Exhibit 99.1

Incannex Healthcare Limited
ABN 93 096 635 246
Appendix 4E
For the year ended 30 June 2023
Information for ASX under listing rule 4.3A
| Reporting Period: | 30 June 2023 | |
| Comparative Period: | 30 June 2022 |
| 2.0 | Results for announcement to the market |
| 2.1 | Revenue |
| 30-Jun-23 | 30-Jun-22 | Amount change | Percentage change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Revenues from ordinary activities | - | - | - | nmf |
| 2.2 | Loss for the year |
| 30-Jun-23 | 30-Jun-22 | Amount change | Percentage change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Loss from ordinary activities after tax | (19,979,558 | ) | (14,903,909 | ) | (5,075,649 | ) | 34 | |||||||||
| 2.3 | Net loss for the year |
| 30-Jun-23 | 30-Jun-22 | Amount change | Percentage change | |||||||||||||
| $ | $ | $ | % | |||||||||||||
| Loss from ordinary activities after tax | (19,979,558 | ) | (14,903,909 | ) | (5,075,649 | ) | 34 | |||||||||
| 2.4 | Dividends |
No dividends have been paid, declared or proposed in respect of the year ended 30 June 2023 (2022: Nil).
2.6 Results for the year
Refer to the attached financial statements and review of operations in the Directors’ Report for an explanation of the results for the year.
3 Statement of profit and loss and other comprehensive income
Refer to attached financial statements.
4 Statement of financial position
Refer to attached financial statements.
5 Statement of changes in equity
Refer to attached financial statements.
6 Statement of cash flows
Refer to attached financial statements.
7 Details of dividends and distribution payments
Not applicable.
8 Dividend and distribution reinvestment
Not applicable
9 Net tangible asset per security
| Net tangible asset per ordinary security | 30-Jun-23 | 30-Jun-22 | ||||||
| Net tangible assets | 31,411,440 | 35,869,075 | ||||||
| Number of shares on issue at reporting date | 1,587,010,366 | 1,292,334,028 | ||||||
| Net tangible asset per ordinary security | 1.98 cents | 2.78 cents | ||||||
The net tangibles asset backing per security of 1.98 cents presented above is inclusive of right-of-use assets and lease liabilities. The net tangible asset per security, at 30 June 2023, would reduce to 1.93 cents (2022: no change) if right-of use assets were excluded, and lease liabilities were included in the calculation.
10 Controlled entities
The consolidated financial statements include the financial statements of Incannex Healthcare Limited (‘IHL’) and its wholly owned subsidiaries Incannex Pty Ltd (‘IXPL’) and Psychennex Pty Ltd (‘PXPL’). IXPL is incorporated in Australia and IHL owns 100% of the issued ordinary shares in IXPL (2022: 100%). PXPL is incorporated in Australia and IHL owns 100% of the issued ordinary shares in PXPL (2022: 100%).
11 Joint ventures and associates
Not applicable.
12 Other information
Not applicable.
13 Foreign entities
Not applicable.
14 Commentary on results
15 Audit
The figures in this report are based on the attached Financial Report which is audited.
16 Not applicable
17 Audit Opinion
The independent audit report is not subject to any modified opinion, emphasis of matter or other matter paragraph.
| /s/ Troy Valentine |
Troy Valentine
Chairman
Melbourne, Victoria, 30 August 2023

Incannex Healthcare Limited
ABN 93 096 635 246
Annual Financial Report
For the year ended 30 June 2023
Incannex Healthcare Limited
TABLE OF CONTENTS
| Page | |
| Corporate Information | 1 |
| Chairman’s Letter | 2 |
| Directors’ Report | 4 |
| Business Activities and Outlook | 6 |
| Remuneration Report | 27 |
| Auditor’s Independence Declaration | 34 |
| Statement of Comprehensive Income | 35 |
| Statement of Financial Position | 36 |
| Statement of Changes In Equity | 37 |
| Statement of Cash Flows | 38 |
| Notes to the Financial Statements | 39 |
| Directors’ Declaration | 63 |
| Independent Auditor’s Report | 64 |
| Corporate Governance Statement | 71 |
| Securities Exchange Information | 78 |
i
Incannex Healthcare Limited
CORPORATE INFORMATION
Incannex Healthcare Limited
ABN 93 096 635 246
Directors
Mr Joel Latham (Managing Director & CEO)
Mr Troy Valentine (Non-Executive Chairman)
Mr Peter Widdows (Non-Executive Director)
Dr George Anastassov (Non-Executive Director)
Robert B. Clark (Non-Executive Director) (Appointed 17/08/2022)
Company Secretary
Madhukar Bhalla
Registered Office
Level 23, South Tower Rialto
525 Collins Street
Melbourne Victoria 3000
Principal Place of Business
Suite 15 Level 12
401 Docklands Drive
Docklands Victoria 3008
Share Register
Automic Pty Ltd
Level 5 126 Phillip Street
Sydney NSW 2000
Phone: +61 2 9698 5414
Auditors
PKF Brisbane Audit
Level 6, 10 Eagle St
Brisbane 4000, Queensland
Securities Exchange Listing
ASX Limited (Australian Securities Exchange)
Home Exchange: Melbourne Victoria
ASX Codes: IHL
1
Incannex Healthcare Limited
CHAIRMAN’S MESSAGE
On behalf of the Board of Directors, I am pleased to present the Annual Report of Incannex Healthcare Limited (“Incannex” or “IHL”) for the financial year ended 30 June 2023.
The hard work of our team throughout the year elucidated major opportunities to advance our diversified product development pipeline despite challenging capital market conditions for the broader biotech sector.
Financial decisions that Incannex made in 2021 and 2022 to ensure that the Company remained well funded has ensured that we are able to continue our extensive research and development plans unabated.
Operationally we’ve witnessed amazing progress in the clinical development of our portfolio of drug candidate assets. Most biotech companies only have one lead candidate, however, we have three major efficacy trials underway over three different drugs or therapies that have the potential to influence patient treatment protocols and unlock significant shareholder value upon the release of positive trial results.
In our IHL-42X program to treat obstructive sleep apnoea, our phase 2 proof of concept trial demonstrated that the main symptom measure of sleep apnea was more than halved on average for the participants in this trial, truly outstanding results.
After considerable work by the broader team throughout the year, Incannex has successfully opened its Investigational New Drug file after approval from the US Food and Drug Administration, truly a landmark achievement for the company. Incannex is now at the point whereby we will imminently undertake a major multi- site phase 2/3 pivotal clinical trial to assess IHL-42X for potential registration and marketing approval in the United States.
It was also another important year of development for IHL-675A, our multi-use cannabinoid drug candidate for inflammatory disorders. Various pre-clinical assessments of IHL-675A have demonstrated a superior response to inflammation to CBD administer alone, which is highly encouraging to us from a marketability and economic perspective.
Successful clinical results in the Phase 1 trial for IHL-675A were precursory to the extensive multi-site Phase 2 trial that was commenced in February to assess IHL-675A for use in the treatment of pain and function in patients with rheumatoid arthritis.
IHL-675A comprises cannabidiol and hydroxychloroquine. Both compounds are currently used to treat arthritis. By conducting studies on our unique proprietary combination formulation, we intend to open a major new market for prescribing health professionals to help the growing population of sufferers of rheumatoid arthritis.
With the benefit of our partnership with Monash University, Incannex has proved itself to be a sophisticated and advanced participant in the global psychedelic therapy sector. The PsiGAD phase 2 clinical trial assessing the use of our psilocybin assisted psychotherapy treatment protocol for generalised anxiety disorder nears completion with the final readout of results expected in Q1 of 2024.
Psychedelic therapies continue to garner attention from all walks of life and we are delighted to have established Clarion Clinics as the first dedicated clinic in Australia to provide crucial and potentially transformative psychedelic psychotherapy programs for people with PTSD and treatment resistant depression. We’re confident that Incannex has the most experienced people in country to undertake the significant endeavour to create a commercial psychedelic therapy clinic.
From a corporate perspective, the board of directors is unanimous in its decision to redomicile Incannex to the United States. If approved by our shareholders in a general meeting, the effect will be that all our shareholders will become holders of Incannex shares on the Nasdaq exchange. The Nasdaq is internationally respected and one of the largest exchanges in the world. It is a suitable marketplace for the ambitious goals of our company to develop multiple proprietary pharmaceutical products for the prescribing community of healthcare professionals.
2
Incannex Healthcare Limited
I would like to thank CEO and managing director Mr Joel Latham and the entire Incannex team for their energy and commitment that they bring to Incannex each and every day. Their work ethic, motivation and commitment to Incannex and the development of our clinical assets is something that as a shareholder I am extremely grateful. Lastly, I thank our shareholders. From a clinical perspective Incannex has made great strides throughout the year, a year that has been challenging in financial markets. Inevitably success in the clinic and ultimately as a business is largely due to the support of the company via its shareholders, we very much appreciate that support and look forward to our exciting journey together throughout FY2024.
| /s/ Troy Valentine |
Troy Valentine
Chairman
Melbourne, Victoria, 30th August 2023
3
Incannex Healthcare Limited
DIRECTORS’ REPORT
Your directors submit the annual financial report of Incannex Healthcare Limited (“IHL” or “the Company”) and its wholly owned subsidiaries (’the Group”) for the financial year ended 30 June 2023. In order to comply with the provisions of the Corporations Act 2001, the Directors report as follows:
DIRECTORS
The names of directors who held office during or since the end of the year and until the date of this report are as follows. Directors were in office for this entire period unless otherwise stated. No director served as a director of any other listed company during the period of three years immediately before the end of the financial year.
Mr Joel Latham – Managing Director & Chief Executive Officer
Appointed 24 July 2019
Joel Latham is the CEO and Managing Director of Incannex Healthcare and is responsible for the Company’s commercial operations, strategic decision- making, and oversight of all clinical development assets. Joel has over 15 years commercial management and executive experience, working for a range multi-national publicly traded companies.
Mr Troy Valentine – Non-Executive Chairman
B.Comm
Appointed 12 December 2017
Troy Valentine has been Chairman of the Board of Directors since December 2017. Mr. Valentine is a finance professional with managerial and Board experience spanning over 27 years. He commenced his career with Australian brokerage firm Hartley Poynton (now Euroz Hartley’s Limited) in 1994 before moving to Patersons Securities (now Canaccord Genuity) in 2000 and subsequently became an Associate Director. During his time at Patersons, he was responsible for managing both retail and institutional accounts. Mr. Valentine has significant corporate and capital raising experience, especially with start-ups and small to mid-cap size companies.
He is currently a director of Australian boutique corporate advisory firm Alignment Capital Pty Ltd, which he co-founded in 2014.
Mr Peter Widdows – Non-Executive Director
ACA (ICAEW), BTec, MAICD
Appointed 1 March 2018
Peter Widdows is the former Regional CEO of the H. J. Heinz corporation, with responsibility for a large portion of Asia and Australasia. He has extensive experience in Australian and international consumer goods markets and has worked as a senior executive/CEO in numerous geographies, including Europe, the USA and Asia/Pacific. Mr Widdows has a strong track record of driving profitable growth in both small and large companies and turning around poor performing businesses.
He is the current Non-executive Chair of Sunny Queen Australia Ltd - Australia’s largest shell egg and egg based meal producer and a Non-Executive Director of Youi Holdings Ltd - A general insurance company.
Dr George Anastassov – Non-Executive Director
Appointed 29 June 2022
Dr Anastassov is responsible for APIRx commercial operations, strategic decision-making, and oversight of all clinical development assets. He is one of the developers of the first-in-the world cannabinoid-containing chewing gum-based delivery system among a number of other systems and formulations. Previously, he was CEO and co- founder of AXIM Biotechnologies, which achieved an all-time-high market capitalization of approximately US$1.2B.
4
Incannex Healthcare Limited
Robert Clark – Non-Executive Director
Appointed 17 August 2022
Robert Clark is currently the Vice President, Regulatory Affairs for Novo Nordisk in the United States. He joined Novo Nordisk in 2012 after spending over 20 years at Pfizer in roles of increasing responsibility in the regulatory field. Robert has over 35 years of US and global regulatory experience. Under his leadership, his regulatory teams have received US FDA approvals for a large number of medicines across various therapeutic areas.
COMPANY SECRETARY
Madhukar Bhalla
Appointed 7 July 2021
Madhukar “Madhu” is an experienced company secretary who has previously worked with multiple ASX-listed companies and is proficient in corporate governance, company administration, financial management, and corporate law. Madhu also has significant business and management experience having previous job titles including general manager and corporate administrator. Madhu was the managing director of Colortype Press for a period of 8 years until 2004. There, he was responsible for the overall management of the business, including marketing, contracting, procurement and directing over 30 employees.
DIRECTORS’ MEETINGS
The number of meetings of Directors held during the year, and the number of meetings attended by each director were as follows:
| Name | Number of meetings eligible to attend | Number of meetings attended | ||||||
| Mr Troy Valentine | 16 | 16 | ||||||
| Mr Peter Widdows | 16 | 16 | ||||||
| Mr Joel Latham | 16 | 16 | ||||||
| Dr George Anastassov | 16 | 16 | ||||||
| Mr Robert Clark | 13 | 13 | ||||||
PRINCIPAL ACTIVITIES
During the financial year, the principal activity of the Company was research and development associated with medicinal cannabinoid and psychedelic pharmaceutical products and therapies.
REVIEW OF OPERATIONS AND SIGNIFICANT CHANGES IN STATE OF AFFAIRS
Operating result for the year
The Group’s loss for the year to 30 June 2023 after income tax was $19,979,558 (2022 Loss of $14,903,909).
CASH RESOURCES
At 30 June 2023, the Group had total funds, comprising cash at bank and on hand of $33,363,228 (2022: $37,500,93) the majority of which is held in Australian dollars. Total current assets at year-end stand at $34,685,887 (2022: $37,879,608).
5
Incannex Healthcare Limited
BUSINESS ACTIVITIES AND OUTLOOK
IHL-675A: Incannex’s proprietary anti-inflammatory drug product
Incannex are developing IHL-675A, a proprietary fixed dose combination products that contains cannabidiol (CBD) and hydroxychloroquine sulphate (HCQ), for the treatment of inflammatory conditions. Inflammatory conditions occur when the body’s immune system attacks its own tissues and organs causing inflammation, pain, discomfort, and damage to the effected tissues. Inflammatory diseases include rheumatoid arthritis which mostly affects joints, colitis and Crohn’s disease which affect the gastrointestinal tract, and asthma and chronic obstructive pulmonary disease which affect the respiratory system. Although there are anti-inflammatory drugs available, many patients still experience substantial pain and reduced function even when taking the marketed drugs, and some of the approved drugs have associated safety concerns.
CBD and HCQ have anti-inflammatory activity when used independently. Incannex hypothesized that the combination of CBD and HCQ would be synergistic. That is, the combination of the two drugs would reduce inflammation to a greater extent than would be predicted for the combination based on their activities when used independently. The hypothesis of synergistic anti-inflammatory activity was confirmed in a series of preclinical studies including human peripheral blood mononuclear cells and animal models of inflammatory diseases including arthritis, inflammatory bowel disease and inflammatory lung disease. The results of these preclinical models gave Incannex the confidence to develop a unique fixed dose combination product for assessment in clinical trials with the goal of regulatory approval by bodies including the United States Food and Drug Administration (FDA) and Australian Therapeutic Goods Administration (TGA).
Phase 1 clinical trial assessing the safety, tolerability and pharmacokinetics of IHL-675A
To assess the safety, tolerability, and pharmacokinetics of IHL-675A Incannex ran a Phase 1 clinical trial. The key endpoints of the trial were the adverse events reported and the plasma levels of the active pharmaceutical ingredients (APIs), CBD and HCQ, and their major metabolites over a 28-day period. IHL-675A was compared to the reference listed drugs for CBD and HCQ, Epidiolex and Plaquenil respectively, across all endpoints. The trial included three cohorts of twelve participants each (total n = 36), with equal evaluations applied across all three groups. Participants were monitored for adverse events and had blood samples collected for pharmacokinetic analysis over a 28-day period. The study was conducted at CMAX Clinical Research in South Australia and managed by Avance Clinical.
In July 2022, Incannex received approval from the Bellberry Human Research Ethics Committee (HREC) to conduct the Phase 1 clinical trial investigating IHL-675A. Recruitment of participants for the trial commenced in August 2022. Dosing of participants was completed in October 2022.
Safety and Tolerability Results
IHL-675A was well tolerated, with no adverse events of concern and no serious adverse events reported (Figure 1). The same number of treatment related treatment emergent adverse events (TEAEs) were reported for IHL- 675A as for Epidiolex. Treatment-related TEAEs included abdominal pain, dizziness, fatigue, frequent bowel movements, headache and somnolence. All TEAEs were minor with the exception of one incidence of moderate severity abdominal cramps which resolved soon after onset.
6
Incannex Healthcare Limited
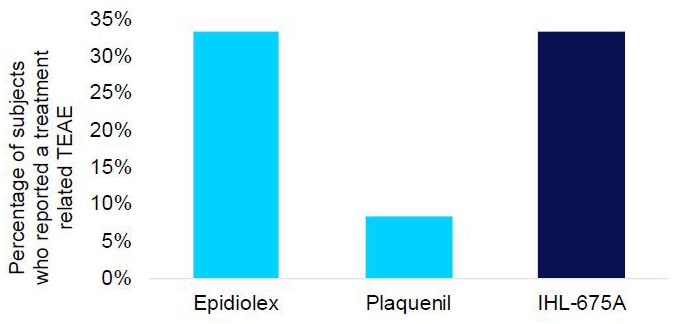
Figure 1. Percentage of subjects who reported a treatment related treatment emergent adverse event in each of the treatment groups of the IHL-675A Phase 1 clinical trial.
CBD Pharmacokinetic Results
Comparison of the average pharmacokinetics of CBD in participants administered IHL-675A compared to those administered Epidiolex revealed that the CBD was taken up from IHL-675A more quickly and reached a higher maximum concentration than from Epidiolex (Figure 2). The average maximum concentration (Cmax) of CBD from IHL-675A was 1.57 times higher than for Epidiolex. The time to reach the maximum concentration (Tmax) was 26% faster for IHL-675A than Epidiolex. CBD administered in IHL-675A was also cleared more quickly than Epidiolex. The half-life (t1/2) of CBD from IHL-675A was 13% faster than Epidiolex. The total exposure (AUCinf) was similar for CBD administered as IHL-675A and Epidiolex. These patterns are trends at this point (p >0.05). Similar results were observed for CBD metabolites 7-COOH-CBD and 7-OH-CBD. Pharmacokinetic parameters are presented in Table 1.
7
Incannex Healthcare Limited

Figure 2. Average plasma concentrations of CBD over time for the IHL-675A and Epidiolex treatment groups in the IHL-675A Phase 1 clinical trial.
8
Incannex Healthcare Limited
Table 1. CBD and metabolite PK parameters from IHL-675A Phase 1 study
| IHL-675A | Epidiolex | |||||||||||||||||||||||||||||||||
| Cmax | Tmax | AUCinf | T1/2 | Cmax | Tmax | AUCinf | T1/2 | |||||||||||||||||||||||||||
| (ng/mL) | (hr) | (hr*ng/mL) | (hr) | (ng/mL) | (hr) | (hr*ng/mL) | (hr) | |||||||||||||||||||||||||||
| CBD | Mean | 207.04 | 2.13 | 841.08 | 220.17 | 131.89 | 2.88 | 725.9 | 231.22 | |||||||||||||||||||||||||
| SD | 117.44 | 0.91 | 358.63 | 53.85 | 61.92 | 1.21 | 223.98 | 56.45 | ||||||||||||||||||||||||||
| Min | 72.6 | 1.02 | 391 | 113.84 | 45.6 | 1.5 | 355 | 144.41 | ||||||||||||||||||||||||||
| Max | 472 | 4 | 1699 | 301.17 | 241 | 6 | 1121 | 305.88 | ||||||||||||||||||||||||||
| 7-OH-CBD | Mean | 55.24 | 2.17 | 389.18 | 40.54 | 21.06 | 3 | 262.27 | 21.15 | |||||||||||||||||||||||||
| SD | 34.58 | 0.94 | 214.49 | 52.79 | 9.15 | 1.22 | 103.95 | 10.05 | ||||||||||||||||||||||||||
| Min | 14.9 | 1.02 | 220 | 10.78 | 7.7 | 1.5 | 149 | 10.54 | ||||||||||||||||||||||||||
| Max | 116 | 4 | 950 | 202.58 | 38.4 | 6 | 448 | 49.36 | ||||||||||||||||||||||||||
| 7-COOH-CBD | Mean | 479.75 | 2.83 | 18753.9 | 167.87 | 362.17 | 4.97 | 16268 | 153.68 | |||||||||||||||||||||||||
| SD | 218.74 | 1.2 | 8979.02 | 95.47 | 299.63 | 1.3 | 11069.2 | 92.41 | ||||||||||||||||||||||||||
| Min | 209 | 1.5 | 11445 | 46.03 | 116 | 2.5 | 4475 | 18.47 | ||||||||||||||||||||||||||
| Max | 921 | 6 | 43714 | 332.65 | 1180 | 6.05 | 42018 | 317.68 | ||||||||||||||||||||||||||
Hydroxychloroquine Pharmacokinetic Results
Comparison of the average pharmacokinetics of hydroxychloroquine in participants administered IHL-675A compared to those administered Plaquenil revealed that hydroxychloroquine was taken up more slowly from IHL-675A than from Plaquenil but the two drugs had a similar maximum plasma concentration (Figure 3). The time to reach the maximum concentration (Tmax) for HCQ administered as IHL-675A was 46% slower than for Plaquenil. The hydroxychloroquine clearance and total exposure was similar for the two drugs. These patterns are trends at this point (p >0.05). Plasma concentrations of hydroxychloroquine metabolites desethylhydroxychloroquine, bisdesethylhydroxychloroquine and desethylchloroquine were detected only at low levels (<2 ng/mL) at all points in the study. Pharmacokinetic parameters are presented in Table 2.
