Exhibit 99.1

1 Corporate Presentation March 2022 Nasdaq: NRSN

Trademarks in this presentation are the property of their respective owners and used for informational and educational purposes only. 2 Disclaimer This presentation and oral statements made regarding the subject of this presentation contain "forward - looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements contained in this presentation other than statements of historical facts, including our business strategy and plans and objectives for future operations, including our financial performance, are forward looking statements. The words " anticipate"," believe," "continue," "estimate," "expect," "intend," "may," "will" and similar expressions are intended to identify forward looking statements. We have based these forward looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short term and long term business operations and objectives, and financial needs. Forward looking statements made in this presentation include statements about the market for therapeutics targeting neurodegenerative diseases and its opportunities for our product candidates; our expectations regarding our competitive advantages; the planned development timeline of our product candidates; and characterizations of the pre - clinical and clinical trial results of our product candidates. Forward looking statements are subject to a number of risks and uncertainties and represent our views as of the date of the presentation. The future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. You should not rely on these statements as representing our views in the future. More information about the risks and uncertainties affecting the Company is contained under the heading "Risk Factors" in the Company's final prospectus filed with the Securities and Exchange Commission on December 10, 2021. We undertake no obligation or duty to update information contained in these forward looking statements, whether as a result of new information, future events or otherwise.
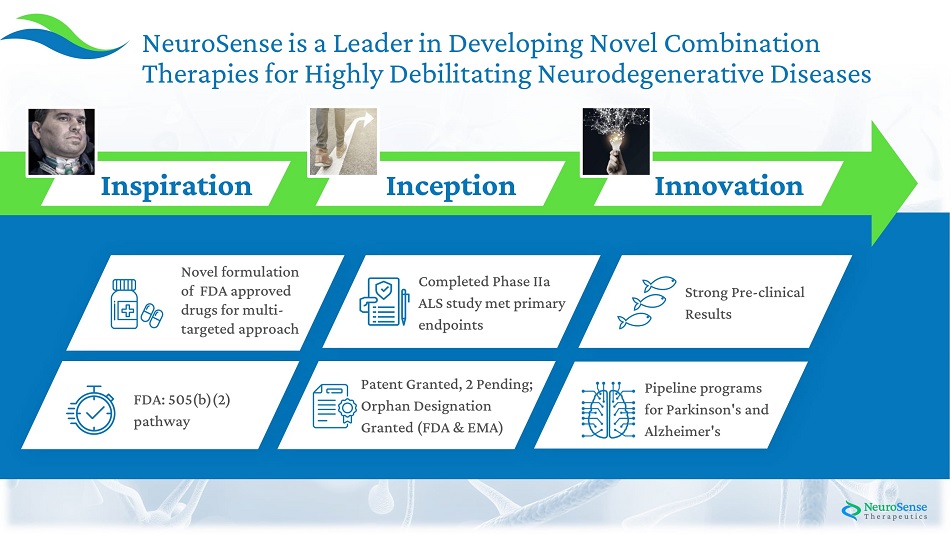
3 S t r ong Pr e - clini c al Results Compl e t e d Ph a se I I a A LS study m e t pri m a r y endpoints Pa t e nt G ra nte d , 2 Pending; Orphan Designation G ra nte d (FD A & EMA) N o v e l f ormul a t ion of F D A approv e d drugs f or m ulti - t a rg e t e d approach FDA: 50 5 ( b ) ( 2 ) pathway Pipe line programs f or Par k inson's and Alzheimer's NeuroSense is a Leader in Developing Novel Combination Therapies for Highly Debilitating Neurodegenerative Diseases Inspiration I n cep tion Innovation
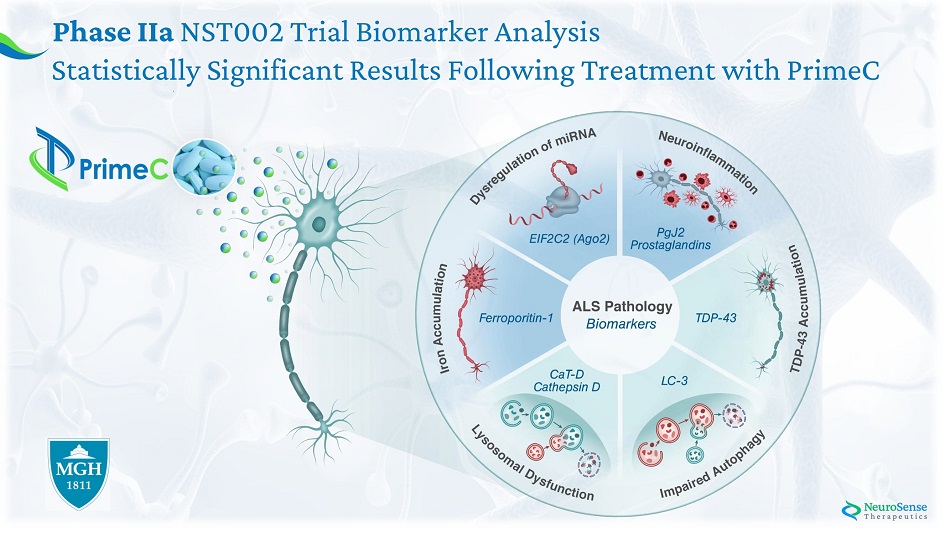
Phase IIa NST002 Trial Biomarker Analysis Statistically Significant Results Following Treatment with PrimeC
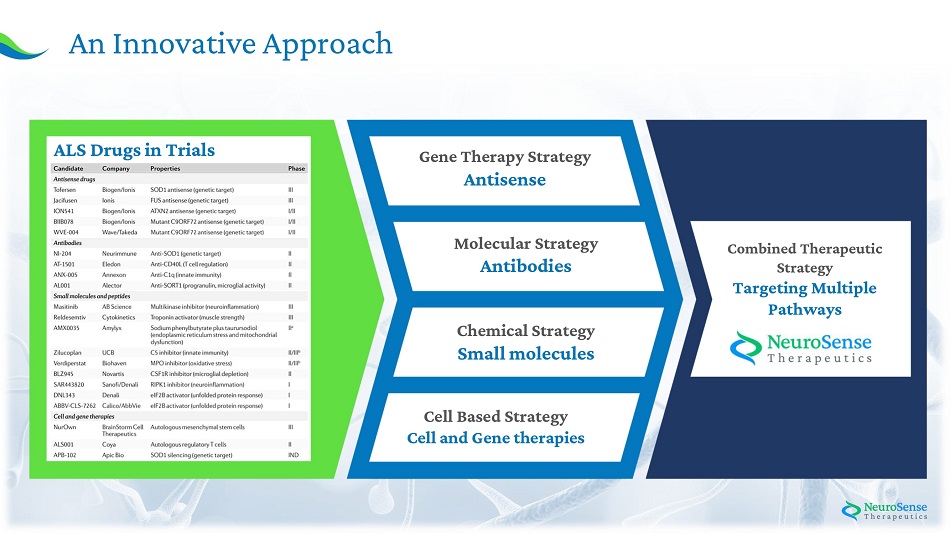
5 A n Inno v ative A ppro a ch Gene Therapy Str a tegy Antisense M olecular Str a tegy Antibodies Chemica l Strategy Sma l l molecu l es Cel l B a s ed St r ate g y Cel l an d Gene therapies Combine d Thera p e u ti c Strategy Targeting M ult i pl e Pathways ALS Drug s in Tria ls

