinary shares (other than with respect to 416,666 ordinary shares) have agreed during the 12-month period following the date of this Prospectus to substantially similar lock-up provisions, subject to certain exceptions. Please refer to the sections titled “Shares Eligible for Future Sale” and “Underwriting” for more information.
| |
| Risk Factors | Investing in our ordinary shares involves risks. Please refer to the section titled “Risk Factors” beginning on page 26 of this Prospectus for a discussion of factors you should carefully consider before deciding to invest in our ordinary shares. |
The foregoing discussion and tables above are based on 8,527,450 ordinary shares outstanding as of the date hereof, and excludes:
| ● | 528,823 ordinary shares issuable upon the conversion of convertible loan, with a conversion price of U.S.$2.04 per share; | |
| ● | 1,279,117 ordinary shares reserved for future issuance under our Incentive Plan, which was adopted January 18, 2023 and to be effective upon the consummation of this offering, as well as any future increases in the number of ordinary shares reserved for issuance under our Incentive Plan. |
Unless otherwise indicated, all information in this Prospectus assumes:
| ● | no exercise by the underwriters of their option to purchase up to 361,855 additional ordinary shares from us to cover over-allotments and/or warrants to purchase up to 120,618 ordinary shares, if any; and | |
| ● | an initial public offering price of U.S.$4.50 per share. |
(1) Effective January 17, 2023, we implemented a 1-for-380.83 reverse split of our ordinary shares, pursuant to which shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of that date.
| 24 |
SUMMARY OF CONSOLIDATED FINANCIAL INFORMATION
The following summary of consolidated financial information as of December 31, 2020 and 2021 and June 30, 2022 and for the years ended December 31, 2020 and 2021 and for the six months ended June 30, 2021 and 2022 are derived from our audited consolidated financial statements and unaudited interim condensed consolidated financial statements, respectively, included elsewhere in this Prospectus. Our consolidated financial statements are prepared and presented in accordance with IFRS. Our historical results are not necessarily indicative of results expected for future periods. The following summary of consolidated financial information should be read in conjunction with the sections entitled “Presentation of Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements of our Group, including the notes thereto, included elsewhere in this Prospectus.
The following table presents our summary of consolidated statements of profit or loss and comprehensive loss data for the financial years ended December 31, 2020 and 2021 and for the six months ended June 30, 2021 and 2022:
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||||||
| 2020 | 2021 | 2021 | 2022 | |||||||||||||||||||||
| S$ | S$ | U.S.$ | S$ | S$ | U.S.$ | |||||||||||||||||||
| Other operating income | 127,456 | 154,610 | 111,206 | 49,405 | 149,570 | 107,581 | ||||||||||||||||||
| Other (losses)/gains - net | (573,856 | ) | 3,185 | 2,291 | (209,533 | ) | (150,136 | ) | (107,988 | ) | ||||||||||||||
| R&D expenses | (1,038,091 | ) | (1,090,623 | ) | (784,452 | ) | (522,881 | ) | (604,043 | ) | (434,470 | ) | ||||||||||||
| General and administrative expenses | (345,616 | ) | (788,801 | ) | (567,360 | ) | (284,721 | ) | (306,457 | ) | (220,425 | ) | ||||||||||||
| Finance expenses | (105,519 | ) | (117,696 | ) | (84,655 | ) | (57,668 | ) | (62,042 | ) | (44,625 | ) | ||||||||||||
| Share of results of associate | - | (212,578 | ) | (152,901 | ) | - | (22,283 | ) | (16,027 | ) | ||||||||||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||||||
| Income tax expenses | - | - | - | - | - | - | ||||||||||||||||||
| Loss for the year/period | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||||||
| Other comprehensive income: | ||||||||||||||||||||||||
| Foreign currency translation loss, net of tax | (6,616 | ) | (22,628 | ) | (16,275 | ) | (25,669 | ) | (37,208 | ) | (26,763 | ) | ||||||||||||
| Total comprehensive loss | (1,942,242 | ) | (2,074,531 | ) | (1,492,146 | ) | (1,051,067 | ) | (1,032,599 | ) | (742,717 | ) | ||||||||||||
| Loss for the year/period attributable to equity holders of the Company | (1,935,440 | ) | (2,051,650 | ) | (1,475,689 | ) | (1,025,270 | ) | (995,287 | ) | (715,879 | ) | ||||||||||||
| Loss per share attributable to equity holders of the Company | ||||||||||||||||||||||||
| Basic and diluted | (0.33 | ) | (0.30 | ) | (0.21 | ) | (0.16 | ) | (0.13 | ) | (0.09 | ) | ||||||||||||
The following table presents our summary of consolidated balance sheets data as of December 31, 2020 and 2021 and as of June 30, 2022:
| As of December 31, | As of June 30, | |||||||||||||||||||
| 2020 | 2021 | 2022 | ||||||||||||||||||
| S$ | S$ | U.S.$ | S$ | U.S.$ | ||||||||||||||||
| Cash and cash equivalents | 885,272 | 2,512,768 | 1,807,357 | 1,487,161 | 1,069,669 | |||||||||||||||
| Property, plant and equipment | 2,301,025 | 2,495,791 | 1,795,146 | 2,684,305 | 1,930,738 | |||||||||||||||
| Total assets | 3,815,299 | 6,082,527 | 4,374,974 | 5,153,398 | 3,706,680 | |||||||||||||||
| Convertible loans | 2,323,423 | 2,310,757 | 1,662,056 | 2,451,595 | 1,763,357 | |||||||||||||||
| Total liabilities | 3,339,519 | 3,490,923 | 2,510,914 | 3,594,393 | 2,585,336 | |||||||||||||||
| Total equity | 475,780 | 2,591,604 | 1,864,060 | 1,559,005 | 1,121,344 | |||||||||||||||
| 25 |
An investment in our ordinary shares involves a high degree of risk. You should carefully consider the following risk factors, as well as all of the other information contained in this Prospectus, before making an investment decision. The risks described below are not the only ones facing us. The occurrence of any of the following risks, or of additional risks and uncertainties not presently known to us or that we currently believe to be immaterial, could significantly harm our business, financial condition, results of operations and growth prospects. In such case, the trading price of shares of our ordinary shares could decline, and you may lose part or all of your investment.
RISKS RELATED TO OUR FINANCIAL POSITION
We do not have a long operating history and we do not currently have any product approved for commercial sale.
We are a pre-clinical biopharmaceutical development stage company and we do not have any products approved for commercial sale as of the date of this Prospectus. We are focused on developing human cells as therapeutics and our technologies are new and unproven. Since our incorporation in 2018, we have invested most of our resources in developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, constructing our centralized cGMP Facility, hiring and training staff, raising capital and providing general and administrative support for these operations. Consequently, we do not have a meaningful operating history upon which our business and prospects may be evaluated, and predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history or a history of successfully developing and commercializing drug products. We have not yet demonstrated our ability to overcome many of the risks and uncertainties frequently encountered by companies in the rapidly evolving biopharmaceutical clinical trial industry. If we do not successfully address these risks, our business, financial condition, results of operations and growth prospects will be materially and adversely affected. At present time, we manufacture limited quantities of cells for researchers and institutions on a non-profit, cost recovery basis for the purpose of research.
We have incurred losses since our incorporation and we expect to incur significant losses for the foreseeable future, which raise substantial doubt on our ability to continue as a going concern. Our ability to continue as a going concern is dependent on being able to obtain sufficient additional funding to finance our operations.
Since our incorporation in 2018, we have incurred losses. As of December 31, 2020, 2021 and June 30, 2022, we have accumulated losses of S$3.02 million, S$5.07 million and S$6.06 million (approximately U.S.$4.36 million), respectively. As the Group has accumulated losses and net cash outflows from operating activities, our certifying accountant, WWC, P.C. had raised substantial doubt about our ability to continue as a going concern. Given that we will continue to invest most of our resources in developing our product candidates, we expect to continue to incur increasing losses for the foreseeable future. In addition, we anticipate a substantial increase in our expenses if, and as, we seek to:
| 26 |
| ● | initiate clinical development of our proprietary product candidates, CTM-N2D, iPSC-gdNKT and CTM-GDT; | |
| ● | advance additional product candidates to clinical trials, including CTM-N2D, iPSC-gdNKT and CTM-GDT; | |
| ● | discover and develop additional product candidates; | |
| ● | establish and validate our own clinical-scale and commercial-scale cGMP facilities; | |
| ● | initiate or develop a MAA, or equivalent in the relevant countries, for CTM-N2D, iPSC-gdNKT and CTM-GDT and/or seek marketing approvals for any of our other product candidates that successfully complete clinical trials; | |
| ● | engage, on an as needed basis, third-party contractors including CROs and CMOs to conduct clinical trials; | |
| ● | maintain, expand and protect our intellectual property portfolio; | |
| ● | acquire or in-license other product candidates and technologies; | |
| ● | incur additional costs associated with operating as a public company; and | |
| ● | increase our employee headcount and related expenses to support these activities. |
We may not be successful in any or all of these activities and even if we are successful, we may not generate revenues that are significant enough to generate profit. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all. Further, if we are unable to continue as a going concern, we may have to discontinue operations and liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, which would cause our shareholders to lose all or a part of their investment.
We will require additional capital, which, if available, may cause dilution to our shareholders, restrict our operations and/or require us to relinquish rights to our product candidates.
As of the date of this Prospectus, we have financed our operations primarily from private equity financing and issuance of convertible loans. We intend to use the proceeds from this offering to, among other uses, advance CTM-N2D, iPSC-gdNKT and CTM-GDT through clinical development. Developing pharmaceutical products and conducting pre-clinical studies and clinical trials are expensive. As of December 31, 2020, 2021, and June 30, 2022, we had cash and cash equivalents of S$885,272, S$2.51 million and S$1.49 million (approximately U.S.$.1.07 million), respectively. Our R&D expenses increased from S$1.04 million for the financial year ended December 31, 2020 to S$1.09 million (approximately U.S.$784,452) for the financial year ended December 31, 2021 and increased from S$522,881 for six months ended June 30, 2021 to S$604,043 (approximately U.S.$434,470) for the six months ended June 30, 2022. During the period from April to June 2021, the Company raised an aggregate of S$4 million (approximately U.S.$2.88 million) gross proceeds from the sale of our equities. The Company raised capital in an aggregate of S$1.22 million (approximately U.S.$879,415) between September and October 2022. In future, we will require additional cash funding to continue to execute our strategic plan and fund operations from time to time.
Until and unless we can achieve substantial revenue from our products to offset our expenses, we expect to finance our operations through the proceeds from this offering, a combination of equity offerings and debt financings, and potentially through additional license and development agreements or strategic partnerships with third parties. We may not be able to obtain sufficient financing or financing on terms which are reasonable to us. In addition, market volatility resulting from the COVID-19 pandemic or other factors could adversely impact our ability to obtain sufficient financing as and when needed. We have no commitments for any additional financing, and will likely be required to raise such financing through the sale of additional securities, which, in the case of equity securities, may occur at prices lower than the offering price of our ordinary shares in this offering. If we sell equity or equity-led securities, our current shareholders, including investors in this offering, may be diluted, and the terms may include liquidation or other preferences that are senior to or otherwise adversely affect the rights of our shareholders. Moreover, if we obtain debt financing, we may need to allocate a significant percentage of our operating cash flow to paying principal and interest on such debt and we may need to comply with operating restrictions, such as limitations on incurring additional debt, which could hinder our future ability to acquire, sell or license intellectual property rights. This consequently could impede our business operations. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares to decline.
| 27 |
If we obtain additional financing through licensing or collaboration arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to our business and operations. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our efforts to obtain additional financing for our business operations may also divert our time and attention from our day-to-day activities, which may impair or delay the development of our product candidates. In addition, our cash flow requirements may fluctuate as a result of factors currently unknown to us including, but not limited to, any unforeseen costs we may incur as a result of pre-clinical study or clinical trial delays due to the COVID-19 pandemic amongst other causes, and we may need to seek additional financing sooner than anticipated. If we are unable to obtain sufficient financing on a timely basis or at all, we may have to significantly curtail or stop one or more of our research or development programs.
Any acquisitions or strategic collaborations are capital intensive, may dilute our shareholders’ equity interest, cause us to incur debt or assume contingent liabilities and/or subject us to other risks.
From time to time, we may contemplate undertaking various acquisitions and strategic collaborations, including licensing or acquiring complementary drugs, intellectual property rights, technologies or businesses. Any potential acquisition or strategic partnership we may undertake involves several risks and challenges, including, but not limited to:
| ● | increased operating expenditure and cash flow requirements; | |
| ● | taking on clinical trial indebtedness or contingent or unknown liabilities; | |
| ● | assimilation of operations, intellectual property and drugs of an acquired company, including difficulties associated with integrating new personnel; | |
| ● | the diversion of our management’s time and focus from our existing drug programs and initiatives in pursuing such a strategic partnership, merger or acquisition; | |
| ● | retention of key employees, the loss of key personnel, and uncertainties in maintaining crucial business relationships; | |
| ● | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or product candidates and regulatory approvals; and | |
| ● | our inability to generate revenue from acquired drugs, intellectual property rights, technologies, and/or businesses sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
If we undertake acquisitions or strategic partnerships, we may issue securities which will dilute our shareholders’ equity interests, take on debt obligations, incur significant one-off expenditures or acquire intangible assets that could result in substantial future amortization expense. Moreover, we may not identify suitable acquisition or strategic partnership opportunities, and this inability could impair our growth or limit access to technology or drugs that may be important to the development of our business.
Our business and the business and operations of our research partners and/or other third parties with whom we conduct business are subject to the effects of health epidemics, including the current ongoing COVID-19 pandemic globally, in regions where we or third parties on which we rely have business operations.
The outbreak of the contagious disease, COVID-19, which was declared as a pandemic by the World Health Organization, has disrupted economic activity and business operations worldwide. Our headquarter is located in Singapore, which is subject to the ministerial advisories on work-from-home measures, restrictions on workplace capacity and other safe management measures which may change from time to time to mitigate the impact of the COVID-19 pandemic. Our cGMP Facility is located in Johor, Malaysia, which, as of the date of this Prospectus, is no longer subject to COVID-19 restrictions, save for the standard operating procedure on matters such as operating hours, ventilation, hand hygiene, premise cleanliness, COVID-19 screening test, case management of symptomatic individuals, confirmed cases of COVID-19 and close contacts, and wearing of face masks, implemented by the Malaysian Government as part of Malaysia’s transition phase to endemic.
| 28 |
Notwithstanding so, even if restrictions are lifted in the Singapore and/or Malaysia, similar or further restrictions could be subsequently implemented due to the constantly evolving COVID-19 situation. The effects of quarantines, stay-at-home, executive and similar government orders, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, in Singapore, Malaysia and other countries, could negatively impact our business operations and the operations of third parties we rely on, vendors disrupt or delay the enrollment of patients in these sites. Furthermore, these restrictions may delay any regulatory reviews by the relevant regulatory and health authorities, including related to the clinical trial submission for our CTM-N2D, iPSC-gdNKT and CTM-GDT product candidates, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our usual business operations.
In addition, the COVID-19 pandemic has significantly disrupted financial markets around the world and could continue to restrict the level of economic activity, and may limit our ability to access capital, which could adversely impact our liquidity now or in the future. A recession or market correction resulting from the spread of COVID-19 could materially affect our business and the value of our ordinary shares.
As a result of the COVID-19 pandemic or other pandemic, epidemic or outbreak of an infectious disease, we may experience disruptions that could severely impact our business, pre-clinical studies and clinical trials, including but not limited to:
| ● | delay or difficulties in enrolling donors for our clinical trials; | |
| ● | delays or difficulties in enrolling patients for our clinical trials; | |
| ● | delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; | |
| ● | diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials; | |
| ● | interruption of key clinical trial activities, such as clinical trial site data monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others or interruption of clinical trial subject visits and study procedures, which may impact the integrity of subject data and clinical study endpoints; | |
| ● | interruption or delays in the operations of the HSA and/or other relevant health and regulatory authorities, which may impact review and approval timelines; | |
| ● | interruption of, or delays in receiving, supplies of our product candidates and other required materials from our contract manufacturing organizations due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems; | |
| 29 |
| ● | interruptions in pre-clinical studies due to restricted or limited operations at our cGMP Facility; | |
| ● | increase in cost of operations due to rising logistical and shipping cost; | |
| ● | increase in cost of operations due to rising consumable price; | |
| ● | increase in costs of conducting clinical trials due to additional compliance requirements; | |
| ● | limitations on employee resources that would otherwise be focused on the conduct of our pre-clinical studies and clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; and | |
| ● | interruption or delays to our discovery and clinical activities, which in turn, could cause us to fail to meet milestone deadlines required to maintain intellectual property licenses upon which we rely. |
The full impact of the COVID-19 pandemic is highly uncertain as the COVID-19 pandemic is still evolving rapidly around the world as of the date of this Prospectus and there can be no assurance or certainty that the spread of COVID-19 will be contained in the near future. We are currently unable to determine the full extent of potential delays or impacts arising from the COVID-19 pandemic on our business, our clinical trials, healthcare systems or the global economy as a whole, but these delays could have a material adverse impact on our operations.
RISKS RELATED TO OUR BUSINESS IN THE BIOPHARMACEUTICAL INDUSTRY
Our business depends upon the success of our CTM-N2D, iPSC-gdNKT and CTM-GDT product candidates in obtaining regulatory approval and being commercialized.
The success of our business depends on our ability to utilize our proprietary technologies to manufacture our product candidates obtain regulatory approval for such product candidates, and commercialize the approved products. Our product candidates have not yet been evaluated in humans and may never become commercialized. All our product candidates developed from our proprietary technology platforms will require significant additional clinical and non-clinical development, review and approval by the relevant health and regulatory authorities in the various jurisdictions, substantial capital and time investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before they can be successfully commercialized. If any of our product candidates encounter safety or efficacy issues, developmental delays or regulatory issues or other roadblocks, it could impact the development plans for our other product candidates.
Our research on utilizing CTM-N2D, CTM-GDT and iPSC-gdNKT to treat cancer is novel, and we must overcome significant challenges such as obtaining required regulatory approvals in order to develop, commercialize and manufacture our product candidates.
Cell-based therapies are still considered novel and a new medical science in South-east Asia. The CTGTP Regulations in Singapore became effective on March 1, 2021. Malaysia’s CGTP regulations became effective on January 1, 2021. It can be expected that such regulations will be updated as more experience and expertise are gained by regulators, clinicians and the scientific community. Although future regulations may hinder or be detrimental to cell-based therapies, the medical community generally considers that such cellular “living” medicine has the potential to enhance patient treatment modalities for many diseases. Reflecting this, Singapore set up a national agency in April 2020 known as ACTRIS comprised of, inter alia, all the national universities, hospitals, research institutions and funding agencies in Singapore. More information on ACTRIS can be found in page 120 of this Prospectus.
Our R&D efforts are focused on utilizing CTM-N2D, iPSC-gdNKT and CTM-GDT as immunotherapies for cancers. To date, HSA has approved only one cell-based cancer therapy for commercialization (i.e. Kymriah® by Novartis (Singapore) Pte Ltd) and as far as we are aware, no gamma delta T cell-based therapy has been approved for commercial use by HSA and/or any other relevant regulatory authority in any of the key markets. The processes and requirements imposed by HSA and/or other relevant regulatory authorities in multiple jurisdictions may cause delays and additional costs in obtaining approvals for marketing authorization for our product candidates. Because our CTM-N2D, iPSC-gdNKT and CTM-GDT products are novel, and cell-based therapies are relatively new, regulatory agencies may lack the necessary experience to assess our product candidates. As such, this may increase the regulatory review process and period, including the time it takes for HSA and/or other relevant regulatory authorities to review our clinical trial application if and when submitted, such as the review of the Phase I clinical trial as described in paragraph “Development Status of CTM-N2D Therapy” in the section entitled “Business” which was submitted to the regulatory authorities in respect of CTM-N2D for the treatment of relapsed or refractory solid tumors of different types. Such regulatory delays may thereby increase our development costs and cause further delays in the commercialization of our CTM-N2D, iPSC-gdNKT and CTM-GDT products. Additionally, commercializing novel immuno-oncology therapies will create significant challenges for us, including but not limited to:
| 30 |
| ● | educating medical personnel regarding the potential side-effect profile of our cell products and, as the clinical program progresses, on observed side effects with the immune-oncology therapy; | |
| ● | training a sufficient number of medical personnel on how to properly handle and administer our cell products, especially in our planned solid tumor trial wherein the cells are given through a procedure by trained medical doctors; | |
| ● | enrolling sufficient numbers of patients in clinical trials; | |
| ● | developing a reliable and safe and an effective means of manufacturing our cell products; | |
| ● | manufacturing our cells on a large scale and in a cost-effective manner; | |
| ● | sourcing starting material suitable for clinical and commercial manufacturing; and | |
| ● | establishing sales and marketing capabilities, as well as developing a manufacturing process and distribution network to support the commercialization of any approved products. |
We must be able to overcome these challenges in order for us to develop, manufacture and commercialize our product candidates utilizing CTM-N2D, iPSC-gdNKT and CTM-GDT in each geographical market. As such, we remain open to exploring the possibility of conducting clinical trials in other countries.
Our pre-clinical studies may experience delays or may not progress to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these programs on a timely basis or at all.
In order to obtain HSA and/or other relevant regulatory authority approval to market a new biological product we must demonstrate proof of safety, purity and potency or efficacy in humans. We will have to conduct adequate and well-controlled clinical trials in order to meet high safety standards and requirements. We cannot guarantee the timely completion or successful outcome of our pre-clinical testing and studies and cannot determine whether the regulatory authority will accept our proposed clinical programs or if the outcome of our pre-clinical testing and studies will ultimately support the further development of our programs. As a result, we cannot be certain that we can submit any clinical trial or similar applications for our pre-clinical programs on the timelines we expect, if at all, and we cannot be sure that submission of clinical trials or similar applications will result in the regulatory authority or other regulatory authorities allowing clinical trials to begin.
Conducting pre-clinical testing is a prolonged, time-consuming and costly process. The period of pre-clinical testing may vary substantially depending on the type, complexity and novelty of the program, and may often take several years or more for each program. Any delays in pre-clinical testing and studies conducted by us or potential future partners may cause us to incur additional operating expenses. The commencement and rate of completion of pre-clinical studies and clinical trials for a product candidate may be delayed by many factors, including, for example:
| ● | inability to generate sufficient pre-clinical or other in vivo or in vitro data to support the commencement of clinical trials; | |
| ● | delays in reaching a consensus with regulatory agencies on clinical trial design; and | |
| ● | the relevant regulatory authorities not allowing us to rely on previous clinical trial findings of safety and efficacy for other similar but approved products and published scientific literature. |
Moreover, as standards for pre-clinical assessment are constantly evolving and may change rapidly, even if an agreement with HSA and/or other relevant regulatory authorities on a pre-clinical trial proposal has been made, HSA and/or other relevant regulatory authorities may not accept the proposed clinical trial submission, in which case patient enrollment would be put on partial or complete hold and treatment of enrolled patients could be temporarily put on hold as the product candidate is re-assessed. Even if we commence clinical trials, we cannot guarantee the success of our clinical trials or development efforts.
| 31 |
The results of pre-clinical studies and early-stage clinical trials may not be indicative of future results in future clinical trials. Initial success in any clinical trials may not be indicative of future results obtained at the completion of such trials in later stage trials.
The results of our pre-clinical studies may not be indicative of future results of clinical trials, and the results of any early-stage clinical trials we commence may not be indicative of the results of the later-stage clinical trials. For example, pre-clinical models as applied to cell therapy in oncology are not sufficiently representative of the clinical setting, and thus cannot always accurately predict clinical activity nor identify all potential risks, and may not provide sufficient guidance as to appropriate dose or administration regimen of a given therapy. In addition, initial success in clinical trials may not be indicative of results obtained when such trials are completed. Interim data from clinical trials that we may conduct are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary data are required to undergo audit and verification procedures that may result in the final data being materially different from the preliminary data we previously announced. Negative differences between preliminary or interim data and final data could materially adversely affect the prospects of any product candidate that is impacted by such data updates.
Clinical trials involve a lengthy and costly process with an uncertain outcome, and we may encounter substantial delays due to a variety of reasons outside our control.
Pre-clinical and clinical trials are costly, time consuming and subject to substantial uncertainty in respect of the outcome. Due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables, our product candidates may fail at any time during the trial. The results from pre-clinical testing or early clinical trials of a product candidate may not accurately indicate or ensure the outcome of later phases of clinical trials of our product candidates. HSA and/or other relevant regulatory authorities may suspend or terminate our clinical trials of any of our product candidate(s) at any time for various reasons, including, but not limited to, a perception that humans participating in such trials are exposed to unacceptable health risks or adverse side effects, or other adverse initial experiences or clinical trial findings. HSA and/or other relevant regulatory authorities may also require us to conduct additional pre-clinical studies or clinical trials due to negative or inconclusive results, or may fail to approve the raw materials, manufacturing processes or facilities of third-party manufacturers upon which we rely, and/or change their approval policies or regulations or their prior guidance to us during clinical development in a manner rendering our clinical data insufficient or unsuitable for approval. In addition, data obtained from our pre-clinical and clinical trials may not be deemed adequate to support the submission of the relevant regulatory filings in our key markets. We cannot guarantee that any pre-clinical studies or clinical trials that we may plan or initiate will be conducted as planned or completed on schedule, if at all.
We may fail at any stage in one or more of our clinical trials. We may face circumstances which prevent successful initiation, timely completion, or the desired results of our pre-clinical studies and clinical trials including, but not limited to:
| ● | delays and challenges in obtaining requisite regulatory approvals to commence pre-clinical studies and clinical trials on humans; | |
| ● | delays in reaching agreement on acceptable terms with prospective clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different trial sites; | |
| ● | our ability to recruit sufficient patients for our clinical trials in a timely manner or at all; | |
| ● | delays in sufficiently developing, characterizing or conducting a manufacturing process suitable for advanced clinical trials; | |
| ● | delays in reaching an agreement with HSA and/or other relevant regulatory authorities on the planning, design or implementation of our pre-clinical studies and clinical trials; | |
| ● | changes in regulatory requirements or guidance that may require us to amend or submit new clinical protocols, or the introduction of unanticipated requirements; |
| 32 |
| ● | changes in the standard of care on which a clinical development plan was based, which consequently may require new or additional trials; | |
| ● | lack of quality of our product candidates or other materials necessary to conduct pre-clinical studies or clinical trials of our product candidates; | |
| ● | clinical trials of our product candidates may have negative or inconclusive results, and as such, we may, or HSA and/or other relevant regulatory authorities may require us, to conduct additional clinical trials or abandon product development programs; | |
| ● | inability of enrolled patients in foreign countries to adhere to our clinical protocol and process due to differences in healthcare services or cultural customs, or additional administrative burdens associated with foreign regulatory schemes; and | |
| ● | failure of ourselves or any third-party manufacturers, contractors or suppliers to comply with regulatory requirements, maintain adequate quality controls, or be able to provide sufficient product supply to conduct and complete pre-clinical studies or clinical trials of our product candidates. |
In addition, the COVID-19 pandemic may increase the likelihood that we face such difficulties or delays in initiating, enrolling, conducting or completing our planned and ongoing pre-clinical studies and clinical trials, as applicable. If the COVID-19 pandemic continues to prevent or otherwise obstruct regulatory authorities’ conduct of regular inspections, reviews or other regulatory activities, HSA’s (or other relevant regulatory authorities) timely review and process our regulatory filings could be severely affected. If we experience delays in the initiation, enrollment or completion of any pre-clinical study or clinical trial of our product candidates, or if any pre-clinical studies or clinical trials of our product candidates are canceled, the commercial prospects of our product candidates may be materially and adversely affected, and our ability to generate product revenues from any of these product candidates will be delayed or not realized at all. In addition, any delays in completing our clinical trials may increase our costs and hold up our product candidate development and approval process.
Our business is highly reliant on the success of our product candidates, in particular CTM-N2D, iPSC-gdNKT and CTM-GDT, and we may fail to develop CTM-N2D, iPSC-gdNKT and CTM-GDT successfully or to obtain regulatory approval for them.
We cannot assure that CTM-N2D, iPSC-gdNKT and CTM-GDT will be safe and effective or will obtain the requisite approvals and licenses for commercialization, on a timely basis or at all. Although some of our employees have prior exposure to clinical trials, regulatory compliance and cGMP manufacturing, we have not previously completed any clinical trials or submitted a marketing approval application to HSA, or similar regulatory approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that CTM-N2D, iPSC-gdNKT and CTM-GDT will be successful in clinical trials or receive the requisite regulatory approvals for the development, commercialization and marketing of CTM-N2D, iPSC-gdNKT and CTM-GDT. HSA (or other relevant regulatory authorities) can delay, limit or refuse to grant approval of any of our product candidates for many reasons. For further details about such reasons, please refer to the risk factor entitled “Clinical trials involve a lengthy and costly process with an uncertain outcome, and we may encounter substantial delays due to a variety of reasons outside our control”. Any delay in obtaining, or inability to obtain, requisite regulatory approvals will delay or hinder our ability to successfully commercialize CTM-N2D, iPSC-gdNKT and CTM-GDT. This may have a material and adverse impact on our business, financial condition, results of operations and growth prospects.
Furthermore, as CTM-N2D is our lead product candidate and CTM-GDT is based on the same core proprietary technology, if any of our clinical trials of CTM-N2D encounter safety, efficacy or manufacturing problems, development delays, regulatory issues or other problems, our development plans for CTM-N2D and all of our other product candidates in our pipeline could be significantly hindered, which could materially and adversely affect our business, financial condition, results of operations and growth prospects.
| 33 |
In the event we manage to obtain clinical proof-of-concept from our CTM-N2D Phase I trials for relapsed or refractory solid tumors of different types, we may develop CTM-N2D for additional indications. We may not be able to advance any of these clinical trial indications through the development process. Even if we receive regulatory approval to market CTM-N2D for the treatment of any of these additional clinical trial indications, any such additional clinical trial indications may not be successfully commercialized, widely accepted in the marketplace or found to be more effective than other commercially available alternatives. If we are unable to successfully develop and commercialize CTM-N2D for these additional clinical trial indications, our commercial opportunities will be limited.
Enrollment and retention of patients for clinical trials is costly and time-consuming and could be delayed, made more difficult or rendered impossible by multiple factors outside our control.
Identifying and qualifying suitable patients to participate in our clinical trials is vital to our development of our product candidates. We need the enrollment of a sufficient number of patients suitable to undergo such clinical trials, including patients who are suffering from the disease that the product candidate is intended to treat and who meet other eligibility criteria for any clinical trial of a new product candidate. The rate of patient enrollment, which is a pivotal factor in determining the timing of clinical trials, is dependent on many factors beyond our control, including:
| ● | our ability to locate and set up clinical trial sites; | |
| ● | the size and nature of the patient population; | |
| ● | the design and eligibility criteria of the clinical trial; | |
| ● | the physical proximity of patients to clinical sites; | |
| ● | the patient referral practices and protocols of medical practitioners; | |
| ● | changing medical practice patterns or guidelines related to the clinical trial indications we are investigating; | |
| ● | competing clinical trials or approved therapies which are seen as an attractive alternative to patients and their medical practitioners; | |
| ● | perceived risks and benefits of the product candidate under study, including as a result of adverse effects observed in similar or competing therapies; | |
| ● | our ability to obtain and maintain consent to our clinical trials from patients which may be affected as a result of various reasons, including but not limited to, patients’ unwillingness to participate in our clinical trials due to the ongoing COVID-19 pandemic; | |
| ● | the risk that enrolled patients will drop out of our clinical trial or die before completion of the clinical trial; | |
| ● | our patients failing to complete a clinical trial or returning for post-treatment follow-up; and | |
| ● | our ability to manufacture or obtain the requisite materials for a patient and clinical trial. |
In addition, we will compete with many other ongoing clinical trials in sourcing for and recruiting patients for our expected clinical trials. Our clinical trials may also compete with other clinical trials for product candidates that are in a similar cellular immunotherapy area as our product candidates. This competition could reduce the number and profiles of available patients, as some patients may opt to enroll in a clinical trial conducted by one of our competitors instead of ours. Since the number of qualified clinical investigators is limited, we may conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial site. If we are unable to enroll a sufficient number of patients in our clinical trials in a timely manner, completion of our clinical trials may be delayed or may not be achieved, which would prevent us from commercializing our product candidates which in turn, could also cause us to fail to meet milestone deadlines required to maintain intellectual property licenses upon which we rely.
If any of our product candidates, or any competing product candidates, cause severe adverse effects (such as the development of severe or fatal cytokine release syndrome, neurotoxicity or graft-versus-host disease) we may be required to delay or discontinue further clinical development.
Our product candidates may have undesirable side effects that could result in us or HSA and/or other relevant regulatory authorities requiring us to interrupt, delay or discontinue clinical trials and could result in a more restrictive label than anticipated or the delay or denial of regulatory approvals. Our clinical trials may demonstrate a high and unacceptable level of severity and prevalence of side effects or unexpected characteristics.
| 34 |
As of the date of this Prospectus, our evaluation of our product candidates is limited to pre-clinical studies. As such, there can be no guarantee that any toxicity, and/or other adverse events, will not occur in human subjects during clinical trials.
While scientific data suggests that gamma delta T cell-based therapies may be better-tolerated as compared to alpha beta T cell-based therapies due to biologic differences between these cell types, there can be no assurance that patients will not experience cytokine release syndrome, neurotoxicity, GvHD or other serious adverse events. Severe adverse events associated with our product candidates, CTM-N2D, iPSC-gdNKT and CTM-GDT may also develop, since targeting NKG2DLs is not yet a well-characterized modality. NKG2D targets multiple ligands, and the landscape of ligand expression is currently not fully understood. For example, there are risks that NKG2DLs may be expressed on either known or an as-yet-underappreciated population of healthy cells. Therefore, such cells may also be targeted by CTM-N2D, iPSC-gdNKT and CTM-GDT and lead to adverse events of unknown frequency and severity. Such adverse events may cause delays in completion of our clinical programs. If unacceptable side effects arise in the development of our product candidates such that there is no longer a positive benefit risk, our clinical trials may be suspended or terminated, or the regulatory authorities may deny approval of our product candidates for any or all targeted clinical trial indications. Treatment-related side effects could also affect patient enrollment or the successful completion clinical trial by enrolled patients or result in potential product liability claims. In addition, these side effects may not be adequately identified or managed by the relevant medical personnel, and inadequate training in identifying or managing the potential side effects of our product candidates could result in patient injury or death.
Public perception of cell-based immuno-oncology therapies for treating cancer may impact public perception of our company and product candidates, or our business and operations.
Our platform utilizes a relatively new technology involving the human T cells and utilization of those modified cells in other clinical trial individuals. Public perception of our product candidates may be influenced by claims, such as that cell-based immunotherapy is unsafe, unethical, or immoral and, consequently, the public or the medical community may not be ready to accept our product candidates. Negative public reaction to and scrutiny of cell-based immunotherapy in general could result in governments implementing tighter regulations and stricter labeling requirements of cell-based immunotherapy products, including any of our product candidates, and could result a drop in the demand for any products we may develop. Adverse public attitudes may also adversely impact our ability to enroll suitable patients for our clinical trials. More restrictive government regulations or negative public opinion could have an adverse effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop.
We may not identify or develop other product candidates and may fail to capitalize on programs or product candidates that may present a greater commercial opportunity or for which there is a greater likelihood of success.
Our business is largely based on our ability to identify, develop and commercialize successful product candidates. A key element of our strategy is to discover and develop additional product candidates based upon our core proprietary technology platform through our internal research programs. We may also explore strategic collaborations for the discovery of new product candidates. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. In addition, targets for different cancers may require changes to our core proprietary technology platform, which may delay development or prevent the manufacture our product candidates. Our research programs may initially suggest positive results in identifying potential product candidates, but fail to produce successful product candidates for clinical development for many reasons, including without limitation the following:
| ● | the research methodology(ies) and/or technology platform(s) we use may not be accurate in identifying potential product candidates; | |
| ● | competitors may develop alternatives that render our product candidates obsolete or less attractive; |
| 35 |
| �� | we may choose to discontinue development if we determine that clinical results do not show promise; | |
| ● | product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights; | |
| ● | a product candidate may be shown to have harmful side effects or other characteristics that clinical trials indicate it is unlikely to be safe, effective or otherwise does not meet applicable regulatory criteria; and | |
| ● | a product candidate may not be accepted as safe and effective by patients and/or the medical professionals. |
Due to our limited resources, we have to focus our time and resources on the development of specific types of treatment, or treatment for a specific type of cancer, and we may forego or delay pursuing opportunities with certain programs or product candidates or for clinical trial indications which may be shown in the future to have greater commercial potential. Our estimates regarding the potential market for our product candidates may not be accurate, and if we do not correctly identify and accurately assess the commercial potential for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Alternatively, we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
If any of these events occur, we may be forced to delay or discontinue our development efforts with respect to a particular product candidate or we may fail to develop a potentially successful product candidate.
Although we intend to design the clinical trials for our product candidates, we plan to rely on third parties to assist us in conducting our clinical trials.
Although we manufacture our product candidates in our cGMP Facility instead of engaging and relying on an external CMO, we rely on third parties such as CROs, CMOs and specialist medical professionals, including but not limited to oncologists and hematologists, to assist us in other aspects of conducting clinical trials for our product candidates. As a result, many important aspects of our clinical development, including their conduct and timing, will not be within our direct control. Our reliance on third parties to conduct future clinical trials will also result in less direct control over the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with external parties can also be challenging, potentially leading to gaps in expectations and difficulties in coordinating activities. External parties may:
| ● | have staffing difficulties; | |
| ● | fail to comply with contractual obligations; | |
| ● | experience regulatory compliance issues; | |
| ● | undergo changes in priorities or become financially distressed; and/or | |
| ● | form relationships with other entities, some of which may be our competitors. |
If third parties whom we contract with do not perform our clinical trials in a satisfactory manner, breach their obligations to us and/or fail to comply with applicable legislative and regulatory requirements, we would be unable to rely on clinical data collected by these third parties and may be required to repeat, extend the duration of, or increase the size of any clinical trials we conduct, which could significantly delay the development and/or commercialization of our product candidates and significantly increase our costs.
If any of our relationships with these third parties deteriorate or terminate, we may not be able to arrange with alternative third parties on commercially reasonable terms, or at all. If third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain are compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any clinical trials such third parties are associated with may be extended, delayed or terminated, and we may not be able to obtain marketing approval for or successfully commercialize our product candidates. As a result, we believe that our financial results and the commercial prospects for our product candidates in the subject clinical trial would be materially and adversely affected, our costs could increase and our ability to generate revenue could be significantly delayed.
| 36 |
If third parties that we may rely on for our clinical trials do not successfully satisfy their contractual obligations, comply with regulatory requirements or meet targeted timelines, we may not be able to obtain requisite marketing approval for or commercialize our product candidates.
We are unable to independently conduct clinical trials though we intend to design our clinical trials for our product candidates and manufacture our product candidates in our cGMP Facility instead of engaging and relying on an external CMO. We remain dependent on medical institutions, clinical investigators, contract laboratories, and other third parties, such as CROs and CMOs to conduct or otherwise support other aspects of our clinical trials for our product candidates. In particular, in clinical trials, we are largely dependent on these parties for execution of clinical trials for our product candidates and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that our clinical trials are conducted in compliance with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on third parties will not relieve us of our regulatory responsibilities. For any violations of laws and regulations during the conduct of our clinical trials, we could be subject to untitled letters, warning letters or enforcement action that may include civil penalties up to and including criminal prosecution.
We and such third parties will be required to comply with the applicable legislations, regulations and other requirements in the relevant jurisdictions in conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate, and that the trial patients are adequately informed of the potential risks of participating in clinical trials and their rights are protected. These regulations are enforced by the relevant governmental and regulatory authorities through regular inspections of clinical trial sponsors, principal investigators and trial sites. If we and/or these third parties fail to comply with applicable Good Clinical Practice and other regulations of the relevant jurisdictions, the clinical data generated in our clinical trials may be perceived as unreliable and the governmental and regulatory authorities may require us to conduct additional clinical trials before approving our marketing application filings. We cannot guarantee that, upon inspection or review, the governmental or regulatory authorities will determine that any of our future clinical trials are in compliance with applicable legislations, regulations and other requirements. In addition, our clinical trials must be conducted with product candidates produced under cGMP regulations. Our failure or the failure of these third parties to comply with these regulations may require us to conduct clinical trials again, which would delay the marketing approval process and could also subject us to enforcement action. The COVID-19 pandemic and government measures taken in response have also had a significant impact on our third-party contractors, and we expect that they will face further disruption, which may affect our commencement and execution of our pre-clinical studies and clinical trials.
As a result, we do not have direct control over several crucial aspects of our clinical development, including their conduct and timing. Our reliance on third parties to conduct future clinical trials will also result in limited direct control over the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. Working with external parties can also be challenging, which may potentially lead to mistakes as well as difficulties in coordinating activities. External parties may:
| ● | have staffing difficulties; | |
| ● | fail to comply with contractual obligations; | |
| ● | experience legal and/or regulatory compliance issues; | |
| ● | undergo changes in priorities or become financially distressed; and/or | |
| ● | form relationships with other entities, some of which may be our competitors, which may undermine our clinical development. |
If third parties do not satisfactorily conduct our clinical trials, breach their contractual obligations to us and/or violate regulatory requirements, we cannot rely on clinical data collected by these third parties and may have to repeat, extend the duration of, or increase the size of any clinical trials we conduct, which could significantly delay commercialization and involve significantly greater expenditures.
If any of our relationships with these third parties deteriorate or terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. If third parties do not successfully carry out their contractual obligations or meet targeted timelines, if they have to be replaced with alternative third parties or if the quality or accuracy of the clinical data they obtain are compromised due to any breach of our clinical protocols, regulatory requirements or for other reasons, any clinical trials such third parties are associated with may be extended, delayed or terminated, and we may not be able to obtain the requisite marketing approval for or successfully commercialize our product candidates. As a result, we believe that our financial results and the commercial prospects for our product candidates in the clinical trials would be adversely affected, and our costs will be increased thereby affecting our ability to commercialize our products and to generate revenues.
| 37 |
If we fail to successfully compete with academic institutions and other biopharmaceutical companies that are developing similar product candidates or alternatives to cellular immunotherapy product candidates, our business will be materially and adversely affected.
The field of immunotherapy for treatment of cancers is characterized by intense and dynamic competition to develop new technologies and proprietary therapies. Any product candidates that we developed or are in the process of developing will have to compete with existing therapies and new therapies that may from time to time become available in the future. We face competition from various sources, including well-funded biopharmaceutical companies, established biopharmaceutical companies, as well as public and private research institutions. The areas of competition include relevant scientific and management human resources, funding for product development, establishing clinical study sites and clinical subjects participating in the trials.
Our known biopharmaceutical competitors working on allogeneic CAR-T therapies currently include Allogene Therapeutics, Inc. (NASDAQ:ALLO), Astellas Pharma Inc. (TSE:4503.T), Bristol-Myers Squibb (NYSE:BMY), Celyad Oncology SA (NASDAQ:CYAD), Fate Therapeutics, Inc. (NASDAQ:FATE), Gilead Sciences, Inc. (NASDAQ:GILD), NANTKWEST, INC.(NASDAQ:NK), Novartis International AG (NYSE:NVS), Surface Oncology, Inc. (NASDAQ:SURF), Takeda Pharmaceutical Company Limited (NYSE:TAK) and numerous other biopharmaceutical companies.
We are aware of the recent developments of our potential competitors for allogeneic CAR gamma delta T- cell therapy. Based on publicly disclosed information, in June 2022 Acepodia Biotech, Inc. announced FDA clearance of Investigational New Drug application for an Anti-CD20 armed allogeneic gamma delta T-cell therapy to treat non-Hodgkin’s Lymphoma, at the same time that Immatics N.V. (NASDAQ: IMTX) and Bristol Myers Squibb (NYSE: BMY) announced the expanded strategic alliance to develop multiple allogeneic off-the-shelf TCR-T and/or CAR-T gamma delta cell therapy programs. In April 2022, Adicet Bio, Inc. (NASDAQ: ACET) received FDA fast track designation for gamma-delta T-cell therapy for advanced lymphoma which subsequently reported emerging positive Phase I safety and efficacy data for relapsed or refractory B-cell Non-Hodgkin’s Lymphoma in June 2022. In addition, numerous academic institutions are conducting pre-clinical and clinical research in these areas, as well as with other white blood cell types including gamma delta T cells.
Our existing competitors in the field of gamma delta T cell therapy are listed in Table 2 of the section entitled “Competition” in this Prospectus. We are aware of the recent development of our potential competitor for allogeneic gamma delta T- cell therapy. Based on publicly disclosed information, in March 2022, FDA granted Orphan Drug Designation to TC BioPharm (Holdings) PLC (NASDAQ: TCBP) investigative allogeneic unmodified gamma delta T-cell product, for use as a potential therapeutic option in patients with relapsed/refractory acute myeloid leukemia. As of date of this Prospectus, we are not aware of any approved Phase III trial for gamma delta T cell-based cancer immunotherapy. Our gamma delta T cell product candidate may also face competition from other cell-based immunotherapy approaches derived from NK cells and T cells.
Our existing competitors in the field of iPSC-derived immune cells include Fate Therapeutics, Inc. (NASDAQ:FATE), Takeda-CiRA joint program (Takeda Pharmaceutical Company Limited (NYSE:TAK), Centre for iPS Cell Research and Application, Kyoto University) and Century Therapeutics, Inc. (NASDAQ:IPSC). As of the date of this Prospectus, Takeda-CiRA joint program is in its pre-clinical stage, while Fate Therapeutics, Inc. (NASDAQ:FATE) has various ongoing Phase I clinical trials for iPSC-derived NK cells.
Many of our existing or potential competitors may have greater financial and other resources, larger teams of R&D staffs, and more experienced capabilities in researching, developing and testing products than we do. Many of these companies also have more experience in conducting clinical trials, obtaining relevant regulatory approvals, and manufacturing, marketing and distributing therapeutic products. Smaller or pre-clinical stage companies like us may successfully compete by establishing collaborative relationships with larger pharmaceutical companies or academic institutions. Accordingly, our competitors may be more successful than us in obtaining approval for treatments and achieving widespread market acceptance. Our competitors’ treatments may be more clinically effective, or more effectively marketed, than any treatment we may commercialize and they may render our treatments obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our treatments.
Mergers and acquisitions in the industry may result in even greater resources being concentrated in a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical study sites and subjects for clinical studies. Other small or early-stage companies may also prove to be strong competitors, particularly those with collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new therapies enter the market and advanced technologies become available from time to time. We expect that any treatments which we develop and commercialize will need to compete on, among other things, efficacy, safety, convenience of administration and delivery, price, the level of generic competition and the availability of reimbursement from government and other third-party payors.
Our ability to commercialize our proprietary cell products could be reduced or eliminated if our competitors develop and commercialize products that are potentially more suitable, more effective, have a better safety profile, are more convenient or are less expensive than our products. Our competitors also may obtain relevant regulatory approvals for their products more rapidly than we may be able to obtain approval for ours, which could result in our competitors obtaining a head start and establishing a frontrunner position before we are ready to commercialize. If we are not able to compete effectively against our existing and potential competitors, our business, financial condition, results of operations and growth prospects may be materially and adversely affected. Please refer to the section entitled “Competition” for further details.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
As of the date of this Prospectus, we have twenty-seven (27) full-time employees. We are required to continue expanding our managerial, operational, quality, manufacturing, finance, sales and other resources in order to manage our operations and clinical trials, continue our development activities and eventually commercialize our product candidates. Our existing management and personnel, systems and facilities may not be sufficient to manage and support our future growth. Our growth strategy requires that we:
| 38 |
| ● | discover new product candidates, develop the process and analytical methods for clinical trials and submissions and applications to the relevant regulatory authorities, complete the required clinical trial studies for each, and receive approval from the regulatory authorities to initiate clinical trials for such product candidates; | |
| ● | manage our clinical trials effectively; | |
| ● | identify, recruit, retain, incentivize and integrate additional employees; | |
| ● | continue to maintain and renew qualification of our in-house clinical cGMP Facility; and | |
| ● | continue to improve our operational, financial and management controls, reports systems and procedures. |
If we are unable to attract skilled employees, increase the size of our organization or manage our future growth effectively, it will impair our ability to execute our business strategy and our business, financial condition, results of operations and growth prospects will be materially and adversely affected.
If we fail to attract and retain senior management, clinical, and key scientific personnel, we may be unable to successfully develop our product candidates, conduct our clinical trials and commercialize our product candidates.
Our business is partially reliant on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. Our senior management is crucial for our business and operations. The loss of any of our senior management could delay, hinder or prevent the successful development of our product pipeline, initiation or completion of our planned clinical trials or the commercialization of our future product candidates. We have employment and/or service agreements with all of our senior management team members.
Competition for qualified and experienced personnel in the biopharmaceuticals field is high due to the limited number of individuals with the skillset and experience required by our clinical trial industry. We will need to hire additional personnel to manage and support the expansion of our clinical development and any commercial activities we may commence. We may not be able to attract and retain suitable and qualified personnel on acceptable terms, or at all. If we cannot employ and retain the requisite qualified personnel for the operation of our business, our operation, financial condition, results of operations and growth prospects would be materially and adversely affected. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers have intellectual property rights to their research output.
We may incur substantial liabilities as a result of product liability lawsuits, if any, against us and this could limit commercialization of any product candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in clinical trials and may face an even greater risk if we commercialize any product candidate that we may develop. If we cannot successfully defend ourselves against claims that any such product candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| ● | decreased demand for any product candidate that we may develop; | |
| ● | loss of revenue; | |
| ● | substantial monetary awards to trial participants or patients; | |
| ● | significant time and costs to defend the related litigation; | |
| ● | withdrawal of clinical trial participants; | |
| ● | increased insurance costs; | |
| ● | the inability to commercialize any product candidate that we may develop; and | |
| ● | loss of reputation and significant negative publicity and media attention. |
Any such outcomes could materially and adversely affect our business, financial condition, results of operations and growth prospects.
| 39 |
Currently, we have no insurance coverage, and even if we obtain insurance policies, they may be inadequate, may not cover all of our potential liabilities and may potentially expose us to unrecoverable risks.
As of the date of this Prospectus, we do not have any insurance coverage for potential liabilities arising from our business as we have not begun commercialization nor clinical trials. We do not carry insurance for all categories of risk that our business may encounter. We anticipate that we will need to obtain appropriate product or clinical trial liability insurance coverage each time we commence a clinical trial and if we successfully commercialize any product candidate. Our license agreements include broad obligations to indemnify our licensors against losses, damages, costs, or expenses or claims for compensation arising of the products we are developing or licensed cell line we will use in connection with our product. Insurance availability, coverage terms and pricing continue to vary with market conditions. We will endeavor to obtain appropriate insurance coverage for insurable risks that we identify from time to time. However, we may fail to accurately predict, assess or quantify insurable risks. We may not be able to obtain appropriate insurance coverage and insurers may not respond as we intend to cover insurable events that may occur. Any significant uninsured liability may require us to pay substantial amounts, which would materially and adversely affect our business, financial condition, results of operations and growth.
In addition, although we are dependent on certain key management personnel, we do not have any key man life insurance policies in place in respect of any such individual. Therefore, if any of our key management personnel passes away or becomes disabled, we will not receive any compensation to assist with the absence of such key management personnel. The loss of such key management personnel could materially and adversely affect our business, financial condition, results of operations and growth prospects.
Moving forward, we intend to maintain a Directors’ and officers’ insurance policy pursuant to which our Directors and officers are insured against liability for actions taken in their capacities as Directors and officers. Although we intend to maintain such insurance, any such claim that may be brought could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance, or that is in excess of the limits of the insurance coverage. Moreover, in future, we may not be able to maintain insurance coverage, which is already high, at a reasonable cost or in sufficient amounts to protect us against losses.
Our business involves the use of biohazardous materials and we and any third-party manufacturers and suppliers we may engage are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these biohazardous materials.
Our R&D activities and those of any third-party manufacturers and suppliers we may engage involve the controlled storage, use and disposal of biohazardous materials. We and our manufacturers and suppliers may thus be subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these biohazardous materials. In some cases, these biohazardous materials and various wastes resulting from their use are stored at our manufacturers’ facilities pending their use and disposal.
We cannot completely prevent the risk of contamination despite our best efforts, and any contamination could result in an obstruction of our R&D efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers and suppliers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot be absolutely certain that this is always the case and/or fully eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other relevant authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, subject to frequent developments and are increasingly stringent over time. We cannot anticipate the impact of such changes and cannot be certain of our future compliance with such developments. We have not obtained any biological or hazardous waste insurance coverage as of the date of this Prospectus. Any contamination by biohazardous materials resulting from our operations could require us to suspend and/or cease our operations and therefore materially and adversely affect our business, financial condition, results of operations and growth prospects.
We are exposed to information technology and cyber security risks and disruption of service.
Our businesses and operations rely heavily on information technology as, amongst other things, we handle, store, manage and transmit data relating to our day-to-day activities not limited to manufacturing, product analysis and data, product candidates and trials. While we have taken steps internally to back up critical data on a service provided by a reputable third-party vendor, as well as storing local copies within the Company, a risk of failure beyond our control still exists. We are therefore exposed to risks of cyber security threats, data and data privacy breaches, as well as other network security and stability risks. The scale and sophistication of cyber security threats have increased especially in recent times. We are also reliant on a number of third-party vendors to provide adequate and timely software and hardware support, that could have a material adverse effect on our systems. Disruptions to our information technology systems, whether resulting from cyber-attacks, a failure by a key vendor or otherwise, that can cause interruptions to our network and services, may result in penalties. While we have established policies and frameworks, as described in the section titled “Intellectual Property – Trade Secrets” of this prospectus, we cannot assure you that such policies and frameworks are sufficient or that our business, financial condition, results of operations and prospects would not be adversely affected by such information technology and cyber security threats, data privacy breaches as well as other network security and stability risks.
RISKS RELATED TO MANUFACTURING OF CELL PRODUCTS
Our cGMP Facility may be affected by circumstances beyond our control, such as natural disasters, and we may be required to cease operations and manufacturing of our product candidates
We rely on the continued operations and maintenance of our cGMP Facility for the manufacture of our product candidates. The manufacturing of our product candidates in our cGMP Facility may be frustrated, disrupted, delayed or prevented by circumstances beyond our control, including without limitation earthquakes, fires, floods, explosion, accidents, disruptions to the electrical supply (including increased prices that may be prohibitive) leading to its shutdown, or other natural disasters. If any such event occurs, this could cause our equipment to malfunction and/or break down, and we may be forced to cease operations at our cGMP Facility notwithstanding that we have installed our own standby generator to try to mitigate such risks, until we manage to fully restore it in proper working order and condition. This will impede and delay our pre-clinical testing and progress of our proposed clinical trials and increase our costs and expenses, which will materially and adversely affect our business and operations.
| 40 |
In addition, as our cGMP Facility is subject to rigorous testing and standards in Malaysia, we may be subject to unannounced audit checks (or routine testing) from time to time by the relevant authorities in Malaysia, including the NPRA and others. If we fail to comply with any of the standards required of us, or if we do not comply with any of the Malaysian authorities’ directions to us within a specified time period, we may be required to suspend or cease our operations at our cGMP Facility. If this happens, we may be subject to warnings or penalties imposed by the Malaysian authorities or we may lose our license to operate the cGMP Facility. This would similarly impede and delay our pre-clinical testing and progress of our proposed clinical trials and increase our costs and expenses, which would materially and adversely affect our business, operations and prospects. As of the date of this Prospectus, our Group has not been subject to any penalties issued by the Malaysian authorities for any non-compliance with the standards required for the operation and maintenance of a cGMP Facility in Malaysia.
Our current cGMP Facility may not be sufficient to handle the large-scale commercial manufacture and production of product candidates
As of the date of this Prospectus, we are a pre-clinical biopharmaceutical company with three product candidates: CTM-N2D, CTM-GDT and iPSC-gdNKT. Our cGMP Facility is equipped to undertake the manufacture and production of our product candidates for pre-clinical and clinical trial purposes. However, our current cGMP Facility may not have the capabilities and resources to undertake and facilitate large-scale commercial production of our product candidates. In the event that any of our product candidates reach the commercialization stage, we will need to incur additional expenses to build another facility which is equipped to handle large-scale manufacturing or outsource manufacturing and/or production.
Our manufacturing process is complex, and we may encounter challenges in production of our product candidates, which would delay or hinder our ability to provide a sufficient supply of our product candidates for clinical trials or our products for patients, if approved.
Our product candidates are human cells, and the process of manufacturing such product candidates, as well as engineered K562 cells, is complex, highly regulated and involves numerous risks. Manufacturing our product candidates involves harvesting blood cells from a donor, isolating the gamma delta T cells, activating and expanding the gamma delta T cells, introducing a mRNA to be expressed on the gamma delta T cells, fill and finish and eventually shipping and infusing the cell product into the patient’s body. As a result of these complexities, the cost of manufacturing our cellular product candidates is generally higher than traditional small-molecule chemical compounds or biologics, and such manufacturing process is less reliable and harder to reproduce.
Our manufacturing process is subject to product loss or failure, or product variation that may negatively impact patient outcomes, due to logistical issues associated with supply chain disruptions, the collection of starting material from the donor, shipping such material to the manufacturing site, shipping the final product back to the clinical trial recipient, preparing the product for administration, infusing the patient with the product, manufacturing issues or different product characteristics resulting from the differences in donor starting materials, variations between reagent lots, interruptions in the manufacturing process, contamination, equipment or reagent failure, improper installation or operation of equipment, vendor or operator error, inconsistency in cell growth and variability in product characteristics.
Apart from the transport of material from the donor, we may also face disruptions in the supply of other materials, especially those which have to be imported from other countries, which are essential parts of the manufacturing process. For example, we have to use a special cell culture vessel for storage of the CTM-N2D product candidate, for which we have currently only been able to identify one supplier who sends it from the United States. We also use a special cell culture medium in the manufacturing process of the CTM-N2D product candidate which has to be kept in a special freezer and therefore special arrangements have to be made for its courier. In this regard, medical courier is a lot more expensive than other third-party service providers. Therefore, in the event that there are any delays in these deliveries, or increased prices for the material and/or delivery, the manufacturing processes may be affected as the delay and/or increased costs may be prohibitive.
| 41 |
Slight deviations from our normal manufacturing processes could result in a decrease in production yields, product defects and other supply disruptions. If, for any reason in our CTM-N2D, iPSC-gdNKT and CTM-GDT clinical studies, we damage and/or lose the starting material for a manufactured product for any of our patients at any point in the process, the manufacturing process for that patient would need to be repeated from the beginning and the resulting delay could require restarting the manufacturing process, or could result in such patient withdrawing from our clinical trial. If microbial, viral and/or other contaminations are discovered in any of our product candidates or in any of the manufacturing facilities in which our products or other materials are manufactured, such manufacturing facilities may need to be closed for an extended period of time to investigate and salvage the contamination. We may have to maintain a chain of identity in which materials are pegged to a specific donor as they move from the donor to the manufacturing facility, through the manufacturing process and back to the clinical trial recipient. Maintaining a chain of identity is challenging and complex and requires a high degree of accuracy and precision, and failure to do so could result in adverse patient outcomes, loss of product or regulatory action, including withdrawal of our products from the market, if licensed. Any failure in the foregoing processes could render a batch of product unusable, affect the regulatory approvals of such product candidate, cause us to incur fines or penalties or prejudice our reputation and that of our product candidates.
We may revise our manufacturing process for various reasons, such as to control costs, achieve scale, decrease processing time, and increase manufacturing success rate. As a result of changes made to our manufacturing process during the course of clinical development, we may need to demonstrate the comparability of the product used in earlier clinical phases or at earlier portions of a trial to the product used in later clinical phases or later portions of the trial. Changes to our manufacturing process made before or after commercialization could require us to show the comparability of the resulting product to the product candidate used in the clinical trials using earlier processes. We may need to collect additional non-clinical or clinical data from any modified process prior to obtaining marketing approval for the relevant product candidate produced with such modified process. If such data are not ultimately comparable to the data obtained in earlier trials or an earlier stage of the same trial in terms of safety and/or efficacy, we may be required to make further changes to our manufacturing process and/or undertake additional clinical testing, either of which could significantly delay the clinical development or commercialization of the associated product candidate, which would materially and adversely affect our business, financial condition, results of operations and growth prospects.
We are highly reliant on certain key suppliers for certain steps of our manufacturing processes.
Our manufacturing processes for CTM-N2D, iPSC-gdNKT and CTM-GDT depend on the use of cell culture vessel, and other reagents, some of which might only be available from certain key suppliers. In addition, some of these reagents, at the time of procurement, typically expire after approximately four to six months. Due to the short expiration period, we are unable to store the reagents in large quantities for future needs to mitigate against the risk of shortage due to disruption of the supply chain.
Further, although many of the reagents and consumables we require for our manufacturing process are available from more than one commercial supplier, we have not yet confirmed the suitability of the use of all such reagents and consumables in our manufacturing process. Even if we are able to replace any raw materials or consumables with alternatives, such alternatives may be more expensive, result in lower yields or not be as suitable for our purposes. In addition, some of the raw materials that we use are complex materials, which is likely to be harder to obtain substitutes for. Therefore, supply disruptions could result in significant delays and additional regulatory submissions and prevent us from being able to manufacture our product candidates due to the unsuitability of the substituted reagent or consumable that we are able to obtain.
In addition, some of the reagents that we use may be restricted by limited use label licenses and may be for research use and not for commercial application of any kind. We may need to replace certain reagents in order for us to commercialize our products in the future. While there are alternatives available, such alternatives may be more expensive, may result in lower yields or may not be as suitable for our purpose. These alternative reagents may also face regulatory challenges and review and potentially result in significant delays in our clinical trials and future developments.
Any disruption in supply of these reagents could result in significant delays in our clinical trials, which would materially and adversely affect our business, financial condition, results of operations and growth prospects.
| 42 |
We rely on the storage of our cell bank for the engineered K562 cells and peripheral blood mononuclear cells, and any damage or loss to them would cause delays in our clinical trials and may materially and adversely affect our business.
The cell bank of the engineered K562 cells we license and our peripheral blood mononuclear cells are stored in liquid nitrogen tanks at our cGMP Facility. If these materials are damaged or lost at our cGMP Facility, including by the loss or malfunction of these liquid nitrogen tanks, or by damage from fire, power loss or other natural disasters, we would need to establish replacement master and working cell banks of the engineered K562 cells and peripheral blood mononuclear cells, which would impact clinical supply and delay our clinical trials or treatment of patients. If we are unable to find replacement storage facilities and/or re-manufacture the engineered K562 cells timeously, we may incur significant additional expenses and liability to patients whose treatment is delayed, which would materially and adversely affect our business. If we are unable to source for peripheral blood mononuclear cells which are suitable for manufacturing from healthy donors on a timely basis, we may incur significant additional expenses and liability to patients whose treatment is delayed, which would materially and adversely affect our business. Our K562 Cell License for NK cell expansion also requires us to broadly indemnify our licensor against any loss, damages, costs, expenses, or other claim for compensation in connection with the license or our licensed products under the license.
Delays in obtaining renewal of regulatory approvals for our cGMP Facility and licenses could delay our development plans and thereby limit our ability to generate revenues.
We believe that in-house cGMP manufacturing capability is important to facilitate clinical product supply, lower the risk of manufacturing disruptions and enable more cost-effective manufacturing. We have an existing cGMP Facility in Johor Bahru, Malaysia that will allow us to supply the product candidates needed for our clinical trials. In the event that our product candidates are approved for commercial use, we also plan to build an additional facility for the commercial-scale manufacture of our product candidates in the future. The design, construction, qualification and regulatory approvals for such facilities require substantial capital and technical expertise and any delay would limit our development activities and our opportunities for growth.
Furthermore, our cGMP Facility is subject to ongoing, periodic inspection by the NPRA to ensure compliance with cGMP and other relevant laws and regulations. Any failure to follow and document our adherence to these regulations or other regulatory requirements may lead to significant delays in the availability of products for clinical use or may result in the termination of or a hold on a clinical study. The Malaysian authorities may also make unannounced checks and audits of our cGMP Facility. Failure or omission to comply with relevant laws or regulations could also result in sanctions being imposed on us, including fines, injunctions, civil penalties, a requirement to suspend or put on hold one or more of our clinical trials, failure of regulatory authorities to grant marketing approval of our drug candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of drug candidates, operating restrictions and criminal prosecutions, any of which could materially adversely affect our business, financial condition, results of operations and growth prospects.
We also may encounter problems with regard to the following:
| ● | complying with legislation and regulations governing donor traceability, manufacturing, release of product candidates and other requirements from regulatory authorities outside Malaysia; | |
| ● | achieving adequate or clinical-grade materials that fulfill regulatory agency standards and/or specifications with consistent and acceptable production yield and costs; | |
| ● | bacterial, fungal or viral contamination in our cGMP Facility; and/or | |
| ● | shortage in qualified personnel, raw materials and/or key contractors. |
Our product candidates, if approved by the relevant regulatory authorities, may require significant commercial supply to meet market demand. In these cases, we may need to significantly increase or “scale up”, our production process. If we fail to develop sufficient manufacturing capacity and/or experience, whether internally or with a third party, in time or at all, or fail to manufacture our product candidates economically or at reasonable scale or volume or in accordance with cGMP, or if the cost of the increase or “scale-up” in our production process is not economically feasible, our development programs and commercialization of any approved products will be materially adversely affected and we may not be able to produce a sufficient quantity of our product candidates required to meet future demand and our business, financial condition, results of operations and growth prospects may be materially adversely affected.
| 43 |
The optimal donor and manufacturing parameters for our product candidates may have not been definitively established, which may hinder our ability to optimize our product candidates or to address any safety or efficacy issues that may arise.
If any of our clinical trials reveal issues with the safety or efficacy of any of our product candidates, modification of the donor selection criteria or the manufacturing process may be necessary to address such issues. However, we have not fully characterized or identified how donor characteristics and manufacturing process parameters affect the optimal cancer cell killing ability for our CTM-N2D and CTM-GDT product candidates for in vitro and animal efficacy studies or how such potency differences may translate into efficacy to be seen in human clinical trials, including both the proportion of patients who achieve a meaningful clinical response, and the duration of any such clinical responses. As a result, our ability to improve our manufacturing process or product potency, safety, or efficacy according to such parameters is limited and may require significant trial and error, which may cause us to incur significant costs or could result in significant delays to the clinical development and eventual commercialization of our product candidates.
We have not yet developed a validated methodology of freezing and thawing large quantities of CTM-N2D, iPSC- gdNKT and CTM-GDT product candidates, which may be required for the storage and overseas distribution of our product candidates.
We have not yet developed a validated method of freezing and thawing CTM-N2D, iPSC-gdNKT and CTM-GDT, which can be frozen and thawed in smaller quantities, in large quantities without damage, in a cost-efficient manner and without degradation over time. We may encounter difficulties not only in developing freezing and thawing methodologies for large scale use, but also in obtaining the necessary regulatory approvals for using such methodologies in treatment. If we cannot adequately demonstrate that our frozen product candidates are similar to our product candidates in unfrozen form to the satisfaction of the HSA and/or other regulatory authorities, we could face substantial delays in obtaining regulatory approvals from the HSA and/or other regulatory authorities. If we are unable to develop a validated method of freezing CTM-N2D, iPSC-gdNKT and CTM-GDT for shipping purposes, our ability to promote adoption and standardization of our products candidates, as well as achieve economies of scale by centralizing our production facility, will be limited. Even if we are able to successfully freeze and thaw CTM-N2D, iPSC-gdNKT and CTM-GDT in large quantities, we will also need to develop a cost-effective and reliable distribution and logistics network, which we may be unable to accomplish.
Furthermore, we have not yet demonstrated long-term stability of cryopreserved CTM-N2D, iPSC-gdNKT and CTM-GDT and therefore are not certain if we will be able to store the cryopreserved cells for extended periods of time. If we are unable to demonstrate such long-term stability, we will need to reduce the manufacturing batch size to ensure that the material we produce will be used before it expires. In that case, the scaling of our production processes will not deliver the efficiencies we expect, and the cost per dose of our product candidates will be substantially higher.
Due to various reasons (including the foregoing), we have not yet established the long-term stability of our cryopreserved CTM-N2D, iPSC-gdNKT and CTM-GDT and we may not be able to commercialize CTM-N2D, iPSC-gdNKT and CTM-GDT on a large scale or in a cost-effective manner. If any of our product candidates is found to be unstable, we will have to conduct more frequent manufacturing runs, which could cause us to incur significant additional expenses. Currently, we have identified a potential collaboration partner to develop cryopreservation method for our product candidates.
We believe we may require an updated and validated protocol for commercial-scale expansion and manufacturing of gamma delta T cells for conducting pivotal trials and for commercialization of our product candidates, if approved.
Future clinical trials that we conduct, as well as any potential commercialization of our product candidates when approved, will depend on the reliability, safety and efficacy of our processes for expanding, modifying and manufacturing CTM-N2D, iPSC-gdNKT and CTM-GDT at scale. Our efforts to scale up production of our CTM-N2D, iPSC-gdNKT and CTM-GDT in anticipation of future clinical trials or commercialization may reveal, an inability to overcome biology or may otherwise encounter challenges, including scrutiny from regulatory authorities. To the extent we encounter any such difficulties, our ability to conduct additional clinical trials or to scale for commercialization will be hindered or prevented, which would have an adverse effect on our business. Currently, we are in the midst of identifying potential collaboration partners to develop methods for the scaling up manufacturing of our product candidates.
| 44 |
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure full protection of our proprietary rights. If our patent position does not adequately protect our product candidates, others could compete against us more directly, which would harm our business, possibly materially.
Our commercial success will depend in part on obtaining and maintaining proprietary rights including patent protection and trade secret protection of our current product candidates and future product candidates, the processes used to manufacture them and the methods for using them, as well as successfully asserting and defending these proprietary rights against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell or importing our product candidates is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
The patent positions of biotechnology and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in pharmaceutical patents has emerged to date in the U.S. or in foreign jurisdictions outside of the U.S., including Singapore. Changes in either the patent laws or interpretations of patent laws in the U.S. and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be enforced in the patents that may be issued from the applications we currently licensed or may in the future own or license from third parties. Further, if any patents we obtain or license are deemed invalid and unenforceable, our ability to commercialize or license our product candidates or technology could be adversely affected.
Others may file patent applications covering products and technologies that are similar, identical, or competitive to ours or important to our business. We cannot be certain that any patent application owned by a third party will not have priority over patent applications filed or in-licensed by us, or that we or our licensors will not be involved in patent office proceedings such as interference, derivation, opposition, reexamination, inter partes review, reissue, post grant review or invalidity proceedings before U.S. or non-U.S. patent offices. Such proceedings are also expensive and time consuming.
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| ● | others may be able to make products that are similar to our product candidates, but that are not covered by the claims of our patents or our licensed patents; | |
| ● | any patents that we obtain from licensing or otherwise may not provide us with any competitive advantages; | |
| ● | any granted patents that we rely upon may be held invalid or unenforceable as a result of legal challenges by third parties; and | |
| ● | the patents of others may have an adverse effect on our business. |
We are dependent on licensed intellectual property. If we were to lose our rights to licensed intellectual property, we may not be able to continue developing or commercializing our product candidates, if approved. If we breach any of the agreements under which we license the use, development and commercialization rights to our product candidates or technology from third parties or, in certain cases, we fail to meet certain development deadlines, we could lose license rights that are important to our business.
We do not currently own any patents, and we are heavily reliant upon a number of license agreements under which we are granted rights to intellectual property that are important to our business and we may need or choose to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose on us, various development, regulatory and/or commercial diligence obligations, payment of milestones and/or royalties and other obligations. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the licenses, in which event we would not be able to market products covered by the licenses. Our business could suffer, for example, if any current or future licenses terminate, if the licensors fail to abide by the terms of the licenses, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including:
| ● | the scope of rights granted under the license agreements and other interpretation-related issues; | |
| ● | whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |
| ● | our right to sublicense patent and other rights to third parties; | |
| ● | our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations; | |
| ● | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; | |
| ● | our right to transfer or assign the license; and | |
| ● | the effects of termination. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
| 45 |
We have entered into several licenses to support our various projects. Termination of any of these license agreements would have a material adverse impact on our ability to develop and commercialize derived products under each respective agreement.
We have entered into several licenses to support our various projects. We may enter into additional licenses to third-party intellectual property that are necessary or useful to our business. Our current licenses and any future licenses that we may enter into impose various royalty payment, milestone, and other obligations on us. Under some license agreements, we may not control prosecution of the licensed intellectual property, or may not have the first right to enforce the intellectual property. In those cases, we may not be able to adequately influence patent prosecution or enforcement, or prevent inadvertent lapses of coverage due to failure to pay maintenance fees. If we fail to comply with any of our obligations under a current or future license agreement, the licensor may allege that we have breached our license agreement, and may accordingly seek to terminate our license. Termination of any of our current or future licenses could result in our loss of the right to use the licensed intellectual property, which could materially adversely affect our ability to develop and commercialize a product candidate or product, if approved, as well as harm our competitive business position and our business prospects. Under some license agreements, termination may also result in the transfer of or granting in rights under certain of our intellectual property and information related to the product candidate being developed under the license, such as regulatory information.
The agreements under which we have licensed intellectual property or technology or may license intellectual property or technology in the future to or from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we have licensed or may license prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
In addition, if our licensor fails to abide by the terms of any of the licenses, if the licensor fails to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms, our business could suffer. Moreover, our licensor may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing, misappropriating or otherwise violating the licensor’s rights.
Similarly, if we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may have to seek alternative options, such as developing new product candidates with design-around technologies, which may require more time and investment, or abandon development of the relevant research programs or product candidates and our business, financial condition, results of operations and prospects could suffer.
Some of the intellectual property covered by our licenses concern patent applications. We cannot assure investors that any of the currently pending or future patent applications will result in granted patents, nor can we predict how long it will take for such patents to be granted.
Some of the intellectual property covered by our current licenses from the licensor concern certain, specified patent rights (including patent applications and Patent Cooperation Treaty patent applications). While to some extent and for at least a certain period of time, the licensor has agreed to assume responsibility for the preparation, filing, prosecution and maintenance of patent applications covered by the licensed patent rights, we cannot be certain as to when or if final patents will be issued for those patent applications covered by the licensed patent rights. However, the licensor may not successfully prosecute certain patent applications, the prosecution of which the licensor controls, under which we are only a licensee and on which our business substantially depends. Even if patents issue from these applications, there is no assurance that the patents will be free from defects or survive validity or enforceability challenges, the licensors may fail to maintain these patents, may decide not to pursue litigation against third-party infringers, may fail to prove infringement or may fail to defend against counterclaims of patent invalidity or unenforceability.
Moreover, it is possible that the licensed pending patent applications will not result in granted patents, and even if such pending patent applications are granted as patents, they may not provide a basis for intellectual property protection of commercially viable products or may not provide us with any competitive advantages. Further, it is possible that, for any of the patents that may be granted in the future, others will design around the licensed patent rights or identify methods for preventing or treating diseases that do not concern the rights covered by our licenses. Further, we cannot assure investors that other parties will not challenge any patents granted to the licensor or that courts or regulatory agencies will hold licensor’s patents to be valid or enforceable. We cannot guarantee investors that, if required to defend the covered patents, we will have the funds to or be successful in defending challenges made against the licensed patents. Any successful third-party challenge to the licensed patents could result in the unenforceability or invalidity of such patents, or to such patents being interpreted narrowly or otherwise in a manner adverse to our interests. Our ability to establish or maintain a technological or competitive advantage over our competitors may be diminished because of these uncertainties.
Even if patents are issued based on patent applications to which we have been granted a license, because the patent positions of pharmaceutical and biotechnology products are complex and uncertain, we cannot predict the scope and extent of patent protection for our product candidates.
Any patents that may be issued based on patent applications that we have been granted licenses to will not ensure sufficient protection with respect to our activities for a number of reasons, including without limitation the following:
| ● | any issued patents may not be broad or strong enough to prevent competition from other identical or similar products; | |
| ● | if patents are not issued or if issued patents expire, there would be no protections against competitors making generic equivalents; | |
| ● | there may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim; | |
| ● | there may be other patents existing, now or in the future, in the patent landscape for our product candidates that we seek to commercialize or develop, if any, that will affect our freedom to operate; | |
| ● | if patents that we have been granted licenses to are challenged, a court could determine that they are not valid or enforceable; | |
| ● | a court could determine that a competitor’s technology or product does not infringe patents that we have been granted licenses to; | |
| ● | patents to which we have been granted licenses could irretrievably lapse due to failure to pay fees or otherwise comply with regulations, or could be subject to compulsory licensing; and | |
| ● | if we encounter delays in our development or clinical trials, the period of time during which we could market our products under patent protection would be reduced. |
| 46 |
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the United States Patent and Trademark Office (USPTO) and foreign Intellectual Property Offices in several stages over the term of the patent. Maintenance fees are also due for pending patent applications in some countries. The USPTO and various foreign intellectual property offices require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to office actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
The life of patent protection is limited, and third parties could develop and commercialize products and technologies similar or identical to ours and compete directly with us after a patent licensed to us expires, which could materially and adversely affect our ability to commercialize our products and technologies.
The life of a patent and the protection it affords is limited. For example, in the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. In Europe, the expiration of an invention patent is 20 years from its filing date. Even if we successfully obtain patent protection for an approved product candidate, it may face competition from biosimilar medications. Manufacturers of other drugs may challenge the scope, validity or enforceability of the patents underlying our technology in court or before a patent office, and the patent holder may not be successful in enforcing or defending those intellectual property rights and, as a result, we may not be able to develop or market the relevant product candidate exclusively, which would materially adversely affect any potential sales of that product.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, the patent pending applications licensed to us may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Even if we believe that the patents involved are eligible for certain (and time-limited) patent term extensions, there can be no assurance that the applicable authorities, including the FDA and the USPTO, and any equivalent regulatory authority in other countries, will agree with our assessment of whether such extensions are available, and such authorities may refuse to grant extensions to such patents, or may grant more limited extensions than requested. For example, depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, one or more of the U.S. patents licensed to us may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Action of 1984, or Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. However, we may not be granted an extension or may be granted a shorter extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patent, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than requested. If we are unable to obtain patent term extension or term of any such extension is less than requested, our competitors may obtain approval of competing products following our patent expiration, and our business could be harmed. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The patent pending applications licensed to us for our product candidates are expected to expire on various dates. Upon the expiration, we or our licensor will not be able to assert such licensed patent rights against potential competitors, which would materially adversely affect our business, financial condition, results of operations and prospects.
We may need to license intellectual property from third parties, and such licenses may not be available on commercially reasonable terms or may not be available at all.
There may be intellectual property rights existing now, or in the future, relevant to our product candidates that we seek to commercialize or develop, if any, that may affect our ability to commercialize such product candidates. Although the Company is not aware of any such intellectual property rights, a third party may hold intellectual property rights, including patent rights, which are important or necessary to the development or manufacture of our product candidates. Even if all our main product candidates are covered by patents, it may be necessary for us to use the patented or proprietary technology of third parties to commercialize our product candidates, in which case we would be required to obtain licenses from these third parties. Such licenses may not be available on commercially reasonable terms, or at all, and we could be forced to accept unfavorable contractual terms. In that event, we may be required to expend significant time and resources to redesign our technology, product candidates, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, our business could be harmed.
The licensing or acquisition of third-party intellectual property rights is a competitive area, and several more established companies may pursue strategies to license or acquire third party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license intellectual property rights to us. We also may be unable to license or acquire third party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. If we are unable to successfully obtain rights to required third party intellectual property rights or maintain the existing intellectual property rights we have, we may have to abandon development of the relevant project or product candidate, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
| 47 |
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our product candidates.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. We are not aware of any third-party proprietary rights that our planned products will infringe or misappropriate, but we have not conducted any freedom to operate study as we are in the earliest stages of development. We thus cannot guarantee that our product candidates, or manufacture or use of our product candidates, will not infringe third-party patents. Furthermore, a third party may claim that we are using technologies covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and scientific personnel. Some of these third parties may be better capitalized and have more resources than us. There is a risk that a court would decide that we are infringing the third party’s patents and would order us to stop the activities covered by the patents. In that event, we may not have a viable way around the patent and may need to halt commercialization of our product candidates. In addition, there is a risk that a court will order us to pay the other party damages for having violated the other party’s patents. In addition, we may be obligated to indemnify our licensors and collaborators against certain intellectual property infringement claims brought by third parties, which could require us to expend additional resources. The pharmaceutical and biotechnology industries have produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform.
If we are sued for patent infringement, we would need to demonstrate that our product candidates or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving invalidity is difficult. For example, in the U.S., proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and diversion of management’s time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, which may not be available, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may incur substantial monetary damages, encounter significant delays in bringing our product candidates to market and be precluded from manufacturing or selling our product candidates.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than us or the third parties from whom we license intellectual property because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
In addition to the possibility of litigation relating to infringement claims asserted against us, we may become a party to other patent litigation and other proceedings, including inter partes review proceedings, post grant review proceedings, derivation proceedings declared by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future technologies or product candidates or products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace.
Competitors may infringe or otherwise violate our intellectual property, including patents that may issue to or be licensed by us. As a result, we may be required to file claims in an effort to stop third-party infringement or unauthorized use. Any such claims could provoke these parties to assert counterclaims against us, including claims alleging that we infringe their patents or other intellectual property rights, and/or that any of our intellectual property, including licensed intellectual property, is invalid and/or unenforceable. This can be prohibitively expensive, particularly for a company of our size, and time-consuming, and even if we are successful, any award of monetary damages or other remedy we may receive may not be commercially valuable. In addition, in an infringement proceeding, a court may decide that our asserted intellectual property is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our intellectual property does not cover the third party’s technology. An adverse determination in any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing as patents.
If the breadth or strength of our patent or other intellectual property rights is compromised or threatened, it could allow third parties to exploit and, in particular, commercialize our technology or products or result in our inability to exploit and/or commercialize our technology and products without infringing third-party intellectual property rights. Further, third parties may be dissuaded from collaborating with us.