9
Incannex Healthcare Limited

Figure 3. Average plasma concentrations of hydroxychloroquine over time for the IHL-675A and Plaquenil treatment groups in the IHL-675A Phase 1 clinical trial.
10
Incannex Healthcare Limited
Table 2. Hydroxychloroquine and metabolite PK parameters from IHL-675A Phase 1 study
| IHL-675A | Plaquenil | |||||||||||||||||||||||||||||||||
| Cmax | Tmax | AUCinf | T1/2 | Cmax | Tmax | AUCinf | T1/2 | |||||||||||||||||||||||||||
| (ng/mL) | (hr) | (hr*ng/mL) | (hr) | (ng/mL) | (hr) | (hr*ng/mL) | (hr) | |||||||||||||||||||||||||||
| HCQ | Mean | 54.71 | 5.59 | 2986 | 182.62 | 55.52 | 3.46 | 3430.8 | 251.6 | |||||||||||||||||||||||||
| SD | 23.85 | 2.51 | 1244.46 | 93.7 | 24.81 | 1.94 | 1104.38 | 73.65 | ||||||||||||||||||||||||||
| Min | 22 | 2 | 800 | 35.68 | 26.1 | 1 | 2073 | 163.92 | ||||||||||||||||||||||||||
| Max | 105 | 12.03 | 4217 | 311.57 | 124 | 6 | 5888 | 421.51 | ||||||||||||||||||||||||||
| DESETHYL- | Mean | 1.38 | 81.08 | NA | NA | 1.29 | 17.46 | NA | NA | |||||||||||||||||||||||||
| HYDROXY- | SD | 1.24 | 183.01 | NA | NA | 1.04 | 35.04 | NA | NA | |||||||||||||||||||||||||
| CHLOROQUINE | Min | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||
| Max | 4.4 | 673.83 | 0 | 0 | 3.3 | 123.93 | 0 | 0 | ||||||||||||||||||||||||||
| DESETHYL- | Mean | 0.8 | 7.77 | NA | NA | 0.42 | 5.59 | NA | NA | |||||||||||||||||||||||||
| CHLOROQUINE | SD | 0.72 | 13.03 | NA | NA | 0.84 | 13.58 | NA | NA | |||||||||||||||||||||||||
| Min | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
| Max | 2 | 49.05 | 0 | 0 | 2.9 | 49.07 | 0 | 0 | ||||||||||||||||||||||||||
| BISDESETHYL- | Mean | 0 | 0 | NA | NA | 0 | 0 | NA | NA | |||||||||||||||||||||||||
| HYDROXY- | SD | 0 | 0 | NA | NA | 0 | 0 | NA | NA | |||||||||||||||||||||||||
| CHLOROQUINE | Min | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||
| Max | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
NA- metabolite not detected at levels sufficient to calculate PK parameter
Interpretation of the results from the phase 1 clinical trial.
IHL-675A is well tolerated in healthy volunteers. Adverse events for IHL-675A were consistent with what was observed, and has been publicly reported, for Epidiolex and Plaquenil. Both active pharmaceutical ingredients, CBD and HCQ, are absorbed from IHL-675A. Trends in PK profiles indicate that the uptake of CBD may be more rapid for IHL-675A than Epidiolex and uptake of HCQ may be slower for IHL-675A than Plaquenil. This could be advantageous for IHL-675A. CBD provides immediate relief for inflammation and pain whereas HCQ is a slower acting molecule and provides extended relief.
The safety and pharmacokinetic data from this Phase 1 clinical trial in healthy volunteers adds to the company’s confidence in proceeding with assessment of IHL-675A in patients with inflammatory diseases, with the initial focus on rheumatoid arthritis.
11
Incannex Healthcare Limited
Phase 2 clinical trial assessing the effects of IHL-675A on pain and function in patients with rheumatoid arthritis.
In February 2023, Incannex announced that it had commenced a Phase 2 clinical trial to assess the safety and efficacy of IHL-675A on pain and function in patients with rheumatoid arthritis. In this trial rheumatoid arthritis patients will receive one of IHL-675A, CBD, HCQ or placebo for 24 weeks. The treatments will be double blinded, meaning neither the investigators nor patients will know which treatment an individual is receiving. The study will be managed by Avance Clinical, an Australian and US CRO (Avance), who will identify and onboard 8-13 clinical trial sites with expertise in rheumatoid arthritis to conduct patient recruitment and assessments. Avance Clinical will manage the sites and study conduct, ensure that the data is of the necessary quality, and conduct the analysis of data collected across all the trial sites.
The trial will include 128 participants who meet the eligibility criteria. Participants will be randomised to one of 4 arms: either IHL-675A, CBD alone, HCQ alone or placebo. The primary endpoint for the study is pain and function relative to baseline determined via the score on the RAPID3 assessment at 24 weeks. Participants will also record their pain and function outcomes daily, by completing questionnaires on pain, fatigue, joint stiffness and quality of life, using an electronic Patient Reported Outcomes device (similar to completing a questionnaire on an electronic tablet). The participants will attend monthly visits at the clinical trial site, where blood tests, and physical examinations will monitor additional safety and efficacy outcomes including inflammatory biomarkers. The trial will also include a sub-study examining joint damage via MRI. Subjects will be assessed for eligibility in the MRI study based on their Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) at screening.
The results of this study will establish the safety and efficacy of IHL-675A in rheumatoid arthritis and will be a critical component of future regulatory applications, including contributing to the combination rule assessment in the FDA505(b)2 new drug application (NDA) dossier.
After the end of financial year, in July 2023, Incannex received approval from the Human Research Ethics Committee (HREC) for its lead site, Emeritus Research in Camberwell, VIC, to conduct the Phase 2 clinical trial investigating the effect of IHL-675A on pain and reduced function in patients with rheumatoid arthritis. Site selection, approval and HREC submission is ongoing and Incannex anticipates that approval for the remaining sites will be received over the coming months.
IHL-42X: Incannex’s proprietary drug product for treatment of obstructive sleep apnea
Incannex are developing IHL-42X, a proprietary combination of dronabinol and acetazolamide for treatment of obstructive sleep apnea (OSA). OSA is a disease of sleep disordered breathing where the upper airway repeatedly completely or partially collapses during sleep. This disrupts airflow, reduces oxygen uptake and leads to poor sleep quality. Presentation of OSA often includes snoring and waking up gasping for air. The immediate consequences of OSA are daytime sleepiness, negative impacts on mood and cognitive function, including an increased risk of traffic accidents. Long term, patients with untreated OSA have an increased risk of cardiovascular disease, deficits in executive function and mental health issues such as depression and anxiety. Current standard of care for OSA is the use of positive airway pressure (PAP) devices, such as CPAP. Although these devices are effective, patient compliance is less than 50% due to issues with discomfort, inconvenience, cost and safety concerns. There are no approved pharmacotherapies for treatment of OSA.
Incannex hypothesized that the combination of dronabinol, a synthetic form of THC, and acetazolamide, a carbonic anhydrase inhibitor that is used for the treatment of a range of indications would be an effective treatment for OSA. This hypothesis was based on the published observations that each of the drugs had a benefit in patients with OSA. However, the therapeutic effect of the drugs when used alone was limited and there were concerns with side effects at therapeutic doses. IHL-42X is designed to reduce the dose of each component drug, increasing the therapeutic effect, and reducing the side effects.
12
Incannex Healthcare Limited
IHL-42X proof of concept clinical trial.
In June 2022, Incannex unveiled promising outcomes from a comprehensive analysis of its Phase 2 clinical trial evaluating the efficacy of IHL-42X in treating patients with obstructive sleep apnea (OSA). Particularly, the low dose of IHL-42X demonstrated superior safety and effectiveness metrics when compared to higher doses. Notably, low-dose IHL-42X led to an average reduction of 50.7% in apnea-hypopnea index (AHI) among trial participants (Figure 4), with 25% experiencing a substantial reduction of over 80% (Figure 5). AHI is the main measure used to diagnose and monitor OSA. A reduction in the AHI indicates an improvement in the disease, which is anticipated to lead to improved sleep quality and decreased daytime sleepiness.
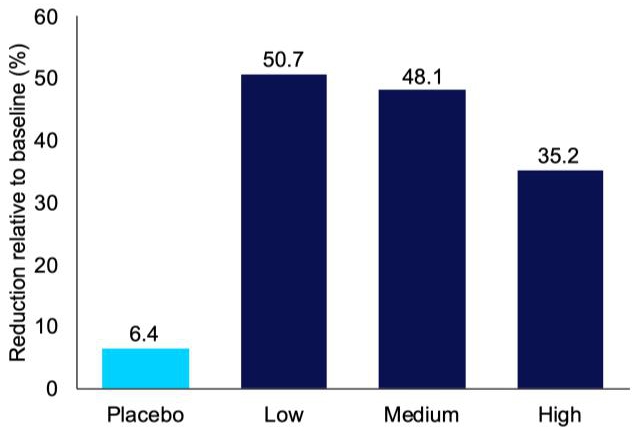
Figure 4. Average reduction in apnea hypopnea index (AHI) for each treatment period, relative to baseline, in the IHL-42X proof of concept phase 2 clinical trial.
13
Incannex Healthcare Limited

Figure 5. Proportion of patients in each IHL-42X proof of concept treatment period who experienced a reduction in AHI of >50% and >80% relative to baseline.
IHL-42X also improved other aspects of OSA. The oxygen desaturation index, which is a measure similar to AHI, but instead measures the number of times there is insufficient blood oxygen levels or desaturation events, dropped by an average of 59.7% (Figure 6). The improvement in AHI and ODI culminated in improved patient reported sleep quality (Figure 7).
14
Incannex Healthcare Limited
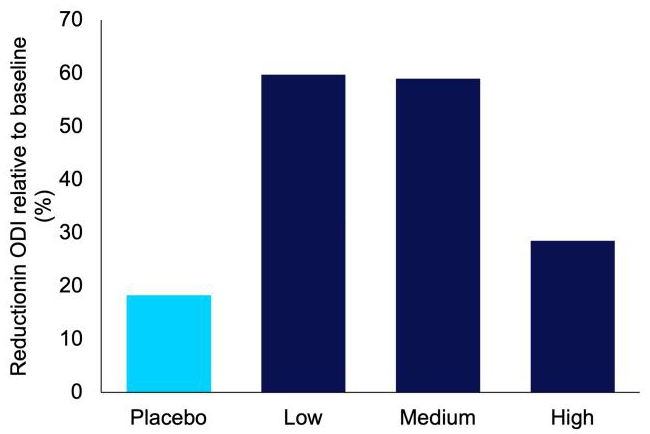
Figure 6. Average reduction in oxygen desaturation index (ODI) for each treatment period, relative to baseline, in the IHL-42X proof of concept phase 2 clinical trial.
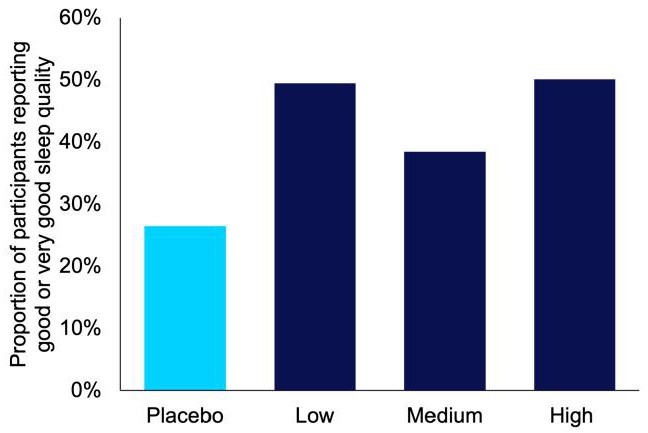
Figure 7. Proportion of patients in each IHL-42X proof of concept treatment period who reported good or very good sleep quality.
The proof-of-concept study also returned encouraging results from a safety perspective. Of significance, the compound was well-tolerated, with fewer treatment-emergent adverse events in the low-dose group compared to the placebo (Figure 8). Another important result was that low-dose IHL-42X resulted in THC blood concentrations below thresholds for impaired driving (1 ng/mL) on the morning following administration (Figure 9). None of the samples in the low dose treatment period had a THC concentration of greater than 0.45 ng/mL.
15
Incannex Healthcare Limited
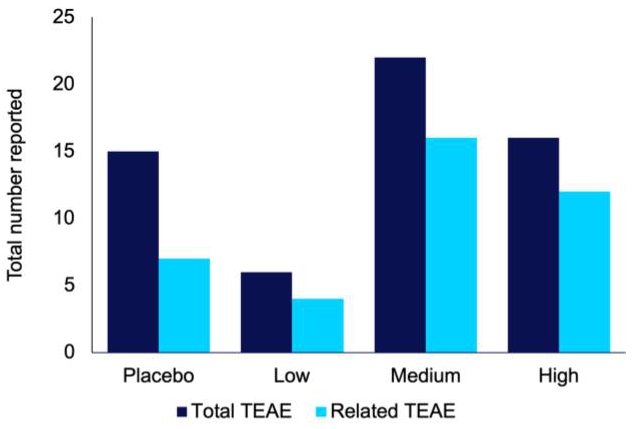
Figure 8. Total number of treatment emergent adverse events (TEAE), and TEAE that were probably or possibly related to the treatment, reported during each IHL-42X treatment period.
16
Incannex Healthcare Limited
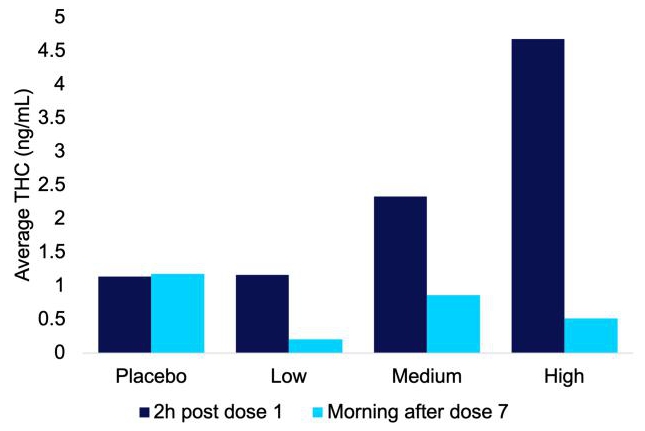
Figure 9. Average THC concentrations in plasma samples collected the during each of the treatment periods of the IHL-42X proof of concept clinical trial. The average is calculated for samples for which there was THC detected. In the placebo treatment period this was a single sample.
The safety and efficacy data from the IHL-42X proof-of-concept study confirmed the Incannex’s hypothesis that the combination of dronabinol and acetazolamide would be an effective treatment for sleep apnea and has given the company the confidence to continue to develop IHL-42X towards regulatory approval.
Expanded patent position for IHL-42X
In December 2022, Incannex announced that it had filed an additional provisional patent application for protection of IHL-42X. This patent application was based on data from a further analysis of the data from the IHL-42X phase 2 proof of concept clinical trial where IHL-42X was shown to have a dose dependent effect on loop gain and low dose IHL-42X had a statistically significant effect on airway collapsibility. This provided some explanation as to why IHL-42X at low dose was observed to be more effective at reducing AHI than medium or high doses.
Bioavailability/bioequivalence clinical trial
In November 2022, Incannex announced that it had engaged CMAX Clinical Research and Novotech CRO to undertake a bioequivalence/bioavailability (BA/BE) clinical trial for IHL-42X. The BA/BE study focuses on assessing the pharmacokinetics and tolerability of IHL-42X’s active pharmaceutical ingredients (APIs), dronabinol (THC) and acetazolamide, in comparison to FDA reference listed drugs Marinol and Taro acetazolamide tablets respectively. The study will also investigate the effect of food on IHL-42X tolerability and pharmacokinetics. The BA/BE study involves 116 participants and will evaluate the concentrations of APIs and metabolites in blood samples over 48 hours. This study design adheres to FDA recommendations for bioequivalence studies. The outcomes of the BA/BE trial will be a crucial component of a forthcoming New Drug Application (NDA), serving as a bridging mechanism to the reference listed drugs, thereby facilitating regulatory approval via the FDA505(b)2 regulatory pathway.
Approval was received in July 2023 from Bellberry HREC for the conduct of the BA/BE clinical trial.
17
Incannex Healthcare Limited
Phase 2/3 clinical trial investigating IHL-42X in patients with OSA
The next step in the development of IHL-42X is a global Phase 2/3 clinical trial investigating the effect of the drug product in patients with OSA who are non-compliant, intolerant or naïve to positive airway pressure devices, such as CPAP. This study will include at least 385 patients across 45 clinical trial sites located across the world. Feedback from the FDA in a preIND meeting guided the design of this clinical trial. Efficacy of the drug will be assessed by a co-primary endpoint consisting of change in AHI from baseline and change in functional outcomes of sleep score from baseline at 52 weeks. Secondary and exploratory endpoints will include other PSG and sleep parameters, change in cognitive function and a range of safety and efficacy focused biomarkers.
Appointment of lead principal investigators for Phase 2/3 trial.
In June 2023, Incannex announced that Dr John D Hudson of FutureSearch Trials of Neurology, Austin, Texas and Dr Russell Rosenberg, of Neurotrials Research Inc, Atlanta Georgia, had been recruited as co-Lead Principal Investigators for the IHL-42X Phase 2/3 Study.
J. Douglas Hudson, MD, is board certified in Neurology and Sleep Medicine. He serves as the Principal Investigator for FutureSearch Trials of Neurology, Austin, Texas. Dr. Hudson has supervised over 300 clinical trials over the past 20 years mostly related to neurological and sleep disorders and has been a national and international speaker for these disorders.
Dr. Hudson completed his neurology residency at the University of Iowa and was Austin’s first board certified sleep specialist. Past activities include founding the Austin Neurological Clinic and Sleep Medicine Consultants. He held the position of President of the Texas Neurological Society, with a Lifetime Achievement Award and President of the Capital Area American Heart Association.
FutureSearch Trials consists of two clinical research facilities in Austin and Dallas, Texas which have been in operation for over 15 years. The Austin site where Dr. Hudson is the Principal Investigator focuses on clinical research studies for treatment of neurological, pain and sleep disorders and features an on-site sleep lab. Regarding the IHL-42X trial, Dr Hudson said: “Clinical trials for novel formulations of medication are newsworthy for any specialty and sleep medicine is no exception.
Obstructive Sleep Apnea, affecting millions of people, remains under treated. This is due in part to patients not being diagnosed, and in part due to poor patient compliance with current therapeutic modalities. While unheard of a few years ago, oral medications to help reduce the cause of OSA, are now undergoing further investigation. This is more than exciting, it could prove to be life-changing for many patients.”
Dr. Rosenberg is currently Chief Science Officer and CEO of NeuroTrials Research in Atlanta, Georgia. Dr. Rosenberg, a native of St. Louis, obtained his doctorate in clinical and research psychology from The Ohio State University and received specialized training in sleep disorders medicine and research at Rush Presbyterian - St. Luke’s Medical Center in Chicago. He has more than 35 years’ experience in clinical sleep medicine and research, acting as an investigator in over 300 clinical trials including 14 in OSA and 211 in other sleep related disorders. He is a Board-Certified Sleep Specialist and Fellow of the American Academy of Sleep Medicine. Dr. Rosenberg is former Chair and spokesperson for the National Sleep Foundation (NSF) and has appeared frequently on local and national television news shows including the Today Show, Good Morning America, CNN, and MSNBC.
Neurotrials Research Inc is a clinical research facility in Atlanta, Georgia that has been in operation for over 25 years. Neurotrials Research is focused on delivery of trials in neurology/CNS and sleep indications. Regarding the IHL-42X phase 2/3 clinical trial Dr Rosenberg said: “Incannex has developed a sound, rational, scientific protocol to determine the efficacy and safety of IHL-42X in subjects with obstructive sleep apnea.” “Many sleep apnea patients cannot adhere to positive airway pressure therapy, use it for an inadequate period at night or just refuse it. Having a safe, effective pharmacological option for obstructive sleep apnea will be a positive addition to the treatment landscape as it will offer those that struggle to adhere to positive airway pressure therapy an alternative therapy.”
18
Incannex Healthcare Limited
Investigational New Drug Application
Subsequent to the end of the financial year, Incannex submitted an investigational new drug (IND) application to the US FDA for review. The IND dossier compiled by the Incannex team included comprehensive modules on the safety and efficacy of IHL-42X and its component active pharmaceutical ingredients. It also includes detailed information on the development, manufacturing, quality and stability of the IHL-42X drug product, as well as the clinical protocol and investigator information for the Phase 2/3 IND opening clinical trial.
The modules of the IND were:
| ● | Module 1 – Administrative Information and Prescribing Information |
| ● | Module 2 – Nonclinical/Clinical Overviews and Summaries |
| ● | Module 3 – Quality data |
| ● | Module 4 – Nonclinical Study Reports and Key Literature References |
| ● | Module 5 – Clinical Study Reports, Clinical Protocol and Investigator Information |
Submitting and clearing an IND with the FDA is crucial for companies to gain regulatory approval, conduct clinical trials, and engage in scientific dialogue with FDA whilst they progress investigational drugs through the stages of development in the United States. The FDA review process for an IND application involves evaluation of the scientific, clinical, and safety aspects to ensure that the proposed clinical trial meets regulatory requirements.
FDA completed their review of the IND package and Incannex received confirmation from the agency that the IND opening study may proceed. That is, the IND application has cleared.
Clearance of the IND application is a critical milestone that is required to conduct clinical trial in the United States. Incannex are now working with Fortrea, the CRO engaged to manage the Phase 2/3 clinical trial to prepare institutional review board applications for the lead trial sites, complete the selection and approval of the remaining trials sites, and further prepare for patient recruitment and dosing for the clinical trial.
