Table of Contents
Filed pursuant to Rule 424(b)(3)
Registration No. 333-266510
Frazier Lifesciences Acquisition Corporation
Two Union Square, 601 Union St., Suite 3200
Seattle, Washington 98101
Dear Frazier Lifesciences Acquisition Corporation Shareholder:
You are cordially invited to attend an extraordinary general meeting of Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company (“FLAC”), which will be held on November 15, 2022 at 10:30 a.m. Eastern time, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025 (the “General Meeting”), or at such other time, on such other date and at such other place to which the General Meeting may be adjourned.
On July 25, 2022, FLAC, NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“NewAmsterdam Pharma”), NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (which will be converted into a Dutch public limited liability company (naamloze vennootschap)) (“Holdco”) prior to or promptly following the closing of the Business Combination (defined below), and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and wholly owned subsidiary of Holdco (“Merger Sub”), entered into a business combination agreement (as may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), pursuant to which certain transactions will occur, and in connection therewith, Holdco will become the ultimate parent company of NewAmsterdam Pharma, and Merger Sub will merge with and into FLAC, with FLAC surviving the merger as a wholly owned subsidiary of Holdco (the “Merger” and together with the other transactions contemplated under the Business Combination Agreement, the “Business Combination”). In connection with the Business Combination, Holdco will be converted into a Dutch public limited liability company (naamloze vennootschap), thereby changing its name to NewAmsterdam Pharma Company N.V.
At the General Meeting, FLAC shareholders will be asked to consider and vote upon: (i) a proposal, as an ordinary resolution, to adopt and approve the Business Combination Agreement, a copy of which is attached to the accompanying proxy statement/prospectus as Annex A, and the transactions contemplated thereby, including the Business Combination (the “Business Combination Proposal” or “Proposal No. 1”), (ii) a proposal, as a special resolution, to authorize and approve the Merger in accordance with the relevant provisions of the Cayman Islands Companies Act and the plan of merger pursuant to which the Merger will be effected (the “Plan of Merger”), and the transactions contemplated thereby (the “Merger Proposal” or “Proposal No. 2”), and (iii) a proposal, as an ordinary resolution, to adjourn the General Meeting to a later date or dates, if necessary to permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal or the Merger Proposal at the General Meeting or to allow reasonable time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting (the “Adjournment Proposal” or “Proposal No. 3” and, together with the Business Combination Proposal and the Merger Proposal, the “Transaction Proposals”).
As further described in the accompanying proxy statement/prospectus, subject to the terms and conditions of the Business Combination Agreement, in connection with the consummation of the Business Combination, among other things:
| • | The shareholders of NewAmsterdam Pharma (“Participating Shareholders”) will contribute all outstanding shares in the capital of NewAmsterdam Pharma to Holdco in exchange for the issuance of ordinary shares in the share capital of Holdco (“Holdco Shares”) (the “Exchange”); |
Table of Contents
| • | Immediately after giving effect to the Exchange, the legal form of Holdco will be converted from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) (the “Holdco Reorganization”), provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing (as defined below); |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco (the “Surviving Company”); |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding warrant to purchase a FLAC Class A Ordinary Share (defined below) will become a warrant to purchase one Holdco Share, on the same contractual terms (“Holdco Warrants”); |
| • | Each NewAmsterdam Pharma option that is outstanding and unexercised (“NewAmsterdam Pharma Options”) will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will change its jurisdiction of incorporation by deregistering as a Cayman Islands exempted company and continuing and domesticating as a corporation incorporated under the laws of the State of Delaware (the “Domestication”); and |
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders (including Amgen and MTPC (each defined below) for this purpose) and holders of NewAmsterdam Pharma Options who were directors, officers, employees or consultants of NewAmsterdam Pharma as of the date of the Business Combination Agreement (the “Participating Optionholders”) and who are at the time of achievement of such milestone still providing services to Holdco or its subsidiaries, 1,886,137 additional Holdco Shares (the “Earnout Shares”), which in the case of the Participating Optionholders will take the form of awards of restricted stock units under Holdco’s long-term incentive plan. The development milestone consists of the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the period beginning on the Closing Commencement Date and ending on the date that is five years after the Final Closing Date. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within five years of the Merger. |
In connection with the foregoing and concurrently with the execution of the Business Combination Agreement, FLAC and Holdco entered into subscription agreements (the “Subscription Agreements”) with certain investors (the “PIPE Investors”), pursuant to which the PIPE Investors agreed to subscribe for and purchase from Holdco, and Holdco agreed to issue and sell to such PIPE Investors, an aggregate of 23,460,000 Holdco Shares at $10.00 per share for gross proceeds of $234.6 million (the “PIPE Financing”) on the date upon which the closing of the Business Combination is completed. The Holdco Shares to be issued pursuant to the Subscription Agreements have not been registered under the U.S. Securities Act of 1933, as amended (the “Securities Act”), in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. Holdco will grant the PIPE Investors certain registration rights in connection with the PIPE Financing. The PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination. The Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the aggregate gross cash proceeds received from the PIPE Financing and from the trust account established by FLAC containing the proceeds of FLAC’s initial public offering being at least $250 million, after giving effect to any redemptions of FLAC Class A Ordinary Shares in connection with the Business Combination.
Table of Contents
In addition, in connection with the execution of the Business Combination Agreement, FLAC, NewAmsterdam Pharma and Holdco entered into a support agreement (the “Sponsor Support Agreement”) with the holders of all issued and outstanding FLAC Class B Ordinary Shares, including Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (the “Sponsor” and, together with the holders of all other issued and outstanding FLAC Class B Ordinary Shares, the “FLAC Initial Shareholders”), pursuant to which the FLAC Initial Shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them; and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. As of June 30, 2022, 14,301,000 FLAC Class A Ordinary Shares were outstanding. The FLAC Class A Ordinary Shares include (i) 13,800,00 FLAC Class A Ordinary Shares issued in FLAC’s initial public offering (“FLAC Public Shares”) and (ii) 501,000 shares issued to the Sponsor as part of a private placement which closed concurrently with FLAC’s initial public offering (“FLAC Private Placement Shares”) which do not hold redemption rights. As of June 30, 2022, the FLAC Initial Shareholders held 501,000 FLAC Class A Ordinary Shares, representing 3.5% of the outstanding FLAC Class A Ordinary Shares and 3,450,000 FLAC Class B Ordinary Shares (the “Founder Shares”). Redemption rights were provided to the holders of FLAC Public Shares in connection with the Business Combination. For the avoidance of doubt, the FLAC Class B Ordinary Shares and the FLAC Private Placement Shares, have no redemption rights attached. FLAC and certain other of its investors, including affiliates of the Sponsor, representing a total of 1,500,000 FLAC Class A Ordinary Shares, each also entered into support agreements (the “Investor Support Agreements”), the form of which is included herein as Annex F, pursuant to which such FLAC shareholder agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions and (b) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination.
It is anticipated that, following the closing of the Business Combination: (i) FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) will receive approximately 14.9% of Holdco Shares and 14.9% of the voting rights of Holdco, (ii) the PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) (some of whom are also NewAmsterdam Pharma shareholders) will receive approximately 22.0% of Holdco Shares and 22.0% of the voting rights of Holdco, (iii) the FLAC Initial Shareholders and their affiliates (including the Sponsor) will receive approximately 11.0% of Holdco Shares and 11.0% of the voting rights of Holdco and (iv) the Participating Shareholders (excluding Holdco Shares issued pursuant to the PIPE Financing, but including the Holdco Shares issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”) will receive approximately 52.2% of Holdco Shares and 52.2% of the voting rights of Holdco. These ownership levels in Holdco assume that (i) no FLAC Class A Ordinary Shares are elected to be redeemed by the FLAC public shareholders, (ii) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (iii) none of the Holdco Warrants have been exercised, (iv) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (v) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) have been issued, (vi) an aggregate of 8,656,330 Holdco Shares have been issued to Amgen and MTPC pursuant to each of their respective profit rights referenced above, (vii) none of the NewAmsterdam Pharma Options have been exercised prior to the closing of the Business Combination, and (viii) no options of Holdco (the “Holdco Options”) or awards that may be issued under Holdco’s long-term incentive plan following the closing of the Business Combination have been exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by
Table of Contents
either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (iv) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. If all of the Holdco Warrants, NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options were exercisable and immediately exercised upon completion of the Business Combination on a 1:1 basis for Holdco Shares, FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) would hold in aggregate approximately 12.3% of Holdco Shares on a fully diluted basis and 12.3% of the voting rights in aggregate, the FLAC Initial Shareholders would hold approximately 9.1% of Holdco Shares and 9.1% of the voting rights in aggregate and the Participating Optionholders would hold approximately 4.0% of Holdco Shares and 4.0% of the voting rights in aggregate; however, the Holdco Warrants are subject to restrictions on the timing of their exercise and may also be exercisable on a cashless basis by reference to the fair market value of the Holdco Shares, and these percentages are therefore indicative only.
In addition to the Business Combination Proposal and the Merger Proposal, FLAC shareholders are being asked to consider and vote upon a proposal to adjourn the General Meeting to a later date or dates, if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal (Proposal No. 1) or the Merger Proposal (Proposal No. 2). The Adjournment Proposal (Proposal No. 3) will only be presented to FLAC shareholders in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal or the Merger Proposal or for any other reason in connection with, the Business Combination Agreement. Each of these proposals is more fully described in this proxy statement/prospectus, which each shareholder is encouraged to read carefully.
The FLAC Class A Ordinary Shares, FLAC Public Units (defined below) and FLAC Public Warrants (defined below) are currently listed on Nasdaq under the symbols “FLAC,” “FLACU” and “FLACW,” respectively. Upon the closing of the Business Combination, the FLAC securities will be delisted from Nasdaq. Holdco has applied to list the Holdco Shares and Holdco Public Warrants on Nasdaq under the symbols “NAMS” and “NAMSW” respectively, upon the closing of the Business Combination. FLAC cannot assure you that the Holdco Shares or Holdco Public Warrants will be approved for listing on Nasdaq.
Investing in Holdco’s securities involves a high degree of risk. See “Risk Factors” beginning on page 69 of the accompanying proxy statement/prospectus for a discussion of information that should be considered in connection with an investment in Holdco’s securities. Holdco is an “emerging growth company” and a “foreign private issuer” under applicable United States federal securities laws and will be subject to reduced public company reporting requirements.
The accompanying proxy statement/prospectus serves as a:
| • | proxy statement for the General Meeting of FLAC shareholders being held on November 15, 2022, where FLAC shareholders will vote on, among other things, proposals to adopt, approve and authorize each of the Business Combination Agreement and the Plan of Merger, and the transactions contemplated thereby (the “Transactions”); and |
| • | prospectus for the Holdco Shares and Holdco Public Warrants that FLAC shareholders, NewAmsterdam Pharma shareholders and FLAC warrant holders will receive in the Business Combination. |
Pursuant to FLAC’s amended and restated memorandum and articles of association, FLAC is providing its public shareholders with the opportunity to redeem, upon the closing of the Business Combination, FLAC Class A Ordinary Shares then held by them for cash equal to their pro rata share of the aggregate amount then on deposit (as of two business days prior to the closing of the Business Combination) in the trust account established by FLAC containing the proceeds of FLAC’s initial public offering (the “FLAC IPO”) and from certain private placements occurring simultaneously with the FLAC IPO for the benefit of FLAC’s public shareholders (the “Trust Account”). Any such redemptions will take effect as repurchases under the FLAC amended and restated memorandum and articles of association (the “FLAC Shareholder Redemption”). The per-share amount that FLAC will distribute to
Table of Contents
investors who properly redeem their FLAC Class A Ordinary Shares will not be reduced by the aggregate deferred underwriting commission of approximately $4.8 million that FLAC will pay to the underwriters of the FLAC IPO or transaction expenses incurred in connection with the Business Combination. For illustrative purposes, based on the fair value of marketable securities held in the Trust Account of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting, the estimated per FLAC Class A Ordinary Share redemption price would have been approximately $10.04. The redemption rights include the requirement that a shareholder must identify itself in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Public shareholders may elect to redeem their shares even if they vote in favor of the Business Combination Proposal or the Merger Proposal. A public shareholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming in the aggregate his, her or its FLAC Public Shares or, if part of such a group, the group’s FLAC Public Shares, in excess of 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO (i.e., in excess of 2,070,000 FLAC Class A Ordinary Shares). Each redemption of a FLAC Public Share by FLAC’s public shareholders will reduce the amount in the Trust Account.
The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. Holders of outstanding FLAC Public Warrants do not have redemption rights in connection with the Business Combination. Unless otherwise specified, the information in the accompanying proxy statement/prospectus assumes that (i) no FLAC Class A Ordinary Shares are elected to be redeemed by the public shareholders, (ii) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (iii) none of the Holdco Warrants have been exercised, (iv) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (v) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (vi) an aggregate of 8,656,330 Holdco Shares have been issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” (vii) none of the NewAmsterdam Pharma Options have been exercised prior to the closing of the Business Combination, and (viii) no Holdco Options or awards that may be issued under Holdco’s long-term incentive plan following the closing of the Business Combination, have been exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (iv) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. For more information about the factors that affect the assumptions above, please see the section entitled “The Business Combination—Ownership of Holdco Following the Business Combination.”
Each of the FLAC Initial Shareholders has agreed, for no additional consideration, to waive their redemption rights with respect to any Founder Shares or any FLAC Public Shares they may hold in connection with a shareholder vote to approve a proposed initial business combination, including the Business Combination. 501,000 outstanding FLAC Class A Ordinary Shares issued to the Sponsor in a private placement will be excluded from the pro rata calculation used to determine the per-share redemption price. Currently, the FLAC Initial Shareholders collectively own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares. The FLAC Initial Shareholders have agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of the Business Combination and the Transactions. The Founder Shares are subject to transfer restrictions. The FLAC amended and restated memorandum and articles of association includes a conversion adjustment which provides that the Founder Shares will automatically convert at the time of the Business Combination into a number of FLAC Class A Ordinary Shares one day after the closing of the Business Combination, at a conversion rate that entitles the FLAC Initial Shareholders to continue to own, in the aggregate, approximately 19% of the issued and outstanding FLAC Ordinary Shares after giving effect to the PIPE Financing. However, the FLAC Initial Shareholders have agreed to waive such conversion
Table of Contents
adjustment pursuant to the Sponsor Support Agreement. As a result, each remaining Founder Share will be exchanged for one Holdco Share at the closing of the Business Combination, such that the FLAC Initial Shareholders will hold approximately 11.0% (on a fully diluted basis) of the total number of Holdco Shares outstanding after the consummation of the Business Combination.
FLAC is providing the accompanying proxy statement/prospectus and accompanying proxy card to its shareholders in connection with the solicitation of proxies to be voted at the General Meeting and at any adjournments or postponements of the General Meeting. Information about the General Meeting, the Business Combination, the Merger and other related business to be considered by the FLAC shareholders at the General Meeting is included in the accompanying proxy statement/prospectus. Whether or not you plan to attend the General Meeting, all FLAC shareholders are urged to read carefully the accompanying proxy statement/prospectus, including the Annexes and the accompanying financial statements of FLAC and NewAmsterdam Pharma carefully and in their entirety. In particular, you are urged to carefully read the section entitled “Risk Factors” beginning on page 69 of the accompanying proxy statement/prospectus.
At a meeting of a special committee of the FLAC Board (the “FLAC Special Committee”), comprised solely of disinterested and independent directors and established because of the conflict of interests of certain members of the FLAC Board (as more particularly described in “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination”), the FLAC Special Committee unanimously adopted resolutions concluding and finding the Business Combination to be advisable and fair to, and in the best interests of FLAC and its unaffiliated shareholders and recommending the full FLAC Board to authorize the execution, delivery and performance of the Business Combination Agreement and the consummation of the Business Combination, and to present the Business Combination Agreement and the Business Combination to the FLAC shareholders for their approval.
After careful consideration, the FLAC Board, acting upon the unanimous recommendation of the FLAC Special Committee, has approved the Business Combination Agreement, the Business Combination and the Merger, and recommends that FLAC shareholders vote “FOR” the Business Combination Proposal, “FOR” the Merger Proposal, and “FOR” all other proposals presented to FLAC shareholders in the accompanying proxy statement/prospectus. When you consider the FLAC Board and the FLAC Special Committee’s recommendation of these proposals, you should keep in mind that certain FLAC directors and officers have interests in the Business Combination that may conflict with your interests as a shareholder. Please see the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination” in the accompanying proxy statement/prospectus for additional information.
Approval of the Merger Proposal requires a special resolution under Cayman Islands law, being the affirmative vote of at least a two-thirds majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. Approval of the Business Combination Proposal and the Adjournment Proposal each require the affirmative vote of holders of a majority of the FLAC Ordinary Shares that are entitled to vote and are voted at the General Meeting.
Your vote is very important. Whether or not you plan to attend the General Meeting, please vote as soon as possible by following the instructions in the accompanying proxy statement/prospectus to ensure that your shares are represented at the General Meeting. If you hold your shares in “street name” through a bank, broker or other nominee, you will need to follow the instructions provided to you by your bank, broker or other nominee to ensure that your shares are represented and voted at the General Meeting. The transactions contemplated by the Business Combination Agreement, including the Merger, will be consummated only if both the Business Combination Proposal and the Merger Proposal are approved at the General Meeting. The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in the accompanying proxy statement/prospectus.
If you sign, date and return your proxy card without indicating how you wish to vote, your proxy will be voted “FOR” each of the proposals presented at the General Meeting. If you fail to return your proxy card or fail
Table of Contents
to instruct your bank, broker or other nominee how to vote, and do not attend the General Meeting in person, the effect will be, among other things, that your shares will not be counted for purposes of determining whether a quorum is present at the General Meeting. Broker non-votes and abstentions will be counted in connection with the determination of whether a valid quorum is established but will have no effect on the Business Combination Proposal or the Merger Proposal. If you are a shareholder of record and you attend the General Meeting and wish to vote virtually or in person at the General Meeting, you may withdraw your proxy and vote virtually or in person at the General Meeting.
TO EXERCISE YOUR REDEMPTION RIGHTS, YOU MUST DEMAND THAT FLAC REDEEM YOUR SHARES FOR A PRO RATA PORTION OF THE FUNDS HELD IN THE TRUST ACCOUNT AND TENDER YOUR SHARES TO THE TRANSFER AGENT AT LEAST TWO BUSINESS DAYS PRIOR TO THE INITIALLY SCHEDULED VOTE AT THE GENERAL MEETING. THE REDEMPTION RIGHTS INCLUDE THE REQUIREMENT THAT A SHAREHOLDER MUST IDENTIFY HIMSELF, HERSELF OR ITSELF IN WRITING AS A BENEFICIAL HOLDER AND PROVIDE HIS, HER OR ITS LEGAL NAME, PHONE NUMBER AND ADDRESS TO THE TRANSFER AGENT IN ORDER TO VALIDLY REDEEM HIS, HER OR ITS SHARES. YOU MAY TENDER YOUR SHARES BY EITHER DELIVERING YOUR SHARE CERTIFICATE TO THE TRANSFER AGENT OR BY DELIVERING YOUR SHARES ELECTRONICALLY USING DEPOSITORY TRUST COMPANY’S DWAC (DEPOSIT WITHDRAWAL AT CUSTODIAN) SYSTEM. IF THE BUSINESS COMBINATION IS NOT COMPLETED, THEN THESE SHARES WILL NOT BE REDEEMED FOR CASH. IF YOU HOLD THE SHARES IN STREET NAME, YOU WILL NEED TO INSTRUCT THE ACCOUNT EXECUTIVE AT YOUR BANK OR BROKER TO WITHDRAW THE SHARES FROM YOUR ACCOUNT IN ORDER TO EXERCISE YOUR REDEMPTION RIGHTS.
On behalf of the FLAC Board, I would like to thank you for your support of FLAC and look forward to a successful completion of the Business Combination.
| Sincerely, | ||||
| October 18, 2022 | /s/ James N. Topper | |||
James N. Topper Chief Executive Officer and Director | ||||
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED THE TRANSACTIONS DESCRIBED IN THIS PROXY STATEMENT/PROSPECTUS, PASSED UPON THE MERITS OR FAIRNESS OF THE BUSINESS COMBINATION OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THE ACCOMPANYING PROXY STATEMENT/PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
The accompanying proxy statement/prospectus is dated October 18, 2022, and is expected to be first mailed or otherwise delivered to FLAC shareholders on or about October 20, 2022.
Table of Contents
ADDITIONAL INFORMATION
No person is authorized to give any information or to make any representation with respect to the matters that this proxy statement/prospectus describes other than those contained in this proxy statement/prospectus, and, if given or made, the information or representation must not be relied upon as having been authorized by Holdco, FLAC or NewAmsterdam Pharma. This proxy statement/prospectus does not constitute an offer to sell or a solicitation of an offer to buy securities or a solicitation of a proxy in any jurisdiction where, or to any person to whom, it is unlawful to make such an offer or a solicitation. Neither the delivery of this proxy statement/prospectus nor any distribution of securities made under this proxy statement/prospectus will, under any circumstances, create an implication that there has been no change in the affairs of Holdco, FLAC or NewAmsterdam Pharma since the date of this proxy statement/prospectus or that any information contained herein is correct as of any time subsequent to such date.
Table of Contents
NOTICE OF EXTRAORDINARY GENERAL MEETING
OF FRAZIER LIFESCIENCES ACQUISITION CORPORATION
TO BE HELD ON NOVEMBER 15, 2022
TO THE SHAREHOLDERS OF FRAZIER LIFESCIENCES ACQUISITION CORPORATION:
NOTICE IS HEREBY GIVEN that an extraordinary general meeting of the shareholders (the “General Meeting”) of Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company (“FLAC”), will be held on November 15, 2022 at 10:30 a.m. Eastern time, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. You will need the 12-digit meeting control number that is printed on your proxy card to enter the General Meeting. FLAC recommends that you log in at least 15 minutes before the General Meeting to ensure you are logged in when the General Meeting starts. We strongly encourage you to participate by such means and not attend in person. The General Meeting will be held for the following purposes:
| • | Proposal No. 1—The Business Combination Proposal—RESOLVED, as an ordinary resolution (the “Business Combination Proposal” or “Proposal No. 1”), that FLAC’s entry into the business combination agreement, dated as of July 25, 2022 (as may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), by and among FLAC, NewAmsterdam Pharma Company B.V. a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”), NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”), and NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“NewAmsterdam Pharma”), a copy of which is attached to the accompanying proxy statement/prospectus as Annex A, pursuant to which certain transactions will occur, and in connection therewith, Holdco will become the ultimate parent company of NewAmsterdam Pharma, and Merger Sub will merge with and into FLAC, with FLAC surviving the merger as a wholly owned subsidiary of Holdco (the “Merger” and together with the other transactions contemplated under the Businss Combination Agreement, the “Business Combination”) and Holdco shall be converted into a Dutch public limited liability company (naamloze vennootschap), thereby changing its name to NewAmsterdam Pharma Company N.V., and the consummation of the transactions contemplated thereby (the “Transactions”), shall be confirmed, ratified and approved in all respects; |
| • | Proposal No. 2—The Merger Proposal—RESOLVED, as a special resolution (the “Merger Proposal” or “Proposal No. 2”) that the Merger and the plan of merger in the form tabled to the General Meeting (a draft of which is attached to the accompanying proxy statement/prospectus as Annex B, the “Plan of Merger”) pursuant to which Merger Sub will merge with and into FLAC so that FLAC will survive the Merger as a wholly owned subsidiary of Holdco, and all the undertakings, property and liabilities of Merger Sub will vest in FLAC by virtue of such Merger pursuant to the Cayman Islands Companies Act, and the consummation of the Merger and the remaining transactions contemplated thereby, be authorized, approved and confirmed in all respects; and FLAC be authorized to enter into the Plan of Merger; |
| • | Proposal No. 3—The Adjournment Proposal—RESOLVED, as an ordinary resolution (the “Adjournment Proposal” or “Proposal No. 3” and, together with the Business Combination Proposal and the Merger Proposal, the “Transaction Proposals”), to adjourn the General Meeting, in order (i) to solicit additional proxies from FLAC shareholders for, or otherwise in connection with, the Business Combination Proposal, the Merger Proposal or for any other reason in connection with, the Business Combination Agreement or (ii) to allow reasonable time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting. |
The record date for the General Meeting for FLAC shareholders that hold their shares in “street name” is September 30, 2022 for FLAC shareholders holding their shares in “street name,” and only shareholders at the
Table of Contents
close of business on that date may vote at the General Meeting or any adjournment thereof. For the avoidance of doubt, the record date does not apply to FLAC shareholders that hold their shares in registered form and are registered as shareholders in FLAC’s register of members. FLAC shareholders that hold their shares in registered form are entitled to one vote on each proposal presented at the General Meeting for each FLAC Ordinary Share held on the date of the General Meeting.
As further described in the accompanying proxy statement/prospectus, subject to the terms and conditions of the Business Combination Agreement, in connection with the Business Combination, among other things:
| • | The Participating Shareholders will contribute all outstanding shares in the capital of NewAmsterdam Pharma to Holdco in exchange for the issuance of Holdco Shares (the “Exchange”); |
| • | Immediately after giving effect to the Exchange, the legal form of Holdco will be converted from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) (the “Holdco Reorganization”), provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing (as defined below); |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco (the “Surviving Company”); |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding warrant to purchase a FLAC Class A Ordinary Share (defined below) will become a warrant to purchase one Holdco Share, on the same contractual terms (“Holdco Warrants”); |
| • | Each NewAmsterdam Pharma option that is outstanding and unexercised (“NewAmsterdam Pharma Options”) will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will change its jurisdiction of incorporation by deregistering as a Cayman Islands exempted company and continuing and domesticating as a corporation incorporated under the laws of the State of Delaware (the “Domestication”); and |
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders (including Amgen and MTPC (each defined below) for this purpose) and holders of NewAmsterdam Pharma Options who were directors, officers, employees or consultants of NewAmsterdam Pharma as of the date of the Business Combination Agreement (the “Participating Optionholders”) and who are at the time of achievement of such milestone still providing services to Holdco or its subsidiaries, 1,886,137 additional Holdco Shares (the “Earnout Shares”), which in the case of the Participating Optionholders will take the form of awards of restricted stock units under Holdco’s long-term incentive plan. The development milestone consists of the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the period beginning on the Closing Commencement Date and ending on the date that is five years after the Final Closing Date. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within five years of the Merger. |
In connection with the foregoing and concurrently with the execution of the Business Combination Agreement, FLAC and Holdco entered into Subscription Agreements with the PIPE Investors, pursuant to which the PIPE Investors agreed to subscribe for and purchase, and Holdco agreed to issue and sell to such PIPE Investors, an aggregate of 23,460,000 Holdco Shares at $10.00 per share for gross proceeds of $234.6 million (the “PIPE Financing”) substantially concurrently with the closing of the Business Combination is completed. The Holdco Shares to be issued pursuant to the Subscription Agreements have not been registered under the U.S.
Table of Contents
Securities Act, in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. Holdco will grant the PIPE Investors certain registration rights in connection with the PIPE Financing. The PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination. The Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the aggregate gross cash proceeds received from the PIPE Financing and from the trust account established by FLAC containing the proceeds of FLAC’s initial public offering being at least $250 million, after giving effect to any redemptions of FLAC Class A Ordinary Shares in connection with the Business Combination. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy the minimum cash condition, even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights.
In addition, in connection with the execution of the Business Combination Agreement, FLAC, NewAmsterdam Pharma and Holdco entered into the Sponsor Support Agreement with the FLAC Initial Shareholders, pursuant to which the FLAC Initial Shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them; and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. FLAC and certain other of its investors, including affiliates of the Sponsor, representing FLAC Class A Ordinary Shares, also entered into Support Agreements (the “Investor Support Agreements”), the form of which is included herein as Annex F, pursuant to which the FLAC shareholder agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions and (b) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. The above matters are more fully described in the accompanying proxy statement/prospectus, which also includes, as Annex A, a copy of the Business Combination Agreement. You are urged to carefully read the accompanying proxy statement/prospectus in its entirety, including the Annexes and accompanying financial statements of FLAC and NewAmsterdam Pharma. Capitalized terms that are not otherwise defined have the meaning given to such terms in the accompanying proxy statement/prospectus.
Pursuant to FLAC’s amended and restated memorandum and articles of association, FLAC is providing its public shareholders with the opportunity to redeem, upon the closing of the Business Combination, FLAC Class A Ordinary Shares then held by them for cash equal to their pro rata share of the aggregate amount then on deposit (as of two business days prior to the closing of the Business Combination) in the trust account established by FLAC containing the proceeds of FLAC’s initial public offering (the “FLAC IPO”) and from certain private placements occurring simultaneously with the FLAC IPO for the benefit of FLAC’s public shareholders (the “Trust Account”). Any such redemptions will take effect as repurchases under the FLAC amended and restated memorandum and articles of association (the “FLAC Shareholder Redemption”). The per-share amount that FLAC will distribute to investors who properly redeem their FLAC Class A Ordinary Shares will not be reduced by the aggregate deferred underwriting commission of approximately $4.8 million that FLAC will pay to the underwriters of the FLAC IPO or transaction expenses incurred in connection with the Business Combination. For illustrative purposes, based on the fair value of marketable securities held in the Trust Account of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting, the estimated per FLAC Class A Ordinary Share redemption price would have been approximately $10.04. The redemption rights include the requirement that a shareholder must identify itself in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Public shareholders may elect to redeem their shares even if they vote in favor of the Business Combination Proposal or the Merger Proposal. A public shareholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its FLAC Public Shares or, if part of such a group, the group’s FLAC Public Shares, in excess of 15% of the
Table of Contents
FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO (i.e., in excess of 2,070,000 FLAC Class A Ordinary Shares). Each redemption of a FLAC Public Share by FLAC’s public shareholders will reduce the amount in the Trust Account.
The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. Holders of outstanding FLAC Public Warrants do not have redemption rights in connection with the Business Combination. Unless otherwise specified, the information in the accompanying proxy statement/prospectus assumes that: (i) no FLAC Class A Ordinary Shares are elected to be redeemed by the public shareholders, (ii) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (iii) none of the Holdco Warrants have been exercised, (iv) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (v) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (vi) an aggregate of 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” (vii) none of the NewAmsterdam Pharma Options have been exercised and (viii) no options of Holdco or awards that may be issued under Holdco’s long-term incentive plan following the closing of the Business Combination have been exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (iv) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. For more information about the factors that affect the assumptions above, please see the section entitled “The Business Combination—Ownership of Holdco Following the Business Combination.”
Each of the FLAC Initial Shareholders has agreed, for no additional consideration, to waive their redemption rights with respect to any of the Founder Shares or any FLAC Public Shares they may hold in connection with a shareholder vote to approve a proposed initial business combination, including the Business Combination. 501,000 outstanding FLAC Class A Ordinary Shares issued to the Sponsor in a private placement will be excluded from the pro rata calculation used to determine the per-share redemption price. Currently, the FLAC Initial Shareholders own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares. The FLAC Initial Shareholders have agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of the Business Combination and the transactions contemplated thereby. The Founder Shares are subject to transfer restrictions. The FLAC amended and restated memorandum and articles of association includes a conversion adjustment which provides that the Founder Shares will automatically convert at the time of the Business Combination into a number of FLAC Class A Ordinary Shares one day after the closing of the Business Combination, at a conversion rate that entitles the FLAC Initial Shareholders to continue to own, in the aggregate, approximately 19% of the issued and outstanding FLAC Ordinary Shares after giving effect to the PIPE Financing. However, the FLAC Initial Shareholders have agreed to waive such conversion adjustment pursuant to the Sponsor Support Agreement. As a result, each remaining Founder Share will be exchanged for one Holdco Share at the closing of the Business Combination, such that the FLAC Initial Shareholders will hold approximately 11% (on a fully diluted basis) of the total number of Holdco Shares outstanding after the consummation of the Business Combination.
The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in the accompanying proxy statement/prospectus.
Approval of the Merger Proposal requires a special resolution under Cayman Islands law, being the affirmative vote of at least a two-thirds majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. Approval of the Business Combination Proposal and the Adjournment Proposal each require the affirmative vote of holders of a majority of the FLAC Ordinary Shares that are entitled to vote and are voted at the General Meeting.
Table of Contents
After careful consideration, the FLAC Board recommends that FLAC shareholders vote “FOR” the Business Combination Proposal, “FOR” the Merger Proposal, and “FOR” all other proposals presented to FLAC shareholders in the accompanying proxy statement/prospectus.
| By Order of the Board of Directors | ||||
| October 18, 2022 | /s/ James N. Topper | |||
James N. Topper Chief Executive Officer and Director | ||||
Table of Contents
| 1 | ||||
| 6 | ||||
| 8 | ||||
| 31 | ||||
SELECTED HISTORICAL FINANCIAL INFORMATION OF NEWAMSTERDAM PHARMA | 56 | |||
| 58 | ||||
SUMMARY UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | 60 | |||
| 66 | ||||
| 69 | ||||
| 153 | ||||
| 160 | ||||
| 161 | ||||
| 162 | ||||
| 163 | ||||
| 199 | ||||
| 215 | ||||
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | 237 | |||
| 257 | ||||
BUSINESS OF NEWAMSTERDAM PHARMA AND CERTAIN INFORMATION ABOUT NEWAMSTERDAM PHARMA | 259 | |||
| 306 | ||||
| 325 | ||||
FLAC’S MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 342 | |||
| 348 | ||||
| 358 | ||||
| 371 | ||||
| 376 | ||||
| 385 | ||||
| 405 | ||||
| 407 | ||||
| 407 | ||||
| 407 | ||||
| 407 | ||||
| 408 | ||||
| 408 | ||||
| 408 | ||||
| 408 | ||||
| F-1 | ||||
| A-1 | ||||
| B-1 | ||||
| C-1 | ||||
| D-1 | ||||
| E-1 | ||||
| F-1 | ||||
| G-1 | ||||
| H-1 | ||||
| I-1 | ||||
| J-1 |
Table of Contents
ABOUT THIS PROXY STATEMENT/PROSPECTUS
This document, which forms part of a registration statement on Form F-4 filed with the U.S. Securities and Exchange Commission (the “SEC”) by Holdco, constitutes a prospectus of Holdco under Section 5 of the Securities Act of 1933, as amended (the “Securities Act”), with respect to the Holdco securities to be issued to FLAC shareholders and NewAmsterdam Pharma shareholders, if the business combination described below is consummated. This document also constitutes a notice of meeting and a proxy statement under Section 14(a) of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), with respect to the general meeting of FLAC shareholders at which FLAC shareholders will be asked to consider and vote upon proposals to adopt and approve the Business Combination Agreement and the transactions contemplated thereby, including the Business Combination, and to adopt and approve the Merger, along with the Merger Documents, including the Plan of Merger, by the approval and adoption of the Business Combination Proposal and the Merger Proposal, respectively, among other matters.
CONVENTIONS WHICH APPLY TO THIS PROXY STATEMENT/PROSPECTUS
In this proxy statement/prospectus, unless otherwise specified or the context otherwise requires:
| • | “$,” “USD” and “U.S. dollar” each refer to the United States dollar; and |
| • | “€,” “EUR” and “Euro” each refer to the Euro. |
The historical financial information was translated from USD to EUR using the historical closing exchange rate, as at June 30, 2022, of $1.0469 per EUR.
IMPORTANT INFORMATION ABOUT U.S. GAAP AND IFRS
FLAC’s financial statements included in this proxy statement/prospectus have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for financial information and pursuant to the rules and regulations of the SEC.
NewAmsterdam Pharma’s audited financial statements included in this proxy statement/prospectus have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”).
For additional information, see the section entitled “General Information—Presentation of Financial Information.”
SERVICE MARKS AND TRADE NAMES
The NewAmsterdam Pharma name, logos and other service marks of NewAmsterdam Pharma appearing in this prospectus are the property of NewAmsterdam Pharma. Solely for convenience, some of the service marks, logos and trade names referred to in this proxy statement/prospectus are presented without the ™ and SM symbols, but such references are not intended to indicate, in any way, that Holdco will not assert, to the fullest extent under applicable law, its rights or the rights of the applicable licensors to these service marks and trade names. This proxy statement/prospectus contains additional trademarks, service marks and trade names of others. All trademarks, service marks and trade names appearing in this proxy statement/prospectus are, to Holdco’s knowledge, the property of their respective owners. Holdco does not intend Holdco’s use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of Holdco by, any other companies.
1
Table of Contents
SELECTED DEFINITIONS
Unless otherwise stated in this proxy statement/prospectus or the context otherwise requires, references to:
| • | “Aggregate Cash Proceeds” are to the sum of (a) the estimated cash proceeds to be actually received by Holdco or an affiliate thereof in respect of the PIPE Financing plus (b) the amount of cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption; |
| • | “Amgen” are to Saga Investments Coöperatief U.A., an affiliate of Amgen Inc.; |
| • | “Antitrust Laws” are to laws that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition through merger or acquisition; |
| • | “Business Combination” are to the transactions contemplated by the Business Combination Agreement; |
| • | “Business Combination Agreement” are to that certain business combination agreement, dated July 25, 2022 (as may be amended, supplemented or otherwise modified from time to time), by and among FLAC, Holdco, Merger Sub and NewAmsterdam Pharma; |
| • | “Cayman Islands Companies Act” are to the Companies Act (As Revised) of the Cayman Islands as the same may be amended from time to time; |
| • | “Closing” are to the closing of the transactions contemplated by the Business Combination Agreement; |
| • | “Closing Commencement Date” are to the date on which the first Closing step occurs, being no later than the third business day following the satisfaction or waiver of the conditions to the Business Combination, in each case pursuant to and in accordance with the terms of the Business Combination Agreement; |
| • | “Code” are to the United States Internal Revenue Code of 1986, as amended; |
| • | “COVID-19” are to the novel coronavirus known as SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemic or disease outbreaks; |
| • | “Domestication” are to the transfer by way of continuation and deregistration of FLAC from the Cayman Islands and the continuation and domestication of FLAC as a corporation incorporated in the State of Delaware; |
| • | “DGCL” are to the Delaware General Corporation Law; |
| • | “DCGC” are to the Dutch Corporate Governance Code; |
| • | “Earnout Shares” are to 1,886,137 Holdco Shares that will be issued to Participating Shareholders, Participating Optionholders, Amgen and MPTC, only upon the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the Earnout Period, with such Earnout Shares being issuable to Participating Optionholders only if the relevant optionholder continues to provide services to Holdco or one of its subsidiaries through the date of the achievement that causes such Earnout Shares to become issuable and being subject to the same vesting schedule as the corresponding options; |
| • | “Earnout Period” are to the period beginning on the Closing Commencement Date and ending on the date that is five years after the Final Closing Date; |
| • | “Exchange” are to the exchange for the contribution by the Participating Shareholders of all outstanding shares in the capital of NewAmsterdam Pharma in exchange for Holdco Shares; |
| • | “Exchange Act” are to the Securities Exchange Act of 1934; |
| • | “Final Closing Date” are to the date on which the Domestication becomes effective; |
| • | “FLAC” are to Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company; |
| • | “FLAC Articles of Association” are to the amended and restated articles of association of FLAC; |
2
Table of Contents
| • | “FLAC Board” are to FLAC’s board of directors; |
| • | “FLAC Class A Ordinary Shares” are to the Class A ordinary shares, par value $0.0001 per share, of FLAC; |
| • | “FLAC Class B Ordinary Shares” or “Founder Shares” are to the 3,450,000 Class B ordinary shares, par value $0.0001 per share, of FLAC outstanding as of the date of this proxy statement/prospectus that were initially issued to the Sponsor in a private placement in October 2020 prior to the FLAC IPO and of which 150,000 shares were transferred to each of FLAC’s directors other than the chairman (30,000 shares each) in November 2020 (as adjusted by the share sub-division described herein); |
| • | “FLAC Initial Shareholders” are to the Sponsor and each other holder of Founder Shares upon the consummation of the FLAC IPO; |
| • | “FLAC IPO” are to FLAC’s initial public offering that was consummated on December 11, 2020; |
| • | “FLAC Ordinary Shares” are to the FLAC Class A Ordinary Shares and the FLAC Class B Ordinary Shares; |
| • | “FLAC Private Placement Shares” are to the FLAC Class A Ordinary Shares that were issued to the Sponsor as part of the FLAC Private Placement Units; |
| • | “FLAC Private Placement Units” are to the private placement units that were issued to the Sponsor in a private placement simultaneously with the closing of the FLAC IPO, which are identical to the units sold in the FLAC IPO, subject to certain limited exceptions; |
| • | “FLAC Private Placement Warrants” are to the private placement warrants that were issued to the Sponsor as part of the FLAC Private Placement Units, which are substantially identical to the public warrants sold as part of the units in the FLAC IPO, subject to certain limited exceptions; |
| • | “FLAC Public Units” are to the units of FLAC, each unit representing one Class A Ordinary Share and one-third of one warrant to acquire one Class A Ordinary Share, that were offered and sold by FLAC in the FLAC IPO or acquired in the secondary market; |
| • | “FLAC Public Shares” are to the FLAC Class A Ordinary Shares issued in the FLAC IPO, whether acquired in the FLAC IPO or acquired in the secondary market; |
| • | “FLAC Public Warrants” are to the redeemable warrants to purchase FLAC Class A Ordinary Shares that were issued by FLAC in the FLAC IPO; |
| • | “FLAC Special Committee” are to the special committee of the FLAC Board, comprised of independent directors Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher and Krishna R. Polu; |
| • | “FLAC Units” are to the FLAC Public Units and the FLAC Private Placement Units; |
| • | “Frazier” are to Frazier Life Sciences Management, L.P., an affiliate of the Sponsor; |
| • | “Frazier Life Sciences” are the investment team managing all of the Frazier life sciences-affiliated investments and the life sciences-focused entities, companies and funds; |
| • | “FLAC Warrants” are to the FLAC Public Warrants and the FLAC Private Placement Warrants; |
| • | “General Meeting” are to the extraordinary general meeting of FLAC, which will be held on November 15, 2022, at 10:30 a.m., Eastern time, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025, or at such other time or on such other date to which the General Meeting may be adjourned; |
| • | “Group Companies” are to Holdco, NewAmsterdam Pharma and their respective subsidiaries; |
| • | “Holdco” are to NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands with its corporate seat in Naarden and registered office at Gooimeer 2-35, 1411 DC Naarden, the Netherlands and registered with the Dutch trade register under number 86649051 (which will be converted into a public limited liability company (naamloze vennootschap), thereby changing its name to “NewAmsterdam Pharma Company N.V.,” prior to or promptly following Closing); |
3
Table of Contents
| • | “Holdco Articles of Association” are to the articles of association of Holdco, as they are expected to be in effect prior to or promptly following Closing and included as an English translation of the official Dutch text as Annex I hereto; |
| • | “Holdco Board” are to the board of directors of Holdco; |
| • | “Holdco General Meeting” are to the general meeting of shareholders of Holdco. |
| • | “Holdco LTIP” are to Holdco’s long-term incentive plan adopted with effect from as of the Final Closing Date; |
| • | “Holdco Options” are to the options to purchase Holdco Shares; |
| • | “Holdco Private Placement Warrants” are to the warrants to acquire Holdco Shares on substantially equivalent terms and conditions as the FLAC Private Placement Warrants; |
| • | “Holdco Public Warrants” are to the warrants to acquire Holdco Shares on substantially equivalent terms and conditions as the FLAC Public Warrants; |
| • | “Holdco Warrants” are to the Holdco Public Warrants and Holdco Private Placement Warrants; |
| • | “Holdco Shares” are to the ordinary shares in the share capital of Holdco; |
| • | “IASB” are to the International Accounting Standards Board; |
| • | “IFRS” are to the International Financial Reporting Standards as issued by the IASB; |
| • | “IRS” are to the U.S. Internal Revenue Service; |
| • | “Merger” are to the merger of Merger Sub with and into FLAC, with FLAC surviving such merger as a wholly owned subsidiary of Holdco; |
| • | “Merger Documents” are to all documentation and declarations, including but not limited to, the Plan of Merger, required under the Cayman Islands Companies Act in connection with the Merger, to be duly executed and properly filed with the Cayman Islands Registrar of Companies, in accordance with the relevant provisions of the Cayman Islands Companies Act; |
| • | “Effective Date” are to the date on which the Merger becomes effective; |
| • | “Merger Sub” are to NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and wholly owned subsidiary of Holdco prior to the consummation of the Business Combination; |
| • | “MTPC” are to Mitsubishi Tanabe Pharma Corporation; |
| • | “Nasdaq” are to the Nasdaq Stock Market LLC; |
| • | “NewAmsterdam Pharma” are to NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands with its corporate seat in Naarden and registered office at Gooimeer 2-35, 1411 DC Naarden, the Netherlands, and registered with the Dutch trade register under number 76133141; |
| • | “NewAmsterdam Pharma Board” are to the board of directors of NewAmsterdam Pharma; |
| • | “NewAmsterdam Pharma Option” are to options to purchase shares of NewAmsterdam Pharma prior to the Closing; |
| • | “Participating Shareholders” are to the shareholders of NewAmsterdam Pharma immediately prior to the Exchange; |
| • | “Participating Optionholders” are to the holders of NewAmsterdam Pharma Options who were directors, officers, employees or consultants of NewAmsterdam Pharma as of the date of the Business Combination Agreement; |
| • | “Plan of Merger” are to the plan of merger in the form tabled to the General Meeting (a draft of which is attached as Annex B hereto), pursuant to which the Merger will be effectuated; |
4
Table of Contents
| • | “PIPE Financing” are to the transactions contemplated by the Subscription Agreements, pursuant to which the PIPE Investors have collectively committed to subscribe for 23,460,000 Holdco Shares for a purchase price of $10.00 per share, for aggregate gross proceeds of $234.6 million; |
| • | “PIPE Investors” are to the investors in the PIPE Financing; |
| • | “pro forma” are to giving pro forma effect to the Business Combination, including the Merger and the PIPE Financing; |
| • | “Sarbanes-Oxley Act” are to the Sarbanes-Oxley Act of 2002; |
| • | “SEC” are to the U.S. Securities and Exchange Commission; |
| • | “Securities Act” are to the Securities Act of 1933, as amended; |
| • | “Series A Preferred Shares” are to the convertible preferred series A shares in the share capital of NewAmsterdam Pharma; |
| • | “Sponsor” are to Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company; |
| • | “Subscription Agreements” are to the subscription agreements, entered into by FLAC, Holdco and each of the PIPE Investors in connection with the PIPE Financing; |
| • | “Transactions” are to the transactions contemplated by the Business Combination Agreement and the ancillary documents, including the Business Combination, Domestication and the Merger; |
| • | “Transfer Agent” or “Continental” are to Continental Stock Transfer & Trust Company, FLAC’s transfer agent; |
| • | “Trust Account” are to the trust account established at the consummation of the FLAC IPO that holds the proceeds of the FLAC IPO and from certain private placements occurring simultaneously with the FLAC IPO for the benefit of FLAC’s public shareholders and is maintained by Continental Stock Transfer & Trust Company, acting as trustee; and |
| • | “Trust Agreement” are to the investment management trust account agreement FLAC, dated and Continental. |
5
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement/prospectus contains forward-looking statements. Forward-looking statements provide Holdco’s and FLAC’s current expectations or forecasts of future events. Forward-looking statements include statements about Holdco’s and FLAC’s expectations, beliefs, plans, objectives, intentions, assumptions and other statements that are not historical facts. Words or phrases such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will” and “would,” or similar words or phrases, or the negatives of those words or phrases, may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. Examples of forward-looking statements in this proxy statement/prospectus include, but are not limited to, statements regarding Holdco’s disclosure concerning NewAmsterdam Pharma’s operations, cash flows, financial position and dividend policy.
Forward-looking statements appear in a number of places in this proxy statement/prospectus including, without limitation, in the sections titled “NewAmsterdam Pharma’s Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “FLAC’s Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business of FLAC and Certain Information About FLAC” and “Business of NewAmsterdam Pharma and Certain Information About NewAmsterdam Pharma.” Forward-looking statements in this proxy statement/prospectus and in any document incorporated by reference in this proxy statement/prospectus may include, for example, statements about:
| • | FLAC’s ability to complete the Business Combination or, if FLAC does not consummate such Business Combination, any other initial business combination; |
| • | the satisfaction or waiver (if applicable) of the conditions to the Business Combination, including the following closing conditions, among others, (i) approval of the Business Combination and related agreements and transactions by the respective shareholders of FLAC and NewAmsterdam Pharma, (ii) effectiveness of the registration statement of which this proxy statement/prospectus forms a part, (iii) receipt of approval for listing on Nasdaq of Holdco Shares and Holdco Public Warrants to be issued in connection with the Merger, (iv) that Holdco have at least $5,000,001 of net tangible assets upon Closing, (v) that the proceeds actually received from the Trust Account (net of the aggregate amount of cash required to satisfy any exercise by FLAC shareholders of their right to have FLAC redeem their FLAC Class A Ordinary Shares in connection with the Business Combination (the “Cash Redemption Amount”)) and the Aggregate Cash Proceeds be at least $250 million and (vi) the absence of any injunctions; |
| • | the occurrence of any other event, change or other circumstances that could give rise to the termination of the Business Combination Agreement; |
| • | Holdco’s public securities’ potential liquidity and trading; |
| • | Holdco’s ability to raise financing in the future; |
| • | projected financial information, including assumptions about the efficacy of Holdco’s product candidate, reimbursement and anticipated market size and market opportunity; |
| • | Holdco’s dependence on the success of its product candidate, obicetrapib, including obtaining of regulatory approval to market obicetrapib; |
| • | Holdco’s ability to attract and retain senior management and key scientific personnel; |
| • | Holdco’s limited experience in marketing or distributing products; |
| • | managing the risks related to Holdco’s international operations; |
| • | Holdco’s ability to achieve the broad degree of physician adoption and use and market acceptance necessary for commercial success; |
| • | Holdco’s reliance on third parties for all aspects of the manufacturing of obicetrapib for clinical trials; and |
| • | Holdco’s efforts to obtain, protect or enforce its patents and other intellectual property rights related to Holdco’s product candidate. |
6
Table of Contents
Forward-looking statements are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions that could cause actual results to differ materially from those expected or implied by the forward-looking statements. Actual results could differ materially from those anticipated in forward-looking statements for many reasons, including the factors described in the section titled “Risk Factors” in this proxy statement/prospectus. Accordingly, you should not rely on these forward-looking statements, which speak only as of the date of this proxy statement/prospectus. Holdco and FLAC undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this proxy statement/prospectus or to reflect the occurrence of unanticipated events. You should, however, review the factors and risks FLAC describes in its reports it files from time to time with the SEC and that Holdco describes in the reports it will file from time to time with the SEC after the closing of the Business Combination.
In addition, statements that “Holdco believes” and similar statements reflect Holdco’s beliefs and opinions on the relevant subject. These statements are based on information available to Holdco as of the date of this proxy statement/prospectus. And while Holdco believes that information provides a reasonable basis for these statements, that information may be limited or incomplete. Holdco’s statements should not be read to indicate that it has conducted an exhaustive inquiry into, or review of, all relevant information. These statements are inherently uncertain, and you are cautioned not to unduly rely on these statements.
Although Holdco believes the expectations reflected in the forward-looking statements were reasonable at the time made, it cannot guarantee future results, level of activity, performance or achievements. Moreover, FLAC, Holdco nor any other person assumes responsibility for the accuracy or completeness of any of these forward-looking statements. You should carefully consider the cautionary statements contained or referred to in this section in connection with the forward-looking statements contained in this proxy statement/prospectus and any subsequent written or oral forward-looking statements that may be issued by Holdco or persons acting on its behalf.
7
Table of Contents
QUESTIONS AND ANSWERS FOR SHAREHOLDERS OF FLAC
The questions and answers below highlight only selected information from this proxy statement/prospectus and only briefly address some commonly asked questions about the General Meeting and the proposals to be presented at the General Meeting, including with respect to the proposed Business Combination. The following questions and answers do not include all the information that is important to FLAC’s shareholders. Shareholders are urged to read carefully this entire proxy statement/prospectus, including the Annexes and the other documents referred to herein, to fully understand the proposed Business Combination and the voting procedures for the General Meeting, which will be held on November 15, 2022 at 10:30 a.m., Eastern time, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. Unless the context otherwise requires, any reference in the section below to “we,” “us” or “our” refers to FLAC.
| Q: | Why am I receiving this proxy statement/prospectus? |
| A: | FLAC shareholders are being asked to consider and vote upon: (i) a proposal to adopt the Business Combination Agreement and approve the Transactions, including the Business Combination, (ii) a proposal to adopt and approve the Merger, along with Merger Documents, including the Plan of Merger and (iii) a proposal to adjourn the General Meeting if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal or the Merger Proposal. FLAC has entered into the Business Combination Agreement, providing for, among other things, the Merger and the Exchange. |
These transactions are collectively referred to as the Business Combination. You are being asked to vote on the Business Combination Proposal and the Merger Proposal. A copy of the Business Combination Agreement is attached to this proxy statement/prospectus as Annex A. You are also being asked to vote on the Adjournment Proposal to adjourn the General Meeting to a later date or dates, if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement.
This proxy statement/prospectus and its Annexes contain important information about the proposed Business Combination and the other matters to be acted upon at the General Meeting. You should read this proxy statement/prospectus and its Annexes carefully and in their entirety.
Your vote is important. You are encouraged to submit your proxy as soon as possible after carefully reviewing this proxy statement/prospectus and its Annexes.
| Q: | When and where is the General Meeting? |
| A: | The General Meeting will be held on November 15, 2022, at 10:30 a.m. Eastern time, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025, or at such other time or on such other date to which the General Meeting may be adjourned. |
| Q: | What are the specific proposals on which I am being asked to vote at the General Meeting? |
| A: | FLAC shareholders are being asked to approve the following proposals: |
| 1. | Business Combination Proposal—To adopt and approve the Business Combination Agreement and the transactions contemplated thereby, including the Business Combination (Proposal No. 1); |
| 2. | Merger Proposal—To adopt and approve the Merger and the Plan of Merger and the transactions contemplated thereby (Proposal No. 2); and |
| 3. | Adjournment Proposal—To consider and vote upon a proposal to adjourn the General Meeting to a later date or dates, if necessary, to (i) permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement and (ii) allow reasonable time for the filing or mailing of any supplemental or amended |
8
Table of Contents
| disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting (Proposal No. 3). |
The Adjournment Proposal (Proposal No. 3) will only be presented to FLAC shareholders if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with, the Business Combination Agreement.
| Q: | Are the proposals conditioned on one another? |
| A: | The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in this proxy statement/prospectus. |
It is important for you to note that in the event the Business Combination Proposal and the Merger Proposal do not receive the requisite vote for approval, then FLAC will not consummate the Business Combination. If FLAC does not consummate the Business Combination and fails to complete an initial business combination by December 11, 2022, or such later date as may be approved by FLAC’s shareholders, FLAC will be required to dissolve and liquidate the Trust Account by returning the then-remaining funds in such Trust Account to its public shareholders, absent any extension.
| Q: | Why is FLAC proposing the Business Combination? |
| A: | FLAC is a blank check company incorporated on October 7, 2020 as a Cayman Islands exempted company for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. While FLAC may pursue an initial business combination opportunity in any business, industry, sector or geographical location, FLAC intends to capitalize on the ability of its management team to identify promising opportunities in the biotechnology sector. |
FLAC has identified several criteria and guidelines it believes are important for evaluating acquisition opportunities. FLAC has sought to acquire companies with some or all of the following characteristics: (1) therapeutics-focused business, (2) preclinical through commercial stage assets, (3) a company with minimal additional equity required to achieve significant defined clinical, regulatory or commercial milestones, (4) management teams that have requisite experience and expertise, (5) a product focus, (6) near value inflection points, (7) proven performers, (8) potential for liquidity through mergers and acquisitions and (9) capital efficiency.
Based on its due diligence investigations of NewAmsterdam Pharma and the industry in which it operates, including the financial and other information provided by NewAmsterdam Pharma in the course of negotiations, FLAC believes that NewAmsterdam Pharma meets the criteria and guidelines listed above. Please see the section entitled “The Business Combination—The FLAC Board’s Reasons for the Business Combination” for additional information.
Although the FLAC Board believes that the Business Combination with NewAmsterdam Pharma presents a unique business combination opportunity and is in the best interests of FLAC and its shareholders, the FLAC Board did consider the following potentially material negative factors in arriving at that conclusion:
| • | FLAC shareholders would be subject to the execution risks associated with Holdco if they retained their public shares following the closing of the Business Combination, which were different from the risks related to holding public shares of FLAC prior to the Closing; |
| • | risks associated with successful implementation of Holdco’s long-term business plan and strategy; |
| • | risks associated with Holdco realizing the anticipated benefits of the Business Combination on the timeline expected, or at all, including due to factors outside of the parties’ control such as new regulatory requirements or changes to existing regulatory requirements in the biotechnology and biopharmaceuticals industries; and |
9
Table of Contents
| • | the potential negative impact of the ongoing and evolving COVID-19 pandemic and related macroeconomic uncertainty, as well as geopolitical uncertainties. |
These factors are discussed in greater detail in the section entitled “The Business Combination—The FLAC Board’s Reasons for the Business Combination,” as well as in the section entitled “Risk Factors.”
| Q: | Why is FLAC providing shareholders with the opportunity to vote on the Business Combination? |
| A: | The approval of the Business Combination is required under the FLAC Articles of Association, and the Merger requires the approval of FLAC shareholders under Cayman Islands law. Such approvals are also conditions to the closing of the Business Combination under the Business Combination Agreement. Additionally, under the FLAC Articles of Association, FLAC must provide all holders of FLAC Public Shares with the opportunity to have their public shares redeemed upon the consummation of its initial business combination either in conjunction with a tender offer or in conjunction with a shareholder vote. For business and other reasons, FLAC has elected to provide its shareholders with the opportunity to have their public shares redeemed in connection with a shareholder vote rather than a tender offer. The redemption rights include the requirement that a shareholder must identify itself, at least two business days prior to the General Meeting, in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Therefore, FLAC is seeking to obtain the approval of its shareholders of the Business Combination and also allow its public shareholders to effectuate redemptions of their FLAC Public Shares in connection with the closing of the Business Combination in accordance with the FLAC Articles of Association. |
| Q: | What other matters will be brought before the General Meeting? |
| A: | In addition to consideration of the proposals described above, FLAC shareholders will have the opportunity to consider the financial statements of FLAC for the fiscal year ended December 31, 2021 and the fiscal quarter ended June 30, 2022 and ask questions of FLAC’s management. |
| Q: | How will the COVID-19 pandemic impact in-person voting at the General Meeting? |
| A: | You are strongly encouraged to participate in the General Meeting virtually and not attend in person. FLAC is sensitive to the public health and travel concerns FLAC’s shareholders may have and recommendations that public health officials may issue in light of the evolving COVID-19 pandemic. As a result, FLAC may impose additional procedures or limitations on meeting attendees or may decide to hold the meeting in a different location. FLAC plans to announce any such updates on its proxy website, and FLAC’s shareholders are encouraged to check this website prior to the General Meeting if you plan to attend. |
| Q: | What impact will the COVID-19 pandemic have on the Business Combination? |
| A: | It is difficult to predict the impact of the ongoing COVID-19 pandemic on the business of FLAC, NewAmsterdam Pharma and Holdco, and there is no guarantee that efforts by FLAC, NewAmsterdam Pharma and Holdco to address the adverse impacts of COVID-19 on their respective businesses, if any, will be effective. The extent of such impact will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of new strains of COVID-19 and actions taken to contain the pandemic or its impact, among others. If FLAC or NewAmsterdam Pharma experience business disruptions and are unable to recover on a timely basis, the Business Combination and Holdco’s business, financial condition and results of operations following the completion of the Business Combination may be adversely affected. Each of FLAC, NewAmsterdam Pharma and Holdco may also incur additional costs to remedy damages caused by any such disruptions, which could adversely affect its financial condition and results of operations. |
| Q: | What will happen in the Business Combination? |
| A: | Pursuant to the Business Combination Agreement, and upon the terms and subject to the conditions set forth therein, FLAC and NewAmsterdam Pharma will effect a transaction that would replicate the economics of a merger of FLAC and NewAmsterdam Pharma through a merger and separate equity contribution and |
10
Table of Contents
| exchange, which is collectively referred to as the Business Combination. To effect the Business Combination, among other things, (i) the Exchange will be effected and (ii) the Merger will be effected. As a result of the Business Combination, Holdco will be the ultimate parent company of NewAmsterdam Pharma (following the Exchange), NewAmsterdam Pharma’s direct and indirect subsidiaries and FLAC. Please see the section entitled “The Business Combination” for additional information. |
On a pro forma basis as at June 30, 2022, assuming the consummation of the Business Combination and the PIPE Financing, NewAmsterdam Pharma estimates that Holdco would have approximately €413.3 million in cash assuming no redemptions by FLAC’s shareholders, and €296.0 million in cash assuming maximum redemptions by FLAC’s shareholders, which NewAmsterdam Pharma believes will be sufficient to fund its anticipated level of operations through 2026, which includes the forecasted completion of its Phase 3 trials. See the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” for more information.
Holdco has applied to list the Holdco Shares and Holdco Public Warrants on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively, upon the closing of the Business Combination.
We cannot assure you that the Holdco Shares or Holdco Public Warrants will be approved for listing on Nasdaq. Holdco is also eligible to be treated as an “emerging growth company” under the JOBS Act. As an emerging growth company, Holdco may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including presenting only limited selected financial data, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, and to the extent that Holdco no longer qualifies as a “foreign private issuer,” reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. See “Risk Factors—Risks Related to Ownership of Holdco Securities—Holdco is eligible to be treated as an “emerging growth company, and Holdco cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the Holdco Shares less attractive to investors, which could have a material and adverse effect on Holdco, including growth prospects, because Holdco may rely on these reduced disclosure requirements.” In addition, Holdco will be a “foreign private issuer” and as a foreign private issuer, Holdco will be subject to certain different U.S. securities laws than domestic U.S. issuers. Holdco will be exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders. As a foreign private issuer, Holdco will be exempt from a number of rules under the U.S. securities laws and will be permitted to file less information with the SEC than a U.S. domestic registrant. This may limit the information available to holders of the Holdco Shares and Holdco Public Warrants. See “Risk Factors—Risks Related to Ownership of Holdco Securities—As Holdco will be a “foreign private issuer” and intends to follow certain home country corporate governance practices, its shareholders may not have the same protections afforded to shareholders of companies that are subject to all Nasdaq corporate governance requirements.”
| Q: | How has the announcement of the Business Combination affected the trading price of FLAC’s Class A Ordinary Shares? |
| A: | On July 22, 2022, the last trading date before the public announcement of the Business Combination, the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants closed at $9.93, $9.90 and $0.22, respectively. On October 17, 2022, the trading date immediately prior to the date of this proxy statement/prospectus, the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants closed at $10.18, $10.00 and $0.70, respectively. |
| Q: | Following the Business Combination, will FLAC’s securities continue to trade on a stock exchange? |
| A: | No. FLAC anticipates that, following consummation of the Business Combination, the FLAC Class A Ordinary Shares, FLAC Public Units, and FLAC Public Warrants will be delisted from Nasdaq and FLAC will be deregistered under the Exchange Act. However, Holdco has applied to list the Holdco Shares and Holdco Public Warrants on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively, upon the closing of the Business Combination. |
11
Table of Contents
| Q: | Is the Business Combination the first step in a “going private” transaction? |
| A: | No. FLAC does not intend for the Business Combination to be the first step in a “going private” transaction. One of the primary purposes of the Business Combination is to provide a platform for Holdco and NewAmsterdam Pharma to access the U.S. public markets. |
| Q: | Will the management of NewAmsterdam Pharma change in the Business Combination? |
| A: | Following the Closing, the management of NewAmsterdam Pharma will consist solely of NewAmsterdam Pharma’s current management team immediately prior to the Closing, including individuals selected by NewAmsterdam Pharma that join its management team between the date hereof and the Closing. |
| Q: | Who will the directors and executive officers of Holdco be after the Business Combination? |
| A: | Other than as described below, following the Closing, Holdco’s executive officers will be Michael Davidson, M.D., Marc Ditmarsch, M.D., Lina Gugucheva, John Kastelein, M.D., Douglas Kling, and Louise Kooij. In the third quarter of 2022, after the announcement of the Business Combination, in light of its plans to become a public company listed on Nasdaq, NewAmsterdam Pharma determined to seek to appoint in the first half of 2023 a new chief financial officer of Holdco with deep equity capital markets experience and strong relationships with U.S. investors, including biotechnology investors. NewAmsterdam Pharma has evaluated several candidates for the role, including David Topper, the Chief Financial Officer and a director and shareholder of FLAC. If appointed to the role of chief financial officer of Holdco, David Topper would become an employee of Holdco and receive customary compensation and benefits for his service in such role. However, no final determinations have been made to date. In the event a new chief financial officer is appointed for Holdco, Ms. Kooij is expected to become Holdco’s chief accounting officer. |
Pursuant to the Business Combination Agreement, effective immediately upon Closing, the Holdco Board will be comprised of up to nine members, comprised of one executive director and eight non-executive directors, of which two non-executive directors will be designated by FLAC and one executive director and up to six non-executive directors will be designated by NewAmsterdam Pharma.
For additional information, please see the section entitled “Management of Holdco Following the Business Combination.”
| Q: | What will FLAC shareholders receive in the Business Combination? |
| A: | Upon consummation of the Merger, each issued and outstanding FLAC Ordinary Share immediately prior to the Effective Date will be subject to the terms and conditions of the Business Combination Agreement and the Plan of Merger and will be canceled and extinguished in exchange for a claim for a corresponding Holdco Share and such claim will be contributed into Holdco against the issuance of a Holdco Share. |
| Q: | What will FLAC warrant holders receive in the Business Combination? |
| A: | As a result of the Merger, each FLAC Warrant that is outstanding immediately prior to the Effective Date will automatically cease to represent a right to acquire FLAC Class A Ordinary Shares and will represent a right to acquire Holdco Shares on the same contractual terms and conditions as were in effect immediately prior to the Effective Date under the terms of the Warrant Assignment, Assumption and Amendment Agreement (the “Warrant Assumption Agreement”), between Continental, Holdco and FLAC to be entered into pursuant to the Business Combination Agreement. Each such converted warrant will represent the right to acquire the number of Holdco Shares equal to the number of FLAC Class A Ordinary Shares subject to each such FLAC Warrant immediately prior to the Effective Date, for an exercise price of $11.50 per one Holdco Share, and will expire on the five year anniversary of the Final Closing Date. |
| Q: | What will FLAC unit holders receive in the Business Combination? |
| A: | In connection with the consummation of the Business Combination, the FLAC Public Units will automatically separate into their component parts. |
12
Table of Contents
| Q: | What will NewAmsterdam Pharma Shareholders receive in the Business Combination? |
| A: | Upon consummation of the Exchange, holders of NewAmsterdam Pharma ordinary shares will receive Holdco Shares. They will also be entitled to receive Earnout Shares if and when a certain clinical development milestone is achieved during the Earnout Period. See “The Business Combination Agreement and Ancillary Documents—Consideration to NewAmsterdam Pharma Shareholders in the Business Combination” for information on the consideration to be received by NewAmsterdam Pharma shareholders. |
| Q: | What is the PIPE Financing? |
| A: | In connection with the Business Combination and concurrently with the execution of the Business Combination Agreement, FLAC and Holdco entered into the Subscription Agreements with the PIPE Investors pursuant to which the PIPE Investors agreed to subscribe for and purchase, and Holdco agreed to issue and sell to such PIPE Investors, a total of 23,460,000 Holdco Shares in exchange for an aggregate purchase price of $234.6 million. |
| Q: | What are the material differences, if any, in the terms and price of securities issued at the time of the FLAC IPO as compared to the securities that will be issued as part of the PIPE Financing at the closing of the Business Combination? |
| A: | FLAC Public Units were the units issued at the time of the FLAC IPO consisting of FLAC Class A Ordinary Shares and FLAC Public Warrants, at an offering price per security of $10.00. At the closing of the Business Combination, the FLAC Ordinary Shares will convert into Holdco Shares and the FLAC Public Warrants will convert into Holdco Public Warrants. The PIPE Investors will receive Holdco Shares at a price per share of $10.00 as part of the PIPE Financing at the Closing of the Business Combination, and will therefore hold the same security as the holders of FLAC Class A Ordinary Shares immediately prior to the Business Combination. No PIPE Investor will receive any Holdco Public Warrants in its capacity as such. |
| Q: | Will the Sponsor or any of FLAC’s directors, officers or affiliates participate in the PIPE Financing? |
| A: | Frazier Life Sciences X, L.P., the sole member of the Sponsor, Frazier Life Sciences XI, L.P., Frazier Life Sciences Public Fund, L.P. and Frazier Life Sciences Overage Fund, L.P., each an affiliate of Frazier Healthcare Partners (“Frazier”), an affiliate of the Sponsor, have committed to purchase 4,500,000 Holdco Shares (for a purchase price of $10.00 per share) in the PIPE Financing, concurrently and in connection with the closing of the Business Combination. This transaction will be on the same economic terms as the other investors who have agreed to purchase shares in the PIPE Financing pursuant to certain subscription agreements dated July 25, 2022. No other director, officer or affiliate of FLAC will participate in the PIPE Financing. See the section entitled “Certain Relationships and Related Party Transactions—FLAC Relationships and Related Person Transactions—PIPE Subscription” for additional information regarding such funds and their participation in the PIPE Financing. |
| Q: | Will any of NewAmsterdam Pharma’s sponsors, shareholders, directors, officers or their affiliates participate in the PIPE Financing? |
| A: | Certain shareholders of NewAmsterdam Pharma have committed to purchase 4,250,000 Holdco Shares (for a purchase price of $10.00 per share) in the PIPE Financing, concurrently and in connection with the closing of the Business Combination. This transaction will be on the same economic terms as the other investors who have agreed to purchase shares in the PIPE Financing pursuant to certain subscription agreements dated July 25, 2022. No other sponsor, director, officer or affiliate of NewAmsterdam Pharma will participate in the PIPE Financing. See the section entitled “Certain Relationships and Related Party Transactions—NewAmsterdam Pharma Relationships and Related Person Transactions—PIPE Subscription” for additional information regarding such funds and their participation in the PIPE Financing. |
| Q: | What equity stake will the current shareholders of FLAC, the PIPE Investors and the current equityholders of NewAmsterdam Pharma hold in Holdco after the closing of the Business Combination? |
| A: | It is anticipated that, upon completion of the Business Combination: (i) FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) will receive approximately 14.9% of Holdco Shares and 14.9% of the voting rights, (ii) the PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) (some of whom are also NewAmsterdam Pharma shareholders) will receive approximately 22.0% of |
13
Table of Contents
| Holdco Shares and 22.0% of the voting rights, (iii) the FLAC Initial Shareholders and their affiliates (including the Sponsor) will receive approximately 11.0% of Holdco Shares and 11.0% of the voting rights, and (iv) the Participating Shareholders (excluding Holdco Shares issued pursuant to the PIPE Financing, but including the Holdco Shares issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”)) will receive approximately 52.2% of Holdco Shares. |
These ownership levels in Holdco assume the following: (A) no FLAC Class A Ordinary Shares are elected to be redeemed by the public shareholders, (B) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (C) none of the Holdco Warrants have been exercised, (D) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (E) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (F) an aggregate of 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to each of their respective profit rights referenced above, (G) none of the NewAmsterdam Pharma Options have been exercised prior to the Closing and (H) no Holdco Options or awards that may be issued under Holdco’s long-term incentive plan following the Closing have been exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (D) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated.
If all of the Holdco Warrants, NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options were exercisable and immediately exercised upon completion of the Business Combination on a 1:1 basis for Holdco Shares, FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) would hold in aggregate approximately 12.3% of Holdco Shares on a fully diluted basis, the FLAC Initial Shareholders (including the Sponsor) would receive approximately 9.1% of Holdco Shares and 9.1% of the voting rights in aggregate and the Participating Optionholders would receive approximately 4.0% of Holdco Shares and 4.0% of the voting rights in aggregate; however, the Holdco Warrants are subject to restrictions on the timing of their exercise and may also be exercisable on a cashless basis by reference to the fair market value of the Holdco Shares, and these percentages are therefore indicative only.
The ownership percentages with respect to Holdco following the Business Combination do not take into account, unless otherwise expressly stated, the Holdco Warrants, but do include Founder Shares, which will be exchanged for Holdco Shares at the closing of the Business Combination on a 1:1 basis. If the actual facts are different than these assumptions, the ownership percentages in Holdco will be different.
For more information, please see the sections entitled “The Business Combination—Ownership of Holdco Following the Business Combination” and “Unaudited Pro Forma Condensed Combined Financial Information.”
| Q: | Will Holdco obtain new financing in connection with the Business Combination and are there any arrangements to help ensure that Holdco will have sufficient funds to consummate the Business Combination? |
| A: | Yes. Holdco intends to obtain new equity financing through a private placement of Holdco Shares in the PIPE Financing. Holdco intends to use the Aggregate Cash Proceeds for general corporate purposes. The PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination. Unless waived by FLAC or NewAmsterdam Pharma, the Business Combination Agreement provides that each party’s obligation to consummate the Business Combination is conditioned on Holdco having net tangible assets worth at least $5,000,001 immediately after the Closing after giving effect to the amount of cash in the Trust Account (net of the Cash Redemption Amount) together with the proceeds from the PIPE Financing. The committed PIPE subscriptions are sufficient to satisfy this condition if fully funded. Unless waived by NewAmsterdam Pharma, the Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million. The committed |
14
Table of Contents
| PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy this condition, even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights. |
| Q: | Why is FLAC proposing the Adjournment Proposal? |
| A: | FLAC is proposing the Adjournment Proposal to allow the FLAC Board to adjourn the General Meeting to a later date or dates, (i) in order to solicit additional proxies from FLAC shareholders in favor of, or in connection with, the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement or (ii) to allow reasonable time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting. The Adjournment Proposal will only be presented to FLAC shareholders if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with, the Business Combination Agreement. Please see the section entitled “Adjournment Proposal” for additional information. |
| Q: | What happens if I sell my FLAC Ordinary Shares before the General Meeting? |
| A: | The record date for the General Meeting for FLAC shareholders that hold their shares in “street name” is earlier than the date that the Business Combination is expected to be completed. If you transfer your FLAC Ordinary Shares after the record date for FLAC shareholders that hold their shares in “street name,” but before the General Meeting, unless the transferee obtains from you a proxy to vote those shares, you will retain your right to vote at the General Meeting. However, you will not be able to seek redemption of your FLAC Class A Ordinary Shares because you will no longer be able to deliver them for cancellation upon consummation of the Business Combination. If you transfer your FLAC Ordinary Shares prior to the record date for FLAC shareholders that hold their shares in “street name,” you will have no right to vote those shares at the General Meeting or redeem those shares for a pro rata portion of the proceeds held in the Trust Account. |
| Q: | What constitutes a quorum at the General Meeting? |
| A: | One or more shareholders who together hold more than 50% of the issued and outstanding FLAC Ordinary Shares entitled to vote at the General Meeting must be present, in person or represented by proxy, at the General Meeting to constitute a quorum and in order to conduct business at the General Meeting. Broker non-votes and abstentions will be counted as present for the purpose of determining a quorum. The FLAC Initial Shareholders, who currently owns approximately 19% of the issued and outstanding FLAC Ordinary Shares in the aggregate, will count towards this quorum. In the absence of a quorum, the General Meeting will be adjourned to such time or place as is determined by the FLAC Board. As of the record date for the General Meeting for FLAC shareholders that hold their shares in “street name,” the presence of 8,875,501 FLAC Ordinary Shares would be required to achieve a quorum. |
| Q: | What vote is required to approve the proposals presented at the General Meeting? |
| A: | The approval of the Business Combination Proposal requires the affirmative vote of holders of a majority of FLAC Ordinary Shares that are entitled to vote and are voted at the General Meeting, the approval of the Merger Proposal requires the affirmative vote of holders of at least two-thirds of the FLAC Ordinary Shares that are entitled to vote and are voted at the General Meeting. A FLAC shareholder’s failure to vote by proxy or to vote in person at the General Meeting will not be counted towards the number of FLAC Ordinary Shares required to validly establish a quorum, and if a valid quorum is otherwise established, such failure to vote will have no effect on the outcome of any vote on the Business Combination Proposal or the Merger Proposal. Broker non-votes and abstentions will be counted in connection with the determination of whether a valid quorum is established but will have no effect on the Business Combination Proposal or the Merger Proposal. |
The approval of the Adjournment Proposal requires the affirmative vote of holders of a majority of the FLAC Ordinary Shares that are entitled to vote and are voted at the General Meeting. Accordingly, a FLAC shareholder’s failure to vote by proxy or to vote in person at the General Meeting will not be counted towards
15
Table of Contents
the number of FLAC Ordinary Shares required to validly establish a quorum, and if a valid quorum is otherwise established, such failure to vote will have no effect on the outcome of any vote on the Adjournment Proposal. Broker non-votes and abstentions will be counted in connection with the determination of whether a valid quorum is established but will have no effect on the Adjournment Proposal.
The FLAC Initial Shareholders, including the Sponsor, have agreed to vote their Founder Shares and any FLAC Public Shares in favor of the Business Combination Proposal, the Merger Proposal, the Adjournment Proposal and any other proposals that may be submitted to the General Meeting or otherwise to the FLAC shareholders as may be required to approve any of the transactions contemplated under the Business Combination Agreement.
| Q: | What happens if the Business Combination Proposal or the Merger Proposal are not approved? |
A: If the Business Combination Proposal or the Merger Proposal are not approved and FLAC does not consummate the Business Combination, or another initial business combination, by December 11, 2022, absent any extension, FLAC will be required to dissolve and liquidate the Trust Account.
| Q: | How many votes do I have at the General Meeting? |
| A: | FLAC shareholders that hold their shares in “street name” are entitled to one vote on each proposal presented at the General Meeting for each FLAC Ordinary Share held of record as of September 30, 2022, the record date for the General Meeting. As of the close of business on the record date, there were 17,751,000 outstanding FLAC Ordinary Shares. For the avoidance of doubt, the record date does not apply to FLAC shareholders that hold their shares in registered form and are registered as shareholders in FLAC’s register of members. FLAC shareholders that hold their shares in registered form are entitled to one vote on each proposal presented at the General Meeting for each FLAC Ordinary Share held on the date of the General Meeting. |
| Q: | What is the recommendation of the FLAC Board as to each of the Proposals? |
| A: | The FLAC Board, acting upon the unanimous recommendation of the FLAC Special Committee, has approved the Business Combination Agreement, the Business Combination and the Merger, and recommends that FLAC shareholders vote “FOR” the Business Combination Proposal, “FOR” the Merger Proposal, and “FOR” all other proposals presented to FLAC shareholders in the accompanying proxy statement/prospectus. |
| Q: | How will the FLAC Initial Shareholders and FLAC’s other current directors and officers vote? |
| A: | Prior to the FLAC IPO, FLAC entered into agreements with the FLAC Initial Shareholders (including the Sponsor), pursuant to which the FLAC Initial Shareholders have agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of a proposed initial business combination, including the Business Combination. As of the record date, the FLAC Initial Shareholders owned 3,450,000 Founder Shares and no FLAC Public Shares, representing approximately 19% of the FLAC Ordinary Shares entitled to vote at the General Meeting. |
In addition, in connection with the entry into the Business Combination Agreement, FLAC and Holdco entered into the Sponsor Support Agreement with the FLAC Initial Shareholders and NewAmsterdam Pharma, pursuant to which the FLAC Initial Shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them; and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. As of June 30, 2022, certain affiliates of the Sponsor held 1,501,000 FLAC Class A Ordinary Shares, representing approximately 10.5% of the outstanding FLAC Class A Ordinary Shares, including 501,000 FLAC Private Placement Shares which do not have redemption rights.
16
Table of Contents
| Q: | What interests do the Sponsor, FLAC Initial Shareholders and FLAC’s other current officers and directors have in the Business Combination? |
| A: | The Sponsor, FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from or in addition to (and which may conflict with) your interests. You should take these interests into account in deciding whether to approve the Business Combination Proposal and the Merger Proposal. These interests include, among other things, the interests listed below: |
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed not to redeem any Founder Shares or FLAC Public Shares held by them in connection with a shareholder vote to approve a proposed initial business combination; |
| • | the Sponsor paid an aggregate of $25,000 for 2,875,000 Founder Shares. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. Such securities will have a significantly higher value at the time of the Business Combination which, if unrestricted and freely tradable, such Founder Shares would be valued at approximately $34,051,500 (based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022), but, given the restrictions on such shares, FLAC believes such shares have less value. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then FLAC will cease all operations except for the purpose of winding up, redeeming all of the public shares for cash and, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceeding to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In such event, Founder Shares collectively owned by the FLAC Initial Shareholders would be worthless because following the redemption of the public shares, FLAC would likely have few, if any, net assets; |
| • | the Sponsor paid an aggregate of $5,010,000 for its 501,000 FLAC Private Placement Units (with an aggregate fair market value of $4,944,870, based on the closing price of FLAC Public Units on June 30, 2022) and that the component FLAC Private Placement Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, paid an aggregate of $10 million for its 1,000,000 FLAC Public Units in the FLAC IPO (with an aggregate fair market value of $9,870,000, based on the closing price of FLAC Public Units on June 30, 2022), and has agreed to waive any redemption rights with respect to such securities in connection with the Business Combination. In addition, the component FLAC Public Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, and Frazier Life Sciences XI, L.P., Frazier Life Sciences Public Fund, L.P. and Frazier Life Sciences Overage Fund, L.P., each an affiliate of Frazier, an affiliate of the Sponsor, have committed to purchasing 4,500,000 Holdco Shares at a price per share of $10.00 as part of the PIPE Financing; and have agreed to waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination; |
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed to waive their rights to liquidating distributions from the Trust Account with respect to any Founder Shares held by them if FLAC fails to complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | the Investor Rights Agreement will be entered into by the FLAC Initial Shareholders; |
| • | the Holdco Shares to be received by the FLAC Initial Shareholders in connection with the Merger will be subject to certain lock-up periods; |
17
Table of Contents
| • | the continued indemnification of FLAC’s existing directors and officers and the continuation of FLAC’s directors’ and officers’ liability insurance after the Business Combination; |
| • | the Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to shareholders rather than liquidate; |
| • | the Sponsor and its affiliates can earn a positive rate of return on their investment, even if other FLAC shareholders experience a negative rate of return in the post-business combination company; |
| • | FLAC has the right to select two individuals, one of which is expected to be Jamie Topper, to be nominated for election to the initial Holdco Board, who must be reasonably acceptable to NewAmsterdam Pharma and qualify as “independent” directors for purposes of Nasdaq rules; |
| • | the Sponsor, FLAC’s officers and directors, and their respective affiliates will lose their entire investment in FLAC (which is estimated to be approximately $34.5 million, based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022) and will not be reimbursed for any out-of-pocket expenses (which are currently $0) if an initial business combination is not consummated by December 11, 2022, absent any extension; |
| • | the potential hire of David Topper, the Chief Financial Officer and a director and shareholder of FLAC, as the chief financial officer of Holdco in the first half of 2023; |
| • | the Sponsor (including its representatives and affiliates) and FLAC’s officers and directors, are, or may in the future become, affiliated with entities that are engaged in a similar business to Holdco and/or NewAmsterdam Pharma. The representatives and affiliates of the Sponsor, and certain of FLAC’s officers and directors, are in the business of making investments in companies, and may acquire and hold interests in businesses that compete directly or indirectly with Holdco and/or NewAmsterdam Pharma. Certain of FLAC’s officers and directors also have time and attention requirements for investment funds of which they and affiliates of the Sponsor are the investment managers. The Sponsor and FLAC’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to FLAC completing its initial business combination; and |
| • | if the Trust Account is liquidated, including in the event FLAC is unable to complete an initial business combination within the required time period, the Sponsor has agreed to indemnify FLAC to ensure that the proceeds in the Trust Account are not reduced below $10.00 per FLAC Public Share, or such lesser per public share amount as is in the Trust Account on the liquidation date, by the claims of prospective target businesses with which FLAC has entered into an acquisition agreement or claims of any third party for services rendered or products sold to FLAC, but only if such a vendor or target business has not executed a waiver of any and all rights to seek access to the Trust Account. |
See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of additional considerations in connection with the Business Combination.
| Q: | Did the FLAC Board obtain a third-party valuation or fairness opinion in determining whether or not to proceed with the Business Combination? |
| A: | Yes. FLAC retained Lincoln International LLC (“Lincoln”) to provide a fairness opinion to the FLAC Board. On July 24, 2022, Lincoln delivered its opinion to the FLAC Board as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement. For a description of the opinion issued by Lincoln to the FLAC Board, please see “The Business Combination—Opinion of the Financial Advisor to the FLAC Board.” |
| Q: | What happens if I vote against the Business Combination Proposal or the Merger Proposal? |
| A: | If you vote against the Business Combination Proposal or the Merger Proposal but the Business Combination Proposal and the Merger Proposal still obtain the affirmative vote of holders of (i) at least the majority of FLAC Ordinary Shares with respect to the Business Combination Proposal and (ii) two-thirds of |
18
Table of Contents
| FLAC Ordinary Shares with respect to the Merger Proposal, that are entitled to vote and are voted at the General Meeting, then the Business Combination Proposal and the Merger Proposal will be approved, respectively, and, assuming the satisfaction or waiver of the other conditions to closing, the Business Combination will be consummated in accordance with the terms of the Business Combination Agreement. |
If you vote against the Business Combination Proposal or the Merger Proposal and the Business Combination Proposal or the Merger Proposal do not obtain the affirmative vote of holders of (i) at least the majority of FLAC Ordinary Shares with respect to the Business Combination Proposal or (ii) two-thirds of FLAC Ordinary Shares with respect to the Merger Proposal, that are entitled to vote and are voted at the General Meeting, then the Business Combination Proposal and the Merger Proposal, respectively, will fail and FLAC will not consummate the Business Combination. If FLAC does not consummate the Business Combination, it may continue to try to complete a business combination with a different target business until December 11, 2022 or such later date as may be approved by FLAC’s shareholders. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then it will be required to dissolve and liquidate the Trust Account by returning the then-remaining funds in such account to its public shareholders.
| Q: | How do the FLAC Public Warrants differ from the FLAC Private Placement Warrants and what are the related risks for any holders of FLAC Public Warrants following the Business Combination? |
| A: | The FLAC Private Placement Warrants are identical to the FLAC Public Warrants in all material respects, except that the FLAC Private Placement Warrants (i) will not be transferable, assignable or salable until 30 days after the completion of the initial business combination, (ii) will not be redeemable by FLAC (except as described in the notes to FLAC’s financial statements included elsewhere in this proxy statement/ prospectus) so long as they are held by the Sponsor or its permitted transferees, and (iii) may be exercised for cash or on a cashless basis. The Sponsor, or its permitted transferees, have the option to exercise the FLAC Private Placement Warrants on a cashless basis. If the FLAC Private Placement Warrants are held by holders other than the Sponsor or its permitted transferees, the FLAC Private Placement Warrants will be redeemable by FLAC in all redemption scenarios and exercisable by the holders on the same basis as the FLAC Public Warrants. |
Following the Business Combination, Holdco may redeem the Holdco Warrants prior to their exercise at a time that is disadvantageous to you, thereby significantly impairing the value of such warrants. Holdco will have the ability to redeem all outstanding Holdco Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, provided that the closing price of the Holdco Shares equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading day period ending on the third trading day prior to the date on which a notice of redemption is sent to the warrantholders (the “Reference Value”). Holdco will not redeem the Holdco Warrants as described above unless a registration statement under the Securities Act covering the Holdco Shares issuable upon exercise of such warrants is effective and a current prospectus relating to those Holdco Shares is available throughout the 30-day redemption period. If and when the Holdco Warrants become redeemable by Holdco, it may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding Holdco Warrants could force you (i) to exercise your Holdco Warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, (ii) to sell your Holdco Warrants at the then-current market price when you might otherwise wish to hold your Holdco Warrants or (iii) to accept the nominal redemption price which, at the time the outstanding Holdco Warrants are called for redemption, is likely to be substantially less than the market value of your Holdco Warrants.
In addition, Holdco will have the ability to redeem the outstanding Holdco Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.10 per warrant if, among other things, the Reference Value of the Holdco Shares equals or exceeds $10.00 per share but is less than $18.00 per share (as adjusted for share sub-divisions, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like) and the Holdco Private Placement Warrants have also been called for redemption, subject to certain limitations as set out in the Warrant Assumption Agreement. Recent trading prices for the FLAC Class A Ordinary Shares have not exceeded the $10.00 per share threshold at which the
19
Table of Contents
Holdco Warrants would become redeemable. During any 30-day redemption period, the holders will be able to exercise their Holdco Warrants prior to redemption for a number of Holdco Shares determined based on the redemption date and the fair market value of the Holdco Shares. Please see the notes to FLAC’s financial statements included elsewhere in this proxy statement/prospectus. The value received upon exercise of the Holdco Warrants (1) may be less than the value the holders would have received if they had exercised their Holdco Warrants at a later time where the underlying share price is higher and (2) may not compensate the holders for the value of the Holdco Warrants.
In each case, Holdco may only call the Holdco Warrants for redemption upon a minimum of 30 days’ prior written notice of redemption to each holder, provided that holders will be able to exercise their Holdco Warrants prior to the time of redemption and, at Holdco’s election, any such exercise may be required to be on a cashless basis.
| Q: | Do I have redemption rights? |
| A: | Pursuant to the FLAC Articles of Association, holders of FLAC Public Shares other than the Sponsor and any FLAC directors or officers may elect to have their shares redeemed for cash at the applicable redemption price per share calculated in accordance with the FLAC Articles of Association. As of June 30, 2022, this would have amounted to approximately $10.00 per share. If a holder of FLAC Public Shares exercises its redemption rights, then such holder will be exchanging its FLAC Class A Ordinary Shares for cash and will not own Holdco Shares following the closing of the Business Combination. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to the Transfer Agent in accordance with the procedures described herein. The redemption rights include the requirement that a shareholder must identify itself, at least two business days prior to the General Meeting, in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Notwithstanding the foregoing, a holder of the FLAC Public Shares individually, or, together with any affiliate of his or her or any other person with whom he or she is acting in concert or as a “group” (as defined in Section 13 of the Exchange Act) will be restricted from seeking redemption rights with respect to more than 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO. Accordingly, all FLAC Public Shares in excess of the 15% threshold beneficially owned by a public shareholder or group will not be redeemed for cash unless consented to by FLAC. |
FLAC has no specified maximum redemption threshold under the FLAC Articles of Association, other than the aforementioned 15% threshold. Each redemption of FLAC Class A Ordinary Shares by FLAC public shareholders will reduce the amount in the Trust Account, which held marketable securities with a fair value of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting. Unless waived by FLAC or NewAmsterdam Pharma, the Business Combination Agreement provides that each party’s obligation to consummate the Business Combination is conditioned on Holdco having net tangible assets worth at least $5,000,001 immediately after the Closing after giving effect to the amount of cash in the Trust Account (net of the Cash Redemption Amount) together with the proceeds from the PIPE Financing. The committed PIPE subscriptions are sufficient to satisfy this condition if fully funded. Unless waived by NewAmsterdam Pharma, the Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy this condition, even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights. The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. In no event will FLAC redeem its FLAC Class A Ordinary Shares in an amount that would cause its net tangible assets to be less than $5,000,001, as provided in the FLAC Articles of Association. FLAC shareholders who wish to redeem their public shares for cash must refer to and follow the procedures set forth in the section entitled “General Meeting of FLAC—Redemption Rights” in order to properly redeem their public shares.
Holders of FLAC Public Warrants or FLAC Private Placement Shares will not have redemption rights with respect to such securities.
20
Table of Contents
| Q: | Can the FLAC Initial Shareholders redeem their Founder Shares in connection with consummation of the Business Combination? |
| A: | No. The FLAC Initial Shareholders, including the Sponsor, have agreed, for no additional consideration, to waive their redemption rights with respect to their Founder Shares and any FLAC Public Shares they may hold in connection with the consummation of the Business Combination. |
| Q: | What happens if a substantial number of the public shareholders vote in favor of the Business Combination Proposal and exercise their redemption rights? |
| A: | FLAC’s public shareholders are not required to vote against the Business Combination Proposal in order to exercise their redemption rights. Accordingly, the Business Combination may be consummated even though the funds available from the Trust Account and the number of public shareholders are reduced as a result of redemptions by public shareholders. |
If a public shareholder exercises its redemption rights, such exercise will not result in the loss of any warrants that it may hold. Assuming that 100% or 12,300,000 FLAC Class A Ordinary Shares held by FLAC’s public shareholders were redeemed, the 4,600,000 retained outstanding FLAC Public Warrants would have had an aggregate value of $368,000 on June 30, 2022.
If a substantial number of, but not all, FLAC public shareholders exercise their redemption rights, any non-redeeming shareholders would experience dilution to the extent such warrants are exercised and additional Holdco Shares are issued. The FLAC Initial Shareholders, including the Sponsor, have agreed not to redeem any Founder Shares or FLAC Public Shares held by them in connection with a shareholder vote to approve a proposed initial business combination. A public shareholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its FLAC Public Shares or, if part of such a group, the group’s FLAC Public Shares, in excess of 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO (i.e., in excess of 2,070,000 FLAC Class A Ordinary Shares).
Additionally, as a result of redemptions, the trading market for the Holdco Shares may be less liquid than the market for the FLAC Class A Ordinary Shares was prior to consummation of the Business Combination and Holdco may not be able to meet the listing standards for Nasdaq or another national securities exchange.
The below sensitivity table shows the potential impact of possible redemptions on the pro forma book value per share of the shares owned by non-redeeming shareholders in the No Redemption, Low Redemption (which assumes that 33% of FLAC Public Shares held by public shareholders are redeemed), High Redemption (which assumes that 66% of FLAC Public Shares held by public shareholders are redeemed) and Maximum Redemption scenarios (which assumes that 12,261,482 FLAC Public Shares are redeemed, which number represents the maximum number of FLAC Public Shares that may be redeemed such that the closing condition requiring the Aggregate Cash Proceeds equal or exceed $250 million can still be satisfied). Pursuant to the Sponsor Support Agreement and the Investor Support Agreements, shareholders holding 1,500,000 FLAC Class A Ordinary Shares have agreed not to redeem their shares.
21
Table of Contents
Shareholders | Assuming No Redemption(1) | Assuming Low Redemption(1) | Assuming High Redemption(1) | Assuming Maximum Redemption(1) | ||||||||||||||||||||||||||||
| Ownership in shares | Equity % | Ownership in shares | Equity % | Ownership in shares | Equity % | Ownership in shares | Equity % | |||||||||||||||||||||||||
NewAmsterdam Pharma shareholders(2) | 44,914,642 | 52.15 | % | 44,914,642 | 54.84 | % | 44,914,642 | 57.82 | % | 44,914,642 | 60.81 | % | ||||||||||||||||||||
FLAC public shareholders(3) | 12,800,000 | 14.86 | % | 8,576,000 | 10.47 | % | 4,352,000 | 5.60 | % | 538,518 | 0.73 | % | ||||||||||||||||||||
FLAC Initial Shareholders, including the Sponsor and affiliates(4) | 9,451,000 | 10.97 | % | 9,451,000 | 11.54 | % | 9,451,000 | 12.17 | % | 9,451,000 | 12.80 | % | ||||||||||||||||||||
PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) | 18,960,000 | 22.01 | % | 18,960,000 | 23.15 | % | 18,960,000 | 24.41 | % | 18,960,000 | 25.67 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Total Holdco Shares Outstanding(5) | 86,125,642 | 100 | % | 81,901,642 | 100 | % | 77,677,642 | 100 | % | 73,864,160 | 100 | % | ||||||||||||||||||||
Additional Sources of Future Dilution | ||||||||||||||||||||||||||||||||
Earnout Shares | 1,886,137 | 2.19 | % | 1,886,137 | 2.30 | % | 1,886,137 | 2.43 | % | 1,886,137 | 2.55 | % | ||||||||||||||||||||
Holdco Public Warrants(6) | 4,600,000 | 5.34 | % | 4,600,000 | 5.62 | % | 4,600,000 | 5.92 | % | 4,600,000 | 6.23 | % | ||||||||||||||||||||
Holdco Private Placement Warrants(7) | 167,000 | 0.19 | % | 167,000 | 0.20 | % | 167,000 | 0.21 | % | 167,000 | 0.23 | % | ||||||||||||||||||||
Holdco Options(8) | 11,901,209 | 13.82 | % | 10,708,459 | 13.10 | % | 10,159,339 | 13.10 | % | 9,763,120 | 13.22 | % | ||||||||||||||||||||
| (1) | The 501,000 FLAC Private Placement Shares may not be redeemed. Pursuant to the Sponsor Support Agreement, the Investor Support Agreements and the FLAC Articles of Association, FLAC’s directors and officers and certain existing FLAC investors have agreed not to redeem an aggregate of 1,500,000 FLAC Public Shares. |
| (2) | (a) Excludes 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) and (b) includes an aggregate of 8,656,330 Holdco Shares issuable to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement.” |
| (3) | Includes all FLAC Public Shares other than 1,000,000 FLAC Class A Ordinary Shares held by an affiliate of the Sponsor. |
| (4) | Includes (i) 4,500,000 Holdco Shares acquired in the PIPE Financing and (ii) 4,801,000 of Holdco Shares acquired by the Sponsor and certain of its affiliates in connection with the Business Combination. |
| (5) | The percentage presented in each scenario reflects the percentage of the total Holdco Shares outstanding, excluding Holdco Shares underlying Holdco Warrants, Holdco Shares underlying NewAmsterdam Pharma Options, Holdco Shares underlying Holdco Options and the Earnout Shares. |
| (6) | Based on the 4,600,000 FLAC Public Warrants outstanding on the date hereof. |
| (7) | Based on the 167,000 FLAC Private Warrants outstanding on the date hereof. |
| (8) | Based on 4,185,358 Holdco Options (or 1,964,287 NewAmsterdam Pharma Options outstanding on the date hereof), and (i) in the No Redemption scenario, up to approximately 7,715,849 Holdco Options expected to be awarded prior to the Closing, (ii) in the Low Redemption scenario, up to approximately 6,523,099 Holdco Options expected to be awarded prior to the Closing, (iii) in the High Redemption scenario, up to approximately 5,973,979 Holdco Options expected to be awarded prior to the Closing and (iv) in the Maximum Redemption scenario up to approximately 5,577,760 Holdco Options expected to be awarded prior to the Closing. The Holdco Options which Holdco expects to award in connection with the Closing are currently expected to be awarded to certain executive officers and directors. See the section entitled “Management of Holdco Following the Business Combination—Holdco LTIP” for more information. |
The level of redemption also impacts the effective underwriting fee incurred in connection with the FLAC IPO. In a no redemption scenario, based on the approximately $138,120,000 in the Trust Account as of June 30, 2022, approximately $4.8 million in deferred underwriting fees from the FLAC IPO represents an effective deferred underwriting fee of approximately 3.5% as a percentage of cash in the Trust Account. In a maximum redemption scenario, the effective underwriting fee would be approximately 47.9% as a percentage of the cash in the Trust Account. The below sensitivity table shows the potential impact of redemptions on the effective deferred underwriting commission as a percentage of the cash left in the Trust Account.
22
Table of Contents
| ($ in thousands) | Assuming No Redemption | Assuming Low Redemption(1) | Assuming High Redemption(2) | Assuming Maximum Redemption(3) | ||||||||||||
Redemptions ($) | $ | — | $ | 40,999,590 | $ | 81,999,180 | $ | 122,614,820 | ||||||||
Redemptions (Shares) | — | 4,099,959 | 8,199,918 | 12,261,482 | ||||||||||||
Total Deferred Underwriting Commission ($) | $ | 4,830,000 | $ | 4,830,000 | $ | 4,830,000 | $ | 4,830,000 | ||||||||
Effective Deferred Underwriting Commission (as a percentage of cash left in Trust Account post redemptions) | 3.5 | % | 5.0 | % | 8.6 | % | 31.2 | % | ||||||||
Total Underwriting Commission ($) | $ | 7,590,000 | $ | 7,590,000 | $ | 7,590,000 | $ | 7,590,000 | ||||||||
Effective Total Underwriting Commission (as a percentage of cash left in Trust Account post redemptions) | 5.5 | % | 7.8 | % | 13.5 | % | 49.0 | % | ||||||||
| (1) | Assumes 33% of the 12,300,000 FLAC Public Shares held by public shareholders are redeemed. |
| (2) | Assumes 66% of the 12,300,000 FLAC Public Shares held by public shareholders are redeemed. |
| (3) | Assumes that 12,261,482 FLAC Public Shares are redeemed, which number represents the maximum number of FLAC Public Shares that may be redeemed such that the closing condition requiring the aggregate proceeds from the Trust Account and the PIPE Financing equal $250 million can still be satisfied). |
| Q: | Is there a limit on the number of shares I may redeem? |
| A: | Yes. A public shareholder individually, or, together with any affiliate of such shareholder or any other person with whom such shareholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), may not redeem FLAC Public Shares in excess of an aggregate of 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO without FLAC’s consent. Accordingly, all FLAC Class A Ordinary Shares in excess of aforementioned 15% threshold owned by a holder will not be redeemed for cash without FLAC’s consent. On the other hand, a public shareholder who holds less than 15% of the FLAC Public Shares may redeem all of the FLAC Public Shares held by such shareholder for cash. |
FLAC Class B Ordinary Shares cannot be redeemed by the holders in connection with the Business Combination.
In no event is your ability to vote all of your shares (including those shares held by you in excess of 15% of the shares sold in the FLAC IPO) for or against the Business Combination restricted.
FLAC has no specified maximum redemption threshold under the FLAC Articles of Association, other than the aforementioned 15% threshold. Each redemption of a FLAC Public Share by FLAC public shareholders will reduce the amount in the Trust Account, which held marketable securities with a fair value of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting. Unless waived by FLAC or NewAmsterdam Pharma, the Business Combination Agreement provides that each party’s obligation to consummate the Business Combination is conditioned on Unless waived by FLAC or NewAmsterdam Pharma, the Business Combination Agreement provides that each party’s obligation to consummate the Business Combination is conditioned on Holdco having net tangible assets worth at least $5,000,001 immediately after the Closing and after giving effect to the amount of cash in the Trust Account (net of the Cash Redemption Amount) together with the proceeds from the PIPE Financing. The committed PIPE subscriptions are sufficient to satisfy this condition if fully funded. Unless waived by NewAmsterdam Pharma, the Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy this condition, even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights. The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. In no event will FLAC redeem its FLAC Class A Ordinary Shares in an amount that would cause its net tangible assets to be less than $5,000,001, as provided in the FLAC Articles of Association.
| Q: | Will how I vote affect my ability to exercise redemption rights? |
| A: | No. You may exercise your redemption rights whether you vote your FLAC Class A Ordinary Shares for or against, or whether you abstain from voting on, the Business Combination Proposal, the Merger Proposal or |
23
Table of Contents
| any other proposal described by this proxy statement/prospectus. As a result, the Business Combination Agreement and the Plan of Merger can be approved by shareholders who will redeem their shares and no longer remain shareholders, leaving shareholders who choose not to redeem their shares holding shares in a company with a potentially less-liquid trading market, fewer shareholders, potentially less cash and the potential inability to meet the listing standards of Nasdaq. |
| Q: | How do I exercise my redemption rights? |
| A: | In order to exercise your redemption rights, you must (i) if you hold FLAC Public Units, separate the underlying FLAC Class A Ordinary Shares and FLAC Public Warrants, and (ii) prior to 5:00 p.m., Eastern time, on November 10, 2022 (two business days before the initial date of the General Meeting), tender your shares physically or electronically and identify yourself in writing as a beneficial holder and provide your legal name, phone number and address to the Transfer Agent in order to validly redeem your shares and submit a request in writing that FLAC redeem your FLAC Class A Ordinary Shares for cash to the Transfer Agent at the following address: |
Continental Stock Transfer & Trust Company
1 State Street
New York, New York 10004
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
You do not have to be a record date holder in order to exercise your redemption rights. FLAC shareholders seeking to exercise their redemption rights and opting to deliver physical certificates should allot sufficient time to obtain physical certificates from the Transfer Agent and time to effect delivery. It is FLAC’s understanding that FLAC shareholders should generally allot at least two weeks to obtain physical certificates from the Transfer Agent. However, FLAC does not have any control over this process and it may take longer than two weeks. FLAC shareholders who hold their shares in “street name” will have to coordinate with their bank, broker or other nominee to have the shares certificated or delivered electronically.
FLAC shareholders seeking to exercise their redemption rights, whether they are registered holders or hold their shares in “street name” are required to either tender their certificates to the Transfer Agent prior to the date set forth in this proxy statement/ prospectus, at least two business days prior to the vote on the Business Combination Proposal at the General Meeting, or to deliver their shares to the Transfer Agent electronically using Depository Trust Company’s (“DTC”) Deposit/Withdrawal At Custodian (“DWAC”) system, at such shareholder’s option. The requirement for physical or electronic delivery prior to the General Meeting ensures that a redeeming shareholder’s election to redeem is irrevocable once the Business Combination is approved.
Any demand for redemption, once made, may be withdrawn at any time until the vote is taken with respect to the Business Combination. If you delivered your shares for redemption to the Transfer Agent and decide within the required timeframe not to exercise your redemption rights, you may request that the Transfer Agent return the shares (physically or electronically). The redemption rights include the requirement that a shareholder must identify himself, herself or itself in writing as a beneficial holder and provide his, her or its legal name, phone number and address to the Transfer Agent in order to validly redeem his, her or its shares. You may make such request by contacting the Transfer Agent at the phone number or address listed under the question “Who can help answer my questions?” below.
If you hold FLAC Public Units registered in your own name, you must deliver the certificate for such FLAC Public Units to the Transfer Agent with written instructions to separate such FLAC Public Units into FLAC Class A Ordinary Shares and FLAC Public Warrants. This must be completed far enough in advance to permit the mailing of the public share certificates back to you so that you may then exercise your redemption rights upon the separation of the public shares from the FLAC Public Units.
If a broker, dealer, commercial bank, trust company or other nominee holds your FLAC Public Units, you must instruct such nominee to separate your FLAC Public Units. Your nominee must send written instructions by facsimile to the Transfer Agent. Such written instructions must include the number of FLAC Public Units to be split and the nominee holding such FLAC Public Units. Your nominee must also initiate electronically, using
24
Table of Contents
DTC’s DWAC system, a withdrawal of the relevant units and a deposit of an equal number of FLAC Class A Ordinary Shares and FLAC Public Warrants. This must be completed far enough in advance to permit your nominee to exercise your redemption rights upon the separation of the public shares from the FLAC Public Units. While this is typically done electronically on the same business day, you should allow at least one full business day to accomplish the separation. If you fail to cause your FLAC Public Units to be separated in a timely manner, you will likely not be able to exercise your redemption rights.
There is a nominal cost associated with the above-referenced tendering process and the act of certificating the shares or delivering them through the DWAC system. The Transfer Agent will typically charge a tendering broker a fee and it is in the broker’s discretion whether or not to pass this cost on to the redeeming shareholder. However, this fee would be incurred regardless of whether or not shareholders seeking to exercise redemption rights are required to tender their shares, as the need to deliver shares is a requirement to exercising redemption rights, regardless of the timing of when such delivery must be effectuated.
| Q: | What are the U.S. federal income tax consequences of exercising my redemption rights? |
| A: | The U.S. federal income tax consequences of exercising your redemption rights depend on your particular facts and circumstances. See the sections entitled “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the FLAC Shareholder Redemption to U.S. Holders of FLAC Class A Ordinary Shares” and “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the FLAC Shareholder Redemption to Non-U.S. Holders of FLAC Class A Ordinary Shares.” If you are a holder of FLAC Class A Ordinary Shares contemplating exercise of your redemption rights, you are urged to consult your tax advisor to determine the tax consequences thereof. |
| Q: | What are the U.S. federal income tax consequences to me of the Merger? |
| A: | Subject to the limitations and qualifications described in “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities” and “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to Non-U.S. Holders of FLAC Securities” below, the parties to the Merger have agreed to report the Merger, taken together with certain related transactions, as both a transaction described under Section 351 of the Code and as a “reorganization” within the meaning of Section 368 of the Code. If the Merger is treated in this manner for U.S. federal income tax purposes, you will not recognize gain or loss for U.S. federal income tax purposes on the receipt of Holdco Shares for FLAC Class A Ordinary Shares and Holdco Public Warrants for FLAC Public Warrants in connection with the Merger. However, there are significant factual and legal uncertainties as to whether the Merger will qualify as a reorganization. Accordingly, counsel expresses no opinion that the Merger will qualify as a reorganization under Section 368(a) of the Code, and neither FLAC nor NewAmsterdam Pharma intends to request a ruling from the IRS regarding the U.S. federal income tax treatment of the Merger. In addition, no assurance can be given that the IRS will not challenge the Merger’s qualification as a reorganization or that a court will not sustain such a challenge by the IRS, in which case you could recognize gain for U.S. federal income tax purposes on the receipt Holdco Public Warrants for FLAC Public Warrants in connection with the Merger. If the Merger does not qualify as a “reorganization” within the meaning of Section 368 of the Code, you generally will recognize gain or loss for U.S. federal income tax purposes on the receipt of Holdco Public Warrants for FLAC Public Warrants in connection with the Merger. |
| Q: | If I am a FLAC warrant holder, can I exercise redemption rights with respect to my FLAC Public Warrants? |
| A: | No. The holders of FLAC Public Warrants have no redemption rights with respect to such warrants. |
| Q: | Do I have appraisal rights or dissenters’ rights if I object to the proposed Business Combination? |
| A: | Shareholders of FLAC may be entitled to give notice to FLAC prior to the General Meeting that they wish to dissent to the Merger and to receive payment of fair market value for his or her shares if they follow the procedures set out in the Cayman Islands Companies Act, noting that any such dissenters’ rights may be |
25
Table of Contents
| limited pursuant to Section 239 of the Cayman Islands Companies Act which states that no such dissenters’ rights will be available in respect of shares of any class for which an open market exists on a recognized stock exchange at the expiry date of the period allowed for written notice of an election to dissent provided that the merger consideration constitutes shares of any company which at the effective date of the merger are listed on a national securities exchange. It is FLAC’s view that such fair market value would equal the amount which shareholders would obtain if they exercise their redemption rights as described herein. |
| Q: | What happens to the funds held in the Trust Account upon consummation of the Business Combination? |
| A: | If the Business Combination is consummated, the funds held in the Trust Account will be used to: (i) pay FLAC public shareholders who properly exercise their redemption rights, (ii) pay approximately $4.8 million in deferred underwriting commissions to the underwriters of the FLAC IPO, and (iii) pay certain other fees, costs and expenses (including regulatory fees, legal fees, accounting fees, printer fees and other professional fees) that were incurred by FLAC and other parties to the Business Combination Agreement in connection with the Business Combination pursuant to the terms of the Business Combination Agreement (which are currently estimated to be approximately $20 million, excluding the deferred underwriting fees). Any remaining funds will be used by Holdco for general corporate purposes, and thereafter, the Trust Account will terminate except as otherwise provided therein. |
| Q: | What conditions must be satisfied to complete the Business Combination? |
| A: | There are a number of closing conditions in the Business Combination Agreement, including the approval by FLAC shareholders of the Business Combination Proposal and the Merger Proposal. For a summary of the conditions that must be satisfied or waived prior to completion of the Business Combination, please see the section entitled “The Business Combination Agreement and Ancillary Documents—Conditions to Closing of the Business Combination.” |
| Q: | What happens if the Business Combination Agreement is terminated or the Business Combination is not consummated? |
| A: | There are certain circumstances under which the Business Combination Agreement may be terminated. Please see the section entitled “The Business Combination Agreement and Ancillary Documents” for information regarding the parties’ specific termination rights. |
If FLAC does not consummate the Business Combination, it may continue to try to complete a business combination with a different target business until December 11, 2022. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then FLAC will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to us to pay for FLAC income taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public shareholders’ rights as shareholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceed to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In the event of such distribution, it is possible that the per share value of the residual assets remaining available for distribution (including Trust Account assets) will be less than the initial public offering price per unit in the FLAC IPO. Please see the section entitled “Risk Factors—Risks Related to FLAC” for additional information.
Holders of Founder Shares have waived any right to any liquidation distribution with respect to such shares. In addition, there will be no redemption rights or liquidating distributions with respect to the FLAC Public Warrants and the FLAC Private Placement Warrants, which will expire worthless if FLAC fails to complete an initial business combination by December 11, 2022, absent any extension.
26
Table of Contents
| Q: | When is the Business Combination expected to be completed? |
| A: | The closing of the Business Combination is expected to occur in the second half of 2022. See the section entitled “The Business Combination Agreement and Ancillary Documents—Conditions to Closing of the Business Combination” for a description of the conditions which much be satisfied or waived prior to the closing. The Business Combination Agreement may be terminated by FLAC or NewAmsterdam Pharma if the closing of the Business Combination has not occurred on or prior to December 11, 2022 (the “Termination Date”). |
| Q: | What do I need to do now? |
| A: | You are urged to read carefully and consider the information contained in this proxy statement/ prospectus, including the Annexes, and to consider how the Business Combination will affect you as a shareholder. You should then vote as soon as possible in accordance with the instructions provided in this proxy statement/prospectus and on the enclosed proxy card or, if you hold your shares through a brokerage firm, bank or other nominee, on the voting instruction form provided by the broker, bank or nominee. |
| Q: | How do I vote? |
| A: | We will be hosting the General Meeting via live webcast at www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. If you hold your shares in “street name” and were a holder of record of FLAC Ordinary Shares on September 30, 2022, the record date for the General Meeting, you may vote with respect to the proposals by attending the General Meeting virtually or in person, or by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. For the avoidance of doubt, the record date does not apply to FLAC shareholders that hold their shares in registered form and are registered as shareholders in FLAC’s register of members. All holders of shares in registered form on the day of the General Meeting are entitled to vote at the General Meeting. |
Voting by Mail. By signing the proxy card and returning it in the enclosed prepaid and addressed envelope, you are authorizing the individuals named on the proxy card to vote your shares at the General Meeting in the manner you indicate. You are encouraged to sign and return the proxy card even if you plan to attend the General Meeting so that your shares will be voted if you are unable to attend the General Meeting. If you receive more than one proxy card, it is an indication that your shares are held in multiple accounts. Please sign and return all proxy cards to ensure that all of your shares are voted. Votes submitted by mail must be received by 11:59 p.m. Eastern time, on November 14, 2022.
Voting at the General Meeting. We will be hosting the General Meeting via live webcast at www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. You are strongly encouraged to participate in the General Meeting virtually and not attend in person. A summary of the information you need to attend the General Meeting online is provided below:
| • | Instructions on how to attend and participate via the Internet, including how to demonstrate proof of stock ownership, are posted at www.cstproxy.com/flac/2022. |
| • | Assistance with questions regarding how to attend and participate via the Internet will be provided at www.cstproxy.com/flac/2022 on the day of the General Meeting. |
| • | The General Meeting starts at 10:30 a.m. Eastern time. |
| • | You will need your 12-digit control number to enter the General Meeting. |
| • | Shareholders may submit questions while attending the General Meeting via internet. |
If your shares are registered in your name with Continental Stock Transfer and Trust Company and you attend the General Meeting and plan to vote virtually, you must visit www.cstproxy.com/flac/2022, enter the 12-digit control number included on your proxy card or notice of the General Meeting and click on the “Click here to preregister for the online meeting” link at the top of the page. Just prior to the start of the General Meeting you will need to log back into the General Meeting site using your control number. Pre-registration is recommended but is not required in order to attend. Beneficial shareholders (those holding shares through a
27
Table of Contents
stock brokerage account or by a bank or other holder of record) who wish to attend the virtual meeting must obtain a legal proxy by contacting their account representative at the bank, broker, or other nominee that holds their shares and e-mail a copy (a legible photograph is sufficient) of their legal proxy to proxy@continentalstock.com. Beneficial shareholders who e-mail a valid legal proxy will be issued a 12-digit meeting control number that will allow them to register to attend and participate in the virtual meeting. After contacting Continental Stock Transfer & Trust Company, a beneficial holder will receive an e-mail prior to the General Meeting with a link and instructions for entering the virtual meeting. Beneficial shareholders should contact Continental Stock Transfer & Trust Company at least five business days prior to the General Meeting date in order to ensure access.
If you attend the General Meeting and plan to vote in person, you will be provided with a ballot at the General Meeting. If your shares are registered directly in your name, you are considered the shareholder of record and you have the right to vote in person at the General Meeting. If you hold your shares in “street name,” which means your shares are held of record by a broker, bank or other nominee, you should follow the instructions provided by your broker, bank or nominee to ensure that votes related to the shares you beneficially own are properly counted. In this regard, you must provide the record holder of your shares with instructions on how to vote your shares or, if you wish to attend the General Meeting and vote in person, you will need to bring to the General Meeting a legal proxy from your broker, bank or nominee authorizing you to vote these shares. For additional information, please see the section entitled “General Meeting of FLAC.”
To be admitted to the General Meeting, you will need a form of photo identification and valid proof of ownership of FLAC Ordinary Shares or a valid legal proxy. If you have a legal proxy from a shareholder of record, you must bring a form of photo identification and the legal proxy to the General Meeting. If you have a legal proxy from a “street name” shareholder, you must bring a form of photo identification, a legal proxy from the record holder (that is, the bank, broker or other holder of record) to the “street name” shareholder that is assignable, and the legal proxy from the “street name” shareholder to you. Shareholders may appoint only one proxy holder to attend on their behalf. Shareholders that hold their shares in registered form on the date of the General Meeting are entitled to attend and vote at the General Meeting.
| Q: | What will happen if I abstain from voting or fail to vote at the General Meeting? |
| A: | At the General Meeting, a properly executed proxy marked “ABSTAIN” with respect to a particular proposal will be counted as present for purposes of determining whether a quorum is present. For purposes of approval, broker non-votes and abstentions will have no effect on the Business Combination Proposal, the Merger Proposal or the Adjournment Proposal. |
| Q: | What will happen if I sign and return my proxy card without indicating how I wish to vote? |
| A: | Signed and dated proxies received by FLAC without an indication of how the shareholder intends to vote on a proposal will be voted “FOR” each proposal presented to the shareholders. The proxyholders may use their discretion to vote on any other matters which properly come before the General Meeting. |
| Q: | If I am not going to attend the General Meeting, should I return my proxy card instead? |
| A: | Yes. Whether you plan to attend the General Meeting or not, please read the enclosed proxy statement/ prospectus carefully, and vote your shares by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. |
You are strongly encouraged to participate in the General Meeting virtually and not attend in person. FLAC is sensitive to the public health and travel concerns FLAC’s shareholders may have and recommendations that public health officials may issue in light of the evolving COVID-19 pandemic. As a result, FLAC may impose additional procedures or limitations on meeting attendees or may decide to hold the meeting in a different location. We intend to hold the General Meeting via live webcast at www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. We plan to announce relevant updates on our proxy website, and we encourage you to check this website prior to the General Meeting if you plan to attend.
28
Table of Contents
| Q: | If my shares are held in “street name,” will my broker, bank or nominee automatically vote my shares for me? |
| A: | No. Under the rules of various national and regional securities exchanges, your broker, bank, or nominee cannot vote your shares with respect to non-discretionary matters unless you provide instructions on how to vote in accordance with the information and procedures provided to you by your broker, bank, or nominee. |
FLAC believes that all of the proposals presented to the shareholders at this General Meeting will be considered non-discretionary and, therefore, your broker, bank, or nominee cannot vote your shares without your instruction on any of the proposals presented at the General Meeting. If you do not provide instructions with your proxy card, your broker, bank, or other nominee may deliver a proxy card expressly indicating that it is NOT voting your shares. This indication that a broker, bank, or nominee is not voting your shares is referred to as a “broker non-vote.” Broker non-votes will be counted for the purposes of determining the existence of a quorum but will not be counted for purposes of determining the number of votes cast at the General Meeting. Your broker, bank or other nominee can vote your shares only if you provide instructions on how to vote. You should instruct your broker, bank or other nominee to vote your shares in accordance with directions you provide.
| Q: | May I change my vote after I have mailed my signed proxy card? |
| A: | Yes. You may change your vote by sending a later-dated, signed proxy card to FLAC’s Secretary at FLAC’s address listed below so that it is received by FLAC’s Secretary prior to the General Meeting or attend the General Meeting and vote. You also may revoke your proxy by sending a notice of revocation to FLAC’s Secretary, which must be received by FLAC’s Secretary prior to the General Meeting. |
| Q: | What should I do if I receive more than one set of voting materials? |
| A: | You may receive more than one set of voting materials, including multiple copies of this proxy statement/prospectus and multiple proxy cards or voting instruction cards. For example, if you hold your shares in more than one brokerage account, you will receive a separate voting instruction card for each brokerage account in which you hold shares. If you are a holder of record and your shares are registered in more than one name, you will receive more than one proxy card. Please complete, sign, date and return each proxy card and voting instruction card that you receive in order to cast your vote with respect to all of your shares. |
| Q: | Who will solicit and pay the cost of soliciting proxies for the General Meeting? |
| A: | FLAC will pay the cost of soliciting proxies for the General Meeting. FLAC has engaged Morrow Sodali LLC to assist in the solicitation of proxies for the General Meeting. FLAC has agreed to pay Morrow Sodali LLC a fee of $30,000, plus disbursements, and will reimburse it for its reasonable out-of-pocket expenses and indemnify it and its affiliates against certain claims, liabilities, losses, damages and expenses. FLAC will also reimburse banks, brokers and other custodians, nominees and fiduciaries representing beneficial owners of FLAC Ordinary Shares for their expenses in forwarding soliciting materials to beneficial owners of FLAC Ordinary Shares and in obtaining voting instructions from those owners. The directors, officers and employees of FLAC may also solicit proxies by telephone, by facsimile, by mail, on the Internet, or in person. They will not be paid any additional amounts for soliciting proxies. |
| Q: | Who can help answer my questions? |
| A: | If you have questions about the proposals or if you need additional copies of this proxy statement/prospectus or the enclosed proxy card you should contact: |
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union St., Suite 3200
Seattle, WA 9810
(206) 621-7200
Attention: Corporate Secretary
29
Table of Contents
You may also contact the proxy solicitor for FLAC at:
Individuals, please call toll-free: (800) 662-5200
Banks and brokerage, please call: (203) 658-9400
Email: FLAC.info@investor.morrowsodali.com
To obtain timely delivery, FLAC shareholders must request the materials no later than November 7, 2022, or five business days prior to the General Meeting.
You may also obtain additional information about FLAC from documents filed with the SEC by following the instructions in the section entitled “Where You Can Find More Information.”
If you intend to seek redemption of your FLAC Public Shares, you will need to send a letter demanding redemption and deliver your FLAC Public Shares (either physically or electronically) to the Transfer Agent prior to the General Meeting in accordance with the procedures detailed under the question “How do I exercise my redemption rights?” If you have questions regarding the certification of your position or delivery of your FLAC Public Shares, please contact the Transfer Agent:
Continental Stock Transfer & Trust Company
1 State Street, 30th Floor
New York, New York 10004
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
30
Table of Contents
SUMMARY OF THIS PROXY STATEMENT/PROSPECTUS
This summary highlights selected information from this proxy statement/prospectus and does not contain all of the information that is important to you. To better understand the proposals to be submitted for a vote at the General Meeting, including the Business Combination, you should read this proxy statement/prospectus, including the Annexes and other documents referred to herein, carefully and in their entirety. The Business Combination Agreement is the legal document that governs the Business Combination and the other transactions that will be undertaken in connection with the Business Combination. The Business Combination Agreement is also described in detail in this proxy statement/prospectus in the section entitled “The Business Combination Agreement and Ancillary Documents.”
The Parties to the Business Combination
Holdco
Holdco is a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) that was incorporated on June 10, 2022 to facilitate the consummation of the Business Combination. To date, Holdco has not conducted any material activities other than those incident to its formation and the pending Business Combination and only has nominal assets consisting of cash and its interest in Merger Sub. Accordingly, no financial statements of Holdco have been included in this proxy statement/prospectus. Prior to the Effective Date, or, if agreed to by NewAmsterdam Pharma and FLAC, promptly following the PIPE Financing, Holdco’s corporate form will be converted to a Dutch public limited liability company (naamloze vennootschap) and its name will be changed to NewAmsterdam Pharma Company N.V. Upon consummation of the Businss Combination, Holdco will become the ultimate parent entity of NewAmsterdam Pharma. See “Summary—Expected Accounting Treatment” for information on Holdco’s related accounting. Holdco has applied to list the Holdco Shares and Holdco Public Warrants under the Exchange Act and on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively, upon the closing of the Business Combination.
Holdco’s principal executive office is located at Gooimeer 2-35, 1411 DC Naarden, The Netherlands, and its telephone number is +31 (0) 35 206 2971.
NewAmsterdam Pharma
NewAmsterdam Pharma was incorporated pursuant to the laws of the Netherlands as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) under the name NewAmsterdam Pharma B.V. with its corporate seat in Naarden, the Netherlands on October 17, 2019. NewAmsterdam Pharma changed its name to NewAmsterdam Pharma Holding B.V. in October 2021. NewAmsterdam Pharma’s principal subsidiary, Dezima Pharma B.V. (“Dezima”), a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid), was incorporated on August 31, 2012, pursuant to the laws of the Netherlands with its corporate seat in Naarden, the Netherlands. Dezima changed its name to NewAmsterdam Pharma B.V. in October 2021.
It is anticipated that, following the closing of the Business Combination: (i) FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) will receive approximately 14.9% of Holdco Shares and 14.9% of the voting rights of Holdco, (ii) the PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) (some of whom are also NewAmsterdam Pharma shareholders) will receive approximately 22.0% of Holdco Shares and 22.0% of the voting rights of Holdco, (iii) the FLAC Initial Shareholders and their affiliates (including the Sponsor) will receive approximately 11.0% of Holdco Shares and 11.0% of the voting rights of Holdco and (iv) the Participating Shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing, but including the Holdco Shares issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion
31
Table of Contents
and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”) will receive approximately 52.2% of Holdco Shares and 52.2% of the voting rights of Holdco. These ownership levels in Holdco assume that (i) no FLAC Class A Ordinary Shares are elected to be redeemed by the FLAC public shareholders, (ii) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (iii) none of the Holdco Warrants have been exercised, (iv) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (v) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) have been issued, (vi) an aggregate of 8,656,330 Holdco Shares have been issued to Amgen and MTPC (each defined below) pursuant to each of their respective profit rights referenced above, (vii) none of the NewAmsterdam Pharma Options have been exercised prior to the Closing, and (viii) no Holdco Options or awards that may be issued under Holdco’s LTIP following the Closing will be exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (iv) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. If all of the Holdco Warrants, NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options were exercisable and immediately exercised upon completion of the Business Combination on a 1:1 basis for Holdco Shares, FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) would receive in aggregate approximately 12.3% of Holdco Shares on a fully diluted basis, and the FLAC Initial Shareholders (including the Sponsor) would receive in aggregate approximately 9.1% of Holdco Shares on a fully diluted basis; however, the Holdco Warrants are subject to restrictions on the timing of their exercise and may also be exercisable on a cashless basis by reference to the fair market value of the Holdco Shares, and these percentages are therefore indicative only.
NewAmsterdam Pharma’s principal executive office is located at Gooimeer 2-35, 1411 DC Naarden, The Netherlands, and its telephone number is +31 (0) 35 206 2971. NewAmsterdam Pharma’s website is www.newamsterdampharma.com. NewAmsterdam Pharma’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
FLAC
FLAC is a blank check company incorporated on October 7, 2020 as a Cayman Islands exempted company for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. On December 11, 2020, FLAC consummated its initial public offering, generating gross proceeds of $138 million, and a concurrent private placement to the Sponsor, generating gross proceeds of approximately $5.01 million.
The FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants are currently listed on Nasdaq under the symbols “FLACU,” “FLAC” and “FLACW,” respectively.
FLAC’s principal executive office is located at Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101, and its telephone number is (206) 621-7200. FLAC’s website is www.frazierlifesciencesacquisition.com. FLAC’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
Merger Sub
Merger Sub is a Cayman Islands exempted company and wholly owned subsidiary of Holdco that was incorporated on June 30, 2022 to facilitate the consummation of the Business Combination. As part of the
32
Table of Contents
Business Combination, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco.
The mailing address of Merger Sub’s registered office is Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman KY1-1104, Cayman Islands.
The Business Combination
General
On July 25, 2022, FLAC, NewAmsterdam Pharma, Holdco, and Merger Sub entered into the Business Combination Agreement, which provides for, among other things, the following transactions:
| • | The Participating Shareholders will contribute all outstanding shares in the capital of NewAmsterdam Pharma to Holdco in exchange for the issuance of Holdco Shares (the “Exchange”); |
| • | Immediately after giving effect to the Exchange, the legal form of Holdco will be converted from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) (the “Holdco Reorganization”), provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing; |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco (the “Surviving Company”); |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding warrant to purchase a FLAC Class A Ordinary Share (defined below) will become a warrant to purchase one Holdco Share, on the same contractual terms (“Holdco Warrants”); |
| • | Each NewAmsterdam Pharma option that is outstanding and unexercised (“NewAmsterdam Pharma Options”) will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will change its jurisdiction of incorporation by deregistering as a Cayman Islands exempted company and continuing and domesticating as a corporation incorporated under the laws of the State of Delaware (the “Domestication”); and |
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders (including Amgen and MTPC (each defined below) for this purpose) and holders of NewAmsterdam Pharma Options who were directors, officers, employees or consultants of NewAmsterdam Pharma as of the date of the Business Combination Agreement (the “Participating Optionholders”) and who are at the time of achievement of such milestone still providing services to Holdco or its subsidiaries, 1,886,137 additional Holdco Shares (the “Earnout Shares”), which in the case of the Participating Optionholders will take the form of awards of restricted stock units under Holdco’s long-term incentive plan. The development milestone consists of the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the period beginning on the Closing Commencement Date and ending on the date that is five years after the Final Closing Date. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within five years of the Merger. |
33
Table of Contents
For more information about the Business Combination, please see the sections entitled “The Business Combination” and “The Business Combination Agreement and Ancillary Documents.” A copy of the Business Combination Agreement is attached to this proxy statement/prospectus as Annex A.
Organizational Structure
The following diagram illustrates the organizational structure of FLAC immediately prior to the Business Combination.
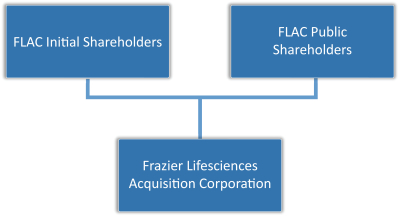
34
Table of Contents
The following diagram illustrates the organizational structure of NewAmsterdam Pharma and Holdco immediately prior to the Business Combination.
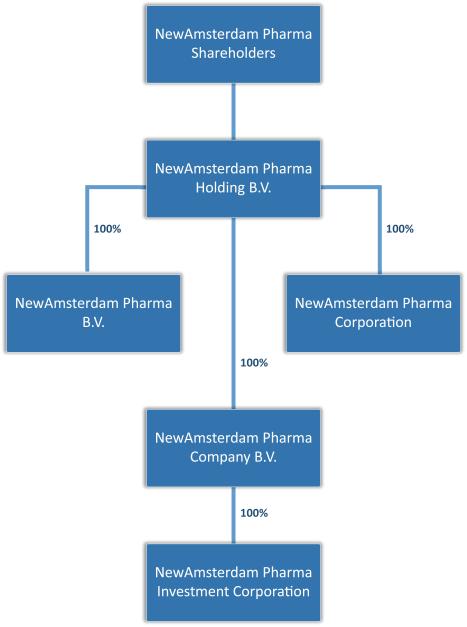
The following diagram illustrates the structure of Holdco immediately following the Business Combination. This diagram assumes that (A) no FLAC Class A Ordinary Shares are elected to be redeemed by FLAC’s public shareholders, (B) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (C) none of the Holdco Warrants have been exercised, (D) Participating Shareholders represent 100% of the issued and outstanding shares of NewAmsterdam Pharma, (E) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (F) an aggregate of 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled
35
Table of Contents
“NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” (G) none of the NewAmsterdam Pharma Options have been exercised prior to the Closing, and (H) no Holdco Options or awards that may be issued under Holdco’s LTIP following the Closing will be exercised. If these assumptions are not correct, then the shareholdings set forth in the diagram below would change. For more information about factors that affect the assumptions above, please see the section entitled “The Business Combination—Ownership of Holdco Following the Business Combination.”
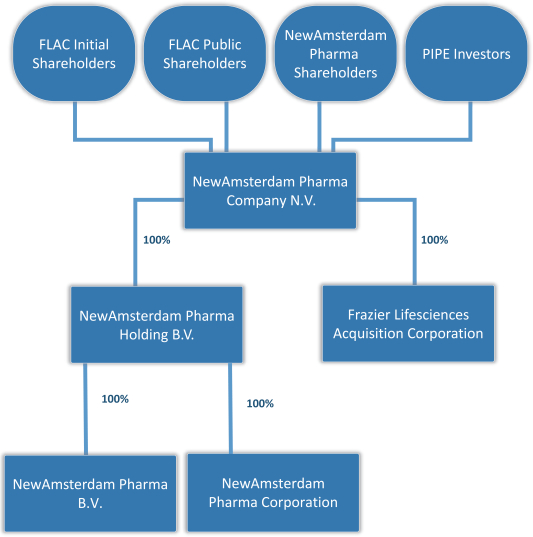
Effect of the Business Combination on Existing FLAC Equity
Subject to the terms and conditions of the Business Combination Agreement, the Business Combination will result in, among other things, the following:
| • | each FLAC Class A Ordinary Share will be exchanged for one fully paid and non-assessable Holdco Share; |
| • | each Founder Share will be exchanged for one fully paid and non-assessable Holdco Share; and |
36
Table of Contents
| • | each FLAC Warrant will become a Holdco Warrant, on the same terms and conditions as those applicable to the respective FLAC Warrants. |
Consideration to NewAmsterdam Pharma Shareholders in the Business Combination
Subject to the terms and conditions of the Business Combination Agreement, the consideration to be received by the NewAmsterdam Pharma shareholders in connection with the Business Combination will be (i) an aggregate number of Holdco Shares determined by using an exchange ratio (the “Exchange Ratio”) equal to (A) $491,000,000 divided by (B) $10.00 multiplied by (C) a fraction of which the numerator is one and the denominator is the fully-diluted number of NewAmsterdam Pharma shares outstanding immediately prior to the Exchange (the fully diluted basis assumes, solely for purposes of this calculation, that shares of NewAmsterdam Pharma and not of Holdco would be issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”) multiplied by (D) the number of NewAmsterdam Pharma shares outstanding immediately prior to the Exchange (subject to rounding differences to account for fractional entitlements of the NewAmsterdam Pharma shareholders) and (ii) 1,886,137 Earnout Shares if and when a certain clinical development milestone is achieved during the Earnout Period.
Each NewAmsterdam Pharma Option outstanding immediately prior to the consummation of the Exchange will remain outstanding and, to the extent unvested, will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such options will become options to purchase, and will when exercised be settled in Holdco Shares. The exercise of each option will be made in Holdco Shares based on the Exchange Ratio. Additionally, the exercise price of each converted option will be determined by dividing the exercise price per share (or depository receipt for a share) of each option to purchase shares (or depository receipts for shares) of NewAmsterdam Pharma by the Exchange Ratio. The Earnout Shares payable to eligible Participating Optionholders will be delivered in the form of awards of restricted stock units under Holdco’s long-term incentive plan.
Ownership of Holdco Following the Business Combination
It is anticipated that, upon completion of the Business Combination: (i) FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) will receive approximately 14.9% of Holdco Shares, (ii) the PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) (some of whom are also NewAmsterdam Pharma shareholders) will receive approximately 22.0% of Holdco Shares, (iii) the FLAC Initial Shareholders and their affiliates (including the Sponsor) will receive approximately 11.0% of Holdco Shares, and (iv) the former NewAmsterdam Pharma shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing, but including the Holdco Shares issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”)) will receive approximately 52.2% of Holdco Shares. These levels of ownership assume (A) no FLAC Class A Ordinary Shares are elected to be redeemed by FLAC’s public shareholders, (B) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (C) none of the Holdco Warrants have been exercised, (D) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (E) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (F) an aggregate of 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to each of their respective profit rights referenced above, (G) none of the NewAmsterdam Pharma Options have been exercised prior to the Closing, and (H) no Holdco Options or awards that may be issued under Holdco’s LTIP following the Closing will be exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in
37
Table of Contents
the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (D) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. If all of the Holdco Warrants, NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options were exercisable and immediately exercised upon completion of the Business Combination on a 1:1 basis for Holdco Shares, FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) would receive in aggregate approximately 12.3% of Holdco Shares on a fully diluted basis, and the FLAC Initial Shareholders (including the Sponsor) would receive in aggregate approximately 9.1% of Holdco Shares on a fully diluted basis; however, the Holdco Warrants are subject to restrictions on the timing of their exercise and may also be exercisable on a cashless basis by reference to the fair market value of the Holdco Shares, and these percentages are therefore indicative only.
In addition to the assumptions made in the preceding paragraph, the factors that will determine the ownership percentages upon consummation of the Business Combination include:
| • | if there are any redemptions by public shareholders of FLAC Public Shares in connection with the Business Combination, it would reduce the aggregate ownership of the public shareholders and increase the aggregate ownership of the other shareholder groups described above; and |
| • | if the PIPE Investors do not fund the PIPE Financing in full, it would reduce the aggregate ownership of the PIPE Investors and increase the aggregate ownership of the other shareholder groups described above commensurately. |
For further information related to the determination of the number of Holdco Shares to be issued to the NewAmsterdam Pharma shareholders upon completion of the Business Combination, please see the section entitled “The Business Combination Agreement and Ancillary Documents—Consideration to NewAmsterdam Shareholders in the Business Combination.”
The ownership percentages with respect to Holdco following the Business Combination do not take into account, unless otherwise expressly stated, the Holdco Warrants, but do include Founder Shares, which will be exchanged for Holdco Shares at the closing of the Business Combination on a 1:1 basis. If the actual facts are different than these assumptions, the ownership percentages in Holdco will be different.
Conditions to Closing of the Business Combination
The respective obligations of each party to the Business Combination Agreement to consummate the Business Combination are subject to the satisfaction, or written waiver by the party for whose benefit such condition exists, at or prior to the Closing of the following conditions, among others:
| • | any applicable waiting period under any applicable Antitrust Law will have expired or terminated, and any consent pursuant to any applicable Antitrust Law will have been obtained; |
| • | no order or law issued by any court of competent jurisdiction or other governmental entity or other legal restraint or prohibition preventing the consummation of the Transactions will be in effect; |
| • | this registration statement/proxy statement must have become effective in accordance with the provisions of the Securities Act, no stop order has been issued by the SEC and remains in effect with respect to this registration statement/proxy statement, and no proceeding seeking such a stop order has been threatened or initiated by the SEC and remains pending; |
| • | the approval, at the General Meeting, of the Business Combination Proposal and the Merger Proposal in accordance with FLAC’s governing documents will have been obtained; |
38
Table of Contents
| • | the approval of the Business Combination Agreement and the Transactions by the shareholders of NewAmsterdam Pharma, in accordance with NewAmsterdam Pharma’s governing documents, will have been obtained; |
| • | (i) Holdco’s initial listing application with Nasdaq in connection with the listing of Holdco Shares will have been approved such that, immediately following the Closing, Holdco will satisfy any applicable initial and continuing listing requirements of Nasdaq, (ii) Holdco will not have received any notice of non-compliance therewith, and (iii) the Holdco Shares will have been approved for listing on Nasdaq subject to official notice of issuance; |
| • | the Holdco Board will be comprised of the individuals appointed in accordance with the Business Combination Agreement; and |
| • | after giving effect to the Transactions (including the PIPE Financing), Holdco will have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) immediately after the Effective Date. |
The obligations of the parties to the Business Combination Agreement to consummate the Business Combination are subject to additional conditions, as described more fully below in the section entitled “The Business Combination Agreement and Ancillary Documents—Conditions to Closing of the Business Combination.”
Related Agreements
This section describes certain additional agreements entered into or to be entered into in connection with the Business Combination Agreement. For additional information, see “The Business Combination Agreement and Ancillary Documents.”
Company Support Agreement
In connection with the execution of the Business Combination Agreement, Holdco, NewAmsterdam Pharma, FLAC, Merger Sub, and certain existing shareholders of NewAmsterdam Pharma (which shareholders only include directors, executive officers and shareholders with voting securities in NewAmsterdam Pharma representing 5% or more of all such voting securities) entered into the Company Support Agreement (included herein as Annex E) pursuant to which, among other things, each such existing shareholder of NewAmsterdam Pharma (a) granted or will grant, as applicable, NewAmsterdam Pharma (or a designee thereof) with a power of attorney permitting and directing NewAmsterdam Pharma to execute on behalf of such shareholder a Dutch deed of issue to effect the Exchange with respect to the shares of NewAmsterdam Pharma held by such shareholder, (b) undertook or will undertake, as applicable, vis-à-vis NewAmsterdam Pharma, Holdco, FLAC and each other existing shareholder of NewAmsterdam Pharma to take all necessary or desirable actions in connection with the transactions set forth in the Business Combination Agreement, (c) agreed to vote in favor of the approval of the Business Combination Agreement, the Exchange and any other matters necessary or reasonably requested by NewAmsterdam Pharma to consummate the transactions contemplated in the Business Combination Agreement, and (d) agreed to certain customary covenants to support the Business Combination (including restrictions on the sale, disposition or transfer of the shares of NewAmsterdam Pharma held by him, her or it). It is anticipated that 24,549,052 Holdco Shares, or approximately 36% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to the Company Support Agreement.
Sponsor Support Agreement
In connection with the execution of the Business Combination Agreement, the FLAC Initial Shareholders, FLAC, Holdco and NewAmsterdam Pharma entered into a Support Agreement, included herein as Annex D (the
39
Table of Contents
“Sponsor Support Agreement”), pursuant to which the FLAC Initial Shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them, and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. 3,450,000 FLAC Class B Ordinary Shares are subject to the Sponsor Support Agreement.
Investor Support Agreements
In connection with the execution of the Business Combination Agreement, certain FLAC shareholders and FLAC entered into Support Agreements, the form of which is included herein as Annex F (the “Investor Support Agreements”), pursuant to which each shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions and (b) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. 1,500,000 FLAC Class A Ordinary Shares are subject to the Investor Support Agreements.
Subscription Agreements
In connection with the execution of the Business Combination Agreement, Holdco and FLAC entered into subscription agreements with PIPE Investors, the form of which is included herein as Annex C (the “Subscription Agreements”), and pursuant to which the PIPE Investors agreed to subscribe for and purchase and Holdco agreed to issue and sell to such PIPE Investors, 23,460,000 Holdco Shares (the “Private Placement Shares”), for an aggregate of $234.6 million in proceeds. The Private Placement Shares to be issued pursuant to the Subscription Agreements have not been registered under the Securities Act, in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder without any form of general solicitation or general advertising.
The Subscription Agreements provide that Holdco is required to file with the SEC, within 30 days after the consummation of the transactions contemplated by the Business Combination Agreement, a registration statement covering the resale of the Private Placement Shares and to use its commercially reasonable efforts to have such registration statement declared effective as soon as practicable after the filing thereof but no later than the earlier of (i) 60 calendar days after the filing thereof (or, in the event the SEC notifies Holdco that it will review such registration statement, 90 calendar days following the filing thereof) and (ii) five business days after the date Holdco is notified (orally or in writing, whichever is earlier) by the SEC that such registration statement will not be “reviewed” or will not be subject to further review.
The closing of the PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination and related transactions.
Investor Rights Agreement
At the closing of the Business Combination, Holdco will enter into an Investor Rights Agreement with the FLAC Initial Shareholders and certain NewAmsterdam Pharma shareholders (the “Investor Rights Agreement”),
40
Table of Contents
the form of which is included herein as Annex G, providing for, among other things, subject to the terms thereof, customary registration rights, including demand and piggy-back rights subject to cut-back provisions. Holdco has agreed to file a registration statement to register the Holdco Shares covered by the Investor Rights Agreement no later than 30 days following consummation of the Business Combination.
Pursuant to the Investor Rights Agreement, certain NewAmsterdam Pharma shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination, subject to certain limited exceptions. In addition, the FLAC Initial Shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares they receive in connection with the Business Combination for a period beginning on the Final Closing Date and ending one year after the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the indicated time periods with respect to 50% of the Holdco Shares the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination, on the earlier of the date that (i) the closing price of a Holdco Share equals or exceeds $12.00 per share (subject to certain adjustments) for any 20 trading days within any 30-day trading period commencing at least 150 days after the Final Closing Date of the Business Combination, or (ii) Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The restrictions on the remaining 50% of the Holdco Shares each of the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination will end prior to the periods indicated above on the date that Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The share transfer restrictions above will not apply with respect to sales to cover withholding taxes due upon vesting of equity awards and, in the case of directors or officers of Holdco, with respect to the sale of up to 10% of the Holdco Shares held by each of them. It is anticipated that 48,865,642 Holdco Shares, or approximately 58% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to the Investor Rights Agreement following the closing of the Business Combination.
Lock-Up Agreement
At the closing of the Business Combination, certain NewAmsterdam Pharma shareholders who are not party to the Investor Rights Agreement (after giving effect to the Merger and the PIPE Financing) will enter into lock-up agreements in the form included herein as Annex H (each, a “Lock-Up Agreement”), pursuant to which, among other things, they will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end on the earlier of the date that (i) the closing price of a Holdco Share equals or exceeds $12.00 per share (subject to certain adjustments) for any 20 trading days within any 30-day trading period commencing at least 150 after the Final Closing Date of the Business Combination, or (ii) Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The share transfer restrictions above will not apply with respect to sales to cover withholding taxes due upon vesting of equity awards and, in the case of directors or officers of Holdco, with respect to the sale of up to 10% of the Holdco Shares held by each of them. It is anticipated that 268,472 Holdco Shares, or less than 1% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to a Lock-Up Agreement. The terms of a Lock-Up Agreement will also apply Holdco Shares underlying options held by the parties to a Lock-Up Agreement.
41
Table of Contents
Warrant Assumption Agreement
At the Closing, Holdco, FLAC and Continental will enter into a Warrant Assignment, Assumption and Amendment Agreement (the “Warrant Assumption Agreement”), in the form attached hereto as Exhibit 4.1, pursuant to which the parties will agree that, as part of the Merger, each FLAC Public Warrant and FLAC Private Placement Warrant that is outstanding immediately prior to the Effective Date will cease to represent a right to acquire FLAC Class A Ordinary Shares and will automatically represent, immediately following the Merger, a right to acquire Holdco Shares on the same contractual terms and conditions as were in effect immediately prior to Merger under the original warrant agreement, dated December 8, 2020, between FLAC and Continental (the “Original Warrant Agreement”), including that the warrant holders are deemed to have consented to an exclusive forum provision requiring all claims to be brought before the courts of the State of New York or the United States District Court for the Southern District of New York other than suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America are the sole and exclusive forum.
The General Meeting of FLAC’s Shareholders
Date, Time and Place of General Meeting
The General Meeting of FLAC will be held at 10:30 a.m. Eastern time, on November 15, 2022, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. You will need the 12-digit meeting control number that is printed on your proxy card to enter the General Meeting. FLAC recommends that you log in at least 15 minutes before the General Meeting to ensure you are logged in when the General Meeting starts.
You are strongly encouraged to participate in the General Meeting virtually and not attend in person. FLAC is sensitive to the public health and travel concerns FLAC’s shareholders may have and recommendations that public health officials may issue in light of the evolving COVID-19 pandemic. As a result, FLAC may impose additional procedures or limitations on meeting attendees or may decide to hold the meeting in a different location.
Proposals
At the General Meeting, FLAC shareholders will be asked to consider and vote upon:
| (i) | a proposal, as an ordinary resolution (the “Business Combination Proposal”), to adopt and approve the Business Combination Agreement, a copy of which is included herein as Annex A, and the transactions contemplated thereby, including the Business Combination (Proposal No. 1); |
| (ii) | a proposal, as a special resolution (the “Merger Proposal”) to authorize and approve the Merger and the Plan of Merger, a copy of which is included herein as Annex B, pursuant to which Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco in accordance with the relevant provisions of the Cayman Islands Companies Act, and the remaining transactions contemplated thereby (Proposal No. 2); and |
| (iii) | a proposal, as an ordinary resolution (the “Adjournment Proposal”), to adjourn the General Meeting to a later date or dates, if necessary, to, among other things, permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with, the Business Combination Agreement at the General Meeting (Proposal No. 3). |
Please see the sections entitled “Business Combination Proposal,” “Merger Proposal” and “Adjournment Proposal.”
42
Table of Contents
Voting Power; Record Date
For FLAC shareholders holding their shares in “street name,” only holders of record at the close of business on September 30, 2022, the record date for the General Meeting, will be entitled to vote at the General Meeting. Each FLAC shareholder that holds its shares in “street name” is entitled to one vote for each FLAC Ordinary Share that such shareholder owned as of the close of business on the record date. If a FLAC shareholder’s shares are held in “street name” or are in a margin or similar account, such shareholder should contact its broker, bank or other nominee to ensure that votes related to the shares beneficially owned by such shareholder are properly counted. On the record date, there were 17,751,000 FLAC Ordinary Shares outstanding, of which 13,800,000 are FLAC Public Shares, 501,000 are FLAC Private Placement Shares held by the Sponsor and 3,450,000 are Founder Shares held by the Sponsor and the other FLAC Initial Shareholders. For the avoidance of doubt, the record date does not apply to FLAC shareholders that hold their shares in registered form and are registered as shareholders in FLAC’s register of members. FLAC shareholders that hold their shares in registered form are entitled to one vote on each proposal presented at the General Meeting for each FLAC Ordinary Share held on the date of the General Meeting.
Vote of the FLAC Initial Shareholders
Prior to the FLAC IPO, FLAC entered into agreements with the FLAC Initial Shareholders, pursuant to which each FLAC Initial Shareholder agreed to vote any FLAC Ordinary Shares owned by them in favor of an initial business combination. These agreements apply to the FLAC Initial Shareholders, including the Sponsor, as it relates to the Founder Shares and the requirement to vote all of the Founder Shares in favor of the Business Combination Proposal and for all other proposals presented to FLAC shareholders in this proxy statement/prospectus. As of the record date, the FLAC Initial Shareholders own 3,450,000 Founder Shares, representing approximately 19% of the FLAC Ordinary Shares then outstanding and entitled to vote at the General Meeting.
Each of the FLAC Initial Shareholders has, for no additional consideration, waived any redemption rights in connection with the Business Combination. The Founder Shares held by the FLAC Initial Shareholders have no redemption rights upon the liquidation of FLAC and will be worthless if no business combination is effected by FLAC by December 11, 2022, absent any extension. However, the FLAC Initial Shareholders are entitled to redemption rights upon the liquidation of FLAC with respect to any public shares they may own. See “The Business Combination Agreement and Ancillary Documents—Sponsor Support Agreement” in the accompanying proxy statement/prospectus for more information related to the Sponsor Support Agreement.
Quorum and Required Vote of FLAC Shareholders
A quorum of FLAC shareholders is necessary to hold a valid meeting. A quorum will be present at the General Meeting if one or more shareholders who together hold not less than a majority of the issued and outstanding ordinary shares entitled to vote at the General Meeting are represented in person or by proxy at the General Meeting. As of the record date for the General Meeting, 8,875,501 ordinary shares would be required to achieve a quorum.
The proposals presented at the General Meeting require the following votes:
| (i) | Business Combination Proposal: The approval of the Business Combination Proposal requires an ordinary resolution under Cayman Islands law, being the affirmative vote of a majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
| (ii) | Merger Proposal: The approval of the Merger Proposal requires a special resolution under Cayman Islands law, being the affirmative vote of at least a two-thirds majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
43
Table of Contents
| (iii) | Adjournment Proposal: The approval of the Adjournment Proposal requires an ordinary resolution under Cayman Islands law, being the affirmative vote of a majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in this proxy statement/prospectus.
Recommendation to Shareholders of FLAC
The FLAC Board and the FLAC Special Committee believe that each of the proposals to be presented at the General Meeting are in the best interest of FLAC and its shareholders and unanimously recommends that its shareholders vote “FOR” the Business Combination Proposal, “FOR” the Merger Proposal, and “FOR” the Adjournment Proposal, in each case, if presented to the General Meeting.
In reaching its decisions with respect to the Business Combination, the FLAC Board and the FLAC Special Committee reviewed various industry and financial data and the due diligence and evaluation materials provided by NewAmsterdam. Also, Lincoln delivered its opinion to the FLAC Board, as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement. See “The Business Combination—Opinion of the Financial Advisor to the FLAC Board.”
The existence of financial and personal interests of one or more of FLAC’s directors may result in a conflict of interest on the part of such director(s) between what he, she or they may believe is in the best interests of FLAC and its shareholders and what he, she or they may believe is best for himself or themselves in determining to recommend that shareholders vote for the proposals. In addition, FLAC’s officers have interests in the Business Combination that may conflict with your interests as a shareholder. See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of these considerations.
Interests of FLAC’s Directors and Executive Officers in the Business Combination
In considering the recommendation of the FLAC Board to vote in favor of the Business Combination, FLAC shareholders should be aware that aside from their interests as shareholders, the Sponsor, FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from, or in addition to, those of other FLAC shareholders generally. The FLAC Board was aware of and considered these interests, among other matters, in evaluating and negotiating the Business Combination, and in recommending to FLAC shareholders that they approve the Business Combination Proposal. FLAC shareholders should take these interests into account in deciding whether to approve the Business Combination Proposal.
These interests include, among other things, the interests listed below:
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed not to redeem any Founder Shares or FLAC Public Shares held by them in connection with a shareholder vote to approve a proposed initial business combination; |
| • | the Sponsor paid an aggregate of $25,000 for 2,875,000 Founder Shares. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. Such securities will have a significantly higher |
44
Table of Contents
value at the time of the Business Combination which, if unrestricted and freely tradable, such Founder Shares would be valued at approximately $34,051,500 (based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022), but, given the restrictions on such shares, FLAC believes such shares have less value. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then FLAC will cease all operations except for the purpose of winding up, redeeming all of the public shares for cash and, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceeding to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In such event, Founder Shares collectively owned by the FLAC Initial Shareholders would be worthless because following the redemption of the public shares, FLAC would likely have few, if any, net assets; |
| • | the Sponsor paid an aggregate of $5,010,000 for its 501,000 FLAC Private Placement Units (with an aggregate fair market value of $4,944,870, based on the closing price of FLAC Public Units on June 30, 2022) and that the component FLAC Private Placement Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, paid an aggregate of $10 million for its 1,000,000 FLAC Public Units in the FLAC IPO (with an aggregate fair market value of $9,870,000, based on the closing price of FLAC Public Units on June 30, 2022), and has agreed to waive any redemption rights, including with respect to the FLAC Class A Ordinary Shares underlying such FLAC Public Units purchased in the FLAC IPO, in connection with the Business Combination. In addition, the component FLAC Public Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, and Frazier Life Sciences XI, L.P., Frazier Life Sciences Public Fund, L.P. and Frazier Life Sciences Overage Fund, L.P., each an affiliate of Frazier, an affiliate of the Sponsor, have committed to purchasing 4,500,000 Holdco Shares at a price per share of $10.00 as part of the PIPE Financing and have agreed to waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination; |
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed to waive their rights to liquidating distributions from the Trust Account with respect to any Founder Shares held by them if FLAC fails to complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | the Investor Rights Agreement will be entered into by the FLAC Initial Shareholders; |
| • | the Holdco Shares to be received by the FLAC Initial Shareholders in connection with the Merger will be subject to certain lock-up provisions for a period of one year, subject to exceptions as described herein; |
| • | the continued indemnification of FLAC’s existing directors and officers and the continuation of FLAC’s directors’ and officers’ liability insurance after the Business Combination; |
| • | the Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to shareholders rather than liquidate; |
| • | the Sponsor and its affiliates can earn a positive rate of return on their investment, even if other FLAC shareholders experience a negative rate of return in the post-business combination company; |
| • | FLAC has the right to select two individuals, one of which is expected to be Jamie Topper, to be nominated for election to the initial Holdco Board, who must be reasonably acceptable to NewAmsterdam Pharma and qualify as “independent” directors for purposes of Nasdaq rules; |
45
Table of Contents
| • | the Sponsor, FLAC’s officers and directors, and their respective affiliates will lose their entire investment in FLAC (which is estimated to be approximately $34.5 million, based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022) and will not be reimbursed for any out-of-pocket expenses (which are currently $0) if an initial business combination is not consummated by December 11, 2022, absent any extension; |
| • | the potential hire of David Topper, the Chief Financial Officer and a director and shareholder of FLAC, as the chief financial officer of Holdco in the first half of 2023; |
| • | the Sponsor (including its representatives and affiliates) and FLAC’s officers and directors, are, or may in the future become, affiliated with entities that are engaged in a similar business to Holdco and/or NewAmsterdam Pharma. The representatives and affiliates of the Sponsor, and certain of FLAC’s officers and directors, are in the business of making investments in companies, and may acquire and hold interests in businesses that compete directly or indirectly with Holdco and/or NewAmsterdam Pharma. Certain of FLAC’s officers and directors also have time and attention requirements for investment funds of which they and affiliates of the Sponsor are the investment managers. The Sponsor and FLAC’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to FLAC completing its initial business combination; and |
| • | if the Trust Account is liquidated, including in the event FLAC is unable to complete an initial business combination within the required time period, the Sponsor has agreed to indemnify FLAC to ensure that the proceeds in the Trust Account are not reduced below $10.00 per FLAC Public Share, or such lesser per public share amount as is in the Trust Account on the liquidation date, by the claims of prospective target businesses with which FLAC has entered into an acquisition agreement or claims of any third party for services rendered or products sold to FLAC, but only if such a vendor or target business has not executed a waiver of any and all rights to seek access to the Trust Account. |
In addition, in connection with the execution of the Business Combination Agreement, FLAC, NewAmsterdam Pharma and Holdco entered into the Sponsor Support Agreement with the FLAC Initial Shareholders, pursuant to which the FLAC Initial Shareholders have agreed to vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions. Currently, the FLAC Initial Shareholders collectively own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares.
As a result of the aforementioned actual or potential conflicts of interests, the FLAC Board formed the FLAC Special Committee, comprised solely of disinterested and independent directors, for the purpose of evaluating the proposed Business Combination and determining whether the Business Combination Agreement and the proposed Business Combination are in the best interests of FLAC and its unaffiliated shareholders. The FLAC Special Committee is composed of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher and Krishna R. Polu.
In addition, FLAC retained Lincoln, a third-party valuation firm, to provide a fairness opinion to the FLAC Board. Lincoln has delivered its opinion to the FLAC Board as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement.
See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of additional considerations in connection with the Business Combination.
46
Table of Contents
The FLAC Board’s Reasons for the Business Combination
FLAC was formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. The FLAC Board sought to do this by utilizing the networks and industry experience of both the Sponsor and the FLAC Board and management to identify, acquire and operate one or more businesses. The members of the FLAC Board and management have extensive transactional experience, particularly in the biotechnology, pharmaceutical and life sciences industries, including with both high growth private companies and publicly traded companies.
The FLAC Board, in evaluating the transaction with NewAmsterdam Pharma, consulted with Goodwin Procter LLP (“Goodwin”), Campbells LLP (“Campbells”), Houthoff Coöperatief U.A. (“Houthoff”), Wilson Sonsini Goodrich & Rosati, Professional Corporation (“WSGR”), Lincoln and its other advisors. In reaching its resolution (i) determining it is in the best interests of FLAC and its shareholders to approve the execution and delivery of the Business Combination Agreement and the ancillary agreements (including the Plan of Merger) and the transactions contemplated by each of the foregoing (including the Merger); (ii) approving the execution and delivery of the Business Combination Agreement, the ancillary agreements and the transactions contemplated thereby (including the Exchange and the Merger); and (iii) recommending that the FLAC shareholders vote to approve the Business Combination Agreement, the ancillary agreements and the transactions contemplated thereby, the FLAC Board considered and evaluated a number of factors, including, but not limited to, the factors discussed below. The FLAC Board viewed its decision as being based on all of the information available and the factors presented to and considered by it. This explanation of the FLAC Board’s reasons for the Business Combination and all other information presented in this section is forward-looking in nature and, therefore, should be read in light of the factors discussed under “Special Note Regarding Forward-Looking Statements.”
The FLAC Board considered a number of factors pertaining to the Business Combination as generally supporting its decision to enter into the Business Combination Agreement and the transactions contemplated thereby, including but not limited to, the following material factors:
| • | Transformative Late-Stage Product Candidate. NewAmsterdam Pharma’s lead candidate, obicetrapib, has clinical data from three Phase 2 trials in high-risk CVD patients, and is currently in four Phase 3 trials and Phase 2b trials as both a monotherapy and a combination therapy, with multiple potential near-term milestones. The FLAC Board believes that NewAmsterdam Pharma is well-positioned to develop obicetrapib with the potential to be a low-dose, once-daily oral CETP inhibitor for lowering LDL-C, if approved. |
| • | Significant Addressable Market with Growth Opportunities. The FLAC Board considered the fact that obicetrapib aims to address an area of significant unmet need, with approximately 30 million patients in the United States and EU5 with residual LDL-C elevation. NewAmsterdam Pharma is also exploring the role CETP inhibition may have in other indications such as Alzheimer’s disease and diabetes, which represent significant potential commercial opportunities, if validated. |
| • | Experienced Cardiometabolic Management Team. NewAmsterdam Pharma is led by a world-class team of industry veterans, including some of the world’s preeminent cardiometabolic experts. Dr. Michael Davidson, NewAmsterdam Pharma’s Chief Executive Officer, is a seasoned executive and leading expert in the field of lipidology, and Dr. John Kastelein, NewAmsterdam Pharma’s founder and Chief Scientific Officer, has authored widely-published clinical research on the development of novel therapies for CVD and the genetic basis of dyslipidemia, referring to low or elevated lipid levels. The FLAC Board believes that the cardiometabolic experience of the NewAmsterdam Pharma management team positions the company for success. |
| • | Attractive Entry Valuation and Cash Runway. NewAmsterdam Pharma will have an anticipated initial pro forma enterprise value of $326.0 million, based on the NewAmsterdam Pharma’s current cash balance, the PIPE Financing and FLAC’s cash in the Trust Account, which pro forma cash amount the parties believe will be sufficient to fund operations of NewAmsterdam Pharma through 2026. The FLAC Board believes this opportunity is attractive relative to relevant comparable life sciences |
47
Table of Contents
companies. The FLAC Board believes the valuation of NewAmsterdam Pharma is favorable to investors seeking long-term return potential and that the $326.0 million enterprise value presents a compelling entry point valuation for a business with a late-stage, novel product candidate and significant barriers to entry. |
| • | Backed by Top Tier Healthcare Investors. Funds affiliated with Frazier, Forbion, Morningside Ventures, and other investors have committed to investments of a total of $234.6 million through an oversubscribed and upsized PIPE Financing. The FLAC Board believes that that this strong investor commitment reflects the potential opportunity created by a transaction with NewAmsterdam Pharma; however, prospective investors should not rely on the past investment decisions of such investors, as such investors may have different risk tolerances and, for those shares not subscribed for in connection with the PIPE Financing, may receive their shares at a significant discount to the market price. |
| • | Long-Term Alignment. The FLAC Board and the FLAC Special Committee considered that the structure of the Business Combination provides for significant long-term alignment among FLAC, NewAmsterdam Pharma’s management team and the existing NewAmsterdam Pharma shareholders. Both the Sponsor and all of the NewAmsterdam Pharma shareholders have agreed to certain lock-up provisions, and shareholders of NewAmsterdam Pharma are also entitled to receive additional Earnout Shares upon the achievement of a certain clinical development milestone during the Earnout Period. As such, the parties to the Business Combination are expected to be aligned on the goal of driving long-term value for the shareholders of NewAmsterdam Pharma. Pursuant to the terms of the Investor Rights Agreement, certain NewAmsterdam Pharma shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination, subject to certain limited exceptions. In addition, the FLAC Initial Shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares they receive in connection with the Business Combination for a period beginning on the Final Closing Date and ending one year after the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the indicated time periods with respect to 50% of the Holdco Shares the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders upon the occurrence of certain specified trading milestones or change of control events. See the section entitled “The Business Combination Agreement and Ancillary Documents—Investor Rights Agreement” for more detail on the terms of the lock-up provisions. At the closing of the Business Combination, certain NewAmsterdam Pharma shareholders who are not party to the Investor Rights Agreement (after giving effect to the Merger and the PIPE Financing) will enter into a Lock-Up Agreement, pursuant to which, among other things, they will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the six month period upon the occurrence of certain specified trading milestones or change of control events. See the section entitled “The Business Combination Agreement and Ancillary Documents—Lock-Up Agreement” for more detail on the terms of the lock-up provisions. |
| • | Fairness Opinion. Lincoln delivered an opinion to the FLAC Board to the effect that, as of the date of such opinion, and subject to and based on the assumptions, limitations, qualifications, conditions and other matters set forth therein, the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement, is fair to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement), from a financial point of view. |
48
Table of Contents
| • | Compelling Opportunity. The FLAC Board and the FLAC Special Committee determined, after an extensive review of other business combination opportunities reasonably available to FLAC, that the proposed Business Combination represents a compelling business combination for FLAC based upon the process utilized to evaluate and assess other potential targets and the criteria that FLAC adopted in its search for a business combination target as detailed above and in other sections of this prospectus/proxy statement. |
| • | The Role of the Independent Directors. In connection with the Business Combination, the FLAC Special Committee, which is composed solely of independent directors, evaluated the proposed terms of the Business Combination, including the Business Combination Agreement and the related agreements, and unanimously approved the Business Combination Agreement and the related agreements and the Transactions, including the Business Combination. |
| • | Terms of the Business Combination Agreement. The FLAC Board and the FLAC Special Committee reviewed and considered the terms of the Business Combination Agreement and the other related agreements, including the parties’ conditions to their respective obligations to complete the transactions contemplated therein, including the limited number of conditions and the relative size of the minimum cash condition of $250 million as compared to the $234.6 million in committed PIPE Financing and the $15 million subject to non-redemption agreements. See “The Business Combination Agreement and Ancillary Documents” for detailed discussions of the terms and conditions of these agreements. |
| • | Results of Due Diligence. The FLAC Board and the FLAC Special Committee considered the scope of the due diligence investigation conducted by FLAC and its outside advisors and evaluated the results thereof and information available to it related to NewAmsterdam Pharma, including: |
| • | diligence on the life sciences market; |
| • | extensive meetings and calls with NewAmsterdam Pharma’s management team regarding its operations and the proposed transaction; |
| • | WSGR’s review and findings relating to NewAmsterdam Pharma’s material intellectual property; |
| • | the commercial findings of FLAC’s advisors; |
| • | the in-depth review of NewAmsterdam’s science and the potential market opportunity, including relative to other market participants, by FLAC, members of the FLAC Special Committee and FLAC’s advisors; |
| • | NewAmsterdam Pharma’s overall sophistication and its readiness to function as a public company, including as a result of its management team; and |
| • | review of materials related to NewAmsterdam Pharma made available, including with respect to financial statements, material contracts, key metrics and performance indicators, benefit plans, intellectual property matters, labor matters, information technology, privacy and personal data, litigation information, environmental matters, the FDA, the EMA) and other regulatory matters and other legal and business matters. |
The FLAC Board also considered a variety of uncertainties and risks and other potentially negative factors concerning the Business Combination, including, but not limited to, the following:
| • | Benefits Not Achieved. The risk that the potential benefits of the Business Combination may not be fully achieved, or may not be achieved within the expected timeframe. |
| • | Business Risks. The risk that the future financial performance of NewAmsterdam Pharma may not meet the FLAC Board of the FLAC Special Committee’s expectations due to factors both in and outside of NewAmsterdam Pharma’s control, including its dependence on obicetrapib, its only product candidate; expectations regarding the timing, initiation, implementation and success of its planned and ongoing clinical trials for obicetrapib and related implications. |
49
Table of Contents
| • | Clinical-Stage Company Risks. The risk that NewAmsterdam Pharma is a clinical-stage company that has incurred net losses since its inception, and anticipates that it will continue to incur significant losses for the foreseeable future, and that NewAmsterdam Pharma has yet to commercialize its product candidate. In addition, the FLAC Board weighed the fact that prior attempts to develop CETP inhibitors were unsuccessful against the fact that NewAmsterdam Pharma’s preclinical and clinical data suggest potentially important distinctions between obicetrapib and its predecessors. |
| • | Liquidation of FLAC. The risks and costs to FLAC if the Business Combination is not completed, including the risk of diverting management focus and resources to other businesses combination opportunities, which could result in FLAC being unable to effect a business combination by December 11, 2022, absent any extension, and force FLAC to liquidate. |
| • | Exclusivity. The fact that the Business Combination Agreement includes an exclusivity provision that prohibits FLAC from soliciting other business combination proposals, so long as the Business Combination Agreement is in effect. |
| • | Shareholder Vote and Minimum Cash Condition. The risk that FLAC’s shareholders may fail to provide the respective votes necessary to effect the Business Combination, and the risk that the minimum cash condition depends on the actions or inactions of parties over whom FLAC and NewAmsterdam Pharma have no control. |
| • | Limitations of Review. The risk that, while FLAC’s management team performed an extensive due diligence review of NewAmsterdam Pharma, there may have been relevant NewAmsterdam Pharma information not considered by FLAC’s management team and accordingly, FLAC may not have properly valued NewAmsterdam Pharma. |
| • | Closing Conditions. The fact that, in addition to the minimum cash conditions discussed above, the completion of the Business Combination is conditioned on the satisfaction of certain other closing conditions, that are also not within FLAC’s control. |
| • | Litigation. The possibility of litigation challenging the Business Combination or that an adverse judgment granting permanent injunctive relief could delay or indefinitely enjoin consummation of the Business Combination. |
| • | Fees and Expenses. The fees and expenses associated with completing the Business Combination. |
| • | Other Risks. Various other risks associated with the Business Combination, the business of FLAC and the business of NewAmsterdam Pharma described under the section entitled “Risk Factors.” |
In addition to considering the factors described above, the FLAC Board and the FLAC Special Committee also considered that certain of the officers and directors of FLAC may have interests in the Business Combination as individuals that are in addition to, and that may be different from, the interests of FLAC’s shareholders. See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination.” The FLAC Special Committee reviewed and considered these interests during the negotiation of the Business Combination and in evaluating and approving, as members of the FLAC Board and the FLAC Special Committee, the Business Combination Agreement and the transactions contemplated therein, including the Business Combination.
The FLAC Board concluded that the potential benefits that it expected FLAC and its shareholders to achieve as a result of the Business Combination outweighed the potentially negative factors associated with the Business Combination. Accordingly, the FLAC Board determined that the Business Combination Agreement, the Business Combination and the Merger, were advisable, fair to, and in the best interests of, FLAC and its shareholders.
50
Table of Contents
Redemption Rights
Pursuant to the FLAC Articles of Association, holders of FLAC Public Shares other than the Sponsor and any FLAC directors or officers may elect to have their shares redeemed for cash at the applicable redemption price per share calculated in accordance with the FLAC Articles of Association. As of June 30, 2022, this would have amounted to approximately $10.00 per share. If a holder of FLAC Public Shares exercises its redemption rights, then such holder will be exchanging its FLAC Class A Ordinary Shares for cash and will not own Holdco Shares following the closing of the Business Combination. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to the Transfer Agent in accordance with the procedures described herein. The redemption rights include the requirement that a shareholder must identify itself, at least two business days prior to the General Meeting, in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Notwithstanding the foregoing, a holder of the FLAC Public Shares individually, or, together with any affiliate of his or her or any other person with whom he or she is acting in concert or as a “group” (as defined in Section 13 of the Exchange Act) will be restricted from seeking redemption rights with respect to more than 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO. Accordingly, all FLAC Public Shares in excess of the 15% threshold beneficially owned by a public shareholder or group will not be redeemed for cash unless consented to by FLAC. 1,500,000 FLAC Class A Ordinary Shares are subject to the Investor Support Agreements.
FLAC has no specified maximum redemption threshold under the FLAC Articles of Association, other than the aforementioned 15% threshold. Each redemption of a FLAC Public Shares by FLAC public shareholders will reduce the amount in the Trust Account, which held marketable securities with a fair value of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting. The Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on Holdco having net tangible assets worth at least $5,000,001 immediately after the Closing after giving effect to the amount of cash in the Trust Account (net of the Cash Redemption Amount) together with the proceeds from the PIPE financing. The committed PIPE subscriptions are sufficient to satisfy this condition if fully funded. Unless waived by NewAmsterdam Pharma, the Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the aggregate gross cash proceeds received from the Trust Account (net of the Cash Redemption Amount) and the Aggregate Cash Proceeds being at least $250 million. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy this condition, even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights. The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. The Sponsor has agreed not to redeem any Founder Shares or FLAC Public Shares held by it in connection with a shareholder vote to approve a proposed initial business combination. FLAC shareholders who wish to redeem their FLAC Public Shares for cash must refer to and follow the procedures set forth in the section entitled “General Meeting of FLAC—Redemption Rights” in order to properly redeem their public shares.
Holders of FLAC Public Warrants or FLAC Private Placement Shares will not have redemption rights with respect to such securities.
Comparison of Shareholder Rights
Until consummation of the Domestication, Cayman Islands law and the FLAC Articles of Association will continue to govern the rights of FLAC shareholders. After consummation of the Merger, Dutch law and the Holdco Articles of Association will govern the rights of Holdco shareholders.
There are certain differences in the rights of FLAC shareholders prior to the Business Combination and the rights of Holdco shareholders after the Business Combination. Please see the section entitled “Comparison of Corporate Governance and Shareholder Rights.”
51
Table of Contents
Appraisal or Dissenters’ Rights
Shareholders of FLAC may be entitled to give notice to FLAC prior to the General Meeting that they wish to dissent to the Merger and to receive payment of fair market value for their shares if they follow the procedures set out in the Cayman Islands Companies Act, noting that any such dissention rights may be limited pursuant to Section 239 of the Cayman Islands Companies Act which states that no such dissention rights will be available in respect of shares of any class for which an open market exists on a recognized stock exchange at the expiry date of the period allowed for written notice of an election to dissent provided that the merger consideration constitutes inter alia shares of any company which at the effective date of the merger are listed on a national securities exchange. It is FLAC’s view that such fair market value would equal the amount which shareholders would obtain if they exercise their redemption rights as described herein.
Proxy Solicitation
Proxies may be solicited by mail, telephone or in person. FLAC has engaged Morrow Sodali LLC to assist in the solicitation of proxies.
If a shareholder grants a proxy, it may still vote its shares at the General Meeting if it revokes its proxy before the General Meeting. A shareholder also may change its vote by submitting a later-dated proxy as described in the section entitled “General Meeting of FLAC—Revoking Your Proxy.”
Certain Information Relating to Holdco
Listing of Holdco Shares and Holdco Public Warrants on Nasdaq
Holdco Shares and Holdco Public Warrants are not currently traded on a stock exchange. Holdco has applied to list the Holdco Shares and Holdco Public Warrants on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively, upon the closing of the Business Combination. We cannot assure you that the Holdco Shares or Holdco Public Warrants will be approved for listing on Nasdaq.
Delisting and Deregistration of FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants
FLAC and Holdco anticipate that, following consummation of the Business Combination, the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants will be delisted from Nasdaq and subsequently deregistered under the Exchange Act.
Emerging Growth Company
Holdco is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Holdco will remain an “emerging growth company” until the earliest to occur of (i) the last day of the fiscal year (a) following the fifth anniversary of the closing of the Business Combination, (b) in which Holdco has total annual gross revenue of at least $1.07 billion or (c) in which Holdco is deemed to be a large accelerated filer, which means the market value of Holdco Shares held by non-affiliates exceeds $700 million as of the last business day of Holdco’s prior second fiscal quarter, and (ii) the date on which Holdco issued more than $1.0 billion in non-convertible debt during the prior three-year period. Holdco intends to take advantage of exemptions from various reporting requirements that are applicable to most other public companies, whether or not they are classified as “emerging growth companies,” including, but not limited to, an exemption from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that Holdco’s independent registered public accounting firm provide an attestation report on the effectiveness of its internal control over financial reporting and reduced disclosure obligations regarding executive compensation.
52
Table of Contents
Foreign Private Issuer
As a “foreign private issuer,” Holdco will be subject to different U.S. securities laws than domestic U.S. issuers. The rules governing the information that Holdco must disclose differ from those governing U.S. corporations pursuant to the Exchange Act. Holdco will be exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders. Those proxy statements are not expected to conform to Schedule 14A of the proxy rules promulgated under the Exchange Act. As a “foreign private issuer,” Holdco will be exempt from a number of rules under the U.S. securities laws and will be permitted to file less information with the SEC than a U.S. company. In addition, as a “foreign private issuer,” Holdco’s officers and directors and holders of more than 10% of the issued and outstanding Holdco Shares, will be exempt from the rules under the Exchange Act requiring insiders to report purchases and sales of ordinary shares as well as from Section 16 short swing profit reporting and liability.
Tax Considerations
Subject to the limitations and qualifications described in “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities” and “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to Non-U.S. Holders of FLAC Securities” below, the parties to the Merger have agreed to report the Merger, taken together with certain related transactions, as both a transaction described under Section 351 of the Code and as a “reorganization” within the meaning of Section 368 of the Code. If the Merger is treated in this manner for U.S. federal income tax purposes, you will not recognize gain or loss for U.S. federal income tax purposes on the receipt of Holdco Shares for FLAC Class A Ordinary Shares and Holdco Public Warrants for FLAC Public Warrants in connection with the Merger. However, there are significant factual and legal uncertainties as to whether the Merger will qualify as a reorganization, and, notwithstanding the position that the parties have agreed to report, no assurance can be given that the IRS will not challenge the Merger’s qualification as a reorganization or that a court will not sustain such a challenge by the IRS, in which case you could recognize gain for U.S. federal income tax purposes on the receipt Holdco Public Warrants for FLAC Public Warrants in connection with the Merger.
Holders of FLAC Ordinary Shares and FLAC Public Warrants should read carefully the information included under “Material Tax Considerations” for a discussion of the material U.S. federal income tax consequences of the Business Combination, including the Merger and the receipt of cash pursuant to the exercise of redemption rights with respect to the FLAC Class A Ordinary Shares, and the material U.S. federal income tax consequences of the ownership and disposition of Holdco Shares and Holdco Public Warrants after the Business Combination and the material Cayman Islands and material Dutch tax consequences of the acquisition, ownership and disposition of Holdco Shares and Holdco Public Warrants.
Holders of FLAC Ordinary Shares and FLAC Public Warrants are urged to consult their tax advisors to determine the tax consequences to them (including the application and effect of any state, local or other income and other tax laws) of the Business Combination, including the material U.S. federal income tax consequences and the material Cayman Islands and material Dutch tax consequences of the acquisition holding, redemption and disposal of Holdco Shares or acquisition, holding, exercise or disposal of Holdco Public Warrants.
Expected Accounting Treatment
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS. Under this method of accounting, FLAC will be treated as the “acquired” company for accounting purposes. As FLAC does not meet the definition of a business under IFRS 3 – Business Combinations (“IFRS 3”), the net assets of FLAC will be stated at historical cost, with no goodwill or other intangible assets recorded. As a result of the Business Combination and related transactions, the existing shareholders of NewAmsterdam Pharma will continue to retain control through their majority ownership of Holdco.
53
Table of Contents
NewAmsterdam Pharma has been determined to be the accounting acquirer based on an evaluation of the following facts and circumstances:
| • | NewAmsterdam Pharma’s shareholders will have the largest voting interest in Holdco under both the No Redemption Scenario and Maximum Redemption Scenario; |
| • | NewAmsterdam Pharma’s senior management will be the senior management of Holdco; |
| • | The business of NewAmsterdam Pharma will comprise the ongoing operations of Holdco; and |
| • | NewAmsterdam Pharma is the larger entity, in terms of substantive operations and employee base. |
As FLAC does not meet the definition of a business in accordance with IFRS 3, the Business Combination is accounted for within the scope of IFRS 2 – Share-based Payment (“IFRS 2”). Any excess of the fair value of Holdco Shares issued to FLAC Shareholders over the fair value of FLAC’s identifiable net assets acquired represents compensation for the service of a stock exchange listing for its shares provided by FLAC and is expensed as incurred.
Risk Factors Summary
You should carefully read this proxy statement/prospectus and especially consider the factors discussed in the section entitled “Risk Factors,” which include, but are not limited to, the following:
Risks Related to NewAmsterdam Pharma’s Limited Operating History, Financial Condition and Capital Requirements
| • | We are a clinical-stage company with limited operating history, no approved products and no historical product revenues, which makes it difficult to assess our future prospects and financial results. We have incurred net losses since our inception, and anticipate that we will continue to incur significant losses for the foreseeable future. We may never generate any product revenue or become profitable or, if we achieve profitability, may not be able to sustain it. |
Risks Related to NewAmsterdam Pharma’s Product Development, Regulatory Approval and Commercialization
| • | We are dependent on the success of our only product candidate, obicetrapib, and cannot guarantee that obicetrapib will successfully complete clinical development, receive regulatory approval or, if approved, be successfully commercialized. |
| • | We have never obtained approval for any product candidate, and may be unable to do so successfully. |
| • | Clinical drug development involves a lengthy and expensive process with uncertain outcomes, and results of earlier studies and trials may not be predictive of future trial results, and our clinical trials may fail to adequately demonstrate the safety and efficacy of obicetrapib. |
| • | The regulatory approval processes of the FDA, the EMA and other comparable regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for obicetrapib, our business will be substantially harmed. |
| • | Obicetrapib may produce undesirable side effects that we may not have detected in our previous preclinical studies and clinical trials. This could prevent us from gaining approval or market acceptance, including broad physician adoption, for our product candidate if approved, or from maintaining such approval and acceptance, and could substantially increase commercialization costs and even force us to cease operations. |
| • | Even if we receive regulatory approval for obicetrapib or our future product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in |
54
Table of Contents
significant additional expenses, limit or withdraw regulatory approval and subject us to penalties if we fail to comply with applicable regulatory requirements. |
| • | Obicetrapib, if approved, will face significant competition from competing therapies and our failure to compete effectively may prevent us from achieving significant market penetration. |
Risks Related to Ownership of Holdco Securities
| • | Holdco does not intend to pay dividends for the foreseeable future. Accordingly, you may not receive any return on investment unless you sell your Holdco Shares for a price greater than the price you paid for the FLAC Class A Ordinary Shares. |
| • | Holdco is eligible to be treated as an “emerging growth company,” and Holdco cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the Holdco Shares less attractive to investors, which could have a material and adverse effect on Holdco, including growth prospects, because Holdco may rely on these reduced disclosure requirements. |
| • | Holdco is a foreign private issuer and, as a result, will not be subject to certain U.S. securities laws, including proxy rules, and will be subject to Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those of a U.S. domestic public company, which may limit the information available to holders of the Holdco Shares and Holdco Warrants. |
Risks Related to FLAC
| • | FLAC’s proximity to its liquidation date expresses substantial doubt about its ability to continue as a “going concern.” |
| • | The Sponsor and FLAC’s other directors, executive officers, advisors and their affiliates may elect to purchase shares from FLAC public shareholders, which may influence a vote on the Business Combination and reduce the public “float” of FLAC’s Class A Ordinary Shares or FLAC Public Warrants. |
| • | You will not have any rights or interests in funds from the Trust Account, except under certain limited circumstances. To liquidate your investment, therefore, you may be forced to sell your FLAC Public Units, FLAC Class A Ordinary Shares or FLAC Public Warrants, potentially at a loss. |
Risks Related to the Business Combination
| • | The consummation of the Business Combination is subject to a number of conditions, including regulatory approvals, and if those conditions are not satisfied or waived, the Business Combination may not be completed. |
| • | The FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from, or are in addition to, those of other FLAC shareholders in recommending that FLAC shareholders vote in favor of approval of the Business Combination Proposal and approval of the other proposals described in this proxy statement/prospectus. |
| • | The exercise of discretion by FLAC’s or NewAmsterdam Pharma’s directors and officers in agreeing to changes to the terms of or waivers of closing conditions in the Business Combination Agreement may result in a conflict of interest when determining whether such changes to the terms of the Business Combination Agreement or waivers of conditions are appropriate and in the best interests of the public shareholders of FLAC or the shareholders of Holdco. |
| • | The Sponsor and FLAC’s officers and directors own shares of FLAC that will be worthless and may incur reimbursable expenses that may not be reimbursed or repaid if the Business Combination is not approved. Such interests may influence their decision to approve and, in the case of the FLAC Board, recommend, the Business Combination. |
55
Table of Contents
SELECTED HISTORICAL FINANCIAL INFORMATION OF NEWAMSTERDAM PHARMA
The following tables set forth selected historical financial information and operating data for NewAmsterdam Pharma as at June 30, 2022 and for the six months ended June 30, 2022 and 2021, and as at and for the years ended December 31, 2021 and 2020. You should read the following summary historical financial information and operating data in conjunction with the sections entitled “NewAmsterdam Pharma’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”, NewAmsterdam Pharma’s unaudited condensed consolidated financial statements and related notes, and NewAmsterdam Pharma’s consolidated financial statements and related notes, all included elsewhere in this proxy statement/prospectus. The consolidated statement of profit or loss data for the six months ended June 30, 2022 and 2021, and for the years ended December 31, 2021 and 2020, the consolidated statement of financial position data as at June 30, 2022 and December 31, 2021 and 2020, and the consolidated cash flow data for the six months ended June 30, 2022 and 2021, and for the years ended December 31, 2021 and 2020 have been derived from NewAmsterdam Pharma’s audited consolidated financial statements appearing elsewhere in this proxy statement/prospectus. NewAmsterdam Pharma’s historical results may not be indicative of the results that may be achieved in the future.
Consolidated Statement of Profit or Loss Data
| For the six months ended June 30, | For the year ended December 31, | |||||||||||||||
| 2022 | 2021 | 2021 | 2020 | |||||||||||||
(€ in thousands, except per share amounts) | ||||||||||||||||
Revenue | 93,500 | — | — | — | ||||||||||||
Research and development expenses | (30,588 | ) | (8,553 | ) | (25,032 | ) | (4,045 | ) | ||||||||
General and administrative expenses | (9,294 | ) | (1,401 | ) | (4,803 | ) | (1,384 | ) | ||||||||
Total operating expenses | (39,882 | ) | (9,954 | ) | (29,835 | ) | (5,429 | ) | ||||||||
Finance income | 10 | — | 9 | — | ||||||||||||
Finance expense | (185 | ) | (137 | ) | (216 | ) | (344 | ) | ||||||||
Net foreign exchange gain | 1,070 | 609 | 1,443 | 24 | ||||||||||||
Profit (loss) before tax | 54,513 | (9,482 | ) | (28,599 | ) | (5,749 | ) | |||||||||
Income tax expense | — | — | — | — | ||||||||||||
Profit (loss) for the period | 54,513 | (9,842 | ) | (28,599 | ) | (5,749 | ) | |||||||||
Basic and diluted profit (loss) per share (€) | ||||||||||||||||
Basic | 3.20 | (0.86 | ) | (2.53 | ) | (1.15 | ) | |||||||||
Diluted | 2.87 | (0.86 | ) | (2.53 | ) | (1.15 | ) | |||||||||
Consolidated Statement of Financial Position Data
| As at June 30, | As at December 31, | |||||||||||
| 2022 | 2021 | 2020 | ||||||||||
(€ in thousands) | ||||||||||||
Assets | ||||||||||||
Property, plant and equipment | 175 | 190 | 12 | |||||||||
Loan receivable | 728 | 718 | — | |||||||||
Non-current assets | 903 | 908 | 12 | |||||||||
Trade receivables | 115,000 | — | — | |||||||||
Prepayments and other receivables | 12,474 | 5,782 | 1,358 | |||||||||
Cash and cash equivalents | 89,478 | 53,092 | 7,861 | |||||||||
Current assets | 216,952 | 58,874 | 9,219 | |||||||||
Total assets | 217,855 | 59,782 | 9,231 | |||||||||
56
Table of Contents
| As at June 30, | As at December 31, | |||||||||||
| 2022 | 2021 | 2020 | ||||||||||
(€ in thousands) | ||||||||||||
Equity | ||||||||||||
Share capital | 163,556 | 83,876 | 2,500 | |||||||||
Other reserves | 1,029 | 591 | — | |||||||||
Accumulated loss | 19,837 | (34,676 | ) | (6,077 | ) | |||||||
Total equity | 184,422 | 49,791 | (3,577 | ) | ||||||||
Liabilities | ||||||||||||
Lease liability | 90 | 111 | — | |||||||||
Deferred revenue | 7,440 | — | — | |||||||||
Non-current liabilities | 7,530 | 111 | — | |||||||||
Loans and borrowings | — | — | 11,650 | |||||||||
Lease liability | 61 | 53 | — | |||||||||
Deferred revenue | 14,060 | — | — | |||||||||
Trade and other payables | 11,782 | 9,827 | 1,158 | |||||||||
Total current liabilities | 25,903 | 9,880 | 12,808 | |||||||||
Total liabilities | 33,433 | 9,991 | 12,808 | |||||||||
Total equity and liabilities | 217,855 | 59,782 | 9,231 | |||||||||
Consolidated Cash Flow Data
| For the six months ended June 30, | For the year ended December 31, | |||||||||||||||
| 2022 | 2021 | 2021 | 2020 | |||||||||||||
(€ in thousands) | ||||||||||||||||
Net cash flows used in operating activities | (44,642 | ) | (8,771 | ) | (25,164 | ) | (5,970 | ) | ||||||||
Net cash flows used in investing activities | (2 | ) | (7 | ) | (20 | ) | (13 | ) | ||||||||
Net cash provided by financing activities | 79,647 | 69,000 | 68,990 | 11,320 | ||||||||||||
Foreign exchange differences | 1,383 | 609 | 1,425 | 24 | ||||||||||||
Cash and cash equivalents at beginning of period | 53,092 | 7,861 | 7,861 | 2,500 | ||||||||||||
Cash and cash equivalents at end of period, net of overdraft | 89,478 | 68,691 | 53,092 | 7,861 | ||||||||||||
57
Table of Contents
SELECTED HISTORICAL FINANCIAL INFORMATION OF FLAC
The summary selected historical statements of operations data of FLAC for the period from October 7, 2020 (inception) through December 31, 2020 and the year ended December 31, 2021 and the balance sheet data as of December 30, 2020 and December 31, 2021 are derived from FLAC’s audited annual financial statements included elsewhere in this proxy statement/prospectus. The summary selected historical condensed statements of operations data of FLAC for the six months ended June 30, 2022 and the condensed balance sheet data as of June 30, 2022 are derived from FLAC’s unaudited interim financial statements included elsewhere in this proxy statement/prospectus. You should read the following summary financial information in conjunction with the section entitled “FLAC’s Management’s Discussion and Analysis of Financial Condition and Results of Operations” and FLAC’s financial statements and related notes appearing elsewhere in this proxy statement/prospectus.
FLAC has neither engaged in any operations nor generated any revenue to date. FLAC’s only activities from inception through June 30, 2022 were organizational activities and those necessary to complete the FLAC IPO and identifying a target company for a business combination. FLAC does not expect to generate any operating revenue until after the consummation of the Business Combination.
| Six Months Ended June 30, 2022 | Six Months Ended June 30, 2021 | Year Ended December 31, 2021 | For The Period From October 7, 2020 (Inception) Through December 31, 2020 | |||||||||||||
General and administrative expenses | $ | 2,275,402 | $ | 482,480 | $ | 1,246,281 | $ | 120,884 | ||||||||
Administrative expenses —related party | 60,000 | 60,000 | 120,000 | 6,774 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (2,335,402 | ) | (542,480 | ) | (1,366,281 | ) | (127,658 | ) | ||||||||
Other income (expenses): | ||||||||||||||||
Interest income from investments held in Trust Account | 115,968 | 11,466 | 16,158 | 851 | ||||||||||||
Change in fair value of derivative warrant liabilities | 2,431,170 | 2,049,810 | 4,528,650 | 619,710 | ||||||||||||
Break-up fee from terminated agreement | — | — | 1,000,000 | — | ||||||||||||
Financing costs—derivative warrant liabilities | — | — | — | (451,450 | ) | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income | $ | 211,736 | $ | 1,518,796 | $ | 4,178,527 | $ | 41,453 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Weighted average number of Class A ordinary shares —basic and diluted | 14,301,000 | 14,301,000 | 14,301,000 | 3,492,105 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted net income per share, Class A | $ | 0.01 | $ | 0.09 | $ | 0.24 | $ | 0.01 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Weighted average number of Class B ordinary shares —basic | 3,450,000 | 3,450,000 | 3,450,000 | 3,109,884 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Weighted average number of Class B ordinary shares —diluted | 3,450,000 | 3,450,000 | 3,450,000 | 3,450,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic net income per share, Class B | $ | 0.01 | $ | 0.09 | $ | 0.24 | $ | 0.01 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Diluted net income per share, Class B | $ | 0.01 | $ | 0.09 | $ | 0.24 | $ | 0.01 | ||||||||
|
|
|
|
|
|
|
| |||||||||
58
Table of Contents
| June 30, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
Balance Sheet Data: | ||||||||||||
Cash | $ | 615,440 | $ | 1,226,716 | $ | 1,365,094 | ||||||
Total assets | 138,898,139 | 139,505,058 | 139,869,628 | |||||||||
Total liabilities | 7,045,949 | 7,864,604 | 12,407,701 | |||||||||
Class A ordinary shares subject to possible redemption, $0.0001 par value; 13,800,000 shares issued and outstanding at redemption value of $10.00 per share | 138,032,977 | 138,000,000 | 138,000,000 | |||||||||
Total shareholders’ (deficit) equity | (6,180,787 | ) | (6,359,546 | ) | (10,538,073 | ) | ||||||
59
Table of Contents
SUMMARY UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial information is provided to aid you in your analysis of the financial aspects of the Business Combination. The following unaudited pro forma condensed combined financial information presents the historical financial information of FLAC and NewAmsterdam Pharma adjusted to give effect to the Business Combination and related transactions and assumes that the Business Combination Proposal is approved and all shares issuable upon closing of the transactions contemplated by the Business Combination Agreement will be Holdco Shares. The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.”
The unaudited pro forma condensed combined statement of financial position as at June 30, 2022, gives pro forma effect to the Business Combination as if it had been consummated as at that date. The unaudited pro forma condensed combined statements of profit or loss for the year ended December 31, 2021 and the six month ended June 30, 2022, give pro forma effect to the Business Combination as if it had occurred as at January 1, 2021. The unaudited pro forma condensed combined financial information has been presented for illustrative purposes only and is not necessarily indicative of the financial position and results of operations that would have been achieved had the Business Combination and related transactions occurred on the dates indicated. Further, the unaudited pro forma condensed combined financial information may not be useful in predicting the future financial condition and results of operations of Holdco.
The unaudited pro forma condensed combined statement of profit or loss and other comprehensive income or for the year ended December 31, 2021 has been prepared using the following:
| • | the consolidated statement of operations for the year ended December 31, 2021 derived from the audited statement of operations for the year ended December 31, 2021 and for the period from October 7, 2020 (inception) through December 31, 2020 of FLAC and the related notes thereto included in this proxy statement/prospectus. The consolidated financial statements of FLAC have been prepared under U.S. GAAP with the U.S. dollar as its reporting currency; and |
| • | the consolidated statement of profit or loss and other comprehensive loss for the year ended December 31, 2021 derived from the audited consolidated financial statements as at December 31, 2021 and 2020 and for the two years in the period ending December 31, 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus. The consolidated financial statements of NewAmsterdam Pharma have been prepared in accordance with IFRS with Euros as its presentation currency. |
The unaudited pro forma condensed combined statement of financial position as at June 30, 2022 and the unaudited pro forma condensed combined statement of profit or loss for the six months ended June 30, 2022 have been prepared using the following:
| • | the unaudited condensed balance sheet as at June 30, 2022 and the unaudited condensed statement of operations for the six months ended June 30, 2022 derived from the condensed balance sheet as at June 30, 2022 and December 31, 2021 and the unaudited statement of operations for the six months ended June 30, 2022 and 2021 of FLAC and the related notes thereto included in this proxy statement/prospectus. The consolidated financial statements of FLAC have been prepared under U.S. GAAP with the U.S. dollar as its reporting currency; and |
| • | the unaudited condensed consolidated statement of financial position of NewAmsterdam Pharma as at June 30, 2022 and the unaudited condensed consolidated statement of profit or loss and other comprehensive loss for the six months ended June 30, 2022 are derived from the unaudited condensed consolidated financial statements as at and for the six months ended June 30, 2022 and 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus. The unaudited condensed consolidated financial statements of NewAmsterdam Pharma have been prepared in accordance with International Accounting Standard 34 – Interim Financial Reporting with Euros as its presentation currency. |
60
Table of Contents
The unaudited pro forma condensed combined financial information is based on the historical consolidated financial statements of NewAmsterdam Pharma, prepared in accordance with International Financial Reporting Standards as issued by IFRS with Euros as its reporting currency, and the historical financial statements of FLAC, prepared in accordance with U.S. GAAP with the U.S. dollar as its reporting currency. The unaudited pro forma condensed combined financial information gives effect to adjustments required to convert FLAC historical financial information to IFRS and its reporting currency to Euros.
You should not rely on the unaudited pro forma condensed combined financial information as being indicative of the historical results that would have been achieved had the companies always been combined or the future results that NewAmsterdam Pharma will experience. FLAC and NewAmsterdam Pharma have not had any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies. The unaudited pro forma condensed combined financial information gives effect to those transactions contemplated in the Business Combination Agreement and those transactions which occur as a direct result of the consummation of the Business Combination. No effect has been given to events which occurred subsequent to the end of the period presented herein which do not occur as a direct result of the consummation of the Business Combination, including:
| • | Receipt of the non-refundable, non-creditable, upfront amount of €115 million from Menarini. |
For more information regarding significant events which occurred following the end of the period presented refer to “Note 15—Events After the Reporting Period” in the accompanying NewAmsterdam Pharma unaudited condensed consolidated financial statements as at and for the six months ended June 30, 2022 and 2021.
The unaudited pro forma condensed combined financial information has been prepared assuming two alternative levels of redemption using the assumptions below.
| • | No Redemption Scenario (“Scenario 1”): This presentation assumes that no FLAC shareholders exercise their redemption rights with respect to their shares of FLAC Class A Ordinary Shares for a pro rata share of cash in the Trust Account. |
| • | Maximum Redemption Scenario (“Scenario 2”): This presentation assumes that 12,261,482 shares of FLAC Class A Ordinary Shares are redeemed for their pro rata share of cash in the Trust Account in connection with the exercise of their redemption rights. In connection with the execution of the Business Combination Agreement, certain other holders of FLAC Class A Ordinary Shares agreed to waive their right to redeem such shares which total 1,500,000 FLAC Class A Ordinary Shares. 501,000 FLAC Class A Ordinary Shares acquired by the Sponsor in a private placement which occurred at the time of the FLAC IPO are not entitled to be redeemed in connection with the Business Combination. FLAC Class B Ordinary Shares do not have redemption rights. The Business Combination Agreement requires, at a minimum, the proceeds of the PIPE Financing plus the balance of the cash held in the Trust Account, after giving effect to redemption of FLAC Class A Ordinary Shares, to be at least $250 million. In order to meet this minimum, holders of an additional 38,518 FLAC Class A Ordinary Shares which are not subject to an agreement to waive redemption rights must choose not to exercise their redemption rights. This scenario gives effect to the redemption of 12,261,482 FLAC Class A Ordinary Shares for aggregate redemption payments of €117.2 million ($122.7 million) at a redemption price of approximately €9.56 ($10.01, converted at a rate of $1.0469 per EUR) per share based on the historical balance of investments held in the Trust Account as of June 30, 2022. |
The foregoing scenarios are for illustrative purposes only as FLAC does not have, as of the date of this proxy statement/prospectus, a meaningful way of providing any certainty regarding the number of redemptions by FLAC stockholders that may actually occur. If the actual redemptions are different than these assumptions, then the amounts and shares outstanding in the unaudited pro forma condensed combined financial information will be different and those changes could be material. See the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” for more information.
61
Table of Contents
PRO FORMA CONDENSED COMBINED STATEMENT OF FINANCIAL POSITION AS AT
JUNE 30, 2022
(UNAUDITED)
(in EUR thousands unless otherwise denoted)
| Scenario 1: No Redemption Scenario | Scenario 2: Maximum Redemption Scenario | |||||||||||||||||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| EUR | USD | EUR(1) | FN | FN | FN | |||||||||||||||||||||||||||||||||||||||
ASSETS | ||||||||||||||||||||||||||||||||||||||||||||
Non-Current Assets | ||||||||||||||||||||||||||||||||||||||||||||
Property, plant and equipment | 175 | — | — | — | — | 175 | — | 175 | ||||||||||||||||||||||||||||||||||||
Loan receivable | 728 | — | — | — | (728 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Investments held in Trust Account | — | 138,133 | 131,945 | — | (131,945 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Intangible assets | — | — | — | — | 82,686 | (8 | ) | 82,686 | — | 82,686 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total non-current assets | 903 | 138,133 | 131,945 | — | (49,987 | ) | 82,861 | — | 82,861 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Current Assets | ||||||||||||||||||||||||||||||||||||||||||||
Trade receivables | 115,000 | — | — | — | — | (4 | ) | 115,000 | — | 115,000 | ||||||||||||||||||||||||||||||||||
Prepayments and other receivables | 12,474 | — | — | 143 | (2 | ) | 4,019 | (10 | ) | 16,636 | — | 16,636 | ||||||||||||||||||||||||||||||||
Cash and cash equivalents | 89,478 | 615 | 588 | — | 323,190 | (4 | ) | 413,256 | (117,235 | ) | (12 | ) | 296,021 | |||||||||||||||||||||||||||||||
Prepaid expenses | — | 150 | 143 | (143 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total current assets | 216,952 | 765 | 731 | — | 327,209 | 544,892 | (117,235 | ) | 427,657 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total Assets | 217,855 | 138,898 | 132,676 | — | 277,222 | 627,753 | (117,235 | ) | 510,518 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
62
Table of Contents
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(1) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
EQUITY AND LIABILITIES | ||||||||||||||||||||||||||||||||||||||||||||
Commitments and Contingencies | ||||||||||||||||||||||||||||||||||||||||||||
Class A ordinary share subject to possible redemption, $0.0001 par value; 13,800,000 shares issued and outstanding at redemption value of $10.00 per share | — | 138,033 | 131,849 | (131,849 | ) | (3 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||||||||||||||||||||||
Share capital | 163,556 | — | — | * | — | 433,921 | (5 | ) | 597,477 | (79,929 | ) | (13 | ) | 517,548 | ||||||||||||||||||||||||||||||
Other Reserves | 1,029 | — | — | — | — | 1,029 | — | 1,029 | ||||||||||||||||||||||||||||||||||||
Preference shares, $0.0001 par value; 1,000,000 shares authorized; none issued or outstanding at December 31, 2021 and 2020 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Class A ordinary shares, $0.0001 par value; 479,000,000 shares authorized; 501,000 shares issued and outstanding (excluding 13,800,000 shares subject to possible redemption | — | — | * | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
Class B ordinary shares, $0.0001 par value; 20,000,000 shares authorized; 3,450,000 shares issued and outstanding | — | — | * | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
Additional paid-in capital | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Retained earnings | 19,837 | (6,181 | ) | (5,904 | ) | — | (21,897 | ) | (6 | ) | (7,964 | ) | (37,306 | ) | (14 | ) | (45,270 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total equity | 184,422 | (6,181 | ) | (5,904 | ) | — | 412,024 | 590,542 | (117,235 | ) | 473,307 | |||||||||||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||||||||||||||||||||||
Deferred revenue | 7,440 | — | — | — | — | 7,440 | — | 7,440 | ||||||||||||||||||||||||||||||||||||
Lease liability | 90 | — | — | — | — | 90 | — | 90 | ||||||||||||||||||||||||||||||||||||
Deferred underwriting commissions | — | 4,830 | 4,614 | — | (4,614 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Derivative warrant liabilities | — | 381 | 365 | — | — | (11 | ) | 365 | — | 365 | ||||||||||||||||||||||||||||||||||
Derivative earnout liability | — | — | — | — | 6,592 | (9 | ) | 6,592 | — | 6,592 | ||||||||||||||||||||||||||||||||||
Loans and borrowings | — | — | — | 131,849 | (3 | ) | (131,849 | ) | (7 | ) | — | — | — | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total non-current liabilities | 7,530 | 5,211 | 4,979 | 131,849 | (129,871 | ) | 14,487 | — | 14,487 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
63
Table of Contents
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(1) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
Current liabilities | ||||||||||||||||||||||||||||||||||||||||||||
Accounts payable | — | 51 | 48 | (48 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Accrued expenses | — | 1,784 | 1,704 | (1,704 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Loans and borrowings | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Lease liability | 61 | — | — | — | — | 61 | — | 61 | ||||||||||||||||||||||||||||||||||||
Trade and other payables | 11,782 | — | — | 1,752 | (2 | ) | (4,931 | ) | (4 | ) | 8,603 | — | 8,603 | |||||||||||||||||||||||||||||||
Deferred revenue | 14,060 | — | — | — | — | 14,060 | — | 14,060 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total current liabilities | 25,903 | 1,835 | 1,752 | — | (4,931 | ) | 22,724 | — | 22,724 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total liabilities | 33,433 | 7,046 | 6,731 | 131,849 | (134,802 | ) | 37,211 | — | 37,211 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total equity and liabilities | 217,855 | 138,898 | 132,676 | — | 277,222 | 627,753 | (117,235 | ) | 510,518 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
| * | The financial statements of FLAC are presented in whole dollars whereas the historical financial information of FLAC in the above unaudited condensed combined statement of financial position is presented initially in thousands of dollars. FLAC Class A Ordinary Shares and FLAC Class B Ordinary Shares at December 31, 2021 are $50 and $345, respectively, as seen in FLAC’s balance sheet as of December 31, 2021 and 2020 which is found elsewhere within this proxy statement/prospectus. As a result of rounding these amounts are shown as zero. These amounts would be reclassified into share capital as part of adjustment (2) below and eliminated in adjustment (5) below. |
64
Table of Contents
PRO FORMA CONDENSED COMBINED STATEMENT OF PROFIT OR LOSS
FOR THE YEAR ENDED DECEMBER 31, 2021
(UNAUDITED)
(in EUR thousands unless otherwise denoted)
| Scenario 1: No Redemption Scenario | Scenario 2: Maximum Redemption Scenario | |||||||||||||||||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical (1) U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(2) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
Research and development expenses | (25,032 | ) | — | — | — | — | (25,032 | ) | — | (25,032 | ) | |||||||||||||||||||||||||||||||||
General and administrative expenses | (4,803 | ) | (1,366 | ) | (1,155 | ) | — | (30,160 | ) | (4 | ) | (36,118 | ) | (37,306 | ) | (5 | ) | (73,424 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total operating expenses | (29,835 | ) | (1,366 | ) | (1,155 | ) | — | (30,160 | ) | (61,150) | (37,306 | ) | (98,456 | ) | ||||||||||||||||||||||||||||||
Finance income | 9 | — | — | — | — | 9 | — | 9 | ||||||||||||||||||||||||||||||||||||
Finance expense | (216 | ) | — | — | — | — | (216 | ) | — | (216 | ) | |||||||||||||||||||||||||||||||||
Interest income from investments held in Trust Account | — | 16 | 14 | — | (14 | ) | (3 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Change in fair value of derivative warrant liabilities | — | 4,529 | 3,828 | — | — | 3,828 | — | 3,828 | ||||||||||||||||||||||||||||||||||||
Break-up fee from terminated agreement | — | 1,000 | 845 | — | — | 845 | — | 845 | ||||||||||||||||||||||||||||||||||||
Net foreign exchange gain | 1,443 | — | — | — | — | 1,443 | — | 1,443 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Loss before tax | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
Income tax expense | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Loss for the period | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Attributable to: | ||||||||||||||||||||||||||||||||||||||||||||
Equity holders of the Company | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Earnings / (loss) per share, basic and diluted: | ||||||||||||||||||||||||||||||||||||||||||||
Class A ordinary shares | 0.24 | |||||||||||||||||||||||||||||||||||||||||||
Class B ordinary shares | 0.24 | |||||||||||||||||||||||||||||||||||||||||||
Ordinary shares | (2.53 | ) | (0.64 | ) | (1.25 | ) | ||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||
65
Table of Contents
The following tables set forth selected historical comparative share information for NewAmsterdam Pharma and FLAC and unaudited pro forma condensed combined per share information after giving effect to the Business Combination, assuming two redemption scenarios as follows:
| • | Assuming No Redemptions: This presentation assumes that no FLAC shareholders exercise their redemption rights with respect to their shares of FLAC Class A Ordinary Shares for a pro rata share of cash in the Trust Account. |
| • | Assuming Maximum Redemptions: This presentation assumes that 12,261,482 shares of FLAC Class A Ordinary Shares which are not subject to the Company Support Agreement, or the Investor Support Agreements are redeemed for their pro rata share of cash in the Trust Account in connection with the exercise of their redemption rights. In connection with the execution of the Business Combination Agreement certain other holders of FLAC Class A Ordinary Shares agreed to waive their right to redeem such shares which total 1,500,000 FLAC Class A Ordinary Shares. 501,000 FLAC Class A Ordinary Shares acquired by the Sponsor in a private placement which occurred at the time of the FLAC IPO are not entitled to be redeemed in connection with the Business Combination. FLAC Class B Ordinary Shares do not hold redemption rights. The Business Combination Agreement requires, at a minimum, the proceeds of the PIPE Financing plus the balance of the cash held in trust, after giving effect to redemption of FLAC Class A Ordinary Shares, to be at least $250 million. In order to meet this minimum, holders of an additional 38,518 FLAC Class A Ordinary Shares which are not subject to an agreement to waive redemption rights must choose not to exercise their redemption rights. This scenario gives effect to 12,261,482 FLAC Class A Ordinary Shares for aggregate redemption payments of €117.2 million ($122.7 million) at a redemption price of approximately €9.56 ($10.01, converted at a rate of $1.0469 per EUR) per share based on the historical balance of investments held in the Trust Account as of December 31, 2021. |
The pro forma book value information reflects the Business Combination as if it had occurred on June 30, 2022 and assumes that 23,460,000 Holdco Shares are issued in the PIPE Financing. The net profit (loss) per share information gives pro forma effect to the Business Combination as if it had occurred on January 1, 2021. The tables below reflect an assumed Exchange Ratio of 2.1307. If the actual facts are different than these assumptions, the below numbers will be different. These figures do not take into account the number of Holdco Warrants to purchase Holdco Shares that will be outstanding immediately following the completion of the Business Combination.
This information is only a summary and should be read together with the selected historical financial information included elsewhere in this proxy statement/prospectus and the historical financial statements of NewAmsterdam Pharma and FLAC and related notes that are included elsewhere in this proxy statement/prospectus. The unaudited pro forma combined per share information of NewAmsterdam Pharma and FLAC is derived from, and should be read in conjunction with, the unaudited pro forma condensed combined financial statements and related notes included elsewhere in this proxy statement/prospectus.
The unaudited pro forma combined net profit (loss) per share information below does not purport to represent the net profit (loss) per share which would have occurred had the companies been combined during the periods presented, nor net profit (loss) per share for any future date or period. The unaudited pro forma combined book value per share information below does not purport to represent what the value of NewAmsterdam Pharma and FLAC would have been had the companies been combined during the periods presented.
66
Table of Contents
As at and for the six months ended June 30, 2022
| Pro Forma Condensed Combined Per Share Information | ||||||||||||||||
| NewAmsterdam Pharma | FLAC(4) | No Redemptions | Maximum Redemptions | |||||||||||||
| (in €) | ||||||||||||||||
Book value per share (basic)(1) | 10.84 | — | 6.86 | 6.41 | ||||||||||||
Book value per share (diluted)(1) | 9.72 | — | 6.58 | 6.10 | ||||||||||||
Book value per share—FLAC Class A Ordinary Shares (basic and diluted) | — | 8.90 | (5) | — | — | |||||||||||
Book value per share—FLAC Class B Ordinary Shares (basic and diluted) | — | (0.40 | )(6) | — | — | |||||||||||
Net profit attributable to equity holders of parent per share (basic) | 3.20 | (2) | — | 0.65 | 0.76 | |||||||||||
Net profit attributable to equity holders of parent per share (diluted) | 2.87 | (3) | — | 0.62 | 0.72 | |||||||||||
Net income per share—FLAC Class A Ordinary Shares | — | 0.01 | — | — | ||||||||||||
Net income per share—FLAC Class B Ordinary Shares | — | 0.01 | — | — | ||||||||||||
Cash dividends per share | — | — | — | — | ||||||||||||
Cash dividends per share—FLAC Class A Ordinary Shares | — | — | — | — | ||||||||||||
Cash dividends per share—FLAC Class B Ordinary Shares | — | — | — | — | ||||||||||||
As at and for the year ended December 31, 2021
| Pro Forma Condensed Combined Per Share Information | ||||||||||||||||
| NewAmsterdam Pharma | FLAC(4) | No Redemptions | Maximum Redemptions | |||||||||||||
| (in €) | ||||||||||||||||
Book value per share (basic and diluted)(1) | 4.40 | — | — | — | ||||||||||||
Book value per share—FLAC Class A Ordinary Shares (basic and diluted) | — | 8.23 | (5) | — | — | |||||||||||
Book value per share—FLAC Class B Ordinary Shares (basic and diluted) | — | (0.40 | )(6) | — | — | |||||||||||
Net loss attributable to equity holders of parent per share (basic and diluted) | (2.53 | )(2) | — | (0.64 | ) | (1.25 | ) | |||||||||
Net income per share—FLAC Class A Ordinary Shares | — | 0.20 | — | — | ||||||||||||
Net income per share—FLAC Class B Ordinary Shares | — | 0.20 | — | — | ||||||||||||
Cash dividends per share | — | — | — | — | ||||||||||||
Cash dividends per share—FLAC Class A Ordinary Shares | — | — | — | — | ||||||||||||
Cash dividends per share—FLAC Class B Ordinary Shares | — | — | — | — | ||||||||||||
67
Table of Contents
| (1) | Book value per share (basic) represents total equity divided by basic number of shares outstanding. Book value per share (diluted) represents total equity dividend by diluted number of shares outstanding. For more information on the diluted shares, please refer to “Unaudited Pro Forma Condensed Combined Financial Information.” |
| (2) | Immediately prior to the Exchange, 17,016,872 NewAmsterdam Pharma weighted average shares were outstanding (before dilution). Immediately after the Exchange, the Participating Shareholders (including Amgen and MTPC for this purpose) will hold 44,914,642 Holdco Shares. If net profit (loss) per share were calculated using 44,914,642 shares, net profit (loss) per share would be 1.21 for the six months ended June 30, 2022 ((0.64) for the year ended December 31, 2021). |
| (3) | Immediately prior to the Exchange, 18,981,159 NewAmsterdam Pharma weighted average shares were outstanding (after dilution). Immediately after the Exchange, the Participating Shareholders (including Amgen and MTPC for this purpose) will hold 49,100,000 Holdco Shares. If net profit per share were calculated using 49,100,000 shares, net profit per share would be 1.11 for the six months ended June 30, 2022. |
| (4) | FLAC historically prepared its financial statements in accordance with U.S. GAAP with the U.S. Dollar as its reporting currency. Per share amounts reported for FLAC reflects its historical financial results reported under U.S. GAAP and are reported in Euro. The historical financial statements of FLAC are presented in U.S. Dollar. The historical financial information was translated from U.S. Dollars to Euros using the historical exchange rates as described in the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” included elsewhere in this proxy statement/prospectus. |
| (5) | Book value per share — FLAC Class A Ordinary Shares represents investments held in Trust Account minus deferred underwriting commissions divided by total FLAC Class A Ordinary Shares outstanding. |
| (6) | Book value per share — FLAC Class B Ordinary Shares represents net assets except for investments held in Trust Account and deferred underwriting commissions divided by total FLAC Class B Ordinary Shares outstanding. |
68
Table of Contents
You should carefully consider the following risk factors, together with all of the other information included in this proxy statement/prospectus, before you decide whether to vote or instruct your vote to be cast to approve the relevant proposals described in this proxy statement/prospectus. These risk factors are not exhaustive and investors are encouraged to perform their own investigation with respect to each of FLAC and NewAmsterdam Pharma’s business, financial condition and prospects. The occurrence of one or more of the events or circumstances described in these risk factors, alone or in combination with other events or circumstances, may adversely affect the ability to complete or realize the anticipated benefits of the Business Combination, and may have a material adverse effect on the business, cash flows, financial condition and results of operations of Holdco following the Business Combination. The risks discussed below may not prove to be exhaustive and are based on certain assumptions made by Holdco, FLAC and NewAmsterdam Pharma which later may prove to be incorrect or incomplete. Holdco, FLAC and NewAmsterdam Pharma may face additional risks and uncertainties that are not presently known to such entity, or that are currently deemed immaterial, which may also impair their business or financial condition.
Risks Related to NewAmsterdam Pharma
Unless the context otherwise requires, any reference in the below subsection of this proxy statement/prospectus to “NewAmsterdam Pharma,” “we,” “our,” and “us” refer to NewAmsterdam Pharma, together with its subsidiaries prior to the consummation of the Business Combination; and “Holdco” refers to NewAmsterdam Pharma Company N.V., together with its subsidiaries after completion of the Business Combination, in each case unless the context otherwise requires.
Risks Related to NewAmsterdam Pharma’s Limited Operating History, Financial Condition and Capital Requirements
We are a clinical-stage company with limited operating history, no approved products and no historical product revenues, which makes it difficult to assess our future prospects and financial results. We have incurred net losses since our inception, and anticipate that we will continue to incur significant losses for the foreseeable future. We may never generate any product revenue or become profitable or, if we achieve profitability, may not be able to sustain it.
We are a clinical-stage biopharmaceutical company with a limited operating history upon which you can evaluate our business and prospects. Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of uncertainty. Our operations to date have been limited to developing and undertaking clinical trials of our product candidate, obicetrapib. We are not profitable and have not generated product revenue from operations. We have historically incurred net losses since we commenced operations in October 2019, including net losses of €28.6 million for the year ended December 31, 2021. Despite having made a profit in the six months ended June 30, 2022, we expect our costs to fund research and development activities for the full fiscal year 2022 to exceed our revenue arising from the Menarini License. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, considering the current research and development stage of our activities, as we do not have products approved for commercial sale. Our ability to ultimately achieve recurring product revenues and profitability is dependent upon our ability to successfully complete the development of obicetrapib and obtain necessary regulatory approvals for, and successfully manufacture, market and commercialize, our product together or with partners.
We believe that we will continue to expend substantial resources in the foreseeable future for the clinical development of obicetrapib or any additional product candidates and indications that we may choose to pursue in the future. These expenditures will include costs associated with research and development, conducting preclinical studies and clinical trials, and payments for third-party manufacturing and supply, as well as sales and marketing of obicetrapib or any of our future product candidates if they are approved for sale by regulatory authorities. Because the outcome of any clinical trial is highly uncertain, we cannot reasonably estimate the
69
Table of Contents
actual amounts necessary to successfully complete the development and commercialization of obicetrapib and any other drug candidates that we may develop in the future. Other unanticipated costs may also arise.
Our future capital requirements depend on many factors, including:
| • | the timing of, and the costs involved in, clinical development and obtaining regulatory approvals for our product candidate; |
| • | changes in regulatory requirements during the development phase that can delay or force us to stop our activities related to obicetrapib or any of our future product candidates; |
| • | the cost of commercialization activities if obicetrapib is approved for sale, including marketing, sales and distribution costs; |
| • | the cost of third-party manufacturing of our product candidate; |
| • | the number and characteristics of any other product candidates we develop or acquire; |
| • | our ability to establish and maintain strategic collaborations, licensing or other commercialization arrangements, and the terms and timing of such arrangements; |
| • | the extent and rate of market acceptance of any future approved products; |
| • | the expenses needed to attract and retain skilled personnel; |
| • | the costs associated with being a public company; |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including potential litigation costs and the outcome of such litigation; |
| • | the timing, receipt and amount of sales of, or royalties on, future approved products, if any; |
| • | any product liability or other lawsuits related to obicetrapib or any future product; |
| • | scientific breakthroughs in the field of treatment for cardio metabolic diseases that could significantly diminish the need for our product candidate or make it obsolete; and |
| • | changes in reimbursement policies that could have a negative impact on our future revenue stream. |
Even if the Business Combination is completed, we may require substantial additional financing to achieve our goals, and a failure to obtain this capital when needed and on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development, commercialization efforts or other operations.
Since our inception, almost all of our resources have been dedicated to the clinical development of obicetrapib. While we have been successful in the past in obtaining financing, we expect to continue to spend substantial amounts to continue the clinical development of our product candidate. At June 30, 2022, we had cash and cash equivalents of €89.5 million. On a pro forma basis at June 30, 2022, assuming the consummation of the Business Combination and the PIPE Financing, we estimate that Holdco would have €413.3 million in cash and cash equivalents assuming no redemptions by FLAC’s shareholders, and €296.0 million in cash and cash equivalents assuming maximum redemptions by FLAC’s shareholders, which we believe will be sufficient to fund our anticipated level of operations into 2026.
We will require additional capital to pursue clinical activities, complete clinical trials, and obtain regulatory approval for and commercialize obicetrapib. In addition, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity, convertible debt or debt financings, third-party funding, marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or a combination of these approaches. Even if we believe that we will have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
70
Table of Contents
Any additional fundraising efforts may divert the attention of our management from day-to-day activities, which may adversely affect our ability to develop and commercialize obicetrapib. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may negatively impact the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us or the possibility of such issuance may cause the market price of Holdco Shares to decline. The incurrence of indebtedness could result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than would be desirable and we may be required to relinquish rights to some of our technologies, intellectual property or future product candidates or otherwise agree to terms unfavorable to us, any of which may harm our business, financial condition, operating results and prospects.
If adequate funds are not available to us on a timely basis, we may be required or choose to:
| • | delay, limit, reduce or terminate clinical trials or other development activities for obicetrapib or any of our future product candidates; |
| • | delay, limit, reduce or terminate our other research and development activities; or |
| • | delay, limit, reduce or terminate our establishment or expansion of manufacturing, sales and marketing or distribution capabilities or other activities that may be necessary to commercialize obicetrapib or any of our future product candidates. |
We may also be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could harm our business, financial condition and results of operations.
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
While we believe that, after giving effect to the Business Combination. our existing cash and cash equivalents will be sufficient to fund our operations through 2026, unless and until we can generate substantial product revenues, we expect to finance our future cash needs through public or private equity offerings, debt financings, collaborations, strategic alliances, license agreements and marketing or distribution arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. To the extent that we raise such additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring and distributing dividends, and may be secured by all or a portion of our assets.
If we raise funds by entering into collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish additional valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through public or private equity offerings, debt financings, collaborations, strategic alliances, license agreements, or marketing or distribution arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or cease operations altogether.
71
Table of Contents
Risks Related to NewAmsterdam Pharma’s Product Development, Regulatory Approval and Commercialization
We are dependent on the success of our only product candidate, obicetrapib, and cannot guarantee that obicetrapib will successfully complete clinical development, receive regulatory approval or, if approved, be successfully commercialized.
We have invested almost all of our efforts and financial resources in the research and development of obicetrapib. Our future success depends on our ability to develop, commercialize, market and sell obicetrapib. However, obicetrapib has yet to receive marketing approval from the FDA, the EMA or other comparable regulatory authorities. Our ability to generate revenues in the future is substantially dependent on our ability to develop, obtain regulatory approval for, and then successfully commercialize obicetrapib. We currently generate no revenue from the sale of any products, and we may never be able to develop or commercialize a marketable product.
Obicetrapib’s marketability and commercialization are subject to significant risks associated with successfully completing current and future clinical trials, including:
| • | our ability to successfully complete our clinical trials, including timely patient enrollment and acceptable safety and efficacy data and our ability to demonstrate the safety and efficacy of obicetrapib; |
| • | our ability to implement strategies to minimize the impact of the COVID-19 pandemic to our business, including with respect to initiating, enrolling, conducting or completing our planned and ongoing clinical trials of obicetrapib and addressing any potential disruption or delays to the supply of our product candidates; |
| • | unless we have received a deferral or waiver, our ability to complete successfully any pediatric clinical trials agreed pursuant to the Pediatric Research Equity Act (“PREA”) or its European Union (“EU”) equivalent; |
| • | that the Phase 3 clinical trials, even if successfully completed, will be sufficient to support a New Drug Application (“NDA”) submission; |
| • | the prevalence and severity of adverse events associated with obicetrapib; |
| • | whether we are required by the FDA, the EMA or other comparable regulatory authorities to conduct additional preclinical studies or clinical trials, and the scope and nature of such studies or trials, prior to approval to market our product, such as a cardiovascular outcomes trial; |
| • | the timely receipt of necessary marketing approvals from the FDA, the EMA and other comparable regulatory authorities, including pricing and reimbursement determinations; |
| • | the ability to successfully commercialize obicetrapib, if approved, for marketing and sale by the FDA, the EMA or other comparable regulatory authorities; |
| • | our ability and the ability of our third party manufacturing partners to timely and satisfactorily manufacture quantities of obicetrapib at quality levels necessary to meet regulatory requirements and at a scale sufficient to meet anticipated demand at a cost that allows us to achieve profitability; |
| • | our success in educating healthcare providers and patients about the benefits, risks, administration and use of obicetrapib, if approved; |
| • | acceptance of obicetrapib, if approved, as safe and effective by patients and the healthcare community; |
| • | the maintenance of an acceptable safety profile of our product following any approval; |
| • | the availability, perceived advantages, relative cost, safety and efficacy of alternative and competing treatments for the indications addressed by obicetrapib; |
72
Table of Contents
| • | entering into, on favorable terms, any collaboration, licensing or other arrangements that may be necessary or desirable to develop, manufacture or commercialize obicetrapib; |
| • | the effectiveness of our and any current or future collaborators’ marketing, sales and distribution strategy, and operations; and |
| • | our ability to obtain, protect and enforce our intellectual property rights with respect to obicetrapib. |
Many of these clinical, regulatory and commercial risks are beyond our control. Accordingly, we cannot assure you that we will be able to advance obicetrapib successfully through clinical development, or to obtain regulatory approval of or commercialize obicetrapib or any future product candidates. If we fail to achieve these objectives or overcome the challenges presented above, we could experience significant delays or an inability to successfully commercialize obicetrapib. Accordingly, we may not be able to generate sufficient revenues through the sale of obicetrapib to enable us to continue our business.
We have never obtained approval for any product candidate, and may be unable to do so successfully.
As a company, we have never progressed a product candidate through to regulatory approval. We have not previously submitted an NDA, an MAA or any similar drug approval filing to the FDA, the EMA or any comparable regulatory authority for any product candidate, and we cannot be certain that obicetrapib will be successful in clinical trials or receive regulatory approval. Further, obicetrapib may not receive regulatory approval even if it is successful in clinical trials. Even if we successfully obtain regulatory approvals to market our product candidate, our revenues will be dependent, to a significant extent, upon the size of the markets in the territories for which we gain regulatory approval and have commercial rights or share in revenues from the exercise of such rights. If the markets for patient subsets that we are targeting are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
Further, our clinical trials may require more time and incur greater costs than we anticipate. We cannot be certain that our planned clinical trials will begin or conclude on time, if at all. Large-scale trials require significant financial and management resources. Third-party clinical investigators do not operate under our control. Any performance failure on the part of such third parties could delay the clinical development of obicetrapib or delay or prevent us from obtaining regulatory approval or commercializing obicetrapib or future product candidates, depriving us of potential product revenue and resulting in additional losses.
Clinical drug development involves a lengthy and expensive process with uncertain outcomes, and results of earlier studies and trials may not be predictive of future trial results, and our clinical trials may fail to adequately demonstrate the safety and efficacy of obicetrapib.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. A failure of one or more of our clinical trials can occur at any time during the clinical trial process. We do not know whether future clinical trials, if any, will begin on time, need to be redesigned, enroll an adequate number of patients on time or be completed on schedule, if at all. Clinical trials can be delayed, suspended or terminated for a variety of reasons, including failure to:
| • | obtain allowance from the FDA or comparable foreign regulatory authorities in order to commence a trial; |
| • | identify, recruit and train suitable clinical investigators; |
| • | reach agreement on acceptable terms with prospective contract research organizations (“CROs”), and clinical trial sites, and have such CROs and sites effect the proper and timely conduct of our clinical trials; |
| • | obtain and maintain institutional review board (“IRB”) approval, or comparable ethics committee (“EC”), approval in foreign jurisdictions, at each clinical trial site; |
73
Table of Contents
| • | identify, recruit and enroll suitable patients to participate in a trial; |
| • | have a sufficient number of patients complete a trial or return for post-treatment follow-up; |
| • | ensure patient compliance with the trial protocols; |
| • | ensure clinical investigators and clinical trial sites observe trial protocol or continue to participate in a trial; |
| • | address any patient safety concerns that arise during the course of a trial; |
| • | address any conflicts with new or existing laws or regulations; |
| • | add a sufficient number of clinical trial sites; |
| • | manufacture sufficient quantities at the required quality of obicetrapib for use in clinical trials; or |
| • | raise sufficient capital to fund a trial. |
Product candidates like obicetrapib in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and earlier clinical trials. In addition to the safety and efficacy traits of any product candidate, clinical trial failures may result from a multitude of factors including flaws in trial design, dose selection, placebo effect and patient enrollment criteria. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials, and it is possible that we will as well. Based upon negative or inconclusive results, we may decide, or regulators may require us, to conduct additional clinical trials or preclinical studies. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval.
We may also encounter delays if a clinical trial is suspended or terminated by us or the IRBs or ECs of the institutions in which such trials are being conducted, the trial’s data safety monitoring board (the “DSMB”), the FDA, the EMA or other comparable regulatory authorities. Such authorities may suspend or terminate one or more of our clinical trials due to a number of factors, including our failure to conduct the clinical trial in accordance with relevant regulatory requirements or clinical protocols, inspection of the clinical trial operations or trial site by the FDA, the EMA or other comparable regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, a finding that the participants are being exposed to an unacceptable benefit-risk ratio, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
In addition, disruptions caused by the COVID-19 pandemic may increase the likelihood that we encounter such difficulties or delays in initiating, enrolling, conducting or completing our planned and ongoing clinical trials, as applicable. If we experience delays in the initiation, enrollment or completion of any clinical trial of obicetrapib, or if any clinical trials of obicetrapib are cancelled, the commercial prospects of obicetrapib may be materially adversely affected, and our ability to generate product revenues will be delayed or not realized at all. In addition, any delays in completing our clinical trials may increase our costs and slow down our product candidate development and approval process. Any of these delays may significantly harm our business and financial condition. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of obicetrapib.
74
Table of Contents
We depend on enrollment of subjects in our clinical trials for obicetrapib. If we experience delays or difficulties enrolling subjects in our clinical trials, our research and development efforts and business, financial condition and results of operations could be materially adversely affected.
If we experience delays or difficulties in the enrollment of subjects in future clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented. Trial costs have increased significantly following the COVID-19 pandemic and there may be delays in patient enrollment. The enrollment of subjects depends on many additional factors, including:
| • | the subject eligibility criteria defined in the protocol; |
| • | the general willingness of subjects to enroll in the trial; |
| • | patient compliance with the trial protocols; |
| • | the sample size of the subjects required for analysis of the trial’s primary endpoints; |
| • | the proximity of subjects to trial sites; |
| • | the design of the trial; |
| • | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | clinicians’ and subjects’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new therapies that may be approved for the indications we are investigating; |
| • | the clinical site’s ability to obtain and maintain subject consents; and |
| • | clinical trial participants may not comply with clinical trial protocol procedures and instructions. |
Our clinical trials may also compete with other clinical trials for product candidates that seek to treat cardio metabolic diseases, and this competition will reduce the number and types of subjects available to us, because some subjects who might have opted to enroll in our trials may instead opt to enroll in a clinical trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we may conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of subjects who are available for our clinical trials in such clinical trial sites. Further, if patients are unwilling to enroll in our clinical trials because of the COVID-19 pandemic and restrictions on travel or healthcare institution policies or other impacts of the COVID-19 pandemic, the timeline for recruiting patients, conducting studies and obtaining regulatory approval of our product candidate may be delayed.
Delays in subject enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of obicetrapib.
Interim, “topline” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose preliminary or “topline” data from our clinical trials, which is based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or clinical trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the “topline” or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. “Topline” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, “topline” data should be viewed with caution until the final data are available.
75
Table of Contents
Additionally, we may also disclose interim data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available or as patients from our clinical trials continue other treatments for their disease. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects. Further, disclosure of interim data by us or by our competitors could result in volatility in the price of our Holdco Shares.
Further, others, including regulatory authorities and collaboration or regional partners, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of our particular program, the approvability or commercialization of obicetrapib or any future product candidate and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is material or otherwise appropriate information to include in our disclosure.
If the interim, “topline,” or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, obicetrapib may be harmed, which could significantly harm our business, financial condition, results of operations and prospects.
The regulatory approval processes of the FDA, the EMA and other comparable regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for obicetrapib, our business will be substantially harmed.
The research, development, testing, manufacturing, labeling, packaging, approval, promotion, advertising, storage, recordkeeping, marketing, distribution, post-approval monitoring and reporting, and export and import of drug products are subject to extensive regulation by the FDA, the EMA and other comparable regulatory authorities in other countries. These regulations differ from country to country. We have not yet obtained regulatory approval to market obicetrapib in the United States or any other country, but plan to seek approval of obicetrapib in the United States, the EU, the United Kingdom, Japan and China. To gain approval to market obicetrapib, we must provide clinical trial data that adequately demonstrate the safety and efficacy of the product for the intended indication.
We cannot be certain of the timely completion or outcome of any of our future preclinical testing and studies, if any, on obicetrapib. We cannot be sure that the FDA, local regulatory authorities in the EU or other comparable regulatory authorities (including the Medicines and Healthcare products Regulatory Agency in the United Kingdom (“MHRA”), the Japan Pharmaceuticals and Medical Devices Agency in Japan (“PMDA”) and the China National Medical Products Administration in China (“NMPA”)) will accept the outcome of our preclinical testing and studies as sufficient to support the submission of an IND, clinical trial authorizations (“CTAs”) or similar applications for any of our programs which may result in us being unable to submit INDs, CTAs or similar applications or result in FDA, local regulatory authorities in the EU or other comparable regulatory authority refusing to allow clinical trials to begin. Furthermore, Phase 3 clinical trials often produce unsatisfactory results even though prior clinical trials were successful. Moreover, the results of clinical trials may be unsatisfactory to the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities even if we believe those clinical trials to be successful. The FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may suspend one or all of our clinical trials or require that we conduct additional clinical, preclinical, manufacturing, validation or drug product quality studies and submit that data before considering or reconsidering any NDA or comparable foreign regulatory application that we may submit. Depending on the extent of these additional studies, approval of any applications that we submit may be significantly delayed or may cause the termination of such programs, or may require us to expend more resources than we have available. The FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities can delay, limit or deny approval of our product candidate for many reasons, including:
| • | our inability to satisfactorily demonstrate that obicetrapib is safe and effective for the target indication; |
76
Table of Contents
| • | the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may disagree with our clinical trial protocol, the interpretation of data from preclinical studies or clinical trials, or adequate conduct and control of clinical trials; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities for approval; |
| • | the population studied in the clinical trials may not be sufficiently broad or representative to assess safety in the patient population for which we seek approval; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities for approval; |
| • | our inability to demonstrate that clinical or other benefits of obicetrapib outweigh any safety or other perceived risks; |
| • | determination by the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities that additional preclinical studies or clinical trials are required; |
| • | the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may fail to approve of the formulation, labeling or the specifications of obicetrapib; |
| • | the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may fail to accept the manufacturing processes or facilities of third-party manufacturers with which we contract; |
| • | the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies or such processes or facilities may not pass a pre-approval inspection; |
| • | the potential for approval policies or regulations of the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities to significantly change or differ from another in a manner rendering our clinical data insufficient for approval; or |
| • | resistance to approval from the FDA’s advisory committee for any reason including safety or efficacy concerns. |
Even if we eventually complete clinical testing and receive approval of any regulatory filing for obicetrapib, the FDA, the EMA or other comparable regulatory authorities (including the MHRA in the United Kingdom, the PMDA in Japan and the NMPA in China) may grant approval contingent on the performance of costly and potentially time-consuming additional post-approval clinical trials or subject to restrictive risk mitigation or surveillance requirements. The FDA, the EMA or other comparable regulatory authorities may also approve obicetrapib for a more limited indication or a narrower patient population than we originally requested, and the FDA may not approve the labeling that we believe is necessary or desirable for the successful commercialization of obicetrapib. To the extent we seek regulatory approval in other foreign countries, we may face challenges similar to those described above with regulatory authorities in applicable jurisdictions.
We and our collaborator(s) are not permitted to market or promote obicetrapib before we receive regulatory approval from the FDA, the EMA, the MHRA, the PMDA, the NMPA or comparable regulatory authorities in other countries, and we may never receive such regulatory approval for obicetrapib to allow us to successfully commercialize our product. If we do not receive regulatory approval with the necessary conditions to allow successful commercialization, we will not be able to generate revenue from obicetrapib in the United States or other countries in the foreseeable future, or at all. Any delay in obtaining, or inability to obtain, applicable regulatory approval for obicetrapib would delay or prevent commercialization of our obicetrapib and could thus negatively impact our business, results of operations and prospects.
77
Table of Contents
Our ongoing clinical trials are subject to delays or failures, which could result in increased costs to us and could delay, prevent or limit our ability to obtain regulatory approval for obicetrapib, which could have an adverse impact on our business.
In addition to our Phase 3 lipid-lowering clinical trials for obicetrapib, we are currently conducting a cardiovascular outcomes trial (“CVOT”), in patients with atherosclerotic cardiovascular disease (“ASCVD”), and a Phase 2a clinical trial of obicetrapib in patients suffering from Alzheimer’s disease. The completion of this clinical trial or any of our other ongoing or future clinical studies may be delayed for a number of reasons, including:
| • | the FDA, EMA or any other regulatory authority may not agree to the clinical trial design or overall program; |
| • | the FDA, EMA or any other regulatory authority may place a clinical trial on hold; |
| • | delays in reaching or failing to reach agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
| • | inadequate quantity or quality of a product candidate or other materials necessary to conduct clinical studies; |
| • | difficulties or delays obtaining IRB or EC approval to conduct a clinical trial at a prospective site or sites; |
| • | severe or unexpected drug-related side effects experienced by patients in a clinical trial, including instances of muscle pain or weakness or other side effects; |
| • | reports from preclinical or clinical testing of other cardio metabolic therapies that raise safety or efficacy concerns; and |
| • | difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the clinical trial, lack of efficacy, side effects, personal issues or loss of interest. |
In addition, a clinical trial may be suspended or terminated by us, the FDA, the EMA, the IRBs or ECs at the sites where the IRBs or ECs are overseeing a clinical trial, a DSMB overseeing the clinical trial at issue or any other regulatory authorities due to a number of factors, including, among others:
| • | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| • | inspection of the clinical trial operations or clinical trial sites by the FDA, EMA or any other regulatory authorities that reveals deficiencies or violations that require us to undertake corrective action, including the imposition of a clinical hold; |
| • | unforeseen safety issues; |
| • | changes in government regulations or administrative actions; |
| • | problems with clinical supply materials; and |
| • | lack of adequate funding to continue the clinical trial. |
Any such delays in our clinical trials could result in increased costs to us and delay, prevent or limit our ability to obtain regulatory approvals.
Obicetrapib may produce undesirable side effects that we may not have detected in our previous preclinical studies and clinical trials. This could prevent us from gaining approval or market acceptance, including broad physician adoption, for our product candidate if approved, or from maintaining such approval and acceptance, and could substantially increase commercialization costs and even force us to cease operations.
As with most pharmaceutical products, use of obicetrapib may be associated with side effects or adverse events that can vary in severity and frequency. Side effects or adverse events associated with the use of
78
Table of Contents
obicetrapib may be observed at any time, including in clinical trials or once a product is commercialized, and any such side effects or adverse events may negatively affect our ability to obtain regulatory approval or market obicetrapib. We cannot assure you that we will not observe drug-related serious adverse events in the future or that the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities will not determine them to be as such. Side effects such as toxicity or other safety issues associated with the use of obicetrapib could require us to perform additional studies or halt development or sale of obicetrapib or expose us to product liability lawsuits, which will harm our business.
Furthermore, our current Phase 3 clinical trials for obicetrapib involve a larger patient base than that previously studied, and the commercial marketing of obicetrapib, if approved, will further expand the clinical exposure of the drug to a wider and more diverse group of patients than those participating in the clinical trials, which may identify undesirable side effects caused by our product that were not previously observed or reported.
The FDA, the EMA and other comparable regulatory authority regulations require that we report certain information about adverse medical events if our product may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date upon which we become aware of the adverse event as well as the nature and severity of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our product. If we fail to comply with our reporting obligations, the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authority could take action including enforcing a hold on or cessation of clinical trials, withdrawal of approved drugs from the market, criminal prosecution, the imposition of civil monetary penalties or seizure of our product.
Additionally, in the event we discover the existence of adverse medical events or side effects caused by obicetrapib, a number of other potentially significant negative consequences could result, including:
| • | our inability to file an NDA or similar application for obicetrapib because of insufficient benefit-risk profile, or the denial of such application by the FDA, the EMA or other comparable regulatory authorities; |
| • | the FDA, the EMA or other comparable regulatory authorities suspending or withdrawing their approval of the product; |
| • | the FDA, the EMA or other comparable regulatory authorities requiring the addition of labeling statements, such as warnings or contraindications or distribution and use restrictions; |
| • | the FDA, the EMA or other comparable regulatory authorities requiring us to issue specific communications to healthcare professionals, such as letters alerting them to new safety information about our product, changes in dosage or other important information; |
| • | the FDA, the EMA or other comparable regulatory authorities issuing negative publicity regarding the affected product, including safety communications; |
| • | our being limited with respect to the safety-related claims that we can make in our marketing or promotional materials; |
| • | our being required to change the way the product is administered, conduct additional preclinical studies or clinical trials, or restrict or cease the distribution or use of the product; and |
| • | our being sued and held liable for harm caused to patients. |
Any of these events could prevent us from achieving approval or market acceptance of obicetrapib and could substantially increase commercialization costs or even force us to cease operations. We cannot assure you that we will resolve any issues related to any product-related adverse events to the satisfaction of the FDA, the EMA or other comparable regulatory authority in a timely manner or ever, which could harm our business, prospects and financial condition.
79
Table of Contents
We conduct clinical trials for our product candidates outside the United States, and the FDA and comparable foreign regulatory authorities may not accept data from such trials, in which case our development plans in the U.S. and applicable foreign jurisdictions may be delayed, which could materially harm our business.
Our ongoing clinical trials are being undertaken both within and outside the United States, and we intend to conduct portions of our future clinical trials outside the United States. The acceptance of clinical trial data by the FDA, EMA or other comparable foreign regulatory authority from clinical trials conducted outside of their respective jurisdictions may be subject to certain conditions, or may not be accepted at all. In cases where data from foreign clinical trials are intended to serve as the basis for marketing approval in the United States, the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; and (ii) the trials were performed by clinical investigators of recognized competence and pursuant to good clinical practice (“GCP”) regulations. Additionally, the FDA’s clinical trial requirements, including sufficient size of patient populations and statistical powering, must be met. Many foreign regulatory authorities have similar approval requirements. In cases where data from foreign clinical trials are intended to serve as the basis for marketing authorizations in the EU, the EMA and/or local regulatory authorities in EU Member States require that such clinical trials follow the principles that are equivalent to the clinical trial requirements set out under relevant EU legislation, including with respect to ethical and GCP standards. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. There can be no assurance that any United States or foreign regulatory authority would accept data from clinical trials conducted outside of its applicable jurisdiction. If the FDA, EMA or any applicable foreign regulatory authority does not accept such data, it would result in the need for additional clinical trials, which would be costly and time-consuming and delay aspects of our business plan, and which may result in our product candidates not receiving approval or clearance for commercialization in the applicable jurisdiction.
Disruptions at the FDA and other regulatory agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA to review and clear or approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory and policy changes, the FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other agencies may also slow the time necessary for new medical devices or modifications to be cleared or approved by government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory authorities, such as the FDA, have had to furlough critical FDA employees and stop critical activities.
Separately, in response to the COVID-19 pandemic, on March 10, 2020, the FDA announced its intention to postpone most inspections of foreign manufacturing facilities, and on March 18, 2020, the FDA temporarily postponed routine surveillance inspections of domestic manufacturing facilities. Subsequently, in July 2020, the FDA resumed certain on-site inspections of domestic manufacturing facilities subject to a risk-based prioritization system, which it utilized to assist in determining when and where it was safest to conduct prioritized domestic inspections. In May 2021, the FDA outlined a detailed plan to move toward a more consistent state of inspectional operations, and in July 2021, the FDA resumed standard inspectional operations of domestic facilities and was continuing to maintain this level of operation as of September 2021. More recently, the FDA has continued to monitor and implement changes to its inspectional activities to ensure the safety of its employees and those of the firms it regulates as it adapts to the evolving COVID-19 pandemic. Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in
80
Table of Contents
response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Even if we receive regulatory approval for obicetrapib or our future product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expenses, limit or withdraw regulatory approval and subject us to penalties if we fail to comply with applicable regulatory requirements.
Any regulatory approvals that we receive for obicetrapib or future product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, risk mitigation and surveillance to monitor the safety and efficacy of the product candidate, and we may be required to include labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings. Such requirements could negatively impact us by reducing revenues or increasing expenses, and cause the approved product not to be commercially viable. Absence of long-term safety data may further limit the approved uses of our product, if any.
If the FDA, the EMA or other comparable regulatory authority approves obicetrapib, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration requirements and continued compliance with current good manufacturing practices (“cGMPs”) and GCPs for any clinical trials that we conduct post-approval. For certain commercial prescription drug products, manufacturers and other parties involved in the supply chain must also meet chain of distribution requirements and build electronic, interoperable systems for product tracking and tracing and for notifying the FDA of counterfeit, diverted, stolen and intentionally adulterated products or other products that are otherwise unfit for distribution in the United States. The EU similarly has in force falsified medicines rules, which require appropriate packaging, labeling, registration and tracking of certain medicinal products to ensure the detection of counterfeit medicinal products, and associated reporting requirements. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | suspension or imposition of restrictions on operations, including costly new manufacturing requirements; |
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary product recalls; |
| • | fines, untitled or warning letters or holds on clinical trials; |
| • | refusal by the FDA, the EMA or other comparable regulatory authority to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
Moreover, if obicetrapib is approved, our product labeling, advertising and promotion will be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about drug products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling.
81
Table of Contents
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize obicetrapib, and harm our business, financial condition and results of operations.
In addition, the policies of the FDA, the EMA, the MHRA, the PMDA, the NMPA and other comparable regulatory authorities may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of obicetrapib. Costs arising out of any regulatory developments could be time-consuming and expensive and could divert management resources and attention and, consequently, could adversely affect our business, financial condition and results of operations. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
We are developing obicetrapib in combination with other therapies, and safety or supply issues with combination products may delay or prevent development and approval of our combination product candidates.
We are developing obicetrapib in combination with one or more approved therapies. For example, we are evaluating obicetrapib in combination with ezetimibe, including the combination on top of high intensity statin therapy. Even if any product candidate we develop were to receive marketing approval or be commercialized for use in combination with other existing therapies, we would continue to be subject to the risks that the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities could revoke approval of the therapy used in combination with our product or that safety, efficacy, manufacturing or supply issues could arise with any of those existing therapies. If the therapies we use in combination with our product candidate are replaced as the standard of care for the indications we choose for any of our product candidate, the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities may require us to conduct additional clinical trials. The occurrence of any of these risks could result in our own product, if approved, being removed from the market or being less successful commercially.
We also may evaluate our product candidate or any future product candidates in combination with one or more therapies that have not yet been approved for marketing by the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities. We will not be able to market and sell any product candidate we develop in combination with an unapproved therapy if that unapproved therapy does not ultimately obtain marketing approval. In addition, unapproved therapies face the same risks described with respect to our product candidate currently in development, including the potential for serious adverse effects, delay in their clinical trials and lack of FDA, EMA, MHRA, PMDA or NMPA approval.
If the FDA, the EMA, the MHRA, the PMDA, the NMPA or other comparable regulatory authorities do not approve these other therapies or revoke their approval of, or if safety, efficacy, manufacturing or supply issues arise with, the therapies we choose to evaluate in combination with our product candidates, we may be unable to obtain approval of or market any such product candidate.
If we are not successful in our efforts to discover and develop additional product candidates, we may be unable to grow our business.
We may elect to build a pipeline of product candidates and progress these product candidates through clinical development for the treatment of a variety of diseases. We also intend to evaluate additional potential indications for obicetrapib and may choose to in-license or acquire other product candidates or commercial products to treat patients suffering from other cardio metabolic diseases with significant unmet medical needs. Even if we are successful in building our pipeline, the potential product candidates that we identify may not be suitable for clinical development, including as a result of being shown to have harmful side effects or other characteristics that indicate that they are unlikely to be products that will receive marketing approval and achieve market acceptance. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or
82
Table of Contents
be more effective than other commercially available alternatives. We may opportunistically pursue a strategy that would entail in-licensing additional product candidates. We may also become reliant on the research efforts of third parties for any such product candidates that we do not intend to conduct preclinical studies or early-stage clinical trials for. If we do not successfully develop and begin to commercialize product candidates, we will face difficulty in obtaining product revenues in future periods, which could result in significant harm to our financial position and potential for growth and adversely affect the price of the Holdco Shares.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and management resources, we are currently primarily focused on the development of obicetrapib for cardio metabolic diseases and we may forego or delay pursuit of opportunities with other product candidates or for other indications for obicetrapib that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial product candidates or profitable market opportunities. Our spending on current and future development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
Even if we obtain and maintain approval for our current and future product candidates from a regulatory authority in one or more jurisdictions, we may nevertheless be unable to obtain approval for our product candidates outside of those jurisdictions, which would limit our market opportunities and could harm our business.
Approval of a product candidate by one regulatory authority in any jurisdiction does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions. Even if one regulatory authority grants marketing approval for a product candidate, comparable regulatory authorities of other countries also must approve the manufacturing and marketing of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and more onerous than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for any product candidates, if approved, is also subject to approval. Obtaining approval for obicetrapib or any future product candidate in the EU from the European Commission following the opinion of the EMA or in other foreign jurisdictions, if we choose to submit a marketing authorization application there, would be a lengthy and expensive process. Even if a product candidate is approved, the FDA, the EMA or other foreign regulatory authorities, as the case may be, may limit the indications for which the drug may be marketed, require extensive warnings on the drug labeling or require expensive and time-consuming additional clinical trials or reporting as conditions of approval. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of obicetrapib or any future product candidate in certain countries.
Obicetrapib, if approved, will face significant competition from competing therapies and our failure to compete effectively may prevent us from achieving significant market penetration.
The biopharmaceutical industry is intensely competitive and subject to rapid and significant technological change. Our potential competitors include large and experienced companies that enjoy significant competitive advantages over us, such as greater financial, research and development, manufacturing, personnel and marketing resources, greater brand recognition and more experience and expertise in obtaining marketing approvals from the FDA, the EMA and other comparable regulatory authorities. These companies may develop new drugs to treat the indications that we target, or seek to have existing drugs approved for use for the treatment of the indications that we target.
83
Table of Contents
We are aware that DalCor Pharmaceuticals (“DalCor”) may continue to develop its cardio metabolic disease-modifying product candidate. We believe, however, that their focus will be on developing in a small genetic subpopulation unlike our focus on patients at high cardiovascular risk with residual elevation of LDL. If obicetrapib is approved, our main competitor will be the current therapies on the market, such as PSCK9 injectables from Amgen Inc., Regeneron Pharmaceuticals, Inc. and Novartis International AG.
Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in this industry. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis products that are more effective or less costly than our product candidate.
Any approved products may fail to achieve the degree of market acceptance by physicians, patients, hospitals, healthcare payors and others in the medical community necessary for commercial success.
Even if we obtain FDA, EMA or other foreign regulatory approvals for our product candidate, the commercial success of obicetrapib will depend significantly on their broad adoption and use by physicians for approved indications. The degree and rate of physician and patient adoption of obicetrapib, if approved, will depend on a number of factors, including:
| • | the clinical indications for which obicetrapib is approved; |
| • | the prevalence and severity of adverse side effects; |
| • | the pricing and extent to which the costs of obicetrapib are reimbursed by third-party payors, and patients’ willingness to pay for obicetrapib; |
| • | physicians’ satisfaction with, and acceptance by the medical community and patients of, the efficacy and safety results of obicetrapib results as demonstrated in clinical trials; |
| • | patient satisfaction with the results and administration of obicetrapib and overall treatment experience, including relative convenience, ease of use and avoidance of, or reduction in, adverse side effects; |
| • | the extent to which physicians recommend obicetrapib to patients; |
| • | physicians’ and patients’ willingness to adopt new therapies in lieu of other products or treatments; |
| • | the timing of market introduction of obicetrapib as well as competitive products; |
| • | the convenience of prescribing and initiating patients on obicetrapib; |
| • | relative convenience and ease of administration of obicetrapib; |
| • | the cost of treatment, safety and efficacy in relation to alternative treatments, including any similar generic treatments; |
| • | the revenues and profitability that obicetrapib will offer physicians as compared to alternative therapies; and |
| • | the effectiveness of our sales and marketing efforts. |
If obicetrapib is approved for use but fails to achieve the broad degree of physician adoption and market acceptance necessary for commercial success, we will not be able to generate significant revenues, and we may not become or remain profitable.
84
Table of Contents
Risks Related to NewAmsterdam Pharma’s Collaboration With or Reliance on Third Parties
We currently contract with third-party contractors, and in some cases, a single contractor, for all aspects of the manufacturing of obicetrapib for clinical trials, and expect to continue to do so to support commercial scale production of obicetrapib, if approved. There are significant risks associated with contracting with third-party suppliers, including their ability to meet the increased need that may result from our potential commercialization efforts. This increases the risk that we will not have sufficient quantities of obicetrapib or be able to obtain such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We currently rely on third-party contract manufacturers and suppliers for all of our required raw materials, active ingredients and finished products for our clinical trials. Because there are a limited number of suppliers for the raw materials that we use to manufacture our product candidate, we may need to engage alternate suppliers to prevent a possible disruption of the manufacture of the materials necessary to produce our product candidate for our clinical trials, and if approved, ultimately for commercial sale. We do not have any control over the availability of raw materials. If we or our manufacturers are unable to purchase these raw materials on acceptable terms, at sufficient quality levels or in adequate quantities, if at all, the development and commercialization of our product candidate or any future product candidates would be delayed, or there would be a shortage in supply, which would impair our ability to meet our development objectives for our product candidates or generate revenues from the sale of any approved products. We also currently rely on a single supplier for our active ingredient and finished product for obicetrapib. While we believe that alternative sources of supply exist, there can be no assurance that we will be able to quickly establish additional or replacement sources if needed, and a reduction or interruption in supply could adversely affect our ability to manufacture our product candidate in a timely or cost-effective manner.
We expect to continue to rely on these or other subcontractors and suppliers to support our commercial requirements if obicetrapib or any of our other product candidates is approved for marketing by the FDA, the EMA or other comparable regulatory authorities. We plan to continue to rely on third parties for the raw materials, compounds and components necessary to produce our product candidates and for our clinical trials.
Our continuing reliance on third-party contract manufacturers and suppliers entails a number of risks, including reliance on the third party for regulatory compliance and quality assurance, the possible breach of the manufacturing or supply agreement by the third party, and the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. In addition, third-party contract manufacturers and suppliers may not be able to comply with cGMP requirements, or similar regulatory requirements outside the United States. If any of these risks transpire, we may be unable to timely retain alternate subcontractors or suppliers on acceptable terms and with sufficient quality standards and production capacity, which may disrupt and delay our clinical trials or the manufacture and commercial sale of our product candidate, if approved.
Our failure or the failure of our third-party contract manufacturers and suppliers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of obicetrapib or any of our other product candidates that we may develop. Any failure or refusal to supply or any interruption in supply of the components for obicetrapib or any other product candidates or products that we may develop could delay, prevent or impair our clinical development or commercialization efforts.
The manufacture of pharmaceutical products is complex and manufacturers often encounter difficulties in production. If we or any of our third-party manufacturers encounter any difficulties, our ability to provide obicetrapib or any future product candidates for clinical trials, or to patients if approved, and the development or commercialization of obicetrapib or any future product candidates could be delayed or stopped.
The manufacture of pharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We and our
85
Table of Contents
contract manufacturers must comply with cGMP requirements. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production and contamination controls. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
We cannot assure you that any stability or other issues relating to the manufacture of obicetrapib or any future product candidate will not occur in the future. As the manufacturing processes are scaled up, they may reveal manufacturing challenges or previously unknown impurities that could require resolution in order to proceed with our planned clinical trials and obtain regulatory approval for the commercial marketing of our products. In the future, we may identify manufacturing issues or impurities that could result in delays in the clinical program and regulatory approval for obicetrapib or any future product candidate, increases in our operating expenses or failure to obtain or maintain approval for obicetrapib or any future product candidate. Our reliance on third-party manufacturers entails risks, including the following:
| • | the inability to meet our product candidate specifications, including product formulation, and quality requirements consistently; |
| • | a delay or inability to procure or expand sufficient manufacturing capacity; |
| • | manufacturing and product quality issues, including those related to scale-up of manufacturing; |
| • | costs and validation of new equipment and facilities required for scale-up; |
| • | a failure to comply with cGMP and similar quality standards; |
| • | the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms; |
| • | termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; |
| • | the reliance on a limited number of sources, and in some cases, single sources for key materials, such that if we are unable to secure a sufficient supply of these key materials, we will be unable to manufacture and sell obicetrapib in a timely fashion, in sufficient quantities or under acceptable terms; |
| • | the lack of qualified backup suppliers for those materials that are currently or in the future purchased from a sole or single source supplier; |
| • | operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier; |
| • | resource constraints, including as a result of labor disputes or unstable political environments; |
| • | carrier disruptions or increased costs that are beyond our control; and |
| • | the failure to deliver our products under specified storage conditions and in a timely manner. |
If we or our third-party manufacturers were to encounter any of these difficulties, and in particular where we rely on a single manufacturer, our ability to provide obicetrapib or any future product candidate to patients in clinical trials and products to patients, once approved, would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the initiation or completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. These events could impact our ability to obtain regulatory approval or successfully commercialize obicetrapib or any future product candidate. Some of these events could be the basis for FDA, EMA or other comparable regulatory authorities’
86
Table of Contents
action, including injunction, recall, seizure, or total or partial suspension of production. Any adverse developments affecting clinical or commercial manufacturing of obicetrapib or any future product candidate may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our product candidates. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. Accordingly, failures or difficulties faced at any level of our supply chain could materially adversely affect our business and delay or impede the development and commercialization of obicetrapib or any future product candidate and could have a material adverse effect on our business, prospects, financial condition and results of operations.
We rely, and expect to continue to rely, on third parties and consultants to assist us in conducting our clinical trials, including our Phase 3 clinical trials for obicetrapib. If these third parties or consultants do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize obicetrapib, if approved.
We do not have the ability to independently conduct many of our clinical trials. We rely on medical institutions, clinical investigators, contract laboratories and other third parties, such as CROs, to conduct clinical trials on obicetrapib. Third parties play a significant role in the conduct of our clinical trials and the subsequent collection and analysis of data. These third parties are not our employees and, except for remedies available to us under our agreements, we have limited ability to control the amount or timing of resources that any such third party will devote to our clinical trials. If our CROs or any other third parties upon which we rely for administration and conduct of our clinical trials do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements, or for other reasons, or if they otherwise perform in a substandard manner, our clinical trials may be extended, delayed, suspended or terminated, and we may not be able to complete development of, obtain regulatory approval for, or successfully commercialize obicetrapib.
We and the third parties upon whom we rely are required to comply with GCP, which are regulations and guidelines enforced by regulatory authorities around the world for products in clinical development. Regulatory authorities enforce these GCP regulations through periodic inspections of clinical trial sponsors, principal investigators and clinical trial sites. If we or our third parties fail to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and our submission of marketing applications may be delayed or the regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, a regulatory authority will determine that any of our clinical trials comply or complied with applicable GCP regulations. In addition, our clinical trials must be conducted with material produced under current cGMP regulations, which are enforced by regulatory authorities. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be impacted if our CROs, clinical investigators or other third parties violate federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
The COVID-19 pandemic and government measures taken in response may also have an impact on our CROs, including due to travel or quarantine policies or prioritization of resources toward the pandemic, and any disruption in their performance would affect our ability to complete our clinical trials.
In order for our clinical trials to be carried out effectively and efficiently, it is imperative that our CROs and other third parties communicate and coordinate with one another. Moreover, our CROs and other third parties may also have relationships with other commercial entities, some of which may compete with us. Our CROs and other third parties may terminate their agreements with us immediately under certain circumstances, such as upon 30 days’ notice or immediately upon a material breach. If our CROs or other third parties conducting our clinical trials do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical trial protocols or GCPs, or for any
87
Table of Contents
other reason, we may need to conduct additional clinical trials or enter into new arrangements with alternative CROs, clinical investigators or other third parties. We may be unable to enter into arrangements with alternative CROs on commercially reasonable terms, or at all. Switching or adding CROs, clinical investigators or other third parties can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which can impact our ability to meet our desired clinical development timelines. Although we carefully manage our relationship with our CROs, clinical investigators and other third parties, there can be no assurance that we will not encounter such challenges or delays in the future or that these delays or challenges will not have a negative impact on our business, prospects, financial condition or results of operations.
We currently intend to rely on our collaboration with Menarini, for the commercialization of obicetrapib, if approved, in certain European areas. Failure or delay of Menarini to fulfill all or part of its obligations to us under the Menarini License, a breakdown in collaboration between the parties or a complete or partial loss of this relationship could materially harm our business if obicetrapib is approved in the relevant jurisdictions.
While we currently plan to commercialize our own products, if approved, in the United States, we entered into the Menarini License with Menarini, an exclusive license agreement, on June 23, 2022 to obtain and maintain regulatory approvals, commercialize and undertake local development, in each case with respect to obicetrapib either as a sole active ingredient product or in a fixed dose combination with ezetimibe for any use, in certain areas of Europe, as set forth in the Menarini License. Our collaboration with Menarini is critical in these areas on our own business, as we do not currently have the internal capacity to market, sale and distribute obicetrapib, if approved, in Europe. Pursuant to the Menarini License, Menarini is responsible for communications with regulatory authorities for the commercialization and local development of obicetrapib in certain areas of Europe, if approved, and other collaborative activities. Menarini must commercialize obicetrapib pursuant to a commercialization plan agreed between the parties and is obligated to use commercially reasonable efforts to commercialize obicetrapib so as to maximize net sales, provided that Menarini has sole discretion to set the price of the products.
Either party has the right in certain circumstances to terminate the collaboration pursuant to the terms of the Menarini License, including in the case (i) of a material breach by the other party, (ii) that a relevant regulatory authority prohibits Menarini to pursue the commercialization of obicetrapib due to safety or efficacy concerns, or (iii) of insolvency of either party. If Menarini delays or fails to perform its obligations under the Menarini License, such as a delay in the anticipated commercial launch, disagrees with our interpretation of the terms of the collaboration or terminates the Menarini License, the commercialization of obicetrapib, if approved, could be significantly adversely affected and our prospects in Europe will be materially harmed.
We may not be able to meet our obligations under the Menarini License. Additionally, if we do not reach certain milestones as set forth in the Menarini License, we will not receive the milestone payments, which could require us to seek funding additional capital to complete clinical trials.
Menarini has also entered into collaborations with third parties addressing targets and disease indications outside the scope of our collaboration. As a result, Menarini may have competing interests with respect to their priorities and resources. We may have disagreements with Menarini with respect to the interpretation of the Menarini License, use of resources or otherwise that could cause our relationship with Menarini to deteriorate. As a result, Menarini may reduce their focus on, and resources allocated to, our commercialization, potentially delaying or terminating our ability to commercialize obicetrapib in Europe, if approved. However, as stated above, Menarini must commercialize obicetrapib pursuant to a commercialization plan agreed between the parties and is obligated to use commercially reasonable efforts to commercialize obicetrapib so as to maximize net sales. Additionally, should we decide to move forward with development of a combination of obicetrapib with a certain inhibitor in the areas of Europe covered by the Menarini License for patients suffering from diabetes, we will need to offer Menarini the opportunity to co-develop that product with us, provided that if Menarini does, we will negotiate with Menarini the economics and other terms in respect of such co-development and the subsequent commercialization of such combination product in such areas of Europe. If
88
Table of Contents
Menarini does not wish to co-develop such combination product, that would prevent our ability to, and our ability to license or authorize a third party to, seek regulatory approval for or promote such combination product, in the areas of Europe covered by the Menarini License.
Should our Menarini License be terminated, we will need to either build marketing, sales, distribution, managerial and other non-technical capabilities or contract with third parties to obtain these capabilities in Europe.
We have limited experience in marketing or distributing products and no internal capability to do so, and an inability to market, distribute and commercialize obicetrapib once approved would prevent us from achieving significant sales and reduce the commercial value of obicetrapib. If we are unable to establish sales, marketing and distribution capabilities for obicetrapib, if approved, or our future product candidates, or enter into sales, marketing and distribution agreements with third parties, we may not be successful in commercializing our product candidates, if and when they are approved.
We do not have a sales or marketing infrastructure and have limited experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any product candidate for which we may obtain marketing approval, we will need to establish a sales and marketing organization or enter into collaboration, distribution and other marketing arrangements with one or more third parties to commercialize such product candidate. In the United States, we intend to build a commercial organization to target areas with the greatest incidence of high cardiovascular risk with residual elevation of LDL and recruit experienced sales, marketing and distribution professionals. The development of sales, marketing, and distribution capabilities will require substantial resources, will be time-consuming and could delay any product launch. We may decide to work with regional specialty pharmacies, distributors and/or multi-national pharmaceutical companies to leverage their commercialization capabilities to commercialize any product candidate for which we may obtain regulatory approval outside of the United States or certain areas of Europe.
If the commercial launch of a product candidate for which we recruit a sales force and establish marketing and distribution capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization costs. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. In addition, we may not be able to hire a sales force in the United States that is sufficient in size or has adequate expertise to target the areas that we intend to target. If we are unable to establish a sales force and marketing and distribution capabilities, our operating results may be adversely affected.
Factors that may inhibit our efforts to commercialize our drugs on our own include:
| • | our inability to recruit, train, and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future products; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage compared to companies with more extensive product lines; |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization; and |
| • | unforeseen costs and limitations with regard to setting up a distribution network. |
If we are unable to establish our own sales, marketing and distribution capabilities in the United States and other jurisdictions in which obicetrapib or any future product candidates are approved, other than in the jurisdictions covered by the Menarini License, we will be required to enter into arrangements with third parties to perform these services. As a result, our revenues and profitability, if any, are likely to be lower than if we were to sell, market and distribute any product candidates that we develop ourselves. We may not be successful in entering into arrangements with third parties to sell, market and distribute our product candidates or may be unable to do so on terms that are favorable to us. We likely will have limited control over such third parties, and
89
Table of Contents
any of them may fail to devote the necessary resources and attention to sell and market our product candidates effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing any product candidates.
We expect to enter into collaborations with third parties for the development or commercialization of obicetrapib or future product candidates, which involve risks that could impact our liquidity, increase our expenses and present significant distractions to our management, and we may not be able to capitalize on the market potential of obicetrapib or any future product candidate if our collaborations are not successful.
In addition to the Menarini License, we may utilize a variety of types of collaboration, distribution and other marketing arrangements with other third parties relating to the development or commercialization, once approved, of obicetrapib or future product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities and efforts to successfully perform the functions assigned to them in these arrangements.
Any future collaborations that we enter into may pose a number of risks, including the following:
| • | collaborators have significant discretion in determining the amount and timing of efforts and resources that they will apply to these collaborations; |
| • | collaborators may not perform their obligations as expected; |
| • | product candidates developed by collaborators may not perform sufficiently in clinical trials to be determined to be safe and effective, thereby delaying or terminating the drug approval process and reducing or eliminating milestone payments to which we would otherwise be entitled if the product candidates had successfully met their endpoints and/or received FDA or EMA approval; |
| • | collaborators may not pursue development and commercialization of our product candidates that receive marketing approval or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| • | product candidates discovered in collaboration with us may be viewed by our collaborators as competitive with their own product candidates or products, which may cause collaborators to cease to devote resources to the commercialization of our product candidates; |
| • | a collaborator with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of such product or products; |
| • | disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities for us with respect to product candidates, or might result in litigation or arbitration, any of which would divert management attention and resources, be time-consuming and expensive; |
90
Table of Contents
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation; |
| • | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and |
| • | collaborations may be terminated for the convenience of the collaborator and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates. |
Collaboration agreements may not lead to the development or commercialization of product candidates in the most efficient manner, or at all. If any future collaborations that we enter into do not result in the successful development and commercialization of products or if one of our collaborators terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our development of our product candidates could be delayed and we may need additional resources to develop our product candidates. All of the risks relating to product development, regulatory approval and commercialization described in this proxy statement/prospectus also apply to the activities of our collaborators.
Additionally, subject to its contractual obligations to us, if a collaborator of ours were to be involved in a business combination, it might deemphasize or terminate the development or commercialization of any product candidate licensed to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and our perception in the business and financial communities could be harmed.
If in the future we acquire or in-license technologies or product candidates, we may incur various costs, may have integration difficulties as a result of becoming subject to onerous contractual requirements or restrictions, and may experience other risks that could harm our business and results of operations.
In the future, we may acquire or in-license additional product candidates and technologies. Any product candidate or technologies we in-license or acquire will likely require additional development efforts prior to commercial sale, including extensive preclinical or clinical testing, or both, and approval by the FDA, the EMA and other comparable regulatory authorities, if any. All product candidates are prone to risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate, or product developed based on in-licensed technology, will not be shown to be sufficiently safe and effective for approval by regulatory authorities. If intellectual property related to product candidates or technologies we in-license is not adequate, we may not be able to commercialize the affected products even after expending resources on their development. In addition, we may not be able to manufacture economically or successfully commercialize any product candidate that we develop based on acquired or in-licensed technology that is granted regulatory approval, and such products may not gain wide acceptance or be competitive in the marketplace. Moreover, integrating any newly acquired or in-licensed product candidates could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may be materially harmed.
Our employees and independent contractors, including principal investigators, CROs, consultants and vendors, may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us or harm our reputation.
We are exposed to the risk that our employees, independent contractors, clinical investigators, CROs, consultants and vendors may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct, breach of contract or disclosure of unauthorized activities to us that violates regulations of the FDA, the EMA or other comparable regulatory authorities, including those laws requiring the reporting of true, complete and accurate information; manufacturing standards; federal, state and foreign healthcare fraud and abuse laws; or laws that require the reporting of financial information or data accurately.
91
Table of Contents
Specifically, research, sales, marketing, education and other business arrangements in the healthcare industry are subject to extensive laws intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, education, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We intend to adopt a code of business conduct and ethics, but it is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws. If any such actions are instituted against us, even if we are successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business. Violations of such laws subject us to numerous penalties, including, but not limited to, the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
If we, or our third-party manufacturers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of its business.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts, business operations and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance.
Risks Related to NewAmsterdam Pharma’s Business and Strategy
The continuing outbreak of COVID-19 around the world, and any future pandemics, may adversely affect our business and that of our suppliers, CROs or other third parties relevant to our business.
The COVID-19 pandemic is impacting worldwide economic activity, particularly economic activity in the United States, and poses the risk that we or our employees, contractors, suppliers, or other partners may be prevented or delayed from conducting business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities. The continued prevalence of COVID-19 and the measures taken by the governments of countries affected could disrupt our supply chain and manufacturing, cause diversion of healthcare resources away from the conduct of preclinical and clinical trial matters to focus on pandemic concerns, limit travel in a manner that interrupts key clinical trial activities, such as
92
Table of Contents
clinical trial site initiations and monitoring, delay regulatory filings with regulatory authorities in affected areas or adversely affect our ability to obtain regulatory approvals. These disruptions could also affect other facets of our business, including but not limited to:
| • | our ability to recruit employees; |
| • | the ability of our employees to travel; |
| • | the ability of our CROs to conduct our clinical trials; |
| • | our ability to import materials; and |
| • | our ability to export materials to our CROs and other third-parties. |
The COVID-19 pandemic and mitigation measures also may have an adverse impact on global economic conditions, which could adversely impact our business, financial condition or results of operations. Additionally, the COVID-19 pandemic has resulted in significant financial market volatility and uncertainty. A continuation or worsening of the levels of market disruption and volatility seen in the recent past as a result of the COVID-19 outbreak could have an adverse effect on our ability to access capital and on the market price of our ordinary shares. In December 2020, we put in place a number of protective measures in response to the COVID-19 pandemic. These measures included a shift to virtual study and home health care visits, adding COVID-19 contingency measures to our clinical trial protocols and direct to subject study drug shipmnets. We have revisited these measures on a regular basis throughout the pandemic and we will continue to do so. Although restrictions imposed by governmental authorities have eased, if conditions worsen, we may need to implement new restrictive measures that could adversely affect our business.
If we fail to manage our growth effectively, our business could be disrupted.
As of June 30, 2022, we had nine full-time employees and seven consultants, of which three are based at our headquarters in the Netherlands. We expect to continue to expand our development, quality, sales, managerial, operational, finance, marketing and other resources in order to manage our operations and clinical trials, continue our development activities and commercialize obicetrapib, if approved. Our management, personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our expansion strategy requires that we:
| • | manage our clinical trials effectively; |
| • | identify, recruit, retain, incentivize and integrate additional employees; |
| • | manage our internal development efforts effectively while carrying out our contractual obligations to third parties; and |
| • | continue to improve our operational, financial and management controls, reporting systems and procedures. |
Due to our limited financial resources and our limited experience in managing a larger public company, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage expansion could delay the execution of our development and strategic objectives, or disrupt our operations; and if we are not successful in commercializing our product candidate, either on our own or through collaborations with one or more third parties, our revenues will suffer and we would incur significant additional losses.
93
Table of Contents
If obicetrapib or our future product candidates receive approval for marketing, and we are found to have improperly promoted off-label use, or if physicians misuse our products, we may become subject to prohibitions on the sale or marketing of our product, significant sanctions and product liability claims, and our image and reputation within the industry and marketplace could be harmed.
The FDA, the EMA or other comparable regulatory authorities strictly regulate the promotional claims that may be made about prescription drug products, such as obicetrapib, if approved. In particular, a product may not be promoted for uses that are not approved by FDA, the EMA or other comparable regulatory authorities as reflected in the product’s approved labeling. For example, if we receive marketing approval for obicetrapib for cardio metabolic disease, physicians, in their professional medical judgment, may nevertheless prescribe obicetrapib to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label use, we may become subject to significant liability under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and other statutory authorities, such as laws prohibiting false claims for reimbursement. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. If we become the target of such an investigation or prosecution based on our marketing and promotional practices, we could face similar sanctions, which would harm our business. In addition, management’s attention could be diverted from our business operations, significant legal expenses could be incurred and our reputation could be damaged. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we are deemed by the FDA to have engaged in the promotion of our products for off-label use, we could be subject to prohibitions on the sale or marketing of our products or significant fines and penalties, and the imposition of these sanctions could also affect our reputation with physicians, patients and caregivers, and our position within the industry.
Physicians may also misuse our products or use improper techniques, potentially leading to adverse results, side effects or injury, which may lead to product liability claims. If our products are misused or used with improper technique, we may become subject to costly litigation. Product liability claims could divert management’s attention from our core business, be expensive to defend, and result in sizable damage awards against us that may not be covered by insurance. We currently carry product liability insurance covering our clinical trials with policy limits that we believe are customary for similarly situated companies and adequate to provide us with coverage for foreseeable risks. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Furthermore, the use of our products for conditions other than those approved by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients. If we cannot successfully manage the promotion of obicetrapib or any future product candidate, if approved, we could become subject to significant liability, which would harm our reputation and negatively impact our financial condition.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of obicetrapib or any future products we may develop.
We face an inherent risk of product liability as a result of the clinical testing of obicetrapib and will face an even greater risk if we commercialize it or any future product. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for our product candidate or any future product candidates we develop; |
| • | injury to our reputation and significant negative media attention; |
94
Table of Contents
| • | withdrawal of clinical trial participants or delay or cancellation of clinical trials; |
| • | costs to defend the related litigation, which may be only partially recoverable even in the event of successful defenses; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to clinical trial participants or patients; |
| • | regulatory investigations, product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenues; |
| • | exhaustion of any available insurance and our capital resources; and |
| • | the inability to commercialize our product, if approved. |
Our inability to obtain and maintain sufficient product liability insurance at an acceptable cost and scope of coverage to protect against potential product liability claims could prevent or inhibit the commercialization of products we may develop. We currently carry general clinical trial product liability insurance in an amount that we believe is adequate to cover the scope of our ongoing clinical programs. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions and deductibles, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Moreover, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses. If and when we obtain approval for marketing obicetrapib or any other product candidate, we intend to expand our insurance coverage to include the commercialization of obicetrapib or any other approved product that we may have; however, we may be unable to obtain this liability insurance on commercially reasonable terms.
If we fail to attract and retain senior management and key scientific personnel, we may be unable to successfully develop our product candidate, conduct our clinical trials and commercialize our product candidate or any other products we may develop.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. We believe that our future success is highly dependent upon the contributions of members of our senior management, as well as our senior scientists and other members of our management team, especially our Chief Executive Officer, Dr. Michael Davidson, our Chief Scientific Officer, Dr. John Kastelein, and our Chief Operating Officer, Douglas Kling. We are not aware of any present intention of any of these individuals to leave our company. The loss of services of any of these individuals, though, could delay or prevent the successful development of our product pipeline, completion of our planned clinical trials or the commercialization of obicetrapib. Although we have agreements with our officers and employees, these agreements do not prevent them from terminating their employment or service arrangement with us as described in the agreements.
Although we have not historically experienced unique difficulties in attracting and retaining qualified employees, we could experience such problems in the future. For example, competition for qualified personnel in the pharmaceutical field is intense due to the limited number of individuals who possess the skills and experience required by our industry. Many of the other pharmaceutical companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles, diverse opportunities including for career advancement and a longer history in the industry than we do. We will need to hire additional personnel as we expand our clinical development and commercial activities. Some of these characteristics may be more appealing to high quality candidates than what we have to offer. We may not be able to attract and retain quality personnel on acceptable terms, or at all. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other
95
Table of Contents
confidential information, or that their former employers own their research output. Moreover, we are domiciled and predominantly based in the Netherlands, which may make it difficult to hire necessary U.S.-based personnel.
Misclassification or reclassification of our independent contractors or employees could increase our costs and adversely impact our business.
Our workers are classified as either employees or independent contractors, and if employees, as either exempt from overtime or non-exempt (and therefore overtime eligible). The tests governing whether a service provider is an independent contractor or an employee are typically highly fact sensitive and can vary by governing law. Laws and regulations that govern the status and misclassification of independent contractors are also subject to divergent interpretations by various authorities, which can create uncertainty and unpredictability. Regulatory authorities and private parties have recently asserted within several industries that some independent contractors should be classified as employees and that some exempt employees should be classified as nonexempt based upon the applicable facts and circumstances and their interpretations of existing rules and regulations. If we are found to have misclassified employees as independent contractors or non-exempt employees as exempt, we could face penalties and have additional exposure under tax (including federal and state tax), workers’ compensation, unemployment benefits, labor, employment and tort laws, including for prior periods, as well as potential liability for employee overtime and benefits and tax withholdings. Legislative, judicial or regulatory (including tax) authorities could also introduce proposals or assert interpretations of existing rules and regulations that would change the classification of a number of independent contractors doing business with us from independent contractor to employee and a number of exempt employees to non-exempt. A reclassification in either case could result in an increase in employment-related costs such as wages, benefits and taxes. The costs associated with employee misclassification, including any related regulatory action or litigation, could therefore have an adverse effect on our results of operations and our financial position.
Under applicable employment laws, we may not be able to enforce covenants not to compete.
We generally include non-competition provisions as part of our agreements with our officers, employees and consultants. These agreements generally prohibit our officers, employees or consultants, if they cease working for us, from competing directly with us or working for our competitors for a limited period. We may be unable to enforce these provisions under the laws of the jurisdictions in which our officers, employees or consultants work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former officers, employees or consultants developed while working for us.
We expect to expand our development, regulatory and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, manufacturing, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Our international operations subject us to various risks, and our failure to manage these risks could adversely affect our results of operations and we may be exposed to significant foreign exchange risk.
We face significant operational risks as a result of doing business internationally, such as:
| • | fluctuations in foreign currency exchange rates; |
96
Table of Contents
| • | differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls; |
| • | potentially adverse and/or unexpected tax consequences, including penalties due to the challenge by tax authorities on the basis of transfer pricing and liabilities imposed from inconsistent enforcement, as well as compliance with potentially conflicting and changing tax laws of taxing jurisdictions, the complexity and adverse consequences of such tax laws, and potentially adverse tax consequences due to changes in such tax laws; |
| • | potential changes to the accounting standards, which may influence our financial situation and results; |
| • | becoming subject to the different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; |
| • | reduced protection of, or significant difficulties in enforcing, intellectual property rights in certain countries; |
| • | difficulties in attracting and retaining qualified personnel; |
| • | restrictions imposed by local labor practices and laws on our business and operations, including unilateral cancellation or modification of contracts; |
| • | rapid changes in global government, economic and political policies and conditions, political or civil unrest or instability, terrorism or epidemics and other similar outbreaks or events, and potential failure in confidence of our suppliers or customers due to such changes or events; and |
| • | tariffs, trade protection measures, import or export licensing requirements, trade embargoes and other trade barriers. |
Additionally, we incur portions of our expenses, and may in the future derive revenues, in currencies other than the euro, in particular, the U.S. dollar. As a result, we are exposed to foreign currency exchange risk as our results of operations and cash flows are subject to fluctuations in foreign currency exchange rates. We currently do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the euro. Therefore, for example, an increase in the value of the euro against the U.S. dollar could be expected to have a negative impact on our revenue and earnings growth as U.S. dollar revenue and earnings, if any, would be translated into euros at a reduced value. We cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows.
Negative economic conditions, including as a result of commodity price inflation or supply chain constraints, the COVID-19 pandemic and the war in Ukraine, may adversely impact our results of operations.
An unforeseen production shortage resulting from any events, including interruptions to business operations and supply chain disruption as a result of worldwide economic and political disruptions including the impacts of military conflict between Russia and Ukraine, and widespread health crises, such as the COVID-19 pandemic, affecting raw material and or intermediate supply or manufacturing capabilities abroad and domestically could adversely impact our business. For example, our supply chain may be disrupted, limiting our ability to manufacture our product candidates for our clinical trials and research and development operations, or our cost base may be increased. Furthermore, economic growth is expected to slow, including due to supply chain disruption, the recent surge in inflation and related actions by central banks and geopolitical conditions, with a significant risk of recession in many parts of the worlds in the near term. This may also prolong tight credit markets and potentially cause such conditions to become more severe. These issues, along with the re-pricing of credit risk and the difficulties currently experienced by financial institutions, may make it difficult to obtain financing.
97
Table of Contents
Our expectations about our business, future performance and other matters are subject to significant risks, assumptions, estimates and uncertainties. As a result, our expectations regarding cash and cash burn, market size and market share, clinical trial completions, regulatory submissions and potential regulatory approvals, and our expectations regarding efficacy levels and benefits of our product candidates, may differ materially from actual results.
The estimates and assumptions included in this proxy statement/prospectus include, among others: expectations regarding our cash runway; estimates of the total addressable market for cardio metabolic disease patients with significant unmet need; assumptions regarding our ability to obtain reimbursement for our product candidate, if approved; assumptions regarding performance under existing partner agreements, including the Menarini License; and assumptions regarding our ability to obtain regulatory approval. These estimates and assumptions are subject to various factors beyond our control, including, for example, changes in the supply of drug products required for our clinical trials, increased costs for such drugs, changes in the regulatory or competitive environment, delays in our clinical trials or in obtaining regulatory approvals, lower than expected rates of reimbursement on our product candidate, if approved, the impact of global health crises (including the COVID-19 pandemic), the imposition or heightening of sanctions or other economic or military measures in relation to the current Russia-Ukraine conflict, and changes in our executive team. Accordingly, our future financial condition and results of operations may differ materially from our estimates.
We may undertake strategic acquisitions in the future and any difficulties from integrating such acquisitions could adversely affect our share price, operating results and results of operations.
We may acquire companies, businesses and products that complement or augment our existing business. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources, result in loss of key personnel and could prove to be more difficult or expensive than we predict. The diversion of our management’s attention and any delay or difficulties encountered in connection with any future acquisitions we may consummate could result in the disruption of our on-going business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. Moreover, we may need to raise additional funds through public or private debt or equity financing, or issue additional shares, to acquire any businesses or products, which may result in dilution for shareholders or the incurrence of indebtedness.
As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from acquisitions we may consummate in the future or have consummated in the past, whether as a result of unidentified risks or liabilities, integration difficulties, regulatory setbacks, litigation with current or former employees and other events, our business, results of operations and financial condition could be adversely affected. If we acquire product candidates, we will also need to make certain assumptions about, among other things, development costs, the likelihood of receiving regulatory approval and the market for such product candidates. Our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions.
In addition, we will likely experience significant charges to earnings in connection with our efforts, if any, to consummate acquisitions. For transactions that are ultimately not consummated, these charges may include fees and expenses for investment bankers, attorneys, accountants and other advisors in connection with our efforts. Even if our efforts are successful, we may incur, as part of a transaction, substantial charges for closure costs associated with elimination of duplicate operations and facilities and acquired in-process research and development charges. In either case, the incurrence of these charges could adversely affect our results of operations for particular periods.
98
Table of Contents
Cyberattacks or other failures in the telecommunications or information technology systems used by us or our third-party vendors, contractors or consultants, could result in information theft, compromise, or other unauthorized access, data corruption and significant disruption of our business operations, and could harm our reputation and subject us to liability, lawsuits and actions from governmental authorities.
Despite the implementation of security measures, our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage from cybersecurity threats, including computer viruses, harmful code and unauthorized access, cyber-attacks (including ranswomware), hacking, theft, phishing, employee error, denial-of-service attacks, social engineering schemes, sophisticated nation-state and nation-state-supported actors unauthorized accesses, natural disasters, fire, terrorism, war and telecommunication and electrical failures. If a disruption event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs, and / or otherwise jeopardize the performance of our software and information technology systems, and could expose us to financial and reputational harm. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of obicetrapib could be delayed.
Successful and attempted attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. As a result of the ongoing COVID-19 pandemic, we may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. Even if identified, we may be unable to adequately investigate or remediate incidents or breaches due to attackers increasingly using tools and techniques that are designed to circumvent controls, to avoid detection, and to remove or obfuscate forensic evidence.
We and certain of our service providers are from time to time subject to actual and attempted cyberattacks and security incidents. While we do not believe that we have experienced any such material system failure or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our marketing approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties for the manufacture of our drug candidate and to conduct clinical trials, and similar events relating to their information technology systems could also have a material adverse effect on our business. To the extent that any disruption or security incident were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development and commercialization of our future drug candidates could be delayed. Similarly, if an actual or attempted security incident were to occur, in additional to reputational damage, we could face investigations and fines from regulators, as well as litigation.
Risks Related to NewAmsterdam Pharma’s Intellectual Property
We may not be successful in obtaining all of the necessary intellectual property rights to allow us to develop and commercialize our product candidate, obicetrapib. If our efforts to obtain, protect or enforce our patents and other intellectual property rights related to our product candidates and technologies are not adequate, including due to the risk that we are unaware of prior art that may affect the validity of our patents, we may not be able to compete effectively in our market and we otherwise may be harmed.
Our future commercial success depends, in part, on our ability to obtain and maintain patent and other proprietary protection for commercially important inventions, to obtain and maintain know-how related to our business, including our product candidates, to defend and enforce our intellectual property rights, in particular
99
Table of Contents
our patent rights, to preserve the confidentiality of our trade secrets, and to operate without infringing, misappropriating, or violating the valid and enforceable patents and other intellectual property rights of third parties. Our ability to preclude or restrict third parties from making, using, selling, offering to sell, or importing competing molecules to our products may depend on the extent to which we have rights under valid and enforceable patents and trade secrets that cover these activities.
We seek to protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we enter into confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, collaborators, CROs, contract manufacturers, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. Further, we may not be aware of all third-party intellectual property rights potentially relating to our product candidates. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
While we have sought and continue to actively seek patent protection for obicetrapib, our patent coverage is limited, and we can provide no assurance that any of our current or future patent applications will result in issued patents or that any issued patents will provide us with any competitive advantage.
The patent applications that we own or license may fail to result in issued patents in the United States or granted patents in foreign jurisdictions. Our ability to obtain and maintain valid and enforceable patents depends on various factors, including determination that our patent claims are patentable over prior art. We may be subject to a third-party preissuance submission of prior art to the United States Patent and Trademark Office (the “USPTO”) or foreign patent offices, and such prior art may prevent issuance of claims that would provide us with a competitive advantage. We cannot be certain that we and respective patent offices have identified all relevant prior art at the time of issuance, and later identification of undiscovered prior art may provide basis for later invalidating our issued patent claims. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to either (i) file any patent application related to the treatment of cardio metabolic disease or Alzheimer’s disease using obicetrapib or (ii) conceive and reduce to practice any of the compositions or methods claimed in our patents or patent applications, including patents or patent applications related to obicetrapib and any of our future product candidates.
Patent applications and patents granted from them are complex, lengthy and highly technical documents that are often prepared under time constraints and may not be free from errors. The existence of errors in a patent may have an adverse effect on the patent, its scope and its enforceability. Even if our pending and future patent applications issue as patents in relevant jurisdictions, they may not issue in a form that will provide us with any meaningful protection for our technology or product candidates, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Even if our pending and future patent applications issue as patents in relevant jurisdictions, changes in law or in interpretation of existing law may provide a basis for competitors to challenge the validity and/or enforceable scope of our patents.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has, in recent years, been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of patent rights are highly uncertain. Our pending and future owned patent applications may not result in patents being issued which protect our technology or product candidates, effectively prevent others from commercializing competitive technologies and product or otherwise provide any competitive advantage. In addition, the scope of claims of an issued patent can be reinterpreted after issuance, and changes in either the patent laws or interpretation of the patent laws in the
100
Table of Contents
United States and other jurisdictions may diminish the value of our patent rights or narrow the scope of our patent protection.
Additionally, limitations on the scope of our intellectual property rights may limit our ability to prevent third parties from designing around such rights and competing against us. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or product candidates in a non-infringing manner. Other parties may compete with us, for example, by independently developing or obtaining competing solid forms of obicetrapib, including crystalline forms and alternative salts of obicetrapib, or by independently developing or obtaining competing synthetic processes for synthesis of obicetrapib or synthetic intermediates that allow competitors to design around our patent claims but which result in the same active ingredient.
In addition, our competitors may seek to invalidate our patents. We may become involved in proceedings brought by competitors in the USPTO or applicable foreign offices challenging our patent rights, such as inter partes review, post grant review, derivation proceedings, interference proceedings, opposition proceedings, revocation proceedings or ex parte reexamination. Patent offices may take a different view on patentability during post-grant challenges than during initial examination, and courts in litigation may take a different view about validity than did the respective patent office. An adverse determination in any such submission, proceeding or litigation could result in loss of exclusivity, patent claims being narrowed, invalidated or held unenforceable, in whole or in part, or could result in limits of the scope or duration of the patent protection of our technologies or product candidates, all of which could limit our ability to stop others from using or commercializing similar or identical product candidates or technology to compete directly with us, without payment to us.
Furthermore, even if they are not challenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. To meet such challenges, which are part of the risks and uncertainties of developing and marketing product candidates, we may need to evaluate third-party intellectual property rights and, if appropriate, to seek licenses for such third-party intellectual property or to challenge such third-party intellectual property, which may be costly and may or may not be successful, which could also have an adverse effect on the commercial potential for obicetrapib and any of our other product candidates.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent prosecution process. When related patents are pursued concurrently in multiple jurisdictions, international treaties may impose additional procedural, documentary, fee payment and other provisions. Additionally, when inventions are made by joint inventors of different nationalities, or where inventive acts were performed in multiple countries, concurrent and potentially conflicting requirements imposed by the laws of multiple jurisdictions may be applicable. We may have failed to adhere to all such provisions during examination of our patent applications or following issuance.
Periodic maintenance or annuity fees and various other governmental fees on any issued patent and/or pending patent applications are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of a patent or patent application. Our outside counsel have systems in place to remind us to pay these fees, and we rely on our outside counsel and their third-party vendors to pay these fees. While an inadvertent lapse may sometimes be cured by payment of a late fee or by other means in accordance with the applicable rules, there are many situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications directed to our product candidates, our competitors might be able to enter the market earlier than should otherwise have been the case, which could harm our business, financial condition, results of operations, and prospects.
101
Table of Contents
Uncertainty and instability resulting from the conflict between Russia and Ukraine could negatively impact our ability to maintain our patents in Russia.
Sanctions imposed on Russia by the United States and the European Union have made it difficult to pay required annual fees, or annuities, to maintain pending patent applications and granted patents in Russia, increasing the risk that our patents may not grant in Russia or, having granted, will lapse through nonpayment of annuities. In addition, the Russian government issued a decree in March 2022 that owners of Russian patents from countries that Russia considers to be unfriendly are no longer entitled to any compensation for compulsory licensing of their patents, increasing the risk that our competitors will be granted a compulsory license under our Russian patents, allowing them to infringe without making any payments to us.
We may receive only limited protection, or no protection, from our issued patents and patent applications and such patents could be narrowed, found invalid or unenforceable if challenged in court or before administrative bodies.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after its earliest priority US utility application was filed. Various extensions may be available; however the life of a patent, and the protection it affords, is limited. Without patent protection for our product candidates, we may be open to competition from generic versions of our product candidates. If we encounter delays in our clinical trials or regulatory approval of obicetrapib, the period of time during which we could market obicetrapib under patent protection could be reduced.
The patent application process, also known as patent prosecution, is expensive and time consuming, and we or any future licensors and licensees may not be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or any future licensors or licensees will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, these and any of our patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, etc., although we are unaware of any such defects that we believe are of material import. If we or any future licensors or licensees fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid and unenforceable. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
The strength of patents in the pharmaceutical field involves complex legal and scientific questions and can be uncertain. This uncertainty includes changes to the patent laws through either legislative action to change statutory patent law or court action that may reinterpret existing law in ways affecting the scope or validity of issued patents. The USPTO or other foreign patent offices may change their interpretation of existing statutes or regulations with potential retroactive effects. The patent applications that we own or in-license may fail to result in issued patents in the United States or foreign countries with claims that cover our product candidates. Even if patents do successfully issue from the patent applications that we own or in-license, third parties may challenge the validity, enforceability or scope of such patents, which may result in such patents being narrowed, invalidated or held unenforceable. For example, patents granted by the European Patent Office may be challenged, also known as opposed, by any person within nine months from the publication of their grant. In addition, post grant review in the USPTO begins with a third party filing a petition on or prior to the date that is 9 months after the grant of the patent or issuance of a reissue patent. Third parties can also challenge a patent in the USPTO by way of inter partes review, ex parte reexamination, derivation, or interference proceedings. Any successful challenge to our patents could deprive us of exclusive rights necessary for the successful commercialization of our product candidates. Furthermore, even if they are unchallenged, our patents may not adequately protect our product candidates, provide exclusivity for our product candidates, or prevent others from designing around our claims. If
102
Table of Contents
the breadth or strength of protection provided by the patents we hold or pursue with respect to our product candidates is challenged, it could dissuade companies from collaborating with us to develop, or threaten our ability to commercialize our product candidates.
If we do not obtain patent term extension for our product candidates, if needed, our business may be harmed.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments to the FDCA, a company may file an abbreviated new drug application (“ANDA”) seeking approval of a generic version of an approved innovator product. Depending upon the timing, duration and specifics of any FDA marketing approval of our product candidates and our technology, one or more of our U.S. patents that we may own in the future may be eligible for limited patent term extension under Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval. Only one patent may be extended and only those claims covering the approved product, a method for using it or a method for manufacturing it may be extended. The application for the extension must be submitted prior to the expiration of the patent for which extension is sought. A patent that covers multiple products for which approval is sought can only be extended in connection with one of the approvals. However, we may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may not be able to protect our intellectual property rights throughout the world, or we may choose not to pursue patent rights in jurisdictions that later become important to our business, thus harming our ability to compete in those jurisdictions.
Filing, prosecuting, maintaining, and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive. In countries in which we elect to pursue patent rights, the requirements for patentability may differ, particularly in developing countries. For example, China often applies a heightened requirement for patentability, with heightened requirements for experimental data in the patent application. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as laws in the United States. For example, some foreign countries do not permit claims to therapeutic methods.
Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection in order to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but enforcement against infringing activities is inadequate. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert
103
Table of Contents
claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful.
In addition, some countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, we may have limited remedies if our patents are infringed or if we are compelled to grant a license to our patents to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we own or license. Finally, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in foreign intellectual property laws.
Changes in U.S. or foreign patent law, including changes in patent office interpretation of applicable rules and statutes, changes effected by judicial holdings, and changes effected by legislation, including changes that may have retroactive effect, could diminish the value of patents in general and our patents in particular, thereby impairing our ability to protect our products.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly on obtaining and enforcing patents. Obtaining and enforcing patents in the pharmaceutical industry involves both technological and legal complexity, and therefore, is costly, time-consuming and inherently uncertain. In addition, the Leahy-Smith America Invents Act (the “AIA”) which was passed on September 16, 2011, resulted in significant changes to the U.S. patent system. Further, U.S. Supreme Court rulings in recent years have either narrowed the scope of patent protection available in certain circumstances or weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained.
The significant changes to U.S. patent law under the AIA include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. For our U.S. patent applications that contain or contained at any time a claim not entitled to priority before March 16, 2013, there is a greater level of uncertainty in the patent law. The USPTO has developed and continues to develop regulations and procedures to govern administration of the AIA, and many of the substantive changes to patent law associated with the AIA. The AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could harm our business and financial condition. It is not clear what other, if any, impact the AIA will have on the operation of our business.
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application, but circumstances could prevent us from promptly filing patent applications on our inventions. Furthermore, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our technology and the prior art allow our technology to be patentable over the prior art. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to either (i) file any patent application related to our product candidates or (ii) invent any of the inventions claimed in our patents or patent applications.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and provide opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued from applications filed before March 16, 2013. Because of a lower evidentiary standard necessary to invalidate a patent claim in USPTO proceedings compared to the evidentiary standard in United States federal court, a third party could potentially provide evidence in a USPTO
104
Table of Contents
proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Depending on decisions by the U.S. Congress, the federal courts, the USPTO, and foreign patent offices, the laws and regulations governing patents could change in unpredictable ways, including with potential retroactive effect, that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive and time consuming, with no certainty of success, and could delay or prevent the development and commercialization of our products and product candidates, or put our patents and other proprietary rights at risk.
Third parties may infringe or misappropriate our intellectual property, including our existing patents and patents that may issue to us in the future. As a result, we may be required to file infringement claims to stop third-party infringement or unauthorized use. Further, we may not be able to prevent misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Generic drug manufacturers may develop, seek approval for, and launch generic versions of our products. If we file an infringement action against such a generic drug manufacturer, that company may challenge the scope, validity or enforceability of our patents, requiring us to engage in complex, lengthy and costly litigation or other proceedings.
For example, if we initiated legal proceedings against a third party to enforce a patent covering our product candidates, the defendant could counterclaim that the patent covering our product candidates is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent.
In addition, within and outside of the United States, there has been a substantial amount of litigation and administrative proceedings, including inter partes review, post grant review, interference or derivation proceedings, and ex parte reexamination proceedings before the USPTO or other comparable proceedings in various foreign jurisdictions, regarding patent and other intellectual property rights in the pharmaceutical industry. These proceedings bring uncertainty to the possibility of challenges to our patents in the future, including challenges by competitors who perceive our patents as blocking entry into the market for their products, and the outcome of such challenges.
Such litigation and administrative proceedings could result in revocation of our patents or amendment of our patents such that they do not cover our product candidates. They may also put our pending patent applications at risk of not issuing, or issuing with limited and potentially inadequate scope to cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. Additionally, it is also possible that prior art of which we are aware, such as may arise during preclinical studies and clinical trials, but which we do not believe affects the validity or enforceability of a claim, may, nonetheless, ultimately be found by a court of law or an administration panel to affect the validity or enforceability of a claim. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a negative impact on our business.
Enforcing our intellectual property rights through litigation would be very expensive, particularly for a company of our size, time-consuming, and inherently uncertain. Some of our competitors may be able to sustain the costs of litigation more effectively than we can because of greater financial resources. Patent litigation and other proceedings may also divert technical and management personnel from their normal responsibilities.
105
Table of Contents
Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could harm our business, financial condition or results of operations.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, during the course of litigation or administrative proceedings, there could be public announcements of the results of hearings, motions or other interim proceedings or developments or public access to related documents. If investors perceive these results to be negative, the market price for Holdco Shares could be significantly harmed.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to the protection afforded by patents, we may also rely on trade secret protection or confidentiality agreements to protect proprietary know-how, technology and other proprietary information that may not be patentable or that we elect not to patent, processes for which patents may be difficult to obtain or enforce, and any other elements of our product candidates, and our product development processes (such as manufacturing and formulation technologies) that involve proprietary know-how, information or technology that is not covered by patents. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, eroding our competitive position in the market.
Trade secrets, confidential information, and know-how can be difficult to protect. We seek to protect these trade secrets and other proprietary technology, in part, by requiring all of our employees, consultants, advisors, and any other third parties that have access to our proprietary know-how, information or technology to execute confidentiality agreements upon the commencement of their relationships with us. We cannot be certain that we have or will obtain these agreements in all circumstances and we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary information. If the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secrets.
Despite our efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets. Adequate remedies may not exist in the event of unauthorized use or disclosure of our trade secrets. In addition, in some situations, these confidentiality agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants, or advisors have previous employment or consulting relationships. To the extent that our employees, consultants or contractors use any intellectual property owned by third parties in their work for us, disputes may arise as to the rights in any related or resulting know-how and inventions. Any misappropriation or unauthorized disclosure of our trade secrets could have an adverse effect on our business, impact our ability to establish or maintain a competitive advantage in our market, or otherwise harm our business, operating results and financial condition.
Furthermore, trade secret protection and confidentiality agreements do not prevent competitors from independently developing substantially equivalent information and techniques and we cannot guarantee that our competitors will not independently develop substantially equivalent information and techniques. The FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all.
There is an increasing trend in the EU towards greater transparency and, while the manufacturing or quality information contained in an EU marketing authorization application (“MAA”) is currently generally protected as confidential information, the EMA and national regulatory authorities may disclose much of the nonclinical and clinical information in MAAs, including the full clinical study reports, in response to freedom of information requests after the marketing authorization has been granted. Similarly, as of January 31, 2022 under the EU
106
Table of Contents
Clinical Trials Regulation (EU) No 536/2014, the EU clinical trials information system allows the public to access MAA data submitted to the EMA or national regulatory authorities (excluding any commercially confidential information). There may be a risk that information that we consider to be trade secrets or other proprietary information becomes publicly available, including to our competitors, under such transparency requirements in the EU.
Third-party claims alleging intellectual property infringement may adversely affect our business, and we may be subject to lawsuits claiming that we infringe, misappropriate or otherwise violate the intellectual property rights of third parties, which could be expensive and time consuming, delay or prevent the development and commercialization of our products and product candidates, or subject future sales to royalty payments, which could damage our business.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties, for example, the intellectual property rights of competitors. Our research, development and commercialization activities may be subject to claims that we infringe or otherwise violate patents owned or controlled by third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our activities related to our product candidates may give rise to claims of infringement of the patent rights of others. We cannot assure you that our product candidates will not infringe existing or future patents. We may not be aware of patents that have already issued that a third party might assert are infringed by our product candidates. It is also possible that patents of which we are aware, but which we do not believe are relevant to our product candidates, could nevertheless be found to be infringed by our product candidates. Nevertheless, we are not aware of any issued patents that we believe would prevent us or our licensee(s) from marketing our product candidates, if approved. There may also be patent applications that have been filed but not published that, when issued as patents, could be asserted against us. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that we may be subject to claims of infringement of the intellectual property rights of third parties.
Third parties making claims against us for infringement or misappropriation of their intellectual property rights may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit. Defense of these claims, regardless of their merit, would cause us to incur substantial expenses and, and would be a substantial diversion of management time and employee resources from our business. In the event of a successful claim of infringement against us by a third party, we may have to (i) pay substantial damages, including treble damages and attorneys’ fees if we are found to have willfully infringed the third party’s patents; (ii) obtain one or more licenses from the third party; (iii) pay royalties to the third party; and/or (iv) redesign any infringing products. Redesigning any infringing products may be impossible or require substantial time and monetary expenditure. Further, we cannot predict whether any required license would be available at all or whether it would be available on commercially reasonable terms. In the event that we could not obtain a license, we may be unable to further develop and commercialize our product candidates, which could harm our business significantly. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. Furthermore, even in the absence of litigation, we may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
107
Table of Contents
Defending ourselves in litigation is very expensive, particularly for a company of our size, and time-consuming. Some of our competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than we can because of greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could harm our business, financial condition or results of operations.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information or alleged trade secrets of third parties or competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise improperly used or disclosed confidential information or trade secrets of these third parties or our employees’ former employers. Further, we may be subject to ownership disputes in the future arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. We may also be subject to claims that former employees, consultants, independent contractors, collaborators or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging our right to and use of confidential and proprietary information. If we fail in defending any such claims, in addition to paying monetary damages, we may lose our rights therein. Such an outcome could have a negative impact on our business. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees. Any litigation or the threat thereof may adversely affect our ability to hire employees. A loss of key personnel or their work product could hamper or prevent our ability to commercialize product candidates, which could have an adverse effect on our business, results of operations and financial condition. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of litigation proceedings could adversely affect our ability to compete in the marketplace.
We may not be able to build name recognition in our markets of interest if our trademarks and trade names are not adequately protected and our business may be adversely affected.
Our future trademark applications in the United States and other foreign jurisdictions may not be allowed or may be subsequently opposed. Once filed and registered, our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. As a means to enforce our trademark rights and prevent infringement, we may be required to file trademark claims against third parties or initiate trademark opposition proceedings. This can be expensive and time-consuming, particularly for a company of our size. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations or prospects.
108
Table of Contents
Disputes over intellectual property subject to the Menarini License may materially impact our ability to commercialize obicetrapib.
The licensing of intellectual property in the Menarini License is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to the Menarini License, including:
| • | the scope of rights granted under the Menarini License and other interpretation-related issues; |
| • | the extent to which Menarini’s technology and processes infringe our intellectual property that is not subject to the Menarini License; |
| • | claims that our technology infringes third-party intellectual property; |
| • | the sublicensing of patent and other rights; |
| • | our diligence obligations and what activities satisfy those diligence obligations; and |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property. |
If disputes over intellectual property we have licensed to Menarini prevent or impair our ability to maintain the Menarini License on acceptable terms, we may be unable to successfully develop and commercialize obicetrapib.
Risks Related to Government Regulation of NewAmsterdam Pharma
Current and future legislation affecting the healthcare industry, including healthcare reform, may impact our business generally and may increase limitations on reimbursement, rebates and other payments, which could adversely affect third-party coverage of our products, our operations and/or how much or under what circumstances healthcare providers will prescribe or administer obicetrapib, if approved.
The United States and some foreign jurisdictions are considering or have enacted a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell obicetrapib profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
For example, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “ACA”), a law intended, among other things, to broaden access to health insurance, improve quality of care, and reduce or constrain the growth of healthcare spending.
Provisions of the ACA relevant to the pharmaceutical industry include the following:
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, not including orphan drug sales; |
| • | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| • | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
109
Table of Contents
| • | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| • | new requirements to report annually certain financial arrangements with physicians and teaching hospitals; as defined in the ACA and its implementing regulations, including reporting any payment or “transfer of value” provided to physicians and teaching hospitals and any ownership and investment interests held by physicians and their immediate family members during the preceding calendar year; |
| • | expansion of healthcare fraud and abuse laws, including the federal civil False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance; and |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research. |
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an Executive Order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The Executive Order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administrations or other efforts, if any, to challenge repeal or replace the ACA, will impact our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, in August 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of an amount greater than $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to healthcare providers of up to 2.0% per fiscal year. These reductions went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. Following the suspension, a 1% payment reduction occurred beginning April 1, 2022 through June 30, 2022, and the 2% payment reduction resumed on July 1, 2022. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. On May 30, 2018, the Right to Try Act was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA permission under the FDA expanded access program. There is no obligation for a pharmaceutical manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act.
Additionally, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drugs. Further, the U.S. House of Representatives recently formed an
110
Table of Contents
Affordable Drug Pricing Task Force to advance legislation intended to control pharmaceutical drug costs and investigate pharmaceutical drug pricing, and the U.S. Senate has requested information from certain pharmaceutical companies in connection with an investigation into pharmaceutical drug pricing practices. If healthcare policies or reforms intended to curb healthcare costs are adopted, or if we experience negative publicity with respect to the pricing of obicetrapib, if approved, or any future product or the pricing of pharmaceutical drugs generally, the prices that we charge for any approved products may be limited, our commercial opportunity may be limited and/or our revenues from sales of our products may be negatively impacted.
If we obtain regulatory approval and commercialization of obicetrapib or any of our future product candidates, these laws may result in additional reductions in healthcare funding, which could have an adverse effect on our customers and accordingly, our financial operations. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of obicetrapib or our future product candidates may be.
Although we cannot predict the full effect on our business of the implementation of existing legislation or the enactment of additional legislation pursuant to healthcare and other legislative reform, we believe that legislation or regulations that would reduce reimbursement for, or restrict coverage of, obicetrapib, if approved, or any of our future products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could adversely affect our business by reducing our ability to generate revenues, raise capital, obtain licenses and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact product sales.
Although we cannot predict the full effect on our business of the implementation of existing legislation or the enactment of additional legislation pursuant to healthcare and other legislative reform, we believe that legislation or regulations that would reduce reimbursement for, or restrict coverage of, obicetrapib, if approved, or any of our future products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could adversely affect our business by reducing our ability to generate revenues, raise capital, obtain licenses and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact product sales.
Our relationships with healthcare professionals, independent contractors, clinical investigators, CROs, consultants and vendors in connection with our current and future business activities may be subject to federal, state and foreign healthcare fraud and abuse laws, false claims laws, transparency laws, government price reporting, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties.
We may currently be or may become subject to various federal, state and foreign healthcare laws, including those intended to prevent healthcare fraud and abuse.
The federal Anti-Kickback Statute prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid Remuneration has been broadly defined to include anything of value, including, but not limited to, cash, improper discounts, and free or reduced price items and services.
Much like the federal Anti-Kickback Statute prohibition in the United States, the provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medicinal products is also prohibited in the EU. The provision of benefits or advantages to
111
Table of Contents
physicians is governed by the national anti-bribery laws of EU Member States. Infringement of these laws could result in substantial fines and imprisonment. Payments made to physicians in certain EU Member States must be publicly disclosed. Moreover, agreements with physicians often must be the subject of prior notification and approval by the physician’s employer, his or her competent professional organization and/or the regulatory authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Federal false claims laws, including the federal False Claims Act (the “FCA”) and civil monetary penalties law impose penalties against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval that are false or fraudulent or making a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government. The FCA has been used to, among other things, prosecute persons and entities submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. The FCA includes a whistleblower provision that allows individuals to bring actions on behalf of the federal government and share a portion of the recovery of successful claims.
Many states have similar fraud and abuse statutes and regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. State and federal authorities have aggressively targeted medical technology companies for, among other things, alleged violations of these anti-fraud statutes, based on improper research or consulting contracts with doctors, certain marketing arrangements that rely on volume-based pricing, off-label marketing schemes, and other improper promotional practices.
The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) among other things, imposes criminal liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services.
Our operations will also be subject to the federal transparency requirements under the ACA, which require certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to annually report to the Centers for Medicare & Medicaid Services (“CMS”) an agency within the U.S. Department of Health and Human Services (“HHS”) information related to payments and other transfers of value provided to physicians, teaching hospitals, certain ownership and investment interests held by physicians and their immediate family members and certain non-physician providers (physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists and anesthesiologist assistants, and certified-nurse midwives). We may also be subject to state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and/or state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidelines promulgated by the federal government.
We may also be subject to federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products, and similar laws in other jurisdictions.
We are subject to stringent privacy laws, information security policies and contractual obligations governing the use, processing, and cross-border transfer of personal information and our data privacy and security practices.
We receive, generate and store sensitive information, including employee and patient data, and are subject to a variety of federal, state, local and foreign laws and regulations that apply to the collection, use, retention, protection, disclosure, transfer and other processing of data in the jurisdictions in which we operate, including comprehensive regulatory systems in the United States and the EU. Legal requirements relating to data
112
Table of Contents
processing continue to evolve and may result in ever-increasing public scrutiny and escalating levels of enforcement, sanctions and increased costs of compliance. An actual or perceived failure to comply with laws and regulations governing personal information could result in government investigations and enforcement actions against us, fines, claims for damages by affected third parties, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
EU data protection laws including the General Data Protection Regulation 2016/679 (“GDPR”) impose strict requirements relating to the processing of personal data, including special protections for “special categories of personal data” which includes, without limitation, health and genetic information of data subjects residing in the EU. The GDPR also generally prohibits the transfer of personal information from the EU to the United States and most other foreign jurisdictions unless the parties to the transfer have implemented specific safeguards to protect the transferred personal information. There is uncertainty regarding how to ensure that transfers of personal information from the EU to the United States comply with the GDPR. As such, any transfers by us, or our vendors, of personal information from the EU may not comply with EU data protection laws; may increase our exposure to the GDPR’s heightened sanctions for violations of its cross-border data transfer restrictions; and may reduce demand for our services from companies subject to EU data protection laws. Loss of our ability to transfer personal information from the EU may also require us to increase our data processing capabilities in those relevant jurisdictions at significant expense.
Similar privacy and data security requirements are either in place or have been proposed in the United States. There are numerous data protection laws that may be applicable to our activities, and a range of enforcement agencies at both the state and federal levels that can review companies for privacy and data security concerns based on general consumer protection laws. The Federal Trade Commission and state Attorneys General are aggressive in reviewing privacy and data security protections for consumers. New laws also are being considered or have been implemented at both the state and federal levels.
Further, regulations promulgated pursuant to HIPAA imposes privacy, security and breach notification obligations on health plans, healthcare clearinghouses and certain healthcare providers, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities, and their covered subcontractors. HIPAA establishes privacy and security standards that limit the use and disclosure of protected health information (“PHI”) and requires the implementation of administrative, physical and technological safeguards to protect the privacy of PHI and ensure the confidentiality, integrity and availability of electronic PHI. Most healthcare providers, including research institutions from which we obtain patient health information, are subject to HIPAA. We do not believe that we are currently acting as a covered entity or business associate under HIPAA and thus are not directly subject to its requirements. However, any person may be prosecuted under HIPAA’s criminal provisions either directly or under aiding-and-abetting or conspiracy principles. Consequently, depending on the facts and circumstances, we could face substantial criminal penalties if we knowingly receive individually identifiable health information from a HIPAA-covered healthcare provider or research institution that has not satisfied HIPAA’s requirements for disclosure of individually identifiable health information.
Complying with the GDPR and other U.S. and foreign data protection laws and regulations may cause us to incur substantial operational costs or require us to change our business practices in a manner adverse to our business. Moreover, complying with these various laws could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Despite our efforts to bring our practices into compliance with these laws and regulations, we may not be successful in our efforts to achieve compliance either due to internal or external factors such as resource allocation limitations or a lack of vendor cooperation. Failure to comply with U.S. and international data protection laws and regulations could result in government enforcement actions (which could include civil or criminal penalties), other administrative actions or litigation. For example, the GDPR sets out substantial fines for breaches of the data protection rules, increased powers for regulators, enhanced rights for individuals, and new rules on judicial remedies and collective redress. Any inability to adequately address privacy concerns, even
113
Table of Contents
if unfounded, or comply with applicable privacy or data protection laws, regulations and policies, could result in additional cost and liability to us, damage our reputation, inhibit sales and adversely affect our business, results of operations and financial condition.
Our marketing efforts may be subject to a variety of regulations.
We may choose to conduct marketing activities, directly and indirectly, via text (SMS) messages, email, and/or through other online and offline marketing channels. Numerous foreign, federal, and state regulations may govern such marketing activities, including the Telemarketing Sales Rule, the Telephone Consumer Protection Act (“TCPA”), state and federal Do-Not-Call regulations and other state telemarketing laws, federal and state privacy laws, the CAN-SPAM Act, and the Federal Trade Commission Act and its accompanying regulations and guidelines, among others. These laws not only allow action to be brought by regulatory agencies, but some of these laws, like the TCPA, allow private individuals to bring litigation against companies for breach of these laws. If we conduct marketing activities regulated by these laws, then we may depend on third-party partners to comply with these laws. Any lawsuit brought by private individuals, or action by a regulatory agency, for an actual or alleged violation of applicable law or regulation by us or our third-party partners may have an adverse effect on our business, results of operations, and financial condition.
We could be adversely affected by violations of the FCPA and other worldwide anti-bribery laws, export and import controls, sanctions, embargoes, and anti-money laundering laws and regulations.
Various of our activities may be subject to anti-bribery, export control and import laws and regulations, including the U.S. Foreign Corrupt Practices Act (“FCPA”) the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the jurisdiction in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. These laws are complex and far-reaching in nature, and, as a result, we cannot assure you that our internal control policies and procedures will protect us from reckless or negligent acts committed by our intermediaries, or that we would not be required in the future to alter one or more of our practices to be in compliance with these laws or any changes in these laws or the interpretation thereof. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. Furthermore, because we engage, and expect to continue to engage, third parties in connection with our clinical trials and other development and commercialization activities, we can be held liable for the corrupt or other illegal activities of our personnel, agents or collaborators, even if we do not explicitly authorize or have prior knowledge of such activities. Other U.S. companies in the biopharmaceutical field have faced criminal penalties under the FCPA for allowing their agents to deviate from appropriate practices in doing business with individuals in the public or private sector.
Any violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction, involve significant costs and expenses, including legal fees, and could result in a material adverse effect on our business, prospects, financial condition, or results of operations or our reputation. We could also suffer severe penalties, including substantial criminal and civil penalties, imprisonment, disgorgement, reputational harm and other remedial measures.
It may be difficult for us to profitably sell obicetrapib or any future product candidate in the United States, if approved, if coverage and reimbursement for these products is limited by government authorities and/or third-party payor policies.
Market acceptance and sales of obicetrapib and our other product candidates, if approved, will depend on the coverage and reimbursement policies of government authorities and third-party payors, in addition to any
114
Table of Contents
healthcare reform measures that may affect reimbursement. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will cover and establish reimbursement and co-payment levels. Such authorities and other third-party payors are increasingly challenging the prices charged for healthcare products, examining the cost effectiveness of drugs in addition to their safety and efficacy, and limiting or attempting to limit both coverage and the level of reimbursement for prescription drugs. We cannot be sure that coverage will be available for obicetrapib or our other product candidates, if approved, or, if coverage is available, the level of reimbursement.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by CMS, an agency within the U.S. Department of HHS, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors often follow CMS. It is difficult to predict what CMS as well as other payors will decide with respect to reimbursement.
Reimbursement may impact the demand for, and/or the price of, any product for which we obtain marketing approval. Assuming we obtain coverage for a given product by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover all or a significant portion of the cost of our products. Therefore, coverage and adequate reimbursement is critical to new product acceptance. Coverage decisions may depend upon clinical and economic standards that disfavor new drug products when more established or lower cost therapeutic alternatives are already available or subsequently become available. There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA, the EMA or other comparable regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution.
Reimbursement by a third-party payor may depend upon a number of factors including the third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide supporting scientific, clinical and cost effectiveness data for the use of our products to the payor. Further, no uniform policy requirement for coverage and reimbursement for drug products exists among third-party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process may require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. We may not be able to provide data sufficient to gain acceptance with respect to coverage and/or sufficient reimbursement levels.
We cannot be sure that coverage or adequate reimbursement will be available for obicetrapib or any of our future product candidates, if approved. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our future products, which would in turn negatively affect revenues from any future sales. If reimbursement is not available, or is available only to limited levels that are not commercially attractive
115
Table of Contents
to us or our collaborators, we may not be able to commercialize obicetrapib or our other product candidates, or achieve profitability, even if approved.
Marketing and reimbursement regulations may materially affect our ability to market and receive coverage for our products in foreign jurisdictions.
We intend to seek approval to market our current and future product candidates in the United States, the EU and selected foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions for our product candidates, we will be subject to rules and regulations in those jurisdictions. In some foreign countries, particularly certain EU Member States, the pricing of drugs is subject to governmental control and other market regulations which could put pressure on the pricing and usage of our product candidates. In these countries, pricing negotiations with governmental authorities can take considerable time after obtaining marketing approval of a product candidate. In addition, market acceptance and sales of our product candidates will depend significantly on the availability of adequate coverage and reimbursement from third-party payors for our product candidates and may be affected by existing and future healthcare reform measures.
In the EU, the requirements governing drug pricing and reimbursement vary widely between Member States. Some Member States provide that products may be marketed only after a reimbursement price has been agreed. Some Member States may require the completion of additional studies that compare the cost effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. Moreover, at the national level, Member States may restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. Member States may approve a specific price for a product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other Member States allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many Member States have increased the amount of discounts required on pharmaceuticals and these efforts could continue as Member States attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the EU. The downward pressure on health care costs in general, particularly prescription products, has become significant. As a result, increasingly high barriers are being erected to the entry of new products in the marketplace. Political, economic and regulatory developments in the EU may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various Member States and parallel trade (arbitrage between low-priced and high-priced Member States) can further reduce prices. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results based rules of reimbursement may apply. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries. Historically, products launched in the EU do not follow price structures of the United States and generally prices tend to be significantly lower. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If pricing is set at unsatisfactory levels or if reimbursement of our products is unavailable or limited in scope or amount, our revenues from sales and the potential profitability of any of our product candidates in those countries would be negatively affected.
FLAC is subject to and Holdco will be subject to changing law and regulations regarding regulatory matters, corporate governance and public disclosure that have increased both FLAC’s costs and the risk of noncompliance and will increase both Holdco’s costs and the risk of non-compliance.
FLAC is and Holdco will be subject to rules and regulations by various governing bodies, including, for example, the SEC, which are charged with the protection of investors and the oversight of companies whose securities are publicly traded, and to new and evolving regulatory measures under applicable law. FLAC’s efforts
116
Table of Contents
to comply with new and changing laws and regulations have resulted in and Holdco’s efforts to comply likely will result in, increased general and administrative expenses.
Moreover, because these laws, regulations and standards are subject to varying interpretations, their application in practice may evolve over time as new guidance becomes available. This evolution may result in continuing uncertainty regarding compliance matters and additional costs necessitated by ongoing revisions to Holdco’s disclosure and governance practices. If FLAC fails to address and comply with these regulations and any subsequent changes, FLAC may be subject to penalty and FLAC and Holdco’s business may be harmed.
Legislative or regulatory healthcare reforms in the United States or abroad may make it more difficult and costly for us to obtain regulatory clearance or approval of obicetrapib or any of our future product candidates now or in the future and to produce, market and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress or by governments in foreign jurisdictions that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture, and marketing of regulated products or the reimbursement thereof. In addition, FDA, EMA or other comparable regulatory authority regulations and guidance are often revised or reinterpreted by the FDA, the EMA or other comparable regulatory authorities in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of obicetrapib or any of our other product candidates now or in the future. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| • | changes to manufacturing methods; |
| • | change in protocol design; |
| • | additional treatment arm (control); |
| • | recall, replacement, or discontinuance of one or more of our products; and |
| • | additional recordkeeping. |
Each of these would likely entail substantial time and cost and could harm our business and our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for obicetrapib or any future products would harm our business, financial condition and results of operations.
Risk Related to Holdco’s Financial Position
Holdco has no operating or financial history and its results of operations may differ significantly from the unaudited pro forma financial data included herein.
Holdco was recently incorporated and has no operating history and no revenues. New Amsterdam Pharma’s unaudited pro forma condensed combined statement of profit or loss for the year ended December 31, 2021, included elsewhere in this proxy statement/prospectus combines FLAC’s historical audited results of operations for the year ended December 31, 2021 with NewAmsterdam Pharma’s historical audited results of operations for the year ended December 31, 2021, and gives pro forma effect to the Business Combination as if it had been consummated as of January 1, 2021. New Amsterdam Pharma’s unaudited pro forma condensed combined statement of profit or loss for the six-months ended June 30, 2022, included elsewhere in this proxy statement/prospectus combines FLAC’s historical unaudited statements of operations for the six months ended June 30, 2022 with NewAmsterdam Pharma’s historical unaudited results of operations for the six months ended June 30, 2022, and gives pro forma effect to the Business Combination as if it had been consummated as at January 1, 2021. New Amsterdam Pharma’s unaudited pro forma condensed combined statement of financial position as at June 30, 2022, combines FLAC’s historical unaudited condensed balance sheets as at June 30, 2022 and NewAmsterdam Pharma’s consolidated statements of financial position at June 30, 2022 and gives pro forma effect to the Business Combination as if it had been consummated on such date.
117
Table of Contents
The unaudited pro forma condensed combined financial statements are presented for illustrative purposes only, are based on certain assumptions, address a hypothetical situation and reflect limited historical financial data. Therefore, the unaudited pro forma condensed combined financial statements are not necessarily indicative of the results of operations and financial position that would have been achieved had the Business Combination been consummated on the dates indicated above, or Holdco’s future consolidated results of operations or financial position. Accordingly, Holdco’s business, assets, cash flows, results of operations and financial condition may differ significantly from those indicated by the unaudited pro forma condensed combined financial information included in this proxy statement/prospectus.
Our ability to use our tax losses to offset future taxable income may be subject to certain limitations.
Holdco’s ability to utilize tax losses and tax loss carryforwards is conditioned upon it attaining profitability and generating taxable income. We have incurred significant tax losses since inception and it is anticipated that we will continue to incur significant losses. As of December 31, 2021, we reported unused tax losses of $173.5 million. Additionally, our ability to utilize tax losses and tax loss carryforwards to offset future taxable income may be subject to certain limitations. In this respect, pursuant to Article 20a of the Dutch Corporate Income Tax Act 1969, the tax loss carryforwards of NewAmsterdam Pharma and its Dutch subsidiary NewAmsterdam Pharma B.V. (formerly Dezima Pharma B.V.) can no longer be offset against future taxable profits if the ultimate ownership in the taxpayer has changed by an amount equal to or greater than 30%, unless certain counter evidence rules are met. We believe and have taken the position that the tax losses of NewAmsterdam Pharma B.V. available for carry forward have not been forfeited as a result of the change of ownership back in 2020, when NewAmsterdam Pharma acquired all shares in the capital of NewAmsterdam Pharma B.V., and that the tax losses of NewAmsterdam Pharma and NewAmsterdam Pharma B.V. will not be forfeited as a result of the Business Combination. On May 25, 2022, we filed a ruling request with the Dutch Tax Authorities to confirm that the change of ownership described above did not result in the loss of the tax losses of NewAmsterdam Pharma B.V. available for carry forward at that time. However, as of the date of this proxy statement/prospectus, the Dutch Tax Authorities have not yet decided on our request. We currently expect, but can in no way guarantee or enforce, that the Dutch Tax Authority will grant our request. Pursuant to the Dutch corporate income tax return filed for the fiscal year ended December 31, 2020, the tax loss carryforward of NewAmsterdam Pharma amounts to $373,842 and the tax loss carryforward of NewAmsterdam Pharma B.V. amounts to $151,292,396. As of January 1, 2021, NewAmsterdam Pharma and NewAmsterdam Pharma B.V. are included in a so-called fiscal unity for Dutch corporate income tax purposes. Any pre-fiscal unity losses incurred at the level of NewAmsterdam Pharma and NewAmsterdam Pharma B.V. can only be offset against future taxable profits of the relevant entity itself (and not against the total taxable profits of the fiscal unity). The amount of tax losses of the fiscal unity for the fiscal year ended December 31, 2021 is not yet clear, since the Dutch corporate income tax return for the fiscal year ended December 31, 2021 has yet to be filed. As a result of the foregoing, the exact amount of tax loss carryforwards in the Netherlands is not yet known. Furthermore, as at January 1, 2022, tax losses can be carried back one year and carried forward indefinitely in the Netherlands. However, both the carry back and carry forward tax loss relief will be limited to 50% of the taxable profit to the extent it exceeds EUR 1 million, calculated per financial year. As a result of transitional law, tax losses incurred in the financial years that started on or after January 1, 2013 (our oldest tax loss year at the date of this proxy statement/prospectus) and that are still available for carry forward as of January 1, 2022 also fall under the new scheme that entered into effect on January 1, 2022 and will therefore be indefinite.
As Holdco is a holding company with no operations, it relies on operating subsidiaries to provide it with funds necessary to meet its financial obligations.
Holdco is a holding company that does not conduct any business operations of its own. As a result, Holdco will be largely dependent upon cash dividends and distributions and other transfers, including for dividends or payments in respect of any indebtedness Holdco may incur, from its subsidiaries to meet its obligations. Any agreements governing the indebtedness of Holdco’s subsidiaries may impose restrictions on its subsidiaries’ ability to pay dividends or other distributions to Holdco. Each of Holdco’s subsidiaries is a distinct legal entity,
118
Table of Contents
and under certain circumstances legal and contractual restrictions may limit Holdco’s ability to obtain cash from such subsidiaries. The deterioration of the earnings from, or other available assets of, Holdco’s subsidiaries for any reason could also limit or impair their ability to pay dividends or other distributions to Holdco.
The PFIC status of FLAC and/or Holdco could result in adverse U.S. federal income tax consequences to U.S. Holders.
We believe Holdco and its non-U.S. subsidiaries, including NewAmsterdam Pharma, will be treated as passive foreign investment companies (each, a “PFIC”) for U.S. federal income tax purposes for the taxable year including the Business Combination and may be treated as PFICs for future taxable years. In addition, FLAC was treated as a PFIC for its taxable year ending in 2021 and is also likely treated as a PFIC in the current taxable year. A non-U.S. corporation is classified as a PFIC for any tax year if at least 75% of its gross income is “passive income” or at least 50% of the value of its assets, determined on the basis of a quarterly averages, is attributable to assets that produce or are held for the production of “passive income.”
Passive income generally includes dividends, interest, royalties, rents (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce passive income. Cash is a passive asset for PFIC purposes, even if held as working capital. For this purpose, a non-U.S. corporation is generally treated as owning a proportionate share of the assets and earning a proportionate share of the income of any other corporation in which it owns, directly or indirectly, at least 25% (by value) of the stock. Accordingly, Holdco will be treated as owning the cash and other cash-equivalent items of FLAC.
Based on the composition of the income and assets of Holdco and its subsidiaries, it is expected that NewAmsterdam Pharma, Holdco and other non-U.S. subsidiaries will be PFICs for the 2022 taxable year and may be PFICs for future taxable years. The determination of whether Holdco or any of its non-U.S. subsidiaries is a PFIC is made annually and thus subject to change, and it generally cannot be made until the end of the taxable year.
A U.S. Holder (as defined in this proxy statement/prospectus in the section entitled “Material Tax Considerations—Material U.S. Federal Income Tax Considerations”) generally will be subject to additional U.S. federal income taxes and interest charges on the gain from a sale of Holdco Shares or Holdco Public Warrants and on receipt of an “excess distribution” with respect to Holdco Shares or any of its non-U.S. subsidiaries. A U.S. Holder of stock of a PFIC generally may mitigate these adverse U.S. federal income tax consequences, however, by making a “qualified electing fund” election or a “mark-to-market” election. If Holdco determines that it and/or any of its subsidiaries is a PFIC for any taxable year, Holdco intends to provide a U.S. Holder such information as the IRS may require, including a PFIC Annual Information Statement, in order to enable the U.S. Holder to make and maintain a “qualified electing fund” election with respect to Holdco and/or such non-U.S. subsidiaries, but there can be no assurance that Holdco will be able to timely provide such required information. U.S. Holders generally will not be able to make a qualified electing fund election solely with respect to Holdco Warrants.
In addition, if FLAC or NewAmsterdam Pharma was a PFIC at any time during the holding period of a U.S. Holder of FLAC Securities or shares of NewAmsterdam Pharma, as applicable, pursuant to proposed Treasury Regulations promulgated under Section 1291(f) (the “Proposed PFIC Regulations”), such holder will recognize gain (but not loss) pursuant to the Business Combination unless:
| • | such U.S. Holder made a qualified electing fund election (“QEF election”) with respect to its shares of NewAmsterdam Pharma or FLAC, as applicable for the first taxable year in which such U.S. Holder held (or was deemed to hold) such shares in which NewAmsterdam Pharma or FLAC was a classified as a PFIC and has continued to properly satisfy the requirements of such QEF election in all subsequent years; |
| • | such U.S. Holder made a QEF election along with an applicable purging election and has continued to properly satisfy the requirements of such QEF election in all subsequent years; or |
| • | Holdco is a PFIC during the taxable year when the Business Combination is completed. |
119
Table of Contents
As discussed more fully below under “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities—Additional Requirements for Tax Deferral” and “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Exchange to U.S. Holders of NewAmsterdam Pharma Shares—Additional Requirements for Tax Deferral,” NewAmsterdam Pharma and FLAC were each treated as PFICs in prior taxable years, and NewAmsterdam Pharma, FLAC and Holdco are expected to be treated as PFICs for the current taxable year, which includes the Business Combination. If this is the case, (i) if the Exchange qualifies as an exchange described in Section 351(a) of the Code (subject to complying with the requirements of Section 367(a), if applicable), a U.S. Holder of shares of NewAmsterdam Pharma will not be required to recognize gain on the exchange of shares of NewAmsterdam Pharma for Holdco Shares pursuant to the Business Combination and (ii) if the Merger qualifies as a transaction governed by Section 351 of the Code or is treated as a “reorganization” within the meaning of Section 368 of the Code (subject to complying with the requirements of Section 367(a), if applicable and, with respect to holders of FLAC Public Warrants, subject to the considerations discussed under “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities—Characterization of the Merger”), a U.S. Holder of FLAC Securities will not be required to recognize gain on the exchange of FLAC Securities for Holdco Securities pursuant to the Business Combination.
However, if the Exchange were not to qualify as an exchange described in Section 351(a) of the Code, or if the Merger were not to qualify as an exchange described in Section 351(a) of the Code or a “reorganization” within the meaning of Section 368 of the Code, any income or gain recognized by such a U.S. Holder as a result of the exchange would generally be subject to a special tax and interest charge, under the rules described more fully below under “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities.”
Please see the section entitled “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities” for a more detailed discussion with respect to Holdco’s PFIC status and the potential tax consequences of the Merger. Prospective U.S. Holders of Holdco Shares or Holdco Public Warrants are urged to consult their tax advisors regarding the possible application of the PFIC rules to them.
The Merger may not qualify as a “reorganization” under Section 368(a) of the Code, potentially causing a U.S. Holder to recognize gain for U.S. federal income tax purposes in connection with the exchange of FLAC Public Warrants.
The parties have agreed to report the Merger as both a transaction described under Section 351 of the Code and as part of a “reorganization” within the meaning of Section 368 of the Code. If the Merger is treated in this manner for U.S. federal income tax purposes, a U.S. Holder of FLAC Public Warrants will not recognize any gain or loss on the deemed transfer of FLAC Public Warrants in connection with the Merger. However, as described under “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities —Tax Consequences of the Merger Under Section 368 of the Code,” there are significant factual and legal uncertainties as to whether the Merger will qualify as a reorganization. For example, under Section 368 of the Code, the acquiring corporation must continue, either directly or indirectly through certain controlled corporations, either a significant line of the acquired corporation’s historic business or use a significant portion of the acquired corporation’s historic business assets in a business. However, there is an absence of guidance directly on point as to how provisions of Section 368 of the Code apply in the case of an acquisition of a corporation with only investment-type assets, such as FLAC. In addition, reorganization treatment could be adversely affected by events or actions that occur prior to or at the time of the Merger, some of which are outside of FLAC’s control (including, for example, the magnitude of the FLAC Shareholder Redemption). Therefore, although the parties have agreed (subject to the limitations described under “Material Tax Considerations—Material U.S. Federal Income Tax Considerations—
120
Table of Contents
U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities—Tax Consequences of the Merger Under Section 368 of the Code”) to report the Merger as a reorganization, counsel is unable to opine on the treatment of the Merger as a reorganization. Thus, the closing of the Merger is not conditioned upon the receipt of an opinion of counsel, and counsel is unable to opine on the treatment of the Merger as a reorganization, and neither FLAC nor NewAmsterdam Pharma intends to request a ruling from the IRS regarding the U.S. federal income tax treatment of the Merger. Accordingly, notwithstanding the position that the parties have agreed to report, no assurance can be given that the IRS will not challenge the Merger’s qualification as a reorganization or that a court will not sustain such a challenge by the IRS.
If, as of the Closing, any requirement for Section 368(a) of the Code is not met, then a U.S. Holder who owns FLAC Public Warrants may recognize gain (but not loss) in an amount equal to the lesser of (i) the amount of gain realized by such holder (generally, the excess of (x) the sum of the fair market values of the Holdco Public Warrants treated as received by such holder and the Holdco Shares received by such holder over (y) such holder’s aggregate adjusted tax basis in the FLAC Public Warrants and FLAC Public Shares treated as having been exchanged therefor) and (ii) the fair market value of the Holdco Public Warrants treated as having been received by such holder in such exchange.
Prospective U.S. Holders of Holdco Shares or Holdco Public Warrants are urged to consult their tax advisors regarding the possible U.S. federal income tax consequences of the Merger to them, including the consequences of the Merger failing to be respected as a reorganization under Section 368(a) of the Code.
Risks Related to Ownership of Holdco Securities
Unless the context otherwise requires, any reference in the below section of this proxy statement/prospectus to “we,” “us” or “our” refers to Holdco following the Business Combination.
Upon Closing, Holdco will be a Dutch public company. The rights of Holdco’s shareholders may be different from the rights of shareholders in companies governed by the laws of the Cayman Islands and may not protect investors in a similar fashion afforded by incorporation in the jurisdiction of the Cayman Islands.
Upon Closing, Holdco will be a public company (naamloze vennootschap) under Dutch law. Holdco’s corporate affairs are governed by its articles of association, the rules of the Holdco Board, Holdco’s other internal rules and policies and by Dutch law. There can be no assurance that Dutch law will not change in the future or that it will serve to protect shareholders in a similar fashion afforded under corporate law principles in the Cayman Islands, which could adversely affect the rights of Holdco’s shareholders.
The rights of shareholders and the responsibilities of Holdco’s directors may be different from the rights and obligations of shareholders and directors in companies governed by the laws of the Cayman Islands. In the performance of their duties, Holdco’s directors are required by Dutch law to consider the interests of Holdco, its shareholders, its employees and other stakeholders, in all cases with due regard to the principles of reasonableness and fairness. It is possible that some of these stakeholders will have interests that are different from, or in addition to, your interests as a shareholder.
For more information on relevant provisions of Dutch corporation law and of the Holdco Articles of Association, see “Description of Holdco Securities” and “Comparison of Corporate Governance and Shareholder Rights.”
The market price and trading volume of the Holdco Shares may be volatile and could decline significantly following the Business Combination.
The stock markets, including Nasdaq on which Holdco has applied to list the Holdco Shares and Holdco Public Warrants to be issued in the Business Combination under the symbols “NAMS” and “NAMSW,” respectively, have from time to time experienced significant price and volume fluctuations. There may not be an active trading market for Holdco Shares, which may make it difficult to sell such shares. Even if an active, liquid and orderly trading market develops and is sustained for the Holdco Shares and Holdco Public Warrants following the Business
121
Table of Contents
Combination, the market price of the Holdco Shares and Holdco Public Warrants may be volatile and could decline significantly. In addition, the trading volume in the Holdco Shares and Holdco Public Warrants may fluctuate and cause significant price variations to occur. If the market price of the Holdco Shares and Holdco Public Warrants decline significantly, you may be unable to resell your shares or warrants at or above the market price of the Holdco Shares and Holdco Public Warrants as of the date immediately following the consummation of the Business Combination. We cannot assure you that the market price of the Holdco Shares and Holdco Public Warrants will not fluctuate widely or decline significantly in the future in response to a number of factors, including, among others, the following:
| • | the realization of any of the risk factors presented in this proxy statement/prospectus; |
| • | additions and departures of key personnel; |
| • | failure to comply with the requirements of Nasdaq; |
| • | failure to comply with the Sarbanes-Oxley Act or other laws or regulations; |
| • | future issuances, sales, resales or repurchases or anticipated issuances, sales, resales or repurchases, of Holdco Shares including due to the expiration of contractual lock-up agreements; |
| • | publication of research reports about Holdco; |
| • | failure to meet expectations of investors or securities analysts; |
| • | the performance and market valuations of other similar companies; |
| • | new laws, regulations, subsidies, or credits or new interpretations of existing laws applicable to Holdco; |
| • | commencement of, or involvement in, litigation involving Holdco; |
| • | broad disruptions in the financial markets, including sudden disruptions in the credit markets; |
| • | speculation in the press or investment community; |
| • | actual, potential or perceived control, accounting or reporting problems; |
| • | actual or anticipated differences in Holdco’s estimates, or in the estimates of analysts, for Holdco’s revenues, results of operations, liquidity or financial condition; |
| • | changes in accounting principles, policies and guidelines; and |
| • | other events or factors, including those resulting from infectious diseases, health epidemics and pandemics (including the ongoing COVID-19 pandemic), natural disasters, war, acts of terrorism or responses to these events. |
In the past, securities class-action litigation has often been instituted against companies following periods of volatility in the market price of their shares. This type of litigation could result in substantial costs and divert Holdco’s management’s attention and resources, which could have a material adverse effect on us.
There can be no assurance that the Holdco Shares will be approved for listing on Nasdaq or that Holdco will be able to comply with the continued listing standards of Nasdaq.
In connection with the consummation of the Business Combination, Holdco has applied to list its ordinary shares and warrants on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively. Holdco’s continued eligibility for listing will depend, among other items, on the number of FLAC Ordinary Shares that are exchanged for Holdco Shares. If, after listing, Holdco fails to satisfy the continued listing requirements of Nasdaq such as the minimum closing bid price requirement, Nasdaq may take steps to delist its securities. Such a delisting would likely have a negative effect on the price of the securities and would impair your ability to sell or purchase the securities when you wish to do so. In such a delisting, Holdco and its shareholders could face significant material adverse consequences including:
| • | a limited availability of market quotations for the combined company’s securities; |
| • | reduced liquidity for the combined company’s securities; |
122
Table of Contents
| • | a determination that the combined company’s stock is a “penny stock” which will require brokers trading in the combined company’s stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for shares of the combined company’s stock; |
| • | a limited amount of analyst coverage; and |
| • | a decreased ability to issue additional securities or obtain additional financing in the future. |
In the event of a delisting, Holdco can provide no assurance that any action taken by it to restore compliance with listing requirements would allow its securities to become listed again, stabilize the market price or improve the liquidity of its securities, prevent its securities from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements. Additionally, if Holdco’s securities are not listed on, or become delisted from, Nasdaq for any reason, and are quoted on the OTC Bulletin Board, an inter-dealer automated quotation system for equity securities that is not a national securities exchange, the liquidity and price of Holdco’s securities may be more limited than if it were quoted or listed on Nasdaq or another national securities exchange. You may be unable to sell your securities unless a market can be established or sustained.
The future exercise of registration rights may adversely affect the market price of Holdco’s Shares.
Pursuant to the Investor Rights Agreement, the FLAC Initial Shareholders and certain NewAmsterdam Pharma shareholders, and their permitted transferees, will be able to demand that Holdco register their securities on a resale registration statement no later than 30 days following the consummation of the Business Combination. The registration and availability of such a significant number of securities for trading in the public market, subject to applicable lock-up restrictions, may have an adverse effect on the market price of Holdco Shares. Sales of a substantial number of shares of Holdco Shares pursuant to the resale registration statement in the public market could occur at any time the registration statement remains effective and applicable lock-up restrictions expire. In addition, certain registration rights holders can request underwritten offerings to sell their securities. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of Holdco Shares. In addition, the existence of the registration rights may make the Business Combination more costly or difficult to conclude. An aggregate of 73,325,642 Holdco Shares, or approximately 85%, will have registration rights following the closing of the Business Combination pursuant to the Investor Rights Agreement and the Subscription Agreement.
If, following the Business Combination, securities or industry analysts do not publish or cease publishing research or reports about Holdco, its business, or its market, or if they change their recommendations regarding the Holdco Shares adversely, then the price and trading volume of the Holdco Shares could decline.
The trading market for the Holdco Shares will be influenced by the research and reports that industry or financial analysts publish about its business. Holdco does not control these analysts, or the content and opinions included in their reports. As a new public company, Holdco may be slow to attract research coverage and the analysts who publish information about the Holdco Shares will have had relatively little experience with Holdco, which could affect their ability to accurately forecast Holdco’s results and make it more likely that Holdco fails to meet their estimates. In the event Holdco obtains industry or financial analyst coverage, if any of the analysts who cover Holdco issues an inaccurate or unfavorable opinion regarding it, Holdco Shares’ price would likely decline. If one or more of these analysts cease coverage of Holdco or fail to publish reports on it regularly, Holdco’s visibility in the financial markets could decrease, which in turn could cause its share price or trading volume to decline.
Holdco does not intend to pay dividends for the foreseeable future. Accordingly, you may not receive any return on investment unless you sell your Holdco Shares for a price greater than the price you paid for the FLAC Class A Ordinary Shares.
Holdco has never declared or paid any cash dividends on its shares. It currently intends to retain all available funds and any future earnings for use in the operation of its business and does not anticipate paying any
123
Table of Contents
dividends on the Holdco Shares in the foreseeable future. Consequently, you may be unable to realize a gain on your investment except by selling such shares after price appreciation, which may never occur.
The Holdco Board may only pay dividends and other distributions from Holdco’s reserves to the extent Holdco’s shareholders’ equity (eigen vermogen) exceeds the sum of our paid-in and called-up share capital plus the reserves it must maintain under Dutch law or the Holdco Articles of Association and (if it concerns a distribution of profits) after adoption of its statutory annual accounts by its general meeting from which it appears that such dividend distribution is allowed. Subject to those restrictions, any future determination to pay dividends or other distributions from Holdco’s reserves will be at the discretion of its board and will depend upon a number of factors, including its results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the Holdco Board deems relevant.
Under the Holdco Articles of Association, the Holdco Board may decide that all or part of the profits shown in Holdco’s adopted statutory annual accounts will be added to its reserves. After reservation of any such profits, any remaining profits will be at the disposal of the general meeting at the proposal of the Holdco Board for distribution on Holdco Shares, subject to applicable restrictions of Dutch law. The Holdco Board is permitted, subject to certain requirements and applicable restrictions of Dutch law, to declare interim dividends without the approval of Holdco’s general meeting. Dividends and other distributions will be made payable no later than a date determined by Holdco. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable will lapse and any such amounts will be considered to have been forfeited to us (verjaring).
Holdco is eligible to be treated as an “emerging growth company,” and Holdco cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the Holdco Shares less attractive to investors, which could have a material and adverse effect on Holdco, including growth prospects, because Holdco may rely on these reduced disclosure requirements.
Holdco qualifies as an emerging growth company within the meaning of the Securities Act, and if Holdco takes advantage of certain exemptions from disclosure requirements available to emerging growth companies, this could make Holdco’s securities less attractive to investors and may make it more difficult to compare Holdco’s performance with other public companies.
Holdco is eligible to be treated as an emerging growth company, as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised financial accounting standards until such time as those standards apply to private companies. Holdco intends to take advantage of this extended transition period under the JOBS Act for adopting new or revised financial accounting standards.
Holdco will remain an “emerging growth company” until the earliest to occur of (i) the last day of the fiscal year (a) following December 8, 2025, the fifth anniversary of the FLAC IPO, (b) in which Holdco has total annual gross revenue of at least $1.07 billion or (c) in which Holdco is deemed to be a large accelerated filer, which means the market value of Holdco Shares that is held by non-affiliates exceeds $700 million as of the last business day of our prior second fiscal quarter, and (ii) the date on which Holdco has issued more than $1.0 billion in non-convertible debt during the prior three-year period.
For as long as Holdco continues to be an emerging growth company, it may also take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including presenting only limited selected financial data, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, and to the extent Holdco no longer qualified as a “foreign private issuer,” reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. As a result, its shareholders may not have access to certain information that they may deem important.
124
Table of Contents
Holdco cannot predict if investors will find Holdco Shares less attractive because it may rely on these exemptions. If some investors find Holdco Shares less attractive as a result, there may be a less active trading market for Holdco Shares and the price of Holdco Shares may be more volatile. Further, there is no guarantee that the exemptions available to Holdco under the JOBS Act will result in significant savings. To the extent that Holdco chooses not to use exemptions from various reporting requirements under the JOBS Act, it will incur additional compliance costs, which may impact Holdco’s financial condition.
Holdco’s management team has limited experience managing a public company.
Most members of Holdco’s management team have limited or no experience managing a publicly traded company, interacting with public company investors, and complying with the increasingly complex laws, rules and regulations that govern public companies. As a public company, we are subject to significant obligations relating to reporting, procedures and internal controls, in both the United States and the Netherlands, and Holdco’s management team may not successfully or efficiently manage such obligations. These obligations and scrutiny will require significant attention from Holdco’s management and could divert their attention away from the day-to-day management of Holdco’s business, which could adversely affect Holdco’s business, financial condition and results of operations. Immediately after the Exchange, the legal form of Holdco will be converted from a private company with limited liability to a public limited liability company in the Netherlands. Such conversion will impose additional burdens on Holdco’s management team.
Past performance by Frazier Healthcare Partners, including members of FLAC’s management team, may not be indicative of future performance of an investment in Holdco.
Past performance by Frazier Healthcare Partners and by FLAC’s management team is not a guarantee of success with respect to the Business Combination. You should not rely on the historical record of Frazier Healthcare Partners or FLAC’s management team as indicative of the future performance of an investment in Holdco or the returns Holdco will, or is likely to, generate going forward.
Investors may have difficulty enforcing civil liabilities against Holdco or the members of the Holdco Board.
Holdco is organized and existing under the laws of the Netherlands and, as such, Dutch private international law governs the rights of Holdco’s shareholders and the civil liability of Holdco’s directors and executive officers are governed in certain respects by the laws of the Netherlands. The ability of Holdco’s shareholders in certain countries other than the Netherlands to bring an action against Holdco or Holdco’s directors and executive officers may be limited under applicable law. In addition, substantially all of Holdco’s assets are located outside the United States.
As a result, it may not be possible for shareholders to effect service of process within the United States upon Holdco or Holdco’s directors and executive officers or to enforce judgments against them in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the United States. In addition, it is not clear whether a Dutch court would impose civil liability on Holdco or any of Holdco’s directors and executive officers in an original action based solely upon the federal securities laws of the United States brought in a court of competent jurisdiction in the Netherlands.
As of the date of this proxy statement/prospectus, the United States and the Netherlands do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. With respect to choice of court agreements in civil or commercial matters, the Hague Convention on Choice of Court Agreements has entered into force for the Netherlands, but has not entered into force for the United States. Accordingly, a judgment rendered by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized and enforced by the competent Dutch courts. However, if a person has obtained a judgment rendered by a court in the United States that is enforceable under the laws of the United States and files a claim with the competent Dutch court, the Dutch court will in principle give binding effect to that foreign judgment if (i) the jurisdiction of the foreign court
125
Table of Contents
was based on a ground of jurisdiction that is generally acceptable according to international standards, (ii) the judgment by the foreign court was rendered in legal proceedings that comply with the Dutch standards of proper administration of justice including sufficient safeguards (behoorlijke rechtspleging), (iii) binding effect of such foreign judgment is not contrary to Dutch public order (openbare orde) and (iv) the judgment by the foreign court is not incompatible with a decision rendered between the same parties by a Dutch court, or with a previous decision rendered between the same parties by a foreign court in a dispute that concerns the same subject and is based on the same cause, provided that the previous decision qualifies for recognition in the Netherlands. However, even if such a foreign judgment is given binding effect, a claim based on that foreign judgment may still be rejected if the foreign judgment is not or no longer formally enforceable.
Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against Holdco or Holdco’s directors, representatives or certain experts named herein who are residents of the Netherlands or countries other than the United States any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws.
The Holdco Articles of Association provide that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act and the Exchange Act, which could limit the ability of securityholders of Holdco to choose a favorable judicial forum for disputes with Holdco or Holdco’s directors, officers or employees.
The Holdco Articles of Association provide that, unless Holdco consents in writing to the selection of an alternative forum, the sole and exclusive forum for any complaint asserting a cause of action arising under the Securities Act or the Exchange Act, to the fullest extent permitted by applicable law, shall be the U.S. federal district courts. This choice of forum provision may increase a securityholder’s cost and limit the securityholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with Holdco or Holdco’s directors, officers or other employees, which may discourage lawsuits against Holdco and Holdco’s directors, officers and other employees. Holdco’s shareholders will not be deemed to have waived Holdco’s compliance with the U.S. federal securities laws and the rules and regulations thereunder as a result of Holdco’s exclusive forum provision. Any person or entity purchasing or otherwise acquiring any of the Holdco Shares or other securities, whether by transfer, sale, operation of law or otherwise, will be deemed to have notice of and have irrevocably agreed and consented to this provision. There is uncertainty as to whether a court would enforce such provision. The Securities Act provides that state courts and federal courts will have concurrent jurisdiction over claims under the Securities Act, and the enforceability of similar choice of forum provisions in other companies’ charter documents has been challenged in legal proceedings. It is possible that a court could find this type of provisions to be inapplicable or unenforceable, and if a court were to find this provision in the Holdco Articles of Association to be inapplicable or unenforceable in an action, Holdco may incur additional costs associated with resolving the dispute in other jurisdictions, which could have adverse effect on Holdco’s business and financial performance.
Each of Sponsor and NewAmsterdam Pharma’s current shareholders will own a significant portion of Holdco Shares and will have representation on the Holdco Board after Closing. Sponsor and NewAmsterdam Pharma’s current shareholders may have interests that differ from those of other shareholders.
Upon the completion of the Business Combination, approximately 14.9% of Holdco Shares will be owned by the FLAC Initial Shareholders and approximately 22.0% of Holdco Shares will be owned by the PIPE Investors (excluding the FLAC Initial Investors and affiliates of the Sponsor). These levels of ownership interests assume that (i) no FLAC Class A Ordinary Shares are elected to be redeemed by the FLAC public shareholders, (ii) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (iii) none of the Holdco Warrants have been exercised, (iv) Participating Shareholders represent 100% of the issued and outstanding shares of NewAmsterdam Pharma, (v) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) have been issued, (vi) an aggregate of 8,656,330 Holdco Shares have been issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of
126
Table of Contents
Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”; (vii) none of the NewAmsterdam Pharma Options have been exercised (prior to Closing) and (viii) no Holdco Options or awards that may be issued under Holdco’s long-term incentive plan following the Closing have been exercised. For more information about factors that affect the assumptions above, please see the section entitled “The Business Combination—Ownership of Holdco Following the Business Combination.” In addition, two of Holdco’s non-executive director nominees will be designated by FLAC. As a result, FLAC and the current Holdco shareholders may be able to significantly influence the outcome of matters submitted for director action, subject to obligation of the Holdco Board to act in the interest of all of Holdco’s stakeholders, and for shareholder action, including the designation and appointment of the Holdco Board and approval of significant corporate transactions, including business combinations, consolidations and mergers. The influence of FLAC and the current Holdco shareholders over Holdco’s management could have the effect of delaying or preventing a change in control or otherwise discouraging a potential acquirer from attempting to obtain control of Holdco, which could cause the market price of Holdco Shares to decline or prevent Holdco’s shareholders from realizing a premium over the market price for Holdco Shares. Additionally, FLAC is controlled by the Sponsor, which is in the business of making investments in companies and which may from time to time acquire and hold interests in businesses that compete directly or indirectly with Holdco or that supply Holdco with goods and services. The Sponsor may also pursue acquisition opportunities that may be complementary to (or competitive with) Holdco’s business, and as a result those acquisition opportunities may not be available to Holdco. The Sponsor’s investors in Holdco Shares should consider that the interests of FLAC and the current Holdco shareholders may differ from their interests in material respects.
If Holdco fails to maintain an effective system of internal control over financial reporting, Holdco may not be able to accurately report its financial results or prevent fraud. As a result, shareholders could lose confidence in Holdco’s financial and other public reporting, which is likely to negatively affect Holdco’s business and the market price of Holdco Shares.
Holdco’s management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with IFRS. As a result of becoming a public company, Holdco will be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by our management on, among other things, the effectiveness of Holdco’s internal control over financial reporting, beginning with Holdco’s annual report filed with the SEC for the first fiscal year ending December 31, 2023. This assessment will need to include disclosures of any material weaknesses identified by Holdco’s management in Holdco’s internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be detected or prevented on a timely basis. Holdco is in the very early stages of the costly and challenging process of planning the activities necessary to perform the evaluation needed to comply with Section 404. Effective internal control over financial reporting is necessary for Holdco to provide reliable financial reports and prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in Holdco’s implementation could cause Holdco to fail to meet its reporting obligations. In addition, any testing conducted by Holdco, or any testing conducted by Holdco’s independent registered public accounting firm, may reveal deficiencies in Holdco’s internal control over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to Holdco’s financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in Holdco’s reported financial information, which is likely to negatively affect Holdco’s business and the market price of Holdco Shares.
In connection with the preparation of NewAmsterdam Pharma’s financial statements at and for the years ended December 31, 2021 and 2020, we identified material weaknesses in the design of our internal control over financial reporting across the principles for each component of the COSO framework at the entity level (i.e. control environment, risk assessment, monitoring, information & communication and control activities) and
127
Table of Contents
accordingly, across our business and IT processes. Specifically, the material weaknesses that we identified, individually or in the aggregate, included the following:
| • | our lack of consistent and documented risk assessment procedures and control activities related to our financial reporting, among which a sufficient level of management review and approval, manual processes, roles and responsibilities, and adequate application and controls over information technology; and |
| • | failure to maintain a sufficient complement of personnel commensurate with our accounting and reporting requirements as we continue to grow as a company, and ability to: (i) design and maintain formal accounting policies, procedures and controls over the fair presentation of our financial statements; (ii) analyze, record and disclose complex accounting matters timely and accurately; and (iii) design and maintain controls over the preparation and review of journal entries and financial statements, including maintaining appropriate segregation of duties. |
Prior to the completion of the Business Combination, we have been a private company with limited accounting personnel to adequately execute our accounting processes and other supervisory resources with which to address our internal control over financial reporting. To address these material weaknesses, we will need to add personnel and continue to develop and implement new financial processes. We have taken steps to remediate the material weakness described above, including by engaging consultants to assist management in developing internal control procedures and anticipate hiring additional qualified accounting and financial reporting personnel, and further evolving our accounting processes and policies. We will not be able to fully remediate these material weaknesses until these steps have been completed and have been operating effectively for a sufficient period of time. The remediation measures we engage in may be time consuming and costly and there is no assurance that these initiatives will ultimately have the intended effects.
Holdco will be required to disclose changes made in its internal controls and procedures on an annual basis and its management will be required to assess the effectiveness of these controls annually. However, for as long as Holdco is an “emerging growth company” under the JOBS Act, its independent registered public accounting firm will not be required to attest to the effectiveness of Holdco’s internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act. An independent assessment of the effectiveness of Holdco’s internal controls could detect problems that Holdco’s management’s assessment might not. Undetected material weaknesses in Holdco’s internal controls could lead to financial statement restatements and require Holdco to incur the expense of remediation.
Holdco may redeem your unexpired warrants prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
Holdco has the ability to redeem all outstanding Holdco Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, if, among other things, the Reference Value equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like). Please see “Description of Holdco Securities—Share Capital—Holdco Warrants—Redemptions of Holdco Warrants for cash when the price per Holdco Share equals or exceeds $18.00.” If and when the Holdco Warrants become redeemable by Holdco, Holdco may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding Holdco Warrants as described above could force you to (i) exercise your Holdco Warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, (ii) sell your Holdco Warrants at the then-current market price when you might otherwise wish to hold your Holdco Warrants or (iii) accept the nominal redemption price which, at the time the outstanding Holdco Warrants are called for redemption, is likely to be substantially less than the market value of your Holdco Warrants. None of the Holdco Private Placement Warrants will be redeemable by Holdco so long as they are held by the Sponsor or their permitted transferees.
In addition, Holdco has the ability to redeem the outstanding Holdco Warrants at any time after they become exercisable and prior to their expiration, at a price of $0.10 per warrant if, among other things, the Reference
128
Table of Contents
Value equals or exceeds $10.00 per share (as adjusted for share sub-divisions, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like) and the former holders of FLAC Private Placement Warrants have also been called for redemption, subject to certain limitations as set forth in the Warrant Assumption Agreement. In such a case, the holders will be able to exercise their Holdco Warrants prior to redemption for a number of Holdco Shares determined based on the redemption date and the fair market value of the Holdco Shares. Please see “Description of Holdco Securities—Holdco Warrants—Redemptions of Holdco Warrants for cash when the price per Holdco Share equals or exceeds $10.00.” The value received upon exercise of the Holdco Warrants (1) may be less than the value the holders would have received if they had exercised their Holdco Warrants at a later time where the underlying share price is higher and (2) may not compensate the holders for the value of the Holdco Warrants, including because the number of ordinary shares received is capped at 0.361 shares of the Holdco Shares per warrant (subject to adjustment) irrespective of the remaining life of the warrants.
Warrants will become exercisable for Holdco Shares, which would increase the number of shares eligible for future resale in the public market and result in dilution to shareholders.
Outstanding Holdco Warrants to purchase an aggregate of 4,767,000 shares of Holdco Shares will become exercisable in accordance with the terms of the Warrant Assumption Agreement. The Holdco Warrants will become exercisable at any time commencing on the later of 30 days after the completion of the Business Combination and 12 months from the closing of the FLAC IPO. The exercise price of the Holdco Warrants will be $11.50 per share. To the extent such warrants are exercised, additional Holdco Shares will be issued, which will result in dilution to the holders of Holdco Shares and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of Holdco Shares. However, there is no guarantee that the Holdco Warrants will ever be in the money prior to their expiration, and as such, the warrants may expire worthless.
The terms of the Holdco Public Warrants may be amended in a manner adverse to a holder if holders of at least 65% of the then outstanding Holdco Public Warrants approve of such amendment.
The Warrant Assumption Agreement provides that (i) the terms of the Holdco Warrants may be amended without the consent of any holder for the purpose of (a) curing any ambiguity or correct any mistake, including to conform the provisions of the Warrant Assumption Agreement to the description of the terms of such warrants and the Warrant Assumption Agreement set forth in this proxy statement/prospectus, or defective provision, (b) amending the definition of “Ordinary Cash Dividend” as contemplated by and in accordance with the Warrant Assumption Agreement or (c) adding or changing any provisions with respect to matters or questions arising under the Warrant Assumption Agreement as the parties to the Warrant Assumption Agreement may deem necessary or desirable and that the parties deem to not adversely affect the rights of the registered holders of such warrants under the Warrant Assumption Agreement and (ii) all other modifications or amendments require the vote or written consent of at least 65% of the then outstanding Holdco Public Warrants; provided that any amendment that solely affects the terms of the Holdco Private Placement Warrants or any provision of the Warrant Assumption Agreement solely with respect to the Holdco Private Placement Warrants will require at least 50% of the then outstanding Holdco Private Placement Warrants.
Accordingly, FLAC or Holdco may amend the terms of the Holdco Public Warrants in a manner adverse to a holder if holders of at least 65% of the then outstanding Holdco Public Warrants approve of such amendment. Although the ability to amend the terms of the Holdco Public Warrants with the consent of at least 65% of the then outstanding Holdco Public Warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, shorten the exercise period or decrease the number of ordinary shares purchasable upon exercise of a warrant.
129
Table of Contents
The Warrant Assumption Agreement will designate the courts of the State of New York or the United States District Court for the Southern District of New York as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by holders of Holdco Warrants, which could limit the ability of Holdco Warrant holders to obtain a favorable judicial forum for disputes with FLAC.
The Warrant Assumption Agreement will provide that, subject to applicable law, (i) any action, proceeding or claim against us arising out of or relating in any way to the Warrant Assumption Agreement, including under the Securities Act, will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and (ii) that Holdco will irrevocably submit to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim.
Notwithstanding the foregoing, these provisions of the Warrant Assumption Agreement will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America are the sole and exclusive forum. Any person or entity purchasing or otherwise acquiring any interest in any of the Holdco Warrants will be deemed to have notice of and to have consented to the forum provisions in the Warrant Assumption Agreement. If any action, the subject matter of which is within the scope of the forum provisions of the Warrant Assumption Agreement, is filed in a court other than a court of the State of New York or the United States District Court for the Southern District of New York (a “foreign action”) in the name of any holder of Holdco Warrants, such holder will be deemed to have consented to: (x) the personal jurisdiction of the state and federal courts located in the State of New York in connection with any action brought in any such court to enforce the forum provisions (an “enforcement action”), and (y) having service of process made upon such warrant holder in any such enforcement action by service upon such warrant holder’s counsel in the foreign action as agent for such Holdco Warrant holder.
This choice-of-forum provision may limit a Holdco Warrant holder’s ability to bring a claim in a judicial forum that it finds favorable for disputes, which may discourage such lawsuits. Alternatively, if a court were to find this provision of the Warrant Assumption Agreement inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, Holdco may incur additional costs associated with resolving such matters in other jurisdictions, which could materially and adversely affect its business, financial condition and results of operations and result in a diversion of the time and resources of management and the Holdco Board.
If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports, about Holdco’s business, Holdco’s stock price and trading volume could decline.
The market for Holdco Shares will depend in part on the research and reports that securities or industry analysts publish about Holdco or its business. Securities and industry analysts do not currently, and may never, publish research on Holdco. If no securities or industry analysts commence coverage of Holdco, the market price and liquidity for Holdco Shares could be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover Holdco downgrade their opinions about Holdco Shares, publish inaccurate or unfavorable research about Holdco, or cease publishing about Holdco regularly, demand for Holdco Shares could decrease, which might cause its share price and trading volume to decline significantly.
Future issuances of equity securities may adversely affect Holdco, including the market price of Holdco Shares and may be dilutive to existing shareholders.
In the future, Holdco may issue equity ranking senior to Holdco Shares. Such securities will generally have priority upon liquidation and may be governed by an instrument containing covenants restricting its operating flexibility. Additionally, any convertible or exchangeable securities that Holdco issues in the future may have rights, preferences and privileges more favorable than those of Holdco Shares. Because Holdco’s decision to issue equity in the future will depend on market conditions and other factors beyond Holdco’s control, it cannot predict or estimate the amount, timing, nature or success of Holdco’s future capital raising efforts. As a result, future capital raising efforts may reduce the market price of Holdco Shares and be dilutive to existing shareholders.
130
Table of Contents
Holdco’s shareholders may not have any preemptive rights in respect of future issuances of Holdco Shares, and, as a result, may experience substantial dilution upon future issuances of Holdco Shares or grants of rights to subscribe for such shares.
In the event of an issuance of Holdco Shares or a grant of rights to subscribe for Holdco Shares, subject to certain exceptions, each shareholder will have a pro rata pre-emption right in proportion to the aggregate nominal value of such holder’s Holdco Shares. These pre-emption rights may be restricted or excluded by a resolution of the general meeting or by another corporate body designated by the general meeting. Prior to Closing, the Holdco Board will be authorized for a period of five years from the completion of the Holdco Reorganization to issue shares or grant rights to subscribe for shares up to Holdco’s authorized share capital from time to time and to limit or exclude pre-emption rights in connection therewith. This could cause existing shareholders to experience substantial dilution of their interest in Holdco.
Holdco is not obligated to, and does not, comply with all best practice provisions of the Dutch Corporate Governance Code.
Upon Closing, Holdco will be subject to the DCGC. The DCGC contains principles and best practice provisions on corporate governance that regulate relations between the board and the general meeting and matters in respect of financial reporting, auditors, disclosure, compliance and enforcement standards. The DCGC is based on a “comply or explain” principle. Accordingly, companies must disclose in their statutory annual reports whether they comply with the provisions of the DCGC. If a company subject to the DCGC does not comply with those provisions, that company would be required to give the reasons for such non-compliance. Holdco does not comply with all best practice provisions of the DCGC.
See “Description of Holdco Securities.” This may affect your rights as a shareholder and you may not have the same level of protection as a shareholder in a Dutch company that fully complies with the DCGC.
Provisions of the Holdco Articles of Association or Dutch corporate law might deter acquisition bids for Holdco that might be considered favorable and prevent, delay or frustrate any attempt to replace or dismiss directors.
Under Dutch law, various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law.
In addition, certain provisions of the Holdco Articles of Association may make it more difficult for a third-party to acquire control of us or effect a change in the composition of the Holdco Board. These include:
| • | a provision that the Holdco directors can only be appointed on the basis of a binding nomination prepared by the Holdco Board which can only be overruled by a two-thirds majority of votes cast representing more than half of Holdco’s issued share capital; |
| • | a provision that Holdco’s directors can only be dismissed by the general meeting by a two-thirds majority of votes cast representing more than half of Holdco’s issued share capital, unless the dismissal is proposed by the Holdco Board in which latter case a simple majority of the votes cast would be sufficient; |
| • | a provision allowing, among other matters, the former chairperson of the Holdco Board or Holdco’s former Chief Executive Officer to manage Holdco’s affairs if all of Holdco’s directors are dismissed and to appoint others to be charged with our affairs, including the preparation of a binding nomination for Holdco’s directors as discussed above, until new directors are appointed by the general meeting on the basis of such binding nomination; and |
| • | a requirement that certain matters, including an amendment of the Holdco Articles of Association, may only be resolved upon by Holdco’s general meeting if proposed by the Holdco Board. |
Dutch law also allows for staggered multi-year terms of Holdco’s directors, as a result of which only part of Holdco’s directors will be subject to appointment or re-appointment in any given year.
131
Table of Contents
Furthermore, in accordance with the Dutch Corporate Governance Code (the “DCGC”), shareholders who have the right to put an item on the agenda for Holdco’s general meeting or to request the convening of a general meeting will not exercise such rights until after they have consulted the Holdco Board. If exercising such rights may result in a change in Holdco’s strategy (for example, through the dismissal of one or more of Holdco’s directors), the Holdco Board must be given the opportunity to invoke a reasonable period of up to 180 days to respond to the shareholders’ intentions. If invoked, the Holdco Board must use such response period for further deliberation and constructive consultation, in any event with the shareholder(s) concerned and exploring alternatives. At the end of the response time, the Holdco Board will report on this consultation and the exploration of alternatives to Holdco’s general meeting. The response period may be invoked only once for any given general meeting and will not apply (i) in respect of a matter for which a response period has been previously invoked or (ii) if a shareholder holds at least 75% of Holdco’s issued share capital as a consequence of a successful public bid.
Moreover, the Holdco Board can invoke a cooling-off period of up to 250 days when shareholders, using their right to have items added to the agenda for a general meeting or their right to request a general meeting, propose an agenda item for Holdco’s general meeting to dismiss, suspend or appoint one or more directors (or to amend any provision in the Articles of Association dealing with those matters) or when a public offer for Holdco is made or announced without Holdco’s support, provided, in each case, that the Holdco Board believes that such proposal or offer materially conflicts with the interests of Holdco and its business. During a cooling-off period, Holdco’s general meeting cannot dismiss, suspend or appoint directors (or amend the provisions in the Holdco Articles of Association dealing with those matters) except at the proposal of the Holdco Board. During a cooling-off period, the Holdco Board must gather all relevant information necessary for a careful decision-making process and at least consult with shareholders representing 3% or more of Holdco’s issued share capital at the time the cooling-off period was invoked, as well as with Holdco’s Dutch works council (if Holdco or, under certain circumstances, any of its subsidiaries would have one). Formal statements expressed by these stakeholders during such consultations must be published on Holdco’s website to the extent these stakeholders have approved that publication. Within one week following the last day of the cooling-off period, the Holdco Board must publish a report in respect of its policy and conduct of affairs during the cooling-off period on Holdco’s website. This report must remain available for inspection by shareholders and others with meeting rights under Dutch law at Holdco’s office and must be tabled for discussion at the next general meeting. Shareholders representing at least 3% of Holdco’s issued share capital may request the Enterprise Chamber (Ondernemingskamer) of the Amsterdam Court of Appeal (the “Enterprise Chamber”), for early termination of the cooling-off period. The Enterprise Chamber must rule in favor of the request if the shareholders can demonstrate that:
| • | the Holdco Board, in light of the circumstances at hand when the cooling-off period was invoked, could not reasonably have concluded that the relevant proposal or hostile offer constituted a material conflict with the interests of Holdco and its business; |
| • | the Holdco Board cannot reasonably believe that a continuation of the cooling-off period would contribute to careful policy-making; or |
| • | other defensive measures, having the same purpose, nature and scope as the cooling-off period, have been activated during the cooling-off period and have not since been terminated or suspended within a reasonable period at the relevant shareholders’ request (i.e., no “stacking” of defensive measures). |
As a “foreign private issuer,” Holdco will be exempt from a number of rules under the U.S. securities laws and will be permitted to file less information with the SEC than a U.S. domestic public company, which may limit the information available to holders of the Holdco Shares and Holdco Warrants.
Holdco is a “foreign private issuer,” as such term is defined in Rule 405 under the Securities Act. Because Holdco qualifies as a “foreign private issuer” under the Exchange Act, it is exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (1) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the
132
Table of Contents
Exchange Act, (2) the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time and (3) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, although it is subject to Dutch laws and regulations with regard to certain of these matters and intends to furnish comparable quarterly information on Form 6-K. In addition, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year and U.S. domestic issuers that are large accelerated filers are required to file their annual report on Form 10-K within 60 days after the end of each fiscal year. As long as Holdco is eligible for the foreign private issuer exemption, it will not be required to obtain shareholder approval for certain dilutive events, such as the establishment or material amendment of certain equity-based compensation plans, it will not be required to provide detailed executive compensation disclosure in its periodic reports, and it will be exempt from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Also, as a “foreign private issuer,” Holdco will be permitted to follow home country practice in lieu of certain corporate governance rules of the Nasdaq, including those that require listed companies to have a majority of independent directors and independent director oversight of executive compensation, nomination of directors and corporate governance matters. As long as Holdco relies on the foreign private issuer exemption, a majority of its board will not be required to be independent directors and its compensation committee will not be required to be composed entirely of independent directors. Accordingly, holders of Holdco Shares may not have the same protections afforded to shareholders of listed companies that are subject to all of the applicable corporate governance requirements.
Holdco may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, Holdco is a “foreign private issuer,” and therefore is not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to Holdco on June 30, 2023. In the future, Holdco would lose its foreign private issuer status if more than 50% of its outstanding voting securities are owned by U.S. residents and any of the following three circumstances applies: (i) the majority of Holdco’s executive officers or directors are U.S. citizens or residents, (ii) more than 50% of Holdco’s assets are located in the United States or (iii) Holdco’s business is administered principally in the United States. If Holdco loses its foreign private issuer status, it will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. Holdco would also have to mandatorily comply with U.S. federal proxy requirements, and its officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, it would lose its ability to rely upon exemptions from certain corporate governance requirements under the listing rules of Nasdaq. As a U.S. listed public company that is not a foreign private issuer, Holdco would incur significant additional legal, accounting and other expenses that it will not incur as a foreign private issuer.
As Holdco will be a “foreign private issuer” and intends to follow certain home country corporate governance practices, its shareholders may not have the same protections afforded to shareholders of companies that are subject to all Nasdaq corporate governance requirements.
As a “foreign private issuer,” following the closing of the Business Combination, Holdco will have the option to follow certain home country corporate governance practices rather than those of Nasdaq, provided that it discloses the requirements it is not following and describes the home country practices it is following. As a result, in accordance with the listing requirements of Nasdaq we will rely on home country governance
133
Table of Contents
requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of Nasdaq. In accordance with Dutch law and generally accepted business practices, the Holdco Articles of Association do not provide quorum requirements generally applicable to general meetings. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting shares. Although we must provide shareholders with an agenda and other relevant documents for our general meetings, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands, thus our practice will vary from the requirement of Nasdaq Listing Rule 5620(b). As permitted by the listing requirements of Nasdaq, we have also opted out of the requirements of Nasdaq Listing Rule 5605(b)(2), which requires that independent directors have regularly scheduled meetings at which only independent directors are present. In addition, we have opted out of shareholder approval requirements, as included in the Nasdaq Listing Rules, for the issuance of securities in connection with certain events such as the acquisition of shares or assets of another company (if, due to the potential issuance of ordinary shares, the ordinary shares would have upon issuance voting power equal to or in excess of 20% of the voting power outstanding before the issuance of stock), the establishment of or amendments to equity-based compensation plans for employees, a change of control of our company and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. For an overview of our corporate governance principles, see “Comparison of Corporate Governance and Shareholder Rights.” Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to these Nasdaq requirements.
Dutch and European insolvency laws are substantially different from U.S. insolvency laws and may offer Holdco shareholders less protection than they would have under U.S. insolvency laws.
As a Dutch public limited liability company, Holdco is subject to Dutch insolvency laws in the event any insolvency proceedings are initiated against us including, among other things, Regulation (EU) 2015/848 of the European Parliament and of the Council of May 20, 2015 on insolvency proceedings. Should courts in another EU Member State determine that Holdco’s centre of main interests (COMI) is situated in that member state, the courts in that member state will in principle have jurisdiction over the insolvency proceedings initiated against Holdco and the insolvency laws of that member state will in principle apply to Holdco, in accordance with and subject to such EU regulations. Insolvency laws in the Netherlands or the relevant other European member state, if any, may offer our shareholders less protection than they would have under U.S. insolvency laws and make it more difficult for Holdco’s shareholders to recover the amount they could expect to recover in a liquidation under U.S. insolvency laws.
Risks Related to FLAC
Unless the context otherwise requires, any reference in the below subsection of this proxy statement/prospectus to “we,” “us” or “our” refers to FLAC prior to the consummation of the Business Combination. The following discussion should be read in conjunction with the other sections of this proxy statement/prospectus and our financial statements and accompanying notes, and other financial information included elsewhere within this proxy statement/prospectus. This discussion includes forward-looking information regarding our business, results of operations and cash flows and contractual obligations and arrangements that involves risks, uncertainties and assumptions. Our actual results may differ materially from any future results expressed or implied by such forward-looking statements as a result of various factors, including, but not limited to, those discussed in the sections of this proxy statement/prospectus entitled “Special Note Regarding Forward-Looking Statements” and “FLAC’s Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
134
Table of Contents
FLAC cannot assure you that its diligence review has identified all material risks associated with the Business Combination, and you may be less protected as an investor from any material issues with respect to NewAmsterdam Pharma’s business, including any material omissions or misstatements contained in the registration statement or proxy statement/prospectus relating to the Business Combination than an investor in an initial public offering.
Before entering into the Business Combination Agreement, FLAC performed a due diligence review of NewAmsterdam Pharma and its business and operations; however, FLAC cannot assure you that its due diligence review identified all material issues, and certain unexpected risks may arise and previously known risks may materialize in a manner not consistent with its preliminary risk analysis. Therefore, as an investor in the Business Combination, you may be exposed to future losses, impairment charges, write-downs, write offs or other charges that could have a significant negative effect on NewAmsterdam Pharma’s financial condition, results of operations and the share price of its securities, which could cause you to lose some or all of your investment without certain recourse against any underwriter that may be available in an underwritten public offering.
The Sponsor will control the election of the FLAC Board until consummation of an initial business combination and hold a substantial interest in FLAC. As a result, the Sponsor will elect all of FLAC’s directors and may exert a substantial influence on actions requiring a shareholder vote, potentially in a manner that you do not support.
The Sponsor and the other FLAC Initial Shareholders collectively own approximately 19% of the issued and outstanding FLAC Ordinary Shares. In addition, the Sponsor is entitled to elect all of FLAC’s directors prior to the initial business combination. Holders of FLAC Class B Ordinary Shares have the exclusive right prior to FLAC’s initial business combination to elect FLAC’s directors. Accordingly, as holders of the FLAC Class A Ordinary Shares, FLAC’s public shareholders will not have the right to vote on the election of directors prior to consummation of the Business Combination. These provisions of the FLAC Articles of Association may only be amended by a special resolution passed by holders representing a two-thirds majority of the FLAC Class B Ordinary Shares. As a result, holders of FLAC Public Shares will not have any influence over the election of directors of FLAC involved in selecting NewAmsterdam Pharma as the counterparty in the Business Combination and recommending in favor of the proposals included in this proxy statement/prospectus.
In addition, as a result of their substantial interest in FLAC, the Sponsor (and the other FLAC Initial Shareholders) may exert a substantial influence on other actions requiring a shareholder vote, potentially in a manner that FLAC shareholders do not support, including amendments to the FLAC Articles of Association and approval of major corporate transactions, including the Business Combination. Accordingly, the FLAC Initial Shareholders will exert significant influence over actions requiring a shareholder vote at least until the completion of a business combination.
The Sponsor and FLAC’s other directors, executive officers, advisors and their affiliates may elect to purchase shares from FLAC public shareholders, which may influence a vote on the Business Combination and reduce the public “float” of FLAC’s Class A Ordinary Shares or FLAC Public Warrants.
The Sponsor and FLAC’s other directors, executive officers, advisors or their affiliates may purchase FLAC Class A Ordinary Shares or FLAC Public Warrants in privately negotiated transactions or in the open market prior to the completion of the Business Combination, although they are under no obligation to do so. However, other than as expressly stated herein, they have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds in the Trust Account will be used to purchase public shares or warrants in such transactions. Such a purchase may include a contractual acknowledgement that such shareholder, although still the record holder of the FLAC Class A Ordinary Shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights.
In the event that the Sponsor or FLAC’s other directors, executive officers, advisors or their affiliates purchase shares in privately negotiated transactions from public shareholders who have already elected to
135
Table of Contents
exercise their redemption rights, such selling shareholders would be required to revoke their prior elections to redeem their shares. The purpose of any such transaction could be to (1) vote in favor of the Business Combination and thereby increase the likelihood of obtaining shareholder approval of the Business Combination, (2) reduce the number of public warrants outstanding or vote such warrants on any matters submitted to the warrant holders for approval in connection with the Business Combination or (3) satisfy a closing condition in an agreement with a partner that requires us to have a minimum net worth or a certain amount of cash at the closing of the Business Combination, where it appears that such requirement would otherwise not be met. Any such purchases of our securities may result in the completion of the Business Combination that may not otherwise have been possible. In addition, if such purchases are made, the public “float” of the FLAC Class A Ordinary Shares or FLAC Public Warrants may be reduced and the number of beneficial holders of FLAC Public Shares may be reduced, which may make it difficult to maintain or obtain the quotation, listing or trading of our securities on a national securities exchange. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent such purchasers are subject to such reporting requirements.
You will not have any rights or interests in funds from the Trust Account, except under certain limited circumstances. To liquidate your investment, therefore, you may be forced to sell your FLAC Public Units, FLAC Class A Ordinary Shares or FLAC Public Warrants, potentially at a loss.
FLAC’s public shareholders are entitled to receive funds from the Trust Account only upon the earlier to occur of: (i) the completion of an initial business combination, and then only in connection with those FLAC Class A Ordinary Shares that such shareholder properly elected to redeem, subject to the limitations described herein, (ii) the redemption of any public shares properly tendered in connection with a shareholder vote to amend and the FLAC Articles of Association to modify the substance or timing of FLAC’s obligation to redeem 100% of FLAC Public Shares if FLAC does not consummate an initial business combination by December 11, 2022 and (iii) the redemption of FLAC Public Shares if FLAC is unable to consummate an initial business by December 11, 2022, subject to applicable law and as further described herein. In no other circumstances will a public shareholder have any right or interest of any kind in the Trust Account. Holders of FLAC Public Warrants will not have any right to the proceeds held in the Trust Account with respect to the FLAC Public Warrants. Accordingly, to liquidate your investment, you may be forced to sell your FLAC Public Units, FLAC Class A Ordinary Shares or FLAC Public Warrants, potentially at a loss.
If FLAC is unable to complete the Business Combination or another business combination by December 11, 2022 (or such later date as may be approved by FLAC’s shareholders), FLAC will cease all operations except for the purpose of winding up, redeeming 100% of the outstanding public shares for cash and, subject to the approval of its remaining shareholders and its board of directors, dissolving and liquidating. In such event, third parties may bring claims against FLAC and, as a result, the proceeds held in its trust account could be reduced and the per-share liquidation price received by shareholders could be less than $10.00 per public share.
If FLAC is unable to complete the Business Combination, it may not be able to find a suitable partner business and consummate a business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders. FLAC’s ability to complete its business combination may be negatively impacted by general market conditions, volatility in the capital and debt markets and the other risks described herein. For example, the COVID-19 pandemic and the conflict in Ukraine may negatively impact businesses FLAC may seek to acquire, including as a result of global supply chain issues, increased market volatility, decreased market liquidity and third-party financing being unavailable on acceptable terms. If FLAC has not consummated a business combination by December 11, 2022, absent any extension, FLAC will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account, including interest earned on the funds held in the trust account and not previously released to us to pay our income taxes, if any (less up to $100,000 of interest to pay dissolution expenses), divided by the number of the then- outstanding public shares, which redemption will completely extinguish public shareholders’
136
Table of Contents
rights as shareholders (including the right to receive further liquidation distributions, if any), and (iii) as promptly as reasonably possible following such redemption, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, liquidate and dissolve, subject in the case of clauses (ii) and (iii), to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. The FLAC Articles of Association provides that, if FLAC winds up for any other reason prior to the consummation of a business combination, FLAC will follow the foregoing procedures with respect to the liquidation of the Trust Account as promptly as reasonably possible but not more than ten business days thereafter, subject to applicable Cayman Islands law. In either such case, FLAC public shareholders may receive only $10.00 per public share, or less than $10.00 per public share, on the redemption of their shares, and our warrants will expire worthless.
FLAC’s proximity to its liquidation date expresses substantial doubt about its ability to continue as a “going concern.”
In connection with FLAC’s assessment of going concern considerations in accordance with the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” FLAC’s management has determined that mandatory liquidation and subsequent dissolution raises substantial doubt about FLAC’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should FLAC be required to liquidate after December 11, 2022. The financial statements do not include any adjustment that might be necessary if FLAC is unable to continue as a going concern.
If the Business Combination is not completed, new potential target businesses may have leverage over FLAC in negotiating a business combination and FLAC’s ability to conduct due diligence on a business combination as it approaches its dissolution deadline may decrease, which could undermine FLAC’s ability to complete a business combination on terms that would produce value for FLAC’s shareholders.
If FLAC is unable to complete this Business Combination, any new potential target business with which FLAC enters into negotiations concerning a business combination will be aware that FLAC must complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders. Consequently, a potential target may obtain leverage over FLAC in negotiating a business combination, knowing that FLAC may be unable to complete a business combination with another target business by December 11, 2022, absent any extension. This risk will increase as FLAC gets closer to the timeframe described above. In addition, FLAC may have limited time to conduct due diligence and may enter into a business combination on terms that FLAC would have rejected upon a more comprehensive investigation.
Because of FLAC’s limited resources and the significant competition for business combination opportunities, if this Business Combination is not completed, it may be more difficult for FLAC to complete an initial business combination. In addition, resources could be wasted in researching acquisitions that are not completed, which could materially adversely affect subsequent attempts to locate and acquire or merge with another business. If FLAC is unable to complete an initial business combination by December 11, 2022, FLAC’s public shareholders may receive only approximately $10.00 per share, on the liquidation of the Trust Account (or less than $10.00 per share in certain circumstances where a third party brings a claim against FLAC that the Sponsor is unable to indemnify), and the FLAC Public Warrants will expire worthless.
If FLAC is unable to complete this Business Combination, FLAC would expect to encounter intense competition from other entities having a business objective similar to its business objective, including private investors (which may be individuals or investment partnerships), other blank check companies and other entities, domestic and international, competing for the types of businesses FLAC could acquire. Many of these individuals and entities are well-established and have extensive experience in identifying and effecting, directly or indirectly, acquisitions of companies operating in or providing services to various industries. Many of these competitors possess greater technical, human and other resources or more local industry knowledge than FLAC does and FLAC’s financial resources will be relatively limited when contrasted with those of many of these competitors. While FLAC believes there are numerous target businesses
137
Table of Contents
FLAC could potentially acquire with the net proceeds of the FLAC IPO and the sale of the FLAC Private Placement Units, FLAC’s ability to compete with respect to the acquisition of certain target businesses that are sizable will be limited by FLAC’s available financial resources. This inherent competitive limitation may give others an advantage in pursuing the acquisition of certain target businesses.
FLAC anticipates that, if FLAC is unable to complete this Business Combination, the investigation of other specific target business and the negotiation, drafting and execution of relevant agreements, disclosure documents and other instruments will require substantial management time and attention and substantial costs for accountants, attorneys and others. If FLAC decides not to complete a specific business combination, the costs incurred up to that point for the proposed transaction likely would not be recoverable. Furthermore, if FLAC reaches an agreement relating to a specific target business, FLAC may fail to complete such business combination (including the Business Combination described in this proxy statement/prospectus) for any number of reasons including those beyond FLAC’s control. Any such event will result in a loss to FLAC of the related costs incurred which could materially adversely affect subsequent attempts to locate and acquire or merge with another business.
If FLAC does not complete this Business Combination and is unable to complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders, FLAC’s public shareholders may receive only approximately $10.00 per share on the liquidation of the Trust Account (or less than $10.00 per share in certain circumstances where a third party brings a claim against FLAC that the Sponsor is unable to indemnify) and the FLAC Public Warrants will expire worthless.
The FLAC Warrants are accounted for as a liability, which could have an adverse effect on the future market price of the FLAC Class A Ordinary Shares and FLAC Warrants.
Upon the consummation of the FLAC IPO and the concurrent sale of the FLAC Private Placement Warrants on December 11, 2020, FLAC issued an aggregate of 4,767,000 warrants, comprising the 4,600,000 warrants included in the units and the 167,000 private placement warrants, including the underwriters’ over-allotment option which was exercised in full. FLAC is accounting for its warrants as a liability and is recording the warrants at fair value upon issuance. FLAC is required to determine the fair value of its warrants at the end of each quarter and any changes in fair value are reflected on FLAC’s statement of operations and balance sheet. Consequently, any increase in the fair value of the warrant liability will have an adverse effect on FLAC’s net income and shareholders’ equity, which could in turn have an adverse effect on the market price of FLAC’s Class A Ordinary Shares and warrants, or the market price of our Class A Ordinary Shares and warrants of any successor following the Business Combination.
FLAC identified a material weakness in its internal control over financial reporting, which was subsequently remediated. If FLAC fails to maintain an effective system of internal controls, the accuracy and timing of its financial reporting may be adversely affected.
FLAC’s management is responsible for establishing and maintaining adequate internal control over financial reporting designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. FLAC’s management is likewise required, on a quarterly basis, to evaluate the effectiveness of our internal controls and to disclose any changes and material weaknesses identified through such evaluation of those internal controls. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of FLAC’s annual or interim financial statements will not be prevented or detected on a timely basis.
FLAC identified a material weakness in its internal control over financial reporting related to the accounting for certain complex financial instruments related to the improper classification of the FLAC Ordinary Shares subject to possible redemption at the closing of the FLAC IPO and the restatement of its earnings per share calculation. As a result of this material weakness, FLAC’s management concluded that our internal control over financial reporting was not effective as of March 31, 2022. This material weakness resulted in a material
138
Table of Contents
misstatement of the initial carrying value of FLAC’s Ordinary Shares subject to possible redemption and the restatement of FLAC’s earnings per share calculation for the affected periods. FLAC determined that it was appropriate to restate its previously issued financial statements included in its Annual Report on Form 10-K for the period ended December 31, 2021 and interim financial statements included in its Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2021, June 30, 2021 and September 30, 2021.
To respond to this material weakness, FLAC has devoted, and plans to continue to devote, significant effort and resources to the remediation and improvement of its internal control over financial reporting. While FLAC has processes to identify and appropriately apply applicable accounting requirements, FLAC plans to enhance these processes to better evaluate its research and understanding of the nuances of the complex accounting standards that apply to its financial statements. The elements of FLAC’s remediation plan can only be accomplished over time, and FLAC can offer no assurance that these initiatives will ultimately have the intended effects. The foregoing actions, which FLAC believes remediated the material weakness in internal control over financial reporting, were completed as of the date of June 30, 2022.
Any failure to maintain such internal control could adversely impact FLAC’s ability to report its financial position and results from operations on a timely and accurate basis. If FLAC’s financial statements are not accurate, investors may not have a complete understanding of FLAC’s operations. Likewise, if FLAC’s financial statements are not filed on a timely basis, FLAC could be subject to sanctions or investigations by the stock exchange on which FLAC’s ordinary shares is listed, the SEC or other regulatory authorities. In either case, there could result a material adverse effect on FLAC. Ineffective internal controls could also cause investors to lose confidence in FLAC’s reported financial information, which could have a negative effect on the trading price of our stock.
FLAC can give no assurance that any additional material weaknesses will not arise in the future due to a failure to implement and maintain adequate internal control over financial reporting or circumvention of these controls. In addition, even if FLAC is successful in strengthening its controls and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors or to facilitate the fair presentation of FLAC’s financial statements.
If third parties bring claims against FLAC, the proceeds held in the Trust Account could be reduced and the per-share redemption amount received by shareholders may be less than $10.00 per share.
FLAC’s placing of funds in the Trust Account may not protect those funds from third-party claims against FLAC. Although FLAC will seek to have all vendors, service providers (other than FLAC’s independent registered public accounting firm), prospective partner businesses or other entities with which FLAC does business execute agreements with FLAC waiving any right, title, interest or claim of any kind in or to any monies held in the Trust Account for the benefit of FLAC’s public shareholders, such parties may not execute such agreements, or even if they execute such agreements, they may not be prevented from bringing claims against the Trust Account, including, but not limited to, fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain advantage with respect to a claim against FLAC’s assets, including the funds held in the Trust Account. If any third party refuses to execute an agreement waiving such claims to the monies held in the Trust Account, FLAC’s management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be significantly more beneficial to FLAC than any alternative.
Examples of possible instances where FLAC may engage a third party that refuses to execute a waiver include the engagement of a third-party consultant whose particular expertise or skills are believed by FLAC’s management to be significantly superior to those of other consultants that would agree to execute a waiver or in cases where FLAC’s management is unable to find a service provider willing to execute a waiver. In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with FLAC and will not seek recourse against the Trust Account for any reason. Upon redemption of FLAC’s public shares, if FLAC is unable to complete a
139
Table of Contents
business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders, or upon the exercise of a redemption right in connection with the Business Combination, FLAC will be required to provide for payment of claims of creditors that were not waived that may be brought against FLAC within the ten years following redemption. Accordingly, the per-share redemption amount received by FLAC’s public shareholders could be less than the $10.00 per share initially held in the Trust Account, due to claims of such creditors.
The Sponsor has agreed that it will be liable to FLAC if and to the extent any claims by a third party (excluding its independent registered public accounting firm) for services rendered or products sold to FLAC, or a prospective partner business with which FLAC has discussed entering into a transaction agreement, reduce the amounts in the Trust Account to below the lesser of (i) $10.00 per public share and (ii) the actual amount per share held in the Trust Account as of the date of the liquidation of the Trust Account if less than $10.00 per public share due to reductions in the value of the trust assets, in each case net of the interest that may be withdrawn to pay our tax obligations (up to $100,000), provided that such liability will not apply to any claims by a third party or prospective partner business who executed a waiver of any and all rights to seek access to the Trust Account nor will it apply to any claims under our indemnity of the underwriters of the FLAC IPO against certain liabilities, including liabilities under the Securities Act. Moreover, in the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. However, FLAC has not asked the Sponsor to reserve for such indemnification obligations, nor has FLAC independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations and FLAC believe that the Sponsor’s only assets are securities of FLAC. The Sponsor may not be able to satisfy those obligations. None of FLAC’s officers or directors will indemnify FLAC for claims by third parties including, without limitation, claims by vendors and prospective partner businesses. As a result, if any such claims were successfully made against the Trust Account, the funds available for a business combination and redemptions could be reduced to less than $10.00 per public share. In such event, FLAC may not be able to complete a business combination, and FLAC shareholders would receive such lesser amount per share in connection with any redemption of public shares.
FLAC’s directors may decide not to enforce the indemnification obligations of the Sponsor, resulting in a reduction in the amount of funds in the Trust Account available for distribution to FLAC’s public shareholders.
In the event that the proceeds in the Trust Account are reduced below the lesser of (i) $10.00 per share or (ii) the actual amount per share held in the Trust Account as of the date of the liquidation of the Trust Account if less than $10.00 per public share due to reductions in the value of the trust assets, in each case net of the interest which may be withdrawn to pay taxes, and the Sponsor asserts that it is unable to satisfy its obligations or that it has no indemnification obligations related to a particular claim, FLAC’s independent directors would determine whether to take legal action against the Sponsor to enforce its indemnification obligations. While FLAC currently expects that its independent directors would take legal action on its behalf against the Sponsor to enforce its indemnification obligations to FLAC, it is possible that FLAC’s independent directors in exercising their business judgment may choose not to do so in any particular instance. If FLAC’s independent directors choose not to enforce these indemnification obligations, the amount of funds in the Trust Account available for distribution to FLAC’s public shareholders may be reduced below $10.00 per share.
If, before distributing the proceeds in the Trust Account to its public shareholders, FLAC files a bankruptcy or insolvency petition or an involuntary bankruptcy or insolvency petition is filed against FLAC that is not dismissed, the claims of creditors in such proceeding may have priority over the claims of its shareholders and the per-share amount that would otherwise be received by its shareholders in connection with its liquidation may be reduced.
If, before distributing the proceeds in the Trust Account to its public shareholders, FLAC files a bankruptcy or insolvency petition or an involuntary bankruptcy or insolvency petition is filed against FLAC that is not dismissed, the proceeds held in the Trust Account could be subject to applicable bankruptcy law, and may be
140
Table of Contents
included in FLAC’s bankruptcy estate and subject to the claims of third parties with priority over the claims of FLAC’s shareholders. To the extent any bankruptcy claims deplete the Trust Account, the per-share amount that would otherwise be received by FLAC shareholders in connection with its liquidation may be reduced.
If, after FLAC distributes the proceeds in the Trust Account to its public shareholders, FLAC files a bankruptcy petition or an involuntary bankruptcy petition is filed against FLAC that is not dismissed, a bankruptcy court may seek to recover such proceeds, and the members of the FLAC Board may be viewed as having breached their fiduciary duties to FLAC’s creditors, thereby exposing the members of the FLAC Board and FLAC to claims of punitive damages.
If, after FLAC distributes the proceeds in the Trust Account to its public shareholders, FLAC files a bankruptcy petition, an involuntary bankruptcy petition is filed against FLAC that is not dismissed or enters into an insolvent liquidation, any distributions received by shareholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court or liquidator could seek to recover all amounts received by FLAC’s shareholders. In addition, the FLAC Board may be viewed as having breached its fiduciary duty to FLAC’s creditors and/or having acted in bad faith, thereby exposing itself and FLAC to claims of punitive damages, by paying public shareholders from the Trust Account prior to addressing the claims of creditors. Furthermore, FLAC’s directors may be viewed as having breached their fiduciary duties to FLAC or its creditors and/or may have acted in bad faith, thereby exposing themselves and FLAC to claims, by paying public shareholders from the Trust Account prior to addressing the claims of creditors. Claims may be brought against FLAC for these reasons. FLAC and its directors and officers who knowingly and willfully authorized or permitted any distribution to be paid out of FLAC’s share premium account while it was unable to pay its debts as they fall due in the ordinary course of business would be guilty of an offense and may be liable for a fine of $18,292.68 and imprisonment for five years in the Cayman Islands.
Were FLAC considered to be a “foreign person,” it might not be able to complete an initial business combination with a U.S. target company if such initial business combination is subject to U.S. foreign investment regulations and review by a U.S. government entity such as the Committee on Foreign Investment in the United States (“CFIUS”), or ultimately prohibited.
Certain federally licensed businesses in the United States, such as broadcasters and airlines, may be subject to rules or regulations that limit foreign ownership. In addition, CFIUS is an interagency committee authorized to review certain transactions involving foreign investment in the United States by foreign persons in order to determine the effect of such transactions on the national security of the United States. Were FLAC considered to be a “foreign person” under such rules and regulations, any proposed business combination between FLAC and a U.S. business engaged in a regulated industry or which may affect national security could be subject to such foreign ownership restrictions and/or CFIUS review. The scope of CFIUS was expanded by the Foreign Investment Risk Review Modernization Act of 2018 (“FIRRMA”) to include certain non-controlling investments in sensitive U.S. businesses and certain acquisitions of real estate even with no underlying U.S. business. FIRRMA, and subsequent implementing regulations that are now in force, also subject certain categories of investments to mandatory filings. If a potential initial business combination with a U.S. business falls within the scope of foreign ownership restrictions, FLAC may be unable to consummate an initial business combination with such business. In addition, if a potential business combination falls within CFIUS’s jurisdiction, FLAC may be required to make a mandatory filing or determine to submit a voluntary notice to CFIUS, or to proceed with the initial business combination without notifying CFIUS and risk CFIUS intervention, before or after closing the initial business combination. The Sponsor is a U.S. entity, and the managing member of the Sponsor is a U.S. person. The Sponsor is not controlled by and does not have substantial ties with a non-U.S. person. However, if CFIUS has jurisdiction over FLAC’s initial business combination, CFIUS may decide to block or delay such initial business combination, impose conditions to mitigate national security concerns with respect to such initial business combination or order FLAC to divest all or a portion of a U.S. business of the combined company if FLAC had proceeded without first obtaining CFIUS clearance. If FLAC were considered to be a “foreign
141
Table of Contents
person,” foreign ownership limitations, and the potential impact of CFIUS, may limit the attractiveness of a transaction with FLAC or prevent FLAC from pursuing certain initial business combination opportunities that FLAC believes would otherwise be beneficial to FLAC and its shareholders. As a result, in such circumstances, the pool of potential targets with which FLAC could complete an initial business combination could be limited and we may be adversely affected in terms of competing with other SPACs which do not have similar foreign ownership issues.
Moreover, the process of government review, whether by CFIUS or otherwise, could be lengthy. Because FLAC has only a limited time to complete its initial business combination, its failure to obtain any required approvals within the requisite time period may require it to liquidate. If FLAC liquidates, its public shareholders may only receive $10.00 per share, and its warrants will expire worthless. This will also cause you to lose any potential investment opportunity in a target company and the chance of realizing future gains on your investment through any price appreciation in the combined company.
Because FLAC is incorporated under the laws of the Cayman Islands, you may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited.
FLAC is an exempted company incorporated under the laws of the Cayman Islands. As a result, it may be difficult for FLAC public shareholders to effect service of process within the United States upon FLAC’s directors or executive officers, or enforce judgments obtained in the United States courts against FLAC’s directors or officers.
FLAC’s corporate affairs are governed by and the FLAC Articles of Association, the Cayman Islands Companies Act and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of FLAC’s directors to FLAC under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, the decisions of whose courts are of persuasive authority, but are not binding on a court in the Cayman Islands. The rights of FLAC’s shareholders and the fiduciary responsibilities of FLAC’s directors under Cayman Islands law are different from what they would be under statutes or judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a different body of securities laws as compared to the United States, and certain states, such as Delaware, may have more fully developed and judicially interpreted bodies of corporate law. In addition, shareholders of Cayman Islands companies may not have standing to initiate a shareholders derivative action in a federal court of the United States.
Shareholders of Cayman Islands exempted companies like FLAC have no general rights under Cayman Islands law to inspect corporate records or to obtain copies of the register of members of these companies. FLAC’s directors have discretion under the FLAC Articles of Association to determine whether or not, and under what conditions, its corporate records may be inspected by its shareholders, but are not obliged to make them available to its shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
FLAC has been advised by Campbells LLP, its Cayman Islands legal counsel, that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against FLAC judgments of courts of the United States predicated upon the civil liability provisions of the federal securities laws of the United States or any state; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against FLAC predicated upon the civil liability provisions of the federal securities laws of the United States or any state, so far as the liabilities imposed by those provisions are penal in nature. In those circumstances, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to
142
Table of Contents
be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). A Cayman Islands Court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
As a result of all of the above, FLAC public shareholders may have more difficulty in protecting their interests in the face of actions taken by FLAC’s founding team, members of the FLAC Board or controlling shareholders than they would as public shareholders of a United States company.
FLAC shareholders may have limited remedies if their shares suffer a reduction in value following the Business Combination.
Any shareholders who choose to remain shareholders following a business combination could suffer a reduction in the value of their shares. Such shareholders are unlikely to have a remedy for such reduction in value, unless they are able to successfully claim that the reduction was due to the breach by FLAC’s officers or directors of a duty of care or other fiduciary duty owed to them, or if they are able to successfully bring a private claim under securities laws that the proxy statement relating to a business combination contained an actionable material misstatement or material omission.
Risks Related to the Business Combination
The consummation of the Business Combination is subject to a number of conditions, and if those conditions are not satisfied or waived, the Business Combination may not be completed.
The Business Combination is subject to a number of conditions, including the expiration of the waiting period under, or obtaining any consent pursuant to any applicable Antitrust Laws. FLAC and NewAmsterdam Pharma believe that no such waiting periods or consents under applicable Antitrust Laws apply with respect to or are required to be obtained by FLAC or NewAmsterdam Pharma. However, there are no assurances that all conditions to the Business Combination will be satisfied or that the conditions will be satisfied in the time frame expected.
If the conditions to the Business Combination are not met (and are not waived, to the extent waivable), either FLAC or NewAmsterdam Pharma may, subject to the terms and conditions of the Business Combination Agreement, terminate the Business Combination Agreement. See the section of this proxy statement/prospectus titled “The Business Combination Agreement and Ancillary Documents—Termination.”
The FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from, or are in addition to, those of other FLAC shareholders in recommending that FLAC shareholders vote in favor of approval of the Business Combination Proposal and approval of the other proposals described in this proxy statement/prospectus.
When considering the FLAC Board’s recommendation that FLAC shareholders vote in favor of the approval of the Business Combination Proposal, FLAC shareholders should be aware that aside from their interests as shareholders, the FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from, or in addition to, those of other FLAC shareholders generally. These interests include, among other things, the interests listed below:
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed not to redeem any Founder Shares or FLAC Public Shares held by them in connection with a shareholder vote to approve a proposed initial business combination; |
| • | the Sponsor paid an aggregate of $25,000 for 2,875,000 Founder Shares. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, |
143
Table of Contents
Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. Such securities will have a significantly higher value at the time of the Business Combination which, if unrestricted and freely tradable, such Founder Shares would be valued at approximately $34,051,500 (based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022), but, given the transfer restrictions on such shares, FLAC believes such shares have less value. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then FLAC will cease all operations except for the purpose of winding up, redeeming all of the public shares for cash and, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceeding to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In such event, Founder Shares collectively owned by the FLAC Initial Shareholders would be worthless because following the redemption of the public shares, FLAC would likely have few, if any, net assets; |
| • | the Sponsor paid an aggregate of $5,010,000 for its 501,000 FLAC Private Placement Units (with an aggregate fair market value of $4,944,870, based on the closing price of FLAC Public Units on June 30, 2022) and that the component FLAC Private Placement Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, paid an aggregate of $10 million for its 1,000,000 FLAC Public Units in the FLAC IPO (with an aggregate fair market value of $9,870,000, based on the closing price of FLAC Public Units on June 30, 2022), and has agreed to waive any redemption rights, including with respect to the FLAC Class A Ordinary Shares underlying such FLAC Public Units purchased in the FLAC IPO, in connection with the Business Combination. In addition, the component FLAC Public Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, and Frazier Life Sciences XI, L.P., Frazier Life Sciences Public Fund, L.P. and Frazier Life Sciences Overage Fund, L.P., each an affiliate of Frazier, an affiliate of the Sponsor, have committed to purchasing 4,500,000 Holdco Shares at a price per share of $10.00 as part of the PIPE Financing and have agreed to waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination; |
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed to waive their rights to liquidating distributions from the Trust Account with respect to any Founder Shares held by them if FLAC fails to complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | the Investor Rights Agreement will be entered into by the FLAC Initial Shareholders; |
| • | the Holdco Shares to be received by the FLAC Initial Shareholders in connection with the Merger will be subject to certain lock-up periods; |
| • | the continued indemnification of FLAC’s existing directors and officers and the continuation of FLAC’s directors’ and officers’ liability insurance after the Business Combination; |
| • | the Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to shareholders rather than liquidate; |
| • | the Sponsor and its affiliates can earn a positive rate of return on their investment, even if other FLAC shareholders experience a negative rate of return in the post-business combination company; |
144
Table of Contents
| • | FLAC has the right to select two individuals, one of which is expected to be Jamie Topper, to be nominated for election to the initial Holdco Board, who must be reasonably acceptable to NewAmsterdam Pharma and qualify as “independent” directors for purposes of Nasdaq rules; |
| • | the fact that the Sponsor, FLAC’s officers and directors, and their respective affiliates will lose their entire investment in FLAC (which is estimated to be approximately $34.5 million, based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022) and will not be reimbursed for any out-of-pocket expenses (which are currently $0) if an initial business combination is not consummated by December 11, 2022, absent any extension; |
| • | the potential hire of David Topper, the Chief Financial Officer and a director and shareholder of FLAC, as the chief financial officer of Holdco in the first half of 2023; |
| • | the Sponsor (including its representatives and affiliates) and FLAC’s officers and directors, are, or may in the future become, affiliated with entities that are engaged in a similar business to Holdco and/or NewAmsterdam Pharma. The representatives and affiliates of the Sponsor, and certain of FLAC’s officers and directors, are in the business of making investments in companies, and may acquire and hold interests in businesses that compete directly or indirectly with Holdco and/or NewAmsterdam Pharma. Certain of FLAC’s officers and directors also have time and attention requirements for investment funds of which they and affiliates of the Sponsor are the investment managers. The Sponsor and FLAC’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to FLAC completing its initial business combination; and |
| • | if the Trust Account is liquidated, including in the event FLAC is unable to complete an initial business combination within the required time period, the Sponsor has agreed to indemnify FLAC to ensure that the proceeds in the Trust Account are not reduced below $10.00 per FLAC Public Share, or such lesser per public share amount as is in the Trust Account on the liquidation date, by the claims of prospective target businesses with which FLAC has entered into an acquisition agreement or claims of any third party for services rendered or products sold to FLAC, but only if such a vendor or target business has not executed a waiver of any and all rights to seek access to the Trust Account. |
In addition, in connection with the execution of the Business Combination Agreement, FLAC, NewAmsterdam Pharma and Holdco entered into the Sponsor Support Agreement with the FLAC Initial Shareholders, pursuant to which the FLAC Initial Shareholders have agreed to vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions. Currently, the FLAC Initial Shareholders collectively own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares.
See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of additional considerations in connection with the Business Combination.
The exercise of discretion by FLAC’s or NewAmsterdam Pharma’s directors and officers in agreeing to changes to the terms of or waivers of closing conditions in the Business Combination Agreement may result in a conflict of interest when determining whether such changes to the terms of the Business Combination Agreement or waivers of conditions are appropriate and in the best interests of the public shareholders of FLAC or the shareholders of Holdco.
In the period leading up to the closing of the Business Combination, other events may occur that, pursuant to the Business Combination Agreement, would require FLAC or NewAmsterdam Pharma to agree to amend the Business Combination Agreement, to consent to certain actions or to waive rights that FLAC or NewAmsterdam Pharma is entitled to under those agreements. Such events could arise because of changes in the course of NewAmsterdam Pharma’s business, a request by the FLAC shareholders, FLAC, NewAmsterdam Pharma shareholders or NewAmsterdam Pharma to undertake actions that would otherwise be prohibited by the terms of
145
Table of Contents
the Business Combination Agreement or the occurrence of other events that would have a material adverse effect on FLAC or NewAmsterdam Pharma’s business and would entitle FLAC or NewAmsterdam Pharma to terminate the Business Combination Agreement. In any of such circumstances, it would be in the discretion of FLAC or NewAmsterdam Pharma, acting through their respective boards, to grant its consent or waive its rights. The existence of the financial and personal interests of FLAC’s and NewAmsterdam Pharma’s directors described elsewhere in this proxy statement/prospectus may result in a conflict of interest on the part of one or more of the directors between what he or she may believe is best for FLAC and the public shareholders of FLAC, or in the case of NewAmsterdam Pharma, NewAmsterdam Pharma’s business having considered the interests of its stakeholders, and what he or she may believe is best for himself or herself or his or her affiliates in determining whether or not to take the requested action. As of the date of this proxy statement/prospectus, neither FLAC nor NewAmsterdam Pharma believes there will be any changes or waivers that their respective directors and officers would be likely to make after shareholder approval of the Business Combination has been obtained. While certain changes could be made without further shareholder approval, if there is a change to the terms of the Business Combination that would have a material impact on the shareholders, FLAC will be required to circulate a new or amended proxy statement/prospectus or supplement thereto and resolicit the vote of the FLAC public shareholders with respect to the Business Combination Proposal.
The unaudited pro forma condensed combined financial information included in this proxy statement/prospectus is illustrative and the actual financial condition and results of operations after the Business Combination may differ materially.
The unaudited pro forma financial information included in this proxy statement/prospectus is presented for illustrative purposes only and is not necessarily indicative of what Holdco’s actual financial position or results of operations would have been had the Business Combination been completed on the date(s) indicated. The preparation of the pro forma financial information is based upon available information and certain assumptions and estimates that FLAC and NewAmsterdam Pharma currently believe are reasonable and does not constitute any representation, estimate or projection of any party. The unaudited pro forma condensed combined information does not purport to indicate the results that would have been obtained had the Business Combination and related transactions actually been completed on the assumed date or for the periods presented, or which may be realized in the future. The pro forma adjustments are based on the information currently available and the assumptions and estimates underlying the pro forma adjustments are described in the accompanying notes. Actual results may differ materially from the assumptions within the accompanying unaudited pro forma condensed combined financial information.
SVB Securities LLC has multiple roles in the Business Combination, which give rise to potential conflicts of interest if the Business Combination is consummated.
SVB Securities LLC (“SVB Securities”) has been engaged by FLAC as a placement agent in connection with the PIPE Financing and by NewAmsterdam Pharma to serve as its financial and capital markets advisor in connection with the Business Combination. Any fees paid to SVB Securities by NewAmsterdam Pharma for its role as financial and capital markets advisor will be reduced by any fees received by SVB Securities in connection with its role as placement agent for FLAC. Because SVB Securities will receive its placement agent fee upon the closing of the PIPE Financing from FLAC and its financial advisor fee from NewAmsterdam Pharma upon the Closing, investors should be aware of the potential conflicts of interest owing to SVB Securities’ multiple roles in the Business Combination.
FLAC and NewAmsterdam Pharma will incur significant transaction and transition costs in connection with the Business Combination.
FLAC and NewAmsterdam Pharma have both incurred and expect to incur significant, non-recurring costs in connection with consummating the Business Combination and operating as a public company following the consummation of the Business Combination. FLAC and NewAmsterdam Pharma may also incur additional costs to
146
Table of Contents
retain key employees. Except as set forth in the Business Combination Agreement, all expenses incurred in connection with the Business Combination Agreement and the Transactions (including the Business Combination), including all legal, accounting, financial advisors and other fees, expenses and costs, will be for the account of the party incurring such fees, expenses and costs. However, in the event that the Business Combination is completed, FLAC will pay, or cause to be paid, remaining NewAmsterdam Pharma and FLAC expenses.
FLAC’s transaction expenses as a result of the Business Combination are currently estimated at approximately $20 million, excluding approximately $4.8 million in deferred underwriting commissions to the underwriters of the FLAC IPO. As a public company, Holdco will incur significant increased costs and its management team, who has limited experience overseeing a public operating company, will be required to devote substantial time to new compliance initiatives.
During the pre-closing period, NewAmsterdam Pharma and FLAC are prohibited from entering into certain transactions that might otherwise be beneficial to NewAmsterdam Pharma, FLAC or their respective shareholders.
Until the earlier of the consummation of the Business Combination or termination of the Business Combination Agreement, NewAmsterdam Pharma and FLAC are subject to certain limitations on the operations of their businesses, each as summarized under the section entitled “The Business Combination—Covenants of the Parties.” The limitations on NewAmsterdam Pharma’s and FLAC’s conduct of their businesses during this period could have the effect of delaying or preventing other strategic transactions and may, in some cases, make it impossible to pursue business opportunities that are available only for a limited time.
The Sponsor and FLAC’s officers and directors own shares of FLAC that will be worthless and may incur reimbursable expenses that may not be reimbursed or repaid if the Business Combination is not approved. Such interests may influence their decision to approve and, in the case of the FLAC Board, recommend, the Business Combination.
FLAC’s officers and directors and/or their affiliates beneficially own shares and units that they purchased in connection with the FLAC IPO. FLAC’s executive officers, directors and their affiliates have no redemption rights with respect to these securities in the event a business combination is not effected by December 11, 2022 or such later date as may be approved by FLAC’s shareholders. Therefore, if the Business Combination or another business combination is not consummated within the required time period, such securities will be worthless. Additionally, FLAC’s officers, directors and their affiliates are entitled to reimbursement of out-of-pocket expenses incurred by them in connection with certain activities on FLAC’s behalf, such as identifying and investigating possible business targets and business combinations. These expenses will be repaid upon completion of the Business Combination. However, if FLAC fails to consummate a business combination, they will not have any claim against the Trust Account for reimbursement. Accordingly, FLAC may not be able to reimburse these expenses if the transactions are not completed.
As of June 30, 2022, FLAC’s officers, directors and their affiliates had incurred no reimbursable expenses, but may incur expenses prior to the General Meeting.
These financial interests may have influenced the decision of FLAC’s directors and officers to approve the Business Combination and to continue to pursue such Business Combination. In considering the recommendations of FLAC’s Board to vote for the Business Combination Proposal, the Merger Proposal and other proposals, its shareholders should consider these interests.
Potential legal proceedings in connection with the Business Combination, the outcomes of which are uncertain, could delay or prevent the completion of the Business Combination, which litigation risk may be increased because certain directors of FLAC are affiliated with us.
In connection with a proposed business combination, it is not uncommon for lawsuits to be filed against companies involved and/or their respective directors and officers alleging, among other things, that this proxy
147
Table of Contents
statement/prospectus contains false and misleading statements and/or omits material information concerning the business combination. It is possible that one or more additional legal actions may arise in connection with the Business Combination and defending such lawsuits could require us to incur significant costs and draw the attention of our management team away from a proposed business combination. Further, the defense or settlement of any lawsuit or claim that remains unresolved at the time the Business Combination is consummated may adversely affect Holdco’s business, financial condition, results of operations and cash flows. Such legal proceedings could delay or prevent the Business Combination from becoming effective within an agreed upon timeframe.
The ability to successfully effect the Business Combination and Holdco’s ability to successfully operate the business thereafter will be largely dependent upon the efforts of certain key personnel of Holdco. The loss of such key personnel could negatively impact the operations and financial results of Holdco.
The ability to successfully effect the Business Combination is dependent upon the efforts of FLAC and NewAmsterdam Pharma’s key personnel. Some of FLAC’s key personnel may remain with Holdco in senior management or advisory positions following the Business Combination and it is likely that some or all of the management of the NewAmsterdam Pharma will remain in place. While Holdco intends to closely scrutinize any individuals that it engages after the Business Combination, we cannot assure you that Holdco’s assessment of these individuals will prove to be correct. These individuals may be unfamiliar with the requirements of operating a company regulated by the SEC, which could cause us to have to expend time and resources helping them become familiar with such requirements.
A departure of Holdco’s key personnel could negatively impact the operations and profitability of the post-combination business. The role of Holdco’s key personnel upon the completion of the Business Combination cannot be ascertained at this time.
Although we contemplate that certain members of NewAmsterdam Pharma’s management team will remain associated with the NewAmsterdam Pharma following the Business Combination, it is possible that members of the management of NewAmsterdam Pharma will not wish to remain in place. The loss of key personnel could negatively impact the operations and profitability Holdco.
FLAC may be forced to close the Business Combination even if FLAC determines it is no longer in its shareholders’ best interest.
FLAC’s public shareholders are protected from a material adverse event of NewAmsterdam Pharma arising between the date of the Business Combination Agreement and the Closing primarily by the right to redeem their public shares for a pro rata portion of the funds held in the Trust Account, calculated as of two business days prior to the consummation of the Business Combination.
FLAC is also restricted from seeking, soliciting, negotiating or consummating any alternative business combination while the Business Combination Agreement is still in effect. If the Business Combination’s benefits do not meet the expectations of investors or securities analysts, the market price of FLAC’s securities or, following the consummation of the Business Combination, the Holdco Shares, may decline but we may still be forced to close the Business Combination.
Since the Sponsor, executive officers and directors of FLAC will lose their entire investment in FLAC if FLAC’s initial business combination is not completed, a conflict of interest may arise in determining whether a particular business combination target is appropriate for FLAC’s initial business combination.
On October 7, 2020, the Sponsor purchased the Founder Shares for a purchase price of $25,000. Prior to the initial investment in FLAC of $25,000 by the Sponsor, FLAC had no assets, tangible or intangible. The Founder Shares will be worthless if FLAC does not complete an initial business combination. In addition, the Sponsor also purchased 501,000 Private Placement Units at a price of $10.00 per Private Placement Unit that will also be worthless if FLAC does not complete the Business Combination. The personal and financial interests of FLAC’s
148
Table of Contents
executive officers and directors may influence their motivation in identifying and selecting a target business combination, completing an initial business combination and influencing the operation of the business following the Business Combination.
Neither the Group Companies and their shareholders nor FLAC and its shareholders will have the protection of any indemnification, escrow, price adjustment or other provisions that allow for a post-closing adjustment to be made to the total Business Combination consideration in the event that any of the representations and warranties made by either party in the Business Combination Agreement ultimately proves to be materially inaccurate or incorrect.
The representations and warranties made by the Group Companies and FLAC to each other in the Business Combination Agreement will not survive the consummation of the Business Combination. As a result, the Group Companies and their shareholders and FLAC and its shareholders will not have the protection of any indemnification, escrow, price adjustment or other provisions that allow for a post-closing adjustment to be made to the total Business Combination consideration if any representation or warranty made by the Group Companies or FLAC in the Business Combination Agreement proves to be materially inaccurate or incorrect. Accordingly, to the extent such representations or warranties are incorrect, FLAC would have no indemnification claim with respect thereto and its financial condition or results of operations could be adversely affected. Additionally, neither FLAC nor the Group Companies will have any right after the closing of the Business Combination to make damages claims against the other party or its shareholders for breaches of representations, warranties or covenants made by the Group Companies or FLAC in the Business Combination Agreement.
Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect our business, including our ability to negotiate and complete the Business Combination.
We are subject to laws and regulations enacted by national, regional and local governments. In particular, we will be required to comply with certain SEC and other legal requirements. Compliance with, and monitoring of, applicable laws and regulations may be difficult, time consuming and costly. Those laws and regulations and their interpretation and application may also change from time to time and those changes could have a material adverse effect on our business, investments and results of operations. In addition, a failure to comply with applicable laws or regulations, as interpreted and applied, could have a material adverse effect on our business, including our ability to negotiate and complete our initial business combination, and results of operations.
On March 30, 2022, the SEC issued proposed rules that would, among other items, impose additional disclosure requirements in business combination transactions involving special purpose acquisition companies (“SPACs”) and private operating companies; amend the financial statement requirements applicable to business combination transactions involving such companies; update and expand guidance regarding the general use of projections in SEC filings, as well as when projections are disclosed in connection with proposed business combination transactions; increase the potential liability of certain participants in proposed business combination transactions; and impact the extent to which SPACs could become subject to regulation under the Investment Company Act of 1940. These rules, if adopted, whether in the form proposed or in revised form, may materially adversely affect our business, including our ability to negotiate and complete the Business Combination and may increase the costs and time related thereto.
Risks Related to Redemption Rights
The ability of shareholders to exercise redemption rights with respect to a large number of outstanding shares of FLAC Class A Ordinary Shares could increase the probability that the Business Combination would be unsuccessful and that shareholders would have to wait for liquidation to redeem their public shares.
At the time FLAC entered into the Business Combination Agreement, it did not know how many shareholders would exercise their redemption rights, and therefore, it structured the Business Combination based on its expectations as to the number of public shares that will be submitted for redemption. If a larger number of
149
Table of Contents
public shares are submitted for redemption than it initially expected, this could lead to a failure to consummate the Business Combination, a failure to maintain the listing of its securities on Nasdaq or another national securities or a lack of liquidity, which could impair Holdco’s ability to fund its operations and adversely affect its business, financial condition and results of operations.
FLAC does not have a specified maximum redemption threshold. The absence of such a redemption threshold may make it possible for FLAC to complete a business combination with which a substantial majority of its shareholders do not agree.
The FLAC Articles of Association do not provide a specified maximum redemption threshold, except that in no event will FLAC redeem FLAC Public Shares in an amount that would cause its net tangible assets to be less than $5,000,001 (so that FLAC does not then become subject to the SEC’s “penny stock” rules). As a result, FLAC may be able to complete the Business Combination even though a substantial majority of its public shareholders do not agree with the transaction and have redeemed their shares or have entered into privately negotiated agreements to sell their shares to the Sponsor or FLAC’s officers, directors, advisors or any of their affiliates. If the Business Combination is not consummated, FLAC will not redeem any shares, all FLAC Class A Ordinary Shares submitted for redemption will be returned to the holders thereof, and FLAC instead may search for an alternate business combination.
If you or a “group” of shareholders of which you are a part are deemed to hold an aggregate of more than 15% of the FLAC Class A Ordinary Shares included in the Public Units sold in the FLAC IPO, you (or, if a member of such a group, all of the members of such group in the aggregate) will lose the ability to redeem all such shares in excess of 15% of the FLAC Class A Ordinary Shares included in the Public Units sold in the FLAC IPO.
A public shareholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its FLAC Public Shares or, if part of such a group, the group’s FLAC Public Shares, in excess of 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO. In order to determine whether a shareholder is acting in concert or as a group with another shareholder, FLAC will require each public shareholder seeking to exercise redemption rights to certify to FLAC whether such shareholder is acting in concert or as a group with any other shareholder. Such certifications, together with other public information relating to share ownership available to FLAC at that time, such as Schedule 13D, Schedule 13G and Section 16 filings under the Exchange Act, will be the sole basis on which FLAC makes the above-referenced determination. Your inability to redeem any such excess shares will reduce your influence over FLAC’s ability to consummate the Business Combination and you could suffer a material loss on your investment in FLAC if you sell such excess shares in open market transactions. Additionally, you will not receive redemption distributions with respect to such excess shares if FLAC consummates the Business Combination. As a result, you will continue to hold that number of FLAC Public Shares aggregating to more than 15% of the FLAC Class A Ordinary Shares sold in the FLAC IPO and, in order to dispose of such excess shares, would be required to sell your FLAC Class A Ordinary Shares in open market transactions, potentially at a loss. There is no assurance that the value of such excess shares will appreciate over time following the Business Combination or that the market price of the FLAC Class A Ordinary Shares will exceed the per-share redemption price. Notwithstanding the foregoing, shareholders may challenge FLAC’s determination as to whether a shareholder is acting in concert or as a group with another shareholder in a court of competent jurisdiction.
However, FLAC’s shareholders’ ability to vote all of their shares (including such excess shares) for or against the Business Combination is not restricted by this limitation on redemption.
There is no guarantee that a shareholder’s decision to continue to hold their public shares following the Business Combination will put the shareholder in a better future economic position than if they decided to redeem their public shares for a pro rata portion of the proceeds held in the Trust Account, and vice versa.
There is no assurance as to the price at which a FLAC shareholder may be able to sell its FLAC Public Shares in the future following the completion of the Business Combination or any alternative business combination. Certain events following the consummation of any initial business combination, including the
150
Table of Contents
Business Combination, may cause an increase in the share price, and may result in a lower value realized now than a shareholder of FLAC might realize in the future had the shareholder not redeemed its shares. Similarly, if a shareholder does not redeem its shares, the shareholder will bear the risk of ownership of the FLAC Public Shares after the consummation of any initial business combination, and there can be no assurance that a shareholder can sell its shares in the future for a greater amount than the redemption price set forth in this proxy statement/prospectus. A shareholder should consult the shareholder’s tax and/or financial advisor for assistance on how this may affect his, her or its individual situation.
Shareholders of FLAC who wish to redeem their FLAC Public Shares for a pro rata portion of the Trust Account must comply with specific requirements for redemption, which may make it difficult for them to exercise their redemption rights prior to the deadline. If shareholders fail to comply with the redemption requirements specified in this proxy statement/prospectus, they will not be entitled to redeem their FLAC Class A Ordinary Shares for a pro rata portion of the funds held in the Trust Account.
FLAC public shareholders who wish to redeem their FLAC Public Shares for a pro rata portion of the Trust Account must, among other things (i) submit a request in writing and (ii) tender their certificates to the Transfer Agent or deliver their shares to the Transfer Agent electronically through the DWAC system at least two business days prior to the General Meeting. In order to obtain a physical stock certificate, a shareholder’s broker and/or clearing broker, DTC and the Transfer Agent will need to act to facilitate this request. Shareholders should generally allot at least two weeks to obtain physical certificates from the Transfer Agent. However, because FLAC does not have any control over this process or over the brokers it may take significantly longer than two weeks to obtain a physical stock certificate. If it takes longer than anticipated to obtain a physical certificate, shareholders who wish to redeem their shares may be unable to obtain physical certificates by the deadline for exercising their redemption rights and thus will be unable to redeem their shares.
Shareholders electing to redeem their shares will receive their pro rata portion of the Trust Account less franchise and income taxes payable, calculated as of two business days prior to the anticipated consummation of the Business Combination. Please see the section entitled “General Meeting of FLAC—Redemption Rights” for additional information on how to exercise your redemption rights.
If a shareholder fails to receive notice of FLAC’s offer to redeem our public shares in connection with the Business Combination, or fails to comply with the procedures for tendering its shares, such shares may not be redeemed.
FLAC will comply with the proxy rules or tender offer rules, as applicable, when conducting redemptions in connection with our initial business combination. Despite FLAC’s compliance with these rules, if a shareholder fails to receive FLAC’s proxy solicitation or tender offer materials, as applicable, such shareholder may not become aware of the opportunity to redeem its shares. In addition, the proxy solicitation or tender offer materials, as applicable, that FLAC will furnish to holders of FLAC’s public shares in connection with the Business Combination will describe the various procedures that must be complied with in order to validly redeem or tender public shares. In the event that a shareholder fails to comply with these procedures, its shares may not be redeemed.
If FLAC is unable to consummate a business combination by December 11, 2022 the public shareholders may be forced to wait beyond such date before redemption from the Trust Account.
If FLAC is unable to consummate a business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders, FLAC will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest (less up to $100,000 of interest to pay dissolution expenses and net of taxes payable), divided by the number of then outstanding public shares, which redemption will completely extinguish public shareholders’ rights as shareholders (including the right to receive further liquidation distributions, if any), and
151
Table of Contents
(iii) as promptly as reasonably possible following such redemption, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, liquidate and dissolve, subject in the case of clauses (ii) and (iii) to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In that case, FLAC shareholders may be forced to wait beyond the initial 24 months before the redemption proceeds of the Trust Account become available to them and they receive the return of their pro rata portion of the proceeds from the Trust Account. FLAC has no obligation to return funds to shareholders prior to the date of the redemption or liquidation, unless it consummates a business combination prior thereto and only then in cases where shareholders have properly sought to redeem their FLAC Class A Ordinary Shares. Only upon the redemption or any liquidation will public shareholders be entitled to distributions if FLAC is unable to complete a business combination.
In the event that a significant number of FLAC Class A Ordinary Shares are redeemed, Holdco Shares may become less liquid following the Business Combination.
If a significant number of FLAC Class A Ordinary Shares are redeemed, Holdco may be left with a significantly smaller number of shareholders. As a result, trading in the Holdco Shares following the Business Combination may be limited and your ability to sell your shares in the market could be adversely affected.
152
Table of Contents
General
FLAC is furnishing this proxy statement/prospectus to FLAC’s shareholders as part of the solicitation of proxies by the FLAC Board for use at the General Meeting of FLAC to be held on November 15, 2022, and at any adjournment or postponement thereof. This proxy statement/prospectus is first being furnished to FLAC’s shareholders on or about October 20, 2022 in connection with the vote on the proposals described in this proxy statement/prospectus. This proxy statement/prospectus provides FLAC’s shareholders with information they need to know to be able to vote or instruct their vote to be cast at the General Meeting.
Date, Time and Place
The General Meeting of FLAC will be held at 10:30 a.m. Eastern time, on November 15, 2022, via live webcast at the following address: www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. You will need the 12-digit meeting control number that is printed on your proxy card to enter the General Meeting. FLAC recommends that you log in at least 15 minutes before the General Meeting to ensure you are logged in when the General Meeting starts.
You are strongly encouraged to participate in the General Meeting virtually and not attend in person. FLAC is sensitive to the public health and travel concerns FLAC’s shareholders may have and recommendations that public health officials may issue in light of the evolving COVID-19 pandemic. As a result, FLAC may impose additional procedures or limitations on meeting attendees or may decide to hold the meeting in a different location.
Purpose of the FLAC General Meeting
At the General Meeting, FLAC is asking holders of its ordinary shares to consider and vote upon three separate proposals:
| • | a proposal to approve by ordinary resolution and adopt the Business Combination Agreement and the consummation of the transactions contemplated thereby, including the Business Combination (the “Business Combination Proposal”); |
| • | a proposal to approve by special resolution and adopt the Merger and the Plan of Merger and the consummation of the transactions contemplated thereby (the “Merger Proposal”); and |
| • | a proposal to approve by ordinary resolution the adjournment of the General Meeting to a later date or dates, if necessary, to, among other things, permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement at the General Meeting (the “Adjournment Proposal”). |
The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in this proxy statement/prospectus.
Recommendation to Shareholders of FLAC
The FLAC Board believes that each of the proposals to be presented at the General Meeting is in the best interest of FLAC and its shareholders and unanimously recommends that its shareholders vote “FOR” the Business Combination Proposal, “FOR” the Merger Proposal, and “FOR” the Adjournment Proposal, in each case, if presented to the General Meeting.
The existence of financial and personal interests of one or more of FLAC’s directors may result in a conflict of interest on the part of such director(s) between what he, she or they may believe is in the best interests of FLAC and its shareholders and what he, she or they may believe is best for himself or themselves in determining
153
Table of Contents
to recommend that shareholders vote for the proposals. In addition, FLAC’s officers have interests in the Business Combination that may conflict with your interests as a shareholder. See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of these considerations.
Voting Power; Record Date
For FLAC shareholders holding their shares in “street name,” only holders of record at the close of business on September 30, 2022, the record date for the General Meeting, will be entitled to vote at the General Meeting. Each FLAC shareholder that holds its shares in “street name” is entitled to one vote for each FLAC Ordinary Share that such shareholder owned as of the close of business on the record date. If a FLAC shareholder’s shares are held in “street name” or are in a margin or similar account, such shareholder should contact its broker, bank or other nominee to ensure that votes related to the shares beneficially owned by such shareholder are properly counted. On the record date, there were 17,751,000 FLAC Ordinary Shares outstanding, of which 13,800,000 are FLAC Public Shares, 501,000 are FLAC Private Placement Shares held by the Sponsor and 3,450,000 are Founder Shares held by the Sponsor and the FLAC Initial Shareholders. For the avoidance of doubt, the record date does not apply to FLAC shareholders that hold their shares in registered form and are registered as shareholders in FLAC’s register of members. FLAC shareholders that hold their shares in registered form are entitled to one vote on each proposal presented at the General Meeting for each FLAC Ordinary Share held on the date of the General Meeting.
Quorum
A quorum of FLAC shareholders is necessary to hold a valid meeting. A quorum will be present at the General Meeting if one or more shareholders who together hold not less than a majority of the issued and outstanding FLAC Ordinary Shares entitled to vote at the General Meeting are represented in person or by proxy at the General Meeting. As of the record date for the General Meeting, 8,875,501 FLAC Ordinary Shares would be required to achieve a quorum.
Vote of the FLAC Initial Shareholders
Prior to the FLAC IPO, FLAC entered into agreements with the FLAC Initial Shareholders, including the Sponsor, pursuant to which each FLAC Initial Shareholder agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of an initial business combination, including the Business Combination and for all other proposals presented to FLAC shareholders in this proxy statement/prospectus. As of the record date, the FLAC Initial Shareholders own 3,450,000 Founder Shares, representing approximately 19% of the FLAC Ordinary Shares then outstanding and entitled to vote at the General Meeting.
Each of the FLAC Initial Shareholders has, for no additional consideration, waived any redemption rights in connection with the Business Combination. The Founder Shares held by the FLAC Initial Shareholders have no redemption rights upon the liquidation of FLAC and will be worthless if no business combination is effected by FLAC by December 11, 2022 or such later date as may be approved by FLAC’s shareholders. However, the FLAC Initial Shareholders are entitled to redemption rights upon the liquidation of FLAC with respect to any FLAC Public Shares they may own. See “The Business Combination Agreement and Ancillary Documents—Sponsor Support Agreement” in the accompanying proxy statement/prospectus for more information related to the Sponsor Support Agreement.
Vote Required for Approval
The proposals presented at the General Meeting require the following votes:
| (i) | Business Combination Proposal: The approval of the Business Combination Proposal requires an ordinary resolution under Cayman Islands law, being the affirmative vote of a majority of the |
154
Table of Contents
| votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
| (ii) | Merger Proposal: The approval of the Merger Proposal requires a special resolution under Cayman Islands law, being the affirmative vote of at least a two-thirds majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
| (iii) | Adjournment Proposal: The approval of the Adjournment Proposal requires an ordinary resolution under Cayman Islands law, being the affirmative vote of a majority of the votes cast by the holders of the issued ordinary shares present in person or represented by proxy at the General Meeting and entitled to vote on such matter. |
The closing of the Business Combination is conditioned upon the approval of the Business Combination Proposal and the Merger Proposal. The Adjournment Proposal is not conditioned on the approval of any other proposal set forth in this proxy statement/prospectus.
It is important for you to note that, in the event that the Business Combination Proposal or the Merger Proposal does not receive the requisite vote for approval, FLAC will not consummate the Business Combination. If FLAC does not consummate the Business Combination and fails to complete an initial business combination by December 11, 2022, absent any extension FLAC will be required to dissolve and liquidate the Trust Account by returning the then remaining funds in such account to the public shareholders.
Abstentions and Broker Non-Votes
Proxies that are marked “abstain” and proxies relating to “street name” shares that are returned to FLAC but marked by brokers as “not voted” will be treated as shares present for purposes of determining the presence of a quorum on all matters. Abstentions and broker non-votes, while considered present for the purposes of establishing a quorum, will not count as votes cast at the General Meeting, and otherwise will have no effect on a particular proposal. If a shareholder does not give the broker voting instructions, under applicable self-regulatory organization rules, its broker may not vote its shares on “non-routine” proposals.
None of the proposals at the General Meeting are routine matters. As such, without your voting instructions, your brokerage firm cannot vote your shares on any proposal to be voted on at the General Meeting.
Voting Your Shares
Shareholders of Record
If you hold your shares in “street name” and are an FLAC shareholder of record, you may vote by mail or at the General Meeting. Each FLAC Ordinary Share that you own in your name entitles you to one vote on each of the proposals for the General Meeting. Your one or more proxy cards show the number of FLAC Ordinary Shares that you own.
There are two ways to vote your ordinary shares at the General Meeting:
| • | You can vote by signing and returning the enclosed proxy card. If you vote by proxy card, your “proxy,” whose name is listed on the proxy card, will vote your shares as you instruct on the proxy card. If you sign and return the proxy card but do not give instructions on how to vote your shares, your shares will be voted as recommended by the FLAC Board “FOR” the Business Combination Proposal and “FOR” the Merger Proposal, in each case, if presented to the General Meeting. Votes received after a matter has been voted upon at the General Meeting will not be counted. |
155
Table of Contents
| • | You can attend the General Meeting and vote. We will be hosting the General Meeting via live webcast at www.cstproxy.com/flac/2022 and in person at 70 Willow Road, Suite 200, Menlo Park, CA, 94025. |
A summary of the information you need to attend the General Meeting online is provided below:
| • | Instructions on how to attend and participate via the Internet, including how to demonstrate proof of stock ownership, are posted at www.cstproxy.com/flac/2022. |
| • | Assistance with questions regarding how to attend and participate via the Internet will be provided at www.cstproxy.com/flac/2022 on the day of the General Meeting. |
| • | The General Meeting starts at 10:30 a.m. Eastern time. |
| • | You will need your 12-digit control number to enter the General Meeting. |
| • | Shareholders may submit questions while attending the General Meeting via internet. |
If you attend the General Meeting and plan to vote in person, you will receive a ballot when you arrive. However, if your shares are held in the name of your broker, bank or another nominee, you must get a valid legal proxy from the broker, bank or other nominee. That is the only way FLAC can be sure that the broker, bank or nominee has not already voted your shares.
Beneficial Owners
If your shares are held in an account at a brokerage firm, bank or other nominee, then you are the beneficial owner of shares held in “street name” and this proxy statement/prospectus is being sent to you by that broker, bank or other nominee. The broker, bank or other nominee holding your account is considered to be the shareholder of record for purposes of voting at the General Meeting. As a beneficial owner, you have the right to direct your broker, bank or other nominee regarding how to vote the shares in your account by following the instructions that the broker, bank or other nominee provides you along with this proxy statement/prospectus. As a beneficial owner, if you wish to vote at the General Meeting, you will need to bring to the General Meeting a legal proxy from your broker, bank or other nominee authorizing you to vote those shares. Please see “—Attending the General Meeting” below for more details.
Attending the General Meeting
Only FLAC shareholders on the record date (if the shares are held in “street name”) or their legal proxy holders may attend the General Meeting. To be admitted to the General Meeting, you will need a form of photo identification and valid proof of ownership of FLAC Ordinary Shares or a valid legal proxy. If you have a legal proxy from a shareholder of record, you must bring a form of photo identification and the legal proxy to the General Meeting. If you have a legal proxy from a “street name” shareholder, you must bring a form of photo identification, a legal proxy from the record holder (that is, the bank, broker or other holder of record) to the “street name” shareholder that is assignable, and the legal proxy from the “street name” shareholder to you. Shareholders may appoint only one proxy holder to attend on their behalf. Shareholders that hold their shares in registered form on the date of the General Meeting are entitled to attend and vote at the General Meeting. A summary of the information you need to attend the Annual General Meeting online is provided below:
| • | Instructions on how to attend and participate via the Internet, including how to demonstrate proof of stock ownership, are posted at www.cstproxy.com/flac/2022. |
| • | Assistance with questions regarding how to attend and participate via the Internet will be provided at www.cstproxy.com/flac/2022 on the day of the Annual General Meeting. |
| • | The General Meeting starts at 10:30 a.m. Eastern time. |
| • | You will need your 12-digit control number to enter the General Meeting. |
| • | Shareholders may submit questions while attending the General Meeting via internet. |
156
Table of Contents
Revoking Your Proxy
If you are a FLAC shareholder and you give a proxy, you may revoke it at any time before it is exercised by doing any one of the following:
| • | you may send another proxy card with a later date; |
| • | you may notify us in writing before the General Meeting that you have revoked your proxy; or |
| • | you may attend the General Meeting, revoke your proxy, and vote, as indicated above. |
No Additional Matters
The General Meeting has been called only to consider the approval of the Business Combination Proposal and the Merger Proposal, and if necessary, to, among other things, permit further solicitation and vote of proxies if there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal or the Merger Proposal, the Adjournment Proposal. Under the FLAC Articles of Association, other than procedural matters incident to the conduct of the General Meeting, no other matters may be considered at the General Meeting if they are not included in this proxy statement/prospectus, which serves as the notice of the General Meeting.
Who Can Answer Your Questions About Voting Your Shares
If you are a shareholder and have any questions about how to vote or direct a vote in respect of your ordinary shares, you may call Morrow Sodali LLC, our proxy solicitor, by calling (800) 662-5200, or banks and brokers can call collect at (203) 658-9400, or by emailing FLAC.info@investor.morrowsodali.com.
Redemption Rights
Pursuant to the FLAC Articles of Association, holders of FLAC Public Shares other than the Sponsor and any FLAC directors or officers may demand that such shares be redeemed in exchange for a pro rata share of the aggregate amount on deposit in the Trust Account, less taxes payable, calculated as of two business days prior to the consummation of the Business Combination. If demand is properly made and the Business Combination is consummated, these shares, immediately prior to the Business Combination, will cease to be outstanding and will represent only the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account which holds the proceeds of the FLAC IPO and a portion of the proceeds from the sale of the FLAC Private Placement Warrants (calculated as of two business days prior to the consummation of the Business Combination, less taxes payable). For illustrative purposes, based on the fair value of marketable securities held in the Trust Account of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting, the estimated per share redemption price would have been approximately $10.04.
In order to exercise your redemption rights, you must:
| • | if you hold FLAC Public Units, separate the underlying FLAC Class A Ordinary Shares and FLAC Public Warrants; |
| • | prior to 5:00 p.m. Eastern time, on November 10, 2022 (two business days before the initially scheduled General Meeting), identify yourself in writing as a beneficial holder and provide your legal name, phone number and address to the Transfer Agent in order to validly redeem your shares and tender your shares physically or electronically and submit a request in writing that FLAC redeem your public shares for cash to Continental Stock Transfer & Trust Company, the Transfer Agent, at the following address: 1 State Street, New York, New York 10004; and |
| • | deliver your public shares either physically or electronically through DTC’s DWAC system to the Transfer Agent at least two business days before the initially scheduled General Meeting. Shareholders seeking to exercise their redemption rights and opting to deliver physical certificates should allot sufficient time to obtain physical certificates from the Transfer Agent and time to effect delivery. Shareholders should generally allot at least two weeks to obtain physical certificates from the Transfer Agent. However, it may take longer than two weeks. Shareholders who hold their shares in street name |
157
Table of Contents
will have to coordinate with their bank, broker or other nominee to have the shares certificated or delivered electronically. If you do not submit a written request and deliver your public shares as described above, your shares will not be redeemed. |
You do not have to be a record date holder in order to exercise your redemption rights. Shareholders seeking to exercise their redemption rights, whether they are registered holders or hold their shares in “street name,” are required either to tender their certificates to the Transfer Agent prior to the date set forth in this proxy statement/prospectus, at least two business days prior to the initially scheduled vote on the Business Combination Proposal at the General Meeting, or to deliver their shares to the Transfer Agent electronically using DTC’s DWAC system, at such shareholder’s option. The requirement for physical or electronic delivery prior to the General Meeting ensures that a redeeming shareholder’s election to redeem is irrevocable once the Business Combination is approved.
If you hold FLAC Public Units registered in your own name, you must deliver the certificate for such units to the Transfer Agent with written instructions to separate such units into FLAC Class A Ordinary Shares and FLAC Public Warrants. This must be completed far enough in advance to permit the mailing of the public share certificates back to you so that you may then exercise your redemption rights upon the separation of the FLAC Public Shares from the FLAC Public Units.
If a broker, dealer, commercial bank, trust company or other nominee holds your FLAC Public Units, you must instruct such nominee to separate your units. Your nominee must send written instructions by facsimile to the Transfer Agent. Such written instructions must include the number of units to be split and the nominee holding such units. Your nominee must also initiate electronically, using DTC’s DWAC system, a withdrawal of the relevant units and a deposit of an equal number of FLAC Class A Ordinary Shares and FLAC Public Warrants. This must be completed far enough in advance to permit your nominee to exercise your redemption rights upon the separation of the public shares from the FLAC Public Units. While this is typically done electronically on the same business day, you should allow at least one full business day to accomplish the separation. If you fail to cause your FLAC Public Units to be separated in a timely manner, you will likely not be able to exercise your redemption rights.
Each redemption of a FLAC Public Share by FLAC’s public shareholders will reduce the amount in the Trust Account, which held marketable securities with a fair value of approximately $138.6 million as of September 30, 2022, the record date for the General Meeting. Unless waived by FLAC or Holdco, the Business Combination Agreement provides that each party’s obligation to consummate the Business Combination is conditioned on Holdco having net tangible assets worth at least $5,000,001 immediately after the Closing after giving effect to the amount of cash in the Trust Account (net of the Cash Redemption Amount), together with the proceeds of the PIPE financing. The committed PIPE subscriptions are sufficient to satisfy this condition if fully funded. The Sponsor has agreed not to redeem any Founder Shares or FLAC Public Shares held by it in connection with a shareholder vote to approve a proposed initial business combination. A public shareholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its FLAC Public Shares or, if part of such a group, the group’s FLAC Public Shares, in excess of 15% of the outstanding FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO (i.e., in excess of 2,070,000 FLAC Class A Ordinary Shares). Each redemption of a FLAC Public Share by FLAC’s public shareholders will reduce the amount in the Trust Account. The Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million.
Prior to exercising redemption rights, FLAC shareholders should verify the market price of the FLAC Class A Ordinary Shares, as shareholders may receive higher proceeds from the sale of their FLAC Class A Ordinary Shares in the public market than from exercising their redemption rights if the market price per share is higher than the redemption price. There is no assurance that you will be able to sell your FLAC Class A Ordinary Shares in the open market, even if the market price per share is higher than the redemption price stated above, as there may not be sufficient liquidity in the FLAC Class A Ordinary Shares when you wish to sell your shares.
158
Table of Contents
If you exercise your redemption rights, your FLAC Class A Ordinary Shares will cease to be outstanding immediately prior to the Business Combination and will only represent the right to receive a pro rata share of the aggregate amount then on deposit in the Trust Account. You will no longer own those shares and you will not receive any Holdco Shares in the Business Combination. You will have no right to participate in, or have any interest in, the future growth of Holdco, if any. You will be entitled to receive cash for your FLAC Class A Ordinary Shares only if you properly and timely demand redemption.
Holders of FLAC Public Warrants or FLAC Private Placement Shares will not have redemption rights with respect to such securities.
If the Business Combination is not approved and FLAC does not consummate an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders, FLAC will be required to dissolve and liquidate the Trust Account by returning the then remaining funds in such account to the public shareholders and all of FLAC’s warrants will expire worthless, unless such period is extended.
Shareholders of FLAC may be entitled to give notice to FLAC prior to the General Meeting that they wish to dissent to the Merger and to receive payment of fair market value for his or her shares if they follow the procedures set out in the Cayman Islands Companies Act, noting that any such dissention rights may be limited pursuant to Section 239 of the Cayman Islands Companies Act which states that no such dissention rights shall be available in respect of shares of any class for which an open market exists on a recognized stock exchange at the expiry date of the period allowed for written notice of an election to dissent provided that the merger consideration constitutes inter alia shares of any company which at the effective date of the merger are listed on a national securities exchange. It is FLAC’s view that such fair market value would equal the amount which shareholders would obtain if they exercise their redemption rights as described herein.
Appraisal Rights or Dissenters’ Rights
Shareholders of FLAC may be entitled to give notice to FLAC prior to the General Meeting that they wish to dissent to the Merger and to receive payment of fair market value for his or her shares if they follow the procedures set out in the Cayman Islands Companies Act, noting that any such dissenters’ rights may be limited pursuant to Section 239 of the Cayman Islands Companies Act which states that no such dissenters’ rights will be available in respect of shares of any class for which an open market exists on a recognized stock exchange at the expiry date of the period allowed for written notice of an election to dissent provided that the merger consideration constitutes shares of any company which at the effective date of the merger are listed on a national securities exchange. It is FLAC’s view that such fair market value would equal the amount which shareholders would obtain if they exercise their redemption rights as described herein.
Proxy Solicitation Costs
FLAC is soliciting proxies on behalf of the FLAC Board. This solicitation is being made by mail but also may be made by telephone or in person. FLAC and its directors, officers and employees may also solicit proxies in person, by telephone or by other electronic means. FLAC will bear the cost of the solicitation.
FLAC has hired Morrow Sodali LLC to assist in the proxy solicitation process, who FLAC will pay a fee of $30,000 plus disbursements. Such fee will be paid with non-trust account funds. FLAC will also reimburse Morrow Sodali LLC for its reasonable out-of-pocket expenses and indemnify it and its affiliates against certain claims, liabilities, losses, damages and expenses.
FLAC will ask banks, brokers and other institutions, nominees and fiduciaries to forward the proxy materials to their principals and to obtain their authority to execute proxies and voting instructions. FLAC will reimburse them for their reasonable out-of-pocket expenses.
159
Table of Contents
Overview
FLAC is asking its shareholders to authorize the adoption of, and approve, the Business Combination Agreement and the Transactions, including the Business Combination.
FLAC shareholders should carefully read this proxy statement/prospectus in its entirety for more detailed information concerning the Business Combination Agreement, which is attached as Annex A to this proxy statement/prospectus. Please see the sections entitled “The Business Combination” and “The Business Combination Agreement and Ancillary Documents” for additional information and a summary of certain terms of the Business Combination and the Business Combination Agreement. FLAC shareholders are urged to read carefully the Business Combination Agreement in its entirety before voting on this proposal.
Vote Required for Approval
The Business Combination is conditioned on the approval of the Business Combination Proposal at the General Meeting.
This Business Combination Proposal (and consequently, the Business Combination Agreement and the Transactions, including the Business Combination) will be adopted and approved only if the FLAC shareholders approve an ordinary resolution which requires the affirmative vote of the holders of at least a majority of the issued FLAC Ordinary Shares present or represented by proxy at a quorate General Meeting and entitled to vote. Broker non-votes and abstentions will have no effect on the outcome of the vote on the Business Combination Proposal.
The FLAC Initial Shareholders have agreed to vote any FLAC Ordinary Shares owned by them in favor of the Business Combination Proposal. As of the date hereof, the FLAC Initial Shareholders own approximately 19% of the issued FLAC Ordinary Shares and have not purchased any public shares, but may do so at any time. In addition, certain shareholders of FLAC, including affiliates of the Sponsor, representing 1,500,000 FLAC Class A Ordinary Shares, have entered into Investor Support Agreements which requires them to vote their shares in favor of the Business Combination.
Resolution
The full text of the resolution to be passed is as follows:
RESOLVED, as an ordinary resolution, that FLAC’s entry into the Business Combination Agreement, dated as of July 25, 2022 (as may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), by and among FLAC, NewAmsterdam Pharma Company B.V. a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”), NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”), and NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“NewAmsterdam Pharma”), a copy of which is attached to the accompanying proxy statement/prospectus as Annex A, pursuant to which certain transactions will occur, and in connection therewith, Holdco will become the ultimate parent company of NewAmsterdam Pharma, and Merger Sub will merge with and into FLAC, with FLAC surviving the merger as a wholly owned subsidiary of Holdco (the “Merger” and together with the other transactions contemplated under the Businss Combination Agreement, the “Business Combination”) and Holdco shall be converted into a Dutch public limited liability company (naamloze vennootschap), thereby changing its name to “NewAmsterdam Pharma Company N.V.,” and the consummation of the transactions contemplated thereby, shall be confirmed, ratified and approved in all respects.
Recommendation of the FLAC Board
THE FLAC BOARD RECOMMENDS
THAT FLAC SHAREHOLDERS VOTE “FOR”
THE BUSINESS COMBINATION PROPOSAL.
160
Table of Contents
Overview
FLAC is asking its shareholders to authorize and approve the Plan of Merger and the consummation of the Merger and the remaining transactions contemplated thereby.
FLAC shareholders should carefully read this proxy statement/prospectus in its entirety for more detailed information concerning the Merger Documents, including the Plan of Merger between FLAC and Merger Sub, which will be substantially in the form to the accompanying proxy statement/prospectus attached as Annex B. Please see the sections entitled “The Business Combination” and “The Business Combination Agreement and Ancillary Documents” for additional information and a summary of certain terms of the Merger. FLAC shareholders are urged to read carefully the Plan of Merger in its entirety before voting on this proposal.
Vote Required for Approval
The Business Combination is conditioned on the approval of the Merger Proposal at the General Meeting.
This Merger Proposal will be adopted and approved only if the FLAC shareholders approve a special resolution which requires the affirmative vote of the holders of at least two-thirds of the votes cast by the holders of issued FLAC Ordinary Shares present in person or represented by proxy at a quorate General Meeting and entitled to vote. Broker non-votes and abstentions will have no effect on the outcome of the vote on the Merger Proposal.
The FLAC Initial Shareholders have agreed to vote any FLAC Ordinary Shares owned by them in favor of the Merger Proposal. As of the date hereof, the FLAC Initial Shareholders own approximately 19% of the issued FLAC Ordinary Shares and have not purchased any public shares, but may do so at any time. In addition, certain shareholders of FLAC, including affiliates of the Sponsor, representing 1,500,000 FLAC Class A Ordinary Shares, have entered into Investor Support Agreements which requires them to vote their shares in favor of the Merger and Plan of Merger.
Resolution
The full text of the resolution to be passed is as follows:
RESOLVED, as a special resolution, that the Merger and the plan of merger in the form tabled to the General Meeting (a draft of which is attached to the accompanying proxy statement/prospectus as Annex B, the “Plan of Merger”) pursuant to which Merger Sub will merge with and into FLAC so that FLAC will survive the Merger as a wholly owned subsidiary of Holdco, and all the undertakings, property and liabilities of Merger Sub will vest in FLAC by virtue of such Merger pursuant to the Cayman Islands Companies Act, and the consummation of the Merger and the remaining transactions contemplated thereby, be authorized, approved and confirmed in all respects; and FLAC be authorized to enter into the Plan of Merger.
Recommendation of the FLAC Board
THE FLAC BOARD RECOMMENDS
THAT FLAC SHAREHOLDERS VOTE “FOR”
THE MERGER PROPOSAL.
161
Table of Contents
FLAC is proposing the Adjournment Proposal to allow the FLAC Board to adjourn the General Meeting to a later date or dates, if necessary, (A) in order to solicit additional proxies from FLAC shareholders in favor of, or in connection with, the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement or (B) to allow reasonable time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting. The Adjournment Proposal will only be presented to FLAC shareholders in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Business Combination Proposal or the Merger Proposal.
Vote Required for Approval
The Adjournment Proposal will be adopted and approved only if the FLAC shareholders approve an ordinary resolution which requires a majority of the votes cast by holders of the issued FLAC Ordinary Shares present in person or represented by proxy at a quorate General Meeting and entitled to vote. Broker non-votes and abstentions will have no effect on the outcome of the vote on the Adjournment Proposal.
The FLAC Initial Shareholders have agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of the Adjournment Proposal, if presented, to permit further solicitation of proxies because there is not sufficient votes for the Business Combination Proposal or the Merger Proposal. As of the date hereof, the FLAC Initial Shareholders own approximately 19% of the issued FLAC Ordinary Shares and have not purchased any FLAC Public Shares, but may do so at any time.
Resolution
The full text of the resolution to be passed is as follows:
RESOLVED, as an ordinary resolution, to adjourn the General Meeting, in order (i) to solicit additional proxies from FLAC shareholders for, or otherwise in connection with, the Business Combination Proposal, the Merger Proposal or for any other reason in connection with the Business Combination Agreement or (ii) to allow reasonable time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable law and for such supplemental or amended disclosure to be disseminated and reviewed by FLAC shareholders prior to the General Meeting.
Recommendation of the FLAC Board
THE FLAC BOARD UNANIMOUSLY RECOMMENDS
THAT FLAC SHAREHOLDERS VOTE “FOR”
THE ADJOURNMENT PROPOSAL, IF PRESENTED.
162
Table of Contents
General
On July 25, 2022, FLAC, NewAmsterdam Pharma, Holdco, and Merger Sub entered into the Business Combination Agreement, which provides for, among other things, the following transactions:
| • | The Participating Shareholders will effect the Exchange; |
| • | Immediately after giving effect to the Exchange, the parties will effect the Holdco Reorganization, provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing; |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as a wholly owned subsidiary of Holdco (the “Surviving Company”); |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding FLAC Warrant will become a Holdco Warrant; |
| • | Each NewAmsterdam Pharma Option will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will effect the Domestication; and |
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders (including Amgen and MTPC for this purpose) and Participating Optionholders and who are at the time of achievement of such milestone still providing services to Holdco or its subsidiaries, 1,886,137 Earnout Shares, which in the case of the Participating Optionholders will take the form of awards of restricted stock units under Holdco’s long-term incentive plan. The development milestone consists of the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the Earnout Period. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within the Earnout Period. |
For more information about the transactions contemplated in the Business Combination Agreement, please see the section entitled “The Business Combination Agreement and Ancillary Documents.” The Business Combination Agreement is incorporated by reference into this proxy statement/prospectus, a copy of which is attached to this proxy statement/prospectus as Annex A.
Effect of the Business Combination on Existing FLAC Equity
Subject to the terms and conditions of the Business Combination Agreement, the Business Combination will result in, among other things, the following:
| • | each FLAC Class A Ordinary Share will be exchanged for one fully paid and non-assessable Holdco Share; |
| • | each Founder Share will be exchanged for one fully paid and non-assessable Holdco Share; and |
| • | each FLAC Warrant will become a Holdco Warrant, on the same terms and conditions as those applicable to the respective FLAC Warrants. |
163
Table of Contents
Consideration to NewAmsterdam Pharma Shareholders in the Business Combination
Subject to the terms and conditions of the Business Combination Agreement, the consideration to be received by the NewAmsterdam Pharma shareholders (including Amgen and MTPC for this purpose) in connection with the Business Combination will be (i) an aggregate number of Holdco Shares determined by using an exchange ratio (the “Exchange Ratio”) equal to (A) $491,000,000 divided by (B) $10.00 multiplied by (C) a fraction of which the numerator is one and the denominator is the fully-diluted number of NewAmsterdam Pharma shares outstanding immediately prior to the Exchange (the fully diluted basis assumes, solely for purposes of this calculation, that shares of NewAmsterdam Pharma and not of Holdco would be issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”), multiplied by (D) the number of NewAmsterdam Pharma shares outstanding immediately prior to the Exchange (subject to rounding differences to account for fractional entitlements of the NewAmsterdam Pharma shareholders) and (ii) 1,886,137 Earnout Shares if and when a certain clinical development milestone is achieved during the Earnout Period.
Each NewAmsterdam Pharma Option outstanding and unexercised immediately prior to the consummation of the Exchange will remain outstanding and, to the extent unvested, will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares. Furthermore, the exercise of each NewAmsterdam Pharma Option will be made in Holdco Shares based on the Exchange Ratio. Additionally, the exercise price of each NewAmsterdam Pharma Option will be determined by dividing the exercise price per share (or depository receipt for a share) of each option to purchase shares (or depository receipts for shares) of NewAmsterdam Pharma by the Exchange Ratio. The Earnout Shares payable to eligible Participating Optionholders will be delivered in the form of awards of restricted stock units under Holdco’s long-term incentive plan.
Aggregate Holdco Proceeds
The aggregate proceeds received by Holdco through the Merger, Exchange and PIPE Financing will be used for general corporate purposes after the Business Combination.
Conditions to Closing of the Business Combination
Conditions to Each Party’s Obligations
The respective obligations of each party to the Business Combination Agreement to consummate the Business Combination are subject to the satisfaction, or waiver by the party for whose benefit such condition exists, at or prior to the Closing of the following conditions:
| • | any applicable waiting period under any applicable Antitrust Law will have expired or terminated, and any consent pursuant to any applicable Antitrust Law will have been obtained; |
| • | no order or law issued by any court of competent jurisdiction or other governmental entity or other legal restraint or prohibition preventing the consummation of the Transactions will be in effect; |
| • | the registration statement—of which this proxy statement/prospectus forms a part—will have become effective in accordance with the provisions of the Securities Act, no stop order has been issued by the SEC and remains in effect with respect to the registration statement of which this proxy statement/prospectus forms a part, and no proceeding seeking such a stop order has been threatened or initiated by the SEC and remains pending; |
| • | the approval at the General Meeting, of the Business Combination Proposal and the Merger Proposal in accordance with FLAC’s governing documents, will have been obtained; |
164
Table of Contents
| • | the approval of the Business Combination Agreement and the Transactions by the shareholders of NewAmsterdam Pharma, in accordance with NewAmsterdam Pharma’s governing documents, will have been obtained; |
| • | (i) Holdco’s initial listing application with Nasdaq in connection with the listing of Holdco Shares will have been approved such that, immediately following the Closing, Holdco will satisfy any applicable initial and continuing listing requirements of Nasdaq, (ii) Holdco will not have received any notice of non-compliance therewith, and (iii) the Holdco Shares and Holdco Public Warrants to be issued in connection with the Transactions will have been approved for listing on Nasdaq subject to official notice of issuance; |
| • | the Holdco Board will be comprised of the individuals appointed in accordance with the Business Combination Agreement and as described in the section entitled “Management of Holdco Following the Business Combination”; and |
| • | after giving effect to the Transactions (including the PIPE Financing), Holdco will have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) immediately after the Effective Date. |
Other Conditions to FLAC’s Obligations
In addition to the conditions described above, the obligation of FLAC to consummate the Business Combination is subject to the satisfaction, or waiver by FLAC, at or prior to the Closing of the following conditions:
| • | (i) the representations and warranties of NewAmsterdam Pharma regarding its organization and qualification, indebtedness and authorized share capital, authority, absence of a material adverse effect and broker fees in connection with the Transactions must be true and correct in all material respects (without giving effect to any materiality qualifications included in such representations and warranties) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date); (ii) the representations and warranties of NewAmsterdam Pharma regarding its capitalization must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects (except for de minimis inaccuracies) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), and (iii) the other representations and warranties of NewAmsterdam Pharma must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not constitute a Company Material Adverse Effect; |
| • | NewAmsterdam Pharma must have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by NewAmsterdam Pharma under the Business Combination Agreement at or prior to the Closing; |
| • | since the date of the Business Combination Agreement, no Company Material Adverse Effect will have occurred and be continuing; and |
| • | at or prior to the Closing, NewAmsterdam Pharma will have delivered, or caused to be delivered, to FLAC: (i) a certificate executed by an authorized officer of NewAmsterdam Pharma, dated as of the Closing Commencement Date, to the effect that the conditions described in the three bullets above are satisfied and (ii) the Investor Rights Agreement duly executed by NewAmsterdam Pharma and the NewAmsterdam Pharma shareholders contemplated to be parties thereto. |
165
Table of Contents
Other Conditions to NewAmsterdam Pharma’s Obligations
In addition to the conditions described above, the obligations of NewAmsterdam Pharma to consummate the Business Combination are subject to the satisfaction, or waiver by NewAmsterdam Pharma, at or prior to the Closing of the following conditions:
| • | the Aggregate Cash Proceeds will be equal to or greater than $250 million; |
| • | the representations and warranties of FLAC regarding its organization and qualification, authority and brokers in connection with the Transactions must be true and correct in all material respects (without giving effect to any materiality qualifications included in such representations and warranties) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be true and correct as of such earlier date); (ii) the representations and warranties of FLAC regarding its capitalization must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects (except for de minimis inaccuracies) as of the date of the Business Combination Agreement and as of the Closing Commencement Date, (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), and (iii) the other representations and warranties of FLAC and Holdco contained in Business Combination Agreement must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not cause a FLAC Material Adverse Effect; |
| • | FLAC must have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by FLAC under the Business Combination Agreement at or prior to the Closing; and |
| • | at or prior to the Closing, FLAC will have delivered, or caused to be delivered, the following documents to NewAmsterdam Pharma: (i) a certificate executed by an authorized officer of FLAC, dated as of the Closing Commencement Date, to the effect that the conditions described in the three bullets above are satisfied; and (ii) the Investor Rights Agreement duly executed by the Sponsor and the affiliates of FLAC and the Sponsor. |
Ownership of Holdco Following the Business Combination
It is anticipated that, upon completion of the Business Combination: (i) FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) will receive approximately 14.9% of Holdco Shares, (ii) the PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) (some of whom are also NewAmsterdam Pharma shareholders) will receive approximately 22.0% of Holdco Shares, (iii) the FLAC Initial Shareholders and their affiliates (including the Sponsor) will receive approximately 11.0% of Holdco Shares, and (iv) the former NewAmsterdam Pharma shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing, but including the Holdco Shares issued to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”) will receive approximately 52.2% of Holdco Shares. These levels of ownership assume (A) no FLAC Class A Ordinary Shares are elected to be redeemed by FLAC’s public shareholders, (B) 23,460,000 Holdco Shares are issued to the PIPE Investors in connection with the PIPE Financing, (C) none of the Holdco Warrants have been exercised, (D) Participating Shareholders representing 100% of the issued and outstanding shares of NewAmsterdam Pharma participated in the Exchange, (E) none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued, (F) an aggregate of 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to each of
166
Table of Contents
their respective profit rights referenced above, (G) none of the NewAmsterdam Pharma Options have been exercised prior to the Closing, and (H) no Holdco Options or awards that may be issued under Holdco’s LTIP following the Closing will be exercised. While all Participating Shareholders will contribute all of their outstanding shares in the capital of NewAmsterdam Pharma to Holdco in the Exchange by either individually executing a deed of issuance and contribution or through NewAmsterdam Pharma’s execution of such a deed on their behalf, we have included the participation of the Participating Shareholders in the Exchange as an assumption in clause (D) above because NewAmsterdam Pharma will be required to confirm and/or effectuate the executions of all such deeds before the Business Combination can be consummated. If all of the Holdco Warrants, NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options were exercisable and immediately exercised upon completion of the Business Combination on a 1:1 basis for Holdco Shares, FLAC’s public shareholders (excluding any Holdco Shares issued pursuant to the PIPE Financing) would receive in aggregate approximately 12.3% of Holdco Shares on a fully diluted basis, and the FLAC Initial Shareholders (including the Sponsor) would receive in aggregate approximately 9.1% of Holdco Shares on a fully diluted basis; however, the Holdco Warrants are subject to restrictions on the timing of their exercise and may also be exercisable on a cashless basis by reference to the fair market value of the Holdco Shares, and these percentages are therefore indicative only.
In addition to the assumptions made in the preceding paragraph, the factors that will determine the ownership percentages upon consummation of the Business Combination include:
| • | if there are any redemptions by public shareholders of FLAC Public Shares in connection with the Business Combination, it would reduce the aggregate ownership of the public shareholders and increase the aggregate ownership of the other shareholder groups described above; and |
| • | if the PIPE Investors do not fund the PIPE Financing in full, it would reduce the aggregate ownership of the PIPE Investors and increase the aggregate ownership of the other shareholder groups described above commensurately. |
For further information related to the determination of the number of Holdco Shares to be issued to the NewAmsterdam Pharma shareholders upon completion of the Business Combination, please see the section entitled “The Business Combination Agreement and Ancillary Documents—Consideration to NewAmsterdam Pharma Shareholders in the Business Combination.”
The ownership percentages with respect to Holdco following the Business Combination do not take into account, unless otherwise expressly stated, the Holdco Warrants, but do include Founder Shares, which will be exchanged for Holdco Shares at the closing of the Business Combination on a 1:1 basis. If the actual facts are different than these assumptions, the ownership percentages in Holdco will be different.
The following table illustrates varying ownership levels in Holdco immediately following the consummation of the Business Combination, assuming:
| • | one of the four scenarios: (a) no redemptions by the holders of FLAC Public Shares (“No Redemption”), (b) low redemption (which assumes that 33% of FLAC Class A Ordinary Shares held by public shareholders are redeemed) (“Low Redemption”), (c) high redemption (which assumes that 66% of FLAC Class A Ordinary Shares held by public shareholders are redeemed) (“High Redemption”) or (d) the maximum number of redemptions by the public shareholders (which assumes that 12,261,482 FLAC Public Shares are redeemed, which number represents the maximum number of FLAC Public Shares that may be redeemed such that the closing condition requiring the Aggregate Cash Proceeds equal to $250 million can still be satisfied). Pursuant to the Sponsor Support Agreement and the Investor Support Agreements, shareholders holding 1,500,000 FLAC Class A Ordinary Shares have agreed not to redeem their shares (assuming that 23,460,000 Holdco Shares are issued in connection with the PIPE Financing) (“Maximum Redemption”); |
| • | that the amount in the Trust Account is $138.1 million (which was the approximate value of the Trust Account as of June 30, 2022, not taking into account $4.8 million of deferred underwriting fees to be paid); |
167
Table of Contents
| • | that Participating Shareholders represent 100% of the issued and outstanding shares of NewAmsterdam Pharma and, other than the issuance of Holdco Shares to Amgen and MTPC described immediately below, there are no “change of control” payments made or required to be made by NewAmsterdam Pharma; |
| • | the issuance of an aggregate of 8,656,330 Holdco Shares to Amgen and MTPC pursuant to their profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”; |
| • | that none of the Holdco Warrants have been exercised; |
| • | that PIPE Investors fund the PIPE Financing in full in accordance with the Subscription Agreements; |
| • | that none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued; and |
| • | that neither the exercise of any NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options, nor the exercise of any awards that may be issued under Holdco’s LTIP following the closing of the Business Combination, have been taken into account. |
The following summarizes the number of Holdco Shares outstanding under the three redemption scenarios:
Shareholders | Assuming No Redemption(1) | Assuming Low Redemption(1) | Assuming High Redemption(1) | Assuming Maximum Redemption(1) | ||||||||||||||||||||||||||||
| Ownership in shares | Equity % | Ownership in shares | Equity % | Ownership in shares | Equity % | Ownership in shares | Equity % | |||||||||||||||||||||||||
NewAmsterdam Pharma shareholders(2) | 44,914,642 | 52.15 | % | 44,914,642 | 54.84 | % | 44,914,642 | 57.82 | % | 44,914,642 | 60.81 | % | ||||||||||||||||||||
FLAC public shareholders(3) | 12,800,000 | 14.86 | % | 8,576,000 | 10.47 | % | 4,352,000 | 5.60 | % | 538,518 | 0.73 | % | ||||||||||||||||||||
FLAC Initial Shareholders, including the Sponsor and affiliates(4) | 9,451,000 | 10.97 | % | 9,451,000 | 11.54 | % | 9,451,000 | 12.17 | % | 9,451,000 | 12.80 | % | ||||||||||||||||||||
PIPE Investors (excluding the FLAC Initial Shareholders and affiliates of the Sponsor) | 18,960,000 | 22.01 | % | 18,960,000 | 23.15 | % | 18,960,000 | 24.41 | % | 18,960,000 | 25.67 | % | ||||||||||||||||||||
Total Holdco Shares(5) | 86,125,642 | 100 | % | 81,901,642 | 100 | % | 77,677,642 | 100 | % | 73,864,160 | 100 | % | ||||||||||||||||||||
Additional Sources of Future Dilution | ||||||||||||||||||||||||||||||||
Earnout Shares | 1,886,137 | 2.19 | % | 1,886,137 | 2.30 | % | 1,886,137 | 2.43 | % | 1,886,137 | 2.55 | % | ||||||||||||||||||||
Holdco Public Warrants(6) | 4,600,000 | 5.34 | % | 4,600,000 | 5.62 | % | 4,600,000 | 5.92 | % | 4,600,000 | 6.23 | % | ||||||||||||||||||||
Holdco Private Placement Warrants(7) | 167,000 | 0.19 | % | 167,000 | 0.20 | % | 167,000 | 0.21 | % | 167,000 | 0.23 | % | ||||||||||||||||||||
Holdco Options currently outstanding(8) | 11,901,209 | 13.82 | % | 10,708,459 | 13.10 | % | 10,159,339 | 13.10 | % | 9,763,120 | 13.22 | % | ||||||||||||||||||||
| (1) | The 501,000 FLAC Private Placement Shares may not be redeemed. Pursuant to the Sponsor Support Agreement, the Investor Support Agreements and the FLAC Articles of Association, FLAC’s directors and officers and certain existing FLAC investors have agreed not to redeem an aggregate of 1,500,000 FLAC Public Shares. |
| (2) | (a) Excludes 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) and (b) includes an aggregate of 8,656,330 Holdco Shares issuable to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement.” |
| (3) | Includes all FLAC Public Shares other than 1,000,000 FLAC Class A Ordinary Shares held by an affiliate of the Sponsor. |
| (4) | Includes (i) 4,500,000 of Holdco Shares acquired in the PIPE Financing and (ii) 4,801,000 of Holdco Shares acquired by the Sponsor and certain of its affiliates in connection with the Business Combination. |
| (5) | The percentage presented in each scenario reflects the percentage of the total Holdco Shares outstanding, excluding Holdco Warrants, Holdco Options and the Earnout Shares. |
168
Table of Contents
| (6) | Based on the 4,600,000 FLAC Public Warrants outstanding on the date hereof. |
| (7) | Based on the 167,000 FLAC Private Warrants outstanding on the date hereof. |
| (8) | Based on 4,185,358 Holdco Options (or 1,964,287 NewAmsterdam Pharma Options outstanding on the date hereof), and (i) in the No Redemption scenario, up to approximately 7,715,849 Holdco Options expected to be awarded prior to the Closing, (ii) in the Low Redemption scenario, up to approximately 6,523,099 Holdco Options expected to be awarded prior to the Closing, (iii) in the High Redemption scenario, up to approximately 5,973,979 Holdco Options expected to be awarded prior to the Closing and (iv) in the Maximum Redemption scenario up to approximately 5,577,760 Holdco Options expected to be awarded prior to the Closing. The Holdco Options which Holdco expects to award in connection with the Closing are currently expected to be awarded to certain executive officers and directors. See the section entitled “Management of Holdco Following the Business Combination—Holdco LTIP” for more information. |
The level of redemption also impacts the effective underwriting fee incurred in connection with our IPO. In a No Redemption scenario, based on the approximately $138.1 million in the Trust Account as of June 30, 2022, FLAC’s $4.8 million in deferred underwriting fees represents an effective deferred underwriting fee of approximately 3.5% as a percentage of cash in the Trust Account. In a Maximum Redemption scenario, the effective underwriting fee would be approximately 31.0% as a percentage of the amount raised in the FLAC IPO (based on the assumption that the FLAC shareholders that are party to the Sponsor Support Agreement and Investor Support Agreements would not redeem their FLAC Ordinary Shares).
Background of the Business Combination
The following chronology summarizes the key meetings and events that led to the signing of the Business Combination Agreement. The following chronology does not purport to catalogue every conversation or correspondence among representatives of FLAC, NewAmsterdam Pharma, and the other various parties. In light of the ongoing COVID-19 pandemic and concerns and requirements around social distancing, travel and other COVID-19 protocols, most meetings were held virtually (including by telephone, teleconference and web-based video and meeting systems) where all participants could hear and be heard. In addition to formal FLAC Board and FLAC Special Committee meetings, the members of management had informal discussions with the FLAC Board and FLAC Special Committee members throughout the process. Further, the FLAC Board routinely held executive sessions among the independent directors without management in attendance.
FLAC is a blank check company incorporated on October 7, 2020 as a Cayman Islands exempted company and formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses. The potential Business Combination was the result of an extensive search for potential transactions utilizing the network and prior experience of the Sponsor and its affiliates, FLAC’s management team and the FLAC Board. The terms of the Business Combination Agreement were the result of arm’s length negotiations among the representatives of FLAC and NewAmsterdam Pharma.
On December 11, 2020, FLAC completed the FLAC IPO of 13,800,000 FLAC Public Units at a price of $10.00 per unit generating gross proceeds of $138,000,000 before underwriting discounts and expenses. Each unit consists of one FLAC Class A Ordinary Share and one-third of one FLAC Public Warrant. Each whole warrant entitles the holder thereof to purchase one FLAC Class A Ordinary Share for $11.50 per share, subject to certain adjustments. Simultaneous with the closing of the FLAC IPO, FLAC completed the private sale of 501,000 FLAC Private Placement Units at a price of $10.00 per unit to the Sponsor. In connection with the FLAC IPO, Credit Suisse Securities (USA) LLC (“Credit Suisse”) acted as the sole book-running manager, Goodwin Procter LLP (“Goodwin”) acted as U.S. legal advisor to FLAC and Campbells LLP (“Campbells”) acted as Cayman Islands legal advisor to FLAC. An aggregate amount of $4,830,000 will be payable to Credit Suisse (in its role as sole book-running manager) for deferred underwriting commissions solely in the event that FLAC completes an initial business combination, subject to the terms of the underwriting agreement.
In addition, as discussed below, Credit Suisse, Jefferies LLC (“Jefferies”) and William Blair & Company, L.L.C. (“William Blair”) were engaged by FLAC as placement agents, financial advisors, and capital markets advisors to FLAC in connection with the Business Combination and SVB Securities LLC (“SVB Securities”) was engaged by FLAC as placement agent in connection with the PIPE Financing, for which additional fees of $11 million in the aggregate will be paid upon the closing of the Business Combination.
No discussions regarding a potential business combination with any potential target were held prior to the completion of the FLAC IPO. Following the completion of the FLAC IPO, FLAC’s management team, led by
169
Table of Contents
Jamie Topper and David Topper (its Chief Executive Officer and Chief Financial Officer, respectively) commenced an active search for potential business combination targets in the life sciences sector. While FLAC’s management team was open to pursuing an initial business combination opportunity in any business, industry, sector or geographical location, it intended to capitalize on the ability of its management team to identify promising opportunities in the biotechnology sector and looked to identify targets that met some or all of the following criteria: (i) a compelling thesis targeting large addressable markets with significant unmet need; (ii) attractive assets at any stage of the product life cycle, from the preclinical stage through the commercial stage, with near-term value inflection points; (iii) a robust business and financial plan, with minimal additional capital expected to be required post-closing of the initial business combination; (iv) a competent and experienced management team; (v) a business that would benefit from a partnership with FLAC to access FLAC’s operational, commercial and financial expertise and its extensive networks, industry contacts and business relationships; and (vi) a business combination that would likely be well received by public investors so that the company would be expected to have continued access to the public capital markets in the future. FLAC’s management team believed that a target company having some combination of these attributes would offer attractive risk-adjusted equity returns for FLAC’s shareholders.
Between December 2020 and April 2022 (when FLAC entered exclusive discussions with NewAmsterdam Pharma), FLAC’s management team inquired about, was provided information on, or discussed the potential suitability of, over 85 different potential business combination targets with attributes that matched some or all of FLAC’s target criteria. These potential targets operated in a broad range of therapeutic areas, including oncology, immunology, cardiology, neurology and genetic diseases. During this process, FLAC’s management team determined to engage in preliminary discussions with approximately 42 of these potential targets which FLAC’s management team thought would be the most viable given the science, management team or potential market interest.
During this time, FLAC’s management team held regular reviews of investment opportunities with the FLAC Board. At these meetings, FLAC’s management team provided an update to the FLAC Board on its evaluations of, discussions with, and due diligence of prospective business combination targets, including a detailed review of several high priority opportunities with the FLAC Board. Discussions with the FLAC Board focused on asset potential, market sizing, management team, execution and operational capability, prior financial backing and readiness to transact. At these meetings, FLAC’s management team made recommendations on next steps for further evaluating these high priority opportunities which, based on preliminary due diligence, demonstrated to FLAC’s management team breakthrough potential, strong management teams, deep financial backing, and a large global market opportunity. Based on feedback from these FLAC Board discussions, FLAC engaged in varying levels of additional evaluations, discussions and due diligence with these prospective business combination targets based on, among other factors, interest of, and due diligence access granted by, the potential target and terms on which these potential targets would be willing to consider a potential transaction (such as the equity valuation ascribed to the potential target). In some cases, FLAC Board members joined FLAC management for follow up engagement with the management of the prospective targets.
Based on input from the FLAC Board, FLAC’s management team’s business judgment and the interest level of the potential target for further engagement and assessment, FLAC identified approximately 16 business combination targets as high priority opportunities as FLAC learned more about their science, research and intellectual property. FLAC entered into non-disclosure agreements with these potential targets, including with NewAmsterdam Pharma, to receive access to confidential evaluation materials, conduct due diligence, and engage in further preliminary discussions. Such non-disclosure agreements contained, among other things, customary non-disclosure and non-use provisions and a customary trust account waiver pursuant to which the counterparty waived any right, title, interest or claim in the Trust Account and agreed not to seek recourse against the Trust Account for any reason. None of these non-disclosure agreements had standstill provisions.
The FLAC team’s due diligence efforts (in concert with FLAC’s advisors) included, in many instances, meetings with the senior management of the prospective targets and their respective advisors, investigation and review of (depending on the prospective target): science and competitive differentiation; business plan, historical performance; pro forma capitalization; cash runway for the combined company; macroeconomic trends impacting the business and the industry in which each prospective target operates; competitive positioning versus
170
Table of Contents
comparable companies in the applicable industry; growth opportunities; performance history of the senior management team; potential impact to the prospective target from trends in the overall economy and industry in which the prospective target operates; regulatory environment; and benefits / challenges related to such prospective target engaging in a potential transaction with FLAC and becoming a public company.
None of these discussions with potential business combination targets proceeded beyond the initial diligence stages, other than discussions with NewAmsterdam Pharma and two other potential business combination targets, referred to herein as Company A and Company B, as discussed in more detail below. In most cases, following these additional discussions and due diligence, FLAC ultimately determined not to pursue each of these potential business combination opportunities either because (i) FLAC concluded that the target business would not be a suitable business combination opportunity for FLAC based on, among other factors, further due diligence indicating that the target business did not meet enough of the criteria FLAC had established (as described above) and the terms on which the potential target would be willing to consider a potential transaction; (ii) FLAC did not meet the valuation expectations of the potential target; or (iii) the potential target pursued an alternative transaction or strategy.
From December 2020 to January 2021, representatives of FLAC, including Jamie Topper, and representatives from NewAmsterdam Pharma, including Michael Davidson, Chief Executive Officer of NewAmsterdam Pharma, and John Kastelein, Chief Scientific Officer of NewAmsterdam Pharma, met on several occasions to discuss NewAmsterdam Pharma’s clinical program and financing plans. During these initial discussions, representatives of NewAmsterdam Pharma conveyed that it planned to prioritize pursuit of alternative financing transactions in the private market.
On January 14, 2021, NewAmsterdam Pharma announced its completion of a $196 million Series A financing, reflecting a valuation of €267.7 million.
On January 29, 2021, the FLAC Board held a meeting, at which members of FLAC’s management team and Goodwin were present, during which FLAC’s management team reviewed the status of potential business combination opportunities with the FLAC Board, including NewAmsterdam Pharma. The FLAC Board discussed the pros and cons of each opportunity. Following such review, FLAC’s Board and management team determined that NewAmsterdam Pharma, despite its encouraging science and impressive management team, was not a priority target at that time due to its recent completion of a private financing round, which meant that it did not have an immediate need for financing, as well as the early stage of NewAmsterdam Pharma’s clinical program given the data published at such time.
The first potential target which FLAC proceeded beyond initial diligence stages with was a preclinical-stage precision medicines company focused on oncology (“Company A”). Company A was already known to FLAC’s management team prior to the FLAC IPO due to Frazier Life Sciences’ involvement in the founding and early financing of Company A. In April 2021, Company A emerged as a priority target based on Company A’s science, management team, market and intellectual property, after which FLAC determined to execute a non-disclosure agreement with Company A to continue discussions. Beginning in May 2021, FLAC conducted extensive due diligence on Company A, including multiple discussions with the FLAC Board, management team and advisors, as well multiple discussions with Company A’s management team. In July and August 2021, FLAC and its advisors began working closely with Company A on the terms of a potential business combination. During this period, FLAC also continued to perform diligence on other potential business combination targets, including Company B.
Between August 3 and August 25, 2021, the FLAC Board held meetings, at which members of FLAC’s management team and Goodwin were present, to discuss the submission of a non-binding letter of intent to acquire Company A. In recognition of the prior investment of Frazier Life Sciences in Company A, related Board positions, and the resulting economic interest of affiliates of the Sponsor in both FLAC and Company, the FLAC Board resolved on August 4, 2021 to establish the FLAC Special Committee, comprised solely of disinterested and independent directors, to provide independent oversight to the proposed business combination process, evaluate the deal terms from an independent perspective, engage outside advisors to diligence Company A or other potential business combination targets, and consider and, if appropriate, engage a third-party valuation firm to provide financial advisory services and deliver a fairness opinion related to a potential business combination with Company A or otherwise. The FLAC Special Committee was and is comprised of independent directors
171
Table of Contents
Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher and Krishna R. Polu. At these meetings, the Board authorized FLAC’s management team to continue to negotiate the terms of the non-binding letter of intent with Company A.
On August 13, 2021, after internal discussion among FLAC’s management team and members of the FLAC Board, and with the support of the FLAC Board and FLAC Special Committee, FLAC submitted a non-binding letter of intent to Company A for consideration. Over the next several days, FLAC and Company A engaged in discussions regarding the deal structure and terms, including a PIPE financing, and exchanged revised drafts of the letter of intent, which included a mutual exclusivity provision valid for 60 days, but with a limited exception for Company A to continue ongoing discussions with another third party for an alternative transaction, subject to a $1.0 million break-up fee payable to FLAC if Company A entered into a definitive agreement for the alternative transaction with such third party. On September 13, 2021, with the prior unanimous approval of the FLAC Board, based on the recommendation of the FLAC Special Committee, FLAC and Company A executed the letter of intent (the “Company A LOI”).
Following entry into the Company A LOI, representatives of FLAC, members of the FLAC Special Committee, Company A, and their respective legal and financial advisors conducted confirmatory and legal diligence in the subsequent weeks through October 2021, including calls with experts and third-party consultants, to further assess the science, manufacturing, commercial opportunity, and the target product profile for Company A’s product candidates. On October 28, 2021, with the prior approval of the FLAC Board (and based on the recommendation of the FLAC Special Committee), FLAC and Company A executed an amendment to the Company A LOI to extend the exclusivity period, including the exception for the alternative transaction, through December 31, 2021. In the subsequent weeks through November 2021, representatives of FLAC, members of the FLAC Special Committee, Company A, and their respective legal and financial advisors continued discussions regarding the proposed business combination, including the PIPE financing, documentation and legal and business due diligence. In late November 2021, Company A informed FLAC that it was proceeding with the alternative acquisition proposal rather than engaging further with FLAC as a potential business combination target, and discussions with Company A regarding a business combination with FLAC were terminated. As a result of such termination, Company A paid a break-up fee of $1.0 million to FLAC as required pursuant to the Company A LOI.
The other potential target which FLAC proceeded beyond initial diligence stages with was a clinical-stage oncology company (“Company B”). On May 4, 2021, representatives of Credit Suisse arranged a meeting between representatives of FLAC and Company B to discuss a potential business combination with FLAC, after which FLAC determined that one of its affiliates execute a non-disclosure agreement with Company B to continue discussions. Between June 14, 2021 and July 31, 2021, FLAC conducted extensive due diligence on Company B, including multiple discussions with members of the FLAC Board and of the FLAC Special Committee, FLAC’s management team and advisors, and also had multiple discussions with Company B’s management team. During this period, Company B informed FLAC of its plans to prioritize pursuit of alternative financing transactions in the private market, rather than a business combination.
Once the Company A LOI and exclusivity obligations with respect to Company A were terminated in November 2021, FLAC and its advisors refocused and concentrated their efforts on NewAmsterdam Pharma, which had completed a private financing round and had advanced its clinical program in the period since its initial discussions with FLAC had ceased at the end of January 2021. FLAC’s management team and the FLAC Board considered NewAmsterdam Pharma as the most compelling opportunity after considering the preliminary diligence findings, due to its science, management team, market and intellectual property. In January 2022, FLAC and its advisors began working closely with NewAmsterdam Pharma on a potential business combination.
On January 13 and 14, 2022, representatives of FLAC, including Jamie Topper, and representatives of NewAmsterdam Pharma, including Michael Davidson and Lina Gugucheva, Chief Business Officer of NewAmsterdam Pharma, attended a meeting, where Dr. Davidson and Ms. Gugucheva provided a brief update on NewAmsterdam Pharma’s current business, which, importantly, included news of additional clinical data that had been generated since the prior interactions with FLAC and which supported the potential of NewAmsterdam Pharma’s product candidate to reduce LDL-C levels on top of high-intensity statin therapy, and further distinguished it from prior clinical-stage CETP inhibitors, mitigating prior concerns of FLAC’s management
172
Table of Contents
team as to the early stage of NewAmsterdam Pharma’s clinical program. At this meeting, Dr. Davidson and Ms. Gugucheva also presented on NewAmsterdam Pharma’s history, current business and future vision, and answered questions from FLAC’s management team. Following that meeting, the parties agreed to move forward with executing a non-disclosure agreement to engage in further discussions.
On January 19, 2022, the FLAC Board held a meeting, at which all members of FLAC’s management team and representatives of Goodwin were present, to discuss the submission of a non-binding letter of intent to acquire NewAmsterdam Pharma. At this meeting, Jamie and David Topper reported to the FLAC Board on the recent discussions with NewAmsterdam Pharma, and the FLAC Board engaged in a discussion about the potential benefits and risks of pursuing a deal with NewAmsterdam Pharma, including the science and the size of the addressable market and growth opportunities, the talent on the management team and challenges attributable to clinical stage companies. In light of the recent developments and discussions with NewAmsterdam Pharma, including the recent clinical data read-out, the FLAC Board authorized FLAC’s management team to pursue the submission and negotiation of a non-binding letter of intent with NewAmsterdam Pharma. Also at this meeting, the FLAC Board determined that, although there were no known actual or potential conflicts of interests between any of the FLAC Board members or either of NewAmsterdam Pharma or Company B, it would not disband the FLAC Special Committee in case the FLAC Special Committee could be helpful with diligence, discussions and progressing negotiations.
On January 24, 2022, FLAC and NewAmsterdam Pharma executed a non-disclosure agreement, on terms substantially similar to those that were entered into with other potential targets approached by FLAC. On January 25, 2022, NewAmsterdam Pharma granted FLAC access to an electronic data room containing certain materials relating to NewAmsterdam Pharma’s commercial products, clinical data and trials, and platform, pipeline, and intellectual property, for purposes of FLAC’s preliminary business and financial due diligence on NewAmsterdam Pharma. Subsequently, representatives of FLAC and NewAmsterdam Pharma held various due diligence calls to discuss NewAmsterdam Pharma’s leadership team, commercial products, commercial strategy including strategic collaborations, regulatory paths and plans, pipeline and platform, competitive positioning and barriers, valuation, financials and information on its expected future cash burn.
On February 21, 2022, representatives of FLAC, including David Topper and Gordon Empey, FLAC’s Vice President and General Counsel of FLAC, and representatives of NewAmsterdam Pharma, including Ms. Gugucheva and Louise Kooij, Chief Financial Officer of NewAmsterdam Pharma, attended a meeting to discuss NewAmsterdam Pharma’s capitalization table and the proposed terms of a non-binding letter of intent to be submitted to NewAmsterdam Pharma.
On February 22, 2022, after internal discussion among all members of FLAC’s management team and FLAC Board, and with the unanimous support of the FLAC Board, FLAC submitted a non-binding letter of intent to enter into a transaction with NewAmsterdam Pharma, on terms reflecting: (i) a pre-money equity valuation of approximately $367.25 million and a PIPE financing that, combined with the FLAC IPO proceeds, would total approximately $695.0 million; (ii) a PIPE financing of $150.0 million, with (x) funds affiliated with the Sponsor committing approximately $50.0 million in such transaction and (y) existing NewAmsterdam Pharma shareholders or their affiliates committing approximately $50.0 million in such transaction; (iii) a post-combination company board of directors, with the size and composition to be mutually agreed upon by FLAC and NewAmsterdam Pharma; and (iv) a binding exclusivity period of up to 60 days. The pre-money equity valuation of $367.25 million reflected a price per share equivalent to NewAmsterdam Pharma’s Series A purchase price, which is consistent with FLAC’s step-up analysis, the results of which determined that average and median step-ups for comparable biotechnology company initial public offerings in the first quarter of 2022 was 1.0x. The $367.25 million valuation was calculated by (a) converting the Series A post-money valuation of €267.7 million to dollars, based on a historical exchange rate of $1.00 to €1.13 as of February 22, 2022, then (b) adding the value of the shares to be granted to Amgen and MTPC pursuant to their profit rights (as described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”) that would be triggered in connection with a potential business combination, entitling Amgen and MTPC to 10.0% and 7.63%, respectively, of the fully diluted shares of NewAmsterdam Pharma.
173
Table of Contents
On March 4, 2022, following a series of discussions with representatives of NewAmsterdam Pharma, including Dr. Davidson and Ms. Gugucheva, regarding NewAmsterdam Pharma’s growth potential and clinical success outlook, members of the FLAC Board and management team met to discuss a potential increase in its valuation of NewAmsterdam Pharma in light of NewAmsterdam Pharma’s receipt of strategic interest from unrelated third parties. After internal discussion, the FLAC Board and management team determined to include additional contingent consideration with an earnout structure in order to bolster the long-term alignment between investor and management incentives. FLAC subsequently submitted a revised letter of intent to NewAmsterdam Pharma, which reflected additional earnout consideration to NewAmsterdam Pharma shareholders of 4,000,000 shares in the post-combination company, subject to the achievement of certain clinical milestones.
On March 10, 2022, the NewAmsterdam Pharma Board held a meeting, at which SVB Securities was present, to discuss the terms of the revised letter of intent.
On March 14, 2022, NewAmsterdam Pharma delivered a further revised letter of intent to FLAC, along with a note that support for the PIPE financing would be important to NewAmsterdam Pharma before NewAmsterdam Pharma would enter into exclusivity, and which revised letter of intent reflected, among other things: (i) an increase in additional earnout consideration to 4,856,137 shares in the post-combination company; (ii) a decrease in the PIPE size to $100 million; (iii) a requirement that proceeds from the Trust Account and other cash of FLAC at closing of the business combination be at least $69 million (not taking the PIPE into account); (iv) the Sponsor and its affiliates agreeing to a forfeiture of up to 50% of its Founder Shares pro rata to the level of redemptions of the FLAC Public Shares in connection with the business combination; and (v) subject in each case to certain exceptions, a lock-up period of (a) six months for any NewAmsterdam Pharma shareholder who is a director, executive officer, employee or holder of “restricted securities” under Rule 144 and (b) one year for the Sponsor and its affiliates.
On March 21, 2022, the FLAC Board held a meeting, at which all members of FLAC’s management team and representatives of Goodwin were present, to discuss the terms of the revised letter of intent provided by NewAmsterdam Pharma and a potential counter proposal, including whether or not to pursue exclusivity at that time. The FLAC Board discussed the pros and cons of agreeing to engage with NewAmsterdam Pharma on an exclusive basis. The FLAC Board authorized management to continue discussions with NewAmsterdam Pharma, but noted the risks related to pursuing only one potential target for a protracted period of time, including because the FLAC Board continued to consider Company B a viable alternative given that Company B had expressed renewed interest in a potential business combination with FLAC as opposed to private financing, and encouraged the management team to continue discussions with Company B in parallel.
On March 23, 2022, the FLAC Board held a meeting at which all members of FLAC’s management team and representatives of Goodwin were present, to discuss the status of the potential business combinations with NewAmsterdam Pharma and Company B, including the submission of a non-binding letter of intent to Company B. The FLAC Board discussed the pros and cons of pursuing a deal with Company B, including anticipated necessary valuation to reach agreement with Company B and relative stage of development of their clinical program. At this meeting, the FLAC Board authorized management to submit a non-binding proposal to Company B.
On March 23, 2022, FLAC delivered a further revised letter of intent to NewAmsterdam Pharma, with the following revisions, among others: (i) a change in the PIPE and Trust Account proceeds condition, providing for minimum aggregate proceeds from both the PIPE and Trust Account of at least $140 million; (ii) revising the forfeiture of Founder Shares so that at closing of a business combination, 25% of the Founder Shares held by the Sponsor would be forfeited in case such aggregate cash proceeds were less than $170 million; (iii) a condition that FLAC would only be required to close if cash held by NewAmsterdam Pharma were at least €105 million, subject to certain permitted adjustments; and (iv) a proposal that the board of directors of the combined company be comprised of seven to nine members, of which two would be designated by the Sponsor.
On March 28, 2022, FLAC submitted a non-binding letter of intent to Company B for consideration and engaged in discussions with Company B regarding potential transaction value and deal structure and terms.
174
Table of Contents
On March 28, 2022, representatives of NewAmsterdam Pharma, including Dr. Davidson and Ms. Gugucheva and representatives of FLAC, including Jamie and David Topper and Gordon Empey, met to review the structure and terms of the proposed collaboration between NewAmsterdam and Menarini, through which Menarini would have exclusive commercialization rights on NewAmsterdam Pharma’s product candidate in the EU.
On April 2, 2022, NewAmsterdam Pharma submitted its proposed revisions to the letter of intent for FLAC’s consideration, including the following: (i) that the valuation in a potential business combination would have to be increased from the initial $367.25 million to also include the cash proceeds received by NewAmsterdam Pharma from the upfront payment under the Menarini collaboration transaction; (ii) eliminating the forfeiture of Sponsor shares; (iii) eliminating the closing condition relating to Aggregate Cash Proceeds of $140 million and once again reinstating a condition that the PIPE proceeds be $100 million; and (iv) eliminating the condition to FLAC’s closing obligations relating to NewAmsterdam’s balance of cash of at least €105 million at closing.
On April 7, 2022, all members of the FLAC’s management team reviewed the status of the potential business combinations with each of NewAmsterdam Pharma and Company B with the members of the FLAC Board, including developments in Jamie and David Topper’s dialogue with each potential target and the terms proposed to be addressed in a further revised letter of intent to NewAmsterdam Pharma. The FLAC Board discussed the pros and cons of each opportunity, including their concern that, given the early stage of Company B’s assets, a business combination with Company B was likely to present greater risk, both in terms of execution of a potential business combination, and thereafter with the combined company. Following such discussion, the FLAC Board determined that FLAC should continue its negotiations with both NewAmsterdam Pharma and Company B, including submitting a further revised letter of intent to NewAmsterdam Pharma on the terms discussed at this meeting. The FLAC management team and the FLAC Board noted that NewAmsterdam Pharma was a more compelling opportunity due to its progress in clinical research, science, management team and intellectual property, as well as its current needs for funding, which made the proposed business combination with FLAC and an accompanying PIPE financing an attractive opportunity. In light of the heightened execution and valuation risks with Company B, and given that NewAmsterdam Pharma continued to be the most attractive opportunity for FLAC, the FLAC Board authorized management to agree to a period of exclusivity with NewAmsterdam Pharma, and indicated that it would support a decision by management to cease discussions with Company B.
Thereafter, on April 7, 2022, FLAC submitted a further revised letter of intent to NewAmsterdam Pharma, accepting the latest changes proposed by NewAmsterdam Pharma and adding a binding $1.0 million break-up fee payable by NewAmsterdam Pharma for a material breach of its exclusivity obligations.
On April 14, 2022, after further discussions and consideration, FLAC’s management team determined not to pursue a business combination with Company B due to the heightened execution and valuation risks. In particular, management had concerns about Company B’s ability to meet FLAC’s timeline to consummate a business combination and the early-stage nature of the company’s assets, which could lead to protracted uncertainty and risk regarding the limitations of its data and the lack of near-term value inflection points.
Also on April 14, 2022, representatives of FLAC, including Jamie and David Topper, presented FLAC’s proposal to the NewAmsterdam Pharma Board.
On April 15, 2022, NewAmsterdam Pharma and FLAC finalized and executed a letter of intent (the “Final LOI”), which reflected the terms contained in FLAC’s last proposal, with the following relevant changes: (i) the break-up fee changed into a mutual obligation to reimburse expenses of the other party up $1.0 million in case of breach by a party of its exclusivity obligations, (ii) the pre-money equity valuation was specified as $491.0 million, reflective of the sum of the previously presented $367.0 million and the contemplated upfront proceeds from a potential Menarini collaboration deal, (iii) the exclusivity obligations expressly excluded NewAmsterdam Pharma’s negotiations with Menarini and certain other permitted disclosures or commercial transactions contemplated by NewAmsterdam Pharma at the time and (iv) the inclusion of a pool for grants of future equity awards by the combined company equal to 15% of its shares outstanding after closing of the proposed business combination. The Final LOI provided for an exclusivity period of up to 60 days.
175
Table of Contents
On April 21, 2022, representatives of FLAC, NewAmsterdam Pharma, Credit Suisse, Jefferies and SVB Securities and their respective legal advisors held an organizational call, led by representatives of Credit Suisse, to discuss the proposed business combination, including the timeline, various workstreams, investor targets for the PIPE financing, and the investor management presentation to be provided to potential PIPE investors.
Also on April 21, 2022, NewAmsterdam Pharma granted FLAC and its advisors access to an expanded electronic data room containing additional information relating to NewAmsterdam Pharma for FLAC’s due diligence review. The materials in the data room reviewed by FLAC and its advisors included, among other things, documents relating to NewAmsterdam Pharma’s corporate governance and capital structure, employees, material contractual relationships, real property, intellectual property, regulatory compliance and tax. Between April 21, 2022 and July 24, 2022, FLAC and its strategic, financial, legal, tax and other advisors conducted further business, financial, tax, legal, regulatory and intellectual property due diligence with respect to NewAmsterdam Pharma, in each case, based on information available in the data room (including through oral and written responses from the management team of NewAmsterdam Pharma) and customary due diligence calls with NewAmsterdam Pharma’s management team and its advisors, including calls with experts and third-party consultants. In addition, FLAC engaged a number of advisors to assist with various aspects of the proposed business combination, including Houthoff as Dutch counsel and WSGR as intellectual property counsel. During this period, representatives and advisors of FLAC and NewAmsterdam Pharma held various calls and meetings to discuss various aspects of the proposed business combination and PIPE financing, including the proposed transaction structure, due diligence matters, and the preparation of materials, including the investor management presentation.
On April 27, 2022, Goodwin delivered an initial draft of the subscription agreement relating to the PIPE Financing to Kirkland & Ellis LLP, as legal advisor to the placement agents (“Kirkland & Ellis”) and Covington & Burling LLP, as legal advisor to NewAmsterdam Pharma (“Covington”). In the subsequent weeks, Goodwin, Kirkland & Ellis and Covington continued to negotiate and exchange drafts of the subscription agreement.
On May 2, 2022, NewAmsterdam Pharma retained Moelis & Company LLC (“Moelis”) in connection with certain potential licensing transactions and M&A transactions with operating companies. The approximate fee payable to Moelis in connection with its services payable upon consummation of the Transactions is estimated to be €1 million.
On May 4, 2022, the FLAC Board held a meeting, at which members of FLAC’s management team and Goodwin were present, during which FLAC’s management team discussed the status of the proposed business combination with NewAmsterdam Pharma and the proposed PIPE Financing, including research analyst coverage and the status and read-outs of business and legal due diligence, noting that no red flags had been identified. Given recent regulatory and litigation developments in the SPAC market, the members of the FLAC Special Committee suggested that the FLAC Board engage a third-party valuation and advisory firm to conduct independent financial analyses and deliver a fairness opinion as to the fairness, from a financial point of view, to the unaffiliated holders of FLAC Shares of the consideration to be received by them in connection with the proposed business combination. The FLAC Board noted that it was not required to obtain a third-party valuation or fairness opinion in connection with the determination to approve the proposed business combination but did so as part of its due diligence and evaluation of it.
Between May 13, 2022 and June 24, 2022, representatives of Covington, Kirkland & Ellis, and Goodwin reviewed and commented on the investor presentation for potential investors in the proposed PIPE Financing prepared by NewAmsterdam Pharma and FLAC. The investor presentation outlined the proposed business combination and included information regarding NewAmsterdam Pharma, which was refined through several rounds of review and comment amongst FLAC’s management team, NewAmsterdam Pharma’s management team and their respective counsel and advisors.
On May 23, 2022, FLAC executed an engagement letter with Credit Suisse, pursuant to which Credit Suisse was engaged as lead placement agent in connection with the PIPE Financing, and financial advisor and capital markets advisor to FLAC.
176
Table of Contents
Also on May 23, 2022, FLAC and NewAmsterdam Pharma entered into a letter agreement extending the exclusivity period under the Final LOI until July 1, 2022.
On May 24, 2022, Goodwin delivered an initial draft of the Business Combination Agreement to Covington, substantially reflecting the terms agreed to in the Final LOI, which draft FLAC had reviewed and discussed with Goodwin in detail.
On May 27, 2022, the FLAC Board held a meeting, at which all members of FLAC’s management team and representatives of Goodwin were present. At this meeting, members of FLAC management and the FLAC Special Committee reported to the full FLAC Board on the recent discussions with various financial advisors and recommended that the FLAC Board engage Lincoln to conduct financial analyses and deliver a fairness opinion to the FLAC Board. The FLAC Board recommended Lincoln based on its industry experience as a nationally recognized investment banking firm with substantial experience in transactions similar to the Transactions. The FLAC Board authorized Mr. Baltera and FLAC’s management team to engage Lincoln on the terms discussed at the meeting.
On May 31, 2022, FLAC entered into an engagement letter with Lincoln, pursuant to which Lincoln would provide financial advisory services and deliver a fairness opinion in connection with the business combination with NewAmsterdam Pharma.
On June 7, 2022, the FLAC Board held a meeting, at which all members of FLAC’s management team and representatives of Goodwin were present, during which FLAC’s management team discussed the status of the proposed business combination with NewAmsterdam Pharma and the proposed PIPE Financing, including investor outreach. The FLAC Board continued to express its support for FLAC’s pursuit of a business combination with NewAmsterdam Pharma.
On June 16, 2022, Covington delivered a revised draft of the Business Combination Agreement to Goodwin, with numerous revisions to, among other things, the proposed representations, warranties and covenants the parties.
During the ensuing weeks, the parties and their legal counsel continued negotiations of not only the Business Combination Agreement and form of subscription agreement, but also exchanged drafts, negotiated and finalized the forms of various ancillary documents, including the Company Support Agreement, Sponsor Support Agreement, the Holdco LTIP, form of Investor Rights Agreement, form of Lock-Up Agreement and form of Warrant Assumption Agreement.
On June 21, 2022, the FLAC Board held a meeting, at which all members of FLAC’s management team and representatives of Goodwin were present, during which FLAC’s management team discussed the status of the proposed business combination with NewAmsterdam Pharma and the proposed PIPE Financing, including a decrease in the committed R&D funding portion of the payments proposed to be made to NewAmsterdam Pharma in its transaction with Menarini and a corresponding increase in the deferred portion of such payments, which changes impacted FLAC’s valuation analysis of NewAmsterdam Pharma. Based on such changes, FLAC’s management team proposed to revise the terms of the business combination with NewAmsterdam Pharma to (i) increase the size of the PIPE Financing from $100 million to $150 million and (ii) reduce the aggregate value of the Earnout Shares from approximately $48.6 million to approximately $18.9 million or lower. The FLAC Board then engaged in extensive discussions and deliberations with FLAC’s management. Among other things, the FLAC Board asked questions pertaining to legal and business due diligence, valuation, feedback from the proposed PIPE Investors, risks, timing and process to closing. Following such discussion, FLAC’s independent directors held an executive session to further discuss the merits of the proposed business combination. After further discussions and deliberations, the FLAC Board approved the revised terms of the business combination with NewAmsterdam Pharma and directed FLAC’s management to proceed with negotiating the proposed terms.
On June 21, 2022, the NewAmsterdam Pharma Board held a meeting, during which NewAmsterdam Pharma’s management discussed a potential revised valuation of NewAmsterdam Pharma, the revised terms of the business combination with FLAC and entry into the Menarini License.
On June 23, 2022, NewAmsterdam Pharma entered into the Menarini License, pursuant to which NewAmsterdam Pharma granted Menarini an exclusive license to commercialize obicetrapib, if approved, in the majority of European countries, either as a monotherapy or as part of a fixed dose combination with ezetimibe,
177
Table of Contents
for cardiovascular diseases. NewAmsterdam Pharma subsequently issued a press release announcing the Menarini License on June 28, 2022.
On June 26, 2022, Goodwin delivered a revised draft of the Business Combination Agreement to Covington, which decreased the number of Earnout Shares payable in the transaction from 4,856,137 to 1,886,137, consistent with the change in valuation discussed by the parties, included a revised closing condition that the aggregate proceeds of the PIPE Financing be at least $150 million and various changes to the proposed representations, warranties and covenants in the Business Combination Agreement.
Between June 27, 2022 and July 24, 2022, NewAmsterdam Pharma, FLAC and their respective advisors held a number of virtual meetings with third-party investors regarding the PIPE Financing. Five of the investors were existing FLAC shareholders and six were existing NewAmsterdam Pharma shareholders. Of those, four existing FLAC shareholders, in addition to Frazier Healthcare Partners, and three existing NewAmsterdam Pharma shareholders ultimately decided to participate in the PIPE Financing. Other investors were selected and contacted during this period, following consultation with the placement agents, based on such investors’ perceived interest in NewAmsterdam Pharma. In addition, during this period, NewAmsterdam Pharma, FLAC and their advisors regularly held meetings to debrief on investor meetings and develop a capital allocation strategy for the PIPE Financing. There are material relationships between certain PIPE investors and:
| • | the Sponsor, namely (a) preexisting arm’s length commercial relationships between certain individuals affiliated with the Sponsor (and/or entities affiliated with them) and certain PIPE Investors, and (b) the fact that certain privately held entities affiliated with Frazier Life Sciences will participate in the PIPE Financing as investors, and Frazier Life Sciences is affiliated with the Sponsor; |
| • | NewAmsterdam Pharma, given that such investors are current NewAmsterdam Pharma shareholders, and some of them currently have the right to appoint directors to NewAmsterdam Pharma’s board; and |
| • | FLAC, on account of (a) such investors’ existing shareholdings in FLAC, and (b) the fact that such investors have relationships with certain of FLAC’s officers and directors. |
On June 30, 2022, FLAC and NewAmsterdam Pharma executed a letter agreement to (i) extend the exclusivity period under the Final LOI until July 31, 2022; (ii) memorialize their agreement to reduce the number of Earnout Shares to 1,886,137; and (iii) increase the PIPE transaction size to $150 million, with (x) funds affiliated with the Sponsor committing approximately $40.0 million in such transaction and (y) existing NewAmsterdam Pharma shareholders or their affiliates committing approximately $30.0 million in such transaction.
Also on June 30, 2022, FLAC executed engagement letters with each of Jefferies, SVB Securities and William Blair (together with Credit Suisse, the “placement agents”), pursuant to which Jefferies, SVB Securities and William Blair were engaged as placement agents to FLAC in connection with the PIPE Financing and Jefferies and William Blair were also engaged as financial advisor and capital markets advisor to FLAC. Prior to FLAC’s engagement of SVB Securities as a placement agent, FLAC was advised of the potential conflicts of interest arising out of the dual role of SVB Securities as a placement agent to FLAC and a financial and capital markets advisor to NewAmsterdam Pharma (SVB Securities having been appointed as financial and capital markets advisor to NewAmsterdam Pharma on May 31, 2022), and considered the benefits and risks thereof to FLAC. Each of NewAmsterdam Pharma and FLAC consented to the engagement of SVB Securities as a placement agent and a financial and capital markets advisor to NewAmsterdam Pharma. FLAC and NewAmsterdam Pharma agreed that this syndicate of banks was well-suited for their roles given their respective experience in the life sciences industry and with transactions of this nature, and their respective levels of familiarity with FLAC, NewAmsterdam Pharma and their respective teams and investor bases. To date, FLAC and NewAmsterdam Pharma have not received notice from any of their respective placement agents, financial advisors, or capital markets advisors indicating they may cease involvement in the Business Combination.
Between July 7, 2022 and July 19, 2022, Covington and Goodwin exchanged various drafts of the Business Combination Agreement, aiming to finalize the terms of the various provisions therein. During this period, representatives of FLAC, including Jamie and David Topper, and representatives of NewAmsterdam Pharma,
178
Table of Contents
including Dr. Davidson and Ms. Gugucheva, held discussions to agree on the period of five years after which the Earnout Shares would no longer be payable, but that in certain circumstances where the combined public company went through a change of control, the Earnout Shares would become immediately payable. The parties also agreed to reduce the pool for grant of equity awards from 15% to 13% of the outstanding shares of Holdco after the Merger, but with the inclusion of an evergreen provision whereby such pool will be increased by 5% of such shares on annual basis. Representatives of Goodwin and Covington also held discussions during such period to resolve a number of issues in the drafting of the Business Combination Agreement.
On July 19, 2022, Bloomberg published an article reporting that NewAmsterdam Pharma was in discussions with FLAC to go public in a transaction that valued NewAmsterdam Pharma at over $700 million.
From July 20, 2022 to July 24, 2022, representatives of Covington, Kirkland & Ellis and Goodwin negotiated the terms of the subscription agreements with the various investors in the PIPE Financing and their respective legal counsel. During this time, due to investor demand, FLAC, NewAmsterdam Pharma and their financial advisors determined to increase the size of the PIPE Financing from $150 million to $234.6 million.
Also during this time, FLAC, NewAmsterdam Pharma and their respective legal counsels finalized the drafts of the Business Combination Agreement and the ancillary agreements, including after considering input of certain investors in the PIPE Financing. During these discussions and after discussions with the members of the FLAC Board, FLAC agreed to include a closing condition in the Business Combination Agreement that NewAmsterdam Pharma would only be required to consummate the business combination if the Aggregate Cash Proceeds, were equal to or greater than $250 million, replacing the previous condition that required only the aggregate cash proceeds of the PIPE Financing to be no less than $150 million. Also during these discussions, certain investors, including funds affiliated with Frazier and Bain Capital Life Sciences, agreed to enter into the Investor Support Agreements, under which such investors agreed not to redeem their FLAC Public Shares, representing an aggregate of approximately $15 million.
On July 24, 2022, agreements were reached with each of the potential investors to the PIPE Financing to execute a subscription agreement simultaneously with the execution of the Business Combination Agreement and the ancillary agreements.
On July 24, 2022, a meeting of the FLAC Board was held with representatives of Lincoln and Goodwin and all members of FLAC’s management team in attendance. Lincoln presented its methodology overview and financial analyses conducted in connection with the proposed business combination in support of Lincoln’s fairness opinion. Thereafter, Lincoln rendered its written opinion that the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement was fair, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement). For a detailed discussion of Lincoln’s opinion, see “ —Opinion of the Financial Advisor to the FLAC Board” below. Representatives from Goodwin reminded the FLAC Board of its fiduciary duties in connection with a business combination transaction, which had been discussed with the FLAC Board throughout the process. Based on the factors cited in “ —The FLAC Board’s Reasons for the Business Combination,” including the size and interest from investors in the PIPE Financing and based on the recommendation of the FLAC Special Committee at this meeting, the FLAC Board unanimously resolved that the proposed business combination is in the best interests of FLAC and its shareholders, and adopted, among other things, resolutions (i) determining that it is in the best interests of FLAC and its shareholders to approve the execution and delivery of the Business Combination Agreement and the ancillary agreements (including the Plan of Merger) and the transactions contemplated thereby (including the Merger); (ii) adopting the Business Combination Agreement and ancillary agreements (including the Plan of Merger) and approving FLAC’s execution, delivery and performance of the same and the consummation of the transactions contemplated thereby (including the PIPE Financing and the Merger); and (iii) recommending that the FLAC shareholders vote in favor of the Business Combination Proposal and the Merger Proposal.
179
Table of Contents
On July 25, 2022, NewAmsterdam Pharma, as sole shareholder of Holdco, and the Holdco Board adopted, among other resolutions, resolutions (i) determining that it is in the best interests of Holdco and its stakeholders to approve the execution and delivery of the Business Combination Agreement and the various ancillary documents and the transactions contemplated by each of the foregoing (including the Merger); and (ii) approving the execution and delivery of the Business Combination Agreement, the ancillary documents and the transactions contemplated thereby (including the Exchange and the Merger).
On July 25, 2022, the NewAmsterdam Pharma Board adopted, among other resolutions, resolutions (i) determining that it is in the best interests of NewAmsterdam Pharma and its stakeholders to approve the execution and delivery of the Business Combination Agreement and the ancillary documents and the transactions contemplated by each of the foregoing; (ii) approving the execution and delivery of the Business Combination Agreement, the ancillary documents and the transactions contemplated thereby (including the Exchange), and (iii) recommending that the NewAmsterdam Pharma shareholders vote to approve the Business Combination Agreement, the ancillary documents and the transactions contemplated thereby.
On July 25, 2022, the parties entered into the Business Combination Agreement and certain ancillary agreements and the PIPE Investors executed definitive documentation with respect to the PIPE Financing, which provided for binding subscriptions to purchase an aggregate of 23,460,000 Holdco Shares at $10.00 per share for an aggregate purchase price of $234.6 million.
On July 25, 2022, FLAC and NewAmsterdam Pharma issued a joint press release announcing the execution of the Business Combination Agreement and FLAC filed a Current Report on Form 8-K, with the Business Combination Agreement, the Company Support Agreement, the Sponsor Support Agreement, the form of the Investor Support Agreement, the form of the Subscription Agreement, the press release and an investor presentation included as exhibits, which filing was also filed pursuant to Rule 425 under the Securities Act.
In the third quarter of 2022, after the announcement of the Business Combination, in light of its plans to become a public company listed on Nasdaq, NewAmsterdam Pharma determined to seek to appoint in the first half of 2023 a new chief financial officer of Holdco with deep equity capital markets experience and strong relationships with U.S. investors, including biotechnology investors. NewAmsterdam Pharma evaluated several candidates for a potential public company chief financial officer role, including one candidate that reached final stages of the NewAmsterdam Pharma Board’s evaluation but was not unanimously supported by the NewAmsterdam Pharma Board. David Topper of FLAC submitted himself for consideration as a candidate in mid-September 2022 and NewAmsterdam Pharma and the NewAmsterdam Pharma Board are continuing to evaluate his candidacy. No final determinations have been made to date.
The FLAC Board’s Reasons for the Business Combination
The FLAC Board and the FLAC Special Committee, in evaluating the transaction with NewAmsterdam Pharma, consulted with Goodwin, Campbells and its other advisors. In reaching its resolution (i) determining it is in the best interests of FLAC and its shareholders to approve the execution and delivery of the Business Combination Agreement and the ancillary agreements (including the Plan of Merger) and the transactions contemplated by each of the foregoing (including the Merger); (ii) approving the execution and delivery of the Business Combination Agreement, the ancillary agreements and the transactions contemplated thereby (including the Exchange and the Merger); and (iii) recommending that the FLAC shareholders vote to approve the Business Combination Agreement, the ancillary agreements and the transactions contemplated thereby, the FLAC Board considered and evaluated a number of factors, including, but not limited to, the factors discussed below. The FLAC Board viewed its decision as being based on all of the information available and the factors presented to and considered by it. This explanation of FLAC’s reasons for the Business Combination and all other information presented in this section is forward-looking in nature and, therefore, should be read in light of the factors discussed under “Special Note Regarding Forward-Looking Statements.”
The members of the FLAC Board are well qualified to evaluate the transaction with NewAmsterdam Pharma. They have extensive transactional experience, particularly in the biotechnology, pharmaceutical and life sciences industries, including with both high growth private companies and publicly traded companies. The
180
Table of Contents
members of the FLAC Board also recognized that FLAC’s management team and the other representatives of FLAC had substantial experience in evaluating the operating and financial merits of, or advising, companies similar to NewAmsterdam Pharma.
The FLAC Board considered a number of factors pertaining to the Business Combination as generally supporting its decision to enter into the Business Combination Agreement and the transactions contemplated thereby, including but not limited to, the following material factors:
| • | Transformative Late-Stage Product Candidate. NewAmsterdam Pharma’s lead candidate, obicetrapib, has clinical data from three Phase 2 trials in high-risk CVD patients, and is currently in four Phase 3 trials and Phase 2b trials as both a monotherapy and a combination therapy, with multiple potential near-term milestones. The FLAC Board believes that NewAmsterdam Pharma is well-positioned to develop obicetrapib with the potential to be a low-dose, once-daily oral CETP inhibitor for lowering LDL-C, if approved. |
| • | Significant Addressable Market with Growth Opportunities. The FLAC Board considered the fact that obicetrapib aims to address an area of significant unmet need, with approximately 30 million patients in the United States and EU5 with residual LDL-C elevation. NewAmsterdam Pharma is also exploring the role CETP inhibition may have in other indications such as Alzheimer’s disease and diabetes, which represent significant potential commercial opportunities, if validated. |
| • | Experienced Cardiometabolic Management Team. NewAmsterdam Pharma is led by a world-class team of industry veterans, including some of the world’s preeminent cardiometabolic experts. Dr. Michael Davidson, NewAmsterdam Pharma’s Chief Executive Officer, is a seasoned executive and leading expert in the field of lipidology, and Dr. John Kastelein, NewAmsterdam Pharma’s founder and Chief Scientific Officer, has authored widely-published clinical research on the development of novel therapies for CVD and the genetic basis of dyslipidemia, referring to low or elevated lipid levels. The FLAC Board believes that the deep cardiometabolic experience of the NewAmsterdam Pharma management team positions the company for success. |
| • | Attractive Entry Valuation and Cash Runway. NewAmsterdam Pharma will have an anticipated initial pro forma enterprise value of $326.0 million, based on the NewAmsterdam Pharma’s current cash balance, the PIPE Financing and FLAC’s cash in trust, which pro forma cash amount the parties believe will be sufficient to fund operations of NewAmsterdam Pharma through 2026. The FLAC Board believes this opportunity is attractive relative to relevant comparable life sciences companies. The FLAC Board believes the valuation of NewAmsterdam Pharma is favorable to investors seeking long-term return potential and that the $326.0 million enterprise value presents a compelling entry point valuation for a business with a late-stage, novel product candidate and significant barriers to entry. |
| • | Backed by Top Tier Healthcare Investors. Funds affiliated with Frazier, Forbion, Morningside Ventures and other investors have committed to investments of a total of $234.6 million through an oversubscribed and upsized PIPE Financing. The FLAC Board believes that that this strong investor commitment reflects the potential opportunity created by a transaction with NewAmsterdam Pharma; however, prospective investors should not rely on the past investment decisions of such investors, as such investors may have different risk tolerances and, for those shares not subscribed for in connection with the PIPE Financing, may receive their shares at a significant discount to the market price. |
| • | Long-Term Alignment. The FLAC Board and the FLAC Special Committee considered that the structure of the Business Combination provides for significant long-term alignment among FLAC, NewAmsterdam Pharma’s management team and the existing NewAmsterdam Pharma shareholders. Both the Sponsor and all of the NewAmsterdam Pharma shareholders have agreed to certain lock-up provisions, and shareholders of NewAmsterdam Pharma are also entitled to receive the Earnout Shares upon the achievement of a certain clinical development milestone during the Earnout Period. As such, the parties to the Business Combination are expected to be aligned on the goal of driving long-term value for the shareholders of NewAmsterdam Pharma. Pursuant to the terms of the Investor Rights Agreement, certain NewAmsterdam Pharma shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination |
181
Table of Contents
for six months from the Final Closing Date of the Business Combination, subject to certain limited exceptions. In addition, the FLAC Initial Shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares they receive in connection with the Business Combination for a period beginning on the Final Closing Date and ending one year after the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the indicated time periods with respect to 50% of the Holdco Shares the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders upon the occurrence of certain specified trading milestones or change of control events. See the section entitled “The Business Combination Agreement and Ancillary Documents—Investor Rights Agreement” for more detail on the terms of the lock-up provisions. At the closing of the Business Combination, certain NewAmsterdam Pharma shareholders who are not party to the Investor Rights Agreement (after giving effect to the Merger and the PIPE Financing) will enter into a Lock-Up Agreement, pursuant to which, among other things, they will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the six month period upon the occurrence of certain specified trading milestones or change of control events. See the section entitled “The Business Combination Agreement and Ancillary Documents—Lock-Up Agreement” for more detail on the terms of the lock-up provisions. |
| • | Fairness Opinion. Lincoln delivered an opinion to the FLAC Board to the effect that, as of the date of such opinion, and subject to and based on the assumptions, limitations, qualifications, conditions and other matters set forth therein, the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement, is fair to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement), from a financial point of view. |
| • | Compelling Opportunity. The FLAC Board and the FLAC Special Committee determined, after an extensive review of other business combination opportunities reasonably available to FLAC, that the proposed Business Combination represents a compelling business combination for FLAC based upon the process utilized to evaluate and assess other potential targets and the criteria that FLAC adopted in its search for a business combination target as detailed above and in other sections of this prospectus/proxy statement. |
| • | The Role of the Independent Directors. In connection with the Business Combination, the FLAC Special Committee, which is composed solely of independent directors, evaluated the proposed terms of the Business Combination, including the Business Combination Agreement and the related agreements, and unanimously approved the Business Combination Agreement and the related agreements and the transactions contemplated thereby, including the Business Combination. |
| • | Terms of the Business Combination Agreement. The FLAC Board and the FLAC Special Committee reviewed and considered the terms of the Business Combination Agreement and the other related agreements, including the parties’ conditions to their respective obligations to complete the transactions contemplated therein, including the limited number of conditions and the relative size of the minimum cash condition of $250 million as compared to the $234.6 million in committed PIPE Financing and the $15 million subject to non-redemption agreements. See “—The Business Combination Agreement and Ancillary Documents” for detailed discussions of the terms and conditions of these agreements. |
| • | Results of Due Diligence. The FLAC Board and the FLAC Special Committee considered the scope of the due diligence investigation conducted by FLAC, certain members of the FLAC Special Committee and its outside advisors and evaluated the results thereof and information available to it related to NewAmsterdam Pharma, including: |
| • | diligence on the life sciences market; |
| • | extensive meetings and calls with NewAmsterdam Pharma’s management team regarding its operations and future plans and the proposed transaction; |
182
Table of Contents
| • | WSGR’s findings relating to NewAmsterdam Pharma’s material intellectual property; |
| • | The commercial findings of FLAC’s advisors; |
| • | the in-depth review of NewAmsterdam’s science and the potential market opportunity, including relative to other market participants, by FLAC, members of the FLAC Special Committee and FLAC’s advisors; |
| • | NewAmsterdam Pharma’s overall sophistication and its readiness to function as a public company, including as a result of its management team; and |
| • | review of materials related to NewAmsterdam Pharma made available, including with respect to financial statements, material contracts, key metrics and performance indicators, benefit plans, intellectual property matters, labor matters, information technology, privacy and personal data, litigation information, environmental matters, FDA, EMA and other regulatory matters and other legal and business matters. |
The FLAC Board also considered a variety of uncertainties and risks and other potentially negative factors concerning the Business Combination, including, but not limited to, the following:
| • | Benefits Not Achieved. The risk that the potential benefits of the Business Combination may not be fully achieved, or may not be achieved within the expected timeframe. |
| • | Business Risks. The risk that the future financial performance of NewAmsterdam Pharma may not meet the FLAC Board’s or the FLAC Special Committee’s expectations due to factors both in and outside of NewAmsterdam Pharma’s control, including its dependence on obicetrapib, its only product candidate; expectations regarding the timing, initiation, implementation and success of its planned and ongoing clinical trials for obicetrapib and related implications. |
| • | Clinical-Stage Company Risks. The risk that NewAmsterdam Pharma is a clinical-stage company that has incurred net losses since its inception, and anticipates that it will continue to incur significant losses for the foreseeable future, and that NewAmsterdam Pharma has yet to commercialize its product candidate. In addition, the FLAC Board weighed the fact that prior attempts to develop CETP inhibitors were unsuccessful against the fact that NewAmsterdam Pharma’s preclinical and clinical data suggest potentially important distinctions between obicetrapib and its predecessors. |
| • | Liquidation of FLAC. The risks and costs to FLAC if the Business Combination is not completed, including the risk of diverting management focus and resources to other businesses combination opportunities, which could result in FLAC being unable to effect a business combination by December 11, 2022 (or such later date as may be approved by FLAC’s shareholders) and could force FLAC to liquidate. |
| • | Exclusivity. The fact that the Business Combination Agreement includes an exclusivity provision that prohibits FLAC from soliciting other business combination proposals so long as the Business Combination Agreement is in effect. |
| • | Shareholder Vote and Minimum Cash Condition. The risk that FLAC’s shareholders may fail to provide the respective votes necessary to effect the Business Combination, and the risk that the minimum cash condition depends on the actions or inactions of parties over whom FLAC and NewAmsterdam Pharma have no control. |
| • | Limitations of Review. The risk that, while FLAC’s management team performed an extensive due diligence review of NewAmsterdam Pharma, there may have been relevant NewAmsterdam Pharma information not considered by FLAC’s management team and accordingly, FLAC may not have properly valued NewAmsterdam Pharma. |
| • | Closing Conditions. The fact that, in addition to the minimum cash conditions discussed above, the completion of the Business Combination is conditioned on the satisfaction of certain other closing conditions, that are also not within FLAC’s control. |
| • | Litigation. The possibility of litigation challenging the Business Combination or that an adverse judgment granting permanent injunctive relief could delay or indefinitely enjoin consummation of the Business Combination. |
183
Table of Contents
| • | Fees and Expenses. The fees and expenses associated with completing the Business Combination. |
| • | Other Risks. Various other risks associated with the Business Combination, the business of FLAC and the business of NewAmsterdam Pharma described under the section entitled “Risk Factors.” |
In addition to considering the factors described above, the FLAC Board and the FLAC Special Committee also considered that certain of the officers and directors of FLAC may have interests in the Business Combination as individuals that are in addition to, and that may be different from, the interests of FLAC’s shareholders. See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination.” The FLAC Board and FLAC Special Committee reviewed and considered these interests during the negotiation of the Business Combination and in evaluating and approving, as members of the FLAC Board and the FLAC Special Committee, the Business Combination Agreement and the transactions contemplated therein, including the Business Combination.
The FLAC Board concluded that the potential benefits that it expected FLAC and its shareholders to achieve as a result of the Business Combination outweighed the potentially negative factors associated with the Business Combination. Accordingly, the FLAC Board determined that the Business Combination Agreement, the Business Combination and the Merger, were advisable, fair to, and in the best interests of, FLAC and its shareholders.
Certain Engagements in Connection with the Transactions
Credit Suisse, Jefferies and William Blair were engaged by FLAC as placement agents, financial advisors, and capital markets advisors to FLAC in connection with the Business Combination, and will receive customary advisory fees in connection therewith. As further described below, Credit Suisse also acted as the sole book-running manager for the FLAC IPO and will be entitled to receive deferred underwriting commissions upon the consummation of the Business Combination. Furthermore, SVB Securities was engaged by FLAC as placement agent in connection with the PIPE Financing and by NewAmsterdam Pharma as financial and capital markets advisor in connection with the Business Combination, and, as further described below, may receive advisory fees from each of FLAC and NewAmsterdam Pharma.
Credit Suisse, Jefferies, SVB Securities and William Blair (together with their affiliates) are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, wealth management, investment research, principal investing, lending, financing, hedging, market making, brokerage and other financial and non-financial activities and services. In addition, Credit Suisse, Jefferies, SVB Securities and William Blair (together with their affiliates) may provide investment banking and other commercial dealings to FLAC, NewAmsterdam Pharma and their respective affiliates in the future, for which they would expect to receive customary compensation. In addition, in the ordinary course of their business activities, Credit Suisse, Jefferies, SVB Securities and William Blair and their affiliates, officers, directors and employees may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of FLAC, NewAmsterdam Pharma or their respective affiliates. Credit Suisse, Jefferies, SVB Securities and William Blair and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
In addition, NewAmsterdam Pharma retained Moelis in connection with certain potential licensing transactions and M&A transactions with operating companies. Moelis is entitled to customary advisory fees in connection with its advisory role, with the fees associated with its advisory role relating to other potential transactions that were ultimately not pursued by the Company payable upon the closing of the Business Combination and the fees associated with its role as advisor in connection with the Menarini License having been paid.
184
Table of Contents
Involvement of Sole Book-Running Manager of the FLAC IPO
Credit Suisse served as the sole book-running manager for the FLAC IPO and will be entitled to receive $4,830,000 of deferred underwriting commissions therefrom in connection with the consummation of FLAC’s initial business combination. Credit Suisse has agreed to waive its rights to the deferred underwriting commissions held in the Trust Account solely in the event that FLAC does not complete its initial business combination prior to the end of the completion window (subject to any extension period thereof), and in such a circumstance, will not receive any of such funds. Such funds will instead be included in the liquidation distribution to FLAC’s public shareholders of the proceeds held in the Trust Account.
As discussed under “—Background of the Business Combination,” Credit Suisse is serving as FLAC’s lead placement agent in connection with the PIPE Financing, and as financial advisor and capital markets advisor to FLAC in connection with the Business Combination. Credit Suisse’s receipt of the deferred underwriting commissions is not dependent on its provision of services in connection with the Business Combination.
Engagement of Financial and Capital Markets Advisors in the Transactions
Dual Role of SVB Securities in the Transactions
As discussed under “—Background of the Business Combination,” SVB Securities was engaged by NewAmsterdam Pharma as financial and capital markets advisor in connection with the Business Combination on May 31, 2022. Pursuant to the terms of SVB Securities’ engagement with NewAmsterdam Pharma, SVB Securities has agreed to provide NewAmsterdam Pharma with financial and capital markets advice and assistance in connection with the Business Combination, including providing financial and capital markets advice on the potential business combination with FLAC and providing other customary financial and capital markets advisory and investment banking services.
On June 30, 2022, SVB Securities was subsequently engaged by FLAC as a placement agent in connection with the PIPE Financing. Prior to FLAC’s engagement of SVB Securities as a placement agent, FLAC was advised of the potential conflicts of interest arising out of the dual role of SVB Securities as a placement agent to FLAC and a financial and capital markets advisor to NewAmsterdam Pharma, and considered the benefits and risks thereof to FLAC. Each of NewAmsterdam Pharma and FLAC consented to the engagement of SVB Securities as a placement agent and a financial and capital markets advisor to NewAmsterdam Pharma. FLAC and NewAmsterdam Pharma agreed that this syndicate of banks was well-suited for their roles given their respective experience in the life sciences industry and with transactions of this nature, and their respective levels of familiarity with FLAC, NewAmsterdam Pharma and their respective teams and investor bases.
Any fees paid to SVB Securities by NewAmsterdam Pharma for its role as financial and capital markets advisor will be reduced by any fees received by SVB Securities in connection with its role as placement agent for FLAC. Because SVB Securities will receive its placement agent fee upon the closing of the PIPE Financing from FLAC and its financial advisor fee from NewAmsterdam Pharma upon the Closing, investors should be aware of the potential conflicts of interest owing to SVB Securities’ multiple roles in the Business Combination.
Engagement of Financial Advisor to the FLAC Board
FLAC retained Lincoln to provide a fairness opinion to the FLAC Board in connection with the Business Combination. Lincoln received customary fees from FLAC for its services, in the amount of $750,000, $50,000 of which was paid upon Lincoln’s retention, and the balance of which is payable upon the earlier of termination of the Transactions in accordance with the Business Combination Agreement or the consummation of the Transactions. No portion of Lincoln’s fee was contingent upon the conclusion reached in its opinion. On July 24, 2022, Lincoln delivered its opinion to the FLAC Board, as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement.
185
Table of Contents
In selecting Lincoln, the Board considered, among other things, the fact that Lincoln is a reputable investment banking firm with substantial experience advising companies. Lincoln, as part of its investment banking business, is continuously engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes.
Opinion of the Financial Advisor to the FLAC Board
FLAC retained Lincoln to provide a fairness opinion to the FLAC Board as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement. FLAC selected Lincoln because Lincoln is a reputable investment banking firm with substantial experience advising companies. Lincoln, as part of its investment banking business, is continuously engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes.
On July 24, 2022, Lincoln delivered its opinion to the FLAC Board to the effect that, as of the date of such opinion, and subject to and based on the assumptions, limitations, qualifications, conditions and other matters set forth therein, the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement, is fair to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement), from a financial point of view.
Lincoln’s opinion was directed to the board of directors of FLAC (in its capacity as such) and only addressed the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement and did not address any other terms, aspects or implications of the Transactions, or any agreements, arrangements or understandings entered into in connection with the Transactions. The summary of Lincoln’s opinion in this proxy statement/prospectus is qualified in its entirety by reference to the full text of its written opinion, which is attached as Annex J to this proxy statement/prospectus and which describes the assumptions, limitations, qualifications, conditions and other matters considered by Lincoln in connection with the preparation of its opinion. Neither Lincoln’s opinion nor the summary of its opinion and the related analyses set forth in this proxy statement/prospectus are intended to be, and do not constitute, advice or a recommendation to the Board or any security holder as to how they should act or vote with respect to any matter relating to the Transactions (including as to whether they should redeem any FLAC Public Shares in the FLAC Shareholder Redemption (as defined in the Business Combination Agreement). Security holders are urged to read the entire opinion carefully in connection with their consideration of the Transactions.
In connection with rendering its opinion, Lincoln, among other things:
| 1) | Reviewed the audited income statements for NewAmsterdam Pharma for the fifteen-month period ended December 31, 2020 and balance sheet as of December 31, 2020 provided to Lincoln by FLAC; |
| 2) | Reviewed the audited income statements for NewAmsterdam Pharma for the twelve-month period ended December 31, 2021 and balance sheet as of December 31, 2021 provided to Lincoln by FLAC; |
| 3) | Reviewed a draft of the Business Combination Agreement dated as of July 19, 2022; |
| 4) | Reviewed the License Agreement between NewAmsterdam Pharma B.V. and A. Menarini International Licensing S.A. dated as of June 23, 2022; |
| 5) | Reviewed NewAmsterdam Pharma’s PIPE presentation dated as of June 2022; |
| 6) | Discussed the business, financial outlook and prospects of NewAmsterdam Pharma and its addressable market, as well as the terms and circumstances surrounding the Transactions, with management of NewAmsterdam Pharma and FLAC; |
186
Table of Contents
| 7) | Reviewed certain financial and other information for NewAmsterdam Pharma, and compared that data and information with certain financial, stock trading and corresponding data and information for companies with publicly traded securities that Lincoln deemed relevant, none of which are directly comparable to NewAmsterdam Pharma; |
| 8) | Reviewed certain financial and other information for NewAmsterdam Pharma and the Transactions, and compared that data and information with certain financial and corresponding data and information for companies that have been subject to change of control merger and acquisition transactions that Lincoln deemed relevant, none of which is directly comparable to NewAmsterdam Pharma and the Transactions; and |
| 9) | Considered such other information and financial, economic and market criteria and analyses that Lincoln deemed relevant. |
In performing its analyses and rendering its opinion with respect to the Transactions, Lincoln, with FLAC management’s consent, relied upon and assumed the accuracy and completeness of all of the financial, accounting, legal, tax and other information Lincoln reviewed, and Lincoln did not assume any responsibility for the independent verification of, nor did Lincoln independently verify, any of such information. Lincoln relied upon the assurances of the management of FLAC that the information provided was complete and correct in all material respects and did not contain any untrue statement of a material fact or omit to state a material fact necessary that would have made such information materially incomplete or misleading in the light of the circumstances under which statement was made. Lincoln assumed that the Transactions will be consummated in a timely manner that complies in all respects with all applicable federal and state statutes, rules and regulations. Lincoln also assumed that at the consummation of the Transactions, Holdco will be fully funded through the completion of its Phase 3 clinical trials of obicetrapib. Lincoln further assumed that in the course of obtaining any necessary regulatory and third-party consents, approvals and agreements for the Transactions, no modification, delay, limitation, restriction, or condition will be imposed that will have an adverse effect on FLAC or the Transactions. Lincoln also assumed that the Transactions will be consummated in accordance with the terms outlined by FLAC and other documents made available to Lincoln, without waiver, modification or amendment of any term, condition or agreement therein that is material to Lincoln’s analysis. Lincoln assumed that there was no material change in the assets, liabilities, business, condition (financial or otherwise), results of operations, or prospects of NewAmsterdam Pharma since the date of the most recent information was made available to Lincoln. Lincoln further assumed that the final terms of the Transactions will not vary materially from those set forth in the copies or drafts, as applicable, reviewed by Lincoln and that the final versions of all documents conform in all material respects to the drafts reviewed by Lincoln.
Lincoln’s opinion was necessarily based on financial, economic, market and other conditions as they exist on and the information made available to Lincoln as of July 24, 2022. Although subsequent developments may affect its opinion, Lincoln does not have any obligation to update, revise or reaffirm its opinion.
Lincoln did not evaluate NewAmsterdam Pharma’s solvency and was not requested to make, and did not make, an independent evaluation or appraisal of the assets or liabilities (contingent, derivative, off-balance sheet or otherwise) of NewAmsterdam Pharma or any of its subsidiaries, nor was Lincoln furnished with any such evaluations or appraisals. Lincoln was not requested to, nor did Lincoln participate in the negotiation or structuring of the Transactions. Lincoln was not requested to, nor did Lincoln seek, alternative candidates for the Transactions.
Lincoln’s opinion (i) did not address the underlying business decision of the FLAC Board or FLAC to proceed with or effect the Transactions or the relative merits of the Transactions as compared to other transaction structures, transactions or business strategies that may be available to FLAC or the effect of any other transaction in which FLAC might engage, and did not address whether the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement is the best possibly attainable under the circumstances, (ii) did not and does not constitute advice or a recommendation to the Board or any security holder as to how they should act or vote with respect to any matter relating to the Transactions (including as to whether they should redeem any FLAC Public Shares in the FLAC Shareholder Redemption (as
187
Table of Contents
defined in the Business Combination Agreement), and (iii) addressed only the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement and did not address any other terms, aspects or implications of the Transactions, or any agreements, arrangements or understandings entered into in connection with the Transactions. Lincoln expressed no opinion as to the fairness of any portion or aspect of the Transactions to (i) the holders of any class of securities, creditors or other constituencies of FLAC, or any other party, except as expressly set forth in its opinion, or (ii) any one class or group of FLAC’s security holders, creditors or other constituencies vis-à-vis any other class or group of FLAC’s security holders, creditors or other constituents (including, without limitation, the allocation of any Aggregate Share Consideration (as defined in the Business Combination Agreement) among or within such classes or groups of security holders, creditors or other constituents). The decision as to whether to proceed with the Transactions or any related transaction depends on an assessment of various factors, many of which are unrelated to the financial analyses on which Lincoln’s opinion is based.
Lincoln expressed no opinion as to what the market price or value of the stock of FLAC or Holdco would be after the announcement of the Transactions. Lincoln’s opinion was not to be construed as a valuation opinion, credit rating, solvency opinion, an analysis of FLAC’s or Holdco’s credit worthiness, as tax advice, or as accounting advice. Lincoln also expressed no opinion about the amount or nature of any compensation or equity arrangement to be given to FLAC’s or Holdco’s officers, directors or employees, or class of such persons, in connection with the Transactions relative to the Aggregate Share Consideration (as defined in the Business Combination Agreement) in the Transactions.
Set forth below is a summary of the material financial analyses reviewed by Lincoln with the FLAC board of directors on July 24, 2022 in connection with rendering its opinion. The following summary, as noted below, does not purport to be a complete description of the analysis performed by Lincoln. The order of the individual analyses described, and the results of these analyses, do not represent relative importance or weight given to these analyses by Lincoln.
Summary of Lincoln’s Financial Analysis
The following is a summary of the material financial analyses prepared and reviewed with the FLAC Board in connection with Lincoln’s opinion, dated July 24, 2022. The summary set forth below does not purport to be a complete description of the financial analyses performed or factors considered by, and underlying the opinion of, Lincoln, nor does the order of the financial analyses described represent the relative importance or weight given to those financial analyses by Lincoln. Lincoln may have deemed various assumptions more or less probable than other assumptions, so the ranges resulting from any particular portion of the analyses summarized below should not be taken to be Lincoln’s view of the actual value of NewAmsterdam Pharma, FLAC or Holdco. Some of the summaries of the financial analyses set forth below include information presented in tabular format. In order to fully understand the financial analyses, the tables must be read together with the text of each summary, as the tables alone do not constitute a complete description of the financial analyses performed by Lincoln. Considering the data in the tables below without considering all financial analyses or factors or the full narrative description of such analyses or factors, including the methodologies and assumptions underlying such analyses or factors, could create a misleading or incomplete view of the processes underlying Lincoln’s financial analyses and its opinion.
In performing its analyses, Lincoln made numerous assumptions with respect to industry performance, general business and economic conditions and other matters, many of which are beyond the control of FLAC, NewAmsterdam Pharma, Holdco, or any other parties to the Transactions. None of FLAC, NewAmsterdam Pharma, Holdco or Lincoln or any other person assumes responsibility if future results are materially different from those discussed. Any estimates contained in these analyses are not necessarily indicative of actual values or predictive of future results or values, which may be significantly more or less favorable than as set forth below. In addition, analyses relating to the value of FLAC or Holdco do not purport to be appraisals or reflect the prices at which FLAC or Holdco stock may actually
188
Table of Contents
be sold. Accordingly, the assumptions and estimates used in, and the results derived from, the financial analyses are inherently subject to substantial uncertainty. Except as otherwise noted, the following quantitative information, to the extent that it is based on market data, is based on market data as it existed on or before July 22, 2022 and is not necessarily indicative of current market conditions.
Transaction Enterprise Value
Using information obtained from management of FLAC and NewAmsterdam Pharma, for purposes of the financial analysis described below, Lincoln derived an implied enterprise value of Holdco pro forma for the Transactions of $391 million. Lincoln first calculated an implied aggregate equity value of Holdco pro forma for the Transactions of $867 million assuming (i) an aggregate value of Holdco Shares to be exchanged for the issued and outstanding shares of NewAmsterdam Pharma of $491 million, (ii) a minimum capital raise of $288 million from both the FLAC Trust Account and the PIPE Financing, (iii) the achievement and announcement of positive Phase 3 data for both of the “BROOKLYN” and “BROADWAY” Phase 3 trials of obicetrapib resulting in the issuance of the Earnout Shares pursuant to the Business Combination Agreement, and (iv) value for the Holdco Warrants of $30 million based on a Black-Scholes Pricing Model. The above values were based on a cash value per Holdco Share of $10.00. Lincoln then subtracted net cash of $477 million based on (i) NewAmsterdam Pharma balance sheet cash of $91 million as of June 30, 2022, (ii) upfront cash proceeds of $117 million pursuant to the Menarini License, (iii) the minimum capital raise of $288 million from both the FLAC Trust Account and the PIPE Financing and (iv) $20 million in estimated transaction expenses in the Transactions. Neither FLAC nor NewAmsterdam prepared financial projections and none were provided to Lincoln or other investors. As a result, Lincoln did not rely upon a discounted cash flow analysis.
Selected Public Companies Analysis
Lincoln reviewed and compared certain information related to the following selected publicly traded biopharmaceutical drug development companies focused on cardiovascular, cardiopulmonary and chronic kidney diseases with operations similar to NewAmsterdam Pharma for its analysis based on its experience and professional judgment (which companies are referred to as the “selected public companies” in this summary of Lincoln’s opinion). Although none of the selected public companies is directly comparable to NewAmsterdam Pharma, the companies listed below were chosen by Lincoln because, among other reasons, they are publicly traded early-stage biopharmaceutical drug development companies with certain operational, business or financial characteristics that, for purposes of Lincoln’s analysis, may be considered similar to those of NewAmsterdam Pharma.
However, because none of the selected public companies is identical or directly comparable to NewAmsterdam Pharma, Lincoln believed that it was inappropriate to, and therefore did not, rely solely on the quantitative results of the selected public company analysis. Accordingly, Lincoln also made qualitative judgments, based on its experience and professional judgment, concerning differences between the operational, business and/or financial characteristics of NewAmsterdam Pharma and the selected public companies that could affect their public trading values in order to provide a context in which to consider the results of the quantitative analysis.
Using publicly available information obtained from SEC filings and other publicly available data sources as of July 22, 2022, Lincoln calculated, for each selected public company, such selected public company’s enterprise value (calculated as the equity value (determined using the treasury stock method and taking into account outstanding in-the-money options, warrants, restricted share units and other convertible securities) plus the book value of debt and certain liabilities less cash, cash equivalents and certain non-operating assets), which is referred to, with respect to the selected public companies, as “Enterprise Value”.
189
Table of Contents
The selected public companies considered in this analysis are summarized below:
Selected Public Company | Enterprise Value ($ in millions) | |||
Aerovate Therapeutics, Inc. | $ | 321 | ||
Akero Therapeutics, Inc. | $ | 213 | ||
Amarin Corporation plc | $ | 157 | ||
Esperion Therapeutics, Inc. | $ | 667 | ||
Lexicon Pharmaceuticals, Inc. | $ | 407 | ||
Madrigal Pharmaceuticals, Inc. | $ | 916 | ||
NGM Biopharmaceuticals, Inc. | $ | 884 | ||
Reata Pharmaceuticals, Inc. | $ | 672 | ||
Silence Therapeutics plc | $ | 199 | ||
Tricida, Inc. | $ | 573 | ||
Verve Therapeutics, Inc. | $ | 1,297 | ||
Median | $ | 573 | ||
Mean | $ | 573 | ||
Lincoln analyzed the selected public companies based on their similarity to NewAmsterdam Pharma, primarily in terms of certain quantitative and qualitative factors, including, but not limited to: the total number of compounds in development, the development stage of such compounds, the estimated commercialization year, the estimated patent protection term of the value-driving drug in the United States, the estimated patient population size, and the estimated peak revenue potential, based on consensus equity research analyst estimates, public filings and Lincoln’s experience and professional judgment. Taking into account such quantitative and qualitative factors, Lincoln observed the illustrative enterprise value range of the selected public companies of $157 million to $1,297 million, the mean and median enterprise value of the selected companies of $573 million and the enterprise value of Esperion Therapeutics, Inc. (as further discussed below) of $667 million.
Selected Precedent Transaction Analysis
Lincoln reviewed and compared certain information relating to the following selected transactions involving biopharmaceutical drug development companies focused on cardiovascular, cardiopulmonary, thrombosis, and chronic kidney diseases similar to the Transactions for its analysis based on its experience and professional judgment (which transactions are referred to as the “selected transactions” in this summary of Lincoln’s opinion). Although none of the selected transactions is directly comparable to the Transactions, the transactions listed below were chosen by Lincoln because, among other reasons, their total number of compounds, the development stage of such compounds and the comparability of such compounds to obicetrapib or other factors, for purposes of Lincoln’s analysis, may be considered similar to the Transactions.
However, because none of the selected transactions used in this analysis is identical or directly comparable to the Transactions, Lincoln believed that it was inappropriate to rely solely on the quantitative results of the selected transaction analysis. Accordingly, Lincoln also made qualitative judgments, based on its experience and professional judgment, concerning differences between the operational, business and/or financial characteristics of NewAmsterdam Pharma and each target company as well as the Transactions and the selected transactions that could affect the transaction values of each in order to provide a context in which to consider the results of the quantitative analysis.
Using publicly available information obtained from SEC filings and other data sources as of the time of the announcement of the relevant transactions, Lincoln calculated, for each selected transaction, the transaction value (calculated as the offer value (determined using the treasury stock method and taking into account outstanding in-the-money options, warrants, restricted stock units, performance stock units and other convertible securities), plus the book value of debt and certain liabilities less cash and cash equivalents) implied for each target company based on the consideration payable in the applicable selected transaction, in each case excluding any contingent payments, which amount is referred to, with respect to the selected transactions, as “Enterprise Value”.
190
Table of Contents
The selected transactions considered in this analysis are summarized below:
Date Announced | Target | Acquirer | Enterprise Value ($ in millions) | |||||
Nov-20 | Emisphere Technologies, Inc. | Novo Nordisk A/S | $ | 1,338 | ||||
Aug-20 | Akcea Therapeutics, Inc. | Ionis Pharmaceuticals, Inc. | $ | 1,758 | ||||
June-20 | Corvidia Therapeutics, Inc. | Novo Nordisk A/S | $ | 2,100 | ||||
May-20 | Portola Pharmaceuticals, Inc. | Alexion Pharmaceuticals, Inc. | $ | 1,457 | ||||
Nov-19 | The Medicines Company | Novartis AG | $ | 9,300 | ||||
June-16 | Aegerion Pharmaceuticals, Inc. | QLT Inc. (nka: Novelion Therapeutics Inc.) | $ | 261 | ||||
Nov-15 | ZS Pharma, Inc. | AstraZeneca PLC (through its wholly owned subsidiary, Zeneca, Inc.) | $ | 2,495 | ||||
Nov-15 | Cardioxyl Pharmaceuticals, Inc. | Bristol-Myers Squibb Company | $ | 2,075 | ||||
Sep-15 | Dezima Pharma B.V. | Amgen Inc. | $ | 1,550 | ||||
Jan-15 | Trophos SA | Roche Holding AG | $ | 541 | ||||
May-13 | Omthera Pharmaceuticals, Inc. | AstraZeneca PLC (through its wholly owned subsidiary, Zeneca, Inc.) | $ | 497 | ||||
Median | $ | 1,550 | ||||||
Mean | $ | 2,125 | ||||||
Lincoln analyzed the selected transactions based on their similarity to the Transactions, primarily in terms of certain quantitative and qualitative factors, including, but not limited to: the total number of compounds in development, the development stage of such compounds, the comparability of such compounds to obicetapib, public filings and Lincoln’s experience and professional judgment. Taking into account such quantitative and qualitative factors, Lincoln observed the illustrative enterprise value range of the selected transactions of $261 million to $9,300 million, the mean enterprise value of the selected transactions of $2,125 million, the median enterprise value of the selected transactions of $1,550 million, and the acquisition of The Medicines Company by Novartis (the selected transaction, which, similar to the Transaction, involved the development of a drug that was widely viewed to be the future secondary LDL-C lowering market leader, as further discussed below) for $9,300 million.
Lookback Analysis (Esperion)
Lincoln reviewed the historical implied enterprise values of Esperion Therapeutics, Inc., the developer of Nexletol, from October 2014 through July 2015, when Nexletol was viewed to be the future secondary LDL-C lowering market leader during its Phase 2 trials, based on the closing trading prices of Esperion Therapeutics, Inc. common stock. During such period, the volume weighted average enterprise value of Esperion Therapeutics, Inc. was $1,575 million.
Lookback Analysis (The Medicines Company)
Lincoln reviewed the historical implied enterprise values of The Medicines Company, the developer of Leqvio, from November 2017 through November 2018, immediately after the launch of its Phase 3 trial when it was viewed to be the future secondary LDL-C lowering market leader, based on the closing trading prices of The Medicines Company common stock. During such period, the volume weighted average enterprise value of The Medicines Company was $2,771 million.
Miscellaneous
During the two years preceding the date of Lincoln’s opinion, Lincoln and its affiliates have not had other investment banking relationships with NewAmsterdam Pharma or its affiliates, or any investment banking
191
Table of Contents
relationships with Holdco or its affiliates, for which Lincoln was paid for its services, other than in connection with its opinion. In addition, Lincoln and its affiliates have not had other investment banking relationships with FLAC or its affiliates, for which Lincoln was paid for its services, other than in connection with its opinion. Lincoln received customary fees from FLAC for its services, in the amount of $750,000, $50,000 of which was paid upon Lincoln’s retention, and the balance of which is payable upon the earlier of termination of the Transactions in accordance with the Business Combination Agreement or the consummation of the Transactions. No portion of Lincoln’s fee was contingent upon the conclusion reached in its opinion. In addition, FLAC has agreed to indemnify Lincoln and certain related parties against certain liabilities, and to reimburse Lincoln for certain expenses, arising in connection with or as a result of its engagement. Lincoln and its affiliates provide a range of investment banking and financial services and, in that regard, Lincoln and its affiliates may in the future provide, investment banking and other financial services to FLAC, Holdco and each of their respective affiliates, for which Lincoln and its affiliates would expect to receive compensation.
Interests of Certain Persons in the Business Combination
Interests of FLAC’s Directors and Executive Officers in the Business Combination
In considering the recommendation of the FLAC Board to vote in favor of the Business Combination, FLAC shareholders should be aware that aside from their interests as shareholders, the Sponsor, FLAC Initial Shareholders and FLAC’s other current officers and directors have interests in the Business Combination that are different from, or in addition to, those of other FLAC shareholders generally. The FLAC Board was aware of and considered these interests, among other matters, in evaluating and negotiating the Business Combination, and in recommending to FLAC shareholders that they approve the Business Combination Proposal. FLAC shareholders should take these interests into account in deciding whether to approve the Business Combination Proposal.
These interests include, among other things, the interests listed below:
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed not to redeem any Founder Shares or FLAC Public Shares held by them in connection with a shareholder vote to approve a proposed initial business combination; |
| • | the Sponsor paid an aggregate of $25,000 for 2,875,000 Founder Shares. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding. Such securities will have a significantly higher value at the time of the Business Combination. If unrestricted and freely tradable, such Founder Shares would be valued at approximately $34,051,500 (based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022), but, given the restrictions on such shares, FLAC believes such shares have less value. If FLAC fails to complete an initial business combination by December 11, 2022, absent any extension, then FLAC will cease all operations except for the purpose of winding up, redeeming all of the public shares for cash and, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceeding to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to FLAC’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. In such event, Founder Shares collectively owned by the FLAC Initial Shareholders would be worthless because following the redemption of the public shares, FLAC would likely have few, if any, net assets; |
| • | the Sponsor paid an aggregate of $5,010,000 for its 501,000 FLAC Private Placement Units (with an aggregate fair market value of $4,944,870, based on the closing price of FLAC Public Units on June 30, 2022) and the component FLAC Private Placement Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
192
Table of Contents
| • | Frazier Life Sciences X, L.P., the sole member of the Sponsor, paid an aggregate of $10 million for its 1,000,000 FLAC Public Units in the FLAC IPO (with an aggregate fair market value of $9,870,000, based on the closing price of FLAC Public Units on June 30, 2022), and has agreed to waive any redemption rights, including with respect to the FLAC Class A Ordinary Shares underlying such FLAC Public Units purchased in the FLAC IPO, in connection with the Business Combination. In addition, the component FLAC Public Warrants will expire worthless if a business combination is not consummated by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | Frazier Life Sciences X, L.P. and certain other funds affiliated with Frazier, an affiliate of the Sponsor, have committed to purchasing 4,500,000 Holdco Shares at a price per share of $10.00 as part of the PIPE Financing and have agreed to waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination; |
| • | the FLAC Initial Shareholders and FLAC’s other current officers and directors have agreed to waive their rights to liquidating distributions from the Trust Account with respect to any Founder Shares held by them if FLAC fails to complete an initial business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders; |
| • | the Investor Rights Agreement will be entered into by the FLAC Initial Shareholders; |
| • | the Holdco Shares to be received by the FLAC Initial Shareholders in connection with the Merger will be subject to certain lock-up provisions for a period of one year, subject to exceptions as described herein; |
| • | the continued indemnification of FLAC’s existing directors and officers and the continuation of FLAC’s directors’ and officers’ liability insurance after the Business Combination; |
| • | the Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to shareholders rather than liquidate; |
| • | the Sponsor and its affiliates can earn a positive rate of return on their investment, even if other FLAC shareholders experience a negative rate of return in the post-business combination company; |
| • | FLAC has the right to select two individuals, one of which is expected to be Jamie Topper, to be nominated for election to the initial Holdco Board, who must be reasonably acceptable to NewAmsterdam Pharma and qualify as “independent” directors for purposes of Nasdaq rules; |
| • | the Sponsor, FLAC’s officers and directors, and their respective affiliates will lose their entire investment in FLAC (which is estimated to be approximately $48.9 million, based on the closing price of FLAC Class A Ordinary Shares on June 30, 2022) and will not be reimbursed for any out-of-pocket expenses (which are currently $0) if an initial business combination is not consummated by December 11, 2022, absent any extension; |
| • | the potential hire of David Topper, the Chief Financial Officer and a director and shareholder of FLAC, as the chief financial officer of Holdco in the first half of 2023; |
| • | the Sponsor (including its representatives and affiliates) and FLAC’s officers and directors, are, or may in the future become, affiliated with entities that are engaged in a similar business to Holdco and/or NewAmsterdam Pharma. The representatives and affiliates of the Sponsor, and certain of FLAC’s officers and directors, are in the business of making investments in companies, and may acquire and hold interests in businesses that compete directly or indirectly with Holdco and/or NewAmsterdam Pharma. Certain of FLAC’s officers and directors also have time and attention requirements for investment funds of which they and affiliates of the Sponsor are the investment managers. The Sponsor and FLAC’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to FLAC completing its initial business combination; and |
| • | if the Trust Account is liquidated, including in the event FLAC is unable to complete an initial business combination within the required time period, the Sponsor has agreed to indemnify FLAC to ensure that the proceeds in the Trust Account are not reduced below $10.00 per FLAC Public Share, or such lesser |
193
Table of Contents
per public share amount as is in the Trust Account on the liquidation date, by the claims of prospective target businesses with which FLAC has entered into an acquisition agreement or claims of any third party for services rendered or products sold to FLAC, but only if such a vendor or target business has not executed a waiver of any and all rights to seek access to the Trust Account. |
In addition, in connection with the execution of the Business Combination Agreement, FLAC, NewAmsterdam Pharma and Holdco entered into the Sponsor Support Agreement with the FLAC Initial Shareholders, pursuant to which the FLAC Initial Shareholders have agreed to vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions. Currently, the FLAC Initial Shareholders collectively own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares.
As a result of the aforementioned actual or potential conflicts of interests, the FLAC Board formed the FLAC Special Committee, comprised solely of disinterested and independent directors, for the purpose of evaluating the proposed Business Combination and determining whether the Business Combination Agreement and the proposed Business Combination are in the best interests of FLAC and its unaffiliated shareholders. The FLAC Special Committee is composed of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher and Krishna R. Polu. In addition, FLAC retained Lincoln, a third-party valuation firm, to provide a fairness opinion to the FLAC Board. Lincoln has delivered its opinion to the FLAC Board as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined in the Business Combination Agreement) of the Aggregate Share Consideration (as defined in the Business Combination Agreement) to be issued by Holdco pursuant to the Business Combination Agreement.
See the section entitled “Business of FLAC and Certain Information About FLAC—Directors, Executive Officers and Corporate Governance—Conflicts of Interest” for a further discussion of additional considerations in connection with the Business Combination.
Redemption Rights
Pursuant to the FLAC Articles of Association, holders of FLAC Public Shares other than the Sponsor and any FLAC directors or officers may elect to have their shares redeemed for cash at the applicable redemption price per share calculated in accordance with the FLAC Articles of Association. As of June 30, 2022, this would have amounted to approximately $10.00 per share. If a holder of FLAC Public Shares exercises its redemption rights, then such holder will be exchanging its FLAC Class A Ordinary Shares for cash and will not own shares of Holdco following the closing of the Business Combination. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to the Transfer Agent in accordance with the procedures described herein. The redemption rights include the requirement that a shareholder must identify itself, at least two business days prior to the General Meeting, in writing as a beneficial holder and provide its legal name, phone number and address to the Transfer Agent in order to validly redeem its shares. Notwithstanding the foregoing, a holder of FLAC Public Shares individually, or, together with any affiliate or any other person with whom it is acting in concert or as a “group” (as defined in Section 13 of the Exchange Act) will be restricted from seeking redemption rights with respect to more than 15% of the FLAC Class A Ordinary Shares included in the FLAC Public Units sold in the FLAC IPO. Accordingly, all FLAC Public Shares in excess of the 15% threshold beneficially owned by a holder of FLAC Public Shares or group will not be redeemed for cash unless consented to by FLAC.
FLAC has no specified maximum redemption threshold under the FLAC Articles of Association, other than the aforementioned 15% threshold. Each redemption of a FLAC Public Shares by FLAC public shareholders will reduce the amount in the Trust Account, which held marketable securities with a fair value of approximately $138.1 million as of June 30, 2022. The Business Combination Agreement provides that (i) the parties’ obligations to consummate the Business Combination are conditioned on Holdco having at least $5,000,001 net
194
Table of Contents
tangible assets upon Closing and after giving effect to the Transactions (including the PIPE Financing and the FLAC Shareholder Redemption) and (ii) NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy the condition described in (ii), even if holders of up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights. The conditions to closing in the Business Combination Agreement are for the sole benefit of the parties thereto and may be waived by such parties. The Sponsor has agreed not to redeem any Founder Shares or FLAC Public Shares held by it in connection with a shareholder vote to approve a proposed initial business combination. FLAC shareholders who wish to redeem their FLAC Public Shares for cash must refer to and follow the procedures set forth in the section entitled “General Meeting of FLAC—Redemption Rights” in order to properly redeem their public shares.
Holders of FLAC Public Warrants or FLAC Private Placement Shares will not have redemption rights with respect to such securities.
Sources and Uses for the Business Combination
The following tables summarize the sources and uses for funding the Business Combination:
Sources & Uses
(No Redemption Scenario - Assuming No Redemptions of the Outstanding Class A Shares by FLAC Shareholders)
Sources (USD, millions) | Uses (USD, millions) | |||||||
FLAC Trust Account(1) | $ | 138 | Transaction Expenses settled in cash(3) | $ 20 | ||||
PIPE Financing | $ | 235 | Additional Cash on Balance Sheet | $473 | ||||
Menarini License upfront payment | $ | 120 | ||||||
NewAmsterdam Pharma Equity Rollover(2) | $ | 491 | NewAmsterdam Pharma Equity Rollover(2) | $491 | ||||
|
|
| ||||||
Total Sources | $ | 984 | Total Uses | $984 | ||||
|
|
| ||||||
| (1) | As of June 30, 2022. |
| (2) | Assumes (i) that Participating Shareholders represent 100% of the issued and outstanding shares of NewAmsterdam Pharma; (ii) that there are no “change of control” payments made or required to be made by NewAmsterdam Pharma or Holdco (other than the payments described to Amgen and MTPC in the clause which follows this one); (iii) that none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued; (iv) the issuance of an aggregate of 8,656,330 Holdco Shares to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” and (v) that neither the exercise of any NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options, nor the exercise of any awards that may be issued under the Holdco LTIP following the closing of the Business Combination, have been taken into account. |
| (3) | Estimated fees and expenses on Closing. |
195
Table of Contents
Sources & Uses
(Maximum Redemption Scenario - Assuming Redemptions of 12,261,482(1) of the Outstanding FLAC Public Shares by FLAC Shareholders)
Sources (USD, millions) | Uses (USD, millions) | |||||||||
FLAC Trust Account(2) | $138 | Transaction Expenses settled in cash (4) | $ 20 | |||||||
PIPE Financing | $235 | Additional Cash on Balance Sheet | $350 | |||||||
Menarini License upfront payment | $120 | |||||||||
NewAmsterdam Pharma Equity Rollover(3) | $491 | NewAmsterdam Pharma Equity Rollover(3) | $491 | |||||||
Redemption of Class A Shares | $123 | |||||||||
|
|
|
| |||||||
Total Sources | $984 | Total Uses | $984 | |||||||
|
|
|
| |||||||
| (1) | Assumes (i) the maximum number of redemptions by the public shareholders (assuming that 23,460,000 Holdco Shares are issued in connection with the PIPE Financing), (ii) that the amount in the Trust Account is $138.1 million (which was the approximate value of the Trust Account as of June 30, 2022, not taking into account $4.8 million of deferred underwriting fees to be paid) and (iii) 1,540,000 are not redeemed, which is the number of FLAC Public Shares subject to the Sponsor Support Agreement and the Investor Support Agreements and the minimum number of FLAC Public Shares needed to satisfy the Closing condition that the Aggregate Cash Proceeds equal at least $250 million. |
| (2) | As of June 30, 2022. |
| (3) | Assumes (i) that Participating Shareholders represent 100% of the issued and outstanding shares of NewAmsterdam Pharma; (ii) that there are no “change of control” payments made or required to be made by NewAmsterdam Pharma or Holdco (other than the payments described to Amgen and MTPC in the clause which follows this one); (iii) that none of the 1,886,137 Earnout Shares (including those issuable to Amgen and MTPC) will be issued; (iv) the issuance of an aggregate of 8,656,330 Holdco Shares to Amgen and MTPC pursuant to each of their respective profit rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” and (v) that neither the exercise of any NewAmsterdam Pharma Options (prior to the Closing) and Holdco Options, nor the exercise of any awards that may be issued under Holdco’s LTIP following the closing of the Business Combination, have been taken into account. |
| (4) | Estimated fees and expenses on Closing. |
The approximate aggregate fees payable to Credit Suisse, Jefferies, SVB Securities and William Blair in connection with the Transactions are estimated to be $11.0 million. Credit Suisse will be paid an additional $4.83 million representing the deferred underwriting commissions, conditioned upon the completion of the Business Combination.
Certain Information Relating to Holdco
Listing of Holdco Public Shares and Holdco Public Warrants on Nasdaq
Holdco Shares and Holdco Public Warrants are not currently traded on a stock exchange. Holdco has applied to list the Holdco Shares and Holdco Public Warrants on Nasdaq under the symbols “NAMS” and “NAMSW,” respectively, upon the closing of the Business Combination.
Restrictions on Resales
All Holdco Shares and Holdco Public Warrants received by holders of FLAC Public Shares in the Business Combination are expected to be freely tradable, except that Holdco Shares and Holdco Public Warrants received in the Business Combination by persons who become affiliates of Holdco for purposes of Rule 144 under the Securities Act may be resold by them only in transactions permitted by Rule 144, or as otherwise permitted under the Securities Act. Persons who may be deemed affiliates of Holdco generally include individuals or entities that control, are controlled by or are under common control with, Holdco and may include the directors and executive officers of Holdco, as well as its principal shareholders.
196
Table of Contents
Delisting of FLAC Class A Ordinary Shares and Deregistration of FLAC
FLAC and Holdco anticipate that, following consummation of the Business Combination, the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants will be delisted from Nasdaq and subsequently deregistered under the Exchange Act.
Emerging Growth Company
Holdco is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Holdco will remain an “emerging growth company” until the earliest to occur of (i) the last day of the fiscal year (a) following the fifth anniversary of the closing of the Business Combination, (b) in which Holdco has total annual gross revenue of at least $1.07 billion or (c) in which Holdco is deemed to be a large accelerated filer, which means the market value of Holdco Shares held by non-affiliates exceeds $700 million as of the last business day of Holdco’s prior second fiscal quarter, and (ii) the date on which Holdco issued more than $1.0 billion in non-convertible debt during the prior three-year period. Holdco intends to take advantage of exemptions from various reporting requirements that are applicable to most other public companies, whether or not they are classified as “emerging growth companies,” including, but not limited to, an exemption from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that Holdco’s independent registered public accounting firm provide an attestation report on the effectiveness of its internal control over financial reporting and reduced disclosure obligations regarding executive compensation.
Foreign Private Issuer
As a “foreign private issuer,” Holdco will be subject to different U.S. securities laws than domestic U.S. issuers. The rules governing the information that Holdco must disclose differ from those governing U.S. corporations pursuant to the Exchange Act. Holdco will be exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements to shareholders. Those proxy statements are not expected to conform to Schedule 14A of the proxy rules promulgated under the Exchange Act. As a foreign private issuer, Holdco will be exempt from a number of rules under the U.S. securities laws and will be permitted to file less information with the SEC than a U.S. company. In addition, as a “foreign private issuer,” Holdco’s officers and directors and holders of more than 10% of the issued and outstanding Holdco Shares, will be exempt from the rules under the Exchange Act requiring insiders to report purchases and sales of ordinary shares as well as from Section 16 short swing profit reporting and liability.
Comparison of Shareholder Rights
Until consummation of the Domestication Merger, Cayman Islands law and the FLAC Articles of Association will continue to govern the rights of FLAC shareholders. After consummation of the Merger, Dutch law and the Holdco Articles of Association will govern the rights of the former FLAC shareholders, as they will receive Holdco Shares as consideration at the time of the Merger.
There are certain differences in the rights of FLAC shareholders prior to the Business Combination and the rights of Holdco shareholders after the Business Combination. Please see the section entitled “Comparison of Corporate Governance and Shareholder Rights.”
Certain Tax Consequences of the Business Combination
Please see the section entitled “Material Tax Considerations.”
Accounting Treatment of the Business Combination
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS. Under this method of accounting, FLAC will be treated as the “acquired” company for accounting purposes. As FLAC
197
Table of Contents
does not meet the definition of a business under IFRS 3—Business Combinations (“IFRS 3”), the net assets of FLAC will be stated at historical cost, with no goodwill or other intangible assets recorded. As a result of the Business Combination and related transactions, the existing shareholders of NewAmsterdam Pharma will continue to retain control through their majority ownership of Holdco.
NewAmsterdam Pharma has been determined to be the accounting acquirer based on an evaluation of the following facts and circumstances:
| • | NewAmsterdam Pharma’s shareholders will have the largest voting interest in Holdco under both the No Redemption Scenario and Maximum Redemption Scenario; |
| • | NewAmsterdam Pharma’s senior management will be the senior management of Holdco; |
| • | The business of NewAmsterdam Pharma will comprise the ongoing operations of Holdco; and |
| • | NewAmsterdam Pharma is the larger entity, in terms of substantive operations and employee base. |
As FLAC does not meet the definition of a business in accordance with IFRS 3, the Business Combination is accounted for within the scope of IFRS 2—Share-based Payment (“IFRS 2”). Any excess of the fair value of Holdco Shares issued to FLAC Shareholders over the fair value of FLAC’s identifiable net assets acquired represents compensation for the service of a stock exchange listing for its shares provided by FLAC and is expensed as incurred.
Appraisal Rights or Dissenters’ Rights
Shareholders of FLAC may be entitled to give notice to FLAC prior to the General Meeting that they wish to dissent to the Merger and to receive payment of fair market value for their shares if they follow the procedures set out in the Cayman Islands Companies Act, noting that any such dissenters’ rights may be limited pursuant to Section 239 of the Cayman Islands Companies Act which states that no such dissenters’ rights will be available in respect of shares of any class for which an open market exists on a recognized stock exchange at the expiry date of the period allowed for written notice of an election to dissent provided that the merger consideration constitutes shares of any company which at the effective date of the merger are listed on a national securities exchange. It is FLAC’s view that such fair market value would equal the amount which shareholders would obtain if they exercise their redemption rights as described herein.
198
Table of Contents
THE BUSINESS COMBINATION AGREEMENT AND ANCILLARY DOCUMENTS
This section of the proxy statement/prospectus describes the material provisions of the Business Combination Agreement, but does not purport to describe all of the terms of the Business Combination Agreement. The following summary is qualified in its entirety by reference to the complete text of the Business Combination Agreement, which is attached as Annex A hereto. You are urged to carefully read the Business Combination Agreement in its entirety because it is the primary legal document that governs the Business Combination. The legal rights and obligations of the parties to the Business Combination Agreement are governed by the specific language of the Business Combination Agreement, and not this summary.
The Business Combination Agreement contains representations, warranties and covenants that the respective parties made to each other as of the date of the Business Combination Agreement or other specific dates. The assertions embodied in those representations, warranties and covenants were made for purposes of the Business Combination Agreement among the respective parties and are subject to important qualifications and limitations agreed to by the parties in connection with negotiating the Business Combination Agreement. The representations, warranties and covenants in the Business Combination Agreement are also modified in important part by the underlying disclosure schedules, which are referred to herein as the “Schedules.” The Schedules are not filed publicly. The representations and warranties in the Business Combination Agreement are also subject to a contractual standard of materiality different from that generally applicable to shareholders and are used for the purpose of allocating risk among the parties rather than establishing matters as facts. Holdco, FLAC, and NewAmsterdam Pharma do not believe that the Schedules contain information that is material to an investment decision. Moreover, certain representations and warranties in the Business Combination Agreement may not have been or may not be, as applicable, accurate as of any specific date and do not purport to be accurate as of the date of this proxy statement/prospectus. Accordingly, no person should rely on the representations and warranties in the Business Combination Agreement or the summaries thereof in this proxy statement/prospectus as characterizations of the actual state of facts about Holdco, FLAC, and NewAmsterdam Pharma or any other matter.
General Description of the Business Combination Agreement
General
On July 25, 2022, FLAC, NewAmsterdam Pharma, Holdco, and Merger Sub entered into the Business Combination Agreement, which provides for, among other things, the following transactions:
| • | The Participating Shareholders will participate in the Exchange; |
| • | Immediately after giving effect to the Exchange, the parties will effect the Holdco Reorganization, provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing; |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC as the Surviving Company; |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding FLAC Warrant will become a Holdco Warrant; |
| • | Each NewAmsterdam Pharma Option will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will effect the Domestication; and |
199
Table of Contents
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders (including Amgen and MTPC for this purpose) and the Participating Optionholders and who are at the time of achievement of such milestone still providing services to Holdco or its subsidiaries, 1,886,137 Earnout Shares, which in the case of the Participating Optionholders will take the form of awards of restricted stock units under Holdco’s long-term incentive plan. The development milestone consists of the achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the Earnout Period. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within the Earnout Period. |
Effect of the Business Combination on Existing FLAC Equity
Subject to the terms and conditions of the Business Combination Agreement, the Business Combination will result in, among other things, the following:
| • | each FLAC Class A Ordinary Share will be exchanged for one fully paid and non-assessable Holdco Share; |
| • | each Founder Share will be exchanged for one fully paid and non-assessable Holdco Share; and |
| • | each FLAC Warrant will become a Holdco Warrant, on the same terms and conditions as those applicable to the respective FLAC Warrants. |
Consideration to NewAmsterdam Pharma Shareholders in the Business Combination
Subject to the terms and conditions of the Business Combination Agreement, the consideration to be received by the NewAmsterdam Pharma shareholders in connection with the Business Combination will be (i) an aggregate number of Holdco Shares valued at $491 million and allocated among such equityholders by using the Exchange Ratio and (ii) 1,886,137 Earnout Shares if and when a certain clinical development milestone is achieved during the Earnout Period. Each NewAmsterdam Pharma Option outstanding immediately prior to the consummation of the Exchange will remain outstanding and, to the extent unvested, will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares. The exercise of each option will be made in Holdco Shares based on the Exchange Ratio. Additionally, the exercise price of each converted option will be determined by dividing the exercise price per share (or depository receipt for a share) of each option to purchase shares (or depository receipts for shares) of NewAmsterdam Pharma by the Exchange Ratio. The Earnout Shares payable to eligible Participating Optionholders will be delivered in the form of awards of restricted stock units under Holdco’s long-term incentive plan.
Aggregate Holdco Proceeds
The aggregate proceeds received by Holdco through the PIPE Financing and from the Trust Account (after giving effect to the FLAC Shareholder Redemption) will be used for general corporate purposes after the Business Combination.
Material Adverse Effect
Under the Business Combination Agreement, certain representations and warranties of FLAC, Holdco, and NewAmsterdam Pharma are qualified in whole or in part by materiality thresholds. In addition, certain representations and warranties of FLAC, Holdco, and NewAmsterdam Pharma are qualified in whole or in part by a material adverse effect standard for purposes of determining whether a breach of such representations and warranties has occurred. Pursuant to the Business Combination Agreement, “Company Material Adverse Event”
200
Table of Contents
means any change, event, effect, occurrence or state of facts that, individually or in the aggregate with any other change, event, effect, occurrence or state of facts, has had or would reasonably be expected to have a material adverse effect on (a) the business, results of operations or financial condition of NewAmsterdam Pharma or its subsidiaries, taken as a whole, or (b) the ability of NewAmsterdam Pharma to consummate the Transactions. In the case of clause (a), none of the following will be taken into account in determining whether a Company Material Adverse Effect has occurred or is reasonably likely to occur: any adverse change, event, effect or occurrence arising after the date of the Business Combination Agreement from or related to (i) changes in general business or economic conditions in or affecting the United States or the Netherlands, or changes therein, or the global economy generally, (ii) any national or international political or social conditions in the United States, the Netherlands, or any other country, including the engagement by the United States, the Netherlands, or any other country in hostilities, whether or not pursuant to the declaration of a national emergency or war, or the occurrence in any place of any military or terrorist attack, sabotage or cyberterrorism, or any escalation of the foregoing, (iii)��changes in conditions of the financial, banking, capital or securities markets generally in the United States, the Netherlands, or any other country or region in the world, or changes therein, including changes in interest rates in the United States, the Netherlands, or any other country and changes in exchange rates for the currencies of any countries, (iv) changes or proposed changes in any applicable laws or IFRS or the interpretation thereof, (v) any change, event, effect, occurrence or state of facts that is generally applicable to the industries or markets in which NewAmsterdam Pharma or its subsidiaries operate, (vi) the execution or public announcement of the Business Combination Agreement or the pendency or consummation of the Transactions, including their impact on the relationships, contractual or otherwise, of NewAmsterdam Pharma or its subsidiaries with employees, customers, investors, contractors, lenders, suppliers, vendors, partners, licensors, licensees, payors or other third parties related thereto (the exception in this clause (vi) will not apply to the representations and warranties in the Business Combination Agreement relating to certain third-party consents, to the extent that their purpose is to address the consequences resulting from the public announcement or pendency or consummation of the Transactions or the condition to Closing pertaining to the accuracy of such representations and warranties), (vii) any failure by NewAmsterdam Pharma or its subsidiaries to meet, or changes to, any internal or published budgets, projections or forecasts in and of itself (although the underlying facts and circumstances resulting in such failure may be taken into account to the extent not otherwise excluded from this definition pursuant to clauses (i) through (vi) or (viii)), (viii) the taking of any action required by or expressly permitted by the Business Combination Agreement or any ancillary document, or the failure to take any action that is prohibited by the Business Combination Agreement or any ancillary document, (ix) any action taken by, or at the express written request of an authorized signatory of FLAC, the Sponsor or any of their respective affiliates, or (x) any hurricane, tornado, flood, earthquake, tsunami, natural disaster, mudslides, wild fires, epidemics, pandemics or quarantines, acts of God or other natural disasters or comparable events in the United States, the Netherlands or any other country or region in the world, or any escalation of the foregoing, including, for the avoidance of doubt, COVID-19 and any law, directive, pronouncement, guideline or recommendation issued by any governmental entity, the Centers for Disease Control and Prevention, the World Health Organization or any industry group providing for business closures, changes to business operations, “sheltering-in-place” or other restrictions that relate to, or arise out of, an epidemic, pandemic or disease outbreak (including the COVID-19 pandemic). A any change, event, effect, occurrence or state of facts resulting from a matter described in any of the foregoing clauses (i) through (v) or (x) may be taken into account in determining whether a Company Material Adverse Effect has occurred or is reasonably likely to occur to the extent such change, event, effect, occurrence or state of facts has a disproportionate adverse effect on NewAmsterdam Pharma or its subsidiaries, taken as a whole, relative to other participants operating in the industries or markets in which NewAmsterdam Pharma or its subsidiaries operate.
Pursuant to the Business Combination Agreement, a “FLAC Material Adverse Effect” means any change, event, effect, occurrence or state of facts that, individually or in the aggregate with any other change, event, effect, occurrence or state of facts, has had or would reasonably be expected to have a material adverse effect on (a) the business, results of operations or financial condition of FLAC or (b) the ability of FLAC to consummate the Transactions. In the case of clause (a), none of the following will be taken into account in determining whether a FLAC Material Adverse Effect has occurred or is reasonably likely to occur: any adverse change, event, effect or occurrence arising after the date of the Business Combination Agreement from or related to
201
Table of Contents
(i) changes in general business or economic conditions in or affecting the United States, or changes therein, or the global economy generally, (ii) any national or international political or social conditions in the United States or any other country, including the engagement by the United States or any other country in hostilities, whether or not pursuant to the declaration of a national emergency or war, or the occurrence in any place of any military or terrorist attack, sabotage or cyberterrorism, (iii) changes in conditions of the financial, banking, capital or securities markets generally in the United States or any other country or region in the world, or changes therein, including changes in interest rates in the United States or any other country and changes in exchange rates for the currencies of any countries, (iv) changes or proposed changes in any applicable laws or IFRS or the interpretation thereof, (v) any change, event, effect, occurrence or state of facts that is generally applicable to the industries or markets in which FLAC operates, (vi) the execution or public announcement of the Business Combination Agreement or the pendency or consummation of the Transactions, including their impact on the relationships, contractual or otherwise, of FLAC with employees, customers, investors, contractors, lenders, suppliers, vendors, partners, licensors, licensees, payors or other third parties related thereto (the exception in this clause (vi) will not apply to the representations and warranties in the Business Combination Agreement relating to certain third-party consents, to the extent that their purpose is to address the consequences resulting from the public announcement or pendency or consummation of the Transactions or the condition to Closing pertaining to the accuracy of such representations and warranties), (vii) any failure by FLAC to meet, or changes to, any internal or published budgets, projections or forecasts in and of itself (although the underlying facts and circumstances resulting in such failure may be taken into account to the extent not otherwise excluded from this definition pursuant to clauses (i) through (vi) or (viii)), (viii) the taking of any action required by or expressly permitted by the Business Combination Agreement or any ancillary document or the failure to take any action that is prohibited by the Business Combination Agreement or any ancillary document, (ix) any action taken by, or at express written request of an authorized signatory of NewAmsterdam Pharma or any of its affiliates, or (x) any hurricane, tornado, flood, earthquake, tsunami, natural disaster, mudslides, wild fires, epidemics, pandemics or quarantines, acts of God or other natural disasters or comparable events in the United States or any other country or region in the world, or any escalation of the foregoing, including, for the avoidance of doubt, COVID-19 and any law, directive, pronouncement, guideline or recommendation issued by any governmental entity, the Centers for Disease Control and Prevention, the World Health Organization or any industry group providing for business closures, changes to business operations, “sheltering-in-place” or other restrictions that relate to, or arise out of, an epidemic, pandemic or disease outbreak (including the COVID-19 pandemic). Any change, event, effect, occurrence or state of facts resulting from a matter described in any of the foregoing clauses (i) through (v) or (x) may be taken into account in determining whether a FLAC Material Adverse Effect has occurred or is reasonably likely to occur to the extent such Event has a disproportionate adverse effect on FLAC relative to other “SPACs” operating in the industries in which FLAC operates.
Closing and Effective Date of the Business Combination
The Closing will take place as promptly as practicable, but no later than the third business day, following the satisfaction (or, to the extent permitted by applicable law, waiver) of the conditions described below under the section entitled “Conditions to Closing of the Business Combination” (other than those conditions that by their nature are to be satisfied at the Closing, but subject to satisfaction or waiver of such conditions). The Closing will take place electronically. Any closing deliverables which need to be notarized by a Dutch civil-law notary will be executed by the applicable persons in the Netherlands at the offices of NautaDutilh N.V.
Conditions to Closing of the Business Combination
Conditions to Each Party’s Obligations
The respective obligations of each party to the Business Combination Agreement to consummate the Business Combination are subject to the satisfaction, or waiver by the party for whose benefit such condition exists, at or prior to the Closing of the following conditions:
| • | any applicable waiting period under any applicable Antitrust Law will have expired or terminated, and any consent pursuant to any applicable Antitrust Law will have been obtained; |
202
Table of Contents
| • | no order or law issued by any court of competent jurisdiction or other governmental entity or other legal restraint or prohibition preventing the consummation of the Transactions will be in effect; |
| • | the registration statement—of which this proxy statement/prospectus forms a part—will have become effective in accordance with the provisions of the Securities Act, no stop order has been issued by the SEC and remains in effect with respect to the registration statement of which this proxy statement/prospectus forms a part, and no proceeding seeking such a stop order has been threatened or initiated by the SEC and remains pending; |
| • | the approval at the General Meeting, of the Business Combination Proposal and the Merger Proposal in accordance with FLAC’s governing documents, will have been obtained; |
| • | the approval of the Business Combination Agreement and the Transactions by the shareholders of NewAmsterdam Pharma, in accordance with NewAmsterdam Pharma’s governing documents, will have been obtained; |
| • | (i) Holdco’s initial listing application with Nasdaq in connection with the listing of Holdco Shares will have been approved such that, immediately following the Closing, Holdco will satisfy any applicable initial and continuing listing requirements of Nasdaq, (ii) Holdco will not have received any notice of non-compliance therewith, and (iii) the Holdco Shares and Holdco Public Warrants to be issued in connection with the Transactions will have been approved for listing on Nasdaq subject to official notice of issuance; |
| • | the Holdco Board will be comprised of the individuals appointed in accordance with the Business Combination Agreement and as described in the section entitled “Management of Holdco Following the Business Combination”; and |
| • | after giving effect to the Transactions (including the PIPE Financing), Holdco will have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) immediately after the Effective Date. |
Other Conditions to FLAC’s Obligations
In addition to the conditions described above, the obligation of FLAC to consummate the Business Combination is subject to the satisfaction, or waiver by FLAC, at or prior to the Closing of the following conditions:
| • | (i) the representations and warranties of NewAmsterdam Pharma regarding its organization and qualification, indebtedness and authorized share capital, authority, absence of a material adverse effect and broker fees in connection with the Transactions must be true and correct in all material respects (without giving effect to any materiality qualifications included in such representations and warranties) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date); (ii) the representations and warranties of NewAmsterdam Pharma regarding its capitalization must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects (except for de minimis inaccuracies) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), and (iii) the other representations and warranties of NewAmsterdam Pharma must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not constitute a Company Material Adverse Effect; |
203
Table of Contents
| • | NewAmsterdam Pharma must have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by NewAmsterdam Pharma under the Business Combination Agreement at or prior to the Closing; |
| • | since the date of the Business Combination Agreement, no Company Material Adverse Effect will have occurred and be continuing; and |
| • | at or prior to the Closing, NewAmsterdam Pharma will have delivered, or caused to be delivered, to FLAC: (i) a certificate executed by an authorized officer of NewAmsterdam Pharma, dated as of the Closing Commencement Date, to the effect that the conditions described in the three bullets above are satisfied and (ii) the Investor Rights Agreement duly executed by NewAmsterdam Pharma and the NewAmsterdam Pharma shareholders contemplated to be parties thereto. |
Other Conditions to NewAmsterdam Pharma’s Obligations
In addition to the conditions described above, the obligations of NewAmsterdam Pharma to consummate the Business Combination are subject to the satisfaction, or waiver by NewAmsterdam Pharma, at or prior to the Closing of the following conditions:
| • | the Aggregate Cash Proceeds will be equal to or greater than $250 million; |
| • | the representations and warranties of FLAC regarding its organization and qualification, authority and brokers will be true and correct in all material respects (without giving effect to any materiality qualifications included in such representations and warranties) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be true and correct as of such earlier date); (ii) the representations and warranties of FLAC regarding its capitalization will be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects (except for de minimis inaccuracies) as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), and (iii) the other representations and warranties of FLAC and Holdco contained in Business Combination Agreement must be true and correct (without giving effect to any materiality qualifications included in such representations and warranties) in all respects as of the date of the Business Combination Agreement and as of the Closing Commencement Date (except that if any such representation and warranty is made of an earlier date, it must be so true and correct as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not cause a FLAC Material Adverse Effect; |
| • | FLAC must have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by FLAC under the Business Combination Agreement at or prior to the Closing; and |
| • | at or prior to the Closing, FLAC will have delivered, or caused to be delivered, the following documents to NewAmsterdam Pharma: (i) a certificate duly executed by an authorized officer of FLAC, dated as of the Closing Commencement Date, to the effect that the conditions described in the three bullets above are satisfied; and (ii) the Investor Rights Agreement duly executed by the Sponsor and certain affiliates of FLAC and the Sponsor. |
Frustration of Closing Conditions
Neither FLAC nor NewAmsterdam Pharma may rely on the failure of any condition described above to be satisfied if such failure was proximately caused by such party’s failure to use reasonable best efforts to cause the Closing to occur or such party’s breach of the Business Combination Agreement.
204
Table of Contents
Representations and Warranties
In the Business Combination Agreement, NewAmsterdam Pharma made customary representations and warranties to FLAC relating to, among other things: organization and qualification; its capitalization and of its subsidiaries; authorization; financial statements; absence of undisclosed liabilities; consents, approvals and no violations to contracts or organizational documents; licenses and permits; material contracts; absence of certain changes; impact of COVID-19; absence of business activities by Holdco and Merger Sub; litigation; compliance with laws; employee benefits; environmental matters; intellectual property; labor and employment matters; insurance policies; tax matters; broker fees; real and personal property; affiliate transactions; data privacy and security; international trade and anti-corruption matters; information supplied for inclusion in the registration statement—of which this proxy statement/prospectus forms a part; health and drug regulatory compliance; Investment Company Act; and the absence of undisclosed arrangements relating to the PIPE Financing.
In the Business Combination Agreement, FLAC made customary representations and warranties to NewAmsterdam Pharma relating to, among other things: organization and qualification; authorization; receipt of opinion from its financial advisor; consents, approvals and no violations to contracts or organizational documents; broker fees; information supplied for inclusion in the registration statement—of which this proxy statement/prospectus forms a part; its capitalization; SEC filings; the Trust Account; affiliate transactions; litigation; compliance with laws; internal controls; financial statements; absence of undisclosed liabilities; tax matters; and international trade and anti-corruption matters.
Covenants of the Parties
Covenants of NewAmsterdam Pharma, Holdco and Merger Sub
NewAmsterdam Pharma, Holdco, and Merger Sub agreed to certain covenants under the Business Combination Agreement, including, among others, the following:
| • | Subject to certain exceptions provided in the Business Combination Agreement, prior to the Closing, NewAmsterdam Pharma will, and will cause its subsidiaries to, operate their business in the ordinary course in all material respects and in accordance with all applicable law and will use commercially reasonable best efforts to maintain and preserve intact in all material respects their business organization, assets, properties and material business relations. |
| • | Subject to certain exceptions provided in the Business Combination Agreement (including actions contemplated thereunder and certain actions in the ordinary course), prior to the Closing, NewAmsterdam Pharma will, and will cause its subsidiaries to, not do any of the following without FLAC’s consent (such consent, other than in the case of certain items expressly noted in the Business Combination Agreement, not to be unreasonably withheld, conditioned or delayed): |
| • | declare, set aside, make or pay any dividend or distribution; |
| • | split, combine, reclassify, recapitalize or otherwise amend any terms of any shares or series of NewAmsterdam Pharma’s equity securities |
| • | merge, consolidate, combine or amalgamate any Group Company with any Person (as defined in the Business Combination Agreement); |
| • | acquire or purchase any business entity or organization; |
| • | adopt amendments to the governing documents of NewAmsterdam Pharma or its subsidiaries or the shareholders agreement of NewAmsterdam Pharma; |
| • | dispose of any material assets or properties of NewAmsterdam Pharma or its subsidiaries, except in the ordinary course of business; |
| • | dispose of or subject to a lien (i) any equity securities of NewAmsterdam Pharma and its subsidiaries, or (ii) any options, warrants, rights of conversion or other rights or arrangements |
205
Table of Contents
obligating NewAmsterdam Pharma and its subsidiary to issue, deliver or sell any of their equity securities; |
| • | incur indebtedness in excess of $2 million; |
| • | amend, modify, cancel or waive any debts; |
| • | enter into, amend, modify, terminate or waive any material benefit or right under any material contract; |
| • | make loans, advances or capital contributions, other than intercompany loans or capital contributions or ordinary course reimbursements of employee expenses; |
| • | amend, modify, adopt, enter into or terminate any material employee benefit plan; materially increase the compensation or benefits payable to any current or former director, manager, officer, employee, or contingent worker earning annual compensation in excess of $150,000, or increase the aggregate annual compensation or benefits payable of any of them to be greater than $150,000; take any action to accelerate any payment, right to payment, or benefit, or the funding of any payment, right to payment or benefit, payable or to become payable to any current or former director, manager, officer, employee or contingent worker; waive or release any material noncompetition, non-solicitation, no-hire, nondisclosure or other restrictive covenant obligation of any of those individuals; pay any special bonus or special remuneration to any director, officer or employee; terminate or furlough the employment of any director, officer, management-level or key employee; or enter into a settlement agreement with any current or former director, officer, or employee; |
| • | make or change any material tax election outside the ordinary course of business; |
| • | enter into any settlement, conciliation or similar contract involving the payment by NewAmsterdam Pharma or any of its subsidiaries in excess of $2 million in the aggregate or that imposes material non-monetary obligations; |
| • | authorize, recommend, propose or announce an intention to adopt a plan of complete or partial liquidation, dissolution, restructuring, recapitalization, reorganization or similar; |
| • | change their methods of accounting in any material respect; |
| • | enter into any contract providing for the payment of any brokerage fee, finders’ fee or other commission in connection with the Transactions; |
| • | make any change of control payment; |
| • | become a party to, establish, adopt, amend, commence participation in or enter into any collective bargaining or other labor union contract; |
| • | fail to keep current and in full force and effect, or to comply in all material respects with the requirements of, any material permit or regulatory permit; |
| • | create or incur any material lien; |
| • | enter into any new material line of business or operations, or discontinue any material line of business or operations; or |
| • | enter into any contract to take, or cause to be taken, any of the actions set forth above. |
| • | NewAmsterdam Pharma must obtain the approval of its shareholders of the execution, delivery and performance of the Business Combination Agreement and the consummation of the Transactions, and cause them to deliver the applicable documents under Dutch law required to effect the Exchange, or otherwise take legally permissible action as a result of which NewAmsterdam Pharma will act on behalf of each of its applicable shareholders in order to take such actions. |
206
Table of Contents
| • | Holdco will, as the sole shareholder of Merger Sub, approve and adopt the Business Combination Agreement, any ancillary document to which Merger Sub is or will be a party, and the Transactions as promptly as reasonably practicable (and in any event within one business day) following the date of the Business Combination Agreement. |
| • | NewAmsterdam Pharma will cause Holdco to, and Holdco will use its reasonable best efforts to cause the Holdco Shares issuable in connection with the Business Combination Agreement to be approved for listing on Nasdaq. |
| • | Subject to certain exceptions, prior to the Closing, Holdco will take all action necessary such that effective as of the Holdco Reorganization, the Holdco Board will consist of the individuals appointed in accordance with the Business Combination Agreement and as described in the section entitled “Management of Holdco Following the Business Combination,” with the parties to agree on the duration of the initial term to which each such Holdco Board member will be elected. |
| • | As promptly as reasonably practicable, NewAmsterdam Pharma will deliver to FLAC and Holdco the audited financial statements of NewAmsterdam Pharma as of December 31, 2021 and the unaudited financial statements as of June 30, 2022 and June 30, 2021, as required to be included in the registration statement of which this proxy statement/prospectus forms a part and any other filings to be made by FLAC and Holdco with the SEC in connection with the Transactions. |
| • | Subject to certain exceptions, prior to the Closing, Holdco will not take any action, or engage in any activities or business, nor incur any liabilities or obligations, other than (a) those that are incident to its organization, (b) the execution of the Business Combination Agreement or any ancillary document to which it is or will be a party, (c) those that are required by the SEC or Nasdaq in connection with the Transactions, (d) those that are expressly contemplated by the Business Combination Agreement or any ancillary document or (e) those that are consented to in writing by FLAC (such consent not to be unreasonably withheld, conditioned or delayed). |
| • | Prior to the effectiveness of the registration statement of which this proxy statement/prospectus forms a part, the Holdco Board will approve and adopt the Holdco LTIP, and the Holdco LTIP will reserve the number of shares of Holdco Shares for grant and issuance thereunder as described in the section entitled “Management of Holdco Following the Business Combination—Holdco LTIP.” |
Covenants of FLAC
FLAC agreed to certain covenants under the Business Combination Agreement, including, among others, the following:
| • | Subject to certain exceptions provided in the Business Combination Agreement, prior to the Closing, FLAC will, and will cause its subsidiaries to, operate their business in the ordinary course in all material respects and in accordance with all applicable law and will use commercially reasonable best efforts to maintain and preserve intact in all material respects the business organization, assets, properties and material business relations. |
| • | Subject to certain exceptions provided in the Business Combination Agreement (including actions contemplated thereunder and certain actions in the ordinary course), prior to the Closing, FLAC will, and will cause its subsidiaries to, not do any of the following without NewAmsterdam Pharma’s consent (such consent, other than in the case of certain items expressly noted in the Business Combination Agreement, not to be unreasonably withheld, conditioned or delayed): |
| • | adopt any amendments, supplements, restatements or modifications to the Investment Management and Trust Agreement, Warrant Assumption Agreement or the governing documents of FLAC or any of its subsidiaries; |
| • | declare, set aside, make or pay any dividend or distribution; |
207
Table of Contents
| • | split, combine or reclassify any of its capital stock or other equity securities or issue any other security in respect of, in lieu of or in substitution for shares of its capital stock; |
| • | incur indebtedness in excess of $2 million; |
| • | make any loans, advances or capital contributions in any other person, other than to, or in, FLAC or any of its subsidiaries; |
| • | issue any equity securities of FLAC or any of its subsidiaries or grant any additional options, warrants or stock appreciation rights with respect to equity securities of the forgoing of FLAC or any of its wholly owned subsidiaries; |
| • | enter into, renew, modify or revise any contract with a related party; |
| • | make or change any material tax election; |
| • | engage in any activities or business, or incur material liabilities, other than as permitted by the Business Combination Agreement; |
| • | authorize, recommend, propose or announce an intention to adopt a plan of complete or partial liquidation or dissolution; |
| • | enter into any contract providing for the payment of any brokerage fee, finders’ fee or other commission in connection with the Transactions; |
| • | incorporate any new direct or indirect subsidiary or enter into any new material line of business; |
| • | enter into any settlement, conciliation or similar contract involving the payment by FLAC or any of its subsidiaries in excess of $2 million in the aggregate or that imposes material non-monetary obligations; |
| • | change FLAC’s methods of accounting in any material respect; |
| • | enter into or materially amend any agreement with, or distribute any assets or property to, any of its officers, directors, employees, partners, shareholders or other affiliates; or |
| • | enter into any contract to take, or cause to be taken, any of the actions set forth above. |
| • | As promptly as practicable after the registration statement of which this proxy statement/prospectus forms a part is declared effective under the Securities Act, FLAC will (i) duly give notice of, convene and hold, the General Meeting for the purposes of obtaining the approval of FLAC’s shareholders of the Business Combination Agreement, the Transactions and the proposals contemplated thereby; and (ii) through the FLAC Board, recommend to its shareholders the adoption and approval of the Business Combination Agreement, the Transactions and the proposals contemplated thereby. |
| • | At the Closing, FLAC will deliver the applicable documents, opinions and notices to Continental pursuant to the Trust Agreement and make all appropriate arrangements to cause Continental to (i) pay as and when due all amounts payable to the public shareholders of FLAC pursuant to the FLAC Shareholder Redemption and the amounts due to the underwriters of the FLAC IPO for their deferred underwriting commissions as set forth in the Trust Agreement and (ii) pay all remaining amounts then available in the Trust Account to Holdco in accordance with the Trust Agreement. After such payments are made, the Trust Account will terminate. |
Mutual Covenants of the Parties
The parties agreed to certain mutual covenants under the Business Combination Agreement, including, among others, the following:
| • | They will use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary or advisable to consummate and make effective as promptly as practicable the Transactions. |
208
Table of Contents
| • | They will (i) use reasonable best efforts to promptly obtain, file with or deliver to, as applicable, any consents of any governmental entities or other persons that FLAC and NewAmsterdam Pharma determine are necessary, proper or advisable to consummate the Transactions and (ii) make any appropriate filings or take, or cause to be taken, any required actions pursuant to any Antitrust Laws with respect to the Transactions as promptly as reasonably practicable. |
| • | Subject to certain specified restrictions and conditions, each of FLAC and NewAmsterdam Pharma will provide to the other party and their representatives during normal business hours reasonable access to each other’s and their respective subsidiaries’ directors, officers, books and records, in a manner so as not to interfere with their normal business operations. |
| • | Each of FLAC and NewAmsterdam Pharma will notify the other party of, and keep each other reasonably informed regarding, any shareholder demands or proceedings related to the Business Combination Agreement, the ancillary documents or any matters relating thereto commenced against such party or its representatives. |
| • | Each of FLAC and NewAmsterdam Pharma will keep confidential certain information being provided in connection with the Business Combination Agreement and the consummation of the Transactions. |
| • | None of the parties will make any public announcements with respect to the Business Combination Agreement or the Transactions prior to the Closing without the prior written consent of FLAC and NewAmsterdam Pharma. |
| • | An agreement as to the intended tax treatment of the Transactions, as described under “Material Tax Considerations,” and to use reasonable best efforts to so qualify for such intended tax treatment, to file all tax returns consistent with, and take no position inconsistent with (whether in audits, tax returns or otherwise), to cooperate in connection with the filing of relevant tax returns and not take any action that would reasonably be expected to prevent or impede such intended tax treatment. |
| • | Each of FLAC and NewAmsterdam Pharma will not, and will cause its respective representatives not to, directly or indirectly, solicit, initiate or engage in discussions or negotiations with, or provide any non-public information to or enter into any agreement with any person concerning, respectively, (i) any acquisition of NewAmsterdam Pharma or of 20% or more of its and its controlled affiliates’ assets or businesses or 20% or more of any class of its voting securities, or (ii) any transaction constituting a “business combination” (as defined in the FLAC Articles of Association) or any other acquisition by FLAC of another person or a material portion of the assets or businesses of another person or any equity, debt or other investment in FLAC or any of its controlled affiliates. FLAC and NewAmsterdam Pharma also agreed not to prepare or take any steps in connection with an offering of any of their respective securities, and to cease any and all existing activities, discussions or negotiations that would reasonably be expected to lead to any of the foregoing transactions, as applicable. |
| • | The parties will prepare and mutually agree upon, and Holdco will file with the SEC, the registration statement of which this proxy statement/prospectus forms a part is declared effective under the Securities Act, and use their reasonable best efforts to, with respect to such registration statement: (i) cause it to comply in all material respects with applicable rules and regulations; (ii) promptly notify the other of, cooperate with each other with respect to and respond promptly to any comments of the SEC or its staff; (iii) have it declared effective under the Securities Act as promptly as practicable after it is filed with the SEC; and (iv) keep it effective through the Closing in order to permit the consummation of the Transactions. |
| • | Each of FLAC and NewAmsterdam Pharma have agreed that all rights to indemnification, advancement or exculpation now existing in favor of the directors and officers of FLAC and NewAmsterdam Pharma, as provided in their respective governing documents or otherwise in effect as of immediately prior to the Effective Date, will survive for a period of six years following the Effective Date. |
209
Table of Contents
| • | Subject to certain exceptions, at or prior to the Closing, (i) FLAC will purchase a “tail” policy providing liability insurance coverage for certain of its respective directors and officers with respect to matters occurring on or prior to the Effective Date and (ii) Holdco will use reasonable best efforst to obtain and maintain, in its own liability insurance policies, “prior acts” coverage for the directors and officers of NewAmsterdam Pharma with respect to matters occurring on or prior to the Effective Date and, if after using such efforts, such coverage is not secured, then, subject to certain exceptions, NewAmsterdam must purchase a “tail” policy providing liability insurance coverage for its respective directors and officers with respect to matters occurring on or prior to the Effective Date. |
| • | Each of FLAC and Holdco will use its respective reasonable best efforts to take, or to cause to be taken, all actions required, necessary or that it otherwise deems to be proper or advisable to consummate the PIPE Financing as contemplated by the Subscription Agreements on the terms described therein. |
| • | Prior to the Closing or the termination of the Business Combination Agreement in accordance with its terms, the parties will not make any offer of securities in the European Union in connection with the Transactions other than in accordance with the provisions of the Regulation (EU) 2017/1129 of the European Parliament and of the Council of June 14, 2017 on the prospectus to be published when securities are offered to the public or admitted to trading on a regulated market (including any relevant delegated regulations). |
| • | Prior to the Closing, NewAmsterdam Pharma may elect that Holdco adopt, effective as of the Closing, an employee stock purchase plan allowing eligible employees to purchase Holdco Shares at periodic intervals with their accumulated payroll deductions (the “ESPP”), providing for (i) a maximum number of Holdco Shares authorized for sale not to exceed a specified percentage of the aggregate number of Holdco Shares outstanding immediately following the Merger, subject to annual increases of not more than a percentage of such aggregate number of Holdco Shares, (ii) purchase and offering periods to be implemented from time to time, (iii) a purchase price per Holdco Share of no less than the lower of (a) 85% of the closing trading price per Holdco Share on the first day of an applicable offering period or (b) 85% of the closing trading price per Holdco Share on the applicable purchase date, and (iv) not more than $25,000 worth of Holdco Shares being available for purchase by an individual employee per year. In case NewAmsterdam so elects that Holdco adopt the ESPP, FLAC and NewAmsterdam will in good faith agree upon the terms of the ESPP, including the percentages referred to above in this paragraph. |
Survival of Representations and Warranties
The representations, warranties, agreements and covenants in the Business Combination Agreement terminate on the Effective Date, except for the covenants that under the Business Combination Agreement by their terms contemplate performance after the Closing.
Termination
The Business Combination Agreement may be terminated at any time prior to Closing under the following customary and limited circumstances:
| • | by mutual written consent of FLAC and NewAmsterdam Pharma; |
| • | by either FLAC or NewAmsterdam Pharma, if: |
| • | the Transactions have not been consummated on or prior to the Termination Date. If the registration statement of which this proxy statement/prospectus forms a part is not declared effective by November 1, 2022, then the Termination Date will be automatically extended until February 9, 2023. This termination right will not be available to a party if such party’s breach of any of its covenants or obligations under the Business Combination Agreement will have proximately caused the failure to consummate the Transactions on or before the Termination Date; |
210
Table of Contents
| • | any governmental entity has issued an order or taken any other action permanently enjoining, restraining or otherwise prohibiting the Transactions and such order or other action has become final and nonappealable; or |
| • | the General Meeting has been held (including any adjournment or postponement thereof), has concluded, FLAC’s shareholders have duly voted and the approval of FLAC’s shareholders of the Business Combination Agreement, the Transactions and the proposals contemplated thereby was not obtained; |
| • | by FLAC, if any of the representations or warranties made by NewAmsterdam Pharma are not true and correct or if NewAmsterdam Pharma or Holdco fails to perform any of its respective covenants or agreements under the Business Combination Agreement (including an obligation to consummate the Closing) such that the conditions to the obligations of FLAC relating to such respective matters, as described in the section entitled “Conditions to Closing of the Business Combination” above, would not be satisfied and the breach (or breaches) is (or are) not cured or cannot be cured within the earlier of (i) 30 days after written notice thereof, and (ii) the Termination Date (as described above). This termination right will not be available if FLAC is in breach of the Business Combination Agreement so as to prevent the conditions to NewAmsterdam Pharma’s obligations described in the bullet below to be satisfied; or |
| • | by NewAmsterdam Pharma, if any of the representations or warranties made by FLAC are not true and correct or if FLAC fails to perform any of its covenants or agreements under the Business Combination Agreement (including an obligation to consummate the Closing) such that the conditions to the obligations of NewAmsterdam Pharma relating to such respective matters, as described in the section entitled “Conditions to Closing of the Business Combination” above, would not be satisfied and the breach (or breaches) is (or are) not cured or cannot be cured within the earlier of (i) 30 days after written notice thereof, and (ii) the Termination Date (as described above). This termination right will not be available if NewAmsterdam Pharma is in breach of the Business Combination Agreement so as to prevent the conditions to FLAC’s obligations described in the bullet above to be satisfied; or |
Expenses
The fees and expenses incurred in connection with the Business Combination Agreement, the ancillary documents and the Transactions, including the fees and disbursements of counsel, financial advisors and accountants, will be paid by the party incurring such fees or expenses. However, if the Closing occurs, then FLAC will pay, or cause to be paid, all unpaid FLAC and NewAmsterdam Pharma expenses as of such time. FLAC and NewAmsterdam Pharma also agreed that each of them will, subject to preceding sentence, 50% of all the filing fees incurred in connection with making any filings required to obtain the applicable approvals under antitrust laws and 50% of all filing fees and associated expenses incurred in connection with filing the registration statement of which this proxy statement/prospectus forms a part and obtaining approval of Nasdaq for listing of the Holdco Shares.
Governing Law
The Business Combination Agreement is governed by and construed in accordance with the laws of the State of Delaware, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the law of any jurisdiction other than the State of Delaware (except that the Cayman Islands Companies Act will apply to the Merger and applicable Dutch law will apply to the Exchange and the Holdco Reorganization).
Amendments
The Business Combination Agreement may be amended or modified only by a written agreement executed and delivered by (a) if prior to Closing, each of FLAC and NewAmsterdam Pharma and (b) if after the Closing, each of Holdco and the Sponsor.
211
Table of Contents
Ancillary Documents
This section describes the material provisions of certain additional agreements that were entered into concurrently with, or will be entered into pursuant to (as applicable) the Business Combination Agreement, which are referred to herein as the “Ancillary Documents,” but does not purport to describe all of the terms thereof. The following summary is qualified in its entirety by reference to the complete text of each of the Ancillary Documents, which are included herein as annexes, other than the Warrant Assumption Agreement, which is attached hereto as Exhibit 4.1. Shareholders and other interested parties are urged to read such Ancillary Documents in their entirety prior to voting on the proposals presented at the General Meeting.
Company Support Agreement
In connection with the execution of the Business Combination Agreement, Holdco, NewAmsterdam Pharma, FLAC, Merger Sub, and certain existing shareholders of NewAmsterdam Pharma (which shareholders only include directors, executive officers and shareholders with voting securities in NewAmsterdam Pharma representing 5% or more of all such voting securities) entered into the Company Support Agreement (included herein as Annex E) pursuant to which, among other things, each such existing shareholder of NewAmsterdam Pharma (a) granted or will grant, as applicable, NewAmsterdam Pharma (or a designee thereof) with a power of attorney permitting and directing NewAmsterdam Pharma to execute on behalf of such shareholder a Dutch deed of issue to effect the Exchange with respect to the shares of NewAmsterdam Pharma held by such shareholder, (b) undertook or will undertake, as applicable, vis-à-vis NewAmsterdam Pharma, Holdco, FLAC and each other existing shareholder of NewAmsterdam Pharma to take all necessary or desirable actions in connection with the transactions set forth in the Business Combination Agreement, (c) agreed to vote in favor of the approval of the Business Combination Agreement, the Exchange and any other matters necessary or reasonably requested by NewAmsterdam Pharma to consummate the transactions contemplated in the Business Combination Agreement, and (d) agreed to certain customary covenants to support the Business Combination (including restrictions on the sale, disposition or transfer of the shares of NewAmsterdam Pharma held by him, her or it). It is anticipated that 24,549,052 Holdco Shares, or approximately 36% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to the Company Support Agreement.
Sponsor Support Agreement
In connection with the execution of the Business Combination Agreement, the FLAC Initial Shareholders, FLAC, Holdco and NewAmsterdam Pharma entered into the Sponsor Support Agreement, included herein as Annex D, pursuant to which the FLAC Initial Shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them; and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. 3,450,000 FLAC Class B Ordinary Shares are subject to the Sponsor Support Agreement.
Investor Support Agreements
In connection with the execution of the Business Combination Agreement, certain FLAC shareholders and FLAC entered into the Investor Support Agreements, the form of which is included herein as Annex F, pursuant to which each shareholders have agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the
212
Table of Contents
Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions and (b) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination. 1,500,000 FLAC Class A Ordinary Shares are subject to the Investor Support Agreements.
Subscription Agreements
In connection with the execution of the Business Combination Agreement, Holdco and FLAC entered into the Subscription Agreements with the PIPE Investors, the form of which is included herein as Annex C, and pursuant to which the PIPE Investors agreed to subscribe for and purchase and Holdco agreed to issue and sell to such PIPE Investors, 23,460,000 Private Placement Shares, for an aggregate of $234.6 million in proceeds. The Private Placement Shares to be issued pursuant to the Subscription Agreements have not been registered under the Securities Act, in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder without any form of general solicitation or general advertising.
The Subscription Agreements provide that Holdco is required to file with the SEC, within 30 days after the consummation of the transactions contemplated by the Business Combination Agreement, a registration statement covering the resale of the Private Placement Shares and to use its commercially reasonable efforts to have such registration statement declared effective as soon as practicable after the filing thereof but no later than the earlier of (i) 60 calendar days after the filing thereof (or, in the event the SEC notifies Holdco that it will review such registration statement, 90 calendar days following the filing thereof) and (ii) five business days after the date Holdco is notified (orally or in writing, whichever is earlier) by the SEC that such registration statement will not be “reviewed” or will not be subject to further review.
The closing of the PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination and related transactions.
Investor Rights Agreement
At the closing of the Business Combination, Holdco will enter into the Investor Rights Agreement with the FLAC Initial Shareholders and certain NewAmsterdam Pharma shareholders, the form of which is included herein as Annex G, providing for, among other things, subject to the terms thereof, customary registration rights, including demand and piggy-back rights subject to cut-back provisions. Holdco has agreed to file a registration statement to register the Holdco Shares covered by the Investor Rights Agreement no later than 30 days following consummation of the Business Combination.
Pursuant to the Investor Rights Agreement, certain NewAmsterdam Pharma shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination, subject to certain limited exceptions. In addition, the FLAC Initial Shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares they receive in connection with the Business Combination for a period beginning on the Final Closing Date and ending one year after the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the indicated time periods with respect to 50% of the Holdco Shares the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination, on the earlier of the date that (i) the closing price of a Holdco Share equals or exceeds $12.00 per share (subject to certain adjustments) for any 20 trading days within any 30-day trading period commencing at least 150 days after the Final Closing Date of the Business Combination, or (ii) Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The restrictions on the remaining 50% of the Holdco Shares
213
Table of Contents
each of the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination will end prior to the periods indicated above on the date that Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The share transfer restrictions above will not apply with respect to sales to cover withholding taxes due upon vesting of equity awards and, in the case of directors or officers of Holdco, with respect to the sale of up to 10% of the Holdco Shares held by each of them. It is anticipated that 48,865,642 Holdco Shares, or approximately 58% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to the Investor Rights Agreement.
Lock-Up Agreement
At the closing of the Business Combination, certain NewAmsterdam Pharma shareholders who are not party to the Investor Rights Agreement (after giving effect to the Merger and the PIPE Financing) will enter into Lock-Up Agreements in the form included herein as Annex H, pursuant to which, among other things, they will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end on the earlier of the date that (i) the closing price of a Holdco Share equals or exceeds $12.00 per share (subject to certain adjustments) for any 20 trading days within any 30-day trading period commencing at least 150 days after the Final Closing Date of the Business Combination, or (ii) Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The share transfer restrictions above will not apply with respect to sales to cover withholding taxes due upon vesting of equity awards and, in the case of directors or officers of Holdco, with respect to the sale of up to 10% of the Holdco Shares held by each of them. It is anticipated that 268,472 Holdco Shares, or less than 1% of the Holdco Shares outstanding following the closing of the Business Combination, will be subject to a Lock-Up Agreement. The terms of a Lock-Up Agreement will also apply Holdco Shares underlying options held by the parties to a Lock-Up Agreement. As described above, it is anticipated that 48,865,642 Holdco Shares, or approximately 58% of the Holdco Shares outstanding following the closing of the Business Combination, will also be subject to a lock-up pursuant to the Investor Rights Agreement.
Warrant Assumption Agreement
At the Closing of the Business Combination, Holdco, FLAC and Continental Stock and Transfer & Trust Company will enter into the Warrant Assumption Agreement, in the form attached hereto as Exhibit 4.1, pursuant to which they will agree that, as part of the Merger, each FLAC Public Warrant and FLAC Private Placement Warrant that is outstanding immediately prior to the Effective Date will cease to represent a right to acquire FLAC Class A Ordinary Shares and will automatically represent, immediately following the Merger, a right to acquire Holdco Shares on the same contractual terms and conditions as were in effect immediately prior to Merger under the Original Warrant Agreement, including that the warrant holders are deemed to have consented to an exclusive forum provision requiring all claims to be brought before the courts of the State of New York or the United States District Court for the Southern District of New York other than suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America are the sole and exclusive forum.
214
Table of Contents
Material U.S. Federal Income Tax Considerations
The following discussion is a summary of material U.S. federal income tax consequences of the Business Combination applicable to beneficial owners of FLAC Class A Ordinary Shares or FLAC Public Warrants (for purposes of this section only, collectively, the “FLAC Securities”) and U.S. Holders (as defined below) of ordinary shares of NewAmsterdam Pharma (for purposes of this section only, “NewAmsterdam Pharma Shares”). If you are a beneficial owner of FLAC Securities, this section addresses material U.S. federal income tax considerations to you (i) of electing to have your FLAC Class A Ordinary Shares redeemed for cash if the Merger is completed, (ii) of exchanging of FLAC Securities for Holdco Shares or Holdco Public Warrants (for purposes of this section only, collectively, the “Holdco Securities”) pursuant to the Merger and (iii) of owning and disposing of Holdco Securities acquired pursuant to the Merger. If you are a U.S. Holder of NewAmsterdam Pharma Shares, this section addresses material U.S. federal income tax consequence to you (i) of exchanging of your NewAmsterdam Pharma Shares for Holdco Securities in connection with the Exchange and (ii) owning and disposing of Holdco Securities received in connection with the Exchange.
This discussion addresses only U.S. Holders that hold FLAC Securities or NewAmsterdam Pharma Shares, as applicable, and, if they participate in the Business Combination, that will hold Holdco Securities as a capital asset (generally property held for investment) after the Merger. It does not discuss all aspects of U.S. federal income taxation that might be relevant to holders in light of their particular circumstances or status, including the alternative minimum tax, the Medicare contribution tax on net investment income, or holders who are subject to special rules, including:
| • | brokers or dealers; |
| • | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| • | tax-exempt organizations, qualified retirement plans, individual retirement accounts or other tax deferred accounts; |
| • | qualified foreign pension funds and entities wholly owned by one or more qualified foreign pension funds; |
| • | banks or other financial institutions, underwriters, or insurance companies; |
| • | real estate investment trusts or regulated investment companies; |
| • | Non-U.S. Holders (as defined below) who are U.S. expatriates or former long-term residents of the United States, or who are present in the United States for a period or periods aggregating 183 days or more in a taxable year; |
| • | except as specifically discussed below under the headings “—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities—Additional Requirements for Tax Deferral” and “U.S. Federal Income Tax Consequences of the Exchange to U.S. Holders of NewAmsterdam Pharma Shares Shares—Additional Requirements for Tax Deferral,” persons that own (directly or indirectly, including by attribution) 5% or more (by vote or value) of the FLAC Securities, NewAmsterdam Pharma Shares or any class of Holdco Securities; |
| • | persons that have at any time within the past five years owned (directly or indirectly, including by attribution) 5% or more (by vote or value) of the FLAC Securities or NewAmsterdam Pharma Shares; |
| • | persons holding FLAC Securities, NewAmsterdam Pharma Shares or Holdco Securities as part of a straddle, hedging or conversion transaction, constructive sale, or other arrangement involving more than one position; |
| • | persons required to accelerate the recognition of any item of gross income with respect to FLAC Securities or NewAmsterdam Pharma Shares as a result of such income being recognized on an applicable financial statement; |
215
Table of Contents
| • | U.S. Holders whose functional currency is not the U.S. dollar; |
| • | persons that received FLAC Securities, NewAmsterdam Pharma Shares or Holdco Securities as compensation for services; |
| • | persons who purchase Holdco Securities as part of the PIPE Financing; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (or beneficial owners of partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes); |
| • | corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | S corporations; or |
| • | controlled foreign corporations or passive foreign investment companies. |
If a partnership (including any entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds FLAC Securities, NewAmsterdam Pharma Shares or Holdco Securities, the tax treatment of a partner in such partnership will depend upon the status of the partner and the activities of the partnership (or entity or arrangement treated as a partnership for U.S. federal income tax purposes). Partners in such a partnership should consult their tax advisors regarding the U.S. federal income tax consequences of the Business Combination with respect to any FLAC Securities or NewAmsterdam Pharma Shares held by such partnership and of such partnership’s holding Holdco Securities after the Business Combination.
This discussion is based on the Code, its legislative history, existing and proposed Treasury regulations promulgated under the Code (the “Treasury Regulations”), published guidance by the IRS and court decisions, all as of the date hereof, and does not take into account proposed changes in such tax laws. These laws are subject to change, possibly on a retroactive basis. This discussion is necessarily general and does not address any tax considerations other than U.S. federal income taxation, including the effect of U.S. federal estate and gift tax, or any state, local or non-U.S. tax laws to a holder of FLAC Securities, NewAmsterdam Pharma Shares or Holdco Securities. FLAC has not sought and does not intend to seek any rulings from the IRS regarding the U.S. federal tax consequences of the Business Combination. In addition, this discussion also assumes that the transactions comprising the Business Combination will be respected, for U.S. federal income tax purposes, as occurring in the order in which they are effected. There is no assurance that the IRS will not take positions concerning the tax consequences of the Business Combination that are different from those discussed below, or that any such different positions would not be sustained by a court.
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of FLAC Securities or NewAmsterdam Pharma Shares, as applicable, before the Business Combination, or of Holdco Securities received in exchange therefor in connection with the Business Combination and that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a domestic corporation; |
| • | an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
| • | a trust if (A) its administration which is subject to the primary supervision of a U.S. court and one or more U.S. persons (within the meaning of the Code) have the authority to control all substantial decisions of the trust or (B) a valid election under applicable Treasury Regulations is in effect to treat the trust as a U.S. person. |
A “Non-U.S. Holder” is a beneficial owner of FLAC Securities or NewAmsterdam Pharma Shares before the Business Combination, or of Holdco Securities received in exchange therefor in connection with the Business Combination and that is, for U.S. federal income tax purposes:
| • | a non-resident alien individual (other than certain former citizens and residents of the United States); |
216
Table of Contents
| • | a foreign corporation or any other foreign organization taxable as a foreign corporation for U.S. federal income tax purposes; or |
| • | a foreign estate or trust, the income of which is not subject to U.S. federal income tax on a net income basis. |
ALL HOLDERS OF FLAC SECURITIES OR NEWAMSTERDAM PHARMA SHARES SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES OF THE BUSINESS COMBINATION AND CONSIDERATIONS RELATING TO THE ACQUISITION, OWNERSHIP AND DISPOSITION OF HOLDCO SECURITIES, INCLUDING THE EFFECTS OF U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX LAWS.
U.S. Federal Income Tax Consequences of the FLAC Shareholder Redemption to U.S. Holders of FLAC Class A Ordinary Shares
Application of Section 302 of the Code to the FLAC Shareholder Redemption
The parties intend to treat the FLAC Shareholder Redemption as a transaction occurring separately from the Merger, and the remainder of this discussion assumes so.
In the event that a U.S. Holder of FLAC Class A Ordinary Shares exercises its right to have its FLAC Class A Ordinary Shares redeemed pursuant to the FLAC Shareholder Redemption, the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale of such stock pursuant to Section 302 of the Code or whether the U.S. Holder will be treated as receiving a corporate distribution. Whether that redemption qualifies for sale treatment will depend largely on the total number of shares of FLAC Class A Ordinary Shares treated as held by the U.S. Holder (including any stock constructively owned by the U.S. Holder as a result of, among other things, owning FLAC Public Warrants or Holdco Securities) relative to all of the shares of FLAC Class A Ordinary Shares both before and after the FLAC Shareholder Redemption. The redemption of stock generally will be treated as a sale of the stock (rather than as a corporate distribution) if the redemption is “substantially disproportionate” with respect to the U.S. Holder, results in a “complete termination” of the U.S. Holder’s interest in FLAC or is “not essentially equivalent to a dividend” with respect to the U.S. Holder. These tests are explained more fully below.
In determining whether any of the foregoing tests are satisfied, a U.S. Holder takes into account not only stock actually owned by the U.S. Holder, but also shares of FLAC Class A Ordinary Shares that are constructively owned by such U.S. Holder. A U.S. Holder may constructively own, in addition to stock owned directly, stock owned by certain related individuals and entities in which the U.S. Holder has an interest or that have an interest in such U.S. Holder, as well as any stock the U.S. Holder has a right to acquire by exercise of an option, which would generally include FLAC Class A Ordinary Shares that could be acquired pursuant to the exercise of FLAC Public Warrants. In order to meet the substantially disproportionate test, the percentage of FLAC’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately following the FLAC Shareholder Redemption of FLAC Class A Ordinary Shares must, among other requirements, be less than 80% of the percentage of FLAC’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately before the FLAC Shareholder Redemption. Prior to the FLAC Shareholder Redemption, FLAC Class A Ordinary Shares might not be treated as voting stock for this purpose, and, consequently, the substantially disproportionate test may not be applicable. There will be a complete termination of a U.S. Holder’s interest if either all the shares of FLAC Class A Ordinary Shares actually and constructively owned by the U.S. Holder are redeemed or all the shares of FLAC Class A Ordinary Shares actually owned by the U.S. Holder are redeemed and the U.S. Holder is eligible to waive, and effectively waives in accordance with specific rules, the attribution of stock in FLAC owned by certain family members and the U.S. Holder does not constructively own any other stock in FLAC. The FLAC Shareholder Redemption of the FLAC Class A Ordinary Shares will not be essentially equivalent to a dividend if a U.S. Holder’s redemption results in a “meaningful reduction” of the U.S. Holder’s proportionate interest in FLAC. Whether the FLAC Shareholder Redemption will result in a meaningful reduction in a U.S. Holder’s proportionate interest in FLAC will depend on the particular facts and circumstances. However, the IRS has indicated in a published ruling
217
Table of Contents
that even a small reduction in the proportionate interest of a small minority shareholder in a publicly-held corporation who exercises no control over corporate affairs may constitute such a “meaningful reduction.”
U.S. Federal Income Tax Consequences to U.S. Holders of the FLAC Shareholder Redemption Treated as a Sale of FLAC Class A Ordinary Shares
Subject to the discussion below under the heading “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities,” if the FLAC Shareholder Redemption qualifies as a sale of stock by the U.S. Holder under Section 302 of the Code, the U.S. Holder generally will be required to recognize gain or loss in an amount equal to the difference, if any, between the amount of cash received and the tax basis of the shares of FLAC Class A Ordinary Shares redeemed. Such gain or loss should be treated as capital gain or loss if such shares were held as a capital asset on the date of the FLAC Shareholder Redemption. Any such capital gain or loss will generally be long-term capital gain or loss if the U.S. Holder’s holding period for such FLAC Class A Ordinary Shares exceeds one year. Long-term capital gains recognized by non-corporate U.S. Holders may be taxed at reduced rates. It is unclear, however, whether the redemption rights of a U.S. Holder with respect to the FLAC Class A Ordinary Shares may suspend the running of the applicable holding period for this purpose. If the running of the holding period is suspended, then non-corporate U.S. Holders may not be able to satisfy the one-year holding period requirement for long-term capital gain treatment, in which case any gain on a sale or taxable disposition of the FLAC Class A Ordinary Shares would be subject to short-term capital gain treatment and would be taxed at regular ordinary income tax rates. The deductibility of capital losses is subject to limitations. A U.S. Holder’s tax basis in such holder’s shares of FLAC Class A Ordinary Shares generally will equal the cost of such shares. A U.S. Holder that purchased FLAC units would have been required to allocate the cost between the shares of FLAC Class A Ordinary Shares and the FLAC Public Warrants comprising the units based on their relative fair market values at the time of the purchase.
U.S. Federal Income Tax Consequences to U.S. Holders of the FLAC Shareholder Redemption Treated as a Distribution on FLAC Class A Ordinary Shares
If the FLAC Shareholder Redemption does not qualify as a sale of stock under Section 302 of the Code, then the U.S. Holder will be treated as receiving a corporate distribution. Subject to the discussion below under the heading “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities,” the gross amount of any such distribution generally will be taxable to a U.S. Holder as ordinary dividend income on the date such distribution is actually or constructively received, but only to the extent that the distribution is paid out of FLAC’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Any such dividends generally will not be eligible for the dividends received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, dividends will be taxed at the preferential long-term capital gains rate (see “—U.S. Federal Income Tax Consequences to U.S. Holders of the FLAC Shareholder Redemption Treated as a Sale of FLAC Class A Ordinary Shares” above), provided the applicable holding period is met, if FLAC Class A Ordinary Shares are readily tradable on an established securities market in the United States (which they will be if the FLAC Class A Ordinary Shares are traded on the Nasdaq) and certain other requirements are met, including that FLAC is not classified as a passive foreign investment company during the taxable year in which the dividend is paid or the preceding taxable year. There can be no assurance that FLAC Class A Ordinary Shares will be considered readily tradable on an established securities market in future years. U.S. Holders should consult their tax advisors regarding the potential availability of the lower rate for any dividends paid with respect to FLAC Class A Ordinary Shares. Dividends that FLAC Class A Ordinary Shares distributes generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income” for foreign tax credit limitation purposes.
A U.S. Holder should consult its tax advisors as to the tax consequences to it of the FLAC Shareholder Redemption.
218
Table of Contents
U.S. Federal Income Tax Consequences of the FLAC Shareholder Redemption to Non-U.S. Holders of FLAC Class A Ordinary Shares
The characterization for U.S. federal income tax purposes of the FLAC Shareholder Redemption for a Non-U.S. Holder of FLAC Class A Ordinary Shares will generally correspond to the U.S. federal income tax characterization of the redemption of a U.S. Holder’s FLAC Class A Ordinary Shares described above under “—Application of Section 302 of the Code to the FLAC Shareholder Redemption,” and the consequences of the FLAC Shareholder Redemption for such Non-U.S. Holder will be as described below under “—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to Non-U.S. Holders” based on such characterization.
U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities
Uncertainty Regarding the U.S. Federal Income Tax Characterization of the Merger
Subject to the discussion below under the headings “—Tax Consequences of the Merger Under Section 368 of the Code,” “—U.S. Federal Income Tax Treatment of the Earnout Shares” and “—Additional Requirements for Tax Deferral” the parties to the Merger have agreed to report the Merger, taken together with certain related transactions, as a transaction described under Section 351 of the Code (the “Intended Tax Treatment”).
If the Merger qualifies for the Intended Tax Treatment, and subject to the discussion below under the headings “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities” and “—Additional Requirements for Tax Deferral,” a U.S. Holder that exchanges its FLAC Class A Ordinary Shares in the Merger for Holdco Shares generally will not recognize any gain or loss on such exchange (except as described below under the heading “—Additional Requirements for Tax Deferral”). In such case, the aggregate adjusted tax basis of the Holdco Shares received by a U.S. Holder in the Merger should be equal to the adjusted tax basis of the FLAC Class A Ordinary Shares surrendered in the Merger in exchange therefor. The holding period of the Holdco Shares received should include the holding period of the FLAC Class A Ordinary Shares surrendered in the Merger in exchange therefor.
If the Merger does not qualify under Section 351 of the Code (or any other tax-deferral provision of the Code, including Section 368 as discussed below under “—Tax Consequences of the Merger Under Section 368 of the Code”), and subject to the discussion below regarding the conversion of FLAC Public Warrants, then a FLAC public shareholder that is a U.S. Holder generally will recognize gain or loss, if any, in an amount equal to the difference, if any, between (i) the fair market value of the Holdco Securities received and (ii) such U.S. Holder’s adjusted tax basis in the FLAC Securities exchanged therefor. Any such gain or loss so recognized will be capital gain or loss, and will be long-term capital gain or loss only if such U.S. Holder’s holding period for the FLAC Class A Ordinary Shares exceeds one year at the time of the Merger. Long-term capital gains of non-corporate U.S. Holders (including individuals) are currently eligible for preferential U.S. federal income tax rates. The deductibility of capital losses is subject to limitations. A U.S. Holder’s holding period in the Holdco Shares (and the Holdco Public Warrants, if any, as applicable) received in the Merger would not include the holding period for the FLAC Class A Ordinary Shares (and the FLAC Public Warrants, if any) exchanged therefor.
The appropriate U.S. federal income tax treatment of the disposition of FLAC Public Warrants in exchange for Holdco Public Warrants in connection with the Merger is uncertain. It is possible that a U.S. Holder of FLAC Public Warrants could be treated as exchanging such FLAC Public Warrants for Holdco Public Warrants. If so treated, a U.S. Holder could be required to recognize gain or loss in such deemed exchange in an amount equal to the difference between the fair market value of the Holdco Public Warrants held by such U.S. Holder immediately following the Merger and the adjusted tax basis of the FLAC Public Warrants held by such U.S. Holder immediately prior to the Merger. Alternatively, it is also possible that a U.S. Holder of FLAC Public Warrants could be treated as transferring its FLAC Public Warrants and shares of FLAC Class A Ordinary Shares to Holdco for Holdco Public Warrants and Holdco Shares in an exchange governed by Section 351 of the Code. If so treated, a U.S. Holder should be required to recognize gain (but not loss) in an amount equal to the lesser of (i) the amount of gain realized by such holder (generally, the excess of (x) the sum of the fair market values of the Holdco Public Warrants treated as received by such holder and the Holdco Shares received by such holder
219
Table of Contents
over (y) such holder’s aggregate adjusted tax basis in the FLAC Public Warrants and FLAC Class A Ordinary Shares treated as having been exchanged therefor) and (ii) the fair market value of the Holdco Public Warrants treated as having been received by such holder in such exchange. However, if the deemed transfer of the FLAC Public Warrants also qualifies as part of a “reorganization” within the meaning of Section 368 of the Code, a U.S. Holder of FLAC Public Warrants generally should not recognize any gain or loss on such deemed transfer of FLAC Public Warrants, and such U.S. Holder’s basis in the Holdco Public Warrants deemed received should be equal to the U.S. Holder’s basis in its FLAC Public Warrants deemed transferred. The requirements for qualification of the Merger as a reorganization are more stringent in certain respects than the requirements for qualification as an exchange under Section 351 of the Code, and may not be satisfied (see discussion immediately below under “—Tax Consequences of the Merger Under Section 368 of the Code”).
The statements set forth in this section “U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities,” insofar as they address the material U.S. federal income tax considerations for U.S. Holders of FLAC Securities in connection with the treatment of the Merger as a transaction which qualifies under Section 351 of the Code, represent the opinion of Goodwin Procter LLP. In addition, as discussed below under “Tax Consequences of the Merger Under Section 368 of the Code,” Goodwin Procter LLP is unable to opine as to whether the Merger qualifies as a “reorganization” under Section 368 of the Code. Such opinion is filed as Exhibit 8.1 to the registration statement of which this proxy statement/prospectus forms a part and is based on customary assumptions, representations and covenants. If any of the assumptions, representations or covenants on which the opinion is based is or becomes incorrect, incomplete, inaccurate or is otherwise not complied with, the validity of the opinion described above may be adversely affected and the tax consequences of the Merger could differ from those described herein. An opinion of counsel is not binding on the IRS or any court, and there can be no certainty that the IRS will not challenge the conclusions reflected in the opinion or that a court would not sustain such a challenge.
Uncertainty Regarding the Treatment of the Merger as a “Reorganization” Under Section 368 of the Code
Subject to the discussion below under the heading “—Additional Requirements for Tax Deferral,” the parties have agreed to report the Merger as qualifying as part of a “reorganization” within the meaning of Section 368 of the Code. If the Merger is treated in this manner for U.S. federal income tax purposes, a U.S. Holder of FLAC Public Warrants generally will not recognize any gain or loss on such deemed transfer of FLAC Public Warrants, and such U.S. Holder’s basis in the Holdco Public Warrants deemed received should be equal to the U.S. Holder’s basis in its FLAC Public Warrants deemed transferred. There are significant factual and legal uncertainties as to whether the Merger will qualify as a reorganization. For example, under Section 368 of the Code, the acquiring corporation must continue, either directly or indirectly through certain controlled corporations, either a significant line of the acquired corporation’s historic business or use a significant portion of the acquired corporation’s historic business assets in a business. However, there is an absence of guidance directly on point as to how provisions of Section 368 of the Code apply in the case of an acquisition of a corporation with only investment-type assets, such as FLAC. In addition, reorganization treatment could be adversely affected by events or actions that occur prior to or at the time of the Merger, some of which are outside of FLAC’s control (including, for example, the magnitude of the FLAC Shareholder Redemption). Therefore, although the parties have agreed (subject to the limitations described in the following paragraph) to report the Merger as a reorganization, counsel is unable to opine on the treatment of the Merger as a reorganization. Thus, the closing of the Merger is not conditioned upon the receipt of an opinion of counsel, and counsel is unable to opine on the treatment of the Merger as a reorganization under Section 368 of the Code, and neither FLAC nor NewAmsterdam Pharma intends to request a ruling from the IRS regarding the U.S. federal income tax treatment of the Merger. Accordingly, no assurance can be given that the IRS will not challenge the Merger’s qualification as a reorganization or that a court will not sustain such a challenge by the IRS.
The parties to the Merger currently intend to report the exchange of FLAC Public Warrants for Holdco Public Warrants as qualifying as part of a “reorganization” within the meaning of Section 368 of the Code, unless either (i) FLAC determines, in consultation with Goodwin Procter LLP (or other nationally recognized tax counsel), that the Merger does not qualify as a reorganization or (ii) the parties are otherwise required by a “determination” that is final within the meaning of Section 1313 of the Code.
220
Table of Contents
If, as of the Closing, any requirement to qualify as a reorganization under Section 368(a) of the Code is not met, then a U.S. Holder who owns FLAC Public Warrants would recognize gain (but not loss) in an amount equal to the lesser of (i) the amount of gain realized by such holder (generally, the excess of (x) the sum of the fair market values of the Holdco Public Warrants treated as received by such holder and the Holdco Shares received by such holder over (y) such holder’s aggregate adjusted tax basis in the FLAC Public Warrants and FLAC Public Shares treated as having been exchanged therefor) and (ii) the fair market value of the Holdco Public Warrants treated as having been received by such holder in such exchange.
U.S. Federal Income Tax Treatment of the Earnout Shares
Subject to the discussions above under the headings “—Characterization of the Merger” and “—Tax Consequences of the Merger Under Section 368 of the Code,” the parties have agreed to report the Merger as a transaction governed by Section 351 of the Code and a “reorganization” within the meaning of Section 368 of the Code. If the issuance of the Earnout Shares is not treated as stock such shares may be treated as taxable boot and could cause the Merger to fail to qualify as a reorganization. However, FLAC does not currently expect the Earnout Shares to fail to qualify as stock for these purposes since the parties intend for U.S. federal income tax purposes to treat the issuance of Earnout Shares as complying with certain operating rules as set forth in Rev. Proc. 77-37, amended by Rev. Proc. 84-42, 1984-1 C.B. 521.
Additional Requirements for Tax Deferral
Subject to Section 367(a) of the Code and the Treasury Regulations promulgated thereunder impose additional requirements for a U.S. Holder to qualify for tax-deferred treatment under Section 351 of the Code with respect to the exchange of FLAC Securities for Holdco Securities. A U.S. Holder who will own five percent or more of either the total voting power or the total value of the stock of Holdco (directly, indirectly or constructively) immediately after the Business Combination (a “5 Percent Holder”) will recognize gain (but not loss) on the exchange of its FLAC Securities for Holdco Securities pursuant to the Business Combination unless such a 5 Percent Holder enters into a “gain recognition agreement” with the IRS in accordance with applicable Treasury Regulations. Any 5 Percent Holders are urged to consult their tax advisors regarding the time and manner of entering into such a gain recognition agreement.
The treatment of U.S. Holders of FLAC Securities could be materially different from that described above if FLAC is treated as a PFIC at any time during the holding period of a U.S. Holder of FLAC Securities. FLAC was treated as a PFIC for its taxable year ending in 2021 and is also likely treated as a PFIC in the current taxable year. However, the application of the PFIC rules is subject to uncertainty in several respects and, therefore, FLAC cannot provide any assurance regarding its PFIC status for any prior or current taxable years. Pursuant to the Proposed PFIC Regulations, if FLAC was a PFIC at any time during the holding period of a U.S. Holder, such holder will recognize gain (but not loss) pursuant to the Business Combination unless:
| • | such U.S. Holder made a QEF election with respect to its shares of FLAC for the first taxable year in which such U.S. Holder held (or was deemed to hold) such shares in which FLAC was a classified as a PFIC and has continued to properly satisfy the requirements of such QEF election in all subsequent years; |
| • | such U.S. Holder made a QEF election along with an applicable purging election and has continued to properly satisfy the requirements of such QEF election in all subsequent years; or |
| • | Holdco is a PFIC during the taxable year when the Business Combination is completed. |
As discussed more fully below under “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities,” Holdco is expected to be treated as a PFIC for the current taxable year, which includes the Business Combination. If this is the case, a U.S. Holder of FLAC Securities will not be required to recognize gain on the exchange of FLAC Securities for Holdco Securities pursuant to the Business Combination, provided that the Merger qualifies as a transaction governed by Section 351 of the Code or is treated as a “reorganization” within the meaning of Section 368 of the Code (subject to complying with the requirements of
221
Table of Contents
Section 367(a), if applicable and, with respect to holders of FLAC Public Warrants, subject to the considerations discussed under “—Characterization of the Merger” above).
However, if the Merger does not qualify as an exchange described in Section 351(a) of the Code or a “reorganization” within the meaning of Section 368 of the Code, any income or gain recognized by such a U.S. Holder as a result of the Merger generally will be subject to a special tax and interest charge, under the rules described more fully below under “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities” (substituting “FLAC Class A Ordinary Shares” for “Holdco Shares”). With respect to the mark-to-market election, it is expected that FLAC Class A Ordinary Shares, which are listed on Nasdaq, will qualify as marketable shares for the PFIC rules purposes, but there can be no assurance that FLAC Class A Ordinary Shares will be “regularly traded” for purposes of these rules.
If the Proposed PFIC Regulations were finalized in their current form, they would be effective for transactions occurring on or after April 1, 1992. Because the Proposed PFIC Regulations have not yet been adopted in final form, they are not currently effective, and there is no assurance that they will be adopted in the form and with the effective date proposed. The IRS has announced that, in the absence of final Treasury Regulations, taxpayers may apply reasonable interpretations of the Code provisions applicable to PFICs and that it considers the rules set forth in the Proposed PFIC Regulations to be reasonable interpretations of those Code provisions.
U.S. Federal Income Tax Consequences of the Merger to Non-U.S. Holders of FLAC Securities
The U.S. federal income tax consequences of the Merger to Non-U.S. Holders generally will correspond to the U.S. federal income tax consequences described under “—U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of FLAC Securities,” above, except that the PFIC rules will not apply to any Non-U.S. Holder and, to the extent the Merger results in a taxable exchange of FLAC Securities, the consequences for a Non-U.S. Holder of recognizing gain in such a taxable exchange would be the same as the consequences of recognizing gain on a sale or other disposition of Holdco Securities described below under the heading “—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to Non-U.S. Holders.”
U.S. Federal Income Tax Consequences of the Exchange to U.S. Holders of NewAmsterdam Pharma Shares
Characterization of the Exchange
Subject to the discussion in the following paragraph and the discussions under the heading “—Additional Requirements for Tax Deferral” below, the parties have agreed to report the Exchange as a transaction described in Section 351(a) of the Code. If the Exchange is treated in this manner, it will result in a tax-free exchange by Participating Shareholders of their NewAmsterdam Pharma Shares for Holdco Shares pursuant to Section 351(a) of the Code.
Holdco does not intend to seek, and has not sought, any rulings from the IRS regarding the U.S. federal income tax consequences of the Exchange. Accordingly, there can be no assurance that the exchange of NewAmsterdam Pharma Shares for Holdco Shares will qualify as an exchange described in Section 351(a) of the Code or that the IRS will not assert, or that a court will not sustain, a position contrary to the conclusions set forth below.
Subject to the limitations and qualifications set forth herein, the remainder of this discussion assumes that the Exchange qualifies as an exchange described in Section 351(a) of the Code.
U.S. Federal Income Tax Consequences for U.S. Holders of NewAmsterdam Pharma Shares
Subject to the discussion below under the heading “—Additional Requirements for Tax Deferral,” a U.S. Holder of NewAmsterdam Pharma Shares will not recognize gain or loss upon the exchange of NewAmsterdam Pharma Shares for Holdco Shares pursuant to the Exchange. A U.S. Holder’s aggregate tax basis in the Holdco Shares received in exchange for NewAmsterdam Pharma Shares will equal the U.S. Holder’s aggregate tax basis in the NewAmsterdam Pharma Shares surrendered in the Exchange. The holding period of such Holdco Shares received in the Exchange will include the holding period of NewAmsterdam Pharma Shares surrendered in exchange therefor. For purposes of determining the tax bases and holding periods for Holdco Shares received in
222
Table of Contents
the Exchange, U.S. Holders who acquired different blocks of NewAmsterdam Pharma Shares at different times or for different prices must allocate their bases and holding periods in their NewAmsterdam Pharma Shares ratably over the Holdco Shares received in the Business Combination.
Additional Requirements for Tax Deferral
Section 367(a) of the Code and the Treasury Regulations promulgated thereunder impose additional requirements for a U.S. Holder to qualify for tax-deferred treatment under Section 351 of the Code with respect to the exchange NewAmsterdam Pharma Shares for Holdco Shares. A U.S. Holder that is a 5 Percent Holder will recognize gain (but not loss) on the exchange of its NewAmsterdam Pharma Shares for Holdco Shares pursuant to the Business Combination unless such a 5 Percent Holder enters into a “gain recognition agreement” with the IRS in accordance with applicable Treasury Regulations. Any 5 Percent Holders are urged to consult their tax advisors regarding the time and manner of entering into such a gain recognition agreement.
If NewAmsterdam Pharma was a PFIC at any time during the holding period of a U.S. Holder of NewAmsterdam Pharma Shares, pursuant to the Proposed PFIC Regulations, such holder will recognize gain (but not loss) pursuant to the Business Combination unless:
| • | such U.S. Holder made a QEF election with respect to its NewAmsterdam Pharma shares for the first taxable year in which such U.S. Holder held (or was deemed to hold) such shares in which NewAmsterdam Pharma was a classified as a PFIC and has continued to properly satisfy the requirements of such QEF election in all subsequent years; |
| • | such U.S. Holder made a QEF election along with an applicable purging election and has continued to properly satisfy the requirements of such QEF election in all subsequent years; or |
| • | Holdco is a PFIC during the taxable year when the Business Combination is completed. |
NewAmsterdam Pharma was treated as a PFIC in prior taxable years, and NewAmsterdam Pharma as well as Holdco are expected to be treated as PFICs for the current taxable year, which includes the Business Combination. If this is the case, and the Exchange were to qualify as an exchange described in Section 351(a) of the Code (subject to complying with the requirements of Section 367(a), if applicable), a U.S. Holder of NewAmsterdam Pharma Shares will not be required to recognize gain on the exchange of NewAmsterdam Pharma Shares for Holdco Shares pursuant to the Business Combination.
However, if the Exchange were not to qualify as an exchange described in Section 351(a) of the Code, any income or gain recognized by such a U.S. Holder as a result of the Exchange would generally be subject to a special tax and interest charge, under the rules described more fully below under “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities” (substituting “NewAmsterdam Pharma Shares” for “Holdco Shares”). The mark-to-market election is not expected to be applicable to NewAmsterdam Pharma Shares.
If the Proposed PFIC Regulations were finalized in their current form, they would be effective for transactions occurring on or after April 1, 1992. Because the Proposed PFIC Regulations have not yet been adopted in final form, they are not currently effective, and there is no assurance that they will be adopted in the form and with the effective date proposed. The IRS has announced that, in the absence of final Treasury Regulations, taxpayers may apply reasonable interpretations of the Code provisions applicable to PFICs and that it considers the rules set forth in the Proposed PFIC Regulations to be reasonable interpretations of those Code provisions.
U.S. Federal Income Tax Consequences if the Exchange Fails to Qualify as an Exchange Described in Section 351(a) of the Code
As discussed above under “—Additional Requirements for Tax Deferral,” if the Exchange were not to qualify as an exchange described in Section 351(a) of the Code, any income or gain recognized by such a U.S. Holder as a result of the Exchange would generally be subject to a special tax and interest charge, under the rules described more fully below under “—Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities” (substituting “NewAmsterdam Pharma Shares” for “Holdco Shares”).
223
Table of Contents
These rules would not apply to a U.S. Holder that has made a QEF election with respect to its NewAmsterdam Pharma Shares (either (a) for the first taxable year in which such U.S. holder held, or was deemed to hold, such shares in which NewAmsterdam Pharma was classified as a PFIC or (b) along with a purging election) and continued to properly satisfy the requirements of such QEF election or who has not held NewAmsterdam Pharma Shares for any taxable year when NewAmsterdam Pharma was classified as a PFIC. If the Exchange failed to qualify as an exchange described in Section 351(a) of the Code and NewAmsterdam Pharma were not treated as a PFIC for the taxable year including the Exchange, such a U.S. Holder of NewAmsterdam Pharma Shares would recognize gain or loss equal to the difference, if any, between (i) the fair market value of the Holdco Shares received in exchange for NewAmsterdam Pharma Shares surrendered in the Exchange and (ii) such U.S. Holder’s adjusted tax basis in such surrendered NewAmsterdam Pharma Shares. Any such gain or loss would be long-term capital gain or loss if such U.S. Holder’s holding period in the NewAmsterdam Pharma Shares surrendered in the Exchange exceeds one year as of the closing date of the Exchange. Long-term capital gains of non-corporate taxpayers are taxed at reduced U.S. federal income tax rates. The deductibility of capital losses is subject to limitations.
U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders
Application of Passive Foreign Investment Company Rules to U.S. Holders of Holdco Securities
Holdco is expected to be treated as a PFIC for the current taxable year, which includes the Business Combination, and may be treated as a PFIC for future taxable years. A non-U.S. corporation will be classified as a PFIC for U.S. federal income tax purposes if either (i) at least 75% of its gross income in a taxable year, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income or (ii) at least 50% of its assets in a taxable year (ordinarily determined based on fair market value and averaged quarterly over the year), including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived in the active conduct of a trade or business) and gains from the disposition of passive assets. A separate determination must be made after the close of each taxable year as to whether a foreign corporation was a PFIC for that year. Once a foreign corporation is treated as a PFIC it is, with respect to a shareholder during the time it qualifies as a PFIC, and subject to certain exceptions, always treated as a PFIC with respect to such shareholder, regardless of whether it satisfied either of the qualification tests in subsequent years.
There are three separate taxation regimes that could apply to a U.S. Holder of Holdco Shares under the PFIC rules, which are (i) the excess distribution regime (which is the default regime), (ii) the QEF regime, and (iii) the mark-to-market regime (each discussed below). A U.S. Holder who holds (actually or constructively) stock in a foreign corporation during any year in which such corporation qualifies as a PFIC is subject to U.S. federal income taxation under one of these three regimes. The effect of the PFIC rules on a U.S. Holder will depend upon which of these regimes applies to such U.S. Holder. Moreover, dividends paid by a PFIC are generally not eligible for the lower rates of taxation applicable to qualified dividend income (“QDI”) under any of the foregoing regimes.
Excess Distribution Regime
A U.S. Holder that does not make a QEF election or a mark-to-market election, as described below, will be subject to the default “excess distribution regime” under the PFIC rules with respect to (i) any gain realized on a sale or other disposition (including a pledge) of Holdco Shares, and (ii) any “excess distribution” received on the U.S. Holder’s Holdco Shares (generally, any distributions in excess of 125% of the average of the annual distributions on Holdco Shares during the preceding three years or the holding period, whichever is shorter). Generally, under this excess distribution regime: the gain or excess distribution will be allocated ratably over the period during which the U.S. Holder held the Holdco Shares; the amount allocated to the current taxable year, will be treated as ordinary income; and the amount allocated to prior taxable years will be subject to the highest tax rate in effect for that taxable year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year.
224
Table of Contents
The tax liability for amounts allocated to years prior to the year of disposition or excess distribution will be payable generally without regard to offsets from deductions, losses and expenses. In addition, gains (but not losses) a U.S. Holder realizes on the sale of Holdco Shares cannot be treated as capital gains, even if the U.S. Holder holds the shares as capital assets. Further, no portion of any distribution will be treated as QDI.
QEF Regime
A QEF election is effective for the taxable year for which the election is made and all subsequent taxable years and may not be revoked without the consent of the IRS. If a U.S. Holder makes a timely QEF election with respect to its direct or indirect interest in a PFIC, the U.S. Holder will be required to include in income each year a portion of the ordinary earnings and net capital gains of the PFIC as QEF income inclusions, even if amount is not distributed to the U.S. Holder. Thus, the U.S. Holder may be required to report taxable income as a result of QEF income inclusions without corresponding receipts of cash. Holders of Holdco Shares that are U.S. Holders subject to U.S. federal income tax should not expect that they will receive cash distributions from Holdco sufficient to cover their respective U.S. tax liability with respect to such QEF income inclusions. In addition, as discussed below, U.S. Holders of Holdco Public Warrants will not be able to make a QEF election with respect to their Holdco Public Warrants.
The timely QEF election also allows the electing U.S. Holder to: (i) generally treat any gain recognized on the disposition of its shares of the PFIC as capital gain; (ii) treat its share of the PFIC’s net capital gain, if any, as long-term capital gain instead of ordinary income; and (iii) either avoid interest charges resulting from PFIC status altogether, or make an annual election, subject to certain limitations, to defer payment of current taxes on its share of PFIC’s annual realized net capital gain and ordinary earnings subject, however, to an interest charge on the deferred tax computed by using the statutory rate of interest applicable to an extension of time for payment of tax. In addition, net losses (if any) of a PFIC will not pass through to an electing U.S. Holder and may not be carried back or forward in computing such PFIC’s ordinary earnings and net capital gain in other taxable years. Consequently, a U.S. Holder may over time be taxed on amounts that as an economic matter exceed our net profits.
A U.S. Holder’s tax basis in Holdco Shares will be increased to reflect QEF income inclusions and will be decreased to reflect distributions of amounts previously included in income as QEF income inclusions. No portion of the QEF income inclusions attributable to ordinary income will be treated as QDI. Amounts included as QEF income inclusions with respect to direct and indirect investments generally will not be taxed again when distributed. U.S. Holders should consult their tax advisors as to the manner in which QEF income inclusions affect their allocable share of Holdco’s income and their basis in their Holdco Shares.
Holdco intends to determine its PFIC status at the end of each taxable year and intends to satisfy any applicable record keeping and reporting requirements that apply to a QEF, including providing to U.S. Holders, for each taxable year that it determines it is or, in its reasonable determination, may be a PFIC, a PFIC Annual Information Statement containing information necessary for U.S. Holders to make a QEF Election with respect to Holdco. Holdco will provide such information electronically.
U.S. Holders of Holdco Public Warrants will not be able to make a QEF election with respect to their warrants. As a result, if a U.S. Holder sells or otherwise disposes of such Holdco Public Warrants (other than upon exercise of such Holdco Public Warrants for cash) and Holdco was a PFIC at any time during the U.S. Holder’s holding period of such Holdco Public Warrants, any gain recognized generally will be treated as an excess distribution, taxed as described above under “—Excess Distribution Regime.” If a U.S. Holder that exercises such Holdco Public Warrants properly makes and maintains a QEF election with respect to the newly acquired Holdco Shares (or has previously made a QEF election with respect to Holdco Shares), the QEF election will apply to the newly acquired Holdco Shares. Notwithstanding such QEF election, the adverse tax consequences relating to PFIC shares, adjusted to take into account the current income inclusions resulting from the QEF election, will continue to apply with respect to such newly acquired Holdco Shares (which generally will be deemed to have a holding period for purposes of the PFIC rules that includes the period the U.S. Holder held the Holdco Public Warrants), unless the U.S. Holder makes a purging election under the PFIC rules. Under one type of purging election, the U.S. Holder will be deemed to have sold such shares at their fair market value and any gain recognized on such deemed sale will be treated as an excess distribution, as described above.
225
Table of Contents
Another type of purging election is available if Holdco is also a “controlled foreign corporation” under the Code. Under this election, Holdco will be deemed to have made a distribution to the U.S. Holder of such U.S. Holder’s pro rata share of Holdco’s earnings and profits as determined for U.S. federal income tax purposes.
Mark-to-Market Regime
Alternatively, a U.S. Holder may make an election to mark marketable shares in a PFIC to market on an annual basis. PFIC shares generally are marketable if: (i) they are “regularly traded” on a national securities exchange that is registered with the Securities Exchange Commission or on the national market system established under Section 11A of the Exchange Act; or (ii) they are “regularly traded” on any exchange or market that the Treasury Department determines to have rules sufficient to ensure that the market price accurately represents the fair market value of the stock. It is expected that Holdco Shares, which are expected to be listed on Nasdaq, will qualify as marketable shares for the PFIC rules purposes, but there can be no assurance that Holdco Shares will be “regularly traded” for purposes of these rules. Pursuant to such an election, an electing U.S. Holder would include in each year as ordinary income the excess, if any, of the fair market value of such stock over its adjusted basis at the end of the taxable year. A U.S. Holder may treat as ordinary loss any excess of the adjusted basis of the stock over its fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the election in prior years. A U.S. Holder’s adjusted tax basis in the PFIC shares will be increased to reflect any amounts included in income, and decreased to reflect any amounts deducted, as a result of a mark-to-market election. Any gain recognized on a disposition of Holdco Shares will be treated as ordinary income and any loss will be treated as ordinary loss (but only to the extent of the net amount of income previously included as a result of a mark-to-market election). A mark-to-market election only applies for the taxable year in which the election was made, and for each subsequent taxable year, unless the PFIC shares ceased to be marketable or the IRS consents to the revocation of the election. U.S. Holders should also be aware that the Code and the Treasury Regulations do not allow a mark-to-market election with respect to stock of lower-tier PFICs that is nonmarketable. There is also no provision in the Code, Treasury Regulations or other published authority that specifically provides that a mark-to-market election with respect to the stock of a publicly-traded holding company (such as Holdco) effectively exempts stock of any lower-tier PFICs from the negative tax consequences arising from the general PFIC rules. We advise U.S. Holders to consult their tax advisor to determine whether the mark-to-market tax election is available to them and the consequences resulting from such election. U.S. Holders of Holdco Public Warrants will not be able to make a mark-to-market election with respect to their warrants.
PFIC Reporting Requirements
A U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year of the U.S. Holder generally is required to file an IRS Form 8621 with such U.S. Holder’s U.S. federal income tax return and provide such other information as the IRS may require. Failure to file IRS Form 8621 for each applicable taxable year may result in substantial penalties and result in the U.S. Holder’s taxable years being open to audit by the IRS until such forms are properly filed.
U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders if Holdco is not a PFIC
Distributions on Holdco Shares
The treatment of U.S. Holders of Holdco Securities could be materially different from that described above if Holdco is not treated as a PFIC for the taxable year including the Business Combination. If Holdco is not treated as a PFIC for the taxable year including the Business Combination, the gross amount of any distribution on Holdco Shares generally will be taxable to a U.S. Holder as ordinary dividend income on the date such distribution is actually or constructively received, but only to the extent that the distribution is paid out of Holdco’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because Holdco does not maintain, nor is it required to maintain, calculations of its earnings and profits under
226
Table of Contents
U.S. federal income tax principles, it is currently expected that any distributions generally will be reported to U.S. Holders as dividends. Any such dividends generally will not be eligible for the dividends received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, dividends will be taxed at the preferential long-term capital gains rate (see “—Sale, Exchange, Redemption or Other Taxable Disposition of Holdco Securities” below), provided the applicable holding period is met, if Holdco Shares are readily tradable on an established securities market in the United States (which they will be if the Holdco Shares are traded on the Nasdaq) and certain other requirements are met, including that Holdco is not classified as a passive foreign investment company during the taxable year in which the dividend is paid or the preceding taxable year. There can be no assurance that Holdco Shares will be considered readily tradable on an established securities market in future years. U.S. Holders should consult their tax advisors regarding the potential availability of the lower rate for any dividends paid with respect to Holdco Shares.
A U.S. Holder must include any Dutch tax withheld from the dividend payment in the gross amount of the dividend even if the holder does not in fact receive it. The dividend is taxable to the holder when the holder receives the dividend, actually or constructively. The amount of the dividend distribution includible in a U.S. Holder’s income will be the U.S. dollar value of the Euro payments made, determined at the spot Euro/U.S. dollar rate on the date the dividend distribution is includible in income, regardless of whether the payment is in fact converted into U.S. dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend payment is included in income to the date the payment is converted into U.S. dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Dividends that Holdco distributes generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income” for foreign tax credit limitation purposes. The rules relating to the determination of the foreign tax credit limitation are complex, and U.S. Holders should consult their tax advisor to determine whether and to what extent they will be entitled to a credit for Dutch withholding taxes imposed in respect of any dividend Holdco distributes.
Sale, Exchange, Redemption or Other Taxable Disposition of Holdco Securities
If Holdco is not treated as a PFIC for the taxable year including the Business Combination, a U.S. Holder generally will recognize gain or loss on any sale, exchange, redemption (subject to the discussion below) or other taxable disposition of Holdco Securities in an amount equal to the difference between (i) the amount realized on the disposition and (ii) such U.S. Holder’s adjusted tax basis in such securities. Any gain or loss recognized by a U.S. Holder on a taxable disposition of Holdco Securities generally will be capital gain or loss and will be long-term capital gain or loss if the holder’s holding period in such shares and/or warrants exceeds one year at the time of the disposition. Preferential tax rates may apply to long-term capital gains of non-corporate U.S. Holders (including individuals). The deductibility of capital losses is subject to limitations. Any gain or loss recognized by a U.S. of a redemption of Holdco Shares, such redemption will be subject to Section 302 of the Code as described above under “—U.S. Federal Income Tax Consequences of the FLAC Shareholder Redemption to U.S. Holders of FLAC Class A Ordinary Shares.”
Exercise or Lapse of a Holdco Public Warrant
Except as discussed below with respect to the cashless exercise of a Holdco Public Warrant, if Holdco is not treated as a PFIC for the taxable year including the Business Combination, a U.S. Holder generally will not recognize gain or loss upon the exercise of a Holdco Public Warrant for cash. A U.S. Holder’s tax basis in a Holdco Share received upon exercise of a Holdco Public Warrant generally should be an amount equal to the sum of (i) the U.S. Holder’s tax basis in the Holdco Public Warrant exchanged therefor and (ii) the exercise price. If the exercise price is paid in Euro, a U.S. Holder’s tax basis in respect of the exercise price will be the U.S. dollar value of the amount in Euro paid on exercise, determined at the spot rate on the date of exercise. The U.S. Holder’s holding period for a Holdco Share received upon exercise of a Holdco Public Warrant will begin
227
Table of Contents
on the date following the date of exercise (or possibly the date of exercise) of the Holdco Public Warrant and will not include the period during which the U.S. Holder held the Holdco Public Warrant. If a Holdco Public Warrant is allowed to lapse unexercised, a U.S. Holder generally will recognize a capital loss equal to such holder’s tax basis in the Holdco Public Warrant.
The tax consequences of a cashless exercise of a Holdco Public Warrant are not clear under current U.S. federal income tax law. A cashless exercise may be tax-deferred, either because the exercise is not a realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either case, a U.S. Holder’s basis in the Holdco Share received would equal the holder’s basis in the Holdco Public Warrants exercised therefor. If the cashless exercise were treated as not being a realization event, it is unclear whether a U.S. Holder’s holding period for the Holdco Share would be treated as commencing on the date of exercise of the warrants or the day following the date of exercise of the warrants. If the cashless exercise were treated as a recapitalization, the holding period of the Holdco Share would include the holding period of the Holdco Public Warrants exercised therefor.
It is also possible that a cashless exercise of a Holdco Public Warrant could be treated in part as a taxable exchange in which gain or loss would be recognized. In such event, a U.S. Holder would recognize gain or loss with respect to the portion of the exercised Holdco Public Warrants treated as surrendered to pay the exercise price of the Holdco Public Warrants (the “surrendered warrants”). The U.S. Holder would recognize capital gain or loss with respect to the surrendered warrants in an amount generally equal to the difference between (i) the fair market value of the Holdco Public Warrants deemed surrendered and (ii) the U.S. Holder’s tax basis in the surrendered warrants. In this case, a U.S. Holder’s tax basis in the Holdco Shares received would equal the U.S. Holder’s tax basis in the Holdco Public Warrants exercised (meaning, the Holdco Public Warrants disposed of by the U.S. Holder in the cashless exercise, other than the surrendered warrants) and the exercise price of such Holdco Public Warrants. It is unclear whether a U.S. Holder’s holding period for the Holdco Shares would commence on the date of exercise of the warrants or the day following the date of exercise of the warrants.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise of warrants, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be approved by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise of Holdco Public Warrants.
U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to Non-U.S. Holders
A Non-U.S. Holder of Holdco Securities will not be subject to U.S. federal income tax or, subject to the discussion below under the heading “—Information Reporting and Backup Withholding,” U.S. federal withholding tax on any dividends (including constructive dividends) received on Holdco Securities or any gain recognized on a sale or other disposition of Holdco Securities (including, any distribution to the extent it exceeds the adjusted basis in the Non-U.S. Holder’s Holdco Securities) unless such dividend or gain (i) is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States, and (ii) if required by an applicable tax treaty, is attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States. Any such dividends and gains that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base in the United States) generally will be subject to U.S. federal income tax at the same regular U.S. federal income tax rates applicable to a comparable U.S. Holder and, in the case of a corporate Non-U.S. Holder, also may be subject to an additional branch profits tax at a 30% rate or a lower applicable tax treaty rate.
The U.S. federal income tax treatment of a Non-U.S. Holder’s exercise of a Holdco Public Warrant, or the lapse of a Holdco Public Warrant held by a Non-U.S. Holder, generally will correspond to the U.S. federal income tax treatment of the exercise or lapse of a warrant held by a U.S. Holder, as described under “—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders—U.S. Federal Income Tax Consequences of Ownership and Disposition of Holdco Securities to U.S. Holders if Holdco is not a PFIC—Exercise or Lapse of a Holdco Public Warrant,” above, although to the extent a cashless exercise
228
Table of Contents
results in a taxable exchange, the consequences for a Non-U.S. Holder of recognizing gain in such a taxable exchange would be the same as the consequences of recognizing gain on a sale or other disposition of Holdco Securities described in the preceding paragraphs above regarding a Non-U.S. Holder’s sale or other disposition of Holdco Securities.
Additional Reporting Requirements
U.S. Holders who are individuals and certain entities will be required to report information with respect to such U.S. Holder’s investment in “specified foreign financial assets” on IRS Form 8938 (Statement of Specified Foreign Financial Assets), subject to certain exceptions (including an exception for Holdco Securities held in accounts maintained at certain financial institutions). An interest in Holdco Securities constitutes a specified foreign financial asset for these purposes. Persons who are required to report specified foreign financial assets and fail to do so may be subject to substantial penalties and the period of limitations on assessment and collection of U.S. federal income taxes will be extended in the event of a failure to comply. U.S. Holders are urged to consult their tax advisors regarding the foreign financial asset and other reporting obligations and their application to the ownership and disposition of Holdco Securities.
Information Reporting and Backup Withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries are subject to information reporting, and may be subject to backup withholding. Backup withholding generally will not apply, however, to a U.S. Holder if (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding. A Non-U.S. Holder generally will eliminate the requirement for information reporting and backup withholding by providing certification of its non-U.S. status, under penalties of perjury, on a duly executed applicable IRS Form W-8 or by otherwise establishing an exemption.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a holder will be allowed as a credit against such holder’s U.S. federal income tax liability and a holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for a refund with the IRS and furnishing any required information.
Material Dutch Tax Considerations
The following is a general summary of material Dutch tax consequences of the acquisition, ownership and disposition of Holdco Shares and Holdco Public Warrants. This summary does not purport to describe all possible tax considerations or consequences that may be relevant to a holder or prospective holder of Holdco Shares or Holdco Public Warrants and does not purport to describe the tax consequences applicable to all categories of investors, some of which (such as trusts or similar arrangements) may be subject to special rules. For Dutch tax law purposes, a holder of Holdco Shares or Holdco Public Warrants may include an individual or entity not holding the legal title to such Holdco Shares or Holdco Public Warrants, but to whom, or to which, the Holdco Shares or Holdco Public Warrants are, or the income thereof is, nevertheless attributed based either on the individual or entity owning a beneficial interest in the Holdco Shares or Holdco Public Warrants or on specific statutory provisions. These include statutory provisions attributing the Holdco Shares or Holdco Public Warrants to an individual who, or who has directly or indirectly inherited from a person who was, the settlor, grantor or similar originator of a trust, foundation or similar entity that holds the Holdco Shares or Holdco Public Warrants.
This summary is based on the tax laws of the Netherlands, published regulations thereunder and published authoritative case law, all as in effect on the date hereof, and all of which are subject to change, possibly with retroactive effect. Where the summary refers to “the Netherlands” or “Dutch” it refers only to the part of the Kingdom of the Netherlands located in Europe.
This discussion is for general information purposes only and is not Dutch tax advice or a complete description of all Dutch tax consequences relating to the acquisition, ownership and disposition of Holdco Shares
229
Table of Contents
or Holdco Public Warrants. In view of its general nature, this summary should be treated with corresponding caution. Holders or prospective holders of Holdco Shares and Holdco Public Warrants should consult their tax advisor regarding the Dutch tax consequences relating to the acquisition, ownership and disposition of Holdco Shares and Holdco Public Warrants in light of their particular circumstances.
Please note that this summary does not describe the Dutch tax consequences for:
| i. | a holder of Holdco Shares or Holdco Public Warrants who has a substantial interest (aanmerkelijk belang) or deemed substantial interest (fictief aanmerkelijk belang) in Holdco under the Dutch Income Tax Act 2001 (Wet inkomstenbelasting 2001). Generally speaking, a holder of securities in a company is considered to hold a substantial interest in such company, if such holder alone or, in the case of individuals, together with such holder’s partner (as defined in the Dutch Income Tax Act 2001) or any relatives by blood or marriage in the direct line (including foster children), directly or indirectly, holds (i) an interest of 5% or more of the total issued and outstanding capital of that company or of 5% or more of the issued and outstanding capital of a certain class of shares of that company; or (ii) rights to acquire, directly or indirectly, such interest; or (iii) certain profit sharing rights in that company that relate to 5% or more of the company’s annual profits or to 5% or more of the company’s liquidation proceeds. A deemed substantial interest may arise if a substantial interest (or part thereof) in a company has been disposed of, or is deemed to have been disposed of, on a non-recognition basis; |
| ii. | a holder of Holdco Shares or Holdco Public Warrants, if the Holdco Shares or Holdco Public Warrants held by such holder qualify or qualified as a participation (deelneming) for purposes of the Dutch Corporate Income Tax Act 1969 (Wet op de vennootschapsbelasting 1969) and subsequently the participation exemption (deelnemingsvrijstelling) is applicable with respect to the Holdco Shares or Holdco Public Warrants. Generally, a holder’s shareholding of 5% or more in a company’s nominal paid-up share capital qualifies as a participation. A holder may also have a participation if (a) such holder does not have a shareholding of 5% or more but a related entity (statutorily defined term) has a participation or (b) the company in which the shares are held is a related entity (statutorily defined term). |
| iii. | pension funds, investment institutions (fiscale beleggingsinstellingen) and tax exempt investment institutions (vrijgestelde beleggingsinstellingen) (each as defined in the Dutch Corporate Income Tax Act 1969) and other entities that are, in whole or in part, not subject to or exempt from Dutch corporate income tax as well as entities that are exempt from corporate income tax in its country of residence, such country of residence being another state of the European Union, Norway, Liechtenstein, Iceland or any other state with which the Netherlands has agreed to exchange information in line with international standards; |
| iv. | a holder of Holco Shares or Holdco Public Warrants who is an individual for whom the Holdco Shares or Holdco Public Warrants or any benefit derived from the Holdco Shares or Holdco Public Warrants are attributable to a membership or a management to a supervisory board, an employment relationship or a deemed employment relationship, or is otherwise considered a remuneration for activities performed by such holder or certain individuals related to such holder (as defined in the Dutch Income Tax Act 2001); and |
| v. | a holder of Holco Shares or Holdco Public Warrants who is not considered the beneficial owner (uiteindelijk gerechtigde) of Holdco Shares or Holdco Public Warrants or the benefits derived from or realized with these Holdco Shares or Holdco Public Warrants. |
Withholding Tax
Dividends distributed by Holdco generally are subject to Dutch dividend withholding tax at a rate of 15%.
Generally, Holdco is responsible for the withholding of such dividend withholding tax at its source; the Dutch dividend withholding tax is for the account of the holder of Holdco Shares or Holdco Public Warrants.
The expression “dividends distributed” includes, among other things:
| i. | distributions in cash or in kind, deemed and constructive distributions and repayments of paid-in capital not recognized for Dutch dividend withholding tax purposes; |
| ii. | liquidation proceeds, proceeds of redemption of Holdco Shares or proceeds of the repurchase of Holdco Shares by Holdco or one of its subsidiaries or other affiliated entities in excess of the average paid-in capital as recognized for Dutch dividend withholding tax purposes; |
230
Table of Contents
| iii. | an amount equal to the par value of Holdco Shares issued or an increase of the par value of Holdco Shares, to the extent that it does not appear that a contribution, recognized for Dutch dividend withholding tax purposes, has been made or will be made; and |
| iv. | partial repayment of the paid-in capital, recognized for Dutch dividend withholding tax purposes, if and to the extent Holdco has net profits (zuivere winst), unless (i) the general meeting has resolved in advance to make such repayment and (ii) the par value of the Holdco Shares concerned has been reduced by an equal amount by way of an amendment of Holdco’s articles of association. The term “net profits” includes anticipated profits that are yet to be realized. |
In addition to the above, it cannot be excluded that payments in consideration for a redemption of Holdco Public Warrants or a full or partial cash settlement of the Holdco Public Warrants fall within the scope of the aforementioned “dividends distributed” and are therefore to such extent subject to Dutch dividend withholding tax at a rate of 15%. As of today, no authoritative case law of the Dutch courts has been published in this respect.
The exercise of a Holdco Public Warrant does in our view not give rise to Dutch dividend withholding tax, except to the extent (i) the exercise price, paid in cash, is below the nominal value of a Holdco Share (currently, the nominal value per Holdco Share is €0.12 and the exercise price is $11.50) and (ii) such difference is not charged against Holdco’s share premium reserve recognized for purposes of Dutch dividend withholding tax. If any Dutch dividend withholding tax due is not effectively withheld for the account of the relevant holder of a Holdco Public Warrant, Dutch dividend withholding tax shall be due by Holdco on a grossed-up basis, meaning that the Dutch dividend withholding tax basis shall be equal to the amount referred to in the preceding sentence multiplied by 100/85. Exceptions and relief from Dutch dividend withholding tax may apply as set forth in the below paragraphs.
Corporate legal entities who are resident or deemed to be resident of the Netherlands for Dutch corporate income tax purposes (“Dutch Resident Entities”) generally are entitled to an exemption from, or a credit for, any Dutch dividend withholding tax against their Dutch corporate income tax liability. The credit in any given year is, however, limited to the amount of Dutch corporate income tax payable in respect of the relevant year with an indefinite carry forward of any excess amount. Individuals who are resident or deemed to be resident of the Netherlands for Dutch income tax purposes (“Dutch Resident Individuals”) generally are entitled to a credit for any Dutch dividend withholding tax against their Dutch income tax liability and to a refund of any residual Dutch dividend withholding tax. The above generally also applies to holders of Holdco Shares or Holdco Public Warrants that are neither resident nor deemed to be resident of the Netherlands if the Holdco Shares or Holdco Public Warrants are attributable to a Dutch permanent establishment of such non-resident holder.
A holder of Holdco Shares or Holdco Public Warrants resident of a country other than the Netherlands may, depending on such holder’s specific circumstances, be entitled to exemptions from, reductions of, or full or partial refunds of, Dutch dividend withholding tax under Dutch national tax legislation or a double tax treaty in effect between the Netherlands and such other country. In 2017, the Netherlands signed the Multilateral Instrument (“MLI”). Since the MLI entered into force in July 2019, the MLI applies to covered tax agreements concluded by the Netherlands. A covered tax agreement is a double tax treaty that is in force between parties to the MLI and for which both parties have made a notification that they wish to modify the double tax treaty using the MLI. A principle purpose test (“PPT”) is part of the MLI in the majority of these covered tax agreements. The PPT disallows treaty benefits if obtaining a treaty benefit is the main or one of the main reasons for an arrangement or transaction, unless the granting of these treaty benefits is in line with the purpose of the relevant treaty provision.
Dividend stripping. Pursuant to legislation to counteract “dividend stripping,” a reduction, exemption, credit or refund of Dutch dividend withholding tax is denied if the recipient of the dividend is not the beneficial owner as described in the Dutch Dividend Withholding Tax Act 1965 (Wet op de dividendbelasting 1965). This legislation generally targets situations in which a shareholder retains its economic interest in shares but reduces the withholding tax costs on dividends by a transaction with another party. The recipient of the dividends is not required to be aware that a dividend stripping transaction took place for these rules to apply. The Dutch State Secretary of Finance takes the position that the definition of beneficial ownership introduced by this legislation will also be applied in the context of a double taxation convention.
231
Table of Contents
Conditional withholding tax on dividends as of January 1, 2024. As of January 1, 2024, a Dutch conditional withholding tax will be imposed on dividends distributed by Holdco to entities related (gelieerd) to Holdco (within the meaning of the Dutch Withholding Tax Act 2021; Wet bronbelasting 2021), if such related entity:
| i. | is considered to be resident (gevestigd) in a jurisdiction that is listed in the yearly updated Dutch Regulation on low-taxing states and non-cooperative jurisdictions for tax purposes (Regeling laagbelastende staten en niet-coöperatieve rechtsgebieden voor belastingdoeleinden) (a “Listed Jurisdiction”); or |
| ii. | has a permanent establishment located in a Listed Jurisdiction to which the Holdco Shares or Holdco Public Warrants are attributable; or |
| iii. | holds the Holdco Shares or Holdco Public Warrants with the main purpose or one of the main purposes of avoiding taxation for another person or entity and there is an artificial arrangement or transaction or a series of artificial arrangements or transactions; or |
| iv. | is not considered to be the beneficial owner of the Holdco Shares or Holdco Public Warrants in its jurisdiction of residences because such jurisdiction treats another entity as the beneficial owner of the Holdco Shares or Holdco Public Warrants (a hybrid mismatch); or |
| v. | is not resident in any jurisdiction (also a hybrid mismatch); or |
| vi. | is a reverse hybrid (within the meaning of Article 2(12) of the Dutch Corporate Income Tax Act 1969), if and to the extent (x) there is a participant in the reverse hybrid which is related (gelieerd) to the reverse hybrid, (y) the jurisdiction of residence of such participant treats the reverse hybrid as transparent for tax purposes and (z) such participant would have been subject to the Dutch conditional withholding tax in respect of dividends distributed by Holdco without the interposition of the reverse hybrid, all within the meaning of the Dutch Withholding Tax Act 2021. |
The Dutch conditional withholding tax on dividends will be imposed at the highest Dutch corporate income tax rate in effect at the time of the distribution (rate in 2022 is 25.8%). The Dutch conditional withholding tax on dividends will be reduced, but not below zero, by any regular Dutch dividend withholding tax withheld in respect of the same dividend distribution. As such, based on the currently applicable rates, the overall effective tax rate of withholding the regular Dutch dividend withholding tax (as described above) and the Dutch conditional withholding tax on dividends will not exceed the highest corporate income tax rate in effect at the time of the distribution (rate in 2022 is 25.8%).
Taxes on Income and Capital Gains
Dutch Resident Entities. Generally, any income derived or deemed to be derived from the Holdco Shares or Holdco Public Warrants held by a Dutch Resident Entity or any capital gain or loss realized on the disposal or deemed disposal of Holdco Shares or Holdco Public Warrants by a Dutch Resident Entity is subject to Dutch corporate income tax at a rate of 15% with respect to taxable profits up to €395,000 and 25.8% with respect to taxable profits in excess of that amount (rates and brackets for 2022).
Dutch Resident Individuals. Any income derived or deemed to be derived from the Holdco Shares or Holdco Public Warrants held by a Dutch Resident Individual or any capital gain or loss realized on the disposal or deemed disposal of the Holdco Shares or Holdco Public Warrants by a Dutch Resident Individual is taxable at the progressive Dutch income tax rates (with a maximum of 49.5% in 2022), if:
| i. | the Holdco Shares or Holdco Public Warrants are attributable to an enterprise from which the holder of Holdco Shares or Holdco Public Warrants derives a share of the profit, whether as an entrepreneur (ondernemer) or as a person who has a co-entitlement to the net worth (medegerechtigd tot het vermogen) of such enterprise without being a shareholder (as defined in the Dutch Income Tax Act 2001); or |
| ii. | such income qualifies as income from miscellaneous activities (resultaat uit overage werkzaamheden), as defined in the Dutch Income Tax Act 2011, which includes situations in which the holder of Holdco Shares or Holdco Public Warrants is considered to perform activities with respect to the Holdco Shares or Holdco Public Warrants that go beyond normal asset management (normaal, actief vermogensbeheer). |
232
Table of Contents
If the above-mentioned conditions i. and ii. do not apply to the Dutch Resident Individual, such individual will be taxed annually under the regime for savings and investments (inkomen uit sparen en beleggen), in which case the Dutch Resident Individual will be subject to Dutch income tax on a deemed return, regardless of the actual income derived or gains realized. The deemed return will be calculated by applying the applicable deemed return percentage (with a maximum of 5.53% (in 2022)) on the Dutch Resident Individual’s net investment assets (rendementsgrondslag) for the year, insofar the individual’s net investment assets for the year exceed a statutory threshold (heffingvrij vermogen). The deemed return on the Dutch Resident Individual’s net investment assets for the year is taxed at a flat rate of 31% (rate for 2022). Actual income, gains or losses in respect of the Holdco Shares or Holdco Public Warrants are as such not subject to Dutch income tax.
The net investment assets for the year are the fair market value of the investment assets less the allowable liabilities on January 1 of the relevant calendar year. The Holdco Shares and Holdco Public Warrants are included as investment assets.
Based on a decision of the Dutch Supreme Court (Hoge Raad) of 24 December 2021 (ECLI:NL:HR:2021:1963), the system of taxation for savings and investments based on a deemed return may under specific circumstances contravene with Section 1 of the First Protocol to the European Convention on Human Rights in combination with Section 14 of the European Convention on Human Rights. On June 28, 2022 the Dutch State Secretary of Finance has issued a decree (which decree will be implemented in Dutch tax law as of 2023 pursuant to the ‘Law on the restoration of rights box 3’ (Wet rechtsherstel box 3) and applies to the calendar year 2022) amending the regime for taxation of savings and investments as in effect on the date hereof to comply with this Dutch Supreme Court ruling. On the basis of the decree as published on June 28, 2022 (and the aforementioned new law) the tax will be levied at the lowest outcome of the following two calculation methods:
Method 1. Under method 1, the annual taxable benefit from a Dutch Resident Individual’s assets and liabilities taxed under this regime, including the Holdco Shares and Holdco Public Warrants, is based on a deemed return (ranging from 1.82% and 5.53% in 2022) of the positive balance of the fair market value of those assets, including the Holdco Shares and Holdco Public Warrants, and the fair market value of these liabilities.
Method 2. Under method 2, the annual taxable benefit from a Dutch Resident Individual’s assets and liabilities taxed under this regime, including the Holdco Shares and Holdco Public Warrants, is based on the actual allocation of the Dutch Resident Individual’s assets and liabilities over the following three categories: (i) bank savings, (ii) other investments, including the Holdco Shares and Holdco Public Warrants, and (iii) liabilities. The tax is calculated as follows:
| i. | a deemed return on the fair market value of the actual amount of bank savings; plus |
| ii. | a deemed return on the fair market value of the actual amount of other investments, including the Holdco Shares and Holdco Public Warrants; minus |
| iii. | a deemed return on the fair market value of the actual amount of liabilities. |
Under the second method, the statutory threshold is divided pro-rata over the three assets and liabilities categories mentioned above. At the date hereof, the deemed returns under (i) to (iii) above have not yet been definitively determined for the year 2022.
Holders of Holdco Shares and Holdco Public Warrants are advised to consult their own tax advisor to ensure that the tax is levied in accordance with the decision of the Dutch Supreme Court.
Non-residents of the Netherlands. A holder of Holdco Shares or Holdco Public Warrants that is neither a Dutch Resident Entity nor a Dutch Resident Individual (“Non-Resident Holder”) will not be subject to Dutch corporate income tax or Dutch income tax in respect of income derived or deemed to be derived from the Holdco Shares or Holdco Public Warrants, provided that:
| i. | such Non-Resident Holder does not derive profits from an enterprise or deemed enterprise (as defined in the Dutch Income Tax Act 2001 and the Dutch Corporate Income Tax Act 1969) which enterprise is, in whole or in part, carried out through a permanent establishment, a deemed permanent establishment |
233
Table of Contents
| or a permanent representative in the Netherlands and to which enterprise or part of an enterprise the Holdco Shares or Holdco Public Warrants are attributable or deemed to be attributable; |
| ii. | such Non-Resident Holder does not have an interest in (including an entitlement to a share in the profits or co-entitlement to the worth of), other than by way of holding a security, an enterprise or deemed enterprise (as defined in the Dutch Income Tax Act 2001 and the Dutch Corporate Income Tax Act 1969) which is effectively managed in the Netherlands and to which the Holdco Shares or Holdco Public Warranties are attributable or deemed to be attributable; and |
| iii. | in the event that the Non-Resident Holder is an individual, such holder does not derive income or capital gains that are taxable as income from miscellaneous activities (resultaat uit overage werkzaamheden), as defined in the Dutch Income Tax Act 2001, which includes situations in which the Non-Resident Holder is considered to perform activities with respect to the Holdco Shares or Holdco Public Warrants that go beyond normal asset management (normaal, actief vermogensbeheer). |
Gift and Inheritance Taxes
Residents of the Netherlands. Gift or inheritance taxes will arise in the Netherlands with respect to a transfer of Holdco Shares or Holdco Public Warrants by way of a gift by, or on the death of, a holder of Holdco Shares or Holdco Public Warrants who is resident or deemed resident of the Netherlands at the time of the gift or such holder’s death.
Non-residents of the Netherlands. No gift or inheritance taxes will arise in the Netherlands with respect to a transfer of Holdco Shares or Holdco Public Warrants by way of a gift by, or on the death of, a holder of Holdco Shares or Holdco Public Warrants who is neither resident nor deemed to be resident of the Netherlands, unless:
| i. | in the case of a gift of a Holdco Share or Holdco Public Warrant by an individual who at the date of the gift was neither resident nor deemed to be resident of the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident of the Netherlands; |
| ii. | in the case of a gift of a Holdco Share or Holdco Public Warrant is made under a condition precedent, the holder of the Holdco Share or Holdco Public Warrant is resident or is deemed to be resident of the Netherlands at the time the condition is fulfilled; or |
| iii. | the transfer is otherwise construed as a gift or inheritance made by, or on behalf of, a person who, at the time of the gift or death, is or is deemed to be resident of the Netherlands. |
For purposes of Dutch gift and inheritance taxes, amongst others, a person that holds the Dutch nationality will be deemed to be resident of the Netherlands if such person has been a resident of the Netherlands at any time during the ten years preceding the date of the gift or such person’s death. Additionally, for purposes of Dutch gift tax, amongst others, a person not holding the Dutch nationality will be deemed to be resident of the Netherlands if such person has been a resident of the Netherlands at any time during the twelve months preceding the date of the gift. Applicable tax treaties may override deemed residency.
Value Added Tax (“VAT”)
No Dutch VAT will be payable by a holder of Holdco Shares or Holdco Public Warrants in respect of any payment in consideration for the ownership or disposition of the Holdco Shares or Holdco Public Warrants.
Real Property Transfer Tax
Under circumstances, the Holdco Shares or Holdco Public Warrants could, for the purposes of Dutch real property transfer tax (overdrachtsbelasting), be treated as real property (fictieve onroerende zaken) located in the Netherlands, in which case this tax could be payable upon acquisition of the Holdco Shares or Holdco Public Warrants.
234
Table of Contents
The Holdco Shares and Holdco Public Warrants will generally not be treated as real property if at the time of, or at any time during the year preceding, the acquisition of the Holdco Shares or Holdco Public Warrants:
| i. | our assets do not and did not include real property situated in the Netherlands; or |
| ii. | our assets only include and included real property, situated either in or outside the Netherlands, that we do not and did not hold, and currently do not intend to hold, predominantly as a financial investment. |
Real property as referred to under i. and ii. above includes legal ownership and more limited legal rights over the property (rights in rem) (zakelijke rechten) as well as contractual rights that give us economic exposure to the value of such real property, and certain participations or interests in entities that are treated as real property.
Holdco’s assets do not include and have not included real property situated in the Netherlands as described above. Consequently, no Dutch real property transfer tax becomes payable upon an acquisition of the Holdco Shares or Holdco Public Warrants.
Stamp Duties
No Dutch documentation taxes (commonly referred to as stamp duties) will be payable by a holder of Holdco Shares or Holdco Public Warrants in respect of any payment in consideration for the ownership or disposition of the Holdco Shares or Holdco Public Warrants.
Material Cayman Islands Tax Considerations
Prospective investors should consult their professional advisors on the possible tax consequences of buying, holding or selling any Shares under the laws of their country of citizenship, residence or domicile.
Cayman Islands Taxation
The following is a discussion on certain Cayman Islands income tax consequences of an investment in shares of a Cayman Islands company. The discussion is a general summary of present law, which is subject to prospective and retroactive change. It is not intended as tax advice, does not consider any investor’s particular circumstances, and does not consider tax consequences other than those arising under Cayman Islands law. On this basis, the following discussion is the opinion of Campbells LLP, Cayman Islands counsel.
Under Existing Cayman Islands Laws
Payments of dividends and capital in respect of shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of interest and principal or a dividend or capital to any holder of shares, as the case may be, nor will gains derived from the disposal of the Shares be subject to Cayman Islands income or corporation tax. The Cayman Islands currently has no income, corporation or capital gains tax and no estate duty, inheritance tax or gift tax.
No stamp duty is payable in respect to the issue of shares or on an instrument of transfer in respect of a share. However, an instrument of transfer in respect of our securities, including our warrants, is stampable if executed in or brought into the Cayman Islands.
FLAC has been incorporated under the laws of the Cayman Islands as an exempted company with limited liability and, as such, has applied for and obtained an undertaking from the Financial Secretary of the Cayman Islands in the following form:
The Tax Concessions Law
Undertaking as to Tax Concessions
In accordance with the Tax Concessions Law the following undertaking is hereby given to Frazier Lifesciences Acquisition Corporation (the “Company”).
(a) that no Law which is hereafter enacted in the Islands imposing any tax to be levied on profits, income, gains or appreciations shall apply to the Company or its operations; and
235
Table of Contents
(b) in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax shall be payable:
(i) on or in respect of the shares, debentures or other obligations of the Company; or
(ii) by way of the withholding in whole or part, of any relevant payment as defined in the Tax Concessions Law.
These concessions shall be for a period of twenty years from the 27th day of October 2020.
236
Table of Contents
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Introduction
The following unaudited pro forma condensed combined financial information is provided to aid you in your analysis of the financial aspects of the Business Combination. The following unaudited pro forma condensed combined financial information presents the historical financial information of FLAC and NewAmsterdam Pharma adjusted to give effect to the Business Combination and related transactions and assumes that the Business Combination Proposal is approved and all shares issuable upon closing of the transactions contemplated by the Business Combination Agreement will be Holdco Shares. The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.”
The unaudited pro forma condensed combined statement of financial position as at June 30, 2022, gives pro forma effect to the Business Combination as if it had been consummated as of that date. The unaudited pro forma condensed combined statements of profit or loss for the year ended December 31, 2021 and the six months ended June 30, 2022, give pro forma effect to the Business Combination as if it had occurred as of January 1, 2021.
The unaudited pro forma condensed combined financial information has been presented for illustrative purposes only and is not necessarily indicative of the financial position and results of operations that would have been achieved had the Business Combination and related transactions occurred on the dates indicated. Further, the unaudited pro forma condensed combined financial information may not be useful in predicting the future financial condition and results of operations of the post-combination company. The actual financial position and results of operations may differ significantly from the pro forma amounts reflected herein due to a variety of factors. The unaudited pro forma adjustments represent management’s estimates based on information available as of the date of the unaudited pro forma condensed combined financial information and is subject to change as additional information becomes available and analyses are performed. This pro forma financial information should be read in conjunction with FLAC’s and NewAmsterdam Pharma’s respective financial statements and related notes thereto included elsewhere in this proxy statement/prospectus, “NewAmsterdam Pharma’s Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “FLAC’s Management’s Discussion and Analysis of Financial Condition and Results of Operation,” “Selected Historical Financial Information of NewAmsterdam Pharma,” “Selected Historical Financial Information of FLAC” and other financial information included elsewhere in this proxy statement/prospectus.
The unaudited pro forma condensed combined statement of profit or loss for the year ended December 31, 2021 has been prepared using the following:
| • | the consolidated statement of operations for the year ended December 31, 2021 derived from the audited statement of operations for the year ended December 31, 2021 and for the period from October 7, 2020 (inception) through December 31, 2020 of FLAC and the related notes thereto included in this proxy statement/prospectus. The consolidated financial statements of FLAC have been prepared under U.S. GAAP with the U.S. dollar as its reporting currency; and |
| • | the consolidated statement of profit or loss and other comprehensive loss for the year ended December 31, 2021 derived from the audited consolidated financial statements as at December 31, 2021 and 2020 and for the two years in the period ending December 31, 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus. The consolidated financial statements of NewAmsterdam Pharma have been prepared in accordance with IFRS with Euros as its presentation currency. |
The unaudited pro forma condensed combined statement of financial position as at June 30, 2022 and the unaudited pro forma condensed combined statement of profit or loss for the six months ended June 30, 2022 have been prepared using the following:
| • | the unaudited condensed balance sheet as at June 30, 2022 and the unaudited condensed statement of operations for the six months ended June 30, 2022 derived from the condensed balance sheet as at |
237
Table of Contents
June 30, 2022 and December 31, 2021 and the unaudited statement of operations for the six months ended June 30, 2022 and 2021 of FLAC and the related notes thereto included in this proxy statement/prospectus. The consolidated financial statements of FLAC have been prepared under U.S. GAAP with the U.S. dollar as its reporting currency; and |
| • | the unaudited condensed consolidated statement of financial position of NewAmsterdam Pharma as at June 30, 2022 and the unaudited condensed consolidated statement of profit or loss and other comprehensive loss for the six months ended June 30, 2022 derived from the unaudited condensed consolidated financial statements as at and for the six months ended June 30, 2022 and 2021 and the related notes thereto included elsewhere in this proxy statement/prospectus. The unaudited condensed consolidated financial statements of NewAmsterdam Pharma have been prepared in accordance with International Accounting Standard 34 - Interim Financial Reporting with Euros as its presentation currency. |
The unaudited pro forma condensed combined financial information gives effect to adjustments required to convert FLAC historical financial information to IFRS and its reporting currency to Euros.
Description of the Transaction
As further described elsewhere in this proxy statement/prospectus, subject to the terms and conditions of the Business Combination Agreement, upon consummation of the Business Combination, among other things:
| • | The shareholders of NewAmsterdam Pharma will contribute all 17,016,872 outstanding shares in the capital of NewAmsterdam Pharma, of which, prior to the Exchange, 2,500,000 were voting ordinary shares, 2,785,714 were non-voting ordinary shares and 11,731,158 were Series A Preferred Shares, to Holdco in exchange for the issuance of 44,914,642 ordinary shares in the share capital of Holdco (including the 8,656,330 Holdco Shares to be issued to Amgen and MTPC as described below); |
| • | Immediately after giving effect to the Exchange, the parties will effect the Holdco Reorganization, provided that NewAmsterdam Pharma and FLAC may agree to effect the Holdco Reorganization promptly following the PIPE Financing; |
| • | After giving effect to the Exchange, Merger Sub will merge with and into FLAC, with FLAC as the Surviving Company; |
| • | In connection with the Merger, each issued and outstanding ordinary share of FLAC will be canceled and extinguished in exchange for a claim for a Holdco Share, and such claim will then be contributed into Holdco against the issuance of a corresponding Holdco Share; |
| • | Immediately following the Merger, each outstanding FLAC Private Warrant and FLAC Public Warrant will, by the terms of the Warrant Assumption Agreement, become a warrant to purchase one Holdco Share, on the same contractual terms which will result in the issuance of 4,767,000 Holdco Warrants; |
| • | Each NewAmsterdam Pharma Option will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in, Holdco Shares; |
| • | Promptly following the Merger, the Surviving Company will change its jurisdiction of incorporation by deregistering as a Cayman Islands exempted company and continuing and domesticating as a corporation incorporated under the laws of the State of Delaware (the “Domestication”); |
| • | In addition to the transactions described above, 8,656,330 Holdco Shares will be issued to Amgen and MTPC pursuant to their profit rights granted upon the acquisition of Dezima as described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement”; and |
238
Table of Contents
| • | Following the Merger, upon the achievement of a certain clinical development milestone, Holdco will issue to the Participating Shareholders, Amgen, MTPC and the Participating Optionholders, the Earnout Shares, which in the case of the Participating Optionholders will take the form of awards of restricted stock units under the Holdco LTIP. The development milestone consists of achievement and public announcement of Positive Phase 3 Data (as defined in the Business Combination Agreement) for each of NewAmsterdam Pharma’s BROADWAY clinical trial and BROOKLYN clinical trial at any time during the Earnout Period. As a result, no Earnout Shares will be issuable if the applicable milestone is not achieved within the Earnout Period. |
In connection with the foregoing and concurrently with the execution of the Business Combination Agreement, FLAC and Holdco entered into Subscription Agreements with certain investors, pursuant to which the PIPE Investors agreed to subscribe for and purchase from Holdco, and Holdco agreed to issue and sell to such PIPE Investors, an aggregate of 23,460,000 Holdco Shares at $10.00 per share for gross proceeds of $234.6 million substantially concurrently with the closing of the Business Combination. The PIPE Financing is contingent upon, among other things, the closing of the Business Combination. The Business Combination Agreement provides that NewAmsterdam Pharma’s obligation to consummate the Business Combination is conditioned on the Aggregate Cash Proceeds being at least $250 million. The committed PIPE subscriptions, if fully funded, and expected cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption, are sufficient to satisfy this condition, even if up to approximately 88% of the issued and outstanding FLAC Public Shares exercise their redemption rights.
As of June 30, 2022, 14,301,000 FLAC Class A Ordinary Shares were outstanding. The FLAC Class A Ordinary Shares include (i) 13,800,00 FLAC Public Shares and (ii) 501,000 FLAC Private Placement Shares which do not hold redemption rights. As of June 30, 2022, 3,450,000 FLAC Class B Ordinary Shares were outstanding and held by the FLAC Initial Shareholders (the “Founder Shares”). Redemption rights were provided to FLAC Public Shares shareholders in connection with the Business Combination. For the avoidance of doubt, the Class B Ordinary Shares and the FLAC Private Placement Shares, have no redemption rights attached. FLAC and certain other of its investors, including affiliates of the Sponsor, representing a total of 1,500,000 FLAC Public Shares, each also entered into Investor Support Agreements, pursuant to which such FLAC shareholder agreed to (a) vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; and (b) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination.
Anticipated Accounting for the Business Combination
The Business Combination will be accounted for as a capital reorganization in accordance with IFRS. Under this method of accounting, FLAC will be treated as the “acquired” company for accounting purposes. As FLAC does not meet the definition of a business under IFRS 3 – Business Combinations (“IFRS 3”), the net assets of FLAC will be stated at historical cost, with no goodwill or other intangible assets recorded. As a result of the Business Combination and related transactions, the existing shareholders of NewAmsterdam Pharma will continue to retain control through their majority ownership of Holdco.
NewAmsterdam Pharma has been determined to be the accounting acquirer based on an evaluation of the following facts and circumstances:
| • | NewAmsterdam Pharma’s shareholders will have the largest voting interest in Holdco under both the No Redemption Scenario and Maximum Redemption Scenario; |
| • | NewAmsterdam Pharma’s senior management will be the senior management of Holdco; |
| • | The business of NewAmsterdam Pharma will comprise the ongoing operations of Holdco; and |
| • | NewAmsterdam Pharma is the larger entity, in terms of substantive operations and employee base. |
239
Table of Contents
As FLAC does not meet the definition of a business in accordance with IFRS 3, the Business Combination is accounted for within the scope of IFRS 2 – Share-based Payment (“IFRS 2”). Any excess of the fair value of Holdco Shares issued to FLAC Shareholders over the fair value of FLAC’s identifiable net assets acquired represents compensation for the service of a stock exchange listing for its shares provided by FLAC and is expensed as incurred.
Basis of Pro Forma Presentation
The adjustments presented on the pro forma condensed combined financial statements have been identified and presented to provide an understanding of NewAmsterdam Pharma upon consummation of the Business Combination for illustrative purposes.
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786 “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” Release No. 33-10786 replaces the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”) and present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). We have elected not to present Management’s Adjustments and will only be presenting Transaction Accounting Adjustments in the following unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information is for illustrative purposes only. The financial results may have been different had the companies always been combined. You should not rely on the unaudited pro forma condensed combined financial information as being indicative of the historical results that would have been achieved had the companies always been combined or the future results that NewAmsterdam Pharma will experience. FLAC and NewAmsterdam Pharma have not had any historical relationship prior to the Business Combination. Accordingly, no pro forma adjustments were required to eliminate activities between the companies. The unaudited pro forma condensed combined financial information gives effect to those transactions contemplated in the Business Combination Agreement and those transactions which occur as a direct result of the consummation of the Business Combination. No effect has been given to events which occurred subsequent to the end of the period presented herein which do not occur as a direct result of the consummation of the Business Combination, including:
| • | Receipt of the non-refundable, non-creditable, upfront amount of €115 million from Menarini. |
For more information regarding significant events which occurred following the end of the period presented refer to “Note 15—Events After the Reporting Period” in the accompanying NewAmsterdam Pharma unaudited condensed consolidated financial statements as at and for the six months ended June 30, 2022 and 2021.
The unaudited pro forma condensed combined financial information has been prepared assuming two alternative levels of redemption using the assumptions below.
| • | No Redemption Scenario (“Scenario 1”): This presentation assumes that no FLAC shareholders exercise their redemption rights with respect to their shares of FLAC Class A Ordinary Shares for a pro rata share of cash in the Trust Account. |
| • | Maximum Redemption Scenario (“Scenario 2”): This presentation assumes that 12,261,482 shares of FLAC Class A Ordinary Shares are redeemed for their pro rata share of cash in the Trust Account in connection with the exercise of their redemption rights. In connection with the execution of the Business Combination Agreement, certain other holders of FLAC Class A Ordinary Shares agreed to waive their right to redeem such shares which total 1,500,000 FLAC Class A Ordinary Shares. 501,000 FLAC Class A Ordinary Shares acquired by the Sponsor in a private placement which occurred at the time of the FLAC IPO are not entitled to be redeemed in connection with the Business Combination. FLAC Class B Ordinary Shares do not have redemption rights. The Business Combination Agreement requires, at a minimum, the proceeds of the PIPE Financing plus the balance of the cash held in the |
240
Table of Contents
Trust Account, after giving effect to redemption of FLAC Class A Ordinary Shares, to be at least $250 million. In order to meet this minimum, holders of an additional 38,518 FLAC Class A Ordinary Shares which are not subject to an agreement to waive redemption rights must choose not to exercise their redemption rights. This scenario gives effect to the redemption of 12,261,482 FLAC Class A Ordinary Shares for aggregate redemption payments of €117.2 million ($122.7 million) at a redemption price of approximately €9.56 ($10.01, converted at a rate of $1.0469 per EUR) per share based on the historical balance of investments held in the Trust Account as of June 30, 2022. |
The foregoing scenarios are for illustrative purposes only as FLAC does not have, as of the date of this proxy statement/prospectus, a meaningful way of providing any certainty regarding the number of redemptions by FLAC Stockholders that may actually occur. If the actual redemptions are different than these assumptions, then the amounts and shares outstanding in the unaudited pro forma condensed combined financial information will be different and those changes could be material.
The following table summarizes the pro forma weighted average number of Holdco Shares outstanding for each of the year ended December 31, 2021 and the six months ended June 30, 2022 under the two alternatives presented above:
| Year ended December 31, 2021 | ||||||||||||||||
| No Redemption Scenario | Maximum Redemption Scenario | |||||||||||||||
| (Shares) | Ownership% | (Shares) | Ownership% | |||||||||||||
Former NewAmsterdam Pharma Shareholders(1) | 44,914,642 | 52 | % | 44,914,642 | 61 | % | ||||||||||
Former FLAC Class A Ordinary Shares(2) | 14,301,000 | 17 | % | 2,039,518 | 3 | % | ||||||||||
Former FLAC Class B Ordinary Shares | 3,450,000 | 4 | % | 3,450,000 | 4 | % | ||||||||||
PIPE Investors(3) | 23,460,000 | 27 | % | 23,460,000 | 32 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted(4) pro forma weighted average number of shares outstanding | 86,125,642 | 100 | % | 73,864,160 | 100 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||
| (1) | Includes an aggregate of 8,656,330 shares issued to Amgen and MTPC pursuant to 2020 Profit Rights as described in “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement.” |
| (2) | Includes 501,000 FLAC Private Placement Shares held by the Sponsor and 1,000,000 FLAC Class A Ordinary Shares held by affiliates of the Sponsor. |
| (3) | Includes 4,500,000 Holdco Shares subscribed for by affiliates of the Sponsor in the PIPE Financing. |
241
Table of Contents
| (4) | Excludes 1,886,137 Earnout Shares, 4,185,358 Holdco Options and 4,600,000 and 167,000 Public Warrants and Private Warrants, respectively, as their impact is anti-dilutive. |
| Six months ended June 30, 2022 | ||||||||||||||||
| No Redemption Scenario | Maximum Redemption Scenario | |||||||||||||||
| (Shares) | Ownership% | (Shares) | Ownership% | |||||||||||||
Former NewAmsterdam Pharma Shareholders(1) | 44,914,642 | 52 | % | 44,914,642 | 61 | % | ||||||||||
Former FLAC Class A Ordinary Shares(2) | 14,301,000 | 17 | % | 2,039,518 | 3 | % | ||||||||||
Former FLAC Class B Ordinary Shares | 3,450,000 | 4 | % | 3,450,000 | 4 | % | ||||||||||
PIPE Investors(3) | 23,460,000 | 27 | % | 23,460,000 | 32 | % | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic pro forma weighted average number of shares outstanding | 86,125,642 | 100 | % | 73,864,160 | 100 | % | ||||||||||
Holdco Options(4) | 3,666,279 | 3,666,279 | ||||||||||||||
Diluted(5) pro forma weighted average number of shares outstanding | 89,791,921 | 77,530,439 | ||||||||||||||
| (1) | Includes an aggregate of 8,656,330 shares issued to Amgen and MTPC pursuant to 2020 Profit Rights as described in “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement.” |
| (2) | Includes 501,000 FLAC Private Placement Shares held by the Sponsor and 1,000,000 FLAC Class A Ordinary Shares held by affiliates of the Sponsor. |
| (3) | Includes 4,500,000 Holdco Shares subscribed for by affiliates of the Sponsor in the PIPE Financing. |
| (4) | Calculated based upon the exercise of 4,185,358 Holdco Options at an exercise price of $1.22 and an average market closing price of $9.82 for the period from January 1, 2022 to June 30, 2022. |
| (5) | Excludes 1,886,137 Earnout Shares and 4,600,000 and 167,000 Public Warrants and Private Warrants, respectively. |
242
Table of Contents
PRO FORMA CONDENSED COMBINED STATEMENT OF FINANCIAL POSITION AS AT
JUNE 30, 2022
(UNAUDITED)
(in EUR thousands unless otherwise denoted)
| Scenario 1: No Redemption Scenario | Scenario 2: Maximum Redemption Scenario | |||||||||||||||||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| EUR | USD | EUR(1) | FN | FN | FN | |||||||||||||||||||||||||||||||||||||||
ASSETS | ||||||||||||||||||||||||||||||||||||||||||||
Non-Current Assets | ||||||||||||||||||||||||||||||||||||||||||||
Property, plant and equipment | 175 | — | — | — | — | 175 | — | 175 | ||||||||||||||||||||||||||||||||||||
Loan receivable | 728 | — | — | — | (728 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Investments held in Trust Account | — | 138,133 | 131,945 | — | (131,945 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Intangible assets | — | — | — | — | 82,686 | (8 | ) | 82,686 | — | 82,686 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total non-current assets | 903 | 138,133 | 131,945 | — | (49,987 | ) | 82,861 | — | 82,861 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Current Assets | ||||||||||||||||||||||||||||||||||||||||||||
Trade receivables | 115,000 | — | — | — | — | (4 | ) | 115,000 | — | 115,000 | ||||||||||||||||||||||||||||||||||
Prepayments and other receivables | 12,474 | — | — | 143 | (2 | ) | 4,019 | (10 | ) | 16,636 | — | 16,636 | ||||||||||||||||||||||||||||||||
Cash and cash equivalents | 89,478 | 615 | 588 | — | 323,190 | (4 | ) | 413,256 | (117,235 | ) | (12 | ) | 296,021 | |||||||||||||||||||||||||||||||
Prepaid expenses | — | 150 | 143 | (143 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total current assets | 216,952 | 765 | 731 | — | 327,209 | 544,892 | (117,235 | ) | 427,657 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total Assets | 217,855 | 138,898 | 132,676 | — | 277,222 | 627,753 | (117,235 | ) | 510,518 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(1) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
EQUITY AND LIABILITIES | ||||||||||||||||||||||||||||||||||||||||||||
Commitments and Contingencies | ||||||||||||||||||||||||||||||||||||||||||||
Class A ordinary share subject to possible redemption, $0.0001 par value; 13,800,000 shares issued and outstanding at redemption value of $10.00 per share | — | 138,033 | 131,849 | (131,849 | ) | (3 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||||||||||||||||||||||
Share capital | 163,556 | — | —* | — | 433,921 | (5 | ) | 597,477 | (79,929 | ) | (13 | ) | 517,548 | |||||||||||||||||||||||||||||||
Other Reserves | 1,029 | — | — | — | — | 1,029 | — | 1,029 | ||||||||||||||||||||||||||||||||||||
Preference shares, $0.0001 par value; 1,000,000 shares authorized; none issued or outstanding at December 31, 2021 and 2020 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
243
Table of Contents
| NewAmsterdam Pharma Historical IFRS | FLAC Historical U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(1) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
Class A ordinary shares, $0.0001 par value; 479,000,000 shares authorized; 501,000 shares issued and outstanding (excluding 13,800,000 shares subject to possible redemption | — | —* | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Class B ordinary shares, $0.0001 par value; 20,000,000 shares authorized; 3,450,000 shares issued and outstanding | — | —* | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Additional paid-in capital | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Retained earnings | 19,837 | (6,181 | ) | (5,904 | ) | — | (21,897 | ) | (6 | ) | (7,964 | ) | (37,306 | ) | (14 | ) | (45,270 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total equity | 184,422 | (6,181 | ) | (5,904 | ) | — | 412,024 | 590,542 | (117,235 | ) | 473,307 | |||||||||||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||||||||||||||||||||||
Deferred revenue | 7,440 | — | — | — | — | 7,440 | — | 7,440 | ||||||||||||||||||||||||||||||||||||
Lease liability | 90 | — | — | — | — | 90 | — | 90 | ||||||||||||||||||||||||||||||||||||
Deferred underwriting commissions | — | 4,830 | 4,614 | — | (4,614 | ) | (4 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Derivative warrant liabilities | — | 381 | 365 | — | — | (11 | ) | 365 | — | 365 | ||||||||||||||||||||||||||||||||||
Derivative earnout liability | — | — | — | — | 6,592 | (9 | ) | 6,592 | — | 6,592 | ||||||||||||||||||||||||||||||||||
Loans and borrowings | — | — | — | 131,849 | (3 | ) | (131,849 | ) | (7 | ) | — | — | — | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total non-current liabilities | 7,530 | 5,211 | 4,979 | 131,849 | (129,871 | ) | 14,487 | — | 14,487 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Current liabilities | ||||||||||||||||||||||||||||||||||||||||||||
Accounts payable | — | 51 | 48 | (48 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Accrued expenses | — | 1,784 | 1,704 | (1,704 | ) | (2 | ) | — | — | — | — | |||||||||||||||||||||||||||||||||
Loans and borrowings | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Lease liability | 61 | — | — | — | — | 61 | — | 61 | ||||||||||||||||||||||||||||||||||||
Trade and other payables | 11,782 | — | — | 1,752 | (2 | ) | (4,931 | ) | (4 | ) | 8,603 | — | 8,603 | |||||||||||||||||||||||||||||||
Deferred revenue | 14,060 | — | — | — | — | 14,060 | — | 14,060 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total current liabilities | 25,903 | 1,835 | 1,752 | — | (4,931 | ) | 22,724 | — | 22,724 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total liabilities | 33,433 | 7,046 | 6,731 | 131,849 | (134,802 | ) | 37,211 | — | 37,211 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total equity and liabilities | 217,855 | 138,898 | 132,676 | �� | — | 277,222 | 627,753 | (117,235 | ) | 510,518 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
| * | The financial statements of FLAC are presented in whole dollars whereas the historical financial information of FLAC in the above unaudited condensed combined statement of financial position is |
244
Table of Contents
| presented initially in thousands of dollars. FLAC Class A Ordinary Shares and FLAC Class B Ordinary Shares at June 30, 2022 are $50 and $345, respectively, as seen in FLAC’s balance sheet as of June 30, 2022 which is found elsewhere within this proxy statement/prospectus. As a result of rounding these amounts are shown as zero. These amounts would be reclassified into share capital as part of adjustment (2) below and eliminated in adjustment (5) below. |
Pro Forma Adjustments to the Unaudited Condensed Combined Statement of Financial Position
The adjustments included in the unaudited condensed combined statement of financial position as of June 30, 2022 are as follows:
IFRS Policy and Presentation Alignment
| (1) | The historical financial information of FLAC was prepared in accordance with U.S. GAAP and presented in USD. The historical financial information was translated from USD to EUR using the historical closing exchange rate, as of June 30, 2022, of $1.0469 per EUR. |
| (2) | Reflects adjustments to align FLAC historical financial statements with the presentation of NewAmsterdam Pharma financial statements. |
| (3) | Reflects the U.S. GAAP to IFRS conversion adjustment related to the reclassification of FLAC’s historical commitments and contingencies (FLAC Class A Ordinary Shares subject to possible redemption) into non-current liabilities (Loans and borrowings). Under U.S. GAAP shares of FLAC Class A Ordinary Shares are classified as temporary equity because they are redeemable at the sole discretion of the shareholder. As FLAC shareholders have the right to require FLAC to redeem the FLAC Class A Ordinary Shares and FLAC has an irrevocable obligation to deliver cash or another financial instrument for such redemption, this is reclassified from temporary equity under U.S. GAAP to other liabilities under IFRS. |
Transaction Accounting Adjustments
| (4) | Reflects adjustments to cash based upon Scenario 1 for the following items: |
| a. | Proceeds from the release of the cash held in trust which becomes available upon consummation of the Business Combination; |
| b. | The issuance of 23,460,000 Holdco Shares to PIPE investors in exchange for €224.1 million ($234.6 million, converted at a rate of $1.0469 per EUR); |
| c. | Proceeds from the repayment of a loan made by NewAmsterdam Pharma to an executive which is classified on the NewAmsterdam Pharma balance sheet as a loan receivable. This loan was repaid on July 19, 2022; |
| d. | Payment of the deferred underwriting commission incurred in connection with the initial public offering of FLAC; |
| e. | Payment of estimated transaction costs incurred in connection with the Business Combination and PIPE Financing, of which €4.9 million was included in trade and other payables at June 30, 2022; and |
245
Table of Contents
| f. | Payment of expected premium for directors’ and officers’ (“D&O”) insurance which must be obtained according to the terms of the Business Combination Agreement. The offsetting entry is seen in Prepaids and other receivables. |
In EUR thousands | ||||
Proceeds from cash held in trust account | 131,945 | |||
Proceeds from PIPE | 224,090 | |||
Proceeds from repayment of outstanding loan to NewAmsterdam Pharma executive | 728 | |||
Payment of deferred underwriting commission | (4,614 | ) | ||
Payment of estimated transaction costs incurred after December 31, 2021 in connection with the Business Combination and PIPE Financing | (23,959 | ) | ||
Payment of expected premium for D&O insurance | (5,000 | ) | ||
|
| |||
Total Cash Adjustment | 323,190 | |||
|
| |||
| (5) | Reflects adjustments to share capital based upon Scenario 1 for the following items: |
| a. | The issuance of 23,460,000 Holdco Shares to PIPE investors in exchange for €224.1 million ($234.6 million, converted at a rate of $1.0469 per EUR); |
| b. | The fair value of 17.8 million Holdco Shares issued in exchange for 14.3 million shares of FLAC Class A Ordinary Shares and 3.5 million shares of FLAC Class B Ordinary Shares. The fair value of such shares is calculated in the table in adjustment 6b below; |
| c. | As described in “Note 11—Asset Acquisition of NewAmsterdam Pharma B.V.” in the accompanying NewAmsterdam Pharma Holding B.V. consolidated financial statements and “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement.” NewAmsterdam Pharma entered into a share purchase agreement with Amgen to acquire the assets and liabilities of Dezima which was determined to be an asset acquisition under IFRS. As part of the asset acquisition, NewAmsterdam Pharma entered into a contingent earnout, or profit right, with Amgen and a similar arrangement with MTPC. Per the contingent earnout, in the event the company raises more than €100 million in an initial public offering, Amgen and MTPC are entitled to an aggregate number of shares equivalent to 17.6% of the pre-public offering valuation. In total 44.9 million Holdco shares will be issued upon the closing of the transactions contemplated by the Business Combination Agreement in exchange for all of the outstanding equity of NewAmsterdam Pharma, of which 8.7 million will be issued directly to Amgen and MTPC. The fair value per Holdco Share is assumed to equal €9.55 ($10.00, converted at a rate of $1.0469 per EUR) as defined in the Business Combination Agreement; and |
| d. | The portion of expected transaction costs incurred in connection with the Business Combination which is capitalized within share capital related to the issuance of new shares. Estimated transaction costs related to the Business Combination total €24.0 million, of which, €3.8 million qualifies for capitalization within share capital and the remainder is expensed. Through June 30, 2022, €4.9 million of transaction costs have been incurred, of which €1.0 million has been capitalized within prepayments and other receivables and reclassified into share capital as described in Adjustment 10 below. An additional €18.9 million of transaction costs are expected, of which €16.2 million are expensed. Costs which qualify for capitalization, which include legal and advisor fees directly related to the equity issuance, are allocated among all Holdco Shares issued with the portion related to those shares issued to FLAC shareholders and in connection with the PIPE being capitalized. |
246
Table of Contents
The table below sets forth the amounts for each item described above and the total share capital adjustment amount:
In EUR thousands | ||||
Issuance of 23.5 million Holdco Shares to PIPE investors | 224,090 | |||
Issuance of 17.8 million Holdco Shares to FLAC shareholders | 130,985 | |||
Issuance of 8.7 million Holdco Shares in connection with Amgen and MTPC profit rights | 82,685 | |||
Portion of transaction costs incurred after December 31, 2021 which is capitalized within share capital | (3,839 | ) | ||
|
| |||
Total Share Capital Adjustment | 433,921 | |||
|
| |||
In addition to the transactions listed above, the following transactions are considered:
| e. | 36.2 million shares of Holdco will be issued in exchange for all of the outstanding shares in NewAmsterdam Pharma, excluding the 8.7 million which are issued directly to Amgen and MTPC pursuant to each of their respective profit rights. The entry to record the elimination of NewAmsterdam Pharma historical share capital and the establishment of Holdco share capital offset resulting in a net impact of zero. |
| f. | Each NewAmsterdam Pharma Option that is outstanding and unexercised will remain outstanding, and to the extent unvested, such option will continue to vest in accordance with its applicable terms, and at the time of the Exchange, such NewAmsterdam Pharma Options will become options to purchase, and will when exercised be settled in Holdco Shares. The NewAmsterdam Pharma Options have equal terms and value before and after the Exchange and any entry to record the Exchange as it relates to the options has a net impact of zero. |
| (6) | Reflects adjustments to retained earnings based upon Scenario 1 for the following items: |
| a. | The elimination of historical FLAC retained earnings; |
| b. | The recording of an expense in accordance with IFRS 2 for the excess of the value of the Holdco Shares issued to FLAC stockholders over the fair value of FLAC’s identifiable net assets acquired, representing a listing cost. |
The share-based compensation related to the listing cost is calculated as:
| In EUR thousands, unless otherwise denoted | ||||||||
| Scenario 1 | Scenario 2 | |||||||
Fair Value of NewAmsterdam Pharma(1) [A] | 462,411 | 462,411 | ||||||
Equity interest in Holdco that will be issued to FLAC shareholders at the closing of the transactions contemplated by the Business Combination Agreement(2) [B] | 28.3 | % | 10.9 | % | ||||
Deemed fair value of shares issued by Holdco to FLAC shareholders [A] * [B] | 130,985 | 50,361 | ||||||
Less: FLAC net assets(3) | 125,945 | 8,711 | ||||||
|
|
|
| |||||
Share-based compensation for listing cost | 5,040 | 41,650 | ||||||
|
|
|
| |||||
| (1) | Calculated as the Purchase Price per the Business Combination Agreement of €469 million ($491 million, converted at a rate of $1.0469 per EUR) less the fair value of the Earnout Shares allocated to shareholders as seen in adjustment 6d below. |
| (2) | Equity interest is calculated based upon the shares issued to FLAC shareholders as seen in the pro forma share table above, excluding those Holdco Shares which are to be issued to PIPE investors. |
| (3) | Net assets of FLAC are calculated as assets minus liabilities based upon the audited financial statements of FLAC as at June 30, 2022 and converted from USD to EUR at a rate of $1.0469 per EUR. Scenario 2 |
247
Table of Contents
| reflects the reduction of net assets in the amount of the payment to redeeming shareholders for their pro-rate portion of the cash held in trust as seen in adjustment (10) below. |
| c. | The portion of expected transaction costs incurred after June 30, 2022 in connection with the Business Combination which is expensed. Estimated transaction costs related to the Business Combination total €24.0 million, of which, €4.9 million has been incurred through June 30, 2022. Of the remaining estimated transaction costs, €16.2 million are expensed. Costs which do not qualify for capitalization and are expensed as incurred include marketing fees and legal and advisor fees not directly related to the equity issuance. Costs which qualify for capitalization, are allocated among all Holdco Shares issued with the portion related to those shares issued to former NewAmsterdam Pharma shareholders being expensed. The remaining portion of qualifying costs are capitalized within share capital as seen in adjustment (5) above; and |
| d. | The recording of the fair value of the Earnout Shares which are allocated to Participating Shareholders (including Amgen and MTPC for this purpose) but excludes the Participating Optionholders. As per the terms of the Business Combination Agreement upon the achievement the certain clinical development milestone during the Earnout Period, Holdco will issue to the Participating Shareholders (including Amgen and MTPC for this purpose) and Participating Optionholders in total an additional 1,886,137 Holdco Shares. The Earnout Shares allocated to Participating Optionholders will take the form of restricted stock units under Holdco’s long-term incentive plan. The Participating Optionholders will vest in their restricted stock units so long as they provide continued service through the achievement of the clinical development milestone. For the avoidance of doubt, if the clinical development milestone is not achieved during the Earnout Period, but a Participating Optionholder has completed their service requirement, they will not be considered vested in the restricted stock units. There is no immediate financial impact with respect to the Participating Optionholders awards due to the uncertainty of achieving the clinical development milestone. No such service condition exists for the Shareholders awards. The Earnout Shares allocated to the Shareholders are accounted for under IAS 32 – Financial Instruments as a deemed dividend and recorded at fair value as a liability on the balance sheet as seen in adjustment (9) below. |
The table below sets forth the amounts for each item described and the total accumulated loss adjustment amount:
In EUR thousands | ||||
Elimination of historical FLAC accumulated loss | 5,904 | |||
Expense arising under IFRS 2 for the excess of the fair value of shares issued to FLAC stockholders over and above the fair value of FLAC’s identifiable net assets | (5,040 | ) | ||
Portion of transaction costs incurred after June 30, 2022 which is expensed | (16,169 | ) | ||
Fair value of the earnout shares allocated to shareholders | (6,592 | ) | ||
|
| |||
Total Accumulated Loss Adjustment | (21,897 | ) | ||
|
| |||
| (7) | Reflects adjustment to loans and borrowings to give effect to the exchange of all 13.8 million FLAC Class A Ordinary Shares subject to possible redemption for 13.8 million Holdco Shares as described in Scenario 1. The impact to share capital is included in entry 5b above. |
| (8) | Reflects the recognition of an intangible asset in connection with an aggregate of 8,656,330 Holdco Shares to be issued to Amgen and MTPC pursuant to each of their respective profit rights described in footnote 5c above. As described in “Note 11—Asset Acquisition of NewAmsterdam Pharma B.V.” in the accompanying NewAmsterdam Pharma Holding B.V. annual consolidated financial statements, NewAmsterdam Pharma has made an accounting policy election to record asset acquisitions under the cost accumulation model. The value per Holdco Share is assumed to equal €9.56 ($10.01, converted at a rate of $1.0469 per EUR) as defined in the Business Combination Agreement. |
| (9) | Upon the achievement a certain clinical development milestone during the Earnout Period, Holdco will issue 1,886,137 Earnout Shares to the Participating Shareholders (including Amgen and MTPC for this purpose) |
248
Table of Contents
| and Participating Optionholders. 1,725,358 Earnout Shares and 160,778 Earnout Shares are allocated to Participating Shareholders and Participating Optionholders, respectively. The Earnout Shares allocated to Participating Optionholders will take the form of restricted stock units under Holdco’s long-term incentive plan. The Participating Optionholders will vest in their restricted stock units so long as they provide continued service through the achievement of the clinical development milestone. For the avoidance of doubt, if the clinical development milestone is not achieved during the Earnout Period, but a Participating Optionholder has completed their service requirement, they will not be considered vested in the restricted stock units. There is no immediate financial impact with respect to the Participating Optionholders awards due to the uncertainty of achieving the clinical development milestone. No such service condition exists for the Participating Shareholders awards. The Earnout Shares allocated to the Participating Shareholders are accounted for under IAS 32 – Financial Instruments as a deemed dividend and recorded at fair value as a liability on the balance sheet as they fail the fixed-for-fixed criteria based on the terms and conditions of the Business Combination Agreement. |
| (10) | Reflects adjustments to prepayments and other receivables for the following items: |
| a. | Payment of expected premium for directors’ and officers’ (“D&O”) insurance which must be obtained according to the terms of the Business Combination Agreement. The offsetting entry is seen in Prepaids and other receivables. |
| b. | Reclassification of transaction costs incurred as of the balance sheet date which qualify for capitalization. Qualifying costs were capitalized within prepayments and other receivables and are reclassified into share capital at the closing of the transactions contemplated by the Business Combination Agreement. |
| In EUR thousands | ||||
Payment of D&O insurance premium | 5,000 | |||
Reclassification of previously capitalized transaction costs into equity | (981 | ) | ||
|
| |||
Total Prepayments and other receivables Adjustment | 4,019 | |||
|
| |||
| (11) | As a result of the closing of the transactions contemplated by the Business Combination Agreement all outstanding FLAC Warrants will cease to represent a right to acquire FLAC Class A Ordinary Shares and will represent a right to acquire Holdco Shares on the same contractual terms. The entry to record the deemed cancellation of FLAC Warrants and deemed issuance of Holdco Warrants results in a net impact of zero as the warrants were replaced with equal terms. |
| (12) | Reflects the payment of €9.56 ($10.01, converted at a rate of $1.0469 per EUR) per share for the redemption of 12.3 million FLAC Class A Ordinary Shares as described in Scenario 2. |
| (13) | Reflects additional adjustments to share capital based upon Scenario 2 for the following items: |
| a. | A reduction in share capital of €80.6 million equal to the difference between the deemed fair value of shares issued to FLAC shareholders in Scenario 1 and Scenario 2 as calculated in the table seen in adjustment 6c above; and |
| b. | Adjustment to the allocation between expense and share capital of qualifying transaction costs. In scenario 1 approximately 52% of qualifying transaction costs are expensed which represents the portion related to the proportion of Holdco Shares issued to former NewAmsterdam Pharma shareholders. In Scenario 2 former NewAmsterdam Pharma shareholders are issued approximately 61% of Holdco Shares due to the redemption of 12.3 million FLAC Class A Ordinary Shares which are not converted to Holdco Shares and as a result, approximately 61% of the qualifying costs are expensed in Scenario 2. |
249
Table of Contents
The table below set forth the amounts for each item described and the total share capital adjustment amount:
In EUR thousands | ||||
Reduction of share capital for the fair value of 12.3 million shares of FLAC Class A Ordinary Shares redeemed in Scenario 2. | (80,624 | ) | ||
Adjustment to allocation between expense and share capital of qualifying transaction costs | 695 | |||
|
| |||
Total Share Capital Adjustment | (79,929 | ) | ||
|
| |||
| (14) | Reflects additional adjustments to retained earnings based upon Scenario 2 for the following items: |
| a. | Incremental expense arising under IFRS 2 related to the listing service. The additional expense is equal to the difference between the share-based compensation expense for listing cost in Scenario 1 and Scenario 2 as calculated in the table seen in adjustment 6c above; and |
| b. | Adjustment to the allocation between expense and share capital of qualifying transaction costs. In scenario 1 approximately 52% of qualifying transaction costs are expensed which represents the portion related to the proportion of Holdco Shares issued to former NewAmsterdam Pharma shareholders. In scenario 2 former NewAmsterdam Pharma shareholders are issued approximately 61% of Holdco Shares due to the redemption of 12.3 million FLAC Class A Ordinary Shares which are not converted to Holdco Shares. |
The table below set forth the amounts for each item described and the total accumulated loss adjustment amount:
In EUR thousands | ||||
Incremental expense under IFRS 2 for the listing service | (36,611 | ) | ||
Adjustment to the allocation between expense and share capital of qualifying transaction costs | (695 | ) | ||
|
| |||
Total Retained Earnings Adjustment | (37,306 | ) | ||
|
| |||
The unaudited pro forma condensed combined financial information for both Scenario 1 and 2 does not reflect the income tax effects of the pro forma adjustments based on the statutory rate in effect for the historical periods presented given the combined entity has incurred significant losses since inception and does not expect to generate taxable income for the foreseeable future, including in fiscal year 2022.
250
Table of Contents
PRO FORMA CONDENSED COMBINED STATEMENT OF PROFIT OR LOSS
FOR THE YEAR ENDED DECEMBER 31, 2021
(UNAUDITED)
(in EUR thousands unless otherwise denoted)
| Scenario 1: No Redemption Scenario | Scenario 2: Maximum Redemption Scenario | |||||||||||||||||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical (1) U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(2) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
Research and development expenses | (25,032 | ) | — | — | — | — | (25,032 | ) | — | (25,032 | ) | |||||||||||||||||||||||||||||||||
General and administrative expenses | (4,803 | ) | (1,366 | ) | (1,155 | ) | — | (30,160 | ) | (4 | ) | (36,118 | ) | (37,306 | ) | (5 | ) | (73,424 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total operating expenses | (29,835 | ) | (1,366 | ) | (1,155 | ) | — | (30,160 | ) | (61,150 | ) | (37,306 | ) | (98,456 | ) | |||||||||||||||||||||||||||||
Finance income | 9 | — | — | — | — | 9 | — | 9 | ||||||||||||||||||||||||||||||||||||
Finance expense | (216 | ) | — | — | — | — | (216 | ) | — | (216 | ) | |||||||||||||||||||||||||||||||||
Interest income from investments held in Trust Account | — | 16 | 14 | — | (14 | ) | (3 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Change in fair value of derivative warrant liabilities | — | 4,529 | 3,828 | — | — | 3,828 | — | 3,828 | ||||||||||||||||||||||||||||||||||||
Break-up fee from terminated agreement | — | 1,000 | 845 | — | — | 845 | — | 845 | ||||||||||||||||||||||||||||||||||||
Net foreign exchange gain | 1,443 | — | — | — | — | 1,443 | — | 1,443 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Loss before tax | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
Income tax expense | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Loss for the period | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Attributable to: | ||||||||||||||||||||||||||||||||||||||||||||
Equity holders of the Company | (28,599 | ) | 4,179 | 3,532 | — | (30,174 | ) | (55,241 | ) | (37,306 | ) | (92,547 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Earnings / (loss) per share, basic and diluted: | ||||||||||||||||||||||||||||||||||||||||||||
Class A ordinary shares | 0.24 | |||||||||||||||||||||||||||||||||||||||||||
Class B ordinary shares | 0.24 | |||||||||||||||||||||||||||||||||||||||||||
Ordinary shares | (2.53 | ) | (0.64 | ) | (1.25 | ) | ||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||
Pro Forma Adjustments to the Unaudited Condensed Combined Statement of Profit or Loss
The adjustments included in the unaudited condensed combined statement of profit or loss for the year ended December 31, 2021 are as follows:
IFRS Policy and Presentation Alignment
| (1) | Certain presentation differences exist in the line items included within the historical NewAmsterdam Pharma statement of profit or loss when compared to the historical FLAC statement of operations. The following presentation differences exist between the historical FLAC statement of operations and the FLAC historical information included within the pro forma condensed combined statement of profit or loss. |
| • | Administrative expenses – related party have been combined with general and administrative expenses to be presented as one figure; |
| • | Loss from operations is shown as total operating expenses; |
251
Table of Contents
| • | Loss before tax is calculated above, but is not included within the historical FLAC statement of operations. The amount is equal to FLAC’s historical net income as there is no income tax presented within the historical FLAC statement of operations; and |
| • | Net income is shown as loss for the period. |
| (2) | The historical financial information of FLAC was prepared in accordance with U.S. GAAP and presented in USD. The historical financial information was translated from USD to EUR using the average exchange rate over the period from January 1, 2021 through December 31, 2021, of $1.1830 per EUR. |
Transaction Accounting Adjustments
| (3) | Reflects the removal of interest income from investments held in the Trust Account. |
| (4) | Reflects adjustments to general and administrative expenses based upon Scenario 1 for the following items: |
| a. | The portion of expected transaction costs incurred after December 31, 2021 in connection with the Business Combination which are expensed. Costs which qualify for capitalization are allocated among all Holdco Shares issued with the portion related to those shares issued to former NewAmsterdam Pharma shareholders being expensed. Under this allocation method 52% and 61% of qualifying costs are expensed in Scenario 1 and Scenario 2, respectively, as seen in adjustment 5d and 13b in the unaudited condensed combined statement of financial position included above. This expense is not expected to have a continuing impact on the combined results. |
| b. | Adjustment in accordance with IFRS 2 for the excess of the value of Holdco Shares issued in exchange for the outstanding equity of FLAC over the net identifiable assets acquired. This results in a charge of (i) €5.0 million in Scenario 1 and (ii) €41.6 million in Scenario 2, resulting in an incremental expense of €36.6 million. See adjustment 6c to the pro forma condensed combined statement of financial position above for details regarding the calculation of the share-based compensation expense for the listing cost. This expense is not expected to have a continuing impact on the combined results. |
| c. | Expense related expected annual premium for additional D&O insurance which must be obtained according to the terms of the Business Combination Agreement. |
The table below set forth the amounts for each item described and the total general and administrative expense adjustment amount:
In EUR thousands | ||||
Portion of transaction costs incurred after December 31, 2021 which is expensed | (20,120 | ) | ||
Expense arising under IFRS 2 for the excess of the fair value of shares issued to FLAC stockholders over and above the fair value of FLAC’s identifiable net assets | (5,040 | ) | ||
Additional expense for expected D&O insurance costs | (5,000 | ) | ||
|
| |||
Total General and Administrative Expense Adjustment – Scenario 1 | (30,160 | ) | ||
|
| |||
| (5) | Reflects additional adjustments to general and administrative expenses based upon Scenario 2 for the following items: |
| a. | The portion of expected transaction costs incurred after December 31, 2021 in connection with the Business Combination which are expensed. Costs which qualify for capitalization are allocated among all Holdco Shares issued with the portion related to those shares issued to former |
252
Table of Contents
| NewAmsterdam Pharma shareholders being expensed. Under this allocation method 52% and 61% of qualifying costs are expensed in Scenario 1 and Scenario 2, respectively, as seen in adjustment 5d and 13b in the unaudited condensed combined statement of financial position included above. This expense is not expected to have a continuing impact on the combined results. |
| b. | Additional adjustment in accordance with IFRS 2 for the excess of the value of Holdco Shares issued in exchange for the outstanding equity of FLAC over the net identifiable assets acquired. This results in a charge of (i) €5.0 million in Scenario 1 and (ii) €41.6 million in Scenario 2, resulting in an incremental expense of €36.6 million. See adjustment 6c to the pro forma condensed combined statement of financial position above for details regarding the calculation of the share-based compensation expense for the listing cost. This expense is not expected to have a continuing impact on the combined results. |
The table below set forth the amounts in Scenario 2 for each item described and the additional general and administrative expense adjustment amount:
In EUR thousands | ||||
Adjustment to the allocation between expense and share capital of qualifying transaction costs | (695 | ) | ||
Incremental expense under IFRS 2 for the listing service | (36,611 | ) | ||
|
| |||
Additional General and Administrative Expense Adjustment – Scenario 2 | (37,306 | ) | ||
|
| |||
The unaudited pro forma condensed combined financial information for both Scenario 1 and 2 does not reflect the income tax effects of the pro forma adjustments based on the statutory rate in effect for the historical periods presented given the combined entity has incurred significant losses since inception and does not expect to generate taxable income for the foreseeable future, including in fiscal year 2022.
253
Table of Contents
PRO FORMA CONDENSED COMBINED STATEMENT OF PROFIT OR LOSS
FOR THE SIX MONTHS ENDED JUNE 30, 2022
(UNAUDITED)
(in EUR thousands unless otherwise denoted)
| Scenario 1: No Redemption Scenario | Scenario 2: Maximum Redemption Scenario | |||||||||||||||||||||||||||||||||||||||||||
| NewAmsterdam Pharma Historical IFRS | FLAC Historical (1) U.S. GAAP | IFRS Policy and Presentation Alignment | Transaction Accounting Adjustments | Pro Forma Combined | Additional Transaction Accounting Adjustments | Pro Forma Combined | ||||||||||||||||||||||||||||||||||||||
| USD | EUR(2) | FN | FN | FN | ||||||||||||||||||||||||||||||||||||||||
Revenue | 93,500 | — | — | — | — | 93,500 | — | 93,500 | ||||||||||||||||||||||||||||||||||||
Research and development expenses | (30,588 | ) | — | — | — | — | (30,588 | ) | — | (30,588 | ) | |||||||||||||||||||||||||||||||||
General and administrative expenses | (9,294 | ) | (2,335 | ) | (2,137 | ) | — | 1,451 | (4 | ) | (9,980 | ) | — | (9,980 | ) | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Total operating expenses | (39,882 | ) | (2,335 | ) | (2,137 | ) | — | 1,451 | (40,568) | — | (40,568 | ) | ||||||||||||||||||||||||||||||||
Finance income | 10 | — | — | — | — | 10 | — | 10 | ||||||||||||||||||||||||||||||||||||
Finance expense | (185 | ) | — | — | — | — | (185 | ) | — | (185 | ) | |||||||||||||||||||||||||||||||||
Interest income from investments held in Trust Account | — | 116 | 106 | — | (106 | ) | (3 | ) | — | — | — | |||||||||||||||||||||||||||||||||
Change in fair value of derivative warrant liabilities | — | 2,431 | 2,225 | — | — | 2,225 | — | 2,225 | ||||||||||||||||||||||||||||||||||||
Break-up fee from terminated agreement | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Net foreign exchange gain | 1,070 | — | — | — | — | 1,070 | — | 1,070 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Profit before tax | 54,513 | 212 | 194 | — | 1,345 | 56,052 | — | 56,052 | ||||||||||||||||||||||||||||||||||||
Income tax expense | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Profit for the period | 54,513 | 212 | 194 | — | 1,345 | 56,052 | — | 56,052 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Attributable to: | ||||||||||||||||||||||||||||||||||||||||||||
Equity holders of the Company | 54,513 | 212 | 194 | — | 1,345 | 56,052 | — | 56,052 | ||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Earnings / (loss) per share: | ||||||||||||||||||||||||||||||||||||||||||||
Class A ordinary shares – basic and diluted | 0.01 | |||||||||||||||||||||||||||||||||||||||||||
Class B ordinary shares – basic and diluted | 0.01 | |||||||||||||||||||||||||||||||||||||||||||
Ordinary shares – Basic | 3.20 | 0.65 | 0.76 | |||||||||||||||||||||||||||||||||||||||||
Ordinary shares – Diluted | 2.87 | 0.62 | 0.72 | |||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
The adjustments included in the unaudited condensed combined statement of profit or loss for the six months ended June 30, 2022 are as follows:
IFRS Policy and Presentation Alignment
| (1) | Certain presentation differences exist in the line items included within the historical NewAmsterdam Pharma statement of profit or loss when compared to the historical FLAC statement of operations. The following presentation differences exist between the historical FLAC statement of operations and the FLAC historical information included within the pro forma condensed combined statement of profit or loss. |
| • | Administrative expenses – related party have been combined with general and administrative expenses to be presented as one figure; |
| • | Loss from operations is shown as total operating expenses; |
| • | Loss before tax is calculated above, but is not included within the historical FLAC statement of operations. The amount is equal to FLAC’s historical net income as there is no income tax presented within the historical FLAC statement of operations; and |
254
Table of Contents
| • | Net income is shown as loss for the period. |
| (2) | The historical financial information of FLAC was prepared in accordance with U.S. GAAP and presented in USD. The historical financial information was translated from USD to EUR using the average exchange rate over the period from January 1, 2022 through June 30, 2022, of $1.0929 per EUR. |
Transaction Accounting Adjustments
| (3) | Reflects the removal of interest income from investments held in the Trust Account. |
| (4) | Reflects adjustments to general and administrative expenses for the following items: |
| a. | Expense related expected annual premium for additional D&O insurance which must be obtained according to the terms of the Business Combination Agreement. |
| b. | Elimination of transaction costs expensed in the current period which are recognized in the transaction accounting adjustments within the pro forma income statement for the year ended December 31, 2021. |
The table below set forth the amounts for each item described and the total general and administrative expense adjustment amount:
| In EUR thousands | ||||
Additional expense for expected D&O insurance costs | (2,500 | ) | ||
Elimination of transaction costs incurred in the current period | 3,951 | |||
|
| |||
Total General and Administrative Expense Adjustment | 1,451 | |||
|
| |||
The unaudited pro forma condensed combined financial information for both Scenario 1 and 2 does not reflect the income tax effects of the pro forma adjustments based on the statutory rate in effect for the historical periods presented given the combined entity has incurred significant losses since inception and does not expect to generate taxable income for the foreseeable future, including in fiscal year 2022.
Net Earnings/(Loss) Per Share
Represents the net loss per share calculated using the historical weighted average shares outstanding, and the issuance of additional shares in connection with the Business Combination, assuming the shares were outstanding since January 1, 2021. As the Business Combination and related equity transactions are being reflected as if they had occurred at the beginning of the periods presented, the calculation of weighted average shares outstanding for basic and diluted net income (loss) per share assumes that the shares issuable relating to the Business Combination have been outstanding for the entirety of the period presented. If the maximum number of shares are redeemed, this calculation is retroactively adjusted to eliminate such shares for the entire period.
255
Table of Contents
The unaudited pro forma condensed combined financial information has been prepared assuming two alternative levels of redemption into cash of FLAC Class A Ordinary Shares for the year ended December 31, 2021 and the six months ended June 30, 2022:
| Year ended December 31, 2021 | ||||||||
| No Redemption Scenario | Maximum Redemption Scenario | |||||||
Net loss attributable to equity holders of the company (in EUR thousands) | (55,421 | ) | (92,547 | ) | ||||
Basic and diluted pro forma weighted average number of shares outstanding(1) | 86,125,642 | 73,864,160 | ||||||
Net loss per share attributable to equity holders of the company, basic and diluted | (0.64 | ) | (1.25 | ) | ||||
| (1) | Excludes 1,886,137 Earnout Shares, 4,185,358 Holdco Options and 4,600,000 and 167,000 Public Warrants and Private Warrants, respectively. |
| Six months ended June 30, 2022 | ||||||||
| No Redemption Scenario | Maximum Redemption Scenario | |||||||
Earnings attributable to equity holders of the company (in EUR thousands) | 56,052 | 56,052 | ||||||
Basic pro forma weighted average number of shares outstanding1 | 86,125,642 | 73,864,160 | ||||||
Diluted pro forma weighted average number of shares outstanding2 | 89,791,921 | 77,530,439 | ||||||
Earnings per share attributable to equity holders of the company, basic | 0.65 | 0.76 | ||||||
Earnings per share attributable to equity holders of the company, diluted | 0.62 | 0.72 | ||||||
| (1) | Excludes 1,886,137 Earnout Shares, 4,185,358 Holdco Options and 4,600,000 and 167,000 Public Warrants and Private Warrants, respectively. |
| (2) | Excludes 1,886,137 Earnout Shares and 4,600,000 and 167,000 Public Warrants and Private Warrants, respectively. |
256
Table of Contents
BUSINESS OF HOLDCO BEFORE THE BUSINESS COMBINATION
The information provided below pertains to the business of Holdco before the Business Combination. As of the date of this proxy statement/prospectus, Holdco has not conducted any material activities other than those incident to its formation and to the matters contemplated by the Business Combination Agreement and the Subscription Agreements, such as the making of certain required securities law filings, the establishment of Merger Sub and the preparation of this proxy statement/prospectus. Upon the terms and subject to the conditions of the Business Combination Agreement, FLAC and NewAmsterdam Pharma will effect a transaction, the result of which Holdco will become the ultimate parent of NewAmsterdam Pharma and FLAC. For information about Holdco’s management, stock ownership and corporate governance following the Business Combination, please see the section entitled “Management of Holdco Following the Business Combination.”
Incorporation
Holdco was incorporated as a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) on June 10, 2022 with an issued share capital of EUR 0.12. Prior to consummation of the Business Combination, the Holdco General Meeting of shareholders will resolve to convert Holdco’s corporate form into a Dutch public limited liability company (naamloze vennootschap). To date, Holdco has not conducted any material activities other than those incident to its formation and the pending Business Combination and only has nominal assets consisting of cash and its interest in Merger Sub.
Articles of Association
Prior to or promptly following the consummation of the Business Combination, Holdco’s current articles of association will be amended and restated in their entirety to be in the form of the Holdco Articles of Association contemplated by the Business Combination Agreement and attached as an English translation of the official Dutch text as Annex I to this proxy statement/prospectus. Holdco’s current articles of association may be amended in accordance with its terms at any time prior to consummation of the Business Combination, with the consent of FLAC, or at any time after consummation of the Business Combination. Please see the section entitled “Description of Holdco Securities.”
Name
Holdco is registered with the Commercial Register of the Netherlands Chamber of Commerce under the registration number 86649051 and the legal name NewAmsterdam Pharma Company B.V. Prior to or promptly following the consummation of the Business Combination, Holdco’s legal name will be changed to NewAmsterdam Pharma Company N.V. as a result of the amendment of Holdco’s articles of association.
Official Seat
Holdco’s official seat (statutaire zetel) is in Naarden, the Netherlands and its business address is Gooimeer 2-35, 1411 DC Naarden, The Netherlands. The mailing address of Holdco’s principal executive office after the closing of the Business Combination will be at Gooimeer 2-35, 1411 DC Naarden, The Netherlands.
Financial Year
Holdco’s financial year is currently the calendar year. In connection with the Business Combination, NewAmsterdam Pharma and FLAC may change the fiscal year of Holdco.
Subsidiaries
Merger Sub, a newly incorporated Cayman Islands exempted company, is a wholly owned subsidiary of Holdco. As of the date of this proxy statement/prospectus, Merger Sub has not conducted any material activities other than those incident to its formation and to the matters contemplated by the Business Combination Agreement.
257
Table of Contents
Sole Shareholder
NewAmsterdam Pharma is currently the sole shareholder of Holdco. NewAmsterdam Pharma shareholders will become shareholders of Holdco pursuant to the Exchange. In connection with the Business Combination, FLAC shareholders will become shareholders of Holdco pursuant to the Merger.
Management Board
Holdco is currently managed by a board with one director. Currently, the director of Holdco is LouFré Management B.V.
Legal Proceedings
As of the date of this proxy statement/prospectus, Holdco was not party to any material legal proceedings. In the future, Holdco may become party to legal matters and claims arising in the ordinary course of business, the resolution of which Holdco does not anticipate would have a material adverse impact on its financial position, results of operations or cash flows.
Properties
Holdco currently does not own or lease any physical property.
Employees
Holdco currently has no employees.
258
Table of Contents
BUSINESS OF NEWAMSTERDAM PHARMA AND CERTAIN INFORMATION ABOUT NEWAMSTERDAM PHARMA
Unless the context otherwise requires, any reference in the below section of this proxy statement/prospectus to“we,” “our,” and “us” refer to NewAmsterdam Pharma, together with its subsidiaries prior to the consummation of the Business Combination; and to Holdco following the consummation of the Business Combination.
Overview
We are a clinical-stage biopharmaceutical company developing oral, non-statin medicines for patients at high risk of cardiovascular disease (“CVD”) with residual elevation of low-density lipoprotein cholesterol (“LDL-C” or “LDL”), for whom existing therapies are not sufficiently effective or well-tolerated. There exists a significant unmet need for a potent, cost-effective and convenient LDL-lowering therapy as an adjunct to statins, a class of lipid-lowering medications that are the current standard of care for high-risk CVD patients with high cholesterol. Our lead product candidate, obicetrapib, is a next-generation, oral, low-dose cholesteryl ester transfer protein (“CETP”) inhibitor, that is currently in four ongoing Phase 3 and Phase 2b clinical trials as both a monotherapy and a combination therapy with ezetimibe for lowering LDL-C and preventing major adverse cardiovascular events (“MACE”).
CVD is a leading cause of death worldwide and the top cause of death in the United States. ASCVD is primarily caused by atherosclerosis, which involves the build-up of fatty material within the inner walls of the arteries. Atherosclerosis is the primary cause of heart attacks, strokes and peripheral vascular disease. One of the most important risk factors for ASCVD is hypercholesterolemia, which refers to elevated LDL-C levels within the body, commonly known as high cholesterol.
A significant proportion of patients with high cholesterol do not achieve acceptable LDL-C levels using statins alone. We estimate that there are more than 30 million patients in the United Kingdom, Germany, France, Spain and Italy (collectively, “EU5”) and in the United States who are not achieving LDL-lowering goals on the current standard of care. Existing non-statin treatment options have been largely unable to address the needs of patients with high cholesterol due to modest efficacy, prohibitive pricing, or an inconvenient and painful injectable administration route. It is estimated that over 75% of ASCVD outpatients prefer oral drugs to injectable therapies.
Our product candidate, obicetrapib, is a next-generation, oral, low-dose CETP inhibitor that we are developing to potentially overcome the limitations of current LDL-lowering treatments. We believe that obicetrapib has the potential to be a once-daily oral CETP inhibitor for lowering LDL-C, if approved. In our Phase 2b ROSE trial, obicetrapib demonstrated a 51% lowering of LDL-C from baseline at a 10 mg dose level on top of high-intensity statins. In all three of our Phase 2 trials, TULIP, ROSE and OCEAN, evaluating obicetrapib as a monotherapy or a combination therapy, we observed statistically significant LDL-lowering activity combined with generally moderate side effects and no drug-related, treatment-emergent serious adverse events (“AEs”). Obicetrapib has demonstrated strong tolerability in more than 600 patients with low or elevated lipid levels (“dyslipidemia”) in our clinical trials to date. Obicetrapib is also expected to be relatively low in cost to manufacture compared to most other branded LDL-lowering therapies on the market with high efficacy. We believe that the estimated low cost of goods for obicetrapib will enable favorable pricing and position it to significantly improve patient access to a high efficacy LDL-lowering therapy compared to existing non-statin treatments. Furthermore, we believe that obicetrapib’s oral delivery, demonstrated activity in low doses, chemical properties and tolerability make it well-suited for combination approaches. We are developing a fixed dose combination of obicetrapib 10 mg and ezetimibe 10 mg, which we believe will demonstrate even greater potency.
Lowering of LDL-C, and particularly ApoB-containing lipoproteins, has been associated with MACE benefit in trials of LDL-lowering drugs, including the REVEAL study with the CETP inhibitor, anacetrapib. We are performing a CVOT to reconfirm this relationship.
259
Table of Contents
Our goal is to develop and commercialize a LDL-lowering monotherapy and combination therapy, which offers the cost and convenience advantages of a once-daily oral pill, and fulfills the significant unmet need for an effective and convenient LDL-lowering therapy. If we obtain marketing approval, we intend to commercialize obicetrapib for patients suffering from CVD independently and with strategic partners in certain jurisdictions. We have partnered with A. Menarini International Licensing S.A., part of Menarini Group (“Menarini”), to provide them with the exclusive rights to commercialize obicetrapib 10 mg either as a sole active ingredient product or in a fixed dose combination with ezetimibe in the majority of European countries, if approved. Subject to receipt of marketing approval, our current plan is to pursue development and commercialization of obicetrapib in the United States ourselves, and to consider additional partners for jurisdictions outside of the United States and the European Union (“EU”), including in Japan, China and the United Kingdom.
The following table summarizes our current clinical programs:
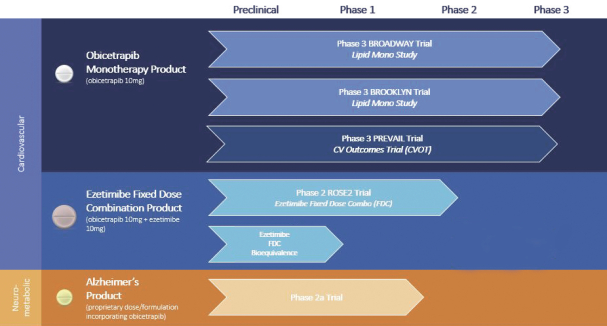
We are conducting two Phase 3 pivotal trials, BROADWAY and BROOKLYN, to evaluate obicetrapib as a monotherapy used as an adjunct to maximally tolerated lipid-lowering therapies to potentially enhance LDL-lowering for high-risk CVD patients. We began enrolling patients in BROADWAY in January 2022 and in BROOKLYN in July 2022. We also commenced our Phase 3 PREVAIL CVOT in March 2022, which is designed to assess the potential of obicetrapib to reduce occurrences of MACE, including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and non-elective coronary revascularization. We expect to report data from our Phase 3 BROADWAY trial and our Phase 3 BROOKLYN trial in 2024. We expect to report data from our Phase 3 PREVAIL CVOT in 2026 and from our Phase 2b ROSE2 trial in 2023. We also expect to report data from our Phase 2 dose-finding study of obicetrapib as an adjunct to stable statin therapy in patients with dyslipidemia in Japan and our Phase 2a clinical trial evaluating obicetrapib in patients with early Alzheimer’s disease in 2023.
We plan to seek approval of obicetrapib in the United States, the EU, Japan, China and the United Kingdom. We are executing multiple Phase 3 trials simultaneously, with clinical plans that incorporate feedback from the FDA, the EMA, PMDA and NMPA, including our Phase 3 BROADWAY trial and PREVAIL CVOT, which both launched in the first quarter of 2022.
We are also investigating obicetrapib as a fixed dose combination with ezetimibe, a non-statin oral LDL-lowering therapy, and plan to seek approval for this fixed dose combination in parallel with obicetrapib monotherapy. In our Phase 2b ROSE2 trial, we are evaluating the efficacy and safety of obicetrapib plus
260
Table of Contents
ezetimibe compared to obicetrapib and placebo alone. A fixed dose combination tablet will be evaluated in two bioequivalence studies a pilot bioequivalence study and a potentially pivotal bioequivalence study. In the pilot bioequivalence study, which we commenced in September 2022, the bioavailability of two fixed dose combination formulations are being compared to the concomitant administration of obicetrapib and ezetimibe. After the pilot bioequivalence study, which we expect to complete in the second half of 2023, one final fixed dose combination formulation will be selected for a potentially pivotal bioequivalence study, to evaluate whether the fixed dose combination is bioequivalent to the concomitant administration of obicetrapib and ezetimibe. In parallel to the potentially pivotal bioequivalence study, a 12-week, phase 3 efficacy and safety study will be conducted with the fixed dose combination. This study will compare the safety and efficacy of the fixed dose combination compared to placebo, obicetrapib and ezetimibe. We expect that both the potentially pivotal bioequivalence study and the phase 3 efficacy and safety study, along with the PREVAIL CVOT study, will be described in the fixed dose combination product label, if approved.
We believe that CETP inhibition may also play a role in other indications by potentially mitigating the risk of developing diseases such as Alzheimer’s disease or diabetes. Evidence suggests that cholesterol accumulation in the brain is a precursor to Alzheimer’s disease. For example, rodents lack the CETP gene and are resistant to Alzheimer’s disease. In early preclinical studies, when the human CETP gene is knocked into a mouse, the cholesterol content of the mouse brain was observed to increase by 25% and when combined with the gene for the amyloid precursor protein, hypothesized to be a driver of Alzheimer’s disease, the risk of developing Alzheimer’s disease may greatly increase. In a preclinical study, we observed that CETP inhibition promoted cholesterol removal from the brain and improved cognition. We commenced a Phase 2a trial in early 2022 in patients with early Alzheimer’s disease and the apolipoprotein E4 (“ApoE4”) mutation to evaluate the pharmacodynamic and pharmacokinetic effects, safety and tolerability of obicetrapib. Clinically demonstrated anti-diabetic benefits have been observed with CETP inhibition in Phase 3 CVOTs that, if seen in obicetrapib, would differentiate it from current treatment alternatives, especially statins. We are planning preclinical studies to examine the potential of obicetrapib for patients suffering from diabetes and have included the onset of diabetes as an endpoint in our PREVAIL CVOT, as measured by AEs indicating Type 2 diabetes, initiation of anti-diabetes medication after confirmed diabetes diagnosis or high levels of hemoglobin A1c and fasting plasma glucose.
Our Management Team and Investors
We are led by a world-class team of industry veterans, including some of the world’s preeminent cardiometabolic experts. Dr. Michael Davidson, our Chief Executive Officer and a member of our Executive Board, is a leading expert in the field of lipidology and is a seasoned executive who served as founder and Chief Executive Officer of Corvidia Therapeutics, Inc. and founder and Chief Medical Officer of Omthera Pharmaceuticals, Inc. In addition, Dr. Davidson is board-certified in internal medicine, cardiology and clinical lipidology and has extensive experience designing, managing and evaluating clinical research. Dr. John Kastelein, our founder and Chief Scientific Officer and a member of our Executive Board, is Emeritus Professor of Medicine at the Department of Vascular Medicine at the Academic Medical Center of the University of Amsterdam. Dr. Kastelein was a co-founder of uniQure N.V. and Xenon Pharmaceuticals Inc. His clinical research, on the development of novel therapies for CVD and the genetic basis of dyslipidemia is widely published, and he serves as the Chief Executive Officer of the Vascular Research Network, a site maintenance organization comprising dozens of hospitals in the Netherlands that are involved in clinical trials for cardiometabolic disease. Douglas Kling, our Chief Operating Officer, is an expert in the development of drugs to treat dyslipidemia and CVD, and has managed clinical operations at both Corvidia Therapeutics, Inc. and Omthera Pharmaceuticals, Inc.
In addition, we are backed by leading life sciences investors, including Forbion and Morningside Ventures. Prospective investors should not rely on the past investment decisions of our investors, as our investors may have different risk tolerances and may have received their shares in prior offerings at a significant discount to the market price. See “Certain Relationships and Related Person Transactions—Holdco Relationships and Related Person Transactions” for more information.
261
Table of Contents
Cardiovascular Disease and Hyperlipidemia
Market Overview
According to the World Health Organization, CVD is a leading cause of death globally and was responsible for approximately 19.05 million deaths, or approximately 32% of all global deaths, in 2020. Hyperlipidemia, more commonly known as high cholesterol, has been observed to nearly double the risk of developing CVD compared to those with normal total cholesterol levels. In the United States alone, approximately 38% of adults have high cholesterol, defined as total blood cholesterol equal to or exceeding 200 mg/dL. One in 311 individuals globally are affected by genetically elevated cholesterol levels from birth, and the average age of diagnosis is approximately 44 years globally, reflecting decades of high cholesterol and resulting damage to the blood vessels.
Hypercholesterolemia underlies significant morbidity and mortality. In the United States and EU5, there are an estimated 231 million adults with hypercholesterolemia, of which we estimate 84 million are currently being treated with prescription medications. Despite the broad availability of a variety of statins capable of significantly reducing LDL-C, many patients are unable to effectively manage their cholesterol and reach their LDL-lowering goals using statins alone and therefore require additional treatment options.
We estimate that there are approximately 30 million patients in the United States and EU5 who are not achieving LDL-lowering goals on current standard of care. While there are several non-statin treatment options available, including ezetimibe, bempedoic acid (Nexletol/Nexlizet) and injectable PCSK9 inhibitors, less than one million patients are currently being treated with these medications due to product limitations relating to relatively low efficacy, side effects or market access hurdles. We believe that a potent, cost-effective, convenient, safe and well-tolerated low-dose oral medication to reduce LDL-C could fulfill this unmet need. Based on internal company estimates derived from primary market research and secondary literature, we believe that such a medication could represent a global peak annual sales potential of three to four billion dollars.
Unmet Medical Need
Elevation in LDL-C is a primary causal factor for ASCVD, and CVD outcomes in high-risk populations improve as the level of LDL-C achieved on therapy decreases. LDL-C is generally understood to be one of the most modifiable risk factors of ASCVD. Results from the Cholesterol Treatment Trialists’ Collaboration have shown that lowering LDL using statin therapy reduces the risk of major vascular events (heart attacks, stroke or coronary revascularization procedures) by approximately 22% for each 40 mg/dL (one mmol/L) reduction in LDL-C achieved. A similar relationship has also been documented in non-statin CVOTs for ezetimibe, two PCSK9 inhibitors, evolocumab and alirocumab, and CETP inhibitor, anacetrapib. These trials have provided evidence that absolute LDL-C reduction and duration of therapy form a consistent model for predicting improved outcomes in patients with established ASCVD.
Despite the broad consensus regarding the causality of LDL-C for ASCVD and the CVD benefits of lipid-lowering therapies, there remains a significant clinical need for improved therapeutic regimens to achieve the goals stated by the American Heart Association (“AHA”) and American College of Cardiology (“ACC”) and other expert panel guidelines for lowering blood cholesterol. Although multiple classes of LDL-lowering therapies are available, there were 4.5 million deaths attributable to high LDL-C worldwide in 2020, representing a 19% increase in total number of deaths compared with 2010, based on the 2022 AHA Heart Disease and Stroke Statistical Update.
Multiple surveys have demonstrated that approximately seven out of ten patients who have been prescribed statin therapy have not reached their LDL-C goals. In a recent observational registry study in patients with ASCVD enrolled between 2016 and 2018 who primarily used statin monotherapies, only one in three patients achieved the AHA/ACC guideline-recommended LDL-C concentration of lower than 70 mg/dL.
We believe an improved LDL-C lowering therapy, particularly a well-tolerated oral therapy that may be preferred by patients, is necessary to achieve the targets established by expert panels. Patients who are at high risk for future CVD and are unable to achieve their LDL-C goals despite current standard of care are in need for an alternative oral therapy.
262
Table of Contents
Our Solution: Enhanced LDL-C Lowering Through CETP Inhibition with Obicetrapib
We believe CETP inhibition with obicetrapib has the potential, if approved, to provide patients and physicians with a new oral therapy option to robustly reduce LDL-C. Obicetrapib is designed to be a next-generation, oral, low-dose CETP inhibitor with powerful LDL-lowering capability, with the potential to be delivered at a low cost to patients and payors compared with other adjuncts to statin therapy. We are developing obicetrapib as both a monotherapy and a combination therapy with ezetimibe, and have structured our obicetrapib program to overcome the safety, potency, trial design and commercial viability limitations of prior CETP inhibitors. Further, we believe that obicetrapib’s oral delivery, demonstrated activity in low doses, chemical properties and potential tolerability make it well-suited for combination approaches.
Obicetrapib has intrinsic properties, such as ionizable features, substantially reduced lipophilicity, good solubility and neutral pH, that we believe give it more favorable properties as a drug candidate compared to prior CETP inhibitors. We have observed a strong tolerability profile for obicetrapib in an aggregate of over 600 patients with dyslipidemia from Phase 1 through Phase 2b clinical trials. We are conducting two Phase 3 pivotal trials, BROADWAY and BROOKLYN, to evaluate obicetrapib as a monotherapy used as an adjunct to maximally tolerated lipid-lowering therapies to potentially enhance LDL-lowering for high-risk CVD patients. We began enrolling patients in BROADWAY in January 2022 and in BROOKLYN in July 2022. We also commenced our Phase 3 PREVAIL CVOT in March 2022, which is designed to assess the potential of obicetrapib to reduce occurrences of MACE, including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and non-elective coronary revascularization. We are also investigating obicetrapib as a fixed dose combination with ezetimibe in our Phase 2b ROSE2 trial, and plan to seek approval for this fixed dose combination in parallel with obicetrapib monotherapy. We are also conducting a Phase 2 dose-finding study of obicetrapib as an adjunct to stable statin therapy in patients with dyslipidemia in Japan.
We believe obicetrapib has the potential to significantly impact the existing treatment paradigm for patients with high cholesterol contributing to CVD, and that the key differentiating attributes of our product candidate include the following:
| • | Enhanced LDL-C reduction capability. We believe obicetrabib’s physical, pharmacokinetic and biopharmaceutical properties position it to potentially demonstrate more favorable potency and enhanced LDL-lowering capability than previous CETP inhibitors. For high-risk patient groups with high cholesterol levels that are not adequately controlled on statins, obicetrapib has been observed in clinical trials to lower LDL-C both as a monotherapy and a combination therapy with an approved cholesterol-lowering medication. In our Phase 2b ROSE clinical trial, we observed a median LDL-C reduction capability of 51% in patients treated with 10 mg obicetrapib on top of high-intensity statins. |
| • | Promising tolerability profile. Patients are often non-compliant with existing cholesterol-lowering therapies, particularly statins, due to their side effect profiles, which results in suboptimal treatment outcomes and disease progression. In all three of our Phase 2 clinical trials of obicetrapib, we observed statistically significant LDL-lowering activity combined with generally moderate side effects and no drug-related, treatment-emergent serious AEs. In addition, clinically demonstrated anti-diabetic benefits have been observed with CETP inhibition in Phase 3 CVOTs, that, if seen in obicetrapib, could make it a potentially attractive adjunct for patients who are concerned about the risks of diabetes associated with statin therapy. |
| • | Convenience. We believe that obicetrapib’s simple once-daily, low-dose oral formulation can improve patient adherence, thereby amplifying its cholesterol-lowering impact. Additionally, unlike injectable PCSK9 inhibitors, obicetrapib can be combined with other treatments. |
| • | Low cost of goods and patient access. We expect that the estimated low cost of goods sold for obicetrapib will enable favorable pricing compared to other drugs on the market. In addition, payor confidence is essential to ensuring access for patients. Based on the LDL-lowering activity of obicetrapib, we believe payors will perceive the LDL-lowering capability of obicetrapib to be on par with PCSK9 inhibitors, which are administered by injection, and to exceed the LDL-lowering |
263
Table of Contents
capabilities of other existing oral therapies, and will ultimately prefer obicetrapib to existing treatment alternatives. |
| • | Effect on other predictors of disease risk. Like other types of LDL-lowering therapies, i.e. statins and PCSK9 inhibitors, CETP inhibition enhances the removal of apolipoprotein B (“ApoB”), a protein found in lipoprotein particles that contributes to atherosclerosis. However, unlike statins, based on observations from our Phase 2 clinical trials, obicetrapib also decreases the presence of lipoprotein(a) (“Lp(a)”), an important biomarker for CVD risk reduction. |
Lowering LDL-C Through CETP Inhibition
Hyperlipidemia, and in particular hypercholesterolemia or high cholesterol, is a major risk factor for atherosclerosis, which involves the build-up of fatty material within the inner walls of the arteries. This is because LDL-C is a “package” of cholesterol contained within a particle that contains ApoB. ApoB is prone to becoming trapped in the walls of arteries, leading to a build-up of fatty material, which in turn elicits a pro-inflammatory response and causes the arterial walls to stiffen. Left untreated, these deposits of fatty material can suddenly break and form a clot that causes a heart attack or a stroke. Lp(a) functions in the circulation as a “sink” for oxidized phospholipids and is also prone, like ApoB, to become trapped in the arterial wall, attached to proteoglycans of the extracellular matrix. Subsequently, these particles build up and contribute to plaque formation and inflammation. Because of the tendency of LDL-C to build up in the arteries, LDL-C is often referred to as “bad cholesterol” and is a leading risk factor for CVD.
LDL-C and ApoB levels are mainly regulated by the liver through a surface protein known as the LDL receptor. Most current LDL-lowering therapies work by increasing the number of LDL receptors, and thereby increasing the clearance of LDL-C from the blood. Statins are the current standard of care for high-risk CVD patients with high cholesterol. Statins reduce cholesterol in the blood by blocking a key enzyme, HMG-CoA-Reductase, necessary for the synthesis of cholesterol, which reduces the amount of cholesterol made by the liver and upregulates the LDL receptor, resulting in lower blood cholesterol. PCSK9 inhibitors, another LDL-lowering treatment alternative, also increase the presence of LDL receptors by inhibiting proprotein convertase subtilisin/kexin type 9 (“PCSK9”), an enzyme involved in the degradation of LDL receptors. Two other LDL-lowering therapies, ezetimibe and Nexletol/Nexlizet, also work by upregulating LDL receptors.
CETP is a plasma glycoprotein produced in the liver that circulates in the blood primarily bound to a high-density lipoprotein cholesterol (“HDL-C” or “HDL”) particle which can also attach to an LDL particle and form a bridge to transfer cholesterol from HDL to LDL, as shown in the figure below. CETP inhibitors, including obicetrapib, reduce LDL-C and increase HDL-C in a two-step process. In the first step, CETP inhibitors obstruct the transfer of cholesterol from HDL to LDL, immediately reducing the concentration of LDL-C in the body and increasing the concentration of HDL-C. This change in concentrations of lipid particles then produces the second step of causing the liver to produce more LDL receptors. The LDL receptor increase results in more LDL-C particles and ApoB being cleared from the bloodstream to be excreted by the digestive tract. In animal models, CETP inhibition has been shown to block the transfer of cholesterol from HDL to LDL and to upregulate LDL receptors, thereby reducing the development of atherosclerosis and risk of ASCVD.
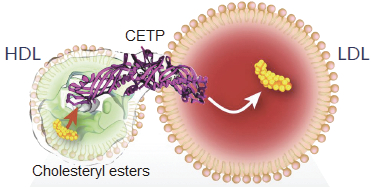
264
Table of Contents
Although it was previously believed that the HDL-raising effects of CETP inhibition would be its primary contributor to decreased CVD risk, LDL-reduction is now known to be the most significant factor for lowering CVD risk. In a population with CETP loss of function genotypes, a 16% reduction in CVD risk was observed for every 10 mg/dL decrease in LDL-C levels. The relationship between genomic loss of CETP function and lower LDL-C levels and CVD risk is consistent with other mechanisms of genomic LDL-C reduction such as HMG-CoA reductase (statins), NPC1L1 (ezetimibe), ATP-citrate lyase (Nexletol/Nexlizet) and PCSK9 (PCSK9 inhibitors). Multiple genetic studies provide support that specific mutations associated with lifelong lower LDL-C levels reduce the risk of CVD.
CETP inhibition also increases the removal of ApoB. Apolipoproteins are proteins that are involved in packaging different types of large lipid-particle complexes that store cholesterol in the body. As shown in the figure below, the primary effect of CETP inhibition is a reduced rate of transfer of cholesteryl esters from HDL into triglyceride-rich lipoproteins, including LDL, which in turn leads to an increased concentration of cholesteryl esters in HDL and the formation of larger HDL particles. Consequently, cholesteryl esters are depleted in LDL and other ApoB-containing lipoproteins, enhancing their clearance from the body.
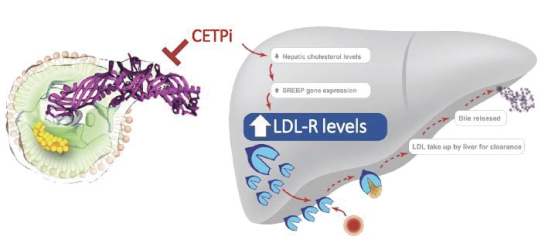
Limitations of Current Non-Statin Therapies
Increased attention by physicians to aggressive LDL-lowering for high-risk CVD patients has led to the increased use of non-statin medicines with LDL-lowering capabilities, including ezetimibe, Nexletol/Nexlizet and PCSK9 inhibitors, either on their own or in conjunction with statins.
However, the needs of high-risk CVD patients with high cholesterol remain largely unaddressed by current non-statin treatment options, which have only modest efficacy, are highly priced, or are inconveniently administered through an injection. In a cross-sectional study of over 20 thousand patients on lipid-lowering medication, current treatments including statins, ezetimibe, PCSK9 inhibitors or a combination of the foregoing resulted in fewer than 3% of patients reaching recommended cholesterol goals of lower than 1.8 mmol/L (70 mg/dL).
| • | Ezetimibe. Non-statin cholesterol-lowering medications, such as ezetimibe, function by preventing the absorption of cholesterol in the intestines by blocking the NPC1L1 protein. Although these drugs are administered at a low dose, which contributes to their safety and tolerability, and are generic and broadly available, they have been shown to only moderately reduce LDL-C. Ezetimibe has been observed to reduce LDL-C by approximately 25% compared to baseline. We believe this relatively modest efficacy supports why relatively few patients and prescribers prefer to utilize this these medications. |
| • | Nexletol/Nexlizet. The other currently available oral non-statin, Nexletol/Nexlizet, which also inhibits an enzyme involved in cholesterol synthesis, shows only modest improvement in lowering LDL-C. |
265
Table of Contents
Nexletol/Nexlizet has been observed to reduce LDL-C by approximately 15% compared to baseline. Along with its relatively modest efficacy, Nexletol/Nexlizet’s label contains safety warnings that include tendon rupture and gout. Given that Nexletol/Nexlizet’s efficacy profile is comparable to generic ezetimibe, payors are reluctant to cover its branded price, thus limiting its access. |
| • | PCSK9 Inhibitors. The PCSK9 inhibitors on the market are injectable, monoclonal antibodies and small interfering RNA that have been observed to reduce LDL-C levels by approximately 50% compared to baseline. While PCSK9 inhibitors have demonstrated their effectiveness at reducing LDL-C when used alone and as an adjunct to statin therapy, we believe their injectable route of administration makes them inconvenient for patients, and their access is further limited by their associated high cost and low rates of prescription approval by payors. It is estimated that over 75% of ASCVD outpatients prefer oral drugs to injectable therapies. |
Limitations of Prior Attempts to Develop CETP Inhibitors
As described above, CETP inhibitors, including obicetrapib, are designed to work by blocking the transfer of cholesteryl esters from HDL to LDL, thereby directly reducing LDL-C levels in the body. We believe that CETP inhibitors can improve upon existing therapies by providing a combination of potent LDL-C lowering activity and tolerability, the ability to be administered orally and favorable pricing.
Other CETP inhibitors have reached varying stages of clinical development, but none have been approved or successfully resulted in a potent, safe and well-tolerated low-dose oral medication. We believe that the prior CETP inhibitor programs did not select optimal compounds or clinical trial designs because they focused on exploring the prominent increase in HDL-C rather than the potential for lowering LDL-C. Nevertheless, anacetrapib, the latest of the prior CETP inhibitors, provided clinical support that the absolute reduction in LDL-C over time by CETP inhibition confers a predictable benefit in the prevalence of adverse cardiovascular outcomes.
The focus on HDL-C raising by developers of prior CETP inhibitors likely resulted in the selection of chemical compounds that were not optimized for LDL-C lowering, which we believe in turn resulted in only modest reductions to CVD risk. In addition to selecting compounds that were not optimized to achieve LDL-C lowering, we believe misunderstandings about the benefits of HDL-C led to insufficient caution around compound-specific blood pressure toxicity signals, which were observed in clinical trials. For example, one CETP inhibitor, torcetrapib, resulted in off-target toxicity and increased blood pressure and aldosterone, as seen early in Phase 2 clinical trials. While another CETP inhibitor, dalcetrapib, had a favorable tolerability profile compared to torcetrapib, the drug had no LDL-lowering activity and therefore no effect on reducing adverse cardiovascular outcomes.
Prior CETP inhibitor programs also used CVOTs designed to evaluate patients with controlled rather than uncontrolled LDL-C levels. We believe the focus on HDL-C led to enrollment of patients with overly low LDL-C baseline levels, minimizing the potential to observe relative reductions in CVD risk, and that specific trials were too short for the full magnitude of MACE benefits to be observed based on the modest reduction in LDL-C achieved. For example, evacetrapib, which also had a favorable tolerability profile, used a CVOT with a median duration of only two years, which was too short to demonstrate the drug’s potential to reduce CVD risk over time, and it too had only a modest observed LDL-lowering capability. Similar CVOTs with other agents that lowered LDL-C by a similar magnitude required at least three years to demonstrate a MACE benefit, including CETP inhibitor anacetrapib.
In a Phase 3 REVEAL CVOT with approximately 30,000 patients with ASCVD receiving intensive atorvastatin therapy, for anacetrapib, its MACE benefits were observed to be predicted by the magnitude of the LDL-C reduction, suggesting that CETP inhibitors behave like statins in reducing MACE. The REVEAL trial began enrollment in August 2011 and completed its long-term follow-up in April 2019. A median four-year follow-up of the REVEAL trial showed that CETP inhibition resulted in a nine percent reduction in MACE (first major coronary event, a composite of coronary death, myocardial infarction or coronary revascularization) compared to placebo. We believe the REVEAL results provide clinical support showing that the absolute reduction in LDL-C over time by CETP inhibition confers a predictable benefit in the prevalence of adverse
266
Table of Contents
cardiovascular outcomes, as measured by MACE. However, due to a very low baseline level of LDL-C (61 mg/dl), the trial showed only a modest absolute LDL-C lowering of 11 mg/dl (17%). In addition, anacetrapib’s commercial viability was limited by its lipophilicity, which caused it to accumulate in fat tissue over time.
We selected obicetrapib for its LDL-lowering activity and safety profile and designed our CVOT to avoid the shortcomings of prior CETP inhibitor programs and to ultimately fulfill the unmet need of patients with and at risk of CVD. Obicetrapib has intrinsic properties, such as ionizable features, substantially reduced lipophilicity, good solubility and neutral pH, that we believe give it more favorable physical, pharmacokinetic and biopharmaceutical properties as a drug candidate compared to other CETP inhibitors.
In 2022, Merck & Co. (“Merck”) announced, through publication in a respected medicinal chemistry journal, the successful design of MK-8262, a CETP inhibitor with a novel molecular structure that could lead to improved LDL lowering potency at a lower dose compared to previous CETP inhibitors. Parameters that were focused on for optimization of MK-8262 were polarity and lipophilicity, oral bioavailability, better IC50 towards lower dosages and consequently better pharmacodynamics on HDL and LDL levels. Based on the published results, the program resulted in a compound that was taken through Phase 1 trials and which displayed a number of favorable characteristics in in-vitro and in-vivo studies. We believe obicetrapib shares important similarities with a number of the features of this compound, which we believe helps to explain the stronger potency at a significantly lower dose we have observed in clinical studies of obicetrapib compared to prior CETP inhibitors. Merck has announced that the compound was not advanced further due to broader strategic reasons and not due to any adverse safety or efficacy findings.
Our Strategy
Our goal is to develop and commercialize potentially transformative oral therapies for patients suffering from cardiometabolic diseases rooted in abnormal cholesterol metabolism for which existing therapies are unsuccessful or not well-tolerated.
The core elements of our strategy to achieve our goal are the following:
| • | Advance the clinical development of obicetrapib as a next-generation oral, low-dose, once-daily LDL-lowering treatment as a monotherapy and a combination therapy with ezetimibe. We are conducting two Phase 3 pivotal trials: BROADWAY, which began enrolling patients in January 2022, and BROOKLYN, which began enrolling patients in July 2022, to evaluate obicetrapib as a monotherapy used as an adjunct to maximally tolerated lipid-lowering therapies to potentially enhance LDL-lowering for high-risk CVD patients. We also commenced our Phase 3 PREVAIL CVOT in March 2022, designed to assess obicetrapib’s potential to reduce occurrences of MACE, including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke and non-elective coronary revascularization, and a Phase 2b trial, ROSE2, to investigate obicetrapib as a fixed dose combination with ezetimibe. We are also conducting a Phase 2 dose-finding study of obicetrapib as an adjunct to stable statin therapy in patients with dyslipidemia in Japan. We expect to report data from our Phase 3 BROADWAY trial and our Phase 3 BROOKLYN trial in 2024. We expect to report data from our Phase 3 PREVAIL CVOT in 2026 and from our Phase 2b ROSE2 trial in 2023. We also expect to report data from our Phase 2 dose-finding study of obicetrapib as an adjunct to stable statin therapy in patients with dyslipidemia in Japan in the second half of 2023. |
| • | Obtain marketing approval from regulatory agencies. We currently plan to seek approval of obicetrapib in the United States, the EU, Japan, China and the United Kingdom. We are executing multiple Phase 3 trials simultaneously, with clinical plans that incorporate feedback from the FDA, EMA, PMDA and NMPA. We have also initiated a Phase 2 study specifically in Japan and are including sufficient numbers of patients in Japan to support approval in those markets on the same timelines as the U.S. and Europe. |
| • | Commercialize obicetrapib for the treatment of cardiometabolic disease. We are focused on selecting optimal partners in targeted geographies at the right time in obicetrapib’s development and |
267
Table of Contents
commercialization process. If we obtain marketing approval, both independently and, in certain jurisdictions through collaborations, we intend to commercialize obicetrapib for patients suffering from cardiometabolic disease. We have partnered with Menarini to exclusively commercialize obicetrapib 10 mg either as a sole active ingredient product or in a fixed dose combination with ezetimibe in the majority of European countries, if approved. Subject to receipt of marketing approval, our current plan is to pursue development and commercialization of obicetrapib in the United States ourselves, and to consider additional partners for jurisdictions outside of the United States and the EU, including in Japan, China and the United Kingdom. |
| • | Continue advancing the clinical development of obicetrapib for the treatment of Alzheimer’s disease. Evidence observed in our preclinical studies suggests that cholesterol accumulation in the brain may be a precursor to Alzheimer’s disease. For example, rodents lack the CETP gene and are resistant to Alzheimer’s disease. In early preclinical studies, when the human CETP gene was knocked into a mouse, the cholesterol content of the mouse brain was observed to increase by 25% and when combined with the gene for the amyloid precursor protein, hypothesized to be a driver of Alzheimer’s disease, the risk of developing Alzheimer’s disease may greatly increase. In a preclinical study, we observed that CETP inhibition promoted cholesterol removal from the brain and improved cognition. We commenced a Phase 2a clinical trial in early 2022 in patients with early Alzheimer’s disease and the ApoE4 mutation to evaluate the pharmacodynamic and pharmacokinetic effects, safety and tolerability of obicetrapib. We currently expect to report data from this trial in 2023. Should the data from this trial warrant it, we intend to continue advancing this product candidate through the clinical development pathway. |
| • | Explore the potential of CETP inhibitors for use in other indications. We believe that CETP inhibition, by markedly increasing HDL-C and lowering LDL-C, may also have a role to play in other indications by potentially mitigating the risk of developing diseases such as diabetes, which led to an estimated 1,500,000 deaths globally in 2019, in addition to CVD and Alzheimer’s disease. Clinically demonstrated anti-diabetic benefits have been observed with CETP inhibition in Phase 3 CVOTs that, if seen in obicetrapib, would differentiate it from current treatment alternatives, especially statins. We are planning preclinical studies examining the potential of obicetrapib for patients suffering from diabetes and have included the onset of diabetes as an endpoint in our CVOT. |
Clinical Development Plan
We are conducting two Phase 3 pivotal trials – our BROADWAY and BROOKLYN trials – designed to measure obicetrapib’s ability to reduce LDL-C as a monotherapy administered as an adjunct to maximally tolerated lipid-modifying therapy. Following our end of Phase 2 meeting with the FDA in the fourth quarter of 2021, we also commenced our Phase 3 PREVAIL CVOT for obicetrapib as a monotherapy administered as an adjunct to maximally tolerated lipid-modifying therapy in early 2022. In our Phase 2b ROSE2 trial, we are evaluating the effect of a fixed dose combination of obicetrapib 10 mg with ezetimibe 10 mg on top of high-intensity statin therapy on reduction in LDL-C. We commenced a Phase 2 dose-finding study of obicetrapib as an adjunct to stable statin therapy in patients with dyslipidemia in Japan.
Based on the lipid-modifying effects of CETP inhibition we have observed in our clinical trials for obicetrapib to date, we have conducted preclinical assessments of obicetrapib to test its potential to stimulate similar protein-modulating activity in the brain for the potential prevention and treatment of Alzheimer’s disease. Following a Type B meeting in June 2021, the FDA confirmed that our preclinical data are sufficient to support a proposed clinical trial of obicetrapib for this indication, and we commenced a Phase 2a clinical trial in early 2022 in patients with early Alzheimer’s disease to evaluate the pharmacodynamic and pharmacokinetic effects, safety and tolerability of obicetrapib.
268
Table of Contents
We have set forth below our current obicetrapib clinical development pipeline.

