AS SUBMITTED TO THE SECURITIES AND EXCHANGE COMMISSION ON NOVEMBER 14, 2022
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933

VALUEONE, INC.
(Exact name of Registrant as specified in its charter)
Nevada | | 8731 | | 83-2466129 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) |
30 Corporate Park, Suite 315
Irvine, CA 92606
(nnn)xxx-yyyy
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Tea Hyen Shin, President
30 Corporate Park, Suite 315
Irvine, CA 92606
(nnn) xxx-yyyy
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
John E. Dolkart, Esq. Dolkart Law PC
100 Pine Street, Suite 1250
San Francisco, CA 94111
(415) 707-2717
Approximate Date of Proposed Sale to the Public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
| | | |
Non-accelerated filer | x | Smaller reporting company | x |
| | | |
| | Emerging growth company | x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. x
CALCULATION OF REGISTRATION FEE
Title of each Class of Securities to be Registered | | Shares to be Registered(1) | | | Proposed Maximum Aggregate Offering Price Per Share(2) | | | Maximum Aggregate Offering Price(1)(2) | | | Amount of Registration Fee(2) | |
Shares of Common Stock, par value $.001, issuable to each stockholder of the Company holding restricted shares equal to 100% of their beneficial ownership | | | 19,100,000 | | | $ | 0.50 | | | $ | 9,550,000 | | | $ | | |
Shares of Common Stock, par value $.001. | | | 5,000,000 | | | $ | 0.50 | | | $ | 2,500,000 | | | $ | | |
| | | | | | | | | | | | | | | | |
TOTAL | | | 24,100,000 | | | | | | | $ | 12,050,000 | | | $ | $1,327.91 | |
| (1) | Pursuant to Rule 416 under the Securities Act, this registration statement shall be deemed to cover additional securities (i) to be offered or issued in connection with any provision of any securities purported to be registered hereby pursuant to terms which provide for a change in the amount of securities being offered or issued to prevent dilution resulting from stock splits, stock dividends, or similar transactions and (ii) of the same class as the securities covered by this registration statement issued or issuable prior to completion of the distribution of the securities covered by this registration statement as a result of a split of, or a stock dividend on, the registered securities. |
| (2) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) promulgated under the Securities Act of 1933, as amended. |
This Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
DATED November 14, 2022
Prospectus
24,100,000 Shares of Common Stock
VALUEONE, INC.
This prospectus (the "Prospectus") covers 24,100,00 shares of our common stock (the "Shares") of which 19,100,00 may be offered for resale or otherwise disposed of by each our record and beneficial stockholders in an amount equal to 100% of the shares of our common stock that each stockholder owns (the "Selling Stockholders") and 5,000,000 shares that may be offered for resale or otherwise disposed of by qualified investors who may purchase shares of our common stock in connection with a financing in which we are seeking to raise and additional $2,500,000 USD. This is the initial public offering of our common stock.
We will not receive any proceeds from the sale or other disposition of the securities by the Selling Stockholders. However, we may receive up to approximately $2,500,000 in gross proceeds upon the purchase of the to-be-offered 5,000,000 shares. We will use such proceeds, if and when received, for acquisitions, research and development and working capital. We do not have any planned acquisitions at this time. The prices at which the Selling Stockholders may sell the shares will be determined by the prevailing market price for the shares or in negotiated transactions. See "Selling Stockholders" for additional information regarding the sales process available to the Selling Stockholders.
We are an "emerging growth company" under the federal securities laws and will be subject to reduced public company reporting requirements as set forth on page 19 of this prospectus. There is a limited established market for our stock. The offering price of our shares has been determined arbitrarily by us. The price does not bear any relationship to our assets, book value, earnings, or other established criteria for valuing a privately held company. In determining the number of shares to be offered and the offering price, we took into consideration our capital structure and the amount of money we would need to implement our business plans. Accordingly, the offering price should not be considered an indication of the actual value of our securities.
Our common stock is not currently quoted on any national securities exchange. We plan to list our common stock on the OTC Markets Group, Inc. "Pink Current Information Tier" and apply with FINRA for the symbol "VLUS" upon the approval of this The closing of this offering is contingent upon the successful listing of the Company on the OTC Markets Group, Inc. ("OTC").
Investing in our securities involves a high degree of risk. See "Risk Factors" beginning on page 15 in this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is November 14, 2022
ADDITIONAL INFORMATION
You should rely only on the information contained or incorporated by reference in this prospectus and in any accompanying prospectus supplement. No one has been authorized to provide you with different information. The shares are not being offered in any jurisdiction where the offer is not permitted. You should not assume that the information in this prospectus or any prospectus supplement is accurate as of any date other than the date on the front of such documents.
We have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the Shares offered hereby, but only under the circumstances and in the jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of Shares. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: We have not done anything that would permit this offering or possession or distribution of this prospectus or any applicable free writing prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus and any applicable free writing prospectus must inform themselves, and observe any restrictions relating to, the offering of the Units and the distribution of this prospectus outside the United States.
TABLE OF CONTENTS
| Page No. |
PROSPECTUS SUMMARY | 8 |
| |
THE OFFERING | 13 |
| |
RISK FACTORS | 15 |
| |
USE OF PROCEEDS | 22 |
| |
MARKET FOR REGISTRANT's COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 22 |
| |
DILUTION | 22 |
| |
MANAGEMENT's DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 24 |
| |
BUSINESS | 25 |
| |
MANAGEMENT | 35 |
| |
EXECUTIVE COMPENSATION | 40 |
| |
PRINCIPAL AND SELLING SECURITYHOLDERS | 40 |
| |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE | 41 |
| |
DESCRIPTION OF SECURITIES | 41 |
| |
SHARES ELIGIBLE FOR FUTURE SALE | 43 |
| |
PLAN OF DISTRIBUTION | 45 |
| |
LEGAL MATTERS | 46 |
| |
EXPERTS | 46 |
| |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | F-1 |
CAUTIONARY NOTE TO INVESTORS
This Prospectus qualifies the distribution of securities of an entity that presently derives all of its revenue, if any, from to be implemented business plans for the distribution of medical testing devices in the United States and in North American and European Markets.
The United States and the Republic of Korea (aka "South Korea") are parties to the Intellectual Property Rights (IPR) Chapter of the U.S.-South Korea trade agreement which contains state-of-the-art protections spanning all types of intellectual property, and requirements to join key multilateral IPR agreements. It also contains strong enforcement provisions to ensure that American intellectual property rights are efficiently and effectively protected in South Korea.
Any intellectual property protected by ValueOne, South Korea, will seek to have reciprocal rights acknowledged by both the United States Patent and Trademark Office ("USPTO") and the Korean Intellectual Property Office ("KIPO").
ValueOne is the strategic joint-venture partner of ValueOne, Inc., a Republic of Korea corporation. The South Korean corporation holds all intellectual property in this prospectus and provides the exclusive right to use the same to ValueOne. The two companies are working together cooperatively to develop new intellectual property pursuant to a Joint Venture Agreement for the Development Ownership; and Control of Intellectual Property and Work Product, Exhibit 1.5 hereto.
Depending on which joint venture partner the primary developer of any new intellectual property is the partners will register the intellectual property in the appropriate jurisdiction and then seek reciprocal registration in any remaining jurisdictions required.
ValueOne does not have any approval or authority to operate in such markets but has applied to do so in South Korea. Upon approval of the South Korean government to manufacture, sell and distribute its devices, the United States corporation will seek reciprocal approval from relevant United States government agencies.
Trademarks
This prospectus contains references to trademarks and service marks and to those belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the © or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies' trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by any other companies.
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere in this prospectus. This summary may not contain all of the information that may be important to you. You should read this entire prospectus carefully, including the sections entitled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our historical financial statements and related notes included elsewhere in this prospectus. In this prospectus, unless otherwise noted, the terms "the Company," "we," "us," and "our" refer to ValueOne, Inc., a Nevada corporation.
The Company
Background
ValueOne, Inc. was incorporated in the State of Nevada as a for-profit Company on May 30, 2018 and adopted the calendar year-end. The Company operates as the exclusive joint-venture partner of ValueOne, Inc. of South Korea.
ValueOne,Inc.is aSouthKoreanbiotechnologycompany founded in Seoul by Tea Hyen Shin 2015. Withonly $50,000 USD in seed capital the company has been able to develop a suite of at home in vitromedical testing devices, with plans for its own clinical testing laboratory and globaldistributionnetwork.
The South Korean parent company has granted an exclusive license to Value One, Inc. ofNevada as part of a strategic joint venture agreement whereby Value One Nevada will act as the primary advertising, marking, distribution and assist in the co-development of researchanddevelopmentfor newmarkets ofproductsdesignedin SouthKorea.
The two joint venture partner companies will work cooperatively to develop these medical testing devicesand establish a supply chain for distribution, sales and marketing in the United Statesand South Korea. An important part of the Company's success relies on the existence ofa clinical laboratory formedicalanddiagnostic testing.
No determination has been made on whether this facility will be acquired or developed from scratch. This will require the retainer of qualified medical doctors and laboratorytechnical personnel to staff the lab to preform testing in support of the company'srangeof athometestingproducts.
Capital Structure
The Company amended its articles of incorporation on May 10, 2021 increasing its total authorized common shares from two million (2,000,000) at $0.001 par value to two hundred million (200,000,000) at $0.001 par value with twenty million (20,000,000) preferred shares at par value of $0.001. On May 19, 2021, one million (1,000,000) shares of Series A Preferred Shares are designated with a par value of $0.001, which carry voting rights senior to the Common Stock and will not be subject to any reverse splits or deductions.
The Preferred Series A shall rank senior to the Common Stock as to dividends and upon liquidation, dissolutions or winding up. On or about May 26, 2021 the 1,000,000 Series A Preferred Shares were issued to our president, chief executive, chairman and controlling shareholder Tea Hyen Shin in return for $1,000 USD cash payment by him.
Overview
We are a Nevada corporation and the joint-venture partner of ValueOne, Inc. of South Korea who holds valuable intellectual property in the medical and biometric testing industry. ValueOne, Inc. intends to develop, market, and distribute medical testing devices and new technologies with exclusive rights to do so in North America.
In or about January 2021 the Company entered into an Exclusive Joint Venture Agreement for the Ownership, Development and Control of Intellectual Property and Work Product. ValueOne is a development stage company that does not currently have any substantial business operations and has not generated any revenues. The Company will not be profitable until it derives sufficient revenues and cash flows from the marketing of its products.
We believe that, if we obtain the proceeds from this offering, the Company will be able to implement its business plan and conduct business pursuant to the business plan for at least the next twelve months. Our operations to date have been devoted primarily to business plan design, start-up and development activities, which include the following:
Development of the ValueOne, Inc., North American business plan.
Defining initial short-term and long-term marketing efforts including scouting locations, and designing and developing a network of affiliates who will assist in the implementation of our business plan.
Research and Development to develop intellectual property and biological testing systems for the at-home use market.
Implementaion of business strategies and techniques. This includes hourly rates and the number of hours required to develop our business plan. These numbers have been incorporated into our Use of Proceeds budget.
Beginning of due diligence on our planned distribution of medical testing kits in North American and later in Europe.
ValueOne never been party to any bankruptcy, receivership or similar proceeding, nor has it undergone any material reclassification, merger, consolidation, purchase or sale of a significant amount of assets not in the ordinary course of business.
We currently do not have trademarks, patents, or any other types of protection on our business plans. Please refer to "Description of Our Business." We have no revenues since inception, have sustained losses since inception, do not have any employees, other than Tea Hyen Shin, our CEO and Chairman of the Board of Directors.
We may not generate sufficient revenues even if we are able to gain market acceptance of our business plan. Accordingly, we will be dependent on future financings in order to maintain our operations and continue our research and development. Please refer to "Risk Factors."
Voting Control
The voting control of our common stock is possessed by Tea Hyen Shin, our Chief Executive Officer and Chairman of the Board of Directors, who was issued 1,000,000 shares, or 100%, of our Preferred Series A Stock for $1,000 in or about May 2021. Mr. Shin also holds 6.5 million shares of our Common Stock, or 34% of the outstanding common shares.
After this Offering, assuming all of the shares in this Offering are sold, which there can be no guarantee, Mr. Shin will still retain 26.9% ownership and control in the Company. When factoring the preferred and common shares together, Mr. Shin will hold 94.2% pre-sale and 92.1% post-sale voting rights effectively controlling the corporation with a super-majority quorum.
Recent Reverse Split
On January 3, 2022 our board of directors approved a reverse split of 2:1 for all common shares.
Our Corporate Strategy
We intend to enter the at-home in-vitro medical testing market in the United States, with potential expansion to other markets such as Canada, Mexico, the United Kingdom and European Union.
We operate through a Joint Venture Agreement with ValueOne, Inc. of South Korea. We plan to work cooperatively to develop new intellectual property and work product to further develop our current line of at-home in-vitro testing kits. The primary location where this will occur at least initially is in Seoul, South Korea at ValueOne, Inc. of South Korea. Our company will benefit from access to this intellectual property and the exclusive license to sell, market and distribute the same. Our kits focus on the in-home diagnosis of sexually transmitted diseases.
The key elements of our growth strategy are:
Research and Development of New Products - We can leverage our relationship with our joint venture partner to tap the biotechnology pipeline from South Korea to introduce new in-vitro testing and other medical products designed for home-use to larger markets.
Entry into Strategic Partnerships - We plan to use our increased market presence in the United States to develop new strategic connections for advertising, marketing, distribution, sales and for the growth of us a brand name for home-use medical and testing products.
| | Develop Additional Revenue Streams - Our kits focus on the in-home diagnosis of sexually transmitted diseases, but with additional investment capital we can develop other testing kits for a broader range of infectious diseases. Further Develop Our Technology and Intellectual Property -We plan to use capital raised from the offering described in this prospectus to fund additional research and development of our IP portfolio and to if beneficial acquire other technology and IP from third parties consistent with our business plans and models. Promotion of Our Brand and Name Recognition -We plan to use capital raised from the offering described in this prospectus to fund development of our brand name and recognition on the internet and in the North American market place for in-vitro and home-use medical testing kits and devices. Securing Additional Capital to Finance Manufacturing and Testing Facilities-We plan to use capital raised from the offering described in this prospectus to fund future offerings of our common stock to raise additional funds for manufacturing and testing facilities although no firm plans exist at this time. |
Industry Analysis
In Vitro Diagnostics is used for the purposes of diagnosis and prognosis of diseases, determination of health conditions, determination of effectiveness of disease treatment, and prevention. It is a medical device that tests using tissue cells, blood, urine, feces, saliva, etc. collected from the human body.
In vitro diagnostic device technology is divided into a total of eight (8) sub-fields as follows, and there are differences in diagnosable diseases. Considering the global market share and average annual growth rate, among the eight sub-fields, the current main ones are immunochemical diagnostics, molecular diagnostics, point-of-care diagnostics, and self-diagnostic blood glucose measurements.
Fields | Description |
Molecular Diagnostics | DNA and RNA containing genetic information of the human body or virus are tested and used to test for immunodeficiency virus (HIV), human papilloma virus (HPV), cancer genes, and genetic diseases |
Immunochemical Diagnosis | Detects the presence of specific small molecules in a sample and uses antigen-antibody reaction to diagnose and track various diseases such as cancer markers, infectious diseases, thyroid function, anemia, allergies, pregnancy, and substance abuse. |
Point-of-Care (POCT) | It enhances the therapeutic effect by allowing the patient to immediately test what was previously tested in the fields of immunology and clinical chemistry. It is used for blood gas test, myocardial infarction test, blood coagulation test, etc. |
Self-Diagnosis of Blood Glucose Measurement | Used by diabetic patients for self-diagnosis of blood sugar levels. |
Blood Diagnosis | A test that studies blood and bone marrow. It is a field that tests blood cells such as red blood cells, white blood cells, platelets, and hemoglobin. It is used for diagnosing or treating leukemia, anemia, autoimmune disease, etc., and tracking and monitoring anticoagulation treatment. |
Clinical Microbiological Diagnosis | It is used for diagnosing and tracking diseases caused by infection by diagnosing microbial infection by testing serum, plasma, and urine, and determining the appropriate antibiotic and dosage. |
Histopathology Diagnosis | Used for diagnosing and tracking diseases caused by infection by finding the source of infection using human tissue. |
Hemostasis Diagnosis | Diagnosis by analyzing body fluids or living tissues to observe cancer tissues or cells |
Our Market Opportunity
As of 2019 the US home medical testing market was valued at $250 million and expected to double by 2024. This assessment was conducted by Cardinal Health and occurred pre-Covid. With the onset of the Covid-19 pandemic consumers were increasingly asked to perform at-home medical tests not only to determine if they were infected with the Covid-19 virus but to perform other medical tests that were unable to be performed in medical offices. Major corporations such as Amazon have sought to exploit this market. We seek to leverage access to South Korean bio-medical and technologies to exploit North American market opportunities in home medical testing.
