UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended September 30, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report
For the transition period from to
Commission file number: 001-42256
WORK Medical Technology Group LTD
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
Cayman Islands
(Jurisdiction of incorporation or organization)
Floor 23, No. 2 Tonghuinan Road
Xiaoshan District, Hangzhou City, Zhejiang Province
The People’s Republic of China
+86-571-82613568
(Address of principal executive offices)
Shuang Wu, Chief Executive Officer
Telephone: +86-571-82613568
Email: wushuang@workmedtech.com
Floor 23, No.2 Tonghuinan Road
Xiaoshan District, Hangzhou City, Zhejiang Province
The People’s Republic of China
+86-571-82613568
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Ordinary Shares | | WOK | | The Nasdaq Stock Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
An aggregate of 14,591,942 ordinary shares, par value $0.0005 per share, as of September 30, 2024.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ☐ No ☒
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | | Emerging growth company | ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☒ | International Financial Reporting Standards as issued by the
International Accounting Standards Board ☐ | Other ☐ |
| * | If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐ |
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☐ No ☐
TABLE OF CONTENTS
INTRODUCTION
In this annual report on Form 20-F, unless the context otherwise requires, references to:
| ● | “China” or the “PRC” are to the People’s Republic of China, including the special administrative regions of Hong Kong, Macau and Taiwan. The term “Chinese” has a correlative meaning for the purpose of this annual report. When used in the case of laws, regulations and rules, of “China” or the “PRC”, it refers to only such laws, regulations and rules of mainland China. When used in the case of government, governmental authorities, regulatory agencies, courts, jurisdictions, tax, entities, enterprises, individuals and residents of “China” or the “PRC” or “Chinese”, it refers to only such government, governmental authorities, regulatory agencies, courts, jurisdictions, tax, entities, enterprises, individuals and residents of mainland China; |
| ● | “Class I medical device” are to a medical device with a low level of risks and whose safety and effectiveness can be ensured through routine administration, pursuant to the Regulations on the Supervision and Administration of Medical Devices (as amended in 2021) (the “2021 Medical Device Regulation”); |
| ● | “Class II medical device” are to a medical device with moderate risks that must be strictly controlled and regulated to ensure its safety and effectiveness, pursuant to the 2021 Medical Device Regulation; |
| ● | “Class III medical device” are to a medical device with relatively high risks that must be strictly controlled and regulated through special measures to ensure its safety and effectiveness, pursuant to the 2021 Medical Device Regulation; |
| ● | “Code” are to the United States Internal Revenue Code of 1986, as amended; |
| ● | “Exchange Act” are to the Securities Exchange Act of 1934, as amended; |
| ● | “FDA” are to the U.S. Food and Drug Administration; |
| ● | “Group” are to the WORK Medical Technology Group LTD, Work Medical Technology Group Limited, and the PRC subsidiaries, collectively; |
| ● | “Hangzhou Hanshi” are to Hangzhou Hanshi Medical Equipment Co., Ltd.; |
| ● | “Hangzhou Shanyou” are to Hangzhou Shanyou Medical Equipment Co., Ltd.; |
| ● | “Hangzhou Woli” are to Hangzhou Woli Medical Treatment Technology Co., Ltd.; |
| ● | “Hangzhou Youshunhe” are to Hangzhou Youshunhe Technology Co., Ltd.; |
| ● | “Huangshan Saitumofei” are to Huangshan Saitumofei Medical Technology Co., Ltd.; |
| ● | “Hunan Saitumofei” are to Hunan Saitumofei Medical Treatment Technology Co., Ltd.; |
| ● | “mainland China” or “Chinese mainland” are to the People’s Republic of China, excluding, solely for the purpose of this annual report, the special administrative regions of Hong Kong, Macau and Taiwan. The term “mainland Chinese” has a correlative meaning for the purpose of this annual report; |
| ● | “Nasdaq” are to the Nasdaq Capital Market; |
| ● | “Ordinary Shares” are to ordinary shares of the Company, par value $0.0005 per share; |
| ● | “PFIC” are to a passive foreign investment company; |
| ● | “PRC subsidiaries” are to Work Hangzhou, Hangzhou Shanyou, Shanghai Chuqiang, Hangzhou Hanshi, Hangzhou Woli, Shanghai Saitumofei, and Hunan Saitumofei, collectively; |
| ● | “RMB” or the “Renminbi” are to the legal currency of China; |
| ● | “SEC” are to the United States Securities and Exchange Commission; |
| ● | “Securities Act” are to the Securities Act of 1933, as amended; |
| ● | “Shanghai Chuqiang” are to Shanghai Chuqiang Medical Equipment Co., Ltd.; |
| ● | “Shanghai Saitumofei” are to Shanghai Saitumofei Medical Treatment Technology Co., Ltd.; |
| ● | “US$,” “USD,” “U.S. dollars,” “$,” and “dollars” are to the legal currency of the United States; |
| ● | “Work BVI” are to Work Medical Technology Group Limited; |
| ● | “WFOE” or “Work Age” are to Work Age (Hangzhou) Medical Treatment Technology Co., Ltd, which is a limited liability company formed in China; and |
| ● | “Work Hangzhou” are to Work (Hangzhou) Medical Treatment Technology Co., Ltd. |
This annual report on Form 20-F includes our audited consolidated financial statements for the fiscal years ended September 30, 2024 and 2023. In this annual report, we refer to assets, obligations, commitments, and liabilities in our consolidated financial statements in United States dollars. These dollar references are based on the exchange rate of RMB to United States dollars, determined as of a specific date or for a specific period. Changes in the exchange rate will affect the amount of our obligations and the value of our assets in terms of United States dollars which may result in an increase or decrease in the amount of our obligations and the value of our assets.
This annual report contains translations of certain RMB amounts into U.S. dollars at specified rates. Unless otherwise stated, the following exchange rates are used in this annual report:
| | | September 30, |
| US$ Exchange Rate | | 2024 | | 2023 | | 2022 |
| At the end of the year - RMB | | RMB7.0176 to $1.00 | | RMB7.2960 to $1.00 | | RMB7.1135 to $1.00 |
| Average rate for the year - RMB | | RMB7.2043 to $1.00 | | RMB7.0824 to $1.00 | | RMB6.5532 to $1.00 |
Part I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not Applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
ITEM 3. KEY INFORMATION
In this annual report, unless otherwise stated, the terms “we,” “us,” “our,” “Work Cayman,” “our Company,” and the “Company” refer to WORK Medical Technology Group LTD, an exempted company limited by shares incorporated under the laws of the Cayman Islands and not a Chinese operating company. Work Cayman has no operations of its own and conducts all of its operations through the PRC subsidiaries, namely, Work (Hangzhou) Medical Treatment Technology Co., Ltd. (“Work Hangzhou”), our wholly owned PRC subsidiary, and its subsidiaries, Hangzhou Shanyou Medical Equipment Co., Ltd. (“Hangzhou Shanyou”), Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”), Hangzhou Hanshi Medical Equipment Co., Ltd. (“Hangzhou Hanshi”), Hangzhou Woli Medical Treatment Technology Co., Ltd. (“Hangzhou Woli”), Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”), Huangshan Saitumofei Medical Technology Co., Ltd. (“Huangshan Saitumofei”), and Hunan Saitumofei Medical Treatment Technology Co., Ltd. (“Hunan Saitumofei”) (collectively referred to herein as “the PRC subsidiaries”). The operations of the PRC subsidiaries could affect other parts of our business.
Investors in our Ordinary Shares should be aware that they will not directly hold equity interests in the PRC subsidiaries but rather are purchasing equity solely in WORK Medical Technology Group LTD, a Cayman Islands holding company, which indirectly owns a majority of the equity interests in such PRC subsidiaries. The Chinese regulatory authorities could disallow this structure, which would likely result in a material change in our operations and/or a material change in the value of the securities we are registering for sale, including that it could cause the value of such securities to significantly decline or become worthless. For risks facing our Company as a result of our organizational structure and doing business in China, see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China.” We do not currently use a variable interest entity (“VIE”) structure.
In addition, as we conduct all of our operations through the PRC subsidiaries in China, we and the PRC subsidiaries are subject to legal and operational risks associated with being based in China, including risks related to the legal, political and economic policies of the Chinese government, the relations between China and the United States, or Chinese or United States regulations, which risks could result in a material change in the PRC subsidiaries’ operations and/or cause the value of our Ordinary Shares to significantly decline or become worthless and affect our ability to offer or continue to offer securities to investors. Recently, the PRC government initiated a series of regulatory actions and made a number of public statements on the regulation of business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts in anti-monopoly enforcement. We do not believe, as advised by our PRC counsel, AllBright Law Offices (Fuzhou), that we or the PRC subsidiaries are directly subject to these regulatory actions or statements, as neither we nor the PRC subsidiaries have implemented any monopolistic behavior, and the PRC subsidiaries’ business does not implicate cybersecurity, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess the personal information of over one million users, nor are we or the PRC subsidiaries involved in any type of restricted industries. On September 8, 2006, the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (the “M&A Rules”) which were jointly adopted by six PRC regulatory agencies came into effect. The M&A Rules include, among other things, provisions that purport to require that offshore special purpose vehicles (each, an “SPV”) that are controlled by PRC entities or individuals and that have been formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, obtain the approval of the China Securities Regulatory Commission (the “CSRC”) prior to the listing and trading of any such SPV’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures regarding its approval of overseas listings by SPVs. Since the Company is neither controlled by PRC entities or individuals, nor formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, we believe, as advised by our PRC counsel, AllBright Law Offices (Fuzhou), that the Company is not an SPV, and, therefore, the M&A Rules do not apply to us, and we do not need to obtain the approval from the CSRC under the M&A Rules. However, substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore SPVs.
Furthermore, on March 31, 2023, the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Administrative Measures”) and relevant supporting guidelines (collectively, the “New Administrative Rules Regarding Overseas Listings”) issued by the CSRC came into force. These rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. According to the New Administrative Rules Regarding Overseas Listings, among other things, Chinese domestic companies conducting overseas securities offering and listing activities, either in direct or indirect form, shall complete filing procedures with the CSRC pursuant to the requirements of Trial Administrative Measures within three working days following their submission of initial public offering (“IPO”), listing applications, or completion of subsequent securities offerings. The New Administrative Rules Regarding Overseas Listings apply to both IPO and subsequent securities offerings of a company in the same overseas market where it has previously offered and listed securities. Under these rules, companies like ours must file with the CSRC within three working days after completing a post-IPO securities offering. On the same day, the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Confidentiality and Archives Administration Provisions”) promulgated by the CSRC became effective. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Furthermore, a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators, and individuals, shall fulfill due procedures in compliance with applicable regulations. Our Ordinary Shares began trading on the Nasdaq Capital Market under the symbol “WOK” on August 23, 2024, and on August 26, 2024, the Company completed its IPO. We believe, as advised by our PRC counsel, Allbright Law Offices (Fuzhou), that our IPO did not involve the leaking of any state secret or working secret of government agencies, or the harming of national security and public interests. However, we may be required to perform additional procedures in connection with the provision of accounting archives. The specific requirements of the relevant procedures are currently unclear, and we cannot be certain whether we will be able to perform the relevant procedures. We also believe, based on the advice of our PRC counsel, AllBright Law Offices (Fuzhou), that (i) because we are not an SPV which acquires PRC domestic companies’ equity with our shares prior to the listing of our Ordinary Shares on Nasdaq, we were not required to submit an application to the CSRC for its approval of the IPO and the listing and trading of our Ordinary Shares with Nasdaq under the M&A Rules; (ii) as we are regarded as a domestic company to be indirectly listed on overseas markets under the Trial Administrative Measures, we were required to complete the filing procedures with the CSRC in accordance with the Trial Administrative Measures with respect to our IPO and on December 21, 2023, we completed all the required filing procedures with the CSRC with respect to our IPO; (iii) neither we nor the PRC subsidiaries are subject to cybersecurity review with the Cyberspace Administration of China (the “CAC”), pursuant to the Measures for Cybersecurity Review (2021 version), which became effective on February 15, 2022, since the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess personal information of over one million users; (iv) the Company is currently not required to obtain permission or approval from any of the PRC central or local governmental authorities, except for completing the filing procedures with the CSRC, and it has not received any denial to list on a U.S. exchange. However, in the event that we conduct subsequent offerings, we could be subject to new filing requirements with the CSRC. In the event that filings with the CSRC are required, we cannot assure you that we can complete the filing procedures, obtain the approvals, or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on the operations of the PRC subsidiaries in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside of China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our Ordinary Shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless; and (v) there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations and future PRC laws and regulations, and there can be no assurance that the relevant government agencies will not take a view that is contrary to, or otherwise different from, the conclusions stated above. If the relevant government agencies take a view that is contrary to, or otherwise different from, the foregoing conclusions, it could have a material adverse effect on the PRC subsidiaries’ business, operating results and reputation, as well as the trading price of our Ordinary Shares. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay our subsequent securities offerings and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for our subsequent securities offerings and affect our ability to offer or continue to offer securities to investors outside China” on page 32; “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations” on page 27; and “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Additional compliance procedures may be required in connection with our subsequent securities offerings, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 30.
However, since these statements and regulatory actions by the PRC government are newly published and official guidance and related implementation rules have yet to be fully issued, it is highly uncertain what the potential impact such modified or new laws and regulations will have on the PRC subsidiaries’ daily business operations, the ability to accept foreign investments and list on a U.S. exchange. Moreover, the Standing Committee of the National People’s Congress (the “SCNPC”) or other PRC regulatory authorities may in the future promulgate laws or regulations or implementing rules that require our Company, or any of our subsidiaries to obtain regulatory approval from Chinese authorities before listing in the U.S. Although the Company is currently not required to obtain permission or approval from any of the PRC central or local governmental authorities, except for completing the filing procedures with the CSRC, and it has not received any denial to list on a U.S. exchange, the PRC subsidiaries’ operations could be adversely affected, directly or indirectly; our ability to offer, or continue to offer, securities to investors would be potentially hindered; and the value of our securities might significantly decline or be worthless, by existing or future laws and regulations relating to the business of the PRC subsidiaries or our industry or by intervention or interruption by PRC governmental authorities, if we or the PRC subsidiaries (i) do not receive or maintain such permissions or approvals, (ii) inadvertently conclude that such permissions or approvals are not required, (iii) applicable laws, regulations, or interpretations change and we or the PRC subsidiaries are required to obtain such permissions or approvals in the future, or (iv) due to any intervention or interruption by PRC governmental with little advance notice. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China” beginning on page 27 for a discussion of these legal and operational risks and other information that should be considered before making a decision to purchase our securities.
Moreover, the Chinese government may exert substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and our subsequent securities offerings at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could decline in value significantly or become worthless. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and our subsequent securities offerings at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless” on page 29.
In addition, although Work Medical Technology Group (China) Limited (“Work Medical Technology”), our Hong Kong subsidiary, is an investment holding company, the legal and operational risks associated with operating in mainland China may also apply to the future activities (if any) in Hong Kong of Work Medical Technology, to the extent that they are made applicable to such entity and its anticipated operations. Work Medical Technology, as of the date of this annual report, has yet to commence operations and is expected to be limited to operating as an investment holding company in the future without any substantive or data-related operations in Hong Kong. However, such operations may be affected if Hong Kong adopts rules, regulations or policy guidance with respect to currency exchange control. Furthermore, as of the date of this annual report, we do not expect that any regulatory actions related to data security or anti-monopoly concerns in Hong Kong will impact the Company’s ability to conduct its business, accept foreign investments, or list on a U.S. or foreign exchange because we have never had and do not plan to have any material operations in Hong Kong. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—The enactment of Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region and Hong Kong Autonomy Act of U.S. could impact our Hong Kong holding subsidiary” on page 42.
Hong Kong was established as a special administrative region of the PRC in accordance with Article 31 of the Constitution of the PRC. The Basic Law of the Hong Kong Special Administrative Region of the PRC (the “Basic Law”) was adopted and promulgated on April 4, 1990, and became effective on July 1, 1997, when the PRC resumed the exercise of sovereignty over Hong Kong. Pursuant to the Basic Law, Hong Kong is authorized by the National People’s Congress of the PRC to exercise a high degree of autonomy and enjoy executive, legislative, and independent judicial power, under the principle of “one country, two systems,” and the PRC laws and regulations shall not be applied in Hong Kong except for those listed in Annex III of the Basic Law (which is confined to laws relating to national defense, foreign affairs, and other matters that are not within the scope of autonomy of Hong Kong). While the National People’s Congress of the PRC has the power to amend the Basic Law, the Basic Law also expressly provides that no amendment to the Basic Law shall contravene the established basic policies of the PRC regarding Hong Kong. As a result, the national laws of the PRC not listed in Annex III of the Basic Law do not apply to our Hong Kong subsidiary, Work Medical Technology. However, there is no assurance that certain PRC laws and regulations, including existing laws and regulations and those enacted or promulgated in the future, will not be applicable to Work Medical Technology due to changes in the current political arrangements between mainland China and Hong Kong or other unforeseeable reasons. The application of such laws and regulations may have a material adverse impact on Work Medical Technology, as relevant authorities may impose fines and penalties upon Work Medical Technology, delay or restrict the repatriation of the proceeds from our securities offerings into mainland China and Hong Kong, and any failure by us to fully comply with any such new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our Ordinary Shares to significantly decline in value or in extreme cases, become worthless.
Furthermore, as more stringent criteria have been imposed by the U.S. Securities and Exchange Commission (the “SEC”) and the Public Company Accounting Oversight Board (the “PCAOB”) recently, our securities may be prohibited from trading on a national exchange or over-the-counter under the Holding Foreign Companies Accountable Act (“the HFCA Act”), if the PCAOB is unable to inspect our auditors for two consecutive years. As a result, an exchange may determine to delist our securities. Pursuant to the HFCA Act, if the PCAOB is unable to inspect an issuer’s auditors for two consecutive years, the issuer’s securities are prohibited to trade on a U.S. stock exchange. The PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. Furthermore, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. On June 22, 2021, United States Senate passed the Accelerating Holding Foreign Companies Accountable Act. On December 29, 2022, legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years, as was formerly required under the HFCA Act before such amendment, to two consecutive years. According to the Consolidated Appropriations Act, any foreign jurisdiction could be the reason why the PCAOB does not have complete access to inspect or investigate a company’s auditor. As it was originally enacted, the HFCA Act applied only if the PCAOB’s inability to inspect or investigate was due to a position taken by an authority in the foreign jurisdiction where the relevant public accounting firm is located. As a result of the Consolidated Appropriations Act, the HFCA Act now also applies if the PCAOB’s inability to inspect or investigate the relevant accounting firm is due to a position taken by an authority in any foreign jurisdiction. The denying jurisdiction does not need to be where the accounting firm is located. As of the date of this annual report, our auditor WWC, P.C., is not on the list published by the PCAOB subject to the determinations as to inability to inspect or investigate completely, as announced by the PCAOB on December 16, 2021, and it is based in the U.S. and is registered with the PCAOB and subject to PCAOB inspection, having its latest inspection completed in 2023. However, recently developments with respect to audits of China-based companies, create uncertainty about the ability of our auditor, to fully cooperate with the PCAOB’s request for audit workpapers without the approval of the Chinese authorities. In the event it is later determined that the PCAOB is unable to inspect or investigate completely the Company’s auditor because of a position taken by an authority in a foreign jurisdiction, then such lack of inspection could cause trading in the Company’s securities to be prohibited under the HFCA Act, and ultimately result in a determination by a securities exchange to delist the Company’s securities. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment, even making it worthless. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC (the “MOF”), and the PCAOB signed a Statement of Protocol (the “Protocol”), which sets out specific arrangements on conducting inspections and investigations over relevant audit firms within the jurisdiction of the PRC and the U.S, including the audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. Notwithstanding the foregoing, in the event it is later determined that the PCAOB is unable to inspect or investigate completely our auditor, then such lack of inspection could cause our securities to be delisted from the stock exchange. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Our Ordinary Shares may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two.” on page 40.
As a holding company, we may rely on dividends and other distributions on equity paid by the PRC subsidiaries for our cash and financing requirements. If any of the PRC subsidiaries incur debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to us. Subject to certain contractual, legal, and regulatory restrictions, and our internal cash management policy, cash and capital contributions may be transferred among our Cayman Islands holding company and our subsidiaries. If needed, our Cayman Islands holding company can transfer cash to the PRC subsidiaries through loans and/or capital contributions, and the PRC subsidiaries can transfer cash to our Cayman Islands holding company through issuing dividends or other distributions. Our finance department supervises cash management, following the instructions of our management. Our finance department is responsible for establishing our cash operation plan and coordinating cash management matters among our subsidiaries and departments. Each subsidiary or department initiates a cash request by putting forward a cash demand plan, which explains the specific amount and timing of cash requested and submitting it to our finance department. The finance department reviews the cash demand plan and prepares a summary for the management of our Company. Management examines and approves the allocation of cash based on the sources of cash and the priorities of the needs. Other than the above, we currently do not have other cash management policies or procedures that dictate how funds are transferred. As of the date of this annual report, none of the PRC subsidiaries have made any dividends or other distributions of earnings to the Company or any U.S. investors. As of the date of this annual report, the Company has transferred the net proceeds in the amount of $7,373,839 from our IPO through Work BVI to Work Medical Technology, from such amount, $5,404,654 of the net proceeds remained available after reimbursing PRC subsidiaries for expenses advanced from them in connection with the IPO. In the fiscal year ended September 30, 2023, and 2022, there was no cash transferred from the Cayman Islands holding company to its PRC subsidiaries. Funds also transferred among five PRC subsidiaries, Hangzhou Shanyou, Hangzhou Hanshi, Hangzhou Woli, Shanghai Saitumofei, and Work Hangzhou for operational purposes. The aggregate principal amounts of funds transferred among the PRC subsidiaries were $2,459,263, $8,262,606, and $84,234 for the fiscal years ended September 30, 2024, 2023, and 2022, respectively. As of the date of this annual report, there have been no other transfers or distributions made or dividends paid among the Company and its subsidiaries, except as described above. In the future, cash proceeds raised from overseas financing activities, including subsequent securities offerings, may be transferred by us to the PRC subsidiaries via capital contribution or shareholder loans, as the case may be. See also “Item 8. Financial Information—A. Consolidated Statements and Other Financial Information—Dividend Policy” and our audited consolidated financial statements for the fiscal years ended September 30, 2024 and 2023.
Cash transfers from our Cayman Islands holding company are subject to applicable PRC laws and regulations on loans and direct investment. We may rely on dividends from our subsidiaries in China for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us, and as a holding company, we will be dependent on receipt of funds from our Hong Kong subsidiary, Work Medical Technology. Current PRC regulations permit our indirect PRC subsidiaries to pay dividends to Work Medical Technology only out of their respective accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entity in China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund, although the amount to be set aside, if any, is determined at the discretion of its board of directors. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. Therefore, we may experience difficulties in complying with the administrative requirements necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries and affiliates in the PRC incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenue from our or their operations, we may be unable to pay dividends on our Ordinary Shares.
Cash dividends, if any, on our Ordinary Shares will be paid in U.S. dollars. Work Medical Technology may be considered a non-resident enterprise for tax purposes, so that any dividends our PRC subsidiaries pay to Work Medical Technology may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10%. See “Item 10. Additional Information—E. Taxation—People’s Republic of China Taxation.”
In order for us to pay dividends to our shareholders, we will rely on payments made from Work Hangzhou and its subsidiaries to WFOE and from WFOE to Work Medical Technology, and then to our Company. According to the EIT Law, such payments from subsidiaries to parent companies in China are subject to the PRC enterprise income tax at a rate of 25%. In addition, if Work Age or its subsidiaries or branches incur debt on their own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
Pursuant to the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be lowered to 5% if a Hong Kong resident enterprise owns no less than 25% of a PRC project. The 5% withholding tax rate, however, does not automatically apply and certain requirements must be satisfied, including without limitation that (a) the Hong Kong project must be the beneficial owner of the relevant dividends; and (b) the Hong Kong project must directly hold no less than 25% share ownership in the PRC project during the 12 consecutive months preceding its receipt of the dividends. In current practice, a Hong Kong project must obtain a tax resident certificate from the Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to any dividends paid by our PRC subsidiaries to its immediate holding company, Work Medical Technology. As of the date of this annual report, we have not applied for the tax resident certificate from the relevant Hong Kong tax authority. Work Medical Technology intends to apply for the tax resident certificate if and when Work Age plans to declare and pay dividends to Work Medical Technology.
To the extent cash is located in the PRC or within a PRC domiciled entity and may need to be used to fund operations outside of the PRC, the funds may not be available due to limitations placed on us and our subsidiaries by the PRC government. To the extent cash in and assets of the business is in the PRC or a PRC entity, the funds and assets may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of us or our subsidiaries by the PRC government to transfer cash and assets.
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Summary of Risk Factors
Investing in our securities involves significant risks. You should carefully consider all of the information in this annual report before investing in our securities. Below is a summary of the principal risks we face. These risks are discussed more fully under “Item 3. Key Information—D. Risk Factors.”
Risks Related to the PRC Subsidiaries’ Business and Industry (for a more detailed discussion, see “Item 3. Key Information — D. Risk Factors — Risks Related to the PRC Subsidiaries’ Business and Industry”)
Risks and uncertainties related to the PRC Subsidiaries’ business and industry include, but are not limited to, the following:
| | ● | The PRC subsidiaries’ operating history may not be indicative of our future growth or financial results and the PRC subsidiaries may not be able to sustain their historical growth rates. |
| | ● | Failure to maintain the quality and safety of the PRC subsidiaries’ products could have a material and adverse effect on the PRC subsidiaries’ and our reputation, financial condition and results of operations. |
| | ● | The PRC subsidiaries may experience significant liability claims or complaints from customers, doctors and patients, litigation and regulatory investigations and proceedings, such as claiming in relation to medical device safety, or adverse publicity involving their products, which could adversely affect the PRC subsidiaries’ and our financial condition and results of operations. |
| | ● | The decreased demand for masks and in the unit price of masks could reduce our revenue, which could adversely affect the PRC subsidiaries’ and our results of operations. |
| | ● | The PRC subsidiaries face the risk of fluctuations in the cost, availability and quality of their raw materials, which could adversely affect their results of operations, and thus, adversely affect the Group as a whole. |
| | ● | A significant interruption in the operations of the PRC subsidiaries’ third-party suppliers and other business partners could potentially disrupt their operations. |
| | ● | The PRC subsidiaries do not have long term contracts with their suppliers and the suppliers can reduce order quantities or terminate sales to the PRC subsidiaries at any time. |
| | ● | Overall tightening of the labor market, increases in labor costs or any possible labor unrest may adversely affect the PRC subsidiaries’ business and results of operations, which may also adversely affect the Group as a whole. |
| | | |
| | ● | The PRC subsidiaries’ industry is intensely competitive. The PRC subsidiaries may face competition from, and they may be unable to compete successfully against, new entrants and established companies with greater resources. |
| | ● | The continuing development of the PRC subsidiaries’ products depends upon the PRC subsidiaries’ maintaining strong working relationships with their customers, distributors and sales agents. |
| | ● | Technological changes may adversely affect sales of the PRC subsidiaries’ products and may cause their products to become obsolete. |
| | ● | Consolidation in the medical device industry could have an adverse effect on the PRC subsidiaries’ revenue and results of operations. |
| | ● | If the PRC subsidiaries fail to identify, acquire and develop other products, they may be unable to grow their business. |
| | ● | If the PRC subsidiaries are not able to implement their strategies to achieve their business objectives, their business operations and financial performance will be adversely affected. |
| | ● | If the PRC subsidiaries fail to adopt new technologies to meet evolving customer needs or emerging industry standards, their business may be materially and adversely affected. |
| | ● | If the PRC subsidiaries sell medical devices to non-profit medical institutions through public bid invitation, their sales prices will be limited based on the results of the bidding. |
| | ● | Changes to the PRC subsidiaries’ payment terms with both customers and suppliers may materially and adversely affect their operating cash flows. |
| | ● | Our success depends on our management team and other key personnel, the loss of any of whom could disrupt our operations. |
| | ● | The PRC subsidiaries may not be able to adequately protect and maintain their intellectual property. |
| | ● | The PRC subsidiaries’ introduction of new technologies and products may increase the likelihood that third parties will assert claims that the PRC subsidiaries’ products infringe upon their proprietary rights. |
| | ● | The PRC subsidiaries may not be able to prevent others from unauthorized use of their intellectual property, which could harm their business and competitive position and adversely affect the Group as a whole. |
| | ● | Economic recessions could have a significant, adverse impact on the PRC subsidiaries’ business. |
| | ● | Changes in U.S. and international trade policies, particularly with regard to China, may adversely impact the PRC subsidiaries’ business and operating results, which may adversely affect the Group as a whole. |
| | ● | The PRC subsidiaries do not have any product liability, business interruption, or property insurance and they may incur liabilities that are not covered by insurance, which could expose them to significant costs and business disruption. |
| | ● | If we do not obtain substantial additional financing, our ability to execute on our business plan as outlined in this annual report will be impaired. |
| | ● | Pandemics and epidemics, natural disasters, terrorist activities, political unrest, and other outbreaks could disrupt the PRC subsidiaries’ delivery and operations, which could materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations. |
| | ● | The PRC subsidiaries’ international sales are subject to a variety of risks that could adversely affect their profitability and operating results, which may adversely affect the profitability and operating results of the Group. |
| | ● | The PRC subsidiaries are subject to a variety of environmental laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws or if they are responsible for releases of contaminants to the environment, which could adversely affect the Group as a whole. |
| | ● | The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole. |
| | ● | The PRC subsidiaries are subject to a variety of construction laws, and they could incur liability if they fail to comply with such laws, which could adversely affect their operations. |
| | ● | The PRC subsidiaries’ rights to use their leased driveways, warehouses, and a parking area could be challenged by governmental authorities, due to the lessor’s failure to obtain the property ownership certificate as required by law, and their rights to use the leased collectively managed construction land could be challenged by governmental authorities, due to the lessor’s failure to comply with legal procedures related to land lease. Failure to comply with administrative or regulatory requirements with respect to property leased by the PRC subsidiaries may disrupt their usage and occupancy rights and could result in penalties and dispossession from such properties, which may disrupt their operations. |
| | ● | If the PRC subsidiaries fail to timely renew their medical device licenses or registration certificates, it could adversely affect the PRC subsidiaries’ and our reputation, financial condition and results of operations. |
| | ● | The PRC subsidiaries have entered into a number of related party transactions in the ordinary course of their business, and may continue to enter into related party transactions in the future. |
| | ● | If our PRC subsidiaries cannot collect timely payments from their customers, their business and operations, and the Group’s financial condition and results of operations may be materially and adversely affected. |
| | ● | Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors. |
| | ● | Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers who are critical and necessary to the production of our PRC subsidiaries’ products and our PRC subsidiaries’ ability to create relationships with new suppliers. |
Risks Related to Doing Business in China (for a more detailed discussion, see “Item 3. Key Information—D. Risk Factors — Risks Related to Doing Business in China”)
We face risks and uncertainties relating to doing business in the PRC in general, including, but not limited to, the following:
| | ● | Changes in the political and economic policies of the PRC government or in relations between China and the United States may materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations and may result in the PRC subsidiaries’ inability to sustain their growth and expansion strategies. |
| | ● | There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations. |
| | ● | The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and subsequent securities offerings at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless. |
| | ● | Additional compliance procedures may be required in connection with our subsequent securities offerings, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless. |
| | ● | You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our directors and officers named in the annual report based on foreign laws. |
| | ● | You may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management named in this annual report based on Hong Kong laws. |
| | ● | Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay our securities offerings and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for our securities offerings and affect our ability to offer or continue to offer securities to investors outside China. |
| | ● | PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for the PRC subsidiaries to pursue growth through acquisitions. |
| | ● | PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or the PRC subsidiaries to liability or penalties, limit our ability to inject capital into the PRC subsidiaries or limit the PRC subsidiaries’ ability to increase their registered capital or distribute profits. |
| | ● | PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or the PRC subsidiaries to liability or penalties, limit our ability to inject capital into the PRC subsidiaries or limit the PRC subsidiaries’ ability to increase their registered capital or distribute profits. |
| | ● | PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of our IPO and subsequent securities offerings to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business. |
| | ● | We rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries to fund offshore cash and financing requirements and any limitation on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities. |
| | ● | We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income. |
| | ● | Dividends payable to our foreign investors and gains on the sale of our Ordinary Shares by our foreign investors may be subject to PRC tax. |
| | ● | We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies. |
| | ● | Restrictions on currency exchange may limit our ability to utilize our revenue effectively. |
| | ● | Fluctuations in exchange rates could result in foreign currency exchange losses to us and may reduce the value of, and amount in U.S. Dollars of dividends payable on, our shares in foreign currency terms. |
| | ● | Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties. |
| | ● | Our Ordinary Shares may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two. |
| | ● | The enactment of Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region and Hong Kong Autonomy Act of U.S. could impact our Hong Kong holding subsidiary. |
Risks Relating to Our Ordinary Shares and the Trading Market (for a more detailed discussion, see “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Ordinary Shares and the Trading Market”)
In addition to the risks described above, we are subject to general risks and uncertainties relating to our Ordinary Shares and the trading market, including, but not limited to, the following:
| | ● | Our share price may be volatile and could decline substantially, which could result in substantial losses to our investors. |
| | | |
| | ● | We may issue additional Ordinary Shares or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our Ordinary Shares. |
| | ● | We currently do not expect to pay dividends on our Ordinary Shares in the foreseeable future. |
| | ● | If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about us or our business, our Ordinary Share price and trading volume could decline. |
| | ● | Our Chief Operating Officer and Liwei Zhang have substantial influence over our Company. Their interests may not be aligned with the interests of our other shareholders, and they could prevent or cause a change of control or other transactions. |
| | ● | A sale or perceived sale of a substantial number of our Ordinary Shares may cause the price of our Ordinary Shares to decline. |
| | ● | We incur substantial increased costs being a public company. |
| | ● | There can be no assurance that we will not be a passive foreign investment company (“PFIC”) for United States federal income tax purposes for any taxable year, which could subject United States holders of our Ordinary Shares could be subject to adverse United States federal income tax consequences. |
| | ● | For as long as we are an emerging growth company, we will not be required to comply with certain reporting requirements, including those relating to accounting standards and disclosure about our executive compensation, that apply to other public companies. |
| | ● | Our ability to produce accurate financial statements have been materially adversely affected by our failure to establish proper internal financial reporting controls. If we fail to establish and maintain proper internal financial reporting controls in a reasonably timely manner, our ability to produce accurate financial statements or comply with applicable regulations may continue to be impaired. |
| | ● | As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders. |
| | ● | As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards. |
| | ● | We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses. |
| | ● | The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States. |
| | ● | You may be unable to present certain proposals before annual general meetings or extraordinary general meetings not called by shareholders. |
| | ● | The obligation to disclose information publicly may put us at a disadvantage to competitors that are private companies. |
| | ● | Our shareholders may be held liable for claims by third parties against us to the extent of distributions received by them upon redemption of their shares. |
| | ● | Our board of directors may decline to register transfers of Ordinary Shares in certain circumstances. Certain provisions in our amended and restated articles of association may discourage, delay, or prevent a change in control. |
Risks Related to the PRC Subsidiaries’ Business and Industry
The PRC subsidiaries’ operating history may not be indicative of our future growth or financial results and the PRC subsidiaries may not be able to sustain their historical growth rates.
The PRC subsidiaries’ operating history may not be indicative of their future growth or financial results. There is no assurance that the PRC subsidiaries will be able to grow their revenue in future periods. Their growth rates may decline for any number of possible reasons, and some of them are beyond their control, including decreasing customer demand, increasing competition, declining growth of the medical device industry in general, emergence of alternative business models, or changes in government policies or general economic conditions. The PRC subsidiaries expect to continue to expand their sales network and product offerings to bring greater convenience to their customers and to increase their customer base and number of transactions. However, the execution of their expansion plan is subject to uncertainty and the total number of items sold and number of transacting customers may not grow at the rate they expect for the reasons stated above. If their growth rates decline, investors’ perceptions of their business and prospects may be adversely affected and the market price of our Ordinary Shares could decline.
Failure to maintain the quality and safety of the PRC subsidiaries’ products could have a material and adverse effect on the PRC subsidiaries’ and our reputation, financial condition and results of operations.
The quality and safety of the PRC subsidiaries’ products, whether self-manufactured or out-sourced, are critical to our success. As a medical device manufacturer with a history of over 20 years, quality and safety are always the core values, as medical devices are directly used for the human body and thus essential to the human health. The PRC subsidiaries pay close attention to quality control, monitoring each step in the process from procurement to production and from warehouse to delivery. Yet, maintaining consistent product quality depends significantly on the effectiveness of their quality control system, which in turn depends on a number of factors, including, but not limited to the design of a quality control system, employee training to ensure that employees adhere to and implement the quality control policies and procedures and the effectiveness of monitoring any potential violation of their quality control policies and procedures. Despite such quality control management system, the PRC subsidiaries cannot eliminate the risks of errors, defects or failures. They may fail to detect or cure defects as a result of a number of factors, many of which are outside their control. Please refer to “Item 4. Information on the Company—B. Business Overview—Quality Control” for more information.
In addition, the quality of the products or services provided by the PRC subsidiaries’ suppliers or business partners is subject to factors beyond the PRC subsidiaries’ control, including the effectiveness and the efficiency of their quality control system, among others. There can be no assurance that the PRC subsidiaries’ suppliers or business partners may always be able to adopt appropriate quality control systems and meet the PRC subsidiaries’ stringent quality control requirements in respect of the products or services they provide. Any failure of suppliers or business partners to provide satisfactory products or services could harm the PRC subsidiaries’ reputation and adversely impact the PRC subsidiaries’ operations. In addition, the PRC subsidiaries may be unable to receive sufficient compensation from suppliers and business partners for the losses caused by them.
As of the date of this annual report, the PRC subsidiaries are unaware of material quality accidents.
The PRC subsidiaries may experience significant liability claims or complaints from customers, doctors and patients, litigation and regulatory investigations and proceedings, such as claiming in relation to medical device safety, or adverse publicity involving their products, which could adversely affect the PRC subsidiaries’ and our financial condition and results of operations.
The PRC subsidiaries face an inherent risk of liability claims or complaints from customers, doctors and patients. The PRC subsidiaries take those complaints and claims seriously and endeavor to reduce such complaints by implementing various remedial measures. Nevertheless, we cannot assure you that they can successfully prevent or address all complaints.
Any complaints or claims against the PRC subsidiaries, even if meritless and unsuccessful, may divert management attention and other resources from the PRC subsidiaries’ business and adversely affect their business and operations. Customers may lose confidence in the PRC subsidiaries and their brands, which may adversely affect their business and results of operations. Furthermore, negative publicity including but not limited to negative online reviews on social media and crowd-sourced review platforms, industry findings or media reports related to medical device quality and safety, public health concerns, illness, injuries, whether or not accurate, and whether or not concerning the PRC subsidiaries’ products, can adversely affect the PRC subsidiaries’ business, results of operations and reputation.
The PRC subsidiaries face potential liability, expenses for legal claims, and harm due to the nature of their business. For example, customers could assert legal claims against them in connection with personal injuries or illness related to the use of medical devices they sell. The PRC government, media outlets and public advocacy groups have been increasingly focused on customer protection in recent years. Selling defective products may expose the PRC subsidiaries to liabilities associated with customer protection laws. The PRC subsidiaries may be responsible for compensation for a customer’s loss even if personal injuries or illness are not caused by them. Thus, they may also be held liable if their suppliers or other business partners fail to comply with applicable product quality and safety related rules and regulations. Though they can ask the responsible parties for indemnity after that, their reputation could still be adversely affected.
The PRC subsidiaries may face additional exposure to claims and lawsuits. These claims could divert management time and attention away from their business and result in significant costs to investigate and defend, regardless of the merits of the claims. In some instances, they may elect or be forced to pay substantial damages if they are unsuccessful in their efforts to defend against these claims, which could harm their business, financial condition, and results of operations. In addition, the PRC subsidiaries’ directors, management and employees may from time to time be subject to litigation and regulatory investigations and proceedings or otherwise face potential liability and expense in relation to medical device quality and safety, commercial, labor, employment, securities or other matters, which could adversely affect their and respective reputations and the Group’s financial condition and results of operations.
Moreover, the PRC subsidiaries’ products may be subject to recall by competent authorities if they fail to comply with certain quality requirements. Recalls may be accompanied by claims or lawsuits for breaches of contract, for the inability to provide products of a certain quality on time, for which the PRC subsidiaries may elect or be forced to pay substantial damages if they are unsuccessful in their efforts to defend against these claims or lawsuits, and thus, could harm their business, and the Group’s financial condition and results of operations.
On November 16, 2020, Hangzhou Shanyou was required to recall 20,000 disposable medical masks by Heilongjiang Provincial Drug Administration, because the mask strings failed to comply with an elastic requirement.
The PRC subsidiaries have not, as of the date of this annual report, been notified that they will be subject to any punishment, nor are they aware of any risk, currently or in the foreseeable future, regarding these recalls by the relevant government authorities, however, there is no assurance that the relevant government authorities will not later determine that a basis does exist for punishment with respect to recalled products.
As of the date of this annual report, the PRC subsidiaries are not aware of any warning, investigations, prosecutions, disputes, claims or other proceedings in respect of customer protection rights or breaches of contract, nor have they been punished or can foresee any punishment to be made by any government authorities of the PRC or any in any overseas jurisdiction. However, there is no assurance that such entities will not find a basis in the future for investigations, prosecutions, disputes, claims or other proceedings with respect to customer protection rights or breaches of contract with respect to the PRC subsidiaries’ products, which could adversely harm their business, and the Group’s financial condition and results of operations.
The decreased demand for masks and in the unit price of masks could reduce our revenue, which could adversely affect the PRC subsidiaries’ and our results of operations.
Since China lifted travel restrictions and quarantine requirements in December 2022, the demand for masks and the sales volume and unit price of masks substantially decreased. With the reduced demand for masks and unit price of masks, our net revenue from sales of masks decreased from $10,619,035, which accounted for approximately 53.87% of our total net revenue, for the fiscal year ended September 30, 2022, to $5,091,331, or approximately 37.53% of our total net revenue, for the fiscal year ended September 30, 2023. Furthermore, our net revenue from sales of masks decreased to $1,559,750, or approximately 13.56% of our total net revenue, for the fiscal year ended September 30, 2024.
While we do not expect this trend to continue in future financial periods, we estimate that the demand and unit price of masks have since stabilized. To mitigate these adverse effects, we have been strengthening our marketing efforts, focusing on medical devices other than masks. Nevertheless, if such trend continues, it could further reduce our revenue from sales of masks, thereby decreasing our total revenue, which could adversely affect the PRC subsidiaries’, the Group’s financial condition and our results of operations.
The PRC subsidiaries face the risk of fluctuations in the cost, availability and quality of their raw materials, which could adversely affect their results of operations, and thus, adversely affect the Group as a whole.
The cost, availability and quality of the PRC subsidiaries’ principal raw materials, such as meltblown, non-woven fabrics, steel spring, OPP membrane, and methyl silicone oil, are important to their operations. If the cost of raw materials increases due to policy changes, large market price fluctuation or any other reason, the business and results of operations of the PRC subsidiaries could be adversely affected, as well as the Group’s financial condition and results of operations.
Lack of availability of raw materials, whether due to shortages in supply, delays or interruptions in processing, failure of timely delivery or otherwise, could interrupt the PRC subsidiaries’ operations and adversely affect their financial results, which could adversely affect the Group as a whole.
Defective raw materials could subject the PRC subsidiaries to product liability claims or legal actions, which could adversely affect their and the Group’s financial condition and results of operations.
A significant interruption in the operations of the PRC subsidiaries’ third-party suppliers and other business partners could potentially disrupt their operations.
The PRC subsidiaries have limited control over the operations of their third-party suppliers and other business partners and any significant interruption in their operations may have an adverse impact on the PRC subsidiaries’ operations. For example, a significant interruption in the operations of their supplier’s manufacturing facilities could cause delay or termination of shipment of the raw materials to them, which may cause delay or termination of shipment of ordered products to their customers, resulting in damage to their customer relationships. If the PRC subsidiaries are unable to adequately address the impact of the interruptions of operations of their third-party suppliers, their business operations and financial results may be materially and adversely affected, and the Group’s financial condition and results of operations may be materially and adversely affected accordingly.
Although the PRC subsidiaries believe that they could establish alternate sources from other suppliers for most of their raw materials, any delay in locating and establishing relationships with other sources could result in shortages or back orders for such raw materials. There can be no assurance that such replacement suppliers will provide the raw materials that are needed by the PRC subsidiaries in the quantities that they request or at the prices that they are willing to pay. Any shortage in quantities or increase in prices could adversely affect the PRC subsidiaries’ and the Group’s financial condition and results of operations.
Moreover, in February 2022, the Russian Federation and Belarus commenced a military action with the country of Ukraine. As a result of this action, various nations, including the United States, have instituted economic sanctions against the Russian Federation and Belarus. Additionally, on October 7, 2023, Hamas, a U.S. designated terrorist organization, launched a series of coordinated attacks from the Gaza Strip onto Israel. On October 8, 2023, Israel formally declared war on Hamas, and the armed conflict is ongoing as of the date of this annual report. Since the PRC subsidiaries’ raw and auxiliary materials are purchased from certified and qualified suppliers in China, their supply has been very stable for many years and are easily sourced due to the PRC subsidiaries’ geographical locations. The prices of raw materials used in the PRC subsidiaries’ operations are not volatile and they, along with the PRC subsidiaries’ supply chain, have not been impacted by the recent conflict between Russia and Ukraine or the war between Isarel and Hamas. However, the impact of these actions and related sanctions on the world economy and the specific impact on the Company’s financial condition, results of operations and cash flows are not determinable as of the date of this annual report.
The PRC subsidiaries do not have long-term contracts with their suppliers and the suppliers can reduce order quantities or terminate sales to the PRC subsidiaries at any time.
The PRC subsidiaries do not have long-term contracts with their suppliers. At any time, their suppliers can reduce the quantities of products that sell to the PRC subsidiaries, or cease selling products to the PRC subsidiaries altogether. Such reductions or terminations could have a material adverse impact on the PRC subsidiaries’ revenue, profits and financial condition, which may also adversely affect the Group as a whole.
Overall tightening of the labor market, increases in labor costs or any possible labor unrest may adversely affect the PRC subsidiaries’ business and results of operations, which may also adversely affect the Group as a whole.
The PRC subsidiaries’ business requires a substantial number of personnel. Any failure to retain stable and dedicated labor by them may lead to disruption to their business operations. Although they have not experienced any labor shortages as of the date of this annual report, they have observed an overall tightening and increasingly competitive labor market. The PRC subsidiaries have experienced, and expect to continue to experience, increases in labor costs due to increases in salary, social benefits, and employee headcount. The PRC subsidiaries compete with other companies in their industry and other labor-intensive industries for labor, and they may not be able to offer competitive remuneration and benefits compared to their competitors. If they are unable to manage and control their labor costs, their business, financial condition, and results of operations, along with the Group’s financial condition and results of operations may be materially and adversely affected.
The PRC subsidiaries’ industry is intensely competitive. The PRC subsidiaries may face competition from, and they may be unable to compete successfully against, new entrants and established companies with greater resources.
The medical device industry is intensely competitive and includes thousands of companies, both domestically and internationally. As more medical device companies seek to outsource more of the design, prototyping and manufacturing of their products, the PRC subsidiaries will face increasing competitive pressures to grow their business in order to maintain their competitive position, and they may encounter competition from, and lose customers to, other companies with design, technological and manufacturing capabilities similar to theirs. Some of their potential competitors may have greater name recognition, greater operating revenue, larger customer bases, longer customer relationships and greater financial, technical, personnel and marketing resources than they have. If they are unsuccessful competing with their competitors for their existing and prospective customers’ business, their financial condition and results of operation may be adversely affected, and the Group’s financial condition and results of operation may be accordingly adversely affected.
Furthermore, increased competition may reduce the PRC subsidiaries’ market share and profitability and require them to increase their sales and marketing efforts and capital commitment in the future, which could negatively affect their results of operations or force them to incur further losses, which could also adversely affect the Group as a whole. Although the PRC subsidiaries have continuously grown their customer base, there is no assurance that they will be able to continue to do so in the future against current or future competitors, and such competitive pressures may have a material adverse effect on their and the Group’s business, financial condition and results of operations.
The continuing development of the PRC subsidiaries’ products depends upon the PRC subsidiaries’ maintaining strong working relationships with their customers, distributors and sales agents.
The research, development, marketing and sale of the PRC subsidiaries’ current products and potential new and improved products or future product indications for which we receive regulatory clearance or approval depend upon the PRC subsidiaries’ maintaining working relationships with their customers, distributors and sales agents. See “Item 4. Information on the Company—B. Business Overview—Research and Development.” The PRC subsidiaries rely on those professionals to provide them with considerable knowledge and experience regarding the research, development, marketing and sale of their products. Distributors and sales agents assist them in marketing and sales, as well as collecting customers’ feedback and advice related to their products. Researchers at hospital and medical institution customers keep them informed of the latest requirements and research and development (“R&D”) results. If the PRC subsidiaries cannot maintain strong working relationships with these professionals and continue to receive their advice and input, the development, improvement and marketing of the PRC subsidiaries’ products could suffer, which could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition and results of operations.
Technological changes may adversely affect sales of the PRC subsidiaries’ products and may cause their products to become obsolete.
The medical device market is characterized by extensive research and development and rapid technological changes. Technological progress or new developments in the industry could adversely affect sales of the PRC subsidiaries’ products. Products could be rendered obsolete because of future innovations by competitors or others, which would have a material adverse effect on the business, financial condition and results of operations of the PRC subsidiaries and the Group.
Consolidation in the medical device industry could have an adverse effect on the PRC subsidiaries’ revenue and results of operations.
Many medical device companies are consolidating to create new companies with greater market power. As the medical device industry consolidates, competition to provide goods and services to industry participants will become more intense. These industry participants may try to use their market power to negotiate price concessions or reductions for the PRC subsidiaries’ products. If the PRC subsidiaries reduce their prices because of consolidation in the healthcare industry, their revenue would decrease, which could have a material adverse effect on their and our business, financial condition and results of operations.
If the PRC subsidiaries fail to identify, acquire and develop other products, they may be unable to grow their business.
As a significant part of their growth strategy, the PRC subsidiaries intend to develop and commercialize additional products through their research and development program or by acquiring additional technologies and patents from third parties. The success of this strategy depends upon their ability to identify, select and acquire the technologies and patents on terms that are acceptable to them.
Any patents and technology the PRC subsidiaries identify or acquire may require additional development efforts prior to commercial manufacturing and sale, including approval or clearance by the applicable regulatory authorities. All products are prone to the risks of failure inherent in medical device product development, including the possibility that the product will not be shown to be sufficiently safe and effective for approval or clearance by regulatory authorities. In addition, we cannot assure you that any such products that are approved or cleared will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace.
If the PRC subsidiaries are unable to develop suitable potential products through internal research programs or by obtaining patents or technologies from third parties, it could have a material adverse effect on the PRC subsidiaries and our business, financial condition and results of operations.
If the PRC subsidiaries are not able to implement their strategies to achieve their business objectives, their business operations and financial performance will be adversely affected.
The PRC subsidiaries’ business plan and growth strategy are based on currently prevailing circumstances and the assumption that certain circumstances will or will not occur, as well as the inherent risks and uncertainties involved in various stages of development. However, there is no assurance that the PRC subsidiaries will be successful in implementing their strategies or that their strategies, even if implemented, will lead to the successful achievement of their objectives. If they are not able to successfully implement their strategies, their business operations and financial performance will be adversely affected, which will adversely affect the Group as a whole.
If the PRC subsidiaries fail to adopt new technologies to meet evolving customer needs or emerging industry standards, their business may be materially and adversely affected.
To remain competitive, the PRC subsidiaries must continue to stay abreast of the constantly evolving industry trends and to enhance and improve their technology accordingly. Success will depend, in part, on the ability of the PRC subsidiaries to identify, develop or acquire leading technologies useful in their business. There can be no assurance that they will be able to use new technologies effectively or meet customer’s requirements. If they are unable to adapt in a cost-effective and timely manner in response to changing market conditions or customer preferences, whether for technical, legal, financial or other reasons, their business may be materially and adversely affected, and may adversely affect the Group’s financial condition and results of operations.
If the PRC subsidiaries sell medical devices to non-profit medical institutions through public bid invitation, their sales prices will be limited based on the results of the bidding.
In China, the procurement of medical devices by non-profit medical institutions is subject to specific procurement procedures. According to relevant laws and regulations, all non-profit medical institutions under all levels of government and state-owned enterprises from different industries shall participate in the centralized procurement of medical devices. The centralized procurement of medical devices must follow the basic principles of openness, fairness, impartiality and good faith, and the procurement method is mainly based on public bid invitation. Therefore, if the PRC subsidiaries sell medical devices to non-profit medical institutions through public bid invitation, their sales prices will be limited based on the results of the bidding, which may reduce their profitability, which result would adversely impact the profitability of the Group.
Changes to the PRC subsidiaries’ payment terms with both customers and suppliers may materially and adversely affect their operating cash flows.
The PRC subsidiaries may experience significant pressure from their suppliers to reduce the number of days of their accounts payable. At the same time, the PRC subsidiaries may experience pressure from their customers to extend the number of days before paying their accounts receivable. Any failure to manage their accounts payable and accounts receivable may have a material adverse effect on their and our business, financial condition and results of operations.
Our success depends on our management team and other key personnel, the loss of any of whom could disrupt our operations.
Our future success will depend in substantial part on the continued service of the members of our senior management, in particular, those identified under the section titled “Management.” The loss of the services of one or more of our key personnel could impede implementation of our business plan and result in reduced profitability. We do not carry key person life insurance on any of our officers or employees. Our future success will also depend on the PRC subsidiaries’ continued ability to attract, retain and motivate highly qualified technical sales and marketing customer support. Because of the rapid growth of the economy in China, competition for qualified personnel is intense. We cannot assure you that we will be able to retain our key personnel or that we will be able to attract, assimilate or retain qualified personnel in the future. Any loss of key personnel or failure to attract, assimilate or retain qualified personnel could disrupt our operations.
The PRC subsidiaries may not be able to adequately protect and maintain their intellectual property.
Our success will depend on the PRC subsidiaries’ ability to continue to develop and market their products. The PRC subsidiaries have been granted 30 patents in mainland China relating to their products and have 13 pending patent applications. No assurance can be given that such patents will not be challenged, invalidated, infringed or circumvented, or that such intellectual property rights will provide a competitive advantage to the PRC subsidiaries. Also, litigation may be necessary to enforce their intellectual property rights or determine the validity and scope of the proprietary rights of others.
The outcome of such potential litigation may not be favorable and any success in litigation may not be able to adequately protect their rights. Such litigation may be costly and divert management attention away from the PRC subsidiaries’ business. An adverse determination in any such litigation would impair their intellectual property rights and may harm their business, prospects and reputation, as well as the business and prospects of the Group. Enforcement of judgments in China is uncertain and even if they are successful in such litigation, it may not provide them with an effective remedy.
The PRC subsidiaries’ introduction of new technologies and products may increase the likelihood that third parties will assert claims that the PRC subsidiaries’ products infringe upon their proprietary rights.
The rapid technological changes that characterize our industry require that the PRC subsidiaries quickly implement new processes and components with respect to their products. Often with respect to recently developed processes and components, a degree of uncertainty exists as to who may rightfully claim ownership rights in such processes and components. Uncertainty of this type increases the risk that claims alleging that such components or processes infringe upon third party rights may be brought against the PRC subsidiaries. Although Hangzhou Shanyou takes, and will continue to take, steps to ensure that its new products do not infringe upon third party rights, if its products or manufacturing processes are found to infringe upon third party rights, it may be subject to significant liabilities and be required to change its manufacturing processes or be prohibited from manufacturing certain products, which could have a material adverse effect on its and our operations and financial condition, as well as the Group as a whole.
The PRC subsidiaries may be required to defend against charges of infringement of patent or other proprietary rights of third parties. Although patent and other intellectual property disputes in our industry have often been settled through licensing or similar arrangements, such defense could require the PRC subsidiaries to incur substantial expense and to divert significant resources of their technical and management personnel and could result in their loss of rights to develop or make certain products or require them to pay monetary damages or royalties to license proprietary rights from third parties. Furthermore, we cannot be certain that the necessary licenses would be available to the PRC subsidiaries on acceptable terms, if at all. Accordingly, an adverse determination in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent the PRC subsidiaries from manufacturing and selling certain of their products. Any such litigation, whether successful or unsuccessful, could result in substantial costs to the PRC subsidiaries and diversions of their resources, either of which could adversely affect their business, and the financial condition and results of operations of the Group.
The PRC subsidiaries may not be able to prevent others from unauthorized use of their intellectual property, which could harm their business and competitive position and adversely affect the Group as a whole.
The PRC subsidiaries rely on a combination of trademark, fair trade practice, patent, copyright and trade secret protection laws in China, as well as confidentiality procedures, to protect their intellectual property rights. The PRC subsidiaries are careful to remain current in their annual patent fee payments. They regard their trademark, patents, know-how, proprietary technologies, and similar intellectual property as critical to our success. The PRC subsidiaries may become an attractive target to intellectual property attacks in the future with the increasing recognition of their brands. Any of their intellectual property rights could be challenged, invalidated, circumvented or misappropriated, or such intellectual property may not be sufficient to provide them with competitive advantages. In addition, there can be no assurance that (i) all of their intellectual property rights will be adequately protected, or (ii) their intellectual property rights will not be challenged by third parties or found by a judicial authority to be invalid or unenforceable. Intellectual property protection may not be sufficient in China. Accordingly, the PRC subsidiaries may not be able to effectively protect their intellectual property rights or to enforce their contractual rights in China. In addition, policing any unauthorized use of their intellectual property is difficult, time-consuming and costly and the steps taken may be inadequate to prevent the misappropriation of their intellectual property. In the event that the PRC subsidiaries resort to litigation to enforce their intellectual property rights, such litigation could result in substantial costs and a diversion of their managerial and financial resources. We can provide no assurance that the PRC subsidiaries will prevail in such litigation. In addition, their trade secrets may be leaked or otherwise become available to, or be independently discovered by, their competitors. Any failure in protecting or enforcing their intellectual property rights could have a material adverse effect on their and our business, financial condition and results of operations.
Economic recessions could have a significant, adverse impact on the PRC subsidiaries’ business.
Our revenue is generated from sales of medical devices, both domestically and internationally, and we anticipate that revenue from such sales will continue to represent the substantial portion of our total revenue in the near future. The PRC subsidiaries’ sales and earnings can also be affected by changes in the general economy.
The medical device industry historically has experienced cyclical fluctuations in financial results due to economic recessions, downturns in business cycles of the PRC subsidiaries’ customers, interest rate fluctuations, and other economic factors beyond the PRC subsidiaries’ control. Deterioration in the economic environment subjects their business to various risks, which may have a material and adverse impact on their operating results, causes them not to reach their long-term growth goals, and, thus, adversely affects the Group as a whole. For example, a downturn in the economy could directly affect the discretionary spending power of their customers and in turn, depress the number of orders for their products.
Changes in U.S. and international trade policies, particularly with regard to China, may adversely impact the PRC subsidiaries’ business and operating results, which may adversely affect the Group as a whole.
The U.S. government has recently made statements and taken certain actions that may lead to potential changes to the U.S. and international trade policies, including recently-imposed tariffs affecting certain products manufactured in China. It is unknown whether and to what extent new tariffs (or other new laws or regulations) will be adopted, or the effect that any such actions would have on the PRC subsidiaries or the industry and customers. Although cross-border business currently contributes a small portion of the PRC subsidiaries’ business, as they continue to sell their products internationally in the future, any unfavorable government policies on international trade, such as capital controls or tariffs, may affect the demand for their products and services, impact the competitive position of their products or prevent them from being able to sell products in certain countries. If any new tariffs, legislation and/or regulations are implemented, or if existing trade agreements are renegotiated or, in particular, if the U.S. government takes retaliatory trade actions due to the recent U.S.-China trade tension, such changes could have an adverse effect on the PRC subsidiaries’ and the Group’s financial condition and results of operations.
The PRC subsidiaries do not have any product liability, business interruption, or property insurance and they may incur liabilities that are not covered by insurance, which could expose them to significant costs and business disruption.
