Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
____________________
FORM 20-F
____________________
(Mark One)
| o | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the fiscal year ended December 31, 2023
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
OR
| o | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
Date of event requiring this shell company report
Commission File Number: 001-41421
____________________
ALVOTECH
(Exact name of Registrant as specified in its charter)
____________________
| Not applicable | Grand Duchy of Luxembourg | ||||
| (Translation of Registrant’s name into English) | (Jurisdiction of incorporation or organization) | ||||
9, Rue de Bitbourg,
L-1273 Luxembourg,
Grand Duchy of Luxembourg
(Address of principal executive offices)
Robert Wessman
Sæmundargata 15-19, 102
Reykjavík, Iceland
+354 422 4500
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
| Ordinary Shares | ALVO | Nasdaq Stock Market LLC | ||||||||||||
| Warrants | ALVOW | Nasdaq Stock Market LLC | ||||||||||||
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
____________________
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual company report: 266,821,844 Ordinary Shares and 10,363,094 Warrants to purchase Ordinary Shares.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | o | Accelerated filer | x | Non-accelerated filer | o | ||||||||||||
| Emerging growth company | x | ||||||||||||||||
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. o
†The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
US GAAP o | International Financial Reporting Standards as issued | Other o | |||||||||
by the International Accounting Standards Board ® | x | ||||||||||
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow. Item17 o Item18 o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
Table of Contents
TABLE OF CONTENTS
B. | |||||||||||
i
Table of Contents
ii
Table of Contents
GENERAL INFORMATION
Unless context otherwise requires, all references in this Annual Report on Form 20-F (“Annual Report”) to “Alvotech,” the “Company,” “we,” “us” and “our” refer to Alvotech and, where appropriate, its consolidated subsidiaries.
This Annual Report includes trademarks, tradenames and service marks, certain of which belong to us and others that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this Annual Report appear without the ®, ™ and SM symbols, but the absence of those symbols is not intended to indicate, in any way, that we will not assert our rights or that the applicable owner will not assert its rights to these trademarks, tradenames and service marks to the fullest extent under applicable law. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 20-F (including information incorporated by reference herein, the “Annual Report”)
contains or may contain forward-looking statements as defined in Section 27A of the Securities Act of 1933, as amended
(the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that
involve significant risks and uncertainties. All statements other than statements of historical facts are forward-looking
statements. These forward-looking statements include information about our possible or assumed future results of
operations or our performance. Words such as “may,” “might,” “will,” “could,” “would,” “should,” “expects,” “intends,”
“plans,” “believes,” “anticipates,” “estimates,” “potential,” “continue,” “ongoing,” “targets”, “possible,” “project,” and
“predict” and variations of such words and similar expressions are intended to identify the forward-looking statements.
Unless otherwise stated or unless the context otherwise requires, references to “Alvotech” or the “Company” are to the
registrant named “Alvotech”, previously known as Alvotech Lux Holdings S.A.S. and its subsidiaries after the
consummation of the business combination between Alvotech Holdings S.A., Oaktree Acquisition Corp. II and Alvotech
(the “Business Combination”), whereas references to “Alvotech Holdings” are to Alvotech Holdings S.A. and its
subsidiaries prior to the consummation of the Business Combination (the “Closing”) on 15 June 2022 (the “Closing
Date”). Forward-looking statements in this Annual Report may include, for example, statements about:
•Development and projections relating to our competitors and industry, including the estimated growth of the industry;
•The timing of, and our ability to obtain and maintain regulatory approval for our product candidates of the U.S. Food and Drug Administration (the “FDA”), European Commission and comparable national or regional authorities;
•The timing of the announcement of clinical study results, the commencement of patient studies, regulatory applications, approvals and market launches;
•Our expectations regarding regulatory review and interactions, including the timing and results of the facility inspection by the FDA or other foreign regulatory authorities;
•Our financial performance;
•Changes in our strategy, future operations, financial position, estimated revenues and losses, projected costs, prospects and plans;
•Our strategic advantages and the impact those advantages will have on future financial and operational results;
•Our expansion plans and opportunities,
•Our ability to grow our business in a cost-effective manner;
•The implementation, market acceptance and success of our business model;
•Developments and projections relating to our competitors and industry, including the estimated growth of the industry;
•Our approach and goals with respect to technology;
•Our expectations regarding our ability to obtain and maintain intellectual property protection and not infringe on the rights of others;
•Changes in applicable laws or regulations;
1
Table of Contents
•The outcome of any known and unknown litigation and regulatory proceedings;
•Our ability to maintain the listing of our ordinary shares, with a nominal value of $0.01 per share (“Ordinary Shares ”), or warrants (the “Warrants”) on The Nasdaq Stock Market LLC (“Nasdaq”) and the Nasdaq Main Market in Iceland (“Nasdaq Iceland Main Market”);
•Our ability to comply with all applicable laws and regulations;
•Our ability to successfully launch our products in certain markets after obtaining regulatory approval for such market;
•Our estimates of expenses and profitability;
•Our ability to raise additional adequate funds through equity or debt financing;
•Our ability to identify and successfully develop new product candidates;
•Our relationship with third party providers for clinical and non-clinical studies, supplies, and manufacturing of our products;
•Our ability to manage our manufacturing risks;
•The impact of worsening or unpredictable macroeconomic conditions, including rising inflation, interest rates and cost of energy, and general market conditions, global geopolitical tension, including regions affected by Russia's invasion of Ukraine and conflicts in the Middle East, or public health emergencies, on the business, financial position, strategy and anticipated milestones; and
•Our relationship with partners for the commercialization of our product candidates.
These forward-looking statements are based on information available as of the date of this Annual Report, and current expectations, forecasts and assumptions, and involve a number of judgments, risks and uncertainties. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date, and we do not undertake any obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
You should not place undue reliance on these forward-looking statements in deciding to invest in our securities. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include:
•The outcome of any legal proceedings that may be instituted against us or others following the Business Combination;
•The outcome of any legal or regulatory proceedings;
•The ability to raise substantial additional funding, which may not be available on acceptable terms or at all;
•The ability to maintain the listing of Ordinary Shares on The Nasdaq Stock Market LLC and Nasdaq Iceland Main Market;
•Changes in applicable laws or regulations;
•The effects of public health emergencies on our business;
•The inherent uncertainty of projected financial information with respect to us, and the possibility that the assumption underlying such projects ultimately prove incorrect;
•The effects of competition on our future business;
•Our position in the market against current and future competitors;
•Our expansion into new products, services, technologies or geographic regions;
•Our ability to implement business plans, forecasts and other expectations, and identify and realize additional opportunities and to continue as a going concern;
•The risk of downturns and the possibility of rapid change in the highly competitive industry in which we operate;
2
Table of Contents
•The risk that we and our current and future commercial partners are unable to successfully develop, seek regulatory approval for, and commercialize our products or services, or experience significant delays in doing so;
•The risk that we may never achieve or sustain profitability;
•The risk that we may need to raise additional capital to execute our business plan, which may not be available on acceptable terms or at all;
•The risk that we experience difficulties in managing our growth and expanding operations;
•The risk that we have identified a material weakness in our internal control over financial reporting which, if not corrected, could affect the reliability of our financial statements;
•The risk that we are unable to secure or protect our intellectual property;
•The risk that estimated growth of the industry does not occur, or does not occur at the rates or timing we have assumed based on third-party estimates and on our own internal analyses; and
•The possibility that we may be adversely affected by other economic, business, and/or competitive factors.
You should refer to the section titled “Item 3.D Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Annual Report.
3
Table of Contents
PART I
Item 1. Identity of Directors, Senior Management and Advisers.
Not applicable.
Item 2. Offer Statistics and Expected Timetable.
Not applicable.
Item 3. Key Information.
A.[Reserved]
B.Capitalization and indebtedness.
Not applicable.
C.Reasons for the offer and use of proceeds.
Not applicable.
D. Risk factors.
An investment in our securities carries a significant degree of risk. In addition to the other information contained in this Annual Report on Form 20-F, including the matters addressed under the heading “Forward-Looking Statements,” you should carefully consider the following risk factors in deciding whether to invest in our securities. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances, may have a material adverse effect relating to our business, financial condition, and results of operations and future prospects, in which event the market price of our securities could decline, and you could lose part or all of your investment. Additional risks and uncertainties of which we are not presently aware or that we currently deem immaterial could also affect our business operations and financial condition.
Summary Risk Factors
Our business is subject to a number of risks and uncertainties. If any of the following risks are realized, our business, financial condition and results of operations could be materially and adversely affected. You should carefully review and consider the full discussion of our risk factors in this section titled “Risk Factors” in Part I, Item 3.D. of this Annual Report. Set forth below is a summary list of the principal risk factors as of the date of the filing of this Annual Report:
•We have a limited operating history in a highly regulated environment on which to assess our business, have incurred significant losses since inception and anticipate that we may continue to incur significant losses for the immediate future.
•We have substantial indebtedness and expect to continue to use leverage in executing our business strategy, which could have important consequences on our business and adversely affect the return on our assets.
•We may need to raise substantial additional funding from shareholders or third parties. This additional funding may not be available on acceptable terms or at all. Failure to obtain such necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
•The regulatory approval processes of the FDA, European Commission, Health Canada and comparable other national or regional authorities are lengthy and time consuming and we cannot give any assurance that marketing authorization applications for any of our product candidates will receive regulatory approval.
•Our product candidates may cause unexpected side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following regulatory approval, if granted.
•Our commercial products will remain subject to continuous subsequent regulatory obligations and scrutiny.
•We rely on third parties to conduct our nonclinical and clinical studies, to manufacture aspects of clinical and commercial supplies of our product candidates, and to store critical components of our product candidates. If these third parties do not successfully carry out their contractual duties, or are not compliant with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates.
4
Table of Contents
•We are subject to a multitude of risks related to manufacturing. Any adverse developments affecting the manufacturing operations of our biosimilar products could substantially increase our costs and limit supply for our products, or could affect the approval status of our products.
•Our biosimilar product candidates, if approved, will face significant competition from the reference products, from other biosimilar products that reference the same reference products including those which may have regulatory exclusivities, and from other medicinal products approved for the same indication(s) as the reference products. Our failure to effectively compete may prevent us from achieving significant market penetration and expansion.
•We currently have no marketing and sales organization. We are dependent on our partners for the commercialization of our biosimilar products in certain markets, and their failure to commercialize in those markets could have a material adverse effect on our business and operating results.
•If we, or one of our partners, infringe or are alleged to infringe the intellectual property rights of third parties, our business could be harmed.
•Our recurring losses raise substantial doubt as to our ability to continue as a going concern.
•We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise are unable to develop and maintain an effective system of internal controls in the future, we may not be able to produce timely and accurate financial statements or comply with applicable laws and regulations, which may adversely affect investor confidence in us and, as a result, the value of Ordinary Shares.
•Future issuances of debt securities and equity securities, including by selling shareholders with resales covered by effective shelf registration statements, may adversely affect us, including the market price of our Ordinary Shares and may be dilutive to existing shareholders.
Risks Related to Our Financial Position and Need for Capital
We have a limited operating history in a highly regulated environment on which to assess our business, have incurred significant losses since inception and anticipate that we may continue to incur significant losses for the immediate future.
We are a biotech company with a limited operating history. We have incurred losses in each year since inception in 2013, including losses of $551.7 million, $513.6 million, and $101.5 million for the years ended 31 December 2023, 2022, and 2021, respectively. We had an accumulated deficit of $2,205.8 million as of 31 December 2023.
We have devoted substantially all of our financial resources to identify and develop our product candidates, including conducting, among other things, analytical characterization, process development and manufacture, formulation and clinical studies and providing general and administrative support for these operations. To date, we have financed our operations primarily through the sale of equity securities, debt financing by way of shareholder loans (convertible and non-convertible) and the issuance of bond instruments (convertible and non-convertible) to third party investors and related parties, as well as through milestone payments received under certain license and development agreements with our partners, for example Teva Pharmaceuticals International GmbH (“Teva”), STADA Arzneimittel AG (“STADA”), and Mercury Pharma Group Limited (“Advanz”). The amount of our future net losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings or strategic collaborations. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk.
There can be no guarantee that we will receive regulatory approval for our product candidates in any country. If we obtain regulatory approval to market a biosimilar product candidate, our future revenue will depend upon the therapeutic indications for which approval is granted, the size of any markets in which our product candidates may receive approval and our ability to achieve sufficient market acceptance, pricing, reimbursement from third-party payors and adequate market share for our product candidates in those markets. However, even if one or more of our product candidates gain regulatory approval and is commercialized, we may never become profitable.
We expect to continue to incur significant expenses, which could lead to increasing operating losses for the immediate future. We anticipate that our expenses will increase substantially if and as we:
•prepare for commercial launch of our products which have received approval;
•continue our analytical, nonclinical and clinical development of our product candidates;
•incur costs associated with being a public company;
5
Table of Contents
•expand the scope of our current clinical studies for our product candidates;
•advance our programs into more expensive clinical studies;
•initiate additional analytical, nonclinical, clinical or other studies for our product candidates;
•change or add contract manufacturers, clinical research service providers, testing laboratories, device suppliers, legal service providers or other vendors or suppliers;
•establish a sales and marketing infrastructure;
•seek to identify, assess, acquire and/or develop other biosimilar product candidates or products that may be complementary to our products;
•make upfront, milestone, royalty or other payments under any license agreements;
•seek to create, maintain, protect, expand and enforce our intellectual property portfolio;
•engage legal counsel and technical experts to help evaluate and avoid infringing any valid and enforceable intellectual property rights of third parties;
•engage in litigation including patent litigation with reference product companies or others that may hold patents allegedly infringed by us;
•seek to attract and retain skilled personnel;
•create additional infrastructure to support our operations as a public company and our product development and planned future commercialization efforts; and
•experience any delays or encounters issues with any of the above, including but not limited to failed studies, conflicting results, safety issues, supply chain issues, and other delays, whether or not due to public health emergencies, litigation or regulatory challenges that may require longer follow-up of existing studies, additional major studies or additional supportive studies in order to obtain regulatory approval.
Further, the net losses we incur may fluctuate significantly from quarter-to-quarter and year-to-year such that a period-to-period comparison of our results of operations may not be a good indication of our future performance quarter-to-quarter and year-to-year due to factors including the timing of clinical trials, any litigation that we may file or that may be filed against us, the execution of collaboration, licensing or other agreements, and the timing of any payments we make or receive thereunder.
Our product revenues have been limited and we may never be profitable.
Although we have received upfront payments, milestone and other contingent payments and/or funding for development from some of our collaboration and license agreements, and some product revenue since we launched AVT02 in Canada, Europe and Australia in 2022 and 2023, we generated limited revenue from product sales. Our ability to generate revenue and achieve profitability depends on the ability of our strategic collaboration partners, to successfully commercialize our approved biosimilars and our ability to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, as well as successfully commercialize, our biosimilar candidates. We cannot predict if and when we will begin generating revenue from product sales outside of Canada, Europe and Australia, as this depends heavily on our success in many areas, including but not limited to:
•launching and commercializing product candidates for which we obtain regulatory approval, either directly or with collaboration partners or distributors;
•obtaining adequate third-party payor coverage and reimbursements for our approved products;
•obtaining market acceptance of biosimilar pharmaceuticals as viable treatment options;
•addressing any competing technological and market developments, including the development of new formulations of the originator biologic or new biologics which can be used to treat the indications for approved biosimilars or biosimilar candidates;
•completing analytical, nonclinical and clinical development of our product candidates;
•developing and testing of our product formulations;
•obtaining and retaining regulatory approvals for product candidates for which we complete clinical studies;
•developing a sustainable and scalable manufacturing process for any approved product candidates that is compliant with regulatory manufacturing requirements;
6
Table of Contents
•establishing and maintaining supply and manufacturing relationships with third parties that can conduct the process and provide adequate (in amount and quality) products to support clinical development and the market demand for our product candidates, if approved;
•identifying, assessing and developing (or acquiring/in-licensing) new product candidates;
•negotiating favorable or commercially reasonable terms in any collaboration, licensing or other arrangements into which we may enter;
•maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how;
•attracting, hiring and retaining qualified personnel; and
•the result of potential litigation including patent litigation with reference product companies or others that may allege infringement by us.
Even if product candidates that we develop are approved for commercial sale, we may incur significant costs in order to manufacture and commercialize any such product. Our expenses could increase beyond our expectations if we are required by the FDA, the European Commission, the European Medicines Agency (the "EMA"), other comparable regulatory authorities, domestic or foreign, or by any unfavorable outcomes in intellectual property litigation filed against us, to change our manufacturing processes or assays or to perform clinical, nonclinical, analytical or other types of studies in addition to those that we currently anticipate. In cases where we are successful in obtaining regulatory approvals to market one or more of our product candidates, our revenue will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory approval, the timing of our entry into a particular market or territory, the number of biosimilar competitors in such markets and whether any have regulatory exclusivity, the national laws governing substitution, the accepted price for the product, the ability to get reimbursement at any price, the nature and degree of competition from the reference product and other biosimilar companies (including competition from large pharmaceutical companies entering the biosimilar market that may be able to gain advantages in the sale of biosimilar products based on brand recognition and/or existing relationships with customers and payors), our ability to manufacture sufficient quantities of the product of sufficient quality and at a reasonable cost and whether we own (or has partnered to own) the commercial rights for that territory. If the market for our product candidates (or its share of that market) is not as significant as we expect, the regulatory approval is narrower in scope than we expect (e.g., for a narrow indication or set of indications) or the reasonably accepted population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from sales of such products, even if approved. If we are unable to successfully complete development and obtain regulatory approval for our product candidates in significant markets, our business may suffer. Additionally, if we are not able to generate substantial revenue from the sale of any approved products or the costs necessary to generate revenues increase significantly, we may never become profitable.
Our operating and financial results are subject to concentration risk.
Our operational and financial results are subject to concentration risk. Our success will depend significantly on the development of a limited number of product candidates, their regulatory approval in a limited number of jurisdictions and their commercialization by a limited number of commercial partners. Even if we are successful in developing and commercializing all of these products, our revenue will be dependent on a limited number of products that would account for a significant majority of our revenues. This concentration risk would increase to the extent we are successful in developing and commercializing fewer products as we would be dependent on a lower number of products for the significant majority of our revenues. Unfavorable changes or the non-occurrence of certain anticipated events with respect to any of these limited number of products, jurisdictions or commercial partners may disproportionally affect our global results. As of 31 December 2023, we have only generated product revenue through sales of AVT02 in Canada, Australia and nineteen select European markets since 2022 through certain commercialization partners. See also “—We are dependent on our partners, such as Teva, STADA and Advanz for the commercialization of our biosimilars and biosimilar candidates in certain major markets, and their failure to commercialize in those markets could have a material adverse effect on our revenue, business and operating results.”
We may be unable to generate sufficient cash flow to satisfy our significant debt service obligations, which would adversely affect our financial condition and results of operations.
Our ability to make principal and interest payments on and to refinance our indebtedness will depend on our ability to generate cash in the future. This, to a certain extent, is subject to general economic, financial, competitive, legislative, regulatory and other factors that may be beyond our control. If our business does not generate sufficient cash flow, if currently anticipated costs and revenues are not realized on schedule, in the amounts projected or at all, or if future borrowings are not available to us in amounts sufficient to enable us to pay our indebtedness or to fund our other liquidity
7
Table of Contents
needs, our financial condition and results of operations may be adversely affected. Furthermore, our debt obligations are secured by substantially all of our intellectual property. If we cannot service our debt payments under the senior bonds
issued by Alvotech Holdings on 14 December 2018, as amended and restated on 16 November 2022 (the “Senior Bonds"), the bondholders may take possession, sell, exchange, license or otherwise dispose of our intellectual property, which we have pledged as collateral for the Senior Bonds. If we cannot generate sufficient cash flow to make scheduled principal and interest payments on our debt obligations in the future, we may need to refinance all or a portion of our indebtedness on or before maturity, sell assets, delay capital expenditures or seek additional equity. If we are unable to refinance any of our indebtedness on commercially reasonable terms or at all or to effect any other action relating to our indebtedness on satisfactory terms or at all, we may be forced to reduce or discontinue operations or seek protection of the bankruptcy laws, our business may be harmed and our securityholders may lose some or all of their investment.
We may need to raise substantial additional funding from shareholders or third parties. This additional funding may not be available on acceptable terms or at all. Failure to obtain such necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
Developing our product candidates is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates through clinical studies.
As of 31 December 2023, we had cash and cash equivalents, excluding restricted cash, of $11.2 million. In February 2024, we accepted an offer for the sale of 10,127,132 Ordinary Shares for gross proceeds of approximately $166 million.
However, even with the aforementioned cash as of 31 December 2023 and the proceeds from the February 2024 private placement, management has determined that there is a material uncertainty that may cast significant doubt about our ability to continue as a going concern. The audited consolidated financial statements appearing elsewhere in this Annual Report on Form 20-F have been prepared on a going concern basis without adjustments that might result from the outcome of this uncertainty and the report of our independent registered public accounting firm thereon includes an explanatory paragraph to that effect.
We may require significant additional funding to obtain regulatory approval for, and to successfully commercialize, our product candidates. In addition, our operating plans may change as a result of many factors that are currently unknown to us, and we may need to seek additional funding sooner than planned. Our future funding requirements will depend on many factors, including but not limited to:
•the scope, rate of progress, results and cost of our analytical studies, clinical studies, nonclinical testing and other related activities;
•the cost of manufacturing clinical supplies and establishing commercial supplies, of our product candidates and any products that we may develop;
•the number and characteristics of product candidates that we pursue;
•the cost, timing and outcomes of regulatory approvals;
•the cost and timing of establishing sales, marketing and distribution capabilities and launching our products that have received regulatory approval;
•the terms and timing of any collaborative, licensing and other arrangements that we may establish, including any milestone and royalty payments thereunder;
•the cost, timing and outcomes of any litigation that we may file or that may be filed against us by third parties; and
•the product revenue, if any, derived from our sales of approved products.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us or the possibility of such issuance may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute the share ownership of our existing shareholders. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an
8
Table of Contents
earlier stage than otherwise would be desirable and to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or for specific strategic considerations. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all. In addition, the perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations.
If we are unable to obtain sufficient funding on a timely basis and on acceptable terms and continue as a going concern, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidates or to otherwise reduce or discontinue our operations. In general, we may be unable to expand our operations or otherwise capitalize on business opportunities, and defend against and prosecute litigation necessary to commercialize our product candidates as desired, which could materially affect our business, financial condition and results of operations. If we are ultimately unable to continue as a going concern, we may have to seek the protection of bankruptcy laws or liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, and it is likely that our securityholders will lose all or a part of their investment.
We may not be able to obtain sufficient funding on a timely basis, on acceptable terms, or at all.
Given the recent inflation, interest rates and volatility on the capital markets, we may be unable to raise sufficient funding on a timely basis, on acceptable terms, or at all. Failure to obtain additional funding could have a material adverse effect on our business, prospects and financial condition and may require us to significantly curtail, delay or discontinue one or more of our development programs or the commercialization of any product candidates or to otherwise reduce or discontinue our operations. Even if we were able to obtain additional funding, the terms on which such funding could be obtained may be unfavorable to us and our securityholders, including higher interest rates, which would burden us with higher debt service obligations and may impact our prospects of becoming profitable, a lower share price at which new equity would be issued, which may cause significant dilution to existing shareholders, and/or we may be required to provide additional incentives to potential investors, such as penny warrants, which also may cause significant dilution to existing shareholders. See also “—Future issuances of debt securities and equity securities may adversely affect us, including the market price of our Ordinary Shares and may be dilutive to existing shareholders,” and “—We have substantial indebtedness and expect to continue to use leverage in executing our business strategy, which could have important consequences on our business and adversely affect the return on our assets.”
We have substantial indebtedness and expect to continue to use leverage in executing our business strategy, which could have important consequences on our business and adversely affect the return on our assets.
As of 31 December 2023, we had $960.2 million in outstanding indebtedness, consisting of $549.4 million in Senior Bonds, $155.9 million in Tranche A and Tranche B Convertible Bonds issued by Alvotech on 20 December 2022 (the "2022 Convertible Bonds"), $80.7 million in the convertible bonds issued by Alvotech to Aztiq Pharma Partners S.à r.l. ("Aztiq") on 16 November 2022 (also known as the "Aztiq Convertible Bond"), $76.6 million from loans from Alvogen, and $97.6 million in bank loans, including the mortgage on our Reykjavik facility and loans to help finance equipment purchases. In addition, we may incur additional indebtedness in order to finance our operations, make acquisitions or to repay existing indebtedness. Our board of directors will consider a number of factors when evaluating our level of indebtedness and when making decisions regarding the incurrence of new indebtedness, including the purchase price of assets to be acquired with debt financing, the estimated market value of our assets and the ability of particular assets, and our ability as a whole, to generate cash flow to cover the expected debt service. Our articles of incorporation do not contain a limitation on the amount of debt we may incur, and the board of directors may change our target debt levels at any time without the approval of shareholders.
This substantial indebtedness, as well as any future indebtedness we may incur, could have important consequences for our business and holders of our securities, including:
•making it more difficult for us to satisfy our obligations with respect to our debt or to our trade or other creditors;
•causing us to pay higher interest rates upon refinancing indebtedness if interest rates rise;
•increasing our vulnerability to adverse economic or industry conditions;
9
Table of Contents
•limiting our ability to obtain additional financing to fund capital expenditures and acquisitions, particularly when the availability of financing in the capital markets is limited;
•requiring a substantial portion of our cash flows from operations for the payment of interest on our debt and reducing our ability to use our cash flows to fund working capital, capital expenditures, acquisitions, stock repurchases, and general corporate requirements;
•limiting our flexibility in planning for, or reacting to, changes in our business and the homebuilding industry; and
•placing us at a competitive disadvantage to less leveraged competitors.
We cannot assure you that our business will generate sufficient cash flow from operations or that future borrowings will be available to us through capital markets financings or under our debt or credit facilities or otherwise in an amount sufficient to enable us to pay our indebtedness, or to fund our other liquidity needs. If we cannot service our debt, we may have to take actions such as selling assets, seeking additional debt or equity or reducing or delaying capital expenditures, strategic acquisitions, investments and alliances. We cannot assure you that any such actions, if necessary, could be effected on commercially reasonable terms, or at all, or on terms that would be advantageous to our securityholders or on terms that would not require us to breach the terms and conditions of our existing or future debt agreements
We may need to refinance all or a portion of our indebtedness and cannot assure you that refinancing will be available on commercially reasonable terms, or at all.
As of 31 December 2023, we had $960.2 million in outstanding indebtedness, that matures between June 2025 and December 2029. We may need to refinance all or a portion of our indebtedness, on or before its maturity.
We cannot assure you that we will be able to refinance any of our indebtedness on commercially reasonable terms, or at all. . If we are unable to refinance any of our indebtedness on commercially reasonable terms or at all or to effect any other action relating to our indebtedness on satisfactory terms or at all, we may be forced to reduce or discontinue operations or seek protection of the bankruptcy laws, our business may be harmed and our securityholders may lose some or all of their investment.
As a European public company with limited liability with registered office in Luxembourg, we will be subject to the sustainability disclosure requirements set out in the EU Corporate Sustainability Reporting Directive and the disclosure requirements set out in the EU Taxonomy Regulation.
On 18 June 2020 the EU adopted Directive 2020/852/EU (the "Taxonomy Regulation"). The goal of the Taxonomy Regulation was to create a uniform, credible, science-based system to classify activities that are environmentally sustainable by determining (among other conditions) if they substantially contribute to specific environmental objectives. The broader objective of the EU Taxonomy is to help financial market participants and other relevant actors to identify which economic operators carry out environmentally sustainable economic activities, and make it easier for these relevant actors to raise funding for these activities.
On 5 January 2023, the EU adopted Directive 2022/2464/EU (the “Corporate Sustainability Reporting Directive”), which amends the non-financial reporting requirements set out in Directive 2013/34/EU (the “Accounting Directive”). The CSRD introduces new mandatory reporting obligations that will require in-scope entities to publish audited sustainability information in their Management Reports addressing environmental, social and governance (“ESG”) matters in line with new mandatory European sustainability reporting standards (“ESRS”) that will be adopted by the European Commission through secondary legislation.
The EU Taxonomy Regulation took effect on 1 June 2023. The CSRD requirements will become effective in stages, based on the characteristics of undertakings, with earliest application from 1 January 2024 (reporting in 2025 on 2024 data). As a European public company with limited liability with registered office in Luxembourg, we will fall under the scope of application of the new sustainability-related reporting requirements. This will involve setting up processes to gather the relevant data, conduct materiality assessments and prepare a CSRD-compliant report, which will likely be a time-consuming and costly exercise.
Certain disclosures for large EU reporting entities are mandatory, even if the entity considers that there are no material impacts, risks or opportunities. Materiality’ under the CSRD must be assessed following the double materiality principle. Double materiality means that the reporting entity should consider both financial materiality (i.e., sustainability matters which from the investor perspective are material to the company’s development, performance and position) and impact materiality (i.e., the impact of corporate activity on sustainability matters from the perspective of citizens,
10
Table of Contents
consumers, employees etc.). Impacts, risks and opportunities are material if they satisfy one or both of these materiality tests.
The disclosure requirements under the CSRD will apply alongside the EU Taxonomy Regulation, which (a) creates a classification system to determine when an economic activity qualifies as “environmentally sustainable” and (b) requires companies in scope of the EU Accounting Directive, including those brought into scope by the CSRD, to disclose the proportion of turnover, capital and operational expenditure directed towards activities that qualify as “environmentally sustainable” (this information should be disclosed even if the contribution is none).
All EU reporting entities must have the sustainability section of their management report audited by a third-party accredited auditor to confirm that it has been prepared in accordance with the relevant ESRS and Article 8 of the Taxonomy Regulation. By Luxembourg Law of 23 July 2016 the non-financial statement may be disclosed either in the management report or in a separate report, under the condition that this separate report is published together with the management report or made available within six months after the balance sheet date on the given entity’s website.
The disclosures set out in the CSRD and the EU Taxonomy Regulation should be also considered together with the adjusted proposal of the EU Directive on Corporate Sustainability Due Diligence (“CSDDD”), which has been agreed by the European Council on 15 March 2024. Whilst the final text of the CSDDD remains to be voted on by the European Parliament and formalized by the European Commission by April 2024, the agreed proposal of the CSDDD sets out new due diligence duties for large companies with (a) more than 1000 employees and (b) a net worldwide turnover of over EUR 450 million generated in the last financial year for which financial statements have been prepared. The due diligence obligations under the agreed proposal of the CSDDD shall also apply to companies entering into franchising or licensing agreements in return for royalties of more than EUR 22.5 million, provided that the company has a net worldwide turnover of over EUR 80 million generated in the last financial year for which financial statements have been prepared. The requirements of this directive may apply to us.
The CSDDD will impose substantive due diligence obligations and also influence the information gathering process required by entities that are also subject to the CSRD. It will also have an impact on the mandatory disclosures to be made under the CSRD on the entity’s due diligence process (which will need to show compliance with the CSDDD if the entity is subject to both the CSRD and CSDDD).
Once the CSDDD has been adopted, EU Member States, including Luxembourg, will have two years to transpose the Directive into national law.
A disruption at our main manufacturing facility could materially and adversely affect our business, financial condition and results of operations.
On 16 November 2022, we acquired the Reykjavik manufacturing and research facility through the purchase of the shares in Fasteignafélagið Sæmundur hf. (“Saemundur”) from ATP Holdings ehf., a related party. Simultaneously, we entered into a loan facility for $48.8 million with Landsbankinn hf., secured with a first priority mortgage over the facility, resulting in the extinguishment of the old loan on the manufacturing and research facility. As owners of the manufacturing and research facility, we are responsible for the maintenance, upkeep and improvements of the facility, for obtaining and maintaining all permits related to the facility and activities therein, and a significant disruption at the facility, whether it be due to fire, natural disaster, power loss, intentional acts of vandalism, climate change, war, terrorism, insufficient quality, or cyber-attacks could materially and adversely affect our business. In addition, failure to make timely payments under the loan facility with Landsbankinn hf. may lead disruptions of our manufacturing facility and to the loss of the facility and equipment therein.
Covenants under our existing debt instruments, and any future debt arrangements may result in the acceleration of outstanding indebtedness and limit the manner in which we operate.
Our existing debt instruments, including the Senior Bonds, the Aztiq Convertible Bond and the 2022 Convertible Bonds, contain customary terms and covenants, as well as customary events of default, including but not limited to defaults related to payment compliance, undertaking and covenant compliance, bankruptcy and insolvency proceedings, judgments against the Company, and delisting events.
In addition, these bonds contain, and any future indebtedness we incur may contain, various negative covenants that restrict or may restrict, among other things, our ability to:
•incur additional indebtedness, guarantee indebtedness or issue disqualified shares or preferred shares;
11
Table of Contents
•declare or pay dividends on, repurchase or make distributions in respect of, capital stock or make other restricted payments;
•make certain investments or acquisitions;
•create certain liens;
•enter into agreements restricting certain subsidiaries’ ability to pay dividends or make other intercompany transfers;
•consolidate, merge, sell or otherwise dispose of all or substantially all of our assets and the assets of our restricted subsidiaries;
•enter into certain transactions with affiliates;
•sell, transfer or otherwise convey certain assets; and
•conduct our business and may be unable to engage in favorable business activities, repurchase our ordinary shares or finance future operations or capital needs.
Servicing these bonds requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. If we are unable to make our installment payments in cash, we may be forced to issue a significant number of Ordinary Shares which could dilute existing shareholders. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Risks Related to the Development of Our Product Candidates
The regulatory review and approval processes of the FDA, European Commission and comparable national or regional authorities are lengthy, time consuming and have uncertain outcomes. If we and our collaboration partners are unable to obtain regulatory approval for our product candidates, our business will be substantially harmed. We cannot give any assurance that marketing authorization applications for any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized.
Our future success is dependent on our ability to develop, obtain regulatory approval for, and then commercialize and obtain adequate third-party coverage and reimbursement for one or more product candidates. We currently only have marketing authorization for AVT02 in the United States, the EEA (comprising the 27 EU Member States, Norway,
Liechtenstein, and Iceland), the UK, Switzerland, Canada, Australia and Saudi Arabia. We also received regulatory
approval for AVT04 in Japan, Canada, and the EEA. We do not have marketing authorization for other product candidates
or in other countries, and may never be able to develop or commercialize a marketable product other than AVT02 and
AVT04 in those countries.
The research, development, testing, manufacturing, labeling, packaging, approval, promotion, advertising, storage, marketing, distribution, post-approval monitoring and reporting and export and import of biologic products are subject to extensive regulation by the FDA and other regulatory authorities in the United States, by the European Commission, the EMA and the National Competent Authorities of the EEA countries, and by other comparable regulatory authorities in other countries, which regulations differ from country to country. Neither we nor any collaboration partner is permitted to market our product candidates before receiving market authorization/approval from the appropriate regulatory authorities.
The time required to seek and obtain market authorization/approval by the FDA and comparable foreign regulatory authorities is unpredictable, may take many years following the completion of clinical studies and depends upon numerous factors. In addition, approval requirements, regulations, or considerations with respect to the type and amount of clinical, nonclinical and analytical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, which may cause delays in the submission of an application for marketing authorization/approval, the authorization or approval, or the decision not to approve an application. Other than the regulatory approval received in the United States, 52 countries, including the EEA, the UK, Switzerland, Canada, Australia, Israel, Morocco, Egypt, Saudi Arabia, South Africa and part of Latin America for AVT02 and in Japan, Canada
12
Table of Contents
and the EEA for AVT04, neither we nor any collaboration partner has obtained regulatory approval for any of our product candidates in the United States, the EEA or in additional other countries where we or our partners have commercial rights, and it is possible that none of our current or future product candidates will ever obtain regulatory approval.
These lengthy approval processes, as well as the unpredictability of the results of analytical, nonclinical, and clinical studies, may result in our failure to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, prospects and financial condition. Moreover, any delays in the commencement or completion of product testing could significantly impact our product development costs and could result in the need for additional financing. For example, our clinical trials must use reference products as comparators, and such supplies may not be available on a timely basis to support such trials.
Most of our product candidates are in varying stages of development and will require additional clinical development, management of analytical, nonclinical, clinical and manufacturing activities, regulatory approval, adequate manufacturing supplies, commercial organization and significant marketing efforts before we may generate any revenue from product sales. Since 2021, we, directly or through our partners, received regulatory approval for AVT02 in 52 countries. The countries where AVT02 has been approved include European countries (such as the EEA, the UK, Switzerland and several Balkan states), Canada, Australia, Israel, Morocco, Egypt, Saudi Arabia, South Africa and parts of Latin America. In February 2024, the FDA approved AVT02 in the United States. AVT02 is currently marketed in 21 countries: in Europe, Canada and Australia. In 2023 AVT04 was approved in Japan and Canada, and, in January 2024, in the EEA. In November 2023, we announced that the FDA had accepted a resubmitted BLA for AVT04 for review. We anticipate that the FDA's review will be completed in April 2024. We have completed, or are expecting to complete, clinical studies for AVT03, AVT05, and AVT06 in 2024, while AVT23 has recently entered a confirmatory efficacy study. In addition we currently have five biosimilar candidates in pre-clinical development.
We cannot be certain that any of our product candidates will receive additional regulatory approval. If we and our collaboration partners do not receive regulatory approvals for enough of our product candidates in sufficiently large markets, we may not be able to continue our operations.
Applications for our product candidates could fail to receive regulatory approval for many reasons, including but not limited to the following:
•the data collected from analytical, nonclinical, or clinical studies of our product candidates may not be sufficient to support an application for regulatory approval as a biosimilar;
•the FDA or comparable supranational, national or regional regulatory authorities may disagree with the design or implementation, or sufficiency of our analytical, nonclinical, or clinical studies;
•the FDA or comparable regulatory authorities may disagree with our interpretation of data from analytical and bioanalytical studies, nonclinical studies or clinical studies;
•we may be unable to provide adequate scientific justification to the FDA or comparable regulatory authorities for extrapolation of a product candidate to each proposed indication;
•the FDA or comparable regulatory authorities may identify significant deficiencies with the manufacturing processes, test procedures and specifications, facilities or third-party manufacturers with which we contract for clinical and commercial supplies. For example, prior to receiving approval for AVT02 from the FDA, we received multiple CRLs, noting certain deficiencies related to the Reykjavik facility and stating that satisfactory resolution of the deficiencies was required before the FDA approved AVT02 and before the FDA may approve AVT04;
•the regulatory exclusivity held by a competing manufacturer; and
•the approval requirements, policies, or regulations of the FDA or comparable regulatory authorities may significantly change in a manner rendering our clinical, nonclinical, analytical, or chemistry, manufacturing, and control data insufficient for approval.
In addition, if we change the regulatory pathway through which we intend to seek approval of any of our product candidates, we may have to conduct additional clinical trials, which may delay our ability to submit a marketing application for the product. Even if we or our collaboration partners were to obtain approval for any of our product candidates, the FDA or comparable regulatory authorities may limit the scope of such approval, e.g., for fewer or more limited indications than those for which we have sought licensure, may grant approval contingent on the completion of costly additional clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
13
Table of Contents
The UK’s withdrawal from the EEA on 31 January 2020, commonly referred to as Brexit, has created significant uncertainty and such uncertainty may make it more difficult for us to achieve regulatory approval in the UK.
Pursuant to the formal withdrawal arrangements agreed between the United Kingdom and the EU, the UK, was subject to a transition period until 31 December 2020, or the Transition Period, during which EU rules continued to apply. The UK and the EU have signed an EU-UK Trade and Cooperation Agreement, or TCA, which became provisionally applicable on 1 January 2021 and entered into force on 1 May 2021. This agreement provides details on how some aspects of the UK and EU’s relationship will operate going forward though many uncertainties remain. The TCA primarily focuses on ensuring free trade between the EEA and the UK in relation to goods, including medicinal products. Although the body of the TCA includes general terms which apply to medicinal products, greater detail on sector-specific issues is provided in an annex to the TCA. The annex provides a framework for the recognition of GMP inspections and for the exchange and acceptance of official GMP documents.
Among the changes that will now occur is that Great Britain (England, Scotland and Wales) will be treated as a third country. Northern Ireland will continue to follow the EU regulatory rules. The TCA also encourages, although it does not oblige, the parties to consult one another on proposals to introduce significant changes to technical regulations or inspection procedures. Among the areas of absence of mutual recognition are batch testing and batch release.
The UK has unilaterally agreed to accept EEA batch testing and batch release. However, the EEA continues to apply EU laws that require batch testing and batch release to take place in the EEA territory. This means that medicinal products that are tested and released in the UK must be retested and re-released when entering the EEA market for commercial use.
As regards marketing authorizations, Great Britain will have a separate regulatory submission process, approval process and a separate national MA. Northern Ireland will, however, continue to be covered by the marketing authorizations granted by the European Commission. For example, the scope of a marketing authorization for a medicinal product granted by the European Commission or by the competent authorities of EEA countries will no longer encompass Great Britain (England, Scotland and Wales). In these circumstances, a separate marketing authorization granted by the UK competent authorities is required to place medicinal products on the market in Great Britain.
On 27 February 2023, the UK Government and the European Commission reached a political agreement on the so-called “Windsor Framework”. The Framework is intended to revise the Northern Ireland Protocol to address some of the perceived shortcomings in its operation. The agreement was adopted at the Withdrawal Agreement Joint Committee on 24 March 2023. If the changes are adopted in the form proposed, medicinal products to be placed on the market in the UK will be authorized solely in accordance with UK laws. Northern Ireland would be reintegrated back into a UK-only regulatory environment under the authority of the MHRA with respect to all medicinal products. The implementation of the Windsor Framework would occur in stages, with new arrangements relating to the supply of medicinal products into Northern Ireland anticipated to take effect in 2025.
Since a significant proportion of the regulatory framework in the UK applicable to our business and our product candidates is derived from EU Directives and Regulations, Brexit, following the Transition Period, could materially impact the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our product candidates in the UK or the EU, now that UK legislation has the potential to diverge from EU legislation. All of these changes could increase our costs and otherwise adversely affect our business. Any delay in obtaining, or an inability to obtain, any regulatory approvals for our product candidates, as a result of Brexit or otherwise, would prevent us from commercializing our product candidates in the UK or the EEA and restrict our ability to generate revenue and achieve and sustain profitability. In addition, we may be required to pay taxes or duties or be subjected to other hurdles in connection with the importation of our product candidates into the EEA. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approvals for our pro in the UK or the EEA for our product candidates, or incur significant additional expenses to operate our business, which could significantly and materially harm or delay our ability to generate revenues or achieve profitability of our business. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK.
As a result of the foregoing, among other factors, there can be no assurance that we would be able to achieve our plan to commercialize our product candidates on our expected timeline, or at all.
14
Table of Contents
If we are not able to demonstrate biosimilarity of our biosimilar product candidates to the satisfaction of the FDA or comparable national or regional regulatory authorities, we will not obtain regulatory approval for commercialization of our biosimilar product candidates and our future results of operations and ability to generate revenue would be adversely affected.
Our future results of operations depend, to a significant degree, on our ability to obtain regulatory approval for and to commercialize our biosimilar product candidates. Any inability to obtain regulatory approval could impact and delay the development timeline of our product candidates. To obtain regulatory approval for the commercialization of these product candidates, we will be required to demonstrate to the satisfaction of the FDA or comparable national regulatory authorities, among other things, that our proposed products are highly similar to biological reference products already approved by the applicable regulatory authority pursuant to approved marketing applications/authorizations, notwithstanding minor differences in clinically inactive components, and that they have no clinically meaningful differences as compared to the marketed biological products in terms of the safety, purity and potency of the products. Each individual jurisdiction may apply different criteria to assess biosimilarity, based on of the data that can be interpreted subjectively in some cases.
It is uncertain if regulatory authorities will grant the reference biosimilar product candidates the same labeling than the labeling approved for the reference product if the reference biosimilar product candidates are approved. For example, an infliximab (Remicade) biosimilar molecule was approved in the EEA with the same label as the reference product, but it did not receive approval initially for the same labeling reference in Canada. A similar outcome could occur with respect to one or more of our product candidates.
In the event that the FDA or comparable regulatory authorities require us to generate additional data, including by conducting additional clinical trials or other lengthy processes or otherwise change their criteria and requirements for the approval of biosimilar products, the approval and commercialization of our proposed biosimilar products could be delayed or prevented. Delays in the commercialization of or the inability to obtain regulatory approval for these products could adversely affect our operating results by restricting or significantly delaying the introduction of new biosimilars.
The structure of complex proteins used in protein-based therapeutics is inherently variable and highly dependent on the processes and conditions used to manufacture them. If we are unable to develop manufacturing processes that demonstrate that our product candidates are highly similar to their reference products, and within a range of variability considered acceptable by regulatory authorities, we may not be able to obtain regulatory approval for our products.
Protein-based therapeutics are inherently heterogeneous and their structures are highly dependent on the manufacturing process and conditions. Products from one manufacturing facility can differ from those produced in another facility. Similarly, physicochemical differences can also exist among different lots produced within a single facility. The physicochemical complexity and size of biologic therapeutics can create significant technical and scientific challenges in the context of their replication as biosimilar products.
The inherent variability in protein structure from one production lot to another is a fundamental consideration with respect to establishing biosimilarity to a reference product to support regulatory approval requirements. For example, the glycosylation of the protein, meaning the manner in which sugar molecules are attached to the protein backbone of a therapeutic protein when it is produced in a living cell, is critical to half-life (how long the drug stays in the body), efficacy and even safety of the therapeutic and is therefore a key consideration for biosimilarity. Defining and understanding the variability of a reference product in order to match its glycosylation profile requires significant skill in cell biology, protein purification and analytical protein chemistry. Variations in the glycosylation profile and other analytical characterizations important for determining biosimilarity to the reference product molecule are risks unique to biosimilar manufacturers.
There are extraordinary technical challenges in developing complex protein-based therapeutics that not only must achieve an acceptable degree of similarity to the reference product in terms of relevant quality attributes such as glycosylation patterns, but also the ability to develop manufacturing processes that can replicate the necessary structural characteristics within an acceptable range of variability sufficient to satisfy regulatory authorities.
For example, the manufacturing process of our products may be susceptible to non-ideal product variability without well-characterized and well-controlled master and working cell banks. A cell bank is a collection of ampoules of uniform composition stored under defined conditions, each containing an aliquot of a single pool of cells. The master cell bank is generally derived from the selected cell clone containing the expression construct that has been encoded to produce the protein of interest, such as a specific monoclonal antibody with a defined amino acid sequence. This unique aliquot of cells allows for a consistent high quality biologic medicine to be produced. The working cell bank is derived by expansion of one or more ampoules of the master cell bank and is used for routine manufacturing. Both the master cell bank and working cell bank are central to obtaining regulatory approval for manufacturing and marketing biologic medicine. The quality of the manufactured biologic product is dependent on the quality of the cells used for its manufacturing, and having
15
Table of Contents
a sufficient supply of master and working cell banks is important for a consistent manufacturing process. Should our cell banks be compromised, we would be unable to produce usable products for patients in any market.
Given the challenges caused by the inherent variability in protein production, we may not be successful in our application for approval of our products if regulators conclude that we have not demonstrated that our product candidates are highly similar to their reference products, or that the processes we use to manufacture our products are unable to produce the products within an acceptable range of variability (including situations where the reference product sponsor changes its manufacturing process and such changes impact the characteristics of the product).
Additionally, the foregoing factors complicate scaling of our manufacturing capabilities. To the extent that we are unable to scale our manufacturing capabilities to produce sufficient quantities of our products at the required specifications and at an acceptable cost, we may be unable to meet demand for our approved product candidates and our business, financial condition, reputation and results of operations may suffer.
Clinical drug development involves a lengthy and expensive process, and we may encounter substantial delays in our clinical studies or may fail to demonstrate safety, purity and efficacy/potency to the satisfaction of applicable regulatory authorities. Additionally, the impact of public health emergencies or the occurrence of unforeseen geopolitical events such as the Russia-Ukraine conflict, the Middle Eastern conflicts and the resulting instability in these regions, may delay the conduct and completion of clinical studies.
Before obtaining regulatory approval from regulatory authorities for the sale of our product candidates, we (and/or our collaboration partners) must conduct clinical studies to demonstrate the safety, purity, and potency (safety and efficacy) of the product candidates in humans.
Clinical studies are expensive and can take many years to complete, and their outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of preclinical studies, including comparative analytical assessments of our product candidates, may not be predictive of the results of clinical studies. The success of clinical studies cannot be predicted.
We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. As a result of public health emergencies, such as the COVID-19 pandemic, and/or the occurrence of unforeseen geopolitical events, such as the Russia-Ukraine conflict and the Middle Eastern conflicts, and the resulting instability in these regions, timelines could be extended. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include but are not limited to:
•inability to generate sufficient preclinical, toxicology or other in vivo or in vitro data to support the initiation of human clinical studies;
•delays in reaching a consensus with regulatory authorities on study design;
•delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites;
•delays in obtaining required Institutional Review Board, or IRB, approval or Ethics Committee positive opinion as part of the single decision on the authorization of the clinical trial issued by EU Member States including input from the national competent authorities and Ethics Committee in relation each clinical study site;
•imposition of a clinical hold by regulatory authorities, after review of an investigational new drug, or IND, application or amendment or equivalent application or amendment, or an inspection of its clinical study operations or study sites or as a result of adverse events reported during a clinical trial;
•delays in administering studies as a result of adverse events or complaints;
•delays in recruiting suitable or sufficient numbers of patients to participate in its clinical studies sponsored by us or our partners;
•difficulty collaborating with patient groups and investigators;
•failure by its CROs, clinical study sites, other third parties or us to adhere to clinical study requirements;
•failure to perform in accordance with the FDA’s good clinical practices requirements or applicable regulatory guidelines and good clinical practice requirements in other countries;
16
Table of Contents
•delays in having patients complete participation in a study or return for post-treatment follow-up, or patients dropping out of a study;
•occurrence of adverse events associated with the product candidate that are viewed to outweigh its potential benefits;
•difficulties justifying the scientific relevance of non-U.S. comparators for use in studies intended to support regulatory approval by FDA;
•questions with regard to the scientific justification for extrapolation of findings across indications;
•changes in regulatory requirements or policies that require amending or submitting new clinical protocols;
•the cost of clinical studies of its product candidates being greater than what we anticipate;
•clinical studies of our product candidates producing negative or inconclusive results, which may result in us deciding or regulators requiring us to conduct additional clinical studies or to abandon product development programs;
•delays in manufacturing, testing, releasing, validating or importing/exporting and/or distributing sufficient stable quantities of our product candidates and reference products for use in clinical studies or the inability to do any of the foregoing;
•staffing shortages and limitation on the movement of people as a result of public health emergencies, the Russia-Ukraine conflict, the various conflicts in the Middle East, and the resulting instability in the regions, and local, national or international governmental restrictions imposed or enforced as a result of these or other health-related or geopolitical events; and
•delays or interruptions to preclinical studies, clinical trials, our receipt of services from third-party service providers or our supply chain due to public health emergencies or the occurrence of unforeseen geopolitical events such as the Russia-Ukraine conflict and the conflicts in the Middle East, and the resulting instability in these regions, or otherwise.
Any inability to successfully complete analytical, nonclinical, or clinical development could result in additional costs to us or impair our ability to achieve regulatory approval and generate revenue. Even if we are successful, the regulatory approval processes and action dates of the FDA, EMA and the European Commission and comparable regulatory authorities may be delayed or continue to be delayed due to impact of public health emergencies or other emergencies in the world. As a result, we may be delayed in obtaining regulatory approvals for our products.
In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions. If we intend to alter the manufacturing process for a particular product candidate, we will need to provide data to the FDA and comparable regulatory authorities demonstrating the comparability of the pre- and post-change product candidate. If we are unable to make that demonstration to the FDA or comparable regulatory authorities, we could face significant delays or fail to obtain regulatory approval to market the product, which could significantly harm our business, prospects and financial condition.
Our product candidates may cause unexpected side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label or result in significant negative consequences following regulatory approval, if granted.
As with most pharmaceutical products, use of our product candidates could be associated with side effects or adverse events which can vary in severity (from minor reactions to death) and frequency (infrequent or prevalent). Side effects or adverse events associated with the use of our product candidates may be observed at any time, including in clinical trials or when a product is commercialized. Undesirable or unexpected side effects caused by our product candidates that must be reported to the FDA or other regulators could cause us or regulatory authorities to interrupt, delay or halt clinical studies and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable regulatory authorities. Results of our studies could reveal a high and unacceptable severity and prevalence of side effects or other safety issues and, if different from the severity and prevalence of side effects for the reference products, could preclude the demonstration of biosimilarity. Such adverse event findings also could require us or our collaboration partners to perform additional studies or halt development or sale of these product candidates or expose us to product liability lawsuits which will harm our business, prospects and financial condition. In such an event, we may be precluded from seeking licensure through the regulatory pathway for biosimilars, or could be required by the FDA or other comparable regulatory authorities to conduct additional animal or human studies regarding the safety and efficacy of our product candidates which we have not planned or anticipated or our studies could be suspended, varied or terminated, and the FDA or comparable regulatory authorities could order us to cease further development of or deny, vary, or
17
Table of Contents
withdraw approval of our product candidates for any or all intended indications. There can be no assurance that we will resolve any issues related to any product-related adverse events to the satisfaction of the FDA or any comparable regulatory authority in a timely manner, if ever, which could harm our business, prospects and financial condition.
Drug-related side effects could affect patient recruitment for clinical trials, the ability of enrolled patients to complete our studies or result in potential product liability claims against which we would need to mount a defense. We currently carry product liability insurance, and we are required to maintain clinical trial insurance pursuant to certain of our license agreements. We believe our product liability insurance coverage is sufficient in light of our current clinical programs; however, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. A successful product liability claim or series of claims brought against us could adversely affect the results of our operations and business. In addition, regardless of merit or eventual outcome, product liability claims may result in impairment of our business reputation, withdrawal of clinical study participants, costs due to related litigation, distraction of management’s attention from our primary business, initiation of investigations by regulators, substantial monetary awards to patients or other claimants, the inability to commercialize our product candidates and decreased demand for our product candidates, if approved for commercial sale.
Additionally, if one or more of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused by such products (or caused by the reference products or other biosimilars based on the applicable reference products), a number of potentially significant negative consequences could result, including but not limited to:
•regulatory authorities may suspend, withdraw or vary approvals of such product;
•regulatory authorities may request or require that the product be recalled or removed from the market;
•regulatory authorities may require additional warnings on the label or otherwise require labeling to be updated or narrowed;
•we may be required to agree to a Risk Evaluation and Mitigation Strategy (“REMS”), or a shared system REMS, or comparable foreign strategy, which could include a medication guide for distribution to patients outlining the risks of side effects, a communication plan for healthcare providers and/or other elements to assure safe use;
•we could be sued and potentially held liable for harm caused to patients; and
•our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, prospects and financial condition.
As our product candidates receive approval, regulatory authorities including the FDA, European Commission, Icelandic Medicines Agency ("IMA"), EMA, National Competent Authorities of EEA countries and other comparable foreign regulatory authorities regulations will require that we regularly report certain information, including information about adverse events that may have been caused by or contributed by those products. The timing of adverse event reporting obligations would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we fail to comply with our reporting obligations, the FDA, European Commission, the IMA, the EMA, the National Competent Authorities of EEA countries or other comparable foreign regulatory authorities could take action that may include criminal prosecution, the imposition of civil monetary penalties, seizure of our products, or suspension or variation of market approval, and delay in approval or clearance of future products.
As a condition to granting marketing authorization or approval of a product, the FDA or other comparable foreign regulatory authorities may require additional clinical trials or other studies. The results generated in these trials could result in the loss of regulatory approval, changes in labeling, and/or new or increased concerns about the side effects, efficacy or safety. Regulatory authorities in countries outside the United States often have similar regulations and may impose comparable requirements. Post-marketing studies, whether conducted by us or by others, whether mandated by regulatory authorities or conducted voluntarily, and other emerging data about products, such as adverse event reports, may also adversely affect the availability or commercial potential of our products.
18
Table of Contents
Our reliance on certain participants for our clinical trials could cause delays in ongoing studies or the development of our products if such participants prove to be too limited or a substantial portion of participants in the studies withdraw.
In order to be successful and pursue market authorization for our products in various countries, we must be able to gather health data on the basis of populations from around the world. To the extent participants in clinical trials are too limited to certain populations, our clinical research may be adversely affected. Additionally, we depend on the willingness of these volunteers to participate in studies, and there is always the risk that they may no longer be willing to participate or revoke the consents necessary for us to process their medical data. For example, due to reasons beyond our control, including public health emergencies, the Russia-Ukraine conflict, the conflict in the Middle East, and the resulting instability in the region, participants and our key employees and advisors may no longer be able to travel or cross country borders to participate in our studies. If, for any reason, a substantial portion of participants in the studies were to withdraw their consent or discontinue their participation, we may not be able to continue our clinical studies for some or all of our product candidates which may cause delays in the development or approval of our product candidates. If our ability to gather and use sufficient data is impaired, we also may not be able to fulfill some contractual obligations with our partners.
The development, manufacture and commercialization of biosimilar products under various regulatory pathways pose unique risks related to regulatory approvals across various jurisdictions.
U.S. Regulatory Framework for Biosimilars
We and our collaboration partners intend to pursue market authorization globally. In the United States, an abbreviated pathway for approval of biosimilar products was established by the Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), as part of the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act (together, the “PPACA”). The BPCIA established this abbreviated pathway under section 351(k) of the Public Health Service Act (the “PHSA”) for biological products shown to be biosimilar to or interchangeable with an FDA-licensed reference biological product. Subsequent to the enactment of the BPCIA, the FDA has issued numerous guidance documents explaining its current thinking regarding the demonstration of biosimilarity and interchangeability as well as the submission and review of such BLA. Market success of biosimilar products will depend on demonstrating to patients, physicians, payors and relevant authorities that such products are similar in quality, safety and efficacy as compared to the reference product. If biosimilar product applications do not continue to be approved and the markets in which we operate do not widely accept the commercialization of biosimilar products, our business will be harmed. How the BPCIA is applied and interpreted by the FDA may have a material impact on our chances of obtaining FDA approval for our biosimilar product candidates, and our business operations after obtaining approval.
We will continue to analyze and incorporate into our product development plans any additional final regulations issued by the FDA, pharmacy substitution policies enacted by state governments and other applicable requirements. The costs of development and approval, along with the probability of success for our biosimilar product candidates, will be dependent upon application of any laws and regulations issued by the relevant regulatory authorities. The costs of developing our products may increase due to uncertainties or changes in guidance provided by regulatory authorities like the FDA, and we may not have adequate funding and resources to pursue market authorization for all of our biosimilar products.
Biosimilar products may also be subject to extensive patent clearances and patent infringement litigation, which may delay and could prevent the commercial launch of a product. Moreover, the PHSA prohibits the FDA from filing an application for a biosimilar candidate to a reference product for four years of the date of first licensure of the reference product by the FDA, and from approving an application for a biosimilar candidate for 12 years from the date of first licensure of the reference product. For example, the FDA would not be able to approve a BLA submitted for a biosimilar that references a specific drug until 12 years after the date of first licensure of the BLA, i.e., the date that reference product BLA was approved. Depending on the product, that regulatory exclusivity period may be further extended by a six-month pediatric exclusivity. The US regulatory exclusivity in the case of AVT02, a biosimilar to Humira (adalimumab), would be 31 December 2014, in the case of AVT04, a biosimilar candidate to Stelara (ustekinumab), would be 25 September 2021, in the case of AVT05, a biosimilar candidate to Simponi and Simponi Aria (golimumab), would be 24 April 2021, and in the case of AVT06, a biosimilar candidate to Eylea (aflibercept), would be 18 May 2024. Interchangeable biosimilar approvals may also be blocked by periods of first interchangeable exclusivity ranging from 12 to 42 months in duration.
19
Table of Contents
Regulatory Framework for Biosimilars Outside the United States
The European Commission approved the first biosimilar medicinal product in 2006. Since then the European Commission and the EMA have acquired extensive experience in the review and approval of biosimilars, and developed guidelines related to the authorization procedure for these products, including data requirements needed to support approval.
The EU provides opportunities for data and market exclusivity related to certain types of marketing authorizations. Upon grant of related marketing authorization, innovative medicinal products generally benefit from eight years of data exclusivity and 10 years of market exclusivity. Data exclusivity, if granted, prevents regulatory authorities in the EEA from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EEA until 10 years have elapsed from the initial marketing authorization of the reference product in the EEA. The overall ten year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity.
A new pharmaceutical form does not trigger a new data exclusivity. It could trigger orphan exclusivity, provided, however, that the targeted disease is a rare disease and that the new pharmaceutical form meets the high threshold for being considered as bringing a significant benefit to patients.
Other regions, including Canada, China, Japan and Korea, also have their own legislation outlining a regulatory pathway for the approval of biosimilars. In some cases, other countries have either adopted European Union guidance (Singapore and Malaysia) or are following guidance issued by the World Health Organization (Cuba and Brazil). While there is overlap in the regulatory requirements across regions, there are also some areas of non-overlap. Additionally, we cannot predict whether countries that we may wish to market in, which do not yet have an established or tested regulatory framework could decide to issue regulations or guidance and/or adopt a more conservative viewpoint than other regions. Therefore, it is possible that even if we obtain agreement from one health authority to an accelerated or optimized development plan, we will need to defer to the most conservative view to ensure global harmonization of the development plan. Also, for regions where regulatory authorities do not yet have sufficient experience in the review and approval of a biosimilar product, these authorities may rely on the approval from another region (for example, the United States), which could delay its approval in that region. In addition, regulatory approval may be delayed as a result of laws in any applicable jurisdiction that provide for stay of regulatory approval related to patent coverage and subsequent litigation.
If other companies’ biosimilar candidates for certain reference products are determined to be interchangeable before ours, or our biosimilar product candidates for these same reference products are not determined to be interchangeable, our business could be negatively impacted.
The FDA may determine that a proposed biosimilar product is “interchangeable” with a reference product, meaning that the biosimilar product may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product, if the application includes sufficient information to show that the product is biosimilar to the reference product and that it can be expected to produce the same clinical result as the reference product in any given patient. In addition, if the biosimilar product may be administered more than once to a patient, the applicant must demonstrate that the risk in terms of safety or diminished efficacy of alternating or switching between the biosimilar product candidate and the reference product is not greater than the risk of using the reference product without such alternation or switch. To make a final determination of biosimilarity or interchangeability, the FDA may require additional confirmatory information beyond what we plan to initially submit in our applications for approval, such as more in-depth analytical characterization, animal testing or further clinical studies. Provision of sufficient information for approval may prove difficult and expensive.
We cannot predict whether any of our biosimilar product candidates will meet regulatory requirements for approval as a biosimilar product or as an interchangeable product.
The concept of “interchangeability” is important because, in the United States for example, the first biosimilar approved as interchangeable with a particular reference product for any condition of use is eligible for a period of market exclusivity during which time the FDA cannot approve a second or subsequent biosimilar product interchangeable with that reference product for any condition of use. The relevant period of exclusivity will end upon the earlier of: (1) one year
20
Table of Contents
after the first commercial marketing of the first interchangeable product; (2) 18 months after resolution of a patent infringement suit instituted under 42 U.S.C. § 262(l)(6) against the applicant that submitted the application for the first interchangeable product, based on a final court decision regarding all of the patents in the litigation or dismissal of the litigation with or without prejudice; (3) 42 months after approval of the first interchangeable product, if a patent infringement suit instituted under 42 U.S.C. § 262(l)(6) against the applicant that submitted the application for the first interchangeable product is still ongoing; or (4) 18 months after approval of the first interchangeable product if the applicant that submitted the application for the first interchangeable product has not been sued under 42 U.S.C. § 262(l)(6). Thus, a determination that another company’s product is interchangeable with the reference biologic made before we obtain approval of our corresponding biosimilar product candidates may delay the potential approval of our products as interchangeable with the reference product, which could materially adversely affect the results of operations and delay, prevent or limit our ability to generate revenue. Even if we are awarded interchangeable exclusivity for a product, that award may be challenged by third parties. Any successful challenge to our exclusivity will negatively impact our ability to market and sell the related product.
In the EEA, the approval of a biosimilar for marketing is based on a positive opinion issued by the EMA and a related decision issued by the European Commission. The regulatory approval is valid throughout the entire EEA. However, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for marketing authorization. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product. In addition, rules governing the interchangeability, switching and substitution of a reference medicinal products by its biosimilar are provided by the national law of individual EEA countries, and many of them do not permit the automatic substitution of a reference medicinal products by its biosimilar. Therefore, even if we obtain regulatory approval for one of our product candidates in the EEA, we may not receive a positive decision from the National Competent Authorities of EEA countries in relation to the interchangeability, switching or substitution of a reference products with our approved product candidate in one or more EEA countries, thereby restricting our ability to market our products in those jurisdictions.
Our commercial products will remain subject to continuous subsequent regulatory obligations and scrutiny.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for pharmacovigilance, manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies (if any) and submission of other post-market information, including both federal and state requirements in the United States and equivalent requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to comply with extensive FDA, and comparable foreign regulatory authority requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices (“cGMP”), regulations. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any marketing authorization application. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we or our collaboration partners receive for our product candidates may be subject to limitations on the approved conditions of use for which the product may be marketed or to the conditions of approval or may contain requirements for potentially costly additional data generation, including clinical trials. We will be required to report certain adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities, and to conduct surveillance to monitor the safety and efficacy of the product candidate. Any new legislation addressing drug safety or biologics or biosimilars issues could result in delays in product development, approval or commercialization or increased costs to assure compliance.
We will have to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions that vary throughout the world and must be consistent with the information in the product’s approved label. As such, we may not promote our products in ways that are not consistent with FDA-approved labeling, e.g., for indications or uses for which they do not have approval. Equivalent limitations are provided both at EU level and national level in the individual EU Member States.
If our product candidates are approved, the company must submit new or supplemental applications and obtain prior approval for certain changes to the licensed approved, therapeutic indications, product labeling and manufacturing process. These changes may require submission of substantial data packages that may include clinical data.
21
Table of Contents
If a regulatory authority discovers previously unknown problems with a biosimilar product (or with the reference product or related biosimilars) such as adverse events of unanticipated severity or frequency, or if there are problems with the facility where the product is manufactured or the regulatory authority disagrees with the advertising, promotion, marketing or labeling of a product, such regulatory authority may impose restrictions on that product or us. If we fail to comply with applicable regulatory requirements, a regulatory authority such as FDA may, among other things:
•issue warning or untitled letters;
•refer a case to the U.S. Department of Justice, or comparable authorities, to impose civil or criminal penalties;
•begin proceedings to suspend or withdraw regulatory approval;
•issue an import alert;
•suspend our ongoing clinical studies or put our investigational new drug application (“IND”) on clinical hold;
•refuse to approve pending applications (including supplements to approved applications) submitted by us;
•ask us to initiate a product recall; or
•refer a case to the U.S. Department of Justice, or comparable authorities, to seize and forfeit products or obtain an injunction imposing restrictions on our operations.
Failure to comply with EU and EU Member State laws that govern conduct of clinical trials, manufacturing approval, marketing authorization of medicinal products and marketing of such products, both before and after grant of the marketing authorization, or with other applicable regulatory requirements may result in administrative, civil or criminal penalties. These penalties could include delays or refusal to authorize the conduct of clinical trials, or to grant marketing authorization, product withdrawals and recalls, product seizures, suspension, withdrawal or variation of the marketing authorization, total or partial suspension of production, distribution, manufacturing or clinical trials, operating restrictions, injunctions, suspension of licenses, fines and criminal penalties.
Any government investigation of alleged violations of law or regulations could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, our value and our operating results will be adversely affected.
Adverse events involving a reference product, or other biosimilars of such reference product, may result in negative publicity for our biosimilar product or ultimately result in the removal of our biosimilar product from the market.
In the event that use of a reference product, or another biosimilar for such reference product, results in unanticipated side effects or other adverse events, it is likely that our biosimilar product candidate will be viewed comparably and may become subject to the same scrutiny and regulatory actions as the reference product or other biosimilar, as applicable. Accordingly, we may become subject to, for example, safety labeling change orders, clinical holds, voluntary or mandatory product recalls or other regulatory actions for matters outside of our control that affect the reference product, or other biosimilars, as applicable, potentially until we are able to demonstrate to the satisfaction of our regulators that our biosimilar product candidate is not subject to the same issues leading to the regulatory action as the reference product or other biosimilar, as applicable. Any recall or safety alert or safety labeling change relating to our product (either voluntary or required by regulatory bodies) could ultimately result in the removal of our product from the market. Any recall could result in significant cost as well as negative publicity that could reduce overall demand for our products.
We are highly dependent on the services of our key executives and personnel, and if we are not able to retain these members of our management or recruit additional management, clinical and scientific personnel, our operations and future performance will suffer.
We are highly dependent on the principal members of our management and scientific and technical staff. The loss of service of any of our management or key scientific and technical staff could harm our business, prospects and financial condition. In addition, we will need to expand and effectively manage our managerial, scientific, operational, financial and other resources in order to successfully pursue our clinical development and commercialization efforts. The pharmaceutical industry has experienced a high rate of turnover of management personnel in recent years. If we are not able to retain our management and to attract, retain and motivate on acceptable terms, additional qualified personnel necessary for the continued development of our business, we may not be able to sustain our operations or grow.
22
Table of Contents
Our future performance will also depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our product candidates, harming future regulatory approvals, sales of our product candidates and results of operations. Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or any of our employees.
We have been and will need to continue to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of 31 December 2023, we had 1,026 employees, including 27 contractors. Additionally, we rely on a number of temporary workers from time to time, as needed. As our development and commercialization plans and strategies develop, we expect to need additional managerial, operational, sales, marketing, financial, legal and other resources. Our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. In addition, our success depends on our ability to attract and retain a talented workforce with a specialized set of skills. A significant part of our employees are expatriates and may need to obtain work visas in the country of operations. Changes to immigration laws or other restrictions on the movement of persons might make it more difficult for us to attract and retain talented employees. Our expected growth could also require significant capital expenditures and may divert financial resources from other projects, such as the development of our current and potential future product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected and our ability to generate and/or grow revenue could be reduced and our ability to implement business strategy may suffer. Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Risks Related to Our Reliance on Third Parties
We rely on third parties to conduct our nonclinical and clinical studies and perform other tasks. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plans to continue to rely upon third-party CROs to monitor and manage data for our ongoing nonclinical and clinical programs. We rely on these parties for execution of our nonclinical and clinical studies and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors are required to comply with relevant practices that may include cGMP, current good clinical practices (“cGCP”) and Good Laboratory Practices (“GLP”), which are regulations and guidelines required by the FDA, and comparable foreign regulatory authorities for all of our product candidates in clinical development. Regulatory authorities monitor these regulations through periodic inspections of study sponsors, principal investigators, study sites and other contractors. If we, any of our CROs, service providers or investigators fail to comply with applicable regulations or cGCPs, the data generated in our nonclinical and clinical studies may be deemed unreliable and the FDA, European Commission, EMA or comparable regulatory authorities may require us to perform additional nonclinical and clinical studies before approving our marketing applications. We cannot provide assurance that upon inspection by a given regulatory authority, such regulatory authority will determine that any clinical investigator for any of our clinical studies comply with cGCP regulations. In addition, our clinical studies must be conducted with product produced in compliance with cGMP regulations. Failure to comply with these regulations by us or any of the participating parties may require us to generate new data, repeat clinical studies, and potentially undergo re-inspection, which would delay the regulatory approval process. Further, if any accidents occur or there are process mistakes at the facilities of CROs or other vendors that handle reference products, there may be product loss which could further delay our nonclinical and clinical programs. Moreover, our business may be implicated if our CRO or any other participating parties violate federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws whether in the United States or equivalent foreign laws and obligations.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under the agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our on-going nonclinical and clinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the
23
Table of Contents
data they obtain is compromised due to the failure to adhere to protocols, regulatory requirements, delays caused by public health emergencies or for other reasons, our clinical studies may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. CROs may also generate higher costs than anticipated. As a result, the results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Switching or adding additional CROs involves additional costs and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet desired clinical development timelines. There can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We partly rely on third parties to manufacture clinical and commercial supplies of our product candidates and to store critical components of our product candidates (including procuring and providing reference product). Our business could be harmed if those third parties fail to provide us with sufficient quantities of product candidates or fail to do so at acceptable quality levels, prices and agreed upon time frame.
We partly rely on third-party manufacturers (contract manufacturing organizations, or “CMOs”) to manufacture and supply our product candidates for our preclinical and clinical studies. We also rely on third parties to manufacture nonclinical and clinical supplies of our product candidates, to store critical components of our product candidates and perform various services related to the product candidates’ compliance with regulatory requirements. Successfully transferring complicated manufacturing techniques to contract manufacturing organizations and scaling up these techniques for commercial quantities is time consuming, and we may not be able to achieve such transfer or do so in a timely manner. Moreover, the availability of contract manufacturing services for protein-based therapeutics is highly variable and there are periods of relatively abundant capacity alternating with periods in which there is little available capacity. If our need for contract manufacturing services increases during a period of industry-wide production capacity shortage, we may not be able to produce our product candidates on a timely basis or on commercially viable terms. Moreover, our manufacturing processes utilize single-use processing technology to manufacture drug substance and drug product. Although we will plan accordingly and generally does not begin a clinical study unless we believe we have a sufficient supply of a product candidate to complete such study, any significant delay, whether due to supply chain interruptions in connection with public health emergencies or otherwise, or discontinuation in the supply of a product candidate for an ongoing clinical study due to the need to replace a third-party manufacturer could considerably delay completion of our clinical studies, product testing and potential regulatory approval of our product candidates, which could harm our business and results of operations.
Reliance on third-party manufacturers entails additional risks, including reliance on the third party for regulatory compliance and quality assurance, the possible breach of the manufacturing agreement by the third party and the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. In addition, commercial manufacturing must be produced in compliance with cGMP regulations. Failure to comply by any CMO may require us to generate new data, repeat clinical studies, and potentially undergo re-inspection, which would delay the regulatory approval process. In addition, if a CMO does not comply with cGMP, our failure or the failure of our third-party manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, license suspension or revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates or any other product candidates or products that we may develop. Any failure or refusal to supply the components for our product candidates that we may develop could delay, prevent or impair our clinical development or commercialization efforts. If our contract manufacturers were to breach or terminate their manufacturing arrangements with us, the development or commercialization of the affected products or product candidates could be delayed, which could have an adverse effect on our business. Any change in our manufacturers could be costly because the commercial terms of any new arrangement could be less favorable and the expenses relating to the transfer of necessary technology and processes could be significant. In addition, any changes in our manufacturers could necessitate generation of new data and pre-license facility inspections. Changes made during the pendency of a BLA before FDA, or during the marketing authorization application, could result in delay in approval of the BLA or the marketing authorization.
If any of our product candidates are approved, in order to produce the quantities necessary to meet anticipated market demand, any contract manufacturer that we engage may need to increase manufacturing capacity. If we are unable to produce our product candidates in sufficient quantities to meet the requirements for the launch of these products or to meet future demand, our revenue and gross margins could be adversely affected. Although we believe that we will not have any material supply issues, we cannot be certain that we will be able to obtain long-term supply arrangements for our product candidates or materials used to produce them on acceptable terms, if at all. If we are unable to arrange for third-
24
Table of Contents
party manufacturing, or to do so on commercially reasonable terms, we may not be able to complete development or commercialization of or products.
Our reliance also extends to in-licensing. For AVT23, a biosimilar candidate referencing Xolair (omalizumab), which we have in-licensed, both manufacturing and clinical studies involve external stakeholders. Any failure on their part to meet regulatory requirements or maintain necessary drug availability puts us at risk of delays and potential breaches of our downstream commercial commitments.
In addition, we engage external transport companies to ship our products between the different supply points used to manufacture the finished product. Delays in shipment, damage of materials during shipment or any other events leading to late delivery or not full amount of ordered quantities could have a significant impact on project timelines, stock on markets and sales.
From time to time we, or our suppliers located in Europe, may require materials and equipment originating in Asia. Conflicts in the Middle East, which have in recent months disrupted shipments through the Suez Canal, may cause delays in shipments originating in Asia or cause the cost of materials originating in Asia to rise unexpectedly. This may cause delays or disruption in our operations or impair the ability of our suppliers to ship materials and equipment to us on time and on budget.
We have entered into collaborations with third parties in connection with the development of certain of our product candidates. Even if we believe that the development of our technology and product candidates is promising, our partners may choose not to proceed with such development if we materially deviate from the original program timelines, the contractual terms, or breach the contractual terms.
We have or may have future collaborations with various partners for the development and commercialization of some of our biosimilar candidates. Our existing and future agreements with our collaboration partners are generally subject to termination by the counterparty under certain circumstances. Accordingly, even if we believe that the development of certain product candidates is worth pursuing, our partners may choose not to continue with such development, if we materially deviate from the original program timelines, the contractual terms, or breach the contractual terms. If any of our collaborations are terminated, we may be required to devote additional resources to the development of our product candidates or seek a new collaboration partner, and the terms of any additional collaborations or other arrangements that we establishes may not be favorable to us, available under commercially reasonable terms or available at all.
We are also at risk that our collaborations or other arrangements may not be successful. Factors that may affect the success of our collaborations include the following:
•our collaboration partners may incur financial, legal or other difficulties that force them to limit or reduce their participation in our joint projects;
•our collaboration partners may be pursuing alternative technologies or developing alternative products that are competitive to our technology and products, either on their own or in partnership with others;
•our collaboration partners may terminate the collaborations, which could make it difficult for us to attract new partners or adversely affect our reputation in the business and financial communities; and
•our collaboration partners may pursue higher priority programs or change the focus of their development programs, which could affect their commitment to us.
If we cannot maintain successful collaborations, our business, financial condition and operating results may be adversely affected.
We are dependent on our partners, such as Teva, STADA and Advanz for the commercialization of our biosimilars and biosimilar candidates in certain major markets, and their failure to commercialize in those markets could have a material adverse effect on our revenue, business and operating results.
We do not currently have direct sales, marketing, and distribution capabilities. Instead, we have chosen to market and commercialize our products through partnerships with multiple regional partners. For example, Teva is responsible for commercialization of, among others, AVT02 and our biosimilar candidates AVT04, AVT05, AVT06 and AVT16 in the United States, Advanz for commercialization of, among others, our biosimilar candidates AVT05 and AVT16 in Europe, and STADA for commercialization of, among others, AVT02 and AVT04 and our AVT06 biosimilar candidate in Europe. If our commercial partners fail to exercise commercially reasonable efforts to market and sell our products in their respective licensed jurisdictions (timely or at all) or are otherwise ineffective in doing so, our business will be harmed and
25
Table of Contents
we may not be able to adequately remedy the harm through negotiation, litigation, arbitration or termination of the license agreements. Moreover, any disputes with our collaboration partners concerning the adequacy of their commercialization efforts will substantially divert the attention of our senior management from other business activities, and will require us to incur substantial legal costs to fund litigation or arbitration proceedings, and perhaps lead to delayed license-related payments to us.
We are subject to a multitude of risks related to manufacturing. Any adverse developments affecting the manufacturing operations of our biosimilar products could substantially increase costs and limit supply.
The process of manufacturing our products is complex, highly regulated and subject to several risks, including but not limited to:
•raw material and/or consumable shortages from external suppliers;
•product loss due to contamination, equipment failure, or operator error;
•equipment installation and qualification failures, equipment breakdowns, labor shortages, natural disasters, power failures and numerous other factors associated with the manufacturing facilities in which our products are produced;
•disruption of supply chains for critical and specialized raw materials, delays in regulatory inspections of supplies, manufacturing and testing facilities; and
•inventory shortages, lack of spare parts, or reduced manufacturing capacities due to local or global events such as disruptions of air traffic, maritime transport, volcanic eruptions, earthquakes, pandemics and international conflict.
Even minor deviations from normal manufacturing processes for any of our products could result in reduced production yields, product defects and other supply disruptions; additionally, FDA will inspect our manufacturing facilities for these issues, and ensure that the processes are satisfactory, before it licenses a BLA made at these facilities. If microbial, viral or other contaminations are discovered in our products or in the manufacturing facilities in which our products are made, manufacturing facilities for an extended period of time to investigate and remedy the contamination, and any such findings pre-licensure could impact FDA’s ability to license a BLA. Further, any defects or contaminations, or inadequate disclosure relating to the risk of using our products post-approval could lead to recalls or safety alerts, or other enforcement action by regulatory authorities.
Any adverse developments affecting manufacturing operations for our products may result in shipment delays, inventory shortages, lot failures, withdrawals or recalls or other interruptions in the supply of our products. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives.
We operate our main R&D and manufacturing facility in Iceland which is an island with a relatively limited number of ports of entry by sea or air which may impact logistics. Geologic activity, in particular volcanic eruptions could impact transport and cause disruptions to our supply chain or ability to export product.
Our main R&D and manufacturing facility is located in Reykjavik. Our operations dependent on supplies which are shipped in either by sea or air and we transport our products to our clients by similar means.
Recently, volcanic activity has increased in the region near Reykjavik. While this activity is localized and is not expected to pose a direct threat to our facility and operations, logistics could be disrupted as a consequence of these earthquakes or volcanic eruptions. Volcanic activity could suddenly increase in other parts of Iceland which are geologically active.
Major natural disasters, which could include strong earthquakes, can damage the electrical grid, district heating systems or infrastructure which is vital to a well-functioning transport and supply system.
While our facilities are designed to withstand earthquakes and volcanic activity are not expected to impact our operations directly, it is possible that any impact to third-parties, damage to infrastructure or the disruption of air traffic and other logistics, could have an impact on our ability to maintain stable operations and therefore cause delays in our development timelines, increase costs or and cause a loss of revenue.
26
Table of Contents
We currently engage single suppliers for some manufacture, clinical trial services, formulation development and product testing of our product candidates. The loss of any of these suppliers or vendors could materially and adversely affect our business.
The biologic drug substance used in all of our programs is currently manufactured at the facility of Alvotech hf. in Reykjavik. In addition, we rely on certain single third-party suppliers for services, such as safety device assembly and associated finished packaging. Prior to engaging any contract manufacturer for services, we perform a qualification of the site, including a verification of our status with regard to the relevant regulations. In addition, we perform regular audits as per our contractor management procedures once the contractor is qualified. Prior to any approval inspection, we engage external partners to help prepare for a successful inspection. We cannot be certain that identifying and establishing relationships with such would not result in significant delay in the development of our product candidates. Additionally, we may not be able to enter into arrangements with alternative vendors on commercially reasonable terms or at all. A delay in the development of our product candidates or having to enter into a new agreement with a different third-party on less favorable terms than what we have with our current suppliers could have a material adverse impact upon on our business.