6 Experienced Leadership H e a d o f Scie n tif ic Progra m Shira n Zimr i , PhD C MO Fere n c Tr a cik , MD C EO , Board M e mber Alo n B e n - Noon C h airm a n of t h e Board M a rk Le u chtenberger C FO O r Eisenberg Niva R usse k - B l u m , PhD VP Disco v e ry & IP G e n e rator VP B D Ne d ir a S a lzman H e a d o f A L S P rogram Av it a l Pushett
7 Scientific Adviso r y Boa r d P r of. Jerem y S h e f ne r ( Cha i r) • Senior VP at the Barrow Neurological Institute • C h a i r o f t h e D e p a r t m e n t o f N e ur olog y Dr. Jins y A n d rews • As s o c i a t e P r o fe sso r o f N e ur olog y , D i v i s i o n of Neuromuscular Medicine, Columbia University • Director of Neuromuscular Clinical Trials Prof . M e r i t Cu d kow i c z • C h ie f o f N e ur olog y a t Ma ss G e n e r a l a n d D i r e c t o r , S e a n M . H e a l e y & AM G C e n t e r f o r A L S • P r o fe sso r o f N e ur olog y a t H a r v a rd Me d i c a l S c h oo l Dr. Je f f e ry R o se n f e ld • Professor of Neurology and Associate Chairman of Neurology at Loma L i n d a U n i v e r s i t y S c h oo l o f Me d i c i n e • Medical Director of Center for Restorative Neurology at Loma Linda University Prof . Or l a Ha r di m a n • Head of Academic Unit of Neurology at Trinity College Dublin and Consultant Neurologist at Beaumont • Co - Chair of the European Consortium to Cure ALS and Chair o f t h e S c i e n t ific C o mmi tt e e o f ENC A L S
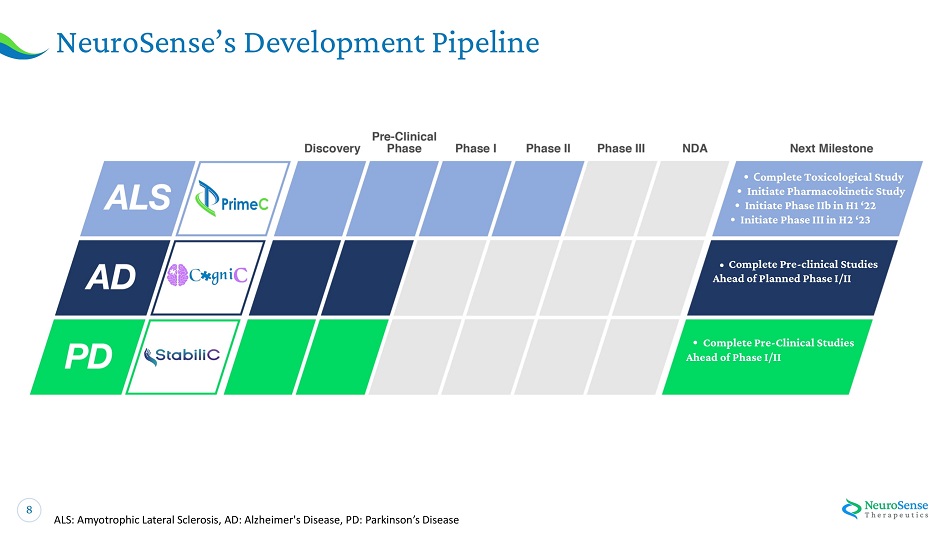
8 NeuroS e ns e ’ s De v el o p men t Pipel ine ALS: Amyotrophic Lateral Sclerosis, AD: Alzheimer's Disease, PD: Parkinson’s Disease • C omple t e Toxicological Stud y • Init i at e Pharma co k i n e ti c S tu d y • Init i at e Phase I I b in H 1 ‘ 22 • Init i at e P h a s e I I I in H 2 ‘ 23 • C omple t e Pr e - cli n ical Studie s A h e a d o f P la n n e d P h a s e I / II • C omple t e P r e - C li n ical Studie s A h e a d of Phase I / II