The PRC subsidiaries do not maintain product liability, business interruption or property insurance. Moreover, they do not carry any key-man life insurance of any variety. The PRC subsidiaries provide social security insurance, including pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan for their employees.
In the event the PRC subsidiaries were to incur substantial losses or liabilities due to fire, explosions, floods, other natural disasters or accidents, or any product liability claims or business interruption, their results of operations would be materially and adversely affected. Any such occurrences could have an adverse effect on the PRC subsidiaries and the Group’s financial condition and results of operations.
If we do not obtain substantial additional financing, our ability to execute on our business plan as outlined in this annual report will be impaired.
Our plans for business expansion and development are dependent upon our raising significant additional capital. Our plans call for significant new investments in research and development, marketing, expanded productions capacity, and working capital for raw materials and other items. Management estimates that our capital needs for expansion will be approximately $30 million. Although we expect the proceeds of our IPO and our net earnings to substantially fund our planned growth and development, our management will be required to properly and carefully administer and allocate these funds. Should our capital needs be higher than estimated, or should additional capital be required after the close of our IPO, we will be required to seek additional investments, loans, or debt financing, or conduct subsequent securities offerings to fully pursue our business plans. Such additional investment may not be available to us on terms which are favorable or acceptable, and we cannot guarantee you that the proceeds of a subsequent security offering will adequately meet our capital needs. Should we be unable to meet our full capital needs, our ability to fully implement our business plan will be impaired.
Pandemics and epidemics, natural disasters, terrorist activities, political unrest, and other outbreaks could disrupt the PRC subsidiaries’ delivery and operations, which could materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
Global pandemics, epidemics in China or elsewhere in the world, or fear of spread of contagious diseases, such as Ebola virus disease (EVD), coronavirus disease 2019 (COVID-19), Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), H1N1 flu, H7N9 flu, and avian flu, as well as hurricanes, earthquakes, tsunamis, or other natural disasters could disrupt the PRC subsidiaries’ business operations, reduce or restrict their supply of products and services, incur significant costs to protect their employees and facilities, or result in regional or global economic distress, which may materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations. Actual or threatened war, terrorist activities, political unrest, civil strife, and other geopolitical uncertainty could have a similar adverse effect on the PRC subsidiaries’ and our business, financial condition, and results of operations. Any one or more of these events may impede the PRC subsidiaries’ production and delivery efforts and adversely affect their sales results, or even for a prolonged period of time, which could materially and adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
Beginning in 2020, the COVID-19 pandemic resulted in quarantines, travel restrictions, and temporary closure of stores and facilities in China and elsewhere. China lifted its travel restrictions and quarantine requirements in December 2022. The extent of the impact of COVID-19 on the Company’s future financial results will be dependent on future developments, such as the length and severity of any resurgence, future government actions in response to COVID-19 on the global economy and capital markets, among many other factors, all of which remain highly uncertain and unpredictable. Given this uncertainty, the Company is currently unable to quantify the expected impact of COVID-19 on its future operations, financial condition, liquidity, and results of operations.
The PRC subsidiaries are also vulnerable to natural disasters and other calamities. We cannot assure you that the PRC subsidiaries are adequately protected from the effects of fire, floods, typhoons, earthquakes, power loss, telecommunications failures, break-ins, war, riots, terrorist attacks, or similar events. Any of the foregoing events may give rise to interruptions, damage to the PRC subsidiaries’ property, delays in production, breakdowns, system failures, technology platform failures, or internet failures, which could cause the loss or corruption of data or malfunctions of our manufacturing facilities, as well as adversely affect the PRC subsidiaries’ and our business, financial condition, and results of operations.
The PRC subsidiaries’ international sales are subject to a variety of risks that could adversely affect their profitability and operating results, which may adversely affect the profitability and operating results of the Group.
The PRC subsidiaries sell disposable medical devices internationally. For the fiscal year ended September 30, 2024, 2023, and 2022, their international sales accounted for 15%, 7%, and 7%, respectively, of our revenue. Although they take measures to minimize risks inherent to their international sales, the following risks may have a negative effect on their profitability and operating results, impair the performance of their foreign sales or otherwise disrupt their business, which may adversely affect the profitability and operating results of the Group:
| ● | Fluctuations in the value of currencies could cause exchange rates to change and impact their profitability; |
| ● | Greater difficulty in collecting accounts receivable and longer payment cycles, which can be more common in their international sales, could adversely impact their operating results over a particular fiscal period; |
| ● | According to the PRC regulations, enterprises that export medical devices are required to ensure that the exported medical devices meet the legal requirements of the importing country. The PRC subsidiaries strive to meet the legal requirements of medical devices of foreign jurisdictions, including with respect to product quality, however, changes in foreign regulations, export duties, taxation and limitations on imports or exports could increase their operational costs, impose fines or restrictions on their ability to carry on their business or expand their international sales. |
| ● | Loss of goods caused by long-distance sea transportation, extreme weather, third-party logistics companies, which could increase their operational costs; and |
| ● | No product liability insurance has been bought for their products, and thus, they may incur considerable damages if their costumers sue them for product defects and obtain favorable judgments. |
The PRC subsidiaries are subject to a variety of environmental laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws or if they are responsible for releases of contaminants to the environment, which could adversely affect the Group as a whole.
Our operating subsidiaries are all located in the PRC. Chinese laws impose various environmental controls on the management, handling, generation, manufacturing, transportation, storage, use and disposal of wastewater, solid waste, and other materials used or generated in the manufacturing of Hangzhou Shanyou’s products. If the PRC subsidiaries fail to comply with any present or future environmental laws, they could be subject to fines, corrective actions, other liabilities or the suspension of production, which could adversely affect the Group.
The PRC subsidiaries endeavor to adhere to environmental protection and governance requirements and have formulated a systematic environmental protection management system in the treatment of wastewater and solid waste in accordance with national requirements. However, changes in environmental laws may result in costly compliance requirements or otherwise subject them to future liabilities.
In particular, with the further expansion of Hangzhou Shanyou’s production capacity, its pollutant emissions will increase, resulting in the increase of the environmental protection spending and the difficulty of environmental protection management, which could have an adverse effect on the PRC subsidiaries’ and the Group’s financial condition and results of operations.
With respect to one construction project, Hangzhou Shanyou failed to file the required Environmental Impact Registration Form for the records. According to the PRC laws and regulations, Hangzhou Shanyou is required to file the Environmental Impact Registration Form of construction project for the records. Its failure to meet such requirement may result in a penalty of up to RMB50,000 imposed by local authorities, which it does not expect will have a material impact on its and our business, financial condition and results of operations.
Moreover, Hangzhou Shanyou failed to obtain the relevant authorities’ approval of an Environmental Impact Statement before construction of facilities for its production lines, and failed to inspect the production lines to determine if they complied with relevant regulations of environmental protection and did not make the Inspection and Acceptance Reports of Environmental Protection upon completion of such construction. According to PRC laws and regulations, before construction, Hangzhou Shanyou was required to obtain the approval of the relevant authorities after reviewing its Environmental Impact Statement. Upon the completion of construction, it is required to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection. Hangzhou Shanyou’s failure to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection may result in an order to complete relevant procedures, and a penalty of between RMB0.2 million and RMB1 million, which may be imposed by local authorities. If Hangzhou Shanyou fails to complete relevant procedures within the stipulated period, the local authorities may impose a penalty of between RMB1 million and RMB2 million. Furthermore, if material environmental pollution or ecological damage is caused by such failure, Hangzhou Shanyou may be ordered to stop production or stop using the affected production lines by the PRC regulatory authorities. Moreover, its non-compliance in failing to inspect its production lines and making Inspection and Acceptance Reports of Environmental Protection may be recorded in Credibility Archive, a list that record companies’ non-compliance with environmental matters that can be accessed by the public, according to Interim Measures for Environmental Protection Acceptance of Completion of Construction Projects. Being recorded in Credibility Archive results in disclosure of such non-compliance to the public, which may adversely affect the reputation of Hangzhou Shanyou, and adversely affect the Group as a whole. As of the date of the annual report, Hangzhou Shanyou believes it has obtained the approval for its Environmental Impact Statement by the relevant authorities, and it has completed the procedures for the inspection of its production lines; however, despite the completion of the required procedures, there is no assurance that Hangzhou Shanyou will not be penalized for not completing the above procedures in a timely manner, and any penalty could adversely affect the reputation of Hangzhou Shanyou, which could adversely affect the business, financial condition and results of operations of the Group.
The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole.
Companies operating in China are required to comply with a variety of fire protection laws, such as having an examination of fire protection design, inspection and acceptance of fire protection, record-filing and random inspection of construction projects. According to relevant laws and regulations with respect to construction projects, construction projects are subject to inspection and acceptance for fire protection. For certain construction projects, such as Hangzhou Shanyou’s production lines, a project facility owner shall provide fire protection design drawings to the relevant authorities before commencing construction and prepare the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, and such authorities shall conduct a random inspection thereof. In case of failure to provide fire protection design drawings that meet the requirements of construction, the relevant authorities shall not issue a construction license. If a project facility owner places into service a construction project which was required to undergo a fire protection inspection and acceptance and the preparation of an Inspection and Acceptance Report of Fire Protection, but has not undergone such inspection and acceptance and failed to prepare an Inspection and Acceptance Report of Fire Protection, or has failed the inspection and acceptance, or failed to suspend the use of such construction project for which such project facility owner prepared the Inspection and Acceptance Report of Fire Protection for the records of the competent authorities but is later found to be unqualified in a random inspection, such project facility owner will be ordered to stop using the affected construction project, stop production or business operations and a fine of between RMB30,000 and RMB300,000 may be imposed by competent departments. If a project facility owner has undergone a fire protection inspection and acceptance and has already prepared an Inspection and Acceptance Report of Fire Protection but only fails to submit the report to the competent authorities for the records, such project facility owner will be subject to a fine of RMB5,000. Please see “Item 4. Information on the Company—B. Business Overview—Regulation—Fire Prevention Management” on page 88.
As of the date of this annual report, Hangzhou Shanyou, the PRC subsidiary that manages all production lines, has not provided fire protection design drawings before construction nor has it prepared the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, which may result in it being ordered to stop the use of its production lines and it being subject to a fine of between RMB30,000 and RMB300,000. If it makes up the inspection and acceptance and prepares Inspection and Acceptance Reports of Fire Protection but does not submit them to the competent authorities for the records, it will be subject to a fine of RMB5,000. Since all of its products are manufactured by operation of such production lines, any such development could materially and adversely affect the Group’s business, financial condition and results of operations.
The PRC subsidiaries are subject to a variety of construction laws, and they could incur liability if they fail to comply with such laws, which could adversely affect their operations.
According to relevant laws and regulations, any company engaged in the construction and decoration of various types of buildings and their attached facilities, including the installation of their supporting lines, pipelines and equipment, prior to the commencement of construction, should apply to the competent department of the local government for a construction license. Those who have not obtained the construction license shall be ordered by the license-issuing authority that has jurisdiction to stop the construction, to correct such lapse within a time limit, and pay a fine of between 1% to 2% of the contract price of the construction project. Furthermore, the project facility owner shall, within 15 days from the date of the completion and acceptance inspection of the construction project, submit the inspection and acceptance report to the competent administrative department of construction or other relevant departments for the record. If it fails to do so, it shall be ordered to make corrections and pay a fine of between RMB0.2 million to RMB0.5 million. Where the project facility is placed into service without being subject to completion inspection and acceptance, the project facility owner shall be ordered to make corrections and pay a fine of between 2% to 4% of the contract price of the project. If any loss is caused as a consequence of the failure to obtain the construction license, it shall also be liable for compensation.
As of the date of this annual report, as to the production lines of Hangzhou Shanyou, which were placed into service without having been subject to completion inspection and acceptance, it did not obtain a construction license prior to the commencement of construction nor undergone the completion inspection and acceptance nor did it prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities, therefore, Hangzhou Shanyou could be fined (i) an amount equal to 1% to 2% of the contract price of the production lines construction (approximately $16,500 to $33,000), for its failure to obtain a construction license prior to the commencement of construction, (ii) plus a fine of between RMB0.2 million to RMB0.5 million (approximately $28,000 to $70,000) for its failure to prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities and make adequate rectification, and (iii) a fine of between 2% to 4% of the contract price of the production lines construction (approximately $33,000 to $66,100), for its failure to complete inspection and acceptance before placing the production lines into service. As a result, the aggregate amount of potential fine could be between $77,500 to $169,100. While we anticipate that the possibility of being fined is relatively low, since Hangzhou Shanyou has not been subject to such fine historically, and the estimated maximum fine amount is relatively immaterial, being less than 2% of our consolidated revenue in the fiscal year ended September 30, 2024, we cannot assure you that the production lines will not be ordered to be suspended and rectified, which could affect Hangzhou Shanyou’s production, and therefore could materially and adversely affect both its and our business, financial condition and results of operations. Moreover, we cannot assure that the safety of the production lines has been adequately addressed, since they have not been subject to completion inspection and acceptance. If any personal injury or property loss is caused by the production lines, Hangzhou Shanyou would be liable for compensation, which could materially and adversely affect both its and our reputation, business, financial condition and results of operations.
The PRC subsidiaries’ rights to use their leased driveways, warehouses, and a parking area could be challenged by governmental authorities, due to the lessor’s failure to obtain the property ownership certificate as required by law, and their rights to use the leased collectively managed construction land could be challenged by governmental authorities, due to the lessor’s failure to comply with legal procedures related to land lease. Failure to comply with administrative or regulatory requirements with respect to property leased by the PRC subsidiaries may disrupt their usage and occupancy rights and could result in penalties and dispossession from such properties, which may disrupt their operations.
The PRC subsidiaries lease several properties for various operational purposes. Non-compliance with administrative or regulatory requirements by the lessors or our PRC subsidiaries regarding these rental properties could lead to disruptions in usage and occupancy rights, potential penalties, and dispossession from the rental properties, which may disrupt the PRC subsidiaries’ operations.
As of the date of this annual report, the PRC subsidiaries have leased three offices, two driveways, two warehouses, and a parking area. However, the property ownership certificates for the two driveways, two warehouses, and the parking area have not been provided by the lessors of such properties to the PRC subsidiaries. This poses a risk of these sites being identified as illegally constructed, or the PRC subsidiaries lacking the legal right to use these properties. If these properties are determined to have been illegally constructed and are ordered to be demolished, or the PRC subsidiaries are determined to lack the legal right to use these properties, the PRC subsidiaries may be at risk of not being able to continue to use such properties. Moreover, for those properties that the PRC subsidiaries are determined to lack the legal right to use, the leases of these properties may be invalidated or terminated due to challenges by entitled third parties, which may extinguish our PRC subsidiaries’ rights to continued usage and occupancy of these properties, and their operations may be disrupted.
Moreover, the land leased by the PRC subsidiaries for warehouses is classified as collectively managed construction land. Under PRC laws and regulations, the lease of such land, which is duly registered according to law, can be granted by the landowner to an entity, but requires approval from over two-thirds of the collective economic organization’s members or villagers. If this requirement is not met, competent authorities may mandate rectification within a prescribed time-frame, which would result in the termination of the lease and tenant’s dispossession from the leased property. As of the date of this annual report, the lessors of our PRC subsidiaries’ leased land have not furnished valid property ownership certificates or obtained the necessary consent from the required percentage of collective members or villagers. This non-compliance poses a risk that the lessors may be ordered to rectify the situation, which would terminate the lease and extinguish the PRC subsidiaries’ rights to continued usage of the leased land and their operations may be disrupted.
In addition, lease agreements of some of the PRC subsidiaries’ leased properties are not registered and filed with the competent PRC government authorities as required by applicable PRC laws and regulations. Pursuant to the PRC laws, such registration and filing shall be accompanied by valid property ownership certificates provided by the lessors of such properties, which have yet to be provided, as stated above. The PRC subsidiaries could face fines of up to RMB10,000 for each such unregistered property, should the PRC subsidiaries fail to rectify the non-compliance within the time-frame prescribed by the relevant authorities.
Although the PRC subsidiaries have not received any notices from the lessors or competent departments, we cannot assure you that the lessors will provide valid property ownership certificates, nor can we assure you that the properties mentioned above will not be determined to have been illegally constructed and be ordered to be demolished.
Additionally, we cannot guarantee that the lessors of such land will not be required to rectify any violations, nor can we assure that these lessors possess the legal right to enter into the lease agreements. If the PRC subsidiaries’ expectations are wrong, they may need to find new facilities for one or more of their needs, and bear additional costs. Any consequential loss of any of the leased properties mentioned above would require additional expense and divert management’s time and attention to find an alternative location, and would disrupt the PRC subsidiaries’ business operations, which could materially and adversely impact our business, financial condition, and results of operations.
If the PRC subsidiaries fail to timely renew their medical device licenses or registration certificates, it could adversely affect the PRC subsidiaries’ and our reputation, financial condition and results of operations.
As a medical device manufacturer with all of our operating subsidiaries located in the PRC, all of the PRC subsidiaries’ manufacturing and sales activities must comply with relevant laws and regulations of China and other countries. Pursuant to the Administrative Measures for the Registration and Record-filing of Medical Devices promulgated on August 26, 2021 and effective on October 1, 2021, as amended from time to time, Class I medical devices are subject to recordation administration with Class II and Class III medical devices subject to registration administration. The U.S. Food, Drug and Cosmetic Act stipulates that manufacturer from foreign country which intend to sell its products in the U.S. should register with the FDA and list its medical devices with FDA. The PRC subsidiaries are in the business of manufacturing and sales of Class I and II medical devices and sales of III medical devices. If they fail to timely record or register their medical devices, both their and our financial condition and results of operations will be adversely affected.
The PRC subsidiaries have entered into a number of related party transactions in the ordinary course of their business, and may continue to enter into related party transactions in the future.
In the ordinary course of the PRC subsidiaries’ business, they have entered into transactions with related parties. For the fiscal years ended September 30, 2024, 2023, and 2022, the total revenues of the Group were $11,506,440, $13,565,951, and $19,711,290, respectively, while the revenues generated by sales to related parties were $577,370, $1,064,336, and $212,975, respectively, which accounted for 5%, 8%, and 0.04% of the total revenues, respectively. For the fiscal years ended September 30, 2024, 2023, and 2022, the total borrowed money of the Group was $13,394,778, $9,107,082, and 7,248,367, respectively, while the money borrowed from related parties was $nil, $nil, and $nil, respectively, which accounted for 0%, 0%, and 0%, respectively.
Based on the foregoing analysis, we conclude that the PRC subsidiaries have not relied on a large number of related party transactions to conduct their ordinary business transactions. Nevertheless, past experience may not reflect the future. We cannot guarantee that the PRC subsidiaries will not continue to enter into related party transactions in the future. In addition, there can be no assurance that they have achieved and will achieve the most favorable terms with related parties for each related party transaction. Furthermore, there can be no assurance that the above-mentioned transactions or any future related party transactions that they may enter into, individually or in the aggregate, will not have an adverse effect on their business, and their and the Group’s financial condition and results of operations. Further, the transactions with the related parties may potentially involve conflicts of interests. Additionally, there can be no assurance that any disputes that may arise among the PRC subsidiaries and related parties will be resolved in the PRC subsidiaries’ favor. For details, see “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
If our PRC subsidiaries cannot collect timely payments from their customers, their business and operations, and the Group’s financial condition and results of operations may be materially and adversely affected.
Our PRC subsidiaries could not collect timely payments from some of their customers due to such customers’ lack of working capital, and, as a result, the Group made a significantly greater amount of bad debt provision in the fiscal year ended September 30, 2022, based on credit-worthiness and financial condition of the distributor customers. Despite the recovering economy since late 2022 and management’s increased efforts in payment collections, we cannot assure you that our PRC subsidiaries will be able to collect timely payments as expected. See “Item 5. Operating And Financial Review and Prospects—B. Liquidity and Capital Resources.” Our PRC subsidiaries’ failure to collect timely payments in future financial periods could have a material and adverse effect on their business, and their and the Group’s financial condition and results of operations.
Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors.
Our PRC subsidiaries rely in part on third-party distributors to sell their products. For the fiscal year ended September 30, 2024, 2023, and 2022, the PRC subsidiaries had approximately 953, 892, and 867 domestic distributors, and 29, 22, and 37 exporting distributors, respectively. See “Item 4. Information on the Company—B. Business Overview—Marketing and Sales—Distribution Network.”
As our PRC subsidiaries sell and distribute their products through their distributors, and any one of the following events could cause fluctuations or reductions in our revenue that could have an adverse effect on our PRC subsidiaries’ and our financial condition and results of operations:
| ● | reduction, delay or cancelation of orders from one or more of the distributors; |
| ● | selection or increased sales by the distributors of our PRC subsidiaries’ competitors’ products; |
| ● | failure to renew distribution agreements and maintain relationships with the existing distributors; |
| ● | failure to establish relationships with new distributors on favorable terms; or |
| ● | inability to timely identify and appoint additional or replacement distributors upon the loss of one or more of the distributors. |
Our PRC subsidiaries may not be able to compete successfully against larger and better-funded sales and marketing campaigns of some of their current or future competitors, especially if these competitors provide their distributors with more favorable arrangements. We cannot assure you that our PRC subsidiaries will not lose any of their distributors to their competitors, which could cause them to lose some or all of their favorable arrangements with such distributors and may result in the termination of their relationships with other distributors. In addition, our PRC subsidiaries may not be able to successfully manage their distributors and the cost of any consolidation or further expansion of their distribution and sales network may exceed the revenue generated from these efforts. There can be no assurance that our PRC subsidiaries will be successful in detecting any non-compliance by their distributors with the provisions of their distribution agreements. Non-compliance by the distributors could, among other things, negatively affect our brand, demand for our PRC subsidiaries’ products, and our PRC subsidiaries’ relationships with other distributors. Furthermore, if the sales volumes of our PRC subsidiaries’ products to consumers are not maintained at a satisfactory level or if distributor orders fail to track consumers’ demand, our PRC subsidiaries’ distributors may not place orders for new products from our PRC subsidiaries, or may decrease the quantity of the distributors’ usual orders. The occurrence of any of these factors could result in a significant decrease in the sales volume of our PRC subsidiaries’ products, and therefore, adversely affect both our PRC subsidiaries’ and our financial condition and results of operations.
Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers who are critical and necessary to the production of our PRC subsidiaries’ products and our PRC subsidiaries’ ability to create relationships with new suppliers.
As of the fiscal years ended September 30, 2024, 2023, and 2022, the PRC subsidiaries had a total of 141, 143, and 135 suppliers, respectively. For the fiscal year ended September 30, 2024, the top three significant suppliers represented approximately 15%, 10%, and 7% of the total supplies purchased, respectively. For the fiscal year ended September 30, 2023, the top three significant suppliers represented approximately 13%, 11%, and 9% of the total supplies purchased, respectively. For the fiscal year ended September 30, 2022, the top three significant suppliers represented approximately 16%, 11%, and 7% of the total supplies purchased, respectively. See “Item 4. Information on the Company—B. Business Overview —Supplier.”
Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers and enter into new supplier agreements. Our PRC subsidiaries rely on suppliers to provide raw materials for the products. If our PRC subsidiaries’ suppliers experience substantial financial difficulties, cease operations, or otherwise face business disruptions, our PRC subsidiaries would be required to take measures to ensure raw materials remain available. Any supply chain disruption could affect our PRC subsidiaries’ ability to provide medical devices, and could increase the production costs and negatively affect both our PRC subsidiaries’ and our financial condition and results of operations.
Risks Related to Doing Business in China
Changes in the political and economic policies of the PRC government or in relations between China and the United States may materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations and may result in the PRC subsidiaries’ inability to sustain their growth and expansion strategies.
All of the operations are conducted in the PRC and a majority of our revenue is sourced from the PRC. Accordingly, our financial condition and results of operations are affected to a significant extent by economic, political and legal developments in the PRC or changes in government relations between China and the United States or other governments. There is significant uncertainty about the future relationship between the United States and China with respect to trade policies, treaties, government regulations and tariffs.
The PRC economy differs from the economies of most developed countries in many respects, including the extent of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the PRC government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets, and the establishment of improved corporate governance in business enterprises, a substantial portion of productive assets in China is still owned by the government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. The PRC government also exercises significant control over China’s economic growth by allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy, regulating financial services and institutions and providing preferential treatment to particular industries or companies.
While the PRC economy has experienced significant growth in the past four decades, growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall PRC economy, but may also have a negative effect on the PRC subsidiaries. The PRC subsidiaries’ financial condition and results of operation could be materially and adversely affected by government control over capital investments or changes in tax regulations that are applicable to the PRC subsidiaries. In addition, the PRC government has implemented in the past certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity.
In July 2021, the SEC has imposed enhanced disclosure requirements on China-based companies seeking to register securities with the SEC. As all of the operations are based in China, any future Chinese, U.S. or other rules and regulations that place restrictions on capital raising or other activities by China based companies could adversely affect the PRC subsidiaries’ business and results of operations. If the business environment in China deteriorates from the perspective of domestic or international investment, or if relations between China and the United States or other governments deteriorate, the Chinese government may intervene with the PRC subsidiaries’ operations and business in China and United States, as well as the market price of our Ordinary Shares, may also be adversely affected.
There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
All of the operations are conducted in the PRC, and are governed by PRC laws, rules and regulations. The PRC subsidiaries are subject to laws, rules and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws, rules and regulations governing economic matters in general. The overall effect of legislation over the past four decades has significantly enhanced the protections afforded to various forms of foreign investment in China. However, China has not developed a fully integrated legal system, and recently enacted laws, rules and regulations may not sufficiently cover all aspects of economic activities in China or may be subject to significant degrees of interpretation by PRC regulatory agencies. In particular, because these laws, rules and regulations are relatively new, and because of the limited number of published decisions and the nonbinding nature of such decisions, and because the laws, rules and regulations often give the relevant regulator significant discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties and can be inconsistent and unpredictable. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, and which may have a retroactive effect. As a result, we or the PRC subsidiaries may not be aware of the violation of these policies and rules until after the occurrence of the violation.
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we or the PRC subsidiaries enjoy than in more developed legal systems. These uncertainties may impede the PRC subsidiaries’ ability to enforce the contracts they have entered into and could materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations.
On July 6, 2021, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly issued the “Opinions on Severely Cracking Down on Illegal Securities Activities According to Law,” or the Opinions, and made the Opinions available to the public. The Opinions emphasized the need to strengthen the administration over illegal securities activities, and the need to strengthen the supervision over overseas listings by Chinese companies. Effective measures, such as promoting the construction of relevant regulatory systems will be taken to deal with the risks and incidents of China-concept overseas listed companies, and cybersecurity and data privacy protection requirements and similar matters. The Opinions remain unclear on how the law will be interpreted, amended and implemented by the relevant PRC governmental authorities, but the Opinions and any related implementing rules to be enacted may subject us and the PRC subsidiaries to compliance requirements in the future.
On July 10, 2021, the Cyberspace Administration of China issued a revised draft of the Measures for Cybersecurity Review for public comments, which required that, among others, in addition to “operator of critical information infrastructure,” any “data processor” controlling personal information of no less than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review, and further elaborated the factors to be considered when assessing the national security risks of the relevant activities. On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should be subject to cybersecurity review.
On November 14, 2021, the Cyberspace Administration of China released the Regulations on Network Data Security (draft for public comments) and will accept public comments until December 13, 2021. The draft Regulations on Network Data Security provide that if a data processor that processes personal data of more than one million users intends to list overseas, it shall apply for a cybersecurity review. In addition, data processors that process important data or are listed overseas shall carry out an annual data security assessment on their own or by engaging a data security services institution, and the data security assessment report for the prior year should be submitted to the local cyberspace affairs administration department before January 31 of each year. We do not believe, as advised by our PRC counsel, AllBright Law Offices (Fuzhou), that we or any of the PRC subsidiaries may be considered to be an “operator of critical information infrastructure”, or “online platform operators,” as mentioned above; however, Measures for Cybersecurity Review (2021 version) were recently adopted and the Regulations on Network Data Security (draft for public comments) are in the process of being formulated and it remains unclear how the Opinions will be interpreted, amended and implemented by the relevant PRC governmental authorities.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. These Rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. According to the New Administrative Rules Regarding Overseas Listings, among other things, Chinese domestic companies conducting overseas securities offering and listing activities, either in direct or indirect form, shall complete filing procedures with the CSRC pursuant to the requirements of the Trial Administrative Measures within three working days following their submission of initial public offering (“IPO”), listing applications, or completion of subsequent securities offerings. The New Administrative Rules Regarding Overseas Listings apply to both IPO and subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities. Under these rules, companies like ours must file with the CSRC within three working days after completing a post-IPO securities offering. As we are regarded as a domestic company to be indirectly listed on overseas markets under the Trial Administrative Measures, we were required to complete the filing procedures with the CSRC in accordance with the Trial Administrative Measures with respect to our IPO and on December 21, 2023, we completed all the required filing procedures with the CSRC with respect to our IPO. See “Item 3. Key Information—D. Risk Factors—Risks Related to doing business in China—Additional compliance procedures may be required in connection with our subsequent securities offerings, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 30.
Furthermore, if the CSRC or other regulatory agencies later promulgate new rules or explanations requiring that we obtain their approvals for any follow-on offering, we may be unable to obtain such approvals which could significantly limit or completely hinder our ability to offer or continue to offer securities to our investors.
Furthermore, the PRC government authorities may strengthen oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers like us. Such actions taken by the PRC government authorities may intervene or influence the PRC subsidiaries’ operations at any time, which are beyond our control. Therefore, any such action may adversely affect the PRC subsidiaries’ operations and significantly limit or hinder our ability to offer or continue to offer securities to you and reduce the value of such securities.
Uncertainties regarding the enforcement of laws and the fact that rules and regulations in China can change quickly with little advance notice, along with the risk that the Chinese government may intervene or influence the PRC subsidiaries’ operations at any time, or may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers could result in a material change in the PRC subsidiaries’ and our operations, financial performance and/or the value of our Ordinary Shares or impair our ability to raise money.
The PRC government exerts substantial influence over the manner in which the PRC subsidiaries conduct their business activities. The PRC government may also intervene or influence the PRC subsidiaries’ operations and subsequent securities offerings at any time, which could result in a material change in the PRC subsidiaries’ operations and our Ordinary Shares could significantly decline in value or become worthless.
Except for completing the filing procedures with the CSRC, we are currently not required to obtain any other approval from Chinese authorities to list on U.S exchanges. However, if the Company or any of the PRC subsidiaries were required to obtain approval in the future and were denied permission from Chinese authorities to list on U.S. exchanges, we will not be able to continue listing on U.S. exchange, continue to offer securities to investors, or materially affect the interest of the investors and cause significantly depreciation of our price of Ordinary Shares.
The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those authority relating to taxation, environmental regulations, land use rights, property and other matters. The central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our and the PRC subsidiaries’ part to ensure compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in the operations in China.
The Chinese cybersecurity regulator announced on July 2, 2021, that it had begun an investigation of Didi Global Inc. (NYSE: DIDI) and two days later ordered that the company’s app be removed from smartphone app stores. Similarly, the PRC subsidiaries’ business segments may be subject to various government and regulatory interference in the regions in which the PRC subsidiaries operate. The PRC subsidiaries could be subject to regulation by various political and regulatory entities, including various local and municipal agencies and government sub-divisions. The PRC subsidiaries may incur increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to comply.
Furthermore, it is uncertain when and whether we or the PRC subsidiaries will be required to obtain permission from the PRC government to list on U.S. exchanges in the future, and even when such permission is obtained, whether it will be denied or rescinded. Although neither we nor any of the PRC subsidiaries is currently required to obtain permission from any of the PRC central or local government, except for completing the filing procedures with the CSRC, and neither we nor any of the PRC subsidiaries has received any denial to list on a U.S. exchange, the PRC subsidiaries’ operations could be adversely affected, directly or indirectly, by existing or future laws and regulations relating to the PRC subsidiaries’ business or industry, which may also adversely affect the Group as a whole. Recent statements by the Chinese government indicating an intent, and the PRC government may take actions to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless.
Additional compliance procedures may be required in connection with our subsequent securities offerings, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which became effective on March 31, 2023. These rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. According to the New Administrative Rules Regarding Overseas Listings, among other things, Chinese domestic companies conducting overseas securities offering and listing activities, either in direct or indirect form, shall complete filing procedures with the CSRC pursuant to the requirements of Trial Administrative Measures within three working days following their submission of initial public offering (“IPO”), listing applications, or completion of subsequent securities offerings. The New Administrative Rules Regarding Overseas Listings apply to both IPOs and subsequent securities offerings of the company in the same overseas market where it has previously offered and listed securities. Under these rules, companies like ours must file with the CSRC within three working days after completing a post-IPO securities offering. As we are regarded as a domestic company to be indirectly listed on overseas markets under the Trial Administrative Measures, we were required to complete the filing procedures with the CSRC in accordance with the Trial Administrative Measures with respect to our IPO and on December 21, 2023, we completed all the required filing procedures with the CSRC with respect to our IPO. We are advised by our PRC counsel, AllBright Law Officers (Fuzhou), that the Company is currently not required to obtain permission or approval from any of the PRC central or local governmental authorities, except for completing the filing procedures with the CSRC, and it has not received any denial to list on a U.S. exchange. However, in the event that we conduct subsequent offerings, we could be subject to new filing requirements with the CSRC. In the event that filings with the CSRC are required, we cannot assure you that we can complete the filing procedures, obtain the approvals, or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure to do so would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on our operations in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our Ordinary Shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless. Furthermore, on February 24, 2023, the CSRC promulgated the Confidentiality and Archives Administration Provisions, which also became effective on March 31, 2023. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall institute a sound confidentiality and archives administration system, and take necessary measures to fulfill confidentiality and archives administration obligations. They shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Therefore, a domestic company that plans to, either directly or through its overseas listed entity, publicly disclose or provide to relevant individuals or entities including securities companies, securities service providers and overseas regulators, any documents and materials that contain state secrets or working secrets of government agencies, shall first obtain approval from competent authorities according to law, and file with the secrecy administrative department at the same level. The above-mentioned documents and materials that, if leaked, will be detrimental to national security or public interest, therefore, the domestic company shall strictly fulfill relevant procedures stipulated by applicable regulations. Furthermore, a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. Working papers produced in mainland China by securities companies and securities service providers in the process of undertaking businesses related to overseas offering and listing by domestic companies shall be retained in mainland China. Where such documents need to be transferred or transmitted to areas outside of mainland China, relevant approval procedures stipulated by regulations shall be followed. We believe, as advised by our PRC counsel, Allbright Law Offices (Fuzhou), that our IPO did not involve the leaking of any state secret or working secret of government agencies, or the harming of national security and public interests. However, we may be required to perform additional procedures in connection with the provision of accounting archives. The specific requirements of the relevant procedures are currently unclear, and we cannot be certain whether we will be able to perform the relevant procedures.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our directors and officers named in the annual report based on foreign laws.
We are an exempted company incorporated under the laws of the Cayman Islands. Our operations are conducted by the PRC subsidiaries in China, and most of our assets are located in China. In addition, most of our directors and senior executive officers reside within China for a significant portion of the time and are PRC nationals. As a result, it may be difficult for our shareholders to effect service of process upon us or those persons inside China. In addition, China does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the Cayman Islands and many other countries and regions. Therefore, recognition and enforcement in China of judgments of a court in any of these non-PRC jurisdictions in relation to any matter not subject to a binding arbitration provision may be difficult or impossible.
Shareholder claims that are common in the United States, including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the Unities States have not been efficient in the absence of mutual and practical cooperation mechanism. According to Article 177 of the PRC Securities Law which took effect in March 2020, overseas securities regulatory authorities may not carry out investigations and evidence collection directly within the territory of mainland China, and that no Chinese entity or individual is allowed to provide any documents or materials related to securities business activities to overseas agencies without prior consent of the securities regulatory authority of the State Council and the competent departments of the State Council.
You may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management named in this annual report based on Hong Kong laws.
You may incur additional costs and face procedural obstacles in effecting service of legal process, enforcing foreign judgments or bringing actions in Hong Kong against us or our management, as judgments entered in the United States can be enforced in Hong Kong only at common law. To enforce a judgment of the United States in Hong Kong, it must be a final judgment rendered conclusively upon the merits of the claim, for a liquidated amount in a civil matter and not in respect of taxes, fines, penalties, or similar charges, the proceedings in which the judgment was obtained were not contrary to natural justice, and the enforcement of the judgment is not contrary to public policy of Hong Kong. Such a judgment must be for a fixed sum and must also come from a “competent” court, as determined by the private international law rules applied by the Hong Kong courts.
Furthermore, foreign judgments of United States courts will not be directly enforced in Hong Kong. There are currently no treaties or other arrangements providing for reciprocal enforcement of foreign judgments between Hong Kong and the United States. However, the common law of Hong Kong permits an action to be brought upon a foreign judgment. A foreign judgment itself may form the basis of a cause of action, since the judgment may be regarded as creating a debt between the parties to such action. The defenses that are available to a defendant in a common law action brought on the basis of a foreign judgment include lack of jurisdiction, breach of natural justice, fraud, and contrary to public policy. However, a separate legal action for debt must be commenced in Hong Kong in order to recover such debt from the judgment debtor. A foreign judgment of the United States predicated solely upon the federal securities laws of the United States or the securities laws of any State or territory within the United States may be enforceable in Hong Kong, but will be subject to the conditions with regard to enforcement of such judgments being met, including but not limited to the foregoing requirements.
Any requirement to obtain prior approval under the M&A Rules and/or any other regulations promulgated by relevant PRC regulatory agencies in the future could delay our securities offerings and failure to obtain any such approvals, if required, could have a material adverse effect on the PRC subsidiaries’ and our business, operating results and reputation, as well as the trading price of our Ordinary Shares, and could also create uncertainties for our securities offerings and affect our ability to offer or continue to offer securities to investors outside China.
On August 8, 2006, six PRC regulatory agencies, including the Ministry of Commerce (the “MOFCOM”), the State-Owned Assets Supervision and Administration Commission (“SASAC”), the State Administration of Taxation (“SAT”), the State Administration of Industry and Commerce (“SAIC”), the CSRC, and the State Administration of Foreign Exchange (“SAFE”), jointly adopted the M&A Rules, which came into effect on September 8, 2006 and were amended on June 22, 2009. The M&A Rules include, among other things, provisions that purport to require that offshore special purpose vehicle formed for the purpose of an overseas listing of securities in a PRC entity obtain the approval of the CSRC prior to the listing and trading of any such special purpose vehicle’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures regarding its approval of overseas listings by special purpose vehicles. However, substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore special purpose vehicles.
While the application of the M&A Rules remains unclear, we believe, based on the advice of our PRC counsel, AllBright Law Offices (Fuzhou), that (i) since the Company is neither controlled by PRC entities or individuals, nor formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, we believe that the Company is not an SPV, and therefore, the M&A Rules do not apply to us, and we do not need to obtain the approval from the CSRC under the M&A Rules; and (ii) there are substantial uncertainties regarding the interpretation and application of PRC laws and regulations and future PRC laws and regulations, and there can be no assurance that the relevant government agencies will take a view that is contrary to, or otherwise different from, the conclusion stated above. Substantial uncertainty remains regarding the scope and applicability of the M&A Rules to offshore special purpose vehicles. If the CSRC or other PRC regulatory body subsequently determines that we need to obtain the CSRC’s approval for our subsequent securities offerings or if the CSRC or any other PRC government authorities promulgates any interpretation or implements rules that would require us to obtain CSRC or other governmental approvals for our subsequent securities offerings under the M&A Rules, we may face adverse actions or sanctions by the CSRC or other PRC regulatory agencies. In any such event, these regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in China, limit the PRC subsidiaries operating privileges in China, delay or restrict the repatriation of the proceeds from our subsequent securities offerings into the PRC or take other actions that could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition, results of operations, reputation and prospects, as well as our ability to complete subsequent securities offerings. The CSRC or other PRC regulatory agencies may also take actions requiring us, or making it advisable for us, to halt subsequent securities offerings before settlement and delivery of Ordinary Shares. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that such settlement and delivery may not occur in subsequent securities offerings. See “Item 4. Information on the Company—B. Business Overview—Regulations—M&A Rules and Regulation on Overseas Listings” on page 94.
In addition, the security review rules issued by the MOFCOM that took effect in September 2011 specify that if a merger and acquisition of domestic enterprise by a foreign investor falls within the M&A safety review scope, the foreign investor shall file an application for M&A safety review to the Ministry of Commerce. The M&A safety review scope is as follows: foreign investors’ M&A of domestic military industry enterprises and military industry support enterprises, enterprises around key and sensitive military facilities, and other units which have impact on national defense security; and foreign investors’ M&A of domestic enterprises, which have impact on the national security, in fields of important agricultural products, important energy and resources, important infrastructure, important transport service, key technology and major equipment manufacturing, etc. and such M&A may result in foreign investors’ acquirement of actual control over the enterprises. The rules prohibit any activities attempting to bypass a security review, including by holding on agency basis, trust, multi-tier reinvestment, leasing, loan, control by agreement, overseas transactions, etc.
The PRC subsidiaries are not operating in an industry that prohibits or limits foreign investment. As a result, the PRC subsidiaries are not required to obtain any permission from Chinese authorities to operate, other than those permissions a domestic company in China will need to engage in businesses similar to the PRC subsidiaries’. Such licenses and permissions include a Business License, Medical Device Registration Certificate, Medical Device Production Certificate, Medical Device Selling Certificate, Record Form of Medical Device Products, Certificate of Class I medical device production recordation, Certificate of Class II medical device selling recordation, Record Registration Form for Foreign Trade Business Operators, and Certificate of the Customs of the People’s Republic of China on Registration of a Customs Declaration Entity. The PRC subsidiaries have obtained the above licenses and permissions to conduct their business in China. However, the PRC government may take actions to exert more oversight and control over offerings by China-based issuers conducted overseas and/or foreign investment in such companies, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors outside China and cause the value of our securities to significantly decline or become worthless.
In the future, the PRC subsidiaries may grow their business by acquiring complementary businesses. Complying with the requirements of the above-mentioned regulations and other relevant rules to complete such transactions, if required, could be time-consuming, and any required approval processes, including obtaining approval from the MOFCOM or its local counterparts may delay or inhibit the PRC subsidiaries’ ability to complete such transactions. It is unclear whether the PRC subsidiaries’ business would be deemed to be in an industry that raises “national defense security” or “national security” concerns. However, the MOFCOM or other government agencies may publish explanations in the future determining that the PRC subsidiaries’ business is in an industry subject to the security review, in which case the PRC subsidiaries’ future acquisitions in the PRC, may be closely scrutinized or prohibited. The PRC subsidiaries’ abilities to expand the business or maintain or expand market share through future acquisitions would as such be materially and adversely affected. Furthermore, according to the M&A Rules, if a PRC entity or individual plans to merge or acquire its related PRC entity through an overseas company legitimately incorporated or controlled by such entity or individual, such a merger and acquisition will be subject to examination and approval by the MOFCOM. There is a possibility that the PRC regulators may promulgate new rules or explanations requiring that the PRC subsidiaries obtain the approval of the MOFCOM or other PRC governmental authorities for the PRC subsidiaries’ completed or ongoing mergers and acquisitions. There is no assurance that, if the PRC subsidiaries plan to make an acquisition, they can obtain such approval from the MOFCOM or any other relevant PRC governmental authorities for their mergers and acquisitions, and if the PRC subsidiaries fail to obtain those approvals, they may be required to suspend their acquisition and be subject to penalties. Any uncertainties regarding such approval requirements could have a material adverse effect on the PRC subsidiaries’ and our business, results of operations and corporate structure.
In addition, on July 6, 2021, the relevant PRC government authorities made public the Opinions on Strictly Cracking Down Illegal Securities Activities in Accordance with the Law. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision on overseas listings by China-based companies and proposed to take effective measures, such as promoting the construction of relevant regulatory systems to deal with the risks and incidents faced by China-based overseas-listed companies. Pursuant to the Opinions, Chinese regulators are required to accelerate rulemaking related to the overseas issuance and listing of securities, and update the existing laws and regulations related to data security, cross-border data flow, and management of confidential information. Numerous regulations, guidelines and other measures are expected to be adopted under the umbrella of or in addition to the Cybersecurity Law and Data Security Law.
On July 10, 2021, the Cyberspace Administration of China issued the Measures for Cybersecurity Review (Revision Draft for Comments) for public comments, which proposes to authorize the relevant government authorities to conduct cybersecurity review on a range of activities that affect or may affect national security, including listings in foreign countries by an operator that possess the personal data of more than one million users. The operators refer to operators of critical information infrastructure and data processors. On November 14, 2021, the Cyberspace Administration of China issued the Regulations on Network Data Security (draft for public comments) which set forth cyber data security compliance requirements in greater detail. On December 28, 2021, the Measures for Cybersecurity Review (2021 version) were promulgated and took effect on February 15, 2022, which provide that any “online platform operators” controlling personal information of more than one million users which seeks to list in a foreign stock exchange should also be subject to cybersecurity review. Neither we nor the PRC subsidiaries do expect to be subject to cybersecurity review, because the PRC subsidiaries currently engage in the manufacture and sale of medical devices and neither we nor the PRC subsidiaries possess personal information of over one million users. As of the date of this annual report, neither we nor the PRC subsidiaries have been involved in any investigations on cybersecurity review initiated by the Cyberspace Administration of China, and neither we nor the PRC subsidiaries have received any warning, sanction, or penalty in such respect. However, the Measures for Cybersecurity Review were recently adopted and the Regulations on Network Data Security (draft for public comments) are in the process of being formulated and the Opinions remain unclear on how they will be interpreted, amended and implemented by the relevant PRC governmental authorities. On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. Where a domestic company seeks to directly offer and list securities on overseas markets, the issuer shall file with the CSRC. Where a domestic company seeks to indirectly offer and list securities on overseas markets, the issuer shall designate a major domestic operating entity, which shall, as the domestic responsible entity, file with the CSRC. Initial public offerings or listings on overseas markets shall be filed with the CSRC within 3 working days after the relevant application is submitted overseas. See “Item 3. Key Information—D. Risk Factors—Risks Related to doing business in China—Additional compliance procedures may be required in connection with our subsequent securities offerings, due to the promulgation of the new filing-based administrative rules for overseas offering and listing by domestic companies in China, which could significantly limit or completely hinder our ability to offer or continue to offer our Ordinary Shares to investors and could cause the value of our Ordinary Shares to significantly decline or become worthless” on page 30. Furthermore, if the CSRC or other regulatory agencies later promulgate new rules or explanations requiring that we or the PRC subsidiaries obtain their prior approvals or ex-post record-filing for our IPO and any follow-on offerings, we or the PRC subsidiaries may be unable to obtain such approvals or record-filing which could significantly limit or completely hinder our ability to offer or continue to offer securities to our investors.
Except for the filing procedures with the CSRC, as of the date of this annual report, we do not believe we or the PRC subsidiaries are required to obtain any permission from any PRC governmental authorities to offer securities to foreign investors. We have been closely monitoring regulatory developments in China regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings, including our IPO and offering securities to foreign investors. As of the date of this annual report, neither we nor the PRC subsidiaries have received any inquiry, notice, warning, sanctions or regulatory objection to our IPO from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty regarding the enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital markets activities. If it is determined in the future that the approval of the CSRC, the Cyberspace Administration of China or any other regulatory authority is required for our IPO, we or the PRC subsidiaries may face sanctions by the CSRC, the Cyberspace Administration of China or other PRC regulatory agencies. These regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in China, limit the PRC subsidiaries ability to pay dividends outside of China, limit the PRC subsidiaries’ operations in China, delay or restrict the repatriation of the proceeds from our subsequent securities offerings into China or take other actions that could have a material adverse effect on the PRC subsidiaries’ and our business, financial condition, results of operations and prospects, as well as the trading price of our securities. In addition, if the Cyberspace Administration of China or other PRC regulatory agencies later promulgate new rules requiring that we or the PRC subsidiaries obtain their approvals for our IPO, we or the PRC subsidiaries may be unable to obtain such approval or a waiver of such approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding such an approval requirement could have a material adverse effect on the trading price of our securities.
Hong Kong does not have a comprehensive cyber or data security law. The Personal Data (Privacy) Ordinance (Chapter 486 of the Laws of Hong Kong) (“PDPO”) is the main legislation in Hong Kong which aims to protect the privacy of individuals in relation to personal data, and to regulate the collection, holding, processing, or use of personal data based on a set of data protection principles. The PDPO regulates the conduct of a data user, i.e. any person who, either alone or jointly or in common with other persons, controls the collection, holding, processing or use of personal data.
Pursuant to Section 33 of the PDPO, the PDPO is applicable to the collection and processing of personal data if such activities take place in Hong Kong, or if the personal data is collected by a data user whose principal place of business is in Hong Kong. Contravention with the data protection principals set out in PDPO may entitle the Privacy Commissioner for Personal Data to issue a written notice directing the data user to remedy and prevent recurrence of any contravention. As of the date of this annual report, we have neither collected nor processed any personal data in Hong Kong, and our Hong Kong subsidiary, Work Medical Technology, has not collected or processed any personal data. Therefore, we believe the PDPO does not currently have any material impact on our Hong Kong subsidiary’s business, or our business, financial condition or our subsequent securities offerings. However, if (i) we or our Hong Kong subsidiary do not comply with any privacy laws or regulations, or any compromise of security that results in the unauthorized release or transfer of personally identifiable information or users’ personal data, or (ii) applicable laws, regulations, or interpretations thereof change and we or our Hong Kong subsidiary become subject to any requirements for additional permissions or approvals in the future, we or our Hong Kong subsidiary may have to expend significant time and costs to procure them. If we or our Hong Kong subsidiary is unable to do so, on commercially reasonable terms, in a timely manner or otherwise, we or our Hong Kong subsidiary may become subject to governmental enforcement actions, litigation or public statements against us or our Hong Kong subsidiary by customers or others, and our and our Hong Kong subsidiary’s and business, reputation, financial condition, and results of operations may be materially and adversely affected.
In addition, although Work Medical Technology, our Hong Kong subsidiary, is an investment holding company, the legal and operational risks associated with operating in mainland China may also apply to the future limited activities (if any) in Hong Kong of Work Medical Technology, to the extent that they are made applicable to such entity and its anticipated operations. Work Medical Technology, as of the date of this annual report, has yet to commence operations, and is expected to be limited to operating as an investment holding company in the future without any substantive or data-related operations in Hong Kong. However, such operations may be affected if Hong Kong adopts rules, regulations or policy guidance with respect to currency exchange control. Furthermore, as of the date of this annual report, we do not expect that any regulatory actions related to data security or anti-monopoly concerns in Hong Kong will impact the Company’s ability to conduct its business, accept foreign investments, or list on a U.S. or foreign exchange, because we have never had and do not plan to have any material operations in Hong Kong.
PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for the PRC subsidiaries to pursue growth through acquisitions.
Under the Anti-Monopoly Law, companies undertaking acquisitions relating to businesses in China must notify the State Council’s anti-monopoly law enforcement authority, in advance of any transaction where the parties’ revenue in the China market exceed certain thresholds and the buyer would obtain control of, or decisive influence over, the target, while under the M&A Rules, the approval of MOFCOM must be obtained in circumstances where overseas companies established or controlled by PRC entities or residents acquire domestic companies affiliated with such PRC entities or residents. Applicable PRC laws, rules and regulations also require certain merger and acquisition transactions to be subject to security review. Due to the level of our revenue, the PRC subsidiaries’ proposed acquisition of control of, or decisive influence over, any company with revenue within China of more than RMB400 million in the year prior to any proposed acquisition would be subject to the State Council’s anti-monopoly law enforcement authority’s merger control review. As a result, many of the transactions the PRC subsidiaries may undertake could be subject to the State Council’s anti-monopoly law enforcement authority’s merger review. Complying with the requirements of the relevant regulations to complete such transactions could be time-consuming, and any required approval processes, including approval from the State Council’s anti-monopoly law enforcement authority, may delay or inhibit the PRC subsidiaries’ ability to complete such transactions, which could affect the PRC subsidiaries’ ability to expand their business or maintain market share, and which may adversely affect the Group as a whole. If the practice of the State Council’s anti-monopoly law enforcement authority and MOFCOM remains unchanged, the PRC subsidiaries’ ability to carry out their investment and acquisition strategy may be materially and adversely affected and there may be significant uncertainty as to whether the PRC subsidiaries will be able to complete large acquisitions in the future in a timely manner or at all, which could adversely affect the expansion goals of the Group.
PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or the PRC subsidiaries to liability or penalties, limit our ability to inject capital into the PRC subsidiaries or limit the PRC subsidiaries’ ability to increase their registered capital or distribute profits.
SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or the SAFE Circular 37, on July 4, 2014, which replaced the former circular commonly known as “SAFE Circular 75” promulgated by SAFE on October 21, 2005. SAFE Circular 37 requires PRC residents to register with local branches of SAFE in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle.” SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as increase or decrease of capital contributed by PRC individuals, share transfer or exchange, merger, division or other material event. In the event that a Chinese mainland shareholder-holding interests in a special purpose vehicle fails to fulfill the required SAFE registration, the PRC subsidiaries of that special purpose vehicle may be prohibited from making profit distributions to the offshore parent and from carrying out subsequent cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital into its PRC subsidiary. Moreover, failure to comply with the various SAFE registration requirements described above could result in liability under PRC law for evasion of foreign exchange controls.
We have notified substantial beneficial owners of Ordinary Shares who we know are PRC residents of their filing obligation, and are aware that all substantial beneficial owners have completed the necessary registration with the local SAFE branch or qualified banks as required by SAFE Circular 37. However, we may not at all times be aware of the identities of all of our beneficial owners who are PRC residents. We do not have control over our beneficial owners and cannot assure you that all of our PRC-resident beneficial owners will comply with SAFE Circular 37 and subsequent implementation rules. The failure of our beneficial owners who are PRC residents to register or amend their SAFE registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation rules, or the failure of future beneficial owners of our Company who are PRC residents to comply with the registration procedures set forth in SAFE Circular 37 and subsequent implementation rules, may subject such beneficial owners or the PRC subsidiaries to fines and legal sanctions. Furthermore, since it is unclear how SAFE Circular 37, and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant PRC government authorities, we cannot predict how these regulations will affect the PRC subsidiaries’ business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to the PRC subsidiaries and limit the PRC subsidiaries’ ability to distribute dividends to our Company. These risks may have a material adverse effect on our business, financial condition and results of operations.
PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay us from using the proceeds of our IPO and subsequent securities offerings to make loans or additional capital contributions to the PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.
We are an offshore holding company conducting our operations in China through the PRC subsidiaries. We may make loans to PRC subsidiaries subject to the approval from governmental authorities and limitation of amount, or we may make additional capital contributions to our subsidiaries in China.
Any loans to our WFOE in China, which is treated as a foreign-invested enterprise under PRC law, are subject to PRC regulations and foreign exchange loan registrations. For example, loans by us to our WFOE in China to finance its activities cannot exceed statutory limits and must be registered with the local counterpart of SAFE. In addition, a foreign invested enterprise shall use its capital pursuant to the principle of authenticity and self-use within its business scope. The capital of a foreign invested enterprise shall not be used for the following purposes: (i) directly or indirectly used for payment beyond the business scope of the enterprise or the payment prohibited by relevant laws and regulations; (ii) directly or indirectly used for investment in securities investments other than banks’ principal-secured products unless otherwise provided by relevant laws and regulations; (iii) the granting of loans to non-affiliated enterprises, except where it is expressly permitted in the business license; and (iv) paying the expenses related to the purchase of real estate that is not for self-use (except for the foreign-invested real estate enterprises).
SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming the Administration of Foreign Exchange Settlement of Capital of Foreign-invested Enterprises, or SAFE Circular 19, effective on June 2015, in replacement of the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, the Notice from the State Administration of Foreign Exchange on Relevant Issues Concerning Strengthening the Administration of Foreign Exchange Businesses, and the Circular on Further Clarification and Regulation of the Issues Concerning the Administration of Certain Capital Account Foreign Exchange Businesses. Although SAFE Circular 19 allows RMB capital converted from foreign currency-denominated registered capital of a foreign-invested enterprise to be used for equity investments within China, it also reiterates the principle that RMB converted from the foreign currency-denominated capital of a foreign-invested company may not be directly or indirectly used for purposes beyond its business scope. Thus, it is unclear whether SAFE will permit such capital to be used for equity investments in China in actual practice. SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or SAFE Circular 16, effective on June 9, 2016, which reiterates some of the rules set forth in SAFE Circular 19, but changes the prohibition against using RMB capital converted from foreign currency-denominated registered capital of a foreign-invested company to issue RMB entrusted loans to a prohibition against using such capital to issue loans to non-associated enterprises.
Violations of SAFE Circular 19 and SAFE Circular 16 could result in administrative penalties. SAFE Circular 19 and SAFE Circular 16 may significantly limit our ability to transfer any foreign currency we hold, including the net proceeds from our IPO and subsequent securities offerings, to our WFOE, which may adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business in China.
On October 23, 2019, SAFE issued the Circular on Further Promoting Cross-border Trade and Investment Facilitation, or SAFE Circular 28, which took effect on the same day. SAFE Circular 28, subject to certain conditions, allows foreign-invested enterprises whose business scope does not include investment, or non-investment foreign-invested enterprises, to use their capital funds to make equity investments in China. On December 4, 2023, SAFE issued the Circular of the State Administration of Foreign Exchange on Further Deepening Reforms to Facilitate Cross-Border Trade and Investment, which stipulated that domestic equity transferors (including institutions and individuals) can directly remit to the capital project settlement account the equity transfer consideration funds paid in foreign currency by domestic entities, as well as the foreign exchange funds raised by domestic enterprises listed overseas. The funds in the capital project settlement account can be independently settled and utilized. Since SAFE Circular 28 was issued only recently, its interpretation and implementation in practice are still subject to substantial uncertainties.
In light of the various requirements imposed by PRC regulations on loans to and direct investment in PRC entities by offshore holding companies, and the fact that the PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans to PRC subsidiaries in or future capital contributions by us to our WFOE in China. As a result, uncertainties exist as to our ability to provide prompt financial support to the PRC subsidiaries when needed. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds we expect to receive from our IPO and subsequent securities offerings and to capitalize or otherwise fund the PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand the PRC subsidiaries’ business.
We rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries to fund offshore cash and financing requirements and any limitation on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities.
We are a holding company and rely to a significant extent on dividends and other distributions on equity paid by our subsidiaries for our offshore cash and financing requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders, fund inter-company loans, service any debt we may incur outside of China and pay our expenses. The laws, rules and regulations applicable to the PRC subsidiaries permit payments of dividends only out of their retained earnings, if any, determined in accordance with applicable accounting standards and regulations.
Under PRC laws, rules and regulations, each of our subsidiaries incorporated in mainland China is required to set aside at least 10% of its after-tax profits each year, after making up for previous years’ accumulated losses, if any, to fund certain statutory reserves, until the aggregate amount of such fund reaches 50% of its registered capital. As a result of these laws, rules and regulations, our subsidiaries incorporated in mainland China are restricted in their ability to transfer a portion of their respective net assets to their shareholders as dividends. As of September 30, 2024 and 2023, these restricted assets were $972,494 and $972,494, respectively.
Limitations on the ability of the PRC subsidiaries to make remittance to pay dividends to us could limit our ability to access cash generated by the operations of those entities, including to make investments or acquisitions that could be beneficial to our businesses, pay dividends to our shareholders or otherwise fund and conduct our business.
We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.
Under the PRC Enterprise Income Tax Law and its implementing rules, both of which came into effect on January 1, 2008 and were last amended on December 29, 2018, enterprises established under the laws of jurisdictions outside of China with “de facto management bodies” located in China may be considered PRC tax resident enterprises for tax purposes and may be subject to the PRC enterprise income tax at the rate of 25% on their global income. “De facto management body” refers to a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise. The SAT issued the Notice Regarding the Determination of Chinese-Controlled Offshore-Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, or the SAT Circular 82, on April 22, 2009. SAT Circular 82 provides certain specific criteria for determining whether the “de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although Circular 82 only applies to offshore enterprises controlled by PRC enterprises, not those controlled by individuals or foreign enterprises, the determining criteria set forth in SAT Circular 82 may reflect the SAT’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises. If we were to be considered a PRC resident enterprise, we would be subject to PRC enterprise income tax at the rate of 25% on our global income, and our profitability and cash flow may be materially reduced as a result of our global income being taxed under the Enterprise Income Tax Law. We believe that none of our entities outside of mainland China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
Dividends payable to our foreign investors and gains on the sale of our Ordinary Shares by our foreign investors may be subject to PRC tax.