Our Competitive Advantage
We can leverage access to the South Korean biotechnology market with strategic connections for distribution across larger markets, i.e. the United States. With access to United States capital markets we finance continued research and development of intellectual property in South Korea and the United States all with the security of shared management and mutual benefit vis a vis the Joint Venture Agreement.
Summary of Risk Factors
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flows and prospects that you should consider before making a decision to invest in our Common Stock. These risks are discussed more fully in "Risk Factors" beginning on page 15 of this prospectus. These risks include, but are not limited to, the following:
º We have a history of net losses, and we expect to continue to incur losses for the foreseeable future. If we achieve profitability, we may not be able to sustain it;
º The commercial success of our in-development and future diagnostic tests and services and our revenue growth depends upon attaining significant market acceptance among payers providers, clinics, patients, and biopharmaceutical companies;
º If we fail to retain our distribution partners and, as we grow, fail to increase our sales and marketing capabilities or develop broad awareness of our in-development and future diagnostic tests in a cost-effective manner, we may not be able to generate revenue growth;
º If we cannot maintain our current relationships, or enter into new relationships, including with biopharmaceutical companies, our revenue prospects could be reduced;
º We currently depend upon third-party suppliers and expect to depend on third party suppliers in the future, including contract manufacturers and single source suppliers, making us vulnerable to supply problems and price fluctuations;
º Natural or man-made disasters and other similar events, including the COVID-19 pandemic, may significantly disrupt our business, and negatively impact our business, financial condition and results of operations;
º Any failure to offer high-quality support for our in-development and future diagnostic tests and services may adversely affect our relationships with providers and negatively impact our reputation among patients and providers, which may adversely affect our business, financial condition and results of operations;
º We may face additional costs, loss of revenue, significant liabilities, harm to our brand, decreased use of our products or services and business disruption if there are any disruptions in our information technology systems as a result of security or data privacy breaches or other unauthorized or improper access through us or third-parties under domestic and foreign privacy and data laws;
º Our results of operations will be materially harmed if we are unable to accurately forecast customer demand for, and utilization of, our diagnostic tests and manage our inventory;
º Cost-containment efforts of our customers, purchasing groups and governmental purchasing organizations could have a material adverse effect on our future sales and profitability;
º Litigation and other legal proceedings, including costs of insurance and others, may adversely affect our business;
º Our Officers and Directors are currently employed by our joint venture partner ValueOne, Inc., of South Korea and are physically located there in Seoul.. As our business plan is implemented and our company grows we may need to hire new officers or directors who are located in the United States. This may require additional capital to fund such executive employment agreements.
º We will need to raise additional capital to fund our existing operations, develop our platform, commercialize new diagnostic tests and/or expand our operation;
º Failure to obtain or maintain adequate coverage and reimbursement for in-development or future diagnostic tests could limit our ability, and that of our collaborators, to fully commercialize our in-development or future diagnostic tests, which would decrease our ability to generate revenue;
º Healthcare reform measures could hinder or prevent the commercial success of our in-development or future diagnostic tests;
º Compliance with United States Food and Drug Administration ("FDA") regulations can be costly and time-consuming, and violations can lead to severe fines, administrative or judicial sanctions, and other penalties that could be disruptive to our business;
º
º If we are ultimately unable to obtain any necessary or desirable FDA and comparable foreign regulatory authorities' approvals or clearances, or if such approvals or clearances are significantly delayed, our business will be substantially harmed;
º Delays or failures in our clinical trials will prevent us from commercializing any in-development, modified or new diagnostic tests and will adversely affect our business, operating results and prospects;
º Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results;
º
º We and our third-party suppliers must comply with environmental, health and safety laws and regulations, which can be expensive, disruptive, and restrict how we do business;
º Our success may be impaired if we are unable to obtain, maintain and protect our intellectual property rights and confidentiality of trade secrets;
º Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our in-development and future diagnostic tests, products and services;
º We may become involved in lawsuits to protect or enforce our patents, the patents of our licensors or other intellectual property rights, which could be expensive, time consuming and unsuccessful;
º Patent terms may be inadequate to protect our competitive position on our in-development and future diagnostic tests, products and services for an adequate amount of time;
º There has been no prior public market for our Shares and an active trading market may not develop;
º If we are unable to execute our current operating plans, our results of operations may be materially adversely affected, and we may not be able to continue as a going concern, resulting in our securities having little or no value at all.
º We expect that the price of our Shares will fluctuate substantially, and you may not be able to sell the shares you purchase in this offering at or above the offering price;
º If you purchase our Shares in this offering, you will incur immediate and substantial dilution in the book value of your shares;
º A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our Shares to drop significantly, even if our business is doing well;
º Our directors, officers and principal stockholders beneficially own in the aggregate 35.4% of the Company's current outstanding Common Stock entitled to vote and may take actions that may not be in the best interests of our other stockholders;
º When factoring our Preferred Series A Stock in our cumulative voting rights, our directors, officers, and principal stockholders beneficially own in the aggregate 94.2% of the Company's total voting rights, a super-majority quorum, under Nevada law.
º The significant voting power held by the Company's directors, officers and principal stockholders may affect the market value of shares with different voting rights.
Corporate Information
We were incorporated in the State of Nevada on May 30, 2018. Our executive office is located at 30 Corporate Park, Suite 315 Irvine, CA 92606 and our telephone number is +1 (954) 253-5840. Our internet website is www.valueone.us. The information on, or that can be accessed through, our website is not part of this prospectus, and you should not rely on any such information in making the decision whether to purchase our common stock.
The Offering
Common Stock to be Sold | Up to 19,100,000 shares of our common stock representing 100% of the shares of our common stock that each of our stockholders owns and 5,000,000 shares of our common stock to be sold to qualified investors at $0.50 per share to qualified persons in connection with a financing in which we are seeking to raise $2,500,000. |
| |
Common Stock Outstanding before the Offering Common Stock Outstanding after the Offering | 19,100,000 as of November 14, 2022(1) 24,100,000 |
| |
Use of Proceeds | This is a resale prospectus to register shares of the Selling Stockholders but we may receive up to approximately $2,500,000 in gross proceeds upon the cash purchase of the additional securities to be sold. We intend to use the net proceeds from the additional securities for (i) potential mergers and acquisitions, (ii) research and development; (iii) repayment of debt and (iv) general working capital. The expected uses of the net proceeds from the exercise of the warrants represent our intentions based upon our current plans and business conditions. The precise uses, amounts and timing of the application of proceeds have yet to be determined by our management and may differ, in some or all respects, from those enumerated above. The amounts used for each purpose and timing of our actual expenditures may also vary significantly depending on numerous factors. See "Use of Proceeds." We will not receive any of the proceeds from the sale or other disposition of the securities by the Selling Stockholders. We have agreed to bear the expenses relating to the registration of the shares of our common stock held by the Selling Stockholders. See "Use of Proceeds". |
| |
Dividend Policy | We have never declared any cash dividends on our common stock. We currently intend to retain all available funds and any future earnings for use in financing the growth of our business and do not anticipate paying any cash dividends for the foreseeable future. See "Dividend Policy". |
| |
| |
Risk Factors _________________________________ | You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the "Risk Factors" section beginning on page 15 of this prospectus before deciding whether or not to invest in our common stock. ___________ |
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC registering under the Securities Act the common stock being offered under this prospectus. This prospectus, which is a part of such registration statement, does not include all of the information contained in the registration statement and its exhibits. For further information regarding us, the Selling Securityholders and our common stock, you should consult the registration statement and its exhibits.
Statements contained in this prospectus concerning the provisions of any documents are summaries of those documents and are not necessarily complete, and we refer you to the documents filed with the SEC for more information. The registration statement and any of its amendments, including exhibits filed as a part of the registration statement or an amendment to the registration statement, are available for inspection and copying as described below.
We will distribute annual reports to our stockholders, including financial statements audited and reported on by an independent registered public accounting firm. Any or all reports and other documents we will file with the SEC, as well as any or all of the documents incorporated by reference in this prospectus or the registration statement we filed with the SEC registering for sale the shares of our common stock being offered pursuant to this prospectus, are available at the SEC's website www.sec.gov, as well as our website www.valueone.us. If you do not have internet access, requests for copies of such documents should be directed to Tea Hyen Shin, the Company's CEO, at ValueOne, Inc., 30 Corporate Park, Suite 315 Irvine, CA 92606.
RISK FACTORS
Investing in our common stock involves a high degree of risk. Prospective investors should carefully consider the risks described below, together with all of the other information included or referred to in this prospectus, before purchasing shares of our common stock. There are numerous and varied risks that may prevent us from achieving our goals. If any of these risks actually occurs, our business, financial condition or results of operations may be materially adversely affected. In such case, the trading price of our common stock could decline and investors in our common stock could lose all or part of their investment.
Risks Related to Our Company
Since we have a limited operating history in our industry, it is difficult for potential investors to evaluate our business.
Our short operating history makes it difficult for potential investors to evaluate our business or prospective operations. As an early stage company, we are subject to all the risks inherent in the financing, expenditures, operations, complications and delays inherent in a new business. Accordingly, our business and success face risks from uncertainties faced by developing companies in a competitive environment. There can be no assurance that our efforts will be successful or that we will ultimately be able to attain profitability.
We have a history of operating losses and we may need additional financing to meet our future long-term capital requirements.
We have a history of losses and may continue to incur operating and net losses for the foreseeable future. We have not achieved sustainable profitability on an annual basis. We may not be able to reach a level of revenue to achieve profitability. If our revenues grow slower than anticipated, or if operating expenses exceed expectations, then we may not be able to achieve profitability in the near future or at all, which may depress our stock price.
We may not be able to raise capital when needed, if at all, which would force us to delay, reduce or eliminate our acquisition of suitable target companies and could cause our business plan to fail.
We will need substantial additional funding to pursue our acquisition of companies and business units that meet our desired standards. There are no assurances that future funding will be available on favorable terms or at all. The failure to fund our operating and capital requirements could have a material adverse effect on our business, financial condition and results of operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our acquisition strategy. Any of these events could significantly harm our business, financial condition and prospects.
We may acquire other assets or businesses, or form collaborations or make investments in other companies or technologies that could harm our operating results, dilute our stockholders' ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of businesses and assets or enter into strategic alliances and collaborations, to initiate and then expand our operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any such transaction, any of which could have a detrimental effect on our financial condition, results of operations and cash flows. We have limited experience with acquiring other companies and assets and limited experience with forming strategic alliances and collaborations. We may not be able to find suitable acquisition candidates, and if we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business and we may incur additional debt or assume unknown or contingent liabilities in connection therewith. Integration of an acquired company or assets may also disrupt ongoing operations, require the hiring of additional personnel and the implementation of additional internal systems and infrastructure, especially the acquisition of commercial assets, and require management resources that would otherwise focus on developing our existing business. We may not be able to find suitable strategic alliance or collaboration partners or identify other investment opportunities, and we may experience losses related to any such investments.
To finance any acquisitions or collaborations, we may choose to issue debt or equity securities as consideration. Any such issuance of securities would dilute the ownership of our stockholders. If the price of our Common Stock is low or volatile, we may not be able to acquire other assets or companies or fund a transaction using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
Our future success depends on our ability to retain our CEO and to attract, retain and motivate qualified personnel.
Our future business and results of operations depend in significant part upon the continued contributions of our CEO and his business acumen and strategic connections. If we lose his service or if he fails to perform in his current position, or if we are not able to attract and retain skilled personnel as needed, our business could suffer.
Because we do not have an audit or compensation committee, shareholders will have to rely on the entire Board of Directors to perform these functions.
We do not have an audit or compensation committee comprised of independent directors. Indeed, we do not have any audit or compensation committee. These functions are performed by the Board of Directors as a whole. Thus, there is a potential conflict in that board members who are also part of management will participate in discussions concerning management compensation and audit issues that may affect management decisions.
We expect to face intense competition, often from companies with greater resources and experience than we have.
To acquire qualified companies, we are likely to face competition from companies that have substantially greater financial, technological, managerial and research and development resources and experience than we have. In addition, if we are successful in closing our acquisition of one or more target companies, these acquired companies are likely to face competition for their service and product offerings from large and well-established companies that have greater marketing and sales experience and capabilities than we have. If we are unable to compete successfully, we may be unable to grow, sustain our revenue or be successful in achieving our business plan.
Current global financial conditions have been characterized by increased volatility which could negatively impact our business, prospects, liquidity and financial condition.
Current global financial conditions and recent market events have been characterized by increased volatility and the resulting tightening of the credit and capital markets has reduced the amount of available liquidity and overall economic activity. We cannot guaranty that debt or equity financing, the ability to borrow funds or cash generated by operations will be available or sufficient to meet or satisfy our initiatives, objectives or requirements. Our inability to access sufficient amounts of capital on terms acceptable to us for our operations will negatively impact our business, prospects, liquidity and financial condition.
We are growing the size of our organization, and we may experience difficulties in managing any growth we may achieve.
As of the date of this prospectus, we have one full-time employee, our CEO, Tea Hyen Shin. As our acquisition plans proceed and development and commercialization plans and strategies develop, we expect to need additional development, managerial, operational, sales, marketing, financial, accounting, legal, and other resources. Future growth would impose significant added responsibilities on members of management. Our management may not be able to accommodate those added responsibilities, and our failure to do so could prevent us from effectively managing future growth, if any, and successfully growing our Company.
Our industry is subject to rapid change, which could make our solutions and the diagnostic tests we develop and services we offer, obsolete. If we are unable to continue to innovate and improve our diagnostic tests and services, we could lose customers or market share.
Our industry is characterized by rapid changes, including technological and scientific breakthroughs, frequent new product introductions and enhancements and evolving industry standards, all of which could make our current diagnostic tests and others we are developing obsolete. Our future success will depend on our ability to keep pace with the evolving needs of our customers on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of scientific and technological advances. In recent years, there have been numerous advances in technologies relating to the diagnosis and treatment of cancer. There have also been advances in methods used to analyze very large amounts of molecular information. We must continuously enhance our offerings and develop new and improved diagnostic tests to keep pace with evolving standards of care. If we do not leverage or scale our sample and data biobank, discover new diagnostic biomarkers or applications or update our diagnostic tests to reflect new scientific knowledge, including about prostate cancer biology and other cancers in our product development pipeline, information about new cancer therapies or relevant clinical trials, our diagnostic tests could become obsolete and sales of our current diagnostic tests and any new tests we develop could decline or fail to grow as expected. This failure to make continuous improvements to our diagnostic tests to keep ahead of those of our competitors could result in the loss of customers or market share that would adversely affect our business, financial condition and results of operations. The development of new liquid biopsy technologies could negatively impact demand for our products.
Our potential for rapid growth and our entry into new markets make it difficult for us to evaluate our current and future business prospects, and we may be unable to effectively manage any growth associated with these new markets, which may increase the risk of your investment and could harm our business, financial condition, results of operations and cash flow.
Our entry into new markets as we acquire new businesses may place a significant strain on our resources and increase demands on our executive management, personnel and systems, and our operational, administrative and financial resources may be inadequate. We may also not be able to effectively manage any expanded operations or achieve planned growth on a timely or profitable basis, particularly if the number of customers using our technology significantly increases or their demands and needs change as our business expands. If we are unable to manage expanded operations effectively, we may experience operating inefficiencies, the quality of our products and services could deteriorate, and our business and results of operations could be materially adversely affected.
If we are unable to develop and maintain our brand and reputation for our service and product offerings, our business and prospects could be materially harmed.
Our business and prospects depend, in part, on developing and then maintaining and strengthening our brand and reputation in the markets we will serve for the companies we acquire. If problems arise with our future products or services, our brand and reputation could be diminished. If we fail to develop, promote and maintain our brand and reputation successfully, our business and prospects could be materially harmed.
We may not maintain sufficient insurance coverage for the risks associated with our future business operations.
Risks associated with our prospective businesses and operations include, but are not limited to, claims for wrongful acts committed by our officers, directors, and other representatives, the loss of intellectual property rights, the loss of key personnel, risks posed by natural disasters and risks of lawsuits from customers who are injured from or dissatisfied with our services. Any of these risks may result in significant losses. We cannot provide any assurance that our insurance coverage will be sufficient to cover any losses that we may sustain, or that we will be able to successfully claim our losses under our insurance policies on a timely basis or at all. If we incur any loss not covered by our insurance policies, or the compensated amount is significantly less than our actual loss or is not timely paid, our business, financial condition and results of operations could be materially and adversely affected.
