Exhibit 10.6
Certain confidential information contained in this document, marked by brackets and asterisk, has been
omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K, because it (i) is not material and (ii) would be
competitively harmful if publicly disclosed
Agreement
This Agreement (the “Agreement”) is made and entered into this 30th day of May, 2022 (“Effective Date”) by and between Polyrizon Ltd., Company Number 513637025 located at 5 Hatidhar St. R’annana, Israel. (“Polyrizon”), and SciSparc Ltd., Company No. 513581652 located at 20 Raul Walenberg St., Tel Aviv, Israel (“SciSparc”). (Collectively referred to as “Parties” and each may be referred individually as a “Party”).
| WHEREAS | Polyrizon operates and holds the know-how, inter-alia, in the field of creating formulations for therapeutic delivery Products (“Polyrizon Field”); and |
| WHEREAS | SciSparc operates and holds the know-how, inter-alia, in the field of developing unique cannabinoid technologies for treatment of central nervous system disorders (“SciSparc Field”); and |
| WHEREAS | The Parties wish to enter into this Agreement and to define the terms and conditions for the co-development of a formulation for intranasal application of SCI-160 (HU-433) combined with PEA (endocannabinoid palmitoylethanolamide) for central nervous system (“CNS”) disorders (the “Product”) and the manufacturing, use, commercialization and sales efforts of the Product, all in accordance with and subject to the provisions of this Agreement; |
NOW, THEREFORE, in consideration of the mutual promises and covenants set forth herein, the parties hereby agree as follows:
| 1. | Definitions and interpretation |
In this Agreement:
| 1.1. | Unless otherwise specified, words importing the singular include the plural, words importing any gender include every gender, and words importing persons include bodies corporate and unincorporated; and in each case vice versa. |
| 1.2. | Reference to Clauses and other provisions are references to Clauses and other provisions of this Agreement. |
| 1.3. | The headings shall not affect the interpretation of this Agreement. |
| 1.4. | The expressions “hereunder”, “hereto”, “herein”, “hereof” and similar expressions relate to this entire Agreement and not to any particular provision thereof. |
| 2. | Co-Development and Joint Business Efforts |
| 2.1. | The purpose of this Agreement is to set out the terms and conditions under which the Parties will fully co-develop the Product. As such, the Parties shall use their best efforts to carry out their respective obligations with respect to the co-development activities as set forth in this Agreement and in accordance with the agreed Specifications (as defined below). The Parties shall at all times during the Term of this Agreement cooperate and collaborate in good faith with respect to the co- development activities and implementation of the Development Plan (as defined below) and thereafter with respect to the marketing and sales efforts. |
| 2.2. | As of the execution of this Agreement the Parties have appointed the following representatives to the joint project team (the “Steering Committee”): |
| 2.2.1. | Polyrizon’s representative shall be Polyrizon’s CEO and/or CRO. |
| 2.2.2. | SciSparc’s representative shall be SciSparc’s CEO and/or CTO. |
| 2.3. | The Steering Committee shall manage the joint development activities and will jointly supervise over the business and development processes of the project in order to meet the detailed development plan set out in Annex A to this Agreement (the “Development Plan”). |
| 2.4. | In addition to the above, the Steering Committee shall set out and establish the specifications of the Product and of each Work Order (the “Specifications”). |
| 2.5. | It is hereby agreed that the Development Plan will be executed by both Parties signing detailed work orders which will specify the required tasks and timelines (the “Work Order”). |
| 2.6. | It is hereby agreed that, in the event a Party is required to provide certain information, material or other input to the other Party in order to complete a Work Order and fails to do so, the deadline for such Work Order will be postponed in correspondence with the delay caused by such failing party. |
| 2.7. | Each Party agrees and undertakes to provide its representatives to the Steering Committee with full authority to make any decision required in order to facilitate the advancement of the collaboration. |
| 2.8. | It is hereby agreed that all decisions with regards to the Product will be made unanimously by decision of the parties’ representatives to the Steering Committee, provided however, that the decisions will be subject to each Party’s internal corporate governance requirements and board approvals if applicable. |