Interference or derivation proceedings brought by the USPTO or its foreign counterparts may be necessary to determine the priority or inventorship of inventions with respect to our licensed patents or patent applications, and we may also become involved in other proceedings, such as re-examination proceedings, before the USPTO or its foreign counterparts. Due to the substantial competition in the pharmaceutical space, the number of such proceedings may increase. This could delay the prosecution of our pending patent applications or impact the validity and enforceability of any future patents that we may obtain. In addition, any such litigation, submission or proceeding may be resolved adversely to us and, even if successful, may result in substantial costs and distraction to our management.
| 48 |
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and product could be significantly diminished.
We also rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its transparency initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may be subject to claims that our employees or consultants have wrongfully used or disclosed alleged trade secrets.
As is common in the biotechnology and pharmaceutical industries, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees or consultants have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we could lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Our intellectual property may not be sufficient to protect our product candidates from competition, which may negatively affect our business as well as limit our partnership or acquisition appeal.
We may be subject to competition despite the existence of intellectual property we license or may in the future own. We can give no assurances that our intellectual property rights will be sufficient to prevent third parties from designing around patents we own or license and developing and commercializing competitive products. The existence of competitive products that avoid our intellectual property could materially adversely affect our operating results and financial condition. Furthermore, limitations, or perceived limitations, in our intellectual property may limit the interest of third parties to partner, collaborate or otherwise transact with us, if third parties perceive a higher than acceptable risk to commercialization of our product candidates or future product candidates.
We may elect to sue a third party, or otherwise make a claim, alleging infringement or other violation of patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights that we either own or license from a third party. If we do not prevail in enforcing our intellectual property rights in this type of litigation, we may be subject to:
| ● | paying monetary damages related to the legal expenses of the third party; | |
| ● | facing additional competition that may have a significant adverse effect on our product pricing, market share, business operations, financial condition, and the commercial viability of our product; and | |
| ● | restructuring our company or delaying or terminating select business opportunities, including, but not limited to, R&D, clinical trial, and commercialization activities, due to a potential deterioration of our financial condition or market competitiveness. |
A third party may also challenge the validity, enforceability or scope of the intellectual property rights that we license or own and the result of these challenges may narrow the scope or claims of or invalidate patents that are integral to our product candidates in the future. There can be no assurance that we will be able to successfully defend patents we own or license in an action against third parties due to the unpredictability of litigation and the high costs associated with intellectual property litigation, amongst other factors.
Intellectual property rights may be less extensive and enforcement may be more difficult in jurisdictions outside of the U.S. Therefore, we may not be able to protect our intellectual property and third parties may be able to market competitive products that may use some or all of our intellectual property.
Changes to patent law, including the Leahy-Smith America Invests Act of 2011 and other future article of legislation, may substantially change the regulations and procedures surrounding patent applications, issuance of patents and prosecution of patents. We can give no assurances that the patents of ours or our licensor’s can be defended or will protect us against future intellectual property challenges, particularly as they pertain to changes in patent law and future patent law interpretations
| 49 |
If any of our license agreements with ATPL is terminated, we could lose our rights to key components enabling our core proprietary CAR gamma delta T cell technology platform.
We are highly reliant on three licenses granted to us pursuant to the Patent License, K562 Cell License for NK cell expansion, and Know-How License by ATPL, a wholly-owned subsidiary of A*STAR and the leading R&D agency in Singapore. A*STAR is the leading body for biomedical research initiatives among the research community in Singapore. Please refer to the section entitled “Intellectual Property” for further information on these licenses. Our lead product candidates, CTM-N2D, iPSC-gdNKT and CTM-GDT, are derived from the technology we licensed from ATPL. If any of these three license agreements is terminated for any reason, we would lose the rights to use the licensed intellectual property rights that may be material or necessary to the development and/or production of our product candidates, which could impede or prevent our successful commercialization of such product candidates and materially adversely affect our business, financial condition, results of operations and growth prospects.
Furthermore, our Patent License is field-specific and has been granted to us in the field of immunotherapy, including stem cell therapy and treatment and immunotherapy drugs using any licensed patents that issue from and claim priority with and from the two Patent Cooperation Treaty applications in the Patent License.
If the licenses granted to us by ATPL are affected in any manner, it could delay our development and commercialization of our product candidates, which in turn could materially adversely affect our business, financial condition, results of operations and growth prospects.
Our development and commercialization rights to our current and future product candidates and technology are subject, in part, to the terms and conditions of patent licenses granted to us by others.
We do not have the right to control the preparation, filing, prosecution, maintenance, enforcement and defense of patents and patent applications in respect of the technology(ies) that we license from ATPL. ATPL is responsible for patent prosecution and maintenance costs in a limited number of countries and territories. Therefore, we cannot guarantee that these patent applications and patents issued therefrom will be prepared, filed, prosecuted, maintained, enforced and defended in a manner consistent with our best interests. If ATPL fails to prosecute, maintain, enforce and defend any of such patent applications or patents, or lose rights to any of those patents or patent applications, the patent rights we have licensed may be reduced or eliminated, and our right to develop and commercialize any of our products that are the subject of such licensed rights could be impaired. Additionally, we may be required to reimburse ATPL for their expenses related to the prosecution, maintenance, enforcement and defense of patents and patent applications that we license from them.
We may not be able to meet our commercialization milestones in our patent license agreements with ATPL, and if our license agreement is terminated as a result, our clinical trials and business will be significantly affected.
Any breach or non-compliance of any term in any of our patent license agreements with ATPL (such as our failure to meet any of the commercialization milestones relating to different phases of clinical trials or otherwise) may result in termination. Failure by us to renew or otherwise maintain the required patent licenses, or cancellation, suspension or revocation of any of our patent licenses pursuant to our patent license agreements with ATPL may result in the interruption of our clinical trials and operations if we are not able to use the patent licenses and this could potentially affect our working relationship with ATPL and/or A*STAR, which would have a materially adverse effect on our business, operations and future prospects. We may not be able to identify another licensor able and willing to license such technology(ies) to us, and we may be required to recreate our product candidates altogether which would significantly increase our costs and expenses required to operate. This would also severely delay any potential commercialization of our products and we may not be able to create a new product candidate suitable for pre-clinical or clinical trials or even commercialization.
We have entered into three addenda for our Patent License that extends the deadlines to meet the commercialization milestones. Failure to meet these extended milestone deadlines may result in termination of the license. Additionally, in the second addendum CytoMed agreed to take over responsibility for all patent prosecution maintenance costs beginning January 2022.
| 50 |
Any patents that we have licensed from ATPL will expire, and when they expire, our business may be affected.
Our development of our product candidates is reliant on some of the technologies licensed to us pursuant to the patent licenses from ATPL as described in the section entitled “Intellectual Property”. When the patents licensed from ATPL, assuming they are issued from the pending patent applications reach the end of their lives, we may not be able to continue to preclude others from making, using or selling, any of our product candidates that are approved.
We may not be able to obtain and enforce our intellectual property rights throughout the world.
Filing, prosecuting, enforcing and defending the patents rights relating to our product candidates which have been licensed to us in every country in the world would likely be prohibitively expensive. ATPL is responsible for patent prosecution and maintenance costs in a limited number of countries and territories, and if we want to pursue protection in countries other than those for which ATPL is obligated, we will be responsible for the prosecution and maintenance costs. The extent of patent protection can vary in different countries. In addition, we have entered into three addenda for our Patent License that extends the deadlines to meet the commercialization milestones. Failure to meet these extended milestone deadlines may result in termination of the Patent License. Additionally, in the second addendum CytoMed agreed to take over responsibility for all patent prosecution and maintenance costs beginning January 2022.
The requirements for patentability may also differ in certain countries, particularly in developing countries; thus, even in countries where we do pursue patent protection, there can be no assurance that any patents will be issued by the relevant authorities in relation to our product candidates. Moreover, our ability to protect and enforce our intellectual property rights may be adversely affected by developments in international and foreign intellectual property legislation, regulations and policies.
Additionally, the laws of certain countries may not afford the same level of intellectual property protection as the laws of Singapore or other countries. Many companies have encountered significant problems in protecting and defending their intellectual property rights in certain foreign jurisdictions. It may be more difficult to enforce our patents license rights and other intellectual property rights in the legal systems of some countries, including India and China. This could make it difficult for us to obtain an injunction to stop third party infringement and/or misappropriation of our patents and/or our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. Consequently, we may not be able to prevent third parties from utilizing our technologies in certain countries outside Singapore or from selling or importing products made from our inventions in and into Singapore and/or other jurisdictions.
It is possible that competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop and market their own products and export such products to jurisdictions where we have patent protection, if our patent protection against infringement is not sufficiently robust. These products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing with us. Further, our Patent License gives ATPL the right but not the obligation to prosecute and defend any and all infringements of any licensed patent that may issue. We are obligated to obtain ATPL’s consent to pursue enforcement of our licensed patents or defend any claims asserting the invalidity of these patents (or control of such enforcement or defense) of such patent rights in all relevant jurisdictions. Proceedings to enforce our patent rights, whether or not successful, could result in substantial costs and divert our efforts and resources from other aspects of our business.
Moreover, such proceedings could put any patents at issue in such proceedings at risk of being revoked, invalidated or interpreted narrowly and our licensed patent applications at risk of not being issued and could provoke third parties to assert claims against us. We may not be successful in any lawsuits or other patent office proceedings involving the licenses granted to us and the damages or other remedies awarded, if any, may not be sufficient to compensate us for our losses and expenses and/or otherwise be commercially meaningful. Furthermore, while we intend to seek to protect the patent rights under the license agreements granted to us and if necessary, enforce rights of any patents that issue, in major markets for our products, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our products, if approved. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
| 51 |
If our patent protection is not sufficiently robust, our competitors could develop and commercialize products and technology similar or identical to ours.
There is intense competition in the market for cell therapy, and the market is also subject to rapid technological change. Our business depends largely on our ability to maintain a competitive edge in the development and protection of technologies and products for use in these fields.
There have been several complex legal and factual questions relating to patents of biopharmaceutical companies which has, in recent years, been the subject of much litigation. As a result, there are considerable uncertainties relating to the issuance, scope, validity, enforceability and commercial value of our licensed patent rights. Our pending and future patent applications may not result in patents being issued that protect our technology or product candidates or effectively prevent others from commercializing similar or identical technologies and product candidates.
The process to seek and obtain patent protection is costly, time-consuming and complex, and we may not be able to file, prosecute, obtain, maintain, enforce or license all required or desirable patents or patent applications at a reasonable cost or in a timely manner. We may also fail to identify patentable aspects of our R&D output before it is too late to obtain patent protection.
There may be changes in the scope of patent claims before a patent is issued, and after issuance of the patent. Even if the patent applications we license issue as patents, the patents may not be in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us or otherwise provide us with any competitive advantage. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative products in a manner which does not infringe on our patents.
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of third-party patents, which could materially and adversely affect our ability to develop, manufacture and market our product candidates.
There are many patents issued or applied for in the biopharmaceutical industry, and we may not be aware of patents or patent applications held by others which are relevant to our business and our product candidates.
Furthermore, after issuance of a patent, the scope of patent claims depends on an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be inaccurate, and we may incorrectly determine that our product candidates are not covered by a third-party patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in any relevant jurisdiction that we consider relevant may also be incorrect. If we fail to correctly identify or interpret the scope of third-party patents, we may be subject to infringement claims. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims and/or lawsuits. If we fail in any such dispute, in addition to being forced to pay monetary damages, we may be temporarily or permanently prohibited from commercializing our product candidates. We may also be forced to attempt to redesign our product candidates in a manner that no longer infringes third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to the development and commercialization of our product candidates.
| 52 |
We may face claims for infringing, misappropriating or otherwise violating intellectual property rights of third parties or engaging in unfair competition, which would be expensive and time-consuming, and could hinder the successful development and/or commercialization our product candidates.
The success of our business is affected by our ability to develop, manufacture and market our technology and use our technology without infringing the proprietary rights of third parties. As the relevant pharmaceutical and biotechnology industries expand and more patents are issued, we face greater risks of patents being issued to third parties that relate to our product candidates and technology of which we are not aware or that we may need to circumvent or overcome to continue our business operations as currently contemplated. As a result, our technology and any future product candidates that we commercialize could be alleged to infringe patent rights and other proprietary rights of third parties, which may require costly litigation and, if we are not successful, could require us to pay substantial damages and/or limit our ability to commercialize our product candidates.
We may have to defend ourselves against litigation claims in which the scope, enforceability and validity of third-party proprietary rights are at issue, or to establish our proprietary rights. Regardless of whether any such claims that we are infringing patents or other intellectual property rights have merit, such claims can be time consuming, divert management attention and financial resources and are costly to evaluate and defend.
If a license is available from a third party who contends any product candidates for which we obtain approval infringe or misappropriate third party intellectual property rights, we may have to pay substantial license fees, annual minimum fees, royalties, upfront fees, or milestone fees, or grant cross-licenses to our intellectual property rights. We may also have to modify our product candidates so they do not infringe third-party intellectual property rights, which may not be possible or may require additional regulatory approvals, or substantial monetary expenditures and time, during which our product candidates may not be available for manufacture, use, or sale.
We may fail to identify unauthorized use of our intellectual property and enforce our intellectual property rights against infringement, and may incur substantial costs as a result of bringing litigation or other proceedings to protect our intellectual property rights.
Identifying unauthorized use of our intellectual property is challenging and expensive. From time to time, we will review products from our competitors to determine potential infringement of our rights if any. We may not be able to identify unauthorized use of, or take appropriate steps to enforce, our intellectual property rights. Any failure by us to identify any unauthorized use of our intellectual property rights by third parties could result in competitors offering products that incorporate our product features, which could in turn reduce demand for our products.
We may also, from time to time, seek to enforce our intellectual property rights against infringers when we determine that a successful outcome is probable and may result in an increase in the value of our intellectual property.
If we or our licensor choose to enforce our licensed patent rights against a party, we or our licensor may face a counterclaim that our patent is invalid and/or unenforceable. Our patents may be challenged in legal proceedings. Proceedings to challenge patents are also available internationally, including, for example, opposition proceedings and nullity actions. In general, grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion may include an allegation that someone connected with the prosecution of the patent withheld relevant information, or made a misleading statement, during prosecution. Third parties may also raise similar claims, even outside the context of litigation. We are unable to predict the outcome of litigation suits. With respect to the validity question, for example, we cannot guarantee that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we may lose at least part, and perhaps all, of the patent protection on our product candidates.
In addition, such lawsuits and proceedings are costly and would take up considerable time and resources and divert the attention of managerial and scientific personnel from our business operations even if we were successful in preventing the infringement of such patents. Litigation is inherently unpredictable, and there is a risk that the court will decide that such patents are not valid and that we do not have the right to stop the other party from using the technologies. Furthermore, some of our competitors have significantly greater resources and thus may be able to sustain the costs of complex patent litigation more effectively than us. There is also the risk that, even if the validity of such patents is upheld, the court will refuse to grant an injunction against the other party on the ground that there is no infringement of our intellectual property rights.
| 53 |
There could also be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results negatively on us, it could materially adversely affect the price of our ordinary shares. Additionally, any uncertainties resulting from the initiation and continuation of any litigation could materially and adversely affect our ability to raise the funds necessary to continue our operations.
Developments in U.S. patent law or the patent law of other jurisdictions present uncertainties in our ability to obtain patents and diminish the value of patents in general, thereby affecting our ability to protect our current and any future product candidates.
The U.S. Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. For example, in recent years, the U.S. Supreme Court has modified certain tests used by the USPTO in awarding patents, which may reduce our chances of obtaining the requisite patents and increase the likelihood of a challenge of any patents we obtain or license. Similarly, there have been international developments in how the patent laws in their respective jurisdictions are interpreted. Those changes, as well as any future changes, may materially and adversely affect our patent rights and our ability to obtain issued patents.
Developments in either the patent laws or interpretation of the patent laws in the United States could result in greater uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Under the Leahy-Smith America Invents Act, or the America Invents Act, assuming that all other conditions for patentability are satisfied, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention.
The America Invents Act also includes a number of significant changes that affect the prosecution of patent applications and patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO-administered post-grant proceedings, including post-grant review, inter partes review and derivation proceedings. The implementation of the America Invents Act could result in greater uncertainties and expenses relating to the prosecution of our patent applications and the enforcement or defense of patents issued therefrom, all of which could materially and adversely affect our business, financial condition, results of operations and growth prospects.
Further, the U.S. Supreme Court has made judgments on several patent cases in recent years, with a trend towards either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. This results in greater uncertainty with respect to the value of patents, if and once obtained. Depending on actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce patents. Similarly, developments in patent law and regulations in other countries or jurisdictions, changes in the enforcement authorities or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our ability to obtain new patents or to enforce patents, which in turn could materially adversely affect our business, financial condition, results of operations and growth prospects.
We may fail to obtain or enforce assignments of intellectual property rights from our employees and contractors.
Although we generally require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning all such intellectual property to us, we may fail to obtain an enforceable agreement with each party who in fact conceives or develops intellectual property that we regard as our own. Furthermore, our assignment agreements may be breached, and we may be forced to bring or defend claims to determine the ownership of what we regard as our intellectual property, and we may not be successful in such claims. If we fail in any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights which could materially and adversely affect our business, financial condition, results of operations and growth prospects. Even if we are successful in defending against such claims, litigation could result in substantial costs and diversion of management’s and other employees’ attention from our business operations.
| 54 |
If we fail to prevent disclosure of trade secrets and other proprietary information, the value of our technology and products could be materially diminished.
We may face difficulties in protecting our trade secrets from being disclosed or shared with third parties. We rely on trade secrets to protect our proprietary information and technologies, especially where we do not believe patent protection is appropriate or obtainable, or where the enforcement of such patents would be challenging. We rely in part on confidentiality agreements with our employees, consultants, contractors, outside scientific collaborators and other advisors to protect our trade secrets and other proprietary information. We cannot guarantee that we have entered into such agreements with each party that may have had access to our proprietary information or technologies, or that such agreements, even if in place, will not be circumvented. These agreements may not effectively prevent disclosure of proprietary information or technology and may not provide an adequate remedy in the event of unauthorized disclosure of such information or technology. In addition, others may independently discover our trade secrets and proprietary information, in which case we may have no right to prevent them from using such trade secrets or proprietary information to compete with us. We may need to engage in expensive and time-consuming litigation to enforce and determine the scope of our proprietary rights. Our failure to obtain or maintain trade secret protection could materially and adversely affect our business, financial condition, results of operations and growth prospects.
RISKS RELATED TO COMMERCIALIZATION
We have no experience as a company in obtaining regulatory approval for a drug.
As a company, we have not yet obtained regulatory approval for, or commercialized, any drug as of the date of this Prospectus. It is possible that the HSA and/or other relevant health and regulatory authorities may refuse to accept any or all of our planned NDAs for substantive review or may conclude after review of our data that our application is insufficient to obtain regulatory approval for any current or future product candidates. If the HSA and/or other relevant health and regulatory authorities does not approve any of our planned NDAs, we may need to conduct additional costly clinical, non-clinical or manufacturing validation studies before the HSA will reconsider our application(s). Depending on the extent of these or any other HSA and/or other relevant health and regulatory authorities-required studies, approval of any NDA or other application that we submit may be significantly delayed, possibly for several years, or may require us to expend more resources than we have available. Any failure or delay in obtaining regulatory approvals would prevent us from commercializing any of our product candidate, generating revenues and achieving and sustaining profitability. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the HSA and/or other relevant health and regulatory authorities to approve any NDA or other application that we submit. If any of these outcomes occur, we may be forced to abandon the development of our product candidates, which would materially adversely affect our business and could potentially cause us to cease operations. We may face similar risks for our applications in foreign jurisdictions.
If any of our product candidates are approved for marketing and commercialization and we have not developed or secured third-party marketing, sales and distribution capabilities, we will be unable to successfully commercialize such products and may not be able to generate product revenue.
We currently do not have any sales, marketing or distribution organizational experience or capabilities. We will need to develop internal sales, marketing and distribution capabilities to commercialize any product candidate to obtain regulatory authority approval, which would be costly and time-consuming, or enter into partnerships with third parties to achieve the same. If we decide to directly market any approved products, we will have to invest substantial financial and managerial resources in a marketing and sales team with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties to market products or decide to co-promote products with partners, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we can enter into such arrangements on acceptable terms or at all. In entering into third-party marketing or distribution arrangements, any product revenue is contingent on the efforts of the third parties and we cannot guarantee that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for any approved product. If we fail to commercialize any product approved in the future, if any, either on our own or through third parties, our business, financial condition, results of operations and growth prospects could be materially adversely affected.
| 55 |
If we fail to establish biopharmaceutical collaborations on commercially reasonable terms, or at all, we may have to change our development and commercialization plans.
The advancement of our product candidates and development programs and the potential commercialization of our current and future product candidates will require substantial additional financing. For some of our programs, we may partner with pharmaceutical and biotechnology companies to develop and commercialize such product candidates. Any such collaborations may incur non-recurring and other charges, increase our near and long-term expenditures, require us to issue securities that dilute our existing shareholders, or disrupt our management and business.
We face intense competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Any definitive agreement for other partnerships is contingent, among other things, on our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors, including the design or results of clinical trials, the progress of our clinical trials, the likelihood of approval by the relevant regulatory authorities, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and clinical trial industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar clinical trial indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidates. Further, we may fail to enter into a strategic partnership or other alternative arrangements for future product candidates because they may be deemed to be premature for collaborations and third parties may not be satisfied with the level of safety and efficacy of our product candidates. Any delays in entering into new collaborations or strategic partnership agreements related to any product candidate we develop could hinder the development and commercialization of our product candidates, which would materially and adversely affect our business prospects, financial condition, and results of operations.
We have entered enter into collaborations with third parties to develop or commercialize our product candidates, our prospects with respect to those product candidates will depend significantly on the success of those collaborations.
The risks of our present and future collaboration with third parties to develop and/or commercialize our product candidates include without limitation:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; | |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; | |
| ● | our agreements with collaborators include agreements that may not provide us with sole ownership of all intellectual property rights resulting from the collaborations and do not address all issues that may arise from joint ownership; | |
| ● | current and future collaboration agreements may not provide us with sufficient ownership or control of all intellectual property rights; | |
| ● | collaborators may not properly enforce, maintain or defend our intellectual property rights or may use our proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation, or other intellectual property proceedings; | |
| ● | disputes may arise between a collaborator and us that cause the delay or termination of the research, development or commercialization of the product candidates, or that result in costly litigation or arbitration that diverts management attention and resources; | |
| ● | if a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished or terminated; | |
| ● | collaboration agreements may restrict our right to pursue new product candidates; and | |
| ● | we may become involved in disputes over the terms of our collaboration agreements and intellectual property rights generated from our collaborations. |
| 56 |
In the event of disagreements and/or conflicts between our collaborators and us, our collaborators may act in a hostile and/or vexatious manner which could hinder the implementation of our strategies. Future collaborators may develop, either alone or with others, products in related fields that are competitive with our products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or to which the collaborators have rights, may result in the withdrawal of support for our product candidates. Non-competition clauses in our agreements with our collaborators may prevent us from entering into collaborations with their competitors and/or being able to obtain timely regulatory approvals. Our collaborators may also terminate their agreements with us prematurely and/or fail to devote sufficient resources to the development and commercialization of products. Any of these events could hinder and severely undermine our product development efforts.
As a result, if we enter into collaboration agreements and strategic partnerships or out-license our intellectual property, products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will be able to achieve a level of revenue or specific net income that justifies such transaction.
Our product candidates could be subject to regulatory limitations following approval, if such approval is granted.
Subsequent to obtaining approval of a product candidate, if any, we must adhere to all applicable government legislation and regulations regarding the manufacture, labeling, marketing, distribution and promotion of biologic products, which are subject to change from time to time. We must adhere to labeling protocols of HSA and/or other relevant regulatory authorities, which prohibit promoting “off-label uses”. We may not be able to obtain the labeling claims necessary or desirable to successfully commercialize our products, including CTM-N2D, iPSC-gdNKT, CTM-GDT or other product candidates in development from time to time.
The HSA and/or other relevant regulatory authorities could impose substantial restrictions on the use of an approved product including restricting its use to limited clinical centers as well as through the product label, as well as on advertising, promotional and distribution activities associated with such approved product. The HSA and/or other relevant regulatory authorities could also condition their approval on the performance of post-approval clinical trials, patient monitoring or testing, which could be time-consuming and expensive. If the results of such post-marketing trials are not satisfactory, the HSA and/or other relevant regulatory authorities could withdraw marketing authorization or may impose conditions on continued marketing and/or impose or require further commitments from us or our partners that may be expensive and/or time-consuming to fulfill.
In addition, if side effects are identified after any of our products are on the market, if manufacturing problems occur subsequent to regulatory approval, or if we, our manufacturers or our partners fail to comply with regulatory requirements, including those mentioned above, we or our partners could be subject to the following:
| ● | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned clinical trials; | |
| ● | restrictions on such products’ manufacturing processes; | |
| ● | changes to the product label; | |
| ● | restrictions on the marketing of a product; | |
| ● | restrictions on product distribution; | |
| ● | requirements to conduct post-marketing clinical trials; | |
| ● | warnings from the regulatory authority; | |
| ● | withdrawal of the product from the market; | |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; | |
| ● | recall of products; | |
| ● | fines, restitution or disgorgement of profits or revenue; | |
| ● | suspension or withdrawal of regulatory approvals; | |
| ● | refusal to permit the import or export of our products; | |
| ● | product seizure; | |
| 57 |
| ● | injunctions; and/or | |
| ● | imposition of civil or criminal penalties. |
Any one or a combination of the above could obstruct market acceptance of the affected product, or could substantially increase the costs and expenses of commercializing such product, which in turn could delay or prevent us from generating any revenue or profit from the sale of such product and could materially and adversely affect our business, financial condition, results of operations and growth prospects.
A summary of the material regulations in Singapore and Malaysia applicable to our operations to our knowledge are set out in the section titled “Regulation”. However, as the biopharmaceutical industries in Singapore and Malaysia are only being regulated in recent times, there is a possibility that the biopharmaceutical industries may become more heavily regulated and we may be subject to further legislative, regulatory and compliance requirements.
Our product candidates could be subject to more stringent limitations and procedures imposed by third-party users, including hospitals, than those imposed by regulatory authorities.
Even if we manage to obtain the necessary licenses, permits and approvals for our products, we may also be required to comply with policies, limitations and other procedures imposed by third-party users, including hospitals, of our products. Such policies, limitations and other procedures may change from time to time, depending on factors including but not limited to perceived risks and benefits of our products, public sentiment towards the biopharmaceutical industries and relevant government legislation, regulations and policies. This may restrict the distribution of our products and/or increase our compliance costs, which may materially and adversely affect our financial performance and financial condition.
There may be limited market opportunities for our product candidates, if approved, and if such market opportunities are smaller than we anticipated, our revenues and business could be materially and adversely affected.
Cancer therapies are sometimes categorized as first-line, second-line, or third-line, and the regulatory authorities often approve new therapies initially only for third-line use. When cancer is detected early enough, first-line therapy, usually chemotherapy, hormone therapy, surgery, radiation therapy or a combination of these, may be sufficient as a cure for cancer. Second and third-line therapies are administered to patients when prior therapy is not effective. Our initial planned clinical trials are expected to enroll patients who have received these standard therapies in order to first evaluate whether the product is safe and whether there is any anti-cancer activity. We currently are unable to ascertain whether CTM-N2D, iPSC-gdNKT and CTM-GDT will be safe for use in humans and whether CTM-N2D, iPSC-gdNKT and CTM-GDT will demonstrate any anti-cancer activity. Subsequently, we plan to conduct additional clinical trials depending on the anti-cancer activity we note in the initial clinical trials. If the anti-cancer activity is sufficient, we may initially seek approval of any product candidates we develop as a therapy for patients who have received one or more prior standard therapies. Subsequently, for those products that prove to be sufficiently beneficial, if any, we would expect to seek approval potentially in earlier lines of therapy, but there is no guarantee that product candidates we develop, even if approved for later lines of therapy, would be approved for earlier lines of therapy, and, prior to any such approvals, we may have to conduct additional clinical trials.
There may not be as many patients who have the type of cancers we are targeting. Additionally, the potentially addressable patient population for our current or future product candidates may be limited. Potentially addressable patient populations for our product candidates are only estimates. These estimates could prove to be inaccurate as there may not be as many potential patients in Singapore and elsewhere as estimated. It may also be that such patients may not be otherwise amenable to treatment with our product candidates, or suitable patients could become increasingly difficult to identify and access, any of which could materially and adversely affect our business, financial condition, results of operations and growth prospects.
| 58 |
The commercial success of any of our product candidates will depend upon such product candidate’s level of market acceptance by physicians, patients, third-party payors and others in the medical community.
Even if requisite approvals are obtained from HSA and/or other regulatory authorities, the commercial success of our product candidates will depend, in part, on the acceptance by physicians, patients and healthcare payors of cell therapy products in general, and our product candidates in particular, as medically necessary, cost-effective and safe. Physicians, patients, healthcare payors, professionals and others in the medical community may not approve of any product candidates that we commercialize. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of cell therapy products and, in particular, our product candidates, if approved for commercial sale, will depend on several factors, including without limitation:
| ● | the efficacy and safety of such product candidates as demonstrated in clinical trials; | |
| ● | the potential and perceived advantages of product candidates over alternative treatments; | |
| ● | the cost of treatment relative to alternative treatments; | |
| ● | the clinical trial indications for which the product candidate is approved by the HSA and/or other relevant regulatory authorities; | |
| ● | the willingness of physicians to prescribe new therapies; | |
| ● | the willingness of the target patient population to try new therapies; | |
| ● | the prevalence and severity of any side effects; | |
| ● | product labeling or product insert requirements imposed by HSA and/or other relevant regulatory authorities, including any limitations or warnings contained in a product’s approved labeling; | |
| ● | relative convenience and ease of administration; | |
| ● | the timing of market introduction of competitive products; | |
| ● | adverse publicity concerning our product candidates or favorable publicity about competing products and treatments; | |
| ● | sufficient third-party payor coverage, any limitations in terms of center or personnel training requirement imposed by third parties and adequate reimbursement; | |
| ● | limitations or warnings contained in the regulatory authority-approved labeling for our product candidates; | |
| ● | any regulatory authority requirement to undertake a risk evaluation and mitigation strategy; | |
| ● | the effectiveness of our sales, marketing and distribution efforts; and | |
| ● | potential product liability claims. |
Even if a product candidate displays a favorable efficacy and safety profile in pre-clinical studies and clinical trials, market acceptance of the product will not be fully known until after such product is launched. Our product candidates may not achieve broad market acceptance.
Furthermore, the policies of HSA and/or other relevant regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay marketing approval of a product. We cannot determine the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in Singapore or abroad. If we are late or fail to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we fail to maintain regulatory compliance, we may lose any marketing approval that we may have previously obtained and as a result, we may not be able to achieve or sustain profitability.
There are several uncertainties relating to the insurance coverage and reimbursement status of newly approved products on the market. Failure to obtain or maintain adequate coverage and reimbursement for our product candidates, if approved, could hinder our ability to market such products and to generate product revenue.
The expenses incurred as a result of the administration of any of our cell therapy product candidates is anticipated to be significant, when and if regulatory approval is obtained. We expect that coverage and reimbursement by government and private payors will be essential for most patients to be able to afford these treatments. Accordingly, sales of our products, if approved, will depend substantially, both domestically and internationally, on the extent to which the costs of our product candidates will be reimbursed by government authorities, private health coverage insurers and/or other third-party payors. Coverage and reimbursement by a third-party payor could depend upon several factors, including the third-party payor’s determination that use of our product is (i) a covered benefit under its health plan, (ii) safe, effective and medically necessary, (iii) appropriate for the specific patient, (iv) cost-effective and (v) neither experimental nor investigational.
| 59 |
Obtaining coverage and reimbursement for a product from third-party payors is a time-consuming and expensive process that could require us to provide supporting scientific, clinical and cost-effectiveness data. We may not be able to provide adequate data to obtain acceptance with respect to coverage and reimbursement. If coverage and reimbursement are not available, or are available only at limited levels, we may fail to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be sufficient to generate a sufficient return on our investment.
There is significant uncertainty relating to third-party coverage and reimbursement of newly approved drug products and/or treatments.
Outside of Singapore, international operations may vary significantly by country and our products may be subject to extensive government price controls and other market regulations. Increasing focus on cost-containment initiatives in the European Union, Canada and other countries could result in pricing pressure. In many countries, the prices of medical products are governed by several price control mechanisms as part of national health systems. The post-approval process to secure pricing and reimbursement for a product may be time-consuming depending on the country. In general, the prices of medical and therapeutic products under such systems may be substantially lower than in Singapore. Other countries allow companies to fix their own prices for such products, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside Singapore, the reimbursement for our products may be lower than that of Singapore and may be insufficient to generate commercially viable and sustainable product revenues and profits.
Moreover, any efforts by government and third-party payors in Singapore and abroad to limit and/or reduce healthcare costs could limit coverage and the level of reimbursement for our product candidates.
There is no certainty that the medical costs of our treatment for suitable patients will be covered by insurance. As our product candidates are novel, the treatment and medical costs may not be covered by medical and/or hospitalization insurances obtained by these patients. If so, the costs of treatment with our product candidates may be prohibitively high, and deter suitable patients from seeking help resulting in a low take-up rate for our product candidates. This will materially and adversely affect our business, growth and future prospects of our Group.
Healthcare reform initiatives and other administrative and legislative proposals may affect our business.
We cannot anticipate the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action in Singapore, Malaysia and/or any other relevant jurisdiction. If we or any third parties we may engage are late or fail to adapt to developments in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been previously obtained and we may not achieve or sustain profitability. Furthermore, future price controls or other changes in pricing regulation or negative publicity related to the pricing of cell therapy products could restrict the amount that we are able to charge for our cell therapy products, which could render our product candidates, if approved, commercially unviable and materially and adversely affect our ability to raise additional capital on acceptable terms.
| 60 |
Obtaining and maintaining marketing approval or commercialization of our product candidates in one jurisdiction does not mean that we will be successful in obtaining marketing approval of our product candidates in other jurisdictions.
Approval procedures are different among different jurisdictions and can involve requirements and administrative review periods significantly different from, and more extensive than, those in Singapore, Malaysia and/or other relevant jurisdictions including additional pre-clinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside Singapore, Malaysia and/or other relevant jurisdictions, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, we may have to obtain approval for the prices that we intend to charge for our products.
If we market products approved in jurisdictions outside Singapore, Malaysia and/or other relevant jurisdictions, we expect that we will be subject to additional risks in commercialization, including without limitation:
| ● | variations in regulatory requirements for approval of therapies in different jurisdictions; | |
| ● | decreased scope of protection of intellectual property rights; | |
| ● | unanticipated changes in tariffs, trade barriers and other regulatory requirements; | |
| ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; | |
| ● | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; | |
| ● | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; | |
| ● | foreign reimbursement, pricing and insurance regimes; | |
| ● | workforce uncertainty in countries where labor unrest is more common; | |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and | |
| ● | business interruptions resulting from geopolitical actions, including war and terrorism, natural disasters including earthquakes, typhoons, floods and fires, and other public health crises, illnesses, epidemics or pandemics, such as the potential impact of the COVID-19 pandemic. |
We do not have any prior experience in these areas. Any of the foregoing difficulties, if encountered, could materially and adversely affect our business, financial condition, results of operations and growth prospects.
RISKS RELATED TO OUR ORDINARY SHARES AND THIS OFFERING
There has been no public market for our ordinary shares prior to this offering, and you may not be able to resell our shares at or above the price you paid, or at all.
We have applied to list our ordinary shares on the Nasdaq, but we cannot guarantee an active trading market for our ordinary shares following this offering. If an active trading market for our ordinary shares does not develop after this offering, the market price and liquidity of our ordinary shares will be materially and adversely affected. You may not be able to sell your shares quickly or at the market price in this case. Negotiations between us and the underwriters will determine the offering price for our ordinary shares and the offering price may bear no relationship to the market price for our ordinary shares after this offering. In addition, the market price of our ordinary shares may decline below the offering price. Furthermore, an inactive market may also impair our ability to raise capital by selling shares of our ordinary shares and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of ordinary shares as consideration.
| 61 |
The market price for our ordinary shares may be volatile, which could contribute to the loss of all or part of your investment.
Prior to this offering, there has not been a public market for our ordinary shares. Accordingly, the initial public offering price for our ordinary shares may not be indicative of the price that will continue to prevail in the trading market, if any, following this offering. Assuming that there is an active market for our ordinary shares, the trading price of our ordinary shares following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control.
Factors affecting the trading price of our ordinary shares may include, but are not limited to:
| ● | our decision on whether or not to initiate a clinical study or to terminate an existing clinical study; | |
| ● | adverse regulatory decisions, including failure to receive regulatory approval for our products; | |
| ● | success or failure of competitive products, immunotherapy or cellular therapies more generally; | |
| ● | adverse developments concerning our manufacturers or our strategic partnerships; | |
| ● | adverse safety or other clinical results, such as those that have occurred in the past or that may occur in the future, related to cellular therapies being developed by other companies that are or may be perceived to be similar to our cellular therapies; | |
| ● | operating and share price performance of other companies that investors deem comparable to us; | |
| ● | sales of substantial amounts of ordinary shares by our Directors, executive officers or significant shareholders or the perception that such sales could occur; | |
| ● | general economic and political conditions such as recessions, interest rates, fuel prices, elections, drug pricing policies, international currency fluctuations, acts of war or terrorism, and other public health crises, illnesses, epidemics or pandemics, such as the potential impact of the COVID-19 pandemic; and | |
| ● | other factors discussed in these risk factors. |
Any of or a combination of the above factors could materially and adversely affect your investment in our ordinary shares, and our ordinary shares may trade at prices significantly below the initial public offering price, which could contribute to a loss of all or part of your investment. In such circumstances the trading price of our ordinary shares may not recover and may experience a further decline.
In addition, broad market and clinical trial industry factors could materially and adversely affect the market price of our ordinary shares, irrespective of our operating performance. The stock market in general, and Nasdaq and the market for biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. We cannot predict the trading prices and valuations of these stocks, and of ours. For example, the trading prices for common stock of other biopharmaceutical and biotechnology companies have been highly volatile as a result of the COVID-19 pandemic. As the COVID-19 pandemic continues to rapidly evolve, the extent to which the outbreak may impact our business, pre-clinical studies and clinical trials is contingent on future developments, which are highly uncertain and cannot be accurately predicted. We may face a loss of investor confidence in the market for biopharmaceutical stocks or the stocks of other companies which investors perceive to be similar to us, the opportunities in the biopharmaceutical market or the stock market in general, which may decrease our share price regardless of our business, financial condition, results of operations or growth prospects.
We may face securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a period of volatility or decline in the market price of its securities. We are likely to face this risk as pre-clinical biopharmaceutical companies have experienced significant share price volatility in recent years. If we face such litigation, we may have to pay substantial costs (including legal costs) and this would also require a diversion of management’s attention and resources, which could materially and adversely affect our business, financial condition, results of operation and growth prospects.
| 62 |
If securities analysts do not publish research or reports about our business or if they publish negative reports or downgrade our shares, the price of our ordinary shares could decline.
The trading market for our ordinary shares will rely in part on the research and reports that financial analysts publish about us, our business, our markets and our competitors. We have no control over the research and reports that these financial analysts publish. If we and our ordinary shares are not discussed by securities analysts after the consummation of this offering, this may materially and adversely affect the market price of our ordinary shares. Furthermore, if any securities analyst chooses to downgrade our shares or issue unfavorable commentary on us or our business, our share price would likely decline. If one or more of these security analysts ceases coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our ordinary shares could decrease, which in turn could cause our share price or trading volume to decline and may also impair our ability to expand our business with existing customers and attract new customers.
You will experience immediate and substantial dilution in the net tangible assets of the shares you purchase in this offering.
If you purchase our ordinary shares in this offering, you will experience immediate and substantial dilution, as the initial public offering price of our ordinary shares will be substantially greater than the net tangible assets per share of our ordinary shares.
Assuming (i) an initial public offering price of U.S.$ 4.50 per share, the midpoint of the price range set forth on the cover page of this Prospectus, (ii) that the number of shares offered by us, as set forth on the cover page of this Prospectus, remains the same and (iii) no exercise of the underwriters’ option to purchase additional shares, if you purchase our ordinary shares in this offering, you will suffer immediate and substantial dilution of approximately U.S.$3.39 per share. Further, giving effect to the same assumptions, investors purchasing ordinary shares in this offering will contribute approximately 58.60% of the total amount invested by shareholders since our incorporation, but will own only approximately 21.03% of the ordinary shares outstanding after giving effect to this offering. If the underwriters exercise their option to purchase additional shares, or if outstanding options to purchase our ordinary shares are exercised, you will experience additional dilution. For a further description of the dilution that you will experience immediately after this offering, please refer to the section entitled “Dilution”.
A significant portion of our total outstanding shares are eligible to be sold into the market in the near future, which could cause the market price of our ordinary shares to drop significantly.
Sales of a substantial number of shares of our ordinary shares in the public market, or the perception in the market that the holders of a large number of shareholders intend to sell ordinary shares, could reduce the market price of our ordinary shares. Following consummation of this offering, we will have 11,468,642 ordinary shares outstanding, based on the number of ordinary shares outstanding as of the date of this Prospectus and assuming conversion of all outstanding convertible loans into ordinary shares. This is inclusive of our shares offered in this offering, which may be resold in the market immediately without any restrictions, unless purchased by our affiliates. In addition, 1,337,631 ordinary shares may be sold without restriction under Rule 144 of the Securities Act and are not subject to any contractual restrictions. Substantially all of the remaining 7,718,642 ordinary shares will be restricted as a result of securities laws, market standoff provisions or lock-up agreements, but will become eligible to be sold after this offering as described in the section titled “Shares Eligible for Future Sale”.
We also intend to register all ordinary shares subject to equity awards issued or reserved for future issuance under our equity compensation plans on a registration statement on Form S-8. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates under Rule 144 under the Securities Act and the market standoff provisions and lock-up agreements described above. Any sales of securities by these shareholders could have a negative impact on the trading price of our ordinary shares.
| 63 |
Concentration of ownership of our ordinary shares among our existing Directors, executive officers, and principal shareholders may prevent new investors from influencing significant corporate decisions.
Following this offering, our Directors and executive officers, and entities affiliated with them, as well as holders of more than 5% of our outstanding ordinary shares, in the aggregate will beneficially own a percentage of our ordinary shares, after giving effect to the issuance of ordinary shares in this offering but without giving effect to any purchases by such persons or entities in the offering. These shareholders, acting together, will be able to control or significantly influence resolutions requiring shareholder approval, including the election and removal of our Directors and approval of any merger, consolidation or sale of all or substantially all of our assets. Some of these persons and entities may have an interest in purchasing additional ordinary shares in this offering, which would increase their ownership percentage.
Some of these persons or entities may have interests different from yours. For example, because many of these shareholders purchased their shares at prices substantially below the price at which shares are being sold in this offering and have held their shares for a longer period, they may be more interested in selling our company to an acquirer than other investors, or they may want us to pursue strategies that deviate from the interests of other shareholders.
We are an “emerging growth company” under the JOBS Act, and a “smaller reporting company” as of the date of this Prospectus and we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. As a result of the reduced disclosure and governance requirements applicable to emerging growth companies and smaller reporting companies, our ordinary shares may be less attractive to investors.
We intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding an advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
We intend to take advantage of these reporting exemptions until we are no longer an “emerging growth company”. We are expected to remain as an “emerging growth company” until the earlier of: (1) the last day of the financial year following the fifth anniversary of the consummation of this offering; (2) the last day of the financial year during which we have total annual gross revenue of at least U.S.$1.235 billion or (3) the date on which we are deemed to be a “large accelerated filer” under the rules of the SEC, which means the market value of our ordinary shares that is held by non-affiliates is of U.S.$700 million or more as of the prior June 30; and (4) the date on which we have issued more than U.S.$1 billion in non-convertible debt during the prior three-year period.
We have taken advantage of reduced reporting requirements in this Prospectus. In particular, in this Prospectus, we have not included all of the executive compensation related information that would be required if we were not an “emerging growth company”. In addition, Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. An “emerging growth company” can, therefore, delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have an irrevocable election to take advantage of the benefits of this extended transition period.
We are also a “smaller reporting company” as of the date of this Prospectus, meaning that the market value of our ordinary shares held by non-affiliates plus the proposed aggregate amount of gross proceeds to us as a result of this offering is less than U.S.$700 million and our annual revenue is less than U.S.$100 million during the most recently completed financial year. We may continue to be a “smaller reporting company” after consummation of this offering if either (i) the market value of our ordinary shares held by non-affiliates is less than U.S.$250 million or (ii) our annual revenue is less than U.S.$100 million during the most recently completed financial year and the market value of our ordinary shares held by non-affiliates is less than U.S.$700 million. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company”, which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in this Prospectus and in our periodic reports and proxy statements. We cannot predict if investors will find our shares less attractive if we rely on such exemptions. If some investors find our shares less attractive as a result, there may be a less active trading market for our shares and our share price may be more volatile.
| 64 |
We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempted from certain provisions applicable to U.S. domestic public companies.
Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K; | |
| ● | the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; | |
| ● | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and | |
| ● | the selective disclosure rules by issuers of material nonpublic information under Regulation FD. |
We will be required to file an annual report on Form 20-F within four months of the end of each financial year. Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections and/or information that would be made available to you if you were investing in a U.S. domestic issuer.
We will have significant operating expenditures as a public company, and our management will be required to devote substantial time and effort to planning and implementing new compliance initiatives.
As a public company, we expect to have significant legal, accounting and other expenditure (including related insurance) that we did not previously incur as a private company. In addition, the Sarbanes-Oxley Act and rules of the SEC and those of Nasdaq have imposed various requirements on public companies including that we establish and maintain effective disclosure and financial controls. Our management and other personnel will need to devote a substantial amount of time and effort to planning and implementing these compliance initiatives. Moreover, these rules and regulations have increased and will continue to increase our legal and financial compliance costs and will make some activities more laborious, time-consuming and expensive.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls over financial reporting and disclosure controls and procedures. In particular, we are required to assess our systems and procedures, and test our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. In addition, we will be required to have an independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting the later of our second annual report on Form 20-F or the first annual report on Form 20-F following the date on which we are no longer an emerging growth company unless we are a smaller reporting company. We are required to comply with Section 404 of the Sarbanes-Oxley Act, which will involve substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. If we do not comply with the requirements of Section 404 in a timely manner, or if we or our clinical trial independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, there may be a decrease in the market price of our ordinary shares and we may be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities, which would require additional financial and management resources.
| 65 |
We are required to prepare timely and accurate financial statements to implement our business plan and ensure compliance with Section 404. We expect that we will need to continue developing existing procedures and controls, and implement new operational and financial systems, to effectively conduct our business. Any delay in the implementation of, or disruption in the transition to, new or enhanced systems, procedures or controls, may cause our operations to suffer, and we may be unable to determine that our internal control over financial reporting is effective and to obtain an unqualified report on internal controls from our auditors as required under Section 404 of the Sarbanes-Oxley Act. This, in turn, could materially adversely affect the trading prices for our ordinary shares and our ability to access the capital markets.
We may lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur additional legal, accounting and other expenses.
In order to maintain our current status as a foreign private issuer, either (1) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the United States or (2) (a) a majority of our executive officers or directors must not be U.S. citizens or residents, (b) more than 50 percent of our assets cannot be located in the United States and (c) our business must be administered principally outside the United States. If we lost this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC rules and Nasdaq listing standards. The regulatory and compliance costs to us under U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance. These rules and regulations could also make it more difficult for us to attract and retain qualified Board members.
If our system of internal control over financial reporting is not effective and robust, our financial results may not be accurately reported and we may not be able to prevent fraud. As a result, shareholders could deem our financial and other public reporting unreliable, which would materially and adversely affect our business and the trading price of our ordinary shares.
We need effective and robust internal controls over financial reporting in order to present reliable and accurate financial reports and prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could result in us breaching our reporting obligations. When we lose our status both as an emerging growth company and a smaller reporting company, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting. There are complex rules governing the standards that must be met for management to assess our internal control over financial reporting which require significant documentation, testing and possible remediation. Any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed material weaknesses or that may require prospective or retroactive revisions to our financial statements or us to determine other areas for further attention or improvement. Inadequate internal controls could also cause investors to lose confidence in our reported financial information, which could materially and adversely affect the trading price of our ordinary shares.
Our disclosure controls and procedures are not risk-free and may not fully prevent and/or identify errors or acts of fraud.
Upon the consummation of this offering, we will have to satisfy periodic reporting requirements of the Exchange Act. We have planned our disclosure controls and procedures to reasonably ascertain that information required to be disclosed in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, certainty that the objectives of the control system are met.
We are subject to inherent limitations including the possibility that judgments in decision-making can be faulty, and that breakdowns and/or non-compliance can occur due to simple error or oversight. For example, our Directors and/or executive officers could inadvertently fail to disclose a new relationship or arrangement causing us to fail to make any related party transaction disclosures. Additionally, our disclosure controls can possibly be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls as misstatements due to error or fraud may occur and not be detected in time, if at all.
| 66 |
Our results of operations may be affected by changes to, or interpretations of financial accounting standards may affect our results of operations, which may require us to change our business practices.
Our financial statements are prepared in accordance with IFRS. These accounting principles are subject to interpretation by the Financial Accounting Standards Board, the SEC and various bodies formed to interpret and create accounting rules and regulations. Changes in accounting rules can have a significant effect on our reported financial results and may affect our reporting of transactions which may be completed before the announcement of such a change. Changes to those rules or the questioning of current practices may materially adversely affect our financial results, including those contained in this filing, or the way we conduct our business.
Our ability to use our loss carryovers and certain other tax attributes may be limited.
As described above under the heading “We have incurred losses since our incorporation and we expect to incur significant losses for the foreseeable future”, we have incurred losses since our incorporation and anticipate that we will continue to incur significant losses for the foreseeable future. Under the Income Tax Act and subject to the agreement with the Comptroller, a corporation is generally allowed a deduction for trade losses, carried forward indefinitely from a prior taxable year. Under that provision, we can carry forward our trade losses to offset our future taxable income, if any, until such trade losses are fully utilized, subject to the fulfilment of certain conditions.
Furthermore, if a corporation undertakes substantial change in the shareholders (which is generally defined as changes of the owner of 50% or more of the company’s (or its ultimate parent company’s) total number of issued shares) as at the relevant dates (i.e. the last day of the year in which the loss was incurred and the first day of the year of assessment in which such loss is to be claimed), Section 37(12) of the Income Tax Act limits the corporation’s ability to use / carry forward the unutilized trade losses and donations to reduce its tax liability for periods after the substantial shareholder change. Our issuance of ordinary shares pursuant to this offering may result in a limitation under Section 37(12) of the Income Tax Act, either separately or in combination with certain prior or subsequent shifts in the ownership of our ordinary shares. As a result, our ability to carry forward of our trade losses to reduce our future income tax liability may be subject to limitations. This could result in increased income tax liability for us if we generate taxable income in a future period.
The use of our tax attributes will also be limited to the extent that we do not generate positive taxable income in future tax periods. To the extent our ability to utilize our trade losses and other tax assets going forward is limited, in part or altogether, our tax liability for future periods may be greater than expected, and our business, financial condition, results of operations and growth prospects may be materially adversely affected.
We have broad discretion in our use of net proceeds from this offering.
We currently intend to use the net proceeds from this offering for our working capital and general corporate purposes, which may include further funding for our operating costs as a public company. We may also use the proceeds to advance the clinical development of our product candidates. For a further description of our use of proceeds from this offering and the concurrent private placement, please refer to the section entitled “Use of Proceeds”. That said, we have broad discretion in our use of the net proceeds from this offering. As a result, investors will be relying only on limited information about our specific intentions for the use of the net proceeds of this offering. We may apply the net proceeds for purposes that do not yield a significant return or any return at all for our shareholders. In addition, we may use the net proceeds from this offering in a manner that does not produce income or results in a loss in value.
We do not intend to pay any cash dividends to our shareholders for the foreseeable future.
We intend to invest our future profits, if any, to fund our growth and development. In addition, we may enter into debt agreements, the terms of which may prevent us from issuing and paying dividends to our shareholders. As a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future. We cannot guarantee that our ordinary shares will appreciate in value or even maintain the price at which our shareholders have purchased our ordinary shares. Investors seeking cash dividends should not purchase our ordinary shares.
| 67 |
RISKS RELATED TO INVESTMENTS IN SINGAPORE COMPANIES
We are incorporated in Singapore, and our shareholders may have more difficulty in protecting their interests than they would as shareholders of a corporation incorporated in the United States.
Our corporate affairs are governed by, inter alia, our constitution and by the laws governing companies incorporated in Singapore. The rights of our shareholders and the responsibilities of the members of our Board under Singapore law may be different from those applicable to a corporation incorporated in the United States. Therefore, our public shareholders may have more difficulty protecting their interests in connection with actions taken by us, our management, members of our Board or our controlling shareholders than they would as shareholders of a corporation incorporated in the United States. For example, controlling shareholders in corporations incorporated in Delaware are subject to fiduciary duties while controlling shareholders in Singapore companies are not subject to such duties.
In addition, subject to the Singapore Companies Act and our constitution, only persons who are registered as shareholders in our register of members are recognized under Singapore law as our shareholders. Only registered shareholders have legal standing to institute shareholder actions against us or otherwise seek to enforce their rights as shareholders. Investors in our ordinary shares who are not specifically registered as shareholders in our register of members (for example, where such shareholders hold ordinary shares indirectly through the DTC) are required to be registered as shareholders in our register of members in order to institute or enforce any legal proceedings or claims against us, our Directors and/or our executive officers relating to shareholder rights. The administrative process of becoming a registered shareholder could result in delays prejudicial to any such legal proceeding or enforcement action. Please refer to the section entitled “Comparison of Shareholder Rights” for a discussion of certain differences between Singapore and Delaware corporation law.
Our shareholders may have more difficulty transferring their shares in the Company.
The transfer of our shares is subject to restrictions under our constitution and by the laws governing companies incorporated in Singapore. Under Singapore law, a public company shall not register a transfer of shares unless a proper instrument of transfer has been delivered to the company. In addition, our constitution provides that our Directors may decline to lodge a notice of transfer of shares with ACRA if (a) the shares are not fully paid shares, (b) the Directors do not approve of the transferee or (c) the Company has a lien on the shares. As such, it may be difficult for you to transfer your shares in the Company.
It may be difficult for you to enforce any judgment obtained in the United States against us, our Directors and officers and/or our affiliates.
A majority of our Directors and officers reside outside the United States. In addition, a majority of our assets and the assets of such persons are located outside the United States. As a result, it may be difficult to enforce in the United States any judgment obtained in the United States against us or any of such persons, including judgments based on the civil liability provisions of the U.S. securities laws. In addition, in original actions brought in courts in jurisdictions located outside the United States, it may be difficult for investors to enforce liabilities based upon U.S. securities laws.
As of the date of this Prospectus, there is no treaty between the United States and Singapore providing for the reciprocal recognition and enforcement of judgments in civil and commercial matters and a final judgment for the payment of money rendered by any federal or state court in the United States based on civil liability, whether or not predicated solely upon the federal securities laws, would, therefore, not be automatically enforceable in Singapore. It is not clear whether a Singapore court may impose civil liability on us, our Directors and/or officers who reside in Singapore in an action brought in the Singapore courts against us and/or such persons with respect to a violation solely of the federal securities laws of the United States.
| 68 |
In addition, holders of book-entry interests in the ordinary shares (for example, where such shareholders hold ordinary shares indirectly through the DTC) will be required to be registered shareholders as reflected in our register of members in order to have standing to bring a shareholder action and, if successful, to enforce a foreign judgment against us, our Directors or our executive officers in the Singapore courts. Any such enforcement action would be subject to applicable Singapore laws. The administrative process of becoming a registered shareholder could result in delays that could be prejudicial to any legal proceeding or enforcement action. In making a determination as to enforceability of a judgment of a state court or a federal court of the United States, the Singapore courts would have regard to, among others, whether the judgment was final and conclusive, given by a court of law of competent jurisdiction, expressed to be for a fixed sum of money, whether it was procured by fraud, or in breach of principles of natural justice, or whether the enforcement thereof would be contrary to public policy.
Accordingly, there can be no assurance that the Singapore courts would enforce against us, our Directors and/or our officers, judgments obtained in the United States which based on the civil liability provisions of the federal securities laws of the United States.
Subject to the general authority to allot and issue new ordinary shares as may be approved by our shareholders pursuant to the Singapore Companies Act and our constitution, our Directors may allot and issue new ordinary shares from time to time on such terms and conditions and for such purposes as may be determined by our Board of Directors in its sole discretion. Any issuance of new shares would dilute the percentage ownership of existing shareholders and could adversely impact the market price of our ordinary shares.
Under Singapore law, we may only allot and issue new ordinary shares with prior approval of our shareholders in a general meeting. Subject to the general authority to allot and issue new ordinary shares provided by our shareholders, the provisions of the Singapore Companies Act, and our constitution, we may allot and issue new ordinary shares on such terms and conditions as our Directors may think fit to impose. Such terms and conditions may be adverse to the rights of holders of our ordinary shares. Any additional issuances of new ordinary shares could dilute the percentage ownership of our existing shareholders and may adversely impact the market price of our ordinary shares.
Because new issuances of ordinary shares are subject to restrictions in respect of the percentage thereof under the Singapore Companies Act and the requisite shareholders’ approval, if a sufficient number of shares have not been approved for issuance in respect of one or more transactions in any given year, we may be delayed in raising capital through equity offerings or delayed or prevented from consummating an acquisition using our ordinary shares. Assuming shareholders have approved the issuance of new shares, we may seek to raise capital in the future, including to fund acquisitions, future investments and other growth opportunities. We may, for these and other purposes, issue additional ordinary shares or securities convertible into ordinary shares. Any additional issuances of new ordinary shares could dilute the percentage ownership of our existing shareholders and may also adversely impact the market price of our ordinary shares.
We are subject to the laws of Singapore, which differ in certain material respects from the laws of the United States.
As a company incorporated in Singapore, we are required to comply with the laws of Singapore, some of which are capable of extra-territorial application, as well as our constitution. In particular, we are required to comply with certain provisions of the Securities and Futures Act 2001 of Singapore, which prohibit certain forms of market conduct and information disclosures, and impose criminal and civil penalties on corporations, directors and officers in respect of any breach of such provisions. In addition, the Singapore Take-over Code specifies, among other things, the circumstances wherein a general offer shall be taken upon a change in control of a Singapore-incorporated public company and the manner and price at which voluntary and mandatory general offers are to be made.
| 69 |
The laws of Singapore and of the United States differ in certain significant respects. The rights of our shareholders and the obligations of our Directors and officers under Singapore law may be different from those applicable to U.S. corporations, including corporations incorporated in Delaware, in material respects, and our shareholders may have more difficulty and less clarity in protecting their interests in connection with actions taken by us, our management, members of our Board and/or our controlling shareholders than would otherwise apply to U.S. corporations, including those incorporated in Delaware. Please refer to the section titled “Comparison of Shareholder Rights” for a discussion of certain differences between Singapore and Delaware corporation law.
In addition, the application of Singapore law, in particular, the Singapore Companies Act may, in certain circumstances, impose more restrictions on us, our shareholders, Directors and officers than would otherwise be applicable to U.S. corporations, including those incorporated in Delaware. For example, the Singapore Companies Act requires a director to act with reasonable degree of diligence in the discharge of the duties of his office and, in certain circumstances, imposes criminal liability for specified contraventions of particular statutory requirements or prohibitions. In addition, pursuant to the provisions of the Singapore Companies Act, two or more shareholders holding 10% or more of the total number of issued shares as at the date of the deposit of the relevant requisition, carrying the right of voting at general meetings (disregarding paid-up shares held as treasury shares) may require our Directors to convene an extraordinary general meeting.
Singapore tax laws may differ from the tax laws of other jurisdictions.
Singapore tax laws may differ from the tax laws of other jurisdictions, including the United States. For example, Singapore does not impose tax on capital gains. However, there are no specific laws or regulations which deal with the characterization of whether a gain is income or capital in nature. Gains arising from the disposal of our ordinary shares may be construed to be of an income nature and subject to Singapore income tax, especially if they arise from or are otherwise connected with the activities which the IRAS regards as carrying on a trade or business in Singapore. In addition, holders of our ordinary shares may for the purposes of Singapore income tax be required to recognize gains or losses (not being gains or losses in the nature of capital) even though no sale or disposal of our ordinary shares is made. Prospective investors should consult their tax advisors concerning the overall tax consequences of purchasing, owning and disposing of our shares. Please also refer to the section entitled “Tax Considerations”.
Singapore take-over laws contain provisions that may vary from those in other jurisdictions.
The Singapore Take-over Code applies to, among others, corporations with a primary listing of their equity securities in Singapore. While the Singapore Take-over Code is drafted with, among others, listed public companies in mind, unlisted public companies with more than fifty (50) shareholders and net tangible assets of S$5.0 million or more, must also observe the letter and spirit of the general principles and rules of the Singapore Take-over Code, wherever this is possible and appropriate. Public companies with a primary listing overseas may apply to the SIC to waive the application of the Singapore Take-over Code. As of the date of this Prospectus, no application has been made to SIC to waive the application of the Singapore Take-over Code in relation to us.
In this regard, the Singapore Take-over Code contains certain provisions that may possibly delay, deter or prevent a future take-over or change in control of us. Under the Singapore Take-over Code, except with the consent of SIC, any person acquiring an interest, whether by a series of transactions over a period of time or not, either on his own or together with parties acting in concert with him, in 30% or more of our voting shares is required to extend a take-over offer for all remaining voting shares in accordance with the procedural and other requirements under the Singapore Take-over Code.
Except with the consent of SIC, such a take-over offer is also required to be made if a person holding between 30% and 50% (both inclusive) of our voting shares, either on his own or together with parties acting in concert with him, acquires additional voting shares representing more than 1% of our voting shares in any six (6) month period. While the Singapore Take-over Code seeks to ensure an equality of treatment among shareholders in take-over or merger situations, its provisions could substantially impede the ability of the shareholders to benefit from a change of control and, as a result, may adversely affect the market price of the ordinary shares and the ability to realize any benefit from a potential change of control.
| 70 |
Our operations are affected by the changes in existing Malaysian laws and regulations, and the adoption of new Malaysian laws and regulations and/or the changes in interpretation of the Malaysian laws and regulations as well as possible inconsistencies between the various Malaysian laws and regulations and/or the corresponding interpretation
Our operations in Malaysia are regulated by the laws and regulations of Malaysia, including those relating to the corporate, investment, marketing, labor, environmental protection, occupational health and safety, and taxation matters. The legal and regulatory regimes in Malaysia may be uncertain and subject to unforeseen changes. At times, the interpretation or application of laws and regulations in Malaysia may be unclear. Government policies, regulations and guidelines issued and imposed by the relevant authorities may change from time to time. We may have to incur significant capital and maintenance expenditures to comply with laws and regulations. The failure to discharge our obligations could result in the imposition of fines and penalties, damage to our reputation, delays in production or the temporary or permanent closure of our operations. In addition, existing laws, regulations or policies may become stricter or more strictly enforced. Our operations and business may face investigation, scrutiny or evaluation. The adoption of new laws and regulations or any modification to the existing laws and regulations may result in additional expenses to comply with the new laws.
While we have compliance procedures in place to ensure compliance with new legislation and every effect is taken to ensure the requirements of any new legislation are met, there is no certainty on the approach which will be taken by the relevant regulators, and we may incur additional compliance costs with the introduction of new or amended regulations. We have no control over such conditions and developments and there can be no assurance that such conditions and developments will not have a material adverse effect on our business. As a result, we may face new liabilities, reduced operating hours, additional investment requirements, or delays in the opening or expansion of operations. We may also be compelled to conduct preventive or remedial actions. These may result in increased costs. Such costs, liabilities, or disruptions in operations may materially and adversely affect our business.
We are subject to the political, economic and social conditions in the jurisdiction of Malaysia
Our cGMP Facility is located in Malaysia and our operations are substantially based in Malaysia and is therefore sensitive to the social, economic, political and legal conditions in Malaysia. Developments in Malaysia such as changes in Malaysian government policies, currency and interest rates, inflation, capital restrictions, price and wage controls, unemployment rate, taxes and duties will materially and/or adversely affect our business. We have no control over such conditions and developments and there is no assurance that such conditions and developments will not occur. These changes, if they occur, will materially and/or adversely affect our Malaysian business and operations and thus our business in general.
We are subject to the foreign exchange legislation and regulations in the jurisdiction of Malaysia
Local and foreign investors are subject to the FX Notices in Malaysia. The FSA and the IFSA govern the foreign exchange control framework in Malaysia. Under the FSA and the IFSA, the BNM has issued FX Notices. These FX Notices embody the BNM’s general permissions and directions. They set out the circumstances in which residents and non-residents must seek the specific approval of BNM to remit funds to and from Malaysia.
The FX Notices are reviewed regularly according to changing circumstances. As of the date of this Prospectus, foreign investors are allowed to repatriate from Malaysia, funds including any income earned or proceeds from divestments of Ringgit Asset (including any property in Malaysia), provided that the repatriation is made in foreign currency and the conversion of Malaysian Ringgit into foreign currency is undertaken in accordance with the FX Notices. Should the repatriation of funds be restricted in the future, this will limit our ability to extract the profits from our Malaysian business operations. In addition, our Malaysian subsidiaries may be subject to restrictions on the borrowing of foreign currency or from non-residents, which may affect our ability to raise funds in the future should the need arise. “Ringgit Asset” is defined in the FX Notices as:
| ● | Ringgit-denominated securities or Islamic securities issued in Malaysia by a resident; | |
| ● | Ringgit-denominated securities or Islamic securities issued by a non-resident as approved in writing by the Bank; | |
| ● | Ringgit-denominated Financial Instrument or Islamic Financial Instrument as approved in writing by the Bank; | |
| ● | Ringgit deposit with a Financial Institution in Malaysia including deposit-like instrument with only Ringgit delivery at the inception and maturity; or | |
| ● | any property in Malaysia. |
| 71 |
The relevant rules and regulations on foreign exchange control in Malaysia may also be subject to change from time to time. If there is any adverse change in the foreign exchange rules and regulations relating to the borrowing or repatriation of foreign currency, our business may be materially and adversely affected.
Regulatory risks in relation to environmental hazards, production safety and the occurrence of accidents in Malaysia
As CytoMed Malaysia’s operation in Malaysia involves handling and storing biohazardous materials or articles and sensitive equipment, CytoMed Malaysia is subject to the OSHA. The necessary facilities and measures to comply with OSHA would lead to additional costs of operation.
In the event of occurrence of accidents which causes personal injury of the staff, we may be subject to legal proceedings which may in turn result in civil or criminal liabilities if the legal proceeding taken against us turn out to be successful. Although employees who suffered injuries (including death) in the course of employment are able to claim for benefits under the SOSCO and the EIS, we as employers are not automatically immunized against any claims by virtue of contributions made towards SOSCO and EIS. In fact, courts in Malaysia have recently held that employees are allowed to claim for aggravated and exemplary damages for gross negligence on the part of employers despite having already received compensation from SOSCO.
In such circumstances, CytoMed Malaysia may also be subject to investigation by the relevant authority and if such occurrence of accidents is found to be a breach of any of the conditions of our licenses, permits and approvals, our licenses, permits and approvals for the operation of our business in Malaysia may be revoked. This could cause harm to our reputation and our financial condition may suffer. That said, claims for compensation may be mitigated by us procuring the relevant insurance or workmen’s compensation policies.
We may be subject to costs and risks associated with the monitoring, rehabilitation and compliance with environmental laws and regulations
We are subject to environmental laws and regulations, under which we are required to take steps and/or omit certain steps for the protection and preservation of the environment, including control of pollution and the conservation and management of land. Compliance with environmental laws and regulations will increase our costs and may also hinder, delay or prevent our activities, depending on what is permitted and how these requirements are interpreted and implemented by the relevant regulatory authorities. In addition, economic development and improvements in living standards may increase public awareness of environmental protection. Thus, environmental laws and regulations may become more stringent and/or more stringently enforced. If so, we may not be able to comply with these environmental laws and regulations, and thus may be subject to penalties and liabilities under environmental laws and regulations.
We are subject to regulations governing foreign workers in the event of employment of such foreign workers
In the event our operations in Malaysia require foreign workers, such foreign workers are regulated by the Malaysian government authorities which set a limit on the number of foreign workers which we may hire and also impose levies on each foreign worker hired by our Malaysian companies. Hence, any changes in governmental policies with respect to foreign workers in Malaysia, such as to decrease the number of foreign workers permissible to be employed by the Malaysian companies or to increase the levy payable by Malaysian companies for foreign works may materially and adversely affect our business. Any increase in demand and thus competition for foreign workers may also increase our labor costs.
Mechanism for enforcement of foreign judgments in Malaysia is limited to certain jurisdictions
In Malaysia, foreign judgments may be enforced without the need to commence a fresh action, provided that the foreign judgment in question is granted by a “reciprocating country”. The REJA provides for the mechanism to enforce foreign judgments, which is by way of ex-parte registration with the High Court of Malaya. To be eligible for registration, the foreign judgment must, amongst others, be final between parties, must provide for a sum of money to be payable (monetary judgment), the judgment must not be contrary to the public policy of Malaysia, the judgment was not obtained fraudulently or in a manner contrary to the rules of natural justice, and was pronounced by a specific court from one of the reciprocating countries set out in REJA. At the time of registration, the applicant must show that the foreign judgment was issued or pronounced within 6 years prior, has not been satisfied in full by the judgment debtor and is capable of being enforced or executed in the court of original jurisdiction. Upon registration, the judgment given by the High Court of Malaya will be treated as if it is a judgment given by the court of original jurisdiction.
The reciprocating countries under REJA are United Kingdom, Hong Kong, Singapore, New Zealand, Republic of Sri Lanka, India (excluding State of Jammu and Kashmir, State of Manipur, Tribal areas of State of Assam, Scheduled areas of the States of Madras and Andhra) and Brunei Darussalam. As mentioned, the foreign judgment eligible for registration under REJA must be one given by the specific court as prescribed. For example, in the case of Singapore, only the judgment made by the High Court of Singapore can be enforced in Malaysia through REJA.
As the mechanism provided under REJA is limited to monetary judgments in certain jurisdictions, enforcement of foreign judgments which do not fall within the ambit of REJA requires the initiation of a fresh suit and action in court.
| 72 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Prospectus contains “forward-looking statements”. Forward-looking statements are based on our beliefs and assumptions and on information currently available to us, and include, without limitation, statements regarding our business, financial condition, strategy, results of operations, certain of our plans, objectives, assumptions, expectations, prospects and beliefs and statements regarding other future events or prospects. Forward-looking statements include all statements that are not historical facts and can be identified by the use of forward-looking terminology such as the words “believe”, “expect”, “plan”, “intend”, “seek”, “anticipate”, “estimate”, “predict”, “potential”, “assume”, “continue”, “may”, “will”, “should”, “could”, “shall”, “risk” or the negative of these terms or similar expressions that are predictions of or indicate future events and future trends.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. We caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, the development of the industry in which we operate and the effect of acquisitions on us may differ materially from those made in or suggested by the forward-looking statements contained in this Prospectus. In addition, even if our results of operations, financial condition and liquidity, the development of the industry in which we operate and the effect of acquisitions on us are consistent with the forward-looking statements contained in this Prospectus, those results or developments may not be indicative of results or developments in subsequent periods.
��
Factors that may cause our actual results to differ materially from those expressed or implied by the forward-looking statements in this Prospectus include, but are not limited to, the risks described under “Risk Factors”. For example, factors that could cause actual results to vary from projected results include without limitation the following:
| ● | our plans to develop and commercialize our product candidates; | |
| ● | the initiation, timing, progress and results of our current and future pre-clinical studies and clinical trials and our R&D programs; | |
| ● | our expectations regarding the impact of the ongoing COVID-19 pandemic on our business, our industry and the economy; | |
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; | |
| ● | our ability to successfully acquire or obtain licenses for additional product candidates on reasonable terms; | |
| ● | our ability to establish and maintain collaborations and/or obtain additional funding; | |
| ● | our ability to obtain regulatory approval for our current and future product candidates from the HSA and/or other relevant regulatory authorities; | |
| ● | our expectations regarding the potential market size and the rate and degree of market acceptance of our current and future product candidates; | |
| ● | our ability to fund our working capital requirements and expectations regarding the sufficiency of our capital resources; | |
| ● | our intellectual property position; | |
| ● | developments in and/or disputes concerning our intellectual property or other proprietary rights; | |
| ● | our expectations regarding government and third-party payor coverage and reimbursement; | |
| ● | our ability to compete in the markets we are in; | |
| ● | the impact of government laws and regulations and liabilities thereunder; | |
| ● | our expected use of proceeds from this offering; | |
| ● | our need to hire additional personnel and our ability to attract and retain such personnel; | |
| ● | developments relating to our competitors and our industry; | |
| ● | we are incorporated in Singapore, and our shareholders may have more difficulty protecting their interests than they would as shareholders of a corporation incorporated in the United States; and | |
| ● | other risk factors as discussed under the section titled “Risk Factors”. |
Forward-looking statements speak only as of the date they are made, and we do not undertake any obligation to update them in light of new information or future developments or to release publicly any revisions to these statements in order to reflect later events or circumstances or to reflect the occurrence of unanticipated events.
| 73 |
We estimate that we will receive net proceeds from the sale of ordinary shares of approximately U.S.$9.31 million, based upon an assumed initial public offering price of U.S.$4.50 per share, the midpoint of the range set forth on the cover page of this Prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses. If the underwriters’ option to purchase 361,855 additional ordinary shares is exercised in full, we estimate that we will receive net proceeds of approximately U.S.$10.82 million, after deducting estimated underwriting discounts and commissions and estimated offering expenses.
Each U.S.$1.00 increase (or decrease, as the case may be) in the assumed initial public offering price of U.S.$4.50 per share, the midpoint of the range set forth on the cover page of this Prospectus, would increase (or decrease, as the case may be) the net proceeds to us from this offering by approximately U.S.$2.24 million, assuming the number of shares offered, as set forth on the cover page of this Prospectus, remains the same and after deducting estimated underwriting discounts and commissions. Similarly, each increase (or decrease, as the case may be) of 1 million shares in the number of ordinary shares would increase (or decrease, as the case may be) the net proceeds to us from this offering by approximately U.S.$4.19 million, assuming that the assumed initial public offering price remains the same, and after deducting estimated underwriting discounts and commissions.
We intend to use the net proceeds of this offering in the following manner after we complete the remittance process:
| ● | Approximately U.S.$2 million or 21.5% to advance the clinical development of CTM-N2D including the CTA application, the initiation of a Phase I clinical trial, and up to the stage of commencing a Phase II clinical trial; |
| ● | Approximately U.S.$2 million or 21.5% to continue technology development of iPSC-gdNKT; | |
| ● | Approximately U.S.$1 million or 10.7% to advance the clinical development of CTM-GDT including the CTX application, for a Phase I clinical trial in Malaysia; and |
| ● | the remainder to fund other R&D activities, manufacturing expansion, working capital and general corporate purposes; |
The precise amounts and percentage of proceeds we will allocate to particular categories of activity will depend on prevailing market and business conditions as well as particular opportunities that may arise from time to time. This expected use of our net proceeds from this offering set out above represents our intentions based upon our current plans and business conditions as of the date of this Prospectus, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including any unforeseen cash needs. Similarly, the priority of our prospective uses of proceeds will depend on developing business and market conditions. Accordingly, our management will have flexibility and broad discretion in applying the net proceeds of the offering. If an unforeseen event occurs or business and market conditions change, we may use the proceeds of this offering differently than as described in this Prospectus.
| 74 |
We have never declared nor paid any cash dividends on our ordinary shares, and we do not currently intend to declare or pay any cash dividends in the foreseeable future. We currently intend to retain all available funds and any future earnings, if any, to fund the growth and development of our business. Any future decision to declare and pay cash dividends will be made at the discretion of our Board, subject to applicable laws and organizational documents. Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
| 75 |
The following table sets forth our cash and our capitalization as of June 30, 2022 on:
| ● | an actual basis giving effect to the 1-for-380.83 reverse split as if it had occurred on June 30, 2022; and | |
| ● | a pro forma basis to reflect (i) the above; (ii) the issuance of 486,485 ordinary shares; and (iii) the issuance and conversion of 611,813 ordinary shares for convertible loans; and | |
| ● | a pro forma as adjusted basis to further reflect (i) the above; and (ii) the issuance and sale of 2,412,369 ordinary shares by us in this offering at an initial public offering price of U.S.$4.50 per ordinary share, assuming the underwriters do not exercise the over-allotment option, after deducting underwriting discounts and estimated offering expenses payable by us. |
The pro forma information below is illustrative only, and our capitalization following the completion of this offering is subject to adjustment based on the actual net proceeds to us from the offering. You should read this table together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this Prospectus.
| As of June 30, 2022 | ||||||||||||||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted | ||||||||||||||||||||||
| S$ | U.S.$ | S$ | U.S.$ | S$ | U.S.$ | |||||||||||||||||||
| Cash and cash equivalents | 1,487,161 | 1,069,669 | 2,709,811 | 1,949,083 | 15,295,890 | 11,001,862 | ||||||||||||||||||
| Total liabilities | 3,594,393 | 2,585,336 | 1,142,798 | 821,980 | 1,142,798 | 821,980 | ||||||||||||||||||
| Equity | ||||||||||||||||||||||||
| Ordinary shares, 7,957,975 shares outstanding on an actual basis; 9,056,273 shares outstanding on a pro forma basis; and 11,468,642 shares outstanding on an as adjusted basis (assuming 2,412,369 ordinary shares to be issued in this offering with no exercise of over-allotment option) | 7,690,355 | 5,531,436 | 12,413,000 | 8,928,289 | 25,589,613 | 18,405,821 | ||||||||||||||||||
| Other reserves | (68,188 | ) | (49,046 | ) | (68,188 | ) | (49,046 | ) | (68,188 | ) | (49,046 | ) | ||||||||||||
| Accumulated losses | (6,062,619 | ) | (4,360,655 | ) | (7,111,019 | ) | (5,114,738 | ) | (7,858,098 | ) | (5,652,088 | ) | ||||||||||||
| Non-controlling interest | (543 | ) | (391 | ) | (543 | ) | (391 | ) | (543 | ) | (391 | ) | ||||||||||||
| Total equity | 1,559,005 | 1,121,344 | 5,233,250 | 3,764,114 | 17,662,784 | 12,704,296 | ||||||||||||||||||
| Total capitalization | 1,559,005 | 1,121,344 | 5,233,250 | 3,764,114 | 17,662,784 | 12,704,296 | ||||||||||||||||||
| Net tangible assets | 1,533,593 | 1,103,066 | 5,207,838 | 3,745,836 | 17,637,372 | 12,686,018 | ||||||||||||||||||
| Shares outstanding | 7,957,975 | 7,957,975 | 9,056,273 | 9,056,273 | 11,468,642 | 11,468,642 | ||||||||||||||||||
| Net tangible assets per share | 0.19 | 0.14 | 0.58 | 0.41 | 1.54 | 1.11 | ||||||||||||||||||
| 76 |
If you invest in our ordinary shares, your interest will be diluted to the extent of the difference between the initial public offering price per share and the net tangible assets per share after this offering.
Our historical net tangible assets as of June 30, 2022 were S$1.53 million (approximately U.S.$1.10 million), or S$0.19 (approximately U.S.$0.14) per share of our ordinary shares, as adjusted to reflect a 1-for 380.83 reverse split of our ordinary shares, effective January 17, 2023. Our historical net tangible assets represent our total tangible assets less total liabilities. Historical net tangible assets per share is our historical net tangible book deficit divided by the number of shares of our ordinary shares outstanding as of June 30, 2022.
Pro forma net tangible assets represent our total tangible assets less total liabilities, divided by the pro forma number of shares of our ordinary shares outstanding. As of June 30, 2022, after giving effect to the conversion for convertible loans, our pro forma net tangible assets were S$5.21 million (approximately U.S.$3.75 million), or S$0.58 (approximately U.S.$0.41) per share. After giving effect to the sale and issuance of 2,412,369 shares of our ordinary shares at an assumed initial public offering price of U.S.$4.50 per share of ordinary shares, and after deducting the underwriting discount and commissions and estimated offering expenses payable by us, but assuming no exercise of the ordinary shares offered hereby to the underwriters, our pro forma as adjusted net tangible assets as of June 30, 2022 would have been approximately S$17.64 million (approximately U.S.$12.69 million), or S$1.54 (approximately U.S.$1.11) per share. This represents an immediate increase in pro forma as adjusted net tangible assets of U.S.$0.69 per share to our existing stockholders and an immediate dilution of U.S.$3.39 per share to new investors participating in this offering.