Obicetrapib for Cardiovascular Disease
There is broad scientific consensus that elevation in LDL-C is a primary causal factor for ASCVD, and CVD outcomes in high-risk populations improve as the level of LDL-C achieved on therapy decreases. A study published in the Journal of the American Medicine in 2017 found that genetic variants related to lower LDL-C levels were significantly associated with a lower risk of CVD. Specifically, the study concluded that the quantum of reduced genetic risk for CVD associated with CETP mutations was almost identical to the genetic risk of CVD observed in patients with genetically reduced levels of the proteins targeted by statins, PCSK9 inhibitors and ezetimibe. We believe the consistency of benefit across genotypes observed in all target genes is predictive of the clinical efficacy of CETP-induced LDL-lowering on CVD.
The direct correlation between LDL-C reduction and decrease in atherosclerotic cardiovascular events has been documented for both statin as well as non-statin therapies in CVOTs for ezetimibe, the PCSK9 inhibitors evolocumab and alirocumab, and for the CETP inhibitor anacetrapib. Most notably, a median four-year follow-up of the REVEAL Phase 3 study of anacetrapib showed that CETP inhibition resulted in a nine percent reduction in MACE (first major coronary event, a composite of coronary death, myocardial infarction or coronary
269
Table of Contents
revascularization) compared to placebo. However, due to a very low baseline level of LDL-C (61 mg/dl), the trial showed only a modest absolute LDL-C lowering of 11 mg/dl (17%). After approximately six and a half years of total follow-up, the REVEAL clinical trial showed additional MACE reduction of 20%. The table below shows the reduction in MACE and each component of MACE in the in-trial and post-trial periods.
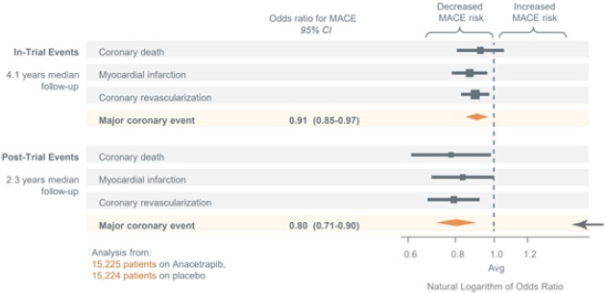
We believe the REVEAL results provide clinical support that the absolute reduction in LDL-C over time by CETP inhibition confers a predictable benefit in the prevalence of adverse cardiovascular outcomes, as measured by MACE. Specifically, the decrease in MACE observed in the REVEAL study of anacetrapib is consistent with the findings of The Cholesterol Treatment Trialists’ (“CTT”) Collaboration, illustrated in the graphic below. The CTT collaboration conducted a meta-analysis of 26 statin clinical trials and showed that there is a consistent, linear decrease in MACE for every absolute unit of non-HDL (which is primarily composed of LDL-C) cholesterol reduction. The MACE reduction observed in REVEAL falls on the meta-regression line – specifically, the CTT metaregression line predicts that an absolute reduction of LDL-C of 11 mg/dl, as seen in REVEAL, would correspond to the 9% reduction in MACE observed in REVEAL.
270
Table of Contents
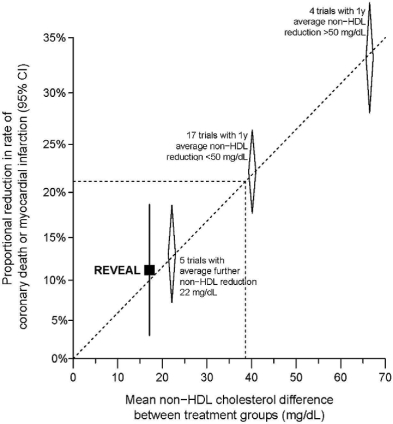
The graphic above presents a linear prediction of MACE benefit based on a meta-analysis of 26 prior statin clinical trials. Actual results may differ materially.
With these learnings in mind, we are executing a clinical development plan for obicetrapib focused on patients with high baseline LDL-C and that is designed to support a broad CVD label, if successful. To date, we have completed seven Phase 1 trials and three Phase 2 trials of obicetrapib. We are conducting two Phase 3 lipid trials and a third Phase 3 CVOT, and a fourth Phase 2b trial is ongoing.
Ongoing Clinical Trials for Cardiovascular Disease
Phase 3 BROADWAY and BROOKLYN Lipid Trials
We are conducting two Phase 3 pivotal trials designed to measure obicetrapib’s LDL-lowering capability and plan to enroll an aggregate of 2,700 patients across both studies who require additional LDL-lowering on top of their maximum tolerated lipid-modifying therapies. Our BROADWAY trial (TA-8995-302), which began enrolling patients in January 2022, is expected to enroll approximately 2,400 patients in the United States, Europe and Asia with heterozygous familial hypercholesterolemia (“HeFH”) (individuals genetically predisposed to very high cholesterol) or established ASCVD, and who have baseline LDL-C of at least 70 mg/dL, and an additional risk enhancer in participants with an LDL-C level below 100 mg/dL (including other abnormal biometrics, a recent myocardial infarction or Type 2 diabetes). Our BROOKLYN trial (TA-8995-301), which began enrolling patients in July 2022, is expected to enroll an anticipated 300 HeFH patients in the United States, Canada, Europe and Africa who have baseline LDL-C of at least 70 mg/dL. Obicetrapib will be administered in a once-daily 10 mg dose as an adjunct to diet (for regulatory purposes in the EU) and maximally tolerated lipid-modifying therapy, for a 52-week treatment period. Such lipid-modifying therapies include statins or, for statin-intolerant patients, ezetimibe, Nexletol/Nexlizet, PCSK9 inhibitors, or fibrates (a class of drugs which increase HDL-C without significantly reducing LDL-C).
The primary endpoint of both trials is percent change from baseline in LDL-C compared to placebo after 12 weeks. Secondary endpoints will also include percent changes from baseline compared to placebo after 12 weeks
271
Table of Contents
in Lp(a), ApoB, HDL-C, non-HDL-C (representing total cholesterol minus HDL-C), LDL-C from baseline to placebo after 180 days and 52 weeks, and, for BROADWAY, total cholesterol and triglycerides and MACE from baseline to 30 days after the last dose. We also expect to evaluate the safety and tolerability profile of obicetrapib in a broadly representative population of adult males and females of all ages, including elderly and very elderly participants, assessed by AEs, vital signs, clinical laboratory values and electrocardiogram (“ECG”) measurements as well as to evaluate the effects of obicetrapib on blood pressure.
Phase 3 PREVAIL Cardiovascular Outcomes Trial
We have also initiated our PREVAIL trial (TA-8995-304), our Phase 3 CVOT, to evaluate the effects of 10 mg obicetrapib in participants with ASCVD on MACE (cardiovascular death, myocardial infarction, stroke and non-elective coronary revascularization). We expect to enroll 9,000 participants at sites in the United States, Canada, Europe, Asia, and Australia with established ASCVD and an LDL-C level of at least 70 mg/dL, and an additional risk enhancer in participants with an LDL-C level below 100 mg/dL, whose ASCVD is not adequately controlled despite maximally tolerated lipid-modifying therapies. The planned median study follow-up is expected to be 36 months, and the treatment period will continue until the last participant has been followed for a minimum of 2.5 years after the last patient has been randomized or until the target number of 959 primary endpoint events (i.e., cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or non-elective coronary revascularization) have occurred, whichever is later.
We have designed our PREVAIL trial based on insights gained from analyzing failures of prior CVOTs for other CETP inhibitors. Our trial design targets higher baseline LDL-C patients, which we believe creates potential for greater observed absolute LDL-C reduction, particularly given the observed median LDL-lowering activity of 51% in our Phase 2b ROSE clinical trial. We are focused on patients with high LDL-C levels and who have at least one other risk enhancer (including recent myocardial infarction, Type 2 diabetes, high triglyceride levels or low HDL-C), compared to prior CVOTs for CETP inhibitors that enrolled patients with low baseline LDL-C. We are planning for longer duration of follow-up to maximize opportunities to observe MACE reduction, with all patients to be followed for a minimum of three years. We believe that the inclusion of a high-risk patient population with established ASCVD and other risk enhancers increases the likelihood that the trial will accrue sufficient primary endpoint events over time and potentially result in a strong relative risk reduction in the treatment arm.
Phase 2b ROSE2 Trial
In our ongoing Phase 2b ROSE2 trial, we are evaluating the effect of a fixed dose combination of obicetrapib 10 mg with ezetimibe 10 mg on top of high-intensity statin therapy on reduction in LDL-C levels. This randomized, double-blind placebo-controlled trial among 114 patients with dyslipidemia is designed to compare a once-daily oral 10 mg dose of obicetrapib alone and a combination therapy with 10 mg of ezetimibe to placebo for 12 weeks. The primary endpoint is percent change in LDL-C levels from baseline. We currently expect to report data from this trial in 2023.
Phase 2 Japan Trial
In our ongoing Phase 2 trial in Japan, we are evaluating the effects of three doses of obicetrapib (2.5 mg, 5 mg, and 10 mg) on LDL-C levels including efficacy, safety and tolerability. This is a randomized, double-blind, placebo controlled trial that is expected to enroll 108 adult Japanese patients with dyslipidemia. The trial is being conducted at hospitals and clinics across Japan. The primary endpoint is the percent change from baseline to end of treatment (day 56) in LDL-C for each obicetrapib group compared to placebo. We currently expect to report data from this trial in the second half of 2023.
Completed Phase 2 Clinical Trials
We have completed three Phase 2 trials of obicetrapib for the treatment of cardiometabolic disease. In all three trials obicetrapib was observed to robustly lower LDL-C and increase HDL-C from baseline across various treatment settings. Obicetrapib was also observed to be well-tolerated compared to placebo, in both the 5 mg and 10 mg doses and as a combination therapy with ezetimibe. The majority of treatment-emergent adverse events (“TEAEs”) were mild or moderate in severity and there were no drug-related, treatment-emergent serious AEs. The graphs below summarize the results of the three Phase 2 trials.
272
Table of Contents
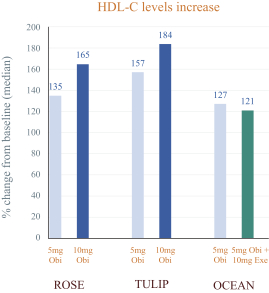 | 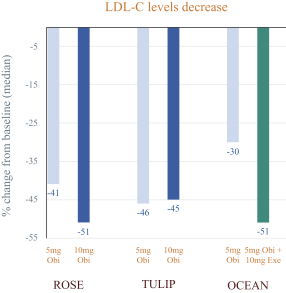 |
In our Phase 2b ROSE trial, we observed that obicetrapib has robust LDL-lowering capability as an adjunct to high-intensity statins at both 5 mg and 10 mg dosages. Based on our ROSE trial, we are using a 10 mg dosage for our Phase 3 trials. In our Phase 2a TULIP trial, we observed that a daily dose of up to 10 mg of obicetrapib alone significantly reduced LDL-C and increased HDL-C. Based on observations from our Phase 2b OCEAN trial, we believe that obicetrapib is at least additive for LDL lowering as a combination therapy with ezetimibe.
The table below summarizes the trial designs of the three Phase 2 trials we have completed.
Trial | Design | Patients | Obicetrapib Formulation | |||
TULIP (TA-8995-03) | Randomized, double-blind placebo-controlled trial to evaluate the percent changes in LDL-C and HDL-C levels | 364 patients with mild dyslipidemia not on lipid-altering therapy at screening | 1, 2.5, 5 or 10 mg alone and as a combination therapy with statins | |||
ROSE (TA-8995-201) | Randomized, double-blind placebo-controlled trial to evaluate LDL-C reduction | 114 patients with mild dyslipidemia already receiving high-intensity statin therapy | 5 mg or 10 mg | |||
OCEAN (TA-8995-303) | Randomized, double-blind placebo-controlled trial to evaluate LDL-C reduction | 112 patients with mild dyslipidemia | 5 mg alone and as a combination therapy with 10 mg ezetimibe |

