UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F/A
(Amendment No. 4)
☒ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended________________________
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report . . . . . . . . . . . . . . . . . . .
For the transition period from_________ to _________
Commission file number: __________________
ADASTRA HOLDINGS LTD.
(Exact name of Registrant as specified in its charter)
Not applicable
(Translation of Registrant's name into English)
British Columbia, Canada
(Jurisdiction of incorporation or organization)
5451 - 275 Street
Langley, British Columbia V4W 3X8
Canada
(Address of principal executive offices)
Michael Forbes
5451 - 275 Street
Langley, British Columbia V4W 3X8, Canada
Telephone: 778-715-5011
Facsimile: 844-874-9893
Michael@adastraholdings.ca
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
Title of each class | Trading
Symbols(s) | Name of each exchange on which registered |
Not Applicable | Not Applicable | Not Applicable |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
Common Shares Without Par Value
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.: Not Applicable
Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report.: Not applicable.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act:
☐ Yes ☒ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☐ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☐ Yes ☒ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☐ Yes ☒ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of "large accelerated filer, "accelerated filer," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated Filer | ☐ | Non-accelerated filer | ☒ |
| | | | Emerging Growth Company | ☐ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP | ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board | ☒ | Other | ☐ |
If "Other" has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☐ No
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This registration statement contains forward-looking statements. Forward-looking statements are projections in respect of future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as "may", "should", "intend", "expect", "plan", "anticipate", "believe", "estimate", "predict", "potential", or "continue" or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, including the risks in the section entitled "Risk Factors", uncertainties and other factors, which may cause our company's or our industry's actual results, levels of activity or performance to be materially different from any future results, levels of activity or performance expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity or performance. Except as required by applicable law, including the securities laws of the United States and Canada, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
ABOUT THIS REGISTRATION STATEMENT
As used in this registration statement, the terms "we", "us" and "our" refer to Adastra Holdings Ltd., a British Columbia corporation, and its direct and indirect subsidiaries, unless otherwise specified.
Our financial statements and other financial information are prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, or "IFRS", in Canadian dollars. None of our consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles, and our financial statements may therefore not be comparable to financial statements of United States companies.
In this registration statement, the terms "dollar", "$" or "CAD$" refer to Canadian dollars and the term, "US$" refers to United States dollars.
The share and per share information in this registration statement, other than in our consolidated financial statements and the notes thereto, or where referred to as "pre-consolidated", reflects a consolidation of our issued and outstanding common shares on the basis of one new common share for three old common shares which was completed on April 9, 2021.
PART I
Item 1. Identity of Directors, Senior Management and Advisers
A. Directors and Senior Management
The following table sets forth the names, business addresses and functions of our directors and senior management:
Name | Business Address | Function |
Michael Forbes | 275 Street, Langley, British Columbia, Canada | As our Chief Executive Officer and Chairman of the board of directors, Mr. Forbes is responsible for strategic planning and operations, as well as managing our relations with our lawyers, regulatory authorities and investor community; as a director, Mr. Forbes supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Forbes is a member of our audit committee. |
Oliver Foeste | 275 Street, Langley, British Columbia, Canada | As our Chief Financial Officer, Mr. Foeste is responsible for the management and supervision of all financial aspects of our business. Mr. Foeste assists in strategic planning, oversees capital planning and capital raising, budgeting, financial reporting and risk management. In performing his duties, Mr. Foeste maintains relationships with our auditors, legal counsel, banks, and other stakeholders. |
Donald Dinsmore | 275 Street, Langley, British Columbia, Canada | As our Chief Operating Officer, Mr. Dinsmore is responsible for the execution of strategic plans and operations management of the business, Mr. Dinsmore assists in strategic planning, supervises production, quality, sales, marketing, and regulatory compliance. In the fulfilment of his duties, he maintains relationships with suppliers, customers, legal counsel, and other stakeholders. As our Corporate Secretary, Mr. Dinsmore is responsible for maintaining our corporate records. As a director, Mr. Dinsmore supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
George Routhier | 759 Highway 511, Perth, Ontario, Canada | As an independent director, Mr. Routhier supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Routhier is a member of our audit committee. |
Paul Morgan | 100 388 Harbour Road, Victoria, British Columbia, Canada | As an independent director, Mr. Morgan supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Morgan is a member of our audit committee. |
B. Advisors
Not applicable.
C. Auditors
Our auditors are Davidson & Company LLP, located at 1200 - 609 Granville Street, Vancouver, British Columbia V7Y 1G6, Canada. Davidson & Company LLP is registered with both the Canadian Public Accountability Board and the U.S. Public Company Accounting Oversight Board.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
A. [Reserved]
B. Capitalization and Indebtedness
The table below sets forth our capitalization and indebtedness as of September 30, 2021. You should read this table in conjunction with our audited and unaudited consolidated financial statements, together with the accompanying notes and the other information appearing under the heading "Item 5. Operating and Financial Review and Prospects".
| September 30, 2021 (Unaudited) $ |
Cash | 1,207,688 |
Total Current Assets | 4,867,598 |
Total Assets | 40,638,028 |
Current Liabilities (Unsecured) | 1,884,738 |
Current Liabilities (Secured) | 3,498,728 |
Non-Current Liabilities (Unsecured) | 84,240 |
Non-Current Liabilities (Secured) | - |
Share Capital | 41,833,128 |
Reserves | 5,461,270 |
Subscriptions received | 135,000 |
Deficit | (12,259,076) |
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
An investment in our common shares involves a number of very significant risks. You should carefully consider the following risks and uncertainties in addition to other information in this registration statement in evaluating our company and our business before purchasing our common shares. Our business, operating results and financial condition could be seriously harmed due to any of the following risks. The risks described below are not the only ones facing our company. Additional risks not presently known to us may also impair our business operations. You could lose all or part of your investment due to any of these risks.
Risks Related to Our Business
Our activities are subject to regulation by governmental authorities.
Our activities are subject to regulation by governmental authorities. Achievement of our business objectives are contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all regulatory approvals, where necessary, for the sale of our products and services. We cannot predict the time required to secure all appropriate regulatory approvals for our products and services, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and services and could have a material adverse effect on our business, results of operations and financial condition.
Doing business in the cannabis industry leaves our company subject to possible regulatory risks.
The cannabis industry is a new industry which is highly regulated, highly competitive and evolving rapidly and psychedelics are illegal substances other than when used for scientific or medical purposes. As such, new risks may emerge, and management may not be able to predict all such risks or be able to predict how such risks may result in actual results differing from the results contained in any forward-looking statements.
Psychedelics are Schedule III substances under the Controlled Drugs and Substances Act for which the extraction of raw materials from, or involvement in production activities are regulated federally in Canada. In order to develop our business and undertake research and development, the Company is applying for a dealer license for psychedelics which, if obtained, will allow the Company to possess and formulate psychedelics. While we anticipate being successful in this application, there is no guarantee that we will obtain a dealer license to possess and formulate psychedelics. If we are successful in this application there remain uncertainties with respect to how government regulations will impact our business with respect to psychedelics. The Company is in the process of assessing this impact. At present a dealer license holder can only trade with another individual who has the same license. Their activities are limited in trade and they cannot provide substances to individuals or companies as Health Canada has yet to approve psychedelics as a drug and it is currently illegal to possess psychedelics in Canada without a dealer license or other exemption. We are confident that our application will be successful, however, if in the unlikley event we are unable to obtain a dealer license, this will serverly limit our ability to undertake research and development in psychedelics.
These industries are subject to extensive controls and regulations, which may significantly affect the financial condition of market participants. The plain packaging requirements and restrictions on promotion of cannabis and the restrictions on promotion of illegal substances in Canada may limit the ability to effectively advertise and promote our products and business. The marketability of any product may also be affected by numerous factors that are beyond the control of the investee companies and which cannot be predicted, such as changes to government regulations, including those relating to taxes and other government levies which may be imposed. Changes in government levies, including taxes, could reduce the investee companies' earnings and could make future capital investments or the investee companies' operations uneconomic. The cannabis industry is also subject to numerous legal challenges, which may significantly affect the financial condition of market participants, and which cannot be reliably predicted.
The impact of various legislative regimes on our business plans and operations is uncertain. There is no guarantee that the applicable legislation regulating the manufacture, distribution, sale and promotion of cannabis will create or allow for the growth opportunities we currently anticipate.
Acquisitions or other consolidating transactions in our industry could have adverse effects on our company.
Acquisitions or other consolidating transactions in our industry could have adverse effects on our company. We could lose strategic relationships if our partners are acquired by or enter into agreements with a competitor, causing us to lose access to distribution, content and other resources. The relationships between our company and our strategic partners may deteriorate and cause an adverse effect on the business. We could lose customers if competitors or user of competing technology consolidate with our current or potential customers. Furthermore, our current competitors could become larger players in the market or new competitors could form from consolidations. Any of the foregoing events could put our company at a competitive disadvantage, which could cause our company to lose customers, revenue, and market share. Consolidation in the industry could also force our company to divert greater resources to meet new or additional competitive threats, which could harm our operating results.
COVID-19 Pandemic has and will continue to affect our business.
COVID-19 outbreak continues to rapidly evolve and is causing business disruptions across the entire global economy and society. We have taken various measures to prioritize the health and safety of our employees, customers and partners, including restricted work travel and site access; improved safety & hygiene; and the requirement of nonessential staff members to work remotely. As a manufacturer of consumable and medicinal products, our practice is always to operate to global pharma-quality standards to the best of our abilities with strict hygiene practices and mandated personal protective equipment. The extent of the impact on COVID-19 on our operational and financial performance will depend on various developments, including the duration and magnitude of the outbreak, and the impact on customers, employees and vendors, all of which are uncertain and cannot be predicted at this point.
We could have issues of conflicts of interest with our directors and officers.
Certain of the directors and officers of our company are also directors and officers of other companies, and conflicts of interest may arise between their duties as officers and directors of our company and as officers and directors of such other companies.
Global economic conditions could materially adversely impact demand for our products and services.
Our operations and performance depend significantly on economic conditions. The COVID-19 global pandemic and resulting government health regulations have resulted in significant reductions in global economic output and have negatively impacted global economic conditions. The ultimate impact and duration of current negative global economic conditions are highly uncertain. Uncertainty about global economic conditions could result in customers postponing purchases of our products and services in response to tighter credit, unemployment, negative financial news and/or declines in income or asset values and other macroeconomic factors, which could have a material negative effect on demand for our products and services and, accordingly, on our business, results of operations or financial condition.
We have ongoing need for financing.
Our ability to continue operations will be largely reliant on our continued attractiveness to equity investors. We are expected to incur operating losses as we continue to expend funds to develop our business operations. Our continued development will require substantial additional financing. The failure to raise such capital could result in the delay or indefinite postponement of current business objectives or the going out of business. The primary source of funding available to our company will consist of equity financing. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favorable. In addition, from time to time, we may enter into transactions to acquire assets or the shares of other corporations. These transactions may be financed wholly or partially with debt, which may temporarily increase our debt levels above industry standards.
We lack business diversification.
The prospects for our success are dependent upon the future performance and market acceptance of our facilities, products, processes, and services. Unlike certain entities that have the resources to develop and explore numerous product lines, operating in multiple industries or multiple areas of a single industry, we do not have the ability to immediately diversify or benefit from the possible spreading of risks or offsetting of losses. Again, the prospects for our success are dependent upon the development or market acceptance of a very limited number of facilities, products, processes or services.
We may encounter issues with managing growth.
Any expansion of our business may place a significant strain on our financial, operational and managerial resources. There can be no assurance that we will be able to implement and subsequently improve our operations and financial systems successfully and in a timely manner in order to manage any growth we experience. There can be no assurance that we will be able to manage growth successfully. Any ability of our company to manage growth successfully could have a material adverse effect on our business, financial condition and results of operations.
We rely on key consultants.
There can be no assurance that any of our consultants will remain with our company or that, in the future, they will not organize competitive businesses or accept opportunities with companies competitive with us. We depend on a number of key officers and directors the loss of any one of whom could have an adverse effect on our company.
We may become subject to litigation.
We may be forced to litigate, enforce, or defend our intellectual property rights, protect our trade secrets, or determine the validity and scope of other parties' proprietary rights. Such litigation would be a drain on the financial and management resources of our company which may affect our operations and business. Furthermore, because the content of most of our intellectual property concerns cannabis and other activities that are not legal in some state jurisdictions, we may face additional difficulties in defending our intellectual property rights. We may become party to litigation from time to time in the ordinary course of business which could adversely affect our business. Should any litigation in which we become involved in be determined against our company such a decision could adversely affect our ability to continue operating and the market price for our common shares and could use significant resources. Even if we are involved in litigation and wins, litigation can redirect significant company resources.
We may be subject to product recalls.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labelling disclosure. If any of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. We may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention. Although we have detailed procedures in place for testing our products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if we are subject to a recall, the image of our company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for our products and could have a material adverse effect on our results of operations and financial condition. Additionally, product recalls may lead to increased scrutiny of our operations by regulatory agencies, requiring further management attention, potential loss of applicable licenses and potential legal fees and other expenses.
We could have issues with product liability due to the nature of the Company's products.
As a distributor of products designed to be ingested by humans, our company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused significant loss or injury. In addition, the sale of our products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of our products alone or in combination with other medications or substances could occur. We may be subject to various product liability claims, including, among others, that our products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against our company could result in increased costs, could adversely affect our company's reputation with clients and consumers generally, and could have a material adverse effect on our business, prospects, financial condition and results of operations. There can be no assurances that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could have a material adverse effect on our business, prospects, financial condition and results of operations.
We rely on third party relationships.
We are dependent on several third-party relationships and their related costs, including raw materials and supplies related to their growing operations, as well as electricity, water and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain from third-party relationships could materially impact our financial condition and operating results. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on our business, financial condition and operating results.
The third parties with which we do business may perceive that they are exposed to reputational risks as a result of our cannabis business activities, Failure to establish or maintain business relationships could have a material adverse effect on our company.
Our advertising and promotional expenditures may not be effective or efficient.
Our future growth and profitability will depend on the effectiveness and efficiency of advertising and promotional expenditures, including our ability to (i) create greater awareness of its products; (ii) determine the appropriate creative message and media mix for future advertising expenditures; and (iii) effectively manage advertising and promotional costs in order to maintain acceptable operating margins. There can be no assurance that advertising and promotional expenditures will result in revenues in the future or will generate awareness of our technologies or services. In addition, no assurance can be given that we will be able to manage our advertising and promotional expenditures on a cost-effective basis.
Being a public company requires significant obligations.
Our Company incurs significant legal, accounting, insurance and other expenses as a result of being a public Company, which may negatively impact our performance and could cause our results of operations and financial condition to suffer. Compliance with applicable securities laws in Canada and the rules and policies of the Canadian Securities Exchange constitutes a significant expense, including legal and accounting costs, and makes some activities more time-consuming and costly. Reporting obligations as a public company and our anticipated growth may place a strain on our financial and management systems, processes and controls, as well as on personnel.
Our company may rely on intellectual property.
Our success may depend in part on the ability to protect ideas and technology. Even if we move to protect technology with trademarks, patents, copyrights or by other means, we are not assured that competitors will not develop similar technology, business methods or that they will be able to exercise their legal rights. Other countries may not protect intellectual property rights to the same standards as does Canada. Actions taken to protect or preserve intellectual property rights may require significant financial and other resources such that said actions have a meaningfully impact the ability to successfully grow our business.
Fraudulent or illegal activity could occur within our company.
Our Company is exposed to the risk that its employees, independent contractors and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to our company that violates: (i) government regulations; (ii) manufacturing standards; (iii) federal and provincial healthcare fraud and abuse laws and regulations; or (iv) laws that require the true, complete and accurate reporting of financial information or data. It is not always possible for our company to identify and deter misconduct by its employees and other third parties, and the precautions taken by our company to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting our company from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against our company, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of the Issuer's operations, any of which could have a material adverse effect on our business, financial condition and results of operations.
We cannot ensure product viability.
If the products our company sells are not perceived to have the effects intended by the end user, our business may suffer. Many of our products contain innovative ingredients or combinations of ingredients. There is little long-term data with respect to efficacy, unknown side effects and/or interaction with individual human biochemistry. Moreover, there is little long-term data with respect to efficacy, unknown side effects and/or its interaction with individual animal biochemistry. As a result, our products could have certain side effects if not taken as directed or if taken by an end user that has certain known or unknown medical conditions.
We rely on the success of our quality control systems.
The quality and safety of our products are critical to the success of our business and operations. As such, it is imperative that our (and our service provider's) quality control systems operate effectively and successfully. Quality control systems can be negatively impacted by the design of the quality control systems, the quality training program, and adherence by employees to quality control guidelines. Although we strive to ensure that all of our service providers have implemented and adhere to high caliber quality control systems, any significant failure or deterioration of such quality control systems could have a material adverse effect on our business and operating results.
The cannabis industry and market are relatively new in Canada and this industry and market may not continue to exist or grow as anticipated.
Our company operates its business in a relatively new industry and market. In addition to being subject to general business risks, we must continue to build brand awareness in this industry and market through significant investments in its strategy, its production capacity, quality assurance and compliance with regulations. In addition, there is no assurance that the industry and market will continue to exist and grow as currently estimated or anticipated or function and evolve in the manner consistent with management's expectations and assumptions. Any event or circumstance that adversely affects the cannabis industry and market could have a material adverse effect of our business, financial conditions and results of operations.
The cannabis industry is difficult to quantify, and investors will be reliant on their own estimates of the accuracy of market data.
Because the cannabis industry is in an emerging stage with uncertain boundaries, there is a lack of information about comparable companies available for potential investors to review in deciding about whether to invest in our company and, few, if any, established companies whose business model our company can follow or upon whose success our company can build. Accordingly, investors will have to rely on their own estimates in deciding about whether to invest in our company. There can be no assurance that our estimates are accurate or that the market size is sufficiently large for our business to grow as projected, which may negatively impact our financial results.
The cannabis industry is very competitive.
The marijuana production industry is competitive in all of its phases. We face strong competition from other companies in connection with such matters. Many of these companies have greater financial resources, operational experience and technical capabilities than us. As a result of this competition, we may be unable to maintain our operations or develop them as currently proposed, on terms we consider acceptable or at all. Consequently, the revenues, operations and financial condition of our company could be materially adversely affected.
Because of early stage of the industry in which we operate, we may face additional competition from new entrants. If the number of users of marijuana in Canada increases, the demand for products will increase and we expect that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, we will require a continued high level of investment in research and development, marketing, sales and client support. We may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis which could materially and adversely affect the business, financial condition and results of operations.
Consolidation that may intensify competition.
The cannabis industry is undergoing rapid growth and substantial change, which has resulted in an increase in competitors, consolidation and formation of strategic relationships. Acquisitions or other consolidating transactions could harm our company in a number of ways, including by losing strategic partners if they are acquired by or enter into relationships with a competitor, losing customers, revenue and market share, or forcing our company to expend greater resources to meet new or additional competitive threats, all of which could harm our operating results.
The processing of cannabis includes risks inherent in an agricultural business including the risk of crop loss, sudden changes in environmental conditions, equipment failure, product recalls and others.
Our business will be subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks. There can be no assurance that natural elements will not have a material adverse effect on our products.
The cannabis industry could be subject to negative customer perception.
We believe the cannabis industry is highly dependent upon consumer perception regarding the medical benefits, safety, efficacy and quality of the cannabis distributed for medical purposes to such consumers. Consumer perception can be significantly influenced by scientific research or findings, regulatory investigations, litigation, political statements both in Canada and in other countries, media attention and other publicity (whether or not accurate or with merit) regarding the consumption of cannabis products for medical or recreational purposes, including unexpected safety or efficacy concerns. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for cannabis and the business, results of operations and financial condition of our company. Our dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity (whether or not accurate or with merit), could have an adverse effect on any demand for our services and products which could have a material adverse effect on our business, financial condition and results of operations. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis for medical purposes in general, or associating the consumption of cannabis with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers' failure to consume such products legally, appropriately or as directed.
The mushroom and mushroom-derived products could be subject to negative customer perception.
We are dependent upon consumer perception of mushrooms and mushroom-derived products. The public may associate our mushrooms and mushroom-derived products with illegal psychoactive mushrooms, which are prohibited substances, which may negatively impact our future revenues once we obtain the necessary licensing.
General healthcare regulation may affect our businesses.
Healthcare service providers in Canada are subject to various governmental regulation and licensing requirements and, as a result, our businesses operate in an environment in which government regulations and funding play a key role. The level of government funding directly reflects government policy related to healthcare spending, and decisions can be made regarding such funding that are largely beyond the businesses' control. Any change in governmental regulation, delisting of services, and licensing requirements relating to healthcare services, or their interpretation and application, could adversely affect the business, financial condition and results of operations of these business units. In addition, we could incur significant costs in the course of complying with any changes in the regulatory regime. Non‐compliance with any existing or proposed laws or regulations could result in audits, civil or regulatory proceedings, fines, penalties, injunctions, recalls or seizures, any of which could adversely affect the reputation, operations or financial performance of our company.
We rely on physicians and other healthcare professionals.
We rely on the availability of physicians and other healthcare professionals to provide services at our facilities. If physicians and other healthcare professionals were unable or unwilling to provide these services in the future due to any sort of reason, this would cause interruptions in our business until mitigated accordingly. As such, vacancies and disabilities relating to our current medical staff may cause interruptions in our business and result in lower revenues.
As we expand our operations, we may encounter difficulty in securing the necessary professional medical and skilled support staff to support our expanding operations. There is currently a shortage of certain medical physicians in Canada and this may affect our ability to hire physicians and other healthcare practitioners in adequate numbers to support our growth plans, which may adversely affect the business, financial condition and results of operations.
Security breaches and other disruptions to our information technology networks and systems could substantially interfere with our operations and could compromise the confidentiality of our proprietary information.
We rely upon information technology systems and networks, some of which are managed by third parties, to process, transmit and store electronic information, and to manage or support a variety of business processes and activities, including supply chain management, manufacturing, invoicing and collection of payments from our customers. Additionally, we collect and store sensitive data, including intellectual property, proprietary business information, the proprietary business information of our suppliers, as well as personally identifiable information of our employees, in data centers and on information technology systems. The secure operation of these information technology systems, and the processing and maintenance of this information, is critical to our business operations and strategy. Despite security measures and business continuity plans, our information technology systems and networks may be vulnerable to damage, disruptions or shutdowns due to attacks by hackers or breaches due to errors or malfeasance by employees, contractors and others who have access to our networks and systems, or other disruptions during the process of upgrading or replacing computer software or hardware, hardware failures, software errors, third-party service provider outages, power outages, computer viruses, telecommunication or utility failures or natural disasters or other catastrophic events. The occurrence of any of these events could compromise our systems and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability or regulatory penalties under laws protecting the privacy of personal information, disrupt operations and reduce the competitive advantage we hope to derive from our investment in technology. Our insurance coverage may not be available or adequate to cover all the costs related to significant security attacks or disruptions resulting from such attacks.
If consumers elect to produce cannabis for their own purposes under the cannabis regulations it could reduce the addressable market for our products.
Under the Cannabis regulations, three options are available for an individual to obtain cannabis for medical purposes: (i) registering with a holder of a license to sell for medical purposes and purchasing products from that entity; (ii) register with Health Canada to produce a limited amount of cannabis for their own medical purposes; or (iii) designate someone else to produce cannabis for them. In addition, the Cannabis Act (as defined herein) permits households to grow a maximum of four cannabis plants for recreational purposes. Subject to further regulation by provincial and municipal governments it is possible that production of cannabis by consumers for medical or recreational purposes could significantly reduce the addressable market for our products and could have a material adverse effect on our business, financial condition or results of operations.
We face competition from illicit dispensaries and the illicit market.
We also face competition from illicit dispensaries and the illicit market more broadly. Participants in these markets are unlicensed and unregulated. They may be selling cannabis and cannabis products with higher concentrations of active ingredients and using classes of cannabis that are not yet regulated for sale in Canada. Various Canadian cities have seen an influx in the number of illicit dispensaries. Any inability or unwillingness of law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation and sale of cannabis and cannabis products could result in the perpetuation of the illicit market for cannabis and/or have a material adverse effect on the perception of cannabis use. Any or all these events could have a material adverse effect on our business, prospects, financial condition and results of operations.
Risks Related to Our Company
We may need to raise additional funds to support our business operations or to finance future acquisitions, including through the issuance of equity or debt securities, which could have a material adverse effect on our ability to grow our business.
If we do not generate sufficient cash from operations or do not otherwise have sufficient cash and cash equivalents to support our business operations or to finance future acquisitions, we may need to raise additional capital through the issuance of debt or equity securities. We may not be able to raise cash in future financing on terms acceptable to us, or at all.
Financings, if available, may be on terms that are dilutive to our shareholders, and the prices at which new investors would be willing to purchase our securities may be lower than the current price of our common shares. The holders of new securities may also receive rights, preferences or privileges that are senior to those of existing holders of our common shares. If new sources of financing are required but are insufficient or unavailable, we would be required to modify our plans to the extent of available funding, which could harm our ability to grow our business.
The report of our independent registered public accounting firm for the year ended December 31, 2020 includes a "going concern" explanatory paragraph.
The report of our independent registered public accounting firm on our consolidated financial statements as of and for the year ended December 31, 2020 includes an explanatory paragraph indicating that there is substantial doubt about our ability to continue as a going concern. If we are unable to raise sufficient capital when needed, our business, financial condition and results of operations will be materially and adversely affected, and we will need to significantly modify our operational plans to continue as a going concern. If we are unable to continue as a going concern, we might have to liquidate our assets and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our consolidated financial statements. The inclusion of a going concern explanatory paragraph by our auditors, our lack of cash resources and our potential inability to continue as a going concern may materially adversely affect our share price and our ability to raise new capital or to enter into critical contractual relations with third parties.
We have no operating experience as a publicly traded company in the U.S.
We have no operating experience as a publicly traded company in the U.S. Although the individuals who now constitute our management team have experience managing a publicly traded company, there is no assurance that the past experience of our management team will be sufficient to operate our company as a publicly traded company in the United States, including timely compliance with the disclosure requirements of the Securities and Exchange Commission. These requirements will place significant strain on our management team, infrastructure and other resources. In addition, our management team may not be able to successfully or efficiently manage our company as a U.S. public reporting company that is subject to significant regulatory oversight and reporting obligations.
We lack operating history.
We have only started to carry on our current business since 2019. We are therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources. Our failure to meet any of these conditions could have a materially adverse effect on our company and may force us to reduce, curtail, or discontinue operations. There is no assurance that we will be successful in achieving a return on shareholders' investment and the likelihood of success must be considered in light of the early stage of operations. We may not successfully address all of the risks and uncertainties or successfully implement our existing and new products and services. If we fail to do so, it could materially harm our business and impair the value of our common shares, resulting in a loss to shareholders. Even if we accomplish these objectives, we may not generate the anticipated positive cash flows or profits. No assurance can be given that we can or will ever be successful in our operations and operate profitably.
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of British Columbia, Canada. Some of our directors and officers, and the experts named in this registration statement, are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, it may be difficult for holders of our common shares who reside in the United States to effect service within the United States upon our directors and officers who are not residents of the United States. It may also be difficult for holders of our common shares who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors and officers under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or our directors or officers predicated upon the civil liability provisions of the United States federal securities laws or the securities or "blue sky" laws of any state within the United States or (ii) would enforce, in original actions, liabilities against us or our directors or officers predicated upon the United States federal securities laws or any such state securities or "blue sky" laws.
As a foreign private issuer, we are not subject to certain United States securities law disclosure requirements that apply to a domestic United States issuer, which may limit the information that would be publicly available to our shareholders.
As a foreign private issuer, we will be exempt from certain rules under the Securities Exchange Act of 1934 that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Securities Exchange Act of 1934. In addition, our officers, directors and principal shareholders will be exempt from the reporting and "short-swing" profit recovery provisions of Section 16 of the Securities Exchange Act of 1934. Moreover, we are not required to file periodic reports and financial statements with the Securities and Exchange Commission as frequently or as promptly as a company that files as a U.S. domestic issuer whose securities are registered under the Securities Exchange Act of 1934, nor are we generally required to comply with the Securities and Exchange Commission's Regulation FD, which restricts the selective disclosure of material non-public information. For as long as we are a "foreign private issuer" we intend to file our annual financial statements on Form 20-F and furnish our quarterly interim financial statements on Form 6-K to the Securities and Exchange Commission. However, the information we file or furnish is not the same as the information that is required in annual and quarterly reports on Form 10-K or Form 10-Q for U.S. domestic issuers. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a U.S. domestic issuer.
Risks Related to Our Common Shares
Because we can issue additional common shares, our shareholders may experience dilution in the future.
We are authorized to issue an unlimited number of common shares without par value. Our board of directors has the authority to cause us to issue additional common shares without consent of our shareholders. The issuance of any such securities may result in a reduction of the book value or market price of our common shares. Given the fact that we have not achieved profitability or generated positive cash flow historically, and we operate in a capital-intensive industry with significant working capital requirements, we may be required to issue additional common equity or securities that are dilutive to existing common shares in the future in order to continue its operations. Our efforts to fund our intended business plan may result in dilution to existing shareholders. Further, any such issuances could result in a change of control or a reduction in the market price for our common shares.
Volatility in our common share price may subject us to securities litigation.
The market for our common shares may have, when compared to seasoned issuers, significant price volatility, and we expect that our share price may continue to be more volatile than that of a seasoned issuer for the foreseeable future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management's attention and resources away from the day-to-day business operations.
The market price of our common shares may be volatile and may fluctuate in a way that is disproportionate to our operating performance.
Our common shares are listed on the Canadian Securities Exchange. Trading of shares on the Canadian Securities Exchange is often characterized by wide fluctuations in trading prices, due to many factors that may have little to do with our operations or business prospects.
The volume of trading in our common shares has been low and the share price has fluctuated significantly. This volatility could depress the market price of our common shares for reasons unrelated to operating performance. The market price of our common shares could decline due to the impact of any of the following factors upon the market price of our common shares:
● sales or potential sales of substantial amounts of our common shares;
● announcements about us or about our competitors;
● litigation and other developments relating to our company or those of our suppliers or our competitors;
● conditions in the automobile industry;
● governmental regulation and legislation;
● variations in our anticipated or actual operating results;
● change in securities analysts' estimates of our performance, or our failure to meet analysts' expectations;
● change in general economic conditions or trends;
● changes in capital market conditions or in the level of interest rates; and
● investor perception of our industry or our prospects.
Many of these factors are beyond our control. The stock markets in general, and the market price of shares of companies in the cannabis industries, have historically experienced extreme price and volume fluctuations. These fluctuations often have been unrelated or disproportionate to the operating performance of these companies. These broad market and industry factors could reduce the market price of our common shares, regardless of our actual operating performance.
A prolonged and substantial decline in the price of our common shares could affect our ability to raise further working capital, thereby adversely impacting our ability to continue operations.
A prolonged and substantial decline in the price of our common shares could result in a reduction in the liquidity of our common shares and a reduction in our ability to raise capital. Because we plan to acquire a significant portion of the funds, we need in order to conduct our planned operations through the sale of equity securities, a decline in the price of our common shares could be detrimental to our liquidity and our operations because the decline may cause investors not to choose to invest in our shares. If we are unable to raise the funds, we require for all our planned operations and to meet our existing and future financial obligations, we may be forced to reallocate funds from other planned uses and may suffer a significant negative effect on our business plan and operations, including our ability to develop new products and continue our current operations. As a result, our business may suffer, and we may go out of business.
Because we do not intend to pay any cash dividends on our common shares in the near future, our shareholders will not be able to receive a return on their shares unless they sell them.
We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common shares in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of our board of directors, and will depend upon, among other things, the results of operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend. Unless we pay dividends, our shareholders will not be able to receive a return on their shares unless they sell them.
Our common shares are subject to penny stock rules. Trading of our common shares may be restricted by the Securities and Exchange Commission's penny stock regulations, which may limit a shareholder's ability to buy and sell our common shares.
Our common shares are penny stock. The Securities and Exchange Commission has adopted Rule 15g-9 which generally defines "penny stock" to be any equity security that has a market price (as defined) less than US$5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and "accredited investors". The term "accredited investor" refers generally to institutions with assets in excess of US$5,000,000 or individuals with a net worth in excess of US$1,000,000 or annual income exceeding US$200,000 or US$300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the Securities and Exchange Commission which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer's account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer's confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the common shares that are subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in, and limit the marketability of, our common shares.
The Financial Industry Regulatory Authority sales practice requirements may also limit a shareholder's ability to buy and sell our common shares.
In addition to the "penny stock" rules promulgated by the Securities and Exchange Commission, the Financial Industry Regulatory Authority or FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer's financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common shares, which may limit your ability to buy and sell our shares.
We may be classified as a "passive foreign investment company," which may have adverse U.S. federal income tax consequences for U.S. shareholders.
Generally, for any taxable year in which 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our common shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company ("PFIC") for U.S. federal income tax purposes. Our status as a PFIC is a fact-intensive determination made on an annual basis, and we cannot provide any assurance regarding our PFIC status for the taxable year ending December 31, 2020 or for future taxable years.
If we are a PFIC for any year during a non-corporate U.S. shareholder's holding period of our common shares, then such non-corporate U.S. shareholder generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called "excess distribution" received on our common shares, as ordinary income, rather than as capital gain, and the preferential tax rate applicable to dividends received on our common shares would not be available. Interest charges would also be added to the taxes on gains and distributions realized by all U.S. holders.
A U.S. shareholder may avoid these adverse tax consequences by making a timely and effective "qualified electing fund" election ("QEF election"). A U.S. shareholder who makes a QEF election generally must report, on a current basis, its share of our ordinary earnings and net capital gains, whether or not we distribute any amounts to our shareholders. The QEF election is available only if our company characterized as a PFIC provides a U.S. shareholder with certain information regarding its earnings and capital gains as required under applicable U.S. Treasury regulations. In the event we become a PFIC, U.S. Holders should be aware that we might not satisfy the recordkeeping requirements that apply to a QEF or supply U.S. Holders with information such U.S. Holders require to report under the QEF rules in the event that our company is a PFIC for any tax year.
A U.S. shareholder may also mitigate the adverse tax consequences by timely making a mark-to-market election. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the increase in the fair market value of the common shares and deduct from gross income the decrease in the value of such shares during each of its taxable years. A mark-to-market election may be made and maintained only if our common shares are regularly traded on a qualified exchange. Whether our common shares are regularly traded on a qualified exchange is an annual determination based on facts that, in part, are beyond our control. Accordingly, a U.S. shareholder might not be eligible to make a mark-to-market election to mitigate the adverse tax consequences if we are characterized as a PFIC.
Each U.S. shareholder should consult their own tax advisors with respect to the possibility of making these elections and the U.S. federal income tax consequences of the acquisition, ownership and disposition of our common shares. This paragraph is qualified in its entirety by the discussion in the section of this registration statement entitled "Certain Material United States Federal Income Tax Considerations." In addition, our PFIC status may deter certain U.S. investors from purchasing our common shares, which could have an adverse impact on the market price of our common shares.
Item 4. Information on the Company
A. History and Development of the Company
We are a corporation incorporated under the Business Corporations Act (British Columbia) in British Columbia, Canada under the name "Adastra Holdings Ltd." with an authorized share structure of unlimited number of common shares without par value. Our principal place of business is located at 5451 - 275 Street, Langley, British Columbia V4W 3X8, Canada and our telephone number is (778) 715-5011. Our registered and records office is located at 800 - 885 West Georgia Street, Vancouver, British Columbia V6C 3H1, Canada.
We are an extraction and processing solutions company. Our mission is to develop and deploy large-scale cannabis and hemp extraction technologies and provide turn-key processing solutions to help licensed standard and micro-cultivators maximize the value of every harvest. We are also applying for a dealers licence for mushroom and mushroom-derived products in preparation for licencing of psychedelics in Canada, and operate a research-based multidisciplinary centre with physicians and healthcare professionals providing medical cannabis therapies.
History of the Company
Our Company was incorporated under the laws of the province of British Columbia on October 14, 1987. On December 19, 2019, our company changed its name to "Adastra Labs Holdings Ltd." from "Arrowstar Resources Ltd." (our company prior to this change of name, "Arrowstar"). On March 19, 2021, our company changed its name to "Phyto Extractions Inc." from "Adastra Labs Holdings Ltd.". On April 9, 2021, our company completed a consolidation of its common shares (on the basis of one (1) post consolidated common share for every three (3) pre-consolidation shares). On September 1, 2021, our company changed its name to "Adastra Holdings Ltd." from "Phyto Extractions Inc.".
On December 20, 2019, Arrowstar acquired all of the issued and outstanding common shares of Adastra Labs Holdings (2019) Ltd. ("Adastra"), a private British Columbia cannabis extraction and processing solutions company incorporated on June 18, 2018. The transaction was accounted for as a reverse acquisition, with Adastra being identified as the accounting acquirer and the net assets of Arrowstar at the date of the transaction are deemed to have been acquired by Adastra. At the time of the transaction, we changed our financial year end from April 30 to December 31.
On August 31, 2021, we acquired all of the issued and outstanding common shares of 1225140 B.C. Ltd., doing business as PerceiveMD, (“PerceiveMD”), pursuant to the terms of a share purchase agreement dated August 10, 2021. PerceiveMD owns a private British Columbia research-based multidisciplinary center for medical cannabis and other therapies. We intend to employ physicians and other healthcare professionals at our clinics related to the operations of PerceiveMD who will provide consultations to patients regarding the benefits of using cannabis-based products. Patients will be seen by a licensed physician and prescribed cannabis-based products.
At this time, we cannot fulfill any prescriptions ourselves as we do not hold the medical licence required from Health Canada and the College of Physicians and Surgeons. Instead, the prescription will be fulfilled by a licensed producer. We are preparing documents for the medical licence application, which will be submitted shortly, and we expect this to be approved in approximately three months. We are confident that our application will be successful, however, if, in the unlikely event, we are unsuccessful in this application we will continue to operate under our current model with prescriptions fulfilled by a licensed producer.
On September 17, 2021, we acquired all of the issued and outstanding common shares of 1204581 B.C. Ltd. ("1204581BC") pursuant to the terms of a share purchase agreement dated September 15, 2021. 1204581BC is the owner of intellectual property rights for the Phyto Extractions Brand.
Development of the Company
On October 18, 2018, Adastra submitted applications with Health Canada for the licenses under "An Act respecting cannabis and to amend the Controlled Drugs and Substances Act, the Criminal Code and other Acts" (the "Cannabis Act"), which licenses will designate Adastra and Chemia Analytics Inc. ("Chemia"), a wholly owned subsidiary of Adastra, as a licensed processor and a licensed cannabis tester, respectively.
On January 9, 2019, Adastra entered into a lease agreement with 1178562 B.C. Ltd., the holder of the property. On June 12, 2019, Adastra acquired all of the shares of 1178562 B.C. Ltd.
In mid-February 2019, Adastra finalized its plans for the retrofit of the building located at 5451 275th Street, Langley, British Columbia V4W 3X8, Canada (the "Facility"). In late February/early March 2019, Adastra submitted materials to the Township of Langley for re-zoning and commenced the retrofit of the Facility. On March 15, 2019, Adastra completed demolition and re-insulation of the Facility. On April 14, 2019, Adastra received unanimous Township of Langley Council approval to proceed with rezoning of the Facility. On May 10, 2019, Adastra received a status letter from Health Canada advising that there are no critical concerns at this time. On May 27, 2019, Adastra received final approval from the Township of Langley for re-zoning of the Facility. On June 10, 2019, Adastra had commenced the post-demolition portion of the retrofit of the Facility. In late November 2019, Adastra completed the retrofit of the Facility and on December 2, 2019, Adastra submitted its evidence package for its Cannabis (processing, sales) license application to Health Canada.
On October 25, 2019, Chemia received its analytical testing license from Health Canada. On March 16, 2020, Health Canada issued a Standard Processing License on the Facility to Adastra. Health Canada issued an Analytical Testing License to our facility owned by Chemia. The license became effective March 17, 2020. We received an amendment to our Standard Processing License authorizing the sale of cannabis extract, cannabis edibles and cannabis topicals on March 13, 2021, and our research license for organoleptic testing on April 16, 2021.
B. Business Overview
We are an integrated Canadian cannabis company focused on extraction and associated analytical testing. We, through one of our wholly owned subsidiaries, have a license to produce cannabis extracts under the Cannabis Act (LIC-SRIM66H586-2020). In addition, we, through one of our wholly owned subsidiaries, have been granted a license by Health Canada to conduct analytical testing for the cannabis industry (LIC-WOUX7802CE-2019). The Facility is a purpose-built facility with a view to be in compliance with European Union ("EU") Good Manufacturing Practices ("GMP") standards, which will allow us, subject to obtaining any necessary permits to export the products from the Facility to international destinations where cannabis is nationally legal for medical or adult usage purposes. We intend to export only to authorized recipients / countries - initially the EU for medical cannabis extracts once GMP accredited. There will be no US exports unless there is a change in US federal law. The Facility was completed in the quarter ended March 31, 2020 and became operational in April 2020.
Cannabis
The terms cannabis and marijuana are used interchangeably in Canada. The two main types of cannabis/marijuana are the Sativa and Indica plants, with hybrid strains being created when the genetics of each are crossed. Within each type of cannabis, there are hundreds of different phytochemical compounds, including many different cannabinoids (the most common being detla-9-tetrahydrocannabinol ("THC")), which is the psychoactive ingredient, and cannabidiol ("CBD"), which is responsible for many of the non-psychoactive effects of medical marijuana.
Cannabis can be used for either recreational or medicinal purposes and typically comes in the form of dried plant, powder form, resin or oil. Cannabis for medical use was legalized in Canada in 2001. Cannabis for recreational use was legalized in Canada in 2018.
Regulatory Framework
Cannabis was legalized in Canada for medicinal use in 2001 and has been a commonly prescribed medication since then. The production, distribution and sale of medicinal and adult-use cannabis is tightly controlled by the Canadian federal government. In 2013, Health Canada introduced the commercial cannabis licensed producer program under the Marijuana for Medical Purposes Regulations ("MMPR") program. In 2015, the Supreme Court of Canada found certain elements of the MMPR unconstitutional which led to the development of the "ACMPR" (Access to Cannabis for Medical Purposes Regulations), specifically medical cannabis patients having the right to use oils and derivative forms of cannabis. In August 2016, the MMPR was replaced by the ACMPR. The ACMPR program as it relates to commercial production is very similar to the MMPR.
The Canadian federal Cannabis Act came into force on October 17, 2018, to create a new legal regime for non-medicinal, or recreational, cannabis and to continue the legal regime for medicinal cannabis. Medicinal cannabis continues to be subject to different rules than recreational cannabis. The production and sale of medicinal cannabis is governed strictly by the federal government, whereas the regime for recreational cannabis is created by federal, provincial and municipal regulations.
The federal government regulates cannabis through the Cannabis Act, and governs the rules and regulations regarding:
● Minimum legal age requirement for recreational cannabis customers.
● Types of cannabis products available for sale. The sale of certain amounts of dried cannabis, fresh cannabis, cannabis oils, cannabis plants and cannabis plant seeds are legal as of October 17, 2018. Edibles containing cannabis and cannabis concentrates became legally available for sale October 17, 2019.
● Packaging and labelling requirements for products.
● Restrictions on promotional activities, displays and dispensing.
● Legalization of possession in public of 30 grams or less of dried cannabis, or the equivalent amount of other legal types of cannabis products, for someone 18 years of age or older, while maintaining a criminal offence for possession of more than that amount.
● Legalization of growing up to four cannabis plants in a private residence, while maintaining offences for having more than that or having them in a public place.
● Creating a licensing regime and requirements for the commercial production of cannabis and its wholesale sale to provincial and territorial distributors.
● Authorizing the provinces and territories to each legislate to create a new regulatory regime in their respective jurisdictions for the distribution and sale of recreational cannabis to persons 18 years of age or older.
● Maintaining offences for production, distribution or selling of cannabis outside of the newly regulated cannabis industry (with some exceptions).
● Continuing the separate legal regime for growing and selling medicinal cannabis to medical patients.
Under the regime created by the Cannabis Act, the federal government licenses and regulates the growing, processing and production of cannabis products for commercial purposes, and each province and territory controls the distribution and sale of recreational cannabis in their jurisdiction. Each jurisdiction is adopting unique legislation to address these issues. Only cannabis products that are grown or produced by a federally licensed producer may be sold or purchased in the provinces and territories. The distribution model of each province will not adversely affect our company as a business-to-business provider.
Each provincial or territorial government is responsible for its own regime for the distribution and sale of recreational cannabis in its jurisdiction. For example, in Saskatchewan, retail cannabis is sold by private retailers, in Quebec, by a government-operated public entity and, in British Columbia, by a combination of public and private retailers. Each province and territory also has the power to add, within its jurisdiction, more restrictive regulations from some of those in the federal legislation, including:
● Lowering the limit of cannabis permissible for personal recreational possession and consumption.
● Adding any rules and regulations to those for home grown recreational cannabis.
● Restricting the consumption of recreational cannabis in public spaces.
● Raising the minimum age for possession and consumption of cannabis.
Municipalities have the powers given to them by provincial statutes. Municipalities are given some authority over land use regulation and have the power to prohibit and regulate certain uses through zoning by-laws. Generally, the use carried out at any commercial real property must fit within the applicable zoning by-laws or the municipality can stop or prevent the use from being carried out on that real property.
Psychedelics are Schedule III substances under the Controlled Drugs and Substances Act for which the extraction of raw materials from, or involvement in production activities are regulated federally in Canada. In order to develop our business and undertake research and development, the Company is applying for a dealer license for psychedelics which, if obtained, will allow the Company to possess and formulate psychedelics. At present a dealer license holder can only trade with another individual who has the same license. Their activities are limited in trade and they cannot provide substances to individuals or companies as Health Canada has yet to approve psychedelics as a drug and it is currently illegal to possess psychedelics in Canada without a dealer license or other exemption. While we anticipate being successful in this application, there is no guarantee that we will obtain a dealer license to possess and formulate psychedelics. If we are successful in this application there remain uncertainties with respect to how government regulations will impact our business with respect to psychedelics. The Company is in the process of assessing this impact.
Current Status of Cannabis License Applications
The ACMPR was repealed when the Cannabis Act and the cannabis regulations came into effect on October 17, 2018. Under the new regime, when an applicant applies for a license there are four sub-categories to choose from:
(a) Cannabis (cultivation, processing, sales);
(b) Industrial Hemp;
(c) Research; and
(d) Analytical Testing.
On October 18, 2018, Adastra submitted two applications with Health Canada for the licences under the Cannabis Act. This included an application for Adastra under the first category: Cannabis (cultivation, processing, sales) and an application for Chemia under the fourth category: (Analytical Testing). The Cannabis license that Adastra has applied for permit it to process and sell its products for either medical or recreational use. Chemia has been granted an Analytical Testing license by Health Canada effective October 25, 2019. Adastra has been granted a Standard Processing License for production of cannabis extracts and sale of cannabis extracts to other Health Canada licensed companies) by Health Canada on March 13, 2020. Adastra has also been granted a Research License for organoleptic testing on April 16, 2021 from Health Canada.
Our business depends on these three licenses and the loss of any one of them could have a material adverse effect on our business, results of operations and financial condition.
Other Intellectual Property
Pursuant to the acqusition of 1204581BC the Company becames the owner of intellectual property rights for the Phyto Extractions Brand. The Company benefits from the brand awareness of the Phyto Extractions Brand to market both the current and potential future cannabis products.
Facility
On January 9, 2019, Adastra entered into a lease agreement with 1178562 B.C. Ltd., the holder of the property. Adastra has subsequently acquired all the common shares of 1178562 B.C. Ltd. The Facility is a 13,035 square foot building that was a former food manufacturing facility.
Existing Facility Floorplan 13,035 sq. ft.
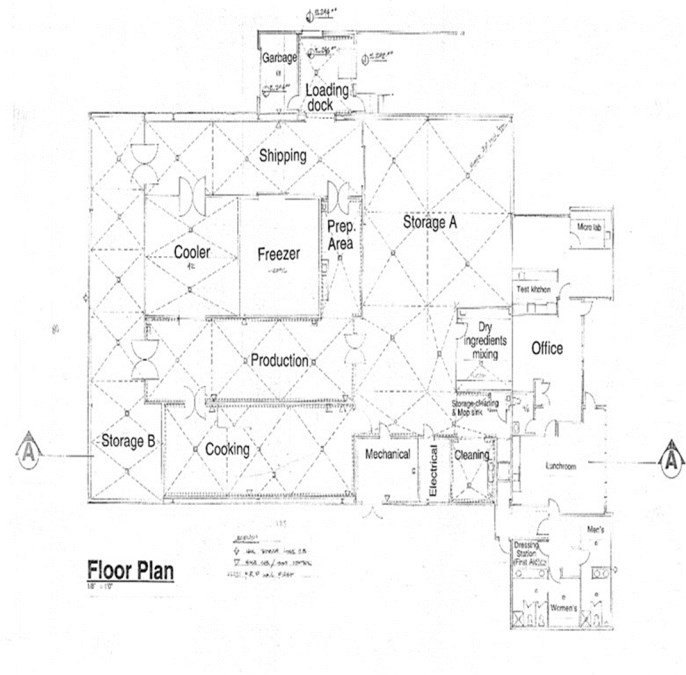
The site was chosen with two important considerations in mind. First, the current design and use of the facility as a food manufacturing facility for 20 years ensures the infrastructure and familiarity of Health Canada regulators with the Facility are optimal for conversion to a GMP cannabis extraction and analytical testing operation. Second, the strategic location in the Lower Mainland of British Columbia allows Adastra and Chemia to service licensed producers and other cannabis industry operations with easy access.