The following table illustrates this dilution on a per share basis and reflects the reverse split effective January 17, 2023:
| U.S.$ | U.S.$ | |||||||
| Assumed initial public offering price per share | 4.50 | |||||||
| Historical net tangible assets per share as of June 30, 2022 | 0.14 | |||||||
| Pro forma increase in net tangible assets per share as of June 30, 2022 attributable to the pro forma transactions described above | 0.28 | |||||||
| Pro forma net tangible assets per share as of June 30, 2022 before giving to effect this offering | 0.41 | |||||||
| Increase per share attributable to this offering | 0.69 | |||||||
| Pro forma net tangible assets per share, as adjusted to give effect to this offering | 1.11 | |||||||
| Dilution in pro forma net tangible assets per share to new investors | 3.39 | |||||||
If the underwriters exercise in full their option to purchase up to 361,855 additional shares of ordinary shares from us, the pro forma as adjusted net tangible assets per share after giving effect to this offering would be U.S.$1.12 per share, representing an immediate increase to existing shareholders of U.S.$0.01 per share, and dilution to new investors participating in this offering of U.S.$3.38 per share.
| 77 |
The following table summarizes on the pro forma as adjusted basis described above, the differences between the number of shares purchased from us on an as converted basis, the total consideration paid and the weighted-average price per share paid by existing shareholders and by investors purchasing shares in this offering at the assumed initial public offering price of U.S.$4.50 per share, the midpoint of the price range set forth on the cover page on this Prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us:
| Shares Purchased | Total Consideration | Average Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| % | U.S.$ | % | U.S.$ | |||||||||||||||||
| Existing shareholders | 9,056,273 | 78.97 | 7,669,571.32 | 41.40 | 0.85 | |||||||||||||||
| New investors | 2,412,369 | 21.03 | 10,855,660.50 | 58.60 | 4.50 | |||||||||||||||
| Total | 11,468,642 | 100.00 | 18,525,231.82 | 100.00 | 1.62 | |||||||||||||||
A U.S.$1.00 increase or decrease in the assumed initial public offering price of U.S.$4.50 per share, the midpoint of the price range set forth on the cover page of this Prospectus, would increase or decrease the total consideration paid by new investors by U.S.$2.41 million and, in the case of an increase, would increase the percentage of total consideration paid by new investors to 63.37% and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors to 52.40%, assuming that the number of shares offered by us, as set forth on the cover page of this Prospectus, remains the same. Similarly, an increase or decrease of 1 million shares of ordinary shares offered by us would increase or decrease the total consideration paid by new investors by U.S.$4.50 million and, in the case of an increase, would increase the percentage of total consideration paid by new investors to 66.69% and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors to 45.32%, assuming that the assumed initial public offering price remains the same.
If the underwriters exercise their option to purchase additional shares in full, our existing shareholders would own 76.55% and our new investors would own 20.39% of the total number of shares of our ordinary shares outstanding upon the completion of this offering.
The foregoing discussion and table above gives effect to the pro forma transactions described and excludes:
● 1,279,117 ordinary shares reserved for future issuance under our Incentive Plan, which was adopted by the Company on January 18, 2023 and to be effective upon the consummation of this offering, as well as any future increases in the number of ordinary shares reserved for issuance under our Incentive Plan; and
● 120,618 ordinary shares underlying the warrants to be issued to the representative of the underwriters in connection with this offering.
To the extent that any outstanding warrants are exercised, new options or other equity awards are issued under our equity incentive plans, or we issue additional shares in the future, there will be further dilution to new investors participating in this offering.
| 78 |
SELECTED CONSOLIDATED FINANCIAL INFORMATION
The following selected consolidated financial information as of December 31, 2020 and 2021 and June 30, 2022 and for the years ended December 31, 2020 and 2021 and for the six months ended June 30, 2021 and 2022 have been derived from our audited consolidated financial statements and unaudited interim condensed financial statements, respectively, included elsewhere in this Prospectus. Our consolidated financial statements are prepared and presented in accordance with IFRS. Our historical results are not necessarily indicative of results expected for future periods. The following selected consolidated financial information should be read in conjunction with the sections entitled “Presentation of Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements of CytoMed, including the notes thereto, included elsewhere in this Prospectus.
The following table presents our selected consolidated statements of profit or loss and comprehensive loss data for the financial years ended December 31, 2020 and 2021 and for the six months ended June 30, 2021 and 2022:
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||||||
| 2020 | 2021 | 2021 | 2022 | |||||||||||||||||||||
| S$ | S$ | U.S.$ | S$ | S$ | U.S.$ | |||||||||||||||||||
| Other operating income | 127,456 | 154,610 | 111,206 | 49,405 | 149,570 | 107,581 | ||||||||||||||||||
| Other (losses)/gains - net | (573,856 | ) | 3,185 | 2,291 | (209,533 | ) | (150,136 | ) | (107,988 | ) | ||||||||||||||
| R&D expenses | (1,038,091 | ) | (1,090,623 | ) | (784,452 | ) | (522,881 | ) | (604,043 | ) | (434,470 | ) | ||||||||||||
| General and administrative expenses | (345,616 | ) | (788,801 | ) | (567,360 | ) | (284,721 | ) | (306,457 | ) | (220,425 | ) | ||||||||||||
| Finance expenses | (105,519 | ) | (117,696 | ) | (84,655 | ) | (57,668 | ) | (62,042 | ) | (44,625 | ) | ||||||||||||
| Share of results of associate | - | (212,578 | ) | (152,901 | ) | - | (22,283 | ) | (16,027 | ) | ||||||||||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||||||
| Income tax expenses | - | - | - | - | - | - | ||||||||||||||||||
| Loss for the year/period | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||||||
| Other comprehensive income: | ||||||||||||||||||||||||
| Foreign currency translation loss, net of tax | (6,616 | ) | (22,628 | ) | (16,275 | ) | (25,669 | ) | (37,208 | ) | (26,763 | ) | ||||||||||||
| Total comprehensive loss | (1,942,242 | ) | (2,074,531 | ) | (1,492,146 | ) | (1,051,067 | ) | (1,032,599 | ) | (742,717 | ) | ||||||||||||
| Loss for the year/period attributable to equity holders of the Company | (1,935,440 | ) | (2,051,650 | ) | (1,475,689 | ) | (1,025,270 | ) | (995,287 | ) | (715,879 | ) | ||||||||||||
| Loss per share attributable to equity holders of the Company | ||||||||||||||||||||||||
| Basic and diluted | (0.33 | ) | (0.30 | ) | (0.21 | ) | (0.16 | ) | (0.13 | ) | (0.09 | ) | ||||||||||||
LOSS PER SHARE
| December 31, | June 30, | |||||||||||||||
| 2020 | 2021 | 2021 | 2022 | |||||||||||||
| Weighted average number of ordinary shares used in computing basic loss | 5,897,651 | 6,934,824 | 6,543,850 | 7,672,622 | ||||||||||||
| Weighted average number of ordinary shares used in computing diluted loss | 5,897,651 | 6,934,824 | 6,543,850 | 7,672,622 | ||||||||||||
The following table presents our selected consolidated balance sheets data as of December 31, 2020 and 2021 and as of June 30, 2022:
| As of December 31, | As of June 30, | |||||||||||||||||||
| 2020 | 2021 | 2022 | ||||||||||||||||||
| S$ | S$ | U.S.$ | S$ | U.S.$ | ||||||||||||||||
| Cash and cash equivalents | 885,272 | 2,512,768 | 1,807,357 | 1,487,161 | 1,069,669 | |||||||||||||||
| Property, plant and equipment | 2,301,025 | 2,495,791 | 1,795,146 | 2,684,305 | 1,930,738 | |||||||||||||||
| Total assets | 3,815,299 | 6,082,527 | 4,374,974 | 5,153,398 | 3,706,680 | |||||||||||||||
| Convertible loans | 2,323,423 | 2,310,757 | 1,662,056 | 2,451,595 | 1,763,357 | |||||||||||||||
| Total liabilities | 3,339,519 | 3,490,923 | 2,510,914 | 3,594,393 | 2,585,336 | |||||||||||||||
| Total equity | 475,780 | 2,591,604 | 1,864,060 | 1,559,005 | 1,121,344 | |||||||||||||||
| 79 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the notes thereto, included elsewhere in this Prospectus, as well as the sections titled “Presentation of Financial Information” and “Selected Consolidated Financial Information”. The following discussion and analysis include forward-looking statements. These forward-looking statements are subject to risks, uncertainties and other factors that could cause our actual results to differ materially from those expressed or implied by the forward-looking statements. Factors that could cause or contribute to these differences include, but are not limited to, those discussed elsewhere in this Prospectus. Please refer to the sections titled “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors” for further details.
Overview
We are a pre-clinical biopharmaceutical company focused on the R&D of allogeneic, “off-the-shelf” cell-based immunotherapies for treatment of human cancers. The development of our novel technologies has been inspired by the clinical success of existing CAR-T cells in treating hematological malignancies as well as the current clinical limitations and commercial challenges in extrapolating the CAR-T principle into treatment of solid tumors. All of our product candidates are designed to be allogeneic, meaning they are produced using cells from a different person than the patient treated, as well as on an “off-the-shelf” basis, unlike existing autologous cell therapies. Built on our proprietary platform technologies, we are developing three product candidates: CTM-N2D, iPSC-gdNKT and CTM-GDT.
Since our incorporation in 2018, we have devoted most of our resources to support our product candidate development efforts, building our intellectual property portfolio, developing our supply chain, conducting business planning, constructing our centralized cGMP Facility, hiring and training staff, raising capital and providing general and administrative support for these operations. We do not have any product candidates approved for commercial sale and have not generated any revenue. To date, we have funded our operations primarily from private equity financing and issuance of convertible loans.
We recorded a loss of S$1.94 million and S$2.05 million (approximately U.S.$1.48 million) for the financial years ended December 31, 2020 and 2021, respectively, and S$1.03 million for the six months ended June 30, 2021 and S$995,391 (approximately U.S.$715,954) for the six months ended June 30, 2022. We expect to continue to incur losses for the foreseeable future in connection with our ongoing activities. As of December 31, 2020, 2021 and June 30, 2022, we had accumulated losses of S$3.02 million, S$5.07 million and S$6.06 million (approximately U.S.$4.36 million), respectively and our net tangible assets were S$430,532, S$2.56 million and S$1.53 million (approximately U.S.$1.10 million), respectively. Our ability to generate product revenue sufficient to achieve profitability will depend on the successful development and commercialization of one or more of our product candidates.
We expect that our expenses and capital requirements will increase significantly in connection with our ongoing activities as we continue to develop and seek regulatory approvals for our product candidates, engage in other R&D activities to expand our pipeline of product candidates, maintain and expand our intellectual property portfolio, and ultimately establish a sales organization and operate as a public company. Our loss for the year may fluctuate significantly from quarter-to-quarter and year-to-year, depending on the timing of our clinical trials, and our expenditures on other R&D activities.
| 80 |
We will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations or other strategic transactions. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue the development and commercialization of one or more of our product candidates.
Based on our current operating plan, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient to fund our operations for at least the next 2 years from the date of this Prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. Please refer to the section titled “Liquidity and Capital Resources”.
COVID-19 Pandemic
The ongoing COVID-19 pandemic, which has spread worldwide and continues to rapidly evolve as of the date of this Prospectus, may continue to affect our ability to initiate and complete pre-clinical studies, delay the initiation of our planned clinical trials or future clinical trials, disrupt regulatory activities, and have other adverse effects on our business, results of operations, and financial condition. In addition, the COVID-19 pandemic has caused substantial disruption in the global financial markets and may adversely impact economies worldwide, both of which could result in adverse effects on our business and operations and our ability to raise additional funds to support our operations.
As the ultimate duration of the COVID-19 pandemic, disruption to our business and related financial impact in the future are still highly uncertain, it poses operational challenges to our business, especially production and supply chain management. We are continuing to monitor the COVID-19 situation closely.
We are following, and will continue to follow, recommendations and precautionary measures imposed by the relevant governments of the countries in which we operate. We expect to continue to take actions as may be required or recommended by government authorities or as we determine are in the best interests of our employees and other business partners in light of the COVID-19 pandemic.
As of the date of this Prospectus, we cannot be certain what the overall impact of the COVID-19 pandemic will be on our business, and it has the potential to adversely affect our business, financial condition, results of operations and prospects.
Financial Operations Overview
Revenue
Since our incorporation, we have not generated any revenue and do not expect to generate any revenue from the commercial sale of products in the foreseeable future. As of the date of this Prospectus, we have no therapeutic products approved for sale commercially. If our development efforts for one or more of our product candidates are successful and result in regulatory approval, or if we enter into collaboration with third parties, we may generate revenue from a combination of product sales or payments from collaboration in the future.
Other Operating Income
Other operating income primarily consists of government grants received in view of the COVID-19 pandemic and support in relation to the technology innovation, research income and interest income.
Other (Losses)/Gains – Net
Other (losses)/gains – net, consist of the fair value changes on convertible loans and the net currency exchange losses.
| 81 |
Expenses
R&D Expenses
Our R&D expenses consist primarily of costs incurred for our research and pre-clinical activities, which include:
| ● | salaries, welfare benefits and other related expenses for the employees engaged in research functions; | |
| ● | depreciation of property, plant and equipment associated with research activities; | |
| ● | costs of manufacturing product candidates and consultants engaged in research, pre-clinical studies and potential future clinical trials; | |
| ● | expenses related to the upkeep of the facilities we use, which include expenses for maintenance of facilities and laboratory equipment; | |
| ● | costs related to regulatory compliance; and | |
| ● | royalty payments for our intellectual property used in research activities. |
We expense research costs as incurred. Non-refundable advance payments for goods or services to be received in the future for use in R&D activities are recorded as prepaid expenses. The prepaid amounts are expensed as the related goods are delivered or the services are performed. We allocate our direct R&D expenses across each product candidate.
Our R&D activities are central to our business. We expect that our R&D expenses will continue to increase in absolute amounts in the foreseeable future as we continue pre-clinical development for our product candidates and continue our advances in scientific research and product development.
General and Administrative Expenses
General and administrative expenses consist mainly of salaries, welfare benefits and other related expenses for employees engaged in finance, operations and administrative functions. General and administrative expenses also include, inter alia, professional fees for legal, auditing, tax and consulting services, depreciation and other expenses that are not attributable to R&D activities.
We expect that our general and administrative expenses will increase in the near-term as we continue to build a team to support our administrative, accounting and finance efforts. Following this offering, we expect to incur increased expenses associated with being a public company, including costs of accounting, audit, legal, regulatory and tax compliance services, director and officer insurance costs, and investor and public relations costs.
Finance Expenses
Finance expenses consisted of interest expenses on our convertible loans issued in financial year 2020 that were still outstanding, interest expenses on bank borrowing, third party loan and lease liabilities.
Share of results of associate
Share of results of associate consisted of the share of post-acquisition results of an associate incorporated in Malaysia during the financial year and its impairment loss.
Results of Operations
Six Months Ended June 30, 2021 and 2022
The following table summarizes our consolidated results of operations for the six months ended June 30, 2021 and 2022:
| Six Months Ended June 30, | ||||||||||||||||
| 2021 | 2022 | Change | ||||||||||||||
| S$ | S$ | U.S.$ | S$ | |||||||||||||
| Other operating income | 49,405 | 149,570 | 107,581 | 100,165 | ||||||||||||
| Other losses – net | (209,533 | ) | (150,136 | ) | (107,988 | ) | 59,397 | |||||||||
| R&D expenses | (522,881 | ) | (604,043 | ) | (434,470 | ) | (81,162 | ) | ||||||||
| General and administrative expenses | (284,721 | ) | (306,457 | ) | (220,425 | ) | (21,736 | ) | ||||||||
| Finance expenses | (57,668 | ) | (62,042 | ) | (44,625 | ) | (4,374 | ) | ||||||||
| Share of results of associate | - | (22,283 | ) | (16,027 | ) | (22,283 | ) | |||||||||
| Loss before income tax | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | (30,007 | ) | ||||||||
| Income tax expense | - | - | - | - | ||||||||||||
| Loss for the period | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | (30,007 | ) | ||||||||
Other Operating Income
Other operating income increased by S$100,165 (approximately U.S.$72,046) from S$49,405 for the six months ended June 30, 2021 to S$149,570 (approximately U.S.$107,581) for the six months ended June 30, 2022, mainly due to an increase of a government grant income of S$50,000 (approximately U.S.$35,963) from Enterprise Singapore under the Startup SG Tech - Proof-of-concept which is a scheme to help the Company to develop and commercialize their innovations.
Other Losses - Net
Other losses - net, decreased from S$209,533 for the six months ended June 30, 2021 to S$150,136 (approximately U.S.$107,988) for the six months ended June 30, 2022, primarily pertaining to a decrease of S$55,890 (approximately U.S.$58,377) in fair value of the convertible loans from S$196,728 to S$140,838 (approximately U.S.$101,300).
R&D Expenses
R&D expenses were S$522,881 for the six months ended June 30, 2021, compared to S$604,043 (approximately U.S.$434,470) for the six months ended June 30, 2022. The increase of S$81,162 (approximately U.S.$58,377) was primarily attributable to an increase of S$31,517 (approximately U.S.$ 22,670) in laboratory supplies and services for research, pre-clinical activities and facility-related expenses, higher employee benefits of S$66,560 (approximately U.S.$ 47,875) resulting from increase in R&D personnel headcount and employee salary rates, and also contributed by the increase of S$19,034 (approximately U.S.$13,691) in depreciation of property, plant and equipment. The increase was partially offset by a decrease of S$27,450 (approximately U.S.$19,744) in professional fees associated with research activities.
General and Administrative Expenses
General and administrative expenses were S$284,721 and S$306,457 (approximately U.S.$220,425) for the six months ended June 30, 2021 and 2022, respectively. The increase of S$21,736 (approximately U.S.$15,634) was mainly driven by an increase of S$18,538 (approximately U.S.$13,334) in employee benefits, higher depreciation of S$10,794 (approximately U.S.$7,764) and higher expenses on IT, general repair and maintenance, utilities and purchase of tools and supplies of S$8,869 (approximately U.S.$6,379). The increase was partially offset by a decrease of S$16,823 (approximately U.S.$12,100) in professional fees primarily pertaining to the offering expenses.
Finance Expenses
Finance expenses increased by S$4,374 (approximately U.S.$3,146) from S$57,668 for the six months ended June 30, 2021 to S$62,042 (approximately U.S.$44,625) for the six months ended June 30, 2022. This resulted from additional interest incurred of S$5,207 (approximately U.S.$3,745) from a third-party loan drawn down in October 2021. The increase was partially offset by a decrease of S$627 (approximately U.S.$451) in bank loan interest and a decrease of S$186 (approximately U.S.$134) in interest on lease liabilities.
Share of results of associate
Share of results of associate recognized by S$22,283 (approximately U.S.$16,027) for the six months ended June 30, 2022, which pertain to the share of loss from the associate during the period.
Years Ended December 31, 2020 and 2021
The following table summarizes our consolidated results of operations for the years ended December 31, 2020 and 2021:
| Year Ended December 31, | ||||||||||||||||
| 2020 | 2021 | Change | ||||||||||||||
| S$ | S$ | U.S.$ | S$ | |||||||||||||
| Other operating income | 127,456 | 154,610 | 111,206 | 27,154 | ||||||||||||
| Other (losses)/gains – net | (573,856 | ) | 3,185 | 2,291 | 577,041 | |||||||||||
| R&D expenses | (1,038,091 | ) | (1,090,623 | ) | (784,452 | ) | (52,532 | ) | ||||||||
| General and administrative expenses | (345,616 | ) | (788,801 | ) | (567,360 | ) | (443,185 | ) | ||||||||
| Finance expenses | (105,519 | ) | (117,696 | ) | (84,655 | ) | (12,177 | ) | ||||||||
| Share of results of associate | - | (212,578 | ) | (152,901 | ) | (212,578 | ) | |||||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (116,277 | ) | ||||||||
| Income tax expense | - | - | - | - | ||||||||||||
| Loss for the year | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | (116,277 | ) | ||||||||
| 82 |
Other Operating Income
Other operating income increased by S$27,154 (approximately U.S.$19,531) from S$127,456 for the financial year ended December 31, 2020 to S$154,610 (approximately U.S.$111,206) for the financial year ended December 31, 2021, mainly due to the increases in research income of S$54,373 (approximately U.S.$39,109) and interest income from fixed deposits placed with financial institution of S$3,369 (approximately U.S.$2,423). The increase was partially offset by a decrease in government grant income of S$24,620 (approximately U.S.$17,708) mainly pertaining to the various support measures from the Singapore and Malaysia governments in view of the COVID-19 pandemic being provided during the financial year 2020.
Other (Losses)/Gains - Net
We recorded other losses of S$573,856 for the financial year ended December 31, 2020, compared to other gains of S$3,185 (approximately U.S.$2,291) for the financial year ended December 31, 2021. The change was primarily pertaining to the fair value on the convertible loans from loss of S$573,423 (approximately U.S.$412,446) for the financial year ended December 31, 2020 to a gain of S$12,666 (approximately U.S.$9,110) for the financial year ended December 31, 2021.
R&D Expenses
R&D expenses were S$1.04 million for the financial year ended December 31, 2020, compared to S$1.09 million (approximately U.S.$784,452) for the financial year ended December 31, 2021. The increase of S$52,532 (approximately U.S.$37,785) was primarily attributable to the increase in laboratory supplies and services of S$145,990 (approximately U.S.$105,006) for research, pre-clinical activities and facility-related expenses, and higher employee benefits of S$57,415 (approximately U.S.$41,297) resulting from the continued growth in headcount. The increase was partially offset by the decrease in professional fees of S$139,128 (approximately U.S.$100,070) pertaining to the clinical trial preparations.
General and Administrative Expenses
General and administrative expenses were S$345,616 and S$788,801 (approximately U.S.$567,360) for the financial years ended December 31, 2020 and 2021, respectively. The increase of S$443,185 (approximately U.S.$318,769) was due mainly to the professional fees of S$377,430 (approximately U.S.$271,474) in relation to this offering and valuation service fee for the convertible loans, increase in employee benefits of S$37,811 (approximately U.S.$27,196) and higher depreciation on renovation and computer equipment of S$12,597 (approximately U.S.$9,061).
Finance Expenses
Finance expenses increased by S$12,177 (approximately U.S.$8,759) from S$105,519 for the financial year ended December 31, 2020 to S$117,696 (approximately U.S.$84,655) for the financial year ended December 31, 2021. This resulted from a drawdown of convertible loan of S$500,000 since August 2020.
Share of results of associate
Share of results of associate recognized by S$212,578 (approximately U.S.$152,901) for the financial year ended December 31, 2021, which consist of the impairment of investment in an associate of S$215,297 (approximately U.S.$ 154,857), partially offset by the share of post-acquisition profit of S$2,719 (approximately U.S.$1,956).
| 83 |
Liquidity and Capital Resources
Since our incorporation, we have not generated any revenue from commercial sales of product and have incurred losses and negative cash flows from our operations and expect these conditions to continue for the foreseeable future. We have not yet commercialized any of our product candidates, which are in various phases of pre-clinical development, and we do not expect to generate revenue from commercial sales of any products for the foreseeable future.
Our loss was S$1.94 million and S$2.05 million (approximately U.S.$1.48 million) for the financial years ended December 31, 2020 and 2021, respectively and S$1.03 million for the six months ended June 30, 2021 and S$995,391 (approximately U.S.$715,954) for the six months ended June 30, 2022, respectively. As of December 31, 2021 and June 30, 2022, we had accumulated losses of S$5.07 million and S$6.06 million (approximately U.S.$4.36 million), respectively and net cash used in operating activities of S$632,439 and S$484,798 (approximately U.S.$348,701), respectively. We expect that our expenses and capital requirements will increase significantly in connection with our ongoing activities as we continue to develop and seek regulatory approvals for our product candidates, engage in other R&D activities to expand our pipeline of product candidates, maintain and expand our intellectual property portfolio, and ultimately establish a sales organization and operate as a public company.
As of December 31, 2021 and June 30, 2022, we had cash and cash equivalents of S$2.51 million and S$1.49 million (approximately U.S.$1.07 million), respectively. To date, we have funded our operations primarily through private equity financing and issuance of convertible loans. We issued an aggregate of S$1.75 million (approximately U.S.$1.26 million) convertible loans in 2020. Between April and June 2021, we raised an aggregate of S$4 million (approximately U.S.$2.88 million) gross proceeds from the sale of our equities. The Company raised capital in an aggregate of S$1.22 million (approximately U.S.$879,415) between September and October 2022.
Based on our current operating plans, we believe that the net proceeds from this offering, together with our current resources, will be sufficient to meet our current and anticipated working capital requirements and capital expenditures for at least the next 2 years from the date of this Prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect.
Cash Flows
The following table summarizes our cash flows for the year/period presented:
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||||||
| 2020 | 2021 | 2021 | 2022 | |||||||||||||||||||||
| S$ | S$ | U.S.$ | S$ | S$ | U.S.$ | |||||||||||||||||||
| Net cash used in operating activities | (849,443 | ) | (1,712,985 | ) | (1,232,096 | ) | (632,439 | ) | (484,798 | ) | (348,701 | ) | ||||||||||||
| Net cash used in investing activities | (513,054 | ) | (823,666 | ) | (592,437 | ) | (255,123 | ) | (422,072 | ) | (303,583 | ) | ||||||||||||
| Net cash from/(used in) financing activities | 1,883,522 | 4,173,396 | 3,001,794 | 3,906,863 | (100,404 | ) | (72,218 | ) | ||||||||||||||||
| Net changes in cash and cash equivalents | 521,025 | 1,636,745 | 1,177,261 | 3,019,301 | (1,007,274 | ) | (724,502 | ) | ||||||||||||||||
| Cash and cash equivalents at beginning of financial year/period | 370,942 | 885,272 | 636,749 | 885,272 | 2,512,768 | 1,807,357 | ||||||||||||||||||
| Effect of foreign currency translation on cash and cash equivalents | (6,695 | ) | (9,249 | ) | (6,653 | ) | (8,613 | ) | (18,333 | ) | (13,186 | ) | ||||||||||||
| Cash and cash equivalents at end of the financial year/period | 885,272 | 2,512,768 | 1,807,357 | 3,895,960 | 1,487,161 | 1,069,669 | ||||||||||||||||||
| 84 |
Operating Activities
During the six months ended June 30, 2021, net cash used in operating activities was S$632,439. This was primarily attributable to the loss for the year of S$1.03 million, adjusted for non-cash charges that primarily included depreciation and amortization of S$161,158 and fair value loss on convertible loans of S$196,728, and S$57,668 in interest expenses, S$870 in interest income and a S$4,819 net change in working capital.
During the six months ended June 30, 2022, net cash used in operating activities was S$484,798 (approximately U.S.$348,701). This was primarily attributable to the loss for the year of S$995,391 (approximately U.S.$715,954), adjusted for non-cash charges that primarily included depreciation and amortization of S$190,984 (approximately U.S.$137,369) and fair value loss on convertible loans of S$140,838 (approximately U.S.$101,300), and S$62,042 (approximately U.S.$44,625) in interest expenses, S$1,468 (approximately U.S.$1,056) in interest income, S$22,283 (approximately U.S.$16,027) in share of results of associate and a S$91,049 (approximately U.S.$65,489) net change in working capital.
During the financial year ended December 31, 2020, net cash used in operating activities was S$849,443. This was primarily attributable to the loss for the year of S$1.94 million, adjusted for non-cash charges of S$865,073 for depreciation and amortization, fair value loss on convertible loans and unrealized currency translation gains, and S$105,519 in interest expenses and a S$115,591 net change in working capital.
During the financial year ended December 31, 2021, net cash used in operating activities was S$1.71 million (approximately U.S.$1.23 million). This was primarily attributable to the loss for the year of S$2.05 million (approximately U.S.$1.48 million), adjusted for non-cash charges of S$377,892 (approximately U.S.$271,806) for depreciation and amortization, fair value gain on convertible loans and unrealized currency translation losses, and S$117,696 (approximately U.S.$84,655) in interest expenses, S$3,369 (approximately U.S.$2,423) in interest income, S$212,578 (approximately U.S.$152,901) in share of results of associate and a S$369,248 (approximately U.S.$265,587) net change in working capital.
Investing Activities
Net cash used in investing activities during the six months ended June 30, 2021 was S$255,123, mainly arose from the purchase of plant and equipment of S$13,810 and the balance payment for equity investment in an associate of S$241,313.
Net cash used in investing activities during the six months ended June 30, 2022 was S$422,072 (approximately U.S.$303,583), used in purchase of plant and equipment.
Net cash used in investing activities during the financial year ended December 31, 2020 was S$513,054, mainly arose from the purchase of plant and equipment of S$244,725 and advanced payment for the equity investment in an associate of S$247,899.
Net cash used in investing activities during the financial year ended December 31, 2021 was S$823,666 (approximately U.S.$592,437), mainly due to the purchase of property, plant and equipment of S$582,354 (approximately U.S.$418,869) and the balance payment for acquisition of an associate for S$241,312 (approximately U.S.$173,568).
Financing Activities
During the six months ended June 30, 2021, net cash generated from financing activities was S$3.91 million mainly due to the proceeds from issuance of ordinary shares of S$2 million and proceeds from issuance of shares application monies of S$2 million. This was partially mitigated by the interest payment of S$57,668 and repayment of bank borrowings and lease liabilities of S$35,469.
.
During the six months ended June 30, 2022, net cash used in financing activities was S$100,404 (approximately U.S.$72,218) due to the interest payment of S$62,042 (approximately U.S.$44,625) and repayment of bank borrowings and lease liabilities of S$38,362 (approximately U.S.$27,593).
During the financial year ended December 31, 2020, net cash generated from financing activities was S$1.88 million mainly due to the proceeds from convertible loan of S$1.50 million and proceeds from issuance of ordinary shares of S$500,000. This was partially mitigated by the interest payment of S$105,519.
During the financial year ended December 31, 2021, net cash generated from financing activities was S$4.17 million (approximately U.S.$3 million) mainly due to the proceeds from issuance of ordinary shares of S$2 million (approximately U.S.$1.44 million), proceeds from share application monies of S$2 million (approximately U.S.$1.44 million), and proceeds from a third party loan of S$350,000 (approximately U.S.$251,744). This was partially offset by the interest payment of S$117,696 (approximately U.S.$84,655) and repayment of bank borrowings of S$51,452 (approximately U.S.$37,008).
Funding Requirements
Our plan of operation is to continue implementing our business strategy, continue R&D of CTM-N2D and our other product candidates and continue to expand our research pipeline and our internal R&D capabilities. We expect our expenses to increase substantially in connection with our ongoing activities, particularly as we advance the pre-clinical activities of our product candidates. In addition, we expect to incur additional costs associated with operating as a public company following the consummation of this offering. Our future capital requirements will depend on many factors, including:
| ● | the scope, timing, progress, costs, and results of discovery, pre-clinical development, and clinical trials for our current and future product candidates; | |
| ● | the number of clinical trials required for regulatory approval of our current and future product candidates; | |
| ● | the costs, timing, and outcome of regulatory review of any of our current and future product candidates; | |
| ● | the cost of manufacturing clinical and commercial supplies of our current and future product candidates; | |
| 85 |
| ● | the costs and timing of future commercialization activities, including manufacturing, marketing, sales, and distribution, for any of our product candidates for which we receive marketing approval; | |
| ● | the costs and timing of preparing, filing, and prosecuting patent applications, maintaining and enforcing our intellectual property rights, and defending any intellectual property-related claims, including any claims by third parties that we are infringing on their intellectual property rights; | |
| ● | the cost of maintaining our own R&D and centralized cGMP Facility and future facility expansion plans; | |
| ● | our ability to maintain existing, and establish new, strategic collaborations, licensing, or other arrangements and the financial terms of any such agreements, including the timing and amount of any future milestone, royalty, or other payments due under any such agreement; | |
| ● | the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval; | |
| ● | the expenses to attract, hire and retain, skilled personnel; | |
| ● | the costs of operating as a public company; | |
| ● | our ability to establish a commercially viable pricing structure and obtain approval for coverage and adequate reimbursement from third-party and government payors; | |
| ● | addressing any potential interruptions or delays resulting from factors related to the ongoing COVID-19 pandemic; | |
| ● | the effect of competing technological and market developments; and | |
| ● | the extent to which we acquire or invest in businesses, products and technologies. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. As of December 31, 2021 and June 30, 2022, we had cash and cash equivalents of S$2.51 million and S$1.49 million (approximately U.S.$1.07 million). Based on our operating plans, we believe that the net proceeds from this offering, together with our existing cash, will be sufficient to fund our operations for at least the next 2 years. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect.
Until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our operations from the sale of additional equity or debt financings, or other capital which comes in the form of strategic collaborations, licensing, or other arrangements. In the event that additional financing is required, we may not be able to raise it on terms favorable to us, or at all. If we raise additional funds through the issuance of equity or convertible debt securities, it may result in dilution to our existing shareholders. Debt financing or preferred equity financing, if available, may result in increased fixed payment obligations, and the existence of securities with rights that may be senior to those of our ordinary shares. If we incur indebtedness, we could become subject to covenants that would restrict our operations.
If we raise funds through strategic collaboration, licensing or other arrangements, we may relinquish significant rights or grant licenses on terms that are not favorable to us. Our ability to raise additional funds may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic and otherwise. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves.
| 86 |
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of June 30, 2022:
| Payment Due by Period | ||||||||||||||||||||
| Total | Less than 1 year | Between 1 and 2 years | Between 2 and 5 years | Over 5 years | ||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | ||||||||||||||||
| Contractual Obligations: | ||||||||||||||||||||
| Bank borrowings (1)(2) | 698,114 | 58,583 | 58,583 | 175,749 | 405,199 | |||||||||||||||
| Third party loan (3) | 350,000 | 350,000 | - | - | - | |||||||||||||||
| Convertible bonds (2)(3) | 1,791,589 | 1,791,589 | - | - | - | |||||||||||||||
| Lease liabilities (2) | 19,901 | 8,529 | 8,529 | 2,843 | - | |||||||||||||||
| Commitment: | ||||||||||||||||||||
| Minimum royalty commitments | 169,773 | 4,458 | 4,458 | 38,342 | 122,515 | |||||||||||||||
| (1) | Includes scheduled principal payments on bank borrowings outstanding as of June 30, 2022. |
| (2) | Includes interest payments. |
| (3) | The amounts disclosed in the table above are based on the contractual undiscounted cash flows. |
Other than as shown above, we did not have any significant capital and other commitments, long term obligations or guarantees as of June 30, 2022.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with IFRS. The preparation of our financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, costs and expenses and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in greater detail in Note 2 to our financial statements in this Prospectus, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our financial statements.
R&D Expenses
We record as expenses all costs incurred in conducting research and pre-clinical activities in the periods in which such costs are incurred. R&D expenses include employees’ benefits, laboratory supplies, fees paid to entities that conduct certain quality testing activities as well as facility-related expenses.
As part of the process of preparing our financial statements, we are required to estimate our accrued R&D expenses. We make estimates of our accrued expenses as of each balance sheet date in the financial statements based on facts and circumstances known to us at that time. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we adjust the accrual or the amount of prepaid expenses accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are too high or too low in any particular period. To date, there have not been any material adjustments to our prior estimates of accrued R&D expenses.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements and do not have any holdings in variable interest entities.
| 87 |
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to our financial statements in this Prospectus.
Quantitative and Qualitative Disclosures about Market Risks
We are exposed to market risks in the ordinary course of our business. These risks primarily include currency risk and interest rate risk.
Currency risk
We operate in Asia with dominant operations in Singapore and Malaysia. We regularly transact in currencies other than our respective functional currencies (“foreign currencies”). Currency risk arises when transactions are denominated in foreign currencies other than functional currency. In addition, we are exposed to currency translation risk on the net assets in foreign operations.
Interest rate risk
As of December 31, 2021 and June 30, 2022, we had cash and cash equivalents of S$2.51 million and S$1.49 (approximately U.S.$1.07 million). Our exposure to interest rate sensitivity is impacted by changes in the underlying Malaysia bank interest rates but is minimal. We have not entered into investments for trading or speculative purposes.
Emerging Growth Company Status
We are an “emerging growth company” under the JOBS Act. The JOBS Act, permits that an “emerging growth company” may take advantage of the extended transition period for complying with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have elected to avail ourselves of delayed adoption of certain accounting standards. Accordingly, our financial statements may not be comparable to the financial statements of public companies that comply with such new or revised accounting standards. We intend to rely on other exemptions provided by the JOBS Act, including without limitation, not being required to comply with the auditor attestation requirements of Section 404(b) of Sarbanes-Oxley Act.
We will remain an emerging growth company until the earliest of (i) the last day of the financial year in which we have more than U.S.$1.235 billion in annual revenue, (ii) the date we qualify as a “large accelerated filer” as defined in Rule 12b-2 under Exchange Act, which would occur if the market value of our ordinary shares held by non-affiliates exceeded U.S.$700 million, (iii) the issuance, in any three-year period, by us of more than U.S.$1 billion in non-convertible debt securities, and (iv) the last day of the financial year ending after the fifth anniversary of our initial public offering.
| 88 |
Overview
We are a pre-clinical biopharmaceutical company focused on harnessing our proprietary technologies into creating novel cell-based immunotherapies for treatment of human cancers. The development of our novel technologies has been inspired by the clinical success of existing CAR-T cells in treating hematological malignancies as well as the current clinical limitations and commercial challenges in extrapolating the CAR-T principle into treatment of solid tumors.
We believe that the current development of CD19-targeting CAR-T cells in treating B-cell malignancies signifies that cellular immunotherapy is becoming one of the pillars in cancer care. However, we believe that it remains challenging to apply the current CAR-T principle into treatments of other type of cancers, in particular solid tumors, due to a variety of reasons, including (i) the reliance on the limited cell quality and quantity of patients, (ii) the lack of suitable surface cancer antigens and their recognition system and (iii) the risk of cancer escape because of a single antigen-targeting strategy. To this end, we have established two novel patient blood cell-independent platform technologies to manufacture “off-the-shelf” cell-based cancer immunotherapies: one of which depends on healthy donor blood cells and the other of which depends on iPSC. Such platform technologies and the resulting product candidates exploit the multiple antigen recognition systems of NK cells and gamma delta T cells in the human body and so as to recognize and treat a broad range of cancers. Built on the proprietary platform technologies that we licensed, we are developing three product candidates: CTM-N2D, iPSC-gdNKT and CTM-GDT. CTM-N2D is our lead product candidate and it consists of expanded gamma delta T cells grafted with NKG2DL-targeting CAR to enhance anti-cancer cytotoxicity. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s acknowledgement on January 6, 2023 that we had submitted the relevant documents to meet the approval conditions of the CTA initially granted in July 2022 relating to the use of our CTM-N2D for the aforementioned trial by HSA, and no further action was required.
Pursuant to the Clinical Study Agreement, the National University Hospital Singapore will conduct the ANGELICA Trial using our lead product candidate CTM-N2D, with Dr. Raghav Sundar, Consultant, Department of Hematology-Oncology of National University Hospital Singapore, being the principal investigator of the trial. Unless terminated earlier, the Clinical Study Agreement shall continue until completion of the trial. National University Hospital Singapore has obtained approval from the Singapore National Healthcare Group Domain Specific Review Boards in February 2023.
Pursuant to the Clinical Study Agreement, Company has agreed to (i) provide funding and supply at no cost to National University Hospital Singapore such quantities of the CTM-N2D as may be required for the ANGELICA Trial, (ii) to provide all relevant clinical information, (iii) where applicable, to provide an instruction manual/guide, product labels, storage, handling and safety instructions and advice which are required for the safe and proper use of CTM-N2D and the proper planning and conduct of the trial. In March 2023, we obtained and provided to National University Hospital Singapore a clinical trial insurance certificate required to conduct the trial in Singapore.
In all publications generated from the ANGELICA Trial, both Company and National University Hospital Singapore would be granted co-authorship. All the study results shall be jointly owned by both parties. Discoveries or inventions that relate to any enhancements or modifications to the CTM-N2D arising from the conduct of the ANGELICA Trial, whether conceived or reduced to practice either solely by or jointly with National University Hospital Singapore, will be the joint property of National University Hospital Singapore and Company.
Either party may terminate the Clinical Study Agreement at any time upon providing at least thirty (30) days’ prior written notice to the other party. National University Hospital Singapore may terminate the Clinical Study Agreement immediately in the event National University Hospital Singapore and/or the principal investigator believes on reasonable grounds that continuing the said trial poses an unacceptable risk to the rights, interests, safety or well-being of human subjects, or if a serious adverse event occurs which necessitates the discontinuance of the ANGELICA Trial.
We are looking at recruiting our first patient in the second half of 2023. Please refer to the sections entitled “Business – Our Solutions and Product Candidates: CTM-N2D, iPSC-gdNKT and CTM-GDT” and “Business – CTM-N2D Therapy – Development Status of CTM-N2D Therapy” in this Prospectus for further details on the application process and progress. We expect to expand our pipeline further in Phase II trials of CTM-N2D therapy for specific cancer indications. Our second product candidate iPSC-gdNKT utilizes iPSC as a starting material to generate gdNKT, which is a synthetic hybrid of a gamma delta T cell and a NK cell. The hybrid cells express receptors of both cells which potentially allow the gdNKT cells to recognize and treat a broad range of cancers. This product has been undergoing pre-clinical process development since the fourth quarter of 2022, and is targeting to commence pre-clinical studies after 2024. Our third product candidate, CTM-GDT consists of expanded allogeneic gamma delta T cells and exploits the potential of these cells to recognize and treat a broad range of cancers. We are looking to submit a CTX application for Phase I clinical trial to NPRA, Malaysia after 2023.
As at the date of this Prospectus, we are a holding company incorporated in Singapore which oversees our operations in Malaysia. We conduct our business activities primarily through our direct wholly-owned subsidiary in Malaysia, CytoMed Malaysia, but may be commencing more research and clinical trial activities in Singapore through CytoMed moving forward. Other than CytoMed Malaysia, our subsidiaries have minimal operations as at the date of this Prospectus.
We also have an operating cGMP Facility in Johor, Malaysia (which is near Singapore) which has been built in accordance with the international PIC/S GMP standards to manufacture cell therapy products to support clinical trials. In addition, we have a well-trained operations team who conduct all essential cGMP activities including manufacture, quality control, quality assurance and documentation. We manufacture our product candidates in our cGMP Facility instead of engaging and relying on an external CMO. As of the date of this Prospectus, our current product pipeline and progress is summarized in Figure 1.
| 89 |
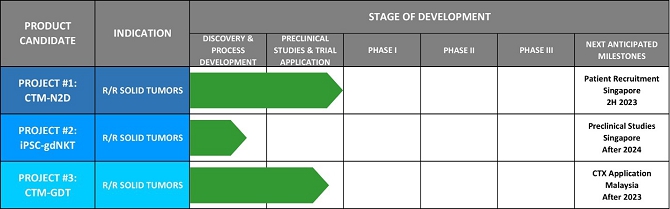
Figure 1. Pipeline, milestone, and progress.
Strategy
Currently, there are vast unmet medical needs in cancer treatment, in particular for solid tumors. Our goal is to develop novel “off-the-shelf” cell therapies to improve the quality of life of cancer patients and to increase their overall survival. We believe that our uniquely designed platform technologies and product development strategies are expected to strengthen our capability to achieve such goal. We intend to focus on developing our two “off-the-shelf, allogeneic” platform technologies based on healthy donor blood cells and iPSCs respectively. Key elements of our strategies are as follows:
Uniquely Designed Platform Technologies to Develop Novel Cell Therapies for Cancers
Currently, many companies employ αβ T cells as main effector cells. Some companies attempt to graft NK receptors, or γδ TCRs to potentially expand antigen recognition. However, the use of αβ T cells limits such therapy to autologous use. Some companies have employed gene editing technologies to enable the use of αβ T cells allogeneically, but additional gene editing increases complexity in manufacturing and increases cost. Furthermore, genetically engineered cells may pose potential safety issue due to chances of insertional mutagenesis, leading to secondary cancers. Innate immune cells, such as γδ T cells, can be used in an allogeneic setting without requiring additional gene editing, therefore making it an ideal cell type for allogeneic therapy.
Another potential cell source is iPSC. iPSC can replicate limitlessly and can be differentiated into cell of choice. iPSC can be differentiated into innate immune cells such as NK and γδ T cells so these cells can be used for allogeneic cancer immunotherapy. As such, we employ γδ T cell derived from blood of healthy donors to generate CTM-N2D and CTM-GDT. We also use iPSC to generate iPSC-gdNKT, a hybrid between NK and γδ T cells to increase the number of surface receptors on the immune cell surface for increased antigen recognition.
We believe we have uniquely designed our platform technologies based on the following: (a) the targeted cancer antigens are ubiquitous stress-induced antigens, such as NKG2DLs and PAg; (b) the cancer antigen-recognition receptors are the built-in recognition system of innate immune cells such as γδ TCR and the grafted NKG2DL-targeting CAR; and (c) the cytotoxic effector cells are lymphocytes of innate immune system, for example, NK cells and gamma delta T cells. Such platforms allow us to use healthy donor blood cells and unlimited iPSCs as starting materials to manufacture CTM-N2D and iPSC-gdNKT product candidates, respectively. Our product candidates generated using such platform technologies possess the following desirable features: (1) “off-the-shelf”; (2) broad-spectrum; and (3) for many types of cancer patients. More importantly, both our healthy donor blood cell and iPSC-based technologies are amenable to genetic modification and thus open the possibility to further functional enhancement and indication specification.
| 90 |
In-house Development of the Technologies into Cell Therapies for Cancers
The cell therapy products currently in our pipeline are based on the patent-pending, core proprietary technologies licensed from A*STAR, Singapore’s lead R&D agency in the public sector. The key inventors of these proprietary technologies licensed to us from A*STAR, Dr. ZENG Jieming and Dr. WANG Shu, have been actively involved as technology inventors in the product development of these proprietary technologies since 2018. Dr. ZENG Jieming joined us in 2019 as a full-time employee, serving as our Chief Scientific and Medical Officer to oversee product development and manufacturing. Dr. TAN Wee Kiat, a scientist who is familiar with the CTM-N2D technology, has been employed by us on a full-time basis as our Chief Operating Officer since 2019.
The direct involvement of the inventors and key scientists during process and product development has provided valuable in-depth understanding of the technologies and experience, which are crucially important for timely and cost-efficient translation of these proprietary technologies into therapeutic products. Such benefit has been manifested by the speedy completion of our cGMP Facility and staff training. These well-trained staff members are critical to the success of quality product manufacturing and new product development.
In-house Manufacture of the Cell Therapy Products for Cancer Treatments
We believe that our in-house cGMP manufacturing capability will enable us to maintain not only the quality and availability of cell therapy products, which is crucial for our clinical success, but also allow us to manufacture cell therapy products more cost-effectively, which will be crucial for commercial viability. As such, we have constructed and fully equipped our own all-in-one cGMP Facility in Johor, Malaysia. Built in 2019, our cGMP Facility was internally validated by completion of manufacturing runs in 2020 and externally audited in 2021 by the NPRA, which is also a member of PIC/S. Our cGMP Facility also includes a quality control laboratory to support release of cell products and an R&D lab to conduct staff training and process development. We believe our current cGMP Facility is sufficient to support our planned Phase I and Phase II clinical trials.
Continuous Exploration of Enabling Technologies to Advance Cell Therapy Products
Both our healthy donor blood cell and iPSC-based technologies are open platforms, and we will continue to explore enabling technologies that may further enhance these platforms to generate more novel cell therapy for cancers. Such efforts may further expand and fine-tune our product pipeline for various cancer indications. Currently, Puricell is designated to explore iPSC-based enabling technologies for enhancing cell-based therapies. We are also exploring opportunities with potential partners from A*STAR to further develop proprietary manufacturing capabilities that may further increase manufacturing flexibility and scalability.
Background of Cellular Immunotherapy for Cancer and CAR-T Cell Therapy
Cellular Immunotherapy for Cancer and CAR-T Cell Therapy
Harnessing the components of the immune system to treat cancer is promising in providing targeted therapy that may reduce morbidity and mortality caused by conventional non-targeted treatments, such as chemotherapy and radiation. Immunotherapy of cancer may involve and utilize components like cytokines, antibodies, cancer vaccines and immune cells. In terms of strategy, cancer immunotherapy may be categorized into two major types: One relies largely on the patient’s own immune responses to cancers, for example, non-specific immune stimulation using cytokines, vaccination with cancer antigens, and immune checkpoint blockage to treat immunogenic cancers. The mechanism of these treatments is to stimulate or unleash the patient’s own immune cells in the body to fight cancer. In contrast, the other strategy is less reliant on the patient’s own immune response, for example, adoptive cell therapy. Typically, in adoptive cell therapy, the immune cells are derived from the cancer patient’s blood and are then genetically modified to enhance their anti-cancer activity and expanded into a clinically relevant number before reinfusion back to the patient (Figure 2). The mechanism of such cellular immunotherapy is to directly send in an army of cultured killer cells into the patient’s body to eliminate cancer. Technological progresses in antigen recognition, genetic modification, and immune cell expansion have paved the way to recent success of adoptive therapy using CAR-T for some hematologic malignancies (Figure 2).
| 91 |
CAR is a genetically engineered artificial receptor to recognize surface cancer antigen. It consists of three domains, namely the intracellular, transmembrane, and extracellular domain. The intracellular domain serves to activate cytotoxicity of effector cells to kill target cells. The transmembrane domain anchors the CAR molecule on the surface of effector cells. The extracellular domain is responsible for recognizing antigen of interest. Through expressing CAR, cytotoxic effector cells such as T cells may be redirected to specifically recognize a cancer-specific or cancer-associated antigen (Figure 2). With certain level of specificity, these CAR-T may recognize and destroy the cancer cells while sparing the healthy cells. The principle of CAR-T strategy has been applied to treat B-cell malignancies by targeting CD19, a B-cell lineage marker. The potency of such CD19-targeting CAR-T have been successfully demonstrated in multiple clinical trials (Kochenderfer et al. 2015; Lee et al. 2015; Locke et al. 2017; Maude et al. 2014; Neelapu et al. 2017; Schuster et al. 2017).

Figure 2. Manufacturing and mechanism of action of current CAR-T cell therapy. In this figure, “aAPC” refers to an artificial Antigen Presenting Cell.
Limitations of the Current CAR-T Cell Therapy for Cancer
Current CAR-T cell immunotherapy treatment for cancer is typically manufactured from patient’s alpha beta T cells. Using lentiviral vector, the gene of a CAR that binds to a certain surface protein on patient’s cancer cells is integrated into the genome of alpha beta T cells. Such generated CAR-T cells can then be used to treat certain cancers. The principle of CAR-T has been successfully demonstrated for treating B-cell malignancies in multiple clinical trials (Kochenderfer et al. 2015; Lee et al. 2015; Locke et al. 2017; Maude et al. 2014; Neelapu et al. 2017; Schuster et al. 2017). Such trials have led to the first two CD19-targeting CAR-T cell therapies approved by the FDA. One is tisagenlecleucel (marketed by Novartis International AG (NYSE:NVS)in the U.S. as Kymriah®) for treating acute lymphoblastic leukemia. The other is axicabtagene ciloleucel (marketed by Gilead Sciences, Inc. (NASDAQ:GILD) in the U.S. as Yescarta®)) for treating Non-Hodgkin’s lymphoma. These approved therapies target CD19, which is only expressed in normal B cells and their malignant counterparts. Such progress suggests that CD19-targeting CAR-T therapy is a breakthrough in cancer immunotherapy. However, to replicate the success of CD19-targeting CAR-T therapies in treating other types of cancers, particularly solid tumors, remains challenging. The limitations of current CAR-T strategy are manifold:
| 92 |
| i. | Lack of Suitable Surface Antigen for Solid Tumors |
Conceptually, the CAR-T principle is simple: grafting a CAR into T cells (typically alpha beta T cells) to redirect T cells for cancer recognition. Practically, however, the lack of suitable surface antigen limits the extrapolation of CAR-T principle into treatment of other cancers. Current CAR-T rely on the grafted CARs as the only mechanism to recognize cancer cells. The antigen targeted by CAR must be a surface antigen expressed on cancer cells but not on normal cells. The lack of surface antigens that can be safely targeted without causing clinically unmanageable side effects is a limitation in expanding CAR-T therapy beyond B-cell malignancies.
CD19, the target of the current CAR-T cell therapies is a B-cell lineage surface antigen that expresses in B-cell malignancy and normal B cells, but not other blood cell lineage. Although CD19-targeting CAR-T also kill normal B cells and cause B-cell depletion, such side effect is still clinically manageable. However, it is not easy to find a suitable surface antigen like CD19 in cancers other than for acute lymphoblastic leukemia. A limited number of other potential targets are being tested in clinical trials, such as targeting BCMA to treat multiple myeloma (Ali et al. 2016; Raje et al. 2019). In solid tumors, finding specific surface targets is even more challenging since most known tumor antigens are intracellular and thus not targetable by CAR, which targets surface antigens only.
Selecting and targeting a suitable surface antigen is of crucial importance to develop CAR-T therapy for other types of cancer. Targeting a cancer-specific antigen may provide “on-target on-cancer” therapeutic effect that outweighs its “on-target off-cancer” side effect and thus benefit the cancer patients. The delicate balance between potential therapeutic effects and unwanted side effects of the CAR-T technology is determined by the specificity of the selected cancer antigen (Figure 3).
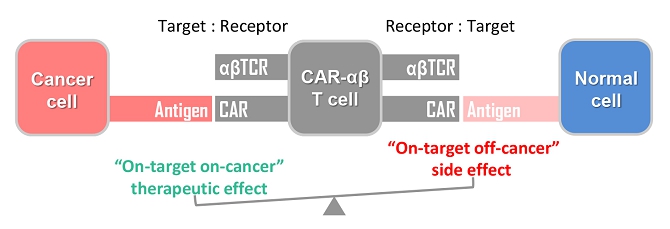
Figure 3. Product feature of current CAR-T cell therapy.
| ii. | Targeting Single Antigen |
Currently, available CAR-T therapy can only recognize a single antigen. This is mainly due to the following technical reasons: (a) the lack of specific surface antigens in cancers; (b) the difficulties to graft multiple types of CARs in T cells using lentiviral vectors; and (c) the use of scFv of antibody as an antigen-binding domain in grafting CAR. The specificity of antibody determines that scFv-based CAR can only bind and target a single antigen. Such limitation poses a risk of cancer escape due to antigen loss, in which CAR-T cells would no longer be able recognize the cancer cells. We believe such risk has been proven in clinical studies (Jacoby et al. 2016; Yu et al. 2017).
| 93 |
| iii. | Complicated Manufacturing Process |
Current CAR-T manufacturing technologies pose another challenge to extend CAR-T therapy to a wide range of cancers (Levine et al. 2017). To manufacture CAR-T for B-cell malignancies, a large amount of patient’s blood cells is collected through an invasive leukapheresis procedure. Typically, such cancer patients would usually have gone through multiple lines of chemotherapy. As a result, the number and quality of blood cells especially alpha beta T cells, onto which the CARs are to be expressed, may already have been compromised. Such poor starting material makes it difficult to manufacture good quality CAR-T products. Failure in manufacturing can exclude the patients from CAR-T therapy. Moreover, lentiviral vectors are used to express the CAR in T cells and the manufacture of GMP-grade lentiviral vectors itself is a complicated process. To ensure the production of high-quality lentiviral vectors with minimal variability, several rounds of filtration and testing is required during manufacturing of a series of multiple sub-batches (Levine et al. 2017).
| iv. | Undesirable Product Features |
The above-mentioned technology and manufacturing limitations substantially restrict the widespread application of current CAR-T therapies and CAR-T products. There are several undesirable features of current CAR-T products:
Inaccessible: Patients may be excluded from CAR-T therapy due to the failure of blood tests and manufacturing feasibility tests. Certain patients (e.g., pediatric patients) are not eligible for CAR-T therapy due to the limited blood cells that can be collected from such patients.
Highly personalized “made-to-order” product and not “off-the-shelf”: Existing manufacturing processes of the current CAR-T products rely solely on patient’s own blood cells. Therefore, the CAR-T products are highly personalized and not “off-the-shelf”. They are not readily available if cancer patients require urgent treatment.
Safety concerns: The contamination of the starting material (patient’s blood cells) by existing cancer cells and the use of lentiviral vector to graft the CAR are not only challenging to manufacture but also pose the risk of secondary cancer, which has been described in a clinical study (Ruella et al. 2018). Due to the potential risk of insertional mutagenesis caused by lentiviral vectors, a minimum of 15-year post-treatment follow-up is required for current CAR-T products.
Expensive: It has previously been reported that Kymriah® has a one-time price of U.S.$475,000 for acute lymphoblastic leukemia (Cancer Discov, 7: OF1). The list price of Yescarta® is U.S.$373,000 (Cancer Discov, 8:5-6). These prices do not cover additional costs of hospital admission which is required in order to administer the agent and the potential treatment of side effects. Despite the clinical successes of CD19-targeting CAR-T therapies, current CAR-T therapies may be out of reach for most of the population.
Our Solutions and Product Candidates: CTM-N2D, iPSC-gdNKT and CTM-GDT
Due to the obvious limitations of the current CAR-T technologies, we believe it is imperative to explore different cytotoxic cell sources, cancer-targeting strategies and production schemes for other types of cancers, in particular, solid tumors. To this end, we have designed and established three novel patient blood cell-independent platform technologies, in which we use healthy donor blood cells and induced pluripotent stem cells to develop and manufacture “off-the-shelf” cell-based cancer immunotherapies. Our current product candidates in the pipeline include CTM-N2D, iPSC-gdNKT and CTM-GDT.
CTM-N2D
To produce CTM-N2D, blood cells from healthy donors are used as starting material. Through a proprietary expansion technology, high purity gamma delta T cells are obtained as effector cells. Through an mRNA electroporation technology, the expanded gamma delta T cells are further grafted with NKG2DL-targeting CAR to enhance anti-cancer cytotoxicity. Such manufacturing process is depicted in Figure 4 and this CTM-N2D technology has also been published in two peer-reviewed papers by its inventors (Cytotherapy, 2018, 20: 420; Molecular Therapy: Oncolytics, 2020, 17: 421.)
| 94 |
The cutting-edge features of the technology are as follows: (i) the use of healthy donor blood cells instead of patient blood cells as starting material for manufacturing to solve the product availability issue; (ii) the use of gamma delta T cells instead of alpha beta T cells as effector cells to expand the product applicability to a large population of patients; and (iii) the use of a NKG2DL-targeting CAR instead of the conventional single antigen-targeting CAR to expand the product indications to a wide range of cancer types.
Our CAR-gamma delta T cell technology is ready for Phase I clinical trial, being that we have translated the CAR-gamma delta T cell technology into a cell therapy named as CTM-N2D for further evaluation in a clinical trial, the ANGELICA Trial, with National University Hospital Singapore. We had submitted a full clinical trial dossier to HSA in April 2020. A pre-submission meeting was held with HSA in July 2020. In January 2021, HSA informed that they had reviewed our submission and, suggested that we formally submit a CTA application. CTM-N2D consists of culture-expanded cells and is considered a Class 2 CTGTP, hence, a CTA application is required for clinical trial. On March 10, 2023, we entered the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s confirmation on January 6, 2023 that we had satisfied the approval conditions of the CTA initially granted in July 2022, apart from continued compliance with the HPA and its subsidiary legislation, relating to the use of our CTM-N2D for the aforementioned trial by HSA. The proposed clinical trial is an open-label, single-centre, dose escalation, Phase I study. The study objectives are to determine safety, activity, and safe dosage of haploidentical or allogeneic NKG2DL-targeting CAR-gamma delta T cells, in which the proposed plan is to administer CTM-N2D in four weekly doses to patients with relapsed or refractory solid tumors of different types.
Concurrently, we have also been awarded a grant from Enterprise Singapore not exceeding S$250,000 in relation to the conduct of research on developing cryopreservation techniques for the export of CTM-N2D. As at the date of this prospectus, we have received an initial cash advancement of S$50,000.
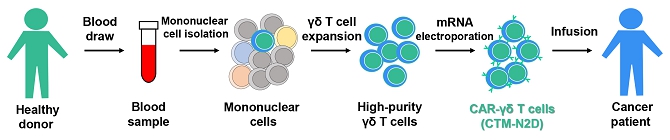
Figure 4. Manufacturing process of CAR-gamma delta T cells (CTM-N2D therapy).
iPSC-gdNKT
To exploit the cancer recognition systems of both gamma delta T cells and NK cells, we developed a proprietary induced pluripotent stem cell-derived gamma delta NKT cell platform for cancer treatment. To produce gamma delta NKT cells, iPSC lines have been derived from gamma delta T cells. The derived gamma delta T-iPSCs are used as starting material to generate gamma delta NKT cells via “NK cell-promoting” differentiation. Such a synthetic strategy to derive gamma delta NKT cells is depicted in Figure 5 and two peer-reviewed publications by the technology inventors (Stem Cell Reports, 9: 1796, 2017; PLoS ONE, 14: e0216815, 2019)
| 95 |
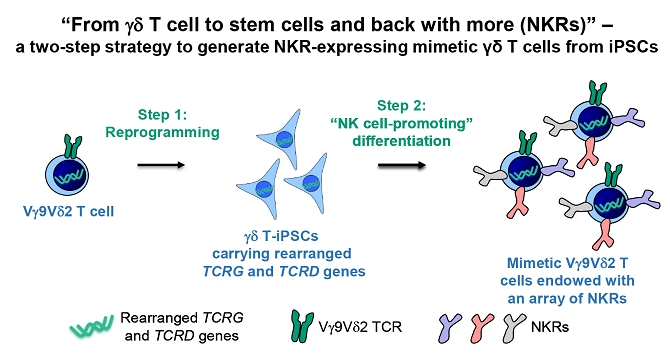
Figure 5. Synthetic strategy of iPSC-derived gamma delta NKT cells (iPSC-gdNKT therapy).
The potential novel features of the technology are as follows: (i) the use of gamma delta T-iPSCs, our proprietary cell source as starting material for manufacturing to make the production patient/donor blood cell-independent, which may ultimately solve the starting material availability issue; (ii) using a differentiation process to derive gamma delta NKT cells so that genetic modification is unnecessary, which makes manufacturing simpler and the products potentially more suitable for the patients; (iii) gamma delta NKT cells are innate lymphocytes in nature and applicable to allogeneic setting since these cells are unlikely to cause GvHD; and (iv) gamma delta NKT cells possess cancer recognition systems of gamma delta T cells and NK cells and this unique array of built-in receptors enables gamma delta NKT cells to recognize a wide spectrum of cancer types, including solid tumor and leukemia.
Extensive in vitro studies have demonstrated that gamma delta NKT cells are highly cytotoxic at recognizing and killing a “broad-spectrum” of cancers with great specificity (Zeng et al. 2019, a publication by the inventors of the technology). We are in the process of further evaluating gamma delta NKT cells in cancer treatment in pre-clinical studies. iPSC-gdNKT is considered a Class 2 CTGTP and hence a CTA application would be required prior to commencing clinical trial in Singapore. This product has been undergoing pre-clinical process development since fourth quarter of 2022, and is targeting to commence pre-clinical studies after 2024
CTM-GDT
Adoptive transfer of gamma delta T cells as cancer treatment has been investigated in clinical studies. Typically, a patient’s own gamma delta T cells have been first expanded in vitro using zoledronic acid and IL-2. These expanded autologous gamma delta T cells were then administrated back to the same patients in multiple doses. The clinical efficacy has been variable, which may be due to the variable quality and quantity of administrated patient-derived gamma delta T cells.
Compared with autologous gamma delta T cells expanded from patient’s own blood cells, allogeneic gamma delta T cells expanded from blood cells of healthy donors may provide a more cytotoxic cell source. Moreover, unlike autologous gamma delta T cells, the number of allogeneic gamma delta T cells that can be obtained during manufacturing is not restricted by the starting materials from the patient himself. Hence, we believe that it is valuable to further explore the potential cytotoxicity of allogeneic gamma delta T cells in clinical trials. To this end, we are also developing allogeneic gamma delta T cells into CTM-GDT therapy for cancer treatment (Figure 6). CTM-GDT is considered a CGTP in Malaysia according to NPRA and we intend to manufacture the investigational product locally. Therefore, a CTX application to NPRA is required prior to commencing clinical trial in Malaysia. We are looking to submit a CTX application for Phase I clinical trial to NPRA, Malaysia after 2023.
| 96 |

Figure 6. Manufacturing process of allogeneic γδ T cells (CTM-GDT therapy).
CTM-N2D Therapy
Background of CAR-gamma delta T Cell Technology
In our CAR-gamma delta T cell technology, we have used NKG2DL-targeting CAR to recognize a broad range of cancers and harnessed gamma delta T cells to carry out the anti-cancer effector functions. The rationale for using these two components is as follows:
| i. | NKG2D and NKG2DLs as Targets |
NKG2D is a naturally occurring receptor that is expressed on the cell surface of NK cells, CD8+ T cells and gamma delta T cells. NKG2D recognizes eight NKG2DLs, namely MICA/B and ULBP 1-6 (Marcus et al. 2014). These ligands are usually expressed at low levels or non-existent on the cell surface of healthy cells, but their expressions can be upregulated upon undergoing stress, infection, and malignant transformation. During tumorigenesis, DNA damage responses (such as genotoxic stress and stalled DNA replication) and poorly regulated cell proliferation may both induce NKG2DL expression on the cell surface of cancer cells. Since these biological changes are the hallmark of cancers, it is not surprising that increased expression of NKG2DLs in cancer cells has been found in patients with many different types of cancers (Table 1) (Demoulin et al. 2017). The ubiquitous expression of NKG2DLs makes them interesting targets in cancer treatment. As demonstrated by our inventors of this technology targeting NKG2DLs is achievable by exploiting the ligand-binding domain of NKG2D and integrating such domain into a NKG2DL-targeting CAR (Cytotherapy, 2018, 20: 420; Molecular Therapy: Oncolytics, 2020, 17: 421.). Using such NKG2DL-targeting CAR, it is possible to target multiple NKG2DLs and thus develop a CAR-T therapy that can be used in a wide range of cancers with lower risk of cancer escape.
| ii. | Gamma delta T Cells as Effector Cells |
As of the date of this Prospectus, most of the existing CAR-T therapies for cancer use alpha beta T cells as effector cells. As a result, such alpha beta T cells require the patient-specific autologous paradigm of CAR-T cell production and application (Fesnak, June, and Levine 2016; Newick et al. 2017). To manufacture CAR-T cells, patient’s own alpha beta T cells are expanded and genetically modified using lentiviral vector to express CAR. Such alpha beta T cell-based products are made strictly for autologous use only. They are not usable in an allogeneic setting for other recipients because the alpha beta T cells may recognize host’s normal cells through their TCRs and result in GvHD. Furthermore, manufacturing CAR-T is not feasible for cancer patients who suffer from profound lymphopenia due to previous chemotherapy and/or radiotherapy (Allen et al. 2017).
| 97 |

Table 1. Expression of NKG2DLs in various types of cancers (adapted from Demoulin B et al. 2017).
To make such alpha beta T cell-based CAR-T products accessible to a broad range of cancer patients and usable in an allogeneic setting, manufacturing CAR-T cells using donor alpha beta T cells while disrupting alpha beta TCR expression in alpha beta T cells using sophisticated genetic modification has been proposed as a solution to manufacture “universal” CAR-T cells (Marcus and Eshhar 2014; Torikai and Cooper 2016). However, such strategy will require additional complexities in the already complicated manufacturing process. We believe that such a strategy will be a serious challenge to the robustness, efficiency, and safety of genetic engineering technologies as well as the capability of quality control of such heavily modified products. Hence, from the viewpoint of manufacturing, we think that using other effector cells, such as gamma delta T cells, is more desirable.
| 98 |
Gamma delta T cells are a distinct subset of CD3+ T cells that express heterodimer of gamma delta TCR chains. In the bloodstream, approximately 1%-5% of the T cell population are gamma delta T cells which express the variable-gene segments Vγ9 and Vδ2, and thus are known as Vγ9Vδ2 T cells (Tyler et al. 2015). Biologically, gamma delta T cells differ strikingly from alpha beta T cells. Through gamma delta TCRs, gamma delta T cells recognize and interact in a non-MHC restricted fashion with various antigens, including PAgs that are produced during metabolic dysregulation in cancer cells (Tyler et al. 2015; Kabelitz 2016; Legut, Cole, and Sewell 2015). Like NK cells, gamma delta T cells also express NKG2D as a co-stimulatory receptor. Once activated, gamma delta T cells can exert cytotoxic effector functions to kill target cells by secreting cytotoxic molecules like granzyme and perforin and to activate anti-cancer immunity by secreting proinflammatory cytokines like IFN-γ and TNF (Figure 7).

Figure 7. Anti-cancer activities of gamma delta T cells.
Gamma delta T cells are vital in immune surveillance against cancer development. Gamma delta T cells feature an inflammatory migration profile (Brandes et al. 2003), being capable of homing to epithelial and mucosal tissues and infiltrating a broad range of human malignancies (Fournie et al. 2013). A study of approximately 18,000 human tumors across a panel of 39 tumor types has identified the presence of intra-tumoral gamma delta T cells as the most significant indicator for favorable prognosis, which is positively correlated with patient survival (Figure 8) (Gentles et al. 2015). A recent study has reported the computational identification of abundance of Vγ9Vδ2 T cells in approximately 10,000 cancer biopsies from 50 types of hematological and solid malignancies (Tosolini et al. 2017). Infiltrating Vγ9Vδ2 T cells are prominent in blood cancers (including B cell-acute lymphoblastic leukemia, acute promyelocytic leukemia (M3-AML), and chronic myeloid leukemia) and in solid tumors (including cancers of prostate, esophagus, cervix, head and neck, lung, pancreas and breast). These findings strongly indicate the therapeutic value of gamma delta T cells in cancer treatment.
| 99 |

Figure 8. Intratumoral gamma delta T cell frequency is positively correlated with cancer patient survival (adapted from Gentles AJ et al, 2015).
Adoptive transfer of Vγ9Vδ2 T cells has been used to treat cancers in several early-phase clinical trials. Such therapy was well-tolerated and linked to positive clinical outcomes (Fournie et al. 2013; Kobayashi and Tanaka 2015). GvHD was not reported in these clinical studies, suggesting that Vγ9Vδ2 T cells are a safe effector cell source for developing CAR-T therapies. In pre-clinical studies, gamma delta T cells have been redirected with CARs to target cancers. It was reported that peripheral blood Vγ9Vδ2 T cells transduced with retroviral vectors encoding either GD2 or CD19-specific CARs display antigen-specific IFN-γ secretion and cytotoxicity against cancer cells (Rischer et al. 2004). This is a demonstration that CAR-expressing gamma delta T cells may serve as potent and specific anti-cancer effector cells. Moreover, by comparing the potency of three different T-cell populations (Epstein-Barr virus-specific cytotoxic T lymphocytes (EBV-CTLs), cytokine-induced killer (CIK) cells, and Vγ9Vδ2 T cells) as effector cells after modifying them with CD33-targeting CARs, it has been found that Vγ9Vδ2 T cells are as potent as the other two population in terms of in vitro cytotoxicity (Pizzitola et al. 2011). Using polyclonal γδ T cells, it has been shown that gamma delta T cells expressing CD19-targeting CARs can reduce CD19+ leukaemia xenografts in mice (Deniger et al. 2013).
We believe the data described above strongly supports the combined use of NKG2DL-targeting CAR as cancer-recognizing receptor and gamma delta T cells (Vγ9Vδ2 T cells) as effector cells to generate CTM-N2D as a novel cancer therapy to treat a wide range of cancers. Such therapy becomes even more attractive because the built-in and grafted receptors are unlikely to recognize normal cells and cause GvHD which enables its application in allogeneic setting.
| 100 |
Product Design and Manufacturing of CTM-N2D
| i. | Product Design and Features of CTM-N2D Therapy |
The principle of CTM-N2D therapy is similar to that of the current CAR-T cell technology. However, since different components have been integrated into CTM-N2D, the key features of CTM-N2D are quite different from those of conventional CAR-T cells. In CTM-N2D, NKG2DL-targeting CAR is used to redirect the gamma delta T cells for cancer recognition (Figure 9). As such, CTM-N2D may recognize multiple stress-induced cancer antigens via the built-in receptors and the grafted NKG2DL-targeting CAR. Moreover, CTM-N2D can be produced from healthy donor blood due to the use of gamma delta T cells as effector cells. Thus, we believe CTM-N2D may be a potential solution to overcome the limitations of current CAR-T cell technology and for treating various cancers in patients.
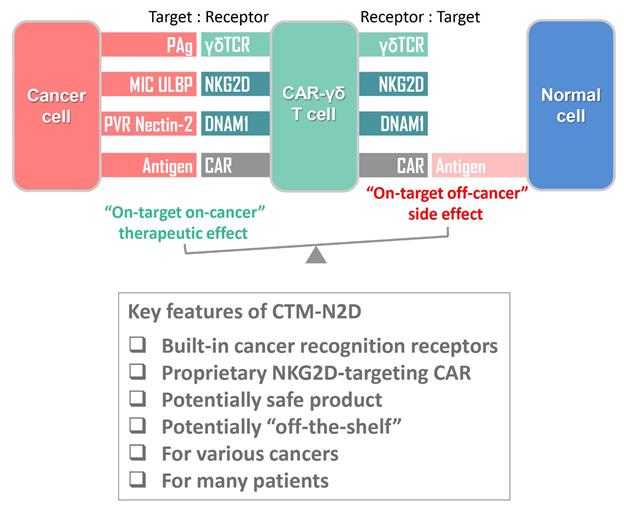
Figure 9. Product design and features of CTM-N2D therapy.
| ii. | Manufacturing of CTM-N2D |
To produce CTM-N2D, blood cells from healthy donors (either haploidentical or allogeneic) will be used as starting material. Through a proprietary expansion technology, high purity gamma delta T cells can be obtained. Typical morphology of the expanded gamma delta T cells is shown in Figure 10. Through an mRNA electroporation technology, these gamma delta T cells are further grafted with a NKG2DL-targeting CAR to potentially enhance the potential anti-cancer activity. The manufacturing process of CTM-N2D is depicted in Figure 4.
| 101 |
Figure 10. Typical morphology of expanded gamma delta T cells.
Potential Advantages of CTM-N2D Therapy over current CAR-T products
We believe CTM-N2D has the potential to overcome the afore-mentioned limitations of current CAR-T products. The following are the unique features of CTM-N2D that we believe make it potentially feasible for treating solid tumors:
| i. | Targeting Stress-induced Cancer Antigens |
Stress-induced surface molecules are a category of cancer antigens that are under-utilized in current cancer therapy. For example, NKG2DLs are a group of stress-induced cancer antigens, which include ULBP1-6 and MICA/B (8 types of antigens) (Marcus et al. 2014). PAg is expressed on infected or transformed cells (Bonneville and Scotet 2006). PVR (CD155) and Nectin-2 (CD112) are expressed in cancer cells and their recognitions by DNAM-1 are essential for cell-mediated cytotoxicity (Pende et al. 2005; Carlsten et al. 2007; El-Sherbiny et al. 2007). Below are the unique features of these stress-induced cancer antigens:
Suitable targets: Unlike the lineage-specific antigens (e.g. CD19 of B-cell lineage) targeted by current CAR-T technology that are also expressed in normal cells (targeting these antigens may cause “on-target off-cancer” toxicity), the above-described stress-induced signals represent a restricted set of conserved endogenous surface molecules that are commonly observed in many types of cancer cells. These antigens are constantly being monitored by the innate immune cells of the body during cancer immunosurveillance and thus are potentially more suitable targets (Marcus et al. 2014).
Essential targets: Unlike lineage markers that are non-essential for cancer cells, this type of cancer antigen is induced by dysregulated mevalonate pathway, dysregulated proliferation and/or DNA-damage response, which are the hallmarks of cancer cells (Gober et al. 2003; Raulet, Marcus, and Coscoy 2017). Targeting such essential antigens may reduce the risk of cancer escape through immunoediting (Dunn, Old, and Schreiber 2004).
| 102 |
Ubiquitous targets: Since this type of cancer antigen is induced by common dysregulation biological processes of cancer cells, the antigens tend to be ubiquitously expressed by cancer cells. They appear in a wide range of cancers from leukemia to solid tumors. For example, it is well-studied that NKG2DLs are expressed in many tumor cell lines and primary tumors of diverse tissue-origins (Table 1) (Demoulin et al. 2017).
| ii. | Targeting Multiple Stress-induced Cancer Antigens |
To overcome cancer escape due to antigen loss, simultaneously targeting multiple antigens is critical. However, such a feature remains to be developed for CAR-T platforms due to the lack of suitable targets and the limited genetic payload (Lim and June 2017; Roybal and Lim 2017).
In contrast, CTM-N2D therapy is believed to be able to overcome these limitations by (a) targeting multiple stress-induced cancer antigens via the built-in receptors on gamma delta T cells, e.g., γδ TCR, NKG2D, DNAM-1; and (b) targeting eight known NKG2DLs expressed by cancer cells such as MICA/B, ULBP1-6 via the grafted NKG2DL-targeting CAR. We believe that simultaneously targeting these ubiquitous ligands not only reduces the risk of cancer escape but also broadens the spectrum of cancer recognition.
| iii. | Simpler Manufacturing Process |
CTM-N2D is based on gamma delta T cells instead of the traditional alpha beta T cells as cytotoxic effector cells. This significantly changes the production process and final product features. There is no using of leukapheresis and lentiviral vector during the production of CTM-N2D. The manufacturing process involves simple blood withdrawal, mononuclear cell isolation, specific gamma delta T cell expansion and mRNA electroporation.
| iv. | Unique Product Features |
The use of CTM-N2D technology may enable the following unique product features:
Targeting various types of cancers: CTM-N2D is armed with built-in receptors and grafted NKG2DL-targeting CAR to recognize ubiquitous cancer antigens. CTM-N2D may potentially recognize various types of cancers.
Applicable to a large diverse pool of patients: CTM-N2D is a donor gamma delta T cell-based product. Gamma delta T cells are unlikely to cause GvHD in recipients in an allogeneic setting and thus applicable in many patients. Hence, CTM-N2D can potentially be made and administered to a large diverse pool of patients.
Accessible: The production is based on healthy donor blood cells. By not relying on the patient’s own blood cells, the patient has a low risk of exclusion from CTM-N2D therapy due to production failure.
“Off-the-shelf”: Manufacturing of CTM-N2D starts with healthy donor’s blood cells. It has the potential to be “pre-made” and used in many patients. This “off-the-shelf” feature is favorable to meet the urgent medical need of cancer patients.
Potentially safe: Due to the use of healthy donor blood cells as starting materials and the use of mRNA electroporation to graft the CAR, there is a lower risk of secondary cancer caused by the product.
Pre-clinical Studies of CTM-N2D
We believe that the feasibility of CAR-grafted gamma delta T cells has been demonstrated in our previous publications (Du et al. 2016; Xiao et al. 2018; Ang et al. 2020). These publications suggest the feasibility of combining expansion of gamma delta T cells and mRNA electroporation to produce CAR-grafted gamma delta T cells on a large-scale basis. Depending on the type of targeted cancer antigen, different CAR constructs can be used to redirect the gamma delta T cells. To generate CTM-N2D, we use a CAR construct that targets NKG2DLs (Xiao et al. 2018; Ang et al. 2020).
CTM-N2D has been evaluated by in vitro cytotoxicity assay against cancer cell lines. As shown in Figure 11, gamma delta T cells grafted with NKG2Dz-γδT were significantly more cytotoxic than the controls, which are the gamma delta T cells subjected to electroporation using mGFP-γδ T. Although gamma delta T cells express natural NKG2D protein (NKG2Dp-γδT) and also showed better cytotoxicity than the controls, they were not as cytotoxic as NKG2Dz-γδT. This result suggests that NKG2DL-targeting CAR is a better construct in activating the cytotoxicity of gamma delta T cells.
| 103 |
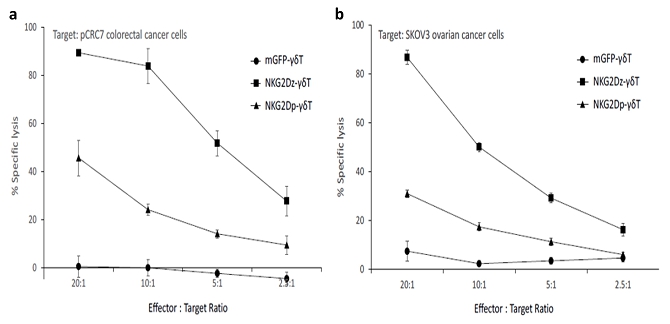
Figure 11. In vitro cytotoxicity of CTM-N2D against cancer cell lines. (a) CTM-N2D against pCRC7, a colorectal cancer cell line; (b) CTM-N2D against SKOV3, an ovarian cancer cell line. mGFP-γδT; gamma delta T cells subjected to electroporation using mRNA encoding mGFP; NKG2Dp-γδT: gamma delta T cells express natural NKG2D protein; NKG2Dz-γδT: gamma delta T cells grafted with NKG2DL-targeting CAR, also known as CTM-N2D.
To exploit the cancer recognition capability of gamma delta T cells through gamma delta TCR, zoledronic acid has been used to upregulate the PAg expression in cancer cells and sensitize the cancer cells to gamma delta T cell-mediated cytotoxicity. As demonstrated in Figure 12, zoledronic acid (known in trade name as Zometa) sensitized the cancer cells to killing by gamma delta T cells and may further enhance the cytotoxicity of CTM-N2D.
To evaluate the cytotoxicity of CTM-N2D in vivo, a xenograft mouse model was established using human ovarian cancer cell line SKOV3 in non-obese diabetic scid gamma mice. Seven days later, 1x107 cells of CTM-N2D were given as treatment twice a week for a total of 3 weeks. Figure 13 shows how the injection of unmodified gamma delta T cells significantly reduces the cancer growth, while the injection of CTM-N2D significantly reduces the cancer burden and prolongs the survival of these cancer-grafted mice.
| 104 |
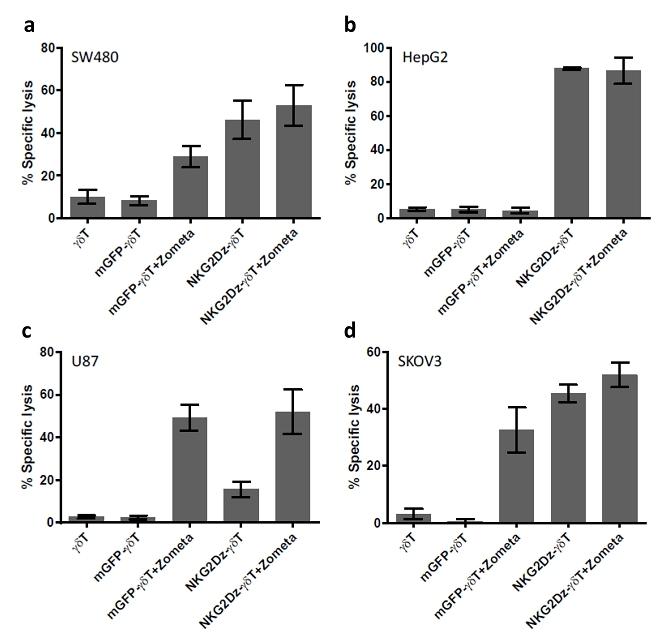
Figure 12. In vitro cytotoxicity of gamma delta T cells and CTM-N2D against Zometa-sensitized cancer cell lines. Cytotoxicity against (a) SW480, a colorectal cancer cell line; (b) HepG2, a hepatocellular carcinoma cell line; (c) U87, a glioblastoma cell line; and (d) SKOV3, an ovarian cancer cell line.