| 3. | Information and Reports |
| 3.1. | The Parties will prepare quarterly reports to be provided to the Steering Committee which shall include all development activities and any other relevant activities carried out by each party in furtherance of the joint project, and all relevant information required by the Steering Committee and reasonably necessary in order to ascertain and analyse the status of the joint project. |
| 3.2. | In addition, each Party shall be entitled to receive any information required by such Party, in connection with this Agreement and the Product, from the Steering Committee. |
| 4. | Costs and Expenses; Royalties. |
| 4.1. | The Parties hereby agree that SciSparc shall bear all costs and expenses incurred in the development and establishment of the joint business activities and in the co-development of the Product. |
| 4.2. | SciSparc shall pay Polyrizon the agreed upon amount set out in each signed Work Order. |
| 4.3. | Development Milestone Fee. |
| 4.3.1. | SciSparc shall pay to Polyrizon a total of up to two million and five hundred and fifty thousand dollars ($2,550,000.00) (the “Development Milestone Fee”) in consideration for and conditioned upon the completion of the milestones as set forth below (“Milestone”). Polyrizon will issue to SciSparc an invoice for the portion of the Development Milestone Fee then due and SciSparc shall pay the Development Milestone Fee in accordance with the following: |
| 4.3.1.1. | First Milestone – Preclinical safety studies. SciSparc shall preform preclinical safety tests on the Product. Upon a successful preclinical safety test SciSparc shall pay Polyrizon an amount of US$ 80,000. |
| 4.3.1.2. | Second Milestone – Phase I Clinical trial. SciSparc shall preform preclinical safety tests on the Product. Upon a successful preclinical safety test SciSparc shall pay Polyrizon an amount of US$ 120,000. |
| 4.3.1.3. | Third Milestone – Phase IIa Clinical Trial. SciSparc shall preform phase IIa clinical trials on the Product. Upon completion of phase IIa clinical trial SciSparc shall pay Polyrizon an amount of US$ 150,000. |
| 4.3.1.4. | Fourth Milestone – Phase IIb Clinical Trial. SciSparc shall preform phase IIb clinical trials on the Product Upon completion of phase IIb clinical trial SciSparc shall pay Polyrizon an amount of US$ 200,000 |
| 4.3.1.5. | Fifth Milestone – Phase III Clinical Trial. SciSparc shall preform phase III clinical trials on the Product Upon completion of phase III clinical trial SciSparc shall pay Polyrizon an amount of US$ 500,000. |
| 4.3.1.6. | Sixth Milestone – Regulatory Approval in U.S. Upon receipt of the approval of the FDA SciSparc shall pay Polyrizon an amount of US$ 750,000. |
| 4.3.1.7. | Seventh Milestone – Regulatory Approval in the EU. Upon receipt of the approval of the relevant EU regulatory body SciSparc shall pay Polyrizon an amount of US$ 750,000. |
It is agreed that SciSparc shall notify Polyrizon within three business days as of the completion of each Milestone that is carried out by SciSparc. In addition, it is hereby agreed that the Steering Committee shall provide updates to Polyrizon as to the progress of each Milestone that is being carried out by SciSparc.
Upon the completion of each Milestone: (i) SciSparc shall pay to Polyrizon the applicable Development Milestone Fee as set out in Section 4.3.1-4.3.7 within 14 days as of SciSparc’s notice of completing each applicable Milestone, unless the fee for such Milestone is to be converted according to the provisions of Section 4.7.2 below. And (ii) Polyrizon shall transfer to SciSparc the Work Product (as defined below) developed created or made during the applicable Milestone.
| 4.4. | License Fee-Royalties. In addition to the Development Milestone Fee, and in consideration of the license rights granted to SciSparc as set forth under section 6 herein, SciSparc shall pay to Polyrizon the following royalties (the “Royalties”): |
| 4.4.1. | For sales of the Product by SciSparc, SciSparc shall pay Polyrizon an amount equal to 3.25% of the Net Income actually received by SciSparc. |
| 4.4.2. | For sales of the Product by a sublicensee of SciSparc, SciSparc shall pay Polyrizon an amount equal to 35% of the income actually received by SciSparc from the sublicensee. |
For the purpose of this Agreement the Term “Net Income” means the profit actually earned by SciSparc from the sale of the product less the expenses paid in the process of selling the Product. For the purpose of determining the Net Income under this Agreement the Parties shall agree in good faith the type of expenses that shall be taken into account. . For avoidance of doubt it is hereby agreed that salaries of employees of SciSparc and on-going payments to sales advisors shall not be considered expenses to be deducted.