Proposed Facility Layout
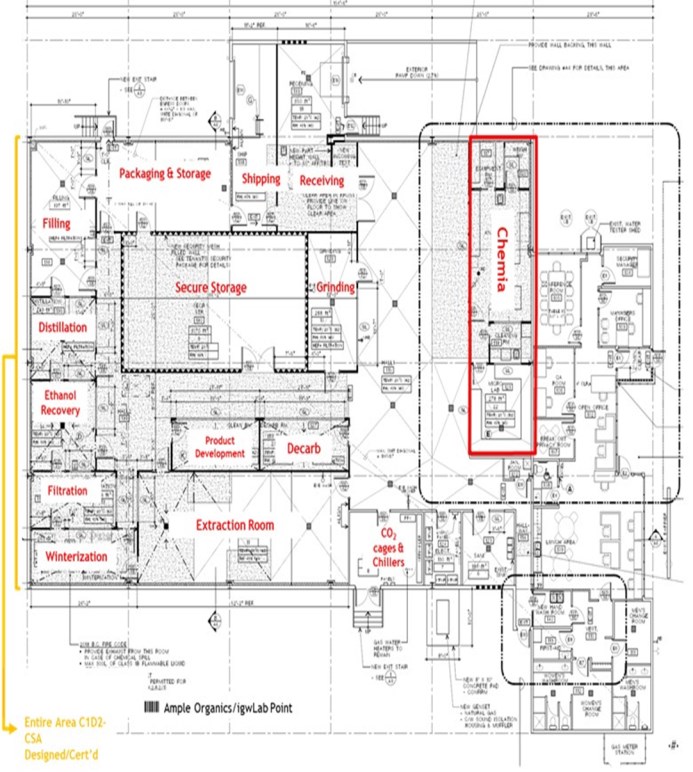
The Facility modifications are under design with a cross-functional team of cannabis industry consultants, equipment manufacturer design input, and construction and design contractors.
Revised Floorplan
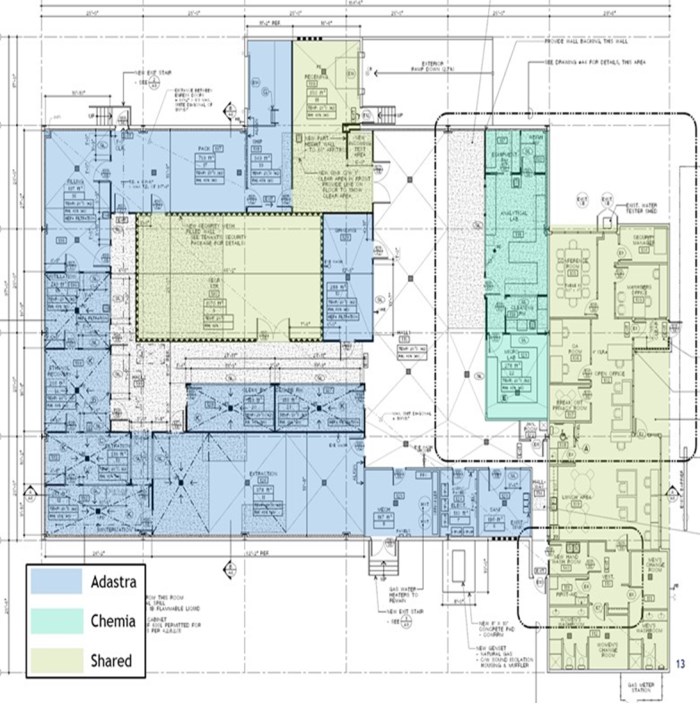
Adastra and Chemia operate together at the Facility. Some portions of the Facility restrict access to Adastra or Chemia.
We completed renovation of the Facility during the quarter ended March 31, 2020 at a cost of $3,900,000.
Extraction Equipment
Adastra has selected Supercritical CO2 extraction equipment and has agreed to purchase an extraction system that includes the ExtraktLAB Series 140 CO2 extractor along with the necessary additional equipment and software required for its operation of the extractor for an agreed price of $756,900. These units have a capacity of processing 422 pounds of cannabis biomass per day with a crude cannabis oil yield of 11-19% based on the cannabis flower/trim combinations intended to be used as extraction biomass.
We purchased and setup extraction, processing and testing equipment during the quarter ended March 31, 2020. We fully outfitted our centralized facility with equipment necessary to process cannabis at cost of $1,200,000.
The ability to further process cannabis crude to distillate, a high potency cannabinoid product containing over 90% active ingredient was contingent on the purchase of distillation equipment. Adastra purchased two Germany made wiped-film short path distillation units, one for $362,950 from Root Sciences, paid in cash, and the other for $386,215 from CapSure Consulting Ltd., paid through the issuance of 471,685 shares at a fair value price of $0.84 per common share. The common shares issued were subject to a hold period of four months and one day from the date of issuance. Together these machines refine cannabis crude a rate of 6.61 lbs per minute and provide a bulk salable product or the input into consumer-packaged goods such as edibles, topical and vaporizer cartridges.
Adastra also invested in two RoboCap RL-300s from ATG Pharma. Both filling machines were purchased for a total of $114,469.96 and commissioned in June 2020. These assets fill vaporizer cartridges, small bottles and candy molds. Together the two machines are capable of producing 45,000 vaporizer cartridges per day.
Adastra expanded its operational capabilities in October 2020 through the purchase of a high-capacity EV-Mass 30 Cryogenic Ethanol Extraction system from Evolved Extractions for $760,440.24. This extraction system can process more than 900 lbs of cannabis biomass per day with a yield of 11-19% to crude with fewer post-processing steps than Supercritical CO2 extraction.
Beginning in December 2020 Adastra began to retrofit the Facility to allow for the use of butane and propane. This construction, completed in February 2021, permitted the installation of MeP70XT Hydrocarbon Extractor from ExtractionTek Solutions LLC. This extractor and ancillary equipment was purchased for a total of $284,009.71 and has allowed for the production of rare, high margin Butane Hash Oil products including shatter, waxes, crumble, live resin, diamonds & sauce and more. This extractor can extract over 400 lbs of cannabis biomass per day.
Laboratory Equipment
Chemia will be required to perform all cannabis testing specified in the Cannabis Act regulations. Chemia expects to require the following specific equipment in addition to other miscellaneous equipment:
• 1x LCMS-8060 Triple Quadrupole Detector Package
• 1x GCMS-TQ8050 w/EI ion source Smart PackGE
• 1x ICPMS-2030 Mass Spectrometer
• 1x Multiwave GO Microwave Digestion System
• 1x Cannabis Analyzer Base Package: LC-2030C
Operations
Principal Products
We are a health and wellness company focused on the processing of cannabis and cannabis analytical testing. We provide processing solutions to licensed cultivators of cannabis and hemp, and supply cannabis extracts to qualified Canadian and international business-to-business partners under their own brand.
To meet growing requirements, we intend to produce our cannabis extractions for both recreational and medical use.
In addition to providing at-cost analytical testing services to Adastra, Chemia provides full-suite cannabis analytical testing services to 3rd parties throughout the Province of British Columbia lower mainland.
Our team can provide consulting and testing services for product formulation and development for cannabis product developers.
Our revenue is generated through bulk cannabis extracts sales, processed cannabis extract wholesales and toll processing contracts within Canada. Revenues of $3,639,012 for the nine months ended September 30, 2021 consisted of bulk distillate sales of $2,077,639, packaged shatter sales of $1,177,895,toll processing revenue of $40,283 and other revenue of $343,195. Revenues of $2,499,355 for the year ended December 31, 2020 consisted of bulk distillate sales of $1,974,908, and toll processing revenue of $524,447.
Extraction
To meet growing requirements, we intend to produce our cannabis extractions for both recreational and medical use:
● Indica and Sativa strains and combinations of both - for various medicinal purposes including anxiety, insomnia, pain, nausea and cancer treatment.
● Premium extracts are expected to have a higher market value but only when they provide a truly unique experience.
Specific products developed by us will be initially limited by those currently permitted in the medicinal market and recreational market.
Cannabis is a powerful stimulus that provides a unique experience to each user. With each variation comes a completely different experience. We understand the power that cannabis can have over people and the difference each strain can make. From pain-relief strains to euphoric "outer" body experiences, we anticipate having unique extracts for the desire of every consumer.
We plan to process, sell and distribute high quality cannabis extracts currently permitted by Health Canada under the Cannabis Act. We plan to increase our product line offering for additional products to be manufactured per Health Canada regulations as permitted under the Cannabis Act.
We plan to develop special product lines, each with its unique identifier and use to allow customers to use cannabis based on why they want to consume it. We expect that each product line will have a different strain, giving customers the ability to choose the kind of effect they need and want.
Indica, Sativa and Hybrid strains are used to treat a wide variety of ailments. Each type of strain extract will be derived of a unique blend of premium cannabis plant material. We also intend to create a line of single-origin and handcrafted cannabis extract products.
Our planned products, some of which are subject to legalization and/or obtaining necessary additional licenses (noted with "*"), are projected to include:
- Bulk Low THC Cannabis Oil for tinctures;
- Bulk CBD Oil without THC*;
- Consumer packaged Low THC Cannabis Oil*;
- Consumer packaged CBD Oil without THC*;
- Bulk High THC Cannabis Oil (distillate);
- Bulk Vaporizing pen formulations;
- Bulk water-soluble (nano-emulsion) cannabis oils and distillate;
- Bulk THC distillate Cannabis Gel Capsules (two-part);
- Consumer packaged THC Cannabis Oil Tinctures;
- Consumer packaged Vaporizing pens;
- Consumer packaged THC distillate Cannabis Gel Capsules;
- Bulk and consumer packaged hydrocarbon extracts;
- Bulk and consumer packaged Cannabis infused cosmetics and topics*; and
- Bulk and consumer dried flower*.
We depend on the brand licensing agreement to CannMart Labs Inc. to bring our products to market in Canada. Per this agreement, CannMart Labs Inc. has an exclusive license to use the Company's brands in association with development, manufacture, production, promotion, marketing, packaging, distribution and sale of the following types of products:
- Cannabis vaporizing pen cartridges and batteries;
- Cannabis capsules;
- Cannabis tincture bottles and tincture jars;
- Cannabis syringes;
- Cannabis terpene extracts (terp sauce); and
- Cannabis concentrates in solid form such as shatter, wax, live resin, crystals (diamonds) produced with Butane Hash Oil (BHO) or Propane Hash Oil (PHO) processes.
Per this brand licensing agreement, CannMart Labs Inc. fulfills sales orders of the Company's products across Canada and pays the Company 50% of gross profits earned from these sales on a quarterly basis. In return, the Company assists in the marketing of the Company's products to increase sales. This agreement is set to terminate during 2023 and as at September 30, 2021, the Company has earned over $2,200,000 from the agreement. We also intend to brand our products using proprietary trademarks to be acquired by or developed in house.
In addition, we anticipate we will provide other Health Canada cannabis license holders in the industry (Standard Cultivators or other Standard Processors) the ability to process cannabis into cannabis oils, distillate or other extract related formulations as a B2B service for a fee.
No part of our business is reasonably expected to be affected in the current financial year by either the renegotiation or termination of any contract.
Cannabis and hemp biomass is widely available in the Canadian market, noting that there can be raw material price volality, with prices also varying depending on strain as well as quantity and quality of product available. Overall, due to certain oversupply of non-craft cannabis and hemp biomass in the Canadian markets, the price of cannabis and hemp biomass has generally reduced over the last couple years.
Production Flow
The production flow of the Facility was designed to maximize the use of the existing production area layout while meeting all the requirements for Cannabis regulations.
Super Critical CO2 Production Flow Path
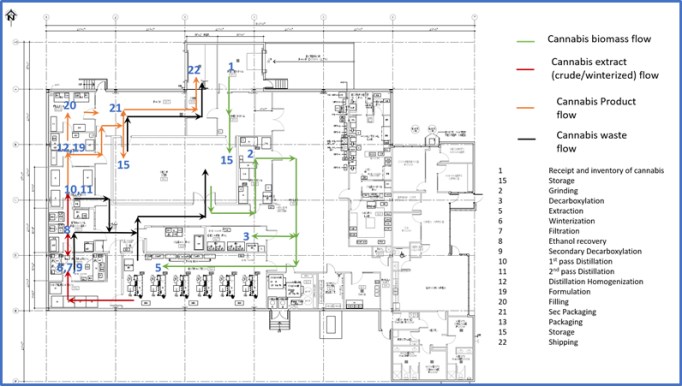
The flow path above depicts the receiving process whereby dried cannabis flower and trim is brought into our extraction facility for supercritical CO2 extraction, cryogenic ethanol extraction and hydrocarbon extraction. The first step involves a receipt moisture test, weighing and input into the inventory control system.
Following successful receipt, dried cannabis is then transferred into a temperature and humidity controlled secure vault until it is withdrawn for extraction. During the production process, dried cannabis is checked out of the secured vault (15) and enters production.
When CO2 extraction is to be performed, cannabis is transported to one of two grinding stations where it is milled to a fine consistency (2) to maximize surface area and compactness to improve extraction efficiency.
Following grinding the cannabis biomass is transferred to the Decarboxylation room (3) where it is heated in a vacuum and terpenes are extracted by a cold trap. These terpenes will be re-introduced to the extracted cannabis oil. The cannabis biomass is then extracted with supercritical CO2 (5) where crude cannabis oil is produced.
The cannabis crude oil is then mixed with ethanol and stored for 24-48 hours during winterization (4) after which it is filtered using large stainless-steel filters under a vacuum to remove waxes and lipids (6,7,8).
The remaining cannabis oil-ethanol mixture is processed through a rotary evaporator to remove and recycle all ethanol (8). The remaining cannabis oil then undergoes another distillation process in the Short Path Distillation equipment to further refine the oil to its purest form (10,11). If required, the cannabis distillate can be formulated to meet Cannabis Act regulations. Finally, the refined and properly dosed cannabis oil is filled into bottles (8) and packaged (9) for sale. The cannabis product can be sent to shipping (10A) or secure storage (10B).
Spent biomass is removed from the CO2 extraction equipment (1) and transported to a caged storage area (2). Spent biomass is THC- and cannabinoid-free and will be disposed of through 3rd party composting (3).
Cryogenic Ethanol Extraction Production Workflow
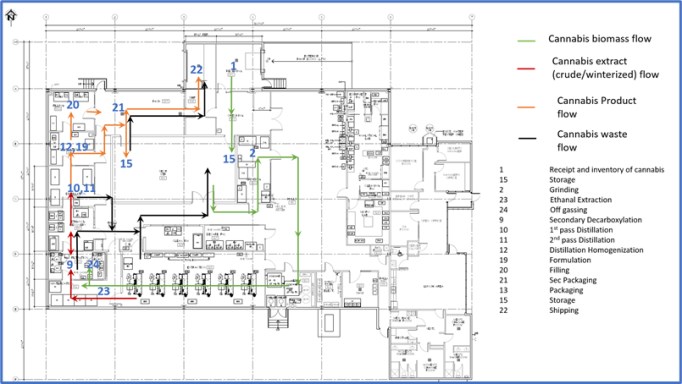
When Cryogenic Ethanol extraction is to be performed, cannabis is transported to the Grinding room (2) to be filled into extraction bags. No milling is required.
Once filled into bags, the cannabis moves into the Winterization Room (23) for ethanol extraction. Cannabis crude is generated and flows into the Filtration room (9) for secondary decarboxylation and devolatilization.
The decarboxylated cannabis crude then undergoes another distillation process in the Short Path Wiped Film Distillation equipment to further refine the oil to its purest form (10,11). If required, the cannabis distillate oil can be formulated diluted to meet Cannabis Act regulations. Finally, the refined and properly dosed cannabis oil is filled into bottles (8) and packaged (9) for sale. The cannabis product can be sent to shipping (10A) or secure storage (10B).
Spent biomass is removed from the Cryogenic Ethanol Extraction equipment (23) and transported to a caged storage area (2). Spent biomass is THC- and cannabinoid-free and will be disposed of through 3rd party composting (3).
Hydrocarbon Extraction Production Flow
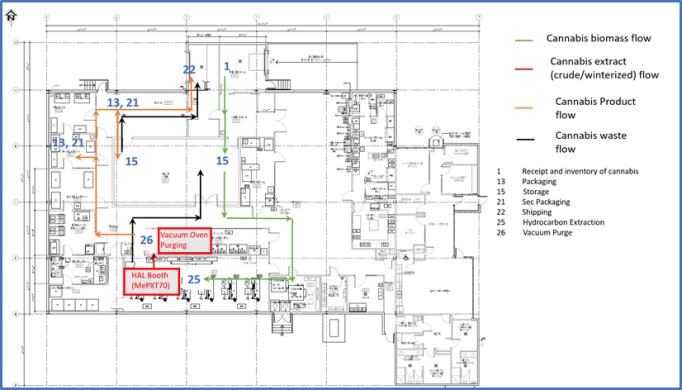
When Hydrocarbon extraction is to be performed, cannabis is transported to the HAL booth (25) for extraction.
Once filled into bags, the cannabis is extracted (25) and a cannabis extract is produced.
The cannabis extract flows into Product Development (26) for hydrocarbon purge using vacuum ovens. Bulk purged extract can then be packaged (9) for sale. The cannabis product can be sent to shipping (10A) or secure storage (10B).
Spent biomass is removed from the Hal Booth (25) and transported to a caged storage area (2). Spent biomass is THC- and cannabinoid-free and will be disposed of through 3rd party composting (3).
Testing
Chemia provides QA/QC and compliance testing services for licensed producers of cannabis and cannabis products under Health Canada regulations. Chemia also provides in-process testing services to help cultivators and processors optimize their processes. For QA/QC and compliance testing, any company holding a valid Health Canada cultivation or micro-cultivation, or processing license can send Chemia their sample. In addition to that, any dispensary representative, independent patient, and anyone else who wants to learn the chemistry and composition of their cannabis products can send samples for testing.
Chemia expects to provide the following testing services:
- Potency Testing
o Fast, high sensitivity testing to determine the potency of cannabis products. Chemia's tests will determine the levels of THCA/THC and CBDA/CBD and their total equivalency. Potency can be determined on fresh/dried flower, oils, extracts and concentrates.
- Microbial Contamination
o Chemia will be outfitted to complete all Health Canada mandated microbial tests on fresh/dried flower, oils, extracts and concentrates. Including amounts of total yeast and mold, total aerobic microbial counts, bile-tolerant gram-negative bacteria, E. coli, and Salmonella. Upon request testing for S. aureus, and P. aeruginosa will be conducted.
- Aflatoxins Analysis
o Chemia's method is capable of fulfilling Health Canada requirements for the analysis of aflatoxins on dried cannabis flower, oils and extracts. Chemia will test for aflatoxins B1, B2, G1 and G2.
- Heavy Metal Analysis
o Cannabis is known to accumulate heavy metals in its tissues. As required by Health Canada, its lab can efficiently and accurately test dried flower, oil and extracts for lead (Pb), cadmium (Cd), mercury (Hg) and arsenic (As). Additional metal screening for trace elements can also be performed as needed.
- Pesticide Screening
o Regulations for pesticide use on cannabis are strict. Chemia will use a combination of high-tech analytical techniques for pesticides screening. This screening can be done for fresh/dried flower, oils, extracts and concentrates.
- Residual Solvent Analysis
o To ensure the safety of customer's products and maintain compliance with Health Canada regulations, Chemia has developed tests to determine the quantity of residual solvents in cannabis oils, extracts and concentrates. Chemia can determine the quantity of methanol, ethanol, acetone, isopropanol (isopropyl alcohol), acetonitrile, hexane, heptane and pentane.
- Loss On Drying
o Before sale in Canada the moisture content of all dried cannabis flower must be reported. Chemia's tests will provide a customer with the percent moisture of customer's products.
- Foreign Matter
o As per Health Canada regulations Chemia will visually inspect customer's cannabis products for the presence of foreign material.
- Cannabinoid Profiling
o In addition to potency testing, Chemia can quantify 14 unique cannabinoids in cannabis products. The list of cannabinoids includes Tetrahydrocannabinolic acid (THCA), Δ9-Tetrahydrocannabinol (Δ9-THC), Cannabidiolic acid (CBDA), Cannabidiol (CBD), Cannabinol (CBN), Cannabigerolic acid (CBGA), Cannabigerol (CBG), (±)-Cannabichromenic acid (CBCA), (±)-Cannabichromene (CBC), Tetrahydrocannabivarinic acid (THCVA), Tetrahydrocannabivarin (THCV), Cannabidivarinic acid (CBDVA), Cannabidivarin (CBDV) and Cannabigerovarinic acid (CBGVA).
- Terpene Analysis
o Flavour and aroma of cannabis come from its terpene profile. That's why Chemia can quantify 39 terpenes commonly detected in cannabis. Chemia's tests can determine the quantities of the monoterpenes, alpha-Pinene, Camphene, Sabinene, beta-Pinene, beta-Myrcene, alpha-Phellandrene, (1S)-(+)-3-Carene, alpha-Terpinene, (R)-(+)-Limonene, Eucalyptol, Ocimene, gamma-Terpinene, Sabinene Hydrate, L-(-)-Fenchone, Terpinolene, Linalool, (1R)-Endo-(+)-Fenchyl Alcohol, (1S)-(-)-Camphor, (-)-Isopulegol, Isoborneol, (+/-)-Borneol, Hexahydrothymolalpha-Terpineol,gamma-Terpineol, Geranyl Acetate, (+)-Pulegone, Geraniol, Nerol. CAI can also test for sesquiterpenes including, alpha-Cedrene, trans-Caryophyllene, (-)-Caryophyllene Oxide, alpha-Humulene, Valencene, cis-Nerolidol, trans-Nerolidol, Guaiol, (+)-Cedrol and (-)-Alpha-Bisabolol.
Although Chemia will be required to perform all cannabis testing specified in the Cannabis Act regulations, it is permitted to stage purchase of expensive analytical equipment and certify the various tests in a staggered manner as a way of reducing capital expenditures. Further, Chemia plans to finance used equipment that has been certified from the manufacturer to reduce the overall cost and allow for the 3rd party testing revenue to reduce the cost of equipment financing.
Principal Markets
There are two broad segments, medical use and recreational. There is growth and greater acceptance in the medical use of CBD products for pain management, appetite control and cell generation involved in the treatment of cancer, MS, epilepsy and AIDS.
Clients are to include direct retail as well as wholesaling to distributors. The regulations also allow the export of products, subject to obtaining the necessary permitting. To maintain a stable supply in the market that meets the demand level, we expect to utilize a variety of distribution channels. Our online presence will also help to connect with the B2B customers. We will also be targeting other businesses that are licensed by Health Canada for product testing, white labelling products and toll processing.
The retail market for cannabis products is still developing and there is a need for more participants within the industry. Health practitioners and doctors play a key role by introducing their patients to cannabis as an alternative treatment. We anticipate designing marketing campaigns to create relationships with health care professionals including education, forums, training, printed literature, and media. Healthcare professionals employed at our clinics will provide consultations to patients and may market our products to them depending on the patients’ needs. The Company aims to create relationships with healthcare professionals to support the marketing of our products, mainly through the use of social media. In April 2021, the Company also entered into a marketing services agreement with Hybrid Financial Ltd. to assist in all aspects of a marketing campaign for the Company.
Within the recreational use segment, we view the following areas where there are potential growth opportunities:
• 25-to-35-YEAR-OLD URBAN PROFESSIONALS: The 25-to-35 "yuppie" market prefer the upscale branding approach.
• ATHLETES: This target group is a blend of medical and recreational use, seeking CBD products for cell generation and healing properties.
• BABY BOOMERS: This was the original "hippie" generation who likely experimented in their youth. Now, largely with grown children, not needing to set moral standards, or frankly seeing the benefits of legalizing an industry to protect users from indiscriminate processors, they have become more accepting of legalization.
Competitive Conditions
We compete with other licensed cannabis processors and cannabis testers in Canada. We anticipate that competition from new participants into the market will increase in the short-to-mid-term, as existing applications in queue with Health Canada are processed and approved. However, we also believe that many current and prospective applicants will not be able to obtain the necessary funding to conduct the necessary construction activities necessary to submit an initial application or in the case of current applicants, final application. Consolidation in this industry has already started, and management of our company believes that it will likely continue and increase as more producers and vendors are licensed by Health Canada under the Cannabis Act.
Seasonality
Our business is not subject to seasonality fluctuations.
Employees
We have 29 employees as of the date of this registration statement.
C. Organizational Structure
We have one direct wholly owned subsidiary, Adastra, a British Columbia corporation. Adastra has six wholly owned direct and indirect subsidiaries incorporated in British Columbia, Canada: (1) Adastra Labs Inc., for the extraction and concentrates production facility; (2) Chemia Analytics Inc., for a cannabis testing and analysis laboratory; (3) 1178562 B.C. Ltd., the owner of the Facility; (4) Adastra Brands Inc., a holding company for future branding of cannabis products which has no activities to date; (5) PerceiveMD, a direct subsidiary of Adastra Labs Inc. and the owner of a multidisciplinary medical clinic focusing on treatments with cannabis and psychedelics; and (6) 1204581, a direct subsidiary of Adastra Labs Inc. and the owner of intellectual property rights for the Phyto Extractions brand.
Although Adastra and Chemia are co-located at the Facility in Langley, British Columbia, Canada, they are separate legal entities to allow Chemia to provide objective production testing for Adastra at cost and keep these analytical testing activities separate from Adastra production. We through Adastra and Chemia have acquired two licenses from Health Canada to become a legal processor of cannabis in Canada through Adastra and to be a licensed cannabis analytical testing laboratory with Chemia.
D. Property Plant and Equipment
Through our wholly owned subsidiary, 1178562 B.C. Ltd., we own the Facility. See "Item 4.B Business Overview - Facility."
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited financial statements for the years ended December 31, 2020, the eight-month period ended December 31, 2019, and the period from June 18, 2018 to April 30, 2019 and related notes, included in this registration statement. Our audited consolidated financial statements included in this registration statement were prepared in accordance with International Financial Reporting Standards ("IFRS"), as adopted by the International Accounting Standards Board.
The preparation of the consolidated financial statements in conformity with these accounting principles requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent liabilities at the financial statement date and reported amounts of revenue and expenses during the reporting period. On an on-going basis we review our estimates and assumptions. The estimates were based on historical experience and other assumptions that we believe to be reasonable under the circumstances. Actual results are likely to differ from those estimates or other forward-looking statements under different assumptions or conditions, but we do not believe such differences will materially affect our financial position or results of operations. Our actual results may differ materially as a result of many factors, including those set forth under the headings entitled "Cautionary Note regarding Forward Looking Statements" and "Risk Factors" herein.
All monetary amounts are expressed in Canadian dollars, except per share amounts or as otherwise indicated.
REVERSE ACQUISITION
On December 20, 2019, Arrowstar acquired all of the issued and outstanding common shares of Adastra, a private British Columbia cannabis extraction and processing solutions company incorporated on June 18, 2018. At the time of the transaction, we changed our financial year end from April 30 to December 31.
This transaction constitutes a reverse take-over (the "RTO") of Arrowstar by Adastra and has been accounted for as a reverse acquisition transaction in accordance with the guidance provided in IFRS 2, Share-based payments. As Arrowstar did not qualify as a business according to the definition in IFRS 3, the reverse take-over does not constitute a business combination; rather it is treated as an issuance of common shares by Adastra for the net assets of Arrowstar, with Adastra as the continuing entity. Accordingly, no goodwill was recorded with respect to the transaction as it does not constitute a business.
For accounting purposes, Adastra was treated as an accounting parent company (legal subsidiary) and Arrowstar has been treated as the accounting subsidiary (legal parent) in the financial statements. As Adastra was deemed to be the acquirer for accounting purposes, its assets, liabilities and operations since incorporation are included in these financial statements at their historical carrying values. Arrowstar's results of operations have been included from December 20, 2019.
| | | December 20, 2019 | |
| Assets and (liabilities) of Arrowstar acquired | | $ | |
| Cash | | 111,496 | |
| Receivables | | 10,802 | |
| Other assets | | 7,504 | |
| Accounts payable | | (356,384 | ) |
| Net liabilities acquired | | (226,582 | ) |
| | | | |
| Consideration paid in RTO of Arrowstar | | | |
| Common shares (7,218,678 common shares at $0.15 fair value per share) | | 1,082,802 | |
| | | | |
| Listing expense | | 1,309,384 | |
The transaction was measured at the fair value of the shares that Adastra would have had to issue to the shareholders of Arrowstar, to give the shareholders of Arrowstar the same percentage equity interest in the combined entity that results from the reverse acquisition had it taken the legal form of Adastra acquiring Arrowstar.
A. Operating Results
Overall Performance
As at September 30, 2021, December 31, 2020 and 2019, we had a mortgage payable liability and were in a negative working capital position. Our focus for the nine-month period ended September 30, 2021, included the expansion of our centralized processing facility in Langley, British Columbia, Canada. Our focus for the year ended December 31, 2020, included the raise of equity capital, the expansion of our centralized processing facility in Langley, British Columbia, Canada. Our focus for the eight-month period ended December 31, 2019, included the raise of debt and equity capital, the build out of our centralized processing facility in Langley, British Columbia, Canada, efforts towards obtaining a public listing by completing an RTO on the Canadian Securities Exchange, and the continued progression of our applications with Health Canada. We received our Analytical Testing License on October 19, 2019 and received our Standard Processing License on March 16, 2020. These licenses became effective March 17, 2020. We received an amendment to our Standard Processing license authorizing the sale of cannabis extract, cannabis edibles and cannabis topicals on March 13, 2021 and our research license for organoleptic testing on April 16, 2021.
Operations and Facility
As of the date of this registration statement, we intend to focus on the generation of revenue through two primary activities, being wholesale activities and tolling services related to the production of cannabis extracts and related cannabis products.
As of April 23, 2020, we have completed the commissioning and commencement of operations at our 13,035 square feet Langley, British Columbia facility. We currently operate a primary supercritical CO2 extraction line for the production of crude cannabis/hemp extracts, wiped film short path distillation plant for the production of high potency cannabis distillate, a formulation, filling line and packaging line for the manufacture of tincture bottles and vaporizer products as consumer-packaged goods.
At our 13,035 sq. ft. Langley, British Columbia facility which has completed commissioning and commenced operations as at April 23, 2020, we currently operate supercritical CO2 primary extraction line for crude cannabis/hemp oil production, wiped film short path distillation line for production of high grade cannabis distillate, formulation and filling line for tincture bottles and vaporizer products, and packaging for finished packaged goods.
In September 2020, we installed and commissioned our cryo-ethanol extraction production line that triples our cannabis oil production capacity while reducing overall costs. This production line allows the re-purposing of the CO2 supercritical extraction line for other cannabis concentrate products such as high terpene full spectrum extracts.
During the quarter ending December 31, 2020, we installed and commissioned our hydrocarbon extraction production line using propane and butane or other solvent mixes. This production line allows us to extract over 400 kg per day of cannabis biomass into a variety of high value, and rare hydrocarbon cannabis concentrate products.
Wholesale Bulk Extracts Production
During the nine months ended September 30, 2021 and the twelve months ended December 31, 2020, we continued processing our own inventory of dried cannabis through supercritical CO2 and cryo-ethanol extraction lines and distillation lines for the purpose of selling the resulting bulk cannabis concentrates to licensed clients or using it to fulfill contract manufacturing orders, primarily for vaporizing cartridges. We have procured all of our bulk shipments of dried cannabis for our wholesale production lines from various licensed cultivators under the Cannabis Act. During the nine months ended September 30, 2021, we recognized revenue of $3,639,012 and during the twelve months ended December 31, 2020, we recognized revenue of $2,499,355.
Results of Operations
NIne Months Ended September 30, 2021 and September 30, 2020
For the nine months ended September 30, 2021, we had operating expenses of $2,043,174 and loss and comprehensive loss of $1,081,266, compared to operating expenses of $7,524,415 and loss and comprehensive loss of $7,706,337 during the nine months ended September 30, 2020.
Revenues increased to $3,639,012 during the nine months ended September 30, 2021, compared to $1,254,258 during the nine months ended September 30, 2020, from increased processing of cannabis biomass for third party licensed producers and in-house distillate production, and hydrocarbon extraction.
Cost of sales increased to $1,972,771 during the nine months ended September 30, 2021, compared to $1,088,721 during the nine months ended September 30, 2020, and consists of biomass, labour, solvents and an allocation of production overheads such as facility costs and amortization of production equipment.
For the nine months ended September 30, 2021, the Company had operating expenses of $2,043,174 and loss and comprehensive loss of $1,081,266, compared to operating expenses of $7,524,415 and loss and comprehensive loss of $7,706,337 during the nine months ended September 30, 2020.
The decrease in operating expenses and loss and comprehensive loss were the result of the Company's expenses with respect to advertising and promotion, office, professional fees and consulting, and share-based payments having decreased as the Company moved from its startup phase into production and expansion of its business during the nine months ended September 30, 2021, offset by a rise in wages and salary costs. The most significant changes in operating expenses and other expenses were as follows:
- Advertising and promotion decreased to $328,484 during the nine months ended September 30, 2021, compared to $694,380 during the nine months ended September 30, 2020, due to the Company developing a more focused investor awareness campaign and being more established in the market.
- Office expenses decreased to $282,686 during the nine months ended September 30, 2021, compared to $390,335 during the nine months ended September 30, 2020, as the Company moved past its startup phase and into regular production. In the prior period costs were incurred for small tools and equipment as the Company prepared the facility for operations.
- Professional fees and consulting decreased to $404,169 during the nine months ended September 30, 2021, compared to $570,814 during the nine months ended September 30, 2020, primarily due to the decrease of consultants needed to prepare the extraction process, commissioning of equipment and testing.
- Share-based payments decreased to $19,456 during the nine months ended September 30, 2021, compared to $5,332,500 during the nine months ended September 30, 2020, as during the nine months ended September 30, 2021 only 33,333 stock options were granted (YTD 2020 - 4,206,667), all granted options vest immediately.
- Wages and salaries increased to $742,346 during the nine months ended September 30, 2021, compared to $353,050 during the nine months ended September 30, 2020, due to increased hiring activity and production output related to the Langley Facility.
- During the nine months ended September 30, 2021, the Company incurred impairment of property, plant and equipment of $150,000 related to an ERP software in development that the Company determined would not be completed. No impairment of property, plant and equipment was recorded during the nine months ended September 30, 2020.
- During the nine months ended September 30, 2021, the Company recognized a write-down of inventory of $406,312 in accordance with IFRS requirements to value inventory at the lower of cost or net realizable value. No write-down of inventory was recorded during the nine months ended September 30, 2020.
Year Ended December 31, 2020 and Eight Months Ended December 31, 2019
The following table summarizes the results of operations for the year ended December 31, 2020 and the eight months ended December 31, 2019:
| | | December 31, 2020 | | | For the eight months ended
December 31, 2019 | |
| | | $ | | | $ | |
| Expenses | | (7,945,831 | ) | | (1,128,382 | ) |
| Loss and comprehensive loss) | | (7,615,864 | ) | | (2,657,957 | ) |
| | | | | | | |
| Weighted average number of common shares outstanding | | | | | | |
| - basic and diluted | | 39,695,235 | | | 27,495,882 | |
| | | | | | | |
| Loss and comprehensive loss per share | | | | | | |
| - basic and diluted | | (0.19 | ) | | (0.03 | ) |
| | | December 31, 2020 | | | For the eight months ended
December 31, 2019 | |
| | | $ | | | $ | |
| Total assets | | 13,736,950 | | | 11,351,010 | |
| Working capital surplus (deficiency) | | 105,093 | | | (471,084 | ) |
| Total liabilities | | 3,650,794 | | | 6,266,088 | |
| Non-current liabilities | | 60,000 | | | 2,955,511 | |
| Shareholders' equity | | 10,086,156 | | | 5,084,922 | |
| Deficit | | (11,177,810 | ) | | (3,561,946 | ) |
For the year ended December 31, 2020, we had expenses of $7,945,831 and comprehensive loss of $7,615,864, compared to expenses of $1,128,382 and comprehensive loss of $2,657,957.
This increase in expenses and comprehensive loss in 2020 as compared to 2019 was the result of our expenses with respect to office, professional fees and consulting, and wages and salaries have increased as we expand our development efforts relating to the build out of our centralized processing facility and has engaged in further marketing activity in relation to the general development, promotion, and expansion of our business during the year ended December 31, 2020, and relative to the comparative period ended December 31, 2019. More specifically:
- Advertising and promotion increased by approximately $692,441 due to increased investor awareness campaigns initiated by our company in its initial year of production.
- Office expenses increased by approximately $47,333 to $227,271 as initial production commencement has increased operating resources.
- Professional fees and consulting increased by approximately $256,513 to $704,297 primarily due to the increase of consultants needed to prepare the extraction process, commissioning of equipment and testing.
- Wages and salaries increased by approximately $223,814 to $526,327 due to increased hiring activity and production output for the Company in their initial year of operations.
- Share-based payments of $5,332,500 is a non-cash expense. This represents the fair value of 12,500,000 options granted using the Black-Scholes option pricing model: and
- Revenues increase to $2,499,355 from processing of cannabis biomass for third party licensed producers and in-house distillate production.
Our loss and comprehensive loss for the year ended December 31, 2020 was $7,615,864. The incurred amount was offset by revenues of $2,499,355 which represent our year of product sale and production revenue. We incurred $749,258 in advertising and promotion expenses during the year represent our investor awareness campaign. We incurred $5,332,500 in non-cash expenses for stock-based payments for directors, officers, employees and consultants.
Our loss and comprehensive loss for the eight months ended December 31, 2019 was $2,657,957. The incurred amount was due in large part to the increased activity and related expenditures above specific to our efforts to expand the business, build our facility, and obtain a public listing. Approximately $1,300,000 represented the excess of the non-cash consideration paid over the net assets of Arrowstar.
Eight Months Ended December 31, 2019 and the Period from June 18, 2018 to April 30, 2019
The following table summarizes the results of operations for the eight months ended December 31, 2019 and the period from June 18, 2018 to April 30, 2019:
| | | December 31, 2019 | | | April 30, 2019 | |
| | | $ | | | $ | |
| Expenses | | (1,128,382 | ) | | (849,614 | ) |
| Loss and comprehensive loss) | | (2,657,957 | ) | | (903,989 | ) |
| | | | | | | |
| Weighted average number of common shares outstanding | | | | | | |
| - basic and diluted | | 27,495,882 | | | 13,265,014 | |
| | | | | | | |
| Loss and comprehensive loss per share | | | | | | |
| - basic and diluted | | (0.10 | ) | | (0.07 | ) |
With respect to loss and comprehensive loss per share, financial instruments are anti-dilutive.
| | | December 31, 2019 | | | April 30, 2019 | |
| | | $ | | | $ | |
| Total assets | | 11,351,010 | | | 6,254,518 | |
| Working capital surplus (deficiency) | | (471,084 | ) | | (145,048 | ) |
| Total liabilities | | 6,266,088 | | | 2,544,507 | |
| Non-current liabilities | | 2,955,511 | | | - | |
| Shareholders' equity | | 5,084,922 | | | 4,614,000 | |
| Deficit | | (3,561,946 | ) | | (903,989 | ) |
For the eight months ended December 31, 2019, we raised additional equity capital which contributed to greater cash and current assets, we increased equipment purchases which drove an increase in total assets, and we incurred greater expenses overall which contributed to the increase in loss and comprehensive loss.
Our expenses with respect to office, professional fees and consulting, and wages and salaries increased as we expanded our development efforts relating to the build out of our centralized processing facility, attain a public listing, and engaged in further marketing activity in relation to the general development, promotion, and expansion of our business during the period ended December 31, 2019, relative to the comparative period ended April 30, 2019. More specifically:
- Advertising and promotion increased by approximately $56,817 due to increased efforts in, online marketing campaigns, and shareholder outreach to create investor and customer awareness as a newly listed entity;
- Office expenses increased by approximately $65,514 to $179,938 as general and administrative items such as office supplies and products all increased. Additionally, property expenses in the amount of $68,023 was included in this amount. Furthermore, small tools and equipment $53,659 as we prepared the facility for operations and computer and internet expenses of approximately $26,827 were incurred for website subscription costs.
- Professional fees and consulting increased by approximately $193,924 to $447,784 primarily due to greater legal fees related to the Issuer's public listing, and other general corporate matters which increased as a result of becoming a reporting issuer. Additionally, we engaged with consultants to perform various professional services namely the provision of consulting relating to our centralized processing facility and our custom-built extraction facility and equipment commissioning;
- Wages and salaries increased by approximately $2,246 to $302,513 as we hired additional staffing at our facility and head office in various departments to facilitate growth efforts; and
- Listing expense of $1,309,384 is primarily a non-cash expense. This represents the excess of the non-cash consideration paid over the net assets of our company, and the resulting cost of obtaining a public listing.
Our loss and comprehensive loss for the eight months ended December 31, 2019 was $2,657,957. The incurred amount was due in large part to the increased activity and related expenditures above specific to our efforts to expand the business, build our facility, and obtain a public listing. Approximately $1,309,384 represented the excess of the non-cash consideration paid over the net assets of Arrowstar.
Our loss and comprehensive loss for the period from June 18, 2018 to April 30, 2019 was $903,989. The incurred amount was due in large part to the increased activity and related expenditures specific to our efforts to expand the business, build its facility, and obtain a public listing.
B. Liquidity and Capital Resources
Our objectives when managing our liquidity and capital structure are to support further advancement of our business objectives and existing service offerings, as well as to ensure that we are able to meet our financial obligations as they come due.
Cash and working capital
As at September 30, 2021 we had working capital deficit of $515,868. As at December 31, 2020 we had working capital $105,093 (deficit of $471,084 December 31, 2019). On November 16, 2021, the Company announced a private placement financing to raise gross proceeds of $2,500,000 to improve its working capital position. In management's opinion, this will provide sufficient working capital for our present requirements.
Cash flow activity
For the nine months ended September 30, 2021 operating activities consumed $675,235. Financing activities provided $851,215 and consisted of proceeds received on the mortgage renewal, offset by payments of interest on the mortgage payable and related borrowing costs. Investing activities consumed $113,753 and consisted of acquisitions of property, plant and equipment, offset by cash received on the acquisitions of Perceive MD and 1204581 B.C. Ltd, resulting in a net increase in cash of $62,227 and an ending cash balance of $1,207,688.
For the nine months ended September 30, 2020 operating activities consumed $2,257,920. Financing activities provided cash of $3,424,014 and consisted of proceeds from the issuance of unit financing offet by payments of interest on the mortgage payable and related borrowing costs. Investing activities consumed $1,233,830 and consisted of acquisitions of property, plant and equipment, offset by interest income received, resulting in a net decrease in cash of $67,736 and an ending cash balance of $2,309,090.
For the year ended December 31, 2020 operating activities consumed approximately $2,833,750. We invested a further amount of approximately $1,801,014 in extraction equipment and had interest income of $8,303. These amounts were offset by cash flows from financing activities of $3,395,096 resulting in a net decrease in cash of $1,231,365 and an ending cash balance of $1,145,461.
The increase in cash and increase in working capital deficiency as at December 31, 2019 was primarily the result of an approximate $5,694,075 increase in cash flows from financing activities driven by our private placement and convertible debenture offerings. Cash flows from financing activities were partially offset by increase in cash used in operating activities and increase in cash used in investing activities of approximately, $1,433,444 and $4,246,299 which primarily consists of the acquisition of property plant and equipment and equipment deposits respectively.
Capital resource management
We define capital as the components of shareholders' equity. Our objectives when managing capital are to support further advancement of our business objectives, as well as to ensure that we are able to meet our financial obligations as they come due.
We manage our capital structure to maximize our financial flexibility making adjustments to it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. We rely on the expertise of our management to sustain the future development of the business. Management reviews our capital management approach on an ongoing basis and believes that this approach, given the relative size of our company, is reasonable. There were no changes in our approach to capital management during the nine months ended September 30, 2021 and the year ended December 31, 2020. We are not subject to externally imposed capital requirements.
Tabular Disclosure of Contractual Obligations
As of December 31, 2020, we had the following contractual obligations and commitments:
| | | | | | Payments due by period | |
| Contractual obligations | | Total | | | Less than 1
year | | | 1-3 years | | | 3-5 years | | | More than 5
years | |
| | | $ | | | $ | | | $ | | | $ | | | $ | |
| Mortgage payable | | 2,442,830 | | | 2,442,830 | | | - | | | - | | | - | |
| Government loan | | 60,000 | | | - | | | 60,000 | | | - | | | - | |
| Total | | 2,502,830 | | | 2,442,830 | | | 60,000 | | | - | | | - | |
Off-Balance Sheet Arrangements
We have not engaged in any off-balance sheet arrangements such as obligations under guarantee contracts, a retained or contingent interest in assets transferred to an unconsolidated entity, any obligation under derivative instrument or any obligation under a material variable interest in an unconsolidated entity that provides financing, liquidity, market risk or credit risk support to our company or engages in leasing or hedging services with our company.
C. Research and Development
We do not currently have any patents or intellectual property arising from research and development, although we do own intellectual property rights including the Phyto Extractions brand.
D. Trend Information
We do not know of any trends, commitments, events, or uncertainty that are expected to have a material effect on our company's business, financial condition, or results of operations other than as described in the section "Risk Factors" and in the section entitled "Quantitative and Qualitative Disclosures About Market Risk".
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
The following table sets forth the name, office held, age, and functions and areas of experience in our company of each of our directors and senior management:
Name,
Office Held,
Age | Area of Experience and Functions in Our Company |
Michael Forbes Chief Executive Officer and Director Age: 41 | As our Chief Executive Officer and Chairman of the board of directors, Mr. Forbes is responsible for strategic planning and operations, as well as managing our relations with our lawyers, regulatory authorities and investor community; as a director, Mr. Forbes supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Forbes is a member of our audit committee. |
Oliver Foeste Chief Financial Officer Age: 45 | As our Chief Financial Officer, Mr. Foeste is responsible for the management and supervision of all of the financial aspects of our business. Mr. Foeste assists in strategic planning, oversees capital planning and capital raising, budgeting, financial reporting and risk management. In performing his duties, Mr. Foeste maintains relationships with our auditors, legal counsel, banks, and other stakeholders. |
Donald Dinsmore Chief Operating Officer, Corporate Secretary and Director Age: 33 | As our Chief Operating Officer, Mr. Dinsmore is responsible for the execution of strategic plans and operations management of the business, Mr. Dinsmore assists in strategic planning, supervises production, quality, sales, marketing, and regulatory compliance. In the fulfilment of his duties, he maintains relationships with suppliers, customers, legal counsel, and other stakeholders. As our Corporate Secretary, Mr. Dinsmore is responsible for maintaining our corporate records. As a director, Mr. Dinsmore supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
George Routhier Director Age: 68 | As an independent director, Mr. Routhier supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Routhier is a member of our audit committee. |
Paul Morgan Director Age: 40 | As an independent director, Mr. Morgan supervises our management and helps to ensure compliance with our corporate governance policies and standards. Mr. Morgan is a member of our audit committee. |
The following is a brief account of the business experience of each of our directors and senior management.
Michael Forbes, Chief Executive Officer and Director
Michael Forbes became a director effective April 29, 2021 and our Chief Executive Officer effective May 3, 2021. Mr. Forbes is a registered pharmacist. Mr. Forbes has founded five cannabis medical clinics under the Concord Medical Clinic umbrella, built Clarity Cannabis and Honeycomb Cannabis to over 10 locations, and also founded the cannabis licensed production facility, Sitka Weed Works, in Canada. Having medical experience of over 16 years, Mr. Forbes has also built and operated pharmacy chains across British Columbia and Alberta, and founded a dozen medical clinics, including 3 methadone clinics in order to protect the public from drug diversion and increase accessibility to medicine. Mr. Forbes graduated from the University of British Columbia in 2002 with a BSc. in Pharmaceutical Sciences, also achieving additional certification in Hormone Restoration, Age Management Medicine, and more recently, Cannabis Plant Production and Facility Management from Kwantlen Polytechnic University. He was elected to attend the Ivey School of Business for Canada's Top 40 under 40 in 2017 where he received an honorary MBA.
Oliver Foeste, Chief Financial Officer
Oliver Foeste, CPA, CA became our Chief Financial Officer effective July 13, 2021.
Mr. Foeste is the founder and Managing Partner of Invictus Accounting Group LLP and has significant executive, director, finance, and restructuring experience across a number of industry sectors. Prior to founding Invictus Accounting Group LLP, Mr. Foeste was in senior finance and accounting roles at Toronto Stock Exchange, TSX Venture Exchange, and New York Stock Exchange listed issuers, and earned his CPA at Deloitte and a boutique tax advisory firm.
Donald Dinsmore, Chief Operating Officer, Corporate Secretary and Director
Donald Dinsmore is a plant biochemist with a background working with successful research groups at the University of Calgary where he earned his Bachelors and Masters of Science degrees in Plant Biochemistry. Upon completing his degrees he developed his business experience working in privately held software companies and the entertainment industry. In 2017 Mr. Dinsmore pursued his passion in cannabis cultivation and became the Operations Manager for Heart Rock Mountain Farms, CA, USA cannabis growers.
In 2018 Mr. Dinsmore joined Adastra Labs Inc. as the Quality Assurance Person where he was critical in the design, buildout and Health Canada licensing of the facility. Upon commencement of operations he acted as Production Manager developing three extraction lines and contributed to the commercialization and release of dozens of our products.
Following the acquisition of Adastra Labs Inc. and our name change to Phyto Extractions Inc., Mr. Dinsmore assumed the role of director effective April 29, 2021 and Chief Operating Officer and Corporate Secretary effective May 3, 2021.
George Routhier, Director
George Routhier became a director of our company effective December 19, 2019. Mr. Routhier is the CEO of Pipedreemz Inc., a cannabis licensing regulations consulting company responsible for the successful licensing of approximately 30 percent of cannabis Licensed Producers across Canada over the past six years.
Paul Morgan, Director
Paul Morgan became a director of our company effective July 13, 2021. Mr. Morgan is a corporate / commercial lawyer located in Victoria, British Columbia and has been actively practicing in British Columbia since 2013. Mr. Morgan's legal practice has included a particular focus on various segments of the cannabis industry including, but not limited to, retailers and licensed producers of cannabis since approximately 2016.
Family Relationships
There are no family relationships between any of our directors and senior management.
