Exhibit 10.7
**THIS EXHIBIT HAS BEEN REDACTED TO REMOVE INFORMATION THAT IS NOT MATERIAL AND THAT THE REGISTRANT MUST TREAT AS PRIVATE AND CONFIDENTIAL.**
EXCLUSIVE LICENSE AGREEMENT
by and between
FELICITEX THERAPEUTICS, INC.
and
SELVITA S.A.
Exhibit D
TABLE OF CONTENTS
| | | Page |
| ARTICLE I | DEFINITIONS | 1 |
| | | |
| ARTICLE II | SCOPE OF THE AGREEMENT | 13 |
| | | |
| 2.1 | Development and Commercialization of Optioned Compounds and Products | 13 |
| | | |
| 2.2 | Internal or External Development | 13 |
| | | |
| 2.3 | Other Transactions | 13 |
| | | |
| ARTICLE III | LICENSE GRANTS | 13 |
| | | |
| 3.1 | Exclusive License from Selvita to Felicitex | 13 |
| | | |
| 3.2 | Non-Exclusive License from Selvita to Felicitex | 13 |
| | | |
| 3.3 | Sublicensing and Transfer Rights | 14 |
| | | |
| 3.4 | Further Actions | 14 |
| | | |
| 3.5 | Rights Retained by the Parties | 14 |
| | | |
| 3.6 | Good Faith Negotiations on License or (Re-)Transfer of Rights | 14 |
| | | |
| 3.7 | Section 365(n) of the Bankruptcy Code | 14 |
| | | |
| ARTICLE IV | TECHNOLOGY TRANSFER | 15 |
| | | |
| 4.1 | Consultation Without Charge | 15 |
| | | |
| 4.2 | Additional Services | 15 |
| | | |
| ARTICLE V | DEVELOPMENT AND COMMERCIALIZATION | 15 |
| | | |
| 5.1 | Responsibility for Development and Commercialization | 15 |
| | | |
| 5.2 | Diligence Obligation | 15 |
| | | |
| 5.3 | Obligations Under SEL141 Grant | 16 |
| | | |
| 5.4 | Internal or External Development and Commercialization | 16 |
| | | |
| 5.5 | Reports | 17 |
| | | |
| ARTICLE VI | REGULATORY MATTERS | 17 |
| | | |
| 6.1 | Compliance | 17 |
| | | |
| 6.2 | Data Integrity | 17 |
| | | |
| 6.3 | Regulatory Submissions | 18 |
| | | |
| 6.4 | Communications with Authorities | 18 |
| | | |
| 6.5 | Adverse Event Reporting | 18 |
| | | |
| 6.6 | Recalls | 18 |
Exhibit D
TABLE OF CONTENTS
(continued)
| | | Page |
| ARTICLE VII | COMMERCIAL TERMS | 18 |
| | | |
| 7.1 | General Terms for Payments to Selvita with Respect to Optioned Compounds | 18 |
| | | |
| 7.2 | Payments during Internal Development | 20 |
| | | |
| 7.3 | Payments in Course of External Development | 19 |
| | | |
| 7.4 | Market Price | 25 |
| | | |
| 7.5 | Reporting Obligations | 25 |
| | | |
| 7.6 | Accounting; Audit Rights | 25 |
| | | |
| 7.7 | Payments; Conversion | 26 |
| | | |
| 7.8 | Late Payments | 26 |
| | | |
| 7.9 | Withholding or Other Taxes | 26 |
| | | |
| ARTICLE VIII | [INTENTIONALLY OMITTED] | 27 |
| | | |
| ARTICLE IX | INTELLECTUAL PROPERTY RIGHTS | 27 |
| | | |
| 9.1 | Ownership | 27 |
| | | |
| 9.2 | Prosecution and Maintenance of Patents | 27 |
| | | |
| 9.3 | Patent Costs | 29 |
| | | |
| 9.4 | Enforcement of Patents and Know-How | 29 |
| | | |
| 9.5 | Third Party Actions Claiming Infringement | 30 |
| | | |
| ARTICLE X | CONFIDENTIALITY | 30 |
| | | |
| 10.1 | Confidentiality; Exceptions | 30 |
| | | |
| 10.2 | Product Information | 31 |
| | | |
| 10.3 | Authorized Disclosure | 31 |
| | | |
| 10.4 | Press Release | 32 |
| | | |
| 10.5 | Disclosure of Agreement Terms | 32 |
| | | |
| 10.6 | Remedies | 32 |
| | | |
| 10.7 | Publications | 32 |
| | | |
| 10.8 | Republication | 33 |
| | | |
| 10.9 | Return of Confidential Information | 34 |
| | | |
| ARTICLE XI | REPRESENTATIONS AND WARRANTIES | 34 |
| | | |
| 11.1 | Representations and Warranties of Both Parties | 34 |
Exhibit D
TABLE OF CONTENTS
(continued)
| | | Page |
| 11.2 | Representations and Warranties of Selvita | 35 |
| | | |
| 11.3 | Covenants of Felicitex | 35 |
| | | |
| 11.4 | Disclaimer | 35 |
| | | |
| ARTICLE XII | INDEMNIFICATION; INSURANCE | 35 |
| | | |
| 12.1 | Indemnification | 35 |
| | | |
| 12.2 | Indemnification regarding SEL141 Grant | 36 |
| | | |
| 12.3 | Procedure | 36 |
| | | |
| 12.4 | Insurance | 37 |
| | | |
| 12.5 | LIMITATION OF LIABILITY | 37 |
| | | |
| ARTICLE XIII | TERM AND TERMINATION | 38 |
| | | |
| 13.1 | Term | 38 |
| | | |
| 13.2 | Early Termination | 38 |
| | | |
| 13.3 | Effects of Termination and/or Expiry | 38 |
| | | |
| ARTICLE XIV | MISCELLANEOUS | 40 |
| | | |
| 14.1 | Dispute Resolution | 40 |
| | | |
| 14.2 | Arbitration | 40 |
| | | |
| 14.3 | Governing Law | 41 |
| | | |
| 14.4 | Sectoral Sanctions Identification (SSI) List | 41 |
| | | |
| 14.5 | Assignment | 41 |
| | | |
| 14.6 | Performance Warranty | 42 |
| | | |
| 14.7 | Force Majeure | 42 |
| | | |
| 14.8 | Notices | 42 |
| | | |
| 14.9 | Export Clause | 42 |
| | | |
| 14.10 | Waiver | 42 |
| | | |
| 14.11 | Severability | 42 |
| | | |
| 14.12 | Entire Agreement | 42 |
| | | |
| 14.13 | Independent Contractors | 43 |
| | | |
| 14.14 | Headings; Construction; Interpretation | 43 |
| | | |
| 14.15 | Books and Records | 43 |
| | | |
| 14.16 | Further Actions | 43 |
| | | |
| 14.17 | Parties in Interest | 44 |
| | | |
| 14.18 | Performance by Affiliates | 44 |
| | | |
| 14.19 | Counterparts and Language | 44 |
Exhibit D
List of Exhibits
| Exhibit A | Joint Collaboration IP |
| Exhibit B | Optioned Compounds |
| Exhibit C | Selvita Collaboration IP |
| Exhibit D | Procedure for Calculating Structural Similarity |
| Exhibit E | Target |
| Exhibit F | Implementation Statement |
| Exhibit G | Calculation Scheme for Diluted Value Share |
| Exhibit H | Exemplary Calculation of Participation Payments |
| Exhibit I | Press Release |
Exhibit D
EXCLUSIVE LICENSE AGREEMENT
This EXCLUSIVE LICENSE AGREEMENT (this “Agreement”) is entered into and made effective as of this [ ] day of [ ], 20[ ] (the “Effective Date”) by and between Felicitex Therapeutics, Inc., a corporation duly organized under the laws of the State of Delaware, United States having its principal place of business at One Kendall Square Building 200, B2002, Cambridge, Massachusetts 02139, U.S.A. (“Felicitex”), and Selvita S.A., a Polish corporation, having its principal place of business at Park Life Science, ul. Bobrzyńskiego 14, 30-348 Kraków, Poland (“Selvita”). Felicitex and Selvita are each referred to herein by name or as a “Party” or, collectively, as the “Parties.”.
RECITALS
WHEREAS, Selvita and Felicitex each possess certain proprietary technology, intellectual property and expertise with respect to the identification and optimization of small molecule inhibitors for all uses against specified targets, including in the area of cancer;
WHEREAS, Felicitex and Selvita have previously undertaken certain discovery research activities to validate a certain kinase target of interest, “DYRK1A/B”, and to generate new kinase inhibitor drug candidates with high selectivity towards such selected kinase target with defined activity in certain cancer subtypes, with an initial focus on, but not limited to, pancreatic, colon, ovarian, lung and hematopoietic cancers based on targeting cancer cell quiescence;
WHEREAS, Selvita is conducting a novel kinase inhibitor program SEL141 (“SEL141 Program”) for which Selvita has received a grant from the Polish Agency for Enterprise Development (the “SEL141 Grant”); and
WHEREAS, Selvita wishes to grant to Felicitex and Felicitex wishes to receive from Selvita an exclusive, worldwide license on certain of Selvita’s intellectual property rights to further Research, Develop, Manufacture and Commercialize certain “Optioned Compounds” directed to the “Target” for any and all uses in the “Field” in the “Territory” (each as defined below), in particular for the “Research”, “Development”, “Manufacturing” and “Commercialization” (each as defined below) of the “Products” (as defined below).
NOW, THEREFORE, in consideration of the premises and mutual covenants herein contained, and for other good and valuable consideration, the receipt and legal sufficiency of which are hereby acknowledged, accepted and agreed to, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS
As used in this Agreement, the following terms will have the meanings set forth in this Article 1 unless context dictates otherwise:
1.1 “Affiliate” means, with respect to a Person, any other Person which, directly or indirectly through one (1) or more intermediaries, controls, is controlled by or is under common control with such Person, regardless of whether such Affiliate is or becomes an Affiliate on or after the Effective Date. A Person shall be deemed to “control” another Person if it (a) owns, directly or indirectly, beneficially or legally, more than fifty percent (50%) of the outstanding voting securities or capital stock of such other Person, or has other comparable ownership interest with respect to any Person other than a corporation; or (b) has the power, whether pursuant to contract, ownership of securities or otherwise, to direct the management and policies of such other Person.
Exhibit D
1.2 “Business Day” means a day on which banking institutions in Boston, Massachusetts and Krakow, Poland are open for business, excluding any Saturday or Sunday.
1.3 “Calendar Quarter” means a period of three (3) consecutive months ending on the last day of March, June, September, or December, respectively.
1.4 “Calendar Year” means a period of twelve (12) consecutive months beginning on January 1 and ending on December 31.
1.5 “Change-of-Control Event” means (a) a Party (i) merges or consolidates with any Third Party, or (ii) effects any other transaction or series of related transactions involving the transfer of capital stock of a Party to a Third Party, other than a transaction in which a Party or underwriters for a Party sell securities of a Party (A) in a public offering, or (B) directly to bona fide venture capital investors or bona fide institutional investors that routinely make such investments for the potential financial return on such investments and not with any view to acquisition, in the case of each of the foregoing clauses (i) and (ii) such that the stockholders of a Party immediately prior thereto, in the aggregate, no longer beneficially own more than fifty percent (50%) of the outstanding voting securities of the surviving entity or the ultimate parent of the surviving entity, following the closing of such merger, consolidation, other transaction or series of related transactions; or (b) any “person” or “group” (as such terms are defined under Section 13(d) and 14(d) of the United States Securities Exchange Act of 1934) that did not control such Party on the Effective Date obtains control (as defined in Section 1.1) of such Party.
1.6 “Clinical Trial” means a clinical trial in a human subject that has been approved by a Regulatory Authority and is designed to measure the safety or efficacy of a Product. A clinical trial can be a Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, or a study incorporating more than one of these phases.
1.7 “Collaboration IP” means Collaboration Know-How and Collaboration Patents.
1.8 “Collaboration Know-How” means, collectively, Joint Collaboration Know-How and Selvita Collaboration Know-How.
1.9 “Collaboration Patents” means, collectively, Joint Collaboration Patents and Selvita Collaboration Patents.
1.10 “Commercialization” or “Commercialize” means all activities undertaken with respect to a product relating to marketing, promotion (including advertising and detailing), medical affairs activities, medical science liaison activities, sponsored product or continuing medical education activities, post-Regulatory Approval clinical studies (that are not required to obtain or maintain such Regulatory Approval), obtaining pricing and reimbursement approval, in each case with respect to such product, any importing, offering for sale, distribution and sale of such product, identifying, screening or treating patients as potential users of such product, and interacting with Regulatory Authorities regarding the foregoing.
Exhibit D
1.11 “Commercially Reasonable Efforts” means, with respect to the performing Party, the carrying out of obligations of such Party using a diligent level of efforts and resources that a similar situated biopharmaceutical company (taking into consideration size, assets, status (e.g. “start-up” status) and dependency on third party investors) typically devotes to its own owned or licensed products of similar market potential at a similar stage in its development or product live, taking into account scientific and commercial factors, including issues of safety and efficacy, product profile, difficulty in developing or manufacturing a product, competitiveness of alternative products in the marketplace, the patent or other proprietary position of the Optioned Compound, the regulatory requirements involved and the potential profitability for the performing Party of the Optioned Compound marketed or to be marketed. If either Party grants a sublicense or assigns its rights and obligations under this Agreement to an Affiliate, Sublicensee, Third Party Partner or other Third Party as permitted under this Agreement, then, “Commercially Reasonable Efforts” shall be applied with respect to such Affiliate, Sublicensee Third Party Partner or other Third Party, but in no case shall fall below “Commercially Reasonable Efforts” as applicable for the Party granting the sublicense or assigning its rights and obligations.
1.12 “Compound(s)” means a small molecule kinase inhibitor compound(s) directed to a Target. “Small molecule” means a compound with molecular weight in its neutral form of less than or equal to 1000 unified atomic mass units.
1.13 “Control”, “Controls” or “Controlled” means, with respect to any Patent or Know-How or other intellectual property right, possession of the right (whether through ownership or license (other than by operation of this Agreement) or control (as used in Section 1.1) over an Affiliate with such right) to grant the licenses or sublicenses under such intellectual property right or Know-How or Patent as provided herein without violating the terms of any agreement or other arrangement with any Third Party. Notwithstanding the foregoing, an intellectual property right or Know-How or Patent of a Party that is licensed or otherwise acquired from a Third Party after the Effective Date and would otherwise be considered to be under the Control of a Party shall not be deemed to be under the Control of such Party if the application of such definition in the context of any license grants or sublicenses under this Agreement would require the granting Party to make additional payments or royalties to a Third Party in connection with such license or sublicense grants.
1.14 “Cover”, “Covering” or “Covered” means, with respect to a Patent and a product, composition, technology, process or method that, in the absence of ownership of or a license granted under a Valid Claim of such Patent, the Research, Development, Manufacture or Commercialization (including the use, offer for sale, sale or importation) of such product or composition, or the practice of such technology, process or method, would infringe such Valid Claim (or, in the case of a Valid Claim that has not yet issued, would infringe such Valid Claim if it were to issue).
Exhibit D
1.15 “Derivative” means, with respect to an Optioned Compound, a Compound which is a derivative or a modification of such Optioned Compound that is within the Stated Similarity Coefficient for the Target and which satisfies the selectivity and activity criteria for the Target as were established for the Optioned Compound from which such Derivative was identified, generated or optimized, wherein such Derivative includes (a) analogs of such Optioned Compound within the Stated Similarity Coefficient for the relevant Target that are derived by modifying such Optioned Compound in one or more steps by chemical or molecular-genetic means, or (b) structurally novel Compounds within the Stated Similarity Coefficient for the relevant Target that are created from such Optioned Compound by modifying the central core structure or “scaffold” (as is commonly referred to as “scaffold hopping”) of such Optioned Compound, in each case of (a) or (b) where such Compound was not independently developed by an Affiliate or successor of a Party, as can be shown by contemporaneous scientific records.
1.16 “Develop” or “Development” means post-Research pre-clinical development, clinical development, including GLP Toxicology Studies, formulation, Manufacturing process development and scale-up (including active pharmaceutical ingredient and drug product production), Manufacturing process validation, stability testing, analytical testing, quality assurance and quality control, technical support, pharmacokinetic studies, Clinical Trials, interacting with Regulatory Authorities regarding the foregoing, and all other activities relating to seeking, obtaining or maintaining any Regulatory Approvals for a pharmaceutical product from the FDA or any other applicable Regulatory Authority.
1.17 “Direct Disposal” means an outright sale or any other outright transfer to a Third Party of the Optioned Compounds or Collaboration IP which constitute subject matter of this Agreement without any prior Implementation by Felicitex in its own Research and Development business activity, it being understood that the subcontracting of certain scientific activities to Engaged Persons prior to Implementation by Felicitex or the allocation of certain Research or Development studies or activities to academic laboratories prior to Implementation by Felicitex shall not constitute a Direct Disposal.