| 4.5. | SciSparc shall pay such Royalties to Polyrizon within 14 days as of the end of each calendar quarter with respect to Net Income generated in the previous quarter. SciSparc shall keep and maintain, and Polyrizon shall have the right to access and audit, such books and records as reasonably necessary to confirm SciSparc’s Net Income and performance of its payment obligations under this Section 4. |
| 4.6. | Sole Consideration. For the avoidance of doubt, and notwithstanding anything to the contrary, it is explicitly agreed that the Development Milestone Fee and the Royalties set forth herein are the only and exclusive payment due to Polyrizon in consideration for – respectively, the development hereunder, and the license rights granted to SciSparc under this Agreement (both to the Jointly Owend Ip and the Pre-Existing IP of Polyrizon, as further defined below); and that other than the payment set out herein, no other payments or consideration whatsoever shall be due or payable to Polyrizon. |
| 4.7.1. | All amounts payable under this Agreement shall be made by bank-wire transfer in immediately available funds to an account that Polyrizon designates, or such other reasonable method as the Parties may mutually agree upon from time to time. Any payments or portions of payments due under this Agreement that are not paid by the date such payments are due under this Agreement shall bear interest equal to the greater of (a) five percent (5%) per annum, or (b) the maximum rate permitted by law, pro rated to reflect the number of days such payment is delayed. |
| 4.7.2. | Notwithstanding the foregoing in Section 4.7.1, The Parties Agree that the Development Milestone Fee set out in Section 4.3 above may be converted into shares of SciSparc subject to SciSparc receiving the required corporate approvals in advance, and to the consent and written approval executed by both Parties hereto. The Development Milestone Fee eligible for conversion will not exceed the aggregate amount of US$ 350,000. The conversion price will be the average quoted price per share on the month preceding the conversion. |
| 5. | IP; Joint Intellectual Property |
| 5.1. | In this Agreement the following capitalized terms have the meanings set forth below: |
“Intellectual Property” means all intellectual, moral, industrial and/or proprietary property and rights now or hereafter recognized under any applicable law or in equity anywhere in the world, whether issued or pending, registered or unregistered, patentable or unpatentable, including, but not limited to (i) all forms of patents and utility models; (ii) inventions, discoveries, trade secrets and other proprietary rights, including ideas, formulas, compositions (whether patentable or unpatentable and whether or not reduced to practice), know-how, manufacturing and production processes and techniques, research and development information, drawings, specifications, designs, plans, technical data, rights in algorithms, binary codes, business methods, concepts, customers, distributers and suppliers lists, ideas; (vi) computer software (source and object code), modules, libraries, code, or other components, and documentation for the foregoing; (iii) rights associated with works of authorship, including but not limited to copyrights and mask works; (iv) trademarks and service marks, trade names, domain name registration; (v) designs (whether or not capable of registration), design rights; (vi) database rights; (vii) trade secrets and know how; (viii) all rights to confidential or proprietary information; (ix) use cases and business models; and with respect to the intellectual property included in paragraphs (i) to and including (ix) above - any rights analogous to those mentioned herein; all derivative works thereof, any other intellectual property rights, moral rights or industrial property rights not otherwise set forth in (i) through (ix) above; and any current or future applications, renewals, extensions, provisionals, continuations, continuations-in-part, divisions, re-exams and reissues thereof; the right to apply to any of the above; and all of the tangible embodiments thereof.
“Intellectual Property Rights” means all rights, title and interest in and to any Intellectual Property.
“Pre-Existing IP” means any Intellectual Property of a Party existing on the Effective Date, or that is developed or made outside the scope of this Agreement, and without breaching any of the provisions herein and any modifications, derivatives, enhancements or changes thereto subject to the Jointly Owned IP as defined below). For the avoidance of doubt, Polyrizon’s Intellectual Property in the Polyrizon Field is Polyrizon’s Pre-Existing IP; and SciSparc’s Intellectual Property in the SciSparc Field is SciSparc’s Pre-Existing IP.
“Work Product” means all results and outcomes of the project carried out by the Parties under this Agreement and shall include, but not be limited to, software including software source code and object code, and derivative works, inventions, discoveries, improvements, designs, concepts, techniques, methods, Products, content, processes, derivative works, domain names, formulae, specifications, know how, computer software programs, databases, mask works, logos and trade secrets, whether or not patentable, copyrightable or protectable as trade secrets, as well as business plans, file layouts, and manufacturing information. The Steering Committee shall prepare and maintain adequate and current records of all Work Product, created in the course of performance hereunder, and all technical manuals and documentation, created conceived, reduced to practice, authored, developed or delivered by the Parties or their employees, agents, consultants, contractors and representatives including without limitation any third party service providers approved by both Parties, either solely or jointly with others, during and in connection with the performance of this Agreement and co-development of the Product, which records shall be and remain the joint property of both Parties.