B. Compensation
The following table sets forth all direct and indirect compensation paid, payable, awarded, granted, given or otherwise provided, directly or indirectly, by our company or any subsidiary thereof to members of our management and directors of our company, in any capacity, including, for greater certainty, all plan and non-plan compensation, direct and indirect pay, remuneration, economic or financial award, reward, benefit, gift or perquisite paid, payable, awarded, granted, given or otherwise provided to the members of our management or directors of our company for services provided and for services to be provided, directly or indirectly, to our company or any subsidiary thereof for the years ended December 31, 2020 and 2019, other than stock options and other compensation securities:
Table of Compensation Excluding Compensation Securities |
Name and position |
Year
| Salary,
consulting fee,
retainer or
commission | Bonus | Committee
or meeting
fees | Value of
perquisites1 | Value of all
other
compensation | Total
compensation |
| | $ | $ | $ | $ | $ | $ |
Andrew Hale2
Former Chief Executive Officer, President and Director | 2020 2019 | 180,091 120,115 | - - | - - | - - | - - | 180,091 120,115 |
Stephen Brohman3
Former Chief Financial Officer, Corporate Secretary and Director | 2020 2019 | 150,9004 107,2984 | - - | - - | - - | - - | 150,900 107,298 |
Blaine Blaney5
Former Chief Financial Officer and Director | - - | - - | - - | - - | - - | - - | - - |
Georges Routhier6
Director | 2020 2019 | 2,5007 17,5007 | - - | - - | - - | - - | 2,500 17,500 |
Notes:
1 "Perquisites" include perquisites provided to an officer or director that are not generally available to all employees and that, in aggregate, are: (a) C$15,000, if the officer or director's total salary for the financial year is C$150,000 or less, (b) 10% of the officer or director's salary for the financial year if the officer or director's total salary for the financial year is greater than C$150,000 but less than C$500,000, or (c) C$50,000 if the officer or director's total salary for the financial year is C$500,000 or greater.
2 Andrew Hale was appointed Chief Executive Officer, President and a director of our company on December 19, 2019. Mr. Hale resigned from all positions with our company on February 26, 2021.
3 Stephen Brohman was appointed as a director of our company on September 2, 2014, and Chief Financial Officer and Corporate Secretary of our company on December 19, 2019. Mr. Brohman resigned on July 13, 2021.
4 We were charged by Donaldson Brohman Martin CPA Inc., a firm in which Mr. Brohman is a principal, for accounting and tax services, in the amount of $150,900 in 2020 and $107,298 in 2019.
5 Blaine Baily was appointed Chief Financial Officer of our company on June 21, 2005, Secretary of our company on April 5, 2007, and a director of our company on February 3, 2014. Mr. Bailey resigned as Secretary of our company on August 20, 2007, Chief Financial Officer of our company on December 19, 2019, and as a director of our company on March 26, 2021.
6 George Routhier was appointed as a director of our company on December 19, 2019.
7 We were charged by Pipedreemz Inc., a company controlled by Mr. Routhier, for consulting services, in the amount of $2,500 in 2020 and $17,500 in 2019.
Employment, Consulting and Management Agreements
Other than as set out herein, we do not have any employment, consulting or management agreements or arrangements with any of our directors or senior management.
Stephen Brohman, Chief Financial Officer and Director
Donaldson Brohman Martin, CPA Inc. ("DBM CPA"), Chartered Professional Accountants, provided accounting services to our company during the year ended December 31, 2020. Our Chief Financial Officer and director, Stephen Brohman, is a principal of DBM CPA.
Andrew Hale, Former Chief Executive Officer and Director
Our former Chief Executive Officer and director, Andrew Hale received a fee of $180,000 per annum for providing services as Chief Executive Officer of our company. Mr. Hale resigned as our Chief Executive Officer and director effective February 26, 2021.
Benefits Upon Termination
We have no contract, agreement, plan or arrangement, whether written or unwritten, that provides for benefits to our directors or members of our management upon termination of employment of our directors or members of our management.
Stock Options and Other Compensation Securities
The following table sets out all compensation securities granted or issued to members of our management and directors of our company during the year ended December 31, 2020 for services provided, or to be provided, directly or indirectly, to our company or any subsidiary thereof:
Compensation Securities |
Name and position |
Type of
compensation
security
| Number of
Compensation
Securities/Number
of Underlying
Securities /
Percentage of
Class | Date of
Issue or
Grant | Issue,
Conversion
or Exercise
Price | Closing
Price of
Security or
Underlying
Security on
Date of
Grant | Closing
Price of
Security or
Underlying
Security at
Year End | Expiry Date |
| | # | | $ | $ | $ | |
Andrew Hale
Former Chief Executive Officer, President and Director | Stock options | 666,666 / 666,666 /
16% | January 30, 2020 | 1.35 | 1.35 | 1.38 | January 30, 2025 |
| Stock options | 483,333 / 483,333 / 11.6% | August 4, 2020 | 2.34 | 2.38 | 1.38 | August 4, 2025 |
Stephen Brohman
Former Chief Financial Officer, Corporate Secretary and Director | Stock options | 333,333 / 333,333 /
8% | January 30, 2020 | 1.35 | 1.35 | 1.38 | January 30, 2025 |
| Stock options | 250,000 / 250,000 /
6% | August 4, 2020 | 2.34 | 2.38 | 1.38 | August 4, 2025 |
Blaine Blaney
Former Chief Financial Officer and Director | Stock options | 50,000 / 50,000 / 1.2% | January 30, 2020 | 1.35 | 1.35 | 1.38 | January 30, 2025 |
| Stock options | 33,333 / 33,333 / 0.8% | August 4, 2020 | 2.34 | 2.38 | 1.38 | August 4, 2025 |
Georges Routhier
Director | Stock options | 83,333 / 83,333 / 2% | January 30, 2020 | 1.35 | 1.35 | 1.38 | January 30, 2025 |
| Stock options | 33,333 / 33,333 / 0.8% | August 4, 2020 | 2.34 | 2.38 | 1.38 | August 4, 2025 |
Pension, Retirement or Similar Benefits
We have not set aside or accrued any amounts to provide pension, retirement or similar benefits for our directors or members of our management during the year ended December 31, 2020.
C. Board Practices
Term of Office
Each director of our company holds office until the next annual general meeting of our company or until his successor is elected or appointed, unless his office is earlier vacated in accordance with the articles of our company or the provisions of the Business Corporations Act (British Columbia). Each officer of our company is appointed to serve at the discretion of our board of directors.
Committees
We have one committee: the audit committee.
Audit Committee
We have an audit committee comprised of Michael Forbes, who also serves as the Chief Executive Officer and George Routhier, and Paul Morgan, both of whom are independent directors.
We have adopted a charter for our audit committee. According to our audit committee charter, the mandate of our audit committee is to assist our board of directors in fulfilling its financial oversight responsibilities. According to our audit committee charter, our audit committee will review and consider, in consultation with our auditors, the financial reporting process, the system of internal control over financial reporting and the audit process. In performing its duties, our audit committee will maintain effective working relationships with our board of directors, management and external auditors. To effectively perform his or her role, each committee member must obtain an understanding of the principal responsibilities of committee membership as well as our company's business, operations and risks.
Our audit committee has roles and responsibilities including:
● being directly responsible for overseeing the work of external auditors in preparing or issuing the auditor's report, including the resolution of disagreements between management and the external auditors regarding financial reporting and audit scope or procedures;
● considering whether controls are in place over annual and interim financial reporting as well as controls over assets, transactions and the creation of obligations, commitments and liabilities of our company;
● reviewing the financial statements and financial information of our company prior to their release to the public; and
● considering and pre-approving any non-audit services (being services other than services rendered for the audit and review of the financial statements or services that are normally provided by the external auditor in connection with statutory and regulatory filings or engagements) which are proposed to be provided by the external auditors to our company or any subsidiary of our company.
Compensation Committee
We do not have a separately constituted compensation or remuneration committee. Our entire board of directors acts as the compensation committee. Our board of directors is solely responsible for determining the compensation to be paid to our executive officers and evaluating their performance. In addition, our board of directors reviews annually the total compensation package of each of our executives on an individual basis.
Our board of directors has not adopted any specific policies or objective for determining the amount or extent of compensation for directors or officers. There is no policy or target regarding allocation between cash and non-cash elements of our compensation program.
D. Employees
As of December 31, 2018, 2019 and 2020, we had 3, 3 and 27 employees, respectively. Our employees are not members of a labor union.
Our workforce is based out of our corporate office and Facility in Langley, British Columbia, Canada.
The breakdown of full-time employees by main category of activity and geographic location, as at December 31, 2020 is as follows:
Activity | Number of Full-Time
Employees | Location |
Research & Development | 4 | Langley, British Columbia, Canada |
Production | 17 | Langley, British Columbia, Canada |
Sales & Marketing | 1 | Langley, British Columbia, Canada |
General & Administration | 4 | Langley, British Columbia, Canada |
Executives | 1 | Langley, British Columbia, Canada |
E. Share Ownership
As of December 31, 2021, our directors and management beneficially owned the following common shares and options of our company:
Name and Office Held | Number of Common Shares
Owned and Percent of Total
Outstanding Common Shares | Common Shares that the individual has the right to
acquire within 60 days |
# of Shares | % of Class1 | Stock Options | Warrants | Convertible
Debentures |
Michael Forbes
Chief Executive Officer and Director | 1,284,0262 | 1.95% | 300,0005 | - | - |
Oliver Foeste
Chief Financial Officer | - | * | 300,0006 | - | - |
Donald Dinsmore
Chief Operating Officer, Corporate Secretary and Director | 85,597 | * | 233,3343 | - | - |
Paul Morgan
Director | | | 300,0005 | | |
Georges Routhier
Director | - | * | 116,6664 | - | - |
* denotes less than 1% of class of shares owned.
Notes:
1 Based on 65,970,547 common shares of our company issued and outstanding as at Nobember 5, 2021.
2 These common shares are held by MDC Forbes, a private company owned by Mr. Forbes.
3 Consists of 166,667 stock options exercisable at a price of $1.35 per share, for a period expiring on January 30, 2025, and 66,667 stock options exercisable at a price of $2.34 per share for a period expiring on August 4, 2025.
4 Consists of 83,333 stock options exercisable at $1.35 per share, for a period expiring on January 30, 2025, and 33,333 stock options exercisable at a price of $2.34 for a period expiring on August 4, 2025.
5 Consists of 300,000 stock options exercisable at a price of $1.06 per share, for a period expiring on October 25, 2026.
6 Consists of 300,000 stock options exercisable at a price of $1.06 per share, for a period expiring on October 25, 2026. These stock options are held by 1180777 B.C.Ltd, a private company influenced by Mr. Foeste.
Stock Option Plan
We have a stock option plan, which is a "rolling" stock option plan whereby a maximum of 10% of the issued shares of our company, from time to time, may be reserved for issuance pursuant to the exercise of options. The principal purposes of our stock option plan are to provide our company with the advantages of the incentive inherent in share ownership on the part of directors, employees, consultants of our company and our subsidiaries responsible for the continued success of our company and subsidiaries; to create in such persons a proprietary interest in, and a greater concern for, the welfare and success of our company and subsidiaries; to encourage such persons to remain with our company and subsidiaries; and to attract new directors, employees and consultants to our company and our subsidiaries.
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
To the best of our knowledge, there are no shareholders that are beneficial owners of 5% or more of our common shares.
B. Related Party Transactions
Other than as disclosed below, since the beginning of our preceding three financial years ended December 31, 2020 there have been no transactions or loans between our company and:
(a) enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common control with, our company;
(b) associates, meaning unconsolidated enterprises in which we have a significant influence or which have significant influence over our company;
(c) individuals owning, directly or indirectly, an interest in the voting power of our company that gives them significant influence over our company, and close members of any such individual's family (close members of an individual's family are those that may be expected to influence, or be influenced by, that person in their dealings with our company);
(d) key management personnel, that is, those persons having authority and responsibility for planning, directing and controlling the activities of our company, including directors and senior management of our company and close members of such individuals' families; and
(e) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (c) or (d) or over which such a person is able to exercise significant influence, including enterprises owned by directors or major shareholders of our company and enterprises that have a member of key management in common with our company.
During the year ended December 31, 2020, we granted the stock options to the following people who were management personnel or directors as of the date of grant or who are currently management personnel or directors:
Granted to | Grant date | Expiry date | Exercise price | Stock options granted |
| | | $ | # |
Andrew Hale | January 30, 2020 | January 30, 2025 | 1.35 | 666,667 |
Stephen Brohman | January 30, 2020 | January 30, 2025 | 1.35 | 333,333 |
Georges Routhier | January 30, 2020 | January 30, 2025 | 1.35 | 83,333 |
Blaine Bailey | January 30, 2020 | January 30, 2025 | 1.35 | 50,000 |
Donald Dinsmore | January 30, 2020 | January 30, 2025 | 1.35 | 166,667 |
Priyanka Nalawade | January 30, 2020 | January 30, 2025 | 1.35 | 166,667 |
Andrew Hale | August 4, 2020 | August 4, 2025 | 2.34 | 483,333 |
Stephen Brohman | August 4, 2020 | August 4, 2025 | 2.34 | 250,000 |
Georges Routhier | August 4, 2020 | August 4, 2025 | 2.34 | 33,333 |
Blaine Bailey | August 4, 2020 | August 4, 2025 | 2.34 | 33,333 |
Donald Dinsmore | August 4, 2020 | August 4, 2025 | 2.34 | 66,667 |
Priyanka Nalawade | August 4, 2020 | August 4, 2025 | 2.34 | 66,667 |
The aggregate value of transactions and outstanding balances with key management personnel and directors and entities over which they have control or significant influence were as follows:
| | | Transactions for the nine
months ended September 30, | | | Balances outstanding
September 30, | |
| | | 2021 | | | 2020 | | | 2021 | | | 2020 | |
| | | $ | | | $ | | | $ | | | $ | |
| DBM CPA | | 61,091 | | | 114,750 | | | - | | | 12,600 | |
| Andrew Hale | | 47,479 | | | 135,070 | | | - | | | - | |
| Donald Dinsmore | | 52,083 | | | - | | | - | | | - | |
| MDC Forbes | | 25,000 | | | - | | | 15,750 | | | - | |
| Pipedreemz | | - | | | 2,500 | | | - | | | - | |
| Invictus Accounting Group LLP | | 31,263 | | | - | | | 6,996 | | | - | |
| | | 216,916 | | | 252,320 | | | 22,746 | | | 12,600 | |
| | | Transactions for the years
ended December 31, | | | Balances outstanding
December 31, | |
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
| | | $ | | | $ | | | $ | | | $ | |
| DBM CPA | | 150,900 | | | 107,298 | | | 12,915 | | | - | |
| Andrew Hale | | 180,091 | | | 120,115 | | | - | | | - | |
| Pipedreemz | | 2,500 | | | 17,500 | | | - | | | - | |
| | | 333,491 | | | 244,913 | | | 12,915 | | | - | |
All related party balances are unsecured and are due within thirty days without interest.
The above transactions with the key management personnel and directors are included in operating expenses as follows:
Donaldson Brohman Martin CPA Inc.
The amounts consist of the professional fees charged by DBM CPA for the accounting and tax services of Stephen Brohman, our former Chief Financial Officer and director. Mr. Brohman is a principal of DBM CPA, a firm in which he has significant influence.
Andrew Hale
The amounts consist of charges by Andrew Hale, our former Chief Executive Officer and director, for salaries paid.
Pipedreemz Inc.
The amounts consist of the consulting fees charged to our company by Pipedreemz Inc. for advisory services to our company. George Routhier, a director of our company, is the owner of Pipedreemz Inc.
Invictus Accounting Group LLP
The amounts consist of the professional fees charged by Invictus Accounting Group LLP for the accounting and tax services of Oliver Foeste, our Chief Financial Officer. Mr. Foeste is the Managing Partner of Invictus Accounting Group LLP, a firm in which he has significant influence.
Cannabis Supply agreement with Sitka Weed Works Inc
On October 7, 2021 the Company entered into a supply and purchase agreement with Sitka Weed Works. This is a related party transaction because Michael Forbes is a director and controlling shareholder of Sitka Weed Works Inc. Under the terms of the agreement, Sitka Weed Works Inc. will supply cannabis products to the Company on a non-exclusive basis and the Company shall purchase the cannabis products on a non-exclusive basis. These cannabis products will be used by the Company as raw materials for its production lines.
C. Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
A. Consolidated Statements and Other Financial Information
See "Item 18. Financial Statements".
Export Sales
None.
Legal Proceedings
None.
Dividends
We have not declared any dividends since our inception and do not anticipate that we will do so in the foreseeable future. We currently intend to retain future earnings, if any, to finance the development of our business. Any future payment of dividends or distributions will be determined by our board of directors on the basis of our earnings, financial requirements and other relevant factors.
B. Significant Changes
The management of our company is not aware of any significant changes since the date of our most recent annual financial statements that have not been disclosed elsewhere in this registration statement.
Item 9. The Offer and Listing.
A. Offer and Listing Details
Principal Market
Our common shares are listed for trading on the Canadian Securities Exchange under the symbol "XTRX". Our common shares also trade on the Frankfurt Stock Exchange under the symbol "D2EP".
Our common shares are in registered form and the transfer of our common shares is managed by our transfer agent, National Securities Administrators Ltd., located at Suite 702, 777 Hornby Street, Vancouver, British Columbia V6Z 1S4, Canada.
B. Plan of Distribution
Not applicable.
C. Markets
See "Item 9.A Offer Listing and Details".
On July 15, 2013, the Securities and Exchange Commission issued an order pursuant to Section 12(j) of the Securities Exchange Act of 1934 (the "Exchange Act") revoking registration of common shares of Gulfside Industries Ltd. (n/k/a Consolidated Gulfside Resources, Ltd.) registered pursuant to Section 12(g) of the Exchange Act. Gulfside Industries Ltd. was a former name of our company at that time. The registration was revoked because we were delinquent in our periodic filings with the Securities and Exchange Commission having not filed any periodic reports since we filed a Form 10-Q for the period ended March 31, 1995.
Upon this registration statement becoming effective, we may seek a broker-dealer to have quotations for our common shares entered on the OTC Link alternative trading system maintained by OTC Markets Group Inc. There is no assurance that our common shares will trade on the OTC Link or on any other market or exchange in the United States, or if our common shares are traded on a market or exchange in the United States, that a liquid public market for those shares will materialize. There is currently no public market in the United States for our common shares and investors in the United States may have difficulty reselling our common shares.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
Item 10. Additional Information.
A. Share Capital
We are authorized to issue an unlimited number of common shares without par value.
On December 31, 2020, we had 43,334,100 common shares outstanding.
On December 31, 2021, we had 65,970,547 common shares outstanding, subsequent to the issuance of 2,513,720 common shares for the acquisition of PerceiveMD, 20,000,000 common shares for the acquisition of 1204581 and 122,727 common shares from a non-brokered private placement. All of our common shares issued and outstanding were fully paid and non-assessable. There are no shares not representing capital. Our Company or subsidiaries do not own any shares of our company.
On December 31, 2019, we had 36,228,941 common shares issued and outstanding. During the year ended December 31, 2020, we issued a total of 7,105,159 common shares so that we had 43,334,100 common shares issued and outstanding as at December 31, 2020.
More than 10% of our capital has not been paid for with assets other than cash within the past five years.
Warrants
As at December 31, 2020, we had the following outstanding warrants to purchase our common shares:
Expiry date | Exercise price | Warrants outstanding and exercisable |
| $ | # |
January 17, 2022 | 2.25 | 189,934 |
January 27, 2022 | 2.25 | 266,760 |
July 10, 2022 | 2.25 | 200,589 |
July 13, 2022 | 1.50 | 3,882,667 |
July 16, 2022 | 2.25 | 759,605 |
July 28, 2022 | 2.25 | 953,564 |
August 5, 2022 | 2.25 | 161,688 |
December 19, 2022 | 1.80 | 1,921,185 |
Total outstanding | | 8,335,992 |
As at December 31, 2021, we had the following outstanding warrants to purchase our common shares:
Expiry date | Exercise price | Warrants outstanding and exercisable |
| $ | # |
January 17, 2022 | 2.25 | 189,934 |
January 27, 2022 | 2.25 | 266,760 |
July 10, 2022 | 2.25 | 200,589 |
July 13, 2022 | 1.50 | 3,882,667 |
July 16, 2022 | 2.25 | 759,605 |
July 28, 2022 | 2.25 | 953,564 |
August 5, 2022 | 2.25 | 161,688 |
December 19, 2022 | 1.80 | 1,921,185 |
October 18, 2023 | 1.75 | 122,727 |
Total outstanding | | 8,458,719 |
Stock Options
As at December 31, 2020, we had the following outstanding stock options to purchase our common shares:
Expiry date | Exercise price | Stock options outstanding and exercisable |
| $ | # |
June 1, 2022 | 1.35 | 66,667 |
January 30, 2025 | 1.35 | 2,483,333 |
August 5, 2025 | 2.34 | 1,616,667 |
Total outstanding | | 4,166,667 |
As at December 31, 2021, we had the following outstanding stock options to purchase our common shares:
Expiry date | Exercise price | Stock options outstanding and exercisable |
| $ | # |
June 1, 2022 | 1.35 | 66,667 |
January 30, 2025 | 1.35 | 1,750,001 |
August 5, 2025 | 2.34 | 750,000 |
August 4, 2026 | 1.35 | 33,333 |
October 25, 2026 | 1.06 | 900,000 |
November 2, 2026 | 0.95 | 215,000 |
Total outstanding | | 3,715,001 |
Other Convertible Obligations or Other Outstanding Equity-Linked Securities, or Subscription Rights
Other than the warrants and stock options described above, as at December 31, 2020 and as at December 31, 2021, we had no outstanding convertible obligations or other outstanding equity-linked securities.
Issuances of Securities
During the last three years, we have issued the following securities:
Since the Fiscal Year Ended December 31, 2020
On October 28, 2021, we granted an aggregate of 215,000 stock options to certain employees and a consultant for the purchase of up to 215,000 common shares at a price of $0.95 per share. Each stock option is exercisable for a period of five years.
On October 25, 2021, we granted an aggregate of 900,000 stock options to certain directors and officers for the purpose of up to 900,000 common shares at a price of $1.06 per share. Each stock option is exercisable for a period of five years.
On October 18, 2021, we completed a non-brokered private placement whereby we issued 122,727 units at a price of $1.10 per unit for gross proceeds of $135,000. Each unit was comprised of one common share and one transferrable common share purchase warrant with each warrant entitling the holder thereof to acquire one common share at a price of $1.75 per share for two years from the date of the closing. The warrants are subject to an acceleration provision whereby if the daily closing price of the common shares as quoted on the Canadian Securities Exchange closes at or above $2 per share for 50 consecutive trading days, then we may accelerate the expiration date of the warrants to the date that is 30 trading days from the date that notice of such acceleration is given via news release. From and after the new accelerated expiration date, no warrants may be exercised, and all unexercised warrants will be void.
On August 4, 2021, we granted an aggregate of 33,333 stock options to a consultant for the purchase of up to 33,333 common shares at a price of $1.35 per share. Each stock option is exercisable for a period of five years.
On April 9, 2021, we consolidated our issued share capital on a ratio of three old common shares for every one new post-consolidated common share. The share and per share information in this section do not reflect such consolidation.
On August 31, 2021, we issued an aggregate of 2,513,720 common shares to the former shareholders of PerceiveMD in consideration for their respective shares of PerceiveMD.
On September 17, 2021, we issued an aggregate of 20,000,000 common shares to the former shareholders of 1204581 in consideration for their respective shares of 1204581.
Fiscal Year Ended December 31, 2020
On January 17, 2020, we issued 189,934 units on conversion of $250,000 of principal and $6,411 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
On January 27, 2020, we issued 266,760 units on conversion of $350,000 of principal and $10,126 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
On July 10, 2020, we issued 200,589 units on conversion of $250,000 of principal and $20,795 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued
On July 13, 2020, we completed a non-brokered private placement whereby 3,882,667 units were issued at a price of $0.90 per unit for gross proceeds of $3,494,400. Each unit consisted one common share and one common share purchase warrant with each warrant exercisable into one common share at an exercise price of $1.50 until July 13, 2022. Additionally, we settled services and outstanding indebtedness of $543,175 through the issuance of 647,280 common shares at a fair value price of $0.84 per share.
On July 16, 2020, we issued 759,605 units on conversion of $945,000 of principal and $80,467 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
On July 28, 2020, we issued 953,564 units on conversion of $1,182,000 of principal and $105,311 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
On August 5, 2020, we issued 161,688 units on conversion of $200,000 of principal and $18,279 of accrued interest of the convertible debenture. Each unit consisted of one common share of our company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
During the year ended December 31, 2020, we issued 42,967 common shares related to the exercise of 42,967 warrants at an exercise price of $1.80 per share.
Fiscal Year Ended December 31, 2019
On December 20, 2019, we completed a non-brokered private placement whereby 5,892,456 units were issued at a price of $0.45 per unit for gross proceeds of $2,651,605. Each unit consisted of one common share and one common share purchase warrant with each warrant exercisable into one common share at an exercise price of $0.60 for three years from the date of closing December 20, 2022.
On December 20, 2019, we issued 21,656,034 common shares at a price of $0.05 per share to the shareholders of Arrowstar as consideration for the RTO of Arrowstar by Adastra.
Fiscal Year Ended April 30, 2019
On June 20, 2018, we issued 1 common share for $1 on incorporation, this incorporation share was cancelled on February 7, 2019.
On February 7, 2019, we closed a private placement for the issuance of 26,000,000 common shares at a price of $0.005 per share for gross proceeds of $130,000.
On February 28, 2019, we closed a private placement for the issuance of 21,750,000 common shares at a price of $0.02 per share for gross proceeds of $435,000.
On March 7, 2019, we closed a private placement for the issuance of 21,053,333 common shares at a price of $0.075 per share for gross proceeds of $1,579,000.
On March 31, 2019, we closed a private placement for the issuance of 8,203,333 common shares at a price of $0.15 per share for gross proceeds of $1,230,500.
On April 30, 2019, we closed a private placement for the issuance of 4,131,667 common shares at a price of $0.3 per share for gross proceeds of $1,239,500.
B. Memorandum and Articles of Association
Our Company was incorporated on October 14, 1987 under the laws of the Province of British Columbia. Our corporation number is BC0334777. Our Company is governed by the Business Corporations Act (British Columbia) (the "BCBCA"), and its Notice of Articles (which sets forth the name and authorized share structure of our company), and its Articles (which sets forth rules for our company's conduct) are described below.
Objects and Purposes
Our Articles do not contain, and are not required under the laws of British Columbia, to contain a description of the our company's objects or purposes. There are no restrictions in our Notice of Articles or Articles on the business that we may carry on.
Powers of Directors
Our directors are responsible for managing and supervising the management of the affairs and business of our Company and have authority to exercise all such powers of our company that are not required by the BCBCA or our Articles, to be exercised by our shareholders.
Each director holds office until our next annual general meeting or until he or she is removed, dies or his or her office is earlier vacated in accordance with our Articles or with the provisions of the BCBCA. A director appointed or elected to fill a vacancy on our board holds office until our next annual general meeting. Under our Articles, a director is not required to hold any shares of our company to qualify as a director or officer.
The directors are entitled to the remuneration, if any, for acting as directors as the directors may from time to time determine. If the directors so decide, the remuneration of the directors will be determined by the shareholders. That remuneration may be in addition to any salary or other remuneration paid to a director in such director's capacity as an officer or employee of our company. Our Articles do not contain any requirements with respect to the determination of remuneration of the directors in the absence of an independent quorum.
Pursuant to our Articles, any director that holds a material interest in any contract or transaction that is material to our company (a "disclosable interest") must disclose that interest to the board, and abstain from voting to approve such contract or transaction, unless all of our directors have a disclosable interest in that contract or transaction, in which case any or all of the directors may vote. Interested directors may, however, be counted for purposes of determining if a quorum is present at a meeting of the directors at which such contract or transaction is to be considered. Directors or officers with a disclosable interest in a contract or transaction is not liable to account for any profits if the disclosable interest is disclosed and the transaction is approved by the disinterested directors or by our shareholders by way of a special resolution approved by a 2/3 majority of the votes cast. Transactions or contracts in which a director or officer has a disclosable interest are not invalid or void as a result of the interest, even if the necessary disclosures are not made or the approvals are not obtained.
Our Articles provide that our company, if authorized by its directors, may:
● borrow money in the manner and amount, on the security, from the sources and on the terms and conditions that the directors consider appropriate;
● issue bonds, debentures and other debt obligations either outright or as security for any liability or obligation of our company or any other person and at such discounts or premiums and on such other terms as the directors consider appropriate;
● guarantee the repayment of money by any other person or the performance of any obligation of any other person; and
● mortgage, charge, whether by way of specific or floating charge, grant a security interest in, or give other security on, the whole or any part of the present and future assets and undertaking of our company.
Our Articles and the BCBCA do not provide for any mandatory retirement age for directors.
Common Shares
We are authorized to issue an unlimited number of common shares without par value. The holders of our common shares are entitled to vote at all meetings of our shareholders, to receive dividends if, as and when declared by our board of directors and to participate ratably in any distribution of property or assets upon the liquidation, winding-up or other dissolution of our company. Our common shares carry no pre-emptive rights, conversion or exchange rights, redemption, retraction, repurchase, sinking fund or purchase fund provisions. There are no provisions requiring the holders of our common shares to contribute additional capital and there are no restrictions on the issuance of additional securities by our company. There are no restrictions on the repurchase or redemption of our common shares, or the declaration of any dividends or distributions, by our company except to the extent that any such repurchase, redemption, dividend or distribution would render our company insolvent pursuant to the BCBCA.
Any alteration or variation to the special rights and restrictions attached to any class or series of any of our issued and outstanding shares will require approval by a separate resolution passed by a 2/3 of the holders of that class or series.
Meetings of the Shareholders
Meetings of our shareholders may be located anywhere in British Columbia or, if approved by the Issuer's directors, any location outside of British Columbia.
Our Articles and the BCBCA provide that our annual meetings of shareholders must be held at least once in each calendar year and not more than 15 months after the last annual general meeting at such time and place as our directors may determine.
Our directors may, at any time, call a meeting of shareholders. Under the BCBCA, the holders of not less than 5% of the Issuer's issued shares that carry the right to vote at a meeting may requisition our directors to call a meeting of shareholders for the purposes of transacting any business that may be transacted at a general meeting.
Shareholders entitled to vote at meetings are entitled to attend any meeting of shareholders. In addition, the directors, the president, if any, the secretary, if any, and any lawyer or auditor for the Issuer are entitled to attend any meeting of shareholders, but if any of those persons do attend a meeting of shareholders, that person is not to be counted in the quorum, and is not entitled to vote at the meeting, unless that person is a shareholder or proxy holder entitled to vote at the meeting.
Under our Articles, the quorum for the transaction of business at a meeting of our shareholders is two persons that are, or who represent by proxy, shareholders holding at least 5% of the shares entitled to vote at the meeting.
Limitations on Share Ownership
There are no limitations under the BCBCA or our Articles with respect to the rights of non-Canadians to own or vote shares of our company. The Investment Canada Act (Canada) provides for the review of certain significant transactions in Canada by non-Canadians that could be injurious to national security.
Changes in Control
There are no provisions in our Article that would have the effect of delaying, deferring or preventing a change in control of our company, and that would operate only with respect to a merger, acquisition or corporate restructuring involving our company or our subsidiaries.
Disclosures of Share Ownership
There are no provisions in our Articles requiring the disclosure of an ownership interest in our shares above any threshold. In general, under applicable securities regulation in Canada, a person or company who beneficially owns, directly or indirectly, voting securities of an issuer or who exercises control or direction over voting securities of an issuer or a combination of both, carrying more than 10% of the voting rights attached to all the issuer's outstanding voting securities is an insider and must, within 10 days of becoming an insider, file a report in the required form effective the date on which the person became an insider. The report must disclose any direct or indirect beneficial ownership of, or control or direction over, securities of the reporting issuer. Additionally, securities regulation in Canada provides for the filing of a report by an insider of a reporting issuer whose holdings change, which report must be filed within five days from the day on which the change takes place.
The rules in the U.S. governing the ownership threshold above which shareholder ownership must be disclosed are more stringent than those discussed above. Section 13 of the Exchange Act imposes reporting requirements on persons who acquire beneficial ownership (as such term is defined in Rule 13d-3 under the Exchange Act) of more than 5% of a class of an equity security registered under Section 12 of the Exchange Act. In general, such persons must file, within 10 days after such acquisition, a report of beneficial ownership with the Securities and Exchange Commission containing the information prescribed by the regulations under Section 13 of the Exchange Act. This information is also required to be sent to the issuer of the securities and to each exchange where the securities are traded.
Upon the effectiveness of this registration statement, United States federal securities laws will require our company to disclose, in its annual reports on Form 20-F, holders who own 5% or more of our issued and outstanding voting shares.
C. Material Contracts
There are no other contracts, other than those disclosed below and those entered into in the ordinary course of our business, that are material to our company and which were entered into in the last two completed fiscal years or which were entered into before the two most recently completed fiscal years but are still in effect as of the date of this registration statement:
- On May 14, 2019, we entered into an agreement with United Science, LLC for the purchase of various equipment necessary for our business. The total equipment costs totaled $756,900.
- On March 12, 2020, we entered into a supply agreement with CannMart Labs Inc. to supply certain cannabis and marijuana extract and concentrate products to CannMart Labs. This agreement is subject to the condition that both parties receive the required approvals from Health Canada.
- On April 3, 2020, we entered into a toll processing agreement with Acreage Pharms Ltd. Pursuant to this agreement, we agreed to provide standard processing services to Acreage Pharms Ltd. Standard processing, in this agreement, means altering the physical or chemical properties of cannabis and/or hemp by the use of a solvent to produce cannabis or hemp products. This agreement is subject to the condition that both parties receive the required approvals from Health Canada.
- On May 6, 2020, we entered into a toll processing agreement with Muskoka Grown Ltd. Pursuant to this agreement, we agreed to provide standard processing services to Muskoka Grown Ltd. Standard processing, in this agreement, means altering the physical or chemical properties of cannabis and/or hemp by the use of a solvent to produce cannabis or hemp products. This agreement is subject to the condition that both parties receive the required approvals from Health Canada.
- On June 1, 2020, we entered into a toll processing agreement with BC Hop Company Ltd. Pursuant to this agreement, we agreed to provide standard processing services to BC Hop Company Ltd. Standard processing, in this agreement, means altering the physical or chemical properties of cannabis and/or hemp by the use of a solvent to produce cannabis or hemp products. This agreement is subject to the condition that both parties receive the required approvals from Health Canada.
- On August 5, 2020, we entered into an agreement with Evolved Extraction Solutions Ltd. for the purchase of various equipment necessary for our business. The total equipment costs totaled $760,440.
- On August 17, 2020, we entered into a supply agreement with Pure Sunfarms Corp for cannabis products. We agreed to purchase a minimum of 6,000 Kilograms of cannabis product from Pure Sunfarms at a price of $0.20 per gram. This agreement had a term of 6 months.
- On September 4, 2020, we entered into an agreement with ExtractionTek Sales LLC for the purchase of various equipment necessary for our business. The total equipment costs totaled $284,010.
- On October 15, 2020, we entered into a letter of engagement with Proactive Investors Canada who agreed to provide investor relation services involving media promotion and coverage of our company. The letter of engagement contemplates a one year term and we agreed to pay Proactive $22,501 for their services.
- On January 15, 2021, we entered into a toll processing agreement with SynerGenetics Bioscience Inc. Pursuant to this agreement, we agreed to provide standard processing services to SynerGenetics Bioscience Inc. Standard processing, in this agreement, means altering the physical or chemical properties of cannabis and/or hemp by the use of a solvent to produce cannabis or hemp products. This agreement is subject to the condition that both parties receive the required approvals from Health Canada.
- On April 13, 2021, we entered into a marketing services agreement with Hybrid Financial Ltd. Hybrid Financial has agreed to assist in all aspects of a marketing campaign for our company. Hybrid Financial will, among other things, contact investment professionals and provide updates on their marketing services to garner interest in our securities. Hybrid Financial will be paid a cash sum for their services.
- On June 9, 2021, we, Adastra Labs Inc. as purchaser and Phyto Extractions Inc as guarantor, entered into a supply agreement with Pure Sunfarms Corp. Pursuant to this agreement we agreed to purchase bulk amounts of cannabis-related products from Pure Sunfarms at various price points depending on the amount of products purchased.
- On August 10, 2021, we, Adastra Labs Inc. as purchaser and Adastra Holdings Ltd. as a contributor towards the purchase price, entered into a Share Purchase Agreement with Jean Paul Lim and MDC Forbes Inc. for the purchase of all of the issued and outstanding shares of Perceive MD. Pursuant to this agreement, we agreed to pay the shareholders of Perceive MD an amount of Shares equal to $2,290,000.00 as calculated on the basis of the daily volume weighted average of actual trading prices (measured in hundredths of cents) for 10 consecutive trading days immediately prior to the closing date of the acquisition.
- On September 15, 2021, we, Adastra Labs Inc. as purchaser and Adastra Holdings Ltd., entered into a Share Purchase Agreement with the shareholders of 1204581 for the purchase of all the issued and outstanding shares of 1204581. Pursuant to this agreement, we agreed to pay 1204581 shareholders 20,000,000 common shares equal to $19,200,000.
D. Exchange Controls
There are no government laws, decrees or regulations in Canada that restrict the export or import of capital or that affect the remittance of dividends, interest or other payments to non-resident holders of our common shares. However, the remittance of dividends or other payments to United States residents or other non-residents of Canada may be subject to withholding taxes or may be required to be reported under the Proceeds of Crime (Money Laundering) and Terrorist Financing Act.
E. Taxation
Certain Canadian Federal Income Tax Considerations for United States Residents
The following is a summary of certain Canadian federal income tax considerations generally applicable to the holding and disposition of our securities acquired by a holder who, at all relevant times, (a) for the purposes of the Income Tax Act (Canada) (i) is not resident, or deemed to be resident, in Canada, (ii) deals at arm's length with us, and is not affiliated with us, (iii) holds our common shares as capital property, (iv) does not use or hold the common shares in the course of carrying on, or otherwise in connection with, a business carried on or deemed to be carried on in Canada and (v) is not a "registered non-resident insurer" or "authorized foreign bank" (each as defined in the Income Tax Act (Canada)), or other holder of special status, and (b) for the purposes of the Canada-U.S. Tax Convention (the "Tax Treaty"), is a resident of the United States, has never been a resident of Canada, does not have and has not had, at any time, a permanent establishment or fixed base in Canada, and who otherwise qualifies for the full benefits of the Tax Treaty. Holders who meet all the criteria in clauses (a) and (b) above are referred to herein as "U.S. Holders", and this summary only addresses such U.S. Holders.
This summary does not deal with special situations, such as the particular circumstances of traders or dealers, tax exempt entities, insurers or financial institutions, or other holders of special status or in special circumstances. Such holders, and all other holders who do not meet the criteria in clauses (a) and (b) above, should consult their own tax advisors.
This summary is based on the current provisions of the Income Tax Act (Canada), the regulations thereunder in force at the date hereof, the current provisions of the Tax Treaty, and our understanding of the administrative and assessing practices of the Canada Revenue Agency published in writing prior to the date hereof. This summary takes into account all specific proposals to amend the Income Tax Act (Canada) and regulations thereunder publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the "Proposed Amendments") and assumes that such Proposed Amendments will be enacted in the form proposed. However, such Proposed Amendments might not be enacted in the form proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative or assessing practices, whether by legislative, governmental or judicial decision or action, nor does it take into account tax laws of any province or territory of Canada or of any other jurisdiction outside Canada, which may differ significantly from those discussed in this summary.
For the purposes of the Income Tax Act (Canada), all amounts relating to the acquisition, holding or disposition of our securities must generally be expressed in Canadian dollars. Amounts denominated in United States currency generally must be converted into Canadian dollars using the rate of exchange that is acceptable to the Canada Revenue Agency.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular U.S. Holder, and no representation with respect to the Canadian federal income tax consequences to any particular U.S. Holder or prospective U.S. Holder is made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, all prospective purchasers (including U.S. Holders as defined above) should consult with their own tax advisors for advice with respect to their own particular circumstances.
Withholding Tax on Dividends
Amounts paid or credited or deemed to be paid or credited as, on account or in lieu of payment of, or in satisfaction of, dividends on our common shares to a U.S. Holder will be subject to Canadian withholding tax. Under the Tax Treaty, the rate of Canadian withholding tax on dividends paid or credited by us to a U.S. Holder that beneficially owns such dividends and substantiates eligibility for the benefits of the Tax Treaty is generally 15% (unless the beneficial owner is a company that owns at least 10% of our voting stock at that time, in which case the rate of Canadian withholding tax is generally reduced to 5%).
Dispositions
A U.S. Holder will not be subject to tax under the Income Tax Act (Canada) on a capital gain realized on a disposition or deemed disposition of a security, unless the security is "taxable Canadian property" to the U.S. Holder for purposes of the Income Tax Act (Canada) and the U.S. Holder is not entitled to relief under the Tax Treaty.
Generally, our common shares will not constitute "taxable Canadian property" to a U.S. Holder at a particular time unless, at any time during the 60 month period immediately preceding the disposition, more than 50% of the fair market value of such security was derived, directly or indirectly, from one or any combination of: (i) real or immoveable property situated in Canada, (ii) "Canadian resource properties" (as defined in the Income Tax Act (Canada)), (iii) "timber resource properties" (as defined in the Income Tax Act (Canada)), and (iv) options in respect of, or interests in, or for civil law rights in, property described in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain other circumstances set out in the Income Tax Act (Canada), common shares could also be deemed to be "taxable Canadian property".
If our common shares become listed on a "designated stock exchange" as defined in the Income Tax Act (Canada) and are so listed at the time of disposition, our common shares generally will not constitute "taxable Canadian property" of a U.S. Holder at that time unless, at any time during the 60 month period immediately preceding the disposition, the following two conditions are met: (i) the U.S. Holder, persons with whom the U.S. Holder did not deal at arm's length, partnerships in which the U.S. Holder or such non-arm's length person holds a membership interest (either directly or indirectly through one or more partnerships), or the U.S. Holder together with all such persons, owned 25% or more of the issued shares of any class or series of shares of our company; and (ii) more than 50% of the fair market value of the shares of our company was derived directly or indirectly from one or any combination of real or immovable property situated in Canada, Canadian resource properties (as defined in the Income Tax Act (Canada)), timber resource properties (as defined in the Income Tax Act (Canada)) or options in respect of, or interests in, or for civil law rights in, property described in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain other circumstances set out in the Income Tax Act (Canada), common shares could also be deemed to be "taxable Canadian property".
U.S. Holders who may hold common shares as "taxable Canadian property" should consult their own tax advisors with respect to the application of Canadian capital gains taxation, any potential relief under the Tax Treaty, and special compliance procedures under the Income Tax Act (Canada), none of which is described in this summary.
Certain Material United States Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from the acquisition, ownership and disposition of our common shares. This summary applies only to U.S. Holders that acquire our common shares pursuant to this prospectus and does not apply to any subsequent U.S. Holder of our common shares.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder as a result of the acquisition, ownership and disposition of our common shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including specific tax consequences to a U.S. Holder under an applicable tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any particular U.S. Holder. In addition, this summary does not address the U.S. federal alternative minimum, net investment income, U.S. federal estate and gift, U.S. Medicare contribution, U.S. state and local, or non-U.S. tax consequences of the acquisition, ownership or disposition of our common shares. Except as specifically set forth below, this summary does not discuss applicable tax reporting requirements.
Each U.S. Holder should consult its own tax advisor regarding all U.S. federal, U.S. state and local and non-U.S. tax consequences of the acquisition, ownership and disposition of our common shares.
No opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the "IRS") has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership or disposition of our common shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, or contrary to, any position taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of This Disclosure
Authorities
This summary is based on the Internal Revenue Code, as amended (the "Code"), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the "Canada-U.S. Tax Convention"), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date hereof. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis, which could affect the U.S. federal income tax considerations described in this summary. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term "U.S. Holder" means a beneficial owner of our common shares that is for U.S. federal income tax purposes:
• an individual who is a citizen or resident of the U.S.;
• a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the U.S., any state thereof or the District of Columbia;
• an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
• a trust that
a. is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions; or
b. has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations of the acquisition, ownership or disposition of our common shares by U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) broker-dealers, dealers, or traders in securities or currencies that elect to apply a "mark-to-market" accounting method; (d) U.S. Holders that have a "functional currency" other than the U.S. dollar; (e) U.S. Holders that own our common shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquire our common shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold our securities other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); and (h) U.S. Holders that own directly, indirectly, or by attribution, 10% or more, by voting power, of our outstanding stock.
This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Income Tax Act (Canada); (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold our securities in connection with carrying on a business in Canada; (d) persons whose securities in our company constitute "taxable Canadian property" under the Income Tax Act (Canada); or (e) persons that have a permanent establishment in Canada for purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately above, should consult their own tax advisors regarding all U.S. federal, U.S. state and local, and non-U.S. tax consequences (including the potential application and operation of any income tax treaties) relating to the acquisition, ownership or disposition of our common shares.
If an entity or arrangement that is classified as a partnership (or other "pass-through" entity) for U.S. federal income tax purposes holds our common shares, the U.S. federal income tax consequences to such partnership and the partners (or other owners) of such partnership of the acquisition, ownership or disposition of our common shares generally will depend on the activities of the partnership and the status of such partners (or other owners). This summary does not address the U.S. federal income tax considerations for any such partner or partnership (or other "pass-through" entity or its owners). Owners of entities and arrangements that are classified as partnerships (or other "pass-through" entities) for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences of the acquisition, ownership or disposition of our common shares.
Ownership and Disposition of Our Common Shares
Distributions on Our Common Shares
Subject to the "passive foreign investment company" ("PFIC") rules discussed below (see "Tax Consequences if Our Company is a PFIC"), a U.S. Holder that receives a distribution, including a constructive distribution, with respect to our common shares will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of the current or accumulated "earnings and profits" of our company, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated "earnings and profits" of our company, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder's tax basis in our common shares and thereafter as gain from the sale or exchange of such common shares (see "Sale or Other Taxable Disposition of Our Common Shares" below). However, we may not maintain calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by our company with respect to our common shares will constitute a dividend. Dividends received on our common shares generally will not be eligible for the "dividends received deduction" available to U.S. corporate shareholders receiving dividends from U.S. corporations. If our company is eligible for the benefits of the Canada-U.S. Tax Convention or our common shares is readily tradable on an established securities market in the U.S., dividends paid by our company to non-corporate U.S. Holders generally will be eligible for the preferential tax rates applicable to long-term capital gains, provided certain holding period and other conditions are satisfied, including that our company not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Our Common Shares
Subject to the PFIC rules discussed below, upon the sale or other taxable disposition of our common shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount of cash plus the fair market value of any property received and such U.S. Holder's tax basis in the common shares sold or otherwise disposed of. Such capital gain or loss will be long-term capital gain or loss if, at the time of the sale or other taxable disposition, the U.S. Holder's holding period for such security is more than one year. Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
PFIC Status of Our Company
If our company is or becomes a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the ownership and disposition of our common shares. The U.S. federal income tax consequences of owning and disposing of our common shares if our company is or becomes a PFIC are described below under the heading "Tax Consequences if Our Company is a PFIC."
A non-U.S. corporation is a PFIC for each tax year in which (i) 75% or more of its gross income is passive income (as defined for U.S. federal income tax purposes) (the "income test") or (ii) on average for such tax year, 50% or more (by value) of its assets either produces or is held for the production of passive income (the "asset test"). For purposes of the PFIC provisions, "gross income" generally includes sales revenues less cost of goods sold, plus income from investments and from incidental or outside operations or sources, and "passive income" generally includes dividends, interest, certain rents and royalties, and certain gains from commodities or securities transactions. In determining whether or not it is a PFIC, a non-U.S. corporation is required to take into account its pro rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value).
Under certain attribution and indirect ownership rules, if our company is a PFIC, U.S. Holders will generally be deemed to own their proportionate shares of our company's direct or indirect equity interest in any company that is also a PFIC (a "Subsidiary PFIC").
Our Company does not know if it currently is a PFIC or was a PFIC in a prior year and, based on current business plans and financial projections, does not know if it will be a PFIC in subsequent tax years. The determination of PFIC status is inherently factual, is subject to a number of uncertainties, and can be determined only annually after the close of the tax year in question. Additionally, the analysis depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. We might be determined to be a PFIC for the current tax year or any prior or future tax year, and no opinion of legal counsel or ruling from the IRS concerning the status of our company as a PFIC has been obtained or will be requested. U.S. Holders should consult their own U.S. tax advisors regarding the PFIC status of our company.
Tax Consequences if Our Company is a PFIC
If our company is a PFIC for any tax year during which a U.S. Holder owns our common shares, special rules may increase such U.S. Holder's U.S. federal income tax liability with respect to the ownership and disposition of such common shares. If our company meets the income test or the asset test for any tax year during which a U.S. Holder owns our common shares, our company will be treated as a PFIC with respect to such U.S. Holder for that tax year and for all subsequent tax years, regardless of whether our company meets the income test or the asset test for such subsequent tax years, unless the U.S. Holder elects to recognize any unrealized gain in such common shares or makes a timely and effective QEF Election or, if applicable, Mark-to-Market Election.
Under the default PFIC rules:
• any gain realized on the sale or other disposition (including dispositions and certain other events that would not otherwise be treated as taxable events) of our common shares (including an indirect disposition of the stock of any Subsidiary PFIC) and any "excess distribution" (defined as a distribution to the extent it, together with all other distributions received in the relevant tax year, exceeds 125% of the average annual distribution received during the preceding three years) received on our common shares or with respect to the stock of a Subsidiary PFIC will be allocated ratably to each day of such U.S. Holder's holding period for our common shares;
• the amount allocated to the current tax year and any year prior to the first year in which our company was a PFIC will be taxed as ordinary income in the current year;
• the amount allocated to each of the other tax years (the "Prior PFIC Years") will be subject to tax at the highest ordinary income tax rate in effect for the applicable class of taxpayer for that year;
• an interest charge will be imposed with respect to the resulting tax attributable to each Prior PFIC Year, which interest charge is not deductible by non-corporate U.S. Holders; and
• any loss realized on the disposition of our common shares generally will not be recognized.