1.18 “EMA” means the European Medicines Agency, and any successor entity thereto.
1.19 “EU” or “European Union” means all countries that are officially recognized as member states of the European Union at any particular time during the Term.
1.20 “Euro” or “EUR” means the lawful currency of the member states of the European Union that adopt the single currency in accordance with the relevant European Union treaties.
1.21 “European Commission” means the authority within the European Union that has the legal authority to grant Regulatory Approvals in the European Union based on input received from the EMA or other competent Regulatory Authorities.
1.22 “Executive Officers” means Selvita’s Chief Executive Officer and Felicitex’s Chief Executive Officer.
1.23 “Felicitex IP” means Felicitex Know-How and Felicitex Patents.
Exhibit D
1.24 “Felicitex Know-How” means Know-How that: (a) is Controlled by Felicitex as of the Effective Date or thereafter during the Term and (b) is necessary or reasonably useful for the Research, Development, Manufacture or Commercialization of Optioned Compounds and Products in the Field in the Territory.
1.25 “Felicitex Patent(s)” means Patents that: (a) are Controlled by Felicitex as of the Effective Date or thereafter during the Term and (b) claim or are directed to Felicitex Know-How.
1.26 “FDA” means the United States Food and Drug Administration, and any successor entity thereto.
1.27 “Field” means the treatment and remediation of any human or veterinary oncologic disease, disorder or condition.
1.28 “First Commercial Sale” means, on a country-by-country basis, the first sale or other disposition for value of a Product by Felicitex or any of its Affiliates, Sublicensees, Third Party Partners or any other Third Party to an independent or unaffiliated Third Party after all Regulatory Approvals for such Product have been obtained in such country.
1.29 “GAAP” means generally accepted accounting principles in the United States, or internationally, as appropriate, consistently applied; and will mean IFRS at such time as IFRS: (a) becomes the generally accepted accounting standard and applicable laws require that a Party use IFRS or (b) is adopted as the applicable accounting standard of such Party.
1.30 “GLP Toxicology Study” means a toxicology study that is conducted in compliance with the then-current good laboratory practice standards promulgated or endorsed by the FDA, as defined in U.S. 21 C.F.R. Part 58 (or such other comparable regulatory standards in jurisdictions outside the U.S. to the extent applicable to the relevant toxicology study, as they may be updated from time to time) and is required to meet the requirements for filing an IND. For purposes of this Section 1.30, and this Agreement, “GLP” means Good Laboratory Practice for Non-Clinical Laboratory Studies as defined in Part 58 of Title 21 of the U.S. Code of Federal Regulations.
1.31 “IFRS” or “International Financial Reporting Standards” means the set of accounting standards and interpretations and the framework in force on the Effective Date and adopted by the European Union as issued by the International Accounting Standards Board (IASB) and the International Financial Reporting Interpretations Committee (IFRIC), as such accounting standards may be amended from time to time.
1.32 “Implementation” means any expenditure use, Research or Development by Felicitex of the Optioned Compounds or Collaboration IP which constitutes subject matter of this Agreement in its own Research or Development business activity for purposes of Felicitex, which may include, but not limited to, Research and Development activities required for Clinical Trials, INDs, MAAs and Regulatory Approval such as: (i) safety and efficacy studies in vitro, (ii) safety and efficacy studies in in vivo models, (iii) in vivo toxicology studies, (iv) formulation development for Clinical Trials, (v) performance of Clinical Trials or the like.
Exhibit D
1.33 “IND” means: (a) an investigational new drug application submitted to the FDA pursuant to Part 312 of Title 21 of the U.S. Code of Federal Regulations (b) any comparable filing(s) outside the U.S. for the investigation of any product in any other country or group of countries (including an application for Clinical Trial(s) to be submitted to the EMA or other Regulatory Authorities in the EU as further defined in the Clinical Trials Directive (2001/20/EC, as amended) as well as any non-EU equivalent of the foregoing in any other country) and (c) all amendments and supplements thereto.
1.34 “Indication” means any human disease or condition, or sign or symptom of a human disease or condition. For the avoidance of doubt, all variants of a single disease or condition (whether classified by severity or otherwise) in relation to which the Development or Commercialization of a pharmaceutical product does not require a separate Regulatory Approval shall be treated as the same Indication, whereas all variants in relation to which the Development or Commercialization of a pharmaceutical product does require a separate Regulatory Approval shall constitute separate Indications.
1.35 “Initiation” means, with respect to a Clinical Trial, the first dosing of the first human subject enrolled in such Clinical Trial with a Product.
1.36 “Joint Collaboration IP” means Joint Collaboration Know-How and Joint Collaboration Patents.
1.37 “Joint Collaboration Know-How” means the Know-How described in Exhibit A.
1.38 “Joint Collaboration Patent(s)” means the Patents described in Exhibit A, which shall be updated, from time to time, as necessary by the Parties to include any applicable Patents filed or granted after the Effective Date which cover Optioned Compounds or Products.
1.39 “Know-How” means all tangible and intangible:
(a) information, techniques, technology, practices, trade secrets, inventions (whether patentable or not), methods, knowledge, know-how, skill, experience, data, results (including pharmacological, toxicological, pre-clinical and clinical test data and results, research data, reports and batch records), analytical and quality control data, analytical methods (including applicable reference standards), full batch documentation, packaging records, release, stability, storage and shelf-life data, and Manufacturing process information, results or descriptions, software and algorithms; and
(b) compositions of matter, cells, cell lines, assays, animal models and physical, biological or chemical material;
in each case ((a) and (b)) that is not generally known.
1.40 “Law” or “Laws” means all applicable laws, statutes, rules, regulations, orders, judgments, or ordinances having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision.
Exhibit D
1.41 “MAA” or “Marketing Authorization Application” means a regulatory application filed with the EMA seeking Regulatory Approval of a pharmaceutical product, and all amendments and supplements thereto filed with the EMA or any equivalent authority in any other country or regulatory jurisdiction.
1.42 “Major EU Country” means any of the following countries: France, Germany, Italy, Spain or the United Kingdom. “Major EU Countries” means some or all of the foregoing countries.
1.43 “Major Market Countries” means: (a) the United States, (b) Japan and (c) all of the Major EU Countries.
1.44 “Manufacture” or “Manufacturing” means, as applicable, all activities associated with the production, manufacture, supply, processing, filling, packaging, labeling, shipping, and storage of a compound or pharmaceutical product, as the case may be, or any components thereof, manufacture of pre-clinical, clinical and commercial supply, product characterization, quality assurance and quality control development, testing and release.
1.45 “NDA” means a New Drug Application (as more fully described in 21 C.F.R. 314.50 et seq. or its successor regulation) and all amendments and supplements thereto filed with the FDA, or any equivalent filing, including an MAA, in a country or regulatory jurisdiction other than the United States.
1.46 “Net Sales” means: (a) the gross amounts invoiced by Felicitex or any of its Affiliates for sales of Product to independent or unaffiliated Third Party purchasers of such Product or (b) all other revenues, receipts, monies and the fair market value of other consideration collected or received (whether by way of cash or credit or any benefit, advantage or concession) by Felicitex or any of its Affiliates for sales of Products less the following deductions with respect to such sales that are actually incurred and either included in the billing as a line item as part of the gross amount invoiced, or otherwise documented as a deduction in accordance with IFRS/GAAP to be specifically attributable to actual sales of such Product: (i) adequate credits, allowances or customary trade, quantity or cash discounts and refunds, replacements or credits for returned Products, and recalls (ii) sales tax and customs duties imposed in conjunction with such sales, but only to the extent that the selling party is not entitled to a refund of such taxes or duties, (iii) non-US taxes which are deducted or paid, but only to the extent that the selling party is not entitled to a refund of such taxes or duties, and (iv) costs for insurance, packing and transport of Products.
If non-monetary compensation is received for any Product in any country, Net Sales will be calculated based on the average price charged for such Product in such country during the preceding Calendar Quarter, or in the absence of such sales, the fair market value of the Product in such country, as determined by the Parties in good faith.
Net Sales shall be determined on, and only on, the first sale by Felicitex or any of its Affiliates to a Third Party (other than a Sublicensee or a distributor). Sales of an Product between Felicitex and any of its Affiliates for resale shall be excluded from the computation of Net Sales, but the subsequent resale of such Product to a Third Party (other than a Sublicensee or a distributor) shall be included within the computation of Net Sales. For the avoidance of doubt: net sales on sales of Products by Felicitex’s or its Affiliates’ Sublicensees or Third Party Partners (or by their sublicensees or distributors) to other independent or unaffiliated Third Parties are considered “Participation Income” pursuant to Section 7.3.1 and therefore are not reflected as Net Sales pursuant to this Section 1.46.
Exhibit D
If a Product under this Agreement is sold in the form of a Combination Product, then Net Sales for such Combination Product shall be determined on a country-by-country basis by mutual agreement of the Parties in good faith taking into account the perceived relative value contributions of the Product and the other ingredient or component in the Combination Product, as reflected in their respective market prices. In case of disagreement, an independent expert agreed upon by both Parties or, failing such agreement, designated by the International Chamber of Commerce, shall determine such relative value contributions and such determination shall be final and binding upon the Parties. As used in this Section, “Combination Product” means an Product that: (a) contains one or more additional active ingredients (whether co-formulated or co-packaged) that are not Optioned Compounds or (b) is combined with one or more products, devises, pieces of equipment or components.
In the event a Product is “bundled” for sale together with one or more other products in a country (a “Product Bundle”), then Net Sales for such Product sold under such arrangement shall be determined on a country-by-country basis by mutual agreement of the Parties in good faith taking into account the relative value contributions of the Product and the other products in the Product Bundle, as reflected in their individual sales prices. In case of disagreement, an independent expert agreed upon by both Parties or, failing such agreement, the International Chamber of Commerce shall determine such relative value contributions and such determination shall be final and binding upon the Parties
1.47 “Optioned Compound” means: (a) the Compound(s) listed in Exhibit B; (b) any Derivative, enhancement, refinement, invention or improvement of any Compound described in clause (a) above that is first synthesized, identified, conceived, reduced to practice or developed by (or on behalf of) Felicitex or any of its Affiliates, Sublicensees or Third Party Partners after the Effective Date or (c) any salt or prodrug of a Compound described in clause (a) or (b) above.
1.48 “Patents” means: (a) all national, regional and international patents and patent applications, including provisional patent applications, (b) all patent applications claiming priority from any one of the above, including divisionals, continuations, continuations-in-part, (c) any and all patents that have issued or in the future issue from the foregoing patent applications ((a) and (b)), including utility models, petty patents and design patents and certificates of invention, (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications ((a), (b), and (c)), and (e) any similar rights, including so-called pipeline protection or any importation, revalidation, confirmation or introduction patent or registration patent or patent of additions to any of such foregoing patent applications and patents.
Exhibit D
1.49 “Person” means any individual, partnership, joint venture, limited liability company, corporation, firm, trust, association, unincorporated organization, governmental authority or agency, or any other entity not specifically listed herein.
1.50 “Phase 1 Clinical Trial” means a Clinical Trial in which the Product is administered to human subjects at single and multiple dose levels with the primary purpose of determining safety, metabolism, and pharmacokinetic and pharmacodynamic properties of the Product, and which is consistent with 21 U.S. CFR § 312.21(a). For the purposes of the milestone payments under this Agreement, a “Phase 1 Clinical Trial” shall be a Clinical Trial which is submitted to the Regulatory Authority as a Phase 0 Clinical Trial, Phase 1 Clinical Trial or as a Phase 1/2 Clinical Trial.
1.51 “Phase 2 Clinical Trial” means a Clinical Trial of the Product in human patients, the principal purposes of which are to make a preliminary determination that the Product is safe for its intended use, to determine its optimal dose, and to obtain sufficient information about the Product’s efficacy to permit the design of Phase 3 Clinical Trials, and which is consistent with 21 U.S. CFR § 312.21(b). For the purposes of the milestone payments under this Agreement, a “Phase 2 Clinical Trial” shall be a Clinical Trial which is submitted to the Regulatory Authority as a Phase 2 Clinical Trial or as a Phase 2/3 Clinical Trial.
1.52 “Phase 3 Clinical Trial” means a Clinical Trial of the Product in human patients, which trial is designed (a) to establish that the Product is safe and efficacious for its intended use; (b) to define warnings, precautions and adverse reactions that are associated with the Product in the dosage range to be prescribed; and (c) to be, either by itself or together with one or more other Clinical Trials having a comparable design and size, the final human Clinical Trial in support of Regulatory Approval of an MAA or NDA of the Product, and (d) consistent with 21 U.S. CFR § 312.21(c). For the purposes of the milestone payments under this Agreement, a “Phase 3 Clinical Trial” shall be a Clinical Trial which is submitted to the Regulatory Authority as a Phase 3 Clinical Trial.
1.53 “Product” means any therapeutic product comprising or based upon an Optioned Compound, whether or not as the sole active ingredient, and in any dosage form or formulation.
1.54 “Prosecution and Maintenance” or “Prosecute and Maintain” means, with regard to a Patent, the preparation, filing, prosecution and maintenance of such Patent, as well as re-examinations, reissues, appeals, and requests for patent term adjustments with respect to such Patent, together with the initiation or defense of interferences, post-grant reviews, Inter Parties Reviews, Ex Parte Reexam, the initiation or defense of oppositions and other similar proceedings with respect to the particular Patent, and any appeals therefrom. For clarification, “Prosecution and Maintenance” or “Prosecute and Maintain” shall not include any other defense or enforcement actions taken with respect to a Patent.
1.55 “Regulatory Approval” means, with respect to a country in the Territory, the approval, license or authorization of the applicable Regulatory Authority(ies) necessary for the marketing and sale of a pharmaceutical or biopharmaceutical product for a particular indication in such country in the Territory, including any separate pricing or reimbursement approvals, but only to the extent that such approvals are legally required for the marketing and sale of a pharmaceutical product for such indication in such country. For the avoidance of doubt: (a) if the marketing and sale of a pharmaceutical product for a given indication in a given country does not legally require a separate pricing or reimbursement approval, no such approval is required within this definition and (b) if the marketing and sale of a pharmaceutical product for a given indication requires more than one separate pricing or reimbursement approval in a given country, the first pricing or reimbursement approval achieved shall suffice to meet this definition.
Exhibit D
1.56 “Regulatory Authority” means, with respect to a country in the Territory, any national, multinational, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity that regulates or otherwise exercises authority with respect to the Research, Development, Manufacture, Commercialization (including marketing, sale, distribution), use or other exploitation of pharmaceutical products in such country, including the FDA and the EMA, and any successor(s) thereto.
1.57 “Regulatory Dossier” means the technical, medical and scientific registrations, authorizations and approvals (including approvals of NDAs, supplements and amendments, pre-and post- approvals, pricing and Third Party reimbursement approvals, and labeling approvals) of any Regulatory Authority necessary for the Development (including the conduct of Clinical Trials), Manufacture, Commercialization (including distribution, marketing, promotion, offer for sale, use, import, reimbursement, export or sale) of a product in a regulatory jurisdiction, together with all related correspondence to or from any Regulatory Authority and all documents referenced in the complete regulatory chronology for each NDA, including all Regulatory Materials and drug master file(s) (if any).
1.58 “Regulatory Materials” means regulatory applications, notifications, registrations, Regulatory Approvals or other submissions made to or with a Regulatory Authority that are necessary or reasonably desirable in order to Develop, Manufacture, market, sell or otherwise Commercialize a product in a particular country, territory or possession. Regulatory Materials include INDs and NDAs, and amendments and supplements to any of the foregoing, and applications for pricing approvals.
1.59 “Research” means the discovery, research and pre-clinical development prior to the initiation of GLP Toxicology Studies, including identification, characterization, optimization, non-clinical testing, pharmacology studies, toxicology studies prior to initiation of GLP Toxicology Studies, synthesis, chemical analysis, bioanalytical analysis, material performance studies (such as measurements of stability, physical form, dissolution, or visual or spectroscopic analysis, and the like).
1.60 “Sale Transaction” means, with respect to Felicitex or any of its Affiliates, (a) a sale and assignment of all or a part of Felicitex’s rights, title and interest to the “DYRK1A/B” research program to a Third Party, including the licenses granted to it by Selvita under this Agreement in relation to Optioned Compounds and Products, independent of whether such sale transaction concerns solely the assets related to the “DYRK1A/B” research program or also further unrelated assets of Felicitex or (b) a sale and assignment of all or substantially all of its business or assets to a Third Party. For the avoidance of doubt, a Sale Transaction does not include a merger or stock sale of Felicitex.
Exhibit D
1.61 “Selvita Collaboration IP” means Selvita Collaboration Know-How and Selvita Collaboration Patents.