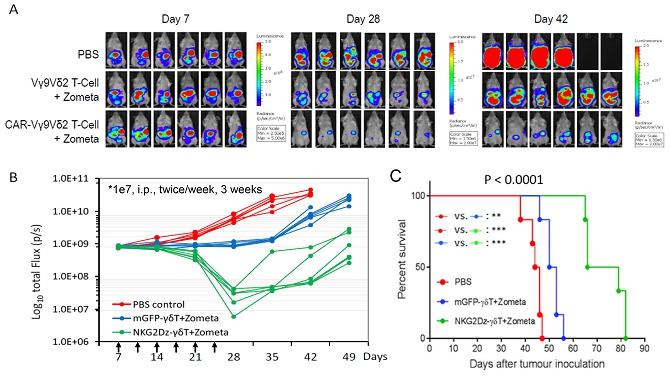
Figure 13. In vivo cytotoxicity of CTM-N2D against human cancer in xenograft mouse model. A human ovarian cancer cell line, SKOV3 was used to established cancer xenograft mouse model. Seven days later, CTM-N2D was given as treatment twice a week for total 3 weeks. Disease progress was monitored by IVIS® in vivo imaging system to show the (A) cancer growth; (B) cancer burden; and (C) survival. mGFP-γδT; gamma delta T cells subjected to electroporation using mRNA encoding mGFP; NKG2Dz-γδT: gamma delta T cells grafted with NKG2DL-targeting CAR, also known as CTM-N2D.
| 105 |
Clinical Studies Relevant to CTM-N2D Therapy
We believe the pre-clinical data described above provide a scientific basis for conducting clinical studies of CTM-N2D therapy. More importantly, we believe a substantial amount of relevant human data strongly supports the use of CTM-N2D therapy to treat solid tumors (Kobayashi et al. 2007; Bennouna et al. 2008; Abe et al. 2009; Nakajima et al. 2010; Noguchi et al. 2011; Sakamoto et al. 2011; Nicol et al. 2011; Kobayashi et al. 2011; Cui et al. 2014; Wada et al. 2014; Wilhelm et al. 2014; Aoki et al. 2017; Alnaggar et al. 2019; Xu et al. 2021). CTM-N2D is NKG2DL-targeting CAR-grafted gamma delta T cells. Adoptive transfer of autologous and allogeneic gamma delta T cells has been used to treat a wide range of cancers in clinical studies for more than a decade without causing severe adverse effects in patients. Regarding targeting NKG2DL, recent pre-clinical and clinical studies using NKG2DL-targeting CAR-alpha beta T cells also suggest that targeting NKG2DLs are well-tolerated. Below is a summary of these clinical findings relevant to CTM-N2D.
| i. | Adoptive Transfer of gamma delta T Cells as Cancer Treatment |
In humans, 1–10% of peripheral blood lymphocytes are gamma delta T cells, of which Vγ9Vδ2 T cells are the major subset. Such Vγ9Vδ2 T cells express Vγ9Vδ2 TCRs to recognize PAg on infected or transformed cells (Bonneville and Scotet 2006). In contrast to human leukocyte antigen (HLA)-dependent antigen recognition of alpha beta TCR, PAg recognition of Vγ9Vδ2 TCR is HLA-independent. Therefore, Vγ9Vδ2 T cells are unlikely to cause GvHD in an allogeneic setting (Lamb and Lopez 2005). Moreover, Vγ9Vδ2 T cells can kill cancer cells even without modification and are being used in clinical trials (Deniger, Moyes, and Cooper 2014; Hoeres et al. 2018).
In clinical trials, an ex vivo approach has been commonly used (Kobayashi et al. 2007; Bennouna et al. 2008; Abe et al. 2009; Nakajima et al. 2010; Noguchi et al. 2011; Sakamoto et al. 2011; Nicol et al. 2011; Kobayashi et al. 2011; Cui et al. 2014; Wada et al. 2014; Wilhelm et al. 2014; Aoki et al. 2017; Alnaggar et al. 2019; Xu et al. 2021). Patient’s own gamma delta T cells were first expanded in vitro using zoledronic acid and IL-2. These expanded autologous gamma delta T cells were then administrated back to the same patients in multiple doses. Such treatments were well-tolerated since no dose-limiting toxicities were observed at a dose level up to 4x109 cells per dose. However, the clinical efficacy has been variable, which may be due to the use of such autologous gamma delta T cells as monotherapy and/or the inherent poor quality of these patient-derived gamma delta T cells.
To study the potential of anti-cancer function of non-autologous gamma delta T cells, haploidentical or allogeneic gamma delta T cells have recently been used in clinical trials (Alnaggar et al. 2019; Wilhelm et al. 2014; Xu et al. 2021). There were no severe adverse events observed in these trials using non-autologous gamma delta T cells. Combining haploidentical gamma delta T cell infusion with lymphodepleting chemotherapy to facilitate graft survival, zoledronic acid to sensitize the cancer cells and IL-2 to support gamma delta T cell survival, improved clinical response has been achieved. Such combination therapy strategy can be extended to our clinical trials.
| ii. | Targeting NKG2DLs with CAR-alpha beta T Cells in Cancer Treatment |
In humans, NKG2DL are identified as MICA/B and ULBP1-6. The expression of NKG2DL is induced by various types of stress, including viral infection and malignant transformation (Raulet et al. 2013; Le Bert and Gasser 2014). Many tumor cell lines and primary tumors of various tissue-origin express NKG2DLs (Demoulin et al. 2017), which provide attractive targets for haematological cancers and solid tumors.
CAR construct has been developed to target NKG2DLs. Currently, NKG2DL-targeting CAR is a fusion of NKG2D protein and T-cell surface glycoprotein CD3 zeta. It has been used to further enhance the anti-cancer potency of alpha beta T cells. These NKG2DL-targeting CAR-alpha beta T cells have been demonstrated in a pre-clinical study to treat pancreatic cancer (Demoulin et al. 2017). A product named NKR-2, based on such technology is currently being developed by Celyad Oncology SA (NASDAQ:CYAD) in clinical trials according to Celyad Oncology SA (NASDAQ:CYAD)’s website. Preliminary data from the dose-escalation segment of this Celyad Oncology SA (NASDAQ:CYAD)clinical study demonstrated that NKR-2 cell was well-tolerated and no toxicity was observed in dose level of 1x109 cells. We believe that such data provides support for evaluating NKG2DL-targeting CAR in the context of gamma delta T cells.
| 106 |
Development Status of CTM-N2D Therapy
We have translated the CAR- gamma delta T cell technology into a trial-ready cell therapy CTM-N2D for further evaluation in a clinical trial. The proposed clinical trial currently being discussed is an open-label, single-center, dose escalation, Phase I study. The study objectives are to determine safety, activity, and safe dosage of haploidentical or allogeneic NKG2DL-targeting CAR-grafted gamma delta T cells, which will be given in four weekly doses to patients with relapsed or refractory solid tumors of different types. We have conducted a pre-submission meeting with HSA. A full clinical trial dossier including trial protocol, Investigator’s Brochure (IB) and Chemistry, Manufacturing, and Control (CMC) has been submitted and reviewed by HSA during the pre-submission meeting, which has concluded that they have no major issues with the documents. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s acknowledgement on January 6, 2023 that we had submitted the relevant documents to meet the approval conditions of the CTA initially granted in July 2022 relating to the use of our CTM-N2D for the aforementioned trial by HSA, and no further action was required. Pursuant to the Clinical Study Agreement, the National University Hospital Singapore will conduct the ANGELICA Trial using our lead product candidate CTM-N2D, with Dr. Raghav Sundar, Consultant, Department of Hematology-Oncology of National University Hospital Singapore, being the principal investigator of the trial. Unless terminated earlier, the Clinical Study Agreement shall continue until completion of the trial. National University Hospital Singapore has obtained approval from the Singapore National Healthcare Group Domain Specific Review Boards in February 2023. Pursuant to the Clinical Study Agreement, Company has agreed to (i) provide funding and supply at no cost to National University Hospital Singapore such quantities of the CTM-N2D as may be required for the ANGELICA Trial, (ii) to provide all relevant clinical information, (iii) where applicable, to provide an instruction manual/guide, product labels, storage, handling and safety instructions and advice which are required for the safe and proper use of CTM-N2D and the proper planning and conduct of the trial. In March 2023, we obtained and provided to National University Hospital Singapore a clinical trial insurance certificate required to conduct the trial in Singapore. In all publications generated from the ANGELICA Trial, both Company and National University Hospital Singapore would be granted co-authorship. All the study results shall be jointly owned by both parties. Discoveries or inventions that relate to any enhancements or modifications to the CTM-N2D arising from the conduct of the ANGELICA Trial, whether conceived or reduced to practice either solely by or jointly with National University Hospital Singapore, will be the joint property of National University Hospital Singapore and Company. Either party may terminate the Clinical Study Agreement at any time upon providing at least thirty (30) days’ prior written notice to the other party. National University Hospital Singapore may terminate the Clinical Study Agreement immediately in the event National University Hospital Singapore and/or the principal investigator believes on reasonable grounds that continuing the said trial poses an unacceptable risk to the rights, interests, safety or well-being of human subjects, or if a serious adverse event occurs which necessitates the discontinuance of the ANGELICA Trial. We target to recruit our first patient in the second half of 2023. The development status of CTM-N2D therapy is depicted in Figure 1.
iPSC-gdNKT Therapy
Background of iPSC-derived gamma delta NKT Cell Technology
As of the date of this Prospectus, most CAR-T therapies for cancers are typically generated from patient’s own blood cells. Such products use alpha beta T cells as effector cells and are highly personalized and not suitable for other recipients. This is because in an allogeneic setting the TCR of alpha beta T cells may recognize host’s normal cells and cause GvHD. In contrast, immunotherapies basing on lymphocytes of innate immune system such gamma delta T cells and NK cells may be generated from donor blood cells. Gamma delta T cells and NK cells do not express alpha beta TCRs and are unlikely to cause GvHD under allogeneic setting. However, both patient blood cells and donor blood cells are limited in cell number and variable in quality. Identifying suitable blood donors and qualifying donated blood cells are necessary to ensure good quality as such cell sources form the starting material for manufacturing cell therapies. Using such limited and variable cell sources as starting material may pose significant challenges to scalability of production and standardization of products. Hence, an unlimited and standardized cell source is more desirable.
In 2007, Professor Yamanaka and his team of scientists from Kyoto University, Japan, discovered that human dermal fibroblast could be converted into pluripotent stem cells through the delivery of four genes using viral vector (Takahashi et al. 2007). Aptly named as the Yamanaka factors, these genes were integrated into the genome of the fibroblast and “reprogrammed” the mature fibroblast back to pluripotent status. Such pluripotent stem cells are “induced” from mature cell and thus named iPSCs. Functionally, iPSCs are capable of self-renewing to provide unlimited cell source and differentiating into any cell types of the body. Moreover, the scientific developments of iPSC technology have enabled the generation of iPSCs via non-viral methods that leave no “genetic footprint” in the genome, thus making iPSCs potentially more suitable for therapeutic applications.
Despite the short history of iPSCs, they are moving quickly into clinical trials. Autologous iPSCs have been used to generate retinal cells to treat blindness in a clinical trial in Japan in 2014 (Mandai, Kurimoto, and Takahashi 2017). There are ongoing trials that are investigating the use of iPSC-derived heart muscle cells for heart disease (Cyranoski 2018) and the use of iPSC-derived neurons for Parkinson’s disease (Takahashi 2020). Besides regenerative medicine, there is an ongoing trial using iPSC-derived mesenchymal stem cells to treat GvHD (Bloor et al. 2020). In late 2018, a U.S. based company gained FDA approval for using iPSC-derived NK cells to treat cancer (Shankar et al. 2020). We believe that human pluripotent stem cells, especially iPSCs, are emerging as a reliable and standardizable starting material to produce cell therapy products.
Our Group has the know-how to use pluripotent stem cells to generate therapeutic cells of various types, including cardiomyocytes, neurons, dendritic cells (Zeng et al. 2007; Du et al. 2010; Zeng et al. 2009; Zeng et al. 2012; Zeng and Wang 2014; Zeng, Wu, and Wang 2015). Recently, we have successfully reprogrammed peripheral blood cells into iPSCs using the nonviral episomal method. Typical morphology of such generated iPSCs is shown in Figure 14. We believe we have further demonstrated the use of iPSCs to generate NK cells and gamma delta NKT cells against cancers (Zeng et al. 2017; Zeng, Tang, and Wang 2019). Typical morphology of such iPSC-derived gamma delta NKT cells is shown in Figure 15. Details of iPSC-derived gamma delta NKT cells are described below.
| 107 |

Figure 14. Typical morphology of iPSCs generated from peripheral blood cells.

Figure 15. Typical morphology of iPSC-derived gamma delta NKT cells.
| 108 |
Product Design and Manufacturing Process of iPSC-gd NKT Cells
| i. | Product Design and Features of iPSC-gdNKT Cells |
To develop a clinically cytotoxic and commercially viable cell-based immunotherapy for cancers, both the product function and its manufacturing need to be taken into consideration.
Among the innate immune cells, gamma delta T cells and NK cells have an array of built-in receptors to recognize cancers, including solid tumors. Such a cancer recognition system possesses several unique features including proven suitability, pattern recognition, targeting essential antigens and targeting ubiquitous antigens (Figure 16). We believe that integrating such cancer recognition system of gamma delta T cells and NK cells into one product, namely gamma delta NKT cells would not only enhance the potential product cytotoxicity but also broaden the indication spectrum (Figure 17).
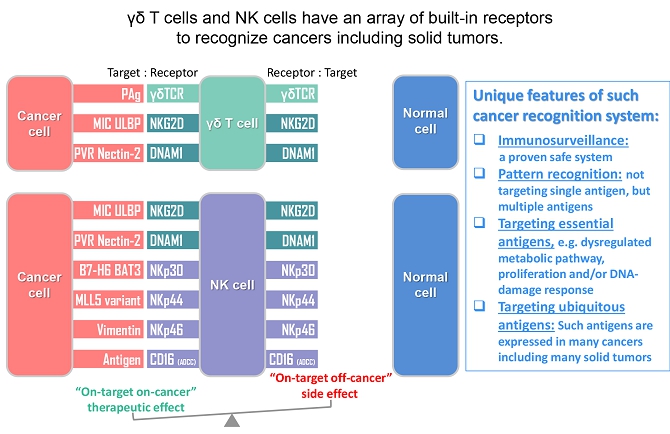
Figure 16. Target recognition system of gamma delta T cells and NK cells and its unique features.

Figure 17. Integrating cancer recognition capability of gamma delta T cells and NK cells into one product, gamma delta NKT cells.
| 109 |
| ii. | Manufacturing Process of iPSC-gdNKT Cells |
Ideally, such a product may be manufactured in large-scale from an unlimited cell source, which is not patient or donor blood cells, without relying on genetic modification. To this end, we are in the process of designing a proprietary manufacturing process to generate gamma delta NKT cells from iPSCs (Figure 18), which includes a one-time conversion of gamma delta T cells into iPSCs (“gamma delta T-iPSCs”) and using gamma delta T-iPSCs as starting material to manufacture gamma delta NKT cells.
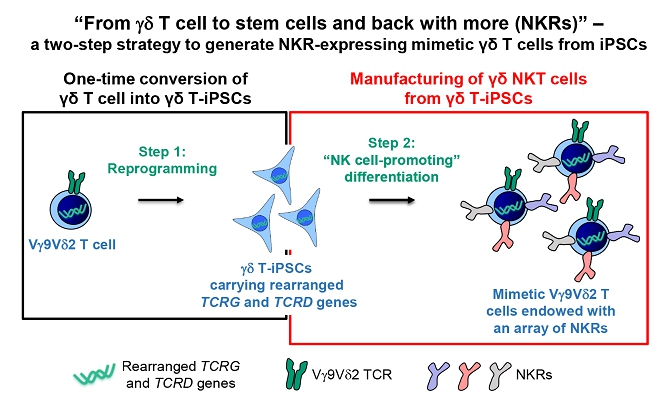
Figure 18. Manufacture of gamma delta NKT cells from gamma delta T-iPSCs.
| 110 |
Successful Generation of iPSCs from gamma delta T Cells (gamma delta T-iPSCs)
Using our optimized reprogramming protocol, we believe we have successfully generated iPSCs from gamma delta T cells and confirmed their derivation from gamma delta T cells (Figure 19). Such generated gamma delta T-iPSCs will serve as unlimited starting material to generate iPSC-derived gamma delta NKT cells.
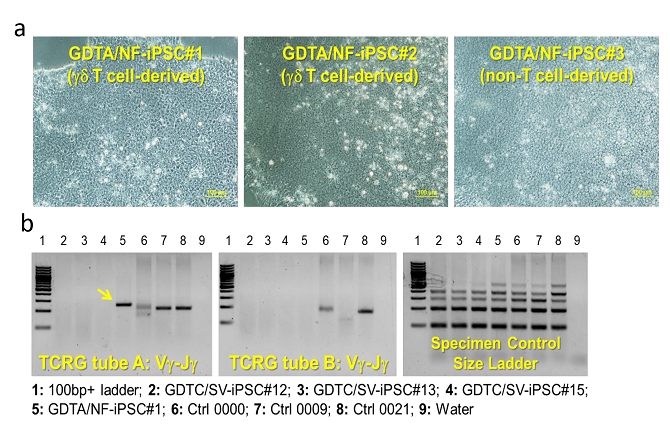
Figure 19. Generation and identification of γδ T-iPSCs. In this figure, “γδ T cell” refers to gamma delta T cell;
“GDTA/NF” means gamma delta T cell aggregate/ nucleofection
Successful Generation of gamma delta NKT Cells from gamma delta T-iPSCs
Using our “NK cell-promoting” differentiation protocol, we have successfully generated gamma delta NKT cells from gamma delta T-iPSCs. Such gamma delta NKT cells express cancer recognition receptors of both gamma delta T cells and NK cells (Figure 20).
Gamma delta NKT Cells Recognize and Kill a Broad-spectrum of Cancer Cells
We tested direct cytotoxicity of gamma delta NKT cells against cancer cells of different origins. These cancer cells were as various as follows: glioblastoma of brain (T98G, U-87); adenocarcinoma, ductal carcinoma and metastatic carcinoma of breast (MCF7, BT-474, MDA-MB-453); Burkitt’s lymphoma (Daudi, Raji); hepatocellular carcinoma (Hep G2); adenocarcinoma of ovary (SK-OV-3); metastatic melanoma of skin (FM-57, Malme-3M); squamous cell carcinoma of tongue (SCC-25); colorectal carcinoma and adenocarcinoma of colon (HCT 116, SW480); multiple myeloma (RPMI 8226); chronic myelogenous leukemia (K562); acute monocytic leukemia (THP-1). Results showed that gamma delta NKT cells efficiently killed these very different cancer cells even at low effector to target (E:T) ratios, suggesting that most surface receptors on gamma delta NKT cells may be involved in cancer recognition. See Figure 21.

Figure 20. Morphology and phenotype of gamma delta NKT cells.
| 111 |
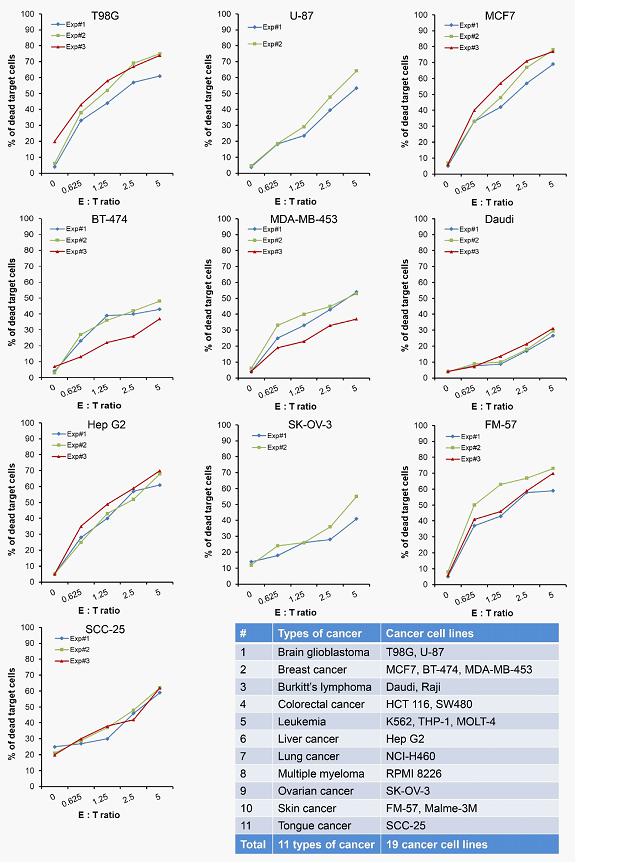
Figure 21. Direct cytotoxicity of gamma delta NKT cells against a broad-spectrum of cancer cells.
Direct cytotoxicity of gamma delta NKT cells against SW480, a colorectal adenocarcinoma cell line, was observed under a live imaging microscope. A 48-hour time-lapse video showed that gamma delta NKT cells eliminated most SW480 cells within first 12 hours, which we believe further confirms the cytotoxicity of this novel type of killer cells. See Figure 22.
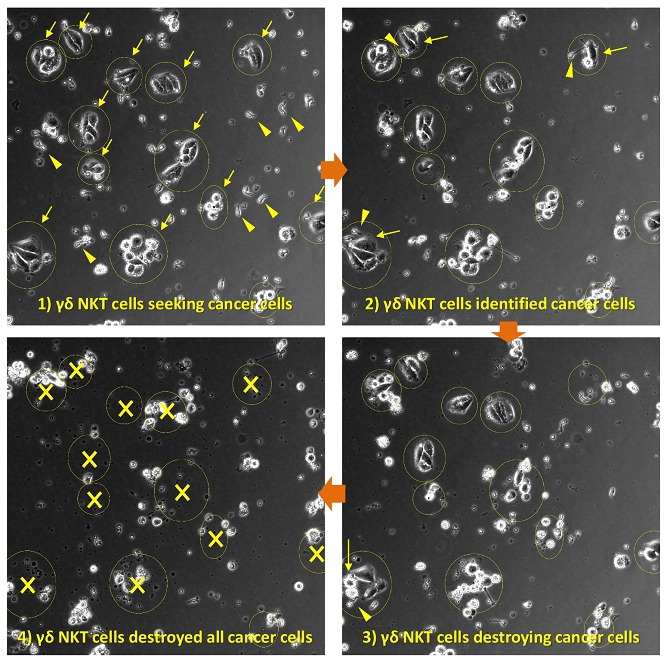
Figure 22. Seek, see, and destroy ‒ unravelling the action of gamma delta NKT cells frame by frame. In the above figure, the arrows indicate cancer cells, and the triangles indicate iPSC-derived gamma delta NKT cells.
Development Status of iPSC-derived gamma delta NKT Cell Technology
Extensive in vitro studies have demonstrated that iPSC-derived gamma delta NKT cells have the potential to recognize and kill a “broad-spectrum” of cancers with great specificity. To realize the potential of iPSC-derived gamma delta NKT cells in cancer treatment, we have been in the process of further evaluating iPSC-derived gamma delta NKT cells in pre-clinical studies since the fourth quarter of 2022, and is targeting to commence pre-clinical studies after 2024.
| 112 |
Property
Administration Office and Research Laboratory in Singapore
We own the leasehold interest of Singapore Laboratory situated at 1 Commonwealth Lane, #08-22, Singapore 149544 (Figure 23), which has an area of approximately 111 square meters housed in an industrial building within walking distance to Biopolis, a strategic district in Singapore designated for technology R&D and where A*STAR is located. The duration of the lease in respect of this property is 30 years commencing from March 1, 2008 and expiring on March 1, 2038, which is expected to be sufficient to complete our projects as stated in Figure 1. We are using the premises as our administration office and laboratory to conduct R&D and support research in our cGMP Facility in Malaysia, having fully fitted it with the necessary facilities and equipment to conduct research on GMP-grade iPSC and other cell type manufacture as well as cryopreservation of our CTM-N2D product for overseas export.






Figure 23. Our Administration Office and Laboratory in Singapore.
cGMP Facility in Malaysia
Our fully equipped, all-in-one cGMP Facility, consisting of office, R&D laboratory, quality control laboratory and cGMP facility is situated at 12 Jalan Permas 9/16, Bandar Baru Permas Jaya, 81750 Johor, Malaysia (Figure 24), and has the capacity to manufacture our cell therapy product candidates, being CTM-N2D, iPSC-gdNKT and CTM-GDT, for use in R&D and Phase I and Phase II clinical trials. We have also successfully completed similar trial production runs for CTM-GDT in the cGMP Facility.
For our CTM-N2D therapy product candidate, we have established standard operating procedures, BMR, in-process quality control tests and finished product quality control tests. Based on the manufacturing runs for CTM-N2D which were completed in 2020, we believe we have completed the validation of the manufacturing process of CTM-N2D and are ready to produce CTM-N2D for our Phase I clinical trial.
Our cGMP Facility is a three-story cluster facility situated on a plot of freehold land owned by us. The total gross floor area of the factory is approximately 8,600 square feet, of which 1,500 square feet is dedicated to our cGMP Facility comprising cleanrooms which support cGMP manufacturing of cell therapy products, a quality control laboratory, a cryostorage room, a control room and a material storage room.

Figure 24. Our cGMP Facility.
| 113 |
Employees
As of date of this Prospectus, we have twenty-seven (27) full-time employees, of which sixteen (16) have a master’s degree or Ph.D. degree. Of these employees, twenty-three (23) employees are engaged in our R&D and manufacturing activities. The rest of our employees carry out office administration and finance functions.
Our operations are currently managed by Chief Scientific and Medical Officer, Dr. ZENG Jieming, and Chief Operating Officer, Dr. TAN Wee Kiat. Pursuant to our employment agreements with Dr. ZENG Jieming and Dr. TAN Wee Kiat, both Dr. ZENG Jieming and Dr. TAN Wee Kiat are required to keep all proprietary and confidential information entrusted to them confidential during and after their employment and all intellectual properties developed by Dr. ZENG Jieming and Dr. TAN Wee Kiat belong exclusively to the Company.
We rely heavily on non-disclosure agreements with our employees to protect our confidential and proprietary information. Both Dr. ZENG Jieming and Dr. WANG Shu are the inventors of our CTM-N2D, CTM-GDT and iPSC-gdNKT cell technologies and the co-founders and shareholders of the Company.
We currently rely on substantial growth activities on our existing R&D team including key aspects of clinical development and manufacturing and only outsource certain quality services and tests and BAS software to the qualified independent organizations, advisors and consultants.
We have generally been able to retain valuable employees, members of our management, scientific and development team. We maintain a good working relationship with our employees and we have not experienced any labor disputes.
For information regarding our executive officers and SAB, please refer to the section entitled “Management”.
| 114 |
Intellectual Property
Our intellectual property rights and proprietary technology are protected through a combination of intellectual property rights protection regimes, including trade secrets and patents, confidentiality and non-disclosure obligations and contracts and contractual provisions in respect of innovations and invention assignments which clarify and address intellectual property ownership. We seek not only to protect our intellectual property rights and proprietary technology in key markets, but also to supplement our intellectual property portfolio to enhance such protection for our technological innovations and branding efforts by filing new patent and trademark applications when and where appropriate. We subscribe to a continuing intellectual property rights protection framework which includes guidelines on R&D, trade secrets, know-how, acquisition, exploitation, monitoring and enforcement of intellectual property rights.
We currently do not own any patents. As of the date of this Prospectus, the Company and/or its subsidiary, Puricell, have entered into three license agreements with ATPL, relating to developing, using, making, having made, manufacturing, distribute, marketing, importing, exporting, and selling our CTM-N2D, iPSC-gdNKT, CTM-GDT or future pipeline product candidates, upon obtaining regulatory approval.
The licenses include the Patent License, Know-How License, and K562 License for NK cell expansion. We and Puricell will rely on these licenses in developing products which we plan to seek and to obtain regulatory approval for and, upon receiving approval, plan to market and sell them worldwide.
Patent License
In June 2018, we entered into an exclusive, worldwide, non-sublicensable, non-transferable and revocable-for-cause license with ATPL under two pending patent applications filed under the Patent Cooperation Treaty (PCT) related to NKG2DL-targeting CAR-gamma delta T cell and iPSC-gdNKT cell technology, and any granted patent(s) and application(s) claiming common priority with and from such applications in the field of immunotherapy, including stem cell therapy, which was subsequently amended by an addendum entered into in December 2020, a second addendum entered into in September 2021, and a third addendum entered into in October 2022 (“Patent License”). Both Dr. ZENG Jieming and Dr. WANG Shu are the inventors of the CTM-N2D, CTM-GDT and iPSC-gdNKT cell technologies described in the Patent License, as well as co-founders and shareholders of the Company. The patent for the iPSC-gdNKT cell technology has been granted protection in Japan to ATPL until February 7, 2038, being 20 years from the filing date of February 7, 2018.
ATPL is not obligated under the Patent License to render any technical assistance, maintenance or support services with respect to the Company. ATPL and its affiliates retained the right to use and develop the licensed patents solely for internal R&D purposes. ATPL made no warranties as to the licensed patents or that use of them would be free from infringement of third party rights.
The Company owns any new versions of, modifications, additions, improvements, upgrades, and development to the licensed technology developed by the Company (“Enhancements”)
Payments to ATPL pursuant to the Patent License include an upfront licensing fee (in the mid-five figure Singapore dollar) and royalties, subject to an annual minimum payment (in the low-five figure Singapore dollar). Royalties which are calculated based on tiered low single-digit percentage of net sales, and an annual minimum royalty, payable until the end of the term of the Patent License. To date, we have paid APTL an aggregate of S$96,300 (approximately U.S.$69,266), including an upfront license fee and annual minimum royalty. Under this Patent License, we are also required to pay an aggregate of S$660,000 (approximately U.S.$474,718) for various milestones related to the clinical development and to meet commercialization milestones of our product candidates.
Unless terminated earlier, the term of the Patent License extends until expiration of the last of the patent rights licensed to us by ATPL. Patents issuing from these patent applications, if any, are not expected to expire before 2038. We may terminate the Patent License at any time upon providing at least thirty (30) days’ prior written notice to ATPL. ATPL may terminate the Patent License in circumstances including a breach by us remaining unremedied by us after thirty (30) days’ notice, including failure to meet development and commercialization milestones, an encumbrance taking possession or a receiver being appointed of any of our property or assets, any voluntary arrangement between us and our creditors, and/or cessation of our business, liquidation or receivership.
The aforementioned addendum in relation to the Patent License was entered into in respect of, amongst others, the NKG2DL-targeting CAR-gamma delta T cell and iPSC-gdNKT cell technology, which granted us the right to sublicense our license rights to our affiliates subject to certain conditions, such as ATPL being informed of any such sublicense within thirty (30) days of the execution of the same and such sublicense being on a non-exclusive basis only. Royalty payments charged under the sublicenses shall not be lower than the royalty payable by us to ATPL under the Patent License.
The aforementioned second addendum extends the deadlines to meet the commercialization milestones in exchange for CytoMed agreeing to take over responsibility for all patent prosecution and maintenance costs beginning January 2022 and includes the correct application number for one of the two licensed PCT patent application in relation to the NKG2DL-targeting CAR-gamma delta T cell.
Pursuant to the aforementioned third addendum, the four milestones have been extended from the second addendum as follows: commencement of Phase 1 clinical trial for fifteen (15) months; commencement of Phase 2 clinical trial for forty-five (45) months; commencement of Phase 3 clinical trial for fifty-seven (57) months; and commercial sale of the Licensed Product for sixty-nine (69) months.
Two Patent-Pending Families
CAR-gdT
One issued patent and 7 pending patent applications made by A*STAR are related to our CTM-N2D and CTM-GDT cells technologies, and include manufacturing process, treatment and compositions of matter claims. The issued patent is the European patent and is licensed from ATPL. The pending patent applications include applications in the United States, China, Malaysia, Singapore, United Kingdom, Germany and France. Of these issued and pending patent applications, none are owned or co-owned by us. The expiration date of the issued patent and pending patent applications are approximately 2038.
iPSC-gdNKT
One issued patent and 7 pending patent applications made by A*STAR are related to our iPSC-gdNKT cell technology, and include manufacturing process, treatment and compositions of matter claims. The issued patent is the Japan patent and is licensed from ATPL. The pending patent applications include applications in the United States, China, Malaysia, Singapore, United Kingdom, Germany and France. Of these issued and pending patent applications, none are owned or co-owned by us. The expiration date of the issued patent and pending patent applications are approximately 2038.
Know-How License
In October 2020, Puricell entered into a license agreement with ATPL, under which ATPL granted Puricell and its affiliates a worldwide, fee bearing, royalty bearing, non-exclusive, non-sublicensable, non-transferable and revocable-for-cause license to use ATPL’s GMP-compatible reprogramming of human blood cells know-how for research, development, and manufacturing of GMP-grade human iPSC derived from the licensed technology and to use, make, have made, manufacture, distribute, market, import, export, sell and have sold GMP-grade human iPSC which incorporate the licensed know-how or which cannot be developed, manufactured, used, sold, performed or provided without infringing ATPL’s rights in the licensed know-how (“Know-How License”). Payments to ATPL pursuant to the Know-How License include a nominal upfront license fee and royalties which are calculated based on a percentage ranging from the low-single digit to mid-single digit of net revenue up to an agreed maximum amount and an annual minimum royalty (in the low-four figure Singapore dollar). To date, we have paid ATPL an aggregate of S$6,420 (approximately U.S.$4,618), including an upfront license fee and annual minimum royalty. The last royalty payment will be on December 15, 2030. The term of the Know-How License is ten (10) years, unless terminated earlier.
| 115 |
ATPL made no warranties concerning the licensed know-how, including that use of the licensed know-how would be free from third party infringement claims.
The Know-How License does not prejudice ATPL and its affiliates from further developing, using, commercializing, and licensing the licensed technology. Except as provided in the Know-How License, Puricell shall not adapt, translate, alter, copy reproduce, deal in, reverse engineer, decompile, disassemble, or create any derivative works based in whole or part on the licensed know-how.
Puricell is required to indemnify ATPL and its affiliates, officers, employees, and scientists against any loss, damages, costs, expenses or other claim for compensation which arises out or in connection with the Know-How License or in any way related to the licensed know-how, except to the extent caused by ATPL’s negligence or willful misconduct.
Puricell is entitled to terminate on provision of at least thirty (30) days’ written notice. ATPL may terminate for a breach by Puricell that remains uncured after 30 days’ notice, an encumbrance takes possession of Puricell or a receiver is appointed of any Puricell property or assets, Puricell makes a voluntary arrangement with its creditors, Puricell goes into liquidation, except for purposes of amalgamation or reconstruction and the resulting licensee agrees to be bound by or assume the license, or Puricell ceases or threatens to cease to carry on its business.
The Know-How License is not to be assigned without ATPL’s prior written consent, but there is a novation provision that permits a new entity that takes over the whole or substantially the whole of ATPL’s operations to take on ATPL’s rights and obligations.
K562 Cell License for NK cell expansion
In December 2020, ATPL granted us and our affiliates, a non-exclusive, royalty-free, fee bearing, non-transferable, non-sublicensable, and revocable-for-cause license to use modified human K562 cells in the territories of Singapore and Malaysia for internal academic, research or investigation only and include the results of such uses in marketing materials, such uses which are important for the development of our future product candidates. Unless terminated earlier, the license has a five (5) year term which may be renewed by mutual agreement on similar terms. To date, we have paid a nominal upfront license fee of S$3,210 (approximately U.S.$2,309) to ATPL pursuant to the K562 Cell License for NK cell expansion.
ATPL retained the right to use all products and/or services the Company creates which contain or incorporate the modified human K562 cells. Any new intellectual property generated by ATPL is owned by ATPL and its affiliates. ATPL also retained the rights of ATPL and its affiliates to use, license, or otherwise commercialize modified human K562 cells.
The K562 Cell License for NK cell expansion includes use restrictions. The licensed product, or any new intellectual property ATPL generates, is owned by ATPL and the Company does not acquire any rights in it. Except for the Company’s affiliates, the cell line shall not be transferred or sublicensed without the express written permission of ATPL.
The K562 Cell License for NK cell expansion does not preclude the Company from commercializing products or services developed by the Company and its affiliates, but only to the extent that any such commercial products shall not incorporate any part of the licensed product or otherwise infringe ATPL intellectual property rights. ATPL acknowledged that the Company and its affiliates’ customers may use commercial products without a sublicense and without making additional payments to ATPL. The Company also agreed not to file any patent or other application claiming rights over the licensed product or the subject of the licensed products, but ATPL agreed that the results of research that the Company solely conducts using the licensed product will be owned by the Company. The Company also must comply with all applicable laws, rules, and regulations that may be required to accept delivery of the K562 cells.
ATPL made no warranties concerning the Licensed Products or K562 cells, including that their use would be free from third party infringement claims. The K562 Cell License for NK cell expansion required the Company to acknowledge that the K562 cells or modifications licensed under the K562 Cell License for NK cell expansion are or may be the subject of a patent application not licensed under the K562 Cell License for NK cell expansion, and that nothing in the K562 Cell License for NK cell expansion grants any implied or express license or right under any patents, know-how, trade secrets, or proprietary rights to the licensed products or any related process for profit-making or commercial purposes.
ATPL shall have the right to prosecute and defend any and all infringements and all benefits obtained therefrom belong to ATPL.
| 116 |
The Company is required to indemnify ATPL and its affiliates, officers, employees, and scientists against any loss, damages, costs, expenses or other claim for compensation which arises out or in connection with the K562 Cell License for NK cell expansion or in any way related to the licensed products or modified human K562 cells, except to the extent caused by ATPL’s negligence or willful misconduct.
Unless earlier terminated, the K562 Cell License for NK cell expansion has a five (5) year term extendable on request.
The Company is entitled to terminate the K562 Cell License for NK cell expansion on provision of at least two (2) months’ written notice. ATPL may terminate for a Company breach that remains uncured after thirty (30) days’ notice, an encumbrance takes possession, or a receiver is appointed to the property or assets of the Company, the Company goes into liquidation, except for purposes of amalgamation or reconstruction and the resulting Licensee agrees to be bound by or assume the K562 Cell License for NK cell expansion, or the Company ceases to carry on its business.
The K562 Cell License for NK cell expansion is not to be assigned without ATPL’s prior written consent, but there is a novation provision that permits a new entity that takes over the whole or substantially the whole of ATPL’s operations to take on ATPL’s rights and obligations.
Kyoto University Technology
We are dedicated to developing cutting-edge induced pluripotent stem cell technology as a source material for allogeneic cancer immunotherapy, and understand we may need a license to intellectual proper rights of Kyoto University. We have notified Kyoto University of our research progress, understand that a license is available, but understand that we do not need to enter into a licensing agreement with Kyoto University until we have begun clinical trials. A license is not yet required for our current stage of R&D.
Other intellectual property protection
We also rely on trade secrets, know-how, continuing technological innovation and our confidential information to develop and maintain our proprietary position and protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and others who may have access to proprietary information, under which they are bound to assign to us inventions made during the term of their employment or term of service.
Trade Secrets
Trade secrets may be afforded some measure of protection by a number of agreements amongst contracting member states and legislation in certain countries, of which remedies available consequent of a breach varies from destruction of products manufactured as a result of illegally obtained proprietary information, compulsory and punitive damages, injunctive relief, provisional relief to prevent infringement and preserve evidence to criminal sanctions. As we rely on trade secrets to protect our proprietary information, know- how and technologies and appreciate that the value of our trade secrets lies in its secrecy, we have and will continuously and consistently explore and take proactive steps to safeguard our trade secrets, relying in part on confidentiality agreements with our employees, and third parties and/or refrain from conducting our business where such protection is wanting.
However, these agreements may be breached, and we may not have adequate remedies for such breaches. In addition, our trade secrets may otherwise be acquired or independently discovered by competitors. To the extent that our contractors, commercial partners, collaborators, employees, and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For more information, please refer to the section titled “Risk Factors—Risks Related to Our Intellectual Property”.
We also seek to preserve the integrity and confidentiality of our data and trade secrets by ensuring constant physical security for our premises, physical and electronic security of our information technology systems and maintaining back-ups of our information.
Trademarks
We also seek to protect our brand through trademark rights. As of the date of this Prospectus, “CytoMed Therapeutics” and our logo has been registered in Singapore on June 21, 2021 and will be due for renewal on June 21, 2031. Our trademark registration application in Malaysia has been registered on June 24, 2021 and will be due for renewal on June 24, 2031.
Domain Names
As of the date of this Prospectus, the Company owns the domain name “Cytomed.sg”, which was registered in 2018 and is due for renewal in 2024.
| 117 |
Competition
The field of immunotherapy for treatment of cancers is characterized by intense and dynamic competition to develop new technologies and proprietary therapies. Any product candidates that we are in the process of developing will have to compete with existing therapies and new therapies that may from time to time become available in the future. We face competition from various sources, including well-funded biopharmaceutical companies, established biopharmaceutical companies, as well as public and private research institutions. The areas of competition include relevant scientific and management human resources, funding for product development, establishing clinical study sites and clinical subjects participating in the trials.
Our known biopharmaceutical competitors working on allogeneic CAR-T therapies currently include Allogene Therapeutics, Inc. (NASDAQ:ALLO), Astellas Pharma Inc. (TSE:4503.T), Bristol-Myers Squibb (NYSE:BMY), Celyad Oncology SA (NASDAQ:CYAD), Fate Therapeutics, Inc. (NASDAQ:FATE), Gilead Sciences, Inc. (NASDAQ:GILD), NANTKWEST, INC.(NASDAQ:NK), Novartis International AG (NYSE:NVS), Surface Oncology, Inc. (NASDAQ:SURF), Takeda Pharmaceutical Company Limited (NYSE:TAK) and numerous other biopharmaceutical companies.
We are aware of the recent developments of our potential competitors for allogeneic CAR gamma delta T- cell therapy. Based on publicly disclosed information, in June 2022, Acepodia Biotech, Inc. announced FDA clearance of Investigational New Drug application for an Anti-CD20 armed allogeneic gamma delta T-cell therapy to treat non-Hodgkin’s Lymphoma, at the same time that Immatics N.V. (NASDAQ: IMTX) and Bristol Myers Squibb (NYSE: BMY) announced the expanded strategic alliance to develop multiple allogeneic off-the-shelf TCR-T and/or CAR-T gamma delta cell therapy programs. In April 2022, Adicet Bio, Inc. (NASDAQ: ACET) received FDA fast track designation for gamma-delta T-cell therapy for advanced lymphoma which subsequently reported emerging positive Phase I safety and efficacy data for relapsed or refractory B-cell Non-Hodgkin’s Lymphoma in June 2022. In addition, numerous academic institutions are conducting pre-clinical and clinical research in these areas, as well as with other white blood cell types including gamma delta T cells.
Our existing competitors in the field of gamma delta T cell therapy are listed in Table 2. We are aware of the recent development of our potential competitor for allogeneic gamma delta T- cell therapy. Based on publicly disclosed information, in March 2022, FDA granted Orphan Drug Designation to TC BioPharm (Holdings) PLC (NASDAQ: TCBP) investigative allogeneic unmodified gamma delta T-cell product, for use as a potential therapeutic option in patients with relapsed/refractory acute myeloid leukemia. As of the date of this Prospectus, we are not aware of any approved Phase III trial for gamma delta T cell-based cancer immunotherapy. Our gamma delta T cell product candidate may also face competition from other cell-based immunotherapy approaches derived from NK cells and T cells.
Our existing competitors in the field of iPSC-derived immune cells include Fate Therapeutics, Inc. (NASDAQ:FATE) and Takeda-CiRA joint program (Takeda Pharmaceutical Company Limited (NYSE:TAK), Centre for iPS Cell Research and Application, Kyoto University) and Century Therapeutics, Inc. (NASDAQ:IPSC). As of the date of this Prospectus, the Takeda-CiRA joint program is in its pre-clinical stage, while Fate Therapeutics, Inc. (NASDAQ:FATE) has various ongoing Phase I clinical trials for iPSC-derived NK cells.
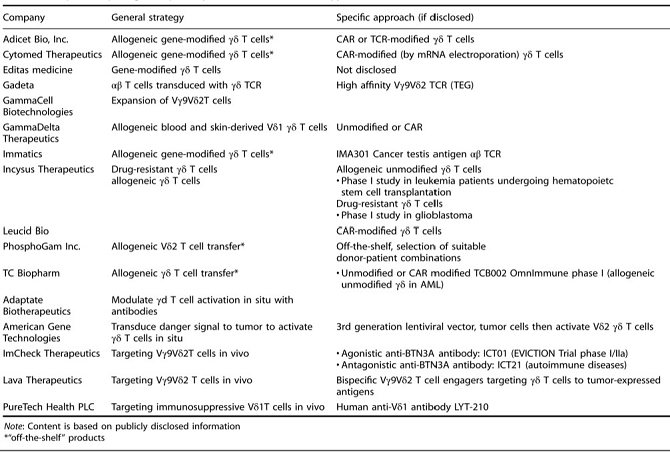
Table 2. Companies exploring γδ T cell-based immunotherapy (adapted from Kabeliz D et al, 2020).
| 118 |
Many of our existing potential competitors may have greater financial and other resources, larger team of R&D staffs, and more experienced capabilities in researching, developing and testing products than we do. Many of these companies also have more experience in conducting clinical trials, obtaining relevant regulatory approvals, and manufacturing, marketing and distributing therapeutic products. Smaller or pre-clinical stage companies like us may successfully compete by establishing collaborative relationships with larger pharmaceutical companies or academic institutions. Accordingly, our competitors may be more successful than us in obtaining approval for treatments and achieving widespread market acceptance. Our competitors’ treatments may be more clinically effective, or more effectively marketed, than any treatment we may commercialize and our competitors’ treatments may render our treatments obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our treatments.
Mergers and acquisitions in the industry may result in resources being further concentrated in a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical study sites and subjects for clinical studies. Other small or early-stage companies may also prove to be strong competitors, particularly those with collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new therapies enter the market and advanced technologies become available from time to time. We expect that any treatments which we develop and commercialize will need to compete on, among other things, efficacy, safety, convenience of administration and delivery and price. Commercialization of any treatments we develop will be affected by the level of generic competition and the availability of reimbursement from government and other third-party payors.
Our ability to commercialize our proprietary cell products could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have a better safety profile, are more convenient or are less expensive than our products. Our competitors also may obtain relevant regulatory approvals for their products more rapidly than we may be able to obtain approval for ours, which could result in our competitors obtaining a head start and establishing a frontrunner position before we are ready to commercialize. If we are not able to compete effectively against our existing and potential competitors, our business, financial condition, results of operations and growth prospects may be materially and adversely affected.
| 119 |
Prospects, Business Strategies and Future Plans
Industry Overview and Prospects
Immunotherapy is gaining recognition and acceptance as an alternative therapy to treatment of cancer, in particular, in cancer patients who do not respond well to conventional cancer treatment such as surgery, chemotherapy or radiation.
CAR-T cell therapy as a form of immunotherapy
Immunotherapy harnesses the immune system’s ability to fight cancer. CAR-T cell therapy is a form of immunotherapy which uses specially altered T-cells to fight cancer. Cancer patients have naturally occurring T-cells which are often capable of targeting cancer cells. These T-cells are a powerful form of immune cells in our human body, and come in several types. The NKT-cells, in particular, are capable of recognizing and eliminating cancer cells in a precise manner. However, the existence of these T-cells alone is not sufficient to eliminate all cancer cells. A potential roadblock is that these T-cells must first become activated before they can effectively eliminate cancer cells, and they must be able to maintain that activity for a sufficiently long period of time in order to sustain an effective anti-cancer response. Another limitation is that these T-cells might not exist in sufficient quantity.
CAR-T cell therapy market
Since the approval of the first CAR-T cell therapeutic treatment in 2017 by the FDA, there has been widespread research and an exponential increase in clinical trial activity in the field of immunotherapy. Between 2017 and 2022, there have been at least 6 CAR-T products that have reached the market, in which the earliest approvals, Kymriah® and Yescarta®, have been commercially available since 2018, and have been infused into many patients worldwide.
However, all approved CAR-T cell therapies thus far take an autologous treatment approach. Autologous CAR-T cells are expensive to produce because they are manufactured on a patient-by-patient basis, and can be hampered by a shortage of CAR-T cells or viral vectors. Hence, current CAR-T cell therapies are usually recommended for end-stage cancer patients who have exhausted all conventional treatment options.
CAR-T cell therapy has been a game-changer in the cancer treatment industry, creating hope that it could usher in a new era of cancer treatment. However, the success stories have typically come from targeting CD19, which is now considered an antigen that holds the key to a limited range of blood cancers. CAR-T cell therapy treatment for other types of cancers, in particular, solid tumors, has been severely limited so far.
Cell Therapy in Singapore and Malaysia
The cell therapy sector in Singapore and Malaysia is at a nascent stage, with only a handful of biopharmaceutical companies within the cellular immunotherapy space in the treatment of cancer. Singapore approved the first CAR-T therapy for cancer treatment in March 2021, Kymriah® by Novartis (Singapore) Pte Ltd, which is the first and only commercially approved CAR-T therapy in Singapore currently in use for certain specific forms of B-cell malignancies. Malaysia has not yet approved any CAR-T therapy for treatment of cancer for commercial use.
Singapore established ACTRIS in April 2020 to meet the potential demand of cell therapy process development and product manufacturing to enable clinical utility. ACTRIS is the national center for facilitating discovery, process development and manufacturing of cellular-based therapeutics in immunotherapy and regenerative medicine. ACTRIS works with other government agencies such as A*STAR and industry players such as hospitals, universities, research organizations and clinicians. ACTRIS is a business unit under CRIS, a wholly-owned subsidiary of the Ministry of Health Singapore.
| 120 |
We intend to capitalize on our close connections with A*STAR to tap into the latest developments and advances in cell-based therapeutics in the field of cancer treatment.
Business Strategies and Future Plans
CytoMed is a pre-clinical biopharmaceutical company and aims to establish itself as one of the leading biopharmaceutical companies in Asia engaged in the immunotherapy R&D, with the goal of providing affordable cell-based cancer immunotherapy for unmet needs. We are headquartered in Singapore with our production facilities in Malaysia for cost efficiency.
We intend to focus on developing our two (2) “off-the-shelf, allogeneic” technology platforms centered on gamma delta T cells and iPSCs respectively. Please refer to the “Business” section for further details.
Our aim is to be amongst the pioneers of cellular immunotherapy treatment for cancer to serve the emerging economies within South-east Asia. We aim to strategically position ourselves as a bridge between the cutting-edge of cellular therapy in the U.S. and our origin amongst emerging economies in South-east Asia, with our headquarter located in Singapore. We believe that the U.S. has the depth and breadth of expertise and experience to accelerate our R&D efforts in cellular therapy. We aim to establish our profile with like-minded biopharmaceutical companies, researchers, scientists and sources of investment capital within the U.S. In addition, we anticipate that cellular science will advance and gain more mainstream acceptance in the future. We are considering conducting research in regenerative medicine and in particular, stem cell application in an aging population in Asia. We believe we are well-placed with the necessary expertise, experience and resources to take advantage of such prospects.
Close connections with scientific and medical community
As described in the section entitled “Management”, we have industry advisors on our Scientific Advisory Board and our key executive officers have the relevant specialized technical and medical expertise in cellular therapy.
Our core technologies are licensed from A*STAR, Singapore’s lead public sector R&D agency. By working closely with research organizations and government bodies, we are continually kept abreast of the latest developments in the field of cellular therapy. We believe that our strategic positioning, experience and expertise will give us an edge to take advantage of the growing and developing cellular therapy market.
In addition, we also seek collaboration, joint-ventures and out-licensing rights to jointly develop our two novel “off-the-shelf” technology platforms using our infrastructure in Singapore and Malaysia with industry players so as to speed up commercialization of our product candidates. In 2021, through our wholly-owned subsidiary, Advance Cancer Centre Pte Ltd, we acquired a 20% equity stake in LMC which operates a licensed private hospital in Malaysia with an Oncology & Haematology Department. LMC is a licensed private hospital offering obstetrics and gynaecological services, minimally invasive surgery, cancer treatment and care, plastic and reconstructive surgery, orthopaedics and clinical research. In 2019, LMC received approval from the National Committee of Clinical Research of Malaysia to set up an independent ethics committee for the purpose of facilitating ethical review and approval of clinical trials on-site. We intend to work with LMC to conduct clinical trials.
| 121 |
In addition, we have acquired the premises at 1 Commonwealth Lane, #08-22, Singapore 149544. Please refer to the section titled “Administration Office and Research Laboratory in Singapore” below for more information. We use the premises, which is fitted with relevant equipment to perform research on GMP-grade iPSC and other cell type manufacture as well as cryopreservation of our CTM-N2D product for overseas delivery, as our administrative office and research laboratory.
We may also explore setting up a joint laboratory in the U.S. depending on the extent of our collaboration with suitable partners there.
Lower business and operating costs in South-east Asia
The relatively lower business and operating costs in South-east Asia should position us well to achieve our vision of developing our donor blood cell-based and iPSC-based platform technologies into off-the-shelf cancer immunotherapies with the ultimate goal to offer lower cost cell therapies as a form of cancer treatment to patients.
We have invested in and completed construction of our own cGMP Facility which is fully equipped with state-of-the-art equipment, which we believe is capable of handling the manufacturing for Phase I and Phase II clinical trials for our clinically-ready CAR T therapy, which is trade-named “CTM-N2D”. Our cGMP Facility has been audited by the NPRA of the Ministry of Health Malaysia, which has determined that our cGMP Facility has a satisfactory level of compliance. We believe our proprietary product candidate, CTM-N2D, is clinically ready to commence clinical trials while our iPSC-gdNKT platform is at the final development stage for clinical trials as of the date of this Prospectus. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s acknowledgement on January 6, 2023 that we had submitted the relevant documents to meet the approval conditions of the CTA initially granted in July 2022 relating to the use of our CTM-N2D for the aforementioned trial by HSA, and no further action was required. We have already recruited a competent team of scientists and technicians and have provided them with the necessary training to manufacture the investigational product for clinical trial. We target to recruit our first patient in the second half of 2023. Our iPSC-gdNKT platform is in the pre-clinical development stage as of the date of this Prospectus.
MSCs as our potential future product candidate
Stem cells are unspecialized cells with the capacity to regenerate and replicate as well as to develop into different types of specialized cells in the body. They are important in the human body as they are able to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat many diseases. Depending on the types of stem cells, different types of stem cells possess different capacity of differentiation.
We are currently researching the use of MSCs, which are multipotential adult stem cells found in the supportive framework of organs and are the building blocks for tissue renewal and repair. MSCs have the ability to differentiate into multiple cell types of mesenchymal lineage, including bone, cartilage, fat, and muscle cells as well as those of non-mesenchymal lineage depending on the environment in which they are cultured. Several studies have reported that the mechanism of MSCs in repairing tissue damage is associated with their immune-modulatory properties. One of MSCs’ vital biological functions, the immune-modulation, allows MSCs to travel to specific injury or inflammation sites in the body, and to modulate immune response to reduce inflammation. A relatable use of MSC’s tissue repair capability and immunomodulation function is demonstrated in lung tissue repair and cytokine release syndrome control in patients with severe COVID-19 disease. COVID-19 patients who have undergone MSCs treatment recovered faster and had less severe cytokine release syndrome.
It is reported that MSC therapies have been approved in several countries including Japan, South Korea and Canada. Some other specific medical conditions under global research include age-related macular degeneration, aging frailty, Alzheimer’s disease, autoimmune disease, cerebral palsy, stroke, spinal cord injury, heart disease and failure.
CytoMed is considering partnering with our associate hospital, LMC, to focus our research on umbilical cord and adipose tissue-derived MSCs as regenerative medicine. We believe that our current facility and professional research team has the capability to culture high quality MSCs for regenerative purposes.
Nevertheless, as of the date of this Prospectus, we have not yet planned or commenced development of MSCs and there is no certainty that we will manufacture any MSCs as one of our product candidates.
| 122 |
Cell-based therapies are still considered novel and a new medical science in South-east Asia. The CTGTP Regulations in Singapore became effective on March 1, 2021. Malaysia’s CGTP regulations became effective on January 1, 2021. We expect that such regulations will be amended as more experience and expertise are gained by both regulators, clinicians and scientific community. Although future regulations may hinder or be detrimental to cell-based therapies, the medical community generally considers that such cellular “living” medicine has the potential to enhance patient treatment modalities for many diseases. Reflecting this, Singapore set up a national agency in April 20, 2020 known as ACTRIS comprising, inter alia, all the national universities, hospitals, research institutions and funding agencies in Singapore. More information on ACTRIS can be found in page 120 of this Prospectus.
Singapore
It is intended that the Singapore Laboratory will be used, amongst others, to conduct research in Singapore after completion of renovations. However, as of the date of this Prospectus, we do not yet have any clinical operations in Singapore and has not commenced any clinical trials in Singapore and the regulations below will only apply if and when CytoMed and/or any of its subsidiaries commences clinical operations and/or clinical trials in Singapore. On March 10, 2023, we entered into the Clinical Study Agreement pursuant to which the ANGELICA Trial will be conducted by the National University Hospital Singapore to evaluate the safety of CTM-N2D in human subjects, following the HSA’s acknowledgement on January 6, 2023 that we had submitted the relevant documents to meet the approval conditions of the CTA initially granted in July 2022 relating to the use of our CTM-N2D for the aforementioned trial by HSA, and no further action was required.
Being a company incorporated in Singapore, we are subject to all relevant laws and regulations of Singapore and may be affected by new laws, regulations and policies which are introduced by the Singapore government from time to time. We have identified the main laws and regulations (apart from those pertaining to general business requirements) that we anticipate may materially affect our operations, the relevant regulatory bodies and the licenses, permits and approvals typically required for the conduct of our business in Singapore.
The following description is a summary of material laws and regulations applicable to our operations. The laws and regulations set out below are not exhaustive and are only intended to provide general information to investors and are neither designed nor intended to be a substitute for professional advice. Prospective investors should consult their own advisers regarding the implication of the relevant laws and regulations on us.
Overview
In Singapore, the health products industry is regulated by the HSA and operates under the oversight of the MOH Singapore. The HSA administers health-related laws and regulations and regulates the health products sector, ensuring that drugs, innovative therapeutics and other health-related products are regulated and meet safety, quality and efficacy standards. Our registered medical professionals are also regulated by the SMC under legislation and regulations including the Medical Registration Act 1997, the Medical Registration Regulations 2010 and the relevant guidelines in Singapore.
Apart from the laws and regulations mentioned below for which HSA or MOH Singapore is the regulatory authority, compliance with the applicable circulars, directives and guidance released by HSA or MOH Singapore from time to time must be ensured as well.
Our business generally falls under the regulatory framework consisting of the HPA, HBRA, and the partially in-force HCSA, with subsidiary legislation and guidelines as promulgated by HSA, MOH Singapore and the SMC.
In particular, human biomedical research is regulated by the HBRA and its sub-legislation, namely, the Human Biomedical Research Regulations 2017 and the Human Biomedical Research (Restricted Research) Regulations 2017. Legislation is supplemented by the Ethics Guidelines for Human Biomedical Research released by the Bioethics Advisory Committee of Singapore.
Apart from the above, it is also important to note privacy issues that may arise out of research or clinical trials are regulated under the PDPA Singapore, if not already covered by subsidiary legislation of the main legislation set out in this section.
| 123 |
HSA
The HSA, established by the HSA Act, operates under the oversight of MOH Singapore. The general functions, objects and duties of the HSA as stated in the HSA Act, include in particular:
| (i) | to regulate the manufacture, import, export, sale, supply, advertisement and use of health products, tobacco products, radioactive materials and irradiating apparatuses in accordance with the applicable written laws; |
| (ii) | to conduct technological assessments of health products for the purpose of determining their quality, safety, efficacy and suitability for consumption and use in Singapore and to advise the Singapore government thereon; |
| (iii) | to provide professional, investigative, analytical and other services in health sciences and chemical metrology (relating to human health) to the Singapore government and to any other person or body (whether in Singapore or elsewhere); and |
| (iv) | to conduct or engage any other person to conduct research in health sciences, and generally to promote the development of health sciences. |
MA
Pharmaceutical products (also known as chemical or biological drugs) were previously regulated under the MA and Poisons Act 1938 of Singapore. They are now regulated as “therapeutic products” under the HPA and the Health Products (Therapeutic Products) Regulations 2016, along with other health products (medical devices and cosmetic products). This is part of the HSA’s initiative to transfer regulatory control of all health products from the MA to the HPA. We will also be required to ensure compliance with circulars, directives and guidance relating to “therapeutic products”, including the Guidance on Therapeutic Product Registration in Singapore released by the HSA in August 2021, where applicable.
Cell, tissue and gene therapy products, in which our business is involved, are classified as a new category of health product under the HPA. This is further elaborated on below.
Import, supply, presentation and advertisement of cell, tissue or gene therapy products
HPA
The HPA, amongst others, regulates the manufacture, import, supply, presentation and advertisement of health products. It was amended by the HPA Order 2021 on March 1, 2021 to introduce a new category of health products, namely, CTGTP in the First Schedule to the HPA. The HPA therefore provides the legislative basis for regulating the manufacture, import, supply, presentation, and advertisement of CTGTP.
Prior to this, regulatory controls for CTGTPs were provided for in the MA.
Our research has been conducted in our laboratories in Malaysia and we are preparing to conduct trials in Singapore. While we intend to manufacture any approved product candidates in Malaysia, we may potentially conduct research, trials, presentations, advertisements, and treatments in Singapore. As our CTM-N2D, iPSC-gdNKT and CTM-GDT cell technologies may be considered as CTGTP, we are therefore mindful of the restrictions under the HPA and its regulations that could require us to obtain approvals and/or licensing in Singapore.
CTGTP Regulations
The CTGTP Regulations are given effect under the HPA, and came into operation on March 1, 2021. They provide for the regulations relating to the manufacture, import, supply, presentation, registration, duties, and obligations of manufacturers, importers and other persons carrying out such activities.
| 124 |
In summary, CTGTPs are health products intended for use in humans for therapeutic, preventive, palliative, or diagnostic purposes. CTGTP can contain any of the following and achieves its primary intended action by pharmacological, immunological, physiological, metabolic or physical means:
| (i) | viable or non-viable human cells or tissues; | |
| (ii) | viable animal cells or tissues; or | |
| (iii) | recombinant nucleic acids. |
Regulation 2 of the CTGTP Regulations states that CTGTPs are risk-stratified into two classes:
| (i) | Class 1 CTGTPs are deemed lower risk and satisfy all of the following criteria: |
| (a) | minimally manipulated; | |
| (b) | intended for homologous use (performing same function and administered at the same anatomical site or histological environment in the recipient as in the donor); and | |
| (c) | not combined or used in conjunction with therapeutic products or medical devices as defined in the First Schedule to the HPA. |
Examples of Class 1 CTGTPs include bone grafts, amniotic membrane, and skin.
| (ii) | Class 2 CTGTPs are deemed higher risk, and are CTGTPs other than Class 1 CTGTPs. Examples include gene modified cells, cells grown on scaffold, culture expanded cells, vectors with therapeutic gene, and xeno-based products. |
The level of control over companies dealing in the activities of manufacture, import and supply (“dealers”) is calibrated to the degree of manipulation of the CTGTP.
| (i) | The CTGTP Regulations provide that CTGTPs that are minimally manipulated are subject to lower controls. |
Dealers dealing in only minimally manipulated CTGTPs are not subject to licensing requirements and need only provide a written notification to HSA prior to the start of the manufacturing, importing or wholesaling activity (“dealer’s notice”). Upon successful submission of a notice, the dealer will be considered a known importer, known wholesaler, or known manufacturer (“known dealer”). Failure to submit a notice is an offence under the CTGTP Regulations. Renewal of a notice is not required, but the dealer must submit an update of the dealer’s notice whenever there are any changes to the information which was provided earlier.
However, a dealer which already has a license for (ii) below does not need to submit a notice for import, wholesale, or manufacture of minimally manipulated CTGTP.
| (ii) | CTGTPs that are more than minimally manipulated require a license. |
Known dealers are required to comply with the respective duties and obligations under the CTGTP Regulations, including to:
| a. | maintain records of manufacture; | |
| b. | maintain records of receipt and supply; | |
| c. | maintain systems of traceability of the CTGTP and its starting and raw materials, including any substances that come into contact with the cells and tissue; | |
| d. | maintain records of defects and adverse effects; | |
| e. | report defects and adverse effects; | |
| f. | notify HSA concerning recall; and | |
| g. | notify HSA before supply of Class 2 CTGTPs. |
| 125 |
In addition, dealers are required to comply with the relevant regulatory guidance where appropriate, which include:
| (i) | For manufacturers – MOH Singapore’s Guidelines for Healthcare Institutions Providing Tissue Banking and HSA’s Guidelines on Good Manufacturing Practice for CTGTPs; and | |
| (ii) | For importers and wholesalers – HSA’s Good Distribution Practice Standard for Medical Devices, ISO 13485, and HSA’s Guidance Notes on Good Distribution Practice. |
Additional guidance can also be sought from other regulatory guidance released by the relevant regulatory authorities, including HSA’s Guidance on Cell, Tissue and Gene Therapy Products Registration in Singapore.
Advertisement Regulations
Advertisements of CTGTPs do not require prior approval from the HSA. However, the advertisements must comply with the principles and requirements as stated in the HPA and Advertisement Regulations. The Advertisement Regulations are given effect under the HPA and came into effect on November 1, 2016. Some of such key principles and requirements are highlighted below.
Advertisements of CTGTPs refer to any information that can promote the sale or use of the CTGTP, and can be in any forms or media including but not limited to publication in a newspaper, magazine, journal or other periodical, display of posters or notices, circulars, brochures, pamphlets, books, letters addressed to individuals or organizational bodies, and/or any other activity intended to introduce, publicize or raise the profile or public awareness or visibility of any CTGTP for the purpose of promoting the sale or use of it.
Advertisements of CTGTPs must be aligned with the intended uses per its registration or notification made to HSA. Importantly, they cannot be directed at the public (i.e. cannot be made available in any public places) and, amongst others, must not:
| (i) | advertize an unregistered or unnotified product; | |
| (ii) | advertize an unregistered indication of a registered or notified product; | |
| (iii) | make any false or misleading claims or representations; | |
| (iv) | make unsubstantiated claims; | |
| (v) | make claims that mislead by emphasis, contrast or omission with regard to the safety, quality or efficacy of the product; | |
| (vi) | make claims that give rise to any unrealistic expectations with regard to the effectiveness of the product; | |
| (vii) | encourage inappropriate or excessive use; | |
| (viii) | suggest guaranteed results without side effects; | |
| (ix) | falsely claim any endorsement by public authority; | |
| (x) | include endorsements or recommendations by any healthcare professional or a person of celebrity, social or professional status; | |
| (xi) | use the names or logos of HSA and any of professional groups; and/or | |
| (xii) | offer refunds, in full or partial amounts, to users of the product. |
Clinical trials and research
In the event that clinical trials, research and/or treatments are undertaken in Singapore, we will be required to obtain and maintain the necessary licenses and approvals, and compliance with the following legislation and regulations (where applicable) must be ensured. Depending on the requirements of the specific clinical trials, research and/or treatments and industry standards from time to time, we will also be required to adhere to international guidelines such as the Guidelines for Good Clinical Practice of the International Conference on Harmonization.
| 126 |
PHMCA
The PHMCA governs the control, licensing and inspection of, among others, medical clinics, clinical laboratories and healthcare establishments for the purpose of ensuring patient safety.
Clinical laboratories, healthcare establishments and medical clinics falling within the respective definitions in the PHMCA (as set out below) must have a license issued by the Director of Medical Services (as referred to in the PHMCA) and must operate in accordance with the terms and conditions of such license.
A clinical laboratory is defined in Section 2 of the PHMCA as any premises used or intended to be used for any type of examination of the human body or of any matter derived therefrom for the purpose of providing information for the diagnosis, prevention or treatment of any disease or for the assessment of the health of any person, or for ascertaining the cause of death or the result of any medical or surgical treatment given to any person, but does not include any such premises: (a) which are maintained by the Singapore Government or the National University of Singapore, (b) which form part of the premises of a licensed private hospital, or (c) which are maintained by a medical practitioner or dentist as part of his medical clinic for the exclusive use of his practice.
A healthcare establishment is defined in Section 2 of the PHMCA as any premises (a) which is used or intended to be used for the provision of any service, or for carrying out any practice or procedure, that is related to the diagnosis, treatment or care of persons suffering from any disease, injury or disability; and (b) which is declared by the Minister (as referred to in the PHMCA), by order published in the Gazette (as referred to in the PHMCA), to be a healthcare establishment for the purposes of the PHMCA, but does not include a private hospital, medical clinic or clinical laboratory (each defined in the PHMCA) or part thereof, or an establishment or conveyance maintained by the Singapore Government or the National University of Singapore.
A medical clinic is defined in the Section 2 of the PHMCA as any premises used or intended to be used (a) for the diagnosis or treatment of persons suffering from, or believed to be suffering from, any disease, injury or disability of mind or body; or (b) for curing or alleviating any abnormal condition of the human body by the application of any apparatus, equipment, instrument or device requiring the use of electricity, heat or light, but does not include any such premises (i) which are maintained by the Singapore Government or the National University of Singapore; or (ii) which form part of the premises of a licensed private hospital (as defined in the PHMCA).
If a medical clinic, clinical laboratory or healthcare establishment is not licensed or used otherwise than in accordance with the terms and conditions of its license, every person having the management or control thereof shall be guilty of an offence and shall be liable on conviction to a fine not exceeding S$20,000 or to imprisonment for a term not exceeding 2 years or to both.
ECEG 2016 and HME 2016
The ECEG 2016 provides guidance for inculcating good medical practice in Singapore based on the fundamental tenets of medical ethics.
In particular, Guideline B6 of the ECEG 2016 stipulates that in general, untested treatments that are not generally accepted by the medical profession can only be offered to patients in two forms: (i) in the context of formal and approved clinical trials (which would be subject to the ethics of research), or (ii) as innovative therapy (which may only be offered when conventional therapy is unhelpful and it is a desperate or dire situation).
Guideline B6.1 of the HME 2016 defines “innovative therapy” as “a completely novel or significantly modified standard therapy with little or nothing in the way of studies or scanty evidence of efficacy, effects or side effects”. Under the HME 2016 and ECEG 2016, doctors may offer such innovative therapy to patients in desperate or dire situations, and where conventional therapy is unhelpful. In such instances, patients’ informed consent must be obtained, failing which the doctor may be struck off the Singapore Registrar of Medical Practitioners.
| 127 |
Guideline B8 of the ECEG 2016 also stipulates that any medical research on human subjects or trials of any treatment on patients must be approved by the Institutional Review Board or Ethics Committee (as referred to in the ECEG 2016) and conform to the Singapore’s Good Clinical Practice guidelines and other existing guidelines on human biomedical research.
CRM Regulations
The CRM Regulations governs the import, manufacture and supply of clinical research materials for use in clinical research studies (including clinical trials). The CRM Regulations is given effect under the HPA and came into effect on November 1, 2016.
CRM refers to any registered or unregistered therapeutic product, medicinal product, medicinal device, applicable CTGTP or placebo, that is manufactured, imported or supplied for the purpose of being used in clinical research, by way of administration to a trial participant in accordance with the research protocol or for a clinical purpose.
The manufacture, import and supply of CRM in Singapore for (i) Class 2 CTGTPs or (ii) Class 1 CTGTPs for which a manufacturer / importer / wholesaler notification has not been made to HSA, must comply with the CRM Regulations.
All local dealers of CRM must only supply the CRM for use in a clinical research, unless the CRM is locally registered and is manufactured, imported or supplied with a valid dealer’s license. The local sponsor of the clinical research must also ensure that the CRM is only used in a research study approved by an institutional review board, and in accordance with the research protocol, and must ensure that any unused CRM is disposed of or exported to another country within 6 months of study conclusion.
A CRM notification is not needed under certain scenarios listed in the CRM Regulations. For products imported solely for export to overseas trial sites, a CRM notification is not applicable and separate approval to import them must be applied for separately.
Additional requirements such as prior approval from the HSA must be complied with if a CRM comprises controlled drugs and psychotropic substances, poisons and radiopharmaceuticals.
Clinical Trials Regulations
Following the HPA Order 2021 coming into effect, which specified and defined CTGTPs as a distinct category of products under the HPA, all Class 2 CTGTPs used in research are subject to the Clinical Trials Regulations with effect from March 1, 2021. Prior to this, the Medicines (Clinical Trials) Regulations 2016 under the MA applied to in-house manufactured Class 2 CTGTPs used in research.
The Clinical Trials Regulations is given effect under the HPA, and came into effect on November 1, 2016. It provides a risk-based approval to the regulation of clinical trials, whereby the requirements and extent of pre-trial regulatory review are risk-stratified according to the local registration status of the investigational product used in the clinical trial. The risk stratification of the clinical trials is intended to improve the overall resource efficiency while ensuring trial participants’ safeties.
A CTA is required for a “higher risk” clinical trial of a locally unregistered therapeutic product or Class 2 CTGTP, or involving an unapproved use of a locally registered therapeutic product or Class 2 CTGTP. On the other hand, a “lower risk” clinical trial of a locally registered product or Class 2 CTGTP that is used in accordance with its approved label will only be required to be notified to HSA through a Clinical Trial Notification (“CTN”). As locally registered products would already have been reviewed by HSA for product registration, CTN submissions will be subjected to a simplified regulatory screening and verification process that leverages the review by the Institutional Review Board (as referred to in the Clinical Trials Regulations). In most instances, this is expected to shorten clinical trial start-up timelines as compared to clinical trials that require authorization.
| 128 |
Further guidance can be sought from HSA’s regulatory guidance, in particular from its Clinical Trials Guidance dated March 1, 2021 on determination of whether a clinical trial requires a CTA, CTN or Clinical Trial Certificate.
HBRA
Generally, the HBRA regulates the conduct of human biomedical research (as defined under Section 3 of the HBRA) conducted under the supervision and control of a research institution and the handling of human tissue for use in research and the research has been reviewed by an institutional review board appointed by that research institution. It first came into force on July 1, 2016 and was implemented in phases, and includes provisions on consent, institutional review boards, savings and transition of research conduct, regulation of human biomedical research, prohibited and restricted human biomedical research, human tissue activities and tissue banks, enforcement powers, etcetera.
Read with the HBRA, subsidiary legislation under the HBRA, namely the Human Biomedical Research Regulations 2017 and Human Biomedical Research (Restricted Research) Regulations 2017, cumulatively this legislation regulates the conduct of human biomedical research and, in particular, subject certain types of research to stricter controls. This includes the introduction of human stem cells (pluripotent or not) into animals. The Ethics Guidelines for Human Biomedical Research, released by the Bioethics Advisory Committee of Singapore, does not have statutory force but operates alongside these subsidiary legislations to provide guidance and emphasize the fundamental principles of solidarity, respect for persons, justice, proportionality, sustainability, beneficence and research integrity.
In particular, the HBRA provides clarity with regard to the roles and responsibilities of parties involved in human biomedical research and the handling of human tissue for use in research. Clinical trials using Class 1 CTGTPs have to ensure compliance with the HBRA, which is regulated by MOH Singapore.
No human biomedical research is permitted to be conducted except under the supervision and control of a research institution with (a) a place of business in Singapore; and (b) at least two (2) individuals ordinarily resident in Singapore who are responsible on behalf of the research institution for the supervision and control of the biomedical research. Any person who contravenes this shall be guilty of an offence and shall be liable on conviction to a fine not exceeding S$50,000 or to imprisonment for a term not exceeding five (5) years or to both.
A research institution is required to submit a notification in the prescribed form and manner before the commencement of the first human biomedical research conducted under that research institution’s supervision and control. It must also submit in the prescribed form a declaration of compliance in respect of all human biomedical research conducted under its supervision and control in the preceding 12 months, or such other period of time as the Director of Medical Services (as referred to in the HBRA) may require. Any person who fails to comply with either of the above shall be guilty of an offence and shall be liable on conviction to a fine not exceeding S$10,000 or to imprisonment for a term not exceeding 12 months or to both.
The HBRA requires that every research institution must, in respect of any human biomedical research which is carried out under its supervision and control, among others:
| (a) | supervise, review and proactively monitor the conduct of the research; |
| (b) | designate a principal person in charge to be responsible for ensuring that the research institution complies with the provisions of the HBRA; |
| (c) | formula and implement appropriate standards, policies and procedures to supervise, review and monitor the conduct of the research; |
| (d) | establish a data and safety monitoring board if its institutional review board considers that it is necessary for the purposes of any particular research proposal; and |
| (e) | investigate any areas of concern and take such remedial measures as appropriate. |
The Second Schedule of the HBRA sets out the research, studies and matters excluded from the definition of human biomedical research, which includes clinical trials of health products and medicinal products conducted in accordance with the HPA or MA, respectively. The applicability of the HBRA therefore depends on the matters that are ultimately the subject of the clinical trials conducted by us.
| 129 |
Conduct in laboratory
Any research facility that uses animals for scientific purposes must obtain a license from the Agri-Food and Veterinary Authority of Singapore. The research facility must also comply with the Guiding Principles for the Care and Use of Animals for Scientific Purposes released by the National Advisory Committee for Laboratory Animal Research, and establish an Institutional Animal Care and Use Committee to oversee and evaluate the animal care and use programs of an institution as required under the Animals and Birds (Care and Use of Animals for Scientific Purposes) Rules given effect under the Animals and Birds Act 1965 of Singapore.
Singapore also adheres to the OECD’s Mutual Acceptance of Data scheme. This is therefore an endorsement that Singapore’s generated research data complies with the OECD’s Principles of Good Laboratory Practice and such data can then be accepted automatically by other OECD countries and facilities the sharing of research.
Third parties with research facilities that use animals for scientific purposes that we may collaborate with for the R&D of our product candidates will be required to obtain the requisite licenses.
HCSA
The HCS Bill was passed in the Singapore Parliament on January 6, 2020. The phased implementation of the HCSA commenced from January 3, 2022, with the final phase targeted to be implemented in end-2023 subject to any deferments by MOH Singapore. The HCSA will thereafter repeal the PHMCA.
MOH Singapore acknowledged the need to replace the PHMCA with the HCSA primarily due to significant changes to the healthcare landscape in Singapore – in particular, from emerging new healthcare services and models (e.g. home and community-based care and telemedicine services) and new technological advancements (e.g. proton beam therapy, cell, tissue and gene therapy, and clinical genetic testing services). The HCSA therefore introduces a services-based licensing regime, replacing the premises-based licensing regime under the PHMCA, and generally groups the licensable healthcare services into 6 broad service categories – hospital services, ambulatory care services, long-term residential care services, non-premises based services, health support services and special services. A provider may be required to have multiple licenses depending on the services provided. License conditions will depend on the type of service provided and some services will require other licenses as a pre-requisite.
Section 3 of the HCS Bill defines “healthcare service” to mean any of the following services, whether or not provided for reward:
| (a) | assessment, diagnosis, treatment, prevention or alleviation of an ailment, a condition, disability, disease, disorder or an injury affecting any part of the human body or mind; |
| (b) | nursing or rehabilitative care of an individual suffering from an ailment, a condition, disability, disease, disorder or an injury mentioned in (a); |
| (c) | conduct of any clinical procedure to change, or that is intended to change, the appearance or anatomy of an individual; |
| (d) | assessment of the health of an individual; or |
| (e) | any other service of a medical or healthcare nature that is prescribed. |
| 130 |
Licensable healthcare services mean any of the healthcare services listed in the First Schedule of the HCS Bill.
CTGT will be a newly regulated service covered under the HCSA as a special service from, pursuant to MOH Singapore’s last announcement as of the date of this Prospectus, March 2023. As such, the specific regulations in relation to the HCSA have not been released.
Notwithstanding that specific regulations in relation to CTGT have yet to be released, the HCS Bill generally states the following likely to be generally relevant to CTGT:
| (i) | for selected services, mandates the appointments of key personnel in addition to the licensee – namely the key appointment holder, Principal Officer, and Clinical Governance Officer (as defined in the HSC Bill) – to strengthen governance and oversight of healthcare services; |
| (ii) | mandates the institution of quality assurance committees and/or clinical ethics committees for select licensees, and/or service review committees for selected services or programs that are deemed higher risk, more complex or of greater public interest; |
| (iii) | enhances MOH Singapore’s powers to gather data for the purposes of patient safety, care, welfare, and public health interest, and to publish information about non-compliant licensees and unlicensed providers; |
| (iv) | places restrictions on licensees’ names to minimize public misperception; |
| (v) | places restrictions on the provision of licensable healthcare services together with other unrelated or unlicensed services at a premise or a conveyance; and |
| (vi) | only a licensee or a person acting on the authority of a HCSA licensee may advertize licensable healthcare services. |
Regulations such as the Healthcare Services (General) Regulations, Healthcare Services (Advertisement) Regulations, and other regulations in relation to the specific services, will have to be complied with when drafted and implemented under the HCSA. Given the upcoming changes to legislation and regulations relating to CTGTs in Singapore, we and/or the hospitals and/or third parties that we may collaborate with will be required to, inter alia, ensure compliance with all applicable legislation and regulations in force, implement controls, obtain the necessary licenses and approvals, depending on factors including the location(s) of our clinical trials and research and healthcare services using CTGTs.
Malaysia
CytoMed Malaysia is a pre-clinical biopharmaceutical company which does not have any clinical operations and has not commenced any clinical trials in Malaysia.