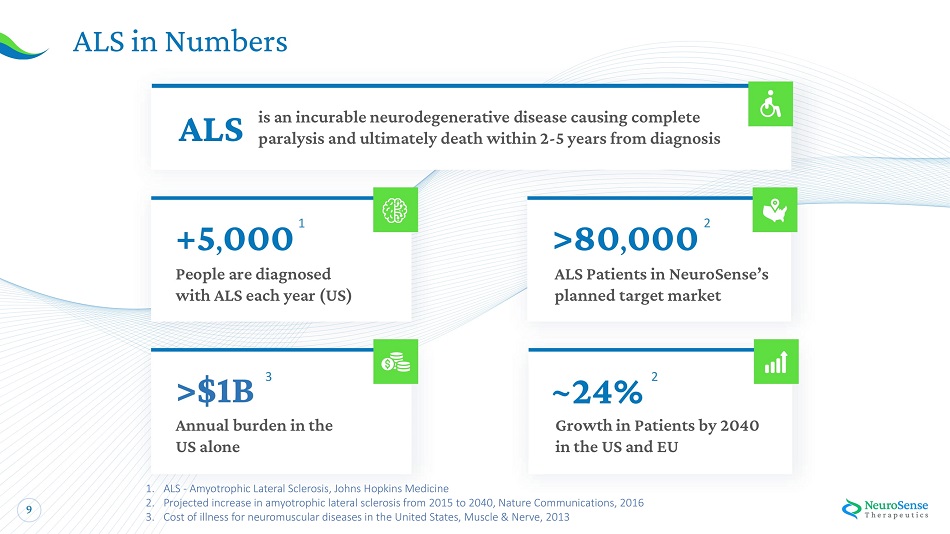
9 AL S in Nu m bers ~24% Gr o wt h i n P a tien ts b y 2 0 40 i n the U S a nd EU ALS i s a n incur a bl e n e urodegen e rative dise a s e ca u sin g c o mpl e te paralysis and ultimately death within 2 - 5 years from diagnosis >$1B A n n u a l burden i n the U S a lone + 5 , 00 0 1 P eo p l e a re d i a gnos ed wit h ALS e a c h y e a r (US) > 80 , 00 0 2 A L S P a tien ts i n Ne u ro S en s e ’ s pl a n n e d target mark e t 2 3 1. ALS - Amyotrophic Lateral Sclerosis, Johns Hopkins Medicine 2. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040, Nature Communications, 2016 3. Cost of illness for neuromuscular diseases in the United States, Muscle & Nerve, 2013

10 The People Behind T he Num b ers Oct. 2016
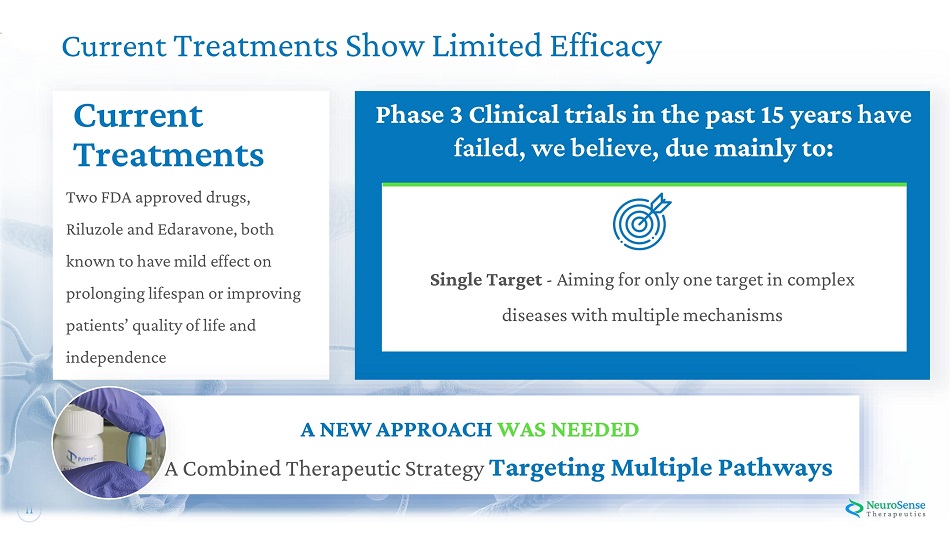
11 Current Treatments Show Limited Efficacy Phase 3 C l ini c a l tri a l s i n th e past 1 5 yea r s have failed , w e belie v e , due mai nl y to: A N E W APPROA C H WA S N E E DED A Combined Therapeutic Strategy Targeting Multiple Pathways Single Target - Aiming for only one target in complex di s e a ses wit h m u l tipl e m e chani s ms Current Treatments Two F D A a p p r o ved dru g s, Ri l u zole a n d Ed a r a v o n e , both k n o w n t o hav e mild eff ect on pro l on g ing l i f es p a n o r i m p r o v i n g patient s ’ qu a l ity o f l i f e a nd independence