A Phase 2a TULIP trial of obicetrapib, which was completed in 2014, was a randomized, double-blind placebo-controlled trial among 364 patients with mild dyslipidemia and not on lipid-altering therapy at screening and involved once-daily oral dosing of obicetrapib up to 10 mg or a placebo alone and as a combination therapy with statins. The primary endpoints were the percent changes in LDL-C and HDL-C levels from baseline to week 12 of the trial, which were met for both doses. The 5 mg dose of obicetrapib resulted in a mean reduction of LDL-C by 45% and increased HDL-C by 161%, compared to placebo. In patients treated with 10 mg obicetrapib plus statin therapy (20 mg atorvastatin or 10 mg rosuvastatin), LDL-C levels were approximately 50% lower and HDL-C levels were approximately 140% higher, respectively, than those observed in patients receiving statin therapy alone.
273
Table of Contents
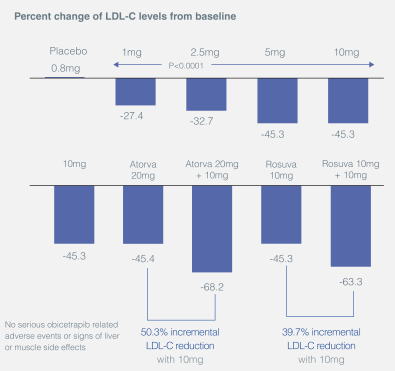
Key secondary endpoints included percent changes in ApoB and apolipoprotein A1 (“ApoA1”). In patients treated with 5 mg obicetrapib, ApoB was reduced by 33.8%, while ApoA1 levels increased by 58.3%. A daily dose of 10 mg obicetrapib on top of statin therapy resulted in an ApoB reduction of 30% and an ApoA1 increase of 54.1% than those observed in patients receiving statin therapy alone. Other secondary endpoints included percent change in ApoE, nascent HDL levels and ABCA-1 efflux. A summary of certain of these results follows:
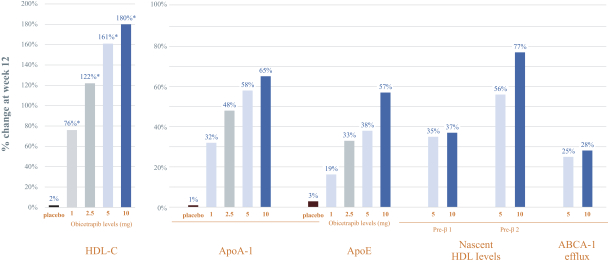
A total of 284 patients experienced at least one TEAE, of which 95 experienced a suspected study drug-related TEAE. For all treatment groups, the most common TEAEs were the common cold and headache (22.9% and 13.2%, respectively). Most TEAEs were mild or moderate in severity with only 13 patients experiencing a severe TEAE, two of which were suspected to be related to the study drug (one subject in the placebo group and one subject in the atorvastatin 20 mg and obicetrapib 10 mg combination). Prevalence, incidence and severity of
274
Table of Contents
TEAEs were similar across all treatment groups. There were eight patients with a treatment-emergent serious adverse event, none of which were study drug related, and no deaths occurred during the study.