Any failure to protect our future intellectual property rights could impair our ability to protect our technology and our brand.
Our success depends in part on our ability to enforce our intellectual property and other proprietary rights of the companies we expect to acquire. We expect to rely upon a combination of trademark and trade secret laws, as well as license and other contractual provisions, to protect our intellectual property and other proprietary rights. These laws, procedures and restrictions provide only limited protection and any of our intellectual property rights may be challenged, invalidated, circumvented, infringed or misappropriated. To the extent that our intellectual property and other proprietary rights are not adequately protected, third parties may gain access to our proprietary information, develop and market solutions similar to ours or use trademarks similar to ours, each of which could materially harm our business. The failure to adequately protect our intellectual property and other proprietary rights could have a material adverse effect on our business, financial condition and results of operations.
The impact of epidemics or pandemics may limit our future business both from the demand and supply sides. Our sale people may not be able to effectively engage with customers due to restrictions on travel, conferences and in-person meetings. Our supply chain may be impacted by production and distribution delays. Due to these factors we may limit future operations to reduce expenses until events support and allow normal business procedures.
Our future acquired businesses and/or operations and the businesses of our potential customers could be materially and adversely affected by the risks, or the public perception of the risks, related to a pandemic or other health crisis, such as the global outbreak of the novel coronavirus (COVID-19).
The growth of the businesses we acquire may, in part, be reliant on the willingness of customers to invest in their products and solutions. The risk, or public perception of the risk, of a pandemic or media coverage of infectious diseases could cause customers to avoid purchases which would delay sales of those products and solutions.
In addition, since we source raw materials used in our products from various U.S. suppliers, the impact of COVID-19 on these suppliers, or any of our other suppliers, distributors and resellers, or transportation or logistics providers, may negatively affect the price and availability of our ingredients and/or packaging materials and impact our supply chain. If the disruptions caused by COVID-19 continue for an extended period of time, our ability to meet the demands of our consumers may be materially impacted.
To date, we have not experienced any reduction in the available supply of our products. Additionally, many of our employees, including members of our management team, have been working remotely as a result of the closure of our offices and warehouses in compliance with local and state regulations in response to the COVID-19 pandemic. If our operations or productivity become, or continue to be, impacted throughout the duration of the COVID-19 outbreak and government-mandated closures, those occurrences may negatively impact our business, financial condition, and cash flow.
The extent to which the COVID-19 pandemic will further impact our business will depend on future developments and, given the uncertainty around the extent and timing of the potential future spread or mitigation and around the imposition or relaxation of protective measures, we cannot reasonably estimate the impact to our business at this time.
Risks Related to the Securities Markets and Ownership of our Equity Securities
The market price of our Common Stock may fluctuate, and you could lose all or part of your investment.
The price of our common stock may decline below the Offering price of the shares following this offering. The stock market in general, and the market price of our common stock will likely be subject to fluctuation, whether due to, or irrespective of, our operating results, financial condition and prospects.
Our financial performance, our industry's overall performance, changing consumer preferences, technologies, government regulatory action, tax laws and market conditions in general could have a significant impact on the future market price of our Common Stock. Some of the other factors that could negatively affect our share price or result in fluctuations in our share price include:
| * | actual or anticipated variations in our periodic operating results; |
| | |
| * | increases in market interest rates that lead purchasers of our common stock to demand a higher investment return; |
| | |
| * | changes in earnings estimates; |
| | |
| * | changes in market valuations of similar companies; |
| | |
| * | actions or announcements by our competitors; |
| | |
| * | adverse market reaction to any increased indebtedness we may incur in the future; |
| | |
| * | additions or departures of key personnel; |
| | |
| * | actions by stockholders; |
| | |
| * | speculation in the media, online forums, or investment community; and |
| | |
| * | our intentions and ability to list our Common Stock on the NYSE MKT and our subsequent ability to maintain such listing. |
| | |
You will experience immediate and substantial dilution as a result of this offering.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of our shares of Common Stock in this offering at an assumed public offering price of $0.50 per share for aggregate gross proceeds of up to $2,500,000 and after deducting the estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $0.001 per share.
We do not know whether an active, liquid and orderly trading market will develop for our securities or what the market price of our securities will be and as a result it may be difficult for you to sell your shares of our Common Stock.
There is currently an illiquid market for our securities. The lack of an active market may impair your ability to sell those securities at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your securities. Further, an inactive market may also impair our ability to raise capital by selling securities and may impair our ability to enter into collaborations or acquire companies or products by using our securities as consideration.
If we do not meet the listing standards of a national securities exchange our investors' ability to make transactions in our securities will be limited, and we will be subject to additional trading restrictions.
Our securities are not currently but will be in the near future traded over-the-counter on the OTC Markets Group, Inc. Pink Current Information or QB Market Tiers, and are not qualified to be listed on a national securities exchange, such as NASDAQ or NYSE. Accordingly, we face significant material adverse consequences, including:
A limited availability of market quotations for our securities;
A reduced liquidity with respect to our securities;
Our shares of common stock are currently classified as "penny stock" which requires brokers trading in our shares of common stock to adhere to more stringent rules, resulting in a reduced level of trading activity in the secondary trading market for our shares of common stock;
A limited amount of news and analyst coverage for our company; and
A decreased ability to issue additional securities or obtain additional financing in the future.
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as "covered securities." Since our Common Stock is traded on the OTC.PINK or OTC.QB, our common stock is a covered security. Although the states are preempted from regulating the sale of our securities, the federal statute allows the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer traded over-the-counter, our common stock would not be a covered security and we would be subject to regulation in each state in which we offer our securities.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our securities will be your sole source of gain for the foreseeable future.
Some provisions of our charter documents and Nevada law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our articles of incorporation and bylaws that will become effective in connection with consummation of this offering, as well as provisions of Nevada law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include provisions that:
Permit our Board of Directors to issue up to 1,000,000 shares of preferred stock, with any rights, preferences and privileges as it may designate, of which we have designated 1,000,000 Series A preferred stock with 10,000 votes per share, which are held by Tea Hyen Shin. These shares vote a preference to common of 200:1.
Provide that all vacancies on our Board of Directors, including as a result of newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
Not provide for cumulative voting rights, thereby allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election;
Provide that special meetings of our stockholders may be called by a majority of the Board of Directors; and
Provide that our Board of Directors is expressly authorized to make, alter or repeal the bylaws.
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors, who are responsible for appointing the members of our management. Any provision of our articles of incorporation or bylaws or Nevada law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock and could also affect the price that some investors are willing to pay for our Common Stock.
The subscription agreement for the purchase of common stock from the Company contains an exclusive forum provision, which will limit investors ability to litigate any issue that arises in connection with the offering anywhere other than the Federal courts in Nevada.
The subscription agreement states that it shall be governed by the local law of the State of Nevada and the United States, and the parties consent to the exclusive forum of the Federal courts in Nevada for any action brought under the Securities Act, the Exchange Act, or any matters derived from the subscription agreement. They will not have the benefit of bringing a lawsuit in a more favorable jurisdiction or under more favorable law than the local law of the State of Nevada. Moreover, we cannot provide any certainty as to whether a court would enforce such a provision. In addition, you cannot waive compliance with the federal securities laws and the rules and regulations thereunder as Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. The combination of both potentially unfavorable forum and the lack of certainty regarding enforceability poses a risk regarding litigation related to the subscription to this offering and should be considered by each investor before signing the subscription agreement. In addition, our exclusive forum provision may result in additional costs related to litigation of the matter for an investor.
Forward Looking Statements
This prospectus contains forward-looking statements within the meaning of the Federal securities laws. These statements relate to future events or future predictions, including events or predictions relating to our future financial performance, and are based on current expectations, estimates, forecasts and projections about us, our future performance, our beliefs and management's assumptions. They are generally identifiable by use of the words "may," "will," "should," "expect," "plan," "anticipate," "believe," "feel," "confident," "estimate," "intend," "predict," "forecast," "potential" or "continue" or the negative of such terms or other variations on these words or comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks described under "Risk Factors" that may cause the Company's or its industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. In addition to the risks described in Risk Factors, important factors to consider and evaluate in such forward-looking statements include: (i) general economic conditions and changes in the external competitive market factors which might impact the Company's results of operations; (ii) unanticipated working capital or other cash requirements including those created by the failure of the Company to adequately anticipate the costs associated with acquisitions and other critical activities; (iii) changes in the Company's corporate strategy or an inability to execute its strategy due to unanticipated changes; and (iv) the failure of the Company to complete any or all of the transactions described herein on the terms currently contemplated. In light of these risks and uncertainties, many of which are described in greater detail elsewhere in this Risk Factors discussion, there can be no assurance that the forward-looking statements contained in this prospectus will in fact transpire.
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither the Company nor any other person assumes responsibility for the accuracy and completeness of such statements. We do not undertake any duty to update any of the forward-looking statements after the date of this prospectus to conform such statements to actual results or changes in our expectations.
Emerging Growth Company Status
The Jumpstart Our Business Startups Act of 2012, or the JOBS Act, permits an "emerging growth company" such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to elect the extended transition period for complying with new or revised accounting standards applicable to public companies. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
USE OF PROCEEDS
This prospectus relates to shares of our common stock that may be offered and sold from time to time by the Selling Stockholders. We will receive no proceeds from the sale of shares of Common Stock by the Selling Stockholders in this offering. We may receive proceeds from additional shares included in the offering. The following table illustrates the amount of net proceeds of up to $2,500,000 we may receive from warrants exercised by the Selling Stockholders. It is possible that we may only receive some or none of the $2,500,000 should there be some or no exercise by the Selling Stockholders of their rights to purchase shares of our common stock under their warrants. In such case, we will reallocate our use of proceeds as the Board of Directors deems to be in the best interests of the Company to effectuate our business plan. The intended use of proceeds is as follows:
| | 100% | | | 75% | | | 50% | 25% | |
Gross Offering Proceeds | | $ | 2,500,000 | | | | $1,875,00 | | | | $1,250,000 | | $625,000 | |
Offering Costs(1) | | | $100,000 | | | | $100,000 | | | | $100,000 | | $100,000 | |
Use of Net Proceeds: | | | | | | | | | | | | | | |
Research and Development | | | $1,000,000 | | | | $750,000 | | | | $500,000 | | $250,000 | |
Acquisitions | | | $500,000 | | | | $375,000 | | | | $250,000 | | $125,000 | |
Working Capital(2) | | | $1,500,000 | | | | $1,125,000 | | | | $750,000 | | $375,000 | |
Debt Reduction | | | $0 | | | | $0 | | | | $0 | | $0 | |
(1) | We expect to spend approximately $100,000 in expenses relating to this offering, including legal, accounting, consulting, travel, printing, administrative filing fees and other miscellaneous costs. |
(2) We use working capital to pay for miscellaneous and general operating expenses, as well as legal and accounting fees, for executive compensation and core consulting services fees. |
The allocation of the use of proceeds among the categories of anticipated expenditures represents management's best estimates based on the current status of our proposed operations, plans, investment objectives, capital requirements, and financial conditions. Future events, including changes in economic or competitive conditions of our business plan or the completion of less than the total offering, may cause us to modify the above-described allocation of proceeds. Our use of proceeds may vary significantly in the event any of our assumptions prove inaccurate. We reserve the right to change the allocation of net proceeds from the Offering as unanticipated events or opportunities arise.
MARKET FOR REGISTRANT's COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Dividend Policy
We have not declared nor paid any cash dividend on our common stock, and we currently intend to retain future earnings, if any, to finance the expansion of our business, and we do not expect to pay any cash dividends in the foreseeable future. The decision whether to pay cash dividends on our common stock will be made by our board of directors, in their discretion, and will depend on our financial condition, results of operations, capital requirements and other factors that our board of directors considers significant.
DILUTION
The sale of shares of our common stock by the Selling Stockholders may have a dilutive impact on our stockholders.
Our net tangible book value as of December 31, 2021 was approximately $(30,450.00) or $(.001) per share. Net tangible book value per share is determined by dividing our total tangible assets, less total liabilities, by the number of shares of common stock outstanding as of December 31, 2021. Dilution with respect to net tangible book value per share represents the difference between the amount per share paid by the Selling Stockholders and the net tangible book value per share of common stock immediately after sale of such shares.
After giving effect to the issuance of 2,500,000 shares of our common stock to the other Selling Stockholders at the assumed average sale price of $0.50 per share, and after deducting estimated offering expenses payable by us, our as adjusted net tangible book value as of December 31, 2021 would have been approximately $(30,450.00), or $(.001) per share.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital
is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
SELLING STOCKHOLDERS
This prospectus relates to the possible resale by the Selling Stockholders of shares of our common stock that represent 100% of the shares of our common stock held by each of our stockholders and shares of common stock. Under the terms of the Stock Purchase Agreement we may sell an additional 5,000,000 common shares netting proceeds of approximately $2,500,000. We are filing the registration statement of which this prospectus is a part pursuant to the provisions of the Purchase Agreement.
The table below sets forth, to our knowledge, information concerning the beneficial ownership of shares of our common stock by the Selling Stockholders as of November 1, 2022. The percentages of shares owned before and after the offering are based on 19,100,00 of common stock outstanding. The information in the table below with respect to the Selling Stockholders has been obtained from the Selling Stockholders. solely on information supplied to us by the Selling Stockholders and assumes the sale of all the shares offered hereby. Other than as described in the footnotes below, the Selling Stockholders have not, within the past three years, had any position, office or other material relationship with us or any of our predecessors or affiliates other than as a holder of our securities, or are broker-dealers or affiliates of a broker-dealer. Information concerning the Selling Stockholders may change from time to time and, if necessary and required, we will amend or supplement this prospectus accordingly.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to shares. Unless otherwise indicated below, to our knowledge, all persons named in the table have sole voting and investment power with respect to their shares of common stock. The inclusion of any shares in this table does not constitute an admission of beneficial ownership for the person named below.
MANAGEMENT's DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion should be read in conjunction with the consolidated financial statements and the related notes contained elsewhere in this prospectus. In addition to historical information, the following discussion contains forward looking statements based upon current expectations that are subject to risks and uncertainties. Actual results may differ substantially from those referred to herein due to a number of factors, including, but not limited to, risks described in the section entitled "Risk Factors" and elsewhere in this prospectus.
General
Our executive offices are located at 30 Corporate Park, Suite 315 Irvine, CA 92606; telephone
(954) 253-5840. Our corporate website address is www.valueone.us
Overview
We are a Nevada corporation based in California operating under an Exclusive Joint Venture Agreement for the Development Ownership and Control of Intellectual Property and Work Product with a South Korean corporation, ValueOne, Inc. Our executive officers and directors are compensated by our South Korean joint venture partner and and do not have compensation plans with the Company but for Tea Hyen Shin who performs services based on an Executive Employment Agreement with a term of three years from August 2021 to August 2024. We are seeking to manufacture and distribute a proprietary line of in-vitro testing equipment designed for in home use.
Results of Operations
We are a start-up company in the early phase of development with limited operations and cash flows. Our operating history constitutes three and a half (3.5) years of losses and payment of basic expenses to maintain our corporation and prepare our business plans.
Liquidity and Capital Requirements
There is no public market for our capital stock and although we are seeking to list our common stock on the OTC Markets Group, Inc. even when listed will constitute a penny-stock and be subjected to heightened scrutiny by broker-dealers and therefore be more illiquid more difficult to deposit, clear and trade by our individual shareholders.
We are seeking to raise an additional $2.5 million dollars through this prospectus and intend on using these funds to finance our operations. If we are unsuccessful in raising additional funds we may have to obtain loans or issue debt to finance our operations.
Based on our historic operations we have low operating costs, however we expect this to increase if we become a public company subject to the periodic reporting requirements of the Securities and Exchange Act of 1934, as amended, and therefore will require additional funds to maintain basic operations.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are reasonably likely to have a current or future effect on our financial condition, revenues, result of operations, liquidity or capital expenditures.
CONDENSED COMBINED FINANCIAL STATEMENTS

The accompanying notes (Index, at F-5) are an integral part of these financial statements.
BUSINESS
Overview
We were incorporated on May 30, 2018 in the State of Nevada. We have undergone several name changes and changes of control since our incorporation. Our founder has been involved in a similar business located in Seoul, South Korea. This is our joint venture partner ValueOne, Inc. of South Korea. Together, pursuant to our Joint Venture Agreement, we intend to enter the at-home in-vitro medical testing market in the United States, with potential expansion to other markets such as Canada, Mexico, the United Kingdom and European Union.