1.62 “Selvita Collaboration Know-How” means the Know-How described in Exhibit C.
1.63 “Selvita Collaboration Patents” means the Patents listed in Exhibit C, which shall be updated as necessary by the Parties to include any applicable Patents filed or granted after the Effective Date which cover Optioned Compounds or Products.
1.64 “Selvita IP” means Selvita Know-How and Selvita Patents.
1.65 “Selvita Know-How” means Know-How that: (a) is Controlled by Selvita as of the Effective Date and (b) is necessary or reasonably useful for the Research, Development, Manufacture or Commercialization of Optioned Compounds and Products against the Target in the Field in the Territory. For purposes of clarity, Selvita Know-How excludes Selvita Collaboration Know-How and Selvita’s interest in any Joint Collaboration Know-How.
1.66 “Selvita Patents” means Patents that: (a) are Controlled by Selvita or its Affiliates as of the Effective Date or thereafter during the Term and (b) claim or are directed to Selvita Know-How. For purposes of clarity, Selvita Patents exclude Selvita Collaboration Patents and Selvita’s interest in any Joint Collaboration Patent.
1.67 “Stated Similarity Coefficient” means Tanimoto coefficient, calculated pursuant to the algorithm as described in Exhibit D, is more than 0.85.
1.68 “Sublicensee” means, with respect to Felicitex, a Third Party to whom Felicitex has granted a license under Know-How or Patents Controlled by it, or a sublicense pursuant to this Agreement, to Research, Develop, Manufacture or Commercialize the Optioned Compounds or Products in the Field, excluding any Third Party acting solely as a distributor.
1.69 “Target” means the target(s) described in Exhibit E.
1.70 “Territory” means the entire world.
1.71 “Third Party” means any Person other than Selvita or Felicitex that is not an Affiliate of Selvita or of Felicitex.
1.72 “Third Party Partner” means, with respect to Felicitex, a Third Party with whom Felicitex has undertaken a Sale Transaction.
1.73 “United States” or “U.S.” means the United States of America and all of its territories and possessions.
1.74 “U.S. Dollar” or “US$” means the legal tender currency of the U.S..
Exhibit D
1.75 “Valid Claim” means:
(a) a claim of an issued patent that has not expired, lapsed, been cancelled or abandoned, or been dedicated to the public, disclaimed, or held unenforceable, invalid, or cancelled by a court or administrative agency of competent jurisdiction in an order or decision from which no appeal has been or can be taken, including through opposition, reexamination, reissue or disclaimer; or
(b) a claim of a pending patent application that has not been finally abandoned and which has been pending for no more than seven (7) years from the date of filing of the earliest priority patent application to which such pending patent application is entitled to claim benefit.
1.76 Additional Definitions. Each of the following definition is set forth in the Sections of this Agreement indicated below:
“Adjusted Value Share” shall have the meaning as defined in Section 7.1.3
“Arbitration Request” shall have the meaning as defined in Section 14.2.1
“Claims” shall have the meaning as defined in Section 12.1
“Confidential Information” shall have the meaning as defined in Section 10.1
“Decreased Value Share” shall have the meaning as defined in Section 7.1.2
“Diluted Value Share” shall have the meaning as defined in Section 7.3.1
“Disclosing Party” shall have the meaning as defined in Section 10.1.
“Engaged Person” shall have the meaning as defined in Section 2.2
“External Development” shall have the meaning as defined in Section 5.4
“Indemnified Party” shall have the meaning as defined in Section 12.1
“Indemnifying Party” shall have the meaning as defined in Section 12.1
“Initial Value Share” shall have the meaning as defined in Section 7.1.2
“Internal Development” shall have the meaning as defined in Section 5.4
“Losses” shall have the meaning as defined in Section 12.1
“Minimum Royalty Rate” shall have the meaning as defined in Section 7.3.2(e)
“Minimum Value Share” shall have the meaning as defined in Section 7.3.2(e)
“Notice Period” shall have the meaning as defined in Section 13.2.1
“Participation Income” shall have the meaning as defined in Section 7.3.1
“Product Information” shall have the meaning as defined in Section 10.2
“Publishing Party” shall have the meaning as defined in Section 10.7.2
“Receiving Party” shall have the meaning as defined in Section 10.1.
“Required Publications” shall have the meaning as defined in Section 10.3.
“Reviewing Party” shall have the meaning as defined in Section 10.7.2
“SEL141 Loss” shall have the meaning as defined in Section 12.2
“Severed Clause” shall have the meaning as defined in Section 14.11
“Term” shall have the meaning as defined in Section 13.1.
Exhibit D
ARTICLE II
SCOPE OF THE AGREEMENT
2.1 Development and Commercialization of Optioned Compounds and Products. Felicitex shall have the sole and exclusive (even as to Selvita) right and responsibility for all Research, Development, Manufacturing and Commercialization activities for all Optioned Compounds and Products against the Target in the Field in the Territory. For this purpose, Selvita shall grant to Felicitex the licenses outlined in Article III hereof. In consideration of the license grants, Felicitex shall pay to Selvita certain milestones, royalties and/or participation payments on basis of Selvita’s Initial Value Share, Decreased Value Share or, as applicable, Adjusted Value Share as defined and outlined in Article VII.
2.2 Internal or External Development. Subject to the provisions of Section 5.4, Felicitex shall be entitled to undertake the Research, Development and Commercialization of Optioned Compounds and Products either internally by itself or by any of its Affiliates (including, for the avoidance of doubt, through Third Parties such as independent contractors or subcontractors, e.g. a Contract Research Organization (“CRO”) (each an “Engaged Person”)) or externally through a Sublicensee (following an out-licensing transaction) or through a Third Party Partner following a Sale Transaction.
2.3 Other Transactions. A Change of Control Event in either Party shall neither effect this Agreement nor the Parties’ rights and obligations hereunder, it being understood that either Party upon a Change of Control Event may not be the surviving entity and that in such case the legal successor will assume such Party’s rights and obligations hereunder.
ARTICLE III
LICENSE GRANTS
3.1 Exclusive License from Selvita to Felicitex. Selvita hereby grants to Felicitex an exclusive, milestone-bearing, royalty-bearing, transferable license, with the right to grant sublicenses through multiple tiers of Sublicensees, under the Selvita Collaboration IP and Selvita’s share in the Joint Collaboration IP, in each case to the extent Covering Optioned Compounds and as necessary or useful to Research, Develop, Manufacture and Commercialize any Optioned Compounds or Products against the Target in the Field in the Territory. Felicitex hereby accepts and acknowledges such exclusive license.
3.2 Non-Exclusive License from Selvita to Felicitex. Selvita hereby grants to Felicitex a non-exclusive, milestone-bearing, royalty-bearing, transferable license, with right to grant sublicenses through multiple tiers of Sublicensees, under the Selvita IP, to the extent Covering Optioned Compounds and as necessary to Research, Develop, Manufacture and Commercialize any Optioned Compounds or Products against the Target in the Field in the Territory and to the extent that such Selvita IP, but for the license granted, would be infringed by the Research, Development, Manufacture and Commercialization of Optioned Compounds and Products against the Target in the Field in the Territory. Felicitex hereby accepts and acknowledges such license.
Exhibit D
3.3 Sublicensing and Transfer Rights. Each sublicense granted under any of the licenses granted by Selvita under this Article III as well as any transfer thereof shall be subject to the conditions outlined in Section 5.4 and shall be consistent with the terms and conditions of this Agreement.
3.4 Further Actions.
3.4.1 Selvita shall take and, to the extent that any rights licensed by Selvita to Felicitex hereunder are Controlled by an Affiliate of Selvita, shall cause its Affiliates to take, all such steps as are necessary to perfect Felicitex’s rights as licensed to it hereunder.
3.4.2 To the extent not already provided prior to the Effective Date, Selvita shall promptly as possible following the Effective Date, provide to Felicitex access to and copies of all documents and materials containing licensed Know-How as shall be reasonably requested by Felicitex and as necessary or reasonably useful to exercise its rights under the license grants in this Article III and, pursuant to the obligations in Section 4.1 hereof, shall provide sufficient consultation time to fully advise and instruct Felicitex with respect to the Know-How.
3.5 Rights Retained by the Parties. Any rights of Selvita or rights of Felicitex, as the case may be, that are not expressly granted to the other Party pursuant to this Agreement shall be retained by such Party.
3.6 Good Faith Negotiations on License or (Re-)Transfer of Rights. If Felicitex, in addition to the rights and licenses granted to it under this Agreement, or Selvita wishes to acquire or license any rights controlled by the other Party in order to pursue Development of Optioned Compounds outside the Field in other therapeutic areas, such as Alzheimer disease, then the Parties shall negotiate in good faith for an agreement with commercially reasonable terms pursuant to which the requesting Party may acquire the necessary rights from the other Party to further Research, Develop, Manufacture and Commercialize the relevant Optioned Compounds in the requested territory outside the Field. For clarity, neither Party shall be under any obligation to agree to enter into any such agreement for the grant of any such rights or licenses to the other Party, beyond the obligation to consider and negotiate any such request in good faith and on commercially reasonable terms and the failure to reach an agreement shall not constitute a breach or violation of this Agreement by either Party.
3.7 Section 365(n) of the Bankruptcy Code. All rights and licenses granted under or pursuant to this Agreement by a Party to the other Party are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties, as licensees of such rights under this Agreement, shall retain and may fully exercise all of their respective rights and elections under the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against either Party under the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction, the Party that is not a party to such proceeding shall be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in the non-subject Party’s possession, shall be promptly delivered to it: (a) upon any such commencement of a bankruptcy proceeding upon the non-subject Party’s written request therefore, unless the Party subject to such proceeding elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under clause (a) above, following the rejection of this Agreement by or on behalf of the Party subject to such proceeding upon written request therefore by the non-subject Party.
Exhibit D
ARTICLE IV
TECHNOLOGY TRANSFER
4.1 Consultation Without Charge. Upon the request of Felicitex, Selvita shall provide to Felicitex consultation and advice as reasonably required with respect to Felicitex’s Development activities with respect to Optioned Compounds and Products against the Target in the Field. Up to twenty (20) consulting hours of work by one (1) full time employee (or, any proportioned amount of hours of work by more than one (1) full time employee) for such consultation and advice, if provided via teleconference or videoconference, shall be provided at no additional cost to Felicitex and, if provided in person at Felicitex’s facilities as may be mutually agreed by the Parties, shall be provided subject to Felicitex’s payment of Selvita’s reasonable and documented travel expenses associated with the provision of such consultation and advice. If Felicitex requests any consultation and advice exceeding the limitations of time and work amount as stated above, Section 4.2 shall apply.
4.2 Additional Services. Subject to mutual agreement by the Parties pursuant to a separate contract, Selvita shall provide to Felicitex consultation, advice or other services at the rate of [**] per full time employee per year (consisting of at least a total of 1,800 hours per year), if the work undertaken does not involve reagents (reagents and outsourcing being invoiced separately), or (b) [**] per full time employee per year (consisting of at least a total of 1,800 hours per year), if the work undertaken involves reagents (no external outsourcing, just procurement for chemistry and biology), or (c) US$[**] per full time employee per year (consisting of at least a total of 1,800 hours per year), if the work undertaken involves reagents and external outsourcing.
ARTICLE V
DEVELOPMENT AND COMMERCIALIZATION
5.1 Responsibility for Development and Commercialization. Subject to its obligations pursuant to Section 5.2 to Section 5.5 below, Felicitex shall have the sole responsibility and discretion for the Development and Commercialization of the Optioned Compounds and the Products.
5.2 Diligence Obligation. Felicitex shall use its Commercially Reasonable Efforts to further Research, Develop and Manufacture at least one (1) Product and to seek Regulatory Approval for and to Commercialize at least one (1) Product in the Major Market Countries following receipt of Regulatory Approval therein.
Exhibit D
5.3 Obligations Under SEL141 Grant. The Parties shall cooperate to meet the requirements under the SEL141 Grant and the related grant agreement with the Polish Agency for Enterprise Development, whereas Felicitex shall be solely responsible for and shall undertake Commercially Reasonable Efforts to meet solely the following requirements:
5.3.1 Implementation. Felicitex shall commence the Implementation of the Optioned Compounds or Collaboration IP which constitute subject matter of this Agreement within six (6) months following the Effective Date.
5.3.2 Implementation Statement. Felicitex shall within one (1) year after the Effective Date deliver to Selvita a written statement in a form and substance as attached to this Agreement as Exhibit F certifying that it has Implemented the Optioned Compounds or Collaboration IP which constitute subject matter of this Agreement in its own Research and Development business activity. Selvita is entitled to reasonably amend Exhibit F on basis of new applicable Polish Laws imposing on Selvita obligations related to the reporting on Implementation of the subject matter of this Agreement, it being understood that any such amendment shall in no event cause obligations of Felicitex different or in addition to those outlined in this Agreement.
5.3.3 No Direct Disposal. With regard to its obligations related to the Implementation, Felicitex in particular shall not undertake a Direct Disposal of the Optioned Compounds or Collaboration IP which constitute the subject matter of this Agreement without any prior Implementation by Felicitex.
5.4 Internal or External Development and Commercialization. Felicitex shall be entitled to undertake the Development and Commercialization of Optioned Compounds and Products either internally by itself or by any of its Affiliates or by any of its Engaged Persons (in each case a “Internal Development”) or externally through a Sublicensee (following an out-licensing transaction) or through a Third Party Partner following a Sale Transaction (in each case an “External Development”), provided that
(a) any of the aforesaid partners to be selected by Felicitex to undertake the Development and Commercialization of Optioned Compounds and Products (either by way of Internal Development or by way of External Development) shall be qualified in Felicitex’s reasonable opinion on the date of engagement, to undertake such Development and Commercialization activities; and
(b) Felicitex shall procure that the contractual rights and obligations of any such partner are consistent with the terms and conditions of this Agreement and that any such partner submits itself to diligence and reporting obligations which are consistent with those of Felicitex under this Agreement and which in particular comply with the obligations outlined in Section 5.5, 6.2, 7.5, 7.6 and 7.9; and
(c) Felicitex prior to entering into any out-licensing transaction or Sale Transaction related (solely or inter alia) to Felicitex’ “DYRK1A/B” research program shall notify Selvita about such envisaged transaction and, promptly upon entering into such transaction, shall disclose to Selvita any and all provisions of the underlying contractual agreement which are required or reasonably useful for Selvita to calculate or audit its payment claims towards Felicitex under Article VII of this Agreement and
Exhibit D
(d) Felicitex, in case of both Internal Development and External Development, shall (except in the case of a Sale Transaction to a non-Affiliate of Felicitex to which Selvita has previously consented (such consent not to be unreasonably withheld) or a merger, sale or acquisition in which it is not the surviving entity) remain responsible towards Selvita for all Development and Commercialization activities outlined in this Agreement as well as for all payment obligations pursuant to Article VII (in case of a Sale Transaction which involves an assignment of this Agreement to a Third Party Partner in form of joint liability together with any assignee of Felicitex), and
(e) Felicitex, in case of External Development following a Sale Transaction which involves an assignment of this Agreement to a Third Party Partner, shall procure that such Third Party Partner submits itself to payment obligations applicable for External Development (pursuant to Section 7.3), as the Parties agree and acknowledge that Development and Commercialization of Optioned Compounds or Products that is undertaken by a Third Party Partner constitutes External Development (not Internal Development, although such Third Party Partner may become an assignee of Felicitex upon a Sale Transaction).
5.5 Reports. In addition to its other reporting obligations under this Agreement, Felicitex (itself or through its Affiliate or Sublicensee or Third Party Partner, as applicable) shall provide Selvita with regular periodic written reports summarizing in reasonable detail the plans, activities and accomplishments of Felicitex (or its Affiliate or Sublicensee or Third Party Partner, as applicable) with respect to the further Research, Development, Manufacturing and Commercialization of Optioned Compounds and Products. Such written reports shall be provided to Selvita at least every Contract Quarter during the Term.
ARTICLE VI
REGULATORY MATTERS
6.1 Compliance. Felicitex shall perform its obligations in good scientific manner and comply in all material respects with all applicable FDA and other current international regulatory requirements and standards, and comparable foreign regulatory standards, and other Laws, including all of the requirements, laws, regulations, terms and obligations applicable to the SEL141 Grant and the related grant agreement with the Polish Agency for Enterprise Development.
6.2 Data Integrity. Felicitex shall maintain, or cause to be maintained, usual and customary records of its Research, Development and Commercialization activities as required by Law, in the usual and customary detail and accuracy and in good scientific manner appropriate for patent and regulatory purposes, and properly reflecting all work done and results achieved in the performance of such activities. Such records shall be retained by Felicitex for at least ten (10) years after the termination of this Agreement, or for such longer period as may be required by Law.
Exhibit D
6.3 Regulatory Submissions. As between Felicitex and Selvita, Felicitex shall own and maintain all regulatory submissions, Regulatory Dossiers, Regulatory Material and Regulatory Approvals for the Products, including all INDs and MAAs.
