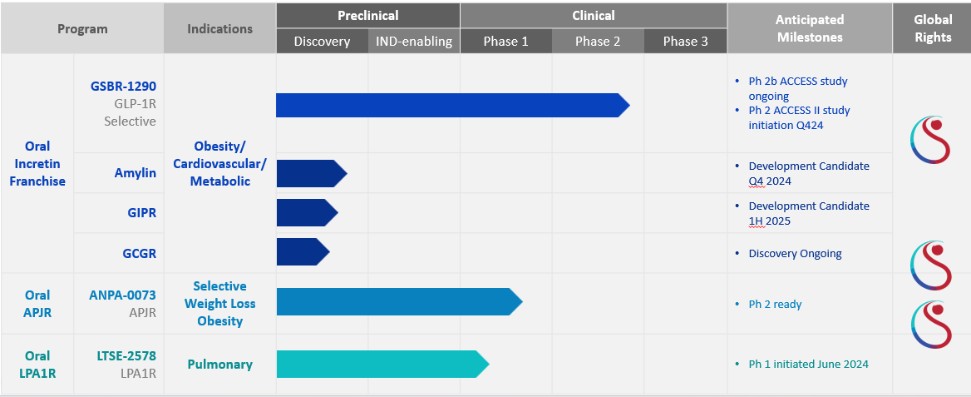
Nordisk, Eli Lilly, AstraZeneca, Sanofi and Kailera Therapeutics, formerly Hercules CM Newco. We are also aware of other GLP-1R plus dual/tri incretin targeting peptides in development by Eli Lilly, Jiangsu Hansoh Pharmaceutical Group, Boehringer Ingelheim, Altimmune, Carmot Therapeutics, Sciwind Biosciences, Novo Nordisk, Viking Therapeutics, Amgen, Merck, Zealand Pharma, D&D Pharmatech, GMAX Biopharma, Jiangsu Hengrui Medicine, BrightGene, Innovent Biologics, PegBio, NeuroBo Pharmaceuticals, Hanmi Phamaceuticals, Progen Holdings, Pep2Tango, Metsera, QL Biopharma, Lexaria Bioscience, Sun Pharmaceutical, Gan & Lee, Innogen and Biomed Industries. Additionally, we are aware of APJR targeted product candidates in development for COVID-19 acute respiratory distress syndrome by CohBar, Inc.; IPF, systemic sclerosis interstitial lung disease, and kidney nephrotic syndrome by Apie Therapeutics; and muscle atrophy by BioAge Labs, Inc. Both Amgen and Bristol Myers Squibb (“BMS”) have APJR targeted product candidates for heart failure. Furthermore, we are aware of LPA1R targeted product candidates in development for IPF by BMS, Horizon Therapeutics (acquired by Amgen in October 2023) and DJS Antibodies; and myelin restoration and neuroinflammation by Pipeline Therapeutics.
Many of our competitors, either alone or with their collaborators, have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than we do. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the timing and scope of marketing approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing products could present superior treatment alternatives, including by being more effective, safer, more convenient, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our product candidates. If we are unable to compete effectively, our opportunity to generate revenue from the sale of our products we may develop, if approved, could be adversely affected.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Any failure to compete effectively could harm our business, financial condition and operating results.
In addition, we and any third-party collaborators are facing increasing competition from companies utilizing AI and other computational approaches for drug discovery. Some of these competitors are involved in drug discovery themselves and/or with partners, and others develop software or as well as other tools utilizing AI which can be used, directly or indirectly, in drug discovery. To the extent these other AI approaches to drug discovery prove to be successful, or more successful, than our and any third-party collaborators’ approach, our business, financial condition and operating results could be adversely affected.
If the market opportunities for any of our product candidates are smaller than we estimate, even assuming approval of a product candidate, our revenue may be adversely affected, and our business may suffer.
The precise incidence and prevalence for all the conditions we aim to address with our product candidates are unknown. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new information may change the estimated incidence or prevalence of these diseases. The total addressable market across all of our product candidates will ultimately depend upon, among other things, the diagnosis criteria included in the final label for each of our product candidates approved for sale for these indications, the availability of alternative treatments and the safety, convenience, cost and efficacy of