The increasing prevalence of STDs is considered to be the major driving factor for industry growth. According to the World Health Organization (WHO), more than one million STDs are acquired every year worldwide. Thus, the demand for effective and accurate diagnostic methods is set to increase, driving market growth. Following to this, companies are actively investing in research activities to develop new testing methods for an accurate diagnosis. The commercial availability of immunochromatographic tests (ICT) all over the world is considered to create lucrative growth opportunities in this market. This testing technique is one of the most sensitive, affordable, and user-friendly advanced method of diagnosis which can provide results within 15-20 minutes.
Rising U.S. FDA approved testing kits is projected to further fuel market development. For instance, in May 2019, the U.S. FDA approved two tests that can detect the presence of Neisseria gonorrhoeae and bacteria Chlamydia trachomatis. The Xpert CT/NG and Aptima Combo 2 Assay are the two devices cleared by the regulatory body for extragenital diagnostic testing via the rectum and throat. In addition, other factors such as favorable government initiatives, growing awareness, and knowledge of sexual diseases coupled with new drug discoveries will support the overall market growth. Conversely, lack of awareness in the developing regions is predicted to impede the industry growth to some extent.
Depending upon types, the worldwide (https://sdki.jp/reports/std-diagnostics-market/53355) STD Diagnostics market is categorized into Syphilis, Chlamydia, Gonorrhea, Chancroid, Herpes Simplex Virus, Human Immunodeficiency Virus, and Human Papilloma Virus testing. Out of these, the Chlamydia testing category accounted for the highest revenue share, over 34% share in 2019. Chlamydia is the most commonly occurring STDs and can be treated by antibiotic medications such as erythromycin and azithromycin. In addition, a rise in the prevalence of this infection among pregnant women is anticipated to increase the rate of chlamydia screening, supporting the segment growth.
On the other hand, Syphilis screening is projected to grow with the fastest growth rate during the study period. As per the Centers for Disease Control and Prevention (CDC), there were more than 115,000 reported new diagnoses of all stages of syphilis in the U.S. alone and the number is increasing. The growing rate of anonymous sex, rising number of unprotected sex, sex with multiple partners and under the influence of several therapeutics will support the growth this category.
In terms of testing devices, the overall industry is broadly bifurcated into laboratory, and point
of care devices. The laboratory devices are further divided into flow cyto meters, thermal cyclers - PCR, differential light scattering machines, lateral flow readers - immune- chromatographic assays, and absorbance microplate reader- enzyme-linked immune-sorbent assay (ELISA). Of the laboratory devices, the Lateral Flow Readers category held the highest, over 26%, revenue share in 2019. Point of care (POC) devices are sub-divided into portable/rapid diagnostic kits, and phone chips (microfluidics + ICT). Among these rapid diagnostic kits accounted for the significant share and is projected to grow with promising CAGR during the forecast period. The integration of advanced technologies, coupled with the growing demand for rapid screening support the segment growth
Based on the testing location, the worldwide STD diagnostics market is divided into PoC, and laboratory testing (private labs, and public health labs). Currently, the laboratory segment captured maximum revenue share owing to the growing number of laboratories, offering STD screening. However, PoCs are anticipated to expand at the fastest growth rate due to its associated benefits compared to laboratories.
Regionally, the overall market is viewed as North America, Middle East & Africa,Asia Pacific, Latin America, and Europe.North Americais a dominating region for STD diagnostics owing to the rising prevalence of this infection coupled with favorable government policies for market development. As per the study of the National Center for Biotechnology Information (NCBI), in the U.S. only, more than 20 million sexually transmitted infections (STIs) occur every year and more than half of the cases occur between 15-24 year age group.
Conversely, the Asia Pacific region is the fastest-growing market, expanding at over 12% CAGR throughout the study scope. The rapid increase in the prevalence of sexual disorders, coupled with increasing government funding and programs are contributing to the APAC market growth.
Among the US medical device market $163.11 billion (USD 163.11 billion) by sector (020), in vitro diagnostic medical devices account for 17,8%, and STD tests such as syphilis, Chlamydia, and gonorrhea are considered post-sexual measures in the UK.
According to a report published by Allied Market Research on the 'Stand-Infectious Disease (STD) Test Market by Disease Type and Test Site: Global Opportunity Analysis and Industry Outlook, 2018-2025', the global STD test market will reach 139.57 million by 2025. Therefore, it is expected to grow at an average annual rate of 5.6% after 2018.
Proprietary In-Vitro Testing Kits
We have, though our joint venture partner, developed a line of proprietary in-vitro medical testing kits for home use to screen for various sexually transmitted diseases. We plan to facilitate the marketing and distribution of these kits in the United States, North America, the United Kingdom and European Union.
12 Month Goals
During the next twelve (12) months we hope to fully fund our offering in connection with this prospectus and launch a follow-up offering. With $2.5 million raised in investment capital we can devote sufficient resources to fund research and development, operating costs, and potential acquisitions. We plan to enter into strategic partnerships in the North American market to facilitate our brand recognition and reach with skilled parties who can assist in critical phases of our business: manufacturing, distribution, diagnostic testing, customer service, etc. If we are unable to meet are capital needs we may be forced to issue debt securities or take on loans at less than favorable rates.
Market Growth
As of 2019 the US home medical testing market was valued at $250 million and expected to double by 2024. This assessment was conducted by Cardinal Health and occurred pre-Covid. With the onset of the Covid-19 pandemic consumers were increasing asked to perform at-home medical tests not only to determine if they were infected with the Covid-19 virus but to perform other medical tests that were unable to be performed in medical offices. Major corporations such as Amazon have sought to exploit this market. We seek to leverage access to South Korean bio-medical and technologies to exploit North American market opportunities in home medical testing.
According to Fortune Business Insight, the global in vitro diagnostics market size was USD 61.2 billion in 2018 and is expected to reach $88 billion USD by 2026, growing at a CAGR of 4.5% during the forecast period.
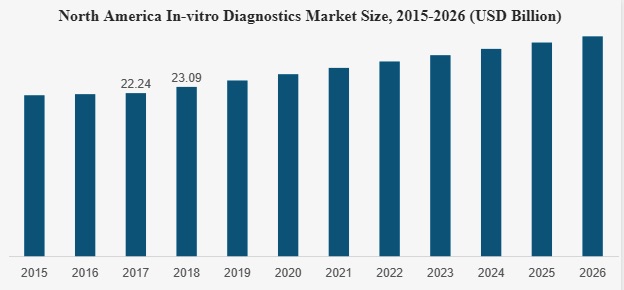
North America generated revenue of $23.09 billion in 2018 and is expected to dominate the global in vitro diagnostics market by 2026.Marketing and Growth Strategy
For operation, the South Korean parent company ValueOne, Inc. of South Korea and Value One, Inc. of Nevada share core functions and conduct business for the global market. ValueOne Korea is focused on research and development and manufacture of testing products and technologies, while ValueOne Nevada is focused on conducting sales and marketing all over the world, including the US market.
In 2021, Value One Korea applied for a patent for its technology in South Korea and signed a contract with Value One Inc for the right to use the technology in the United States and other countries. STD Test Kits, which have completed product development, are in the process of being approved by the South Korean FDA. In the meantime, ValueOne Korea has signed an LOI with an OEM suitable for the production of our products and is negotiating production conditions. Test Kits produced by this OEM manufacturer will be supplied to Value One Inc.
Approval from the South Korean FDA is expected to be completed in early 2023, and at the same time, it is scheduled to apply for approval from the US FDA. FDA approval for sales are expected to be completed by summer 2023, and sales and marketing will proceed in earnest. After FDA approval, ValueOne, Inc. will register the product with Amazon and other online retailers such as Walmart, Walgreens, Target, etc. and starts online marketing. At the same time, we will seek to develop, register, and introduce new products to the US market from our development pipeline in South Korea. For offline sales, we are recruiting a marketing expert who is proficient in the US market and build a marketing strategy and sales network.
We are now in contact with several marketing experts and negotiating terms. Among them, a strong candidate is a person who is in charge of the management of an NGO affiliated with the United Nations in the United States. We are negotiating the terms of the contract for the marketing manager in the US, and it is proceeding positively, so the contract is expected to be completed in 2023.
The first target of offline sales is a Group Purchasing Organization (GPO) in the United States, which will negotiate products and prices after FDA approval. Vizient in Irving Texas, Premier in Charlotte North Carolina, HealthTrust in Nashville Tennessee, Intalere in St. Louis Missouri, which are purchasing South Korea's Covid Test Kit, are top-of-the-line GPOs.
Sales to South Korea, Japan, Southeast Asia, Africa, the Middle East, and Europe are currently being negotiated in advance with several distributors, and in particular, contracts in Africa, the Middle East and Japan are expected to be concluded first.
We are negotiating with the Suez Canal Special Economic Zone management company for overseas sales of our STD Test Kits, and we are also reviewing the diabetes check kit and HIV kit, which are under development. The items under discussion are the Middle East, Europe and Africa, and after sales for a certain period of time, the company wants to transfer technology to a pharmaceutical company in the Suez Canal Special Economic Zone. After the technology transfer, the company is also negotiating to manufacture and produce directly and pay royalties. A contract is expected in early 2023.
We are negotiating with IVAYA BIO & INDUSTRY, a Korean agent for the Japanese Ministry of Health, Labor and Welfare, and our STD Test KIT 15,000 sets per month and HIV KIT 15,000pcs per month to supply and supply our products and other companies' products. BIO & INDUSTRY is requesting supply from Malaysia. Our STD Test KIT completed the test by providing a sample to IVAYA BIO & INDUSTRY, and when FDA approval is completed, we will sign a contract and supply it from 2Q. Prostitutes registered with the Japanese Ministry of Health, Labor and Welfare are being tested for sexually transmitted diseases and AIDS once a month with a PCR test. As the incidence of sexually transmitted diseases increases, the Ministry of Health, Labor and Welfare is trying to more effectively manage prostitutes by testing them once a week with our Self Test Kit.
In 2023, we plan to open a US sales office in Orange County, California and open Southeast Asia ValueOne in Ho Chi Minh, Vietnam to outsource regional marketing and low-cost product development and OEM production From the beginning stage of product development in collaboration with medical institutions, the opinions of specialists in the field were reflected in the design of the product. Initially, it was decided to do an OEM in a specialized manufacturing company located in South Korea that produces medical devices by verifying, inspecting, and managing safety and effectiveness. The company has secured competitiveness with stable quality, low defect rate, and low supply price by establishing an efficient production system.
In the future, we plan to build our own intelligent production plant that improves productivity, quality, and customer satisfaction by applying information and communication technology (ICT) combined with digital automation solutions to production processes such as design, development, manufacturing, and distribution and logistics. We plan to develop products that do not fall under telemedicine and apply them to Smart Factory build to have a small-volume production system.
Current Testing Kits in Development Pipeline
These products are being developed by ValueOne South Korea and will be released in 2022/2023. As their exclusive joint venture partner we will have access to all kits upon approval with appropriate regulatory agencies in the United States and South Korea.
1. Three (3) types of Vaginitis Test Kit -Trichomonas vaginalis: belongs to Trichomonas vaginalis, has flagella at the tip of the cell, accumulates leukocytes in the vagina, the site of infection, and secretes a large amount of purulent vaginal secretions. -Candida albicans: As a shipping yeast-type fungus belonging to the Ascomycete family, it causes skin mycoses due to infection by Candida or infects the vagina, mouth, and rectum to cause infections. -Gardnerellavaginalis: Bacterial vaginitis with a thin cell wall and intermediate characteristics between gonorrhea and microplasma.
2. HIV (Human Immunodeficiency Virus) tester A virus that invades the human body and destroys the immune function of the human body and causes Acquired Immune Deficiency Syndrome (AIDS). Symptoms such as fever, fatigue, headache, weight loss, and loss of appetite appear during the stage of transition to AIDS after the incubation period.
3. HPV-DNA (human papillomavirus) test kit
Most of the HPV infection spreads to the vagina and anus through sexual contact and is transformed into abnormal cells, causing high-risk types (HPV16 and 18) cervical cancer, vaginal cancer, and vulvar cancer. Most people infected with HPV do not have symptoms and do not know they are infected. In connection with this test kit, HPV-DNA (human papillomavirus) test reagent and beta-amyloid protein diagnostic reagent for dementia diagnosis will be additionally developed.
4. Lung cancer 3 types tester kit
When lung cancer develops, certain biochemical substances in the blood increase. This is a diagnostic kit that tests this substance by rapid test (POCT, RAPID). 1) Cyfra-21 test kit : In the case of lung cancer (small cell), the concentration of biochemical substances in the blood is checked. 2) NSE (Neuro-specific enolase) test kit: Check the blood biochemical concentration of lung cancer and neuroendocrine tumors. 3) A test kit that tests Cyfra-21 and NSE at once.
5. Myopathy genetic tester and test reagent
Myopathy is a serious and rare intractable disease designated by the United Nations. It is a chronic, progressive disease that is caused by mutations and gradually becomes incapacitated by the failure of muscle cells to be created beyond the gene for synthesizing muscle proteins.
6. Allergy Test Kit
Externally harmless substances such as dust mites or pollen. It is a kit that tests unnecessary immune and hypersensitivity reactions to antigens.
7. Allergy diagnosis equipment and reagents
Related to cells that protect the body from IgE antibodies or substances that are dangerous to our body. It is a diagnostic device that examines substances present in the bloodstream.
8. Arterial Blood Gas Analysis Diagnostic Device.
This is a test diagnostic device that determines whether the oxygen and carbon dioxide levels in the blood are imbalanced or there is an acid-base imbalance by examining the lung function to see if the exchange of oxygen and carbon dioxide is smooth when there are symptoms of respiratory difficulty. This device diagnoses respiratory and metabolic disorders and kidney disease with arterial blood gas test, ABGA, PH, PO2, PCO2, bicarbonate, and HCO3.
9. Myocardial infarction diagnostic device.
Cardiovascular angiography is used to find infarct vessels and diagnose the extent and site of stenosis, and diagnose non-infarct vessel stenosis. Heart-specific troponin and creatinine kinase (CK-MB) are confirmed by blood tests at the site, and myocardial infarction is diagnosed when the levels are elevated.
10. Point of care testing (POCT) diagnostic device
This is a diagnostic device that measures various health indicators such as blood sugar, electrolyte concentration, cardiovascular markers, cholesterol, drug levels, urinalysis, infectious disease, organ function, and immune response. The POCT portable equipment performs the same tests as the in vitro diagnostic (IVD) system, while enabling precise analysis with fewer body fluid samples and reagents and faster tests. POCT diagnostic equipment is combined with laboratory equipment to increase the convenience and speed of use. It is a method that greatly reduces contamination and errors by saving time and reducing the inconvenience of handling samples by putting sample preparation, cell lysis, nuclear phase purification, amplification, and detection in one cartridge. By reducing the components to the cartridge, it must be made into a compact, performance and durable product, with precision and chemical inactivation. It automates the precise movement of samples and reagents in the cartridge based on hydrodynamics, and increases the reliability and accuracy of the test.
Government Regulation
Our business is subject to governmental regulation through the Food and Drug Administration (FDA) in the United States and South Korea as well as state regulators in the United States.
Healthcare reform measures could hinder or prevent the commercial success of our diagnostic tests.
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system in ways that may harm our future revenues and profitability and the demand for our diagnostic tests. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. Current and future legislative proposals to further reform healthcare or reduce healthcare costs may limit coverage of or lower reimbursement for the procedures associated with the use of our diagnostic tests.
The effect of any healthcare reform initiative implemented in the future could impact our revenue from the sale of our diagnostic tests. For example, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the "ACA"), contains a number of provisions, including those governing enrollments in federal healthcare programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government healthcare programs and will result in the development of new programs.
There have been judicial challenges to certain aspects of the ACA, as well as efforts by Congress to repeal, replace or alter the implementation of certain aspects of the ACA. Most recently, the Supreme Court of the United States granted certiorari on March 2, 2020, for a Texas District Court case, which invalidated the ACA in its entirety because he concluded that the individual mandate was unconstitutional. Although the Supreme Court of the United States reversed the holding and remanded the case back to the Texas District Court, it is unclear how the Texas District Court will rule, as it is unclear how any future decisions, subsequent appeals, and other efforts to challenge, repeal, or replace, or alter the implementation of the ACA will affect our business, financial condition and results of operations.
Congress may continue to pursue significant changes to the current healthcare laws. We face uncertainties that might result from modifications or repeal of any of the provisions of the ACA, including as a result of current and future executive orders and legislative actions. The impact of those changes on us and potential effect on the medical device industry as a whole is currently unknown. Any changes to the ACA are likely to have an impact on our results of operations, and may negatively affect our business, financial condition and results of operations. We cannot predict what other healthcare programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States on our business, financial condition and results of operations.