Under the Enterprise Income Tax Law and its implementation regulations issued by the State Council, a 10% PRC withholding tax is applicable to dividends payable to investors that are non-resident enterprises, which do not have an establishment or place of business in mainland China or which have such establishment or place of business but the dividends are not effectively connected with such establishment or place of business, to the extent such dividends are derived from sources within mainland China. Any gain realized on the transfer of Ordinary Shares by such investors is also subject to PRC tax at a current rate of 10% which in the case of dividends will be withheld at source if such gain is regarded as income derived from sources within mainland China. If we are deemed a PRC resident enterprise, dividends paid on our Ordinary Shares, and any gain realized from the transfer of our Ordinary Shares, may be treated as income derived from sources within mainland China and may as a result be subject to PRC taxation. See “Item 4. B. Business Overview—Regulations—Regulations Relating to Taxation.” Furthermore, if we are deemed a PRC resident enterprise, dividends payable to individual investors who are non-PRC residents and any gain realized on the transfer of Ordinary Shares by such investors may be subject to PRC tax at a current rate of 20%. Any PRC tax liability may be reduced under applicable tax treaties. However, it is unclear whether holders of our Ordinary Shares would be able to claim the benefit of income tax treaties or agreements entered into between China and other countries or areas if we are considered a PRC resident enterprise. If dividends payable to our non-PRC investors, or gains from the transfer of our Ordinary Shares by such investors are subject to PRC tax, the value of your investment in our Ordinary Shares may decline significantly.
We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
On February 3, 2015, the SAT issued the Announcement on Several Issues Concerning the Enterprise Income Tax on Indirect Transfer of Assets by Non-Resident Enterprises, or the SAT Circular 7. The SAT Circular 7 extends its tax jurisdiction to transactions involving the transfer of taxable assets through offshore transfer of a foreign intermediate holding company. In addition, SAT Circular 7 has introduced safe harbors for internal group restructurings and the purchase and sale of equity through a public securities market. SAT Circular 7 also brings challenges to both foreign transferor and transferee (or other person who is obligated to pay for the transfer) of taxable assets. On October 17, 2017, the SAT issued the Announcement on Issues Relating to Withholding at Source of Income Tax of Non-resident Enterprises, or the SAT Circular 37, which came into effect on December 1, 2017. The SAT Circular 37 further clarifies the practice and procedures of the withholding of non-resident enterprise income tax.
Where a non-resident enterprise transfers taxable assets indirectly by disposing of the equity interests of an overseas holding company, which is an Indirect Transfer, the non-resident enterprise as either transferor or transferee, or the PRC entity that directly owns the taxable assets, may report such Indirect Transfer to the relevant tax authority. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding or deferring PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise. Both the transferor and the transferee may be subject to penalties under PRC tax laws if the transferee fails to withhold the taxes and the transferor fails to pay the taxes.
We face uncertainties as to the reporting and other implications of certain past and future transactions where PRC taxable assets are involved, such as offshore restructuring, sale of the shares in our offshore subsidiaries and investments. Our Company may be subject to filing obligations or taxed if our Company is transferor in such transactions, and may be subject to withholding obligations if our Company is transferee in such transactions, under SAT Circular 7 and/or SAT Circular 37. For transfer of shares in our Company that do not qualify for the public securities market safe harbor by investors who are non-PRC resident enterprises, the PRC subsidiaries may be requested to assist in the filing under SAT Circular 7 and/or SAT Circular 37. As a result, we may be required to expend valuable resources to comply with SAT Circular 7 and/or SAT Circular 37 or to request the relevant transferors from whom we purchase taxable assets to comply with these circulars, or to establish that our Company should not be taxed under these circulars, which may have a material adverse effect on our financial condition and results of operations.
Restrictions on currency exchange may limit our ability to utilize our revenue effectively.
All of our revenue is denominated in Renminbi. The Renminbi is currently convertible under the “current account,” which includes dividends, trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans, including loans we may secure from our onshore subsidiaries. Currently, PRC subsidiaries may purchase foreign currency for settlement of “current account transactions,” including payment of dividends to us, without the approval of SAFE by complying with certain procedural requirements. However, the relevant PRC governmental authorities may limit or eliminate our ability to purchase foreign currencies in the future for current account transactions. Since we expect a significant portion of our future revenue will be denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in Renminbi to fund our business activities outside of the PRC or pay dividends in foreign currencies to our shareholders. Foreign exchange transactions under the capital account remain subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities. This could affect our ability to obtain foreign currency through debt or equity financing for our subsidiaries.
Fluctuations in exchange rates could result in foreign currency exchange losses to us and may reduce the value of, and amount in U.S. Dollars of dividends payable on, our shares in foreign currency terms.
The value of the RMB and the Hong Kong dollar against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign exchange policy adopted by the PRC government. In August 2015, the People’s Bank of China, or the PBOC, changed the way it calculates the mid-point price of RMB against the U.S. dollar, requiring the market-makers who submit for reference rates to consider the previous day’s closing spot rate, foreign-exchange demand and supply as well as changes in major currency rates. In 2017, the value of the Renminbi appreciated by approximately 6.3% against the U.S. dollar; and in 2018, the Renminbi depreciated by approximately 5.7% against the U.S. dollar. From the end of 2018 through the end of December 2020, the value of the Renminbi appreciated by approximately 5.10% against the U.S. dollar. It is difficult to predict how market forces or PRC or U.S. government policy, including any interest rate increases by the Federal Reserve, may impact the exchange rate between the RMB and the U.S. dollar in the future. There remains significant international pressure on the PRC government to adopt a more flexible currency policy, including from the U.S. government, which has threatened to label China as a “currency manipulator,” which could result in greater fluctuation of the RMB against the U.S. dollar. However, the PRC government may still at its discretion restrict access to foreign currencies for current account transactions in the future. Therefore, it is difficult to predict how market forces or government policies may impact the exchange rate between the RMB and the U.S. dollar or other currencies in the future. In addition, the PBOC regularly intervenes in the foreign exchange market to limit fluctuations in RMB exchange rates and achieve policy goals. If the exchange rate between RMB and U.S. dollar fluctuates in unanticipated manners, our results of operations and financial condition, and the value of, and dividends payable on, our shares in foreign currency terms may be adversely affected. We may not be able to pay dividends in foreign currencies to our shareholders. Appreciation of RMB to U.S dollar will result in exchange loss, while depreciation of RMB to U.S dollar will result in exchange gain.
Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties.
Companies operating in China are required to participate in various government-mandated employee benefit contribution plans, including certain social insurance, housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentages of salaries, including bonuses and allowances, of the PRC subsidiaries’ employees up to a maximum amount specified by the local government from time to time at locations where the PRC subsidiaries operate their businesses. The requirement of employee benefit contribution plans has not been implemented consistently by the local governments in China, given the different levels of economic development in different locations. Companies operating in China are also required to withhold individual income tax on employees’ salaries based on the actual salary of each employee upon payment. The PRC subsidiaries may be subject to late fees and fines in relation to the underpaid employee benefits and under-withheld individual income tax, which may adversely affect the PRC subsidiaries’ and the Group’s financial condition and results of operations.
As of the date of this annual report, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and the housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the fiscal years of 2024, 2023, and 2022, $300,945, $287,629, and $295,009 of contributions for the social insurance fund, respectively, should have been made but were not. For the fiscal years of 2024, 2023 and 2022, $94,815, $90,620, and $92,945 of contributions for the housing provident fund, respectively, should have been made but were not.
Although the PRC subsidiaries have not received any order or notice from the local authorities, nor any claims or complaints from their current and former employees regarding such non-compliance, we cannot assure you that the PRC subsidiaries will not be subject to any order to rectify non-compliance in the future, nor can we assure you that there will not be any employee complaints regarding social insurance payments or housing provident fund contributions against the PRC subsidiaries, or that the PRC subsidiaries will not receive any claims in respect of social insurance payments or housing provident fund contributions under the PRC laws and regulations. In addition, the PRC subsidiaries may incur additional costs to comply with such laws and regulations by the PRC government or relevant local authorities. Any such development could materially and adversely affect the PRC subsidiaries’ and our business, financial condition and results of operations.
Our Ordinary Shares may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. The HFCA Act states if the SEC determines that a company has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for two consecutive years, the SEC shall prohibit such Ordinary Shares from being traded on a national securities exchange or in the over-the-counter trading market in the U.S.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCA Act. A company will be required to comply with these rules if the SEC identifies it as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCA Act, including the listing and trading prohibition requirements described above. Furthermore, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, and on December 29, 2022, the Consolidated Appropriations Act was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to the Accelerating Holding Foreign Companies Accountable Act, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years, as was formerly required under the HFCA Act before such amendment, to two consecutive years. According to the Consolidated Appropriations Act, any foreign jurisdiction could be the reason why the PCAOB does not have complete access to inspect or investigate a company’s auditor. As it was originally enacted, the HFCA Act applied only if the PCAOB’s inability to inspect or investigate was due to a position taken by an authority in the foreign jurisdiction where the relevant public accounting firm is located. As a result of the Consolidated Appropriations Act, the HFCA Act now also applies if the PCAOB’s inability to inspect or investigate the relevant accounting firm is due to a position taken by an authority in any foreign jurisdiction. The denying jurisdiction does not need to be where the accounting firm is located.
On September 22, 2021, the PCAOB adopted a final rule implementing the HFCA Act, which provides a framework for the PCAOB to use when determining, as contemplated under the HFCA Act, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the HFCA Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. On December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (i) mainland China, and (ii) Hong Kong. Our auditor is not headquartered in mainland China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determination. On August 26, 2022, CSRC, the MOF, and the PCAOB signed a Protocol, which sets out specific arrangements on conducting inspections and investigations over relevant audit firms within the jurisdiction of both the PRC and the U.S., including the audit firms based in mainland China and Hong Kong, taking the first step toward opening access for the PCAOB to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. Notwithstanding the foregoing, in the event it is later determined that the PCAOB is unable to inspect or investigate completely our auditor, then such lack of inspection could cause our securities to be delisted from the stock exchange.
Furthermore, various equity-based research organizations have recently published reports on China-based companies after examining their corporate governance practices, related party transactions, sales practices and financial statements, and these reports have led to special investigations and listing suspensions on U.S. national exchanges. Any similar scrutiny on us, regardless of its lack of merit, could cause the market price of our Ordinary Shares to fall, divert management resources and energy, cause us to incur expenses in defending ourselves against rumors, and increase the premiums we pay for director and officer insurance.
WWC, P.C., the independent registered public accounting firm that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. As of the date of this annual report, our auditor, WWC, P.C., is not on the list published by the PCAOB subject to the determinations as to inability to inspect or investigate completely, as announced by the PCAOB on December 16, 2021, and is based in the U.S., registered with the PCAOB and subject to PCAOB inspection, having its latest inspection completed in 2023. However, the recent developments would add uncertainties to our offering, and we cannot assure you whether Nasdaq or regulatory authorities would apply additional and more stringent criteria to us after considering the effectiveness of our auditor’s audit procedures and quality control procedures, adequacy of personnel and training, or sufficiency of resources, geographic reach or experience as it relates to the audit of our financial statements.
The SEC may propose additional rules or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCA Act. However, some of the recommendations were more stringent than the HFCA Act. For example, if a company’s auditor was not subject to PCAOB inspection, the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCA Act and to address the recommendations in the PWG report. It is unclear when the SEC will complete its rulemaking and when such rules will become effective and what, if any, of the PWG recommendations will be adopted. The implications of this possible regulation in addition to the requirements of the HFCA Act are uncertain. While we understand that there has been dialogue among the CSRC, the SEC and the PCAOB regarding the inspection of PCAOB-registered accounting firms in China, there can be no assurance that we will be able to comply with requirements imposed by U.S. regulators. Such uncertainty could cause the market price of our Ordinary Shares to be materially and adversely affected, and our securities could be delisted and prohibited from being traded on the national securities exchange earlier than would be required by the HFCA Act. If our securities are unable to be listed on another securities exchange by then, such a delisting would substantially impair your ability to sell or purchase our Ordinary Shares when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of our Ordinary Shares. The delisting of our Ordinary Shares, or the threat of their being delisted, may materially and adversely affect the value of your investment, even making it worthless.
Further, new laws and regulations or changes in laws and regulations in both the United States and China could affect our ability to maintain the listing of our Ordinary Shares on Nasdaq, which could materially impair the market for and market price of our Ordinary Shares.
The enactment of Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region and Hong Kong Autonomy Act of U.S. could impact our Hong Kong holding subsidiary.
On June 30, 2020, the Standing Committee of the PRC National People’s Congress adopted the Law of the PRC on Safeguarding National Security in the Hong Kong Special Administrative Region (Hong Kong National Security Law). This law defines the duties and government bodies of the Hong Kong National Security Law for safeguarding national security and four categories of offences — secession, subversion, terrorist activities, and collusion with a foreign country or external elements to endanger national security — and their corresponding penalties. On July 14, 2020, U.S. President Donald Trump signed the Hong Kong Autonomy Act (“HKAA”), into law, authorizing the U.S. administration to impose blocking sanctions against individuals and entities who are determined to have materially contributed to the erosion of Hong Kong’s autonomy. On August 7, 2020, the U.S. government imposed HKAA-authorized sanctions on eleven individuals, including former chief executive of Hong Kong Special Administrative Region Carrie Lam. On October 14, 2020, the U.S. State Department submitted to relevant committees of Congress the report required under HKAA, identifying persons materially contributing to “the failure of the Government of China to meet its obligations under the Joint Declaration or the Basic Law.” The HKAA further authorizes secondary sanctions, including the imposition of blocking sanctions, against foreign financial institutions that knowingly conduct a significant transaction with foreign persons sanctioned under this authority. The imposition of sanctions may directly affect the foreign financial institutions as well as any third parties or customers dealing with any foreign financial institution that is targeted. It is difficult to predict the full impact of the Hong Kong National Security Law and HKAA on Hong Kong and companies located in Hong Kong. If our Hong Kong subsidiary is determined to be in violation of the Hong Kong National Security Law or the HKAA by competent authorities, our business operations, financial position and results of operations could be materially and adversely affected.
Risks Relating to our Ordinary Shares and the Trading Market
Our share price may be volatile and could decline substantially, which could result in substantial losses to our investors.
The trading price of our Ordinary Shares is likely to continue to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, including the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. The securities of some of these companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial price declines in their trading prices. The trading performances of other Chinese companies’ securities after their offerings may affect the attitudes of investors toward Chinese companies listed in the United States in general and consequently may impact the trading performance of our shares, regardless of our actual operating performance.
The market price of our Ordinary Shares may be volatile, both because of actual and perceived changes in the Company’s financial results and prospects, and because of general volatility in the stock market. The factors that could cause fluctuations in our share price may include, among other factors discussed in this section, the following:
| | ● | actual or anticipated variations in the financial results and prospects of the company or other companies in the medical device business; |
| | ● | changes in financial estimates by research analysts; |
| | ● | changes in the market valuations of other medical device companies; |
| | ● | announcements by us or our competitors of new medical devices, expansions, investments, acquisitions, strategic partnerships or joint ventures; |
| | ● | mergers or other business combinations involving us; |
| | ● | additions and departures of key personnel and senior management; |
| | ● | changes in accounting principles; |
| | ● | the passage of legislation or other developments affecting us or our industry; |
| | ● | the trading volume of our Ordinary Shares in the public market; |
| | | |
| | ● | the release of lockup, escrow or other transfer restrictions on our outstanding equity securities or sales of additional equity securities; |
| | ● | potential litigation or regulatory investigations; |
| | ● | changes in economic conditions, including fluctuations in global and Chinese economies; |
| | ● | financial market conditions; |
| | ● | natural disasters, terrorist acts, acts of war or periods of civil unrest; and |
| | ● | the realization of some or all of the risks described in this section. |
The listing of our Ordinary Shares on the Nasdaq Global Market is contingent on our compliance with the Nasdaq Global Market’s conditions for continued listing. A decline in the closing price of our Ordinary Shares could result in a breach of the requirements for listing on the Nasdaq Global Market. If we do not maintain compliance, Nasdaq could commence suspension or delisting procedures in respect of our Ordinary Shares. The commencement of suspension or delisting procedures by an exchange remains at the discretion of such exchange and would be publicly announced by the exchange. If a suspension or delisting were to occur, there would be significantly less liquidity in the suspended or delisted securities. In addition, our ability to raise additional necessary capital through equity or debt financing would be greatly impaired. Furthermore, with respect to any suspended or delisted Ordinary Shares, we would expect decreases in institutional and other investor demand, analyst coverage, market making activity and information available concerning trading prices and volume, and fewer broker-dealers would be willing to execute trades with respect to such Ordinary Shares. A suspension or delisting would likely decrease the attractiveness of our Ordinary Shares to investors and cause the trading volume of our Ordinary Shares to decline, which could result in a further decline in the market price of our Ordinary Shares.
In addition, the stock markets have experienced significant price and trading volume fluctuations from time to time, and the market prices of the equity securities of medical device companies are sometimes subject to sharp price and trading volume changes. These broad market fluctuations may materially and adversely affect the market price of our Ordinary Shares.
We may issue additional Ordinary Shares or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our Ordinary Shares.
We may issue additional Ordinary Shares or our other securities to investors. We may also issue additional Ordinary Shares or other equity securities of equal or senior rank in the future for any reason or in connection with, among other things, future acquisitions or repayment of outstanding indebtedness, without shareholder approval, in a number of circumstances.
Our issuance of additional Ordinary Shares or other equity securities of equal or senior rank would have the following effects:
| | ● | our existing shareholders’ proportionate ownership interest in us will decrease; |
| | ● | the amount of cash available per share, including for payment of dividends in the future, may decrease; |
| | ● | the relative voting strength of each previously outstanding share may be diminished; and |
| | ● | the market price of our Ordinary Shares may decline. |
We currently do not expect to pay dividends on our Ordinary Shares in the foreseeable future.
We currently do not expect to pay dividends on our Ordinary Shares in the foreseeable future. Instead, for the foreseeable future, it is expected that we will continue to retain any earnings to finance the development and expansion of its business, and not to pay any cash dividends on its Ordinary Shares. Consequently, you should not rely on an investment in the Company as a source for any future dividend income.
Subject to the provisions of the Companies Act (Revised) of the Cayman Islands (the "Cayman Companies Act") and any rights attaching to any class or classes of shares under and in accordance with the articles: (a) the directors may declare dividends or distributions out of our funds which are lawfully available for that purpose; and (b) our shareholders may, by ordinary resolution, declare dividends but no such dividend shall exceed the amount recommended by the directors. Subject to the requirements of the Cayman Companies Act regarding the application of a company’s share premium account and with the sanction of an ordinary resolution, dividends may also be declared and paid out of any share premium account. The directors when paying dividends to shareholders may make such payment either in cash or in specie. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our Ordinary Shares will likely depend entirely upon any future price appreciation of our Ordinary Shares. We cannot guarantee that our Ordinary Shares will appreciate in value or even maintain the price at which you purchased the Ordinary Shares. You may not realize a return on your investment in our Ordinary Shares and you may even lose your entire investment in our Ordinary Shares.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about us or our business, our Ordinary Share price and trading volume could decline.
The trading market for our Ordinary Shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on us. If no securities or industry analysts commence coverage of our Company, the trading price for its Ordinary Shares would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade its securities or publish inaccurate or unfavorable research about its business, its stock price would likely decline. If one or more of these analysts cease coverage of our Company or fail to publish reports on our Company, demand for its Ordinary Shares could decrease, which might cause its Ordinary Share price and trading volume to decline.
Our Chief Operating Officer and Liwei Zhang have substantial influence over our Company. Their interests may not be aligned with the interests of our other shareholders, and they could prevent or cause a change of control or other transactions.
As of the date of this annual report, Baiming Yu, our Chief Operating Officer (“COO”), beneficially owns an aggregate of 42.83% of our outstanding Ordinary Shares. Liwei Zhang, the spouse of Baiming Yu, beneficially owns an aggregate of 4.28% of our outstanding Ordinary Shares. Baiming Yu and Liwei Zhang beneficially own an aggregate of 47.11% of our outstanding Ordinary Shares. Moreover, Baiming Yu owns 3.35% of shares and Liwei Zhang owns 1.65% of shares of Hangzhou Shanyou. Because share percentage is aligned with voting power, Baiming Yu and Liwei Zhang do not have substantial influence over Hangzhou Shanyou.
Accordingly, as to Work Cayman, Mr. Yu and Ms. Zhang could have significant influence in determining the outcome of any corporate transaction or other matter submitted to the shareholders for approval, including mergers, consolidations, the appointment of directors and other significant corporate actions. They will also have the power to prevent or cause a change in control. Without the consent of Mr. Yu and Ms. Zhang, we may be prevented from entering into transactions that could be beneficial to us or our minority shareholders. In addition, they could violate their fiduciary duties by diverting business opportunities from us to themselves or others. The interests of Mr. Yu and Ms. Zhang may differ from the interests of our other shareholders. The concentration in the ownership of our Ordinary Shares may cause a material decline in the value of our Ordinary Shares. For more information regarding Mr. Yu, Ms. Zhang, and their affiliated entity, see “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
A sale or perceived sale of a substantial number of our Ordinary Shares may cause the price of our Ordinary Shares to decline.
If our shareholders sell substantial amounts of our Ordinary Shares in the public market, the market price of our Ordinary Shares could fall. Moreover, the perceived risk of this potential dilution could cause shareholders to attempt to sell their shares and investors to short our Ordinary Shares. These sales also make it more difficult for us to sell equity-related securities in the future at a time and price that we deem reasonable or appropriate.
We incur substantial increased costs being a public company.
We incur significant legal, accounting and other expenses as a public company that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and Nasdaq, impose various requirements on the corporate governance practices of public companies.
Compliance with these rules and regulations increases our legal and financial compliance costs and makes some corporate activities more time-consuming and costlier. We have incurred additional costs in obtaining director and officer liability insurance. In addition, we incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers.
We are an “emerging growth company,” as defined in the JOBS Act and will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Ordinary Shares that is held by non-affiliates exceeds $700 million as of the prior March 31, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 in the assessment of the emerging growth company’s internal control over financial reporting and permission to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
After we are no longer an “emerging growth company,” or until five years following the completion of our initial public offering, whichever is earlier, we expect to incur significant additional expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 and the other rules and regulations of the SEC. For example, as a public company, we have been required to increase the number of independent directors and adopt policies regarding internal controls and disclosure controls and procedures.
We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
There can be no assurance that we will not be a passive foreign investment company (“PFIC”) for United States federal income tax purposes for any taxable year, which could subject United States holders of our Ordinary Shares could be subject to adverse United States federal income tax consequences.
A non-United States corporation will be a PFIC for United States federal income tax purposes for any taxable year if either (i) at least 75% of its gross income for such taxable year is passive income or (ii) at least 50% of the value of its assets (based on average of the quarterly values of the assets) during such year is attributable to assets that that produce or are held for the production of passive income. Based on the current and anticipated value of our assets and the composition of our income assets, we are not currently a PFIC under the current PFIC rules for United States federal income tax purposes. However, the determination of whether or not we are a PFIC according to the PFIC rules is made on an annual basis and depends on the composition of our income and assets and the value of our assets from time to time. Therefore, changes in the composition of our income or assets or value of our assets may cause us to become a PFIC. The determination of the value of our assets (including goodwill not reflected on our balance sheet) may be based, in part, on the quarterly market value of Ordinary Shares, which is subject to change and may be volatile.
The classification of certain of our income as active or passive, and certain of our assets as producing active or passive income, and hence whether we are or will become a PFIC, depends on the interpretation of certain United States Treasury Regulations as well as certain IRS guidance relating to the classification of assets as producing active or passive income. Such regulations guidance is potentially subject to different interpretations. If due to different interpretations of such regulations and guidance the percentage of our passive income or the percentage of our assets treated as producing passive income increases, we may be a PFIC in one or more taxable years.
If we are a PFIC for any taxable year during which a United States person holds Ordinary Shares, certain adverse United States federal income tax consequences could apply to such United States person. See “Item 10. Additional Information—E. Taxation—United States Federal Income Taxation—Passive Foreign Investment Companies.”
For as long as we are an emerging growth company, we will not be required to comply with certain reporting requirements, including those relating to accounting standards and disclosure about our executive compensation, that apply to other public companies.
We are classified as an “emerging growth company” under the JOBS Act. For as long as we are an emerging growth company, unlike other public companies, we will not be required to, among other things, (i) provide an auditor’s attestation report on management’s assessment of the effectiveness of our system of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act, (ii) comply with any new requirements adopted by the Public Company Accounting Oversight Board, or the PCAOB, requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, (iii) provide certain disclosure regarding executive compensation required of larger public companies, or (iv) hold nonbinding advisory votes on executive compensation. We will remain an emerging growth company for up to five years, although we will lose that status sooner if we have more than $1.235 billion of revenues in a fiscal year, have more than $700 million in market value of our Ordinary Shares held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period.
To the extent that we rely on any of the exemptions available to emerging growth companies, you will receive less information about our executive compensation and internal control over financial reporting than issuers that are not emerging growth companies. If some investors find our Ordinary Shares to be less attractive as a result, there may be a less active trading market for our Ordinary Shares and our share price may be more volatile.
Our ability to produce accurate financial statements has been materially adversely affected by our failure to establish proper internal control over financial reporting. If we fail to establish and maintain proper internal control over financial reporting in a reasonably timely manner, our ability to produce accurate financial statements or comply with applicable regulations may continue to be impaired.
Our independent registered public accounting firm has not conducted an audit of our internal control over financial reporting. In the course of preparing our consolidated financial statements for the year ended September 30, 2024, we identified a material weakness in our internal control over financial reporting and other control deficiencies as of September 30, 2024. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified to date relates to a lack of accounting staff and resources with appropriate knowledge of generally accepted accounting principles in the United States (“U.S. GAAP”) and SEC reporting and compliance requirements.
Following the identification of the material weakness and control deficiency, we have undertaken certain remedial steps and plan to continue taking remedial measures, including:
| | (i) | recruiting qualified accounting personnel with relevant U.S. GAAP and SEC reporting experience and qualifications to strengthen the financial reporting function and to establish a financial and system control framework; and |
| | (ii) | implementing regular and continuous U.S. GAAP financial reporting training programs for our accounting and financial reporting personnel. |
The implementation of these measures may not fully address the material weakness in our internal control over financial reporting, and we cannot conclude that it has been fully remedied as of the date of this annual report. Our failure to correct the material weakness or our failure to discover and address any other material weaknesses could result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects, as well as the trading price of our Ordinary Shares, may be materially and adversely affected. Moreover, ineffective internal control over financial reporting significantly hinders our ability to prevent fraud.
As a public company, we are subject to Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. We qualify as an “emerging growth company” pursuant to the JOBS Act with less than US$1.235 billion in revenue for our last fiscal year. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, in the assessment of the emerging growth company’s internal control over financial reporting. Moreover, even if management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us.
During the course of documenting and testing our internal control procedures, we may identify other weaknesses and deficiencies in the internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404. Moreover, if we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of our securities. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions.
As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and therefore there may be less publicly available information about us than if we were a U.S. domestic issuer. We are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| | ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K; |
| | ● | the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; and |
| | ● | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and the selective disclosure rules by issuers of material non-public information under Regulation FD. |
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
As a foreign private issuer, we are permitted to take advantage of certain provisions in the Nasdaq listing standards that allow us to follow Cayman Islands law for certain governance matters. Certain corporate governance practices in the Cayman Islands may differ significantly from Nasdaq corporate governance listing standards as, except for general fiduciary duties and duties of care, Cayman Islands law has no corporate governance regime which applies to the Company that prescribes specific corporate governance standards in a manner similar to the Nasdaq listing standards. Currently, we do not intend to rely on home country practice with respect to our corporate governance. However, if we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our Ordinary Shares are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing standards. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange.
The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, by the Cayman Companies Act and by the common law of the Cayman Islands. The rights of shareholders to take action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law in the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands and from English common law. Appeals from the Cayman Islands Courts to the Privy Council (which is the final Court of Appeal for British overseas territories such as the Cayman Islands) are binding on courts in the Cayman Islands. Decisions of the English courts, and particularly the Supreme Court and the Court of Appeal are generally of persuasive authority but are not binding in the courts of the Cayman Islands. Decisions of courts in other Commonwealth jurisdictions are similarly of persuasive but not binding authority. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws relative to the United States. Therefore, our public shareholders may have more difficulty protecting their interests in the face of actions by our management, directors or controlling shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
You may be unable to present proposals before annual general meetings or extraordinary general meetings not called by shareholders.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. These rights, however, may be provided in a company’s amended and restated articles of association. Our amended and restated articles of association allow our shareholders holding shares representing in aggregate not less than 10% of our voting share capital in issue, to requisition a general meeting of our shareholders, in which case our directors are obliged to call such meeting. Advance notice of at least five calendar days is required for the convening of any general meeting of our shareholders. A quorum required for a meeting of shareholders consists of at least one shareholder, present in person or by proxy, holding not less than one third of the issued shares of the class of the Company. Our amended and restated articles of association also allow shareholder to propose a resolution for the appointment of a director by giving notice in writing not less than seven nor more than 42 clear days before the date appointed for a general meeting.
The obligation to disclose information publicly may put us at a disadvantage to competitors that are private companies.
We are a public company in the United States. As a public company, we will be required to file periodic reports with the SEC upon the occurrence of matters that are material to our Company and shareholders. Although we may be able to attain confidential treatment of some of our developments, in some cases, we will need to disclose material agreements or results of financial operations that we would not be required to disclose if we were a private company. Our competitors may have access to this information, which would otherwise be confidential. This may give them advantages in competing with our Company. Similarly, as a U.S. public company, we will be governed by U.S. laws that our competitors, which are mostly private Chinese companies, are not required to follow. To the extent compliance with U.S. laws increases our expenses or decreases our competitiveness against such companies, our public company status could affect our results of operations.
Our shareholders may be held liable for claims by third parties against us to the extent of distributions received by them upon redemption of their shares.
If we are forced to enter into an insolvency liquidation, any distributions received by shareholders could be viewed as an unlawful payment if it was proved that immediately following the date on which the distribution was made, we were unable to pay our debts as they fall due in the ordinary course of business. As a result, a liquidator could seek to recover some or all amounts received by our shareholders. Furthermore, our directors may be viewed as having breached their fiduciary duties to us or our creditors and/or may have acted in bad faith, thereby exposing themselves and our Company to claims, by paying public shareholders from the trust account prior to addressing the claims of creditors. We cannot assure you that claims will not be brought against us for these reasons. We and our directors and officers who knowingly and willfully authorized or permitted any distribution to be paid out of our share premium account* in violation of the Cayman Companies Act, while we were unable to pay our debts as they fall due in the ordinary course of business would be guilty of an offence and may be liable for a monetary fine and to imprisonment for five years in the Cayman Islands.
| * | Where a company issues shares at a premium (i.e., above the par value of the shares), whether for cash or otherwise, a sum equal to the aggregate amount or value of the premiums on those shares shall be transferred to an account, to be called the “share premium account.” |
Our board of directors may decline to register transfers of Ordinary Shares in certain circumstances.
Our board of directors may, in its sole discretion, decline to register any transfer of any Ordinary Share which is not fully paid up or on which we have a lien. Our directors may also decline to register any transfer of any Ordinary Share unless (i) the instrument of transfer is lodged with us, accompanied by the certificate for the shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; (ii) the instrument of transfer is in respect of only one class of shares; (iii) the instrument of transfer is properly stamped, if required; (iv) in the case of a transfer to joint holders, the number of joint holders to whom the share is to be transferred does not exceed four; (v) the shares transferred are free of any lien in favor of us; or (vi) a fee of such maximum sum as the Nasdaq Capital Market may determine to be payable, or such lesser sum as our board of directors may from time to time require, is paid to us in respect thereof.
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal. The registration of transfers may, on 14 days’ notice being given by advertisement in one or more newspapers or by electronic means, be suspended and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any year.
This, however, is unlikely to affect market transactions of the Ordinary Shares purchased by investors in a public offering. Once Ordinary Shares have been listed, the legal title to such Ordinary Shares and the registration details of those Ordinary Shares in the Company’s register of members will remain with the Depository Trust Company. All market transactions with respect to those Ordinary Shares will then be carried out without the need for any kind of registration by the directors, as the market transactions will all be conducted through the Depository Trust Company systems.
Provisions in our amended and restated articles of association may discourage, delay, or prevent a change in control.
Some provisions of our amended and restated articles of association may discourage, delay or prevent a change in control of our Company or management that shareholders may consider favorable, including, among other things, the following:
| ● | provisions that authorize our board of directors to issue shares with preferred, deferred or other special rights or restrictions without any further vote or action by our shareholders; and |
| ● | provisions that restrict the ability of our shareholders to call shareholder meetings. |
Item 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
Our Corporate History and Structure
Work Cayman is a Cayman Islands exempted company incorporated on March 1, 2022. Work BVI is our wholly-owned subsidiary formed in the British Virgin Islands on March 15, 2022. Work Medical Technology is Work BVI’s wholly-owned subsidiary formed in Hong Kong on April 19, 2022. WFOE, is Work Medical Technology’s wholly-owned subsidiary formed in Hangzhou on April 28, 2022. Work Hangzhou is WFOE’s wholly-owned subsidiary formed in Hangzhou on November 10, 2021.
Our operations are primarily conducted by Work Hangzhou and its subsidiaries in China.
Work Hangzhou is a limited liability company formed in China on November 10, 2021 with registered capital of RMB5 million.
Hangzhou Woli, a PRC limited liability company formed on July 29, 2022, with registered capital of RMB15 million, is a wholly owned subsidiary of Work Hangzhou.
Shanghai Saitumofei, a PRC limited liability company formed on May 15, 2019, with registered capital of RMB10 million, is a majority owned subsidiary of Work Hangzhou, of which Work Hangzhou owns approximately 44.20% shares, Jun Ma owns approximately 20.80% shares, Jianyuan Lu owns approximately 17.33% shares, Huangshan Tunxi District Leading Industry Incubation Fund Partnership (Limited Partnership) owns approximately 13.33% shares, and Shanghai Aikerui Medical Technology Co., Ltd. owns approximately 4.33% shares. It currently engages in the research and development of Class II and III medical devices, biological and medical technology.
Hunan Saitumofei, a PRC limited liability company formed on April 2, 2021, with registered capital of RMB2 million, is a wholly owned subsidiary of Shanghai Saitumofei.
Hangzhou Shanyou, a PRC limited liability company formed on April 29, 2002, with registered capital of RMB100 million, is a majority owned subsidiary of Work Hangzhou, of which Work Hangzhou owns 95% shares, our COO, Baiming Yu owns 3.35% shares and Liwei Zhang, spouse of Baiming Yu, owns 1.65% shares. It currently engages in the manufacture and sale of Class I and II medical devices.
Hangzhou Hanshi, a PRC limited liability company formed on July 22, 2019, with registered capital of RMB1 million, is a majority owned subsidiary of Hangzhou Shanyou and engages in the sales of Class II medical devices. The rest of Hangzhou Hanshi’s shareholders are Cheng Peng, Xiuwen Zhang and Zhengyan He, who own 25%, 10% and 5% shares, respectively.
Shanghai Chuqiang, a PRC limited liability company formed on March 12, 2018, with registered capital of RMB2 million, is a wholly owned subsidiary of Hangzhou Shanyou and engages in the sales of Class II medical devices.
Hangzhou Youshunhe, a PRC limited liability company formed on February 27, 2023, with registered capital of RMB1 million, is a majority owned subsidiary of Hangzhou Shanyou. The other shareholder of Hangzhou Youshunhe is Hangzhou Shunshunxin Material Technology Co., Ltd. Hangzhou Youshunhe does not have any operations as of the date of this annual report.
Huangshan Saitumofei, a PRC limited liability company formed on April 30, 2024, with registered capital of RMB1 million, is a fully owned subsidiary of Shanghai Saitumofei. Huangshan Saitumofei does not have any operations as of the date of this annual report.
The following diagram illustrates our corporate structure as of the date of this annual report:
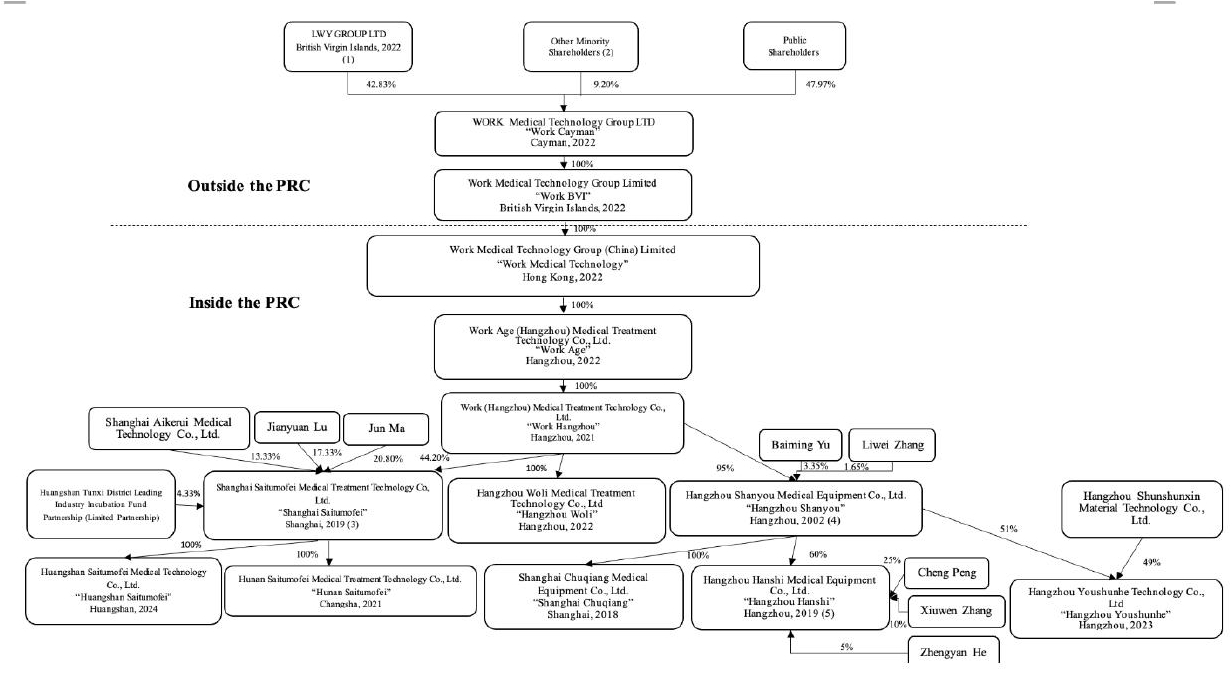
| (1) | Represents 6,250,000 Ordinary Shares held by Baiming Yu, the 100% owner of LWY Group LTD, as of the date of this annual report. |
| (2) | Represents an aggregate of 1,342,500 Ordinary Shares held by 2 shareholders of Work Cayman; namely, JPY GROUP LTD, holding 717,500 Ordinary Shares; and ZLW GROUP LTD, holding 625,000 Ordinary Shares. |
| (3) | The remaining shareholders of Shanghai Saitumofei are Jun Ma, Jianyuan Lu, Huangshan Tunxi District Leading Industry Incubation Fund Partnership (Limited Partnership), and Shanghai Aikerui Medical Technology Co., Ltd., who own 20.80%, 17.33%, 13.33%, and 4.33% shares of Shanghai Saitumofei, respectively. |
| (4) | Our COO, Baiming Yu, owns 3.75% shares of Hangzhou Shanyou, and his spouse, Liwei Zhang, owns 1.35% shares of Hangzhou Shanyou, as of the date of this annual report. |
| (5) | The remaining shareholders of Hangzhou Hanshi are Cheng Peng, Xiuwen Zhang and Zhengyan He, who own 25%, 10% and 5% of shares of Hangzhou Hanshi, respectively. |
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
For details of our principal shareholders’ ownership, please refer to “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
Neither we nor the PRC subsidiaries are operating in an industry that prohibits or limits foreign investment. As a result, neither we nor the PRC subsidiaries are required to obtain any permission from Chinese authorities to operate other than those requisite that a domestic company in China will need to engage in the businesses similar to the that of the PRC subsidiaries. Such licenses and permissions include Business License, Medical Device Registration Certificate, Medical Device Production Certificate, Medical Device Selling Certificate, Record Form of Medical Device Products, Certificate of Class I medical device production recordation, Certificate of Class II medical device selling recordation, Record Registration Form for Foreign Trade Business Operators, and Certificate of the Customs of the People’s Republic of China on Registration of a Customs Declaration Entity. The PRC subsidiaries have obtained the above licenses and permissions to conduct their business in China. However, the PRC government may take actions to exert more oversight and control over offerings by China based issuers conducted overseas and/or foreign investment in such companies, which could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of our securities to significantly decline or become worthless.
We treated Work Hangzhou and its subsidiaries as our consolidated affiliated entities under U.S. GAAP for the fiscal years ended September 30, 2024, 2023, and 2022. We have consolidated the financial results of Work Hangzhou and its subsidiaries in our financial statements in accordance with U.S. GAAP for the same periods.
Work Hangzhou and its subsidiaries contributed to 100% of our consolidated revenue and accounted for 66% of our consolidated total assets and liabilities for the fiscal years ended September 30, 2024, 2023, and 2022. There was no reconciliation performed between the financial position, cash flows and results of operations of Work Hangzhou and us.
Corporate Information
Our principal executive offices are located at Floor 23, No. 2 Tonghuinan Road, Hangzhou City, Zhejiang Province, the PRC, and our telephone number is +86-571-82613568. Our registered office in the Cayman Islands is at the offices of Tricor Services (Cayman Islands) Limited, Second Floor, Century Yard, Cricket Square, P.O. Box 902, Grand Cayman, KY1-1103, Cayman Islands. We maintain a corporate website at http://www.hzsy120.com/. The information contained in, or accessible from, our website or any other website does not constitute a part of this annual report.
The SEC maintains a website at www.sec.gov that contains reports, proxies, and information statements, and other information regarding issuers that file electronically with the SEC using its EDGAR system.
For information regarding our principal capital expenditures, see “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Capital Expenditures.”
B. Business Overview
Our Mission
Our mission is to become a world-leading manufacturer of medical devices, and we are committed to providing professional and reliable medical devices to the world.
Overview
In furtherance of our commitment to providing professional and reliable medical devices, both domestically and internationally, the PRC subsidiaries specialize in the research, development, manufacturing, and sales of medical devices as follow:
Hangzhou Shanyou
Among the PRC subsidiaries, only Hangzhou Shanyou is responsible for production, and only Hangzhou Shanyou has production lines. Hangzhou Shanyou mainly manufactures and sells Class I and II medical devices, including endotracheal tubes, laryngeal mask airways, heat and moisture exchanging filters (HMEF), disposable breathing circuits, nebulizer kits, and yankauer suction sets, etc. In addition to Class I and II medical devices, Hangzhou Shanyou focuses on manufacturing and sales of hygiene products and disposable medical supplies as well, including a filtering half mask and a non-powered air-purifying particle respirator.
Additionally, Hangzhou Shanyou has retained the certificate for selling Class III disposable medical devices. However, as of the date of this annual report, Hangzhou Shanyou has no sale activities for Class III disposable medical devices.
Hangzhou Hanshi
Hangzhou Hanshi mainly focuses on the sales of Class II medical devices.
Shanghai Chuqiang
Shanghai Chuqiang focuses on the sales of Class II medical devices.
Shanghai Saitumofei
Shanghai Saitumofei focuses on the research and development of Class II and III medical devices, and biological and medical technology.
Hunan Saitumofei
Hunan Saitumofei focuses on the research and development of Class I, II and III medical devices.
Hangzhou Woli
Hangzhou Woli focuses on the sales of chemical products.
Competitive Strengths
We believe that the following strengths contribute to our success and are the differentiating factors that set us apart from our peers:
| ● | A Deep Understanding of the Industry. Our COO, Baiming Yu, worked in a hospital as a doctor for nearly a decade, and he has been in the medical device industry for over two decades after he ended his medical practice in hospital. The three-decade experience furnish him with wide connections with people working in this industry, especially in Zhejiang Province, and a deep understanding of their specific needs for medical devices when providing medical services. Our subsidiary, which was founded by Mr. Yu in 2002, Hangzhou Shanyou has been manufacturing medical devices for over 20 years. Given the long-time operating history and interaction with the PRC subsidiaries’ clients, including hospitals, pharmacies and other medical institutions, the PRC subsidiaries have a deep understanding of the medical device industry, which enables them to develop and manufacture suitable and accommodative products tailored to the market. Meanwhile, the PRC subsidiaries’ R&D department has recruited a number of experienced medical professionals with extensive research experience, knowledge of the latest market and clinical needs of medical devices and the ability to develop suitable products to serve the demands efficiently. For more information about the R&D professionals, please refer to “— Research and Development (R&D).” |
| ● | Cost-effective Masks. Hangzhou Shanyou has recently upgraded its production equipment and it is able to manufacture more cost-effective products as a result. After transforming the production lines into fully automated ones, and automatic headband mask machines are put into use, the mask production of each production line has increased from 5,000 per day to 20,000 per day, with consistent product quality, and has decreased the production cost. Due to the boost of production efficiency, Hangzhou Shanyou expanded its production scale and increased the profit margin. |
| ● | Customized and Multifunctional Masks. Masks have been a necessity since the outbreak of COVID-19, and the market expects more from them than their basic function. Hence, customized and multifunctional masks have become increasingly popular over the past couple of years. In Contrast with the competitors that primarily produce masks with the basic function of reducing virus spreading, since the second half of 2021, Hangzhou Shanyou has upgraded its equipment, developing new production lines to produce customized masks with new features, which also can be used in various scenarios. Examples are tight fit masks and foldable masks. For those who work in industries which have stricter hygienic requirements, the PRC subsidiaries provide tight fit masks that reduce the gap between users’ faces and the masks, designed to decrease the possibilities of virus spreading. The PRC subsidiaries also provide foldable masks which reduce condensation on users’ glasses. |
| ● | Wide Distribution Network. The PRC subsidiaries maintain a wide distribution network which allows them to sell their products both domestically and internationally. For the fiscal year ended September 30, 2024, 2023, and 2022, they had approximately 953, 892, and 867 domestic distributors and 29, 22 , and 37 exporting distributors, respectively. With the help of the domestic distributors, the PRC subsidiaries are able to sell their products in 34 provincial level administrative regions of China. The exporting distributors span across six continents through which the PRC subsidiaries can sell their products in over 30 countries, including Australia, Brazil, France, Germany, India, Italy, Japan, Netherlands, Saudi Arabia, South Africa, South Korea, Spain, Switzerland, U.K. and U.S. For more information about the distribution network, please refer to “— Distribution Network.” |
Our Growth Strategies
We intend to develop our business and strengthen brand loyalty by implementing the following strategies:
| ● | Continue to invest in the PRC subsidiaries’ research and department team. We believe that our success to date has in part resulted from the strong research and development capabilities. The R&D team consists of experienced medical professional background with extensive research experience, which allows the PRC subsidiaries to introduce new and more advanced products at competitive prices. We believe the R&D team has strong professional capabilities and medical expertise. Please refer to “— Research and Development (R&D).” We plan to deepen the PRC subsidiaries’ collaboration with medical institutions and invest additional capital in R&D on technology and new products to add new items to the PRC subsidiaries’ product portfolio. |
| ● | Expand the PRC subsidiaries’ sales and distribution network. We intend to expand the PRC subsidiaries’ sales and distribution network to cover new geographic markets, especially those of emerging countries, and cultivate new market demands, to gain bigger market shares in medical consumables and medical equipment markets and access a broader range of customers. We expect to continue to expand the PRC subsidiaries’ sales and distribution channels. In addition, the PRC subsidiaries actively attend expos, professional forums and seminars to keep informed about market trends, which affords them an opportunity to interact with customers and distributors to understand their needs and accommodate their orders. |
| ● | Strengthen the PRC subsidiaries’ quality control system and uphold the commitment to product quality. We intend to uphold the commitment to product quality items to ensure consistently high standards throughout the operations. To reach this goal, we plan to continue to maintain and enhance the PRC subsidiaries’ quality control systems across the entirety of the operations by replacing machines having a low degree of accuracy, organizing monthly training for employees in quality control departments, replacing disqualified suppliers, keeping track of developments of international, national, and local product quality standards, laws, and regulations, and increasing the frequency of quality inspection. |
Our Revenue Model
We generate revenue through the PRC subsidiaries’ manufacturing and sales of medical devices under their own brands. For the fiscal years ended September 30, 2024, 2023 and 2022, we recognized $11,506,440, $13,565,951, and $19,711,290 in revenue, respectively.
The PRC subsidiaries sell medical devices, both domestically and internationally. For the fiscal years ended September 30, 2024, 2023 and 2022, the revenue of domestic sales was $9,823,148, $12,598,261, and $18,291,527, accounting for 85%, 93%, and 93%, respectively, of our revenue, and the revenue of international sales was $1,683,292, $967,690, and $1,419,763, accounting for 15%, 7%, and 7%, respectively, of our revenue.
Products
In the Chinese market, the PRC subsidiaries’ products are sold in 34 provincial-level administrative regions. Internationally, the products are exported to more than 30 countries in Asia, Africa, Europe, North America, South America, and Oceania.
The PRC subsidiaries current product portfolio sold internationally includes 1) Class I disposable medical devices, such as, medical face masks, artery compression tourniquets, endotracheal tube holders, intubating stylets, guedel airways, etc.; 2) Class II disposable medical devices, such as, disposable breathing circuits, laryngeal mask airways, endotracheal tubes, anesthetic kit, oxygen face masks, HMEF, anesthesia masks, laryngoscope blades, yankauer suction sets, and nasal oxygen cannulas; 3) other medical devices, such as KN95 masks and filtering half mask; and 4) medical innovative devices and equipment, such as visualized prostatic dilatation catheter.
As of the date of this annual report, the PRC subsidiaries have a total of 21 medical devices in their product portfolio. All of them are sold domestically and 15 of them are sold internationally.
The 21 products for the fiscal years ended September 30, 2024, 2023, and 2022 were as follows:
Class
Category | | Product Name | | Image | | Own
Brand/OEM/
Resale | | Use | | % of
Sales in the fiscal year ended
September 30,
2024 | | | % of
Sales in
the fiscal
year ended
September 30,
2023 | | | % of
Sales in
the fiscal
year ended
September 30,
2022 | |
| Class I | | Medical Face Masks | |  | | Own Brand/OEM | | Protection, commonly used by healthcare staff. | | | 12.47 | % | | | 16.9 | % | | | 36.45 | % |
| Class I | | FFP2 Masks | |  | | Own Brand | | Protection, used by the general public. | | | 0.00 | % | | | 3.76 | % | | | 4.19 | % |
| Class I | | Artery Compression Tourniquets | |  | | Own Brand | | Stop bleeding. | | | 29.03 | % | | | 20.27 | % | | | 16.03 | % |
| Class II | | Disposable Breathing Circuits | |  | | Own Brand/OEM | | Conduct oxygen/anesthetic gases from machine to patient. | | | 17.32 | % | | | 12.9 | % | | | 9.77 | % |
Note:
| (1) | The KN95 Masks do not fall under Class I, Class II, or Class III medical device classifications. |
In addition to the above products, the PRC subsidiaries are also dedicated to developing new products and technologies, to better accommodate the clinical needs and thus improve medical efficiency. The following product is the example of the latest development.
Visualized Prostatic Dilatation Catheter
Visualized prostatic dilatation catheter is a treatment for patients with benign prostatic hyperplasia. It dilates the hyperplastic prostatic tissue physically while observing the dilatation effect through endoscope. This technology is painless, operation-free, and minimally invasive, without any sequela, which can reduce the operation risk and improves the operation efficiency. The technology has following comparative advantages:
| ● | It replaces the traditional spherical balloon with a columnar balloon which permits the prostate to be compressed more evenly. In addition, the product only dilates the hyperplastic prostate tissue, causing less damage to the urethral mucosa, thus the urethral mucosa will be repaired quickly after operation. In combination, the foregoing features may enhance therapeutic effects with limited complications; |
| ● | The product maintains the position firmly after the dilatation; therefore, it can ensure the dilating effect and stop bleeding by compression. In this way, it releases the external sphincter, and prevents urinary incontinence and massive hemorrhage as well; and |
| ● | The operation process only takes about 15 minutes. During the operation, the water balloon is compressed reducing the likelihood of hemorrhages. |
Hangzhou Shanyou received the Medical Device Registration Certificate for the visualized prostatic dilatation catheter on October 11, 2023, which enables Hangzhou Shanyou to manufacture the product. However, as of the date of this annual report, no visualized prostatic dilatation catheters have been sold, and therefore, no revenue has been generated from the sales of this product.
Licenses and Permissions
The following chart sets forth a summary of the licenses and permissions obtained by the PRC subsidiaries as of the date of this annual report:
| Company | | License/Permission | | Issuing Authority | | Validity(1) |
| Work Age | | Business License | | Xiaoshan District Administration for Market Supervision | | Until April 27, 2052 |
| Work Hangzhou | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| Hangzhou Shanyou | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Medical Device Production Certificate | | Zhejiang Province Medical Products Administration | | Until November 22, 2027 |
| | | Medical Device Operation Certificate | | Hangzhou City Administration for Market Supervision | | Until May 11, 2027 |
| | | Cosmetics production Certificate | | Zhejiang Province Medical Products Administration | | Until August 20, 2029 |
| | | Certificate of Class II Medical Device Operation Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Record Registration Form for Foreign Trade Business Operators | | Foreign Trade Operator Filing and Registration Authority | | Long-term |
| | | Certificate of the Customs of the People’s Republic of China on Registration of Customs Declaration Entity | | Hangzhou Customs District of PRC | | Long-term |
| Company | | License/Permission | | Issuing Authority | | Validity(1) |
| | | Certificate of Class I Medical Device Production Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Medical Device Online Sales Information Filing Form | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Internet Medicine Information Service Qualification Certificate | | Zhejiang Province Medical Products Administration | | Until February 26, 2029 |
| | | High Technology Enterprise Certificate | | Zhejiang Province Department of Science and Technology, Zhejiang Province Department of Finance, Zhejiang Province Taxation Bureau of the State Administration of Taxation | | Until December 8, 2026 |
| | | Medical Device Registration Certificate-Artery Compression Tourniquets | | Zhejiang Province Medical Products Administration | | Until July 21, 2029 |
| | | Medical Device Registration Certificate-Laryngeal Mask Airways | | Zhejiang Province Medical Products Administration | | Until December 26, 2029 |
| | | Medical Device Registration Certificate-Filters/HMEF | | Zhejiang Province Medical Products Administration | | Until April 17, 2027 |
| | | Medical Device Registration Certificate-Endotracheal Tubes | | Zhejiang Province Medical Products Administration | | Until August 8, 2029 |
| | | Medical Device Registration Certificate-General Anesthesia Tracheal Intubation Kit | | Zhejiang Province Medical Products Administration | | Until August 26, 2029 |
| | | Medical Device Registration Certificate-Nasogastric Tubes | | Zhejiang Province Medical Products Administration | | Until July 30, 2029 |
| | | Medical Device Registration Certificate-Disposable Breathing Circuits | | Zhejiang Province Medical Products Administration | | Until May 15, 2028 |
| | | Medical Device Registration Certificate-Guedel Airways | | Zhejiang Province Medical Products Administration | | Until December 14, 2027 |
| | | Medical Device Registration Certificate-Anesthesia Masks | | Zhejiang Province Medical Products Administration | | Until December 14, 2027 |
| | | Medical Device Registration Certificate-Disposable Pulse Oximetry Sensor | | Zhejiang Province Medical Products Administration | | Until June 22, 2027 |
| | | Medical Device Registration Certificate-Intubating Stylets | | Zhejiang Province Medical Products Administration | | Until June 16, 2029 |
| | | Medical Device Registration Certificate-Endotracheal Tube Holders | | Zhejiang Province Medical Products Administration | | Until December 28, 2025 |
| Company | | License/Permission | | Issuing Authority | | Validity(1) |
| | | Medical Device Registration Certificate-Laryngoscope Blades | | Zhejiang Province Medical Products Administration | | Until May 17, 2026 |
| | | Medical Device Registration Certificate-Medical Face Masks | | Zhejiang Province Medical Products Administration | | Until June 8, 2025 |
| | | Medical Device Registration Certificate-Medical Protective Mask | | Zhejiang Province Medical Products Administration | | Until January 4, 2026 |
| | | Medical Device Registration Certificate-Medical Surgical Mask | | Zhejiang Province Medical Products Administration | | Until June 8, 2025 |
| | | Medical Device Registration Certificate-Face Masks (Oxygen Mask, Non-rebreathing Mask, Nebulizer Mask) | | Zhejiang Province Medical Products Administration | | Until April 22, 2026 |
| | | Medical Device Registration Certificate-Arterial Compression Hemostasis Device | | Zhejiang Province Medical Products Administration | | Until July 9, 2029 |
| | | Medical Device Registration Certificate-Disposable Laryngoscopes Blade | | Zhejiang Province Medical Products Administration | | Until June 17, 2029 |
| | | Medical Device Registration Certificate-Disposable Prostate Expansion Catheter | | Zhejiang Province Medical Products Administration | | Until October 10, 2028 |
| | | Class I Medical Device Filing — Disposable Nasal Catheter Carbon Dioxide Detection Connector Tube Kit | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Class I Medical Device Filing — Nasal Oxygen Tube | | Hangzhou City Administration for Market Supervision | | Long-term |
| | | Sanitary Permit for Disinfectant Product Manufacturers | | Health Commission of Zhejiang Province | | Until January 15, 2029 |
| Shanghai Chuqiang | | Business License | | Shanghai Jinshan District Administration for Market Supervision | | Until March 11, 2028 |
| | | Certificate of Class II Medical Device Selling Recordation | | Shanghai Jinshan District Administration for Market Supervision | | Long-term |
| Hangzhou Hanshi | | Business License | | Hangzhou Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Certificate of Class II Medical Device Selling Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| Shanghai Saitumofei | | Business License | | Shanghai Jiading District Administration for Market Supervision | | Until May 14, 2049 |
| Company | | License/Permission | | Issuing Authority | | Validity(1) |
| Hunan Saitumofei | | Business License | | Changsha Wangcheng District Administration for Market Supervision | | Long-term |
| | | Medical Device Registration Certificate-Automatic Blood Cell Morphology Analyzer | | Hunan Province Medical Products Administration | | Until February 26, 2028 |
| Hangzhou Woli | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| | | Certificate of Class II Medical Device Selling Recordation | | Hangzhou City Administration for Market Supervision | | Long-term |
| Hangzhou Youshunhe | | Business License | | Xiaoshan District Administration for Market Supervision | | Long-term |
| Huangshan Saitumofei | | Business License | | Tunxi District Administration for Market Supervision | | Long-term |
Note:
| (1) | According to relevant PRC laws and regulations, the PRC subsidiaries’ certificates, licenses or permits may fail to be renewed or revoked under some circumstance. For certificates related to medical devices, if a certificate holder fails to file a renewal application within the prescribed time or the renewal application does not meet the prescribed requirements, the relevant certificates may not be renewed. If a certificate holder’s production and operation of medical devices are materially in violation of laws and regulations, such certificate holder’s certificates related to such medical devices may be revoked. As for the Business License, before the license expires, the license holder may, at its own discretion, extend the business period and renew the Business License with the competent authority. If the license holder’s operations are materially in violation of relevant laws and regulations, the Business License may be revoked by the competent governmental department. The licensing requirements governing the production and sales of medical devices are constantly evolving within the PRC, and failure to obtain and/or maintain the licenses and permits required to conduct the business may subject applicable PRC subsidiaries to various penalties, including confiscation of revenues, imposition of fines and/or restrictions on the PRC subsidiaries’ business operations, or the discontinuation of the PRC subsidiaries’ operations. Any such disruption in the business operations could materially and adversely affect the PRC subsidiaries’ business, financial condition and results of operations, which could adversely affect the financial condition and results of operations of the Group. We cannot assure you that the PRC subsidiaries will be able to successfully maintain, or complete any required updating and renewal of, these licenses and permits on a timely basis. See “Item 3. Key Information—D. Risk Factors—Risks Related to the PRC subsidiaries’ Business and Industry—If the PRC subsidiaries fail to timely renew their medical device licenses or registration certificates, it could adversely affect the PRC subsidiaries’ and our reputation, financial condition and results of operations.” |
Manufacturing
Hangzhou Shanyou’s production lines are all located in Hangzhou, Zhejiang Province, the PRC. The PRC subsidiaries produce products and stock inventory of raw materials, components and finished goods at the facilities pursuant to the market demand, orders they receive or plan to receive, their production plan and capacity, and procurement information from their distributors. The production model is as follows:
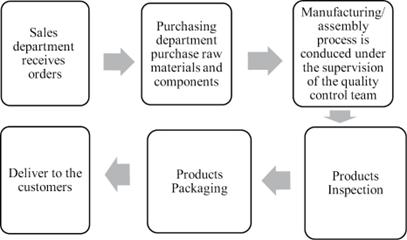
This production process is subject to continuous review and monitoring by the quality control team to ensure that finished products are of the highest quality and meet customer requirements and ISO 13485 medical device quality management systems standard.