12 NeuroSense ' s Lea d Cand i date : P r imeC Two Compoun ds - One Po t ent i al ly Po w erfu l Outcome N e u r oSense ’ s F l a g ship Tr e a t ment A novel formulation , consisting of specific doses of two FDA - approved drugs, Ciprofloxacin & Celecoxib, designed to work synergistically on more than one target : • Re g ul a ti n g microRNA synth esis • Affect i n g ir o n accumul a t ion • Reduci n g neuro i n f l a m m ation 1. Management estimate $ 3. 2 B A n n u a l mark e t potential 1
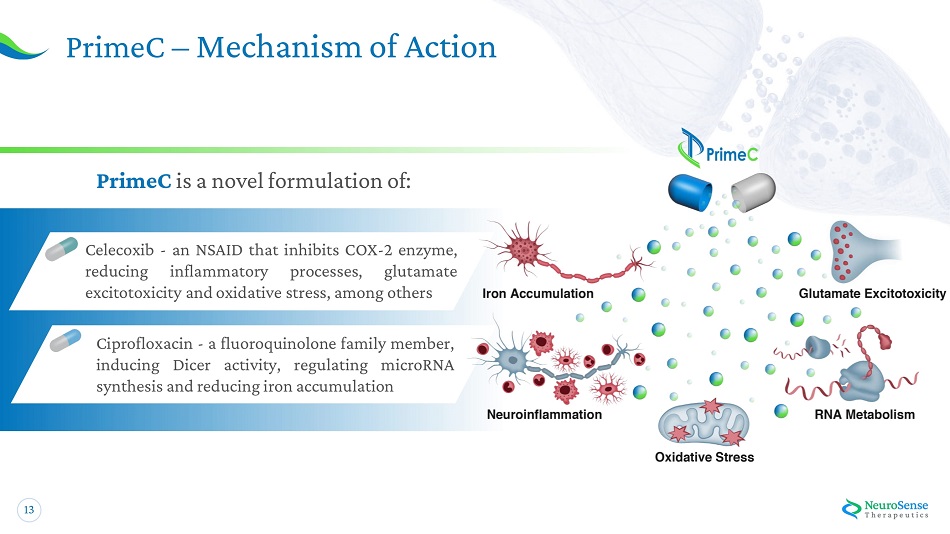
13 Prim e C – Mechan i sm o f A c tion PrimeC is a novel formulation of : Celecoxib - an NSAID that inhibits COX - 2 enzyme, reducing inflammatory processes, glutamate excitotoxicity and oxidative stress, among others Ciprofloxacin - a fluoroquinolone family member, inducing Dicer activity, regulating microRNA synthesis and reducing iron accumulation
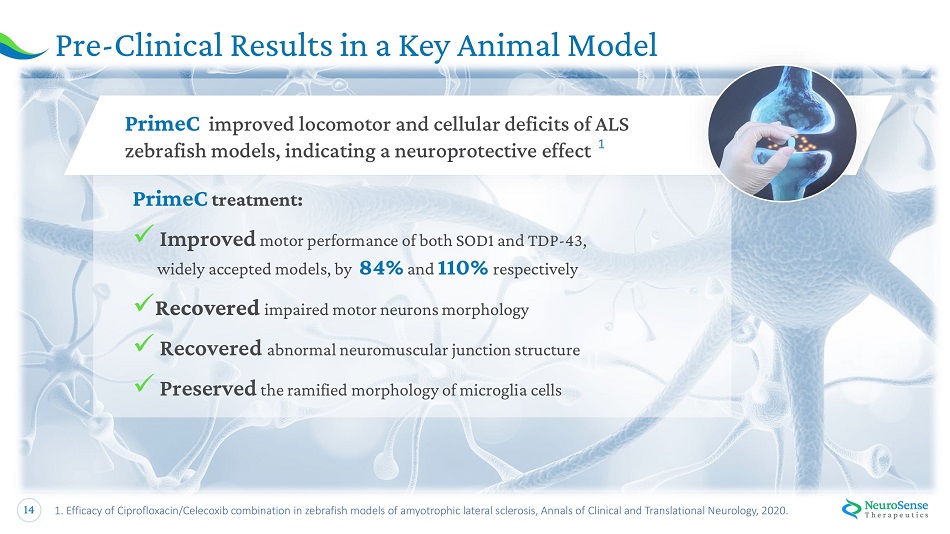
14 P r e - Clinic al Results in a K e y A nima l Model 1. Efficacy of Ciprofloxacin/Celecoxib combination in zebrafish models of amyotrophic lateral sclerosis, Annals of Clinical and Translational Neurology, 2020. Pr i m e C im p rove d loco m o tor and cel l ular def i cits of ALS zebrafish models, indicating a neuroprotective effect 1 Pr i meC tre a t m ent: x Improved motor performance of both SOD1 and TDP - 43, widely accepted models, by 84% and 110% respectively x Recovered impa i r e d motor n e u r o n s morp h ol ogy x Recovered a bnormal n e u r o m u scul a r j u n cti o n str u ct u re x Preserve d t h e r a mifi e d morp h ol og y o f m i c r o g l ia ce ll s
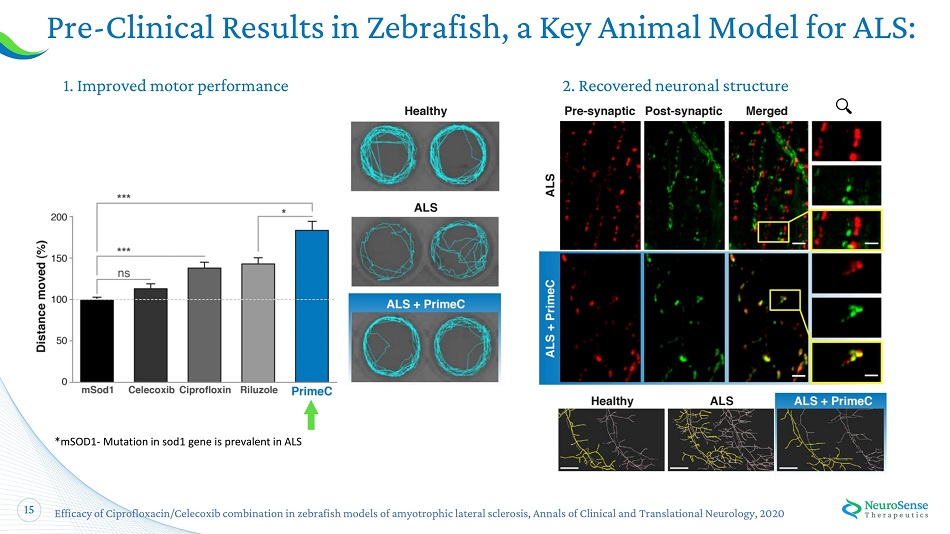