Our failure to obtain or renew certain approvals, licenses, permits and certificates required may result in our inability to continue our operations or may result in enforcement actions with the respective regulatory authorities which would materially and adversely affect our business.
We are required to obtain and maintain various approvals, licenses, permits and certificates from relevant authorities to operate our business. Any failure to obtain any approvals, licenses, permits and certificates necessary for our operations may result in enforcement actions thereunder, including the relevant regulatory authorities ordering us to cease operations, implement potentially costly corrective measures or any other action which could materially disrupt our business operations.
In addition, some of these approvals, permits, licenses and certificates are subject to periodic renewal and/or reassessment by the relevant authorities, and the standards of such renewal and/or reassessment may change from time to time. We cannot give reassurance that we will be able to successfully procure such renewals and/or reassessment when due, and any failure to do so could severely disrupt our business.
Furthermore, if the interpretation or implementation of existing laws and regulations changes or new regulations come into effect requiring us to obtain any additional approvals, permits, licenses or certificates that were previously not required to operate our existing businesses, we cannot provide assurance that we will successfully obtain them, which in turn could restrict the scope of permitted business activities and constrain our drug development and revenue generation capability.
Any of the above developments could have a material adverse effect on our business, financial condition and results of operations.
We and our collaboration partners and contract manufacturers are subject to significant regulation with respect to manufacturing our product candidates. The manufacturing facilities on which we rely may not meet or continue to meet regulatory requirements or may not be able to meet supply demands.
All entities involved in the preparation of therapeutics for clinical studies or commercial sale, including our existing contract manufacturers for our product candidates, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical studies must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We, our collaboration partners or our contract manufacturers must supply all necessary documentation in support of a market application on a timely basis and must adhere to GLP and cGMP regulations enforced by the FDA and other comparable foreign regulatory authorities through their facilities inspection program. Not all contractors supporting our product candidates may be registered or approved for commercial pharmaceutical production. The facilities and quality systems of some or all of our collaboration partners and third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or the associated quality systems for compliance with the regulations applicable to the activities being conducted.
27
Table of Contents
Although we oversee our contract manufacturers, we cannot control the implementation of the manufacturing process by the contract manufacturing partners. If these facilities do not pass a pre-approval plant inspection, regulatory approval of our products may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever.
The regulatory authorities also may, at any time following approval of a product for sale, audit the manufacturing facilities of our collaboration partners and third-party contractors to monitor and ensure compliance with cGMP. Despite our efforts to audit and verify regulatory compliance, one or more of our third-party manufacturing vendors may be found on regulatory inspection by the FDA or other comparable foreign regulatory authorities to be noncompliant with cGMP regulations. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement and that may include the invalidation of drug product lots or processes, the temporary or permanent suspension of a clinical study or commercial sales or import or the temporary or permanent closure of a facility and that may require re-inspection thereby causing delays. In some cases, a product recall may be warranted or required, which would materially affect our ability to supply and market products. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business, prospects and financial condition.
If we, our collaboration partners or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA or other applicable foreign regulatory authority can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new biologic product, or suspension, variation or revocation of an approval. As a result, our business, financial condition and results of operations may be materially harmed.
Additionally, if supply from one approved manufacturer is interrupted, registration of an alternative manufacturer would require submissions of variations to the marking authorization which could result in further delay. The regulatory authorities may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and prior regulatory approval and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could incur higher costs and cause the delay or termination of clinical studies, regulatory submissions, required approvals or commercialization of our product candidates. Furthermore, if our suppliers fail to meet contractual requirements and we is unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical studies may be delayed or we could lose potential revenue from sales of an approved product.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to develop and manufacture our product candidates, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaboration partners, advisors, employees and consultants prior to beginning research or disclosing proprietary information, such as trade secrets. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business.
We may not be successful in our efforts to identify, develop or commercialize additional product candidates.
Although a substantial amount of our effort will focus on the continued testing, potential approval and commercialization of our existing product candidates, the success of our business also depends upon our ability to identify, develop and commercialize additional product candidates (in addition to the lead candidates). Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Our development efforts may fail to yield additional product candidates suitable for development and/or commercialization for a number of reasons, including but not limited to the following:
•we may not be successful in identifying potential product candidates that pass our strict screening criteria;
28
Table of Contents
•we may not be able to overcome technological hurdles to development or a product candidate may not be capable of producing commercial quantities at an acceptable cost or at all;
•we may not be able to assemble sufficient resources to acquire or discover additional product candidates;
•our product candidates may not succeed in analytical, nonclinical, or clinical testing;
•our potential product candidates may fail to show biosimilarity to reference products;
•we may not be successful in overcoming intellectual property obstacles in a timely manner or at all; and
•competitors may develop alternatives that render our product candidates obsolete or less attractive or the market for a product candidate may change such that a product candidate may not justify further development.
If any of these events occur, we may be forced to abandon our development efforts for a program or programs or we may not be able to identify, develop or commercialize additional product candidates, which would have a material adverse effect on our business and could potentially cause us to cease operations.
We rely on certain significant shareholders and affiliated entities for certain key services in the execution of our strategy and business operations.
We have entered into various service agreements with our direct and indirect significant shareholders and related entities, such as Alvogen, Aztiq, Alvogen Malta (Out-Licensing) Ltd. (“Adalvo”) and Floki Invest ehf. (“Floki”). These services include, among others, IT services, corporate administrative, legal, financial, facility management, portfolio and market intelligence research, regulatory compliance, quality audit, and publishing services, and certain administrative and financial services related to our Reykjavik facility. These services are key to our ability to continue to execute on our business strategy and to keep our business operations uninterrupted. Any interruption in the provision of these services may materially harm our business. In addition, because the providers of the services are direct or indirect significant shareholders and related entities, we may not be able or willing to enforce our contractual rights under the service agreements the same way we would if the service providers were unrelated third-party providers. See also “—We currently rely on Alvogen’s ERP solution and other components of Alvogen’s IT infrastructure and will continue to do so for the foreseeable future”.
Risks Related to Our Competition and Industry
Our biosimilar product candidates, if approved, will face significant competition from the reference products, other biosimilars, and from other medicinal products approved for the same indication(s) as the reference products. Our failure to effectively compete may prevent us from achieving significant market penetration and expansion.
We expect to enter highly competitive markets. We expect other companies to seek approval to manufacture and market biosimilars to Humira (AVT02), Prolia/Xgeva (AVT03), Stelara (AVT04), Simponi/Simponi Aria (AVT05), Eylea (AVT06), Entyvio (AVT16), Xolair (AVT23), and Keytruda (AVT33). If other biosimilars to these or other non-reference products in the same therapeutic spaces are approved and successfully commercialized before AVT03, AVT04, AVT05, AVT06, AVT16, AVT23 and AVT33, respectively, we may never achieve significant market share for these products, our revenue would be reduced and, as a result, our business, prospects and financial condition could suffer.
Successful competitors in the market have demonstrated the ability to effectively discover, obtain patents, develop, test and obtain regulatory approvals for products, as well as an ability to effectively commercialize, market and promote approved products. Numerous companies, universities and other research institutions are engaged in developing, patenting, manufacturing and marketing of products competitive with those that we are developing. Many of these potential competitors are large, experienced pharmaceutical companies that enjoy significant competitive advantages, such as substantially greater financial, research and development, manufacturing, personnel and marketing resources. These companies also have greater brand recognition and more experience in conducting preclinical testing and clinical trials of product candidates and obtaining FDA and other regulatory approvals of products.
29
Table of Contents
If an improved version of a reference product, is developed or if the market for the reference product significantly declines, sales or potential sales of our biosimilar product candidates may suffer.
Companies may develop improved versions, treatment regimens, combinations and/or doses of a reference product as part of a life cycle extension strategy and may obtain regulatory approval of the improved version under a new or supplemental BLA, or equivalent foreign procedure, filed with the applicable regulatory authority. Should the company manufacturing the reference product for any of our candidate products succeed in obtaining an approval of an improved biologic product, it may capture a significant share of the market for the reference product in the applicable jurisdiction and significantly reduce the market for the reference product and thereby the potential size of the market for our biosimilar product candidates. In addition, the improved product may be protected by additional regulatory exclusivity or patent rights that may subject our follow-on biosimilar to claims of infringement.
Biologic reference products may also face competition as technological advances are made that may offer patients a more convenient form of administration or increased efficacy or as new products are introduced. As new products are approved that compete with the reference product for our biosimilar product candidates, sales of the reference products may be adversely impacted or rendered obsolete. If the market for the reference product is impacted, we may lose significant market share or experience limited market potential for our approved biosimilar products or product candidates, and the value of our product pipeline could be negatively impacted. As a result of the above factors, our business, prospects and financial condition could suffer.
If efforts by manufacturers of reference products to prevent, delay or limit the use of biosimilars are successful, our business may be negatively affected, including but not limited to the sales of our biosimilar products.
Many manufacturers of reference products have increasingly used legislative, regulatory and other means to prevent or delay regulatory approval and to seek to restrict competition from manufacturers of biosimilars. These efforts may include or have included:
•settling patent lawsuits with biosimilar companies, resulting in such patents remaining an obstacle for biosimilar approval by others;
•submitting Citizen Petitions to request the FDA Commissioner to take administrative action with respect to prospective and submitted biosimilar applications or to elaborate or amend the standard of review for such biosimilar applications;
•appealing denials of Citizen Petitions in U.S. federal district courts and seeking injunctive relief to reverse approval of biosimilar applications;
•restricting access to reference brand products for equivalence and biosimilarity testing that interferes with timely biosimilar development plans;
•attempting to influence potential market share by conducting medical education with physicians, payors, regulators and patients claiming that biosimilar products are too complex for biosimilar approval or are too dissimilar from reference products to be trusted as safe and effective alternatives;
•implementing payor market access tactics that benefit their brands at the expense of biosimilars;
•seeking state law restrictions on the substitution of biosimilar products at the pharmacy without the intervention of a physician or through other restrictive means such as excessive recordkeeping requirements or patient and physician notification;
•seeking federal or state regulatory restrictions, or equivalent foreign restrictions, on the use of the same non-proprietary name as the reference brand product for a biosimilar or interchangeable biologic;
•seeking changes to the U.S. Pharmacopeia, an industry recognized compilation of drug and biologic standards, or equivalent international or foreign standards;
•obtaining new patents covering existing products or processes which could extend patent exclusivity for a number of years or otherwise delay the launch of biosimilars;
•originator could compete with us by manufacturing or commercializing their own proprietary biosimilar product to the reference product they sponsor; and
•influencing legislatures so that they attach special patent extension amendments to unrelated federal legislation.
In 2012, Abbott Laboratories filed a Citizen Petition with the FDA asking the agency to refrain from accepting biosimilar applications under the BPCIA arguing that to approve such applications, without compensation to the reference
30
Table of Contents
product sponsor, would constitute an unconstitutional taking of a reference company’s valuable trade secrets under the fifth amendment of the U.S. constitution. The FDA denied this citizen petition in 2016. Other reference companies may file Citizen Petitions in an effort to restrict or prevent the introduction of biosimilars. If the FDA or a federal court determines that biosimilar applications under the BPCIA should be limited, our business may be negatively impacted.
We face intense competition and rapid technological changes and the possibility that our competitors and originators such as AbbVie and Janssen may develop therapies that are similar, more advanced or more effective than ours, which may adversely affect our financial condition and the ability to successfully commercialize our product candidates.
We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies. Some of the pharmaceutical and biotechnology companies developing biosimilars we expect to compete with include companies such as Celltrion Healthcare Co., Ltd. (“Celltrion”), Coherus, Amgen, Pfizer Inc. (“Pfizer”), Samsung Bioepis, Ltd. (“Samsung Bioepis”), and Sandoz International GmbH (“Sandoz”), as well as other companies. These companies may develop biosimilars or other products in the same therapeutic space as our products. For example, based on publicly available information, we expect AbbVie (the originator), Amgen, Boehringer Ingelheim GmbH, Biocon/Fujifilm, Celltrion, Fresenius Kabi, Pfizer, Samsung Bioepis, Coherus, and Sandoz to be our main competitors for AVT02, a biosimilar to Humira (adalimumab); Janssen (the originator), Amgen, Celltrion, Bio-Thera, Formycon, Dong-A/Meiji Seika, Samsung Bioepis, and Biocon to be our main competitors for AVT04, a biosimilar to Stelara (ustekinumab); Amgen (the originator), Sandoz, Celltrion, Fresenius Kabi, Samsung Bioepis, Gedeon Richter, mAbxience, Biocon, Henlius and Teva to be our main competitors for AVT03, a biosimilar candidate to Prolia / Xgeva (denosumab); Janssen (the originator), and Bio-thera to be our main competitors for AVT05, a biosimilar candidate of Simponi and Simponi Aria (golimumab); and Regeneron/Bayer Health Care (the originator), Amgen, Celltrion, Formycon, Altos, Sam Chun Dang, Samsung Bioepis, Sandoz, and Viatris/Biocon, to be our main competitors for AVT06, a biosimilar candidate to Eylea (aflibercept); and Genentech (the originator), Celltrion and Teva, to be our main competitors for AVT23, a biosimilar candidate to Xolair (omalizumab).
Some of our competitors have substantially greater financial, technical and other resources, such as larger research and development team and experienced marketing and manufacturing organizations. Additional mergers and acquisitions in the pharmaceutical industry may result in even more resources being concentrated in our competitors. As a result, these companies may obtain regulatory approval more rapidly than we are able to and may be more effective in selling and marketing their products. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective or less costly than any product candidate that we may develop; they may also obtain patent protection that could block our products; and they may obtain regulatory approval, product commercialization and market penetration earlier than we do. Additionally, our competitors may have more resources in order to effectively pursue, defend against or settle with regard to potential or ongoing litigation. Biosimilar product candidates developed by our competitors may render our potential product candidates uneconomical, less desirable or obsolete, and we may not be successful in marketing our product candidates against competitors. Competitors may also assert in their marketing or medical education programs that their biosimilar products demonstrate a higher degree of biosimilarity to the reference products than do our or other competitor’s biosimilar products, thereby seeking to influence health care practitioners to select their biosimilar products, versus those of us or other competitors.
Our competitors may also succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective or less costly than our biosimilar candidates. They may also obtain exclusivity by regulators that could block or limit the marketability of our products for shorter or longer periods of time; and they may obtain regulatory approval, achieve commercialization and significant market penetration earlier than we do.
Furthermore, our competitors may develop products that are easier to administer than our products, which could adversely affect our results. Many of our biosimilar candidates need to be administered by a physician. Patients may demonstrate a preference for medications which can be administered by the patient at home or for pharmaceuticals that can be administered more rapidly in the clinic. Our competitors could develop proprietary technology that we are unable to replicate allowing competing medications to be self-administered or for sub cutaneous injection. Development of such alternative and competitive technologies and products may limit our success in commercializing our products.
31
Table of Contents
If we are unable to establish effective sales and marketing capabilities in jurisdictions for which we choose to retain commercialization rights or if we are unable to enter into agreements with third parties to market and sell our product candidates, and we are unable to establish and maintain a marketing and sales organization, we may be unable to generate substantial or any revenue.
We currently have no marketing or sales organization. We have no experience selling and marketing our product candidates. To successfully commercialize any products that may result from our development programs, we will need to develop these capabilities, either on our own or with others. If our product candidates receive regulatory approval, we might establish a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize our product candidates in major markets where we may choose to retain commercialization rights. Doing so will be expensive, difficult and time consuming. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of our products.
Further, given our lack of prior experience in marketing and selling biopharmaceutical products, our initial estimate of the size of the required sales force may be materially more or less than the size of the sales force actually required to effectively commercialize our product candidates. As such, we may be required to hire substantially more sales representatives to adequately support the commercialization of our product candidates or we may incur excess costs as a result of hiring more sales representatives than necessary. With respect to certain geographical markets, we may enter into collaborations with other entities to utilize their local marketing and distribution capabilities, but we may be unable to enter into such agreements on favorable terms, if at all. If our future collaboration partners do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. We expect competition from companies such as Celltrion, Sandoz, Amgen, Pfizer, Fresenius Kabi, Boehringer Ingelheim, Samsung Bioepis, Coherus and Viatris that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third-party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
We may need to enter into alliances with other companies that can provide capabilities and funds for the development and commercialization of our product candidates. If we are unsuccessful in forming or maintaining these alliances on sufficiently favorable terms, our business could be adversely affected.
We expect our manufacturing facility in Reykjavik to be able to scale up its capabilities for commercial production. Nevertheless, we are expected to retain contract manufacturing organization services as a second source of supply, including for business continuity risk mitigation. In addition, because we have limited capabilities for late-stage product development, manufacturing, sales, marketing and distribution, we have found it necessary to enter into alliances with other companies. We entered into a collaboration agreement with Teva for the development and commercialization of AVT02 in the United States. Similarly, we entered into a collaboration agreement with STADA for the development and commercialization of AVT02 in Europe. In the future, we may also find it necessary to form alliances or joint ventures with major pharmaceutical companies to jointly develop and/or commercialize specific biosimilar product candidates. In such alliances, we would expect our collaboration partners to provide substantial capabilities in clinical development, manufacturing, regulatory affairs, sales and marketing. We may not be successful in entering into any such alliances. Even if we do succeed in securing such alliances, we may not be able to maintain them if, for example, development or approval of a product candidate is delayed or sales of an approved product are disappointing. If we are unable to secure or maintain such alliances we may not have the capabilities necessary to continue or complete development of our product candidates and bring them to market, which may have an adverse effect on our business.
In addition to product development and commercialization capabilities, we may depend on our alliances with other companies to provide substantial additional funding for development and potential commercialization of our product candidates. We may not be able to obtain funding on favorable terms from these alliances, and if we are not successful in doing so, we may not have sufficient funds to develop a particular product candidate internally or to bring product candidates to market. Failure to bring our product candidates to market will prevent us from generating sales revenue, and this may substantially harm our business, prospects and financial condition. Furthermore, any delay in entering into these alliances could delay the development and commercialization of our product candidates and reduce their competitiveness even if they reach the market. As a result, our business and operating results may be adversely affected.
32
Table of Contents
The commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Even with the requisite approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our product candidates will depend in part on the medical community, patients and third-party payors accepting our product candidates as medically useful, cost-effective and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors and others in the medical community. The degree of market acceptance of any of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
•the safety and efficacy of the product as demonstrated in clinical studies and through the demonstration of biosimilarity;
•any potential advantages over competing biosimilars and/or other treatments in the same therapeutic space(s);
•the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling;
•the clinical indications for which approval is granted;
•the possibility that a competitor may achieve interchangeability in the United States, and we may not;
•relative convenience and ease of administration;
•the extent to which our product may be more or less similar to the reference product than competing biosimilar product candidates;
•policies and practices governing the naming of biological product candidates;
•prevalence of the disease or condition for which the product is approved;
•the cost of treatment, particularly in relation to competing treatments;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the strength of marketing and distribution support and timing of market introduction of competitive products;
•publicity concerning our products or competing products and treatments;
•the extent to which third-party payors provide adequate third-party coverage and reimbursement for our product candidates, if approved;
•patients’ willingness to pay out-of-pocket in the absence of such coverage and adequate reimbursement; and
•our ability to maintain compliance with regulatory requirements.
Even if a potential biosimilar product is expected to have a highly similar efficacy and safety profile to the reference product, as demonstrated through analytical, nonclinical, and clinical studies, market acceptance of the product will not be fully known until after it is launched and may be negatively affected by a potential poor safety experience and the track record of other biosimilar product candidates. Our efforts to educate the medical community and third-party payors on the benefits of the product candidates may require significant resources, may be under-resourced compared to large well-funded pharmaceutical entities and may never be successful. If our product candidates are approved but fail to achieve an adequate level of acceptance by physicians, patients, third-party payors and others in the medical community, we will not be able to generate sufficient revenue to become or remain profitable.
The third-party coverage and reimbursement status of newly-approved products is uncertain. Failure of our third-party commercial partners to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and generate revenue.
Pricing, coverage and reimbursement of our biosimilar product candidates, if approved, may not be adequate to support our commercial infrastructure. Our per-patient prices may not be sufficient to recover our development and manufacturing costs and potentially achieve profitability. Accordingly, the availability and adequacy of coverage and reimbursement by governmental and private payors are essential for most patients to be able to afford expensive treatments such as our products, if approved. Sales of our product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid for by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations or reimbursed by government authorities, private health insurers and other third-party payors. If coverage and reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved
33
Table of Contents
reimbursement amount may not be adequate to allow us to establish or maintain pricing sufficient to realize a return on investment.
There is significant uncertainty related to third-party coverage and reimbursement of newly approved products. In the United States, third-party payors, including private and governmental payors such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered and reimbursed. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our biosimilar product candidates, if approved. In addition, in the United States, no uniform policy of coverage and reimbursement for biologics exists among third-party payors. Therefore, coverage and reimbursement for biologics can differ significantly from payor to payor. As a result, the process for obtaining favorable coverage determinations often is time-consuming and costly and may require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. Further, coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained, less favorable coverage policies and reimbursement rates may be implemented in the future.
Outside the United States, pharmaceutical companies, products and distributors are generally subject to extensive governmental price controls and other market regulations. We believe the increasing emphasis on cost-containment initiatives in EEA, Canada and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to control healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. Certain cost containment practices may adversely affect our product sales. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes.
If our third-party commercial partners are unable to establish or sustain coverage and adequate reimbursement for any of our product candidates from third-party payors, the adoption of those products and sales revenue will be adversely affected, which, in turn, could adversely affect our ability to market or sell those product candidates, if approved.
Measures to contain healthcare costs, including the U.S. Inflation Reduction Act, may reduce the addressable market for our products, affect the prices that our commercial partners are able to obtain and have a material adverse effect on our business and results of operations.
A number of legislative initiatives in the U.S. and other markets, often intended to contain healthcare costs, may impact our cost of obtaining market approval and ability to successfully commercialize our products. In the U.S. the Inflation Reduction Act (IRA), requires manufacturers of certain drugs to engage in price negotiations with Medicare, with prices that can be negotiated subject to a cap. As the U.S. Secretary of the Department of Health and Human Services (HHS) is given significant flexibility to implement different provisions of the IRA it is currently unclear how this particular legislation will work and what impact it will have on the market for biosimilars in the near future.
If a reference product becomes subject to the IRA negotiation provision and related price cap, this may significantly alter the economics for biosimilars to that product. In 2023, HHS added Stelara, the reference product for AVT04, to the list of drugs for the Centers for Medicare & Medicaid Services (“CMS”) Medicare price negotiations. At this point it is unclear whether this will have any impact on the pricing of Stelara or on the competitive position of biosimilars to Stelara, as the Medicare drug price negotiation program is currently subject to legal challenges.
Any reduction in reimbursement for reference products from Medicare, other government programs in the U.S. or similar cost reimbursement systems in other countries, may result in a price reduction for biosimilars. The implementation of cost containment measures or other healthcare reforms may therefore prevent us from being able to generate revenue, attain profitability or commercialize our product candidates.
34
Table of Contents
In the EU, similar political, economic, and regulatory developments may affect our ability to profitably commercialize our product candidates, if approved. In markets outside of the United States and EU, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
Our biosimilar product candidates, if approved, could face price competition from other biosimilars of the same reference products for the same indication. This price competition could exceed our capacity to respond, detrimentally affecting its market share and revenue as well as adversely affecting the overall financial health and attractiveness of the market for the biosimilar.
We expect to enter highly competitive biosimilar markets. Successful competitors in the biosimilar market have the ability to effectively compete on price through payors and their third-party administrators who exert downward pricing pressure. It is possible our biosimilar competitors’ compliance with price discounting demands in exchange for market share could exceed our capacity to respond in kind and reduce market prices beyond our expectations. Such practices may limit our and our collaboration partners’ ability to increase market share and will also impact profitability.
Risks Related to Our Intellectual Property
If we or one of our partners infringes or is alleged to infringe the intellectual property rights of third parties, our business could be harmed. Avoiding and defending against infringement claims could be expensive and time consuming, which may in turn prevent or delay our development and commercialization efforts.
Our commercial success depends in large part on avoiding infringement of the valid and enforceable patents and proprietary rights of third parties and invalidating or rendering unenforceable other patent and proprietary rights of third parties. There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the pharmaceutical industry, including patent infringement lawsuits, interferences, oppositions and reexamination proceedings before the U.S. Patent and Trademark Office (“USPTO”), and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing product candidates. As the pharmaceutical industry expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights, or other intellectual property rights, of third parties.
Our research, development and commercialization activities may be claimed or held to infringe or otherwise violate patents owned or controlled by other parties. The companies that originated the products for which we intend to introduce biosimilar versions, such as AbbVie, Amgen, Janssen, Genentech and Regeneron as well as other competitors (including other companies developing biosimilars) often have developed worldwide patent portfolios of varying sizes and breadth, many of which are in fields relating to our business, and it may not always be clear to industry participants, including us, which patents cover various types of products, methods of use, methods of manufacturing, etc.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to compositions, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. While we have conducted freedom to operate analyses with respect to our lead product candidates, we cannot guarantee that any of our analyses will ensure that claims will not be brought or won against us, nor can we be sure that we have identified each and every patent and pending application in the United States and abroad that is relevant or necessary to the commercialization of our product candidates. Moreover, because patent applications can take up to 18 months after initial priority filing date to publish and issue, there may be currently pending patent applications with claims not yet filed that may later result in issued patents covering our product candidates. We have not yet completed a freedom-to-operate analysis on products we are evaluating for inclusion in our future biosimilar product pipeline, and therefore we do not know whether or to what extent that development of these products may be influenced by unexpired patents and pending applications.
There may also be patent applications that have been filed but not published and if such applications issue as patents, they could be asserted against us. For example, in most cases, a patent filed today would not become known to industry participants for at least 18 months given patent rules applicable in most jurisdictions which typically do not publish patent applications until 18 months from the application’s prior date. Moreover, we may face claims from non-practicing entities that have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. In addition, coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to convince a judicial authority that our product candidates, products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid and/or unenforceable, and we may not be able to do this. Proving to a judicial authority that a patent claim is invalid or unenforceable can be difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the
35
Table of Contents
presumption of validity enjoyed by issued patents. Also in proceedings before courts in Europe, the burden of proving invalidity of the patent usually rests on the party alleging invalidity. Further, proving the invalidity or unenforceability of a patent claim in the jurisdictions in which we operate may also depend on changes in the relevant law. Attempts to resolve intellectual property disputes may require substantial efforts including, but not limited to, validity challenges in patent offices, court litigation and arbitration. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could have a material adverse effect on us. In addition, we may not have sufficient resources to bring these actions to a desired conclusion.
Third parties could bring claims against us that would cause us to incur substantial expenses to defend against and, if successful against us, could cause us to pay substantial monetary damages if our product candidate is on the market. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit. Ultimately, we could be prevented from commercializing a product or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, We are unable to enter into licenses on commercially acceptable terms or at all. If, as a result of patent infringement claims or to avoid potential claims, we choose or is required to seek licenses from third parties, these licenses may not be available on acceptable terms or at all. Even if we are able to obtain a license, the license may obligate us to pay substantial license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Parties making claims against us may obtain injunctive or other equitable relief, which could effectively delay or block our ability to further develop and commercialize one or more of our product candidates. For example, companies that originated the products for which we intend to introduce biosimilar versions may seek damages for their loss of profits and/or market share. Defense of these claims, regardless of their merit, would likely involve substantial litigation expense and would likely be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may, in addition to being blocked from the market, have to pay substantial monetary damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference, derivation or post-grant proceedings declared or granted by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future products. An unfavorable outcome in any such proceedings could require us to delay or cease using the related technology or to attempt to license rights to it from the prevailing party or could cause us to lose valuable intellectual property rights. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, if any license is offered at all. Litigation or other proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may also become involved in disputes with others regarding the ownership of intellectual property rights. For example, we may jointly develop intellectual property with certain parties, and disagreements may therefore arise as to the ownership of the intellectual property developed pursuant to these relationships. If we are unable to resolve these disputes, we could lose valuable intellectual property rights.
BLA holders may submit applications for patent term extensions in the United States or other jurisdictions where similar extensions are available and/or Supplementary Protection Certificates in the EEA countries, and an equivalent process in Switzerland, seeking to extend certain patent protection which, if approved, may interfere with or delay the launch of one or more of our biosimilar products. Further, patent laws in the various jurisdictions in which we do business are subject to change and any future changes in patent laws may be less favorable for us.
The cost of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Patent litigation and other proceedings may fail, and even if successful, may result in substantial costs and distract our management and other employees. The companies that originated the products for which we intend to introduce biosimilar versions, as well as other competitors (including other biosimilar companies) may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings (either filed against Alvotech or one of its partners) could impair our ability to compete in the applicable marketplace. For example, we were in legal proceedings adverse to AbbVie, and our Canadian partner JAMP continues to be, relating to AVT02.
36
Table of Contents
So called “submarine” patents may be granted to our competitors that may significantly alter our launch timing expectations, reduce our projected market size, cause us to modify our product or process or block us from the market altogether.
The term “submarine” patent has been used in the pharmaceutical industry and in other industries to denote a patent issuing from an application that was not published, publicly known or available (including unfiled continuation, continuation-in-part, and divisional applications, and the like) at a critical time during which development and/or commercial decisions are made. Submarine patents add uncertainty to our business, e.g., because key decisions may be made during a period of time during which a pending applications has not yet published and such applications may only become known after those key decisions have already been made and perhaps even acted on. Submarine patents may issue to our competitors covering key aspects of our biosimilar product candidates or our pipeline candidates and thereby cause significant market entry delay, lead to unexpected licensing fees, defeat our ability to market our products or cause us to abandon development and/or commercialization of a molecule.
The issuance of one or more submarine patents may harm our business by causing substantial delays in our ability to introduce a biosimilar candidate into the U.S. market.
We may not timely identify, or identify at all, relevant patents or may incorrectly interpret the relevance, scope or expiration of a patent which might adversely affect our ability to develop and market our products.
We cannot guarantee that any of our patent searches or analyses, including but not limited to the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are 100% accurate and/or exhaustive, nor can we be certain that we have identified each and every patent and pending application in the United States and abroad that is relevant to or necessary for the commercialization of our product candidates in any jurisdiction (timely or at all). The scope of a patent claim is determined by a judicial authority’s interpretation under controlling law. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect and/or different from that of a judicial authority, which may negatively impact our ability to market our products or pipeline molecules. We may determine that our products are not covered by a third-party patent, but a judicial authority may hold otherwise.
Many patents may cover a marketed product, including but not limited to the composition of the product, methods of use, formulations, cell line constructs, vectors, growth media, production processes and purification processes. The identification of all patents and their expiration dates relevant to the production and sale of a reference product is extraordinarily complex and requires sophisticated legal knowledge in the relevant jurisdiction and interactive monitoring and analyzing of the patent landscape. It may be impossible to identify all patents in all jurisdictions relevant to a marketed product. Our determination of the expiration date of any patent in the United States or abroad that we consider (timely or at all) relevant may be incorrect which may negatively impact our ability to develop and market our products. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market our products.
Legal proceedings that carry risk may occur from time to time, and their outcome may be uncertain.
We have been, and may in the future be involved, directly or through our partners, in various legal proceedings, including patent litigation and challenges, other intellectual property disputes, product liability and other product-related litigation, including personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, commercial, environmental, government investigations, employment, tax litigation and other legal proceedings that arise from time to time in the ordinary course of our business. See, for example, “ —We may be involved in lawsuits to protect or enforce our patents or other intellectual property rights, which could be expensive, time consuming and unsuccessful." In
addition, we were in legal proceedings adverse to AbbVie relating to AVT02, which resulted in settlements (see Item 4.B
“Business Overview—Material Agreements, Partnerships and Suppliers”), and our Canadian partner JAMP continues to
be in proceedings adverse to AbbVie relating to AVT02. Litigation is inherently unpredictable, and excessive verdicts do occur. We could incur judgments and/or enter into settlements, which could require us to make payments to the proceedings’ counterparties or limit or discontinue certain of our activities, or could otherwise have a material adverse effect on our business operations. In addition, even if such legal proceedings are ultimately resolved in our favor, they may be costly and time-consuming to conduct, which may materially adversely affect our business, financial condition and results of operations. The cost and resource requirements, including management attention, associated with conducting such legal proceedings may lead us to settle certain actions on terms that are materially adverse to us, even if we believe that the ultimate resolution of the proceedings is likely to be favorable.
An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if we cannot obtain a license from the prevailing party on commercially reasonable terms. Our defense of litigation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation
37
Table of Contents
could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties or enter into development partnerships that would help us bring our product candidates to market.
We may be involved in lawsuits to protect or enforce our patents or other intellectual property rights, which could be expensive, time consuming and unsuccessful.
We may discover that competitors are infringing one or more of our patents after they issue. Expensive and time-consuming litigation may be required to abate such infringement. Although we are not currently involved in any litigation to enforce patents, if we or one of our collaboration partners, such as Teva or STADA, were to initiate legal proceedings against a third-party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including but not limited to lack of novelty, obviousness, written description or non-enablement. Grounds for an unenforceability assertion could include an allegation that someone involved in the prosecution of the patent withheld relevant or material information related to the patentability of the invention from the USPTO or made a misleading statement during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, and although there are protections in place, there is a risk that some of our confidential information could be compromised by disclosure during any litigation we initiate to enforce our patents. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, they could have a material adverse effect on the price of Ordinary Shares.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers or third parties.
We employ individuals, retains independent contractors and consultants and members on our board of directors or scientific advisory board who were previously employed at universities or other pharmaceutical companies, including our competitors or potential competitors. Although we have several mechanisms in place to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. For example, in March 2021, AbbVie brought a suit, which is now dismissed, against Alvotech hf. alleging that Alvotech hf. misappropriated trade secrets through the hiring of a former AbbVie employee. If we fail in defending against any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs or delay and be a distraction to management and other employees.
If we are unable to obtain and maintain effective intellectual property rights, including patent rights, for our product candidates or any future product candidates, we may not be able to prevent competitors from using technologies we consider important to successful development and commercialization of our product candidates, resulting in loss of any potential competitive advantage our intellectual property rights may have otherwise afforded us.
While our principal focus in matters relating to intellectual property is to avoid infringing the valid and enforceable rights of third parties, we also rely upon a combination of intellectual property protection and confidentiality agreements to protect our own intellectual property related to our product candidates and development programs. Our ability to enjoy any competitive advantages afforded by our own intellectual property depends in large part on our ability to obtain and maintain patents and other intellectual property protection in the United States and in other countries with respect to various proprietary elements of our product candidates, such as, for example, our product formulations and processes for manufacturing our products and our ability to maintain and control the confidentiality of trade secrets and confidential information critical to our business.
We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our products that are important to our business. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. There is no guarantee that any patent application we file will result in an issued patent having claims that protect our products. Additionally, while the basic requirements for patentability are similar across jurisdictions,
38
Table of Contents
each jurisdiction has its own specific requirements for patentability. We cannot guarantee that we will obtain identical or similar, or any, patent protection covering our products in all jurisdictions where we file patent applications.
The patent positions of biopharmaceutical companies generally are highly uncertain and involve complex legal and factual questions for which legal principles remain unresolved. As a result, the patent applications that we own or licenses may fail to result in issued patents with claims that cover our product candidates in the United States or in other foreign countries for many reasons. There is no assurance that all potentially relevant prior art relating to our patents and patent applications have been found, considered or cited during patent prosecution, which can be used to invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patent claims being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competitors from using the technologies claimed in any patents issued to us, which may have an adverse impact on our business.
Patents granted by the European Patent Office may be opposed by any person within nine months from the publication of their grant and, in addition, may be challenged before national courts at any time. From time to time, we may be involved in these anonymous or “straw man” oppositions. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patents and patent applications we hold, license or pursue with respect to our product candidates is threatened, it could threaten our ability to prevent third parties from using the same technologies that we use in our product candidates. In addition, changes to the patent laws of the United States provide additional procedures for third parties to challenge the validity of issued patents based on patent applications filed after 15 March 2013. If the breadth or strength of protection provided by the patents and patent applications we hold or pursue with respect to our current or future product candidates is challenged, then it could threaten our ability to prevent competitive products using our proprietary technology. Further, because patent applications in the United States and most other countries are confidential for a period of time, typically for 18 months after filing, we cannot be certain that we were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our patents or patent applications. Furthermore, for applications filed before 16 March 2013 or patents issuing from such applications, an interference proceeding can be provoked by a third-party or instituted by the USPTO to determine who was the first to invent any of the subject matter covered by the patent claims of our applications and patents. As of 16 March 2013, the United States transitioned to a “first-inventor-to-file” system for deciding which party should be granted a patent when two or more patent applications claiming the same invention are filed by different parties. A third-party that files a patent application in the USPTO before us could therefore be awarded a patent covering our invention.
The change to “first-inventor-to-file” from “first-to-invent” is one of the changes to the patent laws of the United States resulting from the Leahy-Smith America Invents Act (the “Leahy-Smith Act”), signed into law on 16 September 2011. Among some of the other significant changes to the patent laws are changes that limit where a patentee may file a patent infringement suit and provide opportunities for third parties to challenge any issued patent in the USPTO. We have filed patent applications, which are in various stages of prosecution/issuance, and plan to pursue additional applications, covering various aspects of our product candidates (e.g., formulations and bioprocesses). We cannot offer any assurances about which or where, if any, patents will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened or infringed by third parties. Any successful actions by third parties to challenge the validity or enforceability of any patents which may issue to us could deprive us the ability to prevent others from using the technologies claimed in such issued patents. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced.
Our business is based primarily on the timing of our biosimilar product launches to occur after the expiration of relevant patents and/or regulatory exclusivity. We file patent applications directed to our proprietary formulations for our product candidates when we believe securing such patents may afford a competitive advantage. For example, the company that originated Humira (AbbVie) owns patents directed to formulations for these products. We have developed our own proprietary formulations for this product and have filed patent applications covering our formulations. We cannot guarantee that our proprietary formulations will avoid infringement of third-party patents, or that the patent applications filed on our proprietary formulations will be found patentable and/or upheld as valid. Moreover, because competitors may be able to develop their own proprietary product formulations, it is uncertain whether issuance of any of our pending patent applications directed to formulations of ATV02, a biosimilar candidate to Humira (adalimumab), would cover the formulations of any competitors.
We do not consider it necessary for us or our competitors to obtain or maintain a proprietary patent position in order to engage in the business of biosimilar development and commercialization. Hence, while our ability to secure patent
39
Table of Contents
coverage on our own proprietary developments may improve our competitive position with respect to the product candidates we intend to commercialize, we do not view our own patent filings as a necessary or essential requirement for conducting our business nor do we rely on patent filings or the potential for any commercial advantage they may provide us as a basis for our success.
Obtaining and maintaining our patent protection depends on compliance with various procedural requirements, document submissions, actions within prescribed deadlines, overcoming substantial and procedural examination requirements, fee payments and other requirements imposed by governmental patent agencies. Our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
We may not be able to adequately protect our intellectual property rights throughout the world.
Filing, prosecuting, defending and enforcing patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Further, licensing partners may choose not to file patent applications in certain jurisdictions in which we may obtain commercial rights (to the extent those partners have a contractual right to do so), thereby precluding the possibility of later obtaining patent protection in these countries. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States or importing products made using our inventions into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but the ability to enforce our patents is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in obtaining, protecting and defending intellectual property rights in certain non-U.S. jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that it initiates and the damages or other remedies awarded, if any, may not be commercially meaningful. Governments of some foreign countries may force us to license our patents to third parties on terms that are not commercially reasonable or acceptable to us (not timely or not at all). Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license in certain jurisdictions.
Changes in the patent laws of the United States and other jurisdictions in which we do business could diminish the value of patents obtainable in such jurisdictions, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success for any given product could be heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time consuming and inherently uncertain.
Depending on future actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
40
Table of Contents
If we are unable to maintain effective (non-patent) proprietary rights for our product candidates or any future product candidates, we may not be able to compete effectively in our markets.
While we have filed patent applications to protect certain aspects of our own proprietary formulation and process developments, we also rely on trade secret protection and confidentiality agreements to protect proprietary scientific, business and technical information and know-how that is not or may not be patentable or that we elect not to patent. However, confidential information and trade secrets can be difficult to protect. Moreover, the information embodied in our trade secrets and confidential information may be independently and legitimately developed or discovered by third parties without any improper use of or reference to information or trade secrets. We seek to protect the scientific, technical and business information supporting our operations, as well as the confidential information relating specifically to our product candidates by entering into confidentiality agreements with parties to whom we need to disclose our confidential information, for example, our employees, consultants, scientific advisors, board members, contractors, potential collaborators and financial investors. However, we cannot be certain that such agreements have been entered into with all relevant parties, or that any such agreements would not be violated. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. Further, from time-to-time we may be subject to anonymous Freedom of Information Act (“FOIA”), requests. To the extent the company needs to respond to such requests, our management’s attention and the company’s resources may be diverted from normal business operations. As a result of either security breaches or FOIA requests, our confidential information and trade secrets thus may become known by our competitors in ways we cannot prevent or remedy.
Although we require all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. We cannot guarantee that our employees, former employees or consultants will not file patent applications claiming our inventions. Because of the “first-to-file” laws in the United States (and in other jurisdictions), such unauthorized patent application filings may defeat our attempts to obtain patents on our inventions.
We may be subject to claims challenging the inventorship or ownership of our patent filings and other intellectual property.
Although we are not currently aware of any claims challenging the inventorship of our patent applications or ownership of our intellectual property, we may in the future be subject to claims that former employees, collaborators or other third parties have an interest in our patent applications or patents we may be granted or other intellectual property as an inventor or co-inventor. For example, we may have inventorship or ownership disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates, or which result from an improper assignment of ownership. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of or right to use valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be successful in obtaining or maintaining necessary intellectual property rights to our product candidates through acquisitions and in-licenses.
We currently have or are pursuing rights to certain intellectual property, through licenses from third parties for various technologies relevant to the manufacture and commercialization of biologics. Because we may find that our programs require the use of proprietary rights held by third parties, the growth of our business may depend in part on our ability to acquire, in-license or use these proprietary rights. We may be unable to acquire or in-license compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify as necessary for our product candidates. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual
41
Table of Contents
property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, financial resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on investment.
If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, our business and financial condition could suffer.
Our ability to market our products in the United States may be significantly delayed or prevented by the BPCIA patent information exchange mechanism.
The BPCIA created an elaborate and complex, private, pre-litigation patent information exchange mechanism for biosimilars to focus issues for patent litigation and/or facilitate dispute resolution with the reference product sponsor before litigation commences/ends.
The BPCIA provides for a detailed and complex mechanism for exchange of confidential and business-sensitive information between a reference product sponsor and a biosimilar candidate (pre-approval) that is demanding, time-sensitive and, to date, not fully tested and therefore unpredictable. This pre-litigation private information exchange is colloquially known as the “patent dance.”
The patent dance requires the biosimilar applicant to disclose not only the regulatory application but also the applicant’s manufacturing process before litigation (and therefore significantly earlier than would normally be required in patent litigation), has the potential to afford the reference product sponsor an easier path than traditional infringement litigation for developing any factual grounds they may require to support allegations of infringement. The rules established in the BPCIA’s patent dance procedures could place biosimilar firms at a significant disadvantage by affording the reference product sponsor a much easier mechanism for factual discovery, thereby increasing the risk that a biosimilar product could be blocked from the market more quickly than under traditional patent infringement litigation processes and in certain cases could outweigh advantages provided to biosimilar firms by the patent dance.
Preparing for and conducting the patent information exchange, briefing and negotiation process under the BPCIA will require sophisticated legal counseling and extensive planning, all under extremely tight deadlines. We cannot guarantee the outcome of the patent dance will be a successful path to commercialization of our biosimilar products.
It is possible for a biosimilar firm to skip the patent dance before any corresponding patent litigation. But this too could place a biosimilar firm at a significant disadvantage by ceding all control of the number of patents and the timing for the start of litigation to the reference product sponsor, thereby increasing the uncertainty before approval and launch and increasing the chances for possible delays. In certain circumstances, the advantages of participating in the patent dance could outweigh the advantages of skipping the patent dance.
Regardless of whether a biosimilar firm chooses to participate in the patent dance, the BPCIA’s information disclosure procedure adds significantly to expense, complexity, uncertainty, and risk. For example, a biosimilar firm may be subject to an allegation of violating the BPCIA independent of the patent issues, given that what could be a violation still has not been fully vetted. Moreover, the complexity of the patent dance and subsequent biosimilar litigation requires highly qualified law firms and the conflict space for such firms is very crowded, with biosimilar firms competing not only with other biosimilar firms but also with reference product sponsors for the engagement of suitable law firms. It may be difficult for us to secure such legal support if large, well-funded references have already entered into engagements with highly qualified law firms or if the most highly qualified law firms choose not to represent biosimilar applicants due to their long-standing relationships with references.
Our Canadian partner, JAMP, is involved in legal proceedings adverse to AbbVie that may have an impact on our AVT02 product in Canada.
While our legal proceedings adverse to AbbVie related to our biosimilar adalimumab product, AVT02, have been settled or otherwise resolved in the United States, the Netherlands, and Japan, and before the European Patent Office, proceedings between our Canadian partner JAMP and AbbVie are pending in Canada.
On 31 March 2021, AbbVie filed four actions in the Federal Court of Canada (T-557-21, T-559-21, T-560-21 and T-561-21, collectively, the “NOC Actions”) against JAMP Pharma Corporation (“JAMP Pharma”), which is our exclusive Canadian partner for AVT02 (adalimumab solution for injection). No Alvotech entity is a named party in the NOC
42
Table of Contents
Actions. AbbVie is seeking declarations pursuant to the Patented Medicines (Notice of Compliance) Regulations and the Patent Act that JAMP Pharma’s adalimumab solution for subcutaneous injection (the “JAMP Pharma Products”) would directly or indirectly infringe the asserted claims of Canadian Patent Nos. 2,898,009; 2,904,458; 2,504,868; 2,847,142; 2,801,917 and 2,385,745. JAMP Pharma counterclaimed, in each of the four actions, alleging that the asserted claims of each of the six patents are invalid.
On 6 April 2021, JAMP Pharma commenced four actions in the Federal Court of Canada (T-572-21, T-573-21, T-577-21 and T-581-21, collectively, the “Impeachment Actions”) seeking declarations that all claims of Canadian Patent Nos. 2,898,009; 2,904,458; 2,504,868; 2,847,142; 2,801,917 and 2,385,745 are invalid, void and of no force or effect, and declarations that the making, using or selling of the JAMP Pharma Products by JAMP Pharma in Canada will not infringe any valid claim of Canadian Patent Nos. 2,898,009; 2,904,458; 2,504,868; 2,847,142; 2,801,917 and 2,385,745. No Alvotech entity is a named party in the Impeachment Actions.
On 4 June 2021, JAMP Pharma amended its Statements of Claim in the Impeachment Actions to only seek declarations that the specific claims asserted in the NOC Actions are invalid, void and of no force or effect, and declarations that the making, using or selling of the JAMP Pharma Products by JAMP Pharma in Canada will not infringe the asserted claims. AbbVie has counterclaimed for declarations that the asserted claims of the patents are valid and that they will be infringed by JAMP Pharma.
The trial of the Impeachment Actions and the NOC Actions commenced on 14 November 2022, and concluded with closing arguments on 14 December 2022. During the course of the proceedings, the patents-at-issue were limited to Canadian Patent Nos. 2,904,458; 2,504,868; and 2,801,917.
In November 2023, Justice McVeigh issued her trial decision, which invalidated AbbVie’s 868 Patent (dosing to treat Crohn’s disease and UC) and 917 Patent (dosing to treat HS). While AbbVie’s 458 Patent (bufferless formulation) was held to be valid and infringed by JAMP Pharma, Justice McVeigh declined to issue a permanent injunction and instead determined that JAMP Pharma could continue to market AVT02 in Canada and compensate AbbVie by way of a running royalty (to be determined by way of a future trial). AbbVie appealed the trial decision and JAMP Pharma cross-appealed (regarding the validity/infringement of the 458 Patent). The appeal and cross-appeal are in the early stages and are unlikely to be heard before the fourth quarter of 2024. Even if JAMP Pharma is successful in defending against AbbVie’s patent infringement claims, litigation could result in substantial cost and distraction to management and other employees.
In December 2021, Health Canada informed JAMP Pharma that the 40 mg/0.4 mL and 80 mg/0.8 mL presentations of SIMLANDI are not subject to the 24-month statutory stay pursuant to the Patented Medicines (Notice of Compliance) Regulations because AbbVie elected to not market the equivalent high-concentration versions to Canadian patients. In January 2022, JAMP Pharma received notices of compliance for the 40 mg/0.4 mL and 80 mg/0.8 mL presentations of SIMLANDI. AbbVie has commenced applications to judicially review Health Canada’s decision in the Federal Court of Canada, and a hearing took place on 16-17 May 2022. On 17 August 2022, the court issued a decision, finding that Health Canada’s interpretation of the regulations was reasonable and dismissing AbbVie’s applications for judicial review. On 3 October 2022, AbbVie issued a Notice of Appeal. The appeal will be heard by the Federal Court of Appeal on 9 April 2024.
In the event that an appellate court finds in AbbVie’s favor, then market access of SIMLANDI in Canada may be impacted.
Potential patent conflict with Johnson & Johnson ("J&J") related to our biosimilar ustekinumab product, AVT04, have been settled or otherwise resolved in the United States, Japan, Canada, and Europe (Europe being resolved January 2024).
In addition, we, directly or through our partners, may become involved in legal proceedings adverse to other originators or market participants.
Risks Related to Legal and Regulatory Compliance Matters
Recently enacted and future legislation, including healthcare legislative reform measures, may have a material adverse effect on our business and results of operations.
In the United States and some foreign jurisdictions, there have been and continue to be a number of legislative and regulatory changes and proposed changes regarding the healthcare system, including initiatives to contain healthcare costs. For example, in March 2010, the PPACA, was passed, which substantially changed the way health care is financed by both governmental and private insurers and continues to significantly impact the U.S. pharmaceutical industry. The PPACA, among other things, created a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate
43
Table of Contents
Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, added a provision to increase the Medicaid rebate for line extensions or reformulated drugs, established annual fees and taxes on manufacturers of certain branded prescription drugs and promotes a new Medicare Part D coverage gap discount program. The PPACA also includes the BPCIA, which created, among other things, a regulatory framework for the approval of biosimilars and interchangeables.
There have been executive, judicial and Congressional challenges to repeal or replace certain aspects of the PPACA. While Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the PPACA such as removing penalties, starting 1 January 2019, for not complying with the PPACA’s individual mandate to carry health insurance and eliminating the implementation of certain PPACA-mandated fees. Additionally, on 17 June 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the PPACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Further, prior to the U.S. Supreme Court ruling, on 28 January 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the PPACA marketplace, which began 15 February 2021 and remained open through 15 August 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the PPACA. On 16 August 2022, President Biden signed the Inflation Reduction Act of 2022 (the “IRA”) into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in PPACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and through a newly established manufacturer discount program. It is possible that the PPACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges, and the healthcare reform measures of the Biden administration, will impact the PPACA, including the BPCIA.
In addition, other legislative changes have been proposed and adopted in the United States since the PPACA was enacted. For example, on 2 August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect on 1 April 2013 and will stay in effect until 2032, unless additional Congressional action is taken. On 2 January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 which, among other things, further reduced Medicare payments to certain providers, including physicians, hospitals and cancer treatment centers. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Further, there has been heightened governmental scrutiny recently over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. At the federal level, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” which expressed its intent to pursue certain policy initiatives to reduce pharmaceutical prices. For example, the executive order expressed the Biden administration’s support of legislative reforms to lower prescription drug prices, including by allowing Medicare’s negotiation of drug prices. In response to Biden’s executive order, on 9 September 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA directs the HHS Secretary to establish a Drug Price Negotiation Program (the “Program”) to lower prices for certain high-expenditure, single-source prescription drugs and biologics covered under Medicare Part B and Part D that have been approved by the FDA for at least 7 years for prescription drugs and at least 11 years for biologics. Under the Program, the HHS Secretary will publish a list of “selected drugs,” and will then negotiate maximum fair prices (“MFP”) with their manufacturers. The Program will be implemented in stages. Beginning in 2026, 10 Medicare Part D “selected drugs” will be subject to price negotiations. By 2029, and in subsequent years thereafter, the number will increase to 20 drugs and biologics covered under Medicare Part B and Part D. Agreements between HHS and manufacturers will remain in place until a drug or biologic is no longer considered a “selected drug” for negotiation purposes. Manufacturers who do not comply with the negotiated prices set under the Program will be subject to an excise tax based on a percentage of total sales of a “selected drug” up to 95% and potential civil monetary penalties. On 29 August 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, including Stelara, the reference product for AVT04, although the Medicare drug
44
Table of Contents
price negotiation program is currently subject to legal challenges. Further, beginning in October 2023, the IRA will require manufacturers that increase prices of certain Medicare Part B and Part D drugs or biologics at a rate greater than inflation to pay rebates to the Centers for Medicare & Medicaid Services or be subject to civil monetary penalties. The IRA also provides certain incentives for the development and manufacture of biosimilars. For example, the Secretary can grant a one-year delay from price negotiations for biosimilars that have a “high likelihood” of a competing biosimilar product entering the market within the requested delay period. In addition, certain Part B biosimilars qualify for an increase in Medicare payments, to 8% of the 5-year Average Sales Price, from 6% under current law. The HHS Secretary has been directed to promulgate regulations to implement the Program and other IRA health reform measures. In response to the Biden administration’s October 2022 executive order, on 14 February 2023, HHS released a report outlining three new models for testing by the CMS Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future.
At the state level, individual states have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Many EU Member States periodically review their reimbursement procedures for medicinal products, which could have an adverse impact on reimbursement status. We expect that legislators, policymakers and healthcare insurance funds in the EU Member States will continue to propose and implement cost-containing measures, such as lower maximum prices, lower or lack of reimbursement coverage and incentives to use cheaper, usually generic, products as an alternative to branded products, and/or branded products available through parallel import to keep healthcare costs down. Moreover, in order to obtain reimbursement for our products in some EEA countries, including some EU Member States, we may be required to compile additional data comparing the cost-effectiveness of our products to other available therapies. Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including those representing the larger markets. The HTA process is the procedure to assess therapeutic, economic and societal impact of a given medicinal product in the national healthcare systems of the individual country. The outcome of an HTA will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product currently varies between EU Member States.
In December 2021, Regulation No 2021/2282 on Health Technology Assessment, or HTA, amending Directive 2011/24/EU, was adopted in the EU. This Regulation, which entered into force in January 2022 and will apply as of January 2025, is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas. The Regulation foresees a three-year transitional period and will permit EU Member States to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU Member States will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement. If we are unable to maintain favorable pricing and reimbursement status in EU Member States for product candidates that we may successfully develop and for which we may obtain regulatory approval, any anticipated revenue from and growth prospects for those products in the EU could be negatively affected.
In addition, the policies of the FDA, the competent authorities of the EU Member States, the EMA, the European Commission and other comparable regulatory authorities with respect to clinical trials may change and additional government regulations may be enacted. For instance, the regulatory landscape related to clinical trials in the EU recently evolved. The EU Clinical Trials Regulation, or CTR, which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on 31 January 2022. The CTR allows sponsors to make a single submission to both the competent authority and an ethics committee in each EU Member State, leading to a single decision for each EU Member State. The assessment procedure for the authorization of clinical trials has been harmonized as well, including a joint assessment by all EU Member States concerned, and a separate assessment by each EU Member State with respect to specific requirements related to its own territory, including ethics rules. Each EU Member State’s decision is communicated to the sponsor via the centralized EU portal. Once the clinical trial approved, clinical study development may proceed. The CTR foresees a three-year transition period. The extent to which ongoing and new clinical trials will be governed by the CTR varies. For clinical trials in relation to which application for approval was made on the basis of the Clinical Trials Directive before 31 January 2023, the Clinical Trials Directive will continue to apply on a transitional basis until January 31, 2025. By 31 January 2025. all ongoing trials will become subject to the provisions of the CTR.
45
Table of Contents
Compliance with the CTR requirements by us and our third-party service providers, such as CROs, may impact our developments plans.
It is currently unclear to what extent the UK will seek to align its regulations with the EU in the future. The UK regulatory framework in relation to clinical trials is derived from existing EU legislation (as implemented into UK law, through secondary legislation).
On 17 January 2022, the UK Medicines and Healthcare products Regulatory Agency, or MHRA, launched an eight-week consultation on reframing the UK legislation for clinical trials. The UK Government published its response to the consultation on 21 March 2023 confirming that it would bring forward changes to the legislation. These resulting legislative amendments will determine how closely the UK regulations will align with the CTR. In October 2023, the MHRA announced a new Notification Scheme for clinical trials which enables a more streamlined and risk-proportionate approach to initial clinical trial applications for Phase 4 and low-risk Phase 3 clinical trial applications. A decision by the UK not to closely align its regulations with the new approach that will be adopted in the EU may have an effect on the cost of conducting clinical trials in the UK as opposed to other countries and/or make it harder to seek a marketing authorization in the EU for our product candidates on the basis of clinical trials conducted in the UK.
In addition, on 26 April 2023, the European Commission adopted a proposal for a new Directive and Regulation to revise the existing pharmaceutical legislation. If adopted in the form proposed, the recent European Commission proposals to revise the existing EU laws governing authorization of medicinal products may result in a decrease in data and market exclusivity opportunities for our product candidates in the EU and make them open to generic or biosimilar competition earlier than is currently the case with a related reduction in reimbursement status.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies governing clinical trials, our development plans may be impacted.
We may be subject to federal and state healthcare laws, including those governing fraud and abuse, false claims, physician payment transparency and health information privacy and security laws, and comparable foreign law equivalents. If we are unable to comply or have not fully complied with such laws, we could face substantial penalties including administrative, civil and criminal penalties, damages, fines, and exclusion from participation in government health care programs.
Our operations may be subject to various civil and criminal fraud and abuse laws. In the United States, federal fraud and abuse laws include, without limitation, the False Claims Act (“FCA”), the Anti-Kickback Statute (“AKS”), the Exclusions Law, and the Civil Monetary Penalties Law (“CMPL”). Many states have similar state laws. These laws may impact, among other things, our research activities as well as our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
•the federal Anti-Kickback Statute, which prohibits, among other things, any individual or entity from knowingly and willfully soliciting, offering or paying remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce another individual or entity to : (a) refer an individual to a person for the furnishing (or arranging for the furnishing) of any item or service for which payment may be made under a federal health care program; (b) purchase or order any covered item or service; (c) arrange for the purchase or order of any covered item or service; or (d) recommend the purchase or order of any covered item or service;
•federal civil and criminal false claims laws and civil monetary penalties laws, including the FCA and the CMPL, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented false, fictitious, or fraudulent claims for payment to the U.S. government;
•the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of health information that allows identification of individual patients on covered entities, including certain healthcare providers, health plans, and healthcare clearinghouses, and their business associates, independent contractors of a covered entity that perform certain services involving the use or disclosure of individually identifiable health information, as well as their covered subcontractors;
•Federal and state transparency laws and regulations, such as the federal Physician Payments Sunshine Act. The federal Physician Payment Sunshine Act which requires certain manufacturers of drugs, devices,
46
Table of Contents
biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services information related to payments and other transfers of value made by such manufacturers to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physicians assistants and nurse practitioners), and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members in such manufacturers; and
•state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the national or federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; national or state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and national or state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Outside the United States, interactions between pharmaceutical companies and healthcare professionals are also governed by strict laws, such as national anti-bribery laws of European countries, national sunshine rules, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, health care reform legislation has strengthened these laws. For example, in the United States the PPACA, among other things, amended the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes, such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, integrity oversight and reporting obligations, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Moreover, one or more of our commercial partners may be subject to the above law and may be investigated or sued for any one or more of the previous concerns which may in turn materially impact us by virtue of our association with such commercial partner(s).
The international aspects of our business expose us to business, regulatory, political, operational, financial and economic risks.
We currently have international operations and a number of international collaborations. Doing business internationally involves a number of risks, including but not limited to:
•multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
•failure by us or our collaboration partners to obtain and maintain regulatory approvals for the use of our products in various countries;
•additional potentially relevant third-party patent rights;
•complexities and difficulties in obtaining protection and enforcing our intellectual property;
•difficulties in staffing and managing foreign operations by us or our collaboration partners;
•complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems by our collaboration partners;
•limits in our ability or our collaboration partners’ ability to penetrate international markets;
47
Table of Contents
•financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products;
•foreign exchange risk, as we have significant asset and liabilities denominated in foreign currencies (mainly in EUR, GBP, ISK, and CHF), and a 10% fluctuation of the exchange rate of ISK against the USD can significantly impact us;
•natural disasters, political and economic instability, including wars such as the Russia-Ukraine conflict, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions;
•certain expenses including, among others, expenses for travel, translation and insurance; and
•regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, specifically our books and records provisions or its anti-bribery provisions.
We are subject to anti-corruption laws and regulations, export and import controls, and sanctions laws and regulations of the United States and other countries. Compliance with these legal standards could impair our ability to compete in international markets. We could face criminal liability and other serious consequences for violations, which could harm our business, prospects and financial condition.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, and other state and national anti-bribery laws in jurisdictions in which we may conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, CROs, contractors and other collaborators and partners from authorizing, promising, offering, providing, soliciting or receiving, directly or indirectly, improper payments or anything else of value improperly to or from recipients in the public or private sector. We have engaged third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization phase, and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, CROs, contractors and other collaborators and partners, even if we do not explicitly authorize or have actual knowledge of such activities. In addition, the FCPA imposes accounting standards and requirements on publicly traded U.S. corporations and their foreign affiliates, which requires such companies to maintain complete and accurate books and records and maintain a system of internal accounting controls.
We are also subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, as well as by comparable import, export, and sanctions laws and regulations in other jurisdictions. Compliance with applicable regulatory requirements regarding the import and export of our products may create delays in the introduction of our products in international markets or, in some cases, prevent the export our products to some countries or persons altogether.
Furthermore, U.S. export control laws and economic sanctions prohibit the shipment of certain products and services to countries, governments, and persons targeted by U.S. sanctions.
Any changes in the laws and regulations described above, shift in the enforcement or scope of existing laws and regulations, or change in the countries, governments, persons, or technologies targeted by such laws and regulations, could result in decreased ability to export our product candidates internationally. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
A breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of our confidential information in internal systems or those used by third party collaborator partners or other contractors or consultants, could compromise the confidentiality, integrity and availability of our confidential information in information technology systems, network-connected control systems and/or our data, interrupt the operation of our business and/or affect our reputation.
To achieve our business objectives, we rely on sophisticated information technology systems, including software, mobile applications, cloud services and network-connected control systems, some of which are managed, hosted, provided or serviced by third parties. Internal or external events that compromise the confidentiality, integrity and availability of our systems and data may significantly interrupt the operation of our business, result in significant costs and/or adversely affect
48
Table of Contents
our reputation and/or place us at a competitive disadvantage resulting from the improper disclosure or theft of confidential information or intellectual property.
Our information technology systems are highly integrated into our business, including our research and development (“R&D”) efforts, our clinical and commercial manufacturing processes and our product sales and distribution processes. Further, as certain employees are working remotely, our reliance on our and third-party information technology systems has increased substantially and is expected to continue to increase. The complexity and interconnected nature of our systems make them potentially vulnerable to breakdown or other service interruptions. Our systems are subject to frequent attempted cyberattacks. As the cyber-threat landscape evolves, these attacks are growing in frequency, sophistication and intensity and are becoming increasingly difficult to detect. Such attacks could include the use of harmful and virulent malware, including ransomware or other denials of service, that can be deployed through various means, including the software supply chain, e-mail, malicious websites and/or the use of social engineering. Attacks such as those experienced by governmental entities (including those that approve and/or regulate our products, such as the FDA, the European Commission or EMA) and other multi-national companies, including some of our peers, could leave us unable to utilize key business systems or access or protect important data, and could have a material adverse effect on our ability to operate our business, including developing, gaining regulatory approval for, manufacturing, selling and/or distributing our products.
Our systems and possibly those of permissible third parties also contain and utilize a high volume of sensitive data, including intellectual property, trade secrets, financial information, regulatory information, strategic plans, sales trends and forecasts, litigation materials and/or personal information belonging to us, our staff, customers and/or other parties. In some cases, we and/or permissible third parties may use third-party service providers to process, store, manage or transmit such data, which may increase our risk. Intentional or inadvertent data privacy or security breaches (including cyberattacks) or lapses by employees, service providers (including providers of information technology-specific services), nation states (including groups associated with or supported by foreign intelligence agencies), organized crime organizations, “hacktivists” or others, create risks that our sensitive data may be exposed to unauthorized persons, our competitors, or the public.
Domestic and global government regulators, our business partners, suppliers with whom it does business, vendors and law firms that host our documents and information in connection with transactions or proceedings, companies that provide us or our partners with business services and companies that we may acquire may face similar risks, and security breaches of their systems could adversely affect our security, leave us without access to important systems, products, raw materials, components, services or information or expose our confidential data. As a part of our business, we share confidential information with third parties, such as commercial partners, consultants, advisors and vendors. We are at risk of our confidential data being disclosed without our consent or lost if these third parties’ servers or databases experience security breaches of their systems.
We have experienced system downtime and attacks but we do not believe such downtime and attacks have had, either individually or in the aggregate, a material adverse effect on our business or results of operations. We continue to invest in the monitoring, protection and resilience of our critical and/or sensitive data and systems and have a Security Operations Center ("SOC") provider and 24/7 monitoring of our systems. However, there can be no assurances that our efforts will detect, prevent or fully recover systems or data from all breakdowns, service interruptions, attacks, and/or breaches of our systems that could adversely affect our business and operations and/or result in the loss or exposure of critical, proprietary, private, confidential or otherwise sensitive data, which could result in material financial, legal, business or reputational harm or negatively affect our share price. While we maintain cyber-liability insurance, our insurance is not sufficient to cover it against all losses that could potentially result from a service interruption, breach of our systems or loss of critical or sensitive data.
We are also subject to various laws and regulations globally regarding privacy and data protection, including laws and regulations relating to the collection, storage, handling, use, disclosure, transfer and security of personal data. The legislative and regulatory environment regarding privacy and data protection is continuously evolving and developing and the subject of significant attention globally. For example, in the EEA, we are subject to the General Data Protection Regulation (“GDPR”), which became effective in May 2018, imposes strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting and which provides for substantial penalties for non-compliance. Other jurisdictions where we operate have enacted or proposed similar legislation and/or regulations. Failure to comply with these current and future laws could result in significant penalties, liability for damages incurred by individuals whose privacy is violated, and could have a material adverse effect on our business and results of operations.
49
Table of Contents
We and our service providers may be subject to evolving data protection and security laws, including in the EEA and the UK, in relation to certain processing of personal data. The actual or perceived failure to comply with such laws could harm our financial condition and operating results and involve distraction from other aspects of our business.
We are also subject to various laws and regulations globally regarding privacy and data protection, including laws and regulations relating to the collection, storage, handling, use, disclosure, transfer and security of personal data. The legislative and regulatory environment regarding privacy and data protection is continuously evolving and developing and the subject of significant attention globally. For example, in the EEA we are subject to the EU’s General Data Protection Regulation (“ EU GDPR”), which became effective in May 2018, and in the United Kingdom, to the United Kingdom’s GDPR (“UK GDPR”). Both regulations impose strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting and which provides for substantial penalties for non-compliance.
Data privacy and security laws are rapidly evolving, becoming increasingly stringent, and creating regulatory uncertainty. Additionally, related obligations may be subject to interpretations which may vary from one country to another. Preparing for and complying with these obligations requires us to devote significant resources, which may necessitate changes to our services, information technologies, systems, and practices and to those of any third parties that process personal data on our behalf.
Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States, such as the EEA and UK’s standard contractual clauses, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. If there is no lawful manner for us to transfer personal data from the EEA, the UK or other jurisdictions to the United States, or if the requirements for a legally-compliant transfer are too onerous, we could face significant adverse consequences, including the interruption or degradation of our operations, the need to relocate part of or all of our business or data processing activities to other jurisdictions at significant expense, increased exposure to regulatory actions, substantial fines and penalties, the inability to transfer data and work with partners, vendors and other third parties, and injunctions against our processing or transferring of personal data necessary to operate our business. Additionally, companies that transfer personal data out of the EEA and UK to other jurisdictions, particularly to the United States, are subject to increased scrutiny from regulators, individual litigants, and activist groups. Some European regulators have ordered companies to suspend or permanently cease certain transfers out of Europe for allegedly violating the EU GDPR’s cross-border data transfer limitations.
We are also bound by contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful. For example, certain privacy laws, such as the EU and UK GDPR, require our customers to impose specific contractual restrictions on their service providers.
In addition, because of the remote work policies we implemented due to the COVID-19 pandemic, information that is normally protected, including company confidential information, may be less secure. Cybersecurity and data security threats continue to evolve and raise the risk of an incident that could affect our operations or compromise our business information or sensitive personal data, including health data. We may also need to collect more extensive health-related information from our employees to manage our workforce.
Other jurisdictions where we operate have enacted or proposed similar legislation and/or regulations. If we or our third-party partners fail to comply or are alleged to have failed to comply with data protection and privacy laws and regulations, or if we were to experience a data breach involving personal data, we could be subject to government enforcement actions. In addition, under the EU GDPR, companies may face private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests. Any associated claims, inquiries, or investigations or other government actions could lead to unfavorable outcomes that have a material impact on our business including through significant penalties or fines, monetary judgments or settlements including criminal and civil liability for us and our officers and directors, increased compliance costs, delays or impediments in the development of new products, negative publicity, increased operating costs, diversion of management time and attention, or other remedies that harm our business, including orders that we modify or cease existing business practices.
50
Table of Contents
We currently rely on Alvogen’s ERP solution and other components of Alvogen's IT infrastructure and will continue to do so for the foreseeable future.
We currently rely on certain IT infrastructure and software owned and/or operated by Alvogen. A service agreement is in place between us and Alvogen addressing confidentiality, service and fees and other customary matters, and the two companies have entered into an agreement regarding the ownership, access rights and retention of shared data, pursuant to which Alvogen stores our data separate from Alvogen data.
We have signed a separate license agreement for our own ERP system and are in the process of implementing and migrating to the new platform in an environment separate from Alvogen’s. This environment set up is underway and the system is expected to go live during the second half of 2024. However, in the meantime, we are relying on Alvogen’s platform and licenses. In addition, we also use a small number of applications related to ERP, that are licensed through Alvogen. We plan to stop using these applications during the second half of 2024.
We are also currently relying on Alvogen’s Azure (cloud) environment and have moved or migrated the majority of our data into a dedicated separate environment. While our components of the environment have been logically separated from Alvogen’s components and are operated by us, a limited number of Alvogen IT administrators continue to have read-only access to our Azure subscriptions for to monitor usage billing purposes. Although we plan to physically separate the remaining resources and have our ERP platform go live by the end of 2024, following the migration of the Azure environment, there can be no assurance that this project will be successful at all or will be achieved on schedule.
There is a risk that other issues due to the shared infrastructure between the companies have not yet been identified, posing a risk to our business operations which are currently relying on the confidentiality, integrity and availability of critical information systems and our data stored on Alvogen’s IT infrastructure. For more information on the service agreements between us and Alvogen, please see “Item 7.B Related Party Transactions.”
The implementation of an ERP system is a complex and time-consuming project that requires transformations of business and finance processes to reap the benefits of the ERP system. Any such transformation involves risk inherent in the conversion to a new system, including loss of information and potential disruption to normal operations. Delays or the failure to fully implement the ERP system and fully separate the IT infrastructure, or interruptions in service or operational difficulties during or following the full implementation of the ERP system, may adversely impact our financial results and could lead to business disruption and loss of business. In addition, the failure or abandonment of any part of the ERP system could result in a write-off of part or all of the costs that have been capitalized on the project, which could adversely affect our results of operations and financial condition. Further, if the ERP system does not operate as intended, the effectiveness of our internal control over financial reporting could be adversely affected or our ability to assess those controls adequately could be delayed. Significant delays in documenting, reviewing and testing our internal control over financial reporting could cause us to fail to comply with SEC reporting obligations related to our management’s assessment of internal control over financial reporting.
Our IT Governance (ITG) and Information Security Management System (ISMS) may not be sufficient to ensure the effective and efficient use of IT in enabling the organization to achieve business objectives and secure the confidentiality, integrity and availability of critical information technology systems and data.
We currently do not have a fully implemented ITG and ISMS in place. At the end of 2022, we hired an Information Security Officer who reports to the General Counsel to strengthen ISMS. The Information Security Officer will introduce an information security (“InfoSec”) program, which includes revising and updating the ISMS, and, together with the CIO, the ITG. The InfoSec program plans to introduce enhanced policies and procedures. We currently have in place ITIL aligned procedures, covering access management, change management, incident management, business continuity and disaster recovery, which will be further reviewed and revised and aligned to the ISO 27001 framework.
We do not currently have a data retention policy in place. We have established procedures for IT business continuity and disaster management, with restore tests conducted quarterly. The full implementation of ITG and ISMS may not be successfully completed during 2024, or at all, due to lack of capabilities, resources or funding, prioritization, or other reasons.
Some of our critical systems and data are hosted on premise in one data center, without a secondary data center for redundancy. Force majeure events impacting the data center such as fire, flood, earthquake, or power outage can therefore pose a risk to our operation and may compromise the confidentiality, integrity and availability of those systems and data. A new data center has been completed as part of the extension build at Saemundargata 19, Reykjavik, Iceland, which will be taken into use in the Spring of 2024 and implementation of the equipment is in progress.
51
Table of Contents
While we have invested, and continue to invest, in ITG and ISMS, there can be no assurance that our efforts will be sufficient to ensure the effective and efficient use of IT, which could adversely affect our business and operations and/or result in the loss of critical or sensitive data, which could result in financial, legal, business or reputational harm.
Our ISMS may be subject to security breaches or other incidents that could result in misappropriation of funds, disruption to operations, disclosure of commercially or personally sensitive information, legal or regulatory breaches and liability, as well as other costs and reputational damage. Given the increasing sophistication and evolving nature of these threats, the possibility of security breaches occurring in the future cannot be ruled out. An extended failure of critical system components, caused by accidental or malicious actions, including those resulting from a cybersecurity attack, could result in a significant commercial loss, interruption to operations, loss of access to critical data or systems, unfavorable publicity, damage to reputation, regulatory investigations, fines or penalties, litigation or other claims by affected parties and possible financial obligations for liabilities and damages related to the theft or misuse of our information and other business delays or disruptions, any of which could have an adverse effect on our business, financial condition, results of operations and reputation. Further, we may be forced to expend significant financial and operational resources in response to a security breach, including repairing system damage, increasing security protection costs by deploying additional personnel and modifying or enhancing protection technologies, investigating and remediating any information security vulnerabilities and defending against and resolving legal and regulatory claims, all of which could divert resources and the attention of management and key personnel away from business operations and adversely affect our business, financial condition and results of operations. See also “A breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of our confidential information in internal systems or those used by third party collaborator partners or other contractors or consultants, could compromise the confidentiality, integrity and availability of our confidential information in information technology systems, network-connected control systems and/or our data, interrupt the operation of our business and/or affect our reputation.”
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our facilities and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by us and our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage.
We or the third parties upon whom we depend may be adversely affected by earthquakes or other natural disasters, and our business continuity and disaster recovery plans may not adequately protect from a serious disaster. Our manufacturing facility and inventories are located in Reykjavik, Iceland and any severe natural or other disaster or disruption at this site could have a material adverse effect on our financial condition and results of operations.
Our corporate headquarters, manufacturing site and a large part of our R&D division are located in Reykjavik, Iceland. Iceland is geographically isolated and has in the past experienced severe earthquakes and other natural disasters, such as volcanic eruptions. Earthquakes or other natural disasters could severely disrupt our operations or those of our collaboration partners and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure (such as the manufacturing facilities of our third-party providers of power or water supplies) or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently are limited and are unlikely to prove adequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which, particularly when taken together with our current lack of business continuity insurance, could have a material adverse effect on our business.
52
Table of Contents
Our business could be materially disrupted by strikes, work stoppages or other labor actions in Iceland or elsewhere.
Under applicable Icelandic labor laws, members of a labor union are required to participate in a strike called by the labor union or work stoppage called by an employers association. As many of our employees in Iceland are members of Icelandic labor unions, we may be faced with strikes, work stoppages or other labor actions in Iceland which may materially disrupt our business at our headquarters, manufacturing site, and the local part of our R&D division. Work stoppages, strikes or other labor actions at other companies or industries within Iceland, including international air traffic, could also have an adverse effect on our ability to operate and may impact earnings and other key business metrics. In addition, work stoppages, strikes or other labor actions of our employees outside of Iceland may affect our operations at those sites outside of Iceland, and work stoppages, strikes or other labor actions of employees of our vendors, suppliers or partners may affect the performance of our partners, our supply chain, our ability to sell our products and our operations generally.
Iceland’s implementation of EEA rules may not be comprehensive or may be delayed, which may result in certain risks and uncertainty for us and our business.
We have significant assets, including our subsidiary Alvotech hf., in Iceland. Many of our assets and material agreements are therefore governed by Icelandic law and subject to the jurisdiction of the Icelandic courts. As an EEA country, Iceland is obligated to implement important parts of EU law relating to the “four freedoms” within the EU single market. Certain aspects of our operations are subject to laws originating from such implementation. If the Icelandic state fails to draft national legislation which conforms with such EU rules, Icelandic individuals and legal persons may not be able to rely on the relevant EU rules and the Icelandic courts could be restricted from applying them unless the Icelandic legislation can be interpreted in a way which conforms with EU rules. This could negatively affect us or other individuals or legal persons who conduct business with us in Iceland.
We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise are unable to develop and maintain an effective system of internal controls in the future, we may not be able to produce timely and accurate financial statements or comply with applicable laws and regulations, which may adversely affect investor confidence in us and, as a result, the value of Ordinary Shares.
We have identified material weaknesses in the design and operating effectiveness of our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the preparation of the consolidated financial statements covered by this report, we identified material weaknesses as follows: (i) we did not have a sufficient number of trained professionals with an appropriate level of internal control knowledge, training and experience; (ii) we did not consistently operate all controls, specifically related to consistent execution, adequate review procedures, and maintaining documentation to evidence control performance, including assessing the accuracy and completeness of information used in the execution of controls; and (iii) we did not implement effective controls over the segregation of duties and certain information technology general controls for information systems that are relevant to the preparation of our financial statements. These material weaknesses could result in a misstatement of our accounts or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected.
Upon identifying the material weaknesses, we began taking steps intended to address the underlying causes of the control deficiencies in order to remediate the material weaknesses, which included the following activities during 2022 and 2023: (i) hired qualified individuals with strong technical accounting, internal control and SEC reporting experience and continued training control owners to reaffirm expectations as it relates to the control design and execution of such controls, including enhancements to the documentation to evidence the execution of the controls; (ii) enhanced the Company's governance and oversight processes by establishing a formal control governance structure, ensuring clear roles and responsibilities for control oversight, conducting regular meetings to review control performance, and implementing a system for reporting control-related matters to the Audit Committee; (iii) implemented formal documentation of certain policies and procedures, and/or redesigned entity level controls, business process-level controls across all significant accounts and information technology general controls across all relevant domains; (iv) developed and executed a risk-based testing plan to cover all identified controls through a mix of design assessment, independent testing of operating effectiveness and management self-certification. The Company has engaged outside consultants to assist in evaluating our internal controls, develop remediation plans to address control deficiencies identified, and actively measure compliance and remediation progress through a quarterly scorecard; and (v) continued implementation of a new enterprise resource planning (“ERP”) system including the engagement of outside consultants to help design and implement automated controls and enhance our information technology general controls environment as part of the ERP system implementation.
53
Table of Contents
In addition to the above actions, we expect to engage in additional activities to enhance our control environment including but not limited to: (i) complete the implementation of a new ERP system, which includes increased automated functionality and controls for the preparation of the financial statements to prevent, among other things, unauthorized overrides, and enhance user access controls, segregation of duties with the system, and audit trails to track and monitor activities; (ii) implement stronger IT controls to ensure the integrity and security of financial information, including enhancing access and change management controls and implementing regular system monitoring and testing; (iii) continue focusing on consistent control execution, adequate review procedures, and improving control documentation, including the accuracy and completeness of information used in the performance of controls; and (iv) continue engaging outside consultants to assist in evaluating the internal controls, and actively measure compliance and remediation through quarterly scorecard.
We cannot assure that the measures we have taken to date, and are continuing to implement, will be sufficient to remediate the material weaknesses identified and avoid potential future material weaknesses. If the steps we take do not remediate the material weaknesses in a timely manner, we will be unable to conclude that we maintain effective internal control over financial reporting. Accordingly, there could continue to be a reasonable possibility that a material misstatement of our financial statements would not be prevented or detected on a timely basis.
If we fail to remediate our existing material weaknesses, identify new material weaknesses in our internal controls over financial reporting, are unable to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, are unable to conclude that our internal controls over financial reporting are effective, or if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal controls over financial reporting when we are no longer an emerging growth company, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of the Ordinary Shares could be negatively affected. As a result of such failures, we could also become subject to investigations by the stock exchanges on which our securities are listed, the Securities and Exchange Commission (“SEC”), or other regulatory authorities, and become subject to litigation from investors and shareholders, which could harm our reputation and financial condition or divert financial and management resources from our regular business activities.
Risks Related to Ownership of our Ordinary Shares and Warrants and our Status as a Public Company
We have and will incur increased costs as a result of operating as a public company, and our management will devote substantial time to new compliance initiatives.
We will continue to incur significant legal, accounting and other expenses as a public company, and these expenses may increase even more if and when we are no longer an emerging growth company, as defined in Section 2(a) of the Securities Act. As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules adopted, and to be adopted, by the SEC and Nasdaq. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time consuming and costly. The increased costs will increase our net loss. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be forced to accept reduced policy limits or incur substantially higher costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on our board advisors or as executive officers.
The market price and trading volume of our Ordinary Shares and Warrants may be volatile and could decline significantly.