| 5.2. | Each Party shall solely own and shall retain all Intellectual property Rights in its Pre-Existing IP. |
| 5.3. | All Intellectual Property jointly made, developed, conceived, first reduced to practice, fixed in any tangible medium of expression or created by the Parties, during the term of this Agreement and in connection with the co-development of the Product (“Joint Intellectual Property”), as well as any patentable invention resulting from any portion of the Work Product hereunder (“Resulting Patent”) will be the joint property of and the entire right, title and interest is hereby assigned jointly to both Parties, and neither Party may enjoy all rights and privileges or ownership of such joint property without the consent of, or accounting to the other (the Joint Intellectual Property and the Resulting Patent shall be referred separately and collectively, “Jointly Owned IP”). |
| 5.4. | The Parties shall jointly agree on appropriate ways to protect the Joint Intellectual Property over the Work Product of the joint project, including by applying for and enforcing patents, copyrights, mask work rights, and other legal protections for the Work Product in any and all countries. The Parties shall jointly appoint a team that will carry out all activities necessary for the legal protection of their joint rights to the Product and to any Work Product, on behalf of the Parties and execute and file any document needed to for securing any patent, copyright, trademark, trade secret, any applications regarding same or any other right or protection relating to any proprietary information (including Work Product), and to do all other lawfully permitted acts to further the prosecution and issuance of patents, copyrights, trademarks, trade secrets or any other right or protection relating to any proprietary information (including Work Product). |
Party.
| 5.5. | For avoidance of doubt, it is hereby clarified that both Parties will only be entitled to enjoy the Jointly Owned IP in equal parts, and other than the license rights granted hereunder by Polyrizon to SciSparc and other than for the purposes of this Agreement, no Party shall enjoy the Jointly Owned IP rights without the consent of the other Party nor derive any income or profit from the Jointly Owned IP rights without ensuring that the other Party receives its share of such profit or income as shall be agreed between the Parties in advance. |
| 5.6. | The Parties hereby agree that they will not use the other Party’s Pre-Existing IP other than in connection with the Product and/or as licensed under this Agreement, for any purpose without the prior written consent of the other Party. |
| 6. | IP and Product Licenses |
| 6.1. | General. Except as set forth in this Section 6, neither Party grants to the other Party any rights or licenses under its Pre-Existing IP or Intellectual Property Rights. |
| 6.2. | Limited License Granted by Polyrizon. |
| 6.2.1. | Grant of License. Subject to the terms of this Agreement, Polyrizon grants to SciSparc |
| (i) | an exclusive worldwide, nontransferable, perpetual, irrevocable, royalty bearing (under Section 4 above), right and license, under Polyrizon’s rights in the Jointly Owned IP, for, and in conjunction with the Product, and as required to develop, use, make, manufacture commercialize, and to otherwise dispose of the Product without restriction as SciSparc may deem fit; and |
| (ii) | a non-exclusive, worldwide, nontransferable, perpetual, irrevocable, royalty bearing (under Section 4 above), right and license, under Polyrizon’s Pre-Exiting IP incorporated in, or on the basis of, or otherwise required for SciSparc to fully effect its license under subsection (i) above, in conjunction with the Product, the Jointly Owned IP and the Work Product/s. |
| 6.2.2. | The licenses above shall include without limitation the following licenses: |
| (i) | To make, have made, use, offer for sale, sell, import and export the Product; and |
| (ii) | To use, copy, modify, create derivative works of the Product, and any other Work Product as needed to fully effect the right granted above. |
| 6.2.3. | Sublicenses. SciScparc may sublicense the rights in the Product, which shall not thereafter be sublicensable (for the purpose of clarification, a sublicensee of SciSparc will not be able to sublicense the license under this Agreement other than with Polyrizon’s consent); provided however, that to the extent the rights in the Product include a Resulting Patent - a subsequent sublicense by the sublicensee is permitted and shall not be subject to the prior consent of Polyrizon.In the event of sublicensing by SciSparc in accordance with this Section 6, SciSparc shall be fully responsible and liable for the protection of the rights under the sub-license and shall ensure that any such sub-licensee executes a license agreement which includes terms and conditions at least as restrictive as this Agreement for the purpose of protecting the rights being licensed under this Agreement and the Intellectual Property Rights of the Parties. |
If a Party grants a sublicense of the license under this Section 6, such Party shall cause its sublicensee to comply with all obligations of such Party hereunder; provided that such Party shall at all times be fully responsible for the performance of such sublicensee.