15 Pre - Clinical Results in Zebrafish, a Key Animal Model for ALS: 1 . I m p r o ved mot o r perf o rm a nc e 2 . Rec o ve r e d ne u r o na l struct u re Efficacy of Ciprofloxacin/Celecoxib combination in zebrafish models of amyotrophic lateral sclerosis, Annals of Clinical and Translational Neurology, 2020 *mSOD1 - Mutation in sod1 gene is prevalent in ALS

16 Phase IIa Trial Design P r o f . V i v ia n D r or y Phas e IIa NS T 00 2 : Te l A v i v Sour a s k y Me d i c a l Ce n ter Safety Blood test, electrocardiogram ( E C G), u r e a , vital sig n s, a dv e rs e e v e n ts (AE) Efficacy AL S FR S - R - Revis ed A LS F u n ction a l R a tin g S c a le – 0 - 48 FV C - Forc e d Vit a l Capacity Biomarkers E x amination o f k ey el e me n t s for A LS di a g n osi s a s w e ll a s PrimeC me c h a n ism o f a ction NST002 15 patients , 12 months dosing, clinic visit every 3 month s , p h one vi s it eve ry 1 . 5 months
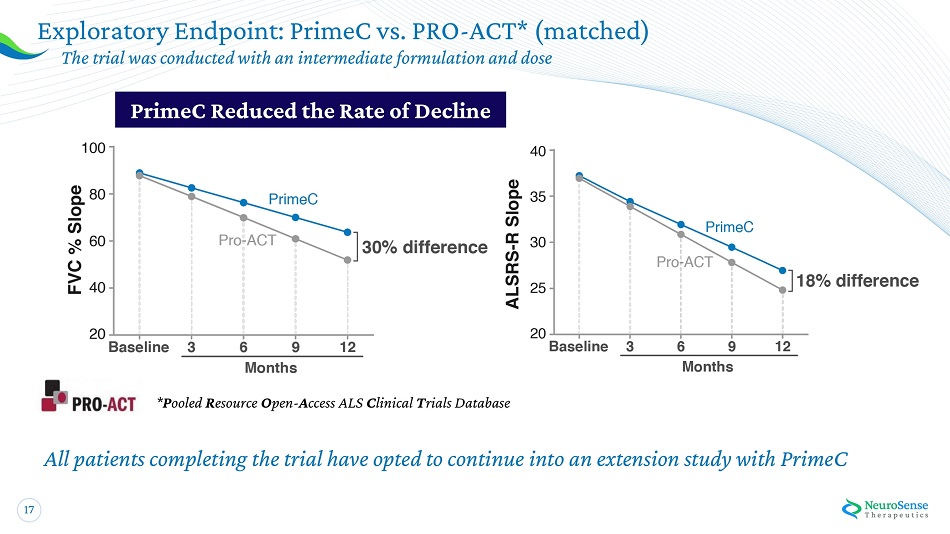
17 * P oo l e d R e so u r ce O pe n - A cc es s ALS C l inical T r ials D ata base All patients completing the trial have opted to continue into an extension study with PrimeC Exploratory Endpoint: PrimeC vs. PRO - ACT* (matched) The tr i al w a s co n d u cted with an i n t erm e diate fo r mu l ation and dose Pr i meC Red u ce d th e Rat e of Decline

18 Phase IIa Clinical Trial: PrimeC intermediate formulation Primary endpoint met, exploratory endpoints showed positive results with PrimeC x Reduced Functional and Respiratory Deterioration x Si g ni f icant changes i n AL S - re l ate d bi o mark e rs x Well Tolerated, No Drug Related SAEs