The stock markets, including Nasdaq and Nasdaq Iceland Main Market on which Ordinary Shares and Warrants are listed under the symbols ALVO and ALVOW, respectively, have from time to time experienced significant price and volume fluctuations. The market price of Ordinary Shares and Warrants may be volatile and could decline significantly. In addition, the trading volume in Ordinary Shares and Warrants may fluctuate and cause significant price variations to occur. Additionally, any substantial amount of trading or sales in Ordinary Shares could make it difficult for us to raise capital through the issuance of debt or equity securities in the future. Generally, securities of biopharmaceutical companies tend to be volatile and experience significant price and volume fluctuations. We cannot guarantee that the market price of Ordinary Shares and Warrants will not fluctuate widely or decline significantly in the future in response to a number of factors, including, among others, the following:
•the realization of any of the risk factors presented in this Annual Report on Form 20-F;
54
Table of Contents
•actual or anticipated differences in our estimates, or in the estimates of analysts, for our revenues, results of operations, liquidity or financial condition;
•regulatory decisions with respect to our product candidates;
•additions and departures of key personnel;
•failure to comply with the requirements of Nasdaq U.S. and Nasdaq Iceland Main Market;
•failure to comply with the Sarbanes-Oxley Act or other laws or regulations in the United States, Luxembourg and Iceland;
•future issuances, sales or resales, or anticipated issuances, sales or resales, of Ordinary Shares;
•publication of research reports about us;
•the performance and market valuations of other similar companies;
•broad disruptions in the financial markets, including sudden disruptions in the credit markets;
•material and adverse impact of public health emergencies and other world emergencies on the markets and the broader global economy;
•speculation in the press or investment community;
•actual, potential or perceived control, accounting or reporting problems; and
•changes in accounting principles, policies and guidelines.
In the past, securities class-action litigation has often been instituted against companies following periods of volatility in the market price of their shares. This type of litigation could result in substantial costs and divert our management’s attention and resources, which could have a material adverse effect on us.
The dual listing of Ordinary Shares may adversely affect the liquidity and value of those ordinary shares.
Our Ordinary Shares are listed on both The Nasdaq Stock Market in the United States (“Nasdaq”) and Nasdaq Iceland Main Market in Iceland. The trading of Ordinary Shares in these markets takes place in different currencies (U.S. dollars on Nasdaq and Icelandic Krona on Nasdaq Iceland Main Market), at different times (resulting from different time zones, different trading days and different public holidays in the United States and Iceland) and with different settlement mechanics. The trading prices of Ordinary Shares on these two markets may differ due to these and other factors. Any decrease in the price of Ordinary Shares on Nasdaq Iceland Main Market could cause a decrease in the trading price of Ordinary Shares on Nasdaq and vice versa. Investors could seek to sell or buy Ordinary Shares to take advantage of any price differences between the markets through a practice referred to as arbitrage. Any arbitrage activity could create unexpected volatility in both the trading prices on one exchange and Ordinary Shares available for trading on the other exchange. Further, the dual listing of Ordinary Shares may reduce the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for Ordinary Shares in the United States.
The listing of Ordinary Shares on Nasdaq Iceland Main Market may result in increased additional compliance risk, which could have a material effect on our business, results of operations and financial condition, or may delay or discourage a takeover attempt.
Our ordinary shares are listed on both the Nasdaq and Nasdaq Iceland Main Market. Nasdaq Iceland Main Market a regulated market in Iceland operated by Nasdaq Iceland, the Icelandic stock exchange. Issuers on Nasdaq Iceland Main Market are subject to the rules of Nasdaq Iceland Main Market and the relevant rules and regulations given the fact that the securities of the issuer are admitted to trading on a regulated market.
As a dual-listed Luxembourg company listed on Nasdaq Iceland Main Market and Nasdaq, we are subject to reporting requirements and certain other applicable requirements under Luxembourg law, U.S. law and Icelandic law, including, but not limited to, Regulation (EU) No 596/2014 of the European Parliament and of the Council of 16 April 2014, on market abuse, as amended (“MAR”), the Directive 2004/109/EC of the European Parliament and of the Council of 15 December 2004 on the harmonization of transparency requirements in relation to information about issuers whose securities are admitted to trading on a regulated market, as amended (the “Transparency Directive”), the Luxembourg law of 23 December 2016, on market abuse, as amended (“Luxembourg Market Abuse Law”), the Luxembourg law of 11 January 2008 on transparency requirements for issuers, as amended (the “Luxembourg Transparency Law”), the Grand-Ducal regulation of 11 January 2008, on transparency requirements for issuers of securities, as amended (the “Luxembourg Transparency Regulation”), Directive 2004/25/EC of the European Parliament and of the Council of 21 April 2004, on
55
Table of Contents
takeover bids, as amended (the “Takeover Directive”) and the Luxembourg law of 19 May 2006, on takeover bids, as amended (the “Luxembourg Takeover Law”).
Transparency Regime
Holders of shares and other financial instruments may be subject to notification obligations pursuant to the Luxembourg Transparency Law. The following description summarizes these obligations. Holders are advised to consult with their own legal advisors to determine whether the notification obligations apply to them.
The Luxembourg Transparency Law and Luxembourg Transparency Regulation provide that, once the Shares are admitted to listing and trading on Nasdaq Iceland Main Market, if a person acquires or disposes of a shareholding in the Company, and if following the acquisition or disposal the proportion of voting rights held by the person reaches, exceeds or falls below one of the thresholds of 5%, 10%, 15%, 20%, 25%, 33 1/3%, 50% and 66 2/3% (each a “Relevant Threshold”) of the total voting rights existing when the situation giving rise to a declaration occurs, such person must simultaneously notify the Company and the Luxembourg Commission de Surveillance du Secteur Financier (the “CSSF”) of the proportion of voting rights held by it further to such event.
A person must also notify the Company and the CSSF of the proportion of his or her voting rights if that proportion reaches, exceeds or falls below a Relevant Threshold as a result of events changing the breakdown of voting rights and on the basis of the information disclosed by the Company.
The same notification requirements apply to a natural person or legal entity to the extent he/she/it is entitled to acquire, to dispose of, or to exercise voting rights in any of the cases or a combination of them stated in Article 9 of the Luxembourg Transparency Law. The notification requirements set out above also apply to a natural person or legal entity that holds, directly or indirectly: (i) financial instruments that, on maturity, give the holder, under a formal agreement, either the unconditional right to acquire or the discretion as to his or her right to acquire the Ordinary Shares, to which voting rights are attached, already issued by the Company; or (ii) financial instruments which are not included in point (i) but which are referenced to the Ordinary Shares referred to in that point and with an economic effect similar to that of the financial instruments referred to in that point, whether or not they confer a right to a physical settlement.
The number of voting rights shall be calculated as specified in Article 12 and 12a of the Luxembourg Transparency Law.
The notification to the Company and the CSSF must be effected promptly, but not later than four trading days after the date on which the shareholder, or the natural person or legal entity referred to above learns of the acquisition or disposal or of the possibility of exercising voting rights, or on which, having regard to the circumstances, should have learned of it, regardless of the date on which the acquisition, disposal or possibility of exercising voting rights takes effect, as specified in the Luxembourg Transparency Law and the related guidelines of the CSSF. Upon receipt of the notification, but not later than three trading days thereafter, the Company must make public all the information contained in the notification as regulated information within the meaning of the Luxembourg Transparency Law.
As long as the notifications have not been made to the Company in the manner prescribed, the exercise of voting rights relating to the shares exceeding the fraction that should have been notified is suspended. The suspension of the exercise of voting rights is lifted as of the moment the shareholder makes the notification.
Where within the fifteen days preceding the date for which the general meeting has been convened, the Company receives a notification or becomes aware of the fact that a notification has to be or should have been made in accordance with the Luxembourg Transparency Law, the board of directors may postpone the general meeting.
Market Abuse Regime
The rules on preventing market abuse set out in the MAR and the Luxembourg Market Abuse Law are applicable to the Company, persons discharging managerial responsibilities within the Company (including the members of the board of directors) (the “PDMRs”), persons closely associated with PDMRs, other insiders and persons performing or conducting transactions in the Company’s financial instruments. Certain important market abuse rules set out in the MAR and the Luxembourg Market Abuse Law that are relevant for investors are described hereunder.
The Company is required to make inside information public. Pursuant to the MAR, inside information is information of a precise nature, which has not been made public, relating, directly or indirectly, to the Company or to one or more financial instruments, and which, if it were made public, would be likely to have a significant effect on the prices of those
56
Table of Contents
financial instruments or on the price of related derivative financial instruments. Unless an exception applies, the Company must without delay publish the inside information by means of a press release and post and maintain it on its website for at least five years. The Company must also provide Nasdaq Iceland and the CSSF with its press release that contains inside information at the time of publication.
It is prohibited for any person to make use of inside information by acquiring or disposing of, for its own account or for the account of a third party, directly or indirectly, financial instruments to which that information relates, as well as an attempt thereto (insider dealing). In addition, it is prohibited for any person to disclose inside information to anyone else (except where the disclosure is made in the normal exercise of an employment, profession or duties) or, whilst in possession of inside information, to recommend or induce anyone to acquire or dispose of financial instruments to which the information relates. Furthermore, it is prohibited for any person to engage in or attempt to engage in market manipulation, for instance by conducting transactions which give, or are likely to give, false or misleading signals as to the supply of, the demand for or the price of a financial instrument.
Non-compliance with the notification obligations under the Market Abuse Regulation, set out in the paragraphs above, is an economic offense and could lead to the imposition of criminal prosecution, administrative fines, imprisonment or other sanctions. Nasdaq Iceland Main Market may impose administrative penalties or a cease-and-desist order under penalty for non-compliance. If criminal charges are pressed, Nasdaq Iceland Main Market is no longer allowed to impose administrative penalties and vice versa, Nasdaq Iceland Main Market is no longer allowed to seek criminal prosecution if administrative penalties have been imposed.
Pursuant to Article 19 of the MAR and the Luxembourg Market Abuse Law, PDMRs must notify the CSSF and the Company of any transactions conducted for his or her own account relating to shares or any debt instruments of the Company or to derivatives or other financial instruments linked thereto.
A PDMR within the Company shall not conduct any transactions on its own account or for the account of a third party, directly or indirectly, relating to the Ordinary Shares or debt instruments of the Company or to derivatives or other financial instruments linked to them during a closed period of 30 calendar days before the announcement of an interim financial report or a year-end report which must be made publicly available.
In addition, pursuant to the MAR and the regulations promulgated thereunder as well as the Luxembourg Market Abuse Law, certain persons who are closely associated with persons discharging managerial responsibilities (PDMRs) as defined in Article 1 (26) of the MAR, are also required to notify the CSSF and the Company of any transactions conducted for their own account relating to shares or any debt instruments of the Company or to derivatives or other financial instruments linked thereto in accordance with MAR.
Takeover Regime and Squeeze-out and Sell-out Procedures
The Takeover Directive has been implemented in Luxembourg in the Luxembourg Takeover Law. The Luxembourg Takeover Law provides that if a person, acting alone or in concert, acquires shares in a company which, when added to any existing holdings of a company’s shares, result in such person having voting rights representing at least 33 1/3% of all of the voting rights attached to the issued and outstanding shares in a company, this person is obliged to make a mandatory takeover bid, at a fair price, for the remaining shares in the company. Where the aforementioned percentage-threshold is met, the person acquiring such voting rights will be deemed to have control over the Issuer in accordance with Luxembourg Takeover Law.
The Luxembourg Takeover Law provides that, when a mandatory or voluntary takeover offer is made to all holders of voting shares in a company and after such offer the offeror holds at least 95% of the capital of that company carrying voting rights and 95% of the voting rights of the company, the offeror may require the holders of the remaining shares to sell those shares to the offeror. The price offered for such shares must be a fair price. The price offered in a voluntary offer would be considered a fair price in the squeeze-out proceedings if 90% of the shares of the company carrying voting rights were acquired in such a voluntary offer, in accordance with Luxembourg Takeover Law. The price paid in a mandatory takeover offer is deemed to be a fair price pursuant to Luxembourg Takeover Law.
The Luxembourg Takeover Law provides that, when a mandatory or voluntary takeover bid is made to all holders of voting shares in a company and if after such offer the offeror (together with any person acting in concert with the offeror) holds shares carrying more than 90% of the voting rights, the remaining shareholders may require that the offeror purchase the remaining shares. The price offered in a voluntary offer would be considered a fair price in the sell-out proceedings if 90% of the shares of the company carrying voting rights were acquired in such a voluntary takeover offer, in accordance with Luxembourg Takeover Law. Where the offeree company has issued more than one class of shares, the right of
57
Table of Contents
squeeze-out and sell-out referred to above can be exercised only in the class in which the relevant threshold has been reached.
Even if there has not been an offer pursuant to the Luxembourg Takeover Law, the Luxembourg law of 21 July 2012 on the squeeze-out and sell-out of securities of companies admitted or having been admitted to trading on a regulated market or which have been subject to a public offer (the “Luxembourg Mandatory Squeeze-Out and Sell-Out Law”) provides that if any individual or legal entity, acting alone or in concert with another, becomes the direct or indirect holder (otherwise than by way of a voluntary or mandatory takeover bid pursuant to the Luxembourg Takeover Law) of shares or other voting securities representing at least 95% of the voting share capital and 95% of the voting rights of a company, (i) such shareholder may require the holders of the remaining shares or other voting securities to sell those remaining securities; and (ii) the holders of the remaining shares or securities may require such shareholder to purchase those remaining shares or other voting securities (the “Mandatory Sell-Out”). The Mandatory Squeeze-Out and the Mandatory Sell-Out must be exercised at a fair price according to objective and adequate methods applying to asset disposals in accordance with the Luxembourg Mandatory Squeeze-Out and Sell-Out Law.
Adherence to the requirements of these rules and regulations may increase our legal, accounting and financial compliance costs, make certain activities more difficult, time consuming and costly, place additional strain on resources and divert management’s attention away from other business matters.
In addition, the applicable legal requirements or the interpretation of such requirements by regulators and courts in each of these jurisdictions may differ or conflict which could expose us to additional costs, sanctions and/or fines. Any of these factors could have a material effect on our business, results of operations and financial condition.
The issuance or resale of a substantial number of Ordinary Shares in the public market could occur at any time. These issuances and sales, or the perception in the market that these issuances or sales may occur, could increase the volatility of the market price of Ordinary Shares or result in a significant decline in the public trading price of Ordinary Shares.
Future issuances of debt securities and equity securities may adversely affect us, including the market price of our Ordinary Shares and may be dilutive to existing shareholders.
Significant additional capital will be needed in the future to continue our planned research, development and business operations. In the future, we may incur debt or issue equity ranking senior to our ordinary shares. Those securities will generally have priority upon liquidation. Such securities also may be governed by an indenture or other instrument containing covenants restricting our operating flexibility. Additionally, any convertible or exchangeable securities that we issue in the future may have rights, preferences and privileges more favorable than those of Ordinary Shares. Because our decision to issue debt or equity in the future will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing, nature or success of our future capital raising efforts. As a result, future capital raising efforts may reduce the market price of Ordinary Shares and be dilutive to existing shareholders.
The sale and issuance of our Ordinary Shares to investors, holders of warrants or convertible bonds will cause dilution to our existing shareholders, and the sale of Ordinary Shares acquired by them, or the perception that such sales may occur, could cause the price of our Ordinary Shares to drop.
On 16 November 2022, we and the bondholders amended and restated certain terms and conditions of existing senior bonds and issued new senior bonds in an aggregate principal amount equal to $70,000,000 (the “Senior Bonds”). Pursuant to the terms of the amended Senior Bonds, we issued 4,198,807 warrants to the bondholders on 31 December 2022. Each new warrant entitles the bondholders, upon exercise, to receive from us one fully paid and non-assessable Ordinary Share, at the exercise price of one cent ($0.01) per share. Pursuant to the terms of the warrant, we registered Ordinary Shares underlying the warrants for resale on 14 July 2023.
In addition, we issued convertible bonds, such as the Aztiq Convertible Bond and the 2022 Convertible Bonds during 2022 and 2023. Under the terms of these convertible bond agreements, bondholders have the right to convert their bonds into Ordinary Shares credited as fully paid on 31 December, 2023, 30 June 2024, or when the bond has been called or put up for optional or mandatory redemption, for a conversion price of $10.00 per share. If the bondholders decide to convert the debt into Ordinary Shares, the share ownership of our existing shareholders will be diluted as a result of the issuance of new Ordinary Shares to the bondholders.
Given the substantial number of Ordinary Shares expected to be registered for potential resale by bondholders, the sale of shares by the bondholders, or the perception in the market that the holders of a large number of shares intend to sell
58
Table of Contents
their shares, could increase the volatility of the market price of Ordinary Shares or result in a significant decline in the public trading price of Ordinary Shares. In addition, if the holders of the Senior Bonds exercise their warrants and/or holders of the Aztiq Convertible Bond and our 2022 Convertible Bonds elect to convert the bonds into ordinary shares and we issue new Ordinary Shares, the existing shareholders will be diluted. Existing shareholders could also experience dilution if we issue Ordinary Shares pursuant to our standby equity purchase agreement, dated as of 18 April 2022, by and among Alvotech and YA II PN, LTD. See also “Our Warrants are exercisable for Ordinary Shares and certain Bonds are convertible into Ordinary Shares, the exercise of which would increase the number of shares eligible for future resale in the public market and result in dilution to our shareholders.”
Following these issuances described above and following the expiration of lock-ups of certain other restricted shareholders and as restrictions on resale end and registration statements are available for use, the market price of our Ordinary Shares could decline if the holders of restricted or locked up shares sell them or are perceived by the market as intending to sell them. As such, sales of a substantial number of Ordinary Shares in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of Ordinary Shares.
We have issued and expect to issue in the future additional Ordinary Shares, including under our Management Incentive Plan. Any such issuances would dilute the interest of our shareholders and likely present other risks.
We have adopted the Alvotech Management Incentive plan in 2022 (the “2022 Plan”) under which restricted stock units (“RSUs”) and options have been granted in 2022 and 2023. Subject to certain vesting and other terms and conditions, the RSUs and options may be settled in Ordinary Shares. If all RSUs and options vest and are exchanged for Ordinary Shares, the combined grants, excluding forfeitures and deliveries, may result in an aggregate of 5,505,941 Ordinary Shares
Ordinary Shares reserved issued under the 2022 Plan become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements, lock-up agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. The aggregate number of Ordinary Shares initially reserved for issuance under the 2022 Plan is 16,802,386 shares. In August 2022, we filed a registration statement on Form S-8 under the Securities Act to register Ordinary Shares or other securities convertible into or exchangeable for Ordinary Shares pursuant to the 2022 Plan, and we may file additional registration statements on Form S-8 in the future.
Accordingly, shares registered under such registration statements may be immediately available for sale in the open market.
Any such issuances of additional Ordinary Shares or securities convertible into Ordinary Shares:
•may significantly dilute the equity interests of our investors;
•may subordinate the rights of holders of Ordinary Shares if securities are issued with rights senior to those afforded Ordinary Shares; and
•may adversely affect prevailing market prices for Ordinary Shares.
We expect to issue a substantial number of Ordinary Shares or other securities convertible into or exchangeable for Ordinary Shares, including under our 2022 Plan.
Our Warrants are exercisable for Ordinary Shares and certain Bonds are convertible into Ordinary Shares, the exercise of which would increase the number of shares eligible for future resale in the public market and result in dilution to our shareholders.
As a result of the Business Combination being consummated, outstanding warrants to purchase an aggregate of
10,916,647 Ordinary Shares became exercisable in accordance with the terms of the warrant agreement, dated 21
September 2020 by and between OACB and Continental Stock Transfer & Trust Company, as warrant agent, governing
OACB’s outstanding warrants, which was assigned to and assumed by Alvotech pursuant to that certain Assignment,
Assumption and Amendment Agreement dated as of 15 June 2022 (the “Warrant Agreement”).
These warrants became exercisable on 15 July 2022. The exercise price of these warrants is $11.50 per share, or approximately $125.5 million, assuming none of the warrants are exercised through “cashless” exercise. To the extent such warrants are exercised, additional Ordinary Shares will be issued, which will result in dilution to the holders of Ordinary Shares and increase the number of shares eligible for resale in the public market. On 8 March 2024, there were 9,943,434
59
Table of Contents
warrants entitling the holders to acquire one Ordinary Share at a price of $11.50 (the "Warrants") outstanding, the last reported sales price of our ordinary shares was $15.60 per share and the last reported sales price of our Public Warrants was $4.45 per warrant. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of Ordinary Shares. See “—The warrants may not continue to be in the money, and they may expire worthless and the terms of the Public Warrants may be amended in a manner adverse to a holder if holders of at least 50% of the then outstanding Public Warrants approve of such amendment.”
In addition, on 16 November 2022, we and the bondholders amended and restated certain terms of existing Senior Bonds and issued new senior bonds in an aggregate principal amount equal to $70.0 million. Pursuant to the terms of the amended Senior Bonds, we were required to use commercially reasonable endeavors to raise new funding through issuance of additional Ordinary Shares (by way of ordinary shares, structured equity and/or preference shares) and/or unsecured convertible bond(s), for net proceeds of at least $75.0 million of net proceeds by 15 December 2022, and are required to raise $150.0 million in net proceeds by 31 March 2023. We failed to raise at least $75.0 million by 15 December 2022, so we were required to grant penny warrants representing 1.5% of the ordinary share capital to the bondholders, and if we had failed to raise at least $150.0 million by 31 March 2023, we would have been required to grant penny warrants representing 1.00% of the ordinary share capital to the bondholders. Since we had not raised $75.0 million of net proceeds by 15 December 2022, we issued 4,198,807 warrants to the bondholders on 31 December 2022. Each new warrant entitles the bondholders, upon exercise, to receive from us one fully paid and non-assessable Ordinary Share, at the exercise price of one cent ($0.01) per share. If the bondholders exercise their warrants and we issue new Ordinary Shares, the existing shareholders will be diluted. Following the issuance of the 2022 Convertible Bonds and the closing of the private placement of Ordinary Shares for gross proceeds of $137.0 million on 10 February 2023, we are not obligated to issue the additional 1.00% warrants to the bondholders.
The Warrants may not continue to be in the money, and they may expire worthless and the terms of the Public Warrants may be amended in a manner adverse to a holder if holders of at least 50% of the then outstanding Public Warrants approve of such amendment.
There is no guarantee that the Warrants will continue to be in the money and, as such, the Warrants may expire
worthless. For example, between 1 January 2023 and 29 February 2024, the last reported sales prices of our ordinary
shares on Nasdaq fluctuated between $6.90 on 29 June 2023 and $17.27 on 26 February 2024.
The Warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and Oaktree Acquisition Corp. II (“OACB”), and were assumed at the time of the Closing by us, pursuant to a warrant assignment, assumption and amendment agreement by and between us, OACB, Continental Stock Transfer & Trust Company, Computershare Inc. and Computershare Trust Company. Computershare is currently the warrant agent. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity, correct any defective provision or correct any mistake, amend the definition of “Ordinary Cash Dividend” or add or change any provisions with respect to matters or questions arising under the warrant as the parties may deem necessary or desirable and that the parties deem shall not adversely affect the rights of the warrant holders, but requires the approval by the holders of at least 50% of the then-outstanding Public Warrants to make any change that adversely affects the interests of the registered holders of Public Warrants. Accordingly, we may amend the terms of the Public Warrants in a manner adverse to a holder if holders of at least 50% of the then-outstanding Public Warrants approve of such amendment and, solely with respect to any amendment to the terms of the Private Placement Warrants or any provision of the warrant agreement with respect to the private placement warrants, 50% of the number of the then outstanding Private Placement Warrants. Although our ability to amend the terms of the Public Warrants with the consent of at least 50% of the then-outstanding Public Warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash, shorten the exercise period or decrease the number of Ordinary Shares purchasable upon exercise of a warrant.
We may redeem the Warrants prior to their exercise at a time that is disadvantageous to the holder, thereby making such warrants worthless.
We may redeem the Warrants prior to their exercise at a time that is disadvantageous to the holder, thereby making such warrants worthless. We have the ability to redeem outstanding Public Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, provided that the closing price of Ordinary Shares equals or exceeds $18.00 per share (as adjusted for share subdivisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading day period ending on the third trading day prior to the date on which a notice of redemption is sent to the warrant holders. We will not redeem the warrants as described above unless a registration statement under the Securities Act covering Ordinary Shares issuable upon exercise of such warrants is effective and a current prospectus relating to those Ordinary Shares is available throughout the 30-day redemption period.
60
Table of Contents
If and when the Public Warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding Public Warrants could force holders (i) to exercise the Public Warrants and pay the exercise price therefor at a time when it may be disadvantageous to do so, (ii) to sell the Public Warrants at the then-current market price when holders might otherwise wish to hold the Public Warrants, or (iii) to accept the nominal redemption price which, at the time the outstanding Public Warrants are called for redemption, is likely to be substantially less than the market value of the Public Warrants.
In addition, we will have the ability to redeem the outstanding Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.10 per warrant if, among other things, the closing price of Ordinary Shares equals or exceeds $10.00 per share (as adjusted for share sub-divisions, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like) on the trading day prior to the date on which a notice of redemption is sent to the warrant holders. Recent trading prices for Ordinary Shares have exceeded the $10.00 per share threshold at which the Warrants would become redeemable. In such a case, the holders will be able to exercise their Warrants prior to redemption for a number of Ordinary Shares determined based on the redemption date and the fair market value of Ordinary Shares.
The value received upon exercise of the Warrants (1) may be less than the value the holders would have received if they had exercised their Warrants at a later time when the underlying share price is higher and (2) may not compensate the holders for the value of the Warrants.
The JOBS Act permits “emerging growth companies” like Alvotech to take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies, which may make our Ordinary Shares less attractive to investors.
We currently qualify as an “emerging growth company” as defined in Section 2(a)(19) of the Securities Act, as modified by the Jumpstart Its Business Startups Act of 2012, which is referred to as the “JOBS Act.” As such, we take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies for as long as it continues to be an emerging growth company, including the exemption from the auditor attestation requirements with respect to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act. As a result, our shareholders may not have access to certain information they deem important.
We cannot predict if investors will find Ordinary Shares less attractive because we rely on these exemptions. If some investors find Ordinary Shares less attractive as a result, there may be a less active trading market and share price for Ordinary Shares may be more volatile. We may incur increased legal, accounting and compliance costs associated with Section 404 of the Sarbanes-Oxley Act.
Risks Related to Investment in a Luxembourg Company and Our Status as a Foreign Private Issuer
As a foreign private issuer, we are exempt from a number of U.S. securities laws and rules promulgated thereunder and is permitted to publicly disclose less information than U.S. public companies must. This may limit the information available to holders of Ordinary Shares.
We qualify as a “foreign private issuer,” as defined in the SEC’s rules and regulations, and, consequently, we are not subject to all of the disclosure requirements applicable to public companies organized within the United States. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, our officers and directors are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. For example, some of our key executives may sell a significant amount of Ordinary Shares and such sales are not required to be disclosed as promptly as public companies organized within the United States would have to disclose. Accordingly, once such sales are eventually disclosed, the price of Ordinary Shares may decline significantly.
Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies. We are also not subject to Regulation FD under the Exchange Act, which prohibits companies from selectively disclosing material nonpublic information to certain persons without concurrently making a widespread public disclosure of such information. Accordingly, there may be less publicly available information concerning Alvotech than there is for U.S. public companies.
As a foreign private issuer, we will file an Annual Report on Form 20-F within four months of the close of each fiscal year ended December 31 and furnish reports on Form 6-K relating to certain material events promptly after we publicly announce these events. However, because of the above exemptions for foreign private issuers, which we rely on,
61
Table of Contents
our shareholders are not afforded the same information generally available to investors holding shares in public companies that are not foreign private issuers.
As a foreign private issuer, we are also permitted to follow home country practice in lieu of certain corporate governance rules of the Nasdaq, including those that require listed companies to have a majority of independent directors and independent director oversight of executive compensation, nomination of directors and corporate governance matters. As of 31 December 2023, three of our eight directors are independent as defined in Nasdaq listing standards and applicable SEC rules. As long as we rely on the foreign private issuer exemption, a majority of our board of directors will not be required to be independent directors and our compensation committee will not be required to be composed entirely of independent directors. Accordingly, holders of our securities may not have the same protections afforded to shareholders of listed companies that are subject to all of the applicable corporate governance requirements.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses. This would subject us to U.S. GAAP reporting requirements which may be difficult for us to comply with.
As a “foreign private issuer,” we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. Under those rules, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to our status on 30 June 2024.
In the future, we could lose our foreign private issuer status if a majority of our Ordinary Shares are held by residents in the United States and any of the following three circumstances applies: (1) the majority of our executive officers or directors are U.S. citizens or residents; (2) more than 50% of our assets are located in the United States; or (3) our business is administered principally in the United States. Although we intend to follow certain practices that are consistent with U.S. regulatory provisions applicable to U.S. companies, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws if we are deemed a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. For example, we would become subject to the Regulation FD, aimed at preventing issuers from making selective disclosures of material information.
We also may be required to modify certain policies to comply with good governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements of Nasdaq that are available to foreign private issuers. For example, Nasdaq’s corporate governance rules require listed companies to have, among other things, a majority of independent board members and independent director oversight of executive compensation, nomination of directors, and corporate governance matters. As a foreign private issuer, we are permitted to follow home country practice in lieu of the above requirements. We intend to follow Luxembourg practice with respect to quorum requirements for shareholder meetings in lieu of the requirement under Nasdaq Listing Rules that the quorum be not less than 33 1/3% of the outstanding voting shares. Under our articles of association, at an ordinary general meeting, there is no quorum requirement and resolutions are adopted by a simple majority of validly cast votes. In addition, under our articles of association, for any resolutions to be considered at an extraordinary general meeting of shareholders, the quorum shall be at least one half of our issued share capital unless otherwise mandatorily required by law and resolutions are adopted with a majority of at least two thirds of the validly cast votes. As long as we rely on the foreign private issuer exemption to certain of Nasdaq’s corporate governance standards, a majority of the directors on our board of directors are not required to be independent directors, our remuneration committee is not required to be comprised entirely of independent directors, and we will not be required to have a nominating and corporate governance committee. Also, we would be required to change our basis of accounting from the International Financial Reporting Standards as adopted by the International Accounting
Standards Board (“IFRS”) to United States generally accepted accounting principles (“U.S. GAAP”), which may be difficult and costly for us to comply with. If we lose our foreign private issuer status and fail to comply with U.S. securities laws applicable to U.S. domestic issuers, we may have to de-list from Nasdaq and could be subject to investigation by the SEC, Nasdaq and other regulators, among other materially adverse consequences.
62
Table of Contents
We are organized under the laws of Luxembourg and a substantial amount of our assets are not located in the United States. It may be difficult to obtain or enforce judgments or bring original actions against us or the members of our board of directors in the United States.
We are organized under the laws of Luxembourg. In addition, a substantial amount of our assets are located in Iceland and elsewhere outside the United States.
Furthermore, some of the members of our board of directors and officers reside outside the United States and a substantial portion of our assets are located in Iceland and elsewhere outside the U.S. Investors may not be able to effect service of process within the United States upon us or these persons or enforce judgments obtained against us or these persons in U.S. courts, including judgments in actions predicated upon the civil liability provisions of the U.S. federal securities laws. Likewise, it also may be difficult for an investor to enforce in U.S. courts judgments obtained against us or these persons in courts located in jurisdictions outside the United States, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws. Awards of punitive damages in actions brought in the United States or elsewhere are generally not enforceable in Luxembourg.
As there is no treaty in force on the reciprocal recognition and enforcement of judgments in civil and commercial matters between the United States and Luxembourg other than arbitral awards rendered in civil and commercial matters, courts in Luxembourg will not automatically recognize and enforce a final judgment rendered by a U.S. court. A valid judgment obtained from a court of competent jurisdiction in the United States may be entered and enforced through a court of competent jurisdiction in Luxembourg, subject to the applicable enforcement procedures (exequatur) as set out in the relevant provisions of the Luxembourg New Civil Procedure Code and in Luxembourg case law. Pursuant to Luxembourg case law, the granting of exequatur is subject to the following requirements:
•the judgment of the U.S. court is final and enforceable (exécutoire) in the United States and has not been fully enforced in the United States and/or in any other jurisdiction;
•the U.S. court had full jurisdiction over the subject matter leading to the judgment (that is, its jurisdiction was in compliance both with Luxembourg private international law rules and with the applicable domestic U.S. federal or state jurisdictional rules);
•the U.S. court applied to the dispute the substantive law which is designated by the Luxembourg conflict of laws rules or, at least, such court’s order must not contravene the principles underlying those rules (based on recent case law and legal doctrine, it is not certain that this condition would still be required for an exequatur to be granted by a Luxembourg court);
•the judgment was granted following proceedings where the counterparty had the opportunity to appear and, if it appeared, to present a defense, and the decision of the foreign court must not have been obtained by fraud, but in compliance with the rights of the defendant;
•the U.S. court acted in accordance with its own procedural laws;
•the judgment of the U.S. court does not contradict an already issued judgment of a Luxembourg court, and
•the decisions and the considerations of the U.S. court must not be contrary to Luxembourg international public policy rules (as such term is interpreted under the laws of Luxembourg) or have been given in proceedings of a tax or criminal nature or rendered subsequent to an evasion of Luxembourg law (fraude à la loi). Awards of damages made under civil liabilities provisions of the U.S. federal securities laws, or other laws, which are classified by Luxembourg courts as being of a penal or punitive nature (for example, fines or punitive damages), might not be recognized by Luxembourg courts. Ordinarily, an award of monetary damages would not be considered as a penalty, but if the monetary damages include punitive damages, such punitive damages may be considered a penalty and therefore not enforceable in Luxembourg.
Similarly, as Alvotech hf., a subsidiary of Alvotech, has significant assets in Iceland, investors may seek to enforce judgments obtained in the United States against Alvotech in Iceland. As there is no treaty in force on the reciprocal recognition and enforcement of judgments in civil and commercial matters between the United States and Iceland other than arbitral awards entered in civil and commercial matters, courts in Iceland will not automatically recognize and enforce a final judgment rendered by a U.S. court. Based on recent Icelandic case law, a valid judgment obtained from a court of competent jurisdiction in the United States will not be directly recognized and enforceable in Iceland. Instead, the judgment creditor would need to issue fresh legal proceedings against the judgment debtor in Iceland in which the U.S. judgment would serve as evidence, in addition to other evidence and legal arguments regarding the merits of the case, which will be adjudicated by the Icelandic courts.
63
Table of Contents
If an original action is brought in Luxembourg or Iceland, without prejudice to specific conflict of law rules, Luxembourg courts or Icelandic courts may refuse to apply the designated law (i) if the choice of such foreign law was not made bona fide or (ii) if the foreign law was not pleaded and proved or (iii) if pleaded and proved, such foreign law is contrary to mandatory Luxembourg or Icelandic laws or incompatible with Luxembourg or Icelandic public policy rules. In an action brought in Luxembourg or Iceland on the basis of U.S. federal or state securities laws, Luxembourg courts or Icelandic courts may not have the requisite power to grant the remedies sought. Also, an exequatur may be refused by a Luxembourg court in respect of punitive damages.
In practice, Luxembourg courts tend not to review the merits of a foreign judgment, although there is no clear statutory prohibition of such review. A contractual provision allowing the service of process against a party to a service agent could be overridden by Luxembourg or Icelandic statutory provisions allowing the valid serving of process against a party in accordance with applicable laws at the domicile of the party. Further, in the event any proceedings are brought in a Luxembourg court in respect of a monetary obligation payable in a currency other than the Euro, a Luxembourg court would have the power to give judgment as an order to pay the obligation in a currency other than the Euro. However, enforcement of the judgment against any party in Luxembourg would be available only in Euros and, for such purposes, all claims or debts would be converted into Euros. Similarly, in the event any proceedings are brought in an Icelandic court in respect of a monetary obligation payable in a currency other than the Icelandic Krona, an Icelandic court would have the power to give judgment as an order to pay the obligation in a currency other than the Icelandic Krona.
In addition, actions brought in a Luxembourg court against Alvotech, the members of our board of directors, our officers, or the experts named herein to enforce liabilities based on U.S. federal securities laws may be subject to certain restrictions. In particular, Luxembourg courts generally do not award punitive damages. Litigation in Luxembourg also is subject to rules of procedure that differ from the U.S. rules, including, with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. Proceedings in Luxembourg would have to be conducted in the French or German language, and all documents submitted to the court would, in principle, have to be translated into French or German. For these reasons, it may be difficult for a U.S. investor to bring an original action in a Luxembourg court predicated upon the civil liability provisions of the U.S. federal securities laws against Alvotech, the members of our board of directors, our officers, or the experts named herein. In addition, even if a judgment against Alvotech, the non-U.S. members of our board of directors, our officers, or the experts named in this Annual Report on Form 20-F based on the civil liability provisions of the U.S. federal securities laws is obtained, a U.S. investor may not be able to enforce it in United States or Luxembourg courts.
Our directors and officers might enter into indemnification agreements with Alvotech. Under such agreements, the directors and officers could be entitled to indemnification from Alvotech to the fullest extent permitted by Luxemburg law against liability and expenses reasonably incurred or paid by him or her in connection with any claim, action, suit, or proceeding in which he or she would be involved by virtue of his or her being or having been a director or officer and against amounts paid or incurred by him or her in the settlement thereof. Luxembourg law permits us to keep directors indemnified against any expenses, judgments, fines and amounts paid in connection with liability of a director towards Alvotech or a third-party for management errors i.e., for wrongful acts committed during the execution of the mandate (mandat) granted to the director by Alvotech, except in connection with criminal offenses, gross negligence or fraud. The rights to and obligations of indemnification among or between Alvotech and any of our current or former directors and officers are generally governed by the laws of Luxembourg and subject to the jurisdiction of the Luxembourg courts, unless such rights or obligations do not relate to or arise out of such persons’ capacities listed above. Although there is doubt as to whether U.S. courts would enforce this indemnification provision in an action brought in the United States under U.S. federal or state securities laws, this provision could make it more difficult to obtain judgments outside Luxembourg or from non-Luxembourg jurisdictions that would apply Luxembourg law against our assets in Luxembourg.
Luxembourg, Icelandic and European Union insolvency and bankruptcy laws are substantially different from U.S. insolvency and bankruptcy laws and may offer our shareholders less protection than they would have under U.S. insolvency and bankruptcy laws.
As a company organized under the laws of Luxembourg and with its registered office in Luxembourg, we are subject to Luxembourg insolvency and bankruptcy laws in the event any insolvency proceedings are initiated against us including, among other things, Council and European Parliament Regulation (EU) 2015/848 of May 20, 2015, on insolvency proceedings (recast). Should courts in another EU Member State determine that the insolvency and bankruptcy laws of that country apply to us in accordance with and subject to such EU regulations, the courts in such EU Member State could have jurisdiction over the insolvency proceedings initiated against us.
64
Table of Contents
We are the parent company of Alvotech hf., our main operating subsidiary. As a company organized under the laws of Iceland and with its registered office in Iceland, Alvotech hf. is subject to Icelandic insolvency and bankruptcy laws in the event any insolvency proceedings are initiated against it.
Insolvency and bankruptcy laws in Luxembourg, Iceland or the relevant other EU Member State, if any, may offer our shareholders less protection than they would have under U.S. insolvency and bankruptcy laws and make it more difficult for them to recover the amount they could expect to recover in a liquidation under U.S. insolvency and bankruptcy laws.
The rights of our shareholders and responsibilities of our directors and officers are governed by Luxembourg or Icelandic law and differ in some respects from the rights and responsibilities of shareholders under other jurisdictions, including jurisdictions in the United States or Iceland.
Our corporate affairs are governed by our articles of association, and by the laws governing companies incorporated in Luxembourg, including the Luxembourg law of August 10, 1915 on commercial companies, as amended (the
“Luxembourg Company Law”). The rights of our shareholders and the responsibilities of our directors and officers under Luxembourg law differ in some respects from those of a company incorporated under other jurisdictions, including jurisdictions in the U.S. corporate laws governing Luxembourg companies may not be as extensive as those in effect in U.S. jurisdictions and the Luxembourg Company Law in respect of corporate governance matters might not be as protective of shareholders as the corporate law and court decisions interpreting the corporate law in Delaware, where the majority of U.S. public companies are incorporated. Further, under Luxembourg law there may be less publicly available information about us than would otherwise be published by or about U.S. issuers. In addition, we anticipate that all of our shareholder meetings will take place in Luxembourg. Our shareholders may have more difficulty in protecting their interests in connection with actions taken by our directors and officers or our principal shareholders than they would as shareholders of a corporation incorporated in a jurisdiction in the United States.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding Ordinary Shares and Warrants adversely, then the price and trading volume of Ordinary Shares and Warrants could decline.
The trading market for Ordinary Shares and Warrants is influenced by the research and reports that industry or securities analysts may publish about us, our business, our market, or our competitors. If any of the analysts who may cover us change their recommendation regarding Ordinary Shares and Warrants adversely, cease to provide coverage or provide more favorable relative recommendations about our competitors, the price of Ordinary Shares and Warrants would likely decline.
Only two majority shareholders may have significant influence over the outcome of matters submitted to shareholders for approval, which may prevent us from engaging in certain transactions.
As of the date hereof, our two largest shareholders, Alvogen and Aztiq, own approximately 71.6% of our Ordinary Shares. As a result of their ownership interest and other contractual rights, these shareholders exercise significant influence over all matters requiring shareholder approval, including the appointment of directors and the approval of significant corporate transactions. Such corporate action might be taken even if other shareholders oppose them. This ownership and control may also have the effect of delaying or preventing a future change in control, impeding a merger, consolidation, takeover or other business combination that may be in the best interest of us and any other shareholder. This ownership and control may be used to prevent us from raising additional funds through the sale of equity which may make it more difficult for us to finance our operations.
We rely on certain significant shareholders and affiliated entities for certain key services in the execution of our strategy and business operations.
We have entered into various service agreements with our direct and indirect significant shareholders and related entities, such as Alvogen, Aztiq, Alvogen Malta (Out-Licensing) Ltd. (“Adalvo”) and Floki Invest ehf. (“Floki”). These services include, among others, IT services, corporate administrative, legal, financial, facility management, portfolio and market intelligence research, regulatory compliance, quality audit, and publishing services, and certain administrative and financial services related to our Reykjavik facility. These services are key to our ability to continue to execute on our business strategy and to keep our business operations uninterrupted. Any interruption in the provision of these services may materially harm our business. In addition, because the providers of the services are direct or indirect significant shareholders and related entities, we may not be able or willing to enforce our contractual rights under the service agreements the same way we would if the service providers were unrelated third-party providers. See also “—We currently
65
Table of Contents
rely on Alvogen’s ERP solution and other components of Alvogen’s IT infrastructure and will continue to do so for the foreseeable future”.
Risks Related to Taxation
If we are treated as a “passive foreign investment company” for any taxable year, U.S. investors could be subject to adverse U.S. federal income tax consequences.
A non-U.S. corporation generally will be treated as a “passive foreign investment company” (“PFIC”) for U.S. federal income tax purposes if either (i) at least 75% of its gross income in a taxable year, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income or (ii) at least 50% of its assets in a taxable year (ordinarily determined based on fair market value and averaged quarterly over the year), including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
Based on our analysis of our income, assets, activities and market capitalization, we believe that we were not treated as a PFIC for our taxable year ended 31 December 2023. However, the determination of whether a non-U.S. corporation is a PFIC is a fact-intensive determination made on an annual basis and the applicable law is subject to varying interpretation. In particular, the characterization of our assets as active or passive may depend in part on our current and intended future business plans, which are subject to change. In addition, the total value of our assets for PFIC testing purposes may be determined in part by reference to the market price of Ordinary Shares from time to time, which may fluctuate considerably. As a result, there can be no assurance with respect to our status as a PFIC for any taxable year, and our U.S. counsel expresses no opinion with respect to our PFIC status for any taxable year.
If we are treated as a PFIC, U.S. investors may be subject to certain adverse U.S. federal income tax consequences, including additional reporting requirements. For further discussion of the PFIC rules and the adverse U.S. federal income tax consequences in the event we are classified as a PFIC, as well as certain elections that may be available to U.S. investors, see “Item 10.E Taxation-Material U.S. Federal Income Tax Considerations for U.S. Holders.” U.S. investors should consult their tax advisors regarding the application of the PFIC rules in their particular circumstances.
If we or any of our subsidiaries is treated as a “controlled foreign corporation,” certain U.S. investors could be subject to adverse U.S. federal income tax consequences.
Generally, under the Internal Revenue Code of 1986, as amended (the "Code"), if a U.S. investor owns or is treated as owning, directly, indirectly, or constructively, 10% or more of the total value or total combing voting power of our stock, the U.S. investor may be treated as a “United States shareholder” with respect to each controlled foreign corporation (“CFC”) in our corporate structure, if any. A non-U.S. corporation generally will be a CFC if United States shareholders own, directly, indirectly, or constructively, 10% or more of the total value or total combined voting power of the stock of such corporation. Because our corporate structure includes a U.S. corporate subsidiary, our non-U.S. corporate subsidiaries, including any non-U.S. corporate subsidiaries that may be formed or acquired in the future, will be treated as CFCs, regardless of whether we are treated as a CFC. A United States shareholder of a CFC may be required to annually report and include in its U.S. taxable income its pro rata share of the CFC’s “Subpart F income”, “global intangible low-taxed income,” and investments of earnings in U.S. property, regardless of whether the CFC makes any distributions to its shareholders. Furthermore, an individual United States shareholder with respect to a CFC generally will not be allowed certain tax deductions and foreign tax credits that are allowed to a corporate United States shareholder. Failure to comply with CFC reporting obligations may also subject a United States shareholder to significant penalties. There can be no assurance that the Company will provide to any United States shareholder information that may be necessary for the United States shareholder to comply with its CFC reporting and tax paying obligations. U.S. investors should consult their tax advisors regarding the application of the CFC rules in their particular circumstances.
Changes in tax laws and unanticipated tax liabilities could adversely affect us.
We are subject to tax in Luxembourg and in other jurisdictions, and significant judgment is required in determining our provision for income taxes. Likewise, we are subject to audit by tax authorities in various jurisdictions. In such audits, our interpretation of tax legislation may be challenged and there would be a potential risk of an adverse effect on our consolidated financial statements.
The integrated nature of our worldwide operations can produce conflicting claims from tax authorities in different countries as to the profits to be taxed in the individual countries, including potential disputes relating to the prices our
66
Table of Contents
subsidiaries charge one another for intercompany transactions, known as transfer pricing. Most of the jurisdictions in which we operate have double tax treaties with other foreign jurisdictions, which provide a framework for mitigating the impact of double taxation, although such mechanisms for resolving such conflicting claims can be expected to be very lengthy.
Our tax liabilities could be adversely affected in the future by a number of factors, including changes in accounting standards, changes in the valuation of deferred tax assets and liabilities, and changes in tax laws such as corporate income tax rates and changes in tax treatment of specific items.
Other international tax measures, such as the Organization for Economic Cooperation and Development’s (“OECD’s”) base erosion and profit shifting (“BEPS”) project and the global minimum taxation regime (“Pillar two”) contribute to increased uncertainty and may adversely affect our tax provision. The BEPS project contemplates changes to numerous international tax principles, as well as national tax incentives, and these changes, when adopted by individual countries, could adversely affect our provision for income taxes. Pillar two, was announced by the EU Council on 12 December 2022, and introduces the minimum taxation component of 15% as part of the OECD’s reform of international taxation. Multinational groups are subjected to these rules upon meeting certain criteria, and we continuously monitor whether compliance with these rules becomes applicable. These rules are fairly new and further guidance is progressively sought, as a result of which it remains difficult to predict the magnitude of the eventual effect of such new rules on our financial results.
We may not be able to fully utilize some of our Icelandic NOL carryforwards.
As of 31 December 2023, Alvotech hf., the Icelandic operational entity, had net operating loss (“NOL”) carryforwards. There can be no certainty that we will generate revenues, in the foreseeable future, if ever, and we may never achieve profitability. These NOL carryforwards could expire unused and be unavailable to offset future income tax liabilities. In the absence of their utilization, any increased liabilities could adversely affect our business, results of operations, financial position and cash flows.
Termination or expiration of governmental programs or tax benefits, could adversely affect us.
Some entities forming part of the group benefit from governmental programs or tax benefits. The termination, change or expiration of governmental programs or tax benefits, or a change in our business, could adversely affect our overall effective tax rate.
Item 4. Information on the Company.
A.History and Development of the Company
Alvotech hf. was founded in 2013 in Reykjavik, Iceland with the aim of creating a highly integrated platform company focused exclusively on developing and manufacturing biosimilars for the global market. Over the past eleven years, we have invested steadily and methodically in building a fully integrated platform, enabling us to control quality, cost and speed to market of our developed products, representing a key competitive advantage in the biosimilar business.
Alvotech, previously known as Alvotech Lux Holdings S.A.S., was incorporated under the laws of the Grand Duchy of Luxembourg on August 23, 2021, as a simplified joint stock company. On 15 June 2022, the legal form of Alvotech changed from a simplified joint stock company (société par actions simplifiée) to a public limited liability company (société anonyme) under Luxembourg law. We own no material assets other than our interests in Alvotech hf. and other subsidiaries and do not operate any business. Our business is conducted through Alvotech hf., our direct, wholly-owned subsidiary and its subsidiaries.
Our principal place of business is at 9, Rue de Bitbourg, L-1273 Luxembourg, Grand Duchy of Luxembourg. The mailing address of our group’s principal executive office is Sæmundargata 15-19, 102 Reykjavík, Iceland, and our telephone number is +354 422 4500. Our principal website address is www.alvotech.com. The information contained on, or accessible through, our websites is not incorporated by reference into this Annual Report, and you should not consider it a part of this Annual Report. The SEC maintains an Internet site that contains reports, proxy information statements and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov. Our agent for service of process in the United States is Alvotech USA Inc., 1602 Village Market Blvd., Suite 280, Leesburg, Virginia 20175.
Our actual capital expenditures for the years ended 31 December 2023, 2022, and 2021 amounted to $33.2 million, $37.9 million and $20.5 million, respectively. These capital expenditures primarily consisted of property, plant and equipment, leasehold improvements, lab equipment, and computer equipment in Iceland.
67
Table of Contents
B.Business Overview
Company Overview
We are a vertically integrated biotech company focused solely on the development and manufacture of biosimilar medicines for patients worldwide. Our purpose is to improve the health and quality of life of patients around the world by improving access to proven treatments for various diseases. Since our inception, we have built our company with key characteristics we believe will help us capture the substantial global market opportunity in biosimilars: a leadership team that has brought numerous successful biologics and biosimilars to market around the world; a purpose-built biosimilars R&D and manufacturing platform; commercial partnerships in global markets; and a diverse, expanding portfolio and pipeline addressing many of the largest disease areas and health challenges globally.
A biosimilar is a biological medicine that is highly similar to and has no clinically meaningful differences from an existing approved biological, or reference product. Much as generics do for off-patent small-molecule drugs, biosimilars provide a cost-effective alternative with no clinically meaningful difference to biologic medicines whose patent exclusivity has expired. Many patient, policy, industry and regulatory organizations share our view that the availability of quality, affordable biosimilars is critical to the long-term sustainability of health systems and medical innovation globally. Cost savings generated by biosimilars can be used to treat more people and to sustain the cost of investment in the next generations of innovative therapies. We see both the discovery of novel therapies, which is the focus of many biopharmaceutical companies, and innovating access to medicines, which is our core focus, as critical to the purpose of the pharmaceutical industry as a whole—to deliver breakthrough, life-changing medicines to as many patients as possible, whoever and wherever they are.
We aim to achieve our mission by becoming a leading supplier of biosimilars globally. To do this, we have built a distinctive and comprehensive platform for developing and manufacturing biosimilars at scale. Our platform is designed to enable us to execute the product development and scale-up process in-house: from identifying therapeutic areas and target product candidates with significant unmet patient and market need through R&D, leveraging gold-standard host cell lines, cell-culture processes and Good Manufacturing Practice (“GMP”) manufacturing, clinical testing, and regulatory approvals. In order to give our products global reach with local expertise, we have formed strategic commercialization partnerships with leading pharmaceutical companies covering global markets. We license our intellectual property to partners in exchange for milestone payments and royalties.
Developing and manufacturing biosimilars is a time-consuming, capital intensive, complex and historically uncertain undertaking. We currently have two approved products and an additional nine product candidates in our portfolio and pipeline targeting serious diseases with unmet patient and market need. Product candidates in our pipeline address reference products treating autoimmune, eye, and bone disorders, as well as cancer.
Our Pipeline
Product selection
We believe that the nature and quality of our platform enable us to innovate and systematically produce high quality biosimilars for treating a broad range of serious diseases. We believe that our ability to generate and capture efficiencies across research and development, manufacturing and commercialization gives us key advantages in quality, cost and speed to market when competing with both originator and other biosimilar companies.
Our fully integrated capabilities provide us wide breadth and flexibility in deciding which biosimilar opportunities to pursue, optimizing the commercial, scientific and medical impact of each program as part of our portfolio. We evaluate a rigorous set of six criteria to select our candidates:
•Competitive situation: Evaluates originator value, brand and longevity, as well as competition from biosimilars and originators alike, on an ongoing basis.
•Launch timing: Aims to be among the first wave of biosimilars to every reference product.
•Portfolio fit: Seeking balance across the portfolio, assesses volume/price ratio and the ability to leverage the breadth of our R&D and manufacturing capabilities.
•Differentiation: Seeks opportunities where platform differentiation can be applied and exploited, for example, in potential for interchangeability (for the U.S. market), delivery device and product presentations.
•Feasibility and cost: Ongoing assessment for technical, clinical, intellectual property and regulatory issues as well as cost and time analysis for CMC, clinical and potential for interchangeability.
68
Table of Contents
•Partner insights: Strategic input from commercial partners taken into account at every stage.
In addition to the above, our platform is built for flexibility that may allow us to expand into other healthcare products areas such as respiratory and primary care products.
Our Pipeline
Through our rigorous product selection and development platform, we have been able to build a pipeline comprising two launched products and nine disclosed biosimilar candidates, covering a variety of therapeutic areas, including autoimmune, eye, and bone disorders, as well as cancer. Our lead program, AVT02, a high concentration formulation biosimilar to Humira, has received regulatory approval in over 50 markets and has been launched in over 20 markets globally. We expect to launch AVT02 in the United States during the first half of 2024. We also have a second approved biosimilar, AVT04, which uses the same SP2/0 host cell line as the reference biologic Stelara. AVT04 has been approved in Japan, Canada and the EEA and was launched in Canada on 1 March 2024. Launches of AVT04 in Japan and Europe are expected, respectively, in Q2 2024 and Q3 2024. We anticipate that the FDA’s review of our BLA for AVT04 will be completed by 16 April 2024. Clinical programs for AVT03, AVT05 and AVT06 are at an advanced stage, and we expect to file marketing applications for these biosimilar candidates in 2024. We are also developing, AVT23, that is in clinical development, as well as AVT16 and AVT33, in addition to three undisclosed programs, all of which are in pre-clinical development.
Our Programs
AVT02, our high-concentration biosimilar to Humira
Humira (adalimumab) inhibits tumor necrosis factor (“TNF”), which is a protein in the body that can cause inflammation. Developed and predominantly marketed by AbbVie, adalimumab is prescribed to treat a variety of inflammatory conditions including, rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis among other indications. Humira is approved and marketed in a high concentration formation (100 mg/mL) across four doses (10 mg, 20 mg, 40 mg, 80 mg) which account for roughly 80% of the U.S. Humira market. In 2023, Humira worldwide net revenues were approximately $14.4 billion. A lower concentration formulation (50 mg/mL) is also approved and marketed across three strengths (10 mg, 20mg, 40mg). Adalimumab has many of the core characteristics we look for in selecting a candidate for development. We are aiming to have the first interchangeable high-concentration citrate-free biosimilar to Humira in the United States. Additionally, adalimumab fits well within our immunology portfolio and manufacturing capabilities. The competitive landscape and broad market opportunity for adalimumab is attractive to us and our commercial partners.
•We have directly or through our partners, received regulatory approval for AVT02 in 52 countries. These include the EEA, UK and Switzerland, Canada, Australia, Israel, Morocco, Egypt, Saudi Arabia, South Africa and parts of Latin America. AVT02 is currently marketed in Europe, Canada and Australia. On 23 February 2024, we received U.S. FDA approval for AVT02 as an interchangeable high-concentration citrate-free biosimilar to Humira, AVT02 also qualifies for exclusivity as a high-concentration biosimilar. We expect to launch AVT02 with our partner Teva in the United States during the first half of 2024.
AVT04, our proposed biosimilar to Stelara
Stelara (ustekinumab) is a human IgG1k monoclonal antibody against the human interleukin-12 and -23 cytokines. Marketed by Janssen, Stelara is prescribed to treat a variety of inflammatory conditions including psoriatic arthritis, Crohn’s disease, ulcerative colitis, plaque psoriasis among other indications. In 2023, global net revenues from Stelara were approximately $10.8 billion.
We are using an SP2/0 host cell line, which is the same manufacturing host cell line as Stelara. Developing our biosimilar in the same host cell line as the originator for a product that requires such a long half-life, de-risks the approval process and creates potential differentiation relative to other biosimilar developers.
In 2023, we announced that AVT04 had been approved in Japan and Canada. In early 2024, we announced that it has also been approved in the EEA. We anticipate that the FDA’s review of our BLA for AVT04 will be completed by 16 April 2024. We also announced that we have reached settlement agreements with the manufacturer of the reference product Stelara, Johnson & Johnson, launched AVT04 in March 2024 in Canada, and expect launches in Q2 2024 in Japan, Q3 2024 in Europe, and in February 2025 in the U.S., pending FDA approval.
69
Table of Contents
AVT06, our proposed biosimilar to Eylea
Eylea (aflibercept) is a recombinant fusion protein formulated for intravitreal administration consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1. Developed and marketed by Bayer and Regeneron, Eylea is prescribed to treat conditions including age-related macular degeneration, macular edema, and diabetic retinopathy.
Both the reference product as well as our proposed biosimilar AVT06 are produced in recombinant Chinese hamster ovary cells. In July 2022, we initiated the confirmatory clinical study for AVT06 and, in January 2024, we announced positive topline results from the confirmatory study.
AVT03, our proposed biosimilar to both Xgeva and Prolia
Xgeva and Prolia have the same active ingredient, denosumab, but the products are approved for different indications, patient populations, doses and frequencies of administration. Denosumab is a human IgG2 monoclonal antibody with affinity and specificity for human RANKL, receptor activator of nuclear factor kappa-B ligand. Developed and predominately marketed by Amgen, Xgeva is prescribed to prevent bone fracture, spinal cord compression or the need for radiation or bone surgery in patients with certain types of cancer, and Prolia is prescribed to prevent bone loss and increase bone mass. In 2023 cumulative global sales of Prolia and Xgeva were approximately $6.2 billion.
Both the reference product as well as our proposed biosimilar AVT03 are produced in recombinant Chinese hamster ovary cells.
AVT03 is in the clinical phase and has been developed to have a high degree of analytical similarity to the originator. Further we have engaged with global regulatory authorities on our development strategy in order to align our program with expectations from regulatory authorities and further limit development risk.
Our clinical program consists of two pharmacokinetic (PK) studies in healthy volunteers and a confirmatory efficacy and safety study in patients with post-menopausal osteoporosis.
In January 2024, we announced the positive top-line results from a PK study for AVT03 compared to Prolia in healthy adult subjects. In August 2023, we announced the initiation of a second PK study, which is still ongoing, comparing AVT03 to Xgeva. In August 2022, we announced the initiation of a confirmatory patient study for AVT03, which is also ongoing. The objective of the confirmatory study is to demonstrate clinical similarity of AVT03 to Prolia in terms of efficacy, safety, immunogenicity, and PK in postmenopausal women with osteoporosis.
AVT05, our proposed biosimilar to Simponi and Simponi Aria
Simponi / Simponi Aria (golimumab), inhibits TNF, which is a protein in the body that can cause inflammation. Simponi / Simponi Aria are prescribed to treat a variety of inflammatory conditions including, RA, psoriatic arthritis, ulcerative colitis among others. Simponi is a sterile solution of golimumab antibody supplied for subcutaneous use. Simponi Aria injection is a sterile solution supplied for intravenous use. We are developing both forms of the product. AVT05 is expressed in an SP2/0 host cell line, which matches the cell used by the developer of the originator. AVT05 is in clinical development. We have developed AVT05 to match the host cell line used by the developer of the originator and we intend to pursue interchangeability designation. In November 2023, we announced positive topline results from our pharmacokinetic study for AVT05. In May 2023, Alvotech announced the initiation of a clinical study to compare the efficacy, safety, and immunogenicity of AVT05 and Simponi in adult patients with moderate to severe rheumatoid arthritis. We expect to announce top-line results from the clinical efficacy study in 2024.
AVT23 (also called ADL018), our proposed biosimilar to Xolair
Xolair (omalizumab) is an antibody that targets free IgE and is used to treat patients with allergic asthma, chronic spontaneous urticaria (CSU) and nasal polyp. Xolair, the only currently approved product containing omalizumab, was first approved in 2003. In 2023, we announced an agreement with Kashiv Biosciences LLC ("Kashiv") to in-license AVT23. The agreement covers all 27 countries of the European Union, the UK, Australia, Canada, and New Zealand. Under terms of the agreement, Alvotech will receive an exclusive license to commercialize AVT23, which will be developed and manufactured by Kashiv.
70
Table of Contents
AVT23 is currently in clinical development. A pharmacokinetic (PK) comparability study has been completed, with results demonstrating that AVT23’s bioavailability, safety, tolerability and immunogenicity are comparable to those of Xolair. A confirmatory clinical efficacy study comparing AVT23 to Xolair is currently ongoing.
We are currently in early phase development for five additional products, three for which the reference biologic remains unnamed, in addition to AVT16, a proposed biosimilar to Entyvio (vedolizumab) and AVT33, a proposed biosimilar to Keytruda (pembrolizumab).
Our Market Opportunity
Background on Biologics
Biologic medicines (biologics) are complex pharmaceutical products that typically contain one or more active substances made by or derived from a biological source. Conventional medicines are typically chemically synthesized small molecules that are easily identified and characterized; in contrast, biologics are large, complex molecules that require unique characterization techniques and generally tend to be sensitive to heat and microbial contamination. The creation innovation and advancement of biologics are the result of cutting-edge research and these medicines have provided novel treatments for a variety of illnesses such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, multiple sclerosis, age-related macular degeneration, diabetic macular edema and numerous types of cancer. Biologics are designed to have very specific effects and to interact with specific targets in the patient’s body, mainly on the outside of cells. A more targeted mechanism of action leads to a greater chance of the medicine having the desired effect against the disease and results in fewer side effects compared to traditional medicines. The effectiveness of biologics has led to an increase of investment in R&D within the pharmaceutical sector for biologic medicines.
Background on Biosimilars
A biosimilar is a biological medicine that is highly similar to and has no clinically meaningful differences from an existing approved biological, or reference product. Biosimilars are approved according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological medicines and typically have the same amino acid sequence.
Biosimilars offer a lower cost alternative to their name-brand reference products, and have no clinically meaningful difference in terms of safety, purity or potency when compared to reference products. Because they are designed to be highly similar to already approved biologics, the success rate for developing biosimilars is considerably higher, and the R&D cost proportionally much lower. While the average originator biologic takes an average of 12 years to develop at a cost of more than $2.5 billion, the average biosimilar can usually be developed six to nine years and at a cost of between $100.0 to 200.0 million. Further, this is significantly different to generics, which are simpler to manufacture, can typically developed in two years or less at a cost of less than $10 million, and without needing clinical trials.
The availability of biologics and their rapidly increasing prices have forced healthcare systems and payors around the world, public and private alike, into difficult tradeoffs in the effort to balance the best quality of care, accessibility, sustainability and cost. As biosimilars provide a more affordable alternative to payors and patients, they offer the potential to improve the accessibility of many life-altering treatments to many more patients. More broadly, lower costs for existing treatments can make healthcare systems more sustainable and free up resources to pay for the next generation of innovative brand-name therapies, and the R&D infrastructure that sustains future drug discovery. In this way, we believe that biosimilars can also help to sustain the global biomedical innovation ecosystem as a whole.
While biosimilars share similarities with generics, there are significant differences, including the complexity of development and manufacturing. For traditional medications, generic products can generally be considered identical to the branded product in form and function. In the case of biologics and biosimilars, the complexity of a biologic molecule means that the biosimilar product is not identical in form to the branded product, and some variability from the branded reference product is considered inherent to the process. However, there is no clinically meaningful functional difference between a biosimilar and the reference product in safety, purity or potency.
71
Table of Contents
Our Strategy
Our strategy is to leverage our integrated platform to develop and manufacture high quality biosimilars and to then work with our global network of partners to commercialize the portfolio and pipeline into markets around the world. We are advancing multiple product candidates towards regulatory approval and intend to launch our portfolio and pipeline into over 90 markets around the world. Our strategy can be summarized by the following;
•Platform: At the heart of our strategy is our fully integrated biosimilars platform. We have a purpose-built facility with a footprint of approximately 280,000 square feet that includes R&D, process, quality, manufacturing and headquarters in Reykjavik, Iceland. Additionally, we have cell line, process, analytics and glycoprotein characterization sites in Germany; a regulatory, legal and government affairs office in the United States; and an R&D, clinical, and regulatory strategy center in Switzerland. This infrastructure and know-how enables us to have a full set of capabilities and control, from analysis of reference products and cell line development through fill-and-finish GMP manufacturing and regulatory approvals. Further, it provides us the ability to innovate efficiencies in every step of the process and project those cost-savings throughout our portfolio. We have demonstrated manufacturing capabilities using both of the two most widely-used host cell lines — Chinese hamster ovary (“CHO”) and SP2/0 — as well as cell culture processes, fed batch and perfusion.
•Portfolio and Pipeline: In addition to two approved biosimilars, we are currently advancing a portfolio and pipeline of nine biosimilar candidates through the development and global regulatory process. Our portfolio and pipeline covers a variety of therapeutic areas, including autoimmune disorders, eye disorders, bone disease, respiratory disease, and cancer. Where possible, we seek to develop differentiated products as is the case with the company’s first launched product, AVT02, a biosimilar to Humira. For the U.S. market, our biosimilar was developed as a high-concentration form, which is the predominant product profile that is marketed by the originator company. Additionally, we sought and have been given an interchangeability designation for AVT02 in the U.S. market, with exclusivity. By end of 2023, we had begun commercialization of AVT02, through our commercial partners, in Canada, Australia, and 19 markets across Europe.
•Commercial Partnerships: We have formed a global network of strategic commercial partnerships to ensure that our products can reach the patients in geographies across the world. Our partners include Teva (US), STADA (EU), Fuji Pharma Co., Ltd (Japan), Cipla/Cipla Gulf/Cipla Medpro (Australia, New Zealand, South Africa/Africa), JAMP Pharma (Canada), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS (Middle East and Africa), Abdi Ibrahim (Turkey), Kamada (Israel), MegaLabs, Stein, Libbs, Tuteur and Saval (Latin America), Advanz Pharma (EEA, U.K., Switzerland, Canada, Australia and New Zealand), among others. Our partners’ deep knowledge of the markets and economic, regulatory, payor and reimbursement landscapes in the countries they serve optimizes our commercial opportunity and ability to reach patients in these markets in a way we could not do on our own. We partner only with trusted, market leaders and develop close strategic relationships with these partners that align our interests and the partners’ interests for success.
•People: As of 31 December 2023, we employ over 1000 people around the world. Over 85% of our workforce is dedicated to manufacturing and development of biosimilars. We seek to attract and retain the highest quality talent in order to achieve our mission and execute our strategy.
•ESG and corporate responsibility: We aim to maintain and further develop our commitment to sustainability and corporate responsibility beyond our fundamental mission of expanding access to medicines while lowering costs for patients. We are developing and implementing a comprehensive environmental, social and governance (“ESG”) framework to collect, monitor and report data that assess our environmental and social impact as well as provide transparent disclosures on governance.
We believe that we have certain intrinsic business and operational qualities that may favorably position us to optimize our ESG impact, including the location of our headquarters and manufacturing in Iceland. This enables us to minimize our environmental impact by conducting our principal operations using nearly 100% renewable energy and in a geography with abundant cold and hot water. We intend to make a difference for patients around the world by working strategically towards increasing patient access to medicines, supporting the sustainability of health systems and, where feasible, conducting clinical trials in areas with relatively lower access to healthcare.
72
Table of Contents
Our Platform
We believe that the nature and quality of our platform enable us to innovate and systematically develop and manufacture biosimilar medicines. We consider this ability, and that our platform can generate and capture efficiencies all along the research and development, manufacturing and sales and marketing chain, to be fundamental advantages when competing with both originator and other biosimilar companies in quality, cost and speed to market.
The challenges of biosimilars development
Making biosimilars—biologic medicines that are highly similar to and without clinically meaningful differences from their reference products in terms of safety, purity and potency—is a fundamentally complex task. It requires, among other things, highly specialized expertise and infrastructure, time, and significant capital. Success in the biosimilar space is largely determined by the ability to make biosimilars efficiently and consistently.
We believe that these same barriers to entry also create opportunities for differentiation. The capital investment, sophisticated infrastructure and scientific/technical expertise required are principal reasons that the biosimilar divisions of large originator biopharmaceutical companies, who have access to all of these, have dominated the sector’s early years. But these biosimilars divisions within larger organizations have competing internal demands for resources, including people, R&D and manufacturing facilities. As a result, biosimilars are often viewed as a secondary business. Such internal competition makes consistent and replicable operational control and efficiencies more difficult and costly to achieve, and biosimilars also tend to receive less focus in marketing and distribution. Conversely, smaller companies may not have all of the internal capabilities needed for development or the capital resources to invest in such capabilities. These constraints may require these smaller companies to outsource key parts of the R&D and manufacturing process, thereby potentially losing control over quality or the ability to innovate and control costs.
Research & Development
Our research and development is solely focused on the development of biosimilar medicines, which require considerable time and substantial financial investment. We intend to continue to commit significant resources in financial and human capital to development activities going forward, with the aim of offering more affordable biologic medicines, globally. We also strive to identify opportunities where a level of differentiation can be applied to the development program to enable improved commercial success.
Biosimilar medicines are highly similar to their reference products and typically have identical primary amino acid structure. They are held to the same high-quality standards as innovative biopharmaceuticals. The ultimate goal in the development of biosimilar medications is to develop therapeutics that are highly similar to and have no clinically meaningful difference from their reference products. In order to demonstrate this, we apply rigorous processes in the development of our product candidates.
A biosimilarity claim must demonstrate totality of evidence with respect to physiochemical characteristics, biologic activity, pharmacokinetics, clinical safety and efficacy, and therapeutic indication. Extensive analytical comparisons to the reference products are conducted, followed by nonclinical and clinical pharmacokinetic (“PK”) and pharmacodynamic (“PD”) studies, as required. Finally, a clinical efficacy and safety study is conducted to resolve any remaining uncertainty that the product is biosimilar. This process is described in more detail below.
Early phase development
In this phase of development it is vital to establish a manufacturing process that delivers highly similar product to the reference product. This starts with cell line development activities, where clones having characteristics similar to the reference product with acceptable productivity are selected. Following this a competitive commercial manufacturing process for drug substance and drug product is developed to deliver a product that is highly similar to the reference product, enabling future investment in GMP manufacturing. Numerous characterization methods are also applied to ensure our biosimilar candidate is highly similar to the reference product in structure and function. Significant time and effort is spent on this similarity evaluation to enable a streamlined clinical program in subsequent development phases with a higher probability of success.
Pre-clinical development and GMP manufacturing
In this phase, the manufacturing process is scaled-up up from small pilot scale batches to commercial scale in a commercial site. The goal is to manufacture product with a high degree of analytical similarity to the reference product while also confirming the highest quality product is produced.
73
Table of Contents
In parallel, regulatory authorities in the U.S., EU, and other geographies are engaged to discuss the overall development strategy, in order to ensure the ultimate submission package is approvable in all major regions. Non-clinical studies may also be conducted as required, based on the individual biosimilar program and alignments with regulatory authorities.
Clinical studies
Clinical studies are conducted in this phase to support product registration. Typically, a PK study is performed to demonstrate PK equivalence of the proposed biosimilar to the approved reference products such as those available in both the U.S. and EU. A global, confirmatory clinical efficacy and safety study is typically also performed to demonstrate that there are no clinically meaningful differences between the proposed biosimilar and the reference product. Depending on the specific program, these two studies may be conducted within one larger study or, conversely, additional small studies may need to be performed to support registration. When both a PK and confirmatory efficacy and safety study is required, we take the calculated risk to execute these studies in parallel (where feasible), which enables the fast track to licensing application submission for the program.
In parallel to the clinical studies being conducted, manufacturing process characterization and validation is completed, in addition to completion of the analytical similarity assessment supporting registration.
Interchangeability
When practical and commercially relevant in the U.S. market and other countries and regions, we seek interchangeability designation such as is the case with our lead product, AVT02, a biosimilar to Humira. Interchangeability is a U.S. regulatory construct and according to the FDA, an interchangeable product will have met additional data requirements and so may be substituted for the reference product without the intervention of a prescriber. The substitution may occur at the pharmacy, much as generic drugs are substituted for brand name drugs, subject to varying U.S. state pharmacy laws. Biosimilars, including those designated as interchangeable products, have the potential to reduce health care costs. The concept of interchangeability for biosimilars was signed into law through the Biologics Price Competition and Innovation Act in 2010. In order to be considered interchangeable, a biosimilar must meet additional requirements, including the execution of a “switching study,” utilizing the reference product and biosimilar product in patients. The vast majority of states have passed laws regarding substitution for interchangeable products.
Submission and approval
The ultimate goal is to submit a globally vetted, high-quality dossier that enables first-pass approval based on the totality of evidence for the comparative analytical, Chemistry, Manufacturing and Controls, (“CMC”), and clinical data. Extrapolation principles also allow for attaining a full label matching the reference product other than indications specifically protected by regulatory exclusivity. We work closely with health authorities through the review process to enable approval at the earliest possible time after dossier submission, ensuring we can remain competitive with market entry.
Manufacturing & Supply
Manufacturing Facilities
Our corporate headquarters, main manufacturing site and a large part of our R&D division are located in Reykjavik, Iceland. This facility provides us with purpose-built GMP and has highly integrated capabilities for producing biosimilars at scale. The facility is currently approximately 140,000 square feet and utilizes single-use technology to manufacture drug substance and drug product. It houses our R&D, quality control and quality assurance teams and has an active and valid GMP certificate issued by the Icelandic Medicines Authority authorizing Investigational Medicinal Product and commercial manufacturing. In December 2020, we broke ground on an expansion of our Reykjavik facility that will double the total footprint, adding another 140,000 square feet. The expansion provides space for R&D activities, and is expected to be completed in 2024. It is also is expected to give additional redundancy in drug product capacity, assembly of combination products and devices, and secondary packaging. Additionally, the expansion will support increased warehousing and other supportive functions. With the expansion of the Reykjavik facility’s manufacturing capabilities, we expect our capabilities to be able to meet the demand for our products, after obtaining regulatory approval and commercial launch, in the near future
74
Table of Contents
Third Party Suppliers, Manufacturers, and Raw Materials
Our manufacturing processes utilize single-use processing technology for both drug substance and drug product. Our manufacturing is therefore reliant on the availability of single-use components to complete production. We source these components from various reputable third-party suppliers. However, the price of these materials and components is subject to market forces and competing demands. Increases in the cost of components would have an adverse effect on our forecasted cost of goods. In certain cases, we may rely on only one approved source for a particular component and shortages may significantly impact our ability to manufacture drug substance and drug product. Finding alternative suppliers may not be possible or cause material delay to development plans or commercial production. We have the ability and are currently evaluating opportunities for redundancies in our manufacturing processes in order to mitigate risk and control costs.
We also require the use of certain reagents and materials in order to develop and produce biologic medicines. We acquire these reagents and materials through reputable third parties that specialize in the production and sourcing of these reagents and materials. These materials are widely available commodities. However, unforeseen shortages in these materials may have an adverse effect on either the price of these materials or could cause delays in our development or commercialization timelines.
AVT02 and certain other products within our pipeline require the use of auto-injector devices. We work closely with our vendor in order to assure availability and manage risk through inventory management and relationship management. Our current arrangement with our supplier utilizes a proprietary design.
Master cell banks and working cell banks are critical components in biologic medicine manufacturing. A cell bank is a collection of ampoules of uniform composition stored under defined conditions, each containing an aliquot of a single pool of cells. The master cell bank is generally derived from the selected cell clone containing the expression construct that has been encoded to produce the protein of interest, such as a specific monoclonal antibody with a defined amino acid sequence. This unique aliquot of cells allows for a consistent high quality biologic medicine to be produced. The working cell bank is derived by expansion of one or more ampoules of the master cell bank and is used for routine manufacturing. Both the master cell bank and working cell bank are central to obtaining regulatory approval for manufacturing and marketing biologic medicine. Without well-characterized and well-controlled master and working cell banks, the manufacturing process could be susceptible to non-ideal product variability. The quality of the manufactured biologic product is dependent on the quality of the cells used for our manufacturing, and having a sufficient supply of master and working cell banks is important for a consistent manufacturing process. The master cell banks and working cell banks for our lead product candidates are produced at either an EU or U.S.-based contract manufacturing organization and then transferred internally to both the Reykjavik site in Iceland and Jülich site in Germany for supply continuity and redundancy. The availability of master cell banks is critical to our ability to manufacture products for the commercial market. Should our cell banks (despite any redundancies) be compromised, we would be unable to produce usable products for patients in any market.
Sales and Marketing
We have launched AVT02 in over 20 markets globally, including in select European countries under the trade name Hukyndra and in Canada under the trade name SIMLANDI. We aim to meet patient demand in our major markets, and to launch in further markets in 2024, including in the United States the first half of 2024. A breakdown of product revenue from the sale of AVT02 is presented by region below.
| USD in thousands | 2023 | 2022 | |||||||||||||||||||||
| Product Revenue | % Total | Product Revenue | % Total | ||||||||||||||||||||
| Europe | 41,166 | 84.5 | % | 14,868 | 59.9 | % | |||||||||||||||||
| North America | 6,638 | 13.6 | % | 9,968 | 40.1 | % | |||||||||||||||||
| Asia and other | 895 | 1.8 | % | — | — | % | |||||||||||||||||
| 48,699 | 100 | % | 24,836 | 100 | % | ||||||||||||||||||
To date, we have chosen to market and commercialize our products through numerous strategic partnerships rather than sell a single global license to an individual commercial partner. By partnering with multiple leading regional partners who would likely be able place a higher value on licenses due to their core market(s) focus, we believe we can achieve higher return for the rights of our products. This also better ensures focus from partners on our portfolio. Additionally, by
75
Table of Contents
partnering with multiple partners, we are able to enhance local market knowledge and expand our geographic reach by mitigating our risk of being dependent on one single partner.
By outsourcing sales and marketing, we believe we are able to realize and leverage the following benefits:
•Global reach: By commercializing through best-in-class partners, we can reach nearly all markets around the world, including key markets in the U.S., Europe, Japan, Canada, Australia, and various international markets across regions such as Latin America and Asia. This global approach provides diversification and opportunities for growth often overlooked by companies that focus solely on the U.S. and Europe.
•Local expertise: Our commercial strategy allows us to leverage the expertise from our partners. Our partners’ expertise in managing numerous local regulatory and commercial landscapes has been built up over many years and would be difficult, to replicate internally across all global markets. We believe our partners will enable us to bring our products to market more effectively, than if we were to pursue a commercial strategy on our own.
•Portfolio scale: Our commercial strategy also allows us to combine our products with larger portfolios (via our partners) which, through the benefit of cross-selling, should enhance the attractiveness of our products. Furthermore, through a portfolio approach, we are able to receive the benefits of our partners established relationships with payors and providers.
•Product selection flexibility: As a company focused only on developing and manufacturing biosimilars, our product selection model is not complicated by an in-house set of innovator products, nor is it confined to specific therapeutic areas. We do not need to make product selection decisions to fit a pre-existing commercial strategy or sales and marketing infrastructure, but rather we can take a flexible approach to product selection, evaluating candidates based on their clinical merits, partner preferences and commercial opportunity. We are able to access markets through an existing network or create a new network through our partnership model in various therapeutic areas and various geographies.
•Platform leveragability: Our commercial strategy also allows for the creation of a highly leverageable platform. Products may be added without significant changes in Sales and Marketing or G&A infrastructure. We believe this leveragability, after achieving critical mass through our launches, can create a company more profitable than we would otherwise be, had we decided to create a global commercial infrastructure and distribute our product through that network.
•Milestones: We expect to receive milestone payments from our partners at the time of signature of the commercial agreement and at various points in time through development and in some cases, post approval. Milestones offset the cost of development and create a shared risk alignment with our partners. We further view milestones as a consistent and repeating revenue opportunity, as we fully expect to continue to add product candidates to our pipeline, and subsequently out-license them in order to maximize the value of our dedicated biosimilar development and manufacturing infrastructure.
As a result of our strategic decision to form commercial partnerships, we do not currently have direct sales, marketing, and distribution capabilities. In order for us to commercialize any product on our own, we would need to either develop an infrastructure to facilitate sales, marketing and distribution or contract with third parties that have the requisite capabilities. Our in-house strategic sales and marketing expertise is currently focused on relationships with our existing partners and finding new partner relationships. As of 31 December 2023, we have contracted with 17 partners to sell, market, and distribute our products in certain agreed upon territories.
Commercial partnerships
We have formed strategic commercialization partnerships with leading pharmaceutical companies covering global markets. A commercialization partnership generally consists of two components. First, under the licensing component, we and the partner agree that we will develop the product candidate and that the partner will have the exclusive right to market, distribute and sell our product in a certain territory once the product has been approved by the relevant regulator. In return, the partner agrees to make certain upfront or milestone payments to us, which can be any or a combination of the following:
•Upfront payments upon the signing of the agreement;
•Milestone payments related to the development of the products, for example upon the completion of a clinical trial with respect to the relevant product candidate;
76
Table of Contents
•Milestone payments related to the regulatory approval process of the products, for example upon submitting an application for approval with or receiving approval from the relevant regulator for the relevant product candidate;
•Milestone payments related to the launch or first commercial sale of the product in the relevant territory; and
•Milestone payments related to achieving sales targets in the territory.
Under the supply component of the partnership agreements, we will generally manufacture, supply and deliver the product to each partner, and the partner will exclusively buy the product from us. The purchase price for each commercial partner, unless specifically noted otherwise in the description of the partnership agreements below, is a royalty of approximately 40% (between 35% and 45%) of the estimated net selling price or an agreed-upon applicable floor price, whichever is higher, for the duration of the agreements. The floor price is a minimum price per unit specific to each presentation to be paid by the commercial partner for the product, and is determined per each presentation and product taking into consideration Cost of Goods of manufacturing, supply and commercial market environment. Under certain partnership agreements, we may be eligible to receive additional royalty payments in periods where sales exceed certain targets. As is customary, the partnerships are concluded for durations of ten to twenty years. We recognized $48.7 million of product revenue and $42.7 million of license and other revenue, resulting from the commercial partnerships, for the year ended 31 December 2023. Refer to Note 5 of the consolidated financial statements included elsewhere in this Form 20-F for further details on the revenue recognized under these agreements.