The following description is a summary of material laws and regulations applicable to our operations in Malaysia. The regulations and policies set out below are not exhaustive and are only intended to provide general information to investors and are neither designed nor intended to be a substitute for professional advice. Prospective investors should consult their own advisers regarding the implication of the laws and regulations on us.
| 131 |
Operation of business
The following laws and regulations are generally applicable to the operation of CytoMed Malaysia’s business in Malaysia:
ICA
Section 3(1) of the ICA provides that, no person shall engage in any manufacturing activity unless he is issued a license in respect of such manufacturing activity. Manufacturing activity is defined under the ICA as the making, altering, blending, ornamenting, finishing or otherwise treating or adapting any article or substance with a view to its use, sale, transport, delivery or disposal and includes the assembly of parts and ship repairing but shall not include any activity normally associated with retail or wholesale trade.
Pursuant to section 3(2) of the ICA, any person who fails to comply with the provision of subsection (1) is guilty of an offence and is liable on conviction to a fine not exceeding RM2,000 or to a term of imprisonment not exceeding 6 months and to a further fine not exceeding RM1,000 for every day during which such default continues.
Local Government Act 1976 and Johor Bahru By-Laws
Pursuant to section 102(s) of the Local Government Act 1976, every local authority may from time to time make, amend and revoke by-laws in respect of all such matters as are necessary or desirable for the maintenance of the health, safety and well-being of the inhabitants or for the good order and government of the local authority area and in particular, amongst others, to control and supervise, by registration, licensing or otherwise, including in proper cases by prohibition, a trade, business or industry which is of an obnoxious nature or which could be a source of nuisance to the public or a class of the public.
Pursuant to by-law 3(1) of the Johor Bahru By-Laws, no person shall use a premise for any trade, business or industrial activity without a business premise license issued pertaining to the premise by the Mayor of Johor Bahru. Under by-law 3(3) of the Johor Bahru By-Laws, any person who contravenes by-law 3(1) shall be guilty of an offence and shall, upon conviction, be liable to a fine of not exceeding RM2,000 or to imprisonment for a term of not exceeding 1 year or to both.
FMA
Section 19(1) of the FMA prescribes that no person shall operate or cause or permit to be operated any machinery in respect of which a certificate of fitness is prescribed, unless there is in force in relation to the operation of the machinery a valid certificate of fitness issued under the FMA.
Operating any machinery without a valid certificate will result in an inspector appointed under the FMA serving upon the person a notice in writing prohibiting the operation of the machinery or may render the machinery inoperative until such time as a valid certificate of fitness is issued. In addition, failure to obtain a valid certificate of fitness is considered an offence under the FMA and shall, on conviction, be liable to a fine not exceeding RM150,000 or to imprisonment for a term not exceeding three (3) years or to both.
Assuming that CytoMed Malaysia is operating a “factory” (as defined in the FMA):
| (a) | section 34(2) of the FMA further provides that no person shall except within the written permission of the inspector begin to use any premises as a factory until one (1) month after he has served on the inspector a written notice in the prescribed form. This is not applicable to any person who takes over a factory from another person if there is no change in the nature of the work carried on in the factory provided that the first person shall within 1 month of such taking over have served on the inspector written notice in the prescribed form; |
| 132 |
| (b) | section 36(1) of the FMA also sets out that no person shall install or caused to be installed any machinery in any factory or any machinery in respect of which a certificate of fitness is prescribed, except with the written approval of the inspector. Where any machinery in respect of which a certificate of fitness is prescribed has been installed, a written notice shall be served by the occupier or owner of the factory on the inspector or a licensee where applicable, who shall within 1 month from the date of receipt of the written notice, inspect the machinery; and |
| (c) | section 38(1) of the FMA sets out that there shall be a register kept in every factory in the prescribed form to be called the ‘general register’ and it shall be entered in or attached to that register all such particulars as may be prescribed. The general register and every other register or record or certificate kept in pursuance of FMA shall be preserved and shall be kept available for inspection by any inspector for at least 2 years or such other period as may be prescribed for any class or description of register or record, after the date of the last entry in the register or record. |
Any person who contravenes section 34(1), 36(1), 38(1) above, shall be guilty of an offence and shall, on conviction, be liable to a fine not exceeding RM100,000 or to imprisonment for a term not exceeding 2 years or both.
Notwithstanding the above, the Factories and Machinery (Repeal) Act 2022 has been enacted but has not come into force as of the date of this Prospectus. Once the Factories and Machinery (Repeal) Act 2022 comes into force, the FMA will be repealed in its entirety and its provisions will be integrated into the OSHA.
OSHA
There are no licenses or certificates issued pursuant to the OSHA. However, there are obligations imposed under the OSHA, including but not limited to:
| (a) | ensure, so far as is practicable, the safety, health and welfare at work of all employees; |
| (b) | without prejudice to the above, the matters to which the duty extends include in particular: |
| (i) | the provision and maintenance of plant and systems of work that are, so far as is practicable, safe and without risks to health; | |
| (ii) | the making of arrangements for ensuring, so far as is practicable, safety and absence of risks to health in connection with the use or operation, handling, storage and transport of plant and substances; | |
| (iii) | the provision of such information, instruction, training and supervision as is necessary to ensure, so far as is practicable, the safety and health at work of employees; | |
| (iv) | so far as is practicable, as regards any place of work under the control of the employer or self-employed person, the maintenance of it in a condition that is safe and without risks to health and the provision and maintenance of the means of access to and egress from it that are safe and without such risks; and | |
| (v) | the provision and maintenance of a working environment for employees that is, so far as is practicable, safe, without risks to health, and adequate as regards facilities for their welfare at work; and |
| (c) | except in such cases as may be prescribed, to prepare and as often as may be appropriate revise a written statement of general policy with respect to the safety and health at work of employees and the organization and arrangements for the time being in force for carrying out that policy, and to bring the statement and any revision of it to the notice of all employees. |
Contravention of any of the above provisions shall, on conviction, be liable to a fine not exceeding RM50,000 or to imprisonment for a term not exceeding 2 years or to both.
Notwithstanding the above, the Occupational Safety and Health (Amendment) Act 2022 has been enacted but has not come into force as of the date of this Prospectus. Amendments to the OSHA include but are not limited to the following:
| (a) | imposition of new duties on principal, employer and self-employed person to ensure the safety of contractors and sub-contractors, to conduct risk assessment, and to develop and implement procedures to deal with emergencies; |
| (b) | requirement to appoint an occupational safety and health coordinator; |
| (c) | grant of right to employees to remove themselves from imminent danger at the workplace; |
| (d) | increased penalties and punishments for employers, self-employed persons, principals and manufacturers who breach their duties under the OSHA; and |
| (e) | joint and several liability of directors and specified office bearers for offences committed by the company or other relevant body. |
| 133 |
EQA
Licensing requirements
Pursuant to section 21 of the EQA, there are acceptable conditions specified for the emissions, discharge or deposit of environmentally hazardous substances, pollutants or wastes or the emission of noise into any area, segment or element of the environment. The EQA provides that no person shall, unless licensed:
| (a) | emit or discharge of any environmentally hazardous substances, pollutants or wastes into the atmosphere; |
| (b) | pollute any soil or surface of any land; |
| (c) | emit, discharge or deposit of any environmentally hazardous substances, pollutants or wastes into any inland waters; or |
| (d) | emit of any noise greater in volume, intensity or quality, |
in contravention of the acceptable conditions specified.
Contravention of the above shall be guilty of an offence and shall not be liable to a fine not exceeding RM100,000 or to imprisonment for a period not exceeding 5 years or to both and to a further fine not exceeding RM1,000 a day (RM500 a day for noise pollution) for every day that the offence is continued after a notice by the Director General, requiring him to cease the act specified therein has been served upon the offender.
Pursuant to section 41 of the EQA, every omission or neglect to comply with, and every act done or attempted to be done contrary to, the provisions of the EQA or any regulations made thereunder or any breach of the conditions and restrictions subject to, or upon which, any license is issued under the EQA or any regulations made thereunder shall be an offence against the EQA and in respect of any such offence for which no penalty is expressly provided the offender shall be liable to a fine not exceeding RM10,000 or to imprisonment for a period not exceeding 2 years or to both.
CytoMed Malaysia has taken steps to manage environmentally hazardous wastes in compliance with the EQA. Notably, CytoMed Malaysia has engaged the services of a biohazard waste collector and has designated a disposal area for the collection of biohazard wastes.
Written notification to the Director General
Generally, an owner of occupier of a premise must not, without giving prior written notification to the Director General under the Clean Air Regulations:
| (a) | carry out any change in operation of his premises; |
| (b) | carry out any work on any premises that may result in a source of emission; |
| (c) | construct on any land, any building or premises designed or used for a purpose that may result in a new source of emission; |
| (d) | make, cause or permit to be made any change of, to, or in any plant, machine or equipment used or installed at the premises that causes a material change in the quantity or quality of emission from an existing source; or |
| (e) | carry out any changes or modifications to an existing air pollution control system. |
The written notification must be submitted to the Director General not less than 30 days before the commencement of such work in such form as determined by the Director General. Regulation 29 of the Clean Air Regulations states that, any person who contravenes or fails to comply with any provisions of the Clean Air Regulations shall be guilty of an offence and shall be liable to a fine not exceeding RM100,000 or to imprisonment for a term not exceeding 2 years or to both.
| 134 |
Laboratory work
The following laws and regulations are applicable to the laboratory work undertaken by CytoMed Malaysia:
PA 1952
The PA 1952 governs the licenses issued to use poisons. Licenses are categorized into Type A, B, C, D and E licenses. The licenses will be subject to terms and conditions as the licensing officer may in his discretion impose, which are not inconsistent with PA 1952 or of any regulations made thereunder, subject to appeal to the Minister of Health of Malaysia charged with the responsibility for medical and health services.
Pursuant to section 32 of the PA 1952, any person guilty of an offence against the PA 1952, which includes failure to obtain license to use poisons, shall be punishable by a fine not exceeding RM3,000 or by imprisonment for a term not exceeding 1 year or both. If the act or omission is in the opinion of the court of such a nature as to amount to willful default or culpable negligence, which endangered or was likely to endanger human life, such person shall be liable, on conviction, to a fine not exceeding RM5,000 or to imprisonment for a term not exceeding 2 years or both.
CytoMed Malaysia currently holds a Type A license, which allows its pharmacist(s) to import, store and deal in all poisons generally by retail.
Prevention and Control of Infectious Diseases Act 1988 and PCID Regulations
Pursuant to the PCID Regulations issued by the Minister of Health of Malaysia under section 31 of the Prevention and Control of Infectious Diseases Act 1988, no person shall import or export human tissues or any part thereof and any pathogenic organisms or substances or any part thereof, unless with the approval of the authorized officer. Upon approval, the authorized officer shall issue a permit.
Pursuant to regulation 9 of the PCID Regulations, any person who fails to comply with any provisions under these PCID Regulations commits an offence and shall on conviction be liable:
| (a) | in respect of a first offence, to imprisonment for a term not exceeding 1 year or to a fine not exceeding RM3,000 or to both; |
| (b) | in respect of a second offence or subsequent offence, to imprisonment for a term not exceeding 3 years or to a fine not exceeding RM5,000 or to both; |
| (c) | in respect of a continuing offence, to a further fine not exceeding RM100 for every day during which such offence continues. |
Development and manufacturing of CGTPs
Subject to any further regulatory interpretation, amendments, guidelines, policies or orders to be issued or taken by the relevant governmental authorities (including the Ministry of Health Malaysia), the following laws and regulations may be applicable to CytoMed Malaysia once CytoMed Malaysia passes the clinical trials and commences operations to develop and manufacture CGTPs:
CDCR
The CDCR is a regulation provided under section 26 of the Sale of Drugs Act 1952 of Malaysia. Under CDCR, a product means a drug in a dosage unit or otherwise, for use wholly or mainly by being administered to one or more human beings or animals for a medicinal purpose or a drug to be used as an ingredient of a preparation for a medicinal purpose.
| 135 |
The Guidelines on CGTPs for CGTPs were issued under regulation 29 of the CDCR. It has come into effect on January 1, 2021 and provides that CGTPs fit within the meaning of medicinal products under the CDCR. In accordance to the CDCR, CGTPs would be classified as biological products, as defined above. As such, the Guidelines on CGTPs should be read in conjunction with CDCR.
The Guidelines on CGTPs addresses the development, manufacturing and quality control as well as non-clinical and clinical development of CGTPs which include somatic cell therapy, tissue engineering and gene therapy products. These guidelines are intended for products entering the registration process at the NPCB. In order to investigate the use of CGTPs in a local clinical trial, an application that reports data from pre-clinical studies on the likely safety and efficacy of the investigational product must be filed by NPCB. An approval for a CTIL or a CTX is mandatory before an unregistered CGTP is administered to human trial subjects in Malaysia. Following January 1, 2021, only CGTPs that are registered with the NPCB can market its products in Malaysia.
The registration of CGTPs under the Guidelines on CGTPs are in line with regulation 7 of the CDCR, which provides that no person shall manufacture, sell, supply, import or possess or administrator any product unless:
| (a) | the product is a registered product; and |
| (b) | the person holds the appropriate license required and issued under CDCR. |
Further, pursuant to regulation 8 of the CDCR, the Drug Control Authority of Malaysia may, on application made in such manner or form as it may require, register any product subject to such conditions as it may impose. Other than the registration of products, the CDCR provides that the following circumstances below may require licenses:
| (a) | manufacturer’s license authorizing the licensee to manufacture the registered products in the premises specified in the license and to sell by wholesale or supply the products; |
| (b) | a wholesaler’s license authorizing the licensee to sell by wholesale or supply the registered products from the address of the business premises specified in the license; |
| (c) | a clinical trial import license authorizing the licensee to import any product for purposes of clinical trials, notwithstanding that the product is not a registered product; and |
| (d) | an import license authorizing the licensee to import and sell by wholesale or supply the registered products from the address of the premises specified in the license. |
Any person who contravenes any of the provisions of the CDCR or any condition of any license issued under the CDCR or any condition subject to which a product is registered under the CDCR commits an offence.
According to the CDCR, all registered products and notified cosmetics are to be manufactured within GMP compliant premises. ‘Manufacturing’ is defined as (a) the making of assembling of the product, (b) the enclosing or packing of the product in any container in a form suitable for administration or application, and the labelling of the container, and (c) the carrying out of any process in the course of any of the foregoing activities.
GMP is a standard imposed by the Ministry of Health Malaysia where manufacturers of registered pharmaceutical/veterinary/health supplements/traditional products and/or notified cosmetics to ensure that the product manufactured is safe, efficacious and of quality. GMP compliance is one of the requirements for product registration and/or notification of cosmetics, as well as to apply for a Manufacturer’s License with the Drug Control Authority. GMP compliant premises should be licensed by the local town council, Department of Environment and Fire and Rescue Department. A GMP Certificate will be issued for the purposes of exporting locally manufactured registered products. It endorses that the local manufacturer complies with the current GMP requirements. These certificates are required by the overseas regulatory agencies for the purpose of product registration in the respective countries.
| 136 |
Any person who contravenes any of the provisions of the CDCR or any condition of any license issued under the CDCR or any condition subject to which a product is registered under the CDCR commits an offence. Additionally, the manufacturer’s license may be revoked by the Director of Pharmaceutical Services (as defined in the CDCR), according to regulation 17(1) of the CDCR if the manufacturer does not comply with GMP standards and requirements.
The Guidance Note for CTGPs Manufacturing Facility in Malaysia issued by NPRA, which only applicable to Class II CGTPs (which are higher risk cell therapy products) is established to facilitate and provide guidance in setting up a manufacturing facility for CGTPs which are regulated under the NPRA.
Among others, the guidance note has set out the types of inspection to be conducted on CGTPs manufacturing facility, which depends on the inspection objective and product category as follows:
| (a) | Pre-certification inspection: an inspection conducted on CGTPs manufacturing facility during the voluntary registration period (before year 2023). |
| (b) | Pre-licensing inspection: an inspection conducted on CGTPs manufacturing facility for the purpose of licensing and product registration. |
| (c) | Pre-approval inspection: an inspection conducted on additional manufacturing line/new sources of cell in an existing inspected CGTPs manufacturing facility. |
| (d) | Routine inspection: a subsequent inspection conducted on existing inspected CGTPs manufacturing facility according to a planned schedule by NPRA. |
| (e) | Verification inspection: an inspection conducted following a punitive action. Depending on the condition, verification inspection can be combined with routine inspection. |
| (f) | Investigation inspection: investigation inspection is an inspection conducted on premises based on complaint received and product recall activity. |
PHFSA
Pursuant to section 3 of the PHFSA, any person intending to establish or maintain private healthcare facilities or services including a private blood bank shall obtain a license from the Ministry of Health Malaysia. Private blood bank is defined to mean any premises, other than a government blood bank, used or intended to be used for collecting, screening, processing, storing or distributing natural human blood or blood product.
Pursuant to section 5 of the PHFSA, failure to obtain a license is an offence and any individual person found guilty shall be liable, on conviction to a fine not exceeding RM300,000 or to an imprisonment for a term not exceeding 6 years or to both and for a continuing offence, to a fine not exceeding RM1,000 for every day or part of a day during which the offence continues after conviction. Where the offence is committed by a body corporate, the penalty is a fine not exceeding RM500,000 and for a continuing offence, a fine not exceeding RM5,000 for every day or part of a day during which the offence continues after conviction. The person responsible for the body corporate shall also be guilty of the offence and shall be liable, on conviction to a fine not exceeding RM300,000 or to imprisonment for a term not exceeding 6 years or to both, and for a continuing offence, to a fine not exceeding RM1,000 for every day or part of a day during which the offence continues after conviction.
| 137 |
Employment matters
The following laws and regulations are generally applicable to employment matters:
EPFA
Pursuant to section 43(1) of the EPFA, it is compulsory for employees and their employers to make monthly contributions on the amount of wages at the rate respectively set out in the Third Schedule of the EPFA to the Employment Provident Fund, which is a statutory retirement fund. The contributions are made to the account of the individual employee.
ESSA
Monthly contributions will also have to be made to SOCSO both by the employer and employees irrespective of the amount of wages as per the rates set out in the Third Schedule of the ESSA. The SOCSO administers:
| (a) | the EIS which provides employees with coverage by way of cash benefits and medical care in the event of any disablement or death due to employment injury; and |
| (b) | the Invalidity Pension Scheme which provides 24-hours coverage to employees against invalidity and death due to any cause before attaining the age of 60 years. |
EISA
The EISA implemented the EIS, effective since January 2018 by the SOCSO. The EISA sets out provisions to provide certain benefits and a re-employment placement program for insured persons in the event of loss of employment which will promote active labor market policies. All private sector employers need to pay monthly contributions for each employee. Civil servants, domestic servants and those who are self-employed are exempted. All employees from 18 to 59 years of age have to contribute. Employees aged between 57 and 60 who have never contributed to the SOCSO are exempted from this protection plan.
The employer is liable to pay interest on such amount at 6% per annum for each day of late contributions in respect of any period during which such amount remains unpaid provided that:
| (a) | if the amount of interest so calculated is less than RM5.00, the interest payable shall be RM5.00 in respect of each month or part of a month; and |
| (b) | if the amount of interest exceeds RM5.00, the interest payable shall be calculated to the next highest multiple of RM5.00 in respect of each such month or part of a month. |
Immigration Regulations 1963
Pursuant to regulation 9 of the Immigration Regulations 1963, an employment pass is required for the entry of any foreign person into Malaysia to take up employment under a contract of service. Such contract must be for a minimum period of 2 years employment with a company or firm approved and under which such person is entitled to a salary of not less than RM1,200 per month. Any person who without reasonable cause fails to comply with any condition imposed in respect of any pass shall be guilty of an offence under regulation 39 and shall be liable on conviction to a term of imprisonment not exceeding 6 months or to a fine not exceeding RM1,000 or to both.
Others
PDPA Malaysia
The PDPA Malaysia applies to any person who processes and has control over the processing of personal data in respect of commercial transactions. Personal data refers to any information that relates directly or indirectly to an individual data subject, who is identified or identifiable from that information or from that and other information in the possession of a data user, including any sensitive personal data and expression of opinion about the data subject; but does not include any information that is processed for the purpose of a credit reporting business carried on by a credit reporting agency under the Credit Reporting Agencies Act 2010 of Malaysia.
Data user refers to a person who either alone or jointly or in common with other persons processes any personal data or has control over or authorizes the processing of any personal data, but does not include a data processor. A data user is required to comply with all the personal data protection principles under the PDPA Malaysia, including the notice and choice principle under section 7 of the PDPA Malaysia, which requires the issuance of written notices in the Malay and English languages informing data subjects that, amongst others, the personal data of the data subject is being processed by or on behalf of the data user and the purposes for which the personal data is being or is to be collected and further processed.
Pursuant to section 5(2) of the PDPA Malaysia, a data user who contravenes the personal data protection principles under the PDPA Malaysia commits an offence and shall, on conviction, be liable to a fine not exceeding RM300,000 or to imprisonment for a term not exceeding 2 years or to both.
Permits, licenses and approvals
As of the date of this Prospectus, we have the necessary licenses, permits and approvals in Malaysia required for the operations of CytoMed Malaysia.
| 138 |
Board of Directors
The following table sets forth information regarding the current members of our Board of Directors as of the date hereof and persons who will become members of our Board of Directors immediately prior to the consummation of this offering.
| Name | Age | Position/Title | ||
Executive Directors CHOO Chee Kong | 63 |
Director and Chairman | ||
| Dr. Lucas LUK Tien Wee | 38 | Director and Chief Clinical Officer | ||
| Dr. ZENG Jieming | 49 | Director Nominee and Chief Scientific and Medical Officer | ||
| Dr. TAN Wee Kiat | 35 | Director Nominee and Chief Operating Officer | ||
| Independent Directors | ||||
| Prof. LOH Yuin Han (1) | 45 | Independent Director Nominee | ||
| Dr. YEW Chak Hua (2) | 41 | Independent Director Nominee | ||
| Mark LEONG Kei Wei (3) | 46 | Independent Director Nominee | ||
| WU Tao Thomas (4) | 57 | Independent Director Nominee | ||
| Dr. TOH Keng Kiat (5) | 81 | Independent Director Nominee |
| (1) | Proposed Member of the Nominating and Corporate Governance Committee. |
| (2) | Proposed Member of the Compensation Committee. |
| (3) | Proposed Audit Committee financial expert, proposed Chair of the Audit Committee and proposed Chair of the Compensation Committee. |
| (4) | Proposed Member of the Audit Committee. |
| (5) | Proposed Chair of the Nominating and Corporate Governance Committee and proposed Member of the Audit Committee. |
Executive Officers
The following table sets forth information regarding our current executive officers and persons who will become our executive officers as of 2023, upon consummation of this offering.
| Name | Age | Position/Title | ||
| CHOO Chee Kong | 63 | Chairman and Director | ||
| Dr. Lucas LUK Tien Wee | 38 | Chief Clinical Officer and Director | ||
| Dr. ZENG Jieming | 49 | Chief Scientific and Medical Officer and Director Nominee | ||
| Dr. TAN Wee Kiat | 35 | Chief Operating Officer and Director Nominee | ||
| GOH Yvonne | 34 | Chief Financial Officer | ||
| TAN Yoong Ying | 34 | Chief Corporate Officer |
The following sets forth certain biographical information with respect to our Directors, director nominees and executive officers. Unless otherwise stated, the business address of our Directors, director nominees and executive officers is 1 Commonwealth Lane, #08-22 Singapore 149544.
Mr. CHOO Chee Kong has been our Chairman since the incorporation of our Company in March 2018. Mr. Choo has over 20 years of experience in corporate finance. He is presently the executive vice chairman of CNMC Goldmine Holdings Limited (CNMC Group) which is listed on the SGX-ST (SGX-ST stock code: 5TP) since 2008 where he is responsible for the formulation of the strategic direction and expansion plans as well as the corporate governance of CNMC Group. He previously worked at DBS Bank Ltd of Singapore from 1986 to 2000. In the year 2000, Mr. Choo started his own corporate advisory and stockbroking boutique, Westcomb Financial Group Limited, which he listed on SGX-ST in 2004 (SGX-ST stock code: 5EC) and served as its Chief Executive Officer from 2000 to 2008. He served as an independent director at SGX-ST listed Second Chance Properties Ltd (SGX-ST stock code: 528) from February 2009 to November 2015 and SGX-ST listed FDS Networks Group Ltd (SGX-ST stock code: F07) from June 2008 to March 2015. He also served as a non-executive director at SGX-ST listed Advance SCT Limited (SGX-ST stock code: 5FH) from May 2012 to June 2015. He started his career in October 1981 as a Project Engineer with Esso Singapore Pte Ltd. Mr. Choo received a Bachelor of Engineering in Mechanical Engineering (First Class Honours) from University of Liverpool (United Kingdom) in July 1981 and a Master in Business Administration from the University of Bradford (United Kingdom) in December 1985. We believe that Mr. Choo’s extensive knowledge of our Company as founder and his experience in executive roles across multiple industries qualifies him to serve on our Board.
| 139 |
In October 2015, Mr. Choo, was publicly reprimanded by the Singapore Exchange for failing to disclose and seek shareholder approval for an interested party transaction which occurred in May 2012 where he was the non-executive director to Advance SCT Limited, the company in question, and also the 50% owner of CNCM Capital Pte Ltd, the other company party to the transaction. There were no fines imposed on him and as of the date of this Prospectus, he remains as an executive director of a SGX-ST listed company, CNMC Goldmine Holdings Limited (CNMC Group) (SGX-ST stock code: 5TP).
On October 5, 2009, Mr. Choo was appointed as a non-executive director and chairman of Falmac Limited (“Falmac”), a company listed on the SGX-ST’s Catalist Board when Falmac was being explored as a potential target for a reverse takeover. However, the reverse takeover exercise did not materialize and Falmac was delisted by the SGX-ST on August 22, 2011. Following the delisting of Falmac, Mr. Choo resigned as a non-executive director and chairman of Falmac on August 29, 2011. Subsequently in or around August 2013, a creditor petitioned to the Singapore High Court to wind up Falmac and the winding up order was granted on May 15, 2014.
Dr. Lucas LUK Tien Wee, M.D., has served as a member of our Board since January 2021 and as our Chief Clinical Officer. He has a keen interest in cellular therapy and has attended numerous certificate courses pertaining to clinical cell therapy. He is our designated Principal Investigator for our registered Phase I Clinical Trials in Malaysia, pertaining to Mesenchymal Stem Cell Therapy and CAR-T Cell therapy. Dr. Lucas also serves as a member of the Science Industry Advisory Committee for PSB Academy’s School of Life Sciences (Singapore), since 2019. He is also the Medical Director and Consultant Obstetrician & Gynaecologist (O&G), and founding Chairman of the Independent Ethics Committee, at LMC, a private licensed hospital in Malaysia since 2019. As an advocate for women’s health, Dr. Lucas has held the position of Secretary for the Royal College of Obstetricians & Gynaecologists (RCOG) International Representative Committee (IRC) – Malaysia, from 2018 until October 2021. He is also actively involved in medical education and has been on board the central planning and training committee for the Malaysian National MRCOG Parallel Pathway Programme for Malaysian doctors training in the specialty of Obstetrics & Gynaecology, since November 2017. He is an invited medical lecturer at Monash University (Johor Campus) since March 2021. Dr. Lucas received his M.D. MRCOG (UK) from Royal College of Obstetricians & Gynaecologists (United Kingdom) in 2016 and MB BCh BAO from Royal College of Surgeons (Ireland) in 2008, and has a Good Clinical Practice qualification. We believe that Dr. Luk’s extensive background in the practice of medicine and academic tenure qualifies him to serve on our Board.
Dr. ZENG Jieming, M.D., Ph.D., who will become a Director upon consummation of this offering, is one of our scientific founders. He has served as our Chief Scientific and Medical Officer overseeing the product development and manufacturing as well as the clinical trial application since August 2019. Equipped with over 20 years of research experience and in-depth knowledge in the field, he has dedicated himself to translating the Company’s platform technologies into clinical applications and therapeutics. Prior to joining CytoMed, he has been working as a research scientist in the A*STAR, Singapore from June 2004 until July 2019. He has been the key scientist of several research projects and the lead authors of 11 original research articles, for which he both carried out and led the research as well as wrote and published the papers. His research has been focused on developing efficient non-viral and viral vectors for gene therapy (Zeng, et al. J Gene Med 6: 1247, 2004; Zeng, et al. Biomaterials 26: 679, 2005; Zeng, et al. Biomaterials 28: 1443, 2007; Zeng, et al. Stem Cells 25: 1055, 2007; Zeng, et al. Molecular Therapy 17: 1585, 2009; Du, Zeng et al. J Biosci Bioeng 109: 1, 2010) as well as developing novel cancer immunotherapies from human pluripotent stem cells (“hPSCs”), including human embryonic stem cells (“hESCs”) and induced pluripotent stem cells (“iPSCs”) (Zeng, et al. The Journal of Immunology 188: 4297, 2012; Zeng, et al. Stem Cells Transl Med 3: 69, 2014; Zeng, et al. Scientific Reports 5: 15262, 2015; Zeng, et al. Stem Cell Reports 9: 1796, 2017; Zeng, et al. PLoS One 14: e02168152019). During his 15-year stint in A*STAR, he successfully generated dendritic cells for cancer vaccination and NK cells for adoptive cell transfer from hPSCs (Zeng, et al. The Journal of Immunology 188: 4297, 2012; Zeng, et al. Stem Cells Transl Med 3: 69, 2014; Zeng, et al. Scientific Reports 5: 15262, 2015; Zeng, et al. Stem Cell Reports 9: 1796, 2017). These hPSC-based immune cell technologies open a new frontier in the development of cell-based cancer immunotherapy. In 2017, he has invented an iPSC-based technology to produce a novel type of synthetic immune cells, γδ NKT cells to recognize and kill a wide range of cancers (Zeng, et al. PLoS One 14: e02168152019) and the technology has been licensed to us in 2018. Dr. Zeng received his Ph.D. from National University of Singapore (Singapore) in 2004 and M.D. from Sun Yat-Sen University (China) in 1996. We believe that Dr. Zeng’s professional achievements in translational medicine qualifies him to serve on our Board.
Dr. TAN Wee Kiat, Ph.D., who will become a Director upon consummation of this offering, has served as our Chief Operating Officer since February 2019. He oversees operations of the Company, including facility management, human resource, manpower allocation, manufacturing, quality control and assurance of cell therapy products, clinical trial operations, R&D, budgeting of operation, compliance and support business development function. Prior to that, he served as the Company’s Chief Production Officer and Chief Technology Officer from July 2018 to January 2019 where he was tasked to develop the Company’s technology into a clinically ready platform for clinical trial application. Dr. Tan also serves as a director of Puricell Lab Pte Ltd since its incorporation in October 2020. Prior to joining CytoMed, he served in Tessa Therapeutics Pte Ltd, a biotechnology company engaged in the use of virus specific T cells to treat cancer, as a research scientist from August 2017 to July 2018. Dr. Tan completed his Ph.D. in 2016 following cancer research at the Institute of Bioengineering and Nanotechnology, A*STAR. He graduated with a Bachelor of Science in 2012, both degrees from the National University of Singapore (Singapore). Dr. Tan has a GCP qualification. We believe that Dr. Tan’s background in biopharmaceutical industry operations and academic research qualifies him to serve on our Board.
| 140 |
Ms. GOH Yvonne has served as our Chief Financial Officer since March 2020. She oversees the functions relating to finance, accounting, reporting and procurement. Prior to joining CytoMed, Ms. Goh served as Finance Manager at SBI Offshore Limited) (SGX-ST stock code: 5PL), a public company listed in Singapore engaged in the oil and gas industry, from September 2017 to February 2020. Prior to assuming the position of Finance Manager at SBI Offshore Limited, Ms. Goh was the Assistant Finance Manager from December 2016 to September 2017, Accountant from May 2016 to November 2016 and Senior Accounts Executive from April 2015 to April 2016. From July 2010 until April 2015, Ms. Goh was the Senior Executive – Finance at Leeden National Oxygen Limited, a welding, gas and safety integration specialist based in Singapore. Prior to that, Ms. Goh held accounting roles in various private companies. Ms. Goh is a Chartered Accountant (CA) of the Institute of Singapore Chartered Accountants (ISCA) since 2016. We believe that Ms. Goh’s broad operational management experience in listed companies and experience in accounting qualifies her to serve as our Chief Financial Officer.
Ms. TAN Yoong Ying has served as our Chief Corporate Officer since June 2018 where she oversees legal compliance, business administration, public and government relation, facility management, human resource and corporate secretarial. She has been a director of all our subsidiaries in Malaysia and Singapore since their incorporation, including CytoMed Malaysia, starting from June 2018, IPSCBank Pte Ltd, IPSC Depository Sdn. Bhd. starting from June 2019, Puricell, starting from October 2020 and Advance Cancer Centre Pte Ltd, starting from February 2020. Prior to these roles with the Company’s subsidiaries, Ms. Tan was a director of Advantage Mining Sdn Bhd from March 2016 until June 2018. From November 2014 to March 2017, she served as the Department Manager of Pulai Mining Sdn Bhd, a private gold mining company in Malaysia, where she participated actively in daily operation management, corporate restructuring and acquisition. Ms. Tan holds a Postgraduate Degree in Legal Practice from BPP Law School (United Kingdom) graduated in 2014, LLM from University of Aberdeen (United Kingdom) in 2012, and a Bachelor Degree in Laws from Jinan University (China) in 2011.
Prof. LOH Yuin Han, Ph.D. who will become a Director upon consummation of this offering has served as a Research Director at A*STAR’s IMCB since April 2021, where he also serves as the Division Director overseeing research in Cell Therapies. Concurrently, he is an Associate Professor (Adjunct) at the NUS Yong Loo Lin School of Medicine, National University of Singapore (NUS) Faculty of Science and NUS Graduate School of Integrative Sciences and Engineering starting from July 2018. Prior to joining IMCB, Prof. Loh was a Postdoctoral fellow at the Harvard Medical School, Boston Children’s Hospital, Division of Pediatric Hematology and Oncology from July 2018 to July 2011. There he was trained under the tutelage of Dr. George Q. Daley, one of the first scientists to successfully convert human skin cells to iPSCs. Prof. Loh’s research focuses on dissecting the mechanisms regulating cell fate changes and developing novel tools for the use of stem cells in clinical cell-based therapies. He was the first in the world to describe the regulatory factors which play essential roles in maintaining the self-renewal and pluripotency of embryonic stem cells (Loh et al. Nature Genetics. 2006), and uncovered the method to reverse the cell fate of human blood cells into reprogrammed stem cells (Loh et al. Cell Stem Cells. 2010), which facilitated the modelling of congenital and somatic hematologic diseases using iPSCs (Agarwal S and Loh YH et al. Nature 2010). He was part of the team which demonstrated the use of synthetic mRNA to reprogramming human fibroblast cells (Warren et al. Cell Stem Cells 2010). The work was selected as one of the Top 10 breakthroughs of the year by Science journal, and the technology was spun off as Moderna Therapeutics. In recent years, his group pioneered the discoveries of endogenous retro-element (Yang et al. Cell 2015) and histone chaperones (Fang et al. Nature Communications 2018) as guardians of embryonic stem cell fates. Prof. Loh’s publications are highly regarded by the research community, and have been cited close to 13,000 times by peers worldwide. His research work has earned him several prestigious national and international accolades including the National Research Foundation Investigatorship Award 2018, Stem Cell Society Singapore Young Investigator Award 2015, World Technology Network Fellowship (Biotechnology) 2012, MIT TR35 Asia Pacific Award 2012, A*STAR Investigatorship Research Award 2011, Singapore Youth Award for Science and Technology 2010 (The highest accolade for youth under 35 year old), Singapore National Academy of Science Young Scientist Award 2009, and Quest Technology Award 1997. He serves on both the International and the Education Committee of the International Society for Stem Cell Research (ISSCR) since January 2018, the Executive Council for the Singapore Association for the Advancement of Science (SAAS) since 2013, and the Executive Committee of the Stem Cell Society Singapore (SCSS) since 2012. From 2003 to 2008, Prof. Loh did his Ph.D. study as a pioneer batch of A*STAR Graduate Scholar at the NUS Graduate School of Integrative Sciences and Engineering, and was awarded the Philip Yeo Award (prize money contributed by Nobel Laureate Sydney Brenner) for the best publication. Prof. Loh studied Biology and graduated with B.Sc. 1st Class Honours in 2003 from the National University of Singapore (Singapore), where he was awarded the HSBC Scholarship. We believe that Prof Loh’s strong academic background in cancer research and his recognition in such fields qualifies him to serve on our Board.
| 141 |
Dr. YEW Chak Hua, M.D., who will become a Director upon consummation of this offering, has served as a medical consultant in the specialty of regenerative medicine in various clinics and centers in Malaysia since March 2020. With more than 15 years medical practice experience, Dr. Yew has a deep interest in cellular therapy and stem cells-derived cytokines to treat various chronic degenerative disease such as glaucoma, diabetes, osteoarthritis, sexual dysfunction, sports injury, renal impairment, Alzheimer’s disease, and stroke. Dr. Yew obtained his Letter of Credentialing and Privileging in Aesthetic Medicine Practice in 2017, and passed the membership from International Council of Ophthalmology (UK) in the same year. From October 2016 to March 2020, he was with Optimax Eye Specialist Centre where he gained vast facial aesthetic surgery skills. He is also trained in oculoplastic surgery. Prior to that, Dr. Yew served as Medical Officer in various hospitals, healthcare group and clinics across Malaysia. Dr. Yew received his M.SC in Regenerative Medicine from UCSI University (Malaysia) in 2014, and M.B.B.S from University of Malaya (Malaysia) in 2007. We believe that Dr. Yew’s record in practice of medical practice and his broad understanding of regenerative medicine qualifies him to serve on our Board.
Mr. Mark LEONG Kei Wei, who will become a Director upon consummation of this offering, presently serves as an independent director and audit committee chairman of four SGX-ST listed companies - LMIRT Management Ltd (SGX-ST stock code: D5IU), the manager of Lippo Malls Indonesia Retail Trust, HS Optimus Holdings Limited (SGX-ST stock code: 504), mDR Limited (SGX-ST stock code: Y3D) and 9R Limited (SGX-ST stock code: 1Y1). He also serves on two ASX listed companies – as an executive chairman of Osteopore Limited (ASX stock code: OSX) and non-executive director of Catalano Seafood Ltd (ASX stock code: CSF). Since March 2021, he has been a director of a financial advisory services firm Auspac Financial Advisory Pty Ltd in Australia, and a director of its Singapore subsidiary Auspac Management Services Pte Ltd since September 2021. Mr. Leong was the Chief Operating Officer of SBI Offshore Limited (SGX-ST stock code: 5PL), a company in the Oil & Gas industry from November 2017 to August 2020. Prior to this, he was a director of Pulai Mining Sdn Bhd, an exploration and mining company, from January 2016 to February 2017. From May 2012 to March 2013, he held the role of Vice President (Finance and Investment) of a family office, Colossus Holdings Pte Ltd. From January 2010 to April 2012, Mr. Leong held the dual role of Chief Development Officer and Deputy Chief Executive Officer of Atos Wellness Ltd (ASX stock code: ATW). Between 2002 and 2009, he undertook Chief Financial Officer roles in two SGX-ST listed companies, Westcomb Financial Group Limited (SGX-ST stock code: 5EC) and a subsidiary within Swiber Holdings Ltd (SGX-ST stock code: BGK). Prior to that, he was a senior auditor with KPMG from August 1999 to October 2001. Mr. Leong is a Fellow of the Association of Chartered Certified Accountants (“ACCA”), a Chartered Accountant of the Institute of Singapore Chartered Accountants (“ISCA”) and a Member of the Singapore Institute of Directors (“SID”). We believe that Mr. Leong’s history of serving as executive officer at a publicly traded companies and his broad accounting experience qualifies him to serve on our Board.
Mr. WU Tao Thomas, who will become a Director upon consummation of this offering, has nearly three decades of experience in finance services. He has served as Chief Executive Officer at Noah Holdings Singapore Pte Ltd (wholly owned subsidiary of Noah Holdings Limited) since June 2019. From September 2017 until August 2018, he was the Managing Director, Head of Asia Pacific Corporate Strategy at Julius Baer. Prior to that, Mr. Wu was the Chief Financial Officer at Xunlei Ltd (NASDAQ stock code: XNET) from November 2013 to September 2017, and Noah Holdings Limited (NYSE stock code: NOAH) from March 2010 to November 2013. He also served as an Independent Director and Audit Committee member of Seven Days Inn (NYSE stock code: SVN) from October 2011 to Oct 2013. From March 2007 to February 2010, Mr. Wu was a senior portfolio manager at Alliance Bernstein L.P. based in San Francisco and New York, where he served many Asian institutional clients and retail partners. Prior to that, Mr. Wu was a senior high yield analyst at Moody’s Investors Services based in New York from February 2005 to March 2007, a senior vice president at the investment banking division of Development Bank of Singapore based in Hong Kong from 2001 to 2005 and a Vice President at the mergers and acquisitions division at J.P. Morgan & Company from 1994 to 2001. Mr. Wu received his Master’s Degree in Public Administration from Syracuse University (United States) in 1992 and his Bachelor’s Degree in Mathematics from Grinnell College (United States) in 1987. We believe that Mr. Wu’s strong background in financial analysis and proven record in management positions in investment management qualifies him to serve on our Board.
Dr. TOH Keng Kiat, M.D., who will become a Director upon consummation of this offering, is a medical doctor by training and practicing haematologist. He is currently practising as a Consultant Haemato-Oncologist, beginning from November 2020, at the International Cancer Specialists Pte Ltd in Singapore and also as the Medical Director, starting from August 2017, of Cryoviva Singapore, a AABB accredited cord blood and cord tissue bank with operations in India, Bangkok, Singapore, the Middle East and Vietnam. Dr. Toh was an Associate Professor of Medicine (Practice) of Monash University Malaysia campus since 2007 and retired in January 2023. He was Medical Director of Singapore’s first private cord blood bank, Cordlife Group Limited (now a SGX-listed company. SGX: P8A), from 2005 to 2008. In 1975, Dr. Toh’s early career was with the Singapore Blood Bank and the Ministry of Health Singapore Department of Haematology at the Singapore General Hospital. He remained a visiting consultant there until 2007 before going into private practice. Dr. Toh was a nominated member of Parliament in the Singapore Government from 1992 to 1994, a member of Rotary International and was President from 1998 to 1999 during the Rotary International Convention in Singapore. Dr. Toh received his FRCP(E) in 1980, M.R.C.P (United Kingdom) in 1970, both from University of Edinburgh (United Kingdom), and M.B.B.S. from University of Singapore in 1965. He is a Fellow of the Royal Colleges of Physicians of London, Edinburgh, Glasgow and the International Society of Haematologists. We believe that Dr. Toh’s decades of experience in haematology and managerial experience as a medical director qualifies him to serve on our Board.
| 142 |
Scientific Advisory Board
Upon consummation of this offering, we will have a Scientific Advisory Board comprised of the following individuals:
| Name | Age | Position | ||
| Dr. Harry ZEMON | 56 | Scientific Advisory Board Nominee | ||
| Dr. TOO Heng Phon | 63 | Scientific Advisory Board Nominee | ||
| Dr. Ahmad Radzi Bin AHMAD BADRUDDIN | 64 | Scientific Advisory Board Nominee | ||
| Dr. WANG Shu | 66 | Scientific Advisory Board Nominee |
The following sets forth certain biographical information with respect to the members of our Scientific Advisory Board:
Dr. Harry ZEMON, M.D., FACS, who will become a member of our SAB upon consummation of this offering, is a practicing surgical oncologist with a focus on solid foregut tumors, and has been in practice for over 20 years. He is board certified by the American Board of Surgery since 2006 and is affiliated with White Plains Hospital (United States) and Greenwich Hospital (United States). Dr. Zemon has been the Chair of Surgery at Westmed Medical Group in Westchester, New York since 2014. Prior to that, he served as attending surgeon in North Shore University Hospital, Lake Success (United States) and Long Island Jewish Medical Center (United States) from 2007 to 2013, and Hudson Valley Surgical Group, Sleepy Hollow (United States) from 2006 to 2008. Dr. Zemon has been a member of American College of Surgeons since 2005. He has authored several peer-reviewed publications and book chapters with the topics focusing on laparoscopic surgery and organs transplantation. Dr. Zemon completed fellowships at the Johns Hopkins University (United States) in surgical oncology in 2006 and cancer immunobiology in 2003. His fellowship in immunobiology focused on adoptive immunotherapy under the guidance of Dr. Jonathan Schneck of the Johns Hopkins’ Schneck’s Immunology and Cancer Immunotherapy Laboratory (United States). He completed his medical school and surgical training at George Washington University School of Medicine (United States) in 1998.
Dr. TOO Heng Phon Ph.D., who will become a member of our SAB upon consummation of this offering, has been a faculty member in the Department of Biochemistry, National University of Singapore since 1994, and was a Scientific Advisor to the Biotransformation Innovation Platform and a Scientist in the Bioprocess Technological Institute, A*STAR since September 2014. His laboratory was funded by Roche Diagnostics (United States & Asia Pacific) and National Institute of Health (United States) to develop qPCR assays for infectious diseases. Recently, his team developed a non-viral gene delivery technology to modify mesenchymal stem cells. There are intellectual property protections of his specific diagnostic and biotechnology platforms with various agencies and with Massachusetts Institute of Technology, United States. He is also a co-founder and Chairman of MiRXES Pte Ltd, a Singapore molecular diagnostic company since 2014. Prior to that, from June 1986 to March 1993, he was a Research & Teaching Fellow at Department of Anesthesiology and Department of Biological Chemistry & Molecular Pharmacology Harvard Medical School where he was a recipient of the Merck Sharpe Dohme Academic Development Fellowship. He also received training in the Medical Research Council, Cambridge (United Kingdom), where he was a Procter & Gamble Fellow from January 1985 to December 1986, Dr. Too was a Fellow of the Singapore Massachusetts Institute of Technology Alliance in the Molecular Engineering of Biological & Chemical Systems program in 2000 and co-chaired the Chemical & Pharmaceutical Engineering program in 2018. Dr. Too received his PhD from Imperial College of Science & Technology (United Kingdom) and Institute of Ophthalmology (United Kingdom) in 1985 and BSC (Hon), ARCS from Imperial College of Science & Technology (United Kingdom) in 1982.
| 143 |
Dr. Ahmad Radzi Bin AHMAD BADRUDDIN, who will become a member of our SAB upon consummation of this offering, has been the Chief Executive Officer and Consultant Clinical Oncologist at the Integrated Oncology Centre in Johor Bahru, Malaysia since April 2012. Dr. Radzi currently serves as the Sessional Consultant Clinical Oncologist both at the Gleneagles Medini Hospital, starting from November 2019, in Nusajaya Johor, Malaysia and at Hospital Pantai Kuala Lumpur, beginning from March 2014, in Kuala Lumpur, Malaysia. He is also concurrently serving as Visiting Consultant Clinical Oncologist both at the Sunway Medical Center, starting from August 2016 in Selangor, Malaysia and at the Oncolife Centre, beginning from July 2017, in Kuala Lumpur, Malaysia. Dr. Radzi was Advisory Board Member for Roche in Malaysia, from 2010 to 2017, for Roche’s chemotherapy treatments, where he was the Chairman for the Tarceva Subcommittee and the Co-Chairman for the Avastin Subcommittee. He was also an Advisory Board Member for AstraZeneca in Malaysia from 2010 until 2017. Dr. Radzi received his Medical Doctor degree from the Universiti Kebangsaan Malaysia (Malaysia) in 1984, his Diploma in Medical Radiotherapy specializing in clinical oncology from the Royal College of Radiologists at the University of London (United Kingdom) in 1989, and his Fellowship of the Faculty of the Royal College of Surgeons, also specializing in clinical oncology, at the Royal College of Surgeons Ireland (Ireland) in 1991.
Dr. WANG Shu, M.D., Ph.D., who will become a member of our SAB upon consummation of this offering, is one of our scientific founders. Dr. Wang is awarded with the prestigious title of Emeritus Professorship on July 1, 2022 for his service as a Professor at the Department of Biological Sciences, National University of Singapore (Singapore) since 2002, specializing in immunotherapy, stem cell therapy, and gene therapy with research emphasis on developing platform technologies applicable to cancer treatment. Prior to that, from May 2003 to June 2019, he served as a Lab Principal Investigator, Principal Research Scientist at the Institute of Bioengineering and Nanotechnology (Singapore), focusing on gene therapy. Dr. Wang has published more than 160 peer-reviewed scientific articles in the gene therapy areas a 58 Google Scholar. His publications have over 12,000 citations worldwide. In recent years, he has been working towards building platform technologies that support the development of novel immune cell-based therapeutics. Powerful cell generation, expansion, and modification technologies used in the production process for cancer immunotherapy have been established. His research focuses in several new cellular therapeutics in the area of chimeric antigen receptor (CAR) adoptive immunotherapy and with potential for use against a broad range of cancers are also under testing currently. Dr. Wang received his M.D. and Ph.D. in Cell Biology and Neurobiology from Medical College, University of Göteborg (Sweden) in 1993.
In December 2017, Dr. WANG Shu was the subject of a formal investigation by A*STAR, Institute of Bioengineering and Nanotechnology, Singapore for not adequately supervising a doctoral student that fabricated data in his research. As a consequence of the findings, it was deemed necessary that five (5) publications which incorporated the doctoral student’s fabricated data and which he co-authored be retracted. Dr. WANG is a professor at the National University of Singapore as of the date of this Prospectus.
Family Relationships
There are no family relationships or other arrangements among our Directors and executive officers.
| 144 |
Director Independence
Our current Board has undertaken a review of the independence of each proposed independent Director. and has determined that Prof. LOH Yuin Han, Dr. YEW Chak Hua, Mark LEONG Kei Wei, WU Tao Thomas and Dr. TOH Keng Kiat do not have any relationship that would interfere with their exercise of independent judgment in carrying out their responsibilities as a Director and that the aforementioned proposed Directors are “independent” as defined under the Nasdaq Listing Rules and SEC rules. We anticipate that our proposed independent Directors will have regularly scheduled meetings at which only independent Directors will be present.
Board Composition
Our Board currently consists of two (2) members as of the date of this Prospectus. We will have nine (9) Directors upon consummation of this offering.
Board Committees
Upon consummation of this offering, our Board expects to establish an audit committee, a compensation committee, and a nominating and corporate governance committee. The composition, duties and responsibilities of these Board committees set out below will be effective upon consummation of this offering. Each Board committee is expected to operate under a written charter to be adopted by our Board, a copy of which will be available on our website upon the consummation of this offering. Our Board may from time to time establish other committees as and when required so as to facilitate the management of our business.
Audit Committee
Prior to the consummation of this offering, we will establish an audit committee of our Board. Our audit committee is expected to be comprised of three (3) independent Directors, namely Mark LEONG Kei Wei, Dr. TOH Keng Kiat and WU Tao Thomas, with Mark LEONG Kei Wei serving as chairperson. Our Board has determined that Mark LEONG Kei Wei is an “audit committee financial expert” as defined by applicable SEC rules and the Nasdaq Listing Rules and regulations.
We will adopt an audit committee charter, which will detail the principal functions of the audit committee, including the following:
| ● | general oversight of the integrity of our financial statements, qualifications and independence of our independent auditors and internal financial and accounting controls; | |
| ● | appointing, approving compensation arrangements, retaining, evaluating, terminating and overseeing our independent registered public accounting firm; | |
| ● | reviewing and discussing with our independent registered public accounting firm its independence from us; | |
| ● | reviewing with our independent registered public accounting firm the matters required to be reviewed by applicable auditing requirements; | |
| ● | approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm; | |
| ● | overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the interim and annual financial statements that we file with the SEC and ACRA; | |
| ● | reviewing and approving related person transactions; | |
| ● | reviewing and monitoring our internal controls, disclosure controls and procedures and compliance with legal and regulatory requirements; and | |
| ● | establishing procedures for the confidential, anonymous submission of concerns regarding questionable accounting, internal accounting controls, and auditing matters. |
| 145 |
Compensation Committee
Prior to the consummation of this offering, we will establish a compensation committee of our Board Our compensation committee is expected to comprise of two (2) independent Directors, namely Mark LEONG Kei Wei and Dr. YEW Chak Hua, with Mark LEONG Kei Wei serving as chairperson.
We will adopt a compensation committee charter, which will detail the principal functions of the compensation committee, including the following:
| ● | reviewing compensation goals, policies, plans and programs for our executive officers; | |
| ● | reviewing and approving the compensation of our executive officers; | |
| ● | reviewing and approving employment agreements and other similar arrangements between us and our executive officers; and | |
| ● | reviewing and recommending to our Board the compensation to be provided to our Directors. |
Nominating and Corporate Governance Committee
Prior to the consummation of this offering, we will establish a nominating and corporate governance committee of our Board. Our nominating and corporate governance committee is expected to consist of two (2) independent Directors namely Dr. TOH Keng Kiat and Prof. LOH Yuin Han, with Dr. TOH Keng Kiat serving as chairperson.
We will adopt a nominating and corporate governance committee charter, which will detail the principal functions of the nominating and corporate governance committee, including the following:
| ● | identifying individuals qualified to become members of our Board, consistent with criteria approved by our Board; | |
| ● | overseeing the organization of our Board to discharge the Board’s duties and responsibilities properly and efficiently; and | |
| ● | reviewing and recommending to our Board appropriate changes to our corporate governance guidelines. |
Compensation Committee Interlocks and Insider Participation
None of the proposed members of the compensation committee is expected to be, nor has been at any time, one of our executive officers or employees. None of our executive officers will serve as a member of the Board or compensation committee of any entity that has one or more executive officers serving as a member of our Board or on our compensation committee.
Code of Business Conduct and Ethics
Prior to the consummation of this offering, we will adopt a Code of Business Conduct and Ethics, which shall apply to our Directors, executive officers and employees. We will file a copy of our Code of Business Conduct and Ethics and our audit, compensation and nominating and corporate governance committee charters as exhibits to the registration statement of which this Prospectus is a part. You will be able to review these documents by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, we will also provide a copy of the Code of Business Conduct and Ethics without charge upon request. We intend to disclose any amendments to or waivers of certain provisions of our Code of Business Conduct and Ethics in a current report on Form 6-K.
The information on or accessed through our website is not incorporated in this Prospectus or the registration statement of which this Prospectus forms a part. You should not consider information contained on our website to be part of this Prospectus or in deciding whether to purchase our ordinary shares. The nominating and corporate governance committee of our Board will be responsible for overseeing our code of business conduct and ethics and any waivers applicable to any Director, executive officer or employee. We intend to disclose any amendments to the codes, or any waivers of their requirements, on our website to the extent required by the applicable rules and exchange requirements.
| 146 |
Duties of Directors
Under Singapore law, directors of a Singapore company owe certain fiduciary duties towards the company, including a duty to act in good faith in the best interests of the company, a duty to act honestly and to use reasonable diligence in the discharge of the duties of their office. Directors generally owe fiduciary duties to the Company, and not to the Company’s shareholders. Our shareholders may not have a direct cause of action against our Directors for any breaches of fiduciary duties. The Company has a right to seek damages if a duty owed by Directors is breached, subject to the applicable laws on quantification of damages.
Limitation on Liability and Indemnification Matters
The constitution of the Company provides for every Director and officer of the Company to be indemnified out of the assets of the Company against any liability (other than any liability referred to in section 172B(1)(a) or (b) of the Singapore Companies Act) incurred by the officer to a person other than the Company attaching to the officer in connection with any negligence, default, breach of duty or breach of trust.
We intend to maintain a Directors’ and officers’ insurance policy pursuant to which our Directors and officers are insured against liability for actions taken in their capacities as Directors and officers. We believe that this is necessary to attract and retain qualified persons as Directors and officers of the Company. Please refer to the section titled “Limitation of Liability of Directors and Officers” for further details.
Foreign Private Issuer Exemptions
In general, under the Nasdaq corporate governance standards, foreign private issuers, as defined by the rules adopted under the Exchange Act, are permitted to follow the corporate governance practices of its country of incorporation instead of the corporate governance practices of the Nasdaq. As a Singapore company, we are subject to various requirements under the Singapore Companies Act. Therefore, while we intend to voluntarily follow most Nasdaq corporate governance rules, we are permitted to comply with Singapore Companies Act requirements instead of Nasdaq corporate governance rules, provided that we disclose which requirements we are not following and the equivalent Singapore Companies Act requirement. Pursuant to this “home country practice exemption” with respect to the following:
| ● | we intend to comply with the Singapore Companies Act which does not require that a majority of our Board of Directors consists of independent Directors, as opposed to the requirement under Section 5605(b)(1) of the Nasdaq Listing Rules; and |
| ● | we intend to follow the quorum requirement for shareholder meetings as permitted under the Singapore Companies Act, and pursuant to our Constitution, which provides that 2 members of the Company personally present shall form a quorum in accordance, instead of the requirement under Section 5620(c) of Nasdaq Listing Rules that a quorum must consist of at least 331/3 percent of the outstanding shares of a listed company’s common voting stock. |
The foreign private issuer exemptions do not modify the independence requirements for the audit committee, and we intend to comply with the requirements of the Sarbanes-Oxley Act and the Nasdaq Listing Rules, which require that our audit committee be composed of at least three (3) Directors, all of whom are independent.
We may in the future decide to use the foreign private issuer exemptions with respect to some or all of the other corporate governance rules.
If at any time we cease to be a “foreign private issuer” under the Nasdaq Listing Rules and rules of the Exchange Act, our Board will take all action necessary to comply with all applicable Nasdaq Listing Rules, including corporate governance rules.
Due to our status as a foreign private issuer and our intention to follow certain home country corporate governance practices, our shareholders will not have the same protections afforded to shareholders of companies that are subject to all the Nasdaq corporate governance standards.
| 147 |
The Company qualifies as an “emerging growth company” and a “smaller reporting company,” both within the meaning of the Securities Act, for purposes of the SEC’s executive compensation disclosure rules. In accordance with those rules, the Company is required to provide a Summary Compensation Table and an Outstanding Equity Awards at Fiscal Year End Table, as well as limited narrative disclosures regarding executive compensation for the Company’s last completed fiscal year. Further, the Company’s reporting obligations extend only to the Company’s “named executive officers,” who are the individuals who served as the Company’s principal executive officer and the Company’s next two other most highly compensated officers at the end of fiscal year 2022. For fiscal year 2023, the Company’s named executive officers (NEOs) are:
| Name | Position / Title | |
| CHOO Chee Kong | Chairman | |
| Dr. ZENG Jieming | Chief Scientific and Medical Officer | |
| Dr. TAN Wee Kiat | Chief Operating Officer |
| 148 |
Summary Compensation Table
The table below sets forth information regarding the total compensation awarded to and earned by the Company’s NEOs for services rendered during the financial years ended December 31, 2022:
| Name and Principal Position | Year | Salary (U.S.$) | Bonus (U.S.$) | All Other Compensation (U.S.$) | Total (U.S.$) | ||||||||||||||
| CHOO Chee Kong, | 2022 | - | - | 5,445 | 5,445 | ||||||||||||||
| Chairman | |||||||||||||||||||
| Dr. ZENG Jieming, | 2022 | 73,506 | 2,158 | - | 75,664 | ||||||||||||||
| Chief Scientific and Medical Officer | |||||||||||||||||||
| Dr. TAN Wee Kiat, | 2022 | 62,864 | 2,158 | - | 65,022 | ||||||||||||||
| Chief Operating Officer | |||||||||||||||||||
(1) Mr. Choo is party to a service agreement with CytoMed Malaysia, dated September 8, 2021, for which he receives a monthly service fee, as described in more detail below under Material Terms of Employment Agreements.
(2) The compensation for Mr. Choo reported above was paid in Malaysian Ringgit and is reported above based on a rate of MYR 4.4075 to U.S.$1.00, the exchange rate in effect as of June 30, 2022, as set forth in the H.10 statistical release of the U.S. Board of Governors of the Federal Reserve System.
(3) The compensation for Drs. Zeng and Tan reported above was paid in Singapore dollars and is reported above based on a rate of S$1.3903 to U.S.$1.00, the exchange rate in effect as of June 30, 2022, as set forth in the H.10 statistical release of the U.S. Board of Governors of the Federal Reserve System.
Narrative to Summary Compensation Table
Material Terms of Employment Agreements
As of the date of this Prospectus, each of our NEOs is a party to an employment agreement or service agreement as set forth below.
CHOO Chee Kong entered into a service agreement with CytoMed Malaysia on September 8, 2021. The initial term of the agreement was for a one-year period, which ended on September 7, 2022. The agreement was automatically renewed for an additional year, which is set to expire on September 7, 2023. Pursuant to the service agreement, Mr. Choo receives a sum of RM 2,000 per month (approximately U.S.$450) and is entitled to reimbursement for all reasonable and approved out-of-pocket expenses incurred in connection with his provision of services. Either party may terminate the agreement at any time by giving three months’ written notice to the other party, and the CytoMed Malaysia has the right to immediately terminate the agreement in the event of misconduct, noncompliance, conviction of certain crimes, or material breach. The agreement contains restrictive covenants governing confidentiality, a non-solicitation and non-interference restriction that applies to employees, clients, customers, and executives for one year following termination, and an intellectual property inventions assignment.
Each of Dr. TAN Wee Kiat and Dr. ZENG Jieming entered into respective employment agreements with the Company commencing on July 17, 2018 and August 1, 2019, respectively. Pursuant to the respective employment agreements, Dr. TAN Wee Kiat and Dr. ZENG Jieming are each entitled to reimbursements for eligible out-of-pocket expenses incurred in connection with their employment. The term of each agreement continues until either party gives the other party at least three months’ notice, or payment of salary-in-lieu, and the Company has the right to immediately terminate in the event of default, misconduct or any breach or non-observance by the employee. The agreements contain restrictive covenants governing confidentiality, a non-solicitation and non-competition restriction that applies to employees, client, customers and executives for one year following termination, and confirmation that all intellectual properties developed during the employments belong exclusively to the Company.
Effective upon consummation of the offering, our NEOs will enter into new service agreements directly with the Company which will supersede the abovementioned current service or employment agreements. The Company intends to offer agreements that will not have a specified term and will provide for employment at will and contain standard confidentiality, invention assignment, non-solicitation and non-competition provisions.
Outstanding Equity Awards at Fiscal Year End Table
There are no outstanding equity awards held by any of our NEOs as of December 31, 2022, and, therefore, no table is included below.
| 149 |
Incentive Plan
For future equity awards, our Board has adopted the Incentive Plan to provide an additional means through which to the grant of awards to attract, motivate, retain and reward selected key employees and other eligible persons, including our NEOs. We have also obtained approval for the Incentive Plan from our shareholders. A summary of our Incentive Plan is set out below.
Shares Subject to the Incentive Plan
A total of 1,279,117 ordinary shares will be available for issuance under the Incentive Plan. If an award granted under the Incentive Plan is forfeited, canceled, settled, or otherwise terminated without a distribution of shares, the shares underlying that award will again become available for issuance under the Incentive Plan. If shares delivered under the Incentive Plan are tendered or withheld to pay the exercise price of a share option or to satisfy withholding taxes, such shares will again become available for issuance under the Incentive Plan.
Administration of the Incentive Plan
Our Board or a committee appointed by the Board will administer the Incentive Plan as the plan administrator and will have broad authority to:
| ● | select participants and determine the types of awards that they are to receive; |
| ● | determine the number of shares that are to be subject to awards and the terms and conditions of awards, including the price (if any) to be paid for the shares or the award and establish the vesting conditions (if applicable) of such shares or awards; |
| ● | cancel, modify or waive our rights with respect to, or modify, discontinue, suspend or terminate any or all outstanding awards, subject to any required consents; |
| ● | construe and interpret the terms of the Incentive Plan and any agreements relating to the Incentive Plan; |
| ● | determine whether awards will be settled in cash, ordinary shares, other securities, other property, or in any combination thereof; |
| ● | prescribe, amend, and rescind rules and regulations relating to the Incentive Plan; and |
| ● | make all other determinations deemed necessary or advisable for administering the Incentive Plan. |
| 150 |
Participation
Employees, officers, directors, members of our SAB and consultants that provide services to us may be selected to receive awards under the Incentive Plan.
Types of Awards
The Incentive Plan permits the granting of awards in the form of share options and restricted shares.
Share Options
A share option entitles the participant to purchase ordinary shares at a fixed exercise price. The exercise price per share will be determined by the plan administrator in the applicable award agreement in its sole discretion at the time of the grant, but the exercise price cannot be less than the closing sales price for our ordinary shares on the grant date. The exercise price may be paid in cash, check, by surrender of ordinary shares already held by the participant, or by cashless or net exercise. The maximum term of each share option shall be fixed by the plan administrator, but in no event shall an option be exercisable more than ten (10) years after the date such option is granted.
Restricted Shares
A restricted share award is an award of ordinary shares that vests in accordance with the terms and conditions established by the plan administrator.
Equitable Adjustments
In the event of a merger, consolidation, recapitalization, share split, reverse share split, reorganization, split-up, spin-off, combination, repurchase, or other change in corporate structure affecting the ordinary shares, the maximum number and kind of shares reserved for issuance or with respect to which awards may be granted under the Incentive Plan will be adjusted to reflect such event, and the plan administrator will make such adjustments as it deems appropriate and equitable in the number, kind and exercise price of ordinary shares covered by outstanding awards made under the Incentive Plan.
Change in Control
In the event of any change in control (as defined in the Incentive Plan), the plan administrator will take any action it deems appropriate, which action may include, without limitation, the following: (i) the continuation of the awards, if the Company is the surviving corporation; (ii) the assumption of the awards by the surviving corporation or its parent or subsidiary; (iii) the substitution by the surviving corporation or its parent or subsidiary of equivalent awards; (iv) accelerated vesting of the award, with all performance objectives and other vesting criteria deemed achieved at targeted levels, and a limited period during which to exercise the awards prior to closing of the change in control, or (v) settlement of any award for the change in control price (less, to the extent applicable, the per share exercise price).
| 151 |
Term
The Incentive Plan, unless terminated, will continue in effect for a term of ten (10) years from January 18, 2023, the date the Incentive Plan was adopted by the Board.
Amendment and Termination
The Board may at any time amend, alter, suspend or terminate the Incentive Plan, although no such action may, without the written consent of the participant, impair the rights of any participant with respect to outstanding awards.
Director Compensation
Director Compensation Table
We have not historically had a formal compensation policy with respect to service on our Board, but we have reimbursed our non-employee Directors for out-of-pocket direct expenses incurred in connection with attending meetings. CHOO Chee Kong and Lucas LUK Tien Wee, who were our non-employee Directors as of December 31, 2022, did not receive any compensation for their service as members of our Board, and, therefore, no table is included below.
Prior to our offering, our Board has executed an Independent Director Appointment Letter with each of our non-employee Directors, which will become effective upon consummation of this offering. Each appointment letter includes reimbursement for out-of-pocket direct expenses incurred in connection with meeting attendance and provides that we will pay each of our non-employee Directors annual cash retainers for service on the Board and for service on each committee on which the Director is a member. The chairperson of each committee will receive a higher annual retainer for such service. These retainers are payable in arrears in four equal installments on the last day of each quarter, provided that the amount of such payment will be pro-rated for any portion of such quarter that the Director is not serving on our Board or the applicable committee. The retainers to be paid to non-employee Directors for service on the Board and for service on each committee of the Board on which the Director is a member are as follows:
| Position | Member Annual Retainer | Chairperson Additional Annual Retainer | ||||||
| Board of Directors | U.S.$ | 12,000 | U.S.$ | 3,600 | ||||
| Audit Committee | U.S.$ | 4,200 | U.S.$ | 2,100 | ||||
| Compensation Committee | U.S.$ | 3,000 | U.S.$ | 1,500 | ||||
| Nominating and Corporate Governance Committee | U.S.$ | 3,600 | U.S.$ | 1,800 | ||||
Subject to shareholders’ approval at a general meeting, the Compensation Committee and the Directors shall have the discretion to increase the non-employee Directors’ compensation. In addition, non-employee Directors will be eligible to participate in the Incentive Plan and may be granted share options and/or restricted shares under the Incentive Plan from time to time.
| 152 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for our Directors and executive officers, which are described elsewhere in this Prospectus, the following includes a summary of transactions since our incorporation on March 9, 2018 and any currently proposed transactions, to which we were or are to be a participant, in which:
| ● | the amount involved exceeded or will exceed the lesser of (1) U.S.$120,000 or (2) 1% of the average of our total assets; and |
| ● | any of our Directors, executive officers or holders of more than 5% of our ordinary shares, or member of the immediate family of, or person sharing the household with, the foregoing persons, had or will have a direct or indirect material interest. |
Related Party Transactions
Personal Guarantee by CHOO Chee Kong
On December 28, 2018, CytoMed Malaysia obtained credit facilities of RM 2 million (approximately U.S.$ 453,772) from Hong Leong Bank Berhad to purchase the property at 12, Jalan Permas 9/16, Bandar Baru Permas Jaya, 81750 Masai, Johor Darul Ta’zim in Malaysia for the construction of the cGMP Facility, which is secured by a personal guarantee from CHOO Chee Kong, our Director and Chairman. This personal guarantee is expected to be discharged in 2024.
Guarantee by CHOO Chee Kong pursuant to the Convertible Loan Agreement with mDR Limited
On December 10, 2019, the Company entered into the mDR Convertible Loan of up to S$1.50 million (approximately U.S.$1.08 million) to fund the abovementioned purchase of the shares in LMC, expenses for the proposed listing, and working capital purposes. As security for this loan, CHOO Chee Kong and Messiah Limited executed personal and corporate guarantees, respectively, in favor of mDR Limited guaranteeing the obligations of the Company under the agreement. CHOO Chee Kong is also the sole director and a shareholder of Messiah Limited.
Subscription of Shares
On June 26, 2019, and September 28, 2020, the then-shareholders of the Company agreed to a waiver of their pre-emption rights to allot and issue certain numbers of shares to Glorious Finance Limited, at the consideration sum of S$1 million (approximately U.S.$719,269) and S$500,000 (approximately U.S.$359,635) respectively.
On February 19, 2021, the then-shareholders of the Company agreed to a waiver of their pre-emption rights to allot and issue certain numbers of shares to Dr Lucas LUK Tien Wee, a director of our Company, at the consideration sum of S$190,355 (approximately U.S.$136,916).
On April 15, 2021, we entered into a subscription agreement with Glorious Finance Limited in relation to its subscription of 237,274 ordinary shares in our Company, at consideration sum of S$500,000 (approximately U.S.$359,635). The subscription of shares was completed on April 22, 2021.
On April 15, 2021, we entered into a subscription agreement with mDR Limited in relation to its subscription of 711,822 ordinary shares in our Company at consideration sum of S$1.50 million (approximately U.S.$1.08 million). The subscription of shares was completed on April 22, 2021. In relation to this agreement, CHOO Chee Kong, our Director and Chairman, and mDR Limited also executed a put option agreement on April 15, 2021, allowing mDR Limited to have the right and option to require CHOO Chee Kong to purchase and acquire a certain number of shares in our Company from mDR Limited.
| 153 |
Landmark Investment Agreement
On January 20, 2020, we entered into an investment agreement with, inter alia, LMC, as varied by a supplemental agreement entered into on January 20, 2021 and a further supplemental letter dated October 28, 2021, to acquire new ordinary shares representing 20.0% of the total number of shares in LMC at a subscription consideration of RM1.50 million (approximately U.S.$340,329). The subscription of shares in LMC was completed on September 6, 2021. Dr Lucas LUK Tien Wee, one of the shareholders of LMC and parties of the investment agreement, was appointed a Director of our Company on January 1, 2021.
As our NEO and Chief Clinical Officer, Dr Lucas LUK Tien Wee is deeply involved in our preparation for clinical trial application in Singapore and Malaysia. As Chief Executive Officer and major shareholder of LMC, Dr Lucas LUK Tien Wee brings valuable expertise, experience and connections to facilitate clinical trial preparation including protocol documentation. With deep interest in research, LMC has been purchasing small quantities of R&D products from us since beginning of 2020. The aggregate sum of such transactions for the financial year ended December 31, 2021 is immaterial. After we become publicly listed on Nasdaq, such transactions will be subject to the Audit Committee’s scrutiny to ensure they are conducted on arm’s length basis.
Joint Venture Share Award
On October 7, 2020, our Group incorporated Puricell as part of our plans to develop regenerative medicine and awarded 5.0% shareholding amounting to a nominal S$50 (approximately U.S.$36) in Puricell to LOH Yuin Han, who will be appointed as our independent Director upon consummation of this offering, and who is a senior staff at the IMCB, a research institute under A*STAR. Depending on milestone achievement, LOH Yuin Han’s stake can increase to 20.0%. Such awards are free of cost to LOH Yuin Han.
Policies and Procedures for Related Party Transactions
We have not yet adopted a formal policy for the review, approval or ratification of related party transactions. Accordingly, the transactions discussed above were not reviewed, approved or ratified in accordance with any such policy.
Prior to the consummation of this offering, we will adopt a code of business conduct and ethics requiring us to avoid, wherever possible, all conflicts of interests, except under guidelines or resolutions approved by our Board of Directors (or the appropriate committee of our Board) or as disclosed in our public filings with the SEC. Under our code of business conduct and ethics, conflict of interest situations will include any financial transaction, arrangement or relationship (including any indebtedness or guarantee of indebtedness) involving the Company. A form of the code of business conduct and ethics that we plan to adopt prior to the consummation of this offering is filed as an exhibit to the registration statement of which this Prospectus is a part.
In addition, our audit committee, pursuant to a written charter that we will adopt prior to the consummation of this offering, will be responsible for reviewing and approving related party transactions to the extent that we enter into such transactions. An affirmative vote of a majority of the members of the audit committee present at a meeting at which a quorum is present will be required in order to approve a related party transaction. A majority of the members of the entire audit committee will constitute a quorum. Without a meeting, the unanimous written consent of all of the members of the audit committee will be required to approve a related party transaction. A form of the audit committee charter that we plan to adopt prior to the consummation of this offering is filed as an exhibit to the registration statement of which this Prospectus is a part. We also require each of our Directors and executive officers to complete a Directors’ and officers’ questionnaire that elicits information about related party transactions.
These procedures are intended to determine whether any such related party transaction impairs the independence of a Director or presents a conflict of interest on the part of a Director, employee or officer.
| 154 |
Principal Shareholders as of the date of this Prospectus
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of the date of this Prospectus for:
| ● | each beneficial owner of 5% or more of our outstanding ordinary shares; | |
| ● | each of our directors and executive officers; and | |
| ● | all of our directors and executive officers as a group. |
The following section and tables set forth certain information with respect to the beneficial ownership of our ordinary shares and as adjusted to reflect the sale of the ordinary shares offered by us in our initial public offering and the 1-for-380.83 reverse split of our ordinary shares effective January 17, 2023.
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include ordinary shares issuable upon the exercise of options that are immediately exercisable or exercisable within sixty (60) days of the date of this Prospectus. Percentage ownership calculations prior to this offering are based on 8,527,450 ordinary shares, outstanding as of the date of this Prospectus. Percentage ownership calculations after this offering are based on 10,939,819 ordinary shares, outstanding after this offering.
Except as otherwise indicated, all of the shares reflected in the table are ordinary shares and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
Except as otherwise indicated in the table below, addresses of our directors, executive officers and named beneficial owners are in care of CytoMed Therapeutics Limited, 1 Commonwealth Lane, #08-22, Singapore 149544.
| Before Offering | After Offering | |||||||||||
Number of shares | %(5) | %(5) | ||||||||||
| Shareholders with 5% or more ordinary shares in the Company: | ||||||||||||
| Glorious Finance Limited | 4,653,604 | 54.57 | % | 42.54 | % | |||||||
| WANG Shu | 883,858 | 10.36 | % | 8.08 | % | |||||||
| mDR Limited (2)(4) | 1,276,237 | 14.09 | % | 11.13 | % | |||||||
| ZENG Jieming | 589,239 | 6.91 | % | 5.39 | % | |||||||
| Directors, Director Nominees and NEOs: | ||||||||||||
| CHOO Chee Kong (1) | 2,969,997 | 34.83 | % | 27.15 | % | |||||||
| ZENG Jieming (3) | 589,239 | 6.91 | % | 5.39 | % | |||||||
| Lucas LUK Tien Wee | 92,563 | 1.09 | % | 0.85 | % | |||||||
| TAN Wee Kiat(3) | - | - | - | |||||||||
| LOH Yuin Han(3) | - | - | - | |||||||||
| YEW Chak Hua(3) | - | - | - | |||||||||
| Mark LEONG Kei Wei(3) | - | - | - | |||||||||
| WU Tao Thomas(3) | - | - | - | |||||||||
| Dr. TOH Keng Kiat (3) | ||||||||||||
| All current directors, director nominees and NEOs as a group (9 persons) | 3,651,799 | 42.83 | % | 33.39 | % | |||||||
| (1) | Consists of 2,559,482 Ordinary Shares held by Glorious Finance Limited (“Glorious Finance”) and 410,515 Ordinary Shares held by EP Capital Inc. (“EP Capital”). Mr. CHOO Chee Kong does not directly hold any shares in our Company but is deemed to beneficially own the shares held by Glorious Finance and EP Capital as indicated in the table above. Mr. CHOO Chee Kong owns 55.0% and 100.0% of shares in Glorious Finance and EP Capital, respectively as of the date of this Prospectus. Glorious Finance is a major shareholder of the Company as of the date of this Prospectus. |
| (2) | mDR Limited is a public listed company on the Mainboard of the SGX-ST. |
| (3) | Indicates that a Director Nominee to be appointed as a Director upon consummation of this offering. |
| (4) | Consists of 747,414 Ordinary Shares held by mDR Limited and 528,823 Ordinary Shares issuable pursuant to the mDR Convertible Loan that is convertible at mDR Limited’s option within sixty (60) days of the date of this Prospectus. Please refer to the section titled “Description of Share Capital – General” for further details. |
| (5) | Rounded to the nearest two decimal places. |
| 155 |
The following description summarizes material terms of our constitution as of the date of this Prospectus. Such summary does not purport to be complete and is subject to, and is qualified in its entirety by reference to, all of the provisions of our constitution, a copy of which has been filed as an exhibit to the registration statement of which this Prospectus forms a part. Prospective investors are urged to read the exhibits for a complete understanding of our constitution.
General
On January 17, 2023, the Company implemented a 1-for-380.83 reverse split of our ordinary shares pursuant to which shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date. The reverse split proportionally reduced the number of authorized share capital.
Upon the consummation of this offering, our issued and outstanding share capital will consist of ordinary shares. Following this offering, we will have 11,468,642 ordinary shares issued and outstanding (or 11,830,497 ordinary shares if the underwriters exercise in full their option to purchase additional shares) assuming conversion of all outstanding convertible loans into ordinary shares. We currently only have one class of issued ordinary shares, which have identical rights in all respects and rank equally with one another. Pursuant to the terms of the mDR Convertible Loan, mDR Limited has a right to convert the said convertible loan into ordinary shares of the Company, or redeem the full sum of the convertible loan, upon and on the terms of the convertible loan agreement. Please refer to the sections titled “Principal and Selling Shareholders” and “Certain Relationship and Related Party Transactions – Related Party Transactions” for further details.
For the purposes of this section, references to “shareholders” mean those shareholders whose names and number of shares are entered in our register of members. Subject to the Singapore Companies Act and our constitution, only persons who are registered in our register of members are recognized under Singapore law as our shareholders. As a result, only registered shareholders have legal standing under Singapore law to institute shareholder actions against us or otherwise seek to enforce their rights as shareholders.
Ordinary Shares
Our ordinary shares have no par or nominal value under Section 62A(1) of the Singapore Companies Act. All shares presently issued are fully paid and existing shareholders are not subject to any calls on shares. Although Singapore law does not recognize the concept of “non-assessability” with respect to newly-issued shares, we note that any subscriber of our ordinary shares who has fully paid up all amounts due with respect to such ordinary shares will not be subject under Singapore law to any personal liability to contribute to our assets or liabilities in such subscriber’s capacity solely as a holder of such ordinary shares, subject to any lifting of the corporate veil by the Singapore courts. We believe this interpretation is substantively consistent with the concept of “non-assessability” under most, if not all, U.S. state corporation laws. Under Singapore laws, we cannot, except in the circumstances permitted by the Singapore Companies Act, grant any financial assistance for the acquisition or proposed acquisition of our own ordinary shares. There are no limitations in our constitution or Singapore law on the rights of shareholders not resident in Singapore to hold or vote in respect of our ordinary shares.
Voting Rights
Each ordinary share is entitled to one vote per share. Pursuant to our constitution, voting at any meeting of shareholders must be by show of hands unless a poll has been demanded prior to or on the declaration of the result of the show of hands by, among others, at least one shareholder present in person or by proxy or by attorney or other duty authorized representative and representing not less than 5% of the total voting rights of all shareholders having the right to vote at the meeting. For a poll, each holder of ordinary shares who is present in person or by proxy or by attorney or other duly authorized representative, has one vote for each ordinary share which he holds or represents. Proxies need not be shareholders.
Subject to the Singapore Companies Act and our constitution, only those shareholders who are registered in our register of members will be entitled to vote at any meeting of shareholders in person or by proxy or by attorney or other duly authorized representative. Therefore, since the ordinary shares offered in this offering are expected to be held through the DTC or its nominee, DTC or its nominee will grant an omnibus proxy to DTC participants holding our ordinary shares in book-entry form. Persons holding through a broker, bank, nominee or other institution that is a direct or indirect participant of DTC will have the right to instruct their broker, bank, nominee or other institution holding these ordinary shares on how to vote such ordinary shares by completing the voting instruction form provided by the applicable broker, bank, nominee, or other institution. Whether voting is by a show of hands or by a poll, the vote of DTC or its nominee will be voted by the chairman of the meeting according to the results of the votes of the DTC participants (which results will reflect the instructions received from persons that own our ordinary shares electronically in book-entry form through DTC). In the case of an equality of votes, whether on a show of hands or on a poll, the chairman of the meeting shall be entitled to a second or casting vote.
| 156 |
Dividends
We may, by ordinary resolution, declare dividends at a general meeting of our shareholders, but no dividend shall be payable except out of our available profits and shall not bear interest against our Company, and the amount of any such dividend shall not exceed the amount recommended by our Board. Subject to our constitution and in accordance with the Singapore Companies Act, our Board may, without the approval of our shareholders, declare and pay interim dividends, but any final dividends the Board declares must be approved by an ordinary resolution at a general meeting of our shareholders. Please refer to the section titled “Dividends and Dividend Policy” for further information on our dividend policy.