IHL-216A: Incannex’s proprietary drug product for treatment of traumatic brain injury
Incannex are developing IHL-216A, a combination of cannabidiol and isoflurane for treatment of traumatic brain injury. The two drugs are both known to have neuroprotective effects that occur through different mechanisms of action. Incannex hypothesized that the combination of the two drugs would provide a synergistic neuroprotective effect. This hypothesis was confirmed in two separate rodent models of TBI, both mild and moderate/severe injury models.
Incannex Successfully Concludes Constructive Pre-IND Meeting with FDA for IHL-216A Concussion and Traumatic Brain Injury (TBI) Treatment
In October, 2022 Incannex completed a productive pre-Investigational New Drug (pre-IND) meeting with the U.S. Food and Drug Administration (FDA) concerning its proprietary drug product IHL-216A, developed for the treatment of Traumatic Brain Injury (TBI) and concussion. The pre-IND meeting followed the submission of a comprehensive package to the FDA in August 2022, encompassing details about the unique formulation, a comprehensive clinical development plan, and specific regulatory inquiries necessary for opening an Investigational New Drug application (IND). The IND is a crucial step for conducting clinical trials in the United States, ensuring that trial designs align with data requirements for eventual FDA marketing approval.
In response to the pre-IND submission, the FDA provided valuable multidisciplinary feedback regarding the clinical development of IHL-216A. Moreover, the FDA provided guidance on the use of the FDA505(b)2 regulatory pathway, wherein certain data required for marketing approval can be drawn from publicly available studies on the components of IHL-216A.
19
Incannex Healthcare Limited
Upscaling Production of cGMP IHL-216A
During August 2022, Incannex engaged Curia Global, Inc. (Curia) to facilitate the advanced development and cGMP-grade manufacturing of IHL-216A, the company’s inhaled proprietary drug targeted for the treatment of concussion and traumatic brain injury (TBI). This decision comes following successful proof-of-concept studies that identified the optimal inhaled formulation for IHL-216A on an experimental scale. Curia is tasked with scaling up the fill-finish manufacturing processes for IHL-216A, ensuring compliance with Current Good Manufacturing Practice (cGMP) standards while also generating vital data concerning product quality and stability. This information is set to support future regulatory submissions.
Inclusion of FDA Regulatory Affairs Expert, Mr. Robert B. Clark, on the Board of Directors
Incannex proudly announced the appointment of Mr. Robert B. Clark to its Board of Directors in August 2022. Mr. Clark is an accomplished pharmaceutical executive with over 38 years of substantial regulatory experience in both the US and the global landscape. This includes extensive roles at Pfizer Inc. and Novo Nordisk A/S, each exceeding two decades. Mr. Clark’s expertise extends to strategic regulatory affairs, FDA and European Medicines Agency (EMA) interactions, pharmaceutical advertising regulations, and matters pertaining to healthcare professionals and sales activities.
Presently serving as Vice President of US Regulatory Affairs at Novo Nordisk, Mr. Clark offers strategic leadership to a team of over 50 regulatory professionals engaged in new medicine development. His guidance influences global executive decisions, encompassing drug development strategies, FDA engagement strategies, compliance oversight, and vigilance regarding emerging US regulatory trends. Notably, his leadership has led to the FDA approval of a notable twelve (12) significant new drugs since 2012, reflecting his effectiveness in navigating complex regulatory processes.
Mr. Clark’s impressive background and contributions align closely with Incannex’s endeavors, particularly given its diverse mix of drug candidates including cannabinoids and psychedelic pharmacotherapies. In his own words, Mr. Clark emphasized his enthusiasm to contribute to Incannex’s broad portfolio of treatments designed to address conditions with limited therapeutic alternatives.
Psychennex
Psychennex is a wholly owned subsidiary of Incannex that houses all research, development and commercial activities related to psychedelic molecules, such as psilocybin and MDMA. This includes the clinical development of the PsiGAD program that uses psilocybin assisted psychotherapy for treatment of generalised anxiety disorder and the Clarion Clinics Group for administration of psychedelic assisted psychotherapy in major depressive disorder and post traumatic stress disorder.
20
Incannex Healthcare Limited
PsiGAD
PsiGAD is Incannex’s proprietary psilocybin assisted psychotherapy program for treatment of generalised anxiety disorder. The program is being developed in collaboration with Dr Paul Liknaitsky of Monash University.
Generalized anxiety disorder (GAD) is a serious psychiatric condition affecting around 4-6% of the population during their lifetime. GAD is characterised by diffuse, excessive, uncontrollable worry that tends to be more frequent and severe than within other anxiety disorders. GAD has a chronic, unremitting course that is associated with a high public burden, and significant consequences for relationships, work, and quality of life. It is a highly comorbid disorder, with estimations of lifetime mental disorder comorbidity as high as 90%. It is most comorbid with major depression, and also commonly comorbid with other anxiety disorders, other mood disorders, and non-psychiatric disorders such as chronic pain and irritable bowel syndrome.
International guidelines for GAD treatment recommend selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs), and pregabalin as first-line options, with benzodiazepines such as diazepam as second-line options. However, these treatments show limited efficacy, problematic side effects, and other limitations. Psilocybin assisted psychotherapy in PsiGAD is designed to provide an alternative to these patients who’s disease is not adequately controlled by the above mentioned treatments. Psilocybin is thought to facilitate and improve the efficacy of psychotherapy by allowing patient to access the root causes of their anxiety, address those causes and build new neural connections that lead to a lasting treatment effect.
PsiGAD1
PsiGAD1 is a proof-of-concept clinical trial investigating safety and efficacy of psilocybin assisted psychotherapy for treatment of GAD that is being led by principal investigator Dr Paul Liknaitsky and an extended team of clinical scientists, physicians and therapists at Monash University. The trial will recruit 72 patients in total across equivalent, triple blind, psilocybin and placebo arms. Each patient will receive two dosing sessions and a number of preparatory and integration psychotherapy sessions. The endpoints of this trial encompass safety, efficacy, and tolerability, while secondary outcomes include assessments of quality of life, functional limitations, and comorbid conditions. The primary efficacy endpoint is change in Hamilton Anxiety Rating Scale six weeks after the second dosing session.
In March 2023, interim analysis of the study data to date was conducted. An independent Data Safety Monitoring Board reviewed the data and recommended no change to the study design and had no concerns with the safety of the PsiGAD trial. Review of the interim data by Incannex, consisting of primary endpoint data from the first twenty nine participants found that there is a high probability (greater than 85% - alpha error 0.05 or 95% confidence level) that the total study will show a statistically significant benefit for the psilocybin treatment arm over the placebo treatment arm. This projection was made by assuming the effect size observed in the interim analysis for 29 participants is representative of the effect size through the remaining 43 participants. The end point used in this modelling was a reduction in Hamilton Anxiety Rating Scale (HAM-A) score at 11 weeks relative to baseline (six weeks post second dose), which is the primary endpoint in the trial. This modelling was completed internally by the company and did not get verified by the DMSB.
Recruitment for the trial has continued throughout the reporting period and final study results are expected in late 2023 or early 2024.
Development and manufacture of cGMP psilocybin drug product
Based on the promising outcome of the interim analysis from PsiGAD1, Psychennex engaged Catalent for development and cGMP manufacture of Psychennex’s own psilocybin drug product in March 2023. This drug product will be used in Psychennex’s future clinical trials and potential wider commercial use. This development project is ongoing.
21
Incannex Healthcare Limited
Clarion Clinics
In March 2023, Incannex announced the intention to open multiple psychedelic-assisted psychotherapy clinics in Australia and overseas under the leadership of Peter Widdows, a long-standing Director of the Company.
Incannex had been developing the commercialisation plans for psychedelic clinics for some time, well before the TGA decision to down-schedule psilocybin for treatment-resistant depression (TRD) and MDMA for Post-Traumatic Stress Disorder (PTSD) was announced. The announcement from TGA led to an expansion and announcement of these plans.
The Company has entered a partnership with Australia’s leading clinical psychedelic professionals, all of whom have extensive experience within clinical psychedelic research, treatment, and training.
Dr Paul Liknaitzky: Co-Founder, Director, Chief Strategy Officer, and Chief Scientific Officer
Paul has played a central role in establishing the clinical psychedelic field in Australia and leads the largest group of psychedelic researchers and clinicians in the country. Paul is the Chief Principal Investigator on a program of psychedelic trials and collaborates on numerous others nationally. He has led the development of psychedelic trial protocols, treatment design, trial coordination, therapist selection and training, and has established active collaborations with an extensive network of international experts and organisations in the field. Paul’s work is focused on developing innovative psychedelic therapies, evaluating benefits, exploring potential drawbacks, predicting treatment response, mitigating risks, understanding therapeutic mechanisms, and translating research into practice.
Professor Suresh Sundram: Co-Founder, Director, Chief Medical Officer, and Head of Psychiatry
Suresh is a Fellow of the Royal Australian and New Zealand College of Psychiatrists and a consultant psychiatrist. He holds senior leadership positions in academic and clinical psychiatry and has published more than 150 scientific articles, books, book chapters, and conference abstracts. He has presented as plenary and invited speaker at international and national conferences, served as Deputy Editor for the Asian Journal of Psychiatry, and as an advisor to the United Nations (UN), and to national and state governments. Prof. Sundram has led over 50 clinical trials and studies in psychiatric disorders. He has extensive experience with the use of psychedelics within psychotherapy and has overseen multiple research projects in this field.
Sean O’Carroll: Co-Founder, Director, and Head of Psychotherapy
Sean is an integrative psychotherapist and academic – specialising in experiential, relational, and transpersonal psychotherapy. Since 2019, he has developed and delivered psychedelic-assisted psychotherapy training for several clinical psychedelic research teams. He has served as lead psychotherapist on two clinical research trials, continues to supervise one of these teams, and works as a psychedelic-assisted psychotherapy consultant within industry, with an emphasis on psychotherapy training and protocol development. Sean began lecturing in transpersonal psychology in 2011 and has over ten years’ experience working with what he calls “psychedelic casualties”. Through the Wild Mind Institute, he offers training for mental health practitioners in psychedelic-assisted psychotherapy, “bad trip” integration, and eco-psychotherapy.
In May 2023, the company announced that it had signed a lease for the first clinic in Abbottsford, a suburb of Melbourne, Victoria. The clinic is designed as a commercial scale prototype, which can be scaled up and replicated to other locations. It will have capacity to treat over 600 patients per year in normal working hours and substantially more in extended hour operations. The company also announced that it had secured an initial supply of psilocybin and MDMA to facilitate commencement of clinical operations.
22
Incannex Healthcare Limited
The Clarion Clinics Advisory board is made up of world leading clinical psychedelics experts.
| ● | Dr. Bill Richards is among the world’s best known psychedelic researchers and practitioners. He has had a multi-decade career at the forefront of psychedelic research, therapy, and training, and is a mentor and trainer to numerous research groups around the world. He co- founded the psychedelic research group at Johns Hopkins University and is the Director of Therapy at Sunstone Therapies in Maryland, US. |
| ● | Dr. Andrea Jungaberle is the Chief Medical Officer of Ovid Clinics in Berlin and co-founder of the MIND Foundation, Europe’s leading psychedelic research and education group. She has conducted and/or supervised psychedelic-assisted psychotherapy for hundreds of patients and works both within Germany’s largest clinical psilocybin trial and within clinical service delivery. |
| ● | Professor Matthew Johnson is one of the world’s most published psychedelic scientists. He has been central in the establishment and leading track record of the Johns Hopkins Center for Psychedelic & Consciousness Research, and his work has contributed to standards in practice within clinical psychedelic science. As a high-profile scientist in his field, he is frequently interviewed by national and international media outlets. |
In August 2023, after the end of the reporting period, Clarion Clinics announced that they were accepting registrations for Psychedelic treatment interest as part of pre-screening in readiness for opening.
APIRx
In August 2022, Incannex announced that it had completed the acquisition of APIRx Pharmaceuticals to aggregate the world’s largest portfolio of patented medicinal cannabinoid drug formulations. Founders of APIRx, Dr George Anastassov and Mr Lekhram Changoer joined the Incannex team as non-executive director and chief technology officer respectively. Twenty-two (22) additional clinical and pre-clinical research and development projects were transferred to Incannex, representing aggregate addressable markets of approximately US$400B per annum. These projects are underpinned by an intellectual property portfolio that includes 19 granted patents and 23 pending patents.
The foremost drug candidates stemming from this acquisition encompass:
| ● | MedChew Dronabinol for chemotherapy-induced nausea and vomiting | |
| ● | MedChew Rx for pain and spasticity in multiple sclerosis patients | |
| ● | CannQuitN and CannQuitO – chewable products merging nicotine and cannabinoids, and cannabinoids and opioid antagonists, targeting smoking cessation and opioid addiction respectively | |
| ● | CheWell – a high-bioavailability chewable tablet intended for use in adolescent drug addiction studies and other applicable indications | |
| ● | CanChew – a patented high-bioavailability and extended-release CBD chewing gum designed for the over-the-counter market | |
| ● | Renecann - topical cannabinoid development candidates addressing various skin conditions. |
Engagement of Eurofins Scientific for development and manufacture of CannQuitO, CannQuitN and Renecann formulations was announced in Nov 2022.
The CannQuit products are combination drug assets with associated granted patents and patent applications that were transferred to Incannex as a result of the acquisition of APIRx Pharmaceuticals, completed in August of 2022. Eurofins will undertake formulation development and manufacture of CannQuit Nicotine (‘CannQuitN’) and CannQuit Opioid (‘CannQuitO’).
CannQuitN combines nicotine and cannabidiol (‘CBD’) within a controlled-release, functional, medicated chewing gum. CannQuitO combines CBD and an off-patent prescription opioid antagonist, and/or partial agonist-antagonist within the formulation. The cGMP grade products manufactured by Eurofins will be used in clinical trials designed to assess the safety and efficacy of the CannQuit products for smoking cessation and the treatment of opioid addiction.
23
Incannex Healthcare Limited
Data collected on the quality and stability of the CannQuit anti-addiction products during the development and manufacturing of the two drug candidates at Eurofins will be key components of future regulatory packages. These data packages include investigational new drug (IND) applications and new drug application (NDA) filings with the US Food and Drug Administration (FDA).
Medicated chewing gums deliver their active ingredients directly into the circulation of the oral mucosa, ensuring that the effects of the ingredients are delivered rapidly, but also in a sustained manner to reduce cravings for longer than other delivery methods. Rapid onset and sustained effect are both qualities desirable for the treatment of addiction disorders. Furthermore, the act of chewing, known as mastication, also has an multi- action, anti-anxiety effect that has been demonstrated in other scientific assessments.
ReneCann is Incannex’s proprietary topical cannabinoid formulation for treatment of dermatological conditions caused by disorders of the immune system, including vitiligo, psoriasis, and atopic dermatitis, otherwise known as eczema. The ReneCann formulation is commercially protected by granted and pending patents acquired by Incannex as part of the APIRx acquisition that was finalised in August of 2022.
The unique formulation combines Cannabigerol (‘CBG’) and Cannabidiol (‘CBD’). CBG is a non-psychoactive cannabinoid with potent anti-inflammatory properties. A previous version of ReneCann was used in an in-human proof of concept study with dosing over a 6-week period. The study was conducted at the Maurits Clinic, The Netherlands, and led by a world-renowned dermatologist Dr. Marcus Meinardi, MD, PhD.
In the study, ReneCann reduced disease scores in patients with each of the target skin diseases. Patients with vitiligo, psoriasis and atopic dermatitis were observed to experience improvements in symptoms of 10%, 33% and 22% respectively.
In particular, the results for study participants with vitiligo are highly encouraging, partly because the incidence of the disease is high at 0.5-1.0% of the global population and treatments for it are limited. Vitiligo is observed when pigment-producing cells (melanocytes) stop producing melanin, causing the loss of skin colour in patches and the discoloured areas generally become larger over time. ReneCann was associated with diffuse re-pigmentation (usually perifollicular or from the borders of the lesion) and efficacy lasted for weeks eventually before depigmentation recurred.
The ReneCann Drug product that is produced by Eurofins CDMO will be used in clinical trials confirming the safety and therapeutic effect of ReneCann in vitiligo, psoriasis, and atopic dermatitis. Data on the quality and stability of ReneCann generated as part of this project at Eurofins will be used in the chemistry and manufacturing control modules of future regulatory packages with the US Food and Drug Administration (FDA). ReneCann also has the potential to be assessed for efficacy in other diseases where topical application may provide a benefit over conventional oral dosed cannabinoid formulations.
Incannex has chosen Quest Pharmaceutical Services (QPS) as its partner for regulatory guidance and clinical trial management for the advancement of the CannQuit™ and Renecann™ product lines designed for addiction and immune-disordered skin diseases. QPS, established in 1995, has evolved into a prominent contract research organization, offering a range of services in bioanalysis, pharmacology, and clinical research. QPS is in the process of drafting pre-Investigational New Drug (pre-IND) submissions for the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) for CannQuit™ and Renecann™ products. Subsequent to regulatory clearance, QPS will take a leading role in overseeing clinical trials, collecting relevant evidence of safety and efficacy.
24
Incannex Healthcare Limited
DIRECTORS’ INTERESTS IN THE COMPANY
As at the date of this report, the interests of the directors in the shares and options of the Company were:
| Director | Number of fully paid ordinary shares | Number of options over ordinary shares | No. of performance rights/shares | |||||||||
| Mr Troy Valentine | 36,651,198 | 5,243,413 | - | |||||||||
| Mr Peter Widdows | 16,573,685 | 1,104,913 | - | |||||||||
| Mr Joel Latham | 23,748,413 | 11,683,227 | - | |||||||||
| Dr George Anastassov | 66,972,077 | - | - | |||||||||
| Mr Robert Clark | - | 5,000,000 | - | |||||||||
DIVIDENDS
No dividends have been paid or declared since the start of the financial year and the directors do not recommend the payment of a dividend in respect of the financial year.
AFTER BALANCE DATE EVENTS
No significant events have occurred since the end of the financial year.
SHARE OPTIONS
The Company has the following options on issue as at the date of the Directors’ Report.
| Expiry Date | Exercise Price | Listed/Unlisted | Number | |||||||||
| 30/06/2025 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 30/06/2026 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 30/06/2027 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 30/06/2025 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 30/06/2026 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 30/06/2027 | $ | 0.05 | Unlisted | 750,000 | ||||||||
| 20/11/2023 | $ | 0.15 | Unlisted | 8,200,000 | ||||||||
| 20/11/2023 | $ | 0.25 | Unlisted | 20,000,000 | ||||||||
| 20/11/2023 | $ | 0.20 | Unlisted | 6,650,000 | ||||||||
| 01/07/2025 | $ | 0.26 | Unlisted | 533,333 | ||||||||
| 01/07/2026 | $ | 0.31 | Unlisted | 533,333 | ||||||||
| 01/07/2027 | $ | 0.35 | Unlisted | 533,334 | ||||||||
| 01/07/2025 | $ | 0.26 | Unlisted | 1,399,999 | ||||||||
| 01/07/2026 | $ | 0.31 | Unlisted | 1,399,999 | ||||||||
| 01/07/2027 | $ | 0.35 | Unlisted | 1,400,002 | ||||||||
| 01/07/2025 | $ | 0.26 | Unlisted | 1,399,999 | ||||||||
| 01/07/2026 | $ | 0.31 | Unlisted | 1,399,999 | ||||||||
| 01/07/2027 | $ | 0.36 | Unlisted | 1,400,002 | ||||||||
| 04/08/2025 | $ | 0.612 | Unlisted | 3,000,000 | ||||||||
| 04/08/2025 | $ | 0.69 | Unlisted | 3,000,000 | ||||||||
| 04/08/2025 | $ | 0.765 | Unlisted | 3,000,000 | ||||||||
| 31/12/2025 | $ | 1.00 | Unlisted | 2,500,000 | ||||||||
| 31/05/2024 | $ | 1.50 | Unlisted | 2,500,000 | ||||||||
| 30/04/2026 | $ | 0.25 | Unlisted | 105,800,651 | ||||||||
Unissued Shares under Option
As at the date of this report, there were 169,150,651 unissued ordinary shares under options (2022: 91,995,314)
Option holders do not have any right, by virtue of the options, to participate in any share issue of the Company or any related body corporate.
25
Incannex Healthcare Limited
Shares issued as a result of the exercise of options
During the financial year there were 2,027 ordinary shares issued as a result of the exercise of options (2022: 207,650,638)
INDEMNIFICATION AND INSURANCE OF DIRECTORS AND OFFICERS
Indemnification
The Company has agreed to indemnify the directors of the Company, against all liabilities to another person (other than the Company or a related body corporate) that may arise from their position as directors of the Company, except where the liability arises out of conduct involving a lack of good faith. The agreement stipulates that the Company will meet the full amount of any such liabilities, including costs and expenses.
Insurance premiums
The Company has arranged directors’ and officers’ liability insurance, for past, present or future directors, secretaries, and executive officers. The insurance cover relates to:
| - | costs and expenses incurred by the relevant officers in defending proceedings, whether civil or criminal and whatever their outcome; and |
| - | other liabilities that may arise from their position, with the exception of conduct involving a wilful breach of duty or improper use of information or position to gain a personal advantage. |
The insurance policies outlined above do not contain details of the premiums paid in respect of individual directors or officers of the Company.
ENVIRONMENTAL REGULATIONS
The Group is not subject to any significant environmental regulation.
26
Incannex Healthcare Limited
REMUNERATION REPORT (AUDITED)
This report, which forms part of the Directors’ Report, outlines the remuneration arrangements in place for the key management personnel of Incannex Healthcare Limited (the “Company”) for the financial year ended 30 June 2023.
The key management personnel of the Company are the Directors of the Company including the Managing Director/Chief Executive Officer.