The amounts in upfront and milestone payments and the royalty rates are negotiated between parties and depend in part on the estimated addressable market for the product and the size of the territory.
As a principal matter, we grant our partners access to the dossier, which includes our dossier of data, information and know-how relating to the relevant products that enable our partners to apply for and obtain marketing authorization in the various territories. Marketing authorizations obtained with the help of the dossier remain with the partners after the expiry of the partnership. Partners only return the marketing authorization to us when we terminate the agreement for cause. Certain partners may also get access to our trademarks.
Our principal partners and partnerships include:
United States—Teva
License and Development Agreement with Teva Pharmaceuticals International GmbH
In August 2020, we entered into a license and development agreement with Teva which was amended in June 2021, and again in February 2023, for the commercialization of certain biosimilar products in certain territories (the “LDA”). Under the LDA, we granted Teva an exclusive license (even as to us and our affiliates), with the right to sublicense through multiple tiers, to use, import, commercialize, and market products containing AVT02, AVT04, AVT05, AVT06, and AVT16 in the United States and each of its territories, districts and possessions, including the Commonwealth of Puerto Rico. Under the LDA, Teva has the exclusive right to reference (i) our registration dossiers of certain biosimilar products for its BLA approval, and (ii) all clinical studies conducted by or on our behalf with respect to the development of certain biosimilar products for purposes of obtaining applicable BLA approvals. Under the LDA, we granted Teva the right of first negotiation for commercialization and marketing rights in certain territories for our future biosimilar products (with some exceptions) for five (5) years from the effective date of the agreement.
The LDA expires on a product-by-product basis ten (10) years from the first commercial sale of a product, subject to possible one-year extensions. Either party may terminate the LDA on a product-by-product basis for any material breach by the other party that is not remedied within a specified time period, if either party reasonably believes that there is a material safety issue with respect to such product, or in certain other circumstances. Teva may terminate the LDA on a product-by-product basis within certain time periods, if Teva reasonably demonstrates a lack of commercial viability for such product in which case we retain already paid milestone payments and are allowed to partner with someone else. Either party may also terminate the LDA upon the insolvency of the other party. The LDA will automatically terminate as a whole upon the termination of the Teva Product Supply Agreement, or in part with respect to any product if the Teva Product Supply Agreement is terminated with respect to such product.
In November 2023, we announced that the FDA had accepted the resubmitted biologics license application for AVT04. We anticipate that the FDA’s review will be completed by 16 April 2024.
77
Table of Contents
On 27 February 2023, Alvotech and Teva signed an amendment to the LDA. As part of that amendment, Alvotech agreed to provide future financial consideration to Teva to assist with the cost of launching and marketing the licensed biosimilar products.
On 24 July 2023, Alvotech announced that it had expanded its strategic partnership agreement with Teva. The expanded agreement pertained to the exclusive commercialization in the U.S. by Teva of two new biosimilar candidates to be developed and manufactured by Alvotech and line extensions of two current biosimilar candidates in the partnership, also to be developed and manufactured by Alvotech. The agreement includes milestone payments, the majority paid following product approvals and upon achieving significant sales milestones. Teva and Alvotech will share profit from the commercialization of the biosimilars. Teva also agreed to acquire Tranche B Convertible Bonds which were issued by Alvotech pursuant to a convertible bond instrument, dated 20 December 2022, for $40 million.
As consideration for the rights granted to Teva under the LDA, Teva made upfront payments of $40.0 million and $37.5 million in development milestone payments up to 31 December 2023. Additionally, we are eligible to receive aggregate payments of up to an additional $552.5 million upon the achievement of certain regulatory, commercial, manufacturing and sales milestones.
In the first quarter of 2024, the FDA approved our BLA supporting interchangeability for AVT02 in the United
States for the treatment of adult rheumatoid arthritis, juvenile idiopathic arthritis, adult psoriatic arthritis, adult ankylosing
spondylitis, Crohn’s disease, adult ulcerative colitis, adult plaque psoriasis, adult hidradenitis suppurativa and adult uveitis.
We expect to launch AVT02 through Teva under the trade name SIMLANDI in the U.S. in the first half of 2024.
Product Supply Agreement with Teva Pharmaceuticals International GmbH
In addition to the LDA, we entered into a product supply agreement with Teva in August 2020 for the exclusive manufacture and supply of each product during such product’s respective product supply term (the “PSA”). Under the PSA, we will manufacture and supply each product exclusively to Teva for marketing in the territory. We will meet purchase orders for the product that have been accepted or deemed accepted by us. Teva has agreed to a minimum order quantity for each product. Subject to some limitations, as consideration for supply of product Alvotech will receive 40% of the value of Teva’s net sales of the products.
The PSA remains in force on a product-by-product basis for 10 years from launch, then continuing until terminated by either party with 12 months’ notice. Either party may terminate the PSA on a product-by-product basis for any material breach by the other party that is not remedied within a specified time period. Either party may terminate the agreement with respect to a product if the BLA approval for a product in the territory is revoked by a regulatory authority due to a health, safety or efficacy concern. With exceptions, Teva may require us to purchase any and all unsold quantities of products ordered by Teva prior to termination. We may terminate the PSA if Teva fails to purchase certain minimum quantities of each product. Additionally, either party may terminate the PSA with respect to a product if a margin split event occurred which results in a negative margin for a period of four (4) consecutive calendar quarters.
Europe—STADA
From August to November of 2019, we entered into similar license and supply agreements (“out-license contracts”) with STADA which were amended in March 2020, pursuant to which we granted STADA exclusive licenses (even as to us and our affiliates) to import, commercialize and market certain products containing AVT02, AVT03, AVT04, AVT05, AVT06, and AVT16 in the European Union and certain other countries. Under the amended agreements, STADA also received joint ownership of certain of our intellectual property covering such products in the EU and certain other countries under certain conditions. Pursuant to the amended agreements, we are required to provide, and STADA is required to obtain, all of STADA’s requirements of the licensed products for a defined period of time. We are also obligated to develop the licensed products, including performing all pre-clinical and clinical activities required to submit grants to obtain marketing authorizations for the licensed products in the EU and certain other countries, whereas STADA is required to use all commercially reasonable efforts to sell, market, import and store the licensed products and we have the right to terminate if STADA does not launch after fulfillment of certain conditions. STADA will remit approximately 40% of its in-market sales to us in the form of sales-based royalties.
Subject to certain conditions, the consideration paid to us is subject to a partial or full refund to STADA on a product-by-product basis if (i) the net sales of a product fall below certain specified thresholds, (ii) the manufacture, marketing or sale of such product would result in patent infringement, or (iii) we materially breach the agreement and fail to cure within 60 days of receiving notice from STADA. The licenses granted to STADA will remain exclusive until the fifth anniversary of STADA’s first sale of a product in a country, on a product-by-product and country-by-country basis.
78
Table of Contents
STADA may extend the exclusivity period up to three times for an additional five years by providing written notice one year prior to the expiration of the exclusivity period. Upon expiration of the exclusivity period for a product in a country, STADA will retain a non-exclusive license to import, commercialize and market such product in the country, and will be granted a worldwide, non-exclusive license to manufacture such product for sale in such country.
In May 2021, we entered into a second amendment of the AVT02 agreement to, among other things, expand the agreement to include an additional product and provide certain additional terms for the development, licensing and commercialization of such product. Under the amended agreement, we granted STADA a perpetual, exclusive license to import, commercialize and market the additional product in the EU and certain other countries. If STADA grants us a non-exclusive license to import, commercialize and market the additional product, we will be required to reimburse a portion of the milestone payments received for the development of the additional product. Upon expiration of the exclusive license of any AVT02 product in a country, STADA will be granted a worldwide, non-exclusive license to manufacture the additional product for sale in such country.
Prior to the completion of development of the additional product, STADA may terminate its rights to the additional product upon 10 days written notice. Upon such termination, we would no longer be eligible for payments for the subsequent completion of milestones for the additional product.
On 19 May 2023, Alvotech entered into three termination agreements (the “Termination STADA Agreements”)
with STADA to terminate the license and supply agreements between Alvotech and STADA pertaining to Alvotech’s
product candidates AVT03, AVT05, and AVT16. Pursuant to the terms of the Termination STADA Agreements, Alvotech repaid the aggregate amount of €17.4 million that Alvotech had previously received from STADA under the
Terminated STADA Agreements. Any and all rights, title and/or interest in respect of the products which became jointly
owned as a result of the Agreements with STADA, excluding any trademarks of STADA and/or any of its affiliates,
fully reverted back to the entire and sole ownership alone by Alvotech. STADA has no further rights or licenses under the
Terminated STADA Agreements. The other agreements between Alvotech and STADA, that pertain to AVT02, AVT04 and AVT06, were not terminated or amended.
Under the terms of these agreements, STADA made upfront payments of $4.9 million and $62.4 million in development milestone payments up to 31 December 2023. Additionally, we are eligible to receive aggregate payments of up to an additional $57.2 million upon the achievement of certain, regulatory, commercial, manufacturing and sales milestones.
Europe - Advanz
On 6 February 2023, Alvotech announced that it had entered into an exclusive agreement with Advanz Pharma, for the commercialization of AVT23, a proposed biosimilar to Xolair (omalizumab). The agreement covers the European Economic Area, UK, Switzerland, Canada, Australia and New Zealand. According to the agreement, Alvotech will be responsible for development and manufacture, while Advanz Pharma will handle registration and commercialization.
On 24 May 2023, Alvotech announced that the two companies had extended their exclusive partnership agreement, adding the supply and commercialization of five biosimilar candidates in Europe. Alvotech will be responsible for development and commercial supply and Advanz Pharma will be responsible for registration and commercialization in Europe. The agreement includes candidate biosimilars to Simponi (golimumab) and Entyvio (vedolizumab) and also includes three additional early-stage, undisclosed biosimilar candidates. In conjunction with this agreement, which includes three biosimilar candidates previously licensed to STADA, Advanz Pharma agreed to make upfront payments of $61 million and agreed to make additional payments for an aggregate amount of up to $287.5 million, upon the achievement of certain development and commercial milestones,
Under the terms of these agreements, Advanz Pharma made upfront payments of $75.3 million and $15.1 million in development milestone payments up to 31 December 2023. Additionally, we are eligible to receive aggregate payments of up to an additional $351.0 million upon the achievement of certain, regulatory, commercial, manufacturing and sales milestones.
Japan – Fuji Pharma
On 2 April 2019, Alvotech and Fuji Pharma entered into a license agreement, as amended on June 23, 2020 to reflect a delay in the development process and therefore, among others, amended and restated the milestone payments, (the “Fuji Pharma AVT04 License Agreement”) and a supply agreement (the “Fuji Pharma AVT04 Supply Agreement”). Under the Fuji Pharma AVT04 License Agreement, Alvotech will develop AVT04 and compile and provide a dossier of data, information and know-how relating to AVT04 to Fuji Pharma. Alvotech retains full ownership of all intellectual property
79
Table of Contents
rights in AVT04 and the AVT04 dossier. Fuji Pharma has the exclusive right to use the dossier to obtain and maintain regulatory approvals for AVT04 and to import, finish, market, promote, sell and distribute AVT04 in Japan. Fuji Pharma made a one-time payment on the signature date of $4.6 million and will make an additional milestone payment to Alvotech upon the launch of the product, subject to certain conditions. If Fuji Pharma achieves annual sales in excess of certain target volumes, it will pay Alvotech an additional royalty on the net sales above the target. Under the Fuji Pharma AVT04 Supply Agreement, Alvotech will manufacture, supply and deliver the AVT04 product. Fuji Pharma will pay Alvotech a royalty or the applicable floor price, whichever is higher, for the duration of the agreement. All invoices are payable within thirty business days, in U.S. dollar and by wire transfer. The agreements terminate 20 years after the first commercial sale of AVT04 in Japan. They can be terminated by either party if the other party: (i) withholds any monies due to the other party for more than two months; (ii) commits or permits any substantial breach of any material term of the agreement; (iii) has a receiver or administrator appointed in respect of any of its assets or enters into any agreement with its creditors; or (iv) goes into liquidation. The agreements can be terminated by Fuji Pharma if (i) a competing product obtains reimbursement approval (Fuji Pharma AVT04 License Agreement) before AVT04 obtains reimbursement approval; (ii) AVT04 does not obtain reimbursement approval by 30 November 2023; or (iii) AVT04 obtains reimbursement approval at the same time two competing products obtain reimbursement approval.
On 18 November 2020, Alvotech and Fuji Pharma entered into four binding term sheets with respect to AVT06, two proposed AVT03 biosimilar products and AVT05. On 10 February 2022, Alvotech and Fuji Pharma expanded their strategic partnership and entered into an additional binding term sheet with respect to a new undisclosed biosimilar candidate currently in early phase development, and in January 2023 we announced the expansion with another undisclosed biosimilar candidate. Under the binding term sheets, Alvotech will develop the product candidates and provide a dossier of data, information and know-how relating to the relevant product to Fuji Pharma. Fuji Pharma has the exclusive right to use the dossier to obtain and maintain regulatory approvals and to import, finish, market, promote, sell and distribute the relevant product in Japan. As of 31 December 2022, Fuji Pharma made one-time payments on the signing dates of the binding term sheets of $3.0 million and agreed to make additional payments upon the achievement of certain regulatory and development milestones. Alvotech and Fuji Pharma will enter into license and supply agreements for each product at a later date, subject to fulfilling of certain conditions related to the development of that product and the absence of the commercial launch of competing products in Japan at that time. Fuji Pharma will exclusively buy the relevant biosimilar candidate from Alvotech at a royalty or the applicable floor price, whichever is higher, for the duration of the agreement. The license and supply agreements will terminate 20 years after the first commercial sale of the relevant product in Japan. They can be terminated by either party in case a party (i) withholds any monies due to the other party for more than two months; (ii) commits or permits any substantial breach of any material term of the agreement; (iii) has a receiver or administrator appointed in respect of any of its assets or enters into any agreement with its creditors; or (iv) goes into liquidation.
In September 2023, Fuji Pharma received marketing approval for AVT04 from the Japanese Ministry of Health, Labor and Welfare. In February 2024, Alvotech announced that, following settlement agreements with the manufacturer of the originator biologics, Johnson & Johnson, marketing of AVT04 in Japan was expected to commence after the upcoming round of National Health Insurance reimbursement price listings, in May 2024.
Canada – JAMP Pharma
JAMP Pharma has a portfolio with more than 290 molecules and is a leader in the pharmaceutical industry in Canada. In December 2019, Alvotech entered into five license and supply agreements with JAMP Pharma with respect to AVT02, AVT03, AVT04, AVT05 and AVT06. Under the terms of the agreements, Alvotech will develop the product candidates and provide the dossier of data, information and know-how relating to the relevant product candidate to JAMP Pharma. Alvotech retains full ownership of all intellectual property rights in the product candidates and the dossiers. JAMP Pharma has the exclusive right and obligation to use the dossier to try to obtain and maintain regulatory approvals for the relevant product and to market, sell, and distribute the products in Canada. Alvotech will manufacture, supply and deliver the product to JAMP Pharma and JAMP Pharma will exclusively buy the relevant biosimilar candidate from Alvotech at a royalty or the applicable floor price, whichever is higher, for the duration of the agreement. If the agreed remittance is less than the floor price, JAMP Pharma has the option to turn the supply price for that product into a profit share arrangement. All invoices are payable within sixty days, in euros and by wire transfer. The agreements terminate 20 year after the first commercial sale of the relevant product and are subject to certain customary early termination rights. They can be terminated by either party if the other party (i) commits or permits any substantial breach of any material term or provision of the agreement; (ii) has a receiver or administrator appointed in respect of any of its assets, or enter into any arrangement or composition with its creditors; or (iii) goes into liquidation. The agreements can be terminated by JAMP Pharma (i) in case of Phase III study failure; (ii) in case the dossier is delayed by more than 12 months from the target date; (iii) if, following the agreed launch date, Alvotech’s formulation of the product or the process used in the manufacture of the product violates any third-party patent in Iceland or Canada; (iv) in case of GMP or quality failures hindering registration or launch in the Canada; (v) if Health Canada rejects or does not provide regulatory approval within 18 months of filing;
80
Table of Contents
(vi) if the results of due diligence performed by JAMP Pharma are not satisfactory; (viii) if 50% of the market for the product is not converted to certain product specifications at the time of launch by JAMP Pharma; or (ix) if Alvotech fails to deliver the launch order for the product within 12 months from the placing of the launch and, due to Alvotech’s non- or late delivery of products, JAMP Pharma is out of stock for more than 12 consecutive months.
In January 2022, Health Canada granted marketing authorization to JAMP Pharma for AVT02. In April 2022, JAMP Pharma launched AVT02, under the trade name SIMLANDI, in Canada. In November 2023, Health Canada granted marketing authorization to JAMP Pharma for AVT04 under the trade name JAMTEKI. JAMP Pharma launched JAMTEKI in Canada on 1 March 2024.
On 29 August 2022, Alvotech and JAMP Pharma entered into additional license and supply agreements on substantially the same terms and thereby expanded their partnership with two additional biosimilar candidates, AVT16 and AVT33.
Under these agreements, JAMP Pharma made upfront payments of $18.0 million and $0.5 million in development milestone payments up to 31 December 2023. Additionally, we are eligible to receive aggregate payments of up to an additional $56.2 million upon the achievement of certain regulatory, commercial, manufacturing and sales milestones.
In addition, Alvotech has commercialization partnerships with, among others, Cipla/Cipla Gulf/Cipla Medpro (Australia, New Zealand, South Africa/Africa), DKSH (Taiwan, Hong Kong, Cambodia, Malaysia, Singapore, Indonesia, India, Bangladesh and Pakistan), YAS (Middle East and Africa), Abdi Ibrahim (Turkey), Kamada (Israel), MegaLabs, Stein, Libbs, Tuteur and Saval (Latin America), and Advanz Pharma (EEA, U.K., Switzerland, Canada, Australia and New Zealand).
Kashiv Biosciences for AVT23
In October 2023, Alvotech announced that it had entered into an exclusive licensing agreement for AVT23 (also called ADL018), a proposed biosimilar to Xolair (omalizumab), which is currently in clinical development. The agreement covers all 27 countries of the European Union, the UK, Australia, Canada, and New Zealand. Under terms of the agreement, Alvotech will receive an exclusive license to commercialize AVT23, which will be developed and manufactured by Kashiv Biosciences ("Kashiv"). Kashiv received an upfront payment of $6.0 million and is eligible for subsequent milestone payments up to $34.0 million and royalties.
Material Agreements, Partnerships and Suppliers
China Joint Venture
In September 2018, Alvotech created a 50-50 joint venture with the Joint Venture Partner, Changchun High & New Technology Industries (Group) Inc. ("CCHN") to develop, manufacture and commercialize Alvotech’s biosimilar medicines in China and for the China market. Pursuant to a joint venture agreement, as amended on 17 February 2019, the Joint Venture Partner is investing $100 million in cash to build a state-of-the-art biologic medicine manufacturing facility in Changchun, and Alvotech is contributing the same value via a combination of additional capital and the granting of market licenses for six of its biosimilar medicines in the China market under a separate technology license contract. These capital contributions are made in installments pursuant to the contribution schedule in the joint venture agreement. There are no other anticipated payments under the joint venture agreement aside from the aforementioned capital contributions.
As of 31 December 2023, Alvotech is in discussions with CCHN to buy back Alvotech's interests in the joint venture. See further information on the accounting considerations for the China JV in note 27 of the consolidated financial statements included elsewhere in this 20-F.
U.S. AbbVie Agreement
On 8 March 2022 Alvotech entered into the AbbVie U.S. Agreement with AbbVie Inc. and AbbVie Biotechnology Ltd with respect to AVT02 for the U.S. market. Pursuant to the settlement component of the AbbVie U.S. Agreement, the parties agreed to stipulate to the dismissal of all claims, counterclaims and potential claims in the pending litigation, with each party to bear its own fees and costs, in the U.S. For more information about the U.S. litigation that was terminated, please refer to “Item 8.A Consolidated Statements and Other Financial Information—Legal Proceedings—U.S. Litigations.” The parties further agreed to release each other from certain claims and demands. Under the licensing component of the AbbVie U.S. Agreement, AbbVie granted Alvotech a license effective 1 July 2023 to make, import, use, distribute, sell and offer for sale AVT02 in the U.S. and a license to manufacture, import and store a reasonable amount of AVT02 in anticipation of the commercial launch of AVT02 in the U.S. Under the agreement, Alvotech may sublicense
81
Table of Contents
certain rights to Teva, as a commercialization partner, and may also sublicense to other parties subject to certain conditions. In return, Alvotech is obligated to pay a royalty to AbbVie in the single-digits of the net sales of AVT02 in the U.S. The agreement does not provide for upfront or milestone payments. The obligation of Alvotech to pay royalties shall terminate on the earlier of (i) 11 February 2025; or (ii) a determination that licensed patents are invalid or unenforceable, at which time the license granted will be deemed fully paid up and irrevocable. Each party has the right to terminate the agreement upon breach of certain terms of the agreement that remains uncured for a certain period of time. Additionally, AbbVie may terminate the agreement if Alvotech takes certain actions concerning the patentability, validity, or enforceability of AbbVie’s patents in the U.S. with respect to AVT02.
European AbbVie Agreement
On 4 April 2022, Alvotech entered into the European AbbVie Agreement with AbbVie Biotechnology Ltd with respect to the sale of AVT02 in Europe and selected markets outside of Europe (the “European AbbVie Agreement”). Pursuant to the settlement component, the parties resolved all intellectual property disputes between Alvotech and AbbVie relating to AVT02 in those territories. For more information about those legal disputes, please refer to “Item 8.A Consolidated Statements and Other Financial Information—Legal Proceedings—Legal Proceedings.” The parties further agreed to release each other from certain claims and demands. Under the licensing component of the European AbbVie Agreement, AbbVie granted Alvotech a license effective immediately to make, import, use, distribute, sell and offer for sale AVT02 in Europe and selected markets outside of Europe. Under the agreement, Alvotech may sublicense certain rights to STADA, as a commercialization partner, and may also sublicense to other parties subject to certain conditions. In return, Alvotech is obligated to pay royalties to AbbVie with respect to certain indications that are covered by AbbVie patents, on an indication-by-indication and territory-by-territory basis. For purposes of calculating royalties due under the agreement, the parties agreed that in any territory, a certain percentage of AVT02 sold in such territory is covered by the indication, bringing the effective royalty rate in the single-digit to low-teens percentage range of net sales of AVT02 in the territories. The agreement does not provide for upfront or milestone payments. The royalty payments terminated or will terminate, on an indication-by-indication basis, on 5 June 2022, 11 April, 2025, and 3 June 2031, respectively, at which time the license granted for that indication will be deemed fully paid up and irrevocable. Alvotech’s royalty obligation will terminate earlier if, on a territory-by-territory and indication-by-indication basis, no valid AbbVie patent rights remain. Each party has the right to terminate the agreement upon breach of certain terms of the agreement that remains uncured for a certain period of time. Additionally, AbbVie may terminate the agreement if Alvotech takes certain actions concerning the patentability, validity, or enforceability of AbbVie’s patents in Europe with respect to AVT02.
For the year ended 31 December 2023, we paid $1.7 million in royalties to AbbVie.
Competition
We believe our focus on biosimilars, investment in our platform, and global market reach endow us with a differentiated set of strategic advantages in the dynamic and competitive biosimilars marketplace. These features include substantial control over quality and capacity allocation; the ability to find and exploit operational and process efficiencies across R&D and manufacturing; and the agility to rapidly, flexibly and efficiently pivot to new opportunities to advance a broad portfolio of product candidates. We believe these advantages expand our opportunity and support our commercial and medical goals of accelerating the development of cost-effective biosimilars that are as close to the reference products as possible, and then getting them to the patients around the world who need them.
The specific characteristics of the competitive landscape for each of our publicly announced product development programs include but are not limited to:
AVT02. We expect AbbVie (the originator) as well as Amgen, Boehringer Ingelheim GmbH, Biocon/FujiFilm, Celltrion, Fresenius Kabi, Pfizer, Samsung Bioepis, Coherus, and Sandoz to be our main competitors for AVT02, a biosimilar product candidate to Humira (adalimumab). Most of these companies have either launched or disclosed development plans for a 50 mg/mL Humira biosimilar in the U.S., EU, or both, as well as in some other global markets. Celltrion, Sandoz and Alvotech are the only companies with regulatory approval in the EU for a 100 mg/mL biosimilar version of adalimumab. In the US, Amgen, Samsung Bioepis, Celltrion and Sandoz received approval from FDA for a 100 mg/mL biosimilar version of adalimumab and Boehringer Ingelheim has a BLA pending FDA approval. Companies that announced plans to seek U.S. interchangeability designation for a 100 mg/mL biosimilar version of adalimumab include Amgen, Samsung Bioepis, and Celltrion. On 23 February 2024, the FDA gave market U.S. approval to AVT02 under the tradename SIMLANDI, and announced that SIMLANDI qualified for interchangeability designation and with exclusivity in the U.S. market for an undetermined length of time.
AVT04. We expect Janssen (the originator) as well as Amgen, Celltrion, Bio-Thera, Formycon, Dong-A/Meiji Seika, Samsung Bioepis and Biocon to be our main competitors for AVT04, a biosimilar candidate to Stelara (ustekinumab), all
82
Table of Contents
of which have disclosed development plans for a Stelara biosimilar. Janssen is also attempting to defend against biosimilar competition by expanding the label for Stelara and by launching follow-on drugs. We have reached settlement agreements with the originator to enable launches in the U.S., Europe, Canada and Japan.
AVT06. We expect Regeneron (the originator) Amgen, Celltrion, Formycon, Altos, Sam Chun Dang, Samsung Bioepis, Sandoz and Viatris/Biocon to be our main competitors for AVT06, a biosimilar candidate to Regeneron’s Eylea (aflibercept). As the originator, Regeneron is currently working to expand the label for Eylea and developing higher-concentration formulations.
AVT03. We expect Amgen (the originator), Sandoz, Celltrion, Fresenius Kabi, Samsung Bioepis, Gedeon Richter, mAbxience, Biocon, Henlius and Teva to be our main competitors for AVT03, a biosimilar candidate to Prolia/Xgeva (denosumab), as they have all disclosed development plans for a Prolia/Xgeva biosimilar.
AVT05. We expect Janssen (the originator), and Bio-Thera to be our main competitors for AVT05, a biosimilar candidate to Janssen’s Simponi (golimumab). The originator, Janssen, is solidifying the reference product’s market position by actively expanding the label and by winning approvals in Japan and China. We believe that the originator’s success in expanding the market for the reference product will prove to be a benefit to AVT05’s commercial positioning.
AVT23. We expect Genentech (the originator), Celltrion and Teva to be our main competitors for AVT23, a biosimilar candidate to Genentech’s Xolair (omalizumab), as they have all disclosed development plans for a Xolair biosimilar. As the originator, Genentech is currently working to expand the label for Xolair.
Intellectual Property
The branded pharmaceutical industry relies on patent protection as one of several means to maintain exclusivity on the market. As a biosimilar-focused company, our success will depend in part on our ability to avoid infringement of, to invalidate, and/or to license any relevant and material intellectual property rights of third parties. We expect all branded companies that market products in which we are developing a biosimilar to vigorously protect what they view as their proprietary rights. We fully understand that efforts to market our products may result in patent litigation, which may determine whether a particular patent at issue is valid and whether We have infringed such a patent. Timelines for resolution to patent disputes are difficult to estimate and are very specific to a particular situation (including, for example, the jurisdiction).
While our principal focus in matters relating to intellectual property is to avoid infringing the valid and enforceable rights of third parties, we also use a combination of intellectual property protection and confidentiality agreements to protect our own intellectual property related to our product candidates and development programs. We strive to protect and enhance the proprietary technologies, inventions and improvements that we believe are important to our business, including by seeking, maintaining, enforcing and defending trademarks, trade secrets, patent rights, and other intellectual property rights for our products and processes, whether developed internally or licensed from third parties.
We are actively building our own intellectual property portfolio around our product candidates and platform technologies, including our manufacturing processes, and intend to identify and obtain, directly or through a license, as appropriate, patents that provide protection to our intellectual property and technology base. With respect to these pending and any future applications, we cannot be sure that patents will be granted in any or all jurisdictions, nor can we be sure that any patents that may be granted to us in the future will be commercially useful in protecting our products. In addition to patents, We also rely on trademarks, trade secrets, know-how, continuing technological innovation, confidentiality agreements, and IP assignment agreements in place with our employees to develop and maintain our proprietary position and ensure the future commercial success of our products.
Regulatory Landscape
Government Regulation and Product Approval
Government authorities at the federal, state and local level in the United States and in other countries extensively regulate, among other things, the research, development, testing, clinical trials manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical products such as those we are developing. The processes for obtaining regulatory approvals in the United States and in other countries, along with subsequent obligation of compliance with applicable statutes and regulations, can vary widely and can require the expenditure of substantial time and financial resources.
83
Table of Contents
FDA Approval Process
All of our current product candidates are subject to extensive pre- and post-market regulation in the United States by the FDA as biological products, or biologics. The Public Health Service Act, or PHSA, the Federal Food, Drug and Cosmetic Act, or FFDCA, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, post-approval changes, and import and export of biologics. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending Biologics License Applications, or BLAs, withdrawal of approvals or revocation or suspension of licenses, clinical holds, warning letters, product recalls, product seizures, injunctions, fines, civil penalties or criminal penalties. The PHSA and its implementing regulations provides FDA authority to immediately suspend licenses in certain situations where FDA determines that there exists a danger to health, and to promulgate and enforce regulations to prevent the introduction or spread of communicable diseases in the United States and between states.
The process required by the FDA before a new biologic may be marketed in the United States is long, expensive and inherently uncertain. In order to establish the safety, purity and potency (effectiveness) of the biologic, biologics development in the United States typically involves, among other things, pre-clinical laboratory and animal tests, the submission to the FDA of an investigational new drug application, or IND, which must become effective before U.S. clinical investigations in humans may commence, and adequate and well-controlled clinical trials to establish the safety, purity and potency of the biologic for the conditions of use for which FDA approval is sought. Developing the data to satisfy FDA approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Pre-clinical tests include laboratory evaluation of product chemistry, formulation and toxicology, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the pre-clinical tests must comply with federal regulations and requirements, including good laboratory practices. An IND must be submitted to the FDA to administer an investigational new drug to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human studies, although the IND must also include safety data, e.g., the results of pre-clinical testing and animal testing assessing the toxicology and pharmacology of the product along with other information, including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long term pre-clinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
An IND must become effective before United States clinical trials may begin. There is generally a 30-day waiting period after the IND submission, after which clinical investigations can begin, unless the FDA notifies the sponsor of concerns or questions related to a clinical hold. If that happens, the sponsor and the FDA must resolve the hold issue(s) before the clinical investigation can begin. Otherwise, the clinical trial proposed in the IND may begin at the conclusion of this 30-day period.
Clinical trials involve the administration of the investigational new drug to volunteers or patients all under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations on good clinical practice, or GCP, including, for example, regulations regarding the protection of human subjects, defining, the roles of clinical trial sponsors, administrators and monitors, and governing protocols detailing the objectives of the trial and, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. Before approving a BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients, among other reasons. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements or may impose other conditions. The study sponsor may also suspend a clinical trial at any time on various grounds, including a determination that the subjects or patients are being exposed to an unacceptable health risk.
Clinical trials to support BLAs for regulatory approval of a reference biologic product under the 351(a) pathway are typically conducted in three sequential phases, but the phases may overlap or be combined. In Phase 1, the biologics are initially introduced into patients or healthy human subjects and the biologic is tested to assess the safety/tolerability, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence of effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the biologic for a particular indication, dosage tolerance and optimum dosage and to identify common adverse effects and safety risks.
84
Table of Contents
If a product candidate demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials are undertaken to obtain additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites. These Phase 3 clinical trials are intended to establish data sufficient to demonstrate substantial evidence of the efficacy and safety of the product to permit the FDA to evaluate the overall benefit-risk relationship of the biologic and to provide adequate information for the labeling of the biologic. Trials conducted outside of the United States under similar, GCP-compliant conditions in accordance with local applicable laws may also be acceptable to the FDA in support of product licensing.
Sponsors of clinical trials for investigational drugs generally must publicly disclose certain clinical trial information, including detailed trial design and trial results in a public database administered by the U.S. Department of Health and Human Services. These requirements are subject to specific timelines and apply to most clinical trials of FDA-regulated products.
After completion of the required clinical testing in accordance with all applicable regulatory requirements, detailed information regarding the investigational product is prepared and submitted to the FDA in the form of a BLA requesting approval to market the product for one or more indications or conditions of use. FDA review and approval of the BLA is required before marketing of the product may begin in the United States. The BLA will include the results of pre-clinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls and must demonstrate the continued safety, purity, and potency (efficacy) of the product based on these data.
Manufacturing controls and conformance to current good manufacturing practices (“cGMPs”) are considered very important for biological products. The BLA must also contain extensive manufacturing information. The FDA will inspect the facility or the facilities at which the biologic is manufactured to ensure conformance to cGMPs. This can include reviewing a firm’s previous compliance history, using information sharing from trusted foreign regulatory partners through mutual recognition agreements and other confidentiality agreements, requesting records “in advance of or in lieu of” facility inspections or voluntarily from facilities and sites, and conducting remote interactive evaluations where appropriate.
The cost of preparing and submitting a BLA is substantial. Under federal law, the submission of most original BLAs is subject to a multi-million dollar application user fee, as well as annual fees, both of which are typically increased annually.
The FDA has agreed to certain performance goals in the review of BLAs. First, the FDA has agreed to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to enable substantive review within 60 days from its receipt of a BLA. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA’s stated goal is to review most original BLA applications for standard review biologics within ten months from the date the application is accepted for filing. Although the FDA often meets its user fee performance goals, the review goal date can be extended in the event of a “major amendment,” or can be extended by requests for additional information or clarification, and FDA review may not occur on a timely basis at all. Additionally, as a result of public health emergencies, such as the COVID-19 pandemic, review timelines may be delayed even further.
The FDA often refers applications for novel biologics or biologics which present difficult questions of safety or efficacy, to an advisory committee — typically a panel that includes clinicians and other experts — for review, evaluation and a recommendation as to whether the application should be approved and/or specific use and approvability questions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations.
The FDA reviews a BLA to determine, among other things, whether a product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency. After the FDA evaluates the BLA, including the facilities listed in the BLA, it issues either an approval letter or a complete response letter. A complete response letter outlines the deficiencies in the submission. Remedying those deficiencies may require substantial additional testing or information in order for the FDA to consider the resubmitted application for approval. If, or when, those deficiencies have been addressed to the FDA’s satisfaction such that a resubmitted BLA is approvable, the FDA will issue an approval letter. The FDA has committed to user fee goals of reviewing such resubmissions in two or six months depending on the type of information included. The FDA approval is never guaranteed, and the FDA may refuse to approve a BLA if applicable regulatory criteria are not satisfied.
Under the PHSA, the FDA will approve a BLA if it determines, among other things, that the product is safe, pure and potent and the facility where the product will be manufactured meets standards designed to ensure that it continues to be safe, pure and potent. An approval letter authorizes commercial marketing of the biologic with specific prescribing information for specific indications. The approval for a biologic may be significantly more limited than requested in the
85
Table of Contents
application, including limitations on the specific conditions of use, which could restrict the commercial value of the product. The FDA may also require that certain contraindications, warnings or precautions be included in the product labeling. In addition, under certain circumstances, the FDA may require a risk evaluation and mitigation strategy, or REMS, as a condition of approval, if necessary to ensure that the benefits of the biologic outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the biologic. Moreover, product approval may include post-marketing commitments and/or post-marketing-requirements, including, for example, pediatric studies, safety monitoring, and Phase 4 trials.
Certain types of biologics may also be subject to official lot release. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official lot release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products, such as viral vaccines, before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency and effectiveness of biological products. After approval of biologics, manufacturers must address any safety issues that arise, may be subject to recalls or a halt in manufacturing under certain circumstances, and are subject to periodic inspection after approval.
Because biologically-sourced raw materials are subject to unique contamination risks, their use may be restricted in some countries.
Abbreviated Licensure Pathway of Biological Products as Biosimilars under 351(k)
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, amended the PHSA and created an abbreviated approval pathway for biological products shown to be highly similar to an FDA-licensed reference biological products. This pathway was established as a way to provide more treatment options, increase access to lifesaving medications, and potentially lower health care costs through competition. Under the 351(k) (biosimilar) approval pathway, an application for licensure of a biosimilar product must include information demonstrating biosimilarity based upon the following (unless a specific element is waived by FDA):
•analytical studies demonstrating that the proposed biosimilar product is highly similar to the approved product notwithstanding minor differences in clinically inactive components;
•animal studies (including the assessment of toxicity and immunogenicity); and
•a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed and intended to be used and for which licensure is sought for the biological product.
In addition, an application submitted under the 351(k) pathway must include information demonstrating that:
•the proposed biosimilar product and reference product utilize the same mechanism of action for the condition(s) of use prescribed, recommended or suggested in the proposed labeling, but only to the extent the mechanism(s) of action are known for the reference product;
•the condition or conditions of use prescribed, recommended or suggested in the labeling for the proposed biosimilar product have been previously approved for the reference product;
•the route of administration, the dosage form and the strength of the proposed biosimilar product are the same as those for the reference product; and
•the facility in which the biological product is manufactured, processed, packed or held meets standards designed to assure that the biological product continues to be safe, pure, and potent.
Biosimilarity, as defined in PHSA §351(i), means that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product. In addition, section 351(k)(4) of the PHSA provides for a designation of “interchangeability” between the reference and biosimilar products if certain additional criteria are met, whereby the biosimilar may be substituted for the
86
Table of Contents
reference product without the intervention of the health care provider who prescribed the reference product. An application seeking licensure as an interchangeable must include information sufficient to demonstrate that:
•the proposed product is biosimilar to the reference product;
•the proposed product is expected to produce the same clinical result as the reference product in any given patient; and
•for a product that is administered more than once to an individual, the risk to the patient in terms of safety or diminished efficacy of alternating or switching between the biosimilar and the reference product is no greater than the risk of using the reference product without such alternation or switch.
As with other biological products, FDA approval of a BLA is required before a biosimilar may be marketed in the United States. Biosimilar BLAs (or “351(k) BLAs”) are not required to duplicate the entirety of the data package used to establish the safety and effectiveness of the reference product. Rather, a 351(k) BLA will be approved based on a demonstration of biosimilarity to the reference product, including the information outlined above, and does not require an independent showing of safety and effectiveness. Because a biosimilar can rely in part on FDA’s previous determination of safety and effectiveness for the reference product for approval, biosimilar applicants generally do not need to conduct as many clinical trials. Biosimilar products also may be approved for an indication without direct studies of the biosimilar in that indication, with sufficient scientific justification for extrapolation. However, the FDA may not approve a 351(k) BLA if there is insufficient information to show that the biosimilar is “highly similar” to the reference product or that there are no clinically meaningful differences between the biosimilar product and the reference product. In addition, as with innovator BLAs, biosimilar BLAs will not be approved unless the product is manufactured in facilities designed to assure and preserve the biological product’s safety, purity and potency.
The process for filing and review of a BLA submitted through the 351(k) pathway is very similar to that of a BLA submitted through the 351(a) pathway, although there is a period of statutory exclusivity during which time the FDA is precluded from filing a 351(k) BLA that references a protected reference product. Subsequently, the FDA will accept the application for filing if it meets the regulatory criteria. The FDA may refuse to file applications that it finds are incomplete. The FDA will treat a biosimilar application or supplement as incomplete if, among other reasons, any applicable user fees assessed under the Biosimilar User Fee Act of 2012 have not been paid. In addition, the FDA may accept an application for filing but deny approval on the basis that the sponsor has not demonstrated biosimilarity, in which case the sponsor may choose to conduct further analytical, preclinical or clinical studies and resubmit the BLA to demonstrate biosimilarity under section 351(k).
The timing of final FDA approval of a biosimilar for commercial distribution depends on a variety of factors, including whether the manufacturer of the branded product is entitled to one or more statutory exclusivity periods, during which time the FDA is prohibited from approving any products that are biosimilar to the branded product. The FDA cannot approve a biosimilar application for 12 years from the date of first licensure of the reference product. A reference product may also be entitled to exclusivity under other statutory provisions. For example, a reference product with orphan drug exclusivity for a particular orphan “disease or condition” may be entitled to seven years of exclusivity, in which case no product that is biosimilar to the reference product may be approved until either the end of the 12-year period provided under §351(k)(7), and no biosimilar may be approved for the orphan disease or condition until the end of the seven-year orphan drug exclusivity period. In certain circumstances, a regulatory exclusivity period can extend beyond the life of a patent and thus block §351(k) applications from being approved on or after the patent expiration date.
The first biological product determined to be interchangeable with a branded reference product for any condition of use is also eligible for a period of exclusivity, during which time the FDA may not determine that another product is interchangeable with the same reference product for any condition of use. This exclusivity period lasts until the earlier of: (1) one year after the first commercial marketing of the first interchangeable product; (2) 18 months after resolution of a patent infringement suit instituted under 42 U.S.C. § 262(l)(6) against the applicant that submitted the application for the first interchangeable product, based on a final court decision regarding all of the patents in the litigation or dismissal of the litigation with or without prejudice; (3) 42 months after approval of the first interchangeable product, if a patent infringement suit instituted under 42 U.S.C. § 262(l)(6) against the applicant that submitted the application for the first interchangeable product is still ongoing; or (4) 18 months after approval of the first interchangeable product if the applicant that submitted the application for the first interchangeable product has not been sued under 42 U.S.C. § 262(l)(6).
Advertising and Promotion
The FFDCA prohibits the marketing, promotion, or advertising of an investigational drug as if it has been demonstrated to be safe and effective for the uses for which it is being studied. Once a BLA is approved, a product will be subject to continuing post-approval regulatory requirements, including, among other things, requirements relating to
87
Table of Contents
recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse events. For instance, the FDA closely regulates the post-approval advertising, marketing and promotion of drugs, including biologics, including, for example, direct-to-consumer advertising, off-label promotion, and industry-sponsored scientific and educational activities. Violations of the FDA’s requirements around advertising, marketing, and promotion of drugs can result in significant enforcement activities, including the issuance of warning letters or untitled letters, which may direct a company to correct deviations from FDA, and federal and state investigations, which can lead to civil and criminal penalties, lawsuits, and prosecutions.
As with all drugs, biologics may be marketed only as consistent with FDA-approved labeling. After approval, most changes require submission and FDA approval supplemental BLA before the change can be implemented. This includes changes to labeling or manufacturing processes (including changes to facilities), which typically require prior approval of a supplement. A supplement for a 351(a) BLA seeking to add a new indication typically requires new clinical data, and the FDA generally uses the same procedures and actions in reviewing BLA supplements with clinical data as it does in reviewing BLAs. There are also continuing reporting requirements for marketed drug products.
Adverse Event Reporting and GMP Compliance
In addition to regular periodic reports following FDA approval of a BLA and compliance with any post-marketing commitments or post-marketing requirements, license-holders also must comply with adverse event reporting requirements and must continue to conform to cGMPs, as described above. Manufacture, packaging, labeling, storage, and distribution procedures must continue to conform to cGMP after approval, and FDA conducts periodic surveillance inspections intended to ensure such ongoing compliance. Biologics manufacturers and their manufacturing subcontractors are generally required to register their establishments with the FDA and certain state agencies. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality control to maintain compliance with cGMP.
Post-approval discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency or issues with manufacturing processes or cGMP compliance, or other failures to comply with regulatory requirements, may lead the FDA to, for example:
•require revisions to approved labeling to add new safety information;
•require post-market studies to assess new safety risks;
•issue fines, warning letters, or untitled letters;
•place post-approval clinical trials on hold;
•detain or refusal to permit the import or export of products;
•request voluntary calls;
•seek injunctions, civil forfeiture, civil money penalties, or other civil relief; or
•seek criminal penalties or prosecution.
Under certain circumstances, FDA may initiate proceedings to suspend or revoke a license or recall the product from the market.
Other Healthcare Laws and Compliance Requirements
Although we currently do not have any products on the market or engage with any licensed health care providers in the United States, our current and future business operations are subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we conduct our business. These laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician sunshine laws and regulations.
The federal Anti-Kickback Statute (“AKS”) prohibits any individual or entity from knowingly and willfully offering or paying “remuneration,” directly or indirectly, overtly or covertly, in cash or in kind to induce another individual or entity to: (a) refer an individual to a person for the furnishing (or arranging for the furnishing) of any item or service for which payment may be made under a federal health care program; (b) purchase or order any covered item or service; (c) arrange for the purchase or order of any covered item or service; or (d) recommend the purchase or order of any covered item or service. It also is illegal under the Anti-Kickback Statute to solicit or receive remuneration for such purposes. “Remuneration” is generally defined to include any transfer of value, in cash or in kind, including gifts or free product,
88
Table of Contents
meals, discounts, rebates, and other price concessions. Courts have broadly construed the AKS to include virtually anything of value given to an individual or entity if one purpose of the remuneration is to influence the recipient’s reason or judgment relating to referrals.
There are statutory exceptions and regulatory safe harbors specifying certain payment practices that will not be considered to violate the AKS. Such exceptions and safe harbors include, among others, protection for payments for personal services and management contracts, and for certain discounts. If a payment practice falls squarely within one of the exceptions or safe harbors, it will be immune from criminal prosecution and civil exclusion under the AKS. Importantly, the failure of an arrangement to fall within a statutory exception or regulatory safe harbor does not mean that it necessarily violates the AKS; however, the legality of such arrangements may be closely scrutinized by federal authorities on a facts and circumstances basis and are not protected.
Additionally, states have enacted similar kickback statutes that may apply to healthcare services reimbursed by private insurance, not just those reimbursed by a federal or state health care program. The specific scope of these laws vary. However, in many instances, activities that are protected from scrutiny under the federal statute would not violate the state statutes.
Further, pursuant to changes made under the PPACA, any claims submitted to Medicare or Medicaid as a result of an illegal kickback constitutes a false or fraudulent claims under the federal False Claims Act (“FCA”). Additionally, the ACA amended the intent requirement of the AKS so that a person or entity no longer needs to have actual knowledge of the AKS, or the specific intent to violate it, to have violated the statute.
The civil false claims laws, including the FCA, prohibits, among other things, knowingly presenting or causing the presentation of a false, fictitious or fraudulent claim for payment to the U.S. government. Actions under the FCA may be brought by the government or as a qui tam action by a private individual in the name of the government. Government enforcement agencies and private whistleblowers have investigated pharmaceutical companies for or asserted liability under the FCA for a variety of alleged promotional and marketing activities, such as providing free products to customers with the expectation that the customers would bill federal programs for the products; providing consulting fees and other benefits to physicians to induce them to prescribe products; and engaging in promotion for unapproved uses. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
The federal Health insurance Portability and Accountability Act of 1996 (“HIPAA”) created additional federal criminal statutes that prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of whether the payor is public or private, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing or covering up by any trick, scheme or device a material fact or making any materially false, fictitious or fraudulent statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Additionally, the ACA amended the intent requirement of some of these criminal statutes under HIPAA so that a person or entity no longer needs to have actual knowledge of the statute, or the specific intent to violate it, to have committed a violation.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. For instance, the federal Physician Payments Sunshine Act (“Sunshine Act”) requires certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with specified exceptions) to report annually information related to specified payments or other transfers of value provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors, other healthcare professionals (such as physician assistants and nurse practitioners) and teaching hospitals and to report annually specified ownership and investment interests held by physicians and their immediate family members.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) and their implementing regulations, impose requirements relating to the privacy, security and transmission of individually identifiable health information held by covered entities and their business associates. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity and their covered subcontractors. HITECH also
89
Table of Contents
created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
Many states have also adopted laws similar to each of the above federal laws, which may be broader in scope and apply to items or services reimbursed by any third-party payor, including commercial insurers. We may also be subject to state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, and/or state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information, state and local laws that require the registration of pharmaceutical sales representatives, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
The shifting commercial compliance environment and the need to build and maintain robust systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may violate one or more of the requirements. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to significant penalties, including, without limitation, administrative, civil, and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment.
International Regulation
In addition to regulations in the United States, a variety of foreign regulations govern clinical trials, marketing authorization procedures and commercial sales and distribution of pharmaceutical products. The approval process varies from country to country and the time to approval may be longer or shorter than that required for FDA approval. In the EU, the approval of a biosimilar for marketing is based on an opinion issued by the European Medicines Agency, or EMA, and a related decision issued by the European Commission. However, the subsequent substitutability of a reference medicinal product for the biosimilar is a decision that is made at the national level on a country-by-country basis in individual EU Member States. Other regions, including Canada, Japan and Korea, also have their own regulatory pathways governing the approval and marketing of biosimilars. Some third countries (such as Singapore and Malaysia) have adopted EU guidance. Other countries (such as (Cuba and Brazil) follow guidance issued by the World Health Organization. While there are some similarities between the regulatory requirements across regions, some areas of substantial difference remain.
Clinical Trials in the EU
In the EU, clinical trials are governed by the Clinical Trials Regulation (EU) No 536/2014, or CTR, which entered into application on 31 January 2022, repealing and replacing the former Clinical Trials Directive 2001/20, or CTD, and related national implementing legislation of EU Member States.
The CTR is intended to harmonize and streamline clinical trial authorizations, simplify adverse-event reporting procedures, improve the supervision of clinical trials and increasing their transparency. Specifically, the Regulation, which is directly applicable in all EU Member States, introduces a streamlined application procedure through a single-entry point, the EU portal, the Clinical Trials Information System, or CTIS; a single set of documents to be prepared and submitted for the application; as well as simplified reporting procedures for clinical trial sponsors. A harmonized procedure for the assessment of applications for clinical trials has been introduced and is divided into two parts. Part I assessment is led by the competent authorities of a reference Member State selected by the trial sponsor and relates to clinical trial aspects that are considered to be scientifically harmonized across EU Member States. This assessment is then submitted to the competent authorities of all concerned Member States in which the trial is to be conducted for their review. Part II is assessed separately by the competent authorities and Ethics Committees in each concerned EU Member State. Individual EU Member States retain the power to authorize the conduct of clinical trials on their territory.
The extent to which on-going clinical trials will be governed by the CTR will depend on the duration of the individual clinical trial. Sponsors could choose to submit a clinical trial application under either the CTD or the CTR until 31 January 2023. For clinical trials in relation to which application for approval was made on the basis of the CTD before 31 January 2023, if authorized, those clinical trials will be governed by the CTD until 31 January 2025. By that date, all ongoing trials will become subject to the provisions of the CTR. The CTR will apply to clinical trials from an earlier date if the clinical trial has already transitioned to the CTR framework.
90
Table of Contents
EU Review and Approval Process
In the EU, medicinal products can only be commercialized after a related marketing authorization, or MA, has been granted. A company may submit a marketing authorization application, or MAA, either on the basis of the centralized, or decentralized procedure or mutual recognition procedure.
To obtain an MA for a product in the EU, which is valid throughout the EEA, an applicant must submit an MAA either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in the EU Member States (decentralized procedure, national procedure or mutual recognition procedure). An MA may be granted only to an applicant established in the EU.
The centralized procedure provides for the grant of a single MA by the European Commission that is valid for all EU Member States. Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for (i) medicinal products derived from biotechnological processes, (ii) products designated as orphan medicinal products, (iii) advanced therapy medicinal products, or ATMPs, and (iv) products with a new active substance indicated for the treatment of HIV/AIDS, cancer, neurodegenerative diseases, diabetes, auto-immune and other immune dysfunctions and viral diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, authorization through the centralized procedure is optional on related approval.
Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use, or CHMP, conducts the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing MA.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product targeting an unmet medical need is expected to be of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts a request for accelerated assessment, the time limit of 210 days will be reduced to 150 days (excluding clock stops). The CHMP can, however, revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
Unlike the centralized authorization procedure, the decentralized MA procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralised Procedures – Human, or CMDh, for review. The subsequent decision of the European Commission is binding on all EU Member States.
The mutual recognition procedure allows companies that have a medicinal product already authorized in one EU Member State to apply for this authorization to be recognized by the competent authorities in other EU Member States. Like the decentralized procedure, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU Member States of the MA of a medicinal product by the competent authorities of other EU Member States. The holder of a national MA may submit an application to the competent authority of an EU Member State requesting that this authority recognize the MA delivered by the competent authority of another EU Member State.
An MA has, in principle, an initial validity of five years. The MA may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State in which the original MA was granted. To support the application, the MA holder must provide the EMA or the competent authority with a consolidated version of the eCTD (Common Technical Document) providing up-to-date data concerning the quality, safety and efficacy of the product, including all variations introduced since the MA was granted, at least nine months before the MA ceases to be valid. The European Commission or the competent authorities of the EU Member States may decide on justified grounds relating to pharmacovigilance, to proceed with one further five-year renewal period for the MA. Once subsequently definitively renewed, the MA shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (for a centralized MA) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid (the so-called sunset clause).
91
Table of Contents
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines, or PRIME, scheme, which provides incentives similar to the breakthrough therapy designation in the U.S. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that target unmet medical needs. Eligible products must target conditions for which there is an unmet medical need (there is no satisfactory method of diagnosis, prevention or treatment in the EU or, if there is, the new medicinal product will bring a major therapeutic advantage) and they must demonstrate the potential to address the unmet medical need by introducing new methods of therapy or improving existing ones. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
In the EU, a “conditional” MA may be granted in cases where all the required safety and efficacy data are not yet available. The European Commission may grant a conditional MA for a medicinal product if it is demonstrated that all of the following criteria are met: (i) the benefit-risk balance of the medicinal product is positive; (ii) it is likely that the applicant will be able to provide comprehensive data post-authorization; (iii) the medicinal product fulfils an unmet medical need; and (iv) the benefit of the immediate availability to patients of the medicinal product is greater than the risk inherent in the fact that additional data are still required. The conditional MA is subject to conditions to be fulfilled for generating the missing data or ensuring increased safety measures. It is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once any pending studies are provided, the conditional MA can be converted into a traditional MA. However, if the conditions are not fulfilled within the timeframe set by the EMA and approved by the European Commission, the MA will cease to be renewed.
An MA may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information, or when generating data may be contrary to generally accepted ethical principles. Like a conditional MA, an MA granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard MA. However, unlike the conditional MA, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data. Although the MA “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually, and the MA will be withdrawn if the risk-benefit ratio is no longer favorable.
Post-approval Requirements
Where an MA is granted in relation to a medicinal product in the EU, the holder of the MA is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission and/or the competent regulatory authorities of the individual EU Member States. The holder of an MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAAs must include a risk management plan, or RMP, describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
In the EU, the advertising and promotion of medicinal products are subject to both EU and EU Member States’ laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals, misleading and comparative advertising and unfair commercial practices. Although general requirements for advertising and promotion of medicinal products are established under EU legislation, the details are governed by regulations in individual EU Member States and can differ from one country to another. For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities in connection with an MA. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply
92
Table of Contents
with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU.
Data and marketing exclusivity
The EU also provides opportunities for market exclusivity. Upon receiving an MA in the EU, innovative medicinal products generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents generic or biosimilar applicants from referencing the innovator’s pre-clinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization during a period of eight years from the date on which the reference product was first authorized in the EU. During the additional two-year period of market exclusivity, a generic or biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar product can be marketed until the expiration of the market exclusivity period. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to authorization, is held to bring a significant clinical benefit in comparison with existing therapies.
In the EU, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for marketing authorization. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product.
Pediatric Development
In the EU, Regulation (EC) No 1901/2006 provides that all marketing authorization applications for new medicinal products must include the results of trials conducted in the pediatric population, in compliance with a pediatric investigation plan, or PIP, agreed with the EMA’s Pediatric Committee, or PDCO. The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the medicinal product for which marketing authorization is being sought. The PDCO may grant a deferral of the obligation to implement some or all of the measures provided in the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults. Furthermore, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data are not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once the marketing authorization is obtained in all EU Member States and study results are included in the product information, even when negative, the product is eligible for a six-month extension to the Supplementary Protection Certificate, or SPC, if any is in effect at the time of authorization or, in the case of orphan medicinal products, a two-year extension of orphan market exclusivity. For other countries outside of the EU, such as certain countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product approval, pricing and reimbursement vary from country to country. In all cases, the clinical trials are to be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Pharmaceutical Coverage, Pricing and Reimbursement
In the U.S. and other countries, sales of our products will depend on the availability and extent of coverage and reimbursement from third-party payors, including government healthcare programs and private insurance plans. Patients who are provided medical treatment for their conditions generally rely on third party payors to reimburse all or part of the costs associated with their treatment. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, or comparable foreign programs and commercial payors are critical to new product acceptance. Governments and private payors continue to pursue initiatives to manage drug utilization and contain costs. These payors are increasingly focused on the effectiveness, benefits, and costs of similar treatments, which could result in lower reimbursement rates for our products or narrower populations for whom payors will reimburse. Continued intense public scrutiny of the price of drugs and other healthcare costs, together with payor dynamics, have limited, and are likely to continue to limit, our ability to set or adjust the price of our products based on their value, which could adversely affect our business.
In the U.S., no uniform product coverage and reimbursement policy exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. Obtaining coverage and reimbursement approval of a product from a government or other third-party payor can be a time-consuming and costly
93
Table of Contents
process that can require provision of supporting scientific, clinical and cost-effectiveness data, with no assurance that coverage or specific levels of reimbursement will be obtained. Third-party payors are increasingly examining the medical necessity and cost-effectiveness of products and services in addition to their safety and efficacy. Accordingly, significant uncertainty exists as to the reimbursement status of newly approved products.
Both private and government payors use formularies to manage access and utilization of drugs. A drug’s inclusion and favorable positioning on a formulary are essential to ensure patients have access to a particular drug. Even when access is available, some patients abandon their prescriptions for economic reasons. Third-party payors continue to institute cost reduction and containment measures that lower drug utilization and/or spending altogether and/or shift a greater portion of the costs to patients. Such measures include, but are not limited to, more-limited benefit plan designs, higher patient co-pays or coinsurance obligations, limitations on patients’ use of commercial manufacturer co-pay payment assistance programs (including through co-pay accumulator adjustment or maximization programs), stricter utilization management criteria before a patient may get access to a drug, higher-tier formulary placement that increases the level of patient out-of-pocket costs and formulary exclusion, which effectively encourages patients and providers to seek alternative treatments or pay 100% of the cost of a drug. The use of such measures by pharmacy benefit managers (“PBMs”) and insurers has continued to intensify and could limit use and sales of our products.
Over the past few years, many PBMs and insurers have consolidated, resulting in a smaller number of PBMs and insurers overseeing a large portion of total covered lives in the United States. As a result, PBMs and insurers have greater market power and negotiating leverage to mandate stricter utilization criteria and/or exclude drugs from their formularies in favor of competitor drugs or alternative treatments. In highly competitive treatment markets, PBMs are also able to exert negotiating leverage by requiring incremental rebates from manufacturers in order for them to gain and/or maintain their formulary position. Moreover, third-party coverage policies and reimbursement rates are dynamic, meaning that our products could be subject to less favorable coverage policies and/or reimbursement rates over time, making prospective reimbursement and coverage status of our products difficult to predict.
In the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Other countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. For example, some EU Member States may approve a specific price for a product, or they may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other EU Member States allow companies to fix their own prices for products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many EU Member States have increased the amount of discounts that pharmaceutical companies are requirement to offer. These efforts could continue as countries attempt to manage healthcare expenditures. The downward pressure on healthcare costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products onto national markets. Political, economic, and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States, and parallel trade (arbitrage between low-priced and high-priced member states), can further reduce prices.
Healthcare Reform
Like third-party payors, the U.S. federal government, state legislatures and foreign governments have continually implemented cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for generic substitution. For example, the IRA, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in PPACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and through a newly established manufacturer discount program. State laws may permit or require substitution of interchangeable products, too, when approved interchangeable products are available in the future. Adoption of price controls and cost-containment measures and adoption of more restrictive policies in jurisdictions with existing controls and measures could further limit our net revenue and results. Decreases in third-party reimbursement for our products or decisions by certain third-party payors to not cover specific products, or implement coverage restrictions (e.g. prior authorization, step-edit requirements) could reduce provider utilization and have a material adverse effect on sales, results of operations and financial condition.
In the U.S. and some other countries, particularly over the past few years, a number of legislative and regulatory proposals have been introduced in an attempt to lower drug prices and restrict or regulate post-approval activities.
94
Table of Contents
In the U.S., in addition to market actions taken by private and government payors, there has been heightened government, media, and public scrutiny over the manner in which drug manufacturers set prices for their marketed products, resulting in several presidential executive orders, Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on 9 September 2021, the U.S. Department of Health and Human Services (“HHS”) released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA directs the HHS Secretary to establish a Drug Price Negotiation Program to lower prices for certain high-expenditure, single-source prescription drugs and biologics covered under Medicare Part B and Part D that have been approved by the FDA for at least 7 years for prescription drugs and at least 11 years for biologics. Under the Program, the HHS Secretary will publish a list of “selected drugs,” and will then negotiate maximum fair prices with their manufacturers. The Program will be implemented in stages. Beginning in 2026, 10 Medicare Part D “selected drugs” will be subject to price negotiations. By 2029, and in subsequent years thereafter, the number will increase to 20 drugs and biologics covered under Medicare Part B and Part D. Agreements between HHS and manufacturers will remain in place until a drug or biologic is no longer considered a “selected drug” for negotiation purposes. Manufacturers who do not comply with the negotiated prices set under the Program will be subject to an excise tax based on a percentage of total sales of a “selected drug” up to 95% and potential civil monetary penalties. On 29 August 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, including Stelara, the reference product for AVT04, although the Medicare drug price negotiation program is currently subject to legal challenges. Further, beginning in October 2023, the IRA will require manufacturers that increase prices of certain Medicare Part B and Part D drugs or biologics at a rate greater than inflation to pay rebates to the Centers for Medicare & Medicaid Services or be subject to civil monetary penalties. The IRA also provides certain incentives for the development and manufacture of biosimilars. For example, the Secretary can grant a one-year delay from price negotiations for biosimilars that have a “high likelihood” of a competing biosimilar product entering the market within the requested delay period. In addition, certain Part B biosimilars qualify for an increase in Medicare payments, to 8% of the 5-year Average Sales Price, from 6% under current law. The HHS Secretary has been directed to promulgate regulations to implement the Program and other IRA health reform measures. In response to the Biden administration’s October 2022 executive order, on 14 February 2023, HHS released a report outlining three new models for testing by the CMS Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future
In this dynamic environment, we are unable to predict which or how many government policy, legislative, regulatory, executive or administrative changes may ultimately be, or effectively estimate the consequences to our business if, enacted and implemented. However, to the extent that these or other federal government initiatives further decrease or modify the coverage or reimbursement available for our products, require that we pay increased rebates or shift other costs to us, limit or affect our decisions regarding the pricing of or otherwise reduce the use of our products, or limit our ability to offer co-pay payment assistance to commercial patients, such actions could have a material adverse effect on our business and results of operations. Individual states have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
In many countries outside the United States, government-sponsored healthcare systems are the primary payors for drugs. With increasing budgetary constraints and/or difficulty in understanding the value of medicines, governments and payors in many countries are applying a variety of measures to exert downward price pressure. These measures can include mandatory price controls; price referencing; therapeutic-reference pricing; increases in mandates; incentives for generic substitution and biosimilar usage and government-mandated price cuts. In this regard, many countries have health technology assessment agencies that use formal economic metrics such as cost-effectiveness to determine prices, coverage and reimbursement of new therapies; and these agencies are expanding in both established and emerging markets. For example, some EEA countries may require the completion of studies that compare the cost-effectiveness of a particular medicinal product candidate to currently available therapies. This Health Technology Assessment, or HTA, process is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. In December 2021 the EU HTA Regulation was adopted. The purpose of the Regulation is to introduce joint clinical assessments at EU level. When it enters into application in 2025 the Regulation will be intended to harmonize the clinical benefit assessment of HTA across
95
Table of Contents
the EU. Many countries also limit coverage to populations narrower than those specified on our product labels or impose volume caps to limit utilization. We expect that countries will continue taking aggressive actions to seek to reduce expenditures on drugs. Similarly, fiscal constraints may also affect the extent to which countries are willing to approve new and innovative therapies and/or allow access to new technologies.
Brexit
The UK, withdrawal from the EU on 31 January 2020, commonly referred to as Brexit, has created significant uncertainty concerning the future relationship between the UK and the EU. The Medicines and Healthcare products Regulatory Agency ("MHRA"), is now the UK’s standalone regulator. On 24 December 2020, the EU and UK reached an agreement in principle on the framework for their future relationship, the EU-UK Trade and Cooperation Agreement, or Agreement. The Agreement primarily focuses on ensuring free trade between the EU and the UK in relation to goods, including medicinal products. Although the body of the Agreement includes general terms which apply to medicinal products, greater detail on sector-specific issues is provided in an Annex to the Agreement.
Among the changes that will now occur are that Great Britain (England, Scotland and Wales) will be treated as a third country. Northern Ireland will, with regard to EU regulations, continue to follow the EU regulatory rules. As part of the Agreement, the EU and the UK will recognize GMP inspections carried out by the other party and the acceptance of official GMP documents issued by the other party. The Agreement also encourages, although it does not oblige, the parties to consult one another on proposals to introduce significant changes to technical regulations or inspection procedures. Among the areas of absence of mutual recognition are batch testing and batch release. The UK has unilaterally agreed to accept EU batch testing and batch release. However, the EU continues to apply EU laws that require batch testing and batch release to take place in the EU territory. This means that medicinal products that are tested and released in the UK must be retested and re-released when entering the EU market for commercial use.
The UK regulatory framework in relation to clinical trials is derived from existing EU legislation (as implemented into UK law, through secondary legislation). However, it is currently unclear to what extent the UK will seek to align its regulations with the EU following entry into application of the Clinical Trials Regulation on 31 January 2022.
Marketing authorizations in the UK are governed by the Human Medicines Regulations (SI 2012/1916), as amended. Since 1 January 2021, an applicant for the EU centralized procedure marketing authorization can no longer be established in the UK. As a result, since this date, companies established in the UK cannot use the EU centralized procedure and instead must follow one of the UK national authorization procedures or one of the remaining post-Brexit international cooperation procedures to obtain a marketing authorization to market products in the UK. All existing EU marketing authorizations for centrally authorized products were automatically converted or grandfathered into UK marketing authorization, effective in Great Britain only, free of charge on 1 January 2021, unless the marketing authorization holder opted-out of this possibility. Northern Ireland currently remains within the scope of EU authorizations in relation to centrally authorized medicinal products. Accordingly, until the Windsor Framework is implemented in Northern Ireland on 1 January 2025, products falling within the scope of the EU centralized procedure can only be authorized through UK national authorization procedures in Great Britain.
The MHRA has also introduced changes to national marketing authorization procedures. This includes introduction of procedures to prioritize access to new medicines that will benefit patients, including a 150-day assessment route, a rolling review procedure and the International Recognition Procedure. Since 1 January, 2024, the MHRA may rely on the International Recognition Procedure, or IRP, when reviewing certain types of marketing authorization applications. This procedure is available for applicants for marketing authorization who have already received an authorization for the same product from a reference regulator. These include the FDA, the EMA, and national competent authorities of individual EEA countries. A positive opinion from the EMA and CHMP, or a positive end of procedure outcome from the mutual recognition or decentralized procedures are considered to be authorizations for the purposes of the IRP.
There is no pre-marketing authorization orphan designation for medicinal products in the UK. Instead, the MHRA reviews applications for orphan designation in parallel to the corresponding marketing authorization application. The criteria are essentially the same as those in the EU, but have been tailored for the market. This includes the criterion that prevalence of the condition in Great Britain, rather than the EU, must not be more than five in 10,000. Upon the grant of a marketing authorization with orphan status, the medicinal product will benefit from up to 10 years of market exclusivity from similar products in the approved orphan indication. The start of this market exclusivity period will be set from the date of first approval of the product in Great Britain.
96
Table of Contents
Data Privacy and Security
We are subject to stringent and evolving United States and foreign laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security, including the EU’s General Data Protection Regulation (“EU GDPR”) and the United Kingdom’s General Data Protection Regulation (“UK GDPR”). New privacy rules are being enacted in the United States and globally, and existing ones are being expanded, updated and strengthened. For example, the EU GDPR which went into effect in May 2018 introduced strict requirements regarding the processing of personal data, including health-related data.
The collection and use of personal health data in the EEA is governed by the EU GDPR, which became effective on 25 May 2018. The EU GDPR applies to any company established in the EEA and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EEA or the monitoring of the behavior of data subjects in the EEA. The EU GDPR enhances data protection obligations for controllers and processors of personal data, including stringent requirements relating to the consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct privacy impact assessments for high-risk processing, limitations on retention of personal data and mandatory data breach notification and privacy by design requirements, and creates direct obligations on service providers acting as data processors. The EU GDPR also imposes strict rules on the transfer of personal data outside of the EEA to countries that do not ensure an adequate level of protection, such as the U.S. Failure to comply with the requirements of the EU GDPR and the related national data protection laws of the EEA countries may result in fines up to 20 million Euros or 4% of a company’s global annual revenues for the preceding financial year, whichever is higher. Moreover, the EU GDPR grants data subjects the right to claim compensation for damages resulting from infringement of the EU GDPR.
Following the United Kingdom’s withdrawal and the expiration of the transition period, from 31 January 2020, companies doing business in the EU and the UK will be obliged to comply with both the GDPR and the UK GDPR. The UK has implemented legislation similar to the EU GDPR, the UK GDPR, including the UK Data Protection Act, which provides for fines of up to the greater of 17.5 million British Pounds or 4% of a company’s worldwide turnover, whichever is higher. Additionally, the relationship between the UK and the EU in relation to certain aspects of data protection law remains unclear following Brexit, including with respect to regulation of data transfers between EU Member States and the UK. On 28 June 2021, the European Commission announced a decision of “adequacy” concluding that the UK ensures an equivalent level of data protection to the EU GDPR, which provides some relief regarding the legality of continued personal data flows from the EEA to the UK. Some uncertainty remains, however, as this adequacy determination must be renewed after four years and may be modified or revoked in the interim. We cannot fully predict how the Data Protection Act, the UK GDPR, and other UK data protection laws or regulations may develop in the medium to longer term nor the effects of divergent laws and guidance regarding how data transfers to and from the UK will be regulated.
97
Table of Contents
C.Organizational Structure
Corporate Structure
The following diagram illustrates our corporate structure as of 31 December 2023.
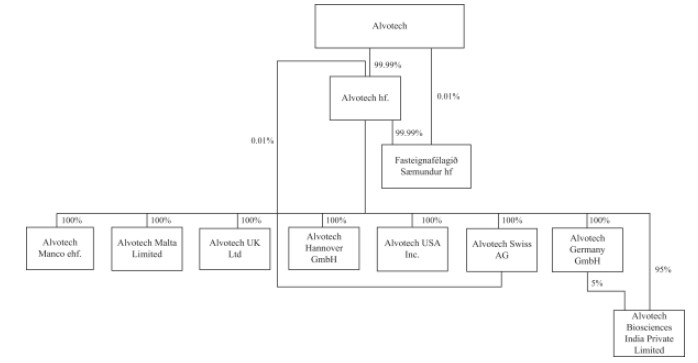
•Alvotech hf., Alvotech Manco ehf., and Fasteignafélagið Sæmundur hf. are incorporated in Iceland;
•Alvotech Malta Limited is incorporated in Malta;
•Alvotech UK Ltd. is incorporated in the United Kingdom;
•Alvotech Hannover GmbH and Alvotech Germany GmbH are incorporated in Germany;
•Alvotech Swiss AG is incorporated in Switzerland;
•Alvotech USA Inc. is incorporated in Virginia, United States; and
•Alvotech Biosciences India Private Limited is incorporated in India.
Alvotech hf. also has a 50% stake in a joint venture, Alvotech & CCHN Biopharmaceutical Limited Liability Company, which is incorporated in China and is not reflected in the above organizational chart. Alvotech is in discussions with CCHN to buy back Alvotech's interests in the joint venture.
D.Property, Plants and Equipment
We believe that our office, research, laboratory and manufacturing facilities, including the ongoing expansion of the Reykjavik facility, are sufficient to meet our current needs. However, as a high-growth company we are constantly evaluating our needs for expanding and or adding to our facilities. We are not aware of, and do not anticipate, environmental issues that may affect our utilization of the facilities described below.
Registered Office in Grand Duchy of Luxembourg
Our registered office is at 9, Rue de Bitbourg, L-1273 Luxembourg, Grand Duchy of Luxembourg, where it has approximately 19 square meters of office space. This location is used for administrative functions only. We are currently leasing this office space. The lease expires in August 2023 but the agreement provides for automatic renewal for one year until termination of the agreement.
98
Table of Contents
Offices and Manufacturing Facility in Iceland
Our corporate headquarters, main manufacturing site and a large part of our R&D division are located in Reykjavik, Iceland. This facility, which we own through a subsidiary, provides us with purpose-built GMP, and has highly integrated capabilities for producing biosimilars at scale. The facility is currently approximately 140,000 square feet and utilizes single-use technology to manufacture drug substance and drug product. It houses our R&D, quality control and quality assurance teams and has an active and valid GMP certificate issued by the Icelandic Medicines Authority authorizing Investigational Medicinal Product and commercial manufacturing. In December 2020, we broke ground on an expansion of our Reykjavik facility that will double the total footprint, adding another 140,000 square feet. The expansion is expected to be completed in 2024 and will give additional redundancy in drug product capacity, assembly of combination products and devices, and secondary packaging. Additionally, the expansion will support increased warehousing and other supportive functions. With the expansion of the Reykjavik facility’s manufacturing capabilities, we expect our capabilities to be able to meet the demand for our products, after obtaining regulatory approval and commercial launch, in the near future. During this expansion, our R&D functions have temporarily moved to another facility in Reykjavik. Permits from the Icelandic EPA (Umhverfisstofnun) and the city of Reykjavik have been granted for the operations in Klettagardar. These facilities have no known additional environmental risks that might impact our operations or utilization of facilities.
Additionally, we have a warehouse of approximately 36,000 square feet in Reykjavik which is used for warehousing, office space and laboratories to sample incoming materials. We are leasing this office space and warehouse until 2038. We also rent office space in Kopavogur, Iceland, for approximately 10,000 square feet, on a lease that expires in 2027. Until the expansion of our Reykjavik facility is completed, we also have short term leases for office space, R&D activities and storage space in Reykjavik, for approximately 57,000 square feet in total, with the leases expiring in 2024.
We hold operational permits from the city of Reykjavik for our facilities in Iceland. The permits address potential environmental impact from our operations. They also address factors that could impact our neighboring communities, such as noise pollution, handling of hazardous substances, air emissions, handling of solid waste and wastewater. We are also required to hold permits from the Icelandic EPA (Umhverfisstofnun) for the use of GMOs in our facilities. We are subject to Icelandic law and regulations, many of whom are set by the Icelandic EPA (Umhverfisstofnun) and the Icelandic Administration of Occupational Safety and Health (Vinnueftirlitið).
Other Offices
We have a facility in Jülich, Germany that focuses on cell line, media, process and analytical development, including tailored clone creation and selection. The Jülich site also serves as a warehouse for supply continuity of master cell banks and working cell banks for our lead product candidates that are produced at contract manufacturing organizations. This facility is approximately 15,000 square feet and is not used for manufacturing. We are holding the space through seven lease agreements, two of which expire in 2024 and provides for automatic renewal until the termination of the agreement, and the other five lease agreements can be terminated at any time with a three-month notice period.
We have a facility in Hannover, Germany that houses our capabilities in analytical glycoprotein characterization. This facility is approximately 14,000 square feet and is not used for manufacturing. We are currently leasing this office space. The lease agreement can be terminated at any time with a 12-month notice period.
Our Virginia, USA office houses our U.S. regulatory, government policy and legal affairs functions. Having recently moved from Arlington, Virginia, to Leesburg, Virginia, the current office is approximately 950 square feet and is not used for manufacturing. We are currently leasing this office space. The lease expires in March 2026.
Our London office houses employees working in London for the Group. Alvotech uses the premises for its employees, strategic meetings and meetings with shareholders and potential investors. The office is approximately 5,500 square feet and the group leases 30% of the premises, containing approximately 1,645 square feet of space. The lease expires in 2028.
Our office in Zurich, Switzerland features our strategic clinical and Medical Affairs R&D center that focuses on late-stage development and regulatory filings. This facility is approximately 3,800 square feet and is not used for manufacturing. We are currently leasing this office space. The lease expires in August 2026.
We have a facility in Bangalore, India that focuses on research and development. This facility is approximately 6,100 square feet and is not used for manufacturing. We are currently leasing this office space. The lease expires in December 2025.
99
Table of Contents
Additionally, we use a small part of a 6,000 square feet office in Malta that for administrative functions. We are currently leasing this office space. The leases expire in August 2025.
Item 4A. Unresolved Staff Comments
Not applicable.
Item 5. Operating and Financial Review and Prospects
You should read the following discussion and analysis of our audited financial condition and results of operations together with our consolidated financial statements appearing elsewhere in this Annual Report on Form 20-F. This Annual Report on Form 20-F contains forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act, including, without limitation, statements regarding our expectations, beliefs, intentions or future strategies that are signified by the words “expect,” “anticipate,” “intend,” “believe,” or similar language. All forward-looking statements included in this Annual Report on Form 20-F are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. In evaluating our business, you should carefully consider the information provided under “Item 3.D. Risk Factors.” Actual results could differ materially from those projected in the forward-looking statements. The terms “Company,” “Alvotech,” “we,” “our” or “us” as used herein refer to Alvotech and its consolidated subsidiaries unless otherwise stated or indicated by context.
All amounts discussed are in U.S. dollars, unless otherwise indicated.
Company Overview
Alvotech is a highly integrated biopharmaceutical company committed to developing and manufacturing high quality biosimilar medicines for patients globally. Our purpose is to improve the health and quality of life of patients around the world by improving access to proven treatments for various diseases. Since our inception, we have built our company with key characteristics we believe will help us capture the substantial global market opportunity in biosimilars: a leadership team that has brought numerous successful biologics and biosimilars to market around the world; a purpose-built biosimilars R&D and manufacturing platform; top commercial partnerships in global markets; and a diverse, expanding pipeline addressing many of the biggest disease areas and health challenges globally. Alvotech is a company committed to constant innovation: we focus our platform, people and partnerships on finding new ways to drive access to more affordable biologic medicines. Alvotech, which was founded in 2013, is led by specialists in biopharmaceutical product creation from around the world that bring extensive combined knowledge and expertise to its mission.
Alvotech currently has two approved biosimilars and an additional nine product candidates in its pipeline for serious diseases with unmet patient and market need. Product candidates in our pipeline address reference products treating autoimmune, eye, and bone disorders, as well as cancer, with combined estimated peak global sales of originator products of more than $130 billion.
•In 2022, Alvotech’s commercial partners launched AVT02 in Canada and Europe and in 2023, in Australia. On 23 February 2024, Alvotech announced the receipt of FDA approval for marketing AVT02 in the U.S. Alvotech expects to launch AVT02 with Teva Pharmaceuticals in the U.S. in the first half of 2024.
•In the fourth quarter of 2023, Alvotech's commercialization partners Fuji Pharma and JAMP Pharma received approval for AVT04, a biosimilar to Stelara (ustekinumab) in Japan and Canada. In January 2024, Alvotech's commercialization partner STADA received approval for AVT04 in the EEA. Alvotech anticipates that the FDA’s review of the BLA for AVT04 will be completed in April 2024.
•Alvotech is in late stage clinical studies for four biosimilar candidates. These are AVT03, a biosimilar candidate to Prolia / Xgeva (denosumab), AVT05, a biosimilar candidate to Simponi and Simponi Aria (golimumab), AVT06, a biosimilar candidate to Eylea (aflibercept), and AVT23, a biosimilar candidate to Xolair (omalizumab).
•Alvotech also has a number of other programs in earlier phases of development that it plans to advance over the coming years. The combined anticipated peak sales for the reference products for these biosimilar candidates in pre-clinical development is over $105 billion. The two most advanced of these, AVT16, a proposed biosimilar to Entyvio (vedolizumab), and AVT33, a proposed biosimilar to Keytruda (pembrolizumab).
100
Table of Contents
Since inception, Alvotech has incurred significant operating losses. Alvotech’s loss for the years ended 31 December 2023, 2022, and 2021 was $551.7 million, $513.6 million, and $101.5 million, respectively. Alvotech’s Adjusted EBITDA was ($291.0) million, ($205.2) million, and ($180.7) million for the years ended 31 December 2023, 2022, and 2021, respectively. Alvotech expects to continue to incur further expenses for the immediate future, as it advances its products through preclinical and clinical development and seeks regulatory approvals, manufactures drug product and drug supply, maintains and expands its intellectual property portfolio, hires additional personnel, and pays for accounting, audit, legal, regulatory and consulting services and costs associated with maintaining compliance with exchange listing rules and the requirements of the SEC, director and officer liability insurance premiums, investor and public relations activities and other expenses associated with operating as a public company. See “Risk Factors We may need to raise substantial additional funding from shareholders or third parties. This additional funding may not be available on acceptable terms or at all. Failure to obtain such necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.”
Factors Affecting Alvotech’s Performance
The pharmaceutical industry is highly competitive and highly regulated. As a result, Alvotech faces a number of industry-specific factors and challenges, which can significantly impact its results. For a more detailed explanation of Alvotech’s business and its risks see “Item 3.D. Risk Factors.”
Competition
The regions in which Alvotech conducts business and the pharmaceutical industry in general is highly competitive. Alvotech faces significant competition from a wide range of companies in a highly regulated industry, including competition from both biosimilar developers and manufacturers as well as competition from branded pharmaceutical developers and manufacturers.
Research and development uncertainty
Research and development within the pharmaceutical industry has a high degree of uncertainty, and likewise there is uncertainty with respect to the probability of success of Alvotech’s biosimilar programs and the timing of the requisite preclinical and clinical steps to achieve regulatory approval of its biosimilar product candidates.