6.4 Communications with Authorities. Felicitex shall be responsible for and act as the sole point of contact for communications with Regulatory Authorities in connection with the Development, Commercialization and Manufacturing of Products. Following the Effective Date, Selvita shall not initiate, with respect to any Product, any meetings or contact with Regulatory Authorities without Felicitex’s prior written consent. To the extent Selvita receives any written or oral communication from any Regulatory Authority relating to a Product, Selvita shall: (a) refer such Regulatory Authority to Felicitex and (b) as soon as reasonably practicable, notify Felicitex and provide Felicitex with a copy of any written communication received by Selvita or, if applicable, complete and accurate minutes of such oral communication.
6.5 Adverse Event Reporting. Felicitex shall comply with any and all Laws that are applicable as of the Effective Date and thereafter during the Term in connection with Product safety data collection and reporting. If Selvita has or receives any information regarding any Adverse Event which may be related to the use of Product, then Selvita shall provide Felicitex with all such information in English promptly. Felicitex shall report to Selvita any material adverse event culminating in death or permanent disability of a patient or subject who is administered a Product. The information exchanged between the Parties pursuant to this Section 6.5 shall be transmitted by e-mail, facsimile or overnight courier to the following address:
| | If to Selvita, | |
| | | |
| | addressed to: | Chief Executive Officer, Selvita, Park Life Science, |
| | | ul. Bobrzyńskiego 14, 30-348 Kraków, Poland |
| | | phone: +48 12 297 47 00 |
| | | fax +48 12 297 47 01 |
| | | |
| | with a copy to: | Chief Operating Officer, Selvita, Park Life Science, |
| | | ul. Bobrzyńskiego 14, 30-348 Kraków, Poland |
| | | phone: +48 12 297 47 00 |
| | | fax +48 12 297 47 01 |
| | | |
| | If to Felicitex, | |
| | | |
| | addressed to: | Chief Executive Officer, Felicitex, |
| | | 27 Strathmore Rd., |
| | | Natick, Massachusetts 02468 |
| | | United States of America |
| | | phone: +01 (919) 213-0025 |
| | | |
| | with copies to: | Boston Law Group, PC |
| | | Attn: Val Gurvits, Esq. |
| | | 825 Beacon Street |
| | | Newton Centre, , MA 02459 |
| | | phone: 617-928-1800 |
| | | email: vgurvits@bostonlawgroup.com |
6.6 Recalls. Felicitex shall have the sole right to determine whether and how to implement a recall or other market withdrawal of the Product.
ARTICLE VII
COMMERCIAL TERMS
7.1 General Terms for Payments to Selvita with Respect to Optioned Compounds and Products. In consideration of the rights granted to Felicitex hereunder, Felicitex shall make the following payments to Selvita in accordance with the terms and conditions of this Article 7, whereas the payment obligations outlined in Section 7.2 apply if and as long as Felicitex undertakes Internal Development and the payment obligations outlined in Section 7.3 apply if Felicitex undertakes External Development.
7.1.1 Initial Value Share. With view to Selvita’s and Felicitex’s contributions to the Research and Development of the Optioned Compounds the Parties agree and acknowledge that Selvita’s share in the value of the subject matter of this Agreement amounts to [**](“Initial Value Share”) [Note to Draft: specific initial value share percentage be completed in final version pursuant to guidelines for calculation of the initial value share (V1 or V2) in Exhibit F, Part A of the RCO Agreement.].
7.1.2 Step-Down Formula. The Parties agree and acknowledge that Felicitex may undertake Development and Commercialization not only with regard to the Optioned Compounds most advanced as of the Effective Date, but that Felicitex may proceed with the Development and Commercialization of any other Optioned Compound for which a Patent with a Valid Claim Covering the Development, Manufacture or Commercialization of such given Optioned Compound may be filed several months after the Effective Date. With a view to Optioned Compounds and only in case that Felicitex (or its Affiliate or Sublicensee or Third Party Partner, as applicable) files the first Patent with a Valid Claim Covering the Development, Manufacture or Commercialization of such Optioned Compounds or related Products after 31 January 2019, Selvita’s Initial Value Share shall be decreased on basis of the following formula (“Decreased Value Share”), whereas the Parties agree that Felicitex (or its Affiliate or Sublicensee or Third Party Partner, as applicable) shall file such first Patent for a given Optioned Compound no later than upon the initiation of GLP Toxicology Studies with regard to such Optioned Compound:
Decreased Value Share =[**] - ([**])*(X-52)/24, where X is the number of months elapsed from 1 October 2014 until the month when the relevant application for the first Patent on such Optioned Compounds or related Products is filed.
Exhibit D
Notwithstanding the aforesiad: If the application for the first Patent on such Optioned Compounds or related Products is filed after 31 January 2021, the Decreased Value Share is zero percent (0%).
For avoidance of doubt: The Step-Down Formula pursuant to this Section 7.1.2 shall be applied only once to a given Optioned Compound.
7.1.3 Value Share Adjustment. Selvita’s Initial Value Share or, if applicable, Decreased Value Share can be modified by up to [**] of its initial value (i.e. it can be [**] or [**] of its initial value), if Felicitex’s costs for pre-clinical Development (IND enabling studies as reflected by invoices from CROs) achieve or exceed [**] U.S. Dollars ([**]) or fall below thereof down to [**] U.S. Dollars (US$[**] In such case, Selvita’s Initial Value Share, or if applicable, Decreased Value Share shall be recalculated on basis of the following formula (“Adjusted Value Share”):
Adjusted Value Share (in percent,%) = V - [V*[**]*(Y-2)/2)], where Y is the direct, evidenced CRO costs for IND-enabling studies in Million U.S. Dollars (US$M) and V is the actual Initial Value Share or, if applicable, Decreased Value Share of Selvita as defined in Section 7.1.1 or, if applicable, Section 7.1.2.
7.2 Payments During Internal Development. If and to the extent that Felicitex undertakes the Development and Commercialization by way of Internal Development, Felicitex shall make the following milestone and royalty payments to Selvita. The milestone and royalty payments reflect the Initial Value Share and therefore are subject to adjustments in case that the Decreased Value Share or Adjusted Value Share applies (any such adjustment to be undertaken pursuant to Section 7.2.1(c), Section 7.2.2(c) and Section 7.2.3(c) below).
7.2.1 Development Milestones for Internal Development. Felicitex shall make the following non-refundable, non-creditable development milestone payments to Selvita. As soon as Felicitex becomes aware that any of the milestone events outlined below has been achieved with respect to an Optioned Compound or Product, Felicitex shall promptly inform Selvita about such occurrence.
(a) Development Milestone Table [Note to Draft: specific milestone payment amounts to be completed in final version pursuant to guidelines for calculation of milestones in Exhibit F, Part B of the RCO Agreement.]
| Milestone Event | Milestone Payment |
| First Indication | Second and any
subsequent
Indication |
| Initiation of the first Phase 2 Clinical Trial | [**] | [**] | |
| Initiation of the first Phase 3 Clinical Trial | [**] | [**] | |
| Regulatory Approval in the U.S. | [**] | [**] | |
| Regulatory Approval in the EU | [**] | [**] | |
| Regulatory Approval in Japan | [**] | [**] | |
| Regulatory Approval in Brazil, Russia, India and China (BRIC; at least 2 out of 4 countries) | [**] | [**] | |
Exhibit D
(b) Further Conditions for Development Milestones. For the avoidance of doubt, the following conditions shall apply to the milestone payments outlined in Section 7.2.1(a) for Development milestone events:
(i) each milestone payment is payable for each Optioned Compound or Product, regardless of the number of Optioned Compounds or Products with which a milestone event is achieved;
(ii) each milestone payment is payable independent from whether the milestone event is achieved by Felicitex or by its Affiliates;
(iii) in the case that the “Initiation of the first Phase 2 Clinical Trial” milestone event is not triggered in the course of Development, the milestone payment for such milestone event shall become due and payable upon the earlier of either (x) the occurrence of the Initiation of the first Phase 3 Clinical Trial or (y) filing of an NDA either in the US, the EU, Japan, or any of the BRIC countries, and, in the case that the “Initiation of the first Phase 3 Clinical Trial” milestone event is not triggered in the course of Development, the milestone payment for such milestone event shall become due and payable upon the filing of an NDA either in the US, the EU, Japan, or any of the BRIC countries; and
(iv) the milestone event “Regulatory Approval in the EU” shall mean Regulatory Approval by EMA or by the European Commission or by any other Regulatory Authority in one of the Major EU Countries, whichever occurs earlier.
(c) Adjustments.
(i) In case a Decreased Value Share is applicable pursuant to Section 7.1.2, the milestone payment amounts indicated in the Development Milestone Table shall be recalculated as follows:
Milestone payment amount multiplied by the Decreased Value Share (in percent) and divided by the Initial Value Share (in percent)
(ii) In case an Adjusted Value Share is applicable pursuant to Section 7.1.3, the milestone payment amounts indicated in the Development Milestone Table shall be recalculated as follows:
Milestone payment amount multiplied by the Adjusted Value Share (in percent) and divided by the Initial Value Share (in percent)
7.2.2 Sales Milestones for Internal Development. Felicitex shall make the following non-refundable, non-creditable sales milestone payments to Selvita. As soon as Felicitex becomes aware that any of the milestone events outlined below has been achieved, Felicitex shall promptly inform Selvita about such occurrence. [Note to Draft: specific milestone payment amounts to be completed in final version pursuant to guidelines for calculation of milestones in Exhibit F, Part B of the RCO Agreement.]
Exhibit D
(a) Sales Milestone Table
Milestone Event
Aggregate Calendar Year Net Sales of all Products
in the Territory exceed | Milestone Payment |
| US[**] | [**] |
| US[**] | [**] |
(b) The sales milestone payments outlined above shall be applicable for each Calendar Year in which the outlined milestone event is achieved with aggregate Net Sales of all Products in such given Calendar Year.
(c) Adjustments.
(i) In case a Decreased Value Share is applicable pursuant to Section 7.1.2, the milestone payment amounts indicated in the Sales Milestone Table shall be recalculated as follows:
Milestone payment amount multiplied by the Decreased Value Share (in percent) and divided by the Initial Value Share (in percent)
(ii) In case an Adjusted Value Share is applicable pursuant to Section 7.1.3, the milestone payment amounts indicated in the Sales Milestone Table shall be recalculated as follows:
Milestone payment amount multiplied by the Adjusted Value Share (in percent) and divided by the Initial Value Share (in percent)
7.2.3 Royalties for Internal Development.
(a) Royalty Rates. During the Royalty Term described below, Felicitex shall pay to Selvita royalties on aggregate Net Sales of Products with the following royalty rates. [Note to Draft: specific royalty rates to be completed in final version pursuant to guidelines for calculation of milestones in Exhibit F, Part C of the RCO Agreement.]
Aggregate Calendar Year Net Sales of Products
in the Territory (in U.S. Dollars) | Royalty Rate |
| US$[**] | [**] |
| US$[**] | [**] |
| Greater than US$[**] | [**] |
Exhibit D
(b) Royalty Term. Royalties on Net Sales of a Product at the rates set forth in Section 7.2.3(a) shall become payable upon the First Commercial Sale of a Product and shall continue to be payable, on a Product-by-Product and country-by-country basis, until expiration of the last Valid Claim of a Selvita Patent or a Collaboration Patent Covering the Development, Manufacturing or Commercialization of the Product in the relevant country. If a Patent that is filed after the Effective Date by Felicitex (or its Affiliate or Sublicensee or Third Party Partner, as applicable) is not a Collaboration Patent, but another Patent Covering the Development, Manufacture or Commercialization of a given Optioned Compound or Product, then in such case the royalty term shall continue until the expiration of the last Valid Claim of such Patent and the Parties agree and acknowledge that in such case, upon expiration of the licensed Selvita Patents and Collaboration Patents, the royalties shall remain payable for the Selvita Know-How and Collaboration Know-How licensed hereunder, but shall be reduced by [**] to reflect the expiry of the licensed Selvita Patents and Collaboration Patents.
(c) Adjustments.
(i) In case a Decreased Value Share is applicable pursuant to Section 7.1.2, the royalty rate indicated in Section 7.2.3(a) shall be recalculated as follows:
Royalty rate multiplied by the Decreased Value Share (in percent) and divided by the Initial Value Share (in percent)
(ii) In case an Adjusted Value Share is applicable pursuant to Section 7.1.3, the royalty rate indicated in Section 7.2.3(a) shall be recalculated as follows:
Royalty rate multiplied by the Adjusted Value Share (in percent) and divided by the Initial Value Share (in percent)
7.3 Payments in Course of External Development.
7.3.1 Participation Income. If and to the extent that Felicitex undertakes Development and Commercialization of Optioned Compounds or Products through External Development, Felicitex shall pay to Selvita a participation payment on Participation Income in an amount equal to Selvita’s Initial Value Share or, if applicable, Decreased Value Share or, if applicable, Adjusted Value Share in such Participation Income. Only in case of prior Internal Development by Felicitex, under the assumption and condition that Felicitex undertakes investments into the Research and Development of Optioned Compounds and Products from its own or any of its Affiliates’ own resources, the applicable Initial Value Share, Decreased Value Share or Adjusted Value Share of Selvita for the Participation Income shall be reduced as described in Exhibit G (“Diluted Value Share”) according the calculation scheme attached as Exhibit G.
Exhibit D
“Participation Income” pursuant to this Section includes all payments payable to Felicitex by such Sublicensee or Third Party Partner in connection with the rights granted or assigned by Felicitex to such Sublicensee or Third Party Partner to Research, Develop, Manufacture and Commercialize the Optioned Compounds or Products, including: (a) all upfront payments, (b) all milestone payments, (c) all royalties (or other recurrent payments calculated on the basis of sales income), (d) all purchase prices and (e) all other revenues, receipts, monies and the fair market value of other consideration directly or indirectly payable to Felicitex, whether by way of cash or credit or any benefit, advantage or concession. Participation Income does not include or apply for (u) non-US taxes which are deducted or paid, but only to the extent that Felicitex is not entitled to a refund of such taxes or duties, (v) sales, use, and/or value added taxes; (w) refunds or rebates; (x) payments for Research, Development and/or Manufacturing services of Felicitex acting as a subcontractor or service provider (to the extent that the compensation is for fair market value); (y) consideration received for the purchase of an equity interest in Felicitex (to the extent that the compensation is for fair market value) and (z) reimbursement of patent costs of Felicitex. If Optioned Compounds or Products are out-licensed or sold together with other compounds or products in the same transaction, then the Participation Income calculations will be determined per Optioned Compound or Product at each component’s fair market value. An exemplary calculation of the participation payment to Selvita is attached hereto as Exhibit H.
7.3.2 Further Conditions for Participation Payments.
(a) If the external Development and Commercialization by Felicitex pursuant to Section 7.3.1 commences after the achievement of one or more milestones by Felicitex in course of an Internal Development, the payment obligations pursuant to Section 7.2 shall apply to such internally achieved milestones and the payment obligations pursuant to Section 7.3 shall apply to all milestone events occurring after commencement of the External Development;
(b) If Felicitex undertakes the Development and Commercialization of Optioned Compounds and Products both through Internal Development and External Development (for example in case of a partial out-licensing for certain indications or for certain territories only), Selvita shall receive both (i) participation payments on Participation Income with view to the Optioned Compounds and Products which are developed through External Development and (ii) the milestone and royalty payments pursuant to Section 7.2.1 to Section 7.2.3 with view to the Optioned Compounds and Products which are developed through Internal Development.
(c) The Diluted Value Share shall apply solely to the calculation of participation payments due by Felicitex to Selvita from External Development pursuant to Section 7.3. For the avoidance of doubt, the Initial Value Share, Decreased Value Share or Adjusted Value Share, not the Diluted Value Share, shall apply to other payments due by Felicitex to Selvita from Internal Development.
(d) Felicitex shall promptly notify Selvita about (i) any invoice that Felicitex issues with regard to the Participation Income to its Sublicensee or Third Party Partner and (ii) any receipt of Participation Income by Felicitex from its Sublicensee or Third Party Partner. Felicitex participation payments to Selvita pursuant to this Section 7.3 are payable upon the earlier of (x) sixty (60) days after Felicitex’s issuing an invoice with regard to the respective Participation Income to its Sublicensee or Third Party Partner or (y) thirty (30) days after the date of the invoice from Selvita which Selvita may issue to Felicitex following Felicitex’ receipt of the Participation Income by Felicitex.