Our Phase 2b ROSE trial, which was completed in August 2021, was a randomized, double-blind placebo-controlled trial among 120 patients with mild dyslipidemia who were already receiving high-intensity statin therapy. The trial involved a once-daily oral dose of obicetrapib at either 5 mg or 10 mg dose level for eight weeks. The primary endpoint of this clinical trial was LDL-C reduction from baseline and was met for both doses. Obicetrapib had a rapid effect, with LDL-C levels dropping dramatically in the first four weeks of the study and remaining relatively steady for the remaining four weeks of the study. At the 5 mg dose level, approximately 20% of patients experienced a decrease in median LDL-C levels of over 60%; at the 10 mg dose level, that percentage nearly doubled. A summary of these statistically significant results is as follows:
Median (min, max) LDL-C levels (mg/dL) at baseline and EoT
Time | Placebo | Obicetrapib 5mg | Obicetrapib 10mg | |||
| 90.0 | 95.0 | 88.0 | ||||
Baseline Median | (63, 204) | (54, 236) | (39, 207) | |||
| N=40 | N=39 | N=40 | ||||
| 86.0 | 53.0 | 49.5 | ||||
EoT Median | (43, 137) | (13, 126) | (23, 83) | |||
| N=39 | N=39 | N=40 | ||||
% Change from Baseline (median) | -6.5 | -41.45 | -50.75 | |||
| (-53.9, 31.6) N=39 | (-71.2, 62.3) N=38* | (-76.9, 15.6) N=40 | ||||
% Change from Baseline LS mean (95% Cl) P-value | -4.76 | -37.98 | -44.15 | |||
| (-11.74, 2.22) | (-44.80, -31.17) | (-50.95, -37.35) | ||||
| 0.1814 | <0.0001 | <0.0001 | ||||
We also observed median percent reductions in ApoB of 24.4% and 29.8%; decreases in non-HDL-C of 38.9% and 44.4%; increases in HDL-C of 135.4% and 165.0%; and decreases in Lp(a) of 33.8% and 56.5%, in each case at the 5 mg and 10 mg doses, respectively. These statistically significant results are summarized as follows:
Percent Change from Baseline to 8 Weeks in Lipid Biomarkers
| Placebo (N=40) | 5 mg (N=40) | 10 mg (N=40) | ||||||||
ApoB | Baseline: | |||||||||
| n | 40 | 40 | 40 | |||||||
| Mean (SD) | 90.8 (18.2) | 91.2 (22.6) | 87.5 (22.0) | |||||||
| Median (min, max) | 87.0 (66, 136) | 88.0 (53, 171) | 82.0 (49, 161) | |||||||
| Percent Change: | ||||||||||
| Mean (SD) | -4.67 (17.7) | -22.62 (21.9) | -27.19 (15.3) | |||||||
| Median (min, max) | -2.60 (-50.0, 28.4) | -24.40 (-58.5, 47.4) | -29.75 (-58.4, 13.0) | |||||||
| LS Mean (SE)1 | -4.13 (2.6) | -22.40 (2.6) | -28.12 (2.6) | |||||||
| p-value | <0.0001 | <0.0001 | ||||||||
Non-HDL-C | Baseline: | |||||||||
| n | 40 | 40 | 40 | |||||||
| Mean (SD) | 125.4 (32.7) | 125.9 (36.4) | 121.4 (37.3) | |||||||
275
Table of Contents
| Placebo (N=40) | 5 mg (N=40) | 10 mg (N=40) | ||||||||
| Median (min, max) | 115.0 (87, 227) | 118.5 (69, 276) | 113.0 (53, 242) | |||||||
| Percent Change: | ||||||||||
| Mean (SD) | -4.22 (20.4) | -34.28 (25.6) | -39.25 (17.6) | |||||||
| Median (min, max) | -3.50 (-50.3, 48.4) | -38.90 (-65.6, 66.3) | -44.40 (-70.2, 22.5) | |||||||
| LS Mean (SE)1 | -3.83 (3.2) | -34.37 (3.2) | -39.86 (3.2) | |||||||
| p-value | <0.0001 | <0.0001 | ||||||||
HDL-C | Baseline: | |||||||||
| n | 40 | 40 | 40 | |||||||
| Mean (SD) | 48.6 (15.7) | 48.5 (13.7) | 49.9 (18.7) | |||||||
| Median (min, max) | 44.5 (19, 99) | 46.5 (24, 79) | 44.0 (25, 138) | |||||||
| Percent Change: | ||||||||||
| Mean (SD) | -6.62 (12.4) | 123.92 (57.7) | 156.41 (52.2) | |||||||
| Median (min, max) | -4.90 (-30.3, 28.6) | 135.40 (-26.4, 212.9) | 164.95 (55.1, 286.3) | |||||||
| LS Mean (SE)1 | -6.98 (6.6) | 122.29 (6.6) | 157.35 (6.5) | |||||||
| p-value | <0.0001 | <0.0001 | ||||||||
Lp(a) | Baseline: | |||||||||
| n | 40 | 40 | 40 | |||||||
| Mean (SD) | 108.2 (123.3) | 117.1 (115.3) | 85.8 (106.4) | |||||||
| Median (min, max) | 45.3 (2.9, 410) | 89.4 (2.8, 354) | 29.9 (2.8, 435) | |||||||
| Percent Change: | ||||||||||
| Mean (SD) | 5.4 (21.2) | -30.0 (31.9) | -43.2 (30.1) | |||||||
| Median (min, max) | 4.00 (-29.6, 45.5) | -33.8 (-84.6, 93.8) | -56.5 (-85.7, 18.3) | |||||||
| LS Mean (SE)1 | 5.06 (4.4) | -30.9 (4.4) | -42.0 (4.3) | |||||||
| p-value | <0.0001 | <0.0001 | ||||||||
| 1 | Least squares (LS) means and p-values (two-sided) are from a mixed model for repeated measures (MMRM) model with treatment, visit and treatment-by-visit as factors and baseline LDL-C as a covariate. p-values from comparison to placebo. For percent change values, n=39 for placebo and obicetrapib 5 mg groups for all, except n=38 for LDL-C and Lp(a) for obicetrapib 5 mg. |
Overall, obicetrapib as an adjunct to high-intensity statin therapy at both doses was observed to be well-tolerated compared to placebo. TEAEs were reported by 15 subjects in the 5 mg group and eight subjects in the 10 mg group, compared with 19 subjects in the placebo group. Some of the TEAEs in the study reported by more than one subject in any treatment group were muscle spasms (none in either of the obicetrapib groups and two subjects in the placebo group), fatigue (two subjects in the 5 mg group, one subject in the 10 mg group, and two subjects in the placebo group), basal cell carcinoma (none in either of the obicetrapib groups and two subjects in the placebo group), nausea (one subject in the 5 mg group and two subjects in the placebo group), Type 2 diabetes mellitus (two subjects in the 5 mg group and none in the 10 mg or placebo groups) and hypertension (two subjects in the 5 mg group and no subjects in either the 10 mg or placebo groups). All other TEAEs were experienced by only one or no subjects in each treatment group. TEAEs that were considered by the investigator to be related to study treatment were reported by three subjects (two subjects in the 5 mg group and one subject in the 10 mg group), compared with four subjects in the placebo group. There were no TEAEs leading to death. One subject in the placebo group had a TEAE leading to discontinuation. The majority of TEAEs were mild and moderate in severity; one subject in the placebo group had a severe TEAE. There were two serious TEAEs, both of which occurred in the placebo group.
Based on our ROSE trial and the enhanced LDL-C reduction capability of a 10 mg dose compared with 5 mg and the safety profile we observed, we selected a 10 mg dose for our Phase 3 lipid trials and CVOT.
276
Table of Contents