A U.S. Holder that makes a timely and effective "mark-to-market" election under Section 1296 of the Code (a "Mark-to-Market Election") or a timely and effective election to treat our company and each Subsidiary PFIC as a "qualified electing fund" (a "QEF") under Section 1295 of the Code (a "QEF Election") may generally mitigate or avoid the PFIC consequences described above with respect to our common shares.
If a U.S. Holder makes a timely and effective QEF Election, the U.S. Holder must include currently in gross income each year its pro rata share of our company's ordinary income and net capital gains, regardless of whether such income and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary income or gains without a corresponding receipt of cash from our company. If our company is a QEF with respect to a U.S. Holder, the U.S. Holder's basis in our common shares will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in our common shares and will not be taxed again as a distribution to a U.S. Holder. Taxable gains on the disposition of our common shares by a U.S. Holder that has made a timely and effective QEF Election are generally capital gains. A U.S. Holder must make a QEF Election for our company and each Subsidiary PFIC if it wishes to have this treatment. To make a QEF Election, a U.S. Holder will need to have an annual information statement from our company setting forth the ordinary income and net capital gains for the year. U.S. Holders should be aware that we might not satisfy the recordkeeping requirements that apply to a QEF or supply U.S. Holders with information such U.S. Holders require to report under the QEF rules in the event that our company is a PFIC for any tax year.
In general, a U.S. Holder must make a QEF Election on or before the due date for filing its income tax return for the first year to which the QEF Election applies. Under applicable Treasury Regulations, a U.S. Holder will be permitted to make retroactive elections in particular circumstances, including if it had a reasonable belief that our company was not a PFIC and filed a protective election. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective QEF Election for our company and any Subsidiary PFIC.
A Mark-to-Market Election may be made with respect to stock in a PFIC if such stock is "regularly traded" on a "qualified exchange or other market" (within the meaning of the Code and the applicable Treasury Regulations). A class of stock that is traded on one or more qualified exchanges or other markets is considered to be "regularly traded" for any calendar year during which such class of stock is traded in other than de minimis quantities on at least 15 days during each calendar quarter. If our common shares are considered to be "regularly traded" within this meaning, then a U.S. Holder generally will be eligible to make a Mark-to-Market Election with respect to such security but not with respect to a Subsidiary PFIC.
When these securities become "regularly traded," a U.S. Holder that makes a timely and effective Mark-to-Market Election with respect to such securities generally will be required to recognize as ordinary income in each tax year in which our company is a PFIC an amount equal to the excess, if any, of the fair market value of such stock as of the close of such taxable year over the U.S. Holder's adjusted tax basis in such stock as of the close of such taxable year. A U.S. Holder's adjusted tax basis in our securities generally will be increased by the amount of ordinary income recognized with respect to such stock. If the U.S. Holder's adjusted tax basis in our securities as of the close of a tax year exceeds the fair market value of such stock as of the close of such taxable year, the U.S. Holder generally will recognize an ordinary loss, but only to the extent of net mark-to-market income recognized with respect to such stock for all prior taxable years. A U.S. Holder's adjusted tax basis in our securities generally will be decreased by the amount of ordinary loss recognized with respect to such stock. Any gain recognized upon a disposition of our common shares or warrants generally will be treated as ordinary income, and any loss recognized upon a disposition generally will be treated as ordinary loss to the extent of the net mark-to-market income recognized for all prior taxable years. Any loss recognized in excess thereof will be taxed as a capital loss. Capital losses are subject to significant limitations under the Code. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective Mark-to-Market Election with respect to our common shares.
Foreign Tax Credit
A U.S. Holder that pays (whether directly or through withholding) Canadian income tax in connection with the ownership or disposition of our common shares may be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder's U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder's income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all creditable foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year.
Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder's U.S. federal income tax liability that such U.S. Holder's "foreign source" taxable income bears to such U.S. Holder's worldwide taxable income. In applying this limitation, a U.S. Holder's various items of income and deduction must be classified, under complex rules, as either "foreign source" or "U.S. source." Generally, dividends paid by a non-U.S. corporation should be treated as foreign source for this purpose, and gains recognized on the sale of securities of a non-U.S. corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to our common shares that is treated as a "dividend" may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution, including a constructive distribution, from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
Receipt of Foreign Currency
The amount of any distribution or proceeds paid in Canadian dollars to a U.S. Holder in connection with the ownership, sale or other taxable disposition of our common shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
Information Reporting; Backup Withholding
Under U.S. federal income tax law, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of "specified foreign financial assets" includes not only financial accounts maintained in non-U.S. financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract that has an issuer or counterparty other than a U.S. person and any interest in a non-U.S. entity. A U.S. Holder may be subject to these reporting requirements unless such U.S. Holder's shares of our common shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938 for specified foreign financial assets, filing obligations relating to the PFIC rules including possible reporting on IRS Form 8621, and any other applicable reporting requirements.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on our common shares, and (b) proceeds arising from the sale or other taxable disposition of our common shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 24%, may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder's correct U.S. taxpayer identification number ("TIN") (generally on Form W-9), (b) furnishes an incorrect U.S. TIN, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. TIN and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules are allowed as a credit against a U.S. Holder's U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from dividend withholding tax or otherwise eligible for a reduced withholding rate.
The discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
Certain Reporting Requirements
A U.S. Holder that acquires common shares generally will be required to file Form 926 with the IRS if (1) immediately after the acquisition such U.S. Holder, directly or indirectly, owns at least 10% of the common shares, or (2) the amount of cash transferred in exchange for common shares during the 12-month period ending on the date of the acquisition exceeds US$100,000. Significant penalties may apply for failing to satisfy these filing requirements. U.S. Holders are urged to contact their tax advisors regarding these filing requirements.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP OR DISPOSITION OF OUR COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
F. Dividends and Paying Agents
There is no dividend restriction; however, we have not declared any dividends since our inception and do not anticipate that we will do so in the foreseeable future. We currently intend to retain future earnings, if any, to finance the development of our business. Any future payment of dividends or distributions will be determined by our board of directors on the basis of our earnings, financial requirements and other relevant factors.
There is no special procedure for non-resident holders to claim dividends. Any remittances of dividends to United States residents and to other non-residents are, however, subject to withholding tax. See "Taxation" above.
G. Statement by Experts
The financial statements of our company as of December 31, 2020, 2019 and 2018 included in this registration statement have been audited by Davidson & Company LLP, located at 1200 - 609 Granville Street, Vancouver, British Columbia V7Y 1G6, Canada, as stated in their reports appearing in this registration statement and have been so included in reliance upon the reports of such firm given upon their authority as experts in accounting and auditing.
H. Documents on Display
Upon the effectiveness of this registration statement, we will be subject to the reporting requirements of the Exchange Act, as applicable to "foreign private issuers" as defined in Rule 3b-4 under the Exchange Act and applicable Canadian securities legislation and, in accordance therewith, will file certain reports with, and furnish other information to, each of the Securities and Exchange Commission and certain securities commissions or similar regulatory authorities of Canada.
As a foreign private issuer, we intend to file our annual financial statements on Form 20-F and furnish our quarterly interim financial statements on Form 6-K to the Securities and Exchange Commission. However, the information we file or furnish is not the same as the information that is required in annual and quarterly reports on Form 10-K or Form 10-Q for U.S. domestic issuers. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a U.S. domestic issuer.
The Securities and Exchange Commission maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Securities and Exchange Commission. Such filings are available to the public over the internet at the Securities and Exchange Commission's website at http://www.sec.gov.
Our corporate website address is https://phytoextractions.ca. The information contained on, or that may be accessed through, our corporate website is not part of, and is not incorporated into, this registration statement.
I. Subsidiary Information
Not applicable.
Item 11. Quantitative and Qualitative Disclosures About Market Risk.
Investing in the common shares of our company involves risk. Prospective investors should carefully consider the risks described below, together with all of the other information included in this prospectus before making an investment decision. If any of the following risks actually occurs, the business, financial condition or results of operations of our company could be harmed. In such an event, the trading price of the common shares could decline and prospective investors may lose part or all of their investment.
Financial instruments - fair value
Our financial instruments consist of cash, trade receivables, deposits, accounts payable and accrued liabilities, mortgage payable and loan payable.
The carrying value of accounts payable and accrued liabilities, and loan payable approximated their fair value because of the short-term nature of these instruments. Mortgage payable approximate fair value due to its market rates of interest charged.
Financial instruments measured at fair value on the consolidated statements of financial position are summarized into the following fair value hierarchy levels:
Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices).
Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
Financial instruments - risk
Our financial instruments are exposed to certain financial risks, including credit risk, liquidity risk and currency risk.
Credit risk
We are exposed to credit risk by holding cash. This risk is minimized by holding the funds in Canadian banks and credit unions or with Canadian governments. We have minimal receivable exposure, and its various refundable credits are due from Canadian governments.
Liquidity risk
Liquidity risk is the risk that we are unable to meet our financial obligations as they come due. We manage this risk by careful management of our working capital to ensure our expenditures will not exceed available resources.
Currency risk
We are not exposed to currency risk.
Economic dependence
Economic dependence risk is the risk of reliance upon a select number of customers which significantly impact the financial performance of our company. During the year ended December 31, 2020, our sales were generated by three customers representing 79%, 16% and 5%.
Item 12. Description of Securities Other than Equity Securities.
Not applicable.
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies.
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds.
Not applicable.
Item 15. Controls and Procedures.
Not applicable.
Item 16 [Reserved]
Not applicable.
Item 16A Audit Committee Financial Expert
Not applicable.
Item 16B. Code of Ethics.
Not applicable.
Item 16C. Principal Accountant Fees and Services.
Not applicable.
Item 16D. Exemptions from the Listing Standards for Audit Committees.
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers.
Not applicable.
Item 16F. Change in Registrant's Certifying Accountant.
Not applicable.
Item 16G. Corporate Governance.
Not applicable.
Item 16H. Mine Safety Disclosure
Not applicable.
PART III
Item 17. Financial Statements.
Not applicable.
Item 18. Financial Statements.
Adastra Holdings Inc.
1204581 B.C. Ltd.
Adastra Holdings Inc. - Pro Forma Financial Information

Asdastra Holdings Ltd.
(formerly Phyto Extractions Inc.)
Condensed Interim Consolidated Financial Statements
For the Three and Nine months ended
September 30, 2021 and 2020
(Expressed in Canadian dollars - Unaudited)
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Condensed Interim Consolidated Statements of Financial Position
As at September 30, 2021 and 2020
(Expressed in Canadian dollars - Unaudited)
| | Note | | September 30, 2021 | | | December 31, 2020 | |
| | | | $ | | | $ | |
| ASSETS | | | | | | | |
| Current assets | | | | | | | |
| Cash | | | 1,207,688 | | | 1,145,461 | |
| Receivables and prepayments | 6 | | 1,517,799 | | | 1,129,189 | |
| Income tax receivable | | | 30,000 | | | - | |
| Inventory | 7 | | 2,077,355 | | | 1,421,237 | |
| Deposits | | | 34,756 | | | - | |
| | | | 4,867,598 | | | 3,695,887 | |
| | | | | | | | |
| Long-term deposits | | | 116,000 | | | 4,000 | |
| Goodwill and intangible assets | 4,5 | | 25,781,422 | | | - | |
| Property, plant, and equipment | 8 | | 9,873,008 | | | 10,037,063 | |
| | | | | | | | |
| Total assets | | | 40,638,028 | | | 13,736,950 | |
| | | | | | | | |
| LIABILITIES | | | | | | | |
| Current liabilities | | | | | | | |
| Accounts payable and accrued liabilities | 10 | | 1,874,313 | | | 1,147,964 | |
| Current portion of lease liability | 12 | | 10,425 | | | - | |
| Mortgage payable | 11 | | 3,498,728 | | | 2,442,830 | |
| | | | 5,383,466 | | | 3,590,794 | |
| | | | | | | | |
| Lease liability | 12 | | 24,240 | | | - | |
| Government loan | | | 60,000 | | | 60,000 | |
| | | | | | | | |
| Total liabilities | | | 5,467,706 | | | 3,650,794 | |
| | | | | | | | |
| EQUITY | | | | | | | |
| Share capital | 9 | | 41,833,128 | | | 15,822,152 | |
| Reserves | 9 | | 5,461,270 | | | 5,441,814 | |
| Subscriptions received | 9 | | 135,000 | | | - | |
| Deficit | | | (12,259,076 | ) | | (11,177,810 | ) |
| Total equity | | | 35,170,322 | | | 10,086,156 | |
| | | | | | | | |
| Total liabilities and equity | | | 40,638,028 | | | 13,736,950 | |
Nature of operations and going concern (Note 1)
Subsequent events (Note 16)
Approved on behalf of the Board of Directors on November 29, 2021:
"Michael Forbes" | | "Donald Dinsmore" |
Director | | Director |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Condensed Interim Consolidated Statements of Loss and Comprehensive Loss
For the three and nine months ended September 30, 2021 and 2020
(Expressed in Canadian dollars, except number of shares - Unaudited)
| | | | Three months ended | | | Nine months ended | |
| | | | | | | September 30, | | | | | | September 30, | |
| | Note | | 2021 | | | 2020 | | | 2021 | | | 2020 | |
| | | | $ | | | $ | | | $ | | | $ | |
| | | | | | | | | | | | | | |
| Revenue | | | 1,808,111 | | | 825,903 | | | 3,639,012 | | | 1,254,258 | |
| | | | | | | | | | | | | | |
| Cost of sales | 7,8 | | (874,433 | ) | | (875,485 | ) | | (1,972,771 | ) | | (1,088,721 | ) |
| Gross profit (loss) | | | 933,678 | | | (49,582 | ) | | 1,666,241 | | | 165,537 | |
| | | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | | |
| Advertising and promotion | | | 193,132 | | | 433,859 | | | 328,484 | | | 694,380 | |
| Accretion | | | - | | | 14,768 | | | - | | | 58,405 | |
| Depreciation | 8 | | 61,583 | | | 21,750 | | | 103,695 | | | 70,261 | |
| Insurance | | | 25,142 | | | 21,442 | | | 62,246 | | | 51,843 | |
| Automobile expenses | | | 5,761 | | | - | | | 5,761 | | | - | |
| Office expenses | | | 132,577 | | | 150,854 | | | 282,686 | | | 390,335 | |
| Professional fees and consulting | 10 | | 196,519 | | | 174,913 | | | 404,169 | | | 570,814 | |
| Research expenses | | | 13,535 | | | - | | | 60,199 | | | - | |
| Share-based payments | 9 | | 19,456 | | | 2,795,200 | | | 19,456 | | | 5,332,500 | |
| Travel | | | 31,759 | | | 1,192 | | | 34,132 | | | 2,827 | |
| Wages and salaries | 10 | | 248,511 | | | 154,063 | | | 742,346 | | | 353,050 | |
| Total operating expenses | | | 927,975 | | | 3,768,041 | | | 2,043,174 | | | 7,524,415 | |
| | | | | | | | | | | | | | |
| Income (loss) from operations | | | 5,703 | | | (3,817,623 | ) | | (376,933 | ) | | (7,358,878 | ) |
| | | | | | | | | | | | | | |
| Other income (expense) | | | | | | | | | | | | | |
| Finance expense | 11,14 | | (98,767 | ) | | (78,096 | ) | | (206,121 | ) | | (350,378 | ) |
| Interest income | | | - | | | - | | | 600 | | | 4,419 | |
| Gain on settlement of accounts payable | | | - | | | - | | | 57,500 | | | - | |
| Impairment of property, plant and equipment | 8 | | - | | | - | | | (150,000 | ) | | - | |
| Realized loss on marketable securities | | | - | | | - | | | - | | | (1,500 | ) |
| Write-down of inventory | 7 | | (114,800 | ) | | - | | | (406,312 | ) | | - | |
| Net loss and comprehensive loss for the period | | | (207,864 | ) | | (3,895,719 | ) | | (1,081,266 | ) | | (7,706,337 | ) |
| | | | | | | | | | | | | | |
| Net loss per share | | | | | | | | | | | | | |
| Basic and diluted | | | (0.00 | ) | | (0.10 | ) | | (0.02 | ) | | (0.20 | ) |
| | | | | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | | | | |
| Basic and diluted | | | 44,908,364 | | | 38,511,533 | | | 48,039,591 | | | 38,473,462 | |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Condensed Interim Consolidated Statements of Changes in Equity
For the nine months ended September 30, 2021 and 2020
(Expressed in Canadian dollars, except number of shares - Unaudited)
| | | Common | | | | | | Debenture | | | | | | Subscriptions | | | | | | | |
| | | shares | | | Share capital | | | reserves | | | Reserves | | | received | | | Deficit | | | Total equity | |
| | | # | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
| December 31, 2019 | | 36,228,941 | | | 8,348,407 | | | 298,461 | | | - | | | - | | | (3,561,946 | ) | | 5,084,922 | |
| Convertible debentures - settlement | | 2,532,140 | | | 3,418,390 | | | (298,461 | ) | | 70,814 | | | - | | | - | | | 3,190,743 | |
| Units issued for cash | | 3,882,667 | | | 3,494,400 | | | - | | | - | | | - | | | - | | | 3,494,400 | |
| Share issue cost | | - | | | (60,100 | ) | | - | | | 38,500 | | | - | | | - | | | (21,600 | ) |
| Shares issued for cash - warrants | | 42,967 | | | 77,340 | | | - | | | - | | | - | | | - | | | 77,340 | |
| Shares issued - services | | 156,894 | | | 131,791 | | | - | | | - | | | - | | | - | | | 131,791 | |
| Shares issued - debt settlement | | 490,491 | | | 411,924 | | | - | | | - | | | - | | | - | | | 411,924 | |
| Share-based payment | | - | | | - | | | - | | | 5,332,500 | | | - | | | - | | | 5,332,500 | |
| Loss for the period | | - | | | - | | | - | | | - | | | - | | | (7,706,337 | ) | | (7,706,337 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| September 30, 2020 | | 43,334,100 | | | 15,822,152 | | | - | | | 5,441,814 | | | - | | | (11,268,283 | ) | | 9,995,683 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Income for the period | | - | | | - | | | - | | | - | | | - | | | 90,473 | | | 90,473 | |
| December 31, 2020 | | 43,334,100 | | | 15,822,152 | | | - | | | 5,441,814 | | | - | | | (11,177,810 | ) | | 10,086,156 | |
| Subscriptions received | | - | | | - | | | - | | | - | | | 135,000 | | | - | | | 135,000 | |
| Shares issued on acquisition of Perceive MD | | 2,513,720 | | | 2,010,976 | | | - | | | - | | | - | | | - | | | 2,010,976 | |
| Shares issued on acquisition of Phyto BrandCo | | 20,000,000 | | | 24,000,000 | | | - | | | - | | | - | | | - | | | 24,000,000 | |
| Share based payments | | - | | | - | | | - | | | 19,456 | | | - | | | - | | | 19,456 | |
| Loss for the period | | - | | | - | | | - | | | - | | | - | | | (1,081,266 | ) | | (1,081,266 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| September 30, 2021 | | 65,847,820 | | | 41,833,128 | | | - | | | 5,461,270 | | | 135,000 | | | (12,259,076 | ) | | 35,170,322 | |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Condensed Interim Consolidated Statements of Cash Flows
For the nine months ended September 30, 2021 and 2020
(Expressed in Canadian dollars - Unaudited)
| | | Nine months ended September 30, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| Operating activities | | | | | | |
| Loss for the period | | (1,081,266 | ) | | (7,706,337 | ) |
| Adjustments for non-cash items: | | | | | | |
| Accretion | | - | | | 58,405 | |
| Depreciation | | 103,695 | | | 70,261 | |
| Depreciation - cost of sales | | 618,451 | | | 318,060 | |
| Finance expense | | 206,121 | | | 343,942 | |
| Interest income | | (600 | ) | | (4,419 | ) |
| Share-based payments | | 19,456 | | | 5,332,500 | |
| Services paid in shares | | - | | | 131,791 | |
| Realized loss on marketable securities | | - | | | 1,500 | |
| Write-down of inventory | | 406,312 | | | - | |
| Impairment of property, plant and equipment | | 150,000 | | | - | |
| | | | | | | |
| Net change in non-cash working capital items: | | | | | | |
| Receivables and prepayments | | 34,691 | | | 271,157 | |
| Income tax receivable | | (4,000 | ) | | - | |
| Inventory | | (1,062,430 | ) | | (1,155,172 | ) |
| Deposits | | (146,756 | ) | | - | |
| Accounts payable and accrued liabilities | | 81,091 | | | 80,392 | |
| Cash used in operating activities | | (675,235 | ) | | (2,257,920 | ) |
| | | | | | | |
| Financing activities | | | | | | |
| Proceeds on private placement of units, net of share issue costs | | - | | | 3,472,800 | |
| Proceeds received on warrant exercises | | - | | | 77,340 | |
| Proceeds received on mortgage renewal | | 1,002,877 | | | - | |
| Borrowing costs on mortgage | | - | | | (18,345 | ) |
| Interest paid on mortgage | | (151,662 | ) | | (147,781 | ) |
| Proceeds on loan | | - | | | 40,000 | |
| Cash provided by financing activities | | 851,215 | | | 3,424,014 | |
| | | | | | | |
| Investing activities | | | | | | |
| Consideration paid on acquisition of Perceive MD | | (10,000 | ) | | - | |
| Cash received from the acquisition of PerceiveMD | | 26,302 | | | - | |
| Cash received from the acquisition of Phyto BrandCo | | 301,966 | | | - | |
| Acquisition of property, plant and equipment | | (432,621 | ) | | (1,238,249 | ) |
| Interest income | | 600 | | | 4,419 | |
| Cash used in investing activities | | (113,753 | ) | | (1,233,830 | ) |
| | | | | | | |
| Net increase (decrease) in cash | | 62,227 | | | (67,736 | ) |
| Cash, beginning of period | | 1,145,461 | | | 2,376,826 | |
| | | | | | | |
| Cash, end of period | | 1,207,688 | | | 2,309,090 | |
Supplemental cash flow information (Note 13)
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
NOTE 1 - NATURE OF OPERATIONS AND GOING CONCERN
Adastra Holdings Ltd. (formerly Phyto Extractions Inc.) (the "Company") was incorporated under the laws of the province of British Columbia on October 14, 1987. The Company is an extraction and processing solutions company. The Company's mission is to develop and deploy large-scale cannabis and hemp extraction technologies and provide turn-key processing solutions to help licensed standard and micro-cultivators maximize the value of every harvest. The Company is listed on the Canadian Securities Exchange ("CSE") under the symbol "XTRX". The Company's registered and records office is 5451 275th Street, Langley City, British Columbia, V4W 3X8.
On October 19, 2019, Health Canada issued an Analytical testing license on the Company's facility to Chemia Analytics Inc., ("Chemia"). Chemia is a wholly owned subsidiary of the Company. On March 13, 2020, Health Canada issued a Standard Processing License to the Company's facility to Adastra labs Inc., ("Labs"). Labs is a wholly owned subsidiary of the Company.
On December 20, 2019, Arrowstar Resources Ltd. ("Arrowstar") acquired all of the issued and outstanding common shares of Adastra Labs Holdings (2019) Ltd. ("Adastra") a private British Columbia cannabis extraction and processing solutions company incorporated on June 18, 2018. The acquisition was completed by entering into a share exchange agreement whereby the parties completed a business combination by way of a transaction that constituted a reverse takeover ("RTO") of the Company by Adastra (the "Transaction"). The Transaction was accounted for as an RTO of Arrowstar by Adastra for accounting purposes, with Adastra being identified as the accounting acquirer, and accordingly, these financial statements are a continuation of Adastra. The net assets of Arrowstar at the date of the RTO are deemed to have been acquired by Adastra (Note 3). These unaudited condensed interim consolidated financial statements (the "financial statements") include the results of operations of Arrowstar since December 20, 2019. The comparative figures are those of Adastra prior to the RTO. At the time of the transaction the Company changed its fiscal year end from April 30 to December 31.
On August 10, 2021, the Company completed the acquisition, indirectly through its wholly-owned subsidiary Adastra Labs Inc. ("Adastra") of all of the issued and outstanding shares of 1225140 B.C. Ltd., doing business as PerceiveMD ("PerceiveMD") from the shareholders of PerceiveMD, pursuant to the terms of a share purchase agreement dated August 10, 2021. In consideration for the acquisition of PerceiveMD, the Company paid to the vendors an aggregate purchase price of $2,020,976 consisting of $10,000 in cash and $2,010,976 by way of the issuance of 2,513,720 common shares in the capital of the Company (see Note 4).
On September 1, 2021, the Company changed its name to Adastra Holdings Ltd. (formerly Phyto Extractions Inc.). Trading of the Company's common shares resumed under the new name and under the same ticker symbol "XTRX" on the Canadian Securities Exchange as of market opened on September 1, 2021. Prior to this on April 9, 2021 the Company changed its name from Adastra Labs Holdings Ltd. to Phyto Extractions Inc. and on December 19, 2019 from Arrowstar Resources Ltd to Adastra Labs Holdings Ltd.
On September 15, 2021, the Company completed its previously-announced acquisition of privately held 1204581 B.C. Ltd., doing business as Phyto Extractions ("Phyto BrandCo"), the owner of the intellectual property rights for the Phyto Extractions brand. At closing, the Company issued 20 million common shares to the former shareholders of Phyto BrandCo based on a deemed share price of $0.96 per share, which resulted in total consideration of $24 million based on the market price of $1.20 on closing (see Note 5). The acquisition is an arm's length transaction, and there is no finder's fee payable on closing.
On April 9, 2021, the Company consolidated its issued share capital on a ratio of three (3) old common shares for every one (1) new post-consolidated common share. All current and comparative references to the number of common shares, weighted average number of common shares, loss per share, stock options and warrants have been restated to give effect to this Share Consolidation.
NOTE 1 - NATURE OF OPERATIONS AND GOING CONCERN (continued)
These condensed interim consolidated financial statements ("interim financial statements) are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue its operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of operations. These interim financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities that might be necessary should the Company be unable to continue in existence.
The Company's ability to continue as a going concern is dependent on its ability to generate positive cash flows from operations, complete additional financings, and/or extend or modify its mortgage payable (Note 11).
As at September 30, 2021, the Company had a working capital deficiency of $515,868 (December 31, 2020 - working capital surplus of $105,093), incurred a net loss of $207,864 for the three months ended September 30, 2021 (2020 - net loss of $3,895,719) and net loss of $1,081,266 for the nine months ended September 30, 2021 (2020 - $7,706,337). These events and conditions indicate a material uncertainty exists that may cast significant doubt on the Company's ability to continue as a going concern. If the going concern assumption were not appropriate for these financial statements it could be necessary to restate the Company's assets and liabilities on a liquidation basis.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of presentation
These interim financial statements have been prepared in conformity with International Accounting Standard 34
- Interim Financial Reporting, using the same accounting policies as detailed in the Company's annual audited financial statements for the year ended December 31, 2020, and do not include all the information required for full annual financial statements in accordance with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards Board ("IASB") and interpretations of the International Financial Reporting Interpretations Committee ("IFRIC"). It is suggested that these financial statements be read in conjunction with the annual audited financial statements.
These interim financial statements have been prepared on an historical cost basis, except for financial instruments measured at fair value. In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information.
All amounts on these financial statements are presented in Canadian dollars which is the functional currency of the Company and its subsidiaries.
(b) Reclassification of prior amounts
The Company has reclassified certain items on the condensed interim consolidated statements of cash flows to conform with current period presentation.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (continued)
(c) Principles of consolidation
These financial statements include the financial information of the Company and its subsidiaries. The financial statements include the accounts of the Company and the following subsidiaries:
| Functional | Ownership |
| currency | percentage |
Adastra Labs Holdings (2019) Ltd. (formerly Adastra Labs Holdings Ltd.) | CAD | 100% |
Adastra Labs Inc. | CAD | 100% |
1178562 | B.C. Ltd. | CAD | 100% |
Adastra Brands Inc. | CAD | 100% |
Chemia Analytics Inc. | CAD | 100% |
1125140 | B.C. Ltd (PerceiveMD) | CAD | 100% |
1204581 | B.C. Ltd. (Phyto BrandCo) | CAD | 100% |
Subsidiaries are entities controlled by the Company and are included in the financial statements from the date that control commences until the date that control ceases. The accounting policies of subsidiaries are changed where necessary to align them with the policies adopted by the Company.
The accounting policies, estimates and critical judgments, methods of computation and presentation applied in these financial statements are consistent with those of the most recent annual audited financial statements and are those the Company expects to adopt in its annual financial statements for the year ended December 31, 2021. Accordingly, these financial statements should be read in conjunction with the Company's most recent annual audited financials.
(d) Standards issued but not yet effective
Certain pronouncements have been issued by the IASB or IFRIC that are effective for accounting periods beginning on or after January 1, 2021. The Company has reviewed these updates and determined that many of these updates are not applicable or consequential to the Company and have been excluded from discussion within these significant accounting policies.
(e) Significant accounting judgements and key sources of estimate uncertainty
The information about significant areas of estimation uncertainty considered by management in preparing these financial statements is as follows
COVID-19 estimation uncertainty
On March 11, 2020, the World Health Organization declared the outbreak of the novel coronavirus (COVID-19) a global pandemic. This has resulted in governments worldwide, including the Canadian government, enacting emergency measures to combat the spread of the virus. These measures, which include social distancing, the implementation of travel bans, and closures of non-essential businesses, have caused material disruption to businesses globally, resulting in an economic slowdown. The production and sale of cannabis have been recognized as essential services across Canada. As at September 30, 2021, the Company has not observed any material impairments of assets or a significant change in the fair value of assets, due to the COVID-19 pandemic.
The situation is dynamic and the ultimate duration and magnitude of the impact of COVID-19 on the economy and the financial effect on our business, financial position and operating results remain unknown at this time. In addition, it is possible that estimates in the Company's financial statements will change in the near term as a result of COVID-19 and the effect of any such changes could be material, which could result in, among other things, impairment of long-lived assets. The Company is closely monitoring the impact of the pandemic on all aspects of its business.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair value of stock options and finders' compensation options
Determining the fair value of stock options and compensatory options (finders' options) requires estimates related to the choice of a pricing model, the estimation of stock price volatility, the fair value of the Company's common shares, the expected forfeiture rate and the expected term of the underlying instruments. Any changes in the estimates or inputs utilized to determine fair value could have a significant impact on the Company's future operating results or on other components of shareholders' equity.
Acquisitions
Determination of whether a set of assets acquired and liabilities assumed constitute the acquisition of a business or asset requires the Company to make certain judgments as to whether or not the assets acquired and liabilities assumed include the inputs, processes and outputs necessary to constitute a business as defined in IFRS 3 - Business Combinations. If an acquired set of assets and liabilities includes goodwill, the set is presumed to be a business. Based on an assessment of relevant facts and circumstances, the Company concluded that the acquisition disclosed in Note 3 was acquisition of assets. The values assigned to common shares and the allocation of the purchase price to the net liabilities in the acquisition are based on numerous estimates and judgements of the relative fair values of net liabilities.
The information about significant areas of judgment considered by management in preparing these financial statements is as follows:
Going concern
The assessment of the Company's ability to continue as a going concern as discussed in Note 1 involves judgment regarding future funding available for its operations and working capital requirements.
NOTE 3 - REVERSE ACQUISITION
As described in Note 1, on December 20, 2019, Arrowstar and Adastra completed the Transaction which constituted a reverse acquisition.
The Transaction resulted in the shareholders of Adastra obtaining control of the combined entity by obtaining control of the voting rights, governance and management decision making processes, and the resulting power to govern the financial and operating policies of the combined entity.
The transaction constitutes an RTO of Arrowstar by Adastra and has been accounted for as a reverse acquisition transaction in accordance with the guidance provided in IFRS 2 - Share-based payments. As Arrowstar did not qualify as a business according to the definition in IFRS 3, the RTO does not constitute a business combination; rather it is treated as an issuance of common shares by Adastra for the net assets of Arrowstar and Arrowstar's public listing, with Adastra as the continuing entity. Accordingly, no goodwill was recorded with respect to the transaction as it does not constitute a business combination.
For accounting purposes, Adastra was treated as an accounting parent company (legal subsidiary) and Arrowstar has been treated as the accounting subsidiary (legal parent) in these financial statements. As Adastra was deemed to be the acquirer for accounting purposes, its assets, liabilities, and operations since incorporation are included in these financial statements at their historical carrying values. Arrowstar's results of operations have been included from December 20, 2019.
NOTE 3 - REVERSE ACQUISITION (continued)
| | | December 20, | |
| | | 2019 | |
| Assets and (liabilities) of Arrowstar acquired | | $ | |
| Cash | | 111,496 | |
| Receivables | | 10,802 | |
| Other assets | | 7,504 | |
| Accounts payable | | (356,384 | ) |
| Net liabilities acquired | | (226,582 | ) |
| | | | |
| Consideration paid in RTO of Arrowstar | | | |
| Common shares (7,218,678 common shares at $0.15 fair value per share) | | 1,082,802 | |
| | | | |
| Listing expense | | 1,309,384 | |
The Transaction was measured at the fair value of the shares that Adastra would have to issue to the shareholders of Arrowstar, to give the shareholders of Arrowstar the same percentage of equity interest in the combined entity that results from the reverse acquisition had it taken the legal form of Adastra acquiring Arrowstar.
NOTE 4 - ACQUISITION OF PERCEIVEMD
On August 10, 2021, the Company acquired, indirectly through its wholly-owned subsidiary, Adastra Labs Inc, all of the issued and outstanding shares of PerceiveMD. At closing, the Company issued 2,513,720 million common shares to the former shareholders of PerceiveMD at a share price on the date of acquisition of $0.80 per share, for total consideration of $2.02 million.
PerceiveMD is a multidisciplinary, patient-focused center providing comprehensive assessments for medical cannabis and other therapies. The acquisition will allow the Company to generate revenue from providing cannabis under medical prescriptions.
The transaction has been accounted for as a business combination under IFRS 3 - Business Combinations. The preliminary allocation of the purchase consideration is as follows, noting that the Company intends to engage valuation experts to assist with the identification and measurement of intangible assets separate from goodwill:
| Assets acquired: | | $ | |
| Cash | | 26,302 | |
| Accounts receivable | | 13,647 | |
| Corporate taxes receivable | | 26,000 | |
| | | 65,949 | |
| Liabilities assumed: | | | |
| Accounts payable and other accrued liabilities | | (19,206 | ) |
| Fair value of net assets acquired | | 46,743 | |
| | | | |
| Purchase consideration | | | |
| Share consideration | | 2,010,976 | |
| Cash consideration | | 10,000 | |
| | | 2,020,976 | |
| | | | |
| Goodwill and intangible assets | | 1,974,233 | |
The carrying value of the assets and liabilities acquired equates to fair value due to their short term nature.
NOTE 4 - ACQUISITION OF PERCEIVEMD (continued)
Included in the consolidated statement of loss and comprehensive loss is $71,532 of revenue and $2,987 of income from PerceiveMD since the acquisition date of August 10, 2021. If the acquisition occurred on January 1, 2021, management estimates that revenue would have increased by $578,571 and net loss would have been decreased by approximately $62,625, respectively.
The Company's acquisition of PerceiveMD constitutes a related party transaction as Michael Forbes, Chief Executive Officer and a director of the Company is also a director and controlling shareholder of PerceiveMD.
NOTE 5 - ACQUISITION OF PHYTO BRANDCO
On September 15, 2021, the Company acquired, indirectly through its wholly-owned subsidiary, Adastra Labs Inc, all of the issued and outstanding shares of Phyto BrandCo., the owner of the intellectual property rights for the Phyto Extractions brand. At closing, the Company issued 20 million common shares to the former shareholders of Phyto BrandCo at a share price on the date of acquisition of $1.20 per share, for total consideration of $24 million.
Phyto BrandCo licenses its intellectual property to Canadian cannabis license holders and collects royalties by selling cannabis consumer packaged goods to provincial distributors and retailers. The Company expects to realize synergies by leveraging Phyto BrandCo's suite of branded products to drive revenue and develop integration efficiencies.
The transaction has been accounted for as a business combination under IFRS 3 - Business Combinations. The preliminary allocation of the purchase consideration is as follows, noting that the Company intends to engage valuation experts to assist with the identification and measurement of intangible assets separate from goodwill:
| Assets acquired: | | $ | |
| Cash | | 301,966 | |
| Accounts receivable | | 255,154 | |
| Prepayments | | 19,500 | |
| Property and equipment | | 85,108 | |
| Trademarks | | 35,930 | |
| | | 697,658 | |
| Liabilities assumed: | | | |
| Accounts payable and other accrued liabilities | | (434,252 | ) |
| Lease liability | | (34,665 | ) |
| | | (468,917 | ) |
| | | | |
| Fair value of net identifiable assets acquired | | 228,741 | |
| | | | |
| Purchase consideration | | | |
| Share consideration | | 24,000,000 | |
| | | 24,000,000 | |
| | | | |
| Goodwill and intangible assets | | 23,771,259 | |
The carrying value of the assets and liabilities acquired equates to fair value due to their short term nature, other than property and equipment and trademarks which are depreciated over their estimated useful economic lives.
The trademarks of $35,930 represent 21 registered trademarks the Company has with the Canadian Intellectual Property Office ("CIPO"). These trademarks have finite lives and are measured at cost less accumulated amortization and impairment losses. These trademarks are amortized on a straight-line basis over their estimated useful lives. Useful lives, residual values, and amortization methods for intangible assets with finite useful lives are reviewed at least annually.
NOTE 5 - ACQUISITION OF PHYTO BRANDCO (continued)
The lease liability represents one lease with a fair value of $34,665 on the date of acquisition, which is the net present value of the minimum future lease payments determined using the following assumptions: (1) remaining number of payments - 36; (2) monthly payment - $1,119; and (3) incremental borrowing rate - 10%.
Included in the consolidated statement of loss and comprehensive loss is $45,776 of revenue and $37,116 of losses from Phyto BrandCo since the acquisition date of September 15, 2021. If the acquisition occurred on January 1, 2020, management estimates that revenue would have increased by $1,325,872 and net loss would have been reduced by approximately $187,803, respectively.
NOTE 6 - RECEIVABLES AND PREPAYMENTS
Receivables and prepayments consisted of the following:
| | | September 30, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| Trade accounts receivable | | 1,325,644 | | | 971,102 | |
| Sales tax recoverable | | - | | | 1,578 | |
| Prepaid expenses | | 192,155 | | | 156,509 | |
| | | 1,517,799 | | | 1,129,189 | |
NOTE 7 - INVENTORY
Inventory consisted of the following:
| | | September 30, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| Extracted cannabis and hemp oils (finished goods) | | 1,199,671 | | | 908,117 | |
| Production work in process - distillate | | 339,210 | | | 273,937 | |
| Dried cannabis and hemp biomass | | 538,474 | | | 239,183 | |
| | | 2,077,355 | | | 1,421,237 | |
Inventory expensed to cost of sales in the three and nine months ended September 30, 2021 was $823,916 and $1,798,780, respectively (three and nine months ended September 30, 2020 - $875,485 and $1,088,721, respectively).
NOTE 8 - PROPERTY, PLANT AND EQUIPMENT
| | | | | | | | | Furniture and | | | Computer | | | Laboratory | | | Extraction | | | Building | | | | |
| | | Land | | | Building | | | equipment | | | software | | | equipment | | | equipment | | | improvements | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
| Cost | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 | | 1,592,232 | | | 1,999,328 | | | 59,442 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,863,759 | | | 8,637,663 | |
| Additions | | - | | | - | | | 25,407 | | | - | | | 149,100 | | | 1,862,707 | | | 64,522 | | | 2,101,736 | |
| December 31, 2020 | | 1,592,232 | | | 1,999,328 | | | 84,849 | | | 12,105 | | | 211,395 | | | 2,911,209 | | | 3,928,281 | | | 10,739,399 | |
| Acquisition of Phyto BrandCo | | - | | | - | | | 85,108 | | | - | | | - | | | - | | | - | | | 85,108 | |
| Additions | | - | | | - | | | 25,201 | | | 150,000 | | | 179,004 | | | 268,778 | | | - | | | 622,983 | |
| Impairment | | - | | | - | | | - | | | (150,000 | ) | | - | | | - | | | - | | | (150,000 | ) |
| September 30, 2021 | | 1,592,232 | | | 1,999,328 | | | 195,158 | | | 12,105 | | | 390,399 | | | 3,179,987 | | | 3,928,281 | | | 11,297,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 | | - | | | 122,679 | | | 7,467 | | | - | | | - | | | - | | | - | | | 130,146 | |
| Depreciation | | - | | | 93,832 | | | 12,936 | | | 1,816 | | | 20,527 | | | 296,978 | | | 146,101 | | | 572,190 | |
| December 31, 2020 | | - | | | 216,511 | | | 20,403 | | | 1,816 | | | 20,527 | | | 296,978 | | | 146,101 | | | 702,336 | |
| Depreciation | | - | | | 70,374 | | | 16,635 | | | 1,542 | | | 55,228 | | | 429,069 | | | 149,298 | | | 722,146 | |
| September, 2021 | | - | | | 286,885 | | | 37,038 | | | 3,358 | | | 75,755 | | | 726,047 | | | 295,399 | | | 1,424,482 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Carrying value | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 | | 1,592,232 | | | 1,782,817 | | | 64,446 | | | 10,289 | | | 190,868 | | | 2,614,231 | | | 3,782,180 | | | 10,037,063 | |
| September 30, 2021 | | 1,592,232 | | | 1,712,443 | | | 158,120 | | | 8,747 | | | 314,644 | | | 2,453,940 | | | 3,632,882 | | | 9,873,008 | |
During the three and nine months ended September 30, 2021, the Company allocated $206,884 and $618,451, respectively (three and nine months ended September 30, 2020 - $185,710 and $318,060, respectively) of depreciation to cost of sales and $74,083 and $103,695, respectively (three and nine months ended September 30, 2020 - $21,750 and $70,261, respectively) to operating expense.
NOTE 9 - SHARE CAPITAL
(a) Authorized
Unlimited number of voting common shares without par value.
(b) Issued share capital
As at September 30, 2021, 65,847,820 common shares were issued and outstanding.
(c) Share issuances
During the nine months ended September 30, 2021, the Company had the following share transactions:
(i) During the nine months ended September 30, 2021, the Company recorded a subscription received for 122,727 units at $1.10 per unit for gross proceeds of $135,000. Each unit will consist of one common share and one transferable common share purchase warrant. These proceeds are included in as subscriptions received and will be applied against a future financing.
(ii) On August 10, 2021, the Company issued 2,513,720 common shares at $0.80 per share for a total consideration of approximately $2.01 million pursuant to the acquisition of PerceiveMD (Note 4).
(iii) On September 15, 2021, the Company issued 20,000,000 common shares at $1.20 per share for total consideration of approximately $24 million pursuant to the acquisition Phyto BrandCo (Note 5).
During the year ended December 31, 2020, the Company had the following share transactions:
(i) On January 17, 2020, the Company issued 189,934 units on conversion of $250,000 of principal and $6,411 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(ii) On January 27, 2020, the Company issued 266,760 units on conversion of $350,000 of principal and $10,126 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(iii) On July 10, 2020, the Company issued 200,589 units on conversion of $250,000 of principal and $20,795 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(iv) On July 13, 2020, the Company completed a non-brokered private placement whereby 3,882,667 units were issued at a price of $0.90 per unit for gross proceeds of $3,494,400. Each unit consists of one common share and one common share purchase warrant with each warrant exercisable into one common share at an exercise price of $1.50 until July 13, 2022.
NOTE 9 - SHARE CAPITAL (continued)
In conjunction with the financing, finders' fees of $21,600 in cash were paid and 24,067 compensation options (each a "Compensation Option") were granted. Each Compensation Option entitles the holder to purchase a unit on the same terms as the offering for a period of two years from the date of issuance. Each underlying common share purchase warrant (a "Compensation Option Warrant") will be subject to the same terms as the warrants attached to the units sold in the financing. If the Compensation Option Warrants are accelerated prior to the exercise of the Compensation Option, each Compensation Option Warrant will expire 30 days after the date of exercise of the Compensation Option. The fair value of the Compensation Option was $38,500 ($1.59 per Compensation Option) and was recognized as a share issuance cost. The fair value of the Compensation Options was estimated using the Black-Scholes option pricing model using the following assumptions: risk-free interest rate of 0.28%, annualized volatility of 100%, expected dividend yield of nil%, and an expected life of 2 years.
Additionally, the Company settled services and outstanding indebtedness of $543,715 through the issuance of 647,385 common shares at a fair value price of $0.84 per common share. The common shares issued in connection with the services and debt settlement are subject to a hold period that expires four months and one day from the date of issuance.
(v) On July 16, 2020, the Company issued 759,605 units on conversion of $945,000 of principal and $80,467 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(vi) On July 28, 2020, the Company issued 953,564 units on conversion of $1,182,000 of principal and $105,311 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(vii) On August 5, 2020, the Company issued 161,688 units on conversion of $200,000 of principal and $18,279 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(viii) During the year ended December 31, 2020, the Company issued 42,967 common shares related to the exercise of 42,967 warrants at an exercise price of $1.80 for proceeds of 77,340.
(d) Escrow shares
The Company entered into an Escrow Agreement in connection with closing the RTO on December 20, 2019, in relation to certain of its common shares which were placed in escrow. Pursuant to the Escrow Agreement the escrowed common shares are subject to a timed-release schedule whereby a 10% portion of the escrow shares will be released beginning on listing date, and 15% every six months thereafter until January 6, 2023.
As at September 30, 2021, 3,900,000 common shares were held in escrow (December 31, 2020 - 6,500,000).
(e) Stock options
The Company has an incentive stock option plan (the "Plan") which provides for the granting of options. Under the Plan the maximum number of stock options issued cannot exceed 10% of the Company's currently issued and outstanding common shares. Options granted under the Plan may have a maximum term of ten years. A participant, who is not a consultant conducting investor relations activities, who is granted an option that is exercisable at the market price at the date of grant, will have their options vest immediately, unless otherwise determined by the Board of Directors. Options granted at below market prices will vest one-sixth every three months.
NOTE 9 - SHARE CAPITAL (continued)
A participant who is a consultant conducting investor relations activities who is granted an option under the Plan will become vested with the right to exercise one-quarter of the option upon conclusion of every three months subsequent to the grant date. All options are to be settled by physical delivery of shares.
During the nine months ended September 30, 2021, the Company granted 33,333 stock options with exercise price of $1.35 to certain Directors, Officers, employees, and consultants. The options expire five years from the date of grant and vest immediately. The fair value of these options was $19,456 ($0.584 per option) and was recognized as a share-based payments.
During the year ended December 31, 2020, the Company had the following grants:
(i) On January 30, 2020, the Company granted 2,523,333 stock options with exercise price of $1.35 to certain Directors, Officers, employees, and consultants. The options expire five years from the date of grant and vest immediately. The fair value of these options was $2,537,300 ($1.02 per option) and was recognized as a share-based payments.
(ii) On June 1, 2020, the Company granted 66,667 stock options with exercise price of $1.35 to an employee. The options expire two years from the date of grant and vest immediately. The fair value of these options was $22,200 ($0.33 per option) and was recognized as share-based payments.
(iii) On August 5, 2020, the Company granted 1,616,667 stock options with exercise price of $2.34 to certain Directors, Officers, employees, and consultants. The options expire five years from the date of grant and vest immediately. The fair value of these options was $2,813,800 ($1.74 per option) and was recognized as a share-based payments.
The fair value of the stock options granted during the nine months ended September 30, 2021 and 2020 was estimated using the Black-Scholes option pricing model using the following assumptions:
| | | September 30, 2021 | | | December 31, 2020 | |
| Risk-free interest rate | | 0.71% | | | 0.34 - 1.29% | |
| Annualized volatility | | 100% | | | 100% | |
| Expected dividend yield | | 0.00% | | | 0.00% | |
| Expected life | | 5 years | | | 2 - 5 years | |
A summary of the changes in the Company's stock options outstanding and exercisable is as follows:
| | | | | | Weight average | |
| | | Stock options | | | exercise price | |
| | | # | | | $ | |
| Outstanding, December 31, 2019 | | - | | | - | |
| Granted | | 4,206,667 | | | 1.73 | |
| Cancelled | | (40,000 | ) | | 1.35 | |
| Outstanding, December 31, 2020 | | 4,166,667 | | | 1.73 | |
| Granted | | 33,333 | | | 1.35 | |
| Cancelled | | (1,599,999 | ) | | 1.89 | |
| Outstanding, September 30, 2021 | | 2,600,001 | | | 1.64 | |
| | | | | | | |
| Exercisable, December 31, 2020 | | 4,166,667 | | | 1.73 | |
| Exercisable, September 30, 2021 | | 2,600,001 | | | 1.64 | |
NOTE 9 - SHARE CAPITAL (continued)
As at September 30, 2021, the Company had stock options outstanding and exercisable as follows:
| Options outstanding | | | Weighted average |
| and exercisable | Exercise price | Expiry date | remaining life (years) |
| # | $ | | |
| 66,667 | 1.35 | June 1, 2022 | 0.67 |
| 1,750,001 | 1.35 | January 30, 2025 | 3.34 |
| 750,000 | 2.34 | August 5, 2025 | 3.85 |
| 33,333 | 1.35 | August 5, 2026 | 4.85 |
| 2,600,001 | | | 3.44 |
(f) Compensation options
As at September 30, 2021, the Company has 24,067 compensation options outstanding and exercisable. These options have an exercise price of $0.50 and expire on July 13, 2022.
(g) Warrants
As an incentive to complete a private placement the Company may issue units which include common shares and common share purchase warrants. Using the residual value method, the Company determines whether a value should be allocated to the warrants attached to private placement units. Finders' warrants may be issued as a private placement share issue cost and are valued using the Black-Scholes option pricing model.