ISO 13485 is a widely used international standard for quality management of the medical device industry. Issued by the International Organization for Standardization (ISO), the ISO 13485 standard is an effective solution to meet the comprehensive requirements for a quality management system in the medical device industry.
In order to the maintain product safety and a high standard of product quality, the PRC subsidiaries implement a strict set of quality control policies and inspection protocols. These policies and protocols are enforced by the quality control team, senior management and officers along every step of the production to post-production process.
Certification of Production and Products
As a medical device manufacturing and sales company, the PRC subsidiaries are subject to extensive government regulation and supervision in China. Pursuant to PRC laws, the PRC subsidiaries must obtain production license or filing for Class I and II disposable medical devices, operation license or filing for Class II and III disposable medical devices, and filing or registration certificates for Class I and Class II disposable medical devices. As of the date of this annual report, the PRC subsidiaries own all such licenses and certificates in China. Specifically, the PRC subsidiaries own a License of Production of Medical Devices, Registration Certificates for 21 Class II medical devices and the Filing of 2 Class I medical devices.
The National Medical Products Administration of the State Council is responsible for developing the classification rules for medical devices and maintaining catalogues of classified devices. Based on information on the production, distribution and use of medical devices, the National Medical Products Administration of the State Council analyzes and evaluates the risk levels of medical devices and adjusts the classification rules and classification catalogues.
“Class I” medical devices in China refer to medical devices with low risk, whose safety and effectiveness can be guaranteed through routine administration. Record-filing parties of domestic Class I medical devices shall submit record-filing materials to the drug regulatory authorities at the level of city divided into districts.
“Class II” medical devices in China refer to medical devices with medium risk, whose safety and effectiveness can be ensured by strict control, examination, and administration. Domestic Class II medical device shall be examined by the drug regulatory authorities of provinces, autonomous regions and centrally-administered municipalities which shall issue the Medical Device Registration Certificate upon approval after examination.
“Class III” in China refer to medical devices with high risk, whose safety and effectiveness can be ensured by strict control, examination, and administration through special measures. Domestic Class III medical devices shall be examined by the National Medical Products Administration (“NMPA”), which shall issue the Medical Device Registration Certificate upon approval after examination. The registration certificate for a medical device is valid for five years and the registrant shall apply to the original registration departments for renewal at least six months prior to its expiration date.
Customers
The PRC subsidiaries have three types of customers, i) direct end-user customers, which include hospitals, pharmacies, and medical institutions, ii) domestic distributor customers that distribute the PRC subsidiaries’ products to end-user customers in China, and iii) export distributor customers that distribute the PRC subsidiaries’ products to end-user customers in Asia, Africa, Europe, North America, South America, and Oceania. The top ten countries and regions outside of mainland China where the products are sold are Saudi Arabia, Germany, Switzerland, Hong Kong, France, Poland, Netherlands, Mexico, Romania, and Russia.
As of the fiscal year ended September 30, 2024, the PRC subsidiaries had a total of 989 customers, of which 7 were direct end-user customers, 953 were domestic distributor customers, and 29 were export distributor customers. As of the fiscal year ended September 30, 2023, the PRC subsidiaries had a total of 1,054 customers, of which 140 were direct end-user customers, 892 were domestic distributor customers, and 22 were export distributor customers. As of the fiscal year ended September 30, 2022, the PRC subsidiaries had a total of 1,058 customers, of which 154 were direct end-user customers, 867 were domestic distributor customers, and 37 were export distributor customers. Since direct-end customers generally place smaller and less frequent orders, the PRC subsidiaries have gradually shifted their focus towards developing and maintaining relationships with distributor customers who are perceived to offer long-term and stable revenue contributions. As a result, while the number of direct-end customers decreased from 140 for fiscal year ended September, 2023, to 7 for the fiscal year ended September 30, 2024 and total customers decreased from 1,054 to 989 over the same period, there was an increase in domestic distributor customers from 892 to 953, and an increase in export distributor customers from 22 to 29.
Distributors usually purchase products from the PRC subsidiaries at a lower price and then resell the products to end customers, both domestically and internationally, at a comparatively higher price and earn the price difference. The PRC subsidiaries source their distributors through multiple channels: (i) industry exhibitions/expos, (ii) online platform, and (iii) distributors’ direct contact. For the fiscal years ended September 30, 2024, 2023 and 2022, the PRC subsidiaries had a total of 953, 892, and 867 domestic distributor customers, and 29, 22, and 37 and are export distributor customers, respectively.
For the fiscal year ended September 30, 2024, the PRC subsidiaries’ top three customers accounted for 6%, 5%, and 4%, respectively, of the revenue. For the fiscal year ended September 30, 2023, the PRC subsidiaries’ top three customers accounted for 9%, 8%, and 6%, respectively, of the revenue. For the fiscal year ended September 30, 2022, the top three customers accounted for 6%, 4%, and 4%, respectively, of the revenue.
The PRC subsidiaries source their customers through multiple channels: (i) referrals from their present customers, (ii) industry exhibitions/expos such as China International Medical Equipment Fair, and (iii) provincial medical conferences.
Through the PRC subsidiaries’ distribution network, their products are sold to customers at all levels in China, and other countries and regions in Asia, Africa, Europe, North America, South America, and Oceania. The PRC subsidiaries target overseas customers mainly via exporting distributors and exhibitions. In order to market their products, gain more market share and secure more quality customers, the PRC subsidiaries frequently participate in, and promote their products at, specific medical device exhibitions/expos.
The PRC subsidiaries do not have long-term written sales agreements with their direct-end user customers. Each direct-end user customer sale is typically governed by a brief purchase-order based sales agreement. The key terms of the sales agreements with direct-end user customers (including those agreements with their top customers) include:
| ● | the product’s name, type, quantity, and price; |
| ● | quality standard — medical device qualifications, including business license, medical device production and operation licenses, medical device registration certificates, inspection report, etc.; |
| ● | delivery time, method and payment terms; |
| ● | breach of contract terms, including remedies, such as refunds and return of products (e.g., direct-end user customers are entitled to refunds and may return the product if the wrong product is delivered or the product does not meet agreed upon quality standards); |
| ● | shipping costs, which are typically borne by the seller; and |
| ● | dispute solutions, including bringing a lawsuit at the local court where the direct-end user customers are located, if negotiations are unsuccessful. |
The PRC subsidiaries have no offices or subsidiaries in foreign countries and currently have no intention to establish any offices in foreign countries in the future. In the course of dealing with overseas customers, the PRC subsidiaries have maintained stable business relationships, however, such business relationships are not memorialized in any long-term agreements, but are rather provided for in short-form order sheets. In respect of their international sales, the risk of loss is borne by the customers upon delivery of the products.
In addition, in the course of dealing with the local distributors overseas, such distributers undertake all obligations with respect to the local rules and regulations and taxes regarding the importation of medical products.
The PRC subsidiaries face an inherent risk of liability claims or complaints from customers. When the products are found to be defective, they are required to recall the products according to usage of trade. When customers file product liability claims against the PRC subsidiaries, conflict of laws and product liability laws of the countries or regions where they are located are applicable. Jurisdiction is determined by the sales agreements, and laws of the countries or regions where the customers are located will determine the issue if the sales agreements are absent of jurisdiction selection clauses.
As of the date of this annual report, the PRC subsidiaries are not aware of any warnings, investigations, prosecutions, disputes, claims or other proceedings in respect of medical devices they manufacture or distribute overseas, nor have they been penalized or can foresee any penalties to be made by any overseas jurisdiction with respect to medical device safety.
Please refer to “— Marketing and Sales — Distribution Network” for more details about the business and sales agreements with the PRC subsidiaries’ distributor customers.
For the fiscal years ended September 30, 2024, 2023, and 2022, revenue generated from domestic distributor customers, domestic direct end-user customers and overseas distributor customers amounted to (i) $9,756,622, $66,526, and $1,683,292, (ii) $11,990,599, $607,662, and $967,690, and (iii) $17,150,575, $1,140,952, and $1,419,763, respectively, constituting (i) 85%, 1%, and 15%, (ii) 90%, 4%, 6%, and (iii) 87%, 6%, and 7%, respectively, of our total revenue.
Suppliers
The PRC subsidiaries source their suppliers through multiple channels: (i) industry exhibitions/expos, (ii) online platform, and (iii) suppliers’ direct contact.
The PRC subsidiaries’ suppliers are those providing raw materials for the manufacturing of the products.
The raw and auxiliary materials include meltblown, non-woven fabrics, steel spring, OPP membrane, methyl silicone oil, etc. All of which are purchased from certified and qualified suppliers in China. The PRC subsidiaries’ raw materials supply has been very stable for many years and are easily sourced due to the PRC subsidiaries’ geographical location. The prices of raw materials are not volatile and they, along with the PRC subsidiaries’ supply chain, have not been impacted by the recent conflict between Russia and Ukraine, as of the date of this annual report. At the beginning of 2020, the price of mask raw materials surged and the logistics were affected by the COVID-19 pandemic.
However, since Jiangsu province and Zhejiang province, where the PRC subsidiaries are located and conduct their business, are the core supply areas of mask raw materials and the COVID-19 pandemic was effectively controlled in these areas, the PRC subsidiaries had sufficient raw materials and the prices were not materially impacted during the COVID-19 pandemic. From late 2022 to early 2023, the Chinese government gradually released controls relating to the COVID-19 pandemic. China has substantially lifted COVID-19 restrictions since then by removing mandatory centralized quarantine, compulsory testing, and sweeping lockdowns. During the fiscal year ended September 30, 2024, COVID-19 did not have a material impact on the PRC subsidiaries’ business. As of the date of this annual report, the raw materials our PRC subsidiaries source from their suppliers are sufficient, and the prices of these raw materials remain stable.
According to the Company, it is a common practice in the industry of medical devices for companies to purchase raw materials from a considerable number of suppliers. As of the date of this annual report, the PRC subsidiaries have a total number of 141 suppliers. Although there are lots of suppliers available in the market, our PRC subsidiaries believe that they have established healthy and stable relationships with their significant suppliers through years of cooperation. These significant suppliers, in the aggregate, accounted for over 32%, 34%, and 27% of their raw material purchases for the fiscal years ended September 30, 2024, 2023, and 2022, respectively. For the fiscal year ended September 30, 2024, the top three significant suppliers represented approximately 15%, 10%, and 7% of the total supplies purchased, respectively. For the fiscal year ended September 30, 2023, the top three significant suppliers represented approximately 13%, 11%, and 9% of the total supplies purchased, respectively. For the year ended September 30, 2022, the top three significant suppliers represented approximately 16%, 11%, and 7% of the total supplies purchased, respectively. See “Item 3. Key Information—D. Risk Factors—Risks Related to the PRC Subsidiaries’ Business and Industry—Our success will be dependent upon our PRC subsidiaries’ ability to maintain relationships with existing suppliers who are critical and necessary to the production of our PRC subsidiaries’ products and to create relationships with new suppliers.” There are no minimum purchase requirements with any of the suppliers, including these significant ones. Each supplier order is typically governed by a brief purchase-order based purchase agreement. The key terms of the procurement agreements (including those agreements with our significant suppliers) include:
| ● | the product’s name, type, quantity, and price; |
| ● | quality terms which are typically expressed with reference to national or industry standards; |
| ● | delivery time, method and payment terms. Shipping costs are the responsibility of the supplier; and |
| ● | breach of contract terms, including refund and return of products, compensatory damages. If the supplier cannot deliver the product within the time agreed, or if the products do not meet the stated quality standard, the supplier must compensate the PRC subsidiaries for losses caused, including treble damages if the products are defective or counterfeit. In the event the PRC subsidiaries cannot timely pay, liquidated damages are due to the supplier. |
Marketing and Sales
The PRC subsidiaries market and sell their products through multiple channels: (i) through their sales team, and (ii) through their distribution network, including their domestic and export distributors.
Sales Team
As of the date of this annual report, the PRC subsidiaries have a sales team of 45 employees. Their sales team provides them with direct access to their customers and is capable of promptly addressing customers’ needs. There are 4 team leaders leading their respective teams to market the PRC subsidiaries’ products, both domestically and internationally.
The PRC subsidiaries’ sales team promotes the products in the following ways: (i) advertising on search engine websites such as Baidu and 360; (ii) participating in online bidding on the provincial drug procurement platforms; (iii) attending exhibitions and expos; (iv) attending medical conferences and introducing the products; (v) developing business relation with local distributors and promoting the products; (vi) conducting online searches to obtain public information of potential customers from enterprise yellow pages or enterprise official websites, establishing contact with them by ascertaining their product demand and purchasing intention via telephone calling or social media applications, such as WeChat and QQ.
The compensation package for the sales team includes vacation, social insurance, fixed base salaries and commissions based on the revenue or collection they achieve. The PRC subsidiaries provide their sales team with regular training and internally developed systems to assist them in quickly becoming proficient and productive sales personnel. The employment agreements with the sales team members include, contract period (fixed time or indefinite duration), job description, occupational hazard protection, termination provisions (termination at will is prohibited), and compliance with the Labor Law of the People’s Republic of China and the Labor Contract Law of the People’s Republic of China in all material aspects.
Distribution Network
According to the Company, it is a common practice in the medical device industry for companies to rely on a considerable number of distributors to sell their products. Distributors usually purchase products from the PRC subsidiaries at a discounted price and then resell the products to end customers, both domestically and internationally.
The PRC subsidiaries’ domestic distributors cover 34 provincial-level administrative regions of PRC for the resale of the products in the Chinese market. Domestic distributors market and distribute the products in the regions where they are located and secured approximately 1,010, 1,032, and 1,021 domestic customers for the PRC subsidiaries for the fiscal years ended September 30, 2024, 2023, and 2022, respectively, including hospitals and medical institutions.
The PRC subsidiaries’ exporting distributors can be classified into two categories: those located outside of China that only distribute the products internationally, and those located in China that distribute the products, both domestically and internationally. The total number of direct and indirect customer relationships established overseas through the exporting distributors was approximately 29, 22, and 37 as of the fiscal years ended September 30, 2024, 2023, and 2022, respectively.
The PRC subsidiaries do not have long-term written agreements with their distributors. Each distributor order is typically governed by a brief purchase-order based sales agreement. The key terms of the distributor purchase agreements include:
| ● | the product’s name, type, quantity, and price; |
| ● | qualifications, including business license, medical device production and operation licenses, medical device registration certificates, and inspection report. (The absence of any of these qualifications will result in termination of the agreements); |
| ● | delivery method and payment terms: shipping costs are typically borne by the PRC subsidiaries and payment shall be made before delivery; |
| ● | risk of loss, which is typically borne by the PRC subsidiaries until delivery; |
| ● | breach of contract terms, including refunds and return of products (e.g., distributors are entitled to refunds and may return a product if the wrong product is delivered, or the product does not meet agreed upon quality standards); and |
| ● | dispute solutions, including bringing a lawsuit at the local court where the distributors are located. |
See “Item 3. Key Information—D. Risk Factors—Risks Related to the PRC Subsidiaries’ Business and Industry—Our PRC subsidiaries rely in part on third-party distributors to place their products into the market and they may not be able to control their distributors.”
Research and Development (“R&D”)
The PRC subsidiaries invest in R&D, aiming to develop new products and improve their existing products to accommodate the market needs. The R&D expenses totaled approximately $302,511, $301,644, and $183,900 for the fiscal years ended September 30, 2024, 2023, and 2022, respectively. R&D expenses mainly consist of applicable personnel, design, sample manufacturing and materials expenses. As of September 30, 2024, the PRC subsidiaries have a total of 13 employees in the R&D department.
The PRC subsidiaries expect that the R&D expenses will increase significantly in the future, as they continue to develop new products, enhance their existing products and technologies, and perform activities related to obtaining additional regulatory approval.
The PRC subsidiaries adhere to a market-oriented R&D approach and actively cooperate with universities, hospitals, medical institutions, and distributors in sorting out their R&D orientation based on the real market demand. The market-oriented R&D approach includes the following:
| | ● | Independent R&D. The PRC subsidiaries place great emphasis on independent R&D. For the fiscal years ended September 30, 2024, 2023, and 2022, the PRC subsidiaries invested $302,511, $301,644, and $183,900 in purchasing tooling, equipment, raw materials, and paying researchers’ salaries, accounting for 2.63%, 2.22%, and 0.93% of our total revenue, respectively. The PRC subsidiaries have expanded their R&D department since 2022 and searched for new research fields, and hence, they expect to increase their ratio of R&D investment to revenue to 6% to 10% in the following three years. Visualized prostatic dilatation catheter is the latest products of the research team. |
| ● | Universities and Hospitals to Factories. Many universities and hospitals have research centers where they develop and patent certain R&D results. The PRC subsidiaries from time to time communicate with research personnel from research centers and keep themselves informed of the latest R&D and patents, and if needed by their customers, purchase patents from the research centers and research, develop and manufacture products with such patents. For instance, Hangzhou Shanyou entered into a patent transfer agreement (“Jiaxing Hospital Agreement”) with The Second Hospital of Jiaxing City (the “Hospital”) on April 1, 2021 (the “Agreement Date”). Set forth below are the material terms of the agreement: |
| ● | Nature of the patent transferred: a cuff device for endotracheal intubation, which can automatically inflate and deflate and monitor the pressure of columnar balloon; |
| ● | Patent expiration date: May 6, 2029; |
| ● | Payment terms: one-time payment of RMB60,000 (US$8,737) shall be paid by Hangzhou Shanyou to the Hospital within 30 days of the Agreement Date. As of the date of this annual report, Hangzhou Shanyou has paid RMB60,000 under the Jiaxing Hospital Agreement; |
| ● | Hangzhou Shanyou’s rights: (i) Hangzhou Shanyou is entitled to own the patent; and (ii) Hangzhou Shanyou is entitled to improve the inventions and creations derived from the patent. The resulting new achievements of substantial or creative technological progress derived from Hangzhou Shanyou’s work shall belong to Hangzhou Shanyou; |
| ● | Hangzhou Shanyou’s obligations: (i) Hangzhou Shanyou is required to complete the patent transfer registration within 90 days of the Agreement Date; and (ii) Hangzhou Shanyou is required to pay liquidated damages of 30% of the total contract price, which is RMB18,000 (US$2,621), to the Hospital, if Hangzhou Shanyou fails to pay the contract price in full on time; |
| ● | The Hospital’s rights: (i) the Hospital is entitled to a one-time payment of RMB60,000; and (ii) after transferring the patent to Hangzhou Shanyou, the Hospital is also entitled to improve the inventions and creations derived from the patent. The resulting new achievements of substantial or creative technological progress derived from the Hospital’s work shall belong to the Hospital; |
| ● | The Hospital’s obligations: (i) the Hospital will furnish Hangzhou Shanyou with all documents submitted to or received from China National Intellectual Property Administration, including patent specification, patent certificate, and the latest payment receipt of patent annual renewal fee, within 60 days since the date the Hospital received the payment of RMB60,000 from Hangzhou Shanyou; and (ii) the Hospital will ensure that the patent transfer will not infringe on any legitimate right of any third party, and the Hospital is required to assume all responsibility if a third party accuses Hangzhou Shanyou of patent infringement; failure to do so will result in a refund to be issued by the Hospital to Hangzhou Shanyou within 30 days; |
| ● | Mutual rights and obligations: (i) for the duration of the Jiaxing Hospital Agreement, any party that makes improvements to the patent should promptly notify the other party; (ii) if both parties collaborate on technical improvements to the patent and if neither party applies for a patent for the improved technology, the other party has a duty of confidentiality regarding the improved technology, and it shall not disclose, license, or transfer the improved technology to others without permission; and (iii) for substantial technological progress jointly made by both parties, the right to apply for a patent is jointly owned by both parties; |
| ● | Termination: the Jiaxing Hospital Agreement can be terminated because of the occurrence of an event of force majeure; and |
| ● | Dispute resolution: any dispute arising from the Jiaxing Hospital Agreement will be resolved by negotiation and mediation. If negotiation and mediation fail, legal action shall be taken in the people’s court where the Hospital is situated. |
In addition, Hangzhou Hanshi entered into a patent transfer agreement with Zhejiang University (Zhejiang University Agreement) on December 26, 2019. Set forth below are the material terms of the agreement:
| ● | Nature of the patent transferred: a new type of expanded visualized prostatic dilatation catheter; |
| ● | Patent expiration date: November 8, 2028; |
| ● | Payment terms: one-time payment of RMB315,000 (US$45,868) shall be paid by Hangzhou Hanshi to Zhejiang University within five days of the date of the Zhejiang University Agreement. As of the date of this annual report, Hangzhou Hanshi paid RMB319,725 (US$46,556) under the Zhejiang University Agreement, of which RMB315,000 is the contract price and the remaining RMB4,725 (US$688) is the patent transfer service fee charged by China Intellectual Property Trading Center for the patent transfer from Zhejiang University to Hangzhou Hanshi; |
| ● | Hangzhou Hanshi’s rights: Hangzhou Hanshi is entitled to own the patent; |
| ● | Hangzhou Hanshi’s obligations: (i) Hangzhou Hanshi will complete the patent transfer registration; and (ii) Hangzhou Hanshi is required to pay Zhejiang University liquidated damages of 1% of the total contract price per day (RMB3,125 (US$455)), if Hangzhou Shanyou’s designated contact person fails to (a) complete the relevant work assigned according to the agreed upon time, method and place; (b) ensure that it will not be difficult or impossible for Hangzhou Hanshi to perform the Zhejiang University Agreement due to personnel changes; and (c) guarantee Hangzhou Hanshi’s performance of the Zhejiang University Agreement in an appropriate time, manner and standard; |
| ● | Zhejiang University’s rights: (i) Zhejiang University is entitled to a one-time payment of RMB315,000; |
| ● | Zhejiang University’s obligations: (i) Zhejiang University will furnish Hangzhou Hanshi with patent application acceptance notice, patent applications documents, and related technical documents before January 10, 2020. Failure to do so will result in a payment of RMB50,000 (US$7,281) in liquidated damages to be paid by Zhejiang University to Hangzhou Hanshi; and (ii) Zhejiang University will ensure that the patent transfer will not infringe on any legitimate right of any third party. Failure to do so will result in a payment of RMB50,000 in liquidated damages to be paid by Zhejiang University to Hangzhou Hanshi; |
| ● | Mutual rights and obligations: (i) neither party shall restrict the other’s technological competition and development; and (ii) both parties are entitled to improve the inventions and creations derived from the patent; |
| ● | Termination: the Zhejiang University Agreement can be terminated, if: (i) an event of force majeure occurs; (ii) changes occur in national industry policy or there is a change in the technical project manager, within six months since the date of the agreement; or (iii) Hangzhou Hanshi fails to pay the contract price for over three months; and |
| ● | Dispute resolution: any dispute arising from the Zhejiang University Agreement should be resolved by negotiation and mediation. If negotiation and mediation fail, legal action shall be taken in the people’s court where Zhejiang University is situated. |
| ● | Customers’ Feedback from Sales Representatives and Distributors. Sales representatives and distributors receive feedback and proposals from end-users from time to time. The PRC subsidiaries review the feedback and proposals on a regular basis. Prior to developing a product improvement or new product, the PRC subsidiaries assess end-users’ feedback from their sales representatives and distributors in order to better identify the changing needs and demands of end-users. |
As of the date of the annual report, the PRC subsidiaries have 30 registered patents in mainland China. Due to the ever-changing market demands, the PRC subsidiaries expect to continue to invest in acquiring new patents and technologies that are tailored to the market’s fast-changing requirements.
Competition
The medical device industry is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. The PRC subsidiaries compete or plan to compete with manufacturers of disposable medical devices. Some of these competitors are large, well-capitalized companies with greater market share, resources and experience than the PRC subsidiaries have. As a consequence, they are able to spend more on product development, marketing, sales and other product initiatives than the PRC subsidiaries can. The PRC subsidiaries also compete with smaller medical device companies that have only one product or a limited range of products. The PRC subsidiaries’ current competitors include Henan Tuoren Medical Device Co., Ltd., Guangzhou Weili Medical Device Co., Ltd., and Zhejiang Sujia Medical Device Co., Ltd. The PRC subsidiaries compete based on factors such as price, value, customer support, brand recognition, reputation, and product functionality, reliability, and compatibility.
Although there can be no assurance that the PRC subsidiaries will be able to continue to compete successfully in the future, we believe that the PRC subsidiaries can compete successfully with these companies by offering products of better quality at comparable prices.
Quality Control
Medical device are medical products directly applied to the human body, which is closely related to the life and health of users. Quality and safety are always the PRC subsidiaries’ core value. Reliable, safe and stable product quality is an important driving factor for maintaining market competitiveness. Through long-term business dealings with major hospitals and medical institutions across China, the PRC subsidiaries believe that they have developed a sophisticated quality control management system in accordance with the requirements of Chinese laws and regulations.
The quality control management system fully covers the whole process of manufacturing, which consists of three parts: raw materials inspection, production check, and product examination.
Raw materials are inspected when they arrive at Hangzhou Shanyou’s manufacturing facilities. Hangzhou Shanyou’s inspectors check their names, sizes, models, quantities, packaging, and supplier qualifications, to make sure they are in accordance with the purchasing order or receipt. After the materials pass the inspection, the inspectors put them on record and issue a notice of inbound, suggesting them to be put in storage. The quality control managers check the raw materials again, and review the record and notice, granting approval for storage if the materials are compatible with the description of the record and notice.
Hangzhou Shanyou has made process flow diagrams for all the products, specifying technological parameters and critical parts for quality control. Quality examiners will inspect the production process, to confirm if the production strictly follows the diagrams.
Hangzhou Shanyou inspects all its products before shipping them to customers. The following will be checked and examined after production and before Hangzhou Shanyou ships its products to customers: 1) whether the production strictly follows the procedures listed on the process flow diagram; 2) whether key and/or special processes of production are verified; 3) whether modifications of production assignments, raw materials, and production techniques, if applicable, are made according to instructions; and 4) whether all raw materials are checked, including their names, sizes, models, quantities, packaging, and supplier qualifications.
Hangzhou Shanyou prioritizes product quality management. It is committed to strengthening the professional ethics and cultivating quality consciousness of its employees and forming a strict quality management system, which it believes is in line with international standards.
The quality control management programs have earned Hangzhou Shanyou a number of quality-related manufacturing designations. In 2020, Hangzhou Shanyou received an EC certification, which certifies the compliance of its quality management system and technical documentation with European regulations for medical devices and the requirements of Annex V of directive 93/42/EEC have been met for its products (such as endotracheal tubes, laryngeal mask airways, laryngoscope blades, face masks and suction catheters).
In the same year, Hangzhou Shanyou also received ISO 13485 system certification for over 20 products, including nasogastric tubes, catheter mounts, intubating stylets, artery compression tourniquets, and endotracheal tube holders, demonstrating their ability to provide such safe and high-quality medical devices that meet ISO requirement.
In 2023, Hangzhou Shanyou also registered 17 products with the FDA (registration number: 3007127332), including a surgical respirator, nasal oxygen cannula, reservoir bag and nasopharyngeal airway, allowing their products to enter the U.S. market. It has 17 products that have passed the inspections administered by the local authorities in Zhejiang province and obtained the registration certificates. Additionally, Hunan Saitumofei has one product that has passed the inspections administered by the local authorities in Hunan province and obtained the registration certificate.
However, despite the quality control management system, Hangzhou Shanyou cannot eliminate the risks of errors, defects, or failures. Hangzhou Shanyou may fail to detect or cure defects as a result of a number of factors, many of which are outside our control, including:
| ● | technical or mechanical malfunctions in the production process; |
| ● | human error or malfeasance by quality control personnel; |
| ● | tampering by third parties; and |
| ● | defective raw materials or equipment. |
Failure to detect quality defects in the products could result in patient injury, customer dissatisfaction, or other problems that could harm Hangzhou Shanyou’s reputation and business, expose it to liability, and adversely affect its revenue and profitability. Relevant PRC laws and regulations were formulated to strengthen the administration of rules pertaining to product quality, as well as to clarify the rules on product liability, protect consumers and maintain social and economic order. Products offered for sale in China must meet the relevant quality and safety standards. Violations of state or industrial standards for health, safety and any other related violations may result in civil liabilities and penalties, such as compensation for damages, fines, suspension, or shutdown of business, as well as confiscation of products illegally produced for sale and the sales proceeds of such products.
On December 9, 2019, Hangzhou Shanyou was required to recall a batch of disposable breathing circuits and arrange internal rectification, since a connection part was insufficiently welded at the machine end and a circuit did not pass the leakage test. Hangzhou Shanyou recalled all of the breathing circuits involved and arranged the internal rectification. After investigation and evaluation, Hangzhou Shanyou confirmed the involved products would not cause harm to human health and all the affected goods were disposed of.
On November 16, 2020, Hangzhou Shanyou was required to recall 20,000 disposable medical masks by Heilongjiang Provincial Drug Administration, because the mask strings failed to comply with an elastic requirement. Hangzhou Shanyou recalled the affected batch of masks.
The PRC subsidiaries’ products are subject to various laws and regulations relating to medical devices with various requirements in details, including but not limited to the registration and filings of medical devices, the production and operation license, the production and quality management. Although the PRC subsidiaries endeavor to stay in compliance with such laws and regulations, we cannot assure you that the PRC subsidiaries comply with relevant laws and regulations at all times. Any such failure may have a material and adverse effect on the PRC subsidiaries’ and our business, financial condition, and results of operations.
As of the date of this annual report, we or the PRC subsidiaries are not aware of any other investigations, prosecutions, disputes, claims or other proceedings in respect of quality issues, nor have we or the PRC subsidiaries been penalized additionally or can foresee any penalty to be made by any related PRC government authorities.
Financing
The PRC subsidiaries enter into financing agreements with banks to finance their operations. Set forth below is a detailed description of the PRC subsidiaries’ financing agreements which were filed as exhibits to our registration statement on Form F-1, initially filed with the SEC on April 24, 2023:
| 1. | A comprehensive credit contract between Hangzhou Shanyou and Bank of Beijing dated September 29, 2020. It is a revolving credit contract with an aggregate loan principal of RMB8 million. The term of each loan drawn under the revolving credit contract is 12 months, and the period of loan withdrawal is 12 months starting on the date of this contract. Hangzhou Shanyou fully repaid the loan on September 5, 2022.* |
| 2. | A loan contract affiliated with the comprehensive credit contract between Hangzhou Shanyou and Bank of Beijing dated September 29, 2020. The loan principal was RMB8 million, with an interest rate determined by the one-year LPR, set the day immediately before the withdrawal date, plus 65 basis points. The withdrawal period was from September 29, 2020 to September 28, 2021, and the loan period was one year from the date of withdrawal, with the final maturity date of September 28, 2022. Hangzhou Shanyou fully repaid the loan on September 6, 2021. |
| 3. | A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated January 26, 2021. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR set the day immediately before the contract date, plus 75 basis points, which was 4.6%. The date of loan withdrawal was January 26, 2021, and Hangzhou Shanyou fully repaid the loan on January 25, 2022. |
| 4. | A loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated December 22, 2016. The loan principal was RMB15 million, with an interest rate of 6.06% per month. The term of the loan was from December 22, 2016 to November 21, 2021. Hangzhou Shanyou fully repaid the loan on November 21, 2021. |
| 5. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated January 6, 2020. The loan principal was RMB4 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 216 basis points. The term of the loan was from January 6, 2020 to January 5, 2021. Hangzhou Shanyou fully repaid the loan on January 5, 2021. |
| 6. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated January 15, 2020. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 216 basis points. The term of loan was from January 15, 2020 to January 14, 2021. Hangzhou Shanyou fully repaid the loan on January 14, 2021. |
| 7. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated March 2, 2020. The loan principal was RMB3.5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 117 basis points. The term of loan was from March 2, 2020 to February 27, 2021. Hangzhou Shanyou fully repaid the loan on February 27, 2021. |
| 8. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated June 28, 2020. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 137 basis points. The term of loan was from June 28, 2020 to June 27, 2021. Hangzhou Shanyou fully repaid the loan on June 27, 2021. |
| 9. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated July 2, 2020. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract, plus 137 basis points. The term of loan was from July 2, 2020 to July 1, 2021. Hangzhou Shanyou fully repaid the loan on July 1, 2021. |
| 10. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 29, 2021. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 100 basis points. The term of loan was from September 29, 2021 to September 23, 2022. Hangzhou Shanyou fully repaid the loan on September 1, 2022. |
| 11. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 29, 2021. The loan principal was RMB19.5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 137 basis points. The term of loan was from September 29, 2021 to September 23, 2022. Hangzhou Shanyou fully repaid the loan on September 7, 2022. |
| 12. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 1, 2022. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 70 basis points. The term of loan was from September 1, 2022, to August 31, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 30, 2023, to extend the maturity date to August 29, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commerical Bank agreed on August 26, 2024, to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 115 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025. |
| 13. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 2, 2022. The loan principal was RMB8 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. The term of loan was from September 2, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 29, 2023, to extend the maturity date to August 28, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 95 basis points. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 26, 2024, to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 105 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025. |
| 14. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 7, 2022. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 105 basis points. The term of loan was from September 7, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 29, 2023, to extend the maturity date to August 28, 2024, and the interest rate was adjusted to be determined by the one-year LPR, set the day immediately before the contract date, plus 95 basis points. On August 26, 2024, Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed to extend the maturity date to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 85 basis points. Hangzhou Shanyou intends to fully repay the loan by August 25, 2025. |
| 15. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated September 7, 2022. The loan principal was RMB19.5 million, with an interest rate determined by the one-year LPR, set on the day immediately before the contract date, plus 105 basis points. The term of loan was from September 7, 2022, to August 30, 2023. Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed on August 30, 2023, to extend the maturity date to August 29, 2024. The interest rate remains the same. On August 29, 2024, Hangzhou Shanyou repaid RMB5 million of the outstanding RMB19.5 million loan to Zhejiang Xiaoshan Rural Commercial Bank. On August 26, 2024, Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank agreed to extend the maturity date of the remaining outstanding RMB14.5 million loan to August 25, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 115 basis points. Hangzhou Shanyou intends to fully repay the remaining outstanding RMB14.5 million loan by August 25, 2025. |
| 16. | A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated March 31, 2023. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 40 basis points. The term of loan was from March 31, 2023, to March 30, 2024. Hangzhou Shanyou fully repaid the loan on March 4, 2024. |
| 17. | A working capital loan contract between Hangzhou Shanyou and China CITIC Bank dated June 28, 2023. The loan principal was RMB9 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 35 basis points. The term of loan was from June 28, 2023, to June 27, 2024. Hangzhou Shanyou fully repaid the loan on June 27, 2024. |
| | 18. | A working capital loan contract between Hangzhou Woli and China CITIC Bank dated December 20, 2023. The loan principal was RMB42 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 75 basis points. The term of loan was from December 20, 2023, to December 19, 2024. Hangzhou Woli repaid RMB22 million and RMB20 million on November 28, 2024 and December 4, 2024, respectively. |
| | | |
| | 19. | A working capital loan contract between Hangzhou Woli and China CITIC Bank dated November 28, 2024. The loan principal was RMB22 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 90 basis points. The term of loan was from November 28, 2024, to June 6, 2025. Hangzhou Woli intends to fully repay the loan by June 6, 2025. |
| | 20. | A working capital loan contract between Hangzhou Woli and China CITIC Bank dated December 4, 2024. The loan principal was RMB20 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 90 basis points. The term of loan was from December 4, 2024, to June 6, 2025. Hangzhou Woli intends to fully repay the loan by June 6, 2025. |
| | | |
| | 21. | A working capital loan contract between Hangzhou Shanyou and Bank of Beijing dated June 29, 2023. The loan principal was RMB10 million, with an interest rate determined by the one-year LPR, set the day immediately before the date of loan drawdown. The term of loan was from June 29, 2023, to June 29, 2024. Hangzhou Shanyou fully repaid the loan on June 28, 2024. |
| | 22. | A working capital loan contract between Hangzhou Shanyou and Bank of Jiangsu dated March 4, 2024. The loan principal was RMB3 million, with an interest rate determined by the one-year LPR, set the day immediately before the contract date, plus 60 basis points. The term of loan was from March 4, 2024, to September 3, 2024. Hangzhou Shanyou and Bank of Jiangsu agreed on September 3, 2024 to extend the maturity date to September 2, 2025, and the interest rate was adjusted to be determined by the one-year LPR, set the date immediately before the contract date, plus 70 basis points. Hangzhou Shanyou intends to fully repay the loan by September 2, 2025. |
| | 23. | A working capital loan contract between Hangzhou Shanyou and China CITIC Bank (Hangzhou) dated July 26, 2024. The loan principal was RMB6 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 35 basis points. The term of loan was from July 26, 2024 to July 19, 2025. Hangzhou Shanyou intends to fully repay the loan by July 19, 2025. |
| | 24. | A working capital loan contract between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank dated July 11, 2024. The loan principal was RMB5 million, with an interest rate determined by the one-year LPR, set on the contract date, plus 75 basis points. Hangzhou Shanyou intends to fully repay the loan by July 10, 2025. |
| * | The loan principal was RMB8 million, with an interest rate determined by the one-year LPR, set the day immediately before the withdrawal day, plus 65 basis points. The withdrawal period was from September 29, 2021 to September 28, 2022, and the loan period was one year from the date of withdrawal. There was not an independent loan contract signed by both parties, and Hangzhou Shanyou fully repaid the loan on September 5, 2022. |
Environmental Matters
Hangzhou Shanyou’s production activities are governed by PRC laws and regulations. The wastewater Hangzhou Shanyou generates is sanitary wastewater, which can be disposed directly into municipal pipelines. The corner wastes generated are cleaned and collected by the cleaning personnel on time, and transported to the municipal garbage disposal site for treatment by the local sanitation department. Solid wastes generated during operation are collected and sent to relevant manufacturers for recycling. If new products are developed in the future, Hangzhou Shanyou will take corresponding environmental protection measures according to relevant laws and regulations.
As of the date of this annual report, except as disclosed in this annual report, neither we nor Hangzhou Shanyou are aware of any warning, investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has Hangzhou Shanyou been punished or can it foresee any punishment to be made by any government authorities of the PRC with regard thereto.
Employees
As of September 30, 2024, 2023, and 2022, the PRC subsidiaries had a total of 216, 216, and 278 full-time employees, respectively. The following table sets forth the number of the full-time employees categorized by areas of operations as of September 30, 2024:
| Function/Department | | Number | |
| Manufacturing | | | 137 | |
| Research and Development | | | 13 | |
| General | | | 15 | |
| Sales and Marketing | | | 47 | |
| Management | | | 17 | |
| Total | | | 229 | |
Our success depends on the PRC subsidiaries’ ability to attract, motivate, train and retain qualified personnel. We believe the PRC subsidiaries offer their employees competitive compensation packages and an environment that encourages self-development and, as a result, have generally been able to attract and retain qualified personnel and maintain a stable core management team.
As required by PRC laws and regulations, the PRC subsidiaries participate in various employee benefit plans that are organized by municipal and provincial governments, including, among other things, pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance, and housing fund plans through a PRC government-mandated benefit contribution plan. The PRC subsidiaries are required under PRC laws to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of their employees. As of the date of this annual report, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the fiscal years of 2024, 2023, and 2022, $300,945, $287,629, and $295,009 of contributions for social insurance fund, respectively, should have been made but were not. For the fiscal years of 2024, 2023, and 2022, $94,815, $90,620, and $92,945 of contributions for the housing provident fund, respectively, should have been made but were not. Please see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties” on page 40.
We believe the PRC subsidiaries maintain a good working relationship with their employees, and the PRC subsidiaries have not experienced any material labor disputes. None of their employees are represented by a labor union.
Properties
The PRC subsidiaries’ headquarters, manufacturing facilities and office spaces are located in Hangzhou, Zhejiang Province, Changsha, Hunan Province, and Shanghai, in the PRC.
Properties the PRC Subsidiaries Own
The PRC subsidiaries own the premises of their headquarters, manufacturing facilities, office spaces, and a warehouse, which cover an aggregate building area of approximately 356,176 square feet, with the breakdown set for in the below table:
| Description/Use | | Owner | | Location | | Area
(square
feet) | |
| Office space & manufacturing facility | | Hangzhou Shanyou | | No.138, Building 1 – 3, Louta Town, Xiaoshan District, Hangzhou | | | 97,003 | |
| Manufacturing facility | | Hangzhou Shanyou | | No.138, Building 5, Louta Town, Xiaoshan District, Hangzhou | | | 103,780 | |
| Manufacturing facility | | Hangzhou Shanyou | | No.138, Building 6, Louta Town, Xiaoshan District, Hangzhou | | | 147,266 | |
| Warehouse(1) | | Hangzhou Shanyou | | No.138, Building 5, Louta Town, Xiaoshan District, Hangzhou | | | 8,127 | |
| Total | | | | | | | 356,176 | |
Notes:
| (1) | As of the date of this annual report, Hangzhou Shanyou has not obtained the property ownership certificate for this warehouse. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—The PRC subsidiaries are subject to a variety of construction laws, and they could incur liability if they fail to comply with such laws, which could adversely affect their operations.” |
Leased Properties
In addition to the above-mentioned properties that the PRC subsidiaries own, they currently lease several properties in Hangzhou, Shanghai and Hunan for an aggregate area of approximately 80,690 square feet for warehouses and office space.
The breakdown of the leased properties is as follows:
| Lessor | | Lessee | | Location | | Area
(Square
Feet) | | | Rent | | Term | | Use |
| Hangzhou Tianxia Weaving Co., Ltd. | | Hangzhou Shanyou | | Building No. 113, Louta Town, Xiaoshan District | | | 21,528 | | | $9,840 (RMB70,000.00) | | November 30, 2024 to May 29, 2025 | | Sterilization Warehouse |
| Hangzhou Xiaoshan Louta Guancun Stock Economic Union | | Hangzhou Shanyou | | Development District | | | 2,828 | | | $345.29 (RMB2,456.25) | | January 1, 2023 to December 31, 2025 | | Driveway |
| Hangzhou Xiaoshan Louta Guancun Stock Economic Union | | Hangzhou Shanyou | | Development District | | | 9,831 | | | $962.96 (RMB6,850.00) | | January 1, 2023 to December 31, 2025 | | Driveway |
| Louta Town Village Committee | | Hangzhou Shanyou | | Development District | | | 45,208 | | | $1,955 (RMB12,600.00) from January 1, 2011 to December 31, 2013 $3,910 (RMB25,200.00) from January 1, 2014 to December 31, 2027 | | January 1, 2011 to December 31, 2027 | | Warehouse and Parking Area |
| Ailiu Real Estate Co., Ltd. | | Shanghai Chuqiang | | Caojing Town, Jinshan District, Shanghai | | | 108 | | | $372 (RMB2,400.00) | | February 27, 2018 to February 26, 2028 | | Office |
| | | | | | | | | | | | | | | |
| Hunan Hua Mao Doctor Medical Technology Co., Ltd. | | Hunan
Saitumofei | | Room 201, 204, Building A42, Lian Dong Jin Yu Industrial Center, Wang Cheng Economic and Technological Development Zone, Changsha | | | 646 | | | $553.99 (RMB4,000) | | November 1, 2024 to October 31, 2029 | | Office and Manufacturing Facility |
| Jufu (Shanghai) Enterprise Management Consulting Co., Ltd. | | Shanghai Saitumofei | | Room 145, Zone A, 1/F, Building 6, No. 4997, Bao’an Highway, Anting Town, Shanghai | | | 114 | | | $689.28 (RMB5,029.7) | | June 1, 2024 to May 31, 2025 | | Office |
| Huangshan Tunxi District State-owned Assets Investment and Operation Co., Ltd. | | Huangshan Saitumofei | | No. 6-2 – 6-3, Xingyu Road, Tunxi District, Huangshan | | | 427 | | | $1,715.03 (RMB12,383) | | August 1, 2024 to July 31, 2025 | | Storefront |
Hangzhou Shanyou’s manufacturing facilities, located in No.138, Louta Town, Xiaoshan District, Hangzhou, have capacity for 200 workers, and their annual production capacity is demonstrated in the following table:
| Medical Devices | | Number
(thousand) | |
| Anesthetic Kits | | | 200 | |
| Endotracheal Tube Holders | | | 1,500 | |
| Disposable Breathing Circuits | | | 1,000 | |
| Nasogastric Tubes | | | 200 | |
| Foreskin Circumcision | | | 500 | |
| Artery Compression Tourniquets | | | 1,000 | |
| HMEF | | | 500 | |
| Intubating Stylets | | | 500 | |
| SPO2 Sensor | | | 500 | |
| Anesthesia Masks | | | 200 | |
| Guedel Airways | | | 2,000 | |
| Nasal Oxygen Cannulas | | | 1,500 | |
| Yankauer Suction Sets | | | 500 | |
| Oxygen Transferring Masks | | | 5,000 | |
| Nebulizer Masks | | | 15,000 | |
| Wireless Analgesic Pumps | | | 50 | |
| Masks | | | 474,500 | |
Intellectual Property
The PRC subsidiaries’ business is dependent on a combination of trademarks, patents, domain names, and other proprietary rights in order to protect the PRC subsidiaries’ intellectual property rights. As of the date of this annual report, the PRC subsidiaries have 16 registered trademarks, 30 registered patents, one domain name and one copyright in mainland China.
Trademarks
Set forth below is a detailed description of the PRC subsidiaries’ registered trademarks:
| Country | | Trademark | | Trademark
Registration
No. | | Trademark
Name | | Trademark
Registration
Date | | Trademark
Classes | | Trademark
Owner | | Trademark
Term |
| China | |  | | 3496763 | | Work 沃克 | | 09/21/2004 | | 10 | | Hangzhou Shanyou | | 09/21/2004 – 09/20/2034 |
| China | |  | | 45207397 | | Work 沃克 | | 04/07/2021 | | 10 | | Hangzhou Shanyou | | 04/07/2021 – 04/06/2031 |
| China | |  | | 36105130 | | Botailong 博泰龙 | | 11/28/2019 | | 10 | | Hangzhou Shanyou | | 11/28/2019 – 11/27/2029 |
| China | |  | | 70105831 | | WORK, sanyou | | 10/28/2023 | | 3 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
| China | |  | | 70112405 | | WORK, sanyou | | 11/07/2023 | | 5 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
| China | |  | | 70113945 | | WORK, sanyou | | 11/07/2023 | | 10 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
| China | |  | | 70117006 | | WORK, sanyou | | 10/28/2023 | | 24 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
| China | |  | | 70107445 | | WORK, sanyou | | 08/28/2023 | | 36 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| China | |  | | 70107427 | | WORK, sanyou | | 08/28/2023 | | 35 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| China | |  | | 70106256 | | WORK, sanyou | | 08/28/2023 | | 44 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| Country | | Trademark | | Trademark
Registration
No. | | Trademark
Name | | Trademark
Registration
Date | | Trademark
Classes | | Trademark
Owner | | Trademark
Term |
| China | |  | | 70104692 | | WORK, sanyou | | 08/28/2023 | | 42 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| China | |  | | 70104659 | | WORK, sanyou | | 10/28/2023 | | 41 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
| China | |  | | 70104259 | | WORK, sanyou | | 08/28/2023 | | 9 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| China | |  | | 70102953 | | WORK, sanyou | | 08/28/2023 | | 16 | | Hanagzhou Shanyou | | 08/28/2023 – 08/27/2033 |
| China | |  | | 70093576 | | WORK, sanyou | | 10/28/2023 | | 21 | | Hanagzhou Shanyou | | 10/28/2023 – 10/27/2033 |
| China | |  | | 70091370 | | WORK, sanyou | | 11/07/2023 | | 11 | | Hanagzhou Shanyou | | 11/07/2023 – 11/06/2033 |
Patents
According to the PRC Patent Law promulgated by the SCNPC on March 12, 1984, and last amended on October 17, 2020 with effect from June 1, 2021, and as advised by our PRC counsel, AllBright Law Offices (Fuzhou), during the patent protection period, a patent can be renewed every year to preserve the exclusive proprietary rights in the patent by paying an annual fee commencing from the year in which the patent right is granted; otherwise, the patent right will be terminated in mainland China. Once a patent is expired, it cannot be renewed, however, the patent can still be used. The PRC subsidiaries closely monitor the payment date of each patent and have, as of the date of this annual report, paid patent fees in a timely manner to maintain the patent’s validity, except for the few patents that the PRC subsidiaries do not intend to continue to own. The Company does not expect that the loss of any particular patent would have a materially adverse effect on the PRC subsidiaries’ business and the Group’s financial condition and results of operations, for the following reasons: i) through the firm relationship with the suppliers and customers, professional brand loyalty and stable sales channels established during the protection period, we believe that the PRC subsidiaries’ products can maintain stable sales performances after the patent protection period; ii) the core customers of the PRC subsidiaries are hospitals at all levels in China, and these hospitals strictly choose their suppliers based on suppliers’ product quality and quantity, timeliness of delivery, reputation, etc.; and iii) the PRC subsidiaries plan to continuously keep investing in R&D and developing new patents. Therefore, after the protection period, even though other manufactures can produce medical devices with the PRC subsidiaries’ patents, it is expected that the PRC subsidiaries will still be able to sell their products to their core customers. In addition, the PRC subsidiaries expect to constantly improve and upgrade existing technologies, which can generate new technical patents, which may preserve their prominence in this field despite any patent losses. Set forth below is a detailed description of registered patents which are all owned by the PRC subsidiaries:
| Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
| China | | ZL201822130552.0 | | A carbon dioxide absorption tank 1 | | 07/31/2020 | | Utility model | | 12/18/2028 | | Hangzhou Shanyou |
| China | | ZL201520791869.2 | | An arterial compression hemostat | | 03/02/2016 | | Utility model | | 10/14/2025 | | Hangzhou Shanyou |
| China | | ZL202021052943.6 | | A new-type anti-allergy mask | | 04/09/2021 | | Utility model | | 06/10/2030 | | Hangzhou Shanyou |
| China | | ZL202021230920.X | | A new-type vaginal dilator | | 05/25/2021 | | Utility model | | 06/29/2030 | | Hangzhou Shanyou |
| China | | ZL201530411996.0 | | Compression hemostat | | 04/13/2016 | | Appearance design | | 10/23/2025 | | Hangzhou Shanyou |
| China | | ZL201721459172.0 | | Hemostat and hemostat module | | 06/28/2019 | | Utility model | | 11/03/2027 | | Hangzhou Shanyou |
| China | | ZL201930731098.1 | | Prostate dilatation catheter (one balloon) | | 10/30/2020 | | Appearance design | | 12/26/2029 | | Hangzhou Hanshi |
| China | | ZL201922387773.0 | | A prostate dilatation catheter | | 02/02/2021 | | Utility model | | 12/26/2029 | | Hangzhou Hanshi |
| Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
| China | | ZL202022331716.3 | | An umbilical cord ligation ring for newborn | | 09/28/2021 | | Utility model | | 10/19/2030 | | Hangzhou Shanyou |
| China | | ZL201922420338.3 | | A new-type prostate dilatation catheter | | 02/02/2021 | | Utility model | | 12/26/2029 | | Hangzhou Hanshi |
| China | | ZL202022231860.X | | A new type of protective mask | | 12/3/2021 | | Utility model | | 10/09/2030 | | Hangzhou Shanyou |
| China | | ZL202022374054.8 | | An umbilical cord ligature | | 11/30/2021 | | Utility model | | 10/22/2030 | | Hangzhou Shanyou |
| China | | ZL202022374829.1 | | A new type of umbilical cord ligation ring for newborns | | 11/30/2021 | | Utility model | | 10/22/2030 | | Hangzhou Shanyou |
| China | | ZL201920630410.2 | | A tracheal intubation can automatically inflate and deflate monitoring pressure cuff device | | 04/04/2020 | | Utility model | | 05/06/2029 | | Hangzhou Shanyou |
| China | | ZL201821836490.9 | | A visual prostate expander | | 05/22/2020 | | Utility model | | 11/08/2028 | | Hangzhou Hanshi |
| China | | ZL202010604739.9 | | A umbilical cord clamping device | | 10/21/2022 | | Invention | | 06/28/2040 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL202221864363.6 | | Filtration mechanism and a new type of mask containing the said filtration mechanism | | 02/03/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
| China | | ZL202221867065.2 | | Filter and novel mask containing the filter | | 02/03/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
| China | | ZL202221867105.3 | | A new type of filter mask | | 03/21/2023 | | Utility model | | 07/17/2032 | | Hangzhou Shanyou |
| China | | ZL201910748225.8 | | An umbilical cord cutting device | | 01/23/2024 | | Invention | | 08/13/2039 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL201910748013.X | | A kind of umbilical cord clamp and its processing process | | 09/29/2023 | | Invention | | 08/13/2039 | | Hangzhou Shanyou |
| China | | ZL201811548926.9 | | A kind of carbon dioxide absorption tank | | 03/19/2024 | | Invention | | 12/17/2038 | | Hangzhou Shanyou |
| China | | ZL202010605813.9 | | A novel umbilical cord clamping device | | 01/23/2024 | | Invention | | 06/28/2040 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL202011602759.9 | | Data labeling processing method, device, and system | | 07/18/2023 | | Invention | | 12/28/2040 | | Shanghai Saitumofei |
| China | | ZL202330142507.0 | | Umbilical cord clamp | | 09/26/2023 | | Appearance design | | 03/22/2038 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL202320646770.8 | | A clamping device for the umbilical cord | | 10/27/2023 | | Utility model | | 03/22/2033 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL202221818091.6 | | A clamping and connecting device for umbilical cords | | 07/21/2023 | | Utility model | | 07/11/2032 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| Country | | Patent No. | | Patent Name | | Patent
Publication
Date | | Patent Type | | Patent
Validity
Period | | Patent Owner |
| China | | ZL202111621435.4 | | A clamping device for umbilical cord clamps | | 07/16/2024 | | Invention | | 12/27/2041 | | Hangzhou Shanyou and Hangzhou Obstetrics and Gynecology Hospital |
| China | | ZL202330861318.9 | | Breathing circuit tube | | 07/16/2024 | | Appearance design | | 12/27/2038 | | Hangzhou Shanyou |
| China | | ZL202323627605.7 | | A breathing circuit | | 11/12/2024 | | Utility model | | 12/28/2033 | | Hangzhou Shanyou |
Domain Name
The PRC subsidiaries own a Certification of Global Top Level Domain Name of “hzsy120.com”, with a registration date of August 15, 2003, and an expiration date of August 15, 2031.
Copyrights
Set forth below is a detailed description of our registered copyright, which is owned by Shanghai Saitumofei:
| Country | | Copyright No. | | Copyright Name | | Copyright
Publication
Date | | Copyright Type | | Copyright
Application Date |
| China | | 2020SR1798799 | | White blood cell classification system based on convolutional neural network V1.0 | | Unpublished | | software copyright | | 12/11/2020 |
The term of protection of this computer software copyright shall be 50 years, expiring on December 31 of the fiftieth year after the first publication of such software. However, if such software has not been published within fifty years from the date on which its development has been completed, it shall be no longer protected under relevant regulations in the mainland China, and the term of protection could not be extended. The owner of software copyright has the right to decide whether to make the software available to the public. Even though the above computer software is unpublished, the rights of software copyright owner are still protected by relevant regulations.
Insurance
The PRC subsidiaries provide social security insurance including pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan for their employees. They do not carry any key-man life insurance or product liability insurance and have not purchased any property insurance or business interruption insurance. The PRC subsidiaries have determined that the costs of insuring for related risks and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical. Please refer to “Item 3. Key Information—D. Risk Factors—Risks Related to the PRC Subsidiaries’ Business and Industry—The PRC subsidiaries do not have any product liability, business interruption, or property insurance and they may incur liabilities that are not covered by insurance, which could expose them to significant costs and business disruption” on page 20.
Legal Proceedings
As of the date of this annual report, neither we nor the PRC subsidiaries are a party to any material legal or administrative proceedings. From time to time, the PRC subsidiaries may be subject to various claims and legal actions arising in the ordinary course of business. Litigation or any other legal or administrative proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of the PRC subsidiaries’ resources, including management’s time and attention. Furthermore, as of the date of this annual report, the PRC subsidiaries are not a party to any international claims or litigation with respect to defective products or other matters.
Regulations
This section summarizes the principal PRC laws, rules and regulations relevant to the PRC subsidiaries’ business and operations and the Hong Kong laws and regulations which are relevant to the business. This summary does not purport to be a complete description of all laws and regulations, which apply to the PRC subsidiaries’ business and operations. Investors should note that the following summary is based on relevant laws and regulations in force as of the date of this annual report, which may be subject to change.
The Classification, Registration and Filing of Medical Devices
According to the Regulations on the Supervision and Administration of Medical Devices (as amended in 2021) (the “2021 Medical Device Regulation”) which was promulgated by the State Council on February 9, 2021 and took effect on June 1, 2021, the CFDA, now known as the NMPA, is in charge of the national supervision and administration of medical devices. The relevant departments under the State Council shall be responsible for the supervision of medical devices within their respective scope of authorities. The food and drug supervision and administration departments of the local governments at the county level and above are responsible for the supervision and administration of medical devices within their own administrative districts.
In China, medical devices have been classified into three categories based on the degree of risk. Class I medical devices shall refer to those devices with low degree of risk and whose safety and effectiveness can be ensured through routine administration. Class II medical devices shall refer to those devices with medium degree of risk and whose safety and effectiveness shall be strictly controlled and administered. Class III medical devices shall refer to those devices with high degree of risk and whose safety and effectiveness must be strictly controlled and administered with special measures.
The products the PRC subsidiaries currently produce and sell in China are Class I and Class II medical devices. As of the date of this annual report, the PRC subsidiaries have complied with the relevant laws and regulations regarding the production and sale of medical devices in all material aspects, and each of the PRC subsidiaries has obtained the licenses required for their respective business operations in accordance with relevant regulations.
Furthermore, according to the 2021 Medical Device Regulation, enterprises that export medical devices should ensure that the exported medical devices meet the legal requirements of the importing country or region.
Pursuant to the Measures for the Administration of Registration and Record-filing of Medical Devices promulgated by the State Administration for Market Regulation (“SAMR”) on August 26, 2021 and came into effect on October 1, 2021, Class II and Class III medical devices are subject to product registration management. Class I medical devices are subject to product filing management. Record-filing parties of domestic Class I medical devices shall submit record-filing materials to the drug regulatory authorities at the cities with subordinate districts. Domestic Class II medical device shall be examined by the drug regulatory authorities of provinces, autonomous regions and centrally-administered municipalities which shall issue the Medical Device Registration Certificate upon approval after examination. Domestic Class III medical devices shall be examined by the NMPA which shall issue the Medical Device Registration Certificate upon approval after examination. The registration certificate for a medical device is valid for five years and the registrant shall apply to the original registration departments for renewal at least six months prior to its expiration date.
According to the Measures for the Administration of Registration and Record-filing of Medical Devices, the clinical evaluation shall be conducted for the registration or record-filing of medical devices, except for circumstances specified under any of the following circumstances: (i) the medical device has a clear working mechanism, finalized design and mature production process, and the marketed medical devices of the same kind have been used in clinical application for years (number unspecified) with no record of serious adverse events, and does not change the general purpose of the medical device; or (ii) any other circumstance where the safety and effectiveness of the medical device can be proved through non-clinical evaluation. On September 16, 2021, the NMPA issued its Announcement on Issuing Catalog of Medical Devices Exempted from Clinical Evaluation, which came into effect on October 1, 2021 and was amended on July 20, 2023.