Exhibit D
(e) In the event that the Participation Income payable to Felicitex by a Sublicensee or Third Party Partner does not suffice to result in participation payments to Selvita that are at least equal to the royalty and milestone payments that Selvita could expect from Felicitex in course of Internal Development (taking into account the market value of the Optioned Compounds or Products as well as actual sales of Products), then Selvita shall be entitled to receive minimum royalties from such Sublicensee or Third Party Partner. Felicitex shall procure that any Sublicensee or Third Party Partner agrees to assume and comply with this payment obligation. If Felicitex’s negotiations with a Sublicensee or Third Party Partner are hindered, endangered or impaired by this Section 7.3.2(e), Felicitex and Selvita shall meet upon request of Felicitex and negotiate in good faith an alternative structure which meets both Parties’ economical interests and is qualified to be more easily accepted by such Sublicensee or Third Party Partner. The minimum royalties shall be calculated on basis of net sales of Products by such Sublicensee or other Third Party Partner (or any of their affiliates or sublicensees, if applicable) to Third Party purchasers, it being understood that “net sales” in this context shall be calculated basically in accordance with the Net Sales definition in this Agreement. The royalty rate for minimum royalties (“Minimum Royalty Rate’) shall be calculated on basis of the basic minimum royalties table and Selvita’s “Minimum Value Share” as follows:
(i) Basic Minimum Royalties Table [Note to Draft: specific malty royalty to be completed in final version pursuant to the guidelines for calculation of milestones in Exhibit F, Part D of the RCO Agreement.]
Aggregate Calendar Year Net Sales of Products
in the Territory (in U.S. Dollars) | Royalty Rate |
| US[**] | |
| [**] | |
| Greater than US[**] | |
(ii) Calculation Formula:
Minimum Royalty Rate = Royalty rate from Basic Minimum Royalties Table*Vmin/Initial Value Share, where Vmin (Minimum Value Share) is calculated as follows:
Minimum Value Share (Vmin) = V - [V*[**]*(Y-2)/2], where Y is the direct evidenced CRO cost for IND-enabling studies in Million U.S. Dollars (USSM) and V is either the Initial Value Share or, if applicable. the Decreased Value Share of Selvita (whichever was applied for determination of the Basic Milestone Royalties Table in Section 7.3.2(e)(i)).
(f) For the avoidance of doubt: Any reference to Felicitex in this Section 7.3 shall apply accordingly to any of its Affiliates, if the External Development is not initiated by Felicitex directly, but by any of its Affiliates.
Exhibit D
7.4 Market Price. The Parties unanimously declare that the payments hereunder correspond to the market price as set by the Parties on the basis of negotiations conducted in good faith, each of the Parties acting independently. The market price set by both Parties provided for in this Agreement corresponds to fair market prices based on similar transactions in the biotechnology industry. All rights and benefits inherent in (or attributable to) the transaction under this agreement have been included in the Agreement. The execution of this Agreement was preceded by the Parties elaborating a thorough analysis of the current situation in the biotech and pharmaceutical industry, with a special consideration for the scientific characteristics of the target, its therapeutic potential, interest of the potential end customers and current stage of Research.
7.5 Reporting Obligations. Commencing with the First Commercial Sale of a Product and continuing until the expiration of Felicitex’s payment obligations under this Article 7, Felicitex (itself or through its Affiliate or Sublicensee or Third Party Partner, as applicable) shall provide to Selvita written reports outlining all conditions and circumstances which are required or reasonably useful for Selvita to calculate or audit its payment claims towards Felicitex under this Agreement. Felicitex shall provide such written reports in reasonable details within sixty (60) days after the end of each Calendar Quarter, in particular outlining: (a) any milestones achieved by Felicitex, its Affiliates, its Sublicensees or its Third Party Partners, (b) Net Sales of Products on a product-by-product and country-by-country basis in the Territory invoiced by Felicitex or its Affiliates during the concerned Calendar Quarter, (c) royalties (or other recurrent payments on basis of sales of Products) invoiced by Felicitex from its Sublicensees or its Third Party Partners during such Calendar Quarter and (d) upfront payments, milestone payments and any other consideration invoiced by Felicitex from its Sublicensees or Third Party Partners in such Calendar Quarter. The information contained in each report under this Section 7.5 shall be considered Confidential Information of Felicitex.
7.6 Accounting; Audit Rights.
7.6.1 Record Keeping. Felicitex agrees to keep, and to require its Affiliates and its Sublicensees and Third Party Partners to keep, full, clear and accurate records of the underlying data relating to the reports and payments required by Article VII for a minimum period of five (5) years after the relevant payment is owed pursuant to this Agreement.
7.6.2 Auditing. Felicitex agrees to permit, and to require its Affiliates and, its Sublicensees and Third Party Partners to permit, upon not less than thirty (30) days’ prior written notice, such books and records, as applicable, to be examined by an independent accounting firm selected by Selvita and reasonably acceptable to the audited party, for the purpose of verifying reports provided by Felicitex (or such other party) under this Agreement. Such audit shall not: (a) be performed more frequently than once in any twelve (12) month period (unless a previous audit during such twelve (12) month period revealed a material discrepancy with respect to such period), (b) be conducted for any Calendar Quarter more than three (3) years after the end of the Calendar Year of which such Calendar Quarter is a part or (c) be repeated for any Calendar Quarter, and shall be conducted under appropriate confidentiality provisions satisfactory to the audited party, for the sole purpose of verifying the accuracy and completeness of all financial, accounting and numerical information and calculations provided under this Agreement. The independent accounting firm shall have the right to make copies of relevant portions of the audited party’s books and records; provided that any such copies shall be the Confidential Information of the audited party, shall be protected by appropriate confidentiality obligations and shall not be shared with Selvita or any other Person. The independent accounting firm will prepare and provide to Felicitex and Selvita a written report stating only whether the reports submitted and amounts paid hereunder were correct or incorrect, and the amounts of any discrepancies. Within thirty (30) days following receipt of such report, any discrepancy amounts (together with accrued interest calculated in accordance with Section 7.8) shall, in case of underpayments, be paid by the owing Party to the other Party, or, in case of overpayments, shall be reimbursed by the owing Party to the other Party.
Exhibit D
7.6.3 Costs of Examination. Such examination is to be made at the expense of Selvita, except if the results of the audit reveal an underpayment of royalties, milestone payments or other amounts to Selvita by Felicitex of [**] or more in any calendar year, in which case the audit fees for such examination shall be paid by Felicitex.
7.7 Payments; Conversion. All payments due under this Agreement shall be made in U.S. Dollars by electronic funds transfer to a bank account designated in writing in the invoice of the receiving Party. If converted into Euro, the conversion shall be based on the exchange rate applicable at close of business Boston time at the last Business Day of the preceding Calendar Quarter. Unless otherwise specified in this Agreement or otherwise agreed by the Parties, each payment hereunder shall be due thirty (30) days after the corresponding invoice date.
7.8 Late Payments. Any amount owed by Felicitex to Selvita under this Agreement that is not paid on or before the date such payment is due shall bear interest at a rate per annum equal to the lesser of: (a) the prime or equivalent rate per annum quoted by The Wall Street Journal, Eastern Edition on the first Business Day after such payment is due, plus one hundred basis points and (b) the highest rate permitted by applicable Law, in either case calculated on the number of days such payments are paid after such payments are due and compounded monthly.
7.9 Withholding or Other Taxes. The Parties agree to cooperate in good faith to provide one another with such documents and certifications as are reasonably necessary to enable Felicitex and Selvita to minimize or recover any tax payment. Felicitex may withhold taxes in the event that revenue authorities within the United States require the withholding of taxes on amounts to be paid hereunder to Selvita under applicable tax treaties between Poland and the United States, and in any such event Felicitex shall deduct such taxes from such payment and such taxes shall be paid by Felicitex to the proper taxing authority of the United States on behalf of Selvita (evidence of which payment to such taxing authority shall be provided promptly by Felicitex to Selvita hereunder). In case that Felicitex, any of its Affiliates, its Sublicensees or any Third Party Partner undertakes a payment to Selvita from any country outside the United States and such payment triggers other or additional taxes (including VAT or withholding taxes) than such a payment made from within the United States would have triggered, then such additional taxes shall be paid to the proper taxing authority outside of the United States by the liable party (whereas, in case of Selvita, Felicitex, its Affiliate, its Sublicensee or Third Party Partner shall make the tax payment on behalf of Selvita (evidence of which payment to such taxing authority shall be provided promptly to Selvita)), provided, however, that in any such event Felicitex, its Affiliates, its Sublicensees or Third Party Partner shall gross-up the relevant payment due to Selvita, so that Selvita receives the same net amount it would have received had the payment been made from the United States (taking into consideration applicable tax treaties between the United States and Poland); provided, further, however, that no such gross-up or grossed-up payment shall be due to Selvita if Felicitex’ headquarters for tax domicile purposes is within the United States.
Exhibit D
ARTICLE VIII
[intentionally omitted]
ARTICLE IX
INTELLECTUAL PROPERTY RIGHTS
9.1 Ownership.
9.1.1 Felicitex IP; Selvita IP. As between the Parties, Selvita shall retain all of its rights, title and interest in, to and under the Selvita IP, except to the extent that Selvita expressly has granted rights and licenses to Felicitex under the Selvita IP pursuant to this Agreement, and Felicitex shall retain all of its rights, title and interest in, to and under the Felicitex IP.
9.1.2 Collaboration IP.
(a) Subject to the rights and licenses expressly granted hereunder by Selvita to Felicitex, Selvita shall be the sole owner of any Selvita Collaboration IP, and Selvita shall retain all of its right, title and interest thereto.
(b) Subject to the rights and licenses expressly granted hereunder by Selvita to Felicitex, the Joint Collaboration IP shall be owned jointly by Felicitex and Selvita, and all rights, title and interest thereto shall be jointly owned by the Parties. Subject to the aforesaid limitations, each Party shall be entitled to practice and license the Joint Collaboration IP without restriction and without consent of the other Party, and each Party hereby waives any right it may have under Laws to require any such consent or accounting.
9.2 Prosecution and Maintenance of Patents.
9.2.1 Selvita Patents. Selvita shall have the sole right (but not the obligation) to Prosecute and Maintain the Selvita Patents; provided however that if Selvita at any time, and for any reason, elects not to Prosecute and Maintain the Selvita Patents, Selvita shall immediately notify Felicitex and thereafter Felicitex shall have the right (but not the obligation) to Prosecute and Maintain the Selvita Patents. In the event that Felicitex elects to Prosecute and Maintain the Selvita Patents, then all cost and expenses associated therewith, on a country by country basis, shall be paid by Felicitex and offset against any royalties payable by Felicitex to Selvita for sales in such relevant country. Unless such consultation is prohibited by a Third Party agreement or would otherwise compromise or jeopardize patent strategy on another Selvita patent or patent application unrelated to this Agreement, Selvita shall provide Felicitex with a reasonable opportunity to substantively comment on Prosecution and Maintenance of any Selvita Patent which Covers or claims Optioned Compounds prior to taking material actions (including the filing of initial applications), and will in good faith consider any actions recommended by Felicitex regarding such Selvita Patents. Felicitex shall have the right to review and make comments on and recommendations in relation to the Prosecution and Maintenance of such Patents; provided that Felicitex does so promptly and consistent with any applicable filing deadlines.
Exhibit D
9.2.2 Felicitex Patents. Felicitex shall have the sole right (but not the obligation) to Prosecute and Maintain the Felicitex Patents; provided however that if Felicitex at any time, and for any reason, elects not to Prosecute and Maintain the Felicitex Patents, Felicitex shall immediately notify Selvita and thereafter Selvita shall have the right (but not the obligation) to Prosecute and Maintain the Felicitex Patents. In the event that Selvita elects to Prosecute and Maintain the Felicitex Patents, then all cost and expenses associated therewith, on a country by country basis, shall be paid by Selvita. Unless such consultation is prohibited by a Third Party agreement or would otherwise compromise or jeopardize patent strategy on another Felicitex patent or patent application unrelated to this Agreement, Felicitex shall provide Selvita with a reasonable opportunity to substantively comment on Prosecution and Maintenance of any Felicitex Patent necessary for commercialization of the Optioned Compounds prior to taking material actions (including the filing of initial applications), and will in good faith consider any actions recommended by Selvita regarding such Felicitex Patents.
9.2.3 Collaboration Patents.
(a) Felicitex shall have the sole right (but not the obligation) to Prosecute and Maintain the Collaboration Patents relating to the Optioned Compounds or Products. No less than thirty (30) days prior to filing of a Patent, Felicitex shall provide to Selvita a copy of the proposed patent application for review by Selvita and shall consider in good faith any comments or concerns raised by Selvita within twenty (20) days following receipt of the patent application. Felicitex shall consult with and keep Selvita informed as to material developments with respect to the Prosecution and Maintenance of Collaboration Patents, including by providing copies of all substantive office actions or any other substantive documents that Felicitex receives from any patent office, including notice of all interferences, reissues, re-examinations, oppositions or requests for patent term extensions. Selvita shall fully cooperate with Felicitex in Prosecution and Maintenance of the Collaboration Patents.
(b) If Felicitex elects not to file or to continue to Prosecute or Maintain a Collaboration Patent, then it shall notify Selvita in writing at least ninety (90) days before any deadline applicable to the Prosecution or Maintenance of such Collaboration Patent, as the case may be, or any other date by which an action must be taken to establish or preserve such Collaboration Patent in such country or possession. In such case, at Selvita’s request, Selvita shall have the right to pursue the filing or support the continued Prosecution or Maintenance of such Collaboration Patent in its own name, through patent counsel of Selvita’s choice and at Selvita’s cost and expense, and in such case the ownership in such Collaboration Patent shall then be assigned to Selvita. Any Collaboration Patent assumed by Selvita in accordance with the foregoing shall, prospectively from the date of such assumption, be excluded from the Collaboration Patent as defined under this Agreement. Under Section 9.2.3(b), Selvita shall be likewise entitled to Prosecute and Maintain at its own expense (including, where possible, in form of a separate Patent, such as a divisional application or a continuation application) any claim to the subject matter of any Collaboration Patent that was disclosed in such Collaboration Patent but not elected by Felicitex for further Prosecution and Maintenance.
Exhibit D
(c) The Parties agree to cooperate fully in the Prosecution and Maintenance of Collaboration Patents under this Agreement. Cooperation shall include (i) executing all papers and instruments, or requiring its employees or contractors to execute such papers and instruments, so as to (aa) effectuate the ownership of intellectual property, (bb) enable Felicitex to Prosecute patent applications, and (cc) obtain and maintain any patent extensions, supplementary protection certificates, and the like with respect to any Collaboration Patents.
9.2.4 United States Law. The determination of whether Know-How discovered, developed, invented, conceived or reduced to practice made by a Party for the purpose of allocating proprietary rights (including Patent or other intellectual property rights) therein, shall, for purposes of this Agreement, be made in accordance with Law in the United States as in effect on the Effective Date.
9.3 Patent Costs. Felicitex shall cover all costs and expenses associated with the Prosecution and Maintenance of Collaboration Patents and Felicitex Patents, including costs of patent litigation. Selvita shall cover all costs and expenses associated with the Prosecution and Maintenance of Selvita Patents, including costs of patent litigation.
9.4 Enforcement of Patents and Know-How.
9.4.1 Notice of Infringement. If any Party learns of an actual or alleged infringement or threatened infringement by a Third Party with respect to any Selvita Patent or Felicitex Patent or Collaboration Patent, it shall promptly notify the other Party of all details regarding such infringement that is reasonably available to such Party.
9.4.2 Right to Bring an Action. Felicitex shall have the first right, but not the obligation, to attempt to resolve any infringement or claim, including by filing an infringement suit, defending against such claim or taking other similar action, with respect to a Collaboration Patent and to compromise or settle any such infringement or claim. If Felicitex does not intend to prosecute or defend such action, Felicitex shall inform Selvita without undue delay and Selvita shall have the right, but not the obligation, to resolve any infringement or claim, including by filing an infringement suit, defending against such claim or taking other similar action, with respect to a Collaboration Patent and to compromise or settle any such infringement or claim. Upon each Party’s request, the other Party shall immediately provide the requesting Party with all relevant documentation of such action. Each Party shall have the right to join an action relating to a Collaboration Patent, at its own expense.
9.4.3 Settlement. Felicitex shall not settle or otherwise compromise any action by admitting that any Collaboration Patent is invalid or unenforceable without prior consulting with Selvita, and, Selvita may not settle or otherwise compromise an action without Felicitex’ prior written consent.
Exhibit D
9.4.4 Reasonable Assistance. The Party not enforcing or defending Collaboration Patents shall provide reasonable assistance to the other Party, including providing access to relevant documents and other evidence and making its employees available, subject to the other Party’s reimbursement of any reasonable out-of-pocket expenses incurred on an ongoing basis by the non-enforcing or non-defending Party in providing such assistance.
9.4.5 Distribution of Amounts Recovered. Any amounts recovered by the Party taking an action pursuant to Section 9.4.2, whether by settlement or judgment, shall be allocated in the following order: (a) to reimburse the Party taking such action for any costs incurred, (b) to reimburse the Party not taking but joining such action for its costs incurred in such action; and (c) the remaining amount of such recovery shall be allocated between the Parties pursuant to Selvita’s Initial Value Share, Decreased Value Share or Adjusted Value Share (whichever is applicable) and Felicitex’s corresponding value share.