Capitalization and Other Rights
Our Board may, with the approval of our shareholders at a general meeting, capitalize any reserves or profits and distribute them as shares, credited as paid-up, to our shareholders in proportion to their shareholdings in accordance with our constitution.
Variation of Rights
Subject to the Singapore Companies Act and every other Singapore statute for the time being in force affecting us, under our constitution, whenever our share capital is divided into different classes of shares, the special rights attached to any class may be varied either with the consent in writing of the holders of 75% of the issued shares of the class or with the sanction of a special resolution passed at a separate general meeting of the holders of the shares of the class (but not otherwise) and may be so varied either while the Company is a going concern or during or in contemplation of a winding-up. Except otherwise provided in our constitution, in each general meeting, the necessary quorum shall be two shareholders present in person.
Representative’s Warrants
We have agreed to, upon the closing of this offering, including upon the closing of any offering of ordinary shares sold to cover over allotments, issue to the representative warrants, or the representative’s warrants, to purchase a number of ordinary shares equal to 5% of the total number of ordinary shares sold in this public offering. The representative’s warrants will be exercisable on a cashless basis at a price equal to the initial offering price to the public. The representative’s warrants are exercisable at any time and from time to time, in whole or in part, during the four-and-half-year period commencing six months after the effective date of the registration statement related to this offering.
The representative’s warrants and the ordinary shares underlying the representative’s warrants have been deemed compensation by the Financial Industry Regulatory Authority, or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The representative, or permitted assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the representative’s warrants or the securities underlying the representative’s warrants, nor will the representative engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the representative’s warrants or the underlying shares for a period of 180 days from the effective date of the registration statement. Additionally, the representative’s warrants may not be sold transferred, assigned, pledged or hypothecated for a 180-day period following the effective date of the registration statement except to any underwriter and selected dealer participating in this offering and their bona fide officers or partners. The representative’s warrants will provide for adjustment in the number and price of the representative’s warrants and the ordinary shares underlying such representative’s warrants in the event of recapitalization, merger, stock split or other structural transaction, or a future financing undertaken by us.
Issuance of New Shares
Under the Singapore Companies Act, notwithstanding anything in our constitution, new shares may be issued only with the prior approval of our shareholders in a general meeting. General approval may be sought from our shareholders in a general meeting for the issuance of shares. Such authority to issue new ordinary shares, if granted by our shareholders, shall continue in force until the earlier of:
| ● | the conclusion of the next annual general meeting after the date on which the approval was given; or |
| ● | the expiration of the period within which the next annual general meeting is required by law to be held (i.e., within four (4) months after the end of each financial year in the case of a public company that is listed or within six (6) months after the end of each financial year in the case of any other company); |
however, any such approval may be revoked or varied by the company in a general meeting.
Subject to this, applicable provisions of the Singapore Companies Act and our constitution, our Board may allot, issue or grant options over or otherwise dispose of new ordinary shares to such persons on such terms and conditions and with the rights and restrictions as they may think fit to impose. Such rights are subject to any condition attached to such issue and the regulations of any stock exchange on which our ordinary shares are listed, as well as U.S. federal and blue sky securities laws applicable to such issue.
| 157 |
Preference Shares
Our constitution provides that, subject to the Singapore Companies Act and our constitution, we may issue shares of a different class with preferential, deferred or other special rights, or restrictions, whether with regards to dividend, voting, return of capital or otherwise, or which do not confer voting rights, as our Board, subject to any ordinary resolution of our Company, determine. The Singapore Companies Act states that no company shall allot any preference shares or convert any issued shares into preference shares unless there are set out in its constitution the rights of the holders of those shares with respect to repayment of capital, participation in surplus assets and profits, cumulative or non-cumulative dividends, voting and priority of payment of capital and dividend in relation to other shares or other classes of preference shares.
The issuance of preference shares could have the effect of decreasing the trading price of our ordinary shares, restricting dividends on our ordinary shares, diluting the voting power of our ordinary shares, impairing the liquidation rights of our ordinary shares, or delaying or preventing a change in control of the Company.
Register of Members
Subject to the Singapore Companies Act and our constitution, only persons who are registered in our register of members are recognized under Singapore law as our shareholders with legal standing under Singapore law to institute shareholder actions against us or otherwise seek to enforce their rights as shareholders.
Our ordinary shares, which are expected to be listed and traded on Nasdaq, are expected to be held through the DTC. Accordingly, DTC or its nominee, Cede & Co., will be the shareholder on record registered in our register of members.
A holder of our ordinary shares held in book-entry interests through DTC or its nominee may become a registered shareholder by exchanging its interest in such shares for certificated ordinary shares and being registered in our register of members in respect of such shares. The procedures by which a holder of book-entry interests held through the facilities of the DTC may exchange such interests for certificated ordinary shares are determined by DTC (including the broker, bank, nominee or other institution that holds the shares within DTC) and VStock, which will act as our transfer agent, in accordance with their internal policies and guidelines regulating the withdrawal and exchange of book-entry interests for certificated ordinary shares.
Under the Singapore Companies Act, if (a) the name of any person is without sufficient cause entered in or omitted from the register of members; or (b) default is made or unnecessary delay takes place in entering in the register of members the fact of any person having ceased to be a member, the person aggrieved or any member or the public company itself, may apply to the Singapore courts for rectification of the register of members. The Singapore courts may refuse the application or may order rectification of the register of members and payment by the public company of any damages sustained by any party to the application. The Singapore courts will not entertain any application for the rectification of a register of members in respect of an entry which was made in the register of members more than 30 years before the date of the application.
Singapore Take-over Code
The Singapore Take-over Code, regulates, among other things, the acquisition of voting shares of Singapore-incorporated public companies and contains certain provisions that may delay, deter or prevent a take-over or change in control of such a public company. In this regard, the Singapore Take-over Code applies to, among others, corporations with a primary listing of their equity securities in Singapore. While the Singapore Take-over Code is drafted with, among others, listed public companies in mind, unlisted public companies with more than 50 shareholders and net tangible assets of S$5 million or more, must also observe the letter and spirit of the general principles and rules of the Singapore Take-over Code, wherever this is possible and appropriate. Public companies with a primary listing overseas may apply to SIC to waive the application of the Singapore Take-over Code. As of the date of this Prospectus, no application has been made to SIC to waive the application of the Singapore Take-over Code in relation to us. We may submit an application to SIC for a waiver from the Singapore Take-over Code so that the Singapore Take-over Code will not apply to us for so long as we are not listed on a securities exchange in Singapore. We will make an appropriate announcement if we submit such application and when the result of such application is known.
| 158 |
Any person acquiring an interest, whether in a single transaction of by a series of transactions over a period of time or not, either on his or her own or together with parties acting in concert with such person, in 30% or more of the voting rights in the Company or any person holding, either on his or her own or together with parties acting in concert with such person, between 30% and 50% (both amounts inclusive) of the voting rights in the Company, and if such person (or parties acting in concert with such person) acquires additional voting shares representing more than 1% of the voting rights in the Company in any six-month period, must immediately, except with the consent of SIC, extend a mandatory take-over offer for all the remaining voting shares in accordance with the provisions of the Singapore Take-over Code. Responsibility for ensuring compliance with the Singapore Take-over Code rests with parties (including company directors) to a take-over or merger and their advisors.
Under the Singapore Take-over Code, “parties acting in concert” comprise individuals or companies who, pursuant to an agreement or understanding (whether formal or informal), cooperate, through the acquisition by any of them of shares in a company, to obtain or consolidate effective control of that company. Certain persons are presumed (unless such presumption is rebutted) to be acting in concert with each other. They are as follows:
| ● | a company, its parent company, subsidiaries and fellow subsidiaries (together, the related companies), the associated companies of any of the company and its related companies, companies whose associated companies include any of these foregoing companies and any person who has provided financial assistance (other than a bank in the ordinary course of business) to any of the foregoing for the purchase of voting rights; |
| ● | a company with any of its directors (together with their close relatives, related trusts and companies controlled by any of the directors, their close relatives and related trusts); |
| ● | a company with any of its pension funds and employee share schemes; |
| ● | a person with any investment company, unit trust or other fund whose investment such person manages on a discretionary basis but only in respect of the investment account which such person manages; |
| ● | a financial or other professional adviser, including a stockbroker, with its client in respect of the shareholdings of the adviser and persons controlling, controlled by or under the same control as the adviser; |
| ● | directors of a company (including their close relatives, related trusts and companies controlled by any of such directors, their close relatives and related trusts) which is subject to an offer or where the directors have reason to believe a bona fide offer for the company may be imminent; |
| ● | partners; and |
| ● | an individual and (i) such person’s close relatives, (ii) such person’s related trusts, (iii) any person who is accustomed to act in accordance with such person’s instructions, (iv) companies controlled by the individual, such person’s close relatives, such person’s related trusts or any person who is accustomed to act in accordance with such person’s instructions and (v) any person who has provided financial assistance (other than a bank in the ordinary course of business) to any of the foregoing for the purchase of voting rights. |
Subject to certain exceptions, a mandatory offer must be in cash or be accompanied by a cash alternative at not less than the highest price paid by the offeror or parties acting in concert with the offeror during the offer period and within the six months prior to its commencement. Where any such shares have been acquired for a consideration other than cash, the SIC should be consulted as to the offer to be made for any class of share capital in respect of which no acquisitions have taken place within the preceding 6 months or when there is more than one class of equity share capital involved.
| 159 |
Under the Singapore Take-over Code, where effective control of a company is acquired or consolidated by a person, or persons acting in concert, a general offer to all other shareholders is normally required. An offeror must treat all shareholders of the same class in an offeree company equally. A fundamental requirement is that shareholders in the company subject to the take-over offer must be given sufficient information, advice and time to enable them to reach an informed decision on the offer. These legal requirements may impede or delay a takeover of our company by a third-party.
Election and Re-election of Directors
We may, by ordinary resolution, remove any Director before the expiration of his or her period of office, notwithstanding anything in our constitution or in any agreement between us and such Director. Where any Director so removed was appointed to represent the interests of any particular class of shareholders the resolution to remove him shall not take effect until his successor has been appointed. We may also, by an ordinary resolution, appoint another person in place of a director removed from office pursuant to the foregoing. Special notice shall be required of any notice to remove any director or to appoint another person in place of a director so removed at the meeting at which he is removed. Upon receipt of notice of an intended resolution to remove a Director, we shall immediately send a copy thereof to the Director concerned, and the Director, whether or not he is a member of our company, shall be entitled to be heard on the resolution at the meeting.
Our constitution provides that our Board shall have the power, at any time, to appoint any person to be a director either to fill a casual vacancy or as an additional Director, provided the total number of Directors must not at any time exceed the number fixed in accordance with our constitution. Any person so appointed by the Directors shall hold office only until the next annual general meeting and shall then be eligible for re-election.
Our constitution provides that at every annual general meeting (subsequent to our first annual general meeting), one-third of our Directors for the time being, or, if their number is not 3 or a multiple of 3, then the number nearest to one-third, must retire from office. We may from time to time by ordinary resolution passed at a general meeting increase or reduce the number of our Directors, and may also determine in what rotation the increased or reduced number is to go out of office.
General Meetings of Shareholders
Subject to the Singapore Companies Act, we are required to hold an annual general meeting of shareholders within four (4) months after the end of each financial year as a public company that is listed or within six months from the end of our financial year as any other type of company. Our Directors may convene an extraordinary general meeting whenever they think fit and they must do so upon the requisition of two (2) of more shareholders holding not less than 10% of the total number of paid-up shares as of the date of deposit of the requisition carrying the right to vote at a general meeting (disregarding paid-up shares held as treasury shares).
The Singapore Companies Act provides that a shareholder is entitled to attend any general meeting and speak on any resolution put before the general meeting. Unless otherwise required by law or by our constitution, voting on resolutions put forth at general meetings is by ordinary resolution, requiring the affirmative vote of a simple majority of the voting rights of the shareholders present in person or represented by proxy at the meeting and entitled to vote on the resolution. An ordinary resolution suffices, for example, for the appointment of directors. A special resolution, requiring the affirmative vote of not less than three-fourths of the voting rights of the shareholders present in person or represented by proxy at the meeting and entitled to vote on the resolution, is necessary for certain matters under Singapore law, including voluntary winding-up, amendments to our constitution, a change of our corporate name and a reduction in the share capital.
We must give at least 21 days’ notice in writing for every general meeting convened for the purpose of passing a special resolution. General meetings convened for the purpose of passing ordinary resolutions generally require at least 14 days’ notice in writing.
| 160 |
Shareholder Minority Rights
The rights of minority shareholders of Singapore companies are protected under Section 216 of the Singapore Companies Act, which gives the Singapore courts a general power to make any order, upon application by any shareholder of a company, as they think fit to remedy any of the following situations:
| ● | the affairs of a company are being conducted or the powers of the board of directors are being exercised in a manner oppressive to, or in disregard of the interests of, one or more of the shareholders, including the applicant; or |
| ● | a company takes an action, or threatens to take an action, or the shareholders pass a resolution, or propose to pass a resolution, which unfairly discriminates against, or is otherwise prejudicial to, one or more of the shareholders, including the applicant. |
Singapore courts have wide discretion as to the remedies they may grant and the remedies listed in the Singapore Companies Act itself are not exclusive. In general, the Singapore courts may:
| ● | direct or prohibit any act or cancel or vary any transaction or resolution; |
| ● | regulate the conduct of the affairs of the company in the future; |
| ● | authorize civil proceedings to be brought in the name of, or on behalf of, the company by a person or persons and on such terms as the court may direct; |
| ● | provide for the purchase of a minority shareholder’s shares by the other shareholders or by the company and, in the case of a purchase of shares by the company, a corresponding reduction of its share capital; or |
| ● | provide that the company be wound up. |
In addition, Section 216A of the Singapore Companies Act allows a complainant (including a minority shareholder) to apply to the Singapore courts for leave to bring an action in a court proceeding or arbitration in the name of and on behalf of the company or intervene in an action in a court proceeding or arbitration to which a company is a party for the purpose of prosecuting, defending or discontinuing the action or arbitration on behalf of a company.
Liquidation or Other Return of Capital
On a winding-up or other return of capital, subject to any special rights attaching to any other class of shares, holders of ordinary shares will be entitled to participate in any surplus assets in proportion to their shareholdings.
Limitation of Liability of Directors and Officers
Under Section 172 of the Singapore Companies Act, any provision exempting or indemnifying the officers of a company (including directors) against any liability that would otherwise attach to them in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. However, a company is not prohibited from: (a) as provided in Section 172A of the Singapore Companies Act, purchasing and maintaining for any such individual insurance against liability incurred by him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company; or (b) as provided in Section 172B of the Singapore Companies Act, indemnifying the individual against liability incurred by him or her to a person other than the company except when the indemnity is against any liability (i) of the individual to pay a fine in criminal proceedings, (ii) of the individual to pay a penalty to a regulatory authority in respect of non-compliance with any requirements of a regulatory nature (howsoever arising), (iii) incurred by the individual in defending criminal proceedings in which he or she is convicted, (iv) incurred by the individual in defending civil proceedings brought by the company or a related company in which judgment is given against him or her, or (v) incurred by the individual in connection with an application for relief under Section 76A(13) or Section 391 of the Singapore Companies Act in which the court refuses to grant him or her relief.
| 161 |
As permitted by the Singapore Companies Act, our constitution provides that every officer of the Company shall be entitled to be indemnified out of our assets against any liability (other than any liability referred to in Section 172B(1)(a) or (b) of the Singapore Companies Act) incurred by the officer to a person other than us attaching to the officer in connection with any negligence, default, breach of duty or breach of trust.
We intend to enter into indemnification agreements with each of our Directors and officers. We will be obliged under these agreements to indemnify these individuals to the fullest extent permitted under Singapore law and our constitution against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified (on terms that the full amount of such advances is to be repaid if the individual is convicted in the relevant proceeding (with such conviction being final), final judgment is given against the individual in the relevant proceeding or, as the case may be, the court refuses to grant the individual relief on the application (with such refusal of relief being final)), save that the Company shall not provide any indemnity (to any extent) to a director or an officer against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the Company save for the circumstances as permitted pursuant to Section 172A and Section 172B of the Singapore Companies Act. These indemnification rights shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our constitution, agreement, vote of shareholders or disinterested directors or otherwise.
We expect to maintain standard policies of insurance that provide coverage (1) for our Directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act and (2) for us with respect to indemnification payments that we may make to such Directors and officers.
Listing
Our ordinary shares are currently not listed on any securities exchange. We have applied to list our ordinary shares on Nasdaq, under the symbol “GDTC”.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is VStock Transfer, LLC. The transfer agent and registrar’s address is 18 Lafayette Place, Woodmere, New York 11598.
Comparison of Shareholder Rights
We are incorporated under the laws of Singapore. The following discussion summarizes some of the material differences between the rights of holders of our ordinary shares and the rights of holders of the common stock of a typical corporation incorporated under the laws of the state of Delaware which result from differences in governing documents and the laws of Singapore and Delaware.
| 162 |
This discussion is not and does not purport to be a complete or comprehensive statement of the rights of holders of our ordinary shares under applicable legislation and regulations in Singapore and our constitution or the rights of holders of the common stock of a typical corporation under applicable Delaware law and a typical certificate of incorporation and bylaws.
| Delaware | Singapore | |
| Board of Directors | ||
| A typical certificate of incorporation and bylaws would provide that the number of directors on the board of directors will be fixed from time to time by a vote of the majority of the authorized directors. Under Delaware law, a board of directors can be divided into classes and cumulative voting in the election of directors is only permitted if expressly authorized in a corporation’s certificate of incorporation. | The constitution of companies will typically state the minimum number of directors as well as provide that directors may be appointed or removed by shareholders via ordinary resolution passed at a general meeting, provided that the number of directors following such appointment or removal is within the minimum (and maximum, if any) number of directors provided in the constitution. Section 145(1) of the Singapore Companies Act requires that there shall be at least one director who is ordinarily resident in Singapore. | |
| Limitation on Personal Liability of Directors | ||
| A typical certificate of incorporation provides for the elimination of personal monetary liability of directors for breach of fiduciary duties as directors to the fullest extent permissible under the laws of Delaware, except for liability (i) for any breach of a director’s loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the Delaware General Corporation Law (relating to the liability of directors for unlawful payment of a dividend or an unlawful stock purchase or redemption) or (iv) for any transaction from which the director derived an improper personal benefit. A typical certificate of incorporation would also provide that if the Delaware General Corporation Law is amended so as to allow further elimination of, or limitations on, director liability, then the liability of directors will be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law as so amended. | Please refer to the section titled “Limitation of Liability of Directors and Officers”. |
| 163 |
| Interested Shareholders | ||
| Section 203 of the Delaware General Corporation Law generally prohibits a Delaware corporation from engaging in specified corporate transactions (such as mergers, stock and asset sales, and loans) with an “interested stockholder” for three years following the time that the stockholder becomes an interested stockholder. Subject to specified exceptions, an “interested stockholder” is a person or group that owns 15% or more of the corporation’s outstanding voting stock (including any rights to acquire stock pursuant to an option, warrant, agreement, arrangement or understanding, or upon the exercise of conversion or exchange rights, and stock with respect to which the person has voting rights only), or is an affiliate or associate of the corporation and was the owner of 15% or more of the voting stock at any time within the previous three years. | There are no comparable provisions in Singapore with respect to public companies which are not listed on the Singapore Exchange Securities Trading Limited. |
| A Delaware corporation may elect to “opt out” of, and not be governed by, Section 203 through a provision in either its original certificate of incorporation, or an amendment to its original certificate or bylaws that was approved by majority stockholder vote. With a limited exception, this amendment would not become effective until 12 months following its adoption. | ||
| Removal of Directors | ||
| A typical certificate of incorporation and bylaws provide that, subject to the rights of holders of any preferred stock, directors may be removed at any time by the affirmative vote of the holders of at least a majority, or in some instances a supermajority, of the voting power of all of the then outstanding shares entitled to vote generally in the election of directors, voting together as a single class. A certificate of incorporation could also provide that such a right is only exercisable when a director is being removed for cause (removal of a director only for cause is the default rule in the case of a classified board). | According to the Singapore Companies Act, directors of a public company may be removed before expiration of their term of office by ordinary resolution (i.e., a resolution requiring the affirmative vote of a simple majority of those shareholders present and voting in person or by proxy), notwithstanding anything in the company’s constitution or in any agreement between the company and such director. Notice of the intention to move such a resolution has to be given to the company not less than 28 days before the meeting at which it is moved. The company shall then give notice of such resolution to its shareholders not less than 14 days before the meeting. Where any director removed in this manner was appointed to represent the interests of any particular class of shareholders or debenture holders, the resolution to remove such director will not take effect until such director’s successor has been appointed.
According to Section 152(8) of the Singapore Companies Act, a director of a public company shall not be removed by, or be required to vacate his office by reason of any resolution, request or notice of the directors or any of them notwithstanding anything in the constitution or any agreement. |
| 164 |
| Filling Vacancies on the Board of Directors | ||
| A typical certificate of incorporation and bylaws provide that, subject to the rights of the holders of any preferred stock, any vacancy, whether arising through death, resignation, retirement, disqualification, removal, an increase in the number of directors or any other reason, may be filled by a majority vote of the remaining directors, even if such directors remaining in office constitute less than a quorum, or by the sole remaining director. Any newly elected director usually holds office for the remainder of the full term expiring at the annual meeting of stockholders at which the term of the class of directors to which the newly elected director has been elected expires. | Our constitution provides that our Board shall have the power, at any time, and from time to time, to appoint any person to be a director either to fill a casual vacancy or as an additional director but the total number of directors must not at any time exceed the number fixed in accordance with our constitution. Any person so appointed by the directors shall hold office only until the next annual general meeting and shall then be eligible for re-election. | |
Amendment of Governing Documents | ||
| Amendment of Certification of Incorporation and Bylaws | Alteration to Constitution | |
Under the Delaware General Corporation Law, amendments to a corporation’s certificate of incorporation require the approval of stockholders holding a majority of the outstanding shares entitled to vote on the amendment.
If a class vote on the amendment is required by the Delaware General Corporation Law, a majority of the outstanding stock of the class is required, unless a greater proportion is specified in the certificate of incorporation or by other provisions of the Delaware General Corporation Law. Under the Delaware General Corporation Law, the board of directors may amend bylaws if so authorized in the certificate of incorporation. The stockholders of a Delaware corporation also have the power to amend bylaws. | Our constitution may be altered by special resolution (i.e., a resolution requiring the affirmative vote of not less than three-fourths majority of the shareholders present in person or represented by proxy at the meeting and entitled to vote on the resolution for which not less than 21 days written notice is given). Any alteration or addition made to our constitution under Section 26(1) of the Singapore Companies Act shall, subject to the Singapore Companies Act, be deemed to form part of our original constitution on and from the date of the special resolution or such later date as is specified in the resolution. Our Board has no power to amend our constitution without the requisite approval of our shareholders. | |
Meetings of Shareholders | ||
| Annual and Special Meetings | Annual General Meetings | |
Typical bylaws provide that annual meetings of stockholders are to be held on a date and at a time fixed by the board of directors.
Under the Delaware General Corporation Law, a special meeting of stockholders may be called by the board of directors or by any other person authorized to do so in the certificate of incorporation or the bylaws. | According to Section 175(1)(b) of the Singapore Companies Act, we are required to hold an annual general meeting of shareholders within six months from the end of our financial year. | |
| Extraordinary General Meetings | ||
Any general meeting other than the annual general meeting is called an “extraordinary general meeting”. Two or more shareholders holding not less than 10% of the total number of issued shares (excluding treasury shares) may call an extraordinary general meeting. In addition, the constitution usually also provides that general meetings may be convened in accordance with the Singapore Companies Act by the directors. | ||
| Notwithstanding anything in the constitution, according to Section 176(1) of the Singapore Companies Act, the directors are required to convene a general meeting if required to do so by requisition (i.e., written notice to directors requiring that a meeting be called) by two (2) or more shareholders holding not less than 10% of the total number of paid-up shares as at the date of the deposit of the requisition carrying the right of voting at general meetings of the company. In addition, our constitution provides that the directors may, whenever they think fit, convene an extraordinary general meeting. |
| 165 |
| Quorum Requirements | Quorum Requirements | |
Under the Delaware General Corporation Law, a corporation’s certificate of incorporation or bylaws can specify the number of shares which constitute the quorum required to conduct business at a meeting, provided that in no event shall a quorum consist of less than one-third of the shares entitled to vote at a meeting.
| Regulation 51(2) of our constitution provides that save as otherwise provided in our constitution, the quorum at any general meeting shall be two (2) or more members present in person or by proxy or as representing a corporation or limited liability partnership which is a member. | |
| Indemnification of Officers, Directors and Employees | ||
Under the Delaware General Corporation Law, subject to specified limitations in the case of derivative suits brought by a corporation’s stockholders in its name, a corporation may indemnify any person who is made a party to any third-party action, suit or proceeding on account of being a director, officer, employee or agent of the corporation (or was serving at the request of the corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise) against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with the action, suit or proceeding through, among other things, a majority vote of a quorum consisting of directors who were not parties to the suit or proceeding, if the person:
● acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation or, in some circumstances, at least not opposed to its best interests; and
● in a criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Delaware corporate law permits indemnification by a corporation under similar circumstances for expenses (including attorneys’ fees) actually and reasonably incurred by such persons in connection with the defense or settlement of a derivative action or suit, except that no indemnification may be made in respect of any claim, issue or matter as to which the person is adjudged to be liable to the corporation unless the Delaware Court of Chancery or the court in which the action or suit was brought determines upon application that the person is fairly and reasonably entitled to indemnity for the expenses which the court deems to be proper. | Under Section 172 of the Singapore Companies Act, any provision purporting to exempt or indemnify (directly or indirectly) a director against any liability for negligence, default, breach of duty or breach of trust in relation to a company will be void. However, a company is not prohibited from: (a) as provided in Section 172A of the Singapore Companies Act, purchasing and maintaining for any director insurance against any such liability incurred by him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company; or (b) as provided in Section 172B of the Singapore Companies Act, indemnifying a director against liability incurred by him or her to a person other than the company except when the indemnity is against any liability (i) of the director to pay a fine in criminal proceedings, (ii) of the director to pay a penalty to a regulatory authority in respect of non-compliance with any requirements of a regulatory nature (howsoever arising), (iii) incurred by the director in defending criminal proceedings in which he or she is convicted, (iv) incurred by the director in defending civil proceedings brought by the company or a related company in which judgment is given against him or her or (v) incurred by the director in connection with an application for relief under Section 76A(13) or Section 391 of the Singapore Companies Act in which the court refuses to grant him or her relief.
In cases where a director is sued by the company, Section 391(1) of the Singapore Companies Act gives the court the power to relieve directors either wholly or partially from the consequences of their negligence, default, breach of duty or breach of trust. In order for relief to be obtained, it must be shown that (i) the director acted reasonably and honestly; and (ii) it is fair, having regard to all the circumstances of the case including those connected with such director’s appointment, to excuse the director. | |
| 166 |
To the extent a director, officer, employee or agent is successful in the defense of such an action, suit or proceeding, the corporation is required by Delaware corporate law to indemnify such person for reasonable expenses incurred thereby. Expenses (including attorneys’ fees) incurred by such persons in defending any action, suit or proceeding may be paid in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of that person to repay the amount if it is ultimately determined that person is not entitled to be so indemnified.
|
However, Singapore case law has indicated that such relief will not be granted to a director who has benefited as a result of his or her breach of trust.
Please refer to the section titled “Limitation of Liability of Directors and Officers”. | |
| Shareholder Approval of Issuance of Shares | ||
| Under Delaware law, the board of directors has the authority to issue, from time to time, capital stock in its sole discretion, as long as the number of shares to be issued, together with those shares that are already issued and outstanding and those shares reserved to be issued, do not exceed the authorized capital for the corporation as previously approved by the stockholders and set forth in the corporation’s certificate of incorporation. Under the foregoing circumstances, no additional stockholder approval is required for the issuance of capital stock. Under Delaware law, stockholder approval is required for (i) any amendment to the corporation’s certificate of incorporation to increase the authorized capital and (ii) the issuance of stock in a direct merger transaction where the number of shares exceeds 20% of the corporation’s shares outstanding prior to the transaction, regardless of whether there is sufficient authorized capital. | Section 161 of the Singapore Companies Act provides that notwithstanding anything in the company’s constitution, the directors shall not exercise any power of the company to issue shares without prior approval of the shareholders in a general meeting. Such authorization may be obtained by ordinary resolution (i.e., a resolution requiring the affirmative vote of a simple majority of the voting rights of those shareholders present and voting in person or by proxy). Such authorization may be confined to a particular exercise of that power or may apply to the exercise of that power generally, and any such approval may be unconditional or subject to conditions. Once this shareholders’ approval is obtained, unless previously revoked or varied by the company in a general meeting, it continues in force until the conclusion of the next annual general meeting or the expiration of the period within which the next annual general meeting after that date is required by law to be held, whichever is earlier; but any approval may be revoked or varied by the company in a general meeting. |
| 167 |
| In addition, a corporation may issue one or more classes of stock or one or more series of stock within any class as shall be stated and expressed in the certificate of incorporation or of any amendment thereto, or in the resolution or resolutions providing for the issue of such stock adopted by the board of directors pursuant to authority expressly vested in it by the provisions of its certificate of incorporation. | ||
| Any stock of any class or of any series thereof may be made convertible into, or exchangeable for, at the option of either the holder or the corporation or upon the happening of a specified event, shares of any other class or classes or any other series of the same or any other class or classes of stock of the corporation, at such price or prices or at such rate or rates of exchange and with such adjustments as shall be stated in the certificate of incorporation or in the resolution or resolutions providing for the issue of such stock adopted by the board of directors. |
| Shareholder Approval of Business Combinations | ||
Generally, under the Delaware General Corporation Law, completion of a merger, consolidation, or the sale, lease or exchange of substantially all of a corporation’s assets or dissolution requires approval by the board of directors and by a majority (unless the certificate of incorporation requires a higher percentage) of outstanding stock of the corporation entitled to vote.
The Delaware General Corporation Law also requires a special vote of stockholders in connection with a business combination with an “interested stockholder” as defined in section 203 of the Delaware General Corporation Law. Please refer to the section titled “Interested Shareholders” above. | The Singapore Companies Act mandates that specified corporate actions require approval by the shareholders in a general meeting, notably:
● notwithstanding anything in the company’s constitution, directors are not permitted to carry into effect any proposals for disposing of the whole or substantially the whole of the company’s undertaking or property unless those proposals have been approved by shareholders in a general meeting;
● subject to the constitution of each amalgamating company, an amalgamation proposal in accordance with the full amalgamation procedures under the Singapore Companies Act that do not require a court order must be approved by the shareholders of each amalgamating company via special resolution at a general meeting and by any person, where any provision in the amalgamation proposal would, if contained in any amendment to the constitution of an amalgamating company or otherwise proposed in relation to that company, require the approval of that person; and
● notwithstanding anything in the company’s constitution, the directors may not, without the prior approval of shareholders in a general meeting, issue shares, including shares being issued in connection with corporate actions. | |
| Shareholder Action Without A Meeting | ||
| Under the Delaware General Corporation Law, unless otherwise provided in a corporation’s certificate of incorporation, any action that may be taken at a meeting of stockholders may be taken without a meeting, without prior notice and without a vote if the holders of outstanding stock, having not less than the minimum number of votes that would be necessary to authorize such action, consent in writing. It is not uncommon for a corporation’s certificate of incorporation to prohibit such action. | Shareholder action by written consent is not permitted for a public listed company. | |
| Shareholder Suits | ||
| Under the Delaware General Corporation Law, a stockholder may bring a derivative action on behalf of the corporation to enforce the rights of the corporation. An individual also may commence a class action suit on behalf of himself or herself and other similarly situated stockholders where the requirements for maintaining a class action under the Delaware General Corporation Law have been met. A person may institute and maintain such a suit only if such person was a stockholder at the time of the transaction which is the subject of the suit or his or her shares thereafter devolved upon him or her by operation of law. Additionally, under Delaware case law, the plaintiff generally must be a stockholder not only at the time of the transaction which is the subject of the suit, but also through the duration of the derivative suit. The Delaware General Corporation Law also requires that the derivative plaintiff make a demand on the directors of the corporation to assert the corporate claim before the suit may be prosecuted by the derivative plaintiff, unless such demand would be futile. | Derivative Actions Section 216A(2) of the Singapore Companies Act has a provision which provides a mechanism enabling shareholders to apply to the court for leave to bring a derivative action or commence an arbitration in the name of and on behalf of the company or intervene in an action or arbitration to which the company is a party to for the purpose of prosecuting, defending or discontinuing the action or arbitration on behalf of the company.
Applications are generally made by shareholders of the company, but courts are given the discretion to allow such persons as they deem proper to apply (e.g., beneficial owner of shares).
It should be noted that this provision of the Singapore Companies Act is primarily used by minority shareholders to bring an action or arbitration in the name and on behalf of the company or intervene in an action or arbitration to which the company is a party for the purpose of prosecuting, defending or discontinuing the action on behalf of the company. |
Class Actions The concept of class action suits, which allows individual shareholders to bring an action seeking to represent a class or classes of shareholders, does not exist in Singapore. However, it is possible as a matter of procedure for a number of shareholders to lead an action and establish liability on behalf of themselves and other shareholders who join in or who are made parties to the action. These shareholders are commonly known as “lead plaintiffs”. |
| 168 |
| Distributions and Dividends; Repurchases and Redemptions | ||
| The Delaware General Corporation Law permits a corporation to declare and pay dividends out of statutory surplus or, if there is no surplus, out of net profits for the fiscal year in which the dividend is declared and/or for the preceding fiscal year as long as the amount of capital of the corporation following the declaration and payment of the dividend is not less than the aggregate amount of the capital represented by the issued and outstanding stock of all classes having a preference upon the distribution of assets. | The Singapore Companies Act provides that no dividends can be paid to shareholders except out of profits.
The Singapore Companies Act does not provide a definition on when profits are deemed to be available for the purpose of paying dividends and this is accordingly governed by case law.
Our constitution provides that no dividend can be paid otherwise than out of profits.
| |
According to Section 76C(1) of the Singapore Companies Act, a company, whether or not it is listed on an approved exchange in Singapore or any securities exchange outside Singapore, make an off-market purchase of its own shares in accordance with an equal access scheme authorized in advance at a general meeting.
According to Section 76D of the Singapore Companies Act, a company may make a selective off-market purchase of its own shares in accordance with an agreement authorized in advance at a general meeting by a special resolution where persons whose shares are to be acquired and their associated persons have abstained from voting.
According to Section 76DA(1) of the Singapore Companies Act, a company may, whether or not it is listed on an approved exchange in Singapore or any securities exchange outside Singapore, make an acquisition of its own shares under a contingent purchase contract which has been authorized in advance at a general meeting by a special resolution.
According to Section 76E of the Singapore Companies Act, a company, where it is listed on a securities exchange, may make an acquisition of its own shares on the securities exchange, in accordance with terms and limits authorized in advance at a general meeting. | ||
| 169 |
A company may also purchase its own shares by an order of a Singapore court.
The total number of ordinary shares that may be acquired by a company during a relevant period may not exceed 20% (or such other prescribed percentage) of the total number of ordinary shares as of the date of the resolution passed to authorize the acquisition of the shares. Where, however, a company has reduced its share capital by a special resolution or a Singapore court has made an order confirming the reduction of share capital of the company, the total number of ordinary shares shall be taken to be the total number of ordinary shares as altered by the special resolution or the order of the court. Payment, including any expenses (including brokerage or commission) incurred directly in the acquisition by the company of its own shares, may be made out of the company’s distributable profits or capital, provided that the company is solvent.
| ||
Financial Assistance for the Acquisition of Shares A public company or a company whose holding company or ultimate holding company is a public company shall not give financial assistance to any person whether directly or indirectly for the purpose of or in connection with: ● the acquisition or proposed acquisition of shares in the company or units of such shares; or ● the acquisition or proposed acquisition of shares in its holding company or ultimate holding company, or units of such shares.
Financial assistance may take the form of a loan, the giving of a guarantee, the provision of security, the release of an obligation, the release of a debt or otherwise.
However, it should be noted that a company may provide financial assistance for the acquisition of its shares or shares in its holding company if it complies with the requirements (including approval by special resolution) set out in the Singapore Companies Act. |
| 170 |
| Transactions with Officers or Directors | ||
| Under the Delaware General Corporation Law, some contracts or transactions in which one or more of a corporation’s directors has an interest are not void or voidable because of such interest provided that some conditions, such as obtaining the required approval and fulfilling the requirements of good faith and full disclosure, are met. Under the Delaware General Corporation Law, either (a) the stockholders or the board of directors must approve in good faith any such contract or transaction after full disclosure of the material facts or (b) the contract or transaction must have been “fair” as to the corporation at the time it was approved. If board approval is sought, the contract or transaction must be approved in good faith by a majority of disinterested directors after full disclosure of material facts, even though less than a majority of a quorum. | Under Section 156 of the Singapore Companies Act, directors and chief executive officers are not prohibited from dealing with the company, but where they have an interest in a transaction with the company, that interest must be disclosed to the board of directors. In particular, every director or chief executive officer who is in any way, whether directly or indirectly, interested in a transaction or proposed transaction with the company must, as soon as is practicable after the relevant facts have come to such director’s or chief executive officer’s knowledge, declare the nature of such director’s or chief executive officer’s interest at a board of directors’ meeting or send a written notice to the company containing details on the nature, character and extent of his or her interest in the transaction or proposed transaction with the company.
There is, however, no requirement for disclosure where the interest of the director or chief executive officer (as the case may be) consists only of being a member or creditor of a corporation which is interested in the transaction or proposed transaction with the company if the interest may properly be regarded as immaterial. Where the transaction or the proposed transaction relates to any loan to the company, a director or chief executive officer shall not be deemed to be interested or to have been at any time interested in the transaction or proposed transaction where the director or chief executive officer has only guaranteed or joined in guaranteeing the repayment of such loan, unless the constitution provides otherwise.
Further, where the transaction or the proposed transaction has been or will be made with or for the benefit of a related corporation (i.e. the holding company, subsidiary or subsidiary of a common holding company), a director or chief executive officer shall not be deemed to be interested or to have been at any time interested in the transaction or proposed transaction where he is a director or chief executive officer (as the case may be) of that corporation, unless the constitution provides otherwise.
In addition, a director or chief executive officer who holds any office or possesses any property which directly or indirectly might create duties or interests in conflict with such director’s or chief executive officer’s duties or interests as director or chief executive officer (as the case may be) is required to declare the fact and the nature, character and extent of the conflict at a meeting of directors or send a written notice to the company setting out the fact and the nature, character and extent of the conflict.
The Singapore Companies Act extends the scope of this statutory duty of a director and chief executive officer to disclose any interests by pronouncing that an interest of a member of a director’s or chief executive officer’s (as the case may be) family (including spouse, son, adopted son, step-son, daughter, adopted daughter and step-daughter) will be treated as an interest of the director or chief executive officer (as the case may be). |
| 171 |
Subject to specified exceptions, the Singapore Companies Act prohibits a company from making a loan or quasi-loan to its directors or to directors of a related corporation, or giving a guarantee or security in connection with such a loan or quasi-loan.
Companies are also prohibited from making loans or quasi-loans to its directors’ spouse or children (whether adopted or natural or step-children), or giving a guarantee or security in connection with such a loan or quasi-loan. | ||
| Dissenters’ Rights | ||
Under the Delaware General Corporation Law, a stockholder of a corporation participating in some types of major corporate transactions may, under varying circumstances, be entitled to appraisal rights pursuant to which the stockholder may receive cash in the amount of the fair market value of his or her shares in lieu of the consideration he or she would otherwise receive in the transaction.
| There is no equivalent provision in Singapore under the Singapore Companies Act. | |
| Cumulative Voting | ||
Under the Delaware General Corporation Law, a corporation may adopt in its bylaws that its directors shall be elected by cumulative voting. When directors are elected by cumulative voting, a stockholder has the number of votes equal to the number of shares held by such stockholder times the number of directors nominated for election. The stockholder may cast all of such votes for one director or among the directors in any proportion.
| There is no equivalent provision under the Singapore Companies Act in respect of companies incorporated in Singapore. | |
| Anti-Takeover Measures | ||
Under the Delaware General Corporation Law, the certificate of incorporation of a corporation may give the board the right to issue new classes of preferred stock with voting, conversion, dividend distribution, and other rights to be determined by the board at the time of issuance, which could prevent a takeover attempt and thereby preclude shareholders from realizing a potential premium over the market value of their shares.
In addition, Delaware law does not prohibit a corporation from adopting a stockholder rights plan, or “poison pill”, which could prevent a takeover attempt and also preclude shareholders from realizing a potential premium over the market value of their shares. | The constitution of a Singapore-incorporated company typically provides that the company may allot and issue new shares of a different class with preferential, deferred, qualified or other special rights as its board of directors may determine with the prior approval of the company’s shareholders in a general meeting.
Under the Singapore Take-over Code, if, in the course of an offer, or even before the date of the announcement of the offer, the board of the offeree company has reason to believe that a bona fide offer is imminent, the board must not, except pursuant to a contract entered into earlier, take any action, without the approval of shareholders at a general meeting, on the affairs of the offeree company that could effectively result in any bona fide offer being frustrated or the shareholders being denied an opportunity to decide on its merits. |
| 172 |
SHARES ELIGIBLE FOR FUTURE SALE
Upon consummation of this offering, we will have issued and outstanding 11,468,642 ordinary shares, (or, 11,830,497 if the underwriters’ exercise in full their option to purchase additional shares to cover over-allotments), and assuming conversion of all outstanding convertible loans into ordinary shares. Of the ordinary shares to be issued and outstanding immediately after the closing of this offering, the 2,412,369 ordinary shares to be sold in this offering (plus 361,855 ordinary shares assuming full exercise of the underwriters’ option to purchase additional shares) and an additional 1,337,631 ordinary shares, will be freely tradable without restriction under the Securities Act by persons other than our “affiliates”, as that term is defined in Rule 144 under the Securities Act. The remaining ordinary shares are “restricted securities” under Rule 144. A substantial portion of these restricted securities will be subject to the provisions of the lock-up agreements referred to below.
Sales of substantial amounts of the ordinary shares in the public market could adversely affect prevailing market prices of the ordinary shares. Prior to this offering, there has been no public market for our ordinary shares in the United States. Although we have applied to list the ordinary shares on the Nasdaq, we cannot assure you that a regular trading market will develop in the ordinary shares in the United States. Please refer to the section titled “Risk Factors — Risks Relating to our Ordinary Shares and this Offering”. We do not know whether a market for our ordinary shares will develop to provide you with adequate liquidity. If our share price fluctuates after this offering, you could lose a significant part of your investment. Furthermore, since no shares will be available for sale from certain of our shareholders shortly after this offering because of the contractual and legal restrictions on resale described below, sales of substantial numbers of ordinary shares in the public market after these restrictions lapse could adversely affect the prevailing market price and our ability to raise equity capital in the future.
After the expiration of any lock-up period, these restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under the Securities Act, which exemptions are summarized below.
Rule 144
Under Rule 144 under the Securities Act, a person (or persons whose shares are aggregated) (1) who is not considered to have been one of our affiliates at any time during the 90 days preceding a sale and (2) who has beneficially owned the shares proposed to be sold for at least six months, including, in certain cases, the holding period of any prior owner other than an affiliate is entitled to sell his or her shares without restriction, subject to the Company’s compliance with the reporting obligations under the Exchange Act.
In general, under Rule 144, beginning 90 days after the date of this Prospectus, a person who is our affiliate and has beneficially owned ordinary shares for at least six months is entitled to sell within any three-month period a number of shares that does not exceed the greater of (1) 1.0% of the number of ordinary shares then issued and outstanding, which is expected to be equal to approximately 114,686 ordinary shares immediately after the consummation of this offering and (2) the average weekly trading volume of the ordinary shares on the Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 in connection with the sale.
Any such sales by an affiliate are also subject to manner of sale provisions, notice requirements and our compliance with Exchange Act reporting obligations.
Rule 701
In general, under Rule 701, a person who purchased shares of our ordinary shares pursuant to a written compensatory plan or contract and who is not deemed to have been one of our affiliates during the immediately preceding 90 days may sell these shares in reliance upon Rule 144, but without being required to comply with the notice, manner of sale, public information requirements or volume limitation provisions of Rule 144. Rule 701 also permits affiliates to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701. None of our shares of our outstanding ordinary shares have been issued in reliance on Rule 701 as a result of exercises of stock options and issuance of restricted shares.
Regulation S
Regulation S under the Securities Act provides that shares owned by any person may be sold without registration in the United States, provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that the Company’s shares may be sold in some other manner outside the United States without requiring registration in the United States.
Lock-up Agreements
We, have agreed with the underwriters, subject to certain exceptions, not to offer, pledge, sell, or dispose of, directly or indirectly, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares during the 6-month period following the date of this Prospectus.
Members of our Board, our executive officers and all shareholders beneficially owning more than 5% of our outstanding ordinary shares (other than with respect to 416,666 ordinary shares), subject to certain exceptions, as of the effective date of this Prospectus have agreed during the 12-month period following the date of this Prospectus to substantially similar lock-up provisions, subject to certain exceptions. Please refer to the section titled “Underwriting” for more information.
Registration Statements on Form S-8
We intend to file one or more registration statements on Form S-8 under the Securities Act with the SEC to register the offer and sale of shares of our Common Stock that are issuable under our Incentive Plan. These registration statements will become effective immediately on filing. Shares covered by these registration statements will then be eligible for sale in the public markets, subject to vesting restrictions, any applicable lock-up agreements and market standoff provisions described below, and Rule 144 limitations applicable to affiliates.
Registration Rights
The Company has not granted registration rights to any party.
| 173 |
The following are material Singaporean and U.S. federal income tax considerations relevant to an investment in our ordinary shares. This discussion does not address all of the tax consequences that may be relevant in light of the investor’s particular circumstances. Potential investors should consult their tax advisers regarding the Singaporean, U.S. federal, state and local, and non-U.S. tax consequences of owning and disposing of our ordinary shares in their particular circumstances.
Material Singaporean Tax Considerations
The statements made herein regarding taxation are general in nature and based on certain aspects of current tax laws of Singapore and administrative guidelines issued by the relevant authorities in force as of the date of this Prospectus and are subject to any changes in such laws or administrative guidelines, or in the interpretation of these laws or guidelines, occurring after such date, which changes could be made on a retrospective basis. These laws and guidelines are also subject to various interpretations and the relevant tax authorities or the courts could later disagree with the explanations or conclusions set out below. The statements below are not to be regarded as advice on the tax position of any holder of our ordinary shares or of any person acquiring, selling or otherwise dealing with our ordinary shares or on any tax implications arising from the acquisition, sale or other dealings in respect of our ordinary shares. The statements made herein do not purport to be a comprehensive or exhaustive description of all of the tax considerations that may be relevant to a decision to acquire, own or dispose of our ordinary shares and do not purport to deal with the tax consequences applicable to all categories of investors, some of which (such as dealers in securities) may be subject to special rules. Prospective holders of our ordinary shares are advised to consult their own tax advisers as to the Singapore or other tax consequences of the acquisition, ownership of or disposal of our ordinary shares. The statements below regarding the Singapore tax treatment of dividends received in respect of our ordinary shares are based on the assumption that the Company is tax resident in Singapore for Singapore income tax purposes. It is emphasized that neither the Company nor any other persons involved in this Prospectus accepts responsibility for any tax effects or liabilities resulting from the subscription for, acquisition, holding or disposal of our ordinary shares.
Individual Income Tax
An individual is a tax resident in Singapore in a year of assessment (“YA”) if, in the year preceding the YA, he was physically present in Singapore or exercised an employment in Singapore (other than as a director of a company) for 183 days or more, or if he resides in Singapore except for such temporary absences therefrom as may be reasonable and not inconsistent with a claim by such individual to be resident in Singapore.
Individual taxpayers who are Singapore tax residents are subject to Singapore income tax on income accruing in or derived from Singapore. All foreign-sourced income received in Singapore on or after January 1, 2004 by a Singapore tax resident individual (except for income received through a partnership in Singapore) is exempt from Singapore income tax if the Comptroller is satisfied that the tax exemption would be beneficial to the individual. Currently, a Singapore tax resident individual is taxed at progressive rates ranging from 0% to 22%. The maximum tax rate will be increased to 24% with effect from the year of assessment 2024.
Non-resident individuals, subject to certain exceptions and conditions, are subject to Singapore income tax on income accruing in or derived from Singapore at the rate of 22%, and 24% with effect from the year of assessment 2024.
Corporate Income Tax
A corporate taxpayer is regarded as resident in Singapore for Singapore tax purposes if the control and management of its business is exercised in Singapore.
| 174 |
Corporate taxpayers who are Singapore tax residents are subject to Singapore income tax on income accruing in or derived from Singapore and, subject to certain exceptions, on foreign-sourced income received or deemed received in Singapore. Foreign-sourced income in the form of dividends, branch profits and service income received or deemed received in Singapore by Singapore tax resident companies on or after June 1, 2003 are exempt from tax if certain prescribed conditions are met, including the following:
| (i) | such income is subject to tax of a similar character to income tax under the law of the jurisdiction from which such income is received; | |
| (ii) | at the time the income is received in Singapore, the highest rate of tax of a similar character to income tax (by whatever name called) levied under the law of the territory from which the income is received on any gains or profits from any trade or business carried on by any company in that territory at that time is not less than 15%; and | |
| (iii) | the Comptroller is satisfied that the tax exemption would be beneficial to the person resident in Singapore. |
Certain concessions and clarifications have also been announced by IRAS with respect to such conditions.
A non-resident corporate taxpayer is subject to income tax on income that is accrued in or derived from Singapore, and on foreign-sourced income received or deemed received in Singapore, subject to certain exceptions.
The corporate tax rate in Singapore is currently 17%. Preferential tax rate(s) may be available under certain tax incentives / schemes. In addition, three-quarters of up to the first S$10,000 of a company’s annual normal chargeable income, and one-half of up to the next S$190,000, is exempt from corporate tax from the YA 2020 onwards. The remaining chargeable income (after the tax exemption) will be fully taxable at the prevailing corporate tax rate.
New start-up companies will also, subject to certain conditions and exceptions, be eligible for tax exemption on three-quarters of up to the first S$100,000 of a company’s annual normal chargeable income, and one-half of up to the next S$100,000, a year for each of the company’s first three YAs from YA 2020 onwards. The remaining chargeable income (after the tax exemption) will be taxed at the applicable corporate tax rate.
Dividend Distributions
All Singapore-resident companies are currently under the one-tier system.
Dividends received in respect of our ordinary shares by either a resident or non-resident of Singapore are not subject to Singapore withholding tax, on the basis that we are a tax resident of Singapore and under the one-tier system.
Under the one-tier system, the tax on corporate profits is final and dividends paid by a Singapore resident company are tax exempt in the hands of a shareholder, regardless of whether the shareholder is a company or an individual and whether or not the shareholder is a Singapore tax resident.
Gains on Disposal of our Ordinary Shares
Singapore does not impose tax on capital gains. There are no specific laws or regulations which deal with the characterization of whether a gain is income or capital in nature. Gains arising from the disposal of our ordinary shares may be construed to be of an income nature and subject to Singapore income tax, especially if they arise from or are otherwise connected with the activities which the IRAS regards as carrying on a trade or business in Singapore.
| 175 |
Holders of our ordinary shares who apply, or who are required to apply, the FRS 39, FRS 109 or SFRS(I) 9 (as the case may be) may for the purposes of Singapore income tax be required to recognize gains or losses (not being gains or losses in the nature of capital) in accordance with the provisions of FRS 39, FRS 109 or SFRS(I) 9 (as modified by the applicable provisions of Singapore income tax law) even though no sale or disposal of our ordinary shares is made.
Holders of our ordinary shares who may be subject to this tax treatment should consult their accounting and tax advisers regarding the Singapore income tax consequences of their acquisition, holding and disposal of our ordinary shares.
Stamp Duty
There is no stamp duty payable on the subscription and issuance for our ordinary shares.
Where our ordinary shares evidenced in certificated form are acquired in Singapore, stamp duty is payable on the instrument of their transfer at the rate of 0.2% of the consideration for, or market value of, our ordinary shares, whichever is higher, and is rounded down to the nearest dollar, subject to a minimum duty of S$1.
Stamp duty is borne by the purchaser unless there is an agreement to the contrary. Where an instrument of transfer is executed outside Singapore or no instrument of transfer is executed, no stamp duty is generally payable on the acquisition of our ordinary shares. However, stamp duty may be payable if the instrument of transfer is executed outside Singapore and is received in Singapore.
If the instrument of transfer has been executed, it has to be stamped within (a) 14 days after signing the document if it is executed in Singapore; or (b) within 30 days after receiving the document in Singapore if the document is executed outside Singapore.
Pursuant to recent amendments to the Stamp Duties Act, stamp duty is payable on certain electronic instruments that effect a transfer of interest in our ordinary shares, where such instruments are regarded or deemed to be executed in Singapore, or executed outside Singapore and received in Singapore. In this regard, an electronic instrument that is executed outside Singapore is received in Singapore if (a) it is retrieved or accessed by a person in Singapore; (b) an electronic copy of it is stored on a device (including a computer) and brought into Singapore; or (c) an electronic copy of it is stored on a computer in Singapore.
On the basis that any transfer instruments in respect of any interests in our ordinary shares (whether traded on Nasdaq or JSE) are executed outside Singapore through the transfer agent(s), share registrar(s) and/or administrative depositary agent(s) in the United States and/or South Africa for registration in our share register(s) and/or administrative depositary register(s) (including branch register(s) of members) maintained in the United States and/or South Africa respectively, no stamp duty should be payable in Singapore on such transfers to the extent that the instruments of transfer (including electronic instruments) are not received in Singapore and all electronic records and any information relating to such transfers are not electronically received by persons in Singapore, stored on any server or device in Singapore or made accessible to any person in Singapore.
Estate Duty
Singapore estate duty was abolished with respect to all deaths occurring on or after February 15, 2008.
| 176 |
GST
The issuance of our ordinary shares in connection with this offering is not subject to GST.
The sale of our ordinary shares by a GST-registered investor belonging in Singapore for GST purposes to another person belonging in Singapore is an exempt supply not subject to GST. Any input GST incurred by the GST-registered investor in making this exempt supply is generally not recoverable from the Singapore Comptroller of GST.
Where our ordinary shares are sold by a GST-registered investor in the course of or furtherance of a business carried on by such investor contractually to and for the direct benefit of a person belonging outside Singapore, the sale should generally, subject to satisfaction of certain conditions, be considered a taxable supply subject to GST at 0%. Any input GST incurred by the GST-registered investor in making such a supply in the course of or furtherance of a business may be fully recoverable from the Singapore Comptroller of GST. Investors should seek their own tax advice on the recoverability of GST incurred on expenses in connection with the purchase and sale of our ordinary shares.
Services consisting of arranging, brokering, underwriting or advising on the issue, allotment or transfer of ownership of our ordinary shares rendered by a GST-registered person to an investor belonging in Singapore for GST purposes in connection with the investor’s purchase, sale or holding of our ordinary shares will be subject to GST at the standard rate of 8.0%. Similar services rendered by a GST registered person contractually to and for the direct benefit of an investor belonging outside Singapore should generally, subject to the satisfaction of certain conditions, be subject to GST at 0%.
Material U.S. Federal Income Tax Considerations
The following are material U.S. federal income tax consequences to the “U.S. Holders” described below of owning and disposing of ordinary shares, but this discussion does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a particular person’s decision to acquire ordinary shares.
This discussion applies only to a U.S. Holder that acquires ordinary shares in this offering and holds the ordinary shares as capital assets for U.S. federal income tax purposes. In addition, it does not describe all of the tax consequences that may be relevant in light of a U.S. Holder’s particular circumstances, including any alternative minimum tax or Medicare contribution tax considerations, or consequences applicable to U.S. Holders subject to special rules, such as:
| ● | certain financial institutions; |
| ● | insurance companies, regulated investment companies, real estate investment trusts and broker-dealers; |
| ● | dealers or traders in securities that use a mark-to-market method of tax accounting; |
| ● | entities subject to the “applicable financial statement” accounting rules under Section 451(b) of the Code; |
| ● | persons holding ordinary shares as part of a straddle, integrated or similar transaction; |
| ● | persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar; |
| ● | entities classified as partnerships for U.S. federal income tax purposes and their partners; |
| ● | tax-exempt entities, “Individual Retirement Accounts” or “Roth IRAs”; |
| ● | persons that own or are deemed to own 10% or more of our stock by voting power or value; |
| ● | persons whose “tax residence” is outside the United States; or |
| ● | persons holding ordinary shares in connection with a trade or business outside the United States. |
| 177 |
If a partnership (or other entity that is classified as a partnership for U.S. federal income tax purposes) owns ordinary shares, the U.S. federal income tax treatment of a partner will generally depend on the status of the partner and the activities of the partnership. Partnerships that intend to own ordinary shares and their partners should consult their tax advisers as to their particular U.S. federal income tax consequences of owning and disposing of ordinary shares.
This discussion is based on the Code, administrative pronouncements, judicial decisions, and final, temporary and proposed United States Treasury regulations, all as of the date hereof, any of which is subject to change, possibly with retroactive effect.
This discussion does not address the effects of any state, local or non-U.S. tax laws, or any U.S. federal tax laws other than income tax laws (such as U.S. federal estate or gift tax laws). U.S. Holders should consult their tax advisers concerning the U.S. federal, state, local and non-U.S. tax consequences of owning and disposing of ordinary shares in their particular circumstances.
Except as described below under the section titled “Passive Foreign Investment Company Rules”, this discussion assumes that we are not, and will not be, a PFIC for any taxable year.
Taxation of Distributions
Subject to the PFIC discussion below, distributions paid on our ordinary shares, other than certain pro rata distributions of ordinary shares, will be treated as dividends to the extent paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Because we do not maintain calculations of our earnings and profits under U.S. federal income tax principles, U.S. Holders generally should expect that all distributions will be treated as dividends. Dividends will not be eligible for the dividends-received deduction generally available to U.S. corporations under the Code. Subject to applicable limitations, dividends paid by “qualified foreign corporations” to certain non-corporate U.S. investors are taxable at a preferential rate applicable to long-term capital gains. For example, one of the applicable limitations is that in order for a dividend paid by a “qualified foreign corporation” to be eligible for the preferential rate, it must be paid with respect to shares that the non-corporate U.S. investor has owned for more than 60 days during the 121-day period beginning 60 days before the date on which the shares become ex-dividend. A non-U.S. corporation is treated as a qualified foreign corporation with respect to dividends paid on stock that is readily tradable on certain U.S. securities markets, such as the Nasdaq, where the ordinary shares offered in this offering will be listed. The preferential rate does not apply if the non-U.S. corporation is a PFIC for the year the dividend is paid or the preceding year. Non-corporate U.S. Holders should consult their tax advisers regarding the availability of the preferential rate and any limitations that may apply in their particular circumstances.
Dividends will be included in a U.S. Holder’s income on the date of receipt. The amount of any dividend income paid in a currency other than the U.S. dollar will be the U.S. dollar amount calculated by reference to the spot rate in effect on the date of receipt, regardless of whether the payment is in fact converted into U.S. dollars on such date. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder generally should not be required to recognize foreign currency gain or loss in respect of the amount received. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. Dividends will be treated as foreign-source income for foreign tax credit purposes, which may be relevant to U.S. Holders in calculating their foreign tax credit limitation. Foreign currency gain or loss generally will be treated as U.S.-source income or loss for foreign tax credit purposes.
| 178 |
Sale or Other Taxable Disposition of Ordinary Shares
Subject to the PFIC discussion below, a U.S. Holder will generally recognize capital gain or loss on a sale or other taxable disposition of ordinary shares, which will be long-term capital gain or loss if, at the time of the sale or disposition, the U.S. Holder has owned the ordinary shares for more than one year. The amount of gain or loss will equal the difference between the amount realized on the sale or disposition and the U.S. Holder’s tax basis in the ordinary shares disposed of, in each case as determined in U.S. dollars. A U.S. Holder’s gain or loss will generally be treated as U.S.-source income or loss for foreign tax credit purposes. U.S. Holders that sell ordinary shares for an amount denominated in a non-U.S. currency should consult their tax advisers regarding the exchange rate at which the amount received should be translated to U.S. dollars, and whether any U.S.-source foreign currency gain or loss may be required to be recognized as a result of the sale. Long-term capital gains recognized by non-corporate U.S. Holders are taxed at a rate that is lower than the rate applicable to ordinary income. The deductibility of capital losses is subject to limitations.
Passive Foreign Investment Company Rules
In general, a non-U.S. corporation is a PFIC for U.S. federal income tax purposes for any taxable year in which (i) 50% or more of the value of its assets (generally determined based on the average of the quarterly values of its gross assets) consists of assets that produce, or are held for the production of, passive income, or (ii) 75% or more of its gross income consists of passive income. For purposes of the above calculations, a non-U.S. corporation that owns, directly or indirectly, at least 25% by value of the shares of another corporation is treated as if it held its proportionate share of the assets of the other corporation and received directly its proportionate share of the income of the other corporation. Passive income generally includes dividends, interest, rents, royalties and gains from the sale or exchange of investment property. Cash is a passive asset for these purposes. Goodwill is generally characterized as an active asset if it is associated with business activities that produce active income.
Based on the current and expected composition of our income and assets and the value of our assets, including the estimated value of our goodwill, we do not expect to be a PFIC for our current taxable year or in the foreseeable future. However, our PFIC status for any taxable year is an annual determination that can be made only after the end of that year, and will depend on the composition of our income and assets and the value of our assets from time to time (including the value of our goodwill, which may be determined in part by reference to the market price of the ordinary shares, which could be volatile). We will hold a substantial amount of cash following this offering and our PFIC status for any taxable year may also depend on how, and how quickly, we use our liquid assets and cash (including cash raised in this offering). Because the value of our goodwill may be determined by reference to our market capitalization, we could become a PFIC for any taxable year if the price of our ordinary shares declines significantly while we hold a substantial amount of cash and financial investments. In addition, the application of the PFIC rules is subject to a number of uncertainties and the proper characterization of some of our income and assets is not entirely clear. Accordingly, there can be no assurance that we will not be a PFIC for our current or any future taxable year.
If we were a PFIC for any taxable year and any entity in which we own equity interests were also a PFIC (a “Lower-tier PFIC”), U.S. Holders would be deemed to own a proportionate amount (by value) of the shares of each Lower-tier PFIC and would be subject to U.S. federal income tax according to the rules described in the next paragraph on (i) certain distributions by the Lower-tier PFIC and (ii) dispositions of shares of the Lower-tier PFIC, in each case as if the U.S. Holders held such shares directly, even though the U.S. Holder would not receive any proceeds of those distributions or dispositions.
In general, if we were a PFIC for any taxable year during which a U.S. Holder held ordinary shares, gain recognized by such U.S. Holder on a sale or other disposition (including certain pledges) of its ordinary shares would be allocated ratably over its holding period. The amounts allocated to the taxable year of the sale or disposition and to any year before we became a PFIC with respect to such U.S. Holder would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the resulting tax liability for each such year. Furthermore, to the extent that distributions received by a U.S. Holder in any year on its ordinary shares exceeded 125% of the average of the annual distributions on the ordinary shares received during the preceding three years or the U.S. Holder’s holding period, whichever is shorter, such distributions would be subject to taxation in the same manner. If we were a PFIC for any taxable year during which a U.S. Holder owned ordinary shares, we would generally continue to be treated as a PFIC with respect to the U.S. Holder for all succeeding years during which the U.S. Holder owned the ordinary shares, even if we ceased to meet the threshold requirements for PFIC status. Certain elections may be available that would result in alternative treatments (such as mark-to-market treatment) of the ordinary shares. U.S. Holders should consult their tax advisers to determine whether any of these elections would be available, and, if so, what the consequences of the alternative treatments would be in their particular circumstances.
| 179 |
If we were a PFIC (or with respect to a particular U.S. Holder were treated as a PFIC) for a taxable year in which we paid a dividend or for the prior taxable year, the preferential tax rate described above with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
We do not intend to provide information necessary for U.S. Holders to make qualified electing fund elections which, if available, would result in tax treatment different from the general tax treatment for PFICs described above.
If we were a PFIC for any taxable year during which a U.S. Holders owned any ordinary shares, the U.S. Holder would generally be required to file annual reports on United States Internal Revenue Service Form 8621. Substantial penalties and other adverse tax consequences may apply for failure to timely file such reports. U.S. Holders should consult their tax advisers regarding the determination of whether we are a PFIC for any taxable year and the potential application of the PFIC rules to their ownership of ordinary shares.
Information Reporting and Backup Withholding
Payments of distributions and sales proceeds that are made within the United States or through certain U.S. related financial intermediaries may be subject to information reporting and backup withholding, unless (i) the U.S. Holder is a corporation or other “exempt recipient” and (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against its U.S. federal income tax liability and may entitle it to a refund, provided that the required information is timely furnished to the Internal Revenue Service.
Certain U.S. Holders who are individuals (or certain specified entities) may be required to report information relating to their ownership of ordinary shares or non-U.S. accounts through which ordinary shares are held on Form 8938 with the Internal Revenue Service. Substantial penalties and other tax consequences may apply for failure to timely file such reports. U.S. Holders should consult their tax advisers regarding their reporting obligations with respect to our ordinary shares.
| 180 |
We are offering the ordinary shares described in this Prospectus through the underwriters listed below. Subject to the terms of the underwriting agreement, the underwriters named below have agreed to buy, severally and not jointly, the number of ordinary shares listed opposite their names below. The underwriters are committed to purchase and pay for all of the shares if any are purchased, other than those shares covered by the over-allotment option described below. The Benchmark Company, LLC is acting as the lead managing underwriter of this offering and representative of the underwriters.
| Name | Number of Shares | |
| The Benchmark Company, LLC | ||
| Axiom Capital Management, Inc. | ||
| Total: | 2,412,369 |
The underwriters have advised us that they propose to initially offer the ordinary shares to the public at a price of U.S.$4.50 per share. The underwriters propose to offer the ordinary shares to certain dealers at the same price less a concession of not more than U.S.$ per share, of which up to U.S.$ per share may be reallowed to other dealers. After the initial offering, these figures may be changed by the underwriters.
The shares sold in this offering are expected to be ready for delivery against payment in immediately available funds on or about 2023, subject to customary closing conditions. The underwriters may reject all or part of any order.
We have granted to the underwriters an option to purchase up to an additional 361,855 ordinary shares from us at the same price to the public, and with the same underwriting discount, as set forth in the table below. The underwriters may exercise this option any time during the 45-day period after the date of this Prospectus, but only to cover over-allotments, if any. To the extent the underwriters exercise the option, the underwriters will become obligated, subject to certain conditions, to purchase the shares for which they exercise the option.
Commissions and Discounts
The table below summarizes the underwriting discounts that we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the over-allotment option. In addition to the underwriting discount, we have agreed to pay up to U.S.$175,000 of the fees and expenses of the underwriters, which may include up to U.S.$150,000 of fees and expenses of counsel to the underwriters. In addition to the foregoing, we have agreed to be responsible for the costs of background checks on our senior management in an amount not to exceed U.S.$7,500. The fees and expenses of the underwriters that we have agreed to reimburse are not included in the underwriting discounts set forth in the table below.
We have paid an expense deposit of U.S.$25,000 to (or on behalf of) the underwriters, which will be applied against the out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering, and will be reimbursed to us to the extent not incurred. We have also agreed to pay a non-accountable expense allowance to the underwriters equal to 1.0% of the gross proceeds received in this offering.
Except as disclosed in this Prospectus, the underwriters have not received and will not receive from us any other item of compensation or expense in connection with this offering considered by FINRA to be underwriting compensation under FINRA Rule 5110. The underwriting discount was determined through an arms’ length negotiation between us and the underwriters.
| Total | No | Full | |||||||||
| Per Share | Exercise | Exercise | |||||||||
| Public offering price | U.S.$ | U.S.$ | U.S.$ | ||||||||
| Underwriting discounts and commissions to be paid by us | U.S.$ | U.S.$ | U.S.$ | ||||||||
| Proceeds, before expenses, to us | U.S.$ | U.S.$ | U.S.$ |
We estimate that the total expenses of this offering, excluding underwriting discounts, will be U.S.$1.31 million.
Right of First Refusal
Until twelve (12) months from the closing of this offering, the representative shall have an irrevocable right of first refusal to act as lead or joint-lead investment banker, lead or joint book-runner and/or lead or joint placement agent, at the representative’s sole discretion, for each and every future public and private equity and debt offerings for the Company, or any successor to or any subsidiary of the Company, including all equity linked financings, on terms customary to the representative and such transactions.
| 181 |
Indemnification
We also have agreed to indemnify the underwriters against certain liabilities, including civil liabilities under the Securities Act, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
No Sales of Ordinary Shares
We, have agreed with the underwriters, subject to certain exceptions, not to offer, pledge, sell, or dispose of, directly or indirectly, any of our ordinary shares or securities convertible into or exchangeable or exercisable for any of our ordinary shares during the 6-month period following the date of this Prospectus.
Members of our Board, our executive officers and all shareholders beneficially owning more than 5% of our outstanding ordinary shares (other than with respect to 416,666 ordinary shares), subject to certain exceptions, as of the effective date of this Prospectus have agreed during the 12-month period following the date of this Prospectus to substantially similar lock-up provisions, subject to certain exceptions.
These lock-up agreements provide limited exceptions, and their restrictions may be waived at any time by the representative.
Determination of Offering Price
The underwriters have advised us that they propose to offer the ordinary shares directly to the public at the estimated initial public offering price range set forth on the cover page of this Prospectus. That price range and the initial public offering price are subject to change as a result of market conditions and other factors. Prior to this offering, no public market exists for our ordinary shares. The initial public offering price of the shares was determined by negotiation between us and the underwriters. The principal factors considered in determining the initial public offering price of the shares included, among others:
| ● | the information in this Prospectus and otherwise available to the underwriters, including our financial information; |
| ● | the history and the prospects for the industry in which we compete; |
| ● | the ability and experience of our management; |
| ● | the prospects for our future earnings; |
| ● | the present state of our development and our current financial condition; |
| ● | the general condition of the economy and the securities markets in the United States at the time of this initial public offering; |
| ● | the recent market prices of, and the demand for, publicly-traded securities of generally comparable companies; and |
| ● | other factors as were deemed relevant. |
We cannot be sure that the initial public offering price will correspond to the price at which our ordinary shares will trade in the public market following this offering or that an active trading market for or ordinary shares will develop or continue after this offering.
Price Stabilization, Short Positions and Penalty Bids
To facilitate this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our ordinary shares during and after the offering. Specifically, the underwriters may create a short position in our ordinary shares for their own accounts by selling more ordinary shares than we have sold to the underwriters. The underwriters may close out any short position by purchasing shares in the open market.
In addition, the underwriters may stabilize or maintain the price of our ordinary shares by bidding for or purchasing shares in the open market and may impose penalty bids. If penalty bids are imposed, selling concessions allowed to broker-dealers participating in this offering are reclaimed if shares previously distributed in this offering are repurchased, whether in connection with stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market price of our ordinary shares at a level above that which might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of our ordinary shares to the extent that it discourages resales of our ordinary shares. The magnitude or effect of any stabilization or other transactions is uncertain. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
| 182 |
In connection with this offering, the underwriters and selling group members may also engage in passive market making transactions in our ordinary shares on the Nasdaq Capital Market. Passive market making consists of displaying bids on the Nasdaq Capital Market limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the SEC limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of our ordinary shares at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our ordinary shares. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
The underwriters or syndicate members may facilitate the marketing of this offering online directly or through one of their respective affiliates. In those cases, prospective investors may view offering terms and a prospectus online and place orders online or through their financial advisors. Such websites and the information contained on such websites, or connected to such sites, are not incorporated into and are not a part of this Prospectus.
Other Relationships
The underwriters and their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. The underwriters may in the future receive customary fees and commissions for these transactions.
In the ordinary course of their various business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Listing
We have applied to list our ordinary shares on The Nasdaq Capital Market under the trading symbol “GDTC”.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is VStock Transfer, LLC. The transfer agent and registrar’s address is 18 Lafayette Place, Woodmere, New York 11598.
Selling Restrictions
No action has been taken in any jurisdiction except the United States that would permit a public offering of our ordinary shares, or the possession, circulation or distribution of this Prospectus or any other material relating to us or our ordinary shares in any jurisdiction where action for that purpose is required. Accordingly, the shares may not be offered or sold, directly or indirectly, and neither this Prospectus nor any other offering material or advertisements in connection with the shares may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
| 183 |
Set forth below is an itemization of the total expenses, excluding the underwriting discounts and commissions and non-accountable expense allowance, which are expected to be incurred in connection with the sale of ordinary shares in this offering. With the exception of the registration fee payable to the SEC, the Nasdaq Capital Market listing fee and the filing fee payable to Financial Industry Regulatory Authority, Inc., or FINRA, all amounts are estimates.
| Expenses | Amount | |||
| U.S.$ | ||||
| SEC registration fee | 1,708.10 | |||
| FINRA filing fee | 2,750.00 | |||
| The Nasdaq Capital Market listing fee | 50,000.00 | |||
| Printing and engraving expenses | 25,000.00 | |||
| Legal fees and expenses | 730,000.00 | |||
| Accounting fees and expenses | 170,000.00 | |||
| Transfer agent and registrar fee and expenses | 2,000.00 | |||
| Miscellaneous costs (1) | 327,000.00 | |||
| Total | 1,308,458.10 | |||
| (1) | Miscellaneous costs include, inter alia, out-of-pocket expenses and the non-accountable expense allowance payable to underwriter. |
The Company will pay all of the expenses of this offering.
| 184 |
We are being represented by Ellenoff Grossman & Schole LLP with respect to certain legal matters of U.S. federal securities. The validity of the ordinary shares and certain other matters of Singapore law will be passed upon for us by Opal Lawyers LLC. Certain matters of Malaysian law will be passed upon for us by Zaid Ibrahim & Co. Certain legal matters in connection with this offering will be passed upon for the underwriters by Sheppard, Mullin, Richter & Hampton LLP, New York, NY.
| 185 |
The financial statements as of December 31, 2020 and 2021, included in this Prospectus have been audited by WWC, P.C., an independent registered public accounting firm, as stated in their report appearing herein. Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
On April 5, 2022, Nexia TS Public Accounting Corporation (“prior auditor”) resigned, and we appointed WWC, P.C., for the audit of our consolidated financial statements which are to be included as part of this prospectus that forms a part of this Registration Statement.
The draft prior auditor’s reports for the financial years ended December 31, 2020 and December 31, 2019 which were included in the confidential filing on September 28, 2021, as amended on November 19, 2021 and December 17, 2021 of our draft registration statement on Form F-1 under the Securities Act of 1933 with the Securities and Exchange Commission, did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles.
During the two years ended December 31, 2020 and the subsequent interim period through April 5, 2022, (i) there were no disagreements (as defined in Item 16F(a)(1)(iv) of Form 20-F between the Company and the prior auditor over any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures, which disagreements if not resolved to the prior auditor’s satisfaction would have caused the prior auditor to make reference to the subject matter of the disagreement in connection with its draft auditor’s reports; and (ii) there were no reportable events (as defined in Item 16F(a)(1)(v) of Form 20-F).
We have requested that our prior auditor furnish a letter addressed to the Securities and Exchange Commission stating whether it agrees with the above statements, and, if not, stating the respects in which it does not agree. Such letter is included as Exhibit 16.1 to this Registration Statement on Form F-1.
During the two years ended December 31, 2021, and the subsequent interim period through April 6, 2022, (i) WWC, P.C. did not have any disagreement with us on any matter of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered with respect to our consolidated financial statement, and no written report was provided to us or oral advice was provided that WWC, P.C. concluded was an important factor considered by us in reaching a decision as to the accounting, auditing or financial reporting issue; (ii) there were no matters that was the subject of a disagreement within the meaning of Item 16F(a)(1)(iv) or a reportable event within the meaning of Item 16F(a)(1)(v).
| 186 |
SERVICE OF PROCESS AND ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of the Republic of Singapore, and substantially all of our assets are located outside the United States. In addition, most of our directors and executive officers are nationals or residents of jurisdictions other than the United States and substantially all of their assets are located outside the United States. As a result, it may be difficult or impossible for you to effect service of process within the United States upon us or these persons, or to enforce judgments obtained in U.S. courts against us or them, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our executive officers and directors.
We have appointed Puglisi & Associates as our agent to receive service of process with respect to any action brought against us in the United States in connection with this offering under the federal securities laws of the United States or of any State in the United States.
An investor may or may not be able to commence an original action against us or our Directors or officers, or any person, before the courts outside the United States to enforce liabilities under United States federal securities laws, depending on the nature of the action.
There is uncertainty as to whether judgments of courts in the United States, based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States, will be recognized or enforced by the Singapore courts, and there is doubt as to whether the Singapore courts will enter judgments in original actions brought in the Singapore courts based solely on the civil liability provisions of these securities laws. An in personam final and conclusive judgment in the federal or state courts of the United States under which a fixed or ascertainable sum of money is payable may generally be enforced as a debt in the Singapore courts under the common law as long as it is established that the Singapore courts have jurisdiction over the judgment debtor. However, the Singapore courts are unlikely to enforce a foreign judgment if (a) the foreign judgment is inconsistent with a prior local judgment that is binding on the same parties; (b) the enforcement of the foreign judgment would contravene the public policy of Singapore; (c) the proceedings in which the foreign judgment was obtained were contrary to principles of natural justice; (d) the foreign judgment was obtained by fraud; or (e) the enforcement of the foreign judgment amounts to the direct or indirect enforcement of a foreign penal, revenue or other public law.
In particular, the Singapore courts may potentially not allow the enforcement of any foreign judgment for a sum payable in respect of taxes, fines, penalties or other similar charges, including the judgments of courts in the United States based upon the civil liability provisions of the securities laws of the United States or any state or territory of the United States. In respect of civil liability provisions of the United States federal and state securities law which permit punitive damages against us and our Directors or executive officers, we are unaware of any decision by the Singapore courts which has considered the specific issue of whether a judgment of a United States court based on such civil liability provisions of the securities laws of the United States or any state or territory of the United States is enforceable in Singapore.
In addition, holders of book-entry interests in our ordinary shares will be required to be registered as shareholders in our register of members in order to have standing to bring a shareholder suit and, if successful, to enforce a foreign judgment against us, our Directors or our executive officers in the Singapore courts, subject to applicable Singapore laws. A holder of book-entry interests in our ordinary shares may become our registered shareholder by exchanging its interest in our ordinary shares for certificated ordinary shares and being registered in our register of members. The administrative process of becoming a registered shareholder could result in delays prejudicial to any legal proceeding or enforcement action.
| 187 |
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement (including amendments and exhibits to the registration statement) on Form F-1 under the Securities Act. This Prospectus, which is part of the registration statement, does not contain all of the information set forth in the registration statement and the exhibits and schedules to the registration statement. For further information, we refer you to the registration statement and the exhibits and schedules filed as part of the registration statement. If a document has been filed as an exhibit to the registration statement, we refer you to the copy of the document that has been filed. Each statement in this Prospectus relating to a document filed as an exhibit is qualified in all respects by the filed exhibit.
The SEC maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers, like us, that file electronically with the SEC.
Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers. Accordingly, we will be required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. Those reports may be inspected without charge at the locations described above.
As a foreign private issuer, we will be exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, Directors and controlling shareholder will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We maintain a website at www.cytomed.sg. Information contained on, or that can be accessed through, our website is not a part of, and shall not be incorporated by reference into, this Prospectus.
| 188 |
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Shareholders of |
| CytoMed Therapeutics Limited |
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial positions of CytoMed Therapeutics Limited and its subsidiaries (the “Company”) as of December 31, 2020 and 2021, and the related consolidated statements of profit or loss and other comprehensive income, changes in equity, and cash flows for each of the years in the two-year period ended December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Emphasis of Matter – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 3 to the consolidated financial statements, the Company has incurred substantial losses and had net cash outflows from operating activities during the years ended December 31, 2020 and 2021. These circumstances give rise to substantial doubt that the Company will continue as a going concern. Management’s plans in regards to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this doubt and uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
WWC, P.C.
Certified Public Accountants
PCAOB ID No. 1171
We have served as the Company’s auditor since 2022.