The continuing efforts of the government, insurance companies, managed care organizations and other payers of healthcare services to contain or reduce costs of healthcare may harm:
- our ability to set a price that we believe is fair for our diagnostic tests;
- our ability to generate revenue and achieve or maintain profitability; and
- the availability of capital.
The ACA substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacts our industry. Future changes in healthcare policy could increase our costs and subject us to additional regulatory requirements that may interrupt commercialization of our current and future solutions. Future changes in healthcare policy could also decrease our revenue and impact sales of and reimbursement for our in development and future diagnostic tests.
We must comply with healthcare fraud and abuse laws.
Various federal and state laws, as well as the laws of foreign countries, prohibit payments to induce the referral, purchase, order or use of healthcare products or services and require medical device companies to limit prevent, and/or monitor, and report certain payments to third-party payers, health care professionals, and other individuals. These healthcare fraud and abuse anti-kickback, public reporting and aggregate spend laws affect our sales, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with cancer treatment providers, hospitals, physicians or other potential purchasers or users, including patients, of medical devices and services. In particular, these laws influence, among other things, how we structure our sales offerings, including discount practices, customer support, education and training programs and physician consulting and other service arrangements. These laws prohibit certain marketing initiatives that are commonplace in other industries. If we were to offer or pay inappropriate inducements for the purchase, order or use of our diagnostic tests or our services, or our arrangements are perceived as inappropriate inducements, we could be subject to claims under various healthcare fraud and abuse laws.
Restrictions under applicable United States federal and state healthcare laws and regulations include the following:
- the federal Anti-Kickback Statute, a criminal law, prohibits, among other things, persons and entities from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, leasing, ordering, or arranging for, referring, or recommending the purchase, lease, order of any good or service for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid;
- the Eliminating Kickbacks in Recovery Act, which prohibits knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in return for the referral of a patient to, or in exchange for an individual using the services of certain entities, including laboratories, if the services are covered by a health care benefit program;
- the Beneficiary Inducement Statute, which prohibits any person, organization, or entity from giving anything of value to a federal health care program beneficiary that is likely to induce or influence the beneficiary's choice of provider, practitioner, or supplier for covered services;
- the federal civil False Claims Act, which may be enforced through civil whistleblower or qui tam actions and is often used to enforce the federal Anti-Kickback Statute and other healthcare laws and regulations, imposes civil penalties and potential exclusion from federal healthcare programs, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or for making a false record or statement material to an obligation to pay the federal government or for knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government;
- ederal criminal statutes created by the Health Insurance Portability and Accountability Act of 1996 ("HIPAA") impose criminal liability for, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private insurance plans, or, in any matter involving a healthcare benefit program, for knowingly and willfully making materially false, fictitious, or fraudulent statements in connection with the delivery of or payment for health care benefits; and
- analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers.
Other federal and state laws, as well as the laws of foreign countries, generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payments to government or commercial payers that are false or fraudulent, or for items or services that were not provided as claimed. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of product candidates and medical devices from government-funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. Moreover, any investigation into our practices could cause adverse publicity and require a costly and time-consuming response. If any physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
Manufacturers can also be held liable under these laws if they are deemed to "cause" the submission of false or fraudulent claims by providing inaccurate billing or coding information to customers, by providing improper financial inducements, or through certain other activities. We attempt to ensure that any billing and coding information we provide for our diagnostic tests emphasizes the need for physicians and other providers to make independent judgments, use accurate and appropriate billing and coding that complies with all applicable payer policies, and document the medical need for their patients as appropriate. Nevertheless, the government may not regard any billing errors that may be made by our customers as inadvertent and may examine our role in providing information to our customers, physicians and patients concerning the benefits and potential coverage of more frequent therapy.
The regulatory clearance or approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming, and unpredictable. If we are ultimately unable to obtain any necessary or desirable regulatory approvals or clearances, or if such approvals or clearances are significantly delayed, our business will be substantially harmed.
The time required and ability to obtain clearance or approval by the FDA and comparable foreign regulatory authorities is unpredictable, typically takes several years following the commencement of clinical studies, and depends upon numerous factors, including the type, complexity, and novelty of our products. In addition, policies, laws, regulations, or the type and amount of clinical data necessary to gain clearance or approval may change during the course of a test's clinical development and may vary among jurisdictions, which may cause delays in the clearance or approval of, or the decision not to approve, an application. Regulatory authorities have substantial discretion in the premarket review process and may refuse to accept any application, decide that our data are insufficient for clearance or approval, require additional clinical or other data, or determine that our manufacturing and quality systems are insufficient or in violation of applicable requirements. Even if we believe our data are sufficient to support regulatory approval, regulatory authorities may disagree that approval is warranted, or may require the generation and submission of additional data or data analyses and significantly delay approval.
Before a new medical device, or a new intended use of, claim for, or significant modification to an existing device, can be marketed in the United States, a company must first submit an application for and receive 510(k) clearance pursuant to a premarket notification submitted under Section 510(k) of the Federal Food, Drug, and Cosmetic Act (FDCA), de-novo classification, or "PMA" approval from the FDA, unless an exemption applies. Products that are approved through a PMA application generally need prior FDA approval before modifications can be made that affect safety or effectiveness, and certain modifications to a 510(k)-cleared device may also require FDA premarket review before the modified product can be marketed. We have not applied for regulatory clearance or approval for any of our products, and it is possible that we will never obtain regulatory clearance or approval.
FDA or other regulators can delay, limit, or deny premarket clearance or approval of a product for many reasons, including but not limited to the following:
- FDA or comparable foreign regulatory authorities may disagree with the design, implementation, or results of, or interpretation of the data from, our clinical studies;
- FDA or comparable foreign regulatory authorities may determine that our product has not been shown to be safe and effective or has other characteristics that preclude us from obtaining marketing authorization or prevent or limit its commercial use (for example, a narrowed indication for use claim);
- the population studied in the clinical program may not be sufficiently broad, generalizable, or representative of the intended target population of our product to assure effectiveness and safety in the population for which we seek authorization or clearance;
- FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from clinical studies or may fail to accept data from clinical studies (or clinical sites), including if we fail to establish the integrity of our data;
- serious or unexpected adverse effects or other performance issues are identified with our products;
- FDA or comparable foreign regulatory authorities may determine that our manufacturing or quality system fails to comply with applicable regulations or otherwise fails to meet the standards necessary to support approval; and
- the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
FDA regulation of our industry generally or our tests specifically could be disruptive to our business.
Our operations are subject to extensive federal, state, local and foreign laws and regulations, including FDA laws and regulations, all of which are subject to change. These laws and regulations are complex and are subject to interpretation by the courts and by government agencies. We believe that we are in material compliance with all statutory and regulatory requirements applicable to us, but there is a risk that one or more government agencies could take a contrary position, or that a private party could file suit under the qui tam provisions of the federal False Claims Act or a similar state law. Such occurrences, regardless of their outcome, could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations, and other private third-party payers.
In the United States, before we can market a new medical device, or a new use of, or claim for, an existing product, we must first receive either 510(k) clearance, PMA approval or approval of a de-novo application from the FDA, unless an exemption applies.
Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
* design, development and manufacturing;
* testing, labeling, including directions for use, processes, controls, quality assurance, packaging, storage, distribution, installation and servicing;
* pre-clinical studies and clinical trials;
* establishment registration and listing;
* test kit safety and effectiveness;
* marketing, sales and distribution;
* recordkeeping procedures;
* advertising and promotion;
* premarket authorization (510(k), PMA, de-novo, Emergency Use Authorization (EUA));
* corrections and removals and recalls;
* post-market surveillance, including reporting of deaths or serious injuries, and malfunctions that, if they were to recur, would be likely to cause or contribute to a death or serious injury; and
* product import and export.
Competition
We face stiff competition and high barriers to market entry. Medical testing kits require technical and scientific expertise and are subject to government regulations. Research and Development of such devices is costly and time consuming. Large competitors are already competing in these markets that we intend to focus on in North America and South Korea.
Intellectual Property and Other Proprietary Rights
We regard our domain names and similar intellectual property as critical to our success. We rely on a combination of laws and contractual restrictions with our employees, customers, suppliers, affiliates and others to establish and protect our proprietary rights. Despite these precautions, it may be possible for a third party to copy or otherwise obtain and use our intellectual property without authorization. We cannot assure you that others will not independently develop similar intellectual property. In addition, effective trademark protection may not be available or may not be sought by us in every country in which our products and services are made available online, including the United States.
From time to time, we may be subject to legal proceedings and claims in the ordinary course of our business, including claims of alleged infringement of the trademarks and other intellectual property rights of third parties by us.
Third parties may, in the future, recruit our employees who have had access to our proprietary technologies, processes and operations. These recruiting efforts expose us to the risk that such employees will misappropriate our intellectual property.
Litigation may be necessary in the future to enforce our intellectual property rights, to protect our trade secrets or to determine the validity and scope of the proprietary rights of others. Any litigation, regardless of outcome or merit, could result in substantial costs and diversion of management and technical resources, any of which could materially harm our business.
COVID-19 Regulations
On March 19, 2020, Governor Gavin Newsom issued a stay at home order to protect the health and well-being of all Californians and to establish a consistent approach across the state to slow the spread of COVID-19. This order went into effect on March 19, 2020 and was rescinded on June 15, 2021. The prior order identified certain services as essential, including food, prescriptions, and healthcare. These services were allowed to continue despite the stay at home order. Because cannabis is an essential medicine for many residents, licensees were permitted to operate so long as their operations complied with local rules and regulations. In response to Governor Newsom's emergency declaration regarding COVID-19, BCC licensees that were unable to comply with specific regulatory requirements were able to request relief from specific licensing requirements pursuant to section 5038 of the Bureau's regulations. Numerous retailers requested and were granted relief from certain regulation to perform curbside pickup for cannabis and cannabis product sales. In light of the uncertainty regarding the spread of COVID-19 variants, some form of the prior stay-at-home order could be reinstituted in California in the future.
Employees
As of March 31, 2021, we have 0 full time employees. None of our employees or personnel are represented by a labor union, and we consider our employee/personnel relations to be good. Competition for qualified personnel in our industry is intense, particularly for software development and other technical staff. We believe that our future success will depend in part on our ability to attract, hire and retain qualified personnel.
Properties
We do not own any properties. We currently rent an office in Irvine, California at a monthly cost of $500 per month on a month-to-month basis ("Company Headquarters"). We believe that the Orange County facility is currently adequate for the purposes of our operations.
Legal Proceedings
From time to time we may be named in claims arising in the ordinary course of business. Currently, there are no legal proceeding that are pending against us or involves us that, in the opinion of our management, could reasonably be expected to have a material adverse effect on our business or financial condition.
MANAGEMENT
Set forth below is certain information regarding our executive officers and directors. Each of the directors listed below was elected to our board of directors to serve until our next annual meeting of stockholders or until his or her successor is elected and qualified. All directors hold office for one-year terms until the election and qualification of their successors. The following table sets forth information regarding the members of our board of directors and our executive officers:
The following tables set forth certain information regarding the beneficial ownership of our common stock as the date of this Registration Statement by (i) each person who, to our knowledge, owns more than 5% of our common stock; (ii) each of our directors and executive officers; and (iii) all of our executive officers and directors as a group. Unless otherwise indicated in the footnotes to the following tables, each person named in the table has sole voting and investment power. The address of each of the directors and executive officers listed below is c/o Agro Capital Management Corp., 2620 Regatta Drive, Suite 102, Las Vegas, Nevada 89128; Tel: (702) 560-2430.
For purposes of this table, there are 19,100,000 shares of common stock subject to options or warrants currently exercisable or exercisable within 60 days of the date of this report are deemed outstanding. Investment decisions are made by Tea Hyen Shin with an address of 30 Corporate Park, Suite 315 Irvine, CA 92606.
Class of Securities | Name and Address | Number of Shares | % of % of Class Total Votes |
Common | Tea Hyen Shin(1) 30 Corporate Park, Suite 315 Irvine, CA 92606 | 6,500,000 | 34% 34% |
| | | | |
Series A Preferred(2) | Tea Hyen Shin(1) 30 Corporate Park, Suite 315 Irvine, CA 92606 | 1,000,000 | 100% 100% |
| | | | |
Common | Seung Hwan Jang(1) 30 Corporate Park, Suite 315 Irvine, CA 92606 | 275,000 | 1.4% 1.4% |
| | | | |
Common | All Officers and Directors as a Group (four persons) | 6,775,000 | 35.4% 35.4% |
(1) Officer and/or director of the Company.
(2) The Series A Preferred have voting rights equal to 200 shares of common stock for each share of Series A Preferred stock. Mr. Shin holds 94.2% pre-sale and 92.1% post-sale (if fully funded) of the Company's cumulative voting rights effectively controlling the corporation with a super-majority quorum.
DIRECTORS AND EXECUTIVE OFFICERS
The following persons are the executive officers and directors of our Company:
Name | | Age | | Position(s) |
Tea Hyen Shin | | 54 | | Chief Executive Officer; and Chairman |
Hyun Il Choi | | 64 | | Director |
Seuong Hwan Jang | | 56 | | Director, Secretary |
Ki Hyoung You | | 56 | | Director |
| | | | |
TEA HYEN SHIN
Mr. Shin has served as our Chief Executive Officer and member of our board of directors since inception. He has been an entrepreneur in South Korea since 2000 and has been involved in various start-up ventures in a variety of industries including but not limited to electronic and communications products, software, bio technology and business consulting in addition to forming various joint ventures. Mr. Shin has experience doing business overseas and in the United States. He can speak and write in both Korean and English.
In 2009, he joined Catch By Gene as Vice President and was dedicated to financing and listing in the US to develop and grow the company. Catch By Gene is a development stage biotechnology company focused on developing and commercializing therapeutic and diagnostic products for the treatment of the hepatitis B virus ("HBV") and detection of the human papillomaviruses ("HPV"). The two products developed by the Company thus far include their CBG HPV-DNA Qualitative assay System and CBG HPV-DNA Identification assay System. Catch By Gene had signed the reverse merger agreement with Clinicares Inc (CCRC.PK), a Nevada Corporation. The Company was traded on the Pinksheets under the symbol CBYG after transaction.
In 2007, BSK & Tech Co., Ltd, which requires Mr Shin's business skills and experience in the US public offering, invited him as Vice President. BSK has Business manufacturing, R&D of Stereoscopic 3 Dimensional Camera Chip and Module, and LCD panels. Company entered into a Merger Agreement with Integrated Software Development Inc.(ISDV.PK), a Nevada Corporation on January, 2008. The symbol was changed and traded as "BSKT".
He started his own business in 2000 by participating in the founding of MB Tech Co., Ltd as co-CEO. In the two years since its establishment, MB Tech Inc has achieved outstanding results, such as developing excellent products, acquiring patents, and contracting with satellite broadcasting companies in North America. The company has achieved most of its sales thus far in Canada with both Star-Choice and Bell. Based on such achievements, Company became listed on the US OTCBB market traded by "MBTT".
Since 1998, he has worked for 3 years as a chief manager of LNB department in at CTI Semiconductor Co. Ltd., an electric device manufacturer. His academic background is in Mechanical Engineering from Choong Buk University.
After graduating from the University, he joined Dongguk Group, a chaebol company in 1990, and started his career as a purchasing manager for 9 years.
HYUN IL CHOI
Mr. Choi is an expert in this field with the experience and knowledge to develop and commercialize Value One technology. From 2014 to 2020, he served as Head of Research Center at TCM biosciences Inc. His main work is Research & development for invitro diagnostic reagents of HPV, STD, COVID19.
Beginning in 2001 and for a period for three years thereafter, he was a senior researcher at PPD biosciences Inc., focusing on invitro diagnostic reagents of HPV, STD and HBV. Before working at the company, he served as a research professor at Yonsei University, a famous Korean medical university, for 6 years from 2005. He holds a Ph.D in Biochemistry from Yonsei University, a bachelors of science degree from Korea University in genetic engineering as well as a masters degree in the same subject. Mr. Choi previously acted as Chief Researcher at the Pathology Research Institute. He also serves as a research professor at the Health Science Institute at Yonsei University.
KI HYOUNG YOU
Mr. You has served as an executive officer since the founding of Value One. Before he joined Value One, he served as Director of Hanedul Co., Ltd., a development business company, since August 2017. He established a plan for the construction of the Cheongju Guksa Industrial Complex located in Cheongju-si, Chungcheongbuk-do, Republic of Korea and made a proposal to Cheongju-si. Since then, he has successfully negotiated with local landowners to promote the project, and the project has been successfully awarded.
As a result of this achievement, he was appointed as the CEO of Hanhaedul Co., Ltd. and is in the process of civil engineering work for the establishment of this industrial complex. Mr You is currently performing both the executive duties of Value One and Hanhae's management roles.