| 7. | Non– Compete and Non-Circumvention |
| 7.1. | Unless it has obtained the prior written consent of the other party, each party hereby undertakes, either alone or jointly, with, through or on behalf of any person, and/or entity directly or indirectly, not to solicit or contact with a view of engagement or employment by any person, any employee, officer or manager of the other Party or any person and/or entity who has been an employee, officer, manager, consultant, of the other Party within the previous two-year period. |
In addition, during the term of this Agreement and for a period of 12 months thereafter, Polyrizon shall refrain from engaging in the development of formulation/s in the field of Cannabinoid Prophylaxis treatment for the prevention of acute postoperative pain, independently for itself, or with or for others.
| 7.2. | Each of these restrictions is an entirely separate and independent restriction on each Party and the validity of one restriction shall not be affected by the validity or unenforceability of another. |
| 7.3. | Each party considers the restrictions in this Section 7 to be reasonable and necessary for the protection of the interests of the joint project. If any such restriction shall be held to be void but would be valid if deleted in part or reduced in application, such restriction shall apply with such deletion or modification as may be necessary to make it valid and enforceable. |
| 7.4. | Unless specifically stated otherwise, the covenants set out in this Section 7 shall continue to apply to the Parties during the term of this Agreement and for a period of 24 months from the date of its termination for any reason whatsoever. The covenants shall be construed during this period by reference to the joint business carried out by the Parties, customers, employees, officers or managers or contracting parties of the Parties as at or during the twelve months period prior to the date of termination. |
| 8.1. | Each of the Parties undertakes to the other that they will not at any time hereafter use or divulge or communicate to any person other than those of their senior executives and those members of their professional advisors who, in each case, need to know any confidential information they may come to know as a result of the performance of this Agreement – such information is defined as confidential (“Confidential Information”) and includes, but is not restricted to, names, contact information, process blueprints, methodologies, proprietary knowledge, know how, information related to the respective business activities of the parties, and/or the joint project, pricing information, sales and marketing information, financial information, business plans, budgets, etc. - and they shall use all reasonable endeavours to prevent the publication or disclosure of any Confidential Information concerning such matters and so that these obligations shall continue to apply during the term of this Agreement and for a period of 5 years thereafter but shall cease to apply to information which shall come into the public domain other than by a breach of this Section 8. |
| 8.2. | No announcement or publicity concerning the terms of this Agreement shall be made or issued by any of the Parties hereto without the prior written approval of the other Party other than as required by Law (including without limitation the U.S. securities laws) or by the rules of any regulatory organisation to which any of the Parties hereto is subject (in which case the Parties shall consult with each other on the form of the announcement if possible under time and legal constraints). |
| 8.3. | Both Parties consider the restrictions comprised in this clause 8 to be reasonable and the provisions herein shall remain in force and be fully applicable in all circumstances in accordance with its terms and in particular shall not be discharged or affected by any breach or repudiation of this Agreement in each case whatever its nature or howsoever caused or arising or by any other matter, circumstance, or thing whatsoever. |
| 9.1. | This Agreement shall be in effect for an unlimited period of time until terminated by either Party in accordance with the provisions of this Section 9 (the “Term”): |
| 9.2. | This Agreement may be terminated at any time by either party upon delivering to the other Party a prior written notice of at least 60 days, provided however, that in the event Polyrizon terminates this Agreement after a Work Order is executed, Polyrizon shall return any fees and expenses actually paid to it by SciSparc in connection with such Work Order and such repayment shall bear simple interest equal to five percent (5%) per annum, and in the event SciSparc terminates this Agreement, that SciSparc pays for any executed Work Order. |
| 9.3. | This Agreement may be terminated by written notice of at least 7 days as a result of a breach of the provisions of this Agreement committed by a Party which was not cured within 7 days as of receipt of notice from the non-breaching party. |
| 9.4. | Upon any termination of this Agreement, or Work Order, as applicable Polyrizon shall transfer to SciSparc all the Work Product developed, created or made in any media and form whatsoever, up to the date of termination. |
| 9.5. | Clauses 5, 6, 7, 8, 9, and 10 shall survive the termination of this Agreement for any reason whatsoever. |