Reliance on commercial partners
Alvotech has partnered with several third parties to commercialize its biosimilar product candidates, once approved by the appropriate regulatory agencies. Alvotech does not currently have the capabilities or the necessary infrastructure to commercialize its products independently.
Impact of Geopolitics and Global Economic Conditions
The Group is subject to additional risks and uncertainties arising from changes to the macroeconomic environment and geopolitical events, including inflation, political instability in particular foreign economies and markets, such as the instability caused by geopolitical conflicts including the war in Ukraine and hostilities in the Middle East, or public health issues or pandemics, such as the COVID-19 pandemic. Global financial markets have experienced volatility and disruption due to macroeconomic and geopolitical events such as rising inflation, the risk of a recession and ongoing conflicts in other countries. In addition, if equity and credit markets deteriorate, including as a result of past and potential future bank failures, it may make any future debt or equity financing more difficult to obtain on favorable terms, and potentially more dilutive to its existing stockholders. The Group cannot predict at this time to what extent it and its collaborators, employees, suppliers, contract manufacturers and/or vendors could potentially be negatively impacted by these events.
The Company believes that inflation will have a general impact on the business in line with overall price increases, increases in the cost of borrowing, and operating in an inflationary economy. We cannot predict the timing, strength, or duration of any inflationary period or economic slowdown or its ultimate impact on the Company. If the conditions in the general economy significantly deviate from present levels and continue to deteriorate it could have a material adverse effect on the Group’s business, financial condition, results of operations and growth prospects.
101
Table of Contents
Components of Operations
Product Revenue
During the year ended 31 December 2023, the Company recognized revenue from product sales resulting from the launch of Alvotech’s AVT02 product, in select European countries, Canada and Australia. The Company expects to continue to recognize product revenue as products are successfully launched into the marketplace.
License and Other Revenue
Alvotech generates a significant portion of its revenue from upfront and milestone payments pursuant to long-term out-license contracts which provide its partners with an exclusive right to market and sell Alvotech’s biosimilar product candidates in a particular territory once such products are approved for commercialization. These contracts typically include commitments to continue development of the underlying compound and to provide supply of the product to the partner upon commercialization.
In the future, revenue may include new out-license contracts and additional milestone payments. Alvotech expects that any revenue it generates will fluctuate from period to period as a result of the timing and amount of license, research and development services, and milestone and other payments.
Operating Expenses
Cost of product revenue
Cost of product revenue includes the cost of inventory sold, labor costs, manufacturing overhead expenses and reserves for expected scrap, as well as shipping and freight costs and royalty costs related to in-license agreements.
Research and development expenses
Research and development expenses consist primarily of costs incurred in connection with Alvotech’s research, development and pre-commercial manufacturing activities prior to commercialization of our products. These costs include:
•personnel expenses, including salaries, benefits and other compensation expenses;
•costs of funding the execution of studies performed both internally and externally;
•costs of purchasing laboratory supplies and non-capital equipment used in designing, developing and manufacturing preclinical study and clinical trial materials;
•expenses related to quality control and other advancement development;
•consultant fees;
•expenses related to regulatory activities, including filing fees paid to regulatory agencies;
•facility costs including rent, depreciation and maintenance expenses;
•fees for maintaining licenses under third party licensing agreements;
•expenses incurred in preparation for commercial launch, such as designing and developing commercial-scale manufacturing capabilities and processes, quality control processes, production asset valuation and other related activities; and
•costs related to amortization, depreciation and impairment losses related to software and property, plant and equipment used in research and development activities.
Expenditures related to research and development activities are generally recognized as an expense in the period in which they are incurred. Due to significant regulatory uncertainties and other uncertainties inherent in the development of pharmaceutical products, Alvotech did not capitalize any research and development expenses as internally developed intangible assets during the years ended 31 December 2023, 2022, and 2021.
Research and development activities will continue to be central to Alvotech’s business model and will vary significantly based upon the success of its programs. Alvotech plans to substantially increase research and development expenses in the near term, as it continues to advance the development of its biosimilar product candidates.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of development, primarily due to the increased size and duration of later-stage clinical trials.
102
Table of Contents
The duration, costs and timing of clinical trials of Alvotech’s products in development and any other product candidates will depend on a variety of factors that include, but are not limited to, the following:
•the number of trials required for approval;
•the per patient trial costs;
•the number of patients who participate in the trials;
•the number of sites included in the trials;
•the countries in which the trials are conducted;
•the length of time required to enroll eligible patients;
•the dose that patients receive;
•the drop-out or discontinuation rates of patients;
•the potential additional safety monitoring or other studies requested by regulatory agencies;
•the duration of patient follow-up;
•the timing and receipt of regulatory approvals; and
•the efficacy and safety profile of the product candidates.
In addition, the probability of success of Alvotech’s products in development and any other product candidate will depend on numerous factors, including competition, manufacturing capability and commercial viability. Alvotech may never succeed in achieving regulatory approval of its product candidates for any indication in any country. As a result of the uncertainties discussed above, the estimated duration and completion costs of any clinical trial that Alvotech conducts is subject to change. Alvotech is also unable to determine with certainty when and to what extent it will generate revenue from the commercialization and sale of products in development or other product candidates, if at all.
General and administrative expenses
General and administrative expenses primarily consist of personnel-related expenses, including salaries, bonuses and other related compensation expenses, and external consulting service costs for corporate and other administrative and operational functions including finance, human resources, information technology and legal, as well as facility-related costs not otherwise included in research and development expenses. These costs relate to the operation of the business and are not related to research and development initiatives. General and administrative costs are expensed as incurred.
Alvotech expects general and administrative expenses to continue to increase as Alvotech increases its headcount and incurs external costs associated with operating as a public company, including expenses related to legal, accounting, tax, consulting services and regulatory matters, maintaining compliance with requirements of exchange listings and of the SEC, director and officer liability insurance premiums and investor relations activities and other expenses associated with operating as a public company. Though expected to increase, Alvotech expects these expenses to decrease as a percentage of revenue in the long-term, as revenue increases.
Share of net loss / profit of joint venture
Alvotech currently holds a 50% ownership interest in the Joint Venture. Alvotech accounts for its ownership interest in the Joint Venture using the equity method of accounting. Under the equity method of accounting, investments in joint ventures are initially recognized at cost and the carrying amount is subsequently adjusted for Alvotech’s share of the profit or loss of the Joint Venture, as well as any distributions received from the Joint Venture. Alvotech’s profit or loss includes its share of the profit or loss of the Joint Venture and, to the extent applicable, other comprehensive income or loss for Alvotech will include its share of other comprehensive income or loss of the Joint Venture. The carrying amount of equity-accounted investments is assessed for impairment and impairment losses will be recognized as impairment loss on investment in joint venture in the statements of profit or loss and other comprehensive income or loss if there is objective evidence of impairment as a result of loss events that have an impact on estimated future cash flows and that can be reliably estimated.
103
Table of Contents
Finance income and finance costs
Finance income consists of changes in the fair value of derivative financial liabilities and interest income. Alvotech recognizes interest income from a financial asset when it is probable that the economic benefits will flow to Alvotech, and the amount of income can be measured reliably.
Finance costs consist of interest expenses related to lease liabilities and borrowings, changes in the fair value of derivative financial liabilities, accretion of Alvotech’s borrowings and amortization of deferred financing fees.
Exchange rate differences
The Group uses the US dollar as its reporting currency and conducts business on a global basis in various currencies. As a result, the Group is exposed to foreign currency exchange movements, primarily in European, Icelandic and UK market currencies, as well as in the Swiss franc.
Gain / Loss on extinguishment of financial liabilities
Alvotech recognizes a gain / loss on extinguishment of financial liabilities in connection with the substantial modification or extinguishment of outstanding financial liabilities. The gain / loss is calculated as the difference between the carrying amount of the liability extinguished and the fair value of the consideration paid.
Income tax benefit
Income tax benefit consists of current tax and deferred tax benefit recorded in the consolidated statement of profit or loss and other comprehensive income or loss.
A.Operating Results
Comparison of the Years Ended 31 December 2023 and 2022
The following table sets forth Alvotech’s results of operations for the years ended 31 December:
| USD in thousands | 2023 | 2022 | |||||||||
| Product revenue | 48,699 | 24,836 | |||||||||
| License and other revenue | 42,735 | 58,193 | |||||||||
| Other income | 1,948 | 1,988 | |||||||||
| Cost of product revenue | (160,856) | (64,095) | |||||||||
| Research and development expenses | (210,827) | (180,622) | |||||||||
| General and administrative expenses | (76,559) | (186,742) | |||||||||
| Operating loss | (354,860) | (346,442) | |||||||||
| Share of net loss of joint venture | (7,153) | (2,590) | |||||||||
| Impairment loss on investment in joint venture | (21,519) | — | |||||||||
| Finance income | 4,823 | 2,549 | |||||||||
| Finance costs | (267,157) | (188,419) | |||||||||
| Exchange rate differences | (5,183) | 10,566 | |||||||||
| (Loss) / gain on extinguishment of financial liabilities | — | (27,311) | |||||||||
| Non-operating (loss) / profit | (296,189) | (205,205) | |||||||||
| Loss before taxes | (651,049) | (551,647) | |||||||||
| Income tax benefit | 99,318 | 38,067 | |||||||||
| Loss for the year | (551,731) | (513,580) | |||||||||
104
Table of Contents
Product revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Product revenue | 48,699 | 24,836 | 23,863 | 96 | |||||||||||||||||||
The Company successfully launched the AVT02 product in Canada and select European countries during the second quarter 2022 and increased sales volume predominantly in Europe during 2023 resulting in $48.7 million of product revenue recognized during the year ended 31 December 2023, almost doubling the product revenue from 2022.
License and other revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| License and other revenue | 42,735 | 58,193 | (15,458) | (26.6) | |||||||||||||||||||
License and other revenue decreased by $15.5 million, or 26.6%, from $58.2 million for the year ended 31 December 2022, to $42.7 million for the year ended 31 December 2023. The decrease in license and other revenue was primarily driven by the recognition of $44.5 million research and development milestone during the same period in the prior year, due to the completion of the AVT04 main clinical program. This was partially offset by the recognition of $31.6 million research and development milestone during 2023 due to the CES completion of the AVT06 program. The remainder of the decrease is mainly due to the net impact of the changes in licensing arrangements during the year ended 31 December 2023.
Other income
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Other income | 1,948 | 1,988 | (40) | (2.0) | |||||||||||||||||||
Other income remained consistent between 31 December 2023, and 31 December 2022. It mainly included in income from a grant received from the Icelandic government to promote research & development activities in Iceland.
Cost of product revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Cost of product revenue | 160,856 | 64,095 | 96,761 | 151 | |||||||||||||||||||
The Company successfully launched AVT02 in select European countries and Canada during the year ended 31 December 2022. As a result, the Company recognized cost of product revenue in the amount of $64.1 million and $160.9 million during the year ended 31 December 2022 and 2023, respectively. Cost of product revenue includes both variable and fixed manufacturing costs associated with commercial manufacturing. Cost of product revenue for the period is disproportionate relative to product revenue due to the timing of new launches and elevated production-related charges,
105
Table of Contents
resulting in higher costs than revenues recognized for the period. Prior to the recognition of cost of product revenues, costs from pre-commercial manufacturing activities were reported as R&D expenses.
Research and development expenses
| Year Ended 31 December | 2022 to 2023 | ||||||||||||||||||||||
| USD in thousands | 2023 | 2022 | $ | % | |||||||||||||||||||
| AVT02 development program expenses | 4,799 | 9,986 | (5,187) | (51.9) | |||||||||||||||||||
| AVT03 development program expenses | 30,714 | 15,667 | 15,047 | 96.0 | |||||||||||||||||||
| AVT04 development program expenses | 7,259 | 23,879 | (16,620) | (69.6) | |||||||||||||||||||
| AVT05 development program expenses | 41,460 | 28,034 | 13,426 | 47.9 | |||||||||||||||||||
| AVT06 development program expenses | 33,109 | 19,044 | 14,065 | 73.9 | |||||||||||||||||||
| Salary and other employee expenses | 41,844 | 52,962 | (11,118) | (21.0) | |||||||||||||||||||
| Depreciation, amortization and impairment | 6,888 | 6,740 | 148 | 2.2 | |||||||||||||||||||
Other research and development expenses (1) | 44,754 | 24,310 | 20,444 | 84.1 | |||||||||||||||||||
| Total research and development expenses | 210,827 | 180,622 | 30,205 | 16.7 | |||||||||||||||||||
(1)Other research and development expenses include other project costs, facility costs and other operating expenses recognized as research and development expenses during the period.
Research and development expenses increased by $30.2 million, or 16.7%, from $180.6 million for the year ended 31 December 2022, to $210.8 million for the year ended 31 December 2023. The increase was primarily driven by a charge of $18.5 million relating to the termination of the co-development agreement with Biosana for AVT23, and a $42.5 million increase in direct program expenses mainly from three biosimilar candidates, AVT03, AVT05 and AVT06, that entered clinical development in 2022. These increases were partially offset by a decrease of $21.8 million primarily related to programs which have completed clinical phase (i.e., AVT02 and AVT04 programs). In addition, upon the launch of AVT02 during the second quarter of 2022, the Company commenced recognizing pre-commercial manufacturing activities as cost of product revenue. As a result, research and development expenses during the year ended 31 December 2022 included $8.8 million of costs relating to AVT02 which have since been recognized as cost of product revenue.
General and administrative expenses
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| General and administrative expense | 76,559 | 186,742 | (110,183) | (59.0) | |||||||||||||||||||
General and administrative expenses decreased by $110.2 million, or 59.0%, from $186.7 million for the year ended 31 December 2022, to $76.6 million for the year ended 31 December 2023. The decrease in general and administrative expenses was primarily attributable to a $83.4 million non-cash share listing expense and $22.9 million of transaction costs as a result of the Business Combination recognized as of 31 December 2022 (see Note 1.1 of the consolidated financial statements included elsewhere in this 20-F for additional information). The Company also incurred $13.4 million of IP-related legal expenses during the year ended 31 December 2022, compared to $2.3 million during the year ended 31 December 2023. This decrease was partially offset by a $3.7 million net increase in other general administrative expenses due to costs from operating as a public company in both the U.S. and Iceland. Lastly, the Company recognized $10.8 million of general and administrative expenses for share-based payments, resulting from the granting of RSUs during the year ended 31 December 2023, against $6.5 million during the year ended 31 December 2022.
106
Table of Contents
Share of net loss of joint venture and impairment loss on investment in joint venture
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Share of net loss of joint venture | 7,153 | 2,590 | 4,563 | 176.2 | |||||||||||||||||||
| Impairment loss on investment in joint venture | (21,519) | — | (21,519) | nm | |||||||||||||||||||
nm = not meaningful, refer to explanation below
Share of net loss of joint venture increased by $4.6 million, or 176.2%, from $2.6 million for the year ended 31 December 2022, to $7.2 million for the year ended 31 December 2023. The increase in losses incurred by the Joint Venture was mainly due to higher depreciation and amortization expense for the year ended 31 December 2023. In 2023, the Group impaired their share of the joint venture, the impairment amounted to $21.5 million, based on discussions between Alvotech and CCHN to buy back Alvotech's interests in the joint venture. The Group estimates the recoverable amount using value in use where the recoverable amount is estimated as the future cash flows expected to arise from dividends to be received from the investment and from its ultimate disposal.
Finance income
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Finance income | 4,823 | 2,549 | 2,274 | 89.2 | |||||||||||||||||||
Finance income increased by 2.3 million, or 89.2%, from $2.5 million for the year ended 31 December 2022, to $4.8 million for the year ended 31 December 2023. The increase in finance income is primarily driven by interest received on cash and cash equivalent held in our bank accounts.
Finance costs
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Finance costs | 267,157 | 188,419 | 78,738 | 41.8 | |||||||||||||||||||
Finance costs increased by $78.7 million, or 41.8%, from $188.4 million for the year ended 31 December 2022, to $267.2 million for the year ended 31 December 2023.The increase in finance costs is primarily related to a $49.2 million increase in interest on debt and borrowings due to the additional financing obtained since 31 December 2022, including the annualized impact of prior year financing, and a $35.4 million increase in fair value of derivative liabilities mainly driven by the OACB Warrants, the Predecessors and OACB Earn Out shares and the Tranche A Conversion Feature, including the additional Tranche A Convertible Bonds secured in 2023. This is partially offset by $16.0 million in charges related to the closing of the Business Combination in 2022.
Exchange rate differences
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Exchange rate differences | (5,183) | 10,566 | (15,749) | (149.1) | |||||||||||||||||||
107
Table of Contents
Exchange rate differences decreased by $15.7 million from a gain of $10.6 million for the year ended 31 December 2022, to a loss of $5.2 million for the year ended 31 December 2023. The decrease was primarily driven by the movements in the exchange rate of foreign currencies, predominantly Icelandic krona and euros.
(Loss) / Gain on extinguishment of financial liabilities
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| (Loss) / Gain on extinguishment of financial liabilities | — | (27,311) | nm | nm | |||||||||||||||||||
nm = not meaningful, refer to explanation below
Alvotech recognized a loss on extinguishment of financial liabilities of $27.3 million during the year ended December 31, 2022, primarily as a result of the following transactions:
•$40.9 million loss resulting from the amendment and upsizing of the Senior Bonds.
•$3.9 million loss resulting from the extinguishment of the lease on the Alvotech facility resulting from the Share Purchase Agreement for the Saemundur manufacturing facility.
•$17.8 million gain resulting from the settlement of related party loans with Aztiq and Alvogen, in which the parties agreed to settle outstanding loan amounts through the issuance of Ordinary Shares.
Income tax benefit
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2022 to 2023 | |||||||||||||||||||||
| 2023 | 2022 | $ | % | ||||||||||||||||||||
| Income tax benefit | 99,318 | 38,067 | 61,251 | 160.9 | |||||||||||||||||||
The income tax benefit increased by $61.3 million for the year ended 31 December 2023, compared to the same period for 2022. This increase was mainly driven by $34.7 million deferred tax credit corresponding to an increase in operating losses and a favorable $26.7 million foreign currency translation of the deferred tax asset recognized on Icelandic tax loss carry-forwards denominated in Icelandic Krona, that the Company expects to fully utilize against future taxable profits.
108
Table of Contents
Comparison of the Years Ended December 31, 2022, and 2021
The following table sets forth Alvotech’s results of operations for the years ended December 31:
| USD in thousands | 2022 | 2021 | |||||||||
| Product revenue | 24,836 | — | |||||||||
| License and other revenue | 58,193 | 36,772 | |||||||||
| Other income | 1,988 | 2,912 | |||||||||
| Cost of product revenue | (64,095) | — | |||||||||
| Research and development expenses | (180,622) | (191,006) | |||||||||
| General and administrative expenses | (186,742) | (84,134) | |||||||||
| Operating loss | (346,442) | (235,456) | |||||||||
| Share of net loss of joint venture | (2,590) | (2,418) | |||||||||
| Finance income | 2,549 | 51,568 | |||||||||
| Finance costs | (188,419) | (117,361) | |||||||||
| Exchange rate differences | 10,566 | 2,681 | |||||||||
| (Loss) / Gain on extinguishment of financial liabilities | (27,311) | 151,788 | |||||||||
| Non–operating (loss) profit | (205,205) | 86,258 | |||||||||
| Loss before taxes | (551,647) | (149,198) | |||||||||
| Income tax benefit | 38,067 | 47,694 | |||||||||
| Loss for the year | (513,580) | (101,504) | |||||||||
Product revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Product revenue | 24,836 | — | 24,836 | nm | |||||||||||||||||||
nm = not meaningful, refer to explanation below
The Company successfully launched the AVT02 product in Canada and select European countries resulting in $24.8 million of product revenue recognized during the year ended 31 December 2022.
License and other revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| License and other revenue | 58,193 | 36,772 | 21,421 | 58.2 | % | ||||||||||||||||||
License and other revenue increased by $21.4 million, or 58.2%, from $36.8 million for the year ended 31 December 2021, to $58.2 million for the year ended 31 December 2022. The company recognized revenue of $44.5 million and $11.6 million resulting from license and milestone payments for AVT04 and AVT05, respectively, for the year ended 31 December 2022. During the year ended 31 December 2021, the Company recognized $20.8 million, $8.6 million, and $7.2 million from license and milestone payments for AVT06, AVT02, and AVT03, respectively.
109
Table of Contents
Other income
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Other income | 1,988 | 2,912 | (924) | 31.7 | % | ||||||||||||||||||
Other income decreased by $0.9 million, or 31.7%, from $2.9 million for the year ended 31 December 2021, to $2.0 million for the year ended 31 December 2022. The decrease in other income was driven by a decrease in income generated from services performed pursuant to Alvotech’s support service arrangements with Alvogen, a related party, during the year ended 31 December 2022, as compared to the year ended 31 December 2021.
Cost of product revenue
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Cost of product revenue | 64,095 | — | 64,095 | nm | |||||||||||||||||||
nm = not meaningful, refer to explanation below
The Company successfully launched AVT02 in select European countries and Canada during the year ended 31 December 2022. As a result, the Company commenced recognizing cost of product revenue in the same period. Cost of product revenue for the year ended 31 December 2022, was $64.1 million, which includes both variable and fixed manufacturing costs associated with commercial manufacturing. Cost of product revenue is disproportionate relative to product revenue due to the timing of new launches, resulting in higher costs than revenues recognized for the period. The Company expects this to normalize as it increases in scale and expands on new product launches. Ultimately, the increase in volumes will result in the absorption of fixed manufacturing costs. Prior to the recognition of cost of product revenues, these costs were reported as research and development expenses as pre-commercial manufacturing activities.
Research and development expenses
Change | |||||||||||||||||||||||
| Year Ended 31 December | 2021 to 2022 | ||||||||||||||||||||||
| USD in thousands | 2022 | 2021 | $ | % | |||||||||||||||||||
| AVT02 development program expenses | 9,986 | 26,610 | (16,624) | 62.5 | |||||||||||||||||||
| AVT03 development program expenses | 15,667 | 6,631 | 9,036 | 136.3 | |||||||||||||||||||
| AVT04 development program expenses | 23,879 | 35,770 | (11,891) | 33.2 | |||||||||||||||||||
| AVT05 development program expenses | 28,034 | 2,822 | 25,212 | nm | |||||||||||||||||||
| AVT06 development program expenses | 19,044 | 11,508 | 7,536 | 65.5 | |||||||||||||||||||
| Salary and other employee expenses | 52,962 | 71,588 | (18,626) | 26.0 | |||||||||||||||||||
| Depreciation, amortization and impairment | 6,740 | 21,764 | (15,024) | 69.0 | |||||||||||||||||||
Other research and development expenses (1) | 24,310 | 13,766 | 10,544 | 76.6 | |||||||||||||||||||
| Total research and development expenses | 180,622 | 191,006 | (10,384) | 5.4 | % | ||||||||||||||||||
nm = not meaningful, refer to explanation below
(1)Other research and development expenses include other project costs, facility costs and other operating expenses recognized as research and development expenses during the period.
Research and development expenses decreased by $10.4 million, or 5.4%, from $191.0 million for the year ended 31 December 2021, to $180.6 million for the year ended 31 December 2022. During the year ended 31 December 2022, the following resulted in an overall decrease to total research and development expenses:
•AVT02 development program expenses decreased by $16.6 million, or 62.5%, as a result of decreased R&D activities. The Company obtained marketing authorization for AVT02 in the EEA, the UK, Switzerland,
110
Table of Contents
Canada, Australia and Saudi Arabia, resulting in the conclusion of pre-launch R&D studies and the recognition of cost of product revenue. As a result, the AVT02 development program expenses decreased during the year ended 31 December 2022. The Company expects these expenses to continue to decrease as the Company seeks to obtain marketing authorization in other jurisdictions, including the US.
•AVT04 development program expenses decreased by $11.9 million, or 33.2%. During the year ended 31 December 2022, the Company completed significant R&D activities related to AVT04. Subsequent to 31 December 2022, in January 2023, Alvotech announced that the FDA had accepted for review a BLA for AVT04 and in November that the FDA had accepted for review its resubmitted BLA for AVT04 with an expected goal date in April 2024. In February 2023, Alvotech announced that the EMA had accepted a Marketing Authorization Application for AVT04, which was approved in February 2024. As a result, the company recognized less R&D expense related to AVT04 as R&D studies entered late stages.
•Salary and other employee expenses decreased by $18.6 million, or 26.0%. This decrease is a result of costs being classified as manufacturing costs subsequent to the Company obtaining marketing authorization for AVT02. Previously, these costs were reported as pre-commercial manufacturing activities within research and development.
•Depreciation, amortization and impairment expenses decreased by $15.0 million, or 69.0%. This decrease is a result of costs being classified as manufacturing costs subsequent to the Company obtaining marketing authorization for AVT02. Previously, these costs were reported as pre-commercial manufacturing activities within research and development.
•The increases in development program expenses of $9.0 million, $25.2 million, and $7.5 million for AVT03, AVT05, and AVT06, respectively, are a result of these biosimilar candidates initiating the clinical phase of development. The Company expects to continue to incur R&D expense as they seek commercialization of these biosimilar candidates.
•Other research and development expenses increased by $10.5 million, or 76.6%. The increase is due to an increase in costs of $4.3 million and $4.2 million for AVT23 and AVT16, respectively.
General and administrative expenses
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| General and administrative expense | 186,742 | 84,134 | 102,608 | 122.0 | |||||||||||||||||||
General and administrative expenses increased by $102.6 million, or 122.0%, from $84.1 million for the year ended 31 December 2021, to $186.7 million for the year ended 31 December 2022. The increase in general and administrative expenses was primarily attributable to the $83.4 million non-cash share listing expense and $10.4 million of additional transaction costs recognized as a result of the Business Combination. See Note 1.1 of the consolidated financial statements included elsewhere in this Form 20-F. The Company also recognized $5.8 million of general and administrative expenses for share-based payments, resulting from the granting of RSUs during the year ended 31 December 2022. Lastly, the company recognized $3.3 million in salary expense related to severance agreements, associated with a management reorganization, and had an increase of $13.6 million on other general administrative expenses related to IT and other third-party services. These increases were offset by $17.4 million less of long-term incentive plan expense recognized during the year ended 31 December 2022.
Share of net loss of joint venture
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Share of net loss of joint venture | 2,590 | 2,418 | 172 | 7.1 | |||||||||||||||||||
Share of net loss of joint venture increased by $0.2 million, or 7.1%, from $2.4 million for the year ended 31 December 2021, to $2.6 million for the year ended 31 December 2022. The increase in the share of net loss of joint venture was due to an increase in losses incurred by the Joint Venture during the year ended 31 December 2022, as compared to 31
111
Table of Contents
December 2021. The increase in losses incurred by the Joint Venture was due to lower interest income combined with higher depreciation and amortization expense for the year ended 31 December 2022.
Finance income
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Finance income | 2,549 | 51,568 | (49.0) | 95.1 | |||||||||||||||||||
Finance income decreased by $49.0 million, or 95.1%, from $51.6 million for the year ended 31 December 2021, to $2.6 million for the year ended 31 December 2022. The decrease in finance income was primarily attributable to $48.7 million in income resulting from a favorable fair value remeasurement of derivative financial liabilities associated with the convertible shareholder loans during the year ended 31 December 2021. In connection with the Business Combination Agreement, on 7 December 2021, the Group’s shareholders entered into the BCA Framework Agreement resulting in the exercise of the conversion, warrant, and funding rights associated with the convertible shareholder loans.
Finance costs
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Finance costs | 188,419 | 117,361 | 71,058 | 60.5 | |||||||||||||||||||
Finance costs increased by $71.1 million, or 60.5%, from $117.4 million for the year ended 31 December 2021, to $188.4 million for the year ended 31 December 2022. The increase in finance costs is primarily related to a $94.2 million increase in finance costs resulting from the change in fair value of derivative liabilities. For the year ended 31 December 2022, the Company recognized finance costs for the following derivatives:
•$48.7 million in finance costs resulting from the increase in fair value of the Predecessor Earn Out Shares;
•$29.9 million in finance costs resulting from the increase in fair value of the Senior Bond Warrants;
•$13.2 million in finance costs resulting from the increase in fair value of the Tranche A Conversion Feature;
•$3.7 million in finance costs resulting from the decrease in fair value of the derivative asset relates to the Senior bond interest feature; and
•$1.4 million in finance costs resulting from the increase in fair value of the OACB Earn Out Shares.
Additionally, the company recognized $13.9 million in finance costs related to the consenting fee and remeasurement of the bonds as result of the terms being amended in association with the closing of the Business Combination with OACB. These increases in finance costs were offset by $35.0 million less of finance costs related to the interest on debt and borrowings. The company incurred less interest costs on borrowings due to the extinguishment of the convertible shareholder loans on 7 December 2021, resulting in less finance costs for the year ended 31 December 2022. During the year ended 31 December 2021, the Company recognized $30.7 million of finance costs related to interest on the convertible shareholders loans.
Exchange rate differences
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Exchange rate differences | 10,566 | 2,681 | 7,885 | 294.1 | |||||||||||||||||||
Exchange rate differences increased by $7.9 million, or 294.1%, from $2.7 million for the year ended 31 December 2021, to $10.6 million for the year ended 31 December 2022. The increase was primarily driven by a change in financial
112
Table of Contents
assets and liabilities denominated in Icelandic Krona and Euros, along with the weakening of the Icelandic Krona compared to the US dollar, during the year ended 31 December 2022.
(Loss) / Gain on extinguishment of financial liabilities
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| (Loss) / Gain on extinguishment of financial liabilities | (27,311) | 151,788 | nm | nm | |||||||||||||||||||
nm = not meaningful, refer to explanation below
Alvotech recognized a loss on extinguishment of financial liabilities of $27.3 million during the year ended 31 December 2022, primarily as a result of the following transactions:
•$40.9 million loss resulting from the amendment and upsizing of the Senior Bonds;
•$3.9 million loss resulting from the extinguishment of the lease on the Alvotech facility resulting from the Share Purchase Agreement for the Saemundur manufacturing facility; and
•$17.8 million gain resulting from the settlement of related party loans with Aztiq and Alvogen, in which the parties agreed to settle outstanding loan amounts through the issuance of Ordinary Shares.
Alvotech recognized a gain on extinguishment of financial liabilities of $151.8 million during the year ended 31 December 2021, in connection with the substantial modification to the terms and conditions of the convertible bonds, as well as the exercise of the conversion, warrant and funding rights associated with the convertible shareholder loans.
Income tax benefit
| Change | |||||||||||||||||||||||
| USD in thousands | Year Ended 31 December | 2021 to 2022 | |||||||||||||||||||||
| 2022 | 2021 | $ | % | ||||||||||||||||||||
| Income tax benefit | 38,067 | 47,694 | (9,627) | 20.2 | |||||||||||||||||||
Income taxes for the year ended 31 December 2022, resulted in an income tax benefit of $38.1 million compared to an income tax benefit of $47.7 million for the year ended 31 December 2021. This decrease in income tax benefit was mainly driven by a $10.1 million foreign currency impact due to the continued weakening of the Icelandic Krona against the US dollar, decreasing the US dollar value of tax loss carry-forwards that Alvotech expects to fully utilize against future taxable profits.
Reconciliation of non-IFRS financial measure
In addition to its operating results, as calculated in accordance with IFRS, Alvotech uses Adjusted EBITDA when monitoring and evaluating operational performance. Adjusted EBITDA is defined as profit or loss for the relevant period, as adjusted for certain items that Alvotech management believes are not indicative of ongoing operating performance. The adjusting items consist of the following:
1.Income tax benefit;
2.Total net finance costs;
3.Depreciation and amortization of property, plant, and equipment, right-of-use assets and intangible assets;
4.Impairment and loss on sale of property, plant, and equipment and other intangible assets;
5.Charge related to contract termination;
6.Long-term incentive plan expense;
7.Share of net loss of joint venture;
8.Impairment loss on investment in joint venture;
113
Table of Contents
9.Exchange rate differences;
10.Share listing expense;
11.Loss (Gain) on extinguishment of financial liabilities; and
12.Transaction costs.
Alvotech believes that this non-IFRS measure assists its shareholders because it enhances the comparability of results each period, helps to identify trends in operating results and provides additional insight and transparency on how management evaluates the business. Alvotech’s executive management team uses this non-IFRS measure to evaluate financial measures to budget, update forecasts, make operating and strategic decisions, and evaluate performance. This non-IFRS financial measure is not meant to be considered alone or as a substitute for IFRS financial measures and should be read in conjunction with Alvotech’s consolidated financial statements prepared in accordance with IFRS. Additionally, this non-IFRS measure may not be comparable to similarly titled measures used by other companies. The most directly comparable IFRS measure to this non-IFRS measure is loss for the year.
The following table reconciles loss for the year to Adjusted EBITDA for the years ended 31 December 2023, 2022, and 2021, respectively:
| USD in thousands | 2023 | 2022 | 2021 | ||||||||||||||
| Loss for the year | (551,731) | (513,580) | (101,504) | ||||||||||||||
| Income tax benefit | (99,318) | (38,067) | (47,694) | ||||||||||||||
| Total net finance costs | 262,334 | 185,870 | 65,793 | ||||||||||||||
| Depreciation and amortization | 24,210 | 20,409 | 18,196 | ||||||||||||||
| Impairment and loss on sale of property, plant and equipment | 365 | — | 2,092 | ||||||||||||||
| Impairment of intangible assets | 1,779 | 2,755 | 3,993 | ||||||||||||||
Charge related to contract termination(5) | 18,500 | — | — | ||||||||||||||
Incentive plan expense(1) | 18,111 | 10,994 | 17,955 | ||||||||||||||
| Share of net loss of joint venture | 7,153 | 2,590 | 2,418 | ||||||||||||||
Impairment loss on investment in joint venture(2) | 21,519 | — | — | ||||||||||||||
| Exchange rate differences | 5,183 | (10,566) | (2,681) | ||||||||||||||
Share listing expense(3) | — | 83,411 | — | ||||||||||||||
| Loss (Gain) on extinguishment of financial liabilities | — | 27,311 | (151,788) | ||||||||||||||
Transactions costs(4) | 918 | 23,695 | 12,503 | ||||||||||||||
| Adjusted EBITDA | (290,977) | (205,178) | (180,717) | ||||||||||||||
(1)Represents expense related to employee incentive plans, reported within cost of product revenue, research and development expenses and general and administrative expenses.
(2)Represents impairment loss on investment in joint venture due to uncertainties around the economic conditions in China. The Group estimated the recoverable amount using value in use where the recoverable amount is estimated as the future cash flows expected to arise from dividends to be received from the investment and from its ultimate disposal.
(3)Represents the share listing expense reported within general and administrative expenses, which was recorded in accordance with IFRS 2 as the excess of the fair value of Alvotech shares issued at the Closing Date over the fair value of OACB’s identifiable net assets acquired.
(4)Represents transaction costs incurred in 2021 and 2022 in connection with the Business Combination and the Icelandic Main Board listing, and, in 2023, with any remaining services reported within general and administrative expenses.
(5)Represents a charge in relation to the termination of the co-development agreement with Biosana for AVT23.
114
Table of Contents
B.Liquidity and Capital Resources
As of 31 December 2023 and 2022, Alvotech had cash and cash equivalents, excluding restricted cash, of $11.2 million and $66.4 million, respectively. Since its inception, Alvotech has incurred operating losses, including net losses of $551.7 million and $513.6 million for the years ended 31 December 2023 and 2022, respectively, and had an accumulated deficit of $2,205.8 million and $1,654.1 million as of 31 December 2023 and 2022, respectively. The Company has financed its activities through successive capital increases, borrowings, and upfront and milestone payments under agreements with its commercial partners. During the year ended 31 December 2023, the Company used $312.2 million cash in operating activities and $46.3 million cash in investing activities, and its financing activities provided $301.3 million cash.
Sources of Liquidity
Alvotech began to generate revenue from product sales in the second quarter of 2022 in conjunction with the commercialization of AVT02 in Canada and select European countries. AVT02 has received regulatory approval in over 50 markets and has been launched in over 20 markets globally to date. Alvotech expects to launch AVT02 in the United States during the first half of 2024. The Company also has a second biosimilar, AVT04, which has been approved in Japan, Canada and the EEA and was launched in Canada in March 2024. Launches of AVT04 in Japan and Europe are expected in Q2 2024 and Q3 2024, respectively. Alvotech anticipates that the FDA’s review of its BLA for AVT04 will be completed by 16 April 2024.
In February and March 2022, Alvotech received $25.0 million from each of Alvogen and Aztiq pursuant to interest free loan advances provided by both significant shareholders, who agreed to settle these outstanding amounts in Ordinary Shares rather than cash in July 2022. The closing of the Business Combination and the PIPE Financing provided the Company with $131.9 million of net proceeds that was used to finance the continuing development and commercialization of its biosimilar product candidates. Additionally, during the year ended 31 December 2022, the Company received $110.0 million in cash proceeds from the loans issued by Alvogen (including the Alvogen Facility), successfully amended and upsized the outstanding Senior Bonds resulting in $57.9 million of net cash proceeds, along with net cash proceeds of $73.4 million from the issuance of the Tranche A and Tranche B 2022 Convertible Bonds and Facility Loans, of which $50.0 million was used to repay amounts drawn under the Alvogen Facility.
In January 2023, the Company issued an additional $10.0 million of Tranche B under the 2022 Convertible Bonds agreement. Holders of the Tranche B Convertible Bonds may elect, at their sole discretion, to convert all or part of the principal amount and accrued interest into Alvotech Ordinary Shares at a conversion price of $10.00 per share on 31 December 2023, 30 June 2024, or upon optional or mandatory redemption of the bonds.
In February 2023, Alvotech completed a private placement for gross proceeds of $137.0 million, and transaction costs of $4.1 million, of its Ordinary Shares at a purchase price of $11.57 per Ordinary Share.
On 6 February 2023, Alvotech announced that it had entered into an exclusive agreement with Advanz Pharma, for the commercialization of AVT23 in the European Economic Area, UK, Switzerland, Canada, Australia and New Zealand. On 24 May 2023, Alvotech announced that the two companies had extended their exclusive partnership agreement, adding the supply and commercialization of five biosimilar candidates in Europe. Alvotech will be responsible for development and commercial supply and Advanz Pharma will be responsible for registration and commercialization in Europe. The agreement includes candidate biosimilars to Simponi (golimumab) and Entyvio (vedolizumab) and also includes three additional early-stage, undisclosed biosimilar candidates. Under the terms of the current agreements with Advanz Pharma, Alvotech received upfront payments of $75.3 million and development milestone payments of $15.1 million up to 31 December 2023. Additionally, Alvotech is eligible to receive aggregate payments of up to an additional $351.0 million upon the achievement of certain, regulatory, commercial, manufacturing and sales milestones.
In July 2023, in conjunction with the Company's expansion of its existing partnership agreement with Teva, Teva also acquired Tranche B Convertible Bonds pursuant to the 2022 Convertible Bonds agreement, in principal amount of $40 million. The expanded agreement pertained to the exclusive commercialization in the U.S. by Teva of two new biosimilar candidates and line extensions of two current biosimilar candidates in the partnership. Additionally, Alvotech received upfront payments of $40.0 million and $37.5 million in development milestone payments up to 31 December 2023 and is eligible to receive aggregate payments of up to an additional $552.5 million upon the achievement of certain regulatory, commercial, manufacturing and sales milestones under the entire partnership with Teva.
Also in July 2023, the Company secured a private placement of the December 2022 Convertible Bonds denominated in Icelandic krona and US dollar for a principal amount of $100 million. As part of this private placement, ATP Holdings ehf., an affiliated of Aztiq, acquired Tranche A Convertible Bonds in principal amount of $30 million.
115
Table of Contents
In February 2024, the Company announced the sale of 10,127,132 Ordinary Shares for an approximate value of $166 million, par value USD 0.01 per share, at a purchase price of $16.41 per Share, or ISK 2,250 per share at foreign exchange rates on February 23, 2024.
For the foreseeable future, Alvotech’s Board of Directors will maintain a capital structure that supports Alvotech’s strategic objectives through managing the budgeting process, maintaining strong investor relations and managing financial risks. Consequently, management and the Board of Directors believe that Alvotech will have sufficient funds, and access to sufficient funds, to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities and commitments in the normal course of business. However, although management continues to pursue these plans, there is no assurance that Alvotech will be successful in obtaining sufficient funding on terms acceptable to Alvotech management to fund continuing operations, if at all. Alvotech’s future capital requirements will depend on many factors, including the following:
•the progress, results, and costs of preclinical studies for any programs that Alvotech may develop;
•the costs, timing, and outcome of regulatory review of program candidates;
•Alvotech’s ability to establish and maintain collaborations, licensing, and other agreements with commercial partners on favorable terms, if at all;
•the achievement of milestones or occurrence of other developments that trigger payments under the agreements that Alvotech has entered into or may enter into with third parties or related parties;
•the extent to which Alvotech is obligated to reimburse clinical trial costs under collaboration agreements, if any;
•the costs of preparing, filing and prosecuting patent applications and maintaining, defending and enforcing Alvotech’s intellectual property rights;
•the extent to which Alvotech acquires or invests in businesses, products, technologies, or other joint ventures;
•the costs of performing commercial-scale manufacturing in-house and, if needed, securing manufacturing arrangements for commercial production of its program candidates; and
•the costs of establishing or contracting for sales and marketing capabilities if Alvotech obtains regulatory approvals to market program candidates.
Cash Flows
Comparison of the Years Ended 31 December 2023 and 2022
| Change | |||||||||||||||||||||||
| Year Ended 31 December | 2022 to 2023 | ||||||||||||||||||||||
| USD in thousands | 2023 | 2022 | $ | % | |||||||||||||||||||
| Cash used in operating activities | ($312,185) | ($312,389) | 204 | (0.1) | |||||||||||||||||||
| Cash used in investing activities | (46,340) | (63,537) | 17,197 | (27.1) | |||||||||||||||||||
| Cash generated from financing activities | 301,319 | 424,910 | (123,591) | (29.1) | |||||||||||||||||||
Operating activities
Net cash used in operating activities decreased by $0.2 million, or 0.1%, from $312.4 million for the year ended 31 December 2022, to $312.2 million for the year ended 31 December 2023. This was primarily driven by a $54.7 million increase in operating cash outflows before considering movements in working capital, $74.2 million increase in cash flows from movements in working capital, and $21.9 million in additional interest paid during 2023 compared to 2022.
The $54.7 million increase in operating cash flow before movements in working capital is mostly due to an increased loss of $38.2 million, $18.5 million allowance for receivables, $21.5 million impairment loss on investment in joint venture and $78.7 million increase in finance costs. This was offset by $61.3 million change in income tax benefit and $83.4 million change in share-listing expense in non-cash expenses. The $74.2 million increase in cash flows from movements in working capital is du to a $21.1 million decrease in cash outflow to inventories, $16.4 million increase in cash inflow from other assets, $31.5 million increase in cash inflow from trade and other payables and other liabilities and a $16.0 million increase in contract liabilities partially offset by a $8.2 million increase in contract assets. Other major change in net cash used in the operating cash flow are due to an increase in paid interests of $21.9 million.
116
Table of Contents
Investing activities
Net cash used in investing activities decreased by $17.2 million, or 27.1%, from $63.5 million for the year ended 31 December 2022, to $46.3 million for the year ended 31 December 2023. The decrease in investing activities was driven by a $4.6 million decrease in cash outflow for the acquisition of property, plant and equipment. Additionally, the Group had a $14.9 million cash outflow in 2022 resulting from the amended bond agreement, whereby Alvotech is required to maintain a minimum of $25.0 million of restricted cash in a separate liquidity account per the terms of their debt agreements. These decreases were partially offset by a $2.1 million increase in cash outflows related to the acquisition of intangible assets as the Group acquired intellectual property rights of AVT23 from Kashiv and continued investing in its SAP project during the year ended December 31, 2023.
Financing activities
Net cash generated from financing activities decreased by $123.6 million, or 29.1%, from $424.9 million for the year ended 31 December 2022, to $301.3 million for the year ended 31 December 2023. In 2023 the Company received $269.8 million in proceeds from new borrowings, thereof $26.9 million from related parties. Additionally, the Group received net $132.7 million from the private placement closed during Q1 2023 and $6.4 million in proceeds from warrants. Repayments of borrowings amounted to $99.4 million and repayments of leases amount to $8.3 million compared to $34.7 million and $11.1 million repayments in 2022, respectively.
Material Cash Requirements for Known Contractual Obligations and Commitments
The following is a description of commitments for known and reasonably likely cash requirements as of 31 December 2023.
Borrowings
Alvotech’s debt consists of interest-bearing borrowings from both financial institutions and related parties. The amount of the outstanding borrowings as of 31 December 2023, was $960.2 million, including payment-in-kind interest. The timing of future payments on the outstanding borrowing amounts, by year, as well as additional information regarding Alvotech’s borrowings and rights conveyed to the lenders, can be found in Note 21 of the audited consolidated financial statements, included elsewhere in this Form 20-F.
Senior Bonds
As of 31 December 2023, the carrying amount of the Senior Bonds was $549.4 million. The Senior Bonds mature in June 2025 and the Group has the option, at any time, to prepay all or any part of the outstanding bonds in exchange for the payment of the redemption premium pursuant to the terms of the agreement.
2022 Convertible Bonds
As of 31 December 2023, the Tranche A and Tranche B Convertible Bonds was $107.1 million and $48.8 million, respectively. Holders of both the Tranche A and Tranche B of the 2022 Convertible Bonds, may elect, at their sole discretion, to convert all or part of the principal amount and accrued interest into Alvotech Ordinary Shares at a conversion price of $10.00 per share on 31 December 2023, 30 June 2024, or upon optional or mandatory redemption of the bonds.
Aztiq Convertible Bond
As of 31 December 2023, the carrying amount of the Aztiq Convertible Bond was $80.7 million. The maturity date of the convertible bond is the later of the (i) 16 November 2025, or (ii) 91 days after the earlier of the full redemption or the final maturity date of the Senior Bonds. Bondholders have the right to convert their outstanding bonds into ordinary shares of Alvotech on 31 December 2023, 30 June 2024, or upon optional or mandatory redemption, for a conversion price of $10.00 per share.
Alvogen Facility
As of 31 December 2023, the carrying amount of the Facility Loans is $48.5 million. Accrued interest on the Facility Loans as of 31 December 2023 is $0.3 million. The first loan includes annuity payments that are due monthly with a final maturity in December 2029 and a variable interest rate of USD Secured Overnight Funding Rate ("SOFR") plus a margin
117
Table of Contents
of 4.75%. The second loan is a bullet loan with a final maturity in December 2027 and a variable interest rate of USD SOFR plus a margin of 3.75%
Other borrowings
On 22 February 2022, the Group entered into a credit facility agreement with Landsbankinn hf. with the ability to draw down an amount up to $8 million. The credit facility is in place to help finance equipment purchases in the future. Per the terms of the credit facility, any borrowings are required to be paid by 1 August 2024 and have a variable interest rate of USD SOFR plus a margin of 4.95%. As of 31 December 2023, the outstanding balance on the credit facility was $7.8 million.
On 22 February 2022, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $3.2 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in March 2029. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $2.5 million.
On 5 August 2022, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $1.8 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in August 2029. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $1.6 million.
On 4 August 2023, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $11.5 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in August 2030. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $11.0 million.
On 14 December 2023, the Group entered into a qualified receivable financing agreement with Landsbankinn hf. for a principal amount of $25.0 million. The qualified receivable financing arrangement has a variable interest rate of USD SOFR plus a margin of 3.50% and a maturity of April 2024. As of 31 December 2023, the outstanding balance on the loan was $25.0 million.
Leases
Alvotech’s future undiscounted payments pursuant to lease agreements totaled $155.4 million as of 31 December 2023, compared to $48.4 million as of 31 December 2022. The timing of these future payments can be found in Note 13 of the audited consolidated financial statements included elsewhere in this Form 20-F.
Other long-term liability to a related party
Alvotech acquired certain rights for the commercialization of its biosimilar Adalimumab product in certain territories in Asia from Lotus Pharmaceutical Co. Ltd., a related party, during the year ended 31 December 2020. Pursuant to the terms of the asset acquisition, Alvotech is required to pay $7.4 million upon the commercial launch of Adalimumab in China which became due on 31 December 2023.
Purchase obligations
For the years ended 31 December 2023, 2022, and 2021, Alvotech did not have any purchase obligations.
While Alvotech does not have legally enforceable commitments with respect to capital expenditures, Alvotech expects to continue to make substantial investments in preparation for commercial launch of its biosimilar product candidates.
Operating Capital Requirements
As of 31 December 2023, the Company had cash and cash equivalents, excluding restricted cash, of $11.2 million. In February 2024, Alvotech accepted an offer for the sale of 10,127,132 Ordinary Shares for gross proceeds of approximately $166 million.
However, even with the aforementioned cash as of 31 December 2023 and the proceeds from the February 2024 private placement, Alvotech’s management has determined that there is a material uncertainty that may cast significant
118
Table of Contents
doubt about its ability to continue as a going concern as described in Note 1.4 of the audited consolidated financial statements included elsewhere in this Annual Report on Form 20-F.
Alvotech’s future funding requirements will depend on many factors as detailed elsewhere in this Annual Report. See the risk factor entitled “We may need to raise substantial additional funding from shareholders or third parties. This additional funding may not be available on acceptable terms or at all. Failure to obtain such necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.” in Item 3.D.
C.Research and Development, Patents and Licenses, etc.
Full details of our research and development activities and expenditures are given in the “Item 4.B. Information on the Company—Business Overview” and “Item 5 Operating and Financial Review and Prospects” sections of this Annual Report on Form 20-F above.
D.Trend Information
Other than as described elsewhere in this Annual Report on Form 20-F, we are not aware of any trends, uncertainties, demands, commitments or events that are reasonably likely to have a material adverse effect on our revenue, income from continuing operations, profitability, liquidity or capital resources, or that would cause our reported financial information not necessarily to be indicative of future operation results or financial condition.
E.Critical Accounting Estimates
Alvotech has prepared its financial statements in accordance with IFRS. The preparation of these financial statements requires Alvotech to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities and related disclosures at the date of the financial statements, as well as revenue and expense recorded during the reporting periods. Alvotech evaluates its estimates and judgments on an ongoing basis. Alvotech bases its estimates on historical experience and other relevant assumptions that it believes to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
An accounting policy is deemed to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, if different estimates reasonably could have been used, or if changes in the estimate that are reasonably possible could materially impact the financial statements. Alvotech’s significant accounting policies are described in more detail in Note 2 of the audited consolidated financial statements as of 31 December 2023, and for the three years ended 31 December 2023, included elsewhere in this Annual Report on Form 20-F.
Revenue recognition
Product revenue
The Company recognizes revenue from the sale of its biosimilar product to commercial partners, identified as the customer, when control is transferred, and the performance obligations have been satisfied. This is when the title passes to the customer, which is upon shipment of the product. At that point, the commercial partner has full discretion over the channel and price to sell the products. Revenue is recognized based on the net selling price from the commercial partners, which is considered to be the transaction price and includes estimated rebates, returns and chargebacks, and other forms of variable consideration recognized by the Customer. Variable consideration is accounted for by the Company only to the extent that it is highly probable that a significant reversal in the revenue recognized will not occur. Variable consideration, which includes any adjustments to the net selling price, is estimated based on the most likely amount method on a contract-by-contract basis. The Company uses historical and market data in determining the most likely amount of variable consideration. These estimates are reviewed each reporting period and involve inherent uncertainty and management’s judgement.
Out-licensing revenue
The consideration to which Alvotech is entitled pursuant to these contracts generally includes upfront payments and payments based upon the achievement of development and regulatory milestones. All contracts include a potential refund obligation whereby Alvotech must refund the consideration paid by the partner in the event of a technical failure or the occurrence of certain other matters that result in partial or full cancellation of the contract. As such, the entire transaction price is comprised of variable consideration, which is estimated using the most likely amount method due to the binary nature of the outcomes under these contracts. Such variable consideration is included in the transaction price only when it
119
Table of Contents
is highly probable that doing so will not result in a significant reversal of cumulative revenue recognized when the underlying uncertainty associated with the variable consideration is subsequently resolved.
The standalone selling prices of the development services and the license to intellectual property are not directly observable and, therefore, are estimated. The standalone selling price of the development services is estimated using the expected cost plus a margin approach, using various data points such as the underlying development budget, contractual milestones, and performance completed at the time of entering into the contract with a partner. The standalone selling price of the license is estimated using the residual approach on the basis that the Alvotech licenses intellectual property for a broad range of amounts and has not previously licensed intellectual property on a standalone basis. Therefore, Alvotech first allocates the transaction price to the development services and subsequently allocates the remainder of the transaction price to the license. Inputs used to determine the standalone selling price of the development services are reviewed by management each reporting period. Changes to these inputs, including changes to the underlying development budget, could impact the timing in which revenue is recognized. The Company has not made any changes to the inputs used in determining the standalone selling price.
Valuation of derivative financial instruments
Alvotech recognized derivative financial liabilities related to warrants, earn out shares and conversion features. The fair values of the derivative liabilities were determined using an option pricing-based approach that incorporated a range of inputs that are both observable and unobservable in nature. The observable and unobservable inputs used in the initial and subsequent fair value measurements relate to (i) the fair value of Ordinary Shares, (ii) the volatility of the Ordinary Shares, (iii) a risk-adjusted discount rate corresponding to the credit risk associated with the repayment of the host debt instruments, and (iv) the probabilities of each derivative being exercised by the holder and the timing of such exercises. The probabilities are determined based on all relevant internal and external information available and are reviewed and reassessed at each reporting date.
The assumptions underlying the valuations represent Alvotech’s best estimates, which involve inherent uncertainties and the application of management’s judgment. As a result, if Alvotech used significantly different assumptions or estimates, its finance costs for prior periods could have been materially different.
Valuation of deferred tax assets
Alvotech recognizes deferred tax assets for all deductible temporary differences and unused tax losses to the extent that it is probable that taxable profits will be available against the deductible temporary differences that can be utilized after consideration of all available positive and negative evidence. Estimation of the level of future taxable profits and the application of relevant jurisdictional tax legislation regarding loss expiry rules, non-deductible expenses, and other guidance are required in order to determine the appropriate carrying value of deferred tax assets.
Alvotech’s estimation of the level of future taxable profits is primarily driven by an evaluation of executed out-license contracts and the expected timing of revenue recognition from such contracts. Alvotech considers the amount of revenues that relate to the various phases of development for its biosimilar product candidates, with greater certainty attributed to revenues earned upon contract execution and before later-stage clinical trials and no certainty attributed to revenues that relate to future sales targets on the basis that such amounts are dependent on events that are not within Alvotech’s control. These forecasts are also evaluated to incorporate potential uncertainty associated with the amount and timing of expected future revenues, driven by factors such as potential competition and the inherent risk associated with biosimilar product development. Changes to these forecasts, and the inputs used in determining the underlying cash flows involve inherent uncertainties and the application of management’s judgement. As a result, if Alvotech used significantly different assumptions or estimates, its valuation of deferred tax assets for current and prior periods could have been materially different.
The carrying amount of deferred tax assets is reviewed at the end of each reporting period and is reduced to the extent it is no longer probable that sufficient taxable profits will be available to allow all or part of the asset to be recovered.
Determination of recoverable amounts of investments in joint venture
After application of the equity method, the Group determines whether it is necessary to recognise an impairment loss on its investment in its joint venture. At each reporting date, the Group determines whether there is objective evidence that the investment in the joint venture is impaired. The Group determined that, considering the uncertainties around the economic conditions in China, there were indicators of potential impairment. The Group calculates the amount of
120
Table of Contents
impairment as the difference between the recoverable amount of the joint venture and its carrying value. The Group estimated the recoverable amount based on estimated future cash flows from the ultimate disposal of the investment.
Item 6. Directors, Senior Management and Employees
A.Directors and senior management.
The following table sets forth the executive officers and directors of Alvotech. Unless otherwise noted, the business address of each of the directors and executive officers of Alvotech is 9, Rue de Bitbourg, L-1273 Luxembourg, Grand Duchy of Luxembourg.
| Name | Age | Title | ||||||||||||
| Executive Officers | ||||||||||||||
| Robert Wessman | 54 | Chief Executive Officer and Executive Chairman of the Board of Directors | ||||||||||||
| Tanya Zharov | 57 | General Counsel | ||||||||||||
| Joseph E. McClellan | 50 | Chief Scientific Officer | ||||||||||||
| Faysal Kalmoua | 48 | Chief Operating Officer | ||||||||||||
| Joel Morales | 46 | Chief Financial Officer | ||||||||||||
| Directors | ||||||||||||||
| Richard Davies | 62 | Director and Deputy Chairman | ||||||||||||
| Tomas Ekman | 56 | Director | ||||||||||||
| Faysal Kalmoua | 48 | Director | ||||||||||||
| Ann Merchant | 59 | Director | ||||||||||||
| Arni Hardarson | 57 | Director | ||||||||||||
| Lisa Graver | 53 | Director | ||||||||||||
| Linda McGoldrick | 68 | Director | ||||||||||||
Executive Officers
Robert Wessman is the founder and has served as Executive Chairman and member of the board of directors of Alvotech since January 2019, and chief executive officer since January 2023. Since November 2018, he has also served as Director at Fuji Pharma and chairman of the board of directors of Lotus Pharmaceuticals and since May 2009, he has served as a member of the board of directors of Aztiq and as a member of the board of directors of Aztiq GP, the general partner of Aztiq Fund I SCSp, a Luxembourg alternative investment fund, and the parent company of Aztiq. Mr. Wessman is also the founder and main partner of the Aztiq group. Mr. Wessman founded Alvogen in July 2009, and served as its Executive Chairman and Chief Executive Officer until June 2022. He continues to serve as Alvogen’s chairman since July 2022. Between 1999 and 2008, Mr. Wessman served as the Chief Executive Officer of Actavis. He has a Bachelor of Science degree in Business Administration from the University of Iceland. We believe Mr. Wessman is qualified to serve on Alvotech’s board of directors due to the perspective he brings as Alvotech’s founder and his experience in top executive positions in the pharmaceutical industry.
Tanya Zharov has served as our General Counsel since January 2023 and Deputy Chief Executive Officer between May 2020 and December 2022. Prior to joining Alvotech, between 2016 and 2020, Ms. Zharov served as Deputy Chief Executive Officer and compliance officer of deCODE genetics. Prior to that, Ms. Zharov held various management positions, including as General Counsel and Deputy Chief Executive Officer at Virding hf from January 2014 to January 2016, as General Counsel and Deputy Chief Executive Officer at Audur Capital from January 2008 to December 2013, as Board Secretary, corporate counsel and Vice President Corporate Governance and Administration at deCODE genetics from July 2003 to December 2007, and as tax partner at PricewaterhouseCoopers from June 1996 to December 1998. Ms. Zharov holds a law degree from the University of Iceland and is a European Patent Attorney.
Joseph E. McClellan has served as our Chief Scientific Officer since October 2019. Prior to joining Alvotech, Mr. McClellan served for over 17 years in various roles at Pfizer Inc., including as Global Head of Biosimilars Development and Medicine/Asset Team Leader of IXIFI (biosimilar infliximab). Mr. McClellan holds a PhD degree in Chemistry, with a focus in Analytical Chemistry and Mass Spectrometry, from the University of Florida, and he was a Postdoctoral Fellow in Mass Spectrometry and Analytical Biochemistry at the Boston University School of Medicine.
121
Table of Contents
Joel Morales has served as our Chief Financial Officer since February 2020 after serving as Chief Financial Officer at our affiliated company Alvogen since 2017. Prior to joining Alvotech he held various positions of increasing responsibility with Endo International plc., from January 2015 to September 2017, with his last position as Senior Vice President of the Generics Business Segment and Global Finance Operations. Prior to that, Mr. Morales spent ten years working for large multinational pharmaceutical companies, including Merck and Schering Plough. Mr. Morales began his career at KPMG as a licensed certified public accountant in the State of New Jersey and has a Bachelor of Science degree in Accounting from Rutgers University.
Faysal Kalmoua has served as Chief Operating Officer since September 2023 and as one of Alvotech’s directors since June 2020. Mr. Kalmoua has also served as a partner of the Aztiq group since June 2022. Between April 2020 and June 2022, Mr. Kalmoua served as Executive Vice President of Portfolio, Business Development and Research and Development for Alvogen Iceland ehf. and Alvogen, Inc. Between November 2015 and March 2020, Mr. Kalmoua served as Executive Vice President of Portfolio for Alvogen, Inc. Prior to joining Alvogen, Mr. Kalmoua served in various management positions for Synthon for nearly 16 years. Mr. Kalmoua holds a Master’s degree in Chemistry from the Radboud University Nijmegen and an executive MBA from Insead.
Non-Executive Directors
Richard Davies has served Deputy Chairman of Alvotech’s board, previously Chairman of Alvotech’s board, and as one of Alvotech’s directors since January 2019. Since November 2018, he has served as Chief Executive Officer of Auregen Bio Therapeutics SA. Prior to joining Auregen Bio Therapeutics, Mr. Davies served as Chief Executive Officer of Bonesupport AB between 2016 and 2018, as Senior Vice President and Chief Commercial Officer of Hospira Inc. between 2012 and 2015, and in various leadership roles at Amgen Inc between 2003 and 2012. Mr. Davies holds an MBA from the University of Warwick and Bachelor of Science in applied chemistry from the University of Portsmouth.
Tomas Ekman has served as one of Alvotech’s directors since January 2019. Since November 2014 he has served as a partner at CVC Capital Partners where he is a member of the CVC Nordics team and is based in Stockholm. Prior to joining CVC in 2014, Mr. Ekman was a partner and Managing Director at 3i, responsible for its Nordic business. Mr. Ekman holds MSc degrees from the University of Strathclyde and Chalmers University of Technology, and an MBA from IMD, Switzerland.
Ann Merchant has served as one of Alvotech’s directors since June 2022. Since 2018, she has served as Vice President for MorphoSys, and as Head of Global Supply Chain since January 2019. Prior to joining MorphoSys, from September 2011 to August 2018, Ms. Merchant served as the President for Schreiner Medipharm. Between 1994 and 2011, Ms. Merchant held various roles at Amgen, including Vice President, Head of International Supply Chain and Site Head between 2007 and 2011. Ms. Merchant holds an MBA from the Henley Business School and a Bachelor of Science in Languages from Georgetown University. We believe Ms. Merchant is qualified to serve on Alvotech’s board of directors because of her experience in executive positions with several pharmaceutical companies and expertise in financial planning, new product launches and creating and executing international strategies to increase market share.
Arni Hardarson has served as one of Alvotech’s directors since June 2022. Mr. Hardarson is a co-founder and partner of the Aztiq group. Between 2009 and June 2022, he served as Deputy to the Chief Executive Officer and General Counsel of Alvogen. Prior to joining Alvogen, Mr. Hardarson was Vice President of Tax and Structure at Actavis, and as partner, member of the executive management committee, and served as a head of tax and legal at Deloitte. Mr. Hardarson holds a Master’s degree in law from the University of Iceland. We believe Mr. Hardarson is qualified to serve on Alvotech’s board of directors because of his extensive expertise in financial and legal matters and his past experience in top executive positions.
Lisa Graver has served as one of Alvotech’s directors since June 2022. Ms. Graver has served in various leadership positions for Alvogen since June 2010, including as President of Alvogen Inc, a subsidiary of Alvogen, since August 2015, as Executive Vice President and Deputy to the Chief Executive Officer of Alvogen Inc. since February 2013, and as Vice president Intellectual Property of Alvogen since June 2010. Prior to joining Alvogen, Ms. Graver was Vice President Intellectual Property and Senior Director Intellectual Property at Actavis Inc. between 2006 and 2008. Ms. Graver holds a BSc in Biology from Lakehead University and a law degree from the Case Western Reserve University School of Law. We believe Ms. Graver is qualified to serve on Alvotech’s board of directors because of her extensive expertise in intellectual property and the pharmaceutical industry.
Linda McGoldrick has served as one of Alvotech’s directors since June 2022. In 1985, Dr. McGoldrick founded, and currently serves as Chairman and Chief Executive Officer of, Financial Health Associates International, a strategic consulting company specializing in healthcare and life sciences. Since January 2020, she has served as the Chief Executive
122
Table of Contents
Officer for 2Enable Health LLC. Prior to joining 2Enable Health LLC, Dr. McGoldrick served as interim CEO at Zillion between June 2019 and December 2019. Over her professional career, Dr. McGoldrick has served in a number of leadership roles, including Senior Vice President and National Development Director for the Healthcare and Life Sciences Industry Practices at Marsh-MMC Companies, International Operations and Marketing Director of Veos plc, and Managing Director Europe for Kaiser Permanente International. In 2018, Dr. McGoldrick was appointed by the Governor of Massachusetts to serve on the state’s Health Information Technology Commission. Dr. McGoldrick has served as a director of numerous publicly traded and private held companies and non-profit organizations in the U.S., UK and Europe, including as director for Compass Pathways since September 2020. In 2012, Dr. McGoldrick was named as one of the Top 100 Corporate Directors of Fortune 100 Companies by the Financial Times. Dr. McGoldrick holds a Master’s Degree in Healthcare from the University of Pennsylvania and an MBA from Wharton. We believe Dr. McGoldrick is qualified to serve on Alvotech’s board of directors because of her extensive expertise in financial matters and the healthcare and life sciences industry.
Diversity of the Board of Directors
The table below provides certain information regarding the diversity of our board of directors as of the date of this Annual Report.
Board Diversity Matrix (As of December 31, 2023)
| Country of Principal Executive Offices | Luxembourg | |||||||||||||||||||||||||
| Foreign Private Issuer | Yes | |||||||||||||||||||||||||
| Disclosure Prohibited under Home Country Law | No | |||||||||||||||||||||||||
| Total Number of Directors | 8 | |||||||||||||||||||||||||
| Female | Male | Non-Binary | Did Not Disclose Gender | |||||||||||||||||||||||
| Part I: Gender Identity | ||||||||||||||||||||||||||
| Directors | 1 | 1 | 0 | 6 | ||||||||||||||||||||||
| Part II: Demographic Background | ||||||||||||||||||||||||||
| Underrepresented Individual in Home Country Jurisdiction | 0 | |||||||||||||||||||||||||
| LGBTQ+ | 0 | |||||||||||||||||||||||||
| Did Not Disclose Demographic Background | 6 | |||||||||||||||||||||||||
Family Relationships
There are no family relationships among any of our executive officers or directors.
B.Compensation
Compensation of Executive Officers
Each of our executive officers has entered into an employment agreement with us for an indefinite period of time. The agreements provide the terms of each individual’s employment or service with us, as applicable.
Each employment agreement contains provisions regarding non-competition, non-solicitation, confidentiality of information and assignment of inventions. The enforceability of the non-competition covenants is subject to limitations. Either we or the executive officer may terminate the applicable executive officer’s employment or service by giving advance written notice to the other party. We may also terminate an executive officer’s employment or services agreement for cause (as defined in the applicable employment or services agreement).
Our executive compensation program reflects its compensation policies and philosophies, as they may be modified and updated from time to time. In addition to a base salary and certain performance-based bonuses, executive officers can be eligible to receive awards under our 2022 equity incentive plan, the Alvotech Management Incentive Plan (the “2022 Plan”), as further described below. Decisions with respect to the compensation of our executive officers, including our named executive officers, are made by the compensation committee of our board of directors.
123
Table of Contents
The following table sets forth information regarding compensation earned by Robert Wessman, our Chief Executive Officer and our other members of the leadership team during the years ended 31 December 2023.
| 2023 | |||||||||||||||||||||||
| Key employees | Salaries and benefits | Pension contribution | Termination benefits | Other long- term benefits | |||||||||||||||||||
| Robert Wessman CEO | 1,491 | 26 | — | — | |||||||||||||||||||
| Other Members of the Leadership Team | 5,020 | 346 | 52 | 9,456 | |||||||||||||||||||
| 6,511 | 372 | 52 | 9,456 | ||||||||||||||||||||
The following table sets forth information regarding compensation earned by Mark Levick, our former Chief Executive Officer until December 31, 2022, and our other executive officers during the years ended 31 December 2022.
| 2022 | |||||||||||||||||||||||
| Key employees | Salaries and benefits | Pension contribution | Termination benefits | Other long- term benefits | |||||||||||||||||||
| Mark Levick CEO | 892 | 162 | 1,157 | — | |||||||||||||||||||
| Other Members of the Leadership Team | 5,400 | 446 | 820 | 5,015 | |||||||||||||||||||
| 6,292 | 608 | 1,977 | 5,015 | ||||||||||||||||||||
Compensation of Directors
At the Annual General Meeting of Alvotech on 6 June 2023 – the shareholders approved the compensation for the board of directors which applies to the independent directors, additionally the shareholders approve a remuneration policy under Lux law.
All vesting of the restricted stock units is subject to the non-employee director’s continuous service on the applicable vesting date. However, for each eligible director who remains in continuous service until immediately prior to the occurrence of a change in control (as such term is defined in the 2022 Plan), the shares subject to his or her then-outstanding restricted stock unit awards will become fully vested immediately prior to the closing of such change in control event.
We will also reimburse our non-employee directors for their reasonable out-of-pocket expenses in connection with attending board and committee meetings.
124
Table of Contents
The following tables sets forth information regarding compensation earned by each of our directors during the years ended 31 December 2023 and 2022:
| Board of Directors’ fee for the year and shares at year end (board fees in thousands and shares in whole amounts). | 2023 | ||||||||||||||||||||||
| Board fees | Pension contribution | Other long-term benefits | Shares at year-end** | ||||||||||||||||||||
| Robert Wessman, Chairman of the board* | — | — | — | — | |||||||||||||||||||
| Richard Davies, Vice-Chairman | 156 | — | 104 | 1,143,713 | |||||||||||||||||||
| Ann Merchant, Board Member | 113 | — | 104 | 10,582 | |||||||||||||||||||
| Árni Harðarson, Board Member* | — | — | — | — | |||||||||||||||||||
| Faysal Kalmoua, Board Member* | — | — | — | — | |||||||||||||||||||
| Linda McGoldrick, Board Member | 81 | — | 104 | 10,582 | |||||||||||||||||||
| Lisa Graver, Board Member | 71 | — | 104 | 10,582 | |||||||||||||||||||
| Tomas Ekman, Board Member* | — | — | — | — | |||||||||||||||||||
| 421 | — | 416 | 1,175,459 | ||||||||||||||||||||
*Waived their board compensation (both cash and equity)
**Direct share ownership
| Board of Directors’ fee for the year and shares at year end (board fees in thousands and shares in whole amounts). | 2022 | ||||||||||||||||||||||
| Board fees | Pension contribution | Other long-term benefits | Shares at year-end** | ||||||||||||||||||||
| Robert Wessman, Chairman of the board | 740 | — | — | — | |||||||||||||||||||
| Richard Davies, Vice-Chairman | 68 | — | — | 1,133,131 | |||||||||||||||||||
| Ann Merchant, Board Member (from 16.6.2022) | 43 | — | — | — | |||||||||||||||||||
| Árni Harðarson, Board Member (from 16.6.2022)* | — | — | — | — | |||||||||||||||||||
| Faysal Kalmoua, Board Member* | — | — | — | — | |||||||||||||||||||
| Linda McGoldrick, Board Member (from 16.6.2022) | 38 | — | — | — | |||||||||||||||||||
| Lisa Graver, Board Member (from 16.6.2022) | 38 | — | — | — | |||||||||||||||||||
| Tomas Ekman, Board Member* | — | — | — | — | |||||||||||||||||||
| Hirofumi Imai, Board member (until 16.6.2022) | — | — | — | — | |||||||||||||||||||
| 927 | — | — | 1,133,131 | ||||||||||||||||||||
*Waived their board compensation (both cash and equity)
**Direct share ownership
Company Management Incentive Plan
On 13 June 2022, our chairman adopted, and our shareholders approved, a new 2022 equity incentive plan, the Management Incentive Plan (the “2022 Plan”).
Awards. The 2022 Plan will provide for the grant of shares, restricted shares units, options or any combination of the foregoing including such other Awards that may be denominated or payable in, value in whole or in part, by reference to or otherwise based upon, or related to, shares (the “Awards”) to our employees, directors, and consultants and any of our affiliates’ employees and consultants.
Authorized Shares. Initially, the maximum number of Ordinary Shares that may be issued under the 2022 Plan after it becomes effective will not exceed 5.79% of our share capital on a fully diluted basis. In addition, the number of Ordinary Shares reserved for issuance under the 2022 Plan may be increased by our board of directors by up to 1% annually over ten (10) years from the date of approval of the 2022 Plan.
125
Table of Contents
Plan Administration. Our board of directors, or any person or persons or committee to whom decision-making authority with respect to the 2022 Plan is delegated by our board of directors (the “Administrator”) will administer the 2022 Plan.
Plan Amendment or Termination. Our board of directors and the Administrator have the authority to amend or, suspend, the 2022 Plan at any time and from time to time, and our board of directors has the authority to terminate the 2022 Plan provided that such action does not materially impair the existing rights of any participant without such participant’s written consent. Certain material amendments also require the approval of our shareholders. No Awards may be granted after the tenth anniversary of the date our board of directors adopted the 2022 Plan. No Awards may be granted under the 2022 Plan while it is suspended or after it is terminated. Rights under any Award granted before suspension or termination of the 2022 Plan shall not be impaired by such suspension or termination.
On 1 December 2022, our Remuneration Committee authorized the grant of restricted stock units (“RSUs”) to certain employees, executive officers and directors under the 2022 Plan. Subject to certain vesting and other terms and conditions, the RSUs may be settled in Ordinary Shares.
The Annual General Meeting of the shareholders approved the Remuneration Policy and the Remuneration of the Alvotech board.
During the year 2023, our Remuneration Committee authorized the grant of a total of 820,602 RSUs to certain employees and executive officers under the 2022 Plan
If all RSUs vest and are exchanged for Ordinary Shares, the combined grants may result in an aggregate of 6,891,717 Ordinary Shares.
Management Share Appreciation Rights Agreements
As part of its long-term incentive program, Alvotech hf. had entered into “phantom share agreements,” which were defined as Share Appreciation Rights (“SARs”) for financial purposes, with certain members of management. The vesting conditions of the SARs under the phantom share agreements were linked to certain milestones in our operations and the payment amounts were determined by the increase in our market value from the grant date of the SARs until the triggering event occurred. The SARs did not give the beneficiaries dividend rights, voting rights or the right to purchase shares of Alvotech but required Alvotech to pay the beneficiaries a cash payment associated with the occurrence of certain designated triggering events. In conjunction with the Business Combination, Alvotech terminated deferred compensation arrangements by entering into settlement agreements with the three former employees and one current employee that had outstanding rights under the phantom share agreements of $38.1 million as of the Closing. Alvotech further agreed with the two other former employees to settle each of their respective claims of $17.5 million, as may be reduced by any applicable tax withholdings, through the allocation of a number of Ordinary Shares by dividing their respective claims by a per share price of $10.00, rounded to the nearest whole share. The shares were allocated to them on 16 June 2023, one year and one day following the Closing which was settled in cash on 16 June 2023.
C.Board Practices
Composition of Our Board of Directors
Our board of directors is currently composed of eight members. In accordance with our articles of association, the board of directors is not divided into classes of directors. Each director was appointed at the closing of the Business Combination on 15 June 2022, to serve as director until the end of the general meeting of shareholders called to approve our annual accounts for the 2024 financial year.
Three of eight directors are independent as defined in Nasdaq listing standards and applicable SEC rules and our board of directors has an independent audit and risk committee, a nominating committee, a compensation committee.
Non-Executive Director Appointment Letters
Our independent non-executive directors are engaged on letters of appointment that set out their duties and responsibilities. The non-executive directors do not receive benefits upon termination or resignation from their respective positions as directors. Under the non-executive director appointment letters, our non-executive directors are entitled to receive annual fees in accordance with our Director Compensation Policy, as discussed in Item 6.B Compensation—Compensation of Directors.
126
Table of Contents
Committees of our Board of Directors
Our board of directors has five standing committees: an audit and risk committee, a compensation committee, a nominating and corporate governance committee, a strategy committee and a Corporate Sustainability Committee. The board has adopted written charters that are available to shareholders on our website at https://investors.alvotech.com/corporate-governance/documents-charters for the audit and risk committee, the compensation committee, and the nominating and corporate governance committees. The reference to our website address in this Annual Report on Form 20-F does not include or incorporate by reference the information on our website into this Annual Report on Form 20-F.
Audit and Risk Committee
The members of our audit and risk committee are Dr. McGoldrick (Chair), Ms. Merchant and Mr. Davies. Each member of our audit and risk committee qualifies as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to audit and risk committee membership. In addition, all audit and risk committee members meet the requirements for financial literacy under applicable SEC and Nasdaq rules and at least one of the audit and risk committee members qualifies as an “audit and risk committee financial expert,” as such term is defined in Item 407(d) of Regulation S-K. The audit and risk committee is responsible for, among other things:
•appointing, compensating, retaining, evaluating, terminating and overseeing our independent registered public accounting firm;
•discussing with our independent registered public accounting firm their independence from management;
•reviewing, with our independent registered public accounting firm, the scope and results of their audit;
•approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm;
•overseeing the financial reporting process and discussing with management and our independent registered public accounting firm the annual financial statements that we file with the SEC;
•overseeing our financial and accounting controls and compliance with legal and regulatory requirements;
•reviewing our policies on risk assessment and risk management;
•reviewing related party transactions; and
•establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters.
Compensation Committee
The members of our compensation committee are Mr. Davies (Chair), Mr. Hardarson and Mr. Ekman. Mr. Davies qualifies as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to compensation committee membership, including the heightened independence standards for members of a compensation committee. The compensation committee is responsible for, among other things:
•reviewing and approving the corporate goals and objectives, evaluating the performance of and reviewing and approving, (either alone or, if directed by the board of directors, in conjunction with a majority of the independent members of the board of directors) the compensation of our chief executive officer;
•overseeing an evaluation of the performance of and reviewing and setting or making recommendations to our board of directors regarding the compensation of our other executive officers;
•reviewing and approving or making recommendations to our board of directors regarding our incentive compensation and equity-based plans, policies and programs;
•reviewing and approving all employment agreement and severance arrangements for our executive officers;
•making recommendations to our shareholders regarding the compensation of our directors; and
•retaining and overseeing any compensation consultants.
127
Table of Contents
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Mr. Davies (Chair), Ms. Merchant and Dr. McGoldrick. The nominating committee is responsible for, among other things:
•identifying individuals qualified to become members of our board of directors, consistent with criteria approved by our board of directors;
•overseeing succession planning for our Chief Executive Officer and other executive officers;
•periodically reviewing our board of directors’ leadership structure and recommending any proposed changes to our board of directors;
•overseeing an annual evaluation of the effectiveness of our board of directors and its committees; and
•developing and recommending to our board of directors a set of corporate governance guidelines.
Corporate Sustainability Committee
The members of our Corporate Sustainability Committee are Ms. Merchant (Chair), Mr. Hardarson and Mr. Wessman. The ESG committee is responsible for, among other things:
•reviewing, monitoring and setting strategy in the area of corporate responsibility;
•overseeing our activities in the area of corporate responsibility that may have an impact on the Company’s reputation and operations;
•periodically assess our compliance obligations;
•monitor and review matters of health and safety and report findings to the broader board; and
•review and evaluate environmental, social and political issues and trends and their relevance to our business and make recommendations to the board regarding those trends and issues.
Strategy Committee
The Strategy committee is responsible for, among other things, reviewing, monitoring and setting strategy for our business. The members of our Strategy committee are Mr. Faysal Kalmoua (Chair), Ms. Lisa Graver and Mr. Wessman.
Risk Oversight
The board of directors is responsible for overseeing our risk management process. The board of directors focuses on
our general risk management strategy, the most significant risks, and oversees the implementation of risk mitigation
strategies by management. The audit and risk committee is also responsible for discussing our policies with respect to risk
assessment and risk management. The board of directors believes its administration of its risk oversight function has not
negatively affected the board of directors’ leadership structure.
Code of Business Conduct
Our board of directors adopted a Code of Business Conduct applicable to the directors, executive officers and team
members that complies with the rules and regulations of Nasdaq and the SEC. The Code of Ethics is available on our
website. In addition, we posted on the Corporate Governance section of our website all disclosures that are required by law
or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the Code of Ethics. The
reference to our website address in this Annual Report does not include or incorporate by reference the information on our
website into this Annual Report.
D.Employees
As of 31 December 2023, we had 1,026 employees, including 27 contractors, 87% of whom were devoted to R&D, quality and technical operations, and 13% to administration and support roles.
128
Table of Contents
Many of our Iceland-based employees are members of Icelandic labor unions and as such the bargaining agreements which these unions enter into with the Icelandic Confederation of Employers, of which Alvotech hf. is a member. We have not experienced any work stoppages and consider our relationship with our employees and the labor unions to be good.
| At 31 December | |||||||||||||||||
| Function: | 2023 | 2022 | 2021 | ||||||||||||||
| Manufacturing | 575 | 512 | 360 | ||||||||||||||
| Administrative | 131 | 129 | 104 | ||||||||||||||
| Research and development | 320 | 306 | 268 | ||||||||||||||
| Total | 1,026 | 947 | 732 | ||||||||||||||
| Geography: | |||||||||||||||||
| Iceland | 839 | 745 | 557 | ||||||||||||||
| European Union | 74 | 79 | 94 | ||||||||||||||
| United States | 14 | 28 | 23 | ||||||||||||||
| Elsewhere | 99 | 95 | 58 | ||||||||||||||
| Total | 1,026 | 947 | 732 | ||||||||||||||
E.Share Ownership
For information regarding the share ownership of our directors and executive officers, see “Item 7.A Major Shareholders” and “Item 6.B Compensation” for a discussion of the 2023 Plan.
F.Disclosure of a registrant’s action to recover erroneously awarded compensation.
Not applicable.
Item 7. Major Shareholders and Related Party Transactions
A.Major Shareholders
The following table sets forth information regarding the beneficial ownership of Ordinary Shares as of 15 February 2024 by:
•each person known by us to be the beneficial owner of more than 5% of Ordinary Shares;
•each of our directors and executive officers; and
•all our directors and executive officers as a group.
Except as otherwise noted herein, the number and percentage of Ordinary Shares beneficially owned is determined in accordance with Rule 13d-3 of the Exchange Act, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any Ordinary Shares as to which the holder has sole or shared voting power or investment power and also any Ordinary Shares which the holder has the right to acquire within 60 days of 15 February 2024 through the exercise of any option, warrant or any other right.