For certain Class III medical devices that are subject to clinical trials with high risk to the human body, approval from the NMPA is required before clinical trials. On September 14, 2020, the NMPA issued the Notice on the List of Class III Medical Devices Subject to Clinical Trial Approval (2020 Revision), which revised the original List of Class III Medical Devices Subject to Clinical Trial Approval and came into effect on September 14, 2020.
According to the Measures for the Administration of Registration and Filing of Medical Devices, the registrants of medical devices shall take the initiative to carry out post-marketing research, further confirm the safety, effectiveness and quality controllability of the medical devices and strengthen the continuous management of the medical devices on the market. In case of any substantial change of the designs, raw materials, production technologies, scopes of application and application methods, etc., of the registered medical device products of Class II or Class III, which may affect the safety or effectiveness of such medical devices, the registrants shall apply to the original registration departments for changing registration.
In addition, Measures for the Administration of Registration and Record-filing of Medical Devices provide details on other aspects such as product development and manufacturing, clinical evaluation, registration system verification, product registration, change of registration, renewal of registration, product filing, etc. It also states special registration procedures, such as the innovative product registration procedure, the priority registration procedure, the contingency registration procedure, etc.
Production and Quality Management of Medical Devices
Pursuant to the 2021 Medical Devices Regulation and the Administrative Measures of Production of Medical Devices (2017), promulgated by the CFDA, amended and effective from November 17, 2017, a producer of medical devices shall satisfy the following conditions: (i) possess production sites, environmental conditions, production equipment and professional technicians that are suitable for such medical device produced; (ii) possess organizations or professional examination staff and examination equipment that carry out quality examination for such medical device produced; (iii) formulate a management system which ensures the quality of such medical device; (iv) have capability of after-sale services suitable for such medical device produced; and (v) satisfy the requirements as prescribed in production R&D and production technique documents.
On March 10, 2022, the SAMR promulgated the Administrative Measures of Production of Medical Devices (2022) (SAMR Notice No.53 [2022]), which came into effective on May 1, 2022 and replace the Administrative Measures of Production of Medical Devices (2017). From May 1, 2022, new applications for medical device production and business activities shall go through licensing or filing according to the relevant provisions of the 2021 Medical Devices Regulation and the Administrative Measures of Production of Medical Devices (2022).
The enterprises engaging in the production of medical devices of Class I shall complete record-filing with NMPA at city level where such entity is located; and an entity engaging in the production of medical devices of Class II or III shall obtain a production permit of medical devices from NMPA at provincial level. The Permit for the Medical Devices Production is valid for 5 years and the registrant shall apply to the original departments that issued such permit for renewal at least 6 months prior to its expiration date.
The Production Measures and the Standards on Production and Quality Management of Medical Devices (the “Standards on Production and Quality Management”) which was promulgated on December 29, 2014 and came into effect on March 1, 2015, stipulates that an enterprise engaging in the production of medical devices shall establish and effectively maintain a quality control system in accordance with the requirements of the Standards on Production and Quality Management. The enterprise engaging in the production of medical devices shall regularly conduct comprehensive self-inspection on the operation of quality management system in accordance with the requirements of the Standards on Production and Quality Management and submit a self-inspection report to the provincial branches of the NMPA or the local branches at the prefectural city level before the end of every year. The enterprise shall establish its procurement control procedures and assess its suppliers by establishing an examination system to ensure the purchased products are in compliance with the statutory requirements. The enterprise shall record the procurement, production and inspection of raw materials. Such records shall be true, accurate, complete and traceable. The enterprise shall apply risk management to the whole process of design and development, production, sales and after-sale services. The measures being adopted shall be applicable to risks associated with the related products.
Pursuant to The Notice of Four Guidelines including On-site Inspection Guidelines for the Standards on Production and Quality Management of Medical Devices promulgated on September 25, 2015 and came into effect on the same date, during the course of on-site verification of the registration of medical devices and on-site inspection of production permit (including changing production permit), the inspection team shall, in accordance with the guidelines, issue recommended conclusions for on-site inspections, which shall be divided into “Passed,” “Failed” or “Reassessment after rectification.” During the supervision and inspection, if it is found that the requirements of the key items or ordinary items that may have direct impact on product quality are not satisfied, the enterprise shall suspend production and go through rectification.
If found that the requirements of the ordinary items are not satisfied, and it does not directly affect product quality, the enterprise shall rectify in a prescribed time. The regulatory authorities shall examine and verify the recommended conclusions and on-site inspection materials submitted by the inspection group and issue the final inspection results.
Clinical trials on medical devices shall be conducted by organizations that possess relevant qualifications as required by the Good Clinical Practice for Medical Devices Trial. According to the Good Clinical Practice for Medical Devices, which became effective on June 1, 2016, the Good Clinical Practice includes full procedures of clinical trial of medical devices, including, among others, the protocol design, conduct, monitoring, verification, inspection, and data collection, recording, analysis and conclusion and reporting procedure of a clinical trial.
Prior to a clinical trial, the applicant shall complete the preclinical study of the investigational medical devices, including product design (structural composition, working principle and mechanism of action, intended use and scope of application, applicable technical requirements) and quality inspection, animal trial and risk analysis, and the result shall support the clinical trial, and the applicant also shall enter into an agreement in writing with the clinical trial organization and researchers regarding matters such as the design of the trial, quality control of the trial, division of responsibility in the trial.
Quality inspection results shall include a self-inspection report and a registration inspection report valid within one year and issued by an eligible inspection institution. Clinical trial of medical devices shall be conducted in two or more clinical trial institutions of medical devices. Before subjects participate in a clinical trial, investigators shall provide the subjects or guardians of persons without or with limited capacity for civil conduct with extensive details about the clinical trial, including known and foreseeable risks and possible adverse events. After full and detailed explanation, the subjects or their guardians and investigators shall sign the informed consent forms with their names and dates. The clinical trial shall be subject to the approval of the ethics committee of clinical trial institution of medical devices. The medical devices included in the List of Class III Medical Devices Requiring Clinical Trial Approval shall also be approved by the NMPA. Before a clinical trial, the applicant shall file with the supervision and administration departments of the provinces, autonomous regions or municipalities where it is located.
On March 30, 2022, the NMPA promulgated the Notice of Releasing Good Clinical Practice for Medical Devices (NMPA Notice No.21 [2022]), which came into effective on May 1, 2022 and replace the Good Clinical Practice for Medical Devices (2016). According to the NMPA Notice No.21 [2022], within the territory of mainland China, the relevant activities of clinical trials of medical devices for the purpose of applying for registration (including in vitro diagnostic reagents) shall comply with the Good Clinical Practice for Medical Devices. The Good Clinical Practice for Medical Devices covers the whole process of medical device clinical trials, including the design, implementation, supervision, inspection, data collection, data recording, data preservation, data analysis, data summary and data reporting of medical device clinical trials.
Laws and Regulations Relating to Medical Devices Operation
Pursuant to the 2021 Medical Device Regulation and the Measures for the Supervision and Administration of Medical Devices Operation, promulgated on July 30, 2014, effective on October 1, 2014 and amended on November 17, 2017, an enterprise engaging in the operation of medical devices shall have business premises and storage conditions suitable for the operation scale and scope, and shall have the quality management system and quality management institution or quality management personnel suitable for the medical devices it operates. An enterprise engaged in the operation of Class II medical devices shall make filing with the local food and drug supervision and administration department at the level of city with districts and provide proofing materials for its satisfying the relevant conditions of engaging in the operation of medical devices, while an enterprise engaged in the operation of Class III medical devices shall apply for an operation permit to the local food and drug supervision and administration department at the level of city with districts and provide proofing materials for its satisfying the relevant conditions of engaging in the operation of such medical devices.
The food and drug supervision and administration department which accepts operation permit application shall grant the Permit for Medical Devices Operation if the enterprise meets the prescribed requirements. The Permit for Medical Devices Operation is valid for five years and may be renewed pursuant to the relevant regulations. An enterprise engaging in the operation of medical devices shall not operate or use any medical device that has not been legally registered, has no qualification certificate, or is outdated, invalid or eliminated.
The Measures for the Supervision and Administration of Medical Devices Operation was amended on March 10, 2022 by the SAMR and took effective on May 1, 2022, according to which there is no need for a registered holder or record holder of medical devices selling its registered or filed medical devices in its domicile or production site to have the operation permit or filing for its operation of medical devices but shall meet the specified operating conditions, while it still needs to have the operation permit or filing if it stores and sells medical devices in other places.
Tender Processes for Medical Devices
According to the Notice on Further Strengthening the Administration of Centralized Procurement of Medical Devices issued on June 21, 2007, all non-profit medical institutions under all levels of government and state-owned enterprises from different industries shall participate in the centralized procurement of medical devices.
The centralized procurement of medical devices must follow the basic principles of openness, fairness, impartiality and good faith, and the procurement method is mainly based on public bid invitation. All localities should formulate and improve centralized procurement procedures and implementation measures for medical devices in accordance with relevant laws and regulations and in light of local actual conditions, to ensure fair and open procedures. Local authorities shall strictly implement the advance announcement of bidding and procurement information, the announcement of bid evaluation methods and the announcement of bidding results. After the results of the bidding are confirmed, the medical institution should be organized to sign the contract in time and supervise the implementation.
According to the Government Procurement Law of the People’s Republic of China (the “Government Procurement Law”) promulgated by SCNPC on June 29, 2002, and last amended on August 31, 2014, and the Regulations for Implementation of the Government Procurement Law of the People’s Republic of China (the “Implementation Regulations”) promulgated by State Council on January 30, 2015 and came into effect on March 1, 2015, the term “government procurement” means the use of fiscal funds by all levels of State authorities, institutions and social organizations to procure goods, projects and services that fall within the catalogue for centralized procurement formulated in accordance with the law or that are above the procurement limits. Government procurement shall combine centralized and decentralized procurement. The scope of centralized procurement shall be defined by the catalogue for centralized procurement promulgated by the people’s governments at provincial level and above. The catalogue for centralized procurement for government procurement items that fall within the central government budget shall be determined and promulgated by the State Council. The catalogue for centralized procurement for government procurement items that fall within the local government budget shall be determined and promulgated by the people’s government or its authorized institution of provinces, autonomous regions and municipalities directly under the central government. Government procurement items that are included in the catalogue for centralized procurement shall be subject to centralized procurement. The limits for government procurement for government procurement items that fall within the central government budget shall be determined and promulgated by the State Council. The limits for government procurement for government procurement items that fall within local government budgets shall be determined and promulgated by the people’s government or its authorized institution of provinces, autonomous regions and municipalities directly under the central government. Government procurement contracts shall be in writing. A supplier shall be prosecuted for legal liability if it sub-contracts the government procurement contract.
In the event that buyers procure goods or services whose budget amount is more than the standard of public bidding amount, the public bidding must be adopted.
As of the date of this annual report, we believe the PRC subsidiaries have been in full compliance with such law in all material respects when the PRC subsidiaries have participated in bidding procedures and, as of the date of this annual report, none have received any notifications with any non-incompliance of the related rules.
Regulations Relating to Advertisements of Medical Devices
The SAMR promulgated the Interim Measures for the Administration of the Examination and Administration of Drugs, Medical Devices, Health Foods, and Formula Foods for Special Medical Purposes (the “Examination Interim Measures”) on December 24, 2019, which came into effect on March 1, 2020. The Examination Interim Measures stipulates that the advertisements for medical devices shall not be released without being reviewed and the contents of a medical device advertisement shall be based on the contents of the registration certificate or filing certificate approved by the drug administrations, or the registered or filed product instructions. Where the medical device advertisement involves the name, scope of application, functional mechanism or structure or composition, etc. of the medical device, the scopes of the registration certificate or filing certificate, or registered or filed product instruction shall not be exceeded. The validity period of the advertisement approval number for drugs, medical devices, health food and formula food for special medical purposes shall be consistent with the shortest validity period of the product registration certificate, filing certificate or production license. If no valid period is prescribed in the product registration certificate, filing certificate or production license, the valid period of the advertisement approval number shall be two years.
Medical Device Product Export
According to the Regulations on Application for Export Certificate of Medical Devices promulgated on January 6, 1996, the State Administration of Medicine (now known as the NMPA) shall examine the safety and legality of medical device products produced by domestic enterprises, and issue export certificates in accordance with international practice to prove that the products have obtained lawful production licenses within the territory of China.
Pursuant to the Regulations on the Administration of Export Sales Certificates of Medical Devices promulgated on June 1, 2015 and came into effect on September 1, 2015, if the registration certificate for a medical device and production permit for a medical device have been obtained in China, or the medical device registration and production filing have been completed, the food and drug supervision and administration department may issue a Medical Device Product Export Sales Certificate to the relevant manufacturing enterprise. The validity term of the Medical Device Product Export Sales Certificate should not exceed the earliest deadline for the various documents submitted by the enterprise in the application materials, and the maximum validity term shall not exceed two years.
According to the Customs Law of the PRC (Amended in 2021) (“Customs Law”) which was passed by the Standing Committee of the National People’s Congress (“SCNPC”) on April 29, 2021, where a consignee or consignor of importing or exporting goods or a customs clearing enterprise handles customs declaration procedures, they shall be subject to registration by customs in accordance with law. Customs clearing personnel shall obtain the occupational qualifications for customs clearances in accordance with law.
According to the Provisions of the Customs of the PRC on the Administration of Registration of Customs Declaration Entities (Revised in 2018), which was promulgated by the General Administration of Customs on May 29, 2018 and became effective as of July 1, 2018, registration of declaring entities shall be divided into the registration of declaring enterprises, for which approval from the relevant competent authority directly under the General Administration of Customs or the authorized customs affiliate shall be the pre-condition to undertake the declaration procedures at the customs and the registration of consignees or consignors of imported or exported goods, for which no additional approvals need to be obtained. On November 19, 2021, the Provisions of the PRC on the Administration of the Recordation of Customs Declaration Entities were promulgated and became effective on January 1, 2022. The Provisions of the Customs of the PRC on the Administration of Registration of Customs Declaration Entities (Revised in 2018) was repealed simultaneously. According to the Administration of the Recordation of Customs Declaration Entities, the registration of customs declaring entities has been completely changed to recordation, and the recordation is implemented for both customs declaration entities and the consignor or consignee of imported and exported goods. As of the date of this annual report, the Certificate of the Customs of the People’s Republic of China on Registration of Customs Declaration Entity, certifying the PRC subsidiaries’ export activities, is valid.
Medical Device Recalls
Pursuant to the Administrative Measures for Medical Device Recalls, which was promulgated on January 25, 2017 and came into effect on May 1, 2017, in accordance with the severity of harm, medical device recalls are divided into: (i) Class I recall, where the circumstances leading to the recall may cause or have caused serious health hazards; (ii) Class II recall, where the circumstances leading to the recall may cause or have caused temporary or reversible health hazards; or (iii) Class III recall where, the circumstances leading to the recall are not likely to cause harm.
Medical device manufacturers shall determine the recall class based on the specific situation and properly design and implement the recall plan based on the recall class and the sale and use of the medical devices. In terms of Class I recall, the recall notice shall be published on the NMPA website and major media. In terms of Class II and Class III recalls, the recall notice shall be published on the website of the food and drug administrative authority of the provinces, autonomous regions or municipalities.
Online Sales of Medical Device
According to the Administration and Supervision Measures of Online Sales of Medical Devices, which was promulgated on December 20, 2017 and came into effect on March 1, 2018, enterprises engaged in online sales of medical devices must be medical device manufacturers and operation enterprises with medical devices production licenses or operation licenses or enterprises which have been filed for record in accordance with laws and regulations, and shall carry out online sales activities of medical devices through their own website or the third-party platform of online medical devices transaction services. The enterprise engaging in online sales of medical devices through its own website shall obtain an Internet Drug Information Services Qualification License according to the law and have the appropriate office space and technical conditions for data backup and failure recovery in line with the business scale. The third-party platform provider shall obtain an Internet Drug Information Services Qualification License in accordance with the law, have the appropriate office space and technical conditions for data backup and failure recovery in line with the business scale, and set up a special medical device network quality and safety management organization or equip medical device quality and safety management personnel.
Environmental Protection
Pursuant to the Environmental Protection Law of the PRC (Amended in 2014) promulgated on April 24, 2014 and became effective on January 1, 2015, the waste discharge licensing system has been implemented in the PRC and entities that discharge medical sewage to water bodies directly or indirectly shall obtain a waste discharge license. Furthermore, installations for the prevention and control of pollution at a construction project must be designed, built and commissioned together with the principal part of the project. Pursuant to the Environmental Impact Assessment Law of the PRC (Amended in 2018) promulgated on October 28, 2002, become effective on September 1, 2003 and last amended on December 29, 2018, the State implements administration by classification on the environmental impact of construction projects according to the level of impact on the environment. The project facility owner shall prepare an environmental impact report, or an environmental impact form or complete an environmental impact registration form (the “Environmental Impact Assessment Documents”) for reporting and filing purpose. If the Environmental Impact Assessment Documents of a construction project have not been reviewed by the approving authority in accordance with the law or have not been granted approval after the review, the project facility owner is prohibited from commencing construction works. For a construction project for which an Environmental Impact Statement is prepared, the project facility may go into production or be delivered for use only after it undergoes an inspection and acceptance has been made by the facility owner.
Pursuant to the Administrative Regulations on Environmental Protection in Construction Projects (Revised in 2017) promulgated on November 29, 1998, and last amended on July 16, 2017, upon the completion of construction, the project facility owner shall inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection. Failure to inspect the production lines to determine if they comply with relevant regulations of environmental protection and make Inspection and Acceptance Reports of Environmental Protection will result in an order to complete relevant procedures, and a penalty between RMB0.2 million and RMB1 million, will be imposed by local authorities. If the project facility owner fails to complete relevant procedures within the stipulated period, the local authorities will impose a penalty between RMB1 million and RMB2 million. Furthermore, if material environmental pollution or ecological damage is caused by such failure, the project facility owner will be ordered to stop production or stop using the construction project by the PRC regulatory authorities. Pursuant to the Interim Measures for Environmental Protection Acceptance of Completion of Construction Projects promulgated on November 20, 2017, and become effective on the same day, if the project facility owner fails to inspect construction projects and make Inspection and Acceptance Reports of Environmental Protection, its non-compliance will be recorded in Credibility Archive and will be disclosed to the public.
Pursuant to the Law of the PRC on Prevention and Control of Environmental Pollution Caused by Solid Wastes (Revised in 2020), promulgated on April 29, 2020 and taken effect on September 1, 2020, all entities and individuals shall take measures to reduce the output of solid waste, promote the comprehensive utilization of solid waste, and reduce the harmfulness of solid waste. Each enterprise generating industrial solid waste shall obtain a discharge permit, establish a sound accountability system for the prevention and control of environmental pollution caused by industrial solid waste in the whole process of generation, collection, storage, transportation, utilization and treatment, set up a management ledger for industrial solid waste to faithfully keep records on the sorts, quantity, whereabouts, storage, utilization and treatment, etc., of industrial solid waste generated, so as to realize the traceability and querying of industrial solid waste, and take measures for the prevention and control of environmental pollution caused by industrial solid waste. Furthermore, the construction of projects which discharge solid waste and the construction of project for storage, use and treatment of solid waste shall be carried out upon the appraisal regarding their effects on environment and in compliance with the relevant state regulations concerning the management of environmental protection in respect of construction projects. The necessary supporting facilities for the prevention and control of environmental pollution caused by solid wastes as specified in the environmental impact assessment documents of the construction project shall be designed, constructed and put into operation simultaneously with the major construction works of the construction project. No construction projects shall be permitted to be put into operation or to use before its facilities for the prevention and control of environmental pollution caused by solid wastes have been inspected and accepted by the competent department of environmental protection that examined and accepted the environmental impact assessment documents. On December 20, 2019, the Catalogue for Classified Administration of Sewage Discharge Permits for Fixed Pollution Sources was promulgated, according to which the state shall implement pollutant discharge registration administration with respect to entities that discharge pollutants in small quantities or discharge pollutants that have little impact on the environment.
Pursuant to the Law of the PRC on Prevention and Treatment of Water Pollution (Amended in 2017) promulgated on June 27, 2017 and taken effect on January 1, 2018, the environmental protection department of the State Council formulates national waste discharge standards. Enterprises that discharge waste into water shall pay a treatment fee. Each of the local environmental protection bureaus is authorized to regulate water pollution within each of their respective jurisdictions by formulating more specific local standards, and may impose penalties for violation, including suspending operations. The water pollutants discharged into the facilities for central treatment of urban sewage shall conform to the national or local standards for the discharge of water pollutants. Furthermore, the environmental impact assessment shall be conducted on new construction, reconstruction and construction expansion projects or other installations on water which directly or indirectly discharge pollutants into the water according to law. The water pollution prevention and treatment facilities of a construction project must be designed, constructed and put into operation simultaneously with the major construction works of the said construction project. The water pollution prevention and treatment facilities shall comply with the requirements of approved or filed environmental impact assessment documents.
Fire Prevention Management
According to the Fire Prevention Law of the PRC promulgated on April 29, 1998, which was amended on October 28, 2008, April 23, 2019, and April 29, 2021, and the Interim Provisions on the Administration of Fire Protection Design Review and Acceptance of Construction Projects promulgated on April 1, 2020 and amended on October 30, 2023, the design and construction of fire prevention features for construction projects shall conform to state’s fire prevention technical standards. Project owners, design entities, construction entities, project supervision entities, etc., shall be responsible for the fire protection design and the quality of the fire protection for construction of the projects. With respect to construction projects, such projects are subject to inspection and acceptance for fire protection. Project facility owners of special construction projects shall file applications with competent authorities for inspection and acceptance for fire protection. These special construction projects include: (1) sports stadiums, halls, showrooms of public exhibition halls or museums, with a gross floor area of more than 20,000 square meters; (2) terminals of civil airports, waiting rooms of passenger transport stations, or waiting rooms of passenger transport docks, with a gross floor area of more than 15,000 square meters; (3) hotels, restaurants, shopping malls or farmers markets, with a gross floor area of more than 10,000 square meters; (4) movie theaters, reading rooms of public libraries, for-profit indoor fitness or leisure venues, outpatient buildings of hospitals, teaching buildings of universities, libraries, canteens, production and processing workshops of labor-intensive enterprises, temples or churches, with a gross floor area of more than 2,500 square meters; (5) children’s rooms, children’s game rooms or other indoor activity spaces of nurseries or a kindergartens, ward buildings of nursing home, welfare centers, hospitals or sanitariums, teaching buildings, libraries or cafeterias of primary or secondary schools, collective dormitories of schools, or staff collective dormitories of labor-intensive enterprises, with a gross floor area of more than 1,000 square meters; (6) song and dance halls, video halls, projection rooms, Karaoke halls, nightclubs, recreation halls, sauna bathrooms, Internet cafes, bars, restaurants, teahouses or cafes with entertainment functions, with a gross floor area of more than 500 square meters; (7) Class I high-rise residential buildings as set forth in the national technical standards of fire protection for construction projects; (8) urban rail transports, tunnels, large-scale power generation, transformation or distribution projects; (9) plants for production, warehouses for storage, or special stations or docks for loading and unloading of inflammable and explosive hazardous materials, or filling stations, supply stations or pressure regulation stations of flammable and explosive gases and liquid; (10) state authority office buildings, electric power dispatching buildings, telecommunication buildings, postal buildings, disaster prevention commanding and dispatching buildings, radio and television buildings or archives buildings; (11) construction projects under any of the circumstances set forth in examples (1) to (6) mentioned above; or (12) public buildings other than examples (10) and (11), and the gross floor area of a single building is more than 40,000 square meters or the height of the building is more than 50 meters.
Apart from these special construction projects mentioned above, for other construction projects, such as Hangzhou Shanyou’s production lines, a project facility owner shall prepare the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, and such authorities shall conduct a random inspection thereof.
If a project facility owner places into service a construction project which was required to undergo a fire protection inspection and acceptance and prepare an Inspection and Acceptance Report of Fire Protection, but has not undergone such inspection and acceptance and has failed to prepare such Inspection and Acceptance Report of Fire Protection, or has failed the inspection and acceptance, or failed to suspend the use of construction project for which such project facility owner prepared the Inspection and Acceptance Report of Fire Protection for the records of the competent authorities but is later found to be unqualified in a random inspection, such project facility owner will be ordered to stop using the affected construction project, stop production or business operations and a fine of between RMB30,000 and RMB300,000 may be imposed by competent departments. If a project facility owner has undergone a fire protection inspection and acceptance and has already prepared an Inspection and Acceptance Report of Fire Protection but fails to submit the report to the competent authorities for the records, such project facility owner will be subject to a fine of RMB5,000.
As of the date of this annual report, Hangzhou Shanyou, the PRC subsidiary that operates all production lines, has not prepared the Inspection and Acceptance Reports of Fire Protection for the records of the competent authorities, which may result in it being ordered to stop the use of its production lines and being subject to a fine of between RMB30,000 and RMB300,000. Since all of its products are manufactured by operation of such production lines, any such development could materially and adversely affect its and our business, financial condition and results of operations. If Hangzhou Shanyou conducts the inspection and acceptance and prepares the Inspection and Acceptance Reports of Fire Protection but does not submit them to the competent authorities for the records, it will be subject to a fine of RMB5,000. See “Item 3. Key Information—D. Risk Factors—Risks Related to the PRC subsidiaries’ Business and Industry—The PRC subsidiaries are subject to a variety of fire protection laws that could be costly for them to comply with, and they could incur liability if they fail to comply with such laws, which could adversely affect the Group as a whole” on page 23.
Construction Management
According to the Administrative Measures for Construction Permits of Building Projects promulgated by the Ministry of Housing and Urban-Rural Development on June 25, 2014, and last amended on March 30, 2021, for construction and decoration works in respect of various housing construction and auxiliary facilities thereof, installation of circuits, pipelines and equipment, as well as construction of infrastructural works for cities and towns, the construction enterprise shall apply for construction permits from the competent department in accordance with the regulations of the Administrative Measures for urban-rural development of housing of the local people’s government at or above county level where the construction is located prior to the commencement of works. It is not necessary to apply for construction permits for construction works with an investment amount less than RMB0.3 million or of which the gross floor area is less than 300 square meters. The administrative authority in charge of housing and urban-rural development of the people’s government of a province, autonomous region or municipality directly under the Central Government may, in accordance with the specific circumstances prevailing in their respective regions, readjust these limits and notify the department under the State Council responsible for construction for its records. Those who have not obtained the construction permits shall be ordered by the license-issuing authority which has jurisdiction to stop the construction, to correct any such lapse within a time limit, and pay a fine of between 1% to 2% of the contract price of the construction project.
According to the Regulations on the Quality Management of Construction Engineering promulgated by the State Council on April 23, 2019, a construction project owner shall, within 15 days from the date of the completion and acceptance inspection of the construction project, submit the inspection and acceptance report of the construction project and the approval documents issued by the planning, public security, fire control, environmental protection and other departments to the construction administrative department or other relevant departments for the record. If it fails to do so, it shall be ordered to make corrections and pay a fine of between RMB0.2 million to RMB0.5 million. Furthermore, where the project facility is placed into service without being subject to completion inspection and acceptance, the construction owner shall be ordered to make corrections and pay a fine of between 2% to 4% of the contract price of the project. Where there is any loss resulting therefrom, such construction owner shall be liable to compensate for any such loss.
As of the date of this annual report, as to the production lines of Hangzhou Shanyou, which were placed into service without having been subject to completion inspection and acceptance, it did not obtain a construction license prior to the commencement of construction nor undergone the completion inspection and acceptance nor did it prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities, therefore, Hangzhou Shanyou could be fined (i) an amount equal to 1% to 2% of the contract price of the production lines construction (approximately $16,500 to $33,000),for its failure to obtain a construction license prior to the commencement of construction, (ii) plus a fine of between RMB0.2 million to RMB0.5 million (approximately $28,000 to $70,000) for its failure to prepare the Inspection and Acceptance Reports of Construction for the records of the competent authorities and make adequate rectification, and (iii) a fine of between 2% to 4% of the contract price of the production lines construction (approximately $33,000 to $66,100), for its failure to complete inspection and acceptance before placing the production lines into service. As a result, the aggregate amount of potential fine could be between $77,500 to $169,100.
Regulation on Foreign Investment Restrictions
Investment activities in mainland China by foreign investors are principally governed by the Catalog of Industries for Encouraging Foreign Investment (2022 Edition), or the Catalog, as promulgated by the Ministry of Commerce of the People’s Republic of China (“MOFCOM”), and the National Development and Reform Commission (“NDRC”) on October 26, 2022, and the Special Administrative Measures for Access of Foreign Investment (2024 Edition), or the Negative List (2024), as promulgated on September 8, 2021. According to the Negative List (2024), our businesses operated in the PRC do not fall into the restricted or prohibited categories.
In addition, a foreign-invested enterprise in the PRC is required to comply with other regulations on its incorporation, operation and changes. On March 15, 2019, the National People’s Congress (“NPC”) adopted the Foreign Investment Law (the “FIL”), which became effective on January 1, 2020. Pursuant to the FIL, PRC will grant national treatment to foreign-invested entities, except for those foreign-invested entities that operate in industries that fall within “restricted” or “prohibited” categories as prescribed in the Negative List (2024) to be released or approved by the State Council.
On December 26, 2019, the State Council promulgated the Implementation Rules to the Foreign Investment Law, which became effective on January 1, 2020. The implementation rules further clarify that the state encourages and promotes foreign investment, protects the lawful rights and interests of foreign investors, regulates foreign investment administration, continues to optimize a foreign investment environment, and advances a higher-level opening. On December 30, 2019, the MOFCOM and SAMR jointly promulgated the Measures for Information Reporting on Foreign Investment, which became effective on January 1, 2020. Pursuant to the Measures for Information Reporting on Foreign Investment, where a foreign investor carries out investment activities in mainland China, directly or indirectly, the foreign investor or the foreign-invested enterprise shall submit the investment information to the competent commerce department.
On February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. The New Administrative Rules Regarding Overseas Listings refine the regulatory system for domestic company’s overseas offering and listing by subjecting both direct and indirect overseas offering and listing activities to the filing-based administration, and clearly defines the circumstances where provisions for direct and indirect overseas offering and listing apply and relevant regulatory requirements.
According to the New Administrative Rules Regarding Overseas Listings, among other things, a domestic company in the PRC that seeks to offer and list securities on overseas markets shall fulfill the filing procedures with the CSRC as per requirement of the Trial Administrative Measures. Where a domestic company seeks to directly offer and list securities on overseas markets, the issuer shall file with the CSRC. If an issuer offers securities on the same overseas market where it has previously offered and listed securities subsequently, filings shall be made with the CSRC within 3 working days after the offering is completed. Upon occurrence of any material event, such as change of control, investigations or sanctions imposed by overseas securities regulatory agencies or other relevant competent authorities, change of listing status or transfer of listing segment, or voluntary or mandatory delisting, after an issuer has offered and listed securities on an overseas market, the issuer shall submit a report thereof to CSRC within three working days after the occurrence and public disclosure of such event. Further, an overseas securities company that serves as a sponsor or lead underwriter for overseas securities offering and listing by domestic companies shall file with the CSRC within 10 working days after signing its first engagement agreement for such business, and submit to the CSRC, no later than January 31 each year, an annual report of its business activities in the previous year associated with overseas securities offering and listing by domestic companies. If an overseas securities company has entered into engagement agreements before the effectuation of the Trial Administrative Measures and is serving in practice as a sponsor or lead underwriter for overseas securities offering and listing by domestic companies, it shall file with the CSRC within 30 working days after the Trial Administrative Measures take effect.
Under the New Administrative Rules Regarding Overseas Listings, a domestic company is prohibited from overseas offering and listing if any of the following circumstances is involved: (1) where such securities offering and listing is explicitly prohibited by provisions in laws, administrative regulations and relevant state rules; (2) where the intended securities offering and listing may endanger national security as reviewed and determined by competent authorities under the State Council in accordance with laws; (3) where the domestic company intending to make the securities offering and listing, or its controlling shareholders and the actual controller, have committed crimes such as corruption, bribery, embezzlement, misappropriation of property or undermining the order of the socialist market economy during the latest three years; (4) where the domestic company intending to make the securities offering and listing is suspected of committing crimes or major violations of laws and regulations, and is under investigation according to law, and no conclusion has yet been made thereof; and (5) where there are material ownership disputes over equity held by the domestic company’s controlling shareholder or by other shareholders that are controlled by the controlling shareholder and/or actual controller. Moreover, a domestic company that seeks to offer and list securities on overseas markets shall abide by certain other regulatory requirements as set out in the New Administrative Rules Regarding Overseas Listings, including, without limitation to, compliance with laws of national secrecy, foreign investment, cybersecurity, data security, cross-border investment and financing, foreign exchange, and other laws and relevant provisions.
The Trial Administrative Measures also provides that if the issuer both meets the following criteria, the overseas securities offering and listing conducted by such issuer will be deemed as indirect overseas offering by PRC domestic companies: (i) 50% or more of any of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent fiscal year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in mainland China, or its main place(s) of business are located in mainland China, or the majority of senior management staff in charge of its business operations and management are PRC citizens or have their usual place(s) of residence located in mainland China. Where an issuer submits an application for initial public offering to competent overseas regulators, such issuer must file with the CSRC within three working days after such application is submitted. The Trial Administrative Measures also require subsequent reports to be filed with the CSRC on material events, such as change of control or voluntary or forced delisting of the issuer(s) who have completed overseas offerings and listings.
Pursuant to the New Administrative Rules Regarding Overseas Listings, the CSRC will conclude the filing procedures and publish the filing results on the CSRC website within 20 working days after receiving the filing documents if the filing documents are complete and in compliance with stipulated requirements. However, during the filing process, the CSRC may request the Company to supply additional documents or may consult with competent authorities, the time for which will not be counted in the 20 working days. We are required to file with the CSRC within three working days after any subsequent securities offering is completed, pursuant to the New Administrative Rules Regarding Overseas Listings.
According to the Trial Administrative Measures, if a domestic company fails to fulfill filing procedures, or offers and lists securities on an overseas market in violation of the measures, the CSRC shall order rectification, issue warnings to such domestic company, and impose a fine of between RMB1 million and RMB10 million. Directly liable persons-in-charge and other directly liable persons shall be warned and each imposed a fine of between RMB0.5 million and RMB5 million. Controlling shareholders and actual controllers of the domestic company that organize or instruct the aforementioned violations shall be imposed a fine of between RMB1 million and RMB10 million.
On February 24, 2023, the CSRC promulgated the Provisions on Strengthening Confidentiality and Archives Administration of Overseas Securities Offering and Listing by Domestic Companies (the “Confidentiality and Archives Administration Provisions”), which also became effective on March 31, 2023. According to the Confidentiality and Archives Administration Provisions, domestic companies that seek overseas offering and listing (either in direct or indirect means) and the securities companies and securities service (either incorporated domestically or overseas) providers that undertake relevant businesses shall institute a sound confidentiality and archives administration system, and take necessary measures to fulfill confidentiality and archives administration obligations. They shall not leak any state secret or working secret of government agencies, or harm national security and public interests. Therefore, a domestic company that plans to, either directly or through its overseas listed entity, publicly disclose or provide to relevant individuals or entities including securities companies, securities service providers and overseas regulators, any documents and materials that contain state secrets or working secrets of government agencies, shall first obtain approval from competent authorities according to laws, and file with the secrecy administrative department at the same level. The above-mentioned documents and materials that, if leaked, will be detrimental to national security or public interest, therefore, the domestic company shall strictly fulfill relevant procedures stipulated by applicable regulations.
Furthermore, the Confidentiality and Archives Administration Provisions stipulates that a domestic company that provides accounting archives or copies of accounting archives to any entities, including securities companies, securities service providers and overseas regulators and individuals, shall fulfill due procedures in compliance with applicable regulations. Working papers produced in mainland China by securities companies and securities service providers in the process of undertaking businesses related to overseas offering and listing by domestic companies shall be retained in mainland China. Where such documents need to be transferred or transmitted to areas outside of mainland China, relevant approval procedures stipulated by regulations shall be followed.
Regulation on Foreign Exchange Control
In 1996, China published The Foreign Currency Administration Regulations, and late on amended on January 14, 1997 and August 5, 2008. This Regulation has been the major one governing the foreign exchange activities in China. Under this Regulation, the Renminbi is convertible for foreign currency account items, including the distribution of dividends, interest payments and trade and service-related foreign exchange transactions. Conversion of Renminbi into foreign currency for capital account items, such as, loans, investment in securities and repatriation of investments, however, is subject to the registration of the State Administration of Foreign Exchange (“SAFE”) or its local counterparts.
Under the Regulation and relevant rules, foreign-invested enterprises may buy, sell and remit foreign currencies at banks authorized to conduct foreign exchange transactions for settlement of currency account transactions after providing valid commercial documents and, in the case of capital account item transactions, only after registration with the SAFE and, as the case may be, other relevant PRC government authorities as required by law.
According to the Overseas Investment Regulation which was issued in 2014, capital investments directed outside of mainland China by domestic or foreign-invested enterprises are also subject to restrictions, which include registration filing with Ministry of Commerce, even though the Notice on Further Improving and Adjusting the Foreign Exchange Management Policy for Capital Account (“No. 2 Notice”) passed in February of 2014 by SAFE has made domestic enterprises much easier releasing foreign currency overseas to foreign companies, including connected companies.
The conversion of Renminbi into foreign currencies, including U.S. dollars, has been based on rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi will be permitted to fluctuate within a band against a basket of certain foreign currencies. The PRC subsidiaries receive a significant portion of their revenue in Renminbi, which is not a freely convertible currency. Under the current structure, our income will be primarily derived from dividend payments from our subsidiaries in China. Even though the PRC subsidiaries may remit the income from China to anywhere deemed desirable, the fluctuation of exchange rate may be a disadvantage to us if Renminbi depreciates.
On January 26, 2017, the SAFE promulgated the Notice on Improving the Check of Authenticity and Compliance to Further Promote Foreign Exchange Control (the “SAFE Circular 3”), which stipulates several capital control measures with respect to the outbound remittance of profit from domestic entities to offshore entities, including (i) under the principle of genuine transaction, banks shall check board resolutions regarding profit distribution, the original version of tax filing records and audited financial statements; and (ii) domestic entities shall hold income to account for previous years’ losses before remitting the profits. Moreover, pursuant to the SAFE Circular 3, domestic entities shall make detailed explanations of the sources of capital and utilization arrangements, and provide board resolutions, contracts and other proof when completing the registration procedures in connection with an outbound investment.
On October 23, 2019, the SAFE promulgated the Circular of the State Administration of Foreign Exchange on Further Promoting Cross-border Trade and Investment Facilitation (the “SAFE Circular 28”), which expressly allows foreign-invested enterprises that do not have equity investments in their approved business scope to use their capital obtained from foreign exchange settlement to make domestic equity investments as long as there is a truthful investment and such investment is in compliance with the foreign investment-related laws and regulations.
On December 4, 2023, SAFE issued the Circular of the State Administration of Foreign Exchange on Further Deepening Reforms to Facilitate Cross-Border Trade and Investment, which stipulated that domestic equity transferors (including institutions and individuals) can directly remit to the capital project settlement account the equity transfer consideration funds paid in foreign currency by domestic entities, as well as the foreign exchange funds raised by domestic enterprises listed overseas. The funds in the capital project settlement account can be independently settled and utilized.
Regulation on Foreign Exchange Registration of Offshore Investment by PRC Residents
In October of 2005, SAFE promulgated a Notification known as “Notification 75,” in which SAFE requires PRC residents to register their direct establishment or indirect control of an offshore entity (referred to in Notification as “special purpose vehicle.”), where such offshore entity was established for the purpose of overseas financing, provided that PRC residents contribute their legally owned assets or equity into such entity. In July of 2014, this Notification was replaced by Notification 37, “Notification on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Returning Investment through Special Purpose Vehicles,” which expanded SAFE oversight scope to include overseas investment registration as well. Meanwhile, Notification 37 also covers more areas such as PRC residents paying capital contribution with overseas assets or equity. Furthermore, Notification 37 requires amendment to the registration where any significant changes with respect to the special purpose vehicle capitalization or structure of the PRC resident itself (such as capital increase, capital reduction, share transfer or exchange, merger or spin off). On February 13, 2015, the SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration Policy on Direct Investment, or Notice 13, which became effective on June 1, 2015 and was amended on December 30, 2019. Under Notice 13, applications for foreign exchange registration of inbound foreign direct investments and outbound overseas direct investments, including those required under Notification 37, will be filed with qualified banks instead of SAFE. The qualified banks will directly examine the applications and accept registrations under the supervision of SAFE.
Regulation on Dividend Distributions
The PRC subsidiary, WFOE, is wholly foreign-owned enterprises under the PRC law. The principal regulations governing the distribution of dividends paid by WFOE include Corporate Law (1993) as lastly amended in December 2023 and became effective on July 1, 2024, the Foreign Investment Law and its Implementing Regulations, and the Enterprise Income Tax Law (2007) as lastly amended in 2018 and its Implementation Regulations (2007) as lastly amended in 2019.
Under these requirements, foreign-invested enterprises may pay dividends only out of their accumulated profit, if any, as determined in accordance with PRC accounting standards and regulations. A PRC company is required to allocate at least 10% of their respective accumulated after-tax profits each year, if any, to fund certain capital reserve funds until the aggregate amount of these reserve funds have reached 50% of the registered capital of the enterprises. A PRC company is not permitted to distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
On March 16, 2007, the NPC enacted the Enterprise Income Tax Law, and on December 6, 2007, the State Council issued the Implementation Regulations on the Enterprise Income Tax Law, both of which became effective on January 1, 2008. The Enterprise Income Tax Law was lately amended on December 29, 2018 and the Implementation Regulations on the Enterprise Income Tax Law was lately amended on April 23, 2019. Under this law and its implementation regulations, dividends payable by a foreign-invested enterprise in the PRC to its foreign investor who is a non-resident enterprise will be subject to a 10% (5% for Hong Kong residents) withholding tax, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a lower withholding tax rate.
M&A Rules and Regulation on Overseas Listings
On August 8, 2006, six PRC regulatory agencies, Ministry of Commerce, the State Assets Supervision and Administration Commission, the State Administration for Taxation, the State Administration for Industry and Commerce, CSRC and SAFE, jointly adopted the Regulation on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or so called the M&A Rules and amended it on June 22, 2009. The M&A Rules purport, among other things, to require that offshore SPVs that are controlled by PRC entities or individuals and that have been formed for overseas listing purposes through acquisitions of PRC domestic interests held by such PRC entities or individuals, obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange. After the FIL and its implementation regulations became effective on January 1, 2020, the provisions of the M&A Rules remain effective to the extent they are not inconsistent with the FIL and its implementation regulations.
Furthermore, on July 6, 2021, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly promulgated the Opinions on Strictly Cracking Down on Illegal Securities Activities in Accordance with the Law, pursuant to which Chinese regulators are required to accelerate rulemaking related to the overseas issuance and listing of securities, and update the existing laws and regulations related to data security, cross-border data flow, and management of confidential information.
Following the promulgation of the Cybersecurity Law and Data Security Law, on February 17, 2023, the CSRC issued the New Administrative Rules Regarding Overseas Listings, which came into force on March 31, 2023. The New Administrative Rules Regarding Overseas Listings provided certain examination and filing mechanisms with respect to a Chinese domestic enterprise’s offering of securities overseas.
As there are still uncertainties regarding the interpretation and implementation of such regulatory guidance in the future, we cannot assure you that we or the PRC subsidiaries will be able to comply with new regulatory requirements relating to our future overseas capital-raising activities and we or the PRC subsidiaries may become subject to more stringent requirements with respect to matters including CSRC approval requirements, data privacy and cross-border investigation and enforcement of legal claims.
While the application of the M&A Rules and the New Administrative Rules Regarding Overseas Listings remains unclear, the CSRC currently has not issued any definitive rule or interpretation concerning what offerings are subject to the CSRC approval procedures; and despite the lack of any definitive rule or interpretation from CSRC, the main purpose of the M&A rule is for national security and national industrial policy and so far none of the Chinese companies that have completed their public listing in the U.S. have obtained such approval; and the PRC subsidiaries’ business operations in China do not belong to a prohibited industry by foreign investment.
However, there is still uncertainty as to how the M&A Rules and the New Administrative Rules Regarding Overseas Listings will be interpreted and implemented in the future. If the CSRC or other PRC regulatory agencies subsequently determine that CSRC approval is required for our subsequent securities offerings, we or the PRC subsidiaries may need to apply for approval from the CSRC and we or the PRC subsidiaries may be subject to penalties and administrative sanctions administered by these regulatory agencies if we fail to obtain such approval. These regulatory agencies may impose fines and penalties on the PRC subsidiaries’ operations in the PRC, limit the PRC subsidiaries’ operating privileges in the PRC, delay or restrict the repatriation of the proceeds from our subsequent securities offerings into the PRC, or take other actions that could materially adversely affect the PRC subsidiaries’ and our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our Ordinary Shares. The CSRC or other PRC regulatory agencies may also take actions requiring us, or making it advisable for us, to halt our subsequent securities offering before settlement and delivery of our Ordinary Shares.
Restriction on Foreign Ownership
The principal regulation governing foreign ownership of businesses in the PRC is Guidance Catalogue for Industrial Structure Adjustments (2024 edition), effective as of February 1, 2024 (the “Catalogue”). The Catalogue classifies the various industries into three categories: encouraged, restricted and prohibited. Risks and uncertainties relating to PRC regulation of medical device businesses include new laws, regulations or policies may be promulgated or announced that will regulate medical device. If these new laws, regulations or policies are promulgated, additional licenses may be required for the PRC subsidiaries’ operations. If the PRC subsidiaries’ operations do not comply with these new regulations after they become effective, or if the PRC subsidiaries fail to obtain any licenses required under these new laws and regulations, the PRC subsidiaries could be subject to penalties and their business operations could be disrupted.
Regulations on Offshore Parent Holding Companies’ Direct Investment in and Loans to their PRC Subsidiaries
China has been open to foreign direct and indirect investments. An offshore company may invest in a PRC company. Such investment is subject to the FIL. Under the FIL, foreign investments no longer need to be approved by Chinese government, but only need to register the investment with Chinese regulatory agency.
However, Chinese government still has foreign exchange control policy. The money transfer in or out of China is still under tight control. So, shareholder loans made by offshore parent holding companies to their PRC subsidiaries are regarded as foreign debts for regulatory purposes, which debts are subject to a number of PRC laws and regulations, including the PRC Foreign Exchange Administration Regulations, Administration Rules on the Settlement, Sale and Payment of Foreign Exchange, the Statistical Monitoring of Foreign Debt Tentative Provisions, and the Provisional Measures on Administration of Foreign Debt.
Pursuant to the Provisional Measures on Administration of Foreign Debt (the “Foreign Debt Measures”) issued by the State Development Planning Commission (revised), Ministry of Finance and SAFE in January 2003 and became effective on March 1, 2003, which was amended on July 26, 2022 and became effective on September 1, 2022, any loans provided by us to the subsidiary in mainland China in foreign currencies shall be classified as foreign debt under the Foreign Debt Measures. According to the Foreign Debt Measures, the sum of cumulative accrued amounts of medium-term to long-term foreign loans and balance amounts of short-term foreign loans taken by a foreign investment enterprise shall be limited to the difference between the total project investment amount approved by the government and the amount of registered capital. Foreign investment enterprises may take foreign loans freely within the scope of difference.
On January 12, 2017, the PBOC issued the Notice of People’s Bank of China on Matters Concerning Macro-prudential Management on All-round Cross-border Financing (the “No. 9 Notice”), which improved the policy framework of the cross-border financing. The No. 9 Notice clarifies the new calculation methods of the upper limit of the risk-weighted balance for all types of cross-border financing, in particular, the upper limit for risk-weighted balance for cross-border financing equals to the capital or the net assets multiplied by the leverage rate of cross-border financing and the macro-prudential adjustment parameters. In the case of the subsidiary in mainland China, the capital or the net assets is calculated at the net assets of each subsidiary, the leverage rate for cross-border financing for an enterprise is 2, and the macro-prudential adjustment parameter is 1 (the “All-Round Mode”). On March 11, 2020, the PBOC and SAFE promulgated the Circular of the People’s Bank of China and the State Administration of Foreign Exchange on Adjusting the Macro-prudential Regulation Parameter for Full-covered Cross-border Financing, which provides that based on the current macro economy and international balance of payments, the macro-prudential regulation parameter as set forth in the Notice 9 is updated from 1 to 1.25. On January 7, 2021, the PBOC and SAFE promulgated the Notice of PBC and SAFE on Adjusting the Macro-prudential Adjustment Parameter for Cross-border Financing of Companies, which provides that the macro-prudential regulation parameter of companies is updated from 1.25 to 1. Currently, the implementation of the foregoing methodologies in cross-border financing have not been formally determined by the PBOC and the SAFE. In the practice, according to the Q&A on Macro-prudential Regulation Parameter for Full-Covered Cross-border Financing (Phase I) (the “Q&A”) issued by the SAFE on May 27, 2017, FIEs shall submit a written filing report to the local foreign exchange bureau when they handle the foreign debt signing filing (registration) for the first time after the issuance of the Q&A, so as to clarify the cross-border financing management mode they choose during the transition period. If the All-Round Mode is selected, the latest audited net assets data shall be reported at the same time. Once the cross-border financing management mode is determined, it shall not be changed. Alternatively, if we choose to use the All-Round Mode, the amount of loans we can make to the subsidiary in mainland China as calculated according to the No. 9 Notice and the Notice of PBC and SAFE on Adjusting the Macro-prudential Adjustment Parameter for Cross-border Financing of Companies will not be more than 2 times of the net assets of such entities.
Moreover, as the debtors of cross-border financing, the subsidiary in mainland China is also required to comply with certain registration formalities for execution of foreign debt contracts with the foreign exchange bureau at the locality within fifteen working days after signing the contracts according to the Notice of State Administration of Foreign Exchange on Promulgation of the Administrative Measures on Registration of Foreign Debt which was promulgated by the SAFE in April 2013 and revised in May 2015.
Pursuant to the Circular of the National Development and Reform Commission on Promoting the Administrative Reform of the Record-filing and Registration System for the Issuance of Foreign Debt by Enterprises promulgated on September 14, 2015 (“Circular 2044”), before the issuance of foreign loans, enterprises shall first apply to the NDRC for record-filing and registration procedures and shall report the information on the issuance to NDRC within 10 business days after completion of each issuance. The term “foreign loan” shall mean RMB-denominated or foreign currency-denominated debt instruments with a maturity of one year or more which are issued overseas by domestic enterprises and their controlled overseas enterprises or branches and for which the principal and interest are repaid as agreed, including bonds issued overseas and long and medium-term international commercial loans, and so forth. On January 5, 2023, the NDRC issued the Administrative Measures for Review and Registration of Medium- and Long-term Foreign Debt of Enterprises, or Foreign Debt Registration Measures, which came into effect on February 10, 2023, and Circular 2044 is, therefore invalid. The Foreign Debt Registration Measures not only further clarifies the scope of debt instruments, including senior debt, perpetual debt, capital debt, medium-term note, convertible bond, exchangeable bond, financial leasing, and commercial loan, but also changes the record-filing and registration system to the review and registration system. According to the Foreign Debt Registration Measures, before incurring foreign debt, an enterprise shall obtain the Certificate of Review and Registration of Enterprise Incurrence of Foreign Debt, or the Certificate of Review and Registration, and complete the review and registration formalities. Without review and registration, no foreign debt may be incurred. An enterprise shall, within ten working days after incurring each foreign debt, report the information of incurring foreign debt to the review and registration authority via the network system, including the main operating indicators of the enterprise and the information about the foreign debt incurred, among others, and report the corresponding information about the foreign debt incurred within ten working days after the expiration of Certificate of Review and Registration. The above provisions shall apply to indirect overseas incurrence of foreign debt by domestic enterprises, which means that enterprises whose main business activities are in China, issuing bonds or borrowing commercial loans overseas in the name of enterprises registered overseas, based on the equity, assets, earnings or other similar rights and interests of the domestic enterprises, are subject to these provisions. However, the NDRC has not issued any other further explanation for the implementation of the Foreign Debt Registration Measures. In practice, the NDRC’s attitude on whether foreign-invested enterprises with foreign loans with a term of more than one year need to register is still not completely unified, and it is generally determined on a case-by-case basis.
Regulations Relating to Employment and Social Welfare
Regulations on Employment
The major PRC laws and regulations that govern employment relationship are the PRC Labor Law, or the Labor Law (issued by the SCNPC on July 5, 1994, came into effect on January 1, 1995 and revised on August 27, 2009 and December 29, 2018, the PRC Labor Contract Law, or the Labor Contract Law, promulgated by the SCNPC on June 29, 2007 and became effective on January 1, 2008, and then amended on December 28, 2012 and became effective on July 1, 2013, and the Implementation Rules of the Labor Contract Law of the PRC, or the Implementation Rules of the Labor Contract Law, issued by the State Council on September 18, 2008 and came into effect on the same day. According to the aforementioned laws and regulations, labor relationships between employers and employees must be executed in written form. The laws and regulations above impose stringent requirements on the employers in relation to entering into fixed-term employment contracts, hiring of temporary employees and dismissal of employees. As prescribed under the laws and regulations, employers shall ensure its employees have the right to rest and the right to receive wages no lower than the local minimum wages. Employers must establish a system for labor safety and sanitation that strictly abide by state standards and provide relevant education to its employees. Violations of the Labor Contract Law and the Labor Law may result in the imposition of fines and other administrative liabilities and/or incur criminal liabilities in the case of serious violations.
Regulations on Social Insurance and Housing Provident Fund
According to the Social Insurance Law of PRC, which issued by the SCNPC on October 28, 2010 and came into effect on July 1, 2011 and was latest revised on December 29, 2018, enterprises and institutions in mainland China shall provide their employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance and other welfare plans. The employer shall apply to the local social insurance agency for social insurance registration within 30 days from the date of its formation. And it shall, within 30 days from the date of employment, apply to the social insurance agency for social insurance registration for the employee. Any employer who violates the regulations above shall be ordered to make correction within a prescribed time limit; if the employer fails to rectify within the time limit, the employer and its directly liable person will be fined. Meanwhile, the Interim Regulation on the Collection and Payment of Social Insurance Premiums, issued by the State Council on January 22, 1999 and came into effect on the same day and was recently revised on March 24, 2019, prescribes the details concerning the social securities.
Apart from the general provisions about social insurance, specific provisions on various types of insurance are set out in the Regulation on Work-Related Injury Insurance, issued by the State Council on April 27, 2003, came into effect on January 1, 2004 and revised on December 20, 2010, the Regulations on Unemployment Insurance, issued by the State Council on January 22, 1999 and came into effect on the same day, the Trial Measures on Employee Maternity Insurance of Enterprises, issued by the Ministry of Labor on December 14, 1994 and came into effect on January 1, 1995. Enterprises subject to these regulations shall provide their employees with the corresponding insurance.
According to the Regulation Concerning the Administration of Housing Provident Fund, implemented since April 3, 1999 and latest amended on March 24, 2019, any newly established entity shall make deposit registration at the housing accumulation fund management center within 30 days as of its establishment. After that, the entity shall open a housing accumulation fund account for its employees in an entrusted bank. Within 30 days as of the date an employee is recruited, the entity shall make deposit registration at the housing accumulation fund management center and seal up the employee’s housing accumulation fund account in the bank mentioned above within 30 days from termination of the employment relationship.
Any entity that fails to make deposit registration of the housing accumulation fund or fails to open a housing accumulation fund account for its employees shall be ordered to complete the relevant procedures within a prescribed time limit. Any entity failing to complete the relevant procedures within the time limit will be fined RMB10,000 to RMB50,000. Any entity that fails to make deposits to the housing provident fund within the time limit or has any shortfall in payment of housing provident fund will be ordered to make the payment or make up the shortfall within the prescribed time limit, otherwise, the housing provident management center is entitled to apply for compulsory enforcement with the People’s Court. As of the date of this annual report, (i) some of the PRC subsidiaries have not completed the social insurance registration and the housing fund registration; (ii) the PRC subsidiaries did not make contributions in the full amount for the social insurance fund and housing provident fund for their employees, as required under the relevant PRC laws and regulations; and (iii) the PRC subsidiaries did not make contributions in the housing fund for some employees. For the fiscal years of 2024, 2023, and 2022, $300,945, $287,629, and $295,009 of contributions for social insurance fund, respectively, should have been made but were not. For the fiscal years of 2024, 2023, and 2022, $94,815, $90,620, and $92,945 of contributions for the housing provident fund, respectively, should have been made but were not. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject the PRC subsidiaries to penalties” on page 40.
Regulations Relating to Taxation
Enterprise income tax
According to the EIT Law, which was promulgated by the SCNPC on March 16, 2007 and last amended and effective on December 29, 2018, and the Enterprise Income Tax Implementation Regulations of the PRC (the “EITIR”), which was promulgated by the State Council on December 6, 2007 and last amended and effective on April 23, 2019, the enterprise income tax of both domestic and foreign-invested enterprises is unified at 25% with certain exceptions. According to the EIT Law, enterprises are classified as “resident enterprises” and “non-resident enterprises.” Pursuant to the EIT Law and the EITIR, PRC resident enterprises typically pay an enterprise income tax at the rate of 25%, while non-PRC resident enterprises without any branches in the PRC should pay an enterprise income tax in connection with their income from the PRC at the tax rate of 10%. Enterprises established under the laws of foreign countries or regions whose “de facto management bodies” (i.e., establishments that carry out substantial and overall management and control over production and operations, personnel, accounting and properties) are located in the PRC are considered as PRC tax resident enterprises, and will generally be subject to enterprise income tax at the rate of 25% of their global income.
Pursuant to the EIT Law, enterprises qualified as “High and New Technology Enterprises” are entitled to a 15% enterprise income tax rate rather than the 25% uniform statutory tax rate. The preferential tax treatment continues as long as an enterprise can retain its “High and New Technology Enterprise” status, which certificate is valid for a period of three years and renewable.
Value-added tax
According to Provisional Regulations on Value-added Tax of the PRC, which were promulgated by the State Council on December 13, 1993 and last amended on November 19, 2017, and the Implementing Rules for the Interim Regulations on Value-added Tax of the PRC promulgated by Ministry of Finance on December 25, 1993 and last amended on November 1, 2011, all enterprises and individuals that engage in the sale of goods, the provision of processing, repair and replacement services, the sale of services, intangible assets or immovable properties and the importation of goods within the territory of the PRC must pay value-added tax.
Dividend Withholding tax
According to the EIT Law and the EITIR, dividends paid by foreign-invested companies to their foreign investors that are non-resident enterprises as defined under the law are subject to withholding tax at a rate of 10%, unless otherwise provided in the relevant tax agreements entered into with the central government of the PRC. Pursuant to the Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation on Income (the “Double Tax Avoidance Arrangement”) promulgated on August 21, 2006, if a Hong Kong resident enterprise is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements under such Double Tax Avoidance Arrangement, the withholding tax rate on the dividends the Hong Kong resident enterprise receives from a PRC resident enterprise may be reduced to 5% from 10% applicable under the EIT Law and the EITIR. However, based on the Notice of the State Taxation Administration on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties promulgated and took into effect on February 20, 2009 by the State Taxation Administration (the “STA”), if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment. Based on the Notice of the State Taxation Administration on the Recognition of Beneficial Owners in Tax Treaties, which was promulgated by STA on February 3, 2018 and came into effect on April 1, 2018, a comprehensive analysis will be used to determine beneficial ownership based on the actual situation of a specific case combined with certain principles, and if an applicant was obliged to pay more than 50% of its income to a third country (region) resident within 12 months of the receipt of the income, or the business activities undertaken by an applicant did not constitute substantive business activities including substantive manufacturing, distribution, management and other activities, the applicant was unlikely to be recognized as a beneficial owner to enjoy tax treaty benefits.
Regulations on Intellectual Property
Trademarks
Trademarks are protected by the PRC Trademark Law adopted in 1982 and lastly amended in 2019, as well as the Implementation Regulation of the PRC Trademark Law adopted by the State Council in 2002 and amended in 2014. The Trademark Office of China National Intellectual Property Administration handles trademark registrations. Trademarks can be registered for a term of ten years and can be repeatedly extended for another ten-year term at the time of expiry. The PRC Trademark Law has adopted a “first-to-file” principle with respect to trademark registration. As of the date of this annual report, the PRC subsidiaries have registered 16 trademarks, all of which are fully owned and in use by us. According to Chinese Trademark Law, if anyone has a dispute over the officially registered trademarks, he can file a petition to the review board of the Trademark Office, requesting a comprehensive review that may result in revoking the registered trademarks. As of the date of this annual report, the PRC subsidiaries have not received any such kind of petition.