Remuneration philosophy
The performance of the Company depends upon the quality of the directors and executives. The philosophy of the Company in determining remuneration levels is to:
| - | set competitive remuneration packages to attract and retain high calibre employees; |
| - | link executive rewards to shareholder value creation; and |
| - | establish appropriate, demanding performance hurdles for variable executive remuneration. |
Remuneration Structure
In accordance with best practice Corporate Governance, the structure of non-executive director and executive remuneration is separate and distinct.
Non-executive director remuneration
The Board seeks to set aggregate remuneration at a level that provides the Company with the ability to attract and retain directors of the highest calibre, whilst incurring a cost that is acceptable to shareholders. The amount of aggregate remuneration apportioned amongst directors is reviewed annually. The Board considers the fees paid to non-executive directors of comparable companies when undertaking the annual review process. Independent advice is obtained when considered necessary to confirm that remuneration is in line with market practice.
Each director receives a fee for being a director of the Company. Non-executive directors may receive performance rights (subject to shareholder approval) as it is considered an appropriate method of providing sufficient reward whilst maintaining cash reserves.
Executive director remuneration
Remuneration consists of fixed remuneration and variable remuneration (comprising short-term and long-term incentive schemes).
Fixed remuneration
Fixed remuneration is reviewed annually by the Board. The process consists of a review of relevant comparative remuneration in the market and internally and, where appropriate, external advice on policies and practices. The Board has access to external, independent advice where necessary.
The fixed remuneration component of key management personnel is detailed in Tables 1 and 2.
27
Incannex Healthcare Limited
Variable remuneration
The objective of the short-term incentive program is to link the achievement of the Group’s operational targets with the remuneration received by the KMP charged with meeting those targets. The total potential short-term incentive available is set at a level so as to provide sufficient incentive to the KMP to achieve the operational targets and such that the cost to the Group is reasonable in the circumstances.
Actual payments granted to each KMP depend on the extent to which specific operating targets set at the beginning of the financial year are met. A short-term incentive remuneration of $426,000 is payable for the financial year ended 30 June 2023 to Joel Latham.
The Company also makes long term incentive payments to reward senior executives in a manner that aligns this element of remuneration with the creation of shareholder wealth. The long-term incentive is provided in the form of performance rights and options over ordinary shares in the Company.
Employee Share Option Plan (ESOP)
The Incannex Healthcare Limited ESOP provides for the directors to set aside shares in order to reward and incentivise employees. Directors will not set aside more than 5% of the total number of issued shares in the Company at the time of the proposed issue. Officers and employees both full and part-time are eligible to participate in the plan.
No shares and options have been issued under the ESOP during the year (2022:1,600,000 shares and 1,600,000 options).
Performance Rights Plan (PRP)
Shareholders approved the Company’s PRP at the Annual General Meeting held on 23 November 2011. The PRP is designed to provide a framework for competitive and appropriate remuneration so as to retain and motivate skilled and qualified personnel whose personal rewards are aligned with the achievement of the Company’s growth and strategic objectives.
No performance rights have been issued under the PRP during the year (2022: nil).
Executive Employment Contracts
For the year ended 30 June 2023, Mr Joel Latham, was appointed as Chief Executive Officer under an employment agreement. The material terms of the agreement are set out as follows:
| - | Commencement date: 1 July 2018 |
| - | Term: No fixed term |
| - | Fixed remuneration: $770,000 per annum, plus $30,000 Board fees, plus superannuation |
| - | Variable remuneration up to 50% of base salary subject to achieving certain performance hurdles. |
| - | Car allowance: $20,000 per annum. |
| - | Termination for cause: no notice period |
| - | Termination without cause: three-month notice period |
28
Incannex Healthcare Limited
Table 1: Remuneration of key management personnel (KMP) for the year ended 30 June 2023
| Short-term (cash-based payments) | Long-term (share-based payments) | Post-employment | ||||||||||||||||||||||||||
| Salary & fees $ | Bonus $ | Other $ | Performance Rights, Shares and Options $ | Superannuation $ | Total $ | Performance Related % | ||||||||||||||||||||||
| Key Management Personnel name | ||||||||||||||||||||||||||||
| Mr Troy Valentine1 | 231,157 | - | 254,000 | 867,331 | 24,271 | 1,376,759 | 63.0 | |||||||||||||||||||||
| Mr Peter Widdows2 | 141,385 | - | 160,000 | - | 14,845 | 316,230 | - | |||||||||||||||||||||
| Mr Joel Latham | 820,000 | 426,000 | - | 1,760,325 | 27,641 | 3,033,966 | 58.0 | |||||||||||||||||||||
| Dr George Anastassov | 175,866 | - | - | - | - | 175,866 | - | |||||||||||||||||||||
| Mr Robert Clark3 | 88,588 | - | - | 87,500 | - | 176,088 | 49.7 | |||||||||||||||||||||
| Total | 1,456,996 | 426,000 | 414,000 | 2,715,156 | 66,757 | 5,078,909 | ||||||||||||||||||||||
| 1) | Mr Valentine was paid $254,000 for consulting fees invoiced to the Company, outside of Director fees. |
| 2) | Mr Widdows was paid $160,000 for consulting fees invoiced to the Company, outside of Director fees. |
| 3) | Mr Clark was appointed on the 17th of August 2022. During the 2023 financial year the Company obtained shareholder approval to issue a Company Acquisition Incentive to Mr Robert Clark on the terms and conditions set out in the Notice of Annual General Meeting dated 21 October 2022. No amount was recognised in the financial statements in the 2023 financial year in relation to the Company Acquisition Incentive. |
29
Incannex Healthcare Limited
Table 2: Remuneration of key management personnel (KMP) for the year ended 30 June 2022
| Short-term (cash-based payments) | Long-term (share-based payments) | Post-employment | ||||||||||||||||||||||||||
| Salary & fees $ | Bonus $ | Other $ | Performance Rights, Shares and Options $ | Superannuation $ | Total $ | Performance Related % | ||||||||||||||||||||||
| Key Management Personnel name | ||||||||||||||||||||||||||||
| Mr Troy Valentine1 | 92,750 | - | 240,000 | 312,538 | 9,275 | 654,563 | 47.8 | |||||||||||||||||||||
| Mr Peter Widdows2 | 84,742 | - | - | - | 8,474 | 93,216 | - | |||||||||||||||||||||
| Mr Joel Latham3 | 533,500 | 245,000 | - | 716,096 | 24,998 | 1,519,594 | 63.3 | |||||||||||||||||||||
| Dr Sud Agarwal4 | 48,000 | - | 90,000 | - | 4,800 | 142,800 | - | |||||||||||||||||||||
| Dr George Anastassov5 | - | - | - | - | - | - | - | |||||||||||||||||||||
| Total | 758,992 | 245,000 | 330,000 | 1,028,634 | 47,547 | 2,410,173 | ||||||||||||||||||||||
| 1) | Remuneration owed to Mr Valentine at 30 June 2022 is $38,750 included in accrued expenses. |
| Mr Valentine was paid $240,000 for consulting fees invoiced to the Company, outside of Director fees. | |
| 2) | Remuneration owed to Mr Widdows at 30 June 2022 is $42,076 included in accrued expenses. |
| 3) | Remuneration owed to Mr Latham at 30 June 2022 is $245,000 included in accrued expenses. |
| 4) | Remuneration owed to Dr Agarwal at 30 June 2022 is $25,300 is included in accounts payable. |
| Dr Agarwal received $90,000 in fees billed through Medical Life Publishing Pty Ltd, for services provided as Chief Medical Officer. Dr Agarwal resigned on the 28th of June 2022. | |
| 5) | Dr Anastassov was appointed on the 28th of June 2022. |
30
Incannex Healthcare Limited
Performance rights
Each performance right is convertible into one ordinary share upon achievement of the performance hurdles. No performance right will vest if the conditions are not satisfied, hence the minimum value of the performance rights yet to vest is nil.
The assessed fair value at grant date of performance rights granted is expensed according to the performance or market-based conditions attached to the performance hurdle. Performance based hurdles are expensed to each reporting period evenly over the period from grant date to vesting date. Market based hurdles are expensed on the grant date unless there is an explicit or implicit service condition. The relevant amount is included in the remuneration table (Table 1) above. Fair values at grant date are independently determined using a trinomial pricing model that takes into account the exercise price, term, the share price at grant date and expected price volatility of the underlying share, barrier price / performance hurdles, the expected dividend yield and the risk- free interest rate. For details on the valuation of performance rights, including assumptions used, refer to note 17 of these financial statements.
There was no Performance rights activity for KMP for the year ended 30 June 2023 (2022: nil).
31
Incannex Healthcare Limited
Key Management Personnel – Option Holdings
The number of options held by Key Management Personnel of the Group during the financial year is as follows:
30 June 2023 - Options
Name |
Balance at |
Other changes | Balance at 30 June 2023 (or on cessation) |
Exercisable | ||||||||||||
| Mr Troy Valentine1 | 2,800,000 | 2,443,413 | 5,243,413 | 3,843,411 | ||||||||||||
| Mr Peter Widdows1 | - | 1,104,913 | 1,104,913 | 1,104,913 | ||||||||||||
| Mr Joel Latham1 | 10,100,000 | 1,583,227 | 11,683,227 | 8,883,226 | ||||||||||||
| Dr George Anastassov | - | - | - | - | ||||||||||||
| Mr Robert Clark2 | - | 5,000,000 | 5,000,000 | - | ||||||||||||
| 1. | Other changes refer to options acquired by taking part in the loyalty option placement during the year. |
| 2. | 5,000,000 share options were issued to Mr Robert Clark approved by shareholders in 2023. |
30 June 2022 – Options
Name |
Balance at |
Other changes | Balance at 30 June 2022 (or on cessation) |
Exercisable | ||||||||||||
| Mr Troy Valentine1 | 7,116,950 | (4,316,950 | ) | 2,800,000 | 466,666 | |||||||||||
| Mr Peter Widdows1 | 657,895 | (657,895 | ) | - | - | |||||||||||
| Mr Joel Latham2 | 4,700,000 | 5,400,000 | 10,100,000 | 4,683,333 | ||||||||||||
| Dr Sud Agarwal3 | 200,000,000 | (200,000,000 | ) | - | - | |||||||||||
| Dr George Anastassov | - | - | - | - | ||||||||||||
| 1. | Other changes refer to conversion of options held to ordinary shares and share options issued to Troy Valentine approved by shareholders in 2022. |
| 2. | 5,400,000 share options were issued to Joel Latham approved by shareholders in 2022. |
| 3. | Dr Agarwal’s change relates to share options that lapsed during the year and conversion of options held. Dr Agarwal resigned on the 28th of June 2022 |
Key Management Personnel – Share Holdings
30 June 2023 - Shares
Name | Balance | Purchases / | Sales / Other Disposals | Balance 30 June | ||||||||||||
| Mr Troy Valentine | 36,651,198 | - | - | 36,651,198 | ||||||||||||
| Mr Peter Widdows | 16,573,685 | - | - | 16,573,685 | ||||||||||||
| Mr Joel Latham | 23,748,413 | - | - | 23,748,413 | ||||||||||||
| Dr George Anastassov1 | - | 66,972,077 | - | 66,972,077 | ||||||||||||
| Mr Robert Clark | - | - | - | - | ||||||||||||
| 1 | The change relates to shares acquired as a former stakeholder of APIRx. |
32
Incannex Healthcare Limited
30 June 2022 – Shares
Name | Balance | Purchases / | Sales / Other Disposals | Balance | ||||||||||||
| Mr Troy Valentine1 | 26,734,248 | 9,916,950 | - | 36,651,198 | ||||||||||||
| Mr Peter Widdows1 | 15,915,790 | 657,895 | - | 16,573,685 | ||||||||||||
| Mr Joel Latham2 | 17,948,414 | 5,800,000 | - | 23,748,413 | ||||||||||||
| Dr Sud Agarwal3 | 66,303,593 | 8,999,500 | - | 75,303,093 | ||||||||||||
| Dr George Anastassov | - | - | - | - | ||||||||||||
| 1 | The change relates to ordinary shares acquired upon conversion of options. |
| 2 | Mr Latham’s changes arise from the conversion of 200,000 share options, and new ordinary shares issued as part of his remuneration packages. |
| 3 | Mr Agarwal’s changes relates to ordinary shares acquired upon conversion of options. Dr Agarwal resigned on the 28th of June 2022 |
END OF REMUNERATION REPORT
NON-AUDIT SERVICES
The Company has not engaged the auditor to perform any non-audit services during the year ended 30 June 2023 (2022: $Nil).
AUDITOR INDEPENDENCE AND NON-AUDIT SERVICES
Section 307C of the Corporations Act 2001 requires our auditors, PKF Brisbane Audit, to provide the directors of the Company with an Independence Declaration in relation to the audit of the annual report. This Independence Declaration is set out on page 34 and forms part of this directors’ report for the year ended 30 June 2023.
Signed in accordance with a resolution of the directors.
| /s/ Troy Valentine |
Troy Valentine
Chairman
Melbourne, Victoria, 30th August 2023
33
 | PKF Brisbane Audit |
| ABN 33 873 151 348 | |
| Level 6, 10 Eagle Street | |
| Brisbane, QLD 4000 Australia | |
| +61 7 3839 9733 | |
| brisbane@pkf.com.au | |
| pkf.com.au |
AUDITOR’S INDEPENDENCE DECLARATION
UNDER SECTION 307C OF THE CORPORATIONS ACT 2001
TO THE DIRECTORS OF INCANNEX HEALTHCARE LIMITED
I declare that, to the best of my knowledge and belief, during the year ended 30 June 2023, there have been no contraventions of:
| (a) | the auditor independence requirements of the Corporations Act 2001 in relation to the audit; and |
| (b) | any applicable code of professional conduct in relation to the audit. |
This declaration is in respect of Incannex Healthcare Limited and the entities it controlled during the year.

PKF Brisbane Audit
| /s/ Liam Murphy |
Liam Murphy
Partner
Brisbane
30 August 2023
PKF Brisbane Pty Ltd is a member of PKF Global, the network of member firms of PKF International Limited, each of which is a separately owned legal entity and does not accept any responsibility or liability for the actions or inactions of any individual member or correspondent firm(s). Liability limited by a scheme approved under Professional Standards Legislation.
34
Incannex Healthcare Limited
CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
For the year ended 30 June 2023
| Consolidated | ||||||||||
| 30 June 2023 | 30 June 2022 | |||||||||
| Notes | $ | $ | ||||||||
| Other income | 3 | 1,376,645 | 788,654 | |||||||
| Total other income | 1,376,645 | 788,654 | ||||||||
| Product costs | - | (6,338 | ) | |||||||
| Administration expense | (568,954 | ) | (280,969 | ) | ||||||
| Advertising and investor relations | (1,852,416 | ) | (2,746,226 | ) | ||||||
| Bad debt expense | - | (134,626 | ||||||||
| Research and development costs | (9,364,796 | ) | (5,371,821 | ) | ||||||
| Compliance, legal and regulatory | (2,632,069 | ) | (3,559,511 | ) | ||||||
| Share based payments | 17 | (3,191,640 | ) | (1,464,550 | ) | |||||
| Occupancy expenses | (124,628 | ) | (112,341 | ) | ||||||
| Depreciation expense | (130,946 | ) | - | |||||||
| Salaries and employee benefit expense | (3,490,754 | ) | (2,016,181 | ) | ||||||
| Total expenses | (21,356,203 | ) | (15,692,563 | ) | ||||||
| Loss before tax | (19,979,558 | ) | (14,903,909 | ) | ||||||
| Income tax | 5 | - | - | |||||||
| Loss after tax | (19,979,558 | ) | (14,903,909 | ) | ||||||
| Other comprehensive income | - | - | ||||||||
| Total comprehensive loss for the year | (19,979,558 | ) | (14,903,909 | ) | ||||||
| Earnings per share | 6 | |||||||||
| Basic loss per share (cents per share) | (1.30 | ) | (1.25 | ) | ||||||
| Diluted loss per share (cents per share) | (1.30 | ) | (1.25 | ) | ||||||
The accompanying notes form part of these financial statements
35
Incannex Healthcare Limited
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
As at 30 June 2023
| Consolidated | ||||||||||
| 30 June 2023 | 30 June 2022 | |||||||||
| Notes | $ | $ | ||||||||
| Assets | ||||||||||
| Current assets | ||||||||||
| Cash and cash equivalents | 8 | 33,363,228 | 37,500,931 | |||||||
| Trade and other receivables | 9 | 287,478 | 294,717 | |||||||
| Other assets | 10 | 1,035,181 | 83,960 | |||||||
| Total current assets | 34,685,887 | 37,879,608 | ||||||||
| Non-current assets | ||||||||||
| Property, plant and equipment | 11 | 443,652 | - | |||||||
| Right-of-use assets | 12 | 743,734 | - | |||||||
| Intangible assets | 13 | 52,717,427 | - | |||||||
| Total non-current assets | 53,904,813 | - | ||||||||
| Total assets | 88,590,700 | 37,879,608 | ||||||||
| Liabilities | ||||||||||
| Current liabilities | ||||||||||
| Trade and other payables | 14 | 3,675,090 | 2,010,533 | |||||||
| Lease liabilities | 12 | 170,656 | - | |||||||
| Total current liabilities | 3,845,746 | 2,010,533 | ||||||||
| Non-current liabilities | ||||||||||
| Lease liabilities | 12 | 616,087 | - | |||||||
| Total non-current liabilities | 616,087 | - | ||||||||
| Total liabilities | 4,461,833 | 2,010,533 | ||||||||
| Net assets | 84,128,867 | 35,869,075 | ||||||||
| Equity | ||||||||||
| Issued capital | 15 | 150,842,248 | 86,586,794 | |||||||
| Reserves | 16 | 12,061,087 | 8,077,191 | |||||||
| Accumulated losses | (78,774,468 | ) | (58,794,910 | ) | ||||||
| Net equity | 84,128,867 | 35,869,075 | ||||||||
The accompanying notes form part of these financial statements
36
Incannex Healthcare Limited
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the year ended 30 June 2023
| Consolidated | Issued Capital | Equity Reserve | Accumulated Losses | Total | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Balance at 30 June 2021 | 45,852,107 | 6,612,641 | (43,891,002 | ) | 8,573,746 | |||||||||||
| Options exercised | 40,274,242 | - | - | 40,274,242 | ||||||||||||
| Share based payments | - | 1,464,550 | - | 1,464,550 | ||||||||||||
| Share placements | 400,000 | - | - | 400,000 | ||||||||||||
| Shares issued to advisors | 450,000 | - | - | 450,000 | ||||||||||||
| Shares issue costs | (389,555 | ) | - | - | (389,555 | ) | ||||||||||
| Comprehensive loss for the year | - | - | (14,903,909 | ) | (14,903,909 | ) | ||||||||||
| Balance at 30 June 2022 | 86,586,794 | 8,077,191 | (58,794,910 | ) | 35,869,075 | |||||||||||
| Options exercised | 2,027 | - | - | 2,027 | ||||||||||||
| Options issued to advisors | - | 684,000 | - | 684,000 | ||||||||||||
| Option placements | - | 108,257 | - | 108,257 | ||||||||||||
| Share based payments | - | 3,191,640 | - | 3,191,640 | ||||||||||||
| Share placements | 13,000,000 | - | - | 13,000,000 | ||||||||||||
| Shares issued to advisors | 2,945,288 | - | - | 2,945,288 | ||||||||||||
| Asset acquisition shares issued | 49,088,139 | - | - | 49,088,139 | ||||||||||||
| Shares issue costs | (780,000 | ) | - | - | (780,000 | ) | ||||||||||
| Comprehensive loss for the year | - | - | (19,979,558 | ) | (19,979,558 | ) | ||||||||||
| Balance at 30 June 2023 | 150,842,248 | 12,061,087 | (78,774,468 | ) | 84,128,867 | |||||||||||
The accompanying notes form part of these financial statements
37
Incannex Healthcare Limited
CONSOLIDATED STATEMENT OF CASH FLOWS
For the year ended 30 June 2023
| Consolidated | ||||||||||
| 2023 | 2022 | |||||||||
| Notes | $ | $ | ||||||||
| Cash flows from operating activities | ||||||||||
| Receipts from customers | - | - | ||||||||
| Receipts from other income | 1,013,879 | 782,383 | ||||||||
| Payments to suppliers and employees | (17,285,861 | ) | (13,596,027 | ) | ||||||
| Interest received and other income | 329,157 | 6,271 | ||||||||
| Net cash (used in) operating activities | 8 | (15,942,825 | ) | (12,807,373 | ) | |||||
| Cash flows from investing activities | ||||||||||
| Payments for the addition of property, plant and equipment | (476,873 | ) | - | |||||||
| Net cash from investing activities | (476,873 | ) | - | |||||||
| Cash flows from financing activities | ||||||||||
| Proceeds from shares issued (net of costs) | 12,330,284 | 41,184,687 | ||||||||
| Repayment of lease liabilities | (54,717 | ) | - | |||||||
| Net cash from financing activities | 12,275,567 | 41,184,687 | ||||||||
| Net decrease in cash and cash equivalents | (4,144,131 | ) | 28,377,314 | |||||||
| Cash and cash equivalents at beginning of the year | 37,500,931 | 9,123,617 | ||||||||
| Effect of exchange rate fluctuations on cash held | 6,428 | - | ||||||||
| Cash and cash equivalents at end of the year | 8 | 33,363,228 | 37,500,931 | |||||||
The accompanying notes form part of these financial statements
38
Incannex Healthcare Limited
NOTES TO THE FINANCIAL STATEMENTS
For the year ended 30 June 2023
1. Significant accounting policies
The principal accounting policies adopted in the preparation of the consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
Nature of Operations
Incannex Healthcare Limited (the “Company”) and its consolidated subsidiaries (collectively, the “Group”) is a clinical stage pharmaceutical development company that is developing unique medicinal cannabis pharmaceutical products and psychedelic medicine therapies. The Company’s common shares trade on the Australian Securities Exchange (“ASX”). The Company’s registered office is at Level 23, South Tower Rialto, 525 Collins Street Melbourne Victoria 3000, Australia.