Except as otherwise indicated, all of the shares reflected in the table are ordinary shares and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
129
Table of Contents
We have based percentage ownership on 266,821,844 Ordinary Shares outstanding as of 15 February 2024.
| Name and Address of Beneficial Owners | Number of Shares | % | |||||||||
Directors and Executive Officers(1) | |||||||||||
| Robert Wessman | — | — | |||||||||
Richard Davies(2) | 1,153,634 | * | |||||||||
| Tomas Ekman | — | — | |||||||||
Ann Merchant(3) | 20,503 | — | |||||||||
| Arni Hardarson | — | — | |||||||||
Lisa Graver(4) | 20,503 | * | |||||||||
Linda McGoldrick(5) | 20,503 | * | |||||||||
Faysal Kalmoua(6) | 39,683 | — | |||||||||
Tanya Zharov(7) | 104,166 | — | |||||||||
Joseph E. McClellan(8) | 277,778 | — | |||||||||
Joel Morales(9) | 208,332 | — | |||||||||
| All Directors and Executive Officers as a group (11 persons) | 1,845,102 | * | |||||||||
| Five Percent Holders Post-Business Combination | |||||||||||
Alvogen Lux Holdings S.à r.l.(10) | 90,005,334 | 33.73 | % | ||||||||
Aztiq Pharma Partners S.à r.l.(4) | 101,165,374 | 37.91 | % | ||||||||
___________
*Indicates beneficial ownership of less than 1% of the total ordinary shares outstanding.
(1)Unless otherwise noted, the business address of each of the directors and executive officers is 9, Rue de Bitbourg, L-1273 Luxembourg, Grand Duchy of Luxembourg.
(2)Consists of 97,880 unvested Earn Out Shares and 9,921 vested RSUs held by Richard Davis.
(3)Consists of 9,921 vested RUSs held by Ann Merchant.
(4)Consists of 9,921 vested RSUs held by Lisa Graver.
(5)Consists of 9,921 vested RSUs held by Linda McGoldrick.
(6)Consists of 39,683 vested RSUs held by Faysal Kalmoua.
(7)Consists of 104,166 vested RSUs held by Tanya Zharov.
(8)Consists of 277,778 vested RSUs held by Joseph McClellan.
(9)Consists of 208,332 vested RSUs held by Joel Morales.
(10)Represents shares held by Alvogen Lux Holdings S.à r.l. (“Alvogen”). Through intermediary holding entities, Alvogen is a wholly-owned subsidiary of Celtic Holdings SCA (“Celtic Holdings”). Investment and voting decisions with respect to the shares held by Alvogen are made by the directors of Celtic Holdings. Carmen Andre, Tomas Ekman, Arni Hardarson, Park Jung Ryun, Christoffer Sjøqvist and Robert Wessman are the directors of Celtic Holdings and may be deemed to have shared voting and dispositive power with respect to the shares held by Alvogen. Carmen Andre, Tomas Ekman, Arni Hardarson, Park Jung Ryun, Christoffer Sjøqvist and Robert Wessman each disclaim any beneficial ownership of any such shares, except to the extent of their pecuniary interest therein, if any. The address of Alvogen is 5, rue Heienhaff, L-1736 Senningerberg, Luxembourg, Grand-Duchy of Luxembourg and the address of Celtic Holdings is 20, avenue Monterey, L-2163 Luxembourg, Grand-Duchy of Luxembourg.
(11)Represents shares held by Aztiq Pharma Partners S.à r.l. (“APP”). APP is a wholly-owned subsidiary of Aztiq Fund I SCSp (“Aztiq Fund”). Investment and voting decisions at Aztiq Fund are made by its general partner, Floki GP S.à r.l. (“Aztiq GP”). Investment and voting decisions with respect to the shares held by APP are made by the members of the board of managers of Aztiq GP. Arni Hardarson, Johann Johannsson, Danny Major, Marc Levebvre and Robert Wessman are members of the board of managers of Aztiq GP and may be deemed to have shared voting and dispositive power with respect to the shares held by APP in Alvotech. Arni Hardarson, Johann Johannsson, Danny Major, Marc Levebvre and Robert Wessman each disclaim any beneficial ownership of any such shares, except to the extent of their pecuniary interest therein, if any. The address of APP is 5, rue Heienhaff, L-1736 Senningerberg, Grand-Duchy of Luxembourg and the address of Aztiq Fund and Aztiq GP is at 4 rue Robert Stumper, L-2557 Luxembourg, Grand-Duchy of Luxembourg.
130
Table of Contents
Voting Rights
The voting rights of the principal shareholders do not differ from the voting rights of other shareholders.
Shareholders in the United States
As of 31 January 2024, to the best of our knowledge 67,081,130 of our outstanding ordinary shares were held by eight shareholders of record in the United States. The actual number of holders is greater than these numbers of record holders, and includes beneficial owners whose ordinary shares are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities.
B.Related Party Transactions
Policies and Procedures for Related Person Transactions
The Board of Directors has adopted a written related person transaction policy that sets forth certain policies and procedures for the review and approval or ratification of transactions involving us in which a related person has or will have a direct or indirect material interest, as determined by the audit and risk committee of the Board. A “related person” for purposes of the policy means: (i) enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common control with, us; (ii) Associates (defined as, unconsolidated enterprises in which we have a Significant Influence or which has Significant Influence over us); (iii) individuals owning, directly or indirectly, an interest in the voting power of us that gives them Significant Influence over us, and close members of any such individual’s family; (iv) key management personnel (i.e., having authority and responsibility for planning, directing and controlling our activities), including Directors and close members of such individuals’ families; and (v) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (iii) or (iv) above or over which such a person is able to exercise Significant Influence, including enterprises owned by our Directors or major shareholders and enterprises that have a member of key management in common with us. “Significant Influence” for purposes of the policy means the power to participate in the financial and operating policy decisions of an enterprise but is less than control over those policies, provided that shareholders beneficially owning a 10% or more interest in the voting power of the enterprise concerned are presumed to have a significant influence on such enterprise.
Pursuant to the policy, each executive director, nominee for the position of executive director and executive officer shall promptly notify the designated contact of any transaction involving us and a related person. The designated contact will present any new related person transactions, and proposed transactions involving related persons, to the Audit and Risk Committee of the Board at its next occurring regular meeting. If the Audit and Risk Committee determines that the related person involved has a direct or indirect material interest in the transaction, and there therefore that the transaction is a related party transaction, the Audit and Risk Committee shall consider all relevant facts and circumstances, including the commercial reasonableness of the terms, the benefit and perceived benefit, or lack thereof, to the Company, opportunity costs of alternate transactions, the materiality and character of the Related Person’s direct or indirect interest, and the actual or apparent conflict of interest of the Related Person. The Audit and Risk Committee will not approve or ratify a Related Person Transaction unless it shall have determined that, upon consideration of all relevant information, the Transaction is in, or not inconsistent with, our best interests . On an annual basis, the Audit and Risk Committee shall review previously approved related person transactions, under the standard described above, to determine whether such transactions should continue. If after the review described above, the Audit and Risk Committee determines not to approve or ratify a related person transaction (whether such transaction is being reviewed for the first time or has previously been approved and is being reviewed), the transaction will not be entered into or continued.
Lease agreements with related parties
Lease agreement with Fasteignafelagid Eyjolfur hf.
The Group entered into a lease agreement with Fasteignafelagid Eyjolfur hf. in April 2023 for a new facility in Iceland with remaining lease terms of approximately 15 years as of 31 December 2023. The building is 140,000 square feet and is currently in construction. The expansion is close to being finalized and is expected to be completed in 2024. Lease liabilities as of 31 December 2023 amount to $69.7 million.
Lease agreement with Flóki Fasteignir ehf. (HRJÁF ehf.)
The Group entered into seven separate lease agreements with Flóki Fasteignir ehf. (HRJAF ehf.) in 2023 for a group of apartment buildings in Iceland used for temporary housing of employees and third-party contractors. The
131
Table of Contents
remaining lease terms leases approximate 8 years, on average, as of 31 December 2023. Lease liabilities as of 31 December 2023 for the new leases amount to $2.6 million.
Lease agreement with Alvogen UK Ltd.
The Group entered into office sublease sharing agreement with Alvogen UK Ltd. in August 2023. The agreement was effective from 1 January 2023 and shall terminate upon the expiration or termination of the lease. The office is approximately 5,500 square feet and the group leases 30% of the premises, containing approximately 1,645 square feet of space. Lease liabilities as of 31 December 2023 amount to $0.6 million.
Lease agreement with Flóki-Art ehf.
The Group entered into an art lease agreement with Flóki-Art ehf. in January 2023, as a result of the Share Purchase Agreement pursuant to which the Group rent pieces of art located in Sæmundargata 15-19, Reykjavik. The remaining lease term for the leased asset is 15 years as of 31 December 2023. Lease liabilities as of 31 December amount to $0.4 million.
Shareholder Loans and Financing
Aztiq Tranche A Convertible Bond
On 31 July 2023, as part of a private placement of subordinated convertible bonds for a principal amount of $100 million, ATP Holdings ehf., an affiliate of Aztiq, acquired Tranche A convertible bonds in an aggregate principal amount equal to $30.0 million (excluding any amount resulting from capitalization of PIK interest accrued) pursuant to the terms thereof) (the “Aztiq Tranche A Convertible Bond”). These Tranche A convertible bonds are ISK denominated and carries an annual payment-in-kind interest rate of 15% per year. The maturity date of the convertible bonds is the later of the (i) 20 December 2025 or (ii) 91 days after the earlier of the full redemption or the final maturity date of the Senior Bonds. Holders of both the Tranche A convertible bonds, may elect, at their sole discretion, to convert all or part of the principal amount and accrued interest into Alvotech Ordinary Shares at a conversion price of $10.00 per share on 31 December 2023, or 30 June 2024, or upon optional or mandatory redemption of the bonds.
As of 31 December 2023, the outstanding carrying amount on the Aztiq Tranche A Convertible Bond was $15.9 million.
C.Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information.
A.Consolidated Statements and Other Financial Information
Our consolidated financial statements are appended at the end of this Annual Report, starting at page F-1.
Dividend Distribution Policy
From the annual net profits of Alvotech, at least 5% shall each year be allocated to the reserve required by applicable laws (the “Legal Reserve”). That allocation to the Legal Reserve will cease to be required as soon and as long as the Legal Reserve amounts to 10% of the amount of the share capital of Alvotech. The legal reserve is not available for distribution.
We do not anticipate paying any cash dividends in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business and product candidates.
In accordance with the Luxembourg law of August 10, 1915, on commercial companies, as amended (“Luxembourg Company Law”), the general meeting of shareholders, by a simple majority vote and based on the recommendation of our board of directors, shall resolve how the remainder of the annual net profits, after allocation to the Legal Reserve, will be disposed of by allocating the whole or part of the remainder to a reserve or to a provision, by carrying it forward to the next following financial year or by distributing it, together with carried forward profits, distributable reserves or share premium to the shareholders, each Ordinary Share entitling to the same proportion in such distributions.
132
Table of Contents
The board of directors may resolve that Alvotech pays out an interim dividend to the shareholders, subject to the conditions of article 461-3 of the Luxembourg Company Law and Alvotech’s articles of association. The board of directors shall set the amount and the date of payment of the interim dividend.
Any share premium, assimilated premium or other distributable reserve may be freely distributed to the shareholders subject to the provisions of the Luxembourg Company Law and Alvotech’s articles of association.
Distributions may be lawfully declared and paid only if our net profits and/or distributable reserves are sufficient under Luxembourg Company Law.
Thus, in case of a dividend payment, each shareholder is entitled to receive a dividend right pro rata according to his or her respective shareholding. The dividend entitlement lapses upon the expiration of a five-year prescription period from the date of the dividend distribution. The unclaimed dividends return to Alvotech’s accounts. However, Alvotech does not anticipate paying cash dividends on our Ordinary shares in the foreseeable future.
A Luxembourg withholding tax of 15% is generally due on dividends and similar distributions made by us to our shareholders, unless a reduced treaty rate or the participation exemption applies. No withholding tax is levied on capital gains and liquidation proceeds
Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our
operations. See also “Item 3. Key Information—D. Risk factors—Legal proceedings that carry risk may occur from time to time, and their outcome may be uncertain” and “Item 3. Key Information—D. Risk factors—Our Canadian partner, JAMP, is involved in legal proceedings adverse to AbbVie that may have an impact on our AVT02 product in Canada.” We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
B.Significant Changes
Please see Note 30. Subsequent Events, included in the audited consolidated financial statements starting at page F-1 included elsewhere in this Form 20-F. Other than the events included in this note, no significant changes have occurred.
Item 9. The Offer and Listing.
A.Offer and Listing Details
Ordinary Shares and Warrants are listed on The Nasdaq Stock Market LLC under the symbols ALVO and ALVOW, respectively. Ordinary Shares are also listed on the Nasdaq Iceland Main Market under the ticker symbol “ALVO” since 8 December 2022, and, prior to that, on the Nasdaq First North Growth Market since 23 June 2022 until their admission to trading to the Nasdaq Iceland Main Market. Prior to 15 June 2022, there was no public trading market for Alvotech’s Ordinary Shares or Warrants.
B.Plan of Distribution
Not applicable.
C.Markets
Ordinary Shares and Warrants are listed on The Nasdaq Stock Market LLC under the symbol “ALVO” and “ALVOW”, respectively, since 16 June 2022. Ordinary Shares are also listed on the Nasdaq Iceland Main Market under the ticker symbol “ALVO” since 8 December 2022 and, prior to that, on the Nasdaq First North Growth Market since 23 June 2022 until their admission to trading to the Nasdaq Iceland Main Market.
D.Selling Shareholders
Not applicable.
133
Table of Contents
E.Dilution
Not applicable.
F.Expenses of the Issue
Not applicable.
Item 10. Additional Information.
A.Share Capital
Not applicable.
B.Memorandum and Articles of Association
A copy of our Amended and Restated Articles of Association have been previously filed as Exhibit 99.3 to our report on Form 6-K filed with the SEC on 6 June, 2023, and is incorporated by reference into this Annual Report.
The information set forth in Exhibit 2.11 is incorporated herein by reference. There are no limitations on the rights to own securities, including the rights of non-resident or foreign shareholders to hold or exercise voting rights on the securities imposed by the laws of Luxembourg or by our Articles.
C.Material Contracts
In addition to the contracts described elsewhere in this Annual Report, the following are summaries of each material contract to which we are a party for the two years preceding the date of this Annual Report. For additional information on our material contracts, please see “Item 4. Information on the Company,” “Item 6. Directors, Senior Management and Employees,” and “Item 7.B Related Party Transactions” of this Annual Report.
Amendment to the Senior Bonds
On 14 December 2018, Alvotech Holdings issued $300.0 million in convertible bonds. The offering included $125.0 million of Tranche A bonds (the “Tranche A Senior Bonds”) that included a guarantee from Alvogen Lux Holdings S.à r.l. (“Alvogen”) and a 10% bonus if the bondholders converted at the time of an initial public offering (“IPO”). In addition, $175.0 million of Tranche B bonds (the “Tranche B Senior Bonds” and, together with the Tranche A Senior Bonds, the “Existing Senior Bonds”, which includes, for the avoidance of doubt, the additional bonds issued in June 2021 as further described below) were issued that did not have a guarantee but included a 25% bonus if the bondholders elected to convert at the time of an Initial Public Offering (the "IPO"). The bonds offered a 15% payment-in-kind interest rate and a put option to sell the bonds back to Alvotech if an IPO had not occurred within three years from the original date of issuance.
On 24 June 2021, holders of the Existing Senior Bonds converted $100.7 million of principal and accrued interest and $4.8 million of additional premium offered by Alvotech Holdings to the bondholders into 455,687 Class A ordinary shares of Alvotech Holdings. Following the conversion, certain bondholders elected to redeem their remaining Existing Senior Bonds for cash, resulting in the payment of $54.1 million in outstanding principal and accrued interest plus an additional $6.1 million of premium that the bondholders elected to be paid in cash. The remaining unconverted and unredeemed Existing Senior Bonds were rolled over into new bonds with an extended maturity of June 2025 and the elimination of conversion rights, among other amendments to the terms and conditions. Such Existing Senior Bonds, including an additional premium of $2.6 million and an extension premium of $8.1 million offered to the bondholders in the form of additional bonds, totaled $280.9 million. Alvotech Holdings also issued an additional $113.8 million of Existing Senior Bonds to one previous bondholder and one new bondholder.
In January and June of 2022, Alvotech Holdings amended the terms of the outstanding bonds. The amendments resulted in the interest rate on the bonds ranging from 7.5% to 10.0%, depending on the amount of aggregate net proceeds following the closing of the business combination by and among Oaktree Acquisition Corp. II, Alvotech Holdings and Alvotech (the “Business Combination”). Additionally, Alvotech made a payment of a $5.0 million consent fee to the bondholders who did not vote against the Business Combination Agreement dated as of 7 December 2021, as amended, by and among OACB, Alvotech Holdings and Alvotech. The payment was made in July 2022. The amendment also included a requirement for Alvotech to maintain a minimum of $25.0 million of restricted cash in a separate liquidity account. As a result of the closing of the Business Combination, there was a change in cash flows on the bonds related to the increase in
134
Table of Contents
interest rate from 7.5% to 10.0%. Alvotech remeasured the carrying value in accordance with IFRS 9 to the present value of the revised cash flows and recognized a $6.5 million loss on the remeasurement of the Existing Senior Bonds.
On 16 November 2022, Alvotech and the bondholders amended and restated certain terms and conditions of the Existing Senior Bonds and issued new senior bonds in an aggregate principal amount equal to $70.0 million (the “New Senior Bonds” and, together with the Existing Senior Bonds, the “Senior Bonds”). The New Senior Bonds were issued subject to the terms of the Existing Senior Bonds, as amended and restated.
The coupon rate applicable to the Senior Bonds is 12.00% per annum, that, subject to certain step down provisions, may be lowered to 11.375% (if Alvotech raises more than $75.0 million but less than $150.0 million in net proceeds from the issuance of new equity) or 10.75% (if Alvotech raises more than $150.0 million in net proceeds from the issuance of new equity). Alvotech shall use commercially reasonable endeavors to procure that the aggregate amount of the net proceeds of all new equity is (i) not less than $75.0 million by 15 December 2022, and (ii) not less than $150.0 million by 31 March 2023, inclusive of any net proceeds raised in (i). This step down provision is subject to certain further conditions, including the FDA approval of a biologics license application for AVT02 on or before 31 March 2023.
For interest accrued until (and including) 15 December 2023, Alvotech has the option to elect that interest accrued in excess of 8.50% per annum be capitalized and added to the outstanding principal amount of the Senior Bonds then outstanding. Interest accrued as of (and including) 16 December 2023 will be payable in cash in arrears on each coupon payment date.
In addition, Alvotech is required to (i) grant the bondholders penny warrants representing 1.5% of its fully diluted ordinary share capital outstanding as at 15 December 2022 if the aggregate amount of the net proceeds of all new equity issuances from November 16, 2022 through 15 December 2022 is less than $75.0 million; and (ii) grant the bondholders penny warrants representing 1.00% of its fully diluted ordinary share capital outstanding as at 31 March 2023 if the aggregate amount of the net proceeds of all new equity issuances from 16 November 2022 through 31 March 2023 (inclusive of any net proceeds raised in (i)) is less than $15.0 million. Each warrant will entitle the bondholders, upon exercise, to receive from Alvotech one fully paid and non-assessable ordinary share of Alvotech, at the exercise price of one cent ($0.01) per share. Since Alvotech had not raised $75.0 million by 15 December 2022, Alvotech issued 4,198,807 warrants to the bondholders on 31 December 2022. Each new warrant entitles the bondholders, upon exercise, to receive from Alvotech one fully paid and non-assessable Ordinary Share, at the exercise price of one cent ($0.01) per share. Pursuant to the terms of the warrant, Alvotech is required to register Ordinary Shares underlying the warrants for resale on or before 15 July 2023. Following the issuance of the 2022 Convertible Bonds and the closing of the private placement of Ordinary Shares for gross proceeds of $137.0 million on 10 February 2023, we are not obligated to issue the additional 1.00% warrants to the bondholders.
The bondholders will be entitled to appoint one observer to receive all information provided to, and attend meetings of, Alvotech’s board of directors (and any committees or groups thereunder).
2022 Convertible Bonds
On December 20, 2022, the Company issued two tranches of convertible bonds, Tranche A is ISK denominated with a principal balance of $59.1 million, of which $3.5 million in cash proceeds were received subsequent to December 31, 2022, and carries an annual payment-in-kind interest rate of 15% per year, while tranche B is USD denominated with a principal balance of $0.6 million and carries an annual payment-in-kind interest rate of 12.5% per year. The maturity date of the convertible bonds is the later of the (i) 20 December 2025 or (ii) 91 days after the earlier of the full redemption or the final maturity date of the Senior Bonds. Holders of both the Tranche A and Tranche B convertible bonds, may elect, at their sole discretion, to convert all or part of the principal amount and accrued interest into Alvotech Ordinary Shares at a conversion price of $10.00 per share on December 31, 2023, June 30, 2024. or when the bond has been called or put up for redemption, including on the maturity date
On 25 January 2023, the Company issued an additional $10.0 million of Tranche B Convertible Bonds.
On 24 July 2023, Alvotech announced that Teva and Alvotech have agreed to expand their existing strategic partnership agreement. As part of the agreement, Teva acquired Tranche B Convertible Bonds in principal amount of $40 million.
On 31 July 2023, Alvotech completed a private placement of Tranche A Convertible Bonds for a total principal amount of $100 million, or approximately ISK 13 billion at current exchange rates. As part of this private placement, ATP Holdings ehf., which is affiliated with Aztiq, acquired Tranche A Convertible Bonds in principal amount of $30 million.
135
Table of Contents
D.Exchange Controls
There are no foreign exchange controls or foreign exchange regulations under the currently applicable laws of the Grand Duchy of Luxembourg.
E.Taxation
Material Luxembourg Tax Considerations
Tax Residency
A holder of Ordinary Shares or Warrants will not become resident, nor be deemed to be resident, in Luxembourg solely by virtue of holding and/or disposing of Ordinary Shares or Warrants or the execution, performance, delivery and/or enforcement of his or her rights thereunder.
Income Tax
For the purposes of this section, a “disposal” may include a sale, an exchange, a contribution, a redemption and any other kind of alienation of Ordinary Shares or Warrants.
Luxembourg Non-Residents
Non-resident holders of Ordinary Shares or Warrants, who have neither a permanent establishment nor a permanent representative in Luxembourg to which or whom Ordinary Shares or Warrants are attributable, are not liable to any Luxembourg income tax, whether they receive payments of dividends or realize capital gains on the disposal of Ordinary Shares or Warrants, except with respect to capital gains realized on a substantial participation before the acquisition or within the first six months of the acquisition thereof, or where the non-resident holder has been a former Luxembourg resident for more than 15 years and has become a non-resident, at the time of transfer, less than five years ago, that are subject to income tax in Luxembourg at ordinary rates (subject to the provisions of any relevant double tax treaty).
Non-resident holders of Ordinary Shares or Warrants having a permanent establishment or a permanent representative in Luxembourg to which or whom Ordinary Shares or Warrants are attributable, must include any income received, as well as any gain realized on the disposal of Ordinary Shares or Warrants, in their taxable income for Luxembourg tax assessment purposes, unless the conditions of the participation exemption regime, as described below, are satisfied. If the conditions of the participation exemption regime are not fulfilled, 50% of the gross amount of dividends received by a Luxembourg permanent establishment or permanent representative are however exempt from income tax. Taxable gains are determined as being the difference between the price for which Ordinary Shares have been disposed of and the lower of their cost or book value.
Under the participation exemption regime (subject to the relevant anti-abuse rules), dividends derived from Ordinary Shares may be exempt from income tax if cumulatively (i) Ordinary Shares are attributable to a qualified permanent establishment (“Qualified Permanent Establishment”) and (ii) at the time the dividend is put at the disposal of the Qualified Permanent Establishment, it holds or commits itself to hold for an uninterrupted period of at least 12 months Ordinary Shares or Warrants representing either (a) a direct participation in the share capital of Alvotech of at least 10% or (b) a direct participation of an acquisition price of at least €1.2 million. A Qualified Permanent Establishment means (a) a Luxembourg permanent establishment of a company covered by Article 2 of the Parent-Subsidiary Directive, (b) a Luxembourg permanent establishment of a capital company (société de capitaux) resident in a State having a double tax treaty with Luxembourg and (c) a Luxembourg permanent establishment of a capital company (société de capitaux) or a cooperative company (société coopérative) resident in an EEA country other than an EU Member State. Liquidation proceeds are assimilated to a received dividend and may be exempt under the same conditions. Ordinary Shares held through a tax transparent entity are considered as being a direct participation proportionally to the percentage held in the net assets of the transparent entity.
Under the participation exemption regime (subject to the relevant anti-abuse rules), capital gains realized on Ordinary Shares or Warrants may be exempt from income tax (save for the recapture rules) if cumulatively (i) Ordinary Shares or Warrants are attributable to a Qualified Permanent Establishment and (ii) at the time the capital gain is realized, the Qualified Permanent Establishment holds or commits itself to hold for an uninterrupted period of at least 12 months Ordinary Shares or Warrants representing either (a) a direct participation in the share capital of Alvotech of at least 10% or (b) a direct participation of an acquisition price of at least €6 million.
136
Table of Contents
Net Worth Tax
A Luxembourg resident as well as a non-resident who has a permanent establishment or a permanent representative in Luxembourg to which Ordinary Shares or Warrants are attributable, are subject to Luxembourg NWT (subject to the application of the participation exemption regime) on such Ordinary Shares or Warrants, except if the holder of Ordinary Shares or Warrants is (i) a resident or non-resident individual taxpayer, (ii) a securitization company governed by the amended law of 22 March 2004 on securitization, (iii) a company governed by the amended law of 15 June 2004 on venture capital vehicles, (iv) a professional pension institution governed by the amended law of 13 July 2005, (v) a specialized investment fund governed by the amended law of 13 February 2007, (vi) a family wealth management company governed by the law of 11 May 2007, (vii) an undertaking for collective investment governed by the amended law of 17 December 2010 or (viii) a reserved alternative investment fund governed by the amended law of 23 July 2016.
However, (i) a securitization company governed by the amended law of 22 March 2004 on securitization, (ii) a company governed by the amended law of 15 June 2004 on venture capital vehicles (iii) a professional pension institution governed by the amended law dated 13 July 2005 and (iv) an opaque reserved alternative investment fund treated as a venture capital vehicle for Luxembourg tax purposes and governed by the amended law of 23 July 2016 remain subject to the minimum NWT.
Other Taxes
Under current Luxembourg tax laws, no registration tax or similar tax is in principle payable by the holder of Ordinary Shares or Warrants upon the acquisition, holding or disposal of Ordinary Shares or Warrants. However, a fixed or ad valorem registration duty may be due upon the registration of Ordinary Shares or Warrants in Luxembourg in the case where Ordinary Shares or Warrants are physically attached to a public deed or to any other document subject to mandatory registration, as well as in the case of a registration of Ordinary Shares or Warrants on a voluntary basis.
No inheritance tax is levied on the transfer of Ordinary Shares or Warrants upon death of a holder in cases where the deceased was not a resident of Luxembourg for inheritance tax purposes at the time of his death.
Gift tax may be due on a gift or donation of Ordinary Shares or Warrants if the gift is recorded in a Luxembourg notarial deed or otherwise registered in Luxembourg.
Material U.S. Federal Income Tax Considerations for U.S. Holders
The following is a discussion of certain material U.S. federal income tax considerations generally applicable to the acquisition, ownership, and disposition of Ordinary Shares by a “U.S. Holder.” This discussion applies only to Ordinary Shares that are held by a U.S. Holder as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not describe all U.S. federal income tax considerations that may be relevant to a U.S. Holder in light of such U.S. Holder’s particular circumstances, nor does it address any state, local, or non-U.S. tax considerations, any non-income tax (such as gift or estate tax) considerations, the alternative minimum tax, the special tax accounting rules under Section 451(b) of the Code, the Medicare contribution tax on net investment income, or any tax consequences that may be relevant to U.S. Holders that are subject to special tax rules, including, without limitation:
•banks or other financial institutions;
•insurance companies;
•mutual funds;
•pension or retirement plans;
•S corporations;
•broker or dealers in securities or currencies;
•traders in securities that elect mark-to-market treatment;
•regulated investment companies;
•real estate investment trusts;
•trusts or estates;
•tax-exempt organizations (including private foundations);
137
Table of Contents
•persons that hold Ordinary Shares as part of a “straddle,” “hedge,” “conversion,” “synthetic security,” “constructive sale,” or other integrated transaction for U.S. federal income tax purposes;
•persons that have a functional currency other than the U.S. dollar;
•certain U.S. expatriates or former long-term residents of the United States;
•persons owning (directly, indirectly, or constructively) 5% (by vote or value) or more of our stock;
•persons that acquired Ordinary Shares pursuant to an exercise of employee stock options or otherwise as compensation;
•partnerships or other entities or arrangements treated as pass-through entities for U.S. federal income tax purposes and investors in such entities;
•“controlled foreign corporations” within the meaning of Section 957(a) of the Code;
•“passive foreign investment companies” within the meaning of Section 1297(a) of the Code; and
•corporations that accumulate earnings to avoid U.S. federal income tax.
If a partnership (including an entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds Ordinary Shares, the tax treatment of a partner in such partnership generally will depend on the status of the partner and the activities of the partnership and the partner. Partnerships holding Ordinary Shares should consult their tax advisors regarding the tax consequences in their particular circumstances.
This discussion is based on the Code, the U.S. Treasury regulations promulgated thereunder, administrative rulings, and judicial decisions, all as currently in effect and all of which are subject to change or differing interpretation, possibly with retroactive effect. Any such change or differing interpretation could alter the tax consequences described herein. Furthermore, there can be no assurance that the Internal Revenue Service (the “IRS”) will not challenge the tax considerations described herein and that a court will not sustain such challenge.
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of Ordinary Shares, that is, for U.S. federal income tax purposes:
•an individual who is a U.S. citizen or resident of the United States;
•a corporation (including an entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia;
•an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
•a trust (i) if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more “United States persons” within the meaning of Section 7701(a)(30) of the Code have the authority to control all substantial decisions of the trust or (B) that has in effect a valid election under applicable U.S. Treasury regulations to be treated as a United States person.
THIS DISCUSSION IS FOR GENERAL INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. U.S. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES OF THE ACQUISTION, OWNERSHIP, AND DISPOSITION OF ORDINARY SHARES IN THEIR PARTICULAR CIRCUMSTANCES.
Distributions on Ordinary Shares
Subject to the PFIC rules discussed below under “—Passive Foreign Investment Company Rules,” distributions on Ordinary Shares generally will be taxable as a dividend for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Such distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the applicable U.S. Holder’s adjusted tax basis in its Ordinary Shares. Any remaining excess will be treated as gain realized on the sale or other taxable disposition of Ordinary Shares and will be treated as described below under “—Sale or Other Taxable Disposition of Ordinary Shares.” The amount of any such distributions will include any amounts required to be withheld by us (or another applicable withholding agent) in respect of any non-U.S. taxes. Any such amount treated as a dividend will be treated as foreign-source dividend income. Any such dividends received by a corporate U.S. Holder generally will not qualify for the dividends-received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations. With respect to non-corporate U.S. Holders, any such dividends generally will be taxed at currently preferential long-term capital gains rates only if (i) Ordinary Shares
138
Table of Contents
are readily tradable on an established securities market in the United States or we are eligible for benefits under an applicable tax treaty with the United States, (ii) we are not treated as a PFIC with respect to the applicable U.S. Holder at the time the dividend was paid or in the preceding year, and (iii) certain holding period and other requirements are met. Any such dividends paid in a currency other than the U.S. dollar generally will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars at that time. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of actual or constructive receipt.
As noted above and subject to applicable limitations, taxing jurisdictions other than the United States may withhold taxes from distributions on Ordinary Shares, and a U.S. Holder may be eligible for a reduced rate of withholding to the extent there is an applicable tax treaty between the applicable taxing jurisdiction and the United States and/or may be eligible for a foreign tax credit against the U.S. Holder’s U.S. federal income tax liability. Recently issued U.S. Treasury regulations, which apply to foreign taxes paid or accrued in taxable years beginning on or after 28 December 2021, may in some circumstances prohibit a U.S. Holder from claiming a foreign tax credit with respect to certain foreign taxes that are not creditable under applicable tax treaties. In lieu of claiming a foreign tax credit, a U.S. Holder may, at such U.S. Holder’s election, deduct foreign taxes in computing such U.S. Holder’s taxable income, subject to generally applicable limitations under U.S. tax law. An election to deduct foreign taxes in lieu of claiming a foreign tax credit applies to all foreign taxes paid or accrued in the taxable year in which such election is made. The foreign tax credit rules are complex and U.S. Holders should consult their tax advisers regarding the application of such rules, including the creditability of foreign taxes, in their particular circumstances.
Sale or Other Taxable Disposition of Ordinary Shares
Subject to the PFIC rules discussed below under “—Passive Foreign Investment Company Rules,” upon any sale or other taxable disposition of Ordinary Shares, a U.S. Holder generally will recognize gain or loss in an amount equal to the difference, if any, between (i) the sum of (A) the amount of cash and (B) the fair market value of any other property received in such sale or disposition and (ii) the U.S. Holder’s adjusted tax basis in the Ordinary Shares. Any such gain or loss generally will be capital gain or loss and will be long-term capital gain or loss if the U.S. Holder’s holding period for such Ordinary Shares exceeds one year. Long-term capital gain recognized by non-corporate U.S. Holders generally will be taxed at currently preferential long-term capital gains rates. The deductibility of capital losses is subject to limitations. For foreign tax credit purposes, any such gain or loss generally will be treated as U.S. source gain or loss.
If the consideration received by a U.S. Holder upon a sale or other taxable disposition of Ordinary Shares is not paid in U.S. dollars, the amount realized will be the U.S. dollar value of such payment calculated by reference to the exchange rate in effect on the date of such sale or disposition. A U.S. Holder may have foreign currency gain or loss to the extent of the difference, if any, between (i) the U.S. dollar value of such payment on the date of such sale or disposition and (ii) the U.S. dollar value of such payment calculated by reference to the exchange rate in effect on the date of settlement.
U.S. Holders should consult their tax advisors regarding the tax consequences of a sale or other taxable disposition of Ordinary Shares, including the creditability of foreign taxes imposed on such sale or disposition by a taxing jurisdiction other than the United States, in their particular circumstances.
Passive Foreign Investment Company Rules
The U.S. federal income tax treatment of U.S. Holders could be materially different from that described above if we are treated as a PFIC for U.S. federal income tax purposes. A non-U.S. corporation generally will be treated as a PFIC for U.S. federal income tax purposes if either (i) at least 75% of its gross income in a taxable year, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income or (ii) at least 50% of its assets in a taxable year (ordinarily determined based on fair market value and averaged quarterly over the year), including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
Based on our analysis of our income, assets, activities and market capitalization, we believe that we were not treated as a PFIC for our taxable year, ended 31 December 2023. However, the determination of whether a non-U.S. corporation is a PFIC is a fact-intensive determination made on an annual basis and the applicable law is subject to varying interpretation. In particular, the characterization of our assets as active or passive may depend in part on our current and intended future business plans, which are subject to change. In addition, the total value of our assets for PFIC testing purposes may be determined in part by reference to the market price of Ordinary Shares from time to time, which may fluctuate
139
Table of Contents
considerably. As a result, there can be no assurance with respect to our status as a PFIC for any taxable year, and our U.S. counsel expresses no opinion with respect to our PFIC status for any taxable year.
Although PFIC status is generally determined annually, if we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder in its Ordinary Shares and the U.S. Holder did not make either a mark-to-market election or a qualifying electing fund (“QEF”) election or, which are referred to collectively as the “PFIC Elections” for purposes of this discussion, for the first taxable year in which we are treated as a PFIC, and in which the U.S. Holder held (or was deemed to hold) Ordinary Shares, or the U.S. Holder does not otherwise make a purging election, as described below, the U.S. Holder generally will be subject to special and adverse rules with respect to (i) any gain recognized by the U.S. Holder on the sale or other taxable disposition of its Ordinary Shares and (ii) any “excess distribution” made to the U.S. Holder (generally, any distributions to the U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by the U.S. Holder in respect of its Ordinary Shares during the three preceding taxable years of the U.S. Holder or, if shorter, the U.S. Holder’s holding period in its Ordinary Shares).
Under these rules:
•the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period in its Ordinary Shares;
•the amount allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess distribution, and to any period in the U.S. Holder’s holding period before the first day of the first taxable year in which we are treated as a PFIC, will be taxed as ordinary income;
•the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in the U.S. Holder’s holding period will be taxed at the highest tax rate in effect for that year and applicable to the U.S. Holder; and
•an additional tax equal to the interest charge generally applicable to underpayments of tax will be imposed on the U.S. Holder with respect to the tax attributable to each such other taxable year of the U.S. Holder.
PFIC Elections
If we are treated as a PFIC and Ordinary Shares constitute “marketable stock,” a U.S. Holder may avoid the adverse PFIC tax consequences discussed above if such U.S. Holder makes a mark-to-market election with respect to its Ordinary Shares for the first taxable year in which the U.S. Holder holds (or is deemed to hold) Ordinary Shares and each subsequent taxable year. Such U.S. Holder generally will include for each of its taxable years as ordinary income the excess, if any, of the fair market value of its Ordinary Shares at the end of such year over its adjusted tax basis in its Ordinary Shares. The U.S. Holder also will recognize an ordinary loss in respect of the excess, if any, of its adjusted tax basis in its Ordinary Shares over the fair market value of its Ordinary Shares at the end of its taxable year (but only to the extent of the net amount of previously included income as a result of the mark-to-market election). The U.S. Holder’s adjusted tax basis in its Ordinary Shares will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of its Ordinary Shares will be treated as ordinary income.
The mark-to-market election is available only for “marketable stock,” generally, stock that is regularly traded on a national securities exchange that is registered with the Securities and Exchange Commission, including the Nasdaq (on which Ordinary Shares are currently listed), or on a foreign exchange or market that the IRS determines has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. As such, such election generally will not apply to any of our non-U.S. subsidiaries, unless the shares in such subsidiaries are themselves “marketable stock.” As such, U.S. Holders may continue to be subject to the adverse PFIC tax consequences discussed above with respect to any lower-tier PFICs, as discussed below, notwithstanding their mark-to-market election with respect to Ordinary Shares.
If made, a mark-to-market election would be effective for the taxable year for which the election was made and for all subsequent taxable years unless Ordinary Shares cease to qualify as “marketable stock” for purposes of the PFIC rules or the IRS consents to the revocation of the election. U.S. Holders should consult their tax advisors regarding the availability and tax consequences of a mark-to-market election with respect to Ordinary Shares in their particular circumstances.
The tax consequences that would apply if we were a PFIC and a U.S. Holder made a valid QEF election would also be different from the adverse PFIC tax consequences described above. In order to comply with the requirements of a QEF election, however, a U.S. Holder generally must receive a PFIC Annual Information Statement from us. If we are determined to be a PFIC for any taxable year, we do not currently intend to provide the information necessary for U.S.
140
Table of Contents
Holders to make or maintain a QEF election. As such, U.S. Holders should assume that a QEF election will not be available with respect to Ordinary Shares.
If we are treated as a PFIC and a U.S. Holder failed or was unable to timely make a PFIC Election for prior periods, the U.S. Holder might seek to make a purging election to rid its Ordinary Shares of the PFIC taint. Under the purging election, the U.S. Holder will be deemed to have sold its Ordinary Shares at their fair market value and any gain recognized on such deemed sale will be treated as an excess distribution, as described above. As a result of the purging election, the U.S. Holder will have a new adjusted tax basis and holding period in Ordinary Shares solely for purposes of the PFIC rules.
Related PFIC Rules
If we are treated as a PFIC and, at any time, has a non-U.S. subsidiary that is treated as a PFIC, a U.S. Holder generally would be deemed to own a proportionate amount of the shares of such lower-tier PFIC, and generally could incur liability for the deferred tax and interest charge described above if we receive a distribution from, or sell or otherwise dispose of all or part of our interest in, such lower-tier PFIC, or the U.S. Holder otherwise was deemed to have sold or otherwise disposed of an interest in such lower-tier PFIC. U.S. Holders should consult their tax advisors regarding the application of the lower-tier PFIC rules in their particular circumstances.
A U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year, may have to file an IRS Form 8621 (whether or not a QEF election or a mark-to-market election is made) and to provide such other information as may be required by the U.S. Treasury Department. Failure to do so, if required, will extend the statute of limitations applicable to such U.S. Holder until such required information is furnished to the IRS and could result in penalties
THE PFIC RULES ARE VERY COMPLEX AND U.S. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE APPLICATION OF SUCH RULES IN THEIR PARTICULAR CIRCUMSTANCES.
Information Reporting and Backup Withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the U.S. Holder’s U.S. federal income tax liability and may entitle the U.S. Holder to a refund, provided that the required information is timely furnished to the IRS.
U.S. Holders should consult their tax advisors regarding the information reporting requirements and the application of the backup withholding rules in their particular circumstances.
THIS DISCUSSION IS FOR GENERAL INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. U.S. HOLDERSSHOULD CONSULT THEIR TAX ADVISORS REGARDING THE U.S. FEDERAL, STATE, AND LOCAL AND NON-U.S. INCOME AND NON-INCOME TAX CONSEQUENCES OF THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF ORDINARY SHARES, INCLUDING THE IMPACT OF ANY POTENTIAL CHANGE IN LAW, IN THEIR PARTICULAR CIRCUMSTANCES.
F.Dividends and Paying Agents
Not applicable.
G.Statement by Experts
Not applicable.
H.Documents on Display
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing
141
Table of Contents
and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm.
We maintain a corporate website at www.alvotech.com. We intend to post our Annual Report on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference.
The Securities and Exchange Commission maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants, such as us, that file electronically with the SEC.
With respect to references made in this Annual Report to any contract or other document of our Company, such references are not necessarily complete, and you should refer to the exhibits attached or incorporated by reference to this Annual Report for copies of the actual contract or document.
I.Subsidiary Information
Not applicable.
J.Annual Report to Security Holders
We intend to submit any annual report provided to security holders in electronic format as an exhibit to a current report on Form 6-K.
Item 11. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks that may result in changes of foreign currency exchange rates and interest rates, as well as the overall change in economic conditions in the countries where we conduct business. As of 31 December 2023, and 2022, we had cash and cash equivalents of $11.2 million and $66.4 million, respectively, excluding restricted cash. Our cash and cash equivalent include both cash in banks and cash on hand. Additional information regarding the Group's management of financial risks relating to its operations can be found in Note 28 of the audited consolidated financial statements, included elsewhere in this Form 20-F.
Foreign currency exchange risk
We are subject to foreign exchange risk in our operations, as some of our financial assets and financial liabilities are denominated in currencies other than the functional currency of our subsidiaries. Any strengthening or weakening of our significant foreign currencies against the USD could impact the measurement of financial instruments in a foreign currency and affect equity. Our significant asset and liabilities denominated in foreign currencies as 31 December 2023, and 31 December 2022 are denominated in EUR, GBP, ISK and CHF. We analyze at the end of each year the sensitivity to foreign currency exchange changes. Specifically, we have performed an analysis to understand the impact of an increase or decrease of a 10% strengthening or weakening of each significant foreign currency, keeping all other variables consistent, as of 31 December 2023. Through this analysis, we note that the only foreign currency that had a material impact was ISK, while all other currencies did not significantly fluctuate. Refer to Note 28 of the consolidated financial statements included elsewhere in this Annual Report on Form 20-F for further information.
Interest rate risk
Our interest-bearing investments and borrowings are subject to interest rate risk. The majority of our borrowings are subject to fixed interest rate. Our exposure to the risk of fluctuations in market interest rates primarily relates to the cash in banks that is denominated with floating interest rates. We analyze at the end of each year the sensitivity to interest rate changes. Specifically, we have performed an analysis to understand the impact of an increase or decrease of a one hundred basis point on the interest rates, keeping all other variables consistent, as of 31 December 2023. Through this analysis, we note that the impacts of the interest rate sensitivity did not have a significant effect on loss before tax.
142
Table of Contents
Credit risk
We are exposed to credit risk from our operating activities, primarily trade receivables, and cash, cash equivalents and deposits held with banks and financial institutions. Cash, cash equivalents and deposits are maintained with high-quality financial institutions in Iceland, Europe and United States. We are also potentially subject to concentrations of credit risk in our trade receivables. Concentrations of credit risk are with respect to trade receivables owed by a limited number of companies comprising our customer base. Our exposure to credit losses is low, however, owing largely to the credit quality of our collaboration partners which are significantly larger than us.
We continually monitor our positions with, and the credit quality of, the financial institutions and corporations, which are counterparts to our financial instruments and do not anticipate non-performance. The maximum default risk corresponds to the carrying amount of the financial assets shown in the statement of financial position. We monitor the risk of a liquidity shortage. The main factors considered here are the maturities of financial assets as well as expected cash flows from equity measures.
Liquidity Risk
Please see Item 5.B and risk factors, including “We may be unable to generate sufficient cash flow to satisfy our significant debt service obligations, which would adversely affect our financial condition and results of operations.” of this Annual Report.
Inflation Risk
We believe that inflation will have a general impact on our business in line with overall price increases, increases in the cost of borrowing, and operating in an inflationary economy. We cannot predict the timing, strength, or duration of any inflationary period or economic slowdown or its ultimate impact on the Company. If the conditions in the general economy significantly deviate from present levels and continue to deteriorate it could have a material adverse effect on the Group’s business, financial condition, results of operations and growth prospects.
Interim Periods
Not applicable.
Safe Harbor
This Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act and as defined in the Private Securities Litigation Reform Act of 1995. See “Special Note Regarding Forward-Looking Statements".
Item 12. Description of Securities Other than Equity Securities.
A.Debt Securities
Not applicable.
B.Warrants and Rights
Not applicable.
C.Other Securities
Not applicable.
D.American Depositary Shares
Not applicable.
143
Table of Contents
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies.
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds.
A.Not applicable.
B.Not applicable.
C.Not applicable.
D.Not applicable.
E.Use of Proceeds.
Not applicable.
Item 15. Controls and Procedures.
A.Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as that term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) that are designed to ensure that information required to be disclosed in the Company’s reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of 31 December 2023.
Based on the material weaknesses described below, our Chief Executive Officer and our Chief Financial Officer have concluded that, as of 31 December 2023, our disclosure controls and procedures were not effective. After giving full
consideration to these material weaknesses, and the additional analyses and other procedures that we performed to ensure
that our consolidated financial statements included in this Annual Report on Form 20-F were prepared in accordance with
IFRS, our management has concluded that our consolidated financial statements present fairly, in all material respects, our
financial position, results of operations and cash flows for the periods disclosed in conformity with IFRS.
B.Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as
such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act and for the
assessment of the effectiveness of our internal control over financial reporting.
Our internal control system was designed to provide reasonable assurance to our management and board of directors
regarding the reliability of financial reporting and the preparation and fair presentation of published financial statements for
external purposes in accordance with IFRS Accounting Standards as issued by the IASB. Because of its inherent
limitations, internal control over financial reporting, no matter how well designed, cannot provide absolute assurance of
achieving financial reporting objectives and may not prevent or detect misstatements. Therefore, even if the internal control
over financial reporting is determined to be effective it can provide only reasonable assurance with respect to financial
statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to
the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the
policies or procedures may deteriorate.
Management has assessed the effectiveness of our internal control over financial reporting as of 31 December 2023
using criteria described in the Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring
Organizations of the Treadway Commission. Based on its assessment, management concluded that our internal control over
financial reporting was not effective due to the identified material weaknesses in internal control over financial reporting as
described below.
144
Table of Contents
Identified Material Weakness
Alvotech has identified material weaknesses in the design and operating effectiveness of its internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
In connection with the preparation of the consolidated financial statements covered by this Annual Report, we identified the following material weaknesses:
(i) the Company did not have a sufficient number of trained professionals with an appropriate level of internal control knowledge, training and experience;
(ii) the Company did not consistently operate all controls, specifically related to consistent execution, adequate review procedures, and maintaining documentation to evidence control performance, including assessing the accuracy and completeness of information used in the execution of controls; and
(iii) the Company did not implement effective controls over the segregation of duties and certain information technology general controls for information systems that are relevant to the preparation of our financial statements.
These material weaknesses could result in a misstatement of Alvotech’s accounts or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected.
Remediation Activities
During the period covered by this Annual Report, we began implementing a remediation plan that is reasonably likely to materially affect, our internal control over financial reporting. This plan includes further developing and implementing formal policies, processes, internal controls and documentation relating to our financial reporting working towards the goal of effective control over financial reporting.
As part of this plan, we began taking steps intended to address the underlying causes of the control deficiencies in order to remediate the material weaknesses, which included the following activities during 2022 and 2023:
(i) Hired qualified individuals with strong technical accounting, internal control and SEC reporting experience and continued training control owners to reaffirm expectations as it relates to the control design and execution of such controls, including enhancements to the documentation to evidence the execution of the controls;
(ii) Enhanced the Company's governance and oversight processes by establishing a formal control governance structure, ensuring clear roles and responsibilities for control oversight, conducting regular meetings to review control performance, and implementing a system for reporting control-related matters to the Audit Committee;
(iii) Implemented formal documentation of certain policies and procedures, and/or redesigned entity level controls, business process-level controls across all significant accounts and information technology general controls across all relevant domains;
(iv) Developed and executed a risk-based testing plan to cover all identified controls through a mix of design assessment, independent testing of operating effectiveness and management self-certification. The Company has engaged outside consultants to assist in evaluating our internal controls, develop remediation plans to address control deficiencies identified, and actively measure compliance and remediation progress through a quarterly scorecard; and
(v) Continued implementation of a new enterprise resource planning (“ERP”) system including the engagement of outside consultants to help design and implement automated controls and enhance our information technology general controls environment as part of the ERP system implementation.
In addition to the above actions, we expect to continue engaging in the following additional remediation measures:
(i) Complete the implementation of a new ERP system, which includes increased automated functionality and controls for the preparation of the financial statements to prevent, among other things, unauthorized overrides, and enhance user access controls, segregation of duties with the system, and audit trails to track and monitor activities;
(ii) Implement stronger IT controls to ensure the integrity and security of financial information, including enhancing access and change management controls and implementing regular system monitoring and testing;
(iii) Continue focusing on consistent control execution, adequate review procedures, and improving control documentation, including the accuracy and completeness of information used in the performance of controls; and
(iv) Continue engaging outside consultants to assist in evaluating the internal controls, and actively measure compliance and remediation through quarterly scorecard.
145
Table of Contents
The Company believes that this structured and phased approach is essential in order to establish effective internal controls over financial reporting in a sustainable manner, which will also enable us to support and adapt to the Company’s continuous growth path. Management may also determine that it is necessary to modify the above-mentioned remediation efforts depending on the circumstances and Company needs. However, we cannot assure that our efforts will be effective, that we will be able to remedy these material weaknesses or that we will be able to prevent any future material weaknesses in our internal control over financial reporting. We plan to continue to address the material weaknesses identified by further improving our internal control over financial reporting, including designing and implementing additional procedures within our finance, manufacturing and supply chain, human resources and information technology departments.
We will not be able to conclude that we have remediated the material weaknesses until all relevant controls are fully implemented and have operated effectively for a sufficient period of time. See also “Item 3. Key Information—D. Risk factors—We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we experience additional material weaknesses in the future or otherwise are unable to develop and maintain an effective system of internal controls in the future, we may not be able to produce timely and accurate financial statements or comply with applicable laws and regulations, which may adversely affect investor confidence in us and, as a result, the value of Ordinary Shares.”
C.Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of the Company’s registered public accounting firm on management’s assessment of the Company’s internal control over financial reporting since we are an emerging growth company.
D.Changes in Internal Control Over Financial Reporting
Refer to “Item 15.A Disclosure Controls and Procedures—Remediation Activities” above for the changes in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) that occurred during the period covered by this Annual Report that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [Reserved]
Item 16A. Audit Committee Financial Expert
Our Board has determined that Dr. McGoldrick (Chair), Ms. Merchant and Mr. Davies each qualify as an “audit committee financial expert” as defined by SEC rules and have the requisite financial sophistication under the applicable rules and regulations of the Nasdaq Stock Market. Dr. McGoldrick (Chair), Ms. Merchant and Mr. Davies are independent as such term is defined in Rule 10A-3 under the Exchange Act and under the listing standards of the Nasdaq Stock Market.
Item 16B. Code of Ethics
Alvotech’s board of directors adopted a Code of Business Conduct applicable to the directors, executive officers and other team members that complies with the rules and regulations of Nasdaq, and Nasdaq Iceland Main Market, and the SEC. The Code of Ethics is available on Alvotech’s website. In addition, Alvotech posted on the Corporate Governance section of its website all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the Code of Ethics. The reference to Alvotech’s website address in this Annual Report on Form 20-F does not include or incorporate by reference the information on Alvotech’s website into this Annual Report on Form 20-F.
Item 16C. Principal Accountant Fees and Services
Deloitte ehf. has served as our independent registered public accountant since 2013 and has audited our consolidated financial statements for the years ended 31 December 2023 and 2022.
146
Table of Contents
The following table shows the aggregate fees for services rendered by Deloitte ehf. to us and our subsidiaries, in the fiscal years ended 31 December 2023 and 2022.
| Year Ended 31 December | |||||||||||
| (in thousands of dollars) | 2023 | 2022 | |||||||||
| Audit Fees | 2,876 | 2,615 | |||||||||
| Audit-Related Fees | 365 | 656 | |||||||||
| Tax Fees | 97 | 20 | |||||||||
| Total | 3,339 | 3,291 | |||||||||
| Auditor Name | Auditor Location | Auditor Firm ID | ||||||||||||
| Deloitte ehf. | Kópavogur, Iceland | 1490 | ||||||||||||
Audit fees. Audit fees consisted of fees for the audit of our annual financial statements and other professional services provided in connection with the statutory and regulatory filings or engagements, including fees for the review of our interim financial information.
Audit-related fees. Audit-related fees included fees for review of our current and historical financial information included in our SEC registration statements, including services that generally only the independent accountant can reasonably provide.
Tax Fees. Tax fees included fees for tax compliance, tax advice, and tax planning.
Audit and Risk Committee Pre-Approval Policies and Procedures
Our audit and risk committee reviews and pre-approves the scope and the cost of audit services related to us and permissible non-audit services performed by the independent auditors. All of the services related to us provided by Deloitte during the last fiscal year have been pre-approved by the audit and risk committee.
Item 16D. Exemptions from the Listing Standards for Audit Committees.
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers.
Not applicable.
Item 16F. Change in Registrant’s Certifying Accountant.
Not applicable.
Item 16G. Corporate Governance.
We are a “foreign private issuer,” as defined by the SEC. As a result, in accordance with Nasdaq rules, we comply with home country governance requirements and certain exemptions thereunder rather than complying with Nasdaq corporate governance standards. While we expect to voluntarily follow most Nasdaq corporate governance rules, we may choose to take advantage of the following limited exemptions:
•Exemption from quorum requirements for shareholder meetings. Luxembourg practice with respect to quorum requirements for shareholder meetings in lieu of the requirement under Nasdaq Listing Rules that the quorum be not less than 33 1/3% of the outstanding voting shares;
•Exemption from the Nasdaq rules applicable to domestic issuers requiring disclosure within four business days of any determination to grant a waiver of the code of business conduct and ethics to directors and officers;
•Exemption from the requirement to obtain shareholder approval for certain issuances of securities, including shareholder approval of share option plans and approval of other securities issuances;
147
Table of Contents
•Exemption from the requirement that our audit and risk committee have review and oversight responsibilities over all “related party transactions,” as defined in Item 7.B of this Annual Report on Form 20-F;
•Exemption from the requirement that a majority of the board of directors must be comprised of Independent Directors as defined in the Nasdaq listing standards. Three of our eight directors are independent as defined in Nasdaq listing standards and applicable SEC rules, and our board of directors has an independent audit and risk committee. In addition, the independence rules applicable to companies listed on the Icelandic Main Market differ from the rules of Nasdaq. One additional director is considered independent under the Icelandic rules but not under the Nasdaq listing rules; and
•Exemption from the requirements related to the composition of our compensation committee and nominating
and corporate governance committee.
Furthermore, Nasdaq Rule 5615(a)(3) provides that a foreign private issuer, such as Alvotech, may rely on home country corporate governance practices in lieu of certain of the rules in the Nasdaq Rule 5600 Series and Rule 5250(d), provided that we nevertheless comply with Nasdaq’s Notification of Noncompliance requirement (Rule 5625), the Voting Rights requirement (Rule 5640) and that we have an audit and risk committee that satisfies Rule 5605(c)(3), consisting of committee members that meet the independence requirements of Rule 5605(c)(2)(A)(ii). Although Alvotech is permitted to follow certain corporate governance rules that conform to Luxembourg requirements in lieu of many of the Nasdaq corporate governance rules, we comply with the Nasdaq corporate governance rules applicable to foreign private issuers.
Accordingly, our shareholders may not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq. We may utilize these exemptions for as long as we continue to qualify as a foreign private issuer.
Item 16H. Mine Safety Disclosure.
Not applicable.
Item 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
Item 16J. Insider Trading Policy.
Not applicable.
Item 16K. Cybersecurity.
Cybersecurity Risk management and strategy
Our cybersecurity risk management program aims to fully identify threats, to present and evaluate them transparently, to mitigate, and manage them proactively. We have developed and implemented a cybersecurity risk management process intended to protect the confidentiality, integrity, and availability of our critical systems and information.
Our cybersecurity risk management process guides us in making cybersecurity risk-informed decisions and provides the basis for evaluating and monitoring the cybersecurity risk profile of the Company. This process provides a shared understanding and promotes a consistent approach to cybersecurity risk management within the Company in line with our information security policy and includes a cybersecurity incident response plan.
As part of our cybersecurity risk management program, we review industry best practices, including the NIST (National Institute of Standards and Technology) Cybersecurity Framework and ISO (International Organization for Standardization) 27001 to manage information security. We periodically conduct ongoing internal and external vulnerability analyses, including simulated attack as well as external testing via a third-party to evaluate the effectiveness of our cybersecurity process and controls.
In an effort to minimize third-party risk, we have established a process to assess the security practices of third-party vendors and service providers and related risks. Our process includes a security assessment informed by vendor questionnaires and contractual security requirements related to data privacy for certain vendors.
148
Table of Contents
The SOC is responsible for investigating all security incidents and alerts including determining the threat type, incident scope and incident severity. Where appropriate, major incidents are escalated according to cybersecurity incident process.
Employee awareness and training are essential to our ability as a company to thwart cyber-attacks. We continuously raise employees’ risk awareness with mandatory, regular online training for all employees and complimentary awareness campaigns.
In 2023, we did not identify any risks from cybersecurity threats, including as a result of any previous cybersecurity incidents, that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial condition. Despite our efforts, we cannot eliminate all risks from cybersecurity threats, or provide assurance that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see “ Risks Related to Legal and Regulatory Compliance Matters - A breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of our confidential information in internal systems or those used by third party collaborator partners or other contractors or consultants, could compromise the confidentiality, integrity and availability of our confidential information in information technology systems, network-connected control systems and/or our data, interrupt the operation of our business and/or affect our reputation.” in Item 3. Key Information – D. Risk Factors.
Cybersecurity Governance
Our board of directors has overall oversight responsibility for our risk management strategy, and delegates information security and related risk management oversight to the Audit and Risk Committee. Members of the audit and risk committee receive regular updates from management, including the CIO and the Cybersecurity and Risks Council, regarding cybersecurity related matters. This includes existing and new cybersecurity risks, how management is addressing, managing and/or mitigating those risks, cybersecurity and data privacy incidents (if relevant), and the status of key information security initiatives.
The Cybersecurity and Risks Council overseas regular review of cybersecurity risk management activities, is responsible for the management of our cyber risk exposure and monitoring the effectiveness of the cybersecurity program, including but not limited to, our cybersecurity tools and controls, and is responsible for establishing and reviewing our risk tolerance for our cyber risk framework.
The cybersecurity and Risks Council includes the CIO, the Director of Corporate Security, the Director of IT Infrastructure and Operations, the Enterprise architect, the Director of Cybersecurity, and Cybersecurity specialist. Those employees have decades of experience in cybersecurity and operations, cybersecurity education, and certifications from various organizations. The Cybersecurity and Risks Council is responsible for mobilizing the Materialization Board, which includes representatives from Legal and Finance, to review identified incident.
149
Table of Contents
PART III
Item 17. Financial Statements.
See pages F-1 through F-66 of this Annual Report.
Item 18. Financial Statements.
Not applicable.
Item 19. Exhibits
EXHIBIT INDEX
| Incorporation By Reference | ||||||||||||||||||||||||||||||||
| Exhibit | Description | Schedule/ Form | File Number | Exhibit | File Date | |||||||||||||||||||||||||||
| 1.1* | 6-K | 001-41421 | 99.30 | 06.06.2023 | ||||||||||||||||||||||||||||
| 2.1 | 8-K | 001-39526 | 4.1 | 09.22.2020 | ||||||||||||||||||||||||||||
| 2.3 | 6-K | 001-41421 | 99.4 | 11.17.2022 | ||||||||||||||||||||||||||||
| 2.4 | 6-K | 001-41421 | 99.5 | 11.17.2022 | ||||||||||||||||||||||||||||
| 2.5 | 20-F | 001-41421 | 2.7 | 06.22.2022 | ||||||||||||||||||||||||||||
| 2.6 | 6-K | 001-41421 | 99.9 | 11.17.2022 | ||||||||||||||||||||||||||||
| 2.7 | 20-F | 001-41421 | 2.11 | 03.01.2023 | ||||||||||||||||||||||||||||
| 2.8 | 20-F | 001-41421 | 2.9 | 03.01.2023 | ||||||||||||||||||||||||||||
| 2.9 | 20-F | 001-41421 | 2.10 | 03.01.2023 | ||||||||||||||||||||||||||||
| 4.1†† | F-4 | 333-261773 | 10.1 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.2†† | F-4 | 333-261773 | 10.2 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.3†† | F-4 | 333-261773 | 10.3 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.4†† | F-4 | 333-261773 | 10.6 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.5†† | F-4 | 333-261773 | 10.7 | 12.20.2021 | ||||||||||||||||||||||||||||
150
Table of Contents
| Incorporation By Reference | ||||||||||||||||||||||||||||||||
| Exhibit | Description | Schedule/ Form | File Number | Exhibit | File Date | |||||||||||||||||||||||||||
| 4.6†† | F-4 | 333-261773 | 10.10 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.7†† | F-4 | 333-261773 | 10.11 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.8†† | F-4 | 333-261773 | 10.12 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.9†† | F-4 | 333-261773 | 10.13 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.10†† | F-4 | 333-261773 | 10.16 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.11†† | F-4 | 333-261773 | 10.17 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.12†† | F-4 | 333-261773 | 10.18 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.13†† | F-4 | 333-261773 | 10.17 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.14+ | F-4 | 333-261773 | 10.22 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.15 | F-4 | 333-261773 | 10.25 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.16†† | F-4 | 333-261773 | 10.26 | 12.20.2021 | ||||||||||||||||||||||||||||
| 4.17†† | F-4 | 333-261773 | 10.29 | 03.14.2022 | ||||||||||||||||||||||||||||
| 4.18†† | F-4 | 333-261773 | 10.31 | 04.19.2022 | ||||||||||||||||||||||||||||
| 4.19†† | F-4 | 333-261773 | 10.34 | 05.02.2022 | ||||||||||||||||||||||||||||
| 4.20# | 20-F | 001-41421 | 4.39 | 06.22.2022 | ||||||||||||||||||||||||||||
151
Table of Contents
| Incorporation By Reference | ||||||||||||||||||||||||||||||||
| Exhibit | Description | Schedule/ Form | File Number | Exhibit | File Date | |||||||||||||||||||||||||||
| 4.21 | F-1 | 333-266136 | 10.37 | 07.14.2022 | ||||||||||||||||||||||||||||
| 4.22 | a6-K | 001-41421 | 99.7 | 11.17.2022 | ||||||||||||||||||||||||||||
| 4.23 | 20-F | 001-41421 | 4.42 | 3/1/2023 | ||||||||||||||||||||||||||||
| 4.24 | 6-K | 001-41421 | 99.1 | 11.17.2022 | ||||||||||||||||||||||||||||
| 4 | 20-F | 001-41421 | 4.46 | 03.01.2023 | ||||||||||||||||||||||||||||
| 4.26†† | 20-F | 001-41421 | 4.47 | 03.01.2023 | ||||||||||||||||||||||||||||
| 4 | 6-K | 001-41421 | 99.4 | 07.12.2023 | ||||||||||||||||||||||||||||
| 8.1* | 20-F | 001-41421 | 8.1 | 03.01.2023 | ||||||||||||||||||||||||||||
| 12.1* | ||||||||||||||||||||||||||||||||
| 12.2* | ||||||||||||||||||||||||||||||||
| 13.1** | ||||||||||||||||||||||||||||||||
| 15.1* | ||||||||||||||||||||||||||||||||
| 97* | ||||||||||||||||||||||||||||||||
| 101.INS* | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document) | |||||||||||||||||||||||||||||||
| 101.SCH* | Inline XBRL Taxonomy Extension Schema Document | |||||||||||||||||||||||||||||||
| 101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||||||||||||||||||||||||
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document | |||||||||||||||||||||||||||||||
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document | |||||||||||||||||||||||||||||||
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |||||||||||||||||||||||||||||||
| 104 | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) | |||||||||||||||||||||||||||||||
__________
*Filed herewith.
**Furnished herewith.
†Certain schedules and exhibits to this Exhibit have been omitted pursuant to Company S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
††Certain confidential portions (indicated by brackets and asterisks) have been omitted from this exhibit.
+Certain schedules and exhibits to this Exhibit have been omitted pursuant to Regulation S-K Item 601(a)(5). The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
#Indicates a management contract or any compensatory plan, contract or arrangement
152
Table of Contents
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this report on its behalf.
20 March 2024
| ALVOTECH | ||||||||
| By: | /s/ Robert Wessman | |||||||
| Name: | Robert Wessman | |||||||
| Title: | Chief Executive Officer | |||||||
153
Table of Contents
Alvotech
_____________________
Consolidated Financial Statements as
of 31 December, 2023 and 2022 and
for the years ended 31 December 2023, 2022, and 2021
Table of Contents
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of Alvotech
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Alvotech and subsidiaries (the "Company") as of December 31, 2023 and 2022, the related consolidated statements of profit or loss and other comprehensive income or loss, changes in equity, and cash flows, for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board and adopted by the EU.
Going concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1.4 to the financial statements, the Company has suffered recurring losses from operations that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1.4. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte ehf.
Kópavogur, Iceland
March 20, 2024
We have served as the Company's auditor since 2013.
F-2
Table of Contents
Consolidated Statements of Profit or Loss and Other Comprehensive Income or Loss for the years ended 31 December 2023, 2022, and 2021
| USD in thousands, except for per share amounts | Notes | 2023 | 2022 | 2021 | |||||||||||||||||||
| Product revenue | 5 | 48,699 | 24,836 | — | |||||||||||||||||||
| License and other revenue | 5 | 42,735 | 58,193 | 36,772 | |||||||||||||||||||
| Other income | 1,948 | 1,988 | 2,912 | ||||||||||||||||||||
| Cost of product revenue | (160,856) | (64,095) | — | ||||||||||||||||||||
| Research and development expenses | (210,827) | (180,622) | (191,006) | ||||||||||||||||||||
| General and administrative expenses | (76,559) | (186,742) | (84,134) | ||||||||||||||||||||
| Operating loss | (354,860) | (346,442) | (235,456) | ||||||||||||||||||||
| Share of net loss of joint venture | 27 | (7,153) | (2,590) | (2,418) | |||||||||||||||||||
| Impairment loss on investment in joint venture | 27 | (21,519) | — | — | |||||||||||||||||||
| Finance income | 7 | 4,823 | 2,549 | 51,568 | |||||||||||||||||||
| Finance costs | 7 | (267,157) | (188,419) | (117,361) | |||||||||||||||||||
| Exchange rate differences | (5,183) | 10,566 | 2,681 | ||||||||||||||||||||
| (Loss) / gain on extinguishment of financial liabilities | 21 | — | (27,311) | 151,788 | |||||||||||||||||||
| Non-operating (loss) / profit | (296,189) | (205,205) | 86,258 | ||||||||||||||||||||
| Loss before taxes | (651,049) | (551,647) | (149,198) | ||||||||||||||||||||
| Income tax benefit | 10 | 99,318 | 38,067 | 47,694 | |||||||||||||||||||
| Loss for the year | (551,731) | (513,580) | (101,504) | ||||||||||||||||||||
| Other comprehensive income / (loss) | |||||||||||||||||||||||
| Item that will be reclassified to profit or loss in subsequent periods: | |||||||||||||||||||||||
| Exchange rate differences on translation of foreign operations | (86) | (6,111) | (305) | ||||||||||||||||||||
| Total comprehensive loss | (551,817) | (519,691) | (101,809) | ||||||||||||||||||||
| Loss per share | |||||||||||||||||||||||
| Basic and diluted loss for the year per share | 11 | (2.43) | (2.60) | (0.92) | |||||||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-3
Table of Contents
Consolidated Statements of Financial Position as of 31 December 2023 and 2022
USD in thousands
| Non-current assets | Notes | 31 December 2023 | 31 December 2022 | ||||||||||||||
| Property, plant and equipment | 12 | 236,779 | 220,594 | ||||||||||||||
| Right-of-use assets | 13 | 119,802 | 47,501 | ||||||||||||||
| Goodwill | 14 | 12,058 | 11,643 | ||||||||||||||
| Other intangible assets | 15 | 19,076 | 25,652 | ||||||||||||||
| Contract assets | 5 | 10,856 | 3,286 | ||||||||||||||
| Investment in joint venture | 27 | 18,494 | 48,568 | ||||||||||||||
| Other long-term assets | 2,244 | 5,780 | |||||||||||||||
| Restricted cash | 16 | 26,132 | 25,187 | ||||||||||||||
| Deferred tax assets | 10 | 309,807 | 209,496 | ||||||||||||||
| Total non-current assets | 755,248 | 597,707 | |||||||||||||||
| Current assets | |||||||||||||||||
| Inventories | 17 | 74,433 | 71,470 | ||||||||||||||
| Trade receivables | 41,292 | 32,972 | |||||||||||||||
| Contract assets | 5 | 35,193 | 25,370 | ||||||||||||||
| Other current assets | 18 | 31,871 | 32,949 | ||||||||||||||
| Receivables from related parties | 25 | 896 | 1,548 | ||||||||||||||
| Cash and cash equivalents | 16 | 11,157 | 66,427 | ||||||||||||||
| Total current assets | 194,842 | 230,736 | |||||||||||||||
| Total assets | 950,090 | 828,443 | |||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-4
Table of Contents
Consolidated Statements of Financial Position as of 31 December 2023 and 2022
USD in thousands
| Equity | Notes | 31 December 2023 | 31 December 2022 | ||||||||||||||
| Share capital | 19 | 2,279 | 2,126 | ||||||||||||||
| Share premium | 19 | 1,229,690 | 1,058,432 | ||||||||||||||
| Other reserves | 20 | 42,911 | 30,582 | ||||||||||||||
| Translation reserve | (1,528) | (1,442) | |||||||||||||||
| Accumulated deficit | (2,205,845) | (1,654,114) | |||||||||||||||
| Total equity | (932,493) | (564,416) | |||||||||||||||
| Non-current liabilities | |||||||||||||||||
| Borrowings | 21 | 922,134 | 744,654 | ||||||||||||||
| Derivative financial liabilities | 28 | 520,553 | 380,232 | ||||||||||||||
| Other long-term liability to related party | 2 | — | 7,440 | ||||||||||||||
| Lease liabilities | 13 | 105,632 | 35,369 | ||||||||||||||
| Long-term incentive plan | 22 | — | 544 | ||||||||||||||
| Contract liabilities | 5 | 73,261 | 57,017 | ||||||||||||||
| Deferred tax liability | 10 | 53 | 309 | ||||||||||||||
| Total non-current liabilities | 1,621,633 | 1,225,565 | |||||||||||||||
| Current liabilities | |||||||||||||||||
| Trade and other payables | 80,563 | 49,188 | |||||||||||||||
| Lease liabilities | 13 | 9,683 | 5,163 | ||||||||||||||
| Current maturities of borrowings | 21 | 38,025 | 19,916 | ||||||||||||||
| Liabilities to related parties | 25 | 9,851 | 1,131 | ||||||||||||||
| Contract liabilities | 5 | 59,183 | 36,915 | ||||||||||||||
| Taxes payable | 925 | 934 | |||||||||||||||
| Other current liabilities | 26 | 62,720 | 54,047 | ||||||||||||||
| Total current liabilities | 260,950 | 167,294 | |||||||||||||||
| Total liabilities | 1,882,583 | 1,392,859 | |||||||||||||||
| Total equity and liabilities | 950,090 | 828,443 | |||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-5
Table of Contents
Consolidated Statements of Cash Flows for the years ended 31 December 2023, 2022, and 2021
USD in thousands
| Cash flows from operating activities | Notes | 2023 | 2022 | 2021 | |||||||||||||||||||
| Loss for the year | (551,731) | (513,580) | (101,504) | ||||||||||||||||||||
| Adjustments for non-cash items: | |||||||||||||||||||||||
| Gain on extinguishment of SARs liability | 22 | — | (4,803) | — | |||||||||||||||||||
| Share-listing expense | 1.1 | — | 83,411 | — | |||||||||||||||||||
| Long-term incentive plan expense | 22 | 78 | 5,492 | 17,955 | |||||||||||||||||||
| Depreciation and amortization | 8 | 24,210 | 20,409 | 18,196 | |||||||||||||||||||
| Impairment of property, plant and equipment | 12 | — | — | 2,092 | |||||||||||||||||||
| Impairment of other intangible assets | 15 | 1,779 | 2,755 | 3,993 | |||||||||||||||||||
| Change in allowance for receivables | 18,500 | — | — | ||||||||||||||||||||
| Change in inventory reserves | 17 | 8,341 | — | — | |||||||||||||||||||
| Loss on disposal of property, plant and equipment | 365 | — | — | ||||||||||||||||||||
| Impairment loss on investment in joint venture | 27 | 21,519 | — | — | |||||||||||||||||||
| Share of net loss of joint venture | 27 | 7,153 | 2,590 | 2,418 | |||||||||||||||||||
| Finance income | 7 | (4,823) | (2,549) | (51,568) | |||||||||||||||||||
| Finance costs | 7 | 267,157 | 188,419 | 117,361 | |||||||||||||||||||
| Loss/(Gain) on extinguishment of financial liabilities | 21 | — | 27,311 | (151,788) | |||||||||||||||||||
| Share-based payments | 23 | 18,033 | 10,317 | — | |||||||||||||||||||
| Exchange rate difference | 5,183 | (10,566) | (2,681) | ||||||||||||||||||||
| Income tax benefit | 10 | (99,318) | (38,067) | (47,694) | |||||||||||||||||||
| Operating cash flow before movement in working capital | (283,554) | (228,861) | (193,220) | ||||||||||||||||||||
| Increase in inventories | 17 | (11,304) | (32,412) | (29,412) | |||||||||||||||||||
| Increase in trade receivables | (8,320) | (3,576) | (28,813) | ||||||||||||||||||||
| Increase / (decrease) in liabilities with related parties | 2,161 | 56 | (453) | ||||||||||||||||||||
| (Increase) / decrease in contract assets | 5 | (17,393) | (9,218) | 15,286 | |||||||||||||||||||
| Increase in other assets | (802) | (17,194) | (4,363) | ||||||||||||||||||||
| Increase in trade and other payables | 31,772 | 16,442 | 14,318 | ||||||||||||||||||||
| Increase in contract liabilities | 5 | 35,396 | 19,396 | 21,470 | |||||||||||||||||||
| (Decrease) / increase in other liabilities | (5,182) | (21,384) | 5,160 | ||||||||||||||||||||
| Cash used in operations | (257,226) | (276,751) | (200,027) | ||||||||||||||||||||
| Interest received | 3,649 | 568 | 16 | ||||||||||||||||||||
| Interest paid | (57,254) | (35,372) | (28,004) | ||||||||||||||||||||
| Income tax paid | (1,354) | (834) | (155) | ||||||||||||||||||||
| Net cash used in operating activities | (312,185) | (312,389) | (228,170) | ||||||||||||||||||||
| Cash flows from investing activities | |||||||||||||||||||||||
| Acquisition of property, plant and equipment | 12 | (33,234) | (37,880) | (20,462) | |||||||||||||||||||
| Disposal of property, plant and equipment | 12 | 133 | 379 | — | |||||||||||||||||||
| Acquisition of intangible assets | 15 | (13,239) | (11,122) | (20,171) | |||||||||||||||||||
| Restricted cash in connection with amended bond agreement | 21 | — | (14,914) | — | |||||||||||||||||||
| Net cash used in investing activities | (46,340) | (63,537) | (40,633) | ||||||||||||||||||||
| Cash flows from financing activities | |||||||||||||||||||||||
| Repayments of borrowings | 21 | (99,367) | (34,714) | (37,496) | |||||||||||||||||||
| Repayments of principal portion of lease liabilities | 13 | (8,269) | (11,147) | (7,350) | |||||||||||||||||||
| Proceeds from new borrowings | 21 | 278,831 | 193,678 | 113,821 | |||||||||||||||||||
| Transaction cost from new borrowings | 21 | (9,004) | — | — | |||||||||||||||||||
| Gross proceeds from private placement equity offering | 19 | 136,879 | — | — | |||||||||||||||||||
| Gross private placement equity offering fee | 19 | (4,141) | — | — | |||||||||||||||||||
| Proceeds from warrants | 28 | 6,390 | — | — | |||||||||||||||||||
| Proceeds on issue of equity shares | 19 | — | — | 185,856 | |||||||||||||||||||
| Transaction costs for amended borrowing agreements | 21 | — | (12,102) | — | |||||||||||||||||||
| Gross proceeds from the PIPE Financing | 1.1 | — | 174,930 | — | |||||||||||||||||||
| Gross PIPE Financing fees paid | 1.1 | — | (5,562) | — | |||||||||||||||||||
| Proceeds from the Capital Reorganization | 1.1 | — | 9,827 | — | |||||||||||||||||||
| Proceeds from loans from related parties | 21 | — | 160,000 | — | |||||||||||||||||||
| Repayment of loans from related parties | 21 | — | (50,000) | — | |||||||||||||||||||
| Net cash generated from financing activities | 301,319 | 424,910 | 254,831 | ||||||||||||||||||||
| Increase / (decrease) in cash and cash equivalents | (57,206) | 48,984 | (13,972) | ||||||||||||||||||||
| Cash and cash equivalents at the beginning of the year | 16 | 66,427 | 17,556 | 31,689 | |||||||||||||||||||
| Effect of movements in exchange rates on cash held | 1,936 | (113) | (161) | ||||||||||||||||||||
| Cash and cash equivalents at the end of the year | 16 | 11,157 | 66,427 | 17,556 | |||||||||||||||||||
Supplemental cash flow disclosures (Note 29)
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-6
Table of Contents
Consolidated Statements of Changes in Equity for the years ended 31 December 2023, 2022, and 2021
USD in thousands
| Share capital | Share premium | Other reserves | Translation reserve | Accumulated deficit | Total equity | ||||||||||||||||||||||||||||||
| At 1 January 2021 | 73 | 166,740 | — | 4,974 | (1,039,030) | (867,243) | |||||||||||||||||||||||||||||
| Loss for the year | — | — | — | — | (101,504) | (101,504) | |||||||||||||||||||||||||||||
| Foreign currency translation differences | — | — | — | (305) | — | (305) | |||||||||||||||||||||||||||||
| Total comprehensive loss | — | — | — | (305) | (101,504) | (101,809) | |||||||||||||||||||||||||||||
| Increase in share capital | 62 | 833,378 | — | — | — | 833,440 | |||||||||||||||||||||||||||||
| At 31 December 2021 | 135 | 1,000,118 | — | 4,669 | (1,140,534) | (135,612) | |||||||||||||||||||||||||||||
| Loss for the year | — | — | — | — | (513,580) | (513,580) | |||||||||||||||||||||||||||||
| Foreign currency translation differences | — | — | — | (6,111) | — | (6,111) | |||||||||||||||||||||||||||||
| Total comprehensive loss | — | — | — | (6,111) | (513,580) | (519,691) | |||||||||||||||||||||||||||||
| PIPE Financing | 175 | 169,193 | — | — | — | 169,368 | |||||||||||||||||||||||||||||
| Settlement of SARs with shares | 35 | 30,267 | — | — | — | 30,302 | |||||||||||||||||||||||||||||
| Capital Reorganization | 1,731 | (173,296) | — | — | — | (171,565) | |||||||||||||||||||||||||||||
| Settlement of related party loans with Ordinary Shares | 50 | 32,150 | — | — | — | 32,200 | |||||||||||||||||||||||||||||
| Recognition of share-based payments | — | — | 14,548 | — | — | 14,548 | |||||||||||||||||||||||||||||
| Recognition of equity component of convertible bonds | — | — | 16,034 | — | — | 16,034 | |||||||||||||||||||||||||||||
| At 31 December 2022 | 2,126 | 1,058,432 | 30,582 | (1,442) | (1,654,114) | (564,416) | |||||||||||||||||||||||||||||
| Loss for the year | — | — | — | — | (551,731) | (551,731) | |||||||||||||||||||||||||||||
| Foreign currency translation differences | — | — | — | (86) | — | (86) | |||||||||||||||||||||||||||||
| Total comprehensive loss | — | — | — | (86) | (551,731) | (551,817) | |||||||||||||||||||||||||||||
| Capital contribution | 118 | 132,618 | — | — | — | 132,736 | |||||||||||||||||||||||||||||
| Vested earn-out shares | 6 | 8,300 | — | — | — | 8,306 | |||||||||||||||||||||||||||||
| Penny warrants exercised | 25 | 27,159 | — | — | — | 27,184 | |||||||||||||||||||||||||||||
| Public warrants exercised | 6 | 7,612 | — | — | — | 7,618 | |||||||||||||||||||||||||||||
| Recognition of share-based payments | — | — | 16,985 | — | — | 16,985 | |||||||||||||||||||||||||||||
| Settlement of RSUs with shares | 8 | 5,095 | (5,781) | — | — | (678) | |||||||||||||||||||||||||||||
| Settlement of SARs with shares | (10) | (9,526) | (4,231) | — | — | (13,767) | |||||||||||||||||||||||||||||
| Recognition of equity component of convertible bonds | — | — | 5,356 | — | — | 5,356 | |||||||||||||||||||||||||||||
| At 31 December 2023 | 2,279 | 1,229,690 | 42,911 | (1,528) | (2,205,845) | (932,493) | |||||||||||||||||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-7
1. General information
Alvotech (the “Parent” or the “Company” or “Alvotech”), previously known as Alvotech Lux Holdings S.A.S., the surviving company after the Business Combination (as defined below) with, among other parties, Alvotech Holdings S.A. (the “Predecessor”), is a Luxembourg public limited company (société anonyme) incorporated and existing under the laws of the Grand Duchy of Luxembourg, having its registered office at 9, rue de Bitbourg, L-1273 Luxembourg, Grand Duchy of Luxembourg and is registered with the Luxembourg Trade and Companies’ Register under number B 258884. The Company was incorporated on 23 August 2021. These consolidated financial statements were approved by the Group’s Board of Directors, and authorized for issue, on 20 March 2024.