Our Phase 2b OCEAN trial, which we completed in June 2021, evaluated the effect of obicetrapib as a combination therapy with ezetimibe on LDL-C levels. This randomized, double-blind placebo-controlled trial among 100 patients with mild dyslipidemia involved once-daily oral 5 mg dose of obicetrapib alone and as a combination therapy with 10 mg of ezetimibe, compared to both placebo and ezetimibe alone, for eight weeks. The primary endpoint of the trial was percent change in LDL-C compared to baseline, which was met. We observed that obicetrapib 5 mg, ezetimibe 10 mg and their combination each significantly reduced LDL-C from baseline and compared with placebo, with statistically significant reductions compared to baseline measured at 34.4%, 14.8% and 52.0%, respectively, compared to a 1.4% reduction in the placebo group. The results are summarized as follows:
Median (min, max) LDL-C levels (mg/dL) at baseline and EOT
Time | Placebo | Ezetimibe 10mg | Obicetrapib 5mg | Obi 5 + Eze 10mg | ||||
| 136.0 | 127.0 | 121.0 | 123.0 | |||||
Baseline Median | (101, 177) | (76, 189) | (82, 153) | (89, 186) | ||||
| (N=24) | (N=27) | (N=27) | (N=27) | |||||
| 138.0 | 105.0 | 86.5 | 63.5 | |||||
EoT Median | (88, 193) | (66, 142) | (38, 137) | (34, 133) | ||||
| (N=25) | (N=24) | (N=26) | (N=24) | |||||
% change from BL median | -2.0 | -14.90 | -30.10 | -51.40 | ||||
| (-24.5, 35.9) | (-46.8, 46.9) | (-56.7, 19.1) | (-69.6, 8.1) | |||||
| (N=27) | (N=25) | (N=25) | (N=24) | |||||
% change from BL LSMeen (95%CI) | 1.40 | -12.86 | -30.70 | -40.95 | ||||
| (-6.03, 8.84) | (-20.29, -5.42) | (-38.21, -23.19) | (-48.73, -33.16) | |||||
p-value | 0.7116 | 0.0007 | <0.0001 | <0.0001 | ||||
We also observed median ApoB reductions of 23.5%, 8.9% and 34.8% for obicetrapib 5 mg, ezetimibe 10 mg and their combination, respectively, compared to 0.9% reduction in the placebo group.
Median (min, max) ApoB levels (mg/dL) at baseline and EOT
Time | Placebo | Ezetimibe 10mg | Obicetrapib 5mg | Obi 5mg + Eze 10mg | ||||
Baseline Median | 105.5 | 103.0 | 102.0 | 105.0 | ||||
| (74, 141) | (79, 133) | (74, 124) | (77, 158) | |||||
| (N=28) | (N=28) | (N=28) | (N=27) | |||||
EoT Median | 107.0 | 94.0 | 75.0 | 73.0 | ||||
| (69, 153) | (59, 137) | (45, 103) | (49, 105) | |||||
| (N=27) | (N=25) | (N=26) | (N=24) | |||||
% change from BL Median | -0.9 | -8.9 | -23.5 | -34.8 | ||||
| (-19.8, 25.4) | (-45.4, 32.3) | (-39.3, 21.2) | (-53.0, 8.9) | |||||
| (N=27) | (N=25) | (N=26) | (N=24) | |||||
Obicetrapib 5 mg alone and as a combination therapy with ezetimibe 10 mg taken once daily for eight weeks displayed a favorable tolerability profile. TEAEs were reported by four subjects in the obicetrapib 5 mg group and nine subjects in the combination group, compared with eight subjects in the ezetimibe 10 mg group and six subjects in the placebo group. The TEAEs in the study reported by more than one subject in any treatment group were diarrhea (no subjects and two subjects in the obicetrapib 5 mg and combination groups, respectively, and two subjects and no subjects in the ezetimibe 10 mg and placebo groups, respectively) and
277
Table of Contents
headache (no subjects and one subject in the obicetrapib 5 mg and combination groups, respectively, and two subjects and one subject in the ezetimibe 10 mg and placebo groups, respectively). All other TEAEs were experienced by one or no subjects in each treatment group. TEAEs that were considered to be related to study treatment were reported by one subject and three subjects in the obicetrapib 5 mg and combination groups, respectively, compared with three subjects and four subjects in the ezetimibe 10 mg and placebo groups, respectively. There were no TEAEs leading to death. One subject in the ezetimibe 10 mg group had a TEAE leading to discontinuation of the study drug, compared to no subjects in the 5 mg group and two subjects in the combination group. The majority of TEAEs were mild and moderate in severity, and one subject in the ezetimibe 10 mg group had a severe TEAE.
Phase 1 Clinical Trials
We have completed seven Phase 1 clinical trials of obicetrapib in healthy patients to date, which are summarized in the below table.
Phase 1 Trial | Design | Treatment / Formulation | Results | |||
| TA-8995-01: Single ascending dose study in healthy Caucasian and Japanese subjects | Randomized, double-blind, single-dose, placebo-controlled trial in healthy men and women. 12 groups of 8 subjects. 2 to 6 randomized to placebo or active treatment. | Single oral dose of 5, 10, 25, 50, 100 and 150 mg obicetrapib capsules, or Single oral dose of placebo | Dose-dependent and sustained inhibition of CETP activity accompanied by a decrease in LDL-C and ApoB and increases in CETP, HDL-C, ApoA-1 and apolipoprotein E (“ApoE”). Pharmacokinetics and pharmacodynamics generally consistent across ethnicity, age and gender. | |||
| TA-8995-E02: Multiple ascending dose study in healthy subjects | Randomized double-blind, placebo-controlled, sequential, multiple ascending-dose design. 5 groups of 12 subjects randomized to placebo or active treatment. Duration of treatment: 28 days of dosing for group 1, 21 days for groups 2-5. | Multiple oral dosages of 5, 10, 2.5, 1, and 25 mg obicetrapib capsules, or Multiple oral dosages of placebo | No safety or tolerability issues observed.
Single and multiple doses of up to 25 mg of obicetrapib did not yield adverse effects on vital signs or ECG changes, nor did clinical laboratory assessments and physical examinations reveal any safety issues. The maximum percent reduction in CETP activity from baseline following the 5 mg and 10 mg doses were 90.9% and 97.6%, respectively. | |||
278
Table of Contents
Phase 1 Trial | Design | Treatment / Formulation | Results | |||
| TA-8995-07: Study to assess the mass balance recovery, pharmacokinetics, metabolism and excretion of 14C-TA-8995 in healthy male subjects | Open label, single oral dose study in 6 subjects. | 10 mL 14C-obicetrapib oral suspension, containing 10 mg and 100 mCi of 14C-obicetrapib | Obicetrapib was steadily absorbed with a median of 4.5 hours to maximum absorption levels. Median half-life was 161 hours. A mean of 63.8% radioactivity was recovered in the feces and 15.4% in the urine, Overall total recovery of radioactivity in excreta approximately 78% of the administered dose. | |||
| TA-8995-04: Study of the electrocardiographic effects of TA-8995 in healthy male and female subjects | 135 subjects randomized to one of 3 study treatments. | Single oral dose of 150 mg obicetrapib capsules, or Single oral dose of placebo, or Single open-label oral dose of 400 mg moxifloxacin | No clinically meaningful effects on any ECG parameter were observed. | |||
| TA-8995-05: A Phase 1, open label study to assess the effects of TA-8995 on the pharmacokinetics of midazolam and digoxin in healthy male subjects | Open label, crossover, fixed sequence study in 16 healthy male subjects. Duration of treatment up to 15 days. | Digoxin 0.25 mg oral tablet on the morning of Days 1 and 13 Midazolam 5 mg oral solution on the morning of Days 2 and 14
obicetrapib 25 mg (2 x 10 mg and 1 x 5 mg) oral capsules on the morning of Day 8 and 10 mg oral capsule on the morning of Days 9 to 15. | No significant effect on digoxin was observed, with a statistically significant decrease in midazolam plasma. Absorption rates of digoxin and midazolam were unaffected by the presence of multiple doses of obicetrapib. | |||
| TA-8995-08: Bioequivalence study of capsule and tablet formulations of TA-8995 in healthy male subjects | Open-label, randomized, 2 treatment period (3 days), cross-over study in 26 subjects | 5 mg obicetrapib orally, either as a capsule or as a tablet in the first treatment period, and vice versa in the second treatment period. | Obicetrapib formulated as a tablet was bioequivalent to obicetrapib formulated as a capsule in terms of overall concentration over time but not in terms of the maximum observed concentration, which varied among study subjects. | |||
| TA-8995-06: A Phase 1 study of the effects of TA-8995 on Lp(a) in male and | Single-center, randomized, double-blind, placebo- | TA-8995 10 mg once daily, TA-8995 2.5 mg | There were statistically significant reductions in Lp(a) in both the | |||
279
Table of Contents
Phase 1 Trial | Design | Treatment / Formulation | Results | |||
| female subjects with elevated Lp(a) | controlled, parallel-group | once daily, or matching placebo once daily | TA-8995 2.5 mg and 10 mg groups, compared with placebo, at week 12 (primary endpoint) and at week 4 (secondary endpoint). There were statistically significant increases in HDL-C, ApoA1, and ApoE levels and decreases in LDL-C and ApoB levels, at week 12, for both the TA-8995 2.5 mg and 10 mg groups, compared with placebo. TA-8995 2.5 mg and 10 mg once daily for 12 weeks was generally well tolerated in subjects with elevated Lp(a) levels. | |||
| TA-8995-09: A randomized, open-label, two-sequence, two-period, two-treatment crossover study to evaluate the effect of food on the bioavailability of obicetrapib tablets in healthy adult subjects | Open-label, single-dose, randomized, 2-sequence, 2-period, 2-treatment crossover study in 30 subjects | 10 mg obicetrapib tablets orally administered either after an overnight fast of at least 10 hours (Treatment T1, fasted) or at 30 minutes after the start of a completed standardized high-fat, high-calorie breakfast that was preceded by an overnight fast of at least 10 hours (Treatment T2, fed). | Based on the plasma concentration data for obicetrapib, the peak and overall systemic exposure were 55-59% greater under fed conditions compared to that of fasted conditions. The least-squares geometric mean of fed versus fasted ratios were 154.87%, 155.42% and 158.53% for AUC0-t, AUC0-∞ and Cmax, respectively. | |||
Obicetrapib for Other Therapeutic Areas
Alzheimer’s disease
According to the World Health Organization, Alzheimer’s disease and other dementias affect approximately 55 million people as of 2021, and this is expected to increase to 78 million in 2030 and 139 million in 2050. Alzheimer’s disease is the most prevalent form of dementia, resulting in the generalized degeneration of the brain.
In a healthy brain, excess cholesterol levels in the neurons and amyloid-beta (“Ab”) peptide removal from cerebral spinal fluid are regulated properly. The brain is the most cholesterol-rich organ in the body; comprising only two percent of the body’s mass, it contains approximately 20% of the body’s cholesterol, which is recycled and redistributed through an ApoE-mediated lipoprotein pathway. Inside populations of cells called astrocytes, ApoE binds
280
Table of Contents
with cholesterol that has been released into the brain by neurons and converts it into a different form of cholesterol that is transported out of the brain into the systemic circulation. In addition to ApoE, the protein associated with HDL, ApoA1, also acts as the brain’s “vacuum cleaner,” by removing toxic cholesterol from peripheral tissue to promote healthy cell function and survival. In addition, small HDL particles that transverse the blood brain barrier remove excess Ab peptides in cerebral spinal fluid for ultimate conversion and transport out of the brain.
Alzheimer’s disease, however, is characterized in part by the aggregation of Ab peptides into amyloid plaques in cerebral spinal fluid, facilitated by the presence of excess cholesterol in cell membranes. Thus, the accumulation of cholesterol in cell membranes and the ineffective clearance of Ab plaques by ApoE and ApoA1 in their HDL forms is associated with the development of Alzheimer’s disease. Importantly, certain forms of ApoE (in particular, ApoE4) are worse at Ab transport than others, such as ApoE2, and are known to be associated with an increased risk of Alzheimer’s disease. Further, CETP activity has been detected in astrocytes, the cells where ApoE bind with cholesterol, indicating the potential for a CETP inhibitor to function in the brain similarly to its lipid-modifying effects in the cardiovascular system. Genetic studies have shown that CETP loss of function mutations mitigate the risk of Alzheimer’s disease in patients with the ApoE4 genotype.
Based on these observations as well as the marked increases of ApoA1 observed in our Phase 2 clinical trials and the increases in ApoE observed in the TULIP trial, we have conducted preclinical assessments of obicetrapib for the prevention and treatment of Alzheimer’s disease. In initial animal testing, we observed, in preliminary data, a statistically significant reduction in Ab42 over the total Ab ratio in the CSF of mice, a biomarker of Alzheimer’s disease activity.
Following a Type B meeting in June 2021, the FDA confirmed that our preclinical data are sufficient to support a proposed clinical trial of obicetrapib for the prevention and treatment of Alzheimer’s disease. We commenced a Phase 2a clinical trial in early 2022 in patients with early Alzheimer’s disease and the ApoE4 mutation to evaluate the pharmacodynamic and pharmacokinetic effects, safety and tolerability of obicetrapib. In this trial, obicetrapib at a 10 mg dose is being administered orally for 24 weeks, and the primary endpoints being evaluated are the change in levels of apolipoproteins in plasma and cerebrospinal fluid from baseline to the end of the 24-week trial period.
Manufacturing and Supply
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on a single contract manufacturer to produce both drug substances and drug products required for our clinical trials. Obicetrapib 5 and 10 mg tablets are manufactured and tested in accordance with Good Manufacturing Practice, at facilities in the United States, Italy and the United Kingdom.
Marketing and Sales
We do not currently have our own marketing, sales or distribution capabilities. In order to commercialize obicetrapib or any future product candidates, if approved for commercial sale, we currently plan to develop our own sales and marketing infrastructure in the United States and have entered, and expect to continue to enter, into arrangements with third parties to perform these services outside of the United States. We may also opportunistically seek strategic collaborations to maximize the commercial opportunities for our future product candidates inside and outside the United States. We have entered into a license agreement with Menarini, pursuant to which Menarini has been granted the exclusive rights to commercialize obicetrapib 10 mg either as a sole active ingredient product or in a fixed dose combination with ezetimibe in the majority of European countries, if approved. As any future product candidates near regulatory approval and potential commercial launch, we plan to assess our options for commercializing each respective product candidate and may choose to commercialize themselves ourselves or with a partner.
Menarini License
On June 23, 2022, we entered into a License Agreement with Menarini (the “Menarini License”), pursuant to which we granted Menarini an exclusive, royalty-bearing, sublicensable license under certain of our
281
Table of Contents
intellectual property and our regulatory documentation to undertake post approval development activities and commercialize multiple brands of obicetrapib in a single unit dose of 10 mg or less, either as a sole active ingredient product or in a fixed dose combination with ezetimibe, for any use in the majority of European countries (the “Menarini Territory”). We retained all rights to obicetrapib in all other territories and in other dosages.
We are solely responsible for conducting the development activities to obtain regulatory approval for obicetrapib. Menarini may conduct market access studies, medical affairs activities, non-registration studies and Phase IV clinical trials in the Menarini Territory. Menarini will be responsible for submitting and obtaining the required regulatory approvals to commercialize obicetrapib (at the licensed dosage) in the Menarini Territory and will own the regulatory approvals, if received. Menarini will also be solely responsible for commercializing obicetrapib (at the licensed dosage), if approved, and will be required to use commercially reasonable efforts to commercialize obicetrapib in the Menarini Territory.
Pursuant to the Menarini License, Menarini made an upfront payment to us of €115.0 million. Menarini has also committed to providing €27.5 million in funding for our research and development activities over several years, together with bearing 50% of any development costs incurred in respect of the pediatric population in the Menarini Territory. We are also eligible to receive up to an additional €863 million upon the achievement of various clinical, regulatory and commercial milestones. If obicetrapib is approved and successfully commercialized by Menarini, we will be entitled to tiered royalties ranging from the low double digits to the mid-twenties as a percentage of net sales in the Menarini Territory, with royalty step-downs in the event of generic entrance or in respect of required third-party intellectual property payments.
The Menarini License will expire on the last to expire royalty term, which is determined on a licensed product-by-licensed product and country-by-country basis, and is the later of (i) the expiration of the last to expire licensed patent that includes a valid claim in the country, (ii) expiration of regulatory exclusivity granted by the prevailing governmental authority for the licensed product in the country or (iii) 12 years from the first commercial sale of the licensed product in the country.
Through June 30, 2022, we have not received any milestone payments from Menarini under the Menarini License.
Intellectual Property
Our future commercial success depends, in part, on our ability to obtain and maintain patent and other proprietary protection for commercially important inventions, to obtain and maintain know-how related to our business, including our product candidates, to defend and enforce our intellectual property rights, in particular our patent rights, to preserve the confidentiality of our trade secrets, and to operate without infringing, misappropriating, or violating the valid and enforceable patents and other intellectual property rights of third parties. Our ability to preclude or restrict third parties from making, using, selling, offering to sell, or importing competing molecules to our products may depend on the extent to which we have rights under valid and enforceable patents and trade secrets that cover these activities.
We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems.
We strive to protect and enhance our proprietary inventions and improvements that we consider commercially important to the development of our business, including by seeking, maintaining, and defending U.S. and foreign patent rights. All of the issued patents and pending patent applications in our patent portfolio are owned by our subsidiary, NewAmsterdam Pharma B.V., Dutch Chamber of Commerce registry number 55971946. As of September 15, 2022, we owned seven issued U.S. patents and nine pending U.S. patent applications. We also owned 100 granted European patents and one pending European patent application, two granted Chinese patents and seven pending Chinese patent applications. In addition, we owned 71 granted patents
282
Table of Contents
and 28 patent pending applications in other foreign jurisdictions, including international applications under the Patent Cooperation Treaty, or PCT.
The patent positions of pharmaceutical companies are generally uncertain and can involve complex legal, scientific, and factual issues. We cannot predict whether any patent applications we pursue will issue as patents in any particular jurisdiction, or whether the claims of any issued patents will provide sufficient proprietary protection from competitors.
In addition, the coverage claimed in a patent application may be significantly reduced before a patent is granted, and its scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our products will be protected or remain protectable by enforceable patents. Moreover, any patents that we license or may own in the future may be challenged, circumvented, or invalidated by third parties. In addition, because of the extensive time required for clinical development and regulatory review of a product candidate we may develop, it is possible that, before our product candidate can be commercialized successfully, any related patents may expire or remain in force for only a short period following commercial launch, thereby limiting the protection such patent would afford the applicable product and any competitive advantage such patent may provide.
For any individual patent, the term depends on the applicable law in the country in which the patent is issued. In most countries where we have patents and patent applications, including the United States, patents have a term of 20 years from the application filing date or earliest claimed nonprovisional priority date. In the United States, the patent term may be shortened if a patent is terminally disclaimed over another patent that expires earlier. The term of a U.S. patent may also be lengthened by a patent term adjustment that is awarded by the USPTO, in order to address administrative delays by the USPTO in examining and granting a patent.
In the United States, the term of a patent that covers an FDA-approved drug may be eligible for patent term extension in order to restore the period of a patent term lost during the premarket FDA regulatory review process. Specifically, the Drug Price Competition and Patent Term Restoration Act of 1984 (the “Hatch-Waxman Act”) permits a patent term extension of up to five years beyond the natural expiration of the patent (but the total patent term, including the extension period, must not exceed 14 years following FDA approval). The patent term extension period granted on a patent covering a product is typically one-half the time between the effective date of a clinical investigation involving human beings is begun and the submission date of an application, plus the time between the submission date of an application and the ultimate approval date. Only one patent applicable to an approved product is eligible for patent term extension, and only those claims covering the approved product, a method for using it, or a method for manufacturing it may be extended. The application for patent term extension must be submitted prior to the expiration of the patent. The USPTO reviews and approves the application for any Patent Term Extension in consultation with the FDA.
Prosecution is a lengthy process, during which the scope of the claims initially submitted for examination by the USPTO and other patent offices may be significantly revised before issuance, if granted at all.
For more information regarding the risks related to our intellectual property, please see “Risk Factors—Risks Related to NewAmsterdam Pharma’s Intellectual Property.”
The issued patents and patent applications for obicetrapib are detailed below.
Obicetrapib First Generation Patents
The patent portfolio for obicetrapib composition of matter includes a first generation patent family directed generally to compounds, pharmaceutical compositions comprising the compounds, and methods of treatment using the compounds and pharmaceutical compositions. We have two granted patents in the United States covering a genus of compounds that includes obicetrapib and claims that more narrowly cover the obicetrapib compound, pharmaceutical compositions, and methods of treatment. We have 17 granted patents in Europe. In Asia, we have one granted patent in China, two granted patents in Japan, one granted patent in the Republic of Korea, one granted patent in Taiwan and one granted patent in Singapore. We have one granted patent in India. In North America outside of the United States, we have one granted patent in Canada and one granted patent in
283
Table of Contents
Mexico. In addition we have 13 granted patents in other foreign jurisdictions. Patent applications are pending in Argentina and Thailand. Patents, and patent applications, if granted, are expected to expire between April 2025 and August 2027, without taking potential patent term extensions into account. The first generation portfolio also includes a patent family covering a method of synthesizing obicetrapib. We have one patent in the United States, five patents in Europe including the United Kingdom, and one patent in Japan. Patents in this family are expected to expire between March 29, 2027 and March 31, 2029, not including patent term extensions.
Obicetrapib Second Generation Patents
Our second generation obicetrapib patent portfolio includes a patent family directed to solid oral dosage forms containing 5 to 10 mg of obicetrapib, including tablet forms, and methods of treatment comprising administration of 1 to 25 mg of obicetrapib daily. We have two granted patents in the United States and a pending application. We have 39 granted patents in Europe. In Asia, we have no granted patents in China, one granted patent in the Republic of Korea, one granted patent in Japan, one granted patent in Taiwan, one granted patent in Singapore and one granted patent in Hong Kong. We have one granted patent in India. In North America outside of the United States, we have one granted patent in Mexico. In addition, we have 16 granted patents in other foreign jurisdictions. Patent applications are pending in Argentina, Brazil, Canada (allowed), China, Hong Kong, Colombia, Costa Rica, Egypt, Libya, Peru, Philippines, Thailand and Venezuela. Patents, and patent applications, if granted, are expected to expire in February 2034, without taking potential patent term extensions or patent term adjustment into account.
We also have a patent family directed to compositions that contain obicetrapib and a statin, methods of treating with compositions that contain obicetrapib and a statin, and in Europe and other foreign jurisdictions, methods of use in which obicetrapib and a statin are separately administered. We have one granted patent in the United States and an application to reissue the patent with additional claims covering separate administration of obicetrapib and a statin. We have no granted patents in Europe. In Asia, we have no granted patents in China, one granted patent in Japan and two granted patent in Taiwan. In North America outside of the United States, we have one granted patent in Mexico. In addition, we have two granted patents in other foreign jurisdictions. Patent applications are pending in China, Hong Kong, Japan, Canada, Europe, Republic of Korea, Thailand and Venezuela. Patents, and patent applications if granted, are expected to expire between February 2034 and August 2035, without taking potential patent term extensions or patent term adjustment into account.
In addition, we have a patent family that claims a synthetic intermediate used in the synthetic process we intend to use commercially, as well as processes to make that intermediate. We have one issued US patent, 39 granted patents in Europe. In Asia, we have one granted patent in China, one granted patent in Hong Kong, one granted patent in Japan, one granted patent in Singapore and one granted patent in Taiwan. In North America outside of the United States, we have one granted patent in Mexico and one granted patent in Canada. In addition, we have 14 granted patents in other foreign jurisdictions. Patent applications are pending in Argentina, Brazil, Canada (allowed), Europe, India, Republic of Korea and Venezuela. Patents, and patent applications if granted, are expected to expire in July 2035, without taking potential patent term extensions or patent term adjustment into account.
Obicetrapib Third Generation Patents
We have one pending US provisional application covering the solid salt form of obicetrapib that we intend to commercialize and the process for its commercial synthesis. Patents, and patent applications if granted, are expected to expire in July 2043, without taking potential patent term extensions or patent term adjustment into account.
We also have patent families, all of which consist of pending patent applications, directed to compositions and methods of use of obicetrapib as a combination therapy with (i) ezetimibe, (ii) statins for use in certain subpopulations of patients, and (iii) SGLT2 inhibitors. Patent applications if granted, are expected to expire respectively in (i) February 2042, (ii) July 2042, and (iii) December 2042, without taking potential patent term extensions or patent term adjustment into account.
284
Table of Contents
In addition, we have a pending international patent application and a pending U.S. provisional application covering methods of using obicetrapib to treat neurodegenerative disease. Any patents that grant from these applications are expected to expire in March 2042 and September 2043, respectively, without taking potential patent term extensions or patent term adjustments into account.
Trade Secrets
We also rely on trade secrets, know-how, confidential information and continuing technological innovation to develop, strengthen and maintain our proprietary position in our field and protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. However, trade secrets can be difficult to protect. While we take measures to protect and preserve our trade secrets, such measures can be breached, and we may not have adequate remedies for any such breach. We seek to protect our proprietary information, in part, using confidentiality agreements and invention assignment agreements with our collaborators, employees and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We cannot guarantee, however, that we have executed such agreements with all applicable counterparties. Furthermore, these agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors and other third parties, or misused by any collaborator to whom we disclose such information. To the extent that our collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For more information regarding the risks related to our intellectual property, please see “Risk Factors—Risks Related to NewAmsterdam Pharma’s Intellectual Property.”
Competition
The biopharmaceutical industry is characterized by intense competition and rapid innovation. Our potential competitors include large pharmaceutical companies, smaller biotechnology and specialty pharmaceutical companies and generic drug companies. Many of our potential competitors have greater financial and technical human resources than we do, as well as greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products, and the commercialization of those products. Accordingly, our potential competitors may be more successful than us in obtaining FDA-approved drugs and achieving widespread market acceptance. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render our product candidates non-competitive or obsolete.
We believe the key competitive factors that will affect the development and commercial success of our obicetrapib product candidate, if approved, will be its enhanced LDL-lowering capability as a monotherapy or as a combination therapy, tolerability profile, convenience of oral dosing, price and availability of reimbursement from governmental and other third-party payors, and effect on other predictors of disease risk.
We are currently developing obicetrapib primarily for the treatment of patients at high cardiovascular risk with elevated levels of LDL-C as an adjunct to statins. If approved, obicetrapib would compete with approved non-statin treatments such as ezetimibe, Nexletol/Nexlizet and PCSK9 inhibitors such as Repatha, Praluent and Leqvio. There are also a number of product candidates in clinical development by third parties, such as Amryt Pharma, Arrowhead Pharmaceuticals, AstraZeneca, CVI Pharmaceuticals, Innovent Biologics, Ionis Pharmaceuticals, Matinas BioPharma, Merck, Novartis, Novo Nordisk, Regeneron Pharmaceuticals and others, that are intended to treat CVD.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control,
285
Table of Contents
approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, and export and import of drug products. We, along with any third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval of our products and product candidates. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Drug Development Process
In the United States, the FDA regulates drugs under the FDCA, as amended, and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. A new drug must be approved by the FDA through the NDA process before it may be legally marketed in the United States, and this process generally involves the following:
| • | completion of preclinical laboratory tests, animal studies, and formulation studies in accordance with FDA’s Good Laboratory Practice requirements and other applicable regulations; |
| • | submission to the FDA of an IND application, which must become effective before human clinical trials may begin and must be updated annually and when certain changes are made; |
| • | approval by an independent IRB, or independent ethics committee at each clinical site before each trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials in accordance with Good Clinical Practices (“GCPs”), to establish the safety and efficacy of the proposed drug for its intended use; |
| • | preparation of and submission to the FDA of an NDA after completion of pivotal trials; |
| • | a determination by the FDA within 60 days of its receipt of an NDA to file the application for review; |
| • | satisfactory completion of an FDA advisory committee review, if applicable; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP, requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| • | potential FDA audit of the nonclinical study and/or clinical study sites that generated data in support of the NDA; and |
| • | FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States. |
Prior to beginning the first clinical trial with a product candidate in the United States, a sponsor must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology and pharmacodynamic characteristics of the product; chemistry, manufacturing and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. Some long-term preclinical testing may continue after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial or not allowing it to commence on the terms originally specified in the IND.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research
286
Table of Contents
subjects provide their informed consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the study until completed. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. Depending on its charter, this group may determine whether a trial may move forward at designated check points based on access to certain data from the trial. The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
A sponsor who wishes to conduct a clinical trial outside of the U.S. may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor must ensure that the clinical trial complies with regulatory requirements if the data is to be used in support of NDA approval. The FDA will accept a well-designed and well-conducted foreign clinical trial not conducted under an IND if the trial was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection, if deemed necessary.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism, excretion and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness. |
| • | Phase 2: The product candidate is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages, and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials. |
| • | Phase 3: The product candidate is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy, and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval and labeling. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA. |
In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. These so-called Phase 4 studies may be conducted after initial marketing approval and may be used to gain additional experience from the treatment of patients in the intended therapeutic indication and are commonly intended to generate additional safety data regarding use of the product in a clinical setting. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing
287
Table of Contents
process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality, and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
While the IND is active and before approval, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected AEs, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
In addition, during the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the new drug.
U.S. Review and Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, preclinical, and other nonclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. Data can come from company-sponsored clinical studies intended to test the safety and effectiveness of a use of the product, or from a number of alternative sources, including studies initiated by independent investigators. To support marketing approval, the data submitted must be sufficient to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. The submission of an NDA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances.
The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once accepted for filing, the FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality, and purity. Under the Prescription Drug User Fee Act (“PDUFA”), guidelines that are currently in effect, the FDA has a goal of ten months from the filing date to complete its initial review and act on a standard NDA for a drug that is a new molecular entity, and of ten months from the date of NDA receipt to review and act on a standard NDA for a drug that is not a new molecular entity. The FDA does not always meet its PDUFA goal dates, and the review process is often extended by FDA requests for additional information or clarification.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates, and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes
288
Table of Contents
and facilities are in compliance with cGMP and adequate to assure consistent production of the product within designated specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs and assure the integrity of the clinical data submitted to the FDA. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates an NDA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter or a Complete Response Letter (“CRL”). An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A CRL indicates that the review cycle of the application is complete and the application is not ready for approval. A CRL will describe all of the deficiencies that the FDA has identified in the NDA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the CRL without first conducting required inspections and/or reviewing proposed labeling. In issuing the CRL, the FDA may recommend actions that the applicant might take to place the NDA in condition for approval, including requests for additional information or clarification. The FDA may delay or refuse approval of an NDA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy (“REMS”), to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, assessment plans, and/or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. The FDA may also require one or more Phase 4 post-marketing studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies or surveillance programs.
In addition, the PREA requires a sponsor to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration. Under PREA, original NDAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current, or fails to submit a request for approval of a pediatric formulation.
U.S. Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates. For example, the Fast Track program is intended to expedite or facilitate the process for reviewing new products that are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a Fast Track designated product has opportunities for more frequent interactions with the applicable FDA review team during product
289
Table of Contents
development and, once an NDA is submitted, the product candidate may be eligible for priority review. A Fast Track-designated product may also be eligible for rolling review, where the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted. Rolling review may occur if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
A product candidate intended to treat a serious or life-threatening disease or condition may also be eligible for Breakthrough Therapy designation to expedite its development and review. A product candidate can receive Breakthrough Therapy designation if preliminary clinical evidence indicates that the product candidate, alone or as a combination therapy with one or more other drugs may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the Fast Track program features, as well as more intensive FDA interaction and guidance beginning as early as Phase 1 and an organizational commitment to expedite the development and review of the product candidate, including involvement of senior managers.
A marketing application for a drug submitted to the FDA for approval, including a product candidate with a Fast Track designation and/or Breakthrough Therapy designation, may be eligible for other types of FDA programs intended to expedite the FDA review and approval process, such as priority review. A product candidate is eligible for priority review if it is designed to treat a serious condition, and if approved, would provide a significant improvement in safety or effectiveness compared to available alternatives for such disease or condition. For new-molecular-entity NDAs, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date, or with respect to non-new-molecular-entity NDAs, within six months of the NDA receipt date.
Additionally, product candidates studied for their safety and effectiveness in treating serious or life-threatening diseases or conditions may utilize an accelerated approval pathway upon a determination that the product has an effect on (1) a surrogate endpoint that is reasonably likely to predict clinical benefit or (2) a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA will generally require the sponsor to perform adequate and well-controlled post-marketing clinical studies to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. Products receiving accelerated approval may be subject to withdrawal of its approval if, for example, the sponsor fails to conduct the required post-marketing studies or if such studies fail to verify the predicted clinical benefit. In addition, for products being considered for accelerated approval, the FDA generally requires, unless otherwise informed by the agency, that all advertising and promotional materials intended for dissemination or publication within 120 days of marketing approval be submitted to the agency for review during the pre-approval review period, which could adversely impact the timing of the commercial launch of the product.
Fast Track designation, Breakthrough Therapy designation, priority review designation, and the accelerated approval pathway do not change the scientific or medical standards for approval or the quality of evidence necessary to support approval, but may expedite the development or review process. Even if a product candidate qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
U.S. Marketing Exclusivity
Market exclusivity provisions under the FDCA can delay the submission or the approval of certain marketing applications. The FDA provides periods of non-patent regulatory exclusivity, which provides the holder of an approved NDA limited protection from new competition in the marketplace. Five years of exclusivity are available to new chemical entities (“NCEs”). An NCE is a drug that contains no active moiety that has been approved by the FDA in any other NDA. An active moiety is the molecule or ion, excluding those
290
Table of Contents
appended portions of the molecule that cause the drug to be an ester, salt, including a salt with hydrogen or coordination bonds, or other noncovalent, or not involving the sharing of electron pairs between atoms, derivatives, such as a complex (i.e., formed by the chemical interaction of two compounds), chelate (i.e., a chemical compound), or clathrate (i.e., a polymer framework that traps molecules), of the molecule, responsible for the physiological or pharmacological activity of the drug substance. During the exclusivity period, the FDA may not accept for review or approve an ANDA, or a 505(b)(2) NDA submitted by another company that contains the same active moiety. An ANDA or 505(b)(2) application, however, may be submitted one year before NCE exclusivity expires if a Paragraph IV certification of patent invalidity, unenforceability, or non-infringement is filed.
The FDCA alternatively provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA, if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the active ingredient for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a 505(b)(1) NDA; however, an applicant submitting a 505(b)(1) NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and efficacy.
The FDA may also grant pediatric exclusivity, which provides a six-month extension to existing regulatory or patent exclusivity. To be eligible for pediatric exclusivity, FDA must issue a Written Request detailing the studies to be performed and the timeframe for their completion. If an applicant agrees to perform the studies as outlined in the Written Request, the applicant must submit study reports at least nine months prior to the expiry of the exclusivity that is to be extended. The study reports must demonstrate that the applicant has met the conditions of the Written Request.
U.S. Post-approval Requirements
Drug products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product, which include restrictions on promoting products for unapproved uses or patient populations (known as “off-label use”) and limitations on industry-sponsored scientific and educational activities. In rare cases, pre-approval of promotional materials may be required. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. Further, for certain modifications to the drug, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain prior FDA approval of a new NDA or NDA supplement, which may require the development and submission of additional data. There also are continuing, annual program fees for any marketed products. Drug manufacturers and their subcontractors involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements. Manufacturers and other parties involved in the drug supply chain for prescription drug products must also comply with product tracking and other tracking requirements and must notify the FDA of counterfeit, diverted, stolen and intentionally adulterated products or products that are otherwise unfit for distribution in the United States. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown
291
Table of Contents
problems with a product, including AEs of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or untitled letters; |
| • | clinical holds on clinical studies; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications or suspension or revocation of product approvals; |
| • | product seizure or detention or refusal to permit the import or export of products; |
| • | consent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs; |
| • | mandated modification of promotional materials and labeling and the issuance of corrective information; |
| • | the issuance of safety alerts, Dear Healthcare Provider letters, press releases, and other communications containing warnings or other safety information about the product; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA closely regulates the marketing, labeling, advertising, and promotion of drug products. A company can make only those claims relating to safety and efficacy, purity, and potency that are approved by the FDA and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising, and potential civil and criminal penalties. Physicians may prescribe, in their independent professional medical judgment, legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA-approved labelling.
Other Healthcare Laws
In the United States, drug manufacturers and sponsors are subject to a number of federal and state healthcare regulatory laws that restrict business practices in the healthcare industry. These laws include, but are not limited to, federal and state anti-kickback, false claims, and other healthcare fraud and abuse laws, as follows:
The U.S. federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting, receiving, or providing any remuneration, directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering, or arranging for, or recommending the purchase, lease, or order of any good, facility, item or service reimbursable, in whole or in part, under Medicare, Medicaid, or other federal healthcare programs. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The federal false claims laws, including the civil False Claims Act, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false, fictitious or fraudulent claim for payment to, or approval by, the federal government, knowingly making, using, or causing to be made or used a
292
Table of Contents
false record or statement material to a false or fraudulent claim to the federal government, or knowingly making a false statement to avoid, decrease, or conceal an obligation to pay money to the U.S. federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Actions under the civil False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Moreover, a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
In addition, the civil monetary penalties statute, subject to certain exceptions, prohibits, among other things, the offer or transfer of remuneration, including waivers of copayments and deductible amounts (or any part thereof), to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of services reimbursable by Medicare or a state healthcare program.
The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), created additional federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their respective implementing regulations, which impose obligations on “covered entities,” including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective “business associates” and their respective subcontractors that create, receive, maintain, or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information.
The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services (“CMMS”), information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors), certain other healthcare professionals including physician assistants and nurse practitioners, and teaching hospitals, and applicable manufacturers and applicable group purchasing organizations to report annually to CMMS ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations extend to include transfers of value made to certain non-physician providers (physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists and anesthesiologist assistants, and certified-nurse midwives).
There are federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, and such reported prices may be used in the calculation of reimbursement and/or discounts on approved products.
Similar state and local laws and regulations may also restrict business practices in the pharmaceutical industry, such as state anti-kickback and false claims laws, which may apply to business practices, including but not limited to, research, distribution, sales, and marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, or by patients themselves; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing information and marketing
293
Table of Contents
expenditures or which require tracking gifts and other remuneration and items of value provided to physicians, other healthcare providers and entities; and state and local laws that require the registration of pharmaceutical sales representatives.
Violations of any of these laws and other applicable healthcare fraud and abuse laws may be punishable by criminal and civil sanctions, including fines and civil monetary penalties, the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid), disgorgement and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on companies. Similar sanctions and penalties, as well as imprisonment, also can be imposed upon executive officers and employees of such companies.
Coverage and Reimbursement
Sales of any pharmaceutical product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. In the United States, no uniform policy exists for coverage and reimbursement for pharmaceutical products among third-party payors. Therefore, decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. The process for determining whether a third-party payor will provide coverage for a product typically is separate from the process for setting the price of such product or for establishing the reimbursement rate that the payor will pay for the product once coverage is approved.
Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the FDA-approved products for a particular indication, or place products at certain formulary levels that result in lower reimbursement levels and higher cost-sharing obligation imposed on patients. One third-party payor’s decision to cover a particular medical product or service does not ensure that other payors will also provide coverage for the medical product or service, and the level of coverage and reimbursement can differ significantly from payor to payor. As a result, the coverage determination process will often require us to provide scientific and clinical support for the use of our products to each payor separately, which can be a time-consuming process, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved.
Moreover, as a condition of participating in, and having products covered under, certain federal healthcare programs, such as Medicare and Medicaid, we are subject to federal laws and regulations that require pharmaceutical manufacturers to calculate and report certain price reporting metrics to the government, such as Medicaid Average Manufacturer Price (“AMP”), and Best Price, Medicare Average Sales Price, the 340B Ceiling Price and Non-Federal AMP reported to the Department of Veteran Affairs, and with respect to Medicaid, pay statutory rebates on utilization of manufacturers’ products by Medicaid beneficiaries. Compliance with such laws and regulations require significant resources and any findings of non-compliance may have a material adverse effect on our revenues.
Healthcare Reform
In the United States and certain foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. In the United States, by way of example, in March 2010, the ACA was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States and significantly affected the pharmaceutical industry. The ACA, among other things, increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs; required collection of rebates for drugs paid by Medicaid managed care organizations; required manufacturers to participate in a coverage gap discount program, under which they must agree to offer point-of-sale discounts (increased to 70%, effective as of January 1, 2019) off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; imposed a non-deductible annual fee on pharmaceutical manufacturers or importers
294
Table of Contents
who sell certain “branded prescription drugs” to specified federal government programs; implemented a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected expanded the types of entities eligible for the 340B drug discount program; expanded eligibility criteria for Medicaid programs; created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and established a Center for Medicare Innovation at CMMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
Since its enactment, there have been judicial, administrative, executive and Congressional legislative challenges to certain aspects of the ACA. For example, on June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the ACA will remain in effect in its current form. Further, prior to the U.S. Supreme Court ruling, President Biden issued an executive order that initiated a special enrollment period from February 15, 2021, through August 15, 2021, for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is possible that the Affordable Care Act will be subject to judicial or Congressional challenges in the future. It is unclear how such challenges and the healthcare reform measures of the Biden administration will impact the ACA.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. Following the suspension, a 1% payment reduction will occur beginning April 1, 2022 through June 30, 2022, and the 2% payment reduction will resume on July 1, 2022. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. By way of example, the American Taxpayer Relief Act of 2021, effective January 1, 2024, would eliminate the statutory cap on rebate amounts owed by drug manufacturers under the Medicaid Drug Rebate Program (“MDRP”), which is currently capped at 100% of the AMP for a covered outpatient drug. Further, based on a recent executive order, the Biden administration expressed its intent to pursue certain policy initiatives to reduce drug prices.
Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
We expect additional state and federal healthcare reform measures to be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
Data Privacy and Security Laws
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-
295
Table of Contents
related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act) that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our partners. In addition, certain state and non-U.S. laws, such as the California Consumer Privacy Act (“CCPA”), the California Privacy Rights Act (“CPRA”) and the GDPR, govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations and other obligations are constantly evolving, may conflict with each other to make compliance efforts more challenging, and can result in investigations, proceedings, or actions that lead to significant penalties and restrictions on data processing.
Regulation and Procedures Governing Approval of Medicinal Products in the EU
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates to the extent we choose to sell any of our product candidates outside of the United States. Whether or not we obtain FDA approval for a product, we or our third-party partners must obtain approval of a product by equivalent competent authorities in foreign jurisdictions before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. As in the United States, post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution would apply to any product that is approved outside the United States.
The process governing the marketing authorization (“MA”), of medicinal products in the EU entails satisfactory completion of preclinical studies and adequate and well-controlled clinical trials to establish the safety, quality and efficacy of the medicinal product for each proposed therapeutic indication.
It also requires the submission to the relevant competent authorities of a marketing authorization application (“MAA”) and granting of an MA by these authorities before the product can be marketed and sold in the EU.
The aforementioned EU rules are generally applicable in the European Economic Area (“EEA”), which consists of the 27 EU member states, as well as Norway, Liechtenstein and Iceland.
Failure to comply with EU and member state laws that apply to the conduct of clinical trials, manufacturing approval, MA of medicinal products and marketing of such products, both before and after grant of the MA, or with other applicable regulatory requirements may result in administrative, civil, or criminal penalties. These penalties could include delays or refusal to authorize the conduct of clinical trials, or to grant MA, product withdrawals and recalls, product seizures, suspension, withdrawal, or variation of the MA, total or partial suspension of production, distribution, manufacturing or clinical trials, operating restrictions, injunctions, suspension of licenses, fines and criminal penalties.
EU Non-Clinical Studies and Clinical Trials
Similar to the United States, the various phases of non-clinical research in the EU are subject to significant regulatory controls.
Non-clinical studies are performed to demonstrate the health or environmental safety of new chemical substances. Non-clinical health and environmental safety studies must be conducted in compliance with the principles of good laboratory practice (“GLP”), as set forth in Directive 2004/10/EC. In particular, non-clinical health and environmental safety studies, both in vitro and in vivo, must be planned, performed, monitored, recorded, reported and archived in accordance with the GLP principles, which define a set of rules and criteria
296
Table of Contents
for a quality system for the organizational process and the conditions for non-clinical studies. These GLP standards reflect the Organization for Economic Co-operation and Development requirements.
Until recently, the Clinical Trials Directive 2001/20/EC, the Directive 2005/28/EC on GCP and the related national implementing provisions of the individual EU member states governed the system for the approval of clinical trials in the EU. As of January 31, 2022, the new Clinical Trials Regulation (EU) No 536/2014 took effect and replaced the Clinical Trials Directive 2001/20/EC. Commission Implementing Regulation (EU) 2017/556 replaces the GCP Directive 2005/28/EC. Pursuant to transitional provisions under the Regulation, trials for which a request for approval was submitted prior to January 31, 2022 may continue under the national implementations of the Directives for a period of up to three years. In addition, for a period of 18 months from January 31, 2022, sponsors may elect which process to follow.
The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the EU. The main characteristics of the regulation include: a streamlined application procedure via a single-entry point, the “EU portal”; a single set of documents to be prepared and submitted for the application, as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed by the competent authorities of all EU member states in which an application for authorization of a clinical trial has been submitted (member states concerned). Part II is assessed separately by each member state concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the concerned EU member state. However, overall related timelines are defined by the Clinical Trials Regulation.
Under either the Clinical Trials Directive or the Clinical Trials Regulation, clinical trials of medicinal products in the EU must be conducted in accordance with EU and national regulations and the International Conference on Harmonization (“ICH”), guidelines on GCP, as well as the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. If the sponsor of the clinical trial is not established within the EU, it must appoint an EU entity to act as its legal representative.
Under the Clinical Trials Directive, the sponsor was obliged to take out a clinical trial insurance policy and/or maintain an appropriate indemnity or compensation scheme for clinical trial subjects, and in most EU member states, the sponsor was liable to provide ‘no fault’ compensation to any study subject injured in the clinical trial. Similarly, the Clinical Trials Regulation prescribes that member states must implement a scheme providing for compensation for damage caused by participation in clinical trials within their territory in the form of insurance, a guarantee, or a similar arrangement that is equivalent as regards its purpose and which is appropriate to the nature and the extent of the risk.
Under the applicable regulatory system, an applicant must obtain prior approval from the competent national authority of the EU member states in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial at a specific study site after the competent ethics committee has issued a related favorable opinion. The application for authorization of a clinical trial must be accompanied by, among other documents, a copy of the trial protocol and an investigational medicinal product dossier containing information about the manufacture and quality of the medicinal product under investigation as prescribed by the Clinical Trials Regulation (EU) No 536/2014 and the Implementing Regulation (EU) 2017/556, as applicable, and further detailed in applicable guidance documents. Any substantial changes to the trial protocol or to other information submitted with the clinical trial application must be notified to or approved by the relevant competent national authorities and ethics committees. Medicinal products used in clinical trials must be manufactured in accordance with GMP.
EU Marketing Authorizations
To obtain an MA for a product in the EU, an applicant must submit an MAA either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in the EU member states (decentralized procedure, national procedure, or mutual recognition procedure). An MA may be granted only to an applicant established in the EU.
297
Table of Contents
The centralized procedure comprises a single application, evaluation and authorization and provides for the grant of a single MA by the European Commission that is valid for all EU member states. Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for (i) medicinal products derived from biotechnological processes, (ii) products designated as orphan medicinal products, (iii) advanced therapy medicinal products and (iv) products with a new active substance indicated for the treatment of HIV/AIDS, cancer, neurodegenerative diseases, diabetes, auto-immune and other immune dysfunctions and viral diseases. For products with a new active substance indicated for the treatment of other diseases and products that are a significant therapeutic, scientific or technical innovation or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use (“CHMP”), is responsible for conducting the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing MA.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product targeting an unmet medical need is expected to be of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. If the CHMP accepts a request for accelerated assessment, the time limit of 210 days will be reduced to 150 days (not including clock stops). The CHMP can, however, revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
Unlike the centralized authorization procedure, the decentralized MA procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU member state in which the product is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU member state prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU member states who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU member state cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralised Procedures—Human for review. The subsequent decision of the European Commission is binding on all EU member states.
The mutual recognition procedure allows companies that have a medicinal product already authorized in one EU member state to apply for this authorization to be recognized by the competent authorities in other EU member states. Like the decentralized procedure, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU member states of the MA of a medicinal product by the competent authorities of other EU member states. The holder of a national MA may submit an application to the competent authority of an EU member state requesting that this authority recognize the MA delivered by the competent authority of another EU member state.
In principle, an MA has an initial validity of five years. The MA may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU member state in which the original MA was granted. To support the application, the MA holder must provide the EMA or the competent authority with a consolidated version of the Common Technical Document, providing up-to-date data concerning the quality, safety and efficacy of the product, including all variations introduced since the MA was granted, at least nine months before the MA ceases to be valid. The European Commission or the competent authorities of the EU member states may decide, on justified grounds relating to pharmacovigilance, to proceed with one further five-year renewal period for the MA. Once subsequently definitively renewed, the MA shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (in case of centralized procedure) or on the market of the authorizing EU member state within three years after authorization ceases to be valid (the so-called sunset clause).
298
Table of Contents
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines (“PRIME”), scheme, which provides incentives similar to the breakthrough therapy designation in the United States. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that show the potential to target unmet medical needs. It permits increased interaction and early dialogue with companies developing promising medicinal products, to optimize their product development plans and speed up their evaluation to help the product reach patients as early as possible. Product developers that benefit from PRIME designation are potentially eligible for accelerated assessment of their MAA although this is not guaranteed. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
In the EU, a “conditional” MA may be granted in cases where all the required safety and efficacy data are not yet available. The conditional MA is subject to conditions to be fulfilled for generating the missing data or ensuring increased safety measures. It is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once the specific obligations under the conditional MA are fulfilled (such as the completion of certain ongoing or new studies) and the complete data confirm that the medicinal product’s benefits continue to outweigh its risks, the conditional MA can be converted into a traditional MA. However, if the specific obligations are not fulfilled within the timeframe set by the EMA, the MA will cease to be renewed.
An MA may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on the efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information or when generating data may be contrary to generally accepted ethical principles. Like a conditional MA, an MA granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard MA. However, unlike the conditional MA, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data on the medicinal product’s efficacy and safety under normal conditions of use. Although the MA “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually and the MA is withdrawn in case the risk-benefit ratio is no longer favorable.
In addition to an MA, various other requirements apply to the manufacturing and placing on the EU market of medicinal products. Manufacture of medicinal products in the EU requires a manufacturing authorization, and import of medicinal products into the EU requires a manufacturing authorization allowing for import. The manufacturing authorization holder must comply with various requirements set out in the applicable EU laws, regulations and guidance. These requirements include compliance with EU GMP standards when manufacturing medicinal products and APIs, including the manufacture of APIs outside of the EU with the intention to import the APIs into the EU. Similarly, the distribution of medicinal products within the EU is subject to compliance with the applicable EU laws, regulations and guidelines, including the requirement to hold appropriate authorizations for distribution granted by the competent authorities of the EU member states. MA holders, manufacturing and import authorization (MIA) holders or distribution authorization holders may be subject to civil, criminal or administrative sanctions, including suspension of manufacturing authorization, in case of non-compliance with the EU or EU member states’ requirements applicable to the manufacturing of medicinal products.
EU Data and Market Exclusivity
The EU provides opportunities for data and market exclusivity related to MAs. Upon receiving an MA, innovative medicinal products are generally entitled to receive eight years of data exclusivity and ten years of market exclusivity. Data exclusivity, if granted, prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar MAA can be submitted, and the
299
Table of Contents
innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until ten years have elapsed from the initial MA of the reference product in the EU. The overall ten-year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the MA holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical entity, and products may not qualify for data exclusivity.
In the EU, there is a special regime for biosimilars products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials regarding comparability must be provided in support of an application for MA.
EU Post-Approval Requirements
Where an MA is granted in relation to a medicinal product in the EU, the holder of the MA is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products.
Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission or the competent regulatory authorities of the individual EU member states. The holder of an MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports (“PSURs”).
All new MAAs must include a risk management plan describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
In the EU, the advertising and promotion of medicinal products are subject to both EU and EU member states’ laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals or organizations, misleading and comparative advertising and unfair commercial practices. Although these general requirements for advertising and promotion of medicinal products are established under EU directives, the details are governed by regulations in each member state and can differ from one country to another. For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics (“SmPC”), as approved by the competent authorities in connection with an MA. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU. There is also a prohibition on the offer or supply of inappropriate inducements to prescribe, subject to exemptions in certain jurisdictions, such as benefits that inexpensive and relevant to the practice of medicine.
Japanese Drug Regulation
Japan is a member of the ICH, and has pharmaceutical law and regulations that are similar in many respects those of the United States and the European Union. Those requirements are embodied in the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (also known as the Pharmaceuticals and Medical Devices Act) and related cabinet orders, Ministerial ordinances, and guidelines.
Clinical trials of medicinal products in Japan must be conducted in accordance with Japanese regulations and the ICH Good Clinical Practice Guidelines (“GCP”). If the sponsor of the clinical trial is not an entity
300
Table of Contents
within Japan, it must appoint a domestic entity to act as its agent and carry out obligations on the overseas sponsor’s behalf. The sponsor must hold a clinical trial insurance policy, and in accordance with industry practice, should establish a compensation policy for the injuries from the trial.
Prior to the commencement of human drug clinical trial, the sponsor must complete a pre-clinical safety evaluation of the investigative product and submit a clinical trial notification, including the clinical trial protocol, to the Ministry of Health Labor and Welfare’s (“MHLW’s”) Pharmaceutical and Medical Device Agency (“PMDA”). This notification must be submitted after obtaining agreement of the investigational review board (“IRB”) in relevant clinical trial institution(s). If the authorities do not raise an issue or comment on the notification application within 30 days, the sponsor may proceed to conclude clinical trial agreement(s) with the site(s) and commence the clinical trial. Any substantial changes to the trial protocol or other information submitted must be cleared by the IRB and notified to the authorities. Medicines used in clinical trials must be manufactured in accordance with Japan’s good manufacturing practices (“GMP”).
Non-clinical studies performed to demonstrate the safety of new chemical or biological substance must be conducted in compliance with the principles of Japanese Good Laboratory Practice rules (“GLP”), which reflect the Organization for Economic Co-operation and Development (“OECD”) requirements. Currently, Japan and EU have a mutual recognition agreement for GLP, and data generated compliant with EU requirements will be accepted by the Japanese authorities. There is no similar agreement with the United States, but this is not a significant issue because of the OECD arrangement.
To market an innovative medicinal product in Japan, domestic or overseas applicant must obtain government approval (or marketing authorization) through a new drug application. If the product is designed for treating certain difficult diseases or those for which the patient population is limited, the applicant may be able to obtain designation as an orphan drug product if it demonstrates unique therapeutic value. There are also expedited programs for (i) truly innovative products with a unique mechanism of action and (ii) products which will satisfy unmet medical needs.
The evaluation of new drug applications is based on PMDA’s assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy. Once PMDA completes its review, the matter is considered by the advisory committee of experts, and the government grants approval upon any positive recommendation from the committee. If foreign data are part of the applicant, a dose response clinical trial for Japanese subjects may be required to ensure that data can be extrapolated to Japan’s population.
Separate from the approval requirement, it is also mandatory that the marketing authorization holder or its partner in Japan possess a distribution license of an appropriate class to commercially distribute the product in Japan. Companies in Japan that actually manufacture drugs must possess a drug manufacturing license, and overseas manufacturers must obtain a manufacturing certification.
People’s Republic of China (“PRC”) Drug Regulation
China heavily regulates the development, approval, manufacturing, and distribution of drugs, including biologics. For purposes of the below description of drug regulation in China, Hong Kong, Macao and Taiwan, which are governed by separate laws, are excluded. The regulatory requirements applicable depend, in part, on whether the drug is made and finished in China, which is referred to as a domestically manufactured drug, or made abroad and imported into China in finished form, which is referred to as an imported drug, as well as the approval or “registration” category of the drug. For both imported and domestically manufactured drugs, China typically requires regulatory approval for a clinical trial application (“CTA”) to conduct clinical trials in China and submit China clinical trial data, prior to submitting an application for marketing approval. For imported drugs, the sponsor and marketing authorization holder must be an overseas company that holds (or will hold) a marketing authorization in another country.
China also prioritizes review and approval of drugs and improvements to drugs (e.g., new indications, routes of administration) that have not yet been approved in any other jurisdiction (i.e., new to the world). In addition, China has created a set of expedited programs for drugs in high priority disease areas and drugs that more effectively treat life-threatening illnesses or that are needed for national emergencies.
301
Table of Contents
The framework law in the drug space in China is the PRC Drug Administration Law (“DAL”). The DAL is implemented by various regulations and rules. The primary drug authority that regulates the life cycle of drugs is the NMPA. The NMPA has its own set of regulations further implementing the DAL. The regulation governing CTAs, marketing approval, and post-approval amendment and renewal is known as the Drug Registration Regulation (“DRR”).
PRC Regulatory Authorities and Recent Government Reorganization
As noted above, NMPA is the drug regulatory agency that implements the laws, regulations, rules, and guidelines governing almost all of the key stages of the life-cycle of pharmaceutical products, including nonclinical studies, clinical trials, marketing approvals, manufacturing, advertising and promotion, distribution, and pharmacovigilance (i.e., post-marketing safety reporting obligations). NMPA’s Center for Drug Evaluation (“CDE”) approves clinical trials and conducts the technical evaluation of each drug and biologic marketing application to assess safety and efficacy. Provincial-level medical products administrations help to enforce these rules, and issue entity licenses to domestic companies, such as drug manufacturing and distribution licenses.
The National Health Commission of the PRC (“NHC”) is China’s primary healthcare regulatory agency. It is responsible for regulating the health care system, including the licensure of medical institutions, which also serve as clinical trial sites, and credentialing of medical personnel.
PRC Breakthrough Therapy Designation by the NMPA
Among other expedited programs, China administers a Breakthrough Therapy Designation. To qualify, a drug must be new to the world, intended to treat a life-threatening disease or one that can seriously impact quality of life, and for which there is no existing therapy in China or a demonstrated substantial improvement over available therapies. Drugs that are designated as breakthrough therapies will receive priority in meeting scheduling, enhanced guidance from CDE to expedite drug development, and may also qualify for other expedited programs, such as priority review and conditional approval.
PRC Non-Clinical Research
The NMPA requires preclinical data to support registration applications for imported and domestic drugs. For domestic laboratories, NMPA oversees an accreditation program pursuant to China’s Good Laboratories Practice. If the pre-clinical research is conducted outside of China, then the applicant must sign and submit a certification with its CTA and marketing application stating that such research was conducted in accordance with applicable good laboratory practice rules.
PRC Clinical Trials and Regulatory Approval
Upon completion of preclinical studies, a sponsor will often need to conduct clinical trials in China to support registration. The materials required for a clinical trial application are substantial even at the CTA stage, including detailed manufacturing information. Drug registration trials in China many only be conducted after obtaining approval of a CTA submitted to CDE, approval of the ethics committee at each accredited hospital site, and human genetic resource approval (“HGR”), which is required for the collection of samples and associated data. CTAs may be approved in 60 business days if there is no comment from CDE, and the other applications can take approximately 3-4 months each. Information about clinical trials must be registered on an NMPA-administered platform and continually updated during the trial, and certain information, not including the protocol, is made publicly available on the platform.
PRC Trial Exemptions and Acceptance of Foreign Data
The NMPA may reduce requirements for clinical trials and data, depending on the drug and the existing data. In some cases, NMPA has granted waivers for all or part of trials and has stated that it will accept data
302
Table of Contents
generated abroad (even if not part of a global study with a site in China), including early phase data, that meets its requirements. According to the Technical Guidance Principles on Accepting Foreign Drug Clinical Trial Data the data from foreign clinical trials must meet China’s authenticity, completeness, accuracy, and traceability requirements, and be obtained consistent with the relevant requirements under the China’s Drug Good Clinical Practice rule (“GCP”). Sponsors must be attentive to potentially meaningful ethnic differences in the subject populations.
PRC Clinical Trial Process and Good Clinical Practices
Pre-market drug clinical trials may have three phases, which may each require a CTA. These clinical trials must be conducted in accordance with a protocol that NMPA, various ethics committees at different sites, and the Office of HGR Administration all clear as part of the aforementioned approvals, and in accordance with applicable drug rules, including China’s Drug GCP, issued jointly by NMPA and NHC. Trials must also be conducted at sites that have received credentials from the NHC and NMPA.
China is a member of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (“ICH”), so its GCP resemble the ICH GCP in a great many respects. However, there are some differences. For example, under China’s GCP the sponsor must provide legal and economic guarantee to the investigator for clinical trial-related injuries, but harm or death caused by medical negligence is excluded. The drug rules contain procedures for amending the clinical trial approval, including obtaining approval for safety-related protocol amendments. NMPA (specifically, its Center for Food and Drug Inspections) has the power to audit trials for GCP compliance during and after the clinical trial.
PRC Drug Marketing Application and Approval
Upon completion of the development process, the applicant may submit a marketing authorization application to CDE. CDE will organize pharmaceutical, medical, and other technical personnel to conduct a review of the safety, efficacy, and quality controllability of the drug based on the application materials submitted, and the results of a verification and inspection (if required). If NMPA decides to approve the drug based on CDE’s opinion, it will issue a drug registration certificate (i.e., a marketing authorization). A marketing authorization must be renewed every five years.
As the marketing authorization holder (“MAH”), a drug company is responsible for the life cycle of the product, including development, production and distribution, post-market studies, routine annual reporting, and the safety monitoring and reporting, among other obligations. The MAH may engage third parties to fulfill some of these obligations, such as appropriately-qualified manufacturers and distributors. If the MAH is overseas, as is required for imported drugs, the MAH must appoint an agent, which must be an entity in China that assists with meeting regulatory obligations. Marketing authorizations can be transferred to entities with the required capacity.
Both investigational and marketed drugs must be made in accordance with China GMPs. Domestic manufacturers must have a drug manufacturing license, and overseas manufacturers must certify that they will make drugs in accordance with GMP and meet their home country’s requirements. Drugs must be distributed in China by licensed drug distributors.
Employees and Human Capital Resources
As of September 15, 2022, we had 11 full-time employees, consisting of clinical, research and development, business development, regulatory, finance and operational personnel, who are employed through our wholly owned subsidiary in the United States, NewAmsterdam Pharma Corporation. None of our employees are subject to a collective bargaining agreement. We consider our relationship with our employees to be good. We have also engaged consultants to assist with our clinical development and regulatory obligations. Our Dutch wholly owned subsidiary, NewAmsterdam Pharma B.V., engages a total of 36 independent contractors. These independent contractors provide a diverse array of services, which includes assisting with our clinical development and regulatory obligations.
303
Table of Contents
NewAmsterdam Pharma Holding B.V. does not directly employ anyone or engage any independent contractors (zelfstandigen zonder personeel). No Works Council or other employee representative body (personeelsvertegenwoordiging) is established within NewAmsterdam Pharma Holding B.V. or NewAmsterdam Pharma B.V.
We recognize that our continued ability to attract, retain and motivate exceptional employees is vital to ensuring our long-term competitive advantage. Our employees are critical to our long-term success and are essential to helping us meet our goals. Among other things, we support and incentivize our employees in the following ways:
| • | Talent development, compensation and retention: We strive to provide our employees with a rewarding work environment, including the opportunity for growth, success and professional development. We provide a competitive compensation and benefits package, including bonus and equity incentive plans, a 401(k) plan—all designed to attract and retain a skilled and diverse workforce. |
| • | Health and safety: We support the health and safety of our employees by providing comprehensive insurance benefits, company-paid holidays, a personal time-off program and other additional benefits which are intended to assist employees to manage their well-being. |
| • | Inclusion and diversity: We are committed to efforts to increase diversity and foster an inclusive work environment that supports our workforce. |
Facilities
Our current corporate headquarters are located in Naarden, the Netherlands, where we lease a small office space pursuant to a lease agreement entered into on July 3, 2020 and which will continue until terminated by either us or the landlord upon one-month prior written notice. In May 2021, we entered into a lease to rent approximately 1,375 square feet of office space in Miami, Florida. The Miami lease will expire on October 31, 2024.
We believe that these existing facilities will be adequate for our near-term needs. If required, we believe that suitable additional or alternative space would be available in the future on commercially reasonable terms.
COVID-19 Impact on Facilities
We have implemented policies that enable our employees to work remotely, and such policies may continue for an indefinite period. We continue to evaluate our protocols and practices as the global response to the COVID-19 pandemic continues to evolve and continue to adhere to local, state and federal guidelines. While we are partially operating virtually in light of the COVID-19 pandemic, we believe our operational needs are being met for the time being. To date, we have not experienced any material impact on our ability to operate our business. We plan to periodically reassess the impact of COVID-19 on our facility needs.
Legal Proceedings
From time to time, we may become involved in material legal proceedings or be subject to claims arising in the ordinary course of our business. We are currently not party to any legal proceedings material to our operations or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by a government authority.
Corporate History
We were incorporated pursuant to the laws of the Netherlands as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) under the name NewAmsterdam Pharma B.V. with a corporate seat in Naarden, the Netherlands on October 17, 2019. We changed our name to NewAmsterdam Pharma Holding B.V. in October 2021. Our principal executive office is at Gooimeer 2-35, 1411 DC, Naarden, the Netherlands. Our principal subsidiary, Dezima Pharma B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid), was incorporated on August 31, 2012, pursuant to the laws of the Netherlands with its corporate seat in Breda, the Netherlands. Dezima changed its name to NewAmsterdam Pharma B.V. in October 2021.
304
Table of Contents
On April 9, 2020, we entered into a share sale and purchase agreement with Amgen to purchase all of the outstanding share capital of Dezima, such that Dezima became our wholly owned subsidiary. As consideration for the sale of the outstanding shares of capital stock in Dezima, we paid Amgen one Euro and granted Amgen the right to receive ordinary shares in the event we closed an initial public offering or the right to receive a portion of the proceeds we would receive upon consummation of certain corporate transactions. We separately entered into a profit right and waiver agreement with MTPC pursuant to which MTPC waived certain historical intellectual property rights and related payment rights in exchange for the right to receive ordinary shares in the event we closed an initial public offering or the right to receive a portion of the proceeds we would receive upon consummation of certain corporate transactions. See the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement” for additional detail.
Since our incorporation in October 2019, we have made $37,706 in principal capital expenditures, relating primarily to the purchase of computers and office equipment. These expenditures related primarily to clinical costs and manufacturing.
305
Table of Contents
NEWAMSTERDAM PHARMA’S MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of NewAmsterdam Pharma’s financial condition and results of operations together with its consolidated financial statements as at December 31, 2021 and for the years ended December 31, 2021 and 2020 and the related notes thereto, the unaudited condensed consolidated financial statements as at June 30, 2022 and for the six months ended June 30, 2022 and 2021 and the related notes thereto, and the unaudited pro forma condensed combined financial information, included elsewhere in this proxy statement/prospectus. The following discussion is based on the NewAmsterdam Pharma’s financial information prepared in accordance with IFRS, which may differ in material respects from generally accepted accounting principles in other jurisdictions, including U.S. GAAP. Some of the information contained in this discussion and analysis or set forth elsewhere in this proxy statement/prospectus, including information with respect to Holdco’s and NewAmsterdam Pharma’s plans and strategy for the business, includes forward-looking statements that involve risks and uncertainties. Please see the section entitled “Special-Note Regarding Forward Looking Statements.” You should also review the section titled “Risk Factors” for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Unless the context otherwise requires, any reference in the section below to “we,” “us” or “our” refers to NewAmsterdam Pharma Holding B.V. and its subsidiaries prior to the Business Combination, which will be the business of Holdco and its consolidated subsidiaries after giving effect to the Business Combination.
Overview
We are a clinical-stage biopharmaceutical company developing oral, non-statin medicines for patients at high risk of CVD with residual elevation of LDL-C, for whom existing therapies are not sufficiently effective or well-tolerated. There exists a significant unmet need for a potent, cost-effective and convenient LDL-lowering therapy as an adjunct to statins, a class of lipid-lowering medications that are the current standard of care for high-risk CVD patients with high cholesterol. Our lead product candidate, obicetrapib, is a next-generation, oral, low-dose CETP inhibitor, that is currently in four ongoing Phase 3 and Phase 2b clinical trials as both a monotherapy and a combination therapy with ezetimibe for lowering LDL-C and preventing MACE.
CVD is a leading cause of death worldwide and the top cause of death in the United States. ASCVD is primarily caused by atherosclerosis, which involves the build-up of fatty material within the inner walls of the arteries. Atherosclerosis is the primary cause of heart attacks, strokes and peripheral vascular disease. One of the most important risk factors for ASCVD is hypercholesterolemia, which refers to elevated LDL-C levels within the body, commonly known as high cholesterol.
A significant proportion of patients with high cholesterol do not achieve acceptable LDL-C levels using statins alone. We estimate that there are more than 30 million patients in the EU5 and in the United States who are not achieving LDL-lowering goals on the current standard of care. Existing non-statin treatment options have been largely unable to address the needs of patients with high cholesterol due to modest efficacy, prohibitive pricing, or an inconvenient and painful injectable administration route. It is estimated that over 75% of ASCVD outpatients prefer oral drugs to injectable therapies.
Our product candidate, obicetrapib, is a next-generation, oral, low-dose CETP inhibitor that we are developing to potentially overcome the limitations of current LDL-lowering treatments. We believe that obicetrapib has the potential to be a once-daily oral CETP inhibitor for lowering LDL-C, if approved. In our Phase 2b ROSE trial, obicetrapib demonstrated a 51% lowering of LDL-C from baseline at a 10 mg dose level on top of high-intensity statins. In all three of our Phase 2 trials, TULIP, ROSE and OCEAN, evaluating obicetrapib as a monotherapy or a combination therapy, we observed statistically significant LDL-lowering activity combined with generally moderate side effects and no drug-related, treatment-emergent serious AEs. Obicetrapib has demonstrated strong tolerability in more than 600 patients with dyslipidemia in our clinical trials to date. Obicetrapib is also expected to be relatively low in cost to manufacture compared to most other branded LDL-lowering therapies on the market with high
306
Table of Contents
efficacy. We believe that the estimated low cost of goods for obicetrapib will enable favorable pricing and position it to significantly improve patient access to a high efficacy LDL-lowering therapy compared to existing non-statin treatments. Furthermore, we believe that obicetrapib’s oral delivery, demonstrated activity in low doses, chemical properties and tolerability make it well-suited for combination approaches. We are developing a fixed dose combination of obicetrapib 10 mg and ezetimibe 10 mg, which we believe will demonstrate even greater potency.
Lowering of LDL-C, and particularly ApoB-containing lipoproteins, has been associated with MACE benefit in trials of LDL-lowering drugs, including the REVEAL study with the CETP inhibitor, anacetrapib. We are performing a CVOT to reconfirm this relationship.
Our goal is to develop and commercialize a LDL-lowering monotherapy and combination therapy, which offers the cost and convenience advantages of a once-daily oral pill, and fulfills the significant unmet need for an effective and convenient LDL-lowering therapy. If we obtain marketing approval, we intend to commercialize obicetrapib for patients suffering from CVD independently and with strategic partners in certain jurisdictions. We have partnered with Menarini, to provide them with the exclusive rights to commercialize obicetrapib either as a sole active ingredient product or in a fixed dose combination with ezetimibe in the majority of European countries, if approved. Subject to receipt of marketing approval, our current plan is to pursue development and commercialization of obicetrapib in the United States ourselves, and to consider additional partners for jurisdictions outside of the United States and the EU, including in Japan, China and the United Kingdom.
Business Impact of the COVID-19 Pandemic and the Conflict in Ukraine
As a result of the spread of the COVID-19 pandemic and the Russian invasion of Ukraine, economic uncertainties have arisen which may negatively affect our financial position, results of operations and cash flows. These uncertainties include, among other things, downturns in the financial markets or in economic conditions, increases in oil prices, inflation, increases in interest rates, supply chain disruptions, and declines in consumer confidence and spending. We have assessed that the COVID-19 pandemic and the conflict in Ukraine did not have a material or direct impact on our operations or financial position. Nevertheless, in light of the ongoing COVID-19 pandemic, we have implemented measures to protect employees and take social responsibility while at the same time attempting to limit any negative effects on our business. The duration of uncertainties and the ultimate financial effects resulting from the ongoing COVID-19 pandemic and the conflict in Ukraine cannot be reasonably estimated at this time. We will continue to monitor these situations very closely and implement further measures if we believe they are required.
Components of our Results of Operations
Revenue
To date, our revenue has solely been derived from our license agreement with Menarini. Pursuant to the Menarini License, we received a non-refundable, non-creditable upfront amount of €115.0 million from Menarini on July 7, 2022, €93.5 million of which was recognized as revenue upon the execution of the Menarini License on June 23, 2022.
Additionally, in partial contribution our costs of development of the licensed products, Menarini may pay us €27.5 million, payable in two equal annual installments. Due to the scientific uncertainties around the commercialization of the licensed products based on the success of clinical trials, out of our control, the fixed €27.5 million is considered constrained at contract execution and is not initially recognized within the transaction price until it becomes highly probable of no significant revenue reversal. At the end of each reporting period, we will assess the probability of significant reversals for any amounts that become likely to be realized prior to recognizing the fixed consideration associated with these payments within the transaction price.
Under the Menarini License, we are also entitled to receive fixed reimbursement payments for our continued development costs, certain cost sharing payments, sales-based royalties, as well as payments based upon the
307
Table of Contents
achievement of defined development, regulatory and commercial milestones. These milestones are contingent payments and represent variable considerations that are not initially recognized within the transaction price. Our ability to receive and generate revenue from these payments is dependent upon a number of factors, including our ability to successfully complete the development of and obtain regulatory approval for obicetrapib within the Menarini Territory. We do not expect to achieve any milestones this year. The uncertainty of achieving these milestones significantly impacts our ability to generate revenue. At the end of each reporting period, we will assess the probability of significant reversals for any amounts that become likely to be realized prior to recognizing the variable consideration associated with these payments within the transaction price.
We do not expect to generate any revenue from product sales for the foreseeable future. Any revenue generated from potential future collaborations may vary due to the many uncertainties in the development of obicetrapib and other factors.
Research and Development Expenses
Research and development expenses are recognized as an expense when incurred and are typically made up of costs from our clinical and preclinical activities, drug development and manufacturing costs, and costs for clinical research organizations (“CROs”) and investigative sites. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data provided by vendors of their actual costs incurred. At each statement of financial position date, we estimate the level of services provided by vendors and the associated expenditure incurred for the services performed.
All such costs are for the purpose of advancing our product candidate to successfully complete clinical development, attain regulatory approval and, if approved, commercialize our product candidate. We have commenced a Phase 3 CVOT trial and two other Phase 3 trials in 2022. Much of the current focus in the Phase 3 trials is on project startup activities, the search for qualified investigator sites and patient enrollment and screening. Research and development expenses consist of the following:
| • | clinical expenses primarily incurred by CROs assisting with our sponsored clinical trials and including clinical investigator costs, patient enrollments and costs of clinical sites; |
| • | manufacturing expenses arising from active pharmaceutical ingredient (“API”) and drug product development as performed by our CMOs, which are used in our clinical trials and research and development activities; |
| • | costs associated with obtaining potential regulatory approval of our product candidate, including preparation and submission of filings, ongoing monitoring and compliance with comments and recommendations provided by regulatory authorities, and regulatory-related advisory fees; |
| • | contracted personnel and employment costs attributed to research and development efforts, which includes management fees, salaries, share-based compensation expenses, bonus plans and payments to contractors who work for us for a fixed number of hours per week or per month; |
| • | preclinical and nonclinical research and development expenses of the product candidate, primarily for costs incurred by CROs assisting with an ongoing two-year rat and hamster carcinogenicity study; and |
| • | other clinical costs such as clinical trial insurance and other consultancy fees. |
We expect our research and development expenses to increase substantially in the future as we advance obicetrapib through clinical trials and pursue regulatory approval. The process of conducting the necessary clinical trials to obtain regulatory approval is costly and time-consuming. Clinical trials generally become larger and more costly to conduct as they advance into later stages and, in the future, we will be required to make estimates for expense accruals related to clinical trial expenses. At this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the development of obicetrapib. See the section entitled “Risk Factors—Risks Related to NewAmsterdam Pharma’s Product Development, Regulatory Approval and Commercialization” for more information regarding the risks associated with clinical development.
308
Table of Contents
General and Administrative Expenses
We recognize general and administrative expenses on the accrual basis when incurred. These expenses mainly relate to consultant fees, employee costs, legal costs, marketing and communication, intellectual property costs due to increased efforts to drug patent development and protection globally, and general overhead costs.
Finance Income
Finance income is recognized using the effective interest rate method and is related to the €709 thousand loan provided to our chief executive officer, Michael Davidson, M.D., pursuant to the loan agreement, dated June 28, 2021 (the “Davidson Loan Agreement”). The principal amount of the Davidson Loan Agreement was used to pay for depository receipts issued by Stichting Administratiekantoor NewAmsterdam Pharma, a Dutch foundation which holds shares on behalf of certain of our shareholders, to Dr. Davidson.
On July 19, 2022, Dr. Davidson repaid the entire principal amount outstanding under the Davidson Loan Agreement, plus outstanding unpaid interest.
Finance Expense
Finance expense is recognized using the effective interest rate method and relates to our negative interest charged on cash balances, interest on the Convertible Loan (up to December 31, 2020) and the interest on lease liabilities (from January 1, 2021 onwards).
Net Foreign Exchange Gain
Our exchange gain relates mainly to cash and cash equivalent balances denominated in foreign currencies, but also to transactions denominated in foreign currencies, mainly in U.S. Dollars, British Pounds, Canadian Dollars and Japanese Yen, which generate exchange gains and losses. As at June 30, 2022, our net exposure to foreign currency risk was €5,333 thousand, compared to €15,914 thousand as at December 31, 2021, mainly related to the U.S. Dollar.
Income Tax
We have a history of losses and therefore have not paid corporate tax. We expect to continue incurring losses as we continue to invest in our clinical and preclinical development programs. Consequently, we do not have any deferred tax assets on our statement of financial position.
Results of Operations
Comparison of results of operations for the six months ended June 30, 2022 and 2021
The following table summarizes our consolidated statements of profit or loss for each period presented:
| For the six months ended June 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Revenue | 93,500 | — | 93,500 | |||||||||
Research and development expenses | (30,588 | ) | (8,553 | ) | (22,035 | ) | ||||||
General and administrative expenses | (9,294 | ) | (1,401 | ) | (7,893 | ) | ||||||
Total operating expenses | (39,882 | ) | (9,954 | ) | (29,928 | ) | ||||||
Finance income | 10 | — | 10 | |||||||||
Finance expense | (185 | ) | (137 | ) | (48 | ) | ||||||
Net foreign exchange gain | 1,070 | 609 | 461 | |||||||||
Profit (loss) before tax | 54,513 | (9,482 | ) | 63,995 | ||||||||
Income tax expense | — | — | — | |||||||||
Profit (loss) for the period | 54,513 | (9,482 | ) | 63,995 | ||||||||
309
Table of Contents
Revenue
Revenue increased by €93.5 million, from nil for the six months ended June 30, 2021 to €93.5 million for the six months ended June 30, 2022. This was driven by the revenue resulting from the partial allocation of the €115.0 million upfront payment received pursuant to the license performance obligation granted upon execution of the Menarini License on June 23, 2022.
Research and Development Expenses
Research and development expenses increased by €22.0 million, or 257.6%, from €8.6 million for the six months ended June 30, 2021 to €30.6 million for the six months ended June 30, 2022. This was largely driven by an increase of €16.8 million in Phase 3 clinical research and development costs, which related to a ramp-up in activities for our two Phase 3 lipid clinical trials (BROADWAY and BROOKLYN) and our CVOT (PREVAIL) trial. In addition, the increase in research and development expenses was also the result of an increase of €2.7 million in chemistry, manufacturing and controls costs due to API campaign costs for the startup of our Phase 3 clinical trials.
We begin separately tracking program expenses at candidate nomination, or when acquired, and accumulate all costs incurred to support each program to date.
| For the six months ended June 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Phase 1 and 2 | (3,850 | ) | (3,316 | ) | (534 | ) | ||||||
Phase 3 | (16,872 | ) | (51 | ) | (16,821 | ) | ||||||
Other | (304 | ) | (24 | ) | (280 | ) | ||||||
Clinical research and development costs | (21,026 | ) | (3,391 | ) | (17,645 | ) | ||||||
Non-clinical research and development costs | (1,264 | ) | (105 | ) | (1,159 | ) | ||||||
Contracted personnel costs | (1,869 | ) | (1,102 | ) | (767 | ) | ||||||
Chemistry, manufacturing and controls (“CMC”) | (6,001 | ) | (3,263 | ) | (2,738 | ) | ||||||
Regulatory | (381 | ) | (682 | ) | 301 | |||||||
Other research and development costs | (47 | ) | (10 | ) | (37 | ) | ||||||
Total research and development expenses | (30,588 | ) | (8,553 | ) | (22,035 | ) | ||||||
General and Administrative Expenses
General and administrative expenses increased by €7.9 million, or 563.4%, from €1.4 million for the six months ended June 30, 2021 to €9.3 million for the six months ended June 30, 2022. This was mainly driven by an increase of €4.8 million in personnel-related costs, the result of increased hiring of individuals across all departments of the company, and an increase of €2.3 million in intellectual property and other legal costs, driven by increased legal costs due to increased efforts related to drug patent development and global patent protection efforts, legal services rendered with respect to internal restructuring, due diligence, and business development.
| For the six months ended June 30, | ||||||||||||
| 2022 | 2021 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Personnel-related costs | (5,450 | ) | (642 | ) | (4,808 | ) | ||||||
Intellectual property and other legal costs | (2,949 | ) | (601 | ) | (2,348 | ) | ||||||
Marketing and communication | (619 | ) | (34 | ) | (585 | ) | ||||||
Facility-related and other costs | (276 | ) | (124 | ) | (152 | ) | ||||||
Total general and administrative expenses | (9,294 | ) | (1,401 | ) | (7,893 | ) | ||||||
310
Table of Contents
Finance Income
Finance income was €0.01 million for the six months ended June 30, 2022. This increase was driven by the accrued finance expense on the loan facility provided to our chief executive officer, Dr. Davidson, in June 2021, which has been fully repaid as described above. We recorded no finance income for the six months ended June 30, 2021.
Finance Expense
Finance expense increased by €0.048 million, from €0.1 million for the six months ended June 30, 2021 to €0.2 million for the six months ended June 30, 2022. This increase was largely driven by an increase in negative interest charged on cash balances.
Net Foreign Exchange Gain
Net foreign exchange gain increased by €0.5 million, from €0.6 million for the six months ended June 30, 2021 to €1.1 million for the six months ended June 30, 2022. This was largely driven by the effect of the appreciation of the U.S. Dollar on cash balances held in U.S. Dollars at the end of the year. The “Foreign Currency Risk” section below includes more information regarding our exposure to foreign currencies.
Income Tax Expense
We generated profit for the six months ended June 30, 2022, however we expect our costs to fund research and development activities for the full fiscal year to exceed our revenue arising from the execution of the Menarini License. As such, we do not expect to generate taxable income for the fiscal year ending December 31, 2022. We recorded no income tax expense for the six months ended June 30, 2022 and 2021.
Profit (loss) for the Period
Profit for the period increased by €64.0 million, from a loss of €9.5 million for the six months ended June 30, 2021 to a profit of €54.5 million for the six months ended June 30, 2022, mainly driven by the execution of the Menarini License.
Comparison of results of operations for the year ended December 31, 2021 and 2020
The following table summarizes our consolidated statements of profit or loss for each period presented:
| For the year ended December 31, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Research and development expenses | (25,032 | ) | (4,045 | ) | (20,987 | ) | ||||||
General and administrative expenses | (4,803 | ) | (1,384 | ) | (3,419 | ) | ||||||
Total operating expenses | (29,835 | ) | (5,429 | ) | (24,406 | ) | ||||||
Finance income | 9 | — | 9 | |||||||||
Finance expense | (216 | ) | (344 | ) | 128 | |||||||
Net foreign exchange gain | 1,443 | 24 | 1,419 | |||||||||
Loss before tax | (28,599 | ) | (5,749 | ) | (22,850 | ) | ||||||
Income tax expense | — | — | — | |||||||||
Total Comprehensive loss for the period, net of tax | (28,599 | ) | (5,749 | ) | (22,850 | ) | ||||||
Research and Development Expenses
Research and developments expenses increased by €21.0 million, or 518.8%, from €4.0 million for the year ended December 31, 2020 to €25.0 million for the year ended December 31, 2021. This was largely driven by an
311
Table of Contents
increase of €9.4 million in clinical research and development costs, which related to clinical trials that were postponed from 2020 to 2021 as a result of various operational considerations, including ensuring FDA comments were properly addressed. In addition to startup activities for our two Phase 3 lipid clinical trials (BROADWAY and BROOKLYN) and our CVOT (PREVAIL), the increase in research and development expenses was also the result of an increase of €7.1 million in chemistry, manufacturing and controls costs due to API and drug product costs incurred for the Phase 2 clinical trials conducted during 2021 and starting API campaign costs for the startup of our Phase 3 clinical trials. The increase in research and development expenses was also the result of an increase of €2.4 million and €1.3 million for contracted personnel and regulatory costs, respectively, related to the above mentioned increase in clinical trials.
We begin separately tracking program expenses at candidate nomination, or when acquired, and accumulate all costs incurred to support each program to date.
| For the year ended December 31, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Phase 1 and 2 | (6,560 | ) | (1,387 | ) | (5,173 | ) | ||||||
Phase 3 | (4,257 | ) | (27 | ) | (4,230 | ) | ||||||
Other | (25 | ) | (36 | ) | 11 | |||||||
Clinical research and development costs | (10,842 | ) | (1,450 | ) | (9,392 | ) | ||||||
Non-clinical research and development costs | (974 | ) | (41 | ) | (933 | ) | ||||||
Contracted personnel costs | (3,363 | ) | (994 | ) | (2,369 | ) | ||||||
Chemistry, manufacturing and controls (“CMC”) | (8,338 | ) | (1,236 | ) | (7,102 | ) | ||||||
Regulatory | (1,452 | ) | (141 | ) | (1,311 | ) | ||||||
Other R&D costs | (63 | ) | (183 | ) | 120 | |||||||
Total research and development expenses | (25,032 | ) | (4,045 | ) | (20,987 | ) | ||||||
General and Administrative Expenses
General and administrative expenses increased by €3.4 million, or 247.0%, from €1.4 million for the year ended December 31, 2020 to €4.8 million for the year ended December 31, 2021. This was driven by an increase of €1.6 million in personnel-related costs due to increased fees, hiring of individuals involved with administrative and quality control activities and share-based payments, an increase of €0.6 million in intellectual property and other legal costs due to increased efforts related to drug patent development and global patent protection efforts, legal services rendered with respect to internal restructuring, due diligence, and business development, an increase of €0.8 million in marketing and communication costs related to initial market pricing research and business development activities and an increase of €0.4 million in facility-related and other costs largely related to an increase in insurance costs due to an increase in coverage for directors and officers.
| For the year ended December 31, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Personnel-related costs | (2,024 | ) | (416 | ) | (1,608 | ) | ||||||
Intellectual property and other legal costs | (1,507 | ) | (885 | ) | (622 | ) | ||||||
Marketing and communication | (845 | ) | (11 | ) | (834 | ) | ||||||
Facility-related and other costs | (427 | ) | (72) | (355 | ) | |||||||
Total general and administrative expenses | (4,803 | ) | (1,384 | ) | (3,419 | ) | ||||||
Finance Income
Finance income was €0.009 million for the year ended December 31, 2021. This increase was driven by the accrued finance expense on the loan facility provided to our chief executive officer, Dr. Davidson, in June 2021,
312
Table of Contents
which has been fully repaid as described above. We recorded no finance income for the year ended December 31, 2020.
Finance Expense
Finance expense decreased by €0.1 million, from €0.3 million for the year ended December 31, 2020 to €0.2 million for the year ended December 31, 2021. This decrease was largely driven by the conversion of €11.7 million of the Convertible Loan and unpaid interest into 1,111,155 Series A Preferred Shares on January 7, 2021, partially offset by an increase in negative interest charged on cash balances and an increase in interest on lease liabilities.
Net Foreign Exchange Gain
Net foreign exchange gain increased by €1.4 million, from €0.02 million for the year ended December 31, 2020 to €1.4 million for the year ended December 31, 2021. This was largely driven by the effect of the appreciation of the U.S. Dollar on cash balances held in U.S. Dollars at the end of the year. The “Foreign Currency Risk” section below includes more information regarding our exposure to foreign currencies.
Income Tax Expense
We recorded no income tax expense for both the year ended December 31, 2020 and December 31, 2021. We have a history of losses and therefore pay no corporate tax.
Loss for the Period
Loss for the period increased by €22.9 million, from a loss of €5.7 million for the year ended December 31, 2020 to a loss of €28.6 million for the year ended December 31, 2021, mainly driven by the increase in research and development expenses.
Liquidity and Capital Resources
We are a clinical-stage biopharmaceutical company and, since inception, we have incurred significant operating losses. Despite having made a profit in the six months ended June 30, 2022, we expect our costs to fund research and development activities for the full fiscal year 2022 to exceed our revenue arising from the Menarini License. Since inception, we have not generated any product revenues or net positive cash flows from operating activities. We will not receive any product revenues or net positive cash flows from operating activities until we successfully develop a product candidate, obtain regulatory approval, and successfully commercialize it. There is no assurance that we will be able to do so.
We have historically funded our operations primarily through the private placements of our Series A Preferred Shares and the sale of convertible notes. As at June 30, 2022, we had cash and cash equivalents of €89.5 million. Cash and cash equivalents are invested in accordance with our investment policy, primarily with a view to capital preservation, liquidity, diversification, and yield, and consist primarily of cash in banks and short-term bank deposits with an original maturity of three months or less, net of outstanding bank overdrafts.
Based on our current operating plan, we believe that our existing cash and cash equivalents, including the upfront payment of €115 million received from the Menarini License in July 2022, will be sufficient to fund our anticipated level of operations until the end of 2023. On a pro forma basis as at June 30, 2022, assuming the consummation of the Business Combination and the PIPE Financing, we estimate that Holdco would have €413.3 million in cash assuming no redemptions by FLAC’s shareholders, and €296.0 million in cash assuming maximum redemptions by FLAC’s shareholders, which we believe will be sufficient to fund our anticipated level of operations through 2026, which includes the forecasted completion of our phase 3 trials. See the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” for more information. We have
313
Table of Contents
based our estimate on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. For example, Holdco may require additional capital resources due to underestimation of the nature, timing and costs of the efforts that will be necessary to complete the development of our product candidate. Holdco may also need to raise additional funds more quickly if it chooses to expand its development activities, its portfolio or if it considers acquisitions or other strategic transactions, including licensing transactions. For more information regarding these risk and factors that could influence our and Holdco’s future capital requirements and the timing thereof, please see the section of this proxy statement/prospectus entitled “Risk Factors.”
Future Funding Requirements
To date, we have devoted substantially all of our resources to organizing and staffing our company, business planning, raising capital, undertaking preclinical studies and conducting clinical trials of obicetrapib. As a result, we are not yet profitable and have incurred losses in each annual period since our inception. This will include the fiscal year ending December 31, 2022, as we expect our costs to fund research and development activities for the full fiscal year to exceed our revenue arising from the Menarini License. As at December 31, 2021, we had an accumulated loss of €34.7 million. After the Business Combination, we expect to continue to incur significant losses for the foreseeable future. We anticipate that our expenses will increase substantially as a result of:
| • | the progress and costs of our discovery, preclinical and non-clinical development; |
| • | the progress and costs of our clinical trials, including costs related to clinical sites, clinical investigators and CROs that are assisting with our sponsored clinical trials, and other research and development activities; |
| • | the costs and timing of obtaining regulatory approval, including the expenses of filing NDAs and MAAs, and the related expenses involved in validating our manufacturing processes; |
| • | the costs associated with any future investigator-sponsored preclinical studies and clinical trials; |
| • | the costs of filing, prosecuting, defending and enforcing any patent applications, claims, patents and other intellectual property rights; |
| • | the costs and timing of obtaining sufficient quantities of our product candidate for clinical trials by establishing production capacities through contracts with contract manufacturing organizations (“CMOs”); |
| • | the terms and timing of any collaborative, licensing and other arrangements that we may establish; |
| • | the potential costs of any delays caused by the COVID-19 pandemic and associated restrictions; |
| • | the costs of preparing for launch and commercialization of our product candidate; and |
| • | the costs of operating as a public company in the United States. |
We may encounter unforeseen expenses, difficulties, complications, delays and other factors that may adversely affect our business. The magnitude of our future net losses will depend on the rate of future growth of our expenses combined with our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our shareholders’ equity and working capital unless and until eliminated by revenue generation and growth.
Until we can generate substantial product revenues, if ever, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, convertible loans, warrants, collaborations, or other means. We may consider raising additional capital to take advantage of favorable market conditions or for other strategic considerations even if we have sufficient funds for planned operations. If we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish certain valuable rights to our product candidates, future revenue streams or research programs or grant licenses on terms that may not be favorable to us. If we
314
Table of Contents
raise additional capital through public or privately placed equity offerings of securities, the terms of these securities or offerings may include liquidation or other preferences that adversely affect our other shareholders’ rights. To the extent that we raise additional funds by issuing and selling equity or equity-linked securities, shareholders will experience dilution. If we raise additional capital through debt financing, we would likely be subject to fixed payment obligations and may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, licensing or selling assets, making capital expenditures or declaring dividends.
Capital may become difficult or impossible to obtain due to poor market or other conditions outside of our control, including possible disruptions caused by the COVID-19 pandemic and Russian hostilities towards Ukraine. If we are unable to raise sufficient additional funds on favorable terms as and when needed, we may be required to delay, reduce, or terminate some or all of our development programs and clinical trials. We may also be required to sell or license to others any of our potential future product candidates that we would prefer to develop and commercialize ourselves. See the section of this proxy statement/prospectus entitled “Risk Factors” for additional detail regarding these risks.
Convertible Loan Agreement
On July 2, 2020, we, as borrower, entered into the Convertible Loan Agreement, granting us up to €17 million across three tranches of €5.7 million each. We drew down on the first tranche on July 2, 2020 and the second tranche on October 12, 2020. We accrued finance expense of €0.3 million over 2020 in connection with the Convertible Loan under the Convertible Loan Agreement. In January 2021, the Convertible Loan Agreement was terminated and the full amount of outstanding principal amount and unpaid interest was converted into 1,111,155 Series A Preferred Shares in connection with the entry into a share subscription agreement with various investors for up to an aggregate purchase price of €160 million (the “Series A Subscription Agreement”). See “—Series A Financing” below for more information.
Series A Financing
On December 30, 2020, we entered into the Series A Subscription Agreement to issue Series A Preferred Shares at a subscription price per share of €14 (the “Subscription Price”) for certain investors and €10.50 (equal to 75% of the Subscription Price) (the “Discounted Subscription Price”) for the parties to the Convertible Loan Agreement as a set off against the Convertible Loan, for up to an aggregate amount of €160 million, occurring in two tranches. In the first tranche, we issued and sold 4,928,573 Series A Preferred Shares for gross proceeds of €69 million. The first tranche closed in January 2021 (the “First Closing”). In accordance with the terms of the Convertible Loan Agreement, €11.7 million of outstanding principal amount and unpaid interest was converted into 1,111,155 Series A Preferred Shares at the First Closing. We were entitled to cause the investors to subscribe for the second tranche Series A Preferred Shares upon the occurrence of certain clinical development and business development milestones. We issued the second tranche of Series A Preferred Shares in February 2022 resulting in gross proceeds to us of €80 million and the issuance of 5,691,430 additional Series A Preferred Shares (the “Milestone Closing”).
The Milestone Closing was contingent on the achievement of certain milestones, namely: at least 50% LDL lowering in the ongoing obicetrapib and ezetimibe Phase 2 combination trial; the hiring of a full-time chief business officer; and confirmation by the FDA that no CVOT would be required for regulatory approval in the United States. After achieving the first two of these milestones, we proceeded with the Milestone Closing as permitted by the terms of the Series A Subscription Agreement. Because the Milestone Closing occurred without having achieved all of the milestones, we were obligated to pursue certain strategic transactions, including a business combination with a special purpose acquisition company.
Menarini License
On June 23, 2022, we entered into the Menarini License, pursuant to which we granted Menarini an exclusive, royalty-bearing, sublicensable license under certain of our intellectual property and our regulatory
315
Table of Contents
documentation to undertake post approval development activities and commercialize multiple brands of obicetrapib, either as a sole active ingredient product or in a fixed dose combination with ezetimibe (the “Licensed Products”), for any use in the Menarini Territory.
We are solely responsible for conducting the development activities to obtain regulatory approval for obicetrapib. Menarini may conduct market access studies, medical affairs activities, non-registration studies and Phase IV clinical trials in the Menarini Territory. Menarini will be responsible for submitting and obtaining the required regulatory approvals to commercialize the Licensed Products in the Menarini Territory and will own the regulatory approvals, if received. Menarini will also be solely responsible for commercializing the Licensed Products, if approved, and will be required to use commercially reasonable efforts to commercialize the Licensed Products in the Menarini Territory.
Pursuant to the Menarini License, Menarini made a non-refundable, non-creditable upfront payment to us of €115 million. Menarini has also committed to providing us €27.5 million in funding for the research and development activities related to the Licensed Products over two years, together with bearing 50% of any development costs incurred in respect of the pediatric population in the Menarini Territory. We are also eligible to receive up to €863 million upon the achievement of various clinical, regulatory and commercial milestones. If obicetrapib is approved, and successfully commercialized by Menarini, we will be entitled to tiered royalties ranging from the low double-digits to the mid-twenties as a percentage of net sales in the Menarini Territory, with royalty step-downs in the event of generic entrance or in respect of required third-party IP payments.
The Menarini License will expire on the last to expire royalty term, which is determined on a licensed product-by-licensed product and country-by-country basis, and is the later of (i) the expiration of the last to expire licensed patent that includes a valid claim in the country, (ii) expiration of regulatory exclusivity granted by the prevailing governmental authority for the Licensed Product in the country or (iii) a specified number of years from the first commercial sale of the licensed product in the country.
In addition, Menarini is expected to purchase obicetrapib and ezetimibe product from us in accordance with a supply agreement to be entered into by Menarini and us (the “Supply Agreement”). We will supply all required quantities of obicetrapib and ezetimibe product for the Menarini Territory as set forth in the Supply Agreement.
Through June 30, 2022, we have received no milestone payments from Menarini under the Menarini Agreement.
Business Combination
In July 2022, we entered into a Business Combination Agreement with FLAC, a special purpose acquisition company sponsored by Frazier Lifesciences Sponsor LLC that is incorporated as a Cayman Islands exempted company, Holdco and Merger Sub. In connection with the Business Combination, we will effect the Exchange. Immediately after giving effect to the Exchange, Holdco will effect the Holdco Reorganization. Following the Holdco Reorganization, Merger Sub will merge with and into FLAC, with FLAC surviving the Merger as the wholly owned subsidiary of Holdco, with Holdco becoming a publicly traded company listed on the Nasdaq in the United States. Our shareholders are expected to have a controlling interest in the combined entity. The transaction is subject to customary and other closing conditions, including the approval of our shareholders and those of FLAC. The transaction is expected to close in the second half of 2022.
Management has assessed the expected accounting treatment of the internal restructuring and transaction on our consolidated financial statements. The internal restructuring will be accounted for as a capital reorganization as we remain the substantive business, and Holdco does not meet the definition of a business pursuant to IFRS 3, Business Combinations. Because our shareholders are expected to hold the controlling interest in the combined entity, and as FLAC does not constitute a business under IFRS 3 Business Combinations, the transaction with FLAC will also be accounted for as a capital reorganization and is in scope of IFRS 2 Share-based Payment. Holdco will issue shares in exchange for the net assets of FLAC. Any difference between the fair value of the shares issued by Holdco and the fair value of FLAC’s identifiable net assets will be treated as costs for the service of obtaining a listing and expensed in the period in which the transaction with FLAC closes.
316
Table of Contents
Cash Flows
Comparison of cash flows for the six months ended June 30, 2022 and 2021
The following table summarizes the primary sources and uses of cash for each period presented:
| For the six months ended June 30, |
| |||||||||||
| 2022 | 2021 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Net cash flows used in operating activities | (44,642 | ) | (8,771 | ) | (35,871 | ) | ||||||
Net cash flows used in investing activities | (2) | (7) | 5 | |||||||||
Net cash flows provided by financing activities | 79,647 | 69,000 | 10,647 | |||||||||
Foreign exchange differences | 1,383 | 609 | 774 | |||||||||
Cash and cash equivalents at beginning of period | 53,092 | 7,861 | 45,231 | |||||||||
Cash and cash equivalents at end of period | 89,478 | 68,691 | 20,787 | |||||||||
Net Cash Flows from Operating Activities
During the six months ended June 30, 2022, net cash used in operating activities was €44.6 million, largely driven by the cash outflows for research and development expenses and general and administrative expenses.
During the six months ended June 30, 2021, net cash used in operating activities was €8.8 million. This was mainly driven by loss for the period of €9.5 million, partially offset by cash inflows for changes in prepayments and other receivables of €0.7 million due to a reduction of prepaid balances related to Chemistry, Manufacturing and Controls (CMC) services, and for changes in trade and other payables of €0.6 million due to an increase in activity related to our Phase 2b clinical trials (OCEAN and ROSE).
Net Cash Flows from Investing Activities
During the six months ended June 30, 2022, net cash used in investing activities was €2 thousand driven by the purchase of equipment.
During the six months ended June 30, 2021, net cash used in investing activities was €7 thousand driven by the purchase of equipment.
Net Cash Flows from Financing Activities
During the six months ended June 30, 2022, net cash generated from financing activities was €79.6 million largely driven by proceeds from the issuance of shares and capital contributions of €79.7 million, resulting from the receipt of the second tranche financing of Series A Preferred Shares on February 18, 2022.
During the six months ended June 30, 2021, net cash generated from financing activities was €69.0 million driven by proceeds from the issuance of shares and capital contribution of €69.0 million, resulting from the receipt of the first tranche financing of Series A Preferred Shares on January 7, 2021.
317
Table of Contents
Comparison of cash flows for the years ended December 31, 2021 and 2020
The following table summarizes the primary sources and uses of cash for each period presented:
| For the year ended December 31, |
| |||||||||||
| 2021 | 2020 | Change | ||||||||||
| (€ in thousands) | ||||||||||||
Net cash flows used in operating activities | (25,164 | ) | (5,970 | ) | (19,194 | ) | ||||||
Net cash flows used in investing activities | (20) | (13) | (7) | |||||||||
Net cash flows provided by financing activities | 68,990 | 11,320 | 57,670 | |||||||||
Foreign exchange differences | 1,425 | 24 | 1,401 | |||||||||
Cash and cash equivalents at beginning of period | 7,861 | 2,500 | 5,361 | |||||||||
Cash and cash equivalents at end of period, net of overdraft | 53,092 | 7,861 | 45,231 | |||||||||
Net Cash Flows from Operating Activities
During the year ended December 31, 2021, net cash used in operating activities was €25.2 million, largely driven by the loss before tax of €28.6 million and changes in prepayments and other receivables of €4.4 million, largely driven by an increase in a value added tax receivable. This was partially offset by changes in trade and other payables of €8.2 million driven by the increase in clinical trials and chemistry, manufacturing and controls activities which drove an increase in payables to CROs and CMOs, and by an increase in a value added tax payable of €2.8 million.
During the year ended December 31, 2020, net cash used in operating activities was €6.0 million driven by the loss before tax of €5.7 million and changes in prepayments and other receivables of €1.4 million largely due to prepayments for chemistry, manufacturing and controls and Phase 1 and 2 clinical trials, partially offset by changes in trade and other payables of €829 thousand driven by general expansion of all clinical trial startup activities and accrued management fees.
Net Cash Flows from Investing Activities
During the year ended December 31, 2021, net cash used in investing activities was €20 thousand driven by the purchase of equipment.
During the year ended December 31, 2020, net cash used in investing activities was €13 thousand driven by the purchase of equipment.
Net Cash Flows from Financing Activities
During the year ended December 31, 2021, net cash generated from financing activities was €69.0 million driven by proceeds from the issuance of shares and capital contributions of €69.0 million.
During the year ended December 31, 2020, net cash generated from financing activities was €11.3 million driven by proceeds from the issuance of the Convertible Loan.
Contractual Obligations and Commitments
Third-Party Service Agreements
We have entered into a variety of agreements and financial commitments in the normal course of business with CROs, CMOs, and other third parties for preclinical and clinical development and manufacturing services. The terms generally provide us with the option to cancel, reschedule and adjust our requirements based on our business needs, prior to the delivery of goods or performance of services. Payments due upon cancellation
318
Table of Contents
generally consist only of payments for services provided or expenses incurred, including non-cancelable obligations of our service providers, up to the date of cancellation. However, some of our service providers also charge cancellation fees upon cancellation. The amount and timing of such payments are not known, but at December 31, 2021 they are estimated to be a maximum of €8.0 million due within one year and €6.1 million due in more than a year.
Leases
We are party to two lease agreements, the Naarden Lease and the office lease agreement with Renaissance Aventura LLC, dated May 24, 2021 (the “Miami Lease”). Under the Naarden Lease, we are obligated to pay €40 thousand per year in rent. The Naarden Lease will continue until terminated by either us or the landlord. Pursuant to the Miami Lease, we are required to pay annual rent ranging from $69 thousand to $75 thousand, increase from the low end of the range to the higher end of the range for each year of the lease. The Miami Lease will expire by its terms on October 31, 2024, unless terminated earlier by either party pursuant to the terms of the Miami Lease.
2020 SPA and Profit Right Agreement
On April 9, 2020, we entered into a share sale and purchase agreement with Amgen (the “2020 SPA”), to purchase all of the outstanding share capital of Dezima, a company whose principal activity was to develop compounds that treat cardiovascular disease related to dyslipidemia. The principal reason for this acquisition was to secure rights to intellectual property and know-how related to the patented drug obicetrapib and the in-process research and development (“IPR&D”). We paid cash consideration of €1 for the share capital of Dezima and agreed to make additional contingent payments to Amgen upon the potential occurrence of certain future events (if they occur) as further described below. In connection with the 2020 SPA, we entered into a profit right and waiver agreement with MTPC (the “Profit Right Agreement”) in consideration for the waiver of certain historical intellectual property rights and related payment rights held by MTPC.
The two events that would trigger the payment of additional consideration to Amgen and MTPC are a traditional underwritten initial public offering (“IPO”) or an exit event, as defined in the 2020 SPA and the Profit Right Agreement. An exit event includes includes a change of control transaction or the transfer of more than 50 percent of our then-outstanding shares. Under the 2020 SPA and the Profit Right Agreement, the aggregate contingent consideration to be paid to Amgen and MTPC resulting from the profit right and IPO share right is calculated as 17.63% of the proceeds, or an aggregate number of shares valued at the IPO price equal to 17.63% of the pre-public offering valuation if an IPO takes place, as applicable. Upon receiving notice of NewAmsterdam Pharma’s intention to pursue an IPO, Amgen would have the right to repurchase all of the outstanding shares of Dezima at a certain specified price.
In addition, Amgen and MTPC were granted rights to match the terms offered by third parties in connection with certain major corporate transactions involving NewAmsterdam Pharma. The Business Combination qualifies as an exit event pursuant to both the 2020 SPA and the Profit Right Agreement. As a result, in connection with the Business Combination and pursuant to side letters entered into by the parties, Amgen and MTPC each will receive their respective profit right payments in the form of Holdco Shares, as follows (for the avoidance of doubt, references below to shares on a “fully diluted basis” assume the exercise of all NewAmsterdam Pharma options outstanding immediately prior to the Exchange and are made after giving effect to the issuance of Holdco Shares to MTPC and Amgen described below):
| (a) | shortly after the Exchange, and prior to the consummation of the Business Combination, a number of Holdco Shares, in the aggregate, equal to (i) 17.63% of the number of shares of NewAmsterdam Pharma outstanding immediately prior to the consummation of the Exchange on a fully diluted basis, multiplied by (ii) the exchange rate applicable to all of holders of NewAmsterdam Pharma shares in the Exchange and then (iii) rounded to the nearest whole number where an entitlement to five-tenths (0.5) of a Holdco Share shall be rounded up; and |
| (b) | if and when the relevant development milestone is achieved, as soon as practicable thereafter, a number of Holdco Shares, in the aggregate, equal to (i) 17.63% of the number of Earnout Shares on a fully |
319
Table of Contents
| diluted basis and then (ii) rounded to the nearest whole number where an entitlement to five-tenths (0.5) of a Holdco share shall be rounded up. |
The payments to Amgen and MTPC described in clauses (a) and (b) above are referred to collectively as the “2020 Profit Rights.” Upon receipt of the 2020 Profit Rights (but in the case of the Earnout Shares, only if the certain clinical development milestone is achieved during the Earnout Period), all rights of Amgen and MTPC under the 2020 SPA and the Profit Right Agreement, respectively, will be extinguished.
Management deems that the events considered in estimating the contingent consideration are uncertain. While the occurrence of the events is within our control, the estimated value of the liability, probability assessments relative to market studies of similar transactions within the pharmaceutical industry, and the likelihood of the occurrence and the timing of the events driving the exercise of the profit right or IPO share right taking place are inherently uncertain. Given that the occurrence of such events is within our control, rather than beyond our control or under the control of the contractual parties, we did not initially recognize a liability.
Menarini License
We will be responsible for the development and commercialization costs related to Licensed Products other than those in the Menarini Territory. In addition, under specified conditions of the agreement, we agreed to bear 50 percent of certain development costs incurred by the other party in the development of the Licensed Products in the Menarini Territory. Please see “—Liquidity and Capital Resources” for a description of the Menarini License.
Quantitative and Qualitative Disclosures about Market Risk
Our principal financial liabilities consist of trade and other payables and loans and borrowings. The main purpose of these financial liabilities is to finance our day-to-day operations. We also have prepayments and other receivables, cash and cash equivalents, and loan receivable that are derived from our operating activities and funding.
We are exposed to market risk, credit risk and liquidity risk. Our senior management oversees the management of these risks. Our board of directors reviews and approves policies for managing each of these risks, which are summarized below.
Market Risk
Market risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk comprises interest rate risk and foreign currency risk.
Interest Rate Risk
The only variable interest-bearing financial instruments are cash and cash equivalents. Changes in interest rates may cause variations in interest income and expense resulting from short-term interest-bearing assets. Management does not expect the short-term interest rates to decrease significantly in the immediate foreseeable future, which limits the interest exposure on our cash and cash equivalents and current financial assets.
320
Table of Contents
The following table demonstrates the sensitivity to a reasonably possible change in interest rates on that portion of financial assets affected. With all other variables held constant, our profit before tax is affected through the impact on floating rate borrowings, as follows:
| Increase /(decrease) in basis points | Effect on profit before tax (€ in thousands) | |||||||
For the year ended December 31, 2021 | ||||||||
Euro | 100 | 380 | ||||||
Euro | (100 | ) | (380 | ) | ||||
U.S. Dollar | 100 | 150 | ||||||
U.S. Dollar | (100 | ) | (150 | ) | ||||
For the year ended December 31, 2020 | ||||||||
Euro | 45 | 32 | ||||||
Euro | (45 | ) | (32 | ) | ||||
Foreign Currency Risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. Our exposure to the risk of changes in foreign exchange rates relates primarily to cash and cash equivalents, and trade and other payables denominated in currencies other than our functional currency. As at December 31, 2021, our net exposure to foreign currency risk was €15.9 million, compared to €691 thousand as at December 31, 2020.
We partly manage our foreign currency risk by selectively holding foreign currency in our cash and cash equivalents, to offset foreign currency exposures from lease liabilities and trade and other payables. We plan to use these cash and cash equivalents to settle future expenses we expect to incur in those foreign currencies.
We are mainly exposed to the currency of U.S. Dollar, British Pounds, Canadian Dollar and Japanese Yen.
The following table demonstrates the sensitivity to a possible change in exchange rates against the Euro with all other variables held constant. Additional sensitivity changes to the indicated currencies are expected to be approximately proportionate. The table shows the effect on our profit before tax (due to changes in the value of monetary assets and liabilities and equity). Our exposure to foreign currency changes for all other currencies is not material. There is no impact to other comprehensive income or equity.
| Effect on profit / (loss) before tax (€ in thousands) | ||||||||
| 10% depreciation | 10% appreciation | |||||||
For the year ended December 31, 2021 | ||||||||
British Pound Sterling | (9 | ) | 15 | |||||
U.S. Dollar | (1,360 | ) | 1,843 | |||||
For the year ended December 31, 2020 | ||||||||
British Pound Sterling | (50 | ) | 61 | |||||
U.S. Dollar | (14 | ) | 14 | |||||
Credit Risk
Credit risk is the risk that a counterparty will not meet its obligations under a financial instrument or customer contract, leading to a financial loss. We are exposed to credit risk primarily from our treasury activities, including deposits with banks and financial institutions and have limited credit risk exposure from our operating activities. We hold available cash in bank accounts with banks which have investment grade credit ratings. Management periodically reviews the creditworthiness of the banks with which it holds assets.
321
Table of Contents
We provided a loan to the CEO which bears interest and is secured. On July 19, 2022, Dr. Davidson repaid the entire outstanding principal amount and unpaid interest under the Davidson Loan Agreement, representing a total of €709 thousand.
We perform research and development activities and do not yet have any sales. Therefore, we are able to reclaim Value Added Tax (“VAT”), which is recoverable from tax authorities. Management periodically reviews the recoverability of the balance of input value added tax and believes it is fully recoverable.
Liquidity risk
We monitor our risk to a shortage of funds using forecasting planning tools. Our objective is to maintain a sufficient level of funding in order to continue our research and development activities, capital obligations, and loans and borrowings. We manage liquidity risk by maintaining adequate reserves, banking facilities and reserve borrowing facilities, by continuously monitoring forecast and actual cash flows, and by matching the maturity profiles of financial assets and liabilities.
Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with IFRS. As a result of becoming a public company, we will be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by our management on, among other things, the effectiveness of our internal control over financial reporting, beginning with our annual report filed with the SEC for the fiscal year ending December 31, 2023. This assessment will need to include disclosures of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be detected or prevented on a timely basis. We are in the very early stages of the costly and challenging process of planning the activities necessary to perform the evaluation needed to comply with Section 404.
In connection with the preparation of our financial statements at December 31, 2021 and for the years ended December 31, 2021 and 2020, we identified material weaknesses in the design of our internal control over financial reporting across the principles for each component of the COSO framework at the entity level (i.e. control environment, risk assessment, monitoring, information & communication and control activities) and accordingly, across our business and IT processes. Specifically, the material weaknesses that we identified, individually or in the aggregate, included the following:
| • | our lack of consistent and documented risk assessment procedures and control activities related to our financial reporting, among which a sufficient level of management review and approval, manual processes, roles and responsibilities, and adequate application and controls over information technology; and |
| • | our failure to maintain a sufficient complement of personnel commensurate with our accounting and reporting requirements as we continue to grow as a company, and ability to: (i) design and maintain formal accounting policies, procedures and controls over the fair presentation of our financial statements, (ii) analyze, record and disclose complex accounting matters timely and accurately, and (iii) design and maintain controls over the preparation and review of journal entries and financial statements, including maintaining appropriate segregation of duties. |
Although several oversight and control activities are performed, not all activities are formalized and documented properly. In addition, where control activities are dependent on information used in a control, we do not perform or document controls to determine the completeness and accuracy of such information. We also do not have controls in place to monitor control activities and identify control deficiencies. Each of these control
322
Table of Contents
deficiencies could result in a misstatement of our accounts or disclosures that would result in a material misstatement of our annual or interim financial statements that would not be prevented or detected, and accordingly, we determined that these control deficiencies constitute material weaknesses.
Prior to the completion of the Business Combination, we have been a private company with limited accounting personnel to adequately execute our accounting processes and other supervisory resources with which to address our internal control over financial reporting. To address these material weaknesses, we will need to add personnel and continue to develop and implement new financial processes. We have begun to take steps to remediate the material weaknesses described above, including by engaging consultants to assist management in developing internal control procedures and anticipate hiring additional qualified accounting and financial reporting personnel, and further evolving our accounting processes and policies. We also intend to build an internal control framework. We will not be able to fully remediate these material weaknesses until these steps have been completed and have been operating effectively for a sufficient period of time.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 until the later of the year following our first annual report required to be filed with the SEC, or the date we are no longer an “emerging growth company” as defined in the JOBS Act, if we take advantage (as we expect to do) of the exemptions contained in the JOBS Act. We will remain an “emerging growth company” for up to five years from the date of the Business Combination, although if the market value of our ordinary shares that are held by non-affiliates exceeds $700 million as of June 30 of any year before that time, we would cease to be an “emerging growth company” as of December 31 of that year. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Our remediation efforts may not enable us to avoid material weaknesses in our internal control over financial reporting in the future.
If we are unsuccessful in building an appropriate internal control environment, we may not be able to prepare and disclose, in a timely manner, our financial statements and other required disclosures, or comply with existing or new reporting requirements. Any failure to report our financial results on an accurate and timely basis could result in sanctions, lawsuits, delisting of our shares from Nasdaq or other adverse consequences that could materially harm our business. If we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed, and investors could lose confidence in our reported financial information. Any of the foregoing occurrences, should they come to pass, could negatively impact the public perception of our company, which could have a negative impact on our share price.
There is also no assurance that we have identified all of our material weaknesses or that we will not in the future have additional material weaknesses. See “Risk Factors—Risks Related to Ownership of Holdco Securities—If Holdco fails to maintain an effective system of internal control over financial reporting, Holdco may not be able to accurately report its financial results or prevent fraud. As a result, shareholders could lose confidence in Holdco’s financial and other public reporting, which is likely to negatively affect Holdco’s business and the market price of Holdco Shares.”
Critical Accounting Estimates
The preparation of the consolidated financial statements has required management to apply accounting policies and methodologies based on complex and subjective judgments, as well as estimates based on past experience and assumptions determined to be reasonable and realistic based on the related circumstances. The
323
Table of Contents
use of these judgements, estimates and assumptions affects the amounts reported in these consolidated financial statements. The final amounts for items for which estimates, and assumptions were made in the consolidated financial statements may differ from those reported in these statements due to the uncertainties that characterize the assumptions and conditions on which the estimates are based.
Research and Development Expense
Research and development costs are recognized as an expense when incurred because the recognition criteria for capitalization are not met and are typically made up of costs from our clinical and preclinical activities, drug development and manufacturing costs, and include costs for CROs and investigative sites. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data provided by vendors of their actual costs incurred. At each balance sheet date, we estimate the level of services performed by the vendors and the associated expenditure incurred for the services provided.
Quantification of the research and development expenses incurred during the period required judgment, because the progress of activities is not directly observable. In estimating progress toward completion of specific tasks, we therefore use non-financial data such as number of patient screenings, patient visits, patient enrollment, clinical site activations and vendor information of actual costs incurred. This data is obtained through reports from outside service providers as to the progress or state of completion of trials or the completion of services and review by our personnel.
Share-Based Payments
We operate equity-settled share-based payment arrangements, under which we receive services from directors, employees and others providing similar services as consideration for equity instruments in NewAmsterdam Pharma.
We determine the fair value of the share-based payment awards at the grant date, including the impact of any market conditions and non-vesting conditions, and recognize an expense for the services received over the service period, with a corresponding increase in equity. For awards with graded-vesting features, each instalment of the award is treated as a separate grant, which means that each instalment is separately expensed over the related vesting period. At the end of each reporting period, service conditions and non-market vesting conditions are taken into account when estimating the number of awards that will ultimately vest.
For options granted under our long-term incentive plan, the total amount to be expensed for services received is determined by reference to the grant date fair value of the options granted as determined using an option valuation model. For indirect share investments, we analyze at each grant date whether the purchase price paid by a participant is in line with the market price of the underlying shares. If a positive difference exists between (i) the actual market value of the shares as determined at the grant date and (ii) the purchase price paid, this results in a fair value to be reported as a share-based payment expense.
Recently Issued and Adopted Accounting Pronouncement
For information on the standards applied for the first time as of January 1, 2021 and 2020 please refer to Note 3 to our consolidated financial statements as at December 31, 2021 provided elsewhere in this proxy statement/prospectus.
324
Table of Contents
BUSINESS OF FLAC AND CERTAIN INFORMATION ABOUT FLAC
FLAC is a special purpose acquisition company incorporated on October 7, 2020 as a Cayman Islands exempted company for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. Although FLAC is not limited to a particular business, industry, sector or geographical location, FLAC focuses on promising opportunities in the biotechnology sector. FLAC has generated no operating revenues to date and does not expect that it will generate operating revenues until it consummates a business combination.
On October 7, 2020, prior to the FLAC IPO, the Sponsor purchased 2,875,000 Founder Shares for an aggregate purchase price of $25,000. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding.
On December 11, 2020, FLAC consummated the FLAC IPO of 13,800,000 FLAC Public Units, including 1,800,000 additional FLAC Public Units to cover over-allotments, with each FLAC Public Unit consisting of one FLAC Class A Ordinary Share and one-third of one FLAC Public Warrant, each whole FLAC Public Warrant entitling the holder thereof to purchase one FLAC Class A Ordinary Share at an exercise price of $11.50 per share, subject to adjustment. The FLAC Public Units were sold at a price of $10.00 per unit, generating gross proceeds of $138 million before underwriting discounts and expenses.
Simultaneously with the closing of the FLAC IPO, FLAC consummated the private placement of 501,000 units, at a price of $10.00 per FLAC Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5 million.
Upon the consummation of the FLAC IPO and the private placement, $138 million of the net proceeds of the FLAC IPO and private placement were deposited in a U.S.-based trust account at J.P. Morgan Chase Bank, N.A., maintained by Continental Stock Transfer & Trust Company, acting as trustee. Transaction costs of the FLAC IPO and the private placement amounted to approximately $8.1 million, inclusive of approximately $4.8 million in deferred underwriting fees and $83,000 to repay to the Sponsor’s borrowings under the $300,000 promissory note payable, which was available to pay accrued offering and formation costs, business, legal and accounting due diligence on prospective acquisitions and continuing general and administrative expenses. Funds held in the Trust Account have been invested only in U.S. government treasury bills with a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act of 1940, as amended (the “Investment Company Act”), which invest only in direct U.S. government obligations. Except with respect to interest earned on the funds in the Trust Account that may be released to FLAC to pay income taxes, if any, the proceeds from the FLAC IPO and the sale of the Private Placement Warrants held in the Trust Account will not be released from the Trust Account (1) to FLAC until the completion of its initial business combination or (2) to FLAC’s public shareholders, until the earliest of: (a) the completion of FLAC’s initial business combination, and then only in connection with those FLAC Class A Ordinary Shares that such shareholders properly elect to redeem, subject to certain limitations, (b) the redemption of any public shares properly tendered in connection with a (i) shareholder vote to amend the FLAC Articles of Association to modify the substance or timing of its obligation to provide holders of its FLAC Class A Ordinary Shares the right to have their shares redeemed in connection with its initial business combination within 24 months from the closing of the FLAC IPO or (ii) with respect to any other provisions relating to shareholders’ rights of holders of FLAC Class A Ordinary Shares or pre-initial business combination activity and (c) the redemption of all of FLAC’s Ordinary Shares if FLAC has not completed its initial business combination within 24 months from the closing of the FLAC IPO (or if such date is further extended at a duly called General Meeting, such later date), subject to applicable law.
The Sponsor
The Sponsor, Frazier Lifesciences Sponsor LLC, is an affiliate of Frazier Healthcare Partners. From 2005 to present, including previously as part of Frazier Healthcare Partners, under the leadership of Managing General
325
Table of Contents
Partners Patrick Heron and James (“Jamie”) Topper, the Frazier Life Sciences team has focused exclusively on life sciences companies and has deployed over $2.1 billion of capital into the life sciences sector with a focus on investing in and creating industry leading biopharmaceutical companies. With its broad range of private and public investment activity and company founding efforts, the Frazier Life Sciences portfolio has been responsible for substantial development activities across 32 drug programs that have resulted in FDA approval since 2010. The Sponsor is controlled by U.S. persons and FLAC does not believe it or its Sponsor constitutes a “foreign person” under CFIUS rules and regulations.
FLAC’s Management Team
FLAC’s management team is led by Jamie Topper, M.D., Ph.D, Managing General Partner at Frazier, who serves as Chairman of the FLAC Board and Chief Executive Officer; David Topper, Partner, Capital Markets at Frazier who serves as Chief Financial Officer; and Gordon Empey, Partner and General Counsel at Frazier Life Sciences, who serves as Vice President and General Counsel. The management team and board members have extensive experience in clinical medicine, drug development, regulatory strategy, and operational and management leadership within academia as well as the healthcare and financial industries. The management team believes that their breadth of experience will bolster their ability to thoroughly evaluate prospective candidates and successfully execute the initial business combination.
FLAC was formed to leverage the extensive experience and track record of the management team with the goal of financing a company that can both develop transformative therapies for patients in need and deliver significant returns to its investors.
Initial Business Combination
FLAC’s initial business combination must occur with one or more target businesses that together have an aggregate fair market value of at least 80% of the net assets held in the Trust Account (excluding the amount of deferred underwriting discounts held in trust and taxes payable on the interest earned on the Trust Account) at the time of signing the agreement to enter into the initial business combination. The FLAC Board has determined that the fair market value of the Business Combination meets the test.
Financial Position
As of June 30, 2022 and December 31, 2021, FLAC had approximately $138.1 million and 138.0 million held in the Trust Account, respectively, which includes approximately $4.8 million of deferred underwriting fees. With the funds available, FLAC offers a target business a variety of options such as creating a liquidity event for its owners, providing capital for the potential growth and expansion of its operations or strengthening its balance sheet by reducing its debt ratio. Because FLAC is able to complete its initial business combination using its cash, debt or equity securities, or a combination of the foregoing, FLAC has the flexibility to use the most efficient combination that will allow it to tailor the consideration to be paid to the target business to fit its needs and desires.
Facilities
FLAC currently maintains its executive offices at Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101. The cost for the use of this space is included in the $10,000 per month fee FLAC pays to the Sponsor for office space, administrative and support services. FLAC considers its current office space adequate for its current operations.
Employees
FLAC currently has three executive officers. These individuals are not obligated to devote any specific number of hours to FLAC’s matters, but they intend to devote as much of their time as they deem necessary to its affairs until FLAC has completed its initial business combination. They have devoted a significant amount of time in identifying NewAmsterdam Pharma and negotiating the terms of the Business Combination. FLAC does not intend to have any full time employees prior to the completion of its initial business combination.
326
Table of Contents
Corporate Information
FLAC was incorporated in October 2020 under the laws of the Cayman Islands. FLAC’s principal executive offices are located at Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101, and its telephone number is (206) 621-7200. FLAC’s website is www.frazierlifesciencesacquisition.com. FLAC’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
Available Information
FLAC files reports, proxy statements and other information with the SEC as required by the Exchange Act. You may access information on FLAC at the SEC website containing reports, proxy statements and other information at: www.sec.gov. Those filings are also available free of charge to the public on, or accessible through, FLAC’s website at www.frazierlifesciencesacquisition.com. A copy of FLAC’s Code of Conduct and Ethics and the charters of its audit committee, compensation committee and nominating and corporate governance committee are posted on FLAC’s website under “Governance Documents.” FLAC’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
Periodic Reporting and Financial Information
The FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants have each been registered under the Exchange Act and FLAC has reporting obligations, including the requirement that it files annual, quarterly and current reports with the SEC. In accordance with the requirements of the Exchange Act, its annual reports contain financial statements audited and reported on by its independent registered public accountants.
FLAC is required to evaluate its internal control procedures as required by the Sarbanes-Oxley Act. The fact that it is a blank check company makes compliance with the requirements of the Sarbanes-Oxley Act particularly burdensome on FLAC as compared to other public companies because a target business with which it seeks to complete its initial business combination may not be in compliance with the provisions of the Sarbanes-Oxley Act regarding adequacy of its internal controls. The development of the internal controls of any such entity to achieve compliance with the Sarbanes-Oxley Act may increase the time and costs necessary to complete any such acquisition.
FLAC has filed a Registration Statement on Form 8-A with the SEC to voluntarily register its securities under Section 12 of the Exchange Act. As a result, FLAC is subject to the rules and regulations promulgated under the Exchange Act.
FLAC is a Cayman Islands exempted company. Exempted companies are Cayman Islands companies conducting business mainly outside the Cayman Islands and, as such, are exempted from complying with certain provisions of the Companies Act. As an exempted company, it applied for and received, a tax exemption undertaking from the Cayman Islands government that, in accordance with Section 6 of the Tax Concessions Act (As Revised) of the Cayman Islands, for a period of 20 years from the date of the undertaking, no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciations will apply to FLAC or FLAC’s operations and, in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax will be payable (i) on or in respect of FLAC’s shares, debentures or other obligations or (ii) by way of the withholding in whole or in part of a payment of dividend or other distribution of income or capital by FLAC to its shareholders or a payment of principal or interest or other sums due under a debenture or other obligation of FLAC.
FLAC is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act. As such, FLAC has been eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including,
327
Table of Contents
but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. FLAC has elected to take advantage of the benefits of this extended transition period.
FLAC would remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of the FLAC IPO, (b) in which it has a total annual gross revenue of at least $1.07 billion, or (c) in which it is deemed to be a large accelerated filer, which means the market value of the FLAC Class A Ordinary Shares that are held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which it has issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Additionally, FLAC is a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. FLAC would remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of the FLAC Ordinary Shares held by non-affiliates exceeds $250 million as of the prior June 30, or (2) its annual revenues exceeded $100 million during such completed fiscal year and the market value of the FLAC Ordinary Shares held by non-affiliates exceeds $700 million as of the prior June 30.
FLAC has made available on its website a PFIC annual information statement to enable U.S. Holders (as defined in the final prospectus related to the FLAC IPO, filed with the SEC on December 10, 2020) to make a QEF election with respect to FLAC’s taxable year ended December 31, 2021, as further described therein. If you are a U.S. Holder of FLAC’s shares, you are urged to consult your tax advisor regarding the advisability of making a QEF election and/or other elections available under the PFIC rules with respect to FLAC Class A Ordinary Shares owned by you, and the procedures necessary to validly make and maintain such elections. FLAC’s website is www.frazierlifesciencesacquisition.com. FLAC’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
Legal Proceedings
There is no material litigation, arbitration or governmental proceeding currently pending against FLAC or any members of its management team in their capacity as such.
Directors, Executive Officers and Corporate Governance
Directors and Executive Officers
As of July 31, 2022, the directors and executive officers of FLAC are as follows:
| NAME | AGE | POSITION | ||
James N. Topper, M.D., Ph.D. | 60 | Chief Executive Officer and Chairman | ||
David Topper | 65 | Chief Financial Officer and Director | ||
Gordon Empey | 53 | Vice President and General Counsel | ||
Robert F. Baltera | 57 | Director | ||
Michael F. Bigham | 65 | Director | ||
Carol G. Gallagher, Pharm.D. | 58 | Director | ||
Krishna R. Polu, M.D | 49 | Director |
328
Table of Contents
James N. Topper, M.D., Ph.D., has served as FLAC’s Chief Executive Officer and Chairman of the FLAC Board since October 2020. Dr. Topper currently serves as a Managing Partner of Frazier Life Sciences. He joined Frazier in 2003 and opened Frazier’s Menlo Park office in the same year. Throughout his 15 years as a Managing Partner, Dr. Topper has invested across over 35 companies encompassing a broad spectrum of life science and biopharmaceutical companies. Dr. Topper has led and served as a board member for many of Frazier’s successful life sciences investments, including Acerta Pharma BV (sold to AstraZeneca), Amunix Pharmaceuticals (sold to Sanofi), Calistoga Pharmaceuticals (co-founder, sold to Gilead Sciences), Mavupharma (sold to AbbVie), Rempex (sold to The Medicines Company), Incline (co-founder, sold to The Medicines Company), Alnara (sold to Lilly), Portola (co-founder, Nasdaq: PTLA), Phathom Pharmaceuticals (Nasdaq: PHAT), CoTherix (sold to Actelion), and Threshold (Nasdaq: THLD). He currently represents Frazier on the boards of Alpine Immune Sciences (Nasdaq: ALPN), AnaptysBio (Nasdaq: ANAB), Lassen Therapeutics, Seraxis Holdings, Inc., Frazier Life Sciences Acquisition Corporation (Nasdaq: FLAC), Enlaza Therapeutics, Inc., Serum, Inc. and Sudo Biosciences, Inc. In 2011 and 2016, Dr. Topper was named to the Midas List of leading venture capitalists, and in 2013, Dr. Topper was recognized by Forbes as a top ten healthcare investor. Dr. Topper received his M.D. and Ph.D. in Biophysics from Stanford and his B.S. from the University of Michigan.
Dr. Topper is the brother of David Topper.
David Topper has served as FLAC’s Chief Financial Officer and on the FLAC Board since October 2020. Mr. Topper has served as Partner, Capital Markets, at Frazier since May 1, 2021. Prior to that, Mr. Topper previously served as Senior Advisor for Capital Markets at Frazier from March 2020 until May 2021. Mr. Topper currently serves as a member of the board of directors of TermGrid, a board observer at CircleUp, and previously served as a member of the board of directors of Amherst Pierpont Securities, Engility Corp., TASC, Affinion Group and MeteoGroup. From 2012 to 2019, Mr. Topper was an Operating Partner at General Atlantic, providing capital markets expertise to portfolio companies. Prior to General Atlantic, Mr. Topper was Co-Head of Equity Capital Markets at J.P. Morgan, where he led many of the firm’s major advisory and capital-raising transactions and worked with the U.S. Treasury and other regulatory agencies on crisis-related issues. He also served as Chairman of the Commitments Committee at J.P. Morgan. Prior to J.P. Morgan, Mr. Topper spent 22 years at Morgan Stanley, where he served as Co-Head of U.S. Equity Capital Markets, Managing Director, and Chairman of the Equity Commitment Committee. Earlier in his career, he held several other senior management positions in Morgan Stanley’s Debt Capital Markets, Leveraged Finance, and Mergers & Acquisitions departments. David received his B.A. from Duke University and his M.B.A. from Stanford Graduate School of Business.
Mr. Topper is the brother of Dr. Topper.
Gordon Empey has served as FLAC’s Vice President and General Counsel since October 2020. Mr. Empey joined the Frazier Life Sciences team in 2017, where he currently serves as a Partner and General Counsel. He has over 20 years of experience as counsel to venture capital investors and life sciences companies. Prior to joining Frazier Life Sciences, Mr. Empey was a partner with Cooley LLP, one of the premier biotechnology and technology law firms, until June 2017. In his legal practice at Cooley, Mr. Empey focused on emerging growth companies, corporate securities and mergers & acquisitions, and worked closely with Frazier Life Sciences on several investments and company creation efforts. Mr. Empey also advised numerous other life sciences venture capital firms and companies on structuring investments and corporate matters. Before joining Cooley, Mr. Empey was Executive Vice President and General Counsel at Radiant Research from May 2004 to August 2007, when it was sold to Covance and Swiss Biosciences. Earlier in his career, Mr. Empey served as an officer in the United States Navy, Judge Advocate General Corps. Mr. Empey received his J.D. from the University of California at Berkeley, Boalt Hall, and his B.A. from Colgate University.
Robert F. Baltera has served on the FLAC Board since October 2020. Mr. Baltera has served as President and Chief Executive Officer and on the board of directors of Cirius Pharmaceuticals since March 2017. Mr. Baltera joined Frazier as an Entrepreneur in Residence in January 2016, where he co-founded Hawkeye Therapeutics, Inc., a search company focused on in-licensing and developing high-quality assets from pharmaceutical companies. Since April 2020, Mr. Baltera has served as the Co-Founder/Executive Chairman of a private biotechnology company, Trestle Biotherapeutics. Mr. Baltera also served as the Executive Chairman of
329
Table of Contents
Mavupharma, Inc. from March 2017 until July 2019. From February 2015 until December 2015, Mr. Baltera served as Chief Executive Officer and a member of the board of directors of Laguna Pharmaceuticals, Inc., a biotechnology company. Mr. Baltera was the Chief Executive Officer of Amira Pharmaceuticals, Inc., a pharmaceutical development company, a position he held from July 2007 through September 2011, when Amira was sold to Bristol-Myers Squibb. Prior to Amira, Mr. Baltera held a number of senior management positions at Amgen (Nasdaq: AMGN) over 17 years, most recently serving as Vice President of Corporate and Contract Manufacturing. Mr. Baltera served on the board of directors of Organovo Holdings, Inc. (Nasdaq: ONVO) from October 2009 to August 2019, and served as Lead Independent Director from June 2014 through August 2016. Mr. Baltera previously served on the board of directors of Xencor, Inc. (Nasdaq: XNCR), a biotechnology development company. He currently serves on the board of directors of Imago BioSciences, Inc. (Nasdaq: IMGO), Panmira Pharmaceuticals, LLC and the San Diego Venture Group. He is also a Business Advisory Panel member of PBS Biotech Inc. Mr. Baltera received his M.B.A. from the Anderson School at the University of California, Los Angeles, and an M.S. in genetics and a B.S. in microbiology from The Pennsylvania State University. Mr. Baltera attended the Director Education and Certification program at the University of California, Los Angeles.
Michael F. Bigham has served on the FLAC Board since October 2020. Mr. Bigham has served as the Executive Chairman of the board of directors of Paratek Pharmaceuticals, Inc. (Nasdaq: PRTK) since June 2019. Prior to that, he was Chief Executive Officer and Chairman of the board of directors of Paratek from October 2014 to June 2019. Mr. Bigham has more than 30 years of senior leadership experience in the biopharmaceutical industry. From January 2003 to November 2015, he was a General Partner at Abingworth LLP, a leading international investment group dedicated to life sciences and healthcare. From November 2015 to December 2018, he served as part-time Executive Partner at the firm. He currently serves on the boards of Ancora Biotech and Nutcracker Therapeutics, both private companies. He has previously served on the board of directors of Inmediata, where he was also Chairman, and Avila Therapeutics, where he was also the founding Chairman and Chief Executive Officer. He has also previously served on the board of directors of Magellan Biosciences, Portola Pharmaceuticals, Supernus Pharmaceuticals (Nasdaq: SUPN), Avedro, Valeritas, Adamas Pharmaceuticals (Nasdaq: ADMS), and TeneoBio. He was formerly Vice Chairman of Corixa Corporation, a public biotechnology company, and was President and Chief Executive of Coulter Pharmaceuticals, a public oncology company, until it merged into Corixa. Previously, he was an early employee at Gilead Sciences (Nasdaq: GILD), where he served in various capacities, including Executive Vice President of Operations and Chief Financial Officer. Before joining Gilead Sciences, he was a Partner at Hambrecht & Quist, where he became Co-Head of Healthcare Investment Banking. Mr. Bigham received his B.S. from the University of Virginia and qualified as a C.P.A. before completing his M.B.A. at Stanford University.
Carol G. Gallagher, Pharm.D., has served on the FLAC Board since October 2020. Dr. Gallagher has served as a Venture Partner at New Enterprise Associates since 2014. Dr. Gallagher has over ten years of experience as a director in public and private companies and over 30 years of experience in biopharmaceutical companies. She is currently a Venture Partner at New Enterprise Associates. Dr. Gallagher is currently a director of Atara Biotherapeutics (Nasdaq: ATRA), where she has served since January 2013, and Certara Inc., where she serves since June 2021. She also serves as a director at, PIONYR Immunotherapeutics, Qpex BioPharma, Recludix, TRex Bio, Turning Point Therapeutics (Nasdaq: TPTX) and Chromacode. She previously served as a director at Annexon, Aragon Pharmaceuticals, Metacrine (Nasdaq: MTCR), Millendo Therapeutics, Seragon Pharmaceuticals, AnaptysBio (Nasdaq: ANAB) and eFFECTOR Therapeutics. From 2008 to 2011, Dr. Gallagher was the President and Chief Executive Officer of Calistoga Pharmaceuticals, which developed the first-in-class cancer therapeutic, CAL-101, and was acquired by Gilead Sciences in 2011. CAL-101 was approved as ZYDELIG in the US and Europe in 2014. Earlier in her career, she held commercial and drug development roles within Eli Lilly (NYSE: LLY), Amgen (Nasdaq: AMGN), Agouron, Pfizer (NYSE: PFE) and Biogen (Nasdaq: BIIB). She studied chemistry at Vanderbilt University and then attained her B.S. and Doctor of Pharmacy degrees from the College of Pharmacy at the University of Kentucky.
Krishna R. Polu, M.D., has served on the FLAC Board since October 2020. Since January 2021, Dr. Polu has served as a principal at Red Tree Venture Capital. Dr. Polu previously served as the Executive Vice President R&D of Equillium, Inc. (Nasdaq: EQ) from January 2020 to December 2020, and additionally served as Chief
330
Table of Contents
Medical Officer from August 2018 to December 2020. Since January 2021, Dr. Polu has served as a principal at Red Tree Venture Capital. Dr. Polu also serves as a member of the board of directors of Goldilocks Therapeutics, where he previously served as a strategic advisor from February 2018 to January 2020. Prior to that, Dr. Polu was an Entrepreneur in Residence at Frazier from February 2017 to August 2018, where he founded Expedition Therapeutics, a search company focused on identifying and in-licensing assets in the kidney and autoimmune therapeutic areas. During this time, he also served as interim Chief Executive Officer of Scout Bio, a company focused on the discovery and development of gene therapies for companion animals. From January 2015 to December 2016, Dr. Polu served as Chief Medical Officer at Raptor Pharmaceuticals, a then-public company focused on rare diseases, until its acquisition by Horizon Pharmaceuticals for $800 million. In that role, he oversaw clinical development, regulatory affairs, pharmacovigilance and medical affairs, and was responsible for securing additional drug approvals for Procysbi in nephropathic cystinosis, supporting product launches for Quinsair in cystic fibrosis, and advancing the pipeline in other rare diseases including Huntington’s disease, cystic fibrosis, bronchiectasis and nontuberculous mycobacteria. Prior to Raptor, Dr. Polu served as Chief Medical Officer at CytomX Therapeutics (Nasdaq: CTMX) and directed preclinical development and translational research efforts for the Probody platform in oncology and helped secure a number of pharma partnerships. Prior to CytomX, he led clinical development and pharmacovigilance activities at Affymax, a then-public biopharmaceutical company, where he was instrumental in securing FDA approval of peginesatide for the treatment of anemia in patients on dialysis. Dr. Polu also held senior level positions in clinical development at Amgen (Nasdaq: AMGN) and was responsible for leading clinical development programs in heart failure, anemia of chronic kidney disease, and diabetes.
Dr. Polu currently serves as an advisor to Trestle Biotherapeutics, Medikine, and Mineralys Therapeutics. Dr. Polu is also the co-founder of Lassen Therapeutics, where he also serves as an advisor. Dr. Polu received his B.A. in Human Biology from Stanford University and his M.D. from the University of Texas Health Science Center, San Antonio. He completed his residency in internal medicine at the University of Colorado followed by a clinical and research fellowship in nephrology at Harvard Medical School at the Brigham and Women’s Hospital and Massachusetts General Hospital. Dr. Polu has co-authored several scientific and clinical publications in the areas of genetics and renal disease.
FLAC believes its board of directors and management team are well positioned to take advantage of the growing set of investment opportunities focused on the biotechnology sector, and that FLAC’s contacts, relationships and investment and operating experience will allow it to generate an attractive transaction for its shareholders.
There are no family relationships between any director or executive officer except that James N. Topper and David Topper are siblings. All of FLAC’s directors and executive officers are U.S. persons.
Number and Terms of Office of Officers and Directors
The FLAC Board is divided into three classes, with only one class of directors being appointed in each year, and with each class (except for those directors appointed prior to FLAC’s first annual general meeting) serving a three-year term. The term of office of the first class of directors, consisting of Robert F. Baltera and Michael F. Bigham, will expire at the first general annual meeting. The term of office of the second class of directors, consisting of Carol G. Gallagher and Krishna R. Polu, will expire at the second annual general meeting. The term of office of the third class of directors, consisting of David Topper and James N. Topper, will expire at the third annual general meeting.
Prior to the completion of an initial business combination, any vacancy on the FLAC Board may be filled by a nominee chosen by holders of a majority of the Founder Shares. In addition, prior to the completion of an initial business combination, holders of a majority of the Founder Shares may remove a member of the FLAC Board for any reason.
331
Table of Contents
Pursuant to an agreement to be entered into on or prior to the closing of the FLAC IPO, the Sponsor, upon and following consummation of an initial business combination, will be entitled to nominate three individuals for appointment to the FLAC Board, as long as the Sponsor holds any securities covered by the registration and shareholder rights agreement.
FLAC’s officers are appointed by the FLAC Board and serve at the discretion of the FLAC Board, rather than for specific terms of office. The FLAC Board is authorized to appoint persons to the offices set forth in and the FLAC Articles of Association as it deems appropriate. The FLAC Articles of Association provides that its officers may consist of one or more chairman of the board, chief executive officer, chief financial officer, chief business officer, president, vice presidents, secretary, treasurer and such other offices as may be determined by the FLAC Board.
Director Independence
Nasdaq listing standards require that a majority of the FLAC Board be independent. An “independent director” is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship with the company which in the opinion of the company’s board of directors, could interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director. The FLAC Board has determined that each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher and Krishna R. Polu are “independent directors” as defined in Nasdaq’s listing standards and applicable SEC rules.
FLAC’s independent directors have had regularly scheduled meetings at which only independent directors are present.
Executive Officer and Director Compensation
None of FLAC’s executive officers or directors has received any cash compensation for services rendered to us. Commencing on the date that FLAC’s securities were first listed on Nasdaq through the earlier of consummation of its initial business combination and its liquidation, FLAC has reimbursed the Sponsor for office space and secretarial and administrative services provided in the amount of $10,000 per month. In addition, the Sponsor, executive officers and directors, or any of their respective affiliates are reimbursed for any out-of-pocket expenses incurred in connection with activities on FLAC’s behalf such as identifying potential partner businesses and performing due diligence on suitable business combinations. FLAC’s audit committee reviews on a quarterly basis all payments that were made by it to the Sponsor, executive officers or directors, or its or their affiliates. Any such payments prior to an initial business combination will be made using funds held outside the trust account. Other than quarterly audit committee review of such reimbursements, FLAC does not expect to have any additional controls in place governing its reimbursement payments to its directors and executive officers for their out-of-pocket expenses incurred in connection with FLAC’s activities on its behalf in connection with identifying and consummating an initial business combination. Other than these payments and reimbursements, no compensation of any kind, including finder’s and consulting fees, will be paid by FLAC to the Sponsor, executive officers and directors, or any of their respective affiliates, prior to completion of an initial business combination.
After the completion of an initial business combination, directors or members of FLAC’s founding team who remain with FLAC may be paid consulting or management fees from the combined company. All of these fees will be fully disclosed to shareholders, to the extent then known, in the proxy solicitation materials or tender offer materials furnished to FLAC’s shareholders in connection with a proposed business combination. FLAC has not established any limit on the amount of such fees that may be paid by the combined company to its directors or members of management. It is unlikely the amount of such compensation will be known at the time of the proposed business combination, because the directors of the post-combination business will be responsible for determining executive officer and director compensation. Any compensation to be paid to FLAC���s executive officers will be determined, or recommended to the FLAC Board for determination, either by a compensation committee constituted solely by independent directors or by a majority of the independent directors on the FLAC Board.
332
Table of Contents
FLAC does not intend to take any action to ensure that members of its founding team maintain their positions with FLAC after the consummation of the initial business combination, although it is possible that some or all of FLAC’s executive officers and directors may negotiate employment or consulting arrangements to remain with it after the initial business combination. The existence or terms of any such employment or consulting arrangements to retain their positions with FLAC may influence its founding team’s motivation in identifying or selecting a partner business but FLAC does not believe that the ability of its founding team to remain with it after the consummation of the initial business combination will be a determining factor in FLAC’s decision to proceed with any potential business combination. FLAC is not party to any agreements with its executive officers and directors that provide for benefits upon termination of employment.
Committees of the Board of Directors
The FLAC Board has three standing committees: an audit committee, a nominating committee and a compensation committee. Each committee operates under a charter that has been approved by the FLAC Board and has the composition and responsibilities described below. The charter of each committee is available on FLAC’s website.
Audit Committee
Robert F. Baltera, Michael F. Bigham and Carol G. Gallagher serve as members of the audit committee. Michael F. Bigham serves as the chairperson of the audit committee. The FLAC Board has determined that each of Robert F. Baltera, Michael F. Bigham and Carol G. Gallagher is independent.
Each member of the audit committee meets the financial literacy requirements of Nasdaq and the FLAC Board has determined that each of Robert F. Baltera and Michael F. Bigham qualifies as an “audit committee financial expert” as defined in applicable SEC rules and has accounting or related financial management expertise.
The audit committee operates pursuant to a charter and is responsible for:
| • | meeting with FLAC’s independent registered public accounting firm regarding, among other issues, audits, and adequacy of FLAC’s and control systems; |
| • | monitoring the independence of the independent registered public accounting firm; |
| • | verifying the rotation of the lead (or coordinating) audit partner having primary responsibility for the audit and the audit partner responsible for reviewing the audit as required by law; |
| • | reviewing financial statements proposed to be included in FLAC’s registration statements and periodic reports and earnings releases; |
| • | inquiring and discussing with management FLAC’s compliance with applicable laws and regulations; |
| • | pre-approving all audit services and permitted non-audit services to be performed by FLAC’s independent registered public accounting firm, including the fees and terms of the services to be performed; |
| • | appointing or replacing the independent registered public accounting firm; |
| • | determining the compensation and oversight of the work of the independent registered public accounting firm (including resolution of disagreements between management and the independent auditor regarding financial reporting) for the purpose of preparing or issuing an audit report or related work; |
| • | establishing procedures for the receipt, retention and treatment of complaints received by FLAC regarding accounting, internal accounting controls or reports which raise material issues regarding its financial statements or accounting policies; |
333
Table of Contents
| • | reviewing and approving all payments made to FLAC’s existing shareholders, executive officers or directors and their respective affiliates. Any payments made to members of FLAC’s audit committee will be reviewed and approved by the FLAC Board, with the interested director or directors abstaining from such review and approval. |
Nominating and Corporate Governance Committee
The members of FLAC’s nominating and corporate governance committee are Michael F. Bigham and Krishna R. Polu. Krishna R. Polu serves as the chairperson of the nominating and corporate governance committee. The FLAC Board has determined that each of Michael F. Bigham and Krishna R. Polu is independent.
The nominating and corporate governance committee is responsible for overseeing the selection of persons to be nominated to serve on the FLAC Board. The nominating and corporate governance committee considers persons identified by its members, management, shareholders, investment bankers and others.
Guidelines for Selecting Director Nominees
The guidelines for selecting nominees are specified in the nominating committee’s charter, which provides that persons to be nominated:
| • | should have demonstrated notable or significant achievements in business, education or public service; |
| • | should possess the requisite intelligence, education and experience to make a significant contribution to the FLAC Board and bring a range of skills, diverse perspectives and backgrounds to its deliberations; and |
| • | should have the highest ethical standards, a strong sense of professionalism and intense dedication to serving the interests of the shareholders. |
The nominating committee considers a number of qualifications relating to management and leadership experience, background and integrity and professionalism in evaluating a person’s candidacy for membership on the FLAC Board. The nominating committee may require certain skills or attributes, such as financial or accounting experience, to meet specific board needs that arise from time to time and will also consider the overall experience and makeup of its members to obtain a broad and diverse mix of board members. The nominating committee does not distinguish among nominees recommended by shareholders and other persons.
Compensation Committee
The members of FLAC’s compensation committee are Carol G. Gallagher and Krishna R. Polu. Carol G. Gallagher serves as chairperson of the compensation committee. The FLAC Board has determined that each of Carol G. Gallagher and Krishna R. Polu is independent.
The compensation committee operates pursuant to a charter, which details the principal functions of the compensation committee, including:
| • | reviewing and approving on an annual basis the corporate goals and objectives relevant to FLAC’s Chief Executive Officer’s compensation, evaluating the Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of the Chief Executive Officer based on such evaluation; |
| • | reviewing and approving the compensation of all of FLAC’s other Section 16 executive officers; |
| • | reviewing FLAC’s executive compensation policies and plans; |
| • | implementing and administering FLAC’s incentive compensation equity-based remuneration plans; |
334
Table of Contents
| • | assisting management in complying with FLAC’s proxy statement and annual report disclosure requirements; |
| • | approving all special perquisites, special cash payments and other special compensation and benefit arrangements for FLAC’s executive officers and employees; |
| • | producing a report on executive compensation to be included in FLAC’s annual proxy statement; and |
| • | reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors. |
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other adviser and is directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Compensation Committee Interlocks and Insider Participation
None of FLAC’s executive officers currently serves, and in the past year has not served, as a member of the compensation committee of any entity that has one or more executive officers serving on the FLAC Board.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, requires FLAC’s officers, directors and persons who beneficially own more than ten percent of FLAC Ordinary Shares to file reports of ownership and changes in ownership with the SEC. These reporting persons are also required to furnish FLAC with copies of all Section 16(a) forms they file. Based solely upon a review of such forms, FLAC believes that during the year ended December 31, 2021, there were no delinquent filers.
Code of Ethics
FLAC adopted a Code of Conduct and Ethics (the “Code of Ethics”) applicable to FLAC’s directors, officers and employees. A copy of the Code of Ethics is available on FLAC’s website. FLAC intends to disclose any amendments to or waivers of certain provisions of FLAC’s Code of Ethics in a Current Report on Form 8-K.
Conflicts of Interest
Under Cayman Islands law, directors and officers owe the following fiduciary duties:
| • | duty to act in good faith in what the director or officer believes to be in the best interests of the company as a whole; |
| • | duty to exercise powers for the purposes for which those powers were conferred and not for a collateral purpose; |
| • | directors should not improperly fetter the exercise of future discretion; |
| • | duty to exercise powers fairly as between different sections of shareholders; |
| • | duty not to put themselves in a position in which there is a conflict between their duty to the company and their personal interests; and |
| • | duty to exercise independent judgment. |
In addition to the above, directors also owe a duty of care which is not fiduciary in nature. This duty has been defined as a requirement to act as a reasonably diligent person having both the general knowledge, skill and experience that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and the general knowledge skill and experience of that director.
335
Table of Contents
As set out above, directors have a duty not to put themselves in a position of conflict and this includes a duty not to engage in self-dealing, or to otherwise benefit as a result of their position. However, in some instances what would otherwise be a breach of this duty can be forgiven and/or authorized in advance by the shareholders provided that there is full disclosure by the directors. This can be done by way of permission granted in the FLAC Articles of Association or alternatively by shareholder approval at general meetings.
Each of FLAC’s officers and directors presently has, and any of them in the future may have additional, fiduciary or contractual obligations to another entity, including private funds under the management of Frazier and their respective portfolio companies, pursuant to which such officer or director is or will be required to present a business combination opportunity to such entity. In addition, existing and future funds managed by Frazier and their respective portfolio companies may compete with FLAC for business combination opportunities and, if such opportunities are pursued by such entities, FLAC may be precluded from pursuing such opportunities. Accordingly, if any of FLAC’s officers or directors becomes aware of a business combination opportunity which is suitable for an entity to which he or she has then-current fiduciary or contractual obligations, he or she will honor his or her fiduciary or contractual obligations to present such business combination opportunity to such entity, and may only decide to present it to FLAC if such entity rejects the opportunity and consummating the same would not violate any restrictive covenants to which such officers and directors are subject. Notwithstanding the foregoing, FLAC may pursue an affiliated joint acquisition opportunity with an entity to which an officer or director has a fiduciary or contractual obligation. Any such entity may co-invest with FLAC in the target business at the time of its initial business combination, or FLAC could raise additional proceeds to complete the acquisition by issuing to such entity a class of equity or equity-linked securities. The FLAC Articles of Association will provide that FLAC renounces its interest in any corporate opportunity offered to any director or officer unless such opportunity is expressly offered to such person solely in his or her capacity as a director or officer of the company and such opportunity is one FLAC is legally and contractually permitted to undertake and would otherwise be reasonable for it to pursue, and to the extent the director or officer is permitted to refer that opportunity to FLAC without violating another legal obligation.
Below is a table summarizing the entities to which FLAC’s executive officers and directors currently have fiduciary duties, contractual obligations or other material management relationships:
INDIVIDUAL | ENTITY | ENTITY’S BUSINESS | AFFILIATION | |||
James N. Topper | Frazier Life Sciences and its affiliated funds | Investment Firm | Managing General Partner | |||
| Frazier Life Sciences Management, L.P., and its affiliated funds | Management Company | Managing General Partner | ||||
| Frazier Lifesciences Sponsor LLC | Investment Firm | Manager | ||||
| Alcresta Therapeutics, Inc. (a Frazier portfolio company) | Biotechnology Company | Board Observer | ||||
| Alpine Immune Sciences, Inc. (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| AnaptysBio, Inc. (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| Lassen Therapeutics (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| Dascena, Inc. (a Frazier portfolio company) | Biotechnology and Precision Medicine Company | Board Observer |
336
Table of Contents
INDIVIDUAL | ENTITY | ENTITY’S BUSINESS | AFFILIATION | |||
| Seraxis Holding, Inc. (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| Sudo Biosciences, Inc. (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| Enlaza Therapeutics, Inc. (a Frazier portfolio company) | Pharmaceutical Research and Development Company | Director | ||||
| Serum, Inc. (a Frazier portfolio company) | Biotechnology Company | Director | ||||
| Sonoma Biotherapeutics Inc. (a Frazier portfolio company) | Biotechnology Company | Board Observer | ||||
David Topper | Frazier Life Sciences and its affiliated funds | Investment Firm | Partner, Capital Markets | |||
| Frazier Lifesciences Sponsor LLC | Investment Firm | Manager | ||||
| CircleUp | Financial Technology Company | Board Observer | ||||
| TermGrid Limited | Software Company | Director | ||||
Gordon Empey | Frazier Life Sciences and its affiliated funds | Investment Firm | Partner and General Counsel | |||
| Frazier Life Sciences Management, L.P., and its affiliated funds | Management Company | Partner and General Counsel | ||||
| Frazier Lifesciences Sponsor LLC | Investment Firm | Manager | ||||
Robert F. Baltera | Frazier Life Sciences and its affiliated funds | Investment Firm | Entrepreneur in Residence | |||
| Cirius Therapeutics, Inc. (a Frazier portfolio company) | Biotechnology Company | President and Chief Executive Officer | ||||
| Imago BioSciences, Inc. | Biotechnology Company | Director | ||||
| Trestle Biotherapeutics Inc. | Biotechnology Company | Co-Founder/Executive Chairman | ||||
| Panmira Pharmaceuticals & FLAP LLC | Biotechnology Company | Director | ||||
| PBS Biotech Inc. | Biotechnology Company | Business Advisory Panel Member | ||||
Michael F. Bigham | Paratek Pharmaceuticals, Inc | Biotechnology Company | Executive Chairman | |||
| Adamas Pharmaceuticals, Inc. | Biotechnology Company | Director | ||||
Carol G. Gallagher | New Enterprise Associates | Venture Capital Firm | Venture Partner | |||
| Atara Biotherapeutics, Inc. | Biotechnology Company | Director | ||||
337
Table of Contents
INDIVIDUAL | ENTITY | ENTITY’S BUSINESS | AFFILIATION | |||
| Certara Inc. | Biotechnology Company | Director | ||||
PIONYR Immunotherapeutics Inc. (a New Enterprise Associates portfolio company) | Biotechnology Company | Director | ||||
| Qpex BioPharma, Inc. | Biotechnology Company | Director | ||||
Recludix Pharma, Inc. (a New Enterprise Associates portfolio company) | Biotechnology Company | Director | ||||
| TRex Bio, Inc. | Biotechnology Company | Director | ||||
| Turning Point Therapeutics, Inc. | Biotechnology Company | Director | ||||
Krishna R. Polu | Equillium, Inc. | Biotechnology Company | Executive Vice President R&D and Chief Medical Officer | |||
| Goldilocks Therapeutics, Inc. | Biotechnology Company | Director | ||||
| Lassen Therapeutics (a Frazier portfolio company) | Biotechnology Company | Co-Founder and Advisor | ||||
| Medikine, Inc. | Biotechnology Company | Advisor | ||||
| Mineralys Therapeutics, Inc. | Biotechnology Company | Advisor | ||||
| Trestle Biotherapeutics, Inc. | Biotechnology Company | Advisor |
Potential investors should also be aware of the following other potential conflicts of interest:
| • | Our executive officers and directors are not required to, and will not, commit their full time to FLAC’s affairs, which may result in a conflict of interest in allocating their time between FLAC’s operations and the consummation of the Business Combination. FLAC does not intend to have any fulltime employees prior to the completion of its initial business combination. Each of FLAC’s executive officers is engaged in several other business endeavors for which he may be entitled to substantial compensation, and FLAC’s executive officers are not obligated to contribute any specific number of hours per week to FLAC’s affairs. |
| • | The Sponsor subscribed to Founder Shares prior to the date of the FLAC IPO and purchased FLAC Private Placement Units in a transaction that closed simultaneously with the closing of the FLAC IPO. The FLAC Initial Shareholders have entered into an agreement with FLAC, pursuant to which they have agreed to waive their redemption rights with respect to their Founder Shares and FLAC Public Shares in connection with (i) the completion of FLAC’s initial business combination and (ii) a shareholder vote to approve an amendment to the FLAC Articles of Association (A) that would modify the substance or timing of FLAC’s obligation to provide holders of FLAC Class A Ordinary Shares the right to have their shares redeemed in connection with FLAC’s initial business combination or to redeem 100% of FLAC Public Shares if FLAC does not complete its initial business combination within 24 months from the closing of the FLAC IPO or (B) with respect to any other provision relating to the rights of holders of FLAC Class A Ordinary Shares or pre-initial business combination activity. Additionally, each of the FLAC Initial Shareholders has agreed to waive its rights to liquidating distributions from the trust account with respect to its Founder Shares if FLAC fails to complete its initial business combination within the required time period. If FLAC does not complete its initial |
338
Table of Contents
business combination within the required time period, the FLAC Private Placement Units and the underlying securities will expire worthless. Except as described herein, the FLAC Initial Shareholders have agreed not to transfer, assign or sell any of their Founder Shares until the earliest of (A) one year after the completion of the FLAC IPO and (B) subsequent to the initial business combination, including the Business Combination, (x) if the closing price of FLAC Class A Ordinary Shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the consummation of the Business Combination, or (y) the date on which FLAC completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of FLAC’s public shareholders having the right to exchange their ordinary shares for cash, securities or other property. With certain limited exceptions, the FLAC Private Placement Units, the FLAC Private Placement Shares, the FLAC Private Placement Warrants and the Class A Ordinary Shares underlying such warrants, will not be transferable until 30 days following the completion of FLAC’s initial business combination. Because each of FLAC’s directors other than the Chairman will own ordinary shares or warrants directly or indirectly, they may have a conflict of interest in determining whether a particular partner business is an appropriate business with which to effectuate FLAC’s initial business combination. |
| • | FLAC’s officers and directors may have a conflict of interest with respect to evaluating a particular business combination if the retention or resignation of any such officers and directors was included by a partner business as a condition to any agreement with respect to FLAC’s initial business combination. |
| • | FLAC is not prohibited from pursuing an initial business combination or subsequent transaction with a company that is affiliated with the Sponsor, founders, officers or directors. While not applicable to the Business Combination, in the event FLAC were to seek to complete its initial business combination with a company that is affiliated with the Sponsor or any of FLAC’s founders, officers or directors, FLAC, or a committee of independent directors, will obtain an opinion from an independent investment banking firm which is a member of FINRA or an independent valuation or accounting firm that such initial business combination or transaction is fair to FLAC from a financial point of view. FLAC is not required to obtain such an opinion in any other context. Furthermore, in no event will the Sponsor or any of FLAC’s existing officers or directors, or any of their respective affiliates, be paid by FLAC any finder’s fee, consulting fee or other compensation prior to, or for any services they render in order to effectuate, the completion of FLAC’s initial business combination. Further, commencing on the date FLAC’s securities were first listed on Nasdaq, FLAC will also reimburse the Sponsor for office space and secretarial and administrative services provided to it in the amount of $10,000 per month. |
FLAC cannot assure that any of the aforementioned conflicts will be resolved in its favor.
See the section entitled “The Business Combination—Interests of Certain Persons in the Business Combination—Interests of FLAC’s Directors and Executive Officers in the Business Combination” for a further discussion of additional considerations in connection with the Business Combination.
Limitation on Liability and Indemnification of Officers and Directors
Cayman Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against willful default, willful neglect, civil fraud or the consequences of committing a crime. The FLAC Articles of Association provides for indemnification of FLAC’s officers and directors to the maximum extent permitted by law, including for any liability incurred in their capacities as such, except through their own actual fraud, willful default or willful neglect. FLAC will enter into agreements with its directors and officers to provide contractual indemnification in addition to the indemnification provided for in the FLAC Articles of Association.
FLAC has purchased a policy of directors’ and officers’ liability insurance that insures its officers and directors against the cost of defense, settlement or payment of a judgment in some circumstances and insures FLAC against its obligations to indemnify its officers and directors.
339
Table of Contents
FLAC’s officers and directors have agreed to waive any right, title, interest or claim of any kind in or to any monies in the Trust Account, and have agreed to waive any right, title, interest or claim of any kind they may have in the future as a result of, or arising out of, any services provided to FLAC and will not seek recourse against the trust account for any reason whatsoever (except to the extent they are entitled to funds from the trust account due to their ownership of public shares). Accordingly, any indemnification provided will only be able to be satisfied by FLAC if (i) FLAC has sufficient funds outside of the trust account or (ii) FLAC consummates an initial business combination.
FLAC’s indemnification obligations may discourage shareholders from bringing a lawsuit against FLAC’s officers or directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against FLAC’s officers and directors, even though such an action, if successful, might otherwise benefit FLAC and FLAC’s shareholders. Furthermore, a shareholder’s investment may be adversely affected to the extent FLAC pays the costs of settlement and damage awards against its officers and directors pursuant to these indemnification provisions.
FLAC believes that these provisions, the insurance and the indemnity agreements are necessary to attract and retain talented and experienced officers and directors.
Executive Compensation
Executive Officer and Director Compensation
In November 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described herein. None of FLAC’s executive officers or directors have received any cash compensation for services rendered to FLAC. Since the consummation of the FLAC IPO and until the earlier of consummation of FLAC’s initial business combination and its liquidation, FLAC will reimburse an affiliate of the Sponsor for office space and secretarial and administrative services provided to FLAC in an amount not to exceed $10,000 per month. In addition, the Sponsor, executive officers and directors, or any of their respective affiliates will be reimbursed for any out-of-pocket expenses incurred in connection with activities on FLAC’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. FLAC’s audit committee reviews on a quarterly basis all payments that were made to the Sponsor, executive officers or directors, or FLAC’s or their affiliates. Any such payments prior to an initial business combination are made using funds held outside the trust account. Other than quarterly audit committee review of such reimbursements, FLAC does not have any additional controls in place governing its reimbursement payments to its directors and executive officers for their out-of-pocket expenses incurred in connection with FLAC’s activities on its behalf in connection with identifying and consummating an initial business combination. Other than these payments and reimbursements, no compensation of any kind, including finder’s and consulting fees, is paid by the company to the Sponsor, executive officers and directors, or any of their respective affiliates, prior to completion of FLAC’s initial business combination.
After the completion of FLAC’s initial business combination, directors or members of its management team who remain with FLAC may be paid consulting or management fees from the combined company. All of these fees will be fully disclosed to shareholders, to the extent then known, in the proxy solicitation materials or tender offer materials furnished to FLAC’s shareholders in connection with a proposed business combination. FLAC has not established any limit on the amount of such fees that may be paid by the combined company to FLAC’s directors or members of management. It is unlikely the amount of such compensation will be known at the time of the proposed business combination, because the directors of Holdco will be responsible for determining executive officer and director compensation. Any compensation to be paid to FLAC’s executive officers will be determined, or recommended to the FLAC Board for determination, either by a compensation committee constituted solely by independent directors or by a majority of the independent directors on the FLAC Board.
FLAC does not intend to take any action to ensure that members of its management team maintain their positions with FLAC after the consummation of FLAC’s initial business combination, although it is possible that
340
Table of Contents
some or all of FLAC’s executive officers and directors may negotiate employment or consulting arrangements to remain with FLAC after its initial business combination. The existence or terms of any such employment or consulting arrangements to retain their positions with FLAC may influence its management’s motivation in identifying or selecting a target business but FLAC does not believe that the ability of its management to remain with FLAC after the consummation of its initial business combination will be a determining factor in its decision to proceed with any potential business combination. FLAC is not party to any agreements with its executive officers and directors that provide for benefits upon termination of employment.
341
Table of Contents
FLAC’S MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of FLAC’s financial condition and results of operations should be read in conjunction with the financial statements and the notes thereto contained elsewhere in this proxy statement/prospectus. Certain information contained in the discussion and analysis set forth below includes forward looking statements that involve risks and uncertainties.
Overview
FLAC is a special purpose acquisition company incorporated on October 7, 2020 as a Cayman Islands exempted company for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses or entities. FLAC has generated no operating revenues to date and does not expect that it will generate operating revenues until it consummates a business combination.
On October 7, 2020, prior to the FLAC IPO, the Sponsor purchased 2,875,000 Founder Shares for an aggregate purchase price of $25,000. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding.
On December 11, 2020, FLAC consummated the FLAC IPO of 13,800,000 FLAC Public Units, including 1,800,000 additional FLAC Public Units to cover over-allotments, with each FLAC Public Unit consisting of one FLAC Class A Ordinary Share and one-third of one FLAC Public Warrant, each whole FLAC Public Warrant entitling the holder thereof to purchase one FLAC Class A Ordinary Share at an exercise price of $11.50 per share, subject to adjustment. The FLAC Public Units were sold at a price of $10.00 per unit, generating gross proceeds of $138 million before underwriting discounts and expenses.
Simultaneously with the closing of the FLAC IPO, FLAC consummated the private placement of 501,000 units, at a price of $10.00 per FLAC Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5 million.
Upon the consummation of the FLAC IPO and the private placement, approximately $138 million of the net proceeds of the FLAC IPO and certain of the proceeds of the private placement were deposited in a U.S.-based trust account at J.P. Morgan Chase Bank, N.A., maintained by Continental Stock Transfer & Trust Company, acting as trustee. Transaction costs of the FLAC IPO and the private placement amounted to approximately $8.11 million, inclusive of approximately $4.83 million in deferred underwriting fees and $83,000 to repay to the Sponsor’s borrowings under the $300,000 promissory note payable, which was available to pay accrued offering and formation costs, business, legal and accounting due diligence on prospective acquisitions and continuing general and administrative expenses. Funds held in the Trust Account have been invested only in U.S. government treasury bills with a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 under the Investment Company Act of 1940, as amended (the “Investment Company Act”), which invest only in direct U.S. government obligations. Except with respect to interest earned on the funds in the Trust Account that may be released to FLAC to pay income taxes, if any, the proceeds from the FLAC IPO and the sale of the Private Placement Warrants held in the Trust Account will not be released from the Trust Account (1) to FLAC until the completion of its initial business combination or (2) to FLAC’s public shareholders, until the earliest of: (a) the completion of FLAC’s initial business combination, and then only in connection with those FLAC Public Shares that such shareholders properly elect to redeem, subject to certain limitations, (b) the redemption of any FLAC Public Shares properly tendered in connection with a (i) shareholder vote to amend the FLAC Articles of Association to modify the substance or timing of its obligation to provide holders of the FLAC Public Shares the right to have their shares redeemed in connection with its initial business combination within 24 months from the closing of the FLAC IPO or (ii) with respect to any other provisions relating to shareholders’ rights of holders of FLAC Class A Ordinary Shares or pre-initial business combination
342
Table of Contents
activity and (c) the redemption of all of FLAC’s Ordinary Shares if FLAC has not completed its initial business combination within 24 months from the closing of the FLAC IPO, subject to applicable law.
If FLAC is unable to complete a business combination within 24 months from the closing of the FLAC IPO, or December 11, 2022, absent any extension, FLAC will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account and not previously released to FLAC to pay for FLAC’s income taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public shareholders’ rights as shareholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of FLAC’s remaining shareholders and the FLAC Board, proceed to commence a voluntary liquidation and thereby a formal dissolution of FLAC, subject in each case to its obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law.
Liquidity and Going Concern
As of June 30, 2022, FLAC had approximately $615,000 in cash and a working capital deficit of approximately $1.1 million.
FLAC’s liquidity needs up to June 30, 2022 had been satisfied through a contribution of $25,000 from the Sponsor to cover for certain expenses on behalf of FLAC in exchange for the issuance of the Founder Shares, the loan of approximately $83,000 pursuant to the note issued to the Sponsor, and the proceeds from the consummation of the private placement not held in the Trust Account. FLAC fully repaid the note to the Sponsor on December 14, 2020. In addition, in order to finance transaction costs in connection with a business combination, the Sponsor or an affiliate of the Sponsor, or certain of FLAC’s officers and directors may, but are not obligated to, provide FLAC working capital loans. To date, there were no amounts outstanding under any working capital loan. In addition, in September 2021, FLAC entered into a term sheet in connection with a proposed initial business combination. On December 30, 2021, upon termination of the term sheet, FLAC received a break-up fee of $1 million.
Based on the foregoing, FLAC’s management believes that FLAC will have sufficient working capital and borrowing capacity from the Sponsor or an affiliate of the Sponsor, or certain of the officers and directors to meet FLAC’s needs through the earlier of the consummation of a Business Combination or one year from this filing. However, in connection with the FLAC’s assessment of going concern considerations in accordance with FASB ASU 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” FLAC’s management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about FLAC’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should FLAC be required to liquidate after December 11, 2022 or such later date as may be approved by FLAC’s shareholders. The condensed financial statements do not include any adjustment that might be necessary if FLAC is unable to continue as a going concern. FLAC’s management intends to complete the Business Combination prior to the liquidation date.
FLAC’s management continues to evaluate the impact of the COVID-19 pandemic and has concluded that the specific impact is not readily determinable as of the date of the unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Results of Operations
FLAC’s entire activity has consisted of preparing for FLAC’s formation and the FLAC IPO, and since the FLAC IPO, its activity has been limited to the search for a prospective initial business combination, including negotiating and preparing for the Business Combination. FLAC will not be generating any operating revenues until the closing and completion of FLAC’s initial Business Combination at the earliest.
343
Table of Contents
For the three months ended June 30, 2022, we had net loss of approximately $1.2 million, which consisted of approximately $2.0 million in general and administrative expenses, and $30,000 in administrative expenses-related party, partially offset by approximately $715,000 in change in fair value of derivative warrant liabilities, and approximately $103,000 in interest income from investments held in trust account.
For the three months ended June 30, 2021, we had a net loss of approximately $1.3 million, which consisted of approximately $275,000 in general and administrative expenses, $30,000 in administrative expenses-related party, approximately $1.0 million in change in fair value of derivative warrant liabilities, offset by approximately $7,000 in interest income from investments held in Trust Account.
For the six months ended June 30, 2022, we had net income of approximately $212,000, which consisted of approximately $2.4 million in change in fair value of derivative warrant liabilities, and approximately $116,000 in interest income from investments held in trust account, offset by approximately $2.3 million in general and administrative expenses, and $60,000 in administrative expenses-related party.
For the six months ended June 30, 2021, we had a net income of approximately $1.5 million, which consisted of approximately $11,000 in interest income from investments held in Trust Account, and approximately $2.0 million in change in fair value of derivative warrant liabilities, offset by approximately $482,000 in general and administrative expenses, and $60,000 in administrative expenses-related party.
Contractual Obligations
Registration and Shareholder Rights
The holders of Founder Shares, FLAC Private Placement Units, FLAC Private Placement Shares, FLAC Private Placement Warrants, and FLAC Class A Ordinary Shares underlying the FLAC Private Placement Warrants and units that may be issued upon conversion of working capital loans, if any, will be entitled to registration rights (in the case of the Founder Shares, only after conversion of such shares into FLAC Class A Ordinary Shares) pursuant to a registration and shareholder rights agreement entered into upon consummation of the FLAC IPO (the “Registration Rights Agreement”). These holders will be entitled to certain demand and “piggyback” registration and shareholder rights. However, the Registration Rights Agreement provides that FLAC will not permit any registration statement filed under the Securities Act to become effective until the termination of the applicable lock-up period for the securities to be registered. FLAC will bear the expenses incurred in connection with the filing of any such registration statements. See the section entitled “Certain Relationships and Related Party Transactions—FLAC Relationships and Related Person Transactions—Registration Rights” for additional information.
Underwriting Agreement
FLAC granted the underwriters a 45-day option from the date of the final prospectus relating to the FLAC IPO to purchase up to 1,800,000 additional units to cover over-allotments, if any, at $10.00 per unit, less underwriting discounts and commissions. The underwriters exercised this option in full on December 11, 2020.
The underwriters were entitled to underwriting discounts of $0.20 per unit, or approximately $2.8 million in the aggregate, paid upon the closing of the FLAC IPO. An additional fee of $0.35 per unit, or approximately $4.8 million in the aggregate will be payable to the underwriters for deferred underwriting commissions. The deferred underwriting commissions will become payable to the underwriters from the amounts held in the Trust Account solely in the event that FLAC completes a business combination, subject to the terms of the underwriting agreement.
Administrative Services Agreement
On December 8, 2020, FLAC entered into an administrative services agreement, pursuant to which it agreed to pay the Sponsor a total of $10,000 per month for office space, utilities and administrative support. At the closing of the Business Combination, such agreement will automatically terminate pursuant to its terms.
344
Table of Contents
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic, including new variant strains of the underlying virus, current or anticipated military conflict, including between Russia and Ukraine, terrorism, sanctions or other geopolitical events as well as adverse developments in the economy and capital markets, including rising energy costs, inflation and interest rates, in the United States and globally, on the industry and has concluded that while it is reasonably possible that these events could have a negative effect on FLAC’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of the unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Critical Accounting Policies
The preparation of unaudited condensed financial statements and related disclosures in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the unaudited condensed financial statements, and income and expenses during the periods reported. Actual results could materially differ from those estimates. FLAC has identified the following as its critical accounting policies:
Class A Ordinary Shares Subject to Possible Redemption
FLAC accounts for the FLAC Class A Ordinary Shares subject to possible redemption in accordance with the guidance in Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity” (“ASC Topic 480”). Shares of FLAC Class A Ordinary Shares subject to mandatory redemption, if any, are classified as liability instruments and are measured at fair value. Shares of conditionally redeemable FLAC Class A Ordinary Shares (including FLAC Class A Ordinary Shares that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within FLAC’s control) are classified as temporary equity. At all other times, shares of FLAC Class A Ordinary Shares are classified as shareholders’ equity. As part of the private placement, FLAC issued 501,000 FLAC Private Placement Shares. These FLAC Private Placement Shares will not be transferable, assignable or salable until 30 days after the completion of FLAC’s initial business combination, as such are considered non-redeemable and presented as permanent equity in FLAC’s balance sheet. The FLAC Class A Ordinary Shares feature certain redemption rights that are considered to be outside of FLAC’s control and subject to the occurrence of uncertain future events. Accordingly, at June 30, 2022 and December 31, 2021, 13,800,000 FLAC Class A Ordinary Shares subject to possible redemption are presented as temporary equity, outside of the shareholders’ deficit section of the accompanying balance sheets.
Under ASC 480-10-S99, FLAC has elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of the reporting period. This method would view the end of the reporting period as if it were also the redemption date of the security. Effective with the closing of the FLAC IPO, FLAC recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Derivative Warrant Liabilities
FLAC does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. FLAC evaluates all of its financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC Topic 480 and ASC Subtopic 815-15 “Derivatives and Hedging-Embedded Derivatives” (“ASC Subtopic 815-15”). The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
345
Table of Contents
The 4,600,000 FLAC Public Warrants and the 167,000 FLAC Private Placement Warrants are recognized as derivative liabilities in accordance with Derivatives and Hedging-Contracts in Entity’s Own Equity (“ASC Subtopic 815-40”). Accordingly, FLAC recognizes the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in FLAC’s statement of operations. The fair value of the FLAC Public Warrants issued in connection with the FLAC IPO and FLAC Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and subsequently, have been measured based on the listed market price of such warrants.
Net Income (Loss) per Ordinary Share
FLAC complies with accounting and disclosure requirements of the FASB ASC Topic 260, “Earnings Per Share.” FLAC has two classes of shares, which are referred to as FLAC Class A Ordinary Shares and FLAC Class B Ordinary Shares. Income and losses are shared pro rata between the two classes of shares. This presentation assumes a business combination as the most likely outcome. Net income per ordinary share is calculated by dividing the net income by the weighted average shares of ordinary shares outstanding for the respective period.
The calculation of diluted net income does not consider the effect of the warrants underlying the units sold in the FLAC IPO (including the consummation of the over-allotment) and the FLAC Private Placement Warrants to purchase an aggregate of 4,767,000 shares of FLAC Class A Ordinary Shares in the calculation of diluted income (loss) per share, because their exercise is contingent upon future events. As a result, diluted net income per FLAC Ordinary Share is the same as basic net income per FLAC Ordinary Share for the three and six months ended June 30, 2022 and 2021. Accretion associated with the redeemable FLAC Class A Ordinary Shares is excluded from earnings per share as the redemption value approximates fair value.
Recent Issued Accounting Standards
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic 820 “Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. The ASU amends ASC 820 to clarify that a contractual sales restriction is not considered in measuring an equity security at fair value and to introduce new disclosure requirements for equity securities subject to contractual sale restrictions that are measured at fair value. The ASU applies to both holders and issuers of equity and equity-linked securities measured at fair value. The amendments in this ASU are effective for the Company in fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. The Company is still evaluating the impact of this pronouncement on the condensed financial statements.
FLAC’s management does not believe that any other recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on the accompanying financial statements.
JOBS Act
The JOBS Act contains provisions that, among other things, relax certain reporting requirements for qualifying public companies. FLAC qualifies as an “emerging growth company” and under the JOBS Act is allowed to comply with new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. FLAC is electing to delay the adoption of new or revised accounting standards, and as a result, FLAC may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, the condensed financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Additionally, FLAC is in the process of evaluating the benefits of relying on the other reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an
346
Table of Contents
“emerging growth company,” FLAC chooses to rely on such exemptions FLAC may not be required to, among other things, (i) provide an auditor’s attestation report on FLAC’s system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (the “PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis) and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of the FLAC IPO or until FLAC is no longer an “emerging growth company,” whichever is earlier.
347
Table of Contents
MANAGEMENT OF HOLDCO FOLLOWING THE BUSINESS COMBINATION
The following information concerning the management of Holdco is based on the provisions of the Holdco Articles of Association, the form of which is attached as an English translation of the official Dutch text as Annex I to this proxy statement/prospectus, and which are expected to be in effect in such form prior to or promptly following Closing. However, the Holdco Articles of Association may be changed at any time prior to consummation of the Business Combination by mutual agreement of FLAC and NewAmsterdam Pharma or after consummation of the Business Combination by amendment in accordance with their terms. If the Holdco Articles of Association are amended, the below summary may cease to accurately reflect the Holdco Articles of Association as so amended.
Board Structure, Directors and Executive Officers
Board Structure
As of the date of this document, Holdco is a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid). Prior to or promptly following the closing of the Business Combination, Holdco will be converted into a Dutch public limited liability company (naamloze vennootschap) with a single-tier board structure (the “Holdco Board”), consisting of one Holdco executive director and up to eight Holdco non-executive directors. There are no family relationships among any of Holdco’s directors.
Board of Directors
The Holdco Board shall consist of up to nine members, comprised of one executive director and eight non-executive directors, of which two non-executive directors will be designated by FLAC and one executive director and six non-executive directors will be designated by NewAmsterdam Pharma. Following the closing of the Business Combination, each of Holdco’s directors will hold office for the term set by the Holdco General Meeting (as set forth in the table below), except in the case of his or her earlier death, resignation or dismissal. Holdco’s directors do not have a retirement age requirement under the Holdco Articles of Association.
The Holdco directors will be appointed by the Holdco General Meeting upon a binding nomination by the Holdco Board. The Holdco General Meeting may at all times overrule a binding nomination by a resolution adopted by a majority of at least two thirds of the votes cast, provided such majority represents more than half of the issued share capital. If the Holdco General Meeting overrules a binding nomination, the Holdco Board will make a new nomination.
The DCGC provides the following best practice recommendations on the terms for tenure of Holdco’s directors:
| • | executive directors should be appointed for a maximum period of four years, without limiting the number of consecutive terms they may serve; and |
| • | non-executive directors should be appointed for two consecutive periods of no more than four years. Thereafter, non-executive directors may be reappointed for a maximum of two consecutive periods of no more than two years, provided that the reasons for any reappointment after an eight-year term of office should be disclosed in our statutory annual report. |
The executive director on the Holdco Board will be appointed for an initial term of four years. The initial non-executive directors on the Holdco Board will be appointed with staggered terms of up to four years. The initial directors were selected pursuant to the Business Combination Agreement.
The Holdco General Meeting may at any time suspend or dismiss a Holdco director. The Holdco General Meeting may only adopt a resolution to suspend or dismiss a Holdco director by a majority of at least two thirds of the votes cast, provided such majority represents more than half of the issued share capital, unless the resolution is adopted at the proposal of the Holdco Board, in which latter case the resolution may be adopted by a simple majority of the votes cast. Please see the section entitled “Comparison of Corporate Governance and Shareholder Rights” for more information on the Holdco Board.
348
Table of Contents
The following table lists the initial Holdco directors whom we anticipate will be appointed to serve on the Holdco Board following the completion of the Business Combination, as well as their ages and the year of expiration of their term as directors and position.
Name | Age | Year in which Term Expires | Position | |||||||||
Executive Director | ||||||||||||
Michael Davidson, M.D. | 65 | 2025 | Chief Executive Officer, Director | |||||||||
Non-Executive Directors | ||||||||||||
John Kastelein, M.D., Ph. D. FESC | 68 | 2023 | Chief Scientific Officer, Director | |||||||||
James N. Topper, M.D., Ph.D. | 60 | 2025 | Director | |||||||||
Juliette Audet | 36 | 2024 | Director | |||||||||
Louis Lange, M.D., Ph.D. | 74 | 2024 | Director | |||||||||
Sander Slootweg | 53 | 2023 | Director | |||||||||
Nicholas Downing, M.D. | 37 | 2023 | Director | |||||||||
The following is a brief summary of the business experience of Holdco’s directors. Unless otherwise indicated, the current business address for each Holdco director is the same as Holdco’s business address: Gooimeer 2-35, 1411 DC Naarden, The Netherlands.
Executive Director
Dr. Michael Davidson. Michael Davidson, M.D., has served NewAmsterdam Pharma as its Chief Executive Officer and an executive director since August 2020. Prior to joining NewAmsterdam Pharma, Dr. Davidson was the founder and Chief Executive Officer of Corvidia Therapeutics, Inc. from January 2016 until April 2018 and the Chief Science/Medical Officer from April 2018 until July 2020, when Corvidia was acquired by Novo Nordisk A/S for up to $2.1 billion. Dr. Davidson, who is a leading expert in the field of lipidology and was named in The Best Doctors in America for the past 15 years, is also currently a professor of medicine and director of the lipid clinic at the University of Chicago. Dr. Davidson co-founded and served as the Chief Medical Officer of Omthera Pharmaceuticals, Inc. in 2008, which was later acquired by AstraZeneca Pharmaceuticals in 2013 for up to $443M. His research background encompasses both pharmaceutical and nutritional clinical trials including extensive research on statins, novel lipid-lowering drugs, and omega-3 fatty acids. Dr. Davidson is board-certified in internal medicine, cardiology, and clinical lipidology and served as President of the National Lipid Association from 2010 to 2011. Dr. Davidson currently serves on the board of directors of Tenax Therapeutics, Inc. (Nasdaq: TENX) and Silence Therapeutics plc (Nasdaq: SLN). Dr. Davidson also serves on the boards of two private biotechnology companies, Sonothera and NanoPhoria Bioscience. Dr. Davidson received his B.A. and M.S. from Northwestern University and his M.D. from The Ohio State University School of Medicine.
We believe Dr. Davidson’s extensive experience in the field of cardiology and his prior management experience provide him the qualifications and skills to serve on the Holdco Board.
Non-Executive Directors
Dr. John Kastelein. John Kastelein, M.D., Ph. D. FESC, co-founded NewAmsterdam Pharma in 2020 and has served as its Chief Scientific Officer and an executive director since January 1, 2020. Dr. Kastelein has also served as the chief executive officer of Vascular Research Network Inc. (“VRN”) since January 2013 and as the Chief Medical Officer of Staten Biotechnology B.V. since January 2018. Dr. Kastelein also serves as emeritus professor of medicine and was the chair of the department of vascular medicine at the Academic Medical Center of the University of Amsterdam. He serves on the advisory board of the Dutch Atherosclerosis Society. In 2011 he received the ZonMw Pearl for his research in the field of gene therapy. Dr. Kastelein also serves on the board of directors of North Sea Therapeutics Inc., VRN and Oxitope Pharma Inc. Dr. Kastelein also serves as an advisor to a number of biotech and pharmaceutical companies. Dr. Kastelein was awarded a doctorate in medicine (with honors) from the University of Amsterdam, trained in internal medicine at the Academic Medical Center of the University of Amsterdam, and trained in lipidology and molecular biology at the University of British Columbia in Vancouver. Dr. Kastelein published his first clinical research on CETP-inhibition in the New England Journal of Medicine in 1997.
349
Table of Contents
We believe Dr. Kastelein’s deep scientific and medical knowledge about NewAmsterdam Pharma’s product candidate and his experience in senior management, provide Dr. Kastelein with the qualifications and skills to serve on the Holdco Board.
Dr. James N. Topper. James N. Topper, M.D., Ph.D., has served as FLAC’s Chief Executive Officer and Chairman of the FLAC Board since October 2020. Dr. Topper currently serves as a Managing Partner of Frazier Life Sciences. He joined Frazier in 2003 and opened Frazier’s Menlo Park office in the same year. Throughout his 15 years as a Managing Partner, Dr. Topper has invested across over 35 companies encompassing a broad spectrum of life science and biopharmaceutical companies. Dr. Topper has led and served as a board member for many of Frazier’s successful life sciences investments, including Acerta Pharma BV (sold to AstraZeneca), Amunix Pharmaceuticals, Inc. (sold to Sanofi), Aptinyx Inc. (Nasdaq: APTX), Calistoga Pharmaceuticals, Inc. (co-founder, sold to Gilead Sciences), Entasis Therapeutics Holdings Inc. (sold to Innoviva), Mavupharma (sold to AbbVie), Rempex (sold to The Medicines Company), Incline (co-founder, sold to The Medicines Company), Alnara (sold to Lilly), Portola, Inc. (co-founder, Nasdaq: PTLA), Phathom Pharmaceuticals Inc. (Nasdaq: PHAT), CoTherix, Inc (sold to Actelion), and Threshold Pharmaceutical, Inc. (Nasdaq: THLD). He currently represents Frazier on the boards of Alpine Immune Sciences (Nasdaq: ALPN), AnaptysBio, Inc. (Nasdaq: ANAB), Lassen Therapeutics, Seraxis Holdings, Inc., Frazier Life Sciences Acquisition Corporation (Nasdaq: FLAC), Enlaza Therapeutics, Inc., Serum, Inc. and Sudo Biosciences, Inc. In 2011 and 2016, Dr. Topper was named to the Midas List of leading venture capitalists, and in 2013, Dr. Topper was recognized by Forbes as a top ten healthcare investor. Dr. Topper received his M.D. and Ph.D. in Biophysics from Stanford and his B.S. from the University of Michigan.
We believe that Dr. Topper’s experience overseeing Frazier’s investments in biotechnology, his experience in senior management positions and his significant knowledge of industry, medical and scientific matters, provide Dr. Topper with the qualifications and skills to serve on the Holdco Board.
Juliette Audet. Juliette Audet has served on the NewAmsterdam Pharma board since 2020. Ms. Audet has been a partner at Forbion Venture Capital since January 2021 and served as a principal at Forbion from October 2019 until December 2020. Prior to joining Forbion, Ms. Audet was a Principal at Novartis Venture Fund based in Cambridge, Massachusetts from January 2018 until July 2019. Ms. Audet currently serves on the board of directors of Mestag Therapeutics Limited. Ms. Audet received an M.B.A., with distinction, from Harvard Business School and her M.Sc in physics from EPFL (Lausanne, Swiss Federal Institute of Technology).
We believe that Ms. Audet’s extensive experience investing in life science companies and her managerial experience provider her the qualifications and skills to serve on the Holdco Board.
Dr. Louis Lange. Louis Lange, M.D., Ph.D., has served on the NewAmsterdam Pharma board since 2021. Dr. Lange previously served as the chief of cardiology and a professor of medicine at the Washington University School of Medicine and was one of the early academicians in molecular cardiology. Dr. Lange founded and served as the chief executive officer and chairman of CV Therapeutics, Inc. (Nasdaq: CVTX) from 1990 until 2019, and as a senior advisor to Gilead Sciences, Inc. from 2009 until 2019, following its acquisition of CV Therapeutics. Dr. Lange currently serves as a general partner with Asset Management Ventures. Dr. Lange also serves on the board of directors of Stealth Biotherapeutics Corp. (Nasdaq:MITO), BioPlus Acquisition Corp. (Nasdaq:BIOS), Epiphany Tech Acquisition Corp., (Nasdaq: EPHY), Amygdala Neurosciences, Inc. and Recardia, Inc. Dr. Lange previously served on the board of directors of Audentes Therapeutics, Inc. (sold to Astellas Pharma Inc.). Dr. Lange has a Bachelor’s degree from the University of Rochester, an M.D. from Harvard University and a Ph.D. in Biological Chemistry, also from Harvard University.
We believe that Dr. Lange’s board experience, medical background and experience as a public company officer, provide Dr. Lange with the qualifications and skills to serve on the Holdco Board.
Sander Slootweg. Sander Slootweg has served on the NewAmsterdam Pharma board since 2020. Mr. Slootweg co-founded Forbion and has served as managing partner since 2006. Mr. Slootweg currently serves on the boards of several of Forbion’s portfolio companies including, Replimune Group Inc., NorthSea Therapeutics B.V., Azafaros
350
Table of Contents
B.V., Xention, Oxyrane Belgium NV and Forbion European Acquisition Corporation (Nasdaq: FRBN). Mr. Slootweg was responsible for several substantial exits: Forbion’s major position in Argenx SE (Nasdaq: ARGX), Dezima’s acquisition by Amgen in 2015 for up to $1.55 billion and the sale of Biovex Group, Inc. to Amgen in 2011 for up to $1 billion. Mr. Slootweg has previously served on the boards of Pulmagen Therapeutics, Fovea Pharmaceuticals SA (sold to Sanofi-aventis in 2009), uniQure N.V. (IPO on Nasdaq in 2014), Argenta Limited (sold to Galapagos NV in 2010), Alantos Pharmaceuticals, Inc. (sold to Amgen in 2007), Impella CardioSystems AG (sold to Abiomed Inc. in 2005), Pieris Pharmaceuticals, Inc. (IPO on Nasdaq in 2015). Before co-founding Forbion, Mr. Slootweg was an investment director at ABN AMRO Capital Life Sciences. Mr. Slootweg holds degrees in business and financial economics from the Free University of Amsterdam and business administration from Nijenrode University, The Netherlands.
We believe that Mr. Slootweg’s experience investing in and serving on multiple boards of life science companies, provide Mr. Slootweg with the qualifications and skills to serve on the Holdco Board.
Dr. Nicholas Downing. Nicholas Downing, M.D., joined Bain Capital Life Sciences in 2018 where he currently serves as a Principal. Prior to joining Bain Capital, Dr. Downing was a resident physician at the Brigham and Women’s Hospital in Boston from 2015 until 2018, where he cared for patients on the inpatient medical service and in the outpatient clinic. Throughout his medical career, Dr. Downing has been an active health policy researcher and is the author of over 40 articles in the peer-reviewed scientific literature. Prior to his medical career, Dr. Downing was a consultant at McKinsey and Company where he worked with clients in the pharmaceutical, hospital and financial services industries on a wide range of strategic problems. Dr. Downing also serves as a director on the board of Kestra Medical Technologies, Ltd., Cardurion Pharmaceuticals, Inc. and River Renal Companies. Dr. Downing graduated from Harvard College magna cum laude with a degree in chemistry. He received an M.D. cum laude from Yale University School of Medicine.
We believe that Dr. Downing’s medical experience, as well as his experience investing and serving on the boards of life science companies provide Dr. Downing with the qualifications and skills to serve on the Holdco Board.
Director and Officer Qualifications
Holdco is not expected to formally establish any specific, minimum qualifications that must be met by each of its directors and other officers. However, Holdco expects generally to evaluate the following qualities: educational background, diversity of professional experience, knowledge of Holdco’s business, integrity, professional reputation, independence, wisdom and the ability to represent the best interests of Holdco’s stakeholders.
The nomination and corporate governance committee will prepare policies regarding director qualification requirements and the process for identifying and evaluating director candidates for adoption by the Holdco Board.
351
Table of Contents
Executive Officers
The following table lists the names, ages and positions of those individuals who we anticipate being appointed as executive officers of Holdco.
Name | Age | Position | ||||
Executive Officer | ||||||
Michael Davidson, M.D. | 65 | Chief Executive Officer | ||||
Marc Ditmarsch, M.D. | 56 | Chief Development Officer | ||||
Lina Gugucheva | 35 | Chief Business Officer | ||||
John Kastelein, M.D. | 68 | Chief Scientific Officer | ||||
Douglas Kling | 49 | Chief Operating Officer | ||||
Louise Kooij | 47 | Chief Financial Officer | ||||
Dr. Michael Davidson. Dr. Davidson’s biography is included above under “—Board of Directors.”
Dr. Marc Ditmarsch. Marc Ditmarsch, M.D., joined NewAmsterdam Pharma in January 2020 as its Vice President Clinical & Operations and was promoted to Chief Development Officer in August 2022. Prior to joining NewAmsterdam Pharma, Dr. Ditmarsch served as an independent consultant for clinical development and operations at Gadeta Biotechnology from 2019 until 2020. From 2016 until 2019, Dr. Ditmarsch served as a country medical director at AstraZeneca and as global product vice president in the global medicines development organization from 2014 until 2016. Dr. Ditmarsch previously worked at F. Hoffmann-La Roche AG and as a vascular and emergency medicine resident surgeon. Dr. Ditmarsch received his M.D. from the faculty of medicine at the Free University in Amsterdam.
Lina Gugucheva. Lina Gugucheva joined NewAmsterdam Pharma in May 2021 as its Chief Business Officer. Ms. Gugucheva has previously served as acting Chief Business Officer at Scorpion Therapeutics, Inc. from May 2020 until April 2021 and at Northern Biologics Inc. (acquired by Boehringer Ingelheim in May 2020) from November 2019 until May 2020. Ms. Gugucheva has also served in multiple roles, including as the Vice President of Business Development & Strategy at IFM Therapeutics, LLC from October 2017 until December 2019, where she led the sale of an IFM subsidiary, IFM Tre, to Novartis in 2019. Ms. Gugucheva began her career as a life sciences transactional attorney, with experience both at the law firm of Covington & Burling LLP and in-house at Royalty Pharma. Ms. Gugucheva holds a J.D. from Harvard Law School and a B.A. in molecular biology and political science from New York University.
Dr. John Kastelein. Dr. Kastelein’s biography is included above under “—Board of Directors.”
Douglas Kling. Douglas Kling joined NewAmsterdam Pharma in March 2021 as its Chief Operating Officer. Prior to joining NewAmsterdam Pharma, Mr. Kling served as the Senior Vice President of Clinical Development at Corvidia Therapeutics, Inc. from December 2017 until February 2021. From March 2015 until November 2017, Mr. Kling served as the Senior Vice President, Clinical Development at Matina BioPharma Holdings, Inc. Mr. Kling earned a B.S. from Duke University and an M.B.A. from Rutgers Business School.
Louise Kooij. Louise Kooij has served as NewAmsterdam Pharma’s chief financial officer since May 2020. Ms. Kooij previosuly spent 14 years working in various finance roles at Genzyme Europe B.V., a multinational biotechnology company. Since May 2020, Ms. Kooij has also served as an independent consultant in the role of chief financial officer to other private biotechnology start ups. From January 2016 to April 2018, Ms. Kooij led Genzyme’s business operations team in Europe and from April 2018 until May 2020, served as the head of Genzyme’s rare disease unit in central and eastern Europe. Ms. Kooij received a master’s degree from Nyenrode Business University and her auditing degree from Hogeschool Markus Verbeek.
In addition, NewAmsterdam Pharma determined to seek to appoint in the first half of 2023 a new chief financial officer of Holdco with deep equity capital markets experience and strong relationships with U.S. investors, including biotechnology investors. NewAmsterdam Pharma has evaluated several candidates for the role, including David Topper, the Chief Financial Officer and a director and shareholder of FLAC. If appointed
352
Table of Contents
to the role of chief financial officer of Holdco, David Topper would become an employee of Holdco and receive customary compensation and benefits for his service in such role. However, no final determinations have been made to date. In the event a new chief financial officer is appointed for Holdco, Ms. Kooij is expected to become Holdco’s chief accounting officer.
Committees of the Holdco Board
Upon the completion of the Business Combination, the Holdco Board will establish three standing committees: an audit committee, compensation committee and nomination and corporate governance committee.
Audit Committee
The audit committee is expected to consist of Louis Lange, Sander Slootweg and Juliette Audet. We expect that Juliette Audet will serve as chairperson of the audit committee. The audit committee will assist the Holdco Board in overseeing Holdco’s accounting and financial reporting processes, the engagement of its independent auditor, and the audits of its financial statements. The Holdco Board has determined that Louis Lange qualifies as an “audit committee financial expert,” as such term is defined in the rules of the SEC.
Holdco intends to rely on the phase-in rules of the SEC and Nasdaq with respect to the independence of Holdco’s audit committee. These rules require that a majority of the members of Holdco’s audit committee meet the independence standard for audit committee membership within 90 days of the effectiveness of the registration statement of which this proxy statement/prospectus forms a part. In addition, all members of Holdco’s audit committee must meet the independence standard for audit committee membership within one year of the effectiveness of the registration statement of which this proxy statement/prospectus forms a part. The audit committee will be governed by a charter that complies with applicable rules of Nasdaq, which charter will be posted on Holdco’s website.
Compensation Committee
The compensation committee is expected to consist of Louis Lange and Nicholas Downing. We expect Louis Lange will serve as chairperson of the compensation committee. The compensation committee will assist the Holdco Board in determining compensation for Holdco’s directors and executive officers. The composition of Holdco’s compensation committee is consistent with the best practice provisions of the DCGC.
The compensation committee will be governed by a charter that will be posted on Holdco’s website.
Nomination and Corporate Governance Committee
The nomination and corporate governance committee is expected to consist of Sander Slootweg, Nicholas Downing and Louis Lange. We expect Sander Slootweg will serve as chairperson of the nomination and corporate governance committee. The nomination and corporate governance committee will assist the Holdco Board in identifying individuals qualified to become Holdco directors consistent with criteria established by Holdco and in developing Holdco’s code of business conduct and ethics.
The nomination and corporate governance committee will be governed by a charter that will be posted on Holdco’s website.
Code of Business Conduct and Ethics
Upon completion of the Business Combination, Holdco intends to adopt a code of business conduct and ethics which outlines the principles of legal and ethical business conduct under which Holdco will do business. This code will apply to all of Holdco’s employees, officers and directors. Holdco’s code of business conduct and ethics will be available on its website after the closing of the Business Combination.
353
Table of Contents
Holdco Board Dividend Policy
Holdco has never declared or paid any cash dividends on its shares. It currently intends to retain all available funds and any future earnings for use in the operation of its business and does not anticipate paying any dividends on the Holdco Shares in the foreseeable future. The Holdco Board may only pay dividends and other distributions from our reserves to the extent Holdco’s shareholders’ equity (eigen vermogen) exceeds the sum of Holdco’s paid-in and called-up share capital plus the reserves it must maintain under Dutch law or the Holdco Articles of Association and (if it concerns a distribution of profits) after adoption of its statutory annual accounts by the Holdco General Meeting from which it appears that such dividend distribution is allowed. Subject to those restrictions, any future determination to pay dividends or other distributions from Holdco’s reserves will be at the discretion of the Holdco Board and will depend upon a number of factors, including its results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the Holdco Board deems relevant.
Under the Holdco Articles of Association, the Holdco Board may decide that all or part of the profits shown in our adopted statutory annual accounts will be added to Holdco’s reserves. After reservation of any such profits, any remaining profits will be at the disposal of the Holdco General Meeting at the proposal of the Holdco Board for distribution on Holdco Shares, subject to applicable restrictions of Dutch law. The Holdco Board is permitted, subject to certain requirements and applicable restrictions of Dutch law, to declare interim dividends without the approval of the Holdco General Meeting. Dividends and other distributions will be made payable no later than a date determined by Holdco. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable will lapse and any such amounts will be considered to have been forfeited to us (verjaring).
Other Corporate Governance Matters
Nasdaq rules provide that foreign private issuers may follow home country practice in lieu of the Nasdaq corporate governance standards, subject to certain exceptions and except to the extent that such exemptions would be contrary to U.S. federal securities laws. The home country practices followed by Holdco in lieu of Nasdaq rules are described below:
| • | In accordance with Dutch law and generally accepted business practices, the Holdco Articles of Association do not provide quorum requirements generally applicable to general meetings. Holdco’s practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires that an issuer provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting shares. |
| • | Although Holdco must provide shareholders with an agenda and other relevant documents for Holdco’s General Meetings, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands, thus Holdco’s practice will vary from the requirement of Nasdaq Listing Rule 5620(b). |
| • | As permitted by the listing requirements of Nasdaq, Holdco has also opted out of the requirements of Nasdaq Listing Rule 5605(b)(2), which requires the independent directors of an issuer have regularly scheduled meetings with just the other independent directors present. |
| • | Holdco will also rely on the exemptions to the SEC and Nasdaq rules with respect to the independence of the Holdco audit committee. These rules require that a majority of the members of Holdco’s audit committee meet the independence standard for audit committee membership within 90 days of the effectiveness of the registration statement of which this proxy statement/prospectus forms a part. In addition, all members of Holdco’s audit committee must meet the independence standard for audit committee members within one year of the effectiveness of the registration statement of which this proxy statement/prospectus forms a part. |
In addition, Holdco has opted out of shareholder approval requirements, as included in the Nasdaq Listing Rules, for the issuance of securities in connection with certain events such as the acquisition of shares or assets of
354
Table of Contents
another company (if, due to the potential issuance of ordinary shares, the ordinary shares would have upon issuance voting power equal to or in excess of 20% of the voting power outstanding before the issuance of stock), the establishment of or amendments to equity-based compensation plans for employees, a change of control and certain private placements. Holdco’s practice varies from the requirements of Nasdaq Rule 5635, which generally requires that an issuer obtain shareholder approval for the issuance of securities in connection with such events.
Compensation
Holdco was formed on June 10, 2022 and as such has not yet paid any compensation to those who will serve as its directors and executive officers following the Business Combination.
Historical Compensation of NewAmsterdam Pharma’s Executive Officers
The amount of compensation, including benefits in kind, accrued or paid to the NewAmsterdam Pharma executive officers who are intended to become Holdco’s executive officers with respect to the year ended December 31, 2021 is described in the table below. We are providing disclosure on an aggregate basis, as disclosure of compensation on an individual basis is not required in Holdco’s home country and is not otherwise publicly disclosed by Holdco.
All executive officers | (in Euros ‘000)(1) | |||
Base Compensation(2) | 484 | |||
Bonuses(3) | 156 | |||
Management Fees(4) | | 1,475 | | |
Options Granted in 2021(5) | 918 | |||
Total Compensation | 3,033 | |||
| (1) | Amounts paid in U.S. dollars have been converted to Euros in accordance with IAS 21—the Effects of Changes in Foreign Exchange Rates. |
| (2) | Base compensation represents the cash compensation paid annually to NewAmsterdam Pharma’s executive officers. |
| (3) | Bonus amounts are fixed per each executive officer’s employment agreement. |
| (4) | Management fees consist of discretionary cash bonuses paid to executive officers serving in a consulting capacity. |
| (5) | The following options were granted to executive officers pursuant to NewAmsterdam Pharma’s long-term incentive plan: 785,001 options for depositary receipts relating to non-voting ordinary shares and (ii) 894,286 options for non-voting ordinary shares, each with an expiration date of no later ten years from the date of grant and an exercise price of EUR 2.48 per option. The aggregate fair value of the options that is set forth in the table above was determined pursuant to the regulations of IFRS 2 “Share-based Payments.” |
Compensation of NewAmsterdam Pharma Directors
The amount of compensation, including benefits in kind, accrued or paid to the NewAmsterdam Pharma directors who are intended to become Holdco’s directors with respect to the year ended December 31, 2021 is described in the table below. We are providing disclosure on an aggregate basis, as disclosure of compensation on an individual basis is not required in Holdco’s home country until after the consummation of the Business Combination and is not otherwise publicly disclosed by Holdco.
All directors | (in Euros ‘000)(1) | |||
Cash Compensation | 31 | |||
Options Granted in 2021(2) | 13 | |||
Total Compensation | 44 | |||
| (1) | Amounts paid in U.S. dollars have been converted to Euros in accordance with IAS 21—the Effects of Changes in Foreign Exchange Rates. |
| (2) | The following options were granted to executive officers pursuant to NewAmsterdam Pharma’s long-term incentive plan: 40,000 options for non-voting ordinary shares, each with an expiration date of no later ten years from the date of grant and an exercise price of EUR 2.48 per option. The aggregate fair value of the options that is set forth in the table above was determined pursuant to the regulations of IFRS 2 “Share-based Payments.” |
355
Table of Contents
Director and Executive Officer Award Grants
In connection with the Closing, Holdco expects to grant Holdco Options to a number of consultants and employees, including certain executive officers and directors. For additional information, see “The Business Combination—Ownership of Holdco Following the Business Combination.”
Director and Executive Officer Compensation Following the Business Combination
Dutch law does not provide for limitations with respect to the aggregate annual compensation paid to Holdco’s directors or executive officers, provided that compensation paid to Holdco directors must be consistent with Holdco’s compensation policy. Such compensation policy will be adopted by the Holdco General Meeting prior to the Closing, to be effective upon Closing. Changes to such compensation policy will require a vote of the Holdco General Meeting by simple majority of votes cast. Following the Closing, the Holdco Board and/or the compensation committee will determine the compensation of individual directors and executive officers with due observance of the compensation policy to the extent applicable. A proposal with respect to compensation schemes in the form of Holdco shares or rights to Holdco shares in which directors may participate (such as the LTIP) is also subject to approval by the Holdco General Meeting by simple majority of votes cast. Such a proposal must set out at least the maximum number of shares or rights to subscribe for shares to be granted to Holdco directors and the criteria for granting or amendment.
Holdco Directors Service Agreements
Holdco expects to enter into services agreements with all of its directors which will regulate their services as directors of Holdco. These services agreements will contain non-competition and non-solicitation arrangements, as well as a requirement to assign and transfer to Holdco any intellectual and industrial property rights originating from the director’s services as a director or inventor of Holdco.
Holdco Director Indemnification Agreements
The Holdco Articles of Association will require Holdco to indemnify its current and former directors to the fullest extent permitted by law, subject to certain exceptions. Holdco expects to enter into indemnification agreements with all of its directors providing for procedures for indemnification and advancements by Holdco of certain expenses and costs relating to claims, suits or proceedings arising from his or her service to Holdco or, at Holdco’s request, service to other entities, as directors or officers to the maximum extent permitted by law.
Holdco LTIP
In connection with the Business Combination, Holdco will establish the Holdco LTIP, to be effective as of the Final Closing Date, pursuant to which Holdco may grant options, restricted stock, restricted stock units, share appreciation rights and other equity and equity-based awards. The maximum number of Holdco Shares underlying awards granted pursuant to the Holdco LTIP (other than awards granted as replacement awards in connection with a merger or business combination) will not exceed (a) 13% of the aggregate number of Holdco Shares outstanding immediately following the Effective Date after giving effect to the Exchange, the exercise of the 2020 Profit Rights described in the section entitled “NewAmsterdam Pharma’s Management Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—2020 SPA and Profit Right Agreement,” the Merger, the PIPE Financing and the other Transactions (but not, for the avoidance of doubt, exercise of any Holdco Warrants), which aggregate number will include the Holdco Shares underlying the NewAmsterdam Pharma Options outstanding immediately prior to the Exchange, plus (b) the maximum number of Earnout Shares that may be issued as restricted stock units under the Holdco LTIP; provided that the number of Holdco Shares initially reserved for grant under the Holdco LTIP shall be increased annually on January 1 of each calendar year, starting on the first January 1 after Closing (or on such closing date, if Closing occurs after January 1, 2023), by five percent (5%) of the then issued and outstanding Holdco Shares or such lower number as may be determined by the Holdco Board.
356
Table of Contents
The Holdco LTIP will be administered by the Holdco Board and its compensation committee. Holdco may grant awards under the Holdco LTIP to its directors, employees, consultants or other advisors. The Holdco Board (or its compensation committee) may condition awards under the Holdco LTIP upon the achievement or satisfaction of performance criteria and will determine the vesting conditions for awards under the Holdco LTIP. The Holdco LTIP will provide for provisions for good leavers and bad leavers as well as for a change in control of Holdco.
Holdco Rollover Option Plan
In connection with the Business Combination, Holdco will also establish a rollover option plan (“Holdco Rollover Plan”), pursuant to which Holdco will assume outstanding NewAmsterdam Pharma Options of certain NewAmsterdam Pharma optionholders who hold their options through entities in exchange for a grant of options to acquires Holdco Shares. The maximum number of Holdco Shares underlying the options covered by the Holdco Rollover Plan is 1,736,545. Any Holdco shares underlying awards granted under the Holdco Rollover Plan that are forfeited, canceled or otherwise terminated shall become available for issuance under the Holdco LTIP.
The Holdco Rollover Plan will be administered by the Holdco Board and its compensation committee. The options subject to the Holdco Rollover Plan will continue on the same terms as the NewAmsterdam Pharma Options for which they are being exchanged. The Holdco Rollover Plan will provide for provisions applicable in the event of a change of control of Holdco.
357
Table of Contents
DESCRIPTION OF HOLDCO SECURITIES
This section of this proxy statement/prospectus includes a description of the material terms of the Holdco Articles of Association and of applicable Dutch law. The following description is intended as a summary only and does not constitute legal advice regarding those matters and should not be regarded as such. The description is qualified in its entirety by reference to the complete text of the Holdco Articles of Association, which are attached as an English translation of the official Dutch text as Annex I to this proxy statement/prospectus. We urge you to read the full text of the Holdco Articles of Association.
General
Holdco was incorporated pursuant to Dutch law on June 10, 2022. Holdco’s corporate affairs are governed by the Holdco Articles of Association, the board rules of the Holdco Board, Holdco’s other internal rules and policies and by Dutch law. Holdco is registered with the Dutch Trade Register under number 86649051. Holdco’s corporate seat is in Naarden, the Netherlands, and Holdco’s office address is Gooimeer 2-35 1411 DC Naarden, the Netherlands.
As of the date of this proxy statement/prospectus, Holdco is a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid). Prior to or promptly following Closing, Holdco will become a Dutch public limited liability company (naamloze vennootschap). Unless otherwise indicated, the descriptions set forth below assumes Holdco has already been converted into a Dutch public limited liability company (naamloze vennootschap) and that the Holdco Articles of Association have been amended to take the form attached as an English translation of the official Dutch text as Annex I to this proxy statement/prospectus.
Share Capital
Authorized Share Capital
As of the Closing, the Holdco Articles of Association will provide for an authorized share capital that will be approximately (but no more than) five (5) times the number of Holdco Shares outstanding immediately following such closing. The Holdco Shares will have a nominal value of €0.12.
Under Dutch law, Holdco’s authorized share capital is the maximum capital that Holdco may issue without amending the Holdco Articles of Association. An amendment of the Holdco Articles of Association would require a resolution of Holdco General Meeting upon proposal by the Holdco Board.
The Holdco Articles of Association will provide that, for as long as any Holdco Shares are admitted to trading on Nasdaq or on any other regulated stock exchange operating in the United States, the laws of the State of New York will apply to the property law aspects of the Holdco Shares reflected in the register administered by Holdco’s transfer agent, subject to certain overriding exceptions under Dutch law.
Holdco Shares
The following summarizes the main rights of holders of Holdco Shares:
| • | each holder of Holdco Shares is entitled to one vote per share on all matters to be voted on by shareholders generally, including the appointment of Holdco directors; |
| • | there are no cumulative voting rights; |
| • | the holders of Holdco Shares are entitled to dividends and other distributions as may be declared from time to time by Holdco out of funds legally available for that purpose, if any; |
| • | upon Holdco’s liquidation and dissolution, the holders of Holdco Shares will be entitled to share ratably in the distribution of all of Holdco’s assets remaining available for distribution after satisfaction of all Holdco’s liabilities; and |
358
Table of Contents
| • | the holders of Holdco Shares have pre-emption rights in case of share issuances or the grant of rights to subscribe for shares, except if such rights are limited or excluded by the corporate body authorized to do so and except in such cases as provided by Dutch law and the Holdco Articles of Association. |
Holdco Warrants
At the Closing, Holdco will enter into the Warrant Assumption Agreement, and pursuant thereto, each of the FLAC Warrants will automatically be converted into a Holdco Warrant, which such Holdco Warrant being subject to the same terms and conditions (including exercisability terms) as were applicable to the corresponding FLAC Warrant immediately prior to the Closing.
Each Holdco Warrant entitles the registered holder to purchase one Holdco Share at a price of $11.50 per share, subject to adjustment as discussed below, at any time commencing 30 days after the consummation of the Business Combination, provided that Holdco has an effective registration statement under the Securities Act covering the Holdco Shares issuable upon exercise of the warrants and a current prospectus relating to them is available (or Holdco permits holders to exercise their warrants on a cashless basis under the circumstances specified in the Warrant Assumption Agreement) and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder. Pursuant to the Warrant Assumption Agreement, a warrant holder may exercise its warrants only for a whole number of Holdco Shares. This means only a whole warrant may be exercised at a given time by a warrant holder. No fractional warrants will be issued and only whole warrants will trade. The Holdco Warrants will expire five years after the Closing, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
Holdco has agreed that as soon as practicable, but in no event later than 20 business days after the Closing, Holdco will use its commercially reasonable efforts to file with the SEC a registration statement covering the Holdco Shares issuable upon exercise of the Holdco Warrants, and Holdco will use its commercially reasonable efforts to cause the same to become effective within 60 business days after the Closing, and to maintain the effectiveness of such registration statement and a current prospectus relating to those Holdco Shares until the Holdco Warrants expire or are redeemed, as specified in the Warrant Assumption Agreement; provided that if the Holdco Shares are at the time of any exercise of a Holdco Warrant not listed on a national securities exchange such that they satisfy the definition of a “covered security” under Section 18(b)(1) of the Securities Act, Holdco may, at its option, require holders of the Holdco Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event Holdco so appoints, Holdco will not be required to file or maintain in effect a registration statement. If a registration statement covering the Holdco Shares issuable upon exercise of the Holdco Warrants is not effective by the 60th day after the Closing, warrant holders may, until such time as there is an effective registration statement and during any period when Holdco has failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption, but Holdco will use its best efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
Redemptions of Holdco Warrants for cash when the price per Holdco Share equals or exceeds $18.00. Once the Holdco Warrants become exercisable, Holdco may call the Holdco Warrants for redemption (except as described herein with respect to the Holdco Private Placement Warrants):
| • | in whole and not in part; |
| • | at a price of $0.01 per Holdco Warrant; |
| • | upon not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| • | if, and only if, the closing price of the Holdco Shares equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which notice of the redemption is given to the warrant holders (the “Reference Value”). |
359
Table of Contents
Holdco will not redeem the Holdco Warrants as described above unless a registration statement under the Securities Act covering the issuance of the Holdco Shares issuable upon exercise of the Holdco Warrants is then effective and a current prospectus relating to those shares is available throughout the 30-day redemption period. If and when the Holdco Warrants become redeemable by Holdco, Holdco may exercise its redemption right even if Holdco is unable to register or qualify the underlying securities for sale under all applicable state securities laws. As a result, Holdco may redeem the warrants as set forth above even if the holders are otherwise unable to exercise the warrants.
Holdco has established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and Holdco issues a notice of redemption of the Holdco Warrants, each warrant holder will be entitled to exercise his, her or its warrant prior to the scheduled redemption date. However, the price of the Holdco Shares may fall below the $18.00 redemption trigger price (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) as well as the $11.50 (for whole Holdco Shares) warrant exercise price after the redemption notice is issued.
Redemption of Holdco Warrants for cash when the price per Holdco Share equals or exceeds $10.00. Once the Holdco Warrants become exercisable, Holdco may redeem the outstanding Holdco Warrants:
| • | in whole and not in part; |
| • | at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption; provided that during such 30 day period holders will be able to exercise their Holdco Warrants on a cashless basis prior to redemption and receive that number of Holdco Shares determined by reference to the table below, based on the redemption date and the “fair market value” of the Holdco Shares (as defined below) except as otherwise described below; provided, further, that if the Holdco Warrants are not exercised on a cashless basis or otherwise during such 30 day period, Holdco will redeem such warrants for $0.10 per share; |
| • | if, and only if, the Reference Value equals or exceeds $10.00 per share (as adjusted for share subdivisions, share dividends, reorganizations, recapitalizations and the like) on the trading day before Holdco sends the notice of redemption to the warrant holders; and |
| • | if the Reference Value is less than $18.00 per share (as adjusted for share subdivisions, share dividends, reorganizations, recapitalizations and the like), the Holdco Private Placement Warrants must also be concurrently called for redemption on the same terms as the outstanding Holdco Public Warrants, as described above. |
The numbers in the table below represent the number of Holdco Shares that a warrant holder will receive upon exercise in connection with a redemption by Holdco pursuant to this redemption feature, based on the “fair market value” of the Holdco Shares on the corresponding redemption date (assuming holders elect to exercise their warrants and such warrants are not redeemed for $0.10 per warrant), determined based on volume-weighted average price of the Holdco Shares as reported during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of warrants, and the number of months that the corresponding redemption date precedes the expiration date of the Holdco Warrants, each as set forth in the table below. Holdco will provide its warrant holders with the final fair market value no later than one business day after the 10-trading day period described above ends.
The share prices set forth in the column headings of the table below will be adjusted as of any date on which the number of Holdco Shares issuable upon exercise of a Holdco Warrant or the exercise price of the Holdco Warrant is adjusted as set forth under the heading “Anti-dilution Adjustments” below. If the number of Holdco Shares issuable upon exercise of a Holdco Warrant is adjusted, the adjusted share prices in the column headings will equal the share prices immediately prior to such adjustment, multiplied by a fraction, the numerator of which is the exercise price of the Holdco Warrant after such adjustment and the denominator of which is the price of the Holdco Warrant immediately prior to such adjustment. In such an event, the number of Holdco Shares in the table below shall be adjusted by multiplying such Holdco Share amounts by a fraction, the numerator of which is
360
Table of Contents
the number of Holdco Shares deliverable upon exercise of a Holdco Warrant immediately prior to such adjustment and the denominator of which is the number of Holdco Shares deliverable upon exercise of a Holdco Warrant as so adjusted.
| Redemption Date (period to expiration of warrants) | Fair Market Value of Class A Ordinary Shares | |||||||||||||||||||||||||||||||||||
| <$10.00 | $11.00 | $12.00 | $13.00 | $14.00 | $15.00 | $16.00 | $17.00 | >$18.00 | ||||||||||||||||||||||||||||
60 months | 0.261 | 0.281 | 0.297 | 0.311 | 0.324 | 0.337 | 0.348 | 0.358 | 0.361 | |||||||||||||||||||||||||||
57 months | 0.257 | 0.277 | 0.294 | 0.310 | 0.324 | 0.337 | 0.348 | 0.358 | 0.361 | |||||||||||||||||||||||||||
54 months | 0.252 | 0.272 | 0.291 | 0.307 | 0.322 | 0.335 | 0.347 | 0.357 | 0.361 | |||||||||||||||||||||||||||
51 months | 0.246 | 0.268 | 0.287 | 0.304 | 0.320 | 0.333 | 0.346 | 0.357 | 0.361 | |||||||||||||||||||||||||||
48 months | 0.241 | 0.263 | 0.283 | 0.301 | 0.317 | 0.332 | 0.344 | 0.356 | 0.361 | |||||||||||||||||||||||||||
45 months | 0.235 | 0.258 | 0.279 | 0.298 | 0.315 | 0.330 | 0.343 | 0.356 | 0.361 | |||||||||||||||||||||||||||
42 months | 0.228 | 0.252 | 0.274 | 0.294 | 0.312 | 0.328 | 0.342 | 0.355 | 0.361 | |||||||||||||||||||||||||||
39 months | 0.221 | 0.246 | 0.269 | 0.290 | 0.309 | 0.325 | 0.340 | 0.354 | 0.361 | |||||||||||||||||||||||||||
36 months | 0.213 | 0.239 | 0.263 | 0.285 | 0.305 | 0.323 | 0.339 | 0.353 | 0.361 | |||||||||||||||||||||||||||
33 months | 0.205 | 0.232 | 0.257 | 0.280 | 0.301 | 0.320 | 0.337 | 0.352 | 0.361 | |||||||||||||||||||||||||||
30 months | 0.196 | 0.224 | 0.250 | 0.274 | 0.297 | 0.316 | 0.335 | 0.351 | 0.361 | |||||||||||||||||||||||||||
27 months | 0.185 | 0.214 | 0.242 | 0.268 | 0.291 | 0.313 | 0.332 | 0.350 | 0.361 | |||||||||||||||||||||||||||
24 months | 0.173 | 0.204 | 0.233 | 0.260 | 0.285 | 0.308 | 0.329 | 0.348 | 0.361 | |||||||||||||||||||||||||||
21 months | 0.161 | 0.193 | 0.223 | 0.252 | 0.279 | 0.304 | 0.326 | 0.347 | 0.361 | |||||||||||||||||||||||||||
18 months | 0.146 | 0.179 | 0.211 | 0.242 | 0.271 | 0.298 | 0.322 | 0.345 | 0.361 | |||||||||||||||||||||||||||
15 months | 0.130 | 0.164 | 0.197 | 0.230 | 0.262 | 0.291 | 0.317 | 0.342 | 0.361 | |||||||||||||||||||||||||||
12 months | 0.111 | 0.146 | 0.181 | 0.216 | 0.250 | 0.282 | 0.312 | 0.339 | 0.361 | |||||||||||||||||||||||||||
9 months | 0.090 | 0.125 | 0.162 | 0.199 | 0.237 | 0.272 | 0.305 | 0.336 | 0.361 | |||||||||||||||||||||||||||
6 months | 0.065 | 0.099 | 0.137 | 0.178 | 0.219 | 0.259 | 0.296 | 0.331 | 0.361 | |||||||||||||||||||||||||||
3 months | 0.034 | 0.065 | 0.104 | 0.150 | 0.197 | 0.243 | 0.286 | 0.326 | 0.361 | |||||||||||||||||||||||||||
0 months | — | — | 0.042 | 0.115 | 0.179 | 0.233 | 0.281 | 0.323 | 0.361 | |||||||||||||||||||||||||||
The exact fair market value and redemption date may not be set forth in the table above, in which case, if the fair market value is between two values in the table or the redemption date is between two redemption dates in the table, the number of Holdco Shares to be issued for each Holdco Warrant exercised will be determined by a straight-line interpolation between the number of Holdco Shares set forth for the higher and lower fair market values and the earlier and later redemption dates, as applicable, based on a 365 or 366-day year, as applicable. For example, if the volume-weighted average price of the Holdco Shares as reported during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of the warrants is $11.00 per share, and at such time there are 57 months until the expiration of the Holdco Warrants, holders may choose to, in connection with this redemption feature, exercise their warrants for 0.277 Holdco Shares for each whole Holdco Warrant. For an example where the exact fair market value and redemption date are not as set forth in the table above, if the volume-weighted average price of the Holdco Shares as reported during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of the warrants is $13.50 per share, and at such time there are 38 months until the expiration of the Holdco Warrants, holders may choose to, in connection with this redemption feature, exercise their Holdco Warrants for 0.298 Holdco Shares for each whole Holdco Warrant. In no event will the Holdco Warrants be exercisable in connection with this redemption feature for more than 0.361 Holdco Shares per warrant (subject to adjustment).
This redemption feature is structured to allow for all of the outstanding Holdco Warrants to be redeemed when the Holdco Shares are trading at or above $10.00 per share, which may be at a time when the trading price of Holdco Shares is below the exercise price of the Holdco Warrants. Holdco has established this redemption feature to provide us with the flexibility to redeem the Holdco Warrants without the warrants having to reach the $18.00 per share threshold set forth above under “Redemptions of Holdco Warrants for cash when the price per Holdco Share equals or exceeds $18.00.” Holders choosing to exercise their Holdco Warrants in connection with a redemption pursuant to this feature will, in effect, receive a number of Holdco Shares for their warrants based
361
Table of Contents
on an option pricing model with a fixed volatility input as of the date of the prospectus relating to the FLAC IPO. This redemption right provides Holdco with an additional mechanism by which to redeem all of the outstanding Holdco Publco Warrants, and therefore have certainty as to its capital structure as the Holdco Warrants would no longer be outstanding and would have been exercised or redeemed. Holdco will be required to pay the applicable redemption price to warrant holders if it chooses to exercise this redemption right and it will allow Holdco to quickly proceed with a redemption of the Holdco Warrants if Holdco determines it is in its best interest to do so. As such, Holdco would redeem the Holdco Warrants in this manner when Holdco believes it is in its best interest to update Holdco’s capital structure to remove the Holdco Warrants and pay the redemption price to the warrant holders. As stated above, Holdco can redeem the warrants when the Holdco Shares are trading at a price starting at $10.00, which is below the exercise price of $11.50, because it will provide certainty with respect to its capital structure and cash position while providing warrant holders with the opportunity to exercise their warrants on a cashless basis for the applicable number of Holdco Shares. If Holdco chooses to redeem the Holdco Warrants when the Holdco Shares are trading at a price below the exercise price of the Holdco Warrants, this could result in the warrant holders receiving fewer Holdco Shares than they would have received if they had chosen to wait to exercise their warrants for Holdco Shares if and when such Holdco Shares were trading at a price higher than the exercise price of $11.50.
No fractional Holdco Shares will be issued upon exercise. If, upon exercise, a holder would be entitled to receive a fractional interest in a Holdco Share, Holdco will round down to the nearest whole number of the number of Holdco Shares to be issued to the holder. If, at the time of redemption, the Holdco Warrants are exercisable for a security other than the Holdco Shares pursuant to the Warrant Assumption Agreement, the Holdco Warrants may be exercised for such security. At such time as the Holdco Warrants become exercisable for a security other than the Holdco Shares, Holdco will use its commercially reasonable efforts to register under the Securities Act the security issuable upon the exercise of the Holdco Warrants.
A holder of a Holdco Warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 9.8% (as specified by the holder) of the Holdco Shares issued and outstanding immediately after giving effect to such exercise.
Anti-dilution Adjustments. If the number of outstanding Holdco Shares is increased by a capitalization or share dividend payable in Holdco Shares, or by a sub-divisions of Holdco Shares or other similar event, then, on the effective date of such capitalization or share dividend, sub-divisions or similar event, the number of Holdco Shares issuable on exercise of each Holdco Warrant will be increased in proportion to such increase in the outstanding Holdco Shares. A rights offering made to all or substantially all holders of Holdco Shares entitling holders to purchase Holdco Shares at a price less than the “historical fair market value” (as defined below) will be deemed a share dividend of a number of Holdco Shares equal to the product of (i) the number of Holdco Shares actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for Holdco Shares) and (ii) one minus the quotient of (x) the price per Holdco Share paid in such rights offering and (y) the historical fair market value. For these purposes, (i) if the rights offering is for securities convertible into or exercisable for Holdco Shares, in determining the price payable for Holdco Shares, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) “historical fair market value” means the volume-weighted average price of Holdco Shares as reported during the 10 trading day period ending on the trading day prior to the first date on which the Holdco Shares trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if Holdco, at any time while the Holdco Warrants are outstanding and unexpired, pay a dividend or make a distribution in cash, securities or other assets to all or substantially all the holders of Holdco Shares on account of such Holdco Shares (or other securities into which the warrants are convertible), other than (a) as described above, (b) any cash dividends or cash distributions which, when combined on a per share basis with all other cash dividends and cash distributions paid on the Holdco Shares during the 365-day period ending on the date of declaration of such dividend or distribution does not exceed $0.50 (as adjusted to appropriately reflect
362
Table of Contents
any other adjustments and excluding cash dividends or cash distributions that resulted in an adjustment to the exercise price or to the number of Holdco Shares issuable on exercise of each Holdco Warrant) but only with respect to the amount of the aggregate cash dividends or cash distributions equal to or less than $0.50 per share, by the amount of cash and/or the fair market value of any securities or other assets paid on each Holdco Share in respect of such event.
If the number of outstanding Holdco Shares is decreased by a consolidation, combination, reverse share sub-division or reclassification of Holdco Shares or other similar event, then, on the effective date of such consolidation, combination, reverse share sub-division, reclassification or similar event, the number of Holdco Shares issuable on exercise of each warrant will be decreased in proportion to such decrease in outstanding Holdco Shares.
Whenever the number of Holdco Shares purchasable upon the exercise of the Holdco Warrants is adjusted, as described above, the Holdco Warrant exercise price will be adjusted by multiplying the warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of Holdco Shares purchasable upon the exercise of the Holdco Warrants immediately prior to such adjustment and (y) the denominator of which will be the number of Holdco Shares so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding Holdco Shares (other than those described above or that solely affects the par value of such Holdco Shares), or in the case of any merger or consolidation of Holdco with or into another corporation (other than a consolidation or merger in which Holdco is the continuing corporation and that does not result in any reclassification or reorganization of Holdco’s issued and outstanding Holdco Shares), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of Holdco as an entirety or substantially as an entirety in connection with which Holdco is dissolved, the holders of the Holdco Warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the Holdco Warrants and in lieu of the Holdco Shares immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of Holdco Shares or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the Holdco Warrants would have received if such holder had exercised their warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of Holdco Shares in such a transaction is payable in the form of Holdco Shares in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Holdco Warrant properly exercises the Holdco Warrant within thirty days following public disclosure of such transaction, the warrant exercise price will be reduced as specified in the Warrant Assumption Agreement based on the Black-Scholes value (as defined in the Warrant Assumption Agreement) of the Holdco Warrant. The purpose of such exercise price reduction is to provide additional value to holders of the warrants when an extraordinary transaction occurs during the exercise period of the Holdco Warrants pursuant to which the holders of the warrants otherwise do not receive the full potential value of the Holdco Warrants.
The Holdco Warrants have been issued in registered form under a Warrant Assumption Agreement between Continental Stock Transfer & Trust Company, as warrant agent, and Holdco. The Warrant Assumption Agreement provides that the terms of the Holdco Warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision or correct any mistake, including to conform the provisions of the Warrant Assumption Agreement to the description of the terms of the Holdco Warrants and the Warrant Assumption Agreement set forth in Exhibit 4.1 hereto, but requires the approval by the holders of at least 65% of the then outstanding Holdco Public Warrants to make any change that adversely affects the interests of the registered holders. You should review a copy of the Warrant Assumption Agreement filed as an exhibit hereto, for a complete description of the terms and conditions applicable to the Holdco Warrants. The warrant holders do not have the rights or privileges of holders of Holdco Shares and any voting rights until they exercise their warrants and receive Holdco Shares. After the issuance of Holdco Shares upon exercise of the Holdco, each holder will be entitled to one vote for each Holdco Share held of record on all matters to be voted on by shareholders.
363
Table of Contents
No fractional shares will be issued upon exercise of the Holdco Warrants. If, upon exercise of the Holdco Warrants, a holder would be entitled to receive a fractional interest in a Holdco Share, Holdco will, upon exercise, round down to the nearest whole number the number of Holdco Shares to be issued to the warrant holder.
Holdco has agreed that, subject to applicable law, any action, proceeding or claim against us arising out of or relating in any way to the Warrant Assumption Agreement will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and Holdco irrevocably submits to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim. This provision applies to claims under the Securities Act but does not apply to claims under the Exchange Act or any claim for which the federal district courts of the United States of America are the sole and exclusive forum.
Shareholders’ Register
Pursuant to Dutch law and the Holdco Articles of Association, Holdco must keep its shareholders’ register accurate and current. The Holdco Board keeps the shareholders’ register and records names and addresses of all holders of registered shares, showing the date on which the shares were acquired, the date of the acknowledgement by or notification of Holdco as well as the amount paid on each share. The register also includes the names and addresses of those with a right of usufruct (vruchtgebruik) on registered shares belonging to another or a pledge (pandrecht) in respect of such shares. The Holdco Shares offered in this offering will be held through DTC. Therefore, DTC or its nominee will be recorded in the shareholders’ register as the holder of those Holdco Shares. The Holdco Shares will be in registered form (op naam). Holdco may issue share certificates (aandeelbewijzen) for registered shares in such form as may be approved by the Holdco Board.
Corporate Objectives
Pursuant to the Holdco Articles of Association, Holdco’s main corporate objectives are:
| • | to develop, conduct research, produce, commercialize, market and sell medicines in general and innovative medicines for cardiovascular diseases in particular; |
| • | to incorporate, to participate in, to finance, to hold any other interest in and to conduct the management or supervision of other entities, companies, partnerships and businesses; |
| • | to provide administrative, technical, financial, economic or other services to other entities, companies, partnerships and businesses; |
| • | to acquire, to manage, to invest, to exploit, to encumber and to dispose of assets and liabilities; |
| • | to furnish guarantees, to provide security, to warrant performance in any other way and to assume liability, whether jointly and severally or otherwise, in respect of obligations of group companies or other parties; and |
| • | to do anything which, in the widest sense, is connected with or may be conducive to the objects described above. |
Limitations on the Rights to Own Securities
Holdco Shares may be issued to individuals, corporations, trusts, estates of deceased individuals, partnerships and unincorporated associations of persons. The Holdco Articles of Association contain no limitation on the rights to own Holdco Shares and no limitation on the rights of non-residents of the Netherlands or foreign shareholders to hold or exercise voting rights.
364
Table of Contents
Limitation on Liability and Indemnification Matters
Under Dutch law, the Holdco directors may be held liable for damages in the event of improper or negligent performance of their duties. They may be held liable for damages to Holdco and to third parties for infringement of the Holdco Articles of Association or of certain provisions of Dutch law. In certain circumstances, they may also incur other specific civil, administrative and criminal liabilities. Subject to certain exceptions, the Holdco Articles of Association provide for indemnification of Holdco’s current and former directors and other current and former officers and employees as designated by the Holdco Board. No indemnification under the Holdco Articles of Association will be given to an indemnified person:
| • | if a competent court or arbitral tribunal has established, without having (or no longer having) the possibility for appeal, that the acts or omissions of such indemnified person that led to the financial losses, damages, expenses, suit, claim, action or legal proceedings as described above are of an unlawful nature (including acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such indemnified person); |
| • | to the extent that his or her financial losses, damages and expenses are covered under insurance and the relevant insurer has settled, or has provided reimbursement for, these financial losses, damages and expenses (or has irrevocably undertaken to do so); |
| • | in relation to proceedings brought by such indemnified person against Holdco, except for proceedings brought to enforce indemnification to which he or she is entitled pursuant to the Holdco Articles of Association, pursuant to an agreement between such indemnified person and Holdco which has been approved by the Holdco or pursuant to insurance taken out by Holdco for the benefit of such indemnified person; and |
| • | for any financial losses, damages or expenses incurred in connection with a settlement of any proceedings effected without Holdco’s prior consent. |
Under the Holdco Articles of Association, the Holdco Board may stipulate additional terms, conditions and restrictions in relation to the indemnification described above.
Federal Forum Provision
The Holdco Articles of Association provide that, unless Holdco consents in writing to the selection of an alternative forum, the sole and exclusive forum for any complaint asserting a cause of action arising under the U.S. Securities Act of 1933, as amended, or the U.S. Securities Exchange Act of 1934, as amended, to the fullest extent permitted by applicable law, will be the U.S. federal district courts.
Shareholders’ Meeting
Holdco General Meetings must be held in the Netherlands in any of the locations specified in the Holdco Articles of Association. The annual Holdco General Meeting must be held within six months of the end of each financial year. Additional extraordinary Holdco General Meetings may also be held, whenever considered appropriate by the Holdco Board and shall be held within three months after the Holdco Board has considered it to be likely that Holdco’s shareholders’ equity (eigen vermogen) has decreased to an amount equal to or lower than half of Holdco’s paid-in and called up share capital, in order to discuss the measures to be taken if so required.
Pursuant to Dutch law, one or more shareholders or others with meeting rights under Dutch law who jointly represent at least one-tenth of Holdco’s issued share capital may request Holdco to convene a general meeting, setting out in detail the matters to be discussed. If the Holdco Board has not taken the steps necessary to ensure that such meeting can be held within six weeks after the request, the proponent(s) may, on their application, be authorized by the competent Dutch court in preliminary relief proceedings to convene a Holdco General Meeting. The court shall disallow the application if it does not appear that the proponent(s) has/have previously requested
365
Table of Contents
the Holdco Board to convene a Holdco General Meeting and the Holdco Board has not taken the necessary steps so that the Holdco General Meeting could be held within six weeks after the request. The application shall also be disallowed if the proponent(s) has/have not demonstrated to have a reasonable interest in the convening of the Holdco General Meeting.
Holdco General Meetings must be convened by an announcement published in a Dutch daily newspaper with national distribution. The notice must state the agenda, the time and place of the meeting, the record date (if any), the procedure for participating in the Holdco General Meeting by proxy, as well as other information as required by Dutch law. The notice must be given at least 15 calendar days prior to the day of the meeting. The agenda for the annual general meeting shall include, among other things, the adoption of Holdco’s statutory annual accounts, appropriation of Holdco’s profits and proposals relating to the composition of the Holdco Board, including the filling of any vacancies. In addition, the agenda shall include such items as have been included therein by the Holdco Board. The agenda shall also include such items requested by one or more shareholders or others with meeting rights under Dutch law representing at least 3% of Holdco’s issued share capital. These requests must be made in writing or by electronic means and received by the Holdco Board at least 60 days before the day of the meeting. No resolutions shall be adopted on items other than those that have been included in the agenda.
In accordance with the DCGC, shareholders who have the right to put an item on the agenda for the Holdco General Meeting or to request the convening of a Holdco General Meeting shall not exercise such rights until after they have consulted the Holdco Board. If exercising such rights may result in a change in Holdco’s strategy (for example, through the dismissal of one or more Holdco directors), the Holdco Board must be given the opportunity to invoke a reasonable period of up to 180 days to respond to the shareholders. If invoked, the Holdco Board must use such response period for further deliberation and constructive consultation, in any event with the shareholder(s) concerned and explore alternatives. At the end of the response time, the Holdco Board shall report on this consultation and the exploration of alternatives to the Holdco General Meeting. The response period may be invoked only once for any given Holdco General Meeting and shall not apply (i) in respect of a matter for which a response period has been previously invoked or (ii) if a shareholder holds at least 75% of Holdco’s issued share capital as a consequence of a successful public bid.
Moreover, the Holdco Board can invoke a cooling-off period of up to 250 days when shareholders, using their right to have items added to the agenda for a Holdco General Meeting or their right to request a Holdco General Meeting, propose an agenda item for the Holdco General Meeting to dismiss, suspend or appoint one or more Holdco directors (or to amend any provision in the Holdco Articles of Association dealing with those matters) or when a public offer for Holdco is made or announced without Holdco’s support, provided, in each case, that the Holdco Board believes that such proposal or offer materially conflicts with the interests of Holdco and its business. During a cooling-off period, the Holdco General Meeting cannot dismiss, suspend or appoint directors (or amend the provisions in the Holdco Articles of Association dealing with those matters) except at the proposal of the Holdco Board. During a cooling-off period, the Holdco Board must gather all relevant information necessary for a careful decision-making process and consult with shareholders representing at least 3% or more of Holdco’s issued share capital at the time the cooling-off period was invoked, as well as with Holdco’s Dutch works council (if Holdco or, under certain circumstances, any of Holdco’s subsidiaries have one). Formal statements expressed by these stakeholders during such consultations must be published on Holdco’s website to the extent these stakeholders have approved that publication. Ultimately one week following the last day of the cooling-off period, the Holdco Board must publish a report on Holdco’s website in respect of its policy and conduct of affairs during the cooling-off period. This report must remain available for inspection by shareholders and others with meeting rights under Dutch law at Holdco’s office and must be tabled for discussion at the next Holdco General Meeting. Shareholders representing at least 3% of our issued share capital may request the Enterprise Chamber for early termination of the cooling-off period. The Enterprise Chamber must rule in favor of the request if the shareholders can demonstrate that:
| • | the Holdco Board, in light of the circumstances at hand when the cooling-off period was invoked, could not reasonably have concluded that the relevant proposal or hostile offer constituted a material conflict with the interests of Holdco and its business; |
366
Table of Contents
| • | the Holdco Board cannot reasonably believe that a continuation of the cooling-off period would contribute to careful policy-making; or |
| • | other defensive measures, having the same purpose, nature and scope as the cooling-off period, have been activated during the cooling-off period and have not since been terminated or suspended within a reasonable period at the relevant shareholders’ request (i.e., no “stacking” of defensive measures). |
The Holdco General Meeting is presided over by the chairperson of the Holdco Board. If no chairperson has been elected or if he or she is not present at the meeting, the Holdco General Meeting shall be presided over by the vice-chairperson of the Holdco Board. If no vice-chairperson has been elected or if he or she is not present at the meeting, the Holdco General Meeting shall be presided over by another person designated in accordance with the Holdco Articles of Association. The Holdco directors may always attend a Holdco General Meeting. In these meetings, they have an advisory vote. The chairperson of the Holdco General Meeting may decide at his or her discretion to admit other persons to the meeting.
All shareholders and others with meeting rights under Dutch law are authorized to attend the general meeting, to address the meeting and, insofar as they have such right, to vote pro rata to his or her shareholding. Shareholders may exercise these rights, if they are the holders of shares on the record date, if any, as required by Dutch law, which is currently the 28th day before the day of the general meeting. Under the Holdco Articles of Association, shareholders and others with meeting rights under Dutch law must notify us in writing or by electronic means of their identity and intention to attend the Holdco General Meeting. This notice must be received by us ultimately on the seventh day prior to the Holdco General Meeting, unless indicated otherwise when such meeting is convened.
Each Holdco Share confers the right on the holder to cast one vote at the Holdco General Meeting. Shareholders may vote by proxy. No votes may be cast at a Holdco General Meeting on Holdco Shares held by Holdco or its subsidiaries or on Holdco Shares for which Holdco or its subsidiaries hold depository receipts. Nonetheless, the holders of a right of usufruct (vruchtgebruik) and the holders of a right of pledge (pandrecht) in respect of Holdco Shares held by Holdco or its subsidiaries in its share capital are not excluded from the right to vote on such Holdco Shares, if the right of usufruct (vruchtgebruik) or the right of pledge (pandrecht) was granted prior to the time such shares were acquired by Holdco or any of its subsidiaries. Neither Holdco nor any of its subsidiaries may cast votes in respect of a Holdco Share on which Holdco or such subsidiary holds a right of usufruct (vruchtgebruik) or a right of pledge (pandrecht). Holdco Shares which are not entitled to voting rights pursuant to the preceding sentences will not be taken into account for the purpose of determining the number of shareholders that vote and that are present or represented, or the amount of the share capital that is provided or that is represented at a Holdco General Meeting.
Decisions of the Holdco General Meeting are taken by a simple majority of votes cast, except where Dutch law or the Holdco Articles of Association provide for a qualified majority or unanimity. Subject to any provision of mandatory Dutch law and any higher quorum requirement stipulated by the Holdco Articles of Association, if Holdco would be subject to the requirement that the Holdco General Meeting can only pass resolutions if a certain part of Holdco’s issued share capital is present or represented at such Holdco General Meeting under applicable securities laws or listing rules, then such resolutions shall be subject to such quorum as specified by such securities laws or listing rules pursuant to the Holdco Articles of Association.
Directors
Appointment of Holdco Directors
Holdco directors will be appointed by the Holdco General Meeting upon binding nomination by the Holdco Board. However, the Holdco General Meeting may at all times overrule a binding nomination by a resolution adopted by at least a two-thirds majority of the votes cast, provided such majority represents more than half of Holdco’s issued share capital. If the Holdco General Meeting overrules a binding nomination, the Holdco Board will make a new nomination.
367
Table of Contents
Prior to the consummation of the Business Combination, the Holdco Board will adopt a diversity policy for the composition of the Holdco Board, as well as a profile for the composition of the Holdco Board, with the assistance of Holdco’s nomination and corporate governance committee. The Holdco Board will make any nomination for the appointment of a Holdco director with due regard to the rules and principles set forth in such diversity policy and profile, as applicable.
At a Holdco General Meeting, a resolution to appoint a Holdco director can only be passed in respect of candidates whose names are stated for that purpose in the agenda of that Holdco General Meeting or in the explanatory notes thereto.
Duties and Liabilities of Holdco Directors
Under Dutch law, the Holdco Board is charged with the management of Holdco, which includes setting Holdco’s policies and strategy, subject to the restrictions contained in the Holdco Articles of Association. The Holdco executive directors manage Holdco’s day-to-day business and operations and implement Holdco’s strategy. The Holdco non-executive directors focus on the supervision on the policy and functioning of the performance of the duties of all Holdco directors and Holdco’s general state of affairs. The Holdco directors may divide their tasks among themselves in or pursuant to internal rules. Each Holdco director has a statutory duty to act in the corporate interest of Holdco and its business. Under Dutch law, the corporate interest extends to the interests of all corporate stakeholders, such as shareholders, creditors, employees, customers and suppliers. The duty to act in the corporate interest of Holdco also applies in the event of a proposed sale or break-up of Holdco, provided that the circumstances generally dictate how such duty is to be applied and how the respective interests of various groups of stakeholders should be weighed.
The Holdco Board is entitled to represent Holdco. The power to represent Holdco also vests in Holdco’s Chief Executive Officer, as well as in any two non-executive directors acting jointly.
Dividends and Other Distributions
Dividends
Holdco has never paid or declared any cash dividends in the past, and Holdco does not anticipate paying any cash dividends in the foreseeable future. Holdco intends to retain all available funds and any future earnings for use in the operation of its business. Under Dutch law, Holdco may only pay dividends and other distributions from its reserves to the extent Holdco’s shareholders’ equity (eigen vermogen) exceeds the sum of its paid-in and called-up share capital plus the reserves Holdco must maintain under Dutch law or the Holdco Articles of Association and (if it concerns a distribution of profits) after adoption of Holdco’s statutory annual accounts by the Holdco General Meeting from which it appears that such dividend distribution is allowed.
Under the Holdco Articles of Association, the Holdco Board may decide that all or part of the profits shown in Holdco’s adopted statutory annual accounts will be added to Holdco’s reserves. After reservation of any such profits, any remaining profits will be at the disposal of the Holdco General Meeting at the proposal of the Holdco Board for distribution on the Holdco Shares, subject to applicable restrictions of Dutch law. The Holdco Board is permitted, subject to certain requirements and applicable restrictions of Dutch law, to declare interim dividends without the approval of the Holdco General Meeting. Dividends and other distributions will be made payable no later than a date determined by the Holdco Board. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable will lapse and any such amounts will be considered to have been forfeited to us (verjaring).
Exchange Controls
Under Dutch law, there are no exchange controls applicable to the transfer to persons outside of the Netherlands of dividends or other distributions with respect to, or of the proceeds from the sale of, shares of a Dutch company, subject to applicable restrictions under sanctions and measures, including those concerning
368
Table of Contents
export control, pursuant to European Union regulations, the Sanctions Act 1977 (Sanctiewet 1977) or other legislation, applicable anti-boycott regulations, applicable anti-money-laundering regulations and similar rules and provided that, under certain circumstances, payments of such dividends or other distributions must be reported to the Dutch Central Bank at their request for statistical purposes. There are no special restrictions in the Holdco Articles or Dutch law that limit the right of shareholders who are not citizens or residents of the Netherlands to hold or vote shares.
Squeeze-Out Procedures
A shareholder who holds at least 95% of Holdco’s issued share capital for his or her own account, alone or together with group companies, may initiate proceedings against Holdco’s other shareholders jointly for the transfer of their Holdco Shares to such shareholder. The proceedings are held before the Enterprise Chamber and can be instituted by means of a writ of summons served upon each of the other shareholders in accordance with the provisions of the Dutch Code of Civil Procedure (Wetboek van Burgerlijke Rechtsvordering). The Enterprise Chamber may grant the claim for squeeze-out in relation to the other shareholders and will determine the price to be paid for the Holdco Shares, if necessary, after appointment of one or three experts who will offer an opinion to the Enterprise Chamber on the value to be paid for the Holdco Shares of the other shareholders. Once the order to transfer becomes final before the Enterprise Chamber, the person acquiring the Holdco Shares shall give written notice of the date and place of payment and the price to the holders of the Holdco hares to be acquired whose addresses are known to him. Unless the addresses of all of them are known to the acquiring person, such person is required to publish the same in a daily newspaper with a national circulation.
Dissolution and Liquidation
Under the Holdco Articles of Association, Holdco may be dissolved by a resolution of the Holdco General Meeting, subject to a proposal of the Holdco Board. In the event of a dissolution, the liquidation shall be effected by the Holdco Board, unless the Holdco General Meeting decides otherwise. During liquidation, the provisions of the Holdco Articles of Association will remain in force as far as possible. To the extent that any assets remain after payment of all of Holdco’s liabilities, any remaining assets shall be distributed to Holdco’s shareholders in proportion to their number of Holdco shares.
Dutch Corporate Governance Code
Upon the consummation of the Business Combination, Holdco will be subject to the DCGC. The DCGC contains principles and best practice provisions on corporate governance that regulate relations between the board of directors and the general meeting and matters in respect of financial reporting, auditors, disclosure, compliance and enforcement standards. The DCGC is based on a “comply or explain” principle. Accordingly, companies must disclose in their statutory annual reports whether they comply with the provisions of the DCGC. If a company subject to the DCGC does not comply with those provisions, that company would be required to give the reasons for such non-compliance. Holdco does not comply with all best practice provisions of the DCGC. As of the date of this document, Holdco’s main deviations from the DCGC are summarized below. In the future, Holdco may deviate from additional provisions of the DCGC, including to follow market practice or governance practices in the United States. The DCGC contains certain independence recommendations for the Holdco Board and its committees. Holdco does not expect to comply with all such recommendations.
Under the Holdco Articles of Association, the Holdco directors are to be appointed on the basis of a binding nomination prepared by the Holdco Board. This means that the nominee will be appointed unless the Holdco General Meeting removes the binding nature of the nomination (in which case a new nomination will be prepared for a subsequent Holdco General Meeting). The Holdco Articles of Association provide that the Holdco General Meeting can only pass such resolution by a two-thirds majority representing more than half of the issued share capital. However, the DCGC recommends that the general meeting can pass such a resolution by simple majority, representing no more than one-third of the issued share capital.
Under the Holdco Articles of Association, the Holdco directors can only be dismissed by the Holdco General Meeting by simple majority, provided that the Holdco Board proposes the dismissal. In other cases, the
369
Table of Contents
Holdco General Meeting can only pass such resolution by a two-thirds majority representing more than half of the issued share capital. The DCGC recommends that the general meeting can pass a resolution to dismiss a director by simple majority, representing no more than one-third of the issued share capital.
The DCGC recommends against providing equity awards as part of the compensation of a non-executive director. However, Holdco may deviate from this recommendation and grant equity awards to the Holdco non-executive directors, consistent with U.S. market practice.
As part of the compensation of Holdco directors, Holdco may grant shares that are not subject to a lock-up period of at least five years after the date of grant and/or grant options without restricting the exercisability of those options during the first three years after the date of grant. In those cases, this would cause additional deviations from the DCGC.
Dutch Financial Reporting Supervision Act
On the basis of the Dutch Financial Reporting Supervision Act (Wet toezicht financiële verslaggeving), or the FRSA, the Dutch Authority for the Financial Markets (Stichting Autoriteit Financiële Markten) (“AFM”), supervises the application of financial reporting standards by Dutch companies whose securities are listed on a Dutch or foreign stock exchange.
Pursuant to the FRSA, the AFM has an independent right to (i) request an explanation from Holdco regarding Holdco’s application of the applicable financial reporting standards if, based on publicly known facts or circumstances, it has reason to doubt that Holdco’s financial reporting meets such standards and (ii) recommend to Holdco the making available of further explanations. If Holdco does not comply with such a request or recommendation, the AFM may request that the Enterprise Chamber order Holdco to (i) make available further explanations as recommended by the AFM, (ii) provide an explanation of the way Holdco has applied the applicable financial reporting standards to Holdco’s financial reports or (iii) prepare or restate Holdco’s financial reports in accordance with the Enterprise Chamber’s orders.
Transfer Agent and Registrar
Upon the consummation of the Business Combination, the transfer agent and registrar for the Holdco Shares will be Continental Stock Transfer & Trust Company.
370
Table of Contents
BENEFICIAL OWNERSHIP OF HOLDCO SECURITIES
The following table sets forth information regarding (i) the actual beneficial ownership of FLAC Ordinary Shares as of September 30, 2022, and (ii) expected beneficial ownership of Holdco Shares immediately following the Business Combination and the PIPE Financing, assuming that no FLAC Ordinary Shares are redeemed, and alternatively the maximum number of FLAC Ordinary Shares are redeemed, by:
| • | each person who is, or is expected to be, the beneficial owner of more than five percent (5%) of the outstanding Holdco Shares post-Business Combination; |
| • | each person who will become an executive officer or director of Holdco post-Business Combination; and |
| • | all expected executive officers and directors of Holdco post-Business Combination, as a group. |
The SEC has defined “beneficial ownership” of a security to mean the possession, directly or indirectly, of voting power and/or investment power over such security. A shareholder is also deemed to be, as of any date, the beneficial owner of all securities that such shareholder has the right to acquire within 60 days after that date through (i) the exercise of any option, warrant or right, (ii) the conversion of a security, (iii) the power to revoke a trust, discretionary account or similar arrangement, or (iv) the automatic termination of a trust, discretionary account or similar arrangement. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, ordinary shares subject to options or other rights (as set forth above) held by that person that are currently exercisable, or will become exercisable within 60 days thereafter, are deemed outstanding, while such shares are not deemed outstanding for purposes of computing percentage ownership of any other person. Each person named in the table has sole voting and investment power with respect to all of the ordinary shares shown as beneficially owned by such person, except as otherwise indicated in the table or footnotes below.
The expected beneficial ownership percentages set forth in the table below do not take into account the issuance of any shares upon the exercise of Holdco Warrants to purchase 4,767,000 Holdco Shares that will remain outstanding following the Business Combination. Beneficial ownership of shares currently owned by holders of NewAmsterdam Pharma shares below are presented after giving effect to the Business Combination and the application of the Exchange Ratio and assumes the illustrative exchange ratio of 2.1307.
The expected beneficial ownership of Holdco Shares post-Business Combination is based on 86,125,642 Holdco Shares issued and outstanding, assuming no redemption, and 73,864,160 Holdco Shares issued and outstanding, assuming the maximum redemption scenario of 12,261,482 redemptions (as more fully described under “Unaudited Pro Forma Condensed Combined Financial Information” herein), and assumes (i) issuance of 23,460,000 Holdco Shares in the PIPE Financing and (ii) that the amount in the Trust Account is $138.1 million (which was the approximate value of the Trust Account as of June 30, 2022). If the actual facts are different than these assumptions, the numbers in the below table will be different. No shareholder has different voting rights than another shareholder except for the obligations imposed on the shareholders subject to the Investor Rights Agreement in connection with the Business Combination as described in the section entitled “The Business Combination Agreement and Ancillary Documents—Investor Support Agreements.”
371
Table of Contents
Unless otherwise indicated, Holdco believes that all persons named in the table below have sole voting and investment power with respect to all shares of capital stock beneficially owned by them. To Holdco’s knowledge, no Holdco Shares beneficially owned by any executive officer, director or director nominee have been pledged as security. Unless otherwise stated, the address for each beneficial owner is Gooimeer 2-35, 1411 DC Naarden, The Netherlands.
| Beneficial Ownership Upon the Completion of the Business Combination and PIPE Financing | ||||||||||||||||
| Assuming No Redemptions | Assuming Maximum Redemption | |||||||||||||||
| Beneficial Owner | Number of Holdco Shares | Percentage of All Holdco Shares | Number of Holdco Shares | Percentage of All Holdco Shares | ||||||||||||
| Directors and Executive Officers of Holdco | ||||||||||||||||
Juliette Audet (1) | 6,635,391 | 7.70 | % | 6,635,391 | 8.98 | % | ||||||||||
Michael Davidson, M.D. (2) | 1,355,018 | 1.56 | % | 1,355,018 | 1.82 | % | ||||||||||
Nicholas Downing, M.D.(3) | — | — | — | — | ||||||||||||
John Kastelein, M.D., Ph.D, FESC (4) | 1,412,667 | 1.62 | % | 1,412,667 | 1.89 | % | ||||||||||
Louis Lange, M.D., Ph.D. | — | — | — | — | ||||||||||||
Sander Slootweg (5) | 19,421,688 | 22.55 | % | 19,421,688 | 26.30 | % | ||||||||||
James N. Topper, M.D., Ph.D. (6) | 9,301,000 | 10.80 | % | 9,301,000 | 12.60 | % | ||||||||||
Marc Ditmarsch, M.D. (7) | 228,606 | * | 228,606 | * | ||||||||||||
Lina Gugucheva (8) | 183,772 | * | 183,772 | * | ||||||||||||
Douglas Kling (9) | 324,677 | * | 324,677 | * | ||||||||||||
Louise Kooij (10) | 155,997 | * | 155,997 | * | ||||||||||||
All executive officers and directors as a group (11 persons) | 39,018,816 | 44.09 | % | 39,018,816 | 51.19 | % | ||||||||||
| Other 5% Shareholders | ||||||||||||||||
Morningside Venture Investments Limited (11) | 5,065,846 | 5.89 | % | 5,065,846 | 6.86 | % | ||||||||||
Entities affiliated with Forbion (12) | 19,421,688 | 22.55 | % | 19,421,688 | 26.30 | % | ||||||||||
Saga Investments Coöperatief U.A. (13) | 4,910,000 | 5.70 | % | 4,910,000 | 7.21 | % | ||||||||||
Stichting Administratiekantoor NewAmsterdam Pharma (14) | 5,326,818 | 6.20 | % | 5,326,818 | 7.21 | % | ||||||||||
Frazier Lifesciences Sponsor LLC and affiliates (15) | 9,301,000 | 10.80 | % | 9,301,000 | 12.60 | % | ||||||||||
Entities affiliated with Bain Capital Life Sciences Investors, LLC (16) | 8,300,000 | 9.64 | % | 8,300,000 | 11.24 | % | ||||||||||
| * | Less than 1% ownership. |
| (1) | Will consist of (i) 6,635,391 Holdco Shares held by Forbion Capital Fund IV Coöperatief U.A. (“Forbion IV”) inclusive of 1,500,000 Holdco Shares subscribed for in connection with the PIPE Financing. The address for Forbion IV is Gooimeer 2-35, 1411 DC Naarden, the Netherlands. Forbion IV Management B.V. (“Forbion IV Management”), the director of Forbion IV, may be deemed to have voting and dispositive power over the Holdco Shares to be held by Forbion IV. Investment decisions with respect to the Holdco Shares to be held by Forbion IV can be made by its investment committee which may delegate such powers to the authorized representatives of Forbion IV Management. Mssrs. Slootweg, van Osch, Mulder, van Houten, van Deventer, Reithinger, Kersten and Boorsma are partners of Forbion IV Management, which acts as the investment advisor to the director of Forbion IV. Ms. Audet and Mr. Slootweg are members of the investment committee of Forbion IV. Forbion IV Management disclaims beneficial ownership of the shares, except to the extent of their pecuniary interest therein. |
372
Table of Contents
| (2) | Will consist of (i) 189,784 Holdco Shares, (ii) options to purchase 556,455 Holdco Shares, exercisable within 60 days of September 30, 2022 and (iii) 608,779 Holdco Shares subject to forfeiture underlying depositary receipts issued by Stichting Administratiekantoor EPNAP (“STAK EPNAP”). STAK EPNAP has sole voting and investment power over the securities described in (iii) while underlying the depositary receipts and are presented here because the depositary receipts can be cancelled by the board of directors of STAK EPNAP at any time as a consequence of which the shareholder will become the beneficial owner of the securities underlying the depositary receipts. |
| (3) | Does not include Holdco Shares held by the Bain Capital Life Sciences Entities (as defined below). Dr. Downing serves as a Principal of Bain Capital Life Sciences Investors, LLC. |
| (4) | Will consist of (i) 268,472 Holdco Shares held by Futurum B.V. (“Futurum”) through NAP PoolCo B.V. (“PoolCo”), (ii) 228,881 Holdco Shares underlying depositary receipts issued by STAK NAP (as defined below) which are held by Futurum through PoolCo and (iii) options to purchase 915,314 Holdco Shares, exercisable within 60 days of September 30, 2022, held by Futurum through PoolCo. STAK NAP has sole voting and investment power over the securities described in (ii) underlying the depositary receipts and are presented here because the depositary receipts can be cancelled by the board of directors of STAK NAP at any time as a consequence of which the shareholder will become the beneficial owner of the securities underlying such depositary receipts. |
| (5) | Will consist of the Holdco Shares described in Note 12. Mr. Slootweg disclaims beneficial ownership of the shares referenced in Note 12, except to the extent of his pecuniary interest therein, if any. |
| (6) | Will consist of the shares described in Note 15. Dr. Topper disclaims beneficial ownership of the shares referenced in Note 15, except to the extent of his pecuniary interest therein, if any. |
| (7) | Will consist of options to purchase 228,606 Holdco Shares, exercisable within 60 days of September 30, 2022, held by Diomedea Medical B.V. through PoolCo. |
| (8) | Will consist of options to purchase 183,772 Holdco Shares, exercisable within 60 days of September 30, 2022. |
| (9) | Will consist of options to purchase 324,677 Holdco Shares, exercisable within 60 days of September 30, 2022. |
| (10) | Will consist of options to purchase 155,997 Holdco Shares, exercisable within 60 days of September 30, 2022, held by LouFré Management B.V. through PoolCo. |
| (11) | Will consist of 5,065,846 Holdco Shares directly and beneficially held by Morningside Venture Investments Limited (“Morningside”), inclusive of 500,000 Holdco Shares subscribed for in connection with the PIPE Financing. Frances Anne Elizabeth Richard, Jill Marie Franklin, Peter Stuart Allenby Edwards and Cheung Ka Ho are the directors of Morningside and share voting and dispositive power with respect to the securities held by Morningside. Each of Ms. Richard, Ms. Franklin, Mr. Edwards and Mr. Ho disclaim beneficial ownership of the Holdco Shares held by Morningside except to the extent of their pecuniary interest therein. The address of Morningside is c/o THC Management Services S.A.M., 2nd Floor, Le Prince de Galles, 3-5 Avenue des Citronniers, MC 98000, Monaco. |
| (12) | Will consist of (i) 6,635,391 Holdco Shares held by Forbion IV, inclusive of 1,500,000 Holdco Shares subscribed for in connection with the PIPE Financing, (ii) 4,543,897 Holdco Shares held by Forbion Growth Opportunities Fund I Coöperatief U.A. (“Forbion Growth”), inclusive of 1,500,000 Holdco Shares subscribed for in connection with the PIPE Financing and (iii) 7,812,300 Holdco Shares held by Forbion Capital Fund II Coöperatief U.A. (“Forbion II”) through PoolCo, including 2,828,380 Holdco Shares underlying depositary receipts issued by STAK NAP. The address for the Forbion entities is Gooimeer 2-35, 1411 DC Naarden, the Netherlands. |
Forbion IV Management, the director of Forbion IV, may be deemed to have voting and dispositive power over the shares held by Forbion IV. Investment decisions with respect to the Holdco Shares to be held by Forbion IV can be made by its investment committee which may delegate such powers to the authorized representatives of Forbion IV Management. Mssrs. Slootweg, van Osch, Mulder, van Houten, van Deventer, Reithinger, Kersten and Boorsma are partners of Forbion IV Management, which acts as the investment advisor to the director of Forbion IV. Mr. Slootweg is a member of the board of directors and is a partner of Forbion IV Management and a member of the investment committee of Forbion IV. Forbion IV Management disclaims beneficial ownership of the shares, except to the extent of their pecuniary interest therein.
373
Table of Contents
Forbion Growth Management B.V. (“Growth Management”), the director of Forbion Growth, may be deemed to have voting and dispositive power over the shares held by Forbion Growth. Investment decisions with respect to the Holdco Shares to be held by Forbion Growth can be made by its investment committee which may delegate such powers to the authorized representatives of Growth Management. Mssrs. Slootweg, van Osch, Mulder, van Houten, van Deventer, Reithinger, Kersten, Joustra and Boorsma are partners of Growth Management, which acts as the investment advisor to the director of Forbion Growth. Mr. Slootweg is a member of the board of directors and is a partner of Growth Management and a member of the investment committee of Forbion Growth. Growth Management disclaims beneficial ownership of the shares, except to the extent of their pecuniary interest therein.
Forbion II Management B.V. (“Forbion II Management”), the director of Forbion II, may be deemed to have voting and dispositive power over the shares held by Forbion II. Investment decisions with respect to the shares held by Forbion II can be made by its investment committee which may delegate such powers to the authorized representatives of Forbion II Management. Mssrs. Slootweg, van Osch, Mulder, van Deventer, Reithinger, and Bergstein are (ex)partners of Forbion II Management, which may act as the investment advisor to the director of Forbion II. Mr. Slootweg is a member of the board of directors and is a partner of Forbion II Management and a member of the investment committee of Forbion II. Forbion II Management and its partners disclaims beneficial ownership of the shares, except to the extent of their pecuniary interest therein. 4,983,920 Holdco Shares are held by Forbion II through PoolCo.
BioGeneration II Management B.V. (“BGM II”) is the director of (1) BioGeneration Ventures II B.V., which, through PoolCo, holds 415,873 Holdco Shares underlying depositary receipts issued by STAK NAP, (2) BGV II Coöperatief U.A. which, through PoolCo, holds 2,269 Holdco Shares underlying depositary receipts issued by STAK NAP, and (3) BioGeneration II Co-Invest B.V. which through PoolCo holds 11,958 Holdco Shares underlying depositary receipts issued by STAK NAP. BGM II is an indirect joint venture between the BGM investment team and the partners of Forbion II Management. Mr. Slootweg is a member of the board of directors and is a partner of Forbion II and a member of the investment committee of BGM II. BGM II disclaims beneficial ownership of the shares, except to the extent of their pecuniary interest therein.
| (13) | The address of the Saga Investments Coöperatief U.A. is Minervum 7061, 4817 ZK Breda, the Netherlands. |
| (14) | Stichting Administratiekantoor NewAmsterdam Pharma (“STAK NAP”) is a Dutch foundation which holds certain securities of NewAmsterdam Pharma on behalf of the holders of depositary receipts issued by STAK NAP, including certain shareholders of NewAmsterdam Pharma and after the Closing, will hold the Holdco Shares following the Exchange. STAK NAP has issued depositary receipts to the participants with each depositary receipt representing the relevant underlying security. Pursuant to STAK NAP’s governing documents, STAK NAP, through its board, has the power to exercise all rights associated with the NewAmsterdam Pharma securities underlying the depositary receipts and after the Closing, the Holdco Shares. As a result, STAK NAP may be deemed to have voting power over such securities. 2,828,380 Holdco Shares owned by Forbion II are underlying the depositary receipts held through STAK NAP. Forbion International Management B.V. (“FIM”) is the sole director of STAK NAP and may be deemed to have voting and dispositive power of the Holdco Shares held by STAK NAP. Mssrs. Slootweg, Van Osch, Reithinger, Mulder, Van Houten and Boorsma are the directors of FIM. FIM and its directors disclaim beneficial ownership of the shares. |
| (15) | Will consist of 3,801,000 Holdco Shares (upon the conversion of (i) 3,300,000 FLAC Class B Ordinary Shares and (ii) 501,000 FLAC Class A Ordinary Shares) held by the Sponsor. The Sponsor is governed by a board of managers, consisting of James N. Topper, David Topper and Gordon Empey. The sole member of the Sponsor is Frazier Life Sciences X, L.P. FHMLS X, L.P. is the general partner of Frazier Life Sciences X, L.P. and FHMLS X, L.L.C. is the general partner of FHMLS X, L.P. Patrick J. Heron and James N. Topper are the members of FHMLS X, L.L.C. Each of Mr. Heron, Mr. James Topper, Mr. David Topper, and Mr. Empey disclaims beneficial ownership of the shares other than to the extent of any pecuniary interest they may have therein, directly or indirectly. |
Also included in the total number is (i) 1,000,000 FLAC Class A Ordinary Shares held by Frazier Life Sciences X, L.P., (ii) 2,000,000 Holdco Shares subscribed for by Frazier Life Sciences X, L.P. in connection with the PIPE Financing, (iii) 500,000 Holdco Shares subscribed for by Frazier Life Sciences XI, L.P. in
374
Table of Contents
connection with the PIPE Financing, (iv) 1,000,000 Holdco Shares subscribed for by Frazier Life Sciences Public Fund L.P., in connection with the PIPE Financing and (v) 1,000,000 Holdco Shares subscribed for by Frazier Life Sciences Public Overage Fund, L.P. in connection with the PIPE Financing. FHMLS XI, L.P. is the general partner of Frazier Life Sciences XI, L.P. and FHMLS XI, L.L.C. is the general partner of FHMLS XI, L.P. Patrick J. Heron and James N. Topper are the members of FHMLS XI, L.L.C. FHMLSP, L.P. is the general partner of Frazier Life Sciences Public Fund, L.P. and FHMLSP, L.L.C. is the general partner of FHMLSP, L.P. Patrick J. Heron, James N. Topper, Albert Cha and James Brush are the members of FHMLSP, L.L.C. FHMLSP Overage, L.P., is the general partner of Frazier Life Sciences Public Overage Fund, L.P. and FHMLSP Overage, L.L.C. is the general partner of FHMLSP Overage, L.P. Patrick J. Heron, James N. Topper, Albert Cha and James Brush are the members of FHMLSP Overage, L.L.C. Each of FHMLS X, L.P., FHMLS X, L.L.C., FHMLS XI, L.P., FHMLS XI, L.L.C., FHMLSP, L.P., FHMLSP, L.L.C., FHMLSP Overage, L.P., FHMLSP Overage, L.L.C., Mr. Cha, Mr. Brush, Mr. Heron and Mr. James Topper disclaims beneficial ownership of the shares other than to the extent of any pecuniary interest they may have therein, directly or indirectly.
| (16) | Will consist of (i) 4,000,000 Holdco Shares held by BCLS II Investco, LP (“BCLS II Investco”) subscribed for in connection with the PIPE Financing, (ii) 4,000,000 Holdco Shares held by BCLS Fund III Investments, LP (“BCLS Fund III”) subscribed for in connection with the PIPE Financing, (iii) 267,429 Holdco Shares held by Bain Capital Life Sciences Fund II, L.P. (“BCLS Fund II”) and (iv) 32,571 Holdco Shares held by BCIP Life Sciences Associates, LP (“BCIPLS” and, together with BCLS II Investco, BCLS Fund III and BCLS Fund II, the “Bain Capital Life Sciences Entities”). Bain Capital Life Sciences Investors, LLC (“BCLSI”) (a) is the manager of Bain Capital Life Sciences Investors II, LLC, which is the general partner of BCLS Fund II, which is the manager of BCLS II Investco (GP), LLC, which is the general partner of BCLS II Investco, (b) is the manager of Bain Capital Life Sciences III General Partner, LLC, which is the general partner of Bain Capital Life Sciences Fund III, L.P., which is the member of BCLS Fund III Investments GP, LLC, which is the general partner of BCLS Fund III, and (c) governs the investment strategy and decision-making process with respect to investments held by BCIPLS. As a result, BCLSI may be deemed to share voting and dispositive power with respect to the securities held by the Bain Capital Life Sciences Entities. The address of the Bain Capital Life Sciences entities is c/o Bain Capital Life Sciences, LP, 200 Clarendon Street, Boston, MA 02116. |
375
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
FLAC Relationships and Related Person Transactions
Promissory Note
On October 7, 2020, Sponsor and FLAC entered into a promissory note (the “Sponsor Promissory Note”) in aggregate principal amount of up to $300,000 to be used for the payment of costs related to the FLAC IPO. The Sponsor Promissory Note was non-interest bearing and principal amount outstanding was payable in full on the earlier of (1) December 31, 2021 and (2) the date of the FLAC IPO. FLAC borrowed $83,000 under the Sponsor Promissory Note and the full amount was repaid on December 14, 2020.
Founder Shares
On October 7, 2020, prior to the FLAC IPO, the Sponsor purchased 2,875,000 Founder Shares for an aggregate purchase price of $25,000. On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of Robert F. Baltera, Michael F. Bigham, Carol G. Gallagher, Krishna R. Polu and David Topper, as adjusted by the share sub-division described below. On December 8, 2020, FLAC effected a share sub-division, resulting in there being an aggregate of 3,450,000 Founder Shares outstanding.
Each of the FLAC Initial Shareholders has agreed, for no additional consideration, to waive such holder’s redemption rights with respect to the Founder Shares and FLAC Public Shares held by the FLAC Initial Shareholders in connection with the consummation of the Business Combination. 501,000 outstanding FLAC Class A Ordinary Shares issued as part of the FLAC Private Placement Units will be excluded from the pro rata calculation used to determine the per-share redemption price. Currently, the FLAC Initial Shareholders own approximately 19% of the issued and outstanding FLAC Ordinary Shares, including all of the Founder Shares. The FLAC Initial Shareholders have agreed to vote any Founder Shares and FLAC Public Shares owned by them in favor of the Business Combination and the Transactions. The Founder Shares are subject to transfer restrictions. The FLAC Articles of Association includes a conversion adjustment which provides that the Founder Shares will automatically convert at the time of the Business Combination into a number of FLAC Class A Ordinary Shares one day after the closing of the Business Combination, at a conversion rate that entitles the FLAC Initial Shareholders to continue to own, in the aggregate, approximately 19% of the issued and outstanding FLAC Ordinary Shares after giving effect to the PIPE Financing. However, the FLAC Initial Shareholders have agreed to waive such conversion adjustment pursuant to the Sponsor Support Agreement. As a result, each remaining Founder Share will be exchanged for one Holdco Share at the closing of the Business Combination, such that the FLAC Initial Shareholders will hold approximately 11% (on a fully diluted basis) of the total number of Holdco Shares outstanding after the consummation of the Business Combination.
Private Placement Units
Simultaneously with the closing of the FLAC IPO, FLAC consummated the private placement of 501,000 units, at a price of $10.00 per FLAC Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5 million. Each FLAC Private Placement Unit consists of one FLAC Class A Ordinary Share and one-third of one FLAC Private Placement Warrant. Each FLAC Private Placement Warrant entitles the holder to purchase one FLAC Class A Ordinary Share for $11.50 per share. If FLAC does not consummate a business combination by December 11, 2022 or receive shareholder approval to extend its term, FLAC will be wound up and the FLAC Private Placement Warrants will expire worthless. The FLAC Private Placement Warrants are substantially similar to the FLAC Public Warrants, except that if held by the Sponsor or its permitted transferees, the FLAC Private Placement Warrants (i) may be exercised for cash or on a cashless basis, (ii) are not subject to being called for redemption (except in certain circumstances when the FLAC Public Warrants are called for redemption and a certain price per FLAC Class A Ordinary Share threshold is met) and (iii) subject to certain limited exceptions, including the FLAC Class A Ordinary Shares issuable upon exercise of the FLAC Private Placement Warrants and the FLAC Private Placement Units, will be subject to transfer
376
Table of Contents
restrictions until 30 days following the consummation of an initial business combination. If the FLAC Private Placement Warrants are held by holders other than the Sponsor or its permitted transferees, the FLAC Private Placement Warrants will be redeemable by FLAC in all redemption scenarios and exercisable by holders on the same basis as the FLAC Public Warrants.
FLAC IPO
Frazier Life Sciences X, L.P. purchased 1,000,000 FLAC Public Units in the FLAC IPO at the public offering price of $10.00 per FLAC Public Unit, generating gross proceeds of approximately $10 million. Each FLAC Public Unit consists of one FLAC Class A Ordinary Share and one-third of one FLAC Public Warrant. Each FLAC Public Warrant entitles the holder to purchase one FLAC Class A Ordinary Share for $11.50 per share. Frazier Life Sciences X, L.P. is the sole member of the Sponsor. FHMLS X, L.P. is the general partner of Frazier Life Sciences X, L.P. and FHMLS X, L.L.C. is the general partner of FHMLS X, L.P. Patrick J. Heron and James N. Topper are the members of FHMLS X, L.L.C. Further, David Topper and Gordon Empey have a pecuniary interest in the general partner of Frazier Life Sciences X, L.P.
Registration and Shareholder Rights
The holders of the Founder Shares, FLAC Private Placement Units, FLAC Private Placement Shares, FLAC Private Placement Warrants, FLAC Class A Ordinary Shares underlying the FLAC Private Placement Warrants and units that may be issued upon conversion of working capital loans, if any, hold registration rights pursuant to the Registration Rights Agreement. The holders of these securities are entitled to make up to three demands that FLAC register the Private Placement Warrants, FLAC Class A Ordinary Shares underlying the Private Placement Warrants and FLAC Class B Ordinary Shares. In addition, the holders have certain unlimited “piggy-back” registration rights with respect to certain registration statements filed by FLAC subsequent to its completion of an initial business combination and rights to require FLAC to register for resale such securities pursuant to Rule 415 under the Securities Act provided that the holders propose to sell securities at an aggregate offering price of at least $10 million. However, the Registration Rights Agreement provides that FLAC will not permit any registration statement filed under the Securities Act to become effective until termination of the applicable lock up period. FLAC will bear the expenses incurred in connection with the filing of any such registration statements.
Further, the holders of the Founder Shares, FLAC Private Placement Units, FLAC Private Placement Shares, FLAC Private Placement Warrants, and FLAC Class A Ordinary Shares underlying the Private Placement Warrants received certain shareholder rights which will become effective upon the consummation of the Business Combination and continue so long as the Sponsor holds any registrable securities. These rights include, the right of the Sponsor to designate up to six individuals to be nominated to serve as directors of the FLAC Board. FLAC is required to use its best efforts to take all necessary and desirable actions so that the Sponsor’s nominees remain on the FLAC Board.
At the closing of the Business Combination, the Registration Rights Agreement will automatically terminate pursuant to its terms and Holdco will enter into the Investor Rights Agreement, pursuant to which the Sponsor will be entitled to certain registration rights with respect to Holdco Shares. The Investor Rights Agreement will provide registration rights to the FLAC Initial Shareholders who will hold an aggregate of 4,951,000 Holdco Shares following the consummation of the Business Combination. For more information about the Investor Rights Agreement, please see the section entitled “The Business Combination Agreement and Ancillary Documents—Investor Rights Agreement.” The 4,500,000 Holdco Shares acquired by affiliates of the Sponsor as described below under the heading “—PIPE Subscription,” will also receive registration rights as provided for in the Subscription Agreement.
Administrative Services Agreement
On December 8, 2020, FLAC entered into an administrative services agreement pursuant to which it agreed to pay the Sponsor a total of $10,000 per month for office space, utilities and administrative support. At the closing of the Business Combination, such agreement will automatically terminate pursuant to its terms.
Letter Agreement
On December 8, 2020, FLAC entered into a letter agreement with the Sponsor and each executive officer and director of FLAC, pursuant to which each of the Sponsor and each executive officer and director of FLAC
377
Table of Contents
has agreed to vote any Founder Shares and FLAC Public Shares held by him, her or it in favor of the Company’s initial business combination; to facilitate the liquidation and winding up of the Company if an initial business combination is not consummated within 24 months of the closing of the FLAC IPO; to certain transfer restrictions with respect to FLAC’s securities; to certain indemnification obligations of the Sponsor; and FLAC has agreed not to enter into a definitive agreement regarding an initial business combination without the prior consent of the Sponsor. At the closing of the Business Combination, the letter agreement will automatically terminate pursuant to its terms. The Sponsor and each executive officer and director of FLAC also agreed to waive any redemption rights, in connection with the Business Combination. The Founder Shares held by the FLAC Initial Shareholders have no redemption rights upon the liquidation of FLAC and will be worthless if no business combination is effected by FLAC by December 11, 2022 or such later date as may be approved by FLAC’s shareholders.
PIPE Subscription
Frazier Life Sciences X, L.P., Frazier Life Sciences XI, L.P., Frazier Life Sciences Public Fund, L.P. and Frazier Life Sciences Overage Fund, L.P. have committed to purchase 4,500,000 Holdco Shares (for a purchase price of $10.00) in the PIPE Financing, concurrently and in connection with the closing of the Business Combination. This transaction will be on the same terms as the other investors who have agreed to purchase shares in the PIPE Financing pursuant to certain subscription agreements dated July 25, 2022. Frazier Life Sciences X, L.P. is the sole member of the Sponsor and Jamie Topper has voting discretion in each of Frazier Life Sciences X, L.P., Frazier Life Sciences Overage Fund, L.P., Frazier Life Sciences XI, L.P. and Frazer Life Sciences Public Fund, L.P. Further, each of Jamie Topper, David Topper and Gordon Empey have a pecuniary interest in the general partner of each of the foregoing funds.
Sponsor Support Agreement
In connection with the execution of the Business Combination Agreement, the FLAC Initial Shareholders, FLAC, Holdco and NewAmsterdam Pharma entered into the Support Agreement, pursuant to which the FLAC Initial Shareholders have agreed to vote (i) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal (as defined in the Business Combination Agreement), (ii) in favor of any other matter reasonably necessary or required to cause the consummation of the Transactions, and (iii) against any proposal that conflicts or materially impedes or interferes with, or would adversely affect or delay the consummation of the Transactions; (b) waive any adjustment to the conversion ratio set forth in FLAC’s amended and restated memorandum and articles of association or any other anti-dilution or similar protection with respect to the FLAC Class B Ordinary Shares held by them; and (c) waive any redemption rights, including with respect to FLAC Class A Ordinary Shares purchased in the FLAC IPO or in the aftermarket, in connection with the Business Combination.
Indemnification of Officers and Directors
Cayman Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against willful default, willful neglect, civil fraud or the consequences of committing a crime. FLAC’s amended and restated memorandum and articles of association provides for indemnification of its officers and directors to the maximum extent permitted by law, including for any liability incurred in their capacities as such, except through their own actual fraud, willful default or willful neglect. FLAC has entered into agreements with each of its directors and officers to provide contractual indemnification in addition to the indemnification provided for in our amended and restated memorandum and articles of association.
In addition, FLAC has purchased a policy of directors’ and officers’ liability insurance that insures its officers and directors against the cost of efense, settlement or payment of a judgment in some circumstances and insures FLAC against its obligations to indemnify its officers and directors.
378
Table of Contents
Policy for Approval of Related Party Transactions
The audit committee of the FLAC Board adopted a charter, providing for the review, approval and/or ratification of “related party transactions,” which are those transactions required to be disclosed pursuant to Item 404 of Regulation S-K as promulgated by the SEC, by the audit committee. At its meetings, the audit committee are provided with the details of each new, existing, or proposed related party transaction, including the terms of the transaction, any contractual restrictions that FLAC has already committed to, the business purpose of the transaction, and the benefits of the transaction to FLAC and to the relevant related party. Any member of the audit committee who has an interest in the related party transaction under review by the audit committee shall abstain from voting on the approval of the related party transaction, but may, if so requested by the chairman of the audit committee, participate in some or all of the audit committee’s discussions of the related party transaction. Upon completion of its review of the related party transaction, the audit committee may determine to permit or to prohibit the related party transaction.
Holdco Relationships and Related Person Transactions
Convertible Loan Agreement
On July 2, 2020, NewAmsterdam Pharma, as borrower, entered into the Convertible Loan Agreement, granting NewAmsterdam Pharma up to €17 million across three tranches of €5.7 million each. NewAmsterdam Pharma drew down on the first tranche on July 2, 2020 and the second tranche on October 12, 2020. In January 2021, the Convertible Loan Agreement was terminated and the full amount of outstanding principal amount and unpaid interest was converted into 1,111,155 shares of NewAmsterdam Pharma’s Series A Preferred Shares in connection with the entry into the Series A Subscription Agreement. The amounts outstanding under the Convertible Loan Agreement were converted into Series A Preferred Shares at a discount to the Series A Preferred Shares subscription price per share of €14 (the “Subscription Price”) representing a price per share equal to approximately €10.50 (equal to 75% of the Subscription Price) (the “Discounted Subscription Price”). See “—Series A Financing” below for more information.
Chief Executive Officer Loan Agreement
On June 28, 2021, NewAmsterdam Pharma entered into a loan agreement with Dr. Davidson (the “Davidson Loan Agreement”) pursuant to which NewAmsterdam Pharma loaned Dr. Davidson €708,571 for the purpose of allowing him to purchase 285,714 depositary receipts from Stichting Administratiekantoor EPNAP described below. The loan under the Davidson Loan Agreement bore interest at a per annum rate of 2.75%. The loan was required to be repaid upon the earlier of (i) the tenth anniversary of the effective date of the Davidson Loan Agreement and (ii) an exit, as defined in the Davidson Loan Agreement. Dr. Davidson had the ability to prepay the outstanding principal and accrued interest, in full or in part, with NewAmsterdam Pharma’s consent. On July 19, 2022, Dr. Davidson repaid the entire outstanding principal amount and all accrued interest under the Davidson Loan Agreement.
Chief Executive Officer Award Agreement
On June 28, 2021, NewAmsterdam Pharma entered into a founder depositary receipt award agreement with Stichting Administratiekantoor EPNAP and Dr. Davidson (the “Davidson Award Agreement”) pursuant to which Stichting Administratiekantoor EPNAP issued 285,714 depositary receipts for NewAmsterdam Pharma’s non-voting convertible shares held by Stichting Administratiekantoor EPNAP (the “Davidson Depositary Receipts”) at an aggregate subscription price of €708,571 to be paid in accordance with the Davidson Loan Agreement. Dr. Davidson retained ownership of 25% of the Davidson Depositary Receipts after twelve months from August 1, 2020 and the remaining 75% vests with 1/36th per month over the following three years.
Series A Financing
On December 30, 2020, NewAmsterdam Pharma entered into the Series A Subscription Agreement to issue Series A Preferred Shares at the Subscription Price for certain investors and the Discounted Subscription Price
379
Table of Contents
for the parties to the Convertible Loan Agreement to set off against the Convertible Loan, for up to an aggregate amount of €160 million, set to occur in two tranches. In the first tranche, NewAmsterdam Pharma issued and sold 4,928,573 Series A Preferred Shares for gross proceeds of €69 million. The first tranche closed in January 2021 (the “First Closing”). In accordance with the terms of the Convertible Loan Agreement, €11.7 million of outstanding principal amount and unpaid interest was automatically converted into 1,111,155 Series A Preferred Shares at the First Closing. NewAmsterdam Pharma was entitled to cause the investors to subscribe for the second tranche Series A Preferred Shares upon the occurrence of certain clinical development and business development milestones. NewAmsterdam Pharma issued the second tranche of Series A Preferred Shares in February 2022 resulting in gross proceeds to us of €80 million and the issuance of 5,691,430 additional Series A Preferred Shares (the “Milestone Closing”).
The table below sets forth the number of Series A Preferred Shares subscribed for by NewAmsterdam Pharma’s directors, executive officers and holders of more than 10% of its capital stock in the Initial Closing. Certain of the related parties listed in the tables below subscribed for Series A Preferred Shares at the Discounted Subscription Price.
| Investor | Number of Series A Preferred Shares | Total Subscription Price | ||||||
Michael Davidson (1) | 64,784 | € | 680,240 | |||||
Forbion Capital Fund II Coöperatief U.A. (1) | 64,784 | € | 680,240 | |||||
Forbion Capital Fund IV Coöperatief U.A.(1) | 981,587 | € | 10,306,667 | |||||
Forbion Capital Fund IV Coöperatief U.A. | 357,143 | € | 5,000,000 | |||||
Forbion Growth Opportunities Fund I Coöperatief U.A. | 714,286 | € | 10,000,000 | |||||
| (1) | The Series A Preferred Shares were received upon the conversion of the outstanding principal amount and interest under the Convertible Loan Agreement into Series A Preferred Shares at the Discounted Subscription Price. |
In February 2022, in connection with the Milestone Closing, NewAmsterdam Pharma’s directors, executive officers and holders of more than 10% of its capital stock subscribed for the number of Series A Preferred Shares set out in the table below. The Milestone Closing was contingent on the achievement of certain milestones, namely: at least 50% LDL lowering in the ongoing obicetrapib and ezetimibe Phase 2 combination trial; the hiring of a full-time chief business officer; and confirmation by the FDA that no CVOT would be required for regulatory approval in the United States. After achieving the first two of these milestones, we proceeded with closing the Milestone Closing on February 17, 2022 as permitted by the terms of the Series A Subscription Agreement. Because the Milestone Closing was closed without having achieved all of the milestones, we were obligated to pursue a strategic transaction, including a business combination with a special purpose acquisition company.
| Investor | Shares of Series A Preferred | Total Subscription Price | ||||||
Michael Davidson | 24,286 | € | 340,000 | |||||
Forbion Capital Fund II Coöperatief U.A. | 24,286 | € | 340,000 | |||||
Forbion Capital Fund IV Coöperatief U.A. | 1,071,429 | € | 15,000,000 | |||||
Forbion Growth Opportunities Fund I Coöperatief U.A. | 714,286 | € | 10,000,000 | |||||
Morningside Venture Investments Limited | 1,071,429 | € | 15,000,000 | |||||
Ascendant BioCapital SPV I, LCC—Series 1 (1) | 357,143 | € | 5,000,000 | |||||
| (1) | Gaurav Gupta was serving on the NewAmsterdam Pharma Board at the time of the Milestone Closing. |
Immediately prior to the Exchange, the Series A Preferred Shares will convert at a 1:1 ratio into shares of NewAmsterdam Pharma ordinary shares and thereafter be contributed in the Exchange for Holdco Shares.
380
Table of Contents
NewAmsterdam Pharma Shareholders’ Agreement
On January 11, 2021, all of the then existing shareholders of NewAmsterdam Pharma entered into the Fully Amended and Restated Shareholders’ Agreement (as amended or supplemented from time to time, the “Shareholder Agreement”). The Shareholder Agreement contains provisions related to the governance of NewAmsterdam Pharma, including memorializing the right of certain investors in NewAmsterdam Pharma to appoint and dismiss members of the NewAmsterdam Pharma Board. Two positions on the NewAmsterdam Pharma Board were reserved for the chief executive officer and chief scientific officer, initially Drs. Michael Davidson and John Kastelein. Jason Dinges, Gaurav Gupta, Sander Slootweg and Juliette Audet were initially appointed by NewAmsterdam Pharma’s investors as non-executive directors on the NewAmsterdam Pharma Board.
The Shareholder Agreement also contains transfer restrictions, preemptive rights, co-sale rights, drag along rights and registration rights, among other matters. The Shareholder Agreement will terminate immediately after giving effect to the Exchange.
PoolCo Shareholders’ Agreements
On March 12, 2021, Wester Investments B.V., BioGeneration Ventures II B.V., BioGeneration II Co-Invest II B.V., BGV II Coöperatief U.A., Forbion Capital Fund II Coöperatief U.A., WestCT B.V., LSP Dementia Fund Coöperatieve U.A., Diomedea Medical B.V., LouFré Holding B.V. incorporated NAP PoolCo B.V. (“PoolCo”). The purpose of this entity is holding ordinary shares, non-voting convertible shares, Series A Preferred Shares in the capital of NewAmsterdam Pharma, including any depositary receipts issued for such shares, or options for these depositary receipts (the “Participations”).
The shareholders of PoolCo, PoolCo, NewAmsterdam Pharma, Stichting Administratiekantoor NewAmsterdam Pharma and Stichting Administratiekantoor EPNAP (the latter two, the “STAKs”) entered into a shareholders agreement (the “PoolCo SHA”) to govern, among others, the participation of the shareholders of PoolCo, their indirect participation in NewAmsterdam Pharma, board composition, transfer restrictions, future issuance of Participations and distributions.
In connection with the consummation of the Business Combination, the PoolCo SHA will be amended (i) to allow and reflect the Exchange, (ii) to reflect the termination of the Shareholder Agreement, and (iii) to incorporate certain other minor amendments.
Intercompany Agreements
In connection with an internal reorganization NewAmsterdam Pharma entered into various intercompany agreements with its subsidiaries, as described below.
Contract Take-Over and Services Agreement
On December 10, 2021, NewAmsterdam Pharma and Dezima entered into a contract take-over agreement (the “Contract Take-Over Agreement”) pursuant to which NewAmsterdam Pharma assigned all of its rights and obligations under its agreements with third parties to Dezima, and Dezima accepted the assignment, so that Dezima became the primary operating entity with the Group Companies. In connection with the transfer of contracts pursuant to the Contract Take-Over Agreement, NewAmsterdam Pharma and Dezima also entered into a service agreement (the “NAP Service Agreement”). The NAP Service Agreement provides that NewAmsterdam Pharma is responsible for providing Dezima administrative support, including, without limitation, financial, information technology and human resources support. NewAmsterdam Pharma provides the services on a cost-plus basis. Either party may terminate the NAP Service Agreement at any time upon one-month’s prior notice.
381
Table of Contents
Intercompany Agreement and IP Assignment
In October 2021, NewAmsterdam Pharma and Dezima entered into an assignment agreement (the “IP Assignment Agreement”) whereby NewAmsterdam assigned to Dezima a pending patent application for obicetrapib. On December 4, 2020, NewAmsterdam Pharma, Dezima and NewAmsterdam Pharma Corporation entered into an intercompany agreement (the “Intercompany Agreement”) pursuant to which various activities, including management, research and development, regulatory affairs and production, among others, were divided among the parties. The agreement specifically provides that Dezima will continue to own all of the intellectual property, including all right, title and interest in and to any and all data, results, inventions, discoveries and material, as well as the intellectual property rights acquired, conceived, discovered, invented, developed, created, made or reduced to practice in the performance of the services under the Intercompany Agreement. The Intercompany Agreement may be terminated by either party upon 180 days’ prior notice and unless terminated, will continue automatically renewing for successive 1-year terms.
Naarden Lease
On March 1, 2020, NewAmsterdam Pharma and an affiliate of Forbion entered into a services agreement (the “Naarden Lease”) pursuant to which the Forbion affiliate leased office space to NewAmsterdam Pharma. NewAmsterdam Pharma pays €3,333 per month in rent. Between March 2020 and September 2020, NewAmsterdam Pharma received a 25% discount on its monthly rent in recognition of the COVID-19 pandemic.
Either party may terminate the Naarden Lease upon one month’s prior notice to the other, and with immediate effect in the event the other party is in serious breach and has not cured within a reasonable period of time, but in no event late than 14 days after receiving notice of such breach.
PIPE Subscription
Forbion Growth Opportunities Fund I Coöperatief U.A., Forbion Capital Fund IV Coöperatief U.A. and Morningside Venture Investments Limited, each of whom is an existing investor in NewAmsterdam Pharma, have committed to purchase an aggregate of 3,500,000 Holdco Shares (for a purchase price of $35 million) in the PIPE Financing, concurrently and in connection with the closing of the Business Combination. This transaction will be on the same terms as the other investors who have agreed to purchase shares in the PIPE Financing pursuant to certain subscription agreements dated July 25, 2022. The 3,500,000 Holdco Shares received by Forbion Growth Opportunities Fund I Coöperatief U.A., Forbion Capital Fund IV Coöperatief U.A. and Morningside Venture Investments Limited will receive registration rights pursuant to the terms of the Subscription Agreement.
Company Support Agreement
In connection with the execution of the Business Combination Agreement, Holdco, NewAmsterdam Pharma, FLAC, Merger Sub, and certain existing shareholders of NewAmsterdam Pharma (which shareholders only include directors, executive officers and shareholders with voting securities in NewAmsterdam Pharma representing 5% or more of all such voting securities) entered into the Company Support Agreement (included herein as Annex E) pursuant to which, among other things, each such existing shareholder of NewAmsterdam Pharma (a) granted or will grant, as applicable, NewAmsterdam Pharma (or a designee thereof) with a power of attorney permitting and directing NewAmsterdam Pharma to execute on behalf of such shareholder a Dutch deed of issue to effect the Exchange with respect to the shares of NewAmsterdam Pharma held by such shareholder, (b) undertook or will undertake, as applicable, vis-à-vis NewAmsterdam Pharma, Holdco, FLAC and each other existing shareholder of NewAmsterdam Pharma to take all necessary or desirable actions in connection with the transactions set forth in the Business Combination Agreement, (c) agreed to vote in favor of the approval of the Business Combination Agreement, the Exchange and any other matters necessary or reasonably requested by NewAmsterdam Pharma to consummate the transactions contemplated in the Business Combination Agreement, and (d) agreed to certain customary covenants to support the Business Combination (including restrictions on the sale, disposition or transfer of the shares of NewAmsterdam Pharma held by him, her or it).
382
Table of Contents
Investor Rights Agreement
At the closing of the Business Combination, Holdco will enter into the Investor Rights Agreement with the FLAC Initial Shareholders and certain NewAmsterdam Pharma shareholders, the form of which is included herein as Annex G, providing for, among other things, subject to the terms thereof, customary registration rights, including demand and piggy-back rights subject to cut-back provisions. Holdco has agreed to file a registration statement to register the Holdco Shares covered by the Investor Rights Agreement no later than 30 days following consummation of the Business Combination.
Pursuant to the Investor Rights Agreement, certain NewAmsterdam Pharma shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares such persons receive in connection with the Business Combination for six months from the Final Closing Date of the Business Combination, subject to certain limited exceptions. In addition, the FLAC Initial Shareholders will agree not to sell, assign, offer to sell, contract, pledge, grant, or otherwise dispose of or enter into any swap or other similar arrangement, with respect to the Holdco Shares they receive in connection with the Business Combination for a period beginning on the Final Closing Date and ending one year after the Final Closing Date of the Business Combination. Notwithstanding the foregoing, the restrictions above will end prior to the indicated time periods with respect to 50% of the Holdco Shares the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination, on the earlier of the date that (i) the closing price of a Holdco Share equals or exceeds $12.00 per share (subject to certain adjustments) for any 20 trading days within any 30-day trading period commencing at least 150 days after the Final Closing Date of the Business Combination, or (ii) Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The restrictions on the remaining 50% of the Holdco Shares each of the NewAmsterdam Pharma shareholders and the FLAC Initial Shareholders, as the case may be, receives in connection with the Business Combination will end prior to the periods indicated above on the date that Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of Holdco’s shareholders having the right to exchange their Holdco Shares for cash, securities or other property, subject to certain limited exceptions. The share transfer restrictions above will not apply with respect to sales to cover withholding taxes due upon vesting of equity awards and, in the case of directors or officers of Holdco, with respect to the sale of up to 10% of the Holdco Shares held by each of them. After the closing of the Business Combination, an aggregate of 44, 914,642 Holdco Shares held by current NewAmsterdam Pharma shareholders (including Amgen and MTPC) will have registration rights pursuant to the terms of the Investor Rights Agreement.
Indemnification Agreements
The Holdco Articles of Association will provide for certain indemnification rights for Holdco’s current and former directors and other current and former officers and employees as designated by the Holdco Board. Holdco also expects to enter into indemnification agreements with each of Holdco’s directors and executive officers providing for procedures for indemnification and advancements by Holdco of certain expenses and costs relating to claims, suits or proceedings arising from his or her service to Holdco or, at Holdco’s request, service to other entities, as officers or directors to the maximum extent permitted by Dutch law and subject to the exceptions provided in such agreements.
Review, Approval or Ratification of Transactions with Related Persons
Upon completion of the Business Combination, Holdco intends to adopt a related party transaction policy that will require the review and, if applicable, approval or ratification of any related party transaction by the Holdco Board, its audit committee or another designated committee consisting solely of independent directors. Holdco’s audit committee will review this related party transaction policy periodically and will recommend changes to the Holdco Board as appropriate.
383
Table of Contents
In addition, under Dutch law and the Holdco Articles of Association, Holdco directors may not take part in any discussion or decision-making that involves a subject or transaction in relation to which he or she has a direct or indirect personal conflict of interest with Holdco. Such a conflict of interest would generally arise if the director concerned is unable to serve Holdco’s interests and the business connected with Holdco with the required level of integrity and objectivity due to the existence of the conflicting personal interest. The Holdco Articles of Association provide that if as a result of conflicts of interests no resolution of the Holdco Board can be adopted, the resolution may nonetheless be adopted by the Holdco Board as if none of the Holdco directors had a conflict of interest. In that latter case, each Holdco director is entitled to participate in the discussion and decision-making process and to cast a vote.
384
Table of Contents
COMPARISON OF CORPORATE GOVERNANCE AND SHAREHOLDER RIGHTS
This section describes the material differences between the rights of FLAC shareholders before the consummation of the Business Combination, and the rights of Holdco shareholders after the Business Combination. These differences in shareholder rights result from the differences between the law of the Cayman Islands and the Netherlands and the respective governing documents of FLAC and Holdco.
This section does not include a complete description of all differences among such rights, nor does it include a complete description of such rights. Furthermore, the identification of some of the differences of these rights as material is not intended to indicate that other differences that may be equally important do not exist. FLAC shareholders are urged to carefully read the relevant provisions of the Cayman Islands Companies Act, the Dutch Civil Code, the Dutch Corporate Governance Code, the FLAC Articles of Association and the Holdco Articles of Association that are expected to be in effect prior to or promptly following Closing. References in this section to the Holdco Articles of Association are references to the form attached as an English translation of the official Dutch text as Annex I hereto. However, the Holdco Articles of Association may be amended at any time prior to consummation of the Business Combination by mutual agreement of FLAC and NewAmsterdam Pharma or after the consummation of the Business Combination by amendment in accordance with its terms. If the Holdco Articles of Association are amended, the below summary may cease to be an accurate reflection of the provisions thereof.
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| Authorized Capital | ||
| FLAC is authorized to issue up to 479,000,000 FLAC Class A Ordinary Shares of a par value of $0.0001 each, (ii) 20,000,000 FLAC Class B Ordinary Shares of a par value of $0.0001 each and (iii) 1,000,000 preference shares of a par value of $0.0001 each. As of June 30, 2022, there were 14,301,000 FLAC Class A Ordinary Shares, 3,450,000 FLAC Class B Ordinary Shares, and no preferred shares issued and outstanding. | As of the Closing, the Holdco Articles of Association will provide for an authorized share capital that will be approximately (but no more than) five (5) times the number of Holdco Shares outstanding immediately following such closing. The Holdco Shares will have a nominal value of €0.12. | |
| Voting Rights | ||
| The FLAC Articles of Association provide that the holders of shares of FLAC shall have one vote for every share of which he or she is the holder. | In accordance with Dutch law and the Holdco Articles of Association, each issued Holdco Share confers the right to cast one vote at the Holdco General Meeting.
The Holdco Articles of Association do not provide for quorum requirements generally applicable to Holdco General Meetings, which is common for Dutch listed N.V. companies.
Resolutions at the Holdco General Meeting can be adopted irrespective of the number of issued Holdco Shares present or represented at such Holdco General Meeting, subject to any provision of mandatory Dutch law. | |
| Appraisal and Dissenters’ Rights | ||
| Under certain circumstances, shareholders may dissent to a merger of a Cayman Islands company by following the procedure set out in the Cayman Islands Companies | Subject to certain exceptions, Dutch law does not recognize the concept of appraisal or dissenters’ rights. However, Dutch law does provide for | |
385
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| Act. Where dissenter rights apply, dissenters to a merger are entitled to receive fair market value for their shares. | squeeze-out procedures as described under “Description of Holdco Securities—Dividends and Other Distributions—Squeeze-Out Procedures.” Also, Dutch law provides for cash exit rights in certain situations for dissenting shareholders of a company organized under Dutch law entering into certain types of mergers. In those situations, a dissenting shareholder may file a claim with the Dutch company for compensation. Such compensation shall then be determined by one or more independent experts. The shares of such shareholder that are subject to such claim will cease to exist as of the moment of entry into effect of the merger. | |
| Dividends | ||
| The directors of FLAC may resolve to pay dividends and other distributions on shares in issue and authorize payment of the dividends or other distributions. Dividends may be paid out of profits, share premium or any other sources permitted under Cayman Islands law. | Under Dutch law, Holdco may only pay dividends and other distributions from its reserves to the extent Holdco’s shareholders’ equity (eigen vermogen) exceeds the sum of Holdco’s paid-in and called-up share capital plus the reserves Holdco must maintain under Dutch law or the Holdco Articles of Association and (if it concerns a distribution of profits) after adoption of Holdco’s statutory annual accounts by Holdco’s General meeting from which it appears that such dividend distribution is allowed. Subject to those restrictions, any future determination to pay dividends or other distributions from Holdco’s reserves will be at the discretion of the Holdco Board and will depend upon a number of factors, including Holdco’s Results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors we deem relevant. See the section “Description of Holdco Securities—Dividends and Other Distributions.”
Dividends and other distributions shall be made payable no later than a date determined by Holdco. Claims to dividends and other distributions not made within five years from the date that such dividends or distributions became payable will lapse and any such amounts will be considered to have been forfeited to Holdco (verjaring). | |
| Purchase and Repurchase of Shares | ||
| Subject to the Cayman Islands Companies Act or applicable stock exchange or other regulatory rules, FLAC may purchase its own shares (including any redeemable shares) in such manner and on such other | Under Dutch law, when issuing shares, a public company such as Holdco may not subscribe for newly issued shares in its own capital. Such company may, however, subject to certain restrictions of Dutch law and its articles of association, acquire shares in | |
386
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| terms as the directors determine at the time of such purchase. | its own capital. A listed public company such as Holdco may acquire fully paid shares in its own capital at any time for no valuable consideration. Furthermore, subject to certain provisions of Dutch law and its articles of association, such company may repurchase fully paid shares in its own capital if (i) the company’s shareholders’ equity (eigen vermogen) less the payment required to make the acquisition does not fall below the sum of paid-in and called-up share capital plus any reserves required by Dutch law or its articles of association and (ii) the aggregate nominal value of shares of the company which the company acquires, holds or on which the company holds a pledge (pandrecht) or which are held by a subsidiary of the company, would not exceed 50% of its then-current issued share capital.
In addition, an acquisition by Holdco of Holdco Shares for a consideration must be authorized by the Holdco General Meeting. Such authorization may be granted for a maximum period of 18 months and must specify the number of Holdco Shares that may be acquired, the manner in which Holdco Shares may be acquired and the price limits within which shares may be acquired. The actual acquisition may only be effected pursuant to a resolution of the Holdco Board.
The Holdco Board will be authorized for a period of 18 months following the completion of the Holdco Reorganization to cause the repurchase of Holdco Shares (or depository receipts for Holdco Shares) by Holdco of up to 10% of Holdco’s issued share capital, for a price per share not exceeding 110% of the average market price of Holdco Shares on Nasdaq (such average market price being the average of the closing prices on each of the five consecutive trading days preceding the date the acquisition is agreed upon by Holdco), provided that, until the Holdco Shares are listed on a stock exchange, the maximum purchase price shall be 110% of the original issue price of the Holdco Shares concerned.
No authorization of the Holdco General Meeting is required if fully paid Holdco Shares are acquired by Holdco with the intention of transferring such Holdco Shares to its employees under an applicable employee share purchase plan. | |
| Redemption Rights | ||
| Upon consummation of the Business Combination, the FLAC Articles of Association provide holders of the FLAC Class A Ordinary Shares with the opportunity to | Holders of Holdco Shares will not have redemption rights in connection with the Business Combination. | |
387
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
redeem their FLAC Class A Ordinary Shares at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account as of two business days prior to the consummation of the Business Combination, including interest (net of taxes payable), divided by the number of then-outstanding FLAC Class A Ordinary Shares, provided that FLAC shall not repurchase FLAC Class A Ordinary Shares in an amount that would cause FLAC’s net tangible assets to be less than $5,000,001.
If FLAC seeks to amend any provision of the FLAC Articles of Association that would affect the substance or timing of FLAC’s obligation to redeem 100% of the public shareholders’ FLAC Class A Ordinary Shares if FLAC has not consummated an initial business combination within twenty-four months after the date of the closing of the FLAC IPO, FLAC must provide public shareholders with the opportunity to redeem their FLAC Class A Ordinary Shares in connection with such vote. FLAC will redeem the public shareholders’ FLAC Class A Ordinary Shares and liquidate if it does not complete a business combination by December 11, 2022 or such later date as may be approved by FLAC’s shareholders.
After consummation of the initial business combination, holders of FLAC Class A Ordinary Shares are not entitled to redemption rights with respect to their FLAC Class A Ordinary Shares. | ||
| Issuance of Shares | ||
| Under Cayman Islands law, and the FLAC Articles of Association, the directors have general and unconditional authority to allot (with or without confirming rights of renunciation), issue, grant options over or otherwise deal with any unissued shares of FLAC to such persons, at such times and on such terms and conditions as they may decide, save that under Article 3.1, the directors may not allot, issue, grant options over otherwise deal with any unissued shares to the extent it may affect the ability of FLAC to carry out a Class B Share Conversion described at Article 12. Without limitation to the preceding, the directors may so deal with the unissued shares of FLAC (a) either at a premium or at par and (b) with without preferred, deferred other special rights or restrictions whether in regard to dividend, voting, return of capital of otherwise. FLAC may issue units of securities in FLAC, which may be comprised of shares, rights, options, warrants or convertible securities of similar nature conferring the | Under Dutch law, a company’s general meeting is the corporate body authorized to resolve on the issuance of shares and the granting of rights to subscribe for shares. The general meeting can delegate such authority to another corporate body of the company for a period not exceeding five years; this authorization may only be extended from time to time for a maximum period of five years and unless stated otherwise in such delegation, this delegation cannot be revoked.
Prior to the consummation of the Business Combination, the Holdco Board will be authorized for a period of five years from the completion of the Holdco Reorganization to issue shares or grant rights to subscribe for shares up to Holdco’s authorized share capital from time to time. Holdco may not subscribe for its own shares on issue. | |
388
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| right upon the holders thereof to subscribe for, purchase or receive any class of shares or other securities in FLAC, on such terms and conditions as the directors may decide. However, Article 38.11 provides that after the issue of public shares (including pursuant to the over-allotment option), and prior to the consummation of a business combination, the directors shall not issue additional shares or any other securities that would entitle the holders thereof to (a) receive funds from the trust account; or (b) vote as a class with the public shares: (i) on a business combination or on any other proposal presented to members prior to or in connection with the completion of a business combination; or (ii) to approve an amendment to the FLAC Articles of Association to: (A) extend the time FLAC has to consummate a business combination beyond twenty- four months after the closing of the IPO or twenty-seven months after the closing of the IPO if FLAC has executed a letter of intent, agreement in principle or definitive agreement or an initial business combination within twenty-four months from the closing of IPO; or (B) amend the foregoing provisions of the FLAC Articles of Association. | ||
| Preemptive Rights | ||
| None. | Under Dutch law, in the event of an issuance of shares, each shareholder will have a pro rata pre-emption right in proportion to the aggregate nominal value of the shares held by such holder (except in case of an issue of shares to employees, against a contribution other than in cash or pursuant to the exercise of a previously acquired right to subscribe for shares). Under the Holdco Articles of Association, the pre-emption rights in respect of newly issued shares may be restricted or excluded by a resolution of the Holdco General Meeting. Another corporate body may restrict or exclude the pre-emption rights in respect of newly issued shares if it has been designated as the authorized body to do so by the Holdco General Meeting. Such designation can be granted for a period not exceeding five years. A resolution of the Holdco General Meeting to restrict or exclude the pre-emption rights or to designate another corporate body as the authorized body to do so requires a majority of not less than two-thirds of the votes cast, if less than one-half of the issued share capital is represented at the meeting.
Prior to the consummation of the Business Combination, the Holdco Board will be authorized for a period of five years from the completion of the Holdco Reorganization to limit or exclude | |
389
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| pre-emption rights in relation to an issuance of shares or a grant of rights to subscribe for shares that the Holdco Board is authorized to resolve upon. See above under “Issuance of Shares.” | ||
| Amendments to Governing Documents | ||
| Amendment of any provision of the FLAC Articles of Association requires a special resolution, meaning a resolution passed by holders of at least two-thirds of the outstanding FLAC Ordinary Shares that are entitled to vote and that do attend and vote at a general meeting. The Sponsor and FLAC’s executive officers and directors have agreed that they will not propose any amendment to the FLAC Articles of Association that would affect the substance or timing of FLAC’s obligation to redeem 100% of its public shares if FLAC does not complete its initial business combination by December 11, 2022 (24 months after the closing of the FLAC IPO), unless FLAC provides public shareholders with the opportunity to redeem their shares upon approval of any such amendment. | At the proposal of the Holdco Board, the Holdco General Meeting may resolve to amend the Holdco Articles of Association. | |
| Number of Directors | ||
| The FLAC Articles of Association provide that, unless otherwise determined by a vote of a majority of the FLAC Ordinary Shares voted, the minimum number of directors shall be one and the maximum shall be ten. | The Holdco Board shall consist of such number of Holdco executive directors and Holdco non-executive directors as the Holdco Board may determine. | |
| Classes of Directors | ||
The FLAC Articles of Association provide the directors shall be divided into three classes: Class I, Class II and Class III. The number of directors in each class shall be as nearly equal as possible.
The Class I directors were elected for a term which expired at FLAC’s first annual general meeting, the Class II directors stand elected for a term expiring at FLAC’s second annual general meeting and the Class III directors stand elected for a term expiring at FLAC’s third annual general meeting. Commencing at FLAC’s first annual general meeting, and at each annual general meeting thereafter, directors elected to succeed those directors whose terms expire shall be elected for a term of office to expire at the third succeeding annual general meeting after their election. | Upon the consummation of the Business Combination each Holdco director will serve staggered terms of up to four years as a result of which only part of the Holdco directors may be subject to appointment or re-appointment in any given year. | |
| Nomination of Directors | ||
| The FLAC Articles of Association provide that shareholders seeking to nominate candidates for election as directors at the annual general meeting must deliver | Under Dutch law, directors are appointed and re-appointed by the general meeting. | |
390
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| notice to the principal executive officers of FLAC not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the scheduled date of the annual general meeting. |
Under the Holdco Articles of Association, the Holdco directors will be appointed by the Holdco General Meeting upon binding nomination by the Holdco Board. However, the Holdco General Meeting may at all times overrule a binding nomination by a resolution adopted by at least a two-thirds majority of the votes cast, provided such majority represents more than half of the issued share capital. If the Holdco General Meeting overrules a binding nomination, the Holdco Board shall make a new nomination. | |
| Election of Directors | ||
| The FLAC Articles of Association provide that prior to the initial business combination, a vote of the majority of the FLAC Class B Ordinary Shares outstanding will be required to appoint any person as director of FLAC. After an initial business combination, a vote of a majority of the FLAC Ordinary Shares outstanding would be required to appoint any person as director of FLAC. The directors of FLAC may appoint any person to be an additional director provided that the appointment does not cause the number of directors to exceed any number fixed as the maximum number of directors. | See above under “Nomination of Directors.” | |
| Removal of Directors | ||
The FLAC Articles of Association provide that a director may be removed if:
(i) they are prohibited by the law of the Cayman Islands from acting as a director; or
(ii) they are made bankrupt or makes an arrangement or composition with his creditors generally; or
(iii) in the opinion of a registered medical practitioner by whom they are being treated they become physically or mentally incapable of acting as a director; or
(iv) they are made subject to any law relating to mental health or incompetence, whether by court order or otherwise; or
(v) without the consent of the other directors, they are absent from meetings of directors for a continuous period of six months; or
(vi) all of the other directors (being not less than two in number) determine that they should be removed as a director, either by a resolution passed by all of the other directors at a meeting of the directors duly | The general meeting shall at all times be entitled to suspend or dismiss a director. Under the Holdco Articles of Association, the Holdco General Meeting may only adopt a resolution to suspend or dismiss a Holdco director by at least a two-thirds majority of the votes cast, provided that such majority represents more than half of Holdco’s issued share capital, unless the resolution is passed at the proposal of the Holdco Board, in which latter case a simple majority of the votes cast is sufficient. If a Holdco director is suspended and the Holdco General Meeting does not resolve to dismiss him or her within three months from the date of such suspension, the suspension shall lapse. | |
391
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
convened and held in accordance with the FLAC Articles of Association or by a resolution in writing signed by all of the other directors.
The FLAC Articles of Association provides that prior to the Business Combination, a vote of a majority of the FLAC Class B Ordinary Shares will be required to remove a director. After the Business Combination, a vote of a majority of the FLAC Ordinary Shares outstanding will be required to remove any person as director of FLAC. | ||
| Filling of Board Vacancies | ||
| The FLAC Articles of Association provide that the directors may appoint any person as a director of FLAC to fill a vacancy. | The Holdco Board can temporarily fill vacancies caused by temporary absence or incapacity of a director without requiring a shareholder vote. If all of the Holdco directors are absent or incapacitated, the authority to temporarily exercise Holdco’s management power shall be vested with the person who most recently ceased to hold office as the chairperson of the Holdco Board, provided that if such former chairperson is unwilling or unable to accept that position, the authority to temporarily exercise Holdco’s management power shall be vested with the person who most recently ceased to hold office as Holdco’s Chief Executive Officer. If such former Chief Executive Officer is also unwilling or unable to accept that position, the authority to temporarily exercise Holdco’s management power shall be vested with one or more persons whom the Holdco General Meeting has designated for that purpose. The person(s) charged with Holdco’s management in this manner may designate one or more persons to be charged with Holdco’s management instead of, or together with, such person(s). | |
| Compensation of Directors | ||
| The FLAC Articles of Association provide that the directors shall determine any compensation of the directors; provided, that no compensation shall be paid to any director prior to the consummation of the initial business combination. | Dutch law does not provide for limitations with respect to the aggregate annual compensation paid to the Holdco directors, provided that such compensation is consistent with Holdco’s compensation policy. Such compensation policy will be adopted by Holdco’s General Meeting prior to the consummation of the Business Combination, to be effective upon Closing. Changes to such compensation policy will require a vote of the Holdco General Meeting by simple majority of votes cast. The Holdco Board determines the remuneration of individual directors with due observance of the compensation policy. A proposal with respect to | |
392
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
remuneration schemes in the form of shares or rights to shares in which Holdco directors may participate is subject to approval by the Holdco General Meeting by simple majority of votes cast. Such a proposal must set out at least the maximum number of shares or rights to subscribe for shares to be granted to the Holdco directors and the criteria for granting or amendment.
Holdco’s compensation policy will authorize the Holdco Board to determine the amount, level and structure of the compensation packages of the Holdco directors at the recommendation of Holdco’s compensation committee. These compensation packages may consist of a mix of fixed and variable compensation components, including base salary, short-term incentives, long-term incentives, fringe benefits, severance pay and pension arrangements, as determined by the Holdco Board. | ||
| Manner of Acting by Board | ||
| The FLAC Articles of Association provide that the affirmative vote by a majority of votes at a meeting of the directors is an act by the FLAC Board. | Each Holdco executive director and each Holdco non-executive director shall have one vote. Resolutions of the Holdco Board shall be passed, irrespective of whether this occurs at a meeting or otherwise, by simple majority, unless the rules applicable to the Holdco Board provide differently. The chairperson of the Holdco Board shall have a casting vote in case of a tied vote at the Holdco Board.
The Holdco executive directors shall not participate in the decision-making concerning:
i. the determination of the compensation of the Holdco executive directors; and
ii. the instruction of an auditor to audit the annual accounts if the Holdco General Meeting has not granted such instruction. | |
| Special Meetings of the Board | ||
| The FLAC Articles of Association provide that a director may, and the secretary of FLAC on the direction of a director shall, call a meeting of the directors by at least five days’ notice to every director. Notice may be waived. | The Holdco Board shall meet as often as any Holdco director deems necessary or appropriate. | |
| Director Action by Written Consent | ||
| The FLAC Articles of Association provide that a resolution in writing signed by all the directors shall be valid and effectual as if it had been passed at a meeting of the directors so long as they receive notice as if the act were being proposed at a meeting. | Resolutions of the Holdco Board may, instead of at a board meeting, be passed in writing, provided that all Holdco directors are familiar with the resolution to be passed and none of them objects to this decision-making process. | |
393
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| Annual Shareholders’ Meetings | ||
| The FLAC Articles of Association provide that to the extent required by the rules and regulations of the national securities exchange upon which the FLAC Ordinary Shares are listed, the SEC and/or any other competent regulatory authority, an annual general meeting of FLAC shall be held no later than one year after the first financial year end occurring after the FLAC IPO, and shall be held in each year thereafter at such time as determined by the directors and FLAC may, but shall not (unless required by the law or the rules and regulations of the national securities exchange upon which the FLAC Ordinary Shares are listed, the SEC and/or any other competent regulatory authority) be obliged to, in each year hold any other general meeting. | Annually, at least one Holdco General Meeting shall be held. This annual Holdco General Meeting must be held within six months of the end of each financial year and must be held in the Netherlands, in any of the locations specified in the Holdco Articles of Association. | |
| Special Shareholders’ Meetings | ||
| The FLAC Articles of Association provides that a general meeting may be called by the directors at any time and shall be called on the proper requisition of a member of FLAC. | Additional extraordinary Holdco General Meetings may also be held, whenever considered appropriate by the Holdco Board and shall be held within three months after the Holdco Board considers it to be likely that Holdco’s shareholders’ equity (eigen vermogen) has decreased to an amount equal to or lower than half of Holdco’s paid-in and called up share capital, in order to discuss the measures to be taken if so required.
Pursuant to Dutch law, one or more shareholders or others with meeting rights under Dutch law who jointly represent at least one-tenth of Holdco’s issued share capital may request Holdco to convene a general meeting, setting out in detail the matters to be discussed. If the Holdco Board has not taken the steps necessary to ensure that such meeting can be held within six weeks after the request, the proponent(s) may, on their application, be authorized by the competent Dutch court in preliminary relief proceedings to convene a Holdco General Meeting. The court shall disallow the application if it does not appear that the proponent(s) has/have previously requested the Holdco Board to convene a Holdco General Meeting and the Holdco Board has not taken the necessary steps so that the Holdco General Meeting could be held within six weeks after the request. The application shall also be disallowed if the proponent(s) has/have not demonstrated to have a reasonable interest in the convening of the Holdco General Meeting.
The agenda shall include such items as have been included therein by the Holdco Board. The agenda shall also include such items requested by one or | |
394
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
more shareholders or others with meeting rights under Dutch law representing at least 3% of Holdco’s issued share capital. These requests must be made in writing or by electronic means and received by the Holdco Board at least 60 days before the day of the meeting. No resolutions shall be adopted on items other than those that have been included in the agenda.
In accordance with the DCGC, shareholders who have the right to put an item on the agenda for the Holdco General Meeting or to request the convening of a Holdco General Meeting shall not exercise such rights until after they have consulted the Holdco Board. If exercising such rights may result in a change in Holdco’s strategy (for example, through the dismissal of one or more Holdco directors), the Holdco Board must be given the opportunity to invoke a reasonable period of up to 180 days to respond to the shareholders’ intentions. If invoked, the Holdco Board must use such response period for further deliberation and constructive consultation, in any event with the shareholder(s) concerned and exploring alternatives. At the end of the response time, the Holdco Board shall report on this consultation and the exploration of alternatives to the Holdco General Meeting. The response period may be invoked only once for any given Holdco General Meeting and shall not apply (i) in respect of a matter for which a response period has been previously invoked or (ii) if a shareholder holds at least 75% of Holdco’s issued share capital as a consequence of a successful public bid.
Moreover, the Holdco Board can invoke a cooling-off period of up to 250 days when shareholders, using their right to have items added to the agenda for a Holdco General Meeting or their right to request a Holdco General Meeting, propose an agenda item for the Holdco General Meeting to dismiss, suspend or appoint one or more Holdco directors (or to amend any provision in the Holdco Articles of Association dealing with those matters) or when a public offer for Holdco is made or announced without Holdco’s support, provided, in each case, that the Holdco Board believes that such proposal or offer materially conflicts with the interests of Holdco and its business. During a cooling-off period, the Holdco General Meeting cannot dismiss, suspend or appoint Holdco directors (or amend the provisions in the Holdco Articles of Association dealing with those matters) except at the proposal of the Holdco Board. During a cooling-off period, the Holdco Board must gather all relevant information necessary for a careful |
395
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
decision-making process and consult with shareholders representing at least 3% or more of Holdco’s issued share capital at the time the cooling-off period was invoked, as well as with Holdco’s Dutch works council (if Holdco or, under certain circumstances, any of Holdco’s subsidiaries would have one). Formal statements expressed by these stakeholders during such consultations must be published on Holdco’s website to the extent these stakeholders have approved that publication. Ultimately one week following the last day of the cooling-off period, the Holdco Board must publish a report in respect of its policy and conduct of affairs during the cooling-off period on Holdco’s website. This report must remain available for inspection by shareholders and others with meeting rights under Dutch law at Holdco’s office and must be tabled for discussion at the next Holdco General Meeting. Shareholders representing at least 3% of Holdco’s issued share capital may request that the Enterprise Chamber terminate the cooling-off period early. The Enterprise Chamber must rule in favor of the request if the shareholders can demonstrate that:
• the Holdco Board, in light of the circumstances at hand when the cooling-off period was invoked, could not reasonably have concluded that the relevant proposal or hostile offer constituted a material conflict with the interests of Holdco and its business;
• the Holdco Board cannot reasonably believe that a continuation of the cooling-off period would contribute to careful policy-making; or
• other defensive measures, having the same purpose, nature and scope as the cooling-off period, have been activated during the cooling-off period and have not since been terminated or suspended within a reasonable period at the relevant shareholders’ request (i.e., no ‘stacking’ of defensive measures). | ||
| Advance Notice Requirements for Shareholder Nominations and Other Proposals | ||
The FLAC Articles of Association provide that members holding at least 40% of the rights to vote at a general meeting may provide a requisition to hold an extraordinary general meeting. Such members’ requisition must:
(i) specify the purpose of the meeting; | Pursuant to Dutch law, one or more shareholders or others with meeting rights under Dutch law who jointly represent at least one-tenth of Holdco’s issued share capital may request Holdco to convene a general meeting, setting out in detail the matters to be discussed in the manner described above under Special Shareholders’ Meetings. | |
396
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
(ii) be signed by or on behalf of each requisitioner; and
(iii) be delivered in accordance with the notice provisions.
The FLAC Articles of Association provide that members seeking to bring business before the annual general meeting must deliver notice to the principal executive officers of FLAC not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the scheduled date of the annual general meeting. |
In addition, pursuant to Dutch law, one or more shareholders or others with meeting rights under Dutch law who jointly represent at least 3% of Holdco’s issued share capital may require the addition of items on the agenda of any Holdco General Meeting, provided such request is made in the manner described above under Special Shareholders’ Meetings.
These rights to request a Holdco General Meeting or to submit agenda items for a Holdco General Meeting are subject to the response period and cooling-off period arrangements discussed above under Special Shareholders’ Meetings. | |
| Notice and Record Date of Shareholders’ Meetings | ||
| The FLAC Articles of Association requires that notice of a general meeting be given not less than five days before the date of the meeting. The notice must state (i) the place, date and hour of the meeting (ii) if the meeting is to be held in two or more places, the technology that will be used to facilitate the meeting; (iii) subject to paragraph (iv), the general nature of the business to be transacted; and (v) if a resolution is proposed as a special resolution, the text of that resolution. | A notice convening a Holdco General Meeting will be made in accordance with Dutch law and in such other manner as may be required to comply with any applicable rules of Nasdaq and any other stock exchange on which Holdco Shares are listed. The record date for the Holdco General Meeting will, if set by the Holdco Board, be 28 days prior to the date of such Holdco General Meeting. | |
| Quorum and Actions | ||
| The FLAC Articles of Association provide that business may only be transacted at a general meeting if a quorum is present, such quorum being one or more shareholders who together hold one-third (or, in respect of any meeting convened to consider a Business Combination, 50%) of the shares entitled to vote as of the record date at such meeting. | Under the Holdco Articles of Association no quorum requirement applies to the Holdco General Meeting generally. | |
| Shareholder Action Without Meeting | ||
| The FLAC Articles of Association provide that action of the shareholders may be taken by unanimous written consent in lieu of a meeting. | Under Dutch law, shareholders’ resolutions may be adopted in writing without holding a meeting of shareholders, provided that (i) the articles of association allow such action by written consent, (ii) the company has not issued bearer shares or, with its cooperation, depository receipts for shares in its capital, and (iii) the resolution is adopted unanimously by all shareholders that are entitled to vote. Although the Holdco Articles of Association allow for shareholders’ resolutions to be adopted in writing, the requirement of unanimity renders the adoption of shareholder resolutions without holding a meeting not feasible for Holdco since it concerns a publicly traded company. | |
397
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| Indemnification of Directors and Officers | ||
The FLAC Articles of Association provide that each current and former director and officer of FLAC (which includes an investment adviser or liquidator) and their personal representatives shall be indemnified against:
(i) all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by the existing or former secretary or officer in or about the conduct of FLAC’s business or affairs or in the execution or discharge of the existing or former secretary’s or officer’s duties, powers, authorities or discretions; and
(ii) without limitation to paragraph (i), all costs, expenses, losses or liabilities incurred by the existing or former secretary or officer in defending (whether successfully or otherwise) any civil, criminal, administrative or investigative proceedings (whether threatened, pending or completed) concerning FLAC or its affairs in any court or tribunal, whether in the Cayman Islands or elsewhere.
No such existing or former secretary or officer, however, shall be indemnified in respect of any matter arising out of his own actual fraud, willful default or willful neglect. | Under Dutch law, the Holdco directors may be held liable for damages in the event of improper or negligent performance of their duties. They may be held liable for damages to Holdco and to third parties for violations of the Holdco Articles of Association or of certain provisions of Dutch law. In certain cases (notably in the event of a personal culpable act of a Holdco director), the Holdco directors may be held liable for damages to Holdco, Holdco shareholders or even third parties. In certain circumstances, the Holdco directors may also incur other specific civil and criminal liabilities. Subject to certain exceptions, the Holdco Articles of Association provide for indemnification of Holdco’s current and former directors and other current and former officers and employees as designated by the Holdco Board. No indemnification under the Holdco Articles of Association shall be given to an indemnified person:
• if a competent court or arbitral tribunal has established, without having (or no longer having) the possibility for appeal, that the acts or omissions of such indemnified person that led to the financial losses, damages, expenses, suit, claim, action or legal proceedings as described above are of an unlawful nature (including acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such indemnified person);
• to the extent that his or her financial losses, damages and expenses are covered under insurance and the relevant insurer has settled, or has provided reimbursement for, these financial losses, damages and expenses (or has irrevocably undertaken to do so);
• in relation to proceedings brought by such indemnified person against Holdco, except for proceedings brought to enforce indemnification to which he or she is entitled under the Holdco Articles of Association, pursuant to an agreement between such indemnified person and Holdco which has been approved by Holdco or pursuant to insurance taken out by Holdco for the benefit of such indemnified person; and | |
398
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
• for any financial losses, damages or expenses incurred in connection with a settlement of any proceedings effected without Holdco’s prior consent.
Under the Holdco Articles of Association, the Holdco Board may stipulate additional terms, conditions and restrictions in relation to the indemnification described above. | ||
| Limitation on Liability of Directors | ||
| The FLAC Articles of Association provide that FLAC may by special resolution release any existing or former director (including alternate director), secretary or other officer of FLAC from liability for any loss or damage or right to compensation which may arise out of or in connection with the execution or discharge of the duties, powers, authorities or discretions of his office; but there may be no release from liability arising out of or in connection with that person’s own actual fraud, willful default or willful neglect. | In principle, the Holdco Articles of Association provide for indemnification of Holdco’s current and former directors and other current and former officers and employees as designated by the Holdco Board. However, the Holdco Articles of Association will not provide for indemnification:
• if a competent court or arbitral tribunal has established, without having (or no longer having) the possibility for appeal, that the acts or omissions of such indemnified person that led to the financial losses, damages, expenses, suit, claim, action or legal proceedings as described above are of an unlawful nature (including acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such indemnified person);
• to the extent that the indemnified person’s financial losses, damages and expenses are covered under insurance and the relevant insurer has settled, or has provided reimbursement for, these financial losses, damages and expenses (or has irrevocably undertaken to do so);
• in relation to proceedings brought by such indemnified person against Holdco, except for proceedings brought to enforce indemnification to which the indemnified person is entitled under the Holdco Articles of Association, pursuant to an agreement between such indemnified person and Holdco which has been approved by Holdco or pursuant to insurance taken out by Holdco for the benefit of such indemnified person; and
• for any financial losses, damages or expenses incurred in connection with a settlement of any proceedings effected without Holdco’s prior consent. | |
399
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
Under the Holdco Articles of Association, the Holdco Board may stipulate additional terms, conditions and restrictions in relation to the indemnification described above. | ||
| Dissolution/Liquidation | ||
| The FLAC Articles of Association provide that in the event that FLAC does not consummate a business combination by twenty-four months after the closing of the FLAC IPO, FLAC shall: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the FLAC Ordinary Shares issued in the FLAC IPO, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account (less taxes payable and up to $100,000 of interest to pay liquidation expenses), divided by the number of then outstanding FLAC Ordinary Shares issued in the FLAC IPO, which redemption will completely extinguish public members’ rights as members (including the right to receive further liquidation distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of FLAC’s remaining members and the FLAC Board, liquidate and dissolve, subject in each case to its obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law. | Under the Holdco Articles of Association, Holdco may be dissolved by a resolution of the Holdco General Meeting, subject to a proposal of the Holdco Board. In the event of a dissolution, the liquidation shall be effected by the Holdco Board, unless the Holdco General Meeting decides otherwise. During liquidation, the provisions of the Holdco Articles of Association will remain in force as far as possible. To the extent that any assets remain after payment of all of Holdco’s liabilities, any remaining assets shall be distributed to Holdco’s shareholders in proportion to their number of Holdco Shares. | |
| Rights of Inspection | ||
| The FLAC Articles of Association provide that no member (not being a director) shall have any right of inspecting any account or book or document of FLAC except as conferred by the Companies Act of the Cayman Islands or authorized by the directors or by FLAC in general meeting. | The Holdco Board must provide the Holdco General Meeting all information that it requires, unless this would be contrary to an overriding interest of Holdco. If the Holdco Board invokes such an overriding interest, it must give reasons to the Holdco General Meeting. | |
| Derivative Shareholder Suits | ||
| FLAC’s Cayman Islands counsel is not aware of any reported class action having been brought in a Cayman Islands court. Derivative actions have been brought in the Cayman Islands courts, and the Cayman Islands courts have confirmed the availability for such actions. In most cases, the company will be the proper plaintiff in any claim based on a breach of duty owed to it, and a claim against (for example) FLAC’s officers or directors usually may not be brought by a shareholder. However, based on English authorities, which would in all likelihood be of persuasive authority and be applied by a | In the event a third-party is liable to a Dutch company, only the company itself can bring a civil action against that party. The individual shareholders do not have the right to bring an action on behalf of the company. Only in the event that the cause for the liability of a third-party to the company also constitutes a tortious act directly against a shareholder, that shareholder has an individual right of action against such third-party in its own name. Dutch law provides for the possibility to initiate such actions collectively, in which a foundation or an association can act as a class representative and | |
400
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
court in the Cayman Islands, exceptions to the foregoing principle apply in circumstances in which:
• a company is acting, or proposing to act, illegally or beyond the scope of its authority;
• the act complained of, although not beyond the scope of the authority, could be effected if duly authorized by more than the number of votes which have actually been obtained; or
• those who control the company are perpetrating a “fraud on the minority.” A shareholder may have a direct right of action against FLAC where the individual rights of that shareholder have been infringed or are about to be infringed. | has standing to commence proceedings and claim damages if certain criteria are met. The court will first determine if those criteria are met. If so, the case will go forward as a class action on the merits after a period allowing class members to opt out from the case has lapsed. All members of the class who are residents of the Netherlands and who did not opt-out will be bound to the outcome of the case. Residents of other countries must actively opt in in order to be able to benefit from the class action. The defendant is not required to file defenses on the merits prior to the merits phase having commenced. It is possible for the parties to reach a settlement during the merits phase. Such a settlement can be approved by the court, which approval will then bind the members of the class, subject to a second opt-out. This new regime applies to claims brought after January 1, 2020 and which relate to certain events that occurred prior to that date. For other matters, the old Dutch class actions regime will apply. Under the old regime, no monetary damages can be sought. Also, a judgment rendered under the old regime will not always bind all individual class members. Even though Dutch law does not provide for derivative suits, Holdco’s directors and officers can still be subject to liability under U.S. securities laws.
The Holdco Articles of Association provide that, unless Holdco consents in writing to the selection of an alternative forum, the sole and exclusive forum for any complaint asserting a cause of action arising under the U.S. Securities Act of 1933, as amended, or the U.S. Securities Exchange Act of 1934, as amended, to the fullest extent permitted by applicable law, shall be the U.S. federal district courts. | |
| Conflict of Interest Transactions | ||
The FLAC Articles of Association provide that, unless permitted by the FLAC Articles of Association, a director may not have a direct or indirect interest or duty which conflicts or may possibly conflict with the interests of FLAC.
However, the FLAC Articles of Association provide that if a director discloses to their fellow directors the nature and extent of any material interest or duty in accordance with the FLAC Articles of Association they may:
(a) be a party to, or otherwise interested in, any transaction or arrangement with FLAC or in which FLAC is or may otherwise be interested; or
(b) be interested in another body corporate promoted by FLAC or in which FLAC is otherwise interested. In particular, the director may be a | Under Dutch law and the Holdco Articles of Association, the Holdco directors shall not take part in any discussion or decision-making that involves a subject or transaction in relation to which he or she has a direct or indirect personal conflict of interest with Holdco. Such a conflict of interest would generally arise if a Holdco director is unable to serve Holdco’s interests and the business connected with it with the required level of integrity and objectivity due to the existence of the conflicting personal interest. The Holdco Articles of Association provide that if as a result of conflicts of interests no resolution of the Holdco can be adopted, the resolution may nonetheless be adopted by the Holdco as if none of the Holdco directors had a conflict of interest. In that latter case, each Holdco director is | |
401
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
director, secretary or officer of, or employed by, or be a party to any transaction or arrangement with, or otherwise interested in, that other body corporate. | entitled to participate in the discussion and decision-making process and to cast a vote.
The DCGC provides the following best practice recommendations in relation to conflicts of interests in respect of Holdco directors:
• A Holdco director should report any conflict of interest or potential conflict of interest in a transaction that is of material significance to Holdco and/or to such person to the chairperson of the Holdco Board without delay and should provide all relevant information in that regard, including the relevant information pertaining to his or her spouse, registered partner or other life companion, foster child and relatives by blood or marriage up to the second degree. If the chairperson of the Holdco Board has a conflict of interest or potential conflict of interest, he or she should report this to the vice-chairperson of the Holdco Board without delay.
• The Holdco Board should decide, outside the presence of the director concerned, whether there is a conflict of interest.
• All transactions in which there are conflicts of interest with directors should be agreed on terms that are customary in the market.
Decisions to enter into transactions in which there are conflicts of interest with Holdco directors that are of material significance to Holdco and/or to the relevant directors should require the approval of the Holdco Board. Such transactions should be published in Holdco’s statutory annual report, together with a description of the conflict of interest. | |
| If a director has made such a disclosure, then they shall not, by reason only of their office, be accountable to FLAC for any benefit that he derives from any such transaction or arrangement or from any such office or employment or from any interest in any such body corporate, and no such transaction or arrangement shall be liable to be avoided on the ground of any such interest or benefit. | ||
| Listing | ||
| The FLAC Public Units, FLAC Public Shares and FLAC Public Warrants trade on Nasdaq. | Holdco will apply to have the Holdco Shares and Holdco Public Warrants trade on Nasdaq. | |
402
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
| Anti-Takeover Provisions | ||
Subject to the provisions of the Companies Act of the Cayman Islands, the FLAC Articles of Association and the rules of Nasdaq, the directors have general and unconditional authority to allot (with or without confirming rights of renunciation), issue, grant options over or otherwise deal with any unissued FLAC Ordinary Shares to such persons, at such times and on such terms and conditions as they may decide, save that the directors may not allot, issue, grant options over or otherwise deal with any unissued Shares to the extent that it may affect the ability of FLAC to carry out a Class B Share Conversion as described in the FLAC Articles of Association.
The directors may so deal with the unissued FLAC Ordinary Shares:
(a) either at a premium or at par; or
(b) with or without preferred, deferred or other special rights or restrictions whether in regard to dividend, voting, return of capital or otherwise.
FLAC may issue rights, options, warrants or convertible securities or securities of similar nature conferring the right upon the holders thereof to subscribe for, purchase or receive any class of FLAC Ordinary Shares or other securities in FLAC at such times and on such terms and conditions as the directors may decide. | Under Dutch law, various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law. In this respect, certain provisions of the Holdco Articles of Association may make it more difficult for a third-party to acquire control of us or effect a change in the composition of the Holdco Board. These include:
• a provision that the Holdco directors can only be appointed on the basis of a binding nomination prepared by the Holdco Board which can only be overruled by a two-thirds majority of votes cast representing more than half of Holdco’s issued share capital;
• a provision that Holdco directors can only be dismissed by the Holdco General Meeting by a two-thirds majority of votes cast representing more than half of Holdco’s issued share capital, unless the dismissal is proposed by the Holdco Board in which latter case a simple majority of the votes cast would be sufficient;
• a provision allowing, among other matters, the former chairperson of the Holdco Board or the former Chief Executive Officer to manage Holdco’s affairs if all of the Holdco directors are dismissed and to appoint others to be charged with Holdco’s affairs, including the preparation of a binding nomination for the Holdco directors as discussed above, until new Holdco directors are appointed by the Holdco General Meeting on the basis of such binding nomination; and
• a requirement that certain matters, including an amendment of the Holdco Articles, may only be resolved upon by the Holdco General Meeting if proposed by the Holdco Board.
Dutch law also allows for staggered multi-year terms of the Holdco directors, as a result of which only part of the Holdco directors may be subject to appointment or re-appointment in any given year. | |
403
Table of Contents
Rights of FLAC Shareholders | Rights of Holdco Shareholders | |
Furthermore, the Holdco Board may, under certain circumstances invoke a reasonable period of up to 180 days to respond to certain shareholder proposals or a statutory cooling-off period of up to 250 days to respond to certain shareholder proposals or a hostile bid. See above under “Shareholders Meeting.” |
404
Table of Contents
PRICE RANGE OF SECURITIES AND DIVIDENDS
FLAC
Price Range of FLAC’s Securities
The FLAC Units, each of which consists of one FLAC Class A Ordinary Share and one-third of one FLAC warrant to acquire one FLAC Class A Ordinary Share, began trading on Nasdaq under the symbol “FLACU” on December 11, 2020. On January 29, 2021, FLAC announced that holders of its FLAC Public Units could elect to separately trade the FLAC Class A Ordinary Shares and FLAC Public Warrants. On January 29, 2021, the FLAC Class A Ordinary Shares and FLAC Public Warrants began trading on Nasdaq under the symbols “FLAC” and “FLACW,” respectively.
On July 22, 2022, the trading date before the public announcement of the Business Combination, the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants closed at $9.93, $9.90 and $0.2235, respectively. As of October 17, 2022, the date immediately prior to the date of this proxy statement/prospectus, the closing price for each FLAC Public Unit, FLAC Class A Ordinary Share, and FLAC Public Warrant was $10.18, $10.00 and $0.70, respectively.
Holders of the FLAC Public Units, FLAC Class A Ordinary Shares and FLAC Public Warrants should obtain current market quotations for their securities. The market price of FLAC’s securities could vary at any time before the Business Combination.
Holders
There are 2 holders of record of the FLAC Public Units, 1 holder of record of the separately traded FLAC Class A Ordinary Shares, and 1 holder of record of the separately traded FLAC Public Warrants.
Dividend Policy
FLAC has not paid any cash dividends on its FLAC Class A Ordinary Shares to date and does not intend to pay cash dividends prior to the completion of the Business Combination.
NewAmsterdam
Price Range of NewAmsterdam Pharma Securities
Historical market price information regarding NewAmsterdam Pharma’s ordinary shares is not provided because they do not have a public market.
Dividend Policy
NewAmsterdam Pharma has never declared or paid any cash dividends on its ordinary shares.
Holdco
Price Range of Holdco’s Securities
Historical market price information regarding Holdco is not provided because, as of the date of this proxy statement/prospectus, there is no public market for the Holdco Shares.
405
Table of Contents
Dividend Policy
Holdco has never declared or paid any cash dividends on its shares. It currently intends to retain all available funds and any future earnings for use in the operation of its business and does not anticipate paying any dividends on the Holdco ordinary shares in the foreseeable future. Consequently, you may be unable to realize a gain on your investment except by selling such shares after price appreciation, which may never occur.
Holdco’s board of directors may only pay dividends and other distributions from its reserves to the extent Holdco’s shareholders’ equity (eigen vermogen) exceeds the sum of our paid-in and called-up share capital plus the reserves it must maintain under Dutch law or its articles of association and (if it concerns a distribution of profits) after adoption of its statutory annual accounts by its general meeting from which it appears that such dividend distribution is allowed. Subject to those restrictions, any future determination to pay dividends or other distributions from Holdco’s reserves will be at the discretion of its board and will depend upon a number of factors, including its results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the Holdco Board deems relevant.
406
Table of Contents
FUTURE SHAREHOLDER PROPOSALS AND NOMINATIONS
Pursuant to the Holdco Articles of Association, any matter of which the discussion or a vote has been requested in writing by one or more persons with meeting rights who, individually or collectively, represent at least 3% percent of the issued share capital shall be included in the convening notice or announced in the same manner, if Holdco has received the substantiated request or a proposal for a resolution no later than on the 60th day prior to that of the general meeting, without prejudice to the Holdco Board’s right to invoke a response period under the DCGC or a cooling-off period under Dutch law (as described in the section entitled “Comparison of Corporate Governance and Shareholder Rights—Advance Notice Requirements for Shareholder Nominations and Other Proposals”).
Shareholders and interested parties may communicate with the FLAC Board, any committee chairperson or the non-management directors as a group by writing to the board or committee chairperson in care of Frazier Lifesciences Acquisition Corporation, Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101. Following the Business Combination, such communications should be sent in care of NewAmsterdam Pharma Company N.V., Gooimeer 2-35, 1411 DC Naarden, The Netherlands, Attn: Secretary. Each communication will be forwarded, depending on the subject matter, to the board of directors, the appropriate committee chairperson or all non-management directors.
NautaDutilh N.V., Dutch counsel to Holdco, has provided a legal opinion for Holdco regarding (i) valid issue, (ii) paying up and (iii) non-assessability of the Holdco Shares offered by this proxy statement/prospectus, based on the assumptions and subject to the qualifications and limitations set out therein. Certain legal matters in connection with this offering relating to U.S. federal law will be passed upon for us by Covington & Burling LLP, New York, New York. Goodwin Procter LLP has provided a legal opinion for Holdco regarding certain U.S. federal income tax consequences. Legal opinions have also been provided by (i) Houthoff Coöperatief U.A. for certain Dutch tax consequences, and (ii) Campbells LLP for certain tax consequences relating to Cayman Islands law, all as set forth in the section titled “Material Tax Considerations” in this proxy statement/prospectus.
The consolidated financial statements of Frazier Lifesciences Acquisition Corporation as of December 31, 2021 and 2020, for the year ended December 31, 2021 and for the period from October 7, 2020 (inception) to December 31, 2020, have been included in this proxy statement/prospectus in reliance upon the report of WithumSmith+Brown, PC, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
The financial statements of NewAmsterdam Pharma Holding B.V. as at December 31, 2021 and 2020, and for each of the two years in the period ended December 31, 2021, included in this proxy statement/prospectus have been audited by Deloitte Accountants B.V., an independent registered public accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
The financial statements of NewAmsterdam Pharma Company B.V. as at June 30, 2022, included in this proxy statement/prospectus have been audited by Deloitte Accountants B.V., an independent registered public accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
407
Table of Contents
Pursuant to the rules of the SEC, FLAC and services that it employs to deliver communications to its shareholders are permitted to deliver to two or more shareholders sharing the same address a single copy of each of FLAC’s annual report to shareholders and FLAC’s proxy statement. Upon written or oral request, FLAC will deliver a separate copy of the annual report to shareholders and/or proxy statement to any shareholder at a shared address to which a single copy of each document was delivered and who wishes to receive separate copies of such documents. Shareholders receiving multiple copies of such documents may likewise request that FLAC delivers single copies of such documents in the future. Shareholders receiving multiple copies of such documents may request that FLAC delivers single copies of such documents in the future. Shareholders may notify FLAC of their requests by calling or writing FLAC at its principal executive offices at Frazier Lifesciences Acquisition Corporation, Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101 or (206) 621-7200.
ENFORCEABILITY OF CIVIL LIABILITY
Holdco is organized under the law of the Netherlands, and certain of the individuals who may be directors and executive officers of Holdco, and certain experts named in this proxy statement/prospectus, reside outside of the United States. All or a substantial portion of the assets of such individuals and of Holdco may be located outside of the United States. As a result, it may not be possible to effect service of process within the United States upon such individuals or Holdco, or to enforce against such individuals or Holdco in United States courts judgments obtained in such courts predicated upon the civil liability provisions of the federal securities laws of the United States. Holdco has been advised by counsel that there is doubt as to the enforceability in the Netherlands, in original actions or in actions for the enforcement of judgments of United States courts, of liabilities predicated solely upon the securities laws of the United States or enforce claims for punitive damages.
The transfer agent for FLAC’s securities is Continental Stock Transfer & Trust Company.
WHERE YOU CAN FIND MORE INFORMATION
FLAC files reports, proxy statements and other information with the SEC as required by the Exchange Act. You may access information on FLAC at the SEC website containing reports, proxy statements and other information at: www.sec.gov. Those filings are also available free of charge to the public on, or accessible through, FLAC’s website at www.frazierlifesciencesacquisition.com. FLAC’s website and the information contained on, or that can be accessed through, the website is not deemed to be incorporated by reference in, and is not considered part of, this proxy statement/prospectus.
If you would like additional copies of this proxy statement/prospectus or FLAC’s other filings with the SEC (excluding exhibits) or if you have questions about the Business Combination or the proposals to be presented at the General Meeting, you should contact FLAC at the following address and telephone number: Frazier Lifesciences Acquisition Corporation, Two Union Square, 601 Union St., Suite 3200, Seattle, WA 98101, Attention: Corporate Secretary, and (206) 621-7200.
You may also obtain additional copies of this proxy statement/prospectus by requesting them from FLAC’s proxy solicitation agent, Morrow Sodali LLC, by calling (800) 662-5200, or banks and brokers can call collect at (203) 658-9400, or by emailing FLAC.info@investor.morrowsodali.com.
Any of the documents you request will be available without charge. If your shares are held in a stock brokerage account or by a bank or other nominee, you should contact your broker, bank or other nominee for additional information.
408
Table of Contents
If you are a FLAC shareholder and would like to request documents, please do so by November 7, 2022, or five business days prior to the General Meeting, in order to receive them before the General Meeting. If you request any documents from FLAC, such documents will be mailed to you by first class mail, or another equally prompt means.
This proxy statement/prospectus is part of a registration statement and constitutes a prospectus of Holdco in addition to being a proxy statement of FLAC for the General Meeting. As allowed by SEC rules, this proxy statement/prospectus does not contain all of the information you can find in the registration statement or the exhibits to the registration statement. Information and statements contained in this proxy statement/prospectus or any Annex to this proxy statement/prospectus are qualified in all respects by reference to the copy of the relevant contract or other annex filed as an exhibit to the registration statement of which this proxy statement/prospectus forms a part, which includes exhibits incorporated by reference from other filings made with the SEC.
All information contained in this document relating to FLAC has been supplied by FLAC and all such information relating to NewAmsterdam Pharma has been supplied by NewAmsterdam Pharma. Information provided by FLAC or NewAmsterdam Pharma does not constitute any representation, estimate or projection of any other party. This document is a proxy statement of FLAC for the General Meeting. FLAC has not authorized anyone to give any information or make any representation about the Business Combination or the parties thereto, including FLAC, that is different from, or in addition to, that contained in this proxy statement/prospectus. Therefore, if anyone does give you information of this sort, you should not rely on it. The information contained in this proxy statement/prospectus speaks only as of the date of this proxy statement/prospectus, unless the information specifically indicates that another date applies.
409
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
Consolidated Financial Statements of Frazier Lifesciences Acquisition Corporation as of and for the years ended December 31, 2021 and 2020 | Page | |||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
Unaudited Interim Financial Statements of Frazier Lifesciences Acquisition Corporation as of and for the six months ended June 30, 2022 and 2021 | ||||
| F-23 | ||||
| F-24 | ||||
| F-25 | ||||
Unaudited Condensed Statement of Cash Flows for the six months ended June 30, 2022 | F-26 | |||
| F-27 | ||||
NEWAMSTERDAM PHARMA HOLDING B.V. AND SUBSIDIARIES
Consolidated Financial Statements of NewAmsterdam Pharma Holding B.V. and Subsidiaries as at December 31, 2021 and for the years ended December 31, 2021 and 2020 | Page | |||
| F-45 | ||||
Consolidated Statements of Profit or Loss and Other Comprehensive Loss | F-46 | |||
| F-47 | ||||
| F-48 | ||||
| F-49 | ||||
| F-50 | ||||
| F-78 | ||||
| F-78 | ||||
| F-79 | ||||
| F-80 | ||||
| F-81 | ||||
Notes to Unaudited Condensed Consolidated Financial Statements | F-82 |
NEWAMSTERDAM PHARMA COMPANY B.V.
| Consolidated Financial Statements of NewAmsterdam Pharma Company B.V. as at June 30, 2022 | Page | |||
| F-95 | ||||
| F-96 | ||||
| F-97 | ||||
F-1
Table of Contents
REPORTOF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Frazier Lifesciences Acquisition Corporation
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Frazier Lifesciences Acquisition Corporation (the “Company”) as of December 31, 2021 and 2020, the related statements of operations, changes in shareholders’ deficit and cash flows for the year ended December 31, 2021 and the period from October 7, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for the year ended December 31, 2021 and the period from October 7, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, if the Company is unable to raise additional funds to alleviate liquidity needs and complete a business combination by December 11, 2022 then the Company will cease all operations except for the purpose of liquidating. The liquidity condition and date for mandatory liquidation and subsequent dissolution raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company’s auditor since 2020.
New York, New York
March 25, 2022
F-2
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
| December 31, | ||||||||
| 2021 | 2020 | |||||||
Assets: | ||||||||
Current assets: | ||||||||
Cash | $ | 1,226,716 | $ | 1,365,094 | ||||
Prepaid expenses | 261,333 | 503,683 | ||||||
|
|
|
| |||||
Total current assets | 1,488,049 | 1,868,777 | ||||||
Investments held in Trust Account | 138,017,009 | 138,000,851 | ||||||
|
|
|
| |||||
Total Assets | $ | 139,505,058 | $ | 139,869,628 | ||||
|
|
|
| |||||
Liabilities, Class A Ordinary Shares Subject to Possible Redemption and Shareholders’ Deficit: | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 167,324 | $ | 162,478 | ||||
Accrued expenses | 54,750 | 74,043 | ||||||
|
|
|
| |||||
Total current liabilities | 222,074 | 236,521 | ||||||
Deferred underwriting commissions | 4,830,000 | 4,830,000 | ||||||
Derivative warrant liabilities | 2,812,530 | 7,341,180 | ||||||
|
|
|
| |||||
Total liabilities | 7,864,604 | 12,407,701 | ||||||
Commitments and Contingencies | ||||||||
Class A ordinary shares subject to possible redemption, $0.0001 par value; 13,800,000 shares issued and outstanding at redemption value of $10.00 per share at December 31, 2021 and 2020 | 138,000,000 | 138,000,000 | ||||||
Shareholders’ Deficit: | ||||||||
Preference shares, $0.0001 par value; 1,000,000 shares authorized; none issued or outstanding at December 31, 2021 and 2020 | — | — | ||||||
Class A ordinary shares, $0.0001 par value; 479,000,000 shares authorized; 501,000 shares issued and outstanding (excluding 13,800,000 shares subject to possible redemption) at December 31, 2021 and 2020 | 50 | 50 | ||||||
Class B ordinary shares, $0.0001 par value; 20,000,000 shares authorized; 3,450,000 shares issued and outstanding at December 31, 2021 and 2020 | 345 | 345 | ||||||
Additional paid-in capital | — | — | ||||||
Accumulated deficit | (6,359,941 | ) | (10,538,468 | ) | ||||
|
|
|
| |||||
Total shareholders’ deficit | (6,359,546 | ) | (10,538,073 | ) | ||||
|
|
|
| |||||
Total Liabilities Class A Ordinary Shares to Possible Redemption and Shareholders’ Deficit | $ | 139,505,058 | $ | 139,869,628 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
| For The Year Ended December 31, 2021 | For The Period From October 7, 2020 (Inception) Through December 31, 2020 | |||||||
General and administrative expenses | $ | 1,246,281 | $ | 120,884 | ||||
Administrative expenses—related party | 120,000 | 6,774 | ||||||
|
|
|
| |||||
Loss from operations | (1,366,281 | ) | (127,658 | ) | ||||
Other income (expenses): | ||||||||
Interest income from investments held in Trust Account | 16,158 | 851 | ||||||
Change in fair value of derivative warrant liabilities | 4,528,650 | 619,710 | ||||||
Break-up fee from terminated agreement | 1,000,000 | — | ||||||
Financing costs—derivative warrant liabilities | — | (451,450 | ) | |||||
|
|
|
| |||||
Net income | $ | 4,178,527 | $ | 41,453 | ||||
|
|
|
| |||||
Weighted average number of Class A ordinary shares-basic and diluted | 14,301,000 | 3,492,105 | ||||||
|
|
|
| |||||
Basic and diluted net income per share, Class A | $ | 0.24 | $ | 0.01 | ||||
|
|
|
| |||||
Weighted average number of Class B ordinary shares-basic | 3,450,000 | 3,109,884 | ||||||
|
|
|
| |||||
Weighted average number of Class B ordinary shares-diluted | 3,450,000 | 3,450,000 | ||||||
|
|
|
| |||||
Basic net income per share, Class B | $ | 0.24 | $ | 0.01 | ||||
|
|
|
| |||||
Diluted net income per share, Class B | $ | 0.24 | $ | 0.01 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
FOR THE YEAR ENDED DECEMBER 31, 2021
| Ordinary Shares | Additional Paid-in Capital | Accumulated Deficit | Total Shareholders’ Deficit | |||||||||||||||||||||||||
| Class A | Class B | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
Balance—December 31, 2020 | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (10,538,468 | ) | $ | (10,538,073 | ) | ||||||||||||||
Net income | — | — | — | — | — | 4,178,527 | 4,178,527 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance—December 31, 2021 | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (6,359,941 | ) | $ | (6,359,546 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
FOR THE PERIOD FROM OCTOBER 7, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| Ordinary Shares | Additional Paid-in Capital | Accumulated Deficit | Total Shareholders’ Deficit | |||||||||||||||||||||||||
| Class A | Class B | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
Balance—October 7, 2020 (inception) | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||
Issuance of Class B ordinary shares to Sponsor | — | — | 3,450,000 | 345 | 24,655 | — | 25,000 | |||||||||||||||||||||
Sale of Private Placement Units, less fair value of derivative warrant liabilities | 501,000 | 50 | — | — | 4,731,060 | — | 4,731,110 | |||||||||||||||||||||
Accretion of Class A ordinary shares subject to possible redemption | — | — | — | — | (4,755,715 | ) | (10,579,921 | ) | (15,335,636 | ) | ||||||||||||||||||
Net income | — | — | — | — | — | 41,453 | 41,453 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance—December 31, 2020 | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (10,538,468 | ) | $ | (10,538,073 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
| For The Year Ended December 31, 2021 | For The Period From October 7, 2020 (Inception) Through December 31, 2020 | |||||||
Cash Flows from Operating Activities: | ||||||||
Net income | $ | 4,178,527 | $ | 41,453 | ||||
Adjustments to reconcile net income to net cash used in operating activities: | ||||||||
General and administrative expenses paid by Sponsor in exchange for issuance of Class B ordinary shares | — | 25,000 | ||||||
General and administrative expenses paid by Sponsor under promissory note | — | 18,200 | ||||||
Interest income from investments held in Trust Account | (16,158 | ) | (851 | ) | ||||
Change in fair value of derivative warrant liabilities | (4,528,650 | ) | (619,710 | ) | ||||
Financing costs—derivative warrant liabilities | — | 451,450 | ||||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses | 242,350 | (503,683 | ) | |||||
Accounts payable | 4,846 | 59,098 | ||||||
Accrued expenses | (19,293 | ) | 29,043 | |||||
|
|
|
| |||||
Net cash used in operating activities | (138,378 | ) | (500,000 | ) | ||||
|
|
|
| |||||
Cash Flows from Investing Activities: | ||||||||
Cash deposited in Trust Account | — | (138,000,000 | ) | |||||
|
|
|
| |||||
Net cash used in investing activities | — | (138,000,000 | ) | |||||
|
|
|
| |||||
Cash Flows from Financing Activities: | ||||||||
Repayment of note payable to related party | — | (82,906 | ) | |||||
Proceeds received from initial public offering, gross | — | 138,000,000 | ||||||
Proceeds received from private placement | — | 5,010,000 | ||||||
Offering costs paid | — | (3,062,000 | ) | |||||
|
|
|
| |||||
Net cash provided by financing activities | — | 139,865,094 | ||||||
|
|
|
| |||||
Net change in cash | (138,378 | ) | 1,365,094 | |||||
Cash—beginning of the period | 1,365,094 | — | ||||||
|
|
|
| |||||
Cash—end of the period | $ | 1,226,716 | $ | 1,365,094 | ||||
|
|
|
| |||||
Supplemental disclosure of noncash financing activities: | ||||||||
Offering costs included in accounts payable | $ | — | $ | 103,380 | ||||
Offering costs included in accrued expenses | $ | — | $ | 45,000 | ||||
Payment of offering costs through note payable | $ | — | $ | 64,706 | ||||
Deferred underwriting commissions | $ | — | $ | 4,830,000 | ||||
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
Note 1—Description of Organization, Business Operations and Basis of Presentation
Organization and General
Frazier Lifesciences Acquisition Corporation (the “Company”) is a blank check company incorporated as a Cayman Islands exempted company on October 7, 2020. The Company was incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses that the Company has not yet identified (“Business Combination”).
As of December 31, 2021, the Company had not yet commenced operations. All activity for the period from October 7, 2020 (inception) through December 31, 2021 relates to the Company’s formation and the initial public offering (the “Initial Public Offering”), which is described below, and after the Initial Public Offering, the search for a target business. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering.
The Company’s Sponsor is Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (“Sponsor”). The registration statement for the Company’s Initial Public Offering was declared effective on December 8, 2020. On December 11, 2020, the Company consummated its Initial Public Offering of 13,800,000 units (each, a “Unit” and collectively, the “Units” and, with respect to the Class A ordinary shares included in the Units being offered, the “Public Shares”), including 1,800,000 additional Units to cover over-allotments (the “Over-Allotment Units”), at $10.00 per Unit, generating gross proceeds of $138.0 million, and incurring offering costs of approximately $8.1 million, inclusive of approximately $4.8 million in deferred underwriting commissions (See Note 6).
Simultaneously with the closing of the Initial Public Offering, the Company consummated the private placement (“Private Placement”) of 501,000 units (each, a “Private Placement Unit” and collectively, the “Private Placement Units”), at a price of $10.00 per Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5.0 million (See Note 4).
Upon the closing of the Initial Public Offering and the Private Placement, approximately $138.0 million ($10.00 per Unit) of the net proceeds of the Initial Public Offering and certain of the proceeds of the Private Placement were placed in a trust account (“Trust Account”) with Continental Stock Transfer & Trust Company acting as trustee and invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which will be invested only in direct U.S. government treasury obligations, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution of the Trust Account as described below.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of its Initial Public Offering and the sale of Private Placement Units, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. The Company’s initial Business Combination must be with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (as defined below) (excluding taxes payable on interest earned) at the time the Company signs a definitive agreement in connection with the initial Business Combination. However, the Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target business or otherwise acquires a controlling interest in the target business sufficient for it not to be required to register as an investment company under the Investment Company Act of 1940, as amended, or the Investment Company Act.
F-7
Table of Contents
The Company will provide the holders of the Public Shares (the “Public Shareholders”) with the opportunity to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a general meeting called to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company will seek shareholder approval of a Business Combination or conduct a tender offer will be made by the Company. The Public Shareholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (initially anticipated to be $10.00 per share, plus any pro rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay its tax obligations). The per-share amount to be distributed to public shareholders who redeem their Public Shares will not be reduced by the deferred underwriting commissions the Company will pay to the underwriters (as discussed in Note 6). These Public Shares will be recorded at a redemption value and classified as temporary equity upon the completion of the Initial Public Offering, in accordance with the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity” (“ASC Topic 480”). In such case, the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 upon such consummation of a Business Combination and a majority of the shares voted are voted in favor of the Business Combination. If a shareholder vote is not required by applicable law or stock exchange listing requirements and the Company does not decide to hold a shareholder vote for business or other reasons, the Company will, pursuant to the Amended and Restated Memorandum and Articles of Association which was adopted by the Company at the consummation of the Initial Public Offering (the “Amended and Restated Memorandum and Articles of Association”), conduct the redemptions pursuant to the tender offer rules of the U.S. Securities and Exchange Commission (the “SEC”), and file tender offer documents with the SEC prior to completing a Business Combination. If, however, a shareholder approval of the transactions is required by applicable law or stock exchange listing requirements, or the Company decides to obtain shareholder approval for business or other reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. Additionally, each public shareholder may elect to redeem their Public Shares irrespective of whether they vote for or against the proposed transaction or whether they were a public shareholder on the record date for the general meeting held to approve the proposed transaction. If the Company seeks shareholder approval in connection with a Business Combination, the holders of the Founder Shares (as defined in Note 5) prior to this Initial Public Offering (the “Initial Shareholders”) agreed to vote their Founder Shares and any Public Shares purchased during or after the Initial Public Offering in favor of a Business Combination. In addition, the Initial Shareholders agreed to waive their redemption rights with respect to their Founder Shares, private placement shares (the “Private Placement Shares”) underlying the Private Placement Units and Public Shares in connection with the completion of a Business Combination. In addition, the Company agreed not to enter into a definitive agreement regarding an initial Business Combination without the prior consent of the Sponsor.
Notwithstanding the foregoing, the Company’s Amended and Restated Memorandum and Articles of Association provides that a public shareholder, together with any affiliate of such shareholder or any other person with whom such shareholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% or more of the Class A ordinary shares sold in the Initial Public Offering, without the prior consent of the Company.
The Company’s Sponsor, officers or directors agreed not to propose an amendment to the Company’s Amended and Restated Memorandum and Articles of Association (A) to modify the substance or timing of the Company’s obligation to allow the redemption of its Public Shares in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination within 24 months from the closing of the Initial Public Offering, or December 11, 2022, agreement in principle or definitive agreement for an initial Business Combination within 24 months from the closing of the Initial Public Offering (the “Combination Period”), or (B) with respect to any other provisions relating to shareholders’ rights or pre-initial Business Combination activity, unless the Company provides the Public Shareholders with the opportunity to redeem their Class A ordinary shares in conjunction with any such amendment.
F-8
Table of Contents
If the Company is unable to complete a Business Combination within the Combination Period, the Company will (i) cease all operations except for the purpose of winding up; (ii) as promptly as reasonably possible, but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its income taxes, if any (less up to $100,000 of interest to pay dissolution expenses), divided by the number of the then-outstanding Public Shares, which redemption will completely extinguish Public Shareholders’ rights as shareholders (including the right to receive further liquidation distributions, if any); and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining shareholders and the board of directors, liquidate and dissolve, subject in the case of clauses (ii) and (iii), to the Company’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law.
In connection with the redemption of 100% of the Company’s outstanding Public Shares for a portion of the funds held in the Trust Account, each holder will receive a full pro rata portion of the amount then in the Trust Account, plus any pro rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay the Company’s taxes payable (less taxes payable and up to $100,000 of interest to pay dissolution expenses). The Initial Shareholders agreed to waive their liquidation rights with respect to the Founder Shares and Private Placement Shares held by them if the Company fails to complete a Business Combination within the Combination Period. However, if the Initial Shareholders should acquire Public Shares in or after the Initial Public Offering, they will be entitled to liquidating distributions from the Trust Account with respect to such Public Shares if the Company fails to complete a Business Combination within the Combination Period. The underwriters agreed to waive their rights to their deferred underwriting commission (see Note 6) held in the Trust Account in the event the Company does not complete a Business Combination within the Combination Period and, in such event, such amounts will be included with the funds held in the Trust Account that will be available to fund the redemption of the Company’s Public Shares. In the event of such distribution, it is possible that the per share value of the residual assets remaining available for distribution in the Trust Account will be less than the $10.00 per share initially held in the Trust Account. In order to protect the amounts held in the Trust Account, the Sponsor agreed that it will be liable to the Company if and to the extent any claims by a third party for services rendered or products sold to the Company, or a prospective target business with which the Company has entered into a written letter of intent, confidentiality or other similar agreement or business combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.00 per Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per share due to reductions in the value of the Trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). In the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have vendors, service providers (except the Company’s independent registered public accounting firm), prospective target businesses or other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account. There can be no guarantee that the Company will be successful in obtaining such waivers from its targeted vendors and service providers.
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-9
Table of Contents
Liquidity and Going Concern
As of December 31, 2021, the Company had approximately $1.2 million in its operating bank account, and working capital of approximately $1.3 million.
The Company’s liquidity needs to date have been satisfied through a contribution of $25,000 from Sponsor to cover for certain expenses in exchange for the issuance of the Founder Shares, the loan of approximately $83,000 from the Sponsor under the Note (as defined in Note 5), and the proceeds from the consummation of the Private Placement not held in the Trust Account. The Company repaid the Note in full on December 14, 2020. In addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, provide the Company Working Capital Loans (as defined in Note 5). As of December 31, 2021 and 2020, there were no amounts outstanding under any Working Capital Loan.
Based on the foregoing, management believes that the Company will have sufficient working capital and borrowing capacity from the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors to meet its needs through the earlier of the consummation of a Business Combination or one year from this filing. However, in connection with the company’s assessment of going concern considerations in accordance with FASB Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about the company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should the Company be required to liquidate after December 11, 2022. The financial statements do not include any adjustment that might be necessary if the company is unable to continue as a going concern. Management intends to complete the Business Combination prior to the liquidation date.
Note 2—Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States of America (“GAAP”) and pursuant to the rules and regulations of the SEC.
Emerging Growth Company
As an emerging growth company, the Company may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the Jumpstart our Business Startups Act of 2021 (the “JOBS Act”) exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard.
F-10
Table of Contents
This may make comparison of the Company’s financial statement with another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. One of the more significant accounting estimates included in these financial statements is the determination of the fair value of the warrant liability. Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no cash equivalents as of December 31, 2021 and 2020.
Investments Held in the Trust Account
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. When the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. When the Company’s investments held in the Trust Account are comprised of money market funds, the investments are recognized at fair value. Trading securities and investments in money market funds are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in interest income from investments held in the Trust Account in the accompanying statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution, which, at times, may exceed the Federal Deposit Insurance Corporation coverage limit of $250,000. As of December 31, 2021 and 2020, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| • | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
F-11
Table of Contents
| • | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| • | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Offering Costs Associated with the Initial Public Offering
Offering costs consisted of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial Public Offering. Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with derivative warrant liabilities are expensed as incurred, presented as other expenses in the statement of operations. Offering costs associated with the Public Shares were charged against the carrying value of the Class A ordinary shares subject to redemption upon the completion of the Initial Public Offering.
Derivative Warrant Liabilities
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company evaluates all of its financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC Topic 480 and ASC Subtopic 815-15 “Derivatives and Hedging—Embedded Derivatives” (“ASC Subtopic 815-15”).
The warrants issued in connection with the Initial Public Offering (the “Public Warrants”) and the Private Placement Warrants (as defined in Note 4) are recognized as derivative liabilities in accordance with “Derivatives and Hedging-Contracts in Entity’s Own Equity” (“ASC Subtopic 815-40”). Accordingly, the Company recognizes the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company’s statement of operations. The fair value of the Public Warrants issued in connection with the Public Offering and Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and have subsequently been measured based on the listed market price of such warrants.
Class A Ordinary Shares Subject to Possible Redemption
The Company accounts for its Class A ordinary shares subject to possible redemption in accordance with the guidance in ASC Topic 480. Class A ordinary shares subject to mandatory redemption (if any) are classified as liability instruments and are measured at fair value. Conditionally redeemable Class A ordinary shares (including Class A ordinary shares that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, Class A ordinary shares are classified as shareholders’ equity. The Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, at December 31, 2021 and 2020, 13,800,000 shares of Class A ordinary shares subject to possible redemption are presented as temporary equity, respectively, outside of the shareholders’ equity section of the Company’s balance sheets.
F-12
Table of Contents
Under ASC 480-10-S99, the Company has elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of the reporting period. This method would view the end of the reporting period as if it were also the redemption date of the security. Effective with the closing of the Initial Public Offering, the Company recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Income Taxes
ASC Topic 740, “Income Taxes” prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company’s management determined that the Cayman Islands is the Company’s only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts for interest and penalties as of December 31, 2021 and 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
There is currently no taxation imposed on income by the Government of the Cayman Islands. In accordance with Cayman federal income tax regulations, income taxes are not levied on the Company. Consequently, income taxes are not reflected in the Company’s financial statements. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
Net Income per Ordinary Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” The Company has two classes of shares, which are referred to as Class A ordinary shares and Class B ordinary shares. Income and losses are shared pro rata between the two classes of shares. Net income per ordinary share is calculated by dividing the net income by the weighted average shares of ordinary shares outstanding for the respective period.
The calculation of diluted net income does not consider the effect of the warrants underlying the Units sold in the Initial Public Offering (including the consummation of the over-allotment) and the Private Placement Warrants to purchase an aggregate of 4,767,000 Class A ordinary shares in the calculation of diluted income per share, because their exercise is contingent upon future events. The Company has considered the effect of Class B ordinary shares that were excluded from the weighted average number of basic shares outstanding as they were contingent on the exercise of over-allotment option by the underwriters. Since the contingency was satisfied, the Company has included these shares in the weighted average number as of the beginning of the period to determine the dilutive impact of these shares. Accretion associated with the redeemable Class A ordinary shares is excluded from earnings per share as the redemption value approximates fair value.
F-13
Table of Contents
The following table reflects presents a reconciliation of the numerator and denominator used to compute basic and diluted net income per share for each class of ordinary shares:
| For The Year Ended December 31, 2021 | For The Period From October 7, 2020 (Inception) Through December 31, 2020 | |||||||||||||||
| Class A | Class B | Class A | Class B | |||||||||||||
Basic and diluted net income per ordinary share: | ||||||||||||||||
Numerator: | ||||||||||||||||
Allocation of net income—basic | $ | 3,366,408 | $ | 812,119 | $ | 21,926 | $ | 19,527 | ||||||||
Allocation of net income—diluted | $ | 3,366,408 | $ | 812,119 | $ | 20,852 | $ | 20,601 | ||||||||
Denominator: | ||||||||||||||||
Basic weighted average ordinary shares outstanding | 14,301,000 | 3,450,000 | 3,492,105 | 3,109,884 | ||||||||||||
Diluted weighted average ordinary shares outstanding | 14,301,000 | 3,450,000 | 3,492,105 | 3,450,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic net income per ordinary share | $ | 0.24 | $ | 0.24 | $ | 0.01 | $ | 0.01 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Diluted net income per ordinary share | $ | 0.24 | $ | 0.24 | $ | 0.01 | $ | 0.01 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Recent Issued Accounting Standards
In August 2020, the FASB issued Accounting Standard Update (the “ASU”) No. 2020-06, Debt-Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings per share calculation in certain areas. The Company early adopted the ASU on January 1, 2021 using a modified retrospective method for transition. Adoption of the ASU did not impact the Company’s financial position, results of operations or cash flows.
The Company’s management does not believe that any recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on the accompanying financial statements.
Note 3—Initial Public Offering
On December 11, 2020, the Company consummated its Initial Public Offering of 13,800,000 Units, including 1,800,000 Over-Allotment Units, at $10.00 per Unit, generating gross proceeds of $138.0 million, and incurring offering costs of approximately $8.1 million, inclusive of approximately $4.8 million in deferred underwriting commissions.
Each Unit consists of one Class A ordinary share and one-third of one Public Warrant. Each whole Public Warrant will entitle the holder to purchase one Class A ordinary share at an exercise price of $11.50 per share, subject to adjustment (see Note 7).
Note 4—Private Placement
Simultaneously with the closing of the Initial Public Offering, the Company consummated the Private Placement of 501,000 Private Placement Units, at a price of $10.00 per Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5.0 million.
F-14
Table of Contents
Each Private Placement Unit consists of one Class A ordinary share and one-third of one redeemable warrant. Each whole private placement warrant underlying the Private Placement Units (the “Private Placement Warrants”) is exercisable for one whole share of Class A ordinary shares at a price of $11.50 per share. A portion of the proceeds from the sale of the Private Placement Warrants to the Sponsor was added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the Private Placement Units will expire worthless. The Private Placement Warrants will be non-redeemable except as described below in Note 7 and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees.
The Sponsor and the Company’s officers and directors agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Units until 30 days after the completion of the initial Business Combination.
Note 5—Related Party Transactions
Founder Shares
On October 7, 2020, the Sponsor paid an aggregate of $25,000 for certain expenses on behalf of the Company in exchange for issuance of 2,875,000 Class B ordinary shares (the “Founder Shares”). On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of the directors other than the Chairman. On December 8, 2020, the Company effected a share sub-division, resulting in an increase in the total number of Founder Shares outstanding from 2,875,000 to 3,450,000 shares. All shares and associated amounts have been retroactively restated to reflect the share sub-division. The Sponsor agreed to forfeit up to an aggregate of 450,000 Founder Shares to the extent that the option to purchase additional units was not exercised in full by the underwriters so that the Founder Shares would represent 20% of the Company’s issued and outstanding shares after the Initial Public Offering. The underwriters fully exercised the over-allotment option on December 11, 2020; thus, these Founder Shares were no longer subject to forfeiture.
The Initial Shareholders agreed not to transfer, assign or sell any of their Founder Shares until the earlier to occur of: (A) one year after the completion of the initial Business Combination or (B) subsequent to the initial Business Combination, (x) if the closing price of Class A ordinary shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the initial Business Combination, or (y) the date on which the Company completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of the Public Shareholders having the right to exchange their ordinary shares for cash, securities or other property.
Related Party Loans
On October 7, 2020, the Sponsor agreed to loan the Company up to $300,000 to be used for the payment of costs related to the Initial Public Offering pursuant to a promissory note (the “Note”). The Note is non-interest bearing, unsecured and due upon the closing of the Initial Public Offering. The Sponsor paid an aggregate of approximately $83,000 to cover for the Company’s expenses under the Note. On December 14, 2020, the Company fully repaid the Note. The facility is no longer available to be drawn.
In addition, in order to fund working capital deficiencies or finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company may repay the Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans may be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans
F-15
Table of Contents
but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1.5 million of such Working Capital Loans may be convertible into private placement units of the post Business Combination entity at a price of $10.00 per unit. The private placement units would be identical to the public units sold, subject to certain limited exceptions. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. As of December 31, 2021 and 2020, the Company had no borrowings under the Working Capital Loans.
Administrative Services Agreement
The Company entered into an agreement that provided that, commencing on the date that the Company’s securities were first listed on the Nasdaq through the earlier of consummation of the initial Business Combination and the liquidation, the Company agreed to pay the Sponsor $10,000 per month for office space, secretarial and administrative services provided to the Company. During the year ended December 31, 2021 and 2020, the Company incurred approximately $120,000 and $7,000 for expenses in connection with the Administrative Services Agreement. As of December 31, 2021 and 2020, there was approximately $-0- and $7,000, respectively, in accrued expenses in connection with such agreement on the accompanying balance sheets.
In addition, the Sponsor, officers and directors, or any of their respective affiliates will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable Business Combinations. The audit committee will review on a quarterly basis all payments that were made by the Company to the Sponsor, officers or directors, or the Company’s or their affiliates. Any such payments prior to an initial Business Combination will be made from funds held outside the Trust Account.
Note 6—Commitments and Contingencies
Registration and Shareholder Rights
The holders of the Founder Shares, Private Placement Units, Private Placement Shares, Private Placement Warrants, Class A ordinary shares underlying the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans (and any Class A ordinary shares issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans) are entitled to registration rights pursuant to a registration and shareholder rights agreement signed upon the effective date of the Initial Public Offering. The holders of these securities are entitled to make up to three demands, excluding short form demands, that the Company registers such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the initial Business Combination. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option from the date of the prospectus relating to the Initial Public Offering to purchase up to 1,800,000 additional Units at the Initial Public Offering price less the underwriting discounts and commissions. The underwriters fully exercised the over-allotment option on December 11, 2020.
The underwriters will be entitled to an underwriting discount of $0.20 per unit, or approximately $2.8 million in the aggregate, paid upon the closing of the Initial Public Offering. In addition, $0.35 per unit, or approximately $4.8 million in the aggregate will be payable to the underwriters for deferred underwriting commissions. The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement.
F-16
Table of Contents
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic, including new variant strains of the underlying virus, current or anticipated military conflict, including between Russia and Ukraine, terrorism, sanctions or other geopolitical events as well as adverse developments in the economy and capital markets, including rising energy costs, inflation and interest rates, in the United States and globally, on the industry and has concluded that while it is reasonably possible that these events could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 7—Class A Ordinary Shares Subject To Possible Redemption
The Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of future events. The Company is authorized to issue 479,000,000 ordinary shares with a par value of $0.0001 per share. Holders of the Company’s Class A ordinary shares are entitled to one vote for each share. As of December 31, 2021 and 2020, there were 14,301,000 Class A ordinary shares issued and outstanding, of which 13,800,000 shares were subject to possible redemption have been classified as temporary equity.
Class A ordinary shares subject to possible redemption reflected on the balance sheet is reconciled on the following table:
Gross proceeds | $ | 138,000,000 | ||
Less: | ||||
Fair value of Public Warrants at issuance | (7,682,000 | ) | ||
Offering costs allocated to Class A ordinary shares subject to possible redemption | (7,653,636 | ) | ||
Plus: | ||||
Accretion on Class A ordinary shares subject to possible redemption amount | 15,335,636 | |||
|
| |||
Class A ordinary shares subject to possible redemption | $ | 138,000,000 | ||
|
|
Note 8—Derivative Warrant Liabilities
As of December 31, 2021 and 2020, the Company has 4,600,000 and 167,000 Public Warrants and Private Placement Warrants, respectively, outstanding.
Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b) 12 months from the closing of the Initial Public Offering; provided in each case that the Company has an effective registration statement under the Securities Act covering the Class A ordinary shares issuable upon exercise of the Public Warrants and a current prospectus relating to them is available and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder (or the Company permits holders to exercise their warrants on a cashless basis under certain circumstances). The Company agreed that as soon as practicable, but in no event later than 20 business days after the closing of the initial Business Combination, the Company will use commercially reasonable efforts to file with the SEC a registration statement covering the Class A ordinary shares issuable upon exercise of the warrants and to maintain a current prospectus relating to those Class A ordinary shares until the warrants expire or are redeemed,
F-17
Table of Contents
as specified in the warrant agreement. If a registration statement covering the Class A ordinary shares issuable upon exercise of the warrants is not effective by the 60th day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the Class A ordinary shares are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, and in the event the Company does not so elect, it will use commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
The warrants have an exercise price of $11.50 per share, subject to adjustments, and will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation. In addition, if (x) the Company issues additional Class A ordinary shares or equity-linked securities for capital raising purposes in connection with the closing of the initial Business Combination at an issue price or effective issue price of less than $9.20 per Class A ordinary share (with such issue price or effective issue price to be determined in good faith by the board of directors and, in the case of any such issuance to the Initial Shareholders or their affiliates, without taking into account any Founder Shares held by the Initial Shareholders or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial Business Combination on the date of the consummation of the initial Business Combination (net of redemptions), and (z) the volume weighted average trading price of Class A ordinary shares during the 10-trading day period starting on the trading day prior to the day on which the Company consummates its initial Business Combination (such price, the “Market Value”) is below $9.20 per share, then the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price, the $18.00 per share redemption trigger price will be adjusted (to the nearest cent) to be equal to 180% of the higher of the Market Value and the Newly Issued Price (and the $10.00 per share redemption trigger price will be adjusted (to the nearest cent) to be equal to the higher of the Market Value and the Newly Issued Price see “Redemption of warrants for cash when the price per class A ordinary share equals or exceeds $18.00” and “Redemption of warrants for Class A ordinary shares when the price per class A ordinary share equals or exceeds $10.00” as described below).
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering, except (i) that the Private Placement Warrants and the Class A ordinary shares issuable upon exercise of the Private Placement Warrants will not be transferable, assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions, (ii) except as described below, the Private Placement Warrants will be non-redeemable so long as they are held by the Sponsor or such its permitted transferees and (iii) the Sponsor or its permitted transferees will have the option to exercise the Private Placement Warrants on a cashless basis and have certain registration rights. If the Private Placement Warrants are held by someone other than the Sponsor or its permitted transferees, the Private Placement Warrants will be redeemable by the Company in all redemption scenarios and exercisable by such holders on the same basis as the Public Warrants.
Redemption of warrants for cash when the price per Class A ordinary share equals or exceeds $18.00:
Once the warrants become exercisable, the Company may call the outstanding warrants for redemption (except as described herein with respect to the Private Placement Warrants):
| • | in whole and not in part; |
| • | at a price of $0.01 per warrant; |
F-18
Table of Contents
| • | upon a minimum of 30 days’ prior written notice of redemption to each warrant holder; and |
| • | if, and only if, the last reported sales price (the “closing price”) of Class A ordinary shares equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to the warrant holders (the “Reference Value”). |
The Company will not redeem the warrants as described above unless a registration statement under the Securities Act covering the issuance of the Class A ordinary shares issuable upon exercise of the warrants is then effective and a current prospectus relating to those Class A ordinary shares is available throughout the 30-day redemption period. If and when the warrants become redeemable by the Company, it may exercise its redemption right even if the Company is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Redemption of warrants for Class A ordinary shares when the price per Class A ordinary share equals or exceeds $10.00:
After the warrants become exercisable, the Company may redeem the outstanding warrants:
| • | in whole and not in part; |
| • | at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of Class A ordinary shares to be determined by reference to an agreed table based on the redemption date and the “fair market value” of Class A ordinary shares; |
| • | if, and only if, the closing price of Class A ordinary shares equals or exceeds $10.00 per Public Share (as adjusted per share subdivisions, share dividends, reorganizations, recapitalizations and the like) on the trading day before the Company sends the notice of redemption to the warrant holders; and |
| • | if the Reference Value is less than $18.00 per share (as adjusted for share splits, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like), then the Private Placement Warrants must also concurrently be called for redemption on the same terms (except as described herein with respect to a holder’s ability to cashless exercise its warrants) as the outstanding Public Warrants as described above. |
The “fair market value” of Class A ordinary shares for the above purpose shall mean the volume weighted average price of Class A ordinary shares during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of warrants. In no event will the warrants be exercisable on a cashless basis in connection with this redemption feature for more than 0.361 Class A ordinary shares per warrant (subject to adjustment).
In no event will the Company be required to net cash settle any warrant. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
Note 9—Shareholders’ Deficit
Preference Shares—The Company is authorized to issue 1,000,000 preference shares with a par value of $0.0001 per share. At December 31, 2021 and 2020, there were no preference shares issued or outstanding.
F-19
Table of Contents
Class A Ordinary Shares—The Company is authorized to issue 479,000,000 Class A ordinary shares with a par value of $0.0001 per share. Holders of the Company’s Class A ordinary shares are entitled to one vote for each share. At December 31, 2021 and 2020, there were 14,301,000 Class A ordinary shares issued and outstanding, of which 13,800,000 shares were subject to possible redemption have been classified as temporary equity (see Note 7).
Class B Ordinary Shares—The Company is authorized to issue 20,000,000 Class B ordinary shares with a par value of $0.0001 per share. On October 7, 2020, the Company issued 2,875,000 Class B ordinary shares. On December 8, 2020, the Company effected a share sub-division, resulting in an increase in the total number of Class B ordinary shares outstanding from 2,875,000 to 3,450,000 shares. All shares and associated amounts have been retroactively restated to reflect the share sub-division. Of the 3,450,000 Class B ordinary shares outstanding, up to 450,000 shares were subject to forfeiture, to the Company by the Initial Shareholders for no consideration to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the Initial Shareholders would collectively own 20% of the Company’s issued and outstanding ordinary shares after the Initial Public Offering (excluding the Private Placement Shares and assuming the initial shareholders do not purchase any units in the Initial Public Offering). The underwriters fully exercised the over-allotment option on December 11, 2020; thus, these 450,000 Class B ordinary shares were no longer subject to forfeiture. At December 31, 2021 and 2020, there were 3,450,000 Class B ordinary shares issued and outstanding period. Class A and Class B ordinary shareholders of record are entitled to one vote for each share held on all matters to be voted on by shareholders. Except as described below, holders of Class A ordinary shares and holders of Class B ordinary shares will vote together as a single class on all matters submitted to a vote of the shareholders except as required by law. Prior to the initial Business Combination, only holders of the Founder Shares will have the right to vote on the appointment of directors. Holders of the Public Shares will not be entitled to vote on the appointment of directors during such time. In addition, prior to the completion of an initial Business Combination, holders of a majority of the Founder Shares may remove a member of the board of directors for any reason. The provisions of the Amended and Restated Memorandum and Articles of Association governing the appointment or removal of directors prior to the initial Business Combination may only be amended by a special resolution passed by holders representing at least two-thirds of the issued and outstanding Class B ordinary shares.
The Class B ordinary shares will automatically convert into Class A ordinary shares on the first business day following the consummation of the initial Business Combination at a ratio such that the number of Class A ordinary shares issuable upon conversion of all Founder Shares will equal, in the aggregate, on an as-converted basis, 20% of the sum of (i) the total number of ordinary shares (excluding the Private Placement Shares) issued and outstanding upon the consummation of the Initial Public Offering, plus (ii) the sum of the total number of Class A ordinary shares issued or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the initial Business Combination (net of any redemptions of Class A ordinary shares by Public Shareholders), excluding any Class A ordinary shares or equity-linked securities exercisable for or convertible into Class A ordinary shares issued, deemed issued, or to be issued, to any seller in the initial Business Combination and any Private Placement Units issued to the Sponsor, members of the founding team or any of their affiliates upon conversion of Working Capital Loans. In no event will the Class B ordinary shares convert into Class A ordinary shares at a rate of less than one-to-one.
Note 10—Fair Value Measurements
The following table presents information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2021 and 2020 and indicates the fair value hierarchy of the valuation techniques that the Company utilized to determine such fair value.
F-20
Table of Contents
December 31, 2021
Description | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Other Unobservable Inputs (Level 3) | |||||||||
Assets: | ||||||||||||
Investments held in Trust Account | $ | 138,017,009 | $ | — | $ | — | ||||||
Liabilities: | ||||||||||||
Derivative warrant liabilities | $ | 2,714,000 | $ | 98,530 | $ | — | ||||||
December 31, 2020
Description | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Other Unobservable Inputs (Level 3) | |||||||||
Assets: | ||||||||||||
Investments held in Trust Account | $ | 138,000,851 | $ | — | $ | — | ||||||
Liabilities: | ||||||||||||
Derivative warrant liabilities | $ | — | $ | — | $ | 7,341,180 | ||||||
Transfers to/from Levels 1, 2, and 3 are recognized at the beginning of the reporting period. The estimated fair value of the Public Warrants of $7,084,000 transferred from a Level 3 measurement to a Level 1 fair value measurement in January 2021, when the Public Warrants were separately listed and traded. The estimated fair value of the Private Warrants of $257,180 was transferred from a Level 3 measurement to a Level 2 fair value measurement as of January 2021, as the transfer of Private Placement Warrants to anyone who is not a permitted transferee would result in the Private Placement Warrants having substantially the same terms as the Public Warrants, the Company determined that the fair value of each Private Placement Warrant is equivalent to that of each Public Warrant.
Level 1 instruments include investments in mutual funds invested in government securities. The Company uses inputs such as actual trade data, benchmark yields, quoted market prices from dealers or brokers, and other similar sources to determine the fair value of its investments.
Other than the transfer of the Public Warrants (Level 3 to Level 1) and Private Placement Warrants (Level 3 to Level 2), there were no transfers to/from Level 1, 2, and 3 in the year ended December 31, 2021.
The change in the fair value of the derivative warrant liabilities for the year ended December 31, 2021 is summarized as follows:
Derivative warrant liabilities at December 31, 2020 | $ | 7,341,180 | ||
Transfer of Public Warrants to level 1 | (7,084,000 | ) | ||
Transfer of Private Warrant to level 2 | (257,180 | ) | ||
Change in fair value of derivative warrant liabilities | — | |||
|
| |||
Derivative warrant liabilities at December 31, 2021 | $ | — | ||
|
|
Note 11 – Break-up Fee
On September 13, 2021, the Company entered into a term sheet in connection with an initial business combination. This term sheet was terminated on December 30, 2021 and in accordance with the term sheet, the Company received a break-up fee of $1 million.
F-21
Table of Contents
Note 12—Subsequent Events
The Company evaluated subsequent events and transactions that occurred up to the date the financial statements were issued. Based upon this review, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
F-22
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
CONDENSED BALANCE SHEETS
| June 30, 2022 | December 31, 2021 | |||||||
| (Unaudited) | ||||||||
Assets: | ||||||||
Current assets: | ||||||||
Cash | $ | 615,440 | $ | 1,226,716 | ||||
Prepaid expenses | 149,722 | 261,333 | ||||||
|
|
|
| |||||
Total current assets | 765,162 | 1,488,049 | ||||||
Investments held in Trust Account | 138,132,977 | 138,017,009 | ||||||
|
|
|
| |||||
Total Assets | $ | 138,898,139 | $ | 139,505,058 | ||||
|
|
|
| |||||
Liabilities, Class A Ordinary Shares Subject to Possible Redemption and Shareholders’ Deficit: | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 50,698 | $ | 167,324 | ||||
Accrued expenses | 1,783,891 | 54,750 | ||||||
|
|
|
| |||||
Total current liabilities | 1,834,589 | 222,074 | ||||||
Deferred underwriting commissions | 4,830,000 | 4,830,000 | ||||||
Derivative warrant liabilities | 381,360 | 2,812,530 | ||||||
|
|
|
| |||||
Total liabilities | 7,045,949 | 7,864,604 | ||||||
Commitments and Contingencies | ||||||||
Class A ordinary shares subject to possible redemption, $0.0001 par value; 13,800,000 shares issued and outstanding at redemption value of approximately $10.00 per share at June 30, 2022 and December 31, 2021 | 138,032,977 | 138,000,000 | ||||||
Shareholders’ Deficit: | ||||||||
Preference shares, $0.0001 par value; 1,000,000 shares authorized; none issued or outstanding at June 30, 2022 and December 31, 2021 | — | — | ||||||
Class A ordinary shares, $0.0001 par value; 479,000,000 shares authorized; 501,000 shares issued and outstanding (excluding 13,800,000 shares subject to possible redemption) at June 30, 2022 and December 31, 2021 | 50 | 50 | ||||||
Class B ordinary shares, $0.0001 par value; 20,000,000 shares authorized; 3,450,000 shares issued and outstanding at June 30, 2022 and December 31, 2021 | 345 | 345 | ||||||
Additional paid-in capital | — | — | ||||||
Accumulated deficit | (6,181,182 | ) | (6,359,941 | ) | ||||
|
|
|
| |||||
Total shareholders’ deficit | (6,180,787 | ) | (6,359,546 | ) | ||||
|
|
|
| |||||
Total Liabilities, Class A Ordinary Shares Subject to Possible Redemption and Shareholders’ Deficit | $ | 138,898,139 | $ | 139,505,058 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-23
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF OPERATIONS
| For The Three Months Ended June 30, | For The Six Months Ended June 30, | |||||||||||||||
| 2022 | 2021 | 2022 | 2021 | |||||||||||||
General and administrative expenses | $ | 1,960,784 | $ | 275,239 | $ | 2,275,402 | $ | 482,480 | ||||||||
Administrative expenses - related party | 30,000 | 30,000 | 60,000 | 60,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (1,990,784 | ) | (305,239 | ) | (2,335,402 | ) | (542,480 | ) | ||||||||
Other income (expenses): | ||||||||||||||||
Interest income from investments held in Trust Account | 102,847 | 7,019 | 115,968 | 11,466 | ||||||||||||
Change in fair value of derivative warrant liabilities | 715,050 | (1,001,070 | ) | 2,431,170 | 2,049,810 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income (loss) | $ | (1,172,887 | ) | $ | (1,299,290 | ) | $ | 211,736 | $ | 1,518,796 | ||||||
|
|
|
|
|
|
|
| |||||||||
Weighted average number of Class A ordinary shares - basic and diluted | 14,301,000 | 14,301,000 | 14,301,000 | 14,301,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted net income (loss) per share, Class A ordinary shares | $ | (0.07 | ) | $ | (0.07 | ) | $ | 0.01 | $ | 0.09 | ||||||
|
|
|
|
|
|
|
| |||||||||
Weighted average number of Class B ordinary shares - basic and diluted | 3,450,000 | 3,450,000 | 3,450,000 | 3,450,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted net income (loss) per share, Class B ordinary shares | $ | (0.07 | ) | $ | (0.07 | ) | $ | 0.01 | $ | 0.09 | ||||||
|
|
|
|
|
|
|
| |||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-24
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2022
| Ordinary Shares | Additional Paid-in Capital | Total Shareholders’ Deficit | ||||||||||||||||||||||||||
| Class A | Class B | Accumulated Deficit | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
Balance - December 31, 2021 | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (6,359,941 | ) | $ | (6,359,546 | ) | ||||||||||||||
Net income | — | — | — | — | — | 1,384,623 | 1,384,623 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance - March 31, 2022 (Unaudited) | 501,000 | 50 | 3,450,000 | 345 | — | (4,975,318 | ) | (4,974,923 | ) | |||||||||||||||||||
Increase in redemption value of Class A ordinary shares subject to possible redemption | — | — | — | — | — | (32,977 | ) | (32,977 | ) | |||||||||||||||||||
Net loss | — | — | — | — | — | (1,172,887 | ) | (1,172,887 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance - June 30, 2022 (Unaudited) | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (6,181,182 | ) | $ | (6,180,787 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
FOR THE THREE AND SIX MONTHS ENDED JUNE 30, 2021
| Ordinary Shares | Additional Paid-in Capital | Total Shareholders’ Deficit | ||||||||||||||||||||||||||
| Class A | Class B | Accumulated Deficit | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
Balance - December 31, 2020 | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (10,538,468 | ) | $ | (10,538,073 | ) | ||||||||||||||
Net income | — | — | — | — | — | 2,818,086 | 2,818,086 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance - March 31, 2021 (Unaudited) | 501,000 | 50 | 3,450,000 | 345 | — | (7,720,382 | ) | (7,719,987 | ) | |||||||||||||||||||
Net loss | — | — | — | — | — | (1,299,290 | ) | (1,299,290 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance - June 30, 2021 (Unaudited) | 501,000 | $ | 50 | 3,450,000 | $ | 345 | $ | — | $ | (9,019,672 | ) | $ | (9,019,277 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-25
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
UNAUDITED CONDENSED STATEMENTS OF CASH FLOWS
| For The Six Months Ended June 30, | ||||||||
| 2022 | 2021 | |||||||
Cash Flows from Operating Activities: | ||||||||
Net income | $ | 211,736 | $ | 1,518,796 | ||||
Adjustments to reconcile net income to net cash used in operating activities: | ||||||||
Interest income from investments held in Trust Account | (115,968 | ) | (11,466 | ) | ||||
Change in fair value of derivative warrant liabilities | (2,431,170 | ) | (2,049,810 | ) | ||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses | 111,611 | 85,863 | ||||||
Accounts payable | (116,626 | ) | (97,798 | ) | ||||
Accrued expenses | 1,774,141 | (22,268 | ) | |||||
Due to related party | — | 1,087 | ||||||
|
|
|
| |||||
Net cash used in operating activities | (566,276 | ) | (575,596 | ) | ||||
|
|
|
| |||||
Cash Flows from Financing Activities: | ||||||||
Offering costs paid | (45,000 | ) | — | |||||
|
|
|
| |||||
Net cash used in financing activities | (45,000 | ) | — | |||||
|
|
|
| |||||
Net change in cash | (611,276 | ) | (575,596 | ) | ||||
Cash - beginning of the period | 1,226,716 | 1,365,094 | ||||||
|
|
|
| |||||
Cash - end of the period | $ | 615,440 | $ | 789,498 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-26
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1-Description of Organization, Business Operations and Basis of Presentation
Organization and General
Frazier Lifesciences Acquisition Corporation (the “Company”) is a blank check company incorporated as a Cayman Islands exempted company on October 7, 2020. The Company was incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses that the Company has not yet identified (“Business Combination”).
As of June 30, 2022, the Company had not yet commenced operations. All activity for the period from October 7, 2020 (inception) through June 30, 2022 relates to the Company’s formation and the initial public offering (the “Initial Public Offering”), which is described below, and after the Initial Public Offering, the search for a target business. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering.
The Company’s Sponsor is Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (“Sponsor”). The registration statement for the Company’s Initial Public Offering was declared effective on December 8, 2020. On December 11, 2020, the Company consummated its Initial Public Offering of 13,800,000 units (each, a “Unit” and collectively, the “Units” and, with respect to the Class A ordinary shares included in the Units being offered, the “Public Shares”), including 1,800,000 additional Units to cover over-allotments (the “Over-Allotment Units”), at $10.00 per Unit, generating gross proceeds of $138.0 million, and incurring offering costs of approximately $8.1 million, inclusive of approximately $4.8 million in deferred underwriting commissions (See Note 6).
Simultaneously with the closing of the Initial Public Offering, the Company consummated the private placement (“Private Placement”) of 501,000 units (each, a “Private Placement Unit” and collectively, the “Private Placement Units”), at a price of $10.00 per Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5.0 million (See Note 4).
Upon the closing of the Initial Public Offering and the Private Placement, approximately $138.0 million ($10.00 per Unit) of the net proceeds of the Initial Public Offering and certain of the proceeds of the Private Placement were placed in a trust account (“Trust Account”) with Continental Stock Transfer & Trust Company acting as trustee and invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act having a maturity of 185 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which will be invested only in direct U.S. government treasury obligations, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution of the Trust Account as described below.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of its Initial Public Offering and the sale of Private Placement Units, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. The Company’s initial Business Combination must be with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (as defined below) (excluding taxes payable on interest earned) at the time the Company signs a definitive agreement in connection with the initial Business Combination. However, the Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target business or otherwise acquires a controlling interest in the target business sufficient for it not to be required to register as an investment company under the Investment Company Act of 1940, as amended, or the Investment Company Act.
F-27
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The Company will provide the holders of the Public Shares (the “Public Shareholders”) with the opportunity to redeem all or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a general meeting called to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company will seek shareholder approval of a Business Combination or conduct a tender offer will be made by the Company. The Public Shareholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (initially anticipated to be $10.00 per share, plus any pro rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay its tax obligations). The per-share amount to be distributed to public shareholders who redeem their Public Shares will not be reduced by the deferred underwriting commissions the Company will pay to the underwriters (as discussed in Note 6). These Public Shares will be recorded at a redemption value and classified as temporary equity upon the completion of the Initial Public Offering, in accordance with the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity” (“ASC Topic 480”). In such case, the Company will proceed with a Business Combination if the Company has net tangible assets of at least $5,000,001 upon such consummation of a Business Combination and a majority of the shares voted are voted in favor of the Business Combination. If a shareholder vote is not required by applicable law or stock exchange listing requirements and the Company does not decide to hold a shareholder vote for business or other reasons, the Company will, pursuant to the Amended and Restated Memorandum and Articles of Association which was adopted by the Company at the consummation of the Initial Public Offering (the “Amended and Restated Memorandum and Articles of Association”), conduct the redemptions pursuant to the tender offer rules of the U.S. Securities and Exchange Commission (the “SEC”), and file tender offer documents with the SEC prior to completing a Business Combination. If, however, a shareholder approval of the transactions is required by applicable law or stock exchange listing requirements, or the Company decides to obtain shareholder approval for business or other reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. Additionally, each public shareholder may elect to redeem their Public Shares irrespective of whether they vote for or against the proposed transaction or whether they were a public shareholder on the record date for the general meeting held to approve the proposed transaction. If the Company seeks shareholder approval in connection with a Business Combination, the holders of the Founder Shares (as defined in Note 5) prior to this Initial Public Offering (the “Initial Shareholders”) agreed to vote their Founder Shares and any Public Shares purchased during or after the Initial Public Offering in favor of a Business Combination. In addition, the Initial Shareholders agreed to waive their redemption rights with respect to their Founder Shares, private placement shares (the “Private Placement Shares”) underlying the Private Placement Units and Public Shares in connection with the completion of a Business Combination. In addition, the Company agreed not to enter into a definitive agreement regarding an initial Business Combination without the prior consent of the Sponsor.
Notwithstanding the foregoing, the Company’s Amended and Restated Memorandum and Articles of Association provides that a public shareholder, together with any affiliate of such shareholder or any other person with whom such shareholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% or more of the Class A ordinary shares sold in the Initial Public Offering, without the prior consent of the Company.
The Company’s Sponsor, officers or directors agreed not to propose an amendment to the Company’s Amended and Restated Memorandum and Articles of Association (A) to modify the substance or timing of the Company’s obligation to allow the redemption of its Public Shares in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination within 24 months from the closing of the Initial Public Offering, or December 11, 2022, agreement in principle or definitive agreement for an initial Business Combination within 24 months from the closing of the Initial Public Offering (the
F-28
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
“Combination Period”), or (B) with respect to any other provisions relating to shareholders’ rights or pre-initial Business Combination activity, unless the Company provides the Public Shareholders with the opportunity to redeem their Class A ordinary shares in conjunction with any such amendment.
If the Company is unable to complete a Business Combination within the Combination Period, the Company will (i) cease all operations except for the purpose of winding up; (ii) as promptly as reasonably possible, but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its income taxes, if any (less up to $100,000 of interest to pay dissolution expenses), divided by the number of the then-outstanding Public Shares, which redemption will completely extinguish Public Shareholders’ rights as shareholders (including the right to receive further liquidation distributions, if any); and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining shareholders and the board of directors, liquidate and dissolve, subject in the case of clauses (ii) and (iii), to the Company’s obligations under Cayman Islands law to provide for claims of creditors and the requirements of other applicable law.
In connection with the redemption of 100% of the Company’s outstanding Public Shares for a portion of the funds held in the Trust Account, each holder will receive a full pro rata portion of the amount then in the Trust Account, plus any pro rata interest earned on the funds held in the Trust Account and not previously released to the Company to pay the Company’s taxes payable (less taxes payable and up to $100,000 of interest to pay dissolution expenses). The Initial Shareholders agreed to waive their liquidation rights with respect to the Founder Shares and Private Placement Shares held by them if the Company fails to complete a Business Combination within the Combination Period. However, if the Initial Shareholders should acquire Public Shares in or after the Initial Public Offering, they will be entitled to liquidating distributions from the Trust Account with respect to such Public Shares if the Company fails to complete a Business Combination within the Combination Period. The underwriters agreed to waive their rights to their deferred underwriting commission (see Note 6) held in the Trust Account in the event the Company does not complete a Business Combination within the Combination Period and, in such event, such amounts will be included with the funds held in the Trust Account that will be available to fund the redemption of the Company’s Public Shares. In the event of such distribution, it is possible that the per share value of the residual assets remaining available for distribution in the Trust Account will be less than the $10.00 per share initially held in the Trust Account. In order to protect the amounts held in the Trust Account, the Sponsor agreed that it will be liable to the Company if and to the extent any claims by a third party for services rendered or products sold to the Company, or a prospective target business with which the Company has entered into a written letter of intent, confidentiality or other similar agreement or business combination agreement, reduce the amount of funds in the Trust Account to below the lesser of (i) $10.00 per Public Share and (ii) the actual amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per share due to reductions in the value of the Trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the Trust Account (whether or not such waiver is enforceable) nor will it apply to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). In the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have vendors, service providers (except the Company’s independent registered public accounting firm), prospective target businesses or other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account. There can be no guarantee that the Company will be successful in obtaining such waivers from its targeted vendors and service providers.
F-29
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these condensed financial statements. The condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
In February 2022, the Russian Federation and Belarus commenced a military action with the country of Ukraine. As a result of this action, various nations, including the United States, have instituted economic sanctions against the Russian Federation and Belarus. Further, the impact of this action and related sanctions on the world economy are not determinable as of the date of these unaudited condensed financial statements and the specific impact on the Company’s financial condition, results of operations, and cash flows is also not determinable as of the date of these unaudited condensed financial statements.
Liquidity and Going Concern
As of June 30, 2022, the Company had approximately $615,000 in its operating bank account, and working capital deficit of approximately $1.1 million.
The Company’s liquidity needs to date have been satisfied through a contribution of $25,000 from Sponsor to cover for certain expenses in exchange for the issuance of the Founder Shares, the loan of approximately $83,000 from the Sponsor under the Note (as defined in Note 5), and the proceeds from the consummation of the Private Placement not held in the Trust Account. The Company repaid the Note in full on December 14, 2020. In addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, provide the Company Working Capital Loans (as defined in Note 5). As of June 30, 2022 and December 31, 2021, there were no amounts outstanding under any Working Capital Loan.
Based on the foregoing, management believes that the Company will have sufficient working capital and borrowing capacity from the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors to meet its needs through the earlier of the consummation of a Business Combination or one year from this filing. However, in connection with the company’s assessment of going concern considerations in accordance with FASB Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about the company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should the Company be required to liquidate after December 11, 2022. The condensed financial statements do not include any adjustment that might be necessary if the company is unable to continue as a going concern. Management intends to complete the Business Combination prior to the liquidation date.
Note 2-Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed financial statements are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States of America (“GAAP”) for financial information and pursuant to the rules and regulations of the SEC. Accordingly, certain disclosures included in the annual financial statements have been condensed or omitted from these financial statements as they are not required for interim financial statements under U.S. GAAP and the rules of the SEC. In the opinion of management, the
F-30
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
unaudited condensed financial statements reflect all adjustments, which include only normal recurring adjustments necessary for the fair statement of the balances and results for the periods presented. Operating results for the three and six months ended June 30, 2022 are not necessarily indicative of the results that may be expected through December 31, 2022, or any future period.
The accompanying unaudited condensed financial statements should be read in conjunction with the audited financial statements and notes thereto included in the Form 10-K filed by the Company with the SEC on March 25, 2022.
Emerging Growth Company
As an emerging growth company, the Company may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the Jumpstart our Business Startups Act of 2021 (the “JOBS Act”) exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard.
This may make comparison of the Company’s unaudited condensed financial statements with another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Use of Estimates
The preparation of unaudited condensed financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed financial statements and the reported amounts of revenues and expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the unaudited condensed financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. One of the more significant accounting estimates included in these condensed financial statements is the determination of the fair value of the warrant liability. Accordingly, the actual results could differ significantly from those estimates.
F-31
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no cash equivalents as of June 30, 2022 and December 31, 2021.
Investments Held in the Trust Account
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. When the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. When the Company’s investments held in the Trust Account are comprised of money market funds, the investments are recognized at fair value. Trading securities and investments in money market funds are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in interest income from investments held in the Trust Account in the accompanying statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution, which, at times, may exceed the Federal Deposit Insurance Corporation coverage limit of $250,000. As of June 30, 2022 and December 31, 2021, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities which qualify as financial instruments under the FASB ASC Topic 820, “Fair Value Measurements” approximates the carrying amounts represented in the balance sheets, primarily due to their short-term nature, except for the derivative warrant liabilities (see Note 8).
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers consist of:
| • | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| • | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| • | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
F-32
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Offering Costs Associated with the Initial Public Offering
Offering costs consisted of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial Public Offering. Offering costs are allocated to the separable financial instruments issued in the Initial Public Offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with derivative warrant liabilities are expensed as incurred, presented as other expenses in the statement of operations. Offering costs associated with the Public Shares were charged against the carrying value of the Class A ordinary shares subject to redemption upon the completion of the Initial Public Offering. The Company classifies deferred underwriting commissions as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
Derivative Warrant Liabilities
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company evaluates all of its financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC Topic 480 and ASC Subtopic 815-15 “Derivatives and Hedging-Embedded Derivatives” (“ASC Subtopic 815-15”). The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The warrants issued in connection with the Initial Public Offering (the “Public Warrants”) and the Private Placement Warrants (as defined in Note 4) are recognized as derivative liabilities in accordance with “Derivatives and Hedging-Contracts in Entity’s Own Equity” (“ASC Subtopic 815-40”). Accordingly, the Company recognizes the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company’s statement of operations. The fair value of the Public Warrants issued in connection with the Public Offering and Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and have subsequently been measured based on the listed market price of such warrants.
Class A Ordinary Shares Subject to Possible Redemption
The Company accounts for its Class A ordinary shares subject to possible redemption in accordance with the guidance in ASC Topic 480. Class A ordinary shares subject to mandatory redemption (if any) are classified as liability instruments and are measured at fair value. Conditionally redeemable Class A ordinary shares (including Class A ordinary shares that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, Class A ordinary shares are classified as shareholders’ equity. The Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, at June 30, 2022 and December 31, 2021, 13,800,000 shares of Class A ordinary shares subject to possible redemption are presented as temporary equity, respectively, outside of the shareholders’ deficit section of the Company’s balance sheets.
Under ASC 480-10-S99, the Company has elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of the reporting
F-33
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
period. This method would view the end of the reporting period as if it were also the redemption date of the security. Effective with the closing of the Initial Public Offering, the Company recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Income Taxes
ASC Topic 740, “Income Taxes” prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company’s management determined that the Cayman Islands is the Company’s only major tax jurisdiction. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts for interest and penalties as of June 30, 2022 and December 31, 2021. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position.
There is currently no taxation imposed on income by the Government of the Cayman Islands. In accordance with Cayman federal income tax regulations, income taxes are not levied on the Company. Consequently, income taxes are not reflected in the Company’s condensed financial statements. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
Net Income (Loss) per Ordinary Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” The Company has two classes of shares, which are referred to as Class A ordinary shares and Class B ordinary shares. Income and losses are shared pro rata between the two classes of shares. This presentation assumes a business combination as the most likely outcome. Net income per ordinary share is calculated by dividing the net income by the weighted average shares of ordinary shares outstanding for the respective period.
The calculation of diluted net income does not consider the effect of the warrants underlying the Units sold in the Initial Public Offering (including the consummation of the over-allotment) and the Private Placement Warrants to purchase an aggregate of 4,767,000 Class A ordinary shares in the calculation of diluted income per share, because their exercise is contingent upon future events. As a result, diluted net income per ordinary share is the same as basic net income per ordinary share for the three and six months ended June 30, 2022 and 2021. Accretion associated with the redeemable Class A ordinary shares is excluded from earnings per share as the redemption value approximates fair value.
F-34
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The following table reflects presents a reconciliation of the numerator and denominator used to compute basic and diluted net income (loss) per ordinary share for each class of ordinary shares:
| For the Three Months Ended June 30, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| Class A | Class B | Class A | Class B | |||||||||||||
Basic and diluted net loss per ordinary share: | ||||||||||||||||
Numerator: | ||||||||||||||||
Allocation of net loss | $ | (944,930 | ) | $ | (227,957 | ) | $ | (1,046,766 | ) | $ | (252,524 | ) | ||||
Denominator: | ||||||||||||||||
Basic and diluted weighted average ordinary shares outstanding | 14,301,000 | 3,450,000 | 14,301,000 | 3,450,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted net loss per ordinary share | $ | (0.07 | ) | $ | (0.07 | ) | $ | (0.07 | ) | $ | (0.07 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
| For the Six Months Ended June 30, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| Class A | Class B | Class A | Class B | |||||||||||||
Basic and diluted net income per ordinary share: | ||||||||||||||||
Numerator: | ||||||||||||||||
Allocation of net income | $ | 170,584 | $ | 41,152 | $ | 1,223,610 | $ | 295,186 | ||||||||
Denominator: | ||||||||||||||||
Basic and diluted weighted average ordinary shares outstanding | 14,301,000 | 3,450,000 | 14,301,000 | 3,450,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted net income per ordinary share | $ | 0.01 | $ | 0.01 | $ | 0.09 | $ | 0.09 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Recent Issued Accounting Standards
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic 820 “Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. The ASU amends ASC 820 to clarify that a contractual sales restriction is not considered in measuring an equity security at fair value and to introduce new disclosure requirements for equity securities subject to contractual sale restrictions that are measured at fair value. The ASU applies to both holders and issuers of equity and equity-linked securities measured at fair value. The amendments in this ASU are effective for the Company in fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance. The Company is still evaluating the impact of this pronouncement on the condensed financial statements.
The Company’s management does not believe that any other recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on the accompanying condensed financial statements.
F-35
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 3-Initial Public Offering
On December 11, 2020, the Company consummated its Initial Public Offering of 13,800,000 Units, including 1,800,000 Over-Allotment Units, at $10.00 per Unit, generating gross proceeds of $138.0 million, and incurring offering costs of approximately $8.1 million, inclusive of approximately $4.8 million in deferred underwriting commissions.
Each Unit consists of one Class A ordinary share and one-third of one Public Warrant. Each whole Public Warrant will entitle the holder to purchase one Class A ordinary share at an exercise price of $11.50 per share, subject to adjustment (see Note 7).
Note 4-Private Placement
Simultaneously with the closing of the Initial Public Offering, the Company consummated the Private Placement of 501,000 Private Placement Units, at a price of $10.00 per Private Placement Unit with the Sponsor, generating gross proceeds of approximately $5.0 million.
Each Private Placement Unit consists of one Class A ordinary share and one-third of one redeemable warrant. Each whole private placement warrant underlying the Private Placement Units (the “Private Placement Warrants”) is exercisable for one whole share of Class A ordinary shares at a price of $11.50 per share. A portion of the proceeds from the sale of the Private Placement Warrants to the Sponsor was added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the Private Placement Units will expire worthless. The Private Placement Warrants will be non-redeemable except as described below in Note 7 and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees.
The Sponsor and the Company’s officers and directors agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Units until 30 days after the completion of the initial Business Combination.
Note 5-Related Party Transactions
Founder Shares
On October 7, 2020, the Sponsor paid an aggregate of $25,000 for certain expenses on behalf of the Company in exchange for issuance of 2,875,000 Class B ordinary shares (the “Founder Shares”). On November 20, 2020, the Sponsor transferred 30,000 Founder Shares to each of the directors other than the Chairman. On December 8, 2020, the Company effected a share sub-division, resulting in an increase in the total number of Founder Shares outstanding from 2,875,000 to 3,450,000 shares. All shares and associated amounts have been retroactively restated to reflect the share sub-division. The Sponsor agreed to forfeit up to an aggregate of 450,000 Founder Shares to the extent that the option to purchase additional units was not exercised in full by the underwriters so that the Founder Shares would represent 20% of the Company’s issued and outstanding shares after the Initial Public Offering. The underwriters fully exercised the over-allotment option on December 11, 2020; thus, these Founder Shares were no longer subject to forfeiture.
The Initial Shareholders agreed not to transfer, assign or sell any of their Founder Shares until the earlier to occur of: (A) one year after the completion of the initial Business Combination or (B) subsequent to the initial Business Combination, (x) if the closing price of Class A ordinary shares equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the initial Business Combination, or
F-36
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
(y) the date on which the Company completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all of the Public Shareholders having the right to exchange their ordinary shares for cash, securities or other property.
Related Party Loans
On October 7, 2020, the Sponsor agreed to loan the Company up to $300,000 to be used for the payment of costs related to the Initial Public Offering pursuant to a promissory note (the “Note”). The Note is non-interest bearing, unsecured and due upon the closing of the Initial Public Offering. The Sponsor paid an aggregate of approximately $83,000 to cover for the Company’s expenses under the Note. On December 14, 2020, the Company fully repaid the Note. The facility is no longer available to be drawn.
In addition, in order to fund working capital deficiencies or finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company may repay the Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans may be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1.5 million of such Working Capital Loans may be convertible into private placement units of the post Business Combination entity at a price of $10.00 per unit. The private placement units would be identical to the public units sold, subject to certain limited exceptions. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. As of June 30, 2022 and December 31, 2021, the Company had no borrowings under the Working Capital Loans.
Administrative Services Agreement
The Company entered into an agreement that provided that, commencing on the date that the Company’s securities were first listed on the Nasdaq through the earlier of consummation of the initial Business Combination and the liquidation, the Company agreed to pay the Sponsor $10,000 per month for office space, secretarial and administrative services provided to the Company. During the three months ended June 30, 2022 and 2021, the Company incurred approximately $30,000 for expenses in connection with the Administrative Services Agreement. During the six months ended June 30, 2022 and 2021, the Company incurred approximately $60,000 for expenses in connection with the Administrative Services Agreement. As of June 30, 2022 and December 31, 2021, we had no amounts payable for such services.
In addition, the Sponsor, officers and directors, or any of their respective affiliates will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable Business Combinations. The audit committee will review on a quarterly basis all payments that were made by the Company to the Sponsor, officers or directors, or the Company’s or their affiliates. Any such payments prior to an initial Business Combination will be made from funds held outside the Trust Account.
F-37
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 6-Commitments and Contingencies
Registration and Shareholder Rights
The holders of the Founder Shares, Private Placement Units, Private Placement Shares, Private Placement Warrants, Class A ordinary shares underlying the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans (and any Class A ordinary shares issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans) are entitled to registration rights pursuant to a registration and shareholder rights agreement signed upon the effective date of the Initial Public Offering. The holders of these securities are entitled to make up to three demands, excluding short form demands, that the Company registers such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the initial Business Combination. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option from the date of the prospectus relating to the Initial Public Offering to purchase up to 1,800,000 additional Units at the Initial Public Offering price less the underwriting discounts and commissions. The underwriters fully exercised the over-allotment option on December 11, 2020.
The underwriters were entitled to an underwriting discount of $0.20 per unit, or approximately $2.8 million in the aggregate, which was paid upon the closing of the Initial Public Offering. In addition, $0.35 per unit, or approximately $4.8 million in the aggregate will be payable to the underwriters for deferred underwriting commissions. The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement.
Note 7-Class A Ordinary Shares Subject To Possible Redemption
The Company’s Class A ordinary shares feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of future events. The Company is authorized to issue 479,000,000 ordinary shares with a par value of $0.0001 per share. Holders of the Company’s Class A ordinary shares are entitled to one vote for each share. As of June 30, 2022 and December 31, 2021, there were 14,301,000 Class A ordinary shares issued and outstanding, of which 13,800,000 shares were subject to possible redemption have been classified as temporary equity.
F-38
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Class A ordinary shares subject to possible redemption reflected on the condensed balance sheets is reconciled on the following table:
Gross proceeds | $ | 138,000,000 | ||
Less: | ||||
Fair value of Public Warrants at issuance | (7,682,000 | ) | ||
Offering costs allocated to Class A ordinary shares subject to possible redemption | (7,653,636 | ) | ||
Plus: | ||||
Accretion on Class A ordinary shares subject to possible redemption amount | 15,335,636 | |||
|
| |||
Accretion on Class A ordinary shares subject to possible redemption, December 31, 2021 | 138,000,000 | |||
Increase in redemption value of Class A ordinary shares subject to possible redemption | 32,977 | |||
|
| |||
Class A ordinary shares subject to possible redemption, June 30, 2022 | $ | 138,032,977 | ||
|
|
Note 8-Derivative Warrant Liabilities
As of June 30, 2022 and December 31, 2021, the Company has 4,600,000 and 167,000 Public Warrants and Private Placement Warrants, respectively, outstanding.
Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b) 12 months from the closing of the Initial Public Offering; provided in each case that the Company has an effective registration statement under the Securities Act covering the Class A ordinary shares issuable upon exercise of the Public Warrants and a current prospectus relating to them is available and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder (or the Company permits holders to exercise their warrants on a cashless basis under certain circumstances). The Company agreed that as soon as practicable, but in no event later than 20 business days after the closing of the initial Business Combination, the Company will use commercially reasonable efforts to file with the SEC a registration statement covering the Class A ordinary shares issuable upon exercise of the warrants and to maintain a current prospectus relating to those Class A ordinary shares until the warrants expire or are redeemed, as specified in the warrant agreement. If a registration statement covering the Class A ordinary shares issuable upon exercise of the warrants is not effective by the 60th day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption. Notwithstanding the above, if the Class A ordinary shares are at the time of any exercise of a warrant not listed on a national securities exchange such that they satisfy the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, and in the event the Company does not so elect, it will use commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
F-39
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The warrants have an exercise price of $11.50 per share, subject to adjustments, and will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation. In addition, if (x) the Company issues additional Class A ordinary shares or equity-linked securities for capital raising purposes in connection with the closing of the initial Business Combination at an issue price or effective issue price of less than $9.20 per Class A ordinary share (with such issue price or effective issue price to be determined in good faith by the board of directors and, in the case of any such issuance to the Initial Shareholders or their affiliates, without taking into account any Founder Shares held by the Initial Shareholders or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial Business Combination on the date of the consummation of the initial Business Combination (net of redemptions), and (z) the volume weighted average trading price of Class A ordinary shares during the 10-trading day period starting on the trading day prior to the day on which the Company consummates its initial Business Combination (such price, the “Market Value”) is below $9.20 per share, then the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the higher of the Market Value and the Newly Issued Price, the $18.00 per share redemption trigger price will be adjusted (to the nearest cent) to be equal to 180% of the higher of the Market Value and the Newly Issued Price (and the $10.00 per share redemption trigger price will be adjusted (to the nearest cent) to be equal to the higher of the Market Value and the Newly Issued Price see “Redemption of warrants for cash when the price per class A ordinary share equals or exceeds $18.00” and “Redemption of warrants for Class A ordinary shares when the price per class A ordinary share equals or exceeds $10.00” as described below).
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering, except (i) that the Private Placement Warrants and the Class A ordinary shares issuable upon exercise of the Private Placement Warrants will not be transferable, assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions, (ii) except as described below, the Private Placement Warrants will be non-redeemable so long as they are held by the Sponsor or such its permitted transferees and (iii) the Sponsor or its permitted transferees will have the option to exercise the Private Placement Warrants on a cashless basis and have certain registration rights. If the Private Placement Warrants are held by someone other than the Sponsor or its permitted transferees, the Private Placement Warrants will be redeemable by the Company in all redemption scenarios and exercisable by such holders on the same basis as the Public Warrants.
Redemption of warrants for cash when the price per Class A ordinary share equals or exceeds $18.00:
Once the warrants become exercisable, the Company may call the outstanding warrants for redemption (except as described herein with respect to the Private Placement Warrants):
| • | in whole and not in part; |
| • | at a price of $0.01 per warrant; |
| • | upon a minimum of 30 days’ prior written notice of redemption to each warrant holder; and |
| • | if, and only if, the last reported sales price (the “closing price”) of Class A ordinary shares equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to the warrant holders (the “Reference Value”). |
The Company will not redeem the warrants as described above unless a registration statement under the Securities Act covering the issuance of the Class A ordinary shares issuable upon exercise of the warrants is then
F-40
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
effective and a current prospectus relating to those Class A ordinary shares is available throughout the 30-day redemption period. If and when the warrants become redeemable by the Company, it may exercise its redemption right even if the Company is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Redemption of warrants for Class A ordinary shares when the price per Class A ordinary share equals or exceeds $10.00:
After the warrants become exercisable, the Company may redeem the outstanding warrants:
| • | in whole and not in part; |
| • | at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of Class A ordinary shares to be determined by reference to an agreed table based on the redemption date and the “fair market value” of Class A ordinary shares; |
| • | if, and only if, the closing price of Class A ordinary shares equals or exceeds $10.00 per Public Share (as adjusted per share subdivisions, share dividends, reorganizations, recapitalizations and the like) on the trading day before the Company sends the notice of redemption to the warrant holders; and |
| • | if the Reference Value is less than $18.00 per share (as adjusted for share splits, share dividends, rights issuances, subdivisions, reorganizations, recapitalizations and the like), then the Private Placement Warrants must also concurrently be called for redemption on the same terms (except as described herein with respect to a holder’s ability to cashless exercise its warrants) as the outstanding Public Warrants as described above. |
The “fair market value” of Class A ordinary shares for the above purpose shall mean the volume weighted average price of Class A ordinary shares during the 10 trading days immediately following the date on which the notice of redemption is sent to the holders of warrants. In no event will the warrants be exercisable on a cashless basis in connection with this redemption feature for more than 0.361 Class A ordinary shares per warrant (subject to adjustment).
In no event will the Company be required to net cash settle any warrant. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
Note 9-Shareholders’ Deficit
Preference Shares-The Company is authorized to issue 1,000,000 preference shares with a par value of $0.0001 per share. At June 30, 2022 and December 31, 2021, there were no preference shares issued or outstanding.
Class A Ordinary Shares-The Company is authorized to issue 479,000,000 Class A ordinary shares with a par value of $0.0001 per share. Holders of the Company’s Class A ordinary shares are entitled to one vote for each share. At June 30, 2022 and December 31, 2021, there were 14,301,000 Class A ordinary shares issued and outstanding, of which 13,800,000 shares were subject to possible redemption have been classified as temporary equity (see Note 7).
F-41
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Class B Ordinary Shares-The Company is authorized to issue 20,000,000 Class B ordinary shares with a par value of $0.0001 per share. On October 7, 2020, the Company issued 2,875,000 Class B ordinary shares. On December 8, 2020, the Company effected a share sub-division, resulting in an increase in the total number of Class B ordinary shares outstanding from 2,875,000 to 3,450,000 shares. All shares and associated amounts have been retroactively restated to reflect the share sub-division. Of the 3,450,000 Class B ordinary shares outstanding, up to 450,000 shares were subject to forfeiture, to the Company by the Initial Shareholders for no consideration to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the Initial Shareholders would collectively own 20% of the Company’s issued and outstanding ordinary shares after the Initial Public Offering (excluding the Private Placement Shares and assuming the initial shareholders do not purchase any units in the Initial Public Offering). The underwriters fully exercised the over-allotment option on December 11, 2020; thus, these 450,000 Class B ordinary shares were no longer subject to forfeiture. At June 30, 2022 and December 31, 2021, there were 3,450,000 Class B ordinary shares issued and outstanding period. Class A and Class B ordinary shareholders of record are entitled to one vote for each share held on all matters to be voted on by shareholders. Except as described below, holders of Class A ordinary shares and holders of Class B ordinary shares will vote together as a single class on all matters submitted to a vote of the shareholders except as required by law. Prior to the initial Business Combination, only holders of the Founder Shares will have the right to vote on the appointment of directors. Holders of the Public Shares will not be entitled to vote on the appointment of directors during such time. In addition, prior to the completion of an initial Business Combination, holders of a majority of the Founder Shares may remove a member of the board of directors for any reason. The provisions of the Amended and Restated Memorandum and Articles of Association governing the appointment or removal of directors prior to the initial Business Combination may only be amended by a special resolution passed by holders representing at least two-thirds of the issued and outstanding Class B ordinary shares.
The Class B ordinary shares will automatically convert into Class A ordinary shares on the first business day following the consummation of the initial Business Combination at a ratio such that the number of Class A ordinary shares issuable upon conversion of all Founder Shares will equal, in the aggregate, on an as-converted basis, 20% of the sum of (i) the total number of ordinary shares (excluding the Private Placement Shares) issued and outstanding upon the consummation of the Initial Public Offering, plus (ii) the sum of the total number of Class A ordinary shares issued or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the initial Business Combination (net of any redemptions of Class A ordinary shares by Public Shareholders), excluding any Class A ordinary shares or equity-linked securities exercisable for or convertible into Class A ordinary shares issued, deemed issued, or to be issued, to any seller in the initial Business Combination and any Private Placement Units issued to the Sponsor, members of the founding team or any of their affiliates upon conversion of Working Capital Loans. In no event will the Class B ordinary shares convert into Class A ordinary shares at a rate of less than one-to-one.
F-42
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 10-Fair Value Measurements
The following table presents information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of June 30, 2022 and December 31, 2021 and indicates the fair value hierarchy of the valuation techniques that the Company utilized to determine such fair value.
June 30, 2022
| Description | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Other Unobservable Inputs (Level 3) | |||||||||
Assets: | ||||||||||||
Investments held in Trust Account | $ | 138,132,977 | $ | — | $ | — | ||||||
Liabilities: | ||||||||||||
Derivative warrant liabilities | $ | — | $ | 381,360 | $ | — | ||||||
December 31, 2021
| Description | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Other Unobservable Inputs (Level 3) | |||||||||
Assets: | ||||||||||||
Investments held in Trust Account | $ | 138,017,009 | $ | — | $ | — | ||||||
Liabilities: | ||||||||||||
Derivative warrant liabilities | $ | 2,714,000 | $ | 98,530 | $ | — | ||||||
Transfers to/from Levels 1, 2, and 3 are recognized at the beginning of the reporting period. The estimated fair value of the Public Warrants of $7,084,000 transferred from a Level 3 measurement to a Level 1 fair value measurement in January 2021, when the Public Warrants were separately listed and traded. The estimated fair value of the Private Warrants of $257,180 was transferred from a Level 3 measurement to a Level 2 fair value measurement in January 2021, as the transfer of Private Placement Warrants to anyone who is not a permitted transferee would result in the Private Placement Warrants having substantially the same terms as the Public Warrants, the Company determined that the fair value of each Private Placement Warrant is equivalent to that of each Public Warrant. The estimated fair value of Public Warrants was transferred from a Level 1 measurement to a Level 2 measurement due to lack of trading activity as of June 30, 2022. There were no other transfers to/from Levels 1, 2, and 3 during the six months ended June 30, 2022.
For the three and six months ended June 30, 2022, the Company recognized a gain in the unaudited condensed statements of operations resulting from a decrease in fair value of the derivative warrant liabilities of approximately $715,000 and $2.4 million, respectively, presented as change in fair value of derivative warrant liabilities in the accompanying unaudited condensed statements of operations.
Level 1 instruments include investments in mutual funds invested in government securities. The Company uses inputs such as actual trade data, benchmark yields, quoted market prices from dealers or brokers, and other similar sources to determine the fair value of its investments.
F-43
Table of Contents
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 11-Subsequent Events
The Company evaluated subsequent events and transactions that occurred up to the date the unaudited condensed financial statements were issued. Based upon this review, except for as noted below, the Company did not identify any other subsequent events that would have required adjustment or disclosure in the unaudited condensed financial statements.
On July 25, 2022, the Company entered into a Business Combination Agreement (as it may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), by and among the Company, NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”), NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“NewAmsterdam Pharma”) and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”).
Concurrently with the execution of the Business Combination Agreement, the Company and Holdco entered into subscription agreements with certain investors (collectively, the “PIPE Investors”), pursuant to which, among other things, such PIPE Investors agreed to subscribe for and purchase, and Holdco agreed to issue and sell to such PIPE Investors, 23,460,000 ordinary shares of Holdco, nominal value EUR 0.12, at $10.00 per share (the “PIPE Shares”), for an aggregate of $234,600,000 in gross proceeds (the “PIPE Financing”). The closing of the PIPE Financing is contingent upon, among other things, the substantially concurrent consummation of the Business Combination and related transactions.
F-44
Table of Contents
REPORTOF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of NewAmsterdam Pharma Holding B.V.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of NewAmsterdam Pharma Holding B.V. and its subsidiaries (the “Company”) as at December 31, 2021 and 2020, the related consolidated statements of profit or loss and other comprehensive loss, changes in equity, and cash flows for each of the
two years in the period ended December 31, 2021 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as at December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the two years ended December 31, 2021 and 2020, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte Accountants B.V.
Rotterdam, The Netherlands
August 3, 2022
We have served as the Company’s auditor since 2020.
F-45
Table of Contents
CONSOLIDATEDSTATEMENTSOFPROFITORLOSS
ANDOTHERCOMPREHENSIVELOSS
for the years ended December 31, 2021 and 2020
(in thousands of Euro, except per share amounts)
| As at | ||||||||||||
| Note | December 31, 2021 | December 31, 2020 | ||||||||||
Research and development expenses | 4 | (25,032 | ) | (4,045 | ) | |||||||
General and administrative expenses | 5 | (4,803 | ) | (1,384 | ) | |||||||
|
|
|
| |||||||||
Total operating expenses | (29,835 | ) | (5,429 | ) | ||||||||
Finance income | 10 | 9 | — | |||||||||
Finance expense | 18-19 | (216 | ) | (344 | ) | |||||||
Net foreign exchange gain | 1,443 | 24 | ||||||||||
Loss before tax | (28,599 | ) | (5,749 | ) | ||||||||
|
|
|
| |||||||||
Income tax expense | 8 | — | — | |||||||||
Loss for the period | (28,599 | ) | (5,749 | ) | ||||||||
|
|
|
| |||||||||
Comprehensive income, net of tax | — | — | ||||||||||
|
|
|
| |||||||||
Total comprehensive loss for the period, net of tax | (28,599 | ) | (5,749 | ) | ||||||||
|
|
|
| |||||||||
Basic and diluted loss per share | ||||||||||||
Ordinary shares | 17 | (2.53 | ) | (1.15 | ) | |||||||
The accompanying notes are an integral part of these financial statements.
F-46
Table of Contents
CONSOLIDATED STATEMENTSOF FINANCIAL POSITION
as at December 31, 2021 and 2020
(in thousands of Euro)
| As of | ||||||||||||
| Note | December 31, 2021 | December 31, 2020 | ||||||||||
Assets | ||||||||||||
Property, plant and equipment | 9 | 190 | 12 | |||||||||
Loan receivable | 10 | 718 | — | |||||||||
|
|
|
| |||||||||
Non-current assets | 908 | 12 | ||||||||||
Current assets | ||||||||||||
Prepayments and other receivables | 6 | 5,782 | 1,358 | |||||||||
Cash and cash equivalents | 14 | 53,092 | 7,861 | |||||||||
|
|
|
| |||||||||
Total current assets | 58,874 | 9,219 | ||||||||||
|
|
|
| |||||||||
Total assets | 59,782 | 9,231 | ||||||||||
|
|
|
| |||||||||
Equity and liabilities | ||||||||||||
Equity | ||||||||||||
Share capital | 15 | 83,876 | 2,500 | |||||||||
Other reserves | 16 | 591 | — | |||||||||
Accumulated loss | (34,676 | ) | (6,077 | ) | ||||||||
|
|
|
| |||||||||
Total equity | 49,791 | (3,577 | ) | |||||||||
|
|
|
| |||||||||
Non-current liabilities | ||||||||||||
Lease liability | 19 | 111 | — | |||||||||
Current liabilities | ||||||||||||
Loans and borrowings | 18 | — | 11,650 | |||||||||
Lease liability | 19 | 53 | — | |||||||||
Trade and other payables | 7 | 9,827 | 1,158 | |||||||||
|
|
|
| |||||||||
Total current liabilities | 9,880 | 12,808 | ||||||||||
|
|
|
| |||||||||
Total equity and liabilities | 59,782 | 9,231 | ||||||||||
|
|
|
| |||||||||
The accompanying notes are an integral part of these financial statements.
F-47
Table of Contents
CONSOLIDATED STATEMENTSOF CHANGESIN EQUITY
for the years ended December 31, 2021 and 2020
(In thousands of Euro except issued shares)
| Number of Shares Outstanding | ||||||||||||||||||||||||||||||||||||
| Note | Voting Ordinary Shares | Non-Voting Ordinary Shares | Series A Preferred Shares | Issued Capital | Share Premium | Other Reserves | Accumulated Loss | Total Equity | ||||||||||||||||||||||||||||
Opening balance at January 1, 2020 | 2,500,000 | — | — | 25 | 2,475 | — | (325 | ) | 2,175 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Issuance of non-voting shares | 15 | — | 2,500,000 | — | 25 | (25 | ) | — | — | — | ||||||||||||||||||||||||||
Total comprehensive loss for the period | 17 | — | — | — | — | — | — | (5,749 | ) | (5,749 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
As at December 31, 2020 | 2,500,000 | 2,500,000 | — | 50 | 2,450 | — | (6,077 | ) | (3,577 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Opening balance at January 1, 2021 | 2,500,000 | 2,500,000 | — | 50 | 2,450 | — | (6,077 | ) | (3,577 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Conversion of convertible debt | 15 | — | — | 1,111,115 | 11 | 11,656 | — | — | 11,667 | |||||||||||||||||||||||||||
Series A — Tranche 1 | 15 | — | — | 4,928,613 | 49 | 68,951 | — | — | 69,000 | |||||||||||||||||||||||||||
Issuance of non-voting shares | 15 | — | 285,714 | — | 3 | 706 | — | — | 709 | |||||||||||||||||||||||||||
Share-based compensation | 16 | — | — | — | — | — | 591 | — | 591 | |||||||||||||||||||||||||||
Total comprehensive loss for the period | 17 | — | — | — | — | — | — | (28,599 | ) | (28,599 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
As at December 31, 2021 | 2,500,000 | 2,785,714 | 6,039,728 | 113 | 83,762 | 591 | (34,676 | ) | 49,791 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-48
Table of Contents
CONSOLIDATED STATEMENTSOF CASH FLOWS
for the years ended December 31, 2021 and 2020
(in thousands of Euro)
| As of | ||||||||||||
| Note | 2021 | 2020 | ||||||||||
Operating Activities: | ||||||||||||
Loss before tax | (28,599 | ) | (5,749 | ) | ||||||||
Non-cash adjustments to reconcile profit before tax to net cash flows: | ||||||||||||
Depreciation and amortization | 9 | 14 | 1 | |||||||||
Finance income | 10 | (9 | ) | — | ||||||||
Finance expense | 18-19 | 2 | 330 | |||||||||
Foreign exchange gain | (1,443 | ) | (24 | ) | ||||||||
Share-based compensation expense | 16 | 1,049 | — | |||||||||
Changes in working-capital: | ||||||||||||
Changes in prepayments and other receivables | (4,424 | ) | (1,357 | ) | ||||||||
Changes in trade and other payables | 8,246 | 829 | ||||||||||
|
|
|
| |||||||||
Net cash flows used in operating activities | (25,164 | ) | (5,970 | ) | ||||||||
|
|
|
| |||||||||
Investing Activities: | ||||||||||||
Purchase of equipment | 9 | (20 | ) | (13 | ) | |||||||
|
|
|
| |||||||||
Net cash flows used in investing activities | (20 | ) | (13 | ) | ||||||||
|
|
|
| |||||||||
Financing Activities: | ||||||||||||
Proceeds from issuance of shares and capital contributions | 15 | 69,000 | — | |||||||||
Proceeds from issuance of borrowings | 18 | — | 11,320 | |||||||||
Payments of lease liabilities | 19 | (10 | ) | — | ||||||||
|
|
|
| |||||||||
Net cash flows provided by financing activities | 68,990 | 11,320 | ||||||||||
|
|
|
| |||||||||
Net increase in cash and cash equivalents | 43,806 | 5,337 | ||||||||||
Foreign exchange differences | 1,425 | 24 | ||||||||||
Cash and cash equivalents at beginning of period | 7,861 | 2,500 | ||||||||||
|
|
|
| |||||||||
Cash and cash equivalents at end of period, net of overdraft | 14 | 53,092 | 7,861 | |||||||||
|
|
|
| |||||||||
The accompanying notes are an integral part of these financial statements.
F-49
Table of Contents
NOTESTO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 — Description of the Business
NewAmsterdam Pharma Holding B.V. and its subsidiaries (together as “NewAmsterdam Pharma” or the “Company”) is a global biotech company focused on the research and development of transformative therapies for cardio-metabolic diseases. The Company was incorporated in The Netherlands on September 17, 2019. The Company’s principal place of business and registered office is Gooimeer 2-35, 1411 DC Naarden, The Netherlands.
On April 9, 2020, NewAmsterdam Pharma Holding B.V. (previous name NewAmsterdam Pharma B.V.) completed the acquisition of the assets and liabilities of NewAmsterdam Pharma B.V. (previous name Dezima Pharma B.V. (“Dezima”)). Dezima was incorporated in The Netherlands on August 31, 2012. On December 4, 2020, NewAmsterdam Pharma B.V. created a wholly owned subsidiary, NewAmsterdam Pharma Corporation (“Corporation”). The Corporation was incorporated in the United States under the laws of Delaware.
With effect from October 25, 2021, the name of NewAmsterdam Pharma B.V. was changed to NewAmsterdam Pharma Holding B.V. In addition, the name of Dezima Pharma B.V. was changed to NewAmsterdam Pharma B.V.
The consolidated financial statements were authorized by the Company’s Board of Directors (the “Board”) for issuance on August 3, 2022.
The consolidated financial statements are presented in Euro (“EUR” or “€”). In these notes, Euro amounts are presented in thousands, except for share and per share amounts and as otherwise indicated. The functional currency of the Company is Euro.
Note 2 — Basis of Preparation of the Consolidated Financial Statements
Basis of Preparation
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) and the interpretations of the IFRS Interpretations Committee (“IFRS IC”) as issued by the International Accounting Standards Board (“IASB”). The consolidated financial statements have been prepared on a historical cost basis, unless otherwise disclosed.
Basis of Consolidation
The consolidated financial statements include the financial statements of the Company and entities controlled by the Company (its subsidiaries). Control is achieved when the Company:
| • | has power over the investee; |
| • | is exposed, or has rights, to variable returns from its involvement with the investee; and |
| • | has the ability to use its power to affect its returns. |
The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control listed above.
The results of the subsidiaries are included in the consolidated statements of profit or loss and consolidated statements of other comprehensive income from the effective date of acquisition up to the date when control ceases to exist. When necessary, adjustments are made to the financial statements of subsidiaries to bring their accounting policies into line with those used by the Company.
All inter-company transactions and unrealized gains on transactions between the Company and its subsidiaries are eliminated.
F-50
Table of Contents
Going Concern
The Company has incurred a net loss since its inception and for all periods presented. Management has assessed it appropriate to prepare these consolidated financial statements on a going concern basis, taking into account the cash and cash equivalents balance of € 53.092 million as at December 31, 2021 and the second tranche of € 80 million capital contribution received in January 2022. See Note 15 for more information regarding the capital structure of the Company. In June 2022, the Company entered into a licensing agreement with A. Menarini International Licensing S.A. (“Menarini”), whereby the Company received an upfront payment of € 115.0 million in July 2022 in exchange for allowing Menarini to locally develop, obtain and maintain regulatory approval, and commercialize finished products containing obicetrapib and ezetimibe. The upfront payment is expected to bring sufficient additional funding until the end of 2023. See Note 21 for more information regarding the agreement with Menarini.
In this respect, management has not identified any material uncertainties that may cast a significant doubt about the Company’s ability to continue to adopt the going concern basis of accounting for a period at least twelve months from the date when the consolidated financial statements are authorized for issue.
Note 3 — Significant Accounting Policies
Significant Accounting Judgments, Estimates and Assumptions
The preparation of these consolidated financial statements has required management to apply accounting policies and methodologies based on complex and subjective judgments, as well as estimates based on past experience and assumptions determined to be reasonable and realistic based on the related circumstances. The use of these judgements, estimates and assumptions affects the amounts reported in these consolidated financial statements. The final amounts for items for which estimates and assumptions were made in the consolidated financial statements may differ from those reported in these statements due to the uncertainties that characterize the assumptions and conditions on which the estimates are based.
The sources of uncertainty identified by the Company are described together with the applicable Note, as follows:
Significant Accounting Judgment / Source of Estimation Uncertainty | Described in | |||
Research and development expense | Note 4 | |||
Share-based payments | Note 16 | |||
Impairment of Property, Plant and Equipment and Intangible Assets
At each reporting date, the Company reviews the carrying amounts of its property, plant and equipment and intangible assets with a definite life to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated to determine the extent of the impairment loss (if any). Where the asset does not generate cash flows that are independent from other assets, the Company estimates the recoverable amount of the cash-generating unit to which the asset belongs. A cash generating unit is the smallest identifiable group of assets that generates cash inflows that are largely independent of the cash inflows from other assets or groups of assets. When a reasonable and consistent basis of allocation can be identified, corporate assets are also allocated to individual cash-generating units, or otherwise they are allocated to the smallest group of cash-generating units for which a reasonable and consistent allocation basis can be identified.
Intangible assets with an indefinite useful life are tested for impairment at least annually and whenever there is an indication at the end of a reporting period that the asset may be impaired.
Recoverable amount is the higher of fair value less costs of disposal and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects
F-51
Table of Contents
current market assessments of the time value of money and the risks specific to the asset for which the estimates of future cash flows have not been adjusted.
If the recoverable amount of an asset (or cash-generating unit) is estimated to be less than its carrying amount, the carrying amount of the asset (or cash-generating unit) is reduced to its recoverable amount. An impairment loss is recognized immediately in profit or loss.
At the end of each reporting period, the Company determines if there is any indication a previously impaired property, plant and equipment, or intangible asset, excluding goodwill, no longer exists. If indicators are identified, the Company determines if the recoverable amount is greater than its carrying amount. If the recoverable amount is greater than its carrying amount, impairment is reversed. Where an impairment loss subsequently reverses, the carrying amount of the asset (or cash-generating unit) is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset (or cash-generating unit) in prior years. A reversal of an impairment loss is recognized immediately in profit or loss to the extent that it eliminates the impairment loss which has been recognized for the asset in prior years.
Segment Reporting
Operating segments are components of the Company that engage in business activities from which it may incur expenses, for which discrete financial information is available and whose operating results are evaluated regularly by the Company’s Chief Operating Decision Maker (“CODM”) to make decisions about resources to be allocated to the segment and assess its performance. The activities of NewAmsterdam Pharma Holding B.V. are considered to be one segment which comprises the research, development and subsequent commercialization of innovative and proprietary compounds that have a potential therapeutic effect in cardiovascular disease. The Board is identified as the CODM and reviews the operating results regularly to make decisions about the resources and to assess overall performance of the Company.
New and Amended IFRS Standards that are Effective for the Current Year
Amendment to IFRS 16 ‘Leases’
IFRS 16 replaced IAS 17 Leases, the current lease accounting standard, and became effective on January 1, 2019. The new lease standard requires assets leased by the Company to be recognized on the consolidated statement of financial position of the Company with a corresponding liability. Expenses related to leases are reported as depreciation and interest expense in the consolidated statement of profit or loss. In addition, on June 30, 2021 the IASB published Covid-19-Related Rent Concessions (Amendment to IFRS 16) amending the standard to provide lessees with an exemption from assessing whether a COVID-19-related rent concession is a lease modification. The related amendment did not have a material impact on the Company.
New Standards, Interpretations and Amendments Not Yet Adopted by the Company
At the date of authorization of these financial statements, the Company has not applied the following new and revised IFRS Standards that have been issued but are not yet effective. The amendments to the standards and interpretations presented are not expected to have a material impact on the consolidated financial statements in future periods.
Amendments to IAS 1 — Presentation of Financial Statements — Classification of liabilities as Current and Non-current (Effective January 1, 2023)
The amendments to IAS 1 affect only the presentation of liabilities as current or non-current in the statement of financial position and not the amount or timing of recognition of any asset, liability, income or expense, or the information disclosed about those items.
F-52
Table of Contents
The amendments clarify that the classification of liabilities as current or non-current is based on rights that are in existence at the end of the reporting period, specify that classification is unaffected by expectations about whether an entity will exercise its right to defer settlement of a liability, explain that rights are in existence if covenants are complied with at the end of the reporting period, and introduce a definition of ‘settlement’ to make clear that settlement refers to the transfer to the counterparty of cash, equity instruments, other assets or services.
Amendments to IFRS 3 — Reference to the Conceptual Framework (Effective January 1, 2022)
The amendments update IFRS 3 so that it refers to the 2018 Conceptual Framework instead of the 1989 Framework. They also add to IFRS 3 a requirement that, for obligations within the scope of IAS 37, an acquirer applies IAS 37 to determine whether at the acquisition date a present obligation exists as a result of past events. For a levy that would be within the scope of IFRIC 21 Levies, the acquirer applies IFRIC 21 to determine whether the obligating event that gives rise to a liability to pay the levy has occurred by the acquisition date. Finally, the amendments add an explicit statement that an acquirer does not recognize contingent assets acquired in a business combination.
Amendments to IAS 16 — Property, Plant and Equipment — Proceeds before Intended Use (Effective January 1, 2022)
The amendments prohibit deducting from the cost of an item of property, plant and equipment any proceeds from selling items produced before that asset is available for use, i.e. proceeds while bringing the asset to the location and condition necessary for it to be capable of operating in the manner intended by management. Consequently, an entity recognizes such sales proceeds and related costs in profit or loss. The entity measures the cost of those items in accordance with IAS 2 Inventories. The amendments also clarify the meaning of ‘testing whether an asset is functioning properly’. IAS 16 now specifies this as assessing whether the technical and physical performance of the asset is such that it is capable of being used in the production or supply of goods or services, for rental to others, or for administrative purposes. If not presented separately in the statement of comprehensive income, the financial statements shall disclose the amounts of proceeds and cost included in profit or loss that relate to items produced that are not an output of the entity’s ordinary activities, and which line item(s) in the statement of comprehensive income include(s) such proceeds and cost. The amendments are applied retrospectively, but only to items of property, plant and equipment that are brought to the location and condition necessary for them to be capable of operating in the manner intended by management on or after the beginning of the earliest period presented in the financial statements in which the entity first applies the amendments. The entity shall recognize the cumulative effect of initially applying the amendments as an adjustment to the opening balance of retained earnings (or other component of equity, as appropriate) at the beginning of that earliest period presented.
Amendments to IAS 37 — Onerous Contracts — Cost of Fulfilling a Contract (Effective January 1, 2022)
The amendments specify that the ‘cost of fulfilling’ a contract comprises the ‘costs that relate directly to the contract’. Costs that relate directly to a contract consist of both the incremental costs of fulfilling that contract (examples would be direct labor or materials) and an allocation of other costs that relate directly to fulfilling contracts (an example would be the allocation of the depreciation charge for an item of property, plant and equipment used in fulfilling the contract). The amendments apply to contracts for which the entity has not yet fulfilled all its obligations at the beginning of the annual reporting period in which the entity first applies the amendments. Comparatives are not restated. Instead, the entity shall recognize the cumulative effect of initially applying the amendments as an adjustment to the opening balance of retained earnings or other component of equity, as appropriate, at the date of initial application.
Amendments to IAS 1 — Presentation of Financial Statements and IFRS Practice Statement 2 — Making Materiality Judgements — Disclosure of Accounting Policies (Effective January 1, 2023)
The amendments change the requirements in IAS 1 with regard to disclosure of accounting policies. The amendments replace all instances of the term ‘significant accounting policies’ with ‘material accounting policy
F-53
Table of Contents
information’. Accounting policy information is material if, when considered together with other information included in an entity’s financial statements, it can reasonably be expected to influence decisions that the primary users of general purpose financial statements make on the basis of those financial statements.
The supporting paragraphs in IAS 1 are also amended to clarify that accounting policy information that relates to immaterial transactions, other events or conditions is immaterial and need not be disclosed. Accounting policy information may be material because of the nature of the related transactions, other events or conditions, even if the amounts are immaterial. However, not all accounting policy information relating to material transactions, other events or conditions is itself material.
The Board has also developed guidance and examples to explain and demonstrate the application of the ‘four-step materiality process’ described in IFRS Practice Statement 2.
Amendments to IAS 8 — Accounting Policies, Changes in Accounting Estimates and Errors — Definition of Accounting Estimates (Effective January 1, 2023)
The amendments replace the definition of a change in accounting estimates with a definition of accounting
estimates. Under the new definition, accounting estimates are “monetary amounts in financial statements that are subject to measurement uncertainty.”
The definition of a change in accounting estimates was deleted. However, the Board retained the concept of changes in accounting estimates in the Standard with the following clarifications:
| • | A change in accounting estimate that results from new information or new developments is not the correction of an error. |
| • | The effects of a change in an input or a measurement technique used to develop an accounting estimate are changes in accounting estimates if they do not result from the correction of prior period errors. |
Amendments to IAS 12 — Income Taxes — Deferred Tax related to Assets and Liabilities arising from a Single Transaction (Effective January 1, 2023)
The amendments introduce a further exception from the initial recognition exemption. Under the amendments, an entity does not apply the initial recognition exemption for transactions that give rise to equal taxable and deductible temporary differences.
Depending on the applicable tax law, equal taxable and deductible temporary differences may arise on initial recognition of an asset and liability in a transaction that is not a business combination and affects neither accounting nor taxable profit. For example, this may arise upon recognition of a lease liability and the corresponding right-of-use asset applying IFRS 16 at the commencement date of a lease.
Following the amendments to IAS 12, an entity is required to recognize the related deferred tax asset and liability, with the recognition of any deferred tax asset being subject to the recoverability criteria in IAS 12.
The amendments apply to transactions that occur on or after the beginning of the earliest comparative period presented. In addition, at the beginning of the earliest comparative period an entity recognizes:
| • | A deferred tax asset (to the extent that it is probable that taxable profit will be available against which the deductible temporary difference can be utilized) and a deferred tax liability for all deductible and taxable temporary differences associated with: |
| • | Right-of-use assets and lease liabilities; or |
F-54
Table of Contents
| • | Decommissioning, restoration and similar liabilities and the corresponding amounts recognized as part of the cost of the related asset. |
| • | The cumulative effect of initially applying the amendments as an adjustment to the opening balance of retained earnings (or other component of equity, as appropriate) at that date. |
Annual Improvements to IFRS Standards 2018-2020
Amendments to IFRS 9 — Financial Instruments (Effective January 1, 2022)
The amendment clarifies that in applying the ‘10 percent’ test to assess whether to derecognize a financial liability, an entity includes only fees paid or received between the entity (the borrower) and the lender, including fees paid or received by either the entity or the lender on the other’s behalf. The amendment is applied prospectively to modifications and exchanges that occur on or after the date the entity first applies the amendment.
Operating Activities of the Company
Note 4 — Research and Development Expense
Accounting Policies
Research and development costs are recognized as an expense when incurred and are typically made up of clinical and preclinical activities, drug development and manufacturing costs, and include costs for clinical research organizations and investigative sites. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as information provided by vendors on their actual costs incurred. At each balance sheet date, the Company estimates the level of services performed by the vendors and the associated expenditure incurred for the services performed.
Quantification of the research and development expenses incurred during the period requires judgment, because the progress of activities is not directly observable. In estimating progress toward completion of specific tasks, the Company therefore uses (non-) financial data such as number of patient screenings, patient visits, patient enrollment, clinical site activations and vendor information of actual costs incurred. This data is obtained through reports from outside service providers as to the progress or state of completion of trials or the completion of services and reviewed by Company personnel.
Research and development expense consisted of the following items for the years ended December 31, 2021 and 2020:
| 2021 | 2020 | |||||||
Clinical R&D Costs | ||||||||
Phase 1 and 2 | (6,560 | ) | (1,387 | ) | ||||
Phase 3 | (4,257 | ) | (27 | ) | ||||
Other | (25 | ) | (36 | ) | ||||
|
|
|
| |||||
Total clinical R&D costs | (10,842 | ) | (1,450 | ) | ||||
Non-clinical R&D costs | (974 | ) | (41 | ) | ||||
Contracted personnel costs | (3,363 | ) | (994 | ) | ||||
Chemistry, Manufacturing and Controls (CMC) | (8,338 | ) | (1,236 | ) | ||||
Regulatory | (1,452 | ) | (141 | ) | ||||
Other R&D costs | (63 | ) | (183 | ) | ||||
|
|
|
| |||||
Total research and development expenses | (25,032 | ) | (4,045 | ) | ||||
|
|
|
| |||||
F-55
Table of Contents
Note 5 — General and Administrative Expenses
Accounting Policies
Expenses are recognized on the accrual basis when incurred by the Company.
General and administrative expenses consisted of the following items for the years ended December 31, 2021 and 2020:
| 2021 | 2020 | |||||||
Contracted personnel costs | (1,618 | ) | (265 | ) | ||||
Travel costs | (136 | ) | — | |||||
Intellectual property | (708 | ) | (498 | ) | ||||
Legal costs | (771 | ) | (382 | ) | ||||
Licenses | (28 | ) | (6 | ) | ||||
Finance and administration | (270 | ) | (151 | ) | ||||
IT | (20 | ) | (2 | ) | ||||
Rent and office services | (110 | ) | (55 | ) | ||||
Marketing and communication | (710 | ) | (11 | ) | ||||
Insurance | (187 | ) | (11 | ) | ||||
Depreciation and amortization | (14 | ) | (1 | ) | ||||
Miscellaneous | (65 | ) | (2 | ) | ||||
Business development | (135 | ) | — | |||||
Board fees | (31 | ) | — | |||||
|
|
|
| |||||
Total general and administrative expenses | (4,803 | ) | (1,384 | ) | ||||
|
|
|
| |||||
Note 6 — Prepayments and Other Receivables
Accounting Policies
Prepayments and other receivables
Prepayments are recognized upon the occurrence of a payment made for an expenditure during one accounting period that will be consumed and incurred in a future period. Prepayments expire and become expenses with the passage of time, use, or events. Value added tax receivable is measured at cost.
Expected credit losses
An expected credit loss allowance is recognized for all receivables measured at amortized cost. This means that an allowance for doubtful debt is recognized for all receivables even though there may not be objective evidence that the trade or other receivable has been impaired.
The Company applies the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance for all other receivables. To measure the expected credit losses, trade receivables have been grouped based on shared credit risk characteristics and the days past due. Trade receivables are written off when there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, the failure of a debtor to engage in a repayment plan with the Company, and a failure to make contractual payments for a period of greater than 120 days past due. Impairment losses on trade receivables and contract assets are presented as net impairment losses within operating profit. Subsequent recoveries of amounts previously written off are credited against the same line item.
F-56
Table of Contents
Prepayments and other receivables consisted of the following items as at December 31, 2021 and 2020:
| 2021 | 2020 | |||||||
Prepayments | ||||||||
Phase 1 and 2 | — | 290 | ||||||
Chemistry, Manufacturing and Controls (CMC) | 21 | 902 | ||||||
Non-clinical R&D costs | 1,111 | 30 | ||||||
Clinical other | 349 | — | ||||||
General and administrative | 11 | 17 | ||||||
|
|
|
| |||||
Total prepayments | 1,492 | 1,239 | ||||||
Value added tax receivable | 4,290 | — | ||||||
|
|
|
| |||||
Total prepayments and other receivables | 5,782 | 1,239 | ||||||
|
|
|
| |||||
The value added tax receivable was received 2022.
Note 7 — Trade and Other Payables
Trade and other payables consisted of the following items as of December 31, 2021 and 2020:
| 2021 | 2020 | |||||||
Accounts payable | 6,219 | 396 | ||||||
Accrued expenses | 375 | 762 | ||||||
Value added tax payable | 2,774 | — | ||||||
Share-based payment liabilities | 458 | — | ||||||
|
|
|
| |||||
Total trade and other payables | 9,827 | 1,158 | ||||||
|
|
|
| |||||
The share-based payment liabilities are described further in Note 16 for share-based payments.
Note 8 — Income Taxes
Accounting Policies
Income taxes
Income tax expense represents the aggregate amount determined on the profit for the period based on current tax and deferred tax.
In cases where the tax relates to items that are charged to other comprehensive income or directly to equity, the tax is also charged respectively to other comprehensive income or directly to equity.
Current tax
The tax currently payable is based on taxable profit for the year. Taxable profit differs from net profit as reported in profit or loss because it excludes items of income or expense that are taxable or deductible in other years and it further excludes items that are never taxable or deductible. The Company’s liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the reporting period.
A provision is recognized for those matters for which the tax determination is uncertain but it is considered probable that there will be a future outflow of funds to a tax authority. The provisions are measured at the best estimate of the amount expected to become payable. The assessment is based on the judgement of tax professionals within the Company supported by previous experience in respect of such activities and in certain cases based on specialist independent tax advice.
F-57
Table of Contents
Deferred taxation
Deferred tax is the tax expected to be payable or recoverable on differences between the carrying amounts of assets and liabilities in the financial statements and the corresponding tax bases used in the computation of taxable profit, and is accounted for using the liability method. Deferred tax liabilities are generally recognized for all taxable temporary differences and deferred tax assets are recognized to the extent that it is probable that taxable profits will be available against which deductible temporary differences can be utilized. Such assets and liabilities are not recognized if the temporary difference arises from the initial recognition (other than in a business combination) of other assets and liabilities in a transaction that affects neither the taxable profit nor the accounting profit. In addition, a deferred tax liability is not recognized if the temporary difference arises from the initial recognition of goodwill.
Deferred tax liabilities are recognized for taxable temporary differences arising on investments in subsidiaries and associates, and interests in joint ventures, except where the Company is able to control the reversal of the temporary difference and it is probable that the temporary difference will not reverse in the foreseeable future. Deferred tax assets arising from deductible temporary differences associated with such investments and interests are only recognized to the extent that it is probable that there will be sufficient taxable profits against which to utilize the benefits of the temporary differences and they are expected to reverse in the foreseeable future.
The carrying amount of deferred tax assets is reviewed at each reporting date and reduced to the extent that it is no longer probable that sufficient taxable profits will be available to allow all or part of the asset to be recovered.
Deferred tax is calculated at the tax rates that are expected to apply in the period when the liability is settled or the asset is realized based on tax laws and rates that have been enacted or substantively enacted at the reporting date.
The measurement of deferred tax liabilities and assets reflects the tax consequences that would follow from the manner in which the Company expects, at the end of the reporting period, to recover or settle the carrying amount of its assets and liabilities.
Deferred tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when they relate to income taxes levied by the same taxation authority and the Company intends to settle its current tax assets and liabilities on a net basis.
Current income tax is the expected tax expense, payable or receivable on taxable income or loss for the period, using tax rates enacted or substantively enacted at reporting date.
The Company has no income tax reported due to losses incurred in both periods presented.
Fiscal Unity in the Netherlands
With an effective date of January 1, 2021, NewAmsterdam Pharma Holding B.V. formed a fiscal unity with NewAmsterdam Pharma B.V. for corporate income tax purposes. A company and its subsidiaries that are part of the fiscal unity are jointly and severally liable for the tax payable by the fiscal unity.
Effective Tax Rate
The table below outlines the reconciliation between the maximum statutory tax rate in the Netherlands (15% for the first € 245,000 and 25% thereafter (2020: 16.5% for the first € 200.000 and 25% thereafter)) and the effective
F-58
Table of Contents
income tax rates for the Company, together with the corresponding amounts, for the years ended December 31, 2021 and 2020:
| 2021 | 2020 | |||||||
Loss before tax | (28,599 | ) | (5,749 | ) | ||||
|
|
|
| |||||
Income tax benefit at statutory tax rate | 7,125 | 1,420 | ||||||
Difference due to the effects of: | ||||||||
Movement in unrecognized deferred tax assets resulting from carried forward losses | (7,125 | ) | (1,420 | ) | ||||
|
|
|
| |||||
Income tax expense: | — | — | ||||||
|
|
|
| |||||
Effective tax rate | 0 | % | 0 | % | ||||
|
|
|
| |||||
Deferred Taxes
Unused tax losses and other credits carried forwards
The Company recognizes a deferred tax asset for unused tax losses, to the extent that it is probable that the deferred tax asset could be utilized against taxable income within the foreseeable future.
As at December 31, 2021, tax losses can be carried forward indefinitely to offset against future taxable income, with utilization of such losses limited to 50% of taxable income in excess of € 1 million in any respective year. As at December 31, 2021, the Company had assessed losses amounting to € 28,599 thousand (2020: € 6,077 thousand). In addition, the Company assumed carry forward losses from the following tax periods, resulting from the fiscal unity of the Company and NewAmsterdam Pharma B.V. as associated with the acquisition of its assets and liabilities:
Year | Tax loss | |||
2012-2013 | 7.097 | |||
2014 | 11,303 | |||
2015 | 27,255 | |||
2016 | 86,547 | |||
2017 | 1,340 | |||
2018 | 2,303 | |||
2019 | 3,019 | |||
2020 | 6,077 | |||
2021 | 28,599 | |||
| 173,540 | ||||
|
| |||
No deferred tax asset was recognized for these assessed losses as the Company has no products approved for sale, no income from collaboration or partnering agreements, and no sources of income in general.
Investing Activities of the Company
Note 9 — Equipment
Accounting Policies
Items of property, plant and equipment are initially recognized at cost. As well as the purchase price, cost includes directly attributable costs and the estimated present value of any future unavoidable costs of dismantling and removing items. The corresponding liability is recognized within provisions. They are subsequently measured at cost less accumulated depreciation and impairment losses.
F-59
Table of Contents
Depreciation is provided on all items of property, plant and equipment so as to write off their carrying value over their expected useful economic lives or in the case of the right of use asset, over the shorter period of lease term and useful life of the underlying asset. Depreciation is provided at the following rates:
| Right | of use asset — straight line over the shorter period of lease term and useful life of the underlying asset |
| Computer | equipment — 20% per annum straight line |
| Right of Use Asset | Computer Equipment | Total | ||||||||||
(i) Cost or valuation | ||||||||||||
At inception | — | — | — | |||||||||
Additions | — | 13 | 13 | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2020 | — | 13 | 13 | |||||||||
|
|
|
|
|
| |||||||
(ii) Accumulated depreciation and impairment | ||||||||||||
At inception | — | — | — | |||||||||
Depreciation | — | 1 | 1 | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2020 | — | 1 | 1 | |||||||||
|
|
|
|
|
| |||||||
(iii) Net book value | ||||||||||||
At inception | — | — | — | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2020 | — | 12 | 12 | |||||||||
|
|
|
|
|
| |||||||
| Right of Use Asset | Computer Equipment | Total | ||||||||||
(i) Cost or valuation | ||||||||||||
At December 31, 2020 | — | 13 | 13 | |||||||||
Additions | 172 | 20 | 192 | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2021 | 172 | 33 | 205 | |||||||||
|
|
|
|
|
| |||||||
(ii) Accumulated depreciation and impairment | ||||||||||||
At December 31, 2020 | — | 1 | 1 | |||||||||
Depreciation | 10 | 4 | 14 | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2021 | 10 | 5 | 15 | |||||||||
|
|
|
|
|
| |||||||
(iii) Net book value | ||||||||||||
At December 31, 2020 | — | 12 | 12 | |||||||||
|
|
|
|
|
| |||||||
At December 31, 2021 | 162 | 28 | 190 | |||||||||
|
|
|
|
|
| |||||||
The Company leases office premises in Florida. Refer to Note 19 for lease liability details related to the right of use asset.
Note 10 — Loans Receivable
Accounting Policies
Loans and receivables are measured at amortized cost using the effective interest method, less any impairment losses. Long-term receivables are discounted to their net present value. Interest receivable is included in financing income.
F-60
Table of Contents
Loans receivable consisted of the following items as at December 31:
| 2021 | 2020 | |||||||
Loan — Michael Davidson | 709 | — | ||||||
|
|
|
| |||||
Accrued finance expense | 9 | — | ||||||
|
|
|
| |||||
Loans and borrowings | 718 | — | ||||||
|
|
|
| |||||
On June 28, 2021, the company provided a loan facility of €709 thousand to the CEO, M. Davidson, for the payment of a purchase price related to the issued depository receipts issued by the Stichting Administratiekantoor NewAmsterdam (the “STAK”) to the CEO. The company, the CEO and the STAK entered into a founder depository receipt award agreement. Refer to Note 21 for details surrounding repayment.
A right of first ranking pledge on the depository receipts is provided as security for the fulfilment of M. Davidson’s obligations under the loan agreement and depository receipt award agreement. All the terms and provisions of the loan agreement are full recourse obligations of M. Davidson, who shall be liable to fully repay the loan, together with all accrued interest thereon on the last business day of the loan term. This condition continues to apply in full if, in the event of an Exit, the relevant proceeds have been applied by M. Davidson towards repayment of the loan and this has not been sufficient to fully repay the loan and all accrued interest thereon.
Exit means a Deemed Liquidation Event as defined in the Shareholders Agreement related to the Company dated January 11, 2021. A Deemed Liquidation Event is the voluntary conversion upon the sale, transfer, license or disposition of all or substantial part of Company assets or capital, or upon an Initial Public Offering.
Interest shall accrue on the principal amount of the loan at a rate per annum of 2.75%. The interest shall accrue from day to day and be added to the principal amount of the loan as from the date that the loan is made available up to the last business day of the loan term. Outstanding principal and interest is due on the earlier of the tenth anniversary of the agreement, the Exit Date or the date M. Davidson ceases to provide services to the Company.
Note 11 — Asset Acquisition of NewAmsterdam Pharma B.V.
Accounting Policies
On April 9, 2020, the Company entered into a purchase agreement with Saga Investments Coöperatief U.A., an affiliate of Amgen (“Amgen”) (the “2020 SPA”), to acquire all of the outstanding share capital of Dezima, a company whose principal activity is to develop compounds that treat cardiovascular disease related to dyslipidemia. The principal reason for this acquisition was to secure the intellectual property, licensing and know-how of the patented drug Obicetrapib and the in-process research and development (“IPR&D”). The Company paid consideration of € 1 for the IPR&D asset and could potentially make an additional contingent payment depending on future exit events (if they occur) as further described below. In connection with the 2020 SPA, the Company and Amgen entered into a profit right and waiver agreement with Mitsubishi Tanabe Pharma Corporation (“MTPC”) (the “Profit Right Agreement”) in consideration for the waiver of certain rights held by MTPC prior to the Dezima transaction.
The aggregate contingent consideration to be paid to Amgen and MTPC would become payable upon a traditional underwritten public offering or an exit event, as defined in the 2020 SPA, and is calculated as either: 26.5% of the proceeds, or an aggregate value of shares equivalent to 26.5% of the pre-public offering valuation if an initial public offering takes place, if the exit event occurs when the Company has received an amount equal to or less than € 100 million in aggregate cumulative financing; or, 17.63% of the proceeds, or an aggregate number of shares equivalent to 17.63% of the pre-public offering valuation if an IPO takes place as applicable, if the Company received an amount greater than € 100 million. The profit right shall lapse if the IPO share right is
F-61
Table of Contents
exercised. The IPO share right shall lapse if, as a result of one or more exit events, the profit right is exercised and all of the assets related IPR&D are sold, leased, transferred, licensed, or otherwise disposed of, or there are no remaining shares. Further, upon an IPO, Amgen would also have the right to repurchase all of the outstanding shares of Dezima at a certain specified price. In addition, Amgen and MTPC were granted rights to match certain major corporate transactions (“Matching Rights”). The transactions contemplated by the Agreement (as defined below in Note 21) qualify as an exit event pursuant to the 2020 SPA.
The IPR&D asset is initially recognized at cost, which is the consideration paid upon the asset acquisition of NewAmsterdam Pharma B.V. The acquired IPR&D asset is regarded as having an indefinite useful life as there is no foreseeable limit to the period over which the asset is expected to generate future cash flows. The profit right and IPO share right are recognized as contingent consideration and are not included in the carrying amount of the asset at acquisition. Likewise, no liability is currently recognized for the contingent consideration. Probability assessments of the contingent consideration will be continually conducted in future periods to determine if a liability should be recognized at fair value and to be remeasured subsequently through profit or loss.
Contingent consideration in asset acquisitions is measured and recognized when payment becomes probable and a reliable estimate can be made. Subsequent changes in the accrued amount of contingent consideration are measured and recognized at the end of each reporting period and upon settlement as an adjustment to the cost basis of the acquired asset or group of assets, or if related to IPR&D where it is not probable that future economic benefits will not flow to the entity, charged to expense.
Management deems that the events considered in estimating the contingent consideration are uncertain and cannot be reliably estimated. While the occurrence of the events are within the control of the Company, the estimated value of the liability, probability assessments relative to market studies of similar transactions within the pharmaceutical industry, and the likelihood of the occurrence and the timing of the events driving the exercise of the profit right or IPO share right taking place are all currently uncertain. Additionally, the occurrence of such events is within the control of the Company, rather than beyond the Company’s control or under the control of the contractual parties. For these reasons, the Company decided to account for the contingent consideration under the cost accumulation model and not initially recognize a liability. As a result of no currently reliable estimate, such disclosure related to the contingent consideration could materially change in future periods if a reliable estimate is able to be made.
Financing Activities of the Company
Note 12 — Financial Risk Management
The Company’s principal financial liabilities consist of trade and other payables and loans and borrowings. The main purpose of these financial liabilities is to finance the Company’s day-to-day operations. The Company also has prepayments and other receivables, cash and cash equivalents, and loan receivable that is derived from the Company’s operating activities and funding.
The Company is exposed to market risk, credit risk and liquidity risk. The Company’s senior management oversees the management of these risks. The Board reviews and agrees policies for managing each of these risks, which are summarized below.
Market Risk
Market risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk comprises interest rate risk and foreign currency risk.
The sensitivity analyses in the following sections relate to the position as at December 31, 2021 and 2020.
F-62
Table of Contents
Interest rate risk
The only variable interest-bearing financial instruments are cash and cash equivalents. Changes in interest rates may cause variations in interest income and expense resulting from short-term interest-bearing assets. Management does not expect the short-term interest rates to decrease significantly in the immediate foreseeable future, which limits the interest exposure on our cash and cash equivalents and current financial assets. Currently the interest rate exposure is not hedged.
Interest rate sensitivity
The following table demonstrates the sensitivity to a reasonably possible change in interest rates on that portion of financial assets affected. With all other variables held constant, the Company’s profit before tax/ Equity is affected through the impact on floating rate borrowings, as follows:
| Increase / (Decrease) in Basis Points | Increase / (Decrease) in Profit Before Tax/Equity | |||||||
2020 | ||||||||
Euro | 45 | (32 | ) | |||||
Euro | (45 | ) | 32 | |||||
2021 | ||||||||
Euro | 100 | (380 | ) | |||||
Euro | (100 | ) | 380 | |||||
US dollar | 100 | (150 | ) | |||||
US dollar | (100 | ) | 150 | |||||
Foreign currency risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. The Company’s exposure to the risk of changes in foreign exchange rates relates primarily to cash and cash equivalents, and trade and other payables denominated in currencies other than the functional currency of the Company. As at December 31, 2021, the Company’s net exposure to foreign currency risk was € 15,914 million (2020: € 691 thousand).
The Company partly manages its foreign currency risk by selectively holding foreign currency in its cash and cash equivalents, to offset foreign currency exposures from lease liabilities and trade and other payables. The Company plans to use these cash and cash equivalents to settle future expenses it expects to incur in those foreign currencies.
Foreign currency sensitivity
The Company is mainly exposed to the currency of United States Dollar, British Pound Sterling, Canadian Dollar and Japanese Yen.
The following table demonstrates the sensitivity to a possible change in exchange rates against the Euro with all other variables held constant. Additional sensitivity changes to the indicated currencies are expected to be approximately proportionate. The table shows the effect on the Company’s profit before tax (due to changes in
F-63
Table of Contents
the value of monetary assets and liabilities and equity). The Company’s exposure to foreign currency changes for all other currencies is not material. There is no impact to other comprehensive income or equity.
| Effect on profit / (loss) before tax | ||||||||
Change in foreign exchange rate against Euro | | 10% depreciation | | 10% appreciation | ||||
2020 | ||||||||
British pound sterling | (50 | ) | 61 | |||||
United States dollar | (14 | ) | 14 | |||||
2021 | ||||||||
British pound sterling | (9 | ) | 15 | |||||
Canadian dollar | — | 1 | ||||||
Japanese yen | (1 | ) | 1 | |||||
United States dollar | (1,360 | ) | 1,843 | |||||
Credit Risk
Credit risk is the risk that a counterparty will not meet its obligations under a financial instrument or customer contract, leading to a financial loss. The Company is exposed to credit risk primarily from its treasury activities, including deposits with banks and financial institutions and has limited credit risk exposure from its operating activities. The Company holds available cash in bank accounts with banks which have investment grade credit ratings. Management periodically reviews the creditworthiness of the banks with which it holds assets.
The Company provided a loan to the Chief Executive Officer which bears interest and is secured. Refer to Note 21 for details surrounding repayment.
The Company performs research and development activities and does not yet have any sales. Therefore, the Company is able to reclaim Value Added Tax (“VAT”) which is recoverable from tax authorities. Management periodically reviews the recoverability of the balance of input value added tax and believes it is fully recoverable.
The Company’s maximum exposure to credit risk for the components of the consolidated statement of financial position at December 31, 2021 and 2020, is the carrying amount as illustrated in Note 6 (excluding prepayments) and Note 7.
Liquidity Risk
The Company monitors its risk to a shortage of funds using forecasting planning tools. The Company’s objective is to maintain a sufficient level of funding in order to continue its research and development activities, capital obligations, and loans and borrowings. The Company manages liquidity risk by maintaining adequate reserves, banking facilities and reserve borrowing facilities, by continuously monitoring forecast and actual cash flows, and by matching the maturity profiles of financial assets and liabilities. The Company believes that it has sufficient access to equity funding in the current reporting period (see Note 15).
F-64
Table of Contents
Maturity profile
The table below summarizes the maturity profile of the Company’s financial liabilities based on contractual undiscounted payments. Amounts disclosed are based on spot rates at the reporting date. Payments related to foreign currency are included at the exchange rates applicable as at December 31, 2021 and 2020.
| On demand | Less than 3 months | 3 - 12 months | More than 1 year | Total | ||||||||||||||||
At December 31, 2020 | ||||||||||||||||||||
Trade and other payables | — | 1,152 | 6 | — | 1,158 | |||||||||||||||
Loans and borrowings | — | 11,650 | — | — | 11,650 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total financial liabilities | — | 12,802 | 6 | — | 12,808 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
At December 31, 2021 | ||||||||||||||||||||
Trade and other payables | — | 5,715 | 880 | — | 6,595 | |||||||||||||||
Value added tax payable | — | 2,774 | — | — | 2,774 | |||||||||||||||
Share-based payment liabilities | 458 | — | — | — | 458 | |||||||||||||||
Lease liability | — | 13 | 40 | 111 | 164 | |||||||||||||||
Loans and borrowings | — | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total financial liabilities | 458 | 8,503 | 920 | 111 | 9,991 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Based on our current operating plan, the Company believes that the existing cash and cash equivalents will be sufficient to fund our anticipated level of operations into the fourth quarter of 2023.
Capital Management
For the purpose of the Company’s capital management, capital includes issued capital, preference shares, share premium contribution and all other equity reserves attributable to the equity holders of the Company. The primary objective of the Company’s capital management is to ensure that it will be able to continue as a going concern. The current cash on hand and the anticipated research activities are the most important parameters in assessing the capital structure.
Note 13 — Financial Assets and Liabilities Fair Value
The Company’s financial assets consist of loan receivable, and cash and cash equivalents. Financial liabilities consist of trade and other payables, loans and borrowings and lease liability. The carrying amount of cash and cash equivalents, trade and other payables approximate their fair value due to their short term nature.
Note 14 — Cash and Cash Equivalents
Accounting policies
Cash and cash equivalents comprise cash and short-term bank deposits with an original maturity of three months or less, net of outstanding bank overdrafts. The carrying amount of these assets is approximately equal to their fair value. Cash and cash equivalents at the end of the reporting period as shown in the consolidated statement of cash flows can be reconciled to the related items in the consolidated reporting position as shown below.
Cash and cash equivalents consisted of the following items as at December 31:
| 2021 | 2020 | |||||||
Cash and cash equivalents at banks | 53,092 | 7,861 | ||||||
|
|
|
| |||||
Cash and cash equivalents | 53,092 | 7,861 | ||||||
|
|
|
| |||||
F-65
Table of Contents
The cash at banks is at full disposal of the Company.
Note 15 — Issued Capital and Reserves
Accounting policies
Equity and reserves
No gain or loss is recognized in profit or loss on the purchase, sale, issue or cancellation of the Company’s own equity instruments. Any difference between the carrying amount and the consideration, if reissued, is recognized in share premium.
Equity instruments
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by the Company are recognized at the proceeds received, net of direct issue costs.
The following table details the number of shares of the Company as at December 31:
| Voting ordinary shares | Non-voting ordinary shares | Series A preferred shares | Total | |||||||||||||
Nominal value | 0.01 | 0.01 | 0.01 | |||||||||||||
Shares issued | ||||||||||||||||
Opening balance as at January 1, 2020 | 2,500,000 | — | — | 2,500,000 | ||||||||||||
Issuance of non-voting shares | — | 2,500,000 | — | 2,500,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Issued shares as at December 31, 2020 | 2,500,000 | 2,500,000 | — | 5,000,000 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Opening balance as at January 1, 2021 | 2,500,000 | 2,500,000 | — | 5,000,000 | ||||||||||||
Convertible loan | — | — | 1,111,115 | 1,111,115 | ||||||||||||
Series A — Tranche I | — | — | 4,928,613 | 4,928,613 | ||||||||||||
Issuance of non-voting shares | — | 285,714 | — | 285,714 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Issued shares as at December 31, 2021 | 2,500,000 | 2,785,714 | 6,039,778 | 11,325,442 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Rights associated to the classes of shares issued by the Company
The Company has both ordinary shares and non-voting ordinary shares which represent the residual interest in the Company. A non-voting ordinary share is an ordinary share in the Company without voting rights.
Series A preferred shares
Series A Preferred Shares carry the same voting rights as ordinary shares. Furthermore, Series A Preferred Shares have a liquidation preference over ordinary shares of the Company. Meaning that in case of a liquidity event, the Series A Preferred Shares shareholders will receive the preference share capital and share premium paid in by the shareholder, increased by any declared but unpaid preference dividends. Any remaining proceeds following the liquidation preference shall be distributed equally among all classes of preference shares and the ordinary shares on equal terms. The Series A Preferred Shares are convertible to ordinary shares on a one-to-one ratio at the option of the holder.
F-66
Table of Contents
The following table details the total share capital and share premium of the Company as at December 31, 2021 and 2020:
| (In Euro) | Voting ordinary shares | Non-Voting ordinary shares | Series A Preferred shares | Share premium | Share capital | |||||||||||||||
Opening balance as at January 1, 2020 | 25,000 | — | — | 2,475,000 | 2,500,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Issuance of non-voting shares Apr 2020 | — | 25,000 | — | (25,000 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As at December 31, 2020 | 25,000 | 25,000 | — | 2,450,000 | 2,500,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As presented in the statement of financial position (in thousands of Euro) | 25 | 25 | — | 2,450 | 2,500 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Opening balance as at January 1, 2021 | 25,000 | 25,000 | — | 2,450,000 | 2,500,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Conversion of convertible debt Jan 2021 | — | — | 11,111 | 11,656,036 | 11,667,147 | |||||||||||||||
Series A — Tranche I Jan 2021 | — | — | 49,286 | 68,950,716 | 69,000,002 | |||||||||||||||
Issuance of non-voting shares Jul 2021 | — | 2,857 | — | 705,714 | 708,571 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As at December 31, 2021 | 25,000 | 27,857 | 60,397 | 83,762,466 | 83,875,720 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As presented in the statement of financial position (in thousands of Euro) | 25 | 28 | 60 | 83,763 | 83,876 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Closing of Series A Preferred Shares — Tranche I and convertible loan
On January 7, 2021, the Company closed a funding round to raise up to € 160 million in equity financing, set to occur in two tranches. The first tranche of € 69 million occurred upon an initial closing on January 7, 2021 for the issue of 4,928,571 Series A preferred shares on the same date. In accordance with the terms of the Convertible Loan Agreement (as defined and described further in Note 18), € 11.7 million in outstanding principal and unpaid interest was converted on the same date of the first tranche into 1,111,155 Series A preferred shares. The second tranche is described in the events after the reporting period (Note 21).
Share-based payments — issuance of non-voting shares
In conjunction with the acquisition of NewAmsterdam Pharma B.V. on April 9, 2020, the Company offered a share-based scheme to former shareholders of NewAmsterdam Pharma B.V. through the STAK which owns the non-voting shares in the Company. The issuance of non-voting shares was recognized in the Company’s share premium reserve. In accordance with the terms of the arrangement, former shareholders of NewAmsterdam Pharma B.V. were granted and distributed Depository Receipts on April 9, 2020, as issued by the STAK, in exchange for the shareholders of NewAmsterdam Pharma B.V. waiving any claims against the Company or NewAmsterdam Pharma B.V. which existed in certain agreements that the Company had assumed upon the acquisition of NewAmsterdam Pharma B.V.
On July 13, 2021, the Company issued 285,714 non-voting ordinary shares with an issue price of € 708,571 to the STAK.
A Depository Receipt, issued by the STAK, has the same economic rights as an ordinary share in the Company and has no voting rights. The Depository Receipts were issued at the nominal value of € 0,01 (one-Euro cent) per Depository Receipt. The fair value of the Depository Receipts has been determined using an implied value approach with reference to the voting ordinary shares.
F-67
Table of Contents
Note 16 — Share-Based Payments
Accounting policies
The Company operates equity-settled share-based payment arrangements, under which the Company and its subsidiaries receive services from directors, employees and others providing similar services as consideration for equity instruments of the Company.
The Company determines the fair value of the share-based payment awards at the grant date, including the impact of any market conditions and non-vesting conditions, and recognizes an expense for the services received over the service period, with a corresponding increase in equity. For awards with graded-vesting features, each instalment of the award is treated as a separate grant, which means that each instalment is separately expensed over the related vesting period. At the end of each reporting period, service conditions and non-market vesting conditions are taken into account when estimating the number of awards that will ultimately vest.
For options granted under the Company’s long term incentive plan (“LTIP”), the total amount to be expensed for services received is determined by reference to the grant date fair value of the options granted as determined using an option valuation model. For indirect share investments, the Company analyses at each grant date whether the purchase price paid by a participant is in line with the market price of the underlying shares. If a positive difference exists between (i) the actual market value of the shares as determined at the grant date and (ii) the purchase price paid, this results in a fair value to be reported as a share-based payment expense.
Long Term Incentive Plan
In July 2021, the Company introduced a long-term incentive plan for employees, officers, and other service providers who qualify as eligible participants (any director, employee, or consultant). Under this equity-settled plan, the Company grants options on Depositary Receipts (“DRs”) relating to non-voting ordinary shares and options on non-voting ordinary shares. On July 8, 2021, the Company granted 262,857 Pre-Series A options, 887,116 First Tranche Options, and 814,312 Second Tranche Options. All options are issued with the same terms as outlined below with the exception of the Second Tranche Options which have additional non-market vesting conditions.
Term
In general, each option under the plan has a four-year vesting period with 25% vesting after one year and the remaining 75% vesting in equal instalments over the next following three years. Additionally, the contractual term is 10 years from grant date.
In the case of a participant who voluntarily leaves and therefore ceases to be an eligible participant (“Good Leaver”), all unvested options will lapse and all vested options that have not yet been exercised or settled must be exercised or settled within 3 months after the Participant became a Good Leaver.
Second-Tranche Vesting Conditions
The Second Tranche Options are subject to the following two vesting conditions:
| • | the Milestone Closing (i.e. the closing of the second tranche of the Series A financing round) having occurred, provided that the Milestone Closing occurs prior to June 1, 2022; and |
| • | the Participant’s employment, management, or advisory agreement having continued uninterrupted through the date of the Milestone Closing. |
If the Milestone Closing does not occur prior to June 1, 2022, the Second Tranche Options will automatically lapse without any consideration becoming due.
F-68
Table of Contents
The milestones which are non-market performance conditions can be described as follows:
| • | no material adverse change has occurred as determined in good faith by the Board with a simple majority, including the investor director majority; |
| • | the representations and warranties set out in the share subscription agreement are true and accurate in all respects on the date of the Milestone Closing subject to an updated disclosure schedule per the date of the Milestone Closing; and |
| • | the satisfaction of each of the following milestones, as determined in good faith by the Board with a simple majority, including the investor director majority, on or prior to the second anniversary of the first closing: |
| • | at least 50% LDL lowering in the ezetimibe/obicetrapib combination trial (the “LDL Milestone”); |
| • | verbal or written confirmation by the U.S. Food and Drug Administration that no CVOT trial is required for U.S. approval following the end of the Phase 2 meeting (the “FDA Milestone”); and |
| • | hiring of a fulltime chief business officer with the unanimous consent of the Board, which consent will not be unreasonable be delayed or withheld (the “CBO Milestone”). |
As of December 31, 2021, the Company satisfied only the LDL Milestone and CBO Milestone. However, in accordance with the clause set out in share subscription agreement, if the LDL Milestone and the CBO Milestone are satisfied, but the FDA Milestone is not, the parties shall proceed with the Milestone Closing. See Note 21 for more information on the closing of the Series A financing round. The Second Tranche Options are considered vested based on the current status of vesting conditions described above.
Liquidity Event or IPO
Upon the occurrence of a liquidity event, the Board may decide that all or part of the unvested awards shall immediately vest and, where relevant, settled in full. All other unvested awards shall be cancelled automatically without compensation for the loss of such awards.
Upon the occurrence of an IPO, the Board may, upon proposal of the remuneration committee, decide that all or part of the unvested awards shall immediately vest and, where relevant, settled in full. Since immediate vesting of unvested awards in such a liquidity event or IPO situation requires a Board decision, accelerated vesting will be taken into account if and when the Board decides so.
Movements of Options During the Year
The changes in the number of options outstanding and their related weighted average exercise prices are as follows:
| 2021 | ||||||||
| Weighted average exercise price | Number of options | |||||||
Outstanding as at January 1 | — | — | ||||||
Granted during the year | 2.48 | 1,964,286 | ||||||
Forfeited during the year | — | — | ||||||
|
|
|
| |||||
Outstanding as at December 31 | 2.48 | 1,964,286 | ||||||
F-69
Table of Contents
As at December 31, 2021, none of the outstanding options are exercisable. The options granted during the year have an exercise price of € 2.48 per option, and will expire on July 6, 2031. The options outstanding as of December 31, 2021 have a remaining contractual life of 9.5 years.
| 2021 | ||||||||
| Expiry date | Exercise price | Number of options | ||||||
July 6, 2031 | 2.48 | 1,964,286 | ||||||
Forfeited during the year | — | — | ||||||
|
|
|
| |||||
Outstanding as at December 31 | 2.48 | 1,964,286 | ||||||
Fair Value of Options Granted
The Black Scholes option pricing formula has been applied for measuring the fair value of the options granted in 2021. The assumptions used to determine the fair value of the options represent management’s best estimates. These estimates involve inherent uncertainties and the application of management’s judgment, specifically as it relates to share value, volatility and expected life. The weighted average fair values and the inputs (ranges) used in the measurement of the fair values of these equity-settled options at the date of grant are summarized below:
| 2021 | ||||
Share value (EUR) | 2.48 | |||
Option exercise price (EUR) | 2.48 | |||
Volatility (%) | 42% - 44% | |||
Expected life (years) | 5.0 - 6.9 | |||
Dividend yield | 0% | |||
Risk-free rate | (0.62%) - (0.58%) | |||
Fair value per option (EUR) | 0.91 - 0.98 | |||
The expected option life is based on management’s best estimate of when the options will be exercised. Expected volatility is estimated by considering historical average share price volatility of a group of comparable companies over a period before the grant date being equal to the expected option life. The expected dividend rate is zero as the Company currently has no history or expectation of declaring dividends on its ordinary shares. The risk-free interest rate is estimated based on the continuous yield on triple A-rated, zero coupon government bonds in the Eurozone with a term to maturity comparable to the (remaining) expected option life.
The fair value of the underlying ordinary shares (the share value above) is estimated by the Company in the absence of a public market for the Company’s equity instruments. The Company relied on an option pricing method (“OPM”) to determine fair value of the ordinary shares. The OPM allocates the overall equity value to the various share classes based on differences in liquidation preferences and participation rights. After the value of the ordinary shares is determined, a discount for lack of marketability (“DLOM”) of 15% is applied to arrive at fair value of the underlying ordinary shares. A DLOM is applied in order to reflect the lack of a recognized market for a privately held interest and the fact that equity interest may not be readily transferrable. A market participant purchasing this equity instrument would recognize this illiquidity associated with the equity instrument which would reduce the overall fair market value.
Share-Based Payment Expenses Recognized for Options Granted
The fair value of the options is expensed over the relevant vesting periods using the graded vesting method, based on management’s estimate of the number of options that will eventually vest. In 2021, the total share-based payment expense recognized for the equity-settled options amounted to € 1,049 million (2020: € 0). This includes share-based payment expense recognized in connection with the vesting of the Second Tranche Options subject to vesting conditions that are considered vested based on the current status of vesting conditions.
F-70
Table of Contents
In addition, the Company recognized € 43 thousand as expenses for the employer social security contributions expected to be payable related to these options. The corresponding liability as of December 31, 2021 amounts to € 43 thousand.
Share Investment Plan
As discussed in Note 15, the Company offers a share-based scheme to former shareholders of NewAmsterdam Pharma B.V. through STAK, a trust which owns non-voting shares in the Company. In July 2021, the Company entered into an arrangement and issued 285,714 non-voting ordinary shares to STAK at an aggregate issue price of EUR 708,571 for which Depository Receipts (“Founder DRs”) were agreed to be issued by STAK to the CEO, M. Davidson (the “Subscriber”), under the LTIP. The vesting and contractual terms for this transaction align with other DRs issued under the LTIP that have been previously disclosed, except as noted below.
The Company provided a loan facility to the Subscriber to pay the purchase price of the Founder DRs subject to the terms and conditions of the loan agreement. This loan is recorded as a loan receivable as it is a full recourse loan. Refer to Note 10 for related discussion and Note 21 for a description of the settlement of the loan.
The Company does not have an obligation to repurchase any vested Founder DRs or otherwise settle vested DRs in cash or repurchase any vested DRs. This element of the Founder DR award is therefore accounted for as an equity-settled share-based payment transaction. As a result, the Company analyses at each grant date whether the purchase price paid by a participant is in line with the market price of the underlying shares. If a positive difference exists between (i) the actual market value of the shares as determined at the grant date and (ii) the purchase price paid, this results in a grant date fair value to be reported as a share-based payment expense, with a corresponding increase in equity.
The Subscriber paid the fair market value of the underlying ordinary shares in the Company at the grant date, each € 2.48 per option. Accordingly, the total fair value of these equity-settled share-based payment awards amounts to nil and there will be no expenses recognized in the income statement related to these investments.
In connection with the award arrangement, if the Subscriber leaves the Company, all unvested Founder DRs will be cancelled automatically, with simultaneous cancellation of the underlying shares, and against payment by the Company to the participant of the lower of the (i) the purchase price paid for such Founder DRs and (ii) the fair market value. As the Founder DRs are delivered immediately on receipt for consideration paid by the Subscriber but remain subject to ongoing vesting conditions, to reflect the consideration payable for the Founder DRs and the potential that the Founder DRs and the potential that the Founder DRs could be repurchased, the Company has recognized the consideration as a financial liability until the Founder DRs have vested, at which time it will be reclassified to equity provided that the Subscriber remains with the Company. This liability is measured at the lower of the price paid for the DRs and their current fair market value. The liability as of the December 31, 2021 amounts to € 457,619.
The movements in the number of Founder DRs outstanding are as follows:
| 2021 | ||||
| Number of options | ||||
Outstanding as at January 1 | — | |||
Granted /purchased during the year | 285,714 | |||
Forfeited during the year | — | |||
|
| |||
Outstanding as at December 31 | 285,714 | |||
|
| |||
The absence of a public market for the Company’s equity instruments requires the Company to estimate the fair value of equity instruments as of each grant date. For further details about the approach applied, please refer to disclosures above regarding how the share value was determined for the purpose of determining the fair value of the options granted under the LTIP.
F-71
Table of Contents
Note 17 — Loss per Share
Loss per share for the period presented has been determined by dividing the loss attributable to shareholders by the weighted average number of shares outstanding during the period. Non-voting ordinary shares and voting ordinary shares are considered to be the same class of equity for purposes of determining the loss attributable per shareholder class per share.
The following table sets forth the computation of basic and diluted loss per share for the year ended December 31, 2021 and 2020:
| (In thousands of Euro, except share and per share amounts) | 2021 | 2020 | ||||||
Loss for the period | (28,599 | ) | (5,749 | ) | ||||
Weighted average number of ordinary shares | 11,325,442 | 5,000,000 | ||||||
Basic and diluted loss per share | (2.53 | ) | (1.15 | ) | ||||
At December 31, 2021 and 2020, outstanding share-based awards (see Note 16) and options from the convertible debt (see Note 17) were excluded from the calculation because their impact would be anti-dilutive. The total number of potential ordinary shares excluded amounted to 1,964,286 (2020: 1,111,115).
Note 18 — Loans and Borrowings
Accounting policies
Loans and borrowings are initially recognized at fair value net of any transaction costs directly attributable to the issue of the instrument. Such interest-bearing liabilities are subsequently measured at amortized cost using the effective interest rate method, which ensures that any finance expense over the period to repayment is at a constant rate on the balance of the liability carried in the consolidated statement of financial position until extinguished on conversion or maturity of the debt. For the purposes of this financial liability, financing expense includes any interest or coupon payable while the liability is outstanding.
Classification as debt or equity
Debt and equity instruments are classified as either financial liabilities or as equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument.
Derecognition of financial liabilities
The Company derecognizes financial liabilities when, and only when, the Company’s obligations are discharged, cancelled or have expired. The difference between the carrying amount of the financial liability derecognized and the consideration paid and payable is recognized in profit or loss.
Loans and borrowings consisted of the following items as at December 31, 2021 and 2020:
| (In thousands of Euro) | 2021 | 2020 | ||||||
Convertible debt | — | 11,320 | ||||||
Accrued finance expense on convertible debt | — | 330 | ||||||
|
|
|
| |||||
Loans and borrowings | — | 11,650 | ||||||
|
|
|
| |||||
Convertible debt
On July 2, 2020, the Company, as borrower, entered into an unsecured convertible loan agreement (the “Convertible Loan Agreement”) which granted the Company up to € 17 million, available over three tranches
F-72
Table of Contents
of € 5.66 million, € 5.66 million and € 5.68 million, respectively, as sourced from three lenders as described in Note 20.
| (In thousands of Euro) | 2020 | |||
Opening balance at inception | ||||
First tranche provided July 2, 2020 | 5,660 | |||
Second tranche provided October 12, 2020 | 5,660 | |||
Total convertible debt proceeds | 11,320 | |||
|
| |||
| 2021 | ||||
Opening balance at January 1, 2021 | 11,320 | |||
Debt converted to equity | (11,320 | ) | ||
Total convertible debt proceeds | — | |||
|
| |||
Interest on the convertible debt is non-compounding and fixed at 8% per annum. Outstanding principal and unpaid interest is due March 15, 2021, or each lender’s relative portion of outstanding principal and unpaid interest is converted based upon one of the following events that occur prior to maturity:
| (1) | Mandatory conversion upon equity investment in the Company exceeding € 85 million (“Qualified Financing”) into the class of shares issued under the Qualified Financing equal to outstanding principal and interest divided by 75% of the Qualified Financing share price; |
| (2) | Voluntary conversion upon equity investment in the Company other than a Qualified Financing (“Non-Qualified Financing”) into the class of shares issued under the Non-Qualified Financing equal to outstanding principal and interest divided by a maximum of 75% of the Non-Qualified Financing share price; or |
| (3) | Liquidation event into shares of a newly created class, entitling the lenders of convertible debt to proceeds from the liquidation event equal to four times the amount paid by the lenders on such shares and such share class having preference to any proceeds payable on classes of shares existing prior to the liquidation event. |
On January 7, 2021, € 11.7 million of the outstanding principal and unpaid interest under the Convertible Loan Agreement was converted into 1,111,155 Series A preferred shares in connection with the Series A financing round described in Note 15.
As the conversion feature results in the conversion of a fixed amount of stated outstanding principal and interest into a variable number of shares, it fails the ‘fixed for fixed’ criterion and therefore is classified as a liability instrument with a bifurcated embedded derivative. However, as at inception and at December 31, 2020 the value of the conversion options was zero.
Note 19 — Lease Liability
Accounting policies
Leased assets
Leased assets are capitalized at the commencement date of the lease and comprise the initial amount, initial direct costs incurred when entering into the lease less any lease incentives. An impairment is undertaken for any right of use lease assets that shows indicators of impairment and an impairment less is recognized against any right of use lase assets that is impaired.
Leased liabilities
The lease liability is measured at the present value of the fixed and variable lease payments net of cash lease incentives that are not paid at the balance date. Lease payments are apportioned between the finance charges and
F-73
Table of Contents
reduction of the lease liability using the incremental borrowing rate implicit in the lease to achieve a constant rate of interest on the remaining balance of the liability. Lease payments for buildings exclude services fees for cleaning and other costs. Lease modifications are accounted for as a new lease with an effective date of the modification.
| 2021 | ||||
Opening balance at January 1, 2021 | — | |||
Additions | 172 | |||
Repayment | (10 | ) | ||
Interest | 2 | |||
Total lease liability | 164 | |||
|
| |||
Current | ||||
Lease liabilities | 53 | |||
Non-current | ||||
Lease liabilities | 111 | |||
With effective date May 29, 2021, the Company leased office premises in Florida. Refer to Note 9 for right of use lease asset details related to the finance lease liability.
Additional Information
Note 20 — Related Parties
As at December 31, 2021 and 2020, the ultimate controlling party of the Company was Forbion Capital Fund II Coöperatief U.A.
The following table provides the total amount of transactions that have been entered into with related parties for the relevant financial year ended December 31, 2021 and 2020.
| Related party relationship | Type of transaction | Purchases from | Amounts owed to | Amounts owed by | ||||||||||||||||
2021 | ||||||||||||||||||||
FCPM III Services B.V. (“Forbion III”) | | Common control | | | Rent and office services | | 20 | 21 | ||||||||||||
Mr. M.H. Davidson | | Exec. director & shareholder | | Receivables | — | — | 718 | ** | ||||||||||||
2020 | ||||||||||||||||||||
FCPM III Services B.V. (“Forbion III”) | | Common control | | | Rent and office services | | 28 | 3 | — | |||||||||||
Forbion Capital Partners | | Common control | | | Due diligence and travel | | 3 | — | — | |||||||||||
Forbion Capital Fund II Coöperatief U.A. (“Forbion II”) | Shareholder | Borrowings | — | 10,291 | * | — | ||||||||||||||
Forbion Capital Fund IV Coöperatief U.A. (“Forbion IV”) | | Common control | | Borrowings | — | 679 | * | — | ||||||||||||
Mr. M.H. Davidson | | Exec. director & shareholder | | Borrowings | — | 679 | * | — | ||||||||||||
| * | Amounts owed to these related parties comprise of outstanding principal and unpaid interest, with further details given in Note 18 which reflects the total of convertible debt due to these three related parties. |
| ** | Amounts receivable from these related parties comprise of outstanding principal and unpaid interest, with further details given in Note 10 which reflects the total loan receivable. |
F-74
Table of Contents
The Company signed a services agreement with Forbion III where the Company could utilize office space and other facilities at the premises of Forbion III. The Services agreement is effective beginning March 2020 and continues for an indefinite period of time. As the lease grants each party the unilateral right to terminate the lease on giving 30 days’ notice to the other party, for any reason and with no significant penalty, management concluded that the agreement did not contain a lease component and the full amount was expensed as General and Administration expenses during 2021 and 2020.
Terms and conditions of transactions with related parties
Transactions with related parties are made on terms equivalent to those that prevail in arm’s length transactions. Outstanding balances at the year-end are unsecured and interest free and settlement generally occurs in cash. There have been no guarantees provided or received for any related party receivables or payables.
Compensation to Directors and Senior Managers of the Company
The Company’s compensation includes base salary, as well as short and long-term incentive schemes.
Key Management Personnel (“KMPs”) are those persons having authority and responsibility for planning, directing and controlling the activities of the Company, including the directors of the company. KMP include the Chief Executive Officer, Chief Financial Officer, Chief Science Officer and Vice President of Research and Development. The following table sets forth the total compensation paid to KMPs during the period:
| 2021 | 2020 | |||||||
Short-term employee benefits | 1,478,641 | 1,302,886 | ||||||
Share-based payments | 740,325 | — | ||||||
|
|
|
| |||||
Total compensation paid to key management personnel | 2,218,966 | 1,302,886 | ||||||
|
|
|
| |||||
Certain non-executive directors of the Company serve as representatives of the Company’s shareholders and as such have of received remuneration from the Company during the period € 26 thousand (2020: € 0).
Note 21 — Events After the Reporting Period
The Company evaluated subsequent events for recognition or disclosure through August 3, 2022, the date these consolidated financial statements were authorized for issuance by the Board.
Closing of Series A financing round
On January 7, 2021, the Company closed a funding round to raise up to € 160 million in equity financing, set to occur in two tranches. The first tranche of € 69 million closed on January 7, 2021 upon the issue of 4,928,571 Series A preferred shares. The second tranche of € 80 million for 5,691,430 Series A preferred shares was subject to the attainment of certain milestone tranche conditions, namely: the LDL Milestone; the FDA Milestone; and the CBO Milestone. As the LDL Milestone and the CBO Milestone were satisfied as of December 31, 2021, the second tranche conditions were considered to be met. The Company received the second tranche financing on February 17, 2022. Because the FDA condition was not satisfied, the Company was obligated to seek additional financing under different scenarios at the discretion of the Company pursuant to the terms of the agreement. The execution of the Menarini Agreement (as defined below) satisfied this requirement as subsequently described in this Note below.
F-75
Table of Contents
Menarini License Agreement
In June 2022, the Company entered into a license agreement with A. Menarini International Licensing S.A. (“Menarini”) to allow Menarini to locally develop, obtain and maintain regulatory approval, and commercialize finished products containing obicetrapib and ezetimibe. Under the license agreement (the “Menarini Agreement”), the Company granted Menarini an exclusive, sub-licensable license in the European Economic Area, United Kingdom and Switzerland (collectively, the “Menarini Territory”) to certain of its patents and know-how, in addition to product trademarks, for the local development and commercialization of compounds by Menarini associated with obicetrapib and ezetimibe (“Licensed Products”), along with other customary licenses between the parties.
Menarini is obligated to pay a non-refundable, non-creditable, upfront amount of € 115.0 million to the Company, which was received in July 2022. Thereafter, in partial contribution to the Company’s costs of development of the Licensed Products, Menarini will pay the Company € 27.5 million, payable in two equal annual installments. The Company will be responsible for the development and commercialization costs related to Licensed Products, excluding local development, regulatory and commercialization costs incurred by Menarini in the Menarini Territory. In addition, the Company and Menarini agree to each bear 50 percent of certain development costs incurred by the other party as specified in the Menarini Agreement.
The Menarini Agreement provides for certain milestone payments from Menarini to the Company upon the achievement of specified development, regulatory and commercial milestones. More specifically, the Company is eligible to receive up to € 863 million upon the achievement of various clinical, regulatory and commercial milestones. If obicetrapib is approved, and successfully commercialized by Menarini, the Company will be entitled to tiered royalties ranging from the teens to the mid-twenties as a percentage of net sales in the Menarini Territory, with royalty step-downs in the event of generic entrance or in respect of required third party IP payments.
The royalty term begins for each Licensed Product on a country-by-country basis upon the first commercial sale of such product in such country and ends on the later of (i) the expiration of the last-to-expire patent that includes a valid claim, (ii) the expiration of regulatory exclusivity in such country for such Licensed Product and (iii) a specified number of years after the first commercial sale of such Licensed Product in such country (the term of the agreement).
In addition, Menarini is expected to purchase obicetrapib and ezetimibe product from us in accordance with a supply agreement to be entered into by Menarini and us (the “Supply Agreement”). The Company will supply all required quantities of obicetrapib and ezetimibe product for the Menarini Territory as set forth in the Supply Agreement. Through the filing date of this Annual Report, the Company has received no milestone payments from Menarini under the Menarini Agreement.
Related Party Receivable
On July 19, 2022, the CEO, M. Davidson, repaid the loan facility of € 709 thousand with the Company including outstanding unpaid interest.
Formation of Holdco and Merger Sub
In June 2022, the Company formed NewAmsterdam Pharma Company B.V., a new Dutch private company with limited liability (“Holdco”) and a wholly-owned subsidiary of the Company. Following the creation of the new entity, also in June 2022, Holdco formed NewAmsterdam Pharma Investment Corporation, a new Cayman-based exempted company (“Merger Sub”), which is a wholly-owned subsidiary of Holdco. The internal restructuring will be accounted for as a capital reorganization as the Company remains the substantive business, and Holdco does not meet the definition of a business pursuant to IFRS 3, Business Combinations. The restructuring was conducted for the purpose of participating in the merger transaction described below. As a result of the internal restructuring, the Company and Merger Sub became wholly-owned subsidiaries of Holdco.
F-76
Table of Contents
Triggering of Exit Events Pursuant to the 2020 SPA
The execution of the Menarini Agreement, described above, and the Agreement between the Company and FLAC, described below, qualified as exit events pursuant to both the 2020 SPA and the Profit Right Agreement, as described in Note 11. On April 28, 2022 the Company and Amgen executed a waiver notice (the “Original Waiver”) requiring Amgen to waive its matching rights pursuant to the 2020 SPA associated with the Menarini Agreement, so long as the Menarini Agreement was executed as of June 1, 2022. Further, the Original Waiver acknowledges that the Menarini Agreement does not trigger payment of the contingent consideration described in Note 11 as no proceeds were issued to shareholders of the Company. On June 10, 2022 the Company and Amgen executed an extension of the Original Waiver (the “Second Waiver”) with the same terms of the Original Waiver except for extending the period of time for when the Menarini Agreement was required to be executed to July 1, 2022. The Menarini Agreement was executed on June 23, 2022 and therefore satisfied the terms of the Second Waiver.
On July 20, 2022, the Company and Amgen and the Company and MTPC each executed additional waiver notices (collectively the “Third Waivers”) stating that the Agreement between the Company and FLAC constituted an additional exit event that triggered payment of the contingent consideration as proceeds are payable to shareholders under the Agreement. The Third Waivers indicate that the contingent consideration will be settled via the profit right. The profit right will be settled in shares of Holdco in lieu of Company shares, and will be issued prior to the closing of the merger with FLAC and the Company (the “Profit Right”). Both Amgen and MTPC also agreeing to waive their right to matching so long as the Agreement is executed before October 31, 2022.
As a result, Amgen will receive 4,910,000 shares of HoldCo with an estimated value of $49,100,000 and MTPC will receive 3,746,330 shares of Holdco with an estimated value of $37,463,300, in addition, each such shareholder will be entitled to receive its pro rata share on additional shares of Holdco, including the earnout shares to be obtained if and when the Company achieves a certain future development milestone (the “Earnout Shares”). Upon the issuance of the HoldCo shares, all rights of Amgen and MTPC under the 2020 SPA and the Profit Right Agreement, respectively, will be extinguished.
Merger between the Company and Frazier Lifesciences Acquisition Corporation
On July 25, 2022, Frazier Lifesciences Acquisition Corporation (“FLAC”), a special purpose acquisition company sponsored by Frazier Lifesciences Sponsor LLC that is incorporated as a Cayman Islands exempted company, Holdco, Merger Sub and the Company entered into a Business Combination Agreement (the “Agreement”). Upon consummation of the merger between FLAC and the Company, Holdco will be a publicly traded company listed on the Nasdaq in the United States. The Company’s shareholders are expected to have a controlling interest in Holdco following the consummation of the business combination transaction. The business combination transaction is subject to customary and other closing conditions, including the approval of the shareholders of both the Company and FLAC. The transaction is expected to close in the fourth quarter of 2022.
Company shareholders are expected to hold the controlling interest in Holdco, and as FLAC does not constitute a business under IFRS 3 Business Combinations, the transaction will also be accounted for as a capital reorganization, and is in scope of IFRS 2 Share-based Payment. Holdco will issue shares in exchange for the net assets of FLAC. Any difference between the fair value of the shares issued by Holdco and the fair value of FLAC’s identifiable net assets will be treated as costs for the service of obtaining a listing and expensed in the period in which the transaction closes.
As a result of the merger, additional financing in the form of a private investment in public entity (“PIPE”) was secured as of July 25, 2022. Holdco has committed to issue 23,460,000 shares with a value of $234.6 million at the closing of the merger.
F-77
Table of Contents
Unaudited Condensed Consolidated Financial Statements
NewAmsterdam Pharma Holding B.V.
CONDENSEDCONSOLIDATEDSTATEMENTOFPROFITORLOSSANDOTHER
COMPREHENSIVEINCOMEORLOSS (UNAUDITED)
for the six months ended June 30
(in thousands of Euro, except per share amounts)
| As at | ||||||||||||
| Note | June 30 2022 | June 30 2021 | ||||||||||
Revenue | 4 | 93,500 | — | |||||||||
Research and development expenses | 5 | (30,588 | ) | (8,553 | ) | |||||||
General and administrative expenses | 6 | (9,294 | ) | (1,401 | ) | |||||||
|
|
|
| |||||||||
Total operating expenses | (39,882 | ) | (9,954 | ) | ||||||||
Finance income | 10 | — | ||||||||||
Finance expense | (185 | ) | (137 | ) | ||||||||
Net foreign exchange gain | 1,070 | 609 | ||||||||||
|
|
|
| |||||||||
Profit (loss) before tax | 54,513 | (9,482 | ) | |||||||||
Income tax expense | 9 | — | — | |||||||||
|
|
|
| |||||||||
Profit (loss) for the period | 54,513 | (9,482 | ) | |||||||||
Other comprehensive income (loss), net of tax | — | — | ||||||||||
|
|
|
| |||||||||
Total comprehensive income (loss) for the period, net of tax | 54,513 | (9,482 | ) | |||||||||
|
|
|
| |||||||||
Basic and diluted earnings per share | ||||||||||||
Basic | 13 | € | 3.20 | € | 0.86 | |||||||
Diluted | 13 | € | 2.87 | € | 0.86 | |||||||
The accompanying notes are an integral part of these financial statements.
F-78
Table of Contents
CONDENSED CONSOLIDATED STATEMENTOF FINANCIAL POSITION
(UNAUDITED)
(in thousands of Euro)
| As at | ||||||||||||
| Note | June 30, 2022 | December 31, 2021 | ||||||||||
Assets | ||||||||||||
Property, plant and equipment | 175 | 190 | ||||||||||
Loan receivable | 728 | 718 | ||||||||||
|
|
|
| |||||||||
Non-current assets | 903 | 908 | ||||||||||
|
|
|
| |||||||||
Current assets | ||||||||||||
Trade receivables | 7 | 115,000 | — | |||||||||
Prepayments and other receivables | 8 | 12,474 | 5,782 | |||||||||
Cash and cash equivalents | 89,478 | 53,092 | ||||||||||
Total current assets | 216,952 | 58,874 | ||||||||||
|
|
|
| |||||||||
Total assets | 217,855 | 59,782 | ||||||||||
|
|
|
| |||||||||
Equity and liabilities | ||||||||||||
Equity | ||||||||||||
Share capital | 11 | 163,556 | 83,876 | |||||||||
Other reserves | 12 | 1,029 | 591 | |||||||||
Retained earnings | 13 | 19,837 | (34,676 | ) | ||||||||
|
|
|
| |||||||||
Total equity | 184,422 | 49,791 | ||||||||||
Non-current liabilities | ||||||||||||
Lease liability | 10 | 90 | �� | 111 | ||||||||
Deferred revenue | 4 | 7,440 | — | |||||||||
|
|
|
| |||||||||
| 7,530 | 111 | |||||||||||
|
|
|
| |||||||||
Current liabilities | ||||||||||||
Lease liability | 10 | 61 | 53 | |||||||||
Deferred revenue | 4 | 14,060 | — | |||||||||
Trade and other payables | 10 | 11,782 | 9,827 | |||||||||
|
|
|
| |||||||||
Total current liabilities | 25,903 | 9,880 | ||||||||||
|
|
|
| |||||||||
Total equity and liabilities | 217,855 | 59,782 | ||||||||||
|
|
|
| |||||||||
The accompanying notes are an integral part of these financial statements.
F-79
Table of Contents
CONDENSED CONSOLIDATED STATEMENTOF CHANGESIN EQUITY (UNAUDITED)
as at June 30, 2022
(in thousands of Euro except issued shares)
| Number of Shares Outstanding | ||||||||||||||||||||||||||||||||||||
| Note | Voting Ordinary Shares | Non-Voting Ordinary Shares | Series A Preferred Shares | Issued Capital | Share Premium | Other Reserves | Retained Earnings | Total Equity | ||||||||||||||||||||||||||||
Opening balance at January 1, 2021 | 2,500,000 | 2,500,000 | — | 50 | 2,450 | — | (6,077 | ) | (3,577 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Convertible loan | — | — | 1,111,115 | 11 | 11,656 | — | — | 11,667 | ||||||||||||||||||||||||||||
Series A—Tranche I | — | — | 4,928,613 | 49 | 68,951 | — | — | 69,000 | ||||||||||||||||||||||||||||
Total comprehensive loss for the period | — | — | — | — | — | — | (9,482 | ) | (9,482 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
As at June 30, 2021 | 2,500,000 | 2,500,000 | 6,039,728 | 110 | 83,057 | — | (15,559 | ) | 67,608 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
As at December 31, 2021 | 2,500,000 | 2,785,714 | 6,039,728 | 113 | 83,763 | 591 | (34,676 | ) | 49,791 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Opening balance at January 1, 2021 | 2,500,000 | 2,785,714 | 6,039,728 | 113 | 83,763 | 591 | (34,676 | ) | 49,791 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Series A—Tranche II | 11 | — | — | 5,691,430 | 57 | 79,623 | — | — | 79,680 | |||||||||||||||||||||||||||
Share-based compensation | 12 | — | — | — | — | — | 438 | — | 438 | |||||||||||||||||||||||||||
Total comprehensive income for the period | 13 | — | — | — | — | — | — | 54,513 | 54,513 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Balance period ended June 30, 2022 | 2,500,000 | 2,785,714 | 11,731,158 | 170 | 163,386 | 1,029 | 19,837 | 184,422 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-80
Table of Contents
CONDENSED CONSOLIDATED STATEMENTOF CASH FLOWS (UNAUDITED)
(in thousands of Euro except issued shares)
| For the six months ended | ||||||||||||
| Note | June 30, 2022 | June 30, 2021 | ||||||||||
Operating Activities: | ||||||||||||
Profit (loss) for the period | 54,513 | (9,482 | ) | |||||||||
Non-cash adjustments to reconcile profit (loss) for the period to net cash flows: | ||||||||||||
Depreciation and amortization | 36 | 2 | ||||||||||
Finance income | (10 | ) | — | |||||||||
Finance expense | 5 | 18 | ||||||||||
Net foreign exchange gain | (1,070 | ) | (609 | ) | ||||||||
Share-based compensation expense | 349 | — | ||||||||||
Changes in operating assets and liabilities: | ||||||||||||
(Increase) in trade receivables | (115,000 | ) | — | |||||||||
(Increase) decrease in prepayments and other receivables | (6,692 | ) | 666 | |||||||||
Increase in trade and other payables | 1,727 | 634 | ||||||||||
Increase in deferred revenue | 21,500 | — | ||||||||||
|
|
|
| |||||||||
Net cash flows used in operating activities | (44,642 | ) | (8,771 | ) | ||||||||
|
|
|
| |||||||||
Investing Activities: | ||||||||||||
Purchase of equipment | (2 | ) | (7 | ) | ||||||||
|
|
|
| |||||||||
Net cash flows used in investing activities | (2 | ) | (7 | ) | ||||||||
|
|
|
| |||||||||
Financing Activities: | ||||||||||||
Proceeds from issuance of shares and capital contributions | 12 | 79,680 | 69,000 | |||||||||
Payments of lease liabilities | (33 | ) | — | |||||||||
|
|
|
| |||||||||
Net cash provided by financing activities | 79,647 | 69,000 | ||||||||||
|
|
|
| |||||||||
Net increase in cash and cash equivalents | 35,003 | 60,222 | ||||||||||
Foreign exchange differences | 1,383 | 609 | ||||||||||
Cash and cash equivalents at beginning of period | 53,092 | 7,861 | ||||||||||
|
|
|
| |||||||||
Cash and cash equivalents at end of period | 89,478 | 68,691 | ||||||||||
|
|
|
| |||||||||
The accompanying notes are an integral part of these financial statements.
F-81
Table of Contents
NOTESTOTHE UNAUDITED CONDENSED
CONSOLIDATED FINANCIAL STATEMENTS
| 1 | GENERALINFORMATION |
NewAmsterdam Pharma Holding B.V. and Subsidiaries (together as “NewAmsterdam” or the “Company”) is a global biotech company focused on the research and development of transformative therapies for cardio-metabolic diseases. The Company was incorporated in The Netherlands on September 17, 2019. The Company’s principal place of business and registered office is Gooimeer 2-35, 1411 DC Naarden, The Netherlands.
The Company’s fiscal year ends on December 31. References to fiscal year 2021 relate to the fiscal year ended December 31, 2021.
| 2 | BASISOFPREPARATION |
These condensed consolidated financial statements and accompanying notes are unaudited and have been prepared in accordance with International Accounting Standard IAS 34 ‘Interim Financial Reporting’. They do not include all of the information required for a complete set of financial statements prepared in accordance with IFRS standards and should be read in conjunction with the annual financial statements for the year ended December 31, 2021.
The unaudited condensed consolidated financial statements have been prepared on a historical cost basis, unless otherwise disclosed. The unaudited condensed consolidated financial statements have been prepared on a basis which assumes that the Company will continue as a going concern, and which contemplates the recoverability of assets and the satisfaction of the liabilities and commitments in the normal course of business.
The unaudited condensed consolidated financial statements are presented in Euro (“EUR” or “€”). In these notes, Euro amounts are presented in thousands, except for share and per share amounts and as otherwise indicated. The functional currency of the Company is Euro. Rounding differences may occur for calculation reasons.
Beginning on January 1, 2022, the Company has presented its value added tax (“VAT”) payable on a net basis against the value added tax receivable included within Prepayments and other receivables as permitted by the Dutch Tax Authorities on the basis that a fiscal unity was formed between NewAmsterdam Pharma Holding B.V. and NewAmsterdam Pharma B.V. for Dutch VAT purposes. A company and its subsidiaries that are part of the fiscal unity are jointly and severally liable for the VAT payable by the fiscal unit. Previous periods were not retrospectively reclassified as the fiscal unity had not yet been approved.
Based on our current operating plan, we believe that the existing cash and cash equivalents will be sufficient to fund our anticipated level of operations for the twelve months following the date of these financial statements.
The unaudited condensed consolidated financial statements have been approved for issue by the Company’s Board of Directors (the “Board”) on September 13, 2022.
| 3 | SIGNIFICANTACCOUNTINGPOLICIES |
Significant Accounting Judgments, Estimates and Assumptions
These condensed consolidated financial statements as at June 30, 2022 and for the six months ended June 30, 2022 and 2021 should be read in conjunction with the annual consolidated financial statements as at and for the years ended December 31, 2021 and 2020 (“annual financial statements”) as the unaudited condensed consolidated financial statements are prepared on a condensed basis in accordance with IAS 34 and not contain all required disclosures under International Financial Reporting Standards as issued by the International
F-82
Table of Contents
Accounting Standards Board. Unless stated otherwise the unaudited condensed consolidated financial statements as at and for the six-month period ended June 30, 2022 and 2021 are presented on a basis consistent with the accounting policies set out in annual financial statements except the accounting policy for IFRS 15 Revenue Recognition is applicable in the six months ended June 30, 2022 arising from the recognition of revenue for the first time during the period and is set out below.
There were no other significant changes in accounting policies, critical accounting judgements and key sources of estimation uncertainty applied by us in these unaudited condensed consolidated financial statements compared to those used in the annual consolidated financial statements as at December 31, 2021.
Revenue Recognition
Under IFRS 15, to determine the recognition of revenue, the Company performs the following five steps:
| 1. | identify the contract(s) with the customer; |
| 2. | identify the performance obligations in the in the contract; |
| 3. | determine the transaction price; |
| 4. | allocate the transaction price to the performance obligations in the contract; and |
| 5. | recognize revenue when (or as) the Company satisfies a performance obligation. |
The Company performs an analysis to identify the performance obligations for its license agreement. Where a license agreement comprises several promises, it must be assessed whether these promises are capable of being distinct within the context of the contract. Promised goods or services are considered distinct when: (i) the customer can benefit from the good or service on its own or together with other readily available resources, and (ii) the promised good or service is separately identifiable from other promises in the contract. In assessing whether promised goods or services are distinct, the Company considers factors such as the stage of development of the underlying intellectual property, the capabilities of the customer to develop the intellectual property on their own and whether the required expertise is readily available. In addition, the Company considers whether the customer can benefit from a promise for its intended purpose without the receipt of the remaining promises, whether the value of the promise is dependent on the unsatisfied promises, whether there are other vendors that could provide the remaining promises, and whether it is separately identifiable from the remaining promises.
Under IFRS 15, the Company applies significant judgment when evaluating (i) whether the obligations under these agreements represent one or more combined performance obligations, (ii) the determination of whether the reimbursable development costs and milestone payments should be included in the transaction price, (iii) the allocation of the transaction price between the identified performance obligations and (iv) the timing of satisfaction of the identified performance obligations.
The Company estimates the transaction price based on the amount of consideration the Company expects to receive for transferring the promised goods or services in the contract. The consideration may include both fixed consideration and variable consideration. At the inception of each arrangement that includes variable consideration, the Company evaluates the amount of the potential payments and the likelihood that the payments will be received. The Company utilizes either the most likely amount method or expected value method to estimate variable consideration to include in the transaction price based on which method better predicts the amount of consideration expected to be received. The amount included in the transaction price is constrained to the amount for which it is highly probable that a significant reversal of cumulative revenue recognized will not occur. At the end of each subsequent reporting period, the Company re-evaluates the estimated variable consideration included in the transaction price and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis in the period of adjustment.
F-83
Table of Contents
After the transaction price is determined, it is allocated to the identified performance obligations based on the estimated standalone selling price. The Company must develop assumptions that require judgement to determine the standalone selling price for each performance obligation identified in the contract. The Company utilizes key assumptions to determine the standalone selling price, which may include other comparable transactions, pricing considered in negotiating the transaction, probabilities of technical and regulatory success and the estimated costs. Certain variable consideration is allocated specifically to one or more performance obligations in a contract when the terms of the variable consideration relate to the satisfaction of the performance obligation and the resulting amounts allocated to each performance obligation are consistent with the amounts the Company would expect to receive for each performance obligation.
Revenue is recognized when the customer obtains control of the goods and/or services as provided in the license agreement. The control can be transferred over time or at a point in time—which results in the recognition of revenue over time or at a point in time. The Company recognizes revenue over time as the customer simultaneously receive the benefits provided by the Company’s performance, satisfied over time. The recognition of revenue over time is based on a pattern that best reflects the satisfaction of the related performance obligation, applying the input method. The input method estimates the satisfaction of the performance obligation as the percentage of total costs that are completed each period compared to the total estimated costs. A cost-based input method of revenue recognition requires management to make estimates of costs to complete the Company’s performance obligation. In making such estimates, significant judgment is required to evaluate assumptions related to cost estimates. The cumulative effect of revisions to estimated costs to complete the Company’s performance obligation will be recorded in the period in which changes are identified and amounts can be reasonably estimated. A significant change in these assumptions and estimates could have a material impact on the timing and amount of revenue recognized in future periods.
Upfront licensing payments are recognized as revenue at the point in time when the Company transfers control of the license only if the license is determined to be a separate performance obligation from other undelivered performance obligations. Contingent development costs and milestone payments are generally included in the transaction price at the amount stipulated in the respective agreement and recognized to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur.
The Company will recognize royalty revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
New Standards, Interpretations and Amendments Not Yet Adopted by the Company
Standards applied for the first time in the current financial statements
The IASB and the IFRS Interpretations Committee have issued amendments to IFRS 3, IAS 16, IAS 37 and IFRS 9 whose application was mandatory for fiscal years beginning on or after January 1, 2022, but which did not have any material effects on the unaudited condensed consolidated financial statements.
Standards to be applied in future periods
The new and amended standards and interpretations that have been published, but not yet effective, include amendments to IAS 1, IAS 8 and IAS 12. The Company intends to adopt these new and amended standards and interpretations, if applicable, when they become effective.
F-84
Table of Contents
Operating Activities of the Company
| 4 | REVENUE |
Revenue consisted of the following for the six months ended June 30:
| 2022 | 2021 | |||||||
License revenue | 93,500 | — | ||||||
|
|
|
| |||||
| 93,500 | — | |||||||
|
|
|
| |||||
On June 23, 2022, the Company entered into a licensing agreement with A. Menarini International Licensing S.A. (“Menarini”) (the “Menarini License”), pursuant to which it granted Menarini an exclusive, royalty-bearing, sublicensable license under certain of its intellectual property and its regulatory documentation to undertake post approval development activities and commercialize multiple brands of obicetrapib, either as a sole active ingredient product or in a fixed dose combination with ezetimibe (the “Licensed Products”), for any use in the majority of European Countries (“Menarini Territory”).
The Company remains responsible for the development and commercialization costs related to Licensed Products, excluding local development, regulatory and commercialization costs incurred by Menarini in the Menarini Territory. In addition, Menarini is expected to purchase the Licensed Products from the Company in accordance with a supply agreement to be negotiated and entered into no later than six months after the execution of the Menarini License.
The Menarini License includes a non-refundable upfront payment, fixed reimbursements for the Company’s continued development costs, payments based upon the achievement of defined development, regulatory and commercial milestones, sales-based royalties, and certain cost sharing payments made by Menarini to the Company and by the Company to Menarini.
The Company has evaluated the Menarini License based on the requirements of IFRS 15 Revenue Recognition and has concluded the following:
The Menarini License includes various licenses under certain of its intellectual property and its regulatory documentation. The various licenses granted under the Menarini License currently in place do not represent distinct performance obligations, as the licenses are highly interrelated and Menarini would likely be unable to derive significant benefits from their access to these licenses on an individual basis.
The Company, considering that (i) there are no material restrictions included in the contract which would prevent Menarini to direct the use of, and obtain substantially all of the remaining benefits and (ii) the majority of the Company’s remaining development activities are in late-stage development and are not expected to significantly affect the functionality of the underlying intellectual property, concludes that the license as of the effective date of the contract has standalone value. As such, the Company concluded that the promise in granting the licenses to Menarini is to provide a right to use the Company’s intellectual property as it exists at the point in time at which the license is granted and therefore, revenue accrued and allocated to this performance obligation has been recognized at a point in time.
The Company has also identified an additional performance obligation that consist of the research and development activities related to the general development and commercialization costs related to Licensed Products. The Company determined that this performance obligation is satisfied over time as Menarini simultaneously receives and consumes the benefits of the services provided as they are performed. This is based on the fact that Menarini is receiving status of the research periodically which allows it to make informed decisions in its local development activities. The Company further considered that the licenses and the R&D services are not highly interdependent or highly interrelated because the Company is able to fulfill its promise to
F-85
Table of Contents
transfer the licenses regardless of fulfilling its promise to perform the remaining R&D services. The Company also concluded that the licenses are considered distinct as Menarini could benefit from the licenses together with readily available resources other than the Company’s R&D services. The Company will utilize a cost-based input method to measure its progress toward completion of its performance obligation and to calculate the corresponding amount of revenue to recognize each period. The Company believes this is the best measure of progress because other measures do not reflect how the Company transfers its performance obligation to Menarini. In applying the cost-based input method of revenue recognition, the Company uses actual clinical study enrollment figures as well as actual costs incurred relative to budgeted costs expected to be incurred attributable to the Menarini Territory for the performance obligation. These costs consist primarily of third-party contract costs relative to the level of patient enrollment in the studies. Revenue will be recognized based on the level of costs incurred relative to the total budgeted costs for the performance obligation.
Considering that the Menarini License has been executed as of June 23, 2022, no revenue has been recognized related to the research and development performance obligation.
As such, two performance obligations for the Menarini License were identified at contract inception, comprising a license to use the Company’s IP (the “license performance obligation”) and a promise to continue the development activities for the licensed compound (the “R&D performance obligation”).
The Company’s assessment of the transaction price included an analysis of amounts it expected to receive, which at contract inception consisted of the non-refundable, upfront payment of €115.0 million that was received by the Company in July 2022 (see Note 15). The Company considers this non-refundable fee of €115.0 million to be the initial transaction price.
The Company has allocated the transaction price to each performance obligation identified on a relative stand-alone selling price basis. The Company has used a combination of methods to calculate the stand-alone selling prices, using the expected cost plus a margin approach to calculate the standalone selling price of the research and development services required in the Menarini License and needed to commercialize obicetrapib in the Menarini Territory and the residual approach to calculate the stand-alone selling price for the license based on the fair value of the total promised goods and services in the Menarini License considering that the Company has not yet established a price for licenses, has not historically sold licenses on a stand-alone basis (i.e., the selling price is uncertain), and the amount allocated is consistent with the allocation objective as the Company believes the stated upfront amount is consistent with a risk-adjusted price that a market participant would be willing to pay for the licenses.
As of June 30, 2022, the Company has allocated €93.5 million to the license performance obligation, recognized as “revenue” in the condensed consolidated statement of profit and €21.5 million to the R&D performance obligation, recognized as “deferred revenue” in the condensed consolidated statement of financial position.
The deferred revenue has been recognized within current and non-current liabilities based on the expected timing of the associated research and development services. €14.1 million has been recognized as current liabilities as the Company expects to perform the associated services within twelve months after the reporting period. The remaining €7.4 million has been recognized as non-current liabilities.
The Company allocated the upfront payment of €115 million to the identified performance obligations based on the combined license performance obligation and recognized the upfront payment as revenue at a point in time upon satisfaction of the performance obligation, which was achieved at the execution of the Menarini License.
Additionally, in partial contribution to the Company’s costs of development of the Licensed Products, Menarini will pay the Company €27.5 million, payable in two equal annual time based installments, together with bearing 50% of any development costs incurred in respect of the pediatric population in the Menarini Territory. Due to the scientific uncertainties around the commercialization of the Licensed Products based on the success of clinical trials, out of the control of the Company, the fixed €27.5 million is considered constrained at contract
F-86
Table of Contents
execution and is not initially recognized within the transaction price until it becomes highly probable of no significant revenue reversal. If certain conditions are not met, then the Company would not have the right to payment. At the end of each reporting period, the Company will assess the probability of significant reversals for any amounts that become likely to be realized prior to recognizing the fixed consideration associated with these payments within the transaction price. To date, the Company has not received any reimbursement payments.
The Menarini License also provides for certain milestone payments from Menarini to the Company upon the achievement of specified development, regulatory and commercial milestones linked to the enhanced value of the license performance obligation. More specifically, the Company is eligible to receive up to an additional €863 million upon the achievement of various clinical, regulatory and commercial milestones These milestones are contingent payments. These milestone payments represent variable consideration that are not initially recognized within the transaction price, due to the scientific uncertainties around the commercialization of the Licensed Products based on the success of clinical trials. At the end of each reporting period, the Company will assess the probability of significant reversals for any amounts that become likely to be realized prior to recognizing the variable consideration associated with these payments within the transaction price. To date, the Company has not received any milestones payments.
Lastly, the Company is entitled to receive tiered royalty payments based on annual aggregate net sales of all Licensed Products in the Menarini Territory, subject to specified reductions upon commercialization. The royalty term begins for each Licensed Product on a country-by-country basis upon the first commercial sale of such product in such country and ends on the later of (i) the expiration of the last-to-expire patent that includes a valid claim, (ii) the expiration of regulatory exclusivity in such country for such Licensed Product and (iii) a specified number of years after the first commercial sale of such Licensed Product in such country (the term of the agreement). In accordance with IFRS 15, the Company recognizes revenue from royalty payments at the later of (i) the occurrence of the subsequent sale; or (ii) the performance obligation to which some or all of the sales-based milestone or royalty payments has been allocated has been satisfied. The Company anticipates recognizing these royalty payments if and when subsequent sales are generated from the Licensed Products.
The Company has incurred in €3 million of costs to obtain the Menarini License related to unavoidable costs from the Company’s financial advisor assisting in the negotiation. These costs have been proportionally allocated to the identified performance obligations on a relative standalone selling price basis as previously discussed. Based on that, €2.4 million has been recognized as “general and administrative expenses” related to the satisfied license performance obligation and €0.6 has been capitalized as “prepayments and other receivables” which will be amortized on a systematic basis that is consistent with the transfer to the customer of the goods or services to which the asset relates.
| 5 | RESEARCHAND DEVELOPMENT EXPENSE |
Research and development expense consisted of the following items for the six months ended June 30:
| 2022 | 2021 | |||||||
Clinical R&D Costs | ||||||||
Phase 1 and 2 | (3,850 | ) | (3,316 | ) | ||||
Phase 3 | (16,872 | ) | (51 | ) | ||||
Other | (304 | ) | (24 | ) | ||||
|
|
|
| |||||
Total clinical R&D costs | (21,026 | ) | (3,391 | ) | ||||
Non-clinical R&D costs | (1,264 | ) | (105 | ) | ||||
Contracted personnel costs | (1,869 | ) | (1,102 | ) | ||||
Chemistry, Manufacturing and Controls (CMC) | (6,001 | ) | (3,263 | ) | ||||
Regulatory | (381 | ) | (682 | ) | ||||
Other R&D costs | (47 | ) | (10 | ) | ||||
|
|
|
| |||||
Total research and development expenses | (30,588 | ) | (8,553 | ) | ||||
|
|
|
| |||||
F-87
Table of Contents
| 6 | GENERALAND ADMINISTRATIVE EXPENSES |
General and administrative expenses consisted of the following items for the six months ended June 30:
| 2022 | 2021 | |||||||
Contracted personnel costs | (1,314 | ) | (492 | ) | ||||
Travel costs | (83 | ) | (3 | ) | ||||
Intellectual property | (699 | ) | (346 | ) | ||||
Legal costs | (2,250 | ) | (255 | ) | ||||
Licenses | — | — | ||||||
Finance and administration | (4,053 | ) | (147 | ) | ||||
IT | (10 | ) | (4 | ) | ||||
Rent and office services | (77 | ) | (52 | ) | ||||
Marketing and communication | (487 | ) | (13 | ) | ||||
Insurance | (8 | ) | (28 | ) | ||||
Depreciation and amortization | (36 | ) | (2 | ) | ||||
Miscellaneous | (105 | ) | (35 | ) | ||||
Business development | (132 | ) | (21 | ) | ||||
Board fees | (40 | ) | (3 | ) | ||||
|
|
|
| |||||
Total general and administrative expenses | (9,294 | ) | (1,401 | ) | ||||
|
|
|
| |||||
| 7 | TRADE RECEIVABLES |
Trade receivables consisted of the following items as at:
| June 30, 2022 | December 31, 2021 | |||||||
License fee receivable | 115,000 | — | ||||||
|
|
|
| |||||
| 115,000 | — | |||||||
|
|
|
| |||||
On July 7, 2022 the Company received the non-refundable, non-creditable, upfront amount of € 115.0 million from Menarini.
| 8 | PREPAYMENTSAND OTHER RECEIVABLES |
Prepayments and other receivables consisted of the following items as at:
| June 30, 2022 | December 31, 2021 | |||||||
Prepayments | ||||||||
Chemistry, Manufacturing and Controls (CMC) | — | 21 | ||||||
Non-clinical R&D costs | 1,102 | 1,111 | ||||||
Clinical other | 9,036 | 349 | ||||||
General and administrative | 750 | 11 | ||||||
|
|
|
| |||||
Total prepayments | 10,888 | 1,492 | ||||||
Deferred issuance costs | 981 | 4,290 | ||||||
Value added tax receivable | 605 | — | ||||||
|
|
|
| |||||
Total prepayments and other receivables | 12,474 | 5,782 | ||||||
|
|
|
| |||||
F-88
Table of Contents
| 9 | INCOME TAXES |
The Company’s effective tax rate for the six months ended June 30, 2022 was 0% (six months ended June 30, 2021: 0%). The Company has generated income for the first time during the period since inception but it is expected to incur losses for the fiscal year ending December 31, 2022, as the Company expects its costs to fund research and development activities for the full fiscal year to exceed its revenue arising from the upfront payment received from Menarini (see Note 4). The Company does not expect to utilize its tax losses for the foreseeable future. As a result, no deferred tax asset is recognized for these losses.
Financing activities of the Company
| 10 | FINANCIAL RISK MANAGEMENT |
The table below summarizes the maturity profile of the Company’s financial liabilities based on contractual undiscounted payments. Payments related to foreign currency are included at the exchange rates applicable as at:
| On demand | Less than 3 months | 3 – 12 months | More than 1 year | Total | ||||||||||||||||
At December 31, 2021 | ||||||||||||||||||||
Trade and other payables | — | 5,715 | 880 | — | 6,595 | |||||||||||||||
Share-based payment liabilities | 458 | — | — | — | 458 | |||||||||||||||
Lease liability | — | 13 | 40 | 111 | 164 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total financial liabilities | 458 | 5,727 | 920 | 111 | 7,217 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| On demand | Less than 3 months | 3 – 12 months | More than 1 year | Total | ||||||||||||||||
At June 30, 2022 | ||||||||||||||||||||
Trade and other payables | — | 11,413 | — | — | 11,413 | |||||||||||||||
Share based payment liabilities | 369 | — | — | — | 369 | |||||||||||||||
Lease liability | — | 10 | 51 | 90 | 151 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total financial liabilities | 369 | 11,423 | 51 | 90 | 11,933 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 11 | ISSUED CAPITALAND RESERVES |
The following table details the number of shares of the Company as at June 30:
| Voting ordinary shares | Non-voting ordinary shares | Series A preferred shares | Total | |||||||||||||
Nominal value | 0.01 | 0.01 | 0.01 | |||||||||||||
Shares issued | ||||||||||||||||
Opening balance as at January 1, 2021 | 2,500,000 | 2,500,000 | — | 5,000,000 | ||||||||||||
Convertible loan | — | — | 1,111,115 | 1,111,115 | ||||||||||||
Series A—Tranche I | — | — | 4,928,613 | 4,928,613 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Issued shares as at June 30, 2021 | 2,500,000 | 2,500,000 | 6,039,728 | 11,039,728 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Issued shares as at December 31, 2021 | 2,500,000 | 2,785,714 | 6,039,728 | 11,325,442 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Opening balance as at January 1, 2022 | 2,500,000 | 2,785,714 | 6,039,728 | 11,325,442 | ||||||||||||
Series A—Tranche II | — | — | 5,691,430 | 5,691,430 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Issued shares as at June 30, 2022 | 2,500,000 | 2,785,714 | 11,731,158 | 17,016,872 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
F-89
Table of Contents
Rights associated to the classes of shares issued by the Company
The Company has both ordinary shares and non-voting ordinary shares which represent the residual interest in the Company. A non-voting ordinary share is an ordinary share in the Company without voting rights.
Series A preferred shares
Series A preferred shares carry the same voting rights as ordinary shares. Furthermore, Series A preferred shares have a liquidation preference over ordinary shares of the Company. Meaning that in case of a Liquidity Event the Series A preferred shares shareholders will receive, the preference share capital and share premium paid in by the shareholder, increased by any declared but unpaid preference dividends. Any remaining proceeds following the liquidation preference shall be distributed to equally among all classes of preference shares and the ordinary shares equal terms. The Preference Series A shares are convertible to ordinary shares on a one-to-one ratio at the option of the holder.
The following table details the total share capital and share premium of the Company as at June 30:
| Date | Voting ordinary shares | Non-Voting ordinary shares | Series A Preferred shares | Share premium | Total share capital and share premium | |||||||||||||||
Opening balance as at January 1, 2021 | 25,000 | 25,000 | — | 2,450,000 | 2,500,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Convertible loan Jan 2021 | — | — | 11,111 | 11,656,036 | 11,667,147 | |||||||||||||||
Series A—Tranche I Jan 2021 | — | — | 49,286 | 68,950,716 | 69,000,002 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As at June 30, 2021 | 25,000 | 25,000 | 60,397 | 83,056,752 | 83,167,149 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As presented in the statement of financial position (in thousands of Euro) | 25 | 25 | 60 | 83,057 | 83,167 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As at December 31, 2021 | 25,000 | 27,857 | 60,397 | 83,762,466 | 83,875,720 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Opening balance as at January 1, 2022 | 25,000 | 27,857 | 60,397 | 83,762,466 | 83,875,720 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Series A—Tranche II Feb 2022 | — | — | 56,914 | 79,623,074 | 79,679,988 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As at June 30, 2022 | 25,000 | 27,857 | 117,311 | 163,385,540 | 163,555,708 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
As presented in the statement of financial position (in thousands of Euro) | 25 | 28 | 117 | 163,386 | 163,556 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Closing of Series A preferred shares—Tranche II
The second tranche of € 80 million for 5,691,430 Series A preferred shares is subject to attainment of certain milestone tranche conditions, namely: at least 50% low-density lipoprotein cholesterol (“LDL”) lowering in the currently ongoing Ezetimibe/Obicetrapib Phase 2 combination trial; confirmation by the U.S. Food and Drug Administration that no CVOT trial is required for U.S. approval following the end of the Phase 2 meeting; and the hiring of a full-time Chief Business Officer (“CBO”) with unanimous consent of the Board.
The LDL and CBO conditions were satisfied as at December 31, 2021. Because the FDA condition was not satisfied, the Company was obligated to seek additional financing under different scenarios at the discretion of the Company pursuant to the terms of the agreement. The execution of the Menarini Agreement satisfied this requirement. Therefore, the milestone tranche conditions were considered to be met. The Company received the second tranche financing on February 18, 2022.
F-90
Table of Contents
| 12 | SHARE-BASED PAYMENTS |
Long term incentive plan
Share-Based Payment Expenses Recognized for Options Granted
The fair value of the options is expensed on a straight-line basis over the relevant vesting periods, based on management’s estimate of the number of options that will eventually vest. For the six months ended June 30, 2022, the total share-based payment expense recognized for the equity-settled options amounted to € 0.349 million (six months ended June 30, 2021: nil). This includes share-based payment expense recognized in connection with the vesting of the Second Tranche Options subject to vesting conditions that are considered vested based on the current status of vesting conditions.
In addition, the Company recognized € 20 thousand as expenses for the employer social security contributions expected to be payable related to these options. The corresponding liability as at June 30, 2022 amounts to € 67 thousand.
Share investment plan
Company has recognized the consideration as a financial liability until the Founder DRs have vested, at which time it will be reclassified to equity provided that the Subscriber remains with the Company. This liability is measured at the lower of the price paid for the Founder DRs and their current fair market value. The liability as at the June 30, 2022 amounts to € 369 thousand (December 31, 2021: €458 thousand).
| 13 | EARNINGS PER SHARE |
Loss per share for the period presented has been determined by dividing the loss attributable to shareholders by the weighted average number of shares outstanding during the period. Non-voting ordinary shares and voting ordinary shares are considered to be the same class of equity for purposes of determining the loss attributable per shareholder class per share.
The following table sets forth the computation of basic and diluted loss per share for the six months ended June 30:
| (In thousands of Euro, except share and per share amounts) | 2022 | 2021 | ||||||
Profit (loss) for the period | 54,513 | (9,482 | ) | |||||
Number of common shares | 17,016,872 | 11,039,728 | ||||||
Number of diluted shares | 18,981,158 | 11,039,728 | ||||||
Basic earnings per share | 3.20 | (0.86 | ) | |||||
Diluted earnings per share | 2.87 | (0.86 | ) | |||||
For the six months ended June 30, 2022, as a result of the profit for the period, in accordance with IAS 33, the effect that would arise if all the outstanding stock options were exercised (represented by 1,964,286 weighted average potentially diluted shares) were taken into consideration in the calculation of diluted profit per share. For the six months ended June 30, 2021, diluted earnings per share is equal to basic earnings per share as there were no potentially dilutive instruments.
For the six months ended June 30, 2022, there are no instruments outstanding that were not included in the calculation of diluted earnings per share because they are antidilutive, that could potentially dilute basic earnings per share further in future periods.
Additional information
| 14 | RELATED PARTIES |
As at June 30, 2022 and December 31, 2021, the ultimate controlling party of the Company was Forbion Capital Fund II Coöperatief U.A.
F-91
Table of Contents
The following table provides the total amount of transactions that have been entered into with related parties for the six months ended June 30, 2022, and 2021:
| Related party relationship | Type of transaction | Purchases from | ||||||||||
2022 | ||||||||||||
FCPM III Services B.V. (“Forbion III”) | Common control | Rent and office services | 20 | |||||||||
2021 | ||||||||||||
FCPM III Services B.V. (“Forbion III”) | Common control | Rent and office services | 20 | |||||||||
The following table provides the positions with related parties for the relevant financial as at June 30, 2022 and December 30, 2021:
| Related party relationship | Type of transaction | Amounts owed by | ||||||||||
2022 | ||||||||||||
Mr. M.H. Davidson | Exec. director & shareholder | Receivables | 728 | |||||||||
2021 | ||||||||||||
Mr. M.H. Davidson | Exec. director & shareholder | Receivables | 718 | |||||||||
| 15 | EVENTS AFTERTHE REPORTING PERIOD |
The Company evaluated subsequent events for recognition or disclosure through September 13, 2022, the date these condensed consolidated financial statements were authorized for issuance by the Board of Directors.
Menarini License upfront payment
On July 7, 2022 the Company received the non-refundable, non-creditable, upfront amount of € 115.0 million from Menarini (see Note 7).
Related Party Receivable
On July 19, 2022 the CEO, M. Davidson, settled the loan facility of € 709 thousand with the Company including outstanding unpaid interest of €19 thousand, for a total of €728 thousand.
Triggering of Exit Events pursuant to the 2020 SPA
On July 20, 2022, the Company and Saga Investments Coöperatief U.A., an affiliate of Amgen (“Amgen”) and the Company and Mitsubishi Tanabe Pharma Corporation (“MTPC”) each executed additional waiver notices (collectively the “Third Waivers”) stating that the Agreement between the Company and FLAC (defined below) constituted an additional exit event that triggered payment of the contingent consideration as proceeds are payable to shareholders under the Agreement. The Third Waivers indicate that the contingent consideration will be settled via the profit right. The profit right will be settled in shares of Holdco in lieu of Company shares, and will be issued prior to the closing of the merger with FLAC and the Company (the “Profit Right”). Both Amgen and MTPC also agreed to waive their right to matching so long as the Agreement is executed before October 31, 2022. The transaction is expected to close in the second half of 2022.
As a result, Amgen will receive 4,910,000 shares of HoldCo with an estimated value of $49,100,000 and MTPC will receive 3,746,330 shares of Holdco with an estimated value of $37,463,300, in addition, each such shareholder will be entitled to receive its pro rata share on additional shares of Holdco, including the earnout shares to be obtained if and when the Company achieves a certain future development milestone (the “Earnout Shares”). Upon the issuance of the HoldCo shares, all rights of Amgen and MTPC under the 2020 SPA and the Profit Right Agreement, respectively, will be extinguished.
F-92
Table of Contents
Merger between the Company and Frazier Lifesciences Acquisition Corporation
On July 25, 2022, Frazier Lifesciences Acquisition Corporation (“FLAC”), a special purpose acquisition company sponsored by Frazier Lifesciences Sponsor LLC that is incorporated as a Cayman Islands exempted company, Holdco, Merger Sub and the Company entered into a Business Combination Agreement (the “Agreement”). Upon the merger between FLAC and Company being consummated, the combined entity with be a publicly traded company listed on the Nasdaq in the United States. The Company’s shareholders are expected to have a controlling interest in the combined entity. The transaction is subject to customary and other closing conditions, including the approval of the shareholders of both the Company and FLAC. The transaction is expected to close in the fourth quarter of 2022.
Company shareholders are expected to hold the controlling interest in the combined entity, and as FLAC does not constitute a business under IFRS 3 Business Combinations, the transaction will also be accounted for as a capital reorganization and is in scope of IFRS 2 Share-based Payment. Holdco will issue shares in exchange for the net assets of FLAC. Any difference between the fair value of the shares issued by Holdco and the fair value of FLAC’s identifiable net assets will be treated as costs for the service of obtaining a listing and expensed in the period in which the transaction closes.
In conjunction with the merger, additional financing in the form of a private investment in public entity (“PIPE”) was secured as of July 25, 2022. HoldCo has committed to issue 23,460,000 with a value of $234,600,000 at the closing of the merger.
NewAmsterdam Pharma Holding B.V.
Naarden, September 13, 2022
F-93
Table of Contents
Table of Contents
REPORTOF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of NewAmsterdam Pharma Company B.V.
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of NewAmsterdam Pharma Company B.V. (the “Company”) as at June 30, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as at June 30, 2022, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provide a reasonable basis for our opinion.
/s/ Deloitte Accountants B.V.
Rotterdam, The Netherlands
September 13, 2022
We have served as the Company’s auditor since 2022
F-95
Table of Contents
NewAmsterdam Pharma Company B.V.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
| Note | June 30, 2022 | |||||||
Assets | ||||||||
Other receivables—related parties | 2 | 1 | ||||||
|
| |||||||
Total assets | 1 | |||||||
|
| |||||||
Equity | ||||||||
Share capital | 3 | (1 | ) | |||||
|
| |||||||
Total equity and liabilities | (1 | ) | ||||||
|
| |||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-96
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF NEW AMSTERDAM PHARMA COMPANY B.V.
Note 1 — Significant accounting policies
General Information
NewAmsterdam Pharma Company B.V. (“the Company”) was incorporated as a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) on June 10, 2022. The Company currently has one wholly-owned subsidiary, NewAmsterdam Pharma Investment Corporation (“Merger Sub”). The Company is registered with the Dutch Trade Register under number 86649051. The Company’s corporate seat is in Naarden, the Netherlands, and the Company’s office address is Gooimeer 2-35 1411 DC Naarden, the Netherlands.
The Company’s parent is NewAmsterdam Pharma Holding B.V. (“NewAmsterdam Pharma”).
The Company was formed for the purpose of becoming the ultimate parent company of NewAmsterdam Pharma, contemporaneous with the closing of the transaction contemplated in the business combination agreement, dated July 25, 2022 (the “Business Combination Agreement”) by and among the Company, NewAmsterdam Pharma, Merger Sub, and Frazier Lifesciences Acquisition Corporation (“FLAC”), a special purpose acquisition company. As described above, the Company currently maintains one directly wholly-owned subsidiary, Merger Sub. Merger Sub was incorporated to facilitate the consummation of the Business Combination Agreement. As of June 30, 2022, Merger Sub had no assets, liabilities or operations. Refer to Note 4 – Subsequent events for additional detail regarding the steps to effectuate the closing of the transaction as outlined in the Business Combination Agreement.
The Company’s fiscal year ends on December 31.
Basis of preparation
These consolidated financial statements and accompanying notes have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IASB”). No annual financial statements have been prepared for the Company as it was created on June 10, 2022. Additionally, separate statements of profit or loss and other comprehensive income or loss, and cash flows have not been presented because there have been no operating activities in the Company during the period of June 10, 2022 to June 30, 2022.
The consolidated financial statements have been prepared on a historical cost basis, unless otherwise disclosed. The consolidated financial statements have been prepared on a basis which assumes that the Company will continue as a going concern, and which contemplates the recoverability of assets and the satisfaction of the liabilities and commitments in the normal course of business.
The consolidated financial statements are presented in Euro (“EUR” or “€”). The functional currency of the Company is Euro. Rounding differences may occur for calculation reasons.
The consolidated financial results of the Company are reported over the period June 10, 2022 until June 30, 2022.
The consolidated financial statements have been approved for issue by the Company’s Board of Directors (the “Board”) on September 13, 2022.
Basis of Consolidation
The consolidated financial statements include the financial statements of the Company and entities controlled by the Company (its subsidiaries). Control is achieved when the Company:
| • | has power over the investee; |
F-97
Table of Contents
| • | is exposed, or has rights, to variable returns from its involvement with the investee; and |
| • | has the ability to use its power to affect its returns. |
The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control listed above.
Other financial assets
Financial instruments are any form of contract that gives rise to a financial asset in one company and a financial liability or equity instrument in another company. A financial asset is recognized on the balance sheet when the entity becomes a party to the contractual terms of the instrument. Other financial assets, including receivables from related parties, are initially recognized at fair value and subsequently measured at amortized cost.
The Company measures the loss allowance for receivables at an amount equal to the probability of default in the next twelve months unless a significant increase in credit risk has occurred in which the loss allowance is measured on the lifetime expected loss. A financial asset is removed from the balance sheet when the contractual right to cash flows from the asset has ceased or been settled.
Equity instruments
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by the Company are recognized at the proceeds received, net of direct issue costs.
Note 2 — Related party transaction with NewAmsterdam Pharma
The Company issued one share of €0.12 to NewAmsterdam Pharma on June 10, 2022.
Note 3 — Share capital
The Company was incorporated on June 10, 2022 and issued 1 ordinary share at €0.12 to NewAmsterdam Pharma. The entire amount of this equity is held by NewAmsterdam Pharma and recorded as a receivable under the caption “Other receivables—related parties” in the Company’s Consolidated Statement of Financial Position. The Company has the ability to issue an unlimited number of additional shares.
Note 4 — Subsequent events
The Company evaluated subsequent events for recognition or disclosure through September 13, 2022, the date these consolidated financial statements were authorized for issuance by the Board of Directors.
Merger between NewAmsterdam Pharma and FLAC
On July 25, 2022, FLAC, a special purpose acquisition company sponsored by Frazier Lifesciences Sponsor LLC that is incorporated as a Cayman Islands exempted company, the Company, Merger Sub and NewAmsterdam Pharma entered into the Business Combination Agreement. Upon consummation of the merger between FLAC and NewAmsterdam Pharma, the Company will be a publicly traded company listed on the Nasdaq in the United States. NewAmsterdam Pharma’s shareholders are expected to have a controlling interest in the Company following the consummation of the Business Combination Agreement. The Business Combination Agreement is subject to customary and other closing conditions, including the approval of the shareholders of both NewAmsterdam Pharma and FLAC. The transaction is expected to close in the fourth quarter of 2022, with the Company converting into a Dutch public company with limited liability, becoming the ultimate parent and listed entity of the merged group.
F-98
Table of Contents
NewAmsterdam Pharma shareholders are expected to hold the controlling interest in the Company, and as FLAC does not constitute a business under IFRS 3 Business Combinations, the transaction will also be accounted for as a capital reorganization and is in scope of IFRS 2 Share-based Payment. The Company will issue shares in exchange for the net assets of FLAC. Any difference between the fair value of the shares issued by the Company and the fair value of FLAC’s identifiable net assets will be treated as costs for the service of obtaining a listing and expensed in the period in which the transaction closes.
As a result of the merger, additional financing in the form of a private investment in public entity (“PIPE”) was secured as of July 25, 2022. The Company has committed to issue 23,460,000 shares with a value of $234.6 million at the closing of the merger. However, the consummation of the transactions contemplated by the Business Combination Agreement is subject to numerous conditions, and there can be no assurances that such conditions will be satisfied.
F-99
Table of Contents
Table of Contents
TABLE OF CONTENTS
| PAGE | ||||||||
ARTICLE 1 CERTAIN DEFINITIONS | A-3 | |||||||
Section 1.1 | Definitions | A-3 | ||||||
ARTICLE 2 MERGER | A-23 | |||||||
Section 2.1 | Transactions | A-23 | ||||||
Section 2.2 | Closing | A-25 | ||||||
Section 2.3 | Withholding | A-25 | ||||||
Section 2.4 | FLAC Warrants | A-26 | ||||||
Section 2.5 | Allocation Schedule | A-26 | ||||||
Section 2.6 | Treatment of Company Equity Awards | A-26 | ||||||
Section 2.7 | Earnout | A-27 | ||||||
Section 2.8 | Exchange of Shares | A-28 | ||||||
ARTICLE 3 REPRESENTATIONS AND WARRANTIES RELATING TO THE GROUP COMPANIES | A-29 | |||||||
Section 3.1 | Organization and Qualification | A-29 | ||||||
Section 3.2 | Capitalization of the Group Companies | A-30 | ||||||
Section 3.3 | Authority | A-31 | ||||||
Section 3.4 | Financial Statements; Undisclosed Liabilities | A-32 | ||||||
Section 3.5 | Consents and Requisite Governmental Approvals; No Violations | A-33 | ||||||
Section 3.6 | Permits | A-33 | ||||||
Section 3.7 | Material Contracts | A-33 | ||||||
Section 3.8 | Absence of Changes | A-35 | ||||||
Section 3.9 | Business Activities | A-35 | ||||||
Section 3.10 | Litigation | A-36 | ||||||
Section 3.11 | Compliance with Applicable Law | A-36 | ||||||
Section 3.12 | Employee Plans | A-36 | ||||||
Section 3.13 | Environmental Matters | A-38 | ||||||
Section 3.14 | Intellectual Property | A-38 | ||||||
Section 3.15 | Labor Matters | A-41 | ||||||
Section 3.16 | Insurance | A-43 | ||||||
Section 3.17 | Tax Matters | A-43 | ||||||
Section 3.18 | Brokers | A-45 | ||||||
Section 3.19 | Real and Personal Property | A-45 | ||||||
Section 3.20 | Transactions with Affiliates | A-46 | ||||||
Section 3.21 | Data Privacy and Security | A-46 | ||||||
Section 3.22 | Compliance with International Trade & Anti-Corruption Laws | A-47 | ||||||
Section 3.23 | Information Supplied | A-48 | ||||||
Section 3.24 | Investigation; No Other Representations | A-48 | ||||||
Section 3.25 | Regulatory Compliance | A-48 | ||||||
Section 3.26 | Investment Company Act | A-50 | ||||||
Section 3.27 | PIPE Financing | A-50 | ||||||
Section 3.28 | EXCLUSIVITY OF REPRESENTATIONS AND WARRANTIES | A-51 | ||||||
ARTICLE 4 REPRESENTATIONS AND WARRANTIES RELATING TO FLAC | A-51 | |||||||
Section 4.1 | Organization and Qualification | A-51 | ||||||
Section 4.2 | Authority | A-51 | ||||||
Section 4.3 | Consents and Requisite Governmental Approvals; No Violations | A-52 | ||||||
Section 4.4 | Brokers | A-52 | ||||||
Section 4.5 | Information Supplied | A-53 | ||||||
A-i
Table of Contents
Section 4.6 | Capitalization of FLAC | A-53 | ||||||
Section 4.7 | SEC Filings | A-53 | ||||||
Section 4.8 | Trust Account | A-54 | ||||||
Section 4.9 | Transactions with Affiliates | A-54 | ||||||
Section 4.10 | Litigation | A-55 | ||||||
Section 4.11 | Compliance with Applicable Law | A-55 | ||||||
Section 4.12 | Business Activities | A-55 | ||||||
Section 4.13 | Internal Controls; Listing; Financial Statements | A-55 | ||||||
Section 4.14 | No Undisclosed Liabilities | A-56 | ||||||
Section 4.15 | Tax Matters | A-57 | ||||||
Section 4.16 | Investigation; No Other Representations | A-58 | ||||||
Section 4.17 | Compliance with International Trade & Anti-Corruption Laws | A-58 | ||||||
Section 4.18 | EXCLUSIVITY OF REPRESENTATIONS AND WARRANTIES | A-59 | ||||||
ARTICLE 5 COVENANTS | A-59 | |||||||
Section 5.1 | Conduct of Business of the Company | A-59 | ||||||
Section 5.2 | Efforts to Consummate | A-62 | ||||||
Section 5.3 | Confidentiality and Access to Information | A-64 | ||||||
Section 5.4 | Public Announcements | A-65 | ||||||
Section 5.5 | Tax Matters | A-66 | ||||||
Section 5.6 | Exclusive Dealing | A-67 | ||||||
Section 5.7 | Preparation of Registration Statement / Proxy Statement | A-68 | ||||||
Section 5.8 | FLAC Shareholder Approval | A-69 | ||||||
Section 5.9 | Required Company Shareholder Approval | A-70 | ||||||
Section 5.10 | Merger Sub Shareholder Approval | A-70 | ||||||
Section 5.11 | Conduct of Business of FLAC | A-70 | ||||||
Section 5.12 | Nasdaq Listing | A-72 | ||||||
Section 5.13 | Trust Account | A-72 | ||||||
Section 5.14 | FLAC Indemnification; Directors’ and Officers’ Insurance | A-72 | ||||||
Section 5.15 | Company Indemnification; Directors’ and Officers’ Insurance | A-73 | ||||||
Section 5.16 | Post-Closing Directors and Officers | A-74 | ||||||
Section 5.17 | PCAOB Financials | A-75 | ||||||
Section 5.18 | Conduct of Business of Holdco | A-76 | ||||||
Section 5.19 | Holdco Equity Incentive Plan | A-76 | ||||||
Section 5.20 | PIPE Subscriptions | A-76 | ||||||
Section 5.21 | EU Securities Regulation | A-77 | ||||||
Section 5.22 | Employee Stock Purchase Plan | A-77 | ||||||
ARTICLE 6 CONDITIONS TO CONSUMMATION OF THE TRANSACTIONS | A-78 | |||||||
Section 6.1 | Conditions to the Obligations of the Parties | A-78 | ||||||
Section 6.2 | Other Conditions to the Obligations of FLAC | A-78 | ||||||
Section 6.3 | Other Conditions to the Obligations of the Company | A-79 | ||||||
Section 6.4 | Frustration of Closing Conditions | A-80 | ||||||
ARTICLE 7 TERMINATION | A-80 | |||||||
Section 7.1 | Termination | A-80 | ||||||
Section 7.2 | Effect of Termination | A-81 | ||||||
ARTICLE 8 MISCELLANEOUS | A-81 | |||||||
Section 8.1 | Non-Survival | A-81 | ||||||
Section 8.2 | Entire Agreement; Assignment | A-81 | ||||||
Section 8.3 | Amendment | A-81 | ||||||
Section 8.4 | Notices | A-82 | ||||||
Section 8.5 | Governing Law | A-83 | ||||||
A-ii
Table of Contents
Section 8.6 | Fees and Expenses | A-83 | ||||||
Section 8.7 | Construction; Interpretation | A-83 | ||||||
Section 8.8 | Exhibits and Schedules | A-84 | ||||||
Section 8.9 | Parties in Interest | A-84 | ||||||
Section 8.10 | Severability | A-84 | ||||||
Section 8.11 | Counterparts; Electronic Signatures | A-84 | ||||||
Section 8.12 | Knowledge of Company; Knowledge of FLAC | A-84 | ||||||
Section 8.13 | No Recourse | A-85 | ||||||
Section 8.14 | Extension; Waiver | A-85 | ||||||
Section 8.15 | Waiver of Jury Trial | A-85 | ||||||
Section 8.16 | Submission to Jurisdiction | A-86 | ||||||
Section 8.17 | Remedies | A-86 | ||||||
Section 8.18 | Trust Account Waiver | A-86 |
ANNEXES AND EXHIBITS
| Annex A | Supporting Company Shareholders | |
| Annex B | PIPE Investors | |
| Annex C | IRA Shareholders | |
| Exhibit A | Form of Company Support Agreement | |
| Exhibit B | Form of Sponsor Support Agreement | |
| Exhibit C | Form of Subscription Agreement | |
| Exhibit D | Form of Investor Rights Agreement | |
| Exhibit E | Form of Lock-Up Agreement | |
| Exhibit F | Form of Holdco Articles of Association | |
| Exhibit G | Form of Surviving Company Certificate of Incorporation | |
| Exhibit H | Form of Surviving Company Bylaws | |
| Exhibit I | Form of Warrant Assumption Agreement | |
| Exhibit J | Form of Holdco Long-Term Incentive Plan | |
A-iii
Table of Contents
BUSINESS COMBINATION AGREEMENT
This BUSINESS COMBINATION AGREEMENT (this “Agreement”), dated as of July 25, 2022, is made by and among NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”), Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company (“FLAC”), NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”), and NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (the “Company”). FLAC, Holdco, Merger Sub and the Company shall be referred to herein from time to time individually as a “Party,” and collectively as the “Parties.” Capitalized terms used but not otherwise defined herein have the meanings set forth in Section 1.1 or elsewhere in this Agreement.
WHEREAS, (a) FLAC is a blank check company that was originally incorporated as a Cayman Islands exempted company on October 7, 2020 and incorporated for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses, (b) Holdco is a newly formed entity that is, as of the date of this Agreement, a wholly owned Subsidiary of the Company and was formed for purposes of consummating the Transactions and (c) Merger Sub is, as of the date of this Agreement, a wholly owned Subsidiary of Holdco that was formed for purposes of consummating the Transactions;
WHEREAS, after the execution of this Agreement and prior to the Effective Date, each Company Shareholder shall contribute and transfer each Company Share held by it to Holdco and Holdco shall accept such contribution and in exchange issue to such holder such number of Holdco Shares that is equal to the Applicable Exchange Consideration Per Share with respect to such Company Share (the foregoing transactions together, the “Company Share Exchange”);
WHEREAS, following the Company Share Exchange, the legal form of Holdco shall be converted from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) on the terms and subject to the conditions set forth in this Agreement (the “Holdco Reorganization”), provided that the Company and FLAC may agree instead to effect the Holdco Reorganization promptly following the PIPE Financing;
WHEREAS, following the Holdco Reorganization and the Required Holdco Shareholder Approval, upon the terms and subject to the conditions of this Agreement and in accordance with the Companies Act (as Revised) (the “Cayman Companies Act”), at the Effective Date, Merger Sub will merge with and into FLAC, with FLAC surviving such merger as a wholly owned subsidiary of Holdco (the “Merger”);
WHEREAS, at the Effective Date, by virtue of the Merger and the Required Holdco Shareholder Approval, and without any further action on the part of any Party or any other Person: (a) the Relevant FLAC Shares shall be automatically cancelled and extinguished in exchange for the Merger Consideration, each issued and outstanding FLAC Warrant shall automatically cease to represent a right to acquire FLAC Class A Shares and shall represent a right to acquire Holdco Shares, and the Merger Consideration will be settled as follows: (i) each holder of Relevant FLAC Shares will be entitled to the Merger Claims, (ii) the Merger Claims will be contributed and transferred to Holdco in exchange for the issuance of Holdco Shares (in each case, upon the terms and subject to the conditions set forth in this Agreement); and (iii) the Surviving Company will issue and allot to Holdco corresponding Equity Securities in the Surviving Company, and (b) the Warrant Exchange shall occur;
WHEREAS, immediately after the Effective Date, the Surviving Company shall domesticate as a Delaware corporation in accordance with Section 388 of the General Corporation Law of the State of Delaware (the “DGCL”) and Section 206 of the Cayman Companies Act (the “Domestication”), on the terms and subject to the conditions set forth in this Agreement;
WHEREAS, in connection with the Transactions, the Parties desire for Holdco to register the Holdco Shares, and the issuance thereof, with the SEC to become a publicly traded company;
A-1
Table of Contents
WHEREAS, pursuant to the Governing Documents of FLAC, FLAC is required to provide an opportunity for its shareholders to have their outstanding FLAC Class A Shares redeemed pursuant to the FLAC Shareholder Redemption on the terms and subject to the conditions set forth therein in connection with obtaining the Required FLAC Shareholder Approval;
WHEREAS, concurrently with the execution of this Agreement, the Company Shareholders listed on Annex A attached hereto (collectively, the “Supporting Company Shareholders”) are entering into a support agreement substantially in the form attached hereto as Exhibit A (the “Company Support Agreement”), with the Company, Holdco, Merger Sub and FLAC, pursuant to which, among other things, each Supporting Company Shareholder (a) granted or will grant, as applicable, the Company (or a designee of the Company) with an irrevocable power of attorney, substantially in the form attached to the Company Support Agreement, permitting and directing the Company (or a designee of the Company), acting on behalf of each such Supporting Company Shareholder, and the proxyholders under such power of attorney to execute (i) the Dutch Deed of Issue Company Share Exchange and (ii) any other Ancillary Documents to which such Supporting Company Shareholder is or will be a party and (b) irrevocably undertook vis-à-vis the Company, Holdco, FLAC and each other Supporting Company Shareholder to perform all necessary or desirable actions in connection with the Transactions to consummate the Company Share Exchange and (c) agreed to certain covenants to support the Transactions, including certain restrictions on the sale, disposition or transfer of the Company Shares held by such Supporting Company Shareholder;
WHEREAS, concurrently with the execution of this Agreement, certain FLAC Shareholders (including Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (the “Sponsor”)) which are the record holders of all the issued and outstanding FLAC Class B Shares, FLAC, the Company and Holdco are entering into a Sponsor Support Agreement substantially in the form attached hereto as Exhibit B (the “Sponsor Support Agreement”), pursuant to which, among other things, such FLAC Shareholders and such principals have agreed (a) to vote in favor of this Agreement and the Transactions (including the Merger), (b) to waive any adjustment to the conversion ratio set forth in the Governing Documents of FLAC or any other anti-dilution or similar protection with respect to the FLAC Class B Shares (whether resulting from the transactions contemplated by the Subscription Agreements or otherwise) and (c) not to redeem their respective shares in FLAC in connection with the Transactions (including the Merger) contemplated hereby and in the Ancillary Documents;
WHEREAS, (a) concurrently with the execution of this Agreement, each of Holdco and FLAC are entering into subscription agreements substantially in the form attached hereto as Exhibit C (each a “Subscription Agreement,” and collectively, the “Subscription Agreements”) with certain investors listed on Annex B attached hereto (each a “PIPE Investor,” and collectively, the “PIPE Investors”) pursuant to which, among other things, the PIPE Investors have agreed to subscribe for and purchase, and Holdco has agreed to issue and sell to the PIPE Investors, substantially concurrently with the Closing, an aggregate number of Holdco Shares set forth in the Subscription Agreements in exchange for an aggregate purchase price of $234,600,000 (which amount, for the avoidance of doubt, includes amounts subscribed for by the Company Shareholders or the Sponsor and its Affiliates), on the terms and subject to the conditions set forth therein (such equity financing hereinafter referred to as the “PIPE Financing”);
WHEREAS, immediately prior to the Closing, in accordance with Section 6.2(d)(ii) and Section 6.3(d)(ii), Holdco, the Sponsor, all other holders of FLAC Class B Shares and the (direct or indirect) Company Shareholders listed on Annex C attached hereto (collectively, the “IRA Shareholders”) shall enter into a registration rights agreement, substantially in the form attached hereto as Exhibit D (the “Investor Rights Agreement”), pursuant to which, among other things, (a) the Sponsor and each IRA Shareholder will agree not to effect any sale or distribution of any Equity Securities of Holdco issued to them pursuant to this Agreement or the Subscription Agreements during the applicable lock-up period described therein and (b) the Sponsor and each IRA Shareholder will be granted certain registration rights with respect to their respective Holdco Shares, in each case, on the terms and subject to the conditions therein;
A-2
Table of Contents
WHEREAS, at the Closing, certain holders of Company Shares who are not party to the Investor Rights Agreement (after giving effect to the Merger and the PIPE Financing) will enter into lock-up agreements substantially in the form attached to this Agreement as Exhibit E (each, a “Lock-Up Agreement”), each of which shall be effective as of the Closing, pursuant to which, among other things, the holders of Company Shares party thereto will agree not to effect any sale or distribution of certain shares of the Company held by them during the applicable lock-up period described therein, and on the terms and subject to the conditions therein;
WHEREAS, effective upon the Effective Date, the appointment of members to the board of directors of Holdco (the “Holdco Board”) as approved in the Required Holdco Shareholder Approval will take effect;
WHEREAS, the board of directors of FLAC (the “FLAC Board”), acting upon the unanimous recommendation of a special committee comprised solely of disinterested and independent directors (the “FLAC Special Committee”), has unanimously (a) determined that the Merger and the other Transactions are fair to, and in the best interests of, FLAC, (b) adopted a resolution approving this Agreement and declaring its advisability and approving the Merger and the other Transactions, and (c) recommended the authorization of the Plan of Merger and the approval of the Transactions by the shareholders of FLAC in order to procure the Required FLAC Shareholder Approval;
WHEREAS, the Holdco Board has (a) determined that this Agreement, the Merger and the other Transactions are in the best interests of Holdco and its business and (b) adopted a resolution approving this Agreement, the Merger and the other Transactions;
WHEREAS, the Company, in its capacity as the sole shareholder of Holdco as of the date of this Agreement, has adopted a resolution approving this Agreement, the Merger and the other Transactions; and
WHEREAS, the board of directors of the Company (the “Company Board”) has duly and unanimously adopted resolutions (i) determining that this Agreement and the Transactions are conducive to the Company’s objects and serve the best interests of the Company, its business and the Company’s stakeholders, (ii) approving the execution, delivery and performance by the Company of this Agreement and the consummation of the Transactions and (iii) resolving to recommend the approval of this Agreement and the Transactions by the holders of Company Shares entitled to vote thereon with the majority necessary pursuant to Company’s Governing Documents (the “Required Company Shareholder Approval”).
NOW, THEREFORE, in consideration of the premises and the mutual promises set forth herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties, each intending to be legally bound, hereby agree as follows:
ARTICLE 1
CERTAIN DEFINITIONS
Section 1.1 Definitions. As used in this Agreement, the following terms have the respective meanings set forth below.
“Additional FLAC SEC Reports” has the meaning set forth in Section 4.7.
“Affiliate” means, with respect to any Person, any other Person who directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term “control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise, and the terms “controlled” and “controlling” have meanings correlative thereto.
“Affordable Care Act” means the U.S. Patient Protection and Affordable Care Act (Pub. L. 111–148).
A-3
Table of Contents
“Aggregate Cash Proceeds” means the sum of the (a) aggregate gross cash proceeds to be received (or deemed received) by Holdco or any of its Affiliates in respect of the PIPE Financing (whether prior to, on or following the Closing Commencement Date) plus (b) the amount of cash available in the Trust Account after giving effect to the FLAC Shareholder Redemption.
“Aggregate Share Consideration” means an amount of Holdco Shares equal to the Purchase Price divided by the Holdco Per Share Value.
“Agreement” has the meaning set forth in the preamble to this Agreement.
“Allocated Company Option Pool” means the number of Company Shares subject to Company Options, or underlying depository receipts for Company Shares subject to Company Options, immediately prior to the Effective Date.
“Allocation Schedule” has the meaning set forth in Section 2.5.
“Ancillary Documents” means the Company Support Agreement, the Sponsor Support Agreement, the Subscription Agreements, the Investor Rights Agreement and the Lock-Up Agreement(s) and each other agreement, document, instrument or certificate contemplated by this Agreement to be executed by the Parties in connection with the Transactions.
“Anti-Corruption Laws” means, collectively, (a) the FCPA; (b) the UK Bribery Act 2010; and (c) any applicable Law enacted in any jurisdiction in connection with or arising under the OECD Convention Combating Bribery of Foreign Public Officials in International Business transactions.
“Antitrust Laws” means Laws that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition through merger or acquisition.
“Applicable Exchange Consideration Per Share” means (a) with respect to each Company Ordinary Share outstanding immediately prior to the Company Share Exchange, the Company Ordinary Share Value, (b) with respect to each Company Preferred Share outstanding immediately prior to the Company Share Exchange, the Company Preferred Share Value and (c) with respect to each Company Non-Voting Share outstanding immediately prior to the Company Share Exchange, the Company Non-Voting Share Value.
“Business” means the researching, developing, testing (whether preclinical or clinical), manufacturing, storing or distributing products, substances or therapies for cardiometabolic and neurometabolic diseases, including cardiovascular disease, hyperlipidemia, diabetes mellitus and Alzheimer’s disease, or any activities, services or products incidental or attendant thereto, in each case as conducted or contemplated to be conducted by the Company as of the date of this Agreement, including the Company’s licenses with third parties.
“Business Combination Proposal” has the meaning set forth in Section 5.8.
“Business Day” means a day, other than a Saturday or Sunday, on which commercial banks in New York, New York, the Cayman Islands, and Amsterdam, the Netherlands are open for the general transaction of business.
“Cayman Companies Act” has the meaning set forth in the recitals to this Agreement.
“Change of Control Payment” means (a) any success, change of control, retention, transaction bonus or other similar payment or amount that may be payable to any Person as a result of or in connection with this Agreement or the Transactions or any other Change of Control Transaction (including any such payments or
A-4
Table of Contents
similar amounts that may become due and payable in connection with such Change of Control Transaction) or (b) any payments made or required to be made pursuant to or in connection with or upon termination of, and any fees, expenses or other payments owing or that will become owing in respect of, any Company Related Party Transaction (in the case of each of clause (a) and (b), regardless of whether paid or payable prior to, at or after the Closing or in connection with or otherwise related to this Agreement or any Ancillary Document).
“Change of Control Transaction” means any transaction or series of related transactions (a) under which any Person(s), directly or indirectly, acquires or otherwise purchases (i) another Person or any of its Affiliates or (ii) all or a material portion of assets, businesses or equity securities of another Person or (b) that results, directly or indirectly, in the shareholders of a Person as of immediately prior to such transaction holding, in the aggregate, less than fifty percent (50%) of the voting shares of such Person (or any successor or parent company of such Person) immediately after the consummation thereof (in the case of each of clause (a) and (b), whether by merger, consolidation, tender offer, recapitalization, purchase or issuance of equity securities, tender offer or otherwise).
“Closing” has the meaning set forth in Section 2.2.
“Closing Commencement Date” has the meaning set forth in Section 2.2.
“Closing Filing” has the meaning set forth in Section 5.4(b).
“Closing Financial Statements” has the meaning set forth in Section 5.17(a).
“Closing Press Release” has the meaning set forth in Section 5.4(b).
“COBRA” means Part 6 of Subtitle B of Title I of ERISA, Section 4980B of the Code and any similar state Law.
“Code” means the U.S. Internal Revenue Code of 1986.
“Company” has the meaning set forth in the preamble to this Agreement.
“Company Acquisition Proposal” means any offer, inquiry, proposal or indication of interest (whether written or oral, and whether binding or non-binding) relating to any transaction or series of related transactions under which any Person(s), directly or indirectly, acquires or otherwise purchases (a) the Company or any of its controlled Affiliates, (b) assets or businesses of the Company or any of its controlled Affiliates that constitute 20% or more of the consolidated revenues, net income or assets of the Company and its controlled Affiliates, taken as a whole or (c) 20% or more of any class of voting Equity Securities of the Company or any of its Controlled Affiliates (in the case of each of clause (a) through (c), whether by merger, consolidation, recapitalization, purchase or issuance of equity securities, tender offer or otherwise). Notwithstanding the foregoing or anything to the contrary herein, none of this Agreement, the Ancillary Documents nor the Transactions shall constitute a Company Acquisition Proposal.
“Company Board” has the meaning set forth in the recitals to this Agreement.
“Company Capitalization Representations” means the representations and warranties set forth in Section 3.2(a).
“Company D&O Persons” has the meaning set forth in Section 5.15(a).
“Company D&O Tail Policy” has the meaning set forth in Section 5.15(c).
“Company Designee” has the meaning set forth in Section 5.16(b).
A-5
Table of Contents
“Company Disclosure Schedules” means the disclosure schedules to this Agreement delivered to FLAC by the Company on the date of this Agreement.
“Company Equity Award” means, as of any determination time, each outstanding Company Option, and each other award to any current or former director, manager, officer, employee, individual independent contractor or other service provider of any Group Company of rights of any kind to receive any Equity Security of any Group Company under any Company Equity Incentive Plan.
“Company Equity Incentive Plans” means the NewAmsterdam Pharma Long-Term Incentive Plan dated July 2021, and each other plan that provides for the award to any current or former director, manager, officer, employee, individual independent contractor or other service provider of any Group Company of rights of any kind to receive Equity Securities of any Group Company or benefits measured in whole or in part by reference to Equity Securities of any Group Company.
“Company Expenses” means, as of any determination time, the aggregate amount of fees, expense, commissions or other amounts incurred by or on behalf of any Group Company that are due and payable and not otherwise expressly allocated to FLAC pursuant to the terms of this Agreement or any Ancillary Document in connection with the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of its covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions, including (a) the fees and expenses of outside legal counsel, accountants, advisors, brokers, investment bankers, consultants, or other agents or service providers of any Group Company, (b) 50% of all the filing fees incurred in connection with making any filings under Section 5.2, (c) 50% of all filing fees and associated expenses incurred in connection with filing the Registration Statement, the Proxy Statement or the Registration Statement / Proxy Statement under Section 5.7, obtaining approval of Nasdaq under Section 5.12 and obtaining the Required FLAC Shareholder Approval (excluding, for the avoidance of doubt, any fees and expenses set forth in (a) above, which shall be paid in accordance with (a) above), (d) any other fees, expenses, commissions or other amounts that are expressly allocated to any Group Company pursuant to this Agreement or any Ancillary Document and (e) Change of Control Payments paid or payable by the Company. Notwithstanding the foregoing or anything to the contrary herein, Company Expenses shall not include any FLAC Expenses.
“Company Financial Statements” has the meaning set forth in Section 3.4(a).
“Company Fundamental Representations” means the representations and warranties set forth in Section 3.1 (Organization and Qualification), Section 3.2(e) and (h) (Capitalization), Section 3.3 (Authority), Section 3.8(a) (No Company Material Adverse Effect) and Section 3.18 (Brokers).
“Company Issuance Rights” means the rights to issuance of Company Ordinary Shares pursuant to the Contracts set forth on Section 1.1(a) of the Company Disclosure Schedules (including as amended and converted into rights to issuance of Holdco Shares pursuant to the Contracts set forth thereon).
“Company IT Systems” means all computer systems, computer software and hardware, communication systems, servers, network equipment and related documentation, in each case, owned, licensed or leased by a Group Company.
“Company Material Adverse Effect” means any Event that, individually or in the aggregate with any other Event, has had or would reasonably be expected to have a material adverse effect on (a) the business, results of operations or financial condition of the Group Companies, taken as a whole, or (b) the ability of the Company to consummate the Transactions; provided, however, that in the case of clause (a), none of the following shall be taken into account in determining whether a Company Material Adverse Effect has occurred or is reasonably likely to occur: (i) changes in general business or economic conditions in or affecting the United States or the Netherlands, or changes therein, or the global economy generally, (ii) any national or international political or social conditions in the United States, the Netherlands, or any other country, including the engagement by the
A-6
Table of Contents
United States, the Netherlands, or any other country in hostilities, whether or not pursuant to the declaration of a national emergency or war, or the occurrence in any place of any military or terrorist attack, sabotage or cyberterrorism, or any escalation of the foregoing, (iii) changes in conditions of the financial, banking, capital or securities markets generally in the United States, the Netherlands, or any other country or region in the world, or changes therein, including changes in interest rates in the United States, the Netherlands, or any other country and changes in exchange rates for the currencies of any countries, (iv) changes or proposed changes in any applicable Laws or IFRS or the interpretation thereof, (v) any Event that is generally applicable to the industries or markets in which any Group Company operates, (vi) the execution or public announcement of this Agreement or the pendency or consummation of the Transactions, including the impact thereof on the relationships, contractual or otherwise, of any Group Company with employees, customers, investors, contractors, lenders, suppliers, vendors, partners, licensors, licensees, payors or other third parties related thereto (provided that the exception in this clause (vi) shall not apply to the representations and warranties set forth in Section 3.5 to the extent that their purpose is to address the consequences resulting from the public announcement or pendency or consummation of the Transactions or the condition set forth in Section 6.2(a) to the extent it relates to such representations and warranties), (vii) any failure by any Group Company to meet, or changes to, any internal or published budgets, projections or forecasts in and of itself (although the underlying facts and circumstances resulting in such failure may be taken into account to the extent not otherwise excluded from this definition pursuant to clauses (i) through (vi) or (viii)), (viii) the taking of any action required by or expressly permitted by this Agreement or any Ancillary Document, or the failure to take any action that is prohibited by this Agreement or any Ancillary Document, (ix) any action taken by, or at the express written request of an authorized signatory of FLAC, Sponsor or any of their respective Affiliates, or (x) any hurricane, tornado, flood, earthquake, tsunami, natural disaster, mudslides, wild fires, epidemics, pandemics or quarantines, acts of God or other natural disasters or comparable events in the United States, the Netherlands or any other country or region in the world, or any escalation of the foregoing, including, for the avoidance of doubt, COVID-19 and any Law, directive, pronouncement, guideline or recommendation issued by any Governmental Entity, the Centers for Disease Control and Prevention, the World Health Organization or any industry group providing for business closures, changes to business operations, “sheltering-in-place” or other restrictions that relate to, or arise out of, an epidemic, pandemic or disease outbreak (including the COVID-19 pandemic); provided however, that any Event resulting from a matter described in any of the foregoing clauses (i) through (v) or (x) may be taken into account in determining whether a Company Material Adverse Effect has occurred or is reasonably likely to occur to the extent such Event has a disproportionate adverse effect on the Group Companies, taken as a whole, relative to other participants operating in the industries or markets in which the Group Companies operate.
“Company Non-Party Affiliates” means, collectively, each Company Related Party and each former, current or future Affiliates, Representatives, successors or permitted assigns of any Company Related Party (other than, for the avoidance of doubt, the Company).
“Company Non-Voting Share Value” means as of immediately prior to the Company Share Exchange, with respect to a Company Non-Voting Share, an amount of Holdco Shares equal to (a) the Aggregate Share Consideration multiplied by (b) a fraction, (i) the numerator of which is one, and (ii) the denominator of which is the number of Fully Diluted Company Shares.
“Company Non-Voting Shares” means the non-voting shares in the share capital of the Company each having a nominal value of EUR 0.01.
“Company Option” means, as of any determination time, each option to purchase Company Shares or depository receipts for Company Shares that is outstanding and unexercised and granted under any Company Equity Incentive Plan.
“Company Option Pool” means an aggregate of 2,390,163 Company Shares.
A-7
Table of Contents
“Company Option Subscription Agreement” means the agreement between the Company and a Company Optionholder pursuant to which the Company allotted to the Company Optionholder one or more Company Options under any Company Equity Incentive Plan.
“Company Optionholder” means each person holding a Company Option as of immediately prior to the Company Share Exchange.
“Company Ordinary Share Value” means, as of immediately prior to the Company Share Exchange, with respect to a Company Ordinary Share, an amount of Holdco Shares equal to (a) the Aggregate Share Consideration multiplied by (b) a fraction, (i) the numerator of which is one, and (ii) the denominator of which is the number of Fully Diluted Company Shares.
“Company Ordinary Shares” means the ordinary shares in the share capital of the Company, each having a nominal value of EUR 0.01.
“Company Owned Intellectual Property” means all Intellectual Property Rights that are owned and either used, held for use or practiced by any Group Company.
“Company Preferred Share Value” means, as of immediately before the Company Share Exchange, with respect to a Company Preferred Share, an amount of Holdco Shares equal to (a) the Aggregate Share Consideration multiplied by (b) a fraction, (i) the numerator of which is one, and (ii) the denominator of which is the number of Fully Diluted Company Shares.
“Company Preferred Shares” means the convertible preferred series A shares in the share capital of the Company, each having a nominal value of EUR 0.01.
“Company Product” means obicetrapib.
“Company Registered Intellectual Property” means all Registered Intellectual Property owned or purported to be owned by, or filed by or in the name of any Group Company, including all Registered Intellectual Property co-owned by any Group Company.
“Company Related Party” has the meaning set forth in Section 3.20.
“Company Related Party Transactions” has the meaning set forth in Section 3.20.
“Company Share Exchange” has the meaning set forth in the recitals to this Agreement.
“Company Shareholders” means, collectively, the holders of Company Shares as of any determination time prior to the Effective Date.
“Company Shareholders Agreement” means the Fully Amended and Restated Shareholders’ Agreement, dated as of January 11, 2021, by and among the Company and the Company Shareholders party thereto, as amended by the Amendment thereto dated March 15, 2021, the Amendment dated July 6, 2021 and the Amendment dated on or about the date hereof.
“Company Shares” means, collectively, the Company Preferred Shares, the Company Ordinary Shares and the Company Non-Voting Shares.
“Company Support Agreement” has the meaning set forth in the recitals to this Agreement.
“Confidentiality Agreement” means that certain Mutual Confidential Disclosure Agreement entered into between the Company and FLAC, dated January 24, 2022.
A-8
Table of Contents
“Consent” means any notice, authorization, qualification, registration, filing, notification, waiver, order, consent or approval to be obtained from, filed with or delivered to, a Governmental Entity or other Person.
“Continental” means Continental Stock Transfer & Trust Company.
“Contingent Worker” means any individual independent contractor, consultant, contractor, sub-contractor, temporary employee, leased employee or other agent used by any Group Company and classified by such Group Company as other than an employee, or compensated other than through wages paid by such Group Company through the Group Company’s payroll function.
“Contract” or “Contracts” means any written or oral agreement, contract, license, lease, obligation, undertaking or other commitment or arrangement that is legally binding upon a Person or any of his, her or its properties or assets.
“Copyrights” has the meaning set forth in the definition of Intellectual Property Rights.
“COVID-19” means the novel coronavirus known as SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemic or disease outbreaks.
“COVID-19 Action” means any inaction or action by the Company, including the establishment of any policy, procedure or protocol, in response to COVID-19 or any COVID-19 Measures (a) that is consistent with the past practice of the Company in response to COVID-19 prior to the date of this Agreement (but only to the extent in compliance with applicable Law), (b) that is consistent with any applicable Law, Order, Proceeding, directive, guideline or recommendation by any Governmental Entity in connection with or in response to COVID-19, or (c) that would, given the totality of the circumstances under which the Company acted or did not act, be unreasonable for FLAC to withhold, condition or delay consent with respect to such action or inaction (whether or not FLAC has a consent right with respect thereto).
“COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, “furlough,” social distancing, shut down, closure, employee time off, employee leave, sequester, business or workplace reopening, or other conditions, restrictions or requirements pursuant to any Law, Order, Proceeding, directive, guideline or recommendation by any Governmental Entity in connection with or in response to COVID-19.
“Current Companies” means the Group Companies, excluding Holdco.
“Designees” has the meaning set forth in Section 5.16(b).
“DGCL” has the meaning set forth in the recitals to this Agreement.
“Domestication” has the meaning set forth in the recitals to this Agreement.
“Dutch Deed of Issue Company Share Exchange” has the meaning set forth in Section 2.1(b)(ii).
“Earnout Period” means the period starting on the Closing Commencement Date and ending on the date that is five (5) years after the Final Closing Date.
“Earnout Pro Rata Share” means, with respect to each recipient of Earnout Shares pursuant to Section 2.7, a percentage equal to the quotient of: (a) the sum of (i) the aggregate number of Holdco Shares that are held by such recipient immediately prior to the Effective Date (including, for the avoidance of doubt, any such Holdco Share issued as a result of the exercise of any Company Issuance Right in connection with and after the Company Share Exchange) plus (ii) the aggregate number of Holdco Shares subject to Rollover Company Options that are
A-9
Table of Contents
held by such recipient immediately prior to the Effective Date; divided by (b) the sum of (i) the aggregate number of Holdco Shares that are held by all Holdco Shareholders immediately prior to the Effective Date (including, for the avoidance of doubt, all Holdco Shares issued as a result of the exercise of any Company Issuance Right in connection with and after the Company Share Exchange) plus (ii) the aggregate number of Holdco Shares subject to all Rollover Company Options held by Eligible Optionholders that are outstanding and unexercised immediately prior to the Effective Date.
“Earnout RSUs” has the meaning set forth in Section 2.7(b).
“Earnout Shares” means an aggregate of 1,886,137 Holdco Shares, which shall be equitably adjusted on account of any subdivision, stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into Holdco Shares, other than as a result of the exercise of Company Issuance Rights), combination, reorganization, reclassification exchange of shares or similar equity restructuring transaction or any changes in the Holdco Shares as a result of a merger, consolidation, reorganization, recapitalization, business combination or similar transaction involving Holdco (excluding, for the avoidance of doubt, the Transactions). For the avoidance of doubt, except where otherwise expressly indicated, references herein to Earnout Shares shall include Earnout RSUs, as applicable.
“Effective Date” has the meaning set forth in Section 2.1(d)(i).
“Eligible Optionholder” means a holder of Rollover Company Options who is a director, manager, officer, employee, or Contingent Worker of a Group Company, in each case as of the date hereof.
“Employee Benefit Plan” means each “employee benefit plan” (as such term is defined in Section 3(3) of ERISA, whether or not subject to ERISA), including each stock option plan, stock purchase plan, bonus or incentive plan, severance pay plan, program or arrangement, deferred compensation arrangement or agreement, employment agreement, compensation plan, program, agreement, or arrangement, change in control plan, program or arrangement, supplemental income arrangement, or vacation plan, in each case that any Group Company maintains, sponsors or contributes to, or has any obligation to contribute to, or with respect to which any Group Company has or may reasonably be expected to have any present or future Liability (including as an ERISA Affiliate).
“Enforceability Exceptions” has the meaning set forth in Section 3.3.
“Environmental Laws” means all Laws and Orders concerning pollution, natural resources, protection of the environment, Hazardous Substances, or human health or safety.
“EPCRS” means the IRS Employee Plans Compliance Resolution System.
“Equity Securities” means any share, share capital, capital stock, partnership, membership, joint venture or similar interest in any Person (including any stock appreciation, phantom stock, profit participation or similar rights), and any option, warrant, right or security (including debt securities) convertible, exchangeable or exercisable therefor.
“ERISA” means the Employee Retirement Income Security Act of 1974.
“ERISA Affiliate” means any entity, trade or business that is, or at any applicable time was, a member of a group described in Section 414(b), (c), (m) or (o) of the Code or Section 4001(b)(1) of ERISA that includes any Group Company.
“ESPP” has the meaning set forth in Section 5.22.
“European Union Member State” means any country that is a member state of the European Union.
A-10
Table of Contents
“Event” means any change, event, effect, occurrence or state of facts.
“Exchange Act” means the U.S. Securities Exchange Act of 1934.
“Exchange Agent” has the meaning set forth in Section 2.8(a).
“Exchange Fund” has the meaning set forth in Section 2.8(b).
“FCPA” means the U.S. Foreign Corrupt Practices Act of 1977.
“FDA” means the U.S. Food and Drug Administration, or any successor agency thereto.
“Federal Securities Laws” means the Exchange Act, the Securities Act and the other U.S. federal securities laws.
“Final Closing Date” means the date on which the Domestication becomes effective.
“FLAC” has the meaning set forth in the preamble to this Agreement.
“FLAC Acquisition Proposal” means any offer, inquiry, proposal or indication of interest (whether written or oral, and whether binding or non-binding) relating to (a) any transaction or series of related transactions under which FLAC or any of its controlled Affiliates, directly or indirectly, (i) acquires or otherwise purchases any other Person(s), (ii) engages in a FLAC Business Combination with any other Person(s) or (iii) acquires or otherwise purchases all or a material portion of the assets or businesses of any other Persons(s) (in the case of each of clause (i), (ii) and (iii), whether by merger, consolidation, recapitalization, purchase or issuance of equity securities, tender offer or otherwise) or (b) any equity, debt or similar investment in FLAC or any of its controlled Affiliates. Notwithstanding the foregoing or anything to the contrary herein, none of this Agreement, the Ancillary Documents or the Transactions shall constitute a FLAC Acquisition Proposal.
“FLAC Board” has the meaning set forth in the recitals to this Agreement.
“FLAC Board Recommendation” has the meaning set forth in Section 5.8.
“FLAC Business Combination” means a “business combination” as such term is defined in the Articles of Association of FLAC as in effect on the date of this Agreement.
“FLAC Capitalization Representations” means the representations and warranties set forth in Section 4.6(a) and Section 4.6(b).
“FLAC Class A Shares” means FLAC’s Class A ordinary shares, par value $0.0001.
“FLAC Class B Shares” means FLAC’s Class B ordinary shares, par value $0.0001.
“FLAC D&O Persons” has the meaning set forth in Section 5.14(a).
“FLAC D&O Tail Policy” has the meaning set forth in Section 5.14(a).
“FLAC Designees” has the meaning set forth in Section 5.16(b).
“FLAC Disclosure Schedules” means the disclosure schedules to this Agreement delivered to the Company by FLAC on the date of this Agreement.
A-11
Table of Contents
“FLAC Expenses” means, as of any determination time, the aggregate amount of fees, expense, commissions or other amounts incurred by or on behalf of, FLAC that are due and payable and not otherwise expressly allocated to the Company pursuant to the terms of this Agreement or any Ancillary Document in connection with the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of its covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions, including (a) the fees and expenses of outside legal counsel, accountants, advisors, brokers, investment bankers, consultants, or other agents or service providers of FLAC, (b) 50% of all the filing fees incurred in connection with making any filings under Section 5.2, (c) 50% of all filing fees and associated expenses incurred in connection with preparing and filing the Registration Statement, the Proxy Statement or the Registration Statement / Proxy Statement under Section 5.7, obtaining approval of Nasdaq under Section 5.12 and obtaining the Required FLAC Shareholder Approval (but excluding for the avoidance of doubt, any fees and expenses set forth in (a) above, which shall be paid in accordance with (a) above), (d) any deferred underwriting commissions and other fees and expenses relating to FLAC’s IPO and (e) any other fees, expenses, commissions or other amounts that are expressly allocated to FLAC pursuant to this Agreement or any Ancillary Document. Notwithstanding the foregoing or anything to the contrary herein, FLAC Expenses shall not include (i) any Company Expenses or (ii) the cost of the FLAC D&O Tail Policy.
“FLAC Financial Advisor” means Lincoln International LLC, financial advisor to the FLAC Special Committee.
“FLAC Financial Statements” has the meaning set forth in Section 4.13(d).
“FLAC Fundamental Representations” means the representations and warranties set forth in Section 4.1 (Organization and Qualification), Section 4.2 (Authority) and Section 4.4 (Brokers).
“FLAC Liabilities” means, as of any determination time, without duplication of any FLAC Expenses, the aggregate amount of Liabilities that would be accrued on a balance sheet, whether such Liabilities are then due and payable by FLAC as of such time.
“FLAC Material Adverse Effect” means any Event that, individually or in the aggregate with any other Event, has had or would reasonably be expected to have a material adverse effect on (a) the business, results of operations or financial condition of FLAC, taken as a whole, or (b) the ability of FLAC or Merger Sub to consummate the Transactions; provided however, that, in the case of clause (a), none of the following shall be taken into account in determining whether a FLAC Material Adverse Effect has occurred or is reasonably likely to occur: any adverse change, event, effect or occurrence arising after the date of this Agreement from or related to (i) changes in general business or economic conditions in or affecting the United States, or changes therein, or the global economy generally, (ii) any national or international political or social conditions in the United States or any other country, including the engagement by the United States or any other country in hostilities, whether or not pursuant to the declaration of a national emergency or war, or the occurrence in any place of any military or terrorist attack, sabotage or cyberterrorism, (iii) changes in conditions of the financial, banking, capital or securities markets generally in the United States or any other country or region in the world, or changes therein, including changes in interest rates in the United States or any other country and changes in exchange rates for the currencies of any countries, (iv) changes or proposed changes in any applicable Laws or IFRS or the interpretation thereof, (v) any Event that is generally applicable to the industries or markets in which FLAC operates, (vi) the execution or public announcement of this Agreement or the pendency or consummation of the Transactions, including the impact thereof on the relationships, contractual or otherwise, of FLAC with employees, customers, investors, contractors, lenders, suppliers, vendors, partners, licensors, licensees, payors or other third parties related thereto (provided that the exception in this clause (vi) shall not apply to the representations and warranties set forth in Section 4.3 to the extent that their purpose is to address the consequences resulting from the public announcement or pendency or consummation of the Transactions or the condition set forth in Section 6.3(b) to the extent it relates to such representations and warranties), (vii) any failure by FLAC to meet, or changes to, any internal or published budgets, projections or forecasts in and of itself
A-12
Table of Contents
(although the underlying facts and circumstances resulting in such failure may be taken into account to the extent not otherwise excluded from this definition pursuant to clauses (i) through (vi) or (viii)), (viii) the taking of any action required by or expressly permitted by this Agreement or any Ancillary Document or the failure to take any action that is prohibited by this Agreement or any Ancillary Document, (ix) any action taken by, or at express written request of an authorized signatory of the Company or any of its Affiliates, or (x) any hurricane, tornado, flood, earthquake, tsunami, natural disaster, mudslides, wild fires, epidemics, pandemics or quarantines, acts of God or other natural disasters or comparable events in the United States or any other country or region in the world, or any escalation of the foregoing, including, for the avoidance of doubt, COVID-19 and any Law, directive, pronouncement, guideline or recommendation issued by any Governmental Entity, the Centers for Disease Control and Prevention, the World Health Organization or any industry group providing for business closures, changes to business operations, “sheltering-in-place” or other restrictions that relate to, or arise out of, an epidemic, pandemic or disease outbreak (including the COVID-19 pandemic); provided, however, that any Event resulting from a matter described in any of the foregoing clauses (i) through (v) or (x) may be taken into account in determining whether a FLAC Material Adverse Effect has occurred or is reasonably likely to occur to the extent such Event has a disproportionate adverse effect on FLAC relative to other “SPACs” operating in the industries in which FLAC operates.
“FLAC Non-Party Affiliates” means, collectively, each FLAC Related Party and each former, current or future Affiliates, Representatives, successors or permitted assigns of any FLAC Related Party (other than, for the avoidance of doubt, FLAC).
“FLAC Related Party” has the meaning set forth in Section 4.9.
“FLAC Related Party Transactions” has the meaning set forth in Section 4.9.
“FLAC SEC Reports” has the meaning set forth in Section 4.7.
“FLAC Shareholder Approval” means, collectively, the Required FLAC Shareholder Approval and the Other FLAC Shareholder Approval.
“FLAC Shareholder Redemption” means the right of the holders of FLAC Class A Shares to redeem all or a portion of their FLAC Class A Shares (in connection with the Transactions or otherwise) as set forth in Governing Documents of FLAC.
“FLAC Shareholders” means holders of FLAC Shares.
“FLAC Shareholders Meeting” has the meaning set forth in Section 5.8.
“FLAC Shares” means (a) prior to the occurrence of the Domestication, collectively, the FLAC Class A Shares and the FLAC Class B Shares and (b) from and after the occurrence of the Domestication, shares of common stock, par value $0.0001 per share, of the Surviving Company. Any reference to the FLAC Shares in this Agreement or any Ancillary Document shall be deemed to refer to clause (a) or clause (b) of this definition, as the context so requires.
“FLAC Special Committee” has the meaning set forth in the recitals to this Agreement.
“FLAC Units” means the units, each consisting of one FLAC Class A Share and one-third of a FLAC Warrant to acquire one FLAC Class A Share.
“FLAC Warrants” means each warrant to purchase one FLAC Class A Share at an exercise price of $11.50 per share, subject to adjustment in accordance with the Warrant Agreement (including, for the avoidance of doubt, each such warrant held by the Sponsor or any other Class B Shareholder).
A-13
Table of Contents
“Foreign Benefit Plan” means each Employee Benefit Plan maintained by any of the Group Companies for the benefit of its current or former employees, officers, directors or other individual service providers employed and located outside of the United States of America (other than any plans, funds or similar programs that are maintained by a Governmental Entity) and which plan is not subject to ERISA or the Code.
“Fully Diluted Company Shares” means, without duplication, as of immediately prior to the Company Share Exchange, the sum of (a) the aggregate number of Company Ordinary Shares issued and outstanding determined on an as-converted to Company Ordinary Share basis (including, for the avoidance of doubt, the number of Company Ordinary Shares issuable upon, or otherwise resulting from, conversion of the Company Preferred Shares and Company Non-Voting Shares, based on the then applicable conversion ratio), and (b) the aggregate number of Company Ordinary Shares (or depository receipts for Company Ordinary Shares) issuable upon the exercise in full or exchange of issued and outstanding options, warrants, awards, convertible securities and any other right to subscribe for Company Ordinary Shares (assuming (i) the conversion into Company Ordinary Shares of any Company Non-Voting Shares issuable upon such exercise, based on the then applicable conversion ratio and (ii) solely for purposes of this definition, the issuance of Company Shares as a result of the exercise of all Company Issuance Rights in connection with the Company Share Exchange), in each case to their maximum extent (including, for the avoidance of doubt, the Allocated Company Option Pool, but excluding the Unallocated Company Option Pool).
“GAAP” means United States generally accepted accounting principles.
“Governing Documents” means the legal document(s) by which any Person (other than an individual) establishes its legal existence or which govern its internal affairs. For example, the “Governing Documents” of a U.S. corporation are its certificate or articles of incorporation and by-laws, the “Governing Documents” of a U.S. limited partnership are its limited partnership agreement and certificate of limited partnership, the “Governing Documents” of a U.S. limited liability company are its operating or limited liability company agreement and certificate of formation, the “Governing Documents” of a Dutch company are its articles of association (statuten), and the “Governing Documents” of a Cayman Islands exempted company are its memorandum and articles of association.
“Governmental Entity” means any United States or non-United States (a) federal, state, local, municipal or other government, (b) governmental or quasi-governmental entity of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal) or (c) body exercising or entitled to exercise any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature, including any arbitral tribunal (public or private).
“Group Companies” means, collectively, the Company, Holdco and their respective Subsidiaries.
“Hazardous Substance” means any hazardous, toxic, explosive or radioactive material, substance, waste or other pollutant that is regulated by, or may give rise to Liability pursuant to, any Environmental Law, including any petroleum products or byproducts, asbestos, lead, polychlorinated biphenyls, per- and poly-fluoroakyl substances, or radon.
“Healthcare Laws” means all Laws relating to healthcare regulatory matters applicable to the Group Companies, including all Laws relating to any federal health care program (as such term is defined in 42 U.S.C. § 1320a-7b(f)), including the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the Stark Anti-Self-Referral Law (42 U.S.C. § 1395nn), the civil False Claims Act (31 U.S.C. § 3729 et seq.), the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)), Sections 1320a-7, 1320a-7a, and 1320a-7b of Title 42 of the United States Code and any comparable self-referral or fraud and abuse laws promulgated by any Governmental Entity, the 21st Century Cures Act (Pub. L. 114-255), the health care fraud criminal provisions under HIPAA, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (42 U.S.C. § 17921 et seq.), and any state or federal Law the purpose of which is to protect the privacy of
A-14
Table of Contents
individually-identifiable patient information, Medicare (Title XVIII of the Social Security Act) and Medicaid (Title XIX of the Social Security Act), the Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, TRICARE (10 U.S.C. Section 1071 et seq.), the Sunshine/Open Payments Law (42 U.S.C. § 1320a-7h) and similar state or foreign Laws related to the reporting of manufacturer payments or transfers of value to health care professionals, in each case including their foreign equivalents.
“HIPAA” means the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.).
“Holdco” has the meaning set forth in the preamble to this Agreement.
“Holdco Board” has the meaning set forth in the recitals to this Agreement.
“Holdco Board Appointments” has the meaning set forth in Section 5.16(a).
“Holdco Equity Incentive Plan” has the meaning set forth in Section 5.19.
“Holdco Per Share Value” means $10.00.
“Holdco Reorganization” has the meaning set forth in the recitals to this Agreement.
“Holdco Shareholders” means, collectively, the holders of Holdco Shares as of any determination time prior to the Effective Date.
“Holdco Shares” means the ordinary shares, nominal value EUR 0.12, in the share capital of Holdco.
“Holdco Warrant” means each warrant to purchase one Holdco Share at a price of $11.50, subject to adjustment.
“IFRS” means International Financial Reporting Standards as promulgated by the International Standards Accounting Board.
“Indebtedness” means, as of any time, without duplication, with respect to any Person, the outstanding principal amount of, accrued and unpaid interest on, fees and expenses arising under or in respect of (a) indebtedness for borrowed money, (b) other obligations evidenced by any note, bond, debenture or other debt security, (c) obligations for the deferred purchase price of property or assets, including “earn-outs” and “seller notes” (but excluding any trade payables arising in the ordinary course of business), (d) reimbursement and other obligations with respect to letters of credit, bank guarantees, bankers’ acceptances or other similar instruments, in each case, solely to the extent drawn, (e) leases required to be capitalized under GAAP or IFRS, as applicable, (f) derivative, hedging, swap, foreign exchange or similar arrangements, including swaps, caps, collars, hedges or similar arrangements, and (g) any of the obligations of any other Person of the type referred to in clauses (a) through (f) above directly or indirectly guaranteed by such Person or secured by any assets of such Person, whether or not such Indebtedness has been assumed by such Person.
“Insurance Policies” has the meaning set forth in Section 3.16.
“Intellectual Property Rights” means all intellectual property rights and related priority rights protected, created or arising under the Laws of the United States or any other jurisdiction or under any international convention, including all (a) patents and patent applications, industrial designs, industrial design registration and design patent rights, including any continuations, divisionals, continuations-in-part and provisional applications and statutory invention registrations, and any patents issuing on any of the foregoing and any reissues, reexaminations, substitutes, supplementary protection certificates, extensions of any of the foregoing
A-15
Table of Contents
(collectively, “Patents”); (b) trademarks, service marks, trade names, service names, brand names, trade dress rights, logos, Internet domain names, corporate names and other source or business identifiers, together with the goodwill associated with any of the foregoing, whether or not registered, and all applications, registrations, extensions and renewals of any of the foregoing (collectively, “Marks”); (c) copyrights and works of authorship, database and design rights, mask work rights and moral rights, whether or not registered or published, and all registrations, applications, renewals, extensions and reversions of any of any of the foregoing (collectively, “Copyrights”); (d) trade secrets, know-how and confidential and proprietary information, including invention disclosures, inventions and formulae, whether patentable or not; (e) rights in or to Software or other technology; and (f) any other intellectual or proprietary rights protectable, arising under or associated with any of the foregoing, including those protected by any Law anywhere in the world.
“Intended Domestication Tax Treatment” has the meaning set forth in Section 5.5(a)(ii).
“Intended Holdco Exchange Tax Treatment” has the meaning set forth in Section 5.5(a)(i).
“Intended Merger Tax Treatment” has the meaning set forth in Section 5.5(a)(iii).
“Intended Tax Treatment” has the meaning set forth in Section 5.5(a)(iii).
“Investment Company Act” means the Investment Company Act of 1940.
“Investor Rights Agreement” has the meaning set forth in the recitals to this Agreement.
“IPO” has the meaning set forth in Section 4.7.
“IRA Shareholders” has the meaning set forth in the recitals to this Agreement.
“IRS” means the U.S. Internal Revenue Service.
“JOBS Act” means the Jumpstart Our Business Startups Act of 2012.
“Latest Balance Sheet Date” has the meaning set forth in Section 3.4(a).
“Law” means any federal, state, local, foreign, national or supranational statute, law (including common law), act, statute, ordinance, treaty, rule, code, regulation or other binding directive or guidance issued, promulgated or enforced by a Governmental Entity having jurisdiction over a given matter.
“Leased Real Property” has the meaning set forth in Section 3.19(b).
“Liability” or “liability” means any and all debts, liabilities and obligations, whether accrued or fixed, absolute or contingent, known or unknown, matured or unmatured or determined or determinable, including those arising under any Law, Proceeding or Order and those arising under any Contract.
“Lien” means any mortgage, pledge, usufruct (vruchtgebruik), security interest, encumbrance, lien, license or sub-license, depository receipt issued for shares with meeting rights (certificaat van een aandeel waaraan vergaderrecht is verbonden), attachment (beslag), charge, or other similar encumbrance or interest (including, in the case of any Equity Securities, any voting, transfer or similar restrictions).
“Malicious Code” means disabling codes or instructions, spyware, Trojan horses, worms, viruses or other Software routines that facilitate or cause unauthorized access to, or disruption, impairment, disablement, or destruction of, Software, information technology systems, data or other materials.
“Marks” has the meaning set forth in the definition of Intellectual Property Rights.
A-16
Table of Contents
“Material Contracts” has the meaning set forth in Section 3.7(a).
“Material Permits” has the meaning set forth in Section 3.6.
“Merger” has the meaning set forth in the recitals to this Agreement.
“Merger Claim” has the meaning set forth in Section 2.1(d)(iv)(A).
“Merger Consideration” has the meaning set forth in Section 2.1(d)(iv)(A).
“Merger Documents” has the meaning set forth in Section 2.1(d)(ii).
“Merger Proposal” has the meaning set forth in Section 5.8.
“Merger Sub” has the meaning set forth in the preamble to this Agreement.
“Multiemployer Plan” has the meaning set forth in Section (3)37 or Section 4001(a)(3) of ERISA.
“Nasdaq” means The Nasdaq Stock Market LLC.
“Non-Party Affiliate” means any Company Non-Party Affiliate or FLAC Non-Party Affiliate.
“Off-the-Shelf Software” means any Software that is made generally and widely available to the public on a commercial basis and is licensed to any of the Group Companies on a non-exclusive basis under standard terms and conditions for a one-time license fee of less than $100,000 per license or an ongoing licensee fee of less than $50,000 per year.
“Officers” has the meaning set forth in Section 5.16(a).
“Order” means any outstanding writ, order, judgment, injunction, decision, determination, award, ruling, subpoena, verdict or decree entered, issued or rendered by any Governmental Entity.
“Other FLAC Shareholder Approval” means the approval of each Other Transaction Proposal by the affirmative vote of the holders of the requisite number of FLAC Shares entitled to vote thereon, whether in person or by proxy at the FLAC Shareholders Meeting (or any adjournment or postponement thereof), in accordance with the Governing Documents of FLAC and applicable Law.
“Other Transaction Proposal” means each Transaction Proposal, other than the Required Transaction Proposals.
“Parties” has the meaning set forth in the preamble to this Agreement.
“Patents” has the meaning set forth in the definition of Intellectual Property Rights.
“PCAOB” means the Public Company Accounting Oversight Board.
“Permits” means any approvals, authorizations, clearances, licenses, registrations, permits or certificates of a Governmental Entity.
“Permitted Liens” means (a) mechanic’s, materialmen’s, carriers’, repairers’ and other similar statutory Liens arising or incurred in the ordinary course of business for amounts that are not yet delinquent or are being contested in good faith by appropriate Proceedings and for which sufficient reserves have been established in
A-17
Table of Contents
accordance with IFRS or GAAP, as applicable, (b) Liens for Taxes, assessments or other governmental charges not yet due and payable as of the Final Closing Date or which are being contested in good faith by appropriate Proceedings and for which sufficient reserves have been established in accordance with IFRS or GAAP, as applicable, (c) encumbrances and restrictions on real property (including easements, covenants, conditions, rights of way and similar restrictions) that do not prohibit or materially interfere with any of the Group Companies’ use or occupancy of such real property, (d) zoning, building codes and other land use Laws regulating the use or occupancy of real property or the activities conducted thereon which are imposed by any Governmental Entity having jurisdiction over such real property and which are not violated by the use or occupancy of such real property or the operation of the businesses of the Group Company and do not prohibit or materially interfere with any of the Group Companies’ use or occupancy of such real property, (e) cash deposits or cash pledges to secure the payment of workers’ compensation, unemployment insurance, social security benefits or obligations arising under similar Laws or to secure the performance of public or statutory obligations, surety or appeal bonds, and other obligations of a like nature, in each case in the ordinary course of business and which are not yet due and payable, (f) grants by any Group Company of non-exclusive rights in Intellectual Property Rights in the ordinary course of business, (g) any Lien, including any netting or set-off, as a result of a fiscal unity (fiscale eenheid) for Dutch tax purposes, and (h) other Liens that do not materially and adversely affect the value, use or operation of the asset subject thereto.
“Person” means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association, trust, joint venture, foundation or other similar entity, whether or not a legal entity.
“Personal Data” means any data or information Processed by or on behalf of a Group Company that identifies a natural person.
“PFIC” means a “passive foreign investment company” within the meaning of Section 1297(a) of the Code.
“PIPE Financing” has the meaning set forth in the recitals to this Agreement.
“PIPE Financing Amount” has the meaning set forth in the recitals to this Agreement.
“PIPE Investors” has the meaning set forth in the recitals to this Agreement.
“Plan of Merger” has the meaning set forth in Section 2.1(d)(ii).
“Positive Phase 3 Data” means, with respect to a clinical trial, (a) the completion of such clinical trial in accordance with its protocol, (b) the completion following data lock of statistical analysis of data generated in such clinical trial in accordance with the applicable protocol, (c) completion of a final study report for such clinical trial, and (d) the determination by the Company that such clinical trial has met its primary endpoint(s) at pre-specified level(s) of statistical significance as set forth in the applicable protocol.
“Pre-Closing FLAC Holders” means the holders of FLAC Shares and FLAC Warrants at any time prior to the Effective Date.
“Prior Acts Coverage” has the meaning set forth in Section 5.15(c).
“Privacy Laws” means applicable Laws in any jurisdiction that govern the receipt, collection, compilation, use, storage, Processing, sharing, safeguarding, security, disposal, destruction, disclosure or transfer of Personal Data, and any such legal requirement governing privacy, data security, data or security breach notification, any penalties and compliance with any order, including Section 5 of the U.S. Federal Trade Commission Act, the U.S. Electronic Communications Privacy Act of 1986, the U.S. Stored Communications Act, the California Online Privacy Protection Act, the California Consumer Privacy Act, the Illinois Biometric Information Privacy
A-18
Table of Contents
Act and other Laws governing the Processing of biometric data, the Massachusetts Data Security Regulations set forth at 201 CMR 17.00, the European Union General Data Protection Regulation 2016/679 (GDPR), the e-Privacy Directive (2002/58/EC) and including any implementing legislation of the foregoing, and all other Laws concerning privacy, data protection, or data security, the U.S. CAN-SPAM Act, the U.S. Telephone Consumer Protection Act and any analogous Laws, in each case to the extent applicable.
“Privacy Requirements” has the meaning set forth in Section 3.21(a).
“Proceeding” means any lawsuit, litigation, action, audit, investigation, examination, claim, complaint, charge, proceeding, suit, arbitration or mediation (in each case, whether civil, criminal or administrative and whether public or private) pending by or before or otherwise involving any Governmental Entity.
“Process” (or “Processed,” “Processes” or “Processing”) means the collection, use, storage, processing, recording, distribution, transfer, import, export, protection (including security measures), disposal or disclosure or other activity regarding data (whether electronically or in any other form or medium).
“Prospectus” has the meaning set forth in Section 8.18.
“Prospectus Regulation” means the Regulation (EU) 2017/1129 of the European Parliament and of the Council of June 14, 2017 on the prospectus to be published when securities are offered to the public or admitted to trading on a regulated market (including any relevant delegated regulations).
“Public Health Laws” means all applicable Laws relating to the development, testing, research (including preclinical, nonclinical and clinical research or studies), manufacture, production, analysis, distribution, approval, importation, exportation, use, handling, quality, packaging, labeling sale or promotion of any drug (including any ingredient or component of the foregoing products), including the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.), the Public Health Service Act (42 U.S.C. § 201 et seq.) or similar federal, state or foreign, Laws.
“Public Shareholders” has the meaning set forth in Section 8.18.
“Public Software” means any Software that contains, includes, incorporates, or has instantiated therein, or is derived in any manner (in whole or in part) from, any Software that is distributed as free software, open source software (e.g., Linux) or similar licensing or distribution models, including under any terms or conditions that impose any requirement that any Software using, linked with, incorporating, distributed with or derived from such Public Software (a) be made available or distributed in source code form; (b) be licensed for purposes of making derivative works; or (c) be redistributable at no, or a nominal, charge.
“Purchase Price” means $491,000,000.
“Real Property Leases” means all leases, sub-leases, licenses or other agreements, in each case, pursuant to which any Group Company leases or sub-leases any real property.
“Registered Domain Names” means internet domain names, including domain names registered with a recognized domain name registry (whether or not Marks).
“Registered Intellectual Property” means all issued Patents, pending Patent applications, registered Marks, pending applications for registration of Marks, registered Copyrights, pending applications for registration of Copyrights and Registered Domain Names.
“Registration Statement / Proxy Statement” means a registration statement on Form F-4 relating to the Transactions and containing a prospectus and proxy statement of FLAC.
A-19
Table of Contents
“Regulatory Permits” means all Permits granted by FDA or any comparable Governmental Entity to any Group Company, including investigational new drug applications, new drug applications, manufacturing approvals and authorizations, or their national or foreign equivalents.
“Relevant FLAC Shares” means the FLAC Shares issued and outstanding immediately prior to the Effective Date and that are held by Pre-Closing FLAC Holders who (a) do not redeem their FLAC Class A Shares for cash pursuant to the FLAC Shareholder Redemption or (b) hold FLAC Class B Shares; provided that “Relevant FLAC Shares” shall exclude any FLAC Shares held by FLAC as treasury shares.
“Representatives” means with respect to any Person, such Person’s Affiliates and its and such Affiliates’ respective directors, officers, managers, employees, members, owners, accountants, consultants, advisors, attorneys, agents and other representatives.
“Required Company Shareholder Approval” has the meaning set forth in the recitals to this Agreement.
“Required FLAC Shareholder Approval” means the approval of each Required Transaction Proposal by the affirmative vote of the holders of the requisite number of FLAC Shares entitled to vote thereon, whether in person or by proxy at the FLAC Shareholders Meeting (or any adjournment or postponement thereof), in accordance with the Governing Documents of FLAC and applicable Law.
“Required Holdco Shareholder Approval” means the adoption of the following resolutions by the Company as Holdco’s sole shareholder (in each case, subject to the applicable terms and conditions set forth in this Agreement, including the chronological and conditional order specified in Section 2.1): (i) to the extent required, the ratification of the execution of this Agreement; (ii) the issuance of Holdco Shares pursuant to the Dutch Deed of Issue Company Share Exchange to the Company Shareholders as part of the Company Share Exchange and, to the extent required, the approval of the contribution in kind of Company Shares as payment for the Holdco Shares so issued and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; (iii) the Holdco Reorganization; (iv) the assumption by Holdco, and issuance, of the Company Issuance Rights as rights to subscribe for Holdco Shares and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; (v) the issuance of Holdco Shares for delivery to the holders of Merger Claims as part of the Merger, and to the extent required, the approval of the contribution in kind and transfer of such Merger Claims as payment for the Holdco Shares so issued and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; (vi) the Holdco Board Appointments; (vii) the ratification of the execution of the Warrant Assumption Agreement, and the authorization of the assumption of FLAC Warrants pursuant to the Warrant Assumption Agreement and issuance of the applicable amount of Holdco Shares pursuant to such Converted Warrants (and, to the extent required, the approval of this contribution in kind as payment for the warrants representing Holdco Shares so issued and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance); (viii) the issuance of Holdco Shares pursuant to the PIPE Financing and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; (ix) the issuance of Holdco Shares or rights to subscribe for Holdco Shares under any Company Equity Incentive Plan pursuant to Section 2.6 and Section 5.18 and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; (x) the issuance of the rights to subscribe for the Earnout Shares pursuant to Section 2.7 and the preclusion of pre-emptive rights for Holdco Shares as part of such issuance; and (xi) the approval and adoption of the Holdco Equity Incentive Plan pursuant to section 2:135(5) of the Dutch Civil Code.
“Required Transaction Proposals” means, collectively, the Business Combination Proposal and the Merger Proposal.
“Rollover Company Option” has the meaning set forth in Section 2.6(a).
“Sanctions and Export Control Laws” means any applicable Law related to (a) import and export controls, including the U.S. Export Administration Regulations, the International Traffic in Arms Regulations, and any
A-20
Table of Contents
other equivalent or comparable export control laws and regulations of other countries, or (b) economic sanctions, including those administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury, the U.S. Department of State, the European Union, any European Union Member State, the United Nations, and Her Majesty’s Treasury of the United Kingdom or (c) anti-boycott measures.
“Sarbanes-Oxley Act” means the U.S. Sarbanes-Oxley Act of 2002.
“Schedules” means, collectively, the Company Disclosure Schedules and the FLAC Disclosure Schedules.
“SEC” means the U.S. Securities and Exchange Commission.
“Securities Act” means the U.S. Securities Act of 1933.
“Securities Laws” means Federal Securities Laws and other applicable foreign and domestic securities or similar Laws.
“Signing Filing” has the meaning set forth in Section 5.4(b).
“Signing Press Release” has the meaning set forth in Section 5.4(b).
“Software” means any and all (a) computer programs, including any and all software implementations of algorithms, models and methodologies, whether in source code or object code; (b) databases and compilations, including any and all data and collections of data, whether machine readable or otherwise; (c) descriptions, flowcharts and other work product used to design, plan, organize and develop any of the foregoing, screens, user interfaces, report formats, firmware, development tools, templates, menus, buttons and icons; and (d) all documentation, including user manuals and other training documentation, related to any of the foregoing.
“Sponsor” has the meaning set forth in the recitals to this Agreement.
“Sponsor Support Agreement” has the meaning set forth in the recitals to this Agreement.
“Subscription Agreement” has the meaning set forth in the recitals to this Agreement.
“Subsidiary” means, with respect to any Person, any corporation, limited liability company, partnership or other legal entity of which (a) if a corporation (including a Dutch B.V. or Dutch N.V.), a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of such Person or a combination thereof, or (b) if a limited liability company, partnership, association or other business entity (other than a corporation), a majority of the partnership or other similar ownership interests thereof is at the time owned or controlled, directly or indirectly, by such Person or one or more Subsidiaries of such Person or a combination thereof and for this purpose, a Person or Persons own a majority ownership interest in such a business entity (other than a corporation) if such Person or Persons shall be allocated a majority of such business entity’s gains or losses or shall be a, or control any, managing director or general partner of such business entity (other than a corporation). The term “Subsidiary” shall include all Subsidiaries of such Subsidiary.
“Supporting Company Shareholders” has the meaning set forth in the recitals to this Agreement.
“Surviving Company” has the meaning set forth in Section 2.1(d)(i).
“Tax” means any U.S. federal, state or local or non-U.S. income, gross receipts, franchise, estimated, alternative minimum, base erosion anti-abuse, diverted profits, digital services, imputed underpayment, sales,
A-21
Table of Contents
use, transfer, value added, excise, stamp, customs, duties, ad valorem, real property, personal property (tangible and intangible), capital stock, social security, unemployment, payroll, wage, employment, severance, occupation, registration, environmental, communication, mortgage, profits, license, lease, service, goods and services, withholding, premium, unclaimed property, escheat, turnover, windfall profits or other taxes of any kind whatever, or any other fees, charges, levies, excises, duties or assessments of any kind in the nature of taxes, whether computed on a separate or combined, unitary or consolidated basis or in any other manner, together with any interest, deficiencies, penalties, additions to tax, or additional amounts imposed by any Governmental Entity with respect thereto, whether disputed or not, and including (a) any secondary Liability for any of the aforementioned and (b) any penalty or charge imposed on account of failure to timely, completely or properly file any Tax Return.
“Tax Return” means returns, information returns, statements, declarations, claims for refund, and reports relating to Taxes, including any schedule or attachment thereto or any amendment thereof, filed or required to be filed with any Governmental Entity.
“Termination Date” has the meaning set forth in Section 7.1(d).
“Transactions” means the transactions contemplated by this Agreement and the Ancillary Documents, including the Holdco Reorganization, the Company Share Exchange, the Merger, and the Domestication.
“Transaction Litigation” has the meaning set forth in Section 5.2(e).
“Transaction Proposals” has the meaning set forth in Section 5.8.
“Triggering Event” means the first date after the Final Closing Date, but within the Earnout Period, on which the Company has achieved and publicly announced Positive Phase 3 Data for each of (a) the Company’s BROADWAY trial of obicetrapib (protocol number: TA-8995-302) and (b) the Company’s BROOKLYN trial of obicetrapib (protocol number: TA-8995-301).
“Trust Account” has the meaning set forth in Section 8.18.
“Trust Account Released Claims” has the meaning set forth in Section 8.18.
“Trust Agreement” has the meaning set forth in Section 4.8.
“Trustee” has the meaning set forth in Section 4.8.
“Unallocated Company Option Pool” means the number of Company Shares equal to the Company Option Pool less the Allocated Company Option Pool.
“Union” has the meaning set forth in Section 3.15(k).
“Unpaid Company Expenses” means the Company Expenses calculated as of immediately prior to the Final Closing Date, in each case, to the extent unpaid as of such time.
“Unpaid FLAC Expenses” means the FLAC Expenses calculated as of immediately prior to the Final Closing Date, in each case, to the extent unpaid as of such time.
“WARN” means the Worker Adjustment Retraining and Notification Act of 1988, as well as analogous applicable foreign, state or local Laws related to plant closings, relocations, mass layoffs and employment losses.
“Warrant Agreement” means the Warrant Agreement, dated as of December 8, 2020, by and between FLAC and the Trustee.
A-22
Table of Contents
“Warrant Assumption Agreement” has the meaning set forth in Section 2.4.
“Willful Breach” means a material breach that is a consequence of an act undertaken or a failure to act by the breaching party with the knowledge that the taking of such act or failure to act would, or would reasonably be expected to, constitute or result in a breach of this Agreement.
ARTICLE 2
MERGER
Section 2.1 Transactions. On the terms and subject to the conditions set forth in this Agreement, the following transactions shall occur in the order set forth in this Section 2.1:
(a) Required Holdco Shareholder Approval. After the execution of this Agreement and at any time prior to the Effective Date, the Company, as the sole shareholder of Holdco, shall adopt the Required Holdco Shareholder Approval.
(b) Company Share Exchange.
(i) Prior to the Effective Date, in accordance with Dutch Law, the Company shall (A) cause each Company Shareholder to effect the Company Share Exchange, whereby each Company Shareholder shall contribute and transfer each Company Share held by it and Holdco shall accept such contribution and transfer and in exchange issue to such holder such number of Holdco Shares that is equal to the Applicable Exchange Consideration Per Share with respect to such Company Share, (B) after the Company Share Exchange, to the extent any Company Issuance Rights shall have been exercised in connection with the Company Share Exchange, issue to such exercising holders, in lieu of Company Shares, such number of Holdco Shares that is equal to the Applicable Exchange Consideration Per Share with respect to the Company Shares that would have been issued to such holders but for the conversion of the Company Share Issuance Rights from rights to subscribe for Company Shares into rights to subscribe for Holdco Shares in connection with the Company Share Exchange (provided that Holdco may issue such Holdco Shares described in this clause (B) immediately after the Holdco Reorganization, to the extent deemed necessary by FLAC and the Company), and (C) immediately after the Company Share Exchange, take all action necessary to repurchase or cancel any Holdco Shares held by the Company, for no consideration.
(ii) In connection with the Company Share Exchange, the Company shall procure that each Company Shareholder will enter with Holdco into one or more notarial deeds of issue of Holdco Shares governed by Dutch Law and notarized by a Dutch civil-law notary, in a form and substance reasonably satisfactory to FLAC (the “Dutch Deed of Issue Company Share Exchange”) under which Holdco will issue to each such Company Shareholder such Holdco Shares in connection with the portion of the consideration to which he, she or it is entitled pursuant to Section 2.1(b)(i), and in fulfilment of such Company Shareholder’s respective obligations under the Dutch Deed of Issue Company Share Exchange to pay up the respective Holdco Shares issued to such Company Shareholder under the Dutch Deed of Issue Company Share Exchange by payment in kind by way of contribution and transfer of the Company Shares held by such Company Shareholder to Holdco.
(c) Holdco Reorganization. After the Company Share Exchange and prior to the Effective Date, Holdco shall effectuate the Holdco Reorganization whereby Holdco shall (i) change its legal form from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) and (ii) amend and restate its Articles of Association substantially in the form set forth on Exhibit F, which, as so amended and restated, shall be the Articles of Association of Holdco until thereafter amended in accordance with the terms thereof and applicable Law; provided that the Company and FLAC may agree instead to effect the Holdco Reorganization promptly following the PIPE Financing (where in such event (A) the order as set forth in this Section 2.1 shall be deemed amended accordingly and (B) each relevant Party commits to execute and provide all additional Closing deliverables related to any of the Transactions, including any required notarizations pursuant to applicable Law).
A-23
Table of Contents
(d) Merger.
(i) The Merger shall become effective on such date as is agreed by FLAC and Merger Sub and specified in the Merger Documents in accordance with the Cayman Companies Act (the date the Merger becomes effective being referred to herein as the “Effective Date”), and on the terms and subject to the conditions set forth in this Agreement and in accordance with the Cayman Companies Act. Following the Effective Date, the separate existence of Merger Sub shall cease and FLAC shall continue as the surviving entity of the Merger (the “Surviving Company”) and shall succeed to and assume all the rights and obligations of Merger Sub in accordance with the Cayman Companies Act.
(ii) On the Closing Commencement Date, FLAC and Merger Sub shall cause a plan of merger, in a form reasonably satisfactory to FLAC, Merger Sub and Holdco (with such modifications, amendments or supplements thereto as may be required to comply with the Cayman Companies Act) (the “Plan of Merger”), along with all other documentation and declarations required under the Cayman Companies Act in connection with such merger, to be duly executed and properly filed with the Cayman Islands Registrar of Companies, in accordance with the relevant provisions of the Cayman Companies Act (together, the “Merger Documents”).
(iii) The Merger shall have the effects as provided in this Agreement, in the Merger Documents and in the applicable provisions of the Cayman Companies Act. Without limiting the generality of the foregoing, and subject thereto, at the Effective Date, all of the assets, properties, rights, privileges, immunities, powers and franchises of Merger Sub shall vest in the Surviving Company and all debts, liabilities, obligations and duties of Merger Sub shall become the debts, liabilities, obligations and duties of the Surviving Company.
(iv) At the Effective Date, by virtue of the Merger and the Required Holdco Shareholder Approval without any further action on the part of any Party or any other Person:
(A) each Relevant FLAC Share issued and outstanding immediately prior to the Effective Date shall be automatically canceled and extinguished in exchange for the right to receive the Merger Consideration, which Merger Consideration will be settled as follows: (1) each holder of Relevant FLAC Shares will be entitled to a claim for a corresponding Holdco Share that is held in the accounts of the Exchange Agent, solely for the benefit of the holders of Relevant FLAC Shares (each, a “Merger Claim” and together, the “Merger Claims”); and (2) the Merger Claims will be contributed and transferred to Holdco by the Exchange Agent for the benefit of the holders of Relevant FLAC Shares in exchange for the issuance of a corresponding number of Holdco Shares (resulting, for the avoidance of doubt, so far as legally possible (subject always to Section 2.8(g)), in each Relevant FLAC Share being exchanged for a Holdco Share), and in fulfillment of each such holder’s respective obligations to pay up such Holdco Shares (together the “Merger Consideration”); and
(B) each FLAC Share held immediately prior to the Effective Date by FLAC as treasury shares shall be canceled and extinguished, and no consideration shall be paid with respect thereto; and
(C) each ordinary share, par value $1.00, of Merger Sub that is issued and outstanding immediately prior to the Effective Date shall be converted into one validly issued, fully paid and non-assessable ordinary share, par value $0.0001, of the Surviving Company (each of which, following the Domestication, shall be converted into one share of common stock, par value $0.0001, of the Surviving Company).
(v) Immediately after the Effective Date, (x) the Governing Documents of Merger Sub shall become the Governing Documents of the Surviving Company, in each case, until thereafter changed or amended as provided therein or by applicable Law and (y) the Directors (as defined in the Governing Documents of Merger Sub) of Merger Sub immediately prior to the Effective Date shall be the Directors of the Surviving Company.
(vi) From and after the Effective Date, the holder(s) of certificates, if any, evidencing ownership of FLAC Shares or FLAC Shares held in book-entry form issued and outstanding immediately prior to the
A-24
Table of Contents
Effective Date shall cease to have any rights with respect to such shares except as otherwise provided for herein or under applicable Law.
(vii) If after the date of this Agreement and prior to the Effective Date, FLAC pays a stock dividend in, splits, combines FLAC Shares into a smaller number of shares, or issues by reclassification any FLAC Shares, then the consideration payable in respect of such FLAC Share in accordance with Section 2.1(d)(iv) will be appropriately adjusted to provide to the holders of the FLAC Shares the same economic effect as contemplated by this Agreement prior to such action, and as so adjusted will, from and after the date of such event, any consideration payable pursuant to Section 2.1(d)(iv).
(viii) Notwithstanding anything herein to the contrary, any FLAC Share redeemed in the FLAC Shareholder Redemption shall be canceled and converted into the right to receive the consideration offered in the FLAC Shareholder Redemption and shall not be entitled to receive any consideration pursuant to Section 2.1(d)(iv).
(e) Domestication. Following the Merger, the Surviving Company shall cause the Domestication to occur in accordance with Section 388 of the DGCL and Section 206 of the Cayman Companies Act. In connection with the Domestication, the Surviving Company shall amend and restate its Governing Documents substantially in the form set forth on Exhibit G and Exhibit H, which, as so amended and restated, shall be the Governing Documents of the Surviving Company until thereafter amended in accordance with the terms thereof and applicable Law.
(f) Board Appointments. Effective upon the Effective Date, the Holdco Board Appointments shall be effected in accordance with Section 5.16.
(g) Nasdaq Listing. Effective upon the Merger and upon satisfaction of all initial listing requirements, the Holdco Shares shall (subject to any restrictions set forth in this Agreement or any Ancillary Documents) become available for listing on Nasdaq in accordance with Section 5.12.
Section 2.2 Closing. On the terms and subject to the conditions set forth in this Agreement, the closing of the Merger (the “Closing”) shall take place electronically by exchange of the closing deliverables by the means provided in Section 8.11 (provided that any Closing deliverables which need to be notarized by a Dutch civil-law notary as a matter of Dutch Law shall be executed at the offices of NautaDutilh N.V., Dutch legal counsel to the Company and Holdco) as promptly as reasonably practicable, but in no event later than the third (3rd) Business Day, following the satisfaction (or, to the extent permitted by applicable Law, waiver) of the conditions set forth in Article 6 (other than those conditions that by their nature are to be satisfied at the Closing, but subject to satisfaction or waiver of such conditions) or at such other place, date or time as FLAC and the Company may agree in writing (the date the Closing commences to occur, the “Closing Commencement Date”).
Section 2.3 Withholding. Holdco and any other applicable withholding agent shall be entitled to deduct and withhold (or cause to be deducted and withheld) from any consideration payable pursuant to this Agreement such amounts as are required to be deducted and withheld under applicable Tax Law. To the extent that amounts are so withheld and remitted to the applicable Governmental Entity, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of which such deduction and withholding was made. Prior to deducting and withholding any amounts pursuant to this Section 2.3, Holdco and any other applicable withholding agent shall use reasonable best efforts to notify the payee five (5) Business Days in advance of any amounts that Holdco or any other applicable withholding agent intends to withhold from any payments hereunder, as well as for the basis pursuant to which Holdco or such other applicable withholding agent intends to withhold under applicable Tax Law. The Parties shall cooperate in good faith to eliminate or reduce any such deduction or withholding (including through the request and provision of any statements, forms or other documents to reduce or eliminate any such deduction or withholding) to the extent reasonably practicable.
A-25
Table of Contents
Section 2.4 FLAC Warrants. As a result of the Merger and without any action of any Party or any other Person (but without limiting the obligations of Holdco pursuant to the penultimate sentence of this Section 2.4), each FLAC Warrant that is outstanding immediately prior to the Effective Date shall automatically cease to represent a right to acquire FLAC Class A Shares and shall automatically represent (pursuant to the execution of, and subject to the terms of, the Warrant Assumption Agreement), immediately following the Effective Date, a right to acquire Holdco Shares (a “Converted Warrant”) on the same contractual terms and conditions as were in effect immediately prior to the Effective Date under the terms of the Warrant Agreement (collectively, the “Warrant Exchange”); provided that each Converted Warrant: (a) shall represent the right to acquire the number of Holdco Shares equal to the number of FLAC Class A Shares subject to each such FLAC Warrant immediately prior to the Effective Date (subject to the provisions of Section 2.1(d)(vii), which shall apply mutatis mutandis); (b) shall have an exercise price of $11.50 per whole warrant required to purchase one Holdco Share (subject to the provisions of Section 2.1(d)(vii), which shall apply mutatis mutandis); and (c) shall expire on the five (5) year anniversary of the Final Closing Date. Holdco shall enter into a warrant assumption agreement as of immediately prior the Effective Date, such assumption agreement to be substantially in the form attached hereto as Exhibit I (the “Warrant Assumption Agreement”). Upon exercise, the Converted Warrants will be contributed to Holdco by the Exchange Agent as contribution in kind and transfer for and on behalf of the holders of the Converted Warrants in exchange for the issuance of a corresponding number of Holdco Shares pursuant to this Section 2.4.
Section 2.5 Allocation Schedule. No later than three (3) Business Days prior to the scheduled Closing Commencement Date, the Company shall deliver to FLAC an allocation schedule (the “Allocation Schedule”) setting forth (a) the number of each class and series of Company Shares held by each Company Shareholder, the number of Company Shares subject to each Company Equity Award (whether directly or indirectly through depository receipts for Company Shares) held by each holder thereof, as well as whether each such Company Equity Award will be vested or unvested as of immediately prior to the Effective Date, and, in the case of the Company Options, the exercise price of thereof, as well as reasonably detailed calculations and vesting schedule with respect to the components and subcomponents thereof, and the number of Company Shares subject to each other warrant, award, convertible security or any other right to subscribe for Company Ordinary Shares held by each holder thereof, and (b) the number of Holdco Shares that each Company Shareholder or holder of any other option, warrant, award, convertible security or any other right to subscribe for Company Ordinary Shares is entitled to receive as a result of Company Share Exchange (including after giving effect to the exercise of any Company Issuance Rights in connection with the Company Share Exchange) and (c) the Earnout Pro Rata Share allocated to each Company Shareholder, Eligible Optionholder or holder of Company Issuance Right, as the case may be, as well as reasonably detailed calculations with respect to the component and subcomponents thereof, and (d) a certification, duly executed by an authorized officer of the Company, that the information and calculations delivered pursuant to clauses (a), (b) and (c) are, and will be as of immediately prior to the Effective Date, (i) true and correct in all respects and (ii) in accordance with the applicable provisions of this Agreement, the Governing Documents of the Company, the Company Shareholders Agreement and applicable Laws and, in the case of the Company Equity Awards, a Company Equity Incentive Plan and any applicable grant or similar agreement with respect to any such Company Equity Award. The Company will review any comments to the Allocation Schedule provided by FLAC or any of its Representatives and consider in good faith and incorporate any reasonable comments proposed by FLAC or any of its Representatives prior to the issuance of any Holdco Shares. Notwithstanding the foregoing or anything to the contrary herein, the aggregate number of Holdco Shares that each Company Shareholder or holder of other Equity Securities (including a holder of Company Issuance Rights) will have a right to receive pursuant to Section 2.1(b) will be (A) rounded down to the nearest whole number in the event that the fractional Holdco Share that otherwise would be so paid is less than five-tenths (0.5) of a Holdco Share and (B) rounded up to the nearest whole number in the event that the fractional Holdco Share that otherwise would be so paid is greater than or equal to five-tenths (0.5) of a Holdco Share.
Section 2.6 Treatment of Company Equity Awards.
(a) Prior to the Company Share Exchange, with effect as of immediately following the Company Share Exchange, the Company shall amend the Company Equity Incentive Plans in respect of each Company
A-26
Table of Contents
Optionholder effective as of the Company Share Exchange as follows: (i) no further Company Options will be issued under any Company Equity Incentive Plans on or after the Effective Date; (ii) existing Company Options will remain outstanding and, to the extent unvested, shall continue to vest in accordance with the applicable Company Option Subscription Agreement; and (iii) except as otherwise set forth in this Agreement, settlement of any award upon exercise of Company Options shall be made in Holdco Shares. The Company shall use commercially reasonably efforts to obtain the approval of such amendments by each Company Optionholder to the extent required by Law and the terms of the Company Equity Incentive Plans or Company Option Subscription Agreement, provided that if the approval of any Company Optionholder is not obtained, the Company Options held by such holder shall remain subject to the terms of the applicable Company Equity Incentive Plans and Company Option Subscription Agreement and the Governing Documents of the Company as amended from time to time. Upon effectiveness of the foregoing amendments, the number of Holdco Shares that are deliverable upon exercise of a Company Option (each, a “Rollover Company Option”) shall be equal to (A) the number of Company Shares subject to such Company Option immediately prior to the Company Share Exchange, multiplied by (B) the Company Ordinary Share Value at an exercise price per Holdco Share equal to (x) the exercise price per Company Ordinary Share of such Company Option immediately prior to the Effective Date, divided by (y) the Company Ordinary Share Value. Each Rollover Company Option shall be subject to the same terms and conditions (including applicable vesting, expiration and forfeiture provisions) that applied to the Company Option immediately prior to the Company Share Exchange, as amended pursuant to this Section 2.6. In the case of any Company Optionholders who are U.S. taxpayers, such conversion shall occur in a manner intended to comply with the requirements of Sections 409A and 424 of the Code.
(b) Prior to the Closing, the Parties shall cooperate in good faith in order to finalize and agree any necessary amendment to the Governing Documents of the Company and the terms and conditions of the Company Equity Incentive Plans, and shall take all such other reasonably necessary or reasonably appropriate actions to cause Holdco Shares to be delivered upon exercise of Rollover Company Options, or otherwise to give effect to the provisions of this Agreement.
Section 2.7 Earnout.
(a) Following the Closing, promptly (but in any event no later than ten (10) Business Days) after the occurrence of the Triggering Event, Holdco shall issue or cause to be issued to the Holdco Shareholders and Eligible Optionholders as of immediately prior to the Effective Date (after giving effect to the issuance of any Holdco Shares as a result of the exercise of any Company Issuance Rights in connection with the Company Share Exchange) their respective Earnout Pro Rata Share of the Earnout Shares. For the avoidance of doubt, the Triggering Event shall only occur once, if at all.
(b) Notwithstanding anything in this Agreement to the contrary, any Earnout Shares issuable under this Section 2.7 in respect of Rollover Company Options shall (i) be issued to the relevant Eligible Optionholder only if such Eligible Optionholder continues to provide services (whether as an employee, director or individual independent contractor) to Holdco or one of its Subsidiaries through the date of the occurrence of the Triggering Event that causes such Earnout Shares to become issuable, and (ii) take the form of restricted stock units issued under the Holdco Equity Incentive Plan (“Earnout RSUs”) and pursuant to Holdco’s form of restricted stock unit grant agreement. The Earnout RSUs shall be subject to the same vesting schedule as the corresponding Rollover Company Options. Any Earnout Shares that are forfeited as a result of an Eligible Optionholder ceasing to provide services through the date of the occurrence of the Triggering Event shall be reallocated to the other recipients of Earnout Shares in accordance with their respective Earnout Pro Rata Shares.
(c) At all times during the Earnout Period, Holdco shall maintain sufficient Holdco Shares available for issuance under its authorized share capital to permit Holdco to satisfy its issuance obligations set forth in this Section 2.7 and shall exert its reasonable best efforts to take all actions required to increase the number of Holdco Shares available for issuance under its authorized share capital if at any time there shall be insufficient Holdco Shares thereunder to satisfy its issuance obligations set forth in this Section 2.7.
A-27
Table of Contents
(d) Notwithstanding anything to the contrary contained herein, no fraction of an Earnout Share will be issued by virtue of the Triggering Event, and each Person who would otherwise be entitled to a fraction of an Earnout Share (after aggregating all fractional Earnout Shares that otherwise would be received by such holder in connection with the occurrence of such Triggering Event) shall instead have the number of Earnout Shares issued to such Person (i) rounded down to the nearest whole number in the event that the fractional Earnout Share that otherwise would be so paid is less than five-tenths (0.5) of an Earnout Share and (ii) rounded up to the nearest whole number in the event that the fractional Earnout Share that otherwise would be so paid is greater than or equal to five-tenths (0.5) of an Earnout Share.
(e) Following the Closing but during the Earnout Period, if (i) Holdco is purchased or acquired pursuant to a Change of Control Transaction and (ii) such Change of Control Transaction occurs at any time after Positive Phase 3 Data has been achieved with respect to the Company’s BROADWAY trial of obicetrapib (protocol number: TA-8995-302) but prior to the occurrence of the Triggering Event, then any Earnout Shares that remain unissued as of immediately prior to the consummation of such Change of Control Transaction shall immediately become issuable and the holders of Holdco Shares as of immediately prior to the Effective Date and the Eligible Optionholders as of immediately prior to the Effective Date who continue to provide services (whether as an employee, director or individual independent contractor) to Holdco or one of its Subsidiaries through the date of the Change in Control shall be entitled to receive their respective Earnout Pro Rata Share of such Earnout Shares prior to the consummation of such Change of Control Transaction. Any Earnout Shares shall be issuable to as specified on the Allocation Schedule, subject to any reallocation made pursuant to Section 2.7(b).
Section 2.8 Exchange of Shares.
(a) Exchange Agent. As promptly as reasonably practicable following the date of this Agreement, but in no event later than five (5) Business Days prior to the Closing Commencement Date, Holdco shall appoint Continental (or its applicable Affiliate) as an exchange agent (the “Exchange Agent”) and enter into an exchange agent agreement with the Exchange Agent, in a form and substance reasonably acceptable to FLAC, for the purpose of effecting the contribution and transfer of the Merger Claims against the issuance of Holdco Shares as contemplated by Section 2.1(d) on the terms and subject to the other conditions set forth in this Agreement. Notwithstanding the foregoing or anything to the contrary herein, in the event that Continental is unable or unwilling to serve as the Exchange Agent, then Holdco and the Company shall, as promptly as reasonably practicable thereafter, but in no event later than the Closing Commencement Date, mutually agree upon an exchange agent (in either case, such agreement not to be unreasonably withheld, conditioned or delayed), Holdco shall appoint and enter into an exchange agent agreement with such exchange agent, who shall for all purposes under this Agreement constitute the Exchange Agent, such agreement to be in a form and substance reasonably acceptable to FLAC.
(b) Exchange Fund. On the Closing Commencement Date (and after the Effective Date), Holdco shall deliver to the Exchange Agent, for the benefit of the holders of the Merger Claims, the number of Holdco Shares (in uncertificated form or book-entry form) sufficient to deliver the applicable Holdco Shares to be issued to the holders of the Merger Claims pursuant to a Dutch deed of issue entered into between Holdco and the Exchange Agent and this Agreement (the “Exchange Fund”). Holdco shall cause the Exchange Agent pursuant to irrevocable instructions, to deliver that portion of the Merger Consideration consisting of Holdco Shares out of the Exchange Fund in accordance with this Agreement. Except as contemplated by this Section 2.8, the Exchange Fund shall not be used for any other purpose.
(c) Exchange Procedures. After the Effective Date, and as promptly as practicable after the surrender to the Exchange Agent of the book-entry shares representing the Relevant FLAC Shares held by such holder for cancellation, and such other documents as may reasonably be required by the Exchange Agent, each holder of Relevant FLAC Shares shall be entitled to receive in exchange therefor, and Holdco shall cause the Exchange Agent to deliver the applicable number of Holdco Shares and each Relevant FLAC Share held immediately prior to the Effective Date shall forthwith be cancelled.
A-28
Table of Contents
(d) Distributions with Respect to Unexchanged FLAC Shares. No dividends or other distributions declared or made after the Effective Date with respect to the Holdco Shares with a record date after the Effective Date shall be paid to the holder of any unsurrendered book-entry shares representing Relevant FLAC Shares until such book-entry shares are surrendered for exchange in accordance with this Section 2.8. Subject to the effect of escheat, tax or other applicable Laws, following surrender of any such book-entry shares, Holdco shall pay or cause to be paid to the holder of the book-entry shares representing Relevant FLAC Shares without interest, (i) promptly, but in any event no later than five (5) Business Days of such surrender, the amount of dividends or other distributions with a record date after the Effective Date and theretofore paid with respect to the Holdco Shares to which a holder of Relevant FLAC Shares would be entitled pursuant to Section 2.1(d)(iv), and (ii) at the appropriate payment date, the amount of dividends or other distributions, with a record date after the Effective Date but prior to surrender and a payment date occurring after surrender, payable with respect to the Holdco Shares to which a holder of Relevant FLAC Shares would be entitled pursuant to Section 2.1(d)(iv).
(e) Termination of Exchange Fund. Any portion of the Exchange Fund that remains undistributed for one (1) year after the Effective Date shall be delivered to Holdco for no consideration, and any holders of Relevant FLAC Shares who have not theretofore surrendered to the Exchange Agent their book-entry shares representing the Relevant FLAC Shares held by such holder for cancellation in accordance with this Section 2.8 shall thereafter look only to Holdco for the applicable consideration for the Merger. Any portion of the Exchange Fund remaining unclaimed by holders of Relevant FLAC Shares, as may be applicable, as of a date which is immediately prior to such time as such amounts would otherwise escheat to or become property of any Governmental Entity shall, to the extent permitted by applicable Law, become the property of Holdco free and clear of any claims or interest of any person previously entitled thereto.
(f) No Liability. None of the Exchange Agent, FLAC, Holdco, the Surviving Company or any of their respective Affiliates shall be liable to any holder of FLAC Shares (or dividends or distributions with respect thereto) or cash delivered to a public official pursuant to any abandoned property, escheat or similar Law in accordance with this Section 2.8.
(g) Fractional Shares. Notwithstanding any other provision of this Agreement, no fractional shares of Holdco Shares will be issued, and the number of Holdco Shares each holder of FLAC Shares or Company Shares, as applicable, is entitled to receive pursuant to Section 2.1 will be (i) rounded down to the nearest whole number in the event that the fractional Holdco Share that otherwise would be so paid is less than five-tenths (0.5) of a Holdco Share and (ii) rounded up to the nearest whole number in the event that the fractional Holdco Share that otherwise would be so paid is greater than or equal to five-tenths (0.5) of a Holdco Share.
ARTICLE 3
REPRESENTATIONS AND WARRANTIES RELATING
TO THE GROUP COMPANIES
Subject to Section 8.8, except as set forth on the Company Disclosure Schedules, the Company hereby represents and warrants to FLAC, in each case, as of the date of this Agreement and as of the Closing Commencement Date (or in the case of representations and warranties that speak of a specified date, as of such specified date), as follows:
Section 3.1 Organization and Qualification.
(a) Each Group Company is a corporation, limited liability company or other applicable business entity duly organized or formed, as applicable, validly existing and in good standing (or the equivalent thereof, if applicable, in each case, with respect to the legal form, jurisdictions that recognize the concept of good standing or any equivalent thereof) under the Laws of its jurisdiction of formation or organization (as applicable), registered seat, registration details, business address and share capital. Section 3.1(a) of the Company Disclosure
A-29
Table of Contents
Schedules sets forth the jurisdiction of formation or organization (as applicable) for each Group Company. Each Group Company has the requisite corporate, limited liability company or other applicable business entity power and authority to own, lease and operate its properties and to carry on its respective portion of the Business as presently conducted, except where the failure to have such power or authority would not be material to the Group Companies, taken as a whole. There are no pending applications for registration (and no resolutions or other actions requiring such registration) in the commercial register or with any other competent authority in respect of any Group Company that have not yet been registered.
(b) True and complete copies of the Governing Documents of the Company and the Company Shareholders Agreement have been made available to FLAC, in each case, as amended and in effect as of the date of this Agreement. The Governing Documents of the Company and the Company Shareholders Agreement are in full force and effect, and the Company is not in breach or violation of any provision set forth in its Governing Documents or in material breach of the Company Shareholders Agreements. The Company Shareholders Agreement and all rights, claims and Liabilities thereunder will terminate upon consummation of the Company Share Exchange.
(c) Each Group Company is duly qualified or licensed to transact business and is in good standing (or the equivalent thereof, if applicable, in each case, with respect to the jurisdictions that recognize the concept of good standing or any equivalent thereof) in each jurisdiction in which the property and assets owned, leased or operated by it, or the nature of the business conducted by it, makes such qualification or licensing necessary, except where the failure to be so duly qualified or licensed and in good standing would not result in a Company Material Adverse Effect.
Section 3.2 Capitalization of the Group Companies.
(a) Section 3.2(a) of the Company Disclosure Schedules sets forth a true and complete statement as of the date of this Agreement of (i) the number and class or series (as applicable) of all of the Equity Securities of the Company issued and outstanding, (ii) the identity of the Persons that are the record and beneficial owners thereof, (iii) with respect to each Company Equity Award, (A) the date of grant, (B) any applicable exercise (or similar) price and (C) the expiration date, and (D) any applicable vesting schedule (including acceleration provisions). All of the Equity Securities of the Company have been duly authorized and validly issued and all of the outstanding Company Shares are fully paid and non-assessable (meaning that the holders of the Company Shares will not by reason of merely being such a holder, be subject to assessment or calls by the Company or its creditors for further payment on such Company Shares). The Equity Securities of the Company (1) were not issued in violation of the Governing Documents of the Company or the Company Shareholders Agreement or any other Contract to which the Company is party or bound, (2) were not issued in violation of any preemptive rights, call option, right of first refusal or first offer, subscription rights, transfer restrictions or similar rights of any Person under the Governing Documents of the Company or any other Contract to which the Company is a party or bound or applicable Laws and (3) have been offered, sold and issued in compliance with Securities Laws. Except as set forth on Section 3.2(a) of the Company Disclosure Schedules and except for the Company Issuance Rights, the Company has no outstanding (x) equity appreciation, phantom equity or profit participation rights or (y) options, restricted stock, phantom stock, warrants, purchase rights, subscription rights, conversion rights, exchange rights, calls, puts, rights of first refusal or first offer or other Contracts that could require the Company to issue, sell or otherwise cause to become outstanding or to acquire, repurchase or redeem any Equity Securities or securities convertible into or exchangeable for Equity Securities of the Company. The Company Equity Incentive Plans are the only equity incentive plans maintained by the Company and all outstanding option, restricted stock and similar awards have been granted under the Company Equity Incentive Plans.
(b) The Equity Securities of the Company are free and clear of all Liens (other than transfer restrictions under applicable Securities Law or under the Company Shareholders Agreement or the Company’s Governing Documents). Except for the Company Shareholders Agreement, there are no voting trusts, proxies or other Contracts with respect to the voting or transfer of the Company’s Equity Securities.
A-30
Table of Contents
(c) Section 3.2(c) of the Company Disclosure Schedules sets forth a true and complete statement of (i) the number and class or series (as applicable) of all of the Equity Securities of each Subsidiary of the Company issued and outstanding and (ii) the identity of the Persons that are the record and beneficial owners thereof. Except for the Subscription Agreements, there are no outstanding (A) equity appreciation, phantom equity, or profit participation rights or (B) options, restricted stock, phantom stock, warrants, purchase rights, subscription rights, conversion rights, exchange rights, calls, puts, rights of first refusal or first offer or other Contracts that could require any Subsidiary of the Company to issue, sell or otherwise cause to become outstanding or to acquire, repurchase or redeem any Equity Securities or securities convertible into or exchangeable for Equity Securities of the Subsidiaries of the Company. There are no voting trusts, proxies or other Contracts with respect to the voting or transfer of any Equity Securities of any Subsidiary of the Company to which the Company or any Subsidiary is a party.
(d) None of the Group Companies owns or holds (of record, beneficially, legally or otherwise), directly or indirectly, any Equity Securities in any Person other than a Group Company or the right to acquire any such Equity Security, and none of the Group Companies are a partner or member of any partnership, limited liability company or joint venture other than a Group Company.
(e) Section 3.2(e) of the Company Disclosure Schedules sets forth a list of all Indebtedness of the Group Companies as of the date of this Agreement, including the principal amount of such Indebtedness, the outstanding balance as of the date of this Agreement, and the debtor and the creditor thereof.
(f) Section 3.2(f) of the Company Disclosure Schedules sets forth a list of all Change of Control Payments of the Group Companies.
(g) Each Company Equity Award was granted in compliance in all material respects with all applicable Laws and all of the terms and conditions of the applicable Company Equity Incentive Plan, and each Company Option has an exercise price per share that is equal to or greater than the fair market value of a Company Share on the date of such grant determined in a manner consistent with Section 409A of the Code.
(h) As of the Closing, (i) the authorized share capital of Holdco will consist only of Holdco Shares, par value EUR 0.12 per share, and (ii) all of the issued and outstanding Holdco Shares when issued in accordance with the terms hereof (A) will be duly authorized, validly issued, fully paid and nonassessable (meaning that the holders of the Holdco Shares will not by reason of merely being such a holder, be subject to assessment or calls by Holdco or its creditors for further payment on such Holdco Shares), (B) will have been issued in compliance in all material respects with applicable Law and (C) will not have been issued in breach or violation of any preemptive rights or Contract to which Holdco is a party or bound.
Section 3.3 Authority. The Company has the requisite corporate, limited liability company or other similar power and authority to execute and deliver this Agreement and each Ancillary Document to which it is or will be a party, to perform its obligations hereunder and thereunder, and to consummate the Transactions. Subject to the receipt of the Required Company Shareholder Approval, the execution and delivery of this Agreement, the Ancillary Documents to which the Company is or will be a party and the consummation of the Transactions have been (or, in the case of any Ancillary Document entered into after the date of this Agreement, will be upon execution thereof) duly authorized by all necessary corporate (or other similar) action on the part of the Company. This Agreement and each Ancillary Document to which the Company is or will be a party has been or will be, upon execution thereof, as applicable, duly and validly executed and delivered by the Company and constitutes or will constitute, upon execution and delivery thereof, as applicable, a valid, legal and binding agreement of the Company (assuming that this Agreement and the Ancillary Documents to which the Company is or will be a party are or will be upon execution thereof, as applicable, duly authorized, executed and delivered by the other Persons party thereto), enforceable against the Company in accordance with its terms (subject to applicable bankruptcy, insolvency, reorganization, moratorium or other Laws affecting generally the enforcement of creditors’ rights and subject to general principles of equity (the “Enforceability Exceptions”)). On or prior to
A-31
Table of Contents
the date of this Agreement, the Company Board has duly and unanimously adopted resolutions (i) determining that this Agreement and the Transactions are conducive to the Company’s objects and serve the best interests of the Company, its business and the Company’s stakeholders, (ii) approving the execution, delivery and performance by the Company of this Agreement and the consummation of the Transactions and (iii) resolving to recommend the approval of this Agreement and the Transactions by the holders of Company Shares entitled to vote thereon with the Required Company Shareholder Approval. Other than the Required Company Shareholder Approval and the entry into a Dutch Deed of Issue Company Share Exchange by each Company Shareholder in connection with the Company Share Exchange, no other corporate action or vote is required under applicable Law, the Governing Documents of the Company or the Company Shareholders Agreements, on the part of the Company or any Company Shareholders, to enter into this Agreement and each Ancillary Document to which it is or will be a party, to perform its obligations hereunder and thereunder, and to consummate the Transactions.
Section 3.4 Financial Statements; Undisclosed Liabilities.
(a) The Company has made available to FLAC a true and complete copy of (i) the audited consolidated statements of financial position of the Current Companies as of December 31, 2020 and 2021 and the related audited consolidated statements of profit or loss and other comprehensive income (loss), changes in equity and cash flows of the Current Companies for the 12-month periods then ended and (ii) the audited consolidated statements of financial position of the Current Companies as of December, 31, 2020 and December 31, 2021 (the “Latest Balance Sheet Date”) and the related audited consolidated statements of profit or loss and other comprehensive income (loss), changes in equity and cash flows of the Current Companies for each of the years then ended (clauses (i) and (ii), collectively, the “Company Financial Statements”), each of which are attached as Section 3.4(a) of the Company Disclosure Schedules. Each of the Company Financial Statements (including the notes thereto) (A) was prepared in accordance with IFRS applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto), (B) fairly presents, in all material respects, the financial position, results of operations and cash flows of the Current Companies as at the date thereof and for the period indicated therein, except as otherwise specifically noted therein and (C) in the case of clause (i), were audited in accordance with the standards of IFRS and contain an unqualified report of the Company’s auditors (other than the qualification related to the Company’s recurring losses from operations and net capital deficiency that raise substantial doubt about its ability to continue as a going concern). When the Closing Financial Statements (including the notes thereto) are delivered following the date of this Agreement in accordance with Section 5.17, each Closing Financial Statement shall (1) be prepared in accordance with IFRS applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto), (2) fairly present, in all material respects, the financial position, results of operations and cash flows of the Group Companies as at the date thereof and for the period indicated therein, except as otherwise specifically noted therein, (3) have been audited in accordance with the standards of the PCAOB and contain an unqualified report of the Company’s auditors and (4) comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates thereof (including Regulation S-X).
(b) No Group Company has any Liabilities of the type required to be set forth on a balance sheet in accordance with IFRS, except (i) as reflected on the Company Financial Statements, (ii) for Liabilities incurred in the ordinary course of business since the Latest Balance Sheet Date (none of which is a Liability for breach of contract, breach of warranty, tort, infringement or violation of Law), (iii) for Liabilities incurred in connection with the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of their respective covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions and (iv) for Liabilities that are not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
(c) The Group Companies have established and maintain systems of internal accounting controls that are designed to provide, in all material respects, reasonable assurance that (i) all transactions are executed in accordance with management’s authorization and (ii) all transactions are recorded as necessary to permit
A-32
Table of Contents
preparation of proper and accurate financial statements in accordance with IFRS and to maintain accountability for the Group Companies’ assets. The Group Companies maintain and, for all periods covered by the Financial Statements, have maintained books and records of the Group Companies in the ordinary course of business that are accurate and complete and reflect the revenues, expenses, assets and liabilities of the Group Companies in all material respects.
(d) Since January 1, 2020, to the Company’s knowledge, no Group Company has received any written complaint, allegation, assertion or claim that there is (i) “significant deficiency” in the internal controls over financial reporting of the Group Companies, (ii) a “material weakness” in the internal controls over financial reporting of the Group Companies to the Company’s knowledge or (iii) fraud, whether or not material, that involves management or other employees of the Group Companies who have a significant role in the internal controls over financial reporting of the Group Companies.
Section 3.5 Consents and Requisite Governmental Approvals; No Violations.
(a) No consent, approval or authorization of, or designation, declaration or filing with, any Governmental Entity is required on the part of the Company (or any Group Company) with respect to the Company’s execution, delivery or performance of its obligations under this Agreement or the Ancillary Documents to which the Company is or will be party or the consummation of the Transactions, except for (i) the filing with the SEC of (A) the Registration Statement / Proxy Statement and the declaration of the effectiveness thereof by the SEC and (B) such reports under Section 13(a) or 15(d) of the Exchange Act as may be required in connection with this Agreement, the Ancillary Documents or the Transactions, (ii) such filings with and approvals of Nasdaq to permit Holdco Shares to be issued in accordance with this Agreement and, along with the Holdco Warrants, to be listed on Nasdaq, (iii) such filings and approvals required in connection with the Domestication, (iv) filing of the Merger Documents under the applicable Law of the Cayman Islands, (v) the Required Holdco Shareholder Approval, (vi) filings under any Antitrust Laws or (vii) any other consents, approvals, authorizations, designations, declarations, waivers or filings, the absence of which would not have a Company Material Adverse Effect.
(b) Neither the execution, delivery or performance by the Company of this Agreement nor the Ancillary Documents to which the Company is or will be a party nor the consummation of the Transactions will, directly or indirectly (with or without due notice or lapse of time or both) will (i) result in any breach of any provision of the Company’s Governing Documents, (ii) result in a violation or breach of, or constitute a default or give rise to any right of termination, Consent, cancellation, amendment, modification, suspension, revocation or acceleration under, any of the terms, conditions or provisions of (A) any Contract to which any Group Company is a party or (B) any Material Permits, (iii) violate, or constitute a breach under, any Order or applicable Law to which any Group Company or any of its properties or assets are bound or (iv) result in the creation of any Lien upon any of the assets or properties (other than any Permitted Liens) or Equity Securities of any Group Company, except, in the case of any of clauses (ii) through (iv) above, as would not have a Company Material Adverse Effect.
Section 3.6 Permits. Each of the Group Companies has all Permits that are required to own, lease or operate its properties and assets and to conduct its business, except where the failure to obtain or hold such Permit would not result in a Company Material Adverse Effect (the “Material Permits”). Except as is not and would not reasonably be expected to be material to the Group Companies, taken as a whole, (i) each Material Permit is in full force and effect in accordance with its terms and (ii) no written notice of revocation, cancellation or termination of any Material Permit has been received by the Group Companies. Each Group Company is, and (where applicable) has been, in all material respects fulfilling and performing its obligations under each of the Material Permits held by it or to which it is bound.
Section 3.7 Material Contracts.
(a) Section 3.7(a) of the Company Disclosure Schedules sets forth a list of the following Contracts to which a Group Company is, as of the date of this Agreement, a party (each Contract required to be set forth on
A-33
Table of Contents
Section 3.7(a) of the Company Disclosure Schedules, together with each of the Contracts entered into after the date of this Agreement that would be required to be set forth on Section 3.7(a) of the Company Disclosure Schedules if entered into prior to the execution and delivery of this Agreement, collectively, the “Material Contracts”):
(i) any Contract relating to Indebtedness of any Group Company or to the placing of a Lien (other than any Permitted Lien) on any material assets or properties of any Group Company;
(ii) any Contract under which any Group Company is lessee of or holds or operates, in each case, any tangible property (other than real property), owned by any other Person, except for any lease or agreement under which the aggregate annual rental payments do not exceed $1,000,000;
(iii) any Contract under which any Group Company is lessor of or permits any third party to hold or operate, in each case, any tangible property (other than real property), owned or controlled by such Group Company, except for any lease or agreement under which the aggregate annual rental payments do not exceed $1,000,000;
(iv) any material joint venture, profit-sharing, partnership, collaboration, co-promotion, commercialization, research and development or other similar Contract (including any such Contract that governs the research, development, ownership, enforcement, use, or other exploitation of any Intellectual Property Rights or other assets, in each case which is material to the Business);
(v) any Contract that (A) limits or purports to limit, in any material respect, the freedom of any Group Company to engage or compete in any line of business or with any Person or in any area, to operate any asset or assets or that would so limit or purport to limit, in any material respect, the operations of Holdco or any of its Affiliates after the Closing, (B) contains any exclusivity, “most favored nation” or similar provisions, obligations or restrictions or (C) contains any other provisions restricting or purporting to restrict the ability of any Group Company to sell, manufacture, develop, commercialize, test or research the Company Products, directly or indirectly through third parties, or to solicit any potential employee or customer in any material respect or that would so limit or purports to limit, in any material respect, Holdco or any of its Affiliates after the Closing;
(vi) any Contract requiring any future capital commitment or capital expenditure (or series of capital expenditures) by any Group Company in an amount in excess of (A) $1,000,000 annually or (B) $2,500,000 over the life of the Contract;
(vii) any Contract requiring any Group Company to guarantee the Liabilities of any Person (other than any other Group Company) or pursuant to which any Person (other than any other Group Company) has guaranteed the Liabilities of a Group Company, in each case in excess of $1,000,000;
(viii) any Contract under which any Group Company has, directly or indirectly, made or agreed to make any loan, advance, or assignment of payment to any Person or made any capital contribution to, or other investment in, any Person other than a Group Company;
(ix) any Contract required to be disclosed on Section 3.20 of the Company Disclosure Schedules;
(x) any Contract with any Person (A) pursuant to which any Group Company may be required to pay milestones, royalties or other contingent payments based on any research, testing, development, regulatory filings or approval, sale, distribution, commercial manufacture or other similar occurrences, developments, activities or events or (B) under which any Group Company grants to any Person any right of first refusal, right of first negotiation, option to purchase, option to license or any other similar rights with respect to any Company Product or any Intellectual Property Rights;
A-34
Table of Contents
(xi) any Contract governing the terms of, or otherwise related to, the employment, engagement or services of any current director, manager, officer, employee, or Contingent Worker of a Group Company (A) whose annual base salary (or, in the case of a Contingent Worker, actual or anticipated annual base compensation) is in excess of $150,000 or (B) that provides for severance or any other post-termination payments or benefits;
(xii) any Contract governing the terms of, or otherwise related to, the employment, engagement or services of any former director, manager, officer, employee or Contingent Worker of a Group Company pursuant to which any Group Company, as of the Closing, has or will have an obligation to pay severance or other post-termination pay;
(xiii) any Contract providing for any Change of Control Payment of the type described in clause (a) of the definition thereof;
(xiv) any collective bargaining agreements and any other agreements executed with a union or similar organization;
(xv) any Contract for the disposition of any material portion of the assets or business of any Group Company or for the acquisition by any Group Company of any material assets or material business of any other Person (other than acquisitions or dispositions made in the ordinary course of business), or under which any Group Company has any continuing obligation with respect to an “earn-out,” contingent purchase price or other contingent or deferred payment obligation;
(xvi) any settlement, conciliation or similar Contract (A) the performance of which would be reasonably likely to involve any payments in excess of $2,000,000 in the aggregate after the date of this Agreement, (B) with a Governmental Entity or (C) that imposes or is reasonably likely to impose, at any time in the future, any material, non-monetary obligations on any Group Company (or Holdco or any of its Affiliates after the Closing); and
(xvii) any other Contract the performance of which requires either (A) annual payments to or from any Group Company in excess of $1,000,000 or (B) aggregate payments to or from any Group Company in excess of $2,500,000 over the life of the Contract and, in each case, that is not terminable by the applicable Group Company without penalty upon less than ninety (90) days’ prior written notice.
(b) (i) Each Material Contract is valid and binding on the applicable Group Company and, to the knowledge of the Company, the counterparty thereto, and is in full force and effect and (ii) the applicable Group Company and, to the knowledge of the Company, the counterparties thereto, are not in material breach of, or default under, any Material Contract. As of the date of this Agreement, no written notice of termination has been received by the Company with respect to any Material Contract, and to the knowledge of the Company, none of the other parties to any Material Contract has indicated to a Group Company that it intends to terminate the Material Contract or to terminate or reduce its business dealings with a Group Company.
Section 3.8 Absence of Changes. During the period beginning on January 1, 2021 and ending on the date of this Agreement, (a) no Company Material Adverse Effect has occurred and (b) except as expressly contemplated by this Agreement, any Ancillary Document or in connection with the Transactions, each Group Company has conducted its business in the ordinary course in all material respects (including, for the avoidance of doubt, recent past practice in light of COVID-19).
Section 3.9 Business Activities.
(a) To the knowledge of the Company, none of the Group Companies’ actions or inactions prior to the date of this Agreement and since January 1, 2020 in response to COVID-19: (i) has resulted in any Group Company experiencing any material business interruption or material losses; or (ii) if taken following the date of this Agreement would constitute a Company Material Adverse Effect.
A-35
Table of Contents
(b) Each of Holdco and Merger Sub was incorporated solely for the purpose of entering into this Agreement, the Ancillary Documents and consummating the Transactions and has not engaged in any activities or business, other than those incident or related to or incurred in connection with its organization, incorporation or formation, as applicable, or the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of its covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions.
Section 3.10 Litigation. As of the date of this Agreement there is, and since October 17, 2019, there has been no Proceeding pending or, to the Company’s knowledge, threatened against any Group Company that, if adversely decided or resolved, has been or would reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole. Neither the Group Companies nor any of their respective properties or assets is subject to any material Order. As of the date of this Agreement, there are no material Proceedings by a Group Company pending against any other Person.
Section 3.11 Compliance with Applicable Law. Each Group Company (a) conducts (and since the Company’s inception has conducted) its business in accordance with all Laws and Orders applicable to such Group Company and is not in violation of any such Law or Order and (b) has not received any written communications from a Governmental Entity that alleges that such Group Company is not in compliance with any such Law or Order, except in each case of clauses (a) and (b), as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
Section 3.12 Employee Plans.
(a) Section 3.12(a) of the Company Disclosure Schedules sets forth a true and complete list of all material Employee Benefit Plans (including, for each such Employee Benefit Plan, whether it is a Foreign Employee Benefit Plan). With respect to each material Employee Benefit Plan, the Group Companies have provided FLAC with true and complete copies of the material documents pursuant to which the plan is maintained, funded and administered.
(b) Each Employee Benefit Plan has been established and administered in all material respects in accordance with applicable Laws and with its terms. No Employee Benefit Plan is, or within the past three years has been, the subject of an application or filing under a government sponsored amnesty, voluntary compliance, or similar program. All material payments or contributions required to have been timely made with respect to all Employee Benefit Plans either have been made or have been accrued in accordance with the terms of the applicable Employee Benefit Plan and applicable Law, except as would result in a material liability to the Company.
(c) No Group Company nor any ERISA Affiliate has since the incorporation of the Company, contributed to, been required to contribute to or has any Liability (including on account of an ERISA Affiliate) with respect to or under (whether contingent or otherwise): (i) a Multiemployer Plan; (ii) a “defined benefit plan” (as defined in Section 3(35) of ERISA, whether or not subject to ERISA) or a plan that is or was subject to Title IV of ERISA Section 412 of the Code or Section 302 of ERISA; (iii) a “multiple employer plan” within the meaning of Section of 413(c) of the Code or Section 210 of ERISA; (iv) a “multiple employer welfare arrangement” as defined in Section 3(40) of ERISA; or (v) any funded welfare benefit plan within the meaning of Section 419 of the Code. No Group Company nor any ERISA Affiliate has ever incurred any liability under Title IV of ERISA that has not been paid in full. No Group Company has any material Liabilities to provide any retiree or post-termination or post-ownership health or life insurance or other welfare-type benefits to any Person other than health continuation coverage pursuant to COBRA or similar Laws and for which the recipient pays the full cost of coverage and no Group Company has ever promised to provide such benefits. No Group Company has any material Liabilities by reason of at any time being considered a single employer under Section 414 of the Code with any other Person.
A-36
Table of Contents
(d) Each Employee Benefit Plan that is intended to be qualified under Section 401(a) of the Code is so qualified and has timely received a favorable determination or approval from the IRS with respect to such qualification, or may rely on an opinion or advisory letter issued by the IRS with respect to a volume submitter or prototype plan adopted in accordance with the requirements for such reliance, or has time remaining for application to the IRS for a determination of the qualified status of such Employee Benefit Plan for any period for which such Employee Benefit Plan would not otherwise be covered by an IRS determination and, to the knowledge of the Company, no event or omission has occurred that would cause any Employee Benefit Plan to lose such qualification or require corrective action to the IRS or EPCRS to maintain such qualification. Since the incorporation of the Company, none of the Group Companies has incurred (whether or not assessed) any material penalty or Tax under Section 4980H, 4980B, 4980D, 6721 or 6722 of the Code.
(e) No Proceeding (other than those relating to routine claims for benefits) is pending or, to the knowledge of the Company, threatened with respect to any Employee Benefit Plan and, to the knowledge of the Company, there is no reasonable basis for any such Proceeding.
(f) Except as required by applicable Law, no Group Company has announced its intention, in any material respect, to modify or terminate any Employee Benefit Plan or adopt any arrangement or program which, once established, would come within the definition of an Employee Benefit Plan.
(g) Neither the execution and delivery of this Agreement nor the consummation of the Transactions will (i) result in any payment or benefit becoming due to or result in the forgiveness of any Indebtedness of any current or former director, manager, officer, employee, individual independent contractor or other service providers of any of the Group Companies, (ii) increase the amount or value of any compensation or benefits payable to any current or former director, manager, officer, employee, individual independent contractor or other service providers of any of the Group Companies, (iii) result in the acceleration of the time of payment or vesting, or trigger any payment or funding of any compensation or benefits to any current or former director, manager, officer, employee, individual independent contractor or other service providers of any of the Group Companies, or (iv) further restrict any rights of the Group Companies to amend or terminate any Employee Benefit Plan.
(h) No amount that could be received (whether in cash or property or the vesting of property) by any “disqualified individual” of any of the Group Companies under any Employee Benefit Plan or otherwise as a result of the consummation of the Transactions could, separately or in the aggregate, be nondeductible under Section 280G of the Code or subjected to an excise tax under Section 4999 of the Code.
(i) The Group Companies have no obligation to make a “gross-up” or similar payment in respect of any taxes that may become payable under Section 4999 or 409A of the Code.
(j) Any transfer of property to a current or former employee who is a U.S. taxpayer which was subject to a substantial risk of forfeiture and which would otherwise have been subject to taxation under Section 83(a) of the Code is, to the Company’s knowledge, covered by a valid and timely filed election under Section 83(b) of the Code, and a copy of such election has been provided to the Company.
(k) Each Employee Benefit Plan that constitutes in any part a nonqualified deferred compensation plan within the meaning of Section 409A of the Code has been operated and maintained in all material respects in operational and documentary compliance with Section 409A of the Code and applicable guidance thereunder. No payment to be made under any Employee Benefit Plan is, or to the knowledge of the Company, will be, subject to the penalties of Section 409A(a)(1) of the Code.
(l) Each Foreign Benefit Plan that is required to be registered or intended to be tax exempt has been registered (and, where applicable, accepted for registration) and is tax exempt and has been maintained in good standing, to the extent applicable, with each applicable Governmental Entity. No Foreign Benefit Plan is a
A-37
Table of Contents
“defined benefit plan” (as defined in ERISA, whether or not subject to ERISA) or has any material unfunded or underfunded Liabilities. All material payments and contributions required to have been made by or on behalf of the Group Companies with respect to all Foreign Benefit Plans either have been timely made or have been accrued in all material respects in accordance with the terms of the applicable Foreign Benefit Plan and applicable Law, including plans or arrangements maintained or sponsored by a Governmental Entity.
Section 3.13 Environmental Matters. Except as would not have a Company Material Adverse Effect:
(a) Each Group Company is (and where still relevant, since the incorporation of the Company, was) in compliance with all applicable material Environmental Laws (including whether applicable to its operations and the use or condition of any real property currently or formerly owned or leased, including the Leased Real Property.
(b) None of the Group Companies has received any written notice or communication from any Governmental Entity or any other Person regarding any actual, alleged, or potential violation or remediation requirement in any respect of, or a failure to comply in any respect with, any Environmental Laws.
(c) There is (and since the incorporation of the Company there has been) no Proceeding pending or, to the Company’s knowledge, threatened in writing against any Group Company pursuant to Environmental Laws.
(d) The Group Companies have not manufactured, released, treated, stored, disposed of, arranged for disposal of, transported or handled, or to the knowledge of the Company, contaminated, or exposed, any Person or any real property currently or formerly owned or leased (including the Leased Real Property) to, any Hazardous Substances.
(e) To the knowledge of the Company, there are no underground storage tanks, landfills, current or former waste disposal areas or polychlorinated biphenyls or any other condition or contamination at or on any material real property currently or formerly owned or leased (including the material Leased Real Property) by a Group Company that require reporting, investigation, cleanup, remediation or any other type of response action by a Group Company pursuant to any Environmental Laws.
(f) The Group Companies have made available to FLAC copies of all material environmental, health and safety reports and documents that are in any Group Company’s possession or control relating to the current or former operations, properties or facilities of the Group Companies.
Section 3.14 Intellectual Property.
(a) Section 3.14(a) of the Company Disclosure Schedules sets forth, as of the date of this Agreement, a true and complete list of all (i) Company Registered Intellectual Property and (ii) material unregistered Marks owned by any Group Company. Section 3.14(a) of the Company Disclosure Schedules lists, for each item of Company Registered Intellectual Property as of the date of this Agreement (A) the record owner(s) of such item, (B) the jurisdictions in which such item has been issued or registered or filed, (C) the issuance, registration or application date, as applicable, for such item, (D) deadlines due prior to December 31, 2022 and (E) whether such Company Registered Intellectual Property is subject to any Contract or other present or contingent material obligation as a result of any funding or support from, or any arrangement with, any Governmental Entity, nonprofit organization or educational institution. The Group Companies have no material in-licensed Intellectual Property Rights.
(b) As of the date of this Agreement, to the Company’s knowledge, all necessary fees and filings with respect to any material Company Registered Intellectual Property have been submitted to the relevant intellectual property office, Governmental Entity or Internet domain name registrar to maintain such material Company Registered Intellectual Property in full force and effect. As of the date of this Agreement, no issuance or
A-38
Table of Contents
registration obtained and no application filed by the Group Companies for any material Intellectual Property Rights has been cancelled, abandoned, allowed to lapse or not renewed, except where such Group Company has decided to cancel, abandon, allow to lapse or not renew such issuance, registration or application. Except as set forth on Section 3.14(b) of the Company Disclosure Schedules, as of the date of this Agreement, there are no material Proceedings, including litigations, interference, re-examination, inter partes review, reissue, opposition, nullity, cancellation or similar administrative proceedings pending that relate to any of the Company Registered Intellectual Property and, to the Company’s knowledge, (i) no such material Proceedings are threatened by any Governmental Entity or any other Person and (ii) as of the date of this Agreement, there are no facts or circumstances that would reasonably be expected to give rise to any such material Proceeding.
(c) A Group Company exclusively owns, as indicated in Section 3.14(a) of the Company Disclosure Schedules, all right, title and interest in and to all material Company Owned Intellectual Property, free and clear of all Liens or obligations to others (other than Permitted Liens). For all Company Registered Intellectual Property, each inventor on the Patent has assigned their rights in writing to a Group Company or to an assignor to a Group Company and such assignments have been or shall be, upon Closing, recorded with the United States Patent and Trademark Office or relevant foreign intellectual property office, as applicable. Except as set forth on Section 3.14(c) of the Company Disclosure Schedules, no Group Company has transferred ownership of, or granted any exclusive license with respect to, any material Company Owned Intellectual Property used in the Business as currently conducted to any other Person. Section 3.14(c) of the Company Disclosure Schedules sets forth a list of all current Company Owned Intellectual Property as of the date of this Agreement to which any Person has been granted any license or covenant not to sue under, or otherwise has received or acquired any right (whether or not exercisable) or interest in, any Company Owned Intellectual Property, other than non-disclosure agreements and nonexclusive licenses granted in the ordinary course of business to vendors or suppliers of any Group Company, including any contract research organizations or contract manufacturing organizations.
(d) To the knowledge of the Company, (i) the Company Owned Intellectual Property constitute all of the material Intellectual Property used in or necessary for the operation of the Group Companies’ respective businesses as currently conducted and presently proposed to be conducted as of the date of this Agreement, and (ii) all Company Owned Intellectual Property will survive the consummation of the Transactions, in each case, without modification, cancellation, termination, suspension of, or acceleration of any right, obligation or payment with respect thereto; provided, however, that the Group Companies’ rights in owned Patents and Patent applications may be subject to ordinary course modifications, cancellations, terminations, suspensions of, accelerations of rights, obligations or payments by the Company in the ordinary course of prosecution in the United States Patent and Trademark Office or a foreign Patent office in a manner consistent with past practices.
(e) Except as set forth on Section 3.14(e) of the Company Disclosure Schedules, the Company Registered Intellectual Property is subsisting and, to the knowledge of the Company, is valid and enforceable (except for applications for Registered Intellectual Property that have not issued). To the Company’s knowledge, all of the Group Companies’ rights in and to Company Registered Intellectual Property and Company Owned Intellectual Property, are valid and enforceable (except for applications for Registered Intellectual Property that have not issued). No representation or warranty in this Section 3.14(e) shall apply to infringement of any Intellectual Property Rights.
(f) Each Group Company’s current and, to the Company’s knowledge, former, employees, consultants, advisors and independent contractors, in each case who independently or jointly contributed to or otherwise participated in the authorship, invention, creation, improvement, modification or development of any material Company Owned Intellectual Property have entered into a valid and enforceable written Contract whereby such Person has (i) agreed to maintain and protect the confidential information of all Group Companies, and (ii) assigned to such Group Company all such Intellectual Property Rights authored, invented, created, improved, modified or developed by such Person in the course of such Person’s employment or other engagement with such Group Company.
A-39
Table of Contents
(g) Each Group Company has taken reasonable steps to safeguard and maintain the secrecy of any trade secrets, know-how and other confidential information included in the Company Owned Intellectual Property. Without limiting the foregoing, to the Company’s knowledge, each Group Company has not disclosed or made available any trade secrets, know-how or confidential information material to the Business to any other Person other than pursuant to a written non-disclosure agreement containing appropriate limitations on use, reproduction and disclosure. To the Company’s knowledge, there has been no violation or unauthorized access to or disclosure of any trade secrets, know-how or confidential information included in the Company Owned Intellectual Property.
(h) None of the Company Owned Intellectual Property is subject to any outstanding Order that restricts in any material respect the use, sale, transfer, licensing or exploitation thereof by the Group Companies or affects in any material respect the validity, use or enforceability of any such Company Owned Intellectual Property, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
(i) To the Company’s knowledge, neither the conduct of the business of the Group Companies nor any Company Product made, used, marketed, licensed, provided, sold, offered for sale, distributed, imported or otherwise exploited by the Group Companies infringes, misappropriates, or otherwise violates any Intellectual Property Rights of any other Person, in each case, except as has not resulted in and would not reasonably be expected to result in, individually or in the aggregate, any material liability or disruption to the Business.
(j) As of the date of this Agreement, there is not and, to the knowledge of the Company, there has not been any material Proceeding pending nor has any Group Company received any written communications (i) alleging that a Group Company has infringed, misappropriated or otherwise violated any Intellectual Property Rights of any other Person, (ii) challenging the validity, enforceability, use or exclusive ownership of any material Company Owned Intellectual Property or (iii) pertaining to an unsolicited offer or demand for any Group Company to take a license under any Patent or consider the applicability of any Patents to any products or services of the Group Companies or to the Business.
(k) To the Company’s knowledge, no Person is infringing misappropriating, misusing, diluting or violating, or has infringed, misappropriated, misused, diluted or violated, any Company Owned Intellectual Property in any material respect. No Group Company is currently investigating or has made any written claim or filed any Proceeding against any Person alleging any infringement, misappropriation or other violation of any Company Owned Intellectual Property, or has invited any Person to take a license under any Company Owned Intellectual Property.
(l) Section 3.14(l) of the Company Disclosure Schedules sets forth all material Public Software that is incorporated or embedded in, or linked to, any proprietary Software of a Group Company by any Group Company as of the date of this Agreement. The Group Companies are in material compliance with all licenses governing such Public Software. No Group Company has accessed, used, modified, linked to, created derivative works from or incorporated into any proprietary Software that constitutes a product or service offered by a Group Company or is otherwise considered Company Owned Intellectual Property and that is distributed or made available for remote access outside of the Group Companies, or is otherwise used in a manner that may trigger or subject such Group Company to any obligations set forth in the license for such Public Software, any Public Software, in whole or in part, in each case in a manner that (i) requires any Company Owned Intellectual Property to be licensed, sold, disclosed, distributed, hosted or otherwise made available, including in source code form or for the purpose of making derivative works, for any reason, (ii) grants, or requires any Group Company to grant, the right to decompile, disassemble, reverse engineer or otherwise derive the source code or underlying structure of any Company Owned Intellectual Property, (iii) limits in any manner the ability to charge license fees or otherwise seek compensation in connection with marketing, licensing or distribution of any Company Owned Intellectual Property or (iv) otherwise imposes any limitation, restriction or condition on the right or ability of any Group Company to use, hold for use, license, host, distribute or otherwise dispose of any Company
A-40
Table of Contents
Owned Intellectual Property, other than compliance with notice and attribution requirements, in each case, except as is not and would not reasonably be expected to materially and adversely affect the value, use, enforceability, or the Group Company’s ownership rights in any Company Owned Intellectual Property.
Section 3.15 Labor Matters.
(a) Section 3.15(a) of the Company Disclosure Schedules contains a true and complete list of pseudonymized data of each employee of each Group Company as of the date of this Agreement, setting forth for each employee: (i) whether classified as exempt or non-exempt for wage and hour purposes; (ii) whether paid on a salary, hourly or commission basis; (iii) the employee’s actual annual base salary (if paid on a salary basis), hourly rate (if paid on an hourly basis), or commission rate (if paid on a commission-only basis), as applicable; (iv) bonus and commission potential; (v) date of hire; (vi) work location; (vii) the entity that employs the individual; and (viii) the total amount of Change of Control Payment to be paid to such employee at the Closing or otherwise in connection with the Transactions.
(b) Section 3.15(b) of the Company Disclosure Schedules contains a true and complete list of pseudonymized data of all Contingent Workers of each Group Company as of the date of this Agreement, setting forth for each such individual: (i) a description of his, her, or its services rendered, (ii) the start date of the engagement, (iii) the applicable notice period, (iv) the service fee and compensation (including bonus entitlements) granted to the individual Contingent Worker, and (v) the primary location (e.g., U.S. state) from which services are performed.
(c) To the Company’s knowledge, none of the Contingent Workers has claimed an employment relationship and no third parties have claimed the reclassification of the contractual relationship between any Contingent Worker and any Group Company.
(d) To the Company’s knowledge, none of the Group Companies engages Contingent Workers who have been hired through third parties that are being or should have been treated for Tax purposes as employees of the Group Companies.
(e) None of the Group Companies provided a proposal, assurance or commitment to any employee or Contingent Worker regarding any change to his/her terms of employment or working conditions or regarding the continuance, introduction, increase or improvement of any benefit or any structural or discretionary arrangement or practice and no negotiations have commenced for any such matter.
(f) None of the Group Companies has established, nor is in the process of establishing a works council or other co-determination body, nor has a legal or contractual obligation to do so and no employee, trade union or other party has requested the establishment of any such body.
(g) Each Group Company currently classifies and has classified for the past three (3) years each of its employees as exempt or non-exempt in compliance with the Fair Labor Standards Act and applicable state, provincial, local and foreign wage and hour Laws, and is and has been otherwise in compliance in all material respects with such Laws. To the extent that any Contingent Workers are or were engaged by any Group Company, such Group Company currently classifies and treats them, and has properly classified and treated them for the past three (3) years, as Contingent Workers (as distinguished from employees) in compliance in all material respects with applicable Law and for the purpose of all Employee Benefit Plans and perquisites.
(h) Each Group Company is, and since the incorporation of the Company has been, in material compliance with all applicable Laws and regulations respecting labor and employment matters, including fair employment practices, pay equity, the classification of independent contractors, the classification of employees and Contingent Workers, workplace safety and health, work authorization and immigration, unemployment compensation, workers’ compensation, accommodation of disabilities, discrimination, harassment,
A-41
Table of Contents
whistleblowing, retaliation, affirmative action, background checks, prevailing wages, terms and conditions of employment, child labor, reductions in force, employee leave and wages and hours, including payment of minimum wages and overtime. No Group Company is delinquent in any payments to any employee or Contingent Worker for any wages, salaries, commissions, bonuses, severance, fees or other direct compensation due with respect to any services performed for it or amounts required to be reimbursed to such employees or Contingent Workers.
(i) Since the incorporation of the Company, (i) no Group Company (A) has or has had any material Liability for any arrears of wages or other compensation for services (including salaries, wage premiums, commissions, fees or bonuses), or any penalty or other sums for failure to comply with any of the foregoing, and (B) has or has had any material Liability for any failure to pay into any trust or other fund governed by or maintained by or on behalf of any Governmental Entity with respect to unemployment compensation benefits, social security, social insurances or other benefits or obligations for any employees of any Group Company (other than routine payments to be made in the ordinary course of business); and (ii) each Group Company has withheld all amounts required by applicable Law or by agreement to be withheld from wages, salaries and other payments to employees or Contingent Workers of each Group Company, except as has not and would not reasonably be expected to result in, individually or in the aggregate, material Liability to the Group Companies.
(j) Since the incorporation of the Company, no Group Company has experienced a “mass layoff” or “plant closing” as defined by WARN, and no Group Company has incurred any material Liability under WARN.
(k) No Group Company is a party to, bound by, or negotiating any collective bargaining agreements, work rules or practices, or other agreements or Contracts with any labor organization, labor union, works council or other Person purporting to act as exclusive bargaining representative (“Union”) of any employees or Contingent Workers with respect to the wages, hours or other terms and conditions of employment of any employee or Contingent Worker, nor is there any duty on the part of any Group Company to bargain with any Union. In the past three (3) years, there has been no actual or, to the Company’s knowledge, threatened unfair labor practice charges, material grievances, arbitrations, strikes, lockouts, work stoppages, slowdowns, picketing, hand billing or other material labor disputes against or affecting any Group Company. To the Company’s knowledge, in the past three (3) years, there have been no labor organizing activities with respect to any employees of any Group Company nor has the Company engaged in any unfair labor practice.
(l) No employee layoff, facility closure or shutdown (whether voluntary or by Order), reduction-in-force, furlough, temporary layoff, material work schedule change or reduction in hours, or reduction in salary or wages, or other workforce changes affecting employees of the Group Companies has occurred within the past six (6) months or is currently contemplated, planned or announced, including as a result of COVID-19 or any applicable employment-related COVID-19 Measure. Except as would not reasonably be expected to result in, individually or in the aggregate, material Liability to the Group Companies, each Group Company has complied in all material respects with (i) all applicable employment-related COVID-19 Measures including all applicable COVID-19 related Laws, regulations, orders and guidance of any Governmental Entity; and (ii) the Families First Coronavirus Response Act (including with respect to eligibility for tax credits under such Families First Coronavirus Response Act) and any other applicable COVID-19 related leave Law.
(m) Except as set forth on Section 3.15(m) of the Company Disclosure Schedules, in the past twelve (12) months (i) no director, officer, or key employee’s employment with any Group Company has been terminated or furloughed for any reason; and (ii) to the knowledge of the Company, no director, officer, or management level or key employee, or group of employees or Contingent Workers, has provided notice of any plans to terminate his, her or their employment or service arrangement with any Group Company.
(n) Currently and within the three (3) years preceding the date of this Agreement, no Group Company has been a party to any Proceeding, settlement, or out-of-court or pre-charge or pre-litigation arrangement, in each case relating to employment or labor matters concerning the employees or Contingent Workers of any
A-42
Table of Contents
Group Company (including those concerning allegations of employment discrimination, retaliation, breach of contract, noncompliance with wage and hour Laws, the misclassification of employees or Contingent Workers, violation of restrictive covenants, sexual or other harassment or misconduct, other unlawful harassment, or unfair labor practices), and no such matters are pending or, to the knowledge of the Company, threatened against any Group Company or any employees or Contingent Workers of any Group Company (in their respective capacity as employees or Contingent Workers of any Group Company), as applicable.
(o) Except as set forth on Section 3.15(o) of the Company Disclosure Schedules, each U.S. employee of each Group Company is employed at-will and no employee is subject to any employment contract with any Group Company, whether oral or written, for a fixed term of employment with any Group Company.
(p) No Group Company reasonably expects any material liabilities with respect to any sexual harassment, or other discrimination, retaliation or policy violation allegations, nor, to the Company’s knowledge, are there any such allegations relating to officers, directors, employees, contractors, or agents of the Group Companies, that, if known to the public, would reasonably be expected to bring material economic harm to the Group Companies.
(q) No Group Company (i) is subject to any affirmative action obligations under any Law, including Executive Order 11246, or (ii) is a government contractor or subcontractor for purposes of any Law with respect to the terms and conditions of employment, including the Service Contracts Act or prevailing wage Laws.
(r) There are no outstanding assessments, payments of damages, penalties, fines, Liens, charges, surcharges, or other amounts due or owing pursuant to any workplace safety and insurance legislation and no Group Company has been reassessed in any material respect under such legislation during the past three (3) years and, to the knowledge of the Company, no audit of any Group Company is currently being performed pursuant to any applicable workplace safety and insurance legislation.
Section 3.16 Insurance. Section 3.16 of the Company Disclosure Schedules sets forth a list of all material policies of fire, liability, workers’ compensation, property, casualty and other forms of insurance owned or held by any Group Company as of the date of this Agreement (the “Insurance Policies”). All Insurance Policies are in full force and effect, all premiums due and payable thereon as of the date of this Agreement have been paid in full as of the date of this Agreement and, to the extent applicable, the Company has not taken any action or failed to take any action that (including with respect to the Transactions), with or without notice, lapse of time or both, would constitute or result in a breach or violation of, or default under, any of the Insurance Policies or would permit or cause the termination, non-renewal or modification thereof or acceleration or creation of any right or obligation thereunder, and true and complete copies of all such Insurance Policies have been made available to FLAC. No claim by any Group Company is pending under any such Insurance Policies as to which coverage has been denied or disputed, or rights reserved to do so, by the underwriters thereof, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
Section 3.17 Tax Matters.
(a) Each Group Company has prepared and filed all income and other material Tax Returns required to have been filed by it, all such Tax Returns are true and complete in all material respects and prepared in compliance in all material respects with all applicable Law, and each Group Company has paid all material Taxes required to have been paid by it regardless of whether shown on a Tax Return, and has paid all assessments and reassessments in respect of Taxes in all material respects. The Company Financial Statements reflect all Liabilities for unpaid Taxes of the Group Companies for periods (or portions of periods) through the date of such statements in accordance with the requirements of IFRS.
(b) Each Group Company has (i) timely withheld and paid to the appropriate Governmental Entity all material amounts required to have been withheld and paid in connection with amounts paid or owing to any
A-43
Table of Contents
employee, individual independent contractor, other service providers, equity interest holder or other third party, (ii) remitted, or will remit on a timely basis, such amounts to the appropriate Governmental Entity and (iii) complied in all material respects with applicable Law with respect to Tax withholding, including all reporting and record keeping requirements.
(c) To the Company’s knowledge, no Group Company is currently the subject of a Tax audit or examination with respect to material Taxes. No Group Company has been informed in writing of a Tax audit or examination or the commencement or anticipated commencement of any Tax audit or examination that has not been resolved or completed, in each case with respect to material Taxes.
(d) No Group Company has consented to extend or waive the time in which any material Tax may be assessed or collected by any Governmental Entity, other than any such extensions or waivers that are no longer in effect or that were extensions of time to file Tax Returns obtained in the ordinary course of business, in each case with respect to material Taxes.
(e) No rulings in respect of Tax or similar Tax agreements have been entered into or issued by any Governmental Entity with respect to a Group Company which agreement or ruling would be effective after the Closing Commencement Date.
(f) No Group Company is or has been a party to any “listed transaction” as defined in Section 6707A of the Code and Section 1.6011-4 of the Treasury Regulations (or any corresponding or similar provision of state, local, or non-U.S. income Tax Law).
(g) There are no Liens for material Taxes on any assets of the Group Companies other than Permitted Liens.
(h) During the two (2)-year period ending on the date of this Agreement, no Group Company was a distributing corporation or a controlled corporation in a transaction purported or intended to be governed by Section 355 of the Code.
(i) No Group Company organized outside of the United States is treated as an “expatriated entity” as defined in Section 7874(a)(2)(A) of the Code, a “surrogate foreign corporation” as defined in Section 7874(a)(2)(B) of the Code or a domestic corporation as a result of the application of Section 7874(b) of the Code, in each case as defined in the Code as in effect on the date of this Agreement.
(j) Since January 1, 2020, no written claim has been made by a Governmental Entity in a jurisdiction where any Group Company does not file Tax Returns that such Group Company is or may be subject to taxation by that jurisdiction.
(k) No Group Company (i) has been a member of an affiliated group filing a consolidated Tax Return (other than a group the common parent of which was a Group Company or any of its current Affiliates) or (ii) has any material Liability for the Taxes of any Person (other than a Group Company or any of its current Affiliates) under Section 1.1502-6 of the Treasury Regulations (or any similar provision of state, local or non-U.S. Law), as a transferee or successor or by Contract (other than any Contract the principal purpose of which does not relate to Taxes).
(l) No Group Company is a party to any Tax allocation, Tax sharing or Tax indemnity or similar agreements (other than in respect of the existing fiscal unities for Dutch tax purposes or one that is included in a Contract entered into in the ordinary course of business that is not primarily related to Taxes) and no Group Company is a party to any joint venture, partnership or other arrangement that is treated as a partnership for U.S. federal income Tax purposes.
A-44
Table of Contents
(m) Each Group Company is a tax resident only in its jurisdiction of organization, incorporation or formation, as applicable.
(n) No Group Company has a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than in its jurisdiction of incorporation.
(o) The Company immediately prior to the Closing will not be treated as an “investment company” within the meaning of Section 351(e) or 368(a)(2)(F) of the Code.
(p) To the knowledge of the Company, (i) no Group Company has been a party to any transaction or series of transactions which is or forms part of a scheme for the avoidance of Tax which can reasonably be considered as such and (ii) none of the Group Companies was required to make any filings to a Tax authority under Council Directive (EU) 2018/822 of 25 May 2018 amending Directive 2011/16/EU for any transaction or arrangement to which the Company is a party.
(q) No Group Company has taken or agreed to take any action not contemplated by this Agreement or any Ancillary Document that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment. To the knowledge of the Company, no facts or circumstances exist, other than any facts or circumstances to the extent that such facts or circumstances exist or arise as a result of or are related to any act or omission of FLAC or any of its Affiliates occurring after the date hereof and not contemplated by this Agreement or any of the Ancillary Documents, that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment.
(r) Notwithstanding anything to the contrary in this Agreement, no Group Company makes any representations as to the amount of, or the limitations on the use after the Closing, of any net operating losses, capital losses, deductions, Tax credits and similar items of the Group Companies.
Section 3.18 Brokers. Except for fees due to the Persons whose names are set forth on Section 3.18 of the Company Disclosure Schedules (which fees shall be the sole responsibility of the Company, except as otherwise provided in Section 8.6), no broker, finder, investment banker or other Person is entitled to any brokerage fee, finders’ fee or other commission in connection with the Transactions based upon arrangements made by or on behalf of the Company or any of its Affiliates for which any of the Group Companies has any obligation. The Company has made available to FLAC true and complete copies of all Contracts pursuant to which it is required to make payments to Persons set forth on Section 3.18 of the Company Disclosure Schedules.
Section 3.19 Real and Personal Property.
(a) Owned Real Property. No Group Company owns any real property.
(b) Leased Real Property. Section 3.19(b) of the Company Disclosure Schedules sets forth a true and complete list (including street addresses) of all real property leased by any of the Group Companies (the “Leased Real Property”) and all Real Property Leases pursuant to which any Group Company is a tenant or landlord (or sub-tenant or sub-landlord) as of the date of this Agreement. True and complete copies of all such Real Property Leases have been made available to FLAC. Each Real Property Lease is in full force and effect and is a valid, legal and binding obligation of the applicable Group Company party thereto, enforceable in accordance with its terms against such Group Company and, to the Company’s knowledge, each other party thereto (subject to the Enforceability Exceptions). There is no material breach or default by any Group Company or, to the Company’s knowledge, any third party under any Real Property Lease, and, to the Company’s knowledge, no event has occurred which (with or without notice or lapse of time or both) would constitute a material breach or default or would permit termination of, or a material modification of or acceleration of rent under any Real Property Lease. No Group Company has leased, subleased, licensed or granted occupancy rights in any parcel or any portion of
A-45
Table of Contents
any parcel of Leased Real Property to any other Person and no other Person has any rights to the use, occupancy or enjoyment thereof pursuant to any lease, sublease, license, occupancy or other agreement, nor has a Group Company assigned its interest under any Real Property Lease to any third party, in each case, in any material respects. The Leased Real Property constitutes all of the material real property used or occupied by the Company in connection with the conduct of the Business.
(c) Personal Property. Each Group Company has good, marketable and indefeasible title to, or a valid leasehold interest in or license or right to use, all of the material tangible assets and properties of the Group Companies reflected in the Company Financial Statements or thereafter acquired by the Group Companies, except for assets disposed of in the ordinary course of business. Such assets and properties are free and clear of any Liens (other than Permitted Liens or Liens that do not materially and adversely affect the operation of the Business).
(d) Tangible Assets. The material tangible assets owned, leased or otherwise used by a Group Company are in good condition (except for ordinary wear and tear).
Section 3.20 Transactions with Affiliates. Section 3.20 of the Company Disclosure Schedules sets forth (a) all Contracts between (x) any Group Company, on the one hand, and (y) any officer, director, employee, partner, member, manager, direct or indirect equityholder or Affiliate of any Group Company (other than, for the avoidance of doubt, any other Group Company) or any child, stepchild, grandchild, parent, stepparent, grandparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law (including in each of the foregoing cases any adoptive relationships) of the foregoing Persons, on the other hand (each Person identified in this clause (y), a “Company Related Party”), other than (i) Contracts with respect to a Company Related Party’s employment with (including benefit plans and other ordinary course compensation from) any of the Group Companies entered into in the ordinary course of business, (ii) Contracts related solely to a Company Shareholder’s or a holder of Company Equity Awards’ status as a holder of Equity Securities of the Company entered into in the ordinary course of business, (iii) Contracts entered into after the date of this Agreement that are either permitted pursuant to Section 5.1(b) or entered into in accordance with Section 5.1(b) and (iv) the Ancillary Documents and any other Contracts that the Group Companies are expressly required to enter into pursuant to this Agreement; and (b) all Contracts that, following the Closing, would be required to be disclosed in FLAC’s filings with the SEC as a “related party transaction” under Federal Securities Laws. No Company Related Party (A) owns any interest in any material asset used in any Group Company’s business, (B) possesses, directly or indirectly, any material financial interest in, or is a director or executive officer of, any Person which is a supplier, lender, partner, lessor, lessee or other material business relation of any Group Company or (C) owes any material amount to, or is owed any material amount by, or has any claim or cause of action against, any Group Company (other than ordinary course accrued compensation, employee benefits, employee or director expense reimbursement or other transactions entered into after the date of this Agreement that are either permitted pursuant to Section 5.1(b) or entered into in accordance with Section 5.1(b)). All Contracts, interests and other matters that are required to be set forth on Section 3.20 of the Company Disclosure Schedules are referred to herein as “Company Related Party Transactions.”
Section 3.21 Data Privacy and Security.
(a) Each Group Company complies, and has at all times since the incorporation of the Company complied in all material respects with all: (i) applicable Privacy Laws; (ii) privacy and data security policies and procedures adopted by the Group Company and applicable contractual obligations relating to the receipt, collection, compilation, use, storage, Processing, sharing, safeguarding, security (technical, physical and administrative), disposal, destruction, disclosure, or transfer (including cross-border) of Personal Data; and (iii) to the extent applicable, Payment Card Industry Data Security Standard and all other applicable requirements of the payment card brands (the items described in the foregoing clauses (i) (ii), and (iii), collectively, the “Privacy Requirements”). The Group Companies have not supplied or provided access to Personal Data Processed by them to a third party for remuneration or other consideration in violation of Privacy Requirements.
A-46
Table of Contents
(b) No Group Company has received written notice, or to the Company’s knowledge, any other notice, of any pending Proceedings, nor has there been any Proceedings since the incorporation of the Company against any Group Company initiated by (i) any Person; (ii) the United States Federal Trade Commission, any state attorney general or similar state official; (iii) any other Governmental Entity, foreign or domestic; or (iv) any regulatory or self-regulatory entity that, in each case of clauses (i) to (iv), allege that any Processing of Personal Data by or on behalf of a Group Company is in violation of any applicable Privacy Requirements or that relate to any data security incidents, ransomware incidents, or violations of any Privacy Requirements and, to the Company’s knowledge, there are no facts or circumstances which could reasonably serve as the basis of any such Proceeding.
(c) Each Group Company has taken reasonable organizational, physical, administrative, and technical measures consistent with standards prudent in the industry in which the Group Company operates to protect all Personal Data and all other data used in the conduct of the business that is owned, controlled, or stored by each Group Company from and against data security incidents or, in the case of Personal Data, other misuse. Each Group Company has implemented reasonable procedures to detect data security incidents and to protect Personal Data against loss and against unauthorized access, use, modification, disclosure, or other misuse.
(d) In connection with each third-party servicing, outsourcing, Processing, or otherwise using Personal Data collected, held, or Processed by or on behalf of the Group Companies, the applicable Group Company has obligated any such third party to comply with applicable Privacy Laws with respect to Personal Data and take appropriate steps to protect and secure Personal Data from data security incidents, in each case to the extent required under Privacy Requirements and except as would not have a Company Material Adverse Effect.
(e) Since the incorporation of the Company, each Group Company has implemented and maintained, consistent with practices reasonable in the industry in which the Group Companies operate, security measures to protect the Company IT Systems used by any Group Company to store, Process or transmit Intellectual Property Rights of the Company or Personal Data from loss, theft, unauthorized access, use, disclosure or modification except as would not have a Company Material Adverse Effect. To the Company’s knowledge, none of the Company IT Systems contain any Malicious Code that materially disrupt or materially adversely affect the functionality of any Company IT Systems or Company Products.
(f) Since the incorporation of the Company and to the Company’s knowledge (i) there has been no unauthorized Processing of Personal Data in the possession or control of any Group Company or, any of the service providers of any Group Company and (ii) there have been no unauthorized intrusions or breaches of security of any Company IT Systems.
(g) The consummation of any of the Transactions will not violate any applicable Privacy Requirements as they currently exist or as they existed at any time during which any of the Personal Data was collected or obtained.
Section 3.22 Compliance with International Trade & Anti-Corruption Laws.
(a) None of the Group Companies, their directors and officers or, to the Company’s knowledge, any of their employees, is or has been, since the incorporation of the Company, (i) a Person named on any Sanctions and Export Control Laws-related list of designated Persons maintained by a Governmental Entity; (ii) located, organized or resident in a country or territory which is itself the subject of or target of any Sanctions and Export Control Laws; (iii) an entity 50% or more owned, directly or indirectly, by one or more Persons described in clause (i) or (ii); or (iv) otherwise engaging in dealings with or for the benefit of any Person described in clauses (i) through (iii) or any country or territory which is itself the subject of or target of any Sanctions and Export Control Laws (at the time of this Agreement, the Crimea, Luhansk People’s Republic, and Donetsk People’s Republic regions of Ukraine, Cuba, Iran, North Korea and Syria), to the extent in violation of Sanctions and Export Control Laws.
A-47
Table of Contents
(b) Since the incorporation of the Company, none of the Group Companies, their directors and officers or, to the Company’s knowledge, any of their employees, has (i) violated or has caused the Company or any of the Group Companies to be in violation of any applicable Anti-Corruption Law, or (ii) made, offered, promised, paid or received any unlawful bribes, kickbacks or improper payments, to any official or employee of any Governmental Entity, or to any domestic or foreign political party or candidate for political office, in violation of Law or for the purpose of influencing any act or decision of such official or of any Governmental Entity to obtain or retain business or direct business to any Person in violation of Law.
(c) Since the incorporation of the Company, none of the Group Companies, their directors and officers or, to the Company’s knowledge, any of their employees has, directly or indirectly, violated any, or been subject to actual or, to the knowledge of the Company, pending or threatened Proceedings, demand letters, settlements or enforcement actions relating to any applicable Anti-Corruption Law.
(d) Since the incorporation of the Company, the Company has complied with all applicable Anti-Corruption Laws.
Section 3.23 Information Supplied. None of the information supplied or to be supplied by or on behalf of the Group Companies prior to the Closing expressly for inclusion in the Registration Statement / Proxy Statement, and actually included in the Registration Statement / Proxy Statement, will, when the Registration Statement / Proxy Statement is declared effective or when the Registration Statement / Proxy Statement is mailed to the Pre-Closing FLAC Holders or at the time of the FLAC Shareholders Meeting, and in the case of any amendment thereto, at the time of such amendment, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they are made, not misleading.
Section 3.24 Investigation; No Other Representations.
(a) The Company, on its own behalf and on behalf of its Representatives, acknowledges and agrees that (i) it has conducted its own independent review and analysis of, and, based thereon, has formed an independent judgment concerning, the business, assets, condition, operations and prospects of FLAC and (ii) it has been furnished with or given access to such documents and information about FLAC and its business and operations as it and its Representatives have deemed necessary to enable it to make an informed decision with respect to the execution, delivery and performance of this Agreement, the Ancillary Documents and the transactions contemplated hereby and thereby.
(b) In entering into this Agreement and the Ancillary Documents to which it is a party, the Company has relied solely on its own investigation and analysis and the representations and warranties expressly set forth in Article 4 and in the Ancillary Documents to which it is a party and no other representations or warranties of FLAC or any other Person, either express or implied, and the Company, on its own behalf and on behalf of its Representatives, acknowledges and agrees that, except for the representations and warranties expressly set forth in Article 4 and in the Ancillary Documents to which it is a party, neither FLAC nor any other Person makes or has made any representation or warranty, either express or implied, in connection with or related to this Agreement, the Ancillary Documents or the transactions contemplated hereby or thereby.
Section 3.25 Regulatory Compliance.
(a) Section 3.25(a) of the Company Disclosure Schedules sets forth, as of the date of this Agreement, a true and complete list of all material Regulatory Permits held by the Group Companies. The Group Companies and the Company Products are in compliance in all material respects with all Regulatory Permits, and, to the Company’s knowledge, no event, circumstance or state of facts has occurred which (with or without due notice or lapse of time or both) would reasonably be expected to result in the failure of a Group Company to be in compliance in all material respects with the terms of any such Regulatory Permit. To the knowledge of the
A-48
Table of Contents
Company, (i) no Governmental Entity is considering limiting, suspending or revoking any Regulatory Permit and (ii) each third party that is a manufacturer, contractor or agent for the Group Companies is in compliance in all material respects with all Regulatory Permits required by all Public Health Laws insofar as they reasonably pertain to the Company Products.
(b) There is no act, omission, event or circumstance of which the Company has knowledge that would reasonably be expected to give rise to or lead to any material Proceeding against any Group Company related to compliance with Public Health Laws, and to the Company’s knowledge, no such Proceedings have been threatened. To the Company’s knowledge, the Group Companies do not have any Liability for failure to comply with any Public Health Laws.
(c) All Company Products are being, whether by the Company or a third party, researched, developed, tested, investigated, manufactured, prepared, packaged, labeled, stored and distributed in compliance in all material respects with the Public Health Laws.
(d) To the knowledge of the Company, all preclinical studies and clinical trials conducted by or on behalf of the Group Companies or involving any Company Products are being and have been conducted in all material respects in accordance with all applicable clinical trial protocols, informed consents and applicable requirements and Public Health Laws, including those of the FDA and any comparable Governmental Entity. The Company has not received any written or, to the Company’s knowledge, oral notice that the FDA or any other Governmental Entity has recommended, initiated, or threatened to initiate any action to suspend, terminate, or otherwise restrict any preclinical studies and clinical trials conducted by or on behalf of the Company or involving any Company Products.
(e) To the knowledge of the Company, as of the date of this Agreement, no Group Company, nor any clinical trial site conducting a clinical trial of any Company Product, is undergoing or has undergone any inspection or any other Governmental Entity investigation related to any Company Product, and the Group Companies have not identified or received written notice of instances or allegations of research misconduct, research fraud, or improper or inaccurate data collection or recording with respect to a Company Product that would compromise or materially affect the integrity, reliability, completeness, or accuracy of the resulting data, or the rights, safety, or welfare of the research participants.
(f) Since the incorporation of the Company, the Group Companies have not distributed any Company Products that were upon their shipment by any Group Company adulterated or misbranded in violation of 21 U.S.C. § 331 or any other Governmental Entity’s jurisdiction. No Company Products have been seized, withdrawn, recalled, detained or subject to a suspension (other than in the ordinary course of business) of research, development, testing, manufacturing or distribution, and, to the Company’s knowledge, there are no facts or circumstances reasonably likely to cause (i) the seizure, denial, withdrawal, recall, or detention, or public health notification or safety alert or suspension of manufacturing or other activity relating to any Company Product or (ii) a termination, seizure or suspension of research, development, testing, clinical investigation, manufacturing, distributing or other activity of any Company Product, in either case ((i) or (ii)), except as would not have a Company Material Adverse Effect. To the Company’s knowledge, there are no pending or threatened Proceedings against the Group Companies in the United States or any other jurisdiction seeking the withdrawal, recall, revocation, suspension, import detention or seizure of any Company Product.
(g) Except as would not have a Company Material Adverse Effect, none of the Group Companies nor, to the Company’s knowledge, any of the Company’s equityholders with five percent (5%) or greater interest, nor any of their respective directors, managers, officers, employees, individual independent contractors or other service providers, including clinical trial investigators, coordinators, monitors, Company Products or services, have been or are currently disqualified, excluded or debarred from, or threatened with or currently subject to an investigation or Proceeding that could result in disqualification, exclusion or debarment under state or federal statutes or regulations, or assessed or threatened with assessment of civil monetary penalties regarding any health
A-49
Table of Contents
care programs of any Governmental Entity, or convicted of any crime regarding health care products or services, or engaged in any conduct that would reasonably be expected to result in any such debarment, exclusion, disqualification, or ineligibility, including, without limitation, (A) debarment under 21 U.S.C. Section 335a or any similar Law, (B) exclusion under 42 U.S.C. Section 1320a-7 or any similar Law or (C) exclusion under 48 CFR Subpart Section 9.4, the System for Award Management Nonprocurement Common Rule. None of the Group Companies nor, to the Company’s knowledge, any of their current or former members, officers, partners, employees, contractors or agents have been subject to any consent decree of, or criminal or civil fine or penalty imposed by, any Governmental Entity related to fraud, theft, embezzlement, breach of fiduciary responsibility, financial misconduct, or obstruction of an investigation of controlled substances. None of the Group Companies nor, to the Company’s knowledge, any of their current or former members, officers, partners, employees, contractors or agents has been (i) subject to any enforcement, regulatory or administrative Proceedings against or affecting the Company or any of its Affiliates relating to or arising under any Healthcare Law and, to the Company’s knowledge, no such enforcement, regulatory or administrative Proceeding has been threatened, or (ii) a party to any corporate integrity agreement, monitoring agreement, deferred prosecution agreement, consent decree, settlement order, or similar agreement imposed by any Governmental Entity. None of the Group Companies nor, to the Company’s knowledge, any of their officers, directors, employees, agents or contractors have received written notice from the FDA, any other Governmental Entity or any health insurance institution with respect to debarment, disqualification or restriction.
(h) All material reports, documents, claims, permits and notices required to be filed, maintained or furnished to the FDA or any other Governmental Entity by the Company or any third party involving Company Products have been so filed, maintained or furnished. To the knowledge of the Company, all such reports, documents, claims, permits and notices were complete and accurate in all material respects on the date filed (or were corrected or supplemented by a subsequent filing).
(i) The Group Companies and, to the Company’s knowledge, their employees, are and have been, since the incorporation of the Company, in compliance in all material respects with all Healthcare Laws and Public Health Laws.
(j) To the knowledge of the Company, no officer or other employee or agent of any Group Company has (i) made any untrue statement of material fact or fraudulent statement to the FDA or any other Governmental Entity; (ii) failed to disclose a material fact required to be disclosed to the FDA or any other Governmental Entity; or (iii) committed an act, made a statement or failed to make a statement that would reasonably be expected to provide the basis for the FDA or any other Governmental Entity to refuse to grant a Regulatory Permit for any Company Product.
(k) There have been no Proceedings, and no such Proceedings are pending or, to the Company’s knowledge, threatened in writing against any Group Company related to product liability for the Company Products.
Section 3.26 Investment Company Act. None of the Group Companies are an “investment company” or a Person directly or indirectly “controlled” by or acting on behalf of a person subject to registration and regulation as an “investment company,” in each case, within the meaning of the Investment Company Act.
Section 3.27 PIPE Financing.
(a) Holdco has delivered to the Company true and complete copies of the Subscription Agreements. As of the date of this Agreement, there are no other agreements, side letters, or arrangements between Holdco and any PIPE Investor relating to the PIPE Financing.
(b) As of the date of this Agreement, no fees, consideration or other discounts are payable or have been agreed to by Holdco with any PIPE Investor in respect of its portion of the PIPE Financing Amount.
A-50
Table of Contents
Section 3.28 EXCLUSIVITY OF REPRESENTATIONS AND WARRANTIES. NOTWITHSTANDING THE DELIVERY OR DISCLOSURE TO FLAC OR ANY OF THEIR RESPECTIVE REPRESENTATIVES OF ANY DOCUMENTATION OR OTHER INFORMATION (INCLUDING ANY FINANCIAL PROJECTIONS OR OTHER SUPPLEMENTAL DATA), EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS ARTICLE 3 OR THE ANCILLARY DOCUMENTS, NONE OF HOLDCO, THE COMPANY, ANY COMPANY NON-PARTY AFFILIATE NOR ANY OTHER PERSON MAKES, AND HOLDCO AND THE COMPANY EXPRESSLY DISCLAIMS, AND FLAC HEREBY AGREES THAT IT IS NOT RELYING ON, ANY REPRESENTATIONS OR WARRANTIES OF ANY KIND OR NATURE, EXPRESS OR IMPLIED IN CONNECTION WITH THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR ANY OF THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY, INCLUDING AS TO THE ACCURACY AND COMPLETENESS OF THE MATERIALS OR ANY OTHER INFORMATION RELATING TO THE BUSINESS AND AFFAIRS OR HOLDINGS OF THE GROUP COMPANIES THAT HAVE BEEN MADE AVAILABLE TO FLAC OR ANY OF ITS REPRESENTATIVES OR IN ANY PRESENTATION OF THE BUSINESS AND AFFAIRS OF THE GROUP COMPANIES BY THE MANAGEMENT OF THE COMPANY OR OTHERS IN CONNECTION WITH THE TRANSACTIONS CONTEMPLATED HEREBY OR BY THE ANCILLARY DOCUMENTS, AND NO STATEMENT CONTAINED IN ANY OF SUCH MATERIALS OR MADE IN ANY SUCH PRESENTATION SHALL BE DEEMED A REPRESENTATION OR WARRANTY HEREUNDER OR OTHERWISE OR DEEMED TO BE RELIED UPON BY FLAC OR ANY FLAC NON-PARTY AFFILIATE IN EXECUTING, DELIVERING AND PERFORMING THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY. EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES EXPRESSLY SET FORTH IN ARTICLE 3 OR THE ANCILLARY DOCUMENTS, IT IS UNDERSTOOD THAT ANY COST ESTIMATES, PROJECTIONS OR OTHER PREDICTIONS, ANY DATA, ANY FINANCIAL INFORMATION OR ANY MEMORANDA OR OFFERING MATERIALS OR PRESENTATIONS, INCLUDING ANY OFFERING MEMORANDUM OR SIMILAR MATERIALS MADE AVAILABLE BY ANY GROUP COMPANY ARE NOT AND SHALL NOT BE DEEMED TO BE OR TO INCLUDE REPRESENTATIONS OR WARRANTIES OF THE COMPANY, ANY COMPANY NON-PARTY AFFILIATE OR ANY OTHER PERSON, AND ARE NOT AND SHALL NOT BE DEEMED TO BE RELIED UPON BY FLAC OR ANY FLAC NON-PARTY AFFILIATE IN EXECUTING, DELIVERING OR PERFORMING THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY.
ARTICLE 4
REPRESENTATIONS AND WARRANTIES RELATING TO FLAC
Subject to Section 8.8, except as set forth (a) on the FLAC Disclosure Schedules or (b) in any FLAC SEC Reports (excluding any disclosures in any “risk factors” section that do not constitute statements of fact, disclosures in any forward-looking statements disclaimers and other disclosures that are generally cautionary, predictive or forward-looking in nature) (it being acknowledged that nothing disclosed in any such FLAC SEC Reports will be deemed to modify or qualify the representations and warranties set forth in Section 4.1, Section 4.2, Section 4.4, Section 4.6, Section 4.7, Section 4.8, Section 4.10, Section 4.12, Section 4.13(c) or Section 4.15), FLAC hereby represents and warrants to the Company, in each case, as of the date of this Agreement and as of the Closing Commencement Date (or in the case of representations and warranties that speak of a specified date, as of such specified date), as follows:
Section 4.1 Organization and Qualification. FLAC is an exempted company duly organized, validly existing and in good standing under the Laws of the Cayman Islands.
Section 4.2 Authority.
(a) FLAC has the requisite exempted company power and authority to execute and deliver this Agreement and each Ancillary Document to which it is or will be a party, to perform its obligations hereunder
A-51
Table of Contents
and thereunder, and to consummate the Transactions. Subject to the receipt of the FLAC Shareholder Approval and the approvals and consents to be obtained by Merger Sub pursuant to Section 5.10, the execution and delivery of this Agreement, the Ancillary Documents to which FLAC is or will be a party and the consummation of the Transactions have been (or, in the case of any Ancillary Document entered into after the date of this Agreement, will be upon execution thereof) duly authorized by all necessary exempted company action on the part of FLAC. This Agreement and each Ancillary Document to which FLAC is or will be a party has been or will be, upon execution thereof, as applicable, duly and validly executed and delivered by FLAC and constitutes or will constitute, upon execution and delivery thereof, as applicable, a valid, legal and binding agreement of FLAC (assuming that this Agreement and the Ancillary Documents to which FLAC is or will be a party are or will be upon execution thereof, as applicable, duly authorized, executed and delivered by the other Persons party thereto), enforceable against FLAC in accordance with its terms (subject to the Enforceability Exceptions).
(b) On or prior to the date of this Agreement, (i) the FLAC Financial Advisor has delivered its opinion (and, if it is in writing, has provided a true, complete and correct copy of such opinion to the FLAC Board dated as of the date thereof) to the effect that, as of the date of such opinion and based upon and subject to the assumptions, limitations, qualifications, conditions and other matters set forth therein, the Aggregate Share Consideration to be issued by Holdco pursuant to this Agreement is fair, from a financial point of view, to the unaffiliated holders (i.e., excluding directors, officers and Affiliates of FLAC and the Sponsor) of Relevant FLAC Shares; and (ii) the FLAC Board has duly and unanimously adopted resolutions (A) determining that this Agreement and the Transactions are advisable and fair to, and in the best interest of, FLAC and the holders of FLAC Shares, (B) approving the execution, delivery and performance by FLAC of this Agreement and the consummation of the Transactions and (C) resolving to recommend the approval of this Agreement and the Transactions by the holders of FLAC Shares entitled to vote thereon.
Section 4.3 Consents and Requisite Governmental Approvals; No Violations.
(a) No consent, approval or authorization of, or designation, declaration or filing with, any Governmental Entity is required on the part of FLAC with respect to FLAC’s execution, delivery or performance of its obligations under this Agreement or the Ancillary Documents to which it is or will be party or the consummation of the Transactions, except for (i) compliance with and filings under any Antitrust Laws, (ii) the filing with the SEC of (A) the Registration Statement / Proxy Statement and the declaration of the effectiveness thereof by the SEC and (B) such reports under Section 13(a) or 15(d) of the Exchange Act as may be required in connection with this Agreement, the Ancillary Documents or the Transactions, (iii) such filings with and approvals of Nasdaq to permit Holdco Shares to be issued in accordance with this Agreement and, along with the Holdco Warrants, to be listed on Nasdaq, (iv) filing of the Merger Documents under the applicable Law of the Cayman Islands, (v) such filings and approvals required in connection with the Domestication, (vi) the approvals and consents to be obtained by Merger Sub pursuant to Section 5.10, (vii) the Required FLAC Shareholder Approval or (viii) any other consents, approvals, authorizations, designations, declarations, waivers or filings, the absence of which would not have a FLAC Material Adverse Effect.
(b) Neither the execution, delivery or performance by FLAC of this Agreement nor the Ancillary Documents to which FLAC is or will be a party nor the consummation by FLAC of the Transactions will, directly or indirectly (with or without due notice or lapse of time or both) will (i) result in any breach of any provision of the Governing Documents of FLAC, (ii) result in a violation or breach of, or constitute a default or give rise to any right of termination, Consent, cancellation, amendment, modification, suspension, revocation or acceleration under, any of the terms, conditions or provisions of any Contract to which FLAC is a party, (iii) violate, or constitute a breach under, any Order or applicable Law to which FLAC or any of its properties or assets are bound or (iv) result in the creation of any Lien upon any of the assets or properties (other than any Permitted Liens) of FLAC, except, in the case of clauses (ii) through (iv) above, as would not have a FLAC Material Adverse Effect.
Section 4.4 Brokers. Except for fees due to the Persons whose names are set forth on Section 4.4 of the FLAC Disclosure Schedules (which fees shall be the sole responsibility of FLAC, except as otherwise provided
A-52
Table of Contents
in Section 8.6), no broker, finder, investment banker or other Person is entitled to any brokerage fee, finders’ fee or other commission in connection with the Transactions based upon arrangements made by or on behalf of FLAC for which FLAC has any obligation.
Section 4.5 Information Supplied. None of the information supplied or to be supplied by or on behalf of FLAC prior to Closing expressly for inclusion in the Registration Statement / Proxy Statement, and actually included in the Registration Statement / Proxy Statement, will, when the Registration Statement / Proxy Statement is declared effective or when the Registration Statement / Proxy Statement is mailed to the Pre-Closing FLAC Holders or at the time of the FLAC Shareholders Meeting, and in the case of any amendment thereto, at the time of such amendment, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they are made, not misleading.
Section 4.6 Capitalization of FLAC.
(a) Section 4.6(a) of the FLAC Disclosure Schedules sets forth a true and complete statement of the number and class or series (as applicable) of the issued and outstanding FLAC Shares and the FLAC Warrants. All outstanding Equity Securities of FLAC have been duly authorized and validly issued and are fully paid and non-assessable. Such Equity Securities (i) were not issued in violation of the Governing Documents of FLAC and (ii) are not subject to any preemptive rights, call option, right of first refusal, subscription rights, transfer restrictions or similar rights of any Person (other than transfer restrictions under applicable Securities Laws or under the Governing Documents of FLAC) and were not issued in violation of any preemptive rights, call option, right of first refusal, subscription rights, transfer restrictions or similar rights of any Person.
(b) Except for the FLAC Warrants and the FLAC Class B Shares, there are no outstanding (A) equity appreciation, phantom equity or profit participation rights or (B) options, restricted stock, phantom stock, warrants, purchase rights, subscription rights, conversion rights, exchange rights, calls, puts, rights of first refusal or first offer or other Contracts that could require FLAC, and, except as expressly contemplated by this Agreement or the Ancillary Documents, or as mutually agreed in writing by the Company and FLAC, there is no obligation of FLAC, to issue, sell or otherwise cause to become outstanding or to acquire, repurchase or redeem any Equity Securities or securities convertible into or exchangeable for Equity Securities of FLAC.
(c) Section 4.6(c) of the FLAC Disclosure Schedules sets forth a list of all Indebtedness of FLAC as of the date of this Agreement, including the principal amount of such Indebtedness, the outstanding balance as of the date of this Agreement, and the debtor and the creditor thereof.
Section 4.7 SEC Filings. FLAC has timely filed or furnished all statements, forms, reports and documents required to be filed or furnished by it prior to the date of this Agreement with the SEC pursuant to Federal Securities Laws since its initial public offering (“IPO”) (collectively, and together with any exhibits and schedules thereto and other information incorporated therein, and as they have been supplemented, modified or amended since the time of filing, the “FLAC SEC Reports”), and, as of the Closing, will have filed or furnished all other statements, forms, reports and other documents required to be filed or furnished by it subsequent to the date of this Agreement with the SEC pursuant to Federal Securities Laws through the Closing (collectively, and together with any exhibits and schedules thereto and other information incorporated therein, and as they have been supplemented, modified or amended since the time of filing, but excluding the Registration Statement / Proxy Statement to be filed by Holdco, the “Additional FLAC SEC Reports”). Each of the FLAC SEC Reports, as of their respective dates, and as of the date of any amendment or filing that superseded the initial filing, complied with, and each of the Additional FLAC SEC Reports, as of their respective dates, and as of the date of any amendment or filing that superseded the initial filing, will comply, in all material respects with the applicable requirements of the Federal Securities Laws (including, as applicable, the Sarbanes-Oxley Act) applicable to the FLAC SEC Reports or the Additional FLAC SEC Reports (for purposes of the Additional FLAC SEC Reports, assuming that the representation and warranty set forth in Section 3.23 is true and correct in all respects with
A-53
Table of Contents
respect to all information supplied by or on behalf of Group Companies expressly for inclusion therein). As of their respective dates of filing, the FLAC SEC Reports did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which they were made or will be made, as applicable, not misleading (for purposes of the Additional FLAC SEC Reports, assuming that the representation and warranty set forth in Section 3.23 is true and correct in all respects with respect to all information supplied by or on behalf of Group Companies expressly for inclusion therein). As of the date of this Agreement, there are no outstanding or unresolved comments in comment letters received from the SEC with respect to the FLAC SEC Reports.
Section 4.8 Trust Account. As of the date of this Agreement, FLAC has an amount in cash in the Trust Account equal to at least $138,000,000. The funds held in the Trust Account are (a) invested in United States “government securities” within the meaning of Section 2(a)(16) of the Investment Company Act, having a maturity of 180 days or less or in money market funds meeting certain conditions under Rule 2a-7 promulgated under the Investment Company Act which invest only in direct U.S. government treasury obligations and (b) held in trust pursuant to that certain Investment Management Trust Agreement, dated December 8, 2020 (the “Trust Agreement”), between FLAC and Continental, as trustee (the “Trustee”). There are no separate agreements, side letters or other agreements or understandings (whether written or unwritten, express or implied) that would cause the description of the Trust Agreement in the FLAC SEC Reports to be inaccurate in any material respect or, to FLAC’s knowledge, that would entitle any Person to any portion of the funds in the Trust Account (other than (i) in respect of deferred underwriting commissions or Taxes, (ii) in respect of a FLAC Shareholder Redemption or (iii) if FLAC fails to complete a FLAC Business Combination within the allotted time period set forth in the Governing Documents of FLAC and liquidates the Trust Account, subject to the terms of the Trust Agreement, FLAC (in limited amounts to permit FLAC to pay the expenses of the Trust Account’s liquidation, dissolution and winding up of FLAC) and then the Pre-Closing FLAC Holders). Prior to the Closing, none of the funds held in the Trust Account are permitted to be released, except in the circumstances described in the Governing Documents of FLAC and the Trust Agreement. FLAC has performed all material obligations required to be performed by it to date under, and is not in material default or delinquent in performance or any other respect (claimed or actual) in connection with the Trust Agreement, and, to the knowledge of FLAC, no event has occurred which, with due notice or lapse of time or both, would constitute such a material default thereunder. As of the date of this Agreement, there are no claims or Proceedings pending with respect to the Trust Account. Since December 8, 2020, FLAC has not released any money from the Trust Account (other than interest income earned on the funds held in the Trust Account to the extent permitted by the Trust Agreement). Upon the consummation of the Transactions, including the distribution of assets from the Trust Account (A) in respect of deferred underwriting commissions or Taxes or (B) in respect of a FLAC Shareholder Redemption, each in accordance with the terms of and as set forth in the Trust Agreement, FLAC shall have no further obligation under either the Trust Agreement or the Governing Documents of FLAC to liquidate or distribute any assets held in the Trust Account, and the Trust Agreement shall terminate in accordance with its terms.
Section 4.9 Transactions with Affiliates. Section 4.9 of the FLAC Disclosure Schedules sets forth all Contracts between (a) FLAC, on the one hand, and (b) any officer, director, employee, partner, member, manager, direct or indirect equityholder (including the Sponsor) or Affiliate of either FLAC or the Sponsor, on the other hand (each Person identified in this clause (b), a “FLAC Related Party”), other than (i) Contracts solely related to a FLAC Related Party’s or a holder of FLAC Warrants’ status as a holder of FLAC Shares or FLAC Warrants, as applicable, in the ordinary course of business, (ii) employment with, or the provision of services to, FLAC entered into in the ordinary course of business (including benefit plans, indemnification arrangements and other ordinary course compensation), (iii) Contracts with respect to Pre-Closing FLAC Holders and (iv) the Ancillary Documents and any other Contracts that FLAC or a FLAC Related Party is expressly required to enter into pursuant to this Agreement. No FLAC Related Party (A) owns any interest in any material asset used in the business of FLAC, (B) possesses, directly or indirectly, any material financial interest in, or is a director or executive officer of, any Person which is a material client, supplier, customer, lessor or lessee of FLAC or (C) owes any material amount to, or is owed material any amount by, FLAC. All Contracts, interests and other
A-54
Table of Contents
matters that are required to be set forth on Section 4.9 of the FLAC Disclosure Schedules are referred to herein as “FLAC Related Party Transactions.”
Section 4.10 Litigation. As of the date of this Agreement, there is no Proceeding pending or, to FLAC’s knowledge, threatened against or involving FLAC that, if adversely decided or resolved, has been or would reasonably be expected to be, individually or in the aggregate, material to FLAC. Neither FLAC nor any of its properties or assets is subject to any material Order. As of the date of this Agreement, there are no material Proceedings by FLAC pending against any other Person.
Section 4.11 Compliance with Applicable Law. FLAC is (and since its organization, incorporation or formation, as applicable, has been) in compliance with all applicable Laws, except as would not have a FLAC Material Adverse Effect. Except as would not be material to FLAC, without limiting the foregoing, FLAC has not violated or, to FLAC’s knowledge, is under investigation with respect to, or have been threatened in writing or charged with or given notice of any violation of any provisions of: (a) Privacy Laws (substituting “FLAC” for “Group Companies” in the definition thereof) and Laws applicable to lending activities; (b) Anti-Corruption Laws; or (c) any Law regulating or covering conduct in, or the nature of, the workplace, including regarding sexual harassment or, on any impermissible basis, a hostile work environment.
Section 4.12 Business Activities. Since its incorporation, FLAC has not conducted any business activities other than activities (i) in connection with or incident or related to its incorporation or continuing corporate (or similar) existence, (ii) in connection with its IPO, (iii) directed toward the accomplishment of a FLAC Business Combination, including those incident or related to or incurred in connection with the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of its covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions or (iv) that are administrative, ministerial or otherwise immaterial in nature. Except as set forth in FLAC’s Governing Documents, there is no Contract binding upon FLAC or to which FLAC is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of it or its Subsidiaries, any acquisition of property by it or its Subsidiaries or the conduct of business by it or its Subsidiaries (including, in each case, following the Closing).
Section 4.13 Internal Controls; Listing; Financial Statements.
(a) Except as not required in reliance on exemptions from various reporting requirements by virtue of FLAC’s status as an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act, or “smaller reporting company” within the meaning of the Exchange Act, since its incorporation, (i) FLAC has established and maintained a system of internal controls over financial reporting (as defined in Rule 13a-15 and Rule 15d-15 under the Exchange Act) sufficient to provide reasonable assurance regarding the reliability of FLAC’s financial reporting and the preparation of the FLAC Financial Statements for external purposes in accordance with GAAP and (ii) FLAC has established and maintained disclosure controls and procedures (as defined in Rule 13a-15 and Rule 15d-15 under the Exchange Act) designed to ensure that material information relating to FLAC is made known to FLAC’s principal executive officer and principal financial officer by others within FLAC.
(b) FLAC has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
(c) Since its incorporation, FLAC has complied in all material respects with all applicable listing and corporate governance rules and regulations of Nasdaq. The issued and outstanding (i) FLAC Units, (ii) FLAC Class A Shares and (iii) FLAC Warrants are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on Nasdaq. As of the date of this Agreement, there is no material Proceeding pending or, to the knowledge of FLAC, threatened against FLAC by Nasdaq or the SEC with respect to any intention by such entity to deregister FLAC Units, FLAC Class A Shares or FLAC Warrants or prohibit or terminate the listing of such units, FLAC Class A Shares or FLAC Warrants on Nasdaq. FLAC has not taken any action that is designed to terminate the registration of FLAC Units, FLAC Class A Shares or FLAC Warrants under the Exchange Act.
A-55
Table of Contents
(d) FLAC’s audited balance sheets as of October 7, 2020, December 31, 2020 and December 31, 2021 and the related audited statements of operations, changes in shareholders’ deficit and cash flows of FLAC for the period from October 7, 2020 to October 7, 2020, the period from October 7, 2020 through December 31, 2020 and the year ended December 31, 2021 (the “FLAC Financial Statements”) (i) fairly present in all material respects the financial position of FLAC as at the respective dates thereof, and the results of its operations, shareholders’ equity and cash flows for the respective periods then ended (subject, in the case of any unaudited interim financial statements, to normal year-end audit adjustments (none of which is expected to be material) and the absence of footnotes), (ii) were prepared in conformity with GAAP applied on a consistent basis during the periods involved (except, in the case of any audited financial statements, as may be indicated in the notes thereto and subject, in the case of any unaudited financial statements, to normal year-end audit adjustments (none of which is expected to be material) and the absence of footnotes), (iii) in the case of the audited FLAC Financial Statements, were audited in accordance with the standards of the PCAOB and (iv) comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates thereof (including Regulation S-X or Regulation S-K, as applicable).
(e) The unaudited consolidated balance sheets of FLAC as of March 31, 2022 and March 31, 2021, June 30, 2022 and June 30, 2021, and the related unaudited statements of operations, changes in shareholders’ deficit and cash flows of FLAC for each of the three-month and six-month periods then ended and the related notes thereto, when delivered following the date of this Agreement in accordance with Section 5.17 (i) will be prepared in accordance with GAAP applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto) and (ii) will fairly present, in all material respects, the financial position, results of operations and cash flows of the FLAC as at the date thereof and for the period indicated therein, except as otherwise specifically noted therein and (iii) will comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates thereof (including Regulation S-X or Regulation S-K, as applicable).
(f) FLAC has established and maintains systems of internal accounting controls that are designed to provide, in all material respects, reasonable assurance that (i) all transactions are executed in accordance with management’s authorization and (ii) all transactions are recorded as necessary to permit preparation of proper and accurate financial statements in accordance with GAAP and to maintain accountability for FLAC’s and its Subsidiaries’ assets. FLAC maintains and, for all periods covered by the FLAC Financial Statements, has maintained books and records of FLAC in the ordinary course of business that are accurate and complete and reflect the revenues, expenses, assets and liabilities of FLAC in all material respects.
(g) Since its incorporation, FLAC has not received any written complaint, allegation, assertion or claim that there is (i) a “significant deficiency” in the internal controls over financial reporting of FLAC to FLAC’s knowledge, (ii) a “material weakness” in the internal controls over financial reporting of FLAC to FLAC’s knowledge or (iii) fraud, whether or not material, that involves management or other employees of FLAC who have a significant role in the internal controls over financial reporting of FLAC.
Section 4.14 No Undisclosed Liabilities. FLAC does not have any Liabilities of the type required to be set forth on a balance sheet in accordance with GAAP consistently applied and in accordance with past practice, except for the Liabilities (a) set forth on Section 4.14 of the FLAC Disclosure Schedules, (b) incurred in connection with the negotiation, preparation or execution of this Agreement or any Ancillary Documents, the performance of its covenants or agreements in this Agreement or any Ancillary Document or the consummation of the Transactions, (c) that are incurred in connection with or incident or related to FLAC’s incorporation, or continuing corporate existence, in each case, which are immaterial in nature, (d) that are incurred in connection with activities that are administrative or ministerial, in each case, which are immaterial in nature or (e) set forth or disclosed in the FLAC Financial Statements included in the FLAC SEC Reports.
A-56
Table of Contents
Section 4.15 Tax Matters.
(a) FLAC has prepared and filed all income and other material Tax Returns required to have been filed by it, all such Tax Returns are true and complete in all material respects and prepared in compliance in all material respects with all applicable Law, and FLAC has paid all material Taxes required to have been paid or deposited by it regardless of whether shown on a Tax Return, and has paid all assessments and reassessments in respect of Taxes in all material respects. The FLAC Financial Statements reflect all Liabilities for unpaid Taxes of FLAC for periods (or portions of periods) through the date of such statements in accordance with the requirements of GAAP.
(b) FLAC has (i) timely withheld and paid to the appropriate Governmental Entity all material amounts required to have been withheld and paid in connection with amounts paid or owing to any employee, individual independent contractor, other service providers, equity interest holder or other third party, (ii) remitted, or will remit on a timely basis, such amounts to the appropriate Governmental Entity and (iii) complied in all material respects with applicable Law with respect to Tax withholding, including all reporting and record keeping requirements.
(c) To the knowledge of FLAC, FLAC is not currently the subject of a Tax audit or examination with respect to material Taxes. FLAC has not been informed in writing of a Tax audit or examination or the commencement or anticipated commencement of any Tax audit or examination that has not been resolved or completed, in each case with respect to material Taxes.
(d) FLAC has not consented to extend or waive the time in which any material Tax may be assessed or collected by any Governmental Entity, other than any such extensions or waivers that are no longer in effect or that were extensions of time to file Tax Returns obtained in the ordinary course of business, in each case with respect to material Taxes.
(e) No rulings in respect of Tax or similar Tax agreements have been entered into or issued by any Governmental Entity with respect to FLAC which agreement or ruling would be effective after the Closing Commencement Date.
(f) FLAC is not, and has not, been a party to any “listed transaction” as defined in Section 6707A of the Code and Section 1.6011-4 of the Treasury Regulations (or any corresponding or similar provision of state, local, or non-U.S. income Tax Law).
(g) During the two (2)-year period ending on the date of this Agreement, FLAC was not a distributing corporation or a controlled corporation in a transaction purported or intended to be governed by Section 355 of the Code.
(h) There are no Liens for Taxes on any assets of FLAC other than Permitted Liens.
(i) FLAC is not treated as an “expatriated entity” as defined in Section 7874(a)(2)(A) of the Code, a “surrogate foreign corporation” as defined in Section 7874(a)(2)(B) of the Code or a domestic corporation as a result of the application of Section 7874(b) of the Code, in each case as defined in the Code as in effect on the date of this Agreement.
(j) FLAC (i) has not been a member of an affiliated group filing a consolidated federal income Tax Return or (ii) has any material Liability for the Taxes of any Person under Section 1.1502-6 of the Treasury Regulations (or any similar provision of state, local or non-U.S. Law), as a transferee or successor or by Contract (other than any Contract the principal purpose of which does not relate to Taxes).
(k) Since January 1, 2020, no written claims have been made by any Governmental Entity in a jurisdiction where FLAC does not file Tax Returns that FLAC is or may be subject to taxation by that jurisdiction.
A-57
Table of Contents
(l) FLAC is not a party to any Tax allocation, Tax sharing or Tax indemnity or similar agreements (other than one that is included in a Contract entered into in the ordinary course of business that is not primarily related to Taxes) and FLAC is not a party to any joint venture, partnership or other arrangement that is treated as a partnership for U.S. federal income Tax purposes.
(m) FLAC is a tax resident only in its jurisdiction of formation.
(n) FLAC does not have a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than its jurisdiction of incorporation.
(o) FLAC immediately prior to the Closing will not be treated as an “investment company” within the meaning of Sections 351(e) and 368(a)(2)(F) of the Code.
(p) FLAC has not taken or agreed to take any action not contemplated by this Agreement or any Ancillary Documents that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment. To the knowledge of FLAC, no facts or circumstances exist, other than any facts or circumstances to the extent that such fact or circumstances exist or arise as a result of or are related to any act or omission by a Group Company or a Company Shareholder or any of their respective Affiliates after the date hereof and in each case not contemplated by this Agreement or any of the Ancillary Documents, that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment.
Section 4.16 Investigation; No Other Representations.
(a) FLAC, on its own behalf and on behalf of its Representatives, acknowledges and agrees that (i) it has conducted its own independent review and analysis of, and, based thereon, has formed an independent judgment concerning, the business, assets, condition, operations and prospects, of the Group Companies and (ii) it has been furnished with or given access to such documents and information about the Group Companies and their respective businesses and operations as it and its Representatives have deemed necessary to enable it to make an informed decision with respect to the execution, delivery and performance of this Agreement, the Ancillary Documents and the Transactions.
(b) In entering into this Agreement and the Ancillary Documents to which it is or will be a party, FLAC has relied solely on its own investigation and analysis and the representations and warranties expressly set forth in Article 3 and in the Ancillary Documents to which it is or will be a party and no other representations or warranties of the Company or any other Person, either express or implied, and FLAC, on its own behalf and on behalf of its Representatives, acknowledges and agrees that, except for the representations and warranties expressly set forth in Article 3 and in the Ancillary Documents to which it is or will be a party, neither the Company, Holdco nor any other Person makes or has made any representation or warranty, either express or implied, in connection with or related to this Agreement, the Ancillary Documents or the Transactions.
Section 4.17 Compliance with International Trade & Anti-Corruption Laws.
(a) Since FLAC’s incorporation, neither FLAC, its directors and officers, nor, to FLAC’s knowledge, any of their Representatives or any other Persons acting for or on behalf of any of the foregoing, is or has been, (i) a Person named on any Sanctions and Export Control Laws-related list of designated Persons maintained by a Governmental Entity; (ii) located, organized or resident in a country or territory which is itself the subject of or target of any Sanctions and Export Control Laws; (iii) an entity owned, directly or indirectly, by one or more Persons described in clause (i) or (ii); or (iv) otherwise engaging in dealings with or for the benefit of any Person described in clauses (i) through (iii) or any country or territory which is itself or has, since FLAC’s incorporation, been the subject of or target of any Sanctions and Export Control Laws (at the time of this Agreement, the Crimea, Luhansk People’s Republic, and Donetsk People’s Republic regions of Ukraine, Cuba, Iran, North Korea, Venezuela, Sudan and Syria).
A-58
Table of Contents
(b) Since FLAC’s incorporation, neither FLAC, its directors and officers, nor, to FLAC’s knowledge, any of their Representatives or any other Persons acting for or on behalf of any of the foregoing has (i) made, offered, promised, paid or received any unlawful bribes, kickbacks or other similar payments to or from any Person, (ii) made or paid any contributions, directly or indirectly, to a domestic or foreign political party or candidate or (iii) otherwise made, offered, received, authorized, promised or paid any improper payment under any Anti-Corruption Laws.
Section 4.18 EXCLUSIVITY OF REPRESENTATIONS AND WARRANTIES. NOTWITHSTANDING THE DELIVERY OR DISCLOSURE TO THE COMPANY OR ANY OF ITS REPRESENTATIVES OF ANY DOCUMENTATION OR OTHER INFORMATION (INCLUDING ANY FINANCIAL PROJECTIONS OR OTHER SUPPLEMENTAL DATA), EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS ARTICLE 4 OR THE ANCILLARY DOCUMENTS, NONE OF FLAC, ANY FLAC NON-PARTY AFFILIATE NOR ANY OTHER PERSON MAKES, AND FLAC EXPRESSLY DISCLAIMS, AND THE COMPANY HEREBY AGREES THAT IT IS NOT RELYING ON, ANY REPRESENTATIONS OR WARRANTIES OF ANY KIND OR NATURE, EXPRESS OR IMPLIED IN CONNECTION WITH THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR ANY OF THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY, INCLUDING AS TO THE ACCURACY AND COMPLETENESS OF THE MATERIALS OR ANY OTHER INFORMATION RELATING TO THE BUSINESS AND AFFAIRS OR HOLDINGS OF FLAC THAT HAVE BEEN MADE AVAILABLE TO THE COMPANY OR ANY OF ITS REPRESENTATIVES OR IN ANY PRESENTATION OF THE BUSINESS AND AFFAIRS OF FLAC BY OR ON BEHALF OF THE MANAGEMENT OF FLAC OR OTHERS IN CONNECTION WITH THE TRANSACTIONS CONTEMPLATED HEREBY OR BY THE ANCILLARY DOCUMENTS, AND NO STATEMENT CONTAINED IN ANY OF SUCH MATERIALS OR MADE IN ANY SUCH PRESENTATION SHALL BE DEEMED A REPRESENTATION OR WARRANTY HEREUNDER OR OTHERWISE OR DEEMED TO BE RELIED UPON BY THE COMPANY OR ANY COMPANY NON-PARTY AFFILIATE IN EXECUTING, DELIVERING AND PERFORMING THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY. EXCEPT FOR THE REPRESENTATIONS AND WARRANTIES EXPRESSLY SET FORTH IN ARTICLE 4 OR THE ANCILLARY DOCUMENTS, IT IS UNDERSTOOD THAT ANY COST ESTIMATES, PROJECTIONS OR OTHER PREDICTIONS, ANY DATA, ANY FINANCIAL INFORMATION OR ANY MEMORANDA OR OFFERING MATERIALS OR PRESENTATIONS, INCLUDING ANY OFFERING MEMORANDUM OR SIMILAR MATERIALS MADE AVAILABLE BY OR ON BEHALF OF FLAC ARE NOT AND SHALL NOT BE DEEMED TO BE OR TO INCLUDE REPRESENTATIONS OR WARRANTIES OF FLAC, ANY FLAC NON-PARTY AFFILIATE OR ANY OTHER PERSON, AND ARE NOT AND SHALL NOT BE DEEMED TO BE RELIED UPON BY THE COMPANY OR ANY COMPANY NON-PARTY AFFILIATE IN EXECUTING, DELIVERING OR PERFORMING THIS AGREEMENT, THE ANCILLARY DOCUMENTS OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY.
ARTICLE 5
COVENANTS
Section 5.1 Conduct of Business of the Company.
(a) From and after the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, the Company shall, and the Company shall cause its Subsidiaries to, except as expressly contemplated by this Agreement or any Ancillary Document, as required by applicable Law, as set forth on Section 5.1 of the Company Disclosure Schedules, or as consented to in writing by FLAC (such consent not to be unreasonably withheld, conditioned or delayed), (i) operate the business of the Group Companies in the ordinary course in all material respects and in accordance with all applicable Law and (ii) use commercially reasonable efforts to maintain and preserve intact in all material respects the business organization, assets, properties and material business relations of the Group Companies, taken as a whole, and to maintain existing relations and goodwill with Governmental Entities and material customers, suppliers, licensors,
A-59
Table of Contents
licensees, distributors, creditors, lessors, and business associates and to keep available the services of the Group Companies’ present officers.
(b) Without limiting the generality of the foregoing, from and after the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, the Company shall, and the Company shall cause its Subsidiaries to, except as expressly contemplated by this Agreement or any Ancillary Document, as required by applicable Law, as set forth on Section 5.1 of the Company Disclosure Schedules or as consented to in writing by FLAC (such consent, other than in the case of Section 5.1(b)(i), Section 5.1(b)(iii), Section 5.1(b)(ix) (but only to the extent relating to any Material Contract of the type described in Section 3.7(a)(ix)) Section 5.1(b)(xiv) or Section 5.1(b)(xv), not to be unreasonably withheld, conditioned or delayed), not do any of the following:
(i) declare, set aside, make or pay a dividend on, or make any other distribution or payment in respect of, any Equity Securities of any Group Company or repurchase any outstanding Equity Securities of any Group Company, other than dividends or distributions, declared, set aside or paid by any of the Company’s Subsidiaries to the Company or any Subsidiary that is, directly or indirectly, wholly owned by the Company, or enter into any agreement with respect to the voting rights of its capital stock;
(ii) reclassify, split, combine, subdivide or redeem, purchase or otherwise acquire, directly or indirectly, any of its capital stock or securities convertible or exchangeable into or exercisable for any shares of its capital stock;
(iii) (A) merge, consolidate, combine or amalgamate any Group Company with any Person or (B) purchase or otherwise acquire (whether by merging or consolidating with, purchasing any Equity Security in or a substantial portion of the assets of, or by any other manner) any Equity Securities, material assets or other materials rights of any corporation, partnership, association or other business entity or organization or division thereof;
(iv) adopt any amendments, supplements, restatements or modifications to any Group Company’s Governing Documents or the Company Shareholders Agreements;
(v) other than pursuant to Contracts to which the Company is a party that are in effect as of the date of this Agreement, transfer, sell, lease, license, mortgage, pledge, surrender, encumber, divest, cancel, abandon or allow to lapse or expire or otherwise dispose of any of its material assets, properties, licenses, operations, rights, product lines, businesses or interests therein, except for (A) sales or other dispositions in the ordinary course of business; (B) sales, leases, or other dispositions of assets with a fair market value not in excess of $250,000 in the aggregate or (C) non-exclusive licenses entered in the ordinary course of business;
(vi) other than the issuance of shares of the Company upon the exercise or conversion of any Company Options outstanding on the date of this Agreement in accordance with the terms of the applicable Company Equity Incentive Plan and the underlying grant, award or similar agreement or the issuance of Company Options covering up to 140,164 Company Ordinary Shares under the applicable Company Equity Incentive Plan, transfer, issue, sell, grant or otherwise directly or indirectly dispose of, or subject to a Lien, (A) any Equity Securities of any Group Company or (B) any options, warrants, rights of conversion or other rights, agreements, arrangements or commitments obligating any Group Company to issue, deliver or sell any Equity Securities of any Group Company;
(vii) incur, create or assume any Indebtedness, other than (A) ordinary course trade payables or (B) Indebtedness in an aggregate amount not to exceed $2,000,000;
(viii) other than in the ordinary course of business, amend, modify, cancel, or waive any debts held by it;
A-60
Table of Contents
(ix) other than amendments or modifications of Material Contracts in the ordinary course of business and that, individually or in the aggregate, are not material, (A) amend, modify or terminate any Material Contract (excluding, for the avoidance of doubt, any expiration or automatic extension or renewal of any such Material Contract pursuant to its terms or entering into additional work orders pursuant to, and in accordance with the terms of, any Material Contract in the ordinary course of business), (B) waive any material benefit or right under any Material Contract or (C) enter into any Contract that would constitute a Material Contract;
(x) make any loans, advances or capital contributions to, or guarantees for the benefit of, or any investments in, any Person, other than (A) intercompany loans or capital contributions between the Company and any of its wholly owned Subsidiaries, or between any such Subsidiaries and (B) the reimbursement of expenses of employees in the ordinary course of business;
(xi) except (x) as required under the terms of any Employee Benefit Plan set forth on Section 3.12(a) of the Company Disclosure Schedules or (y) in the ordinary course of business (it being understood and agreed, for the avoidance of doubt, that in no event shall the exception in this clause (y) be deemed or construed as permitting any Group Company to take any action that is not permitted by any other provision of this Section 5.1(b)), (A) amend, modify, adopt, enter into or terminate any material Employee Benefit Plan of any Group Company or any material benefit or compensation plan, policy, program or Contract that would be an Employee Benefit Plan if in effect as of the date of this Agreement, (B) materially increase the compensation or benefits payable to any current or former director, manager, officer, employee, or Contingent Worker of any Group Company earning annual compensation in excess of $150,000, or increase the aggregate annual compensation or benefits payable to any other current or former director, manager, officer, employee, or Contingent Worker of any Group Company to be greater than $150,000, (C) take any action to accelerate any payment, right to payment, or benefit, or the funding of any payment, right to payment or benefit, payable or to become payable to any current or former director, manager, officer, employee or Contingent Worker of any Group Company, (D) waive or release any material noncompetition, non-solicitation, no-hire, nondisclosure or other restrictive covenant obligation of any current or former director, manager, officer, employee, individual independent contractor or other service provider of any Group Company, (E) pay any special bonus or special remuneration to any director, officer or employee of any Group Company, (F) terminate or furlough the employment of any director, officer, management-level or key employee of any Group Company, or (G) enter into a settlement agreement with any current or former director, officer, or employee of any Group Company;
(xii) make, change or revoke any entity Tax classification or other material election concerning Taxes, settle any material Tax claim or assessment, or consent to any extension or waiver of the limitation period applicable to or relating to any material Tax claim or assessment, other than any such extension or waiver that is obtained in the ordinary course of business;
(xiii) enter into any settlement, conciliation or similar Contract the performance of which would involve the payment by the Group Companies in excess of $2,000,000, in the aggregate, or that imposes, or by its terms will impose at any point in the future, any material, non-monetary obligations on any Group Company;
(xiv) authorize, recommend, propose or announce an intention to adopt, or otherwise effect, a plan of complete or partial liquidation, dissolution, restructuring, recapitalization, reorganization or similar transaction involving any Group Company;
(xv) change any Group Company’s methods of accounting in any material respect, other than changes that are (i) made in accordance with PCAOB standards, (ii) required by changes in applicable Law or IFRS, or (iii) required by such Group Company’s auditors;
(xvi) enter into any Contract with any broker, finder, investment banker or other Person under which such Person is or will be entitled to any brokerage fee, finders’ fee or other commission in connection with the Transactions;
A-61
Table of Contents
(xvii) make any Change of Control Payment that is not set forth on Section 3.2(f) of the Company Disclosure Schedules;
(xviii) become a party to, establish, adopt, amend, commence participation in or enter into any collective bargaining or other labor union Contract;
(xix) fail to keep current and in full force and effect, or to comply in all material respects with the requirements of, any material Permit or any Regulatory Permit;
(xx) create or incur any material Lien (other than Permitted Liens) that is not incurred in the ordinary course of business on any of its assets;
(xxi) enter into any new material line of business or operations, or discontinue any material line of business or any material business operations; or
(xxii) enter into any Contract to take, or cause to be taken, any of the actions set forth in this Section 5.1.
Notwithstanding anything in this Section 5.1 or this Agreement to the contrary, nothing set forth in this Agreement shall give FLAC, directly or indirectly, the right to control or direct (a) the operations of the Group Companies prior to the Closing or (b) any action taken, or omitted to be taken, by any Group Company to the extent such act or omission is reasonably determined by the Company to be necessary to comply with applicable Law or COVID-19 Measures in effect as of the date of this Agreement (which shall in no event be deemed to constitute a breach of this Section 5.1) or any action taken, or omitted to be taken, by any Group Company to the extent reasonably determined that such act or omission is necessary in response to COVID-19 to maintain and preserve in all material respects the business organization, assets, properties and material business relations of the Group Companies, taken as a whole, shall not be deemed to constitute a breach of Section 5.1; provided, however, that (i) in the case of clause (b), the Company shall give FLAC prior written notice of any such act or omission to the extent reasonably practicable, which notice shall describe in reasonable detail the act or omission and the reason(s) that such act or omission is being taken, or omitted to be taken, pursuant to clause (b)and, in the event that it is not reasonably practicable for the Company to give the prior written notice described in this clause (i), the Company shall instead give such written notice to FLAC promptly after such act or omission and (ii) in no event shall clause (b) be applicable to any act or omission of the type described in Section 5.1(b)(i), Section 5.1(b)(ii), Section 5.1(b)(iii), Section 5.1(b)(iv), Section 5.1(b)(vi), Section 5.1(b)(viii), Section 5.1(b)(xii), Section 5.1(b)(xvii) or Section 5.1(b)(xxii) (to the extent related to any of the foregoing).
Section 5.2 Efforts to Consummate.
(a) Subject to the terms and conditions herein provided, each of the Parties shall use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary or advisable to consummate and make effective as promptly as reasonably practicable the Transactions (including (i) the satisfaction, but not waiver, of the closing conditions set forth in Article 6 and, in the case of any Ancillary Document to which such Party will be a party after the date of this Agreement, to execute and deliver such Ancillary Document when required pursuant to this Agreement, (ii) using reasonable best efforts to obtain the PIPE Financing on the terms and subject to the conditions set forth in the Subscription Agreements, and (iii) making all such filings with and obtaining all such approvals of Nasdaq to permit Holdco Shares to be issued in accordance with this Agreement to be listed on Nasdaq) and not to take any action after the date of this Agreement that would reasonably be expected to prevent, materially delay, or materially impair the consummation of the Transactions.
(b) Without limiting the generality of the foregoing, each of the Parties shall use reasonable best efforts to promptly obtain, file with or deliver to, as applicable, any Consents of any Governmental Entities necessary,
A-62
Table of Contents
proper or advisable to consummate the Transactions. Each of the Company, on the one hand, and FLAC, on the other, shall bear 50% of the costs incurred in connection with obtaining such Consents, including any filing or similar fees with respect to any Antitrust Laws; provided that each Party shall bear its out-of-pocket costs and expenses in connection with the preparation of any such Consents. Each Party shall (i) make any appropriate filings or take, or cause to be taken, any required actions pursuant to any applicable Antitrust Laws with respect to the Transactions as promptly as practicable following the date of this Agreement and (ii) respond as promptly as reasonably practicable to any requests by any Governmental Entity for additional information and documentary material that may be requested pursuant to any Antitrust Laws. FLAC shall promptly inform the Company of any communication between FLAC, on the one hand, and any Governmental Entity, on the other hand, and the Company shall promptly inform FLAC of any communication between the Company, on the one hand, and any Governmental Entity, on the other hand, in either case, regarding any of the Transactions. Subject to the terms of the Confidentiality Agreement, the Parties shall provide each other with copies of all material correspondence, filings or communications, including any documents, information and data contained therewith, between them or any of their Representatives, on the one hand, and any Governmental Entity, on the other hand, with respect to this Agreement and the Transactions. Without limiting the foregoing, each Party and their respective Affiliates shall not extend any waiting period, review period or comparable period under any applicable Antitrust Laws or enter into any agreement with any Governmental Entity not to consummate the Transactions, except with the prior written consent of FLAC and the Company. Nothing in this Section 5.2 obligates any Party or any of its Affiliates to agree to (i) sell, license or otherwise dispose of, or hold separate and agree to sell, license or otherwise dispose of, any entities, assets, lines of business or facilities of any Group Company or any entity, asset, line of business or facility of such Party or any of its Affiliates, (ii) terminate, amend or assign existing relationships and contractual rights or obligations, (iii) amend, assign or terminate existing licenses or other agreements, or (iv) enter into new licenses or other agreements. No Party shall agree to any of the foregoing measures with respect to any other Party or any of its Affiliates, except with FLAC’s and the Company’s prior written consent.
(c) From and after the date of this Agreement until the earlier of the Closing or termination of this Agreement in accordance with its terms, FLAC, on the one hand, and the Company, on the other hand, shall give counsel for the Company (in the case of FLAC) or FLAC (in the case of the Company) a reasonable opportunity to review in advance, and consider in good faith the views of the other in connection with, any proposed written communication to any Governmental Entity relating to the Transactions. Each of the Parties agrees not to participate in any substantive meeting or discussion, either in person or by telephone with any Governmental Entity in connection with the Transactions unless it consults with, in the case of FLAC, the Company, or, in the case of the Company, FLAC in advance and, to the extent not prohibited by such Governmental Entity, gives, in the case of FLAC, the Company, or, in the case of the Company, FLAC, the opportunity to attend and participate in such meeting or discussion. The Parties agree to consult and cooperate with one another in connection with any analyses, appearances, presentations, memoranda, briefs, arguments, opinions and proposals made or submitted by or on behalf of any Party in connection with judicial proceedings under or relating to any Antitrust Law.
(d) Notwithstanding anything to the contrary in the Agreement, in the event that this Section 5.2 conflicts with any other covenant or agreement in this Article 5 that is intended to specifically address any subject matter, then such other covenant or agreement shall govern and control solely to the extent of such conflict.
(e) From and after the date of this Agreement until the earlier of the Final Closing Date or termination of this Agreement in accordance with its terms, FLAC, on the one hand, and the Company, on the other hand, shall each notify the other in writing promptly after learning of any shareholder demands or other shareholder Proceedings (including derivative claims) relating to this Agreement, any Ancillary Document or any matters relating thereto (collectively, the “Transaction Litigation”) commenced against, in the case of FLAC, FLAC or any of its Representatives (in their capacity as a representative of FLAC) or, in the case of the Company, any Group Company or any of their respective Representatives (in their capacity as a representative of a Group
A-63
Table of Contents
Company). FLAC and the Company shall each (i) keep the other reasonably informed regarding any Transaction Litigation (to the extent such action would not jeopardize an attorney-client privilege or the attorney work product doctrine), (ii) give the other the opportunity to, at its own cost and expense, participate in the defense, settlement and compromise of any such Transaction Litigation, (iii) consider in good faith the other’s advice with respect to any such Transaction Litigation and (iv) reasonably cooperate with each other, including with respect to the defense, settlement and compromise of any such Transaction Litigation. Notwithstanding the foregoing, the Company shall, subject to and without limiting the covenants and agreements, and the rights of FLAC, set forth in the immediately preceding sentence, control the negotiation, defense and settlement of any such Transaction Litigation; provided, however, that in no event shall the Company, any other Group Company or any of their respective Representatives settle or compromise any Transaction Litigation without the prior written consent of FLAC (such consent not to be unreasonably withheld, conditioned or delayed, it being understood to be reasonable for FLAC to withhold, condition or delay its consent if any such settlement or compromise (A) does not provide for a full, unconditional and irrevocable release of FLAC and each Representative that is the subject of such Transaction Litigation, (B) provides for (x) the payment of cash any portion of which is payable prior to the Closing by FLAC or any Representative thereof or would otherwise constitute a FLAC Liability or (y) any non-monetary, injunctive, equitable or similar relief against FLAC or (C) contains an admission of wrongdoing or Liability by FLAC or any of its Representatives). Without limiting the generality of the foregoing, in no event shall FLAC or any of its Representatives settle or compromise any Transaction Litigation without the Company’s prior written consent.
Section 5.3 Confidentiality and Access to Information.
(a) The information being provided in connection with this Agreement and the consummation of the Transactions is subject to the terms of the Confidentiality Agreements, the terms of which are incorporated herein by reference. Notwithstanding the foregoing or anything to the contrary in this Agreement, in the event that this Section 5.3(a) or the Confidentiality Agreement conflicts with any other covenant or agreement contained herein or in the Ancillary Documents that contemplates the disclosure, use or provision of information or otherwise, then such other covenant or agreement contained herein shall govern and control to the extent of such conflict.
(b) From and after the date of this Agreement until the earlier of the Final Closing Date or the termination of this Agreement in accordance with its terms, upon reasonable advance written notice, the Company shall provide, or cause to be provided, to FLAC and its Representatives during normal business hours reasonable access to the directors, officers, books and records of the Group Companies (in a manner so as to not interfere with the normal business operations of the Group Companies). Notwithstanding the foregoing, none of the Group Companies shall be required to provide to FLAC or any of its Representatives any information (i) if and to the extent doing so would (A) violate any Law to which any Group Company is subject (B) result in the disclosure of any trade secrets of third parties in breach of any Contract with such third party, (C) violate any legally binding obligation of any Group Company with respect to confidentiality, non-disclosure or privacy or (D) jeopardize protections afforded to any Group Company under the attorney-client privilege or the attorney work product doctrine; provided that, in case of each of clauses (A) through (D), the Company shall, and shall cause the other Group Companies to, use commercially reasonable efforts to provide such access or information to the extent it can be provided (or otherwise convey such information regarding the applicable matter as can be conveyed) without violating such privilege, doctrine, Contract, obligation or Law, or (ii) if any Group Company, on the one hand, and FLAC, any FLAC Non-Party Affiliate or any of their Representatives, on the other hand, are adverse parties in a litigation and such information is reasonably pertinent thereto; provided that the Company shall, in the case of clause (i) or (ii), provide prompt written notice of the withholding of access or information on any such basis.
(c) From and after the date of this Agreement until the earlier of the Final Closing Date or the termination of this Agreement in accordance with its terms, upon reasonable advance written notice, FLAC shall provide, or cause to be provided, to the Company and its Representatives during normal business hours
A-64
Table of Contents
reasonable access to the directors, officers, books and records of FLAC (in a manner so as to not interfere with the normal business operations of FLAC). Notwithstanding the foregoing, FLAC shall not be required to provide, or cause to be provided to, the Company or any of its Representatives any information (i) if and to the extent doing so would (A) violate any Law to which FLAC is subject, (B) result in the disclosure of any trade secrets of third parties in breach of any Contract with such third party, (C) violate any legally binding obligation of FLAC with respect to confidentiality, non-disclosure or privacy or (D) jeopardize protections afforded to FLAC under the attorney-client privilege or the attorney work product doctrine; provided that, in case of each of clauses (A) through (D), FLAC shall use commercially reasonable efforts to provide such access or information to the extent it can be provided (or otherwise convey such information regarding the applicable matter as can be conveyed) without violating such privilege, doctrine, Contract, obligation or Law, or (ii) if FLAC, on the one hand, and any Group Company, any Company Non-Party Affiliate, or any of their respective Representatives, on the other hand, are adverse parties in a litigation and such information is reasonably pertinent thereto; provided that FLAC shall, in the case of clause (i) or (ii), provide prompt written notice of the withholding of access or information on any such basis.
(d) The Parties hereby acknowledge and agree that the Confidentiality Agreement shall be automatically terminated effective as of the Closing without any further action by any Party or any other Person.
Section 5.4 Public Announcements.
(a) Subject to Section 5.7 and Section 5.8, none of the Parties or any of their respective Representatives shall issue any press releases or make any public announcements with respect to this Agreement or the Transactions prior to the Closing without the prior written consent of the Company and FLAC; provided, however, that each Party may make any such announcement or other communication (i) if such announcement or other communication is required by applicable Law, in which case the disclosing Party and its Representatives shall use reasonable best efforts to consult with the Company, if the disclosing party is FLAC, or FLAC, if the disclosing party is the Company, to review such announcement or communication and provide the opportunity to comment thereon, and the disclosing Party shall consider such comments in good faith, (ii) to the extent such announcements or other communications contain only information previously disclosed in a public statement, press release or other communication previously approved in accordance with this Section 5.4(a) and (iii) to Governmental Entities or other Persons in connection with any Consents required to be made or obtained under this Agreement, the Ancillary Documents or in connection with the Transactions.
(b) The initial press release concerning this Agreement and the Transactions shall be a joint press release in the form agreed by the Company and FLAC prior to the execution of this Agreement and such initial press release (the “Signing Press Release”) shall be released as promptly as reasonably practicable after the execution of this Agreement on the day thereof. Promptly after the execution of this Agreement, FLAC shall file with the SEC a current report on Form 8-K (the “Signing Filing”) with the Signing Press Release and a description of this Agreement as required by, and in compliance with, Securities Laws, which the Company shall have the opportunity to review and comment upon prior to filing and FLAC shall consider such comments in good faith. The Company, on the one hand, and FLAC, on the other hand, shall, prior to the Closing, mutually agree upon (such agreement not to be unreasonably withheld, conditioned or delayed by either the Company or FLAC, as applicable) a press release announcing the consummation of the Transactions (the “Closing Press Release”), and, on the Final Closing Date, the Parties shall cause the Closing Press Release to be released. Promptly after the Closing (but in any event within four (4) Business Days after the Final Closing Date), Holdco and FLAC shall file with the SEC a current report on Form 6-K and Form 8-K, respectively (the “Closing Filing”) with the Closing Press Release, a description of the Closing and the other information required by Securities Laws, which Holdco and FLAC shall have the opportunity to review, comment upon prior to the Closing, and consent to the filing, of the Closing Filing, which shall be mutually agreed upon by the Company and FLAC prior to the Closing (such agreement not to be unreasonably withheld, conditioned or delayed by either the Company or FLAC, as applicable). In connection with the preparation of each of the Signing Press Release, the Signing Filing, the Closing Press Release and the Closing Filing, each Party shall, upon written
A-65
Table of Contents
request by any other Party, furnish such other Party with all information concerning itself, its directors, officers and equityholders, and such other matters as may be reasonably necessary for such press release or filing.
Section 5.5 Tax Matters.
(a) Tax Treatment.
(i) The Parties agree that the Transactions, including the Company Share Exchange together with the Merger, are undertaken as part of a prearranged integrated plan. The Parties accordingly intend that for U.S. federal income Tax (and applicable U.S. state and local) purposes the Company Share Exchange together with the Merger constitute (A) a Tax-deferred exchange within the meaning of Section 351(a) of the Code and (B) a Tax-deferred exchange that satisfies the exception to Section 367(a)(1) of the Code set forth in the Treasury Regulation Section 1.367(a)-3(b) (together with the treatment of the issuance of the Earnout Shares (other than the Earnout RSUs) under this Section 5.5(a), the “Intended Holdco Exchange Tax Treatment”). The Parties further agree to treat the issuance of the Earnout Shares to the holders of Holdco Shares pursuant to Section 2.7 as complying with Rev. Proc. 84-42, 1984-1 C.B. 521, and the issuance of Earnout Shares shall be effected in accordance with Rev. Proc. 84-42. The Parties shall prepare and file all U.S. Tax Returns consistent with the U.S. Intended Holdco Exchange Tax Treatment and shall not take any inconsistent position on any Tax Return, or during the course of any Proceeding with respect to Taxes, unless otherwise required by a “determination” within the meaning of Section 1313 of the Code.
(ii) The Parties intend that for U.S. federal income Tax (and applicable U.S. state and local Tax) purposes the Domestication constitute a “reorganization” within the meaning of Section 368(a)(1)(F) of the Code (the “Intended Domestication Tax Treatment”). The Parties shall prepare and file all U.S. Tax Returns consistent with the Intended Domestication Tax Treatment and shall not take any inconsistent position on any U.S. Tax Return, or during the course of any Proceeding with respect to Taxes, unless otherwise required by a “determination” within the meaning of Section 1313 of the Code.
(iii) The Parties intend that for U.S. federal income Tax (and applicable U.S. state and local Tax) purposes (A) the Merger constitutes a transaction treated as a “reorganization” within the meaning of Section 368(a) of the Code and (B) the FLAC Shareholder Redemption be treated as a transaction occurring separately from the “reorganization” described in clause (A) of this paragraph ((A) and (B), collectively the “Intended Merger Tax Treatment,” and together with the Intended Holdco Exchange Tax Treatment and the Intended Domestication Tax Treatment, the “Intended Tax Treatment”). The Parties shall prepare and file all U.S. Tax Returns consistent with the Intended Merger Tax Treatment and shall not take any inconsistent position on any U.S. Tax Return, or during the course of any Proceeding with respect to Taxes, unless either (A) FLAC determines, in consultation with Goodwin Procter LLP (or other nationally recognized Tax counsel), that the Merger does not qualify for the Intended Merger Tax Treatment due to an inability to satisfy the requirements under Treasury Regulations Section 1.368-1(d)(3) or (B) otherwise required by a “determination” within the meaning of Section 1313 of the Code.
(iv) The Parties hereby adopt this Agreement as a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a) in respect of the Transactions. The Parties shall not, and shall not permit or cause their respective Affiliates to, take any action, or knowingly fail to take any action, which action or failure to act prevents or impedes, or would reasonably be expected to prevent or impede, the Intended Tax Treatment.
(b) Tax Matters Covenants; Tax Cooperation.
(i) Each of the Parties shall (and shall cause their respective Affiliates to) cooperate fully, as and to the extent reasonably requested by another Party, in connection with the filing of relevant Tax Returns, and any audit, litigation or other proceeding with respect to Taxes. Such cooperation shall include the retention and (upon the other Party’s request) the provision (with the right to make copies) of records and information
A-66
Table of Contents
reasonably relevant to any Tax Proceeding, making employees available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder and making available to the Pre-Closing FLAC Holders information reasonably necessary to compute any income of any such holder (or its direct or indirect owners) arising (i) if applicable, as a result of Holdco’s status as a PFIC or a “controlled foreign corporation” within the meaning of Section 957(a) of the Code for any taxable period ending on or prior to the Closing, including timely providing (A) a PFIC Annual Information Statement to enable such holders to make a “Qualifying Electing Fund” election under Section 1295 of the Code for such taxable period, and (B) information to enable applicable holders to report their allocable share of “subpart F” income under Section 951 of the Code and “GILTI” income under Section 951A of the Code for such taxable period and (ii) under Section 367(b) of the Code and the Treasury Regulations promulgated thereunder.
(ii) Holdco acknowledges that any Pre-Closing FLAC Holder who owns five percent (5%) or more of the shares of Holdco immediately after the Closing, as determined under Section 367 of the Code and the Treasury Regulations promulgated thereunder, may enter into (and cause to be filed with the IRS) a gain recognition agreement in accordance with Treasury Regulations Section 1.367(a)-8. Upon the written request of any Pre-Closing FLAC Holder made following the Final Closing Date, Holdco shall (i) use reasonable best efforts to furnish to such Pre-Closing FLAC Holder such information as it reasonably requests in connection with such Pre-Closing FLAC Holder’s preparation of a gain recognition agreement, and (ii) use reasonable best efforts to provide such Pre-Closing FLAC Holder with the information reasonably requested by such Pre-Closing FLAC Holder for purposes of determining whether there has been a gain “triggering event” under the terms of such Pre-Closing FLAC Holder’s gain recognition agreement.
(iii) Following the Final Closing Date, (A) Holdco shall, or shall cause FLAC to, comply with the Tax reporting obligations of Treasury Regulations 1.368-3 and 1.367(a)-3(c)(6) and (B) Holdco shall not permit FLAC to liquidate or to be treated as liquidating for U.S. federal income Tax purposes.
(iv) Within ninety (90) days after the end of each taxable year of Holdco, Holdco shall (A) determine its status as a PFIC, (B) determine the PFIC status of each of its Subsidiaries that at any time during such taxable year was a “foreign corporation” within the meaning of Section 7701(a) of the Code, but only if Holdco determines that it was a PFIC for such taxable year and (C) make such PFIC status determinations available to the shareholders of Holdco electronically.
Section 5.6 Exclusive Dealing.
(a) From the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, the Company shall not, and shall cause Holdco and the other Group Companies and instruct and use reasonable best efforts to cause its and their respective Representatives not to, directly or indirectly: (i) solicit, initiate, encourage (including by means of furnishing or disclosing information), facilitate, discuss or negotiate, directly or indirectly, any Company Acquisition Proposal; (ii) furnish or disclose any non-public information to any Person in connection with, or that would reasonably be expected to lead to, a Company Acquisition Proposal; (iii) enter into any Contract or other arrangement or understanding regarding a Company Acquisition Proposal; (iv) prepare or take any steps in connection with a public offering of any Equity Securities of any Group Company (or any Affiliate or successor of any Group Company); or (v) otherwise cooperate in any way with, or assist or participate in, or knowingly facilitate or encourage any effort or attempt by any Person to do or seek to do any of the foregoing. The Company agrees to (A) notify FLAC promptly upon receipt of any Company Acquisition Proposal by any Group Company, and to describe the material terms and conditions of any such Company Acquisition Proposal in reasonable detail (including the identity of the Persons making such Company Acquisition Proposal) and (B) keep FLAC reasonably informed on a current basis of any modifications to such offer or information. The Company shall immediately, and shall cause the other Group Companies and its and their respective Representatives to, cease and cause to be terminated any and all existing activities, discussions or negotiations with any Persons (other than FLAC) conducted prior to or as of the date of this Agreement by the Company or any of its Subsidiaries that would reasonably be expected to lead to a Company Acquisition Proposal or the matters described in clause (iv) above, and shall, as promptly as
A-67
Table of Contents
practicable, terminate access by each such Person and its Representatives to any online or other data rooms containing any non-public information in respect of the Company or any of its Subsidiaries for the purpose of permitting such Persons to evaluate a potential Company Acquisition Proposal. For clarity, any actions taken by any of the Representatives of the Group Companies in violation of this Section 5.6(a) will be deemed to be a breach of this Section 5.6(a) by the Group Companies.
(b) From the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, FLAC shall not, and shall instruct and use reasonable best efforts to cause its Representatives not to, directly or indirectly: (i) solicit, initiate, encourage (including by means of furnishing or disclosing information), facilitate, discuss or negotiate, directly or indirectly, any FLAC Acquisition Proposal; (ii) furnish or disclose any non-public information to any Person in connection with, or that would reasonably be expected to lead to, a FLAC Acquisition Proposal; (iii) enter into any Contract or other arrangement or understanding regarding a FLAC Acquisition Proposal; (iv) prepare or take any steps in connection with an offering of any securities of FLAC (or any Affiliate or successor of FLAC); or (v) otherwise cooperate in any way with, or assist or participate in, or knowingly facilitate or encourage any effort or attempt by any Person to do or seek to do any of the foregoing. FLAC agrees to (A) notify the Company promptly upon receipt of any FLAC Acquisition Proposal by FLAC, and to describe the material terms and conditions of any such FLAC Acquisition Proposal in reasonable detail (including the identity of any person or entity making such FLAC Acquisition Proposal) and (B) keep the Company reasonably informed on a current basis of any modifications to such offer or information. FLAC shall immediately, and shall cause its Representatives to, cease and cause to be terminated any and all existing activities, discussions or negotiations with any Persons (other than with the Group Companies) conducted prior to or as of the date of this Agreement by FLAC that would reasonably be expected to lead to a FLAC Acquisition Proposal or the matters described in clause (iv) above, and shall, as promptly as practicable, terminate access by each such Person and its Representatives to any online or other data rooms containing any non-public information in respect of FLAC or any of its Subsidiaries for the purpose of permitting such Persons to evaluate a potential FLAC Acquisition Proposal. For clarity, any actions taken by any of the Representatives of FLAC in violation of this Section 5.6(b) will be deemed to be a breach of this Section 5.6(b) by FLAC.
Section 5.7 Preparation of Registration Statement / Proxy Statement. As promptly as reasonably practicable following the date of this Agreement, FLAC, Holdco and the Company shall prepare and mutually agree upon (such agreement not to be unreasonably withheld, conditioned or delayed by any of the Parties), and Holdco shall file with the SEC, the Registration Statement / Proxy Statement (it being understood that the Registration Statement / Proxy Statement shall include a proxy statement / prospectus of FLAC which will be included therein as a prospectus in connection with the registration under the Securities Act of the Holdco Shares to be issued in the Company Share Exchange and in the Merger and which will be used as a proxy statement for the FLAC Shareholders Meeting to adopt and approve the Transaction Proposals and other matters reasonably related to the Transaction Proposals, all in accordance with and as required by FLAC’s Governing Documents, applicable Law, and any applicable rules and regulations of the SEC and Nasdaq). Each of FLAC, Holdco and the Company shall use its reasonable best efforts to (a) cause the Registration Statement / Proxy Statement to comply in all material respects with the applicable rules and regulations promulgated by the SEC (including, with respect to the Group Companies, the provision of financial statements of, and any other information with respect to, the Group Companies for all periods, and in the form, required to be included in the Registration Statement / Proxy Statement under Securities Laws (after giving effect to any waivers received) or in response to any comments from the SEC); (b) promptly notify the others of the receipt of any comments of the SEC or its staff (with the Parties reasonably cooperating with each other with respect to a prompt response to any such comments); (c) have the Registration Statement / Proxy Statement declared effective under the Securities Act as promptly as reasonably practicable after it is filed with the SEC; and (d) keep the Registration Statement / Proxy Statement effective through the Closing in order to permit the consummation of the Transactions. FLAC, on the one hand, and the Company and Holdco, on the other hand, shall promptly furnish, or cause to be furnished, to the other all information concerning such Party, its Non-Party Affiliates and their respective Representatives that may be required or reasonably requested in connection with any action contemplated by this Section 5.8 or for
A-68
Table of Contents
including in any other statement, filing, notice or application made by or on behalf of Holdco to the SEC or Nasdaq in connection with the Transactions. If any Party becomes aware of any information that should be disclosed in an amendment or supplement to the Registration Statement / Proxy Statement in order to disclose material information or to make the statements included therein, in the light of the circumstances under which they are made, not misleading, then (i) such Party shall promptly inform, in the case of FLAC, the Company and Holdco, or, in the case of the Company and Holdco, FLAC thereof; (ii) such Party shall prepare and mutually agree upon with, in the case of FLAC, the Company and Holdco, or, in the case of the Company and Holdco, FLAC (in either case, such agreement not to be unreasonably withheld, conditioned or delayed), an amendment or supplement to the Registration Statement / Proxy Statement; (iii) Holdco shall file such mutually-agreed upon amendment or supplement with the SEC; and (iv) the Parties shall reasonably cooperate, if appropriate, in mailing such amendment or supplement to the Pre-Closing FLAC Holders. Holdco and the Company shall as promptly as reasonably practicable advise FLAC of the time of effectiveness of the Registration Statement / Proxy Statement, the issuance of any stop order relating thereto or the suspension of the qualification of Holdco Shares for offering or sale in any jurisdiction, and Holdco and the Company shall each use its reasonable best efforts to have any such stop order or suspension lifted, reversed or otherwise terminated. Each of the Parties shall use reasonable best efforts to ensure that none of the information related to it or any of its Non-Party Affiliates or its or their respective Representatives, supplied by or on its behalf for inclusion in the Registration Statement / Proxy Statement will, at the time the Registration Statement / Proxy Statement is initially filed with the SEC, at each time at which it is amended, or at the time it becomes effective under the Securities Act contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they are made, not misleading.
Section 5.8 FLAC Shareholder Approval. As promptly as reasonably practicable following the time at which the Registration Statement / Proxy Statement is declared effective under the Securities Act, FLAC shall (a) give notice of and (b) duly convene and hold, a meeting of its shareholders (the “FLAC Shareholders Meeting”) in accordance with the Governing Documents of FLAC, for the purposes of obtaining the FLAC Shareholder Approval and providing holders of FLAC Class A Shares with the opportunity to elect to effect a FLAC Shareholder Redemption. FLAC shall, through the unanimous approval of its board of directors, recommend to its shareholders that they vote their FLAC Shares in favor of (the “FLAC Board Recommendation”): (i) the adoption and approval of this Agreement and the Transactions (the “Business Combination Proposal”); (ii) adoption and approval of the Merger, along with, in each case, Merger Documents and the transactions contemplated thereby (the “Merger Proposal”); (iii) the adoption and approval of each other proposal that either the SEC or Nasdaq (or the respective staff members thereof) indicates is necessary in its comments to the Registration Statement / Proxy Statement or in correspondence related thereto, if any; (iv) the adoption and approval of each other proposal reasonably agreed by FLAC and the Company as necessary or appropriate in connection with the consummation of the Transactions; and (v) the adoption and approval of a proposal for the adjournment of the FLAC Shareholders Meeting, if necessary, to permit further solicitation of proxies because there are not sufficient votes to approve and adopt any of the foregoing (such proposals in clauses (i) through (v) together, the “Transaction Proposals”); provided that FLAC may postpone or adjourn the FLAC Shareholders Meeting (A) to solicit additional proxies for the purpose of obtaining the FLAC Shareholder Approval, (B) for the absence of a quorum, or (C) to allow reasonable additional time for the filing or mailing of any supplemental or amended disclosures that FLAC has determined, based on the advice of outside legal counsel, is reasonably likely to be required under applicable Law and for such supplemental or amended disclosure to be disseminated and reviewed by the Pre-Closing FLAC Holders prior to the FLAC Shareholders Meeting; provided, further, that, without the consent of the Company, in no event shall FLAC adjourn the FLAC Shareholders Meeting for more than fifteen (15) Business Days later than the most recently adjourned meeting or to a date that is beyond the Termination Date. The FLAC Board Recommendation shall be included in the Registration Statement / Proxy Statement. None of the FLAC Board, FLAC or any committee of the FLAC Board shall withdraw or modify, or propose publicly or by formal action of the FLAC Board, any committee of the FLAC Board or FLAC to withdraw or modify, in a manner adverse to the Company, the FLAC Board Recommendation.
A-69
Table of Contents
Section 5.9 Required Company Shareholder Approval. As promptly as reasonably practicable following the time at which the Registration Statement / Proxy Statement is declared effective under the Securities Act, the Company shall (a) obtain the Required Company Shareholder Approval, (b) cause each Company Shareholder to grant the Company (or a designee of the Company) with an irrevocable power of attorney permitting and directing the Company (or a designee of the Company), acting on behalf of each such Company Shareholder, and the proxyholders under such power of attorney to execute, or otherwise take such legally permissible action as a result of which the Company may act on behalf of each applicable Company Shareholder in order to execute, (i) the Dutch Deed of Issue Company Share Exchange and (ii) any other Ancillary Documents to which such Company Shareholder is or will be a party (to the extent such Company Shareholder has not yet provided such a power of attorney) and (c) cause each Company Shareholder to take, or otherwise take such legally permissible action as a result of which the Company may act on behalf of such Company Shareholder in order to take, all necessary or desirable actions in connection with the Transactions to consummate the Company Share Exchange (and any other Transaction to which such Company Shareholder is a party) in accordance with the terms of this Agreement.
Section 5.10 Merger Sub Shareholder Approval. As promptly as reasonably practicable (and in any event within one (1) Business Day) following the date of this Agreement, Holdco, as the sole shareholder of Merger Sub, will approve and adopt this Agreement, the Ancillary Documents to which such Merger Sub is or will be a party and the Transactions (including the Merger).
Section 5.11 Conduct of Business of FLAC.
(a) From and after the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, FLAC shall, and FLAC shall cause its Subsidiaries to, except as expressly contemplated by this Agreement or any Ancillary Document, as required by applicable Law, (i) operate the business of FLAC in the ordinary course in all material respects and in accordance with all applicable Law, (ii) comply with, and continue performing under, their Governing Documents and the Trust Agreement and (iii) use commercially reasonable efforts to maintain and preserve intact in all material respects the business organization, assets, properties and material business relations of FLAC, taken as a whole.
(b) Without limiting the generality of the foregoing, from and after the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, FLAC shall not, and FLAC shall cause its Subsidiaries to, except as expressly contemplated by this Agreement or any Ancillary Document (including, for the avoidance of doubt, in connection with the Domestication or the PIPE Financing), as required by applicable Law, as set forth on Section 5.11 of the FLAC Disclosure Schedules or as consented to in writing by the Company (such consent, other than in the case of Section 5.11(b)(i), Section 5.11(b)(ii), Section 5.11(b)(iii), Section 5.11(b)(vi), Section 5.11(b)(x) or Section 5.11(b)(xv) not to be unreasonably withheld, conditioned or delayed), not do any of the following:
(i) adopt any amendments, supplements, restatements or modifications to the Trust Agreement, Warrant Agreement or the Governing Documents of FLAC or any of its Subsidiaries;
(ii) declare, set aside, make or pay a dividend on, or make any other distribution or payment in respect of, any Equity Securities of FLAC or any of its Subsidiaries, or repurchase, redeem or otherwise acquire, or offer to repurchase, redeem or otherwise acquire, any outstanding Equity Securities of FLAC or any of its Subsidiaries, as applicable;
(iii) split, combine or reclassify any of its capital stock or other Equity Securities or issue any other security in respect of, in lieu of or in substitution for shares of its capital stock;
(iv) incur, create or assume any Indebtedness, except for Indebtedness in an amount not to exceed $2,000,000 in the aggregate;
A-70
Table of Contents
(v) make any loans or advances to, or capital contributions in, any other Person, other than to, or in, FLAC or any of its Subsidiaries;
(vi) issue any Equity Securities of FLAC or any of its Subsidiaries or grant any additional options, warrants or stock appreciation rights with respect to Equity Securities of the foregoing of FLAC or any of its wholly owned Subsidiaries;
(vii) enter into, renew, modify or revise any FLAC Related Party Transaction (or any Contract or agreement that if entered into prior to the execution and delivery of this Agreement would be a FLAC Related Party Transaction), other than the entry into any Contract with a FLAC Related Party with respect to the incurrence of Indebtedness permitted by Section 5.11(b)(iv);
(viii) make, change or revoke any Tax classification or other material election concerning Taxes, settle any material Tax claim or assessment, or consent to any extension or waiver of the limitation period applicable to or relating to any material Tax claim or assessment, other than any such extension or waiver that is obtained in the ordinary course of business.
(ix) engage in any activities or business or incur any material FLAC Liabilities, other than any activities, businesses or FLAC Liabilities that are otherwise permitted under this Section 5.11 (including, for the avoidance of doubt, any activities or business contemplated by, or Liabilities incurred in connection with, this Agreement, the Company Support Agreement or any Ancillary Document) or consented to by the Company pursuant to this Section 5.11);
(x) authorize, recommend, propose or announce an intention to adopt a plan of complete or partial liquidation or dissolution;
(xi) enter into any Contract with any broker, finder, investment banker or other Person under which such Person is or will be entitled to any brokerage fee, finders’ fee or other commission in connection with the Transactions;
(xii) incorporate, form or organize any new direct or indirect Subsidiary of FLAC or engage in any new line of business that is materially different from the general nature of the businesses of FLAC as of the date of this Agreement;
(xiii) other than in the ordinary course of business, (A) enter into, amend, modify or terminate any material Contract (excluding, for the avoidance of doubt, any expiration or automatic extension or renewal of any such material Contract pursuant to its terms) or (B) waive any material benefit or right under any material Contract;
(xiv) enter into any settlement, conciliation or similar Contract the performance of which would involve the payment by FLAC in excess of $2,000,000, in the aggregate, or that imposes, or by its terms will impose at any point in the future, any material, non-monetary obligations on FLAC;
(xv) change FLAC’s methods of accounting in any material respect, other than changes that are (i) made in accordance with PCAOB standards, (ii) required by changes in applicable Law or GAAP, or (iii) required by FLAC’s auditors;
(xvi) enter into or materially amend any agreement with, or pay, distribute or advance any assets or property to, any of its officers, directors, employees, partners, stockholders or other Affiliates, other than payments or distributions in the ordinary course of business; or
(xvii) enter into any Contract to take, or cause to be taken, any of the actions set forth in this Section 5.11.
A-71
Table of Contents
(c) Notwithstanding anything in this Section 5.11 or this Agreement to the contrary, (i) nothing set forth in this Agreement shall give the Company, directly or indirectly, the right to control or direct the operations of FLAC and (ii) nothing set forth in this Agreement shall prohibit, or otherwise restrict the ability of, FLAC from using the funds held by FLAC outside the Trust Account to pay any FLAC Expenses or FLAC Liabilities or from otherwise distributing or paying over any funds held by FLAC outside the Trust Account to the Sponsor or any of its Affiliates, in each case, prior to the Closing.
Section 5.12 Nasdaq Listing. The Company shall cause Holdco to, and Holdco shall, use its reasonable best efforts to cause Holdco Shares issuable in accordance with this Agreement to be approved for listing on Nasdaq (and FLAC and the Company shall reasonably cooperate in connection therewith), subject to official notice of issuance, as promptly as practicable after the date of this Agreement, and in any event prior to the Final Closing Date and to cause Holdco to satisfy any applicable initial and continuing listing requirements of Nasdaq.
Section 5.13 Trust Account. Upon satisfaction or, to the extent permitted by applicable Law, waiver of the conditions set forth in Article 6 and provision of notice thereof to the Trustee, (a) at the Closing, FLAC shall (i) cause the documents, certificates and notices required to be delivered to the Trustee pursuant to the Trust Agreement to be so delivered, and (ii) make all appropriate arrangements to cause the Trustee to (A) pay as and when due all amounts, if any, payable to the Public Shareholders pursuant to the FLAC Shareholder Redemption, (B) pay the amounts due to the underwriters of the IPO for their deferred underwriting commissions as set forth in the Trust Agreement and (C) immediately thereafter, pay all remaining amounts then available in the Trust Account to FLAC in accordance with the Trust Agreement, and (b) thereafter, the Trust Account shall terminate, except as otherwise provided therein.
Section 5.14 FLAC Indemnification; Directors’ and Officers’ Insurance.
(a) All rights to indemnification or exculpation now existing in favor of the directors and officers of FLAC, as provided in the Governing Documents of FLAC or otherwise in effect as of immediately prior the Effective Date, in either case, solely with respect to any matters occurring on or prior to the Effective Date, shall survive the Transactions and shall continue in full force and effect from and after the Effective Date for a period of six (6) years. FLAC will perform and discharge, or cause to be performed and discharged, all obligations to provide such indemnity and exculpation during such six (6)-year period. To the maximum extent permitted by applicable Law, during such six (6)-year period, FLAC shall advance, or caused to be advanced, expenses in connection with such indemnification as provided in the Governing Documents of FLAC or other applicable agreements as in effect immediately prior the Effective Date. The indemnification and liability limitation or exculpation provisions of the Governing Documents of FLAC shall not, during such six (6)-year period, be amended, repealed or otherwise modified after the Effective Date, in any manner that would materially and adversely affect the rights thereunder of individuals who, as of immediately prior to the Effective Date, or at any time prior to such time, were directors or officers of FLAC (the “FLAC D&O Persons”) entitled to be so indemnified, have their liability limited or be exculpated with respect to any matters occurring on or prior to the Effective Date and relating to the fact that such FLAC D&O Person was a director or officer of FLAC immediately prior the Effective Date, unless such amendment, repeal or other modification is required by applicable Law.
(b) FLAC shall not have any obligation under this Section 5.14 to any FLAC D&O Person when and if a court of competent jurisdiction shall ultimately determine (and such determination shall have become final and non-appealable) that the indemnification of such FLAC D&O Person in the manner contemplated hereby is prohibited by applicable Law.
(c) Prior to the Effective Date, FLAC shall purchase and will cause the Group Companies to maintain, for a period of six (6) years after the Effective Date, without lapses in coverage, a “tail” policy providing directors’ and officers’ liability insurance coverage for the benefit of those Persons who are currently covered by any comparable insurance policies of FLAC as of the date of this Agreement with respect to matters occurring on
A-72
Table of Contents
or prior to the Effective Date (the “FLAC D&O Tail Policy”). Such “tail” policy shall provide coverage on terms (with respect to coverage and amount) that are substantially the same as (and no less favorable in the aggregate to the insured than) the coverage provided under FLAC’s directors’ and officers’ liability insurance policies as of the date of this Agreement; provided that FLAC shall not be obligated to pay a premium for such “tail” policy in excess of 300% of the most recent annual premium paid by FLAC prior to the date of this Agreement. In the event that the premium for the FLAC D&O Tail Policy exceeds 300% of the most recent annual premium paid by FLAC prior to the date of this Agreement, FLAC shall purchase the maximum coverage available for 300% of the most recent annual premium paid by FLAC prior to the date of this Agreement.
(d) If FLAC or any of its successors or assigns shall (i) merge or consolidate with or merge into any other corporation or entity and shall not be the surviving or continuing corporation or entity of such consolidation or merger or (ii) transfer all or substantially all of their respective properties and assets as an entity in one or a series of related transactions to any Person, then in each such case, proper provisions shall be made so that the successors or assigns of FLAC shall assume all of the obligations set forth in this Section 5.14.
(e) The FLAC D&O Persons entitled to the indemnification, liability limitation, exculpation and insurance set forth in this Section 5.14 are intended to be third-party beneficiaries of this Section 5.14. This Section 5.14 shall survive the consummation of the Transactions and shall be binding on all successors and assigns of FLAC.
Section 5.15 Company Indemnification; Directors’ and Officers’ Insurance.
(a) All rights to indemnification or exculpation now existing in favor of the directors and officers of the Group Companies, as provided in the Group Companies’ Governing Documents or otherwise in effect as of immediately prior to the Effective Date, in either case, solely with respect to any matters occurring on or prior to the Effective Date, shall survive the Transactions and shall continue in full force and effect from and after the Effective Date for a period of six (6) years. Holdco will cause the applicable Group Companies to perform and discharge all obligations to provide such indemnity and exculpation during such six (6)-year period. To the maximum extent permitted by applicable Law, during such six (6)-year period, Holdco shall cause the applicable Group Companies to advance expenses in connection with such indemnification as provided in the Group Companies’ Governing Documents or other applicable agreements in effect as of immediately prior to the Effective Date. The indemnification and liability limitation or exculpation provisions of the Group Companies’ Governing Documents shall not, during such six (6)-year period, be amended, repealed or otherwise modified after the Effective Date in any manner that would materially and adversely affect the rights thereunder of individuals who, as of the Effective Date or at any time prior to the Effective Date, were directors or officers of the Group Companies (the “Company D&O Persons”) entitled to be so indemnified, have their liability limited or be exculpated with respect to any matters occurring prior to Closing and relating to the fact that such Company D&O Person was a director or officer of any Group Company prior to the Effective Date, unless such amendment, repeal or other modification is required by applicable Law.
(b) None of the Group Companies shall have any obligation under this Section 5.15 to any Company D&O Person when and if a court of competent jurisdiction shall ultimately determine (and such determination shall have become final and non-appealable) that the indemnification of such Company D&O Person in the manner contemplated hereby is prohibited by applicable Law.
(c) Holdco shall use reasonable best efforts to include and, from and after the Closing, maintain, or cause to be included and so maintained, within Holdco’s directors’ and officers’ liability insurance policy or policies with respect to matters occurring on or after the Effective Date, coverage in commercially reasonable terms, including “prior acts” for the benefit of directors and officers of the Company who are currently covered by any comparable insurance policies as of the date of this Agreement, with respect to matters occurring on or prior to the Effective Date (“Prior Acts Coverage”). If, after reasonable best efforts by Holdco prior to the Closing, Holdco is not able to include such Prior Acts Coverage within Holdco’s directors’ and officers’ liability
A-73
Table of Contents
insurance policy or policies from and after the Closing, then the Company shall purchase, at or prior to the Closing, and Holdco shall maintain, or cause to be maintained, in effect for a period of six (6) years after the Effective Date, without lapses in coverage, a “tail” policy providing directors’ and officers’ liability insurance coverage for the benefit of those Persons who are currently covered by any comparable insurance policies of the Group Companies as of the date of this Agreement with respect to matters occurring on or prior to the Effective Date (the “Company D&O Tail Policy”). Such “tail” policy shall provide coverage on terms (with respect to coverage and amount) that are substantially the same as (and no less favorable in the aggregate to the insured than) the coverage provided under the Group Companies’ directors’ and officers’ liability insurance policies as of the date of this Agreement; provided that none of the Group Companies shall be obligated to pay a premium for such “tail” policy in excess of 300% of the most recent annual premium paid by the Company prior to the date of this Agreement. In the event that the premium for the Company D&O Tail Policy exceeds 300% of the most recent annual premium paid by the Company prior to the date of this Agreement, the Company shall purchase the maximum coverage available for 300% of the most recent annual premium paid by the Company prior to the date of this Agreement.
(d) If the applicable Group Company or any of its successors or assigns (i) shall merge or consolidate with or merge into any other corporation or entity and shall not be the surviving or continuing corporation or entity of such consolidation or merger or (ii) shall transfer all or substantially all of their respective properties and assets as an entity in one or a series of related transactions to any Person, then in each such case, proper provisions shall be made so that the successors or assigns of such Group Company shall assume the appropriate obligations set forth in this Section 5.15.
(e) The Company D&O Persons entitled to the indemnification, liability limitation, exculpation and insurance set forth in this Section 5.15 are intended to be third-party beneficiaries of this Section 5.15. This Section 5.15 shall survive the consummation of the Transactions and shall be binding on all successors and assigns of the Group Companies.
Section 5.16 Post-Closing Directors and Officers.
(a) Holdco shall, subject to applicable Nasdaq listing requirements, take all necessary action and cause that, effective as of the Holdco Reorganization, (i) the Holdco Board shall consist of up to nine (9) directors, with one (1) executive director serving an initial term expiring at the third annual general meeting of Holdco to occur after the Closing and up to eight (8) non-executive directors who shall serve staggered multi-year terms, expiring at the first, second and third annual general meetings of Holdco to occur after the Closing, with the allocation of such terms among such non-executive directors to be determined by mutual agreement between the Company and FLAC following the date of this Agreement; (ii) the members of the Holdco Board are the Persons determined in accordance with Section 5.16(b) (the “Holdco Board Appointments”); (iii) the members of the compensation committee, audit committee and nominating and corporate governance committee of the Holdco Board shall be the non-executive directors determined in accordance with Section 5.16(c); and (iv) the officers of Holdco (the “Officers”) shall be the individuals determined in accordance with Section 5.16(d).
(b) As promptly as practicable following the date of this Agreement, and in any event within sufficient time to allow for customary due diligence and background checks on the designated individuals prior to the mailing of the Registration Statement / Proxy Statement to the Pre-Closing FLAC Holders, (i) FLAC shall identify two (2) individuals to serve as non-executive directors on the Holdco Board who must (A) be reasonably acceptable to the Company to serve as non-executive directors on the Holdco Board immediately after the Holdco Reorganization and (B) qualify as “independent” pursuant to Nasdaq listing standards (the “FLAC Designees”), and (ii) the Company shall identify up to seven (7) individuals to serve as directors, including one executive director and up to six (6) non-executive directors, on the Holdco Board, immediately after the Effective Date (the “Company Designees” and, together with the FLAC Designees, the “Designees”), such Company Designees anticipated to include the individuals identified on Section 5.16(b) of the Company Disclosure Schedules, in all cases subject to applicable listing rules of Nasdaq and applicable Law and subject to customary
A-74
Table of Contents
due diligence and review of background checks. FLAC and the Company will agree in good faith on the initial terms of service for each of the FLAC Designees and Company Designees. Prior to the Effective Date, the Company shall name such Company Designees whose names are not identified on Section 5.16(b) of the Company Disclosure Schedules and may replace any Company Designee whose names are identified on Section 5.16(b) of the Company Disclosure Schedules, in each case with such Company Designee being reasonably acceptable to FLAC, which designation shall be made by written notice and subject to applicable listing rules of Nasdaq and applicable Law and subject to customary due diligence and review of background checks.
(c) Prior to the mailing of the Registration Statement / Proxy Statement to the Pre-Closing FLAC Holders, FLAC and the Company shall designate the Designees to serve as members of the compensation committee, the audit committee and the nominating and corporate governance committee of the Holdco Board, immediately after the Effective Date, subject to applicable listing rules of Nasdaq and applicable Law. In the event that any Designee is unwilling or unable (whether due to death, disability, termination of service or otherwise) to serve as a committee member, then, prior to the mailing of the Registration Statement / Proxy Statement to the Pre-Closing FLAC Holders, FLAC and the Company shall jointly replace such Designee with another Designee to serve as such committee member.
(d) The individuals identified on Section 5.16(d) of the Company Disclosure Schedules shall be the Officers immediately after the Holdco Reorganization, with each such individual holding the title set forth opposite his or her name. In the event that any individual identified on Section 5.16(d) of the Company Disclosure Schedules is unwilling or unable (whether due to death, disability, termination of service or otherwise) to serve as an Officer, then, prior to the mailing of the Registration Statement / Proxy Statement to the Pre-Closing FLAC Holders, the Company may, with the prior written consent to FLAC (such consent not to be unreasonably withheld, conditioned or delayed), replace such individual with another individual to serve as such Officer.
(e) At or prior to the Closing, FLAC shall deliver to the Company and Holdco evidence reasonably acceptable to the Company and Holdco that the members of the FLAC Board and the officers of FLAC, in each case immediately prior to the Closing shall have resigned with effect as of immediately preceding the Effective Date.
(f) Effective as of the Effective Date and as a result of the Merger, the directors and officers of Merger Sub immediately prior to the Effective Date shall be the initial directors and officers of the Surviving Company, each to hold office in accordance with the Governing Documents of the Surviving Company until their respective successors are duly elected or appointed and qualified or their earlier death, resignation or removal.
Section 5.17 PCAOB Financials.
(a) As promptly as reasonably practicable, the Company shall deliver to FLAC and Holdco (i) the final audited Company Financial Statements and the unaudited consolidated statements of financial position of the Group Companies as of June 30, 2022 and June 30, 2021 and the related unaudited consolidated statements of statements of profit or loss and other comprehensive income (loss), changes in equity and cash flows of the Group Companies for each of the six-month periods then ended and the related notes thereto (the “Closing Financial Statements”), and (ii) any other audited or unaudited consolidated statements of financial position and the related audited or unaudited consolidated statements profit or loss and other comprehensive income, changes in equity and cash flows of the Company as of and for a year-to-date period ended as of the end of any other different fiscal quarter (and as of and for the same period from the previous fiscal year) or fiscal year (and as of and for the prior fiscal year), as applicable that is required to be included in the Registration Statement / Proxy Statement and any other filings to be made by FLAC or Holdco with the SEC in connection with the Transactions. All such financial statements (A) will fairly present in all material respects the financial position of the Group Companies as at the date of the statement of financial position, and the results of its operations,
A-75
Table of Contents
shareholders’ equity and cash flows for the respective periods then ended (subject, in the case of any unaudited interim financial statements, to normal year-end audit adjustments (none of which is expected to be material) and the absence of footnotes), (B) will be prepared in conformity with IFRS applied on a consistent basis during the periods involved (except, in the case of any audited financial statements, as may be indicated in the notes thereto and subject, in the case of any unaudited financial statements, to normal year-end audit adjustments (none of which is expected to be material) and the absence of footnotes), (C) in the case of any audited financial statements, will be audited in accordance with the standards of the PCAOB and contain an unqualified report of the Company’s auditor and (D) will comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates thereof (including Regulation S-X).
(b) The Parties shall use their reasonable best efforts (i) to assist, upon advance written notice, during normal business hours and in a manner such as to not unreasonably interfere with the normal operation of any other Party, each other Party in causing to be prepared in a timely manner any other financial information or statements (including customary pro forma financial statements) that are required to be included in the Registration Statement / Proxy Statement and any other filings to be made by FLAC or Holdco with the SEC in connection with the Transactions and (ii) to obtain the consents of its auditors with respect thereto as may be required by applicable Law or requested by the SEC.
Section 5.18 Conduct of Business of Holdco(a) . Except as set forth on Section 5.18 of the Company Disclosure Schedules, from and after the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, Holdco shall not take any action, or engage in any activities or business, nor incur any liabilities or obligations, other than (a) those that are incident to its organization, (b) the execution of this Agreement or any Ancillary Document to which it is or will be a party, (c) those that are required by the SEC or Nasdaq in connection with the Transactions, (d) those that are expressly contemplated by this Agreement or any Ancillary Document (including the enforcement of any of its rights or the performance of any of its obligations under this Agreement or any Ancillary Documents and the consummation of the transactions contemplated hereby or thereby) or (e) those that are consented to in writing by FLAC (such consent not to be unreasonably withheld, conditioned or delayed).
Section 5.19 Holdco Equity Incentive Plan. Prior to the effectiveness of the Registration Statement / Proxy Statement, the Holdco Board shall approve and adopt the Holdco Long-Term Incentive Plan (the “Holdco Equity Incentive Plan”) (subject to its approval by the Holdco Shareholders), substantially in the form attached hereto as Exhibit J, and with any changes or modifications thereto as the Company and FLAC may mutually agree (such agreement not to be unreasonably withheld, conditioned or delayed by either the Company or FLAC, as applicable), in the manner prescribed under applicable Laws, effective as of the Final Closing Date. The Holdco Equity Incentive Plan shall reserve a number of Holdco Shares for grant thereunder equal to the sum of (a) thirteen percent (13%) of the aggregate number of Holdco Shares outstanding immediately following the Effective Date after giving effect to the Company Share Exchange, exercise of the Company Issuance Rights, the Merger, the PIPE Financing and the other Transactions contemplated hereby (but not, for the avoidance of doubt, exercise of any Holdco Warrants), which aggregate number of Holdco Shares in this clause (a) shall be inclusive of Holdco Shares underlying the Rollover Company Options granted pursuant to Section 2.6, plus (b) the maximum number of Holdco Shares that may be issued as Earnout RSUs; provided that the number of Holdco Shares initially reserved for grant under the Holdco Equity Incentive Plan shall be increased annually on January 1 of each calendar year, starting on the first January 1 after Closing (or on the Closing Date, if Closing occurs after January 1, 2023), by five percent (5%) of the then issued and outstanding Holdco Shares or such lower number as may be determined by the Holdco Board. Holdco Shares reserved under the Holdco Equity Incentive Plan pursuant to clause (b) of the preceding sentence shall be available to be issued only with respect to Earnout RSUs and to the extent Earnout RSUs are forfeited or do not become issuable, such Holdco Shares shall not be available for issuance pursuant to other awards.
Section 5.20 PIPE Subscriptions. Unless otherwise approved in writing by FLAC or Holdco, as the case may be, neither Holdco nor FLAC shall permit any amendment or modification to be made to, any waiver (in
A-76
Table of Contents
whole or in part) of, or provide consent to modify (including consent to terminate), any provision or remedy under, or any replacements of, any of the Subscription Agreements, in each case, other than any assignment or transfer contemplated therein or expressly permitted thereby (without any further amendment, modification or waiver to such assignment or transfer provision); provided that, in the case of any such permitted assignment or transfer, the initial party to such Subscription Agreement shall remain bound by its obligations with respect thereto in the event that the transferee or assignee, as applicable, does not comply with its obligations to consummate the purchase of Holdco Shares contemplated thereby. Subject to the immediately preceding sentence and in the event that all conditions in the Subscription Agreements have been satisfied, each of Holdco and FLAC shall use its respective reasonable best efforts to take, or to cause to be taken, all actions required, necessary or that it otherwise deems to be proper or advisable to consummate the transactions contemplated by the Subscription Agreements on the terms described therein, including using its reasonable best efforts to enforce its rights under the Subscription Agreements to cause the PIPE Investors to pay to (or as directed by) Holdco the applicable purchase price under each PIPE Investor’s applicable Subscription Agreement in accordance with its terms. Without limiting the generality of the foregoing, Holdco and FLAC shall give each other prompt written notice: (i) of the receipt of any request from a PIPE Investor for an amendment to any Subscription Agreement; (ii) of any material breach or material default to the knowledge of Holdco or FLAC, as the case may be (or any event or circumstance that, to the knowledge of Holdco or FLAC, as the case may be, with or without notice, lapse of time or both, would be reasonably likely to give rise to any such breach or default) by any party to any Subscription Agreement; (iii) of the receipt by Holdco or FLAC of any written notice or other written communication from any PIPE Investor with respect to any actual or threatened or claimed expiration, lapse, withdrawal, breach, default, termination or repudiation of the Subscription Agreement by such PIPE Investor; and (iv) if Holdco does not expect to receive all or any portion of the applicable purchase price under any PIPE Investor’s Subscription Agreement in accordance with its terms.
Section 5.21 EU Securities Regulation. From and after the date of this Agreement until the earlier of the Closing and the termination of this Agreement, the Parties shall not make any offer of securities in the European Union in connection with the Transactions other than in accordance with the provisions of the Prospectus Regulation. If the Parties determine that a prospectus or a prospectus exemption document (as applicable) may be required to be published in accordance with the provisions of the Prospectus Regulation, each Party shall use its reasonable best efforts to take such actions and to do such things that such Party deems reasonably necessary or desirable, including the delivery or execution of any documents or instruments reasonably required or desirable in order for the Company or Holdco to publish a prospectus or be exempted from the obligation to publish a prospectus or a prospectus exemption document (as applicable) under the Prospectus Regulation.
Section 5.22 Employee Stock Purchase Plan. Upon the election of the Company, the Parties will cooperate in good faith and agree upon the form of an employee stock purchase plan designed to allow eligible employees to purchase Holdco Shares at periodic intervals with their accumulated payroll deductions (the “ESPP”), to be adopted by Holdco prior to the Closing and effective as of the Closing. In case so adopted, the ESPP will (a) with respect to U.S. employees, be intended to be an employee stock purchase plan under Section 423 of the Code, (b) provide for a maximum number of Holdco Shares authorized for sale thereunder not to exceed a specified percentage of the aggregate number of Holdco Shares outstanding immediately following the Effective Date after giving effect to the Company Share Exchange, exercise of the Company Issuance Rights, the Merger, the PIPE Financing and the other Transactions contemplated hereby (but not, for the avoidance of doubt, exercise of any Holdco Warrants), subject to annual increases of not more than a percentage of such aggregate number of Holdco Shares, in each case with such maximum percentages to be agreed between Holdco and FLAC following the date of this Agreement, (c) provide for purchase and offering periods to be implemented from time to time, (d) contemplate a purchase price per Holdco Share thereunder of no less than the lower of (i) 85% of the closing trading price per Holdco Share on the first day of an applicable offering period or (ii) 85% of the closing trading price per Holdco Share on the applicable purchase date, and (e) provide that all of an employee’s ESPP options may not vest and become exercisable with respect to more than $25,000 worth of Holdco Shares (measured as of the date of grant) per year.
A-77
Table of Contents
ARTICLE 6
CONDITIONS TO CONSUMMATION OF THE TRANSACTIONS
Section 6.1 Conditions to the Obligations of the Parties. The obligations of the Parties to consummate the Merger are subject to the satisfaction or, if permitted by applicable Law, waiver by the Party for whose benefit such condition exists, of the following conditions:
(a) the applicable waiting period (and any extension(s) thereof) relating to the Transactions shall have expired or been terminated and any other applicable Consent shall have been obtained (or deemed, by applicable Law, to have been obtained), as applicable, so that the Transactions are deemed to be cleared, approved or consented to under any applicable Antitrust Law;
(b) no Order or Law issued by any court of competent jurisdiction or other Governmental Entity or other legal restraint or prohibition, in each case preventing the consummation of the Transactions, shall be in effect;
(c) the Registration Statement / Proxy Statement shall have become effective in accordance with the provisions of the Securities Act, no stop order suspending the effectiveness of the Registration Statement / Proxy Statement shall have been issued under the Securities Act and shall remain in effect with respect to the Registration Statement / Proxy Statement, and no Proceeding seeking such a stop order shall have been threatened or initiated by the SEC and remain pending;
(d) the Required FLAC Shareholder Approval shall have been obtained;
(e) the Required Company Shareholder Approval shall have been obtained;
(f) (i) Holdco’s initial listing application with Nasdaq in connection with the Transactions shall have been approved such that, immediately following the Closing, Holdco shall satisfy any applicable initial and continuing listing requirements of Nasdaq, (ii) Holdco shall not have received any notice of non-compliance therewith, and (iii) the Holdco Shares and Holdco Warrants to be issued in connection with the Transactions shall have been approved for listing on Nasdaq, subject to official notice of issuance;
(g) the size and composition of Holdco Board shall be as contemplated under Section 5.16; and
(h) after giving effect to the Transactions (including the PIPE Financing and the FLAC Shareholder Redemption), Holdco shall have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) immediately after the Effective Date.
Section 6.2 Other Conditions to the Obligations of FLAC. The obligations of FLAC to consummate the Merger are subject to the satisfaction or, if permitted by applicable Law, waiver by FLAC, of the following further conditions:
(a) (i) the Company Fundamental Representations shall be true and correct in all material respects (except for such representations and warranties that are qualified by their terms by any limitation as to “materiality,” “Company Material Adverse Effect” or similar limitation, which representations and warranties as so qualified shall be true and correct in all respects) as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date (except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all material respects as of such earlier date); (ii) the Company Capitalization Representations shall be true and correct (without giving effect to any limitation as to “materiality” or “Company Material Adverse Effect” or any similar limitation set forth herein) in all respects as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date
A-78
Table of Contents
(except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all respects as of such earlier date), other than de minimis inaccuracies; and (iii) the representations and warranties of the Company set forth in Article 3 (other than the Company Fundamental Representations and the Company Capitalization Representations) shall be true and correct (without giving effect to any limitation as to “materiality” or “Company Material Adverse Effect” or any similar limitation set forth herein) in all respects as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date (except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all respects as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not constitute a Company Material Adverse Effect;
(b) the Company shall have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by the Company under this Agreement at or prior to the Closing;
(c) since the date of this Agreement, no Company Material Adverse Effect shall have occurred and be continuing;
(d) at or prior to the Closing, the Company shall have delivered, or caused to be delivered, to FLAC the following documents:
(i) a certificate duly executed by an authorized officer of the Company, dated as of the Closing Commencement Date, to the effect that the conditions specified in Section 6.2(a), Section 6.2(b) and Section 6.2(c) are satisfied; and
(ii) the Investor Rights Agreement duly executed by the Company and the IRA Shareholders.
Section 6.3 Other Conditions to the Obligations of the Company. The obligations of the Company to consummate the Merger are subject to the satisfaction or, if permitted by applicable Law, waiver by the Company, of the following further conditions:
(a) the Aggregate Cash Proceeds shall be equal to or greater than $250,000,000;
(b) (i) the FLAC Fundamental Representations shall be true and correct in all material respects (except for such representations and warranties that are qualified by their terms by any limitation as to “materiality,” “FLAC Material Adverse Effect” or similar limitation, which representations and warranties as so qualified shall be true and correct in all respects) as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date (except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all material respects as of such earlier date); (ii) the FLAC Capitalization Representations shall be true and correct (without giving effect to any limitation as to “materiality” or “FLAC Material Adverse Effect” or any similar limitation set forth herein) in all respects as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date (except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all respects as of such earlier date), other than de minimis inaccuracies; and (iii) the representations and warranties of FLAC set forth in Article 4 (other than the FLAC Fundamental Representations and the FLAC Capitalization Representations) shall be true and correct (without giving effect to any limitation as to “materiality” or “FLAC Material Adverse Effect” or any similar limitation set forth herein) in all respects as of the date of this Agreement and as of the Closing Commencement Date, as though made on and as of the Closing Commencement Date (except to the extent that any such representation and warranty is made of an earlier date, in which case such representation and warranty shall be true and correct in all material respects as of such earlier date), except where the failure of such representations and warranties to be true and correct, taken as a whole, does not constitute a FLAC Material Adverse Effect;
A-79
Table of Contents
(c) FLAC shall have performed and complied in all material respects with the covenants and agreements required to be performed or complied with by FLAC under this Agreement at or prior to the Closing;
(d) at or prior to the Closing, FLAC shall have delivered, or caused to be delivered, the following documents to the Company:
(i) a certificate duly executed by an authorized officer of FLAC, dated as of the Closing Commencement Date, to the effect that the conditions specified in Section 6.3(a), Section 6.3(b) and Section 6.3(c) are satisfied; and
(ii) the Investor Rights Agreement duly executed by FLAC, the Sponsor and any other FLAC Related Party party thereto.
Section 6.4 Frustration of Closing Conditions. The Company may not rely on the failure of any condition set forth in this Article 6 to be satisfied if such failure was proximately caused by the Company’s failure to use reasonable best efforts to cause the Closing to occur, as required by Section 5.3, or a breach of this Agreement. FLAC may not rely on the failure of any condition set forth in this Article 6 to be satisfied if such failure was proximately caused by FLAC’s failure to use reasonable best efforts to cause the Closing to occur, as required by Section 5.3, or a breach of this Agreement.
ARTICLE 7
TERMINATION
Section 7.1 Termination. This Agreement may be terminated and the Transactions may be abandoned at any time prior to the Closing:
(a) by mutual written consent of FLAC and the Company;
(b) by FLAC, if any of the representations or warranties of the Company set forth in Article 3 shall not be true and correct or if Holdco or the Company has failed to perform or comply with any of their respective covenants or agreements set forth in this Agreement (including an obligation to consummate the Closing) such that the conditions to Closing set forth in either Section 6.2(a) or Section 6.2(b) would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failures to perform or comply with such covenants or agreements, as applicable, is (or are) not cured or cannot be cured within the earlier of (i) thirty (30) days after written notice thereof is delivered to the Company by FLAC, and (ii) the Termination Date; provided, however, that the right to terminate this Agreement pursuant to this Section 7.1(b) shall not be available to FLAC if FLAC is then in breach of this Agreement so as to prevent the conditions to Closing set forth in either Section 6.3(b) or Section 6.3(c) from being satisfied;
(c) by the Company, if any of the representations or warranties of FLAC set forth in Article 4 shall not be true and correct or if FLAC has failed to perform or comply with any of its covenants or agreements set forth in this Agreement (including an obligation to consummate the Closing) such that the conditions to Closing set forth in either Section 6.3(b) or Section 6.3(c) would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failures to perform or comply with such covenants or agreements, as applicable, is (or are) not cured or cannot be cured within the earlier of (i) thirty (30) days after written notice thereof is delivered to FLAC by the Company, and (ii) the Termination Date; provided, however, that the right to terminate this Agreement pursuant to this Section 7.1(c) shall not be available to the Company if the Company is then in breach of this Agreement so as to prevent the conditions to Closing set forth in either Section 6.2(a) or Section 6.2(b) from being satisfied;
(d) by either FLAC or the Company, if the Transactions shall not have been consummated on or prior to December 11, 2022 (the “Termination Date”); provided that if the Registration Statement / Proxy Statement
A-80
Table of Contents
filed pursuant to Section 5.7 is not declared effective by November 1, 2022, then the Termination Date will be automatically extended by sixty (60) days to February 9, 2023; and provided, further, that the right to terminate this Agreement pursuant to this Section 7.1(d) shall not be available to a Party if such Party’s breach of any of its covenants or obligations under this Agreement shall have proximately caused the failure to consummate the Transactions on or before the Termination Date;
(e) by either FLAC or the Company, if any Governmental Entity shall have issued an Order or taken any other action permanently enjoining, restraining or otherwise prohibiting the Transactions and such Order or other action shall have become final and nonappealable; or
(f) by either FLAC or the Company if the FLAC Shareholders Meeting has been held (including any adjournment or postponement thereof), has concluded, the FLAC Shareholders have duly voted and the Required FLAC Shareholder Approval was not obtained.
Section 7.2 Effect of Termination. In the event of the termination of this Agreement pursuant to Section 7.1, this entire Agreement shall forthwith become void (and there shall be no Liability or obligation on the part of the Parties and their respective Representatives) with the exception of Section 5.3, this Section 7.2, Article 8 and Article 1 (to the extent related to the foregoing), each of which shall survive such termination and remain valid and binding obligations of the Parties. Notwithstanding the foregoing, the termination of this Agreement pursuant to Section 7.1 shall not affect (a) any Liability on the part of any Party for actual fraud or Willful Breach of any covenant or agreement set forth in this Agreement prior to such termination or (b) any Person’s Liability under any Subscription Agreement, the Confidentiality Agreement, the Company Support Agreement or the Sponsor Support Agreement to which such Person is a party to the extent arising from a claim against such Person by another Person party to such agreement on the terms and subject to the conditions thereunder.
ARTICLE 8
MISCELLANEOUS
Section 8.1 Non-Survival. The representations, warranties, agreements and covenants in this Agreement shall terminate at the Effective Date, except for those covenants and agreements that, by their express terms, contemplate performance after the Effective Date.
Section 8.2 Entire Agreement; Assignment. This Agreement (together with the Ancillary Documents) constitutes the entire agreement among the Parties with respect to the subject matter hereof and supersedes all other prior agreements and understandings, both written and oral, among the Parties with respect to the subject matter hereof. This Agreement may not be assigned by any Party (whether by operation of law or otherwise) without the prior written consent of (a) FLAC and the Company prior to Closing and (b) Holdco and the Sponsor after the Closing. Any attempted assignment of this Agreement not in accordance with the terms of this Section 8.2 shall be void. Subject to the preceding sentence, this Agreement shall be binding upon and inure to the benefit of the Parties and their respective permitted successors and assigns.
Section 8.3 Amendment. This Agreement may be amended or modified only by a written agreement executed and delivered by (a) FLAC and the Company prior to the Closing and (b) Holdco and the Sponsor after the Closing. This Agreement may not be modified or amended except as provided in the immediately preceding sentence and any purported amendment by any Party or Parties effected in a manner which does not comply with this Section 8.3 shall be void, ab initio. Subject to the foregoing, this Agreement may be amended before or after the approval of this Agreement by the shareholders of the Company, Holdco or FLAC; provided that after any such shareholder approval, no amendment shall be made to this Agreement that by Law requires further approval or authorization by the shareholders of the Company or FLAC without such further approval or authorization.
A-81
Table of Contents
Section 8.4 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given) by delivery in person, by e-mail (having obtained electronic delivery confirmation thereof (i.e., an electronic record of the sender that the e-mail was sent to the intended recipient thereof without an “error” or similar message that such e-mail was not received by such intended recipient)), or by registered or certified mail (postage prepaid, return receipt requested) (upon receipt thereof) to the other Parties as follows:
(a) If to FLAC, to:
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union St., Suite 3200
Seattle, Washington 98101
| Attention: | James N. Topper | |||
| David Topper | ||||
| E-mail: | james@frazierhealthcare.com | |||
| david.topper@frazierhealthcare.com |
with a copy (which shall not constitute notice) to:
Goodwin Procter LLP
100 Northern Avenue
Boston, Massachusetts 02210
| Attention: | Jocelyn M. Arel | |||
| Jacqueline Mercier | ||||
| E-mail: | jarel@goodwinlaw.com | |||
| jmercier@goodwinlaw.com |
(b) If to the Company or, after the Closing, Holdco, to:
c/o NewAmsterdam Pharma B.V.
20803 Biscayne Boulevard
Suite 105
Aventura, FL 33180
| Attention: | Michael Davidson | |||
| E-mail: |
with a copy (which shall not constitute notice) to:
c/o NewAmsterdam Pharma B.V.
c/o NewAmsterdam Pharma Holding B.V.
Gooimeer 2-35
1411 DC Naarden
The Netherlands
| Attention: | Michael Davidson, Chief Executive Officer | |||
| E-mail: |
with a copy (which shall not constitute notice) to:
Covington & Burling LLP
The New York Times Building
620 Eighth Avenue
A-82
Table of Contents
New York, NY 10018
| Attention: | Jack S. Bodner | |||
| Kerry S. Burke | ||||
| Brian K. Rosenzweig | ||||
| Facsimile: | 646-441-9079 | |||
| E-mail: | jbodner@cov.com | |||
| kburke@cov.com | ||||
| brosenzweig@cov.com |
or to such other address as the Party to whom notice is given may have previously furnished to the others in writing in the manner set forth above.
Section 8.5 Governing Law. This Agreement shall be governed by and construed in accordance with the Laws of the State of Delaware, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the law of any jurisdiction other than the State of Delaware (except that the Cayman Companies Act shall apply to the Merger and applicable Dutch Law shall apply to the Company Share Exchange and the Holdco Reorganization).
Section 8.6 Fees and Expenses. Except as otherwise set forth in this Agreement, all fees and expenses incurred in connection with this Agreement, the Ancillary Documents and the Transactions, including the fees and disbursements of counsel, financial advisors and accountants, shall be paid by the Party incurring such fees or expenses; provided that, for the avoidance of doubt, (a) if this Agreement is terminated in accordance with its terms, the Company shall pay, or cause to be paid, all Unpaid Company Expenses and FLAC shall pay, or cause to be paid, all Unpaid FLAC Expenses and (b) if the Closing occurs, then FLAC shall pay, or cause to be paid, all Unpaid Company Expenses and all Unpaid FLAC Expenses.
Section 8.7 Construction; Interpretation. The term “this Agreement” means this Business Combination Agreement together with the Schedules and Exhibits hereto, as the same may from time to time be amended, modified, supplemented or restated in accordance with the terms hereof. The headings set forth in this Agreement are inserted for convenience only and shall not affect in any way the meaning or interpretation of this Agreement. No Party, nor its respective counsel, shall be deemed the drafter of this Agreement for purposes of construing the provisions hereof, and all provisions of this Agreement shall be construed according to their fair meaning and not strictly for or against any Party. Unless otherwise indicated to the contrary herein by the context or use thereof: (a) the words, “herein,” “hereto,” “hereof” and words of similar import refer to this Agreement as a whole, including the Schedules and Exhibits, and not to any particular section, subsection, paragraph, subparagraph or clause set forth in this Agreement; (b) masculine gender shall also include the feminine and neutral genders, and vice versa; (c) words importing the singular shall also include the plural, and vice versa; (d) the words “include,” “includes” or “including” shall be deemed to be followed by the words “without limitation”; (e) references to “$” or “dollar” or “US$” shall be references to United States dollars; (f) the word “or” is disjunctive but not necessarily exclusive; (g) the words “writing,” “written” and comparable terms refer to printing, typing and other means of reproducing words (including electronic media) in a visible form; (h) the word “day” means a calendar day unless Business Day is expressly specified; (i) the word “extent” in the phrase “to the extent” means the degree to which a subject or other thing extends, and such phrase shall not mean simply “if”; (j) all references to Articles, Sections, Exhibits or Schedules are to Articles, Sections, Exhibits and Schedules of this Agreement; (k) the words “provided” or “made available” or words of similar import (regardless of whether capitalized or not) mean, when used with reference to documents or other materials required to be provided or made available to FLAC, any documents or other materials posted to the electronic data room located at www.dfsvenue.com under the project name “Project Yankee” as of 5:00 p.m., Eastern Time, at least one (1) day prior to the date of this Agreement; (l) the expression “ordinary course of business” means in the ordinary and usual course of the Company’s or FLAC’s business, as applicable, consistent with past practice (including, for the avoidance of doubt, recent past practice in light of COVID-19); provided that, notwithstanding anything to the contrary contained in this Agreement, nothing herein shall prevent the Company from taking or failing to take any
A-83
Table of Contents
COVID-19 Actions and (i) no such COVID-19 Actions shall be deemed to violate or breach this Agreement in any way, (ii) all such COVID-19 Actions shall be deemed to constitute an action taken in the ordinary course of business and (iii) no such COVID-19 Actions shall serve as a basis for FLAC to terminate this Agreement or assert that any of the conditions to the Closing contained herein have not been satisfied; (m) all references to any Law will be to such Law as consolidated, replaced, revised, amended or supplemented from time to time, and the rules or regulations thereunder; and (n) all references to any Contract are to that Contract as amended, supplemented or otherwise modified from time to time in accordance with the terms thereof (subject to any restrictions on amendments or modifications set forth in this Agreement). If any action under this Agreement is required to be done or taken on a day that is not a Business Day, then such action shall be required to be done or taken not on such day but on the first succeeding Business Day thereafter. For the avoidance of doubt, in the event of a conflict between the terms of this Agreement and the Company Support Agreement or the Sponsor Support Agreement, the terms of this Agreement shall prevail in each case.
Section 8.8 Exhibits and Schedules. All Exhibits and Schedules, or documents expressly incorporated into this Agreement, shall form an integral part of this Agreement and are hereby incorporated into this Agreement and made a part hereof as if set out in full in this Agreement. The Schedules shall be arranged in sections and subsections corresponding to the numbered and lettered Sections and subsections set forth in this Agreement. Any item disclosed in the Company Disclosure Schedules or in the FLAC Disclosure Schedules corresponding to any Section or subsection of Article 3 (in the case of the Company Disclosure Schedules) or Article 4 (in the case of the FLAC Disclosure Schedules) shall be deemed to have been disclosed with respect to every other section and subsection of Article 3 (in the case of the Company Disclosure Schedules) or Article 4 (in the case of the FLAC Disclosure Schedules), as applicable, where the relevance of such disclosure to such other Section or subsection is reasonably apparent on the face of the disclosure. The information and disclosures set forth in the Schedules that correspond to the section or subsections of Article 3 or Article 4 may not be limited to matters required to be disclosed in the Schedules, and any such additional information or disclosure is for informational purposes only and does not necessarily include other matters of a similar nature.
Section 8.9 Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each Party and its successors and permitted assigns and, except as provided in Section 5.14, Section 5.15, the last sentence of this Section 8.9 and Section 8.13, nothing in this Agreement, express or implied, is intended to or shall confer upon any other Person any rights, benefits or remedies of any nature whatsoever under or by reason of this Agreement. The Sponsor shall be an express third-party beneficiary of Section 8.2, Section 8.3, this Section 8.9 and Section 8.13. Each of the Non-Party Affiliates shall be an express third-party beneficiary of this Section 8.9 and Section 8.13.
Section 8.10 Severability. Whenever possible, each provision of this Agreement will be interpreted in such a manner as to be effective and valid under applicable Law, but if any term or other provision of this Agreement is held to be invalid, illegal or unenforceable under applicable Law, all other provisions of this Agreement shall remain in full force and effect so long as the economic or legal substance of the Transactions is not affected in any manner materially adverse to any Party. Upon such determination that any term or other provision of this Agreement is invalid, illegal or unenforceable under applicable Law, the Parties shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in an acceptable manner in order that the Transactions are consummated as originally contemplated to the greatest extent possible.
Section 8.11 Counterparts; Electronic Signatures. This Agreement and each Ancillary Document may be executed in one or more counterparts, each of which shall be deemed to be an original, but all of which shall constitute one and the same agreement. Delivery of an executed counterpart of a signature page to this Agreement or any Ancillary Document by facsimile, e-mail, or scanned pages shall be effective as delivery of a manually executed counterpart to this Agreement or any such Ancillary Document.
Section 8.12 Knowledge of Company; Knowledge of FLAC. For all purposes of this Agreement, the phrase “to the Company’s knowledge,” “to the knowledge of the Company” and “known by the Company” and
A-84
Table of Contents
any derivations thereof shall mean as of the applicable date, the actual knowledge of the individuals set forth on Section 8.12 of the Company Disclosure Schedules, solely in their respective capacities as directors, officers or employees of the Company, as applicable, assuming reasonable due inquiry and investigation of his or her direct reports. For all purposes of this Agreement, the phrase “to FLAC’s knowledge,” “to the knowledge of FLAC” and “known by FLAC” and any derivations thereof shall mean as of the applicable date, the actual knowledge of the individuals set forth on Section 8.12 of the FLAC Disclosure Schedules, solely in their respective capacities as directors, officers or employees of FLAC, as applicable, assuming reasonable due inquiry and investigation of his or her direct reports. For the avoidance of doubt, none of the individuals set forth on Section 8.12 of the Company Disclosure Schedules or Section 8.12 of the FLAC Disclosure Schedules shall have any personal Liability or obligations regarding such knowledge.
Section 8.13 No Recourse. Except for claims pursuant to any Ancillary Document by any party(ies) thereto against any Non-Party Affiliate, and then solely with respect to claims against the Non-Party Affiliates that are party to the applicable Ancillary Document, each Party agrees on behalf of itself and on behalf of the Company Non-Party Affiliates, in the case of the Company, and the FLAC Non-Party Affiliates, in the case of FLAC, that (a) this Agreement may only be enforced against, and any action for breach of this Agreement may only be made against, the Parties, and no claims of any nature whatsoever arising under or relating to this Agreement, the negotiation hereof or its subject matter, or the Transactions shall be asserted against any Non-Party Affiliate, and (b) none of the Non-Party Affiliates shall have any Liability arising out of or relating to this Agreement, the negotiation hereof or its subject matter, or the Transactions, including with respect to any claim (whether in tort, contract or otherwise) for breach of this Agreement or in respect of any written or oral representations made or alleged to be made in connection herewith, as expressly provided herein, or for any actual or alleged inaccuracies, misstatements or omissions with respect to any information or materials of any kind furnished by the Company, FLAC or any Non-Party Affiliate concerning any Group Company, FLAC, this Agreement or the Transactions, other than in the case of actual fraud.
Section 8.14 Extension; Waiver. Any Party may, at any time prior to the Closing, (a) extend the time for the performance of the obligations or acts of any other Party to be performed hereunder, (b) waive any inaccuracies in the representations and warranties of any other Party that are contained in this Agreement or (c) waive compliance by any other Party with any of the agreements or conditions contained in this Agreement. Any agreement on the part of any such Party to any such extension or waiver shall be valid only if set forth in a written instrument signed on behalf of such Party. Any waiver of any term or condition shall not be construed as a waiver of any subsequent breach or a subsequent waiver of the same term or condition, or a waiver of any other term or condition of this Agreement. The failure of any Party to assert any of its rights hereunder shall not constitute a waiver of such rights.
Section 8.15 Waiver of Jury Trial. EACH PARTY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY PROCEEDING, CLAIM, DEMAND, ACTION, OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR UNDER ANY ANCILLARY DOCUMENT OR (II) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES IN RESPECT OF THIS AGREEMENT OR ANY ANCILLARY DOCUMENT OR ANY OF THE TRANSACTIONS RELATED HERETO OR THERETO OR ANY FINANCING IN CONNECTION WITH THE TRANSACTIONS CONTEMPLATED HEREBY OR ANY OF THE TRANSACTIONS CONTEMPLATED THEREBY, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. EACH PARTY HEREBY AGREES AND CONSENTS THAT ANY SUCH PROCEEDING, CLAIM, DEMAND, ACTION OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE AN ORIGINAL COUNTERPART OF A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF
A-85
Table of Contents
LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) SUCH PARTY MAKES THIS WAIVER VOLUNTARILY AND (D) SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS Section 8.15.
Section 8.16 Submission to Jurisdiction. Each of the Parties irrevocably and unconditionally submits to the exclusive jurisdiction of the Chancery Court of the State of Delaware (or, if the Chancery Court of the State of Delaware declines to accept jurisdiction, any state or federal court within the State of Delaware or, in the event each federal court within the State of Delaware declines to accept jurisdiction, any other Delaware state court), for the purposes of any Proceeding (a) arising under this Agreement or under any Ancillary Document or (b) in any way connected with or related or incidental to the dealings of the Parties in respect of this Agreement or any such Ancillary Document or any of the Transactions (except, in the case of any Ancillary Documents referenced in the foregoing clauses (a) and (b) where such Ancillary Document expressly provides that a different court shall have jurisdiction with respect to matters pertaining to such Ancillary Document, in which case any such Proceedings to the extent arising under such Ancillary Document shall be brought in accordance with the provisions thereof), and irrevocably and unconditionally waives any objection to the laying of venue of any such Proceeding in any such court, and further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such Proceeding has been brought in an inconvenient forum. Subject to the exceptions in the foregoing sentence, each Party irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any Proceeding against such Party (i) arising under this Agreement or under any Ancillary Document or (ii) in any way connected with or related or incidental to the dealings of the Parties in respect of this Agreement or any Ancillary Document or any of the Transactions, (A) any claim that it is not personally subject to the jurisdiction of the courts as described in this Section 8.16 for any reason, (B) that it or its property is exempt or immune from the jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (C) that (1) the Proceeding in any such court is brought against such Party in an inconvenient forum, (2) the venue of such Proceeding against such Party is improper or (3) this Agreement, or the subject matter hereof, may not be enforced against such Party in or by such courts. Each Party agrees that service of any process, summons, notice or document by registered mail to such party’s respective address set forth in Section 8.4 shall be effective service of process for any such Proceeding.
Section 8.17 Remedies. Except as otherwise expressly provided herein, any and all remedies provided herein will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that the Parties do not perform their respective obligations under the provisions of this Agreement (including failing to take such actions as are required of them hereunder to consummate the Transactions) in accordance with their specific terms or otherwise breach such provisions. It is accordingly agreed that the Parties shall be entitled to an injunction or injunctions, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement, in each case, without posting a bond or undertaking and without proof of damages and this being in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement on the basis that the other Parties have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity. Without limiting the foregoing, each Party hereby agrees that service of process upon such Party in any Proceeding contemplated by this Section 8.17 shall be effective if notice is given in accordance with Section 8.4.
Section 8.18 Trust Account Waiver. Reference is made to the final prospectus of FLAC, filed with the SEC (File No. 333-250858) on December 10, 2020 (the “Prospectus”). The Company acknowledges, agrees and
A-86
Table of Contents
understands that FLAC has established a Trust Account containing the proceeds of its IPO and from certain private placements occurring simultaneously with the IPO, including interest accrued from time to time thereon (the “Trust Account”) for the benefit of public shareholders of FLAC (including overallotment shares acquired by FLAC’s underwriters, the “Public Shareholders”), and FLAC may disburse monies from the Trust Account only in the express circumstances described in the Prospectus. For and in consideration of FLAC entering into this Agreement, and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, each of the Company, Holdco, and Merger Sub hereby agrees on behalf of itself and its respective Representatives that, notwithstanding anything to the contrary in this Agreement, none of the Company, Holdco, Merger Sub or their respective Representatives does now or shall at any time hereafter have any right, title, interest or claim of any kind in or to any monies in the Trust Account or distributions therefrom, or make any claim against the Trust Account (including any distributions therefrom), regardless of whether such claim arises as a result of, in connection with or relating in any way to, this Agreement or any proposed or actual business relationship between FLAC or any of its Representatives, on the one hand, and the Company, Holdco, Merger Sub or any of their respective Representatives, on the other hand, or any other matter, and regardless of whether such claim arises based on contract, tort, equity or any other theory of legal liability (any and all such claims are collectively referred to hereafter as the “Trust Account Released Claims”). Each of the Company, Holdco and Merger Sub, on behalf of itself and its respective Representatives, hereby irrevocably waives any Trust Account Released Claims that the Company, Holdco, Merger Sub or any of their Representatives may have against the Trust Account (including any distributions therefrom) now or in the future as a result of, or arising out of, any negotiations, or Contracts with FLAC or its Representatives and will not seek recourse against the Trust Account (including any distributions therefrom) for any reason whatsoever (including for an alleged breach of any agreement with FLAC or its Affiliates), other than for the release of proceeds from the Trust Account upon the consummation of the Merger.
* * * * *
A-87
Table of Contents
IN WITNESS WHEREOF, each of the Parties has caused this Business Combination Agreement to be duly executed on its behalf as of the day and year first above written.
| FRAZIER LIFESCIENCES ACQUISITION CORPORATION | ||
| By: | /s/ James N. Topper | |
| Name: | James N. Topper | |
| Title: | Chief Executive Officer | |
| NEWAMSTERDAM PHARMA HOLDING B.V. | ||
| By: | /s/ Michael H. Davidson | |
| Name: | Michael H. Davidson | |
| Title: | Chief Executive Officer | |
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||
| By: | /s/ Louise Kooij | |
| Name: | LouFré Management B.V. | |
represented by LouFré Holding B.V. represented by Louise Kooij | ||
| Title: | Sole Director | |
| NEWAMSTERDAM PHARMA INVESTMENT CORPORATION | ||
| By: | /s/ Louise Kooij | |
| Name: | LouFré Management B.V. | |
represented by LouFré Holding B.V. represented by Louise Kooij | ||
| Title: | Sole Director | |
[Signature Page to Business Combination Agreement]
A-88
Table of Contents
The Companies Act (As Revised) of the Cayman Islands
Plan of Merger
This plan of merger (the “Plan of Merger”) is made on [ ] between Frazier Lifesciences Acquisition Corporation (the “Surviving Company”) and NewAmsterdam Pharma Investment Corporation (the “Merging Company”).
Whereas the Merging Company is a Cayman Islands exempted company and is entering into this Plan of Merger pursuant to the provisions of Part XVI of the Companies Act (As Revised) (the “Statute”).
Whereas the Surviving Company is a Cayman Islands exempted company and is entering into this Plan of Merger pursuant to the provisions of Part XVI of the Statute.
Whereas the sole director of the Merging Company and the directors of the Surviving Company deem it desirable and in the commercial interests of the Merging Company and the Surviving Company, respectively, that the Merging Company be merged with and into the Surviving Company and that the undertaking, property and liabilities of the Merging Company vest in the Surviving Company (the “Merger”).
Terms not otherwise defined in this Plan of Merger shall have the meanings given to them under the Business Combination Agreement dated July 25, 2022, and made between, amongst others, the Surviving Company and the Merging Company (the “Merger Agreement”) a copy of which is annexed at Annexure 1 hereto.
Now therefore this Plan of Merger provides as follows:
| 1 | The constituent companies (as defined in the Statute) to this Merger are the Surviving Company and the Merging Company. |
| 2 | The surviving company (as defined in the Statute) is the Surviving Company. |
| 3 | The registered office of the Surviving Company is c/o Campbells Corporate Services Limited of Floor 4, Willow House, Cricket Square, Grand Cayman KY1-9010, Cayman Islands. |
| 4 | The registered office of the Merging Company is c/o Maples Corporate Services Limited of PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands. |
| 5 | Immediately prior to the Effective Date (as defined below), the share capital of the Surviving Company will be US$50,000 divided into 479,000,000 Class A Ordinary Shares of a par value of US$0.0001 each, 20,000,000 Class B Ordinary Shares a par value of US$0.0001 each and 1,000,000 Preference Shares with a par value of US$0.0001 each and the Surviving Company will have [ ] Class A Ordinary Shares, [ ] Class B Ordinary Shares and no Preference Shares in issue. |
| 6 | Immediately prior to the Effective Date (as defined below), the share capital of the Merging Company will be US$50,000 divided into 50,000 ordinary shares of a par value of US$1.00 each and the Merging Company will have 1 ordinary share in issue. |
| 7 | The date on which it is intended that the Merger is to take effect is the date that this Plan of Merger is registered by the Registrar in accordance with section 233(13) of the Statute (the “Effective Date”). |
| 8 | The terms and conditions of the Merger, including the manner and basis of converting shares in each constituent company into shares in the Surviving Company, are set out in the Merger Agreement in the form annexed at Annexure 1 hereto. |
| 9 | The rights and restrictions attaching to the shares in the Surviving Company are set out in the Amended and Restated Memorandum and Articles of Association of the Surviving Company in the form annexed at Annexure 2 hereto. |
B-1
Table of Contents
| 10 | The Memorandum and Articles of Association of the Surviving Company shall be amended and restated by the deletion in their entirety and the substitution in their place of the Amended and Restated Memorandum and Articles of Association in the form annexed at Annexure 2 hereto on the Effective Date and the authorised share capital of the Surviving Company after the Merger shall be amended from US$50,000.00 divided into 479,000,000 Class A Ordinary Shares of a par value of US$0.0001 each, 20,000,000 Class B Ordinary Shares a par value of US$0.0001 each and 1,000,000 Preference Shares with a par value of US$0.0001 each to US$50,000 divided into 50,000 shares of a par value of US$1.00 each, by (i) the reclassification of all authorised shares of the Surviving Company of whatever class as shares; (ii) the consolidation of all authorised but unissued shares and all authorised and issued shares of par value of US$0.0001 each in the capital of the Company by a factor of 10,0000. |
| 11 | There are no amounts or benefits which are or shall be paid or payable to any director of either constituent company or the Surviving Company consequent upon the Merger. |
| 12 | The Merging Company has granted no fixed or floating security interests that are outstanding as at the date of this Plan of Merger. |
| 13 | The Surviving Company has granted no fixed or floating security interests that are outstanding as at the date of this Plan of Merger. |
| 14 | The name and address of the sole director of the surviving company (as defined in the Statute) after the Merger is: |
| 14.1 | LouFré Management B.V. of Frans Halslaan 9, 1272 HN, Huizen, Netherlands. |
| 15 | This Plan of Merger has been approved by the board of directors of each of the Surviving Company and the Merging Company pursuant to section 233(3) of the Statute. |
| 16 | This Plan of Merger has been authorised by the shareholders of the Merging Company pursuant to section 233(6) of the Statute. |
| 17 | This Plan of Merger has been authorised by the shareholders of the Surviving Company pursuant to section 233(6) of the Statute by way of resolutions passed at an extraordinary general meeting of the Surviving Company. |
| 18 | At any time prior to the Effective Date, this Plan of Merger may be: |
| 18.1 | terminated by the board of directors of either the Surviving Company or the Merging Company; |
| 18.2 | amended by the board of directors of both the Surviving Company and the Merging Company to: |
| (a) | change the Effective Date provided that such changed date shall not be a date later than the ninetieth day after the date of registration of this Plan of Merger with the Registrar of Companies; and |
| (b) | effect any other changes to this Plan of Merger which the directors of both the Surviving Company and the Merging Company deem advisable, provided that such changes do not materially adversely affect any rights of the shareholders of the Surviving Company or the Merging Company, as determined by the directors of both the Surviving Company and the Merging Company, respectively. |
| 19 | This Plan of Merger may be executed in counterparts. |
| 20 | This Plan of Merger shall be governed by and construed in accordance with the laws of the Cayman Islands. |
In witness whereof the parties hereto have caused this Plan of Merger to be executed on the day and year first above written.
SIGNED by | ) | |||||
Duly authorised for | ) | |||||
and on behalf of | ) | Director | ||||
Frazier Lifesciences Acquisition Corporation | ) |
B-2
Table of Contents
SIGNED by | ) | |||||
Duly authorised for | ) | |||||
and on behalf of | ) | Director | ||||
NewAmsterdam Pharma Investment | ) | |||||
Corporation | ) |
B-3
Table of Contents
Annexure 1
Merger Agreement
B-4
Table of Contents
Annexure 2
Amended and Restated Memorandum and Articles of Association of the Surviving Company
B-5
Table of Contents
THE COMPANIES ACT (AS REVISED)
OF THE CAYMAN ISLANDS
COMPANY LIMITED BY SHARES
AMENDED AND RESTATED
MEMORANDUM AND ARTICLES OF ASSOCIATION
OF
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
(AS ADOPTED BY SPECIAL RESOLUTION DATED [ ])
B-6
Table of Contents
THE COMPANIES ACT (AS REVISED)
OF THE CAYMAN ISLANDS
COMPANY LIMITED BY SHARES
AMENDED AND RESTATED
MEMORANDUM OF ASSOCIATION
OF
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
(AS ADOPTED BY SPECIAL RESOLUTION DATED [ ])
| 21 | The name of the Company is Frazier Lifesciences Acquisition Corporation. |
| 22 | The Registered Office of the Company shall be at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands, or at such other place within the Cayman Islands as the Directors may decide. |
| 23 | The objects for which the Company is established are unrestricted and the Company shall have full power and authority to carry out any object not prohibited by the laws of the Cayman Islands. |
| 24 | The liability of each Member is limited to the amount unpaid on such Member’s shares. |
| 25 | The share capital of the Company is US$50,000 divided into 50,000 shares of a par value of US$1.00 each. |
| 26 | The Company has power to register by way of continuation as a body corporate limited by shares under the laws of any jurisdiction outside the Cayman Islands and to be deregistered in the Cayman Islands. |
| 27 | Capitalised terms that are not defined in this Memorandum of Association bear the respective meanings given to them in the Articles of Association of the Company. |
B-7
Table of Contents
THE COMPANIES ACT (AS REVISED)
OF THE CAYMAN ISLANDS
COMPANY LIMITED BY SHARES
AMENDED AND RESTATED
ARTICLES OF ASSOCIATION
OF
FRAZIER LIFESCIENCES ACQUISITION CORPORATION
(AS ADOPTED BY SPECIAL RESOLUTION DATED [ ])
| 1 | Interpretation |
| 1.1 | In the Articles Table A in the First Schedule to the Statute does not apply and, unless there is something in the subject or context inconsistent therewith: |
“Articles” | means these articles of association of the Company. | |
“Auditor” | means the person for the time being performing the duties of auditor of the Company (if any). | |
“Company” | means the above named company. | |
“Directors” | means the directors for the time being of the Company. | |
“Dividend” | means any dividend (whether interim or final) resolved to be paid on Shares pursuant to the Articles. | |
“Electronic Record” | has the same meaning as in the Electronic Transactions Act. | |
“Electronic Transactions Act” | means the Electronic Transactions Act (As Revised) of the Cayman Islands. | |
“Member” | has the same meaning as in the Statute. | |
“Memorandum” | means the memorandum of association of the Company. | |
“Ordinary Resolution” | means a resolution passed by a simple majority of the Members as, being entitled to do so, vote in person or, where proxies are allowed, by proxy at a general meeting, and includes a unanimous written resolution. In computing the majority when a poll is demanded regard shall be had to the number of votes to which each Member is entitled by the Articles. | |
“Register of Members” | means the register of Members maintained in accordance with the Statute and includes (except where otherwise stated) any branch or duplicate register of Members. | |
“Registered Office” | means the registered office for the time being of the Company. | |
“Seal” | means the common seal of the Company and includes every duplicate seal. | |
B-8
Table of Contents
“Share” | means a share in the Company and includes a fraction of a share in the Company. | |
“Special Resolution” | has the same meaning as in the Statute, and includes a unanimous written resolution. | |
“Statute” | means the Companies Act (As Revised) of the Cayman Islands. | |
“Subscriber” | means the subscriber to the Memorandum. | |
“Treasury Share” | means a Share held in the name of the Company as a treasury share in accordance with the Statute. | |
| 1.2 | In the Articles: |
| (a) | words importing the singular number include the plural number and vice versa; |
| (b) | words importing the masculine gender include the feminine gender; |
| (c) | words importing persons include corporations as well as any other legal or natural person; |
| (d) | “written” and “in writing” include all modes of representing or reproducing words in visible form, including in the form of an Electronic Record; |
| (e) | “shall” shall be construed as imperative and “may” shall be construed as permissive; |
| (f) | references to provisions of any law or regulation shall be construed as references to those provisions as amended, modified, re-enacted or replaced; |
| (g) | any phrase introduced by the terms “including”, “include”, “in particular” or any similar expression shall be construed as illustrative and shall not limit the sense of the words preceding those terms; |
| (h) | the term “and/or” is used to mean both “and” as well as “or.” The use of “and/or” in certain contexts in no respects qualifies or modifies the use of the terms “and” or “or” in others. The term “or” shall not be interpreted to be exclusive and the term “and” shall not be interpreted to require the conjunctive (in each case, unless the context otherwise requires); |
| (i) | headings are inserted for reference only and shall be ignored in construing the Articles; |
| (j) | any requirements as to delivery under the Articles include delivery in the form of an Electronic Record; |
| (k) | any requirements as to execution or signature under the Articles including the execution of the Articles themselves can be satisfied in the form of an electronic signature as defined in the Electronic Transactions Act; |
| (l) | sections 8 and 19(3) of the Electronic Transactions Act shall not apply; |
| (m) | the term “clear days” in relation to the period of a notice means that period excluding the day when the notice is received or deemed to be received and the day for which it is given or on which it is to take effect; and |
| (n) | the term “holder” in relation to a Share means a person whose name is entered in the Register of Members as the holder of such Share. |
| 2 | Commencement of Business |
| 2.1 | The business of the Company may be commenced as soon after incorporation of the Company as the Directors shall see fit. |
B-9
Table of Contents
| 2.2 | The Directors may pay, out of the capital or any other monies of the Company, all expenses incurred in or about the formation and establishment of the Company, including the expenses of registration. |
| 3 | Issue of Shares |
| 3.1 | Subject to the provisions, if any, in the Memorandum (and to any direction that may be given by the Company in general meeting) and without prejudice to any rights attached to any existing Shares, the Directors may allot, issue, grant options over or otherwise dispose of Shares (including fractions of a Share) with or without preferred, deferred or other rights or restrictions, whether in regard to Dividend or other distribution, voting, return of capital or otherwise and to such persons, at such times and on such other terms as they think proper, and may also (subject to the Statute and the Articles) vary such rights. Notwithstanding the foregoing, the Subscriber shall have the power to: |
| (a) | issue one Share to itself; |
| (b) | transfer that Share by an instrument of transfer to any person; and |
| (c) | update the Register of Members in respect of the issue and transfer of that Share. |
| 3.2 | The Company shall not issue Shares to bearer. |
| 4 | Register of Members |
| 4.1 | The Company shall maintain or cause to be maintained the Register of Members in accordance with the Statute. |
| 4.2 | The Directors may determine that the Company shall maintain one or more branch registers of Members in accordance with the Statute. The Directors may also determine which register of Members shall constitute the principal register and which shall constitute the branch register or registers, and to vary such determination from time to time. |
| 5 | Closing Register of Members or Fixing Record Date |
| 5.1 | For the purpose of determining Members entitled to notice of, or to vote at any meeting of Members or any adjournment thereof, or Members entitled to receive payment of any Dividend or other distribution, or in order to make a determination of Members for any other purpose, the Directors may provide that the Register of Members shall be closed for transfers for a stated period which shall not in any case exceed forty days. |
| 5.2 | In lieu of, or apart from, closing the Register of Members, the Directors may fix in advance or arrears a date as the record date for any such determination of Members entitled to notice of, or to vote at any meeting of the Members or any adjournment thereof, or for the purpose of determining the Members entitled to receive payment of any Dividend or other distribution, or in order to make a determination of Members for any other purpose. |
| 5.3 | If the Register of Members is not so closed and no record date is fixed for the determination of Members entitled to notice of, or to vote at, a meeting of Members or Members entitled to receive payment of a Dividend or other distribution, the date on which notice of the meeting is sent or the date on which the resolution of the Directors resolving to pay such Dividend or other distribution is passed, as the case may be, shall be the record date for such determination of Members. When a determination of Members entitled to vote at any meeting of Members has been made as provided in this Article, such determination shall apply to any adjournment thereof. |
B-10
Table of Contents
| 6 | Certificates for Shares |
| 6.1 | A Member shall only be entitled to a share certificate if the Directors resolve that share certificates shall be issued. Share certificates representing Shares, if any, shall be in such form as the Directors may determine. Share certificates shall be signed by one or more Directors or other person authorised by the Directors. The Directors may authorise certificates to be issued with the authorised signature(s) affixed by mechanical process. All certificates for Shares shall be consecutively numbered or otherwise identified and shall specify the Shares to which they relate. All certificates surrendered to the Company for transfer shall be cancelled and subject to the Articles no new certificate shall be issued until the former certificate representing a like number of relevant Shares shall have been surrendered and cancelled. |
| 6.2 | The Company shall not be bound to issue more than one certificate for Shares held jointly by more than one person and delivery of a certificate to one joint holder shall be a sufficient delivery to all of them. |
| 6.3 | If a share certificate is defaced, worn out, lost or destroyed, it may be renewed on such terms (if any) as to evidence and indemnity and on the payment of such expenses reasonably incurred by the Company in investigating evidence, as the Directors may prescribe, and (in the case of defacement or wearing out) upon delivery of the old certificate. |
| 6.4 | Every share certificate sent in accordance with the Articles will be sent at the risk of the Member or other person entitled to the certificate. The Company will not be responsible for any share certificate lost or delayed in the course of delivery. |
| 7 | Transfer of Shares |
| 7.1 | Subject to Article 3.1, Shares are transferable subject to the approval of the Directors by resolution who may, in their absolute discretion, decline to register any transfer of Shares without giving any reason. If the Directors refuse to register a transfer they shall notify the transferee within two months of such refusal. |
| 7.2 | The instrument of transfer of any Share shall be in writing and shall be executed by or on behalf of the transferor (and if the Directors so require, signed by or on behalf of the transferee). The transferor shall be deemed to remain the holder of a Share until the name of the transferee is entered in the Register of Members. |
| 8 | Redemption, Repurchase and Surrender of Shares |
| 8.1 | Subject to the provisions of the Statute the Company may issue Shares that are to be redeemed or are liable to be redeemed at the option of the Member or the Company. The redemption of such Shares shall be effected in such manner and upon such other terms as the Company may, by Special Resolution, determine before the issue of the Shares. |
| 8.2 | Subject to the provisions of the Statute, the Company may purchase its own Shares (including any redeemable Shares) in such manner and on such other terms as the Directors may agree with the relevant Member. |
| 8.3 | The Company may make a payment in respect of the redemption or purchase of its own Shares in any manner permitted by the Statute, including out of capital. |
| 8.4 | The Directors may accept the surrender for no consideration of any fully paid Share. |
| 9 | Treasury Shares |
| 9.1 | The Directors may, prior to the purchase, redemption or surrender of any Share, determine that such Share shall be held as a Treasury Share. |
B-11
Table of Contents
| 9.2 | The Directors may determine to cancel a Treasury Share or transfer a Treasury Share on such terms as they think proper (including, without limitation, for nil consideration). |
| 10 | Variation of Rights of Shares |
| 10.1 | If at any time the share capital of the Company is divided into different classes of Shares, all or any of the rights attached to any class (unless otherwise provided by the terms of issue of the Shares of that class) may, whether or not the Company is being wound up, be varied without the consent of the holders of the issued Shares of that class where such variation is considered by the Directors not to have a material adverse effect upon such rights; otherwise, any such variation shall be made only with the consent in writing of the holders of not less than two thirds of the issued Shares of that class, or with the approval of a resolution passed by a majority of not less than two thirds of the votes cast at a separate meeting of the holders of the Shares of that class. For the avoidance of doubt, the Directors reserve the right, notwithstanding that any such variation may not have a material adverse effect, to obtain consent from the holders of Shares of the relevant class. To any such meeting all the provisions of the Articles relating to general meetings shall apply mutatis mutandis, except that the necessary quorum shall be one person holding or representing by proxy at least one third of the issued Shares of the class and that any holder of Shares of the class present in person or by proxy may demand a poll. |
| 10.2 | For the purposes of a separate class meeting, the Directors may treat two or more or all the classes of Shares as forming one class of Shares if the Directors consider that such class of Shares would be affected in the same way by the proposals under consideration, but in any other case shall treat them as separate classes of Shares. |
| 10.3 | The rights conferred upon the holders of the Shares of any class issued with preferred or other rights shall not, unless otherwise expressly provided by the terms of issue of the Shares of that class, be deemed to be varied by the creation or issue of further Shares ranking pari passu therewith. |
| 11 | Commission on Sale of Shares |
The Company may, in so far as the Statute permits, pay a commission to any person in consideration of that person subscribing or agreeing to subscribe (whether absolutely or conditionally) or procuring or agreeing to procure subscriptions (whether absolutely or conditionally) for any Shares. Such commissions may be satisfied by the payment of cash and/or the issue of fully or partly paid-up Shares. The Company may also on any issue of Shares pay such brokerage as may be lawful.
| 12 | Non Recognition of Trusts |
The Company shall not be bound by or compelled to recognise in any way (even when notified) any equitable, contingent, future or partial interest in any Share, or (except only as is otherwise provided by the Articles or the Statute) any other rights in respect of any Share other than an absolute right to the entirety thereof in the holder.
| 13 | Lien on Shares |
| 13.1 | The Company shall have a first and paramount lien on all Shares (whether fully paid-up or not) registered in the name of a Member (whether solely or jointly with others) for all debts, liabilities or engagements to or with the Company (whether presently payable or not) by such Member or their estate, either alone or jointly with any other person, whether a Member or not, but the Directors may at any time declare any Share to be wholly or in part exempt from the provisions of this Article. The registration of a transfer of any such Share shall operate as a waiver of the Company’s lien thereon. The Company’s lien on a Share shall also extend to any amount payable in respect of that Share. |
B-12
Table of Contents
| 13.2 | The Company may sell, in such manner as the Directors think fit, any Shares on which the Company has a lien, if a sum in respect of which the lien exists is presently payable, and is not paid within 14 clear days after notice has been received or deemed to have been received by the holder of the Shares, or to the person entitled to it in consequence of the death or bankruptcy of the holder, demanding payment and stating that if the notice is not complied with the Shares may be sold. |
| 13.3 | To give effect to any such sale the Directors may authorise any person to execute an instrument of transfer of the Shares sold to, or in accordance with the directions of, the purchaser. The purchaser or their nominee shall be registered as the holder of the Shares comprised in any such transfer, and they shall not be bound to see to the application of the purchase money, nor shall their title to the Shares be affected by any irregularity or invalidity in the sale or the exercise of the Company’s power of sale under the Articles. |
| 13.4 | The net proceeds of such sale after payment of costs, shall be applied in payment of such part of the amount in respect of which the lien exists as is presently payable and any balance shall (subject to a like lien for sums not presently payable as existed upon the Shares before the sale) be paid to the person entitled to the Shares at the date of the sale. |
| 14 | Call on Shares |
| 14.1 | Subject to the terms of the allotment and issue of any Shares, the Directors may make calls upon the Members in respect of any monies unpaid on their Shares (whether in respect of par value or premium), and each Member shall (subject to receiving at least 14 clear days’ notice specifying the time or times of payment) pay to the Company at the time or times so specified the amount called on the Shares. A call may be revoked or postponed, in whole or in part, as the Directors may determine. A call may be required to be paid by instalments. A person upon whom a call is made shall remain liable for calls made upon them notwithstanding the subsequent transfer of the Shares in respect of which the call was made. |
| 14.2 | A call shall be deemed to have been made at the time when the resolution of the Directors authorising such call was passed. |
| 14.3 | The joint holders of a Share shall be jointly and severally liable to pay all calls in respect thereof. |
| 14.4 | If a call remains unpaid after it has become due and payable, the person from whom it is due shall pay interest on the amount unpaid from the day it became due and payable until it is paid at such rate as the Directors may determine (and in addition all expenses that have been incurred by the Company by reason of such non-payment), but the Directors may waive payment of the interest or expenses wholly or in part. |
| 14.5 | An amount payable in respect of a Share on issue or allotment or at any fixed date, whether on account of the par value of the Share or premium or otherwise, shall be deemed to be a call and if it is not paid all the provisions of the Articles shall apply as if that amount had become due and payable by virtue of a call. |
| 14.6 | The Directors may issue Shares with different terms as to the amount and times of payment of calls, or the interest to be paid. |
| 14.7 | The Directors may, if they think fit, receive an amount from any Member willing to advance all or any part of the monies uncalled and unpaid upon any Shares held by that Member, and may (until the amount would otherwise become payable) pay interest at such rate as may be agreed upon between the Directors and the Member paying such amount in advance. |
| 14.8 | No such amount paid in advance of calls shall entitle the Member paying such amount to any portion of a Dividend or other distribution payable in respect of any period prior to the date upon which such amount would, but for such payment, become payable. |
B-13
Table of Contents
| 15 | Forfeiture of Shares |
| 15.1 | If a call or instalment of a call remains unpaid after it has become due and payable the Directors may give to the person from whom it is due not less than 14 clear days’ notice requiring payment of the amount unpaid together with any interest which may have accrued and any expenses incurred by the Company by reason of such non-payment. The notice shall specify where payment is to be made and shall state that if the notice is not complied with the Shares in respect of which the call was made will be liable to be forfeited. |
| 15.2 | If the notice is not complied with, any Share in respect of which it was given may, before the payment required by the notice has been made, be forfeited by a resolution of the Directors. Such forfeiture shall include all Dividends, other distributions or other monies payable in respect of the forfeited Share and not paid before the forfeiture. |
| 15.3 | A forfeited Share may be sold, re-allotted or otherwise disposed of on such terms and in such manner as the Directors think fit and at any time before a sale, re-allotment or disposition the forfeiture may be cancelled on such terms as the Directors think fit. Where for the purposes of its disposal a forfeited Share is to be transferred to any person the Directors may authorise some person to execute an instrument of transfer of the Share in favour of that person. |
| 15.4 | A person any of whose Shares have been forfeited shall cease to be a Member in respect of them and shall surrender to the Company for cancellation the certificate for the Shares forfeited and shall remain liable to pay to the Company all monies which at the date of forfeiture were payable by that person to the Company in respect of those Shares together with interest at such rate as the Directors may determine, but that person’s liability shall cease if and when the Company shall have received payment in full of all monies due and payable by them in respect of those Shares. |
| 15.5 | A certificate in writing under the hand of one Director or officer of the Company that a Share has been forfeited on a specified date shall be conclusive evidence of the facts stated in it as against all persons claiming to be entitled to the Share. The certificate shall (subject to the execution of an instrument of transfer) constitute a good title to the Share and the person to whom the Share is sold or otherwise disposed of shall not be bound to see to the application of the purchase money, if any, nor shall their title to the Share be affected by any irregularity or invalidity in the proceedings in reference to the forfeiture, sale or disposal of the Share. |
| 15.6 | The provisions of the Articles as to forfeiture shall apply in the case of non payment of any sum which, by the terms of issue of a Share, becomes payable at a fixed time, whether on account of the par value of the Share or by way of premium as if it had been payable by virtue of a call duly made and notified. |
| 16 | Transmission of Shares |
| 16.1 | If a Member dies the survivor or survivors (where they were a joint holder) or their legal personal representatives (where they were a sole holder), shall be the only persons recognised by the Company as having any title to the deceased Member’s Shares. The estate of a deceased Member is not thereby released from any liability in respect of any Share, for which the Member was a joint or sole holder. |
| 16.2 | Any person becoming entitled to a Share in consequence of the death or bankruptcy or liquidation or dissolution of a Member (or in any other way than by transfer) may, upon such evidence being produced as may be required by the Directors, elect, by a notice in writing sent by that person to the Company, either to become the holder of such Share or to have some person nominated by them registered as the holder of such Share. If they elect to have another person registered as the holder of such Share they shall sign an instrument of transfer of that Share to that person. The Directors shall, in either case, have the same right to decline or suspend registration as they would have had in the case of a transfer of the Share by the relevant Member before their death or bankruptcy or liquidation or dissolution, as the case may be. |
B-14
Table of Contents
| 16.3 | A person becoming entitled to a Share by reason of the death or bankruptcy or liquidation or dissolution of a Member (or in any other case than by transfer) shall be entitled to the same Dividends, other distributions and other advantages to which they would be entitled if they were the holder of such Share. However, they shall not, before becoming a Member in respect of a Share, be entitled in respect of it to exercise any right conferred by membership in relation to general meetings of the Company and the Directors may at any time give notice requiring any such person to elect either to be registered or to have some person nominated by them registered as the holder of the Share (but the Directors shall, in either case, have the same right to decline or suspend registration as they would have had in the case of a transfer of the Share by the relevant Member before their death or bankruptcy or liquidation or dissolution or any other case than by transfer, as the case may be). If the notice is not complied with within 90 days of being received or deemed to be received (as determined pursuant to the Articles) the Directors may thereafter withhold payment of all Dividends, other distributions, bonuses or other monies payable in respect of the Share until the requirements of the notice have been complied with. |
| 17 | Amendments of Memorandum and Articles of Association and Alteration of Capital |
| 17.1 | The Company may by Ordinary Resolution: |
| (a) | increase its share capital by such sum as the Ordinary Resolution shall prescribe and with such rights, priorities and privileges annexed thereto, as the Company in general meeting may determine; |
| (b) | consolidate and divide all or any of its share capital into Shares of larger amount than its existing Shares; |
| (c) | convert all or any of its paid-up Shares into stock, and reconvert that stock into paid-up Shares of any denomination; |
| (d) | by subdivision of its existing Shares or any of them divide the whole or any part of its share capital into Shares of smaller amount than is fixed by the Memorandum or into Shares without par value; and |
| (e) | cancel any Shares that at the date of the passing of the Ordinary Resolution have not been taken or agreed to be taken by any person and diminish the amount of its share capital by the amount of the Shares so cancelled. |
| 17.2 | All new Shares created in accordance with the provisions of the preceding Article shall be subject to the same provisions of the Articles with reference to the payment of calls, liens, transfer, transmission, forfeiture and otherwise as the Shares in the original share capital. |
| 17.3 | Subject to the provisions of the Statute and the provisions of the Articles as regards the matters to be dealt with by Ordinary Resolution, the Company may by Special Resolution: |
| (a) | change its name; |
| (b) | alter or add to the Articles; |
| (c) | alter or add to the Memorandum with respect to any objects, powers or other matters specified therein; and |
| (d) | reduce its share capital or any capital redemption reserve fund. |
| 18 | Offices and Places of Business |
Subject to the provisions of the Statute, the Company may by resolution of the Directors change the location of its Registered Office. The Company may, in addition to its Registered Office, maintain such other offices or places of business as the Directors determine.
B-15
Table of Contents
| 19 | General Meetings |
| 19.1 | All general meetings other than annual general meetings shall be called extraordinary general meetings. |
| 19.2 | The Company may, but shall not (unless required by the Statute) be obliged to, in each year hold a general meeting as its annual general meeting, and shall specify the meeting as such in the notices calling it. Any annual general meeting shall be held at such time and place as the Directors shall appoint and if no other time and place is prescribed by them, it shall be held at the Registered Office on the second Wednesday in December of each year at ten o’clock in the morning. At these meetings the report of the Directors (if any) shall be presented. |
| 19.3 | The Directors may call general meetings, and they shall on a Members’ requisition forthwith proceed to convene an extraordinary general meeting of the Company. |
| 19.4 | A Members’ requisition is a requisition of Members holding at the date of deposit of the requisition not less than 10% in par value of the issued Shares which as at that date carry the right to vote at general meetings of the Company. |
| 19.5 | The Members’ requisition must state the objects of the meeting and must be signed by the requisitionists and deposited at the Registered Office, and may consist of several documents in like form each signed by one or more requisitionists. |
| 19.6 | If there are no Directors as at the date of the deposit of the Members’ requisition or if the Directors do not within 21 days from the date of the deposit of the Members’ requisition duly proceed to convene a general meeting to be held within a further 21 days, the requisitionists, or any of them representing more than one-half of the total voting rights of all of the requisitionists, may themselves convene a general meeting, but any meeting so convened shall be held no later than the day which falls three months after the expiration of the said 21 day period. |
| 19.7 | A general meeting convened as aforesaid by requisitionists shall be convened in the same manner as nearly as possible as that in which general meetings are to be convened by Directors. |
| 20 | Notice of General Meetings |
| 20.1 | At least five clear days’ notice shall be given of any general meeting. Every notice shall specify the place, the day and the hour of the meeting and the general nature of the business to be conducted at the general meeting and shall be given in the manner hereinafter mentioned or in such other manner if any as may be prescribed by the Company, provided that a general meeting of the Company shall, whether or not the notice specified in this Article has been given and whether or not the provisions of the Articles regarding general meetings have been complied with, be deemed to have been duly convened if it is so agreed: |
| (a) | in the case of an annual general meeting, by all of the Members entitled to attend and vote at the meeting; and |
| (b) | in the case of an extraordinary general meeting, by a majority in number of the Members having a right to attend and vote at the meeting, together holding not less than 95% in par value of the Shares giving that right. |
| 20.2 | The accidental omission to give notice of a general meeting to, or the non receipt of notice of a general meeting by, any person entitled to receive such notice shall not invalidate the proceedings of that general meeting. |
| 21 | Proceedings at General Meetings |
| 21.1 | No business shall be transacted at any general meeting unless a quorum is present. Two Members being individuals present in person or by proxy or if a corporation or other non-natural person by its duly authorised representative or proxy shall be a quorum unless the Company has only one Member entitled |
B-16
Table of Contents
| to vote at such general meeting in which case the quorum shall be that one Member present in person or by proxy or (in the case of a corporation or other non-natural person) by its duly authorised representative or proxy. |
| 21.2 | A person may participate at a general meeting by conference telephone or other communications equipment by means of which all the persons participating in the meeting can communicate with each other. Participation by a person in a general meeting in this manner is treated as presence in person at that meeting. |
| 21.3 | A resolution (including a Special Resolution) in writing (in one or more counterparts) signed by or on behalf of all of the Members for the time being entitled to receive notice of and to attend and vote at general meetings (or, being corporations or other non-natural persons, signed by their duly authorised representatives) shall be as valid and effective as if the resolution had been passed at a general meeting of the Company duly convened and held. |
| 21.4 | If a quorum is not present within half an hour from the time appointed for the meeting to commence or if during such a meeting a quorum ceases to be present, the meeting, if convened upon a Members’ requisition, shall be dissolved and in any other case it shall stand adjourned to the same day in the next week at the same time and/or place or to such other day, time and/or place as the Directors may determine, and if at the adjourned meeting a quorum is not present within half an hour from the time appointed for the meeting to commence, the Members present shall be a quorum. |
| 21.5 | The Directors may, at any time prior to the time appointed for the meeting to commence, appoint any person to act as chairperson of a general meeting of the Company or, if the Directors do not make any such appointment, the chairperson, if any, of the board of Directors shall preside as chairperson at such general meeting. If there is no such chairperson, or if the chairperson shall not be present within 15 minutes after the time appointed for the meeting to commence, or is unwilling to act, the Directors present shall elect one of their number to be chairperson of the meeting. |
| 21.6 | If no Director is willing to act as chairperson or if no Director is present within 15 minutes after the time appointed for the meeting to commence, the Members present shall choose one of their number to be chairperson of the meeting. |
| 21.7 | The chairperson may, with the consent of a meeting at which a quorum is present (and shall if so directed by the meeting) adjourn the meeting from time to time and from place to place, but no business shall be transacted at any adjourned meeting other than the business left unfinished at the meeting from which the adjournment took place. |
| 21.8 | When a general meeting is adjourned for 30 days or more, notice of the adjourned meeting shall be given as in the case of an original meeting. Otherwise it shall not be necessary to give any such notice of an adjourned meeting. |
| 21.9 | A resolution put to the vote of the meeting shall be decided on a show of hands unless before, or on the declaration of the result of, the show of hands, the chairperson demands a poll, or any other Member or Members collectively present in person or by proxy (or in the case of a corporation or other non-natural person, by its duly authorised representative or proxy) and holding at least 10% in par value of the Shares giving a right to attend and vote at the meeting demand a poll. |
| 21.10 | Unless a poll is duly demanded and the demand is not withdrawn a declaration by the chairperson that a resolution has been carried or carried unanimously, or by a particular majority, or lost or not carried by a particular majority, an entry to that effect in the minutes of the proceedings of the meeting shall be conclusive evidence of that fact without proof of the number or proportion of the votes recorded in favour of or against such resolution. |
| 21.11 | The demand for a poll may be withdrawn. |
| 21.12 | Except on a poll demanded on the election of a chairperson or on a question of adjournment, a poll shall be taken as the chairperson directs, and the result of the poll shall be deemed to be the resolution of the general meeting at which the poll was demanded. |
B-17
Table of Contents
| 21.13 | A poll demanded on the election of a chairperson or on a question of adjournment shall be taken forthwith. A poll demanded on any other question shall be taken at such date, time and place as the chairperson of the general meeting directs, and any business other than that upon which a poll has been demanded or is contingent thereon may proceed pending the taking of the poll. |
| 21.14 | In the case of an equality of votes, whether on a show of hands or on a poll, the chairperson shall be entitled to a second or casting vote. |
| 22 | Votes of Members |
| 22.1 | Subject to any rights or restrictions attached to any Shares, on a show of hands every Member who (being an individual) is present in person or by proxy or, if a corporation or other non-natural person is present by its duly authorised representative or by proxy, shall have one vote and on a poll every Member present in any such manner shall have one vote for every Share of which they are the holder. |
| 22.2 | In the case of joint holders the vote of the senior holder who tenders a vote, whether in person or by proxy (or, in the case of a corporation or other non-natural person, by its duly authorised representative or proxy), shall be accepted to the exclusion of the votes of the other joint holders, and seniority shall be determined by the order in which the names of the holders stand in the Register of Members. |
| 22.3 | A Member of unsound mind, or in respect of whom an order has been made by any court, having jurisdiction in lunacy, may vote, whether on a show of hands or on a poll, by their committee, receiver, curator bonis, or other person on such Member’s behalf appointed by that court, and any such committee, receiver, curator bonis or other person may vote by proxy. |
| 22.4 | No person shall be entitled to vote at any general meeting unless they are registered as a Member on the record date for such meeting nor unless all calls or other monies then payable by them in respect of Shares have been paid. |
| 22.5 | No objection shall be raised as to the qualification of any voter except at the general meeting or adjourned general meeting at which the vote objected to is given or tendered and every vote not disallowed at the meeting shall be valid. Any objection made in due time in accordance with this Article shall be referred to the chairperson whose decision shall be final and conclusive. |
| 22.6 | On a poll or on a show of hands votes may be cast either personally or by proxy (or in the case of a corporation or other non-natural person by its duly authorised representative or proxy). A Member may appoint more than one proxy or the same proxy under one or more instruments to attend and vote at a meeting. Where a Member appoints more than one proxy the instrument of proxy shall state which proxy is entitled to vote on a show of hands and shall specify the number of Shares in respect of which each proxy is entitled to exercise the related votes. |
| 22.7 | On a poll, a Member holding more than one Share need not cast the votes in respect of their Shares in the same way on any resolution and therefore may vote a Share or some or all such Shares either for or against a resolution and/or abstain from voting a Share or some or all of the Shares and, subject to the terms of the instrument appointing the proxy, a proxy appointed under one or more instruments may vote a Share or some or all of the Shares in respect of which they are appointed either for or against a resolution and/or abstain from voting a Share or some or all of the Shares in respect of which they are appointed. |
| 23 | Proxies |
| 23.1 | The instrument appointing a proxy shall be in writing and shall be executed under the hand of the appointor or of their attorney duly authorised in writing, or, if the appointor is a corporation or other non natural person, under the hand of its duly authorised representative. A proxy need not be a Member. |
B-18
Table of Contents
| 23.2 | The Directors may, in the notice convening any meeting or adjourned meeting, or in an instrument of proxy sent out by the Company, specify the manner by which the instrument appointing a proxy shall be deposited and the place and the time (being not later than the time appointed for the commencement of the meeting or adjourned meeting to which the proxy relates) at which the instrument appointing a proxy shall be deposited. In the absence of any such direction from the Directors in the notice convening any meeting or adjourned meeting or in an instrument of proxy sent out by the Company, the instrument appointing a proxy shall be deposited physically at the Registered Office not less than 48 hours before the time appointed for the meeting or adjourned meeting to commence at which the person named in the instrument proposes to vote. |
| 23.3 | The chairperson may in any event at their discretion declare that an instrument of proxy shall be deemed to have been duly deposited. An instrument of proxy that is not deposited in the manner permitted, or which has not been declared to have been duly deposited by the chairperson, shall be invalid. |
| 23.4 | The instrument appointing a proxy may be in any usual or common form (or such other form as the Directors may approve) and may be expressed to be for a particular meeting or any adjournment thereof or generally until revoked. An instrument appointing a proxy shall be deemed to include the power to demand or join or concur in demanding a poll. |
| 23.5 | Votes given in accordance with the terms of an instrument of proxy shall be valid notwithstanding the previous death or insanity of the principal or revocation of the proxy or of the authority under which the proxy was executed, or the transfer of the Share in respect of which the proxy is given unless notice in writing of such death, insanity, revocation or transfer was received by the Company at the Registered Office before the commencement of the general meeting, or adjourned meeting at which it is sought to use the proxy. |
| 24 | Corporate Members |
Any corporation or other non-natural person which is a Member may in accordance with its constitutional documents, or in the absence of such provision by resolution of its directors or other governing body, authorise such person as it thinks fit to act as its representative at any meeting of the Company or of any class of Members, and the person so authorised shall be entitled to exercise the same powers on behalf of the corporation which they represent as the corporation could exercise if it were an individual Member.
| 25 | Shares that May Not be Voted |
Shares in the Company that are beneficially owned by the Company shall not be voted, directly or indirectly, at any meeting and shall not be counted in determining the total number of outstanding Shares at any given time.
| 26 | Directors |
There shall be a board of Directors consisting of not less than one person (exclusive of alternate Directors) provided however that the Company may by Ordinary Resolution increase or reduce the limits in the number of Directors. The first Directors of the Company may be determined in writing by, or appointed by a resolution of, the Subscriber.
| 27 | Powers of Directors |
| 27.1 | Subject to the provisions of the Statute, the Memorandum and the Articles and to any directions given by Special Resolution, the business of the Company shall be managed by the Directors who may exercise all the powers of the Company. No alteration of the Memorandum or Articles and no such direction shall invalidate any prior act of the Directors which would have been valid if that alteration had not been made or that direction had not been given. A duly convened meeting of Directors at which a quorum is present may exercise all powers exercisable by the Directors. |
B-19
Table of Contents
| 27.2 | All cheques, promissory notes, drafts, bills of exchange and other negotiable or transferable instruments and all receipts for monies paid to the Company shall be signed, drawn, accepted, endorsed or otherwise executed as the case may be in such manner as the Directors shall determine by resolution. |
| 27.3 | The Directors on behalf of the Company may pay a gratuity or pension or allowance on retirement to any Director who has held any other salaried office or place of profit with the Company or to their surviving spouse, civil partner or dependants and may make contributions to any fund and pay premiums for the purchase or provision of any such gratuity, pension or allowance. |
| 27.4 | The Directors may exercise all the powers of the Company to borrow money and to mortgage or charge its undertaking, property and assets (present and future) and uncalled capital or any part thereof and to issue debentures, debenture stock, mortgages, bonds and other such securities whether outright or as security for any debt, liability or obligation of the Company or of any third party. |
| 28 | Appointment and Removal of Directors |
| 28.1 | The Company may by Ordinary Resolution appoint any person to be a Director or may by Ordinary Resolution remove any Director. |
| 28.2 | The Directors may appoint any person to be a Director, either to fill a vacancy or as an additional Director provided that the appointment does not cause the number of Directors to exceed any number fixed by or in accordance with the Articles as the maximum number of Directors. |
| 29 | Vacation of Office of Director |
The office of a Director shall be vacated if:
| (a) | the Director gives notice in writing to the Company that they resign the office of Director; or |
| (b) | the Director is absent (for the avoidance of doubt, without being represented by proxy or an alternate Director appointed by them) from three consecutive meetings of the board of Directors without special leave of absence from the Directors, and the Directors pass a resolution that they have by reason of such absence vacated office; or |
| (c) | the Director dies, becomes bankrupt or makes any arrangement or composition with their creditors generally; or |
| (d) | the Director is found to be or becomes of unsound mind; or |
| (e) | all of the other Directors (being not less than two in number) determine that the Director should be removed as a Director, either by a resolution passed by all of the other Directors at a meeting of the Directors duly convened and held in accordance with the Articles or by a resolution in writing signed by all of the other Directors. |
| 30 | Proceedings of Directors |
| 30.1 | The quorum for the transaction of the business of the Directors may be fixed by the Directors, and unless so fixed shall be two if there are two or more Directors, and shall be one if there is only one Director. A person who holds office as an alternate Director shall, if their appointor is not present, be counted in the quorum. A Director who also acts as an alternate Director shall, if their appointor is not present, count twice towards the quorum. |
| 30.2 | Subject to the provisions of the Articles, the Directors may regulate their proceedings as they think fit. Questions arising at any meeting shall be decided by a majority of votes. In the case of an equality of votes, the chairperson shall have a second or casting vote. A Director who is also an alternate Director shall be entitled in the absence of their appointor to a separate vote on behalf of their appointor in addition to their own vote. |
B-20
Table of Contents
| 30.3 | A person may participate in a meeting of the Directors or any committee of Directors by conference telephone or other communications equipment by means of which all the persons participating in the meeting can communicate with each other at the same time. Participation by a person in a meeting in this manner is treated as presence in person at that meeting. Unless otherwise determined by the Directors the meeting shall be deemed to be held at the place where the chairperson is located at the start of the meeting. |
| 30.4 | A resolution in writing (in one or more counterparts) signed by all the Directors or all the members of a committee of the Directors or, in the case of a resolution in writing relating to the removal of any Director or the vacation of office by any Director, all of the Directors other than the Director who is the subject of such resolution (an alternate Director being entitled to sign such a resolution on behalf of their appointor and if such alternate Director is also a Director, being entitled to sign such resolution both on behalf of their appointor and in their capacity as a Director) shall be as valid and effectual as if it had been passed at a meeting of the Directors, or committee of Directors as the case may be, duly convened and held. |
| 30.5 | A Director or alternate Director may, or other officer of the Company on the direction of a Director or alternate Director shall, call a meeting of the Directors by at least two days’ notice in writing to every Director and alternate Director which notice shall set forth the general nature of the business to be considered unless notice is waived by all the Directors (or their alternates) either at, before or after the meeting is held. To any such notice of a meeting of the Directors all the provisions of the Articles relating to the giving of notices by the Company to the Members shall apply mutatis mutandis. |
| 30.6 | The continuing Directors (or a sole continuing Director, as the case may be) may act notwithstanding any vacancy in their body, but if and so long as their number is reduced below the number fixed by or pursuant to the Articles as the necessary quorum of Directors the continuing Directors or Director may act for the purpose of increasing the number of Directors to be equal to such fixed number, or of summoning a general meeting of the Company, but for no other purpose. |
| 30.7 | The Directors may elect a chairperson of their board and determine the period for which they are to hold office; but if no such chairperson is elected, or if at any meeting the chairperson is not present within five minutes after the time appointed for the meeting to commence, the Directors present may choose one of their number to be chairperson of the meeting. |
| 30.8 | All acts done by any meeting of the Directors or of a committee of the Directors (including any person acting as an alternate Director) shall, notwithstanding that it is afterwards discovered that there was some defect in the appointment of any Director or alternate Director, and/or that they or any of them were disqualified, and/or had vacated their office and/or were not entitled to vote, be as valid as if every such person had been duly appointed and/or not disqualified to be a Director or alternate Director and/or had not vacated their office and/or had been entitled to vote, as the case may be. |
| 30.9 | A Director but not an alternate Director may be represented at any meetings of the board of Directors by a proxy appointed in writing by that Director. The proxy shall count towards the quorum and the vote of the proxy shall for all purposes be deemed to be that of the appointing Director. |
| 31 | Presumption of Assent |
A Director or alternate Director who is present at a meeting of the board of Directors at which action on any Company matter is taken shall be presumed to have assented to the action taken unless their dissent shall be entered in the minutes of the meeting or unless they shall file their written dissent from such action with the person acting as the chairperson or secretary of the meeting before the adjournment thereof or shall forward such dissent by registered post to such person immediately after the adjournment of the meeting. Such right to dissent shall not apply to a Director or alternate Director who voted in favour of such action.
B-21
Table of Contents
| 32 | Directors’ Interests |
| 32.1 | A Director or alternate Director may hold any other office or place of profit under the Company (other than the office of Auditor) in conjunction with their office of Director for such period and on such terms as to remuneration and otherwise as the Directors may determine. |
| 32.2 | A Director or alternate Director may act on their own or by, through or on behalf of their firm in a professional capacity for the Company and they or their firm shall be entitled to remuneration for professional services as if they were not a Director or alternate Director. |
| 32.3 | A Director or alternate Director may be or become a director or other officer of or otherwise interested in any company promoted by the Company or in which the Company may be interested as a shareholder, a contracting party or otherwise, and no such Director or alternate Director shall be accountable to the Company for any remuneration or other benefits received by them as a director or officer of, or from their interest in, such other company. |
| 32.4 | No person shall be disqualified from the office of Director or alternate Director or prevented by such office from contracting with the Company, either as vendor, purchaser or otherwise, nor shall any such contract or any contract or transaction entered into by or on behalf of the Company in which any Director or alternate Director shall be in any way interested be or be liable to be avoided, nor shall any Director or alternate Director so contracting or being so interested be liable to account to the Company for any profit realised by or arising in connection with any such contract or transaction by reason of such Director or alternate Director holding office or of the fiduciary relationship thereby established. A Director (or their alternate Director in their absence) shall be at liberty to vote in respect of any contract or transaction in which they are interested provided that the nature of the interest of any Director or alternate Director in any such contract or transaction shall be disclosed by them at or prior to its consideration and any vote thereon. |
| 32.5 | A general notice that a Director or alternate Director is a shareholder, director, officer or employee of any specified firm or company and is to be regarded as interested in any transaction with such firm or company shall be sufficient disclosure for the purposes of voting on a resolution in respect of a contract or transaction in which they have an interest, and after such general notice it shall not be necessary to give special notice relating to any particular transaction. |
| 33 | Minutes |
The Directors shall cause minutes to be made in books kept for the purpose of recording all appointments of officers made by the Directors, all proceedings at meetings of the Company or the holders of any class of Shares and of the Directors, and of committees of the Directors, including the names of the Directors or alternate Directors present at each meeting.
| 34 | Delegation of Directors’ Powers |
| 34.1 | The Directors may delegate any of their powers, authorities and discretions, including the power to sub-delegate, to any committee consisting of one or more Directors. They may also delegate to any managing director or any Director holding any other executive office such of their powers, authorities and discretions as they consider desirable to be exercised by that Director, provided that an alternate Director may not act as managing director and the appointment of a managing director shall be revoked forthwith if they cease to be a Director. Any such delegation may be made subject to any conditions the Directors may impose and either collaterally with or to the exclusion of their own powers and any such delegation may be revoked or altered by the Directors. Subject to any such conditions, the proceedings of a committee of Directors shall be governed by the Articles regulating the proceedings of Directors, so far as they are capable of applying. |
B-22
Table of Contents
| 34.2 | The Directors may establish any committees, local boards or agencies or appoint any person to be a manager or agent for managing the affairs of the Company and may appoint any person to be a member of such committees, local boards or agencies. Any such appointment may be made subject to any conditions the Directors may impose, and either collaterally with or to the exclusion of their own powers and any such appointment may be revoked or altered by the Directors. Subject to any such conditions, the proceedings of any such committee, local board or agency shall be governed by the Articles regulating the proceedings of Directors, so far as they are capable of applying. |
| 34.3 | The Directors may by power of attorney or otherwise appoint any person to be the agent of the Company on such conditions as the Directors may determine, provided that the delegation is not to the exclusion of their own powers and may be revoked by the Directors at any time. |
| 34.4 | The Directors may by power of attorney or otherwise appoint any company, firm, person or body of persons, whether nominated directly or indirectly by the Directors, to be the attorney or authorised signatory of the Company for such purpose and with such powers, authorities and discretions (not exceeding those vested in or exercisable by the Directors under the Articles) and for such period and subject to such conditions as they may think fit, and any such powers of attorney or other appointment may contain such provisions for the protection and convenience of persons dealing with any such attorneys or authorised signatories as the Directors may think fit and may also authorise any such attorney or authorised signatory to delegate all or any of the powers, authorities and discretions vested in them. |
| 34.5 | The Directors may appoint such officers of the Company (including, for the avoidance of doubt and without limitation, any secretary) as they consider necessary on such terms, at such remuneration and to perform such duties, and subject to such provisions as to disqualification and removal as the Directors may think fit. Unless otherwise specified in the terms of their appointment an officer of the Company may be removed by resolution of the Directors or Members. An officer of the Company may vacate their office at any time if they give notice in writing to the Company that they resign their office. |
| 35 | Alternate Directors |
| 35.1 | Any Director (but not an alternate Director) may by writing appoint any other Director, or any other person willing to act, to be an alternate Director and by writing may remove from office an alternate Director so appointed by them. |
| 35.2 | An alternate Director shall be entitled to receive notice of all meetings of Directors and of all meetings of committees of Directors of which their appointor is a member, to attend and vote at every such meeting at which the Director appointing them is not personally present, to sign any written resolution of the Directors, and generally to perform all the functions of their appointor as a Director in their absence. |
| 35.3 | An alternate Director shall cease to be an alternate Director if their appointor ceases to be a Director. |
| 35.4 | Any appointment or removal of an alternate Director shall be by notice to the Company signed by the Director making or revoking the appointment or in any other manner approved by the Directors. |
| 35.5 | Subject to the provisions of the Articles, an alternate Director shall be deemed for all purposes to be a Director and shall alone be responsible for their own acts and defaults and shall not be deemed to be the agent of the Director appointing them. |
| 36 | No Minimum Shareholding |
The Company in general meeting may fix a minimum shareholding required to be held by a Director, but unless and until such a shareholding qualification is fixed a Director is not required to hold Shares.
B-23
Table of Contents
| 37 | Remuneration of Directors |
| 37.1 | The remuneration to be paid to the Directors, if any, shall be such remuneration as the Directors shall determine. The Directors shall also be entitled to be paid all travelling, hotel and other expenses properly incurred by them in connection with their attendance at meetings of Directors or committees of Directors, or general meetings of the Company, or separate meetings of the holders of any class of Shares or debentures of the Company, or otherwise in connection with the business of the Company or the discharge of their duties as a Director, or to receive a fixed allowance in respect thereof as may be determined by the Directors, or a combination partly of one such method and partly the other. |
| 37.2 | The Directors may by resolution approve additional remuneration to any Director for any services which in the opinion of the Directors go beyond that Director’s ordinary routine work as a Director. Any fees paid to a Director who is also counsel, attorney or solicitor to the Company, or otherwise serves it in a professional capacity shall be in addition to their remuneration as a Director. |
| 38 | Seal |
| 38.1 | The Company may, if the Directors so determine, have a Seal. The Seal shall only be used by the authority of the Directors or of a committee of the Directors authorised by the Directors. Every instrument to which the Seal has been affixed shall be signed by at least one person who shall be either a Director or some officer of the Company or other person appointed by the Directors for the purpose. |
| 38.2 | The Company may have for use in any place or places outside the Cayman Islands a duplicate Seal or Seals each of which shall be a fax of the common Seal of the Company and, if the Directors so determine, with the addition on its face of the name of every place where it is to be used. |
| 38.3 | A Director or officer, representative or attorney of the Company may without further authority of the Directors affix the Seal over their signature alone to any document of the Company required to be authenticated by them under seal or to be filed with the Registrar of Companies in the Cayman Islands or elsewhere wheresoever. |
| 39 | Dividends, Distributions and Reserve |
| 39.1 | Subject to the Statute and this Article and except as otherwise provided by the rights attached to any Shares, the Directors may resolve to pay Dividends and other distributions on Shares in issue and authorise payment of the Dividends or other distributions out of the funds of the Company lawfully available therefor. A Dividend shall be deemed to be an interim Dividend unless the terms of the resolution pursuant to which the Directors resolve to pay such Dividend specifically state that such Dividend shall be a final Dividend. No Dividend or other distribution shall be paid except out of the realised or unrealised profits of the Company, out of the share premium account or as otherwise permitted by law. |
| 39.2 | Except as otherwise provided by the rights attached to any Shares, all Dividends and other distributions shall be paid according to the par value of the Shares that a Member holds. If any Share is issued on terms providing that it shall rank for Dividend as from a particular date, that Share shall rank for Dividend accordingly. |
| 39.3 | The Directors may deduct from any Dividend or other distribution payable to any Member all sums of money (if any) then payable by the Member to the Company on account of calls or otherwise. |
| 39.4 | The Directors may resolve that any Dividend or other distribution be paid wholly or partly by the distribution of specific assets and in particular (but without limitation) by the distribution of shares, debentures, or securities of any other company or in any one or more of such ways and where any difficulty arises in regard to such distribution, the Directors may settle the same as they think expedient and in particular may issue fractional Shares and may fix the value for distribution of such specific assets |
B-24
Table of Contents
| or any part thereof and may determine that cash payments shall be made to any Members upon the basis of the value so fixed in order to adjust the rights of all Members and may vest any such specific assets in trustees in such manner as may seem expedient to the Directors. |
| 39.5 | Except as otherwise provided by the rights attached to any Shares, Dividends and other distributions may be paid in any currency. The Directors may determine the basis of conversion for any currency conversions that may be required and how any costs involved are to be met. |
| 39.6 | The Directors may, before resolving to pay any Dividend or other distribution, set aside such sums as they think proper as a reserve or reserves which shall, at the discretion of the Directors, be applicable for any purpose of the Company and pending such application may, at the discretion of the Directors, be employed in the business of the Company. |
| 39.7 | Any Dividend, other distribution, interest or other monies payable in cash in respect of Shares may be paid by wire transfer to the holder or by cheque or warrant sent through the post directed to the registered address of the holder or, in the case of joint holders, to the registered address of the holder who is first named on the Register of Members or to such person and to such address as such holder or joint holders may in writing direct. Every such cheque or warrant shall be made payable to the order of the person to whom it is sent. Any one of two or more joint holders may give effectual receipts for any Dividends, other distributions, bonuses, or other monies payable in respect of the Share held by them as joint holders. |
| 39.8 | No Dividend or other distribution shall bear interest against the Company. |
| 39.9 | Any Dividend or other distribution which cannot be paid to a Member and/or which remains unclaimed after six months from the date on which such Dividend or other distribution becomes payable may, in the discretion of the Directors, be paid into a separate account in the Company’s name, provided that the Company shall not be constituted as a trustee in respect of that account and the Dividend or other distribution shall remain as a debt due to the Member. Any Dividend or other distribution which remains unclaimed after a period of six years from the date on which such Dividend or other distribution becomes payable shall be forfeited and shall revert to the Company. |
| 40 | Capitalisation |
The Directors may at any time capitalise any sum standing to the credit of any of the Company’s reserve accounts or funds (including the share premium account and capital redemption reserve fund) or any sum standing to the credit of the profit and loss account or otherwise available for distribution; appropriate such sum to Members in the proportions in which such sum would have been divisible amongst such Members had the same been a distribution of profits by way of Dividend or other distribution; and apply such sum on their behalf in paying up in full unissued Shares for allotment and distribution credited as fully paid-up to and amongst them in the proportion aforesaid. In such event the Directors shall do all acts and things required to give effect to such capitalisation, with full power given to the Directors to make such provisions as they think fit in the case of Shares becoming distributable in fractions (including provisions whereby the benefit of fractional entitlements accrue to the Company rather than to the Members concerned). The Directors may authorise any person to enter on behalf of all of the Members interested into an agreement with the Company providing for such capitalisation and matters incidental or relating thereto and any agreement made under such authority shall be effective and binding on all such Members and the Company.
| 41 | Books of Account |
| 41.1 | The Directors shall cause proper books of account (including, where applicable, material underlying documentation including contracts and invoices) to be kept with respect to all sums of money received and expended by the Company and the matters in respect of which the receipt or expenditure takes place, all sales and purchases of goods by the Company and the assets and liabilities of the Company. Such |
B-25
Table of Contents
| books of account must be retained for a minimum period of five years from the date on which they are prepared. Proper books shall not be deemed to be kept if there are not kept such books of account as are necessary to give a true and fair view of the state of the Company’s affairs and to explain its transactions. |
| 41.2 | The Directors shall determine whether and to what extent and at what times and places and under what conditions or regulations the accounts and books of the Company or any of them shall be open to the inspection of Members not being Directors and no Member (not being a Director) shall have any right of inspecting any account or book or document of the Company except as conferred by Statute or authorised by the Directors or by the Company in general meeting. |
| 41.3 | The Directors may cause to be prepared and to be laid before the Company in general meeting profit and loss accounts, balance sheets, group accounts (if any) and such other reports and accounts as may be required by law. |
| 42 | Audit |
| 42.1 | The Directors may appoint an Auditor of the Company who shall hold office on such terms as the Directors determine. |
| 42.2 | Every Auditor of the Company shall have a right of access at all times to the books and accounts and vouchers of the Company and shall be entitled to require from the Directors and officers of the Company such information and explanation as may be necessary for the performance of the duties of the Auditor. |
| 42.3 | Auditors shall, if so required by the Directors, make a report on the accounts of the Company during their tenure of office at the next annual general meeting following their appointment in the case of a company which is registered with the Registrar of Companies as an ordinary company, and at the next extraordinary general meeting following their appointment in the case of a company which is registered with the Registrar of Companies as an exempted company, and at any other time during their term of office, upon request of the Directors or any general meeting of the Members. |
| 43 | Notices |
| 43.1 | Notices shall be in writing and may be given by the Company to any Member either personally or by sending it by courier, post, telex, fax or email to such Member or to such Member’s address as shown in the Register of Members (or where the notice is given by email by sending it to the email address provided by such Member). Any notice, if posted from one country to another, is to be sent by airmail. |
| 43.2 | Where a notice is sent by courier, service of the notice shall be deemed to be effected by delivery of the notice to a courier company, and shall be deemed to have been received on the third day (not including Saturdays or Sundays or public holidays) following the day on which the notice was delivered to the courier. Where a notice is sent by post, service of the notice shall be deemed to be effected by properly addressing, pre paying and posting a letter containing the notice, and shall be deemed to have been received on the fifth day (not including Saturdays or Sundays or public holidays in the Cayman Islands) following the day on which the notice was posted. Where a notice is sent by telex or fax, service of the notice shall be deemed to be effected by properly addressing and sending such notice and shall be deemed to have been received on the same day that it was transmitted. Where a notice is given by email service shall be deemed to be effected by transmitting the email to the email address provided by the intended recipient and shall be deemed to have been received on the same day that it was sent, and it shall not be necessary for the receipt of the email to be acknowledged by the recipient. |
| 43.3 | A notice may be given by the Company to the person or persons which the Company has been advised are entitled to a Share or Shares in consequence of the death or bankruptcy of a Member in the same manner as other notices which are required to be given under the Articles and shall be addressed to them by name, or by the title of representatives of the deceased, or trustee of the bankrupt, or by any like description at the address supplied for that purpose by the persons claiming to be so entitled, or at the option of the Company by giving the notice in any manner in which the same might have been given if the death or bankruptcy had not occurred. |
B-26
Table of Contents
| 43.4 | Notice of every general meeting shall be given in any manner authorised by the Articles to every holder of Shares carrying an entitlement to receive such notice on the record date for such meeting except that in the case of joint holders the notice shall be sufficient if given to the joint holder first named in the Register of Members and every person upon whom the ownership of a Share devolves because they are a legal personal representative or a trustee in bankruptcy of a Member where the Member but for their death or bankruptcy would be entitled to receive notice of the meeting, and no other person shall be entitled to receive notices of general meetings. |
| 44 | Winding Up |
| 44.1 | If the Company shall be wound up the liquidator shall apply the assets of the Company in satisfaction of creditors’ claims in such manner and order as such liquidator thinks fit. Subject to the rights attaching to any Shares, in a winding up: |
| (a) | if the assets available for distribution amongst the Members shall be insufficient to repay the whole of the Company’s issued share capital, such assets shall be distributed so that, as nearly as may be, the losses shall be borne by the Members in proportion to the par value of the Shares held by them; or |
| (b) | if the assets available for distribution amongst the Members shall be more than sufficient to repay the whole of the Company’s issued share capital at the commencement of the winding up, the surplus shall be distributed amongst the Members in proportion to the par value of the Shares held by them at the commencement of the winding up subject to a deduction from those Shares in respect of which there are monies due, of all monies payable to the Company for unpaid calls or otherwise. |
| 44.2 | If the Company shall be wound up the liquidator may, subject to the rights attaching to any Shares and with the approval of a Special Resolution of the Company and any other approval required by the Statute, divide amongst the Members in kind the whole or any part of the assets of the Company (whether such assets shall consist of property of the same kind or not) and may for that purpose value any assets and determine how the division shall be carried out as between the Members or different classes of Members. The liquidator may, with the like approval, vest the whole or any part of such assets in trustees upon such trusts for the benefit of the Members as the liquidator, with the like approval, shall think fit, but so that no Member shall be compelled to accept any asset upon which there is a liability. |
| 45 | Indemnity and Insurance |
| 45.1 | Every Director and officer of the Company (which for the avoidance of doubt, shall not include auditors of the Company), together with every former Director and former officer of the Company (each an “Indemnified Person”) shall be indemnified out of the assets of the Company against any liability, action, proceeding, claim, demand, costs, damages or expenses, including legal expenses, whatsoever which they or any of them may incur as a result of any act or failure to act in carrying out their functions other than such liability (if any) that they may incur by reason of their own actual fraud or wilful default. No Indemnified Person shall be liable to the Company for any loss or damage incurred by the Company as a result (whether direct or indirect) of the carrying out of their functions unless that liability arises through the actual fraud or wilful default of such Indemnified Person. No person shall be found to have committed actual fraud or wilful default under this Article unless or until a court of competent jurisdiction shall have made a finding to that effect. |
| 45.2 | The Company shall advance to each Indemnified Person reasonable attorneys’ fees and other costs and expenses incurred in connection with the defence of any action, suit, proceeding or investigation involving such Indemnified Person for which indemnity will or could be sought. In connection with any advance of any expenses hereunder, the Indemnified Person shall execute an undertaking to repay the advanced amount to the Company if it shall be determined by final judgment or other final adjudication that such Indemnified Person was not entitled to indemnification pursuant to this Article. If it shall be |
B-27
Table of Contents
| determined by a final judgment or other final adjudication that such Indemnified Person was not entitled to indemnification with respect to such judgment, costs or expenses, then such party shall not be indemnified with respect to such judgment, costs or expenses and any advancement shall be returned to the Company (without interest) by the Indemnified Person. |
| 45.3 | The Directors, on behalf of the Company, may purchase and maintain insurance for the benefit of any Director or other officer of the Company against any liability which, by virtue of any rule of law, would otherwise attach to such person in respect of any negligence, default, breach of duty or breach of trust of which such person may be guilty in relation to the Company. |
| 46 | Financial Year |
Unless the Directors otherwise prescribe, the financial year of the Company shall end on 31st December in each year and, following the year of incorporation, shall begin on 1st January in each year.
| 47 | Transfer by Way of Continuation |
If the Company is exempted as defined in the Statute, it shall, subject to the provisions of the Statute and with the approval of a Special Resolution, have the power to register by way of continuation as a body corporate under the laws of any jurisdiction outside the Cayman Islands and to be deregistered in the Cayman Islands.
| 48 | Mergers and Consolidations |
The Company shall have the power to merge or consolidate with one or more other constituent companies (as defined in the Statute) upon such terms as the Directors may determine and (to the extent required by the Statute) with the approval of a Special Resolution.
B-28
Table of Contents
FORM OF SUBSCRIPTION AGREEMENT
This Subscription Agreement (this “Subscription Agreement”) is being entered into as of the date set forth on the signature page hereto, by and among NewAmsterdam Pharma Company B.V., a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) that will be converted into a Dutch public limited liability company (naamloze vennootschap) in connection with the Transactions (as defined below) (“New NAP”), Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company (“FLAC”), and the undersigned subscriber (the “Investor”), in connection with the Business Combination Agreement, dated as of the date hereof (as may be amended, supplemented or otherwise modified from time to time, the “Transaction Agreement”), by and among FLAC, New NAP, NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”), and NewAmsterdam Pharma Holding B.V., a Dutch private limited company (besloten vennootschap met beperkte aansprakelijkheid) (the “Company”), pursuant to which, among other things, all outstanding shares in the capital of the Company will be contributed and transferred to New NAP against issuance by New NAP of shares to existing Company shareholders (the “Contribution”), New NAP will convert into a Dutch public limited liability company (naamloze vennootschap) (the “Conversion”), Merger Sub will merge with and into FLAC with FLAC surviving the merger as a wholly owned subsidiary of New NAP, New NAP will issue certain shares and warrants to existing FLAC shareholders and warrant holders, respectively, and FLAC will redomesticate to Delaware (the “Redomestication”) (the “Business Combination” and together with the Contribution, the Conversion, the Redomestication and the other transactions contemplated hereby and by the Transaction Agreement, the “Transactions”). In connection with the Transactions, FLAC and New NAP are seeking commitments from interested investors to purchase, immediately following the Transactions and substantially concurrently with the closing of the Business Combination, ordinary shares in the capital of New NAP, par value EUR 0.12 per share (the “Shares”), in a private placement (the “Private Placement”) for a purchase price of $10.00 per share (the “Per Share Purchase Price”). Concurrently with the execution of this Subscription Agreement, FLAC and New NAP are entering into subscription agreements (the “Other Subscription Agreements” and together with this Subscription Agreement, the “Subscription Agreements”) with certain other investors (the “Other Investors” and together with the Investor, the “Investors”), severally and not jointly, pursuant to which the Investors, severally and not jointly, have agreed to purchase on the closing date of the Business Combination, inclusive of the Subscribed Shares (as defined below), an aggregate amount of up to 23,460,000 Shares, at the Per Share Purchase Price. The aggregate purchase price to be paid by the Investor for the Subscribed Shares is referred to herein as the “Subscription Amount.”
In connection therewith, and in consideration of the foregoing and the mutual representations, warranties and covenants, and subject to the conditions, set forth herein, and intending to be legally bound hereby, the Investor, New NAP and FLAC each acknowledge and agree as follows:
The decision of the Investor to purchase the Subscribed Shares pursuant to this Subscription Agreement has been made by the Investor independently of any Other Investor or any other investor and independently of any information, materials, statements or opinions as to the business, affairs, operations, assets, properties, liabilities, results of operations, condition (financial or otherwise) or prospects of FLAC, New NAP, the Company or any of their respective subsidiaries which may have been made or given by any Other Investor or any other investor or by any agent or employee of any Other Investor or any other investor, and neither the Investor nor any of its agents or employees shall have any liability to any Other Investor or any other investor (or any other person not party to this Agreement) relating to or arising from any such information, materials, statements or opinions. Nothing contained herein or in any Other Subscription Agreement, and no action taken by the Investor or any Other Investor pursuant hereto or thereto, shall be deemed to constitute the Investor and Other Investors or other investors as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Investor and Other Investors or other investors are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by this Subscription Agreement and the Other Subscription
C-1
Table of Contents
Agreements. The Investor acknowledges that no Other Investor has acted as agent for the Investor in connection with making its investment hereunder and no Other Investor will be acting as agent of the Investor in connection with monitoring its investment in the Subscribed Shares or enforcing its rights under this Subscription Agreement. The Investor shall be entitled to independently protect and enforce its rights, including without limitation the rights arising out of this Subscription Agreement, and it shall not be necessary for any Other Investor or any other investor to be joined as an additional party in any proceeding for such purpose. The obligations of Investor under this Subscription Agreement are several and not joint with the obligations of any Other Investor or any other investor under the Other Subscription Agreements, and Investor shall not be responsible in any way for the performance of the obligations of any Other Investor under this Subscription Agreement or any other investor under the Other Subscription Agreements.
1. Subscription. The Investor hereby irrevocably subscribes for and agrees to purchase from New NAP and New NAP hereby irrevocably agrees to issue and sell to the Investor, in each case, the number of Shares set forth on the signature page of this Subscription Agreement on the terms and subject to the conditions provided for herein (such Shares, the “Subscribed Shares” and such subscription and issuance, the “Subscription”). The Investor acknowledges and agrees that New NAP reserves the right to accept or reject the Investor’s subscription for the Subscribed Shares for any reason or for no reason, in whole or in part, at any time prior to its acceptance, and the same shall be deemed to be accepted by New NAP only when this Subscription Agreement is signed by a duly authorized person by or on behalf of New NAP; New NAP may do so in counterpart form. The Investor acknowledges and agrees that, as a result of the consummation of the Transactions, the Subscribed Shares that will be purchased by the Investor and issued by New NAP pursuant hereto shall be ordinary shares in the share capital of a Dutch public limited liability company (naamloze vennootschap), provided that, if Dutch law requires the Subscription to occur prior to the Conversion in order to be able to effect the Conversion under Dutch law, Investor shall initially receive ordinary shares in the share capital of a Dutch private limited company (besloten vennootschap met beperkte aansprakelijkheid) that will immediately following the consummation of the Private Placement be converted into the number of ordinary shares in the share capital of a Dutch public limited liability company (naamloze vennootschap) required under this Subscription Agreement.
2. Closing.
(a) The closing of the Subscription contemplated hereby (the “Closing” and the date of which the Closing occurs, the “Closing Date”) shall occur on the date of, and substantially concurrently with and conditioned upon the effectiveness of, the consummation of the Business Combination, provided that the Conversion may also take place after Closing but on the Closing Date. Upon (i) satisfaction or waiver in writing of the conditions set forth in Section 3 of this Subscription Agreement and (ii) delivery of written notice from (or on behalf of) New NAP to the Investor (the “Closing Notice”), that New NAP reasonably expects all conditions to the closing of the Transactions to be satisfied or waived on a date that is not less than ten (10) calendar days from the date on which the Closing Notice is delivered to the Investor, the Investor shall deliver to New NAP, no later than two (2) Business Days prior to the anticipated Closing Date specified in the Closing Notice, (i) the Subscription Amount for the Subscribed Shares by wire transfer of United States dollars in immediately available funds to the account(s) specified by New NAP in the Closing Notice and (ii) if the Conversion takes place after Closing (but on the Closing Date), a duly executed and notarized power of attorney in favor of Dutch legal counsel to New NAP substantially in the form of Exhibit A hereto in order to effect the issuance of the Subscribed Shares pursuant to a notarial deed to that effect (the “PoA”). On the Closing Date, New NAP shall (A) issue the Subscribed Shares to the Investor and cause such Subscribed Shares to be registered in book-entry form in the name of the Investor (or its nominee in accordance with its delivery instructions) or to a custodian designated by the Investor, as applicable, on New NAP’s share register or the register of New NAP’s transfer agent, free and clear of all liens, encumbrances or other restrictions (other than those arising under applicable securities laws or those created by the Investor), and (B) provide evidence to the Investor of such issuance on and as of the Closing Date; provided, however, that New NAP’s obligation to issue the Subscribed Shares to the Investor is contingent upon New NAP having received the Subscription Amount in full and, if applicable, the duly executed and notarized
C-2
Table of Contents
PoA in accordance with this Section 2. For purposes of this Subscription Agreement, “Business Day” shall mean a day, other than a Saturday or Sunday, on which commercial banks in Amsterdam, the Netherlands and New York, New York are open for the general transaction of business, provided that banks shall be deemed to be generally open for the general transaction of business in the event of a “shelter in place” or similar closure of physical branch locations at the direction of any governmental authority if such banks’ electronic funds transfer system (including for wire transfers) are open for use by customers on such day.
(b) In the event the closing of the Transactions does not occur within three (3) Business Days following the anticipated Closing Date identified in the Closing Notice, New NAP shall promptly (but not later than two (2) Business Days thereafter) return the Subscription Amount in full to the Investor by wire transfer of U.S. dollars in immediately available funds to the account from which New NAP received the Subscription Amount, and any book entries shall be deemed cancelled. Notwithstanding such return or cancellation (i) a failure to close on the anticipated Closing Date shall not, by itself, be deemed to be a failure of any of the conditions to Closing set forth in Section 3 herein to be satisfied or waived on or prior to the Closing Date, and (ii) unless and until this Subscription Agreement is terminated in accordance with Section 8 herein, the Investor shall remain obligated (A) to redeliver funds to New NAP and, if applicable, confirm that the PoA has remained in full force and effect, in each case following New NAP’s delivery to the Investor of a new Closing Notice in accordance with this Section 2 and (B) to consummate the Closing upon satisfaction of the conditions set forth in Section 3 herein.
(c) Prior to or at the Closing, the Investor shall deliver to New NAP the legal name of the person in whose name such Subscribed Shares are to be issued and a duly executed Internal Revenue Service Form W-9 or W-8, as applicable.
3. Closing Conditions.
(a) The obligation of each of New NAP, FLAC and the Investor to consummate the Subscription is subject to the satisfaction or, to the extent permitted by applicable law, waiver by the Investor, New NAP or FLAC, as applicable, of the following conditions:
(i) no judgment, order, law, rule or regulation (whether temporary, preliminary or permanent) issued by any court or other governmental authority of competent authority restraining, prohibiting or making illegal the consummation of the Subscription or any other transactions contemplated hereby shall be pending or in effect;
(ii) the Shares shall have been approved for listing, subject to official notice of issuance, on the Stock Exchange (as defined below), and no suspension of the listing or qualification for offering or sale or trading on such Stock Exchange of the Shares shall have occurred and be continuing; and
(iii) (A) all conditions precedent to the consummation of the Transactions under the Transaction Agreement shall have been satisfied (as determined by the parties to the Transaction Agreement and other than those conditions under the Transaction Agreement, which by their nature are to be satisfied at the consummation of the Transactions, including to the extent that any such condition is dependent upon the consummation of the Subscription) or waived and (B) the closing of the Transactions shall be scheduled to occur substantially concurrently with the Closing, provided that the Conversion may also take place after Closing but on the Closing Date.
(b) The obligation of New NAP or FLAC to consummate the Subscription is subject to the satisfaction or, to the extent permitted by applicable law, waiver by New NAP or FLAC, as applicable, of the following conditions:
(i) all representations and warranties of the Investor contained in this Subscription Agreement shall be true and correct in all material respects (other than representations and warranties that are qualified as to materiality, which representations and warranties shall be true in all respects) at and as of the Closing Date (except for representations and warranties expressly made as of a specific date, which shall be true and correct in all material respects (other than representations and warranties that are qualified as to materiality,
C-3
Table of Contents
which representations and warranties shall be true in all respects) as of such date) and the consummation of the Closing shall constitute a reaffirmation by the Investor of each of the representations and warranties of the Investor contained in this Subscription Agreement as of the Closing Date or such earlier date, as applicable, subject to the foregoing qualifiers; and
(ii) the Investor shall have performed, satisfied and complied in all material respects with all covenants, agreements and conditions required by this Subscription Agreement to be performed, satisfied or complied with by it at or prior to the Closing.
(c) The obligation of the Investor to consummate the Subscription is subject to the satisfaction or, to the extent permitted by applicable law, waiver by the Investor of the following conditions:
(i) all representations and warranties of New NAP and FLAC contained in this Subscription Agreement shall be true and correct in all material respects (other than representations and warranties that are qualified as to materiality or New NAP Material Adverse Effect or FLAC Material Adverse Effect (as defined herein), which representations and warranties shall be true and correct in all respects) at and as of the Closing Date (except for representations and warranties expressly made as of a specific date, which shall be true and correct in all material respects (other than representations and warranties that are qualified as to materiality or New NAP Material Adverse Effect or FLAC Material Adverse Effect, which representations and warranties shall be true and correct in all respects) as of such date) and the consummation of the Closing shall constitute a reaffirmation by New NAP and FLAC of each of the representations and warranties of New NAP or FLAC, as applicable, contained in this Subscription Agreement as of the Closing Date or such earlier date, as applicable, subject to the foregoing qualifiers;
(ii) New NAP and FLAC shall have performed, satisfied and complied in all material respects with all covenants, agreements and conditions required by the Subscription Agreement to be performed, satisfied or complied with by it at or prior to the Closing;
(iii) no amendment, modification or waiver of the Transaction Agreement (as the same exists on the date of this Subscription Agreement) shall have occurred, without the Investor’s written consent, that would reasonably be expected to adversely affect the economic benefits that the Investor would reasonably expect to receive under this Subscription Agreement (including any change to the economic terms of the Transactions or to the minimum cash condition set forth in the Transaction Agreement);
(iv) the terms of the Other Subscription Agreements shall not have been materially amended following the date hereof without offering the benefit of any such amendment to the Investor; provided, however, that New NAP may, in its sole discretion, amend the Subscription Amounts in one or more Other Subscription Agreements at a Per Share Purchase Price not less than the same Per Share Purchase Price in this Subscription Agreement;
(v) all consents, waivers, authorizations or orders of, any notice required to be made to, and any filing or registration with, any court or other federal, state, local or other governmental authority, self-regulatory organization (including any required shareholder approvals) or other person in connection with the execution, delivery and performance of this Subscription Agreement (including, without limitation, the issuance of the Shares) required to be made in connection with the Subscription shall have been obtained or made, except where the failure to so obtain or make would not prevent New NAP from consummating the transactions contemplated hereby, including the Subscription; and
(vi) from and after the date hereof, there shall have not occurred any New NAP Material Adverse Effect or FLAC Material Adverse Effect.
4. Further Assurances. At the Closing, New NAP, FLAC and the Investor shall execute and deliver such additional documents and take such additional actions as New NAP, FLAC and the Investor reasonably may deem to be practical and necessary in order to consummate the Subscription as contemplated by this Subscription Agreement; in each case, in accordance with the terms of this Subscription Agreement.
C-4
Table of Contents
5. New NAP and FLAC Representations and Warranties. Each of New NAP, with respect only to the representations and warranties set forth below relating to New NAP and the Company, and FLAC, with respect only to the representations and warranties set forth below relating to FLAC, represents and warrants to the Investor, as of the date hereof and as of the Closing Date, that:
(a) Until the Redomestication, FLAC is an exempted company duly incorporated, validly existing and in good standing under the laws of the Cayman Islands (to the extent such concept exists in such jurisdiction). Immediately following the Redomestication, FLAC will be a corporation organized under the laws of the State of Delaware. FLAC has all power (corporate or otherwise) and authority to own, lease and operate its properties and conduct its business as presently conducted and to enter into, deliver and perform its obligations under this Subscription Agreement. New NAP is duly incorporated as a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) and will be, following the Conversion, validly existing as a Dutch public limited liability company (naamloze vennootschap) and is validly existing under the laws of the Netherlands with all corporate power and authority to own, lease and operate its properties and, conduct its business as presently conducted and to enter into, deliver and perform its obligations under this Subscription Agreement. The Company is duly incorporated as a Dutch private limited liability company (besloten vennootschap met beperkte aansprakelijkheid) and is validly existing under the laws of the Netherlands with all corporate power and authority to own, lease and operate its properties and conduct its business as presently conducted.
(b) As of the Closing Date, the Subscribed Shares will be duly authorized by New NAP and, when issued and delivered to the Investor against full payment therefor in accordance with the terms of this Subscription Agreement, the Subscribed Shares will be validly issued, fully paid and non-assessable (meaning that the holders of the Subscribed Shares will not by reason of merely being such a holder, be subject to assessment or calls by New NAP or its creditors for further payment on such Subscribed Shares) free and clear of any liens or restrictions (other than those arising under applicable securities laws or those created by the Investor), and will not have been issued in violation of or subject to any preemptive or similar rights created under New NAP’s articles of association (as amended as of the Closing Date) or under the laws of the Netherlands.
(c) This Subscription Agreement and the Transaction Agreement have been duly authorized, executed and delivered by New NAP and FLAC and constitute the valid and binding agreements of New NAP and FLAC. Assuming that this Subscription Agreement constitutes the valid and binding agreement of the Investor, this Subscription Agreement is enforceable against each of New NAP and FLAC in accordance with its terms, except as may be limited or otherwise affected by (i) bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other laws relating to or affecting the rights of creditors generally, or (ii) principles of equity, whether considered at law or equity.
(d) The execution, delivery and performance by each of New NAP and FLAC of this Subscription Agreement and the Transaction Agreement and the consummation of the transactions contemplated herein and therein, including the issuance and sale of the Subscribed Shares, do not and will not (1) conflict with or result in a breach or violation of any of the terms or provisions of, or constitute a default under, or result in the creation or imposition of any lien, charge or encumbrance upon any of the property or assets of New NAP, the Company, FLAC or any of their subsidiaries or the Subscribed Shares pursuant to the terms of (i) any indenture, mortgage, deed of trust, loan agreement, lease, license or other agreement or instrument to which New NAP, the Company or any of their subsidiaries is a party or by which New NAP, the Company or any of their subsidiaries is bound or to which any of the property or assets of New NAP is subject that would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, properties, prospects, general affairs, management or financial condition of New NAP, the Company or their subsidiaries, taken as a whole or materially and adversely affect (A) the ability of New NAP and the Company to timely consummate the Transactions, (B) the validity of the issuance of the Subscribed Shares or (C) the legal authority of New NAP and the Company to comply in all material respects with the terms of this Subscription Agreement (each, a “New NAP Material Adverse Effect”); (ii) any indenture, mortgage, deed of trust, loan agreement, lease, license or other agreement or instrument
C-5
Table of Contents
to which FLAC or any of its subsidiaries is a party or by which FLAC or any of its subsidiaries is bound or to which any of the property or assets of FLAC is subject that would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, properties, prospects, general affairs, management or financial condition of FLAC or its subsidiaries, taken as a whole or materially and adversely affect (A) the ability of FLAC to timely consummate the Transactions or (B) the legal authority of FLAC to comply in all material respects with the terms of this Subscription Agreement (each, a “FLAC Material Adverse Effect”); (2) result in any violation of the provisions of the organizational documents of New NAP, the Company or FLAC; or (3) result in any violation of any statute or any law, judgment, order, rule or regulation of any court or governmental agency or body, domestic or foreign, having jurisdiction over New NAP, the Company or FLAC or any of their properties that would reasonably be expected to have, individually or in the aggregate, a New NAP Material Adverse Effect or a FLAC Material Adverse Effect.
(e) FLAC and New NAP have timely made all filings required to be filed by them with the U.S. Securities and Exchange Commission (the “SEC”). As of their respective dates and except to the extent that information contained in any SEC Report has been superseded by a later timely filed SEC Report, all reports, forms, statements, schedules, prospectuses, proxy statements, registration statements and other documents or any amendments related thereto required to be filed by FLAC or New NAP with the SEC (the “SEC Reports”) complied in all material respects with the applicable requirements of the Securities Act of 1933, as amended, (the “Securities Act”), and the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules and regulations of the SEC promulgated thereunder, and none of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The financial statements of FLAC and New NAP, as applicable, included in the SEC Reports comply in all material respects with applicable accounting requirements and the rules and regulations of the SEC with respect thereto as in effect at the time of filing and fairly present in all material respects the financial condition of FLAC and New NAP, as applicable, as of and for the dates thereof and the results of operations and cash flows for the periods then ended, subject, in the case of interim unaudited statements, to normal, year-end audit adjustments, and such consolidated financial statements have been prepared in conformity with applicable accounting requirements (except as may be disclosed therein or in the notes thereto, and except that the interim unaudited financial statements may not contain all footnotes required by applicable accounting requirements). There are no outstanding or unresolved comments in comment letters received by FLAC or New NAP, as applicable, from the staff of the Division of Corporation Finance of the SEC with respect to any of the SEC Reports. A copy of each SEC Report is available to the Investor via the SEC’s EDGAR system.
(f) Other than as contemplated by the Other Subscription Agreements, the Transaction Agreement and any other agreement expressly contemplated by the Transaction Agreement, each of New NAP, the Company and FLAC has not entered, and will not enter, into any side letter, agreement or understanding (written or oral) with any Other Investor or any other investor or potential investor in connection with such Other Investor’s, investor’s or potential investor’s direct or indirect investment in New NAP (other than the side letters entered into, or to be entered into, with Saga Investments Coöperatief U.A. (“Amgen”) and Mitsubishi Tanabe Pharma Corporation (“MTPC”), each related to the share purchase agreement, dated April 9, 2020, and the profit right and waiver agreement, dated April 9, 2020, between Amgen and MTPC, respectively, and the Company and any other side letter or similar agreement relating to the issuance or transfer to any investor of (i) securities of New NAP or (ii) securities to be issued to the direct or indirect securityholders of the Company or FLAC pursuant to the Transaction Agreement). Except for any alternative settlement procedures, eligibility for qualified purchasers to invest, and other than terms particular to the regulatory requirements of such Other Investor or its affiliates or related funds, no Other Subscription Agreement includes (or will include) terms and conditions that are more advantageous to any such Other Investor, investor or potential investor (as compared to this Subscription Agreement). Subject to Section 10(s), the Other Subscription Agreements have not been (and will not be) amended or modified in any material respect following the date of this Subscription Agreement. The Other Subscription
C-6
Table of Contents
Agreements, which are materially identical to this Subscription Agreement in all material respects, reflect not less than the same Per Share Purchase Price in this Subscription Agreement and do not contain any put, anti-dilution, conversion, warrant or other rights to purchase, sell or receive equity or debt securities or cash of New NAP, FLAC, the Company or Merger Sub that are not also in this Subscription Agreement.
(g) Assuming the accuracy of the representations and warranties of the Investor set forth in this Subscription Agreement, neither New NAP, the Company nor FLAC is required to obtain any consent, waiver, authorization or order of, give any notice to, or make any filing or registration with, any court or other federal, state, local or other governmental authority, self-regulatory organization or other person in connection with the execution, delivery and performance by New NAP and FLAC of this Subscription Agreement or the Transaction Agreement (including, without limitation, the issuance of the Subscribed Shares), other than (i) filings with the SEC (including the filing of a Notice of Exempt Offering of Securities on Form D with the SEC, if applicable), (ii) filings required by applicable state securities laws, (iii) the filing of the Registration Statement (as defined below) pursuant to Section 7 of this Subscription Agreement, (iv) filings required by The Nasdaq Stock Market (the “Stock Exchange”), including with respect to obtaining shareholder approval, (v) those contemplated in the Transaction Agreement, (vi) the filing of notification under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, if applicable, and (vii) the failure of which to obtain would not be reasonably likely to have, individually or in the aggregate, a New NAP Material Adverse Effect or a FLAC Material Adverse Effect. FLAC is in material compliance with all applicable laws and rules of the Stock Exchange.
(h) All issued and outstanding Shares have been duly authorized and validly issued by New NAP, are fully paid and are non-assessable (meaning that the holders of the Subscribed Shares will not by reason of merely being such a holder, be subject to assessment or calls by New NAP or its creditors for further payment on such Subscribed Shares). As of the date of this Subscription Agreement, New NAP has one outstanding Share, par value EUR 0.12, which is held by the Company and which will be cancelled without repayment of any amount paid on such Share at Closing. As of the date of this Subscription Agreement, the authorized capital stock of FLAC consists of (i) 479,000,000 Class A ordinary shares, par value $0.0001, of which 14,301,000 shares are outstanding,(ii) 20,000,000 Class B ordinary shares, par value $0.0001, of which 3,450,000 shares are outstanding, (iii) 4,767,000 warrants to purchase Class A ordinary shares of FLAC, with each such warrant exercisable for one whole Class A ordinary share at a price of $11.50 per share, are issued and outstanding and (iii) no preference shares or shares of preferred stock are issued and outstanding. All issued and outstanding capital stock of FLAC has been duly authorized and validly issued, is fully paid are non-assessable. free and clear of all liens or other restrictions (other than those arising under applicable securities laws) and are not subject to preemptive or other similar rights and all outstanding warrants have been duly authorized and validly issued and are not subject to preemptive or similar rights. Except (1) as set forth in this Subscription Agreement and contemplated by the Other Subscription Agreements, the Transaction Agreement and the other agreements and arrangements referred to therein or in the SEC Reports, and (2) for Shares that will be issued at or immediately prior to the Closing to Amgen and MTPC, as of the date hereof, there are no (a) outstanding, and between the date hereof and the Closing, neither New NAP nor FLAC will issue, sell, or cause to be outstanding any, equity interests of New NAP or FLAC, as applicable (or securities of New NAP or FLAC convertible into or exchangeable for equity interests of New NAP or FLAC, as applicable), (b) options, warrants or other rights (including preemptive rights) or agreements, arrangements or commitments of any character, whether or not contingent, of New NAP or FLAC to subscribe for, purchase or acquire from any individual, entity or other person, and no obligation of New NAP or FLAC to issue, any equity interests in New NAP or FLAC (or any securities convertible into or exchangeable or exercisable for such equity interests) other than as disclosed in FLAC’s SEC Reports, (c) equity equivalents or other similar rights of or with respect to New NAP or FLAC, or (d) obligations of New NAP or FLAC to repurchase, redeem or otherwise acquire any of the foregoing securities, shares, options, equity equivalents, interests or rights. There are no securities or instruments issued by or to which New NAP or FLAC is a party containing anti-dilution or similar provisions that will be triggered by the issuance of (i) the Subscribed Shares pursuant to this Subscription Agreement or (ii) the Shares to be issued pursuant to any Other Subscription Agreement. As of the date hereof, neither New NAP
C-7
Table of Contents
nor FLAC owns, directly or indirectly, interests or investments (whether equity or debt) in any person, whether incorporated or unincorporated, other than wholly owned subsidiaries. There are no shareholder agreements, voting trusts or other agreements or understandings to which New NAP or FLAC is a party or by which it is bound relating to the voting of any securities of New NAP or FLAC, as applicable, other than (1) as set forth in the SEC Reports and (2) as contemplated by the Transaction Agreement.
(i) Assuming the accuracy of the Investor’s representations and warranties set forth in Section 6 herein, no registration under the Securities Act is required for the offer and sale of the Subscribed Shares by New NAP to the Investor hereunder. The Subscribed Shares (or any portion thereof) (i) were not offered by any form of general solicitation or general advertising (within the meaning of Regulation D under the Securities Act) and (ii) are not being offered in a manner involving a public offering under, or in a distribution in violation of, the Securities Act or any state securities laws.
(j) Except for such matters as have not had and would not be reasonably likely to have, individually or in the aggregate, a New NAP Material Adverse Effect or a FLAC Material Adverse Effect, there is no (i) action, suit, claim, arbitration or other proceeding, in each case by or before any governmental authority or arbitrator pending, or, to the knowledge of New NAP, the Company or FLAC, threatened against New NAP, the Company or FLAC, as applicable or (ii) judgment, decree, injunction, ruling or order of any governmental entity or arbitrator outstanding against New NAP, the Company or FLAC.
(k) Other than Credit Suisse Securities (USA) LLC, Jefferies LLC, SVB Securities LLC and William Blair & Company, L.L.C. and their respective affiliates (collectively, the “Placement Agents”), neither New NAP, the Company nor FLAC has engaged any broker, finder, commission agent, placement agent or arranger in connection with the sale of the Subscribed Shares, and neither New NAP, the Company nor FLAC is under any obligation to pay any broker’s fee or commission in connection with the sale of the Subscribed Shares other than to the Placement Agents. New NAP and FLAC are solely responsible for the payment of any fees, costs, expenses and commission of the Placement Agents in connection with the sale of the Shares. There is no broker, investment banker, financial advisor, finder or other person which has been retained by or is authorized to act on behalf of New NAP or FLAC who might be entitled to any fee or commission for which the Investor will be liable in connection with the execution of this Subscription Agreement and the consummation of the transactions contemplated hereby.
(l) New NAP is not, and immediately after receipt of payment for the Subscribed Shares will not be, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(m) Neither New NAP, the Company nor FLAC has received any written communication from a governmental authority that alleges that New NAP, the Company or FLAC is not in compliance with or is in default or violation of any applicable law, except where such non-compliance, default or violation would not reasonably be expected to have, individually or in the aggregate, a New NAP Material Adverse Effect or a FLAC Material Adverse Effect.
(n) Upon consummation of the Business Combination, the issued and outstanding Shares will be registered pursuant to Section 12(b) of the Exchange Act and will be listed on the Stock Exchange. As of the date hereof, the issued and outstanding ordinary shares of FLAC are registered pursuant to Section 12(b) of the Exchange Act, and are listed for trading on the Stock Exchange under the symbol “FLAC.”
(o) New NAP and FLAC have furnished to the Investor a true and complete copy of the Transaction Agreement as in effect as of the date hereof.
(p) (i) There has been no action taken by New NAP, the Company or FLAC, or, to the actual knowledge of New NAP, the Company or FLAC, any officer, director, equityholder, manager, employee, agent or representative of New NAP, the Company or FLAC, in each case, acting on behalf of New NAP, the Company or FLAC, in violation of any applicable Anti-Corruption Laws (as defined below), (ii) neither New NAP, the Company nor FLAC has been convicted of violating any Anti-Corruption Laws or subjected to any investigation by a governmental authority for violation of any applicable Anti-Corruption Laws, (iii) neither New NAP, the Company nor FLAC has conducted or initiated any internal investigation or
C-8
Table of Contents
made a voluntary, directed, or involuntary disclosure to any governmental authority regarding any alleged act or omission arising under or relating to any noncompliance with any Anti-Corruption Laws and (iv) neither New NAP, the Company nor FLAC has received any written notice or citation from a governmental authority for any actual or potential noncompliance with any applicable Anti-Corruption Laws. As used herein, “Anti-Corruption Laws” means any applicable laws relating to corruption and bribery, including the U.S. Foreign Corrupt Practices Act of 1977, as amended, the UK Bribery Act 2010, and any similar applicable law that prohibits bribery or corruption.
(q) None of New NAP, the Company, FLAC nor any of their directors is (i) a person or entity named on the List of Specially Designated Nationals and Blocked Persons administered by the U.S. Treasury Department’s Office of Foreign Assets Control (“OFAC”) or in any Executive Order issued by the President of the United States and administered by OFAC (“OFAC List”), or a person or entity prohibited by any OFAC sanctions program, (ii) owned, directly or indirectly, or controlled by, or acting on behalf of, one or more persons that are named on the OFAC List; (iii) organized, incorporated, established, located, resident or born in, or a citizen, national or the government, including any political subdivision, agency or instrumentality thereof, of, Cuba, Iran, North Korea, Syria, the Crimea region of Ukraine or any other country or territory embargoed or subject to substantial trade restrictions by the United States, (iv) a Designated National as defined in the Cuban Assets Control Regulations, 31 C.F.R. Part 515, or (v) a non-U.S. shell bank or providing banking services indirectly to a non-U.S. shell bank. Each of New NAP, the Company and FLAC agrees to provide law enforcement agencies, if requested thereby, such records as required by applicable law, provided that New NAP, the Company or FLAC, as applicable, is permitted to do so under applicable law. To the extent required, it maintains policies and procedures reasonably designed to ensure compliance with OFAC-administered sanctions programs, including for the screening of its investors against the OFAC sanctions programs, including the OFAC List.
(r) Neither New NAP, the Company, FLAC nor any of their subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy, insolvency, reorganization, receivership, liquidation, administration or winding up or failed to pay its debts when due, nor does New NAP, the Company, FLAC or any of their subsidiaries have any knowledge or reason to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or seek to commence an administration.
(s) New NAP, the Company and FLAC acknowledge and agree that, notwithstanding anything herein to the contrary, the Shares may be pledged by Investor in connection with a bona fide margin agreement, provided such pledge shall be (i) pursuant to an available exemption from the registration requirements of the Securities Act or (ii) pursuant to, and in accordance with, a registration statement that is effective under the Securities Act at the time of such pledge, and the Investor effecting a pledge of Shares shall not be required to provide New NAP or FLAC with any notice thereof; provided, however, that neither New NAP, FLAC, the Company or their respective counsels shall be required to take any action (or refrain from taking any action) in connection with any such pledge, other than providing any such lender of such margin agreement with an acknowledgment that, to knowledge of New NAP, FLAC or the Company, as applicable, the Shares are not subject to any contractual prohibition on pledging or lock up, the form of such acknowledgment to be subject to review and comment by New NAP and FLAC in all respects.
6. Investor Representations and Warranties. The Investor represents and warrants to New NAP and FLAC and Placement Agents that:
(a) To the extent applicable, the Investor has been duly formed or incorporated, and is validly existing in good standing (to the extent the concept of good standing is applicable in such jurisdiction) under the laws of its jurisdiction of incorporation or formation and has all power (corporate or otherwise) and authority to own, lease and operate its properties and conduct its business as presently conducted and to enter into, deliver and perform its obligations under this Subscription Agreement.
(b) The Investor (i) is a “qualified institutional buyer” (as defined in Rule 144A under the Securities Act) or institutional “accredited investor” (within the meaning of Rule 501(a) under the Securities Act), in
C-9
Table of Contents
each case, satisfying the applicable requirements set forth on Schedule A, and (ii) is an “institutional account” (as defined in FINRA Rule 4512(c)), and is aware that the sale is being made in reliance on a private placement exemption from registration under the Securities Act and is acquiring the Subscribed Shares only for its own account and not for the account of others, or if the Investor is subscribing for the Subscribed Shares as a fiduciary or agent for one or more investor accounts, the Investor has full investment discretion with respect to each such account, and the full power and authority to make the acknowledgements, representations and agreements herein on behalf of each owner of each such account, and (iv) is not acquiring the Subscribed Shares with a view to, or for offer or sale in connection with, any distribution thereof in violation of the Securities Act. The Investor is not an entity formed for the specific purpose of acquiring the Subscribed Shares. The Investor has completed Schedule A following the signature page hereto and the information contained therein is accurate and complete.
(c) The Investor acknowledges and agrees that the Subscribed Shares are being offered in a transaction not involving any public offering within the meaning of the Securities Act and that the Subscribed Shares have not been registered under the securities laws of the United States or any other jurisdiction except as otherwise required by Section 7 hereof. The Investor acknowledges and agrees that the Subscribed Shares may not be offered, resold, transferred, pledged or otherwise disposed of by the Investor absent an effective registration statement under the Securities Act except (i) to New NAP or a subsidiary thereof, (ii) to non-U.S. persons pursuant to offers and sales that occur outside the United States within the meaning of Regulation S under the Securities Act, (iii) pursuant to Rule 144 promulgated under the Securities Act (“Rule 144”), provided that all of the applicable conditions thereof have been met or (iv) pursuant to another applicable exemption from the registration requirements of the Securities Act, and in each case in accordance with any applicable securities laws of the states and other jurisdictions of the United States, and that any certificates or book entries representing the Subscribed Shares shall contain a restrictive legend to such effect. The Investor acknowledges and agrees that the Subscribed Shares will be subject to transfer restrictions and, as a result of these transfer restrictions, the Investor may not be able to readily offer, resell, transfer, pledge or otherwise dispose of the Subscribed Shares and may be required to bear the financial risk of an investment in the Subscribed Shares for an indefinite period of time. The Investor acknowledges and agrees that the Subscribed Shares may not be eligible for offer, resale, transfer, pledge or disposition pursuant to Rule 144A promulgated under the Securities Act and that Rule 144 will not be available until at least one year from the date that New NAP files a Current Report on Form 8-K following the Closing Date that includes the “Form 10” information required under applicable SEC rules and regulations. The Investor shall not engage in hedging transactions with regard to the Shares unless in compliance with the Securities Act. The Investor acknowledges and agrees that it has been advised to consult legal counsel and tax and accounting advisors prior to making any offer, resale, transfer, pledge or disposition of any of the Subscribed Shares.
(d) The Investor acknowledges and agrees that the Investor is purchasing the Subscribed Shares directly from New NAP. The Investor further acknowledges that there have been no representations, warranties, covenants or agreements made to the Investor by or on behalf of New NAP, FLAC, the Company, the Placement Agents, any of their respective affiliates or any control persons, officers, directors, employees, partners, agents or representatives of any of the foregoing or any other person or entity, expressly or by implication, other than those representations, warranties, covenants and agreements of New NAP and FLAC expressly set forth in this Subscription Agreement.
(e) The Investor’s acquisition and holding of the Subscribed Shares will not constitute or result in a nonexempt prohibited transaction under Section 406 of the Employee Retirement Income Security Act of 1974, as amended, Section 4975 of the Internal Revenue Code of 1986, as amended, or any applicable similar law.
(f) The Investor acknowledges and agrees that the Investor has received, reviewed and understood such financial and other information as the Investor deems necessary in order to make an investment decision with respect to the Subscribed Shares, including, with respect to New NAP and FLAC, the Transactions and the business of the Company and its subsidiaries. Without limiting the generality of the foregoing, the
C-10
Table of Contents
Investor acknowledges that he, she or it has reviewed the SEC Reports. The Investor acknowledges and agrees that the Investor and the Investor’s professional advisor(s), if any, have had the full opportunity to ask such questions, receive such answers and obtain such financial and other information as the Investor and such Investor’s professional advisor(s), if any, have deemed necessary to make an investment decision with respect to the Subscribed Shares. Based on such information as the Investor has deemed appropriate and without reliance upon any Placement Agent, New NAP, FLAC or the Company, the Investor has independently made its own analysis and decision to enter into this Subscription Agreement and consummate the Subscription. Except for (i) the SEC Reports and (ii) the representations, warranties and agreements of New NAP and FLAC expressly set forth in this Subscription Agreement, the Investor is relying exclusively on its own sources of information, investment analysis and due diligence (including professional advice it deems appropriate) with respect to the Transactions, the Subscribed Shares and the business, condition (financial and otherwise), management, operations, properties and prospects of the Company, including but not limited to all business, legal, regulatory, accounting, credit and tax matters.
(g) The Investor became aware of this offering of the Subscribed Shares solely by means of direct contact between the Investor and FLAC, the Company or a representative of FLAC or the Company, and the Subscribed Shares were offered to the Investor solely by direct contact between the Investor and FLAC, the Company or a representative of FLAC or the Company. The Investor did not become aware of this offering of the Subscribed Shares, nor were the Subscribed Shares offered to the Investor, by any other means. The Investor acknowledges that the Subscribed Shares (i) were not offered by any form of general solicitation or general advertising (within the meaning of Regulation D under the Securities Act) and (ii) are not being offered in a manner involving a public offering under, or in a distribution in violation of, the Securities Act, or any state securities laws. The Investor acknowledges that it is not relying upon, and has not relied upon, any statement, representation or warranty made by any person, firm or corporation (including, without limitation, New NAP, FLAC, the Company, the Placement Agents, any of their respective affiliates or any control persons, officers, directors, employees, partners, agents or representatives of any of the foregoing), other than the representations and warranties of New NAP and FLAC contained in Section 5 in this Subscription Agreement, in making its investment or decision to invest in New NAP.
(h) The Investor acknowledges that it is aware that there are substantial risks incident to the purchase and ownership of the Subscribed Shares, including those set forth in SEC Reports. The Investor has exercised its independent judgment in evaluating its investment in the Subscribed Shares, is a sophisticated investor, experienced in investing in private equity transactions and capable of evaluating investment risks independently, both in general and with regard to all transactions and investment strategies involving a security or securities, including the Subscription and the Transactions, and the Investor has sought such accounting, legal and tax advice as the Investor has considered necessary to make an informed investment decision.
(i) Alone, or together with any professional advisor(s), the Investor has adequately analyzed and fully considered the risks of an investment in the Shares and that the Investor is able at this time and in the foreseeable future to bear the economic risk of a total loss of the Investor’s investment in New NAP. The Investor acknowledges specifically that a possibility of total loss of investment exists.
(j) The Investor acknowledges that no federal or state agency has passed upon or endorsed the merits of the offering of the Subscribed Shares or made any findings or determination as to the fairness of this investment.
(k) The execution, delivery and performance by the Investor of this Subscription Agreement are within the powers of the Investor, have been duly authorized by all necessary action and do not and will not violate or constitute or result in a breach or default under or conflict with any law, order, ruling or regulation of any court or other tribunal or of any governmental commission or agency, or any agreement or other undertaking or obligation, to which the Investor is a party or by which the Investor is bound which would reasonably be expected to have a material adverse effect on the legal authority of the Investor to enter into and perform its obligations under this Subscription Agreement, and, if the Investor is not an individual, will not violate any
C-11
Table of Contents
provisions of the Investor’s organizational documents, including, without limitation, its incorporation or formation papers, bylaws, indenture of trust or partnership or operating agreement, as may be applicable. The signature of the Investor on this Subscription Agreement is genuine, and the signatory, if the Investor is an individual, has the legal competence and capacity to execute the same or, if the Investor is not an individual, the signatory has been duly authorized to execute the same, and assuming that this Subscription Agreement constitutes the valid and binding obligation of New NAP and FLAC, this Subscription Agreement constitutes a legal, valid and binding obligation of the Investor, enforceable against the Investor in accordance with its terms except as may be limited or otherwise affected by (i) bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other laws relating to or affecting the rights of creditors generally, and (ii) principles of equity, whether considered at law or equity.
(l) The Investor is not (i) a person or entity named on the List of Specially Designated Nationals and Blocked Persons administered by the OFAC or in any OFAC List, or a person or entity prohibited by any OFAC sanctions program, (ii) a Designated National as defined in the Cuban Assets Control Regulations, 31 C.F.R. Part 515, or (iii) a non-U.S. shell bank or providing banking services indirectly to a non-U.S. shell bank. The Investor agrees to use commercially reasonably efforts to provide law enforcement agencies, if requested thereby, such records as required by applicable law, provided that the Investor is permitted to do so under applicable law. If the Investor is a financial institution subject to the Bank Secrecy Act (31 U.S.C. Section 5311 et seq.), as amended by the USA PATRIOT Act of 2001, and its implementing regulations (collectively, the “BSA/PATRIOT Act”), the Investor, directly or indirectly through a third-party administrator, maintains policies and procedures reasonably designed to comply with applicable obligations under the BSA/PATRIOT Act. To the extent required, the Investor, directly or indirectly through a third-party administrator, maintains policies and procedures reasonably designed for the screening of its investors against the OFAC sanctions programs, including the OFAC List. To the extent required by applicable law, the Investor, directly or indirectly through a third-party administrator, maintains policies and procedures reasonably designed to ensure that the funds held by the Investor and used to purchase the Subscribed Shares were legally derived.
(m) No foreign person (as defined in 31 C.F.R. Part 800.224) in which the national or subnational governments of a single foreign state have a substantial interest (as defined in 31 C.F.R. Part 800.244) will acquire a substantial interest in New NAP as a result of the purchase and sale of Subscribed Shares hereunder such that a declaration to the Committee on Foreign Investment in the United States would be mandatory under 31 C.F.R. Part 800.401, and no foreign person will have control (as defined in 31 C.F.R. Part 800.208) over New NAP from and after the Closing as a result of the purchase and sale of Subscribed Shares hereunder.
(n) The Investor acknowledges that no disclosure or offering document has been prepared by the Placement Agents in connection with the offer and sale of the Subscribed Shares.
(o) The Investor acknowledges and agrees that (a) the Placement Agents are, severally and not jointly, acting solely as placement agents in connection with the Private Placement and are not acting as underwriters or in any other capacity and are not and shall not be construed as fiduciaries for the Investor, the Company or any other person or entity in connection with the Private Placement, (b) the Placement Agents have not made and will not make any representation or warranty, whether express or implied, of any kind or character and have not provided any advice or recommendation in connection with the Private Placement, (c) the Placement Agents will have no responsibility with respect to (i) any representations, warranties or agreements made by any person or entity under or in connection with the Subscription or any of the documents furnished pursuant thereto or in connection therewith, or the execution, legality, validity or enforceability (with respect to any person) or any thereof, or (ii) the business, condition (financial or otherwise), operations, properties or prospects of, or any other matter concerning New NAP, FLAC, the Company, the Private Placement or the Transactions, and (d) the Placement Agents shall have no liability or obligation (including without limitation, for or with respect to any losses, claims, damages, obligations, penalties, judgments, awards, liabilities, costs, expenses or disbursements incurred by the Investor, the
C-12
Table of Contents
Company or any other person or entity), whether in contract, tort or otherwise, to the Investor, or to any person claiming through the Investor, in respect of the Private Placement.
(p) The Investor acknowledges that the Placement Agents may have existing or future business relationships with FLAC, the Company and/or New NAP (including, but not limited to, lending, depository, risk management, advisory and banking relationships) and will pursue actions and take steps that it deems or they deem necessary or appropriate to protect its or their interests arising therefrom without regard to the consequences for a holder of Shares, and that certain of these actions may have material and adverse consequences for a holder of Shares. The Investor acknowledges that the Placement Agents and/or their respective affiliates and affiliates of FLAC and/or New NAP may now or in the future own securities of FLAC and/or New NAP and may purchase securities in connection with the Transaction.
(q) The Investor acknowledges that none of the Placement Agents has acted as the Investor’s financial advisor, tax advisor or fiduciary in connection with the Subscription and this Subscription Agreement. The Investor is aware that Credit Suisse Securities (USA) LLC, Jefferies LLC and William Blair & Company, L.L.C. are acting as New NAP’s financial advisors and equity capital markets advisors and placement agents for the Private Placement, and SVB Securities LLC is also acting as New NAP’s placement agent for the Private Placement. The Investor is aware that SVB Securities LLC has been separately engaged as the Company’s financial and capital markets advisor in connection with the Transactions. The Investor acknowledges that none of the Placement Agents has provided the Investor with any information or advice with respect to the Subscribed Shares and that no such information or advice is necessary or desired. None of the Placement Agents has made or makes any representation as to the Company, New NAP or FLAC or the quality or value of the Subscribed Shares. The Placement Agents may have acquired non-public information with respect to the Company, New NAP or FLAC, which the Investor agrees need not be provided to it. The Investor further acknowledges that none of the Placement Agents have made any independent investigation with respect to the Company, New NAP, FLAC or the Subscribed Shares or the accuracy, completeness or adequacy of any information supplied to any Placement Agent by the Company, New NAP or FLAC. The Placement Agents have not made and do not make any representations express or implied as to FLAC, New NAP, the Company, the Company’s credit quality, or the quality or value of the Subscribed Shares.
(r) The Investor acknowledges that it has not relied on the Placement Agents in connection with its determination as to the legality of its acquisition of the Shares or as to the other matters referred to herein and the Investor has not relied on any investigation that the Placement Agent, any of their affiliates or any person acting on their behalf have conducted with respect to the Shares, FLAC, New NAP or the Company. The Investor further acknowledges that it has not relied on any information contained in any research reports prepared by the Placement Agents or any of their affiliates.
(s) The Investor has or has commitments to have and, at the Closing, will have, sufficient funds to pay the Subscription Amount and consummate the Subscription pursuant to Section 2 herein.
(t) The Investor does not have, as of the date of this Subscription Agreement, and, since the date the Investor was made aware of the Transactions, such Investor has not entered into, any “put equivalent position” as such term is defined in Rule 16a-1 under the Exchange Act or short sale positions with respect to the securities of New NAP or FLAC, as applicable. Notwithstanding the foregoing, if the Investor is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of the Investor’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of the Investor’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Subscribed Shares covered by this Subscription Agreement.
(u) Notwithstanding anything to the contrary set forth herein, the Investor acknowledges and agrees that, subsequent to the date of this Subscription Agreement and prior to the Closing, New NAP and FLAC may enter into one or more additional subscription agreements with other investors with terms and conditions that are not more advantageous to the investor thereunder than the terms and conditions set forth
C-13
Table of Contents
in this Subscription Agreement (other than terms particular to the regulatory requirements of such other investor or its affiliates or related funds that are mutual funds), and entry into such subscription agreements may increase the aggregate amount of Shares being subscribed for in the private placement contemplated by this Subscription Agreement. For the avoidance of doubt, such additional subscription agreements shall reflect not less than the same Per Share Purchase Price and shall, once executed, constitute Other Subscription Agreements for purposes of this Subscription Agreement, mutatis mutandis.
(v) The Investor acknowledges having received and read the Risk Factors (as defined below) in the document titled “Project Yankee – PIPE Risk Factors” located in the folder titled “PIPE Deck + Risk Factors” of the virtual data room related to the Private Placement (the “Risk Factors”).
7. Registration Rights.
(a) Subject to Sections 7(b) and 7(c) herein, New NAP agrees that, within thirty (30) calendar days after the Closing Date (the “Filing Date”), it will file with the SEC (at New NAP’s sole cost and expense) a registration statement registering the resale of the Subscribed Shares (the “Registration Statement”), and New NAP shall use its commercially reasonable efforts to have the Registration Statement declared effective as soon as practicable after the filing thereof, but no later than the earlier of (i) sixty (60) calendar days (or ninety (90) calendar days if the SEC notifies New NAP that it will “review” the Registration Statement) following the earlier of (a) the Filing Date and (b) the initial filing date of the Registration Statement and (ii) five (5) Business Days after New NAP is notified (orally or in writing, whichever is earlier) by the SEC that the Registration Statement will not be “reviewed” or will not be subject to further review (such earlier date, the “Effectiveness Date”); provided, that if such date falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness Date shall be extended to the next Business Day on which the SEC is open for business. New NAP will use its commercially reasonable efforts to provide a draft of the Registration Statement to the Investor for review at least two (2) Business Days in advance of the filing of the Registration Statement; provided that, for the avoidance of doubt, in no event shall New NAP be required to delay or postpone the filing of such Registration Statement as a result of or in connection with the Investor’s review. Any failure by New NAP to file the Registration Statement by the Filing Deadline or to effect such Registration Statement by the Effectiveness Date shall not otherwise relieve New NAP of its obligations to file a Registration Statement as set forth above in this Section 7. Subject to Section 7(b) of this Subscription Agreement, New NAP agrees to use commercially reasonable efforts to cause such Registration Statement, or another shelf registration statement that includes the Subscribed Shares to be sold pursuant to this Subscription Agreement, to remain effective until the earliest of (x) the fourth anniversary of the Closing, (y) the date on which the Investor ceases to hold any Subscribed Shares and (z) the first date on which the Investor is able to sell all of its Subscribed Shares (or shares received in exchange therefor) under Rule 144 within ninety (90) calendar days without limitation as to the manner of sale or the amount of such securities that may be sold and without the requirement for New NAP to be in compliance with the current public information required under Rule 144(c)(i) (or Rule 144(i)(2), as applicable). The Investor agrees to disclose its ownership to New NAP upon its reasonable written request to assist it in making the determination described above. In no event shall the Investor be identified as a statutory underwriter in the Registration Statement; provided, that if the SEC requires that the Investor be identified as a statutory underwriter in the Registration Statement, the Investor will have the option, in its sole and absolute discretion, to either (i) have the opportunity to withdraw its Subscribed Shares from the Registration Statement or (ii) to be included as such in the Registration Statement. Notwithstanding the foregoing, if the SEC prevents New NAP from including any or all of the Subscribed Shares proposed to be registered under the Registration Statement due to limitations on the use of Rule 415 of the Securities Act for the resale of the Subscribed Shares by the applicable shareholders or otherwise, such Registration Statement shall register for resale such number of Subscribed Shares which is equal to the maximum number of Subscribed Shares as is permitted by the SEC. In such event, the number of Shares to be registered for each selling shareholder named in the Registration Statement shall be reduced pro rata among all such selling shareholders and as promptly as practicable after being permitted to register additional Shares under Rule 415 under the Securities Act, New NAP shall amend the Registration Statement or file a
C-14
Table of Contents
new Registration Statement (such amendment or new Registration Statement shall also be deemed to be a “Registration Statement” hereunder) to register such additional Shares and cause such Registration Statement to become effective as promptly as practicable after the filing thereof, but in any event no later than thirty (30) calendar days after the filing of such Registration Statement (the “Additional Effectiveness Date”); provided, that the Additional Effectiveness Date shall be extended to sixty (60) calendar days after the filing of such Registration Statement if such Registration Statement is reviewed by, and comments thereto are provided from, the SEC; provided, further New NAP shall have such Registration Statement declared effective within five (5) Business Days after the date New NAP is notified in writing by the SEC that such Registration Statement will not be “reviewed” or will not be subject to further review. For as long as the Registration Statement shall remain effective pursuant to this Section 7(a), New NAP will use commercially reasonable efforts to (i) qualify the Subscribed Shares for listing on the Stock Exchange and (ii) update or amend the Registration Statement as necessary to include the Subscribed Shares. For as long as the Investor holds the Subscribed Shares, New NAP shall use commercially reasonable efforts to file all reports, and provide all customary and reasonable cooperation, necessary to enable the Investor to resell the Subscribed Shares pursuant to the Registration Statement or Rule 144 (when Rule 144 becomes available to the Investor), as applicable, including providing legal opinions or other documents or instructions required by New NAP’s transfer agent.
(b) Notwithstanding anything to the contrary contained herein, New NAP may delay or postpone filing of such Registration Statement, and from time to time require the Investor not to sell under the Registration Statement or suspend the use or effectiveness of any such Registration Statement, if the board of directors of New NAP reasonably determines in good faith upon the advice of counsel that in order for the Registration Statement to not contain a material misstatement or omission, an amendment thereto would be needed to include information that would at that time not otherwise be required in a periodic report under the Exchange Act, or if such filing or use would materially adversely affect a bona fide business or financing transaction of New NAP or would require premature disclosure of information that could materially adversely affect New NAP and New NAP has a bona fide business purpose for preserving as confidential (each such circumstance, a “Suspension Event”); provided, however, that (i) New NAP may not delay the filing or suspend the use or effectiveness of the Registration Statement for a period of more than forty five (45) consecutive calendar days or more than a total of ninety (90) calendar days, in each case during any 12-month period and (ii) New NAP shall use commercially reasonable efforts to make such Registration Statement available for the sale by the Investor of Subscribed Shares as soon as practicable thereafter. If so directed by New NAP, the Investor will deliver to New NAP or, in the Investor’s sole discretion, destroy all copies of the prospectus covering the Subscribed Shares in the Investor’s possession; provided, however, that this obligation to deliver or destroy all copies of the prospectus covering the Subscribed Shares shall not apply (A) to the extent the Investor is required to retain a copy of such prospectus (x) in order to comply with applicable legal or regulatory requirements or (y) in accordance with a bona fide pre-existing document retention policy or (B) to copies stored electronically on archival servers as a result of automatic data back-up.
(c) New NAP’s obligations to include the Subscribed Shares issued pursuant to this Subscription Agreement (or shares issued in exchange therefor) for resale in the Registration Statement are contingent upon the Investor furnishing in writing to New NAP such reasonable information regarding the Investor, the securities of New NAP held by the Investor and the intended method of disposition of such Subscribed Shares, which shall be limited to non-underwritten public offerings, as shall be reasonably requested in writing by New NAP to effect the registration of such Subscribed Shares, and shall execute such documents in connection with such registration as New NAP may reasonably request that are customary of a selling shareholder in similar situations.
(d) New NAP shall use commercially reasonable efforts, if requested by the Investor, to (i) cause the removal of any restrictive legend related to compliance with the federal securities laws set forth on the Subscribed Shares, (ii) cause its legal counsel to deliver an opinion, if necessary or otherwise required by the transfer agent, to the transfer agent in connection with the instruction under subclause (i) to the effect
C-15
Table of Contents
that removal of such legends in such circumstances may be effected in compliance under the Securities Act and (iii) issue Subscribed Shares without any such legend in certificated or book-entry form or by electronic delivery through The Depository Trust Company, at the Investor’s option, as promptly as reasonably practicable following such request, if the Investor has sold or transferred, or proposes to sell or transfer within five (5) Business Days of such request, Subscribed Shares pursuant to the Registration Statement or in compliance with Rule 144 and (A) the Subscribed Shares are registered for resale under the Securities Act or (B) the Subscribed Shares may be sold by the Investor under Rule 144. New NAP shall use its commercially reasonable efforts to cause the removal of any such legend as promptly as reasonably practicable following receipt of the Investor’s request, provided that the Investor has provided such customary representations and other documentation in connection therewith, including an opinion of counsel (which, for the avoidance of doubt, may be internal counsel), in a form reasonably acceptable to New NAP, to the effect that such sale, assignment or transfer of the Subscribed Shares may be made without registration under the applicable requirements of the Securities Act (provided no such opinion of counsel shall be required in connection with a sale in accordance with Rule 144 or pursuant to a Registration Statement).
(e) At its expense, New NAP shall advise the Investor within two (2) Business Days: (i) when a Registration Statement or any post-effective amendment thereto has become effective; (ii) of any request by the SEC for amendments or supplements to any Registration Statement or the prospectus included therein or for additional information; (iii) of the issuance by the SEC of any stop order suspending the effectiveness of any Registration Statement or the initiation of any proceedings for such purpose; (iv) of the receipt by New NAP of any notification with respect to the suspension of the qualification of the Subscribed Shares included therein for sale in any jurisdiction or the initiation or threatening of any proceeding for such purpose; and (v) subject to the provisions in this Subscription Agreement, of the occurrence of any event that requires the making of any changes in any Registration Statement or prospectus so that, as of such date, the statements therein are not misleading and do not omit to state a material fact required to be stated therein or necessary to make the statements therein (in the case of a prospectus, in the light of the circumstances under which they were made) not misleading. Upon receipt of any written notice from New NAP (which notice shall not contain any material non-public information regarding New NAP) of the occurrence of any of the events in clauses (i)-(v) of the preceding sentence or of a Suspension Event during the period that the Registration Statement is effective or if as a result of a Suspension Event the Registration Statement or related prospectus contains any untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made (in the case of the prospectus) not misleading, the Investor agrees that it will promptly discontinue offers and sales of the Subscribed Shares under the Registration Statement (excluding, for the avoidance of doubt, sales conducted pursuant to Rule 144) until the Investor receives copies of a supplemental or amended prospectus (which New NAP agrees to promptly prepare) that corrects the misstatement(s) or omission(s) referred to above and receives notice that any post-effective amendment has become effective or unless otherwise notified by New NAP that it may resume such offers and sales, and (B) it will maintain the confidentiality of any information included in such written notice delivered by New NAP, except (1) for disclosure to the Investor’s employees, agents and professional advisers who need to know such information and are obligated to keep it confidential, (2) for disclosures to the extent required in order to comply with reporting obligations to its limited partners who have agreed to keep such information confidential and (3) as required by applicable law or subpoena. New NAP shall use its commercially reasonable efforts to obtain the withdrawal of any order suspending the effectiveness of any Registration Statement as soon as reasonably practicable. Upon the occurrence of any of the events in clauses (i)-(v) of the first sentence of this Section 7(e), except for such times as New NAP is permitted hereunder to suspend, and has suspended, the use of a prospectus forming part of a Registration Statement, New NAP shall use its commercially reasonable efforts to as soon as reasonably practicable prepare a post-effective amendment to such Registration Statement or a supplement to the related prospectus (or a report filed with the SEC under the Exchange Act if the Registration Statement permits forward incorporation by reference), or file any other required document so that, as thereafter delivered to purchasers of the Subscribed Shares included
C-16
Table of Contents
therein, such prospectus will not include any untrue statement of a material fact or omit to state any material fact necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading.
(f) For purposes of this Section 7, (i) “Subscribed Shares” shall mean, as of any date of determination, the Subscribed Shares acquired by the Investor pursuant to this Subscription Agreement and any other equity security issued or issuable with respect to such Subscribed Shares by way of stock split, dividend, distribution, recapitalization, merger, exchange, replacement or similar event, and (ii) “Investor” shall include any affiliate of the undersigned Investor to which the rights under this Section 7 have been duly assigned.
(g) Indemnification.
(i) New NAP agrees to indemnify and hold harmless, to the extent permitted by law, the Investor, its directors, officers, employees and agents, and each person who controls the Investor (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and each affiliate of the Investor (within the meaning of Rule 405 under the Securities Act) from and against any and all out-of-pocket losses, claims, damages, liabilities and expenses (including, without limitation, any reasonable and documented outside attorneys’ fees and expenses incurred in connection with defending or investigating any such action or claim) (“Losses”) caused by or based upon any untrue or alleged untrue statement of material fact contained in any Registration Statement, prospectus included in any Registration Statement (“Prospectus”) or preliminary Prospectus or any amendment thereof or supplement thereto or any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein not misleading, except insofar as the same are caused by or contained in any information furnished in writing to New NAP by or on behalf of the Investor, regarding the Investor, expressly for use therein; provided, however, that the indemnification contained in this Section 7(g) shall not apply to amounts paid in settlement of any Losses if such settlement is effected without the consent of New NAP (which consent shall not be unreasonably withheld, conditioned or delayed), nor shall New NAP be liable for any Losses to the extent they arise out of or are based upon a violation which occurs (A) in reliance upon and in conformity with written information furnished by the Investor regarding the Investor, (B) in connection with any failure of such person to deliver or cause to be delivered a prospectus made available by New NAP in a timely manner or (C) in connection with any offers or sales effected by or on behalf of the Investor in violation of Section 7(e) herein after having received notice as set forth in Section 7(e).
(ii) The Investor agrees, severally and not jointly with any other person that is a party to the Other Subscription Agreements, to indemnify and hold harmless New NAP, its directors, officers, employees and agents and each person who controls New NAP (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) against any Losses arising out of or that are based upon any untrue statement of material fact contained in the Registration Statement, Prospectus or preliminary Prospectus or any amendment thereof or supplement thereto or any omission of a material fact required to be stated therein or necessary to make the statements therein not misleading, but only to the extent that such untrue statement or omission, or alleged untrue statement or omission, is contained in any information or affidavit so furnished in writing by the Investor, regarding the Investor, expressly for use in the Registration Statement or a Prospectus; provided, however, that the indemnification contained in this Section 7(g)(ii) shall not apply to amounts paid in settlement of any Losses if such settlement is effected without the consent of such Investor (which consent shall not be unreasonably withheld, conditioned or delayed). In no event shall the liability of such Investor be greater in amount than the dollar amount of the net proceeds received by such Investor upon the sale of the Subscribed Shares giving rise to such indemnification obligation.
(iii) Any person or entity entitled to indemnification herein (the “Indemnified Party”) shall (A) give prompt written notice to the indemnifying party (the “Indemnifying Party”) of any claim with respect to which it seeks indemnification (provided that the failure to give prompt notice shall not
C-17
Table of Contents
impair any person’s or entity’s right to indemnification hereunder to the extent such failure has not actually and materially prejudiced the Indemnifying Party) and (B) unless in such Indemnified Party’s reasonable judgment a conflict of interest between such Indemnified Party and Indemnifying Party may exist with respect to such claim, permit the Indemnifying Party to assume the defense of such claim with counsel reasonably satisfactory to the Indemnified Party. If such defense is assumed, the Indemnifying Party shall not be subject to any liability for any settlement made by the Indemnified Party without its consent. An Indemnifying Party who is not entitled to or elects not to assume the defense of a claim shall not be obligated to pay the fees and expenses of more than one counsel for all parties indemnified by such Indemnifying Party with respect to such claim, unless, in the reasonable judgment of legal counsel to any Indemnified Party, a conflict of interest exists between such Indemnified Party and any other of such Indemnified Parties with respect to such claim. No Indemnifying Party shall, without the consent of the Indemnified Party, consent to the entry of any judgment or enter into any settlement which cannot be settled in all respects by the payment of money (and such money is so paid by the Indemnifying Party pursuant to the terms of such settlement), which settlement shall not include a statement or admission of fault and culpability on the part of such Indemnified Party, and which settlement shall include as an unconditional term thereof the giving by the claimant or plaintiff to such Indemnifying Party of a release from all liability in respect to such claim or litigation.
(iv) The indemnification provided for under this Subscription Agreement shall remain in full force and effect regardless of any investigation made by or on behalf of the Indemnified Party or any officer, director, employee, agent, affiliate or controlling person of such Indemnified Party and shall survive the transfer of the Shares purchased pursuant to this Subscription Agreement.
(v) New NAP shall notify the Investor promptly of the institution, threat or assertion of any proceeding arising from or in connection with the transactions contemplated by Section 7(g)(ii) herein of which New NAP receives notice. The Investor shall notify New NAP promptly of the institution, threat or assertion of any proceeding arising from or in connection with the transactions contemplated by Section 7(g)(i) herein of which the Investor is aware.
8. Termination. This Subscription Agreement shall terminate and be void and of no further force and effect, and all rights and obligations of New NAP, FLAC and the Investor hereunder shall terminate without any further liability on the part of New NAP, FLAC or the Investor in respect thereof, upon the earliest to occur of (i) such date and time as the Transaction Agreement is validly terminated in accordance with its terms, (ii) upon the mutual written agreement of New NAP, FLAC and the Investor to terminate this Subscription Agreement, (iii) sixty (60) days after the Termination Date (as defined in the Transaction Agreement as in effect as of the date hereof), if the Closing has not occurred by such date or (iv) at the election of the Investor, if there has occurred a breach of any representation, warranty, covenant or agreement on the part of New NAP or FLAC set forth in this Subscription Agreement, or if any representation or warranty of New NAP or FLAC shall have become untrue, in either case, such that the conditions set forth in Section 3(a) and 3(c) of this Subscription Agreement are not satisfied or waived (in writing by the person who has the authority to make such waiver) or are not capable of being satisfied on or prior to the Closing and, as a result thereof, the transactions contemplated by this Subscription Agreement will not be and are not consummated at the Closing (the termination events in clauses (i) through (iv), collectively, “Termination Events”); provided that nothing herein will relieve any party from liability for any willful breach hereof prior to the time of termination, and each party will be entitled to any remedies at law or in equity to recover reasonable and documented losses, liabilities or damages arising from any such willful breach. New NAP shall notify the Investor of the termination of the Transaction Agreement promptly after the termination of such agreement. Upon the occurrence of any Termination Event, this Subscription Agreement shall be void and of no further effect and any monies paid by the Investor to New NAP in connection herewith shall promptly (and in any event within one (1) Business Day) following the Termination Event be returned in full to the Investor by wire transfer of U.S. dollars in immediately available funds to the account specified by the Investor, without any deduction for or on account of any tax withholding, charges or set-off, whether or not the Transaction shall have been consummated.
C-18
Table of Contents
9. Trust Account Waiver. The Investor acknowledges that FLAC is a blank check company with the powers and privileges to effect a merger, asset acquisition, reorganization or similar business combination involving FLAC and one or more businesses or assets. The Investor further acknowledges that, as described in FLAC’s prospectus relating to its initial public offering dated December 8, 2020 (the “IPO Prospectus”) available at www.sec.gov, substantially all of FLAC’s assets consist of the cash proceeds of FLAC’s initial public offering and private placement of its securities, and substantially all of those proceeds have been deposited in a trust account (the “Trust Account”) for the benefit of FLAC, its public shareholders and the underwriters of FLAC’s initial public offering. Except with respect to interest earned on the funds held in the Trust Account that may be released to FLAC to pay its tax obligations, if any, the cash and other assets in the Trust Account may be disbursed only for the purposes set forth in the IPO Prospectus. For and in consideration of New NAP and FLAC entering into this Subscription Agreement, the receipt and sufficiency of which are hereby acknowledged, the Investor hereby irrevocably waives any and all right, title and interest, or any claim of any kind it has or may have in the future as a result of, or arising out of, this Subscription Agreement, in or to any monies held in the Trust Account, and agrees not to seek recourse against the Trust Account for such a claim; provided, however, that nothing in this Section 9 shall (x) serve to limit or prohibit the Investor’s right to pursue a claim against FLAC for legal relief against assets held outside the Trust Account, for specific performance or other equitable relief, (y) serve to limit or prohibit any claims that the Investor may have in the future against FLAC’s assets or funds that are not held in the Trust Account (including any funds that have been released from the Trust Account and any assets that have been purchased or acquired with any such funds), or (z) be deemed to limit the Investor’s right, title, interest or claim to any monies held in the Trust Account by virtue of its record or beneficial ownership of FLAC’s Class A Ordinary Shares currently outstanding on the date hereof, pursuant to a validly exercised redemption right with respect to any such Class A Ordinary Shares, except to the extent that the Investor has otherwise agreed in writing with FLAC to not exercise such redemption right.
10. Miscellaneous.
(a) Neither this Subscription Agreement nor any rights that may accrue to the Investor hereunder (other than the Subscribed Shares acquired hereunder, if any) may be transferred or assigned. Notwithstanding the foregoing, the Investor may assign its rights and obligations under this Subscription Agreement (i) to a fund or account managed by the Investor or the same investment manager as the Investor or an affiliate thereof, (ii) at any time to one or more of the Investor’s affiliates, (iii) to another person with the prior written consent of New NAP and FLAC (provided that such assignee(s) agree in writing to be bound by the terms hereof) and (iv) after the Closing, in whole or from time to time in part, to one or more persons in connection with the transfer of Subscribed Shares by the Investor to such person, provided that the Investor complies with all applicable laws and, with respect to any transfer or assignment prior to the Closing, provides written notice of assignment and an updated Schedule B to New NAP and FLAC promptly after such assignment is effected, and such assignee or transferee agrees in writing to be bound by all of the provisions contained herein, makes the representations and warranties in Section 6 of this Subscription Agreement and completes Schedule A hereto.
(b) New NAP may request from the Investor such additional information as New NAP may deem necessary to register the resale of the Subscribed Shares and evaluate the eligibility of the Investor to acquire the Subscribed Shares, and the Investor shall promptly provide such information as may reasonably be requested; provided, that, New NAP agrees to keep any such information provided by the Investor confidential, other than as (i) necessary to include in any Registration Statement pursuant to applicable law, or (ii) may be required by applicable law, rule, regulation or in connection with any legal proceeding or regulatory request; provided, further, that upon receipt of such additional information, New NAP shall be allowed to convey such information to each Placement Agent and such Placement Agent shall keep the information confidential, except as may be required by applicable law, rule, regulation or in connection with any legal proceeding or regulatory request. The Investor acknowledges that New NAP may file a form of this Subscription Agreement with the SEC as an exhibit to a periodic report or a registration statement of New NAP.
C-19
Table of Contents
(c) Each of FLAC, the Investor and New NAP acknowledges that New NAP, FLAC, the Company, the Placement Agents and others will rely on the acknowledgments, understandings, agreements, representations and warranties contained in this Subscription Agreement and that the Company and the Placement Agents are third-party beneficiaries of those provisions. Each of FLAC, the Investor, and New NAP further acknowledge and agree that each of the Placement Agents is a third-party beneficiary with the right to enforce Section 5, Section 6, and Section 10 of this Subscription Agreement on its behalf and not, for the avoidance of doubt, on behalf of FLAC or New NAP, and that each of the Placement Agents will rely on the acknowledgments, understandings, agreements, representations and warranties made by the Investor, FLAC and New NAP contained in this Subscription Agreement. Prior to the Closing, the Investor agrees to promptly notify New NAP, FLAC, the Company and the Placement Agents in writing (including, for the avoidance of doubt, by email) if any of the acknowledgments, understandings, agreements, representations and warranties made by the Investor set forth in Section 6 above are no longer accurate in any material respect (other than those acknowledgments, understandings, agreements, representations and warranties qualified by materiality, in which case the Investor shall notify FLAC, New NAP and the Placement Agents if they are no longer accurate in any respect). The Investor acknowledges and agrees that each purchase by the Investor of Subscription Shares from New NAP will constitute a reaffirmation of the acknowledgments, understandings, agreements, representations and warranties herein (as modified by any such notice) by the Investor as of the time of such purchase.
(d) The Investor acknowledges and agrees that neither it, nor any person or entity acting on its behalf or pursuant to any understanding with the Investor, shall, directly or indirectly, engage in any hedging activities or execute any Short Sales with respect to the securities of FLAC prior to the Closing or the earlier termination of this Subscription Agreement in accordance with its terms. “Short Sales” shall include, without limitation, all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act and all types of direct and indirect stock pledges (other than pledges in the ordinary course of business as part of prime brokerage arrangements), forward sale contracts, options, puts, calls, swaps and similar arrangements (including, without limitation, on a total return basis), and sales and other transactions through non-U.S. broker dealers or foreign regulated brokers. Notwithstanding the foregoing, (i) nothing in this Section 10(d) shall prohibit any entities under common management with the Investor that have no knowledge of this Subscription Agreement or of the Investor’s participation in the transactions contemplated hereby (including the Investor’s controlled affiliates or affiliates) from entering into any Short Sales and (ii) this Section 10(d) shall not apply to ordinary course, non-speculative hedging transactions.
(e) New NAP, FLAC, the Company and the Placement Agents are each entitled to rely upon this Subscription Agreement and each is irrevocably authorized to produce this Subscription Agreement or a copy hereof to any interested party when required by law, governmental authority or self-regulatory organization to do so in any administrative or legal proceeding or official inquiry with respect to the matters covered hereby; provided, however, that the foregoing shall not give the Company or the Placement Agents any rights other than those expressly set forth herein and, without limiting the generality of the foregoing and for the avoidance of doubt, in no event shall the Company be entitled to rely on any of the representations and warranties of New NAP and FLAC set forth in this Subscription Agreement.
(f) All of the agreements, representations and warranties made by each party hereto in this Subscription Agreement shall survive the Closing until the expiration of any applicable statute of limitations.
(g) This Subscription Agreement may not be amended, modified, supplemented or waived or terminated (other than pursuant to the terms of Section 8 above) except by an instrument in writing signed by each of New NAP, FLAC and the Investor. No failure or delay of any of New NAP, FLAC or the Investor in exercising any right or remedy hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of any such right or power, or any abandonment or discontinuance of steps to enforce such right or power, or any course of conduct, preclude any other or further exercise thereof or the exercise of any other right or power. The rights and remedies of New NAP, FLAC and the Investor hereunder are cumulative and are not exclusive of any rights or remedies that they would otherwise have hereunder. Notwithstanding anything to the contrary herein, Section 5, Section 6, Section 10(c), Section 10(d),
C-20
Table of Contents
Section 10(e), this Section 10(g) and Section 11 may not be modified, waived or terminated in a manner that is material and adverse to the Placement Agents without the written consent of the Placement Agents.
(h) This Subscription Agreement (including the schedules hereto) constitutes the entire agreement, and supersedes all other prior agreements, understandings, representations and warranties, both written and oral, among New NAP, FLAC and the Investor, with respect to the subject matter hereof. Except as set forth in Sections 10(c), 10(e) and 10(g) and the last sentence of Section 10(l) with respect to the persons specifically referenced therein, this Subscription Agreement shall not confer any rights or remedies upon any person other than New NAP, FLAC and the Investor, and their respective successors and assigns, and New NAP, FLAC and the Investor acknowledge that such persons so referenced are third party beneficiaries of this Subscription Agreement for the purposes of, and to the extent of, the rights granted to them, if any, pursuant to such provisions.
(i) Except as otherwise provided herein, this Subscription Agreement shall be binding upon, and inure to the benefit of New NAP, FLAC and the Investor and their heirs, executors, administrators, successors, legal representatives, and permitted assigns, and the agreements, representations, warranties, covenants and acknowledgments contained herein shall be deemed to be made by, and be binding upon, such heirs, executors, administrators, successors, legal representatives and permitted assigns.
(j) If any provision of this Subscription Agreement shall be adjudicated by a court of competent jurisdiction to be invalid, illegal or unenforceable, the validity, legality or enforceability of the remaining provisions of this Subscription Agreement shall not in any way be affected or impaired thereby and shall continue in full force and effect.
(k) This Subscription Agreement may be executed in counterparts (including by facsimile or electronic mail or in .pdf) and by different parties in separate counterparts, with the same effect as if all parties hereto had signed the same document. All counterparts so executed and delivered shall be construed together and shall constitute one and the same agreement.
(l) New NAP, FLAC and the Investor acknowledge and agree that irreparable damage would occur in the event that any of the provisions of this Subscription Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of this Subscription Agreement, without posting a bond or undertaking and without proof of damages, to enforce specifically the terms and provisions of this Subscription Agreement in any Chosen Court (as defined below), this being in addition to any other remedy to which New NAP, FLAC or the Investor is entitled at law, in equity, in contract, in tort or otherwise. New NAP, FLAC and the Investor acknowledge and agree that the Company shall be entitled to seek to specifically enforce the Investor’s obligations to fund the Subscription Amount and the provisions of the Subscription Agreement of which the Company is an express third party beneficiary, in each case, on the terms and subject to the conditions set forth herein.
(m) Except as otherwise provided elsewhere in this Subscription Agreement, each party shall pay its own expenses in connection with this Subscription Agreement and the transactions contemplated hereby. Notwithstanding anything herein to the contrary, New NAP shall pay all transfer agent fees (including, without limitation, any fees required for same-day processing of any instruction letter delivered by New NAP and any exercise notice delivered by the Investor), stamp taxes and other taxes and duties levied in connection with the delivery of any Shares to the Investor other than income and capital gains taxes of the Investor that may be incurred in connection with the transactions contemplated hereby.
(n) Any notice or communication required or permitted hereunder to be given or made hereunder shall be in writing and either delivered personally, emailed or sent by overnight mail via a reputable overnight carrier, or sent by certified or registered mail, postage prepaid, and shall be deemed to be given and received (i) when so delivered personally (with written confirmation of receipt), (ii) when sent (with no
C-21
Table of Contents
“bounceback” or notice of non-delivery), if sent by email, or (iii) three (3) Business Days after the date of mailing, in each case, to:
(i) if to the Investor, such addresses set forth on the signature page hereto or to such other address or addresses as the Investor may hereafter designate by notice to New NAP;
(ii) if to New NAP:
NewAmsterdam Pharma Company B.V.
c/o NewAmsterdam Pharma Holding B.V.
Gooimeer 2-35
1411 DC Naarden
The Netherlands
Attention: Louise Kooij
Email:
with a copy (which shall not constitute notice) to:
Covington & Burling LLP
620 Eighth Avenue
New York, NY 10018
(212) 841-1000
Attention: Jack Bodner; Kerry S. Burke; Brian K. Rosenzweig
Email: jbodner@cov.com; kburke@cov.com; brosenzweig@cov.com
(iii) if to FLAC:
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union St., Suite 3200
Seattle, WA 98101
Attention: James N. Topper
Email:
with a copy (which shall not constitute notice) to:
Goodwin Procter LLP
100 Northern Avenue
Boston, MA 02210
Attention: Jocelyn M. Arel, Jacqueline Mercier, Michael R. Patrone
Email: JArel@goodwinlaw.com, JMercier@goodwinlaw.com; MPatrone@goodwinlaw.com
(o) This Subscription Agreement, and any claim or cause of action hereunder based upon, arising out of or related to this Subscription Agreement (whether based on law, in equity, in contract, in tort or any other theory) or the negotiation, execution, performance or enforcement of this Subscription Agreement, shall be governed by and construed in accordance with the laws of the State of Delaware, without giving effect to the principles of conflicts of law thereof.
(p) NEW NAP, FLAC AND THE INVESTOR IRREVOCABLY SUBMIT TO THE EXCLUSIVE JURISDICTION OF THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE AND THE CHANCERY COURT OF DELAWARE (THE “CHOSEN COURTS”), SOLELY IN RESPECT OF THE INTERPRETATION AND ENFORCEMENT OF THE PROVISIONS OF THIS SUBSCRIPTION AGREEMENT AND THE DOCUMENTS REFERRED TO IN THIS SUBSCRIPTION AGREEMENT AND IN RESPECT OF THE TRANSACTIONS CONTEMPLATED HEREBY, AND HEREBY WAIVE, AND AGREE NOT TO ASSERT, AS A DEFENSE IN ANY ACTION, SUIT OR PROCEEDING FOR INTERPRETATION OR ENFORCEMENT HEREOF OR ANY SUCH DOCUMENT THAT IS NOT SUBJECT THERETO OR THAT SUCH ACTION, SUIT OR PROCEEDING MAY NOT BE BROUGHT
C-22
Table of Contents
OR IS NOT MAINTAINABLE IN SAID COURTS OR THAT VENUE THEREOF MAY NOT BE APPROPRIATE OR THAT THIS SUBSCRIPTION AGREEMENT OR ANY SUCH DOCUMENT MAY NOT BE ENFORCED IN OR BY SUCH COURTS, AND NEW NAP, FLAC AND THE INVESTOR IRREVOCABLY AGREE THAT ALL CLAIMS WITH RESPECT TO SUCH ACTION, SUIT OR PROCEEDING SHALL BE HEARD AND DETERMINED BY SUCH A DELAWARE STATE OR FEDERAL COURT. NEW NAP, FLAC AND THE INVESTOR HEREBY CONSENT TO AND GRANT ANY SUCH COURT JURISDICTION OVER THE PERSON OF SUCH PARTIES AND OVER THE SUBJECT MATTER OF SUCH DISPUTE AND AGREE THAT MAILING OF PROCESS OR OTHER PAPERS IN CONNECTION WITH SUCH ACTION, SUIT OR PROCEEDING IN THE MANNER PROVIDED IN THIS SECTION 10(p) OF THIS SUBSCRIPTION AGREEMENT OR IN SUCH OTHER MANNER AS MAY BE PERMITTED BY LAW SHALL BE VALID AND SUFFICIENT SERVICE THEREOF. EACH PARTY ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS SUBSCRIPTION AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES, AND THEREFORE EACH SUCH PARTY HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS SUBSCRIPTION AGREEMENT OR THE TRANSACTIONS CONTEMPLATED BY THIS SUBSCRIPTION AGREEMENT.
EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER; (II) SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THE FOREGOING WAIVER; (III) SUCH PARTY MAKES THE FOREGOING WAIVER VOLUNTARILY AND (IV) SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS SUBSCRIPTION AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVER AND CERTIFICATIONS IN THIS SECTION 10(p).
(q) The headings herein are for convenience only, do not constitute a part of this Subscription Agreement and shall not be deemed to limit or affect any of the provisions hereof. The language used in this Subscription Agreement will be deemed to be the language chosen by the parties hereto to express their mutual intent, and no rules of strict construction will be applied against any party.
(r) If any change in the Shares shall occur between the date hereof and immediately prior to the Closing by reason of any reclassification, recapitalization, stock split (including reverse stock split) or combination, exchange or readjustment of shares, or any stock dividend, the number of Subscribed Shares issued to the Investor, and the Per Share Purchase Price for such Subscribed Shares, shall be appropriately adjusted to reflect such change.
11. Non-Reliance and Exculpation. The Investor acknowledges that it is not relying upon, and has not relied upon, any statement, representation or warranty made by any person, firm or corporation (including, without limitation, the Placement Agents, any of their respective affiliates or any control persons, officers, directors, employees, partners, agents or representatives of any of the foregoing), other than the statements, representations and warranties of New NAP and FLAC expressly contained in this Subscription Agreement, in making its investment or decision to invest in New NAP. The Investor acknowledges and agrees that none of (i) any other investor pursuant to this Subscription Agreement or any Other Subscription Agreement (including such investor’s affiliates or any control persons, officers, directors, employees, partners, agents or representatives of any of the foregoing), (ii) the Placement Agents, their respective affiliates or any control persons, officers, directors, employees, partners, agents or representatives of any of the foregoing, or (iii) any other party to the Transaction Agreement or any Non-Party Affiliate, shall have any liability to the Investor or to any Other Investor pursuant to, arising out of or relating to this Subscription Agreement or any Other Subscription Agreement, the negotiation hereof or thereof or its subject matter, or the transactions contemplated hereby or thereby, including, without limitation, with respect to any action heretofore or hereafter taken or omitted to be
C-23
Table of Contents
taken by any of them in connection with the purchase of the Subscribed Shares or with respect to any claim (whether in tort, contract or otherwise) for breach of this Subscription Agreement or in respect of any written or oral representations made or alleged to be made in connection herewith, as expressly provided herein, or for any actual or alleged inaccuracies, misstatements or omissions with respect to any information or materials of any kind furnished by New NAP, FLAC, the Company, the Placement Agents or any Non-Party Affiliate (as defined below) concerning New NAP, FLAC, the Company, the Placement Agents, any of their respective controlled affiliates, this Subscription Agreement or the transactions contemplated hereby. For purposes of this Subscription Agreement, “Non-Party Affiliates” means each former, current or future officer, director, employee, partner, member, manager, direct or indirect equityholder or affiliate of New NAP, FLAC, the Company, any Placement Agent or any of New NAP’s, the Company’s or the Placement Agents’ controlled affiliates or any family member of the foregoing. The Investor agrees that none of the Placement Agents shall be liable to it (including in contract, tort, under federal or state securities laws or otherwise) for any action heretofore or hereafter taken or omitted to be taken by any of them in connection with the Private Placement. On behalf of the Investor and its affiliates, the Investor releases the Placement Agents in respect of any losses, claims, damages, obligations, penalties, judgments, awards, liabilities, costs, expenses or disbursements related to the Private Placement. The Investor agrees not to commence any litigation or bring any claim against any of the Placement Agents in any court or any other forum which relates to, may arise out of, or is in connection with, the Private Placement. This undertaking is given freely and after obtaining independent legal advice.
12. Disclosure. FLAC shall, by no later than 9:00 a.m., New York City time, on the first (1st) Business Day immediately following the date of this Subscription Agreement, issue one or more press releases or file with the SEC a Current Report on Form 8-K (collectively, the “Disclosure Document”) disclosing all material terms of the transactions contemplated hereby and by the Other Subscription Agreements, the Transactions and any other material, nonpublic information that FLAC, New NAP or any of their respective affiliates has provided to the Investor at any time prior to the filing of the Disclosure Document. Upon the issuance of the Disclosure Document, to the actual knowledge of New NAP and FLAC, the Investor shall not be in possession of any material, non-public information received from New NAP, FLAC or any of their respective officers, directors, employees or agents. Notwithstanding anything in this Subscription Agreement to the contrary, New NAP and FLAC shall not (i) publicly disclose the name of the Investor or any of its affiliates or advisers (or any of their respective affiliates or advisers), or include the name of the Investor or any of its affiliates or advisers in any press release without the prior written consent of the Investor, or (ii) publicly disclose the name of the Investor or any of its affiliates or advisers (or any of their respective affiliates or advisers), or include the name of the Investor or any of its affiliates or advisers (or any of their respective affiliates or advisers) in any filing with the SEC or any regulatory agency or trading market, without the prior written consent of the Investor, except as required by the federal securities laws or pursuant to other routine proceedings of regulatory authorities, or to the extent such disclosure is required by law, at the request of the staff of the SEC or regulatory agency or under the regulations of the Stock Exchange, in which case New NAP or FLAC will provide the Investor with prior written notice (which notice may be by e-mail) of such disclosure under this clause (ii) (provided, that in any such event under this clause (ii), New NAP or FLAC, as applicable, shall use its commercially reasonable efforts to allow the Investor an opportunity to review such public statement, press release, filing or other communication) or (iii) except to the extent such announcements or other communications contain only information that is substantially equivalent to the information that has previously been disclosed in a public statement, press release or other communication without breach by New NAP or FLAC of its obligations under this Section 12, publicly disclose the name of the Investor or any of its affiliates or advisers, or include the name of the Investor or any of its affiliates or advisers.
[SIGNATURE PAGES FOLLOW]
C-24
Table of Contents
IN WITNESS WHEREOF, New NAP has accepted this Subscription Agreement as of the date set forth below.
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||
| By: | ||
LouFré Management B.V. | ||
| represented by LouFré Holding B.V. | ||
| represented by Louise Kooij Director | ||
Date: , 2022
[Signature Page to Subscription Agreement]
C-25
Table of Contents
IN WITNESS WHEREOF, FLAC has accepted this Subscription Agreement as of the date set forth below.
| FRAZIER LIFESCIENCES ACQUISITION CORPORATION | ||
| By: | ||
| Name: James N. Topper | ||
| Title: Chief Executive Officer | ||
Date: , 2022
[Signature Page to Subscription Agreement]
C-26
Table of Contents
IN WITNESS WHEREOF, the Investor has executed or caused this Subscription Agreement to be executed by its duly authorized representative as of the date set forth below.
| Name of Investor: | State/Country of Formation or Domicile: |
| By: |
| |
| Name: |
| |
| Title: |
|
| Name in which Shares are to be registered (if different): | Date: , 2022 | |
| Investor’s EIN: | ||
| Business Address-Street: | Mailing Address-Street (if different): | |
| City, State, Zip: | City, State, Zip: | |
| Attn: | Attn: | |
| Telephone No.: | Telephone No.: | |
| Facsimile No.: | Facsimile No.: | |
| E-Mail: | E-Mail: | |
| Number of Shares subscribed for: | ||
| Aggregate Subscription Amount: $ | Price Per Share: $10.00 | |
You must pay the Subscription Amount by wire transfer of United States dollars in immediately available funds to the account specified by New NAP in the Closing Notice.
[Signature Page to Subscription Agreement]
C-27
Table of Contents
SCHEDULE A
ELIGIBILITY REPRESENTATIONS OF THE INVESTOR
This Schedule must be completed by the Investor and forms a part of the Subscription Agreement to which it is attached. Capitalized terms used and not otherwise defined in this Schedule have the meanings given to them in the Subscription Agreement. The Investor must check the applicable box in either Part A or Part B and the applicable box in Part C below.
| A. | QUALIFIED INSTITUTIONAL BUYER STATUS |
(Please check the applicable subparagraphs):
| ☐ | Investor is a “qualified institutional buyer” (as defined in Rule 144A under the Securities Act (a “QIB”)). |
| ☐ | Investor is subscribing for the Subscribed Shares as a fiduciary or agent for one or more investor accounts, and each owner of such accounts is a QIB. |
*** OR ***
| B. | INSTITUTIONAL ACCREDITED INVESTOR STATUS |
(Please check the applicable subparagraphs):
| ☐ | We are an “accredited investor” (within the meaning of Rule 501(a) under the Securities Act or an entity in which all of the equity holders are accredited investors within the meaning of Rule 501(a) under the Securities Act), and have marked and initialed the appropriate box below indicating the provision under which we qualify as an “accredited investor.” |
| ☐ | We are not a natural person. |
Investor is an institutional “accredited investor” within the meaning of Rule 501(a) under the Securities Act and has checked the appropriate box(es) below indicating the applicable provision under which the Investor qualifies as such:
| ☐ | Investor is an organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, a corporation, Massachusetts or similar business trust, partnership, or limited liability company that was not formed for the specific purpose of acquiring the securities of FLAC being offered in this offering, with total assets in excess of $5,000,000. |
| ☐ | Investor is a “private business development company” as defined in Section 202(a)(22) of the Investment Advisers Act of 1940. |
| ☐ | Investor is a “bank” as defined in Section 3(a)(2) of the Securities Act. |
| ☐ | Investor is a “savings and loan association” or other institution as defined in Section 3(a)(5)(A) of the Securities Act, whether acting in its individual or fiduciary capacity. |
| ☐ | Investor is a broker or dealer registered pursuant to Section 15 of the Exchange Act. |
| ☐ | Investor is an investment adviser registered pursuant to Section 203 of the Investment Advisers Act of 1940 or registered pursuant to the laws of a state. |
| ☐ | Investor is an investment adviser relying on the exemption from registering with the Commission under Section 203(l) or (m) of the Investment Advisers Act of 1940. |
| ☐ | Investor is an “insurance company” as defined in Section 2(a)(13) of the Securities Act. |
| ☐ | Investor is an investment company registered under the Investment Company Act of 1940. |
| ☐ | Investor is a “business development company” as defined in Section 2(a)(48) of the Investment Company Act of 1940. |
Schedule A-1
C-28
Table of Contents
| ☐ | Investor is a “Small Business Investment Company” licensed by the U.S. Small Business Administration under either Section 301(c) or (d) of the Small Business Investment Act of 1958. |
| ☐ | Investor is a “Rural Business Investment Company” as defined in Section 384A of the Consolidated Farm and Rural Development Act. |
| ☐ | Investor is a plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, and such plan has total assets in excess of $5,000,000. |
| ☐ | Investor is an employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974 if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of such act, which is one of the following. |
| ☐ | A bank; |
| ☐ | A savings and loan association; |
| ☐ | A insurance company; or |
| ☐ | A registered investment adviser. |
| ☐ | Investor is an employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974 with total assets in excess of $5,000,000. |
| ☐ | Investor is an employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974 that is a self-directed plan with investment decisions made solely by persons that are accredited investors. |
| ☐ | Investor is a trust with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the securities offered in this offering, whose purchase is directed by a sophisticated person as described in Rule 506(b)(2)(ii) under the Securities Act. |
***AND***
| C. | AFFILIATE STATUS |
(Please check the applicable box)
Investor:
| ☐ | is: |
| ☐ | is not: |
an “affiliate” (as defined in Rule 144) of FLAC or acting on behalf of an affiliate of FLAC.
Schedule A-2
C-29
Table of Contents
SCHEDULE B
SCHEDULE OF TRANSFERS
Investor’s Subscription was in the amount of Shares. The following transfers of a portion of the Subscription have been made:
Date of Transfer or Reduction | Transferee | Number of Subscribed Shares Transferred or Reduced | Investor Revised Subscription Amount | |||
Schedule B as of , 20 , accepted and agreed to as of this day of , 20 by:
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||
| By: |
| |
| Name: | ||
| Title: | ||
Name of Investor: |
|
Signature of Investor: |
| By: |
| |
| Name: | ||
| Title: |
Schedule B-1
C-30
Table of Contents
Exhibit A – PoA
POWER OF ATTORNEY
THE UNDERSIGNED
[Note: authority statement to be included in Exhibit A-1]
[name entity], a [company] under the laws of [jurisdiction], having its registered office at [address], and registered with the [name foreign companies registrar] under registration number [number] (the “Investor”),
HEREBY DECLARES
| 1. | The Investor grants an irrevocable power of attorney to each individual civil law notary, assigned civil law notary, candidate civil law notary, lawyer, notarial assistant and paralegal working with NautaDutilh N.V. (each: an “Attorney”). |
| 2. | The scope of this power of attorney extends to the performance of the following acts for and on behalf of the Investor: |
| a. | to appear before any civil law notary of NautaDutilh N.V. (or one of their deputies) as a party to a notarial deed drawn up by NautaDutilh N.V., pursuant to which NewAmsterdam Pharma Company B.V. (“New NAP”) will issue ordinary shares in its capital to the Investor (the “Subscribed Shares”) and the Investor will acquire the Subscribed Shares in accordance with and subject to the terms and conditions of the Subscription Agreement entered into by and among New NAP, Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company, and the Investor, dated as of [date] (the “Subscription Agreement”); |
| b. | to sign, execute and deliver on behalf of the Investor a private deed of delivery of the Subscribed Shares drawn up by NautaDutilh N.V., pursuant to which the Subscribed Shares will be registered in book-entry form in the name of the Investor (or its nominee in accordance with its delivery instructions) or to a custodian designated by the Investor, as applicable, on New NAP’s share register or the register of New NAP’s transfer agent in accordance with and subject to the terms and conditions of the Subscription Agreement; and |
| c. | upon discussion and in agreement with the Investor, to sign, execute and deliver any private and notarial deed, agreement, statement, declaration, form or other document and to perform any other acts, including acts of disposition (beschikkingshandelingen), on behalf of the Investor that an Attorney considers necessary, useful or advisable in connection with the performance of the matters described above. |
| 3. | Each Attorney is authorised to act also as counterparty to the Investor or as an attorney-in-fact of any such counterparty. |
| 4. | This power of attorney is subject to each Attorney’s right to indemnification for all acts performed under this power of attorney. |
| 5. | The relationship between the Investor and each Attorney under this power of attorney is governed exclusively by the laws of the Netherlands. |
(signature page follows)
Exhibit A
C-31
Table of Contents
Signature page to a power of attorney.
[Investor], [represented by]:
|
| |||||
| Name: | Name: | |||||
| Title: | Title: | |||||
| Date: | Date: |
Please observe the following requirements when executing this power of attorney:
| 1. | A copy of a valid passport of each individual signing this power of attorney must be attached. |
| 2. | Each signature on this power of attorney must be notarized. If notarized outside the Netherlands, this power of attorney must be apostilled. Alternatively, this process can be completed through a video meeting with a representative of NautaDutilh. Please contact NautaDutilh to set this up (contact details below). During such video conference, the signatory has to (i) show his/her original passport, (ii) sign a printed copy of such passport (by wet ink signature) and (iii) sign a printed copy of this power of attorney (by wet ink signature). Please do not sign the printed copy of the passport and/or this power of attorney in advance of the video conference. |
| 3. | This power of attorney must be signed by wet-ink signature (i.e. digital signatures will not be accepted). |
| 4. | If the Investor is an entity not incorporated under Dutch law: a lawyer practicing in the country of incorporation of the Investor must issue the confirmation statement substantially in the form of Exhibit A-1 hereto. If you wish to make changes to this format, please contact NautaDutilh (contact details below). |
| 5. | Following execution, please send a scan copy of the signed documents by e-mail, followed by the originals. Please use the following contact details: NautaDutilh N.V., Attn. Marloes van der Laan, Beethovenstraat 400, 1082 PR Amsterdam, The Netherlands, E-mail: |
| 6. | Please note that additional KYC-documentation may be requested, all to the satisfaction of the notary. |
C-32
Table of Contents
Exhibit A-1
[Letterhead counsel]
NautaDutilh N.V.
Attn. P.C.S. van der Bijl / M.L. van der Laan
Beethovenstraat 400
1082 PR Amsterdam
the Netherlands
[Place], [date]
Dear Sir, Madam,
I am a [lawyer admitted to the bar of] / [notary practicing in] [jurisdiction].
[name entity], a [company] under the laws of [jurisdiction], having its registered office at [address], and registered with the [name foreign companies registrar] under registration number [number] (the “Company”) has requested me to make the following statements with regard to the power of attorney (the “Power of Attorney”) of which a copy is attached hereto.
For the purposes of making these statements I have reviewed the Power of Attorney, the Company’s organisational documents and such other documents as I have deemed necessary.
I hereby state that [signatory/signatories], at the time of the execution of the Power of Attorney, had the power to represent the Company for the purposes of the execution of the Power of Attorney in the name and on behalf of the Company and that the Company has the power to enter into the transactions described in the Power of Attorney.
Yours sincerely,
[Name counsel]
C-33
Table of Contents
SPONSOR SUPPORT AGREEMENT
THIS SPONSOR SUPPORT AGREEMENT (this “Agreement”) is dated as of July 25, 2022 by and among (i) Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (the “Sponsor”), (ii) the other holders of FLAC Class B Shares set forth on Schedule I hereto (the “Other Class B Holders” and, together with the Sponsor, collectively, the “Class B Holders”), (iii) Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman Islands exempted company (“FLAC”), (iv) NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (the “Company”), and (v) NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands, which was formed by the Company for the sole purpose of consummating the transactions contemplated by the Business Combination Agreement (as defined below), and which shall convert into a public limited liability company (naamloze vennootschap) incorporated under the laws of the Netherlands prior to the Merger (as defined below) (“Holdco”). Capitalized terms used but not defined herein shall have the respective meanings ascribed to such terms in the Business Combination Agreement.
RECITALS
WHEREAS, FLAC, Holdco, the Company, and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and a direct wholly owned subsidiary of Holdco (“Merger Sub”), have entered into that certain Business Combination Agreement, dated as of the date hereof (as it may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), pursuant to which, among other things, (i) the Company shall cause each Company Shareholder to contribute and transfer each Company Share held by it to Holdco and Holdco shall accept such contribution and in exchange issue to such holder such number of Holdco Shares that is equal to the Applicable Exchange Consideration Per Share with respect to such Company Share and (ii) Merger Sub will merge with and into FLAC, with FLAC surviving as a wholly owned subsidiary of Holdco, on the terms and subject to the conditions therein (the “Merger”);
WHEREAS, as of the date hereof, each Class B Holder beneficially owns (as defined in Rule 13d-3 under the Exchange Act), and has sole voting power with respect to the number and type of FLAC Shares, and owns the FLAC Warrants, indicated opposite such Holder’s name on Schedule I attached hereto; and
WHEREAS, as an inducement to FLAC and the Company to enter into the Business Combination Agreement and to consummate the transactions contemplated therein, the parties hereto desire to agree to certain matters as set forth herein.
NOW, THEREFORE, in consideration of the foregoing and the mutual agreements contained herein, and intending to be legally bound hereby, the parties hereto hereby agree as follows:
ARTICLE I
COVENANTS
Section 1.1 Agreement to Vote. Each Class B Holder hereby agrees to appear and vote at any duly called meeting of the shareholders of FLAC (or any adjournment or postponement thereof), provide his, her or its written consent in any action by written resolution of the shareholders of FLAC, or in any other circumstance in which the vote, consent or other approval of the shareholders of FLAC is sought, all of such Class B Holder’s FLAC Shares, and in each such case cause all the FLAC Shares held by such Class B Holder to be counted as present thereat for purposes of calculating a quorum, and vote or provide his, her or its consent: (a) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal, (b) in favor of any other matter reasonably necessary or required to the consummation of the Transactions and
D-1
Table of Contents
considered and voted upon by the shareholders of FLAC and (c) against any proposal that conflicts or materially impedes or interferes therewith, including any FLAC Acquisition Proposal, or would adversely affect or delay the consummation of the Transactions.
Section 1.2 Waivers.
(a) Anti-Dilution Protection. Notwithstanding anything to the contrary in any other agreement or contract to which the Class B Holders are bound, the Class B Holders (for themselves and for their successors, heirs and assigns) hereby (but subject to the consummation of the Merger) irrevocably and unconditionally waive, to the fullest extent permitted by Law and the Governing Documents of FLAC, and agree not to exercise, assert or perfect, any rights to adjustment or other anti-dilution protections with respect to the rate at which FLAC Class B Shares held by the Class B Holders convert into Holdco Shares, whether resulting from the Transactions, the Subscription Agreements or otherwise, so that each FLAC Class B Share held by each Class B Holder issued and outstanding as of immediately prior to the Merger shall convert into one Holdco Share on the Effective Date upon consummation of the Merger.
(b) Redemption Rights. Each Class B Holder hereby waives any and all rights to redeem any FLAC Shares (in connection with the Transactions or otherwise) as set forth in the Governing Documents of FLAC, and shall not elect to cause FLAC to redeem any FLAC Shares beneficially owned or owned of record by the Class B Holders (in connection with the Transactions or otherwise).
Section 1.3 No Transfer. During the period commencing on the date hereof and ending on the earlier of (a) the Effective Date and (b) such date and time as the Business Combination Agreement shall be validly terminated in accordance with Section 7.1 thereof, each Class B Holder shall not (i) sell, offer to sell, contract or agree to sell, assign, hypothecate, pledge, create a Lien on, grant any option to purchase, transfer, or otherwise dispose of or agree to dispose of, directly or indirectly, file (or participate in the filing of) a registration statement with the SEC (other than the Registration Statement/Proxy Statement), deposit into a voting trust, grant any proxy or power of attorney with respect to, or establish or increase a put equivalent position or liquidate or decrease a call equivalent position (within the meaning of Section 16 of the Exchange Act) with respect to, any FLAC Shares or FLAC Warrants held by such Class B Holder, (ii) enter into any swap or other arrangement that transfers to another Person, in whole or in part, any of the economic consequences of ownership of any shares of FLAC Shares or FLAC Warrants held by such Class B Holder (clauses (i) and (ii) collectively, a “Transfer”) or (iii) publicly announce any intention to effect any transaction specified in clause (i) or (ii); provided, however, that the foregoing shall not prohibit Transfers from a Class B Holder to and any of such Class B Holder’s Affiliates, so long as, prior to and as a condition to the effectiveness of any such Transfer, such Affiliate executes and delivers to FLAC a joinder to this Agreement in the form attached hereto as Annex A.
Section 1.4 New Shares. In the event that (a) any FLAC Shares, FLAC Warrants or other Equity Securities of FLAC are issued to any Class B Holder or any of its Affiliates after the date of this Agreement pursuant to any stock dividend, stock split, recapitalization, reclassification, combination, conversion or exchange of FLAC Shares or FLAC Warrants of, on or affecting the FLAC Shares or FLAC Warrants owned by the Class B Holders or otherwise, (b) any Class B Holder purchases or otherwise acquires beneficial ownership of any FLAC Shares, FLAC Warrants or other Equity Securities of FLAC after the date of this Agreement, or (c) any Class B Holder acquires the right to vote or share in the voting of any FLAC Shares or other Equity Securities of FLAC after the date of this Agreement (such FLAC Shares, FLAC Warrants or other Equity Securities issued, purchased or acquired as described in any of the foregoing clauses (a) through (c), collectively, the “New Securities”), then (x) the applicable Class B Holder shall notify FLAC, Holdco and the Company in writing and as promptly as practicable of any such New Securities and (y) such New Securities shall be subject to the terms of this Agreement to the same extent as if they constituted the FLAC Shares or FLAC Warrants owned by the Class B Holders as of the date hereof.
D-2
Table of Contents
ARTICLE II
REPRESENTATIONS AND WARRANTIES
Section 2.1 Representations and Warranties of the Class B Holders. Each Class B holder, severally and not jointly, represents and warrants as of the date hereof to FLAC, the Company and Holdco as follows:
(a) Organization; Due Authorization. Such Class B Holder has the full power and authority to execute and deliver this Agreement and to perform such Holder’s obligations hereunder. If such Class B Holder is an entity, it is duly organized, validly existing and in good standing under the Laws of the jurisdiction in which it is incorporated, formed, organized or constituted, and the execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby are within such Class B Holder’s corporate, limited liability company or organizational powers and have been duly authorized by all necessary corporate, limited liability company or organizational actions on the part of such Class B Holder. This Agreement has been duly executed and delivered by such Class B Holder and, assuming due authorization, execution and delivery by the other parties to this Agreement, this Agreement constitutes a legally valid and binding obligation of such Class B Holder, enforceable against such Class B Holder in accordance with the terms hereof (except as enforceability may be limited by the Enforceability Exceptions). If this Agreement is being executed in a representative or fiduciary capacity, the Person signing this Agreement has full power and authority to enter into this Agreement on behalf of the applicable Class B Holder.
(b) Ownership. Such Class B Holder is the record and beneficial owner (as defined in the Exchange Act) of, and has good title to the FLAC Shares and FLAC Warrants set forth on Schedule I attached hereto as are opposite the name of such Class B Holder, and there exist no Liens or any other limitation or restriction (including any restriction on the right to vote, sell or otherwise dispose of such FLAC Shares or FLAC Warrants (other than transfer restrictions under the Securities Act)) affecting any such FLAC Shares or FLAC Warrants, other than Liens pursuant to (i) this Agreement, (ii) FLAC’s Governing Documents, (iii) the Business Combination Agreement, (iv) that certain letter agreement dated December 8, 2020, by and among FLAC, the Sponsor and each of Robert F. Baltera, Michael F. Bigham, Krishna R. Polu, Carol Gallagher and David Topper (the “Voting Letter Agreement”) or (v) any applicable securities Laws. The FLAC Shares and FLAC Warrants set forth on Schedule I attached hereto are the only Equity Securities in FLAC owned of record or beneficially by such Class B Holder on the date of this Agreement, and none of such Class B Holder’s FLAC Shares or FLAC Warrants are subject to any proxy, voting trust or other agreement or arrangement with respect to the voting of such FLAC Shares or FLAC Warrants, except as provided hereunder and under the Voting Letter Agreement. Other than the FLAC Warrants, such Class B Holder does not hold or own any rights to acquire (directly or indirectly) any Equity Securities of FLAC or any securities convertible into, or which can be exchanged for, Equity Securities of FLAC.
(c) No Conflicts. The execution and delivery of this Agreement by such Class B Holder does not, and the performance by the Class B Holder of its obligations hereunder will not, (i) conflict with or result in a violation of the organizational documents of the Class B Holder or (ii) require any consent or approval that has not been given or other action that has not been taken by any Person (including under any Contract binding upon the Class B Holder or the Class B Holder’s FLAC Shares or FLAC Warrants), in each case, to the extent such consent, approval or other action would prevent, enjoin or materially delay the performance by the Class B Holder of its, his or her obligations under this Agreement, the Business Combination Agreement or the transactions contemplated hereby or thereby.
(d) Litigation. There are no Proceedings pending against such Class B Holder, or, to the knowledge of such Class B Holder, threatened against such Class B Holder, before (or, in the case of threatened Proceedings, that would be before) any arbitrator or any Governmental Entity, which in any manner challenges or seeks to prevent, enjoin or materially delay the performance by such Class B Holder of its, his or her obligations under this Agreement, the Business Combination Agreement or the transactions contemplated hereby or thereby.
D-3
Table of Contents
(e) No Brokers. No investment banker, broker, finder, consultant or intermediary or other Person is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission based upon arrangements made by or on behalf of such Class B Holder in connection with its entering into this Agreement.
(f) Acknowledgment. Such Class B Holder understands and acknowledges that each of FLAC, Holdco and the Company is entering into the Business Combination Agreement in reliance upon such Class B Holder’s execution and delivery of this Agreement.
(g) No Other Representations or Warranties. Except for the representations and warranties made by such Class B Holder in this Article II, neither such Class B Holder nor any other Person makes any express or implied representation or warranty to FLAC, Holdco or the Company in connection with this Agreement or the transactions contemplated by this Agreement, and such Class B Holder expressly disclaims any such other representations or warranties.
ARTICLE III
MISCELLANEOUS
Section 3.1 Termination. This Agreement and all of its provisions shall terminate and be of no further force or effect upon the earlier of: (a) the valid termination of the Business Combination Agreement in accordance with Section 7.1 thereof prior to the Closing, (b) the liquidation of FLAC and (c) the written agreement of the Class B Holders, FLAC, Holdco and the Company. Upon such termination of this Agreement, all obligations of the parties under this Agreement will terminate, without any liability or other obligation on the part of any party hereto to any Person in respect hereof or the transactions contemplated hereby, and no party hereto shall have any claim against another (and no Person shall have any rights against such party), whether under contract, tort or otherwise, with respect to the subject matter hereof; provided, however, that the termination of this Agreement shall not relieve any party hereto from liability arising in respect of any breach of this Agreement prior to such termination. This Article III shall survive the termination of this Agreement.
Section 3.2 Governing Law; Venue. This Agreement shall be governed by and construed in accordance with the Laws of the State of Delaware, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the law of any jurisdiction other than the State of Delaware. Each of the parties irrevocably and unconditionally submits to the exclusive jurisdiction of the Chancery Court of the State of Delaware (or, if the Chancery Court of the State of Delaware declines to accept jurisdiction, any state or federal court within the State of Delaware or, in the event each federal court within the State of Delaware declines to accept jurisdiction, any other Delaware state court), for the purposes of any Proceeding (a) arising under this Agreement or (b) in any way connected with or related or incidental to the dealings of the parties in respect of this Agreement, and irrevocably and unconditionally waives any objection to the laying of venue of any such Proceeding in any such court, and further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such Proceeding has been brought in an inconvenient forum. Each party irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any Proceeding against such party (i) arising under this Agreement or (ii) in any way connected with or related or incidental to the dealings of the parties in respect of this Agreement, (A) any claim that it is not personally subject to the jurisdiction of the courts as described in this Section 3.2 for any reason, (B) that it or its property is exempt or immune from the jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (C) that (1) the Proceeding in any such court is brought against such party in an inconvenient forum, (2) the venue of such Proceeding against such party is improper or (3) this Agreement, or the subject matter hereof, may not be enforced against such party in or by such courts. Each Party agrees that service of any process, summons, notice or document by registered mail to such party’s respective address set forth in Section 3.8 below shall be effective service of process for any such Proceeding.
D-4
Table of Contents
Section 3.3 Waiver of Jury Trial. THE PARTIES EACH HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. THE PARTIES EACH HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE AN ORIGINAL COUNTERPART OF A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) EACH SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) EACH SUCH PARTY MAKES THIS WAIVER VOLUNTARILY AND (D) EACH SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 3.3.
Section 3.4 Assignment. This Agreement and all of the provisions hereof will be binding upon and inure to the benefit of the parties hereto and their respective heirs, successors and permitted assigns. Neither this Agreement nor any of the rights, interests or obligations hereunder will be assigned (including by operation of law) without the prior written consent of all of the other parties hereto.
Section 3.5 Specific Performance. The parties hereto agree that irreparable damage may occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the parties hereto shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in the Chancery Court of the State of Delaware (or, if the Chancery Court of the State of Delaware declines to accept jurisdiction, any state or federal court within the State of Delaware or, in the event each federal court within the State of Delaware declines to accept jurisdiction, any other Delaware state court), this being in addition to any other remedy to which such party is entitled at law or in equity, and in each case, without posting a bond or undertaking and without proof of damages. Each of the parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement on the basis that the other parties have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity.
Section 3.6 Amendment. This Agreement may not be amended, changed, supplemented, waived or otherwise modified or terminated, except upon the execution and delivery of a written agreement executed by FLAC, Holdco, the Company and the Class B Holders. Notwithstanding anything to the contrary contained herein, any holder of Class B Shares may become party to this Agreement by executing and delivering a joinder to this Agreement in the form attached hereto as Annex A. In such event, each such Person shall thereafter shall be deemed a Class B Holder for all purposes under this Agreement.
Section 3.7 Severability. If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this Agreement will remain in full force and effect. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
Section 3.8 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given) by delivery in person, by e-mail (having obtained electronic delivery confirmation thereof (i.e., an electronic record of the sender that the e-mail was sent to the intended recipient thereof without an “error” or similar message that such e-mail was not received
D-5
Table of Contents
by such intended recipient)), or by registered or certified mail (postage prepaid, return receipt requested) (upon receipt thereof) to the other parties as follows:
If to FLAC:
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union St., Suite 3200
Seattle, Washington 98101
Attention: James N. Topper
David Topper
E-mail:
with a copy (which will not constitute notice) to:
Goodwin Procter LLP
100 Northern Avenue
Boston, Massachusetts 02210
Attention: Jocelyn M. Arel
Jacqueline Mercier
E-mail: jarel@goodwinlaw.com
jmercier@goodwinlaw.com
If to the Company, Holdco or Merger Sub:
c/o NewAmsterdam Pharma B.V.
20803 Biscayne Boulevard
Suite 105
Aventura, FL 33180
Attention: Michael Davidson
Email:
with a copy (which shall not constitute notice) to:
c/o NewAmsterdam Pharma B.V.
c/o NewAmsterdam Pharma Holding B.V.
Gooimeer 2-35
1411 DC Naarden
The Netherlands
Attention: Michael Davidson, Chief Executive Officer
Email:
and with a copy (which shall not constitute notice) to
Covington & Burling LLP
The New York Times Building
620 Eighth Avenue
New York, New York 10018
Attention: Jack S. Bodner
Kerry S. Burke
Brian K. Rosenzweig
E-mail: jbodner@cov.com
kburke@cov.com
brosenzweig@cov.com
D-6
Table of Contents
If to the Sponsor or any other Class B Holder:
Frazier Lifesciences Sponsor LLC
Two Union Square
601 Union St., Suite 3200
Seattle, Washington 98101
with a copy (which will not constitute notice) to:
Goodwin Procter LLP
100 Northern Avenue
Boston, Massachusetts 02210
Attention: Jocelyn M. Arel
Jacqueline Mercier
E-mail: jarel@goodwinlaw.com
jmercier@goodwinlaw.com
Section 3.9 Counterparts. This Agreement may be executed in two or more counterparts (any of which may be delivered by electronic transmission), each of which shall constitute an original, and all of which taken together shall constitute one and the same instrument.
Section 3.10 Entire Agreement. This Agreement and the agreements referenced herein constitute the entire agreement and understanding of the parties hereto in respect of the subject matter hereof and supersede all prior understandings, agreements or representations by or among the parties hereto to the extent they relate in any way to the subject matter hereof.
Section 3.11 Further Assurances. From time to time and without additional consideration, each Class B Holder shall execute and deliver, or cause to be executed and delivered, such additional transfers, assignments, endorsements, proxies, consents and other instruments, and shall take such further actions, as FLAC, Holdco or the Company may reasonably request for the purpose of carrying out and furthering the intent of this Agreement.
[Signature page follows]
D-7
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| SPONSOR: | ||
| FRAZIER LIFESCIENCES SPONSOR LLC | ||
| By: | /s/ James N. Topper | |
| Name: James N. Topper | ||
| Title: Manager | ||
[Signature Page to Sponsor Support Agreement]
D-8
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| FLAC: | ||
| FRAZIER LIFESCIENCES ACQUISITION CORPORATION | ||
| By: | /s/ James N. Topper | |
| Name: James N. Topper | ||
| Title: Chief Executive Officer | ||
[Signature Page to Sponsor Support Agreement]
D-9
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| OTHER CLASS B HOLDERS: |
/s/ Robert F. Baltera |
| Robert F. Baltera |
/s/ Michael F. Bigham |
| Michael F. Bigham |
/s/ Carol Gallagher |
| Carol Gallagher |
/s/ David Topper |
| David Topper |
/s/ Krishna R. Polu |
| Krishna R. Polu |
[Signature Page to Sponsor Support Agreement]
D-10
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| COMPANY: | ||
| NEWAMSTERDAM PHARMA HOLDING B.V. | ||
| By: | /s/ Michael H. Davidson | |
| Name: Michael H. Davidson | ||
| Title: Chief Executive Officer | ||
[Signature Page to Sponsor Support Agreement]
D-11
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| HOLDCO: | ||||
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||||
| By: | /s/ Louise Kooij | |||
| Name: | LouFré Management B.V. represented by LouFré Holding B.V. | |||
| Title: | Sole Director | |||
[Signature Page to Sponsor Support Agreement]
D-12
Table of Contents
Schedule I
Class B Holders
Name of Class B Holder | Number and Type of FLAC Shares Beneficially Owned | FLAC Warrants Beneficially Owned | ||||||
Sponsor | 3,300,000 Class B Shares | — | ||||||
Robert F. Baltera | 30,000 Class B Shares | — | ||||||
Michael F. Bigham | 30,000 Class B Shares | — | ||||||
Carol Gallagher | 30,000 Class B Shares | — | ||||||
David Topper | 30,000 Class B Shares | — | ||||||
Krishna R. Polu | 30,000 Class B Shares | — | ||||||
[Schedule I to Sponsor Support Agreement]
D-13
Table of Contents
Exhibit A
Form of Joinder Agreement
The undersigned is executing and delivering this joinder agreement (this “Joinder”) pursuant to the Sponsor Support Agreement, dated as of [●], 2022 (as the same may hereafter be amended, the “Sponsor Support Agreement”), by and among (i) Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company, (ii) the other holders of FLAC Class B Shares set forth on Schedule I thereto, (iii) Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman Islands exempted company, (iv) NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands and (v) NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands. Capitalized terms used but not otherwise defined herein shall have the meanings provided in the Sponsor Support Agreement.
By executing and delivering this Joinder, the undersigned hereby agrees to become a party to, to be bound by, and to comply with the Sponsor Support Agreement as a Class B Holder in the same manner as if the undersigned were an original signatory to the Sponsor Support Agreement. For purposes of the Sponsor Support Agreement and Schedule I thereto, the table below sets forth the name of the undersigned Class B Holder, the number and type of FLAC Shares held by such Class B Holder and the number of FLAC Warrants held by such Class B Holder:
Name of Class B Holder | Number and Type of FLAC Shares Beneficially Owned | FLAC Warrants Beneficially Owned | ||
[Name] | [ ] | [ ] |
Accordingly, the undersigned has executed and delivered this Joinder as of the date written below.
| Date: [●], 2022 | ||||||
| By: |
| |||||
| Name: | ||||||
| Title: | ||||||
| Address for Notices: | ||||||
| With copies to: | ||||||
[Exhibit A to Sponsor Support Agreement]
D-14
Table of Contents
E-1