For the fiscal year ended 30 June 2023, the Group incurred a total comprehensive loss after income tax of $19.98 million (2022: $14.9 million) and had net cash outflows from operations of $15.94 million (2022: $12.8 million). The Group held total cash of $33.36 million as of 30 June 2023 (2022: $37.5 million).
New or amended Accounting Standards and Interpretations adopted
The Group has adopted all of the new or amended Accounting Standards and Interpretations issued by the International Accounting Standards Board (‘IASB’) that are mandatory for the current reporting periods.
Any new or amended Accounting Standards or Interpretations that are not yet mandatory have not been early adopted.
Historical cost convention
The consolidated financial statements have been prepared under the historical cost convention, except for, where applicable, the revaluation of financial assets and liabilities at fair value through profit or loss, financial assets at fair value through other comprehensive income and derivative financial instruments.
Critical accounting estimates
The preparation of the consolidated financial statements requires the use of certain critical accounting estimates. It also requires management to exercise its judgement in the process of applying the Group’s accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements, are disclosed in note 2.
Comparatives
Where necessary, comparative information has been reclassified and repositioned for consistency with current year disclosures.
Statement of compliance
These consolidated financial statements were authorised for issue by the Board of Directors in August 2023.
The consolidated financial statements comply with Australian Accounting Standards, which include Australian equivalents to International Financial Reporting Standards (“AIFRS”), in their entirety. Compliance with AIFRS ensures that the financial report also complies with International Financial Reporting Standards (“IFRS”).
39
Incannex Healthcare Limited
Parent entity information
In accordance with AASB 10 (IFRS 10) Consolidated Financial Statements, these consolidated financial statements present the results of the Group only. Supplementary information about the parent entity is disclosed in note 24.
Principles of consolidation
The consolidated financial statements incorporate the assets and liabilities of all subsidiaries of the Company as at 30 June 2023 and 2022 and the results of all subsidiaries for the years then ended. Incannex Healthcare Limited and its subsidiaries together are referred to in these consolidated financial statements as the ‘Group’. Details of all controlled entities are set out in Note 22.
Subsidiaries are all those entities over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Group. They are de-consolidated from the date that control ceases.
Intercompany transactions between entities in the Group are eliminated. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
Where the Group loses control over a subsidiary, it derecognizes the assets including goodwill, liabilities and non- controlling interest in the subsidiary together with any cumulative translation differences recognized in equity. The Group recognizes the fair value of the consideration received and the fair value of any investment retained together with any gain or loss in profit or loss.
Operating segments
Operating segments are presented at note 4 using the ‘management approach’, where the information presented is on the same basis as the internal reports provided to the Chief Executive Officer. The Chief Executive Officer is responsible for the allocation of resources to operating segments and assessing their performance.
Foreign currency translation
The consolidated financial statements are presented in Australian dollars, which is the Company’s functional and presentation currency.
Foreign currency transactions
Foreign currency transactions are translated into Australian dollars using the exchange rates prevailing at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at financial year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in profit or loss.
Revenue recognition
The Company recognizes revenue to depict the transfer of goods and services to clients in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods and services by applying the following steps:
| ● | Identify the contract with a client; |
| ● | Identify the performance obligations in the contract; |
| ● | Determine the transaction price; |
| ● | Allocate the transaction price to the performance obligations; and |
| ● | Recognize revenue when, or as, the Company satisfies a performance obligation. |
40
Incannex Healthcare Limited
Revenue may be earned over time as the performance obligations are satisfied or at a point in time which is when the entity has earned a right to payment, the customer has possession of the asset and the related significant risks and rewards of ownership, and the customer has accepted the asset.
The Company’s arrangements with clients can include multiple performance obligations. When contracts involve various performance obligations, the Company evaluates whether each performance obligation is distinct and should be accounted for as a separate unit of accounting under AASB 15 (IFRS 15), Revenue from Contracts with Customers.
The Company determines the standalone selling price by considering its overall pricing objectives and market conditions. Significant pricing practices taken into consideration include discounting practices, the size and volume of our transactions, our marketing strategy, historical sales, and contract prices. The determination of standalone selling prices is made through consultation with and approval by management, taking into consideration our go-to-market strategy. As the Company’s go-to-market strategies evolve, the Company may modify its pricing practices in the future, which could result in changes in relative standalone selling prices.
The Company disaggregates revenue from contracts with customers based on the categories that most closely depict how the nature, amount, timing and uncertainty of revenue and cash flows are affected by economic factors. During the years ended 30 June 2023 and 2022, the Company recognized revenue from only one such category, being cannabinoid oils sales.
The Company receives payment from its clients after invoicing within the normal 28-day commercial terms. If a client is specifically identified as a credit risk, recognition of revenue is stopped except to the extent of fees that have already been collected.
Other income
Other income is recognized when it is received or when the right to receive it is established. Other income primarily consists of grant income and interest income.
Interest income
Interest revenue is recognized as interest accrues using the effective interest method. This is a method of calculating the amortised cost of a financial asset and allocating the interest income over the relevant period using the effective interest rate, which is the rate that exactly discounts estimated future cash receipts through the expected life of the financial asset to the net carrying amount of the financial asset.
Income tax
The income tax expense or benefit for the period is the tax payable on that period’s taxable income based on the applicable income tax rate for each jurisdiction, adjusted by the changes in deferred tax assets and liabilities attributable to temporary differences, unused tax losses and the adjustment recognized for prior reporting years, where applicable.
Deferred tax assets and liabilities are recognized for temporary differences at the tax rates expected to be applied when the assets are recovered or liabilities are settled, based on those tax rates that are enacted or substantively enacted, except for:
| ● | When the deferred income tax asset or liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and that, at the time of the transaction, affects neither the accounting nor taxable profits; or |
| ● | When the taxable temporary difference is associated with interests in subsidiaries, associates or joint ventures, and the timing of the reversal can be controlled, and it is probable that the temporary difference will not reverse in the foreseeable future. |
41
Incannex Healthcare Limited
Deferred tax assets are recognized for deductible temporary differences and unused tax losses only if it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
The carrying amount of recognized and unrecognized deferred tax assets are reviewed at each reporting date. Deferred tax assets recognized are reduced to the extent that it is no longer probable that future taxable profits will be available for the carrying amount to be recovered. Previously unrecognized deferred tax assets are recognized to the extent that it is probable that there are future taxable profits available to recover the asset.
Deferred tax assets and liabilities are offset only where there is a legally enforceable right to offset current tax assets against current tax liabilities and deferred tax assets against deferred tax liabilities; and they relate to the same taxable authority on either the same taxable entity or different taxable entities which intend to settle simultaneously.
Government grants
Income from government grants is recognized only when the Company has reasonable assurance that the grants will be received, and the conditions of the grants will be complied with. Income from Government grants is recognized on a systematic basis over the periods in which the Company recognizes as expenses the related costs for which the grants are intended to compensate. Government grants relate to Australian Federal Government’s COVID-19 support package of a “Cash Flow Boost” for eligible organisations, supporting small and medium sized organisations.
Current and non-current classification
Assets and liabilities are presented in the statement of financial position based on current and non-current classification.
An asset is classified as current when: it is either expected to be realised or intended to be sold or consumed in the Group’s normal operating cycle; it is held primarily for the purpose of trading; it is expected to be realised within 12 months after the reporting period; or the asset is cash or cash equivalent unless restricted from being exchanged or used to settle a liability for at least 12 months after the reporting period. All other assets are classified as non-current.
A liability is classified as current when: it is either expected to be settled in the Group’s normal operating cycle; it is held primarily for the purpose of trading; it is due to be settled within 12 months after the reporting period; or there is no unconditional right to defer the settlement of the liability for at least 12 months after the reporting period. All other liabilities are classified as non-current.
Deferred tax assets and liabilities are classified as non-current.
Cash
Cash and deposits held at call with financial institutions, other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Trade and other receivables
Trade receivables are initially recognized at fair value and subsequently measured at amortised cost using the effective interest method, less any allowance for expected credit losses. Trade receivables are due for settlement within 30 days.
The Group has applied the simplified approach to measuring expected credit losses, which uses a lifetime expected loss allowance. To measure the expected credit losses, trade receivables have been grouped based on days overdue.
Other receivables are recognized at amortised cost, less any allowance for expected credit losses.
42
Incannex Healthcare Limited
Other financial assets
Other financial assets are initially measured at fair value. Transaction costs are included as part of the initial measurement, except for financial assets at fair value through profit or loss. Such assets are subsequently measured at either amortised cost or fair value depending on their classification. Classification is determined based on both the business model within which such assets are held and the contractual cash flow characteristics of the financial asset unless an accounting mismatch is being avoided.
Financial assets are derecognized when the rights to receive cash flows have expired or have been transferred and the Group has transferred substantially all the risks and rewards of ownership. When there is no reasonable expectation of recovering part or all a financial asset, its carrying value is written off.
Property, plant and equipment
All property, plant and equipment is recognised at historical cost less depreciation. Depreciation is calculated using the straight-line method to allocate their cost or revalued amounts, net of their residual values, over their estimated useful lives or, in the case of leasehold improvements and certain leased plant and equipment, the shorter lease term as follows:
| ● | Buildings | 25-40 years | |
| ● | Machinery | 10-15 years | |
| ● | Vehicles | 3-5 years | |
| ● | Furniture, fittings and equipment | 3-8 years |
Furniture, fittings and equipment include assets in the form of office fit outs. These assets and other leasehold improvements are recognised at their fair value and depreciated over the shorter of their useful life or the lease term, unless the entity expects to use the assets beyond the lease term.
Intangible assets
Patents and trademarks
Separately acquired patents and trademarks are shown at historical cost. Trademarks have an indefinite useful life. Patents have been assessed to have a 13-year useful life. Amortisation shall begin when the patents are available for use. At that point, they will be carried at cost less accumulated amortisation and impairment losses.
Intangible assets with an indefinite useful life or that are not yet available for use are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired.
Research and development
Research costs are expensed in the period in which they are incurred. Development costs are capitalised when it is probable that the project will be a success considering its commercial and technical feasibility; the Group is able to use or sell the asset; the Group has sufficient resources and intent to complete the development; and its costs can be measured reliably. Capitalised development costs are amortised on a straight-line basis over the period of their expected benefit, being their finite life of 10 years. The Company has not capitalised any development costs for the years ended June 30 2023 and 2022.
Right-of-use leased assets
A right-of-use asset is recognised at the commencement date of a lease. The right-of-use asset is measured at cost, which comprises the initial amount of the lease liability, adjusted for, as applicable, any lease payments made at or before the commencement date net of any lease incentives received, any initial direct costs incurred, and an estimate of costs expected to be incurred for dismantling and removing the underlying asset, and restoring the site or asset.
Right-of-use assets are depreciated on a straight-line basis over the unexpired period of the lease or the estimated useful life of the asset, whichever is the shorter. Right-of use assets are subject to impairment or adjusted for any remeasurement of lease liabilities.
43
Incannex Healthcare Limited
The Company has elected not to recognise a right-of-use asset and corresponding lease liability for short-term leases with terms of 12 months or less and leases of low-value assets. Lease payments on these assets are expensed to profit or loss as incurred.
Lease Liabilities
A lease liability is recognised at the commencement date of a lease. The lease liability is initially recognised at the present value of the lease payments to be made over the term of the lease, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, the Group’s incremental borrowing rate. Lease payments comprise of fixed payments less any lease incentives receivable, variable lease payments that depend on an index or a rate, amounts expected to be paid under residual value guarantees, exercise price of a purchase option when the exercise of the option is reasonably certain to occur, and any anticipated termination penalties. The variable lease payments that do not depend on an index or a rate are expensed in the period in which they are incurred.
Lease liabilities are measured at amortised cost using the effective interest method. The carrying amounts are remeasured if there is a change in the following: future lease payments arising from a change in an index, or a rate used; residual guarantee; lease term; certainty of a purchase option and termination penalties. When a lease liability is remeasured, an adjustment is made to the corresponding right-of use asset, or to profit or loss if the carrying amount of the right-of-use asset is fully written down.
Trade and other payables
These amounts represent liabilities for goods and services provided to the Group prior to the end of the financial years and which are unpaid. Due to their short-term nature, they are measured at amortised cost and are not discounted. The amounts are unsecured and are usually paid within 30 days of recognition.
Provisions
Provisions are recognized when the Group has a present (legal or constructive) obligation as a result of a past event, it is probable the Group will be required to settle the obligation, and a reliable estimate can be made of the amount of the obligation. The amount recognized as a provision is the best estimate of the consideration required to settle the present obligation at the reporting date, taking into account the risks and uncertainties surrounding the obligation. If the time value of money is material, provisions are discounted using a current pre-tax rate specific to the liability. The increase in the provision resulting from the passage of time is recognized as a finance cost.
Employee benefits
Short-term employee benefits
Liabilities for wages and salaries, including non-monetary benefits, annual leave and long service leave expected to be settled wholly within 12 months of the reporting date are measured at the amounts expected to be paid when the liabilities are settled.
Other long-term employee benefits
The liability for annual leave and long service leave not expected to be settled within 12 months of the reporting date are measured at the present value of expected future payments to be made in respect of services provided by employees up to the reporting date using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service. Expected future payments are discounted using market yields at the reporting date on corporate bonds with terms to maturity and currency that match, as closely as possible, the estimated future cash outflows.
Retirement benefit obligations
All employees of the Group are entitled to superannuation contributions in accordance with Australian law. Contributions to employees’ nominated superannuation plans are expensed in the period in which they are incurred.
44
Incannex Healthcare Limited
Share-based payments
Equity-settled compensation benefits are provided to employees.
Equity-settled transactions are awards of shares, performance rights or options over shares, that are provided to employees in exchange for the rendering of services.
The cost of equity-settled transactions are measured at fair value on grant date. Fair value is independently determined using Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk free interest rate for the term of the option, together with non-vesting conditions that do not determine whether the Group receives the services that entitle the employees to receive payment. Inputs into the Black-Scholes option pricing models used to calculate fair value are classified as level three inputs under the fair value hierarchy of AASB 13 (IFRS 13). No account is taken of any other vesting conditions.
The cost of equity-settled transactions are recognized as an expense with a corresponding increase in equity over the vesting period. The cumulative charge to profit or loss is calculated based on the grant date fair value of the award, the best estimate of the number of awards that are likely to vest and the expired portion of the vesting period. The amount recognized in profit or loss for the period is the cumulative amount calculated at each reporting date less amounts already recognized in previous periods.
Market conditions are taken into consideration in determining fair value. Therefore, any awards subject to market conditions are considered to vest irrespective of whether or not that market condition has been met, provided all other conditions are satisfied.
If equity-settled awards are modified, as a minimum an expense is recognized as if the modification has not been made. An additional expense is recognized, over the remaining vesting period, for any modification that increases the total fair value of the share-based compensation benefit as at the date of modification.
If the non-vesting condition is within the control of the Group or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the Group or employee and is not satisfied during the vesting period, any remaining expense for the award is recognized over the remaining vesting period, unless the award is forfeited.
If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognized immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new award is treated as if they were a modification.
Fair value measurement
When an asset, liability or equity instrument, financial or non-financial, is measured at fair value for recognition or disclosure purposes, the fair value is based on the price that would be received to sell an asset or an equity instrument or paid to transfer a liability in an orderly transaction between market participants at the measurement date; and assumes that the transaction will take place either: in the principal market; or in the absence of a principal market, in the most advantageous market.
Fair value is measured using the assumptions that market participants would use when pricing the asset, liability or equity instrument, assuming they act in their economic best interests. For non-financial assets, the fair value measurement is based on its highest and best use. Valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, are used, maximising the use of relevant observable inputs and minimising the use of unobservable inputs.
45
Incannex Healthcare Limited
Assets, liabilities and equity instruments measured at fair value are classified into three levels, using a fair value hierarchy that reflects the significance of the inputs used in making the measurements. For assets and liabilities measured at fair value after initial recognition, classifications are reviewed at each reporting date and transfers between levels are determined based on a reassessment of the lowest level of input that is significant to the fair value measurement. The three levels of the fair value hierarchy are described as follows:
| ● | Level 1 — quoted (unadjusted) market prices in active markets for identical assets or liabilities; |
| ● | Level 2 — valuation techniques for which the lowest level input that is significant to the fair value measurement is directly or indirectly observable; and |
| ● | Level 3 — valuation techniques for which the lowest level input that is significant to the fair value measurement is unobservable. |
For recurring and non-recurring fair value measurements, external valuers may be used when internal expertise is either not available or when the valuation is deemed to be significant. External valuers are selected based on market knowledge and reputation. Where there is a significant change in fair value of an asset or liability from one period to another, an analysis is undertaken, which includes a verification of the major inputs applied in the latest valuation and a comparison, where applicable, with external sources of data.
Issued capital
Ordinary shares are classified as equity.
Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds.
46
Incannex Healthcare Limited
Dividends
Dividends are recognized when declared during the financial years.
Loss per share
Basic loss per share
Basic loss per share is calculated by dividing the profit attributable to the owners of Incannex Healthcare Limited, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the financial years, adjusted for bonus elements in ordinary shares issued during the financial years. These values are set out in Note 6.
Diluted loss per share
Diluted loss per share adjusts the figures used in the determination of basic earnings per share to take into account the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares. These values are set out in Note 6.
Goods and Services Tax (‘GST’) and other similar taxes
Revenues, expenses and assets are recognized net of the amount of associated GST, unless the GST incurred is not recoverable from the tax authority. In this case it is recognized as part of the cost of the acquisition of the asset or as part of the expense.
Receivables and payables are stated inclusive of the amount of GST receivable or payable. The net amount of GST recoverable from the tax authority is included in other receivables in the statement of financial position.
Cash flows are presented on a gross basis. The GST components of cash flows arising from investing or financing activities which are recoverable from, or payable to the tax authority, are presented as operating cash flow. Commitments and contingencies are disclosed net of the amount of GST recoverable from, or payable to, the tax authority.
Adoption of new and revised standards
The consolidated entity has adopted all of the new or amended Accounting Standards and Interpretations issued by the Australian Accounting Standards Board (‘AASB’) that are mandatory for the current reporting period. Any new or amended Accounting Standards or Interpretations that are not yet mandatory have not been early adopted.
Accounting standards and interpretations issued but not yet effective
Australian Accounting Standards and Interpretations that have recently been issued or amended but are not yet effective and have not been adopted by the Group for the annual reporting period ended 30 June 2023 and are not material to the disclosure in these accounts.
47
Incannex Healthcare Limited
2. Critical accounting judgements, estimates and assumptions
The preparation of the consolidated financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the consolidated financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
Acquisition of APIRx Pharmaceuticals
The Group has determined that the acquisition of APIRx Pharameticals (“APIRx”) in August 2022 is not deemed a business combination as the acquired set of activities and assets of APIRx did not meet the definition of a business under AASB 3 Business Combinations. Therefore, the transaction has been accounted for as an asset acquisition.
In an asset acquisition, the assets acquired are assigned a carrying amount based on the cost of the transaction and their relative fair values. The cost of the transaction was determined based on the fair value of the shares issued for consideration (in accordance with the shared based payment transactions accounting policy below). No deferred tax will arise in relation to the acquired assets and assumed liabilities as per the initial recognition exemption under AASB 112 Income Taxes.
Furthermore, no goodwill arises on acquisition and transaction costs of the acquisition are included in the capitalised cost of the asset. In determining when a transaction is an asset acquisition and not a business, significant judgment is required to assess whether the assets acquired constitute a business in accordance with AASB 3. Under AASB 3 a business is an integrated set of activities and assets that is capable of being conducted or managed for the purposes of providing a return, and consists of inputs and processes which, when applied to those inputs has the ability to create outputs.
Share-based payment transactions
The Group measures the cost of equity-settled transactions with employees and third parties by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined by using either the trinomial or Black-Scholes model taking into account the terms and conditions upon which the instruments were granted. The accounting estimates and assumptions relating to equity-settled share-based payments would have no impact on the carrying amounts of assets and liabilities within the next annual reporting period but may impact profit or loss and equity.
Intangible assets with an indefinite useful life or that are not yet available for use
The Group tests annually, or more frequently if events or changes in circumstances indicate impairment, whether an intangible asset with an indefinite useful life or an intangible asset that is not yet available for use has suffered any impairment, in accordance with the accounting policy stated in note 1. The recoverable amounts have been determined using the Relief from Royalty method. These calculations require the use of assumptions, including estimated discount rates, royalty rates, and growth rates of the estimated future cash flows. Refer to note 12 for further information.
3. Other income
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| Other income (point in time) | $ | $ | ||||||
| Interest | 362,766 | 6,271 | ||||||
| Refundable R&D tax offset | 1,013,879 | 782,383 | ||||||
| 1,376,645 | 788,654 | |||||||
48
Incannex Healthcare Limited
4. Segment Information
Identification of reportable operating segments
AASB 8 (IFRS 8) Operating Segments requires operating segments to be identified on the basis of internal reports about components of the Group that are regularly reviewed by the Chief Executive Officer in order to allocate resources to the segment and to assess its performance.
The Group’s operating segments have been determined with reference to the monthly management accounts used by the Chief Executive Officer to make decisions regarding the Group’s operations and allocation of working capital. Due to the size and nature of the Group, the Board as a whole has been determined as the Chief Executive Officer.
Based on the quantitative thresholds included in AASB 8 (IFRS 8), for the fiscal year ended 30 June 2023, the Group was organised into three operating segments:
| 1. | Research and develop the use of psychedelic medicine and therapies for the treatment of mental health disorders. This activity commenced during the year. During the current year the operations consisted entirely of research and development activities, including clinical trials. |
| 2. | Research and develop the use of medicinal cannabinoid products. During the year the Group continued to research and develop its products and the range of its products, including further clinical trials. |
| 3. | Corporate operations, consisting of management of the organisation, capital management and management of resources. Revenues consist of finance income and other income. |
The Group has only one geographical segment, namely Australia.