The Company and its subsidiaries (collectively referred to as the “Group”) are a global biotech company specialized in the development and manufacture of biosimilar medicines for patients worldwide. The Group has commercialized a certain biosimilar product and has multiple biosimilar molecules.
1.1 Capital Reorganization
On 15 June 2022 (the “Closing Date”), the Company consummated the capital reorganization with Alvotech Holdings S.A. and OACB (the “Business Combination” or “Capital Reorganization”) pursuant to the business combination agreement, dated as of 7 December 2021, as amended by an amendment agreement dated 18 April 2022 and 7 June 2022 (the “Business Combination Agreement”), by and among the Company, Oaktree Acquisition Corp. II (“OACB”) and the Predecessor. The closing of the Business Combination resulted in the following transactions:
•OACB merged with and into the Company, whereby (i) all of the outstanding ordinary shares of OACB (“OACB Ordinary Shares”) were exchanged for ordinary shares of Alvotech (“Ordinary Shares”) on a one-for-one basis, pursuant to a share capital increase of Alvotech and (ii) all of the outstanding warrants of OACB ceased to represent a right to acquire OACB Ordinary Shares and now represent a right to be issued one Ordinary Share, with Alvotech as the surviving company in the merger. Prior to the merger OACB shares were redeemed, resulting in $9.8 million of cash proceeds from the OACB trust account;
•Alvotech redeemed and canceled the initial shares held by the initial sole shareholder of Alvotech pursuant to a share capital reduction of Alvotech;
•The legal form of Alvotech changed from a simplified joint stock company (société par actions simplifiée) to a public limited liability company (société anonyme) under Luxembourg law; and
•The Predecessor merged with and into the Parent, whereby all outstanding ordinary shares of the Predecessor (“Predecessor Ordinary Shares”) were exchanged for Ordinary Shares, pursuant to a share capital increase of Alvotech, with Alvotech as the surviving company in the merger.
Concurrently with the execution of the Business Combination Agreement, OACB and Alvotech entered into subscription agreements (“Subscription Agreements”) with certain investors (the “PIPE Financing”). On 15 June 2022, immediately prior to the closing of the Business Combination, the PIPE Financing was closed, pursuant to the Subscription Agreements, in which subscribers collectively subscribed for 17,493,000 Ordinary Shares at $10.00 per share for an aggregate subscription price equal to $174.9 million.
As part of the Business Combination, Predecessor shareholders were granted a total of 38,330,000 Ordinary Shares subject to certain vesting conditions (“Predecessor Earn Out Shares”). Former OACB shareholders were granted a total of 1,250,000 Ordinary Shares subject to certain vesting conditions (“OACB Earn Out Shares”). Additionally, as part of the Business Combination the Company assumed the 10,916,647 outstanding warrants (“OACB Warrants”), on substantially the same contractual terms and conditions as were in effect immediately prior to the Business Combination. See Note 28 for further details.
The Business Combination was accounted for as a capital reorganization. Under this method of accounting, OACB was treated as the “acquired” company for financial reporting purposes, with Alvotech Holdings S.A. being the accounting acquirer and accounting predecessor. Accordingly, the capital reorganization was treated as the equivalent of Alvotech issuing shares at the closing of the Business Combination for the net assets of OACB as of the Closing Date, accompanied by a recapitalization. The capital reorganization, which was not within the scope of IFRS 3 since OACB did not meet the definition of a business in accordance with that guidance, was accounted for within the scope of IFRS 2. In accordance with IFRS 2, Alvotech recorded a one-time non-cash share listing expense of $83.4 million, recognized as a general and administrative expense, based on the excess of the fair value of
F-8
Alvotech shares issued, at the Closing Date, over the fair value of OACB’s identifiable net assets acquired. The fair value of shares issued was estimated based on a market price of $9.38 per share as of 15 June 2022.
| Shares | (in 000s) | ||||||||||
| OACB Shareholders | |||||||||||
| Class A Shareholders | 976,505 | ||||||||||
| Class B Shareholders | 5,000,000 | ||||||||||
| OACB Earn Out Shares | 1,250,000 | ||||||||||
| Total Alvotech Shares issued to OACB shareholders | 7,226,505 | ||||||||||
| Fair value of Shares issued to OACB as of 15 June 2022 | $56,060 | ||||||||||
| Fair value of OACB Earn Out Shares issued to OACB as of 15 June 2022 | 9,100 | ||||||||||
| Estimated fair market value | 65,160 | ||||||||||
| Adjusted net liabilities of OACB as of 15 June 2022 | (18,251) | ||||||||||
| Difference – being the share listing expense | 83,411 | ||||||||||
In connection with the Business Combination and PIPE Financing, the Company incurred $28.5 million of transaction costs, which represent legal, financial advisory, and other professional fees in connection with the Business Combination and PIPE Financing, during the year ended December 31, 2022. Of this amount, $5.6 million represented equity issuance costs related to PIPE Financing that were capitalized in share premium. The remaining $22.9 million was recognized as general and administrative expense.
1.2 Information about subsidiaries and joint ventures
| Entity name | Principal activity | Issued and paid capital (presented in whole shares) | Place of establishment | Proportion of ownership and voting power held by Alvotech | |||||||||||||||||||||||||
| 31.12.2023 | 31.12.2022 | ||||||||||||||||||||||||||||
| Alvotech hf | Biopharm. | 3,893,650 | Iceland | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Germany GmbH | Biopharm. | 31,182 | Germany | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Swiss AG | Biopharm. | 153,930 | Switzerland | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Hannover GmbH | Biopharm. | 29,983 | Germany | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Malta Ltd | Group Serv. | 80,450 | Malta | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech USA Inc | Biopharm. | 10 | USA | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech UK Ltd | Group Serv. | 135 | UK | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Manco ehf | Group Serv. | 215,390 | Iceland | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech Biosciences India Private Ltd | Biopharm | 96,113 | India | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Fasteignafelagið Sæmundur hf | Real estate | 12,965,337 | Iceland | 100.00 | % | 100.00 | % | ||||||||||||||||||||||
| Alvotech & CCHN Biopharmaceutical Co. Ltd* | Biopharm. | 110,000,021 | China | 50.00 | % | 50.00 | % | ||||||||||||||||||||||
*Alvotech & CCHN Biopharmaceutical Co. Ltd. is an unconsolidated joint venture (see Note 27).
1.3 Information about shareholders
Significant shareholders of the Company are Aztiq Pharma Partners S.à r.l. (Aztiq) and Alvogen Lux Holdings S.à r.l. (Alvogen), with 37.9% and 33.7% ownership interest as of 31 December 2023, and 40.7% and 35.8% ownership interest as of 31 December 2022, respectively. The remaining 28.4% ownership interest is held by various entities, with no single shareholder holding more than 2.4% ownership interest as of 31 December 2023.
F-9
The remaining 23.5% ownership interest was held by various entities, with no single shareholder holding more than 2.4% ownership interest as of 31 December 2022.
1.4 Going concern
The Group has primarily funded its operations with proceeds from the issuance of ordinary shares and the issuance of loans and borrowings to both related parties and third parties. The Group has also incurred recurring losses since its inception, including net losses of $551.7 million, $513.6 million, and $101.5 million for the years ended 31 December 2023, 2022, and 2021, respectively, and had an accumulated deficit of $2,205.8 million as of 31 December 2023. The Group has not generated positive operational cash flow, largely due to the continued focus on biosimilar product development and expansion efforts.
As of 31 December 2023, the Group had cash and cash equivalents, excluding restricted cash, of $11.2 million and current assets less current liabilities of ($66.1) million.
The Group devotes substantially all of its efforts towards obtaining regulatory approval and raising capital necessary to fund its operations and it is subject to a number of risks associated with clinical research and development, the development of and regulatory approval of commercially viable biosimilar products, the need to raise adequate additional financing necessary to fund the development and commercialization of its biosimilar products.
The Company announced in February 2024 that the U.S. Food and Drug Administration ("FDA") has approved SIMLANDI (adalimumab) injection, as an interchangeable biosimilar to Humira, for the treatment of adult rheumatoid arthritis, juvenile idiopathic arthritis, adult psoriatic arthritis, adult ankylosing spondylitis, Crohn’s disease, adult ulcerative colitis, adult plaque psoriasis, adult hidradenitis suppurativa and adult uveitis. Teva is Alvotech’s strategic partner for the exclusive commercialization of SIMLANDI in the United States. SIMLANDI is the first high-concentration, citrate-free biosimilar to Humira that has been granted an interchangeability status by the FDA, and will qualify for interchangeable exclusivity for the 40mg/0.4ml injection. This approval is an important milestone for the Company to access the U.S. market with a unique positioning. The Company expects to launch AVT02 with its partner Teva in the United States during the first half of 2024.
Additionally, in February 2024, the Company announced it has reached settlement agreements with Johnson & Johnson in Japan, Canada and in the European Economic Area (EEA) for AVT04, a biosimilar to Stelara (ustekinumab). Regulatory approval for AVT04 in these markets has already been granted. Market applications for AVT04 are currently pending in additional global markets, including in the U.S. Market entry of AVT04 in Canada started in March 2024. Launch of AVT04 in Japan is anticipated after the upcoming round of National Health Insurance reimbursement price listings, in May 2024. Entry to the first European markets is expected as soon as possible after the expiration date of the European Supplementary Protection Certificate for Stelara, which is in late July 2024. These approvals represent another significant milestone for the Company to tap into the Stelara market.
The closing of the private placement equity offering in February 2024 provided the Group with gross proceeds of $166 million (net proceeds of $160 million) that is expected to be used to finance general corporate purposes and working capital, to strengthen its production capacity, and to support expected biosimilars launches. As part of the transaction, the Group sold 10,127,132 Ordinary Shares, par value USD 0.01 per share, at a purchase price of $16.41 per share, or ISK 2,250 per share, at foreign exchange rates on 23 February 2024.
Additionally, the Group expects to continue to source its financing during the development of its biosimilar products from existing out-license contracts with commercial partners. In light of these conditions and events management evaluated whether there is substantial doubt about the Group’s ability to continue as a going concern for at least one year after the date that the consolidated financial statements are issued. Based on the cash on hand, funding received, and projected future cash flows, management concluded that the Group has the ability to continue as a going concern for at least one year after the date that the consolidated financial statements are issued. As such, the consolidated financial statements have been prepared on a going concern basis
However, although management continues to pursue these plans, there is no assurance that the Group will be successful in obtaining sufficient funding on terms acceptable to the Group to fund continuing operations, if at all. If financing is obtained, the terms of such financing may adversely affect the holdings or the rights of the Group’s shareholders. The ability to obtain funding, therefore, is outside of management’s control and is a material uncertainty that may cast significant doubt upon the Group’s ability to continue as a going concern.
.
F-10
2. Summary of significant accounting policies
2.1 Basis of preparation
The consolidated financial statements of the Group have been prepared in accordance and in compliance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"), which comprise all standards and interpretations approved by the IASB, and as adopted by the European Union ("EU").
All amendments to IFRSs issued by the IASB that are effective for annual periods that begin on or after 1 January 2023 have been adopted as further described within the footnotes to the consolidated financial statements. The Group has not adopted any standards or amendments to standards in issue that are available for early adoption.
The consolidated financial statements have been prepared on a historical cost basis, except for certain financial assets and financial liabilities which have been measured at fair value. Historical cost is generally based on the fair value of the consideration given in exchange for goods and services. The consolidated financial statements are presented in U.S. Dollar ("USD") and all values are rounded to the nearest thousand unless otherwise indicated.
2.2 Basis of consolidation
The consolidated financial statements incorporate the financial statements of the Company and entities controlled by the Company and its subsidiaries. Control is achieved when the Company:
•has power over the investee;
•is exposed, or has rights, to variable returns from its involvement with the investee; and
•has the ability to use its power to affect its returns.
When the Company has less than a majority of the voting rights of an investee, it has power over the investee when the voting rights are sufficient to give it the practical ability to direct the relevant activities of the investee unilaterally. The Company considers all relevant facts and circumstances in assessing whether or not the Company’s voting rights in an investee are sufficient to give it power, including:
•the size of the Company’s holding of voting rights relative to the size and dispersion of holdings of the other vote holders;
•potential voting rights held by the Company, other vote holders or other parties;
•rights arising from other contractual arrangements; and
•any additional facts and circumstances that indicate that the Company has, or does not have, the current ability to direct the relevant activities at the time that decisions need to be made, including voting patterns at previous shareholders’ meetings.
Consolidation of a subsidiary begins when the Company obtains control over the subsidiary and ceases when the Company loses control of the subsidiary. Specifically, income and expenses of a subsidiary acquired or disposed of during the year are included in the consolidated statements of profit or loss and other comprehensive income or loss from the date the Company gains control until the date when the Company ceases to control the subsidiary. The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control.
All intra-group transactions, balances, income and expenses are eliminated in full in consolidation.
2.3 Investments in joint ventures
To the extent the Group concludes that it does not control, and thus consolidate, a joint venture, the Group accounts for its interest in joint ventures using the equity method of accounting. As such, investments in a joint venture are initially recognized at cost and the carrying amount is subsequently adjusted for the Group’s share of the profit or loss of the joint venture, as well as any distributions received from the joint venture. The Group carries its ownership interest in a joint venture as “Investment in joint venture” on the consolidated statements of financial position. The Group’s profit or loss includes its share of the profit or loss of the joint venture and, to the extent applicable, other comprehensive income or loss for the Group includes its share of other comprehensive income or loss of the joint venture. The Group’s share of a joint venture’s profit or loss in a particular year is presented as “Share of net loss of joint venture” in the consolidated statements of profit or loss and other comprehensive income or loss.
F-11
The carrying amount of equity-accounted investments is assessed for impairment as a single asset. Impairment losses are incurred only if there is objective evidence of impairment as a result of loss events that have an impact on estimated future cash flows and that can be reliably estimated. Losses expected as a result of future events are not recognized. The Group recognized an impairment loss of $21.5 million related to its investment in the joint venture for the year ended 31 December 2023. No impairment losses were recognized in 2022 or 2021.
2.4 Critical accounting judgments and key sources of estimation uncertainty
The preparation of the consolidated financial statements in conformity with IFRS requires Group management to make judgments, estimates and assumptions about the reported amounts of assets, liabilities, income and expenses that are not readily apparent from other sources.
The estimates and associated assumptions are based on information available when the consolidated financial statements are prepared, historical experience and other factors that are considered to be relevant. Judgments and assumptions involving key estimates are primarily made in relation to the measurement and recognition of revenue, the impairment of the investment in the joint venture, the valuation of derivative financial liabilities, the valuation of restricted share units (“RSUs”), and the valuation of deferred tax assets. Apart from those involving estimations, critical accounting judgments include the Group’s evaluation as to whether it controls its joint venture in China and material uncertainties with respect to the Group’s going concern assessment.
Existing circumstances and assumptions may change due to events arising that are beyond the Group’s control. Therefore, actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods.
2.5 Segment reporting
The Group operates and manages its business as one operating segment based on the manner in which the Chief Executive Officer, the Group’s chief operating decision maker, assesses performance and allocates resources across the Group.
2.6 Revenue recognition
Product revenue
The Company recognizes revenue from the sale of its biosimilar product to commercial partners, identified as the customer, when control is transferred, and the performance obligations have been satisfied. This is when the title passes to the customer, which is upon shipment of the product. At that point, the commercial partner has full discretion over the channel and price to sell the products. Revenue is recognized based on the net selling price from the commercial partners, which is considered to be the transaction price and includes estimated rebates, returns and chargebacks, and other forms of variable consideration recognized by the customer. Variable consideration is accounted for by the Company only to the extent that it is highly probable that a significant reversal in the revenue recognized will not occur. Variable consideration, which includes any adjustments to the net selling price, is estimated based on the most likely amount method on a contract-by-contract basis.
Out-licensing revenue
A significant part of the Group’s revenue is generated from long-term out-license contracts which provide the customer with an exclusive right to market and sell products in a particular territory once such products are approved for commercialization. These contracts typically include the Group’s promises to continue development of the underlying compound and to provide supply of the product to the customer upon commercialization. The Group concludes that the license, development services and commercial supply are separate performance obligations. This is because customers generally have the capabilities to perform the necessary development, manufacturing and commercialization activities on their own or with readily available resources and have the requisite expertise in the industry and the territory for which the license has been granted. Further, the intellectual property is generally in a later phase of development at the time the license is granted such that any subsequent development activities performed by the Group are not expected to significantly modify or transform the intellectual property. The fact that the Group is contractually obligated to perform development activities for and provide commercial supply to the
F-12
customer does not impact this conclusion. The Group’s promise to provide commercial supply to its customers is contingent upon the achievement of regulatory approval in the particular territory for which the license has been granted.
The consideration to which the Group is entitled pursuant to these contracts generally includes upfront payments and payments based upon the achievement of development and regulatory milestones. All contracts include a potential refund obligation whereby the Group must refund the consideration paid by the customer in the event of a technical failure or the occurrence of certain other matters that result in partial or full cancellation of the contract. As such, the entire transaction price is comprised of variable consideration, which is estimated using the most likely amount method due to the binary nature of the outcomes under these contracts. Such variable consideration is included in the transaction price only when it is highly probable that doing so will not result in a significant reversal of cumulative revenue recognized when the underlying uncertainty associated with the variable consideration is subsequently resolved. The Group does not account for a significant financing component since a substantial amount of consideration promised by the customer is variable and the amount or timing of that consideration varies on the basis of a future event that is not substantially within the control of either party. Certain contracts also include commercialization milestones upon the first commercial sale of a product in a particular territory, as well as royalties. Commercialization milestones and royalties are accounted for as sales-based royalties; therefore, such amounts are not included in the transaction price and recognized as revenue until the underlying sale that triggers the milestone or royalty occurs.
Upfront payments, when applicable, are received in advance of transferring control of all goods and services. Therefore, a portion of upfront payments is recorded as a contract liability upon receipt. Due to the existence of refund provisions, upfront payments and certain development milestone payments are generally included in the transaction price upon submission of the first clinical trial application to the respective regulatory agency, since it is at this point in time that a significant reversal of cumulative revenue recognized related to such payments is no longer highly probable. Other development and regulatory milestones may not be included in the transaction price until such milestones are achieved due to the degree of uncertainty associated with achieving these milestones. Contract liabilities are presented on the consolidated statements of financial position as either current or non-current based upon forecasted performance. In certain contracts, the Group may transfer control of goods and services, and thus recognize revenue, prior to having the right to invoice the customer. In these circumstances, the Group recognizes contract assets for revenue recognized, and subsequently reclasses the contract asset to trade receivables upon issuing an invoice and the right to consideration is only conditional on the passage of time. Contract assets are presented on the consolidated statements of financial position as either current or non-current based upon the expected timing of settlement.
The standalone selling prices of the development services and the license to intellectual property are not directly observable and, therefore, are estimated. The standalone selling price of the development services is estimated based on the expected costs to be incurred during the development period, using various data points such as the underlying development budget, contractual milestones and performance completed at the time of entering into the contract with a customer. The standalone selling price of the license is estimated using the residual approach on the basis that the Group licenses intellectual property for a broad range of amounts and has not previously licensed intellectual property on a standalone basis. Therefore, the Group first allocates the transaction price to the development services and subsequently allocates the remainder of the transaction price to the license. If the product is still in early phase of development and the constraint on variable consideration has not been resolved, all the transaction price is allocated to the development service.
The standalone selling price of the commercial supply is directly observable and the stated prices in the Group’s supply contracts reflect the standalone selling price of such goods.
The licenses to intellectual property are right of use licenses on the basis that the ongoing development work performed by the Group does not significantly affect the intellectual property to which the customer has rights. Therefore, control of the license transfers to the customer at the point in time when the right to use the license is granted to the customer. The license is generally granted to the customer at the time the contract is executed with the customer.
The Group satisfies its performance obligation related to the development services over time as the Group’s performance enhances the value of the licensed intellectual property controlled by the customer throughout the performance period. The Group recognizes revenue using a cost-based input measure since this measure best reflects the progress of the development services and, therefore, the pattern of transfer of control of the services to the customer. In certain instances, the Group may subcontract services to other parties for which the Group is ultimately responsible. Costs incurred for such subcontracted services are included in the Group’s measure of progress for
F-13
satisfying its performance obligation. Changes in the total estimated costs to be incurred in measuring the Group’s progress toward satisfying its performance obligation may result in adjustments to cumulative revenue recognized at the time the change in estimate occurs.
Upon the achievement of regulatory approval and the commencement of commercial sale of its products, the Group will satisfy its performance obligation related to commercial supply at the point in time when control of the manufactured product is transferred to the customer. Transfer of control for such goods will occur in accordance with the stated shipping terms.
The Group does not incur incremental costs of obtaining a contract with a customer that would require capitalization. Costs to fulfill performance obligations are not incurred in advance of performance and, as such, are expensed when incurred.
Other revenue
Other revenue primarily consists of clinical trial support services rendered by the Group for its customers, which is recognized as the service is provided. Revenue for such services is presented in the consolidated statements of profit or loss and other comprehensive income or loss net of any discounts.
2.7 Cost of product revenue
Cost of product revenue includes the cost of inventory sold, labor costs, manufacturing overhead expenses and reserves for expected scrap, as well as shipping and freight costs and royalty costs related to in-license agreements.
2.8 Research and development expenses
Research and development expenses primarily consist of personnel costs, material and other lab supply costs, facility costs and internal and external costs related to the execution of studies and other development program advancement initiatives. Such expenses also include costs incurred in preparation for commercial launch, such as designing and developing commercial-scale manufacturing capabilities and processes, quality control processes, production asset validation and other related activities. The costs also include amortization, depreciation and impairment losses related to software, property, plant and equipment, and right-of-use assets used in research and development activities and pre-commercial manufacturing and quality control activities.
An internally generated intangible asset arising from the Group’s development is recognized only if the Group can demonstrate: the technical feasibility of completing the intangible asset so that it will be available for use or sale; the intent to complete the intangible asset and use or sell it; how the intangible asset will generate probable future economic benefits; the availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset; and the ability to measure reliably the expenditure attributable to the intangible asset during its development.
The amount initially recognized for internally-generated intangible assets is the sum of the expenditures incurred from the date when the intangible asset first meets the aforementioned recognition criteria. If an internally-generated intangible asset cannot be recognized, the related development expenditure is charged to profit or loss in the period in which it is incurred.
Expenditures related to research and development activities are generally recognized as an expense in the period in which they are incurred. The Company did not capitalize any development expenses as intangible assets during the years ended 31 December 2023, 2022, and 2021 as not all the criteria in paragraph 57 of IAS 38 have been met.
2.9 General and administrative expenses
General and administration expenses primarily consist of personnel-related costs, including salaries and other related compensation expense, for corporate and other administrative and operational functions including finance, human resources, information technology and legal, as well as facility-related costs. These costs relate to the operation of the business and are not related to research and development initiatives.
Expenditures related to general and administration activities are recognized as an expense in the period in which they are incurred.
F-14
2.10 Finance income and finance cost
Finance income consists of changes in the fair value of derivative financial liabilities and interest income. Interest income from a financial asset is recognized when it is probable that the economic benefits will flow to the Group and the amount of income can be measured reliably. Interest income is accrued on a time basis, by reference to the principal outstanding and at the effective interest rate applicable, which is the rate that exactly discounts estimated future cash receipts through the expected life of the financial asset to that asset’s net carrying amount on initial recognition.
Finance cost consists of changes in the fair value of derivative financial liabilities, interest expense related to lease liabilities and borrowings, accretion of borrowings and amortization of deferred debt issue costs.
2.11 Foreign currency translation
The consolidated financial statements are presented in U.S. Dollars, which is the Group’s presentation currency. The Group maintains the financial statements of each entity within the Group in its respective functional currency. The majority of the Group’s expenses are incurred in U.S. Dollars and Icelandic Krona, and the majority of the Company’s cash and cash equivalents are held in a combination of Icelandic Krona, Euros and U.S. Dollars. Transactions in currencies other than the Group’s presentation currency (foreign currencies) are recognized at the rates of exchange prevailing at the dates of the transactions. At the end of each reporting period, monetary items denominated in foreign currencies are retranslated at the rates prevailing at that date. Non-monetary items carried at fair value that are denominated in foreign currencies are retranslated at the rates prevailing at the date when the fair value was determined. Non- monetary items that are measured in terms of historical cost in a foreign currency are not retranslated. Exchange differences on monetary items are recognized in profit or loss in the period in which they arise.
Exchange differences arising on translation of a foreign controlled subsidiary are recognized in other comprehensive income or loss and accumulated in a translation reserve within equity. The cumulative translation amount is reclassified to profit or loss if and when the net investment in the foreign controlled subsidiary is disposed.
2.12 Fair value measurements
The Group measures certain financial liabilities at fair value through profit or loss (FVTPL) at each reporting period. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The Group uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure the fair values of such financial liabilities, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs.
Fair values are categorized into different levels in a fair value hierarchy based on the inputs used in the valuation techniques, as follows:
•Level 1: quoted prices in active markets for identical assets and liabilities;
•Level 2: inputs other than quoted prices that are observable for the asset or liability, either directly (e.g., prices) or indirectly (e.g., derived from prices); and
•Level 3: inputs for the asset or liability that are unobservable.
The carrying amounts of cash and cash equivalents, restricted cash, trade receivables, other current assets, contract assets, trade and other payables and other current liabilities in the Group’s consolidated statements of financial position approximate their fair value because of the short maturities and nature of these instruments.
For liabilities that are measured at fair value on a recurring basis, the Group determines whether transfers have occurred between levels in the fair value hierarchy by reassessing the inputs used in determining fair value at the end of each reporting period.
F-15
2.13 Goodwill and other intangible assets
Goodwill and business combinations
Acquisitions are first reviewed to determine whether a set of assets acquired constitute a business and should be accounted for as a business combination. If the assets acquired do not meet the definition of a business, the Group will account for the transaction as an asset acquisition. If the definition of a business combination is met, the Group will account for the transaction using the acquisition method of accounting. The consideration transferred in a business combination is measured at fair value, which is calculated as the sum of the acquisition-date fair values of the assets transferred by the Group, liabilities incurred by the Group to the former owners of the acquiree and the equity interests issued by the Group in exchange for control of the acquiree. Acquisition-related costs are recognized in the consolidated statements of profit or loss and other comprehensive income or loss as incurred.
Goodwill represents the excess of the purchase price of the business combination over the Group’s interest in the net fair value of the identifiable assets, liabilities, contingent liabilities, the amount of any noncontrolling interests in the acquiree and the fair value of the acquirer’s previously held equity interest in the acquiree. Goodwill is reviewed for impairment at least annually, and whenever there is an indication that the asset may be impaired. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs of disposal and value in use. The value in use calculation is performed using discounted expected future cash flows. The discount rate applied to these cash flows is based on the weighted average cost of capital and reflects current market assessments of the time value of money.
If the initial accounting for a business combination is incomplete by the end of the reporting period in which the business combination occurs, the Group reports provisional amounts for the items for which the accounting is incomplete. Those provisional amounts are adjusted during the measurement period, or as additional assets or liabilities are recognized, to reflect new information obtained about facts and circumstances that existed at the acquisition date that, if known, would have affected the amounts recognized at that date.
The Group did not complete any business combinations during the years ended 31 December 2023. Refer to Note 1.1 for the Business Combination completed during the year ended 31 December 2022.
Other intangible assets
Other intangible assets consist of software, customer relationships, and intellectual property rights. Intangible assets acquired in a business combination are identified and recognized separately from goodwill if they satisfy the definition of an intangible asset and their fair values can be reliably measured. The cost of intangible assets is their fair value at the acquisition date.
Intangible assets with finite useful lives are reported at cost less accumulated amortization and accumulated impairment losses. Amortization is recognized on a straight-line basis over an asset’s estimated useful life. The estimated useful life and amortization method are reviewed at each balance sheet date, with the effect of any changes in estimate being accounted for on a prospective basis. Intangible assets that are subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. The following useful lives are used in the calculation of amortization:
| Software | 3-5 years | ||||
| Customer relationships | 7 years | ||||
| Intellectual property rights* | 10 years | ||||
•From launch date
Intangible assets with indefinite useful lives are reviewed for impairment at least annually, and whenever there is an indication that the asset may be impaired. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs of disposal and value in use. The value in use calculation is performed using discounted expected future cash flows. The discount rate applied to these cash flows is based on the weighted average cost of capital and reflects current market assessments of the time value of money.
F-16
2.14 Income tax
Income tax includes the current tax and deferred tax charge recorded in the consolidated statements of profit or loss and other comprehensive income or loss.
Current tax
The current tax expense is based on taxable profit for the year. Taxable profit differs from ‘profit before tax’ as reported in the consolidated statements of profit or loss and other comprehensive income or loss because it excludes items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Group’s current tax expense is calculated using tax rates that have been enacted or substantively enacted by the end of the reporting period.
Accruals for tax contingencies are made when it is not probable that a tax authority will accept the tax position, based upon management’s interpretation of applicable laws and regulations and the expectation of how the tax authority will resolve the matter. Accruals for tax contingencies are measured using either the most likely amount or the expected value amount depending on which method the entity expects to better predict the resolution of the uncertainty.
Deferred tax
Deferred tax is provided in full for all temporary differences between the carrying amounts of assets and liabilities in the consolidated financial statements and the corresponding tax bases used in the computation of taxable profit, except to the extent the temporary difference arises from:
•The initial recognition of an asset or a liability in a transaction that is not a business combination and that affects neither the taxable profit nor accounting profit;
•The initial recognition of residual goodwill (for deferred tax liabilities only); or
•Investments in subsidiaries, branches, associates and joint ventures, where the Group is able to control the timing of the reversal of the temporary difference and it is not probable that it will reverse in the foreseeable future.
Deferred tax liabilities and assets are measured at the tax rates that are expected to apply in the period in which the liability is settled or the asset realized, based on tax rates and tax laws that have been enacted or substantively enacted by the end of the reporting period. The measurement of deferred tax liabilities and deferred tax assets reflects the tax consequences that would follow from the manner in which the Group expects, at the balance sheet date, to recover or settle the carrying amount of the assets and liabilities.
Deferred tax liabilities are generally recognized for all taxable temporary differences. Deferred tax assets are generally recognized for all deductible temporary differences to the extent that it is probable that taxable profits will be available against which those deductible temporary differences can be utilized. The carrying amount of deferred tax assets is reviewed at the end of each reporting period and reduced to the extent that it is no longer probable that sufficient taxable profits will be available to allow all or part of the asset to be recovered.
Deferred tax is charged or credited to the consolidated statements of profit or loss and other comprehensive income or loss, except when the tax arises from a business combination or it relates to items charged or credited directly to equity, in which case the deferred tax is also taken directly to equity.
Deferred tax assets and liabilities are offset when they relate to income taxes levied by the same taxation authority and the Group intends to settle its current tax assets and liabilities on a net basis in that taxation authority.
2.15 Property, plant and equipment
Property, plant and equipment is recognized as an asset when it is probable that future economic benefits associated with the asset will flow to the Group and the cost of the asset can be measured in a reliable manner. Property, plant and equipment which qualifies for recognition as an asset are initially measured at cost.
The cost of property, plant and equipment includes an asset’s purchase price and any directly attributable costs of bringing the asset to working condition for its intended use.
F-17
Depreciation is calculated and recognized as an expense on a straight-line basis over an asset’s estimated useful life. The estimated useful lives, residual values and depreciation method are reviewed at each balance sheet date, with the effect of any changes in estimate accounted for on a prospective basis. The following useful lives are used in the calculation of depreciation:
| Facility | 40 years | ||||
| Facility equipment | 5-20 years | ||||
| Computer equipment | 3 years | ||||
| Leasehold improvements | 3-15 years | ||||
| Furniture and fixtures | 5 years | ||||
Certain of the Group’s property, plant and equipment assets have been pledged to secure borrowings as further described in Note 21. Significant disposals of pledged assets are subject to lender approval. Upon disposal or retirement of an asset, the difference between the sales proceeds, if applicable, and the carrying amount of the asset is recognized in the consolidated statements of profit or loss and other comprehensive income or loss at the time of disposal or retirement.
At the end of each reporting period, or sooner if events triggering an interim impairment assessment occur, the Group reviews the carrying amounts of its property, plant and equipment to determine whether there is any indication that the value of such assets are impaired. Triggering events that warrant an interim impairment assessment include, but are not limited to, the technical obsolescence of equipment or failure of such equipment to meet regulatory requirements. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss and the carrying amount of the asset is reduced to its recoverable amount, which is the higher of fair value less costs of disposal and value in use.
2.16 Inventories
Inventories, which consist of raw materials and supplies, work in progress and finished goods are stated at the lower of cost or net realizable value. Net realizable value is the expected sales price less completion costs and costs to be incurred in marketing, selling and distributing the inventory. Cost is calculated using the weighted average cost method or the first-in,first-out method, depending on the nature of the inventory.
Inventories include direct costs for raw materials and supplies and, as applicable, direct and indirect labor and overhead expenses that have been incurred to bring inventories to their present location and condition.
If the net realizable value is lower than the carrying amount, a write-down of inventory is recognized for the amount by which the carrying amount exceeds net realizable value.
The Group does not pledge inventories as collateral to secure its liabilities.
2.17 Financial assets
Recognition of financial assets
Financial assets are recognized when the Group becomes a party to the contractual provisions of the instrument. Financial assets are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issue of financial assets, other than financial assets measured at FVTPL, are added to or deducted from the fair value of the financial assets, as appropriate, on initial recognition. Transaction costs directly attributable to the acquisition of financial assets at FVTPL are recognized immediately in profit or loss. There were no transaction costs related to the acquisition of financials assets in 2023, 2022, or 2021. All of the Group’s financial assets are measured at amortized cost as of 31 December 2023 and 2022.
Financial assets measured at amortized cost
Financial assets measured at amortized cost are debt instruments that give rise to contractual cash flows that are solely payments of principal and interest on the principal amount outstanding. The Group’s financial assets measured at amortized cost are trade receivables, certain other current assets, receivables from related parties, restricted cash and cash and cash equivalents.
F-18
Interest income is recognized by applying the effective interest rate, except for short-term receivables when the effect of discounting is immaterial.
Impairment of financial assets
The Group recognizes a loss allowance for expected credit losses ("ECL") on its trade receivables and other debt instruments that are measured at amortized cost. In addition, although contract assets are not financial assets, a loss allowance for ECL are also recognized for such assets. ECL is based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that the Group expects to receive, discounted at an approximation of the original effective interest rate. The amount of ECL is updated at each reporting date to reflect changes in credit risk since initial recognition of the respective financial instrument.
The Group always recognizes lifetime ECL for trade receivables and contract assets. The expected credit losses on these financial assets are estimated using a provision matrix based on the Group’s historical credit loss experience, adjusted for factors that are specific to the debtors, general economic conditions and an assessment of both the current as well as the forecasted direction of conditions at the reporting date, including time value of money where appropriate.
The Group writes off a financial asset when there is no reasonable expectation of recovery, such as information indicating that the debtor is in severe financial difficulty and there is no realistic prospect of recovery. A trade receivable or contract asset that is considered uncollectible is written off against the allowance account. Subsequent recoveries of amounts previously written off are credited against the allowance account. Changes in the carrying amount of the allowance account are recognized in profit or loss. The Group did not write off any trade receivables or contract assets during the years ended 31 December 2023, 2022, and 2021.
The Group estimates impairment for related party receivables on an individual basis. No impairment is recognized for restricted cash or cash and cash equivalents as management has estimated that the effects of any calculated ECL would be immaterial.
Derecognition of financial assets
The Group derecognizes a financial asset when the contractual rights to the cash flows from the asset expire, or when it transfers the financial asset and substantially all the risks and rewards of ownership of the asset to another party. If the Group neither transfers nor retains substantially all the risks and rewards of ownership and continues to control the transferred asset, the Group recognizes its retained interest in the asset as well as an associated liability. If the Group retains substantially all the risks and rewards of ownership of a transferred financial asset, the Group continues to recognize the financial asset and also recognizes a collateralized borrowing for the proceeds received.
On derecognition of a financial asset, the difference between the asset’s carrying amount and the sum of the consideration received and receivable and the cumulative gain or loss that had been recognized in other comprehensive income or loss and accumulated in equity is recognized in profit or loss.
2.18 Financial liabilities
Financial liabilities
The Group’s financial liabilities consist of trade and other payables, certain other current liabilities loans and borrowings, lease liabilities, derivative financial instruments, long-term incentive plans, share appreciation right plans and other long-term liability to a related party. All financial liabilities are initially measured at fair value. Loans and borrowings are recorded net of directly attributable transaction costs and less the value attributable to any embedded derivative financial instruments, if applicable.
The Group derecognizes financial liabilities when, and only when, the Group’s obligations are discharged, cancelled, substantially modified or have expired. Additionally, management elected, as part of its accounting policy, to recognize the difference between the carrying amount of the financial liabilities and the fair value of the consideration paid for the extinguishment in the consolidated statement of profit or loss and other comprehensive income or loss.
F-19
Financial liabilities subsequently measured at amortized cost
After initial recognition, financial liabilities other than derivative financial instruments and awards issued pursuant to long-term incentive plans are subsequently measured at amortized cost using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that discounts all estimated future cash payments through the expected life of the financial liability, or a shorter period if appropriate, to the amortized cost of a financial liability. The effective interest rate includes the effects of any discount or premium on acquisition of the financial liability, as well as any fees or costs incurred upon acquisition.
Financial liabilities subsequently measured at FVTPL
Derivative financial instruments
Certain rights and features pursuant to borrowing arrangements and other contracts may provide the counterparty with one or more financial instruments that need to be evaluated and potentially accounted for separately by the Group. These financial instruments are either embedded in a host instrument or are treated as a separate financial instrument if they are contractually transferable independent from the host instrument. Such rights and features pursuant to the Group’s contracts with both third parties and related parties include earn out rights, conversion rights and warrant rights.
Equity conversion features within host debt instruments that meet the definition of a derivative and have economic and risk characteristics that are not closely related to the host instrument are embedded derivatives that are separated from the host instrument and accounted for separately. As part of the accounting for embedded derivatives or separate financial instruments, management considers the appropriate accounting classification under IAS 32.
Embedded derivatives and separate financial instruments that meet the fixed-for-fixed criteria are classified as equity and initially measured at fair value. Warrant rights that provide the holder with an option to purchase ordinary shares at a specified price or pursuant to a specified formula are generally separate derivative financial instruments that are accounted for as derivative liabilities. Earn Out Shares grant the holder with a variable number of Ordinary Shares based on certain vesting conditions tied to the stock price and are accounted for as derivative liabilities. In the event that the fair value of any derivative liabilities, determined using unobservable inputs, exceeds the transaction price of a borrowing arrangement, the Group records a deferred loss at the inception of the borrowing arrangement for the difference between the fair value of the derivative liabilities and the transaction price of the borrowing arrangement. Such deferred losses are recognized over the term of the related borrowing arrangement using the straight-line method of amortization. The deferred loss is netted against derivative financial liabilities on the consolidated statements of financial position. Amortization of the deferred loss is recognized as a component of “Finance costs” in the consolidated statements of profit or loss and other comprehensive income or loss.
The Group recognized derivative liabilities related to the Predecessor Earn Out Shares, OACB Earn Out Shares and assumed OACB warrants. Additionally, the Group recognized an embedded derivative for the conversion feature associated with the Tranche A Convertible Bonds, as further described in Note 21. These features are liability-classified, rather than equity-classified, because the Group is obligated to issue a variable number of ordinary shares to the holder upon conversion or exercise of the feature. Therefore, these derivative liabilities were initially recorded at fair value and remeasured to fair value at each reporting period with gains and losses arising from changes in the fair value recognized in finance income or finance costs, as appropriate.
The fair values of the derivative liabilities were determined using a valuation approach that incorporated a range of inputs that are both observable and unobservable in nature. The inputs used in the initial and subsequent fair value measurements predominantly relate to (i) the price of the Group’s Ordinary Shares (ii) the volatility of the Group’s Ordinary Shares, (ii) a risky discount rate corresponding to the credit risk associated with the repayment of the host debt instruments, and (iii) the probabilities of each derivative being exercised by the holder and the timing of such exercises. The probabilities are determined based on all relevant internal and external information available and are reviewed and reassessed at each reporting date.
The Group will derecognize any derivative liabilities if and when the rights are exercised by the holders or the time period during which the rights can be exercised expires.
Liabilities to related parties
The majority of the Group’s liabilities to related parties arose from its acquisition of rights for the commercialization of the Group’s biosimilar Adalimumab product in certain territories in Asia from Lotus Pharmaceutical Co. Ltd., a
F-20
related party, during the year ended 31 December 2021. Pursuant to the terms of the asset acquisition, the Group made an upfront payment of $1.9 million and is required to pay $7.4 million upon the commercial launch of Adalimumab in China which became due on 31 December 2023.
Long-term incentive plans
Share appreciation rights
The Group issued to certain current and former employees share appreciation rights ("SARs") that require settlement in connection with the occurrence of specified, future triggering events. Grants occurred from 2015 through 2020. The awards include a combination of vesting conditions, such as service and performance conditions, as well as non-vesting conditions depending on the particular award. The individuals retain their vested awards upon termination of employment with the Group. Settlement amounts are determined by the change in the Group’s market value from the grant date of the SAR until the triggering events occur. The SARs do not expire at a specific date.
Pursuant to the terms of the SAR agreements, management determined that the Group cannot avoid paying cash to settle the awards and, therefore, SARs are liability-classified in the consolidated statements of financial position. Accordingly, SARs were recorded at fair value and were subsequently remeasured each reporting period with the change in fair value reflected as a gain or loss in the consolidated statements of profit or loss and other comprehensive income or loss, as appropriate. The fair value of the SARs was determined using the Black-Scholes-Merton pricing model. In connection with the closing of the Business Combination, the Company reached a settlement agreement for share appreciation rights previously awarded to certain current and former employees. The remaining share appreciation rights were settled through the issuance of fully vested RSUs under the Management Incentive Plan on 1 December 2022.
Employee incentive plan
The Group also sponsors an employee incentive plan for certain qualifying employees. Under the plans, such employees are entitled to cash payments upon achievement of key milestones, such as a research and development milestone or the occurrence of an exit event. The awards include a combination of vesting conditions, such as service and performance conditions, as well as non-vesting conditions depending on the particular award. Since the Group cannot avoid paying cash to settle the awards, the employee incentive plan is liability-classified in the consolidated statements of financial position. Accordingly, awards issued pursuant to the employee incentive plan are recorded at fair value and are subsequently remeasured each reporting period with the change in fair value reflected as a gain or loss in the consolidated statements of profit or loss and other comprehensive income or loss, as appropriate. Employee incentive plan liabilities are presented as either current or non-current on the consolidated statements of financial position based on the anticipated timing of settlement.
The fair value of the employee incentive plan awards is determined by estimating the probability of success in reaching the specified milestones and other levers, such as the anticipated timing of potential milestone achievement.
Management Incentive Plan
The Group can issue share options, restricted share units (“RSUs”), and other share-based awards under the Company’s new incentive plan (the “Management Incentive Plan”) which was approved by the Board in June 2022. Awards issued under the Management Incentive Plan are accounted for in accordance with IFRS 2. Share-based payments are classified as equity-settled share-based payments as the Company intends to settle the awards with equity and has the commercial substance to do so. Share-based payments are measured at the grant date fair value of the instruments issued and recognized over the expected vesting periods. The number of shares expected to vest are reviewed and adjusted at the end of each reporting period such that the amount of expense recognized shall be based on the number of equity instruments that will eventually vest.
2.19 Litigation and other contingencies
The Group may, from time to time, become involved in legal proceedings arising out of the normal course of its operations. For instance, as a developer and manufacturer of biosimilars, the Group may be subject to lawsuits alleging patent infringement or other similar claims filed by the reference product sponsor. Similarly, the Group may utilize patent challenge procedures to challenge the validity, enforceability or infringement of the reference product
F-21
sponsor’s patents. Other parties may also file patent infringement claims against the Group alleging that the Group’s products or manufacturing process techniques infringe their patents.
The Group establishes reserves for specific legal matters when it determines that the likelihood of an unfavorable outcome is probable and the loss is reasonably estimable. When such conditions are not met for a specific legal matter, no reserve is established. Although management currently believes that resolving claims against the Group, including claims where an unfavorable outcome is reasonably possible, will not have a material impact on the liquidity, results of operations, or financial condition of the Group, these matters are subject to inherent uncertainties and management’s view of these matters may change in the future. It is possible that an unfavorable outcome of a lawsuit or other contingency could have a material impact on the liquidity, results of operations, or financial condition of the Group.
Significant judgment is required in both the determination of probability of loss and the determination as to whether the amount of loss can be reasonably estimated. Accruals are based only on information available at the time of the assessment, due to the uncertain nature of such matters. As additional information becomes available, management reassesses potential liabilities related to pending claims and litigation and may revise its previous estimates, which could materially affect the Group’s results of operations in a given period.
The Group maintains liability insurance coverages for various claims and exposures. The Group’s insurance coverage limits its maximum exposure on claims; however, the Group is responsible for any uninsured portion of losses. Management believes that present insurance coverage is sufficient to cover potential exposures.
2.20 Leases
The Group assesses whether a contract is or contains a lease at inception of the contract. The Group recognizes a right-of-use asset and a corresponding lease liability with respect to all lease arrangements in which it is the lessee, except for those with a lease term of twelve months or less and leases of low value assets. For these leases, the Group recognizes the lease payments as an operating expense on a straight-line basis over the term of the lease unless another systematic basis is more representative of the time pattern in which economic benefits from the leased assets are consumed. The Group’s leased assets consist of various real estate, fleet and equipment leases.
Right-of-use assets reflect the initial measurement of the lease liability, lease payments made at or before the lease commencement date and any initial direct costs less lease incentives that may have been received by the Group. These assets are subsequently measured at cost less accumulated depreciation, impairment losses and remeasurements of the underlying lease liability. Right-of-use assets are depreciated over the shorter of the lease term and the useful life of the underlying asset. If a lease transfers ownership of the underlying asset to the Group or the lease includes a purchase option that the Group is reasonably certain to exercise, the related right-of-use asset is depreciated over the useful life of the underlying asset. Depreciation starts at the commencement date of the lease.
Lease liabilities are initially measured at the present value of the lease payments that are not paid at the commencement date, discounted by using the rate implicit in the lease. If this rate cannot be readily determined, the Group uses its incremental borrowing rate, which is the rate of interest that the Group would need to pay to borrow, on a collateralized basis, an amount equal to the lease payments over a similar term in a similar economic environment based on information available at the commencement date of the lease. The lease payments included in the measurement of the lease liability comprise fixed payments (including in-substance fixed payments) less any incentives, variable lease payments that depend on an index or rate, expected residual guarantees and the exercise price of purchase options reasonably certain to be exercised by the Group.
The lease liability is subsequently measured by increasing the carrying amount to reflect interest on the lease liability, using the effective interest method, and by reducing the carrying amount to reflect payments made during the lease term. The Group remeasures the lease liability if the lease term has changed, when lease payments based on an index or rate change or when a lease contract is modified and the modification is not accounted for as a separate lease.
Variable payments that do not depend on an index or rate are not included in the measurement of the lease liability and the right-of-use asset. The related payments are recognized as an expense in the period in which the event or condition that triggers those payments occurs.
As a practical expedient, lessees are not required to separate non-lease components from lease components, and instead account for any lease and associated non-lease components as a single lease component. The Group has used this practical expedient.
F-22
2.21 Loss per share
Holders of the Predecessor Earn Out Shares and OACB Earn Out Shares have equal dividend and participation rights to the ordinary shareholders. However, these participating securities are classified as liabilities and as such, the shares held are not included in the weighted average number of ordinary shares outstanding in the basic loss per share calculation.
The calculation of basic loss per share is based on the loss for the year attributable to ordinary shareholders of the Group and the weighted average number of ordinary shares outstanding during the period.
Diluted loss per share is computed by dividing the loss for the year attributable to ordinary shareholders of the Group by the weighted average number of ordinary shares outstanding in the basic loss per share calculation, both of which are adjusted for the effects of all dilutive potential ordinary shares. Antidilutive effects of potential ordinary shares, which result in an increase in earnings per share or a reduction in loss per share, are not recognized in the computation of diluted loss per share.
3. New accounting standards
New standards and interpretations adopted and effective during the periods
The following new IFRS standards have been adopted by the Group effective 1 January 2023:
IFRS 17 - Insurance Contracts
In May 2017, the IASB issued IFRS 17, Insurance Contracts, which replaces IFRS 4, Insurance Contracts. This standard sets out principles for the recognition, measurement, presentation and disclosure of insurance contracts that are within the scope of IFRS 17. In June 2020, the IASB issued Amendments to IFRS 17, which addresses concerns and implementation challenges that were identified after IFRS 17, Insurance Contracts, was published in 2017. The amendments are effective for annual periods beginning on or after 1 January 2023. IFRS 17 requires fundamental accounting changes to how insurance contracts are measured and accounted for. It introduces the general measurement model, based on a risk-adjusted present value of future cash flows that will arise as the insurance contract is fulfilled. This new measurement model aims to provide relevant information of the future cash flows. The general measurement model is modified for the measurement of reinsurance contracts held, direct participating contracts, and investment contracts with discretionary participation features. Also, while the general measurement model applies to all groups of insurance contracts in scope of IFRS 17, a simplified approach (a premium allocation approach) may be used to measure contracts that meet certain criteria. IFRS 17 also includes new disclosure requirements, providing more clarity and transparency for users of financial statements. The adoption of the standard did not have a material impact on the consolidated financial statements of the Group.
IAS 1 (Amendment) - Disclosure of Accounting Policies
The IASB issued Disclosure of Accounting Policies (Amendments to IAS 1) and IFRS Practice Statement 2 Making Materiality Judgements. The amendments replace the requirement for entities to disclose their significant accounting policies with the requirement to disclose their material accounting policy information. The amendments also include guidance to help entities apply the definition of material in making decisions about accounting policy disclosures. The adoption of these amendments did not have a material impact on the consolidated financial statements of the Group.
IAS 8 (Amendments) - Definition of Accounting Estimates
The IASB issued amendments on IAS 8 to help entities to distinguish between accounting policies and accounting estimates. The amendments clarify how companies distinguish changes in accounting policies from changes in accounting estimates, with a primary focus on the definition of and clarifications on accounting estimates. The distinction between the two is important because changes in accounting policies are applied retrospectively, while changes in accounting estimates are applied prospectively. The amendments further clarify that accounting estimates are monetary amounts in the financial statements and are subject to measurement uncertainty. The amendments also clarify the relationship between accounting policies and accounting estimates by specifying that a company develops accounting estimates to achieve the objective set out by an accounting policy. The amendments are reflected in all financial statements and disclosures of the Group. The adoption of the amendments did not have a material impact on the consolidated financial statements of the Group.
F-23
IAS 12 (Amendments) - Deferred Tax Related to Assets and Liabilities Arising from a Single Transaction
The IASB issued amendments on IAS 12, which clarifies how companies shall account for deferred tax on transactions such as leases and decommissioning obligations, with a focus on reducing diversity in practice. The amendments narrow the scope of the initial recognition exemption in paragraphs 15 and 24 of IAS 12 so that it does not apply to transactions that give rise to equal and offsetting temporary differences. As a result, companies will need to recognize deferred tax assets and a deferred tax liability for temporary differences arising on initial recognition of a lease and a decommissioning provision. The adoption of the amendments did not have a material impact on the consolidated financial statements of the Group.
IAS 12 (Amendments) - International Tax Reform—Pillar Two Model Rules
In March 2022, the OECD released technical guidance on its 15% global minimum tax agreed as the second ‘pillar’ of a project to address the tax challenges arising from digitalisation of the economy. This guidance elaborates on the application and operation of the Global Anti-Base Erosion (GloBE) Rules agreed and released in December 2021 which lay out a co-ordinated system to ensure that multinational enterprises with revenues above €750 million pay tax of at least 15% on the income arising in each of the jurisdictions in which they operate. In May 2023, the IASB issued amendments to IAS12 Income Taxes to introduce a temporary exception to the requirements to recognise and disclose information about deferred tax assets and liabilities related to Pillar Two income taxes. As the Company does not meet the revenue thresholds, this guidance had no impact on the Group's consolidated financial statements.
New and revised IFRS standards in issue but not yet effective
The following new standards are not yet adopted by or effective for the Group and have not been applied in preparing these consolidated financial statements.
IAS 1 (Amendments) – Classification of Liabilities as Current or Non-Current
The IASB issued amendments to IAS 1, which affect the presentation of liabilities as current or non-current in the statement of financial position. The amendment does not impact the amount or timing of recognition of any asset, liability, income or expenses, or the information disclosed about those items. The amendments clarify that the classification of liabilities as current or non-current is based on rights that are in existence at the end of the reporting period, specify that classification is unaffected by expectations about whether an entity will exercise its right to defer settlement of a liability, explain that rights are in existence if covenants are complied with at the end of the reporting period, and introduce a definition of ‘settlement’ to make clear that settlement refers to the transfer to the counterparty of cash, equity instruments, other assets or services. The amendments are applied retrospectively for annual periods beginning on or after 1 January 2024, with early application permitted. The Group currently evaluates the impact of these amendments on the consolidated financial statements.
IAS 1 (Amendments) – Non-current Liabilities with Covenants
These amendments clarify how conditions with which an entity must comply within twelve months after the reporting period affect the classification of a liability. The amendments also aim to improve information an entity provides related to liabilities subject to these conditions. The amendments also respond to stakeholders’ concerns about the classification of such a liability as current or non-current. The amendment is effective for annual periods beginning on or after 1 January 2024. The Group currently evaluates the impact of these amendments on the consolidated financial statements.
IFRS 16 (Amendment) - Lease Liability in a Sale and Leaseback
This amendment adds subsequent measurement requirements for sale and leaseback transactions. This amendment includes requirements for sale and leaseback transactions in IFRS 16 to explain how an entity accounts for a sale and leaseback after the date of the transaction. Sale and leaseback transactions where some or all the lease payments are variable lease payments that do not depend on an index or rate are most likely to be impacted. The amendment is effective for annual periods beginning on or after 1 January 2024. The Group anticipates that the application of this amendment will not have a material impact on the consolidated financial statements.
F-24
4. Segment reporting
As disclosed in Note 2, the Group operates and manages its business as one operating segment.
A significant portion of the Group’s revenue is generated from long-term out-license contracts which provide the customer with exclusive rights to a particular territory, which generally span multiple countries or a particular continent, as well as the Group’s promises to continue development of the underlying compound and to provide supply of the product to the customer upon commercialization. Therefore, based on the nature of the customer agreements, revenue information is not currently available on a country-by-country basis.
Revenue from customers based on the geographic market in which the revenue is earned, which predominantly aligns with the rights conveyed to the Group’s customers pursuant to its out-license contracts, is as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Europe | 63,510 | 39,433 | 20,509 | ||||||||||||||
| North America | 18,306 | 30,780 | 11,660 | ||||||||||||||
| Asia and other | 9,618 | 12,816 | 4,603 | ||||||||||||||
| 91,434 | 83,029 | 36,772 | |||||||||||||||
Non-current assets, excluding financial instruments and deferred tax assets, based on the location of the asset is as follows:
| 2023 | 2022 | ||||||||||
| Europe | 415,659 | 334,837 | |||||||||
| North America | 5,094 | 240 | |||||||||
| Asia and Other | 6,194 | 3,715 | |||||||||
| 426,947 | 338,792 | ||||||||||
Revenue from transactions with individual customers that exceeds ten percent or more of the Group’s total revenue is as follows:
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||
| Revenue | % Total | Revenue | % Total | Revenue | % Total | ||||||||||||||||||||||||||||||
| Customer A | 9,430 | 10.3 | % | 17,940 | 21.6 | % | 10,070 | 27.4 | % | ||||||||||||||||||||||||||
| Customer B | 46,954 | 51.4 | % | 38,376 | 46.2 | % | 18,369 | 50.0 | % | ||||||||||||||||||||||||||
| Customer C | 8,876 | 9.7 | % | 12,840 | 15.5 | % | 1,590 | 4.3 | % | ||||||||||||||||||||||||||
| Customer D | 16,556 | 18.1 | % | — | — | % | — | — | % | ||||||||||||||||||||||||||
5. Revenue
Disaggregated revenue
The following table summarizes the Groups’ revenue from contracts with customers, disaggregated by the type of good or service and timing of transfer of control of such goods and services to customers:
| 2023 | 2022 | 2021 | |||||||||||||||
| Product revenue (point in time revenue recognition) | 48,699 | 24,836 | — | ||||||||||||||
| License revenue (point in time revenue recognition) | 12,177 | 424 | 1,453 | ||||||||||||||
| Development and other service revenue (over time revenue recognition) | 30,558 | 57,769 | 35,319 | ||||||||||||||
| 91,434 | 83,029 | 36,772 | |||||||||||||||
F-25
Reassessment of variable consideration
Subsequent changes to the estimate of the transaction price are generally recorded as adjustments to revenue in the period of change. The Group updates variable consideration estimates on a quarterly basis. The quarterly changes in estimates did not result in material adjustments to the Group’s previously reported revenue or trade receivables during the years ended 31 December 2023, 2022, and 2021.
Contract assets and liabilities
A reconciliation of the beginning and ending balances of contract assets and contract liabilities is shown in the table below:
Contract Assets | Contract Liabilities | ||||||||||
| 31 December 2021 | 19,438 | 74,536 | |||||||||
| Contract asset additions | 29,823 | — | |||||||||
| Amounts transferred to trade receivables | (19,690) | — | |||||||||
| Customer prepayments | — | 46,127 | |||||||||
| Revenue recognized | — | (26,782) | |||||||||
| Foreign currency adjustment | (915) | 51 | |||||||||
| 31 December 2022 | 28,656 | 93,932 | |||||||||
| Contract asset additions | 19,634 | — | |||||||||
| Amounts transferred to trade receivables | (2,412) | — | |||||||||
| Derecognition of contract liability | — | (42,089) | |||||||||
| Customer prepayments | — | 100,555 | |||||||||
| Revenue recognized | — | (23,101) | |||||||||
| Foreign currency adjustment | 171 | 3,147 | |||||||||
| 31 December 2023 | 46,049 | 132,444 | |||||||||
The net increase in contract assets as of 31 December 2023 is due to revenue recognized when the performance obligation has been met which is offset by the transfer of such amounts to trade receivables on the basis that the Group’s right to that consideration is no longer contingent on its performance. The net increase in contract liabilities as of 31 December 2023 is due to customer prepayments in advance of the Group’s performance. As of 31 December 2023, $10.9 million and $35.2 million are recorded as non-current contract assets and current contract assets, respectively. Non-current contract assets will materialize over the next 2 to 3 years. As of 31 December 2023, $73.3 million and $59.2 million are recorded as non-current contract liabilities and current contract liabilities, respectively. Non-current contract liabilities will be recognized as revenue over the next 2 to 6 years as either services are rendered or contractual milestones are achieved, depending on the performance obligation to which the payment relates.
Remaining performance obligations
Due to the long-term nature of the Group’s out-license contracts, the Group’s obligations pursuant to such contracts represent partially unsatisfied performance obligations at year-end. The revenues under existing out-license contracts with original expected durations of more than one year are estimated to be $383.0 million. The Group expects to recognize the majority of these revenues over the next 5 years.
F-26
6. Salaries and other employee expenses
The average number of individuals employed by the Group during the years ended 31 December 2023, 2022, and 2021 was 999, 858, and 645, respectively. The aggregate salary and other employee expenses incurred by the Group for these employees were as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Salary expense | 107,067 | 92,082 | 67,433 | ||||||||||||||
Defined contribution plan expense (1) | 11,518 | 10,052 | 7,694 | ||||||||||||||
| Long-term incentive plan expense | 78 | 5,481 | 17,955 | ||||||||||||||
| Share-based payments (see Note 23) | 18,033 | 10,317 | — | ||||||||||||||
| Other employee expense | 19,718 | 11,670 | 10,274 | ||||||||||||||
| Temporary labor | 8,495 | 5,838 | 6,164 | ||||||||||||||
| 164,909 | 135,440 | 109,520 | |||||||||||||||
(1)Defined contribution plan expense consists of costs incurred by the Group for employees of certain subsidiaries that are required by local laws to participate in pension schemes. These pension schemes are not sponsored or administered by the Group. Pursuant to the requirements of the schemes, the Group is required to contribute a certain percentage of its payroll costs to the pension schemes. Such contributions are charged to the consolidated statements of profit or loss and other comprehensive income or loss as they are incurred in accordance with the rules of the pension schemes.
Salaries and other employee expenses are included within the consolidated statements of profit or loss and other comprehensive income or loss as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Cost of product revenue | 76,908 | 42,501 | — | ||||||||||||||
| Research and development expenses | 44,339 | 52,962 | 71,588 | ||||||||||||||
| General and administrative expenses | 43,662 | 39,977 | 37,932 | ||||||||||||||
| Total salary and other employee expenses | 164,909 | 135,440 | 109,520 | ||||||||||||||
7. Finance income and finance costs
Finance income earned during the years ended 31 December 2023, 2022 and 2021 is as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Changes in the fair value of derivatives (see Note 28) | — | 1,637 | 51,549 | ||||||||||||||
| Interest income from cash and cash equivalents | 4,547 | 556 | 18 | ||||||||||||||
| Other interest income | 276 | 356 | 1 | ||||||||||||||
| 4,823 | 2,549 | 51,568 | |||||||||||||||
F-27
Finance costs incurred during the years ended 31 December 2023, 2022, and 2021 are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Changes in the fair value of derivatives (see Note 28) | 132,333 | 96,981 | 2,804 | ||||||||||||||
| Interest on debt and borrowings | 129,327 | 71,452 | 106,548 | ||||||||||||||
| Consenting fee (see Note 21) | — | 7,430 | — | ||||||||||||||
| Loss on remeasurement of bonds (see Note 21) | — | 6,511 | — | ||||||||||||||
| Interest on lease liabilities (see Note 13) | 3,840 | 6,022 | 6,423 | ||||||||||||||
| Amortization of deferred debt issue costs | 1,657 | 23 | 1,586 | ||||||||||||||
| 267,157 | 188,419 | 117,361 | |||||||||||||||
8. Depreciation, amortization and impairment
Depreciation, amortization and impairment expenses incurred during the years ended 31 December 2023, 2022, and 2021 are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Depreciation and impairment of property, plant and equipment (see Note 12) | 14,353 | 9,807 | 10,666 | ||||||||||||||
| Depreciation of right of use assets (see Note 13) | 8,913 | 9,869 | 8,699 | ||||||||||||||
| Amortization and impairment of intangible assets (see Note 15) | 2,723 | 3,488 | 4,916 | ||||||||||||||
| 25,989 | 23,164 | 24,281 | |||||||||||||||
Depreciation, amortization and impairment expenses are included within the consolidated statements of profit or loss and other comprehensive income or loss as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Cost of product revenue | 15,582 | 10,053 | — | ||||||||||||||
| Research and development expenses | 6,886 | 9,757 | 21,764 | ||||||||||||||
| General and administrative expenses | 3,521 | 3,354 | 2,517 | ||||||||||||||
| Total depreciation, amortization and impairment expense | 25,989 | 23,164 | 24,281 | ||||||||||||||
9. Audit fees
| 2023 | 2022 | 2021 | |||||||||||||||
| Financial Statement audit fees | 2,876 | 2,615 | 5,502 | ||||||||||||||
| Other fees, including tax services | 462 | 676 | 136 | ||||||||||||||
| Total fees | 3,339 | 3,291 | 5,638 | ||||||||||||||
Financial Statements audit fees consist of fees for the audit of our annual financial statements and other professional services provided in connection with the statutory and regulatory filings or engagements, including fees for the review of our interim financial information.
Other fees, including tax services, include fees for review of our current and historical financial information included in our SEC registration statements, fees for tax compliance, tax advice, and tax planning.
Audit fees for 2021 included fees for the audit of the consolidated financial statements for 2019 and 2020.
F-28
10. Income tax
Taxation recognized in the consolidated statements of profit or loss and other comprehensive income or loss during the years ended 31 December 2023, 2022, and 2021 is as follows:
| Current tax | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Direct taxes - current | 1,307 | 1,015 | 706 | ||||||||||||||
| Direct taxes – prior year | (60) | (115) | 491 | ||||||||||||||
| Total current tax | 1,247 | 900 | 1,197 | ||||||||||||||
| Deferred tax | |||||||||||||||||
| Current | (89,847) | (54,236) | (48,414) | ||||||||||||||
| Prior year | (10,718) | 15,269 | (477) | ||||||||||||||
| Total deferred tax | (100,565) | (38,967) | (48,891) | ||||||||||||||
| Total income tax benefit | (99,318) | (38,067) | (47,694) | ||||||||||||||
The prior year deferred tax impact of $10.7 million mainly relates to foreign currency impact on losses denominated in Icelandic krona.
The factors affecting the tax benefit during the years ended 31 December 2023 and 2022 relate to the recognition of a deferred tax asset on accumulated tax losses, as management assessed that it was probable that the accumulated tax losses would be fully utilized in the coming years, as further described below.
There were no accruals for tax contingencies during the years ended 31 December 2023, 2022, and 2021.
The effective tax rate for the year of 15.3% (2022: 6.9%, 2021: 32.0%) is lower than the applicable Luxembourgish statutory rate of corporation tax. The reconciling items between the statutory rate and the effective tax rate are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Tax rate | 24.9 | % | 24.9 | % | 24.9 | % | |||||||||||
| Effect of tax rate in foreign jurisdictions | (3.4 | %) | (2.4 | %) | (8.2 | %) | |||||||||||
| Permanent differences | (6.7 | %) | (8.9 | %) | 30.4 | ||||||||||||
| Non-recognition of tax losses | (1.5 | %) | (3.8 | %) | (15.0 | %) | |||||||||||
| Other items | 2.0 | % | (2.9 | %) | (0.1) | ||||||||||||
| Effective tax rate | 15.3 | % | 6.9 | % | 32.0 | % | |||||||||||
The movement in net deferred taxes during the years ended 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Balance at 1 January | 209,187 | 170,268 | |||||||||
| Deferred tax credited to profit or loss | 100,567 | 38,919 | |||||||||
| Balance at 31 December | 309,754 | 209,187 | |||||||||
| Deferred tax assets | 309,807 | 209,496 | |||||||||
| Deferred tax liabilities | (53) | (309) | |||||||||
Where there is a right of offset of deferred tax balances within the same tax jurisdiction, IAS 12 requires these to be presented after such offset in the consolidated statements of financial position. The closing deferred tax balances included above are after offset; however, the disclosure of deferred tax assets by category below are presented before such offset.
F-29
The amount of deferred tax recognized in the consolidated statements of financial position as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Deferred tax assets attributable to temporary differences in respect of tax losses | 301,375 | 205,290 | |||||||||
| Deferred tax assets attributable to other temporary differences | 11,941 | 6,832 | |||||||||
| Deferred tax liabilities attributable to other temporary differences | (3,562) | (2,935) | |||||||||
| Net deferred tax assets | 309,754 | 209,187 | |||||||||
A deferred tax liability has been recognized in relation to ordinary timing differences arising from depreciation, amortization, other provisions and difference in measurement basis of customer relationships. A deferred tax liability of $3.6 million and $2.9 million has been recognized as of 31 December 2023 and 2022, respectively.
A deferred tax asset has been recognized in relation to ordinary timing differences arising from various provisions, reserves, employee benefits and tax losses carried forward in the Group. The deferred tax asset on tax losses relates to tax losses arising in Iceland, and management considers probable that future forecasted profit associated with product and out-licensing revenue will be available to offset the cumulative tax losses as of 31 December 2023. No deferred tax asset is recognized on tax losses arising in Luxembourg as their recoverability is unlikely to be realized. A deferred tax asset of $309.8 million and $209.5 million is recognized as of 31 December 2023 and 2022, respectively.
These tax losses expire as follows:
| 2024-2026 | 68,821 | ||||
| 2027-2029 | 289,608 | ||||
| Later | 1,155,294 | ||||
| 1,513,723 | |||||
As of December 2023, the Group has total unused tax losses of $1,514 million which is comprised of $1,501 million of accumulated tax losses in Iceland and $13 million accumulated tax losses in Luxembourg.
11. Loss per share
Basic loss per share is computed by dividing loss for the year by the weighted average number of ordinary shares outstanding during the period.
Diluted loss per share is computed by adjusting the calculation of basic loss per share for the effects of dilutive potential ordinary shares from financial instruments that may be converted or exercised into ordinary shares of the Group. For the years ended 31 December 2023 and 2022, 86,745,377 and 148,857,998, respectively, potential ordinary shares pursuant to the RSUs, Senior Bond Warrants, Aztiq Convertible Bond, 2022 Convertible Bonds, OACB Warrants, Predecessor Earn Out Shares, and OACB Earn Out Shares (as defined and discussed in Notes 21 and 28) were excluded in the calculation of diluted loss per share, since the effect of doing so would result in a reduction of loss per share and thus be antidilutive.
For the year ended 31 December 2021, there were no potential ordinary shares pursuant to such agreements as all conversion, warrant and funding rights associated with these agreements had been exercised or otherwise expired (refer to Note 22 for further details). Therefore, the calculation of diluted loss per share did not differ from the calculation of basic loss per share.
F-30
The calculation of basic and diluted loss per share for the years ended 31 December 2023, 2022, and 2021 is as follows (in thousands, except for share and per share amounts):
| 2023 | 2022 | 2021 | |||||||||||||||
| Earnings | |||||||||||||||||
| Loss for the year | (551,731) | (513,580) | (101,504) | ||||||||||||||
| Number of shares | |||||||||||||||||
| Weighted average number of ordinary shares outstanding | 227,256,469 | 197,721,710 | 110,673,309 | ||||||||||||||
| Basic and diluted loss per share | (2.43) | (2.60) | (0.92) | ||||||||||||||
12. Property, plant and equipment
Property, plant and equipment consists of facility and computer equipment, furniture, fixtures and leasehold improvements. Movements within property, plant and equipment during the years ended 31 December 2023 and 2022 are as follows:
| Facility | Facility Equipment | Furniture, fixtures and leasehold improvements | Computer equipment | Total | |||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| Balance at 1 January 2023 | 115,000 | 145,150 | 9,598 | 1,959 | 271,707 | ||||||||||||||||||||||||
| Reclassification of assets | — | 2,771 | (112) | (7) | 2,652 | ||||||||||||||||||||||||
| Additions | — | 29,351 | 1,500 | 518 | 31,369 | ||||||||||||||||||||||||
| Disposals | — | (1,233) | (23) | (136) | (1,392) | ||||||||||||||||||||||||
| Translation difference | — | 679 | (85) | (22) | 572 | ||||||||||||||||||||||||
| Balance at 31 December 2023 | 115,000 | 176,718 | 10,878 | 2,312 | 304,908 | ||||||||||||||||||||||||
| Depreciation | |||||||||||||||||||||||||||||
| Balance at 1 January 2023 | 359 | 46,002 | 3,233 | 1,519 | 51,113 | ||||||||||||||||||||||||
| Reclassification of assets | — | 3,330 | (112) | (7) | 3,211 | ||||||||||||||||||||||||
| Depreciation | 2,875 | 10,572 | 676 | 230 | 14,353 | ||||||||||||||||||||||||
| Disposals | — | (737) | (22) | (136) | (895) | ||||||||||||||||||||||||
| Translation difference | — | 330 | 40 | (23) | 347 | ||||||||||||||||||||||||
| Balance at 31 December 2023 | 3,234 | 59,497 | 3,815 | 1,583 | 68,129 | ||||||||||||||||||||||||
| Net carrying amount | |||||||||||||||||||||||||||||
| Balance at 31 December 2023 | 111,766 | 117,221 | 7,063 | 729 | 236,779 | ||||||||||||||||||||||||
F-31
| Facility | Facility Equipment | Furniture, fixtures and leasehold improvements | Computer equipment | Total | |||||||||||||||||||||||||
| Cost | |||||||||||||||||||||||||||||
| Balance at 1 January 2022 | — | 88,510 | 32,395 | 1,551 | 122,456 | ||||||||||||||||||||||||
| Reclassification of assets | — | 25,486 | (25,486) | — | — | ||||||||||||||||||||||||
| Additions | 115,000 | 35,156 | 2,706 | 357 | 153,219 | ||||||||||||||||||||||||
| Disposals | — | (2,959) | — | — | (2,959) | ||||||||||||||||||||||||
| Translation difference | — | (1,043) | (17) | 51 | (1,009) | ||||||||||||||||||||||||
| Balance at 31 December 2022 | 115,000 | 145,150 | 9,598 | 1,959 | 271,707 | ||||||||||||||||||||||||
| Depreciation | |||||||||||||||||||||||||||||
| Balance at 1 January 2022 | — | 33,853 | 8,614 | 1,459 | 43,926 | ||||||||||||||||||||||||
| Reclassification of assets | — | 5,985 | (5,985) | — | — | ||||||||||||||||||||||||
| Depreciation | 359 | 8,752 | 621 | 75 | 9,807 | ||||||||||||||||||||||||
| Disposals | — | (2,597) | — | — | (2,597) | ||||||||||||||||||||||||
| Translation difference | — | 9 | (17) | (15) | (23) | ||||||||||||||||||||||||
| Balance at 31 December 2022 | 359 | 46,002 | 3,233 | 1,519 | 51,113 | ||||||||||||||||||||||||
| Net carrying amount | |||||||||||||||||||||||||||||
| Balance at 31 December 2022 | 114,641 | 99,148 | 6,365 | 440 | 220,594 | ||||||||||||||||||||||||
On 16 November 2022, the Group entered into a share purchase agreement (the “Share Purchase Agreement”) relating to shares in Fasteignafélagið Sæmundur hf. (“Saemundur”) with ATP Holdings ehf., an affiliate of Aztiq. Pursuant to the Share Purchase Agreement, Alvotech purchased 99.99% of the shares in Saemundur through the issuance the Aztiq Convertible Bond, as defined and discussed in Note 21, and the assumption of debt. At the time of closing, Saemundur’s only asset was the property where Alvotech’s Reykjavik manufacturing and research facility (the “Facility”) are located.
The Share Purchase Agreement was accounted for as an asset acquisition under IFRS 3 as all of the fair value of the gross assets acquired from Saemundur were concentrated in the Alvotech Facility. As a result, the purchase price was determined to be $115.0 million, which consists of $80.0 million related to the fair value of the Aztiq Convertible Bond, $30.0 million in loans assumed by the Company, and $5.0 million associated with the settlement of the pre-existing relationship with Saemundur. The entire purchase price was allocated to the Facility as it was the only asset acquired. Additionally, the Company recognized a $3.9 million loss on the extinguishment of the lease liability related to the Facility. See Note 21 for further details.
The Group pledged $127.4 million and $122.4 million of property, plant and equipment as collateral to secure bank loans with third parties as of 31 December 2023 and 2022, respectively.
F-32
13. Leases
The Group’s leased assets consist of facilities, fleet and equipment pursuant to both arrangements with third parties and related parties. The carrying amounts of the Group’s right-of-use assets and the movements during the years ended 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Right-of-use assets | |||||||||||
| Balance at 1 January | 47,501 | 126,801 | |||||||||
| Adjustments for indexed leases | 7,354 | 10,201 | |||||||||
| New leases | 74,109 | 9,583 | |||||||||
| Cancelled leases | (139) | — | |||||||||
| Derecognition due to acquisition of Alvotech Facility (see Note 12) | — | (88,941) | |||||||||
| Reclassification | (443) | ||||||||||
| Depreciation | (8,913) | (9,869) | |||||||||
| Translation difference | 333 | (274) | |||||||||
| Balance at 31 December | 119,802 | 47,501 | |||||||||
The Group entered into lease agreement with Fasteignafelagid Eyjolfur hf. in April 2023 for a facility expansion in Iceland with remaining lease terms of approximately 15 years as of 31 December 2023. The building is 140,000 square feet and is currently in construction. The expansion is close to being finalized and is expected to be completed in 2024. The lease amount is in substance fixed and is based on construction cost, adjusted monthly. Righ-of-use asset as of 31 December 2023 amounts to $68.5 million.
The Group’s right-of-use assets as of 31 December 2023 and 2022 are comprised of the following:
| 2023 | 2022 | ||||||||||
| Right-of-use assets | |||||||||||
| Facilities | 110,692 | 41,702 | |||||||||
| Fleet | 389 | 339 | |||||||||
| Equipment | 8,721 | 5,460 | |||||||||
| 119,802 | 47,501 | ||||||||||
F-33
At the commencement date of the lease, the Group recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The Group’s lease liabilities and the movements during the years ended 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Lease liabilities | |||||||||||
| Balance at 1 January | 40,532 | 122,140 | |||||||||
| Adjustments for indexed leases | 7,405 | 10,247 | |||||||||
| New leases | 72,882 | 7,458 | |||||||||
| Cancelled leases | (167) | — | |||||||||
| Installment payments | (7,260) | (7,655) | |||||||||
| Derecognition due to acquisition of Alvotech Facility (see Note 12) | — | (80,075) | |||||||||
| Foreign currency adjustment | 1,932 | (11,682) | |||||||||
| Translation difference | (9) | 99 | |||||||||
| Balance at 31 December | 115,315 | 40,532 | |||||||||
| Current liabilities | (9,683) | (5,163) | |||||||||
| Non-current liabilities | 105,632 | 35,369 | |||||||||
The amounts recognized in the consolidated statements of profit or loss and other comprehensive income or loss during the years ended 31 December 2023, 2022, and 2021 in relation to the Group’s lease arrangements are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Depreciation expense from right-of-use assets | |||||||||||||||||
| Facilities | (7,631) | (9,423) | (8,228) | ||||||||||||||
| Fleet | (180) | (119) | (38) | ||||||||||||||
| Equipment | (1,102) | (327) | (433) | ||||||||||||||
| Total depreciation expense from right-of-use assets | (8,913) | (9,869) | (8,699) | ||||||||||||||
| Interest expense on lease liabilities | (3,840) | (6,022) | (6,423) | ||||||||||||||
| Foreign currency difference on lease liability | (1,932) | 11,682 | 3,744 | ||||||||||||||
| Loss from extinguishment of lease agreement (see Note 12) | (28) | (3,859) | — | ||||||||||||||
| Total amount recognized in profit and loss | (14,713) | (8,068) | (11,378) | ||||||||||||||
The maturity analysis of undiscounted lease payments as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Less than one year | 14,637 | 6,000 | |||||||||
| One to five years | 51,053 | 20,160 | |||||||||
| Thereafter | 89,682 | 22,274 | |||||||||
| 155,372 | 48,434 | ||||||||||
The Group’s lease liabilities as of 31 December 2023 and 2022 do not include $0.7 million and $0.1 million, respectively, of costs for short-term leases and low value leases.
F-34
14. Goodwill
The Group’s goodwill balances as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Balance as of 1 January | 11,643 | 12,367 | |||||||||
| Translation difference | 415 | (724) | |||||||||
| Balance as of 31 December | 12,058 | 11,643 | |||||||||
Goodwill is recognized at the Group level, which is determined to be the smallest cash-generating unit. The recoverable amount of the cash-generating unit is determined based on a value in use calculation which uses cash flow projections based on the financial forecast for the period 2024-2030 which reflect the recent business developments of the Group and has been approved by management and the Board of Directors. The Group determined that the terminal growth rate and the discount rate are the key assumptions used in determining the current estimate of value in use.
Cash flows beyond 2030 have been extrapolated using a negative 5% terminal rate in both the 2023 and 2022 value in use calculations, respectively. A discount rate of 25.0% (2022: 27.6%) per annum was used in determining the current estimate of value in use. Since the recoverable amount of the cash-generating unit was substantially in excess of its carrying amount as of 31 December 2023 and 2022, management believes that any reasonably possible change in the key assumptions on which the recoverable amount of the cash-generating unit is based would not cause the carrying amount of the cash-generating unit to exceed its recoverable amount.
There were no goodwill impairment charges recognized in the consolidated statements of profit or loss and other comprehensive income or loss in any prior periods.
15. Other Intangible assets
Other intangible assets consist of software, customer relationships, and licensed intellectual property rights. Movements in intangible assets during the years ended 31 December 2023 and 2022 are as follows:
| Software | Customer relationships | Intellectual property rights | Total | ||||||||||||||||||||
| Cost | |||||||||||||||||||||||
| Balance at 1 January 2023 | 13,684 | 2,181 | 15,000 | 30,865 | |||||||||||||||||||
| Reclassification of assets | 1,002 | — | — | 1,002 | |||||||||||||||||||
| Additions | 4,094 | — | 6,000 | 10,094 | |||||||||||||||||||
| Impairment | (1,779) | — | — | (1,779) | |||||||||||||||||||
| Retirement | — | — | (15,000) | (15,000) | |||||||||||||||||||
| Translation difference | 72 | 90 | — | 162 | |||||||||||||||||||
| Balance at 31 December 2023 | 17,073 | 2,271 | 6,000 | 25,344 | |||||||||||||||||||
| Amortization | |||||||||||||||||||||||
| Balance at 1 January 2023 | 3,343 | 1,870 | — | 5,213 | |||||||||||||||||||
| Amortization | 626 | 318 | — | 944 | |||||||||||||||||||
| Translation difference | 28 | 83 | — | 111 | |||||||||||||||||||
| Balance at 31 December 2023 | 3,997 | 2,271 | — | 6,268 | |||||||||||||||||||
| Net carrying amount | |||||||||||||||||||||||
| Balance at 31 December 2023 | 13,076 | — | 6,000 | 19,076 | |||||||||||||||||||
F-35
| Software | Customer relationships | Intellectual property rights | Total | ||||||||||||||||||||
| Cost | |||||||||||||||||||||||
| Balance at 1 January 2022 | 8,777 | 2,329 | 15,000 | 26,106 | |||||||||||||||||||
| Additions | 7,682 | — | — | 7,682 | |||||||||||||||||||
| Impairment | (2,755) | — | — | (2,755) | |||||||||||||||||||
| Translation difference | (20) | (148) | — | (168) | |||||||||||||||||||
| Balance at 31 December 2022 | 13,684 | 2,181 | 15,000 | 30,865 | |||||||||||||||||||
| Amortization | |||||||||||||||||||||||
| Balance at 1 January 2022 | 2,933 | 1,664 | — | 4,597 | |||||||||||||||||||
| Amortization | 423 | 310 | — | 733 | |||||||||||||||||||
| Translation difference | (13) | (104) | — | (117) | |||||||||||||||||||
| Balance at 31 December 2022 | 3,343 | 1,870 | — | 5,213 | |||||||||||||||||||
| Net carrying amount | |||||||||||||||||||||||
| Balance at 31 December 2022 | 10,341 | 311 | 15,000 | 25,652 | |||||||||||||||||||
Additions during the year ended 31 December 2023 were primarily comprised of licensed intellectual property rights from Kashiv as detailed below.