A summary of the changes in the Company's warrants outstanding and exercisable is as follows:
| | | | | | Weight average | |
| | | Warrants | | | exercise price | |
| | | # | | | $ | |
| Outstanding, December 31, 2019 | | 2,470,552 | | | 2.19 | |
| Issued | | 6,414,807 | | | 1.80 | |
| Exercised | | (42,967 | ) | | 1.80 | |
| Expired | | (506,400 | ) | | 2.76 | |
| Outstanding, December 31, 2020 and September 30, 2021 | | 8,335,992 | | | 1.80 | |
As at September 30, 2021, the Company had warrants outstanding and exercisable as follows:
| Warrants outstanding | | | Weighted average |
| and exercisable | Exercise price | Expiry date | remaining life (years) |
| # | $ | | |
| 189,934 | 2.25 | January 17, 2022 | 0.30 |
| 266,760 | 2.25 | January 27, 2022 | 0.33 |
| 200,589 | 2.25 | July 10, 2022 | 0.78 |
| 3,882,667 | 1.50 | July 13, 2022 | 0.78 |
| 759,605 | 2.25 | July 16, 2022 | 0.79 |
| 953,564 | 2.25 | July 28, 2022 | 0.82 |
| 161,688 | 2.25 | August 5, 2022 | 0.84 |
| 1,921,185 | 1.80 | December 19, 2022 | 1.22 |
| 8,335,992 | | | 0.86 |
NOTE 10 - RELATED PARTY TRANSACTIONS
A number of key management personnel and Directors hold positions in other entities that result in them having control or significant influence over the financial or operating policies of these entities. There were no loans to key management personnel or Directors, or entities over which they have control or significant influence during the periods ended September 30, 2021 or September 30, 2020.
During the period ended September 30, 2021, 33,333 options were granted (2020 - 5,800,000) to Officers and Directors having a fair value on grant of $19,456 (2020 - $2,531,999).
The following related parties transacted with the Company or Company controlled entities during the periods:
(a) Andrew Hale was a Director and the Company's President and CEO. He resigned on March 1, 2021.
(b) Blaine Bailey was a Director and Chairman of the Company's Audit Committee. He resigned on March 26, 2021.
(c) Stephen Brohman was the Company's CFO. He is a principal of Donaldson Brohman Martin CPA Inc. ("DBM CPA") a firm in which he has significant influence. DBM CPA provided the Company with CFO, accounting and tax services. Stephen Brohman resigned on July 14, 2021.
(d) George Routhier is a Company Director. He is the owner of Pipedreemz Inc. ("Pipedreemz"), which provides advisory services to the Company.
(e) Michael Forbes is a Director and the Company's President and CEO. He was appointed on April 29, 2021 and is the owner of MDC Forbes, which provides CEO services to the Company.
(f) Donald Dinsmore is a Director and the Company's COO. He was appointed on April 29, 2021.
(g) Oliver Foeste is the Company's CFO. He was appointed on July 14, 2021 and is the Managing Partner of Invictus Accounting Group LLP which provides the Company with CFO, accounting and tax services.
The aggregate value of transactions with key management personnel and Directors and entities over which they have control or significant influence were as follows:
| | | Three months ended | | | Nine months ended | |
| | | | | | September 30, | | | | | | September 30, | |
| | | 2021 | | | 2020 | | | 2021 | | | 2020 | |
| | | $ | | | $ | | | $ | | | $ | |
| DBM CPA | | - | | | 42,750 | | | 61,091 | | | 114,750 | |
| Andrew Hale | | - | | | 45,021 | | | 47,479 | | | 135,070 | |
| Donald Dinsmore | | 31,250 | | | - | | | 52,083 | | | - | |
| MDC Forbes | | 15,000 | | | - | | | 25,000 | | | - | |
| Pipedreemz | | - | | | - | | | - | | | 2,500 | |
| Invictus Accounting Group LLP | | 31,263 | | | - | | | 31,263 | | | - | |
| | | 77,513 | | | 87,771 | | | 216,916 | | | 252,320 | |
In addition to the above, the Company's acquisition of PerceiveMD constitutes a related party transaction as Michael Forbes, was also a Director and controlling shareholder of PerceiveMD prior to the transaction (See Note 4 for more details).
As at September 30, 2021, the Company had an outstanding accounts payable balance with related parties as follows:
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| MDC Forbes | | 15,750 | | | - | |
| DBM CPA | | - | | | 12,600 | |
| Invictus Accounting Group LLP | | 6,996 | | | - | |
| | | 22,746 | | | 12,915 | |
All related party balances are unsecured and are due within thirty days without interest and incurred in the normal course of business.
NOTE 10 - RELATED PARTY TRANSACTIONS (continued)
The transactions with the key management personnel and Directors are included in operating expenses as follows:
(a) Consulting fees
Includes advisory services of George Routhier, charged to the Company via Pipedreemz, and CEO services by Michael Forbes, charged to the Company via MDC Forbes.
(b) Professional fees
Includes accounting and tax services of the Company's former CFO, Stephen Brohman, charged to the Company via DBM CPA and accounting services of the Company's new CFO, Oliver Foeste, charged to the Company via Invictus Accounting Group LLP.
(c) Wages and salaries
Includes services by Andrew Hale as the prior CEO, and Donald Dinsmore as COO.
NOTE 11 - MORTGAGE PAYABLE
| | | | | | Second | | | | | | Fourth | | | | |
| | | First Mortgage | | | Mortgage | | | Third Mortgage | | | Mortgage | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | |
| December 31, 2019 | | 2,437,175 | | | | | | | | | | | | 2,437,175 | |
| New mortgage (refinancing) | | (2,446,000 | ) | | 2,446,000 | | | - | | | - | | | - | |
| Transaction costs | | | | | (18,345 | ) | | - | | | - | | | (18,345 | ) |
| Finance expense | | 42,457 | | | 178,242 | | | - | | | - | | | 220,699 | |
| Repayments | | (33,632 | ) | | (163,067 | ) | | - | | | - | | | (196,699 | ) |
| December 31, 2020 | | - | | | 2,442,830 | | | - | | | - | | | 2,442,830 | |
| New mortgage (refinancing) | | | | | (2,446,000 | ) | | - | | | 3,500,000 | | | 1,054,000 | |
| Transaction costs | | | | | - | | | (18,345 | ) | | (32,778 | ) | | (51,123 | ) |
| Finance expense | | | | | 35,783 | | | 104,723 | | | 64,177 | | | 204,683 | |
| Repayments | | | | | (32,613 | ) | | (86,378 | ) | | (32,671 | ) | | (151,662 | ) |
| September 30, 2021 | | - | | | - | | | - | | | 3,498,728 | | | 3,498,728 | |
a) On January 7, 2019, the Company entered into a mortgage for $2,446,000 (the "First Mortgage") which bore interest at the rate of 8.25% per annum, calculated monthly. The First Mortgage matured on February 1, 2020 and was renewed as discussed below.
The First Mortgage payable was recorded at amortized cost and bore an effective interest rate of 10.44%.
The carrying value of the First Mortgage at December 31, 2019 was $2,437,175. Included in mortgage payable on initial recognition were the related mortgage transaction costs of $50,755 which were amortized over the term of the First Mortgage using the effective interest rate method.
The Company maintained minimum interest-only payments of $16,816 per month in connection with the First Mortgage. Total interest expense of the First Mortgage during the nine months ended September 30, 2021 was $nil (2020 - $33,632).
NOTE 11 - MORTGAGE PAYABLE (continued)
b) On February 1, 2020, the Company renewed the First Mortgage of $2,446,000 (the "Second Mortgage") which bears interest at the rate of 8.00% per annum, calculated monthly. The Second Mortgage matured on February 1, 2021 and was renewed as discussed below.
The Second Mortgage payable was recorded at amortized cost and bore an effective interest rate of 8.79%.
The carrying value of the Second Mortgage payable as at December 31, 2020 was $2,442,830. Included in mortgage payable on initial recognition were the related mortgage transaction costs of $18,345 which were being amortized over the term of the Second Mortgage using the effective interest rate method.
The Company maintained minimum interest-only payments of $16,307 per month in connection with the Second Mortgage. Total finance expense of the Second Mortgage during the three and nine months ended September 30, 2021 was $nil and $35,783, respectively (2020 - $53,488 and $124,652, respectively).
c) On February 1, 2021, the Company renewed the Second Mortgage of $2,446,000 (the "Third Mortgage") which bears interest at the rate of 8.00% per annum, calculated monthly. The Third Mortgage matures on February 1, 2022, can be repaid before maturity without penalty and is secured by the mortgage property and building improvements. The Third Mortgage payable was recorded at amortized cost (principal value less $18,345 transaction costs) and bares an effective interest rate of 8.79%.
On July 9, 2021, the Third Mortgage was refinanced (Note 10(d)). As a result, the carrying value of the Third Mortgage payable at September 30, 2021 was $nil.
Until refinancing, the Company maintained minimum interest-only payments of $16,307 per month. Total finance expense during the three and nine months ended September 30, 2021 was $33,559 and $104,723, respectively (three and nine months ended September 30, 2020 - $nil and $nil, respectively).
d) On July 9, 2021, the Company refinanced the Third Mortgage and increased the facility to $3,500,000 (the "Fourth Mortgage") which bears interest at the rate of 6.50% per annum, calculated monthly for one year. The mortgage matures on July 1, 2022 and is secured by the mortgage property and building improvements. The Fourth Mortgage payable was recorded at amortized cost (principal value less $32,778 transaction costs) and bares an effective interest rate of 7.39%. The carrying value of the Fourth Mortgage payable at September 30, 2021 was $3,498,728.
The Company maintains minimum interest-only payments of $18,958 per month. As at September 30, 2021 the total non-discounted remaining scheduled payments related to the mortgage including interest payments totaled $3,692,183. Total finance expense during the three and nine months ended September 30, 2021 was $64,177 (three and nine months ended September 30, 2020 - $nil, respectively).
NOTE 12 - LEASE LIABILITY
| | | September 30, | | | December 31, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| Opening balance | | - | | | - | |
| Additions - Phyto BrandCo acquisition | | 34,665 | | | - | |
| Closing balance | | 34,665 | | | - | |
| Less: current portion | | 10,425 | | | - | |
| Long-term portion | | 24,240 | | | - | |
On October 15, 2020, the prior to being acquired by the Company, Phyto BrandCo entered into a four-year lease agreement for a promotional vehicle. The base monthly payment is $1,118.55 with an initial payment of $9,732.26. The incremental borrowing rate used to determine discount the lease liability was 10%.
NOTE 13 - SUPPLEMENTAL CASH FLOW INFORMATION
| | | Nine months ended | |
| | | | | | September 30, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| Non-cash operating activities | | | | | | |
| Share subscription for prepaid expenses | | 135,000 | | | - | |
| Non-cash financing activities | | | | | | |
| Conversion of convertible debentures - debenture reserves | | - | | | 298,461 | |
| Convertible debentures - settlement | | - | | | (3,418,390 | ) |
| Convertible debentures - reserves | | - | | | (70,814 | ) |
| Share issuance costs - Compensation Options | | - | | | (38,500 | ) |
| Non-cash investing activities | | | | | | |
| Equipment purchases included in accounts payable and accrued liabilities | | 190,362 | | | 792,279 | |
| Shares issued for debt settlement | | - | | | 412,024 | |
During the nine months ended September 30, 2021 and 2020 no amounts were paid for income tax expense.
NOTE 14 - CONVERTIBLE DEBENTURES
On October 31, 2019, the Company closed a 2,353,333 convertible debenture unit offering at a price of $1.35 per debenture unit for gross proceeds $3,177,000. Each debenture consisted of a 12% secured convertible debenture with a maturity of two years from the date of issuance. If the holder converted their debenture unit, they were entitled to one common share and one share purchase warrant exercisable at $2.25 per share two years from the date of the convertible debenture closing October 31, 2021 at the holder's discretion.
If the closing price of the Common Shares of the Company was higher than $3.00 for any 10 consecutive trading days, the expiry date of the Warrants may be accelerated to the 30th day after the date of a news release announcing such acceleration. The debentures were secured to the facility subordinate to the mortgage currently on the facility.
As the debentures were convertible into units, the liability and equity components were presented separately on the consolidated statement of financial position. The initial carrying amount of the financial liability was determined by discounting the stream of future payments of interest and principal at a market interest rate of 16% totaling $2,878,539. Using the residual method, the carrying amount of the conversion feature is the difference between the principal amount and the initial carrying value of the financial liability. The equity component was recorded in debenture reserves on the consolidated statement of financial position totaling $298,461. The debentures, net of the equity components were accreted using the effective interest method over the term of the debentures, such that the carrying amount of the financial liability was equal to the principal balance at maturity.
During the year ended December 31, 2020, the Company settled principal plus accrued interest of $3,418,390 relating to the debentures through the issuance of 2,532,140 units. The Company reversed $298,461 from debenture reserves in connection with the settlement.
During the three and nine months ended September 30, 2021, the Company recorded interest expense of $nil and $nil, respectively (2020 - $10,852 and $169,552, respectively) and accretion expense of $nil and $nil, respectively (2020 - $14,768 and $58,405, respectively).
NOTE 15 - FINANCIAL RISK MANAGEMENT
(a) Capital management
The Company defines capital as the components of shareholders' equity. The Company's objectives when managing capital are to support further advancement of the Company's business objectives, as well as to ensure that the Company is able to meet its financial obligations as they come due.
The Company manages its capital structure to maximize its financial flexibility making adjustments to it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. The Company relies on the expertise of the Company's management to sustain the future development of the business. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There were no changes in the
Company's approach to capital management during the nine months ended September 30, 2021. The Company is not subject to externally imposed capital requirements.
(b) Financial instruments - fair value
The Company's financial instruments consist of cash, trade receivables, deposits, accounts payable and accrued liabilities, mortgage payable, lease liability and loan payable.
As at September 30, 2021, the carrying values of cash, receivables and prepayments, deposits, accounts payable and accrued liabilities, and loan payable approximated their fair value because of the short-term nature of these instruments. The mortgage payable and lease liability approximate fair value due to its market rates of interest charged.
Financial instruments measured at fair value on the condensed interim consolidated statements of financial position are summarized into the following fair value hierarchy levels:
Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e., as prices) or indirectly (i.e., derived from prices).
Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
(c) Financial instruments - risk
The Company's financial instruments are exposed to certain financial risks, including credit risk, liquidity risk and interest rate risk.
Credit risk
(a) The Company is exposed to credit risk by holding cash. This risk is minimized by holding the funds in Canadian banks and credit unions or with the Canadian government. The Company has minimal receivable exposure, and its various refundable credits are due from the Canadian government.
Liquidity risk
(b) Liquidity risk is the risk that the Company is unable to meet its financial obligations as they come due. The Company manages this risk by careful management of its working capital to ensure its expenditures will not exceed available resources.
NOTE 15 - FINANCIAL RISK MANAGEMENT (continued)
Interest rate risk
(c) Interest rate risk is the risk that future cash flows will fluctuate as a result of changes in market interest rates. The Company's mortgage payable, lease liability, and convertible debentures carry fixed interest rates and as such, the Company is not exposed to interest rate risk.
Economic dependence
(d) Economic dependence risk is the risk of reliance upon a select number of customers which significantly impact the financial performance of the Company. During the three and nine months ended September 30, 2021, two customers represented approximately 98% and 99% of the Company's revenue (December 31, 2020 - one customer representing 100% of revenue).
NOTE 16 - SUBSEQUENT EVENTS
On October 18, 2021, the Company completed a non-brokered private placement whereby the Company issued 122,727 units at a price of $1.10 per unit for gross proceeds of $135,000. Each unit is comprised of one common share and one transferrable common share purchase warrant with each warrant entitling the holder thereof to acquire one common share at a price of $1.75 per share for two years from the date of the closing. The warrants are subject to an acceleration provision whereby if the daily closing price of the common shares closes at or above $2.00 per share for 50 consecutive trading days, then the Company may accelerate the expiration date of the warrants to the date that is 30 trading days from the date that notice of such acceleration is given via news release. From and after the new accelerated expiration date, no warrants may be exercised, and all unexercised warrants would be void.
On October 25, 2021, the Company granted an aggregate of 900,000 stock options to certain directors and officers for the purchase of up to 900,000 common shares at a price of $1.06 per share. Each stock option is exercisable for a period of five years.
On October 28, 2021, the Company granted an aggregate of 215,000 stock options to certain employees and a consultant for the purchase of up to 215,000 common shares at a price of $0.95 per share. Each stock option is exercisable for a period of five years.
On November 16, 2021, the Company announced a private placement financing to raise gross proceeds of $2,500,000 through the issuance of 2,631,578 units at a price of $0.95 per unit. Each unit will be comprised of one common share and one common share purchase warrant. Each warrant will entitle the holder to acquire one common share of the Company at a price of $1.00 per warrant for a period of three years from the closing of the financing.
Adastra Holdings Ltd.
(formerly Phyto Extractions Inc.)
Consolidated Financial Statements
For the year ended December 31, 2020 and period ended December 31, 2019
(Expressed in Canadian Dollars)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
Adastra Holdings Ltd.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Adastra Holdings Ltd. (the "Company"), as of December 31, 2020 and 2019, and the related consolidated statements of loss and comprehensive loss, changes in equity, and cash flows for the year ended December 31, 2020 and for the eight months ended December 31, 2019, and the related notes (collectively referred to as the "financial statements"). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Adastra Holdings Ltd. as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the year ended December 31, 2020 and for the eight months ended December 31, 2019 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company had a working capital deficit of $105,093 and has incurred losses since inception. These events and conditions indicate a material uncertainty exists that may cast significant doubt on the Company's ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.

Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company's auditor since 2019.
| | /s/ DAVIDSON & COMPANY LLP |
| | |
Vancouver, Canada | Chartered Professional Accountants |
| | |
| November 2, 2021 | |
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Financial Position |
| As at December 31, 2020 and December 31, 2019 |
| | | | December 31, | | | December 31, | |
| | | | 2020 | | | 2019 | |
| | Note | | $ | | | $ | |
| Assets | | | | | | | |
| Current assets | | | | | | | |
| Cash | | | 1,145,461 | | | 2,376,826 | |
| Receivables and prepayments | 4 | | 1,129,189 | | | 461,167 | |
| Marketable securities | 5 | | - | | | 1,500 | |
| Inventory | 6 | | 1,421,237 | | | - | |
| | | | 3,695,887 | | | 2,839,493 | |
| Non-current assets | | | | | | | |
| Deposits | 7 | | 4,000 | | | 4,000 | |
| Property, plant and equipment | 7 | | 10,037,063 | | | 8,507,517 | |
| | | | 10,041,063 | | | 8,511,517 | |
| Total assets | | | 13,736,950 | | | 11,351,010 | |
| | | | | | | | |
| Liabilities and equity | | | | | | | |
| Current liabilities | | | | | | | |
| Accounts payable and accrued liabilities | | | 1,147,964 | | | 873,402 | |
| Mortgage Payable | 13 | | 2,442,830 | | | 2,437,175 | |
| | | | 3,590,794 | | | 3,310,577 | |
| Non-current liabilities | | | | | | | |
| Convertible debenture | 14 | | - | | | 2,955,511 | |
| Government loan | 2 | | 60,000 | | | - | |
| Total liabilities | | | 3,650,794 | | | 6,266,088 | |
| | | | | | | | |
| Equity | | | | | | | |
| Share capital | 8 | | 15,822,152 | | | 8,348,407 | |
| Reserves | | | 5,441,814 | | | 298,461 | |
| Deficit | | | (11,177,810 | ) | | (3,561,946 | ) |
| Total equity | | | 10,086,156 | | | 5,084,922 | |
| Total liabilities and equity | | | 13,736,950 | | | 11,351,010 | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Nature of operations and going concern | 1 | | | | | | |
| Events after the reporting period | 17 | | | | | | |
Approved on behalf of the Board of Directors on November 2, 2021:
"Michael Forbes" | Director | "Donald Dinsmore" | Director |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Changes in Equity |
| For the year ended December 31, 2020 and the eight months ended December 31, 2019 |
| | | Number | | | Share | | | Debenture | | | | | | | | | Total
| |
| | | of shares | | | capital | | | Reserves | | | Reserves | | | Deficit | | | equity (deficiency) | |
| | | # | | | $ | | | $ | | | $ | | | $ | | | $ | |
| May 1, 2019 | | 27,046,111 | | | 4,614,000 | | | - | | | - | | | (903,989 | ) | | 3,710,011 | |
| Convertible debenture - pre RTO | | - | | | - | | | 298,461 | | | - | | | - | | | 298,461 | |
| Reverse acquisition transaction (Note 3): | | | | | | | | | | | | | | | | | | |
| Issuance of shares pursuant to reverse acquisition | | 7,218,678 | | | 1,082,802 | | | - | | | - | | | - | | | 1,082,802 | |
| Private placement units issued | | 1,964,152 | | | 2,651,605 | | | - | | | - | | | - | | | 2,651,605 | |
| Loss and comprehensive loss | | - | | | - | | | - | | | - | | | (2,657,957 | ) | | (2,657,957 | ) |
| December 31, 2019 | | 36,228,941 | | | 8,348,407 | | | 298,461 | | | - | | | (3,561,946 | ) | | 5,084,922 | |
| | | | | | | | | | | | | | | | | | | |
| January 1, 2020 | | 36,228,941 | | | 8,348,407 | | | 298,461 | | | - | | | (3,561,946 | ) | | 5,084,922 | |
| Convertible debentures - settlement | | 2,532,140 | | | 3,418,390 | | | (298,461 | ) | | 70,814 | | | - | | | 3,190,743 | |
| Units issued for cash | | 3,882,667 | | | 3,494,400 | | | - | | | - | | | - | | | 3,494,400 | |
| Share issue costs | | - | | | (60,100 | ) | | - | | | 38,500 | | | - | | | (21,600 | ) |
| Shares issued for cash - warrants | | 42,967 | | | 77,340 | | | - | | | - | | | - | | | 77,340 | |
| Shares issued - services | | 156,894 | | | 131,791 | | | - | | | - | | | - | | | 131,791 | |
| Shares issued - debt settlement | | 490,491 | | | 411,924 | | | - | | | - | | | - | | | 411,924 | |
| Share-based payments | | - | | | - | | | - | | | 5,332,500 | | | - | | | 5,332,500 | |
| Loss and comprehensive loss for the year | | - | | | - | | | - | | | - | | | (7,615,864 | ) | | (7,615,864 | ) |
| December 31, 2020 | | 43,334,100 | | | 15,822,152 | | | - | | | 5,441,814 | | | (11,177,810 | ) | | 10,086,156 | |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Loss and Comprehensive Loss |
| | | | For the year ended | | | For the eight months ended | |
| | | | December 31, 2020 | | | December 31, 2019 | |
| | Note | | $ | | | $ | |
| Revenue | | | 2,499,355 | | | - | |
| Cost of sales | | | 1,713,774 | | | - | |
| Gross profit | | | 785,581 | | | - | |
| Expenses | | | | | | | |
| Advertising and promotion | | | 749,258 | | | 56,817 | |
| Accretion | 14 | | 58,405 | | | 13,259 | |
| Depreciation | 7 | | 92,700 | | | 78,172 | |
| Insurance | | | 69,294 | | | 18,820 | |
| Office expenses | | | 227,271 | | | 179,938 | |
| Professional fees and consulting | 10 | | 704,297 | | | 447,784 | |
| Rent | | | - | | | 10,806 | |
| Research expenses | | | 181,750 | | | - | |
| Share-based payments | 8,10 | | 5,332,500 | | | - | |
| Travel | | | 4,029 | | | 20,273 | |
| Wages and salaries | 10 | | 526,327 | | | 302,513 | |
| Total operating expenses | | | 7,945,831 | | | 1,128,382 | |
| Loss from operating expenses | | | (7,160,250 | ) | | (1,128,382 | ) |
| Interest expense | 13,14 | | (399,248 | ) | | (227,955 | ) |
| Interest income | | | 8,303 | | | 7,764 | |
| Listing expense | 3 | | - | | | (1,309,384 | ) |
| Realized loss on marketable securities | 5 | | (1,500 | ) | | - | |
| Write-down of inventory | 6 | | (63,169 | ) | | - | |
| Loss and comprehensive loss for the year | | | (7,615,864 | ) | | (2,657,957 | ) |
| | | | | | | | |
| Loss per share | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | |
| - basic # | 8 | | 39,695,235 | | | 27,495,882 | |
| - diluted # | 8 | | 39,695,235 | | | 27,495,882 | |
| | | | | | | | |
| Basic loss per share $ | 8 | | (0.19 | ) | | (0.10 | ) |
| Diluted loss per share $ | 8 | | (0.19 | ) | | (0.10 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Cash Flows |
| | | | For the year ended | | | For the eight months ended | |
| | Note | | December 31, 2020 | | | December 31, 2019 | |
| | | | | | | | |
| Operating activities | | | | | | | |
| Loss for the year | | | (7,615,864 | ) | | (2,657,957 | ) |
| Adjustments for non-cash items: | | | | | | | |
| Accretion | | | 58,405 | | | 13,259 | |
| Depreciation | | | 92,700 | | | 78,172 | |
| Interest expense | | | 399,248 | | | 227,955 | |
| Interest income | | | (8,303 | ) | | (7,764 | ) |
| Listing expense | | | - | | | 1,309,384 | |
| Realized loss on marketable securities | | | 1,500 | | | - | |
| Share-based compensation | | | 5,332,500 | | | - | |
| Shares for services | | | 131,791 | | | - | |
| Net change in non-cash working capital items | 12 | | (1,225,727 | ) | | (396,493 | ) |
| | | | (2,833,750 | ) | | (1,433,444 | ) |
| | | | | | | | |
| Financing activities | | | | | | | |
| Issue of units for cash | | | 3,472,800 | | | 2,651,605 | |
| Warrants exercised | | | 77,340 | | | - | |
| Borrowing costs mortgage | | | (18,345 | ) | | - | |
| Interest paid - mortgage | | | (196,699 | ) | | (134,530 | ) |
| Convertible debenture | | | - | | | 3,177,000 | |
| Loan proceeds | | | 60,000 | | | - | |
| | | | 3,395,096 | | | 5,694,075 | |
| | | | | | | | |
| Investing activities | | | | | | | |
| Acquisition of property, plant and equipment | | | (1,801,014 | ) | | (4,365,559 | ) |
| Interest income | | | 8,303 | | | 7,764 | |
| Cash acquired on reverse acquisition | | | - | | | 111,496 | |
| | | | (1,792,711 | ) | | (4,246,299 | ) |
| | | | | | | | |
| Net increase in cash | | | (1,231,365 | ) | | 14,332 | |
| Cash, beginning of year | | | 2,376,826 | | | 2,362,494 | |
| Cash, end of year | | | 1,145,461 | | | 2,376,826 | |
| | | | | | | | |
| Supplemental cash flow information | 12 | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
1. Nature of operations and going concern
Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) (the "Company") was incorporated under the laws of the province of British Columbia on October 14, 1987. The Company is an extraction and processing solutions company. The Company's mission is to develop and deploy large-scale cannabis and hemp extraction technologies and provide turn- key processing solutions to help licensed standard and micro-cultivators maximize the value of every harvest. The
Company is listed on the Canadian Securities Exchange ("CSE") under the symbol "XTRX".
The Company's registered and records office is 5451 275th Street, Langley City, British Columbia, V4W 3X8.
On April 9, 2021, the Company consolidated the Company's issued share capital on a ratio of three (3) old common shares for every one (1) new post-consolidated common share. All current and comparative references to the number of common shares, weighted average number of common shares, loss per share, stock options and warrants have been restated to give effect to this Share Consolidation. Additionally, the Company changed their name to Phyto Extractions Inc.
On October 19, 2019, Health Canada issued a Analytical testing license on the Company's facility to Chemia Analytics Inc., ("Chemia"). Chemia is a wholly owned subsidiary of the Company. On March 13, 2020, Health Canada issued a Standard Processing License on the Company's facility to Adastra labs Inc., ("Labs"). Labs is a wholly owned subsidiary of the Company.
On December 20, 2019, Arrowstar Resources Ltd. ("Arrowstar") acquired all of the issued and outstanding common shares of Adastra Labs Holdings (2019) Ltd. ("Adastra") a private British Columbia cannabis extraction and processing solutions company incorporated on June 18, 2018. The acquisition was completed by entering into a share exchange agreement whereby the parties completed a business combination by way of a transaction that constituted a reverse takeover ("RTO") of the Company by Adastra (the "Transaction"). The Transaction was accounted for as an RTO of
Arrowstar by Adastra for accounting purposes, with Adastra being identified as the accounting acquirer, and accordingly, these financial statements are a continuation of Adastra. The net assets of Arrowstar at the date of the RTO are deemed to have been acquired by Adastra (Note 3). These consolidated financial statements (the "financial statements") include the results of operations of Arrowstar since December 20, 2019. The comparative figures are those of Adastra prior to the RTO. At the time of the transaction the Company changed its financial year end from April 30 to December 31.
These financial statements are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of operations. These financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities that might be necessary should the Company be unable to continue in existence.
The Company's ability to continue as a going concern is dependent on its ability to generate positive cash flows from operations, complete additional financings, and/or extend or modify its mortgage payable (note 13).
As at December 31, 2020, the Company had a working capital (deficit) of $105,093 (December 31, 2019 - ($471,084)) and has incurred losses since inception. These events and conditions indicate a material uncertainty exists that may cast significant doubt on the Company's ability to continue as a going concern. If the going concern assumption were not appropriate for these financial statements it could be necessary to restate the Company's assets and liabilities on a liquidation basis.
2. Significant accounting policies
(a) Basis of presentation
These financial statements have been prepared in accordance with International Financial Reporting Standards and Interpretations (collectively, "IFRS"), as issued by the International Accounting Standards Board ("IASB") and the International Financial Reporting Interpretations Committee ("IFRIC") and have been applied consistently by the
Company and its subsidiaries.
These financial statements have been prepared on an historical cost basis, except for financial instruments which are classified as fair value through profit or loss ("FVTPL"). In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information.
All amounts on these financial statements are presented in Canadian dollars which is the functional currency of the Company and its subsidiaries (note 2(b)).
2. Significant accounting policies (continued)
(b) Principles of consolidation
These financial statements include the financial information of the Company and its subsidiaries. The financial statements include the following entities:
| Adastra Holdings Ltd. (formerly Phyto Extractions Inc.) | Legal parent company |
| | |
| Adastra Labs Holdings (2019) Ltd. (formerly Adastra Labs Holdings Ltd.) | Holding company |
| | |
| Adastra Labs Inc. | Extraction and concentrates
production company |
| | |
| Chemia Analytics Inc. | Cannabis testing an analysis
laboratory company |
| | |
| 1178562 B.C. Ltd. | Facility owner |
| | |
| Adastra Brands Inc. | Intellectual property
company |
Subsidiaries are entities controlled by the Company and are included in the financial statements from the date that control commences until the date that control ceases. The accounting policies of subsidiaries are changed where necessary to align them with the policies adopted by the Company.
These financial statements account for Arrowstar as a controlled entity (accounting acquiree) requiring consolidation since the date of the RTO (Notes 1 and 3), effective December 20, 2019.
(c) Standards issued but not yet effective
Certain pronouncements have been issued by the IASB or IFRIC that are effective for accounting periods beginning on or after January 1, 2021. The Company has reviewed these updates and determined that many of these updates are not applicable or consequential to the Company and have been excluded from discussion within these significant accounting policies.
The preparation of financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at each reporting date and the reported amounts of income and expenses during each reporting period. Estimates and assumptions are continuously evaluated and are based on management's experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
The information about significant areas of estimation uncertainty considered by management in preparing these financial statements is as follows:
Fair value of stock options and finders' compensation options
Determining the fair value of stock options and compensatory options (finders' options) requires estimates related to the choice of a pricing model, the estimation of stock price volatility, the fair value of the Company's common shares, the expected forfeiture rate and the expected term of the underlying instruments. Any changes in the estimates or inputs utilized to determine fair value could have a significant impact on the Company's future operating results or on other components of shareholders' equity.
2. Significant accounting policies (continued)
Acquisitions
Determination of whether a set of assets acquired and liabilities assumed constitute the acquisition of a business or asset requires the Company to make certain judgments as to whether or not the assets acquired and liabilities assumed include the inputs, processes and outputs necessary to constitute a business as defined in IFRS 3 Business Combinations. If an acquired set of assets and liabilities includes goodwill, the set is presumed to be a business. Based on an assessment of relevant facts and circumstances, the Company concluded that the acquisition disclosed in Note 3 was acquisition of assets. The values assigned to common shares and the allocation of the purchase price to the net liabilities in the acquisition are based on numerous estimates and judgements of the relative fair values of net liabilities.
Property, Plant and Equipment
The estimated useful lives of property plant and equipment are reviewed by management and adjusted if necessary. To estimate property, plant and equipment's useful life, management must use its past experience with the same or similar assets, review engineering estimates and industry practices for similar pieces of property, plant and equipment and/or apply statistical methods to assist in its determination of useful life. Additionally, management makes estimates with respect to the fair value of equipment acquired for non-monetary consideration. The Company assesses fair value by comparing market prices for similar types of property, plant and equipment.
The information about significant areas of judgment considered by management in preparing these financial statements is as follows:
Income taxes
Tax provisions are based on enacted or substantively enacted laws. Changes in those laws could affect amounts recognized in profit or loss both in the period of change, which would include any impact on cumulative provisions, and in future periods. Deferred tax assets (if any) are recognized only to the extent it is considered probable that those assets will be recoverable. This involves an assessment of when those deferred tax assets are likely to reverse and a judgment as to whether or not there will be sufficient taxable profits available to offset the tax assets when they do reverse. This requires assumptions regarding future profitability and is therefore inherently uncertain. To the extent assumptions regarding future profitability change, there can be an increase or decrease in the amounts recognized in respect of deferred tax assets as well as the amounts recognized in profit or loss in the period in which the change occurs.
Going concern
The assessment of the Company's ability to continue as a going concern as discussed in Note 1 involves judgment regarding future funding available for its operations and working capital requirements.
2. Significant accounting policies (continued)
Property, Plant and Equipment
Property, plant and equipment is measured at cost less accumulated depreciation and impairment losses. The cost of an item of property, plant and equipment consists of the purchase price, any costs directly attributable to bringing the asset to the location and condition necessary for its intended use and an initial estimate of the costs of dismantling and removing the item and restoring the site on which it is located. No depreciation is recorded in the year of disposal.
Depreciation is recognized over the following terms, intended to depreciate the cost of property, plant and equipment, less its residual values if any, over its estimated useful lives:
Furniture and equipment | 20% declining balance |
Leasehold improvements | 10 years straight line |
Buildings | 20 years straight line |
Extraction equipment | 20% declining balance |
Laboratory equipment | 20% declining balance |
Computer software | 20% declining balance |
Cost includes expenditures that are directly attributable to the acquisition of the asset. Subsequent costs are included in the asset's carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost can be measured reliably. Repairs and maintenance costs are charged to profit or loss during the period they are incurred. Any gain or loss on the disposal or retirement of equipment is recognized in profit or loss.
Share capital
Common shares are classified as shareholders' equity. Transaction costs directly attributable to the issue of common shares are recognized as a deduction from shareholders' equity. Common shares issued for consideration other than cash, are valued based on their market value at the date the shares are issued.
The Company has adopted a residual value method with respect to the measurement of warrants attached to private placement units. The residual value method first allocates value to the more easily measurable component based on fair value and then the residual value, if any, to the less easily measurable component. The Company considers the fair value of common shares issued in the private placements to be the more easily measurable component. The balance, if any, is allocated to the attached warrants. Any fair value attributed to the warrants is recorded as warrant reserve.
Share-based payment transactions
The Company has a stock option plan that provides for the granting of options to Officers, Directors, related company employees and consultants to acquire shares of the Company. The fair value of the options is measured on grant date and is recognized as an expense with a corresponding increase in contributed surplus as the options vest.
Options granted to employees and others providing similar services are measured at grant date at the fair value of the instruments issued. Fair value is determined using the Black-Scholes option pricing model taking into account the terms and conditions upon which the options were granted. The amount recognized as an expense is adjusted to reflect the actual number of share options that are expected to vest. Each tranche in an award with graded vesting is considered a separate grant with a different vesting date and fair value. Each grant is accounted for on that basis.
Options granted to non-employees are measured at the fair value of the goods or services received, unless that fair value cannot be estimated reliably, in which case the fair value of the equity instruments issued is used. The value of the goods or services is recorded at the earlier of the vesting date, or the date the goods or services are received.
Over the vesting period, share-based payments are recorded as an operating expense and as contributed surplus. When options are exercised the consideration received is recorded as share capital and the related share-based payments originally recorded as contributed surplus are transferred to share capital. When an option is cancelled or expires, the initial recorded value is reversed from contributed surplus and credited to deficit.
2. Significant accounting policies (continued)
Loss per share
Basic loss per share is computed by dividing the net loss attributable to common shareholders by the weighted average number of common shares outstanding during the reporting period.
Diluted loss per share is computed similar to basic loss per share except that (i) net loss attributable to common shareholders are adjusted for the dilutive effect of warrants and stock options. Under this method, the Company assumes that outstanding dilutive stock options and warrants were exercised and that the proceeds from such exercises (after adjustment of any unvested portion of stock options) were used to acquire common shares at the average market price during the reporting periods. For the period presented, diluted loss per share equals basic loss per share as there were no dilutive stock options or warrants outstanding.
Financial instruments
Effective June 18, 2018 (date of incorporation), the Company adopted IFRS 9, Financial Instruments ("IFRS 9"). IFRS 9 provides three different measurement categories for non-derivative financial assets - subsequently measured at amortized cost, fair value through profit or loss ("FVTPL") or fair value through other comprehensive income - while all non-derivative financial liabilities are classified as subsequently measured at amortized cost. The category into which a financial asset is placed and the resultant accounting treatment is largely dependent on the nature of the business of the entity holding the financial asset. All financial instruments are initially recognized at fair value.
Financial assets
Measurement
The Company initially recognizes financial assets on the trade date, which is the date that the Company becomes a party to the contractual provisions of the instrument. The Company derecognizes a financial asset when the contractual rights to the cash flows from the asset expire, or it transfers the rights to receive the contractual cash flows in a transaction in which substantially all of the risks and rewards of ownership of the financial asset are transferred. Any interest in such transferred financial assets that is created or retained by the Company is recognized as a separate asset or liability. The Company classifies all of its financial assets, except cash, as subsequently measured at amortized cost. Cash is classified as FVTPL. All financial assets that do not meet the criteria to be recognized as subsequently measured at amortized cost or subsequently measured at fair value through other comprehensive income are classified as FVTPL.
Impairment of financial assets at amortized cost
An 'expected credit loss' impairment model applies which requires a loss allowance to be recognized based on expected credit losses. The estimated present value of future cash flows associated with the asset is determined and an impairment loss is recognized for the difference between this amount and the carrying amount as follows: the carrying amount of the asset is reduced to estimated present value of the future cash flows associated with the asset, discounted at the financial asset's original effective interest rate, either directly or through the use of an allowance account and the resulting loss is recognized in profit or loss for the period.
In a subsequent period, if the amount of the impairment loss related to financial assets measured at amortized cost decreases, the previously recognized impairment loss is reversed through profit or loss to the extent that the carrying amount of the investment at the date the impairment is reversed does not exceed what the amortized cost would have been had the impairment not been recognized.
Financial liabilities
The Company measures all of its financial liabilities as subsequently measured at amortized cost. Financial liabilities are recognized initially at fair value, net of transaction costs incurred, and are subsequently measured at amortized cost. Any difference between the amounts originally received, net of transaction costs, and the redemption value is recognized in profit and loss over the period to maturity using the effective interest method.
2. Significant accounting policies (continued)
Impairment of non-financial assets
The carrying amount of the Company's long-lived assets is reviewed for an indication of impairment at the end of each reporting period. If an indication of impairment exists, the Company makes an estimate of the asset's recoverable amount. Individual assets are grouped for impairment assessment purposes at the lowest level at which there are identifiable cash flows that are largely independent of the cash flows of other groups of assets (cash generating units
"CGU"). The recoverable amount of an asset group is the higher of its fair value less costs to sell and its value in use.
In assessing value in use, the estimated future cash flows are adjusted for the risks specific to the CGU and are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money.
Where the carrying amount of an asset group exceeds its recoverable amount, the CGU is considered impaired and is written down to its recoverable amount. Impairment losses are recognized in profit or loss. Impairment losses recognized in respect of CGUs are allocated to reduce the carrying amounts of the assets in the CGU on a pro rata basis.
An assessment is made at each reporting date as to whether there is any indication that previously recognized impairment losses may no longer exist or may have decreased. If such indication exists, the recoverable amount is estimated. A previously recognized impairment loss is reversed only if there has been a change in the estimates used to determine the asset's recoverable amount. An impairment loss is reversed only to the extent that the asset's carrying amount does not exceed the carrying amount that would have been determined, net of depreciation, if no impairment loss had been recognized.
Income taxes
Income tax comprises current and deferred income taxes. Current income tax and deferred income tax are recognized in profit or loss, except to the extent that they relate to items recognized directly in shareholders' equity.
Current income tax is the expected tax payable or receivable on the taxable profit or loss for the year, using tax rates enacted or substantively enacted at the reporting date, adjusted for any amendments to tax payable in respect of previous years.
Deferred income tax is provided for, based on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized. Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
COVID-19 estimation uncertainty
On March 11, 2020, the World Health Organization declared the outbreak of the novel coronavirus (COVID-19) a global pandemic. This has resulted in governments worldwide, including the Canadian government, to enact emergency measures to combat the spread of the virus. These measures, which include social distancing, the implementation of travel bans, and closures of non-essential businesses, have caused material disruption to businesses globally, resulting in an economic slowdown. The production and sale of cannabis have been recognized as essential services across Canada. As at December 31, 2020, the Company has not observed any material impairments of assets or a significant change in the fair value of assets, due to the COVID-19 pandemic.
The situation is dynamic and the ultimate duration and magnitude of the impact of COVID-19 on the economy and the financial effect on our business, financial position and operating results remain unknown at this time. In addition, it is possible that estimates in the Company's financial statements will change in the near term as a result of COVID-19 and the effect of any such changes could be material, which could result in, among other things, impairment of long-lived assets. The Company is closely monitoring the impact of the pandemic on all aspects of its business.
2. Significant accounting policies (continued)
Inventory
Inventory is valued at the lower of cost and net realizable value. Cost of cannabis and hemp biomass is comprised of initial third-party acquisition costs, plus analytical testing costs. Costs of extracted cannabis and hemp oil inventory are comprised of initial acquisition cost of the biomass and all direct and indirect processing costs including labor related costs, consumables, materials, packaging supplies, utilities, facility costs, analytical testing costs, and production related depreciation. Net realizable value is determined as the estimated selling price in the ordinary course of business less the estimated cost of completion and the estimated costs necessary to make the sale. Packaging and supplies are initially valued at cost and subsequently at the lower of cost and net realizable value.
Cost of sales
Cost of sales represents costs directly related to manufacturing and distribution of the Company's products and services. Primary costs include raw materials, packaging, direct labor, overhead, shipping and the depreciation of production equipment and facilities. Manufacturing overhead and related expenses include salaries, wages, employee benefits, utilities, maintenance and property taxes. The Company recognizes the cost of sales as the associated revenue is recognized.
Revenue
Cannabis concentrate sales revenue earned under fee for service agreements, is recognized at a point in time when the Company is considered to have satisfied its performance obligations. The performance obligations are considered satisfied once all of the following have been met: (i) the manufacturing process (services) are complete; (ii) regulatory quality assurance, and customer quality assurance specifications (acceptance of the finished goods) have been met; and (iii) when the transaction price can be reliably measured in instances of variable consideration or non-monetary consideration.
At times, the Company may enter into contracts with customers where payment for the services provided by the Company is in the form of retention of a certain portion of the finished goods. In such instances, the consideration amount is variable and it's determined based on fair market values for the same or similar goods. As fair market values are readily available for cannabis concentrate, the level of estimation uncertainty is limited.
Revenues are recorded net of discounts but inclusive of freight in the sale of goods. Once the customer has accepted the finished goods, the Company has no obligations for returns, refunds, warranties or similar obligations.
Revenue from the sale of cannabis biomass and oil is recognized at a point in time when the Company has satisfied the performance obligations under the customer contracts. Revenue from toll processing and white label manufacturing, under fee for service agreements, is recognized over time under the customer contracts using a percentage of completion method measured by reference to the completed processing as a percentage of the total product to be processed under contract. Revenues of $2,499,355 for the year ended December 31, 2020 consisted of bulk distillate sales of $1,974,908, and toll processing revenue of $524,447.
Government assistance
Government grants and assistance are recognized as a reduction in the related expense in the period in which the grant or assistance become receivable on all conditions, if any, have been satisfied.
During the year ended December 31, 2020, the Company determined the impact on the revenue of the Company and its subsidiaries, as a result of the COVID-19 pandemic, qualified the Company to apply for the Canadian Emergency
Wage Subsidy ("CEWS") provided by the Government of Canada. As a result, the Company has recorded a reduction in wages and salaries of $10,288 (2019 - $nil).
During the year ended December 31, 2020, the Company qualified for a government-guaranteed bank loan of $60,000 which is interest-free. If the balance of the loan is repaid on or before December 31, 2022, 25% will not have to be repaid ($15,000). Funds can be used to pay non-deferrable operating expenses including payroll.
3. Reverse acquisition
As described in note 1, on December 20, 2019, Arrowstar and Adastra completed the Transaction which constituted a reverse acquisition.
The Transaction resulted in the shareholders of Adastra obtaining control of the combined entity by obtaining control of the voting rights, governance and management decision making processes, and the resulting power to govern the financial and operating policies of the combined entity.
The transaction constitutes an RTO of Arrowstar by Adastra and has been accounted for as a reverse acquisition transaction in accordance with the guidance provided in IFRS 2, Share-based payments. As Arrowstar did not qualify as a business according to the definition in IFRS 3, the RTO does not constitute a business combination; rather it is treated as an issuance of common shares by Adastra for the net assets of Arrowstar and Arrowstars public listing, with Adastra as the continuing entity. Accordingly, no goodwill was recorded with respect to the transaction as it does not constitute a business.
For accounting purposes, Adastra was treated as an accounting parent company (legal subsidiary) and Arrowstar has been treated as the accounting subsidiary (legal parent) in these financial statements. As Adastra was deemed to be the acquirer for accounting purposes, its assets, liabilities and operations since incorporation are included in these financial statements at their historical carrying values. Arrowstar's results of operations have been included from
December 20, 2019.
| | | December 20, | |
| | | 2019 | |
| Net assets (liabilities) of Arrowstar acquired: | | $ | |
| Cash | | 111,496 | |
| Receivables | | 10,802 | |
| Other assets | | 7,504 | |
| Accounts payable | | (356,384 | ) |
| Net liabilities acquired | | (226,582 | ) |
| | | | |
| Consideration paid in RTO of Arrowstar: | $ | | |
| Common shares (fair value 21,656,034 common shares at $0.05 per share) | | 1,082,802 | |
| Total consideration paid | | 1,082,802 | |
| Listing expense | | 1,309,384 | |
The Transaction was measured at the fair value of the shares that Adastra would have had to issue to the shareholders of Arrowstar, to give the shareholders of Arrowstar the same percentage equity interest in the combined entity that results from the reverse acquisition had it taken the legal form of Adastra acquiring Arrowstar.
4. Receivables and prepayments
Receivables and prepayments consist of the following:
| | | December 31, | | | December 31, | |
| | | 2020 | | | 2019 | |
| | | $ | | | $ | |
| Trade accounts receivable | | 971,102 | | | - | |
| Sales tax recoverable | | 1,578 | | | 303,708 | |
| Prepaid expenses | | 156,509 | | | 157,459 | |
| | | 1,129,189 | | | 461,167 | |
5. Marketable securities
On November 8, 2011, the Company received 10,000 shares in Dunnedin Ventures Inc. as part of a property option agreement. The shares were valued at $1,500 at the RTO date and included in other assets. During the year ended December 31, 2020, the Company disposed of the shares for no proceeds and accordingly, realized a loss on disposition in the amount of $1,500.
6. Inventory
Inventory consist of the following:
| | | December 31, | | | December 31, | |
| | | 2020 | | | 2019 | |
| | | $ | | | $ | |
| Extracted cannabis and hemp oils (finished goods) | | 908,117 | | | - | |
| Production work in process - distillate | | 273,937 | | | - | |
| Dried cannabis and hemp biomass | | 239,183 | | | - | |
| | | 1,421,237 | | | - | |
Inventory expensed to cost of sales during the year ended December 31, 2020 was $1,148,692 (2019 - $nil).
7. Property and equipment
| | | | | | | | | Furniture and | | | Computer | | | Laboratory | | | Extraction | | | Building | | | | |
| | | Land | | | Building | | | equipment | | | software | | | equipment | | | equipment | | | improvements | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost | | | | | | | | | | | | | | | | | | | | | | | | |
| May 1, 2019 | | 1,592,232 | | �� | 1,982,512 | | | 11,823 | | | - | | | - | | | - | | | 299,643 | | | 3,886,210 | |
| Additions | | - | | | 16,816 | | | 47,619 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,564,116 | | | 4,751,453 | |
| December 31, 2019 | | 1,592,232 | | | 1,999,328 | | | 59,442 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,863,759 | | | 8,637,663 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | | | | |
| May 1, 2019 | | - | | | 49,563 | | | 2,411 | | | - | | | - | | | - | | | - | | | 51,974 | |
| Depreciation | | - | | | 73,116 | | | 5,056 | | | - | | | - | | | - | | | - | | | 78,172 | |
| December 31, 2019 | | - | | | 122,679 | | | 7,467 | | | - | | | - | | | - | | | - | | | 130,146 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 | | 1,592,232 | | | 1,999,328 | | | 59,442 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,863,759 | | | 8,637,663 | |
| Additions | | - | | | - | | | 25,407 | | | - | | | 149,100 | | | 1,862,707 | | | 64,522 | | | 2,101,736 | |
| December 31, 2020 | | 1,592,232 | | | 1,999,328 | | | 84,849 | | | 12,105 | | | 211,395 | | | 2,911,209 | | | 3,928,281 | | | 10,739,399 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 | | - | | | 122,679 | | | 7,467 | | | - | | | - | | | - | | | - | | | 130,146 | |
| Depreciation | | - | | | 93,832 | | | 12,936 | | | 1,816 | | | 20,527 | | | 296,978 | | | 146,101 | | | 572,190 | |
| December 31, 2020 | | - | | | 216,511 | | | 20,403 | | | 1,816 | | | 20,527 | | | 296,978 | | | 146,101 | | | 702,336 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net book value | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 | | 1,592,232 | | | 1,876,649 | | | 51,975 | | | - | | | - | | | - | | | 3,863,759 | | | 8,507,517 | |
| December 31, 2020 | | 1,592,232 | | | 1,782,817 | | | 64,446 | | | 10,289 | | | 190,868 | | | 2,614,231 | | | 3,782,180 | | | 10,037,063 | |
During the year ended December 31, 2020, the Company allocated $479,490 (2019 - $nil) of depreciation to cost of sales and $92,700 (2019 - $53,867) to operating expense.