The revenues and results of these segments of the Group as a whole are set out in the condensed statement of comprehensive income and the assets and liabilities of the Group as a whole are set out in the condensed statement of financial position. A summary of revenue and expenses for the period and assets and liabilities at the end of the fiscal year for each segment is shown below.
| 30 June 2023 | Psychedelic products | Cannabinoid Products | Corporate | Consolidated | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Revenue from external customers | - | - | - | - | ||||||||||||
| Interest revenue | - | (129 | ) | 362,895 | 362,766 | |||||||||||
| Other revenue | - | 1,013,879 | - | 1,013,879 | ||||||||||||
| Other expenses | (1,092,033 | ) | (9,121,608 | ) | (11,142,562 | ) | (21,356,203 | ) | ||||||||
| Segment loss after income tax | (1,092,033 | ) | (8,107,858 | ) | (10,779,667 | ) | (19,979,558 | ) | ||||||||
| Segment assets | 881,808 | 53,359,216 | 34,349,676 | 88,590,700 | ||||||||||||
| Segment liabilities | (433,278 | ) | (2,395,958 | ) | (1,632,597 | ) | (4,461,833 | ) | ||||||||
| 30 June 2022 | Psychedelic products | Cannabinoid Products | Corporate | Consolidated | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Revenue from external customers | - | - | - | - | ||||||||||||
| Interest revenue | - | 96 | 6,175 | 6,271 | ||||||||||||
| Other revenue | - | 782,383 | - | 782,383 | ||||||||||||
| Other expenses | (883,708 | ) | (4,642,796 | ) | (10,166,059 | ) | (15,692,563 | ) | ||||||||
| Segment loss after income tax | (883,708 | ) | (3,860,317 | ) | (10,159,884 | ) | (14,903,909 | ) | ||||||||
| Segment assets | 56,058 | 263,731 | 37,559,819 | 37,879,608 | ||||||||||||
| Segment liabilities | (354,310 | ) | (577,819 | ) | (1,078,404 | ) | (2,010,533 | ) | ||||||||
49
Incannex Healthcare Limited
5. Income tax
The prima facie income tax benefit on pre-tax accounting loss from operations reconciles to the income tax benefit in the financial statements as follows:
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Accounting loss before tax | (19,979,558 | ) | (14,903,909 | ) | ||||
| Income tax benefit at the applicable tax rate of 25% (2022: 26%) | 4,994,890 | 3,725,977 | ||||||
| Non-deductible expenses | (1,259,881 | ) | (564,872 | ) | ||||
| Non-assessable income | 253,439 | 195,596 | ||||||
| Deferred tax assets not recognized | (3,988,448 | ) | (3,356,701 | ) | ||||
| Income tax benefit | - | - | ||||||
| Unrecognized Deferred Tax Asset | ||||||||
| Deferred tax asset not recognized in the financial statements: | ||||||||
| Unused tax losses | 29,636,125 | 24,845,264 | ||||||
| Net unrecognized tax benefit at 25% (2022: 26%) | 7,409,031 | 6,211,316 | ||||||
The potential deferred tax benefit has not been recognized as an asset in the financial statements because recovery of the asset is not considered probable in the context of AASB 112 Income Taxes (IAS 12).
The benefit will only be realised if:
| a) | the Company derives future assessable income of a nature and of an amount sufficient to enable the benefit to be realised. |
| b) | the Company complies with the conditions for deductibility imposed by the law; and |
| c) | no changes in tax legislation adversely affect the Company in realising the benefit. |
6. Loss per share
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Basic loss per share - cents per share | (1.30 | ) | (1.25 | ) | ||||
| Basic loss per share | ||||||||
| The loss and weighted average number of ordinary shares used in the calculation of basic loss per share is as follows: | ||||||||
| Total comprehensive loss for the year | (19,979,558 | ) | (14,903,909 | ) | ||||
| - Weighted average number of ordinary shares (number) | 1,536,826,010 | 1,191,154,011 | ||||||
The company notes that the diluted loss per share is the same as basic loss per share.
50
Incannex Healthcare Limited
7. Dividends
The Company has not declared a dividend for the year ended 30 June 2023 (2022: $nil).
8. Cash and cash equivalents
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Cash at bank and on hand | 33,363,228 | 37,500,931 | ||||||
| 33,363,228 | 37,500,931 | |||||||
| Cash at bank earns interest at floating rates based on daily bank deposit rates. | ||||||||
| Reconciliation of loss for the year to net cash flows from operating activities: | ||||||||
| Loss after income tax | (19,979,558 | ) | (14,903,909 | ) | ||||
| Non-cash-based expenses: | ||||||||
| Share-based payments | 3,191,640 | 1,464,550 | ||||||
| Depreciation and amortisation | 130,946 | - | ||||||
| Foreign exchange gain | (6,428 | ) | (594,394 | ) | ||||
| Changes in net assets and liabilities: | ||||||||
| (Increase)/Decrease in receivables | 7,240 | (92,320 | ) | |||||
| (Increase)/Decrease in other current assets | (951,221 | ) | 53,447 | |||||
| Increase/(Decrease) in trade payables and accrued expenses | 1,561,321 | 1,111,080 | ||||||
| Increase/(Decrease) in other liabilities | 103,235 | 154,173 | ||||||
| Cash flows used in operations | (15,942,825 | ) | (12,807,373 | ) | ||||
9. Trade and other receivables (current)
| Current | Consolidated | |||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| GST recoverable | 287,478 | 294,717 | ||||||
| 287,478 | 294,717 | |||||||
Expected credit losses
The Group applies the AASB 9 (IFRS 9) simplified model of recognising lifetime expected credit losses for all trade receivables as these items do not have a significant financing component. In measuring the expected credit losses, the trade receivables have been assessed on a collective basis as they possess shared credit risk characteristics. They have been grouped based on the days past due and also according to the geographical location of customers.
10. Other assets (current)
| Prepayments1 | 935,172 | 59,836 | ||||||
| Office rental bond | 100,009 | 24,124 | ||||||
| 1,035,181 | 83,960 |
| 1 | Prepayments consist prepaid clinical trial insurances, prepaid R&D expenditure relating to PsiGAD and IHL-675A clinical trials and scientific, marketing, and adverting subscription services. |
51
Incannex Healthcare Limited
11. Property, plant and equipment (non-current)
| Furniture, fittings, and equipment | Total | |||||||
| $ | $ | |||||||
| Year ended 30 June 2023 | ||||||||
| Opening net book amount | - | - | ||||||
| Additions | 476,873 | 476,873 | ||||||
| Depreciation charge | (33,221 | ) | (33,221 | ) | ||||
| Closing net book amount | 443,652 | 443,652 | ||||||
| At 30 June 2023 | ||||||||
| Cost | 476,873 | 476,873 | ||||||
| Accumulated depreciation and impairment | (33,221 | ) | (33,221 | ) | ||||
| Net book amount | 443,652 | 443,652 | ||||||
12. Intangible assets
Patents |
Trademarks | Other intangibles3 | Total fair value | |||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Year ended 30 June 2023 | ||||||||||||||||
| Opening net book amount | - | - | - | - | ||||||||||||
| Acquisition of assets1 | 22,822,000 | 28,904,000 | 991,000 | 52,717,427 | ||||||||||||
| Amortisation charge2 | - | - | - | - | ||||||||||||
| Closing net book amount | 22,822,000 | 28,904,000 | 991,000 | 52,717,427 | ||||||||||||
At 30 June 2023 | ||||||||||||||||
| Cost | 22,822,000 | 28,904,000 | 991,000 | 52,717,427 | ||||||||||||
| Accumulated amortization and impairment2 | - | - | - | - | ||||||||||||
| Net book amount | 22,822,000 | 28,904,000 | 991,000 | 52,717,427 | ||||||||||||
| 1 | On 4 August 2022, the Company completed the acquisition of APIRx Pharmaceuticals via the issuance of 218,169,506 IHL ordinary shares to the stakeholders of APIRx in an all–scrip transaction. As substantially all of the fair value of the assets acquired in the transaction relates to intangible assets (patents, trademarks, active clinical and pre-clinical research and development projects), the transaction has been determined to be an asset acquisition and not a business combination. In addition to the shares issued to APIRx, the Company issued 13,090,170 IHL ordinary shares & 9,000,000 IHL options to Ryba LLC as part of their engagement terms as lead M&A advisors, which were included in the cost of the assets acquired. The total cost was allocated to the acquired assets on the basis of the assets’ relative fair values. |
| 2 | Patents have been assessed to have a 13-year useful life; trademarks have an indefinite useful life. There has been no amortisation at period end as the assets are not available for use yet. |
| 3 | Other intangibles relates to the fair value of other IP assets acquired, including pending or inactive patents. |
52
Incannex Healthcare Limited
12. Intangible assets (continued)
Impairment testing for intangible assets with indefinite life or that are not yet available for use
The accounting standards state that an impairment test must be performed annually for indefinite life intangible assets such as patents and trademarks. Further, companies must also assess at each reporting date whether there is any indication that the asset may be impaired and, if so, perform an impairment test.
The recoverable amount was determined using the Relief from Royalty (‘RFR’) valuation method. Fair value was measured largely using Level 2 and Level 3 inputs under AASB 13 Fair Value Measurement. The key assumptions are outlined below.
The calculations reflect a thirteen-year revenue forecast and requires the use of assumptions, including estimated royalty rates, tax rate, estimated discount rates and expected useful life.
The thirteen-year revenue forecast is based on the Group’s thirteen-year forecasts relating to acquired drug candidates currently in the pre-clinical and active clinical stages, being Medchew, Chewell, CanQuit O, CanQuit N and Renecann which was presented to the Audit committee. The thirteen-year forecast is based on the expiry date of each of the 20 granted patents, the average useful life for the granted patents is approximately 13 years. Accordingly, the revenue forecast exceeds five years and extends through to the end of 2035. The Company’s confident that the valuation of the Patents and Trademarks are reliable and were based on past experience and the Company’s forecast operating and financial performance. Revenue beyond the thirteen-year period applied a terminal growth rate of 11.20% for revenue growth.
The following key assumptions were used in the Relief from Royalty model:
| Patents | Trademarks | |||||||
| Royalty rate1 | 5.25 | % | 6.25 | % | ||||
| Terminal growth rate2 | N/A | 11.20 | % | |||||
| Post-tax discount rate3 | 42.5 | % | 42.5 | % | ||||
| Discount rate premium4 | 1.00 | % | 1.00 | % | ||||
| Tax rate5 | 30.00 | % | 30.00 | % | ||||
| Compound annual revenue growth rate6 | 10.18 | % | 10.18 | % | ||||
| 1 | The royalty rates (a percentage of gross revenue) used in the valuation models is based on rates observed in the market. |
| 2 | The terminal growth rate is a blended rate based on the relative proportion of revenue generated by each Trademark at the end of the forecast period, and the expected market growth of the drugs market specific to the indications treated by the drug candidates under those Trademarks. |
| 3 | The discount rate applied has been determined with reference to the rates of return expected by venture capitalists investing in early-stage companies based on academic research and empirical evidence. |
| 4 | Intangible assets, by their nature, generally carry more risk than tangible assets and therefore, the return required for tangible assets such as working capital and fixed assets is typically lower than the company discount rate, and the return required for intangible assets is higher than the discount rate. |
| 5 | The tax rate applied in the valuation model is based on the Australian corporate tax rate of 30.0%. |
| 6 | Compounded annual growth rate over 10 years from FY25-35. |
There is no indication of impairment at balance date.
53
Incannex Healthcare Limited
13. Leases
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Amounts recognised in statement of financial position | ||||||||
| Right-of-use assets | ||||||||
| Right-of-use assets1 | 841,460 | - | ||||||
| Depreciation | (97,726 | ) | - | |||||
| 743,734 | - | |||||||
| 1 | For the year ended 30 June 2023, the Group entered into a three new lease agreement for its corporate head office in Sydney, Melbourne office and Clarion Clinic site. The leases have four, five and three-year terms respectively. |
| Lease liabilities | ||||||||
| Current | 170,656 | - | ||||||
| Non-current | 616,087 | - | ||||||
| 786,743 | - | |||||||
| Amounts recognised in statement of comprehensive income | ||||||||
| Depreciation charge of right-of-use assets | 97,726 | - | ||||||
| Net finance expenses | 33,609 | - | ||||||
| 131,335 | - | |||||||
14. Trade and other payables (current)
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Trade payables | 2,707,441 | 1,300,696 | ||||||
| Accrued expenses | 641,031 | 415,449 | ||||||
| Employee leave entitlements | 326,618 | 294,388 | ||||||
| 3,675,090 | 2,010,533 | |||||||
54
Incannex Healthcare Limited
15. Issued capital
| Consolidated | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| 150,842,248 | 86,586,794 | |||||||
| Consolidated | ||||||||||||||||
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
| $ | No. of shares | $ | No. of shares | |||||||||||||
| (a) Ordinary shares - movements during year | ||||||||||||||||
| At start of year | 86,586,794 | 1,292,334,028 | 45,852,106 | 1,068,411,224 | ||||||||||||
| Issues of new shares – placements1 | 13,000,000 | 63,414,635 | 400,000 | 5,000,000 | ||||||||||||
| Issues of new shares – acquistion2 | 49,088,139 | 218,169,506 | - | - | ||||||||||||
| Issues of new shares – employees and directors’ | - | - | - | 10,000,000 | ||||||||||||
| Exercise of options | 2,027 | 2,027 | 40,274,243 | 207,650,638 | ||||||||||||
| Shares in lieu of advisor fees3 | 2,945,288 | 13,090,170 | 450,000 | 1,272,166 | ||||||||||||
| Share issue costs4 | (780,000 | ) | - | (389,555 | ) | - | ||||||||||
| At end of year | 150,842,248 | 1,587,010,366 | 86,586,794 | 1,292,334,028 | ||||||||||||
| 1 | On 9 December 2022, the Group raised $13 million from a placement of 63,414,635 shares with a small consortium of US and international institutional investors with significant healthcare experience in the US, Europe and Asia. |
| 2 | On 4 August 2022, the Company completed the acquisition on APIRx Pharmaceuticals via the issuance of 218,169,506 IHL ordinary shares to the stakeholders of APIRx in an all–scrip transaction. |
| 3 | On 4 August 2022, the Company issued 13,090,170 IHL ordinary shares to Ryba LLC as lead M&A Advisors on the APIRx acquisition. |
| 4 | On 9 December 2022, the Group incurred $780k of share issue cost from Bell Potter relating to the share placement completed during the period. |
16. Reserves
| Equity based premium reserve | Consolidated | |||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Balance at 1 July 2022 | 8,077,191 | 6,612,641 | ||||||
| Options issued to advisors1 | 684,000 | - | ||||||
| Issues of new options – placement2 | 108,257 | - | ||||||
| Equity instruments issued to management and directors3 | 3,191,640 | 1,464,550 | ||||||
| At 30 June 2023 | 12,061,087 | 8,077,191 | ||||||
| 1 | During the year ended 30 June 2023, the Company issued 9,000,000 options to Ryba LLC pursuant to the mandate executed between the companies in November 2021. As the transaction between the Company and APIRx was deemed complete on 04 August 2022 the options were issued. |
| 2 | During the year ended 30 June 2023, the Company issued 105,800,651 options to existing shareholders for nominal amount as part of a loyalty placement offer. |
| 3 | Relates to the amortization of shares and options issued as share-based payments during the current and prior periods. |
The equity based premium reserve is used to record the value of equity issued to raise capital, and for share-based payments.
55
Incannex Healthcare Limited
17. Share based payments
From time to time, the Company may issue equity securities (i.e., shares, options or performance rights) to its employees, directors or advisors to more closely align rewards for performance with the achievement of the Company’s growth and strategic objectives. Where the recipient is a director of the Company, shareholder approval must be sought under the ASX Listing Rules prior to the issue of any equity securities to any director.
Fair value of shares issued
The fair value of shares issued to employees is determined using the closing price of shares on the grant date and expensed over the vesting period. The total fair value of shares issued to employees and directors during the year was $1,866,328 as of 30 June 2023 there was $895,318 of total unrecognized compensation cost related to unvested shares.
Options
The exercise price of options outstanding as of 30 June 2023 and 2022 ranged between $0.35 and $1.50.
As of 30 June 2023, there was $1,325,311 of recognized and $615,452 of total unrecognized compensation cost related to unvested share options, which is expected to be recognized over a weighted-average period of approximately 1.39 years.
The fair values at grant date are independently determined using either a trinomial pricing or Black-Scholes option model that take into account any price to exercise, the term of the options or rights, the share price at grant date, the price volatility of the underlying share and the risk-free interest rate for the term of the options or rights. The expensed fair value in the tables below represents the proportion of the total fair value that has been allocated to the current period with the balance to be expensed in future periods.
The following share options were issued to employees and consultants as share based payments during the year ended 30 June 2023:
| Options | Number | Grant | Expiry Date | Exercise | Total | |||||||||||
| Options granted to Directors | ||||||||||||||||
| Unlisted Options | 2,500,000 | 29-Nov- 22 | 31-May-24 | $ | 1.00 | $ | 57,500 | |||||||||
| Unlisted Options | 2,500,000 | 29-Nov- 22 | 31-May-24 | $ | 1.50 | $ | 30,000 | |||||||||
| Options granted to third parties | ||||||||||||||||
| Unlisted Options | 3,000,000 | 04-Aug- 22 | 04-Aug-25 | $ | 0.61 | $ | 243,000 | |||||||||
| Unlisted Options | 3,000,000 | 04-Aug- 22 | 04-Aug-25 | $ | 0.69 | $ | 228,000 | |||||||||
| Unlisted Options | 3,000,000 | 04-Aug- 22 | 04-Aug-25 | $ | 0.76 | $ | 213,000 | |||||||||
| Total options | 14,000,000 | $ | 771,500 | |||||||||||||
56
Incannex Healthcare Limited
17. Share based payments (continued)
The following share options were issued to employees and consultants as share based payments during the year ended 30 June 2022:
| Options | Number | Grant Date | Expiry Date | Exercise Price | Total fair value | |||||||||||
| Options granted to Directors | ||||||||||||||||
| Unlisted Options | 1,399,999 | 09-Jun-22 | 01-Jul-25 | $ | 0.26 | $ | 298,200 | |||||||||
| Unlisted Options | 1,399,999 | 09-Jun-22 | 01-Jul-26 | $ | 0.31 | $ | 309,400 | |||||||||
| Unlisted Options | 1,400,002 | 09-Jun-22 | 01-Jul-27 | 0.35 | 324,800 | |||||||||||
| Unlisted Options | 1,399,999 | 09-Jun-22 | 01-Jul-26 | 0.26 | 326,200 | |||||||||||
| Unlisted Options | 1,399,999 | 09-Jun-22 | 01-Jul-27 | 0.31 | 334,600 | |||||||||||
| Unlisted Options | 1,400,002 | 09-Jun-22 | 01-Jul-28 | 0.35 | 347,200 | |||||||||||
| Options granted to employees | ||||||||||||||||
| Unlisted Options | 533,333 | 29-Apr-22 | 01-Jul-25 | $ | 0.26 | $ | 139,200 | |||||||||
| Unlisted Options | 533,333 | 29-Apr-22 | 01-Jul-26 | $ | 0.31 | $ | 143,467 | |||||||||
| Unlisted Options | 533,334 | 29-Apr-22 | 01-Jul-27 | $ | 0.35 | $ | 148,800 | |||||||||
| Total options | 10,000,000 | $ | 2,371,867 | |||||||||||||
The fair values at grant date are independently determined using either a trinomial pricing or Black-Scholes option model that take into account any price to exercise, the term of the options or rights, the share price at grant date, the price volatility of the underlying share and the risk-free interest rate for the term of the options or rights. Inputs into the trinomial and Black-Scholes option pricing models used to calculate fair value are classified as level three inputs under the fair value hierarchy of AASB 13 (IFRS 13).