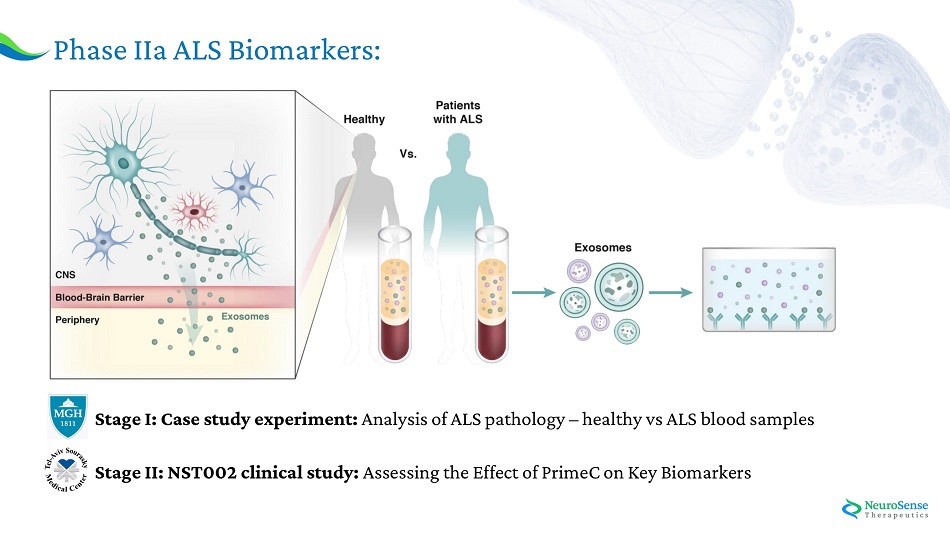
Phase IIa AL S Bioma r ker s : Stage I: Case study experiment: Analysis of ALS pathology – healthy vs ALS blood samples Stage II: NST002 clinical study: Assessing the Effect of PrimeC on Key Biomarkers
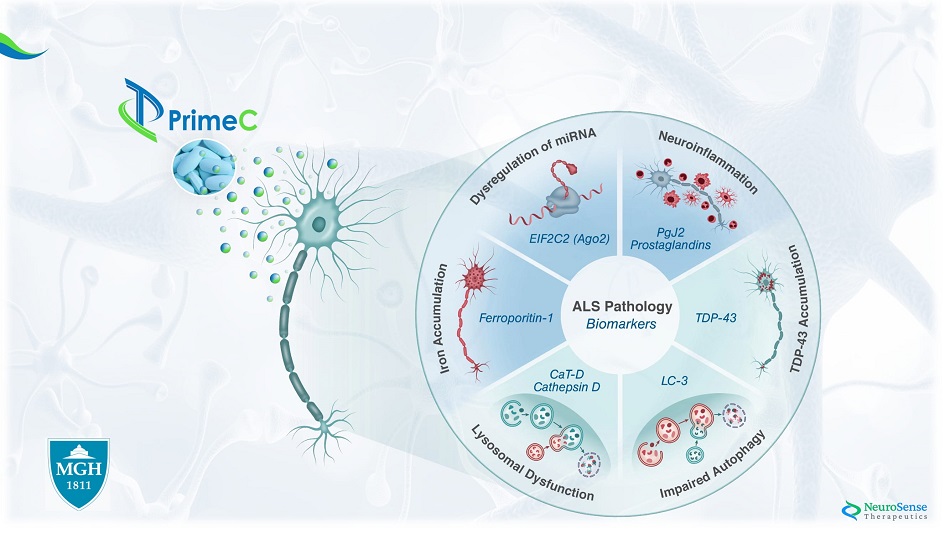
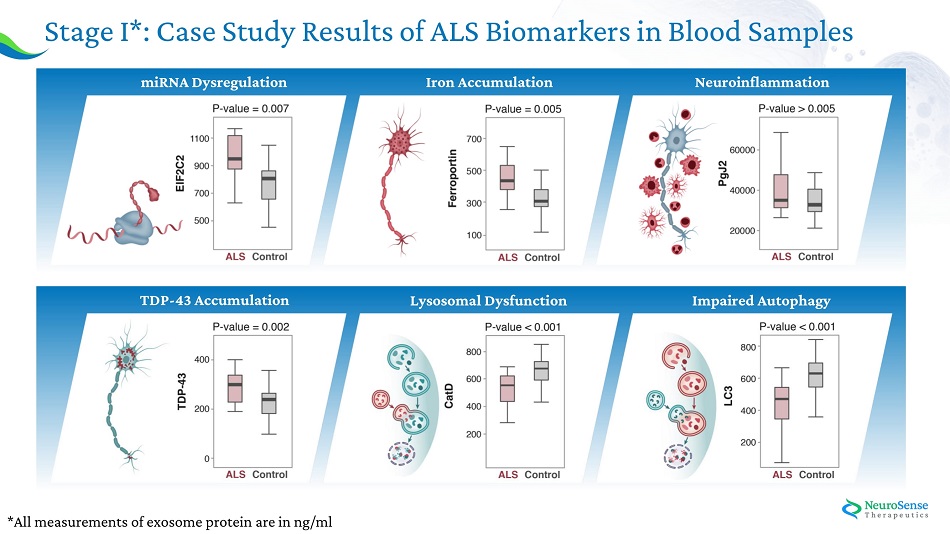
*All measurements of exosome protein are in ng/ml Stage I*: Case Study Results of ALS Biomarkers in Blood Samples Im paired Aut o pha g y L yso s omal D y sfunct ion T D P - 4 3 Acc u m u lat ion m i RN A D y s r egu lat ion Ir o n Acc u m u lat ion Ne u r o infl a mma tion
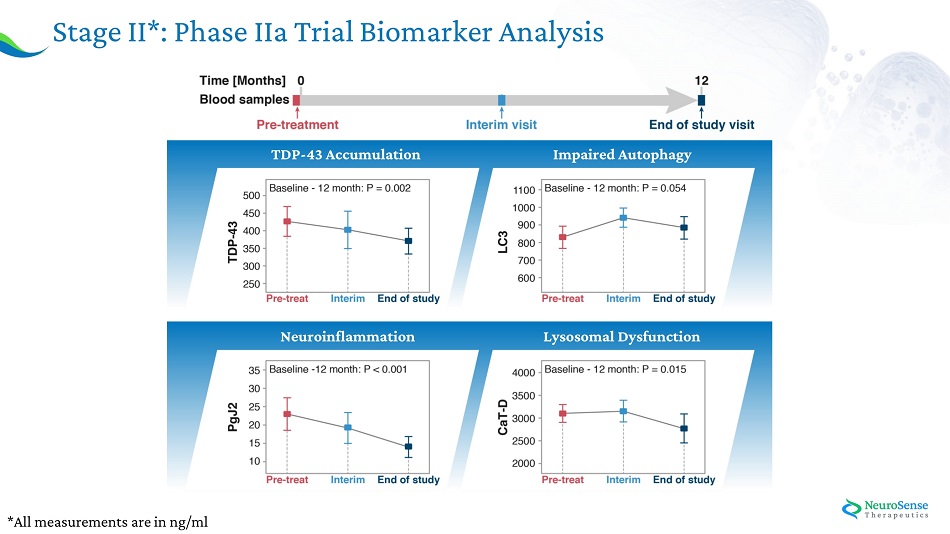
Stage II*: Phase IIa Trial Biomarker Analysis *All measurem e n t s a r e in n g/ml Ne u r o infl a mma tion Im paired Aut o pha g y T D P - 4 3 Acc u m u lat ion L yso s omal D y sfunct ion

23 The biological activity observed in TDP - 43 levels may serve to indicate clinical outcomes, as can be seen from the relative correlation between the reduction in TDP - 43 levels and slower deterioration in ALSFRS - R . Correlation Between Biological Activity and Clinical Outcome
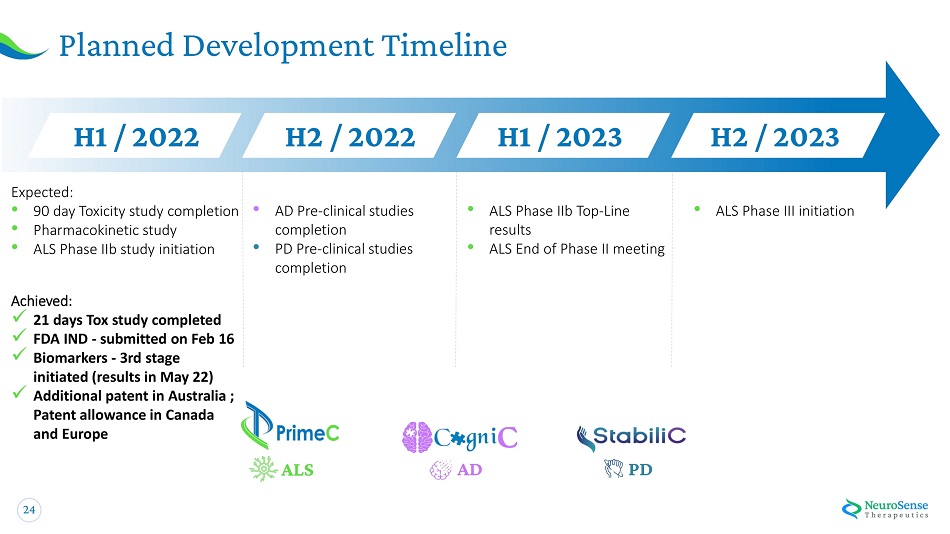
24 Planned Development Timeline • Pharmacokinetic study • ALS Phase IIb study initiation Expected: • 9 0 d a y T o xicity s tudy c o m p le ti o n • • AD Pre - clinical studies completion PD Pre - clinical studies completion • ALS Phase IIb Top - Line results • ALS End of Phase II meeting • ALS Phase III initiation AD PD H 1 / 20 2 2 H 2 / 20 2 2 H 1 / 20 2 3 H 2 / 20 2 3 Achieved: x 21 days Tox study completed x FDA IND - submitted on Feb 16 x Biomarkers - 3rd stage initiated (results in May 22) x Additional patent in Australia ; Patent allowance in Canada and Europe ALS