9.5 Third Party Actions Claiming Infringement.
9.5.1 Notice. If a Party becomes aware of any action of a Third Party claiming an infringement of Third Party intellectual property rights relating to this Agreement, such Party shall promptly notify the other Party of all details regarding such claim or action that is reasonably available to such Party.
9.5.2 Right to Defend. Felicitex shall have the right, at its sole expense, but not the obligation, to defend a Third Party action and to compromise or settle such Third Party action. If Felicitex declines or fails to assert its intention to defend such Third Party action within sixty (60) days after sending (in the event that Felicitex is the notifying Party) or receipt (in the event that Selvita is the notifying Party) of notice under Section 9.5.1, then Selvita shall have the right, but not the obligation, to defend such Third Party action. The Party defending such Third Party action shall have the sole and exclusive right to select counsel for such Third Party action.
9.5.3 Costs, Settlement, Assistance, Recovered Amounts. Section 9.4.3 to Section 9.4.5 shall apply accordingly.
ARTICLE X
CONFIDENTIALITY
10.1 Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, the Parties agree that the receiving Party (the “Receiving Party”) shall keep confidential and shall not, now or at any time thereafter, publish or otherwise disclose or use for any purpose other than as provided for in this Agreement, and to carry out any and all of its obligations under this Agreement, any Know-How or other information and materials, patentable or otherwise, in any form (written, oral, photographic, electronic, magnetic, or otherwise) which is disclosed to it by the other Party (the “Disclosing Party”) or otherwise received or accessed by a Receiving Party in the course of performing its obligations or exercising its rights under this Agreement, including trade secrets, Know-How, inventions or discoveries, proprietary information, formulae, processes, techniques and information relating to a Party’s past, present and future marketing, financial and Development activities of any product or potential product or useful technology of the Disclosing Party and the pricing thereof (collectively, “Confidential Information”), except to the extent that it can be established by the Receiving Party that such Confidential Information:
(a) was in the lawful knowledge and possession of the Receiving Party prior to the time it was disclosed to, or learned by, the Receiving Party, or was otherwise developed independently by the Receiving Party, as evidenced by written records kept in the ordinary course of business, or other documentary proof of actual use by the Receiving Party;
Exhibit D
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the Receiving Party in breach of this Agreement; or
(d) was disclosed to the Receiving Party, other than under an obligation of confidentiality, by a Third Party who had no obligation not to disclose such information to others.
10.2 Product Information. Selvita recognizes that by reason of Felicitex’s exclusive rights under this Agreement, Felicitex has an interest in Selvita’s retention in confidence of certain information of Selvita. Accordingly, until the end of the Term, and for a period of twenty (20) years thereafter, Selvita shall keep confidential, and not publish or otherwise disclose, and not use for any purpose other than to fulfill Selvita’s obligations or exercise Selvita’s rights hereunder any Joint Collaboration IP, Selvita Collaboration Know-How and any Selvita Know How, to the extent that the information pertains specifically to any particular Optioned Compound (the “Product Information”), except to the extent: (a) the Product Information is in the public domain or generally available through no fault of Selvita, (b) such disclosure or use is expressly permitted by the terms and conditions of this Agreement. For the purposes of this Section, each Party shall be deemed to be both Disclosing Party and Receiving Party with regard to Product Information.
10.3 Authorized Disclosure. Except as expressly provided otherwise in this Agreement to the extent necessary or required to fully exercise its rights hereunder, a Receiving Party may use and disclose Confidential Information of the Disclosing Party as follows:
(a) to Regulatory Authorities as required in connection with any filing, application or request for Regulatory Approval; provided, however, that reasonable measures shall be taken to assure confidential treatment of such information;
(b) in response to a valid order of a court of competent jurisdiction or other supra-national, federal, national, regional, state, provincial and local governmental or regulatory body of competent jurisdiction or, if so advised by the Receiving Party’s legal counsel, such disclosure is otherwise required by Law, including by reason of filing with securities regulators; provided, however, that, to the extent practicable, the Receiving Party shall first have given notice to the Disclosing Party and given the Disclosing Party a reasonable opportunity to quash such order or to obtain a protective order or confidential treatment requiring that the Confidential Information and documents that are the subject of such order be held in confidence by such court or agency or, if disclosed, be used only for the purposes for which the order was issued; and provided further that the Confidential Information disclosed in response to such court or governmental order shall be limited to that information which is legally required to be disclosed in response to such court or governmental order;
Exhibit D
(c) to a patent authority as may be reasonably necessary or useful for purposes of obtaining or enforcing a Collaboration Patent; provided, however, that reasonable measures shall be taken to assure confidential treatment of such information, to the extent such protection is available;
(d) in communication with actual or potential investors, lenders, acquirers, merger partners, consultants, advisors, licensees, sublicensees, collaborators or others on a need to know basis, in each case under appropriate confidentiality provisions substantially equivalent to those of this Agreement; or
(e) to the extent mutually agreed to in writing by the Parties or otherwise permitted under this Agreement (including the Parties’ right to involve sub-contractors for their activities under the Research Plan).
10.4 Press Release. On or promptly after the Effective Date, the Parties shall jointly issue a public announcement of the execution of this Agreement in the form attached hereto as Exhibit I. Thereafter, the Parties shall use good faith efforts to agree on joint press releases with respect to material developments relating to the Development or Commercialization of Products.
10.5 Disclosure of Agreement Terms. Except to the extent required by Law or by securities exchange listing requirements (in particular of the Warsaw Stock Exchange) or as otherwise permitted in accordance with Section 10.3(d) and (e) or Section 10.4, neither Party shall make any public announcements concerning this Agreement or the subject matter hereof without the prior written consent of the other, which shall not be unreasonably withheld, conditioned or delayed.
10.6 Remedies. Each Party shall be entitled to seek, in addition to any other right or remedy it may have, at Law or in equity, a temporary injunction, without the posting of any bond or other security, enjoining or restraining the other Party from any violation or threatened violation of this Article 10.
10.7 Publications.
10.7.1 Restrictions on Publication. Felicitex is entitled to publish or publicly disclose the results generated in the course of this Agreement without the prior written consent of Selvita, but only after submission to and review by Selvita pursuant to Section 10.7.2. Selvita is entitled to publish or publicly disclose the results generated in the course of this Agreement after submission to and review by Felicitex pursuant to Section 10.7.2. and subject to the prior written consent of Felicitex. Felicitex acknowledges that Selvita has certain obligations to publish or publicly disclose the results generated in the course of performing the Research Collaborationunder the SEL141 Grant and the related grant agreement with the Polish Agency for Enterprise Development. Felicitex will consider these obligations in a supportive manner.
Exhibit D
10.7.2 Submission; Review. The Party seeking to publish results hereunder (the “Publishing Party”) shall provide the other Party (the “Reviewing Party”) with a copy of such proposed abstract, manuscript, or presentation no less than sixty (60) days thirty (30) days in the case of abstracts) prior to its intended submission for publication. The Reviewing Party shall respond in writing promptly and in no event later than thirty (30) days (ten (10) Business Days in the case of abstracts) after receipt of the proposed material, with one or more of the following:
(a) comments on the proposed material, which the Publishing Party shall consider in good faith;
(b) a specific statement of concern, based upon the need to seek patent protection or to block publication if the Reviewing Party determines that the proposed disclosure is intellectual property that should be maintained as a trade secret to protect an Optioned Compound or any Research or Development activities conducted under this Agreement; or
(c) an identification of the Reviewing Party’s Confidential Information that is contained in the material reviewed.
10.7.3 Patent and Trade Secret Protection. In the event of concern by the Reviewing Party over patent protection or whether maintaining a trade secret would be a priority, the Publishing Party agrees not to submit such publication or to make such presentation that contains such information until the Reviewing Party is given a reasonable period of time, and in no event less than sixty (60) days, to seek patent protection for any material in such publication or presentation which it believes is patentable or to resolve any other issues, or to abandon such proposed publication or presentation if the Reviewing Party reasonably determines in good faith that maintaining such information as a trade secret is a commercially-reasonable priority. Any Confidential Information of the Reviewing Party shall, if requested by the Reviewing Party, be removed.
10.7.4 Use of Name. Except as expressly provided herein, neither Party shall mention or otherwise use the name, logo, or trademark of the other Party or any of its Affiliates (or any abbreviation or adaptation thereof) in any publication, press release, marketing and promotional material, or other form of publicity without the prior written approval of such other Party in each instance. The restrictions imposed by this Section shall not prohibit either Party from making any disclosure identifying the other Party in a disclosure that is required by Law. In addition, either Party may use the other Party’s name, logo or trademark on its own website to identify the other Party as its collaborator, provided that each Party complies with the formatting specifications and requirements provided by the other Party whose identity would be posted.
10.8 Republication. Nothing in this Article 10 shall prohibit either Party from including in future publications, press releases, marketing and promotional materials any materials previously authorized for public disclosure by the other Party.
Exhibit D
10.9 Return of Confidential Information. Upon the effective date of expiration or termination of this Agreement for any reason, either Party may request in writing, and the other Party shall either, with respect to Confidential Information (in the event of termination of this Agreement with respect to one or more terminated countries within the Territory but not in its entirety, solely to the extent relating to such terminated countries within the Territory) to which such first Party does not retain rights under the surviving provisions of this Agreement: (a) promptly destroy all copies of such Confidential Information in the possession of the other Party and confirm such destruction in writing to the requesting Party; or (b) promptly deliver to the requesting Party, at the other Party’s expense, all copies of such Confidential Information in the possession of the other Party; provided, however, that the other Party shall be permitted to retain such Confidential Information for the sole purpose of performing any continuing obligations hereunder or exercising its rights hereunder that survive such termination. Notwithstanding the foregoing, such other Party also shall be permitted to retain one (1) copy of such Confidential Information for archival purposes and such additional copies of, or any computer records or files containing, such Confidential Information that have been created solely by such Party’s automatic archiving and back-up procedures, to the extent created and retained in a manner consistent with such other Party’s standard archiving and back-up procedures, but not for any other use or purpose. All Confidential Information shall continue to be subject to the terms of this Agreement for the period set forth in Section 13.3.2.
ARTICLE XI
REPRESENTATIONS AND WARRANTIES
11.1 Representations and Warranties of Both Parties. Each Party hereby represents, warrants and covenants to the other Party, as of the Effective Date, that:
11.1.1 Such Party is duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
11.1.2 Such Party has taken all necessary action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder;
11.1.3 This Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, binding obligation, enforceable against it in accordance with the terms hereof;
11.1.4 The execution, delivery and performance of this Agreement by such Party does not and will not conflict with any agreement or any provision thereof, or any instrument or understanding, oral or written, to which it is or becomes a party or by which it is or becomes bound, nor violate any Law or regulation of any court, governmental body or administrative or other agency having jurisdiction over such Party;
11.1.5 No government authorization, consent, approval, license, exemption of, or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any Laws currently in effect, is necessary for its execution and delivery of this Agreement.
Exhibit D
11.2 Representations and Warranties of Selvita.
11.2.1 Selvita hereby represents and warrants to Felicitex, as of the Effective Date and for the Term of this Agreement, that it has not previously, and in the future will not, assign(ed), transfer(red), license(d), convey(ed) or otherwise encumber(ed) its right, title or interest in or to any present or future interest, lien or encumbrance in or to the Selvita IP, the Collaboration Patents or the Collaboration Know-How in any manner causing a conflict with the scope of rights and licenses granted by Selvita to Felicitex under this Agreement.
11.2.2 Selvita represents, warrants and covenants that it has fully disclosed to Felicitex the terms and conditions of the SEL141 Grant and instructed Felicitex on the proper completion of its obligations under the SEL141 Grant and will, during the Term of this Agreement, timely assist and work with Felicitex without any additional fee, charge or cost, to insure that it is continuously in compliance with the terms and conditions of the SEL141 Grant.
11.3 Covenants of Felicitex. Felicitex covenants to Selvita that it shall not use in any capacity, in connection with the performance of the activities contemplated by this Agreement, any Person who has been debarred pursuant to Section 306 of the FFDCA, or who is the subject of a conviction described in such section (or subject to a similar sanction of EMA). It agrees to inform Selvita in writing immediately if it or any Person who is performing services hereunder on its behalf is debarred or is the subject of a conviction described in Section 306, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to its knowledge, is threatened, relating to the debarment or conviction of it or any Person performing services hereunder; and
11.4 Disclaimer. Except as otherwise expressly set forth in this Agreement, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, AND EXPRESSLY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. Without limiting the generality of the foregoing, each Party disclaims any warranties with regards to: (a) the success of any study or test commenced under this Agreement or (b) the safety or usefulness for any purpose of the technology or materials, including any Optioned Compounds, it provides or discovers under this Agreement.
ARTICLE XII
INDEMNIFICATION; INSURANCE
12.1 Indemnification. Each Party (each a “Indemnifying Party”) shall indemnify, defend and hold harmless the other Party and its Affiliates, and its and their respective directors, officers, employees and agents (each a “Indemnified Party”), from and against any and all liabilities, damages, losses, costs and expenses, including the reasonable fees of attorneys and other professional Third Parties (collectively, “Losses”), arising out of or resulting from any and all Third Party suits, claims, actions, proceedings or demands (“Claims”) based upon:
(a) the negligence, recklessness or wrongful intentional acts or omissions of the Indemnifying Party or its Affiliates or its Sublicensees and its or their respective directors, officers, employees and agents, in connection with the Indemnifying Party’s performance of its obligations or exercise of its rights under this Agreement;
Exhibit D
(b) any breach of any representation or warranty or covenant made by the Indemnifying Party under Article XI or any other provision under this Agreement;
(c) the Research, Development, Manufacturing and Commercialization of Optioned Compounds that is actually conducted by or on behalf of the Indemnifying Party, its Affiliates or Sublicensees the handling and storage by or on behalf of the Indemnifying Party, its Affiliates or Sublicensees of any chemical agents or other compounds for the purpose of conducting Research or Development or Commercialization by or on behalf of the Indemnifying Party, its Affiliates or Sublicensees or Third Party Partners, including any product liability, personal injury, property damage or other damage; or
(d) any gross negligence, recklessness, wrongful intentional act or omission, failure to comply with any Law, breach of any agreement with a Third Party, or infringement of Patent or other intellectual property rights of any Third Party by the Indemnifying Party, its Affiliates or Sublicensees with respect to the Research, Development, Manufacturing or Commercialization of any Optioned Compound or Product anywhere in the world prior to the Effective Date or under this Agreement;
in each case, provided that, such indemnity shall not apply to the extent that the Indemnified Party itself has an indemnification obligation pursuant to this Section for such Loss, in which event each Party shall indemnify the other to the extent of their respective liability for such Loss.
12.2 Indemnification regarding SEL141 Grant. In case of a breach of Felicitex’s obligations under Section 5.3.1 (Implementation), Section 5.3.2 (Implementation Statement) or Section 5.3.3 (No Direct Disposal), Felicitex shall indemnify, defend and hold harmless Selvita and its Affiliates from and against any and all liability, damage, loss, cost or expense (including reasonable attorneys’ fees) (“SEL141 Loss”) arising out of or resulting from a premature termination of the SEL141 Grant by the Polish Agency for Enterprise Development or by any other competent governmental authority, to the extent such termination and SEL141 Loss is caused due to a breach by Felicitex of its obligations under Sections 5.3.1 to 5.3.3. In the event that Selvita receives notice of any breach, threatened breach or violation of SEL141 Grant by Felicitex, its Affiliates or Sublicensees,, then Selvita shall immediately notify Felicitex and provide Felicitex with the information needed to cure such breach or threatened breach of the SEL141 Grant.
12.3 Procedure.
12.3.1 Notice of Claim. An Indemnified Party seeking indemnification under this Article 12 shall give prompt written notification to the Indemnifying Party of the Third Party Claim for which indemnification may be sought (it being understood and agreed, however, that the failure by an Indemnified Party to give notice of a Third Party Claim as provided in this Section shall not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that such Indemnifying Party is actually prejudiced as a result of such failure to give notice).