Patents
According to the PRC Patent Law promulgated by the SCNPC on March 12, 1984 and last amended on October 17, 2020 with effect from June 1, 2021, and its latest Implementation Rules promulgated by the State Council on December 11, 2023 and took into effect on January 20, 2024, the National Intellectual Property Administration is responsible for administering patents in the PRC. Inventions, utility models, and designs with the features of novelty, inventiveness and practical applicability, are three kinds of patent defined and protected under China’s Patent Law. The State Intellectual Property Office is responsible for examining and approving patent applications. Once the application is approved, the applicants can have their patent under Chinese legal protection for a long term since its application date, which is 20 years for invention and ten years for utility models and designs.
Domain Names
Internet domain name registration and related matters are primarily regulated by the Measures on Administration of Internet Domain Names, which were promulgated by the MIIT on August 24, 2017 and took effect on November 1, 2017, and the Detailed Rules for the Implementation of National Top-level Domain Name Registration, which were promulgated by China Internet Network Information Center and took effect on June 18, 2019. Domain name owners are required to register their domain names and the MIIT is in charge of the administration of PRC internet domain names. The domain name services follow a “first come, first file” principle. The applicants will become the holders of such domain names upon the completion of the registration procedures.
Copyrights
Pursuant to the PRC Copyright Law promulgated by the SCNPC on September 7, 1990 and last amended on November 11, 2020 (the latest revision became effective from June 1, 2021) and the Implementing Regulations of the PRC Copyright Law promulgated by the State Council on August 2, 2002, last amended on January 30, 2013 (the latest revision became effective from March 1, 2013), PRC nationals, legal persons, and other organizations may copyright their works, whether published or not, which works include, among others, works of literature, art, natural science, social science, engineering technology and computer software. Pursuant to the Regulations on the Protection of Computer Software promulgated by the State Council in December 2001, and most recently amended in January 2013, and the Rules for the Registration of Computer Software Copyright, which was promulgated by the China Copyright Office and came into effect in February 2002, anyone who publishes, revises or translates computer software without obtaining the prior approval of the computer software copyright holders shall bear civil liability to the copyright owner as a consequence of harming the copyright. The corporate computer software copyright is valid for a term of 50 years, i.e., until December 31st of the 50th year, starting from the date as of first publication. Computer software copyright owners shall register at the registration institution authorized by the PRC Copyright Office to obtain the computer software copyright registration certificates as primary evidence of the computer software copyright being registered.
Regulations Relating to Anti-Monopoly, Anti-Corruption and Anti-Bribery
On August 30, 2007, the Anti-Monopoly Law, which was promulgated by the SCNPC, and amended on June 24, 2022 which amendment became effective on August 1, 2022, stipulates the regulation of market monopoly. The Anti-Monopoly Law prohibits the conclusion of a monopoly agreement between business operators, the abuse of a dominant market position by a business operator and concentration of undertakings which has or may have an effect of excluding or limiting market competition. The newly revised Anti-Monopoly Law proposes to increase the fines on business operators liable for illegal concentration to “no more than ten percent of the preceding year’s sales revenue of the business operators if the concentration of business operators has or may have an effect of excluding or limiting competition; or a fine of up to RMB5 million if the concentration of business operators does not have an effect of excluding or limiting competition.” Furthermore, the relevant authority may require business operators to declare where there is evidence that the resulting concentration has or may have the effect of eliminating or restricting competition, even if the level of such concentration does not reach the filing threshold. If the business operators fail to make a declaration, the relevant authority will conduct an investigation according to law.
Pursuant to the Anti-Unfair Competition Law of the PRC promulgated by the SCNPC on September 1, 1993 and last amended on April 23, 2019, a business operator shall not resort to bribery to seek a transaction opportunity or competitive advantage by offering money or goods or by any other means, to (i) any employee of the counterparty in a transaction, (ii) any entity or individual entrusted by the counterparty in a transaction to handle relevant affairs, or (iii) any other entity or individual that takes advantage of powers or influence to influence a transaction. A business operator may expressly offer a discount to the counterparty or pay commissions to the intermediaries of a transaction in the course of transaction activities, which shall be properly recorded at both parties’ accounting books. Any commercial bribery committed by an employee of a given business operator will be deemed as conduct of such business operator unless evidence shows that such act is not related to such business operator’s efforts in seeking a transaction opportunity or competitive advantage.
Regulations related to business registration in Hong Kong
Our Hong Kong subsidiary, Work Medical Technology, is an investment holding company and is subject to regulations related to business registration in Hong Kong. The Business Registration Ordinance (Chapter 310 of the Laws of Hong Kong) requires every person carrying on any business to make an application to the Commissioner of Inland Revenue in the prescribed manner for the registration of that business within one month after the commencement of business. The Commissioner of Inland Revenue must register each business for which a business registration application is made and, as soon as practicable after the prescribed business registration fee and levy are paid, issue a business registration certificate or a branch registration certificate for the relevant business or the relevant branch, as the case may be. Any person who fails to apply for business registration shall be guilty of an offence and shall be liable to a fine of HK$5,000 and to imprisonment for 1 year.
Except for the business registration certificate to be issued under the Business Registration Ordinance, we are not required to obtain any industry-specific license, permit, authorization or qualification for our Hong Kong subsidiary. The Commissioner of Inland Revenue issued the business registration certificate for Work Medical Technology which is valid from April 19, 2024, to April 18, 2025.
C. Organizational Structure
See “—A. History and Development of the Company.”
D. Property, Plants and Equipment
See “—B. Business Overview—Properties.”
Item 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
Item 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion of our financial condition and results of operations is based upon and should be read in conjunction with our consolidated financial statements and their related notes included in this annual report. This report contains forward-looking statements. In evaluating our business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” in this annual report. We caution you that our businesses and financial performance are subject to substantial risks and uncertainties.
A. Operating Results
Business Overview
We are a holding company with operating subsidiaries (collectively referred as the “Group”), and with all of our operations and assets in China. Our vision is to provide professional and reliable medical devices, both domestically and internationally. The PRC subsidiaries mainly manufacture and sell Class I and II medical devices, including endotracheal tubes, laryngeal mask airways, heat and moisture exchanging filters (HMEF), disposable breathing circuits, nebulizer kits, and yankauer suction sets. Besides Class I and II medical devices, the PRC subsidiaries also have retained the certificate of selling Class III disposable medical devices.
The PRC subsidiaries have three types of customers, i) direct end-user customers, which include hospitals, pharmacies, and medical institutions, ii) domestic distributor customers that distribute the PRC subsidiaries’ products to end-user customers in China, and iii) export distributor customers that distribute the PRC subsidiaries’ products to end-user customers in Asia, Africa, Europe, North America, South America, and Oceania.
Revenue decreased by $2,059,511, or approximately 15.2%, to $11,506,440 for the year ended September 30, 2024 from $13,565,951 for the year ended September 30, 2023. The decrease was mainly due to the decline in demand and unit price of masks and was offset by the increase of medical devices other than masks and commodity trading.
Revenue decreased by $6,145,339, or approximately 31.2%, to $13,565,951 for the year ended September 30, 2023 from $19,711,290 for the year ended September 30, 2022. The decrease was mainly due to the decline in demand and unit price of masks and medical devices other than masks, and was offset by the increase of commodity trading.
We incurred a net loss of $3,540,409 for the fiscal year ended September 30, 2024, mainly due to the increase of professional consulting fees and the decrease of gross profit caused by insufficient market demand of masks, and our net income was $63,383 and $944,126 for the fiscal years ended September 30, 2023 and 2022, respectively.
Key Factors Affecting Our Results of Operations
We believe the following key factors may affect our financial condition and results of operations:
| ● | the PRC subsidiaries’ ability to effectively and efficiently conduct sales and marketing; |
| ● | the PRC subsidiaries’ ability to acquire sufficient raw materials and key components and obtain equipment and services from their suppliers in suitable quantity and quality; |
| ● | the PRC subsidiaries’ ability to effectively manage inventories; and |
| ● | the PRC subsidiaries’ ability to enhance our operational efficiency. |
Results of Operations
The following table sets forth a summary of our consolidated statements of income for the fiscal years ended September 30, 2024, 2023 and 2022, respectively. This information should be read together with our consolidated financial statements and related notes included elsewhere in this annual report. The results of operations in any period are not necessarily indicative of our future trends.
| | | For the Years Ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Net revenue | | $ | 11,506,440 | | | $ | 13,565,951 | | | $ | 19,711,290 | |
| Cost of revenue | | | (8,638,054 | ) | | | (9,422,967 | ) | | | (15,292,498 | ) |
| Gross profit | | | 2,868,386 | | | | 4,142,984 | | | | 4,418,792 | |
| Selling expenses | | | (2,053,019 | ) | | | (1,567,385 | ) | | | (1,032,685 | ) |
| General and administrative expenses | | | (4,558,041 | ) | | | (1,861,728 | ) | | | (2,563,879 | ) |
| Research and development expenses | | | (302,511 | ) | | | (301,644 | ) | | | (183,900 | ) |
| Total operating expenses | | | (6,913,571 | ) | | | (3,730,757 | ) | | | (3,780,464 | ) |
| (Loss) income from operations | | | (4,045,185 | ) | | | 412,227 | | | | 638,328 | |
| Total other income (loss), net | | | 379,485 | | | | (301,838 | ) | | | 475,233 | |
| (Loss) income before income tax | | | (3,665,700 | ) | | | 110,389 | | | | 1,113,561 | |
| Income tax benefit (expense) | | | 125,291 | | | | (47,006 | ) | | | (169,435 | ) |
| Net (loss) income | | | (3,540,409 | ) | | | 63,383 | | | | 944,126 | |
Net revenue
| | | For the Year Ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Masks | | $ | 1,559,750 | | | $ | 5,091,331 | | | $ | 10,619,035 | |
| Medical devices other than masks | | | 9,414,751 | | | | 7,997,540 | | | | 8,747,886 | |
| Commodity trading | | | 435,728 | | | | 325,429 | | | | 106,643 | |
| Others | | | 96,211 | | | | 151,651 | | | | 237,726 | |
| Net revenue | | $ | 11,506,440 | | | $ | 13,565,951 | | | $ | 19,711,290 | |
Sales decreased by $2,059,511, or approximately 15.2%, to $11,506,440 for the year ended September 30, 2024 from $13,565,951 for the year ended September 30, 2023. The decrease was mainly due to the decline in demand and unit price of masks and was offset by the increase of medical devices other than masks and commodity trading.
Sales decreased by $6,145,339, or 31.2%, to $13,565,951 for the year ended September 30, 2023 from $19,711,290 for the year ended September 30, 2022. The decrease was mainly due to the decline in demand and unit price of masks and medical devices other than masks, and was offset by the increase of commodity trading.
Sales of masks decreased by $3,531,581, or 69.4%, to $1,559,750 for the year ended September 30, 2024 from $5,091,331 for the year ended September 30, 2023. The decrease was mainly because (i) the demand for masks and the sales volume of masks decreased for the year ended September 30, 2024; and (ii) the Company shifted its marketing focus from masks to medical devices other than masks.
Sales of masks decreased by $5,527,704, or 52.1%, to 5,091,331 for the year ended September 30, 2023 from $10,619,035 for the year ended September 30, 2022. The decrease was mainly because (a) as China lifted travel restrictions and quarantine requirements in December 2022, the demand for masks decreased, and accordingly, the sales volume of masks decreased by 87.2% for the year ended September 30, 2023; and partially offset by (b) the average unit sales price of masks for the year ended September 30, 2023 increased 28.3% compared with the previous year, which was attributable to the fact that the Company sold more high priced masks, such as KN95 masks and customized patterned masks, other than regular medical face masks.
Medical devices other than masks are mainly anesthesia and respiratory consumables. The sales of medical devices increased by $1,417,211, or 17.7%, to $9,414,751 for the year ended September 30, 2024 from $7,997,540 for the year ended September 30, 2023. The increase was mainly attributable to the Group’s decision to intensify marketing efforts for medical devices other than masks, which led to higher sales volumes.
Medical devices other than masks decreased by $750,346, or 8.6%, to $7,997,540 for the year ended September 30, 2023 from $8,747,886 for the year ended September 30, 2022. The decrease was mainly due to a slight decrease in market demand.
Commodity trading mainly involves trading in disposable medical articles or raw materials of medical devices. Commodity trading increased by $110,299, or 33.9%, to $435,728 for the year ended September 30, 2024 from $325,429 for the year ended September 30, 2023. The increase was mainly due to the increase of market demand and our business expansion.
Commodity trading increased by $218,786, or 205.2%, to $325,429 for the year ended September 30, 2023 from $106,643 for the year ended September 30, 2022. The increase was mainly due to our business expansion.
Cost of revenue
Cost of revenue primarily includes the cost of materials, direct labor, overhead, and other related incidental expenses that are directly attributable to the principal operations of the PRC subsidiaries. Cost of revenue decreased by $784,913, or approximately 8.3%, to $8,638,054 for the year ended September 30, 2024 from $9,422,967 for the year ended September 30, 2023. The decrease in the cost of revenue was smaller than the decrease in net revenue, mainly because the unit cost of medical devices other than masks was relatively higher than the unit cost of masks; therefore, the increased sales of medical devices other than masks partially offset the decrease in the cost of revenue related to masks.
Cost of revenue decreased by $5,869,531, or approximately 38.4%, to $9,422,967 for the year ended September 30, 2023 from $15,292,498 for the year ended September 30, 2022. The decrease was mainly attributable to (a) the decreased sales for the year ended September 30, 2023; and (b) the sufficient supply of raw materials for mask production in the market, resulting in a low unit price of raw materials.
Gross profit
Gross profit decreased by $1,274,598, or approximately 30.8%, to $2,868,386 for the year ended September 30, 2024 from $4,142,984 for the year ended September 30, 2023. Gross profit margin decreased to 24.9% for the year ended September 30, 2024, as compared to 30.5% for the year ended September 30, 2023. The decrease of gross profit margin was primarily due to the increased sales of medical devices other than masks with relative lower profit margin and the decreased sales of masks with relative higher profit margin for the year ended September 30, 2024.
Gross profit decreased by $275,808, or approximately 6.2%, to $4,142,984 for the year ended September 30, 2023 from $4,418,792 for the year ended September 30, 2022. Gross profit margin increased to 30.5% for the year ended September 30, 2023, as compared to 22.4% for the year ended September 30, 2022. The increase of gross profit margin was primarily attributable to (a) relatively lower unit cost of masks, caused by an overall downward trend of deprecation cost by using a double declining balance method for depreciation of mask production machines; and (b) the increase of commodity trading with relative high profit margin for the year ended September 30, 2023.
Selling expenses
Our selling expenses primarily consist of salaries, welfare expenses as well as marketing and promotion expenses. Our selling and marketing expenses increased by $485,634, or approximately 31.0%, to $2,053,019 for the year ended September 30, 2024 from $1,567,385 for the year ended September 30, 2023, which was primarily due to more marketing and promotion expenses incurred for sales of medical devices other than masks.
The selling and marketing expenses increased by $534,700, or approximately 51.8%, to $1,567,385 for the year ended September 30, 2023 from $1,032,685 for the year ended September 30, 2022, which was primarily because the PRC subsidiaries paid more bonuses to their sales personnel at the end of December 2023 for their constant hard work during the pandemic.
General and administrative expenses
Our general and administrative expenses primarily consist of professional fees, salaries and welfare expenses, depreciation and amortization, allowance for doubtful accounts and office expenses. Our general and administrative expenses increased by $2,696,313, or approximately 144.8%, to $4,558,041 for the year ended September 30, 2024 from $1,861,728 for the year ended September 30, 2023, primarily due to the increase of professional consulting fees for improving our financial reporting as we became a public company.
The general and administrative expenses decreased by $702,151, or approximately 27.4%, to $1,861,728 for the year ended September 30, 2023 from $2,563,879 for the year ended September 30, 2022, which was primarily due to the fact that our PRC subsidiaries could not collect timely payments from some of their customers for the year ended September 30, 2022, and a significant amount of bad debt provision was made, but with the recovering economy since late 2022 and management’s increased efforts in payment collections, we believe that the liquidity risk from our customers has been alleviated, and a reduced bad debt provision was made for the year ended September 30, 2023. The decrease in general and administrative expenses was offset by the increase in salaries and welfare expenses.
Research and development expenses
Our research and development expenses were incurred for the development of medical devices and technologies used for the manufacturing of medical devices. Our research and development expenses increased by $867, or approximately 0.3%, to $302,511 for the year ended September 30, 2024 from $301,644 for the year ended September 30, 2023, which remained relatively stable.
The research and development expenses increased by $117,744, or approximately 64.0%, to $301,644 for the year ended September 30, 2023 from $183,900 for the year ended September 30, 2022. The increase was primarily because we enhanced our collaboration with medical institutions and invested additional capital in R&D on technology and new product.
Other income (loss)
The total net other income was $379,485 for the year ended September 30, 2024, the net other loss was $301,838 for the year ended September 30, 2023, and the total net other income was $475,233 for the year ended September 30, 2022.
The net other income for the year ended September 30, 2024 primarily consisted of (i) $775,181 from the reassessment of a previously accrued disputed payable, now recognized as non-operating income due to recent resolution of the dispute; (ii) subsidy income of $346,571; (iii) interest expenses of $557,571; (iv) other miscellaneous expenses of $196,423; and (v) interest income of $11,727.
The net other expenses for the year ended September 30, 2023 primarily consisted of interest expenses of $392,741.
The net other income for the year ended September 30, 2022 primarily consisted of received government subsidies of $1,186,641, which were partially offset by interest expense of $352,968 and loss on disposal of equipment of $360,409 due to equipment upgrading.
Income tax benefit (expense)
The income tax benefit was $125,291 for the year ended September 30, 2024, and the income tax expense were $47,006 and $169,435 for the years ended September 30, 2023 and 2022, respectively. The change was related to taxable income, primarily due to the increase of loss or decrease of profit before income tax.
Net (loss) income
As a result of the foregoing, our net loss was $3,540,409 for the year ended September 30, 2024, and our net income was $63,383 and $944,126 for the years ended September 30, 2023 and 2022, respectively.
B. Liquidity and Capital Resources
To date, the primary sources of liquidity consist of cash flows from the PRC subsidiaries’ operating activities, capital contributions from shareholders and loans from banks. The Group has financed its operations primarily through capital contributions and revenue from operations. As of September 30, 2024, we had cash of $6,557,605 and a total working capital of $647,030. For the next 12 months from the issuance date of this annual report, we plan to implement measures to meet the cash requirements, including: 1) strictly controlling and reducing expenses; 2) seeking additional credit facilities; and 3) seeking equity financing from shareholders. We believe the above measures will be adequate to meet the operation requirements in the next 12 months.
If we experience an adverse operating environment or incur unanticipated capital expenditure requirements, or if we accelerate our growth, then additional financing may be required. No assurance can be given, however, that additional financing, if required, would be available on favorable terms or at all. Such financing may include the use of additional debt or the sale of additional equity securities. Any financing which involves the sale of equity securities or instruments that are convertible into equity securities could result in immediate and possibly significant dilutions to the existing shareholders.
All of our operations are conducted in China and a majority portion of our revenue, expense, and cash are denominated in Renminbi (RMB). Current foreign exchange and other regulations in the PRC may restrict the PRC entities in their ability to transfer their net assets to us and our subsidiaries. However, we have no present plans to declare any dividends and we plan to retain our retained earnings to continue to grow our business. In addition, these restrictions have no impact on our ability to meet our cash obligations, as all of our current cash obligations are due within the PRC as of the date of this annual report.
We have limited financial obligations denominated in U.S. dollars, thus, the foreign currency restrictions and regulations in the PRC on dividend distribution will not have a material impact on the liquidity, financial condition and results of operations of the Company.
Summary of Cash Flows
The following table summarizes our cash flows for the periods indicated:
| | | For the year ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Net cash (used in) provided by operating activities | | $ | (2,228,087 | ) | | $ | 2,209,736 | | | $ | (2,258,948 | ) |
| Net cash used in investing activities | | | (9,064,079 | ) | | | (583,304 | ) | | | (1,347,165 | ) |
| Net cash provided by (used in) financing activities | | | 15,657,024 | | | | (728,719 | ) | | | 3,566,223 | |
| Effect of exchange rate changes | | | 555,464 | | | | (33,852 | ) | | | (59,819 | ) |
| Net increase in cash, cash equivalents, and restricted cash | | $ | 4,920,322 | | | $ | 863,861 | | | $ | (99,709 | ) |
| Cash, cash equivalents and restricted cash, beginning of period | | | 1,637,283 | | | | 773,422 | | | | 873,131 | |
| Cash, cash equivalents and restricted cash, end of period | | $ | 6,557,605 | | | $ | 1,637,283 | | | $ | 773,422 | |
Operating Activities
Net cash used in operating activities was $2,228,087 for the year ended September 30, 2024, primarily derived from (a) a net loss of $3,540,409, adjusted by depreciation and amortization of $1,410,452, loss on disposal of equipment of $42,504, provision of inventory impairment of $762, provision of allowance for credit loss of $12,157, and deferred tax benefit of $46,044; (b) an increase in advances to suppliers of $1,061,321; (c) a decrease in accrued expenses and other current liabilities of $1,524,631; and (d) a decrease in accounts payable of $2,553,902, and offset by (a) a decrease in accounts receivable of $1,815,361, mainly in line with the decrease of revenue; and (b) a decrease in amounts due from related parties/(partially offset by increase in amounts due to related parties) of $1,775,923, mainly due to the Company’s enhanced collection efforts, resulting in a large amount of repayments from related parties.
Net cash provided by operating activities was $2,209,736 for the year ended September 30, 2023, primarily derived from (a) a net income of $63,383, adjusted by depreciation and amortization of $1,603,129, and addition of allowance for credit loss of $149,503; (b) a decrease of advances to suppliers of $977,318, mainly due to the Group’s request that suppliers speed up delivery of raw materials in response to increasing production capacity; (c) an increase in accounts payable of $864,585, due to the increase in the purchase of raw materials in response to market demand; (d) a decrease in prepaid expenses and other current assets of $1,291,145, which was primarily due to a decrease in interest-free loans to third parties; and (e) an increase in accrued expenses and other liabilities of $1,688,370, due to an increase in VAT tax payable and accrued payroll; and offset by (a) an increase in amounts due (from) to related parties of $3,964,077, mainly due to the advance to a related party for the initial public offering costs and receivable of selling to related parties; (b) an increase of inventories of $400,544, mainly due to the preparation for the increase in future sales; and (c) a decrease of income tax payable of $136,508.
Net cash used in operating activities was $2,258,948 for the year ended September 30, 2022, primarily derived from (a) a decrease in accounts payable of $5,822,547, due to an increase of settlements for the year ended September 30, 2022; (b) an increase in accounts receivable of $4,234,997, due to the liquidation difficulties of our customers under the impact of pandemic; and (c) a decrease in deferred revenue of $751,375, due to a decrease in market demand for masks; which was offset by (a) a net income of $944,126, adjusted by depreciation and amortization of $3,258,267; (b) an increase in accrued expenses and other current liabilities of $917,303, which was mainly due to a decrease in loans to third-parties; and (c) a decrease in inventories of $2,053,675, which was primarily due to the decrease in sales for the year ended September 30, 2022.
Investing Activities
For the year ended September 30, 2024, net cash used in investing activities was $9,064,079, which was derived from purchase of property and equipment of $9,225,094 and purchase of intangible assets of $33,313; offset by disposal of equipment of $194,328.
For the year ended September 30, 2023, net cash used in investing activities was $583,304, which was primarily derived from purchases of fixed assets of $455,391 and purchases of intangible assets of $127,913.
For the year ended September 30, 2022, net cash used in investing activities was $1,347,165, which primarily consisted of purchases of fixed assets of $2,012,546, offset by (a) disposal of equipment of $601,760 due to equipment upgrading; and (b) cash acquired from Shanghai Saitumofei of $63,621.
Financing Activities
For the year ended September 30, 2024, net cash provided by financing activities was $15,657,024, which mainly consisted of (a) net proceeds from our initial public offering of $7,373,839, from such amount, $5,404,654 of the net proceeds remained available after reimbursing PRC subsidiaries for expenses advanced from them in connection with the IPO; (b) proceeds from short-term bank borrowings of $13,394,778; (c) the repayment from related parties of $5,477,054, and loans to third parties of $2,734,478, respectively; (d) the contributions from non-controlling interest of $2,767,032; which were offset by (a) repayment of short-term bank borrowings of $9,369,404; (b) interest-free loans to related parties of $4,574,088; (c) loans to third parties of $1,133,656; (d) payment of offering cost of $613,009; and (e) cash deposited in escrow account of $400,000.
For the year ended September 30, 2023, net cash used in financing activities was $728,719, which mainly consisted of (a) interest-free loans to related parties of $5,669,834; (b) repayment of short-term bank borrowings of $6,000,791; (c) interest-bearing loans to third parties of $2,723,935; and (e) payment of offering cost of $1,356,176; offset by (a) repayment from related parties of $5,891,373; and (b) proceeds from short-term bank borrowings of $9,107,082.
For the year ended September 30, 2022, net cash provided by financing activities was $3,566,223, primarily consisted of (a) proceeds from short-term bank borrowings of $7,248,367; (b) the collection of loans from related parties of $13,260,930; and (c) the collection of interest-free loans from third parties of $829,042, offset by (a) interest-free loans to related parties of $9,493,121; (b) repayment of short-term bank borrowings of $7,248,367; and (c) interest-free loans to third parties of $1,030,628.
Capital Expenditures
Our capital expenditures are primarily incurred for the purpose of purchasing property and building or machinery. Our capital expenditures were $9,258,407, $583,304 and $2,012,546 for the year ended September 30, 2024, 2023 and 2022, respectively. We intend to fund our future capital expenditures with our existing cash balance and net proceeds from our IPO and expect to continue to make capital expenditures to meet the expected growth of our business.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated entities or financial partnerships, including entities sometimes referred to as structured finance or special purpose entities, that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Holding Company Structure
WORK Medical Technology Group LTD, is a holding company with no material operations of its own. We conduct our operations through the PRC subsidiaries in China. As a result, our ability to pay dividends depends upon dividends paid by the PRC subsidiaries. If our existing PRC subsidiaries or any newly formed ones incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to us. In addition, our subsidiaries in mainland China are permitted to pay dividends to us only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Under PRC law, each of our subsidiaries in mainland China is required to set aside at least 10% of its after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of their registered capital. In addition, our subsidiaries in mainland China may allocate a portion of their after-tax profits based on PRC accounting standards to enterprise expansion funds and staff bonus and welfare funds at their discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends. Remittance of dividends by a wholly foreign-owned company out of mainland China is subject to examination by the banks designated by SAFE. The PRC subsidiaries distributed dividends in the fiscal year ended September 30, 2021, and have made no further plans to pay dividends since such date and do not expect to do so unless and until they have generated sufficient accumulated profits and have met the requirements for statutory reserve funds.
Contractual Obligations
Commitments and Contingencies
From time to time, the PRC subsidiaries may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, we do not believe these actions, in the aggregate, will have a material adverse impact on the PRC subsidiaries’ financial position, results of operations or liquidity.
Operating Lease
The Group did not have any operating lease contractual obligations, significant capital and other commitments, long-term obligations, or guarantees as of September 30, 2024, 2023 and 2022.
C. Research and Development, Patents and Licenses, etc.
See “Item 4. Information on the Company—B. Business Overview” and “Item 5. Operating and Financial Review and Prospects—A. Operating Results.”
D. Trend Information
Other than as disclosed elsewhere in this report, we are not aware of any trends, uncertainties, demands, commitments or events for the years ended September 30, 2024, 2023 and 2022 that are reasonably likely to have a material and adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future results of operations or financial conditions.
E. Critical Accounting Policies and Estimates
We prepare our consolidated financial statements in accordance with U.S. GAAP. The preparation of financial statements in conformity with U.S. GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. We continually evaluate these judgments and estimates based on our own experience, knowledge and assessment of current business and other conditions, and our expectations regarding the future based on available information and assumptions that we believe to be reasonable. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies require a higher degree of judgment than others in their application.
When reading our consolidated financial statements, you should consider our selection of critical accounting policies, the judgment and other uncertainties affecting the application of such policies and the sensitivity of reported results to changes in conditions and assumptions. Our critical accounting policies and practices include the following: (i) revenue recognition; (ii) fair value measurement; and (iii) income taxes. See “Note 2—Summary of Significant Accounting Policies” to our consolidated financial statements for the disclosure of these accounting policies. We believe the following accounting estimates involve the most significant judgments used in the preparation of our financial statements.
Income taxes
Deferred tax assets and liabilities are measured using enacted tax rates expected to be applied to taxable income in the years when those temporary differences are expected to be recovered or settled. Deferred tax assets are reduced by a valuation allowance when, based upon the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities. The amounts of allowance over deferred tax assets were $78,210, $59,879, and $12,584 as of September 30, 2024, 2023 and 2022, respectively.
Allowance for credit losses
We maintain an allowance for credit losses by estimating the expected credit and collectability trend of our customers. Accounts receivable is considered past due based on its contractual terms. In estimating the allowance for credit losses, we consider various factors, including historical experience, credit-worthiness of customers, current and reasonable forecasted future economic conditions, aging of the accounts receivable balances, payment patterns, and the forecasted information in pooling basis upon the use of the Current Expected Credit Loss Model, or the CECL Model, in accordance with ASC topic 326—Financial Instruments—Credit Losses. We also consider to provide specific allowance for credit losses for those accounts receivable balances when facts and circumstances have emerged to indicate that these receivables are unlikely to be collected. Changes in these estimates and assumptions could materially affect the quantity of credit losses, which could be material to our financial position and results of operations.
The allowance of credit losses for accounts receivable as of September 30, 2024 and 2023 was $1,003,799 and $1,011,601, respectively. The allowance of credit losses for prepaid expenses and other current assets as of September 30, 2024 and 2023 was $427,351 and $352,935, respectively.
Impairment of Long-Lived Assets
We periodically review our long-lived assets for impairment indicators to identify any events that may lead the carrying value to be unrecoverable. Such events include an historical or projected trend of net cash outflow or a future expectation that we will sell or dispose of a significant asset well before its originally estimated useful life ends. The selection and assessment of qualitative factors used to determine whether it is more likely than not that the fair value of a reporting unit exceeds the carrying value involves significant judgment and estimates, which could be material to our financial position and results of operations. No impairment of long-lived assets was recognized for the years ended September 30, 2024, 2023 and 2022.
Item 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth information regarding our directors and executive officers as of the date of this annual report. Unless otherwise stated, the business address for our directors and executive officers is that of our principal executive offices at Floor 23, No. 2 Tonghuinan Road, Xiaoshan District, Hangzhou City, Zhejiang Province, the PRC.
| Name | | Age | | Position with our Company |
| Shuang Wu | | 42 | | Chief Executive Officer, Director, and Chairman of the Board of Directors |
| Ningfang Liang | | 52 | | Chief Financial Officer |
| Baiming Yu | | 54 | | Chief Operating Officer and Director |
| Xiaoyang Li | | 38 | | Independent Director |
Zhenguo Wu | | 38 | | Independent Director |
| Robert Johnson | | 47 | | Independent Director |
Shuang Wu has served as our Chief Executive Officer (“CEO”) and Chairman of the Board of Directors since August 2022 and our director since March 2022. Mr. Wu has extensive managerial and financial experience. Before joining our Company, from August 2019 to May 2022, he was the Chief Operating Officer of EZGO Technologies Ltd (Nasdaq: EZGO), a company focusing on sales of lithium battery and electric bicycle, and was responsible for making and enforcing business plans. From January 2018 to July 2019, he served as the vice president of Changzhou Hengmao Electricity Technology Co., Ltd., working for business operation and management. He was the General Manager of Investment of Shanghai Dafeng Investment Group Co., Ltd. from June 2015 to December 2017. From November 2011 to December 2014, he served as the assistant of General Manager of Travelex of Auckland Airport, a foreign exchange service provider. From June 2009 to November 2011, he was a financial adviser of Westpac Bank. Mr. Wu received his bachelor’s degree of finance and master’s degree of business from Massey University.
Ningfang Liang has served as our Chief Financial Officer (“CFO”) since August 2022. Mr. Liang has been in the financial field and working for U.S.-listed companies for over 15 years. From December 2008 to June 2011, he was the manager of Sirius International Insurance Group, a former Nasdaq-listed company (Nasdaq: SG), and from June 2016 to June 2022, he rejoined the company and resumed being the manager, responsible for evaluating business performance and consolidated financial results, preparing for SEC filings and recommending changes for business plan from the perspective of finance. From March 2013 to January 2016, and from June 2011 to February 2013, he worked as the Chief Financial Officer of two U.S. public companies respectively, Tantech Holdings Ltd. (Nasdaq: TANH) and China GengSheng Minerals, Inc. (OTCMKTS: CHGS). From December 2006 to December 2008, he worked as a senior accountant in American International Group Inc. (NYSE: AIG). He was a financial analyst of Celgene Corporation, a former Nasdaq-listed company (Nasdaq: CELG), from January 2005 to December 2006. Mr. Liang has a bachelor’s degree of science from Shanghai University of Finance and Economics and an MBA degree from University of Illinois, Urbana-Champaign. He is also a Certified Public Accountant of the states of New Jersey and Illinois.
Baiming Yu has served as our Chief Operating Officer (“COO”) since August 2022 and he has served as our director since August 2024. Mr. Yu has enriched experience in the field of medicine. He is the founder of Hangzhou Shanyou and has been its General Manager since January 2002, responsible for the company’s daily operations and management. Additionally, he served as the General Manager of Hangzhou Yuanqi Biotech Co., Ltd. from January 2012 to December 2018. From August 1993 to December 2001, he was a doctor of The First People’s Hospital of Xiaoshan City. Mr. Yu received his bachelor’s degree of medicine in anesthesia from the former Zhejiang Medical University, which was merged into Zhejiang University in 1998.
Xiaoyang Li has served as our director since August 2024. Dr. Li has been working in Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine since July 2011. He was a Resident Physician initially and later got promoted to be an Attending Physician in June 2016. Since December 2020, he has been the Associate Chief Physician of the hospital. Dr. Li received his master’s degree of clinical medicine and doctor’s degree of internal medicine from Shanghai Jiao Tong University.
Zhenguo Wu has served as our director since January 2025. Mr. Wu has substantial legal professional experience in capital markets. He has been a lawyer and providing capital markets legal advisory services at Watson & Band Law Offices since December 2024. From April 2021 to November 2024, he served as the board secretary and general counsel at Xuhang Holdings Ltd (SUNH). From October 2015 to February 2021, he served as the senior vice president of the investment banking division of Shenwan Hongyuan Securities Underwriting and Sponsoring Co., Ltd. Mr. Wu obtained a bachelor’s degree in English from Tangshan Normal University in 2010 and a master’s degree in law from East China University of Political Science and Law in 2013.
Robert Johnson has served as our director since August 2024. Mr. Johnson has over 15 years of experience in tax and finance. He has been the Financial Controller of BAS Holdings Investments, LLC since February 2020, primarily responsible for review of periodic account and intercompany reconciliations and financial statement reporting and cash, receivables, and payables management for operating companies. From January 2017 to January 2020, he was the controller for family office of Lionstone Development, LLC. From June 2010 to June 2015, he was the controller for family office of BSL Capital, Inc. From September 2005 to June 2010, he served as a senior tax accountant of CBIZ MHM, LLC, and from January 2004 to September 2005, he was a tax accountant of Mallah Furman and Company. Mr. Johnson received his bachelor’s degrees of science in accounting and finance from University of Central Florida, and his MBA degree from University of Miami. He is both a Certified Management Accountant and Certified Public Accountant.
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
Board Diversity Matrix
| Country of Principal Executive Offices: | | China |
| Foreign Private Issuer | | Yes |
| Disclosure Prohibited under Home Country Law | | No |
| Total Number of Directors | | 5 |
| | Female | | Male | | Non-Binary | | Did Not
Disclose
Gender |
| Part I: Gender Identity | |
| Directors | 0 | | 5 | | 0 | | 0 |
| Part II: Demographic Background | |
| Underrepresented Individual in Home Country Jurisdiction | 0 |
| LGBTQ+ | 0 |
| Did Not Disclose Demographic Background | 0 |
Family Relationships
None of our directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
B. Compensation
Since June 1, 2022, we paid Baiming Yu RMB24,000 (approximately $3,529) per month for his services. As of September 30, 2024, the accumulated amount was RMB672,000 (approximately $98,812).
From June 1, 2022, to May 31, 2025, the compensation of our CEO is RMB30,000 (approximately $4,368) per month. From June 1, 2022, to August 31, 2024, the compensation of our CFO was $2,000 per month. From September 1, 2024, to August 31, 2025, the compensation of our CFO is $12,500 per month. The Company has settled the accumulated unpaid compensation in cash in the aggregated amount of $54,000 in August 2024, and henceforth pays compensation to the CEO and CFO on a monthly basis.
As of the date of this annual report, we have not set aside or accrued any amount to provide pension, retirement, or other similar benefits to our directors and executive officers.
C. Board Practices
Board of Directors
Our board of directors, or Board of Directors consists of five directors, including three independent directors. Unless a shareholding qualification for directors is fixed by ordinary resolution passed by the Company’s shareholders, a director is not required to hold any shares in our Company to qualify to serve as a director. A director shall not, as a director, vote in respect of any contract, transaction, arrangement or proposal in which they have an interest which (together with any interest of any person connected with them) is a material interest (otherwise then by virtue of their interests, direct or indirect, in shares or debentures or other securities of, or otherwise in or through, the Company) and if they shall do so their vote shall not be counted, nor in relation thereto shall they be counted in the quorum present at the meeting, but (in the absence of some other material interest than is mentioned below) none of these prohibitions shall apply to: (a) the giving of any security, guarantee or indemnity in respect of: (i) money lent or obligations incurred by them or by any other person for the benefit of the Company or any of its subsidiaries; or (ii) a debt or obligation of the Company or any of its subsidiaries for which a director has assumed responsibility in whole or in part and whether alone or jointly with others under a guarantee or indemnity or by the giving of security; (b) where the Company or any of its subsidiaries is offering securities in which offer the director is or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which the director is to or may participate; (c) any contract, transaction, arrangement or proposal affecting any other body corporate in which he is interested, directly or indirectly and whether as an officer, shareholder, creditor or otherwise howsoever, provided that he (together with persons connected with them) does not to their knowledge hold an interest representing one per cent or more of any class of the equity share capital of such body corporate (or of any third body corporate through which his interest is derived) or of the voting rights available to members of the relevant body corporate; (d) any act or thing done or to be done in respect of any arrangement for the benefit of the employees of the Company or any of its subsidiaries under which they are not accorded as a director any privilege or advantage not generally accorded to the employees to whom such arrangement relates; (e) any matter connected with the purchase or maintenance for any director of insurance against any liability or (to the extent permitted by the Companies Act (Revised) of the Cayman Islands) indemnities in favor of directors, the funding of expenditure by one or more directors in defending proceedings against him or them or the doing of anything to enable such director or directors to avoid incurring such expenditure; or (f) any contract, transaction, arrangement or proposal in which the director has an interest which is not a material interest. The directors may exercise all the powers of the company to borrow money, mortgage its undertaking, property and uncalled capital, and issue debentures or other securities whenever money is borrowed or as security for any obligation of the company or of any third party.
Committees of the Board of Directors
Our board of directors consists of five directors, including three independent directors. We have established an Audit Committee, a Nominating and Corporate Governance Committee and a Compensation Committee. We have adopted a charter for each of the three committees. Each of the committees of our board of directors has the composition and responsibilities described below.
Audit Committee
Xiaoyang Li, Zhenguo Wu, and Robert Johnson serve as members of our Audit Committee with Robert Johnson serving as the chairman of the Audit Committee. All of our Audit Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our board of directors have determined that Robert Johnson possesses accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC. Our Audit Committee oversees our accounting and financial reporting processes and the audits of our financial statements. Our Audit Committee performs several functions, including:
| ● | evaluating the independence and performance of, and assesses the qualifications of, our independent auditor, and engages such independent auditor; |
| ● | approving the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services, and approves in advance any non-audit service to be provided by the independent auditor; |
| ● | monitoring the independence of the independent auditor and the rotation of partners of the independent auditor on our engagement team as required by law; |
| ● | reviewing the financial statements to be included in our Annual Report on Form 20-F and Current Reports on Form 6-K and reviews with management and the independent auditors the results of the annual audit and reviews of our quarterly financial statements; |
| ● | overseeing all aspects of our systems of internal accounting control and corporate governance functions on behalf of the board; |
| ● | reviewing and approving in advance any proposed related-party transactions and report to the full Board on any approved transactions; and |
| ● | providing oversight assistance in connection with legal, ethical and risk management compliance programs established by management and our board of directors, including Sarbanes-Oxley Act implementation, and makes recommendations to our board of directors regarding corporate governance issues and policy decisions. |
Compensation Committee
Xiaoyang Li, Zhenguo Wu, and Robert Johnson serve as members of our Compensation Committee with Xiaoyang Li serving as the chairman of the Compensation Committee. All of our Compensation Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our Compensation Committee is responsible for overseeing and making recommendations to our board of our directors regarding the salaries and other compensation of our executive officers and general employees and providing assistance and recommendations with respect to our compensation policies and practices.
Nominating and Corporate Governance Committee
Xiaoyang Li, Zhenguo Wu, and Robert Johnson serve as members of our Nominating and Corporate Governance Committee, with Zhenguo Wu serving as the chairman of the Nominating and Corporate Governance Committee. All of our Nominating and Corporate Governance Committee members satisfy the “independence” requirements of the Nasdaq listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. Our Nominating and Corporate Governance Committee is responsible for identifying and proposing new potential director nominees to the board of directors for consideration and reviewing our corporate governance policies.
Duties of Directors
Under Cayman Islands law, directors and officers owe the following fiduciary duties:
| (i) | duty to act in good faith in what the director or officer believes to be in the best interests of the company as a whole; |
| (ii) | duty to exercise powers for the purposes for which those powers were conferred and not for a collateral purpose; |
| (iii) | directors should not properly fetter the exercise of future discretion; |
| (iv) | duty not to put themselves in a position in which there is a conflict between their duty to the company and their personal interests; and |
| (v) | duty to exercise independent judgment. |
In addition to the above, directors also owe a duty of care which is not fiduciary in nature. This duty has been defined as a requirement to act as a reasonably diligent person having both the general knowledge, skill and experience that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and the general knowledge skill and experience which that director has.
As set out above, directors have a duty not to put themselves in a position of conflict and this includes a duty not to engage in self-dealing, or to otherwise benefit as a result of their position. However, in some instances what would otherwise be a breach of this duty can be forgiven and/or authorized in advance by the shareholders provided that there is full disclosure by the directors. This can be done by way of permission granted in the memorandum and articles of association or alternatively by shareholder approval at general meetings.
Accordingly, as a result of multiple business affiliations, our officers and directors may have similar legal obligations relating to presenting business opportunities meeting the above-listed criteria to multiple entities. In addition, conflicts of interest may arise when our board evaluates a particular business opportunity with respect to the above-listed criteria. We cannot assure you that any of the afore-mentioned conflicts will be resolved in our favor. Furthermore, each of our officers and directors has pre-existing fiduciary obligations to other businesses of which they are officers or directors.
Our Company has the right to seek damages if a duty owed by our directors is breached. A shareholder may in certain limited exceptional circumstances have the right to seek damages in our name if a duty owed by our directors is breached.
In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association as may be amended from time to time. Our Company has a right to seek damages against any director who breaches a duty owed to us.
Our directors have all the powers necessary for managing, and for directing and supervising, our business affairs.
Terms of Directors and Executive Officers
Unless removed or re-appointed, each director shall be appointed for a term expiring at the next-following annual general meeting, if one is held. At any annual general meeting held, our directors will be elected by an ordinary resolution of our shareholders. At each annual general meeting, each director so elected shall hold office for a one-year term and until the election of their respective successors in office or removed.
A director may be removed by ordinary resolution.
A director may at any time resign or retire from office by giving us notice in writing. Unless the notice specifies a different date, the director shall be deemed to have resigned on the date that the notice is delivered to us.
Subject to the provisions of the articles, the office of a director may be terminated forthwith if:
| (a) | he is prohibited by the law of the Cayman Islands from acting as a director; |
| (b) | he is made bankrupt or makes an arrangement or composition with his creditors generally; |
| (c) | he resigns his office by notice to us; |
| (d) | he only held office as a director for a fixed term and such term expires; |
| (e) | in the opinion of a registered medical practitioner by whom he is being treated he becomes physically or mentally incapable of acting as a director; |
| (f) | he is given notice by the majority of the other directors (not being less than two in number) to vacate office (without prejudice to any claim for damages for breach of any agreement relating to the provision of the services of such director); |
| (g) | he is made subject to any law relating to mental health or incompetence, whether by court order or otherwise; or |
| (h) | without the consent of the other directors, he is absent from meetings of directors for continuous period of six months. |
Qualification
There is currently no shareholding qualification for directors, although a shareholding qualification for directors may be fixed by our shareholders by ordinary resolution.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for an initial term from June 1, 2022, to May 31, 2025.
The executive officers are entitled to a fixed salary, as described below under “Compensation of Directors and Officers,” and to participate in our equity incentive plans, if any and other company benefits, each as determined by the Board from time to time.
We may terminate the executive officer’s employment for cause, at any time, without notice or remuneration, for certain acts, such as conviction or plea of guilty to a felony or grossly negligent or dishonest acts to our detriment, or misconduct or a failure to perform agreed duties. In such case, the executive officer will not be entitled to receive payment of any severance benefits or other amounts by reason of the termination, and his right to all other benefits will terminate, except as required by any applicable law. We may also terminate the executive officer’s employment without cause immediately and without prior written notice upon the removal of the executive officer pursuant to the exercise of any power contained in the memorandum and articles of association of the Company or upon 30 days’ advance written notice. In such case of termination by us, we are required to provide the following severance payments and benefits to the executive officer: a cash payment of three month of base salary as of the date of such termination.
The executive officer may terminate his or her employment at any time with 30 days’ advance written notice if there is any significant change in his or her duties and responsibilities or a material reduction in his or annual salary. In such case, the executive officer will be entitled to receive compensation equivalent to three months of his or her base salary. In addition, if we or our successor terminates the employment agreements upon a merger, consolidation, or transfer or sale of all or substantially all of our assets with or to any other individual(s) or entity, the executive officer shall be entitled to the following severance payments and benefits upon such termination: 1) a lump sum cash payment equal to three months of base salary at a rate equal to the greater of his or her annual salary in effect immediately prior to the termination, or his or her then current annual salary as of the date of such termination; 2) a lump sum cash payment equal to a pro-rated amount of target annual bonus for the fiscal year immediately preceding the termination; 3) payment of premiums for continued health benefits under our health plans for three months following the termination; and 4) immediate vesting of 100% of the then-unvested portion of any outstanding equity awards held by the executive officer. The employment agreements also contain customary restrictive covenants relating to confidentiality and non-competition. The foregoing description of the terms of the employment agreements is qualified in its entirety by reference to the provisions of the employment agreement filed as Exhibit 4.2 to this annual report, which are incorporated by reference herein.
We have also entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we have agreed to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our Company.
Insider Participation Concerning Executive Compensation
Our CEO, Shuang Wu, was making all determinations regarding executive officer compensation from the inception of the Company until our Compensation Committee was established in August 2024.
Code of Business Conduct and Ethics
We have adopted a code of ethics that applies to all of our executive officers, directors and employees in accordance with the rules of Nasdaq and the SEC. The code of ethics codifies the business and ethical principles that govern all aspects of our business. You will be able to review the code of ethics by accessing our public filings at the SEC’s website at www.sec.gov.
Compensation Recovery Policy
We have adopted a compensation recovery policy to provide for the recovery of erroneously-awarded incentive compensation, as required by the Dodd-Frank Wall Street Reform and Consumer Protection Act, final SEC rules and applicable listing standards.
D. Employees
See “Item 4. Information on the Company—B. Business Overview—Employees.”
E. Share Ownership
The following table sets forth information with respect to the beneficial ownership, within the meaning of Rule 13d-3 under the Exchange Act, of our Ordinary Shares as of the date of this annual report for:
| ● | each of our directors and executive officers; and |
| ● | each person known to us to own beneficially more than 5% of our Ordinary Shares. |
Beneficial ownership includes voting or investment power with respect to the securities. Except as indicated below, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all Ordinary Shares shown as beneficially owned by them. Percentage of beneficial ownership of each listed person is based on 14,591,942 Ordinary Shares outstanding as of the date of this annual report.
Information with respect to beneficial ownership has been furnished by each director, officer, or beneficial owner of 5% or more of our Ordinary Shares. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. In computing the number of Ordinary Shares beneficially owned by a person listed below and the percentage ownership of such person, Ordinary Shares underlying options, warrants, or convertible securities are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person.
| | | Ordinary Shares
Beneficially Owned | | | Voting
Power | |
| | | Number | | | % | | | % | |
| Directors and Officers:(1) | | | | | | | | | |
| Shuang Wu | | | — | | | | — | | | | — | |
| Baiming Yu(2) | | | 6,250,000 | | | | 42.83 | % | | | 42.83 | % |
| Ningfang Liang | | | — | | | | — | | | | — | |
| Robert Johnson | | | — | | | | — | | | | — | |
| Zhenguo Wu | | | — | | | | — | | | | — | |
| Xiaoyang Li | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| 5% Shareholders(3): | | | | | | | | | | | | |
| LWY GROUP LTD(2) | | | 6,250,000 | | | | 42.83 | % | | | 42.83 | % |
| (1) | Unless otherwise indicated, the business address of each of the individuals is Floor 23, No. 2 Tonghuinan Road Xiaoshan District, Hangzhou City, Zhejiang Province, the PRC. The business address of Ningfang Liang is 65 Grassman Pl, Berkeley Heights, New Jersey, U.S. The business address of Robert Johnson is 1450 Brickell Avenue 31st Floor, Miami, Florida, U.S. The business address of Zhenguo Wu is Floor 26-27, The Center, No. 989 Changle Road, Xuhui District, Shanghai City, PRC. |
| (2) | The number of Ordinary Shares beneficially owned represents 6,250,000 Ordinary Shares held by LWY GROUP LTD, a British Virgin Islands company, which is 100% owned by Baiming Yu, as of the date of this annual report. |
| (3) | The registered address of each of the shareholders is 2/F, Palm Grove House, P.O. Box 3340, Road Town, Tortola, British Virgin Islands. All shareholders acquired their Ordinary Shares from the Company on March 1, 2022. |
As of the date of this annual report, approximately 47.97% of our issued and outstanding ordinary shares are held in the United States by one record holder CEDE & Company.
We are not aware of any other arrangement that may, at a subsequent date, result in a change of control of our Company.
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
Item 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
The following is a list of related parties with which the Group has transactions:
No. | | Name of Related Parties | | Relationship with the Group |
| a | | Baiming Yu | | COO, Director and a shareholder of the Company |
| b | | Liwei Zhang | | Shareholder of the Company and the spouse of
Baiming Yu |
| c | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
| d | | Baicheng Zhang | | Immediate family member of Liwei Zhang |
| e | | Shuang Wu | | CEO, Director, and Chairman of the Board of Directors |
| f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
| g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
| h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
| i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director before January, 2024 |
| j | | Ming Zhao | | The Supervisor of Shanghai Saitumofei |
| k | | Hangzhou Yizhiying Information Consulting Management Co., Ltd | | Shuang Wu acted as executive director |
| g | | Qijia Yu | | Immediate family member of Baiming Yu |
| | | For the years ended
September 30, | |
| Nature | | 2024 | | | 2023 | | | 2022 | |
| Loan to Hangzhou Shuige(1) | | $ | 1,538,672 | | | $ | 423,585 | | | $ | 1,983,764 | |
| Repayment from Hangzhou Shuige(1) | | | 1,110,448 | | | | 423,585 | | | | 5,889,698 | |
| Loan to Liwei Zhang(1) | | | 319,254 | | | | 303,075 | | | | — | |
| Repayment from Liwei Zhang(1) | | | 330,580 | | | | 362,377 | | | | — | |
| Advance to Baiming Yu(2) | | | 3,705 | | | | 1,187,554 | | | | 17,148 | |
| Reimbursement from Baiming Yu(2) | | | 9,375 | | | | 644,678 | | | | — | |
| Repayment from Baiming Yu(2) | | | 692,074 | | | | — | | | | — | |
| Sales to Hangzhou Qingniu(3) | | | 577,370 | | | | 1,011,587 | | | | 16,524 | |
| Loan to Hangzhou Qingniu(1) | | | 2,716,162 | | | | 4,943,174 | | | | 7,356,760 | |
| Rent income from Hangzhou Qingniu(4) | | | — | | | | 52,749 | | | | 192,636 | |
| Repayment from Hangzhou Qingniu(1)(3) (4) | | | 4,036,026 | | | | 5,105,411 | | | | 7,371,232 | |
| Loan from Xiaoshan Ance(5) | | | — | | | | — | | | | 152,597 | |
| Advance to Shuang Wu(6) | | | 2,054,627 | | | | 3,467,737 | | | | 174,800 | |
| Repayment from Shuang Wu(6) | | | 1,159,929 | | | | 2,022,703 | | | | 851,143 | |
| Advance to Huiyu Chuanggu(7) | | | 447,927 | | | | 2,894,499 | | | | — | |
| Reimbursement from Huiyu Chuanggu(7) | | | 2,077,162 | | | | 1,397,077 | | | | — | |
| Management service fee to Huiyu Chuanggu(8) | | | 1,247,508 | | | | — | | | | — | |
| Rent income from Hangzhou Yuanqi(4) | | | — | | | | — | | | | 3,815 | |
| Collection from Hangzhou Yuanqi(4) | | | — | | | | 706 | | | | 3,815 | |
| Advance to Ming Zhao(9) | | | — | | | | 866 | | | | — | |
| Collection from Ming Zhao(9) | | | — | | | | 866 | | | | 1,958 | |
| Advance from Hangzhou Yizhiying Information Consulting Management Co., Ltd(10) | | | — | | | | — | | | | 149,606 | |
| Repayment to Hangzhou Yizhiying Information Consulting Management Co., Ltd(10) | | | — | | | | — | | | | 61,885 | |
| Advance to Qijia Yu(2) | | | 8,142 | | | | — | | | | — | |
| Repayment from Qijia Yu(2) | | | 2,846 | | | | — | | | | — | |
| (1) | This represented the Group’s interest-free loans, which were due on demand, to these related parties and repayment from these related parties. |
| (2) | It was the advances which were interest-free and due on demand made to the related parties for the Group’s daily operational purposes, and reimbursement from the related parties for the Group’s operational expenses. |
| (3) | It was the receivable for selling medical consumables to this related party and repayment from this related party. |
| (4) | It was the receivable and collection for providing rental service to this related party. |
| (5) | This consisted of the Group’s interest-free loan from this related party for daily operations, which was due on demand. |
| (6) | This consisted of the Group’s advance to this related party with no fixed term of repayment and interest, and the repayment from this related party. |
| (7) | This consisted of the advance to this related party for the Company’s initial public offering costs, including legal fees and accounting fees, and the reimbursement of excessive advance payment from this related party. |
| (8) | It was the management service fee for Huiyu Chuanggu’s intergrated management consulting services during the Company’s IPO process. This fee was charged once at a fixed price only when the Company completed its IPO. |
| | (9) | This consisted of the advance to this related party for the Group’s daily operations. |
| | (10) | This consisted of the Group’s advance from this related party for the Group’s daily operations with no fixed term of repayment and interest. |
Employment Agreements
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Employment Agreements and Indemnification Agreements.”
Material Transactions with Related Parties
(a) Nature of relationships with related parties
| No. | | Name of Related Parties | | Relationship with the Group |
| a | | Baiming Yu | | COO, Director and a shareholder of the Company |
| b | | Liwei Zhang | | Shareholder of the Company and the spouse of Baiming Yu |
| c | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
| d | | Baicheng Zhang | | Immediate family member of Liwei Zhang |
| e | | Shuang Wu | | Chief Executive Officer of the Company |
| f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
| g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
| h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
| i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director |
| j | | Ming Zhao | | Shareholder of Shanghai Saitumofei |
| k | | Hangzhou Yizhiying Information Consulting Management Co., Ltd. | | Shuang Wu acted as executive director |
| l | | Qijia Yu | | Immediate family member of Baiming Yu |
(b) Due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | | As of September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Shuang Wu | | $ | 1,424,989 | | | $ | 795,243 | | | $ | - | |
| Hangzhou Qingniu | | | 855,618 | | | | 1,294,296 | | | | 429,347 | |
| Hangzhou Shuige | | | 421,092 | | | | - | | | | - | |
| Qijia Yu | | | 5,437 | | | | - | | | | - | |
| Huiyu Chuanggu | | | - | | | | 1,453,583 | | | | - | |
| Baiming Yu | | | - | | | | 683,376 | | | | 160,405 | |
| Liwei Zhang | | | - | | | | 11,184 | | | | 70,514 | |
| Baicheng Zhang | | | - | | | | - | | | | 46,012 | |
| Total | | $ | 2,707,136 | | | $ | 4,237,682 | | | $ | 706,278 | |
(c) Due to related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | | As of September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Huiyu Chuanggu | | $ | 1,064,916 | | | $ | - | | | $ | - | |
| Shuang Wu | | | - | | | | - | | | | 623,070 | |
| Xiaoshan Ance | | | 142,499 | | | | 137,061 | | | | 140,578 | |
| Baiming Yu | | | 5,820 | | | | - | | | | - | |
| Ming Zhao | | | 4,207 | | | | 4,048 | | | | 4,151 | |
| Hangzhou Shuige | | | - | | | | 17,818 | | | | 18,275 | |
| Hangzhou Yizhiying Information Consulting Management Co., Ltd | | | - | | | | - | | | | 80,812 | |
| Hangzhou Yuanqi | | | - | | | | 685 | | | | - | |
| Total | | $ | 1,217,442 | | | $ | 159,612 | | | $ | 866,886 | |
(d) Loan guarantee provided by related parties
See “Item 18. Financial Statements-Note 10”.
(e) Prepayment to related party for property purchase
Not applicable.
(f) Share Issuances in March 2022
On March 1, 2022, we issued the following Ordinary Shares, par value $1.00 per share, to certain founding shareholders of Work Cayman:
| Purchaser | | Number of
Ordinary
Shares | | | Consideration | |
| Tricor Services (Cayman Islands) Limited | | | 1 | | | $ | 1 | |
| LWY GROUP LTD | | | 24,999 | | | $ | 24,999 | |
| HJZ GROUP LTD | | | 5,740 | | | $ | 5,740 | |
| SANYOU NO.1 GROUP LTD | | | 3,890 | | | $ | 3,890 | |
| JPY GROUP LTD | | | 2,870 | | | $ | 2,870 | |
| SANYOU NO.2 GROUP LTD | | | 2,500 | | | $ | 2,500 | |
| YQJ GROUP LTD | | | 2,500 | | | $ | 2,500 | |
| ZHF GROUP LTD | | | 2,500 | | | $ | 2,500 | |
| ZLW GROUP LTD | | | 2,500 | | | $ | 2,500 | |
| ZXH GROUP LTD | | | 2,500 | | | $ | 2,500 | |
(g) Share Transfer in March 2022
On March 1, 2022, Tricor Services (Cayman Islands) Limited transferred its one share of Work Cayman to LWY GROUP LTD for $1.
(h) Change in Authorized Share Capital and Share Subdivision in April 2023
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from US$50,000 divided into 50,000 shares of par value US$1.00 each to US$50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
C. Interests of Experts and Counsel
Not applicable.
Item 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report. See “Item 18. Financial Statements.”
Legal Proceedings
As of the date of this annual report, neither we nor the PRC subsidiaries are a party to any material legal or administrative proceedings. From time to time, the PRC subsidiaries may be subject to various claims and legal actions arising in the ordinary course of business. Litigation or any other legal or administrative proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of the PRC subsidiaries’ resources, including management’s time and attention. Furthermore, as of the date of this annual report, the PRC subsidiaries are not a party to any international claims or litigation with respect to defective products or other matters.