From July 2002 to August 2017, he served as Director of Real Estate Division of Jae Sang Law Firm. His main duties include internal operation and risk management, win-win cooperation and negotiation with the residents of the development area, government relationship , dispute resolution, and other fields. He is the owner of the ability to smoothly carry out the planned work of the company based on various experiences.
His first job began in 1989 at Samsung Group. He worked for Samsung Life Insurance, an affiliated company, for over 10 years, providing support for the organization's operations, such as sales management, welfare benefits, and purchasing. Through Samsung's specialized training system, well-organized management work, and systematic organizational experience, he had acquired the ability to demonstrate excellent competencies in his later career.
He earned a bachelors degree from Pyeong Taek University majoring in Business Administration in 1986.
SEOUNG HWAN JANG
Mr. Jang was appointed BOD of Value One in January 2021 and is responsible for sales and marketing. Since 2019, he has established and operated Facite FM Global, which is mainly engaged in coin development and mining.
In 2004, he joined 21C VISION Co., Ltd., a marketing Company, and implemented and operated the education and marketing establishment and sales system. In 2006, he was selected as a marketing director for Hanwoori General Foods, where he established the company's new marketing system with a suitable product planning and effective sales network.
In 2008, he served as Division Chief in the Travel Division of Cherry CNC Co., Ltd. His main duties included development and marketing of travel products. Then in 2010, he joined Mohave, a network marketing company specializing in household goods and home medical devices, where he worked in product development, distribution network, and marketing system. He has extensive experience working in a variety of marketing roles, including food, education, and travel. So, with his experience and ability, he can contribute a lot to Value One's sales and marketing in Korea. Mr. Jang majored in Marketing at Nam Seoul University and graduated in 1985.
Board of Directors and Corporate Governance
When considering whether directors have the experience, qualifications, attributes and skills to enable the Board of Directors to satisfy its oversight responsibilities effectively in light of our business and structure, the Board of Directors focuses primarily on the information discussed in each of the directors' individual biographies as set forth above. With regard to Messrs. Benson and Hicks, the Board considered their day-to-day operational leadership of our company and in-depth knowledge of our business and experience in corporate management that will assist our corporate governance.
The Board of Directors periodically reviews relationships that directors have with our company to determine whether the directors are independent. Directors are considered "independent" as long as they do not accept any consulting, advisory or other compensatory fee (other than director fees) from us, are not an affiliated person of our company or our subsidiaries (e.g., an officer or a greater than 10% stockholder) and are independent within the meaning of applicable United States laws, regulations and the Nasdaq Capital Market listing rules. In this latter regard, the Board of Directors uses the Nasdaq Marketplace Rules (specifically, Section 5605(a)(2) of such rules) as a benchmark for determining which, if any, of our directors are independent, solely in order to comply with applicable SEC disclosure rules.
The Board of Directors has determined that none of our directors is independent within the meaning of the Nasdaq Marketplace Rules cited above, and that we do not have an audit committee financial expert as that term is defined by listing standards of the national securities exchanges and SEC rules, including the rules relating to the independence standards of an audit committee and the non-employee director definition of Rule 16b-3 under the Securities Exchange Act of 1934.
Director or Officer Involvement in Certain Legal Proceedings
Our directors and executive officers were not involved in any legal proceedings as described in Item 401(f) of Regulation S-K in the past ten years.
Directors and Officers Liability Insurance
We do not have directors' and officer' liability insurance. Our officers and directors have indemnification rights under applicable laws, and our articles of incorporation and bylaws.
Committees of the Board of Directors
Currently, our Board of Directors acts as audit, nominating, corporate governance and compensation committees. The Board of Directors has adopted charters relative to its audit committee, compensation committee and nominating committee. Until such time as we add more members to the Board, the entire Board will determine all matters and no committees have been formed. We intend to appoint persons to the board of directors and committees of the board of directors as required to meet the corporate governance requirements of a national securities exchange, although we are not required to comply with these requirements until we are listed on a national securities exchange. We intend to appoint directors in the future so that we have a majority of our directors who will be independent directors, and of which at least one director will qualify as an "audit committee financial expert," prior to a listing on a national securities exchange.
Audit Committee
The audit committee's duties under the terms of its charter are to recommend to our board of directors the engagement of independent auditors to audit our financial statements and to include the terms of its charter review our accounting and auditing principles. The audit committee reviews the scope, timing and fees for the annual audit and the results of audit examinations performed by independent public accountants, including their recommendations to improve the system of accounting and internal controls. The audit committee oversees the independent auditors, including their independence and objectivity. However, the committee members are not acting as professional accountants or auditors, and their functions are not intended to duplicate or substitute for the activities of management and the independent auditors. The audit committee is empowered to retain independent legal counsel and other advisors as it deems necessary or appropriate to assist the audit committee in fulfilling its responsibilities, and to approve the fees and other retention terms of the advisors. The audit committee members possess an understanding of financial statements and generally accepted accounting principles.
Compensation Committee
The compensation committee has certain duties and powers as described in its charter, including but not limited to periodically reviewing and approving our salary and benefits policies, compensation of our executive officers, administering our stock option plans, and recommending and approving grants of stock options under those plans.
Nominating Committee
Under the charter of our nominating and corporate governance committee, the nominating and corporate governance committee considers and makes recommendations on matters related to the practices, policies and procedures of the board of directors and takes a leadership role in shaping our corporate governance. As part of its duties, the nominating and corporate governance committee assesses the size, structure and composition of the board of directors and its committees, coordinates evaluation of board performance and reviews board compensation. The nominating and corporate governance committee also acts as a screening and nominating committee for candidates considered for election to the board of directors.
Compensation Committee Interlocks and Insider Participation
None of our directors or executive officers serves as a member of the board of directors or compensation committee of any other entity that has one or more of its executive officers serving as a member of our board of directors.
Code of Ethics
We have adopted a written code of ethics that applies to all of our directors, officers and employees in accordance with the rules of the SEC, FINRA, and OTC. We will post a copy of our code of ethics on our website, and intend to post amendments to this code, or any waivers of its requirements, as well.
Conflicts of Interest
We comply with applicable state law with respect to transactions (including business opportunities) involving potential conflicts. Applicable state corporate law requires that all transactions involving our company and any director or executive officer (or other entities with which they are affiliated) are subject to full disclosure and approval of the majority of the disinterested independent members of our Board of Directors, approval of the majority of our stockholders or the determination that the contract or transaction is intrinsically fair to us. More particularly, our policy is to have any related party transactions (i.e., transactions involving a director, an officer or an affiliate of our company) be approved solely by a majority of the disinterested independent directors serving on the Board of Directors. We expect to have at least three independent directors serving on the Board of Directors and intend to maintain a Board of Directors consisting of a majority of independent directors.
Indemnification of Directors and Executive Officers
Section 145 of the Nevada Revised Statutes provides for, under certain circumstances, the indemnification of our officers, directors, employees and agents against liabilities that they may incur in such capacities. Below is a summary of the circumstances in which such indemnification is provided.
In general, the statute provides that any director, officer, employee or agent of a corporation may be indemnified against expenses (including attorney' fees), judgments, fines and amounts paid in settlement, actually and reasonably incurred in a proceeding (including any civil, criminal, administrative or investigative proceeding) to which the individual was a party by reason of such status. Such indemnity may be provided if the indemnified person's actions resulting in the liabilities: (i) were taken in good faith; (ii) were reasonably believed to have been in or not opposed to our best interests; and (iii) with respect to any criminal action, such person had no reasonable cause to believe the actions were unlawful. Unless ordered by a court, indemnification generally may be awarded only after a determination of independent members of the Board of Directors or a committee thereof, by independent legal counsel or by vote of the stockholders that the applicable standard of conduct was met by the individual to be indemnified.
The statutory provisions further provide that to the extent a director, officer, employee or agent is wholly successful on the merits or otherwise in defense of any proceeding to which he or she was a party, he or she is entitled to receive indemnification against expenses, including attorney' fees, actually and reasonably incurred in connection with the proceeding.
Indemnification in connection with a proceeding by us or in our right in which the director, officer, employee or agent is successful is permitted only with respect to expenses, including attorney' fees actually and reasonably incurred in connection with the defense. In such actions, the person to be indemnified must have acted in good faith, in a manner believed to have been in our best interests and must not have been adjudged liable to us, unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability, in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expense which the Court of Chancery or such other court shall deem proper. Indemnification is otherwise prohibited in connection with a proceeding brought on our behalf in which a director is adjudged liable to us, or in connection with any proceeding charging improper personal benefit to the director in which the director is adjudged liable for receipt of an improper personal benefit.
Nevada law authorizes us to reimburse or pay reasonable expenses incurred by a director, officer, employee or agent in connection with a proceeding in advance of a final disposition of the matter. Such advances of expenses are permitted if the person furnishes to us a written agreement to repay such advances if it is determined that he or she is not entitled to be indemnified by us.
The statutory section cited above further specifies that any provisions for indemnification of or advances for expenses does not exclude other rights under our certificate of incorporation, by-laws, resolutions of our stockholders or disinterested directors, or otherwise. These indemnification provisions continue for a person who has ceased to be a director, officer, employee or agent of the corporation and inure to the benefit of the heirs, executors and administrators of such persons.
The statutory provision cited above also grants us the power to purchase and maintain insurance policies that protect any director, officer, employee or agent against any liability asserted against or incurred by him or her in such capacity arising out of his or her status as such. Such policies may provide for indemnification whether or not the corporation would otherwise have the power to provide for it.
At present, we do not maintain director' and officer' liability insurance in order to limit the exposure to liability for indemnification of directors and officers, including liabilities under the Securities Act; however, we are in the process of obtaining such insurance.
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the cash and non-cash compensation awarded to or earned by: (i) each individual who served as the principal executive officer and principal financial officer of the Company during the years ended December 31, 2021 and 2020; and (ii) each other individual who received more than $100,000 or 1,000,000 shares of stock in the form of salary and bonus during such year. For purposes of this report, these individuals are collectively the "named executive officers" of our Company.
Name and Position(s) | Years | Salary | Bonus | Stock Awards | Options Awards | Non-equity incentive plan compensation | Non-qualified deferred compensation earnings | All other compensation | Total |
Tea Hyen Shin, Chairman and CEO | 2021 | $0.00 | $0.00 | 5,500,000 | | | | | $0.00 |
2020 | $0.00 | $0.00 | | | | | | $0.00 |
2018 | $0.00 | $0.00 | 1,000,000 | | | | | $0.00 |
Employment and Advisory Agreements
Tea Hyen Shin is currently performing services under a three (3) year agreement for executive services. His base rate is $0.00 per year and 1,000,000 common shares for the term with board of directors discretion to award performance based bonuses of cash or stock or options/warrants for the purchase of stock.
Equity Compensation Plan Information
The Company has no formal plan at this time. Any equity compensation is reviewed, voted on, and approved by the Board of Directors.
PRINCIPAL SECURITYHOLDERS
The following table sets forth certain information as of November 14, 2022, the beneficial ownership of our common stock by the following persons:
| * | each person or entity who, to our knowledge, owns more than 5% of our common stock; |
| * | our executive officers named In the Summary Compensation Table above; |
| * | all of our executive officers and directors as a group. |
Unless otherwise indicated in the footnotes to the following table, each person named in the table has sole voting and investment power and that person's address is c/o ValueOne, Inc., 30 Corporate Park, Suite 315 Irvine, CA 92606. Shares of common stock subject to options, warrants, or other rights currently exercisable or exercisable within 60 days of the date of this prospectus, are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the stockholder holding the options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other stockholder.
Class of Securities | | Name and Address | | # of Common Shares | | | % of Class | |
| | | | | | | | |
Common | | Tea Hyen Shin (1) | | | 6,500,000 | | | | 34.0 | % |
| | | | | | | | | | |
Common | | Ki Hyoung You (1) | | | 0 | | | | 0.0 | % |
| | | | | | | | | | |
Common | | Hyun Il Choi(1) | | | 0 | | | | 0.0 | % |
| | | | | | | | | | |
Common | | Seung Hwan Jang(1) | | | 275,000 | | | | 1.4 | % |
| | | | | | | | | | |
Common | | All Officers and Directors as a Group (five persons) | | | 6,775,000 | | | | 35.4 | % |
| (1) | Officer and/or director of our Company. |
We have agreed to keep such registration effective until all shares of common stock can be sold without registration pursuant to Rule 144 under the Securities Act.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
During the past three years, there have been no transactions, whether directly or indirectly, between the Company and any of its officers, directors or their family members other than three (3) share issuances to the two (2) daughters and one (1) son of Mr. Tea Hyen Shin our founder: (1) Bo Ra Shin 500,000 shares; (2) Bo Ran Shin 500,00 shares; and Seung Ha Shin 500,000 shares each transaction occurring on or about May 10, 2021. See items 3, 4 and 5 in the shareholders list include herein.
DESCRIPTION OF SECURITIES
Authorized Capital Stock
Our authorized capital stock consists of 200,000,000 shares of common stock, par value $0.001 per share, and 20,000,000 shares of "blank check" Preferred Stock, par value $0.001 per share. As of the date of this prospectus, there were 19,100,000 shares of common stock issued and outstanding and 1,000,000 shares of Series A Preferred Stock issued and outstanding.
Issued and Outstanding Capital Stock
The issued and outstanding securities of the Company on the date of this prospectus are as follows:
| * | 19,100,000 shares of common stock; |
| * | 1,000,000 shares of preferred "series A" stock; |
| * | 0 options or warrants; and |
| * | 0 convertible debt instruments. |
Description of Common Stock
The holders of common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of common stock that are present in person or represented by proxy. Except as otherwise provided by law, amendments to the articles of incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of common stock. Our Articles of Incorporation do not provide for cumulative voting in the election of directors. The common stockholders will be entitled to such cash dividends as may be declared from time to time by the Board from funds available. Upon liquidation, dissolution or winding up of the Company, the common stockholders will be entitled to receive pro rata all assets available for distribution to such holders.
Description of Preferred Stock
We are authorized to issue up to 20,000,000 shares of preferred stock, par value $0.001 per share. There are 1,000,000 shares of Series A Preferred Stock are issued and outstanding held by Tea Hyen Shin. These shares vote at a preference of 200:1 or stated differently the 1,000,000 shares of preferred stock vote with the equivalent power of 200,000,000 shares of common stock. Calculated cumulatively with his common shares this gives Tea Hyen Shin over 94% voting control of the Company.
All shares of the designated and the undesignated preferred stock are issuable on such other terms and conditions as the Board may determine at or prior to issuance, without further action of the stockholders. Such preferred shares may or may not be issued in series, convertible into shares of common stock, redeemable by the Company and entitled to cumulative dividends. Other terms and conditions may be imposed at the time of issuance. Should some or all of the outstanding or future issues of any convertible preferred stock be exchanged for shares of common stock, the resulting increase in the number of issued and outstanding common stock may or may not have a depressive effect on the market value of our common stock.
Unless specifically issued without such rights, the holders of preferred stock are entitled to one vote for each share held on all matters submitted to a vote of shareholders. Future issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could be used to discourage an unsolicited acquisition proposal. For instance, the issuance of a series of preferred stock might impede an acquisition or other business combination by including class voting rights that would enable the holder to block such a transaction or facilitate a business combination by including voting rights that would provide a required percentage vote of the stockholders. In addition, under certain circumstances, the issuance of preferred stock could adversely affect the voting power of the holders of our common stock.
Although our Board of Directors is required to make any determination to issue such stock based on its judgment as to the best interests of our shareholders, our Board of Directors could act in a manner that would discourage an acquisition attempt or other transaction that some, or a majority, of the shareholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then market price of such stock. The Board of Directors does not at present intend to seek stockholder approval prior to any issuance of currently authorized stock, unless otherwise required by law or stock exchange rules. We have no present plans to issue any preferred stock.
The Board has designated 1,000,000 shares of Series A Preferred Stock. The holders of the Series A Preferred Stock are entitled to vote at a ratio of 200: 1 with respect to common shareholders on all matters submitted to a vote of the stockholders, including the election of directors. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of common stock that are present in person or represented by proxy. Holders of the Series A Preferred Stock are preferred as to dividends and upon liquidation to the holders of common stock. The holders of Series A Preferred Stock have certain protective provisions, including a prohibition against the Company authorizing shares of capital stock with equal or superior rights to the Series A Preferred Stock and certain additional protective rights to prevent the authorization of additional shares of Series A Preferred Stock or to alter any of the rights of the Series A Preferred Stock unless approved by a majority of the holders of the Series A Preferred Stock.