| 10.1. | This Agreement shall be governed and enforced exclusively in accordance with the laws of the state of Israel in all respects including its construction, interpretation and performance and without regard to its conflict of laws rules and policies. It is hereby agreed that in the event of a dispute arising in connection with this Agreement the parties shall first try to resolve the dispute by a meeting and a discussion between the CEOs of both parties. If the parties fail to amicably resolve the dispute within 14 days of commencement of such discussions, each party may refer the dispute to the authorized courts of Tel Aviv, Israel, which shall have exclusive jurisdiction over all disputes arising in connection with this Agreement. |
| 10.2. | This Agreement supersedes all prior discussions, agreements, and writings and constitutes the entire agreement between the Parties with respect to the subject matter hereof. |
| 10.3. | No waiver or modification of this Agreement will be binding upon any party unless made in writing and signed by a duly authorized representative of such party and no failure or delay in enforcing any right will be deemed a waiver. |
| 10.4. | None of the Parties hereto may assign its rights or obligations under this Agreement, in whole or in part, without the prior written consent of the other Party hereto, provided however that such consent shall not be required in the event of an assignment to a successor in interest in connection with the merger, acquisition, reorganisation or the sale of all or substantially all of the assets of a party. |
| 10.5. | In no event shall either party be liable to the other Party for any consequential, incidental, special or indirect damages, however caused, arising out of or in connection with this Agreement, or of any other obligations relating to this Agreement, whether or not the Party has been advised of the possibility of such damages, unless such damages are a result of wilful misconduct or gross negligence of the damaging party. |
| 10.6. | Nothing in this Agreement shall constitute or be deemed to constitute a partnership or principal and agent relationship between the Parties hereto and neither of them shall have any authority to bind the other in any way. |
| 10.7. | Any right or remedy of the Parties in respect of a breach of any provision of this Agreement shall be in addition and without prejudice to all other rights and remedies of the Parties, and no failure to exercise or delay in exercising or enforcing any right or remedy shall operate to impair or constitute a waiver by that Party of that or any of its other rights or remedies and no single or partial exercise or enforcement of any such right or remedy shall preclude or restrict any other or further exercise or enforcement of any such right or remedy. |
| 10.8. | If any of the provisions of this Agreement is held to be invalid, illegal or unenforceable in any respect under any law, the validity, legality and enforceability of the remainder of this Agreement shall not be affected. |
| 10.9. | A notice given under this Agreement shall be in writing in the English language and shall be sent for the attention of the party, and to the address, electronic address or fax number, given in the preamble to this Agreement and/or notified in writing to the other party, and shall be delivered personally, sent by fax, sent by pre-paid first-class registered post, sent by registered airmail or sent by electronic mail. |
| 10.10. | A notice is deemed to have been received if delivered personally, at the time of delivery, in the case of fax or electronic mail, on the next business day following the day of transmission, in the case of pre-paid first class post, special delivery or registered post, 5 days from the date of posting, in the case of registered airmail, five days from the date of posting. |
In Witness Whereof, the Parties have caused this Agreement to be executed and become effective on the Effective Date.
| /s/ Tomer Izraeli | | Adi Zuloff-Shani |
| Polyrizon Ltd. | | SciSparc Ltd. |
| | | |
| By: | Tomer Izraeli, CEO | | By: | Adi Zuloff-Shani |
Annex A
Development Plan
Nasal spray
Formulation development and technical batch preparation in view of pre-clinical and clinical studies
POLYRIZON LTD
Send to: SciSparc Ltd.
Contact person: Dr. Adi Zuloff-Shani
Email: Adi@scisparc.com
May 22nd, 2022
Work plan proposal
Background:
| 1. | Trap & Target drug delivery technology for intranasal administration |
The nasal cavity is a large, air-filled space above and behind the nose in the middle of the face. Each cavity is the continuation of one of the two nostrils. The nasal cavity is the uppermost part of the respiratory system and provides the nasal passage for inhaled air from the nostrils to the nasopharynx and rest of the respiratory tract. The nasal mucosa, also called respiratory mucosa, lines the entire nasal cavity, from the nostrils to the pharynx. A dynamic layer of mucus overlies the nasal epithelium (the outermost layer of cells of the nasal mucosa).
The nasal sub-mucosa underlies the basement membrane. This layer is made up of glands which secrete watery substances and mucus, nerves, an extensive network of blood vessels and cellular elements like blood plasma. The entire mucosa is highly concentrated with blood vessels and contains large venous-like spaces.