Dividend Policy
As of the date of this annual report, the Company has transferred the net proceeds in the amount of $7,373,839 from our IPO through Work BVI to Work Medical Technology, from such amount, $5,404,654 of the net proceeds remained available after reimbursing PRC subsidiaries for expenses advanced from them in connection with the IPO.
Our board of directors has discretion regarding whether to declare or pay dividends. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. All dividends are subject to certain restrictions under Cayman Islands law, namely that our Company may only pay dividends out of profits or share premium, and provided always that we are able to pay our debts as they fall due in the ordinary course of business. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
As of the date of this annual report, we have never declared or paid cash dividends on our Ordinary Shares. We currently do not have any plans to pay cash dividends. Rather, we currently intend to retain all of our available funds and any future earnings to operate and grow our business.
If we determine to pay dividends on any of our Ordinary Shares in the future, as a holding company, we will depend upon receipt of funds from our British Virgin Islands subsidiary, Work BVI.
Current PRC regulations permit the PRC subsidiaries to pay dividends to Work Medical Technology only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in mainland China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entities in mainland China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of mainland China. Therefore, we may experience difficulties in complying with the administrative requirements necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries and affiliates in mainland China incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenue from our operations, we may be unable to pay dividends on our Ordinary Shares.
Cash dividends, if any, on our Ordinary Shares will be paid in U.S. dollars. Work Medical Technology or any of our subsidiaries outside of China may be considered a non-resident enterprise for tax purposes, so that any dividends the PRC subsidiaries pay to Work Medical Technology or any of our subsidiaries outside of China may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10%. See “Item 10. Additional Information—E. Taxation—Taxation—People’s Republic of China Taxation.”
In order for us to pay dividends to our shareholders, we will rely on payments made from Work Hangzhou’s subsidiaries to Work Hangzhou and from Work Hangzhou to WFOE, and the distribution of such payments to Work Medical Technology, and from Work Medical Technology to Work BVI and then to our Company. According to the PRC Enterprise Income Tax Law (the “EIT Law”), income obtained by enterprises shall pay enterprise income tax including income from equity investment such as dividends and bonuses. In addition, if Work Hangzhou or its subsidiaries or branches incur debt on their own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
Item 9. THE OFFER AND LISTING
A. Offer and Listing Details.
Our Ordinary Shares have been listed on the Nasdaq Global Market since August 23, 2024 under the symbol “WOK.”
B. Plan of Distribution
Not applicable.
C. Markets
Our Ordinary Shares have been listed on the Nasdaq Global Market since August 23, 2024 under the symbol “WOK.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
Item 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We are an exempted company incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our amended and restated memorandum of association and articles of association, the Cayman Companies Act, and the common law of the Cayman Islands.
We incorporate by reference into this annual report the description of our amended and restated memorandum and articles of association, which is filed as Exhibit 1.1 to this annual report on Form 20-F.
Registered Office
Our registered office in the Cayman Islands is at the offices of Harneys Fiduciary (Cayman) Limited, 4th Floor, Harbour Place, 103 South Church Street, P.O. Box 10240, Grand Cayman, KY1-1002, Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees.”
Ordinary Shares
General
Under our amended and restated articles of association, the objects of our Company are unrestricted and we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands. All of our issued and outstanding Ordinary Shares will be fully paid up and non-assessable. Our Ordinary Shares are issued in registered form and are issued when registered in our register of members. Unless the directors determine otherwise, each holder of our Ordinary Shares will not receive a certificate in respect of such Ordinary Shares. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their Ordinary Shares. We may not issue shares or warrants to bearer.
Dividends
Subject to the provisions of the Cayman Companies Act and any rights attaching to any class or classes of shares under and in accordance with the amended and restated articles of association:
| (a) | the directors may declare dividends or distributions out of our funds which are lawfully available for that purpose; and |
| (b) | our shareholders may, by ordinary resolution, declare dividends but no such dividend shall exceed the amount recommended by the directors. |
Subject to the requirements of the Cayman Companies Act regarding the application of a company’s share premium account and with the sanction of an ordinary resolution, dividends may also be declared and paid out of any share premium account. The directors when paying dividends to shareholders may make such payment either in cash or in specie.
Unless provided by the rights attached to a share, no dividend shall bear interest.
Voting Rights
A shareholder may participate in a general meeting in person, by proxy or through the medium of conference telephone, video or any other form of communications equipment provided that all person participating in the meeting are able to hear and speak to each other throughout the meeting.. At any general meeting a resolution put to the vote at the meeting shall be decided on a show of hands, unless a poll is (before or on the declaration of the result of the show of hands) demanded by the chairman of such meeting or by at least two shareholders having the right to vote on the resolutions or one or more shareholders present, in person or by proxy, who individually or together hold not less than ten percent of the voting rights of all those who are entitled to vote on the resolution. Unless a poll is so demanded, a declaration by the chairman as to the result of a resolution and an entry to that effect in the minutes of the meeting, shall be conclusive evidence of the outcome of a show of hands, without proof of the number or proportion of the votes recorded in favor of, or against, that resolution.
If a poll is duly demanded it shall be taken in such manner as the chairman directs and the result of the poll shall be deemed to be the resolution of the meeting at which the poll was demanded.
In the case of an equality of votes, whether on a show of hands or on a poll, the chairman of the meeting at which the show of hands takes place or at which the poll is demanded, shall not be entitled to a second or casting vote.
As a matter of Cayman Islands law, (i) an ordinary resolution requires the affirmative vote of a majority of votes cast by shareholders who, being entitled to do so, attend and vote at a general meeting of the company; and (ii) a resolution is deemed to be a special resolution where it has been approved by at least two-thirds (or any higher threshold specified in a company’s articles of association) of votes cast by shareholders who attend and vote at a general meeting for which notice specifying the intention to propose the resolution as a special resolution has been given. Ordinary resolutions and special resolutions can be passed by a unanimous written resolution of all of the company’s shareholders.
Under Cayman Islands law, some matters, such as amending the memorandum and articles of association, changing the name or resolving to be registered by way of continuation in a jurisdiction outside the Cayman Islands, require the approval of shareholders by a special resolution.
Variation of Rights of Shares
If at any time, our share capital is divided into different classes of shares, the rights attached to any class of shares (unless otherwise provided by the terms of issue of the shares of that class), whether or not our Company is being wound-up, may be varied either with the consent in writing of the holders of two-thirds of the issued shares of that class or with the sanction of a resolution passed by a majority of two-thirds of the votes cast at a separate meeting of the issued shares of that class. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
Alteration of Share Capital
Subject to the Companies Act, our shareholders may from time to time by ordinary resolution:
| ● | increase the share capital by such sum, to be divided into shares of such classes and amount, as the resolution prescribes; |
| ● | consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares; |
| ● | convert all or any of its paid up shares into stock and reconvert the stock into paid up shares of any denomination; |
| ● | sub-divide our existing shares, or any of them into shares of a smaller amount than that fixed by our Articles; provided that in the subdivision the proportion between the amount paid and the amount, if any, unpaid on each reduced share will be the same as it was in case of the share from which the reduced share is derived; and |
| ● | cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so cancelled or, in the case of shares without nominal par value, diminish the number of shares into which our capital is divided. |
Calls on Shares and Forfeiture
Our Board of Directors may from time to time make calls upon shareholders for any amounts unpaid on their Ordinary Shares in a notice served to such shareholders at least 14 clear days prior to the specified time and place of payment. Any Ordinary Shares that have been called upon and remain unpaid are subject to forfeiture. No shareholder shall be entitled to vote at any general meeting unless all calls or other sums payable by such shareholder have been paid. Our Board of Directors may deduct from a dividend or any other amount payable to a person in respect of an Ordinary Share any amount due by that person to the Company on a call or otherwise in relation to an Ordinary Share.
Unclaimed Dividend
A dividend that remains unclaimed for a period of six years after it became due for payment shall be forfeited to, and shall cease to remain owing by, the company.
Forfeiture or Surrender of Shares
Our Board of Directors may from time to time make calls upon shareholders for any amounts unpaid on their Ordinary Shares in a notice served to such shareholders at least 14 clear days prior to the specified time and place of payment. Any Ordinary Shares that have been called upon and remain unpaid are subject to forfeiture. No shareholder shall be entitled to vote at any general meeting unless all calls or other sums payable by such shareholder have been paid. Our Board of Directors may deduct from a dividend or any other amount payable to a person in respect of an Ordinary Share any amount due by that person to the Company on a call or otherwise in relation to an Ordinary Share. In addition, under the Companies Act, our Company may accept the surrender of any fully paid share for no consideration unless, as a result of the surrender, the surrender would result in there being no shares outstanding (other than shares held as treasury shares).
Share Premium Account
The directors shall establish a share premium account and shall carry the credit of such account from time to time to a sum equal to the amount or value of the premium paid on the issue of any share or capital contributed or such other amounts required by the Companies Act.
Redemption and Purchase of Own Shares
Subject to the Companies Act and any rights for the time being conferred on the shareholders holding a particular class of shares, we may by action of our directors:
| 1. | issue shares that are to be redeemed or liable to be redeemed, at our option or the shareholder holding those redeemable shares, on the terms and in the manner our directors determine before the issue of those shares; |
| 2. | with the consent by special resolution of the shareholders holding shares of a particular class, vary the rights attaching to that class of shares so as to provide that those shares are to be redeemed or are liable to be redeemed at our option on the terms and in the manner which the directors determine at the time of such variation; and |
| 3. | purchase all or any of our own shares of any class including any redeemable shares on the terms and in the manner which the directors determine at the time of such purchase. |
We may make a payment in respect of the redemption or purchase of its own shares in any manner authorized by the Companies Act, including out of any combination of capital, our profits and the proceeds of a fresh issue of shares.
When making a payment in respect of the redemption or purchase of shares, the directors may make the payment in cash or in specie (or partly in one and partly in the other) if so authorized by the terms of the allotment of those shares or by the terms applying to those shares, or otherwise by agreement with the shareholder holding those shares.
Under the Companies Act, the redemption or repurchase of any share may be paid out of our Company’s profits or out of the proceeds of a new issue of shares made for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve). If the repurchase proceeds are paid out of our Company’s capital, our Company must, immediately following such payment, be able to pay its debts as they fall due in the ordinary course of business. In addition, under the Companies Act, no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase would result in there being no shares outstanding and (c) unless the manner of purchase (if not so authorized under the articles of association) has first been authorized by a resolution of our shareholders.
Transfer of Shares
Provided that a transfer of Ordinary Shares complies with applicable rules of the SEC, Nasdaq Capital Market, and federal and state security laws of the United States, a shareholder may transfer Ordinary Shares to another person by completing an instrument of transfer in a common form or in a form prescribed by Nasdaq Capital Market or in any other form approved by the directors, executed:
| (a) | where the Ordinary Shares are fully paid, by or on behalf of that shareholder; and |
| (b) | where the Ordinary Shares are partly paid, by or on behalf of that shareholder and the transferee. |
Our Board of Directors may, in its absolute discretion, decline to register any transfer of any Ordinary Share.
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal.
This, however, is unlikely to affect market transactions of the Ordinary Shares purchased by investors in the public offering. Since the Ordinary Shares were listed, the legal title to such Ordinary Shares and the registration details of those Ordinary Shares in the Company’s register of members have remained with VStock Transfer LLC. All market transactions with respect to those Ordinary Shares will then be carried out without the need for any kind of registration by the directors, as the market transactions will all be conducted through the DTC systems.
The registration of transfers may, after compliance with any notice required of the Nasdaq Capital Market, be suspended and the register closed at such times and for such periods as our Board of Directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any calendar year as our board may determine.
Inspection of Books and Records
Holders of our Ordinary Shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements.
General Meetings
As a Cayman Islands exempted company, we are not obliged by the Companies Act to call shareholders’ annual general meetings. Accordingly, we may, but shall not be obliged to, in each year hold a general meeting as an annual general meeting. Any annual general meeting held shall be held at such time and place as may be determined by our board of directors. All general meetings other than annual general meetings shall be called extraordinary general meetings.
The directors may convene general meetings whenever they think fit. General meetings shall also be convened on the written requisition of one or more of the shareholders entitled to attend and vote at our general meetings who (together) hold at least ten percent of the rights to vote at such general meeting in accordance with the notice provisions in the articles, specifying the purpose of the meeting and signed by each of the shareholders making the requisition. If the directors do not convene such meeting for a date not later than 21 clear days’ after the date of receipt of the written requisition, those shareholders who requested the meeting may convene the general meeting themselves within three months after the end of such period of 21 clear days in which case reasonable expenses incurred by them as a result of the directors failing to convene a meeting shall be reimbursed by us.
At least 5 clear days’ notice of an extraordinary general meeting and 5 clear days’ notice of an annual general meeting shall be given to shareholders entitled to attend and vote at such meeting. The notice shall specify the place, the day and the hour of the meeting and the general nature of that business. In addition, if a resolution is proposed as a special resolution, the text of that resolution shall be given to all shareholders. Notice of every general meeting shall also be given to the directors and our auditors.
Subject to the Companies Act and with the consent of the shareholders who, individually or collectively, hold at least 90 percent of the voting rights of all those who have a right to vote at a general meeting, a general meeting may be convened on shorter notice.
A quorum required for any general meeting of shareholders consists of one or more shareholders present or by proxy, holding shares which carry in aggregate not less than one-third of all votes attaching to all of our shares in issue and entitled to vote at such general meeting.
If, within 15 minutes from the time appointed for the general meeting, or at any time during the meeting, a quorum is not present, the meeting, if convened upon the requisition of shareholders, shall be cancelled. In any other case it shall stand adjourned to the same time and place seven days or to such other time or place as is determined by the directors.
The chairman may, with the consent of a meeting at which a quorum is present, adjourn the meeting. When a meeting is adjourned for seven days or more, notice of the adjourned meeting shall be given in accordance with the articles of association.
Directors
We may by ordinary resolution, from time to time, fix the maximum and minimum number of directors to be appointed. Under the articles, we are required to have a minimum of one director and the maximum number of directors shall be unlimited.
Any appointment may be to fill a vacancy or as an additional director.
Unless the remuneration of the directors is determined by the shareholders by ordinary resolution, the directors shall be entitled to such remuneration as the directors may determine.
The shareholding qualification for directors may be fixed by our shareholders by ordinary resolution and unless and until so fixed no share qualification shall be required.
Unless removed or re-appointed, each director shall be appointed for a term expiring at the next-following annual general meeting, if one is held. At any annual general meeting held, our directors will be elected by an ordinary resolution of our shareholders. At each annual general meeting, each director so elected shall hold office for a one-year term and until the election of their respective successors in office or removed.
A director may at any time resign or retire from office by giving us notice in writing. Unless the notice specifies a different date, the director shall be deemed to have resigned on the date that the notice is delivered to us.
Our officers are appointed by and serve at the discretion of our board of directors.
Subject to the provisions of our Articles (which include the removal of a director by ordinary resolution), the office of a director may be terminated forthwith if (a) he is prohibited by the laws of the Cayman Islands from acting as a director, (b) he is made bankrupt or makes an arrangement or composition with his creditors generally, (c) he resigns his office by notice to us, (d) he only held office as a director for a fixed term and such term expires, (e) in the opinion of a registered medical practitioner by whom he is being treated he becomes physically or mentally incapable of acting as a director, (f) he is given notice by the majority of the other directors (not being less than two in number) to vacate office (without prejudice to any claim for damages for breach of any agreement relating to the provision of the services of such director), (g) he is made subject to any law relating to mental health or incompetence, whether by court order or otherwise, or (h) without the consent of the other directors, he is absent from meetings of directors for continuous period of six months.
Each of the compensation committee and the nominating and corporate governance committee shall consist of at least three directors and the majority of the committee members shall be independent within the meaning of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market. The audit committee shall consist of at least three directors, all of whom shall be independent within the meaning of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market and will meet the criteria for independence set forth in Rule 10A-3 or Rule 10C-1 of the Exchange Act.
Powers and Duties of Directors
Under Cayman Islands law, directors and officers owe the following fiduciary duties:
| (i) | duty to act in good faith in what the director or officer believes to be in the best interests of the company as a whole; |
| (ii) | duty to exercise powers for the purposes for which those powers were conferred and not for a collateral purpose; |
| (iii) | directors should not properly fetter the exercise of future discretion; |
| (iv) | duty not to put themselves in a position in which there is a conflict between their duty to the company and their personal interests; and |
| (v) | duty to exercise independent judgment. |
In addition to the above, directors also owe a duty of care which is not fiduciary in nature. This duty has been defined as a requirement to act as a reasonably diligent person having both the general knowledge, skill and experience that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and the general knowledge skill and experience which that director has.
As set out above, directors have a duty not to put themselves in a position of conflict and this includes a duty not to engage in self-dealing, or to otherwise benefit as a result of their position. However, in some instances what would otherwise be a breach of this duty can be forgiven and/or authorized in advance by the shareholders provided that there is full disclosure by the directors. This can be done by way of permission granted in the memorandum and articles of association or alternatively by shareholder approval at general meetings.
Accordingly, as a result of multiple business affiliations, our officers and directors may have similar legal obligations relating to presenting business opportunities meeting the above-listed criteria to multiple entities. In addition, conflicts of interest may arise when our board evaluates a particular business opportunity with respect to the above-listed criteria. We cannot assure you that any of the afore-mentioned conflicts will be resolved in our favor. Furthermore, each of our officers and directors has pre-existing fiduciary obligations to other businesses of which they are officers or directors.
Our Company has the right to seek damages if a duty owed by our directors is breached. A shareholder may in certain limited exceptional circumstances have the right to seek damages in our name if a duty owed by our directors is breached.
In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association as may be amended from time to time. Our Company has a right to seek damages against any director who breaches a duty owed to us.
Our directors have all the powers necessary for managing, and for directing and supervising, our business affairs.
Capitalization of Profits
The directors may resolve to capitalize:
| (a) | any part of our profits not required for paying any preferential dividend (whether or not those profits are available for distribution); or |
| (b) | any sum standing to the credit of our share premium account or capital redemption reserve, if any. |
The amount resolved to be capitalized must be appropriated to the shareholders who would have been entitled to it had it been distributed by way of dividend and in the same proportions.
Liquidation Rights
Upon the winding up of our Company, after the full amount that holders of any issued shares ranking senior to the Ordinary Shares as to distribution on liquidation or winding up are entitled to receive has been paid or set aside for payment, the holders of our Ordinary Shares are entitled to receive any remaining assets of our Company available for distribution as determined by the liquidator. The shareholders may, subject to our Articles and any other sanction required by the Companies Act, pass a special resolution allowing the liquidator to do either or both of the following:
| ● | to divide in specie among the shareholders the whole or any part of our assets and, for that purpose, to value any assets and to determine how the division shall be carried out as between the shareholders or different classes of shareholders; and |
| ● | to vest the whole or any part of the assets in trustees for the benefit of shareholders and those liable to contribute to the winding up. |
The directors have the authority to present a petition for our winding up to the Grand Court of the Cayman Islands on our behalf without the sanction of a resolution passed at a general meeting.
Register of Members
Under the Companies Act, we must keep a register of members and there should be entered therein:
| ● | the names and addresses of our members, and, a statement of the shares held by each member, which: |
| ● | distinguishes each share by its number (so long as the share has a number); |
| ● | confirms the amount paid, or agreed to be considered as paid, on the shares of each member; |
| ● | confirms the number and category of shares held by each member; and |
| ● | confirms whether each relevant category of shares held by a member carries voting rights under the articles of association of the company, and if so, whether such voting rights are conditional |
| ● | the date on which the name of any person was entered on the register as a member; and |
| ● | the date on which any person ceased to be a member. |
Under Cayman Islands law, the register of members of our Company is prima facie evidence of the matters set out therein (i.e., the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a member registered in the register of members is deemed as a matter of Cayman Islands law to have legal title to the shares as set against its name in the register of members. Once our register of members has been updated, the shareholders recorded in the register of members will be deemed to have legal title to the shares set against their name.
If the name of any person is incorrectly entered in or omitted from our register of members or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a member of our Company, the person or member aggrieved (or any member of our Company or our Company itself) may apply to the Grand Court of the Cayman Islands for an order that the register be rectified. The Court may either refuse such application or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
C. Material Contracts
As of the date of this annual report, we have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulations—Other Laws—Foreign Exchange Control.”
E. Taxation
People’s Republic of China Taxation
We are a holding company incorporated in the Cayman Islands and we gain substantial income by way of dividends paid to us from the PRC subsidiaries. The EIT Law and its implementation rules provide that PRC-sourced income of foreign enterprises, such as dividends paid by a subsidiary in mainland China to its equity holders that are non-resident enterprises, will normally be subject to PRC withholding tax at a rate of 10%, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a preferential tax rate or a tax exemption.
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a PRC domestic enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that actually, comprehensively manage and control the production and operation, staff, accounting, property, and other aspects of an enterprise, the only official guidance for this definition currently available is set forth in SAT Circular 82, which provides guidance on the determination of the tax residence status of a PRC-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a PRC enterprise or enterprise group as its primary controlling shareholder. In spite of further to SAT Circular 82, the SAT issued the SAT Bulletin 45, which took effect in September 2011 and was amended in June 2018, but SAT Bulletin 45 only provides for procedures and administration details of determination on resident status and administration on post-determination matters, did not define “de facto management body.” Although Work Cayman does not have a PRC enterprise or enterprise group as our primary controlling shareholder and is therefore not a PRC-controlled offshore incorporated enterprise within the meaning of SAT Circular 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in SAT Circular 82 to evaluate the tax residence status of Work Cayman and its subsidiaries organized outside of mainland China.
According to SAT Circular 82, a PRC-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to PRC enterprise income tax on its worldwide income only if all of the following criteria are met: (i) the places where senior management and senior management departments that are responsible for daily production, operation and management of the enterprise perform their duties are mainly located within the territory of mainland China; (ii) financial decisions (such as money borrowing, lending, financing and financial risk management) and personnel decisions (such as appointment, dismissal and salary and wages) are decided or need to be decided by organizations or persons located within the territory of mainland China; (iii) main property, accounting books, corporate seal, the board of directors and files of the minutes of shareholders’ meetings of the enterprise are located or preserved within the territory of mainland China; and (iv) one half (or more) of the directors or senior management staff having the right to vote habitually reside within the territory of mainland China.
We believe that we do not meet some of the conditions outlined in the immediately preceding paragraph. For example, the key assets and records of Work Cayman, including the resolutions and meeting minutes of our board of directors and the resolutions and meeting minutes of our shareholders, are located and maintained outside mainland China. In addition, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities. Accordingly, we believe that Work Cayman and its offshore subsidiary should not be treated as a “resident enterprise” for PRC tax purposes if the criteria for “de facto management body” as set forth in SAT Circular 82 were deemed applicable to us. As the tax residency status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, however, we will continue to monitor our tax status.
If the PRC tax authorities determine that Work Cayman is a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from any dividends we pay to our shareholders that are non- resident enterprises. In addition, non-resident enterprise shareholders may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of our Ordinary Shares, if such income is treated as sourced from within mainland China. It is unclear whether our non-PRC individual shareholders would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to dividends or gains realized by non-PRC individuals, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. It is also unclear, however, whether our non-PRC shareholders would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. There is no guidance from the PRC government to indicate whether or not any tax treaties between mainland China and other countries would apply in circumstances where a non-PRC company was deemed to be a PRC tax resident, and thus there is no basis for expecting how tax treaty between mainland China and other countries may impact non-resident enterprises. See “Item 3. Key Information—D. Risk Factors—Risk Factors—Risks Related to Doing Business in the PRC—We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.” on page 38.
As a Cayman Islands holding company, we may receive dividends from the PRC subsidiary through offshore subsidiaries. The EIT Law and its implementing rules provide that dividends paid by a PRC entity to a non-resident enterprise for income tax purposes is subject to PRC withholding tax at a rate of 10%, subject to reduction by an applicable tax treaty with China. Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, the withholding tax rate in respect to the payment of dividends by a PRC enterprise to a Hong Kong enterprise may be reduced to 5% from a standard rate of 10% if the Hong Kong enterprise directly holds at least 25% of the PRC enterprise. Pursuant to the Notice of the State Administration of Taxation on the Issues concerning the Application of the Dividend Clauses of Tax Agreements, or SAT Circular 81, a Hong Kong resident enterprise must meet the following conditions, among others, in order to apply the reduced withholding tax rate: (i) it must be a company; (ii) it must directly own the required percentage of equity interests and voting rights in the PRC resident enterprise; and (iii) it must have directly owned such required percentage in the PRC resident enterprise throughout the 12 months prior to receiving the dividends. In October 2019, the SAT promulgated the Administrative Measures for Entitlement to Treaty Benefits for Non-resident Taxpayers, or SAT Circular 35, which became effective on January 1, 2020. SAT Circular 35 provides that non-resident enterprises are not required to obtain pre-approval from the relevant tax authority in order to enjoy the reduced withholding tax. Instead, non-resident enterprises and their withholding agents may, by self-assessment and on confirmation that the prescribed criteria to enjoy the tax treaty benefits are met, directly apply the reduced withholding tax rate, and file necessary forms and supporting documents when performing tax filings, which will be subject to post-tax filing examinations by the relevant tax authorities. Accordingly, we may be able to benefit from the 5% withholding tax rate for the dividends it receives from our operating entities established in mainland China, if we satisfy the conditions prescribed under SAT Circular 82 and other relevant tax rules and regulations. However, according to SAT Circular 82 and SAT Circular 35, if the relevant tax authorities consider the transactions or arrangements we have are for the primary purpose of enjoying a favorable tax treatment, the relevant tax authorities may adjust the favorable withholding tax in the future.
Provided that Work Cayman is not deemed to be a PRC resident enterprise, holders of our Ordinary Shares who are not PRC residents will not be subject to PRC income tax on dividends distributed by us or gains realized from the sale or other disposition of our shares. However, under SAT Bulletin 7, where a non-resident enterprise conducts an “indirect transfer” by transferring taxable assets, including, in particular, equity interests in a PRC resident enterprise, indirectly by disposing of the equity interests of an overseas holding company, the non-resident enterprise, being the transferor, or the transferee or the PRC entity which directly owned such taxable assets may report to the relevant tax authority such indirect transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of reducing, avoiding, or deferring PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC enterprise income tax, and the transferee or other person who is obligated to pay for the transfer is obligated to withhold the applicable taxes, currently at a rate of 10% for the transfer of equity interests in a PRC resident enterprise.
We and our non-PRC resident investors may be at risk of being required to file a return and being taxed under SAT Bulletin 7, and we may be required to expend valuable resources to comply with SAT Bulletin 7, or to establish that we should not be taxed under this Bulletin. See “Item 3. Key Information—D. Risk Factors—Risk Factors—Risks Related to Doing Business in China—We and our shareholders face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.” on page 38.
Hong Kong Taxation
According to Tax (Amendment) (No. 3) Ordinance 2018 published by Hong Kong government, since September 1, 2018, under the two-tiered profits tax rates regime, the profits tax rate for the first HKD2 million of assessable profits has been lowered to 8.25% (half of the rate specified in Schedule 8 to the Inland Revenue Ordinance (IRO)) for corporations. Work HK was not subject to Hong Kong profit tax for any period presented as it did not have assessable profit during the periods presented.
Material Tax Consequences Applicable to U.S. Holders of Our Ordinary Shares
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the Ordinary Shares. For this purpose, a “U.S. Holder” is a holder of Ordinary Shares that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This brief summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our Ordinary Shares. This brief summary generally considers only U.S. Holders that will own our Ordinary Shares as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, and administrative and judicial interpretations thereof, (including with respect to the Tax Cuts and Jobs Act of 2017), all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the IRS with regard to the U.S. federal income tax treatment of an investment in our Ordinary Shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. Holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Ordinary Shares in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our Ordinary Shares as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, Ordinary Shares representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold securities through a partnership or other pass-through entity are not addressed.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our Ordinary Shares, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Taxation of Dividends and Other Distributions on Our Ordinary Shares
Subject to the PFIC rules discussed below, the gross amount of distributions made by us to you with respect to the Ordinary Shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the Ordinary Shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a PFIC for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Because there is no income tax treaty between the United States and the Cayman Islands, clause (1) above can be satisfied only if the Ordinary Shares are readily tradable on an established securities market in the United States. Under U.S. Internal Revenue Service authority, Ordinary Shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on certain exchanges, which presently include the Nasdaq Stock Market. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our Ordinary Shares, including the effects of any change in law after the date of this annual report.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our Ordinary Shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your Ordinary Shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
Taxation of Dispositions of Ordinary Shares
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our Ordinary Shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the securities in U.S. dollars and the amount realized on the disposition in U.S. dollar (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of securities will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Company (PFIC) Consequences
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
| ● | 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| ● | At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Ordinary Shares and how quickly we dispose of the cash raised in our IPO. Accordingly, there can be no assurance that we will not become a PFIC. The Company has no obligation to take measures to avoid becoming a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our Ordinary Shares at a gain: (1) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the securities, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the Ordinary Shares while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. We do not intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year. In addition, we do not intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our Subsidiaries are a PFIC. Therefore, the QEF election will not be available with respect to our Ordinary Shares.
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our Ordinary Shares which are regularly traded on a qualifying exchange, including the Nasdaq Capital Market, can elect to mark the securities to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the securities and the U.S. Holder’s adjusted tax basis in the securities. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our Ordinary Shares during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules.
Information Reporting and Backup Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of securities. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our Ordinary Shares, unless such securities are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts, if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F within four months after the end of each fiscal year. The SEC maintains a website at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing, among other things, the furnishing and content of proxy statements to shareholders, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
Item 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Foreign Exchange Risk
A substantial majority of our operating activities are denominated in RMB and all of our assets and liabilities are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the PBOC or other authorized financial institutions at exchange rates quoted by the PBOC. Approval of foreign currency payments by the PBOC or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices and signed contracts. The value of RMB is subject to changes in central government policies and to international economic and political development affecting supply and demand in the China Foreign Exchange Trading System market.
Credit Risk
Financial instruments that potentially expose us to concentrations of credit risk consist primarily of accounts receivable. We conduct credit evaluations of our customers and generally do not require collateral or other security from them. We evaluate our collection experience and long outstanding balances to determine the need for an allowance for doubtful accounts. We conduct periodic reviews of the financial condition and payment practices of our customers to minimize collection risk on accounts receivable.
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest rate that our deposited cash can earn. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed to material risks due to changes in interest rates. An increase in the applicable interest rate, however, may raise the cost of any debt we may incur in the future.
Inflation Risk
We do not believe that inflation has had a material effect on our business, financial condition, or results of operations. If our raw material costs become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases. Our inability or failure to do so could harm our business, financial condition, and operating results.
Seasonality
Seasonality does not materially affect our business or the results of our operations.
Item 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Not applicable.
Part II
Item 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
Item 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
See “Item 10. Additional Information” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1 (File Number 333- 271474), as amended, which was declared effective by the SEC on August 22, 2024, for our IPO, which was closed on August 26, 2024. We issued and sold an aggregate of 2,000,000 Ordinary Shares, at a price of $4.00 per share. The total gross proceeds received from the IPO, including proceeds from the exercise of the over-allotment option, was $8,367,768. Kingswood Capital Partners, LLC was the underwriter of our IPO.
We incurred approximately $1,969,185 in expenses in connection with our IPO and with the issuance of over-allotment shares. None of the transaction expenses included payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates. None of the net proceeds we received from the IPO were paid, directly or indirectly, to any of our directors or officers or their associates, persons owning 10% or more of our equity securities or our affiliates.
The net proceeds raised from the IPO were approximately $7,373,839 after deducting underwriting discounts, from such amount, $5,404,654 of the net proceeds remained available after reimbursing PRC subsidiaries for expenses advanced from them in connection with the IPO. For the period from the effectiveness of the registration statement on Form F-1 to the date as of this annual report, we used approximately US$0.40 million for funding an indemnification escrow account that may be utilized by the underwriters to fund any bona fide indemnification claims of the underwriters arising during the 12-month period following the closing of the IPO. We still intend to use the remaining proceeds from our IPO in the manner disclosed in our registration statement on Form F-1, as amended (File Number 333-271474).
Item 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon this evaluation, our management has concluded that, as of September 30, 2024, our existing disclosure controls and procedures were ineffective because of a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with U.S. GAAP and includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets, (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are being made only in accordance with authorizations of a company’s management and directors, and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the consolidated financial statements.
Our management, with the participation of our chief executive officer and chief financial officer, conducted an evaluation of the effectiveness of our Company’s internal control over financial reporting as of September 30, 2024 based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO 2013 Framework). Based on this evaluation, we identified one deficiency, which related to a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements, and which we believe to be a material weakness as of September 30, 2024.
As a result of the above material weakness, management has concluded that our internal control over financial reporting was not effective as of September 30, 2024. To remedy our identified material weakness as of September 30, 2024, we have undertaken the remedial steps and also plan to adopt certain measures to improve our internal control over financial reporting as set forth below.
Remediation plan of the Material Weakness in Internal Control over Financial Reporting Reported as of September 30, 2024
As of the date of this annual report, we have not fully addressed the above-referenced weakness. However, we have made progress in implementing remedial measures, including:
| | (i) | recruiting qualified accounting personnel with relevant U.S. GAAP and SEC reporting experience and qualifications to strengthen the financial reporting function and to establish a financial and system control framework; and |
| | (ii) | implementing regular and continuous U.S. GAAP accounting and financial reporting training programs for our accounting and financial reporting personnel. |
Attestation Report of the Registered Public Accounting Firm
This annual report on Form 20-F does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC where domestic and foreign registrants that are non-accelerated filers, which we are, and “emerging growth companies,” which we also are, are not required to provide the auditor attestation report.
Changes in Internal Control over Financial Reporting
As of September 30, 2023, we identified a material weakness in our internal control over financial reporting, which related to a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements.
We implemented the following remedial measures during the fiscal year ended September 30, 2024 to address the material weakness identified as of September 30, 2023:
| | i. | providing U.S. GAAP learning materials for our accounting and financial reporting personnel; and |
| | ii. | attending online U.S. GAAP courses provided by a leading financial education institutions in China. |
Other than as described above, there were no changes in our internal controls over financial reporting that occurred during the period covered by this annual report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [RESERVED]
Item 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Mr. Robert Johnson qualifies as an “audit committee financial expert” as defined in Item 16A of Form 20-F. Mr. Xiaoyang Li, Mr. Zhenguo Wu, and Mr. Robert Jackson satisfy the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules as well as the independence requirements of Rule 10A-3 under the Exchange Act.
Item 16B. CODE OF ETHICS
Our board of directors has adopted a code of business conduct and ethics, which is applicable to all of our directors, officers, and employees. Our code of business conduct and ethics is publicly available on our website.
Item 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered and billed by WWC, P.C.., our independent registered public accounting firm for the periods indicated.
WWC, P.C.
| | | For the Years Ended
September 30, | |
| | | 2023 | | | 2024 | |
| Audit fees (1) | | $ | 240,000 | | | $ | 190,000 | |
| Audit-related fees | | | | | | | | |
| Tax fees | | | | | | | | |
| All other fees | | | | | | | | |
| Total | | $ | 240,000 | | | $ | 190,000 | |
| (1) | Audit fees include the aggregate fees billed for each of the fiscal years for professional services rendered by our independent registered public accounting firm for the audit of our annual financial statements or for the audits of our financial statements and review of the interim financial statements. |
The audit committee of our board of directors has established its pre-approval policies and procedures, pursuant to which the audit committee approved the foregoing audit services provided by WWC, P.C. in fiscal years 2023 and 2024. Consistent with our audit committee’s responsibility for engaging our independent auditors, all audit and permitted non-audit services require pre-approval by the audit committee. The full audit committee approves proposed services and fee estimates for these services. One or more independent directors serving on the audit committee may be delegated by the full audit committee to pre-approve any audit and non-audit services. Any such delegation shall be presented to the full audit committee at its next scheduled meeting. Pursuant to these procedures, the audit committee approved the foregoing audit services provided by WWC, P.C.
Item 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
Item 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
Item 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
Item 16G. CORPORATE GOVERNANCE
Pursuant to the home country rule exemption set forth under Nasdaq Listing Rule 5615, the board of directors of the Company has elected to follow the Company’s home country rules for exemption from the requirements as follows:
| (i) | Nasdaq Listing Rule 5635, which requires a listed company to obtain shareholder approval for certain dilutive events, including: |
| a. | issuance of securities in connection with the acquisition of the stock or assets of another company; |
| b. | issuance of securities that will result in a change of control of the Company; |
| c. | issuance of securities when a stock option or purchase plan or other equity compensation arrangement is established or materially amended; and |
| d. | certain transactions other than a public offering involving issuances of a 20% or greater interest in the Company; and |
| (ii) | Nasdaq Listing Rule 5640, which requires that the voting rights of existing shareholders of publicly traded common stock registered under Section 12 of the Securities Exchange Act of 1934 may not be disparately reduced or restricted through any corporate action or issuance. |
Other than those described above, there are no significant differences between the Company’s corporate governance practices and those followed by U.S. domestic companies under Nasdaq Capital Market corporate governance listing standards.
Item 16H. MINE SAFETY DISCLOSURE
Not applicable.
Item 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
Item 16J. INSIDER TRADING POLICIES.
We have adopted insider trading policies governing the purchase, sale, and other dispositions of our securities by directors, senior management, and employees. A copy of the insider trading policies is filed as an exhibit to this annual report.
Item 16K. CYBERSECURITY.
We have established cybersecurity risk management to identify, assess, and mitigate cybersecurity risks alongside other business risks. The process is in alignment with our strategic objectives and risk appetite. We may engage assessors, consultants, auditors, or other third parties to enhance our cyber security risk management processes. Any cybersecurity incidents are closely monitored for their potential impact on our business strategy, operations, and financial condition. As of the date of this annual report, we have not experienced any cybersecurity incidents that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition. We continuously adapt our business strategy to enhance resilience, strengthen defenses and ensure the sustainability of our operations.
Part III
Item 17. FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18.
Item 18. FINANCIAL STATEMENTS
The consolidated financial statements of WORK Medical Technology Group LTD and its subsidiaries are included at the end of this annual report.
Item 19. EXHIBITS
EXHIBIT INDEX
| Exhibit No. | | Description |
| 1.1* | | Amended and Restated Memorandum of Association and Amended and Restated Articles of Association |
| 2.1 | | Specimen Certificate for Ordinary Shares (incorporated by reference to Exhibit 4.1 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 2.2* | | Description of Securities |
| 4.1 | | Form of Indemnification Agreement with the Registrant’s directors and officers (incorporated by reference to Exhibit 10.1 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.2 | | Form of Director Offer Letter between the Registrant and its directors (incorporated by reference to Exhibit 10.3 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.3 | | English Translation of Form of Procurement Agreement (incorporated by reference to Exhibit 10.4 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.4 | | English Translation of Form of Distribution Agreement (incorporated by reference to Exhibit 10.5 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.5 | | English Translation of Advance Agreement dated October 3, 2021, by and between Hangzhou Shanyou and Baiming Yu (incorporated by reference to Exhibit 10.5 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.6 | | English Translation of Advance Agreement dated October 3, 2021, by and between Hangzhou Shanyou and Liwei Zhang (incorporated by reference to Exhibit 10.6 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.7 | | English Translation of Loan Agreement dated October 9, 2021, by and between Hangzhou Shanyou and Hangzhou Shuige (incorporated by reference to Exhibit 10.7 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.8 | | English Translation of Real Estate Purchasing Agreement dated July 13, 2020, by and between Hangzhou Shanyou and Qijia Yu (incorporated by reference to Exhibit 10.8 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.9 | | English Translation of Medical Devices Purchasing Agreement dated September 30, 2021, by and between Hangzhou Shanyou and Hangzhou Qingniu (incorporated by reference to Exhibit 10.9 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.10 | | English Translation of Comprehensive Credit Agreement dated September 29, 2020, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.11 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.11 | | English Translation of Loan Agreement dated September 29, 2020, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.12 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.12 | | English Translation of Loan Agreement dated January 26, 2021, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.13 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.13 | | English Translation of Loan Agreement dated December 22, 2016, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.14 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.14 | | English Translation of Loan Agreement dated January 6, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.15 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.15 | | English Translation of Loan Agreement dated January 15, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.16 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.16 | | English Translation of Loan Agreement dated March 2, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.17 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.17 | | English Translation of Loan Agreement dated June 28, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.18 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.18 | | English Translation of Loan Agreement dated July 2, 2020, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.19 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.19 | | English Translation of Loan Agreement dated September 29, 2021, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.20 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.20 | | English Translation of Loan Agreement dated September 29, 2021, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.21 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.21 | | English Translation of Loan Agreement dated September 1, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.22 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.22 | | English Translation of Loan Agreement dated September 2, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.23 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.23 | | English Translation of Loan Agreement dated September 7, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.24 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.24 | | English Translation of Loan Agreement dated September 7, 2022, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.25 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.25 | | English Translation of Lease Agreement dated January 1, 2020, by and between Hangzhou Shanyou and Guancuncun Village Committee (incorporated by reference to Exhibit 10.25 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.26 | | English Translation of Lease Agreement dated January 1, 2011, by and between Hangzhou Shanyou and Louta Town Village Committee (incorporated by reference to Exhibit 10.26 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.27 | | English Translation of Lease Agreement dated November 30, 2023, by and between Hangzhou Shanyou ang Hangzhou Tianxia Weaving Co., Ltd. (incorporated by reference to Exhibit 10.27 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.28 | | English Translation of Lease Agreement dated February 27, 2018, by and between Shanghai Chuqiang and Ailiu Real Estate Co., Ltd. (incorporated by reference to Exhibit 10.28 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.29 | | English Translation of Loan Agreement dated March 31, 2023, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.30 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.30 | | English Translation of Patent Transfer Agreement dated April 1, 2021, by and between Hangzhou Shanyou and The Second Hospital of Jiaxing City (incorporated by reference to Exhibit 10.31 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.31 | | English Translation of Patent Transfer Agreement dated December 26, 2019, by and between Hangzhou Hanshi and Zhejiang University (incorporated by reference to Exhibit 10.32 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.32 | | Employment Agreement by and between Shuang Wu and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.32 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.33 | | Employment Agreement by and between Ningfang Liang and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.33 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.34 | | Employment Agreement by and between Baiming Yu and the Registrant dated June 1, 2022 (incorporated by reference to Exhibit 10.34 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.35 | | English Translation of Loan Agreement dated June 28, 2023, by and between Hangzhou Shanyou and China CITIC Bank (incorporated by reference to Exhibit 10.35 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.36 | | English Translation of Loan Agreement dated December 20, 2023, by and between Hangzhou Woli and China CITIC Bank (incorporated by reference to Exhibit 10.36 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.37 | | English Translation of Loan Agreement dated June 29, 2023, by and between Hangzhou Shanyou and Bank of Beijing (incorporated by reference to Exhibit 10.37 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.38 | | Form of Indemnification Escrow Agreement (incorporated by reference to Exhibit 10.38 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.39 | | English Translation of Loan Agreement dated March 4, 2024, by and between Hangzhou Shanyou and Bank of Jiangsu (incorporated by reference to Exhibit 10.39 of our Registration Statement on Form F-1 (File No. 333-271474) initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 4.40 | | English Translation of Loan Agreement dated July 26, 2024, by and between Hangzhou Shanyou and China CITIC Bank (Hangzhou) (incorporated by reference to Exhibit 10.40 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.41 | | English Translation of Loan Agreement dated July 11, 2024, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.41 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.42 | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB10 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commerical Bank (incorporated by reference to Exhibit 10.42 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.43 | | English Translation of Loan Extension Agreement dated August 29, 2023, regarding a loan of RMB8 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.43 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.44 | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB8 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.44 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.45 | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB5 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.45 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.46 | | English Translation of Loan Extension Agreement dated August 26, 2024, regarding a loan of RMB14.5 million, by and between Hangzhou Shanyou and Zhejiang Xiaoshan Rural Commercial Bank (incorporated by reference to Exhibit 10.46 of our Registration Statement on Form F-1 (File No. 333-284006), filed with the Securities and Exchange Commission on December 23, 2024) |
| 4.47* | | English Translation of Loan Extension Agreement regarding RMB22 million of the principal of a loan dated November 28, 2024, by and between Hangzhou Woli and China CITIC Bank |
| 4.48* | | English Translation of Loan Extension Agreement regarding RMB20 million of the principal of a loan dated December 8, 2024, by and between Hangzhou Woli and China CITIC Bank |
| 8.1 | | List of subsidiaries of the Registrant (incorporated by reference to Exhibit 21.1 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 11.1 | | Code of Business Conduct and Ethics of the Registrant (incorporated by reference to Exhibit 99.1 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 12.1* | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 12.2* | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 13.1** | | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 13.2** | | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 15.1* | | Consent of AllBright Law Offices (Fuzhou) |
| 97.1 | | Compensation Recovery Policy (incorporated by reference to Exhibit 99.8 of our Registration Statement on Form F-1 (file No. 333-271474), as amended, initially filed with the Securities and Exchange Commission on April 27, 2023) |
| 97.2* | | Insider Trading Policy |
| 101* | | The following financial statements from the Company’s Annual Report on Form 20-F for the fiscal year ended September 30, 2024, formatted in Inline XBRL: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Income (Loss) And Comprehensive Loss, (iii) Consolidated Statements of Changes in Equity, (iv) Consolidated Statements of Cash Flows, and (v) Notes To Consolidated Financial Statements |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
| * | Filed with this annual report on Form 20-F |
| ** | Furnished with this annual report on Form 20-F |
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | WORK Medical Technology Group LTD |
| | | |
| | By: | /s/ Shuang Wu |
| | | Shuang Wu |
| | | Chief Executive Officer, Director, and |
| | | Chairman of the Board of Directors |
Date: February 14, 2025
INDEX TO FINANCIAL STATEMENTS
WORK MEDICAL TECHNOLOGY GROUP LTD AND SUBSIDIARIES
TABLE OF CONTENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Shareholders of
Work Medical Technology Group Ltd |
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Work Medical Technology Group Ltd and its subsidiaries (collectively the “Company”) as of September 30, 2024 and 2023, and the related consolidated statements of income and comprehensive loss, changes in equity, and cash flows in each of the years in the three-year period ended September 30, 2024, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2024 and 2023, and the results of its operations and its cash flows in each of the years in the three period ended September 30, 2024, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WWC, P.C.
WWC, P.C.
Certified Public Accountants
PCAOB ID No.1171
We have served as the Company’s auditor since December 2021.
San Mateo, California
February 14, 2025

WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED BALANCE SHEETS
(In U.S. dollars, except for otherwise noted)
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Assets | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 6,557,605 | | | $ | 1,596,096 | |
| Restricted cash | | | - | | | | 41,187 | |
| Cash deposited in escrow account | | | 400,000 | | | | - | |
| Accounts receivable, net | | | 1,648,797 | | | | 3,332,322 | |
| Inventories, net | | | 3,183,951 | | | | 4,620,191 | |
| Amounts due from related parties | | | 2,707,136 | | | | 4,237,682 | |
| Advance to suppliers | | | 4,697,029 | | | | 3,469,819 | |
| Prepaid expenses and other current assets, net | | | 1,632,702 | | | | 4,855,381 | |
| Total current assets | | | 20,827,220 | | | | 22,152,678 | |
| | | | | | | | | |
| Property, plant and equipment, net | | | 11,372,485 | | | | 6,626,774 | |
| Intangible assets, net | | | 1,044,062 | | | | 998,450 | |
| Right-of-use assets, net | | | - | | | | 114,127 | |
| Deferred tax assets, net | | | 112,916 | | | | 66,872 | |
| Advance to suppliers for equipment | | | 2,893,880 | | | | - | |
| Total non-current assets | | | 15,423,343 | | | | 7,806,223 | |
| TOTAL ASSETS | | $ | 36,250,563 | | | $ | 29,958,901 | |
| | | | | | | | | |
| Liabilities | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Short-term bank borrowings | | $ | 13,323,643 | | | $ | 8,840,461 | |
| Accounts payable | | | 1,497,152 | | | | 3,961,827 | |
| Deferred revenue | | | 625,326 | | | | 776,210 | |
| Amount due to related parties | | | 1,217,442 | | | | 159,612 | |
| Accrued expenses and other liabilities | | | 3,007,901 | | | | 4,398,596 | |
| Lease liabilities, current | | | - | | | | 49,560 | |
| Income tax payable | | | 508,726 | | | | 736,590 | |
| Total current liabilities | | | 20,180,190 | | | | 18,922,856 | |
| | | | | | | | | |
| Lease liabilities, non-current | | | - | | | | 50,130 | |
| Total non-current liabilities | | | - | | | | 50,130 | |
| TOTAL LIABILITIES | | $ | 20,180,190 | | | $ | 18,972,986 | |
| | | | | | | | | |
| Shareholders’ equity | | | | | | | | |
| Ordinary Shares (par value of $0.0005 per share; 100,000,000 shares authorized as of September 30, 2024 and 2023; 14,591,942 and 12,500,000 shares issued and outstanding as of September 30, 2024 and 2023, respectively)* | | $ | 7,296 | | | $ | 6,250 | |
| Subscription receivable | | | (6,250 | ) | | | (6,250 | ) |
| Additional paid-in capital | | | 6,617,596 | | | | 85,012 | |
| Statutory reserve | | | 899,731 | | | | 887,482 | |
| Retained earnings | | | 6,076,018 | | | | 9,580,585 | |
| Accumulated other comprehensive loss | | | (408,465 | ) | | | (676,828 | ) |
| Total shareholders’ equity | | | 13,185,926 | | | | 9,876,251 | |
| Non-controlling interests | | | 2,884,447 | | | | 1,109,664 | |
| Total equity | | | 16,070,373 | | | | 10,985,915 | |
| TOTAL LIABILITIES AND EQUITY | | $ | 36,250,563 | | | $ | 29,958,901 | |
| * | The shares and per share information are presented on a retroactive basis to reflect the reorganization (Note 1) and share reorganization by way of a subdivision and surrender of shares (Note 12). |
The accompanying notes are an integral part of these consolidated financial statements.
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF INCOME (LOSS) AND COMPREHENSIVE LOSS
(In U.S. dollars, except for otherwise noted)
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Net revenue from third parties | | $ | 10,929,070 | | | $ | 12,501,615 | | | $ | 19,498,315 | |
| Net revenue from related party | | | 577,370 | | | | 1,064,336 | | | | 212,975 | |
| Cost of revenues from third parties | | | (8,346,419 | ) | | | (8,596,872 | ) | | | (15,197,213 | ) |
| Cost of revenue from related party | | | (291,635 | ) | | | (826,095 | ) | | | (95,285 | ) |
| Gross profit | | | 2,868,386 | | | | 4,142,984 | | | | 4,418,792 | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Selling expenses | | | (2,053,019 | ) | | | (1,567,385 | ) | | | (1,032,685 | ) |
| General and administrative expenses | | | (4,558,041 | ) | | | (1,861,728 | ) | | | (2,563,879 | ) |
| Research and development expenses | | | (302,511 | ) | | | (301,644 | ) | | | (183,900 | ) |
| Total operating expenses | | | (6,913,571 | ) | | | (3,730,757 | ) | | | (3,780,464 | ) |
| | | | | | | | | | | | | |
| Other (loss) income: | | | | | | | | | | | | |
| Interest expense | | | (557,571 | ) | | | (392,741 | ) | | | (352,968 | ) |
| Interest income | | | 11,727 | | | | 25,417 | | | | 1,969 | |
| Government subsidies | | | 346,571 | | | | 74,232 | | | | 1,186,641 | |
| Other income(expenses), net | | | 578,758 | | | | (8,746 | ) | | | (360,409 | ) |
| Total other income(loss) | | | 379,485 | | | | (301,838 | ) | | | 475,233 | |
| | | | | | | | | | | | | |
| (Loss) income before income tax expense | | | (3,665,700 | ) | | | 110,389 | | | | 1,113,561 | |
| Income tax benefit (expense) | | | 125,291 | | | | (47,006 | ) | | | (169,435 | ) |
| Net (loss) income | | $ | (3,540,409 | ) | | $ | 63,383 | | | $ | 944,126 | |
| | | | | | | | | | | | | |
| Net (loss) income attributable to non-controlling interest | | | (48,091 | ) | | | (48,647 | ) | | | 78,916 | |
| Net (loss) income attributable to ordinary shareholders | | $ | (3,492,318 | ) | | $ | 112,030 | | | $ | 865,210 | |
| Other comprehensive loss: | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | 453,181 | | | | (268,068 | ) | | | (1,147,710 | ) |
| Total comprehensive loss | | | (3,087,228 | ) | | | (204,685 | ) | | | (203,584 | ) |
| | | | | | | | | | | | | |
| Total comprehensive income (loss) attributable to non-controlling interests | | | 136,727 | | | | (80,165 | ) | | | (164,329 | ) |
| Total comprehensive loss attributable to shareholders | | $ | (3,223,955 | ) | | $ | (124,520 | ) | | $ | (39,255 | ) |
| | | | | | | | | | | | | |
| Weighted average number of Ordinary Shares | | | | | | | | | | | | |
| Basic and Diluted* | | | 12,705,011 | | | | 12,500,000 | | | | 12,500,000 | |
| Basic and diluted (loss) earnings per ordinary share | | | (0.27 | ) | | | 0.01 | | | | 0.07 | |
| * | The shares and per share information are presented on a retroactive basis to reflect the reorganization (Note 1) and share reorganization by way of a subdivision and surrender of shares (Note 12). |
The accompanying notes are an integral part of these consolidated financial statements.
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(In U.S. dollars, except for share and per share data, or otherwise noted)
| | | Ordinary Shares* | | | Subscription | | | Additional
paid-in | | | Statutory | | | Retained | | | Accumulated
other
comprehensive | | | Total
shareholder’s | | | Non-
controlling | | | Total | |
| | | Share | | | Amount | | | receivable | | | capital | | | reserves | | | earnings | | | income (loss) | | | equity | | | interest | | | equity | |
| Balance as of September 30, 2021 | | | 12,500,000 | | | $ | 6,250 | | | $ | (6,250 | ) | | $ | 85,012 | | | $ | 748,529 | | | $ | 8,742,298 | | | $ | 464,187 | | | $ | 10,040,026 | | | $ | 1,354,158 | | | $ | 11,394,184 | |
| Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | 865,210 | | | | — | | | | 865,210 | | | | 78,916 | | | | 944,126 | |
| Provision for statutory reserve | | | — | | | | — | | | | — | | | | — | | | | 102,161 | | | | (102,161 | ) | | | — | | | | — | | | | — | | | | — | |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (904,465 | ) | | | (904,465 | ) | | | (243,245 | ) | | | (1,147,710 | ) |
| Balance as of September 30, 2022 | | | 12,500,000 | | | $ | 6,250 | | | $ | (6,250 | ) | | $ | 85,012 | | | $ | 850,690 | | | $ | 9,505,347 | | | $ | (440,278 | ) | | $ | 10,000,771 | | | $ | 1,189,829 | | | $ | 11,190,600 | |
| Net income(loss) | | | — | | | | — | | | | — | | | | — | | | | — | | | | 112,030 | | | | — | | | | 112,030 | | | | (48,647 | ) | | | 63,383 | |
| Provision for statutory reserve | | | — | | | | — | | | | — | | | | — | | | | 36,792 | | | | (36,792 | ) | | | — | | | | — | | | | — | | | | — | |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (236,550 | ) | | | (236,550 | ) | | | (31,518 | ) | | | (268,068 | ) |
| Balance as of September 30, 2023 | | | 12,500,000 | | | $ | 6,250 | | | $ | (6,250 | ) | | $ | 85,012 | | | $ | 887,482 | | | $ | 9,580,585 | | | $ | (676,828 | ) | | $ | 9,876,251 | | | $ | 1,109,664 | | | $ | 10,985,915 | |
| Initial public offering proceeds net of listing expenses | | | 2,091,942 | | | | 1,046 | | | | — | | | | 5,403,608 | | | | — | | | | — | | | | — | | | | 5,404,654 | | | | — | | | | 5,404,654 | |
| Contribution from non-controlling interest | | | — | | | | — | | | | — | | | | 1,128,976 | | | | — | | | | — | | | | — | | | | 1,128,976 | | | | 1,638,056 | | | | 2,767,032 | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,492,318 | ) | | | — | | | | (3,492,318 | ) | | | (48,091 | ) | | | (3,540,409 | ) |
| Provision for statutory reserve | | | — | | | | — | | | | — | | | | — | | | | 12,249 | | | | (12,249 | ) | | | — | | | | — | | | | — | | | | — | |
| Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 268,363 | | | | 268,363 | | | | 184,818 | | | | 453,181 | |
| Balance as of September 30, 2024 | | | 14,591,942 | | | | 7,296 | | | | (6,250 | ) | | | 6,617,596 | | | | 899,731 | | | | 6,076,018 | | | | (408,465 | ) | | | 13,185,926 | | | | 2,884,447 | | | | 16,070,373 | |
| * | The shares and per share information are presented on a retroactive basis to reflect the reorganization (Note 1) and share reorganization by way of a subdivision and surrender of shares (Note 12). |
The accompanying notes are an integral part of these consolidated financial statements.
WORK MEDICAL TECHNOLOGY GROUP LTD
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In U.S. dollars, except for share and per share data, or otherwise noted)
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | |
| Net (loss) income | | $ | (3,540,409 | ) | | $ | 63,383 | | | $ | 944,126 | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 1,410,452 | | | | 1,603,129 | | | | 3,258,267 | |
| Inventories write-down | | | 762 | | | | 18,320 | | | | (117,327 | ) |
| Gain (loss) on disposal of equipment | | | 42,504 | | | | 8,089 | | | | (10,871 | ) |
| Allowance for credit loss | | | 12,157 | | | | 149,503 | | | | 1,108,999 | |
| Deferred tax benefits | | | (46,044 | ) | | | (94,533 | ) | | | (314,008 | ) |
| Changes in assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable – third parties | | | 1,815,361 | | | | (131,950 | ) | | | (4,234,997 | ) |
| Accounts receivable – related party | | | — | | | | — | | | | 275,993 | |
| Advance to suppliers | | | (1,061,321 | ) | | | 977,318 | | | | (191,774 | ) |
| Amount due (from) to related parties | | | 1,775,923 | | | | (3,964,077 | ) | | | 743,490 | |
| Inventories | | | 1,576,799 | | | | (400,544 | ) | | | 2,053,675 | |
| Operating lease liabilities | | | (2,029 | ) | | | (51,755 | ) | | | (5,212 | ) |
| Prepaid expenses and other current assets | | | 293,683 | | | | 1,291,145 | | | | (194,118 | ) |
| Accounts payable | | | (2,553,902 | ) | | | 864,585 | | | | (5,822,547 | ) |
| Income taxes payable | | | (250,423 | ) | | | 136,508 | | | | 81,428 | |
| Deferred revenue | | | (176,969 | ) | | | 52,245 | | | | (751,375 | ) |
| Accrued expenses and other liabilities | | | (1,524,631 | ) | | | 1,688,370 | | | | 917,303 | |
| Net cash (used in) provided by operating activities | | | (2,228,087 | ) | | | 2,209,736 | | | | (2,258,948 | ) |
| | | | | | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | | | |
| Purchase of intangible assets | | | (33,313 | ) | | | (127,913 | ) | | | — | |
| Purchase of property and equipment | | | (9,225,094 | ) | | | (455,391 | ) | | | (2,012,546 | ) |
| Proceed from disposal of property and equipment | | | 194,328 | | | | — | | | | 601,760 | |
| Cash acquired from Shanghai Saitumofei | | | — | | | | — | | | | 63,621 | |
| Net cash used in investing activities | | | (9,064,079 | ) | | | (583,304 | ) | | | (1,347,165 | ) |
| | | | | | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | | | |
| Proceeds from initial public offering | | | 7,373,839 | | | | — | | | | — | |
| Cash deposited in escrow account | | | (400,000 | ) | | | — | | | | — | |
| Contributions from non-controlling interest | | | 2,767,032 | | | | — | | | | — | |
| Proceeds from short-term borrowings | | | 13,394,778 | | | | 9,107,082 | | | | 7,248,367 | |
| Repayments of short-term borrowings | | | (9,369,404 | ) | | | (6,000,791 | ) | | | (7,248,367 | ) |
| Loans to related parties | | | (4,574,088 | ) | | | (5,669,834 | ) | | | (9,493,121 | ) |
| Repayment from related parties | | | 5,477,054 | | | | 5,891,373 | | | | 13,260,930 | |
| Loans to third parties | | | (1,133,656 | ) | | | (2,723,935 | ) | | | (1,030,628 | ) |
| Repayment from loans to third parties | | | 2,734,478 | | | | 23,562 | | | | 829,042 | |
| Payment of offering cost | | | (613,009 | ) | | | (1,356,176 | ) | | | — | |
| Net cash provided by (used in) financing activities | | | 15,657,024 | | | | (728,719 | ) | | | 3,566,223 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes | | | 555,464 | | | | (33,852 | ) | | | (59,819 | ) |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash and restricted cash | | | 4,920,322 | | | | 863,861 | | | | (99,709 | ) |
| Cash, cash equivalents, and restricted cash, at beginning of year | | | 1,637,283 | | | | 773,422 | | | | 873,131 | |
| Cash, cash equivalents and restricted cash, at end of year | | $ | 6,557,605 | | | $ | 1,637,283 | | | $ | 773,422 | |
| Reconciliation to amounts on consolidated balance sheets: | | | | | | | | | | | | |
| Cash and cash equivalents | | | 6,557,605 | | | | 1,596,096 | | | | 731,178 | |
| Restricted cash | | | - | | | | 41,187 | | | | 42,244 | |
| Total cash, cash equivalents and restricted cash shown in the statement of cash flows | | | 6,557,605 | | | | 1,637,283 | | | | 773,422 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | | | | | |
| Income taxes paid | | | 9,973 | | | $ | 160,162 | | | $ | 340,024 | |
| Interest paid | | | 543,753 | | | $ | 312,035 | | | $ | 312,282 | |
The accompanying notes are an integral part of these consolidated financial statements.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 1. | ORGANIZATION AND PRINCIPAL ACTIVITIES |
WORK Medical Technology Group LTD (the “Company”, “Work Cayman” or “WORK”) was incorporated under the law of the Cayman Islands on March 1, 2022 as an exempted company with limited liability. The Company, together with its subsidiaries (collectively, the “Group”), is engaged in manufacturing and selling medical consumables through its wholly owned subsidiaries in the People’s Republic of China (the “PRC” or “China”). The Company’s Ordinary Shares began trading on the Nasdaq Capital Market under theticker symbol “WOK” on August 23, 2024, and on August 26, 2024, the Company completed its initial public offering of 2,000,000 Ordinary Shares at a price of $4.00 per share (the “IPO”). On August 28, 2024, the underwriter for the IPO exercised its over-allotment option in part to purchase 91,942 Ordinary Shares at a price of $4.00.