Expense for amortization of the Group’s intangible assets is included within the consolidated statements of profit or loss and other comprehensive income or loss as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Cost of product revenue | 318 | 471 | — | ||||||||||||||
| Research and development expenses | 8 | — | 324 | ||||||||||||||
| General and administrative expenses | 618 | 262 | 599 | ||||||||||||||
| 944 | 733 | 923 | |||||||||||||||
At 31 December 2023 and 2022, the Group performed a review of its intangible assets and determined certain software development had been abandoned. In assessing recoverable amount, the Group determined the market for resale was non-existent. Management therefore determined to fully impair the assets, resulting in an impairment charge of $1.8 million and $2.8 million during the years ended 31 December 2023 and 2022, respectively. The impairment charge was recognized as an expense within “General and administrative expense”. For the year ended 31 December 2022, the impairment was recognized as an expense as follows: $2.1 million in "Cost of product revenue" and $0.7 million in "General and administrative expense".
At 31 December 2023, following the termination of the agreement with Biosana, the Group derecognized $15.0 million of other intangible assets relating to intellectual property rights for the co-development and commercialization of AVT23. A corresponding receivable was recognized to reflect the claim against Biosana for full reimbursement. See further information on the receivable in Note 18.
Alvotech entered into an exclusive product licensing and supply agreement with Kashiv for the development and commercialization of AVT23 in September 2023. Under the terms of the agreement, Kashiv granted Alvotech an exclusive right for AVT23 which will be produced using Kashiv’s proprietary process technology and commercialized by Alvotech in specific territories. In exchange, Alvotech made an upfront payment of $3.0 million upon the signing of the agreement, with an additional $3.0 million due upon the beginning of Phase 3 which coincides with the clinical trial application ("CTA") submission.
In addition, Alvotech may be obligated to pay Kashiv up to an aggregate of $25 million (including the $6 million upfront payments mentioned above), payable upon the achievement of various development and regulatory milestones, as well as certain tiered royalty payments up to an aggregate of $15 million based on commercial sales of AVT23. The agreement terminates 10 years after the launch of AVT23 and is subject to certain customary termination rights.
F-36
16. Cash and cash equivalents
Cash and cash equivalents
Cash and cash equivalents include both cash in banks and on hand. Cash and cash equivalents as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Cash and cash equivalents denominated in US dollars | 1,466 | 10,377 | |||||||||
| Cash and cash equivalents denominated in other currencies | 9,691 | 56,050 | |||||||||
| 11,157 | 66,427 | ||||||||||
Restricted cash
Restricted cash relates to cash that may only be used pursuant to certain of the Group’s borrowing arrangements. Therefore, these deposits are not available for general use by the Group. Movements in restricted cash balances during the years ended 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Balance at 1 January | 25,187 | 10,087 | |||||||||
| Additions during the year | — | 14,914 | |||||||||
| Interest income | 945 | 186 | |||||||||
| Balance at 31 December | 26,132 | 25,187 | |||||||||
The Group’s restricted cash is available for use after one year or later.
17. Inventories
The Group’s inventory balances as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Raw materials and supplies | 51,524 | 41,961 | |||||||||
| Work in progress | 33,068 | 29,450 | |||||||||
| Finished goods | 244 | 2,121 | |||||||||
| Inventory reserves | (10,403) | (2,062) | |||||||||
| Balance at 31 December | 74,433 | 71,470 | |||||||||
The increase in inventory from 31 December 2022 to 31 December 2023 is due to the expansion of the commercial launch of certain of the Group’s biosimilar products.
The Group recognised $42.8 million and $20.9 million within cost of goods sold during the years ended 31 December 2023 and 2022, respectively.
During the years ended 31 December 2023, 2022, and 2021, write-down of inventories amounted to $10.4 million, $2.1 million and $1.2 million, respectively, due to product expiration and results from quality control inspections. There were no reversals of inventory write-downs during the years ended 31 December 2023, 2022, and 2021.
F-37
18. Other current assets
The composition of other current assets as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Value-added tax | 8,801 | 6,468 | |||||||||
| Prepaid expenses | 22,035 | 20,601 | |||||||||
| Proceeds receivable from Convertible Bonds (see Note 21) | — | 3,520 | |||||||||
| Derivative asset | — | 851 | |||||||||
| Other short-term receivables | 1,035 | 1,509 | |||||||||
| 31,871 | 32,949 | ||||||||||
During the year, the Group terminated the co-development agreement with Biosana for AVT23 and derecognized $15.0 million of other intangible assets and $3.5 million of prepaid development costs. A receivable of $18.5 million was recognized under other current assets which was fully reserved due to the uncertainty that it would be collected.
19. Share capital
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all liabilities. Equity instruments issued by a Group entity are recognized in the amount of the proceeds received, net of direct issue costs.
Prior to the Capital Reorganization the Group’s equity consisted of Class A and Class B ordinary shares (together the “Predecessor Ordinary Shares”). The Group’s authorized share capital was $99.7 million, consisting of the equivalent of 99,961,829 Class A or Class B ordinary shares with a par value of $0.01 per share. All share capital issued as of 31 December 2023 and 2022 was fully paid.
The Capital Reorganization resulted in the following share capital activity:
•All of the outstanding Predecessor Ordinary Shares were exchanged for 180,600,000 Ordinary Shares and 38,330,000 Predecessor Earn Out Shares;
•976,505 of Class A OACB Ordinary Shares were exchanged for Ordinary Shares;
•6,250,000 of Class B OACB Ordinary Shares were exchanged for 5,000,000 Ordinary Shares and 1,250,000 OACB Earn Out Shares; and
•17,493,000 Ordinary Shares were issued in the PIPE Financing.
No dividends were paid or declared during the years ended 31 December 2023, 2022, and 2021.
Share capital and share premium of the Group’s Ordinary Shares issued as of 31 December 2023, and 2022 are as follows (in thousands, except for share amounts):
| 2023 | 2022 | ||||||||||||||||||||||
| Shares | Share capital and share premium | Shares | Share capital and share premium | ||||||||||||||||||||
| Ordinary Shares | 266,821,844 | 1,231,969 | 252,160,087 | 1,060,558 | |||||||||||||||||||
| Total share capital and share premium | 266,821,844 | 1,231,969 | 252,160,087 | 1,060,558 | |||||||||||||||||||
On 10 February 2023, the Company completed a private placement equity offering for gross proceeds of $137.0 million, and transaction costs of $4.1 million, of its ordinary shares, par value $0.01 per share, at a purchase price of $11.57 per share. The shares were delivered from previously issued ordinary shares held by Alvotech’s subsidiary, Alvotech Manco ehf. As a result of proceeds raised from the private placement offering, the Company extinguished the derivative financial liability related to the Senior Bond Warrants since the Company has not anymore the obligation to issue the penny warrants representing 1.0% of the fully diluted ordinary share capital.
F-38
This was accounted for as an extinguishment of a derivative financial liability in the consolidated statements of profit or loss and other comprehensive income or loss. See Notes 21 and 28 for further information.
Movements in the Group’s Class A and Class B ordinary shares, share capital and share premium during the years ended 31 December 2023, 2022, and 2021 are as follows (in thousands, except for share amounts):
Ordinary Shares | Predecessor Ordinary Shares | Share capital | Share premium | Total | |||||||||||||||||||||||||
| Balance at 1 January 2021 | — | 7,259,139 | 73 | 166,740 | 166,813 | ||||||||||||||||||||||||
| Share issue | — | 6,222,660 | 62 | 833,378 | 833,440 | ||||||||||||||||||||||||
| Balance at 31 December 2021 | — | 13,481,799 | 135 | 1,000,118 | 1,000,253 | ||||||||||||||||||||||||
| Elimination of Predecessor Ordinary Shares (Note 1.1) | — | (13,481,799) | (135) | 135 | — | ||||||||||||||||||||||||
| Issuance of Ordinary Shares (Note 1.1) | 186,576,505 | — | 1,866 | 63,169 | 65,035 | ||||||||||||||||||||||||
| PIPE Financing (Note 1.1) | 17,493,000 | — | 175 | 174,755 | 174,930 | ||||||||||||||||||||||||
| Transaction costs arising on share issue | — | — | — | (5,562) | (5,562) | ||||||||||||||||||||||||
| Predecessor Earn Out Shares (Note 1.1) | 38,330,000 | — | — | (227,500) | (227,500) | ||||||||||||||||||||||||
| OACB Earn Out Shares (Note 1.1) | 1,250,000 | — | — | (9,100) | (9,100) | ||||||||||||||||||||||||
| SARs Settlement (Note 22) | 3,510,582 | — | 35 | 30,267 | 30,302 | ||||||||||||||||||||||||
| Settlement of related party loans with Ordinary Shares | 5,000,000 | — | 50 | 32,150 | 32,200 | ||||||||||||||||||||||||
| Balance at 31 December 2022 | 252,160,087 | — | 2,126 | 1,058,432 | 1,060,558 | ||||||||||||||||||||||||
| Capital contribution | 11,834,061 | — | 118 | 132,618 | 132,736 | ||||||||||||||||||||||||
| Vested earn-out shares | — | — | 6 | 8,300 | 8,306 | ||||||||||||||||||||||||
| Penny warrants (Note 28) | 2,479,962 | — | 25 | 27,159 | 27,184 | ||||||||||||||||||||||||
| Public warrants (Note 28) | 553,552 | — | 6 | 7,612 | 7,618 | ||||||||||||||||||||||||
| Settlement of RSUs with shares (Note 23) | 838,919 | — | 8 | 5,095 | 5,103 | ||||||||||||||||||||||||
| Settlement of SARs with shares (Note 22) | (1,044,737) | — | (10) | (9,526) | (9,536) | ||||||||||||||||||||||||
| Balance at 31 December 2023 | 266,821,844 | — | 2,279 | 1,229,690 | 1,231,969 | ||||||||||||||||||||||||
Alvotech Manco ehf., a subsidiary of Alvotech hf., owns 22,905,618 Ordinary Shares in Alvotech. Such shares are intended for the future issuance of Ordinary Shares under the Management Incentive Plan and other equity offerings.
20. Other reserves
The composition of other reserves as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Equity component of convertible bonds | 21,391 | 16,034 | |||||||||
| Share based payments | 21,520 | 14,548 | |||||||||
| 42,911 | 30,582 | ||||||||||
F-39
21. Borrowings
The Group’s debt consists of interest-bearing borrowings from financial institutions, related parties and third parties. Outstanding borrowings, net of transaction costs, presented on the consolidated statements of financial position as current and non-current as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Senior Bonds | 549,411 | 530,506 | |||||||||
| 2022 Convertible Bonds | 155,914 | 32,441 | |||||||||
| Aztiq Convertible Bond | 80,663 | 65,793 | |||||||||
| Alvogen Facility | 76,556 | 64,588 | |||||||||
| Other borrowings | 97,615 | 71,242 | |||||||||
| Total outstanding borrowings, net of debt issue costs | 960,159 | 764,570 | |||||||||
| Less: current portion of borrowings | (38,025) | (19,916) | |||||||||
| Total non-current borrowings | 922,134 | 744,654 | |||||||||
Convertible shareholder loans
In connection with the Business Combination Agreement (see Note 1.1), on 7 December 2021, the Group’s shareholders entered into the BCA Framework Agreement resulting in the exercise of the conversion, warrant, and funding rights associated with the convertible shareholder loans. As a result, the following issuances of Class A ordinary shares occurred:
•1,522,103 shares from the exercise of warrant and funding rights in exchange for $101.3 million of cash;
•1,137,248 shares from the exercise of warrant rights in exchange for the settlement of $73.7 million of accrued payment-in-kind interest; and
•2,306,555 shares resulting from the conversion of $166.8 million of outstanding principal and accrued payment-in-kind interest.
In connection with these exercises, for the year ended 31 December 2021, the Group recognized finance income of $48.7 million resulting from the remeasurement of the derivative liabilities at the date of extinguishment and a $149.2 million gain on extinguishment of financial liabilities, which primarily reflects the difference between the carrying amount of the pre-transaction convertible shareholder loans and the related derivative financial liabilities and the fair value of the ordinary shares issued. In addition, the gain on extinguishment of financial liabilities includes transaction costs incurred as part of the extinguishment, the acceleration of previously deferred debt issue costs incurred in connection with the issuance of the convertible shareholder loans and the acceleration of previously unamortized accretion of the convertible shareholder loans.
Bonds
On 24 June 2021, holders of the Group’s convertible bonds converted $100.7 million of principal and accrued interest and $4.8 million of additional premium offered by the Group to the bondholders into 455,687 Class A ordinary shares. Following the conversion, certain bondholders elected to redeem their remaining bonds for cash, resulting in the payment of $55.3 million in outstanding principal and accrued interest plus an additional $6.1 million of premium that the bondholders elected to be paid in cash.
The remaining unconverted and unredeemed bonds were replaced with new bonds with an extended maturity of June 2025 and the elimination of conversion rights, among other amendments to the terms and conditions. The Group offered the holders of the replaced bonds an extension premium of $8.1 million for their agreement to extend the maturity of the replaced bonds to June 2025, as well as an additional premium of $2.6 million, both of which were granted to the bondholders in the form of additional bonds. The Group also issued an additional $113.8 million of bonds to one previous bondholder and one new bondholder. On the date of issuance, the fair value and the nominal value of the bonds was $358.8 million and $397.4 million, respectively. The difference between the nominal value and fair value was recognized as a discount that will be amortized over the term of the bonds.
The Group determined that the 24 June 2021 transaction was a substantial modification to its convertible bonds and the associated derivative financial liability and accounted for the transaction as an extinguishment. As a result, the
F-40
Group recognized a gain on extinguishment of financial liabilities of $2.6 million during the year ended 31 December 2021, primarily driven by the difference between the fair value of the post-transaction bonds and the carrying amount of the pre-transaction bonds. The gain on extinguishment of financial liabilities also includes the following:
•Transaction costs and fees incurred as part of the extinguishment;
•The acceleration of previously deferred debt issue costs incurred in connection with the issuance of the pre-transaction bonds; and
•The acceleration of previously unamortized accretion of the pre-transaction bonds.
Prior to the extinguishment of the convertible bonds and as noted above, the bondholders had the option to convert the bonds into Class A ordinary shares up to fourteen days prior to maturity. This conversion right was separately accounted for as a derivative financial liability. During the period from 1 January 2021 to 24 June 2021, there was no change in fair value of the derivative financial liability.
As of 31 December 2021, the carrying amount of the bonds was $363.1 million. Accrued interest on the bonds as of 31 December 2021 is $31.0 million. The Group has the option, at any time, to prepay all or any part of the outstanding bonds. If the Group elects to prepay the bonds within the first three years of the bond agreement, the bondholders are entitled to be paid an additional premium of at least 2.0% of the outstanding principal at the time of such prepayment.
In January and June of 2022, the Group amended the terms of the outstanding bonds. The amendments resulted in the following:
•Following the close of the Business Combination, the interest rate will range from 7.5% to 10.0% depending on the amount of aggregate net proceeds, as defined by the terms of the amended bond agreement;
•A $7.4 million consent fee, recognized as finance costs, paid to the bondholders who did not vote against the Business Combination Agreement;
•The requirement for Alvotech to maintain a minimum of $25.0 million of restricted cash in a separate liquidity account; and
•A decrease in the interest rate to 7.5%, following the closing of the Business Combination, if the Company issues additional shares within six months of the Closing Date, resulting in the Company exceeding the amount of aggregate net proceeds, as defined in the bond agreement.
As a result of the closing of the Business Combination, there was a change in future cash flows on the bonds related to the increase in interest rate from 7.5% to 10.0%. The Company remeasured the carrying value in accordance with IFRS 9 to the present value of the revised cash flows and recognized a $6.5 million loss on the remeasurement of the bonds. The outstanding bonds were subsequently amended as described below.
Senior Bonds
On 16 November 2022, the Group amended and upsized the outstanding bonds by $70.0 million. The amended bond agreement (the “Senior Bonds”) resulted in the following:
•An increase in principal from $455.7 million at the time of the amendment, to $525.7 million;
•An increase in the interest rate, resulting in a range from 10.75% to 12.0% depending on the occurrence of certain events, as defined by the terms of the agreement. The Group accounted for this interest rate feature (the “Senior Bond Interest Rate Feature”) as an embedded derivative, classified as an other current asset in the consolidated statement of financial position as of 31 December 2022;
•Amended the terms of the related party loans from Alvogen, setting forth subordination conditions;
•Contingently issuable penny warrants (exercise price of $0.01) to the bondholders (the “Senior Bond Warrants”) if certain events occur, issuable in two tranches representing 1.5% and 1.0% of the fully diluted ordinary share capital, as defined in the Senior Bonds agreement (see Note 28).
The Group determined that the 16 November 2022 transaction was a substantial modification to its bonds and accounted for the transaction as an extinguishment. As a result, the Group recognized a loss on extinguishment of financial liabilities of $40.9 million, including $12.1 million of transaction costs, during the year ended 31
F-41
December 2022, primarily driven by the difference between the fair value of the post-transaction Senior Bonds and the Senior Bond Warrants and the carrying amount of the pre-transaction bonds. The loss on extinguishment of financial liabilities includes the following:
•Extinguishment of bonds with a carrying value of $440.1 million, including $4.8 million of accrued interest;
•Net cash proceeds of $57.9 million, including transaction costs paid of $12.1 million;
•Recognition of a $4.6 million derivative asset for the Senior Bond Interest Rate Feature;
•Recognition of $528.2 million and $15.4 million representing the fair value of the new Senior Bonds and Senior Bond Warrants (see Note 28), respectively.
As a result of proceeds raised from the private placement offering executed in February 2023, the Company extinguished the liability related to the senior bond warrants since the Company has not anymore the obligation to issue the penny warrants representing the 1.0% Senior Bond Warrants (see Note 28 for further information).
As of 31 December 2023, the carrying amount of the Senior Bonds is $549.4 million, compared to $530.5 million as of 31 December 2022. The Group has the option, at any time, to prepay all or any part of the outstanding bonds in exchange for the payment of the redemption premium pursuant to the terms of the agreement.
The Group has pledged its intellectual property as collateral for the Senior Bonds. Additionally, the Group has pledged the Facility of up to $600 million over the Facility as collateral for the Senior Bonds (2nd lien pledge), as further described in Note 12.
2022 Convertible Bonds
On 20 December 2022, the Group issued two tranches of convertible bonds (the “2022 Convertible Bonds”). Tranche A is ISK denominated with a principal balance of $59.1 million, of which $3.5 million in cash proceeds were received in February 2023, and carries an annual payment-in-kind interest rate of 15% per year, while Tranche B is USD denominated with a principal balance of $0.6 million and carries an annual payment-in-kind interest rate of 12.5% per year. The maturity date of the Convertible Bonds is the later of the (i) 20 December 2025 or (ii) 91 days after the earlier of the full redemption or the final maturity date of the Senior Bonds. Holders of both the Tranche A and Tranche B of the 2022 Convertible Bonds, may elect, at their sole discretion, to convert all or part of the principal amount and accrued interest into Alvotech Ordinary Shares at a conversion price of $10.00 per share on 31 December 2023, 30 June 2024, or upon mandatory or optional redemption of the bonds.
The conversion features (the “Tranche A Conversion Feature” and “Tranche B Conversion Feature”) for both the Tranche A and Tranche B of the 2022 Convertible Bonds were determined to be embedded derivatives as the economic characteristics and risks are not closely related to the debt host. The Group classified the Tranche A Conversion Feature as a liability due to the variability created by conversion rates resulting from the tranche being denominated in ISK and was determined to have a fair value of $24.9 million at issuance date (see Note 28 for further details). The Group classified the Tranche B Conversion Feature as equity due to the conversion price having preservation and passage of time adjustments that meet the fixed-for-fixed criteria.
On 25 January 2023, the Company issued an additional $10.0 million of Tranche B Convertible Bonds. The Tranche B Conversion Feature associated with this additional issuance was determined to have a fair value of $1.4 million at issuance date.
On 24 July 2023, Alvotech announced that Teva and Alvotech have agreed to expand their existing partnership agreement. As part of the agreement, Teva acquired Tranche B Convertible Bonds in principal amount of $40 million. The Tranche B Conversion Feature associated with this additional issuance was determined to have a fair value of $3.9 million at issuance date.
On 31 July 2023, Alvotech completed a private placement of Tranche A Convertible Bonds for a total principal amount of $100 million, or approximately ISK 13 billion at current exchange rates. As part of this private placement, ATP Holdings ehf., an affiliated of Aztiq, acquired Tranche A Convertible Bonds in principal amount of $30 million. The Tranche A Conversion Feature associated with these additional issuances was determined to have a fair value of $45.6 million at issuance date (see Note 28 for further details).
F-42
As of 31 December 2023, the carrying amount of the Tranche A and Tranche B of the 2022 Convertible Bonds is $107.1 million and $48.8 million, respectively.
Aztiq Convertible Bond
On 16 November 2022, the Group issued a convertible bond (also known as the “Aztiq Convertible Bond”) to ATP Holdings ehf, an affiliate of Aztiq, for the Share Purchase Agreement and the acquisition of the Alvotech Facility (See Note 12). The Aztiq Convertible Bond has a principal amount of $80.0 million and carries an interest rate of 12.50% per annum. Interest is payable in six-month intervals and is capitalized and added to the outstanding principal amount of the bonds. The maturity date of the convertible bond is the later of the (i) 16 November 2025 or (ii) 91 days after the earlier of the full redemption or the final maturity date of the Senior Bonds. Bondholders have the right to convert their outstanding bonds into ordinary shares of Alvotech on 30 December 2023, 30 June 2024, or when the bond has been called or put up for mandatory or optional redemption, for a conversion price is $10.00 per share.
The conversion feature (the “Aztiq Conversion Feature”) was determined to be an embedded derivative as the economic characteristics and risks are not closely related to the debt host. The Group classified the Aztiq Conversion Feature as equity due to the conversion price having preservation and passage of time adjustments that meet fixed-for-fixed criteria. As a result, the Group recognized the following related to the Aztiq Convertible Bond:
•$64.0 million related to the debt host;
•$16.0 million related to the Aztiq Conversion Feature; and
•$30.0 million related to the loans (the “Facility Loans”) on the building, which were assumed by the Group as part of the asset acquisition.
In April 2023, we were communicated that ATP Holdings ehf. sold a portion of the Aztiq Convertible Bond to Mitsui & Co., Ltd. ("Mitsui"), a global trading and investment company headquartered in Japan, and Shinhan Healthcare fund 5 ("Shinhan"), a fund established under the laws of the Republic of Korea.
As of 31 December 2023, the carrying amount of the Aztiq Convertible Bond was $80.7 million and includes $15.1 million held by ATP Holdings ehf. The carrying amount of the Aztiq Convertible Bond was $65.8 million as of 31 December 2022.
Alvogen Facility
In connection with an undertaking by Alvotech shareholders to ensure that Alvotech was sufficiently funded through the closing of the Business Combination by providing at least $50.0 million for the operations of the Group, Alvogen and Aztiq provided interest free loan advances to Alvotech. On 22 February 2022, Alvotech borrowed $15.0 million under the facility from Alvogen, as lender. On 29 March 2022, Alvotech withdrew an additional amount of $10.0 million under the facility, for aggregate indebtedness of $25.0 million. On 11 March 2022, Alvotech borrowed $15.0 million under the facility from Aztiq, as lender. On 31 March 2022, Alvotech withdrew an additional amount of $10.0 million under the facility, for aggregate indebtedness of $25.0 million.
On 12 July 2022, the Company entered into settlement agreements with both Aztiq and Alvogen for the $25.0 million in related party loans provided by each party. As a result of the settlement agreements, Aztiq and Alvogen each received 2,500,000 Ordinary Shares. The settlement was accounted for as an extinguishment of financial liabilities. In accordance with IFRS 9, the difference between the fair value of the consideration paid for the settlement, which was determined to be $32.2 million, and the extinguished financial liabilities of $50.0 million was recognized as a gain on the extinguishment of financial liabilities in the consolidated statement of profit or loss and other comprehensive income or loss.
On 11 April 2022, Alvotech entered into a loan agreement with Alvogen, as lender, for a loan of up to $40.0 million bearing an interest rate of 10% per annum. The loan was drawable in two separate installments of $20.0 million each. On 12 April 2022, Alvotech withdrew the first installment of $20.0 million. Alvotech withdrew a second installment of $20.0 million on 9 May 2022 for aggregate indebtedness of $40.0 million.
On 1 June 2022, Alvotech also entered into a loan agreement with Alvogen, as lender, for a loan of $20.0 million bearing an interest rate of 10% per annum. Alvotech withdrew the entire loan amount of $20.0 million on 1 June 2022.
F-43
In connection with the 16 November 2022 Senior Bonds amendment, Alvotech entered into a subordinated loan agreement with Alvogen (the “Alvogen Facility”). As part of the subordinated loan agreement, the Group agreed to the following:
•Rollover the $63.3 million outstanding, which includes $3.3 million of accrued interest, under the Alvogen loans, into the new subordinated loan agreement, and withdraw an additional $50.0 million in loans;
•The interest rate was increased from 10% per annum to 17.5% per annum on the outstanding amounts under the loan facility;
•A repayment date of 91 days after the full redemption or the final maturity date of the Senior Bonds; and
•Contingently issuable penny warrants to the bondholders (the “Alvogen Facility Warrants”) if certain events occur, representing 4.0% of the fully diluted ordinary share capital, as defined in the Alvogen Facility agreement.
The Group determined that the 16 November 2022 transaction was a substantial modification to its related party loans and accounted for the transaction as an extinguishment. As a result, the Group recognized the following:
•Extinguishment of bonds with a carrying value of $63.2 million, including $3.2 million of accrued interest;
•Net cash proceeds of $50.0 million; and
•Recognition of $113.2 million and $1.3 million representing the fair value of the new Alvogen Facility and Alvogen Facility Warrants, respectively.
On 20 December 2022, the Company repaid $50.0 million under the Alvogen Facility, with proceeds from the 2022 Convertible Bonds. As a result, Alvotech extinguished the liability to issue the Alvogen Facility Warrants.
As of 31 December 2023, the carrying amount of the Alvogen Facility is $76.6 million, compared to $64.6 million as of 31 December 2022.
Facility Loans
As noted above, the Group assumed the Facility Loans as part of the asset acquisition for the Facility. On 9 December 2022, the Group extinguished the assumed loans from Arion banki hf., with an outstanding balance of $30.9 million, with two new loans from Landsbankinn hf. for $48.8 million, with variable interest rate, currently 8.3% and 9.3% per annum. The refinancing resulted in net cash proceeds of $17.2 million after transaction costs paid. The Group has pledged the facility as collateral to secure these loans (1st lien pledge), as further described in Note 12.
These two loans were denominated in Icelandic Krona and included a conversion clause to convert them into USD. The conversion of these two loans took place in March 2023.
Under the terms of the loan agreements after conversion, the first loan includes annuity payments that are due monthly with a final maturity in December 2029 and a variable interest rate of USD Secured Overnight Funding Rate ("SOFR") plus a margin of 4.75%. The second loan is a bullet loan with a final maturity in December 2027 and a variable interest rate of USD SOFR plus a margin of 3.75%
The Group determined that conversion to USD of the two loans was a substantial modification to loan agreements and accounted for the transaction as an extinguishment. No gain or loss was recognized as part of the extinguishment.
As of 31 December 2023, the carrying amount of the Facility Loans is $48.5 million.
Other borrowings
In 2015 and 2016, the Group entered into several term loan agreements with a financial institution for a total principal amount of $25.9 million. The loan agreements set forth terms and conditions between the Group and the financial institution, inclusive of certain representations and non-financial covenants. Per the terms of the loan
F-44
agreements, the loans mature throughout 2024, depending on the issuance date of each loan. Interest on the loans is based on variable interest rate of USD SOFR plus a margin of 4.95%, payable on a monthly basis. Interest accrued and unpaid at the end of each interest period increases the principal obligations owed by the Group to the financial institution. As of 31 December 2023, the outstanding balance on the loans is $0.5 million, compared to a balance of $3.2 million as of 31 December 2022.
On 22 February 2022, the Group entered into a credit facility agreement with Landsbankinn hf. with the ability to draw down an amount up to $8 million. The credit facility is in place to help finance equipment purchases in the future. Per the terms of the credit facility, any borrowings are required to be paid by 1 August 2024 and have a variable interest rate of USD SOFR plus a margin of 4.95%. As of 31 December 2023, the outstanding balance on the credit facility was $7.8 million, compared to $13.9 million as of 31 December 2022.
On 22 February 2022, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $3.2 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in March 2029. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $2.5 million, compared to $2.9 million as of 31 December 2022.
On 5 August 2022, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $1.8 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in August 2029. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $1.6 million, compared to $1.8 million as of 31 December 2022.
On 4 August 2023, the Group entered into a loan agreement with Landsbankinn hf. for a principal amount of $11.5 million. The loan is in place to help finance equipment purchases. Per the terms of the loan agreement, annuity payments are due monthly with a final maturity in August 2030. The loan has a variable interest rate of USD SOFR plus a margin of 4.25%. As of 31 December 2023, the outstanding balance on the loan was $11.0 million.
The Group is in compliance with all representations and non-financial covenants required by these agreements. In addition, the Group has pledged equipment as collateral to secure these borrowings, as further described in Note 12.
On 14 December 2023, the Group entered into a qualified receivable financing agreement with Landsbankinn hf. for a principal amount of $25.0 million. The qualified receivable financing arrangement has a variable interest rate of USD SOFR plus a margin of 3.50% and a maturity of April 2024. As of 31 December 2023, the outstanding balance on the loan was $25.0 million. The Group has pledged $25 million of its trade receivables to secure this financing.
Movements in the Group’s outstanding borrowings during the years ended 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Borrowings, net at 1 January | 764,570 | 400,911 | |||||||||
| Recognition of deferred debt issue costs | (6,115) | (2,889) | |||||||||
| Accretion/derecognition of borrowings discount | 15,770 | 35,065 | |||||||||
| Recognition of new borrowings discount | (50,953) | (43,241) | |||||||||
| Proceeds from new borrowings | 275,311 | 467,196 | |||||||||
| Loans from related party converted to equity | — | (50,000) | |||||||||
| Repayments of borrowings | (99,367) | (83,951) | |||||||||
| Accrued interest | 58,212 | 40,424 | |||||||||
| Amortization of deferred debt issue costs | 1,657 | 23 | |||||||||
| Foreign currency exchange difference | 1,075 | 1,032 | |||||||||
| Borrowings, net at 31 December | 960,159 | 764,570 | |||||||||
The weighted-average interest rates of outstanding borrowings for the years ended 31 December 2023, 2022, and 2021 are 12.73%, 12.41%, and 14.83%, respectively.
F-45
The table below details the changes in the Group’s liabilities arising from financing activities, including both cash and non-cash changes. Liabilities arising from financing activities are those for which cash flows were, or future cash flows will be, classified in the Group’s consolidated cash flow statement as cash flows from financing activities.
| 1 January 2023 | Financing Cash flows (a) | Capitalized loan cost changes | Fair value changes, including accretion | Other changes (b) | Foreign exchange impact | 31 December 2023 | |||||||||||||||||
| 2022 Convertible Bonds and Aztiq Convertible Bond | 98,234 | 145,358 | 1,657 | (36,071) | 27,603 | (204) | 236,577 | ||||||||||||||||
| Senior Bonds (including related party) | 530,506 | — | — | 888 | 18,017 | — | 549,411 | ||||||||||||||||
| Other borrowings | 71,242 | 25,102 | — | — | (8) | 1,279 | 97,615 | ||||||||||||||||
| Alvogen Facility | 64,588 | — | — | — | 11,968 | — | 76,556 | ||||||||||||||||
| Borrowings, net | 764,570 | 170,460 | 1,657 | (35,183) | 57,580 | 1,075 | 960,159 | ||||||||||||||||
(a)The cash flows from bank loans, loans from related parties and other borrowings make up the net amount of proceeds from borrowings and repayments of borrowings in the cash flow statement.
(b)Other changes include interest accruals and effects of payments including $3.5 million in cash received from borrowings from the 2022 Convertible Bonds and transaction cost of $9 million paid in 2023.
| 1 January 2022 | Financing Cash flows (a) | Capitalized loan cost changes | Fair value changes, including accretion | Other changes (b) | Foreign exchange impact | Conversion to Equity | Other non-cash movements | 31 December 2022 | |||||||||||||||||||||
| 2022 Convertible Bonds and Aztiq Convertible Bond | — | 55,852 | (2,865) | (40,245) | 1,528 | 444 | — | 83,520 | 98,234 | ||||||||||||||||||||
| Senior Bonds (including related party) | 394,129 | 70,000 | — | 32,069 | 34,308 | — | — | — | 530,506 | ||||||||||||||||||||
| Other borrowings | 6,782 | 33,112 | — | — | 762 | 588 | — | 29,998 | 71,242 | ||||||||||||||||||||
| Alvogen Facility | — | 110,000 | — | — | 4,588 | — | (50,000) | — | 64,588 | ||||||||||||||||||||
| Borrowings, net | 400,911 | 268,964 | (2,865) | (8,176) | 41,186 | 1,032 | (50,000) | 113,518 | 764,570 | ||||||||||||||||||||
(a)The cash flows from bank loans, loans from related parties and other borrowings make up the net amount of proceeds from borrowings and repayments of borrowings in the cash flow statement.
(b)Other changes include interest accruals and payments.
Contractual maturities of principal amounts on the Group’s outstanding borrowings as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Within one year | 38,025 | 19,916 | |||||||||
| Within two years | 867,273 | 3,804 | |||||||||
| Within three years | 4,932 | 696,646 | |||||||||
| Within four years | 37,857 | 3,374 | |||||||||
| Thereafter | 12,072 | 40,830 | |||||||||
| 960,159 | 764,570 | ||||||||||
F-46
22. Long-term incentive plans
Share appreciation rights
Prior to 2020, the Group granted SARs to three former employees. During the year ended 31 December 2021 and 2020, the Group granted SARs to one and two current employees, respectively. There were no new granted SARs in the years ended 31 December 2023 and 2022.
Settlement of SARs
In connection with the closing of the Business Combination, the Company reached a settlement agreement for share appreciation rights previously awarded to certain current and former employees. The rights were settled as follows:
•two former employees will each receive 1,755,291 Ordinary Shares to be issued one year after the Closing Date. In accordance with IFRS 2, the settlements were accounted for as a modification of a share-based payment transaction that changes the awards classification from cash-settled to equity-settled;
•one former employee will receive a $1.5 million cash payment in July 2022; and
•one current employee can elect to receive a cash payment of $1.5 million or 150,000 Ordinary Shares to be issued one year after the Closing Date. The Company recognized the cash settlement option as a liability with a fair value of $0.8 million and the share settlement option as equity with a fair value of $0.7 million.
The settlement agreements resulted in a net $36.8 million decrease in the SARs liability, a $31.0 million increase in equity equal to the fair value of the Ordinary Shares issued to the two former employees, a $1.5 million increase in other current liabilities, and income of $4.3 million in "General and administrative expense" recognized for the difference between the extinguished liabilities and the fair value of consideration paid to the current and former employees. As of 31 December 2022, the Company recognized $0.7 million as an other current liability related to the remaining SARs liability.
All liabilities described above have been fully settled in 2023 as follows:
•Shares were delivered to the two former employees, on a net basis after tax withholding, resulting in a total of 2,465,845 shares which has been reflected accordingly in Note 19.
•The employee who could choose between receiving $1.5 million or 150,000 Ordinary Shares elected to receive the cash payment.
Employee incentive plan
Movements in the Group’s employee incentive plan liabilities during the years ended 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Balance at 1 January | 12,317 | 14,935 | |||||||||
| Additions | 78 | 5,075 | |||||||||
| Payments | (11,736) | (7,693) | |||||||||
| Balance at 31 December prior to reclassification | 659 | 12,317 | |||||||||
| Reclassified to other current liabilities | (659) | (11,773) | |||||||||
| Balance at 31 December | — | 544 | |||||||||
23. Share-based payments
On 1 December 2022, the Renumeration Committee authorized and the Group granted RSUs to employees, executives, and directors granting rights to Ordinary Shares once vesting conditions are met. Compensation expense for RSUs is determined based upon the market price of the Ordinary Shares underlying the awards on the date of grant and expensed over the vesting period, which is generally a 1 to 4-year period, with a 1-year cliff vesting period
F-47
and subsequent monthly vesting, resulting from participants completing a service condition. Movements in RSUs during the year ended 31 December 2023 are as follows:
| 2023 | 2022 | ||||||||||||||||||||||
| RSUs | Weighted Average Fair Value | RSUs | Weighted Average Fair Value | ||||||||||||||||||||
| Outstanding at 1 January | 6,979,482 | $6.72 | — | — | |||||||||||||||||||
| New grants during the year | 820,602 | $8.79 | 7,659,044 | $6.68 | |||||||||||||||||||
| Forfeited during the year | (1,587,929) | $7.11 | — | — | |||||||||||||||||||
| Vested during the year | (2,466,374) | $6.67 | (679,562) | $6.30 | |||||||||||||||||||
| Outstanding at 31 December | 3,745,781 | $7.04 | 6,979,482 | $6.72 | |||||||||||||||||||
The Group recognized $18.0 million and $10.3 million of share-based payment expense during the years ended 31 December 2023 and 2022, respectively, as follows:
| 2023 | 2022 | |||||||
| Cost of product revenue | 3,319 | 1,522 | ||||||
| Research and development expenses | 3,991 | 2,994 | ||||||
| General and administrative expenses | 10,723 | 5,801 | ||||||
| 18,033 | 10,317 | |||||||
24. Litigation
The Group was involved in four litigations (all now dismissed) in the United States adverse to AbbVie arising out of the development of Alvotech’s AVT02 product, and the filing of a biologics license application with the U.S. Food and Drug Administration seeking regulatory approval (the “AbbVie Litigations”).
Alvotech entered into the AbbVie U.S. Agreement with AbbVie Inc. and AbbVie Biotechnology Ltd with respect to AVT02 for the U.S. market. Pursuant to the settlement component of the AbbVie U.S. Agreement, the parties agreed to stipulate to the dismissal of all claims, counterclaims and potential claims in the four U.S. litigations, with each party to bear its own fees and costs. The parties further agreed to release each other from certain claims and demands.
The Group incurred approximately $0.0 million, $8.7 million and $13.5 million in legal expenses during the years ended 31 December 2023, 2022, and 2021, respectively, in preparation for, and/or in relation to, these litigations. Aside from this matter, the Group is not currently a party to any material litigation or similar matters.
25. Related parties
Related parties are those parties which have considerable influence over the Group, directly or indirectly, including a parent company, owners or their families, large investors, key management personnel and their families and parties that are controlled by or dependent on the Group, such as affiliates and joint ventures. Key management personnel include the Group’s executive officers and directors, since these individuals have the authority and responsibility for planning, directing and controlling the activities of the Group. Interests in subsidiaries are set out in Note 1.
Transactions with related parties
A related party transaction is a transfer of resources, services or obligations between the Group and a related party, regardless of whether a price is charged. The Group engages with related parties for both purchased and sold services, loans and other borrowings and other activities.
The Group entered into lease agreement with Fasteignafélagið Eyjólfur hf. in April 2023 for a new facility in Iceland with remaining lease terms of approximately 15 years as of 31 December 2023 (see Note 13). The Group also entered into seventeen separate lease agreements with Flóki Fasteignir ehf. (HRJAF ehf.) throughout 2020, 2021 and 2023 for a group of apartment buildings in Iceland used for temporary housing of employees and third party
F-48
contractors. Two of the leases were terminated during the year ended 31 December 2021. The remaining lease terms for the other fifteen leases approximate 7 years, on average, as of 31 December 2023.
The Group entered into office sublease sharing agreement with Alvogen UK Ltd. in August 2023. The agreement was effective from 1 January 2023 and shall terminate upon the expiration or termination of the Lease.
The Group entered into art lease agreement with Flóki-Art ehf. in January 2023, as a result of the Share Purchase Agreement (see Note 12). The leased asset is located in Sæmundargata 15-19, Reykjavik. The remaining lease term for the leased asset is 15 years, as of 31 December 2023.
The Group provides and receives certain support services through arrangements with Aztiq, Alvogen, and Alvogen Malta (Outlicensing) Ltd. (Adalvo). Services provided to Alvogen consist of finance, administrative, legal and human resource services. Services received from Alvogen primarily consist of marketing, salary processing, and information technology support services. Services received from Adalvo primarily consist of legal, regulatory, supply chain management, and portfolio and market intelligence services.
Purchased service includes rental fees and service expenses, as described above. Rental fees and service expenses with related parties are presented as “General and administrative expenses” or “Research and development expenses” in the consolidated statements of profit or loss and other comprehensive income or loss, depending on the nature of the service performed and expense incurred by the Group. Rental liabilities from lease arrangements with related parties are presented as a component of “Lease liabilities” on the consolidated statements of financial position. Service payables are presented as “Liabilities to related parties” on the consolidated statements of financial position.
Interest includes interest expense on borrowings. Interest expenses on loans from related parties are presented as “Finance costs” in the consolidated statements of profit or loss and other comprehensive income or loss. Borrowings are presented as “Borrowings” and “Current maturities of borrowings” on the consolidated statements of financial position. See Note 21 for further details on the borrowing arrangements with related parties.
Sold service includes services provided to related parties, as described above. Income from related parties for such services are presented as “Other income” in the consolidated statements of profit or loss and other comprehensive income or loss. Amounts receivable for such activities are presented as “Receivables from related parties” on the consolidated statements of financial position. The Group has not recorded bad debt provisions for its receivables from related parties.
F-49
Related party transactions as of and for the year ended 31 December 2023 are as follows:
Purchased service / interest | Sold service | Receivables | Payables/ borrowings | ||||||||||||||||||||
| Alvogen Lux Holdings S.à r.l. – Sister company (a) | 11,968 | — | — | 76,556 | |||||||||||||||||||
| ATP Holdings ehf. - Sister company (a) | 9,193 | — | — | 49,560 | |||||||||||||||||||
| Aztiq Fjárfestingar ehf. – Sister company | — | 4 | — | — | |||||||||||||||||||
| Aztiq Consulting ehf. – Sister company | 178 | 69 | — | 54 | |||||||||||||||||||
| Flóki-Art ehf. - Sister company | 88 | — | — | 422 | |||||||||||||||||||
| Alvogen Iceland ehf. - Sister company | 19 | 1 | — | 484 | |||||||||||||||||||
| Alvogen ehf. - Sister company | — | 152 | 16 | — | |||||||||||||||||||
| Alvogen UK - Sister company | 273 | — | — | 581 | |||||||||||||||||||
| Alvogen Finance B.V. - Sister Company | 3,382 | — | — | 65 | |||||||||||||||||||
| Lotus Pharmaceuticals Co. Ltd. - Sister company (b) | — | 29 | 29 | 7,440 | |||||||||||||||||||
| Lotus International Pte. Ltd. - Sister company | — | 2 | — | — | |||||||||||||||||||
| Alvogen Emerging Markets - Sister company | 108 | — | — | — | |||||||||||||||||||
| Alvogen Inc. - Sister company | 305 | — | — | 284 | |||||||||||||||||||
| Alvotech and CCHT Biopharmaceutical Co., Ltd. (c) | — | — | 758 | 539 | |||||||||||||||||||
| Adalvo Limited - Sister company | 402 | 189 | 86 | 337 | |||||||||||||||||||
| Adalvo UK - Sister company | — | 49 | — | — | |||||||||||||||||||
| Flóki Invest ehf - Sister company | 680 | — | — | 251 | |||||||||||||||||||
| Floki Holdings S.à r.l. - Sister company | 40 | — | — | — | |||||||||||||||||||
| Alvogen Malta Sh. Services - Sister company | — | — | 7 | — | |||||||||||||||||||
| Alvogen Spain SL - Sister company | 14 | — | — | 15 | |||||||||||||||||||
| Norwich Clinical Services Ltd - Sister company | 642 | — | — | 170 | |||||||||||||||||||
| Fasteignafélagið Eyjólfur ehf - Sister company (d) | 3,807 | 102 | — | 69,732 | |||||||||||||||||||
| Flóki fasteignir ehf. - Sister company | 1,682 | — | — | 11,466 | |||||||||||||||||||
| 32,781 | 597 | 896 | 217,956 | ||||||||||||||||||||
(a)The full amount of purchased service relates to interest expenses from long-term liabilities and the full amount of payables / loans are interest-bearing long-term liabilities (see Note 21). In relation to the private placement of Tranche A Convertible Bonds in July, the Company paid underwriters fee to APT Holding amounting to $3.3 million. The underwriter’s fee is accounted for as a transaction cost that is amortized through profit and loss over the life of the instrument.
(b)Payables to Lotus Pharmaceuticals Co. Ltd. consists of the other current liability as further described in Note 2. This other current liability is presented as “Liabilities to related party” on the consolidated statements of financial position.
(c)The amount receivable from Alvotech & CCHN Biopharmaceutical Co., Ltd. relates to amounts due for reference drugs used in research and development studies and certain consulting fees incurred by the Group.
(d)Refer to Note 13 for the details of the new lease.
F-50
Related party transactions as of and for the year ended 31 December 2022 are as follows:
| Purchased service / interest | Sold service | Receivables | Payables/ borrowings | ||||||||||||||||||||
| Alvogen Lux Holdings S.à r.l. – Sister company (a) | 5,415 | — | — | 64,588 | |||||||||||||||||||
| Aztiq Fjárfestingar ehf. – Sister company | 216 | — | — | 20 | |||||||||||||||||||
| Aztiq Consulting ehf. - Sister company | 442 | — | — | 25 | |||||||||||||||||||
| ATP Holdings ehf. - Sister company (a) | 1,254 | — | 765 | 81,254 | |||||||||||||||||||
| Fasteignafélagið Sæmundur hf. - Sister company (e) | 7,189 | — | — | — | |||||||||||||||||||
| Fasteignafélagið Eyjólfur ehf - Sister company | — | 196 | — | — | |||||||||||||||||||
| Alvogen Iceland ehf. - Sister company | 465 | 174 | — | 484 | |||||||||||||||||||
| Alvogen ehf. - Sister company | — | 68 | 1 | — | |||||||||||||||||||
| Lotus Pharmaceuticals Co. Ltd. - Sister company (b) | — | 3 | 2 | 7,440 | |||||||||||||||||||
| Lotus International Pte. Ltd. - Sister company | — | 4 | 3 | — | |||||||||||||||||||
| Alvogen Emerging Markets - Sister company | 98 | — | — | — | |||||||||||||||||||
| Alvogen Korea co. Ltd - Sister company | — | 1 | — | — | |||||||||||||||||||
| Alvogen Inc. - Sister company | 585 | 266 | 12 | 222 | |||||||||||||||||||
| Alvotech and CCHT Biopharmaceutical Co., Ltd. (c) | — | — | 758 | — | |||||||||||||||||||
| Adalvo Limited - Sister company | 1,218 | 106 | — | 349 | |||||||||||||||||||
| Alvogen Malta Sh. Services - Sister company | 603 | — | 7 | — | |||||||||||||||||||
| Alvogen Spain SL - Sister Company | 117 | — | — | — | |||||||||||||||||||
| Norwich Clinical Services Ltd - Sister company | 301 | — | — | 31 | |||||||||||||||||||
| Alvogen Pharma Pvt Ltd - Sister Company | 1,159 | — | — | — | |||||||||||||||||||
| Flóki fasteignir ehf. - Sister company | 1,516 | — | — | 8,876 | |||||||||||||||||||
| L41 ehf. | 26 | — | — | — | |||||||||||||||||||
| Lambhagavegur 7 ehf. (d) | 537 | — | — | — | |||||||||||||||||||
| 21,141 | 818 | 1,548 | 163,289 | ||||||||||||||||||||
(a)The full amount of purchased service relates to interest expenses from long-term liabilities and the full amount of payables / loans are interest-bearing long-term liabilities including discount and accretion (see Note 21).
(b)Payables to Lotus Pharmaceuticals Co. Ltd. consists of the long-term liability as further described in Note 2. This long-term liability is presented as “Other long-term liability to related party” on the consolidated statements of financial position.
(c)The amount receivable from Alvotech & CCHN Biopharmaceutical Co., Ltd. relates to amounts due for reference drugs used in research and development studies and certain consulting fees incurred by the Group.
(d)Lambahagavegur is no longer a related party as it was sold during the year ended 31 December 2023.
(e)Fasteignafélagið Sæmundur hf. was acquired as part of the Share Purchase Agreement, with ATP Holdings ehf., on 16 November 2022. The related party transactions reflect activity until the acquisition date. See Note 12 and Note 21 for further details.
F-51
Related party transactions for the year ended 31 December 2021 are as follows:
| Purchased service / interest | Sold service | ||||||||||
| Alvogen Lux Holdings S.à r.l. – Sister company (a) | 9,383 | — | |||||||||
| Aztiq Pharma Partners S.à r.l. – Sister company (a) | 16,048 | — | |||||||||
| Alvogen Aztiq AB – Sister company (a) | 297 | — | |||||||||
| Aztiq Fjárfestingar ehf. (a) | 120 | — | |||||||||
| Fasteignafélagið Sæmundur hf. - Sister company | 7,762 | — | |||||||||
| Alvogen Iceland ehf. - Sister company | 454 | 2,308 | |||||||||
| Alvogen ehf. - Sister company | 6 | 2 | |||||||||
| Alvogen UK - Sister company | 299 | — | |||||||||
| Lotus Pharmaceuticals Co. Ltd. - Sister company (b) | — | 312 | |||||||||
| Alvogen Emerging Markets - Sister company | 238 | — | |||||||||
| Alvogen Korea co. Ltd - Sister company | — | 9 | |||||||||
| Alvogen Inc. - Sister company | 89 | 654 | |||||||||
| Alvogen Malta Sh. Services - Sister company | 1,216 | 151 | |||||||||
| Alvogen Pharma Pvt Ltd - Sister Company | 491 | — | |||||||||
| HRJÁF ehf - Sister company | 1,415 | — | |||||||||
| L41 ehf. | 29 | — | |||||||||
| Lambhagavegur 7 ehf. | 713 | — | |||||||||
| 39,940 | 3,715 | ||||||||||
(a)The full amount of purchased service relates to interest expenses from long-term liabilities and the full amount of payables / loans are interest-bearing long-term liabilities (see Note 21).
(b)Payables to Lotus Pharmaceuticals Co. Ltd. consists of the long-term liability as further described in Note 2. This long-term liability is presented as “Other long-term liability to related party” on the consolidated statements of financial position.
Commitments and guarantees
The Group does not have any contractual commitments with its related parties other than the receivables, loans and payables previously disclosed.
Key management personnel
At 31 December 2023 and 2022 there are no loans to the members of the Board of Directors and the CEO. In addition, there were no transactions carried out between the Group and members of the Board of Directors nor the CEO in the years ended 31 December 2023 and 2022. The Board of Directors’ remuneration is shown in the table below.
F-52
| Board of Directors’ fee for the year and shares at year end (board fees in thousands and shares in whole amounts). | 2023 | ||||||||||||||||||||||
| Board fees | Pension contribution | Other long-term benefits | Shares at year-end** | ||||||||||||||||||||
| Robert Wessman, Chairman of the board* | — | — | — | — | |||||||||||||||||||
| Richard Davies, Vice-Chairman | 156 | — | 104 | 1,143,713 | |||||||||||||||||||
| Ann Merchant, Board Member | 113 | — | 104 | 10,582 | |||||||||||||||||||
| Árni Harðarson, Board Member* | — | — | — | — | |||||||||||||||||||
| Faysal Kalmoua, Board Member* | — | — | — | — | |||||||||||||||||||
| Linda McGoldrick, Board Member | 81 | — | 104 | 10,582 | |||||||||||||||||||
| Lisa Graver, Board Member | 71 | — | 104 | 10,582 | |||||||||||||||||||
| Tomas Ekman, Board Member* | — | — | — | — | |||||||||||||||||||
| 421 | — | 416 | 1,175,459 | ||||||||||||||||||||
*Waived their board compensation (both cash and equity)
**Direct share ownership
| 2023 | |||||||||||||||||||||||
| Key employees | Salaries and benefits | Pension contribution | Termination benefits | Other long- term benefits | |||||||||||||||||||
| Robert Wessman CEO | 1,491 | 26 | — | — | |||||||||||||||||||
| Other Executive Team Members (9) | 5,020 | 346 | 52 | 9,456 | |||||||||||||||||||
| 6,511 | 372 | 52 | 9,456 | ||||||||||||||||||||
| Board of Directors’ fee for the year and shares at year end (board fees in thousands and shares in whole amounts). | 2022 | ||||||||||||||||||||||
| Board fees | Pension contribution | Other long-term benefits | Shares at year-end** | ||||||||||||||||||||
| Robert Wessman, Chairman of the board | 740 | — | — | — | |||||||||||||||||||
| Richard Davies, Vice-Chairman | 68 | — | — | 1,133,131 | |||||||||||||||||||
| Ann Merchant, Board Member (from 16.6.2022) | 43 | — | — | — | |||||||||||||||||||
| Árni Harðarson, Board Member (from 16.6.2022)* | — | — | — | — | |||||||||||||||||||
| Faysal Kalmoua, Board Member* | — | — | — | — | |||||||||||||||||||
| Linda McGoldrick, Board Member (from 16.6.2022) | 38 | — | — | — | |||||||||||||||||||
| Lisa Graver, Board Member (from 16.6.2022) | 38 | — | — | — | |||||||||||||||||||
| Tomas Ekman, Board Member* | — | — | — | — | |||||||||||||||||||
| Hirofumi Imai, Board member (until 16.6.2022) | — | — | — | — | |||||||||||||||||||
| 927 | — | — | 1,133,131 | ||||||||||||||||||||
*Waived their board compensation (both cash and equity)
**Direct share ownership
| 2022 | |||||||||||||||||||||||
| Key employees | Salaries and benefits | Pension contribution | Termination benefits | Other long- term benefits | |||||||||||||||||||
| Mark Levick CEO | 892 | 162 | 1,157 | — | |||||||||||||||||||
| Other Executive Team Members (9) | 5,400 | 446 | 820 | 5,015 | |||||||||||||||||||
| 6,292 | 608 | 1,977 | 5,015 | ||||||||||||||||||||
F-53
26. Other current liabilities
The composition of other current liabilities as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Unpaid salary and salary related expenses | 31,340 | 15,620 | |||||||||
| Accrued interest | 3,333 | 2,249 | |||||||||
| Accrued vacation leave | 6,075 | 5,025 | |||||||||
| Employee incentive plan | 659 | 12,433 | |||||||||
| Accrued expenses | 21,313 | 18,720 | |||||||||
| 62,720 | 54,047 | ||||||||||
27. Interests in joint ventures
In September 2018, Alvotech hf., a subsidiary of the Group, entered into a joint venture agreement with Changchun High & New Technology Industries (Group) Inc. (the “joint venture partner”, "CCHN") to form a newly created joint venture entity, Alvotech & CCHN Biopharmaceutical Co., Ltd. (the “joint venture” or “JVCO”). The purpose of the JVCO is to develop, manufacture and sell biosimilar products in the Chinese market. The JVCO’s place of business is also the country of incorporation.
| Name of entity | Place of business | Ownership interest | Carrying Amount | ||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||||||||
| Alvotech & CCHN Biopharmaceutical Co., Ltd. | China | 50 | % | 50 | % | 18,494 | 48,568 | ||||||||||||||||||||||
The proportion of ownership interest is the same as the proportion of voting rights held by the Group. Management evaluated whether the Group’s voting rights are sufficient for providing a practical ability to direct the relevant activities and strategic objectives of JVCO unilaterally. As the Group does not hold a majority of the voting rights, the Group does not control JVCO. As a result, the Group’s investment in JVCO is accounted for using the equity method.
The following table provides the change in the Group’s investment in a joint venture during the years ended 31 December 2023 and 2022:
| 2023 | 2022 | ||||||||||
| Balance at 1 January | 48,568 | 55,307 | |||||||||
| Share in losses | (7,153) | (2,590) | |||||||||
| Impairment loss on investment in joint venture | (21,519) | — | |||||||||
| Translation difference | (1,402) | (4,149) | |||||||||
| Balance at 31 December | 18,494 | 48,568 | |||||||||
The Group did not receive any dividends from JVCO during the years ended 31 December 2023, 2022, and 2021. The Group had a $5.0 million commitment to provide a cash contribution to JVCO as of 31 December 2020, which was paid during the year ended 31 December 2021. Similarly, the joint venture partner had a $50.0 million commitment to provide a cash contribution to JVCO as of 31 December 2020, which was also paid during the year ended 31 December 2021. The Group does not have any remaining commitments to JVCO as of 31 December 2023 and 2022. Furthermore, the Group does not have any contingent liabilities relating to its interests in JVCO as of 31 December 2023 or 2022. While there are no significant restrictions resulting from contractual arrangements with JVCO, entities in China are subject to local exchange control regulations. These regulations provide for restrictions on exporting capital from those countries, other than dividends. As of 31 December 2023, it had become clear that there were uncertainties around the economic conditions in China. Accordingly, the Group recorded an impairment loss on its investment in JVCO based on discussions between Alvotech and CCHN to buy back Alvotech´s interest in the joint venture. The Group estimated the recoverable amount using value in use where the recoverable amount is estimated as the future cash flows expected to arise from dividends to be received from the investment and from its ultimate disposal.
F-54
28. Financial instruments
Accounting classification and carrying amounts
Financial assets as of 31 December 2023 and 2022, all of which are measured at amortized cost, are as follows:
| 2023 | 2022 | ||||||||||
| Cash and cash equivalents | 11,157 | 66,427 | |||||||||
| Restricted cash | 26,132 | 25,187 | |||||||||
| Trade receivables | 41,292 | 32,972 | |||||||||
| Other current assets | 1,035 | 5,880 | |||||||||
| Receivables from related parties | 896 | 1,548 | |||||||||
| Other long-term assets | 336 | 4,484 | |||||||||
| 80,848 | 136,498 | ||||||||||
Financial liabilities as of 31 December 2023 and 2022 are as follows:
| 2023 | 2022 | ||||||||||
| Borrowings (measured at amortized cost) | 960,159 | 764,570 | |||||||||
| Derivative financial liabilities (measured at FVTPL) | 520,553 | 380,232 | |||||||||
| Other long-term liability to related party (measured at amortized cost) | — | 7,440 | |||||||||
| Long-term incentive plan (measured at FVTPL) | — | 544 | |||||||||
| Trade and other payables (measured at amortized cost) | 80,563 | 49,188 | |||||||||
| Lease liabilities (measured at amortized cost) | 115,315 | 40,532 | |||||||||
| Liabilities to related parties (measured at amortized cost) | 9,851 | 1,131 | |||||||||
| Other current liabilities | 61,873 | 53,664 | |||||||||
| 1,748,314 | 1,297,301 | ||||||||||
It is management’s estimate that the carrying amounts of financial assets and financial liabilities carried at amortized cost approximate their fair value, with the exception of the Senior Bonds, Aztiq Convertible Bond, 2022 Convertible Bonds, and Alvogen Facility, since any applicable interest receivable or payable is either close to current market rates or the instruments are short-term in nature. Material differences between the fair values and carrying amounts of these borrowings are identified as follows:
| 31 December 2023 | |||||||||||
| Carrying Amount | Fair Value | ||||||||||
| Senior Bonds | 549,411 | 559,867 | |||||||||
| Aztiq Convertible Bond | 80,663 | 84,756 | |||||||||
| 2022 Convertible Bonds | 155,914 | 217,419 | |||||||||
| Alvogen Facility | 76,556 | 82,060 | |||||||||
| 862,544 | 944,102 | ||||||||||
F-55
| 31 December 2022 | |||||||||||
| Carrying Amount | Fair Value | ||||||||||
| Senior Bonds | 530,506 | 535,167 | |||||||||
| Aztiq Convertible Bond | 65,793 | 65,772 | |||||||||
| 2022 Convertible Bonds | 32,441 | 52,463 | |||||||||
| Alvogen Facility | 64,588 | 66,883 | |||||||||
| 693,328 | 720,285 | ||||||||||
Fair value measurements
The following tables illustrate the fair value measurement hierarchy of the Group’s financial instruments measured at fair value on a recurring basis as of 31 December 2023 and 2022:
| 2023 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| Senior Bond Warrants | 19,715 | — | 19,715 | ||||||||||||||||||||
| Tranche A Conversion Feature | — | — | 118,830 | 118,830 | |||||||||||||||||||
| Predecessor Earn Out Shares | — | 349,900 | — | 349,900 | |||||||||||||||||||
| OACB Earn Out Shares | — | 6,200 | — | 6,200 | |||||||||||||||||||
| OACB Warrants | 25,908 | — | — | 25,908 | |||||||||||||||||||
| 45,623 | 356,100 | 118,830 | 520,553 | ||||||||||||||||||||
| 2022 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | ||||||||||||||||||||
| Senior Bond Warrants | — | — | 45,325 | 45,325 | |||||||||||||||||||
| Tranche A Conversion Feature | — | — | 38,055 | 38,055 | |||||||||||||||||||
| Senior Bond Interest Rate Feature (included in other current assets) | — | — | 851 | 851 | |||||||||||||||||||
| Predecessor Earn Out Shares | — | 276,200 | — | 276,200 | |||||||||||||||||||
| OACB Earn Out Shares | — | 10,500 | — | 10,500 | |||||||||||||||||||
| OACB Warrants | 10,152 | — | — | 10,152 | |||||||||||||||||||
| 10,152 | 286,700 | 84,231 | 381,083 | ||||||||||||||||||||
The following table provides a reconciliation of Level 3 financial instruments:
| Senior Bond Warrants | Tranche A Conversion Feature | Senior Bond Interest Rate Feature | |||||||||||||||
| 1 January 2023 | 45,325 | 38,055 | 851 | ||||||||||||||
| Issuance | — | 45,555 | — | ||||||||||||||
| Revaluation | — | 35,220 | — | ||||||||||||||
| Transfer to Level 1 | (45,325) | — | — | ||||||||||||||
| Extinguishment | — | — | (851) | ||||||||||||||
| 31 December 2023 | — | 118,830 | — | ||||||||||||||
F-56
The Group recognized the transfer of the Senior Bond Warrants from Level 3 to Level 1 for $19.7 million (2022: $45.3 million) during the year ended 31 December 2023 due to the lift of the lock-up period for the un-exercised Senior Bond Warrants. The Group did not recognize any transfer of assets or liabilities between levels of the fair value hierarchy during the years ended 31 December 2022 and 2021.
On 16 November 2022, the Group amended and upsized the outstanding bonds by $70.0 million. The amended bond agreement of the Senior Bonds resulted in, among other things, an increase in the interest rate, resulting in a range from 10.75% to 12.0% depending on the occurrence of certain events, as defined by the terms of the agreement (see Note 21). The Group accounted for this interest rate feature (the “Senior Bond Interest Rate Feature”) as an embedded derivative, classified as an "Other current assets" in the consolidated statement of financial position as of 31 December 2022. Since the conditions to adjust the coupon rate have not been met as of 31 March 2023 per the terms of the agreement, the interest rate on the Senior Bonds is now fixed and the embedded derivative previously recorded has been extinguished during the year ended 31 December 2023, resulting in a loss on extinguishment of $0.9 million recorded in finance costs.
Senior Bond Warrants
As noted in Note 21, as a result of proceeds raised from the private placement offering executed in February 2023, the Company extinguished the derivative financial liability related to the senior bond warrants since the Company has not anymore the obligation to issue the 1.0% Senior Bond Warrants, resulting in a gain on extinguishment of $6.5 million. In January and February 2023, the Senior Bond Warrant holders (also known as penny warrant holders) elected to exercise their warrants. As a result, 2,479,962 ordinary shares were issued in exchange for the exercising of the penny warrants. The Company received an immaterial amount of cash and recognized the transaction as an extinguishment of the derivative financial liabilities.
The fair value of the Senior Bond Warrants was derived from the publicly quoted trading price of the Ordinary Shares at the valuation date. As of 31 December 2023, the Company had 1,718,845 warrants with an exercise price of $0.01, representing the 1.5% tranche of Senior Bond Warrants. The Senior Bond Warrants had a fair value of $19.7 million as of 31 December 2023. The change in fair resulted in $8.1 million of finance costs for the year ended 31 December 2023.
Tranche A Conversion Feature
As noted in Note 21, in connection with the Convertible Bonds the Group classified the Tranche A Conversion Feature as an embedded derivative liability due to the variability created by conversion rates resulting from the tranche being denominated in ISK. The conversion feature had a fair value of $118.8 million as of 31 December 2023. The change in fair resulted in $35.2 million of finance costs for the year ended 31 December 2023.
The fair value of the Tranche A Conversion Feature was determined using a lattice model that incorporated inputs and assumptions as further described below. The inputs and assumptions associated with the valuation of the instruments are determined based on all relevant internal and external information available and are reviewed and reassessed at each reporting date.
The following table presents the assumptions and inputs that were used for the model in valuing the Tranche A Conversion Feature:
| 31 December 2023 | 31 December 2022 | ||||||||||
| Stock price | $11.48 | $10.00 | |||||||||
| Conversion price | $10.00 | $10.00 | |||||||||
| Volatility rate | 57.5 | % | 45.0 | % | |||||||
| Risk-free interest rate | 4.2 | % | 4.2 | % | |||||||
| Dividend yield | 0.0 | % | 0.0 | % | |||||||
| Risky yield | 16.3 | % | 19.3 | % | |||||||
F-57
Predecessor Earn Out Shares
As part of the Business Combination, Predecessor shareholders were granted a total of 38,330,000 Ordinary Shares subject to certain vesting conditions (“Predecessor Earn Out Shares”). One half of the Predecessor Earn Out Shares will vest if, at any time during the five years following the closing of the Business Combination, the Alvotech ordinary share price is at or above a volume weighted average price (“VWAP”) of $15.00 per share for any ten trading days within any twenty-trading day period, with the other half vesting at a VWAP of $20.00 per share for any ten trading days within any twenty-trading day period. The Predecessor Earn Out Shares are accounted for as derivative financial liabilities in accordance with IAS 32 and will be subject to ongoing mark-to-market adjustments through the consolidated statement of profit or loss and other comprehensive income or loss. The Predecessor Earn Out Shares had a fair value of $349.9 million as of 31 December 2023, resulting in $73.7 million of finance costs during the year ended 31 December 2023.
The fair value of the Predecessor Earn Out Shares was determined using Monte Carlo analysis that incorporated inputs and assumptions as further described below. The inputs and assumptions associated with the valuation of the instruments are determined based on all relevant internal and external information available and are reviewed and reassessed at each reporting date.
The following table presents the assumptions and inputs that were used for the model in valuing the Predecessor Earn Out Shares:
| 31 December 2023 | 31 December 2022 | ||||||||||
| Number of shares | 38,330,000 | 38,330,000 | |||||||||
| Share price | $11.48 | $10.00 | |||||||||
| Volatility rate | 55.0 | % | 45.0 | % | |||||||
| Risk-free rate | 3.97 | % | 4.05 | % | |||||||
OACB Earn Out Shares
Former OACB shareholders were granted a total of 1,250,000 Ordinary Shares subject to certain vesting conditions (“OACB Earn Out Shares”). One half of the OACB Earn Out Shares will vest if, at any time during the five years following the closing of the Business Combination, the Alvotech ordinary share price is at or above a VWAP of $12.50 per share for any ten trading days within any twenty-trading day period, with the other half vesting at a VWAP of $15.00 per share. On 17 February 2023, the first half of OACB Earn Out Shares vested resulting in the issuance of 625,000 ordinary shares by the Group. The OACB Earn Out Shares are accounted for as derivative financial liabilities in accordance with IAS 32 and will be subject to ongoing mark-to-market adjustments through the consolidated statement of profit or loss and other comprehensive income or loss. The OACB Earn Out Shares had a fair value of $6.2 million as of 31 December 2023, resulting in $4.0 million of finance costs during the year ended 31 December 2023.
The fair value of the OACB Earn Out Shares was determined using a Monte Carlo analysis that incorporated inputs and assumptions as further described below. Assumptions and inputs associated with the valuation of the instruments are determined based on all relevant internal and external information available and are reviewed and reassessed at each reporting date.
The following table presents the assumptions and inputs that were used for the model in valuing the OACB Earn Out Shares:
| 31 December 2023 | 31 December 2022 | ||||||||||
| Number of shares | 625,000 | 1,250,000 | |||||||||
| Share price | $11.48 | $10.00 | |||||||||
| Volatility rate | 55.0 | % | 45.0 | % | |||||||
| Risk-free rate | 3.97 | % | 4.05 | % | |||||||
F-58
OACB Warrants
Additionally, as part of the Business Combination the Company assumed the 10,916,647 outstanding OACB Warrants, on substantially the same contractual terms and conditions as were in effect immediately prior to the Business Combination, including an exercise price of $11.50. Each warrant entitles the holder to purchase one Alvotech ordinary share. During 2023, holders of the OACB warrants exercised their warrant rights for an exercise price of $11.50 for the rights to one ordinary share per warrant. The exercises resulted in the issuance of 553.552 ordinary shares and cash proceeds of approximately $6.3 million. The OACB warrants are accounted for as derivative financial liabilities in accordance with IAS 32 and will be subject to ongoing mark-to-market adjustments through the consolidated statement of profit or loss and other comprehensive income or loss. The OACB warrants had a fair value of $25.9 million as of 31 December 2023. The fair value of the warrants was derived from the publicly quoted trading price at the valuation date. The change in fair value of the OACB Warrants resulted in $17.0 million of finance costs during the year ended 31 December 2023.
Capital management
The capital structure of the Group consists of equity, debt and cash. For the foreseeable future, the Board of Directors will maintain a capital structure that supports the Group’s strategic objectives through managing the budgeting process, maintaining strong investor relations and managing the financial risks of the Group, as further described below. No changes were made in the objectives, policies or processes for managing capital during the years ended 31 December 2023 and 2022.
Financial risk management
The Group’s corporate treasury function provides services across the organization, coordinates access to domestic and international financial markets, monitors and manages the financial risks relating to the Group’s operations through internal risk reports which analyze exposures by degree and magnitude of risks. These risks include market risk (including foreign currency risk and interest rate risk), credit risk and liquidity risk.
Interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Group’s exposure to the risk of fluctuations in market interest rates primarily relates to the cash in bank and borrowings that are subject to floating interest rates.
The following table provides an interest rate sensitivity analysis for the effect on loss before tax:
| 2023 | 2022 | ||||||||||
| Variable-rate financial instruments +100 | (89) | (186) | |||||||||
| Variable-rate financial instruments -100 | 89 | 186 | |||||||||
Foreign currency risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. The Group uses the US dollar as its reporting currency and conducts business on a global basis in various currencies. As a result, the Group is exposed to foreign currency exchange movements, primarily in European, Icelandic and UK market currencies, as well as in the Swiss franc.
F-59
Below are the foreign currencies that have the most significant impact on the Group’s operations.
| Closing rate | Average rate | Change | |||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||||||||
| EUR | 1.105 | 1.061 | 1.091 | 1.052 | 4.1 | % | |||||||||||||||||||||||
| GBP | 1.275 | 1.204 | 1.266 | 1.233 | 5.9 | % | |||||||||||||||||||||||
| ISK | 0.007 | 0.007 | 0.007 | 0.007 | 5.1 | % | |||||||||||||||||||||||
| CHF | 1.188 | 1.071 | 1.156 | 1.047 | 10.9 | % | |||||||||||||||||||||||
| INR | 0.012 | 0.012 | 0.012 | 0.013 | 0.1 | % | |||||||||||||||||||||||
The Group’s assets and liabilities that are denominated in foreign currencies as of 31 December 2023 are as follows:
| Assets | Liabilities | Net assets | |||||||||||||||
| EUR | 36,568 | 46,303 | (9,735) | ||||||||||||||
| GBP | 69 | 3,479 | (3,410) | ||||||||||||||
| ISK | 3,247 | 144,812 | (141,565) | ||||||||||||||
| CHF | 335 | 7,488 | (7,153) | ||||||||||||||
| INR | 167 | 536 | (369) | ||||||||||||||
The Group’s assets and liabilities that are denominated in foreign currencies as of 31 December 2022 are as follows:
| Assets | Liabilities | Net assets | |||||||||||||||
| EUR | 36,420 | 26,514 | 9,906 | ||||||||||||||
| GBP | 111 | 1,538 | (1,427) | ||||||||||||||
| ISK | 49,484 | 109,507 | (60,023) | ||||||||||||||
| CHF | 69 | 7,305 | (7,236) | ||||||||||||||
| INR | 11 | 517 | (506) | ||||||||||||||
A reasonable possible strengthening or weakening of the Group’s significant foreign currencies against the USD would affect the measurement of financial instruments denominated in a foreign currency and affect profit or loss and equity by the amount shown in the sensitivity analysis table below. The analysis assumes that all other variables, such as interest rates, remain constant.
| EUR | GBP | ISK | CHF | INR | |||||||||||||||||||||||||
| Year ended 31 December 2023 | |||||||||||||||||||||||||||||
| -10% weakening | (974) | (341) | (14,156) | (715) | (37) | ||||||||||||||||||||||||
| +10% strengthening | 974 | 341 | 14,156 | 715 | 37 | ||||||||||||||||||||||||
| Year ended 31 December 2022 | |||||||||||||||||||||||||||||
| -10% weakening | (991) | (143) | (6,002) | (724) | (51) | ||||||||||||||||||||||||
| +10% strengthening | 991 | 143 | 6,002 | 724 | 51 | ||||||||||||||||||||||||
F-60
Credit risk
Credit risk it the risk that a counterparty will not fulfill its contractual obligations under a financial instrument contract, leading to a financial loss for the Group. The maximum credit risk exposure for the Group’s financial assets as of 31 December 2023 and 2022 is as follows:
| 2023 | 2022 | ||||||||||
| Cash and cash equivalents | 11,157 | 66,427 | |||||||||
| Restricted cash | 26,132 | 25,187 | |||||||||
| Other assets | 43,559 | 44,884 | |||||||||
| 80,848 | 136,498 | ||||||||||
The Group’s cash and cash equivalents and restricted cash are deposited with high-quality financial institutions. Management believes these financial institutions are financially sound and, accordingly, that minimal credit risk exists. The Group has not experienced any losses on its deposits of cash and cash equivalents and restricted cash yet monitors the credit rating of these financial institutions on a periodic basis.
Other assets primarily consist of other current assets, as described in Note 18, and trade receivables and contract assets recognized in connection with the Group’s performance pursuant to its contracts with customers, all of which are large multinational pharmaceutical companies. In 2023, the Group recognized a receivable of $18.5 million in other current assets following the termination of the co-development agreement with Biosana which was fully reserved as of 31 December 2023 due to the uncertainty of its collection (see Note 18). There are no other significant amounts past due as of 31 December 2023 and 2022 and the Group concludes that any expected credit losses with respect to these assets, except as described above, is immaterial.
Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset.
Contractual maturities of financial assets and liabilities as of 31 December 2023 are as follows:
| Within one year | One to two years | Thereafter | Total | ||||||||||||||||||||
| Financial assets | |||||||||||||||||||||||
| Non-interest bearing | 43,223 | — | — | 43,223 | |||||||||||||||||||
| Variable-interest bearing | 11,157 | — | 26,468 | 37,625 | |||||||||||||||||||
| Total financial assets | 54,380 | — | 26,468 | 80,848 | |||||||||||||||||||
| Financial liabilities | |||||||||||||||||||||||
| Non-interest bearing | 142,436 | — | — | 142,436 | |||||||||||||||||||
| Fixed-interest bearing - Borrowings | 66,309 | 1,101,185 | — | 1,167,494 | |||||||||||||||||||
| Derivative liabilities | — | 520,553 | — | 520,553 | |||||||||||||||||||
| Variable-interest bearing - Borrowings | 44,995 | 10,198 | 65,826 | 121,019 | |||||||||||||||||||
| Total financial liabilities | 253,740 | 1,631,936 | 65,826 | 1,951,502 | |||||||||||||||||||
F-61
Contractual maturities of financial assets and liabilities as of 31 December 2022 are as follows:
| Within one year | One to two years | Thereafter | Total | ||||||||||||||||||||
| Financial assets | |||||||||||||||||||||||
| Non-interest bearing | 40,400 | — | — | 40,400 | |||||||||||||||||||
| Variable-interest bearing | 66,427 | — | 29,671 | 96,098 | |||||||||||||||||||
| Total financial assets | 106,827 | — | 29,671 | 136,498 | |||||||||||||||||||
| Financial liabilities | |||||||||||||||||||||||
| Non-interest bearing | 104,366 | — | 7,984 | 112,350 | |||||||||||||||||||
| Fixed-interest bearing - Borrowings | 45,757 | 66,308 | 896,921 | 1,008,986 | |||||||||||||||||||
| Derivative liabilities | — | — | 380,232 | 380,232 | |||||||||||||||||||
| Variable-interest bearing - Borrowings | 25,259 | 8,036 | 59,109 | 92,404 | |||||||||||||||||||
| Total financial liabilities | 175,382 | 74,344 | 1,344,246 | 1,593,972 | |||||||||||||||||||
Refer to Note 13 for the maturity analysis of the Group’s undiscounted lease payments.
29. Supplemental cash flow information
Supplement cash flow information for the years ended 31 December 2023, 2022, and 2021 is included below (see Note 21 for non-cash movements in borrowings).
| Non-cash investing and financing activities | 2023 | 2022 | 2021 | ||||||||||||||
| Acquisition of property, plant and equipment in trade payables and other current liabilities | 2,266 | 4,131 | 3,812 | ||||||||||||||
| Acquisition of intangibles in trade payables and other current liabilities | 930 | 4,075 | — | ||||||||||||||
| Right-of-use assets obtained through new operating leases | 74,109 | 9,583 | 18,871 | ||||||||||||||
| Purchase of Facility through Aztiq Convertible Bond | — | 115,005 | — | ||||||||||||||
| Non-cash issuance of Aztiq Convertible Bond | — | 80,000 | — | ||||||||||||||
| Equity issued through conversion of borrowings | — | 32,200 | 346,043 | ||||||||||||||
| Acquisition of other intangible assets through financing agreements | — | — | 461 | ||||||||||||||
| Settlement of RSUs with shares | 678 | — | — | ||||||||||||||
| Settlement of SARs with shares | 13,767 | — | — | ||||||||||||||
30. Subsequent events
The Group evaluated subsequent events through 20 March 2024, the date the consolidated financial statements were available to be issued.
On 15 February 2024, the Company announced it has reached settlement agreements with Johnson & Johnson in Japan, Canada and in the EEA for AVT04, a biosimilar to Stelara (ustekinumab). Regulatory approval for AVT04 in these markets has already been granted. Market applications for AVT04 are currently pending in additional global markets, including in the U.S. Alvotech's commercialization partner in Canada, JAMP Pharma, launched AVT04 in Canada on March 1, 2024. Launch of AVT04 in Japan is anticipated after the upcoming round of National Health Insurance reimbursement price listings, in May 2024. Entry to the first European markets is expected as soon as possible after the expiration date of the European Supplementary Protection Certificate (SPC) for Stelara, which is in late July 2024.
On 23 February 2024, the Company announced that the FDA has approved SIMLANDI (adalimumab) injection, as an interchangeable biosimilar to Humira, for the treatment of adult rheumatoid arthritis, juvenile idiopathic arthritis, adult psoriatic arthritis, adult ankylosing spondylitis, Crohn’s disease, adult ulcerative colitis, adult plaque psoriasis,
F-62
adult hidradenitis suppurativa and adult uveitis. In 2023, Humira was one of the highest-grossing pharmaceutical products in the world, with sales in the U.S. of nearly $12.2 billion. Teva is Alvotech’s strategic partner for the exclusive commercialization of SIMLANDI in the United States.
On 26 February 2024, the Company announced the sale of 10,127,132 Ordinary Shares for an approximate value of $166 million (net proceeds of $160 million), par value USD 0.01 per share, at a purchase price of $16.41 per share, or ISK 2,250 per share at the foreign exchange rates on 23 February 2024. The Shares will be delivered to the Investors from previously issued treasury shares held by Alvotech’s subsidiary, Alvotech Manco ehf. The Transaction took place on the Nasdaq Iceland Exchange.
On 12 February 2024, the second tranche of OACB Earn Out Shares vested resulting in the issuance of 625,000 Ordinary Shares. The issuance of Ordinary Shares for the second tranche will be accounted for as an extinguishment of a financial liability in the consolidated statements of profit or loss and other comprehensive income or loss.
On 12 February 2024, the first tranche of Predecessor Earn Out Shares vested resulting in the issuance of 19,165,000 Ordinary Shares. The issuance of Ordinary Shares for the first tranche will be accounted for as an extinguishment of a financial liability in the consolidated statements of profit or loss and other comprehensive income or loss.
Subsequent to 31 December 2023, Senior Bond Warrant holders elected to exercise their warrants. As a result, 1,501,599 Ordinary Shares were issued in exchange for the exercising of the penny warrants. The Company received an immaterial amount of cash and will recognize the transaction as an extinguishment of the derivative financial liabilities. The difference between the equity issued and carrying value of the derivative financial liabilities will be recognized in the consolidated statements of profit or loss and other comprehensive income or loss.
Subsequent to 31 December 2023, holders of the OACB Warrants exercised their warrant rights for an exercise price of $11.50 for the rights to one Ordinary Share per warrant. The exercises result in the issuance of 419,660 Ordinary Shares and cash proceeds of $4.8 million. The Company will recognize the transaction as an extinguishment of the derivative financial liabilities. The difference between the equity issued and carrying value of the derivative financial liabilities will be recognized in the consolidated statements of profit or loss and other comprehensive income or loss.
F-63