SUPPORT AGREEMENT
THIS SUPPORT AGREEMENT (THIS “AGREEMENT”) IS MADE ON JULY 25, 2022 BY AND AMONG
| 1. | Those holders of shares in the capital of the Company (the “Company Shares”) listed in Schedule A attached hereto (the “Company Shareholders”); |
| 2. | NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid), incorporated under the laws of the Netherlands with its corporate seat in Naarden and registered office at Gooimeer 2 35, 1411 DC Naarden, the Netherlands, and registered with the Dutch trade register under number 76133141 (the “Company”); |
| 3. | NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid), incorporated under the laws of the Netherlands with its corporate seat in Naarden and registered office at Gooimeer 2 35, 1411 DC Naarden, the Netherlands, and registered with the Dutch trade register under number 86649051 (“Holdco”); |
| 4. | NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company (“Merger Sub”); and |
| 5. | Frazier Lifesciences Acquisition Corporation, a Cayman Islands exempted company (“FLAC” and, together with the Company Shareholders, the Company, Holdco and Merger Sub, the “Parties”). |
WHEREAS
| A. | Each Company Shareholder has sole voting power with respect to the number and type of Company Shares indicated opposite such Company Shareholder’s name on Schedule A attached hereto. |
| B. | The Company Shareholders and the Company are parties to a Shareholders’ Agreement with regard to their respective shareholdings in the Company, dated 11 January 2021 (as amended or supplemented from time to time, the “SHA”). |
| C. | Concurrently with the effectiveness of this Agreement, the Company, Holdco, Merger Sub and FLAC are entering into a Business Combination Agreement (as amended or supplemented from time to time, the “BCA”), pursuant to and subject to the terms and conditions of which, the Company, Holdco, FLAC and Merger Sub are required, among other matters, to consummate the transactions contemplated thereby (collectively, the “Transactions”), including the following: |
| a) | Company Share Exchange – Each holder of Company Shares will enter into one or more notarial deeds of issue with Holdco (each, a “Deed of Issue”) under which (i) Holdco shall issue to each such holder a number of Holdco ordinary shares (“Holdco Shares”) to which such holder is entitled pursuant to the applicable provisions of the BCA and (ii) in fulfilment of such holder’s respective obligations to pay up the respective Holdco Shares issued to such holder under such Deed of Issue by payment in kind, such holder shall contribute and transfer all of its Company Shares to Holdco (the “Company Share Exchange”). |
| b) | Holdco reorganization – Holdco shall (i) change its legal form from a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) to a public limited liability company (naamloze vennootschap) and (ii) amend and restate its articles of association to be suitable for a company whose shares are listed on The Nasdaq Stock Market LLC (“Nasdaq”). |
Table of Contents