History of the Group and Reorganization
The Company conducts its operations through its PRC subsidiary Hangzhou Shanyou Medical Equipment Co., Ltd. (“Hangzhou Shanyou”) and its subsidiaries.
In preparation for its initial public offering, the Group completed a reorganization on May 6, 2022 (the “Reorganization”), which involved the following steps:
| ● | on November 10, 2021, Work (Hangzhou) Medical Treatment Technology Co., Ltd. (“Work Hangzhou”) was established by Baiming Yu and his spouse, Liwei Zhang, who were the ultimate shareholders of Work Hangzhou; |
| ● | on January 17, 2022, Hangzhou Shanyou newly issued 95% of equity interest to Work Hangzhou. The remaining shareholders of Hangzhou Shanyou are Baiming Yu, with 3.35% of equity interest, and Liwei Zhang, with 1.65% of equity interest; |
| ● | on March 1, 2022, Work Cayman was incorporated and (indirectly) issued Ordinary Shares at par value $1.00 per share to certain founding shareholders. Baiming Yu (“LWY GROUP LTD”) and Liwei Zhang (“ZLW GROUP LTD”), who, following transfer of the initial one subscriber share from Tricor Services (Cayman Islands) Limited to LWY GROUP LTD, indirectly held a 50% and 5% equity interest of Work Cayman, respectively. Certain third parties, as strategic investors, acquired 45% equity shares of the PRC subsidiaries at fair value from Baiming Yu and Liwei Zhang. In exchange, Work Cayman issued the remainder of its 45% Ordinary Shares at par value $1.00 per share to these strategic investors on the day of its incorporation. |
| ● | on March 15, 2022, Work Medical Technology Group Limited (“Work BVI”) was incorporated in the British Virgin Islands as a wholly owned subsidiary of the Company; |
| ● | on April 19, 2022, Work Medical Technology Group (China) Limited (“Work Medical Technology” or “Work HK”) was incorporated in Hong Kong as a wholly owned subsidiary of Work BVI; |
| ● | on April 28, 2022, Work Age (Hangzhou) Medical Treatment Technology Co., Ltd. (“WFOE” or “Work Age”) was established as a wholly owned subsidiary of Work HK in the PRC; and |
| ● | on May 6, 2022, WFOE acquired 100% equity interest of Work Hangzhou. |
On February 21, 2022, Hangzhou Shanyou entered into a share purchase agreement to purchase 60% equity shares of Hangzhou Hanshi Medical Equipment Co., Ltd. (“Hangzhou Hanshi”) from Baiming Yu. Since both Hangzhou Shanyou and Hangzhou Hanshi are under the common control immediately before and after the merger, this transaction was accounted for as a common control merger using merger accounting as if the Reorganization had been consummated at the beginning of the earliest period presented, and no gain or loss was recognized. All the assets and liabilities of Hangzhou Hanshi are recorded at carrying value.
Immediately before and after share issuances and transfer of Work Cayman, Work Hangzhou acquired Hangzhou Shanyou, and WFOE acquired Work Hangzhou. The ultimate shareholders in these entities, who are Baiming Yu and his spouse, Liwei Zhang, did not change. Accordingly, the Reorganization has been treated as a corporate restructuring of entities under common control. Thus, the current capital structure has been retroactively presented in prior periods as if such structure existed at that time, and the entities are presented on a combined basis for all periods to which such entities were under common control. Thus, the results of these subsidiaries are included in the financial statements for years ended September 30, 2024 and 2023. The discussion and presentation of financial statements herein assumes the completion of the Reorganization, which is accounted for retroactively as if it occurred on October 1, 2019, and the equity has been restated to reflect the change, as well.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 1. | ORGANIZATION AND PRINCIPAL ACTIVITIES (cont.) |
The consolidated financial statements reflect the activities of the Company and each of the following entities:
| Name | | Date of
Incorporation | | Place of
incorporation | | Percentage of
effective ownership | | Principal Activities |
| Subsidiaries | | | | | | | | |
| Work BVI | | March 15, 2022 | | BVI | | 100% | | Investment holding |
| Work Medical Technology | | April 19, 2022 | | Hong Kong | | 100% | | Investment holding |
| WFOE | | April 28, 2022 | | PRC | | 100% | | Investment holding |
| Work Hangzhou | | November 10, 2021 | | PRC | | 100% | | Investment holding |
| Hangzhou Shanyou | | April 29, 2002 | | PRC | | 95% | | Produce and sale of medical consumables |
| Hangzhou Hanshi | | July 22, 2019 | | PRC | | 60% owned by Hangzhou Shanyou | | Sale of medical consumables |
| Shanghai Saitumofei Medical Treatment Technology Co., Ltd. (“Shanghai Saitumofei”)* | | May 15, 2019 | | PRC | | 44.2017% as of
September 30, 2024;
51% as of
September 30, 2023 | | Sale of medical consumable |
| Hunan Saitumofei Medical Treatment Technology Co., Ltd (“Hunan Saitumofei”)* | | April 02, 2022 | | PRC | | 100% owned by Shanghai Saitumofei | | Sale of medical consumables |
| Hangzhou Woli Medical Treatment Technology Co., Ltd (“Hangzhou Woli”) | | July 22, 2022 | | PRC | | 100% | | Sale of medical consumables |
| Shanghai Chuqiang Medical Equipment Co., Ltd. (“Shanghai Chuqiang”) | | March 12, 2018 | | PRC | | 100% owned by Hangzhou Shanyou | | Sale of medical consumables |
| Hangzhou Youshunhe Technology Co., Ltd. (“Hangzhou Youshunhe”) | | February 27, 2023 | | PRC | | 51% owned by Hangzhou Shanyou | | Sale of medical consumables |
| Huangshan Saitumofei Medical Treatment Technology* | | April 30, 2024 | | PRC | | 100% owned by Shanghai
Saitumofei | | Research and development |
| * | On May 24, 2024, the original shareholders of Shanghai Saitumofei (“Original shareholders”) signed a Capital injection Agreement with a new shareholder, Tunxi District Huangshan City Leading Industry Incubation Fund Ltd. (“Huangshan Fund”). According to the agreement, the Huangshan Fund intends to invest RMB20 million in cash to acquire a corresponding 13.33% shares in Shanghai Saitumofei. Following the capital injection of Huangshan Fund, the Group’s ownership interest of Shanghai Saitumofei was diluted to 44.2017%. While also on May 24, 2024, the Original shareholders signed a Concerted Action Agreement to ensure that the Group’s majority voting rights (collectively 86.67%) in Shanghai Saitumofei, therefore, the Group is still required to consolidate Shanghai Saitumofei in the reporting entity in the scope of ASC 810 Consolidation. |
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
| (a) | Basis of presentation and principles of consolidation |
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). The accompanying consolidated financial statements include the financial statements of the Company and its subsidiaries. All inter-company balances and transactions are eliminated upon consolidation. For consolidated subsidiaries where the Group’s ownership in the subsidiary is less than 100%, the equity interest not held by the Group is shown as noncontrolling interests.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, related disclosures of contingent assets and liabilities at the balance sheet date, and the reported revenue and expenses during the reported periods in the consolidated financial statements and accompanying notes. Significant accounting estimates include, but not limited to, the allowance for receivable, useful lives of property, plant and equipment and intangible assets, inventories write-downs and the recoverability of long-lived assets. Changes in facts and circumstances may result in revised estimates. Actual results could differ from those estimates, and as such, differences may be material to the consolidated financial statements.
| (c) | Cash and cash equivalents |
Cash and cash equivalents consist of cash on hand and demand deposits. The Group’s demand deposits are held at financial institutions, which have original maturities of less than three months and are unrestricted as to withdrawal and use. Deposits at financial institutions in mainland China and Hong Kong are not FDIC insured, however, those financial institutions are highly solvent and the risk of loss is remote.
Cash that is deposited in the bank but restricted for a specific purpose and therefore not available for immediate and general use by the Group is classified as restricted cash, which is presented separately from cash on the consolidated balance sheets. As of September 30, 2024 and 2023, restricted cash represented cash preserved for guarantee deposit and litigation.
In connection with the closing of the Company’s initial public offering in August 2024, $400,000 of the net proceeds received from the initial public offering was deposited in an escrow account, and the Company is restricted to withdraw therefrom, for twelve months after the closing date of the initial public offering. As of September 30, 2024 and 2023, the balance of the escrow account related to IPO was $400,000 and $nil respectively.
On October 1, 2021, the Group adopted Accounting Standards Update (“ASU”) 2016-02, Lease (FASB ASC Topic 842). The adoption of Topic 842 resulted in the presentation of operating lease right-of-use (“ROU”) assets and operating lease liabilities on the consolidated balance sheet. The Group has elected the package of practical expedients, which allows the Group not to reassess (1) whether any expired or existing contracts as of the adoption date are or contain a lease; (2) lease classification for any expired or existing leases as of the adoption date; and (3) initial direct costs for any expired or existing leases as of the adoption date. Lastly, the Group elected the short-term lease exemption for all contracts with lease terms of 12 months or less.
At inception of a contract, the Group assesses whether a contract is, or contains, a lease. A contract is or contains a lease if it conveys the right to control the use of an identified asset for a period of time in exchange for a consideration. To assess whether a contract is or contains a lease, the Group assesses whether the contract involves the use of an identified asset, whether it has the right to obtain substantially all the economic benefits from the use of the asset and whether it has the right to control the use of the asset.
The right-of-use assets and related lease liabilities are recognized at the lease commencement date. The Group recognizes operating lease expenses for lease payments on a straight-line basis over the lease term.
Operating lease right-of-use of assets
The right-of-use of asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any initial direct costs incurred and less any lease incentive received.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
Lease liabilities
Lease liability is initially measured at the present value of the outstanding lease payments at the commencement date, discounted using the Group’s incremental borrowing rate. Lease payments included in the measurement of the lease liability comprise fixed lease payments, variable lease payments that depend on an index or a rate, amounts expected to be payable under a residual value guarantee and any exercise price under a purchase option that the Group is reasonably certain to exercise.
Lease liability is measured at amortized cost using the effective interest rate method. It is re-measured when there is a change in future lease payments, if there is a change in the estimate of the amount expected to be payable under a residual value guarantee, or if there is any change in the Group assessment of option purchases, contract extensions or termination options.
| (g) | Accounts receivable, net |
Accounts receivable is stated at the original amount less an allowance for credit losses.
Accounts receivable is recognized in the period when the Group has provided goods to its customers and when its right to consideration is unconditional. On January 1, 2023, the Group adopted ASU 2016-13, “Financial Instruments — Credit Losses (Accounting Standards Codification (“ASC”) Topic 326): Measurement on Credit Losses on Financial Instruments”, including certain subsequent amendments, transitional guidance and other interpretive guidance within ASU 2018-19, ASU 2019-04, ASU 2019-05, ASU 2019-11, ASU 2020-02 and ASU 2020-03 (collectively, including ASU 2016-13, “ASC 326”). ASC 326 introduces an approach based on expected losses to estimate the allowance for doubtful accounts, which replaces the previous incurred loss impairment model. The Group’s estimation of allowance for credit losses considers factors such as historical credit loss experience, age of receivable balances, subsequent collection, current market conditions, reasonable and supportable forecasts of future economic conditions, as well as an assessment of receivables due from specific identifiable counterparties to determine whether these receivables are considered at risk or uncollectible.
The Group evaluates its accounts receivable for expected credit losses on a regular basis. The Group maintains an estimated allowance for credit losses to reduce its accounts receivable to the amount that it believes will be collected. The Group considers factors in assessing the collectability of its receivables, such as the age of the amounts due, the customer’s payment history, credit-worthiness and other specific circumstances related to the accounts. The Group adjusts the allowance percentage periodically when there are significant differences between estimated bad debts and actual bad debts. If there is strong evidence indicating that the accounts receivable is likely to be unrecoverable, the Group also makes specific allowance in the period in which a loss is determined to be probable. Accounts receivable balances are written off after all collection efforts have been exhausted.
Inventories, primarily consisting of the raw materials purchased by the Group for the production of medical consumables, work in progress and finished goods, are stated at the lower of cost or net realizable value. Cost of inventory is determined using weighted-average method. Where there is evidence that the utility of inventories, in their disposal in the ordinary course of business, will be less than cost, whether due to physical deterioration, obsolescence, changes in price levels, or other causes, the inventories are written down to net realizable value.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
Advance to suppliers refers to advances for purchase of materials, which is applied against accounts payable when the materials are received. The Group reviews a supplier’s credit history and background information before advancing a payment. If the financial condition of its suppliers were to deteriorate, resulting in an impairment of their ability to deliver goods or provide services, the Group would provide allowance for such amount in the period when it is considered impaired. Advance to suppliers as of September 30, 2024 and 2023 primarily consisted of prepayments for purchasing raw materials.
| (j) | Property, plant and equipment, net |
Property, plant and equipment, except mask production machine, are stated at cost less accumulated depreciation and impairment, if any, and depreciated on a straight-line basis over the estimated useful lives of the assets. Mask production machine is stated at cost less accumulated depreciation and impairment, if any, and depreciated on a double declining balance method over 5 years. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its intended use. Estimated useful lives are as follows:
| Category | | Estimated useful lives |
| Property and buildings | | 10 – 20 years |
| Machinery and equipment | | 3 – 10 years |
| Buildings improvement | | 5 years |
| Vehicle | | 3 – 4 years |
| Office and electric equipment | | 3 – 5 years |
Repair and maintenance costs are charged to expenses as incurred, whereas the cost of renewals and betterment that extends the useful lives of property, plant and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the costs, accumulated depreciation and impairment with any resulting gain or loss recognized in the consolidated statements of income.
| (k) | Intangible assets, net |
Intangible assets are non-monetary assets without physical substance. These items are initially measured at cost and subsequently carried at cost less any accumulated amortization and impairment losses. Intangible assets with finite useful lives are amortized on a straight-line basis over their estimated useful lives, which is as follows:
| Category | | Estimated useful lives |
| Land use rights | | 36 years |
| Patent | | 5 – 10 years |
| Digital factory operation management system | | 10 years |
| Mask customization system | | 5 years |
| (l) | Advance to suppliers for equipment |
Advance to suppliers for equipment refers to advances for purchase of production or R&D equipment, which is applied against accounts payable when the equipment is received. The Group reviews a supplier’s credit history and background information before advancing a payment. If the financial condition of its suppliers were to deteriorate, resulting in an impairment of its ability to deliver equipment, the Group would provide allowance for such amount in the period when it is considered impaired.
| (m) | Impairment of long-lived assets |
The Group reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Group measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Group would recognize an impairment loss, which is the excess of carrying amount over the fair value of the assets, using the expected future discounted cash flows. No impairment of long-lived assets was recognized for the years ended September 30, 2024, 2023 and 2022.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
| (n) | Fair value measurement |
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy prioritizes the inputs used to measure fair value. The hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| ● | Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, quoted market prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable and inputs derived from or corroborated by observable market data. |
| ● | Level 3 — inputs to the valuation methodology are unobservable. |
The level in the fair value hierarchy within which a fair value measurement in its entirety falls is based on the lowest level input that is significant to the fair value measurement in its entirety. In situations where there is little, if any, market activity for the asset or liability at the measurement date, the fair value measurement reflects management’s own judgments about the assumptions that market participants would use in pricing the asset or liability. Those judgments are developed by management based on the best information available in the circumstances.
The carrying amounts of the Group’s financial instruments approximate their fair values because of their short-term nature.
On October 1, 2019, the Group adopted ASC 606 using the modified retrospective approach.
The core principle of the new revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| Step 1: | Identify the contract with the customer |
| Step 2: | Identify the performance obligations in the contract |
| Step 3: | Determine the transaction price |
| Step 4: | Allocate the transaction price to the performance obligations in the contract |
| Step 5: | Recognize revenue when the company satisfies a performance obligation |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
Revenue from sales of self-manufactured masks and medical devices other than masks
The Group sells products to different distributor customers and direct-end user customers, primarily of sales of self-manufactured masks and medical devices other than masks. The Group does not accept returns of products or offer refunds for its customers after the expiration of quality objection periods. The Group usually offers a seven-day quality objection period and any quality deficiencies are determined by the testing of a third-party institution.
The Group grants credit sales for customers and the credit period varies among different customers. The Group normally grants the customers 30 to 180 days to complete their payments after the credit sales, and the length of the credit period is dependent on the customer’s creditworthiness and its transaction experience with the Group. The Group makes its judgment taking all of the facts and circumstances into account, including the PRC subsidiaries’ customary business practices and the knowledge of the customer, in determining whether it is probable that the PRC subsidiaries will collect substantially all of the consideration to which they will be entitled in exchange for the goods or services that the PRC subsidiaries expect to transfer to the customer.
The Group identifies one performance obligation in this business, which is to transfer control of a product to a distributor customer or direct-end user customer upon delivery of the product to the designated place. The revenue is recognized at a point in time when the Group satisfies the performance obligation by transferring the control of the promised product to distributor customers or direct-end user customers. The Group presents the revenue generated from its sales of products on a gross basis as the Group acts as a principal.
Revenue from commodity trading
The Group identifies one performance obligation in commodity trading, which is to transfer control of a product to a customer upon delivery of the product to the designated place. Revenue from commodity trading is recognized on a net basis or gross basis based on whether the Group arranges the provision of products through third parties and control the specified products provided by the third parties before that products are transferred to the customers. The revenue is recognized at a point in time when the Group satisfies performance obligations by arranging the transfer of a promised product to a customer. When the Group acted as an agent, the revenue is measured at fixed consideration which is determined as the difference between the sales price that the Group expects to receive in exchange for arranging promised products to the customer and the settlement price with the third-party suppliers. When the Group acted as a principal, the revenue is measured at fixed consideration which is the sales price that the Group expects to receive in exchange for arranging promised products.
Shipping and handling activities are considered to be fulfilment activities rather than promised services and are not, therefore, considered to be separate performance obligations. The Group’s sales terms provide no right of return outside of a standard quality policy and returns are generally not significant.
Geographic information
The following table disaggregates the Group’s revenue by geographic market for the years ended September 30, 2024, 2023 and 2022:
| | | For the Year Ended
September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Overseas market | | $ | 1,683,292 | | | $ | 967,690 | | | $ | 1,419,763 | |
| China domestic market | | | 9,823,148 | | | | 12,598,261 | | | | 18,291,527 | |
| Net revenue | | $ | 11,506,440 | | | $ | 13,565,951 | | | $ | 19,711,290 | |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
Revenue by product categories
The following table disaggregates the Group’s revenue product categories for the years ended September 30, 2024, 2023 and 2022:
| | | For the Year Ended
September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Masks | | $ | 1,559,750 | | | $ | 5,091,331 | | | $ | 10,619,035 | |
| Medical devices other than masks(1) | | | 9,414,751 | | | | 7,997,540 | | | | 8,747,886 | |
| Commodity trading(2) | | | 435,728 | | | | 325,429 | | | | 106,643 | |
| Others | | | 96,211 | | | | 151,651 | | | | 237,726 | |
| Net revenue | | $ | 11,506,440 | | | $ | 13,565,951 | | | $ | 19,711,290 | |
| (1) | Medical devices other than masks are mainly anesthesia & respiratory consumables. |
| (2) | Commodity trading are mainly trading raw materials of masks and medical devices other than masks. |
Contract balances
Payment terms are established on the Group’s pre-established credit requirements based upon an evaluation of customers’ credit. Contract assets are recognized for in related accounts receivable.
The contract liabilities consist of deferred revenue, which represents the billings or cash received for goods in advance of revenue recognition and is recognized as revenue when all the Group’s revenue recognition criteria are met. The Group’s deferred revenue was $625,326 and $776,210 as of September 30, 2024 and 2023, respectively. During the years ended September 30, 2024 and 2023, the Group recognized $359,496 and $277,561 that was included in deferred revenue balance at September 30, 2023 and 2022, respectively.
Other than deferred revenue, the Group had no other material contract assets, contract liabilities or deferred contract costs recorded on its consolidated balance sheets as of September 30, 2024 and 2023.
Contract costs
For the years ended September 30, 2024 and 2023, the Group did not have any significant incremental costs of obtaining contracts with customers incurred and/or costs incurred in fulfilling contracts with customers within the scope of ASC Topic 606, that shall be recognized as an asset and amortized to expenses in a pattern that matches the timing of the revenue recognition of the related contract.
Government subsidies are recognized as other income when received and all the conditions for their receipt have been met. The government subsidies were paid by cash and have no defined rules and regulations to govern the criteria necessary for the Group to enjoy the benefits.
For the years ended September 30, 2024, 2023 and 2022, the Group received government subsidies of $346,571, $74,232 and $1,186,641, respectively.
Cost of revenue mainly comprised of raw material costs, depreciation, labour cost, shipping and handling costs and other overhead expenses.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
The Group accounts for income taxes under ASC 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities.
The provisions of ASC 740-10-25, “Accounting for Uncertainty in Income Taxes,” prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures. The Group’s operating subsidiaries in the PRC are subject to examination by the relevant tax authorities. According to the PRC Tax Administration and Collection Law, the Law of the PRC on Enterprise Income Tax and other relevant laws and regulations, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or the withholding agent. The statute of limitations is extended to five years under special circumstances, where the underpayment of taxes is more than RMB0.1 million ($15,368). In the case of transfer pricing issues, the statute of limitation is ten years. There is no statute of limitation in the case of tax evasion. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the period incurred.
The Group did not accrue any liability, interest or penalties related to uncertain tax positions in its provision for income taxes line of its consolidated statements of income for the years ended September 30, 2024, 2023 and 2022, respectively.
The Group does not expect that its assessment regarding unrecognized tax positions will materially change over the next 12 months.
| (s) | Value added tax (“VAT”) |
The Group is subject to VAT and related surcharges on revenue generated from sales of products and commodity trading. The Group records revenue net of VAT. This VAT may be offset by qualified input VAT. Net VAT balance between input VAT and output VAT is recorded as VAT payable if output VAT is larger than input VAT and is recorded as VAT recoverable if input VAT is larger than output VAT.
The VAT rate is 13% for taxpayers selling consumer products.
| (t) | Non-controlling Interest |
A non-controlling interest in a subsidiary of the Group represents the portion of the equity (net assets) in the subsidiary not directly or indirectly attributable to the Group. Non-controlling interests are presented as a separate component of equity on the consolidated balance sheets, and net income and other comprehensive loss attributable to non-controlling shareholders are presented as a separate component on the consolidated statements of operations.
| (u) | Foreign currency transactions and translations |
The Group’s principal country of operations is the PRC. The financial position and results of its operations are determined using RMB, the local currency, as the functional currency. The Group’s financial statements are reported using U.S. Dollars (“$”). The results of operations and the consolidated statements of cash flows denominated in foreign currency are translated at the average rate of exchange during the reporting period.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
Assets and liabilities denominated in foreign currencies at the balance sheet date are translated at the applicable rates of exchange in effect at that date. The equity denominated in the functional currency is translated at the historical rate of exchange at the time of capital contribution. Because cash flows are translated based on the average translation rate, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets. Translation adjustments arising from the use of different exchange rates from period to period are included as a separate component of accumulated other comprehensive loss included in consolidated statements of changes in equity. Gains and losses from foreign currency transactions are included in the results of operations.
The value of RMB against $ and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of RMB may materially affect the Group’s financial condition in terms of $ reporting. The following table outlines the currency exchange rates that were used in creating the consolidated financial statements:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Balance sheet items, except for equity accounts | | | 7.0176 | | | | 7.2960 | |
| | | For the Years Ended
September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Items in the statements of income and comprehensive loss, and statements of cash flows | | | 7.2043 | | | | 7.0824 | | | | 6.5532 | |
No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at the rates used in translation.
Comprehensive loss consists of two components, net income and other comprehensive loss. The foreign currency translation gain or loss resulting from translation of the financial statements expressed from RMB to USD is reported in other comprehensive loss in the consolidated statements of income and comprehensive loss.
Earnings per Ordinary Share is calculated in accordance with ASC 260, Earnings per Share. Basic earnings per Ordinary Share is computed by dividing the net income attributable to shareholders of the Company by the weighted average number of Ordinary Shares outstanding during the year. Diluted earnings per Ordinary Share is computed in accordance with the treasury stock method and based on the weighted average number of Ordinary Shares and dilutive Ordinary Share equivalents. Dilutive Ordinary Share equivalents are excluded from the computation of diluted earnings per Ordinary Share if their effects would be anti-dilutive. There is no Ordinary Share equivalent issued to date.
The Group uses the management approach in determining its operating segments. The Group’s chief operating decision maker (“CODM”) identified as the Group’s Chief Executive Officer, relies upon the consolidated results of operations as a whole when making decisions about allocating resources and assessing the performance of the Group. As a result of the assessment made by CODM, the Group has only one reportable segment. The Group does not distinguish between markets or segments for the purpose of internal reporting. As substantially all of the Group’s long-lived assets are located in the PRC, no geographical segments are presented.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.) |
| (y) | Commitments and contingencies |
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. If a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material, is disclosed. Legal costs incurred in connection with loss contingencies are expensed as incurred.
| (z) | Comparative Information |
Certain items in prior years consolidated financial statements have been reclassified to conform to the current year’s presentation to facilitate comparison. These reclassifications had no effect on the reported results of operations or financial position.
| (aa) | Recent accounting pronouncements |
The Company is an “emerging growth company” (“EGC”) as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, EGCs can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies.
In October 2023, the FASB issued ASU 2023-06, “Disclosure Improvements: Codification Amendments in Response to the SEC’s Disclosure Update and Simplification Initiative.” This ASU incorporates certain U.S. Securities and Exchange Commission (SEC) disclosure requirements into the FASB Accounting Standards Codification. The amendments in the ASU are expected to clarify or improve disclosure and presentation requirements of a variety of Codification Topics, allow users to compare entities subject more easily to the SEC’s existing disclosures with those entities that were not previously subject to the requirements, and align the requirements in the Codification with the SEC’s regulations. For entities subject to the SEC’s existing disclosure requirements and for entities required to file or furnish financial statements with or to the SEC in preparation for the sale of or for purposes of issuing securities that are not subject to contractual restrictions on transfer, the effective date for each amendment will be the date on which the SEC removes that related disclosure from its rules. For all other entities, the amendments will be effective in two years later. However, if by June 30, 2027, the SEC has not removed the related disclosure from its regulations, the amendments will be removed from the Codification and not become effective for any entity. The Company does not expect to adopt this guidance early and does not expect the adoption of this ASU to have a material impact on its future consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280)- Improvements to Reportable Segment Disclosures (“ASU 2023-07”), which provides guidance on the enhanced disclosure of significant segment expenses that are regularly provided to the CODM and included within each reported measure of segment profit or loss, on an annual and interim basis. The guidance is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Adoption of this guidance should be applied retrospectively to all prior periods presented. Early adoption is permitted. The Company does not expect to adopt this guidance early and does not expect the adoption of this ASU to have a material impact on its future consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”, which requires disaggregated information about a reporting entity’s effective tax rate reconciliation, as well as information related to income taxes paid to enhance the transparency and decision usefulness of income tax disclosures. The guidance is effective for annual periods beginning after December 15, 2024 on a prospective basis. Early adoption is permitted. The Company does not expect to adopt this guidance early and does not expect the adoption of this ASU to have a material impact on its future consolidated financial statements.
In March 2024, the FASB issued ASU No. 2024-02, Codification Improvements-Amendments to Remove References to the Concepts Statements (“ASU 2024-02”). The amendments in this Update affect a variety of Topics in the Codification. The amendments apply to all reporting entities within the scope of the affected accounting guidance. This update contains amendments to the Codification that remove references to various Concepts Statements. In most instances, the references are extraneous and not required to understand or apply the guidance. In other instances, the references were used in prior statements to provide guidance in certain topical areas. ASU 2024-02 is effective for public business entities for fiscal years beginning after December 15, 2024. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2025. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. The Company does not expect to adopt this guidance early and does not expect the adoption of this ASU to have a material impact on its future consolidated financial statements.
Recent accounting standards that have been issued by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Group does not discuss recent standards that are not anticipated to have an impact on or are unrelated to its consolidated financial position, results of operations, cash flows or disclosures.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 3. | ACCOUNTS RECEIVABLE, NET |
Accounts receivable, net consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Accounts receivable | | $ | 2,652,596 | | | $ | 4,343,923 | |
| Allowance for credit loss | | | (1,003,799 | ) | | | (1,011,601 | ) |
| Accounts receivable, net | | $ | 1,648,797 | | | $ | 3,332,322 | |
The Group recorded reversal of credit loss of $155,467 and $134,846 for the years ended September 30, 2024 and 2023; and recorded credit loss of $108,775, $136,228 and $1,258,039 for the years ended September 30, 2024, 2023 and 2022, respectively.
As of date of issuance of this financial statement, the Group has collected $1,233,131 of outstanding balance as of September 30, 2024.
| | | As of September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Balance at the beginning of the year | | $ | 1,011,601 | | | $ | 1,344,480 | | | $ | 204,826 | |
| Current period addition | | | 108,775 | | | | 136,228 | | | | 1,258,039 | |
| Write-off | | | - | | | | (309,656 | ) | | | - | |
| Reversal | | | (155,467 | ) | | | (134,846 | ) | | | - | |
| Foreign currency translation adjustment | | | 38,890 | | | | (24,605 | ) | | | (118,385.00 | ) |
| Balance at the end of the year | | $ | 1,003,799 | | | $ | 1,011,601 | | | $ | 1,344,480 | |
Inventories consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Raw materials | | $ | 605,154 | | | $ | 741,988 | |
| Work in progress | | | 1,463,866 | | | | 2,030,108 | |
| Finished goods | | | 1,366,573 | | | | 2,089,383 | |
| Less: impairment | | | 251,642 | | | | 241,288 | |
| Inventories, net | | $ | 3,183,951 | | | $ | 4,620,191 | |
Impairment provided for the inventories was $239,715, $18,320 and $102,092 for the years ended September 30, 2024, 2023 and 2022, respectively.
| | | As of September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Balance at the beginning of the year | | $ | 241,288 | | | $ | 229,239 | | | $ | 372,405 | |
| Current period addition | | | 239,715 | | | | 18,320 | | | | 102,092 | |
| Reduction | | | (238,953 | ) | | | - | | | | (222,465 | ) |
| Foreign currency translation adjustment | | | 9,592 | | | | (6,271 | ) | | | (22,793 | ) |
| Balance at the end of the year | | $ | 251,642 | | | $ | 241,288 | | | $ | 229,239 | |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 5. | PREPAID EXPENSES AND OTHER CURRENT ASSETS |
Prepaid expenses and other current assets consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Loans to third parties(1) | | $ | 1,232,268 | | | $ | 3,154,873 | |
| Receivables from third parties(2) | | | 593,327 | | | | 613,580 | |
| Prepaid expenses(3) | | | 143,604 | | | | 24,152 | |
| Deferred offering costs | | | - | | | | 1,356,176 | |
| Others | | | 90,854 | | | | 59,535 | |
| Allowance for doubtful accounts | | | (427,351 | ) | | | (352,935 | ) |
| Total prepaid expenses and other current assets | | $ | 1,632,702 | | | $ | 4,855,381 | |
| (1) | In June 2023, the Company entered into agreements with Hangzhou Bota Commercial Co. Ltd. (“Hangzhou Bota”), to lend RMB19,000,000 (approximately $2,604,167) to Hangzhou Bota, with annualized interest rate ranging from 3.55% to 3.90%, and due in June 2024. The Group has collected all the balance in June 2024. |
In July 2024, the Company entered into agreements with Hangzhou Bota Commercial Co. Ltd. (“Hangzhou Bota”), to lend RMB6,000,000 (approximately $854,993) to Hangzhou Bota, with annualized interest rate at 3.92%, and due in July 2025.
The remaining loans to several third parties for their daily operations are due within one year or on demand.
| (2) | The balance represented receivables from the disposal of equipment to several third parties and other miscellaneous receivables from third parties. The Group has collected $481,361 of these receivables from third parties as of the date of this annual report. |
| (3) | The balance represented the unamortized portion of prepayments made to certain of the Group’s service providers for daily operations. |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 6. | PROPERTY, PLANT AND EQUIPMENT, NET |
Property, plant and equipment, net, consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Property and buildings | | $ | 11,606,914 | | | $ | 5,397,008 | |
| Machinery and equipment | | | 5,367,205 | | | | 12,084,239 | |
| Vehicle | | | 218,326 | | | | 156,119 | |
| Office and electric equipment | | | 143,481 | | | | 137,050 | |
| Buildings improvement | | | 309,994 | | | | - | |
| Subtotal | | | 17,645,920 | | | | 17,774,416 | |
| Less: impairment | | | 914,955 | | | | 2,648,945 | |
| Less: accumulated depreciation | | | 5,358,480 | | | | 8,498,697 | |
| Property, plant and equipment, net | | $ | 11,372,485 | | | $ | 6,626,774 | |
Depreciation expense was $1,336,879, $1,507,792 and $3,209,585 for the years ended September 30, 2024, 2023 and 2022, respectively.
No impairment of long-lived assets was recognized for the years ended September 30, 2024, 2023 and 2022.
Written-off due to disposal of related assets were $1,791,418, $22,327 and $1,531,190 for the years ended September 30, 2024, 2023 and 2022, respectively.
Intangible assets consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Land use rights | | $ | 1,017,360 | | | $ | 978,539 | |
| Patent | | | 143,270 | | | | 137,554 | |
| Digital factory operation management system | | | 171,842 | | | | 103,443 | |
| Mask customization system | | | 38,239 | | | | 36,781 | |
| Subtotal | | | 1,370,711 | | | | 1,256,317 | |
| Less: accumulated amortization | | | 326,649 | | | | 257,867 | |
| Intangible assets, net | | $ | 1,044,062 | | | $ | 998,450 | |
Amortization expenses were $56,923, $53,220 and $48,682 for the years ended September 30, 2024, 2023 and 2022, respectively.
The following table presents future amortization as of September 30, 2024:
| Year ending September 30, | | Amount | |
| 2025 | | $ | 71,087 | |
| 2026 | | | 71,087 | |
| 2027 | | | 65,150 | |
| 2028 | | | 55,066 | |
| 2029 | | | 46,571 | |
| Thereafter | | | 735,101 | |
| | | $ | 1,044,062 | |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 8. | ACCRUED EXPENSES AND OTHER LIABILITIES |
Accrued expenses and other liabilities consisted of the following:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| VAT payable | | $ | 1,862,661 | | | $ | 1,388,754 | |
| Payroll payable | | | 231,113 | | | | 625,802 | |
| Other payable(1) | | | 914,127 | | | | 2,384,040 | |
| Total accrued expenses and other liabilities | | $ | 3,007,901 | | | $ | 4,398,596 | |
| (1) | The balance represented unpaid cancelled investment funds from several investors (FY24: $142,499; FY23: $1,812,370), payables to employees for their reimbursement claims and other miscellaneous payables to third parties. |
Cayman Islands
The Company is incorporated in the Cayman Islands. Under the current laws of the Cayman Islands, the Group is not subject to income or capital gains taxes. In addition, dividend payments are not subject to withholdings tax in the Cayman Islands.
Hong Kong
According to Tax (Amendment) (No. 3) Ordinance 2018 published by Hong Kong government, since September 1, 2018, under the two-tiered profits tax rate regime, the profits tax rate for the first HKD2 million of assessable profits has been lowered to 8.25% (half of the rate specified in Schedule 8 to the Inland Revenue Ordinance (IRO)) for corporations. Work HK was not subject to Hong Kong profit tax for any period presented as it did not have assessable profit during the periods presented.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
PRC
The Group’s subsidiaries in mainland China are all subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws and regulations. The EIT rate for the Group’s operation in mainland China was 25% with the following exceptions.
Hangzhou Shanyou was qualified as a “high and new technology enterprise strongly supported by the State” in November 2017 and renewed in December 2023, and entitled to an EIT rate of 15%, expiring on December 8, 2026.
For qualified small and low-profit enterprises, from January 1, 2023 to December 31, 2027, 25% of the first RMB 3.0 million of the assessable profit before tax is subject to the tax rate of 20%. For the year ended September 30, 2024, there are qualified small and low-profit enterprises in PRC, and thus it was eligible for the above preferential tax rate for small and low-profit enterprises.
The income tax provision consisted of the following components:
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Current income tax (benefit) expenses | | $ | (83,025 | ) | | $ | 141,539 | | | $ | 483,443 | |
| Deferred income tax benefit | | | (42,266 | ) | | | (94,533 | ) | | | (314,008 | ) |
| Total income tax (benefit) expenses | | $ | (125,291 | ) | | $ | 47,006 | | | $ | 169,435 | |
A reconciliation between the Group’s actual provision for income taxes and the provision at the PRC, mainland statutory rate is as follows:
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Income (loss) before income taxes | | $ | (3,665,700 | ) | | $ | 110,389 | | | $ | 1,113,561 | |
| Income tax expenses computed at statutory EIT rate of 25% | | | (916,425 | ) | | | 27,597 | | | | 278,390 | |
| Reconciling items: | | | | | | | | | | | | |
| Effect of preferred tax rate | | | 657,287 | | | | (14,264 | ) | | | (102,578 | ) |
| Additional deduction for R&D expenses | | | (45,377 | ) | | | (45,247 | ) | | | (27,585 | ) |
| Change in valuation allowance | | | 164,582 | | | | 53,964 | | | | 13,660 | |
| Tax effect of non-deductible items | | | 14,642 | | | | 24,956 | | | | 7,548 | |
| Income tax (benefit) expenses | | | (125,291 | ) | | | 47,006 | | | | 169,435 | |
| Effective tax rates | | $ | 3.4 | % | | $ | 42.6 | % | | $ | 15.2 | % |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
Deferred tax assets/liability
As of September 30, 2024 and 2023, the significant components of the deferred tax assets and deferred tax liabilities are summarized below:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Deferred tax assets: | | | | | | |
| Allowance for credit loss | | $ | 213,390 | | | $ | 510,542 | |
| Inventories write-down | | | 37,746 | | | | 36,193 | |
| Impairment of equipment | | | - | | | | 33,768 | |
| Net operating loss carry forwards | | | 78,210 | | | | 52,359 | |
| Deferred tax assets, gross | | | 329,346 | | | | 632,862 | |
| Less: valuation allowance | | | (78,210 | ) | | | (59,879 | ) |
| Deferred tax assets, net | | $ | 251,136 | | | $ | 572,983 | |
| Deferred tax liability: | | | | | | | | |
| Depreciation* | | | 138,220 | | | | 506,111 | |
| Deferred tax liability, net | | | 138,220 | | | | 506,111 | |
| Net deferred tax asset: | | $ | 112,916 | | | $ | 66,872 | |
| * | The PRC income tax laws and regulations allow the Group to deduct certain machinery and equipment’s depreciation once for all at the time of purchase which is in excess of the related depreciation under accounting. |
Uncertain tax positions
The Group evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits associated with the tax positions. As of September 30, 2024 and 2023, the Group did not have any significant unrecognized uncertain tax positions.
As of September 30, 2024 and 2023, summary of the borrowings is as following:
| | | Annual
Interest Rate | | | Maturity
date | | September 30,
2024 | | | September 30,
2023 | |
| Bank of Jiangsu | | | 4.05 | % | | | | March 2024 | | $ | - | | | $ | 411,184 | |
| | | | 4.05 | % | | | | September 2025 | | | 427,497 | | | | - | |
| | | | | | | | | Subtotal | | $ | 427,497 | | | $ | 411,184 | |
| Xiaoshan Rural Commercial Bank | | | 4.40 | % | – 4.70% | | | August 2024 | | $ | - | | | $ | 4,454,496 | |
| | | | 4.35 | % | – 4.50% | | | August 2024 | | | - | | | | 1,370,614 | |
| | | | 4.50 | % | | | | August 2025 | | | 3,491,222 | | | | - | |
| | | | 4.40 | % | | | | August 2025 | | | 1,139,991 | | | | - | |
| | | | 4.20 | % | | | | August 2025 | | | 712,494 | | | | - | |
| | | | 4.20 | % | | | | July 2025 | | | 712,494 | | | | - | |
| | | | | | | | | Subtotal | | $ | 6,056,201 | | | $ | 5,825,110 | |
| Bank of Beijing | | | 3.55 | % | | | | June 2024 | | $ | - | | | $ | 1,370,614 | |
| | | | | | | | | Subtotal | | $ | - | | | $ | 1,370,614 | |
| China Citic Bank | | | 3.90 | % | | | | June 2024 | | | - | | | | 1,233,553 | |
| | | | 3.70 | % | | | | July 2025 | | | 854,993 | | | | - | |
| | | | 4.20 | % | | | | December 2024 | | | 5,984,952 | | | | - | |
| | | | | | | | | Subtotal | | $ | 6,839,945 | | | $ | 1,233,553 | |
| | | | | | | | | Total | | $ | 13,323,643 | | | $ | 8,840,461 | |
Interest expenses were $557,571, $392,741 and $352,968 for the years ended September 30, 2024, 2023 and 2022, respectively. The weighted average interest rates of borrowings outstanding were 4.34%, 4.50% and 4.89% per annum as of September 30, 2024, 2023 and 2022, respectively.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
The borrowings from Xiaoshan Rural Commercial Bank were secured by the Group’s equipments and buildings and guaranteed by Huiyu Holdings Group Co., Ltd. The borrowing from China Citic Bank was secured by Shuang Wu’s building and Baiming Yu.
The Company repaid total amount of the borrowing from China Citic Bank of $5,984,952 in December, 2024.
| 11. | RELATED PARTY TRANSACTIONS |
The following is a list of related parties which the Group has transactions with:
| No. | | Name of Related Parties | | Relationship with the Group |
| a | | Baiming Yu | | COO, Director and a shareholder of the Group |
| b | | Liwei Zhang | | Shareholder of the Group and the spouse of Baiming Yu |
| c | | Huiyu Chuanggu (Hangzhou) Equity Investment Fund Co., Ltd. (“Huiyu Chuanggu”) | | Shuang Wu acted as the executive director and owned 30% equity interests of this related party |
| d | | Baicheng Zhang | | Immediate family member of Liwei Zhang |
| e | | Shuang Wu | | CEO, Director, and Chairman of the Board of Directors |
| f | | Hangzhou Shuige Technology Co., Ltd (“Hangzhou Shuige”) | | 55% equity interests owned by Baiming Yu |
| g | | Hangzhou Qingniu Medical Instrument Co., Ltd (“Hangzhou Qingniu”) | | Owned by immediate family member of Baiming Yu |
| h | | Hangzhou Xiaoshan Ance Building Materials Firm (“Xiaoshan Ance”) | | Owned by Liwei Zhang |
| i | | Hangzhou Yuanqi Biotech Co., Ltd. (“Hangzhou Yuanqi”) | | Baiming Yu acted as director before January, 2024 |
| j | | Ming Zhao | | The Supervisor of Shanghai Saitumofei |
| k | | Hangzhou Yizhiying Information Consulting Management Co., Ltd | | Shuang Wu acted as executive director |
| g | | Qijia Yu | | Immediate family member of Baiming Yu |
Amounts due from related parties
Amounts due from related parties consisted of the following for the periods indicated:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Shuang Wu(1) | | $ | 1,424,989 | | | $ | 795,243 | |
| Hangzhou Qingniu(2) | | | 855,618 | | | | 1,294,296 | |
| Hangzhou Shuige(2) | | | 421,092 | | | | - | |
| Qijia Yu(4) | | | 5,437 | | | | - | |
| Huiyu Chuanggu(3) | | | - | | | | 1,453,583 | |
| Baiming Yu(5) | | | - | | | | 683,376 | |
| Liwei Zhang(2) | | | - | | | | 11,184 | |
| Total | | $ | 2,707,136 | | | $ | 4,237,682 | |
| (1) | The balance represented advances made to the related party for the Group’s daily operational purposes, and reimbursement from the related parties for the Group’s operational expenses. The Group has collected all the amounts from the related party as of the date of the 20-F. |
| (2) | The balance represented an interest-free loan to this related party, which is due on demand. The Group has collected all the amounts from the related party as of the date of the 20-F. |
| (3) | The balance represented the advances made to Huiyu Chuanggu by the Group for its future payment of audit fees and legal expenses which were unsecured, interest-free and repayable on demand. |
| (4) | The balance represented advances made to the related party for the Group’s daily operational purposes. |
| (5) | The balance represented withholding tax receivables related to deemed dividend and advances made to the management for the Group’s daily operational purposes. |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 11. | RELATED PARTY TRANSACTIONS (cont.) |
Amounts due to related parties
Amounts due to related parties consisted of the following for the periods indicated:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Huiyu Chuanggu(1) | | $ | 1,064,916 | | | $ | - | |
| Xiaoshan Ance(2) | | $ | 142,499 | | | $ | 137,061 | |
| Baiming Yu(3) | | | 5,820 | | | | - | |
| Ming Zhao(3) | | | 4,207 | | | | 4,048 | |
| Hangzhou Shuige(2) | | | - | | | | 17,818 | |
| Hangzhou Yuanqi(2) | | | - | | | | 685 | |
| Total | | $ | 1,217,442 | | | $ | 159,612 | |
| (1) | The balance represented the payment on behalf of the Group for its daily operations or the initial public offering costs, including legal fees and accounting fees. |
| (2) | The balance represented interest-free loans from this related party, which was due on demand. |
| (3) | The balance represented advance from this related party for the Group’s daily operations with no fixed term of repayment and interest. |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 11. | RELATED PARTY TRANSACTIONS (cont.) |
| | | For the years ended
September 30, | |
| Nature | | 2024 | | | 2023 | | | 2022 | |
| Loan to Hangzhou Shuige(1) | | $ | 1,538,672 | | | $ | 423,585 | | | $ | 1,983,764 | |
| Repayment from Hangzhou Shuige(1) | | | 1,110,448 | | | | 423,585 | | | | 5,889,698 | |
| Loan to Liwei Zhang(1) | | | 319,254 | | | | 303,075 | | | | — | |
| Repayment from Liwei Zhang(1) | | | 330,580 | | | | 362,377 | | | | — | |
| Advance to Baiming Yu(2) | | | 3,705 | | | | 1,187,554 | | | | 17,148 | |
| Reimbursement from Baiming Yu(2) | | | 9,375 | | | | 644,678 | | | | — | |
| Repayment from Baiming Yu(2) | | | 692,074 | | | | — | | | | — | |
| Selling to Hangzhou Qingniu(3) | | | 577,370 | | | | 1,011,587 | | | | 16,524 | |
| Loan to Hangzhou Qingniu(1) | | | 2,716,162 | | | | 4,943,174 | | | | 7,356,760 | |
| Rent income from Hangzhou Qingniu(4) | | | — | | | | 52,749 | | | | 192,636 | |
| Repayment from Hangzhou Qingniu(1)(3) (4) | | | 4,036,026 | | | | 5,105,411 | | | | 7,371,232 | |
| Loan from Xiaoshan Ance(5) | | | — | | | | — | | | | 152,597 | |
| Advance to Shuang Wu(6) | | | 2,054,627 | | | | 3,467,737 | | | | 174,800 | |
| Repayment from Shuang Wu(6) | | | 1,159,929 | | | | 2,022,703 | | | | 851,143 | |
| Advance to Huiyu Chuanggu(7) | | | 447,927 | | | | 2,894,499 | | | | — | |
| Reimbursement from Huiyu Chuanggu(7) | | | 2,077,162 | | | | 1,397,077 | | | | — | |
| Management service fee to Huiyu Chuanggu(8) | | | 1,247,508 | | | | — | | | | — | |
| Rent income from Hangzhou Yuanqi(4) | | | — | | | | — | | | | 3,815 | |
| Collection from Hangzhou Yuanqi(4) | | | — | | | | 706 | | | | 3,815 | |
| Advance to Ming Zhao(9) | | | — | | | | 866 | | | | — | |
| Collection from Ming Zhao(9) | | | — | | | | 866 | | | | 1,958 | |
| Advance from Hangzhou Yizhiying Information Consulting Management Co., Ltd(10) | | | — | | | | — | | | | 149,606 | |
| Repayment to Hangzhou Yizhiying Information Consulting Management Co., Ltd(10) | | | — | | | | — | | | | 61,885 | |
| Advance to Qijia Yu(2) | | | 8,142 | | | | — | | | | — | |
| Repayment from Qijia Yu(2) | | | 2,846 | | | | — | | | | — | |
| (1) | This represented the Group’s interest-free loans, which was due on demand, to these related parties and repayment from these related parties. |
| (2) | It was the advances which is interest-free and due on demand made to the related parties for the Group’s daily operational purposes, and reimbursement from the related parties for the Group’s operational expenses. |
| (3) | It was the receivable for selling medical consumables to this related party, repayment from this related party. |
| (4) | It was the receivable and collection for providing rental service to this related party. |
| (5) | This consisted of the Group’s interest-free loan from this related party for daily operations, which was due on demand. |
| (6) | This consisted of the Group’s advance to this related party with no fixed term of repayment and interest, and the repayment from this related party. |
| (7) | This consisted of the advance to this related party for the Group’s initial public offering costs, including legal fees and accounting fees, and the reimbursement of excessive advance payment from this related party. |
| (8) | It was the management service fee for Huiyu Chuanggu’s intergrated management consulting services during the Company’s IPO process. This fee was charged once at a fixed price only when the Company completed its IPO. |
| (9) | This consisted of the advance to this related party for the Group’s daily operations. |
| (10) | This consisted of the Group’s advance from this related party for the Group’s daily operations with no fixed term of repayment and interest. |
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
Ordinary Shares
The Company was established as an exempted company under the laws of Cayman Islands on March 1, 2022. The authorized number of Ordinary Shares was 50,000 with par value of $1 per share. The Company issued 50,000 shares to the shareholders at par value of $1 per share.
On April 6, 2023, the shareholders of the Company unanimously passed resolutions effecting the subdivision of the Company’s authorized and issued share capital and the adoption of the amended and restated memorandum of association, pursuant to which, (1) the Company effectuated a 1:2000 share subdivision, whereupon the Company’s authorized share capital was amended from $50,000 divided into 50,000 shares of par value of $1.00 each to $50,000 divided into 100,000,000 Ordinary Shares of a par value of $0.0005 each; and (2) immediately after the share subdivision, the shareholders voluntarily surrendered, on a pro rata basis, a total of 87,500,000 Ordinary Shares of a par value of $0.0005 each, after which, the Company had an aggregate of 12,500,000 Ordinary Shares issued and outstanding.
On August 26 and August 29, 2024, the Company closed its initial public offering (“IPO”) and the sale of the over-allotment shares. The Company issued and sold 2,091,942 Ordinary Shares, including 91,942 Ordinary Shares of the over-allotment shares, at $4.00 per share, for net proceeds of approximately $5,404,654, after (i) deducting underwriting discounts and other related expenses and (ii) reimbursements made to PRC subsidiaries for expenses advanced from them in connection with the Company’s IPO.
Subscription receivable
As of September 30, 2024 and 2023, subscription receivable on the consolidated balance sheets represented the unrecovered consideration of the 12,500,000 and 12,500,000 ordinary shares issued by the Company, respectively.
| 13. | STATUTORY SURPLUS RESERVES AND RESTRICTED NET ASSETS |
A significant portion of the Group’s operations are conducted through its PRC (excluding Hong Kong) subsidiaries, the Group’s ability to pay dividends is primarily dependent on receiving distributions of funds from our subsidiaries. Relevant PRC statutory laws and regulations permit payments of dividends by our subsidiaries only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations, and after it has met the PRC requirements for appropriation to statutory reserves.
The Group is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve are required to be at least 10% of the after-tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entity’s registered capital. Appropriations to the surplus reserve are made at the discretion of the Board of Directors. Paid-in capital of our subsidiaries included in the Group’s consolidated net assets are also non-distributable for dividend purposes.
As a result of these PRC laws and regulations, the Group’s PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to the Group. As of September 30, 2024 and 2023, net assets restricted in the aggregate, which include paid-in capital and statutory reserve funds of the Group’s subsidiaries, that are included in the consolidated net assets were $984,743 and $972,494, respectively.
| 14. | CONCENTRATION OF CREDIT RISK |
Financial instruments that potentially expose the Group to concentrations of credit risk consist primarily of accounts receivable. The Group conducts credit evaluations of its customers, and generally does not require collateral or other security from them. The Group evaluates its collection experience and long outstanding balances to determine the need for an allowance for doubtful accounts. The Group conducts periodic reviews of the financial condition and payment practices of its customers to minimize collection risk on accounts receivable.
There is no single customer that accounted 10% or more of the Group’s total revenue for the twelve months ended September 30, 2024, 2023 and 2022.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 14. | CONCENTRATION OF CREDIT RISK (cont.) |
The following table sets forth a summary of single customers who represented 10% or more of the Group’s total accounts receivable:
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | |
| Percentage of the Group’s total accounts receivable | | | | | | |
| Customer A | | | 24 | % | | | * | |
| Customer B | | | * | | | | 14 | % |
| Customer C | | | * | | | | 13 | % |
| Customer D | | | * | | | | 19 | % |
| * | Represent percentage less than 10% |
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total purchases:
| | | For the years ended | |
| | | September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| Percentage of the Group’s total purchase | | | | | | | | | |
| Supplier A | | | 15 | % | | | * | | | | * | |
| Supplier B | | | 10 | % | | | * | | | | * | |
| Supplier C | | | * | | | | 13 | % | | | * | |
| Supplier D | | | * | | | | 11 | % | | | 11 | % |
| Supplier E | | | * | | | | * | | | | 16 | % |
| * | Represent percentage less than 10% |
The following table sets forth a summary of single suppliers who represented 10% or more of the Group’s total advance to suppliers:
| | | As of September 30, | |
| | | 2024 | | | 2023 | |
| Percentage of the Group’s advance to | | | | | | |
| Supplier E | | | 33 | % | | | * | |
| Supplier F | | | 18 | % | | | 40 | % |
| Supplier G | | | 15 | % | | | 20 | % |
| Supplier H | | | * | | | | 10 | % |
| * | Represent percentage less than 10% |
| 15. | COMMITMENTS AND CONTINGENCIES |
Commitments
As of September 30, 2024 and 2023, the Group has no material purchase commitments or significant leases.
Contingencies
In the ordinary course of business, the Group may be subject to legal proceedings regarding contractual and employment relationships and a variety of other matters. The Group records contingent liabilities resulting from such claims, when a loss is assessed to be probable, and the amount of the loss is reasonably estimable. In the opinion of management, there were no material pending or threatened claims and litigation as of the issuance date of these consolidated financial statements.
WORK MEDICAL TECHNOLOGY GROUP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In U.S. dollars, except share and per share data)
| 16. | Earnings (loss) per share |
The following table sets forth the computation of basic net earnings (loss) per share:
| | | For the fiscal years ended
September 30, | |
| | | 2024 | | | 2023 | | | 2022 | |
| | | $ | | | $ | | | $ | |
| Net earnings (loss) attributable to ordinary shareholders | | | (3,492,318 | ) | | | 112,030 | | | | 865,210 | |
| Weighted average number of ordinary shares outstanding | | | | | | | | | | | | |
| – basic and diluted | | | 12,705,011 | | | | 12,500,000 | | | | 12,500,000 | |
| Basic and diluted earnings (loss) per share | | | (0.27 | ) | | | 0.01 | | | | 0.07 | |
In December 2024, the Company repaid the total amount of the borrowing from China Citic Bank of $5,984,952.
On December 20, 2024, all of the directors of the Company adopted resolutions authorizing the offering and sale of up to 2,500,000 ordinary units, (each, an “Ordinary Unit”) on the Nasdaq Capital Marke, with each Ordinary Unit consisting of:
| (i) | one ordinary share of the Company with a par value of US$0.0005 each; |
| (ii) | one Series A warrant, with each Series A warrant entitling the holder thereof to purchase one Ordinary Share; |
| (iii) | one Series B warrant, with each Series B warrant entitling the holder thereof to purchase one Ordinary Share. |
On February 5, 2025, the Company held the Annual General Meeting of Shareholders (the “Meeting”). At the Meeting, the shareholders of the Company adopted resolutions authorizing the following:
| (i) | as an ordinary resolution, the authorized share capital of the Company was increased from US$50,000 divided into 100,000,000 ordinary shares of par value US$0.0005 each to US$250,000 divided into 500,000,000 ordinary shares of par value US$0.0005 each (the “Share Capital Increase”). |
| (ii) | as an ordinary resolution, subject to and immediately following the Share Capital Increase being effected, the Company will re-designate and re-classify its authorized share capital as follows (the “Share Capital Reorganization”): |
(a) each ordinary share of par value US$0.0005 in issue immediately following the Share Capital Increase, which is expected to be 14,591,942 ordinary shares of par value US$0.0005 each, will be re-designated and re-classified into one Class A ordinary share of par value US$0.0005 each;
(b) 100,000,000 of the remaining authorized but unissued ordinary shares of par value US$0.0005 each will be re-designated and re-classified into one Class B ordinary share of par value US$0.0005 each; and
(c) each of the remaining authorized but unissued ordinary shares of par value US$0.0005 each, which is expected to be 385,408,058 ordinary shares of par value US$0.0005 each, will be re-designated and re-classified into one Class A ordinary share of par value US$0.0005 each,
such that the Company’s authorized share capital will be re-designated and re-classified from US$250,000 divided into 500,000,000 ordinary shares of par value US$0.0005 each to US$250,000 divided into 400,000,000 Class A ordinary shares of par value US$0.0005 each and 100,000,000 Class B ordinary shares of par value US$0.0005 each; and
| (iii) | as a special resolution, that subject to and immediately following the Share Capital Reorganization being effected, the Company will adopt amended and restated memorandum and articles of association, in the form annexed to the proxy statement, in substitution for, and to the exclusion of, the Company’s existing memorandum and articles of association, to reflect the Share Capital Increase, the Share Capital Reorganization and the terms of the Class A ordinary shares and Class B ordinary shares. |
The Group has evaluated subsequent events from September 30, 2024 and through the date of issuance of the consolidated financial statements which is February 14, 2025, and did not identify any subsequent events except disclosed above that would have material financial impact or that required adjustment of the Group’s consolidated financial statements.