Except as otherwise provided by law, amendments to the articles of incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of common stock. Our Articles of Incorporation do not provide for cumulative voting in the election of directors. The common stockholders will be entitled to such cash dividends as may be declared from time to time by the Board from funds available. Upon liquidation, dissolution or winding up of the Company, the common stockholders will be entitled to receive pro rata all assets available for distribution to such holders.
Transfer Agent
Our transfer agent is Transfer Online 512 SE Salmon Street, Portland, Oregon 97214, Tel: +1-503-227-2950 or www.transferonline.com.
Blank Check Preferred Stock
The ability to authorize "blank check" preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our Company.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Nevada Revised Statutes ("NRS") Section 145 provides us with the power to indemnify any of our directors, officers, employees and agents. The person entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was in, or not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have had reasonable cause to believe that his conduct was unlawful.
Under NRS section 145, advances for expenses may be made by agreement if the director or officer affirms in writing that he has met the standards for indemnification and will personally repay the expenses if it is determined that such officer or director did not meet those standards.
Our bylaws include an indemnification provision under which we have the power to indemnify our directors, officers, former directors and officers, employees and other agents (including heirs and personal representatives) against all costs, charges and expenses actually and reasonably incurred, including an amount paid to settle an action or satisfy a judgment to which a director or officer is made a party by reason of being or having been a director or officer of the Company. Our bylaws further provide for the advancement of all expenses incurred in connection with a proceeding upon receipt of an undertaking by or on behalf of such person to repay such amounts if it is determined that the party is not entitled to be indemnified under our bylaws. No advance will be made by the Company to a party if it is determined that the party acting in bad faith. These indemnification rights are contractual, and as such will continue as to a person who has ceased to be a director, officer, employee or other agent, and will inure to the benefit of the heirs, executors and administrators of such a person.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
SHARES ELIGIBLE FOR FUTURE SALE
We have a limited public market for our common stock and a limited number of shares in the public float. Sales of substantial amounts of our common stock in the public market resulting from this offering could adversely affect the prevailing market price and our ability to raise capital in the future.
As of the date of this prospectus, we have 19,100,000 shares of common stock issued and outstanding. Upon the completion of this offering, we will have outstanding up to 24,100,000 common shares with an additional 5,000,000 shares. Of these outstanding shares, all 24,100,000 shares sold by us in the Offering will be freely tradable without restriction or further registration under the Securities Act, unless these shares are purchased by "affiliates" as that term is defined in Rule 144 under the Securities Act. Of the 19,1000,000 shares of our common stock outstanding prior to the completion of this offering and held by existing stockholders, approximately 19,000,000 shares are currently free trading and the remaining are "restricted securities" as that term is defined in Rule 144 under the Securities Act all but approximately 100,000 of which have been held for more than one year. Restricted shares may be sold in the public market only if registered or if they qualify for exemption under Rule 144 or 701 promulgated under the Securities Act, which rules are summarized below, or another exemption.
Rule 144
In general, under Rule 144, as currently in effect, a person who owns shares that were acquired from us or one of our affiliates at least six months prior to the proposed sale is entitled to sell, within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
| * | One percent of the number of shares of common stock then outstanding, which will equal approximately 241,000 shares immediately after this offering; or |
| * | The average weekly trading volume of the common stock on a national securities exchange during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
| * | In addition to these volume limitations, sales of unregistered shares of our common stock in reliance on Rule 144 may only be made by affiliates if such sales: |
| * | are preceded by a notice filing on Form 144; |
| * | are limited to broker's transactions, as such term is defined under Section 4(a)(4) of the Securities Act; and |
| * | only occur at a time when current public information about us is available, which generally would require that we are not delinquent with any of our reports required pursuant to Sections 13 or 15(d) of the Exchange Act. Rule 144 also provides that our affiliates who sell shares of our common stock that are not restricted shares must nonetheless comply with the same restrictions applicable to restricted shares, with the exception of the holding period requirement. |
Under Rule 144, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell such shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144. If the non-affiliate has held the shares for at least one year, then the shares may be sold without regard to the public information provisions of Rule 144. Therefore, unless otherwise restricted, shares held by non-affiliates may be sold immediately upon the expiration of the lock-up agreements.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, consultants or advisors who acquire shares from us in connection with a compensatory stock or option plan or other written agreement will be eligible to resell such shares 90 days after the effective date of this offering in reliance of Rule 144, but without compliance with certain restrictions, including the holding period, contained in Rule 144.
Penny Stock Rules
Broker-dealer practices in connection with transactions in penny stocks are regulated by certain penny stock rules adopted by the SEC. Penny stocks generally are equity securities with a price of less than US $5.00. Penny stock rules require a broker- dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer's account. In addition, the penny stock rules generally require that prior to a transaction in a penny stock, the broker-dealer make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules. Our shares may in the future be subject to such penny stock rules in which care our stockholders would, in all likelihood, as a result of the penny stock rules, find it difficult to sell their securities.
PLAN OF DISTRIBUTION
.
This is a self-underwritten ("best-efforts") offering. This prospectus is part of a registration statement that permits our officers and directors to sell the shares being offered by the Company directly to the public, with no commission or other remuneration payable to them for any shares they may sell. Presently, we expect that our officers and directors will personally contact existing shareholders, friends, family members and business acquaintances and inform them about the offering. In addition, we may market the offering to institutional investors through our officers and directors. We may also offer our shares of common stock through brokers, dealers or agents, although we have no current plans or arrangements to do so. The Company has been contacted by multiple financial institutions, as well as fielded interest from existing shareholders that give the Company assurance as to the marketability of its shares to these identified parties. This offering will terminate on the date which is 270 days from the effective date of this prospectus, although we may close this offering on any date prior if the offering is fully subscribed or upon the vote of our board of directors.
In offering the securities on our behalf, our officers and directors will rely on the safe harbor from broker dealer registration set forth in Rule 3a4-1 under the Exchange Act. The officers and directors will not register as broker-dealers pursuant to Section 15 of the Exchange Act, in reliance upon Rule 3a4-1, which sets forth those conditions under which a person associated with an issuer may participate in the offering of the Issuer's securities and not be deemed to be a broker-dealer. In that regard, we confirm that:
| a. | None of our officers or directors are subject to a statutory disqualification, as that term is defined in Section 3(a)(39) of the Exchange Act; |
| b. | None of our officers or directors will be compensated in connection with their participation by the payment of commissions or other remuneration based either directly or indirectly on transactions in the common stock; |
| c. | None of our officers or directors is or will be, at the time of his participation in the offering, an associated person of a broker-dealer; and |
| d. | Our officers and directors meet the conditions of paragraph (a)(4)(ii) of Rule 3a4-1 of the Exchange Act, in that each (A) primarily perform substantial duties for or on our behalf, other than in connection with transactions in securities, and (B) is not a broker or dealer, or has been an associated person of a broker or dealer, within the preceding 12 months, and (C) has not participated in selling and offering securities for any issuer more than once every 12 months other than in reliance on Paragraphs (a)(4)(i) or (a)(4)(iii) of Rule 3a4-1. |
None of our officers or directors, control persons or affiliates intend to purchase any shares in this offering.
LEGAL MATTERS
John E. Dolkart, Jr., Esq. of Dolkart Law PC located at 100 Pine Street, Suite 1250 San Francisco, CA 94111, will pass upon the validity of the shares of our common stock to be sold in this offering.
EXPERTS
The financial statements of the Company as of and for the years ended December 31, 2021 and 2020, included in this prospectus have been audited by Michael Gillespe and Associates PLCC., an independent registered public accounting firm as set forth in their report, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The SEC maintains an internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, will file periodic reports, proxy statements and other information with the SEC. We also maintain a website at www.valueone.us. Upon completion of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
VALUEONE, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page |
| |
Report of Independent Registered Public Accounting Firm for ValueOne,a> Inc. | F-2 |
Consolidated Balance Sheets - December 31,2021 and 2020. | F-3 |
Consolidated Statements of Stockholder' Equity (Deficit) for the years ended December 31, 2021 and 2020 | F-4 |
Notes to Consolidated Financial Statements | F-5 |
| |
| |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
(included at Exhibit 2.1)
F-2
VALUEONE, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| December 31, 2021 | December 31, 2020 |
| | |
ASSETS |
| | |
CURRENT ASSETS | | |
Cash | $ 40,118 | $ 2,354 |
| | |
TOTAL CURRENT ASSETS | $ 40,118 | $2,354 |
| | |
LIABILITIES AND STOCKHOLDER's (DEFICIT) |
| | |
CURRENT LIABILITIES | | |
Other liabilities | - | >800 |
Due to related party | 9,600 | 5,800 |
| | |
TOTAL CURRENT LIABILITIES | 9,600 | 6,600 |
| | |
| | |
COMMITMENTS AND CONTINGENCIES STOCKHOLDER's (DEFICIT) | | - |
Preferred stock Authorized 20,000,000 shares of preferred stock. $0.001 par value. Issued and outstanding Preferred series A stock: 1,000,000 shares (December 31, 2020 - 0) Common stock | 1,000 | - |
Authorized | | |
200,000,000 shares of common stock, $0.001 par value, | | |
Issued and outstanding | | |
19,100,000 shares of common stock (December 31, 2020 - 1,000,000) | 19,100 | 1,000 |
Additional paid in capital | 28,700 | 1,000 |
Accumulated deficit | (18,282) | (6,246) |
| | |
TOTAL STOCKHOLDER's (DEFICIT) | 30,518 | (4,246) |
| | |
TOTAL LIABILITIES AND STOCKHOLDER's (DEFICIT) | $ 40,118 | $ 2,354 |
| | |
| | |
The accompanying notes are an integral part of these financial statements.
F-3
VALUEONE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share data)
| For the year ended December 31, 2021 | For the year ended December 31, 2020 | |
| | | |
REVENUE | $- | $- | |
| | | |
OPERATING EXPENSES | | | |
General and administrative | $ 6,131 | $ 1,791 | |
Professional fees | 5,905 | 2,300 | |
| | | |
TOTAL OPERATING EXPENSES | (12,036) | (4,091) | |
| | | |
NET LOSS | (12,036) | (4,091) | |
| | | |
| | | | | |
NET LOSS PER COMMON SHARE - BASIC AND DILUTED | $ (0.00) | $ (0.00) |
| | | |
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED | 10,900,274 | 1,000,000 |
VALUEONE, INC.
CONSOLIDATED STATEMENTS OF
STOCKHOLDER' EQUITY (DEFICIT)
(in thousands, except share data)
| Preferred stock | Common Stock | Additional | | |
Number of shares | Amount | Number of shares | Amount | Paid-in Capital | Accumulated Deficit | Total |
| | | | | | | |
Balance, December 31, 2019 | | | 1,000,000 | $ 1,000 | $ 1,000 | $ (2,155) | >$ (155) |
Net loss for the year ending December 31, 2020 | | | - | - | - | (4,091) | (4,091) |
| | | | | | | |
Balance, December 31, 2020 | | | 1,000,000 | $ 1,000 | $ 1,000 | $ (6,246) | $ (4,246) |
May 10, 2021 issued for service | | | 2,000,000 | 2,000 | 2,000 | | 4,000 |
May 26, 2021 issued for cash @$0.0015 | | | 10,500,00 | 10,500 | 21,000 | | 31,500 |
Preferred stock Issued for Cash | 1,000,000 | 1,000 | | | | | 1,000 |
August 1, 2021 issued for compensation | | | 5,000,000 | >5,000 | 5,000 | | 10,000 |
August 1, 2021 issued for salary | | | 500,000 | 500 | (400) | | 100 |
October 11, 2021 issued for service | | | 100,000 | 100 | 100 | | 200 |
| | | | | | | |
Net loss for the year ending December 31, 2021 | | | - | - | - | (12,036) | -(12,036) |
| | | | | | | |
-Balance, December 31, 2021 | -1,000,000 | -1,000 | 19,100,000 | $ 19,100 | $ 28,700 | $ (18,282) | $ 30,518 |
F-4
VALUEONE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the year ending December 31, 2021 | For the year ending December 31, 2020 |
| | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | |
| Net loss for the period | $ (12,036) | $ (4,091) |
| Adjustments to reconcile net loss to net cash used in operating activities | | |
| Common stock issued for service | 14,300 | - |
| Changes in operating assets and liabilities | | |
| Accrued expenses | 3,000 | (120) |
| | | |
| | | |
| NET CASH USED IN OPERATING ACTIVITIES | 5,264 | (4,211) |
| | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | - | - |
| | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | |
| Advances from related party | - | 4,115 |
| Proceeds from sales of preferred stock | 1,000 | |
| Proceeds on sale of common stock | 31,500 | - |
| | | |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 32,500 | 4,115 |
| | | |
| NET INCREASE IN CASH | 37,764 | (96) |
| | | |
| CASH, BEGINNING OF PERIOD | 2,354 | 2,450 |
| | | |
| CASH, END OF PERIOD | $ 40,118 | $ 2,354 |
| | | |
F-5
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 - NATURE OF OPERATIONS AND BASIS OF PRESENTATION
ValueOne, Inc. was incorporated in the State of Nevada as a for-profit Company on May 30, 2018 and adopted the calendar year-end. The Company amended its articles of incorporation on May 10, 2021 increasing its total authorized common shares from two million (2,000,000) at $0.001 par value to two hundred million (200,000,000) at $0.001 par value with twenty million (20,000,000) preferred shares at par value of $0.001.
On May 19, 2021, one million (1,000,000) shares of Series A Preferred Shares are designated with a par value of $0.001, which shall carry voting rights senior to the Common Stock and will not be subject to any reverse splits or deductions. The Preferred Series A shall rank senior to the Common Stock as to dividends and upon liquidation, dissolutions or winding up.
The Company is the joint-venture partner of ValueOne, Inc. of South Korea who holds valuable intellectual property in the medical and biometric testing industry. ValueOne, Inc. intends to develop, market, and distribute medical testing devices and new technologies with exclusive rights to do so in North America.
Going Concern
The financial statements have been prepared, assuming that the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business. To date the Company has generated no revenues from its business operations and has incurred operating losses since inception of $18,282. For the twelve months ended on December 31, 2021, the Company has an operating loss of $12,036.
The Company will require additional funding to meet its ongoing obligations and to fund anticipated operating losses. The ability of the Company to continue as a going concern is dependent on raising capital to fund its initial business plan and ultimately to attain profitable operations. Accordingly, these factors raise substantial doubt as to the Company's ability to continue as a going concern.
The Company intends to continue to fund its business by way of private placements and advances from related parties as may be required. These financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts and classification of liabilities that might result from this uncertainty.
The Company plans to increase working capital by managing its cash flows and expenses, securing financing and increasing revenue. The Company continues to pursue additional channel distribution expansion for its cannabis products to provide even broader access to consumers. Risks include, but are not limited to, the uncertainty of availability of additional financing and the uncertainty of achieving future profitability. Management of the Company intends to raise additional funds through the issuance of equity securities. Failure to generate sufficient cash flows from operations, raise additional capital or reduce certain discretionary spending could have a material adverse effect on the Company's ability to achieve its intended business objectives. These factors raise substantial doubt about the Company's ability to continue as a going concern. The consolidated financial statements do not contain any adjustments that might result from the resolution of any of the above uncertainties.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America ("GAAP"). The accompanying financial statements do not include the accounts of ValueOne, Inc. of South Korea which is merely a joint venture partner and not part of a consolidated holding company corporation. All intercompany transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses recognized during the reported period. Actual results could differ from those estimates.
Reclassification
Certain prior year amounts have been reclassified to conform to the current year presentation. Deferred rent and deferred revenue have been included in accrued liabilities and other current liabilities. The amounts for the prior periods have been reclassified to be consistent with the current year presentation and have no impact on previously reported total assets, total stockholder' deficit or net loss.
Fair Value Measurements
The Company recognizes and discloses the fair value of its assets and liabilities using a hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to valuations based upon unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to valuations based upon unobservable inputs that are significant to the valuation (Level 3 measurements). Each level of input has different levels of subjectivity and difficulty involved in determining fair value.
* Level 1 - Inputs used to measure fair value are unadjusted quoted prices that are available in active markets for the identical assets or liabilities as of the reporting date.
* Level 2 - Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability.
* Level 3 - Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
Fair Value of Financial Instruments
Carrying amounts of certain of the Company's financial instruments, including cash and cash equivalents, prepaid and other current assets, accounts payable, accrued expenses and other liabilities and related party payables approximate fair value due to their short maturities.