San Mateo, California
September 30, 2022, except for Notes 1, 14 and 28 which are dated March 1, 2023

| F-2 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
As of December 31, 2020 and 2021
| Notes | 2020 | 2021 | 2021 | |||||||||||
| S$ | S$ | US$ | ||||||||||||
| ASSETS | ||||||||||||||
| Current assets | ||||||||||||||
| Other receivables | 5 | 583,754 | 768,975 | 553,100 | ||||||||||
| Cash and cash equivalents | 4 | 885,272 | 2,512,768 | 1,807,357 | ||||||||||
| Total current assets | 1,469,026 | 3,281,743 | 2,360,457 | |||||||||||
| Non-current assets | ||||||||||||||
| Investment in associate | 6 | - | 272,970 | 196,338 | ||||||||||
| Property, plant and equipment | 7 | 2,301,025 | 2,495,791 | 1,795,146 | ||||||||||
| Intangible assets | 8 | 45,248 | 32,023 | 23,033 | ||||||||||
| Total non-current assets | 2,346,273 | 2,800,784 | 2,014,517 | |||||||||||
| Total assets | 3,815,299 | 6,082,527 | 4,374,974 | |||||||||||
| LIABILITIES AND EQUITY | ||||||||||||||
| Current liabilities | ||||||||||||||
| Trade and other payables | 9 | 352,394 | 218,733 | 157,328 | ||||||||||
| Contract liabilities | 10 | 6,318 | 13,492 | 9,704 | ||||||||||
| Borrowings | 11 | 35,588 | 385,691 | 277,416 | ||||||||||
| Convertible loans | 12 | 2,323,423 | 2,310,757 | 1,662,056 | ||||||||||
| Lease liabilities | 13 | 7,583 | 7,813 | 5,620 | ||||||||||
| Total current liabilities | 2,725,306 | 2,936,486 | 2,112,124 | |||||||||||
| Non-current liabilities | ||||||||||||||
| Borrowings | 11 | 590,674 | 539,119 | 387,772 | ||||||||||
| Lease liabilities | 13 | 23,539 | 15,318 | 11,018 | ||||||||||
| Total non-current liabilities | 614,213 | 554,437 | 398,790 | |||||||||||
| Total liabilities | 3,339,519 | 3,490,923 | 2,510,914 | |||||||||||
| Capital and reserves | ||||||||||||||
| Share capital | 14 | 3,500,000 | 6,548,960 | 4,710,465 | ||||||||||
| Share application monies | 14 | - | 1,141,395 | 820,970 | ||||||||||
| Translation reserves | (8,352 | ) | (30,980 | ) | (22,283 | ) | ||||||||
| Accumulated losses | (3,015,682 | ) | (5,067,332 | ) | (3,644,776 | ) | ||||||||
| Attributable to equity holders of the Company | 475,966 | 2,592,043 | 1,864,376 | |||||||||||
| Non-controlling interests | (186 | ) | (439 | ) | (316 | ) | ||||||||
| Total equity | 475,780 | 2,591,604 | 1,864,060 | |||||||||||
| Total liabilities and equity | 3,815,299 | 6,082,527 | 4,374,974 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME
For the years ended December 31, 2020 and 2021
| Note | 2020 | 2021 | 2021 | |||||||||||
| S$ | S$ | US$ | ||||||||||||
| Revenue | - | - | - | |||||||||||
| Other operating income | 15 | 127,456 | 154,610 | 111,206 | ||||||||||
| Other (losses)/gains - net | 16 | (573,856 | ) | 3,185 | 2,291 | |||||||||
| Research and development expenses | 17 | (1,038,091 | ) | (1,090,623 | ) | (784,452 | ) | |||||||
| Depreciation of property, plant and equipment | 7 | (86,124 | ) | (98,721 | ) | (71,007 | ) | |||||||
| Amortization of intangible assets | 8 | (507 | ) | (2,512 | ) | (1,807 | ) | |||||||
| Employee benefits expenses | 18 | (130,991 | ) | (168,802 | ) | (121,414 | ) | |||||||
| Finance expenses | 19 | (105,519 | ) | (117,696 | ) | (84,655 | ) | |||||||
| Other expenses | 20 | (127,994 | ) | (518,766 | ) | (373,132 | ) | |||||||
| Share of results of associate | 6 | - | (212,578 | ) | (152,901 | ) | ||||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | ||||||||
| Income tax | 21 | - | - | - | ||||||||||
| Loss for the year | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | ||||||||
| Other comprehensive loss: | ||||||||||||||
| Items that may be reclassified subsequently to profit or loss: | ||||||||||||||
| Foreign currency translation loss | (6,616 | ) | (22,628 | ) | (16,275 | ) | ||||||||
| Total comprehensive loss for the year | (1,942,242 | ) | (2,074,531 | ) | (1,492,146 | ) | ||||||||
| Loss attributable to: | ||||||||||||||
| Equity holders of the Company | (1,935,440 | ) | (2,051,650 | ) | (1,475,689 | ) | ||||||||
| Non-controlling interests | (186 | ) | (253 | ) | (182 | ) | ||||||||
| Total | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | ||||||||
| Total comprehensive loss attributable to: | ||||||||||||||
| Equity holders of the Company | (1,942,056 | ) | (2,074,278 | ) | (1,491,964 | ) | ||||||||
| Non-controlling interests | (186 | ) | (253 | ) | (182 | ) | ||||||||
| Total | (1,942,242 | ) | (2,074,531 | ) | (1,492,146 | ) | ||||||||
| Loss per share for loss attributable to equity holders of the Company: | ||||||||||||||
| - Basic | (0.33 | ) | (0.30 | ) | (0.21 | ) | ||||||||
| - Diluted | (0.33 | ) | (0.30 | ) | (0.21 | ) | ||||||||
LOSS PER SHARE
| December 31, | ||||||||
| 2020 | 2021 | |||||||
| Weighted average number of ordinary shares used in computing basic loss | 5,897,651 | 6,934,824 | ||||||
| Weighted average number of ordinary shares used in computing diluted loss | 5,897,651 | 6,934,824 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the years ended December 31, 2020 and 2021
| Attributable to equity holders of the Company | ||||||||||||||||||||||||||||
| Share capital | Share application monies | Translation reserves | Retained earnings | Total | Non-controlling interests | Total | ||||||||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | S$ | S$ | ||||||||||||||||||||||
| Balance at January 1, 2020 | 3,000,000 | - | (1,736 | ) | (1,080,242 | ) | 1,918,022 | - | 1,918,022 | |||||||||||||||||||
| Total comprehensive loss for the year | - | - | (6,616 | ) | (1,935,440 | ) | (1,942,056 | ) | (186 | ) | (1,942,242 | ) | ||||||||||||||||
| Transaction with owner of the Company recognized directly in equity | ||||||||||||||||||||||||||||
| Issuance of shares | 500,000 | - | - | - | 500,000 | - | 500,000 | |||||||||||||||||||||
| Balance at December 31, 2020 | 3,500,000 | - | (8,352 | ) | (3,015,682 | ) | 475,966 | (186 | ) | 475,780 | ||||||||||||||||||
| Total comprehensive loss for the year | - | - | (22,628 | ) | (2,051,650 | ) | (2,074,278 | ) | (253 | ) | (2,074,531 | ) | ||||||||||||||||
| Transactions with owners of the Company recognized directly in equity | ||||||||||||||||||||||||||||
| Issuance of shares | 2,000,000 | - | - | - | 2,000,000 | - | 2,000,000 | |||||||||||||||||||||
| Issuance of shares – transferred from share application monies | 858,605 | (858,605 | ) | - | - | - | - | - | ||||||||||||||||||||
| Share issued for service | 190,355 | - | - | - | 190,355 | - | 190,355 | |||||||||||||||||||||
| Share application monies received | - | 2,000,000 | - | - | 2,000,000 | - | 2,000,000 | |||||||||||||||||||||
| Total transactions with owners of the Company | 3,048,960 | 1,141,395 | - | - | 4,190,355 | - | 4,190,355 | |||||||||||||||||||||
| Balance at December 31, 2021 | 6,548,960 | 1,141,395 | (30,980 | ) | (5,067,332 | ) | 2,592,043 | (439 | ) | 2,591,604 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CASH FLOWS
For the years ended December 31, 2020 and 2021
| 2020 | 2021 | 2021 | ||||||||||
| S$ | S$ | US$ | ||||||||||
| Operating activities | ||||||||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | (1,475,871 | ) | ||||||
| Adjustments for: | ||||||||||||
| Amortization of intangible assets | 11,207 | 13,212 | 9,503 | |||||||||
| Depreciation of property, plant and equipment | 281,326 | 316,666 | 227,768 | |||||||||
| Fair value loss/(gain) on convertible loans | 573,423 | (12,666 | ) | (9,110 | ) | |||||||
| Share of results of associate | - | 212,578 | 152,901 | |||||||||
| Interest expense | 105,519 | 117,696 | 84,655 | |||||||||
| Interest income | - | (3,369 | ) | (2,423 | ) | |||||||
| Unrealized currency translation (gains)/losses | (883 | ) | 60,680 | 43,645 | ||||||||
| Operating cash flows before movements in working capital | (965,034 | ) | (1,347,106 | ) | (968,932 | ) | ||||||
| Other receivables | (162,050 | ) | (433,116 | ) | (311,526 | ) | ||||||
| Trade and other payables | 277,641 | 63,868 | 45,939 | |||||||||
| Cash used in operations | (849,443 | ) | (1,716,354 | ) | (1,234,519 | ) | ||||||
| Interest received | - | 3,369 | 2,423 | |||||||||
| Income tax paid | - | - | - | |||||||||
| Net cash used in operating activities | (849,443 | ) | (1,712,985 | ) | (1,232,096 | ) | ||||||
| Investing activities | ||||||||||||
| Purchase of property, plant and equipment | (244,725 | ) | (582,354 | ) | (418,869 | ) | ||||||
| Purchase of intangible assets | (20,430 | ) | - | - | ||||||||
| Acquisition of an associate | - | (241,312 | ) | (173,568 | ) | |||||||
| Prepayment for the investment in equity shares | (247,899 | ) | - | - | ||||||||
| Net cash used in investing activities | (513,054 | ) | (823,666 | ) | (592,437 | ) | ||||||
| Financing activities | ||||||||||||
| Proceeds from convertible loans | 1,500,000 | - | - | |||||||||
| Proceeds from issuance of ordinary shares | 500,000 | 2,000,000 | 1,438,538 | |||||||||
| Proceeds from a third party loan | - | 350,000 | 251,744 | |||||||||
| Receipts from share application monies | - | 2,000,000 | 1,438,538 | |||||||||
| Principal payment of bank borrowings | (7,314 | ) | (51,452 | ) | (37,008 | ) | ||||||
| Principal payment of lease liabilities | (3,645 | ) | (7,456 | ) | (5,363 | ) | ||||||
| Interest paid | (105,519 | ) | (117,696 | ) | (84,655 | ) | ||||||
| Net cash from financing activities | 1,883,522 | 4,173,396 | 3,001,794 | |||||||||
| Net increase in cash and cash equivalents | 521,025 | 1,636,745 | 1,177,261 | |||||||||
| Cash and cash equivalents at beginning of year | 370,942 | 885,272 | 636,749 | |||||||||
| Effects of foreign currency translation on cash and cash equivalents | (6,695 | ) | (9,249 | ) | (6,653 | ) | ||||||
| Cash and cash equivalents at end of year | 885,272 | 2,512,768 | 1,807,357 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CASH FLOWS
For the years ended December 31, 2020 and 2021
Significant non-cash transactions affecting the Consolidated Statement of Cash Flows:
During the financial year ended December 31, 2021, the Group had the following significant non-cash transactions:
Issuance of 92,563 ordinary shares for a total consideration of S$190,355 for settlement of a professional fees of the equivalent amount.
| F-7 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 1 | GENERAL |
On March 8, 2018, CytoMed Therapeutics Limited (the former name: CytoMed Therapeutics Pte. Ltd.) (the “Company”) was incorporated in the Republic of Singapore. The Company is a private limited company incorporated and domiciled in Singapore. The address of its registered office is at 1 Commonwealth Lane, #08-22, Singapore 149544. The Company is headquartered in Singapore and conducts its operations domestically and in Malaysia. On January 19, 2023, the Company had converted to a public limited company and the name of the Company has changed from “CytoMed Therapeutics Pte. Ltd.” to “CytoMed Therapeutics Limited” and that the name “CytoMed Therapeutics Limited” has substituted for “CytoMed Therapeutics Pte. Ltd.” wherever “CytoMed Therapeutics Pte. Ltd.” appears in the Constitution of the Company.
The Company’s immediate and ultimate holding corporation is Glorious Finance Limited, incorporated in the British Virgin Islands.
The principal activities of the Company are to carry on the business of innate immune cell-based immunotherapy, pluripotent stem cell-based therapy and undertaking the research and development of immune cell and stem cell-based therapy. The Company conducts its primary operations through its directly held wholly owned subsidiary that is incorporated and domiciled in Malaysia, namely CytoMed Therapeutics (Malaysia) Sdn. Bhd., which is principally engaged in manufacturing innate immune cell-based immunotherapy and pluripotent stem cell-based therapy and consultancy services and undertaking the research and development of immune cell and stem cell-based therapy for advancing cellular immunotherapy to treat cancer.
The principal activities of the subsidiaries of the Company (the “Company” or collectively known as the “Group”) are as follows:
| Name of entity | Principal activities | Country of business / incorporation | Group’s effective equity interest held | |||||||||
| 2020 | 2021 | |||||||||||
| % | % | |||||||||||
| Advance Cancer Centre Pte Ltd | Investment, research and development of medical technologies | Singapore | 100 | 100 | ||||||||
| CytoMed Therapeutics (Malaysia) Sdn Bhd | Research, development and manufacturing of stem cells and innate immune cell-based immuno-therapeutics, research and development of induced pluripotent stem cell-based immuno-therapeutics | Malaysia | 100 | 100 | ||||||||
| Puricell Lab Pte Ltd | Research and development of induced pluripotent stem cell-based biologics and medical technologies | Singapore | 95 | 95 | ||||||||
| IPSCBank Pte Ltd | Stem cell and immune cell banking | Singapore | 100 | 100 | ||||||||
| Held by IPSCBank Pte Ltd | ||||||||||||
| IPSC Depository Sdn Bhd | Stem cell and immune cell banking | Malaysia | 100 | 100 | ||||||||
The principal activities of the associate are disclosed in Note 6 to the financial statements.
| F-8 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
BASIS OF PREPARATION - These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) under the historical cost convention, except as disclosed in the accounting policies below.
The preparation of these financial statements in conformity with IFRS requires management to exercise its judgement in the process of applying the Group’s accounting policies. It also requires the use of certain critical accounting estimates and assumptions. The areas involving a higher degree of judgement or complexity, or areas where estimates and assumptions are significant to the financial statements are disclosed in Note 3.
Adoption of IFRS
The Group has adopted IFRS on January 1, 2019.
On January 1, 2020, the Group adopted the new or amended IFRS and Interpretations of IFRS (“INT IFRS”) that are mandatory for application for the financial year. Changes to the Group’s accounting policies have been made as required, in accordance with the transitional provisions in the respective IFRS and INT IFRS.
BASIS OF ACCOUNTING - The financial statements have been prepared in accordance with the historical cost basis, except as disclosed in the accounting policies below, and are drawn up in accordance with the provisions of the IFRS.
Historical cost is generally based on the fair value of the consideration given in exchange for goods and services.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, regardless of whether that price is directly observable or estimated using another valuation technique. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability which market participants would take into account when pricing the asset or liability at the measurement date. Fair value for measurement and/or disclosure purposes in these financial statements is determined on such a basis.
In addition, for financial reporting purposes, fair value measurements are categorized into Level 1, 2 or 3 based on the degree to which the inputs to the fair value measurements are observable and the significance of the inputs to the fair value measurement in its entirety, which are described as follows:
| ● | Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the entity can access at the measurement date; | |
| ● | Level 2 inputs are inputs, other than quoted prices included within Level 1, that are observable for the asset or liability, either directly or indirectly; and | |
| ● | Level 3 inputs are unobservable inputs for the asset or liability. |
The Company’s policy is to recognize transfers into and transfers out of any of the three levels as of the date of the event or change in circumstances that caused the transfer.
| F-9 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
ADOPTION OF NEW AND REVISED STANDARDS – At the date of authorization of these financial statements, the management anticipates that the adoption of the above/other IFRS and amendments to IFRS in future periods will not have a material impact on the financial statements of the Group in the period of their initial adoption.
NEW AND REVISED IFRS STANDARDS IN ISSUE BUT NOT YET EFFECTIVE - At the date of authorization of these financial statements, the Group and the Company have not adopted the new and revised IFRS, IFRS INT and amendments to IFRS that have been issued but are not yet effective to them. The Group does not anticipate that the adoption of these new and revised IFRS pronouncements in future periods will have a material impact on the Group’s and the Company’s financial statements in the period of their initial adoption.
BASIS OF CONSOLIDATION
| (a) | Consolidation |
Subsidiaries are all entities (including structured entities) over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Group. They are deconsolidated from the date on that control ceases.
In preparing the consolidated financial statements, transactions, balances and unrealized gains on transactions between group entities are eliminated. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment indicator of the transferred asset. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
Non-controlling interests comprise the portion of a subsidiary’s net results of operations and its net assets, which is attributable to the interests that are not owned directly or indirectly by the equity holders of the Company. They are shown separately in the consolidated statement of profit or loss and other comprehensive income, consolidated statement of changes in equity, and consolidated statement of financial positions. Total comprehensive income is attributed to the non-controlling interests based on their respective interests in a subsidiary, even if this results in the non-controlling interests having a deficit balance.
| (b) | Acquisitions |
The acquisition method of accounting is used to account for business combinations entered into by the Group.
The consideration transferred for the acquisition of a subsidiary or business comprises the fair value of the assets transferred, the liabilities incurred and the equity interests issued by the Group. The consideration transferred also includes any contingent consideration arrangement and any pre-existing equity interest in the subsidiary measured at their fair values at the acquisition date.
Acquisition-related costs are expensed as incurred.
| F-10 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are, with limited exceptions, measured initially at their fair values at the acquisition date.
On an acquisition-by-acquisition basis, the Group recognizes any non-controlling interest in the acquiree at the date of acquisition either at fair value or at the non-controlling interest’s proportionate share of the acquiree’s identifiable net assets.
The excess of (a) the consideration transferred, the amount of any non-controlling interest in the acquiree and the acquisition-date fair value of any previous equity interest in the acquiree over the (b) fair value of the identifiable net assets acquired is recorded as goodwill. Please refer to the paragraph “Intangible assets – Goodwill” for the subsequent accounting policy on goodwill.
CONVENIENCE TRANSLATION
Translations of amounts in the consolidated statement of financial position, consolidated statements of profit or loss and other comprehensive income, and consolidated statement of cash flows from SGD into USD as of and for the year ended December 31, 2021 are solely for the convenience of the reader and were calculated at the noon buying rate of USD1 = SGD1.3903, as published in H.10 statistical release of the United States Federal Reserve Board. No representation is made that the SGD amounts could have been, or could be, converted, realized or settled into USD at such rate or at any other rate.
FINANCIAL ASSETS
| (a) | Classification and measurement |
The Group classifies its financial assets at amortized cost.
The classification depends on the Group’s business model for managing the financial assets as well as the contractual terms of the cash flows of the financial assets.
The Group reclassifies debt instruments when and only when its business model for managing those assets changes.
| F-11 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
At initial recognition
At initial recognition, the Group measures a financial asset at its fair value plus, in the case of the financial assets not a fair value through profit or loss, transaction cost that are directly attributable to the acquisition of the financial asset. Transaction costs of financial assets carried at fair value through profit or loss are expensed in profit or loss.
At subsequent measurement
Debt instrument - Debt instruments mainly comprised of cash and cash equivalents and other receivables (excluding prepayments).
Debt instruments that are held for collection of contractual cash flows where those cash flows represent solely payments of principal and interest are measured at amortized cost. A gain or loss on a debt instrument that is subsequently measured at amortized cost and is not part of a hedging relationship is recognized in profit or loss when the asset is derecognized or impaired. Interest income from these financial assets is included in interest income using the effective interest rate method.
| (b) | Impairment |
The Group assesses on a forward-looking basis the expected credit losses associated with its debt financial assets carried at amortized cost. The impairment methodology applied depends on whether there has been a significant increase in credit risk. The note to the financial statement on credit risk details how the Group determines whether there has been a significant increase in credit risk.
| (c) | Recognition and derecognition |
Purchases and sales of financial assets are recognized on trade date – the date on which the Group commits to purchase or sell the asset.
Financial assets are derecognized when the rights to receive cash flows from the financial assets have expired or have been transferred and the Group has transferred substantially all risks and rewards of ownership.
On disposal of a debt instrument, the difference between the carrying amount and the sale proceeds is recognized in profit or loss.
| F-12 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
FINANCIAL LIABILITIES AND EQUITY INSTRUMENTS
| (a) | Classification as debt or equity |
Debt and equity instruments issued by a Group entity are classified as either financial liabilities or as equity in accordance with substance of the contractual arrangements and the definitions of a financial liability and an equity instrument.
| (b) | Equity instruments |
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by a Group are recognized at the proceeds received, net of direct issue costs.
| (c) | Financial liabilities |
Except for convertible loans which are stated at fair value through profit or loss, all other financial liabilities are subsequently measured at amortized cost using the effective interest method.
The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash payments (including all fees and points paid or received that form an integral part of the effective interest rate, transaction costs and other premiums or discounts) through the expected life of the financial liability, or (where appropriate) a shorter period, to the amortized cost of a financial liability.
| (d) | Derecognition of financial liabilities |
The Group derecognizes financial liabilities when, and only when, the Group’s obligations are discharged, cancelled or expired. The difference between the carrying amount of the financial liability derecognized and the consideration paid and payable, including any non-cash assets transferred or liabilities assumed, is recognized in profit or loss.
| (e) | Offsetting financial instruments |
Financial assets and liabilities are offset and the net amount reported in the balance sheet when there is a legally enforceable right to offset and there is an intention to settle on a net basis or realize the asset and settle the liability simultaneously.
INVESTMENT IN AN ASSOCIATE - An associate is an entity over which the Group has significant influence, but not control or joint control, over the financial and operating policies of the entity. Significant influence is presumed to exist when the Group holds 20% or more of the voting power of another entity.
The Group accounts for its investment in the associate using the equity method.
On acquisition of the investment, any excess of the cost of the investment over the Group’s share of the net fair value of the investee’s identifiable assets and liabilities represents goodwill and is included in the carrying amount of the investment. Any excess of the Group’s share of the net fair value of the investee’s identifiable assets and liabilities over the cost of the investment is included as income in the determination of the Group’s share of the associate’s profit or loss in the period in which the investment is acquired.
Under the equity method, the investment in an associate is carried at cost plus post-acquisition changes in the Group’s share of net assets of the associate. Goodwill relating to the associate is included in the carrying amount of the investment and is not tested for impairment separately.
After application of the equity method, the Group determines whether it is necessary to recognize an impairment loss on its investment in its associate. At each reporting date, the Group determines whether there is objective evidence that the investment in the associate is impaired. If there is such evidence, the Group calculates the amount of impairment as the difference between the recoverable amount of the associate and its carrying value, and then recognizes the loss within “Share of results of associate” in the profit or loss.
| F-13 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
PROPERTY, PLANT AND EQUIPMENT
| (a) | Measurement |
| (i) | Property, plant and equipment |
Property, plant and equipment are initially recognized at cost and subsequently carried at cost less accumulated depreciation and accumulated impairment losses.
| (ii) | Components of costs |
The cost of an item of property, plant and equipment initially recognized includes its purchase price and any cost that is directly attributable to bringing the asset to the location and condition necessary for it to be capable of operating in the manner intended by management.
| (b) | Depreciation |
Freehold land is not depreciated. Depreciation on other items of property, plant and equipment is calculated using the straight-line method to allocate their depreciable amounts over their estimated useful lives as followed;
| Building | - | 16 - 60 years | |||
| Laboratory equipment | - | 5 years | |||
| Motor vehicle | - | 5 years | |||
| Furniture, fittings and equipment | - | 3 - 5 years | |||
| Renovation | - | 10 years |
The residual values, estimated useful lives and depreciation method of property, plant and equipment are reviewed, and adjusted as appropriate, at each balance sheet date. The effects of any revision are recognized in profit or loss when the changes arise.
| (c) | Subsequent expenditure |
Subsequent expenditure relating to property, plant and equipment that has already been recognized is added to the carrying amount of the asset only when it is probable that future economic benefits associated with the item will flow to the entity and the cost of the item can be measured reliably. All other repair and maintenance expenses are recognized in profit or loss when incurred.
| F-14 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
INTANGIBLE ASSETS
| (a) | Goodwill |
Goodwill on acquisitions of subsidiaries and businesses, represents the excess of (i) the sum of the consideration transferred, the amount of any non-controlling interest in the acquiree and the acquisition-date fair value of any previous equity interest in the acquiree over (ii) the fair value of the identifiable net assets acquired. Goodwill on subsidiaries is recognized separately as intangible assets are carried at cost less accumulated impairment losses.
| (b) | Acquired intellectual properties licenses |
Intellectual properties licenses acquired are initially recognized at cost and are subsequently carried at cost less accumulated amortization and accumulated impairment losses. These costs are amortized to profit or loss using the straight-line method over 5 years, which is the shorter of their estimated useful lives and periods of contractual rights.
| (c) | Acquired computer software licenses |
Acquired computer software licenses are initially recognized at cost which includes the purchase prices and other directly attributable costs of preparing the asset for its intended use. Costs associated with maintaining the computer software are expensed off when incurred.
Computer software licenses are subsequently carried at cost less accumulated amortization and accumulated impairment losses. These costs are amortized to profit or loss using the straight-line method over their estimated useful lives of 3 years.
The amortization period and amortization method of intangible assets other than goodwill are reviewed at least at each balance sheet date. The effects of any revision are recognized in profit or loss when the changes arise.
IMPAIRMENT OF NON-FINANCIAL ASSETS
| (a) | Goodwill |
Goodwill recognized separately as an intangible asset is tested for impairment annually and whenever there is indication that the goodwill may be impaired.
For the purpose of impairment testing of goodwill, goodwill is allocated to each of the Group’s cash-generating-units (“CGU”) expected to benefit from synergies arising from the business combination.
| F-15 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
An impairment loss is recognized when the carrying amount of a CGU, including the goodwill, exceeds the recoverable amount of the CGU. The recoverable amount of a CGU is the higher of the CGU’s fair value less cost to sell and value-in-use.
The total impairment loss of a CGU is allocated first to reduce the carrying amount of goodwill allocated to the CGU and then to the other assets of the CGU pro-rata on the basis of the carrying amount of each asset in the CGU.
An impairment loss on goodwill is recognized as an expense and is not reversed in a subsequent period.
| (b) | Intangible assets (other than Goodwill) and Property, plant and equipment |
Intangible assets and property, plant and equipment are tested for impairment whenever there is any objective evidence or indication that these assets may be impaired.
For the purpose of impairment testing, the recoverable amount (i.e. the higher of the fair value less cost to sell and the value-in-use) is determined on an individual asset basis unless the asset does not generate cash inflows that are largely independent of those from other assets. If this is the case, the recoverable amount is determined for the CGU to which the asset belongs.
If the recoverable amount of the asset (or CGU) is estimated to be less than its carrying amount, the carrying amount of the asset (or CGU) is reduced to its recoverable amount. The difference between the carrying amount and recoverable amount is recognized as an impairment loss in profit or loss.
An impairment loss for an asset is reversed if, and only if, there has been a change in the estimates used to determine the asset’s recoverable amount since the last impairment loss was recognized. The carrying amount of this asset is increased to its revised recoverable amount, provided that this amount does not exceed the carrying amount that would have been determined (net of any accumulated amortization or depreciation) had no impairment loss been recognized for the asset in prior years.
A reversal of impairment loss for an asset other than goodwill is recognized in profit or loss.
| (c) | Investment in an associate |
The Group determines whether it is necessary to recognize an impairment loss on its investment in its associate. At each reporting date, the Group determines whether there is objective evidence that the investment in the associate is impaired. If there is such evidence, the Group calculates the amount of impairment as the difference between the recoverable amount of the associate and its carrying value, and then recognizes the loss within ‘Share of results of associate’ in the statement of profit or loss.
The annual impairment test of investment in an associate involved significant management assessment and judgement required in determining the assumptions to be used to estimate the recoverable amount. Such recoverable amount is based on the higher of the value in use or fair value less costs of disposal, has been derived from discounted forecast cash flow models, including estimates of futures sales volumes and prices, operating costs, revenue growth rates and the weighted-average cost of capital (discount rate).
TRADE AND OTHER PAYABLES - Trade and other payables represent liabilities for goods and services provided to the Group prior to the end of financial year which are unpaid. They are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). Otherwise, they are presented as non-current liabilities.
Trade and other payables are initially recognized at fair value, and subsequently carried at amortized cost using the effective interest method.
| F-16 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
BORROWINGS - Borrowings are presented as current liabilities unless the Group has an unconditional right to defer settlement for at least 12 months after the balance sheet date, in which case they are presented as non-current liabilities.
| (a) | Borrowings |
Borrowings are initially recognized at fair value (net of transaction costs) and subsequently carried at amortized cost. Any difference between the proceeds (net of transaction costs) and the redemption value is recognized in profit or loss over the period of the borrowings using the effective interest method.
| (b) | Convertible loans |
The component parts of the convertible loans are classified separately as financial liability and equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument. A conversion option that will be settled by the exchange of a fixed amount of cash or another financial asset for a fixed number of the Company’s own equity instruments is an equity instrument. A conversion option that will be settled other than by the exchange of a fixed amount of cash or another financial asset for a fixed number of the Group’s own equity instruments is a conversion option derivatives. Convertible loans issued by the Company are designated at fair value through profit or loss (“FVTPL”) on initial recognition. Convertible loans are measured at fair value with changes in fair value recognized in profit or loss.
Transaction costs that relate to the issue of convertible loans are charged to profit or loss immediately.
| (c) | Borrowing costs |
All borrowing costs are recognized in profit or loss in the period in which they are incurred.
LEASES
When the Group is the lessee
At the inception of the contract, the Group assesses if the contract contains a lease. A contract contains a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. Reassessment is only required when the terms and conditions of the contract are changed.
| F-17 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| ● | Lease liabilities |
The initial measurement of a lease liability is measured at the present value of the lease payments discounted using the implicit rate in the lease, if the rate can be readily determined. If that rate cannot be readily determined, the Group shall use its incremental borrowing rate.
Lease payments include the following:
| - | Fixed payment (including in-substance fixed payments), less any lease incentives receivables; | |
| - | Variable lease payment that are based on an index or rate, initially measured using the index or rate as at the commencement date; | |
| - | Amount expected to be payable under residual value guarantees; | |
| - | The exercise price of a purchase option if is reasonably certain to exercise the option; and | |
| - | Payment of penalties for terminating the lease, if the lease term reflects the Group exercising that option. |
For contracts that contain both lease and non-lease components, the Group allocates the consideration to each lease component on the basis of the relative stand-alone price of the lease and non-lease component. The Group has elected to not separate lease and non-lease component for property leases and account these as one single lease component.
Lease liability is measured at amortized cost using the effective interest method. Lease liability shall be remeasured when:
| - | There is a change in future lease payments arising from changes in an index or rate; | |
| - | There is a change in the Group’s assessment of whether it will exercise an extension option; or | |
| - | There is modification in the scope or the consideration of the lease that was not part of the original term. |
| F-18 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| ● | Short-term and low-value leases |
The Group has elected to not recognized right-of-use assets and lease liabilities for short-term leases that have lease terms of 12 months or less and leases of low value leases. Lease payments relating to these leases are expensed to profit or loss on a straight-line basis over the lease term.
EMPLOYEE BENEFITS - Employee benefits are recognized as an expense, unless the cost qualifies to be capitalized as an asset.
| (a) | Defined contribution plans |
Defined contribution plans are post-employment benefit plans under which the Group pays fixed contributions into separate entities such as the Central Provident Fund on a mandatory, contractual or voluntary basis. The Group has no further payment obligations once the contributions have been paid.
| (b) | Employee leave entitlement |
Employee entitlements to annual leave are recognized when they accrue to employees. A provision is made for the estimated liability for annual leave as a result of services rendered by employees up to the balance sheet date.
PROVISIONS - Provisions are recognized when the Group has a present obligation (legal or constructive) as a result of a past event, it is probable that the Group will be required to settle the obligation, and a reliable estimate can be made of the amount of the obligation.
The amount recognized as a provision is the best estimate of the consideration required to settle the present obligation at the end of the reporting period, taking into account the risks and uncertainties surrounding the obligation. Where a provision is measured using the cash flows estimated to settle the present obligation, its carrying amount is the present value of those cash flows.
When some or all of the economic benefits required to settle a provision are expected to be recovered from a third party, the receivable is recognized as an asset if it is virtually certain that reimbursement will be received and the amount of the receivable can be measured reliably.
| F-19 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
REVENUE RECOGNITION - Revenue is measured based on the consideration to which the Group expects to be entitled in exchange for transferring promised goods or services to the customer, excluding amounts collected on behalf of third parties.
Revenue is recognized when the Group satisfies a performance obligation by transferring promised goods or services to the customer, which is when the customer obtains control of the goods or services. A performance obligation may be satisfied at a point in time or over time. The amount of revenue recognized is the amount allocated to the satisfied performance obligation.
Advance payments received from customers are recognized as contract liabilities as the Group has not yet satisfied its performance obligation. Contract liabilities are recognized as other income when the Group satisfied its performance obligation.
Research income - Research income is recognized at point in time when the Group satisfied its performance obligation by transferring control of promised goods to the customers. The transaction price is the amount of the consideration in the contract (mainly on costs recovery basis) to which the Group expects to be entitled in exchange for transferring the promised goods.
Interest Income - Interest income from a financial asset is accrued on a time basis using the effective interest method, by reference to the principal outstanding and at the effective interest rate applicable, which is the rate that exactly discounts the estimated future cash receipts through the expected life of the financial asset to that asset’s net carrying amount on initial recognition.
RESEARCH AND DEVELOPMENT EXPENSES - R&D expenses are charged to profit or loss in the period in which it occurred. Non-refundable advance payments for goods or services to be received in the future for use in R&D activities are recorded as prepaid expenses. The prepaid amounts are expensed as the related goods are delivered or the services are performed. We allocate our direct R&D expenses across each product candidate.
R&D expenses consist primarily of costs incurred for our research and pre-clinical activities, which include:
| ● | salaries, welfare benefits and other related expenses for the employees engaged in R&D functions; | |
| ● | costs of manufacturing product candidates and consultants engaged in research, pre-clinical studies and potential future clinical trials; | |
| ● | expenses related to the upkeep of the facilities we use, which include expenses for maintenance of facilities and laboratory equipment | |
| ● | costs related to regulatory compliance; and | |
| ● | royalty payments for our intellectual properties used in research activities. |
GOVERNMENT GRANTS AND SUBSIDIES - Grants from the government are recognized as a receivable at their fair value when there is reasonable assurance that the grant will be received and the Group will comply with all the attached conditions.
Government grants receivable are recognized as income over the periods necessary to match them with the related costs which they are intended to compensate, on a systematic basis. Government grants relating to expenses are shown separately as other income.
Grants related to assets are presented as deferred income under trade and other payables.
SEGMENT REPORTING - An operating segment is a component of an entity:
| ● | that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same entity); | |
| ● | whose operating results are regularly reviewed by the entity’s chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance; and | |
| ● | for which discrete financial information is available. |
The Group has identified one operating segment i.e. the business of innate immune cell-based immunotherapy, pluripotent stem cell-based therapy and undertaking the research and development of immune cell and stem cell-based therapy.
The assessment of reportable segments is based upon having similar economic characteristics and if the operating segments are similar in the following respects:
| ● | the nature of the products and services; | |
| ● | the nature of the production processes; | |
| ● | the type or class of customer for their products and services; | |
| ● | the methods used to distribute their products or provide their services; and | |
| ● | if applicable, the nature of the regulatory environment, for example, banking, insurance, or public utilities. |
Reportable segments are distinguished due to their differences in their operations and economics. They are managed separately because they require different business, technological, and marketing strategies. The Group’s Chairman is considered to be the Group’s Chief Operating Decision Maker (“CODM”). The CODM reviews non-financial information, for purposes of allocating resources. Based on the internal financial information provided to the CODM, the Group has determined that the identified operating segment as one reportable segment.
The CODM evaluates the assets and liabilities despite disaggregated financial information being available, the accounting policies used in the determination of the segment amounts are the same as those used in the preparation of the Group’s financial statements.
SHARE CAPITAL - Ordinary shares are classified as equity. Incremental costs directly attributable to the issuance of new ordinary shares are deducted against the share capital account.
SHARE APPLICATION MONIES – Share application monies are recognized as equity when monies received from prospective shareholder pending allotment.
| F-20 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
INCOME TAX - Current income tax for current and prior periods is recognized at the amount expected to be paid to or recovered from the tax authorities, using the tax rates and tax laws that have been enacted or substantively enacted by the balance sheet date. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation and considers whether it is probable that a tax authority will accept an uncertain tax treatment. The Group measures its tax balances either based on the most likely amount or the expected value, depending on which method provides a better prediction of the resolution of the uncertainty.
Deferred income tax is recognized for all temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements except when the deferred income tax arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and affects neither accounting nor taxable profit or loss at the time of the transaction.
A deferred income tax liability is recognized on temporary differences arising on investments in subsidiaries, associates, except where the Group is able to control the timing of the reversal of the temporary difference and it is probable that the temporary difference will not reverse in the foreseeable future.
A deferred income tax asset is recognized to the extent that it is probable that future taxable profit will be available against which the deductible temporary differences and tax losses can be utilized.
Deferred income tax is measured:
| (i) | at the tax rates that are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled, based on tax rates and tax laws that have been enacted or substantively enacted by the balance sheet date; and | |
| (ii) | based on the tax consequence that will follow from the manner in which the Group expects, at the balance sheet date, to recover or settle the carrying amounts of its assets and liabilities. |
Current and deferred income taxes are recognized as income or expense in profit or loss, except to the extent that the tax arises from a business combination or a transaction which is recognized directly in equity. Deferred tax arising from a business combination is adjusted against goodwill on acquisition.
The Group accounts for investment tax credits (for example, productivity and innovation credit) similar to accounting for other tax credits where a deferred tax asset is recognized for unused tax credits to the extent that it is probable that future taxable profit will be available against which the unused tax credits can be utilized.
| F-21 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
FOREIGN CURRENCY TRANSACTIONS
| (a) | Functional and presentation currency |
Items included in the financial statements of each entity in the Group are measured using the currency of the primary economic environment in which the entity operates (“functional currency”). The financial statements are presented in Singapore Dollars, which is the functional currency of the Company.
| (b) | Transactions and balances |
Transactions in a currency other than the functional currency (“foreign currency”) are translated into the functional currency using the exchange rates at the dates of the transactions. Currency exchange differences resulting from the settlement of such transactions and from the translation of monetary assets and liabilities denominated in foreign currencies at the closing rates at the balance sheet date are recognized in profit or loss. Monetary items include primarily financial assets (other than equity investments) and financial liabilities. However, in the consolidated financial statements, currency translation differences arising from borrowings in foreign currencies and net investment in foreign operations, are recognized in other comprehensive income and accumulated in the currency translation reserve.
Foreign exchange gains and losses that relate to borrowings are presented in the profit or loss within “Finance expenses”. All other foreign exchange gains and losses impacting profit or loss are presented in the profit or loss within “Other (losses)/gains - net”.
Non-monetary items measured at fair values in foreign currencies are translated using the exchange rates at the date when the fair values are determined.
| (c) | Translation of Group entities’ financial statements |
The results and financial position of all the Group entities (none of which has the currency of a hyperinflationary economy) that have a functional currency different from the presentation currency are translated into the presentation currency as follows:
| (i) | assets and liabilities are translated at the closing exchange rates at the reporting date; | |
| (ii) | income and expenses are translated at average exchange rates (unless the average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are translated using the exchange rates at the dates of the transactions); and |
| F-22 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (iii) | all resulting currency translation differences are recognized in other comprehensive income and accumulated in the currency translation reserve. These currency translation differences are reclassified to profit or loss on disposal or partial disposal with loss of control of the foreign operation. |
Goodwill and fair value adjustments arising on the acquisition of foreign operations are treated as assets and liabilities of the foreign operations and translated at the closing rates at the reporting date.
CASH AND CASH EQUIVALENTS - For the purpose of presentation in the consolidated statement of cash flows, cash and cash equivalents include cash on hand, deposits with financial institutions which are subject to an insignificant risk of change in value.
RELATED PARTIES
A related party is a person or entity that is related to the Group.
(A) A person or a close member of that person’s family is related to the Group if that person:
(i) has control or joint control over the Group;
(ii) has significant influence over the Group; or
(iii) is a member of the key management personnel of the Company or of a parent of the Company.
(B) An entity is related to the Group if any of the following conditions applies:
(i) The entity and the Company are members of the same group (which means that each parent, subsidiary and fellow subsidiary is related to the others);
(ii) One entity is an associate or joint venture of the other entity (or an associate or joint venture of a member of a group of which the other entity is a member);
(iii) Both entities are joint ventures of the same third party;
(iv) One entity is a joint venture of a third entity and the other entity is an associate of the third entity;
(v) The entity is a post-employment benefit plan for the benefit of employees of either the Group or an entity related to the Group. If the Group is itself such a plan, the sponsoring employers are also related to the Group;
(vi) The entity is controlled or jointly controlled by a person identified in (A);
(vii) A person identified in (A)(i) has significant influence over the entity or is a member of the key management personnel of the entity (or of a parent of the entity); or
(viii) The entity, or any member of a group of which it is a part, provides key management personnel services to the Company or to a parent of the Company.
RESERVES
(i) Translation reserves
Translation reserves represent the foreign currency translation difference arising from the translation of the financial statements of companies within the Group from their functional currency to the Group’s presentation currency.
(ii) Retained earnings
Retained earnings comprise the cumulative net gains and losses recognized in the Group’s consolidated statement of profit or loss.
| 3 | CRITICAL ACCOUNTING JUDGEMENTS AND KEY SOURCES OF ESTIMATION UNCERTAINTY |
In the application of the Company’s accounting policies, which are described in Note 2 to the financial statements, management is required to make judgements, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods.
Key sources of estimation uncertainty
The key assumptions concerning the future and other key sources of estimation uncertainty at the end of the reporting period, that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year, are disclosed below:
| (a) | Impairment assessment for property, plant, and equipment (Note 7) and investment in an associate(Note 6) |
Property, plant and equipment and investment in associate, are tested for impairment when there is any objective evidence or indication that these assets may be impaired. Impairment exists when the carrying value of an asset or CGU exceeds its recoverable amount.
The recoverable amounts of property, plant and equipment and investment in associate have been determined based on higher of the fair value less costs to sell or value-in use (“VIU”) calculations. If the carrying amounts exceed the recoverable amounts, an impairment is recognized to profit or loss for the differences.
| F-23 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
Property, plant and equipment mainly consist of freehold land, building, and laboratory equipment. Management has assessed that there were no objective evidence or indication that the carrying amounts of the Group’s property, plant and equipment may not be recoverable as at the end of reporting date. Accordingly, impairment assessment is not required.
| (b) | Valuation of convertible loans (Note 12) |
The convertible loans issued by the Company are classified as financial liabilities at FVTPL. No quoted prices in an active market are available for these financial liabilities. These financial liabilities were valued by the directors of the Company with reference to valuations carried out by an independent qualified professional valuer. The fair value of these financial liabilities is established by using valuation techniques as set out in the Note to the financial statements. Valuation modals established by the valuer maximise the use of market inputs to the extent possible. Management estimates and assumptions are reviewed periodically and are adjusted if necessary. Should any of the estimates and assumptions changed, it may lead to a change in the fair value to be recognized in profit or loss. As at the end of the reporting periods, management has assessed that the probability of conversion of the convertible loans was anticipated to be 30% whilst the probability of redemption of the convertible loans was anticipated to be 70%.
Going concern
Since the inception the Group incurred substantial losses because the Group is still primarily focused on research and development. It has yet to fully commercialize its products and therapies to generate sustainable re-occurring cash flows. During the years ended December 31, 2020 and 2021, the Group had losses for the year of S$1.94 million and S$2.05 million (approximately U.S.$ 1.48 million), respectively. Additionally, the Group had net cash outflows from operating activities of S$849,443 and S$1.71 million (approximately U.S.$ 1.23 million) for the years ended December 31, 2020, and 2021. These circumstances raise substantial doubt regarding the Group ability to continue as going concern. Management’s plan to address this doubt by continuing procure financing from private investors in the form issuances of rights, ordinary shares, debt, or convertible hybrid instruments to raise cash and working capital for the Group. Management may also contribute their own time at less than market rates for the services. The Group also endeavors to list its ordinary shares on national stock exchange in the United States and concurrently sell additional ordinary an initial public offering that will provide adequate financial resources for management to continue to execute research and development plan and eventually bring a viable suite of oncology products and therapies to the market. Management may not be successful in raising additional funds via the means described above. These financial statements have been prepared on a going concern basis, and not a liquidation basis; accordingly, the accounts may be presented different if a going concern basis was not employed.
| 4 | CASH AND CASH EQUIVALENTS |
For the purpose of the statement of cash flows, cash and cash equivalents comprise the following:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Cash at banks | 885,272 | 2,512,768 | ||||||
| 5 | OTHER RECEIVABLES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Deposits | 43,491 | 15,232 | ||||||
| Due from key management personnel | 261 | 1,259 | ||||||
| Prepayments | 39,899 | 138,014 | ||||||
| Prepaid initial public offering expenses | - | 178,857 | ||||||
| Prepaid consumables | 240,442 | 431,582 | ||||||
| Advances paid for investment in a Malaysia company | 247,899 | - | ||||||
| Sundry receivables | 11,762 | 4,031 | ||||||
| Total | 583,754 | 768,975 | ||||||
Amounts due from key management personnel are generally advances extended to them during the course of business. The balance is unsecured, interest-free, and repayable on demand.
| F-24 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 6 | INVESTMENT IN AN ASSOCIATE |
On January 20, 2020, the Company entered into an Investment Agreement with third parties to acquire 20% of the equity interests in Landmark Medical Centre Sdn Bhd (“LMC”) at a cash consideration of RM1.5 million (approximately S$489,211). The transaction was completed on September 6, 2021.
The Group’s investment in the associate is summarized below:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Beginning of financial year | - | - | ||||||
| Additions | - | 489,211 | ||||||
| Share of post-acquisition profit | - | 2,719 | ||||||
| Impairment of investment in an associate | - | (215,297 | ) | |||||
| Currency realignment | - | (3,663 | ) | |||||
| End of financial year | - | 272,970 | ||||||
Management has assessed the recoverable amount of the investment in associate calculation based on its VIU, using discounted cash flow forecasts covering a five-year period in which the management made judgements over certain key inputs in relation to cash flows, revenue growth rates and discount rate. It was concluded that the fair value less costs of disposal did not exceed the VIU. As a result of this analysis, an impairment loss of S$215,297 is recognized during the year ended December 31, 2021 (2020: Nil).
Key assumptions used to determine the value in use of the investment in associate are as follows:
| % | ||||
| Revenue growth rate | 2.38 - 3.05 | |||
| Discount rate | 13.90 | |||
| Name of entity | Principal activities | Country of business / incorporation | Group’s effective equity interest held | |||||||||
| 2020 | 2021 | |||||||||||
| % | % | |||||||||||
| Landmark Medical Centre Sdn Bhd | Operations of private specialist hospital | Malaysia | - | 20 | ||||||||
The following table illustrates the summarized financial information of the Group’s material associate (and not the Group’s share of those amounts), adjusted for difference in accounting policies between the Group and the associate, if any.
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Current assets | - | 818,177 | ||||||
| Non-current assets | - | 800,979 | ||||||
| Current liabilities | - | (56,776 | ) | |||||
| Non-current liabilities | - | (197,529 | ) | |||||
| Net assets of the associate | - | 1,364,851 | ||||||
| Revenue | - | 746,074 | ||||||
| Loss for the year | - | (35,264 | ) | |||||
Reconciliation of the summarized financial information presented to the carrying amount of the Group’s investment in the associate is as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Net assets | - | 1,364,851 | ||||||
| Group’s equity interest | - | 20 | % | |||||
| Group share of net assets | - | 272,970 | ||||||
| Goodwill | - | 215,297 | ||||||
| Impairment of investment in an associate | - | (215,297 | ) | |||||
| Carrying value | - | 272,970 | ||||||
| F-25 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 7 | PROPERTY, PLANT AND EQUIPMENT |
| Building | Freehold land | Laboratory equipment | Motor vehicle | Furniture, fittings and equipment | Renovation | Total | ||||||||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | S$ | S$ | ||||||||||||||||||||||
| Cost: | ||||||||||||||||||||||||||||
| At January 1, 2020 | 652,096 | 426,637 | 780,247 | 53,943 | 242,242 | 369,669 | 2,524,834 | |||||||||||||||||||||
| Addition | - | - | 206,716 | - | 21,079 | 16,930 | 244,725 | |||||||||||||||||||||
| Currency realignment | 396 | 260 | 1,270 | 33 | 199 | 291 | 2,449 | |||||||||||||||||||||
| At December 31, 2020 | 652,492 | 426,897 | 988,233 | 53,976 | 263,520 | 386,890 | 2,772,008 | |||||||||||||||||||||
| Addition | 491,563 | - | 14,137 | - | 16,579 | 60,075 | 582,354 | |||||||||||||||||||||
| Currency realignment | (11,291 | ) | (7,387 | ) | (57,046 | ) | (934 | ) | (6,147 | ) | (38,098 | ) | (120,903 | ) | ||||||||||||||
| At December 31, 2021 | 1,132,764 | 419,510 | 945,324 | 53,042 | 273,952 | 408,867 | 3,233,459 | |||||||||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||||||||||
| At January 1, 2020 | 9,963 | - | 118,982 | 8,989 | 30,673 | 19,847 | 188,454 | |||||||||||||||||||||
| Addition | 10,832 | - | 168,908 | 10,753 | 55,682 | 35,151 | 281,326 | |||||||||||||||||||||
| Currency realignment | 48 | - | 719 | 49 | 235 | 152 | 1,203 | |||||||||||||||||||||
| At December 31, 2020 | 20,843 | - | 288,609 | 19,791 | 86,590 | 55,150 | 470,983 | |||||||||||||||||||||
| Addition | 18,141 | - | 191,988 | 10,615 | 57,632 | 38,290 | 316,666 | |||||||||||||||||||||
| Currency realignment | (367 | ) | - | (45,055 | ) | (349 | ) | (3,232 | ) | (978 | ) | (49,981 | ) | |||||||||||||||
| At December 31, 2021 | 38,617 | - | 435,542 | 30,057 | 140,990 | 92,462 | 737,668 | |||||||||||||||||||||
| Carrying amount: | ||||||||||||||||||||||||||||
| At December 31, 2020 | 631,649 | 426,897 | 699,624 | 34,185 | 176,930 | 331,740 | 2,301,025 | |||||||||||||||||||||
| At December 31, 2021 | 1,094,147 | 419,510 | 509,782 | 22,985 | 132,962 | 316,405 | 2,495,791 | |||||||||||||||||||||
| F-26 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
Motor vehicle is acquired under finance leasing arrangement. Details of the lease liability is disclosed in Note 13.
As of December 31, 2021, bank borrowing amounting to S$574,810 (2020: S$626,262) is secured by the freehold land and building of the Group with carrying amounts as disclosed above.
Depreciation expense included in the consolidated statement of profit or loss and other comprehensive income is analyzed as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Depreciation of property, plant and equipment (per above) | 281,326 | 316,666 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (195,202 | ) | (217,945 | ) | ||||
| 86,124 | 98,721 | |||||||
| 8 | INTANGIBLE ASSETS |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Goodwill | 355 | 355 | ||||||
| Intellectual properties licenses (Note (a)) | 33,527 | 22,827 | ||||||
| Computer software licenses (Note (b)) | 11,366 | 8,841 | ||||||
| Total | 45,248 | 32,023 | ||||||
| (a) | Intellectual properties licenses |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Cost: | ||||||||
| At beginning of financial year | 53,500 | 62,060 | ||||||
| Additions | 8,560 | - | ||||||
| At end of financial year | 62,060 | 62,060 | ||||||
| Accumulated amortization: | ||||||||
| At beginning of financial year | 17,833 | 28,533 | ||||||
| Amortization charge | 10,700 | 10,700 | ||||||
| At end of financial year | 28,533 | 39,233 | ||||||
| Carrying value | 33,527 | 22,827 | ||||||
| F-27 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (b) | Computer software licenses |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Cost: | ||||||||
| At beginning of financial year | - | 11,873 | ||||||
| Additions | 11,870 | - | ||||||
| Currency realignment | 3 | (18 | ) | |||||
| At end of financial year | 11,873 | 11,855 | ||||||
| Accumulated amortization: | ||||||||
| At beginning of financial year | - | 507 | ||||||
| Amortization charge | 507 | 2,512 | ||||||
| Currency realignment | - | (5 | ) | |||||
| At end of financial year | 507 | 3,014 | ||||||
| Carrying value | 11,366 | 8,841 | ||||||
| (c) | Amortization expense is analyzed as followed: |
| Intellectual properties licenses | 10,700 | 10,700 | ||||||
| Computer software licenses | 507 | 2,512 | ||||||
| Total | 11,207 | 13,212 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (10,700 | ) | (10,700 | ) | ||||
| 507 | 2,512 |
Management has evaluated, assessed and concluded that there is no indication of impairment for the intangible assets and accordingly no impairment allowance is necessary.
| 9 | TRADE AND OTHER PAYABLES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Trade payable | 18,915 | 46,249 | ||||||
| Advances to key management personnel | 12,765 | 1,265 | ||||||
| Sundry payables | 22,658 | 18,038 | ||||||
| Accrued expenses | 286,696 | 144,701 | ||||||
| Deferred grant income | 11,360 | 8,480 | ||||||
| Total | 352,394 | 218,733 | ||||||
The amounts due to key management personnel related to reimbursement of expenses. It is unsecured, interest-free, and repayable on demand.
| F-28 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 10 | CONTRACT LIABILITIES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Contract liabilities | 6,318 | 13,492 | ||||||
Contract liabilities are fees which the Group billed and received consideration ahead of the transfer of goods and services. Management expects that all the unsatisfied performance obligations as of the end of the reporting periods may be recognized as other income within the next twelve months.
There were no contract liabilities as of January 1, 2020. No contract liabilities at December 31, 2020 and December 31, 2019 have been recognized as other income in 2021 and 2020, respectively. Increase in contract liabilities for the years ended December 31, 2021 and 2020 was mainly due to more advances being received from customers for services to be provided in the future.
| 11 | BORROWINGS |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Current | ||||||||
| Bank loan | 35,588 | 35,691 | ||||||
| Third party loan | - | 350,000 | ||||||
| Total – current | 35,588 | 385,691 | ||||||
| Non-current | ||||||||
| Bank loan | 590,674 | 539,119 | ||||||
| Total | 626,262 | 924,810 | ||||||
Note:
| Bank loan: | Principal amount of RM2,000,000 (equivalent to S$660,000) withdrawn from a financial institution, which is charged an effective interest rate of 4.4% per annum and repayable over 180 months in equal monthly instalments of RM15,454. |
The exposure of the borrowing of the Group to interest rate changes and the contractual repayment dates at the balance sheet date are as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| 6 months or less | 17,714 | 17,752 | ||||||
| 6 – 12 months | 17,874 | 17,939 | ||||||
| 1 – 5 years | 158,115 | 159,288 | ||||||
| Over 5 years | 432,559 | 379,831 | ||||||
| Total | 626,262 | 574,810 | ||||||
The loan is secured over building and freehold land (Note 7) and personal guarantees by a director.
| Third party loan: | Principal amount of S$350,000 borrowed from an independent individual, which is charged a fixed interest rate of 3.0% per annum and is repaid in a single lump sum upon the expiry of 12 months from the date of disbursement of the loan. Subject to mutual consent, the loan can be extended for another 6 months, or such other extension period agreed to in writing. The loan is unsecured. |
| F-29 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 12 | CONVERTIBLE LOANS |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Convertible loans | 2,323,423 | 2,310,757 | ||||||
| Loan 1 | Loan 1 | |||||||||||||||
| (Tranche 1) | (Tranche 2) | Loan 2 | Total | |||||||||||||
| S$ | S$ | S$ | S$ | |||||||||||||
| At January 1, 2020 | - | - | - | - | ||||||||||||
| Nominal amount issued | 1,000,000 | 500,000 | 250,000* | 1,750,000 | ||||||||||||
| Fair value adjustment | 325,403 | 160,188 | 87,832 | 573,423 | ||||||||||||
| At December 31, 2020 | 1,325,403 | 660,188 | 337,832 | 2,323,423 | ||||||||||||
| Fair value adjustment | (14,333 | ) | (6,817 | ) | 8,484 | (12,666 | ) | |||||||||
| At December 31, 2021 | 1,311,070 | 653,371 | 346,316 | 2,310,757 | ||||||||||||
* S$250,000 received and accounted as other payables in 2019. This has been reclassified to convertible loan for the financial year ended December 31, 2020.
The convertible loan of S$250,000, where the agreement date started from December 7, 2020, with a maturity of two years from the date of disbursement on September 18, 2019 was extended from September 17, 2021 to November 30, 2022. The convertible loan carries a fixed interest rate of 5% per annum. As of December 31, 2021, the convertible loan was valued at S$346,316 (2020: S$337,832), resulted in a fair value loss of S$8,484 (2020: S$87,832) for the year ended December 31, 2021. The loan is unsecured.
The convertible loan of S$1,500,000, where the agreement date started from December 10, 2019, with a maturity of two years from the first disbursement date on January 6, 2020 was extended for a further one year period on December 31, 2021. The convertible loan carries a fixed interest rate of 5.5%. As of December 31, 2021, the convertible loan was valued at S$1,964,441 (2020: S$1,985,591), resulting in a fair value gain of S$21,150 (2020: fair value loss S$485,591) for the year ended December 31, 2021. As security for this loan, the director Choo Chee Kong and Messiah Limited executed personal and corporate guarantees, respectively, in favor of guaranteeing the obligations of the Company under the agreement. Choo Chee Kong is also the sole director and a shareholder of Messiah Limited. Upon receipt of the redemption notice by the Company issued by the lender during the loan period, on the date falling on each anniversary of the first disbursement date on January 6, 2020, or upon the occurrence of a liquidity event, the Company shall repay such amount of the convertible loan (together with all interest accrued thereon) specified in the redemption notice to the lender within three months from the date of the redemption notice. Therefore, the convertible loan is classified as a current liability.
The convertible loans were stated at fair value which was valued by the directors of the Company with reference to an independent qualified professional valuer.
Key valuation assumptions used to determine the fair value of the convertible loans are as follows:
| % | ||||
| As at December 31, 2020 | ||||
| Risk-free interest rate | 0.87 | |||
| Discount rate for redemption scenario | 15.80 - 16.00 | |||
| Discount rate for conversion scenario | 1.03 | |||
| Conversion discount rate | 50.00 | |||
| As at December 31, 2021 | ||||
| Risk-free interest rate | 0.87 | |||
| Discount rate for redemption scenario | 17.68 - 18.31 | |||
| Discount rate for conversion scenario | 0.56 | |||
| Conversion discount rate | 50.00 | |||
The Group measures the convertible loans at fair value through profit or loss. The fair value measurement of the convertible loans is categorized as Level 3 under the Fair Value Hierarchy with the following details:
| Valuation technique input(s) | Significant unobservable input(s) | Inter-relationship between significant unobservable inputs and fair value measurements | |||
Debt component : Discount cash flow method
| Discount cash flow method | An increase in discount rate would result in a decrease in the fair value measurement of the convertible loans and vice versa.
If the discount rate increases by 5% while holding all other variables constant, the carrying fair value of the convertible loans would decrease by approximately S$9,000 for the financial year ended December 31, 2020 and by approximately S$8,000 for the financial year ended December 31, 2021. | |||
Conversion option component:
Number of Conversion Shares to be issued, Y = A/B * C
| Discount rate per the synthetic rating corporate spread as shown in the above table. | ||||
| A - | Loan conversion amount / (1-discount) | ||||
| B - | Conversion valuation | ||||
| C - | total number of issued Shares as at the date of the Conversion Notice + Y | ||||
During the year, there were no transfers into or out of Level 3.
| F-30 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 13 | LEASE LIABILITIES |
Set out below are the carrying amounts of lease liabilities and the movements during the years:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| At beginning of year | 34,760 | 31,122 | ||||||
| Currency realignment | 7 | (535 | ) | |||||
| Interest charged (Note 19) | 784 | 1,289 | ||||||
| Payment made | (4,429 | ) | (8,745 | ) | ||||
| At end of year | 31,122 | 23,131 | ||||||
Presentation on
Consolidated Statement of Financial Positions:
| Current | 7,583 | 7,813 | ||||||
| Non-current | 23,539 | 15,318 | ||||||
| Total | 31,122 | 23,131 |
The effective interest rate applied to the lease liabilities recognized in the statement of financial position is 4.73% per annum.
Lease liabilities of the Group are secured over a motor vehicle (Note 7).
Total cash outflow for leases amounts to S$10,742 (2020: S$6,854) (including low-value assets rental).
| 14 | SHARE CAPITAL AND SHARE APPLICATION MONIES |
| 2020 | 2021 | |||||||
| Number of shares: | ||||||||
| At beginning of financial year | 5,835,207 | 6,078,341 | ||||||
| Issued | 243,134 | 1,401,404 | ||||||
| At end of financial year | 6,078,341 | 7,479,745 | ||||||
| Paid-up capital (S$): | ||||||||
| At beginning of financial year | 3,000,000 | 3,500,000 | ||||||
| Issued | 500,000 | 2,190,355 | ||||||
| Transfer from share application monies | - | 858,605 | ||||||
| At end of financial year | 3,500,000 | 6,548,960 | ||||||
| Share application monies (S$): | ||||||||
| At beginning of financial year | - | - | ||||||
| Amount received | - | 2,000,000 | ||||||
| Transfer to paid-up capital | - | (858,605 | ) | |||||
| At end of financial year | - | 1,141,395 | ||||||
The paid-up ordinary shares have no par value and carry one vote per share and carry a right to dividends as and when declared by the Company.
Share application monies
Share application monies of S$2,000,000 were received from prospective shareholders in April 2021 and in June 2021 pending allotment of 837,975 additional ordinary shares.
On September 20, 2021, the Company has issued for 359,745 ordinary shares for S$858,605 which was transferred to paid-up capital accordingly.
The newly issued shares rank pari passu in all aspects with the previously issued shares.
| F-31 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
Paid-up capital
For the financial year ended December 31, 2020:
The Company issued 243,134 ordinary shares for a total consideration of S$500,000 of cash to provide funds for the expansion of the Company’s operations.
For the financial year ended December 31, 2021:
The Company issued:
| (i) | 92,563 ordinary shares for a total consideration of S$190,355 for equity-settled share-based payment transactions of a professional fee. The equity-settled share-based payment transaction is measured at the fair value of the service received; | |
| (ii) | 949,096 ordinary shares for a total consideration of S$2,000,000 of cash to provide funds for the expansion of the Group’s operations; and | |
| (iii) | 359,745 ordinary shares for a total consideration of S$858,605 by offsetting the share application monies to provide funds for the expansion of the Group’s operations. |
The newly issued shares rank pari passu in all aspects with the previously issued shares.
On January 17, 2023, the Company effected a 1-for-380.83 reverse share split of the Company’s ordinary shares. Unless indicated or the context otherwise requires, all per share amounts and numbers of ordinary shares in this report have been retrospectively adjusted for the reverse share split, as if such reverse share split occurred on the first day of the years presented. Accordingly, the weighted average number of ordinary shares used in computing basic loss and diluted loss were changed from 2,246,002,833 and 2,640,989,439 to 5,897,651 and 6,934,824 for the years ended December 31, 2020 and 2021, respectively. Also, both basic and diluted loss per share for loss attributable to equity holders of the Company were changed from S$0.0009 and S$0.0008 (U.S.$0.0006) to S$0.33 and S$0.30 (U.S.$0.21) for the years ended December 31, 2020 and 2021, respectively.
| 15 | OTHER OPERATING INCOME |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Jobs Support Scheme | 41,786 | 4,880 | ||||||
| Wage Subsidy Program | 19,686 | 7,385 | ||||||
| Enterprise Development Grant | - | 10,400 | ||||||
| Jobs Growth Incentive | - | 3,750 | ||||||
| Hiring Incentive and Training Programme 2.0 | - | 7,709 | ||||||
| Others | 352 | 3,080 | ||||||
| Research income | 59,468 | 113,841 | ||||||
| Interest income | - | 3,369 | ||||||
| Others | 6,164 | 196 | ||||||
| Total | 127,456 | 154,610 | ||||||
The Jobs Support Scheme of the Singapore Government provides wage support and helps employers retain their local employees during a period of economic uncertainty.
The Wage Subsidy Program is financial assistance introduced in Malaysia paid to employers and helps employers affected economically by the COVID-19 pandemic to continue operations and avoid the loss of jobs and income streams for all enterprises.
| F-32 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
The Enterprise Development Grant (Industry) supports projects that aim to help Small to Medium Enterprises in different stages of their technology innovation journey, to overcome their challenges in terms of resources and research and development capabilities.
The Jobs Growth Incentive of the Singapore Government supports employers to accelerate their hiring of local workforce, so as to create good and long-term jobs for locals.
The Hiring Incentive and Training Programme 2.0 is an economic recovery incentive introduced in Malaysia to promote job creation among employers while increasing employment prospects.
| 16 | OTHER (LOSSES)/GAINS – NET |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Fair value (loss)/gain on convertible loans (Note 12) | (573,423 | ) | 12,666 | |||||
| Currency exchange loss - net | (433 | ) | (9,481 | ) | ||||
| Total | (573,856 | ) | 3,185 | |||||
| 17 | RESEARCH AND DEVELOPMENT EXPENSES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Employee benefits (Note 18) | 342,835 | 400,250 | ||||||
| Depreciation of property, plant and equipment (Note 7) | 195,202 | 217,945 | ||||||
| Amortization of intangible assets (Note 8) | 10,700 | 10,700 | ||||||
| Consumables consumed | 158,130 | 304,120 | ||||||
| Royalty expenses | 10,700 | 10,700 | ||||||
| Professional fees | 202,515 | 63,387 | ||||||
| Electricity expenses | 61,278 | 60,366 | ||||||
| Others | 56,731 | 23,155 | ||||||
| Total | 1,038,091 | 1,090,623 | ||||||
| 18 | EMPLOYEE BENEFITS EXPENSES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Wages and salaries | 407,558 | 491,015 | ||||||
| Defined contribution plans | 55,126 | 66,792 | ||||||
| Other short-term benefits | 11,142 | 11,245 | ||||||
| Total | 473,826 | 569,052 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (342,835 | ) | (400,250 | ) | ||||
| 130,991 | 168,802 | |||||||
| F-33 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 19 | FINANCE EXPENSES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Interest expenses on: | ||||||||
| Bank loan | 22,755 | 19,427 | ||||||
| Third party loan | - | 2,014 | ||||||
| Convertible loans | 81,980 | 94,966 | ||||||
| Lease liabilities (Note 13) | 784 | 1,289 | ||||||
| Total | 105,519 | 117,696 | ||||||
| 20 | OTHER EXPENSES |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Advertising | 6,183 | 2,481 | ||||||
| Entertainment | 2,291 | 310 | ||||||
| Low-value assets rental | 2,425 | 1,997 | ||||||
| Professional fees | 57,411 | 434,841 | ||||||
| Property tax | 3,483 | 4,081 | ||||||
| Printing and stationery | 5,231 | 5,747 | ||||||
| IT expenses | 5,707 | 9,482 | ||||||
| Repair and maintenance | 4,628 | 3,504 | ||||||
| Service fees (Note 26) | 6,625 | 11,660 | ||||||
| Subscription fee | 3,313 | 833 | ||||||
| Transportation and travelling | 1,224 | 867 | ||||||
| Tools and supplies | 1,619 | 1,086 | ||||||
| Water and electricity | 16,046 | 15,766 | ||||||
| Others | 11,808 | 26,111 | ||||||
| Total | 127,994 | 518,766 | ||||||
| 21 | INCOME TAX |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Current tax | - | - |
There is no provision for current income tax as there is no taxable profit for the financial years.
| F-34 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
The tax on the Group’s loss before tax differs from the theoretical amount that would arise using the Singapore standard rate of income tax as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Loss before income tax | (1,935,626 | ) | (2,051,903 | ) | ||||
| Tax calculated at tax rate of 17% | (329,056 | ) | (348,824 | ) | ||||
| Effects of: | ||||||||
| - different tax rates in other countries | (69,479 | ) | 4,990 | |||||
| - deferred tax assets not recognized | 236,754 | 275,944 | ||||||
| - expenses not deductible for tax purposes | 173,669 | 75,271 | ||||||
| - income not subject to tax | (11,888 | ) | (7,381 | ) | ||||
| Income tax expenses | - | - | ||||||
The Group has unused tax losses of S$1,951,000 and S$3,213,000 at December 31, 2020 and 2021, available for offset against future profits, respectively. The tax losses and capital allowances have no expiry except for tax losses amounting to approximately S$1,659,000 (2020: S$1,016,000) which can only be carried forward up to 7 years. No deferred tax asset has been recognized in respect of these tax losses due to the unpredictability of future profit streams.
Unutilized tax losses
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| At beginning of year | 851,000 | 1,951,000 | ||||||
| Addition | 1,100,000 | 1,262,000 | ||||||
| At end of year | 1,951,000 | 3,213,000 | ||||||
| Unrecorded deferred tax benefits @ 17% | 331,670 | 546,210 | ||||||
| 22 | COMMITMENTS |
| (a) | Operating lease commitments |
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Low value leases | 2,425 | 1,997 | ||||||
The Group has elected not to recognize right-of-use assets and lease liabilities for short-term leases that have lease terms of 12 months or less and leases of low value leases. Lease payments relating to these leases are charged to profit or loss on a straight-line basis over the lease term.
| F-35 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (b) | Capital commitments |
Capital expenditures contracted for at the balance sheet date but not recognized in the financial statements are as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Capital investment injection in a Malaysian company | 247,050 | - | ||||||
| (c) | Other commitments |
The Group entered into an exclusive, worldwide, non-sublicensable, non-transferable and revocable-for-cause license with an independent company for the NKG2DL-targeting CAR-gamma delta T cell and iPSC-gdNKT cell technology. As of December 31, 2021, the total future minimum payments under non-cancellable royalty agreements are as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Within 1 year | 4,458 | 4,458 | ||||||
| After 1 year but within 5 years | 42,800 | 42,800 | ||||||
| Over 5 years | 146,056 | 134,285 | ||||||
| Total | 193,314 | 181,543 | ||||||
| 23 | FINANCIAL INSTRUMENTS, FINANCIAL RISKS AND CAPITAL RISKS MANAGEMENT |
| a) | Categories of financial instruments |
The following table sets out the financial instruments as at the end of the reporting period:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Financial assets | ||||||||
| Financial assets at amortized cost: | ||||||||
| Other receivables | 55,514 | 20,522 | ||||||
| Cash and cash equivalents | 885,272 | 2,512,768 | ||||||
| 940,786 | 2,533,290 | |||||||
| Financial liabilities | ||||||||
| Financial liabilities at amortized cost: | ||||||||
| Trade and other payables | 341,034 | 210,253 | ||||||
| Lease liabilities | 31,122 | 23,131 | ||||||
| Borrowings other than convertible loans | 626,262 | 924,810 | ||||||
| 998,418 | 1,158,194 | |||||||
| Financial liabilities at FVTPL: | ||||||||
| Convertible loans | 2,323,423 | 2,310,757 | ||||||
| b) | Financial instruments subject to offsetting, enforceable master netting arrangements and similar agreements |
The Company does not have any financial instruments which are subject to enforceable master netting arrangements or similar netting agreements.
| c) | Financial risk management policies and objectives |
The management of the Company monitors and manages the financial risks relating to the operations of the Company to ensure appropriate measures are implemented in a timely and effective manner. These risks include market risk (including currency risk and interest rate risk), credit risk and liquidity risk.
| F-36 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (i) | Market risk management |
The Group activities are exposed primarily to the financial risks of changes in foreign currency exchange rates and interest rates. Management monitors risks associated with changes in foreign currency exchanges rates and interest rates and will consider appropriate measures should the need arise.
There has been no significant change to the Group’s exposure to market risk or the manner in which it manages and measures the risk.
| (ii) | Foreign currency risk management |
The Group operates in Singapore and Malaysia. Entities in the Group regularly transact in currencies other than their respective functional currencies (“foreign currencies”).
Currency risk arises when transactions are denominated in foreign currencies other than the Group entities’ respective functional currencies.
In addition, the Group is exposed to currency translation risk on the net assets in foreign operations.
The Group’s currency exposure based on the information provided to key management is as follows:
| SGD | MYR | USD | Total | |||||||||||||
| S$ | S$ | S$ | S$ | |||||||||||||
| December 31, 2021 | ||||||||||||||||
| Financial assets | ||||||||||||||||
| Cash and bank balances | 1,738,653 | 364,279 | 409,836 | 2,512,768 | ||||||||||||
| Other receivables | 7,909 | 12,613 | - | 20,522 | ||||||||||||
| Intra-group receivables | 1,089,953 | 12,803 | - | 1,102,756 | ||||||||||||
| 2,836,515 | 389,695 | 409,836 | 3,636,046 | |||||||||||||
| Financial liabilities | ||||||||||||||||
| Trade and other payables | (124,933 | ) | (85,186 | ) | (134 | ) | (210,253 | ) | ||||||||
| Lease liabilities | - | (23,131 | ) | - | (23,131 | ) | ||||||||||
| Borrowings | (2,660,757 | ) | (574,810 | ) | - | (3,235,567 | ) | |||||||||
| Intra-group payables | (1,089,953 | ) | (12,803 | ) | - | (1,102,756 | ) | |||||||||
| (3,875,643 | ) | (695,930 | ) | (134 | ) | (4,571,707 | ) | |||||||||
| Net financial (liabilities)/assets | (1,039,128 | ) | (306,235 | ) | 409,702 | (935,661 | ) | |||||||||
| Add: Net financial assets denominated respective entities’ functional currencies | 1,039,128 | 306,235 | - | 1,345,363 | ||||||||||||
| Currency exposure of financial assets, net of those denominated in the respective entities’ functional currencies | - | - | 409,702 | 409,702 | ||||||||||||
| December 31, 2020 | ||||||||||||||||
| Financial assets | ||||||||||||||||
| Cash and bank balances | 846,110 | 37,686 | 1,476 | 885,272 | ||||||||||||
| Other receivables | 12,155 | 43,359 | - | 55,514 | ||||||||||||
| Intra-group receivables | 701,236 | 11,420 | - | 712,656 | ||||||||||||
| 1,559,501 | 92,465 | 1,476 | 1,653,442 | |||||||||||||
| Financial liabilities | ||||||||||||||||
| Trade and other payables | (74,478 | ) | (266,556 | ) | - | (341,034 | ) | |||||||||
| Lease liabilities | - | (31,122 | ) | - | (31,122 | ) | ||||||||||
| Borrowings | (2,323,423 | ) | (626,262 | ) | - | (2,949,685 | ) | |||||||||
| Intra-group payables | (701,236 | ) | (11,420 | ) | - | (712,656 | ) | |||||||||
| (3,099,137 | ) | (935,360 | ) | - | (4,034,497 | ) | ||||||||||
| Net financial (liabilities)/assets | (1,539,636 | ) | (842,895 | ) | 1,476 | (2,381,055 | ) | |||||||||
| Add: Net financial assets denominated respective entities’ functional currencies | 1,539,768 | 875,835 | - | 2,415,603 | ||||||||||||
| Currency exposure of financial assets, net of those denominated in the respective entities’ functional currencies | 132 | 32,940 | 1,476 | 34,548 |
If the MYR and USD change against the SGD by 5% (2020: 5%) respectively with all other variables including the tax rate being held constant, the effects arising from the net financial liability/asset that are exposed to currency risk will be as follows:
| Increase/(decrease) | ||||||||
| 2021 | 2020 | |||||||
| Loss after tax | Loss after tax | |||||||
| S$ | S$ | |||||||
| MYR against SGD | ||||||||
| - strengthened | - | (1,367 | ) | |||||
| - weakened | - | 1,367 | ||||||
| USD against SGD | ||||||||
| - strengthened | (17,003 | ) | (61 | ) | ||||
| - weakened | 17,003 | 61 | ||||||
| F-37 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (iii) | Interest rate risk management |
The Group is exposed to interest rate risk as the Group has bank loan amounting to S$574,810 (2020: S$626,262) which bear floating interest rates. The interest rates and terms of repayment of the loans are disclosed in the Note 11 to the financial statements. The Group currently does not have an interest rate hedging policy.
The sensitivity analysis below has been determined based on the exposure to interest rate for non-derivative instruments at the end of the reporting period. A 50 basis point increase or decrease is used when reporting interest rate risk internally to key management personnel and represents management’s assessment of the reasonably possible change in interest rates.
If interest rates on loans had been 50 basis points higher/lower and all other variables were held constant, the Group’s loss for the year would increase/decrease by approximately S$2,184 (2020: S$2,380).
| F-38 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (iv) | Credit risk management |
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in a financial loss to us. Our credit risk is primarily attributable to our cash and cash equivalents.
Our credit risk arising from cash and cash equivalents is limited because the counterparties are banks and financial institutions with good credit ratings which we consider to have low credit risk.
| (v) | Liquidity risk management |
Prudent liquidity risk management implies sufficient cash to finance the Group’s and the Company’s operations and development activities. The Group manages the liquidity risk by maintaining a level of cash and cash equivalents deemed adequate to finance the Group’s business operations and development activities. The Group’s objective is to maintain a balance between continuing of funding and flexibility through the use of borrowings.
The table below analyses non-derivative financial liabilities of the Group and the Company into relevant maturity groupings based on the remaining period from the balance sheet date to the contractual maturity date.
| Less than 1 year or on demand | Between 1 and 2 years | Between 2 and 5 years | Over 5 years | Total undiscounted cash flow | ||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | ||||||||||||||||
| December 31, 2021 | ||||||||||||||||||||
| Trade and other payables | 210,253 | - | - | - | 210,253 | |||||||||||||||
| Lease liabilities | 8,740 | 8,740 | 7,283 | - | 24,763 | |||||||||||||||
| Borrowings (excluding lease liabilities) | 2,242,530 | 60,030 | 180,089 | 445,219 | 2,927,868 | |||||||||||||||
| December 31, 2020 | ||||||||||||||||||||
| Trade and other payables | 341,034 | - | - | - | 341,034 | |||||||||||||||
| Lease liabilities | 8,894 | 8,894 | 16,305 | - | 34,093 | |||||||||||||||
| Borrowings (excluding lease liabilities) | 401,920 | 1,561,087 | 183,260 | 514,145 | 2,660,412 | |||||||||||||||
| F-39 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| (vi) | Fair value of financial assets and financial liabilities |
The management considers that the carrying amounts of the Company’s financial assets and financial liabilities approximate their respective fair values due to the relatively short-term maturity of these financial instruments.
The fair values of the Group’s borrowings (other than convertible loans which are disclosed in Note 12 to the financial statements) are determined by using the discounted cash flows method using discount rate that reflects the issuer’s borrowing rate as at the end of the reporting period. The Group’s non-performance risk as at December 31, 2020 and 2021 was assessed to be insignificant.
The fair values of other classes of financial assets and liabilities are disclosed in the respective notes to financial statements.
| (d) | Capital risk management policies and objectives |
The management manages its capital to ensure that the Group will be able to continue as a going concern in order to provide returns for shareholders and benefits for other stakeholders and to maintain an optimal capital structure to reduce cost of capital.
The capital structure of the Company consists of equity attributable to owners of the Company, comprising issued capital and retained earnings as disclosed in the notes to financial statements.
| F-40 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
Management monitors capital based on debt-to-equity ratio. The debt-to-equity ratio is calculated as total debt divided by total equity.
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Total borrowings (Notes 11-13) | 2,980,807 | 3,258,698 | ||||||
| Total equity | 475,780 | 2,591,604 | ||||||
| Debt-to-equity % | 627 | % | 126 | % | ||||
The Group is not subject to externally imposed capital requirements for the financial years ended December 31, 2020 and 2021.
The Group’s overall strategy remains unchanged from prior year.
| 24 | SEGMENT INFORMATION |
Operating segments are identified on the basis of internal reports about components of the Group that are regularly reviewed by the COO (“Chief Operating Officer”) for the purpose of resource allocation and performance assessment. Segment results, assets and liabilities include items directly attributable to a segment as well as those that can be allocated on a reasonable basis. The Group operates in a single business segment which is the business of innate immune cell-based immunotherapy, pluripotent stem cell-based therapy and undertaking the research and development of immune cell and stem cell-based therapy. No operating segments have been aggregated to form the following reportable operating segment.
Geographical information:
Non-current assets (excluding investment in the associate) information based on the location of assets are as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Malaysia | 2,295,086 | 1,985,738 | ||||||
| Singapore | 51,187 | 542,076 | ||||||
| Total | 2,346,273 | 2,527,814 | ||||||
Non-current assets information presented above consist of property, plant and equipment and intangible assets as presented in the consolidated statement of financial position.
| F-41 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 25 | RECONCILIATIONS OF LIABILITIES ARISING FROM FINANCING ACTIVITIES |
| At beginning of year | Proceeds from borrowings | Payments | Interest expenses | Fair value changes | At end of year | |||||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | S$ | |||||||||||||||||||
| 2020 | ||||||||||||||||||||||||
| Bank loan | 633,576 | - | (30,069 | ) | 22,755 | - | 626,262 | |||||||||||||||||
| Convertible loans | - | 1,750,000# | (81,980 | ) | 81,980 | 573,423 | 2,323,423 | |||||||||||||||||
| Lease liabilities | 34,760 | - | (4,422 | )* | 784 | - | 31,122 | |||||||||||||||||
| 2021 | ||||||||||||||||||||||||
| Bank loan | 626,262 | - | (70,879 | ) | 19,427 | - | 574,810 | |||||||||||||||||
| Third party loan | - | 350,000 | (2,014 | ) | 2,014 | - | 350,000 | |||||||||||||||||
| Convertible loans | 2,323,423 | - | (94,966 | ) | 94,966 | (12,666 | ) | 2,310,757 | ||||||||||||||||
| Lease liabilities | 31,122 | - | (9,280 | )* | 1,289 | - | 23,131 | |||||||||||||||||
| # | Includes S$250,000 received and accounted as other payables in 2019. This has been reclassified to convertible loans for the financial year ended December 31, 2020. | |
| * | Includes exchange realignment of S$535 and S$7 for the years ended December 31, 2021 and 2020, respectively. |
| 26 | RELATED PARTY TRANSACTIONS |
Some of the Company’s transactions and arrangements are with related parties and the effect of these on the basis determined between the parties is reflected in these financial statements. The balances are unsecured, interest-free and repayable on demand unless otherwise stated.
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Transactions with related parties: | ||||||||
| Research income from associate | - | 50,205 | ||||||
| Purchase from associate | - | 2,499 | ||||||
| Service fee paid to directors | 6,625 | 11,660 | ||||||
| Balances with related parties: | ||||||||
| Trade payables to associate | - | 1,102 | ||||||
Compensation of directors and key management personnel
The remuneration of directors and key management personnel during the year were as follows:
| 2020 | 2021 | |||||||
| S$ | S$ | |||||||
| Wages and salaries | 255,491 | 273,229 | ||||||
| Defined contribution plans | 35,371 | 37,372 | ||||||
| Other short-term benefits | 2,900 | 3,366 | ||||||
| Total | 293,762 | 313,967 | ||||||
| Analyzed by: | ||||||||
| Directors of the Company | - | - | ||||||
| Other key management personnel | 293,762 | 313,967 | ||||||
| Total | 293,762 | 313,967 | ||||||
| F-42 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2020 and 2021
| 27 | HOLDING COMPANY AND RELATED COMPANY TRANSACTIONS |
The Company is a subsidiary of Glorious Finance Limited, incorporated in the British Virgin Islands, which is also the Company’s ultimate holding company. Related companies in these financial statements refer to members of the ultimate holding company’s group of companies.