| c) | Merger – Merger Sub shall merge with and into FLAC, with FLAC surviving such merger as a wholly owned subsidiary of Holdco (the “Merger”). By virtue of the Merger (i) each of FLAC’s Class A ordinary shares, par value $0.0001 (the “FLAC Class A Shares”), and FLAC’s Class B ordinary shares, par value $0.0001, issued and outstanding immediately prior to the effective time of the Merger (the “Effective Time”) shall be automatically cancelled and extinguished in exchange for one Holdco Share and (ii) each warrant to purchase one or more FLAC Class A Shares that is outstanding immediately prior to the Effective Time shall automatically cease to represent a right to acquire FLAC Class A Shares and shall automatically represent, immediately following the Effective Time, a right to acquire an equivalent number of Holdco Shares pursuant to a warrant assumption agreement to be entered into by Holdco immediately prior the Effective Time. |
| d) | Domestication – Immediately after the Effective Time, the separate existence of Merger Sub shall cease and FLAC shall continue as the surviving entity of the Merger (the “Surviving Company”), and the Surviving Company shall domesticate as a Delaware corporation. |
| e) | Nasdaq listing – Effective upon the Merger and upon satisfaction of all initial listing requirements, the Holdco Shares shall become listed on Nasdaq. |
| f) | PIPE financing – Certain investors shall subscribe for and purchase, and Holdco shall issue and sell to those investors, an aggregate number of Holdco Shares in exchange for a purchase price of $10.00 per Holdco Share, substantially concurrently with the closing of the Merger (the “Closing”). |
| g) | Ancillary Documents – The relevant parties shall enter into such other agreements, documents, instruments or certificates contemplated by the BCA to be executed by the Company, Holdco, FLAC and Merger Sub, as applicable (the “Ancillary Documents”). |
| D. | In order to facilitate the consummation of the Transactions, the Parties are entering into this Agreement. |
THE PARTIES NOW HEREBY AGREE AS FOLLOWS
| 1 | INTERPRETATION |
| 1.1 | References to statutory provisions are to those provisions as they are in force from time to time. |
| 1.2 | Terms that are defined in the singular have a corresponding meaning in the plural and vice versa. |
| 1.3 | Words denoting a gender include each other gender. |
| 1.4 | Except as otherwise required by law, the terms “written” and “in writing” include by use of electronic means of communication. |
| 1.5 | No provision of this Agreement shall be interpreted adversely against a Party solely because that Party was responsible for drafting that particular provision. |
| 1.6 | Although this Agreement has been drafted in the English language, this Agreement pertains to Dutch legal concepts. Any consequence of the use of English words and expressions in this Agreement under any law other than Dutch law shall be disregarded. |
| 1.7 | The words “include”, “included” and “including” are used to indicate that the matters listed are not a complete enumeration of all matters covered. |
| 1.8 | The titles and headings in this Agreement are for construction purposes as well as for reference. No Party may derive any rights from such titles and headings. |
| 1.9 | Capitalized terms used but not defined in this Agreement shall have the respective meanings ascribed to them in the BCA. |
E-2
Table of Contents