The fair value of the equity-settled share options granted is estimated as at the grant date using a Black-Scholes option model taking into account the terms and conditions upon which the options were granted, as follows for the year ended 30 June 2023:
| $1.00 Options | $1.50 Options | $0.612 Options | $0.69 Options | $0.765 Options | ||||||||||||||||||
| 31-May-24 | 31-May-24 | 4-Aug-25 | 4-Aug-25 | 4-Aug-25 | ||||||||||||||||||
| Number | 2,500,000 | 2,500,000 | 3,000,000 | 3,000,000 | 3,000,000 | |||||||||||||||||
| Expected volatility (%) | % | 90 | % | 90 | % | 90 | % | 90 | % | 90 | % | |||||||||||
| Risk-free interest rate (%) | % | 3.18 | % | 3.18 | % | 2.86 | % | 2.86 | % | 2.86 | % | |||||||||||
| Expected life of option (years) | 1.5 | 1.5 | 3.0 | 3.0 | 3.0 | |||||||||||||||||
| Exercise price (cents) | 100 | 150 | 61.2 | 69.0 | 76.5 | |||||||||||||||||
| Grant date share price (cents) | 23.5 | 23.5 | 22.5 | 22.5 | 22.5 | |||||||||||||||||
| Vesting date | 29-Nov-22 | 29-Nov-22 | 4-Aug-22 | 4-Aug-22 | 4-Aug-22 | |||||||||||||||||
57
Incannex Healthcare Limited
17. Share based payments (continued)
The fair value of the equity-settled share options granted is estimated as at the grant date using a Black-Scholes option model taking into account the terms and conditions upon which the options were granted, as follows for the year ended 30 June 2022:
$0.26 | $0.31 | $0.35 | $0.26 | $0.31 | $0.35 | $0.26 | $0.31 | $0.35 | ||||||||||||||||||||||||||||
| 01-Jul-25 | 01-Jul-26 | 01-Jul-27 | 01-Jul-26 | 01-Jul-27 | 01-Jul-28 | 01-Jul-25 | 01-Jul-26 | 01-Jul-27 | ||||||||||||||||||||||||||||
| Number | 1,399,999 | 1,399,999 | 1,400,002 | 1,399,999 | 1,399,999 | 1,400,002 | 533,333 | 533,333 | 533,334 | |||||||||||||||||||||||||||
| Expected volatility (%) | 80 | % | 80 | % | 80 | % | 80 | % | 80 | % | 80 | % | 80 | % | 80 | % | 80 | % | ||||||||||||||||||
| Risk-free interest rate (%) | 3.12 | % | 3.33 | % | 3.33 | % | 3.33 | % | 3.33 | % | 3.33 | % | 2.71 | % | 2.90 | % | 2.90 | % | ||||||||||||||||||
| Expected life of option (years) | 3.06 | 4.06 | 5.06 | 4.06 | 5.06 | 6.07 | 3.18 | 4.18 | 5.18 | |||||||||||||||||||||||||||
| Exercise price (cents) | 26 | 31 | 35 | 26 | 31 | 35 | 26 | 31 | 35 | |||||||||||||||||||||||||||
| Grant date share price (cents) | 35 | 35 | 35 | 35 | 35 | 35 | 41 | 41 | 41 | |||||||||||||||||||||||||||
| Vesting date | 30-Jun-22 | 30-Jun-23 | 30-Jun-24 | 30-Jun-23 | 30-Jun-24 | 30-Jun-25 | 01-Jul-22 | 01-Jul-23 | 01-Jul-24 | |||||||||||||||||||||||||||
The expected life of the options is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
Ordinary shares
There were 218,169,506 IHL ordinary shares issued to the vendors APIRx as part of the asset acquisition, in addition the Company issued 13,090,170 IHL ordinary shares to Ryba LLC as lead M&A advisors. The fair value of the shares was determined with reference to the ASX share price at the date at which the shares were granted. Refer note 12 & 15 for further details.
Performance Rights
Movement in Performance Shares and Performance Rights for the years ended 30 June 2023 & 2022.
58
Incannex Healthcare Limited
18. Remuneration of auditors
| Consolidated | Consolidated | |||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Audit or review of the financial reports of the company | ||||||||
| Amounts received & receivable by the auditor: | ||||||||
| Audit services – PKF Brisbane Audit | 97,750 | 85,000 | ||||||
| Audit services – HLB Mann Judd | - | 23,138 | ||||||
| Audit services – Withum Smith & Brown (US auditor) | - | 357,208 | ||||||
| Other services – Withum Smith & Brown (US auditor) | - | - | ||||||
| 97,750 | 465,346 | |||||||
Withum Smith&Brown, PC were appointed auditors in the US in preparation for listing the Company’s securities in the US.
19. Financial instruments
The Group’s principal financial instruments comprise cash and short-term deposits.
The main purpose of these financial instruments is to raise finance for the Group’s operations. The Group has various other financial liabilities such as trade payables, which arise directly from its operations. It is, and has been throughout the year under review, the Group’s policy that no trading in financial instruments shall be undertaken. The main risks arising from the Group’s financial instruments are cash flow interest rate risk, liquidity risk, and credit risk. The Board reviews and agrees policies for managing each of these risks and they are summarised below.
(a) Interest rate risk
The Group’s exposure to the risk of changes in market interest rates relates primarily to the Group’s short-term deposits with a floating interest rate.
The Group’s exposure to interest rate on financial assets and financial liabilities is detailed in the sensitivity analysis section of this note.
(b) Sensitivity analysis
During 2023, if interest rates had been 50 basis points higher or lower than the prevailing rates realised, with all other variables held constant, there would have been an immaterial change in post-tax result for the year. The impact on equity would have been the same.
(c) Net fair values
The net fair value of cash and cash equivalents and non-interest bearing monetary financial assets and liabilities approximates their carrying value.
(d) Commodity price risk
The Group’s exposure to price risk is minimal.
59
Incannex Healthcare Limited
19. Financial instruments (continued)
(e) Credit risk
There are no significant concentrations of credit risk within the Group.
With respect to credit risk arising from the other financial assets of the Group, which comprise cash and cash equivalents, available-for-sale financial assets and certain derivative instruments, the Group’s exposure to credit risk arises from default of the counter party, with a maximum exposure equal to the carrying amount of these instruments.
Since the Group trades only with recognized third parties, there is no requirement for collateral.
(f) Liquidity risk
The Group’s objective is to maintain a balance between continuity of funding and flexibility through the use of share issues and convertible notes.
The Group’s contractual liabilities at 30 June 2023 were as follows:
Description | Less than 1 month | 1 to 3 months | 3 months to 1 year | 1 to 5 years | Total | |||||||||||||||
| Consolidated | $ | $ | $ | $ | $ | |||||||||||||||
| Payables & accruals | 3,298,131 | 236,514 | 140,445 | - | 3,675,090 | |||||||||||||||
| 3,298,131 | 236,514 | 140,445 | - | 3,675,090 | ||||||||||||||||
The Group’s contractual liabilities at 30 June 2022 were as follows:
Description | Less than 1 month | 1 to 3 months | 3 months to 1 year | 1 to 5 years | Total | |||||||||||||||
| Consolidated | $ | $ | $ | $ | $ | |||||||||||||||
| Payables & accruals | 1,828,527 | - | - | - | 1,828,527 | |||||||||||||||
| 1,828,527 | - | - | - | 1,828,527 | ||||||||||||||||
(g) Capital Management
The Group’s objectives when managing capital are to safeguard its ability to continue as a going concern, so that it may continue to provide returns for shareholders and benefits for other stakeholders. Due to the nature of the Group’s past activities, being mineral exploration, it does not have ready access to credit facilities and therefore is not subject to any externally imposed capital requirements, with the primary source of Group funding being equity raisings and unsecured convertible notes. Accordingly, the objective of the Group’s capital risk management is to balance the current working capital position against the requirements to meet exploration programmes and corporate overheads. This is achieved by maintaining appropriate liquidity to meet anticipated operating requirements, with a view to initiating fund raisings as required.
20. Commitments and contingencies
The Group had no commitments or contingent liabilities as at 30 June 2023.
60
Incannex Healthcare Limited
21. Key Management Personnel compensation and related party disclosure
The Key Management Personnel of Incannex Healthcare Limited during the year were:
Troy Valentine
Peter Widdows
Joel Latham
George Anastassov
Robert Clark (appointed 17 August 2022)
Key management personnel compensation
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Short-term employee benefits | 2,296,996 | 1,333,992 | ||||||
| Post-employment benefits | 66,757 | 47,547 | ||||||
| Share based payments | 2,715,156 | 1,028,634 | ||||||
| Total KMP compensation | 5,078,909 | 2,410,173 | ||||||
Transactions with related entities
Transactions between related parties are on commercial terms and conditions, no more favourable than those available to other parties unless otherwise stated.
During the year, nil (2022: $407,824) fees were paid to Alignment Capital Pty Ltd (“Alignment”), an entity in which Mr Valentine is a director. Alignment was previously engaged by the Company to manage the exercise of IHLOB options program.
During the year, $247,122 (2022: Nil) fees were paid to Cannvalate Pty Ltd (“Cannvalate”), an entity in which Dr Agarwal (KMP in the prior period) is a director. The Company previously entered into a distribution agreement with Cannvalate Pty Ltd whereby the Company had the right to distribute cannabinoid oil products in Australia through Cannvalate’s network.
During the year, Mr Valentine was paid $254,000 (2022: $240,000) for consulting fees invoiced to the Company, outside of his directors’ fees. Mr Widdows was also paid $160,000 (2022: Nil) for consulting fees invoiced to the Company, outside of his directors’ fees.
22. Details of the controlled entity
The consolidated financial statements include the financial statements of Incannex Healthcare Limited (‘IHL’) and its wholly owned subsidiaries Incannex Pty Ltd (‘IXPL’) and Psychennex Pty Ltd (‘PXPL’). IXPL is incorporated in Australia and IHL owns 100% of the issued ordinary shares in IXPL (2022: 100%). PXPL is incorporated in Australia and IHL owns 100% of the issued ordinary shares in PXPL (2022: 100%).
23. Events Subsequent to Reporting Date
No further significant events have occurred since the end of the financial year.
61
Incannex Healthcare Limited
24. Parent entity disclosures
The individual financial statements for the parent entity show the following aggregate amounts.
| Statement of financial position | 2023 | 2022 | ||||||
| Financial Position | $ | $ | ||||||
| Current assets | 33,677,744 | 37,559,819 | ||||||
| Non-Current assets | 671,932 | - | ||||||
| Total assets | 34,349,676 | 37,559,819 | ||||||
| Current liabilities | (1,260,966 | ) | (1,078,404 | ) | ||||
| Non-current liabilities | (371,631 | ) | - | |||||
| Total liabilities | (1,632,597 | ) | (1,078,404 | ) | ||||
| Net assets | 32,717,079 | 36,481,415 | ||||||
| Issued capital | 150,842,248 | 86,586,794 | ||||||
| Reserves | 12,061,087 | 8,077,191 | ||||||
| Accumulated losses | (130,186,256 | ) | (58,182,570 | ) | ||||
| Shareholders’ equity | 32,717,079 | 36,481,415 | ||||||
Contingencies of the Parent Entity
There are no contingent liabilities involving the parent entity (2022: Nil).
Guarantees of the Parent Entity
There are no guarantees involving the parent entity (2022: Nil)
62
Incannex Healthcare Limited
Directors’ Declaration
| 1) | In the opinion of the Directors: |
| a) | the accompanying financial statements, notes and additional disclosures are in accordance with the Corporations Act 2001 including: |
| i) | giving a true and fair view of the Group’s financial position as at 30 June 2023 and of its performance for the year then ended; and |
| ii) | complying with Accounting Standards and Corporations Regulations 2001; and |
| b) | there are reasonable grounds to believe the Company will be able to pay its debts as and when they become due and payable. |
| c) | the financial statements and notes thereto are in accordance with International Financial Reporting Standards issued by the International Accounting Standards Board. |
| 2) | This declaration has been made after receiving the declarations required to be made to the Directors in accordance with Section 295A of the Corporations Act 2001 for the financial year ended 30 June 2023. |
This declaration is signed in accordance with a resolution of the Board of Directors.
| /s/ Troy Valentine |
Troy Valentine
Chairman
Melbourne, Victoria, 30th August 2023
63
 | PKF Brisbane Audit |
| ABN 33 873 151 348 | |
| Level 6, 10 Eagle Street | |
| Brisbane, QLD 4000 Australia | |
| +61 7 3839 9733 | |
| brisbane@pkf.com.au | |
| pkf.com.au |
INDEPENDENT AUDITOR’S REPORT
TO THE MEMBERS OF INCANNEX HEALTHCARE LIMITED
Report on the Financial Report
Opinion
We have audited the accompanying financial report of Incannex Healthcare Limited (the company), which comprises the consolidated statement of financial position as at 30 June 2023, the consolidated statement of comprehensive income, the consolidated statement of changes in equity and the consolidated statement of cash flows for the year then ended, notes comprising a summary of significant accounting policies and other explanatory information, and the directors’ declaration of the company and the consolidated entity comprising the company and the entities it controlled at the year’s end or from time to time during the financial year.
In our opinion the financial report of Incannex Healthcare Limited is in accordance with the Corporations Act 2001, including:
| a) | Giving a true and fair view of the consolidated entity’s financial position as at 30 June 2023 and of its performance for the year ended on that date; and |
| b) | Complying with Australian Accounting Standards and the Corporations Regulations 2001. |
Basis for Opinion
We conducted our audit in accordance with Australian Auditing Standards. Our responsibilities under those standards are further described in the Auditor’s Responsibilities for the Audit of the Financial Report section of our report.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Independence
We are independent of the consolidated entity in accordance with the auditor independence requirements of the Corporations Act 2001 and the ethical requirements of the Accounting Professional and Ethical Standards Board’s APES 110 Code of Ethics for Professional Accountants (including Independence Standards) (the Code) that are relevant to our audit of the financial report in Australia. We have also fulfilled our other ethical responsibilities in accordance with the Code.
PKF Brisbane Pty Ltd is a member of PKF Global, the network of member firms of PKF International Limited, each of which is a separately owned legal entity and does not accept any responsibility or liability for the actions or inactions of any individual member or correspondent firm(s). Liability limited by a scheme approved under Professional Standards Legislation.
64

Key Audit Matters
Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the financial report of the current period. These matters were addressed in the context of our audit of the financial report as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters. For each matter below, our description of how our audit addressed the matter is provided in that context.
1. Acquisition of APIRx Pharmaceuticals
| Why significant | How our audit addressed the key audit matter |
On 4 August 2022, the consolidated entity completed the acquisition of APIRx Pharmaceuticals via the issuance of 218,169,506 IHL ordinary shares to the stakeholders of APIRx in an all–scrip transaction.
Based on independent technical advice the acquisition was determined to be an asset acquisition and not a business combination under the definitions in AASB 3 Business Combinations. As a result of the acquisition the consolidated entity has recognised intangible assets totalling $52.7m:
| Intangible Asset | $ | |||
| Patents | 22,822,185 | |||
| Trademarks | 28,904,234 | |||
| Other IP assets | 991,008 | |||
| 52,717,427 | ||||
The consolidated entity engaged an independent valuation expert to determine the fair value of the consideration paid, identify the intangible assets acquired and to allocate the consideration to the assets acquired relative to their respective fair values.
This is a key audit matter for the following reasons:
| 1. | The acquired assets represent 59.5% of the consolidated entity’s total assets as at 30 June 2023. |
| 2. | The valuation of acquired intangibles assets is complex and requires judgements to be made in adopting key assumptions in the underlying valuation models. This drives additional audit effort in assessing the accounting for the acquisition, the competence of the independent experts used, and the reasonableness of the valuation methodology adopted, and the key assumptions relied upon by management. |
Refer to Notes 1, 2, 12, 15, 16 and 17 to the financial report for a description of the accounting policy, significant estimates and judgements applied, and other details in relation to acquisition.
Our audit procedures included but were not limited to:
| ● | Assessing the reasonableness of the consolidated entity’s determination that the acquired entity does not meet the definition of a business under AASB 3: Business Combinations and has been appropriately accounted for as an asset acquisition; |
| ● | Evaluating the asset acquisition accounting applied by the consolidated entity against the requirements of Australian Accounting Standards; |
| ● | Considering the objectivity, competence, experience and scope of the engagement of the independent experts appointed by the consolidated entity; |
| ● | Evaluating the reasonableness of the valuation methodology used by the consolidated entity and its independent expert to determine the fair value of the consideration paid and intangible assets acquired; and |
| ● | Assessing the adequacy of disclosures in the financial report in accordance with the requirements of Australian Accounting Standards |
65

2. Valuation of share-based payments
| Why significant | How our audit addressed the key audit matter |
During the year ended 30 June 2023, the company issued options and shares (“securities”) to key management personnel, corporate advisers, and the vendors of APIRx Pharmaceuticals, which were accounted for as share-based payments under AASB 2: Share-based Payment.
The consolidated entity engaged an independent valuation expert to assist in determining the fair value of the equity instruments issued as share-based payments.
| Equity Instrument | No. Issued | Fair Value $ | Expensed during FY23 $ | |||||||||
| Shares1 | 218,169,506 | 49,088,139 | - | |||||||||
| Shares2 | 13,090,170 | 2,945,288 | - | |||||||||
| Options2 | 9,000,000 | 684,000 | - | |||||||||
| 52,717,427 | - | |||||||||||
| Options3 | 5,000,000 | 87,500 | 87,500 | |||||||||
| 52,804,927 | 87,500 | |||||||||||
| 1 | Issued to vendors of APIRx Pharmaceuticals in connection with the asset acquisition |
| 2 | Issued to corporate adviser in connection with the asset acquisition |
| 3 | Issued to key management personnel as remuneration |
Total share-based payment expense for the year, including expense recognised in relation to the amortisation of the fair value of securities issued as share-based payments in prior years, totalled $3,191,640.
This is a key audit matter for the following reasons:
| 1. | The company valued the options using the Black Scholes model, where inputs such as volatility and risk-free rate require judgement. |
| 2. | The significance of the share-based payments to the company’s financial position. |
Refer to Notes 1, 2, 15, 16 and 17 to the financial report for a description of the accounting policy, significant estimates and judgements applied, and other details in relation to share-based payments.
Our audit procedures included but were not limited to:
| ● | Obtaining an understanding of the key terms and conditions of the options and shares by inspecting relevant supporting documentation. |
| ● | Considering the objectivity, competence, experience and scope of the engagement of the independent expert appointed by the consolidated entity; |
| ● | Assessing the reasonableness of key inputs into the valuation model used by the independent expert engaged by management. |
| ● | Recalculating the estimated fair value of the options using the Black Scholes option valuation methodology and assumptions assessed as reasonable. |
| ● | Testing the accuracy of the amortisation of share-based payments over the vesting period and the recording of an expense in the statement of profit or loss and an increment to the share-based payment reserve (options) or issued capital (shares). |
| ● | Reviewing the adequacy of the company’s disclosures in respect of the accounting treatment of share-based payments in the financial statements, including the significant judgments involved, and the accounting policy adopted are in accordance with the requirements of Australian Accounting Standards |
66

3. Impairment assessment of intangible assets with an indefinite useful life or that are not yet available for use
| Why significant | How our audit addressed the key audit matter |
The consolidated entity has $52.7m of intangible assets with either an indefinite useful life or that are not yet available for use on its statement of financial position at 30 June 2023.
The consolidated entity tests these assets for impairment at least annually by determining the recoverable amount (the higher of value-in-use or fair value less costs to sell) of the individual assets and comparing the recoverable amounts of the assets to their carrying values.
The consolidated entity has assessed the recoverable amount of the assets based on a fair value model using the relief-from-royalty method that was developed by an independent expert engaged to determine the fair value of the assets in connection with the assets’ acquisition earlier in the financial year.
The key assumptions applied in the model are:
| ● | annual revenue growth rates for the forecast periods; |
| ● | discount rates; |
| ● | royalty rates; and |
| ● | terminal growth rate. |
This is a key audit matter due to:
| 1. | the significance of the balances to the financial statements; and |
| 2. | the level of judgement applied by the consolidated entity in determining the key assumptions used to determine the recoverable amounts. |
Refer to Notes 1, 2, and 12 to the financial report for a description of the accounting policy, significant estimates and judgements applied, and other details in relation to impairment testing for intangible assets with an indefinite useful life or that are not yet available for use.
We considered whether the consolidated entity’s methodology for assessing impairment is in accordance with AASB 136: Impairment of Assets.
Our audit procedures included but were not limited to:
| ● | Evaluating the valuation methodology used by the consolidated entity to determine recoverable amount; |
| ● | Considering the objectivity, competence, experience and scope of the engagement of the independent expert appointed by the consolidated entity to determine the fair value of the assets in connection with the assets’ acquisition earlier in the financial year; |
| ● | Considering management’s assessment regarding any changes required to the key assumptions and forecasts underlying the valuation prepared by management’s independent expert in connection with the assets’ acquisition earlier in the financial year; |
| ● | Confirming that underlying forecasts used in the valuations had been approved by the Board; |
| ● | Assessing the reasonableness of key assumptions; and |
| ● | Assessing the adequacy of the consolidated entity’s disclosures in relation to estimates used to measure the recoverable amounts of the intangible assets, including those at Note 12 relating to key assumptions. |
67

Other Information
The Directors are responsible for the other information. The other information comprises the information included in the consolidated entity’s Annual Report, but does not include the financial report and our auditor’s report thereon.
Our opinion on the financial report does not cover the other information and we do not express any form of assurance conclusion thereon.
In connection with our audit of the financial report, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the financial report or our knowledge obtained in the audit or otherwise appears to be materially misstated. If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard.
Directors’ Responsibilities for the Financial Report
The Directors of the company are responsible for the preparation of the financial report that gives a true and fair view in accordance with Australian Accounting Standards and the Corporations Act 2001 and for such internal control as the Directors determine is necessary to enable the preparation of the financial report that gives a true and fair view and is free from material misstatement, whether due to fraud or error.
In preparing the financial report, the Directors are responsible for assessing the consolidated entity’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the Directors either intend to liquidate the consolidated entity or to cease operations, or have no realistic alternative but to do so.
Auditor’s Responsibilities for the Audit of the Financial Report
Our objectives are to obtain reasonable assurance about whether the financial report as a whole is free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Australian Auditing Standards will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of this financial report.
As part of an audit in accordance with Australian Auditing Standards, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
| ● | Identify and assess the risks of material misstatement of the financial report, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. |
68

| ● | Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the consolidated entity’s internal control. |
| ● | Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the Directors. |
| ● | Conclude on the appropriateness of the Directors’ use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the consolidated entity’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor’s report to the related disclosures in the financial report or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor’s report. However, future events or conditions may cause the consolidated entity to cease to continue as a going concern. |
| ● | Evaluate the overall presentation, structure and content of the financial report, including the disclosures, and whether the financial report represents the underlying transactions and events in a manner that achieves fair presentation. |
| ● | Obtain sufficient appropriate audit evidence regarding the financial information of the entities or business activities within the consolidated entity to express an opinion on the consolidated entity financial report. We are responsible for the direction, supervision and performance of the consolidated entity audit. We remain solely responsible for our audit opinion. |
We communicate with the Directors regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide the Directors with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, actions taken to eliminate threats or safeguards applied.
From the matters communicated with the Directors, we determine those matters that were of most significance in the audit of the financial report of the current period and are therefore the key audit matters. We describe these matters in our auditor’s report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, we determine that a matter should not be communicated in our report because the adverse consequences of doing so would reasonably be expected to outweigh the public interest benefits of such communication.