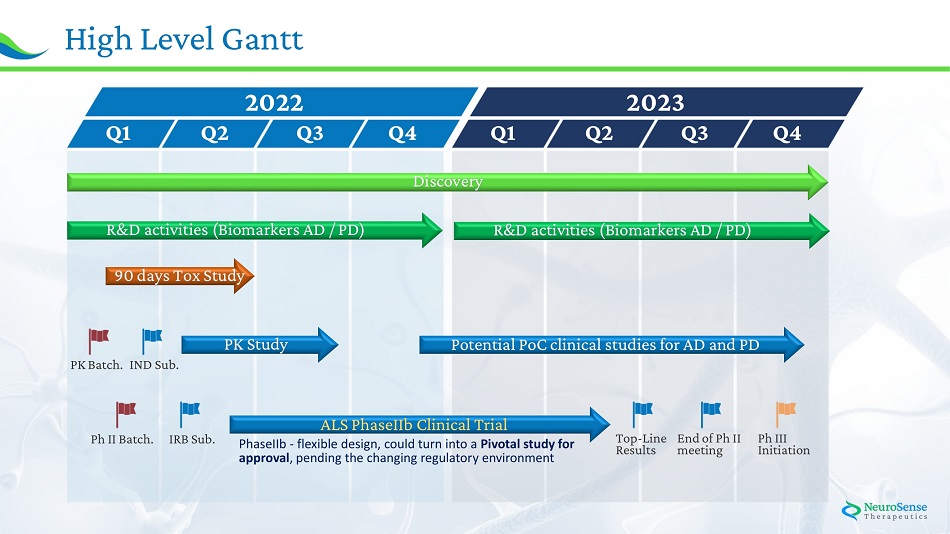
25 Hig h Leve l Gantt Q 1 Q 2 2022 Q 3 Q 4 Q 1 Q 2 2023 Q 3 Q 4 Dis c o ve r y R & D a ctivi tie s (Bi o markers A D / PD) 90 day s T o x St u dy PK Study Po t e n tia l Po C c l inic a l st u die s f o r A D a n d PD PK B a t c h . I N D Su b. I RB Sub. Ph I I Bat c h. T op - L in e E n d o f P h I I Results meeting Ph II I I n i t i ati on AL S P h aseI I b C l inic a l Tri al PhaseIIb - flexible design, could turn into a Pivotal study for approval , pending the changing regulatory environment R & D a ctivi tie s (Bi o markers A D / PD)

26 NRS N – Highlight s Su m mary x Novel formulation addresses multiple targets in a synergistic manner x Promising Phase 2a efficacy results x Statistically significant biomarkers data which corelates to meaningful clinical effect x Patents granted and additional IP coverage (valid till 2038) x Funded expected until completion of Phase 2b ALS study (Q2 2023) x Strong pipeline with short, mid and long term developments in big market indications
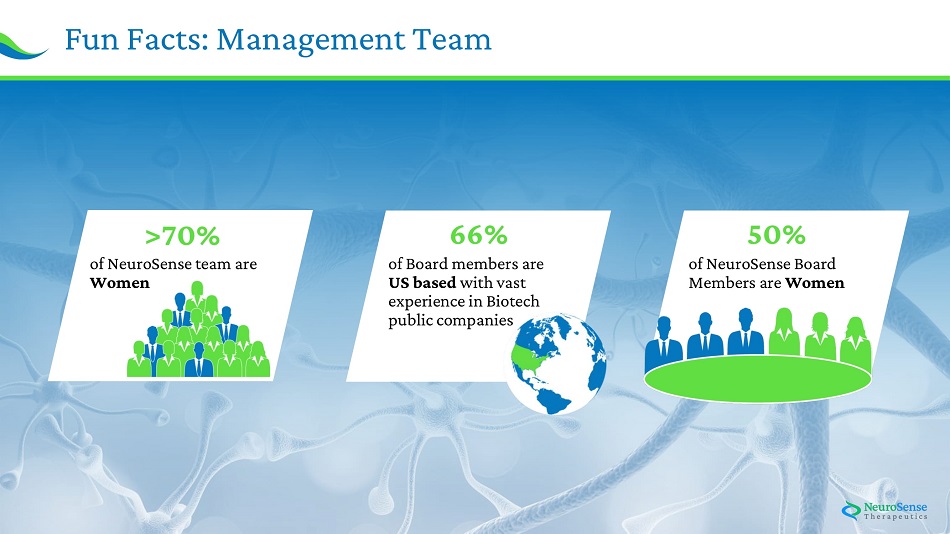
27 Fu n Facts : Managemen t Team 66% o f B o a r d m e mbers a r e US based w i t h v a st experienc e in B i otech p u b l ic c o m p a nies 50% o f N e u ro S e n se B o a rd M e m bers a r e Women < 70% o f N e u r o Sens e t e a m a re Women

28 THAN K YOU! For more information: www.NeuroSense - tx.com i n f o@Neu r oSen s e - tx . c om



