Exhibit D
12.3.2 Assumption of Defense; Participation. Within ninety (90) days after delivery of such notification, the Indemnifying Party may, upon written notice thereof to the Indemnified Party, assume control of the defense of such Third Party Claim with counsel reasonably satisfactory to the Indemnified Party. If the Indemnifying Party does not assume control of such defense, the Indemnified Party shall control such defense and, without limiting the Indemnifying Party’s indemnification obligations, the Indemnifying Party shall reimburse the Indemnified Party for all costs and expenses, including reasonable attorneys’ fees and disbursements, incurred by the Indemnified Party in defending itself within sixty (60) days after receipt of any invoice therefore from the Indemnified Party. The Party not controlling such defense may participate therein at its own expense; provided, however, that, if the Indemnifying Party assumes control of such defense and the Indemnified Party in good faith concludes, based on written advice from outside counsel, that the Indemnifying Party and the Indemnified Party have conflicting interests with respect to such Third Party Claim sufficiently adverse to make unadvisable the representation by the same counsel of both Parties under Law, ethical rules or equitable principles, the Indemnifying Party shall be responsible for the reasonable fees and expenses of a single counsel to the Indemnified Party in connection therewith. The Party controlling such defense shall keep the other Party advised of the status of such Third Party Claim and the defense thereof and shall consider recommendations made by the other Party with respect thereto.
12.3.3 Settlements. The Indemnifying Party shall not agree to any settlement of such Third Party Claim or consent to any judgment in respect thereof that does not include a complete and unconditional release of the Indemnified Party from all liability with respect thereto, that imposes any liability or obligation on the Indemnified Party or that acknowledges fault by the Indemnified Party, without the prior written consent of the Indemnified Party.
12.4 Insurance. Each Party shall maintain, at its cost, self-insurance against liability and other risks associated with its activities and obligations under this Agreement, in such amounts, subject to such deductibles and on such terms as are customary for a company such as the respective Party for the activities to be conducted by it under this Agreement. Each Party shall furnish to the other Party evidence of such self-insurance upon request.
12.5 LIMITATION OF LIABILITY. EXCEPT FOR A BREACH OF ARTICLE 10 OR FOR CLAIMS OF A THIRD PARTY THAT ARE SUBJECT TO INDEMNIFICATION UNDER THIS ARTICLE 12, NEITHER SELVITA NOR FELICITEX, NOR ANY OF THEIR RESPECTIVE AFFILIATES OR SUBLICENSEES, WILL BE LIABLE TO THE OTHER PARTY TO THIS AGREEMENT, ITS AFFILIATES OR ANY OF THEIR SUBLICENSEES FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL OR PUNITIVE DAMAGES OR LOST PROFITS OR ROYALTIES, LOST DATA OR COST OF PROCUREMENT OF SUBSTITUTE GOODS OR SERVICES, WHETHER LIABILITY IS ASSERTED IN CONTRACT, TORT (INCLUDING NEGLIGENCE AND STRICT PRODUCT LIABILITY), INDEMNITY OR CONTRIBUTION, AND IRRESPECTIVE OF WHETHER THAT PARTY OR ANY REPRESENTATIVE OF THAT PARTY HAS BEEN ADVISED OF, OR OTHERWISE MIGHT HAVE ANTICIPATED THE POSSIBILITY OF, ANY SUCH LOSS OR DAMAGE.
Exhibit D
ARTICLE XIII
TERM AND TERMINATION
13.1 Term. The term of this Agreement (“Term”) shall commence on the Effective Date and, unless earlier terminated as provided in this Article 13, shall continue in full force and effect, until the expiry of all payment obligations of Felicitex (or, for the avoidance of doubt, its assignee or legal successor) under this Agreement.
13.2 Early Termination.
13.2.1 Termination for Cause. If either Party breaches any of its material obligations under this Agreement, the Party not in default may give to the breaching Party a written notice specifying the nature of the default, requiring it to cure such breach, and stating its intention to terminate this Agreement if such breach is not cured within ninety (90) days after receipt of such notice (the “Notice Period”). If such breach is not cured within the Notice Period, then the Party not in default, subject to Sections 14.1 and 14.2, shall be entitled to terminate this Agreement by written notice to the other Party. In the event a Party timely files for arbitration pursuant to Section 14.2.1 hereof, then any termination notice shall be automatically stayed, the Notice Period shall be extended, and this Agreement shall remain in full force and effect and the Parties shall continue to fulfill their obligations hereunder.
13.2.2 Termination for Insolvency. Either Party may terminate this Agreement by written notice immediately, if the other Party: (a) becomes insolvent, or (b) a petition of bankruptcy or any similar action under relevant bankruptcy or insolvency proceedings is filed by or against said Party and not dismissed within ninety (90) days thereafter, or (c) a receiver is appointed with respect to any asset of said Party or (d) liquidation proceedings (except solvent and voluntary liquidation for reorganization purposes) are commenced by or against said Party.
13.3 Effects of Termination and/or Expiry.
13.3.1 Licenses to Felicitex. After expiration of the Term (but not after early termination) the licenses granted by Selvita to Felicitex under this Agreement shall turn into perpetual (non-terminable and irrevocable), non-royalty and non-milestone bearing licenses.
13.3.2 Surviving Provisions. Unless explicitly stipulated otherwise in this Agreement, Articles 1 (Definitions), 9 (Intellectual Property Rights), 10 (Confidentiality Obligations), 12 (Indemnification and Insurance) and Sections 14.1 and 14.2 (Dispute Resolution), Section 13.3 (Effects of Termination), 14.3 (Governing Law) hereof shall survive the expiration or termination of this Agreement for any reason. Section 10.2 of Article 10 shall survive for a period of twenty (20) years after the effective date of termination or expiration of this Agreement and all other provisions of Article 10 shall survive for a period of seven (7) years after the effective date of termination or expiration of this Agreement.
Exhibit D
13.3.3 Accrued Liability. Expiration or termination of this Agreement shall not relieve the Parties of any liability that accrued hereunder prior to the effective date of such expiration or termination. In addition, termination of this Agreement shall not preclude either Party from pursuing all rights and remedies it may have hereunder or at Law or in equity with respect to any breach of this Agreement nor prejudice either Party’s right to obtain performance of any obligation.
13.3.4 Obligations upon Termination. Upon termination of this Agreement by either Party, the following shall apply:
(a) Materials and Supplies. Felicitex may, at Selvita’s sole option and discretion: (i) destroy any and all materials relating to or comprising the Products, including clinical or commercial supplies of Products, that are Controlled by Felicitex, any of its Affiliates or any of its Sublicensees or (ii) sell (and cause its Affiliates or Sublicensees to sell) such materials (in whole or in part) to Selvita at a price equal to Felicitex’s costs of goods (transportation and transfer costs will be at Selvita’s cost and expense).
(b) Clinical Trials. To the extent not prohibited by Law, Felicitex shall wind down any ongoing Clinical Trials with respect to the Product, or at Selvita’s sole option and discretion, transfer such Clinical Trials to Selvita at Selvita’s cost and expense (in which case Selvita shall purchase from Felicitex the relevant Clinical Trial supplies of the Product at Felicitex costs of goods.
(c) Regulatory Dossiers. Felicitex shall, upon written request by Selvita and subject to Selvita assuming legal responsibility for any Clinical Trials then ongoing, transfer and assign to Selvita at Selvita’s cost and expense, all Regulatory Dossiers and Regulatory Approvals prepared or obtained by or on behalf of Felicitex prior to the effective date of such termination or expiration, to the extent solely related to Products and transferable, and Felicitex shall have the right to retain one copy of such transferred documentation and Regulatory Approvals for record-keeping purposes, whereas such copy shall be deemed Confidential Information of Selvita subject to the obligations of Article 10 of this Agreement;
(d) Materials and Know-How. Felicitex shall, upon written request of Selvita, return to Selvita or, at Selvita’s option, destroy, at its sole cost and expense, all relevant records and materials in its possession or control containing or comprising the Selvita Know-How and Selvita’s material, or such other Confidential Information of Selvita and Felicitex shall have the right to retain one copy thereof for record-keeping purposes.
(e) Patenting. Felicitex shall: (i) return to Selvita all documents entitling it to act in the name and on behalf of Selvita towards patent registries and (ii) hand over to Selvita the Prosecution and Maintenance of Selvita Collaboration Patents and Joint Collaboration Patents in an orderly and sound manner so that the timely filing of all necessary filings and the duly payment of all applicable fees to the patent registries is secured.
Exhibit D
ARTICLE XIV
MISCELLANEOUS
14.1 Dispute Resolution. If a dispute between the Parties arises under this Agreement, either Party shall have the right to refer such dispute in writing to the respective Executive Officers, and such Executive Officers shall attempt in good faith to resolve such dispute. If the Executive Officers are unable to resolve a given dispute pursuant to this Section within sixty (60) days after referring such dispute to the Executive Officers, or sooner if required by the particular circumstances, either Party may elect to have the given dispute settled by binding arbitration pursuant to Section 14.2.
14.2 Arbitration.
14.2.1 Arbitration Request. If a Party intends to begin an arbitration to resolve a dispute arising under this Agreement, such Party shall, within thirty (3) days following the expiration of the sixty (60) day mediation period referred to in Section 14.1 of this Agreement, provide written notice (the “Arbitration Request”) to the other Party of such intention and a statement of the issues for resolution. From the date of the Arbitration Request and until such time as the dispute has become finally settled, the running of the time periods as to which the other Party must cure a breach of this Agreement shall be suspended as to any breach that is the subject matter of the dispute, however, this Agreement shall remain in full force and effect and the Parties shall continue their obligations hereunder during the pendency of the arbitration(s). Within thirty (30) days after the receipt of the Arbitration Request, the other Party may, by written notice, add additional issues for resolution in a statement of counter-issues. Any arbitration pursuant to this Section will be held in accordance with the International Arbitration Rules of the International Centre for Dispute Resolution (“ICRD”), the international division of the American Arbitration Association, whereas, to the extent legally permissible, the procedure agreed in Section 14.2.2 shall be applied.
14.2.2 Arbitration Procedure. The arbitration shall be held in Boston, Massachusetts, United States unless another location is mutually agreed by the Parties. The arbitration shall be conducted by a single arbitrator knowledgeable in the subject matter at issue in the dispute and acceptable to both Parties; provided that, the Parties may by mutual agreement elect to have the arbitration conducted by a panel of three (3) arbitrators. If the Parties fail to agree on a mutually acceptable arbitrator within thirty (30) days after the Arbitration Request, then the arbitrator shall be selected by the ICRD. The arbitrator may proceed to an award, notwithstanding the failure of either Party to participate in the proceedings. The arbitrator shall, within thirty (30) days after the conclusion of the arbitration hearing, issue a written award and statement of decision describing the essential findings and conclusions on which the award is based, including the calculation of any damages awarded. The arbitrator shall be limited in the scope of his or her authority to resolving only the specific matter which the Parties have referred to arbitration for resolution and shall not have authority to render any decision or award on any other issues. The arbitrator shall be authorized to award compensatory damages, but shall not be authorized to award punitive, special, consequential, or any other similar form of damages, except as provided for in Section 12.5, or to reform, modify or materially change this Agreement. The arbitrator also shall be authorized to grant any temporary, preliminary or permanent equitable remedy or relief the arbitrator deems just and equitable and within the scope of this Agreement, including an injunction or order for specific performance. The Parties hereby expressly agree to waive the right to appeal from the decisions of the arbitrator, and there shall be no appeal to any court or other authority (government or private) from the decision of the arbitrator. Judgment on the award rendered by the arbitrator may be enforced in any court having competent jurisdiction thereof.
Exhibit D
14.2.3 Costs. Each Party shall bear all of its own costs and expenses, including, but not limited to attorneys’ fees, costs, and disbursements arising out of the arbitration, travel, witness fees, consultants, transcripts and the like, and shall pay an equal share of the fees and costs of the arbitrator.
14.2.4 Preliminary Injunctions. Notwithstanding anything in this Agreement to the contrary, a Party may seek a temporary restraining order or a preliminary injunction from any court of competent jurisdiction in order to prevent immediate and irreparable injury, loss, or damage on a provisional basis, pending the award of the arbitrator on the ultimate merits of any dispute.
14.2.5 Confidentiality. All proceedings and decisions of the arbitrator shall be deemed Confidential Information of each of the Parties, and shall be subject to Article 10.
14.3 Governing Law. This Agreement and any dispute arising from the performance or breach hereof shall be governed by and construed and enforced in accordance with the Laws of the State of Delaware excluding any conflicts or choice of law rule or principle that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction. The provisions of the United Nations Convention on Contracts for the International Sale of Goods shall not apply to this Agreement or any subject matter hereof.
14.4 Sectoral Sanctions Identification (SSI) List. Felicitex and Selvita confirm that none of their key personnel or shareholders are on the Sectoral Sanctions Identification (SSI) List of U.S. Treasury Department’s Office of Foreign Assets Control (OFAC) as of the date of the Agreement execution.
14.5 Assignment. Neither Party may assign this Agreement without the consent of the other Party, except as otherwise provided in this Section 14.5. Either Party may assign this Agreement in whole or in part to any Affiliate of such Party without the consent of the other Party; provided that, such assigning Party provides the other Party with written notice of such assignment, the Affiliate agrees in writing to assume performance of all assigned obligations, and the assigning Party shall remain primarily liable for the performance of its obligations under this Agreement by such Affiliate. Further, each Party may assign and transfer this Agreement, and all of its rights and obligations hereunder, without the consent of the other Party, to its successor in interest by way of an acquisition, merger, consolidation, business combination or in connection with the sale of all or substantially all of its business, equity securities or assets; provided that, such assigning or transferring Party provides the other Party with written notice of such assignment and the assignee or transferee agrees in writing to assume performance of all assigned obligations, and the assigning Party shall remain primarily liable for the performance of the assigned obligations under this Agreement by such assignee or transferee (except in the case of a Sale Transaction to a non-Affiliate of Felicitex to which Selvita has previously consented (such consent not to be unreasonably withheld) or a merger, sale or acquisition in which it is not the surviving entity). Any purported assignment in violation of this Section 14.5 shall be null and void. Nothing in this Section 14.5 shall limit or cancel the conditions for Felicitex’s Internal Development or External Development as outlined in Section 5.4.
Exhibit D
14.6 Performance Warranty. Each Party hereby acknowledges and agrees that it shall be responsible for the full and timely performance as and when due under, and observance of all the covenants, terms, conditions and agreements set forth in, this Agreement by its Affiliate(s), Sublicensees and Third Party Partners.
14.7 Force Majeure. No Party shall be held liable or responsible to the other Party nor be deemed to be in default under, or in breach of any provision of, this Agreement for failure or delay in fulfilling or performing any obligation (other than a payment obligation) of this Agreement when such failure or delay is due to force majeure, and without the fault or negligence of the Party so failing or delaying. For purposes of this Agreement, force majeure is defined as causes beyond the control of the Party, including acts of God; material changes in Law; war; civil commotion; destruction of production facilities or materials by fire, flood, earthquake, explosion or storm; labor disturbances; epidemic; and failure of public utilities or common carriers. In such event Selvita or Felicitex, as the case may be, shall immediately notify the other Party of such inability and of the period for which such inability is expected to continue. The Party giving such notice shall thereupon be excused from such of its obligations under this Agreement as it is thereby disabled from performing for so long as it is so disabled for up to a maximum of ninety (90) days, after which time Selvita and Felicitex shall promptly meet to discuss in good faith how to best proceed in a manner that maintains and abides by the Agreement. To the extent possible, each Party shall use reasonable efforts to minimize the duration of any force majeure.
14.8 Notices. Any notice or request required or permitted to be given under or in connection with this Agreement shall be deemed to have been sufficiently given if in writing and personally delivered or sent by, facsimile transmission (receipt verified), or international overnight express courier service (signature required), prepaid, to the Party for which such notice is intended, at the address set forth for such Party below:
| | If to Selvita, | |
| | | |
| | addressed to: | Chief Executive Officer, Selvita S.A., Park Life Science, |
| | | ul. Bobrzyńskiego 14, 30-348 Kraków, Poland |
| | | |
| | with a copy to: | Chief Operating Officer, Selvita S.A., Park Life Science, |
| | | ul. Bobrzyńskiego 14, 30-348 Kraków, Poland |
| | | |
| | If to Felicitex, | |
| | | |
| | addressed to: | Chief Executive Officer, Felicitex, |
| | | 27 Strathmore Rd, |
| | | Natick, Massachusetts 02468 |
| | | United States of America |
| | | |
| | with copies to: | Boston Law Group, PC |
| | | Attn: Val Gurvits, Esq. |
| | | 825 Beacon Street |
| | | Newton Centre, , MA 02459 |
| | | phone: 617-928-1800 |
| | | email: vgurvits@bostonlawgroup.com |
or to such other address for such Party as it shall have specified by like notice to the other Party, provided that notices of a change of address shall be effective only upon receipt thereof. If delivered personally or by facsimile transmission, the date of delivery shall be deemed to be the day on which such notice or request was given, or if such day is not a Business Day, the first Business Day thereafter. If sent by overnight express courier service, the date of delivery shall be deemed to be the second Business Day after such notice or request was deposited with such service.
14.9 Export Clause. Each Party acknowledges that the Laws of the United States restrict the export and re-export of certain commodities and technical data of United States origin. Each Party agrees that it will not export or re-export restricted commodities or the technical data of the other Party in any form without the appropriate United States and foreign government licenses.