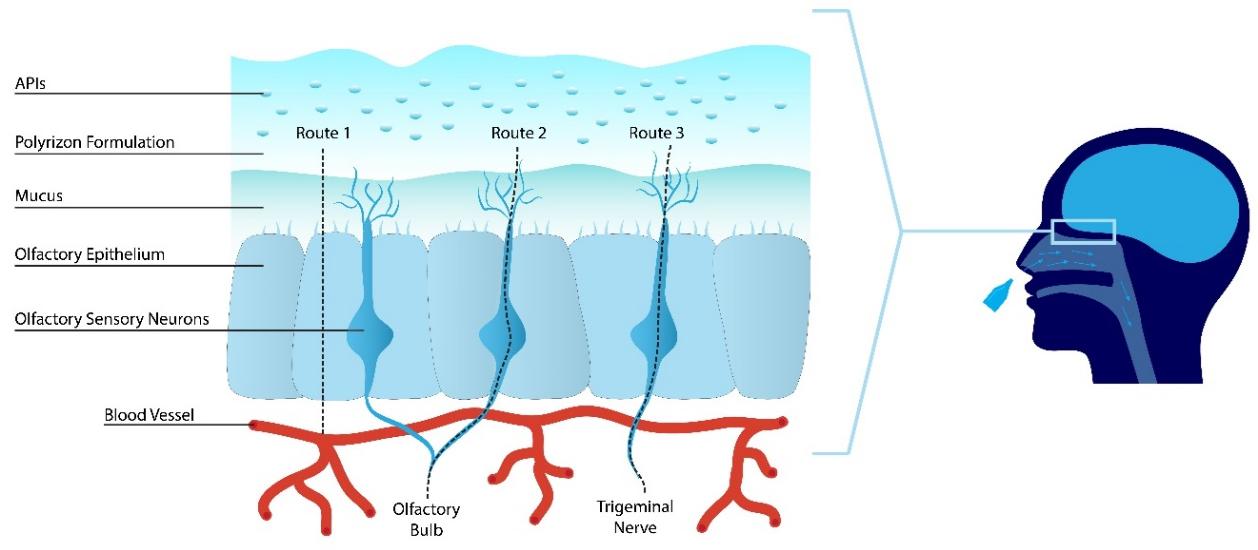
Figure 1: Schematic illustrations of the mucosal tissue (left) and nasal cavity anatomy (right).
The term ‘mucoadhesion’ refers to the adhesion of specific polymers to the surface of the mucosal layer. The mucosal layer is made up of mucus, a viscoelastic fluid, which is secreted by the epithelial cells. A mucoadhesion polymer helps to promote the adhering of a given formulation to the nasal mucosa by physically interacting with the mucosa. Various properties impact the mucoadhesive of polymers, such as: (i) molecular weight; (ii) chain length; (iii) viscosity; (iv) degree of cross-linking; (v) spatial conformation; (vi) flexibility of polymer chains; (vii) concentration; (viii) charge and degree of ionization – anion>cation>non-ionic; (ix) degree of hydration; and (x) pH.
The mechanism of mucoadhesion is characterized by to two steps: contact stage and consolidation stage. The first contact stage is characterized by the initial contact between the polymers and the mucous membrane, with spreading and initiating a deep contact with the mucus layer. In the second consolidation step, the polymers are activated by the presence of moisture and as they hydrate they become mucoadhesive.
Advantages of intranasal drugs delivery
The nasal cavity is an important target for local and systemic drug administration as well as targeting the central nervous system. Due to highly vascularization of the nasal mucosa, liquids or particles that attach to this surface can act either locally or be absorbed into the bloodstream. In addition, the first cranial nerve, or olfactory nerve, is the only point where the central nervous system is exposed to the body’s mucosa, and it is one of six nerves that branch into the nose cavity. This means that medications can be absorbed directly into the brain, bypassing the blood-brain barrier.
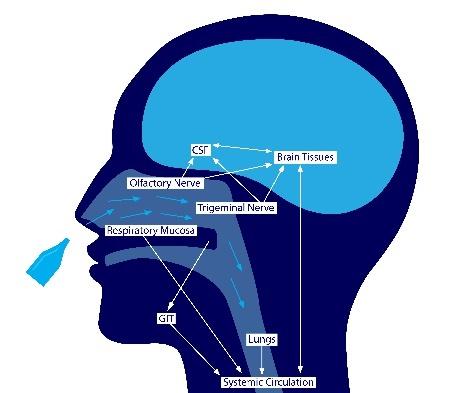
Figure 2: Intranasal drug delivery and potential uptake routes
Although there are many advantages for delivering medicines intranasally, there are also a few drawbacks, such as quick evacuation from the nasal canal, limited bioavailability, and difficulty getting a big enough dosage due to the limited absorption area. Our T&T technology is developed to address the abovementioned drawbacks to further improve the efficiency of intranasal administration.