As at December 31, 2020, the Company had $4,000 in other deposits (December 31, 2019 - $4,000) from the RTO date and were included in deposits.
8. Share capital
Transactions for the issue of share capital during the year ended December 31, 2020:
(i) On January 17, 2020, the Company issued 189,934 units on conversion of $250,000 of principal and $6,411 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(ii) On January 27, 2020, the Company issued 266,760 units on conversion of $350,000 of principal and $10,126 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(iii) On July 10, 2020, the Company issued 200,589 units on conversion of $250,000 of principal and $20,795 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(iv) On July 13, 2020, the Company completed a non-brokered private placement whereby 3,882,667 units were issued at a price of $0.90 per unit for gross proceeds of $3,494,400. Each unit consists one common share and one common share purchase warrant with each warrant exercisable into one common share at an exercise price of $1.50 until July 13, 2022.
In conjunction with the financing, finders' fees of $21,600 in cash were paid and 24,067 compensation options (each a "Compensation Option") were granted. Each Compensation Option entitles the holder to purchase a unit on the same terms as the offering for a period of two years from the date of issuance. Each underlying common share purchase warrant (a "Compensation Option Warrant") will be subject to the same terms as the warrants attached to the units sold in the financing. If the Compensation Option Warrants are accelerated prior to the exercise of the Compensation Option, each Compensation Option Warrant will expire 30 days after the date of exercise of the Compensation Option. The fair value of the Compensation Option was $38,500 ($1.59 per Compensation Option) and was recognized as a share issuance cost. The fair value of the Compensation Options was estimated using the Black-Scholes option pricing model using the following assumptions:
| Assumptions | |
| Risk-free interest rate | 0.28% |
| Expected stock price volatility | 100.00% |
| Expected dividend yield | 0.00% |
| Expected life | 2 years |
Additionally, the Company settled services and outstanding indebtedness of $543,175 through the issuance of 647,280 common shares at a fair value price of $0.84 per common share. The common shares issued in connection with the services and debt settlement are subject to a hold period that expires four months and one day from the date of issuance.
(v) On July 16, 2020, the Company issued 759,605 units on conversion of $945,000 of principal and $80,467 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
8.Share capital (continued)
Transactions for the issue of share capital during the year ended December 31, 2020: (Continued)
(vi) On July 28, 2020, the Company issued 953,564 units on conversion of $1,182,000 of principal and $105,311 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(vii) On August 5, 2020, the Company issued 161,688 units on conversion of $200,000 of principal and $18,279 of accrued interest of the convertible debenture. Each unit consists of one common share of the Company and one share purchase warrant exercisable at $2.25 for two years from the date of conversion. No value was attributed to the warrant component of the units issued.
(viii) During the year ended December 31, 2020, the Company issued 42,967 common shares related to the exercise of 42,967 warrants at an exercise price of $1.80 per share.
Transactions for the issue of share capital during the period ended December 31, 2019:
On December 20, 2019, the Company closed 1,964,152 unit offering at a price of $1.35 per unit for gross proceeds of $2,651,605, with each unit consisting of one (1) common share of the Company and one underlying share purchase warrant exercisable at $1.80 for three years from the date of closing December 20, 2022. The warrants were given a value of $nil using the residual value method.
Escrowed shares
The Company entered into an Escrow Agreement in connection with closing the RTO on December 20, 2019, in relation to certain of its common shares which were placed in escrow. Pursuant to the Escrow Agreement the escrowed common shares are subject to a timed release schedule whereby a 10% portion of the escrow shares will be released beginning on listing date, and 15% every six months thereafter until January 6, 2023.
As at December 31, 2020, 6,500,000 common shares were held in escrow (December 31, 2019 - 8,666,667).
Stock options
The Company has an incentive stock option plan (the "Plan") which provides for the granting of options. Under the Plan the maximum number of stock options issued cannot exceed 10% of the Company's currently issued and outstanding common shares. Options granted under the Plan may have a maximum term of ten years. A participant, who is not a consultant conducting investor relations activities, who is granted an option that is exercisable at the market price at the date of grant, will have their options vest immediately, unless otherwise determined by the Board of Directors. Options granted at below market prices will vest one-sixth every three months.
A participant who is a consultant conducting investor relations activities who is granted an option under the Plan will become vested with the right to exercise one-quarter of the option upon conclusion of every three months subsequent to the grant date. All options are to be settled by physical delivery of shares.
8. Share capital (continued)
Stock options (continued)
On January 30, 2020, the Company granted 2,523,333 stock options with exercise price of $1.35 to certain Directors, Officers, employees and consultants. The options expire five years from the date of grant and vest immediately. The fair value of these options was $2,537,300 ($1.02 per option) and was recognized as a share-based payment expense. The fair value of the stock options was estimated using the Black-Scholes option pricing model using the following assumptions:
| Assumptions | |
| Risk-free interest rate | 1.29% |
| Expected stock price volatility | 100.00% |
| Expected dividend yield | 0.00% |
| Expected life | 5 years |
On June 1, 2020, the Company granted 66,667 stock options with exercise price of $1.35 to an employee. The options expire two years from the date of grant and vest immediately. The fair value of these options was $22,200 ($0.33 per option) and was recognized as a share-based payment expense. The fair value of the stock options was estimated using the Black-Scholes option pricing model using the following assumptions:
| Assumptions | |
| Risk-free interest rate | 0.39% |
| Expected stock price volatility | 100.00% |
| Expected dividend yield | 0.00% |
| Expected life | 2 years |
On August 5, 2020, the Company granted 1,616,667 stock options with exercise price of $2.34 to certain Directors, Officers, employees and consultants. The options expire five years from the date of grant and vest immediately. The fair value of these options was $2,813,800 ($1.74 per option) and was recognized as a share-based payment expense. The fair value of the stock options was estimated using the Black-Scholes option pricing model using the following assumptions:
| Assumptions | |
| Risk-free interest rate | 0.34% |
| Expected stock price volatility | 100.00% |
| Expected dividend yield | 0.00% |
| Expected life | 5 years |
8. Share capital (continued)
Stock options (continued)
A summary of the status of the Company's options as at December 31, 2020 and December 31, 2019 and changes during the year/period then ended is as follows:
| | | Year ended | | | Period ended | |
| | | December 31, 2020 | | | December 31, 2019 | |
| | | | | �� | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | Options | | | exercise price | | | Options | | | exercise price | |
| | | # | | | $ | | | # | | | $ | |
| Options outstanding, beginning of year/period | | - | | | - | | | - | | | - | |
| Granted | | 4,206,667 | | | 1.73 | | | - | | | - | |
| Cancelled | | (40,000 | ) | | 1.35 | | | - | | | - | |
| Options outstanding, end of year/period | | 4,166,667 | | | 1.73 | | | - | | | - | |
As at December 31, 2020, the Company has stock options outstanding and exercisable as follows:
Options | Options | Exercise | | |
outstanding | exercisable | Price | Expiry date | Weighted average |
# | # | $ | | remaining life (years) |
2,483,333 | 2,483,333 | 1.35 | January 30, 2025 | 4.08 |
66,667 | 66,667 | 1.35 | June 1, 2022 | 1.42 |
1,616,667 | 1,616,667 | 2.34 | August 5, 2025 | 4.60 |
4,166,667 | 4,166,667 | | | 4.24 |
Compensation Options
As at December 31, 2020, the Company has Compensation Options outstanding and exercisable as follows:
| Compensation Options | Compensation Options | Exercise | | |
| outstanding | exercisable | Price | Expiry date | Weighted average |
| # | # | $ | | remaining life (years) |
| 24,067 | 24,067 | 0.90 | July 13, 2022 | 1.53 |
| 24,067 | 24,067 | | | 1.53 |
Warrants
As an incentive to complete a private placement the Company may issue units which include common shares and common share purchase warrants. Using the residual value method, the Company determines whether a value should be allocated to the warrants attached to private placement units. Finders' warrants may be issued as a private placement share issue cost and are valued using the Black-Scholes option pricing model.
8. Share capital (continued)
Warrants (continued)
A summary of the status of the Company's warrants as at December 31, 2020 and December 31, 2019 and changes during the year/period then ended is as follows:
| | | Year ended | | | Period ended | |
| | | December 31, 2020 | | | December 31, 2019 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | Warrants | | | exercise price | | | Warrants | | | exercise price | |
| | | # | | | $ | | | # | | | $ | |
| Warrants outstanding, beginning of period | | 2,470,552 | | | 2.19 | | | - | | | - | |
| Replaced on RTO | | - | | | - | | | 506,400 | | | 2.76 | |
| Issued - attached to units | | 6,414,807 | | | 1.80 | | | 1,964,152 | | | 1.80 | |
| Exercised | | (42,967 | ) | | 1.80 | | | - | | | - | |
| Expired | | (506,400 | ) | | 2.76 | | | - | | | - | |
| Warrants outstanding, end of period | | 8,335,992 | | | 1.80 | | | 2,470,552 | | | 2.19 | |
As at December 31, 2020, the Company had warrants outstanding and exercisable as follows:
| Warrants | Warrants | Exercise | | |
| outstanding | exercisable | Price | Expiry date | Weighted average |
| # | # | $ | | remaining life (years) |
| 1,921,185 | 1,921,185 | 1.80 | December 19, 2022 | 1.97 |
| 189,934 | 189,934 | 2.25 | January 17, 2022 | 1.05 |
| 266,760 | 266,760 | 2.25 | January 27, 2022 | 1.07 |
| 200,589 | 200,589 | 2.25 | July 10, 2022 | 1.52 |
| 3,882,667 | 3,882,667 | 1.50 | July 13, 2022 | 1.53 |
| 759,605 | 759,605 | 2.25 | July 16, 2022 | 1.54 |
| 953,564 | 953,564 | 2.25 | July 28, 2022 | 1.57 |
| 161,688 | 161,688 | 2.25 | August 5, 2022 | 1.59 |
| 8,335,992 | 8,335,992 | | | 1.61 |
9. Loss per share
The calculation of basic loss per share for the year ended December 31, 2020 was based on the loss attributable to common shareholders of $7,615,864 (2019 - $2,657,957), and a weighted average number of common shares outstanding of 39,695,235 (2019 - 34,360,992).
All warrants outstanding as at December 31, 2020 and December 31, 2019, were excluded from the diluted weighted average number of common shares calculation as their effect would have been anti-dilutive.
10. Related party payables and transactions
A number of key management personnel and Directors hold positions in other entities that result in them having control or significant influence over the financial or operating policies of these entities. There were no loans to key management personnel or Directors, or entities over which they have control or significant influence during the years ended December 31, 2020 or December 31, 2019.
During the year ended December 31, 2020, 1,933,333 (2019 - nil) options were granted to Officers and Directors having a fair value on grant of $2,531,999 (2019 - $nil).
The following related parties transacted with the Company or Company controlled entities during the periods:
(a) Andrew Hale is a Director and the Company's President and CEO.
(b) Blaine Bailey is a Director and Chairman of the Company's Audit Committee.
(c) Stephen Brohman is the Company's CFO. He is a principal of Donaldson Brohman Martin CPA Inc. ("DBM CPA") a firm in which he has significant influence. DBM CPA provides the Company with accounting and tax services.
(d) George Routhier is a Company Director. He is the owner of Pipedreemz Inc. ("Pipedreemz"), which provides advisory services to the Company.
The aggregate value of transactions and outstanding balances with key management personnel and Directors and entities over which they have control or significant influence were as follows:
| | | Transactions | | | Transactions | | | Balances | | | Balances | |
| | | year ended | | | year ended | | | outstanding | | | outstanding | |
| | | December 31, | | | December 31, | | | December 31, | | | December 31, | |
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
| | | $ | | | $ | | | $ | | | $ | |
| DBM CPA | | 150,900 | | | 107,298 | | | 12,915 | | | - | |
| Andrew Hale | | 180,091 | | | 120,115 | | | - | | | - | |
| Pipedreemz | | 2,500 | | | 17,500 | | | - | | | - | |
| | | 333,491 | | | 244,913 | | | 12,915 | | | - | |
All related party balances are unsecured and are due within thirty days without interest.
The transactions with the key management personnel and Directors are included in operating expenses as follows:
(a) Consulting fees
- Includes the advisory services of Director, George Routhier, charged to the Company by Pipedreemz.
(b) Professional fees
- Includes the accounting and tax services of the Company's CFO, Stephen Brohman, charged to the Company by DBM CPA.
(g) Wages and salaries
- Includes charges by Andrew Hale for salaries paid to Officer.
11. Income taxes
A reconciliation of income taxes at statutory rates with the reported taxes for the year/period ended December 31, 2020 and December 31, 2019, is as follows:
| | | December 31, 2020 | | | December 31, 2019 | |
| | | $ | | | $ | |
| Loss for the period | | (7,615,864 | ) | | (2,657,957 | ) |
| Expected income tax (recovery) | | (2,056,000 | ) | | (718,000 | ) |
| Other | | (79,000 | ) | | 80,000 | |
| Permanent differences | | 1,439,000 | | | 353,000 | |
| Change in recognized deductible temporary differences | | 696,000 | | | 285,000 | |
| Total income tax expense (recovery) | | - | | | - | |
The significant components of the Company's deferred tax assets that have not been included on the consolidated statement of financial position are as follows:
| | | December 31,2020 | | | December 31, 2019 | |
| Deferred tax assets (liabilities) | | | | | | |
| Property and equipment | | 185,000 | | | 37,000 | |
| Non-capital losses available for future period | | 1,037,000 | | | 489,000 | |
| | | 1,222,000 | | | 526,000 | |
| Unrecognized deferred tax assets | | (1,222,000 | ) | | (526,000 | ) |
| Net deferred tax assets | $ | - | | $ | - | |
The Company's unused temporary differences, and unused tax losses that have not been included on the statements of financial position as at December 31, 2020 and December 31, 2019 are as follows:
| | | December 31, 2020 | | | Expiry Date | | | December 31, 2019 | | | Expiry Date | |
| | | $ | | | Range | | | $ | | | Range | |
| Property, plant and equipment | | 683,000 | | | N/A | | | 137,000 | | | N/A | |
| Non-capital loss carry forwards | | 3,840,000 | | | 2038 to 2040 | | | 1,814,000 | | | 2038 to 2039 | |
Tax attributes are subject to review, and potential adjustment, by tax authorities.
12. Supplemental cash flow information
Changes in non-cash operating working capital during the year/period ended December 31, 2020 and December 31,
2019 were comprised of the following:
| | | December 31, | | | December 31, | |
| | | 2020 | | | 2019 | |
| | | $ | | | $ | |
| Receivables and prepayments | | (668,022 | ) | | (411,396 | ) |
| Accounts payable and accrued liabilities | | 384,042 | | | 14,903 | |
| Inventory | | (941,747 | ) | | - | |
| Net change | | (1,225,727 | ) | | (396,493 | ) |
The Company incurred non-cash financing and investing activities during the year/period ended December 31, 2020 and December 31, 2019 as follows:
| | | December 31, | | | December 31, | |
| | | 2020 | | | 2019 | |
| | | $ | | | $ | |
| Non-cash financing activities | | | | | | |
| Conversion of convertible debentures - debenture reserves | | 298,461 | | | - | |
| Convertible debentures - settlement | | (3,418,390 | ) | | - | |
| Convertible debentures - reserves | | (70,814 | ) | | 298,461 | |
| Share issuance costs - Compensation Options | | (38,500 | ) | | - | |
| | | (3,229,243 | ) | | 298,461 | |
| Non-cash investing activities | | | | | | |
| Equipment purchases included in accounts payable and accrued liabilities | | 274,692 | | | 385,894 | |
| Shares issued for debt settlement | | 411,924 | | | - | |
| | | 686,616 | | | 385,894 | |
During the period ended December 31, 2020 no amounts were paid for income tax expenses.
13. Mortgage payable
a) On January 7, 2019, the Company entered into a mortgage for $2,446,000 (the "First Mortgage") which bore interest at the rate of 8.25% per annum, calculated monthly. The First Mortgage matured on February 1, 2020 and was renewed as discussed below.
The First Mortgage payable was recorded at amortized cost and bore an effective interest rate of 10.44%.
The carrying value of the First Mortgage at December 31, 2019 was $2,437,175. Included in mortgage payable on initial recognition were the related mortgage transaction costs of $50,755 which were amortized over the term of the First Mortgage using the effective interest rate method.
The Company maintained minimum interest only payments of $16,816 per month in connection with the First Mortgage. Total interest expense of the First Mortgage during the year ended December 31, 2020 was $42,457 (2019 - $164,242).
b) On February 1, 2020, the Company renewed the First Mortgage of $2,446,000 (the "Second Mortgage") which bears interest at the rate of 8.00% per annum, calculated monthly. The Second Mortgage matures on February 1, 2021 and can be repaid before maturity without penalty and is secured by the mortgage property and building improvements.
13. Mortgage payable (continued)
The Second Mortgage payable was recorded at amortized cost and bares an effective interest rate of 8.79%.
The carrying value of the Second Mortgage payable at December 31, 2020 was $2,442,830. Included in mortgage payable (on initial recognition) were the related mortgage transaction costs of $18,345 which are being amortized over the term of the Second Mortgage using the effective interest rate method.
The Company maintains minimum interest only payments of $16,307 per month. As at December 31, 2020 the total non-discounted remaining scheduled payments related to the mortgage including interest payments, total $2,478,613. Total interest expense during the year ended December 31, 2020 was $178,242 (2019 - $nil).
On February 1, 2021, the Company renewed the mortgage of $2,446,000 which bears interest at the rate of 8.00% per annum, calculated monthly. The mortgage matures on February 1, 2022 and can be repaid before maturity without penalty and is secured by the mortgage property and building improvements.
14. Convertible debentures
On October 31, 2019, the Company closed a 2,353,333 convertible debenture unit offering at a price of $1.35 per debenture unit for gross proceeds $3,177,000. Each debenture consisted of a 12% secured convertible debenture with a maturity of two years from the date of issuance. If the holder converted their debenture unit, they were entitled to one common share and one share purchase warrant exercisable at $2.25 per share exercisable two years from the date of the convertible debenture closing October 31, 2021 at the holder's discretion.
If the closing price of the Common Shares of the Company was higher than $3.00 for any 10 consecutive trading days, the expiry date of the Warrants may be accelerated to the 30th day after the date of a news release announcing such acceleration. The debentures were secured to the facility subordinate to the mortgage currently on the facility.
As the debentures were convertible into units, the liability and equity components were presented separately on the consolidated statement of financial position. The initial carrying amount of the financial liability was determined by discounting the stream of future payments of interest and principal at a market interest rate of 16% totaling $2,878,539. Using the residual method, the carrying amount of the conversion feature is the difference between the principal amount and the initial carrying value of the financial liability. The equity component was recorded in debenture reserves on the consolidated statement of financial position totaling $298,461. The debentures, net of the equity components were accreted using the effective interest method over the term of the debentures, such that the carrying amount of the financial liability was equal the principal balance at maturity.
During the year ended December 31, 2020, the Company settled principal plus accrued interest of $3,418,390 relating to the debentures through the issuance of 2,532,140 units. The Company reversed $298,461 from debenture reserves in connection with the settlement.
The Company recorded interest expense of $169,552 (2019 - $63,713) and accretion expense of $58,405 (2019 - $13,259) related to the convertible debentures during the year ended December 31, 2020. The carrying value of the convertible debentures at December 31, 2020 was $nil (2019 - $2,955,511) and the carrying value of debenture reserves was $nil (2019 - $298,461).
15. Financial risk management Capital management
The Company defines capital as the components of shareholders' equity. The Company's objectives when managing capital are to support further advancement of the Company's business objectives, as well as to ensure that the Company is able to meet its financial obligations as they come due.
The Company manages its capital structure to maximize its financial flexibility making adjustments to it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. The
Company relies on the expertise of the Company's management to sustain the future development of the business.
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There were no changes in the Company's approach to capital management during the year ended December 31, 2020. The Company is not subject to externally imposed capital requirements.
15. Financial risk management (continued)
Financial instruments - fair value
The Company's financial instruments consist of cash, trade receivables, deposits, accounts payable and accrued liabilities, mortgage payable and loan payable.
The carrying value of accounts payable and accrued liabilities, and loan payable approximated their fair value because of the short-term nature of these instruments. Mortgage payable approximate fair value due to its market rates of interest charged.
Financial instruments measured at fair value on the consolidated statements of financial position are summarized into the following fair value hierarchy levels:
Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices).
Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | $ | | | $ | | | $ | | | $ | |
| December 31, 2020 | | | | | | | | | | | | |
| Cash | | 1,145,461 | | | - | | | - | | | 1,145,461 | |
| Deposits | | 4,000 | | | - | | | - | | | 4,000 | |
| | | 1,149,461 | | | - | | | - | | | 1,149,461 | |
| December 31, 2019 | | | | | | | | | | | | |
| Cash | | 2,376,826 | | | - | | | - | | | 2,376,826 | |
| Marketable securities | | 1,500 | | | - | | | - | | | 1,500 | |
| Deposits | | 4,000 | | | - | | | - | | | 4,000 | |
| | | 2,382,326 | | | - | | | - | | | 2,382,326 | |
Financial instruments - risk
The Company's financial instruments are exposed to certain financial risks, including credit risk, liquidity risk and currency risk.
(a) Credit risk
The Company is exposed to credit risk by holding cash. This risk is minimized by holding the funds in Canadian banks and credit unions or with Canadian governments. The Company has minimal receivable exposure, and its various refundable credits are due from Canadian governments.
(b) Liquidity risk
Liquidity risk is the risk that the Company is unable to meet its financial obligations as they come due. The Company manages this risk by careful management of its working capital to ensure its expenditures will not exceed available resources.
(c) Currency risk
The Company is not exposed to currency risk.
Economic dependence
Economic dependence risk is the risk of reliance upon a select number of customers which significantly impact the financial performance of the Company. During the year ended December 31, 2020, the Company's sales were generated by three customers representing 79%, 16% and 5%.
16. Segmented information
The Company has a single reportable segment: the provision of goods and services to the cannabis industry in Canada.
All the Company's revenues are generated in Canada, and its non-current assets are located in Canada.
17. Events after the reporting period
(a) On March 10, 2021, the Company announced the appointment of J. Scott Munro as President, CEO and Director in conjunction with the resignation and retirement of Andrew Hale from said position.
(b) On March 29, 2021, the Company announced the resignation of Blaine Bailey as Director.
(c) On April 30, 2021, the Company announced the resignation of J. Scott Munro as President, CEO and Director.
(d) On May 10, 2021 the Company announced the appointment of Michael Forbes as President, CEO and Director in conjunction with the resignation of J. Scott Munro from said position. Additionally, Donald Dinsmore, Chief Operating Officer of the Company was appointed Director.
(e) During the period subsequent to December 31, 2020, the Company cancelled 1,599,999 stock options and issued a further 933,333 stock options.
(f) On July 6, 2021, 1,300,000 shares were released from escrow.
(g) On July 14, 2021, the Company appointed Oliver Foeste as CFO in conjunction with the resignation of Stephen Brohman. On the same day, the Company also appointed Paul Morgan as a Director of the Company.
(h) On August 1, 2021, the Company refinanced the Third Mortgage and increased the facility to $3,500,000 which bears interest at the rate of 6.50% per annum, calculated monthly for one year. The mortgage matures on July 1, 2022 and is secured by the mortgage property and building improvements.
(i) On August 10, 2021 the Company acquired, through its wholly-owned subsidiary Adastra Labs Inc, all of the issued and outstanding shares in the capital of 1225140 B.C. Ltd., doing business as PerceiveMD for a total contract value of $2,300,000 comprising $10,000 in cash; and $2,290,000 by way of the issuance of unrestricted common shares of the Company. At the time of issuance of these Financial Statements the draft results of this Company are yet to be determined due to the proximity of the issue date to the acquisition. It is thus impracticable to disclosure more detailed information required at this time.
(j) On September 15, 2021 the Company acquired, all of the issued and outstanding shares in the capital of 1204581 B.C. Ltd., doing business as Phyto Extractions for a contract value of $19,200,000 by way of the issuance of 20,000,000 unrestricted common shares of the Company. At the time of issuance of these Financial Statements the draft results of this Company are yet to be determined due to the proximity of the issue date to the acquisition. It is thus impracticable to disclosure more detailed information required at this time.
(k) On October 18, 2021, we completed a non-brokered private placement whereby we issued 122,727 units at a price of $1.10 per unit for gross proceeds of $135,000. Each unit was comprised of one common share and one transferrable common share purchase warrant with each warrant entitling the holder thereof to acquire one common share at a price of $1.75 per share for two years from the date of the closing. The warrants are subject to an acceleration provision whereby if the daily closing price of the common shares as quoted on the Canadian Securities Exchange closes at or above $2 per share for 50 consecutive trading days, then we may accelerate the expiration date of the warrants to the date that is 30 trading days from the date that notice of such acceleration is given via news release. From and after the new accelerated expiration date, no warrants may be exercised, and all unexercised warrants will be void.
(l) On October 25, 2021, we granted an aggregate of 900,000 stock options to certain directors and officers for the purchase of up to 900,000 common shares at a price of $1.06 per share. Each stock option is exercisable for a period of five years.
17. Events after the reporting period (continued)
(m) On October 28, 2021, we granted an aggregate of 215,000 stock options to certain employees and a consultant for the purchase of up to 215,000 common shares at a price of $0.95 per share. Each stock option is exercisable for a period of five years.
Adastra Holdings Ltd.
(formerly Phyto Extractions Inc.)
Consolidated Financial Statements
For the period ended December 31, 2019 and for the period from incorporation on June 18, 2018 to April 30, 2019
(Expressed in Canadian Dollars)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
Adastra Holdings Ltd.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Adastra Holdings Ltd. (the "Company"), as of December 31, 2019 and April 30, 2019, and the related consolidated statements of loss and comprehensive loss, changes in equity, and cash flows for the eight months ended December 31, 2019, and for the period from incorporation on June 18, 2018 to April 30, 2019, and the related notes to the consolidated financial statements (collectively referred to as the "financial statements"). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and April 30, 2019, and the results of its operations and its cash flows for the eight months ended December 31, 2019, and for the period from incorporation on June 18, 2018 to April 30, 2019 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company had a working capital deficit of $471,084 and has incurred losses since inception. These events and conditions indicate a material uncertainty exists that may cast significant doubt on the Company's ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.

Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company's auditor since 2019.
| | /s/ DAVIDSON & COMPANY LLP |
| | |
Vancouver, Canada | Chartered Professional Accountants |
| | |
| November 2, 2021 | |
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Financial Position |
| As at December 31, 2019 and April 30, 2019 |
| | | | December 31, | | | April 30, | |
| | | | 2019 | | | 2019 | |
| | Note | | $ | | | $ | |
| Assets | | | | | | | |
| Current assets | | | | | | | |
| Cash | | | 2,376,826 | | | 2,362,494 | |
| Receivables and prepayments | 4 | | 461,167 | | | 36,965 | |
| Marketable securities | 5 | | 1,500 | | | - | |
| | | | 2,839,493 | | | 2,399,459 | |
| Non-current assets | | | | | | | |
| Deposits | 6 | | 4,000 | | | 20,823 | |
| Property plant and equipment | 6 | | 8,507,517 | | | 3,834,236 | |
| | | | 8,511,517 | | | 3,855,059 | |
| Total assets | | | 11,351,010 | | | 6,254,518 | |
| | | | | | | | |
| Liabilities and equity | | | | | | | |
| Current liabilities | | | | | | | |
| Accounts payable and accrued liabilities | | | 873,402 | | | 137,044 | |
| Mortgage Payable | 12 | | 2,437,175 | | | 2,407,463 | |
| | | | 3,310,577 | | | 2,544,507 | |
| Non-current liabilities | | | | | | | |
| Convertible debenture | 13 | | 2,955,511 | | | - | |
| Total liabilities | | | 6,266,088 | | | 2,544,507 | |
| | | | | | | | |
| Equity | | | | | | | |
| Share capital | 7 | | 8,348,407 | | | 4,614,000 | |
| Debenture reserves | 13 | | 298,461 | | | - | |
| Deficit | | | (3,561,946 | ) | | (903,989 | ) |
| Total equity | | | 5,084,922 | | | 3,710,011 | |
| Total liabilities and equity | | | 11,351,010 | | | 6,254,518 | |
| Nature of operations and going concern | 1 | | | | | | |
Approved on behalf of the Board of Directors on November 2, 2021:
| “Michael Forbes” | | “Donald Dinsmore” |
| Director | | Director |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Changes in Equity |
| For the years ended December 31, 2019 and April 30, 2019 |
| | | Number | | | Share | | | Debenture | | | | | | Total | |
| | | of shares | | | capital | | | Reserves | | | Deficit | | | equity | |
| | | # | | | $ | | | $ | | | $ | | | $ | |
| Issuance of incorporation share | | 1 | | | 1 | | | - | | | - | | | 1 | |
| Cancellation of incorporation share | | (1 | ) | | (1 | ) | | - | | | - | | | (1 | ) |
| Shares issued for cash - private placements | | 81,138,333 | | | 4,614,000 | | | - | | | - | | | 4,614,000 | |
| Loss and comprehensive loss | | - | | | - | | | - | | | (903,989 | ) | | (903,989 | ) |
| April 30, 2019 | | 81,138,333 | | | 4,614,000 | | | - | | | (903,989 | ) | | 3,710,011 | |
| | | | | | | | | | | | | | | | |
| May 1, 2019 | | 81,138,333 | | | 4,614,000 | | | - | | | (903,989 | ) | | 3,710,011 | |
| Convertible debenture - pre RTO | | - | | | - | | | 298,461 | | | - | | | 298,461 | |
| Reverse acquisition transaction (Note 3): | | | | | | | | | | | | | | | |
| Issuance of shares pursuant to reverse acquisition | | 21,656,034 | | | 1,082,802 | | | - | | | - | | | 1,082,802 | |
| Private placement units issued | | 5,892,455 | | | 2,651,605 | | | - | | | - | | | 2,651,605 | |
| Loss and comprehensive loss | | - | | | - | | | - | | | (2,657,957 | ) | | (2,657,957 | ) |
| December 31, 2019 | | 108,686,822 | | | 8,348,407 | | | 298,461 | | | (3,561,946 | ) | | 5,084,922 | |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Loss and Comprehensive Loss |
| | | | For the eight months ended | | | For the period from incorporation on | |
| | | | December 31, 2019 | | | June 18, 2018 to April 30, 2019 | |
| | Note | | $ | | | $ | |
| Expenses | | | | | | | |
| Advertising and promotion | | | 56,817 | | | - | |
| Accretion | 13 | | 13,259 | | | 12,218 | |
| Depreciation | 6 | | 78,172 | | | 51,974 | |
| Insurance | | | 18,820 | | | 3,867 | |
| Management fees | 9 | | - | | | 39,638 | |
| Office expeses | | | 179,938 | | | 114,424 | |
| Professonal fees and consulting | 9 | | 447,784 | | | 253,860 | |
| Rent | | | 10,806 | | | 29,029 | |
| Research expenses | | | - | | | 4,336 | |
| Travel | | | 20,273 | | | 40,001 | |
| Wages and salaries | 9 | | 302,513 | | | 300,267 | |
| Loss from operating expenses | | | (1,128,382 | ) | | (849,614 | ) |
| Interest expense | 12,13 | | (227,955 | ) | | (63,165 | ) |
| Interest income | | | 7,764 | | | 8,790 | |
| Listing expense | 3 | | (1,309,384 | ) | | - | |
| Loss and comprehensive loss for the year | | | (2,657,957 | ) | | (903,989 | ) |
| | | | | | | | |
| Loss per share | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | |
| - basic # | 8 | | 82,487,647 | | | 13,265,014 | |
| - diluted # | 8 | | 82,487,647 | | | 13,265,014 | |
| | | | | | | | |
| Basic loss per share $ | 8 | | (0.03 | ) | | (0.07 | ) |
| Diluted loss per share $ | 8 | | (0.03 | ) | | (0.07 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
| Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) |
| Consolidated Statements of Cash Flows |
| | | | For the eight months ended | | | For the period from incorporation on | |
| | Note | | December 31, 2019 | | | June 18, 2018 to April 30, 2019 | |
| | | | | | | | |
| Operating activities | | | | | | | |
| Loss and comprehensive loss for the year | | | (2,657,957 | ) | | (903,989 | ) |
| Adjustments for non-cash items: | | | | | | | |
| Depreciation | | | 78,172 | | | 51,974 | |
| Accretion | | | 13,259 | | | 12,218 | |
| Interest expense | | | 227,955 | | | 63,165 | |
| Interest income | | | (7,764 | ) | | (8,790 | ) |
| Listing expense | | | 1,309,384 | | | - | |
| Net change in non-cash working capital items | 11 | | (396,493 | ) | | 100,079 | |
| | | | (1,433,444 | ) | | (685,343 | ) |
| | | | | | | | |
| Financing activities | | | | | | | |
| Issue of units for cash | | | 2,651,605 | | | 4,614,000 | |
| Proceeds - mortgage | | | - | | | 2,446,000 | |
| Borrowing costs mortgage | | | - | | | (50,755 | ) |
| Interest paid - mortgage | | | (134,530 | ) | | (63,165 | ) |
| Convertible debenture | | | 3,177,000 | | | - | |
| | | | 5,694,075 | | | 6,946,080 | |
| | | | | | | | |
| Investing activities | | | | | | | |
| Acquisition property plant and equipment | | | (4,365,559 | ) | | (3,886,210 | ) |
| Equipment deposits | | | - | | | (20,823 | ) |
| Interest income | | | 7,764 | | | 8,790 | |
| Cash acquired on reverse acquisition | | | 111,496 | | | - | |
| | | | (4,246,299 | ) | | (3,898,243 | ) |
| | | | | | | | |
| Net increase in cash and cash equivalents | | | 14,332 | | | 2,362,494 | |
| Cash, beginning of year | | | 2,362,494 | | | - | |
| Cash, end of year | | | 2,376,826 | | | 2,362,494 | |
| | | | | | | | |
| Supplemental cash flow information | 11 | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
1. Nature of operations and going concern
Adastra Holdings Ltd. (formerly Phyto Extraction Inc.) (the "Company" or "Arrowstar") was incorporated under the laws of the province of British Columbia on October 14, 1987. The Company is an extraction and processing solutions company. The Company's mission is to develop and deploy large-scale cannabis and hemp extraction technologies and provide turn-key processing solutions to help licensed standard and micro-cultivators maximize the value of every harvest. The Company is listed on the Canadian Securities Exchange ("CSE") under the symbol "XTRX".
The Company's registered and records office is 5451 275th Street, Langley City, British Columbia, V4W 3X8.
On October 19, 2019, Health Canada issued a Analytical testing license on the Company's facility to Chemia Analytics Inc., ("Chemia"). Chemia is a wholly owned subsidiary of the Company. Subsequent to year end, Health Canada issued a Standard Processing License on the Company's facility to Adastra labs Inc., ("Labs"). Labs is a wholly owned subsidiary of the Company.
On December 20, 2019, Arrowstar acquired all of the issued and outstanding common shares of Adastra Labs Holdings
(2019) Ltd. ("Adastra") a private British Columbia cannabis extraction and processing solutions company incorporated on June 18, 2018. The acquisition was completed by entering into a share exchange agreement whereby the parties completed a business combination by way of a transaction that constituted a reverse takeover ("RTO") of the Company by Adastra (the "Transaction"). The Transaction was accounted for as an RTO of Arrowstar by Adastra for accounting purposes, with Adastra being identified as the accounting acquirer, and accordingly, these financial statements are a continuation of Adastra. The net assets of Arrowstar at the date of the RTO are deemed to have been acquired by
Adastra (Note 3). These consolidated financial statements (the "financial statements") include the results of operations of Arrowstar since December 20, 2019. The comparative figures are those of Adastra prior to the RTO. At the time of the transaction the Company changed its financial year end from April 30 to December 31.
These financial statements are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of operations. These financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities that might be necessary should the Company be unable to continue in existence.
The Company's ability to continue as a going concern is dependent on its ability to generate positive cash flows from operations, complete additional financings, and/or extend or modify its mortgage payable (note 12).
As at December 31, 2019, the Company had a working capital deficit of $471,084 (April 30, 2019 - $145,048) and has incurred losses since inception. These events and conditions indicate a material uncertainty exists that may cast significant doubt on the Company's ability to continue as a going concern. If the going concern assumption were not appropriate for these financial statements it could be necessary to restate the Company's assets and liabilities on a liquidation basis.
2. Significant accounting policies
(a) Basis of presentation
These financial statements have been prepared in accordance with International Financial Reporting Standards and
Interpretations (collectively, "IFRS"), as issued by the International Accounting Standards Board ("IASB") and the International Financial Reporting Interpretations Committee ("IFRIC") and have been applied consistently by the
Company and its subsidiaries.
These financial statements have been prepared on an historical cost basis, except for financial instruments which are classified as fair value through profit or loss ("FVTPL"). In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information.
All amounts on these financial statements are presented in Canadian dollars which is the functional currency of the Company and its subsidiaries (note 2(b)).
2. Significant accounting policies (continued)
(b) Principles of consolidation
These financial statements include the financial information of the Company and its subsidiaries. The financial statements include the following entities:
Adastra Holdings Ltd. (formerly Phyto Extractions Inc.) | Legal parent company |
| | |
Adastra Labs Holdings (2019) Ltd. (formerly Adastra Labs Holdings Ltd.) | Holding company |
| | |
Adastra Labs Inc. | Extraction and concentrates
production company |
| | |
Chemia Analytics Inc. | Cannabis testing an analysis
laboratory company |
| | |
1178562 B.C. Ltd. | Facility owner |
| | |
Adastra Brands Inc. | Intellectual property
company |
Subsidiaries are entities controlled by the Company and are included in the financial statements from the date that control commences until the date that control ceases. The accounting policies of subsidiaries are changed where necessary to align them with the policies adopted by the Company.
These financial statements account for Arrowstar as a controlled entity (accounting acquiree) requiring consolidation since the date of the RTO (Notes 1 and 3), effective December 20, 2019.
(c) New accounting standards
The Company adopted the following accounting standards that are effective for accounting periods beginning on or after January 1, 2019:
IFRS 16 - Leases
IFRS 16 specifies how to recognize, measure, present and disclose leases. The new standard provides a single lessee accounting model, requiring lessees to recognize assets and liabilities for all leases unless the lease term is 12 months or less or the underlying asset has a low value. Consistent with its predecessor, IAS 17 the new lease standard continues to require lessors to classify leases as operating or finance. IFRS 16 is to be applied retrospectively for annual periods beginning on or after January 1, 2019.
The most significant effect of the new standard will be the lessee's recognition of the initial present value of unavoidable future lease payments as right-of-use ("ROU") assets and lease liabilities on the statement of financial position, including those for most leases that would formerly have been accounted for as operating leases.
The adoption of IFRS 16 requires the Company to make judgments that affect the valuation of the lease liability and the valuation of the ROU asset. These include determining contracts that are within the scope of IFRS 16; determining the contract term; and determining the interest rate used for the discounting of future cash flows.
The Company does not have any leases and accordingly, there was no impact to the Company's financial statements as a result of adopting this new standard.
New Interpretation IFRIC 23 - Uncertainty over Income Tax Treatments
On June 7, 2017, the IASB issued IFRIC Interpretation 23, Uncertainty over Income Tax Treatments. IFRIC 23 provides guidance on the accounting for current and deferred tax liabilities and assets in circumstances in which there is uncertainty over income tax treatments.
There was no impact to the Company's financial statements as a result of adopting this new standard.
2. Significant accounting policies (continued)
The preparation of financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at each reporting date and the reported amounts of income and expenses during each reporting period. Estimates and assumptions are continuously evaluated and are based on management's experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
The information about significant areas of estimation uncertainty considered by management in preparing these financial statements is as follows:
Acquisitions
Determination of whether a set of assets acquired and liabilities assumed constitute the acquisition of a business or asset requires the Company to make certain judgments as to whether or not the assets acquired and liabilities assumed include the inputs, processes and outputs necessary to constitute a business as defined in IFRS 3 - Business Combinations. If an acquired set of assets and liabilities includes goodwill, the set is presumed to be a business. Based on an assessment of relevant facts and circumstances, the Company concluded that the acquisition disclosed in Note 3 was acquisitions of assets. The values assigned to common shares and the allocation of the purchase price to the net liabilities in the acquisition are based on numerous estimates and judgements of the relative fair values of net liabilities.
Property, Plant and Equipment
The estimated useful lives of property plant and equipment are reviewed by management and adjusted if necessary. To estimate property, plant and equipment's useful life, management must use its past experience with the same or similar assets, review engineering estimates and industry practices for similar pieces of property, plant and equipment and/or apply statistical methods to assist in its determination of useful life. Additionally, management makes estimates with respect to the fair value of equipment acquired for non-monetary consideration. The Company assesses fair value by comparing market prices for similar types of property, plant and equipment.
The information about significant areas of judgment considered by management in preparing these financial statements is as follows:
Income taxes
Tax provisions are based on enacted or substantively enacted laws. Changes in those laws could affect amounts recognized in profit or loss both in the period of change, which would include any impact on cumulative provisions, and in future periods. Deferred tax assets (if any) are recognized only to the extent it is considered probable that those assets will be recoverable. This involves an assessment of when those deferred tax assets are likely to reverse and a judgment as to whether or not there will be sufficient taxable profits available to offset the tax assets when they do reverse. This requires assumptions regarding future profitability and is therefore inherently uncertain. To the extent assumptions regarding future profitability change, there can be an increase or decrease in the amounts recognized in respect of deferred tax assets as well as the amounts recognized in profit or loss in the period in which the change occurs.
Going concern
The assessment of the Company's ability to continue as a going concern as discussed in Note 1 involves judgment regarding future funding available for its operations and working capital requirements.
2. Significant accounting policies (continued)
Property, Plant and Equipment
Property, plant and equipment is measured at cost less accumulated depreciation and impairment losses. The cost of an item of property, plant and equipment consists of the purchase price, any costs directly attributable to bringing the asset to the location and condition necessary for its intended use and an initial estimate of the costs of dismantling and removing the item and restoring the site on which it is located. No depreciation is recorded in the year of disposal.
Depreciation is recognized over the following terms, intended to depreciate the cost of property, plant and equipment, less its residual values if any, over its estimated useful lives:
Furniture and equipment | 20% declining balance |
Leasehold improvements | 10 years straight line |
Buildings | 20 years straight line |
Extraction equipment | 20% declining balance |
Laboratory equipment | 20% declining balance |
Computer software | 20% declining balance |
Cost includes expenditures that are directly attributable to the acquisition of the asset. Subsequent costs are included in the asset's carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost can be measured reliably. Repairs and maintenance costs are charged to profit or loss during the period they are incurred. Any gain or loss on the disposal or retirement of equipment is recognized in profit or loss.
Share capital
Common shares are classified as shareholders' equity. Transaction costs directly attributable to the issue of common shares are recognized as a deduction from shareholders' equity. Common shares issued for consideration other than cash, are valued based on their market value at the date the shares are issued.
The Company has adopted a residual value method with respect to the measurement of warrants attached to private placement units. The residual value method first allocates value to the more easily measurable component based on fair value and then the residual value, if any, to the less easily measurable component. The Company considers the fair value of common shares issued in the private placements to be the more easily measurable component. The balance, if any, is allocated to the attached warrants. Any fair value attributed to the warrants is recorded as warrant reserve.
Share-based payment transactions
The Company has a stock option plan that provides for the granting of options to Officers, Directors, related company employees and consultants to acquire shares of the Company. The fair value of the options is measured on grant date and is recognized as an expense with a corresponding increase in contributed surplus as the options vest.
Options granted to employees and others providing similar services are measured at grant date at the fair value of the instruments issued. Fair value is determined using the Black-Scholes option pricing model taking into account the terms and conditions upon which the options were granted. The amount recognized as an expense is adjusted to reflect the actual number of share options that are expected to vest. Each tranche in an award with graded vesting is considered a separate grant with a different vesting date and fair value. Each grant is accounted for on that basis.
Options granted to non-employees are measured at the fair value of the goods or services received, unless that fair value cannot be estimated reliably, in which case the fair value of the equity instruments issued is used. The value of the goods or services is recorded at the earlier of the vesting date, or the date the goods or services are received.
Over the vesting period, share-based payments are recorded as an operating expense and as contributed surplus. When options are exercised the consideration received is recorded as share capital and the related share-based payments originally recorded as contributed surplus are transferred to share capital. When an option is cancelled or expires, the initial recorded value is reversed from contributed surplus and credited to deficit.
2. Significant accounting policies (continued)
Loss per share
Basic loss per share is computed by dividing the net loss attributable to common shareholders by the weighted average number of common shares outstanding during the reporting period.
Diluted loss per share is computed similar to basic loss per share except that (i) net loss attributable to common shareholders are adjusted for the dilutive effect of warrants and stock options. Under this method, the Company assumes that outstanding dilutive stock options and warrants were exercised and that the proceeds from such exercises (after adjustment of any unvested portion of stock options) were used to acquire common shares at the average market price during the reporting periods. For the period presented, diluted loss per share equals basic loss per share as there were no dilutive stock options or warrants outstanding.
Financial instruments
Effective June 18, 2018 (date of incorporation), the Company adopted IFRS 9, Financial Instruments ("IFRS 9"). IFRS 9 provides three different measurement categories for non-derivative financial assets - subsequently measured at amortized cost, fair value through profit or loss ("FVTPL") or fair value through other comprehensive income - while all non-derivative financial liabilities are classified as subsequently measured at amortized cost. The category into which a financial asset is placed and the resultant accounting treatment is largely dependent on the nature of the business of the entity holding the financial asset. All financial instruments are initially recognized at fair value.
Financial assets
Measurement
The Company initially recognizes financial assets on the trade date, which is the date that the Company becomes a party to the contractual provisions of the instrument. The Company derecognizes a financial asset when the contractual rights to the cash flows from the asset expire, or it transfers the rights to receive the contractual cash flows in a transaction in which substantially all of the risks and rewards of ownership of the financial asset are transferred. Any interest in such transferred financial assets that is created or retained by the Company is recognized as a separate asset or liability. The Company classifies all of its financial assets, except cash, as subsequently measured at amortized cost. Cash is classified as FVTPL. All financial assets that do not meet the criteria to be recognized as subsequently measured at amortized cost or subsequently measured at fair value through other comprehensive income are classified as FVTPL.
Impairment of financial assets at amortized cost
An 'expected credit loss' impairment model applies which requires a loss allowance to be recognized based on expected credit losses. The estimated present value of future cash flows associated with the asset is determined and an impairment loss is recognized for the difference between this amount and the carrying amount as follows: the carrying amount of the asset is reduced to estimated present value of the future cash flows associated with the asset, discounted at the financial asset's original effective interest rate, either directly or through the use of an allowance account and the resulting loss is recognized in profit or loss for the period.
In a subsequent period, if the amount of the impairment loss related to financial assets measured at amortized cost decreases, the previously recognized impairment loss is reversed through profit or loss to the extent that the carrying amount of the investment at the date the impairment is reversed does not exceed what the amortized cost would have been had the impairment not been recognized.
Financial liabilities
The Company measures all of its financial liabilities as subsequently measured at amortized cost. Financial liabilities are recognized initially at fair value, net of transaction costs incurred, and are subsequently measured at amortized cost. Any difference between the amounts originally received, net of transaction costs, and the redemption value is recognized in profit and loss over the period to maturity using the effective interest method.
2. Significant accounting policies (continued)
Impairment of non-financial assets
The carrying amount of the Company's long-lived assets is reviewed for an indication of impairment at the end of each reporting period. If an indication of impairment exists, the Company makes an estimate of the asset's recoverable amount. Individual assets are grouped for impairment assessment purposes at the lowest level at which there are identifiable cash flows that are largely independent of the cash flows of other groups of assets (cash generating units "CGU"). The recoverable amount of an asset group is the higher of its fair value less costs to sell and its value in use. In assessing value in use, the estimated future cash flows are adjusted for the risks specific to the CGU and are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money.
Where the carrying amount of an asset group exceeds its recoverable amount, the CGU is considered impaired and is written down to its recoverable amount. Impairment losses are recognized in profit or loss. Impairment losses recognized in respect of CGUs are allocated to reduce the carrying amounts of the assets in the CGU on a pro rata basis.
An assessment is made at each reporting date as to whether there is any indication that previously recognized impairment losses may no longer exist or may have decreased. If such indication exists, the recoverable amount is estimated. A previously recognized impairment loss is reversed only if there has been a change in the estimates used to determine the asset's recoverable amount. An impairment loss is reversed only to the extent that the asset's carrying amount does not exceed the carrying amount that would have been determined, net of depreciation, if no impairment loss had been recognized.
Income taxes
Income tax comprises current and deferred income taxes. Current income tax and deferred income tax are recognized in profit or loss, except to the extent that they relate to items recognized directly in shareholders' equity.
Current income tax is the expected tax payable or receivable on the taxable profit or loss for the year, using tax rates enacted or substantively enacted at the reporting date, adjusted for any amendments to tax payable in respect of previous years.
Deferred income tax is provided for, based on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized. Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period.
Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists
to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
3. Reverse acquisition
As described in note 1, on December 20, 2019, Arrowstar and Adastra completed the Transaction which constituted a reverse acquisition.
The Transaction resulted in the shareholders of Adastra obtaining control of the combined entity by obtaining control of the voting rights, governance and management decision making processes, and the resulting power to govern the financial and operating policies of the combined entity.