14.10 Waiver. Neither Party may waive or release any of its rights or interests in this Agreement except in writing. The failure of either Party to assert a right hereunder or to insist upon compliance with any term of this Agreement shall not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition. No waiver by either Party of any condition or term in any one or more instances shall be construed as a continuing waiver of such condition or term or of another condition or term. The rights and remedies provided herein are cumulative and do not exclude any other right or remedy provided by Law or otherwise available except as expressly set forth herein.
14.11 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction or otherwise directly or indirectly affects the validity of any other material provision(s) of this Agreement (“Severed Clause”), all other provisions hereof shall remain in full force and effect in such jurisdiction except for such Severed Clause, and such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction. The Parties shall consult and use good faith efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute for such Severed Clause in light of the intent of this Agreement.
14.12 Entire Agreement. This Agreement together with the Exhibits hereto and thereto, set forth all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties with respect to the subject matter of this Agreement and supersede and terminate all prior agreements and understandings between the Parties with respect to the subject matter of this Agreement. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties with respect to the subject matter of this Agreement other than as set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by the respective authorized officers of the Parties.
Exhibit D
14.13 Independent Contractors. Nothing herein shall be construed to create any relationship of employer and employee, agent and principal, partnership or joint venture between the Parties. Each Party is an independent contractor. Neither Party shall assume, either directly or indirectly, any liability of or for the other Party. Neither Party shall have the authority to bind or obligate the other Party and neither Party shall represent that it has such authority.
14.14 Headings; Construction; Interpretation. Headings used herein are for convenience only and shall not in any way affect the construction of or be taken into consideration in interpreting this Agreement. The terms of this Agreement represent the results of negotiations between the Parties and their representatives, each of which has been represented by counsel of its own choosing, and neither of which has acted under duress or compulsion, whether legal, economic or otherwise. Accordingly, the terms of this Agreement shall be interpreted and construed in accordance with their usual and customary meanings, and each of the Parties hereto hereby waives the application in connection with the interpretation and construction of this Agreement of any rule of Law to the effect that ambiguous or conflicting terms or provisions contained in this Agreement shall be interpreted or construed against the Party whose attorney prepared the executed draft or any earlier draft of this Agreement.
Any reference in this Agreement to an Article, Section, subsection, paragraph, clause or Exhibit shall be deemed to be a reference to any Article, Section, subsection, paragraph, clause or Exhibit, of or to, as the case may be, this Agreement. Except where the context otherwise requires, (a) any definition of or reference to any agreement, instrument or other document refers to such agreement, instrument other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein or therein), (b) any reference to any Law refers to such Law as from time to time enacted, repealed or amended, (c) the words “herein,” “hereof” and “hereunder,” and words of similar import, refer to this Agreement in its entirety and not to any particular provision hereof, (d) the words “include,” “includes,” and “including,” shall be deemed to be followed by the phrase “but not limited to,” “without limitation” or words of similar import, (e) the word “or” is used in the inclusive sense (and/or) and (f) the singular shall include the plural, the plural the singular, the use of any gender shall be applicable to all genders. Any reference in this Agreement to “royalty” or “royalties” (whether used in capitalized letters or not) shall include royalties and other recurring or deferred payments payable by a Party to the other Party for compensation or consideration of rights granted hereunder.
14.15 Books and Records. Any financial books and records to be maintained under this Agreement by a Party or its Affiliates or Sublicensees shall be maintained in accordance with GAAP, consistently applied, except that the same need not be audited.
14.16 Further Actions. Each Party shall execute, acknowledge and deliver such further instruments, and do all such other acts, as may be necessary or appropriate in order to carry out the expressly stated purposes and the clear intent of this Agreement.
Exhibit D
14.17 Parties in Interest. All of the terms and provisions of this Agreement shall be binding upon, and shall inure to the benefit of and be enforceable by the Parties hereto and their respective successors and permitted assigns. The covenants and agreements set forth in this Agreement are for the sole benefit of the Parties and their successors, permitted assigns and, with respect to indemnification under Article 12, the indemnitees identified thereunder, and they shall not be construed as conferring any rights on any other Persons.
14.18 Performance by Affiliates. To the extent that this Agreement imposes obligations on Affiliates of a Party, such Party agrees to cause its Affiliates to perform such obligations.
14.19 Counterparts and Language. This Agreement may be signed in counterparts, each and every one of which shall be deemed an original, notwithstanding variations in format or file designation which may result from the electronic transmission, storage and printing of copies from separate computers or printers. Facsimile signatures and signatures transmitted via PDF shall be treated as original signatures. Further, the Parties agree that all conceptive, communications, agreements (including this Agreement) shall be in English and the English language shall control for all purposes.
Signature Page Follows
Exhibit D
IN WITNESS WHEREOF, and intending to be legally bound hereby, the Parties have caused this Agreement to be executed by their duly authorized representatives as of the Effective Date.
| | Selvita S.A. |
| | | | |
| | By: | /s/ Pawel Przewiezlikowski |
| | | Name: | Pawel Przewiezlikowski |
| | | Title: | Chief Executive Officer |
| | | Date: | 31 DEC 2018 |
| | | | |
| | By: | /s/ Miłosz Gruca, PhD |
| | | Name: | Miłosz Gruca, PhD |
| | | Title: | Director of Biology Department Board Member |
| | | Date: | 31 DEC 2018 |
| | | | |
| | Felicitex Inc. |
| | | | |
| | By: | /s/ Maria Vilenchik |
| | | Maria Vilenchik, Ph.D., CEO |
| | | Hereunto Duly Authorized |
| | | Title: | CEO |
| | | Date: | December 27, 2018 |
EXHIBIT A
Joint Collaboration IP
| 1. | Joint Collaboration Know-How |
https://lifescience.app.box.com/file/55424612114
[List of Know-How]
| 2. | Joint Collaboration Patents |
| Patent Number | Filing Date | Priority | Nationalities | Status |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
| [**] | [**] | [**] | [**] | [**] |
EXHIBIT B
Optioned Compounds







EXHIBIT C
Selvita Collaboration IP
1. Selvita Collaboration Know-How
[List of Know-How]
2. Selvita Collaboration Patents
| Patent Number | Filing Date | Priority | Nationalities | Status |
| | | | | |
| | | | | |
Exhibit D
EXHIBIT D
Procedure for Calculating Structural Similarity
Two compounds will be considered as derivatives of each other if their Stated Similarity Coefficient will be >=0.85.
For avoidance of doubt, Stated Similarity Coefficient will be calculated on neutral compounds except in the case of “onium” compounds, formed by substituents other than hydrogen (example: benzalkonium chloride).
The algorithm to calculate the Stated Similarity Coefficients is presented below:
Proposed is to define structural similarity basing on the MACCS fingerprint definition used in a public domain chemoinformatics toolkit “Open Babel” (O’Boyle et al., 2011). Open Babel is an Open Source chemistry toolbox, broadly accepted and used in chemoinformatics. The examples presented were generated using the Open Babel version 2.3.2 (from 17-02-2013), downloaded from their website: http://sourceforge.net/projects/openbabel/ MACCS key fingerprint definition contains a list of SMART queries, and is accessible as a text file on the openbabel installation directory. On Linux it is: /usr/local/share/openbabel/2.3.2/MACCS.txt.
The procedure for the structural similarity is as follow:
1. Generate an SDF formatted file with the query compound
2. Generate an SDF formatted file with the compounds from the compared library
3. Run the openbabel fingerprint similarity procedure, with Tanimoto similarity coefficient, using the command:
obabel query.sdf library.sdf –ofpt -xfMACCS
where: query.sdf and library.sdf are the files created in points 1 and 2.
4. Analyze the output of the program (see example below).
Example: Calculating of similarity of Gefitinib (Iressa) to 20 selected, approved small molecule kinase inhibitors (taken from Figure 1 from the KLIFS article (van Linden, Kooistra, Leurs, de Esch & de Graaf, 2013)
obabel gefitinib.sdf selected_kinase_drugs.sdf –ofpt –xfMACCS
>gefitinib
>gefitinib Tanimoto from gefitinib = 1
Possible superstructure of gefitinib
>erlotinib Tanimoto from gefitinib = 0.6875
>vandetanib Tanimoto from gefitinib = 0.857143
>bosutinib Tanimoto from gefitinib = 0.830769
>lapatinib Tanimoto from gefitinib = 0.666667
>imatinib Tanimoto from gefitinib = 0.513889
>nilotinib Tanimoto from gefitinib = 0.402778
>sorafenib Tanimoto from gefitinib = 0.514286
>regorafenib Tanimoto from gefitinib = 0.514286
>ponatinib Tanimoto from gefitinib = 0.573333
>ruxolitinib Tanimoto from gefitinib = 0.409091
>tofacitinib Tanimoto from gefitinib = 0.577465
>vemurafenib Tanimoto from gefitinib = 0.45977
>dasatinib Tanimoto from gefitinib = 0.602564
>sunitinib Tanimoto from gefitinib = 0.5
>crizotinib Tanimoto from gefitinib = 0.671429
>pazopanib Tanimoto from gefitinib = 0.348315
>axitinib Tanimoto from gefitinib = 0.293333
>dabrafenib Tanimoto from gefitinib = 0.301075
>trametinib Tanimoto from gefitinib = 0.567568
Exhibit D
Definitions:
“Chemical fingerprint” means a string of binary values (0 or 1) used to characterize a molecule. In the presented definition, MACCS structural fingerprint was proposed to describe the compared compound. The MACCS definition of structural keys is frequently used in chemoinformatics, because it allows assigning unambiguously a binary string to the given structure. In the case of other, hashed fingerprints, the particular binary representation may depend on the algorithm used; therefore, the particular result of comparison may depend on the software used.
In the presented examples, MACCS fingerprints are calculated using a publicly accessible program Open Babel. MACCS fingerprint is created using structural key descriptors in which each bit is associated with a SMARTS pattern.
A structural key is a fixed-length bitstring in which each bit is associated with a specific molecular pattern. When a structural key is generated for a molecule, the bitstring encodes whether or not these specific molecular patterns are present or absent in the molecule. The performance of such keys depends on the choice of the fragments used for constructing the keys and the probability of their presence in the searched molecule databases.
“SMARTS” means a language that allows specifying substructures by providing a number of primitive symbols describing atomic and bond properties. Atom and bond primitive specifications may be combined to form expressions by using logical operators. For more information go to: http://www.daylight.com/dayhtml/doc/theory/theory.smarts.html.
“Tanimoto similarity coefficient” means the most commonly used similarity coefficient in chemical informatics. It is often applied to comparison of binary strings, and may be calculated using the equation:
where:
a –the number of “on” features (bits) in structure A)
b – the number of “on” features (bits) in structure B
c – the number of “on” features (bits) common to both fingerprints A and B
The range of Tanimoto coefficient is from 0 to 1, with larger values for more similar compounds.
Exhibit D
Brown and Martin (Brown & Martin, 1997) found that 2D descriptors (in combination with hierarchical clustering) are best at separating actives from inactives, given a particular target. Structural keys, hashed fingerprints and different 3D descriptors were compared and authors concluded that the MACCS structural key descriptor implicitly contains a great deal of information relevant to each type of interaction.
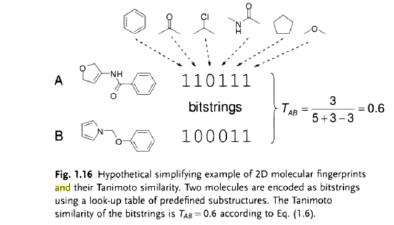
Figure. “Molecular Design: Concepts and Applications” by Gilbert Schneider, Karl-Heinz Baringhaus.
“Stated Tanimoto similarity coefficient” – means Tanimoto coefficient, calculated using MACCS fingerprint representation, is more than 0.85.
References:
Brown, R. D., & Martin, Y. C. (1997). The Information Content of 2D and 3D Structural Descriptors Relevant to Ligand-Receptor Binding. Journal of Chemical Information and Modeling, 37(1), 1–9. doi:10.1021/ci960373c
O’Boyle, N. M., Banck, M., James, C. A., Morley, C., Vandermeersch, T., & Hutchison, G. R. (2011). Open Babel: An open chemical toolbox. Journal of cheminformatics, 3(1), 33. doi:10.1186/1758-2946-3-33
Van Linden, O. P. J., Kooistra, A. J., Leurs, R., de Esch, I. J. P., & de Graaf, C. (2013). KLIFS: A knowledge-based structural database to navigate kinase-ligand interaction space. Journal of medicinal chemistry. doi:10.1021/jm400378w
| | ● | Exemplary analysis of Regorafenib similar molecules based on Tanimoto similarity coefficient (obtained with MACCS fingerprint). |
Exhibit D
Exemplary Tanimoto similarity coefficient matrix (obtained with MACCS fingerprint) - molecular structures of twenty approved small molecule kinase inhibitors (according to Fig.1 from O.P.J. van Linden, et al. (2013) J. Med. Chem. DOI: 10.1021/jm400378w ). Structures of Erlotinib and Vandetanib were switched in the original.
Exhibit D

For convenience – molecular structures from the paper are shown below.
Exhibit D
EXHIBIT E
Target
DYRK1A kinase
DYRK1B kinase
Exhibit D
EXHIBIT F
Implementation Statement
FELICITEX THERAPEUTICS, INC.
27 Strathmore Rd., Natick
Massachusetts 01760
U.S.A.
| | SELVITA S.A. |
| | Bobrzyńskiego 14 |
| | 30-348 Kraków |
| | Poland |
On the basis of Section 5.3 of the Exclusive License Agreement signed between Felicitex Inc. and Selvita SA on [●] we hereby certify that as of the present date we have implemented the results of the research which constitute subject matter of this agreement in the business of Felicitex Inc. through:
| ● | Commencing further research on chemical compounds described in patent application no. [**] by carrying all the required studies to advance the best representatives of the series covered by the application into IND enalbling studies followed by entering FIH studies. |
| | Felicitex Therapeutics Inc. |
| | |
| | By: | /s/ Maria Vilenchik |
| | | Maria Vilenchik, Ph.D., CEO |
| | Date: | December 27, 2018 |
Exhibit D
EXHIBIT G
Calculation Scheme for the Diluted Value Share
Depending on the stage of Development at which Felicitex undertakes External Development, Selvita’s Initial Value Share, Decreased Value Share or Adjusted Value Share, whichever is applicable, shall be diluted pursuant to the following table:
Project progress upon External
Development | | Felicitex share after dilution
of Selvita’s applicable value shares | | Selvita’s dilution
factor (Vd) |
| | | | | |
| IND-ready candidate | | [**] | | [**] |
| | | | | |
| Drug after successful Phase I | | [**] | | [**] |
| | | | | |
| Drug after successful Phase II | | [**] | | [**] |
| | | | | |
| Drug after successful Phase III | | [**] | | [**] |
| | | | | |
| Approved drug | | [**] | | [**] |
Therefore, the Diluted Value Share shall be determined pursuant to the following table:
| Project progress upon External Development | | Diluted Value Share (Vf) |
| | | |
| IND-ready candidate | | Initial Value Share, Decreased Value Share or Adjusted Value Share (whichever is applicable) (Va) |
| Drug after successful Phase I | | Va*[**] |
| Drug after successful Phase II | | Va*[**] |
| Drug after successful Phase III | | Va*[**] |
| Approved drug | | Va*[**] |
Accordingly, the participation payments on Participation Income payable by Felicitex to Selvita shall be calculated on basis of the calculation formula below (which takes into account the dilution pursuant to the table above):
Participation Payment = Vf*(Participation Income), where Vf=Va*(Vd/[**]/ %).
Exhibit D
EXHIBIT H
Exemplary Calculation of Participation Payments
For the avoidance of doubt, the following table provides an exemplary calculation of payments due to Selvita in a possible External Development scenario of Felicitex:
Possible deals by Felicitex with a pharma partner
| Project Progress upon External Development | | Up-front
payment
from the
pharma
partner | | Felicitex’s
share in
the up-
front | | Selvita
share in
the up-
front | | Bio-dollar
values from
the pharma
partner | | Felicitex’s
share in
the bio-
dollar
value | | Selvita
share in
the bio-
dollar
value | | Royalties
from
the
pharma
partner | | Felicitex
royalties
after
payout
to
Selvita | | Selvita
royalties
from
net-
sales
worldwide | | = Selvita share in
Participation
Income received
from the pharma
partner (equal to
Value Share)
(not in addition
to previous
column) – this is
not a royalty rate
but a share in
participation
Income received
from the pharma
partner |
| IND-ready candidate | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] |
| Drug after successful Phase I | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] |
| Drug after successful Phase II | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] |
| Drug after successful Phase III | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] |
| Approved drug | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] | | [**] |
Exhibit D
EXHIBIT I
Press Release
[Draft to be included in final version]
Exhibit D