Property and Equipment
Property, plant, and equipment is stated at cost less accumulated depreciation and amortization. Depreciation and amortization are recognized using the straight‑line method. Repairs and maintenance costs are expensed as incurred. Estimated useful lives in years are as follows:
Description | | Estimated Useful Life |
Furniture | 5 - 7 |
Laboratory equipment | 3 - 5 |
Computer and equipment | 3 - 5 |
Software | 5 |
Revenue Recognition
The Company recognizes revenue from the sales of consumer-packaged goods upon delivery of those goods to its customers in fulfillment of customer orders on a First-in First-out FOB destination basis. Occasionally, the Company also wholesales it products in bulk quantities to other licensed manufacturers or performs manufacturing as a service on behalf of other licensed manufacturers in some cases taking ownership of the inventory and in other cases without taking ownership of the underlying inventory.
Cost of Goods Sold
Costs of goods sold includes direct costs related to the sale of the Company's cannabis products, write-downs of excess and obsolete inventories and amortization of intangible assets.
Shipping and Handling Costs
Shipping and handling costs are expensed as incurred and are included in cost of goods sold.
Research and Development Expenses
Research and development expenses are expensed as incurred and consist primarily of personnel costs, including salaries, benefits, and stock - based compensation, consulting, materials, supplies, and facilities and other overhead allocations.
Marketing Expenses
The Company expenses the costs of marketing, including promotional expenses, as incurred. No marketing expenditures were made in 2020 or 2021.
Income Taxes
The Company accounts for income taxes using the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established to reduce deferred tax assets when management estimates, based on available objective evidence, that it is more likely than not that the benefit will not be realized for the deferred tax assets.
The Company's policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. No interest expense was recognized during the periods presented.
Stock‑Based Compensation
The Company will recognize stock-based compensation in the future for equity awards on a straight-line basis over their vesting periods based on the grant date fair value. The Company estimates the fair value of stock options granted using the Black-Scholes pricing model. This model also requires subjective assumptions, including future stock price volatility and expected time to exercise, which greatly affect the calculated values. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
Comprehensive Loss
Comprehensive loss is the change in equity of an enterprise, except those resulting from stockholder transactions. Accordingly, comprehensive loss includes certain changes in equity that are excluded from net loss. For the year ended December 31, 2020 and year ended December 31, 2019, the Company's comprehensive loss is equal to net loss. There were no components of other comprehensive loss for any of the periods presented.
Net Loss Per Share
Basic net loss per share attributable to common stockholders is calculated based on the weighted-average number of shares of the Company's common stock outstanding during the period. Diluted net loss per share attributable to common stockholders is calculated based on the weighted-average number of shares of the Company's common stock outstanding and other dilutive securities outstanding during the period. The potential dilutive shares of common stock resulting from the assumed exercise of outstanding stock options, warrants and the assumed conversion of preferred stock are determined under the treasury stock method.
Recent Accounting Pronouncements
The Company has implemented all new accounting pronouncements that are in effect and that may impact its financial statements and does not believe that there are any other new pronouncements that have been issued that might have a material impact on its financial position or results of operations except as noted below:
In February 2020, the FASB issued ASU 2020-02, Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842) - Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic 842), which amends the effective date of the original pronouncement for smaller reporting companies. ASU 2016-13 and its amendments will be effective for the Company for interim and annual periods in fiscal years beginning after December 15, 2022. The Company believes the adoption will modify the way the Company analyzes financial instruments, but it does not anticipate a material impact on results of operations. The Company is in the process of determining the effects adoption will have on its consolidated financial statements.
NOTE 3 - COMMON STOCK AND PREFERRED STOCK
The Company's capitalization is two hundred million (200,000,000) common shares at $0.001 par value and twenty million (20,000,000) preferred shares at par value of $0.001. The Company's outstanding capitalization as of the end of December 31, 2021 are 19,100,000 (pre-split 38,200,000) common shares at $0.002 (pre-split $0.001)5000000 par values per share. with 1,000,000 preferred Series A shares at $0.001 par value.
On May 10, 2021, the Company issued 1,500,000 (pre-split 3,000,000) common shares at $0.002 (pre-split $0.001) per share to the ValueOne, Inc. South Korea for the exclusive agreement to use its IP in North America.
On May 10, 2021, the Company issued 500,000 (pre-split 1,000,000) common shares at $0.002 (pre-split $0.001) par value to Black Canyon Consulting, LLC for services rendered.
On May 26, 2021, the Company issued 10,500,000 (pre-split 21,000,000) common shares at $0.003 (pre-split $0.0015) per share to the 44 South Korean individuals.
On August 1, 2021, the Company issued 5,000,000 (pre-split 10,000,000) common shares at $0.002 (pre-split $0.001) per share to the sole director and President of the Company as stock compensation and decrease the loan.
On August 1, 2021, the Company issued 500,000 (pre-split 1,000,000) common shares at $0.0002 (pre-split $0.0001) per share to the sole director and President of the Company as salary.
On October 11, 2021 the Company issued 50,000 (pre-split 100,000) common shares at $0.002 (pre-split $0.001) par value to Capital Advisory, Inc. for services rendered.
On October 11, 2021 the Company issued 50,000 (pre-split 100,000) common shares at $0.002 (pre-split $0.001) par value to Caledonia Capital, Inc. for services rendered.
On May 26, 2021 the Company issued 1,000,000 preferred Series A shares at $0.001 par value to the sole director and President of the Company.
NOTE 4 - RELATED PARTY TRANSACTIONS
As of December 31, 2021, and 2020, the total amount owed to the CEO is $9,600 and $5,800. The amounts due to the related party are unsecured and non- interest-bearing with no set terms of repayment.
NOTE 5 - INCOME TAXES
A reconciliation of the provision for income taxes at the United States federal statutory rate compared to the Company's income tax expense as reported is as follows:
| | December 31, 2021 | | December 31, 2020 |
| | | | |
Net loss before income taxes per financial statements | $ | $ (7,736) | | (4,091) |
Income tax rate | | 21% | | 21% |
Income tax recovery | | (1,625) | | (859) |
Non-deductible | | -- | | - |
Valuation allowance change | | 1,625 | | 859 |
| | | | |
Provision for income taxes | | $- | | - |
The significant component of deferred income tax assets on December 31, 2021 and 2020, is as follows:
| | December 31, 2021 | | December 31, 2020 |
Net operating loss carry-forward | | $ 2,937 | | 1,312 |
Valuation allowance | | (2,937) | | (1,312) |
| | | | |
Net deferred income tax asset | | $- | | |
The amount taken into income as deferred income tax assets must reflect that portion of the income tax loss carryforwards that is more likely-than-not to be realized from future operations. The Company has chosen to provide a full valuation allowance against all available income tax loss carryforwards. The Company has recognized a valuation allowance for the deferred income tax asset since the Company cannot be assured that it is more likely than not that such a benefit will be utilized in future years. The valuation allowance is reviewed annually. When circumstances change and which cause a change in management's judgment about the realizability of deferred income tax assets, the impact of the change on the valuation allowance is generally reflected in current income.
As of December 31, 2021 the Company has no unrecognized income tax benefits. The Company's policy for classifying interest and penalties associated with unrecognized income tax benefits is to include such items as tax expense. No interest or penalties have been recorded during the year ended December 31, 2020 and no interest or penalties have been accrued as of December 31, 2021. As of December 31, 2021, the Company did not have any amounts recorded pertaining to uncertain tax positions.
The tax years from 2019 and forward remain open to examination by federal and state authorities due to net operating loss and credit carryforwards. The Company is currently not under examination by the Internal Revenue Service or any other taxing authorities.
NOTE 6 - SUBSEQUENT EVENTS
The Company has evaluated all events that occurred after the balance sheet date of December 31, 2021 through the date the financial statements were issued. The Company determined that it did not have any reportable subsequent events but for a 1-for-2 reverse common stock split, on January 3, 2022.
OUTSIDE BACK COVER OF PROSPECTUS
We have not authorized any dealer, salesperson or any other person to give any information or to represent anything other than those contained in this prospectus in connection with the offer contained herein, and, if given or made, you should not rely upon such information or representations as having been authorized by ValueOne, Inc. This prospectus does not constitute an offer of any securities other than those to which it relates or an offer to sell, or a solicitation of an offer to buy, to those to which it relates in any state to any person to whom it is not lawful to make such offer in such state. The delivery of this prospectus at any time does not imply that the information herein is correct as of any time after the date of this prospectus.
DEALER PROSPECTUS DELIVERY REQUIREMENT
Until _______________, 2023 [90 days from the date of this prospectus], all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
VALUEONE, INC.
24,100,000 Shares
Common Stock
PROSPECTUS
November 14, 2022
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth the costs and expenses payable by us in connection with the issuance and distribution of the securities being registered. None of the following expenses are payable by the Selling Securityholders. All of the amounts shown are estimates, except for the SEC registration fee.
SEC registration fee | | $ | * | |
Legal fees and expenses | | | * | |
Accounting fees and expenses | | | * | |
Miscellaneous | | | * | |
TOTAL | | $ | * | |
| | | | |
*To be filed by amendment | | | | |
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Nevada Revised Statutes ("NRS") Section 145 provide us with the power to indemnify any of our directors, officers, employees and agents. The person entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was in, or not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have had reasonable cause to believe that his conduct was unlawful.
Under NRS section 145, advances for expenses may be made by agreement if the director or officer affirms in writing that he has met the standards for indemnification and will personally repay the expenses if it is determined that such officer or director did not meet those standards.
Our bylaws include an indemnification provision under which we have the power to indemnify our directors, officers, former directors and officers, employees and other agents (including heirs and personal representatives) against all costs, charges and expenses actually and reasonably incurred, including an amount paid to settle an action or satisfy a judgment to which a director or officer is made a party by reason of being or having been a director or officer of the Company. Our bylaws further provide for the advancement of all expenses incurred in connection with a proceeding upon receipt of an undertaking by or on behalf of such person to repay such amounts if it is determined that the party is not entitled to be indemnified under our bylaws. No advance will be made by the Company to a party if it is determined that the party acting in bad faith. These indemnification rights are contractual, and as such will continue as to a person who has ceased to be a director, officer, employee or other agent, and will inure to the benefit of the heirs, executors and administrators of such a person.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Our Corporate Bylaws at Article IX, provides that the Corporation has accepted a provision indemnifying to the full extent permitted by the law, thereby eliminating or limiting the personal liability of directors, officers, employees or corporate agents for damages for breach of fiduciary duty as a director or officer, but such provision must not eliminate or limit the liability of a director or officer for (a) acts or omissions involving willful misconduct, gross negligence, fraud, or knowing violation of law; or (b) the payments of distributions in violation of Nevada Revised Statutes.
INSOFAR AS INDEMNIFICATION FOR LIABILITIES ARISING UNDER THE SECURITIES ACT OF 1933 MAY BE PERMITTED TO OUR DIRECTORS, OFFICERS AND CONTROLLING PERSONS PURSUANT TO THE FORGOING PROVISIONS OR OTHERWISE, WE HAVE BEEN ADVISED THAT, IN THE OPINION OF THE SECURITIES AND EXCHANGE COMMISSION, SUCH INDEMNIFICATION IS AGAINST PUBLIC POLICY AS EXPRESSED IN THAT ACT AND IS, THEREFORE, UNENFORCEABLE.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
Since March 30, 2018 we have issued the following unregistered securities:
NUMBER | DATE | NAME | AMOUNT | PRICE | SUPPORTING DOCUMENTS |
1. | June 4, 2018 | TEA HYEN SHIN | 1,000,000 | $0.001 | Shin Notes |
2. | May 10, 2021 | VALUEONE, INC. | 1,500,000 | $0.001 | Joint Venture Agreement |
3. | May 10, 2021 | BO RA SHIN | 500,000 | $0.001 | Shin Notes |
4. | May 10, 2021 | BO RAN SHIN | 500,000 | $0.001 | Shin Notes |
5. | May 10, 2021 | SEUNG HA SHIN | 500,000 | $0.001 | Shin Notes |
6. | May 10, 2021 | BLACK CANYON CAPITAL, LLC (John Dolkart, Jr.) | 500,000 | $0.001 | Consulting Services Agreement dated October 7, 2020 |
7-45. | May 26, 2021 May 26, 2021 | The 38 Korean Individuals SEONG OH KWON KYOUNG HA SIN IN HO WOO SEUGH WAN JANG SEONG YONG WOO JAE YONG KWON KUYNG WAN PARK HAE JA KIM OK HAN KIM SEUNG HEE YE JEOUNG OK JE KAL KYONG MI CHO SOUNG JA YUN JEONG HUI HAN JUNG GAB KONG JE CHAN EUM CHAI WON YU SEOUNG SU AN DOH KYOUNG KAN SUN KYOON KIM TAK SOON KWON YOUN HEE LEE JAE KWON LEE YOUNG SOO KIM IN SOO AHN GA HEE KIM MYONG SUN KANG 'MI JA PARK SOON JEONG KIM JI SUK KIM GI TAE NAM DONG IN NOH SEUNG OK WOO JIN HO WOO SE JEONG LEE SU JA LEE WANG YEOL LEE CHANG WON LEE | 21,000,000 1,000,000 1,525,960 1,300,000 300,000 300,000 223,000 200,000 200,000 145,000 100,000 100,000 100,000 53,000 52,000 40,000 30,000 30,000 30,000 24,750 20,240 17,600 15,000 13,000 12,000 12,000 11,950 11,000 10,000 10,000 10,000 10,000 10,000 10,000 10,000 10,000 10,000 10,000 10,000 | $0.0015 $0.0015 | March 23, 2022 Resolution which confirms transaction; amount paid $38,000 with supporting Exhibit A. |
46. | August 1, 2021 | TEA HYEN SHIN | 5,500,000 | $0.001 | Employment Agreement dated August 1, 2021 and Corporate Resolution. |
47. | October 11, 2021 | CAPITAL ADVISORY, INC. (H. Francis Fytton) | 50,000 | $0.001 | Edgar Services Agreement dated October 2021. |
48. | October 11, 2021 | CALEDONIA CAPITAL, INC. (H. Francis Fytton) | 50,000 | | Consulting Services Agreement dated October 2021. |
49. | January 3, 2022 | Reverse Split 1:2 of all Common Shares | 38,200,000 shares became 19,100,000 shares; amounts above adjusted to reflect. | | Corporate Resolution dated January 3, 2022 with majority shareholder approval. |
ITEM 16. Exhibits and Financial Statement Schedules.
See the Exhibit Index immediately preceding the signature page hereto for a list of exhibits filed as part of this registration statement on Form S-1, which Exhibit Index is incorporated herein by reference.
| (b) | Financial Statement Schedules |
All financial statement schedules are omitted because the information called for is not required or is shown either in the consolidated financial statements or in the notes thereto.
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers, or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining any liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (# 230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant. The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iii) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) (i) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(i) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(7) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
EXHIBIT INDEX
Exhibit No. | | Description | |
| | | |
3.1 3.2 3.3 10.1 10.2 10.3 23.1 23.2 | | Articles of Incorporation Bylaws Certificate of Designation of Preferred Series A Shares Joint Venture Agreement Executive Employment Agreement Tea Hyen Shin Subscription Agreement for Purchase of Common Shares Consent of Independent Accounting Opinion of Michael Gillespie | |
| Consent and Legal Opinion of John E. Dolkart, Jr. | |
| | | |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Seoul and Republic of South Korea this 14th day of November 2022.
| VALUEONE, INC. |
| | |
| By: | /s/ Tea Hyen Shin |
| | Name: Tea Hyen Shin |
| | Title: Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Tea Hyen Shin as their true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for them and in their name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by this registration statement that is to be effective on filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as they might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Tea Hyen Shin | | CEO (Principal Financial | | November 14, 2022 |
Tea Hyen Shin | | Officer) and Director | | |
| | | | |
/s/ Ki Hyoung You | | Secretary and Director | | November 14, 2022 |
Ki Hyoung You | | | | |
| | | | |
/s/ Hyun Il Choi | | Director | | November 14, 2022 |
Hyun Il Choi | | | | |
/s/ Seung Hwan Jang | | Director | | November 14, 2022 |
Seung Hwan Jang | | | | |
/s/ John E. Dolkart, Jr. | | Attorney | | November 14, 2022 |
John E. Dolkart, Jr. | | | | |
Exhibit 3.1
Articles of Incorporation
(included under separate cover)
Exhibit 3.2
Bylaws
(included under separate cover)
Exhibit 3.3
Certificate of Designation of Preferred Series A Stock
(included under separate cover)
Exhibit 10.1
Joint Venture Agreement with ValueOne, Inc. of South Korea
(included under separate cover)
Exhibit 10.2
Executive Employment Agreement for Tea Hyen Shin
(included under separate cover)
Exhibit 10.3
Subscription Agreement for the Purchase of Common Shares in ValueOne, Inc.
Exhibit 23.1
(included under separate cover)
Exhibit 23.2
(included under separate cover)