The transaction constitutes an RTO of Arrowstar by Adastra and has been accounted for as a reverse acquisition transaction in accordance with the guidance provided in IFRS 2, Share-based payments. As Arrowstar did not qualify as a business according to the definition in IFRS 3, the RTO does not constitute a business combination; rather it is treated as an issuance of common shares by Adastra for the net assets of Arrowstar and Arrowstars public listing, with Adastra as the continuing entity. Accordingly, no goodwill was recorded with respect to the transaction as it does not constitute a business.
For accounting purposes, Adastra was treated as an accounting parent company (legal subsidiary) and Arrowstar has been treated as the accounting subsidiary (legal parent) in these financial statements. As Adastra was deemed to be the acquirer for accounting purposes, its assets, liabilities and operations since incorporation are included in these financial statements at their historical carrying values. Arrowstar's results of operations have been included from
December 20, 2019.
| | | December 20, | |
| | | 2019 | |
| Net assets (liabilities) of Arrowstar acquired: | | $ | |
| Cash | | 111,496 | |
| Receivables | | 10,802 | |
| Other assets | | 7,504 | |
| Accounts payable | | (356,384 | ) |
| Net liabilities acquired | | (226,582 | ) |
| | | | |
| Consideration paid in RTO of Arrowstar: | | $ | |
| Common shares (fair value 21,656,034 common shares at $0.05 per share) | | 1,082,802 | |
| Total consideration paid | | 1,082,802 | |
| Listing expense | | 1,309,384 | |
The Transaction was measured at the fair value of the shares that Adastra would have had to issue to the shareholders of Arrowstar, to give the shareholders of Arrowstar the same percentage equity interest in the combined entity that results from the reverse acquisition had it taken the legal form of Adastra acquiring Arrowstar.
4. Receivables and prepayments
Receivables and prepayments consist of the following:
| | | December 31, | | | April 30, | |
| | | 2019 | | | 2019 | |
| | | $ | | | $ | |
| Sales tax recoverable | | 303,708 | | | 28,283 | |
| Prepaid expenses | | 157,459 | | | 8,682 | |
| | | 461,167 | | | 36,965 | |
5. Marketable securities
On November 8, 2011, the Company received 10,000 shares in Dunnedin Ventures Inc. as part of a property option agreement. The shares were valued at $1,500 at the RTO date and included in other assets. December 31, 2019 their fair market value remains $1,500.
6. Property and equipment
| | | | | | | | | Furniture and | | | Computer | | | Laboratory | | | Extraction | | | Building | | | | |
| | | Land | | | Building | | | equipment | | | software | | | equipment | | | equipment | | | improvements | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
| Cost | | | | | | | | | | | | | | | | | | | | | | | | |
| June 18, 2018 (incorporation) | | - | | | - | | | - | | | - | | | - | | | - | | | - | | | - | |
| Additions | | 1,592,232 | | | 1,982,512 | | | 11,823 | | | - | | | - | | | - | | | 299,643 | | | 3,886,210 | |
| April 30, 2019 | | 1,592,232 | | | 1,982,512 | | | 11,823 | | | - | | | - | | | - | | | 299,643 | | | 3,886,210 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | | | | |
| June 18, 2018 (incorporation) | | - | | | - | | | - | | | - | | | - | | | - | | | - | | | - | |
| Depreciation | | - | | | 49,563 | | | 2,411 | | | - | | | - | | | - | | | - | | | 51,974 | |
| April 30, 2019 | | - | | | 49,563 | | | 2,411 | | | - | | | - | | | - | | | - | | | 51,974 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost | | | | | | | | | | | | | | | | | | | | | | | | |
| April 30, 2019 | | 1,592,232 | | | 1,982,512 | | | 11,823 | | | - | | | - | | | - | | | 299,643 | | | 3,886,210 | |
| Additions | | - | | | 16,816 | | | 47,619 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,564,116 | | | 4,751,453 | |
| December 31, 2019 | | 1,592,232 | | | 1,999,328 | | | 59,442 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,863,759 | | | 8,637,663 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | | | | | | | | | | | | | | | | |
| April 30, 2019 | | - | | | 49,563 | | | 2,411 | | | - | | | - | | | - | | | - | | | 51,974 | |
| Depreciation | | - | | | 73,116 | | | 5,056 | | | - | | | - | | | - | | | - | | | 78,172 | |
| December 31, 2019 | | - | | | 122,679 | | | 7,467 | | | - | | | - | | | - | | | - | | | 130,146 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net book value | | | | | | | | | | | | | | | | | | | | | | | | |
| April 30, 2019 | | 1,592,232 | | | 1,932,949 | | | 9,412 | | | - | | | - | | | - | | | 299,643 | | | 3,834,236 | |
| December 31, 2019 | | 1,592,232 | | | 1,876,649 | | | 51,975 | | | 12,105 | | | 62,295 | | | 1,048,502 | | | 3,863,759 | | | 8,507,517 | |
Some of the Company's property, plant and equipment was not yet in use as at December 31, 2019 and April 30, 2019. Depreciation is taken when items are in the location and condition necessary for it to be capable of operating in a manner intended by management.
As at December 31, 2019, the Company had paid cash deposits for additional cannabis extraction equipment in the amount of $Nil (April 30, 2019 - $20,823). Other deposits totaled $4,000 (April 30, 2019 - $nil) at the RTO date and were included in deposits.
7. Share capital
Transactions for the issue of share capital during the period ended December 31, 2019:
(i) On December 20, 2019, the Company closed 5,892,456 unit offering at a price of $0.45 per unit for gross proceeds of $2,651,605, with each unit consisting of one (1) common share of the Company and one underlying share purchase warrant exercisable at $0.60 for three years from the date of closing December 20, 2022. The warrants were given a value of $nil using the residual value method.
Transactions for the issue of share capital during the period ended April 30, 2019:
(i) On June 20, 2018, the Company issued 1 common share for $1 on incorporation, the Company cancelled the incorporation share on February 7, 2019;
(ii) On February 7, 2019, the Company closed a private placement for the issuance of 26,000,000 common shares at a price of $0.005 per share for gross proceeds of $130,000;
(iii) On February 28, 2019, the Company closed a private placement for the issuance of 21,750,000 common shares at a price of $0.02 per share for gross proceeds of $435,000;
(iv) On March 7, 2019, the Company closed a private placement for the issuance of 21,053,333 common shares at a price of $0.075 per share for gross proceeds of $1,579,000;
(v) On March 31, 2019, the Company closed a private placement for the issuance of 8,203,333 common shares at a price of $0.15 per share for gross proceeds of $1,230,500; and
(vi) On April 30, 2019, the Company closed a private placement for the issuance of 4,131,667 common shares at a price of $0.30 per share for gross proceeds of $1,239,500.
Escrowed shares
The Company entered into an Escrow Agreement in connection with closing the RTO on December 20, 2019, in relation to certain of its common shares which were placed in escrow. Pursuant to the Escrow Agreement the escrowed common shares are subject to a timed release schedule whereby a 10% portion of the escrow shares will be released beginning on listing date, and 15% every six months thereafter until January 6, 2023.
As at December 31, 2019, 26,000,000 common shares were held in escrow (2019 - nil).
7. Share capital (continued)
Stock options
The Company has an incentive stock option plan (the "Plan") which provides for the granting of options. Under the Plan the maximum number of stock options issued cannot exceed 10% of the Company's currently issued and outstanding common shares. Options granted under the Plan may have a maximum term of ten years. A participant, who is not a consultant conducting investor relations activities, who is granted an option that is exercisable at the market price at the date of grant, will have their options vest immediately, unless otherwise determined by the Board of Directors. Options granted at below market prices will vest one-sixth every three months.
A participant who is a consultant conducting investor relations activities who is granted an option under the Plan will become vested with the right to exercise one-quarter of the option upon conclusion of every three months subsequent to the grant date. All options are to be settled by physical delivery of shares.
During the periods ended December 31, 2019 and April 30, 2019, there were no options granted or outstanding
Warrants
As an incentive to complete a private placement the Company may issue units which include common shares and common share purchase warrants. Using the residual value method, the Company determines whether a value should be allocated to the warrants attached to private placement units. Finders' warrants may be issued as a private placement share issue cost and are valued using the Black-Scholes option pricing model.
A summary of the status of the Company's warrants as at December 31, 2019 and April 30, 2019 and changes during the years then ended is as follows:
| | | Period ended | | | Period ended | |
| | | December 31, 2019 | | | April 30, 2019 | |
| | | | | | Weighted | | | | | | Weighted | |
| | | | | | average | | | | | | average | |
| | | Warrants | | | exercise price | | | Warrants | | | exercise price | |
| | | # | | | $ | | | # | | | $ | |
| Warrants outstanding, beginning of year | | - | | | - | | | - | | | - | |
| Replaced on RTO | | 1,519,200 | | | 0.92 | | | - | | | - | |
| Issued - attached to units | | 5,892,455 | | | 0.60 | | | - | | | - | |
| Warrants outstanding, end of year | | 7,411,655 | | | 0.73 | | | - | | | - | |
As at December 31, 2019, the Company had warrants outstanding and exercisable as follows:
| Warrants | Warrants | Exercise | | |
| outstanding | exercisable | Price | Expiry date | Weighted average |
| # | # | $ | | remaining life (years) |
| 496,100 | 496,100 | 1.00 | February 20, 2020 | 0.14 |
| 1,023,100 | 1,023,100 | 1.50 | April 26, 2020 | 0.32 |
| 5,892,455 | 5,892,455 | 0.60 | December 2, 2022 | 2.97 |
| 7,411,655 | 7,411,655 | | | 2.41 |
8. Loss per share
The calculation of basic loss per share for the period ended December 31, 2019 was based on the loss attributable to common shareholders of $2,657,957 (April 30, 2019 - $903,989), and a weighted average number of common shares outstanding of 103,082,977 (April 30, 2019 - 13,265,014).
All warrants outstanding as at December 31, 2019 and April 30, 2019, were excluded from the diluted weighted average number of common shares calculation as their effect would have been anti-dilutive.
9. Related party payables and transactions
A number of key management personnel and Directors hold positions in other entities that result in them having control or significant influence over the financial or operating policies of these entities. There were no loans to key management personnel or Directors, or entities over which they have control or significant influence during the periods ended December 31, 2019 or April 30, 2019.
The following related parties transacted with the Company or Company controlled entities during the years:
(a) Andrew Hale is a Director and the Company's President and CEO.
(b) Blaine Bailey is a Director and Chairman of the Company's Audit Committee. He controls Promaid Services Ltd. ("Promaid.") which provides the Company with financial services.
(c) Stephen Brohman is the Company's CFO. He is a principal of Donaldson Brohman Martin CPA Inc. ("DBM CPA") a firm in which he has significant influence. DBM CPA provides the Company with accounting and tax services.
(d) George Routhier is a Company Director. He is the owner of Pipedreemz Inc. ("Pipedreemz"), which provides advisory services to the Company.
The aggregate value of transactions and outstanding balances with key management personnel and Directors and entities over which they have control or significant influence were as follows:
| | | Transactions | | | Transactions | | | Balances | | | Balances | |
| | | period ended | | | period ended | | | outstanding | | | outstanding | |
| | | December 31, | | | April 30, | | | December 31, | | | April 30, | |
| | | 2019 | | | 2019 | | | 2019 | | | 2019 | |
| | | $ | | | $ | | | $ | | | $ | |
| DBM CPA | | 107,298 | | | 35,906 | | | - | | | 9,236 | |
| Andrew Hale | | 120,115 | | | 182,224 | | | - | | | - | |
| Pipedreemz | | 17,500 | | | - | | | - | | | - | |
| | | 244,913 | | | 218,130 | | | - | | | 9,236 | |
All related party balances are unsecured and are due within thirty days without interest.
The transactions with the key management personnel and Directors are included in operating expenses as follows:
(a) Consulting fees
- Includes the advisory services of Director, George Routhier, charged to the Company by Pipedreemz.
(b) Professional fees
- Includes the accounting and tax services of the Company's CFO, Stephen Brohman, charged to the Company by DBM CPA.
(g) Wages and salaries
- Includes charges by Andrew Hale for salaries paid to Officer.
10. Income taxes
A reconciliation of income taxes at statutory rates with the reported taxes for the periods ended December 31, 2019 and April 30, 2019, is as follows:
| | | December 31, 2019 | | | April 30, 2019 | |
| | | $ | | | $ | |
| Loss for the period | | (2,657,957 | ) | | (903,989 | ) |
| Expected income tax (recovery) | | (718,000 | ) | | (244,000 | ) |
| Other | | 80,000 | | | 3,000 | |
| Permanent differences | | 353,000 | | | - | |
| Change in recognized deductible temporary differences | | 285,000 | | | 241,000 | |
| Total income tax expense (recovery) | | - | | | - | |
The Company's unused temporary differences, and unused tax losses that have not been included on the statements of financial position as at December 31, 2019 and April 30, 2019 are as follows:
| | | December 31, 2019 | | | Expiry Date | | | April 30, 2019 | | | Expiry Date | |
| | | $ | | | Range | | | $ | | | Range | |
| Property, plant and equipment | | 137,000 | | | N/A | | | 52,000 | | | N/A | |
| Non-capital loss carry forwards | | 1,814,000 | | | 2039 to 2039 | | | 840,000 | | | 2038 | |
Tax attributes are subject to review, and potential adjustment, but tax authorities.
11. Supplemental cash flow information
Changes in non-cash operating working capital during the periods ended December 31, 2019 and April 31, 2019 were comprised of the following:
| | | December 31, | | | April 30, | |
| | | 2019 | | | 2019 | |
| | | $ | | | $ | |
| Receivables and prepayments | | (411,396 | ) | | (36,965 | ) |
| Accounts payable and accrued liabilities | | 14,903 | | | 137,044 | |
| Net change | | (396,493 | ) | | 100,079 | |
The Company incurred non-cash financing and investing activities during the periods ended December 31, 2019 and April 30, 2019 as follows:
| | | December 31, | | | April 30, | |
| | | 2019 | | | 2019 | |
| | | $ | | | $ | |
| Non-cash financing activities | | | | | | |
| Debenture reserves | | 298,461 | | | - | |
| | | 298,461 | | | - | |
| | | | | | | |
| Non-cash investing activities | | | | | | |
| Equipment purchases included in accounts payable | | 385,894 | | | - | |
| | | 385,894 | | | - | |
During the period ended December 31, 2019 amounts paid for interest was $134,530 (April 30, 2019 - $63,165). No amounts were paid for income tax expenses.
12. Mortgage payable
On January 7, 2019, the Company entered a mortgage for $2,446,000 which bears interest at the rate of 8.25% per annum, calculated monthly. The mortgage matures on February 1, 2020 and can be repaid before maturity without penalty and is secured by the mortgage property and building improvements.
The mortgage payable is recorded at amortized cost and bears an effective interest rate of 10.44% as at December 31, 2019 and April 30, 2019.
The carrying value of the mortgage payable at December 31, 2019 and April 30, 2019 was $2,437,175 and $2,407,463. Included in mortgage payable on initial recognition were the related mortgage transaction costs of $50,755 which are amortized over the term of the mortgage using the effective interest rate method.
The Company maintains minimum interest only payments of $16,816 per month. As at December 31, 2019 the total non-discounted remaining scheduled payments related to the mortgage including interest payments, total $2,479,633. Total interest expense during the period ended December 31, 2019 was $164,242 (April 30, 2019 - $63,125).
On February 1, 2020, the Company renewed the mortgage of $2,446,000 which bears interest at the rate of 8.00% per annum, calculated monthly. The mortgage matures on February 1, 2021 and can be repaid before maturity without penalty and is secured by the mortgage property and building improvements.
13. Convertible debenture
On October 31, 2019, the Company closed a 7,060,000 convertible debenture unit offering at a price of $0.45 per debenture unit for gross proceeds $3,177,000. Each debenture consists of a 12% secured convertible debenture with a maturity of two years from the date of issuance. If the holder converts their debenture unit they are entitled to one common share and one share purchase warrant exercisable at $0.75 per share exercisable two years from the date of the convertible debenture closing October 31, 2021 at the holders discretion.
If the closing price of the Common Shares of the Company is higher than $1.00 for any 10 consecutive trading days the expiry date of the Warrants may be accelerated to the 30th day after the date of a news release announcing such acceleration. The debentures will be secured to the facility subordinate to the mortgage currently on the facility.
13. Convertible debenture (continued)
As the debentures are convertible into common shares, the liability and equity components are presented separately on the consolidated statement of financial position. The initial carrying amount of the financial liability was determined by discounting the stream of future payments of interest and principal at a market interest rate of 16% totaling $2,878,539. Using the residual method, the carrying amount of the conversion feature is the difference between the principal amount and the initial carrying value of the financial liability. The equity component is recorded in debenture reserves on the consolidated statement of financial position totaling $298,461. The debentures, net of the equity component are accreted using the effective interest method over the term of the debentures, such that the carrying amount of the financial liability will equal the principal balance at maturity.
The Company recorded interest expense of $63,713 and accretion expense of $13,259 related to the convertible debenture during the period ended December 31, 2019 (April 30, 2019 - $nil).
14. Financial risk management
Capital management
The Company defines capital as the components of shareholders' equity. The Company's objectives when managing capital are to support further advancement of the Company's business objectives, as well as to ensure that the Company is able to meet its financial obligations as they come due.
The Company manages its capital structure to maximize its financial flexibility making adjustments to it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. The
Company relies on the expertise of the Company's management to sustain the future development of the business.
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There were no changes in the Company's approach to capital management during the period ended December 31, 2019. The Company is not subject to externally imposed capital requirements.
Financial instruments - fair value
The Company's financial instruments consist of cash, receivables, marketable securities, deposits, accounts payable and accrued liabilities, mortgage payable and convertible debenture.
The carrying value of receivables, accounts payable and accrued liabilities, and accounts payable approximated their fair value because of the short-term nature of these instruments. Mortgage payable and convertible debenture values approximate fair value due to their market rates of interest charged.
Financial instruments measured at fair value on the consolidated statements of financial position are summarized into the following fair value hierarchy levels:
Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices).
Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
| | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | $ | | | $ | | | $ | | | $ | |
| December 31, 2019 | | | | | | | | | | | | |
| Cash | | 2,376,826 | | | - | | | - | | | 2,376,826 | |
| Marketable securities | | 1,500 | | | - | | | - | | | 1,500 | |
| Deposits | | 4,000 | | | - | | | - | | | 4,000 | |
| | | | | | | | | | | | | |
| April 30, 2019 | | | | | | | | | | | | |
| Cash and cash equivalents | | 2,362,494 | | | - | | | - | | | 2,362,494 | |
| Deposits | | 20,823 | | | - | | | - | | | 20,823 | |
14. Financial risk management (continued)
Financial instruments - risk
The Company's financial instruments are exposed to certain financial risks, including credit risk, interest rate risk, market risk, liquidity risk and currency risk.
(a) Credit risk
The Company is exposed to credit risk by holding cash. This risk is minimized by holding the funds in Canadian banks and credit unions or with Canadian governments. The Company has minimal receivable exposure, and its various refundable credits are due from Canadian governments.
(b) Market risk
The Company is exposed to market risk because of the fluctuating values of its publicly traded marketable securities. The Company has no control over these fluctuations and does not hedge its investments. Based on the December 31, 2019 value of marketable securities every 10% increase or decrease in the share prices of these companies would have impacted loss for the year, up or down, by approximately $70 (April 30, 2019 - $nil) before income taxes.
(c) Liquidity risk
Liquidity risk is the risk that the Company is unable to meet its financial obligations as they come due. The Company manages this risk by careful management of its working capital to ensure its expenditures will not exceed available resources.
(d) Currency risk
The Company is not exposed to currency risk.
1204581 B.C. Ltd.
Condensed Interim Financial Statements
Nine months ended June 30, 2021 and 2020
(Expressed in Canadian Dollars - Unaudited)
1204581 B.C. Ltd.
Condensed Interim Statements of Financial Position
(Expressed in Canadian Dollars - Unaudited)
| | | | June 30, 2021 | | | September 30, 2020 | |
| | Note | | $ | | | $ | |
| Assets | | | | | | | |
| Current assets | | | | | | | |
| Cash | | | 31,204 | | | 26,100 | |
| Receivables and prepayments | 3,13 | | 676,046 | | | 127,062 | |
| | | | 707,250 | | | 153,162 | |
| Non-current assets | | | | | | | |
| Property and equipment | 4 | | 90,561 | | | 5,250 | |
| Intangible assets | 5 | | 34,572 | | | 23,180 | |
| Total assets | | | 832,383 | | | 181,592 | |
| | | | | | | | |
| Liabilities and shareholders' equity | | | | | | | |
| Current liabilities | | | | | | | |
| Accounts payable and accrued liabilities | | | 400,776 | | | 35,171 | |
| Current portion of lease liability | 6 | | 10,169 | | | - | |
| | | | 410,945 | | | 35,171 | |
| Non-current liabilities | | | | | | | |
| Due to shareholders | 8 | | 108,753 | | | 142,599 | |
| Lease liability | 6 | | 26,944 | | | - | |
| Total liabilities | | | 546,642 | | | 177,770 | |
| | | | | | | | |
| Shareholders' equity | | | | | | | |
| Share capital | 7 | | 1 | | | 1 | |
| Retained earnings | | | 285,740 | | | 3,821 | |
| Total shareholders' equity | | | 285,741 | | | 3,822 | |
| Total liabilities and shareholders' equity | | | 832,383 | | | 181,592 | |
Nature of operations and going concern (Note 1)
Subsequent events (Note 14)
Approved on behalf of the Board of Directors on November 26, 2021:
"Michael Forbes" | | "Donald Dinsmore" |
Director | | Director |
| | |
The accompanying notes are an integral part of these condensed interim financial statements
1204581 B.C. Ltd.
Condensed Interim Statements of Income (Loss) and Comprehensive Income (Loss)
(Expressed in Canadian Dollars, except number of shares - Unaudited)
| | | | Nine months ended June 30, | |
| | | | 2021 | | | 2020 | |
| | Note | | $ | | | $ | |
| Revenue | | | | | | | |
| Licensing revenue | 13 | | 1,030,893 | | | 39,537 | |
| | | | | | | | |
| Expenses | | | | | | | |
| Advertising and promotion | | | 279,020 | | | 62,168 | |
| Professional fees and consulting | | | 224,862 | | | 43,175 | |
| Office expenses | | | 175,691 | | | - | |
| Travel | | | 19,291 | | | - | |
| Depreciation | 4 | | 12,770 | | | - | |
| Rent | | | 7,544 | | | - | |
| Automobile expense | | | 7,100 | | | - | |
| Meals and entertainment | | | 6,987 | | | 18 | |
| Insurance | | | 4,673 | | | - | |
| Interest expense | 6 | | 3,078 | | | - | |
| Commission expense | | | 2,000 | | | - | |
| Amortization | 5 | | 2,924 | | | 1,372 | |
| Bank charges | | | 371 | | | 339 | |
| | | | 746,311 | | | 107,072 | |
| Income (loss) from operations | | | 284,582 | | | (67,535 | ) |
| | | | | | | | |
| Other expense | | | | | | | |
| Foreign exchange | | | 2,663 | | | - | |
| | | | | | | | |
| Net income (loss) and comprehensive income (loss) for the period | | | 281,919 | | | (67,535 | ) |
| | | | | | | | |
| Earnings (loss) per share - basic and diluted | | | 3.35 | | | (67.54 | ) |
| | | | | | | | |
| Weighted average number of common shares outstanding - basic and diluted | | | 84,044 | | | 1,000 | |
The accompanying notes are an integral part of these condensed interim financial statements
1204581 B.C. Ltd.
Condensed Interim Statements of Cash Flows
(Expressed in Canadian Dollars - Unaudited)
| | | Nine months ended June 30, | |
| | | 2021 | | | 2020 | |
| | | $ | | | $ | |
| | | | | | | |
| Operating activities | | | | | | |
| Income (loss) for the period | | 281,919 | | | (67,535 | ) |
| | | | | | | |
| Items not affecting cash: | | | | | | |
| Depreciation | | 12,770 | | | - | |
| Amortization | | 2,924 | | | 1,372 | |
| Interest expense | | 3,078 | | | - | |
| | | | | | | |
| Change in non-cash working capital items: | | | | | | |
| Receivables and prepayments | | (548,984 | ) | | (1,277 | ) |
| Accounts payable and accrued liabilities | | 365,605 | | | (7,090 | ) |
| Cash provided by (used in) operating activities | | 117,312 | | | (74,530 | ) |
| | | | | | | |
| Investing activities | | | | | | |
| Purchases of intangible assets | | (14,316 | ) | | (8,020 | ) |
| Purchases of property and equipment | | (44,246 | ) | | - | |
| Cash used in investing activities | | (58,562 | ) | | (8,020 | ) |
| | | | | | | |
| Financing activities | | | | | | |
| Advances to (from) shareholders | | (33,846 | ) | | 89,506 | |
| Payment of interest on lease liability | | (3,078 | ) | | - | |
| Payment of principal on lease liability | | (16,722 | ) | | - | |
| Cash (used in) provided by financing activities | | (53,646 | ) | | 89,506 | |
| | | | | | | |
| Change in cash | | 5,104 | | | 6,956 | |
| Cash, beginning of period | | 26,100 | | | - | |
| Cash, end of period | | 31,204 | | | 6,956 | |
Refer to Note 10 for supplemental cash flow information.
The accompanying notes are an integral part of these condensed interim financial statements
1204581 B.C. Ltd.
Condensed Interim Statements of Changes in Shareholders' Equity
For the nine months ended June 2021 and 2020
(Expressed in Canadian Dollars, except number of shares - Unaudited)
| | | | | | | | | Retained earnings | | | Total Shareholders' | |
| | | Common shares | | | Share capital | | | (deficit) | | | equity (deficiency) | |
| | | # | | | $ | | | $ | | | $ | |
| Balance, September 30, 2019 | | 1,000 | | | 1 | | | (7,319 | ) | | (7,318 | ) |
| Net loss for the period | | - | | | - | | | (67,535 | ) | | (67,535 | ) |
| Balance, June 30, 2020 | | 1,000 | | | 1 | | | (74,854 | ) | | (74,853 | ) |
| Net income for the period | | - | | | - | | | 78,675 | | | 78,675 | |
| Balance, September 30, 2020 | | 1,000 | | | 1 | | | 3,821 | | | 3,822 | |
| Shares issued for private placement | | 99,000 | | | - | | | - | | | - | |
| Net income for the period | | - | | | - | | | 281,919 | | | 281,919 | |
| Balance, June 30, 2021 | | 100,000 | | | 1 | | | 285,740 | | | 285,741 | |
The accompanying notes are an integral part of these condensed interim financial statements
1. NATURE OF OPERATIONS AND GOING CONCERN
1204581 B.C. Ltd. (the "Company") was incorporated under the laws of the province of British Columbia on April 9, 2019. The Company's records office is located at 704 - 595 Howe Street, Vancouver, British Columbia V6C 2T5.
The Company has developed an intellectual property ("IP") portfolio, which includes several issued trademarks (Note 5). The Company commercializes its intellectual property portfolio through IP licensing.
In March 2020, the World Health Organization declared coronavirus COVID-19 a global pandemic. This contagious disease outbreak, which has continued to spread, and any related adverse public health developments, has adversely affected workforces, economies, and financial markets globally, potentially leading to an economic downturn. It is not possible for the Company to predict the duration or magnitude of the adverse results of the outbreak and its effects on the Company's business or results of operations. There are travel restrictions and health and safety concerns that may delay the Company's operational objectives. Operations depend on safeguarding all personnel during the outbreak, which may be prohibitive in certain aspects.
These condensed interim financial statements (the "financial statements") are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. These financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities that might be necessary should the Company be unable to continue in existence.
As at June 30, 2021, the Company had working capital of $296,305 (September 30, 2020 - $117,991) and has been generating positive cash flows from operations. Although the Company has been successful in raising funds through shareholder loans to date, there can be no assurance that adequate or sufficient funding will be available in the future or available under terms acceptable to the Company, or that the Company will generate sufficient revenue and positive cash flows from operations. The continuance of operations is dependent on the Company's continued licensing revenue, generating profitable and cash flow positive commercial operations, and continuing to ob tain financing on acceptable terms. These conditions may cast significant doubt about the Company's ability to continue as a going concern.
2. SIGNIFICANT ACCOUNTING POLICIES
The accounting policies applied in the preparation of these financial statements are consistent with those applied and disclosed in note 2 to the annual audited financial statements for the year ended September 30, 2020. It is suggested that these financial statements be read in conjunction with the annual audited financial statements.
Basis of presentation
These financial statements have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards Board ("IASB") and interpretations of the International Financial Reporting Interpretations Committee ("IFRIC").
These financial statements have been prepared on an historical cost basis, except for financial instruments measured at fair value. In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information. All amounts in these financial statements are presented in Canadian dollars, which is the functional currency of the Company.
Standards issued but not yet effective
Certain pronouncements have been issued by the IASB or IFRIC that are effective for accounting periods beginning on or after January 1, 2021. The Company has reviewed these updates and determined that many of these updates are not applicable or consequential to the Company and have been excluded from discussion within these significant accounting policies.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
Significant accounting judgments, estimates and assumptions
The preparation of financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at each reporting date and the reported amounts of income and expenses during each reporting period. Estimates and assumptions are continuously evaluated and are based on management's experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
The information about significant areas of estimation uncertainty considered by management in preparing these financial statements is as follows:
Useful lives of property and equipment and intangible assets
The estimated useful lives of property and equipment and intangible assets are reviewed by management and adjusted if necessary. To estimate their useful life, management must use its past expe rience with the same or similar assets, review industry practices for similar pieces of equipment and/or apply statistical methods to assist in its determination of useful life.
Collectability of financial assets
The estimate of the collectability of financial assets is subject to estimation as to the credit -risk and likelihood of default by the counterparty. Refer to the disclosure under "Credit risk" within Note 9 for details.
Income taxes
Tax provisions are based on enacted or substantively enacted laws. Changes in those laws could affect amounts recognized in profit or loss both in the period of change, which would include any impact on cumulative provisions, and in future periods. Deferred tax assets (if any) are recognized only to the extent it is considered probable that those assets will be recoverable. This involves an assessment of when those deferred tax assets are likely to reverse and a judgment as to whether there will be sufficient taxable profits available to offset the tax assets when they do reverse. This requires assumptions regarding future profitability and is therefore inherently uncertain. To the extent assumptions regarding future profitability change, there can be an increase or decrease in the amounts recognized in respect of deferred tax assets as well as the amounts recognized in profit or loss in the period in which the change occurs.
Going concern
The assessment of the Company's ability to continue as a going concern as discussed in Note 1 involves judgment regarding future funding available for its operations and working capital requirements.
Commencement of depreciation and amortization of property and equipment and intangible assets
Judgment is used to determine the commencement date of depreciation/amortization of an item of property and equipment or intangible assets. The commencement date is when an item is available for use in the location and condition as intended by management.
3. RECEIVABLES AND PREPAYMENTS
Receivables and prepayments comprise the following:
| | | June 30, 2021 | | | September 30, 2020 | |
| | | $ | | | $ | |
| Trade receivables | | 663,046 | | | 118,062 | |
| Prepaid expenses | | 13,000 | | | 9,000 | |
| | | 676,046 | | | 127,062 | |
4. PROPERTY AND EQUIPMENT
| | | Furniture and | | | | | | | |
| | | equipment | | | Vehicles | | | Total | |
| | | $ | | | $ | | | $ | |
| | | | | | | | | | |
| Cost | | | | | | | | | |
| September 30, 2019 | | - | | | - | | | - | |
| Additions | | 5,833 | | | - | | | 5,833 | |
| September 30, 2020 | | 5,833 | | | - | | | 5,833 | |
| Additions | | 44,246 | | | 53,835 | | | 98,081 | |
| June 30, 2021 | | 50,079 | | | 53,835 | | | 103,914 | |
| | | | | | | | | | |
| Accumulated depreciation | | | | | | | | | |
| September 30, 2019 | | - | | | - | | | - | |
| Depreciation | | 583 | | | - | | | 583 | |
| September 30, 2020 | | 583 | | | - | | | 583 | |
| Depreciation | | 2,676 | | | 10,094 | | | 12,770 | |
| June 30, 2021 | | 3,259 | | | 10,094 | | | 13,353 | |
| | | | | | | | | | |
| Net carrying value | | | | | | | | | |
| September 30, 2020 | | 5,250 | | | - | | | 5,250 | |
| June 30, 2021 | | 46,820 | | | 43,741 | | | 90,561 | |
The right of use asset associated with the lease liability (Note 6) is included above within vehicles.
5. INTANGIBLE ASSETS
| | | Registered | | | | |
| | | trademarks | | | Total | |
| | | $ | | | $ | |
| Cost | | | | | | |
| September 30, 2019 | | 10,276 | | | 10,276 | |
| Additions | | 15,206 | | | 15,206 | |
| September 30, 2020 | | 25,482 | | | 25,482 | |
| Additions | | 14,316 | | | 14,316 | |
| June 30, 2021 | | 39,798 | | | 39,798 | |
| | | | | | | |
| Accumulated amortization | | | | | | |
| September 30, 2019 | | 514 | | | 514 | |
| Amortization | | 1,788 | | | 1,788 | |
| September 30, 2020 | | 2,302 | | | 2,302 | |
| Amortization | | 2,924 | | | 2,924 | |
| June 30, 2021 | | 5,226 | | | 5,226 | |
| | | | | | | |
| Net carrying value | | | | | | |
| September 30, 2020 | | 23,180 | | | 23,180 | |
| June 30, 2021 | | 34,572 | | | 34,572 | |
Trademarks
As at June 30, 2021, the Company has 21 registered trademarks with the Canadian Intellectual Property Office ("CIPO"). These trademarks have finite lives and are measured at cost less accumulated amortization and impairment losses. These intangible assets are amortized on a straight-line basis over their estimated useful lives. Useful lives, residual values, and amortization methods for intangible assets with finite useful lives are reviewed at least annually.
6. LEASE LIABILITY
On October 15, 2020, the Company entered into a four-year lease agreement for a promotional vehicle. The base monthly payment is $1,118.55 with an initial payment of $9,732.26. The incremental borrowing rate used to determine discount the lease liability was 10%.
| | | June 30, 2021 | | | September 30, 2020 | |
| | | $ | | | $ | |
| | | | | | | |
| Opening balance | | - | | | - | |
| Additions | | 53,835 | | | - | |
| Repayments | | (19,800 | ) | | - | |
| Finance costs | | 3,078 | | | - | |
| Ending balance | | 37,113 | | | - | |
| Less: current portion | | (10,169 | ) | | - | |
| Long-term portion | | 26,944 | | | - | |
7. SHAREHOLDERS' EQUITY
The authorized share capital of the Company consists of unlimited common shares without par value. All issued shares are fully paid.
Issued and Outstanding
Nine months ended June 30, 2021
On November 13, 2020, the Company issued 99 common shares valued at $0.001 per share for gross proceeds of $0.10.
On March 16, 2021 the Company underwent a share split at 1,000 to one, increasing the number of common shares from 100 to 100,000. All information with respect to the number of common shares and issuance prices for the periods prior to the share split have been restated to reflect the share split.
Year end September 30, 2020
No transactions for the issue of share capital during the year ended September 30, 2020.
8. RELATED PARTY TRANSACTIONS
Key management personnel include those persons having authority and responsibility for planning, directing and controlling the activities of the Company as a whole. The Company has determined that key management personnel consist of executive and non-executive members of the Company's Board of Directors and Corporate Officers, significant shareholders and companies controlled by them.
Key management personnel compensation
There was no remuneration of key management for the nine months ended June 30, 2021 or June 30, 2020.
Related party balances
Related party balances as at June 30, 2021 and September 30, 2020 are as follows:
| | | June 30, 2021 | | | September 30, 2020 | |
| | | $ | | | $ | |
| | | | | | | |
| Due to shareholder | | 108,753 | | | 142,599 | |
During the nine months ended June 30, 2021, the Company received shareholder advances. These advances are unsecured, non-interest bearing and due on demand.
9. FINANCIAL INSTRUMENTS
Financial instrument - fair value
Financial instruments measured at fair value are classified into one of three levels in the fair value hierarchy according to the relative reliability of the inputs used to estimate the fair values. The three levels of the fair value hierarchy are:
Level 1 - Quoted prices (unadjusted) in active markets for identical assets or liabilities.
Level 2 - Inputs other than quoted prices that are observable for the assets or liability either directly or indirectly.
Level 3 - Inputs for the asset or liability that are not based on observable market data (unobservable inputs).
9. FINANCIAL INSTRUMENTS (continued)
Classification of financial instruments
Financial assets | Initial Recognition | Subsequent measurement |
Cash | Fair value | Amortized cost |
Receivables | Fair value | Amortized cost |
| | |
Financial liabilities | Initial Recognition: | Subsequent measurement |
Accounts payable and accrued liabilities | Fair value | Amortized cost |
Lease liability | Fair value | Amortized cost |
The Company's financial instruments other than cash, approximate their fair values. Cash, under the fair value hierarchy is based on Level 1 quoted prices in active markets for identical assets or liabilities.
Impairment of financial assets
The Company has trade receivables that are subject to the expected credit loss model.
While cash is also subject to the impairment requirements of IFRS 9, the risk is insignificant.
The Company applies the IFRS 9 simplified approach to measure expected credit losses which uses a lifetime expected loss allowance for its trade receivables. The Company applies the general approach using practical expedients to loans receivable which involves recognition at each reporting date of a loss allowance based on a 12-month expected credit loss model without the requirement to re-assess whether any significant increases in credit risk have occurred at each reporting date.
To measure the expected credit losses, trade receivables are grouped by debtor, and each debtor's circumstances are reviewed. The expected loss amount, if any, is based on historical payment profiles, and the corresponding historical credit losses experienced within this period for the debtors.
Amounts are written-off when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, failure of a debtor to engage in a repayment plan with the Company (if applicable), and failure by the debtor to make contractual payments for a period of greater than 120 days past due, or shorter if specific circumstances suggest otherwise.
As at June 30, 2021, the loss allowance was $nil for trade receivables. There has been no historical loss allowance recorded on these items.
Impairment losses are presented as loss provisions within profit or loss. Subsequent recoveries of amounts previously written-off are credited against the same line item.
Financial instruments - risk
Credit risk
Credit risk is the risk of financial loss to the Company if a counterparty to a financial instrument fails to meet its contractual obligations. As at June 30, 2021, credit risk for the Company arises from cash and trade receivables. The carrying amount of these financial assets represents the maximum credit exposure as at June 30, 2021.
Cash is held with a Canadian chartered bank and accordingly, the Company's exposure to credit risk on cash is considered insignificant. As at June 30, 2021, the Company's trade receivables were due from one customer. The Company's trade receivables are current, and management considers the credit risk to be low.
9. FINANCIAL INSTRUMENTS (continued)
Liquidity risk
The Company manages liquidity risk by maintaining an adequate level of cash to meet its ongoing obligations. The Company has been successful in revenue generation and shareholder loans in the past; however, there is no assurance that it will be able to do so in the future.
As at June 30, 2021, the Company had a cash balance of $31,204 (September 30, 2020 - $26,100) to settle accounts payable and accrued liabilities and the current portion of the lease payable totalling of $ 410,945 (September 30, 2020 - $35,171).
Interest rate risk
The Company is not significantly exposed to interest rate risk because it does not have any assets or liabilities subject to variable rates of interest, except for cash held in interest -bearing accounts which poses an insignificant risk exposure.
Economic dependence
Economic dependence risk is the risk of reliance upon a select number of customers which significantly impact the financial performance of the Company. During the nine months ended June 30, 2021, the Company's licensing revenue was generated from a single customer.
10. SUPPLEMENTAL CASH FLOW INFORMATION
The Company did not incur any non-cash investing and financing activities during the nine months ended June 30, 2021 and 2020.
During the nine months ended June 30, 2021 and 2020, no amounts were paid for income tax expenses.
11. CAPITAL RISK MANAGEMENT
The Company defines capital as the components of shareholders' equity. The Company's objectives when managing capital are to support further advancement of the Company's business objectives, as well as to ensure that the Company can meet its financial obligations as they come due.
The Company manages its capital structure to maximize its financial flexibility, adjusting it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. The Company relies on the expertise of the Company's management to sustain the future development of the business. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
There were no changes in the Company's approach to capital management during the nine months ended June 30, 2021. The Company is not subject to externally imposed capital requirements.
12. SEGMENTED INFORMATION
The Company has a single reportable segment: the provision of intellectual property licensing to the cannabis industry in Canada. All the Company's revenues are generated in Canada, and its non-current assets are located in Canada.
13. LICENSING REVENUE
On March 12, 2020 (subsequently amended December 4, 2020), the Company entered into a Licence and Distribution Royalty Agreement (the "Royalty Agreement") with a private Ontario company (the "Licensee"). Pursuant to the Royalty Agreement, the Company granted the Licensee an exclusive Licence for the use of certain registered and pending trademarks (the "Registered trademarks") in exchange for a 50% royalty on the Licensee's gross profit from the sales of said cannabis products. The Royalty Agreement shall terminate upon the second anniversary of the Butane Hash Oil ("BHO") or Propane Hash Oil ("PHO") concentrate products (Shatter, Wax, Live resin, Crystals) becoming available in a Provincial retail outlet. The term shall automatically renew for an additional one-year term provided that the gross sales revenue is equal to or greater than $4,000,000 by the end of the initial term.
During the nine months ended June 30, 2021, the Company recognized $1,030,893 (2020 - $39,537) of licensing revenue in connection with the Royalty Agreement and as at June 30, 2021, there was $663,046 (September 30, 2020 - $118,062) in trade receivables.
14. SUBSEQUENT EVENTS
On September 15, 2021, all the issued and outstanding shares of the Company were acquired by Adastra Labs Inc., whereby the shareholders of the Company received consideration comprising 20,000,000 common shares of Adastra Holdings Ltd., the parent company of Adastra Labs Inc.
1204581 B.C. Ltd.
Financial Statements
September 30, 2020 and for the period from
incorporation on April 9, 2019 to September 30, 2019
(Expressed in Canadian Dollars)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Directors of
1204581 B.C. Ltd.
Opinion on the Financial Statements
We have audited the accompanying statements of financial position of 1204581 B.C. Ltd. (the "Company"), as of September 30, 2020 and 2019, and the related statements of income (loss) and comprehensive income (loss), cash flows, and changes in shareholders' equity (deficiency) for the year ended September 30, 2020 and for the period from incorpora tion on April 9, 2019 to September 30, 2019, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2020 and 2019, and the results of its operations and its cash flows for the year ended September 30, 2020 and for the period from incorporation on April 9, 2019 to September 30, 2019 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, certain events and circumstances raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1, the financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.

Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company's auditor since 2020.
| | /s/ DAVIDSON & COMPANY LLP |
| | |
Vancouver, Canada | Chartered Professional Accountants |
| | |
| November 2, 2021 | |
1204581 B.C. Ltd. Statements of Financial Position As at September 30, 2020 and September 30, 2019 (Expressed in Canadian Dollars) |
| | | | September 30, 2020 | | | September 30, 2019 | |
| | Note | | $ | | | $ | |
| | | | | | | | |
| Assets | | | | | | | |
| Current assets | | | | | | | |
| Cash | | | 26,100 | | | - | |
| Receivables and prepayments | 3,12 | | 127,062 | | | - | |
| | | | 153,162 | | | - | |
| Non-current assets | | | | | | | |
| Due from shareholder | 6 | | - | | | 1 | |
| Property and equipment | 4 | | 5,250 | | | - | |
| Intangible assets | 5 | | 23,180 | | | 9,762 | |
| Total assets | | | 181,592 | | | 9,763 | |
| | | | | | | | |
| Liabilities and shareholders' equity (deficiency) | | | | | | | |
| Current liabilities | | | | | | | |
| Accounts payable and accrued liabilities | | | 35,171 | | | 17,081 | |
| | | | 35,171 | | | 17,081 | |
| Non-current liabilities | | | | | | | |
| Due to shareholders | 7 | | 142,599 | | | - | |
| Total liabilities | | | 177,770 | | | 17,081 | |
| | | | | | | | |
| Shareholders' equity (deficiency) | | | | | | | |
| Share capital | 6 | | 1 | | | 1 | |
| Retained earnings (deficit) | | | 3,821 | | | (7,319 | ) |
| Total shareholders' equity (deficiency) | | | 3,822 | | | (7,318 | ) |
| Total liabilities and shareholders' equity (deficiency) | | | 181,592 | | | 9,763 | |
| | | | | | | | |
| Nature of operations and going concern | 1 | | | | | | |
| Subsequent events | 14 | | | | | | |
Approved on behalf of the Board of Directors on November 2, 2021:
"Michael Forbes" | Director | "Donald Dinsmore" | Director |
| |
The accompanying notes are an integral part of these financial statements.
1204581 B.C. Ltd. Statements of Income (Loss) and Comprehensive Income (Loss) (Expressed in Canadian Dollars) |
| | | | | | | For the period from | |
| | | | | | | incorporation | |
| | | | | | | on April 9, 2019 to | |
| | | | September 30, 2020 | | | September 30, 2019 | |
| | Note | | $ | | | $ | |
| | | | | | | | |
| Revenue | | | | | | | |
| Licensing revenue | 12 | | 212,654 | | | - | |
| | | | | | | | |
| Expenses | | | | | | | |
| Advertising | | | 104,482 | | | - | |
| Amortization | 5 | | 1,788 | | | 514 | |
| Bank charges | | | 367 | | | - | |
| Delivery and freight | | | 756 | | | - | |
| Depreciation | 4 | | 583 | | | - | |
| Meals and entertainment | | | 10 | | | - | |
| Office expense | | | 7,401 | | | - | |
| Professional fees and consulting | | | 78,300 | | | 6,805 | |
| Supplies | | | 7,447 | | | - | |
| Travel | | | 380 | | | - | |
| Loss from operating expenses | | | (201,514 | ) | | (7,319 | ) |
| | | | | | | | |
| Income (loss) and comprehensive income (loss) for the period | | | 11,140 | | | (7,319 | ) |
| | | | | | | | |
| Loss per share | | | | | | | |
| Weighted average number of common shares outstanding: | | | | | | | |
| Basic # | | | 1,000 | | | 1,000 | |
| Diluted # | | | 1,000 | | | 1,000 | |
| | | | | | | | |
| Basic gain (loss) per share | | | 11.14 | | | (7.32 | ) |
| Diluted gain (loss) per share | | | 11.14 | | | (7.32 | ) |
The accompanying notes are an integral part of these financial statements.
1204581 B.C. Ltd. Statements of Cash Flows (Expressed in Canadian Dollars) |
| | | | | | For the period from | |
| | | | | | incorporation | |
| | | | | | on April 9, 2019 to | |
| | | September 30, 2020 | | | September 30, 2019 | |
| | | $ | | | $ | |
| Operating activities | | | | | | |
| Income (loss) for the period | | 11,140 | | | (7,319 | ) |
| Adjustments for: | | | | | | |
| Amortization | | 1,788 | | | 514 | |
| Depreciation | | 583 | | | - | |
| Non-cash working capital items: | | | | | | |
| Receivables and prepayments | | (127,062 | ) | | - | |
| Due to shareholders | | 91,599 | | | | |
| Accounts payable and accrued liabilities | | 18,090 | | | 17,081 | |
| | | (3,862 | ) | | 10,276 | |
| | | | | | | |
| Investing activities | | | | | | |
| Purchases of property and equipment | | (5,833 | ) | | - | |
| Payments for intangible assets | | (15,206 | ) | | (10,276 | ) |
| | | (21,039 | ) | | (10,276 | ) |
| | | | | | | |
| Financing activities | | | | | | |
| Due to shareholders | | 51,001 | | | (1 | ) |
| Proceeds from share capital | | - | | | 1 | |
| | | | | | | |
| | | 51,001 | | | - | |
| Increase in cash | | 26,100 | | | - | |
| Cash, beginning of period | | - | | | - | |
| Cash, end of period | | 26,100 | | | - | |
Refer to note 9 for supplemental cash flow information.
The accompanying notes are an integral part of these financial statements.
1204581 B.C. Ltd. Statements of Changes in Shareholders' Equity (Deficiency) |
For the periods ended September 30, 2020 and September 30, 2019
(Expressed in Canadian Dollars) |
| | | | | | | | | Retained earnings | | | Total Shareholders' | |
| | | Common shares | | | Share capital | | | (deficit) | | | equity (deficiency) | |
| | | # | | | $ | | | $ | | | $ | |
| Issuance of incorporation share | | 1,000 | | | 1 | | | - | | | 1 | |
| Loss and comprehensive loss for the period | | - | | | - | | | (7,319 | ) | | (7,319 | ) |
| September 30, 2019 | | 1,000 | | | 1 | | | (7,319 | ) | | (7,318 | ) |
| | | | | | | | | | | | | |
| October 1, 2019 | | 1,000 | | | 1 | | | (7,319 | ) | | (7,318 | ) |
| Income and comprehensive income for the year | | - | | | - | | | 11,140 | | | 11,140 | |
| September 30, 2020 | | 1,000 | | | 1 | | | 3,821 | | | 3,822 | |
The accompanying notes are an integral part of these financial statements.
1. NATURE OF OPERATIONS AND GOING CONCERN
1204581 B.C. Ltd. (the "Company") was incorporated under the laws of the province of British Columbia on April 9, 2019. The Company's records office is located at 704 - 595 Howe Street, Vancouver, British Columbia V6C 2T5.
The Company has developed an intellectual property ("IP") portfolio, which includes several issued and pending trademarks (Note 5). The Company commercializes its intellectual property portfolio through IP licensing.
In March 2020, the World Health Organization declared coronavirus COVID-19 a global pandemic. This contagious disease outbreak, which has continued to spread, and any related adverse public health developments, has adversely affected workforces, economies, and financial markets globally, potentially leading to an economic downturn. It is not possible for the Company to predict the duration or magnitude of the adverse results of the outbreak and its effects on the Company's business or results of operations. There are travel restrictions and health and safety concerns that may delay the Company's operational objectives. Operations depend on safeguarding all personnel during the outbreak, which may be prohibitive in certain aspects.
These financial statements (the "financial statements") are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. These financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities that might be necessary should the Company be unable to continue in existence.