69

Report on the Remuneration Report
We have audited the Remuneration Report included in the directors’ report for the year ended 30 June 2023. The Directors of the company are responsible for the preparation and presentation of the Remuneration Report in accordance with section 300A of the Corporations Act 2001. Our responsibility is to express an opinion on the Remuneration Report, based on our audit conducted in accordance with Australian Auditing Standards.
Opinion
In our opinion, the Remuneration Report of the consolidated entity for the year ended 30 June 2023 complies with section 300A of the Corporations Act 2001.

PKF BRISBANE AUDIT
| /s/ Liam Murphy |
Liam Murphy
Partner
Brisbane
30 August 2023
70
Incannex Healthcare Limited
CORPORATE GOVERNANCE STATEMENT
Incannex Healthcare’s governance practices guide the Company and its controlled entities’ activities and decision-making to ensure the Company meets stakeholder expectations of sound corporate governance and continuous improvement in company performance.
This Corporate Governance statement reviews the Company’s corporate governance practices against the ASX Corporate Governance Principles and Recommendations – 4th Edition (Corporate Governance Principles). All these practices, unless otherwise stated, were in place as at 24 January 2022.
The Corporate Governance Principles are as follows:
PRINCIPLE 1: Lay solid foundations for management and oversight
PRINCIPLE 2: Structure the board to be effective and add value
PRINCIPLE 3: Instil a culture of acting lawfully, ethically and responsibly
PRINCIPLE 4: Safeguard the integrity of corporate reports
PRINCIPLE 5: Make timely and balanced disclosure
PRINCIPLE 6: Respect the rights of security holders
PRINCIPLE 7: Recognise and manage risk
PRINCIPLE 8: Remunerate fairly and responsibly
Given the differences in size, complexity, history and culture of listed companies, the Corporate Governance Principles adopt an “if not, why not” approach to compliance and disclosure, requiring companies to explain the reasons for any departure from the Corporate Governance Principles recommendations. These explanations are included in section 9 of this statement.
Specific corporate governance policies of the Group are detailed on the Company’s investor website under the ‘Investor Centre’ tab, at https://www.incannex.com.au/investors/. In this statement Incannex Healthcare and its controlled entities together are referred to as the “Group” or “Company”.
PRINCIPLE 1: LAY SOLID FOUNDATIONS FOR MANAGEMENT AND OVERSIGHT
Board Charter and roles and responsibilities
The Board has adopted a Board Charter establishing corporate governance roles and responsibilities within the Group.
Under its Charter, the Board is ultimately responsible to the Company’s shareholders for all matters related to the running of the Company. The Board’s role is to govern the Company rather than to manage it, with the role of Senior Executives and Management to manage the company in accordance with the direction and delegations of the Board.
In general, the Board is responsible for overseeing all policies, practices, management, and operations of the Company, including corporate reporting systems, risk management, remuneration frameworks, governance issues, succession planning, and stakeholder communications. The Board also takes decisions regarding matters of fundamental importance to the Group.
The Board’s focus is to enhance the interests of shareholders and other key stakeholders and to ensure the Group is properly managed. Management is directly accountable to the Board to deliver timely, accurate, and relevant information to enable the Board to perform its responsibilities. Management is also responsible for operating within the relevant directives and the risk appetite established by the Board whilst supporting the Managing Director in executing day-to-day operations.
The respective roles and responsibilities of the Board include:
| ● | providing strategic guidance to the Group, including contributing to the development of and approving the corporate strategy reviewing and approving business plans, the budget, financial plans, and major capital expenditure initiatives |
| ● | overseeing and monitoring: |
| a) | organisational performance and the achievement of the Group’s strategic goals and objectives |
71
Incannex Healthcare Limited
| b) | progress of major capital expenditures and other significant corporate projects including any acquisitions or divestments or clinical trials |
| c) | financial performance including approval of the annual and half-year financial reports and liaison with the Group’s auditors; and |
| d) | effectiveness of the Group’s governance policies and procedures |
| ● | appointment, performance assessment and, if necessary, removal of the Managing Director |
| ● | ratifying the appointment and/or removal and contributing to the performance assessment of members of the Senior Executive team including the CFO, Chief Operating Officer and Company Secretary |
| ● | ensuring there are effective management processes in place and approving major corporate initiatives enhancing and protecting the reputation of the Group |
| ● | overseeing the operation of the Group’s system for compliance and risk management reporting to shareholders |
| ● | ensuring appropriate resources are available to the Senior Executive |
Incannex Healthcare Limited ABN 93 096 635 246 is committed to:
| (a) | complying with its disclosure obligations under the Corporations Act and ASX Listing Rules; |
| (b) | the promotion or investor confidence by ensuring that all investors have equal and timely access to material information concerning the Company, including material information about its financial position, performance, ownership and governance; and |
| © | providing announcements that are accurate, balanced and expressed in a clear and objective manner. The purpose of this policy is to: |
| (a) | raise awareness of the Company’s obligations under the continuous disclosure regime; |
| (b) | establish a process to ensure that information about the Company which may be market sensitive, and which may require disclosure is brought to the attention of the relevant person in a timely manner and is kept confidential; and |
| © | sets outs obligations of Directors, officers, employees and contractors of the Company to ensure that the Company complies with its continuous disclosure obligations. |
Compliance with this policy does not obviate the need for the Company to comply with ‘Annual Report Disclosure’.
1. Responsibilities
1.1 Executive Management
| (a) | Understand the continuous disclosure regulations; and Report potentially material information immediately to either the Company Secretary, the Managing Director or the Chair. |
1.2 Company Secretary
| (a) | Liaise with the Managing Director and/or Chair on information supplied to determine if it needs to be disclosed under continuous disclosure regulations; and |
| (b) | Report the material information to the market. |
72
Incannex Healthcare Limited
2. Policy
| (a) | Executive Management will make themselves aware of the continuous disclosure regulations in the ASX Listing Rules. |
| (b) | In the event that any member of management becomes aware of any fact or circumstance which may give rise to a requirement to disclose such information under the ASX Listing Rules, they will immediately inform either the Company Secretary, the Managing Director or the Chair. |
| (c) | Prior to disclosure, the Company Secretary, in conjunction with the Managing Director and/or the Chair, will review the information to enable a judgement as to the appropriate disclosure to be made. |
| (d) | If there is uncertainty over the requirement to comply with the continual disclosure requirements, then the Company will seek external legal advice. |
| (e) | The Company, through the Company Secretary, will notify the market of any information it is determined is required to be disclosed. |
| (f) | In accordance with ASX Listing Rules, the Company will immediately notify the market of information: |
| (i) | concerning the Company that a reasonable person would expect to have a material effect on the price or value of the Company’s securities; |
| (ii) | that would, or would be likely to, influence persons who commonly invest in securities in deciding whether to acquire or dispose of the Company’s securities; and |
| (iii) | The only exception to this is where the ASX Listing Rules do not require such information to be disclosed. |
| (g) | The Board must receive a copy of all material ASX announcements promptly after they have been made. |
2.2 Internal notification and decision-making concerning the disclosure obligation
The Board has designated the Company Secretary as the person responsible for overseeing and coordinating disclosure of information to the market as well as communicating with the relevant authorities. The Company Secretary will be responsible for ensuring that Company announcements are made in a timely manner and will establish a vetting procedure to ensure that the announcements are factual and do not omit any material information.
The Company Secretary will also ensure that Company announcements are expressed in a clear and objective manner that allows investors to assess the impact of the information when making investment decisions.
To assist the Company Secretary, fulfil the Company’s disclosure requirements, executive staff are responsible for immediately communicating to the Company Secretary any possible continuous disclosure matter concerning the operations of the Company. Executive staff are responsible for ensuring that the information is provided to the Company Secretary as soon as they become aware of it and that it is factual and does not omit any material information. Executive staff will promptly respond to requests from the Company Secretary for further information concerning the possible continuous disclosure matter.
The Company Secretary, after consultation with the Chair and Managing Director, determines whether information should be disclosed to the market.
Before an announcement is released to ASX, the Company must ensure:
| (a) | the Company Secretary has completed its review process; and |
| (b) | the announcement has been circulated to the Board for review; and |
| (c) | the Board has authorised the release of the announcement in writing. |
2.3 Promoting and monitoring compliance
The Company has a Continuous Disclosure Committee, comprising the following:
| (a) | Company Secretary; |
| (b) | General Counsel; |
| (c) | Managing Director; and |
| (d) | The Chair and Non-Executive Directors will form part of the Committee for major announcements |
The purpose of the Continuous Disclosure Committee is to promote and monitor compliance with the Company’s continuous disclosure obligations and to ensure that all employees are aware of this policy. In addition, the Continuous Disclosure Committee is responsible for ensuring that all staff are aware of the type of information that needs to be communicated and their obligation to communicate to the Company Secretary any possible continuous disclosure matter.
73
Incannex Healthcare Limited
A meeting of the Committee may be convened from time to time to consider particular continuous disclosure issues.
On a daily basis, the Company Secretary is charged with monitoring compliance with this policy. As part of that monitoring, all major announcements to the market will be reviewed for compliance with this policy. All public announcements will also be audited for compliance. These compliance reviews will be reported to the Continuous Disclosure Committee as part of their regular review of compliance. Any possible non-compliance will be reported to the Board at its next meeting. The Company Secretary must notify both the Chair and the Managing Director at the earliest opportunity if they believe that a false market in the Company’s securities either exists or has the possibility to exist.
2.4 Measures for seeking to avoid the emergence of a false market in the Company’s securities
The Company recognises that a false market in the Company’s securities may result if the Company provides incomplete information to the market or if the Company fails to respond to market and media speculation that may, or may be likely to, have an impact on the price of the Company’s securities.
While the Company does not, in general, respond to market speculation or rumours unless required to do so by law or other relevant bodies, the Company is committed to disclosing as much information as possible, without harming the Company, to a wide audience of investors through media releases of important milestones, including information that may not strictly be required under continuous disclosure requirements. Information given to the market will also be provided to investors through media releases.
Where appropriate, the Company will request a trading halt to prevent trading in the Company’s securities by an inefficient and uninformed market until the Company can make an announcement to the market.
2.5 Safeguarding confidentiality of corporate information to avoid premature disclosure
All employees are advised of the confidentiality of Company information. In addition, the Company imposes communication blackout periods for financial information between the end of financial reporting periods and the announcement of results to the market. To protect against inadvertent disclosure of price sensitive information, the Company does not hold meetings or briefings to discuss financial information with individual investors, institutional investors, analysts or media representatives during the communication blackout periods, unless such meetings or briefings are the subject of a specific announcement to the market.
2.6 Media contact and comment
The Board has designated the Managing Director or the Chair (where appropriate) to speak to the press on matters associated with the Company. In speaking to the press, the Managing Director or the Chair will not comment on price sensitive information that has not already been disclosed to the market, however, they may clarify previously released information. To assist in safeguarding against the inadvertent disclosure of price sensitive information, the Managing Director or the Chair will be informed of what the Company has previously disclosed to the market on any issue prior to briefing anyone outside the Company.
Subject to the policies of the Board and any committee that the Board may appoint from time to time, the Chair is authorised to comment on:
| (a) | annual and half yearly results at the time of the release of the annual or half yearly report; |
| (b) | resolutions to be put to General Meetings of the Company; |
| (c) | changes in Directors, any matter related to the composition of the Board or Board processes; |
| (d) | any speculation concerning Board meetings or the outcomes of Board meetings; and |
| (e) | other matters specifically related to shareholders. |
Subject to the policies of the Board and any committee that the Board may appoint from time to time, the Managing Director is authorised to comment on:
| (a) | the Company’s future outlook; |
| (b) | any operational matter; |
| (c) | media queries concerning operational issues which reflect either positively or negatively on the Company; |
| (d) | proposed or actual legal actions; and |
| (e) | queries and general discussion concerning the Company’s industry. |
74
Incannex Healthcare Limited
There will be times when Directors and employees will be approached by the media for public comment. On such occasions, the Director(s) or employee(s) should comply with the following:
| (a) | refer the person to the Managing Director or the Chair of the Board as appropriate for comment; |
| (b) | refrain from disclosing any information, documents or other forms of data to the person without the prior consent of the Managing Director or the Chair of the Board; and |
| (c) | report the person who contacted the Director/employee, the reason (explicit or inferred) for the contact and a summary of any other relevant information as soon as possible to the Managing Director or the Chair. |
2.7 External communications including analyst briefings and responses to shareholder questions
The Company discloses its financial and operational results to the market each year/half year/quarter as well as informing the market of other events throughout the year as they occur. Quarterly financial reports, media releases and AGM speeches are all lodged with the relevant authority. As all financial information is disclosed, the Company will only comment on factual errors in information and underlying assumptions when commenting on market analysts’ financial projections, rather than commenting on the projections themselves.
In addition to the above disclosures, the Company does conduct briefings and discussions with analysts and institutional investors. However, price sensitive information will not be discussed unless that particular information has been formally disclosed to the market via an announcement. Slides and investor presentations used in briefings will also be released immediately prior to the briefing to the market.
After the conclusion of each briefing or discussion, it will be reviewed to determine whether any price sensitive information has been inadvertently disclosed. If any price sensitive information was disclosed, it will be announced immediately to the market.
Similarly, when answering shareholder questions, price sensitive information will not be discussed unless that particular information has been formally disclosed to the market via an announcement.
Where a question can only be answered by disclosing price sensitive information, the Company will decline to answer it or take it on notice and announce the information to the market prior to responding.
If any new price sensitive information is to be used in briefing media, institutional investors and analysts or in answering shareholder queries, written materials containing such information will be lodged with the relevant authority prior to the briefing commencing. These briefing materials may also include information that may not strictly be required under continuous disclosure requirements.
This policy will form a component of the induction process for all new employees.
The Company is committed to the full and accurate reporting of its financial results. Consequently, when complying with its periodic disclosure requirements, the Company will provide commentary on its financial results. The purpose of the commentary will be to clarify and balance the information in the financial results.
This commentary will be delivered in a manner that is neutral, free from any bias and easy to understand. This may involve the provision of both positive and negative information about the Company that the Company believes is necessary to keep investors fully informed.
The Company respects the rights of its shareholders and to facilitate the effective exercise of those rights the Company is committed to:
| (a) | communicating effectively with shareholders; |
| (b) | giving shareholders ready access to balanced and understandable information about the Company and corporate proposals; and |
| (c) | making it easy for shareholders to participate in general meetings of the Company. |
75
Incannex Healthcare Limited
2.8 Provision of information
The Company will communicate with shareholders in three main ways:
| (a) | through ASX releases to the market; |
| (b) | through information provided directly to shareholders at general meetings of the Company; and |
| (c) | other market releases. |
It is the Company’s policy to comply with its continuous and periodic disclosure obligations. In accordance with the Company’s continuous disclosure policy, unless exempted by the ASX Listing Rules, the Company will immediately notify the market of information:
| (a) | concerning the Company that a reasonable person would expect to have a material effect on the price or value of the Company’s securities; and |
| (b) | that would, or would be likely to, influence persons who commonly invest in securities in deciding whether to acquire or dispose of the Company’s securities. |
Where practicable the Company will also make available the opportunity for shareholders to participate in new and substantive investor presentations by dial-in or live-stream or by uploading a transcript or recording of the presentation to ASX subsequently. The Company is not required to make available presentations that do not contain new market sensitive information.
“Substantive” presentations include results presentations and the types of presentations given at annual general meetings, investor days or broker conferences.
2.9 Provision of Information to the Board
The Company Secretary is to ensure that a copy of all material market announcements is to be circulated to the Board as soon as is practicable after its release.
2.10 Company website
The Company provides general information about the Company and its operations, details of the Company’s corporate governance policies and procedures and information specifically targeted at keeping the Company’s shareholders informed about the Company on its website.
In particular, where appropriate, after confirmation of receipt by the relevant authority, the following will be posted to the website:
| (a) | relevant announcements made to the market; |
| (b) | media releases; |
| (c) | information provided to analysts or the media during briefings; |
| (d) | the full text of notices of meeting and explanatory material; |
| (e) | information related to general meetings, including the Chair’s address, speeches and voting results; |
| (f) | copies of press releases and announcements for the preceding year; and |
| (g) | copies of annual and half-yearly reports including financial statements for the preceding year. |
Where possible, the website will also be used for web-casting or teleconferencing analyst and media briefings as well as general meetings of the Company. Where the Company does webcast the preceding events, and even where it is not possible to do so, a transcript or summary of the information discussed will be posted to the website.
76
Incannex Healthcare Limited
2.11 Direct communications with shareholders
Throughout the year it may be appropriate for the Company to directly communicate with shareholders. For example, to give shareholders notice of general meetings or to update shareholders by way of a Chair’s letter.
In relation to information that is directly communicated to shareholders, all shareholders have the right to elect to receive all such information by post, facsimile or electronic mail.
2.12 Meetings of the Company
In preparing for general meetings of the Company, the Company will draft the notice of meeting and related explanatory information so that they provide all of the information that is relevant to shareholders in making decisions on matters to be voted on by them at the meeting. This information will be presented clearly and concisely so that it is easy to understand and not ambiguous.
The Company will use general meetings as a tool to effectively communicate with shareholders and allow shareholders a reasonable opportunity to ask questions of the Board of Directors and to otherwise participate in the meeting.
The external auditor of the Company will be asked to attend each annual general meeting and to be available to answer shareholder questions about the conduct of the audit and the preparation and content of the auditor’s report.
2.13 Other information
While the Company aims to provide sufficient information to shareholders about the Company and its activities, it understands that shareholders may have specific questions and require additional information. To ensure that shareholders can obtain all relevant information to assist them in exercising their rights as shareholders, the Company will make available a telephone number and email address for shareholders to make their enquiries.
2.14 Investor Presentations
Where a new and substantive investor or analyst presentation is to be given, the Company will release a copy of the presentation materials on the ASX market announcements platform ahead of the presentation.
3. Review
This policy will be reviewed annually be the Board to ensure it is operating effectively and determine whether any amendments are required.
77
Incannex Healthcare Limited
SECURITIES EXCHANGE INFORMATION
Additional information required by the ASX Limited Listing Rules, and not disclosed elsewhere in this report.
SHAREHOLDINGS
No individual shareholder is recorded as being a substantial shareholder (>5% of the Company’s ordinary share capital).
CLASS OF SHARES AND VOTING RIGHTS
The voting rights attached to the Fully Paid Ordinary shares of the Company are:
| a) | at a meeting of members or classes of members each member entitled to vote may vote in person or by proxy or by attorney; and on a show of hands every person present who is a member has one vote, and |
| b) | on a poll every person present in person or by proxy or attorney has one vote for each ordinary share held. |
Options do not carry any voting rights.
TWENTY LARGEST SHAREHOLDERS (as at 30 August 2023)
| Position | Holder Name | Holding | % IC | |||||||
| 1 | HSBC CUSTODY NOMINEES (AUSTRALIA) LIMITED | 82,707,741 | 5.23 | % | ||||||
| 2 | GEORGE ANASTASSOV | 66,972,077 | 4.23 | % | ||||||
| 3 | PRASCH BV | 63,954,841 | 4.04 | % | ||||||
| 4 | DR SUDHANSHU AGARWAL | 34,080,364 | 2.15 | % | ||||||
| 5 | CANNVALATE PTY LTD | 32,000,000 | 2.02 | % | ||||||
| 6 | MR RAYMOND LAURENCE CARROLL | 30,250,000 | 1.91 | % | ||||||
| 7 | J P MORGAN NOMINEES AUSTRALIA PTY LIMITED | 23,906,440 | 1.51 | % | ||||||
| 8 | BROWNARROWS PTY LTD <EJM A/C> | 23,610,000 | 1.49 | % | ||||||
9 | BNP PARIBAS NOMS PTY LTD DEUTSCHE BANK TCA <DRP> | 22,880,950 | 1.45 | % | ||||||
| 10 | CITICORP NOMINEES PTY LIMITED | 19,087,124 | 1.21 | % | ||||||
| 11 | MR PETER WIDDOWS | 15,973,694 | 1.01 | % | ||||||
| 12 | MR KAIDE WANG | 15,700,000 | 0.99 | % | ||||||
| 13 | IMI LLC | 14,642,234 | 0.93 | % | ||||||
| 14 | BAGBO PTY LTD | 14,516,434 | 0.92 | % | ||||||
| 15 | MR BRIAN PETER BYASS | 14,247,191 | 0.90 | % | ||||||
| 16 | MR JOEL BRADLEY LATHAM | 13,829,129 | 0.87 | % | ||||||
| 17 | GEMINI CAPITALL LLC <THE ARS A/C> | 13,787,086 | 0.87 | % | ||||||
18 | SLADE TECHNOLOGIES PTY LTD <EMBREY FAMILY S/F A/C> | 13,350,000 | 0.84 | % | ||||||
| 19 | ALIGNMENT CAPITAL PTY LTD | 13,194,248 | 0.83 | % | ||||||
| 20 | RYBA LLC | 13,090,170 | 0.83 | % | ||||||
| Total | 541,779,723 | 34.24 | % | |||||||
DISTRIBUTIION OF SHAREHOLDERS (as at 30 August 2023)
| Range | Total Holders | Units | % of Total | |||||||||
| 1 - 1,000 | 589 | 355,819 | 0.02 | % | ||||||||
| 1,001 - 5,000 | 4,139 | 11,903,016 | 0.75 | % | ||||||||
| 5,001 - 10,000 | 2,133 | 16,234,830 | 1.03 | % | ||||||||
| 10,001 - 100,000 | 4,402 | 149,504,411 | 9.45 | % | ||||||||
| 100,001 and above | 1,446 | 1,404,278,944 | 88.75 | % | ||||||||
| Total | 12,709 | 1,582,277,020 | 100.00 | % | ||||||||
There were 4,832 shareholders holding less than a marketable parcel (less than 5,263 shares at $0.095) at 23 August 2023 - a total of 12,798,958 shares. There is no current on-market buy back taking place.
78