The T&T platform delivery technology consist of a mucoadhesive polymers mixture designed to allow a long residence time and an intimate contact with the mucosal tissue for potentially improved delivery of medicines. The T&T platform can be tailored for different molecules to address their specific challenges thus believed to induce improved therapeutic effect. The T&T technology has been designed to enable mucoadhesion and prolonged retention at the deposition site by tailoring the physicochemical properties through composition, concentration and crosslinking of the key polymers of the formulation appears pivotal for the potential development as nasal medicinal product candidates.
SciSparc Ltd is specialty pharmaceutical company developing unique cannabinoid technologies for treatment of central nervous system (CNS) disorders.
Following small animal proof of concept, SciSparc wishes to develop a formulation to potentially improve the intranasal drug delivery.
The main objective of this project is to investigate the feasibility of developing a formulation for nasal drug delivery of two APIs, palmitoylethanolamide (PEA) an HU-433, a proprietary synthetic molecule, both poorly soluble lipophilic molecules (Figure 1).
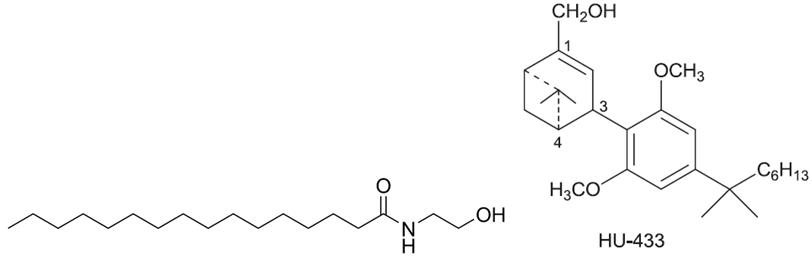
Figure 1. The structure of palmioyethanolamide and HU-433
| APIs / Drug Substance (Combo) | HU-433 - (proprietary synthetic endocannabinoid) Palmitoylethanolamide (PEA) Both solids (powder) |
| Dosage form | Nasal solution (non-sterile) Assuming low viscosity |
| [***] | [***] |
| Primary packaging | TBD if relevant |
| Storage | 15-25°C (TBD stability for HU-433) |
| Development phase | Pre-clinical |
| Destination | Israel |
| Safety data | TBD |
| Batch size | TBD if necessary |
| Timelines Beginning of work | Q2-Q3 2022 |
MSDS of active ingredient(s) should be provided by SciSparc. This offer assumes compounds are classified maximum OEB3 and will be safe to handle within the standard safety precautions of Polyrizon Ltd. If not, they will be handled as if it was a highly potent and the current proposal may need to be revised.
All formulation, manufacturing and analytical data at disposal of SciSparc will be shared with Polyrizon – [***].
The sourcing of raw materials (active compounds and excipients, GMP grade to be provided for clinical production) will be discussed between Polyrizon Ltd. and SciSparc (cost for purchase and shipments are not included).
Associated documentation should be provided.
The supply of primary packaging items (vials with spray pump cap) will be discussed between Polyrizon Ltd. and SciSparc (cost for purchase not included). SciSparc remains responsible for the choice of packaging supplier, in case needed.
1st part: [***]:
[***]
1st part deliverables:
[***]
2nd part: [***]:
[***]
2nd part deliverables:
3rd part:[***]:
3rd part deliverables:
4th part: Permeability
[***]
4th part deliverables:
[***]
6th part: Biological efficacy trials
[***]
[***]
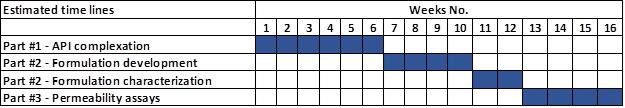
Project estimated timelines:
Starting date: Q2 or Q3 2022, to be confirmed at approval of offer.
Estimated timelines below are indicative only. A planning will be provided at approval of offer. T0 is at receipt of materials from SciSparc (HU-433, PEA and analytical methods).
Financial proposal
Price calculation:
| Program description | Cost Euros
Taxes excluded |
| [***] | [***] |
| [***] | [***] |
| [***] | [***] |
| [***] | [***] |
| [***] | [***] |
| [***] | [***] |
| [***] | [***] |
Payment schedule:
| /s/ Tomer Izraeli | | Adi Zuloff-Shani |
| Polyrizon Ltd. | | SciSparc Ltd. |
| | | |
| By: | Tomer Izraeli, CEO | | By: | Adi Zuloff-Shani |
A-7