As at September 30, 2020, the Company had working capital of $117,991 and has been generating positive cash flows. Although the Company has been successful in raising funds through shareholder loans to date, there can be no assurance that adequate or sufficient funding will be available in the future or available under terms acceptable to the Company, or that the Company will generate sufficient revenue and positive cash flows from operations. The continuance of operations is dependent on the Company's continued licensing revenue, generating profitable and cash flow positive commercial operations, and continuing to obtain financing on acceptable terms. These conditions may cast significant doubt about the Company's ability to continue as a going concern. Subsequent to year end the Company has been fully acquired by Adastra Labs Inc (Note 14).
2. SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
These financial statements have been prepared in accordance with International Financial Reporting Standards
("IFRS"), as issued by the International Accounting Standards Board ("IASB") and interpretations of the International Financial Reporting Interpretations Committee ("IFRIC").
These financial statements have been prepared on an historical cost basis, except for financial instruments measured at fair value. In addition, these financial statements have been prepared using the accrual basis of accounting, except for cash flow information. All amounts in these financial statements are presented in Canadian dollars, which is the functional currency of the Company.
Revenue recognition
Revenue is comprised of intellectual property licensing.
Licensing revenue:
Licensing revenue is a royalty arrangement (Note 12) whereby the Company recognizes revenue from the licensing of its intellectual property for the sale of consumer packaged goods ("CPG") by a third party (the "Licencee"). The Company recognizes revenue as a percentage of the Licencee's gross revenue when the Licencee sells and delivers products to their customers.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
New accounting standards adopted during the year
New standard IFRS 16 - Leases
IFRS 16 - Leases ("IFRS 16") was issued by the IASB on January 13, 2016, replaced IAS 17, Leases. It was effective for annual periods beginning on or after January 1, 2019, with earlier application permitted. IFRS 16 eliminates the current dual accounting model for lessees, which distinguishes between on-balance sheet finance leases and off-balance sheet operating leases. Instead, IFRS 16 requires a single, on-balance sheet accounting model that is similar to current finance lease accounting. Leases become an on-balance sheet liability that attract interest, together with a new asset.
The most significant effect of the new standard is the lessee's recognition of the initial present value of unavoidable future lease payments as right-of-use ("ROU") assets and lease liabilities on the statement of financial position, including those for most leases that would have formerly been accounted for as operating leases.
On adoption, the following practical expedients were permitted by IFRS 16, but were not applicab le to the Company:
- Accounting for leases with a remaining term of less than twelve months as at October 1, 2019, as short-term leases; and
- Accounting for lease payments as an expense for leases of low-value assets.
The modified retrospective approach does not require restatement of prior period comparative financial information and is applied prospectively. The application of IFRS 16 requires the Company to make judgments that affect the valuation of the lease liabilities and the valuation of ROU assets. These include determining contracts that are within the scope of IFRS 16; determining the contract term; and determining the interest rate used for the discounting of future cash flows.
There were no reporting changes as a result of adopting the new interpretation.
New Interpretation IFRIC 23 - Uncertainty over Income Tax Treatments
On June 7, 2017, the IASB issued IFRIC Interpretation 23 - Uncertainty over Income Tax Treatments. The Interpretation provides guidance on the accounting for current and deferred tax liabilities and assets in circumstances in which there is uncertainty over income tax treatments. The Interpretation is applicable for annual periods beginning on or after January 1, 2019. There were no reporting changes as a result of adopting the new Interpretation.
Standards issued but not yet effective
Certain pronouncements have been issued by the IASB or IFRIC that are effective for accounting periods beginning on or after January 1, 2021. The Company has reviewed these updates and determined that many of these updates are not applicable or consequential to the Company and have been excluded from discussion within these significant accounting policies.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
Significant accounting judgments, estimates and assumptions
The preparation of financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the reported amounts of assets, liabilities and contingent liabilities at each reporting date and the reported amounts of income and expenses during each reporting period. Estimates and assumptions are continuously evaluated and are based on management's experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. However, actual outcomes can differ from these estimates.
The information about significant areas of estimation uncertainty considered by management in preparing these financial statements is as follows:
Useful lives of property and equipment and intangible assets
The estimated useful lives of property and equipment and intangible assets are reviewed by management and adjusted if necessary. To estimate their useful life, management must use its past experience with the same or similar assets, review industry practices for similar pieces of equipment and/or apply statistical methods to assist in its determination of useful life.
Collectability of financial assets
The estimate of the collectability of financial assets is subject to estimation as to the credit -risk and likelihood of default by the counterparty. Refer to the disclosure under "Credit risk" within Note 8 for details.
Income taxes
Tax provisions are based on enacted or substantively enacted laws. Changes in those laws could affect amounts recognized in profit or loss both in the period of change, which would include any impact on cumulative provisions, and in future periods. Deferred tax assets (if any) are recognized only to the extent it is considered probable that those assets will be recoverable. This involves an assessment of when those deferred tax assets are likely to reverse and a judgment as to whether there will be sufficient taxable profits available to offset the tax assets when they do reverse. This requires assumptions regarding future profitability and is therefore inherently uncertain. To the extent assumptions regarding future profitability change, there can be an increase or decrease in the amounts recognized in respect of deferred tax assets as well as the amounts recognized in profit or loss in the period in which the change occurs.
Going concern
The assessment of the Company's ability to continue as a going concern as discussed in Note 1 involves judgment regarding future funding available for its operations and working capital requirements.
Commencement of depreciation and amortization of property and equipment and intangible assets
Judgment is used to determine the commencement date of depreciation/amortization of an item of property and equipment/intangible assets. The commencement date is when an item is available for use in the location and condition as intended by management.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
Property and equipment
Property and equipment is measured at cost less accumulated depreciation and impairment losses. The cost of an item of equipment consists of the purchase price, any costs directly attributable to bringing the asset to the location and condition necessary for its intended use, and an initial estimate of the costs of dismantling and removing the item and restoring the site on which it is located, if applicable.
Depreciation is recognized using the following rates/terms, intended to depreciate the cost of equipment, less its residual values, if any, over its estimated useful lives:
Furniture and equipment | 20% declining balance |
Cost includes expenditures that are directly attributable to the acquisition of the asset. Subsequent costs are included in the asset's carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost can be measured reliably. Repairs and maintenance costs are charged to profit or loss during the period they are incurred. Any gain or loss on the disposal or retirement of equipment is recognized in profit or loss.
Intangible assets
Intangible assets with finite lives are measured at cost less accumulated amortization and impairment losses. These intangible assets are amortized on a straight-line basis over their estimated useful lives. Useful lives, residual values, and amortization methods for intangible assets with finite useful lives are reviewed at least annually.
The Company's intangible assets are comprised solely of trademarks which are amortized over a 10-year useful life.
Impairment of non-financial assets
The carrying amount of the Company's long-lived assets is reviewed for an indication of impairment at the end of each reporting period. If an indication of impairment exists, the Company makes an estimate of the asset's recoverable amount. Individual assets are grouped for impairment assessment purposes at the lowest level at which there are identifiable cash flows that are largely independent of the cash flows of other groups of assets (cash generating units "CGU"). The recoverable amount of an asset group is the higher of its fair value less costs to sell and its value in use. In assessing value in use, the estimated future cash flows are adjusted for the risks specific to the CGU and are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money.
Where the carrying amount of an asset group exceeds its recoverable amount, the CGU is considered impaired and is written down to its recoverable amount. Impairment losses are recognized in profit or loss. Impairment losses recognized in respect of CGUs are allocated to reduce the carrying amounts of the assets in the CGU on a pro rata basis.
An assessment is made at each reporting date as to whether there is any indication that previously recognized impairment losses may no longer exist or may have decreased. If such indication exists, the recoverable amount is estimated. A previously recognized impairment loss is reversed only if there has been a change in the estimates used to determine the asset's recoverable amount. An impairment loss is reversed only to the extent that the asset's carrying amount does not exceed the carrying amount that would have been determined, net of depreciation, if no impairment loss had been recognized.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
Share capital
Common shares are classified as shareholders' equity. Transaction costs directly attributable to the issue of common shares are recognized as a deduction from shareholders' equity, as share issue costs. Common shares issued for consideration other than cash, are valued based on their fair value on the date the shares are issued if it is impracticable to estimate reliably the fair value of the goods or services received.
Loss per share
Basic loss per share is computed by dividing the net loss attributable to common shareholders by the weighted average number of common shares outstanding during the reporting period.
Diluted loss per share is computed similar to basic loss per share except that net loss attributable to common shareholders are adjusted for the dilutive effect of warrants and stock options. Under this method, the Company assumes that outstanding dilutive stock options and warrants were exercised and that the proceeds from such exercises (after adjustment of any unvested portion of stock options) were used to acquire common shares at the average market price during the reporting periods. For the periods presented, diluted loss per share equals basic loss per shares as the effect of all dilutive stock options and warrants would have been anti-dilutive.
Income taxes
Income tax comprises current and deferred income taxes. Current income tax and deferred income tax are recognized in profit or loss, except to the extent that they relate to items recognized directly in shareholders' equity.
Current income tax is the expected tax payable or receivable on the taxable profit or loss for the period, using tax rates enacted or substantively enacted at the reporting date, adjusted for any amendments to tax payable in respect of previous periods.
Deferred income tax is provided for, based on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized. Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the period when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period.
Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
2. SIGNIFICANT ACCOUNTING POLICIES (continued)
Financial instruments
For the periods presented, the Company's financial instruments are classified within the following categories: fair value through profit or loss ("FVTPL"), financial assets at amortized cost, and other financial liabilities. The classification depends on the purpose for which the financial assets or liabilities were acquired. Management determines the classification of financial assets and liabilities at initial recognition. The Company accounts for non- derivative financial assets and liabilities as follows:
Recognition
The Company recognizes financial assets and financial liabilities on the date the Company becomes a party to the contractual provisions of the instruments.
Classification
The Company classifies its financial instruments based on the purpose for which they were acquired, in one of the following categories: FVTPL, amortized cost, FVOCI, and other financial liabilities. The fair value and classification of the Company's financial instruments are detailed in Note 8.
Impairment of financial assets
An 'expected credit loss' ("ECL") model applies to financial assets measured at amortized cost. The Company has certain financial assets that are subject to the ECL model (see, "Credit risk" within Note 8).
Financial assets, other than those classified at FVTPL, are assessed for indicators of impairment at each reporting date. Financial assets are impaired when there is objective evidence that one or more events have occurred after the initial recognition of the financial asset (a "loss event"), and that loss event has an impact on the estimated future cash flows of that asset. Objective evidence may include significant financial difficulty of the debtor/obligor and/or delinquency in payment. When impairment has occurred, the cumulative loss is recognized in profit or loss. For financial assets carried at amortized cost, the amount of the impairment loss recognized is the difference between the asset's carrying amount and the present value of estimated future cash flows, discounted at the financial asset's original effective interest rate. Impairment losses may be reversed in subsequent periods.
3. RECEIVABLES AND PREPAYMENTS
Receivables and prepayments comprise the following:
| | | September 30, 2020 | | | September 30, 2019 | |
| | | $ | | | $ | |
| Prepaid expenses | | 9,000 | | | - | |
| Trade receivables | | 118,062 | | | - | |
| | | 127,062 | | | - | |
4. PROPERTY AND EQUIPMENT
| | | Furniture and | | | | |
| | | equipment | | | Total | |
| | | $ | | | $ | |
| | | | | | | |
| Cost | | | | | | |
| September 30, 2019 | | - | | | - | |
| Additions | | 5,833 | | | 5,833 | |
| September 30, 2020 | | 5,833 | | | 5,833 | |
| | | | | | | |
| Accumulated depreciation | | | | | | |
| September 30, 2019 | | - | | | - | |
| Depreciation | | 583 | | | 583 | |
| September 30, 2020 | | 583 | | | 583 | |
| | | | | | | |
| Net book value | | | | | | |
| September 30, 2019 | | - | | | - | |
| September 30, 2020 | | 5,250 | | | 5,250 | |
5. INTANGIBLE ASSETS
| | | Registered | | | | |
| | | trademarks | | | Total | |
| | | $ | | | $ | |
| | | | | | | |
| Cost | | | | | | |
| April 9, 2019 (Incorporation) | | - | | | - | |
| Additionss | | 10,276 | | | 10,276 | |
| September 30, 2019 | | 10,276 | | | 10,276 | |
| Additions | | 15,206 | | | 15,206 | |
| September 30, 2020 | | 25,482 | | | 25,482 | |
| | | | | | | |
| Accumulated amortization | | | | | | |
| April 9, 2019 (Incorporation) | | - | | | - | |
| Amortization | | 514 | | | 514 | |
| September 30, 2019 | | 514 | | | 514 | |
| Amortization | | 1,788 | | | 1,788 | |
| September 30, 2020 | | 2,302 | | | 2,302 | |
| | | | | | | |
| Net book value | | | | | | |
| September 30, 2019 | | 9,762 | | | 9,762 | |
| September 30, 2020 | | 23,180 | | | 23,180 | |
Trademarks
During the year ended September 30, 2020, the Company has 16 registered trademarks with the Canadian Intellectual Property Office ("CIPO") and 6 trademark applications currently in process with CIPO. These trademarks have finite lives and are measured at cost less accumulated amortization and impairment losses. These intangible assets are amortized on a straight-line basis over their estimated useful lives. Useful lives, residual values, and amortization methods for intangible assets with finite useful lives are reviewed at least annually.
6. SHAREHOLDERS' EQUITY
The authorized share capital of the Company consists of unlimited common shares without par value. All issued shares are fully paid.
Issued and Outstanding
No transactions for the issue of share capital during the year ended September 30, 2020.
Transactions for the issue of share capital during the period ended September 30, 2019:
- On April 9, 2019, the Company issued 1,000 common share for $0.001 on incorporation.
Subsequent to year end, the Company completed a share split (Note 11). All information with respect to the number of common shares and issuance prices for the periods prior to the share split have been restated to reflect the share split.
7. RELATED PARTY TRANSACTIONS
Key management personnel include those persons having authority and responsibility for planning, directing and controlling the activities of the Company as a whole. The Company has determined that key management personnel consist of executive and non-executive members of the Company's Board of Directors and Corporate Officers, significant shareholders and companies controlled by them.
Key management personnel compensation
There was no remuneration of key management for the year ended September 30, 2020 or for the period from incorporation on April 9, 2019 to September 30, 2019.
Related party balances
Related party balances as at September 30, 2020 and September 30, 2019 are as follows:
| | | September 30, 2020 | | | September 30, 2019 | |
| | | $ | | | $ | |
| | | | | | | |
| Due to shareholder | | 142,599 | | | - | |
During the year ended September 30, 2020, the Company received shareholder advances. These advances are unsecured, non-interest bearing and due on demand.
8. FINANCIAL INSTRUMENTS
Fair value
Financial instruments measured at fair value are classified into one of three levels in the fair value hierarchy according to the relative reliability of the inputs used to estimate the fair values. The three levels of the fair value hierarchy are:
Level 1 - Unadjusted quoted prices in active markets for identical assets or liabilities
Level 2 - Inputs other than quoted prices that are observable for the assets or liability either directly or indirectly
Level 3 - Inputs that are not based on observable market data
Classification of financial instruments
Financial assets: | Initial Recognition: | Subsequent measurement: |
Cash | Fair value | Amortized cost |
Receivables | Fair value | Amortized cost |
| | | |
Financial liabilities: | Initial Recognition: | Subsequent measurement: |
Accounts payable and accrued liabilities | Fair value | Amortized cost |
The Company's financial instruments other than cash, approximate their fair values. Cash, under the fair value hierarchy is based on Level 1 quoted prices in active markets for identical assets or liabilities.
Economic dependence
Economic dependence risk is the risk of reliance upon a select number of customers which significantly impact the financial performance of the Company. During the year ended September 30, 2020, the Company's licensing revenue was generated from a single customer.
Credit risk
Credit risk is the risk of financial loss to the Company if a counterparty to a financial instrument fails to meet its contractual obligations. As at September 30, 2020, credit risk for the Company arises from cash and trade receivables. The carrying amount of these financial assets represents the maximum credit exposure as at September 30, 2020.
Cash is held with a Canadian chartered bank and accordingly, the Company's exposure to credit risk on cash is considered insignificant. As at September 30, 2020, the Company's trade receivables were due from one customers. The Company's trade receivables are current, and management considers the credit risk to be low.
8. FINANCIAL INSTRUMENTS (continued)
Impairment of financial assets
The Company has the following financial assets that are subject to the expected credit loss model:
- Trade receivables.
While cash is also subject to the impairment requirements of IFRS 9, the risk is insignificant.
The Company applies the IFRS 9 simplified approach to measure expected credit losses which uses a lifetime expected loss allowance for its trade receivables. The Company applies the general approach using practical expedients to loans receivable which involves recognition at each reporting date of a loss allowance based on a 12-month expected credit loss model without the requirement to re-assess whether any significant increases in credit risk have occurred at each reporting date.
To measure the expected credit losses, trade receivables are grouped by debtor, and each debtor's circumstances are reviewed. The expected loss amount, if any, is based on historical payment profiles, and the corresponding historical credit losses experienced within this period for the debtors.
As at September 30, 2020, the loss allowance was $nil for trade receivables. There has been no historical loss allowance recorded on these items.
Amounts are written-off when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, failure of a debtor to engage in a repayment plan with the Company (if applicable), and failure by the debtor to make contractual payments for a period of greater than 120 days past due, or shorter if specific circumstances suggest otherwise.
Impairment losses are presented as loss provisions within profit or loss. Subsequent recoveries of amounts previously written-off are credited against the same line item.
Liquidity risk
The Company manages liquidity risk by maintaining an adequate level of cash to meet its ongoing obligations. The Company has been successful in revenue generation and shareholder loans in the past; however, there is no assurance that it will be able to do so in the future. As at September 30, 2020, the Company had working capital of $117,991 (2019 - working capital deficiency of $7,318), which is considered sufficient to fund future operations and obligations as they come due, and to allow the Company to meet business objectives for at least the next twelve months (note 1).
Market risk
Market risk is the risk that the fair value or future cash flows from a financial instrument will fluctuate because of changes in market prices or prevailing conditions as follows:
Interest rate risk
The Company is not significantly exposed to interest rate risk because it does not have any assets or liabilities subject to variable rates of interest, except for cash held in interest-bearing accounts which poses an insignificant risk exposure.
9. SUPPLEMENTAL CASH FLOW INFORMATION
The Company did not incur any non-cash investing and financing activities during the year ended September 30, 2020 or during the period from incorporation on April 9, 2019 to September 30, 2019.
During the year ended September 30, 2020 or the period from incorporation on April 9, 2019 to September 30, 2019, no amounts were paid for interest or income tax expenses.
10. CAPITAL RISK MANAGEMENT
The Company defines capital as the components of shareholders' equity (deficiency). The Company's objectives when managing capital are to support further advancement of the Company's business objectives, as well as to ensure that the Company can meet its financial obligations as they come due.
The Company manages its capital structure to maximize its financial flexibility adjusting it in response to changes in economic conditions and the risk characteristics of the underlying assets and business opportunities. The Company relies on the expertise of the Company's management to sustain the future development of the business. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
There were no changes in the Company's approach to capital management during the year ended September 30, 2020. The Company is not subject to externally imposed capital requirements.
11.SEGMENTED INFORMATION
The Company has a single reportable segment: the provision of intellectual property licensing to the cannabis industry in Canada. All the Company's revenues are generated in Canada, and its non-current assets are located in Canada.
12. LICENSING REVENUE
On March 12, 2020 (subsequently amended December 4, 2020), the Company entered into a Licence and Distribution Royalty Agreement (the "Royalty Agreement") with a private Ontario company (the "Licensee"). Pursuant to the Royalty Agreement, the Company granted the Licensee an exclusive Licence for the use of certain registered and pending trademarks (the "Registered trademarks") in exchange for a 50% royalty on the Licensee's gross profit from the sales of said cannabis products. The Royalty Agreement shall terminate upon the second anniversary of the Butane Hash Oil ("BHO") or Propane Hash Oil ("PHO") concentrate products (Shatter, Wax, Live resin, Crystals) becoming available in a Provincial retail outlet. The term shall automatically renew for an additional one-year term provided that the gross sales revenue is equal to or greater than $4,000,000 by the end of the initial term.
During the year ended September 30, 2020, the Company recognized licensing revenue of $212,654 (2019 - $nil) in connection with the Royalty Agreement and $118,062 (2019 - $nil) in trade receivables at year end. The trade receivables have been collected subsequent to year end.
13. INCOME TAXES
A reconciliation of income taxes at statutory rates with the reported taxes for the year ended September 30, 2020, and for the period from incorporation on April 9, 2019 to September 30, 2019, is as follows:
| | | | | | For the period from | |
| | | | | | incorporation | |
| | | | | | on April 9, 2019 to | |
| | | September 30, 2020 | | | September 30,2019 | |
| | | $ | | | $ | |
| Income (loss) for the period | | 11,140 | | | (7,319 | ) |
| Expected income tax (recovery) | | 3,000 | | | (2,000 | ) |
| Change in tax resulting from: | | | | | | |
| Permanent differences | | - | | | (1,000 | ) |
| Change in recognized deductible temporary differences and other | | (1,000 | ) | | 3,000 | |
| Adjustment to prior period provision versus statutory tax returns | | (2,000 | ) | | - | |
| Total income tax expense (recovery) | | - | | | - | |
The Company's unused temporary differences, and unused tax losses that have not been included on the statements of financial position as at September 30, 2020, and September 30, 2019, are as follows:
| | | 2020 | | | Expiry Date | | | 2019 | | | Expiry Date | |
| | | $ | | | Range | | | $ | | | Range | |
| Equipment & intangible assets | | 5,000 | | | No expiry | | | 6,000 | | | No expiry | |
| Non-capital loss carry forwards | | 7,000 | | | 2037 to 2040 | | | 7,000 | | | 2037 to 2039 | |
Tax attributes are subject to review, and potential adjustment, but tax authorities.
14. SUBSEQUENT EVENTS
On November 13, 2020, the Company issued 99 common shares valued at $0.001 per share for gross proceeds of $0.10.
On March 16, 2021 the Company underwent a share split at 1,000 to one, increasing the number of common shares from 100 to 100,000.
On September 15, 2021 all the issued and outstanding shares of the Company were acquired by Adastra Holdings Ltd, a British Columbia corporation. The consideration paid was 20,000,000 common shares in Adastra Holdings Ltd.

Adastra Holdings Ltd.
(formerly Phyto Extractions Inc.)
Pro Forma Consolidated Financial Statements
June 30, 2021 and December 31, 2020
(Expressed in Canadian dollars - Unaudited)
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Pro Forma Consolidated Statement of Financial Position
As at June 30, 2021
(Expressed in Canadian dollars – Unaudited)
| | | Adastra
Holdings
Ltd. | | | Phyto
BrandCo | | | Pro Forma
Adjustments | | | Note | | | Pro Forma
Consolidated | |
| | | $ | | | $ | | | $ | | | | | | $ | |
| ASSETS | | | | | | | | | | | | | | | |
| Current assets | | | | | | | | | | | | | | | |
| Cash | | 440,741 | | | 31,204 | | | - | | | | | | 471,945 | |
| Receivables and prepayments | | 558,629 | | | 676,046 | | | (11,160 | ) | | 4c | | | 1,223,515 | |
| Inventory | | 1,936,128 | | | - | | | - | | | | | | 1,936,128 | |
| Deposits | | 28,750 | | | - | | | - | | | | | | 28,750 | |
| | | 2,964,248 | | | 707,250 | | | (11,160 | ) | | | | | 3,660,338 | |
| | | | | | | | | | | | | | | | |
| Long-term deposits | | 122,328 | | | - | | | - | | | | | | 122,328 | |
| Property, plant, and equipment | | 9,995,425 | | | 90,561 | | | - | | | | | | 10,085,986 | |
| Intangible assets | | - | | | 34,572 | | | - | | | | | | 34,572 | |
| Goodwill | | - | | | - | | | 23,605,507 | | | 4a | | | 23,605,507 | |
| Total assets | | 13,082,001 | | | 832,383 | | | 23,594,347 | | | | | | 37,508,731 | |
| | | | | | | | | | | | | | | | |
| LIABILITIES | | | | | | | | | | | | | | | |
| Current liabilities | | | | | | | | | | | | | | | |
| Accounts payable and accrued liabilities | | 1,240,655 | | | 400,776 | | | (11,160 | ) | | 4c | | | 1,630,271 | |
| Mortgage payable | | 2,433,592 | | | - | | | - | | | | | | 2,433,592 | |
| Current portion of lease liability | | - | | | 10,169 | | | - | | | | | | 10,169 | |
| | | 3,674,247 | | | 410,945 | | | (11,160 | ) | | | | | 4,074,032 | |
| | | | | | | | | | | | | | | | |
| Due to shareholders | | - | | | 108,753 | | | (108,753 | ) | | 4b | | | - | |
| Lease liability | | - | | | 26,944 | | | - | | | | | | 26,944 | |
| Government loan | | 60,000 | | | - | | | - | | | | | | 60,000 | |
| Total liabilities | | 3,734,247 | | | 546,642 | | | (119,913 | ) | | | | | 4,160,976 | |
| | | | | | | | | | | | | | | | |
| EQUITY | | | | | | | | | | | | | | | |
| Share capital | | 15,822,152 | | | 1 | | | (1 | ) | | 4a | | | 39,822,152 | |
| | | | | | | | | 24,000,000 | | | 4a | | | - | |
| Reserves | | 5,441,814 | | | - | | | - | | | | | | 5,441,814 | |
| Subscriptions received | | 135,000 | | | - | | | - | | | | | | 135,000 | |
| Retained earnings (deficit) | | (12,051,212 | ) | | 285,740 | | | (285,739 | ) | | | | | (12,051,211 | ) |
| Total equity | | 9,347,754 | | | 285,741 | | | 23,714,260 | | | | | | 33,347,755 | |
| | | | | | | | | | | | | | | | |
| Total liabilities and equity | | 13,082,001 | | | 832,383 | | | 23,594,347 | | | | | | 37,508,731 | |
Approved on behalf of the Board of Directors on January 19, 2021:
"Michael Forbes" | | "Donald Dinsmore" |
Director | | Director |
The accompanying notes are an integral part of these pro forma consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Pro Forma Consolidated Statement of Income (Loss) and Comprehensive Income (Loss)
For the six months ended June 30, 2021
(Expressed in Canadian dollars – Unaudited)
| Adastra
Holdings Ltd. | Phyto
BrandCo (Note 5) | Pro Forma
Adjustment | Note | Pro Forma
Consolidated |
| $ | $ | $ | | $ |
| | | | | |
Revenue | 1,830,901 | 1,030,893 | - | | 2,861,794 |
Cost of sales | 1,098,338 | - | - | | 1,098,338 |
Gross profit | 732,563 | 1,030,893 | - | | 1,763,456 |
| | | | | |
Operating expenses | | | | | |
Advertising and promotion | 135,352 | 242,655 | - | | 378,007 |
Amortization | - | 2,255 | - | | 2,255 |
Depreciation | 42,112 | 8,851 | - | | 50,963 |
Automobile expenses | - | 7,100 | - | | 7,100 |
Insurance | 37,104 | 3,953 | - | | 41,057 |
Office expenses | 150,109 | 170,867 | - | | 320,976 |
Meals and entertainment | - | 3,311 | - | | 3,311 |
Professional fees and consulting | 207,650 | 204,036 | - | | 411,686 |
Supplies | - | (1,125) | - | | (1,125) |
Research expense | 46,664 | - | - | | 46,664 |
Commission expense | - | 2,000 | - | | 2,000 |
Rent | - | 7,544 | - | | 7,544 |
Bank charges | - | 344 | - | | 344 |
Travel | 2,373 | 12,038 | - | | 14,411 |
Wages and salaries | 493,835 | - | (108,753) | 4b | 385,082 |
Income (loss) from operations | (382,636) | 367,064 | 108,753 | | 93,181 |
| | | | | |
Other income (expense) | | | | | |
Finance expense | (107,354) | (4,162) | - | | (111,516) |
Interest income | 600 | - | - | | 600 |
Gain on settlement of accounts payable | 57,500 | - | - | | 57,500 |
Impairment of property, plant and equipment | (150,000) | - | - | | (150,000) |
Write-down of inventory | (291,512) | - | - | | (291,512) |
Foreign exchange | - | (2,663) | - | | (2,663) |
| | | | | |
Net income (loss) and comprehensive income (loss) for the period | (873,402) | 360,239 | 108,753 | | (404,410) |
| | | | | |
Net earnings (loss) per share | | | | | |
Basic and diluted | (0.02) | 4.29 | 0.01 | | (0.01) |
| | | | | |
Weighted average number of shares | | | | | |
Basic and diluted | 43,334,100 | 84,044 | 20,000,000 | | 63,334,100 |
The accompanying notes are an integral part of these pro forma consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Pro Forma Consolidated Statement of Financial Position
As at December 31, 2020
(Expressed in Canadian dollars – Unaudited)
| Adastra
Holdings
Ltd. | Phyto
BrandCo (Note 6) | Pro Forma
Adjustments | Note | Pro Forma
Consolidated |
| $ | $ | $ | | $ |
ASSETS | | | | | |
Current assets | | | | | |
Cash | 1,145,461 | 20,207 | - | | 1,165,668 |
Receivables and prepayments | 1,129,189 | 14,277 | - | | 1,143,466 |
Inventory | 1,421,237 | - | - | | 1,421,237 |
| 3,695,887 | 34,484 | - | | 3,730,371 |
| | | | | |
Long-term deposits | 4,000 | - | - | | 4,000 |
Property, plant, and equipment | 10,037,063 | 55,165 | - | | 10,092,228 |
Intangible assets | - | 23,808 | - | | 23,808 |
Goodwill | - | - | 23,967,872 | 4a | 23,967,872 |
Total assets | 13,736,950 | 113,457 | 23,967,872 | | 37,818,279 |
| | | | | |
LIABILITIES | | | | | |
Current liabilities | | | | | |
Accounts payable and accrued liabilities | 1,147,964 | 39,498 | - | | 1,187,462 |
Mortgage payable | 2,442,830 | - | - | | 2,442,830 |
Current portion of lease liability | - | 9,675 | - | | 9,675 |
| 3,590,794 | 49,173 | - | | 3,639,967 |
�� | | | | | |
Due to shareholders | - | 108,794 | (108,794) | 4b | - |
Lease liability | - | 32,155 | - | | 32,155 |
Government loan | 60,000 | - | - | | 60,000 |
Total liabilities | 3,650,794 | 190,122 | (108,794) | | 3,732,122 |
| | | | | |
EQUITY | | | | | |
Share capital | 15,822,152 | 1 | (1) | 4a | 39,822,152 |
| | | 24,000,000 | 4a | - |
Reserves | 5,441,814 | - | - | | 5,441,814 |
Retained earnings (deficit) | (11,177,810) | (76,666) | 76,667 | | (11,177,809) |
Total equity | 10,086,156 | (76,665) | 24,076,666 | | 34,086,157 |
| | | | | |
Total liabilities and equity | 13,736,950 | 113,457 | 23,967,872 | | 37,818,279 |
The accompanying notes are an integral part of these pro forma consolidated financial statements.
ADASTRA HOLDINGS LTD. (formerly Phyto Extractions Inc.)
Pro Forma Consolidated Statement of Loss and Comprehensive Loss
For the year ended December 31, 2020
(Expressed in Canadian dollars – Unaudited)
| Adastra Holdings Ltd. | Phyto BrandCo (Note 6) | Pro Forma Adjustment | Note | Pro Forma Consolidated |
| $ | $ | $ | | $ |
| | | | | |
Revenue | 2,499,355 | 212,654 | - | | 2,712,009 |
Cost of sales | 1,713,774 | - | - | | 1,713,774 |
Gross profit | 785,581 | 212,654 | - | | 998,235 |
| | | | | - |
Operating expenses | | | | | - |
Advertising and promotion | 749,258 | 140,847 | - | | 890,105 |
Accretion | 58,405 | - | - | | 58,405 |
Amortization | - | 2,736 | - | | 2,736 |
Depreciation | 92,700 | 4,502 | - | | 97,202 |
Delivery and freight | - | 756 | - | | 756 |
Insurance | 69,294 | 720 | - | | 70,014 |
Office expenses | 227,271 | 12,225 | - | | 239,496 |
Meals and entertainment | - | 3,686 | - | | 3,686 |
Professional fees and consulting | 704,297 | 136,509 | - | | 840,806 |
Supplies | - | 8,572 | - | | 8,572 |
Research expense | 181,750 | - | - | | 181,750 |
Share-based payments | 5,332,500 | - | - | | 5,332,500 |
Travel | 4,029 | 7,633 | - | | 11,662 |
Bank charges | - | 411 | - | | 411 |
Wages and salaries | 526,327 | - | (108,794) | 4b | 417,533 |
Loss from operations | (7,160,250) | (105,943) | 108,794 | | (7,157,399) |
| | | | | |
Other income (expenses) | | | | | |
Finance expense | (399,248) | (1,084) | - | | (400,332) |
Interest income | 8,303 | - | - | | 8,303 |
Realized loss on marketable securities | (1,500) | - | - | | (1,500) |
Write-down of inventory | (63,169) | - | - | | (63,169) |
| | | | | |
Net loss and comprehensive loss for the period | (7,615,864) | (107,027) | 108,794 | | (7,614,097) |
| | | | | |
Net loss per share | | | | | |
Basic and diluted | (0.19) | (1.07) | 0.01 | | (0.12) |
| | | | | |
Weighted average number of shares | | | | | |
Basic and diluted | 39,695,235 | 100,000 | 20,000,000 | | 63,334,100 |
The accompanying notes are an integral part of these pro forma consolidated financial statements.
NOTE 1 - BASIS OF PREPARATION
The accompanying unaudited pro forma consolidated financial statements of Adastra Holdings Ltd. (formerly Phyto Extractions Inc.) ("Adastra" or the "Company") have been prepared by management in accordance with International Financial Reporting Standards ("IFRS") from information derived from the financial statements of the Company and the financial statements of 1204581 B.C. Ltd. ("Phyto BrandCo"), to give effect to the proposed transaction (the "Transaction") as described in Note 3.
These unaudited pro forma consolidated financial statements of the Company are compiled from and include, and should be read in conjunction with the following:
a. The audited consolidated annual financial statements of Adastra Holdings Ltd as at December 31, 2020, and for the year then ended.
b. The unaudited consolidated interim financial statements of Adastra Holdings Ltd as at June 30, 2021 and for the six months then ended.
c. The audited annual financial statements of Phyto BrandCo as at September 30, 2020, and for the year then ended.
d. The unaudited interim financial statements of Phyto BrandCo as at June 30, 2021, and for the nine months then ended.
e. The additional information set out in Note 3.
The unaudited pro forma consolidated statements of financial position as at December 31, 2020 and June 30, 2021 have been prepared as if the transactions in note 4 had occurred on each December 31, 2020 and June 30, 2021, respectively. The unaudited pro forma consolidated statement of income (loss) and comprehensive income (loss) for the period ended June 30, 2021 and the year ended December 31, 2020 have been prepared as if the transactions had occurred on June 30, 2021 and December 31, 2020, respectively.
These unaudited pro forma consolidated financial statements are not necessarily indicative of the financial position that would have been achieved if the proposed transactions had been completed on the dates indicated, nor do they purport to project the financial position or results of operations of the combined entity for any future period. In the opinion of the management of the Company and Phyto BrandCo, these unaudited pro forma consolidated statements include all adjustments necessary for the fair presentation, in all material respects, of the transactions described in Note 4. These unaudited pro forma consolidated financial statements do not reflect any cost savings that could result from the combination of the operations of the Company and Phyto BrandCo, as management does not anticipate any material cost savings as a result of the Transaction.
The pro forma adjustments are based in part on estimates, including the fair values of the assets acquired and liabilities assumed, as applicable. For purposes of the pro forma consolidated statement of financial position, it is assumed that there are no tax consequences, and no income tax effect is being recorded. The Company has incurred losses since inception and when combined with Phyto BrandCo is not expected to generate profits in the immediate future, therefore neither entity carries any deferred tax assets in its most recent financial statements.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
The unaudited pro forma consolidated statement of financial position has been compiled using significant accounting policies consistent with those set out in the audited financial statements of the Company for the year ended December 31, 2020 and Phyto BrandCo for the year ended September 30, 2020.
NOTE 3 - DESCRIPTION OF THE TRANSACTION
On September 15, 2021, the Company acquired, indirectly through its wholly-owned subsidiary, Adastra Labs Inc, all of the issued and outstanding shares of Phyto BrandCo., the owner of the intellectual property rights for the Phyto Extractions brand. At closing, the Company issued 20 million common shares to the former shareholders of Phyto BrandCo at a share price on the date of acquisition of $1.20 per share, for total consideration of $24 million.
NOTE 4 - PRO FORMA ADJUSTMENTS AND ASSUMPTIONS
The unaudited pro forma consolidated financial statements reflect the following adjustments:
a) Consideration paid and fair value of net assets acquired
| | | June 30, 2021 | | | December 31, 2020 | |
| Assets acquired: | | $ | | | $ | |
| Cash | | 31,204 | | | 20,207 | |
| Receivables and prepayments | | 676,046 | | | 14,277 | |
| Property, plant and equipment | | 90,561 | | | 55,165 | |
| Trademarks | | 34,571 | | | 23,808 | |
| | | 832,382 | | | 113,457 | |
| Liabilities assumed: | | | | | | |
| Accounts payable and other accrued liabilities | | (400,776 | ) | | (39,499 | ) |
| Lease liability | | (37,113 | ) | | (41,830 | ) |
| | | (437,889 | ) | | (81,329 | ) |
| | | | | | | |
| Fair value of net identifiable assets acquired | | 394,493 | | | 32,128 | |
| | | | | | | |
| Purchase consideration | | | | | | |
| Share consideration | | 24,000,000 | | | 24,000,000 | |
| | | 24,000,000 | | | 24,000,000 | |
| | | | | | | |
| Goodwill and intangible assets | | 23,605,507 | | | 23,967,872 | |
The transaction has been accounted for as a business combination under IFRS 3 - Business Combinations. The preliminary allocation of the purchase consideration is shown above, noting that the Company intends to engage valuation experts to assist with the identification and measurement of intangible assets separate from goodwill. The carrying value of the assets and liabilities acquired equates to fair value due to their short term nature, other than property plant and equipment and trademarks which are depreciated over their estimated useful economic lives.
b) Expense of amounts owed to shareholders
$108,753, owed to shareholders included in the June 30, 2021 and $108,794 included in the December 31, 2020 financial statements of Phyto BrandCo have been expensed to the income statement in both periods as they were waived by the shareholders on acquisition.
c) Elimination of intercompany balances
$11,160 of receivable balances held by the Company owed by Phyto BrandCo have been eliminated from the Pro Forma Consolidated Statement of Financial Position.
NOTE 5 - PHYTO BRANDCO RECONCILIATION TO SIX MONTHS ENDED JUNE 30, 2021
Phyto BrandCo has a September 30 year end. Reconciliation of the Phyto BrandCo results from the nine months ended June 30, 2021 to the six month ended June 30, 2021 is as follows:
| | | Phyto BrandCo nine
months ended June
30, 2021 | | | Less Oct 1, 2020 to
Dec 31, 2020 | | | Phyto BrandCo six
months ended June
30, 2021 | |
| | | $ | | | $ | | | $ | |
| | | | | | | | | | |
| Revenue | | 1,030,893 | | | - | | | 1,030,893 | |
| Cost of Sales | | - | | | - | | | - | |
| Gross Profit | | 1,030,893 | | | - | | | 1,030,893 | |
| | | | | | | | | | |
| Expenses | | | | | | | | | |
| Advertising and promotion | | 279,020 | | | (36,365 | ) | | 242,655 | |
| Amortization | | 2,924 | | | (669 | ) | | 2,255 | |
| Depreciation | | 12,770 | | | (3,919 | ) | | 8,851 | |
| Automobile expenses | | 7,100 | | | - | | | 7,100 | |
| Insurance | | 4,673 | | | (720 | ) | | 3,953 | |
| Office Expenses | | 175,691 | | | (4,824 | ) | | 170,867 | |
| Meals and entertainment | | 6,987 | | | (3,676 | ) | | 3,311 | |
| Professional fees and consulting | | 224,862 | | | (20,826 | ) | | 204,036 | |
| Supplies | | - | | | (1,125 | ) | | (1,125 | ) |
| Commission expense | | 2,000 | | | - | | | 2,000 | |
| Rent | | 7,544 | | | - | | | 7,544 | |
| Bank charges | | 371 | | | (27 | ) | | 344 | |
| Travel | | 19,291 | | | (7,253 | ) | | 12,038 | |
| Income from operations | | 287,660 | | | 79,404 | | | 367,064 | |
| | | | | | | | | | |
| Other income (expense) | | | | | | | | | |
| Finance expense | | (3,078 | ) | | (1,084 | ) | | (4,162 | ) |
| Foreign exchange | | (2,663 | ) | | - | | | (2,663 | ) |
| | | | | | | | | | |
| Net income and comprehensive income for the period | | 281,919 | | | 78,320 | | | 360,239 | |
NOTE 6 - PHYTO BRANDCO RECONCILIATION TO YEAR ENDED DECEMBER 31, 2020
Phyto BrandCo has a September 30 year end. Reconciliation of the Phyto BrandCo Statement of Financial Position as at September 30, 2021 to December 31, 2020 is as follows:
| | | Phyto BrandCo as
at September 30,
2020 | | | Add Oct 1 2020 to
Dec 31, 2020 | | | Phyto BrandCo
as at December 31,
2020 | |
| | | $ | | | $ | | | $ | |
| ASSETS | | | | | | | | | |
| Current assets | | | | | | | | | |
| Cash | | 26,100 | | | (5,893 | ) | | 20,207 | |
| Receivables and prepayments | | 127,062 | | | (112,785 | ) | | 14,277 | |
| | | 153,162 | | | (118,678 | ) | | 34,484 | |
| | | | | | | | | | |
| Property, plant, and equipment | | 5,250 | | | 49,915 | | | 55,165 | |
| Intangible assets | | 23,180 | | | 628 | | | 23,808 | |
| Total assets | | 181,592 | | | (68,135 | ) | | 113,457 | |
| | | | | | | | | | |
| LIABILITIES | | | | | | | | | |
| Current liabilities | | | | | | | | | |
| Accounts payable and accrued liabilities | | 35,171 | | | 4,327 | | | 39,498 | |
| Current portion of lease liability | | - | | | 9,675 | | | 9,675 | |
| | | 35,171 | | | 14,002 | | | 49,173 | |
| | | | | | | | | | |
| Due to shareholders | | 142,599 | | | (33,805 | ) | | 108,794 | |
| Lease liability | | - | | | 32,155 | | | 32,155 | |
| Total liabilities | | 177,770 | | | 12,352 | | | 190,122 | |
| | | | | | | | | | |
| EQUITY | | | | | | | | | |
| Share capital | | 1 | | | - | | | 1 | |
| Retained earnings (deficit) | | 3,821 | | | (80,487 | ) | | (76,666 | ) |
| Total equity | | 3,822 | | | (80,487 | ) | | (76,665 | ) |
| | | | | | | | | | |
| Total liabilities and equity | | 181,592 | | | (68,135 | ) | | 113,457 | |
NOTE 6 - PHYTO BRANDCO RECONCILIATION TO YEAR ENDED DECEMBER 31, 2020 (continued)
Phyto BrandCo has a September 30 year end. Reconciliation of the Phyto BrandCo results from the nine months ended September 30, 2020 to the year ended December 31, 2020 is as follows:
| | | Phyto BrandCo
year ended
September 30,
2020 | | | Less Oct 1, 2019
to Dec 31, 2019 | | | Add Oct 1, 2020
to Dec 31, 2020 | | | Phyto BrandCo
year ended
December 31,
2020 | |
| | | $ | | | $ | | | | | | $ | |
| | | | | | | | | | | | | |
| Revenue | | 212,654 | | | - | | | - | | | 212,654 | |
| Cost of sales | | - | | | - | | | - | | | - | |
| Gross profit | | 212,654 | | | - | | | - | | | 212,654 | |
| | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | |
| Advertising and promotion | | 104,482 | | | - | | | 36,365 | | | 140,847 | |
| Amortization | | 1,788 | | | 279 | | | 669 | | | 2,736 | |
| Depreciation | | 583 | | | - | | | 3,919 | | | 4,502 | |
| Delivery and freight | | 756 | | | - | | | - | | | 756 | |
| Insurance | | - | | | - | | | 720 | | | 720 | |
| Office expenses | | 7,401 | | | - | | | 4,824 | | | 12,225 | |
| Meals and entertainment | | 10 | | | - | | | 3,676 | | | 3,686 | |
| Professional fees and consulting | | 78,300 | | | 37,383 | | | 20,826 | | | 136,509 | |
| Supplies | | 7,447 | | | - | | | 1,125 | | | 8,572 | |
| Travel | | 380 | | | - | | | 7,253 | | | 7,633 | |
| Bank charges | | 367 | | | 17 | | | 27 | | | 411 | |
| Income (loss) from operations | | 11,140 | | | (37,679 | ) | | (79,404 | ) | | (105,943 | ) |
| | | | | | | | | | | | | |
| Other income (expense) | | | | | | | | | | | | |
| Finance expense | | - | | | - | | | (1,084 | ) | | (1,084 | ) |
| | | | | | | | | | | | | |
| Net income (loss) and comprehensive income (loss) for the period | | 11,140 | | | (37,679 | ) | | (80,488 | ) | | (107,027 | ) |
NOTE 7 - PRO FORMA SHARE CAPITAL
Share capital as at June 30, 2021 after giving effect to the pro forma adjustments and assumptions discussed in Note 4 is as follows:
| | | Common Shares | | | Amount | |
| | | # | | | $ | |
| Adastra common shares outstanding as at June 30, 2021 | | 43,334,100 | | | 15,882,152 | |
| Phyto BrandCo common shares outstanding as at June 30, 2021 | | 100,000 | | | 1 | |
| Acquisition adjustment - eliminate Phyto BrandCo common shares | | (100,000 | ) | | (1 | ) |
| Common shares issued on closing of the Transaction | | 20,000,000 | | | 24,000,000 | |
| Total Resulting Issuer Shares | | 63,334,100 | | | 39,882,152 | |
NOTE 7 - PRO FORMA SHARE CAPITAL (continued)
Share capital as at December 31, 2020 after giving effect to the pro forma adjustments and assumptions discussed in Note 4 is as follows:
| | | Common Shares | | | Amount | |
| | | # | | | $ | |
| Adastra common shares outstanding as at December 31, 2020 | | 43,334,100 | | | 15,882,152 | |
| Phyto BrandCo common shares outstanding as at December 31, 2020 | | 100,000 | | | 1 | |
| Acquisition adjustment - eliminate Phyto BrandCo common shares | | (100,000 | ) | | (1 | ) |
| Common shares issued on closing of the Transaction | | 20,000,000 | | | 24,000,000 | |
| Total Resulting Issuer Shares | | 63,334,100 | | | 39,882,152 | |
Basic and Diluted Loss per Share
The loss per share stated on the Pro Forma Consolidated Statement of Income (Loss) and Comprehensive Income (Loss) for the periods ended June 30, 2021, and December 31, 2020 have been computed based on the commons shares outstanding on closing of the Transaction.
For purposes of these pro forma consolidated financial statements, all stock options and warrants have been excluded from the diluted weighted average number of shares calculation, as their effect would have been anti-dilutive.
Item 19. Exhibits.
Exhibit
Number |
Description
|
(1) | Articles of Incorporation and Bylaws |
1.1* | Notice of Articles |
1.2* | Articles |
(4) | Material Contracts |
10.1* | Agreement for the purchase of equipment dated May 14, 2019 with United Science, LLC. |
10.2* | Supply Agreement dated March 12, 2020 with CannMart Labs Inc. |
10.3* | Toll Processing Agreement dated April 3, 2020 with Acreage Pharms Ltd. |
10.4* | Toll Processing Agreement dated May 6, 2020 with Muskoka Grown Ltd. |
10.5* | Toll Processing Agreement dated June 1, 2020 with BC Hop Company Ltd. |
10.6* | Agreement for the purchase of equipment dated August 5, 2020 with Evolved Extraction Solutions Ltd. |
10.7* | Supply Agreement dated August 17, 2020 with Pure Sunfarms Corp. |
10.8* | Agreement for the purchase of equipment dated August 5, 2020 with ExtractionTek Sales LLC. |
10.9* | Letter of Engagement dated October 15, 2020 with Proactive Investors Canada. |
10.10* | Toll Processing Agreement dated January 15, 2021 with SynerGenetics Bioscience Inc. |
10.11* | Market Services Agreement dated April 13, 2021 with Hybrid Financial Ltd. |
10.12* | Supply Agreement dated June 9, 2021 with Pure Sunfarms Corp. |
10.13* | Share Purchase Agreement dated August 10, 2021 with Jean Paul Lim and MDC Forbes Inc. |
10.14* | Share Purchase Agreement dated September 15, 2021 with shareholders of 1204581 B.C. Ltd. |
10.15* | Brand Licensing Agreement dated December 4, 2020 with CannMart Labs Inc. |
(8) | Subsidiaries |
8.1 | Adastra Labs Holdings (2019) Ltd. (incorporated in the Province of British Columbia, Canada) Adastra Labs Inc., (incorporated in the Province of British Columbia, Canada) Chemia Analytics Inc. (incorporated in the Province of British Columbia, Canada) 1178562 B.C. Ltd. (incorporated in the Province of British Columbia, Canada) Adastra Brands Inc. (incorporated in the Province of British Columbia, Canada) 1225140 B.C. Ltd. (incorporated in the Province of British Columbia, Canada) 1204581 B.C. Ltd. (incorporated in the Province of British Columbia, Canada) |
(15) | Additional Exhibits |
15.1* | Consent of Davidson & Company LLP for Adastra Holdings Ltd. |
15.2* | Consent of Davidson & Company LLP for 1204581 B.C. Ltd. |
*Previously filed.
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this registration statement on its behalf.
| ADASTRA HOLDINGS LTD. /s/ Michael Forbes |
| Name: Michael Forbes Title: Chief Executive Officer and Director Date: March 17, 2022 |