Some of the Company’s transactions and arrangements are between members of the group and the effect of these on the basis determined between the parties is reflected in these financial statements. The intercompany balances are unsecured, interest-free and repayable on demand, unless otherwise stated.
| 28 | EVENTS OCCURRING AFTER BALANCE SHEET DATE |
The Company has assessed all events occurred from December 31, 2021, up through March 1, 2023, which is the date that these consolidated financial statements are available to be issued. Other than the events disclosed below, there are not any material subsequent events that would require disclosure in these consolidated financial statements.
On April 18, 2022, the Group had increased its issued share capital to S$7,690,355 by the issuance of 478,230 additional ordinary shares at a consideration sum of S$1,141,395. These newly issued shares rank pari passu in all respects with the previously issued shares.
On October 18, 2022, the Company had increased its share capital to S$8,913,005 and issued additional 486,485 ordinary shares at a consideration of S$1,222,650. These newly issued shares rank pari passu in all respects with the previously issued shares.
The Company intends to undertake a rights issue of new ordinary shares (the “Rights Shares”) in the capital of the Company at an aggregate of S$ 1 million. The Rights Shares, when allotted and issued, shall rank pari passu in all respects with the previously issued shares.
On November 23, 2022, the convertible loan amounting to S$250,000 was extended from December 1, 2022 to the conversion date. On January 26, 2023, the Company converted the convertible loan into 82,990 ordinary shares. These newly issued shares rank pari passu in all respects with the previously issued shares.
On November 23, 2022, the Company entered into a loan agreement with a value of S$300,000. On February 22, 2023, the loan was extended for further one month period or till the date of full repayment, whichever is earlier, commencing from March 4, 2023 and the fixed interest rate changed from 5.0% to 5.5% per annum.
On January 3, 2023, the convertible loan amounting to S$1,500,000 was extended from January 6, 2023 to January 5, 2024.
Effective on January 17, 2023, the registrant implemented a 1-for-380.83 reverse split of its ordinary shares pursuant to which the shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date. As a result, the Company has 8,444,460 ordinary shares issued and outstanding. Unless indicated or the context otherwise requires, all per share amounts and numbers of ordinary shares in this report have been retrospectively adjusted for the reverse share split, as if such reverse share split occurred on the first day of the years presented.
On January 19, 2023, the Company converted to a public limited company and the name of the Company has changed from “CytoMed Therapeutics Pte. Ltd.” to “CytoMed Therapeutics Limited” and that the name “CytoMed Therapeutics Limited” has substituted for “CytoMed Therapeutics Pte. Ltd.” wherever “CytoMed Therapeutics Pte. Ltd.” appears in the Constitution of the Company.
| F-43 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2021 AND JUNE 30, 2022
INDEX
| F-44 |

REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Shareholders of |
| CytoMed Therapeutics Limited |
Results of Review Interim Financial Statements
We have reviewed the accompanying unaudited condensed consolidated statement of financial position of CytoMed Therapeutics Limited and its subsidiaries (collectively the “Company”) as of June 30, 2022, and the related unaudited condensed consolidated statements of profit or loss and other comprehensive income, statements of changes in equity, and statements of cash flows for six-month periods ended June 30, 2021 and 2022, and the related notes (collectively referred to as the “interim financial statements”). Based on our reviews, we are not aware of any material modification that should be made to the accompanying interim financial statements for them to be in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We have previously audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated statements of financial position of the Company as of December 31, 2020 and 2021, and the related statements of profit or loss and other comprehensive income, changes in equity and cash flows for the years then ended (not presented herein); and in our report dated September 30, 2022, except for Notes 1, 14 and 28 which are dated March 1, 2023, we expressed an unqualified opinion on those financial statements. Our report included an emphasis of matter regarding the Company’s ability to continue as a going concern. Our opinion was not modified with respect to this matter. In our opinion, the information set forth in the accompanying condensed consolidated statement of financial position as of December 31, 2021, is fairly stated, in all material respects, in relation to the consolidated statement of financial position from which it has been derived.
Emphasis of Matter - Going Concern
The accompanying interim financial statements have been prepared assuming that the Company will continue as a going concern. As noted above, as of December 31, 2021, there was substantial doubt that the Company would continue as a going concern, As further described in Note 3 to the interim financial statements, the Company has continued to incur substantial losses and had net cash outflows from operating activities during the period ended June 30, 2022. These circumstances did not alleviate the substantial doubt that was outstanding as of December 31, 2021; accordingly, substantial doubt that the Company will continue as a going concern still exists. Management’s plans in regards to these matters are also described in Note 3. The interim financial statements do not include any adjustments that might result from the outcome of this doubt and uncertainty.
Basis for Review Results
These interim financial statements are the responsibility of the Company’s management. We conducted our review in accordance with the standards of the PCAOB. A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with standards of the PCAOB, the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
/s/ WWC, P.C.
WWC, P.C.
Certified Public Accountants
PCAOB ID: 1171
We have served as the Company’s auditor since 2022
San Mateo, California
September 30, 2022, except for Notes 1, 14 and 28 which are dated March 1, 2023

| F-45 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
AS OF DECEMBER 31, 2021 AND JUNE 30, 2022
| Note | December 31, 2021 | June 30, 2022 | June 30, 2022 | |||||||||||
| S$ | S$ | US$ | ||||||||||||
| ASSETS | ||||||||||||||
| Current assets | ||||||||||||||
| Other receivables | 5 | 768,975 | 712,173 | 512,244 | ||||||||||
| Cash and cash equivalents | 4 | 2,512,768 | 1,487,161 | 1,069,669 | ||||||||||
| Total current assets | 3,281,743 | 2,199,334 | 1,581,913 | |||||||||||
| Non-current assets | ||||||||||||||
| Investment in an associate | 6 | 272,970 | 244,347 | 175,751 | ||||||||||
| Property, plant and equipment | 7 | 2,495,791 | 2,684,305 | 1,930,738 | ||||||||||
| Intangible assets | 8 | 32,023 | 25,412 | 18,278 | ||||||||||
| Total non-current assets | 2,800,784 | 2,954,064 | 2,124,767 | |||||||||||
| Total assets | 6,082,527 | 5,153,398 | 3,706,680 | |||||||||||
| LIABILITIES AND EQUITY | ||||||||||||||
| Current liabilities | ||||||||||||||
| Trade and other payables | 9 | 218,733 | 220,122 | 158,326 | ||||||||||
| Contract liabilities | 10 | 13,492 | 13,110 | 9,430 | ||||||||||
| Borrowings | 11 | 385,691 | 382,179 | 274,890 | ||||||||||
| Convertible loans | 12 | 2,310,757 | 2,451,595 | 1,763,357 | ||||||||||
| Lease liabilities | 13 | 7,813 | 7,807 | 5,615 | ||||||||||
| Total current liabilities | 2,936,486 | 3,074,813 | 2,211,618 | |||||||||||
| Non-current liabilities | ||||||||||||||
| Borrowings | 11 | 539,119 | 508,580 | 365,806 | ||||||||||
| Lease liabilities | 13 | 15,318 | 11,000 | 7,912 | ||||||||||
| Total non-current liabilities | 554,437 | 519,580 | 373,718 | |||||||||||
| Total liabilities | 3,490,923 | 3,594,393 | 2,585,336 | |||||||||||
| Capital and reserves | ||||||||||||||
| Share capital | 14 | 6,548,960 | 7,690,355 | 5,531,436 | ||||||||||
| Share application monies | 14 | 1,141,395 | - | - | ||||||||||
| Translation reserves | (30,980 | ) | (68,188 | ) | (49,046 | ) | ||||||||
| Accumulated losses | (5,067,332 | ) | (6,062,619 | ) | (4,360,655 | ) | ||||||||
| Attributable to equity holders of the Company | 2,592,043 | 1,559,548 | 1,121,735 | |||||||||||
| Non-controlling interests | (439 | ) | (543 | ) | (391 | ) | ||||||||
| Total equity | 2,591,604 | 1,559,005 | 1,121,344 | |||||||||||
| Total liabilities and equity | 6,082,527 | 5,153,398 | 3,706,680 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-46 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME
FOR THE SIX MONTHS ENDED JUNE 30, 2021 AND 2022
| Six Months ended June 30, | ||||||||||||||
| Note | 2021 | 2022 | 2022 | |||||||||||
| S$ | S$ | US$ | ||||||||||||
| Revenue | - | - | - | |||||||||||
| Other operating income | 15 | 49,405 | 149,570 | 107,581 | ||||||||||
| Other losses - net | 16 | (209,533 | ) | (150,136 | ) | (107,988 | ) | |||||||
| Research and development expenses | 17 | (522,881 | ) | (604,043 | ) | (434,470 | ) | |||||||
| Depreciation of property, plant and equipment | 7 | (45,254 | ) | (56,048 | ) | (40,314 | ) | |||||||
| Amortization of intangible assets | 8 | (1,256 | ) | (1,254 | ) | (902 | ) | |||||||
| Employee benefits expenses | 18 | (78,992 | ) | (97,530 | ) | (70,150 | ) | |||||||
| Finance expenses | 19 | (57,668 | ) | (62,042 | ) | (44,625 | ) | |||||||
| Other expenses | 20 | (159,219 | ) | (151,625 | ) | (109,059 | ) | |||||||
| Share of results of associate | 6 | - | (22,283 | ) | (16,027 | ) | ||||||||
| Loss before income tax | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||
| Income tax | 21 | - | - | - | ||||||||||
| Loss for the period | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||
| Other comprehensive loss: | ||||||||||||||
| Items that may be reclassified subsequently to profit or loss: | ||||||||||||||
| Foreign currency translation loss | (25,669 | ) | (37,208 | ) | (26,763 | ) | ||||||||
| Total comprehensive loss for the period | (1,051,067 | ) | (1,032,599 | ) | (742,717 | ) | ||||||||
| Loss attributable to: | ||||||||||||||
| Equity holders of the Company | (1,025,270 | ) | (995,287 | ) | (715,879 | ) | ||||||||
| Non-controlling interests | (128 | ) | (104 | ) | (75 | ) | ||||||||
| Total | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||||
| Total comprehensive loss attributable to: | ||||||||||||||
| Equity holders of the Company | (1,050,939 | ) | (1,032,495 | ) | (742,642 | ) | ||||||||
| Non-controlling interests | (128 | ) | (104 | ) | (75 | ) | ||||||||
| Total | (1,051,067 | ) | (1,032,599 | ) | (742,717 | ) | ||||||||
| Loss per share for loss attributable to equity holders of the Company: | ||||||||||||||
| - Basic | (0.16 | ) | (0.13 | ) | (0.09 | ) | ||||||||
| - Diluted | (0.16 | ) | (0.13 | ) | (0.09 | ) | ||||||||
LOSS PER SHARE
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Weighted average number of ordinary shares used in computing basic loss | 6,543,850 | 7,672,622 | ||||||
| Weighted average number of ordinary shares used in computing diluted loss | 6,543,850 | 7,672,622 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-47 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
FOR THE SIX MONTHS ENDED JUNE 30, 2021 AND 2022
| Attributable to equity holders of the Company | ||||||||||||||||||||||||||||
| Share capital | Share application monies | Translation reserves | Accumulated losses | Total | Non-controlling interests | Total equity | ||||||||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | S$ | S$ | ||||||||||||||||||||||
| Balance at January 1, 2021 | 3,500,000 | - | (8,352 | ) | (3,015,682 | ) | 475,966 | (186 | ) | 475,780 | ||||||||||||||||||
| Total comprehensive loss for the period | - | - | (25,669 | ) | (1,025,270 | ) | (1,050,939 | ) | (128 | ) | (1,051,067 | ) | ||||||||||||||||
| Transactions with owners of the Company recognized directly in equity | ||||||||||||||||||||||||||||
| Issuance of shares | 2,000,000 | - | - | - | 2,000,000 | - | 2,000,000 | |||||||||||||||||||||
| Share issued for services | 190,355 | - | - | - | 190,355 | - | 190,355 | |||||||||||||||||||||
| Share application monies received | - | 2,000,000 | - | - | 2,000,000 | - | 2,000,000 | |||||||||||||||||||||
| Total transactions with owners of the Company | 2,190,355 | 2,000,000 | - | - | 4,190,355 | - | 4,190,355 | |||||||||||||||||||||
| Balance at June 30, 2021 | 5,690,355 | 2,000,000 | (34,021 | ) | (4,040,952 | ) | 3,615,382 | (314 | ) | 3,615,068 | ||||||||||||||||||
| Balance at January 1, 2022 | 6,548,960 | 1,141,395 | (30,980 | ) | (5,067,332 | ) | 2,592,043 | (439 | ) | 2,591,604 | ||||||||||||||||||
| Total comprehensive loss for the period | - | - | (37,208 | ) | (995,287 | ) | (1,032,495 | ) | (104 | ) | (1,032,599 | ) | ||||||||||||||||
| Transactions with owners of the Company recognized directly in equity | ||||||||||||||||||||||||||||
| Issuance of shares | 1,141,395 | (1,141,395 | ) | - | - | - | - | - | ||||||||||||||||||||
| Balance at June 30, 2022 | 7,690,355 | - | (68,188 | ) | (6,062,619 | ) | 1,559,548 | (543 | ) | 1,559,005 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-48 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED JUNE 30, 2021 AND 2022
| Six Months ended June 30, | ||||||||||||
| 2021 | 2022 | 2022 | ||||||||||
| S$ | S$ | US$ | ||||||||||
| Operating activities | ||||||||||||
| Loss before income tax | (1,025,398 | ) | (995,391 | ) | (715,954 | ) | ||||||
| Adjustments for: | ||||||||||||
| Amortization of intangible assets | 6,606 | 6,604 | 4,750 | |||||||||
| Depreciation of property, plant and equipment | 154,552 | 184,380 | 132,619 | |||||||||
| Gain on disposal of property, plant and equipment | - | (570 | ) | (410 | ) | |||||||
| Fair value loss on convertible loans | 196,728 | 140,838 | 101,300 | |||||||||
| Share of results of associate | - | 22,283 | 16,027 | |||||||||
| Interest expense | 57,668 | 62,042 | 44,625 | |||||||||
| Interest income | (870 | ) | (1,468 | ) | (1,056 | ) | ||||||
| Unrealized currency translation (gains)/losses | (27,414 | ) | 3,967 | 2,853 | ||||||||
| Operating cash flows before movements in working capital | (638,128 | ) | (577,315 | ) | (415,246 | ) | ||||||
| Other receivables | (119,402 | ) | 56,802 | 40,855 | ||||||||
| Trade and other payables | 124,221 | 34,247 | 24,634 | |||||||||
| Cash used in operations | (633,309 | ) | (486,266 | ) | (349,757 | ) | ||||||
| Interest received | 870 | 1,468 | 1,056 | |||||||||
| Net cash used in operating activities | (632,439 | ) | (484,798 | ) | (348,701 | ) | ||||||
| Investing activities | ||||||||||||
| Purchase of property, plant and equipment | (13,810 | ) | (422,072 | ) | (303,583 | ) | ||||||
| Prepayment for the investment in equity shares | (241,313 | ) | - | - | ||||||||
| Net cash used in investing activities | (255,123 | ) | (422,072 | ) | (303,583 | ) | ||||||
| Financing activities | ||||||||||||
| Proceeds from issuance of ordinary shares | 2,000,000 | - | - | |||||||||
| Proceeds from share application monies | 2,000,000 | - | - | |||||||||
| Principal payment of bank borrowings | (31,240 | ) | (34,051 | ) | (24,492 | ) | ||||||
| Principal payment of lease liabilities | (4,229 | ) | (4,311 | ) | (3,101 | ) | ||||||
| Interest paid | (57,668 | ) | (62,042 | ) | (44,625 | ) | ||||||
| Net cash from/(used in) financing activities | 3,906,863 | (100,404 | ) | (72,218 | ) | |||||||
| Net increase/(decrease) in cash and cash equivalents | 3,019,301 | (1,007,274 | ) | (724,502 | ) | |||||||
| Cash and cash equivalents at beginning of period | 885,272 | 2,512,768 | 1,807,357 | |||||||||
| Effects of foreign currency translation on cash and cash equivalents | (8,613 | ) | (18,333 | ) | (13,186 | ) | ||||||
| Cash and cash equivalents at end of period | 3,895,960 | 1,487,161 | 1,069,669 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
Significant non-cash transactions affecting the Unaudited Interim Condensed Consolidated Statement of Cash Flows:
During the six months ended June 30, 2021, the Group had the following significant non-cash transaction:
Issuance of 92,563 ordinary shares for a total consideration of S$190,355 for settlement of a professional fees of the equivalent amount.
| F-49 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 1 | GENERAL |
On March 8, 2018, CytoMed Therapeutics Limited (the former name: CytoMed Therapeutics Pte. Ltd.) (the “Company”) was incorporated in the Republic of Singapore. The Company is a private limited company incorporated and domiciled in Singapore. The address of its registered office is at 1 Commonwealth Lane, #08-22, Singapore 149544. The Company is headquartered in Singapore and conducts its operations domestically and in Malaysia. On January 19, 2023, the Company had converted to a public limited company and the name of the Company has changed from “CytoMed Therapeutics Pte. Ltd.” to “CytoMed Therapeutics Limited” and that the name “CytoMed Therapeutics Limited” has substituted for “CytoMed Therapeutics Pte. Ltd.” wherever “CytoMed Therapeutics Pte. Ltd.” appears in the Constitution of the Company.
The Company’s immediate and ultimate holding corporation is Glorious Finance Limited, incorporated in the British Virgin Islands.
The principal activities of the Company are to carry on the business of innate immune cell-based immunotherapy, pluripotent stem cell-based therapy and undertaking the research and development of immune cell and stem cell-based therapy. The Company conducts its primary operations through its directly held wholly owned subsidiary that is incorporated and domiciled in Malaysia, namely CytoMed Therapeutics (Malaysia) Sdn. Bhd., which is principally engaged in manufacturing innate immune cell-based immunotherapy and pluripotent stem cell-based therapy and consultancy services and undertaking the research and development of immune cell and stem cell-based therapy for advancing cellular immunotherapy to treat cancer.
The principal activities of the subsidiaries of the Company (the “Company” or collectively known as the “Group”) are as follows:
| Name of entity | Principal activities | Country of business / incorporation | Group’s effective equity interest held | |||||||||
| December 31, 2021 | June 30, 2022 | |||||||||||
| % | % | |||||||||||
| Advance Cancer Centre Pte Ltd | Investment, research and development of medical technologies | Singapore | 100 | 100 | ||||||||
| CytoMed Therapeutics (Malaysia) Sdn Bhd | Research, development and manufacturing of stem cells and innate immune cell-based immuno-therapeutics, research and development of induced pluripotent stem cell-based immuno-therapeutics | Malaysia | 100 | 100 | ||||||||
| Puricell Lab Pte Ltd | Research and development of induced pluripotent stem cell-based biologics and medical technologies | Singapore | 95 | 95 | ||||||||
| IPSCBank Pte Ltd | Stem cell and immune cell banking | Singapore | 100 | 100 | ||||||||
| Held by IPSCBank Pte Ltd | ||||||||||||
| IPSC Depository Sdn Bhd | Stem cell and immune cell banking | Malaysia | 100 | 100 | ||||||||
The principal activities of the associate are disclosed in Note 6 to the financial statements.
| F-50 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
BASIS OF PREPARATION - These unaudited interim condensed financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) under the historical cost convention, except as disclosed in the accounting policies below.
The preparation of these unaudited interim condensed financial statements in conformity with IFRS requires management to exercise its judgement in the process of applying the Group’s accounting policies. It also requires the use of certain critical accounting estimates and assumptions. The areas involving a higher degree of judgement or complexity, or areas where estimates and assumptions are significant to the financial statements are disclosed in Note 3. These unaudited interim condensed consolidated financial statements and the notes accompanying them should be read in conjunction with the Group’s audited consolidated financial statements for the year ended December 31, 2021.
Adoption of IFRS
The Group has adopted IFRS on January 1, 2019.
On January 1, 2020, the Group adopted the new or amended IFRS and Interpretations of IFRS (“INT IFRS”) that are mandatory for application for the financial year. Changes to the Group’s accounting policies have been made as required, in accordance with the transitional provisions in the respective IFRS and INT IFRS.
BASIS OF ACCOUNTING - The unaudited interim condensed financial statements have been prepared in accordance with the historical cost basis, except as disclosed in the accounting policies below, and are drawn up in accordance with the provisions of the IFRS.
Historical cost is generally based on the fair value of the consideration given in exchange for goods and services.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, regardless of whether that price is directly observable or estimated using another valuation technique. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability which market participants would take into account when pricing the asset or liability at the measurement date. Fair value for measurement and/or disclosure purposes in these financial statements is determined on such a basis.
In addition, for financial reporting purposes, fair value measurements are categorized into Level 1, 2 or 3 based on the degree to which the inputs to the fair value measurements are observable and the significance of the inputs to the fair value measurement in its entirety, which are described as follows:
| ● | Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the entity can access at the measurement date; | |
| ● | Level 2 inputs are inputs, other than quoted prices included within Level 1, that are observable for the asset or liability, either directly or indirectly; and | |
| ● | Level 3 inputs are unobservable inputs for the asset or liability. |
The Company’s policy is to recognize transfers into and transfers out of any of the three levels as of the date of the event or change in circumstances that caused the transfer.
| F-51 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Application of new and revised International Financial Reporting Standards (“IFRS”), International Accounting Standards (“IAS”), International Financial Reporting Interpretations Committee (“IFRIC”), Interpretations and Standing Interpretations Committee (“SIC”) Interpretations issued by the International Accounting Standards Board (“IASB”), (collectively, “IFRSs”)
a) Amendments to IFRSs and the new interpretation that are relevant to the Company and mandatorily effective for the current year
| New Standards, Interpretations and Amendments | Effective date issued by IASB | |
| Amendments to IFRS 3, “Reference to the conceptual framework” | January 1, 2022 | |
| Amendments to IAS 16, “Property, plant and equipment: proceeds before intended use” | January 1, 2022 | |
| Annual improvements to IFRS Standards 2018 – 2020 | January 1, 2022 |
The Company has adopted the above new standards, interpretations and amendments as of the effective date. Based on the Company’s assessment, the above standards and interpretations have no significant impact to the Company’s financial condition and financial performance.
b) New standards, interpretations and amendments in issue but not yet effective
New standards, interpretations and amendments in issue but not yet effective are as follows:
| New Standards, Interpretations and Amendments | Effective date issued by IASB | |
| Amendments to IAS 1, “Disclosure of accounting policies” | January 1, 2023 | |
| Amendments to IAS 8, “Definition of accounting estimates” | January 1, 2023 | |
| Amendments to IAS 12, “Deferred tax related to assets and liabilities arising from a single transaction” | January 1, 2023 | |
| Amendments to IAS 1, “Classification of liabilities as current or non-current” | January 1, 2023 | |
| Amendments to IFRS 16, “Lease liability in a sale and leaseback” | January 1, 2024 | |
| Amendments to IFRS 10 and IAS 28, “Sale or contribution of assets between an investor and its associate or joint venture” | To be determined by IASB |
As of June 30, 2022, the Company is undergoing assessment whether the adoption of the above IFRSs have any impact to the Company’s financial position and financial performance.
SIGNIFICANT ACCOUNTING POLICIES - The Group’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended December 31, 2021, included elsewhere in this prospectus. Since the date of those financial statements, there have been no changes to its significant accounting policies.
CONVENIENCE TRANSLATION
Translations of amounts in the unaudited interim condensed consolidated statement of financial position, consolidated statements of profit or loss and other comprehensive income, and consolidated statement of cash flows from SGD into USD as of and for the six months period ended June 30, 2022 are solely for the convenience of the reader and were calculated at the noon buying rate of USD1 = SGD1.3903, as published in H.10 statistical release of the United States Federal Reserve Board. No representation is made that the SGD amounts could have been, or could be, converted, realized or settled into USD at such rate or at any other rate.
| F-52 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 3 | CRITICAL ACCOUNTING JUDGEMENTS AND KEY SOURCES OF ESTIMATION UNCERTAINTY |
In the application of the Company’s accounting policies, which are described in Note 2 to the financial statements, management is required to make judgements, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods.
Key sources of estimation uncertainty
The key assumptions concerning the future and other key sources of estimation uncertainty at the end of the reporting period, that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year, are disclosed below:
| (a) | Impairment assessment for property, plant, and equipment (Note 7) and investment in an associate (Note 6) |
Property, plant and equipment and investment in associate, are tested for impairment when there is any objective evidence or indication that these assets may be impaired. Impairment exists when the carrying value of an asset or CGU exceeds its recoverable amount.
The recoverable amounts of property, plant and equipment and investment in associate have been determined based on higher of the fair value less costs to sell or VIU calculations. If the carrying amounts exceed the recoverable amounts, an impairment is recognized to profit or loss for the differences.
| F-53 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Property, plant and equipment mainly consist of freehold land, building, and laboratory equipment. Management has assessed that there were no objective evidence or indication that the carrying amounts of the Group’s property, plant and equipment may not be recoverable as at the end of reporting date. Accordingly, impairment assessment is not required.
| (b) | Valuation of convertible loans (Note 12) |
The convertible loans issued by the Company are classified as financial liabilities at FVTPL. No quoted prices in an active market are available for these financial liabilities. These financial liabilities were valued by the directors of the Company with reference to valuations carried out by an independent qualified professional valuer. The fair value of these financial liabilities is established by using valuation techniques as set out in the Note 12 to the financial statements. Valuation modals established by the valuer maximize the use of market inputs to the extent possible. Management estimates and assumptions are reviewed periodically and are adjusted if necessary. Should any of the estimates and assumptions changed, it may lead to a change in the fair value to be recognized in profit or loss. As at the end of the reporting periods, management has assessed that the probability of conversion of the convertible loans was anticipated to be 30% whilst the probability of redemption of the convertible loans was anticipated to be 70%.
Going concern
Since the inception the Group incurred substantial losses because the Group is still primarily focused on research and development. It has yet to fully commercialize its products and therapies to generate sustainable re-occurring cash flows. During the six months ended June 30, 2021 and 2022, the Group had losses for the period of S$1.03 million and S$995,391 (approximately U.S.$715,954), respectively. Additionally, the Group had net cash outflows from operating activities of S$632,439 and S$484,798 (approximately U.S.$ 348,701) for the six months ended June 30, 2021 and 2022. These circumstances raise substantial doubt regarding the Group ability to continue as going concern. Management’s plan to address this doubt by continuing procure financing from private investors in the form issuances of rights, ordinary shares, debt, or convertible hybrid instruments to raise cash and working capital for the Group. Management may also contribute their own time at less than market rates for the services. The Group also endeavors to list its ordinary shares on national stock exchange in the United States and concurrently sell additional ordinary an initial public offering that will provide adequate financial resources for management to continue to execute research and development plan and eventually bring a viable suite of oncology products and therapies to the market. Management may not be successful in raising additional funds via the means described above. These financial statements have been prepared on a going concern basis, and not a liquidation basis; accordingly, the accounts may be presented different if a going concern basis was not employed.
| 4 | CASH AND CASH EQUIVALENTS |
For the purpose of the statement of cash flows, cash and cash equivalents comprise the following:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Cash at banks | 2,512,768 | 1,486,845 | ||||||
| Cash on hand | - | 316 | ||||||
| 2,512,768 | 1,487,161 | |||||||
| 5 | OTHER RECEIVABLES |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Deposits | 15,232 | 15,353 | ||||||
| Due from key management personnel | 1,259 | - | ||||||
| Prepayments | 138,014 | 52,725 | ||||||
| Prepaid initial public offering expenses | 178,857 | 156,544 | ||||||
| Prepaid consumables | 431,582 | 474,287 | ||||||
| Goods and Services Tax (GST) refund | - | 4,024 | ||||||
| Sundry receivables | 4,031 | 9,240 | ||||||
| Total | 768,975 | 712,173 | ||||||
Amounts due from key management personnel are generally advances extended to them during the course of business. The balance is unsecured, interest-free, and repayable on demand.
| F-54 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 6 | INVESTMENT IN AN ASSOCIATE |
On January 20, 2020, the Company entered into an investment agreement with third parties, as varied by a supplemental agreement entered into on January 20, 2021 and a further supplemental letter dated October 28, 2021, to acquire 20% of the equity interest in Landmark Medical Centre Sdn Bhd (“LMC”) through its wholly owned subsidiary, Advanced Cancer Centre Pte Ltd, at a cash consideration of RM1.50 million (approximately S$489,211).
The Group’s investment in the associate is summarized below:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Beginning of financial year/period | - | 272,970 | ||||||
| Additions | 489,211 | - | ||||||
| Share of post-acquisition profit | 2,719 | - | ||||||
| Share of loss for the year/period | - | (22,283 | ) | |||||
| Impairment on investment in associate | (215,297 | ) | - | |||||
| Currency realignment | (3,663 | ) | (6,340 | ) | ||||
| End of financial year/period | 272,970 | 244,347 | ||||||
Management has assessed the recoverable amount of the investment in associate calculation based on its VIU, using discounted cash flow forecasts covering a five-year period in which the management made judgements over certain key inputs in relation to cash flows, revenue growth rates and discount rate. It was concluded that the fair value less costs of disposal did not exceed the VIU. As a result of this analysis, an impairment loss of S$215,297 was recognized during the year ended December 31, 2021. As of June 30, 2022, management has evaluated, assessed and concluded that there is no indication of impairment for the investment in an associate and accordingly no impairment is necessary for the six months ended June 30, 2022.
Key assumptions used to determine the value in use of the investment in associate are as follows:
| December 31, 2021 | June 30, 2022 | |||||||
| % | % | |||||||
| Revenue growth rate | 2.38 - 3.05 | 2.38 - 3.05 | ||||||
| Discount rate | 13.90 | 13.90 | ||||||
| Name of entity | Principal activities | Country of business / incorporation | Group’s effective equity interest held | |||||||||
| December 31, 2021 | June 30, 2022 | |||||||||||
| % | % | |||||||||||
| Landmark Medical Centre Sdn Bhd | Operations of private specialist hospital | Malaysia | 20 | 20 | ||||||||
The following table illustrates the summarized financial information of the Group’s material associate (and not the Group’s share of those amounts), adjusted for difference in accounting policies between the Group and the associate, if any.
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Current assets | 818,177 | 662,678 | ||||||
| Non-current assets | 800,979 | 782,784 | ||||||
| Current liabilities | (56,776 | ) | (39,496 | ) | ||||
| Non-current liabilities | (197,529 | ) | (184,233 | ) | ||||
| Net assets of the associate | 1,364,851 | 1,221,733 | ||||||
| Revenue | 746,074 | 230,349 | ||||||
| Loss for the year/period | (35,264 | ) | (111,417 | ) | ||||
Reconciliation of the summarized financial information presented to the carrying amount of the Group’s investment in the associate is as follows:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Net assets | 1,364,851 | 1,221,733 | ||||||
| Group’s equity interest | 20 | % | 20 | % | ||||
| Group share of net assets | 272,970 | 244,347 | ||||||
| Goodwill | 215,297 | 210,110 | ||||||
| Impairment on investment in associate | (215,297 | ) | (210,110 | ) | ||||
| Carrying value | 272,970 | 244,347 | ||||||
| F-55 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 7 | PROPERTY, PLANT AND EQUIPMENT |
| Building | Freehold land | Laboratory equipment | Motor vehicle | Furniture, fittings and equipment | Renovation | Total | ||||||||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | S$ | S$ | ||||||||||||||||||||||
| Cost: | ||||||||||||||||||||||||||||
| At January 1, 2021 | 652,492 | 426,897 | 988,233 | 53,976 | 263,520 | 386,890 | 2,772,008 | |||||||||||||||||||||
| Addition | 491,563 | - | 14,137 | - | 16,579 | 60,075 | 582,354 | |||||||||||||||||||||
| Currency realignment | (11,291 | ) | (7,387 | ) | (57,046 | ) | (934 | ) | (6,147 | ) | (38,098 | ) | (120,903 | ) | ||||||||||||||
| At December 31, 2021 | 1,132,764 | 419,510 | 945,324 | 53,042 | 273,952 | 408,867 | 3,233,459 | |||||||||||||||||||||
| Addition | - | - | 306,892 | - | 29,220 | 85,960 | 422,072 | |||||||||||||||||||||
| Disposal | - | - | - | - | (1,798 | ) | - | (1,798 | ) | |||||||||||||||||||
| Currency realignment | (15,451 | ) | (10,109 | ) | (28,471 | ) | (1,278 | ) | (6,254 | ) | (9,852 | ) | (71,415 | ) | ||||||||||||||
| At June 30, 2022 | 1,117,313 | 409,401 | 1,223,745 | 51,764 | 295,120 | 484,975 | 3,582,318 | |||||||||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||||||||||
| At January 1, 2021 | 20,843 | - | 288,609 | 19,791 | 86,590 | 55,150 | 470,983 | |||||||||||||||||||||
| Addition | 18,141 | - | 191,988 | 10,615 | 57,632 | 38,290 | 316,666 | |||||||||||||||||||||
| Currency realignment | (367 | ) | - | (45,055 | ) | (349 | ) | (3,232 | ) | (978 | ) | (49,981 | ) | |||||||||||||||
| At December 31, 2021 | 38,617 | - | 435,542 | 30,057 | 140,990 | 92,462 | 737,668 | |||||||||||||||||||||
| Addition | 20,166 | - | 103,621 | 5,232 | 31,614 | 23,747 | 184,380 | |||||||||||||||||||||
| Disposal | - | - | - | - | (999 | ) | - | (999 | ) | |||||||||||||||||||
| Currency realignment | (807 | ) | - | (15,404 | ) | (780 | ) | (3,602 | ) | (2,443 | ) | (23,036 | ) | |||||||||||||||
| At June 30, 2022 | 57,976 | - | 523,759 | 34,509 | 168,003 | 113,766 | 898,013 | |||||||||||||||||||||
| Carrying amount: | ||||||||||||||||||||||||||||
| At December 31, 2021 | 1,094,147 | 419,510 | 509,782 | 22,985 | 132,962 | 316,405 | 2,495,791 | |||||||||||||||||||||
| At June 30, 2022 | 1,059,337 | 409,401 | 699,986 | 17,255 | 127,117 | 371,209 | 2,684,305 | |||||||||||||||||||||
| F-56 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Motor vehicle is acquired under finance leasing arrangement. Details of the lease liability is disclosed in Note 13.
As of June 30, 2022, bank borrowing amounting to S$540,759 (December 31, 2021: S$574,810) is secured by the freehold land and building of the Group with carrying amounts as disclosed above.
Depreciation expense included in the consolidated statement of profit or loss and other comprehensive income is analyzed as follows:
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Depreciation of property, plant and equipment | 154,552 | 184,380 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (109,298 | ) | (128,332 | ) | ||||
| 45,254 | 56,048 | |||||||
| 8 | INTANGIBLE ASSETS |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Goodwill | 355 | 355 | ||||||
| Intellectual properties licenses (Note (a)) | 22,827 | 17,477 | ||||||
| Computer software licenses (Note (b)) | 8,841 | 7,580 | ||||||
| Total | 32,023 | 25,412 | ||||||
| (a) | Intellectual properties licenses |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Cost: | ||||||||
| At beginning of financial year/period | 62,060 | 62,060 | ||||||
| At end of financial year/period | 62,060 | 62,060 | ||||||
| Accumulated amortization: | ||||||||
| At beginning of financial year/period | 28,533 | 39,233 | ||||||
| Amortization charge | 10,700 | 5,350 | ||||||
| At end of financial year/period | 39,233 | 44,583 | ||||||
| Carrying value | 22,827 | 17,477 | ||||||
| F-57 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (b) | Computer software licenses |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Cost: | ||||||||
| At beginning of financial year/period | 11,873 | 11,855 | ||||||
| Currency realignment | (18 | ) | (25 | ) | ||||
| At end of financial year/period | 11,855 | 11,830 | ||||||
| Accumulated amortization: | ||||||||
| At beginning of financial year/period | 507 | 3,014 | ||||||
| Amortization charge | 2,512 | 1,254 | ||||||
| Currency realignment | (5 | ) | (18 | ) | ||||
| At end of financial year/period | 3,014 | 4,250 | ||||||
| Carrying value | 8,841 | 7,580 | ||||||
| (c) | Amortization expense is analyzed as followed: |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Intellectual properties licenses | 5,350 | 5,350 | ||||||
| Computer software licenses | 1,256 | 1,254 | ||||||
| Total | 6,606 | 6,604 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (5,350 | ) | (5,350 | ) | ||||
| 1,256 | 1,254 | |||||||
Management has evaluated, assessed and concluded that there is no indication of impairment for the intangible assets and accordingly no impairment allowance is necessary.
| 9 | TRADE AND OTHER PAYABLES |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Trade payable | 46,249 | 70,768 | ||||||
| Advances from key management personnel | 1,265 | 1,265 | ||||||
| Sundry payables | 18,038 | 50,658 | ||||||
| Accrued expenses | 144,701 | 90,391 | ||||||
| Deferred grant income | 8,480 | 7,040 | ||||||
| Total | 218,733 | 220,122 | ||||||
The amounts due to key management personnel related to reimbursement of expenses. It is unsecured, interest-free, and repayable on demand.
| F-58 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 10 | CONTRACT LIABILITIES |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Contract liabilities | 13,492 | 13,110 | ||||||
Contract liabilities are fees which the Group billed and received consideration ahead of the transfer of goods and services. Management expects that all the unsatisfied performance obligations as of the end of the reporting periods may be recognized as other income within the next twelve months.
No contract liabilities at December 31, 2020 have been recognized as other income in 2021. Decrease in contract liabilities for the six months ended June 30, 2022 was mainly due to the contract liabilities being recognized as other income during the period as the goods being delivered to the customers.
| 11 | BORROWINGS |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Current | ||||||||
| Bank loan | 35,691 | 32,179 | ||||||
| Third party loan | 350,000 | 350,000 | ||||||
| Total – current | 385,691 | 382,179 | ||||||
| Non-current | ||||||||
| Bank loan | 539,119 | 508,580 | ||||||
| Total | 924,810 | 890,759 | ||||||
Note:
| Bank loan: | Principal amount of RM2,000,000 (equivalent to S$660,000) withdrawn from a financial institution, which is charged an effective interest rate of 4.4% per annum and repayable over 180 months in equal monthly instalments of RM15,454. |
The exposure of the borrowing of the Group to interest rate changes and the contractual repayment dates at the balance sheet date are as follows:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| 6 months or less | 17,752 | 17,307 | ||||||
| 6 – 12 months | 17,939 | 17,892 | ||||||
| 1 – 5 years | 159,288 | 157,478 | ||||||
| Over 5 years | 379,831 | 348,082 | ||||||
| Total | 574,810 | 540,759 | ||||||
The loan is secured over building and freehold land (Note 7) and personal guarantees by a director.
| Third party loan: | Principal amount of S$350,000 borrowed from an independent individual, which is charged a fixed interest rate of 3.0% per annum and is repaid in a single lump sum upon the expiry of 12 months from the date of disbursement of the loan. Subject to mutual consent, the loan can be extended for another 6 months, or such other extension period agreed to in writing. The loan is unsecured. Subsequent to the period end, the third party loan has been repaid in October 2022. |
| F-59 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 12 | CONVERTIBLE LOANS |
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Convertible loans | 2,310,757 | 2,451,595 | ||||||
| Loan 1 | Loan 1 | |||||||||||||||
| (Tranche 1) | (Tranche 2) | Loan 2 | Total | |||||||||||||
| S$ | S$ | S$ | S$ | |||||||||||||
| At January 1, 2021 | 1,325,403 | 660,188 | 337,832 | 2,323,423 | ||||||||||||
| Fair value adjustment | (14,333 | ) | (6,817 | ) | 8,484 | (12,666 | ) | |||||||||
| At December 31, 2021 | 1,311,070 | 653,371 | 346,316 | 2,310,757 | ||||||||||||
| Fair value adjustment | 87,231 | 42,928 | 10,679 | 140,838 | ||||||||||||
| At June 30, 2022 | 1,398,301 | 696,299 | 356,995 | 2,451,595 | ||||||||||||
The convertible loan of S$250,000, where the agreement date started from December 7, 2020, with a maturity of two years from the date of disbursement on September 18, 2019 was extended from September 17, 2021 to November 30, 2022. The convertible loan carries a fixed interest rate of 5% per annum. As of June 30, 2022, the convertible loan was valued at S$356,995 (December 31, 2021: S$346,316), resulted in a fair value loss of S$10,679 (December 31, 2021: S$8,484) for the period ended June 30, 2022. The loan is unsecured.
The convertible loan of S$1,500,000, where the agreement date started from December 10, 2019, with a maturity of two years from the first disbursement date on January 6, 2020 was extended for a further one year period on December 31, 2021. The convertible loan carries a fixed interest rate of 5.5%. As of June 30, 2022, the convertible loan was valued at S$2,094,600 (December 31, 2021: S$1,964,441), resulting in a fair value loss of S$130,159 (December 31, 2021: fair value gain of S$21,150) for the period ended June 30, 2022. As security for this loan, the director Choo Chee Kong and Messiah Limited executed personal and corporate guarantees, respectively, in favor of guaranteeing the obligations of the Company under the agreement. Choo Chee Kong is also the sole director and a shareholder of Messiah Limited. Upon receipt of the redemption notice by the Company issued by the lender during the loan period, on the date falling on each anniversary of the first disbursement date on January 6, 2020, or upon the occurrence of a liquidity event, the Company shall repay such amount of the convertible loan (together with all interest accrued thereon) specified in the redemption notice to the lender within three months from the date of the redemption notice. Therefore, the convertible loan is classified as a current liability.
The convertible loans were stated at fair value which was valued by the directors of the Company with reference to an independent qualified professional valuer.
Key valuation assumptions used to determine the fair value of the convertible loans are as follows:
| % | ||||
| As at December 31, 2021 | ||||
| Risk-free interest rate | 0.87 | |||
| Discount rate for redemption scenario | 17.68 - 18.31 | |||
| Discount rate for conversion scenario | 0.56 | |||
| Conversion discount rate | 50.00 | |||
| As at June 30, 2022 | ||||
| Risk-free interest rate | 2.31 | |||
| Discount rate for redemption scenario | 15.31 – 16.61 | |||
| Discount rate for conversion scenario | 2.31 | |||
| Conversion discount rate | 50.00 | |||
The Group measures the convertible loans at fair value through profit or loss. The fair value measurement of the convertible loans is categorized as Level 3 under the Fair Value Hierarchy with the following details:
| Valuation technique input(s) | Significant unobservable input(s) | Inter-relationship between significant unobservable inputs and fair value measurements | |||
| Debt component : Discount cash flow method | Discount cash flow method | An increase in discount rate would result in a decrease in the fair value measurement of the convertible loans and vice versa. | |||
| Conversion option component: Number of Conversion Shares to be issued, Y = A/B * C | Discount rate per the synthetic rating corporate spread as shown in the above table. | If the discount rate increases by 5% while holding all other variables constant, the carrying fair value of the convertible loans would decrease by approximately S$8,000 for the financial year ended December 31, 2021 and by approximately S$6,000 for the six months ended June 30, 2022. | |||
| A - | Loan conversion amount / (1-discount) | ||||
| B - | Conversion valuation | ||||
| C - | total number of issued Shares as at the date of the Conversion Notice + Y | ||||
During the period, there were no transfers into or out of Level 3.
| F-60 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 13 | LEASE LIABILITIES |
Set out below are the carrying amounts of lease liabilities and the movements during the year/period:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| At beginning of year/period | 31,122 | 23,131 | ||||||
| Currency realignment | (535 | ) | (516 | ) | ||||
| Interest charged (Note 19) | 1,289 | 503 | ||||||
| Payment made | (8,745 | ) | (4,311 | ) | ||||
| At end of year/period | 23,131 | 18,807 | ||||||
Presentation on
Consolidated Statement of Financial Positions:
| Current | 7,813 | 7,807 | ||||||
| Non-current | 15,318 | 11,000 | ||||||
| Total | 23,131 | 18,807 |
The effective interest rate applied to the lease liabilities recognized in the statement of financial position is 4.73% per annum.
Lease liabilities of the Group are secured over a motor vehicle (Note 7).
Total cash outflow for leases amounts to S$5,233 (December 31,2021: S$10,742) (including low-value assets rental).
| 14 | SHARE CAPITAL AND SHARE APPLICATION MONIES |
| December 31, 2021 | June 30, 2022 | |||||||
| Number of shares: | ||||||||
| At beginning of financial year/period | 6,078,341 | 7,479,745 | ||||||
| Issued | 1,401,404 | 478,230 | ||||||
| At end of financial year/period | 7,479,745 | 7,957,975 | ||||||
| Paid-up capital (S$): | ||||||||
| At beginning of financial year/period | 3,500,000 | 6,548,960 | ||||||
| Issued | 2,190,355 | - | ||||||
| Transfer from share application monies | 858,605 | 1,141,395 | ||||||
| At end of financial year/period | 6,548,960 | 7,690,355 | ||||||
| Share application monies (S$): | ||||||||
| At beginning of financial year/period | - | 1,141,395 | ||||||
| Amount received | 2,000,000 | - | ||||||
| Transfer to paid-up capital | (858,605 | ) | (1,141,395 | ) | ||||
| At end of financial year/period | 1,141,395 | - | ||||||
The paid-up ordinary shares have no par value and carry one vote per share and carry a right to dividends as and when declared by the Company.
Share application monies
Share application monies of S$2,000,000 were received from prospective shareholders in April 2021 and in June 2021 pending allotment of 837,975 additional ordinary shares.
On September 20, 2021, the Company has issued for 359,745 ordinary shares for S$858,605 which was transferred to paid-up capital accordingly.
On April 18, 2022, the Company has issued for 478,230 ordinary shares for S$1,141,395 which was transferred to paid-up capital accordingly.
The newly issued shares rank pari passu in all aspects with the previously issued shares.
| F-61 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Paid-up capital
For the financial year ended December 31, 2021:
The Company issued:
| (i) | 92,563 ordinary shares for a total consideration of S$190,355 for equity-settled share-based payment transactions of a professional fee. The equity-settled share-based payment transaction is measured at the fair value of the service received; | |
| (ii) | 949,096 ordinary shares for a total consideration of S$2,000,000 of cash to provide funds for the expansion of the Group’s operations; and | |
| (iii) | 359,745 ordinary shares for a total consideration of S$858,605 by offsetting the share application monies to provide funds for the expansion of the Group’s operations. |
For the financial period ended June 30, 2022:
The Company issued 478,230 ordinary shares for a total consideration of S$1,141,395 by offsetting the share application monies to provide funds for the expansion of the Group’s operations.
The newly issued shares rank pari passu in all aspects with the previously issued shares.
On January 17, 2023, the Company effected a 1-for-380.83 reverse share split of the Company’s ordinary shares. Unless indicated or the context otherwise requires, all per share amounts and numbers of ordinary shares in this report have been retrospectively adjusted for the reverse share split, as if such reverse share split occurred on the first day of the periods presented. Accordingly, the weighted average number of ordinary shares used in computing basic loss and diluted loss were changed from 2,492,094,624 and 2,921,965,159 to 6,543,850 and 7,672,622 for the six months ended June, 2021 and 2022, respectively. Also, both basic and diluted loss per share for loss attributable to equity holders of the Company were changed from S$0.0004 and S$0.0003 (U.S.$0.0002) to S$0.16 and S$0.13 (US$0.09) for the six months ended June 30, 2021 and 2022, respectively.
| 15 | OTHER OPERATING INCOME |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Jobs Support Scheme | 4,880 | - | ||||||
| Wage Subsidy Program | 1,557 | - | ||||||
| Enterprise Development Grant | 10,400 | 3,600 | ||||||
| Jobs Growth Incentive | - | 4,750 | ||||||
| Hiring Incentive and Training Programme 2.0 | 1,375 | 9,937 | ||||||
| Startup SG Tech - Proof-of-Concept Grant | - | 50,000 | ||||||
| Others | 1,640 | 1,440 | ||||||
| Research income | 28,619 | 74,543 | ||||||
| Interest income | 870 | 1,468 | ||||||
| Others | 64 | 3,832 | ||||||
| 49,405 | 149,570 | |||||||
The Jobs Support Scheme of the Singapore Government provides wage support and helps employers retain their local employees during a period of economic uncertainty.
The Wage Subsidy Program is financial assistance introduced in Malaysia paid to employers and helps employers affected economically by the COVID-19 pandemic to continue operations and avoid the loss of jobs and income streams for all enterprises.
| F-62 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
The Enterprise Development Grant (Industry) supports projects that aim to help Small to Medium Enterprises in different stages of their technology innovation journey, to overcome their challenges in terms of resources and research and development capabilities.
The Jobs Growth Incentive of the Singapore Government supports employers to accelerate their hiring of local workforce, so as to create good and long-term jobs for locals.
The Hiring Incentive and Training Programme 2.0 is an economic recovery incentive introduced in Malaysia to promote job creation among employers while increasing employment prospects.
The Startup SG Tech - Proof-of-concept grant is a scheme to drive the growth of startups based on proprietary technology and to foster the spirit of deep-tech innovation among startups. Under the scheme, qualifying companies may receive early stage funding to help them develop and commercialize their innovations.
| 16 | OTHER LOSSES – NET |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Fair value loss on convertible loans (Note 12) | 196,728 | 140,838 | ||||||
| Currency exchange loss – net | 12,805 | 9,868 | ||||||
| Gain on disposal of property, plant and equipment | - | (570 | ) | |||||
| 209,533 | 150,136 | |||||||
| 17 | RESEARCH AND DEVELOPMENT EXPENSES |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Employee benefits (Note 18) | 179,572 | 246,132 | ||||||
| Depreciation of property, plant and equipment (Note 7) | 109,298 | 128,332 | ||||||
| Amortization of intangible assets (Note 8) | 5,350 | 5,350 | ||||||
| Consumables consumed | 103,035 | 134,552 | ||||||
| Royalty expenses | 5,350 | 4,650 | ||||||
| Professional fees | 64,649 | 37,199 | ||||||
| Electricity expenses | 30,147 | 33,591 | ||||||
| Others | 25,480 | 14,237 | ||||||
| Total | 522,881 | 604,043 | ||||||
| 18 | EMPLOYEE BENEFITS EXPENSES |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Wages and salaries | 222,832 | 295,282 | ||||||
| Defined contribution plans | 29,896 | 37,891 | ||||||
| Other short-term benefits | 5,836 | 10,489 | ||||||
| Total | 258,564 | 343,662 | ||||||
| Less: Accounted under | ||||||||
| “Research and development expenses” (Note 17) | (179,572 | ) | (246,132 | ) | ||||
| 78,992 | 97,530 | |||||||
| F-63 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 19 | FINANCE EXPENSES |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Interest expenses on: | ||||||||
| Bank loan | 9,816 | 9,189 | ||||||
| Third party loan | - | 5,207 | ||||||
| Convertible loans | 47,161 | 47,144 | ||||||
| Lease liabilities (Note 13) | 689 | 503 | ||||||
| Others | 2 | (1 | ) | |||||
| Total | 57,668 | 62,042 | ||||||
| 20 | OTHER EXPENSES |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Advertising | 2,193 | - | ||||||
| Entertainment | 220 | 167 | ||||||
| Low-value assets rental | 1,051 | 922 | ||||||
| Professional fees | 121,383 | 104,560 | ||||||
| Property tax | 1,722 | 3,556 | ||||||
| Printing and stationery | 2,425 | 2,571 | ||||||
| IT expenses | 3,206 | 5,637 | ||||||
| Repairs and maintenance | 1,671 | 3,728 | ||||||
| Service fees (Note 26) | 5,839 | 5,747 | ||||||
| Subscription fee | 749 | 1,228 | ||||||
| Transportation and travelling | 352 | 1,320 | ||||||
| Tools and supplies | 91 | 1,271 | ||||||
| Water and electricity | 7,860 | 9,905 | ||||||
| Freight charges & moving cost | - | 1,156 | ||||||
| Cleaning fee | 2,206 | 2,055 | ||||||
| Others | 8,251 | 7,802 | ||||||
| Total | 159,219 | 151,625 | ||||||
| 21 | INCOME TAX |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Current tax | - | - | ||||||
There is no provision for current income tax as there is no taxable profit for the financial periods.
| F-64 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
The tax on the Group’s loss before tax differs from the theoretical amount that would arise using the Singapore standard rate of income tax as follows:
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Loss before income tax | (1,025,398 | ) | (995,391 | ) | ||||
| Tax calculated at tax rate of 17% | (174,318 | ) | (169,216 | ) | ||||
| Effects of: | ||||||||
| - different tax rates in other countries | (32,987 | ) | (29,073 | ) | ||||
| - deferred tax assets not recognized | 138,873 | 173,028 | ||||||
| - expenses not deductible for tax purposes | 72,012 | 37,810 | ||||||
| - income not subject to tax | (3,580 | ) | (12,549 | ) | ||||
| Income tax expenses | - | - | ||||||
The Group has unused tax losses of S$3,213,000 and S$4,046,000 at December 31, 2021 and June 30, 2022, available for offset against future profits, respectively. The tax losses and capital allowances have no expiry except for tax losses amounting to approximately S$2,107,000 (December 31, 2021: S$1,659,000) which can only be carried forward up to 7 years. No deferred tax asset has been recognized in respect of these tax losses due to the unpredictability of future profit streams.
Unutilized tax losses
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| At beginning of year/period | 1,951,000 | 3,213,000 | ||||||
| Addition | 1,262,000 | 833,000 | ||||||
| At end of year/period | 3,213,000 | 4,046,000 | ||||||
| Unrecorded deferred tax benefits @ 17% | 546,210 | 687,820 | ||||||
| 22 | COMMITMENTS |
| (a) | Operating lease commitments |
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Low value leases | 1,051 | 922 | ||||||
The Group has elected not to recognize right-of-use assets and lease liabilities for short-term leases that have lease terms of 12 months or less and leases of low value leases. Lease payments relating to these leases are charged to profit or loss on a straight-line basis over the lease term.
| F-65 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (b) | Other commitments |
The Group entered into an exclusive, worldwide, non-sublicensable, non-transferable and revocable-for-cause license with an independent company for the NKG2DL-targeting CAR-gamma delta T cell and iPSC-gdNKT cell technology. As of June 30, 2022, the total future minimum payments under non-cancellable royalty agreements are as follows:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Within 1 year | 4,458 | 4,458 | ||||||
| After 1 year but within 5 years | 42,800 | 42,800 | ||||||
| Over 5 years | 134,285 | 122,515 | ||||||
| Total | 181,543 | 169,773 | ||||||
| 23 | FINANCIAL INSTRUMENTS, FINANCIAL RISKS AND CAPITAL RISKS MANAGEMENT |
| a) | Categories of financial instruments |
The following table sets out the financial instruments as at the end of the reporting period:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Financial assets | ||||||||
| Financial assets at amortized cost: | ||||||||
| Other receivables | 20,522 | 28,617 | ||||||
| Cash and cash equivalents | 2,512,768 | 1,487,161 | ||||||
| 2,533,290 | 1,515,778 | |||||||
| Financial liabilities | ||||||||
| Financial liabilities at amortized cost: | ||||||||
| Trade and other payables | 210,253 | 213,082 | ||||||
| Lease liabilities | 23,131 | 18,807 | ||||||
| Borrowings | 924,810 | 890,759 | ||||||
| 1,158,194 | 1,122,648 | |||||||
| Financial liabilities at FVTPL: | ||||||||
| Convertible loans | 2,310,757 | 2,451,595 | ||||||
| b) | Financial instruments subject to offsetting, enforceable master netting arrangements and similar agreements |
The Company does not have any financial instruments which are subject to enforceable master netting arrangements or similar netting agreements.
| c) | Financial risk management policies and objectives |
The management of the Company monitors and manages the financial risks relating to the operations of the Company to ensure appropriate measures are implemented in a timely and effective manner. These risks include market risk (including currency risk and interest rate risk), credit risk and liquidity risk.
| F-66 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (i) | Market risk management |
The Group activities are exposed primarily to the financial risks of changes in foreign currency exchange rates and interest rates. Management monitors risks associated with changes in foreign currency exchanges rates and interest rates and will consider appropriate measures should the need arise.
There has been no significant change to the Group’s exposure to market risk or the manner in which it manages and measures the risk.
| (ii) | Foreign currency risk management |
The Group operates in Singapore and Malaysia. Entities in the Group regularly transact in currencies other than their respective functional currencies (“foreign currencies”).
Currency risk arises when transactions are denominated in foreign currencies other than the Group entities’ respective functional currencies.
In addition, the Group is exposed to currency translation risk on the net assets in foreign operations.
The Group’s currency exposure based on the information provided to key management is as follows:
| SGD | MYR | USD | Total | |||||||||||||
| S$ | S$ | S$ | S$ | |||||||||||||
| June 30, 2022 | ||||||||||||||||
| Financial assets | ||||||||||||||||
| Cash and cash equivalents | 1,044,202 | 86,245 | 356,714 | 1,487,161 | ||||||||||||
| Other receivables | 11,275 | 17,342 | - | 28,617 | ||||||||||||
| Intra-group receivables | 1,090,791 | 12,494 | - | 1,103,285 | ||||||||||||
| 2,146,268 | 116,081 | 356,714 | 2,619,063 | |||||||||||||
| Financial liabilities | ||||||||||||||||
| Trade and other payables | (121,472 | ) | (90,568 | ) | (1,042 | ) | (213,082 | ) | ||||||||
| Lease liabilities | - | (18,807 | ) | - | (18,807 | ) | ||||||||||
| Borrowings including convertible loans | (2,801,595 | ) | (540,759 | ) | - | (3,342,354 | ) | |||||||||
| Intra-group payables | (1,090,791 | ) | (12,494 | ) | - | (1,103,285 | ) | |||||||||
| (4,013,858 | ) | (662,628 | ) | (1,042 | ) | (4,677,528 | ) | |||||||||
| Net financial (liabilities)/assets | (1,867,590 | ) | (546,547 | ) | 355,672 | (2,058,465 | ) | |||||||||
| Add: Net financial assets denominated respective entities’ functional currencies | 1,867,590 | 546,547 | - | 2,414,137 | ||||||||||||
| Currency exposure of financial assets, net of those denominated in the respective entities’ functional currencies | - | - | 355,672 | 355,672 | ||||||||||||
| SGD | MYR | USD | Total | |||||||||||||
| S$ | S$ | S$ | S$ | |||||||||||||
| December 31, 2021 | ||||||||||||||||
| Financial assets | ||||||||||||||||
| Cash and cash equivalents | 1,738,653 | 364,279 | 409,836 | 2,512,768 | ||||||||||||
| Other receivables | 7,909 | 12,613 | - | 20,522 | ||||||||||||
| Intra-group receivables | 1,089,953 | 12,803 | - | 1,102,756 | ||||||||||||
| 2,836,515 | 389,695 | 409,836 | 3,636,046 | |||||||||||||
| Financial liabilities | ||||||||||||||||
| Trade and other payables | (124,933 | ) | (85,186 | ) | (134 | ) | (210,253 | ) | ||||||||
| Lease liabilities | - | (23,131 | ) | - | (23,131 | ) | ||||||||||
| Borrowings including convertible loans | (2,660,757 | ) | (574,810 | ) | - | (3,235,567 | ) | |||||||||
| Intra-group payables | (1,089,953 | ) | (12,803 | ) | - | (1,102,756 | ) | |||||||||
| (3,875,643 | ) | (695,930 | ) | (134 | ) | (4,571,707 | ) | |||||||||
| Net financial (liabilities)/assets | (1,039,128 | ) | (306,235 | ) | 409,702 | (935,661 | ) | |||||||||
| Add: Net financial assets denominated respective entities’ functional currencies | 1,039,128 | 306,235 | - | 1,345,363 | ||||||||||||
| Currency exposure of financial assets, net of those denominated in the respective entities’ functional currencies | - | - | 409,702 | 409,702 | ||||||||||||
If the USD change against the SGD by 5% (2021: 5%) with all other variables including the tax rate being held constant, the effects arising from the net financial liability/asset that are exposed to currency risk will be as follows:
| Increase/(decrease) | ||||||||
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Loss after tax | Loss after tax | |||||||
| S$ | S$ | |||||||
| USD against SGD | ||||||||
| - strengthened | (26,163 | ) | (14,760 | ) | ||||
| - weakened | 26,163 | 14,760 | ||||||
| F-67 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (iii) | Interest rate risk management |
The Group is exposed to interest rate risk as the Group has bank loan amounting to S$ S$540,759 (December 31, 2021: S$574,810) which bear floating interest rates. The interest rates and terms of repayment of the loans are disclosed in the Note 11 to the financial statements. The Group currently does not have an interest rate hedging policy.
The sensitivity analysis below has been determined based on the exposure to interest rate for non-derivative instruments at the end of the reporting period. A 50 basis point increase or decrease is used when reporting interest rate risk internally to key management personnel and represents management’s assessment of the reasonably possible change in interest rates.
If interest rates on loans had been 50 basis points higher/lower and all other variables were held constant, the Group’s loss for the six months ended June 30, 2022 would increase/decrease by approximately S$1,028 (June 30, 2021: S$1,131).
| F-68 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (iv) | Credit risk management |
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in a financial loss to us. Our credit risk is primarily attributable to our cash and cash equivalents.
Our credit risk arising from cash and cash equivalents is limited because the counterparties are banks and financial institutions with good credit ratings which we consider to have low credit risk.
| (v) | Liquidity risk management |
Prudent liquidity risk management implies sufficient cash to finance the Group’s and the Company’s operations and development activities. The Group manages the liquidity risk by maintaining a level of cash and cash equivalents deemed adequate to finance the Group’s business operations and development activities. The Group’s objective is to maintain a balance between continuing of funding and flexibility through the use of borrowings.
The table below analyses non-derivative financial liabilities of the Group and the Company into relevant maturity groupings based on the remaining period from the balance sheet date to the contractual maturity date.
| Less than 1 year | Between 1 and 2 years | Between 2 and 5 years | Over 5 years | Total undiscounted cash flow | ||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | ||||||||||||||||
| June 30, 2022 | ||||||||||||||||||||
| Trade and other payables | 213,082 | - | - | - | 213,082 | |||||||||||||||
| Lease liabilities | 8,529 | 8,529 | 2,843 | - | 19,901 | |||||||||||||||
| Borrowings (excluding lease liabilities) | 1,911,208 | 58,583 | 175,749 | 405,199 | 2,550,739 | |||||||||||||||
| December 31, 2021 | ||||||||||||||||||||
| Trade and other payables | 210,253 | - | - | - | 210,253 | |||||||||||||||
| Lease liabilities | 8,740 | 8,740 | 7,283 | - | 24,763 | |||||||||||||||
| Borrowings (excluding lease liabilities) | 2,242,530 | 60,030 | 180,089 | 445,219 | 2,927,868 | |||||||||||||||
| F-69 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| (vi) | Fair value of financial assets and financial liabilities |
The management considers that the carrying amounts of the Company’s financial assets and financial liabilities approximate their respective fair values due to the relatively short-term maturity of these financial instruments.
The fair values of the Group’s borrowings (other than convertible loans which are disclosed in Note 12 to the financial statements) are determined by using the discounted cash flows method using discount rate that reflects the issuer’s borrowing rate as at the end of the reporting period. The Group’s non-performance risk as at December 31, 2021 and June 30, 2022 was assessed to be insignificant.
The fair values of other classes of financial assets and liabilities are disclosed in the respective notes to financial statements.
| (d) | Capital risk management policies and objectives |
The management manages its capital to ensure that the Group will be able to continue as a going concern in order to provide returns for shareholders and benefits for other stakeholders and to maintain an optimal capital structure to reduce cost of capital.
The capital structure of the Company consists of equity attributable to owners of the Company, comprising issued capital and retained earnings as disclosed in the notes to financial statements.
| F-70 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Management monitors capital based on debt-to-equity ratio. The debt-to-equity ratio is calculated as total debt divided by total equity.
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Total borrowings (Notes 11-13) | 3,258,698 | 3,361,161 | ||||||
| Total equity | 2,591,604 | 1,559,005 | ||||||
| Debt-to-equity % | 126 | % | 216 | % | ||||
The Group is not subject to externally imposed capital requirements for the financial year ended December 31, 2021 and six months period ended June 30, 2022.
The Group’s overall strategy remains unchanged from prior year.
| 24 | SEGMENT INFORMATION |
Operating segments are identified on the basis of internal reports about components of the Group that are regularly reviewed by the COO (“Chief Operating Officer”) for the purpose of resource allocation and performance assessment. Segment results, assets and liabilities include items directly attributable to a segment as well as those that can be allocated on a reasonable basis. The Group operates in a single business segment which is the business of innate immune cell-based immunotherapy, pluripotent stem cell-based therapy and undertaking the research and development of immune cell and stem cell-based therapy. No operating segments have been aggregated to form the following reportable operating segment.
Geographical information:
Non-current assets (excluding investment in the associate) information based on the location of assets are as follows:
| December 31, 2021 | June 30, 2022 | |||||||
| S$ | S$ | |||||||
| Malaysia | 1,985,738 | 1,988,953 | ||||||
| Singapore | 542,076 | 720,764 | ||||||
| Total | 2,527,814 | 2,709,717 | ||||||
Non-current assets information presented above consist of property, plant and equipment and intangible assets as presented in the consolidated statement of financial position.
| F-71 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 25 | RECONCILIATIONS OF LIABILITIES ARISING FROM FINANCING ACTIVITIES |
| At beginning of period | Payments | Interest expenses | Fair value changes | At end of period | ||||||||||||||||
| S$ | S$ | S$ | S$ | S$ | ||||||||||||||||
| June 30, 2021 | ||||||||||||||||||||
| Bank loan | 626,262 | (41,056 | ) | 9,816 | - | 595,022 | ||||||||||||||
| Convertible loans | 2,323,423 | (47,161 | ) | 47,161 | 196,728 | 2,520,151 | ||||||||||||||
| Lease liabilities | 31,122 | (4,918 | ) | 689 | - | 26,893 | ||||||||||||||
| June 30, 2022 | ||||||||||||||||||||
| Bank loan | 574,810 | (43,240 | ) | 9,189 | - | 540,759 | ||||||||||||||
| Third party loan | 350,000 | (5,207 | ) | 5,207 | - | 350,000 | ||||||||||||||
| Convertible loans | 2,310,757 | (47,144 | ) | 47,144 | 140,838 | 2,451,595 | ||||||||||||||
| Lease liabilities | 23,131 | (4,827 | ) | 503 | - | 18,807 | ||||||||||||||
| 26 | RELATED PARTY TRANSACTIONS |
Some of the Company’s transactions and arrangements are with related parties and the effect of these on the basis determined between the parties is reflected in these financial statements. The balances are unsecured, interest-free and repayable on demand unless otherwise stated.
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Transactions with related parties: | ||||||||
| Research income from associate | - | 53,323 | ||||||
| Purchase from associate | - | 1,118 | ||||||
| Service fee paid to directors | 5,839 | 5,747 | ||||||
| Balances with related parties: | ||||||||
| Other receivable from associate | - | 2,843 | ||||||
Compensation of directors and key management personnel
The remuneration of directors and key management personnel during the period were as follows:
| Six Months ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| S$ | S$ | |||||||
| Wages and salaries | 133,674 | 140,897 | ||||||
| Defined contribution plans | 18,453 | 18,931 | ||||||
| Other short-term benefits | 1,680 | 1,675 | ||||||
| Total | 153,807 | 161,503 | ||||||
| Analyzed by: | ||||||||
| Directors of the Company | - | - | ||||||
| Other key management personnel | 153,807 | 161,503 | ||||||
| Total | 153,807 | 161,503 | ||||||
| F-72 |
CYTOMED THERAPEUTICS LIMITED AND SUBSIDIARIES
NOTES TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 27 | HOLDING COMPANY AND RELATED COMPANY TRANSACTIONS |
The Company is a subsidiary of Glorious Finance Limited, incorporated in the British Virgin Islands, which is also the Company’s ultimate holding company. Related companies in these financial statements refer to members of the ultimate holding company’s group of companies.
Some of the Company’s transactions and arrangements are between members of the group and the effect of these on the basis determined between the parties is reflected in these financial statements. The intercompany balances are unsecured, interest-free and repayable on demand, unless otherwise stated.
| 28 | EVENTS OCCURRING AFTER BALANCE SHEET DATE |
The Company has assessed all events occurred from June 30, 2022, up through March 1, 2023, which is the date that these consolidated financial statements are available to be issued. Other than the events disclosed below, there are not any material subsequent events that would require disclosure in these interim financial statements.
On October 18, 2022, the Company had increased its share capital to S$8,913,005 and issued additional 486,485 ordinary shares at a consideration of S$1,222,650. These newly issued shares rank pari passu in all respects with the previously issued shares.
The Company intends to undertake a rights issue of new ordinary shares (the “Rights Shares”) in the capital of the Company at an aggregate of S$1 million. The Rights Shares, when allotted and issued, shall rank pari passu in all respects with the previously issued shares.
On November 23, 2022, the convertible loan amounting to S$250,000 was extended from December 1, 2022 to the conversion date. On January 26, 2023, the Company converted the convertible loan into 82,990 ordinary shares. These newly issued shares rank pari passu in all respects with the previously issued shares.
On November 23, 2022, the Company entered into a loan agreement with a value of S$300,000. On February 22, 2023, the loan was extended for further one month period or till the date of full repayment, whichever is earlier, commencing from March 4, 2023 and the fixed interest rate changed from 5.0% to 5.5% per annum.
On January 3, 2023, the convertible loan amounting to S$1,500,000 was extended from January 6, 2023 to January 5, 2024.
Effective on January 17, 2023, the registrant implemented a 1-for-380.83 reverse split of its ordinary shares pursuant to which the shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date. As a result, the Company has 8,444,460 ordinary shares issued and outstanding. Unless indicated or the context otherwise requires, all per share amounts and numbers of ordinary shares in this report have been retrospectively adjusted for the reverse share split, as if such reverse share split occurred on the first day of the years presented.
On January 19, 2023, the Company converted to a public limited company and the name of the Company has changed from “CytoMed Therapeutics Pte. Ltd.” to “CytoMed Therapeutics Limited” and that the name “CytoMed Therapeutics Limited” has substituted for “CytoMed Therapeutics Pte. Ltd.” wherever “CytoMed Therapeutics Pte. Ltd.” appears in the Constitution of the Company.
| F-73 |
2,412,369 Ordinary Shares
CytoMed Therapeutics Limited
| The Benchmark Company, LLC | Axiom Capital Management, Inc. |
PROSPECTUS
, 2023
Until ______, 2023 (25 days after the date of this Prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 6.
Indemnification of Directors and Officers
Subject to the provisions of the Singapore Companies Act and every other Singapore statute for the time being in force and affecting the Company, our constitution provides that every officer of the Company shall be entitled to be indemnified out of the Company’s assets against any liability (other than any liability referred to in Section 172B(1)(a) or (b) of the Singapore Companies Act) incurred by the officer to a person other than us attaching to the officer in connection with any negligence, default, breach of duty or breach of trust.
Under Section 172 of the Singapore Companies Act, any provision exempting or indemnifying the officers of a company (including directors) against any liability that would otherwise attach to them in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. However, a company is not prohibited from: (a) as provided in Section 172A of the Singapore Companies Act, purchasing and maintaining for any such individual insurance against liability incurred by him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company; or (b) as provided in Section 172B of the Singapore Companies Act, indemnifying the individual against liability incurred by him or her to a person other than the company except when the indemnity is against any liability (i) of the individual to pay a fine in criminal proceedings, (ii) of the individual to pay a penalty to a regulatory authority in respect of non-compliance with any requirements of a regulatory nature (howsoever arising), (iii) incurred by the individual in defending criminal proceedings in which he or she is convicted, (iv) incurred by the individual in defending civil proceedings brought by the company or a related company in which judgment is given against him or her, or (v) incurred by the individual in connection with an application for relief under Section 76A(13) or Section 391 of the Singapore Companies Act in which the court refuses to grant him or her relief.
We will enter into indemnification agreements with our officers and Directors. These indemnification agreements will provide our officers and Directors with indemnification to the maximum extent permitted by the Singapore Companies Act and our constitution, save that the Company shall not provide any indemnity (to any extent) to a Director or an officer against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the Company save for the circumstances as permitted pursuant to Section 172A and Section 172B of the Singapore Companies Act. We also intend to obtain a policy of directors’ and officers’ liability insurance that will insure Directors and officers against the cost of defense, settlement or payment of a judgment under certain circumstances which are permitted under the Singapore Companies Act.
Item 7.
Recent Sales of Unregistered Securities
On June 26, 2019, and September 28, 2020, the then-shareholders of the Company agreed to a waiver of their pre-emption rights to allot and issue certain numbers of shares to Glorious Finance Limited, at the consideration sum of S$1 million (approximately U.S.$719,269) and S$500,000 (approximately U.S.$359,635) respectively.
On February 19, 2021, the then-shareholders of the Company agreed to a waiver of their pre-emption rights to allot and issue certain numbers of shares to Dr Lucas LUK Tien Wee, a director of our Company, at the consideration sum of S$190,355 (approximately U.S.$136,916).
On April 15, 2021, we entered into a subscription agreement with Glorious Finance Limited, as varied by a supplemental letter agreement entered into on September 21, 2022, in relation to its subscription of 237,274 ordinary shares in our Company, at consideration sum of S$500,000 (approximately U.S.$359,635). The subscription of shares was completed on April 22, 2021.
| II-1 |
On April 15, 2021, we entered into a subscription agreement with mDR Limited, as varied by a supplemental letter agreement entered into on September 21, 2022, in relation to its subscription of 711,822 ordinary shares in our Company at consideration sum of S$1.50 million (approximately U.S.$1.08 million). The subscription of shares was completed on April 22, 2021. In relation to this agreement, CHOO Chee Kong, our Director and Chairman, and mDR Limited also executed a put option agreement on April 15, 2021, allowing mDR Limited to have the right and option to require CHOO Chee Kong to purchase and acquire a certain number of shares in our Company from mDR Limited.
In addition, on April 15, 2021, the Company’s initial shareholders approved an increase of authorized share capital to S$7,690,355 (approximately U.S.$5.53 million) by the issue and allotment of 837,975 additional ordinary shares of S$2.39 each at the consideration sum of S$2 million (approximately U.S.$1.44 million) ranking for dividend and in all other respects pari passu with the existing ordinary shares in the Company. The shares were issued under exemptions from registration under Section 4(a)(2) of the Securities Act since they were transactions not involving a public offering. No underwriters were involved in these issuances of ordinary shares.
On April 30, 2021, we entered into a subscription agreement with Jackie Win Kei Lee, as varied by a supplemental letter dated September 21, 2022, in relation to her subscription of 83,871 ordinary shares in our Company at the consideration sum of S$200,175 (approximately U.S.$143,980). The subscription of shares was completed on April 18, 2022.
On April 30, 2021, we entered into a subscription agreement with Lin Zi Tong, as varied by a supplemental letter dated September 21, 2022, in relation to her subscription of 55,914 ordinary shares in our Company at the consideration sum of S$133,450 (approximately U.S.$95,986). The subscription of shares was completed on April 18, 2022.
On April 30, 2021, we entered into a subscription agreement with Shu Fan Frank Lee, as varied by a supplemental letter dated September 21, 2022, in relation to his subscription of 282,816 ordinary shares in our Company at the consideration sum of S$675,000. The subscription of shares was completed on April 18, 2022.
On May 19, 2021, we entered into a subscription agreement with Dynatech Ventures Pte Ltd, as varied by a supplemental letter dated September 21, 2022, in relation to its subscription of 83,798 ordinary shares in our Company at the consideration sum of S$200,000. The subscription of shares was completed on September 20, 2021.
On May 19, 2021, we entered into a subscription agreement with Essex Bio-Investment Limited, as varied by a supplemental letter dated September 21, 2022, in relation to its subscription of 209,494 ordinary shares in our Company at the consideration sum of S$500,000. The subscription of shares was completed on September 20, 2021.
On June 27, 2021, we entered into a subscription agreement with Robert Shengchu Huang, as varied by a supplemental letter dated September 21, 2022, in relation to its subscription of 66,453 ordinary shares in our Company at the consideration sum of S$158,605 (approximately U.S.$114,080). The subscription of shares was completed on September 20, 2021.
On June 28, 2021, we entered into a subscription agreement with I Financial Ventures Group LLC, as varied by a supplemental letter dated September 21, 2022, in relation to its subscription of 55,629 ordinary shares in our Company at the consideration sum of S$132,770 (approximately U.S.$95,497). The subscription of shares was completed on April 18, 2022.
In addition, on August 31, 2022, the Company’s shareholders approved an increase of share capital in two tranches to (i) S$8,690,355 by the issue and allotment of 397,899 additional ordinary shares of S$2.51 each at the consideration sum of S$1 million and (ii) S$9,690,355 by the issue and allotment of 378,800 additional ordinary shares at S$2.64 each at the consideration sum of S$1 million, all ranking for dividend and in all other respects pari passu with the existing ordinary shares in the Company. The Company’s shareholders also approved that authority be given to the Directors, pursuant to Section 161 of the Singapore Companies Act, to allot and issue shares in the Company at any time to such persons and upon such terms and conditions and for such purposes as the Directors may deem fit until the conclusion of the next annual general meeting or the date by which the next annual general meeting is required by law to be held, whichever is earlier. The shares were issued under exemptions from registration under Section 4(a)(2) of the Securities Act since they were transactions not involving a public offering. No underwriters were involved in these issuances of ordinary shares. Subsequently, on October 18, 2022, 221,601 ordinary shares were issued and allotted to Glorious Finance Limited, 35,592 ordinary shares were issued and allotted to mDR Limited, 4,189 ordinary shares were issued and allotted to Dynatech Ventures Pte Ltd, 70,169 ordinary shares were issued and allotted to Robert Shengchu Huang, 10,472 ordinary shares were issued and allotted to Essex Bio-Investment Limited, 3,978 ordinary shares were issued and allotted to Lin Zi Tong, 4,193 ordinary shares were issued and allotted to Jackie Win Kei Lee, 14,141 ordinary shares were issued and allotted to Shu Fan Frank Lee, 2,781 ordinary shares were issued and allotted to I Financial Ventures Group LLC, and 119,369 ordinary shares were issued and allotted to Teoh Teik Kee.
Effective on January 17, 2023, the registrant implemented a 1-for-380.83 reverse split of its ordinary shares pursuant to which the shareholders received one (1) ordinary share for every 380.83 ordinary shares held as of such date. As a result, the Company has 8,444,460 ordinary shares issued and outstanding.
On January 26, 2023, the Company converted the convertible loan of S$250,000 and, accordingly, 82,990 ordinary shares were issued and allotted to Lim Liang Yew Dennis.
Except as disclosed herein, we have not issued any securities for the past three (3) years.
| II-2 |
Item 8.
Exhibits
(a) The following documents are filed as part of this registration statement:
EXHIBIT INDEX:
| II-3 |
| 21.1*** | List of Subsidiaries of the Registrant | |
| 23.1*** | Consent of WWC, P.C., an independent registered public accounting firm | |
| 23.2*** | Consent of Opal Lawyers LLC (included in Exhibits 5.1, 5.2 and 8.1) | |
| 23.3* | Consent of Zaid Ibrahim & Co. (included in Exhibit 5.3) | |
| 23.4*** | Consent of Ellenoff Grossman & Schole LLP (included in Exhibit 8.2) | |
| 24.1*** | Power of Attorney (included in the signature page to this registration statement) | |
| 99.1*** | Consent of Dr. ZENG Jieming | |
| 99.2*** | Consent of Dr. TAN Wee Kiat | |
| 99.3*** | Consent of Prof. LOH Yuin Han | |
| 99.4*** | Consent of Dr. YEW Chak Hua | |
| 99.5*** | Consent of Mark LEONG Kei Wei | |
| 99.6*** | Consent of WU Tao Thomas | |
| 99.7*** | Consent of Dr. TOH Keng Kiat | |
| 99.8*** | Form of Audit Committee Charter | |
| 99.9*** | Form of Compensation Committee Charter | |
| 99.10*** | Form of Nominating and Corporate Governance Committee Charter | |
| 107*** | Calculation of Registration Fee |
| * | Filed herewith. |
| ** | To be filed by amendment. |
| *** | Previously filed. |
| + | Indicates a management contract or compensatory plan. |
| # | Pursuant to Item 601(b)(10)(iv) of Regulation S-K, certain identified information marked with [*****] has been excluded from the exhibit because it is both (i) not material and (ii) the type that the registrant treats as private or confidential. |
(b) Financial Statement Schedules
None.
| II-4 |
Item 9.
Undertakings
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total U.S. dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Securities Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Securities Act if such financial statements and information are contained in periodic reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in the Form F-3.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser:
(i) If the registrant is relying on Rule 430B:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
| II-5 |
(B) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
(ii) If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of to any purchaser in the initial distribution of the securities the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
| II-6 |
(c) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(d) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-7 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Singapore, on March 27, 2023.
| CytoMed Therapeutics Limited | ||
| By: | /s/ CHOO Chee Kong | |
Name: | CHOO Chee Kong | |
| Title: | Director and Chairman | |
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
/s/ CHOO Chee Kong | Director and Chairman | March 27, 2023 | ||
| CHOO Chee Kong | (Principal Executive Officer) | |||
/s/ GOH Yvonne | Chief Financial Officer | March 27, 2023 | ||
GOH Yvonne | (Principal Financial Accounting Officer) | |||
/s/ Lucas LUK Tien Wee | Director | March 27, 2023 | ||
Dr. Lucas LUK Tien Wee |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of CytoMed Therapeutics Limited. has signed this registration statement or amendment thereto in Newark, Delaware on March 27, 2023.
| By: | /s/ Donald J. Puglisi | |
| Name: | Donald J. Puglisi | |
| Title: | Managing Director |
| II-8 |