| 2 | CONDITIONALITY |
| 2.1 | In this Agreement, the “Conditions” are the following conditions precedent: |
| a. | the approval of the BCA by the Company Board; |
| b. | the execution of this Agreement by Company Shareholders collectively constituting the Investor Majority (as defined in the SHA), thereby granting their prior written consent to this Agreement becoming effective; and |
| c. | the execution of the BCA by the Company, Holdco, Merger Sub and FLAC. |
| 3 | UNDERTAKINGS |
| 3.1 | Subject only to the satisfaction of the Conditions, and unless the Expiration Time (as defined below) shall have occurred, each Company Shareholder hereby irrevocably and unconditionally undertakes vis-à-vis each of the Company, Holdco, FLAC and each other Company Shareholder to: |
| a. | appear at any meeting of the holders of Company Shares, or any adjournment or postponement thereof, with respect to the approval of the BCA, any of the Transactions, or any other matters necessary or reasonably requested by the Company for consummation of the Transactions with respect to the Company Shares held by such Company Shareholder, or otherwise cause such Company Shares to be counted as present thereat for purposes of calculating a quorum, and vote (or cause to be voted) (i) in favour of approval of the BCA, the Company Share Exchange, and any other matters necessary or reasonably requested by the Company for consummation of the Transactions, and (ii) against any proposal that conflicts or materially impedes or interferes therewith, including any Company Acquisition Proposal, or would adversely affect or delay the consummation of the Transactions; |
| b. | if so required or applicable, execute and deliver to the Company, a written consent voting all Company Shares held by such Company Shareholder in favour of approval of the BCA, the Company Share Exchange, and any other matters necessary or reasonably requested by the Company for consummation of the Transactions; and |
| c. | take all necessary or desirable actions in connection with the Transactions to consummate the Company Share Exchange (and any other Transaction to which such Company Shareholder is a party) in accordance with the terms of the BCA. |
| 3.2 | Each Company Shareholder hereby irrevocably and unconditionally undertakes vis-à-vis each of the Company, Holdco, FLAC and each other Company Shareholder, to execute and deliver, immediately following the execution of this Agreement by such Company Shareholder, an irrevocable power of attorney substantially in the form attached hereto as Schedule B (the “Power of Attorney”) and to have such Power of Attorney notarized, apostilled or accompanied by confirmations from local counsel in accordance with the instructions set forth underneath the signature block thereof, provided, however, that such Power of Attorney (and the performance of any act pursuant thereto) shall be subject only to the satisfaction of the Conditions. |
| 4 | RESTRICTIONS ON TRANSFER |
| 4.1 | Each Company Shareholder agrees that, prior to the Expiration Time (as defined below), he, she or it shall: |
| a. | only sell, assign, transfer or otherwise dispose of any Company Shares (collectively, a “Transfer”) (i) in compliance with all applicable securities Laws, (ii) in compliance with the Governing Documents of the Company, (iii) in compliance with the BCA and the SHA and (iv) if, prior to such Transfer, each transferee signs a counterpart to this Agreement pursuant to which such transferee agrees to be bound by the terms of this Agreement and to be a “Company Shareholder” hereunder; |
E-3
Table of Contents

| provided that any subsequent transfer of Company Shares by any such transferee shall also be made pursuant to, and in accordance with, all of the provisions of this Clause 4 to the same extent as if each such transferee were a Company Shareholder; and |
| b. | not, directly or indirectly, (i) pledge, encumber or create a Lien on any Company Shares or enter into any contract, option, commitment or other arrangement or understanding with respect to the foregoing; (ii) grant any proxies or powers of attorney or enter into a voting agreement or other arrangement with respect to any of the Company Shares held by such Company Shareholder; (iii) enter into, or deposit any of such Company Shares into, a voting trust or take any other action which would, or would reasonably be expected to, result in a diminution of the voting power represented by any of the Company Shares held by such Company Shareholder; or (iv) commit or agree to take any of the foregoing actions. |
| 4.2 | Each Company Shareholder agrees to promptly notify FLAC in writing of any changes or updates to Schedule A hereto as it relates to such Company Shareholder after the date hereof. |
| 5 | REPRESENTATIONS AND WARRANTIES |
Each Company Shareholder hereby represents and warrants to the Company, Holdco, Merger Sub and FLAC as follows:
| 5.1 | Such Company Shareholder has the full power and authority to execute and deliver this Agreement and to perform such Company Shareholder’s obligations hereunder. |
| 5.2 | This Agreement has been duly executed and delivered by such Company Shareholder and, assuming due authorization, execution and delivered by the other Parties, constitutes a valid, legal and binding agreement with respect to such Company Shareholder, enforceable against such Company Shareholder in accordance with its terms, subject to the Enforceability Exceptions. |
| 5.3 | Such Company Shareholder owns the number of Company Shares indicated opposite such Company Shareholder’s name on Schedule A attached hereto, free and clear of any Liens (other than Liens created by this Agreement, applicable securities Laws, the Company’s Governing Documents, the SHA, and Permitted Liens), and has sole voting and investment power with respect to such Company Shares. None of such Company Shares are subject to any voting trust or other agreement, arrangement or restriction with respect to the voting thereof, and no Person has any right to acquire from such Company Shareholder any of such Company Shares. |
| 5.4 | The execution and delivery of this Agreement by such Company Shareholder, the consummation by such Company Shareholder of the transactions contemplated hereunder and the performance by such Company Shareholder of his, her or its obligations hereunder do not and will not (i) conflict with, or result in any violation or breach of, or default (with or without notice or lapse of time or both) under, any Contract or any judgment to which such Company Shareholder is a party or by which such Holder is bound, or any Law to which such Company Shareholder is subject or, in the event that such Company Shareholder is a corporation, company, partnership, limited liability company, joint venture, association, trust, business trust or other entity, any Governing Document of such Company Shareholder, or (ii) require any consent, approval, qualification, order or authorization of, registration, declaration or filing with, or notice to, any Governmental Entity by such Company Shareholder except for applicable requirements, if any, of the Exchange Act, and except where the failure to obtain such consents, approvals, qualifications, orders or authorizations or registrations, declarations or filings, would not prevent or impair in any material respect the performance by such Company Shareholder of his, her or its obligations under this Agreement. |
| 5.5 | No investment banker, broker, finder, consultant or intermediary or other Person is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission based upon arrangements made by or on behalf of such Company Shareholder in connection with its entering into this Agreement. |
E-4
Table of Contents

| 6 | MISCELLANEOUS PROVISIONS |
| 6.1 | Amendment |
| 6.1.1 | No amendment to this Agreement shall have any force or effect unless it is in writing and signed by all Parties. |
| 6.2 | No rescission or nullification |
| 6.2.1 | To the extent permitted by law, the Parties hereby waive their rights to rescind or nullify or to demand the rescission, nullification or amendment of this Agreement, in whole or in part, on any grounds whatsoever. |
| 6.3 | No transfer, assignment or encumbrance |
| 6.3.1 | No Party may transfer, assign or encumber its contractual relationship, any of its rights or any of its obligations under this Agreement without the prior written approval of the other Party. |
| 6.4 | Term and termination |
| 6.4.1 | This Agreement shall remain in full force and effect for an indefinite period, until the earliest to occur of (such earliest time, the “Expiration Time”) (i) the completion of the Company Share Exchange at the Closing, (ii) such date and time as the BCA shall be terminated pursuant to Article 7 thereof and (iii) upon mutual written agreement of the Parties. |
| 6.4.2 | In the event of the termination of this Agreement pursuant to Clause 6.5, this entire Agreement shall become void (and there shall be no Liability or obligation on the part of the Parties and their respective Representatives), except that the provisions of Clause 1, this Clause 6 and Clause 7 shall remain in full force and effect and survive any termination of this Agreement. |
| 6.5 | Counterparts |
| 6.5.1 | This Agreement may be executed in multiple counterparts, each of which shall be deemed an original and all of which taken together shall constitute one and the same instrument. |
| 6.6 | Notices |
| 6.6.1 | All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given) by delivery in person, by e-mail (having obtained electronic delivery confirmation thereof (i.e., an electronic record of the sender that the e-mail was sent to the intended recipient thereof without an “error” or similar message that such e-mail was not received by such intended recipient)), or by registered or certified mail (postage prepaid, return receipt requested) (upon receipt thereof) to the other Parties as follows: |
| a. | if to the Company, Holdco or Merger Sub: | |
NewAmsterdam Pharma Holding B.V.; | ||
NewAmsterdam Pharma Company B.V.; and | ||
NewAmsterdam Pharma Investment Corporation | ||
Gooimeer 2 35 1411 DC, Naarden, the Netherlands | ||
Attention: Michael Davidson | ||
Louise Kooij | ||
E-mail: | ||
E-5
Table of Contents

with a copy (which shall not constitute notice) to: | ||
Covington & Burling LLP | ||
The New York Times Building 620 Eighth Avenue | ||
New York, NY 10018 | ||
Attention: Jack S. Bodner | ||
Kerry S. Burke | ||
Brian K. Rosenzweig | ||
Facsimile: 646-441-9079 | ||
E-mail: jbodner@cov.com | ||
kburke@cov.com | ||
brosenzweig@cov.com | ||
| b. | if to FLAC: | |
Frazier Lifesciences Acquisition Corporation | ||
Two Union Square | ||
601 Union St., Suite 3200 | ||
Seattle, Washington 98101 | ||
Attention: James N. Topper | ||
David Topper | ||
E-mail: | ||
with a copy (which shall not constitute notice) to: | ||
Goodwin Procter LLP | ||
100 Northern Avenue | ||
Boston, Massachusetts 02210 | ||
Attention: Jocelyn M. Arel | ||
Jacqueline Mercier | ||
E-mail: jarel@goodwinlaw.com | ||
jmercier@goodwinlaw.com | ||
| c. | if to a Company Shareholder, to the address set forth under such Company Shareholder’s signature on the signature page hereto. |
| 6.7 | Capacity as a shareholder |
| 6.7.1 | Notwithstanding anything herein to the contrary, each Company Shareholder signs this Agreement solely in such Company Shareholder’s capacity as a shareholder of the Company, and not in any other capacity, and this Agreement shall not limit or otherwise affect the actions of any affiliate, employee, or designee of the such Company Shareholder or any of its Affiliates in his or her capacity, if applicable, as an officer or director of the Company or any other Person. |
| 7 | GOVERNING LAW AND JURISDICTION |
| 7.1 | This Agreement shall be governed by and construed in accordance with the laws of the Netherlands. |
| 7.2 | The Parties agree that any dispute in connection with this Agreement or any agreement resulting therefrom shall be submitted to the exclusive jurisdiction of the competent court in Amsterdam, the Netherlands. |
(signature page follows)
E-6
Table of Contents

Signature page to the Support Agreement
Forbion Capital Fund IV Coöperatief U.A., represented by:
| /s/ H.A. Slootweg | /s/ G.J. Mulder | |||
Name: Forbion IV Management B.V. Title: Director By: FCPM III Services B.V. Title: Director By: H.A. Slootweg Title: Director |
|
Name: Forbion IV Management B.V. Title: Director By: FCPM III Services B.V. Title: Director By: G.J. Mulder Title: Director |
Address for notices:
Address:
Attention
E-mail:
With copy to:
Forbion Growth Opportunities Fund I Coöperatief U.A., represented by:
| /s/ D.A.F. Kersten | /s/ W.S.J. Joustra | |||
Name: Forbion Growth Management B.V. Title: Director By: D.A.F. Kersten Title: Proxy Holder |
|
Name: Forbion Growth Management B.V. Title: Director By: W.S.J. Joustra Title: Proxy Holder |
E-7
Table of Contents

Signature page to the Support Agreement
NAP PoolCo B.V., represented by:
| /s/ H.A. Slootweg | /s/ G.J. Mulder | |||
Name: Forbion International Management B.V. Title: Director Name: H.A. Slootweg Title: Director |
|
Name: Forbion International Management B.V. Title: Director Name: G.J. Mulder Title: Director |
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-8
Table of Contents

Signature page to the Support Agreement
Morningside Venture Investments Limited, represented by:
| /s/ F.A.E. Richard | /s/ J.M. Franklin | |||
Name: Frances Anne Elizabeth Richard Title: Authorized Signatory |
|
Name: Jill Marie Franklin Title: Authorized Signatory |
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-9
Table of Contents

Signature page to the Support Agreement
Ascendant BioCapital SPV I, LLC – Series 1, represented by Ascendant BioCapital Fund I GP, LLC, in its turn represented by:
| /s/ Guarav Gupta | ||||
Name: G. Gupta Title: Managing Member |
|
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-10
Table of Contents

Signature page to the Support Agreement
Population Health Equity Partners V, L.P., represented by its general partner Population Health Equity Partners V GP, LLC, on its turn represented by:
| /s/ Chris Cox | ||||
Name: C. Cox Title: President | |
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-11
Table of Contents

Signature page to the Support Agreement
Kaiser Permanente Group Trust, represented by Kaiser Foundation Health Plan, Inc. and Investment Committee for the Kiser Permanente Retirement Plans, as named fiduciaries for the plans that participate in the Kaiser Permanente Group Trust:
| /s/ Thomas Lurquin | ||||
Name: T. Lurquin Title: VP - Pensions and Investments | |
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-12
Table of Contents

Signature page to the Support Agreement
NewAmsterdam Pharma Holding B.V., represented by:
| /s/ M.H. Davidson | /s/ J.P.P. Kastelein | |||
Name: M.H. Davidson Title: CEO |
|
Name: Wester Investments B.V. Title: CSO Name: J.J.P. Kastelein Title: Director |
M.H. Davidson
| ||||
/s/ M.H. Davidson |
Address for notices:
Address:
Attention
E-mail:
With copy to:
E-13
Table of Contents

Signature page to the Support Agreement
NewAmsterdam Pharma Company B.V., represented by:
| /s/ Louise Kooij | ||||
Name: LouFré Management B.V. Title: Director Name: LouFré Holding B.V. Title: Director Name: L.F. Kooij Title: Director | ||||
NewAmsterdam Pharma Investment Corporation, represented by:
| ||||
| /s/ Louise Kooij | ||||
Name: LouFré Management B.V. Title: Director Name: LouFré Holding B.V. Title: Director Name: L.F. Kooij Title: Director | | |||
E-14
Table of Contents

Signature page to the Support Agreement
Frazier Lifesciences Acquisition Corporation, represented by:
/s/ James N. Topper |
Name: James N. Topper Title: Chief Executive Officer |
E-15
Table of Contents

SCHEDULE A
[Schedule A]
E-16
Table of Contents

SCHEDULE B
POWER OF ATTORNEY
THE UNDERSIGNED
[Option 1. Principal is a natural person]
[first and given names] [surname], born in [place], [country], on the [day] day of [month] nineteen hundred and [year], residing at [private address], [married/neither married nor registered as partner], holder of a[n] [country][Dutch] passport with number [number] (the “Principal”),
[Option 2. Principal is a legal entity][Note: authority statement to be included in Annex C]
[name entity], a [company] under the laws of [jurisdiction], having its registered office at [address], and registered with the [name foreign companies registrar] under registration number [number] (the “Principal”),
HEREBY DECLARES
| 1. | Capitalised terms used herein have the meanings ascribed thereto in the support agreement to which this power of attorney is a schedule (the “Support Agreement”). |
| 2. | The Principal grants an irrevocable power of attorney to each individual civil law notary, assigned civil law notary, candidate civil law notary, lawyer, notarial assistant and paralegal working with NautaDutilh N.V. (each: an “Attorney”). |
| 3. | The scope of this power of attorney extends to the performance of the following acts for and on behalf of the Principal, in each case in connection with the consummation of the Transactions, involving in particular (a) the Company Share Exchange, (b) the conversion of Holdco into a public company under Dutch law, (c) the Merger, (d) the domestication of the surviving entity of the Merger, (e) the Holdco Shares becoming listed on Nasdaq and (f) the PIPE financing concurrently with Closing, together with all other actions which are necessary, advisable or customary to implement the Transactions: |
| a. | to appear before any (assigned) civil law notary of NautaDutilh N.V. (or one of their deputies) as a party to a notarial deed drawn up by NautaDutilh N.V., pursuant to which Holdco (at that time still in the legal form of a private company with limited liability) will issue shares in its capital to the Principal, and the Principal will acquire shares in the capital of Holdco, by way of a capital increase against contribution in kind of all shares, irrespective of class or designation, held by the Principal at the time of such contribution in the capital of the Company; |
| b. | if applicable, to sign, execute and deliver on behalf of the Principal, in connection with consummation of the Transactions, a private deed of transfer of shares in the capital of Holdco (at that time converted into the legal form of a public company with limited liability) drawn up by NautaDutilh N.V., pursuant to which the Principal’s shares in Holdco’s capital will be transferred by the Principal to the Holdco’s U.S. transfer agent for delivery of those shares in book-entry form on a securities account administered in the Principal’s name, or otherwise at the direction of the Principal; |
| c. | to appear at any meeting of the holders of Company, or any adjournment or postponement thereof, with respect to the approval of the BCA, any of the Transactions, or any other matters necessary or reasonably requested by the Company for consummation of the Transactions with respect to the Company Shares held by the Principal, or otherwise cause the Principal’s Company Shares to be counted as present thereat for purposes of calculating a quorum, and vote (or cause to be voted) (i) in favour of approval of the BCA, the Company Share Exchange, and any other matters necessary or reasonably requested by the Company for consummation of the Transactions, and (ii) against any proposal that conflicts or materially impedes or interferes therewith, including any Company Acquisition Proposal, or would adversely affect or delay the consummation of the Transactions; |
E-17
Table of Contents

| d. | if applicable, to execute and deliver to the Company, a written resolution of the holders of all Company Shares voting in favour of approval of the BCA, the Company Share Exchange, and any other matters necessary or reasonably requested by the Company for consummation of the Transactions; and |
| e. | to sign, execute and deliver any private and notarial deed, agreement, statement, declaration, form or other document and to perform any other acts, including acts of disposition (beschikkingshandelingen), on behalf of the Principal that an Attorney considers necessary, useful or advisable in connection with the Transactions and the performance of the matters described above. |
| 4. | Each Attorney is authorised to act also as counterparty to the Principal or as an attorney-in-fact of any such counterparty. |
| 5. | This power of attorney is granted with full power of substitution and subject to each Attorney’s right to indemnification for all acts performed under this power of attorney. |
| 6. | The relationship between the Principal and each Attorney under this power of attorney is governed exclusively by the laws of the Netherlands. |
(signature page follows)
E-18
Table of Contents

Signature page to a power of attorney.
[Principal], [represented by]:
Name: Title: | |
Name: Title: | ||
| Date: | Date: |
Please observe the following requirements when executing this power of attorney:
| • | A copy of a valid passport of each individual signing this power of attorney must be attached. |
| • | Each signature on this power of attorney must be notarized. If notarized outside the Netherlands, this power of attorney must be apostilled. Alternatively, this process can be completed through a video meeting with a representative of NautaDutilh. Please contact NautaDutilh to set this up (contact details below). During such video conference, the signatory has to (i) show his/her original passport, (ii) sign a printed copy of such passport (by wet ink signature) and (iii) sign a printed copy of this power of attorney (by wet ink signature). Please do not sign the printed copy of the passport and/or this power of attorney in advance of the video conference. |
| • | This power of attorney must be signed by wet-ink signature (i.e. digital signatures will not be accepted). |
| • | If the Principal is an entity not incorporated under Dutch law: a lawyer practicing in the country of incorporation of the Principal must issue the confirmation statement substantially in the form of Annex A hereto. If you wish to make changes to this format, please contact NautaDutilh (contact details below). |
| • | Following execution, please send a scan copy of the signed documents by e-mail, followed by the originals. Please use the following contact details: NautaDutilh N.V., Attn. Marloes van der Laan, Beethovenstraat 400, 1082 PR Amsterdam, The Netherlands, E-mail: |
| • | Please note that additional KYC-documentation may be requested, all to the satisfaction of the notary. |
E-19
Table of Contents

ANNEX A
[Letterhead counsel]
NautaDutilh N.V.
Attn. P.C.S. van der Bijl / M.L. van der Laan
Beethovenstraat 400
1082 PR Amsterdam
the Netherlands
[Place], [date]
Dear Sir,
I am a [lawyer admitted to the bar of] / [notary practicing in] [jurisdiction].
[name entity], a [company] under the laws of [jurisdiction], having its registered office at [address], and registered with the [name foreign companies registrar] under registration number [number] (the “Company”) has requested me to make the following statements with regard to the power of attorney (the “Power of Attorney”) of which a copy is attached hereto.
For the purposes of making these statements I have reviewed the Power of Attorney, the Company’s organisational documents and such other documents as I have deemed necessary.
I hereby state that [signatory/signatories], at the time of the execution of the Power of Attorney, had the power to represent the Company for the purposes of the execution of the Power of Attorney in the name and on behalf of the Company and that the Company has the power to enter into the transactions described in the Power of Attorney.
Yours sincerely,
[Name counsel]]
E-20
Table of Contents
FORM OF INVESTOR SUPPORT AGREEMENT
THIS INVESTOR SUPPORT AGREEMENT (this “Agreement”) is dated as of July 25, 2022 by and among Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman exempted company (“FLAC”), and the shareholder of FLAC whose name appears on the signature page of this Agreement (the “Investor”). Capitalized terms used but not defined herein shall have the respective meanings ascribed to such terms in the Business Combination Agreement (as defined below).
RECITALS
WHEREAS, FLAC, NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (the “Company”), NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands, which was formed by the Company for the sole purpose of consummating the transactions contemplated by the Business Combination Agreement (as defined below), and which shall convert into a public limited liability company (naamloze vennootschap) incorporated under the laws of the Netherlands prior to the Merger (as defined below) (“Holdco”), and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and a direct wholly owned subsidiary of Holdco (“Merger Sub”), have entered into that certain Business Combination Agreement, dated as of the date hereof (as it may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), pursuant to which, among other things, (i) the Company shall cause each Company Shareholder to contribute and transfer each Company Share held by it to Holdco and Holdco shall accept such contribution and in exchange issue to such holder such number of Holdco Shares that is equal to the Applicable Exchange Consideration Per Share with respect to such Company Share and (ii) Merger Sub will merge with and into FLAC, with FLAC surviving as a wholly owned subsidiary of Holdco, on the terms and subject to the conditions therein (the “Merger”);
WHEREAS, as of the date hereof, the Investor beneficially owns (as defined in Rule 13d-3 under the Exchange Act), and has sole voting power with respect to the number and type of FLAC Shares, and owns the FLAC Warrants, as indicated on the signature page hereto;
WHEREAS, as an inducement to FLAC and the Company to enter into the Business Combination Agreement and to consummate the transactions contemplated therein, the parties hereto desire to agree to certain matters as set forth herein.
NOW, THEREFORE, in consideration of the foregoing and the mutual agreements contained herein, and intending to be legally bound hereby, the parties hereto hereby agree as follows:
ARTICLE I
COVENANTS
Section 1.1 Agreement to Vote. The Investor hereby agrees to appear and vote at any duly called meeting of the shareholders of FLAC (or any adjournment or postponement thereof), provide his, her or its written consent in any action by written resolution of the shareholders of FLAC, or in any other circumstance in which the vote, consent or other approval of the shareholders of FLAC is sought, all of such FLAC Shares, and in each case cause all the FLAC Shares held by the Investor to be counted as present thereat for purposes of calculating a quorum, and vote or provide his, her or its consent: (a) in favor of the Business Combination Agreement and the Transactions, including in favor of each Transaction Proposal, (b) in favor of any other matter reasonably
F-1
Table of Contents
necessary or required to the consummation of the Transactions and considered and voted upon by the shareholders of FLAC and (c) against any proposal that conflicts or materially impedes or interferes therewith, including any FLAC Acquisition Proposal, or would adversely affect or delay the consummation of the Transactions.
Section 1.2 No Redemption. The Investor hereby agrees, for the benefit of FLAC, not to redeem, or to submit a request to FLAC’s transfer agent to redeem or otherwise exercise any right to redeem, any FLAC Shares and to reverse and revoke any prior redemption elections made with respect to the FLAC Shares.
Section 1.3 No Transfer. During the period commencing on the date hereof and ending on the earlier of (a) the Effective Date and (b) such date and time as the Business Combination Agreement shall be validly terminated in accordance with Section 7.1 thereof, the Investor shall not (i) sell, offer to sell, contract or agree to sell, assign, hypothecate, pledge, create a Lien on, grant any option to purchase, transfer, or otherwise dispose of or agree to dispose of, directly or indirectly, file (or participate in the filing of) a registration statement with the SEC (other than the Registration Statement/Proxy Statement), deposit into a voting trust, grant any proxy or power of attorney with respect to, or establish or increase a put equivalent position or liquidate or decrease a call equivalent position (within the meaning of Section 16 of the Exchange Act) with respect to, any FLAC Shares or FLAC Warrants held by the Investor, (ii) enter into any swap or other arrangement that transfers to another Person, in whole or in part, any of the economic consequences of ownership of any FLAC Shares or FLAC Warrants held by the Investor (clauses (i) and (ii) collectively, a “Transfer”) or (iii) publicly announce any intention to effect any transaction specified in clause (i) or (ii); provided, however, that the foregoing shall not prohibit Transfers from the Investor to any of the Investor’s Affiliates, so long as, prior to and as a condition to the effectiveness of any such Transfer, such Affiliate executes and delivers to FLAC a joinder to this Agreement in the form attached hereto as Annex A.
Section 1.4 New Shares. In the event that (a) any FLAC Shares, FLAC Warrants or other Equity Securities of FLAC are issued to the Investor or any of its Affiliates after the date of this Agreement pursuant to any stock dividend, stock split, recapitalization, reclassification, combination, conversion or exchange of FLAC Shares or FLAC Warrants of, on or affecting the FLAC Shares or FLAC Warrants owned by the Investor or otherwise, (b) the Investor purchases or otherwise acquires beneficial ownership of any FLAC Shares, FLAC Warrants or other Equity Securities of FLAC after the date of this Agreement, or (c) the Investor acquires the right to vote or share in the voting of any FLAC Shares or other Equity Securities of FLAC after the date of this Agreement (such FLAC Shares, FLAC Warrants or other Equity Securities issued, purchased or acquired as described in any of the foregoing clauses (a) through (c), collectively, the “New Securities”), then (x) the Investor shall notify FLAC in writing and as promptly as practicable of any such New Securities and (y) such New Securities shall be subject to the terms of this Agreement to the same extent as if they constituted the FLAC Shares or FLAC Warrants owned by the Investor as of the date hereof.
ARTICLE II
REPRESENTATIONS AND WARRANTIES
Section 2.1 Representations and Warranties of the Investor. The Investor represents and warrants as of the date hereof to FLAC as follows:
(a) Organization; Due Authorization. The Investor has the full power and authority to execute and deliver this Agreement and to perform the Investor’s obligations hereunder. If the Investor is an entity, it is duly organized, validly existing and in good standing under the Laws of the jurisdiction in which it is incorporated, formed, organized or constituted, and the execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby are within such Investor’s corporate, limited liability company or organizational powers and have been duly authorized by all necessary corporate, limited liability company or organizational actions on the part of such Investor. This Agreement has been duly executed and delivered by the Investor and, assuming due authorization, execution and delivery by the other parties to this
F-2
Table of Contents
Agreement, this Agreement constitutes a legally valid and binding obligation of the Investor, enforceable against the Investor in accordance with the terms hereof (except as enforceability may be limited by the Enforceability Exceptions). If this Agreement is being executed in a representative or fiduciary capacity, the Person signing this Agreement has full power and authority to enter into this Agreement on behalf of the Investor.
(b) Ownership. The Investor is the record and beneficial owner (as defined in the Exchange Act) of, and has good title to the FLAC Shares and FLAC Warrants set forth on the signature page hereto, and there exist no Liens or any other limitation or restriction (including any restriction on the right to vote, sell or otherwise dispose of such FLAC Shares or FLAC Warrants (other than transfer restrictions under the Securities Act)) affecting any such FLAC Shares or FLAC Warrants, other than Liens pursuant to (i) this Agreement, (ii) FLAC’s Governing Documents, (iii) the Business Combination Agreement, or (iv) any applicable securities Laws. The FLAC Shares and FLAC Warrants set forth on the signature page hereto are the only Equity Securities in FLAC owned of record or beneficially by the Investor on the date of this Agreement, and none of the Investor’s FLAC Shares or FLAC Warrants are subject to any proxy, voting trust or other agreement or arrangement with respect to the voting of such FLAC Shares or FLAC Warrants, except as provided hereunder. Other than the FLAC Warrants, the Investor does not hold or own any rights to acquire (directly or indirectly) any Equity Securities of FLAC or any securities convertible into, or which can be exchanged for, Equity Securities of FLAC.
(c) No Conflicts. The execution and delivery of this Agreement by the Investor does not, and the performance by the Investor of its obligations hereunder will not, (i) conflict with or result in a violation of the organizational documents of the Investor or (ii) require any consent or approval that has not been given or other action that has not been taken by any Person (including under any Contract binding upon the Investor or the Investor’s FLAC Shares or FLAC Warrants), in each case, to the extent such consent, approval or other action would prevent, enjoin or materially delay the performance by the Investor of its, his or her obligations under this Agreement, the Business Combination Agreement or the transactions contemplated hereby or thereby.
(d) Litigation. There are no Proceedings pending against the Investor, or, to the knowledge of the Investor, threatened against the Investor, before (or, in the case of threatened Proceedings, that would be before) any arbitrator or any Governmental Entity, which in any manner challenges or seeks to prevent, enjoin or materially delay the performance by the Investor of its, his or her obligations under this Agreement, the Business Combination Agreement or the transactions contemplated hereby or thereby.
(e) No Brokers. No investment banker, broker, finder, consultant or intermediary or other Person is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission based upon arrangements made by or on behalf of the Investor in connection with its entering into this Agreement.
(f) Acknowledgment. The Investor understands and acknowledges that each of FLAC, Holdco and the Company is entering into the Business Combination Agreement in reliance upon the Investor’s execution and delivery of this Agreement.
(g) No Other Representations or Warranties. Except for the representations and warranties made by the Investor in this Article II, neither the Investor nor any other Person makes any express or implied representation or warranty to FLAC, Holdco or the Company in connection with this Agreement or the transactions contemplated by this Agreement, and the Investor expressly disclaims any such other representations or warranties.
ARTICLE III
MISCELLANEOUS
Section 3.1 Termination. This Agreement and all of its provisions shall terminate and be of no further force or effect upon the earlier of: (a) the valid termination of the Business Combination Agreement in accordance with Section 7.1 thereof prior to the Closing, (b) the liquidation of FLAC and (c) the written agreement of the
F-3
Table of Contents
Investor, FLAC, Holdco and the Company. Upon such termination of this Agreement, all obligations of the parties under this Agreement will terminate, without any liability or other obligation on the part of any party hereto to any Person in respect hereof or the transactions contemplated hereby, and no party hereto shall have any claim against another (and no Person shall have any rights against such party), whether under contract, tort or otherwise, with respect to the subject matter hereof; provided, however, that the termination of this Agreement shall not relieve any party hereto from liability arising in respect of any breach of this Agreement prior to such termination. This Article III shall survive the termination of this Agreement.
Section 3.2 Governing Law; Venue. This Agreement shall be governed by and construed in accordance with the Laws of the State of Delaware, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of the law of any jurisdiction other than the State of Delaware. Each of the parties irrevocably and unconditionally submits to the exclusive jurisdiction of the Chancery Court of the State of Delaware (or, if the Chancery Court of the State of Delaware declines to accept jurisdiction, any state or federal court within the State of Delaware or, in the event each federal court within the State of Delaware declines to accept jurisdiction, any other Delaware state court), for the purposes of any Proceeding (a) arising under this Agreement or (b) in any way connected with or related or incidental to the dealings of the parties in respect of this Agreement, and irrevocably and unconditionally waives any objection to the laying of venue of any such Proceeding in any such court, and further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such Proceeding has been brought in an inconvenient forum. Each party irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any Proceeding against such party (i) arising under this Agreement or (ii) in any way connected with or related or incidental to the dealings of the parties in respect of this Agreement, (A) any claim that it is not personally subject to the jurisdiction of the courts as described in this Section 3.2 for any reason, (B) that it or its property is exempt or immune from the jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (C) that (1) the Proceeding in any such court is brought against such party in an inconvenient forum, (2) the venue of such Proceeding against such party is improper or (3) this Agreement, or the subject matter hereof, may not be enforced against such party in or by such courts. Each Party agrees that service of any process, summons, notice or document by registered mail to such party’s respective address set forth in Section 3.8 below shall be effective service of process for any such Proceeding.
Section 3.3 Waiver of Jury Trial. THE PARTIES EACH HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. THE PARTIES EACH HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE AN ORIGINAL COUNTERPART OF A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) EACH SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) EACH SUCH PARTY MAKES THIS WAIVER VOLUNTARILY AND (D) EACH SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 3.3.
Section 3.4 Assignment. This Agreement and all of the provisions hereof will be binding upon and inure to the benefit of the parties hereto and their respective heirs, successors and permitted assigns. Neither this Agreement nor any of the rights, interests or obligations hereunder will be assigned (including by operation of law) without the prior written consent of all of the other parties hereto.
F-4
Table of Contents
Section 3.5 Specific Performance. The parties hereto agree that irreparable damage may occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the parties hereto shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in the Chancery Court of the State of Delaware (or, if the Chancery Court of the State of Delaware declines to accept jurisdiction, any state or federal court within the State of Delaware or, in the event each federal court within the State of Delaware declines to accept jurisdiction, any other Delaware state court), this being in addition to any other remedy to which such party is entitled at law or in equity, and in each case, without posting a bond or undertaking and without proof of damages. Each of the parties agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief when expressly available pursuant to the terms of this Agreement on the basis that the other parties have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity.
Section 3.6 Amendment. This Agreement may not be amended, changed, supplemented, waived or otherwise modified or terminated, except upon the execution and delivery of a written agreement executed by FLAC and the Investor. Notwithstanding anything to the contrary contained herein, any holder of FLAC Shares or FLAC Warrants may become party to this Agreement by executing and delivering a joinder to this Agreement in the form attached hereto as Annex A. In such event, each such Person shall thereafter shall be deemed an Investor for all purposes under this Agreement.
Section 3.7 Severability. If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this Agreement will remain in full force and effect. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
Section 3.8 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given) by delivery in person, by e-mail (having obtained electronic delivery confirmation thereof (i.e., an electronic record of the sender that the e-mail was sent to the intended recipient thereof without an “error” or similar message that such e-mail was not received by such intended recipient)), or by registered or certified mail (postage prepaid, return receipt requested) (upon receipt thereof) to the other parties as follows:
If to FLAC:
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union St., Suite 3200
Seattle, Washington 98101
Attention: James N. Topper
David Topper
E-mail:
with a copy (which will not constitute notice) to:
Goodwin Procter LLP
100 Northern Avenue
Boston, Massachusetts 02210
Attention: Jocelyn M. Arel
Jacqueline Mercier
E-mail: jarel@goodwinlaw.com
jmercier@goodwinlaw.com
F-5
Table of Contents
If to the Investor, to the address or facsimile number set forth for the Investor on the signature page hereto.
Section 3.9 Counterparts. This Agreement may be executed in two or more counterparts (any of which may be delivered by electronic transmission), each of which shall constitute an original, and all of which taken together shall constitute one and the same instrument.
Section 3.10 Entire Agreement. This Agreement and the agreements referenced herein constitute the entire agreement and understanding of the parties hereto in respect of the subject matter hereof and supersede all prior understandings, agreements or representations by or among the parties hereto to the extent they relate in any way to the subject matter hereof.
Section 3.11 Further Assurances. From time to time and without additional consideration, the Investor shall execute and deliver, or cause to be executed and delivered, such additional transfers, assignments, endorsements, proxies, consents and other instruments, and shall take such further actions, as FLAC may reasonably request for the purpose of carrying out and furthering the intent of this Agreement.
[Signature page follows]
F-6
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
| FLAC: | ||
| FRAZIER LIFESCIENCES ACQUISITION CORPORATION | ||
| By: |
| |
| Name: James N. Topper | ||
| Title: Chief Executive Officer | ||
[Signature Page to Investor Support Agreement]
F-7
Table of Contents
| INVESTOR: | ||||
| ||||
| By: |
| |||
| Name: | ||||
| Title: | ||||
| Address: |
| |||
| ||||
| ||||
| FLAC Shares owned: |
| |||
| FLAC Warrants owned: |
| |||
[Signature Page to Investor Support Agreement]
F-8
Table of Contents
Exhibit A
Form of Joinder Agreement
The undersigned is executing and delivering this joinder agreement (this “Joinder”) pursuant to the Investor Support Agreement, dated as of [●], 2022 (as the same may hereafter be amended, the “Investor Support Agreement”), by and among Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman Islands exempted company, and the Investors thereto. Capitalized terms used but not otherwise defined herein shall have the meanings provided in the Investor Support Agreement.
By executing and delivering this Joinder, the undersigned hereby agrees to become a party to, to be bound by, and to comply with the Investor Support Agreement as an Investor in the same manner as if the undersigned were an original signatory to the Investor Support Agreement. For purposes of the Investor Support Agreement and Schedule I thereto, the table below sets forth the name of the undersigned Investor, the number and type of FLAC Shares held by the Investor and the number of FLAC Warrants held by the Investor:
Name of Investor | Number and Type of FLAC Shares Beneficially Owned | FLAC Warrants Beneficially Owned | ||
[Name] | [ ] | [ ] |
Accordingly, the undersigned has executed and delivered this Joinder as of the date written below.
| Date: [●], 2022 | ||||||
| By: |
| |||||
| Name: | ||||||
| Title: | ||||||
| Address for Notices: | ||||||
| With copies to: | ||||||
[Exhibit A to Investor Support Agreement]
F-9
Table of Contents
FORM OF INVESTOR RIGHTS AGREEMENT
THIS INVESTOR RIGHTS AGREEMENT (this “Agreement”), dated as of [●], 2022, is made and entered into by and among NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”) and the parties listed as Investors on Schedule I hereto (collectively with the any person or entity who hereafter becomes a party to this Agreement pursuant to Section 6.2 or Section 6.10 of this Agreement, the “Investors” and each, a “Investor”).
RECITALS
WHEREAS, Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman Islands exempted company (“FLAC”), Holdco, NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (the “Company”), and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and a direct wholly-owned subsidiary of Holdco (“Merger Sub”), have entered into that certain Business Combination Agreement, dated as of [●], 2022 (as it may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), pursuant to which, among other things, (i) each Company Shareholder (as defined in the Business Combination Agreement) of the Company will exchange his, her or its shares of the Company for ordinary shares in the share capital of Holdco (the “Ordinary Shares”) on the terms and subject to the conditions therein (the “Exchange”) and (ii) Merger Sub will merge with and into FLAC, with FLAC surviving as a wholly-owned subsidiary of Holdco, on the terms and subject to the conditions therein (the “Merger”);
WHEREAS, FLAC, Frazier Lifesciences Sponsor LLC, a Cayman Islands limited liability company (the “Sponsor”), and Robert F. Baltera, Michael F. Bigham, Carol Gallagher, David Topper and Krishna R. Polu (together with the Sponsor, the “FLAC Investors”) are party to that certain Registration and Shareholder Rights Agreement, dated as of December 8, 2020 (the “Prior FLAC Agreement”);
WHEREAS, as of the date of this Agreement, the FLAC Investors hold 3,450,000 shares of Class B ordinary shares, par value $0.0001 per share, of FLAC (collectively, the “Founder Shares”);
WHEREAS, the Founder Shares will automatically convert into FLAC’s Class A ordinary shares at the time of the initial Business Combination (as defined in the Prior FLAC Agreement) on a one-for-one basis, subject to adjustment, on the terms and conditions provided in FLAC’s amended and restated memorandum and articles of association, as the same may be amended from time, and will be exchanged for Ordinary Shares in connection with the Merger;
WHEREAS, the Company is party to that certain Fully Amended and Restated Shareholders’ Agreement, dated as of January 11, 2021, as amended by the Amendment dated as of March 15, 2021 and the Second Amendment dated July 6, 2021, by and among the Company and certain investors listed therein (the “Prior Company Agreement” and together with the Prior FLAC Agreement, the “Prior Agreements”);
WHEREAS, certain investors (“Company Investors”) hold ownership interests in the Company, consisting of ordinary shares (“Company Ordinary Shares”) and shares designated as Series A preferred shares (“Company Series A Preferred Shares” and together with Company Ordinary Shares, the “Company Shares”); and
G-1
Table of Contents
WHEREAS, FLAC and the Company desire to terminate the Prior Agreements to provide for the terms and conditions included herein.
NOW, THEREFORE, in consideration of the representations, covenants and agreements contained herein, and certain other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE I
DEFINITIONS
1.1 Definitions. The terms defined in this Article I shall, for all purposes of this Agreement, have the respective meanings set forth below:
“Additional Investor” shall have the meaning given in Section 6.10.
“Additional Investor Ordinary Shares” shall have the meaning given in Section 6.10.
“Adverse Disclosure” shall mean any public disclosure of material non-public information, which disclosure, in the good faith judgment of the Chief Executive Officer or the Chief Financial Officer of Holdco, after consultation with counsel to Holdco, (i) would be required to be made in any Registration Statement or Prospectus in order for the applicable Registration Statement or Prospectus not to contain a Misstatement, (ii) would not be required to be made at such time if the Registration Statement were not being filed, declared effective or used, as the case may be, (iii) is such that Holdco has a bona fide business purpose for not making such information public, and (iv) (a) would be reasonably likely to have an adverse impact on Holdco, (b) could reasonably be expected to have a material adverse effect on Holdco’s ability to effect a material proposed acquisition, disposition, financing, reorganization, recapitalization or similar transaction or (c) relates to information the accuracy of which has yet to be determined by Holdco or which is the subject of an ongoing investigation or inquiry; provided that Holdco takes all reasonable action as necessary to promptly make such determination and conclude such investigation or inquiry.
“Agreement” shall have the meaning given in the Preamble hereto.
“Block Trade” shall have the meaning given in Section 2.4.1.
“Board” shall mean the Board of Directors of Holdco.
“Business Combination Agreement” shall have the meaning given in the Recitals hereto.
“Closing” shall have the meaning given in the Business Combination Agreement.
“Commission” shall mean the U.S. Securities and Exchange Commission.
“Company” shall have the meaning given in the Preamble hereto.
“Company Investor Affiliate” means any Person who directly or indirectly, through one or more intermediaries, Controls, is Controlled by, or is under common Control with, the Company Investor, or in case of an investment fund, its investment manager and/or advisor or an investment fund that is managed and/or advised by an entity that is under common Control with one of the foregoing.
“Company Investors” shall have the meaning given in the Preamble hereto.
G-2
Table of Contents
“Control” means, in relation to any Person, (i) direct, indirect or beneficial ownership of the majority of the voting rights and/or capital interests in such Person, (ii) the power, directly or indirectly, to designate, nominate or remove more than half of the members of the board of directors, management board, supervisory board or similar corporate body of such Person, and/or (iii) the power, directly or indirectly, whether by contract or otherwise, to direct or cause the direction of the management, the affairs, the policies and/or investment decisions of such Person and the terms “Controlled” and “Controlling” have meanings correlative thereto.
“Demanding Investor” shall have the meaning given in Section 2.1.4.
“EDGAR” shall have the meaning given in Section 3.1.3.
“Exchange” shall have the meaning given in the Recitals hereto.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as it may be amended from time to time.
“Final Closing Date” shall have the meaning given in the Business Combination Agreement.
“FLAC” shall have the meaning given in the Preamble hereto.
“FLAC Investors” shall have the meaning given in the Preamble hereto.
“Form F-1 Shelf” shall have the meaning given in Section 2.1.1.
“Form F-3 Shelf” shall have the meaning given in Section 2.1.1.
“Founder Shares” shall have the meaning given in the Preamble hereto.
“Holdco” shall have the meaning given in the Preamble hereto and includes Holdco’s successors by recapitalization, merger, consolidation, spin-off, reorganization or similar transaction.
“Holdco Equity Incentive Plan” shall have the meaning given in the Business Combination Agreement.
“Investor Information” shall have the meaning given in Section 4.1.2.
“Investors” shall have the meaning given in the Preamble hereto, for so long as such person or entity holds any Registrable Securities.
“Joinder” shall have the meaning given in Section 6.10.
“Lock-up” shall have the meaning given in Section 5.1.
“Lock-up Parties” shall mean, as applicable, the Investors and their respective Permitted Transferees.
“Lock-up Period” shall mean:
(A) with respect to each Company Investor:
(i) with respect to 50% of the Ordinary Shares held by such Company Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is six (6) months after the Final Closing Date; (b) the date on which the closing price of an Ordinary Share equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any twenty (20) trading days within any thirty (30)-trading day period commencing at least one hundred and fifty (150) days after the Final Closing Date; or (c) the date on
G-3
Table of Contents
which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property; and
(ii) with respect to the remaining 50% of the Ordinary Shares held by such Company Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is six (6) months after the Final Closing Date; or (b) the date on which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property; and
(B) with respect to each FLAC Investor:
(i) with respect to 50% of the Ordinary Shares held by such FLAC Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is one (1) year after the Final Closing Date; (b) the date on which the closing price of an Ordinary Share equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any twenty (20) trading days within any thirty (30)-trading day period commencing at least one hundred and fifty (150) days after the Final Closing Date; or (c) the date on which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property; and
(ii) with respect to the remaining 50% of the Ordinary Shares held by such FLAC Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is one (1) year after the Final Closing Date; or (b) the date on which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property.
“Lock-up Shares” shall mean the Ordinary Shares and any other equity securities convertible into or exercisable or exchangeable for the Ordinary Shares (including, without limitation, any Private Placement Warrants, existing Company Options (as defined in the Business Combination Agreement) and/or awards issued under the Holdco Equity Incentive Plan) held by the Sponsor or Company Investors immediately following the Closing (other than the PIPE Shares or Ordinary Shares acquired in the public market).
“Maximum Number of Securities” shall have the meaning given in Section 2.1.5.
“Merger” shall have the meaning given in the Recitals hereto.
“Merger Sub” shall have the meaning given in the Recitals hereto.
“Minimum Takedown Threshold” shall have the meaning given in Section 2.1.4.
“Misstatement” shall mean an untrue statement of a material fact or an omission to state a material fact required to be stated in a Registration Statement or Prospectus or necessary to make the statements in a Registration Statement or Prospectus (in the case of a Prospectus, in the light of the circumstances under which they were made) not misleading.
“Ordinary Shares” shall have the meaning given in the Preamble hereto.
“Other Coordinated Offering” shall have the meaning given in Section 2.4.1.
“Permitted Transferees” shall mean with respect to the Investors and their respective Permitted Transferees, (i) prior to the expiration of the Lock-up Period, any person or entity to whom such Investor is
G-4
Table of Contents
permitted to transfer such Registrable Securities prior to the expiration of the Lock-up Period pursuant to Section 5.2 and (ii) after the expiration of the Lock-up Period, any person or entity to whom such Investor is permitted to transfer such Registrable Securities, subject to and in accordance with any applicable agreement between such Investor and/or their respective Permitted Transferees and Holdco and any transferee thereafter.
“Person” means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association, trust, joint venture, investment fund, foundation or other similar entity, whether or not a legal entity.
“Piggyback Registration” shall have the meaning given in Section 2.2.1.
“PIPE Shares” shall mean the Ordinary Shares acquired by any Company Investor in connection with such Company Investor’s participation in the PIPE Financing, as defined in the Business Combination Agreement.
“Prior Agreements” shall have the meaning given in the Preamble hereto.
“Prior Company Agreement” shall have the meaning given in the Preamble hereto.
“Prior FLAC Agreement” shall have the meaning given in the Preamble hereto.
“Private Placement Warrants” shall mean the warrants held by certain FLAC Investors, purchased by such FLAC Investors in the private placement that occurred concurrently with the closing of FLAC’s initial public offering, including any Ordinary Shares issued or issuable upon conversion or exchange of such warrants.
“Prospectus” shall mean the prospectus included in any Registration Statement, as supplemented by any and all prospectus supplements and as amended by any and all post-effective amendments and including all material incorporated by reference in such prospectus.
“Registrable Security” shall mean (i) any outstanding Ordinary Shares and any other equity security (including the Private Placement Warrants and any other warrants to purchase Ordinary Shares and Ordinary Shares issued or issuable upon the exercise or conversion of any other such equity security) of Holdco held by an Investor immediately following the Closing (including any securities distributable pursuant to the Business Combination Agreement), (ii) any Additional Investor Ordinary Shares, and (iii) any other equity security of Holdco issued or issuable with respect to any securities referenced in clause (i) or (ii) above by way of a stock dividend or stock split or in connection with a recapitalization, merger, consolidation, spin-off, reorganization or similar transaction; provided, however, that, as to any particular Registrable Security, such securities shall cease to be Registrable Securities upon the earliest to occur of: (a) a Registration Statement with respect to the sale of such securities shall have become effective under the Securities Act and such securities shall have been sold, transferred, disposed of or exchanged in accordance with such Registration Statement by the applicable Investor; (b) (A) such securities shall have been otherwise transferred (other than to a Permitted Transferee), (B) new certificates for such securities not bearing (or book entry positions not subject to) a legend restricting further transfer shall have been delivered by Holdco and (C) subsequent public distribution of such securities shall not require registration under the Securities Act; (iii) such securities shall have ceased to be outstanding; (iv) such securities may be sold without registration pursuant to Rule 144 promulgated under the Securities Act or any successor rule promulgated under the Securities Act (but with no volume or other restrictions or limitations including as to manner or timing of sale); (v) such securities have been sold without registration pursuant to Section 4(a)(1) of the Securities Act or Rule 145 promulgated under the Securities Act or any successor rules promulgated under the Securities Act and (vi) such securities have been sold to, or through, a broker, dealer or underwriter in a public distribution or other public securities transaction.
“Registration” shall mean a registration, including any related Shelf Takedown, effected by preparing and filing a registration statement, Prospectus or similar document in compliance with the requirements of the
G-5
Table of Contents
Securities Act, and the applicable rules and regulations promulgated thereunder, and such registration statement becoming effective.
“Registration Expenses” shall mean the documented, out-of-pocket expenses of a Registration, including, without limitation, the following:
(A) all registration and filing fees (including fees with respect to filings required to be made with the Financial Industry Regulatory Authority, Inc.) and any national securities exchange on which the Ordinary Shares are then listed;
(B) fees and expenses of compliance with securities or blue sky laws (including reasonable fees and disbursements of outside counsel for the Underwriters in connection with blue sky qualifications of Registrable Securities);
(C) printing, messenger, telephone and delivery expenses;
(D) reasonable fees and disbursements of counsel for Holdco;
(E) reasonable fees and disbursements of all independent registered public accountants of Holdco incurred specifically in connection with such Registration; and
(F) in an Underwritten Offering, reasonable fees and expenses of one (1) legal counsel selected by the majority-in-interest of the Demanding Investors (not to exceed $25,000 without the consent of Holdco).
“Registration Statement” shall mean any registration statement that covers Registrable Securities pursuant to the provisions of this Agreement, including the Prospectus included in such registration statement, amendments (including post-effective amendments) and supplements to such registration statement, and all exhibits to and all material incorporated by reference in such registration statement.
“Requesting Investors” shall have the meaning given in Section 2.1.5.
“Securities Act” shall mean the Securities Act of 1933, as amended from time to time.
“Shelf” shall mean the Form F-1 Shelf, the Form F-3 Shelf or any Subsequent Shelf Registration Statement, as the case may be.
“Shelf Registration” shall mean a registration of securities pursuant to a registration statement filed with the Commission in accordance with and pursuant to Rule 415 promulgated under the Securities Act (or any successor rule then in effect).
“Shelf Takedown” shall mean an Underwritten Shelf Takedown or any proposed transfer or sale using a Registration Statement, including a Piggyback Registration.
“Sponsor” shall have the meaning given in the Preamble hereto.
“Sponsor Member” shall mean a member of Sponsor who becomes party to this Agreement as a Permitted Transferee of Sponsor.
“Subscription Agreement” shall have the meaning given in the Business Combination Agreement.
“Subsequent Shelf Registration Statement” shall have the meaning given in Section 2.1.2.
G-6
Table of Contents
“Transfer” shall mean the (i) sale or assignment of, offer to sell, contract or agreement to sell, hypothecate, pledge, grant of any option to purchase or otherwise dispose of or agreement to dispose of, directly or indirectly, or establishment or increase of a put equivalent position or liquidation with respect to or decrease of a call equivalent position within the meaning of Section 16 of the Exchange Act, and the rules and regulations of the Commission promulgated thereunder, with respect to, any security, (ii) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (iii) public announcement of any intention to effect any transaction specified in clause (i) or (ii).
“Underwriter” shall mean a securities dealer who purchases any Registrable Securities as principal in an Underwritten Offering and not as part of such dealer’s market-making activities.
“Underwritten Offering” shall mean a Registration in which securities of Holdco are sold to an Underwriter in a firm commitment underwriting for distribution to the public.
“Underwritten Shelf Takedown” shall have the meaning given in Section 2.1.4.
“Withdrawal Notice” shall have the meaning given in Section 2.1.6.
ARTICLE II
REGISTRATIONS AND OFFERINGS
2.1 Shelf Registration.
2.1.1 Filing. Within thirty (30) calendar days following the Final Closing Date, Holdco shall submit to or file with the Commission a Registration Statement for a Shelf Registration on Form F-1 (the “Form F-1 Shelf”) or a Registration Statement for a Shelf Registration on Form F-3 (the “Form F-3 Shelf”), if Holdco is then eligible to use a Form F-3 Shelf, in each case, covering the resale of all the Registrable Securities (determined as of two (2) business days prior to such submission or filing) on a delayed or continuous basis and shall use its commercially reasonable efforts to have such Shelf declared effective as soon as practicable after the filing thereof, but no later than the earlier of (i) the sixtieth (60th) calendar day (or ninetieth (90th) calendar day if the Commission notifies Holdco that it will “review” the Registration Statement) following the Final Closing Date and (ii) the tenth (10th) business day after the date Holdco is notified (orally or in writing, whichever is earlier) by the Commission that the Registration Statement will not be “reviewed” or will not be subject to further review (such earlier date, the “Effectiveness Deadline”); provided, however, that if such Effectiveness Deadlines falls on a Saturday, Sunday or other day that the Commission is closed for business, the Effectiveness Deadlines shall be extended to the next business day on which the Commission is open for business. Such Shelf shall provide for the resale of the Registrable Securities included therein pursuant to any method or combination of methods legally available to, and requested by, any Investor named therein. Holdco shall maintain a Shelf in accordance with the terms hereof, and shall prepare and file with the Commission such amendments, including post-effective amendments, and supplements as may be necessary to keep a Shelf continuously effective, available for use to permit the Investors named therein to sell their Registrable Securities included therein and in compliance with the provisions of the Securities Act until such time as there are no longer any Registrable Securities. In the event Holdco files a Form F-1 Shelf, Holdco shall use its commercially reasonable efforts to convert the Form F-1 Shelf (and any Subsequent Shelf Registration Statement) to a Form F-3 Shelf as soon as practicable after Holdco is eligible to use a Form F-3 Shelf. Holdco’ s obligation under this Section 2.1.1, shall, for the avoidance of doubt, be subject to Section 3.4.
2.1.2 Subsequent Shelf Registration. If any Shelf ceases to be effective under the Securities Act for any reason at any time while Registrable Securities are still outstanding, Holdco shall, subject to Section 3.4, use its commercially reasonable efforts to as promptly as is reasonably practicable cause such Shelf to again become
G-7
Table of Contents
effective under the Securities Act (including using its commercially reasonable efforts to obtain the prompt withdrawal of any order suspending the effectiveness of such Shelf), and shall use its commercially reasonable efforts to as promptly as is reasonably practicable amend such Shelf in a manner reasonably expected to result in the withdrawal of any order suspending the effectiveness of such Shelf or file an additional registration statement as a Shelf Registration (a “Subsequent Shelf Registration Statement”) registering the resale of all Registrable Securities (determined as of two (2) business days prior to such filing). If a Subsequent Shelf Registration Statement is filed, Holdco shall use its commercially reasonable efforts to (i) cause such Subsequent Shelf Registration Statement to become effective under the Securities Act as promptly as is reasonably practicable after the filing thereof (it being agreed that the Subsequent Shelf Registration Statement shall be an automatic shelf registration statement (as defined in Rule 405 promulgated under the Securities Act) if Holdco is a well-known seasoned issuer at the time of filing (as defined in Rule 405 promulgated under the Securities Act) at the most recent applicable eligibility determination date) and (ii) keep such Subsequent Shelf Registration Statement continuously effective, available for use to permit the Investors named therein to sell their Registrable Securities included therein and in compliance with the provisions of the Securities Act until such time as there are no longer any Registrable Securities. Any such Subsequent Shelf Registration Statement shall be on Form F-3 to the extent that Holdco is eligible to use such form at the time of filing. Otherwise, such Subsequent Shelf Registration Statement shall be on another appropriate form. Holdco’s obligation under this Section 2.1.2, shall, for the avoidance of doubt, be subject to Section 3.4.
2.1.3 Additional Registrable Securities. Subject to Section 3.4, in the event that any Investor holds Registrable Securities that are not registered for resale on a delayed or continuous basis, Holdco, upon written request of such Investor, shall promptly use its commercially reasonable efforts to cause the resale of such Registrable Securities to be covered by either, at Holdco’s option, any then available Shelf (including by means of a post-effective amendment) or by filing a Subsequent Shelf Registration Statement and cause the same to become effective as soon as practicable after such filing and such Shelf or Subsequent Shelf Registration Statement shall be subject to the terms hereof; provided, however, that Holdco shall only be required to cause such additional Registrable Securities to be so covered twice per calendar year for each of the FLAC Investors and the Company Investors.
2.1.4 Requests for Underwritten Shelf Takedowns. Subject to Section 3.4, following the expiration of the applicable Lock-Up Period, at any time and from time to time when an effective Shelf is on file with the Commission, the Sponsor, or a Company Investor (any of the Sponsor or a Company Investor being in such case, a “Demanding Investor”) may request to sell all or any portion of its Registrable Securities in an Underwritten Offering that is registered pursuant to the Shelf (each, an “Underwritten Shelf Takedown”); provided that Holdco shall only be obligated to effect an Underwritten Shelf Takedown if (i) such offering shall include Registrable Securities proposed to be sold by the Demanding Investor, either individually or together with other Demanding Investors, with a total offering price of at least $25 million in the aggregate or (ii) cover all of the remaining Registrable Securities held by the Demanding Investor (each of the circumstances described in (i) and (ii), the “Minimum Takedown Threshold”). All requests for Underwritten Shelf Takedowns shall be made by giving written notice to Holdco, which shall specify the approximate number of Registrable Securities proposed to be sold in the Underwritten Shelf Takedown. Subject to Section 2.4.4, a majority-in-interest of the Demanding Investors shall have the right to select the Underwriters for such offering (which shall consist of one or more reputable nationally recognized investment banks), subject to Holdco’s prior approval (which shall not be unreasonably withheld, conditioned or delayed). The Sponsor and the Company Investors may demand not more than two (2) Underwritten Shelf Takedowns pursuant to this Section 2.1.4 within any six (6) month period. For the avoidance of doubt, Holdco shall not be required to effect an aggregate of more than four (4) Underwritten Shelf Takedowns pursuant to this Section 2.1.4 in any twelve (12) month period. Notwithstanding anything to the contrary in this Agreement, Holdco may effect any Underwritten Offering pursuant to any then effective Registration Statement, including a Form F-3, that is then available for such offering.
2.1.5 Reduction of Underwritten Offering. If the managing Underwriter or Underwriters in an Underwritten Shelf Takedown, in good faith, advises Holdco, the Demanding Investors and the Investors
G-8
Table of Contents
requesting piggy back rights pursuant to this Agreement with respect to such Underwritten Shelf Takedown (the “Requesting Investors”) (if any) in writing that the dollar amount or number of Registrable Securities that the Demanding Investors and the Requesting Investors (if any) desire to sell, taken together with all other Ordinary Shares or other equity securities that Holdco desires to sell and all other Ordinary Shares or other equity securities, if any, that have been requested to be sold in such Underwritten Offering pursuant to separate written contractual piggy-back registration rights held by any other shareholders, exceeds the maximum dollar amount or maximum number of equity securities that can be sold in the Underwritten Offering without adversely affecting the proposed offering price, the timing, the distribution method, or the probability of success of such offering (such maximum dollar amount or maximum number of such securities, as applicable, the “Maximum Number of Securities”), then Holdco shall include in such Underwritten Offering, before including any Ordinary Shares or other equity securities proposed to be sold by Holdco or by other holders of Ordinary Shares or other equity securities, the Registrable Securities of (i) first, the Demanding Investors that can be sold without exceeding the Maximum Number of Securities (pro rata based on the respective number of Registrable Securities that each Demanding Investor has requested be included in such Underwritten Shelf Takedown and the aggregate number of Registrable Securities that all of the Demanding Investors have requested be included in such Underwritten Shelf Takedown) and (ii) second, to the extent that the Maximum Number of Securities has not been reached under the foregoing clause (i), the Requesting Investors (if any) (pro rata based on the respective number of Registrable Securities that each Requesting Investor (if any) has requested be included in such Underwritten Shelf Takedown and the aggregate number of Registrable Securities that all of the Requesting Investors have requested be included in such Underwritten Shelf Takedown) that can be sold without exceeding the Maximum Number of Securities.
2.1.6 Withdrawal. Prior to the filing of the applicable “red herring” prospectus or prospectus supplement used for marketing such Underwritten Shelf Takedown, a majority-in-interest of the Demanding Investors initiating an Underwritten Shelf Takedown shall have the right to withdraw from such Underwritten Shelf Takedown for any or no reason whatsoever upon written notification (a “Withdrawal Notice”) to Holdco and the Underwriter or Underwriters (if any) of their intention to withdraw from such Underwritten Shelf Takedown; provided that the Sponsor or a Company Investor may elect to have Holdco continue an Underwritten Shelf Takedown if the Minimum Takedown Threshold would still be satisfied by the Registrable Securities proposed to be sold in the Underwritten Shelf Takedown by the Sponsor, the Company Investors or any of their respective Permitted Transferees, as applicable. If withdrawn, a demand for an Underwritten Shelf Takedown shall constitute a demand for an Underwritten Shelf Takedown by the withdrawing Demanding Investor for purposes of Section 2.1.4, unless such Demanding Investor reimburses Holdco for all Registration Expenses with respect to such Underwritten Shelf Takedown (or, if there is more than one Demanding Investor, a pro rata portion of such Registration Expenses based on the respective number of Registrable Securities that each Demanding Investor has requested be included in such Underwritten Shelf Takedown); provided that, if the Sponsor or a Company Investor elects to continue an Underwritten Shelf Takedown pursuant to the proviso in the immediately preceding sentence, such Underwritten Shelf Takedown shall instead count as an Underwritten Shelf Takedown demanded by the Sponsor or such Company Investor, as applicable, for purposes of Section 2.1.4. Following the receipt of any Withdrawal Notice, Holdco shall promptly forward such Withdrawal Notice to any other Investors that had elected to participate in such Underwritten Shelf Takedown. Notwithstanding anything to the contrary in this Agreement, Holdco shall be responsible for the Registration Expenses incurred in connection with an Underwritten Shelf Takedown prior to its withdrawal under this Section 2.1.6, other than if a Demanding Investor elects to pay such Registration Expenses pursuant to clause (ii) of the second sentence of this Section 2.1.6.
2.2 Piggyback Registration.
2.2.1 Piggyback Rights. Subject to Section 2.4.3, following the expiration of the applicable Lock-Up Period, if Holdco or any Investor proposes to conduct a registered offering of, or if Holdco proposes to file a Registration Statement under the Securities Act with respect to the Registration of, equity securities, or securities or other obligations exercisable or exchangeable for, or convertible into equity securities, for its own account or for the
G-9
Table of Contents
account of shareholders of Holdco (or by Holdco and by the shareholders of Holdco including, without limitation, an Underwritten Shelf Takedown pursuant to Section 2.1), other than a Registration Statement (or any registered offering with respect thereto) (i) filed in connection with any employee stock option or other benefit plan, (ii) pursuant to a Registration Statement on Form F-4 or Form S-4 (or similar form that relates to a transaction subject to Rule 145 under the Securities Act or any successor rule thereto), (iii) for an offering of debt that is convertible into equity securities of Holdco, (iv) for a dividend reinvestment plan, or (v) a Block Trade or an Other Coordinated Offering (which shall be subject to Section 2.4), then Holdco shall give written notice of such proposed offering to all of the Investors of Registrable Securities as soon as practicable but not less than ten (10) days before the anticipated filing date of such Registration Statement or, in the case of an Underwritten Offering pursuant to a Shelf Registration, the applicable “red herring” prospectus or prospectus supplement used for marketing such offering, which notice shall (a) describe the amount and type of securities to be included in such offering, the intended method(s) of distribution, and the name of the proposed managing Underwriter or Underwriters, if any, in such offering, and (b) offer to all of the Investors of Registrable Securities the opportunity to include in such registered offering such number of Registrable Securities as such Investors may request in writing within five (5) days after receipt of such written notice (such registered offering, a “Piggyback Registration”). Subject to Section 2.2.2, Holdco shall, in good faith, cause such Registrable Securities to be included in such Piggyback Registration and, if applicable, shall use its commercially reasonable efforts to cause the managing Underwriter or Underwriters of such Piggyback Registration to permit the Registrable Securities requested by the Investors pursuant to this Section 2.2.1 to be included therein on the same terms and conditions as any similar securities of Holdco included in such registered offering and to permit the sale or other disposition of such Registrable Securities in accordance with the intended method(s) of distribution thereof. The inclusion of any Investor’s Registrable Securities in a Piggyback Registration shall be subject to such Investor’s agreement to enter into an underwriting agreement in customary form with the Underwriter(s) selected for such Underwritten Offering.
2.2.2 Reduction of Piggyback Registration. If the managing Underwriter or Underwriters in an Underwritten Offering that is to be a Piggyback Registration, in good faith, advises Holdco and the Investors of Registrable Securities participating in the Piggyback Registration in writing that the dollar amount or number of Ordinary Shares or other equity securities that Holdco desires to sell, taken together with (i) Ordinary Shares or other equity securities, if any, as to which Registration or a registered offering has been demanded pursuant to separate written contractual arrangements with persons or entities other than the Investors of Registrable Securities hereunder, (ii) the Registrable Securities as to which Registration has been requested pursuant to Section 2.2 hereof, and (iii) Ordinary Shares or other equity securities, if any, as to which Registration or a registered offering has been requested pursuant to separate written contractual piggy-back registration rights of persons or entities other than the Investors of Registrable Securities hereunder, exceeds the Maximum Number of Securities, then:
(a) if the Registration or registered offering is undertaken for Holdco’s account, Holdco shall include in any such Registration or registered offering (A) first, Ordinary Shares or other equity securities that Holdco desires to sell, which can be sold without exceeding the Maximum Number of Securities; (B) second, to the extent that the Maximum Number of Securities has not been reached under the foregoing clause (A), the Registrable Securities of Investors exercising their rights to register their Registrable Securities pursuant to Section 2.2.1, pro rata, based on the respective number of Registrable Securities that each Investor has requested be included in such Underwritten Offering and the aggregate number of Registrable Securities that the Investors have requested to be included in such Underwritten Offering, which can be sold without exceeding the Maximum Number of Securities; and (C) third, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (A) and (B), the Ordinary Shares or other equity securities, if any, as to which Registration or a registered offering has been requested pursuant to separate written contractual piggy-back registration rights of persons or entities other than the Investors of Registrable Securities hereunder, which can be sold without exceeding the Maximum Number of Securities;
(b) if the Registration or registered offering is pursuant to a demand by persons or entities other than the Investors of Registrable Securities, then Holdco shall include in any such Registration or registered offering (A) first, the Ordinary Shares or other equity securities, if any, of such requesting persons or entities,
G-10
Table of Contents
other than the Investors of Registrable Securities, which can be sold without exceeding the Maximum Number of Securities; (B) second, to the extent that the Maximum Number of Securities has not been reached under the foregoing clause (A), the Registrable Securities of Investors exercising their rights to register their Registrable Securities pursuant to Section 2.2.1, pro rata, based on the respective number of Registrable Securities that each Investor has requested be included in such Underwritten Offering and the aggregate number of Registrable Securities that the Investors have requested to be included in such Underwritten Offering, which can be sold without exceeding the Maximum Number of Securities; (C) third, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (A) and (B), the Ordinary Shares or other equity securities that Holdco desires to sell, which can be sold without exceeding the Maximum Number of Securities; and (D) fourth, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (A), (B) and (C), the Ordinary Shares or other equity securities, if any, as to which Registration or a registered offering has been requested pursuant to separate written contractual piggy-back registration rights of persons or entities other than the Investors of Registrable Securities hereunder, which can be sold without exceeding the Maximum Number of Securities; and
(c) if the Registration or registered offering and Underwritten Shelf Takedown is pursuant to a request by Investor(s) of Registrable Securities pursuant to Section 2.1 hereof, then Holdco shall include in any such Registration or registered offering securities in the priority set forth in Section 2.1.5.
2.2.3 Piggyback Registration Withdrawal. Any Investor of Registrable Securities (other than a Demanding Investor, whose right to withdraw from an Underwritten Shelf Takedown, and related obligations, shall be governed by Section 2.1.6) shall have the right to withdraw all or any portion of its Registrable Securities from a Piggyback Registration for any or no reason whatsoever upon written notification to Holdco and the Underwriter or Underwriters (if any) of his, her or its intention to withdraw such Registrable Securities from such Piggyback Registration prior to the effectiveness of the Registration Statement filed with the Commission with respect to such Piggyback Registration or, in the case of a Piggyback Registration pursuant to a Shelf Registration, the filing of the applicable “red herring” prospectus or prospectus supplement with respect to such Piggyback Registration used for marketing such transaction. Holdco (whether on its own good faith determination or as the result of a request for withdrawal by persons or entities pursuant to separate written contractual obligations) may withdraw a Registration Statement filed with the Commission in connection with a Piggyback Registration (which, in no circumstance, shall include a Shelf) at any time prior to the effectiveness of such Registration Statement. Notwithstanding anything to the contrary in this Agreement (other than Section 2.1.6), Holdco shall be responsible for the Registration Expenses incurred in connection with the Piggyback Registration prior to its withdrawal under this Section 2.2.3.
2.2.4 Unlimited Piggyback Registration Rights. For purposes of clarity, subject to Section 2.1.6, any Piggyback Registration effected pursuant to Section 2.2 hereof shall not be counted as a demand for an Underwritten Shelf Takedown under Section 2.1.4 hereof.
2.3 Market Stand-off. In connection with any Underwritten Offering of equity securities of Holdco (other than a Block Trade or Other Coordinated Offering), if requested by the managing Underwriters, each Investor that is an executive officer or director of Holdco or an Investor who is participating in the Underwritten Offering, agrees that it shall not Transfer any Ordinary Shares or other equity securities of Holdco (other than those included in such offering pursuant to this Agreement), without the prior written consent of Holdco, during the ninety (90)-day period (or such shorter time agreed to by the managing Underwriters) beginning on the date of pricing of such offering, except as expressly permitted by such lock-up agreement or in the event the managing Underwriters otherwise agree by written consent. Each such Investor agrees to execute a customary lock-up agreement in favor of the Underwriters to such effect (in each case on substantially the same terms and conditions as all such Investors).
G-11
Table of Contents
2.4 Block Trades; Other Coordinated Offerings.
2.4.1 Notwithstanding any other provision of this Article II, but subject to Section 3.4, at any time and from time to time when an effective Shelf is on file with the Commission, if a Demanding Investor wishes to engage in (a) an underwritten registered offering not involving a “roadshow,” an offer commonly known as a “block trade” (a “Block Trade”), or (b) an “at the market” or similar registered offering through a broker, sales agent or distribution agent, whether as agent or principal (an “Other Coordinated Offering”), in each case, (x) with a total offering price of at least $25 million in the aggregate or (y) with respect to all remaining Registrable Securities held by the Demanding Investor, then such Demanding Investor only needs to notify Holdco of the Block Trade or Other Coordinated Offering at least five (5) business days prior to the day such offering is to commence and Holdco shall use its commercially reasonable efforts to facilitate such Block Trade or Other Coordinated Offering; provided that the Demanding Investors representing a majority of the Registrable Securities wishing to engage in the Block Trade or Other Coordinated Offering shall use commercially reasonable efforts to work with Holdco and any Underwriters, brokers, sales agents or placement agents prior to making such request in order to facilitate preparation of the registration statement, prospectus and other offering documentation related to the Block Trade or Other Coordinated Offering.
2.4.2 Prior to the filing of the applicable “red herring” prospectus or prospectus supplement used in connection with a Block Trade or Other Coordinated Offering, a majority-in-interest of the Demanding Investors initiating such Block Trade or Other Coordinated Offering shall have the right to submit a Withdrawal Notice to Holdco, the Underwriter or Underwriters (if any) and any brokers, sales agents or placement agents (if any) of their intention to withdraw from such Block Trade or Other Coordinated Offering. Notwithstanding anything to the contrary in this Agreement, Holdco shall be responsible for the Registration Expenses incurred in connection with a Block Trade or Other Coordinated Offering prior to its withdrawal under this Section 2.4.2.
2.4.3 Notwithstanding anything to the contrary in this Agreement, Section 2.2 shall not apply to a Block Trade or Other Coordinated Offering initiated by a Demanding Investor pursuant to this Agreement.
2.4.4 The Demanding Investor in a Block Trade or Other Coordinated Offering shall have the right to select the Underwriters and any brokers, sales agents or placement agents (if any) for such Block Trade or Other Coordinated Offering (in each case, which shall consist of one or more reputable nationally recognized investment banks).
2.4.5 A Demanding Investor in the aggregate may demand no more than (i) one (1) Block Trade pursuant to this Section 2.4 within any six (6) month period or (ii) two (2) Block Trades or Other Coordinated Offerings pursuant to this Section 2.4 in any twelve (12) month period. For the avoidance of doubt, any Block Trade or Other Coordinated Offering effected pursuant to this Section 2.4 shall not be counted as a demand for an Underwritten Shelf Takedown pursuant to Section 2.1.4 hereof.
ARTICLE III
COMPANY PROCEDURES
3.1 General Procedures. In connection with any Shelf and/or Shelf Takedown, Holdco shall use its commercially reasonable efforts to effect such Registration to permit the sale of such Registrable Securities in accordance with the intended plan of distribution thereof, and pursuant thereto Holdco shall:
3.1.1 prepare and file with the Commission as soon as reasonably practicable a Registration Statement with respect to such Registrable Securities and use its commercially reasonable efforts to cause such Registration Statement to become effective and remain effective, or file a Subsequent Shelf Registration Statement, until all Registrable Securities covered by such Registration Statement are sold in accordance with the intended plan of distribution set forth in such Registration Statement or have ceased to be Registrable Securities;
G-12
Table of Contents
3.1.2 prepare and file with the Commission such amendments and post-effective amendments to the Registration Statement, and such supplements to the Prospectus, as may be reasonably requested by any Investor that holds at least one percent (1%) of the Registrable Securities registered on such Registration Statement or any Underwriter of Registrable Securities or as may be required by the rules, regulations or instructions applicable to the registration form used by Holdco or by the Securities Act or rules and regulations thereunder to keep the Registration Statement effective until all Registrable Securities covered by such Registration Statement are sold in accordance with the intended plan of distribution set forth in such Registration Statement or supplement to the Prospectus or have ceased to be Registrable Securities;
3.1.3 prior to filing a Registration Statement or Prospectus, or any amendment or supplement thereto, furnish without charge to the Underwriters, if any, and the Investors of Registrable Securities included in such Registration, and such Investors’ legal counsel, copies of such Registration Statement as proposed to be filed, each amendment and supplement to such Registration Statement (in each case including all exhibits thereto and documents incorporated by reference therein), the Prospectus included in such Registration Statement (including each preliminary Prospectus), and such other documents as the Underwriters and the Investors of Registrable Securities included in such Registration or the legal counsel for any such Investors may reasonably request in order to facilitate the disposition of the Registrable Securities owned by such Investors; provided that Holdco shall have no obligation to furnish any documents publicly filed or furnished with the Commission pursuant to the Electronic Data Gathering, Analysis and Retrieval System (“EDGAR”). Holdco shall not file any such Registration Statement or Prospectus, or any amendment or supplement thereto, to which a majority-in-interest of the Investors of Registrable Securities included in such Registration or their respective counsels shall reasonably object in writing on a timely basis;
3.1.4 prior to any public offering of Registrable Securities, use its commercially reasonable efforts to (i) register or qualify the Registrable Securities covered by the Registration Statement under such securities or “blue sky” laws of such jurisdictions in the United States as the Investors of Registrable Securities included in such Registration Statement (in light of their intended plan of distribution) may reasonably request (or provide evidence satisfactory to such Investors that the Registrable Securities are exempt from such registration or qualification) and (ii) take such action necessary to cause such Registrable Securities covered by the Registration Statement to be registered with or approved by such other governmental authorities as may be necessary by virtue of the business and operations of Holdco and do any and all other acts and things that may be necessary or advisable to enable the Investors of Registrable Securities included in such Registration Statement to consummate the disposition of such Registrable Securities in such jurisdictions; provided, however, that Holdco shall not be required to qualify generally to do business in any jurisdiction where it would not otherwise be required to qualify or take any action to which it would be subject to general service of process or taxation in any such jurisdiction where it is not then otherwise so subject;
3.1.5 cause all such Registrable Securities to be listed on each national securities exchange on which similar securities issued by Holdco are then listed;
3.1.6 provide a transfer agent or warrant agent, as applicable, and registrar for all such Registrable Securities no later than the effective date of such Registration Statement;
3.1.7 advise each seller of such Registrable Securities, promptly after it shall receive notice or obtain knowledge thereof, of the issuance of any stop order by the Commission suspending the effectiveness of such Registration Statement or the initiation or threatening of any proceeding for such purpose and promptly use its commercially reasonable efforts to prevent the issuance of any stop order or to obtain its withdrawal if such stop order should be issued;
3.1.8 at least three (3) days prior to the filing of any Registration Statement or Prospectus or any amendment or supplement to such Registration Statement or Prospectus (or such shorter period of time as may be (i) necessary in order to comply with the Securities Act, the Exchange Act, and the rules and regulations
G-13
Table of Contents
promulgated under the Securities Act or Exchange Act, as applicable or (ii) advisable in order to reduce the number of days that sales are suspended pursuant to Section 3.4), furnish a copy thereof to each seller of such Registrable Securities or its counsel (excluding any exhibits thereto and any filing made under the Exchange Act that is to be incorporated by reference therein);
3.1.9 notify the Investors at any time when a Prospectus relating to such Registration Statement is required to be delivered under the Securities Act or of the happening of any event as a result of which the Prospectus included in such Registration Statement, as then in effect, includes a Misstatement, and then to correct such Misstatement as set forth in Section 3.4;
3.1.10 in the event of an Underwritten Offering, a Block Trade, an Other Coordinated Offering, or sale by a broker, placement agent or sales agent pursuant to such Registration, in each of the following cases to the extent customary for a transaction of its type, permit a representative of the Investors, the Underwriters or other financial institutions facilitating such Underwritten Offering, Block Trade, Other Coordinated Offering or other sale pursuant to such Registration, if any, and any attorney, consultant or accountant retained by such Investors or Underwriter to participate, at each such person’s or entity’s own expense, in the preparation of the Registration Statement, and cause Holdco’s officers, directors and employees to supply all information reasonably requested by any such representative, Underwriter, financial institution, attorney, consultant or accountant in connection with the Registration; provided, however, that such representatives, Underwriters or financial institutions agree to confidentiality arrangements in form and substance reasonably satisfactory to Holdco, prior to the release or disclosure of any such information;
3.1.11 obtain a “comfort” letter from Holdco’s independent registered public accountants in the event of an Underwritten Offering, a Block Trade, an Other Coordinated Offering or sale by a broker, placement agent or sales agent pursuant to such Registration (subject to such broker, placement agent or sales agent providing such certification or representation reasonably requested by Holdco’s independent registered public accountants and Holdco’s counsel) in customary form and covering such matters of the type customarily covered by “comfort” letters for a transaction of its type as the managing Underwriter or the broker, placement agent or sales agent of such offering or sale may reasonably request, and reasonably satisfactory to a majority-in-interest of the participating Investors;
3.1.12 in the event of an Underwritten Offering, a Block Trade, an Other Coordinated Offering or sale by a broker, placement agent or sales agent pursuant to such Registration, on the date the Registrable Securities are delivered for sale pursuant to such Registration, to the extent customary for a transaction of its type, obtain an opinion, dated such date, of counsel representing Holdco for the purposes of such Registration, addressed to the participating Investors, the broker, placement agents or sales agent, if any, and the Underwriters, if any, covering such legal matters with respect to the Registration in respect of which such opinion is being given as the participating Investors, broker, placement agent, sales agent or Underwriter may reasonably request and as are customarily included in such opinions and negative assurance letters;
3.1.13 in the event of any Underwritten Offering, a Block Trade, an Other Coordinated Offering or sale by a broker, placement agent or sales agent pursuant to such Registration, enter into and perform its obligations under an underwriting or other purchase or sales agreement, in usual and customary form, with the managing Underwriter or the broker, placement agent or sales agent of such offering or sale;
3.1.14 make available to its security holders, as soon as reasonably practicable, an earnings statement covering the period of at least twelve (12) months beginning with the first day of Holdco’s first full calendar quarter after the effective date of the Registration Statement which satisfies the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder (or any successor rule then in effect);
3.1.15 with respect to an Underwritten Offering pursuant to Section 2.1.4, use its commercially reasonable efforts to make available senior executives of Holdco to participate in customary “road show” presentations that may be reasonably requested by the Underwriter in such Underwritten Offering;
G-14
Table of Contents
3.1.16 cooperate with the participating Investors and the Underwriters, if any, to facilitate the timely preparation and delivery of certificates (if such securities are certificated and which shall not bear any restrictive legends unless required under applicable law) representing securities sold under any Registration Statement, and enable such securities to be in such denominations and registered in such names as such Investors or Underwriters may request and keep available and make available to Holdco’s transfer agent prior to the effectiveness of such Registration Statement a supply of such certificates (if such securities are certificated);
3.1.17 cooperate with each participating Investor and Underwriter, if any, and their respective counsels in connection with any filings required to be made with the Financial Industry Regulatory Authority; and
3.1.18 otherwise, in good faith, cooperate reasonably with, and take such customary actions as may reasonably be requested by the participating Investors, consistent with the terms of this Agreement, in connection with such Registration.
Notwithstanding the foregoing, Holdco shall not be required to provide any documents or information to an Underwriter, broker, sales agent or placement agent if such Underwriter, broker, sales agent or placement agent has not then been named with respect to the applicable Underwritten Offering or other offering involving a registration as an Underwriter, broker, sales agent or placement agent, as applicable.
3.2 Registration Expenses. The Registration Expenses of all Registrations shall be borne by Holdco. It is acknowledged by the Investors that the Investors shall bear all incremental selling expenses relating to the sale of Registrable Securities, such as Underwriters’ commissions and discounts, brokerage fees, Underwriter marketing costs, other than as set forth in the definition of “Registration Expenses,” and all reasonable fees and expenses of any legal counsel representing the Investors.
3.3 Requirements for Participation in Registration Statement Offerings. Notwithstanding anything in this Agreement to the contrary, if any Investor does not timely provide Holdco with its requested Investor Information, Holdco may exclude such Investor’s Registrable Securities from the applicable Registration Statement or Prospectus if Holdco determines, based on the advice of counsel, that it is necessary or advisable to include such information in the applicable Registration Statement or Prospectus and such Investor continues thereafter to withhold such information. In addition, no person or entity may participate in any Underwritten Offering or other offering for equity securities of Holdco pursuant to a Registration initiated by Holdco hereunder unless such person or entity (i) agrees to sell such person’s or entity’s securities on the basis provided in any underwriting, sales, distribution or placement arrangements approved by Holdco and (ii) timely completes and executes all customary questionnaires, powers of attorney, indemnities, lock-up agreements, underwriting or other agreements and other customary documents as may be reasonably required under the terms of such underwriting, sales, distribution or placement arrangements. For the avoidance of doubt, the exclusion of an Investor’s Registrable Securities as a result of this Section 3.3 shall not affect the registration of the other Registrable Securities to be included in such Registration.
3.4 Suspension of Sales; Adverse Disclosure; Restrictions on Registration Rights.
3.4.1 Upon receipt of written notice from Holdco that: (i) a Registration Statement or Prospectus contains a Misstatement; (ii) any request by the Commission for any amendment or supplement to any Registration Statement or Prospectus or for additional information or of the occurrence of an event requiring the preparation of a supplement or amendment to such Prospectus so that, as thereafter delivered to the purchasers of the securities covered by such Registration Statement or Prospectus, such Registration Statement or Prospectus will not contain a Misstatement; or (iii) upon any suspension by Holdco, pursuant to a written insider trading compliance program adopted by the Board, of the ability of all “insiders” covered by such program to transact in Holdco’s securities because of the existence of material non-public information, each of the Investors shall forthwith discontinue disposition of Registrable Securities pursuant to such Registration Statement covering such
G-15
Table of Contents
Registrable Securities until (x) in the case of (i) or (ii), it has received copies of a supplemented or amended Prospectus correcting the Misstatement (it being understood that Holdco hereby covenants to prepare and file such supplement or amendment as soon as reasonably practicable after the time of such notice), or until it is advised in writing by Holdco that the use of the Prospectus may be resumed, or (y) in the case of (iii), until the restriction on the ability of “insiders” to transact in Holdco’s securities is removed, and, if so directed by Holdco, each such Investor will deliver to Holdco all copies, other than permanent file copies then in such Investor’s possession, of the most recent Prospectus covering such Registrable Securities at the time of receipt of such notice.
3.4.2 Subject to Section 3.4.4, if the filing, initial effectiveness or continued use of a Registration Statement in respect of any Registration at any time would (i) require Holdco to make an Adverse Disclosure, (ii) require the inclusion in such Registration Statement of financial statements that are unavailable to Holdco for reasons beyond Holdco’s control, or (iii) in the good faith judgment of the Board, such Registration would be detrimental to Holdco and the Board concludes as a result that it is advisable to defer such filing, initial effectiveness or continued use at such time, Holdco may, upon giving prompt written notice of such action to the Investors (which notice shall not specify the nature of the event giving rise to such delay or suspension), delay the filing or initial effectiveness of, or suspend use of, such Registration Statement for the shortest period of time determined in good faith by Holdco to be necessary for such purpose. In the event Holdco exercises its rights under this Section 3.4.2, the Investors agree to suspend, immediately upon their receipt of the notice referred to above, their use of the Prospectus relating to any Registration in connection with any sale or offer to sell Registrable Securities until such Investor receives written notice from Holdco that such sales or offers of Registrable Securities may be resumed, and in each case maintain the confidentiality of such notice and its contents.
3.4.3 Subject to Section 3.4.4, (i) during the period starting with the date thirty (30) days prior to Holdco’s good faith estimate of the date of the filing of, and ending on a date ninety (90) days after the effective date of, a Holdco-initiated Registration and provided that Holdco continues to actively employ, in good faith, all commercially reasonable efforts to maintain the effectiveness of the applicable Shelf Registration Statement, or (ii) if, pursuant to Section 2.1.4, Investors have requested an Underwritten Shelf Takedown and Holdco and Investors are unable to obtain the commitment of underwriters to firmly underwrite such offering, Holdco may, upon giving prompt written notice of such action to the Investors, delay any other registered offering pursuant to Section 2.1.4 or 2.4.
3.4.4 The right to delay or suspend any filing, initial effectiveness or continued use of a Registration Statement pursuant to Section 3.4.2 or a registered offering pursuant to Section 3.4.3 shall be exercised by Holdco, in the aggregate, not more than twice and for not more than sixty (60) consecutive calendar days and for not more than one hundred and twenty (120) total calendar days, during any twelve (12)-month period.
3.5 Reporting Obligations. As long as any Investor shall own Registrable Securities, Holdco, at all times while it shall be a reporting company under the Exchange Act, covenants to file timely (or obtain extensions in respect thereof and file within the applicable grace period) all reports required to be filed by Holdco after the date hereof pursuant to Sections 13(a) or 15(d) of the Exchange Act and to promptly furnish the Investors with true and complete copies of all such filings; provided that any documents publicly filed or furnished with the Commission pursuant to EDGAR shall be deemed to have been furnished or delivered to the Investors pursuant to this Section 3.5. Holdco further covenants that it shall take such further action as any Investor may reasonably request, all to the extent required from time to time to enable such Investor to sell Registrable Securities held by such Investor without registration under the Securities Act within the limitation of the exemptions provided by Rule 144 promulgated under the Securities Act (or any successor rule then in effect). Upon the request of any Investor, Holdco shall deliver to such Investor a written certification of a duly authorized officer as to whether it has complied with such requirements.
G-16
Table of Contents
ARTICLE IV
INDEMNIFICATION AND CONTRIBUTION
4.1 Indemnification.
4.1.1 Holdco agrees to indemnify, to the extent permitted by law, each Investor of Registrable Securities, its officers, directors and agents and each person or entity who controls such Investor (within the meaning of the Securities Act), against all losses, claims, damages, liabilities and reasonable out-of-pocket expenses (including, without limitation, reasonable outside attorneys’ fees) resulting from any untrue Misstatement or alleged Misstatement in any Registration Statement, Prospectus or preliminary Prospectus or any amendment thereof or supplement thereto, except insofar as the same are caused by or contained in any information or affidavit so furnished in writing to Holdco by such Investor expressly for use therein. Holdco shall indemnify the Underwriters, their officers and directors and each person or entity who controls such Underwriters (within the meaning of the Securities Act) to the same extent as provided in the foregoing sentence with respect to the indemnification of the Investor.
4.1.2 In connection with any Registration Statement in which an Investor of Registrable Securities is participating, such Investor shall furnish (or cause to be furnished) to Holdco in writing such information and affidavits as Holdco reasonably requests for use in connection with any such Registration Statement or Prospectus (the “Investor Information”) and, to the extent permitted by law, shall indemnify Holdco, its directors, officers and agents and each person or entity who controls Holdco (within the meaning of the Securities Act) against all losses, claims, damages, liabilities and out-of-pocket expenses (including, without limitation, reasonable and documented outside attorneys’ fees) resulting from any untrue Misstatement or alleged Misstatement in any Registration Statement, Prospectus or preliminary Prospectus or any amendment thereof or supplement thereto, but only to the extent that such Misstatement is contained in (or not contained in, in the case of an omission) any information or affidavit so furnished in writing by or on behalf of such Investor expressly for use therein; provided, however, that the obligation to indemnify pursuant to the foregoing sentence shall be several, not joint and several, among such Investors of Registrable Securities, and the liability of each such Investor of Registrable Securities shall be in proportion to and limited to the net proceeds received by such Investor from the sale of Registrable Securities pursuant to such Registration Statement. The Investors of Registrable Securities shall indemnify the Underwriters, their officers, directors and each person or entity who controls such Underwriters (within the meaning of the Securities Act) to the same extent as provided in the foregoing sentence with respect to indemnification of Holdco.
4.1.3 Any person or entity entitled to indemnification herein shall (i) give prompt written notice to the indemnifying party of any claim with respect to which it seeks indemnification (provided that the failure to give prompt notice shall not impair any person’s or entity’s right to indemnification hereunder to the extent such failure has not materially prejudiced the indemnifying party) and (ii) unless in such indemnified party’s reasonable judgment a conflict of interest between such indemnified and indemnifying parties may exist with respect to such claim, permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party. If such defense is assumed, the indemnifying party shall not be subject to any liability for any settlement made by the indemnified party without its consent (but such consent shall not be unreasonably withheld). An indemnifying party who is not entitled to, or elects not to, assume the defense of a claim shall not be obligated to pay the fees and expenses of more than one counsel for all parties indemnified by such indemnifying party with respect to such claim, unless in the reasonable judgment of any indemnified party a conflict of interest may exist between such indemnified party and any other of such indemnified parties with respect to such claim. No indemnifying party shall, without the consent of the indemnified party, consent to the entry of any judgment or enter into any settlement which cannot be settled in all respects by the payment of money (and such money is so paid by the indemnifying party pursuant to the terms of such settlement) or which settlement includes a statement or admission of fault and culpability on the part of such indemnified party or which settlement does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect to such claim or litigation.
G-17
Table of Contents
4.1.4 The indemnification provided for under this Agreement shall remain in full force and effect regardless of any investigation made by or on behalf of the indemnified party or any officer, director or controlling person or entity of such indemnified party and shall survive the Transfer of Registrable Securities by an Investor. Holdco and each Investor of Registrable Securities participating in an offering also agree to make such provisions as are reasonably requested by any indemnified party for contribution to such party in the event Holdco’s or such Investor’s indemnification is unavailable for any reason.
4.1.5 If the indemnification provided under Section 4.1 from the indemnifying party is unavailable or insufficient to hold harmless an indemnified party in respect of any losses, claims, damages, liabilities and out-of-pocket expenses referred to herein, then the indemnifying party, in lieu of indemnifying the indemnified party, shall contribute to the amount paid or payable by the indemnified party as a result of such losses, claims, damages, liabilities and out-of-pocket expenses in such proportion as is appropriate to reflect the relative fault of the indemnifying party and the indemnified party, as well as any other relevant equitable considerations. The relative fault of the indemnifying party and indemnified party shall be determined by reference to, among other things, whether any action in question, including any Misstatement, was made by (or not made by, in the case of an omission), or relates to information supplied by (or not supplied by in the case of an omission), such indemnifying party or indemnified party, and the indemnifying party’s and indemnified party’s relative intent, knowledge, access to information and opportunity to correct or prevent such action; provided, however, that the liability of any Investor under this Section 4.1.5 shall be limited to the amount of the net proceeds received by such Investor in such offering giving rise to such liability. The amount paid or payable by a party as a result of the losses or other liabilities referred to above shall be deemed to include, subject to the limitations set forth in Sections 4.1.1, 4.1.2 and 4.1.3 above, any legal or other fees, charges or out-of-pocket expenses reasonably incurred by such party in connection with any investigation or proceeding. The parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 4.1.5 were determined by pro rata allocation or by any other method of allocation, which does not take account of the equitable considerations referred to in this Section 4.1.5. No person or entity guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution pursuant to this Section 4.1.5 from any person or entity who was not guilty of such fraudulent misrepresentation.
ARTICLE V
LOCK-UP
5.1 Lock-Up. Subject to Section 5.2 and Section 5.3, each Lock-up Party agrees that it shall not Transfer any Lock-up Shares prior to the end of, in respect of such Lock-up Party, the applicable Lock-up Period (the “Lock-up”).
5.2 Permitted Transferees. The foregoing restrictions set forth in Section 5.1 shall not apply to: (i) 10% of the Ordinary Shares held by each of Holdco’s officers and employees or (ii) Transfers made (a) in the case of an individual, pursuant to a bona fide gift to a member of the individual’s immediate family or to a trust, the beneficiary of which is a member of the individual’s immediate family or an affiliate of such person or entity, or to a charitable organization; (b) in the case of an individual, by virtue of laws of descent and distribution upon death of the individual; (c) in the case of an individual, pursuant to a qualified domestic relations order; (d) in the case of a trust, by distribution to one or more of the permissible beneficiaries of such trust; (e) in the case of a corporation, partnership (whether general, limited or otherwise), limited liability company, trust or other business entity, (x) Transfers to another corporation, partnership, limited liability company, trust or other business entity that controls, is controlled by or is under common control or management with an Investor (including, for the avoidance of doubt, where such Investor is a partnership, to its general partner or a successor partnership or fund, or any other funds managed by such partnership), or (y) as part of a distribution, transfer or other disposition of Ordinary Shares to partners, limited liability company members or stockholders of an Investor; (f) in connection with any bona fide mortgage, encumbrance or pledge to a financial institution in connection with any bona fide loan or debt transaction or enforcement thereunder, (g) in connection with a liquidation, merger, stock exchange,
G-18
Table of Contents
reorganization, tender offer approved by the Board or a duly authorized committee thereof or other similar transaction which results in all of Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property subsequent to the Final Closing Date or (h) to cover withholding taxes upon the vesting of awards issued pursuant to the Holdco Equity Incentive Plan. The parties acknowledge and agree that any Permitted Transferee of a Lock-up Party must enter into a written agreement agreeing to be bound by the terms of this Agreement in form and substance reasonably satisfactory to the Company, including the transfer restrictions set forth in this Article V.
5.3 Notwithstanding the foregoing, except as otherwise agreed to by Holdco and the Sponsor, if any Lock-up Party is granted a release or waiver from the Lock-up provided in this Article V (such party, a “Triggering Investor”), then each other Lock-up Party shall also be granted an early release from its obligations hereunder or under any contractual lock-up agreement with Holdco on the same terms and on a pro-rata basis with respect to such number of Lock-up Shares rounded down to the nearest whole security equal to the product of (i) the total percentage of Lock-up Shares held by the Triggering Investor immediately following the Closing that are being released from the Lock-up agreement multiplied by (ii) the total number of Lock-up Shares held by such other Lock-up Party immediately following the Closing.
ARTICLE VI
MISCELLANEOUS
6.1 Notices. Any notice or communication under this Agreement must be in writing and given by (i) recorded mail, addressed to the party to be notified, postage prepaid and registered or certified with return receipt requested, (ii) delivery in person or by courier service providing evidence of delivery, or (iii) transmission by hand delivery or electronic mail. Each notice or communication that is mailed, delivered, or transmitted in the manner described above shall be deemed sufficiently given, served, sent, and received, in the case of mailed notices, on the third business day following the date on which it is mailed and, in the case of notices delivered by courier service, hand delivery or electronic mail, at such time as it is delivered to the addressee (with the delivery receipt or the affidavit of messenger) or at such time as delivery is refused by the addressee upon presentation. Any notice or communication under this Agreement must be addressed, if to Holdco, to: NewAmsterdam Pharma Company B.V., c/o NewAmsterdam Pharma Holding B.V., Gooimeer 2-35, 1411 DC Naarden, The Netherlands, Attention: Louise Kooij or by email: , and, if to any Investor, at such Investor’s address, electronic mail address as set forth in Holdco’s books and records. Any party may change its address for notice at any time and from time to time by written notice to the other parties hereto, and such change of address shall become effective thirty (30) days after delivery of such notice as provided in this Section 6.1.
6.2 Assignment; No Third Party Beneficiaries.
6.2.1 This Agreement and the rights, duties and obligations of Holdco hereunder may not be assigned or delegated by Holdco in whole or in part.
6.2.2 Subject to Section 6.2.4 and Section 6.2.5, this Agreement and the rights, duties and obligations of an Investor hereunder may be assigned in whole or in part to such Investor’s Permitted Transferees to which it transfers Registrable Securities; provided that with respect to the FLAC Investors and the Company Investors, the rights hereunder that are personal to such Investors may not be assigned or delegated in whole or in part, except that (i) the Sponsor shall be permitted to transfer its rights hereunder as the Sponsor to one or more affiliates or any direct or indirect partners, members or equity holders of the Sponsor (including Sponsor Members), which, for the avoidance of doubt, shall include a transfer of its rights in connection with a distribution of any Registrable Securities held by Sponsor to Sponsor Members (it being understood that no such transfer shall reduce or multiply any rights of the Sponsor or such transferees), and (ii) each of the Company Investors shall be permitted to transfer its rights hereunder as the Company Investors to one or more Company
G-19
Table of Contents
Investor Affiliates or any direct or indirect partners, members or equity holders of such Company Investor (it being understood that no such transfer shall reduce or multiply any rights of such Company Investor or such transferees). Upon a transfer by the Sponsor pursuant to subsection (i) hereof to Sponsor Members, the rights that are personal to the Sponsor shall be exercised by the Sponsor Members only with the consent of the Sponsor’s board of managers in accordance with the Sponsor’s operating agreement.
6.2.3 This Agreement and the provisions hereof shall be binding upon and shall inure to the benefit of each of the parties and its successors and the permitted assigns of the Investors, which shall include Permitted Transferees.
6.2.4 This Agreement shall not confer any rights or benefits on any persons or entities that are not parties hereto, other than as expressly set forth in this Agreement and Section 6.2.
6.2.5 No assignment by any party hereto of such party’s rights, duties and obligations hereunder shall be binding upon or obligate Holdco unless and until Holdco shall have received (i) written notice of such assignment as provided in Section 6.1 hereof and (ii) the written agreement of the assignee, in a form reasonably satisfactory to Holdco, to be bound by the terms and provisions of this Agreement (which may be accomplished by an addendum or certificate of joinder to this Agreement, including the joinder in the form of Exhibit A attached hereto). Any transfer or assignment made other than as provided in this Section 6.2 shall be null and void.
6.3 Counterparts. This Agreement may be executed in multiple counterparts (including PDF counterparts), each of which shall be deemed an original, and all of which together shall constitute the same instrument, but only one of which need be produced.
6.4 Governing Law; Venue. NOTWITHSTANDING THE PLACE WHERE THIS AGREEMENT MAY BE EXECUTED BY ANY OF THE PARTIES HERETO, THE PARTIES EXPRESSLY AGREE THAT (1) THIS AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED UNDER THE LAWS OF THE STATE OF NEW YORK AND (2) THE VENUE FOR ANY ACTION TAKEN WITH RESPECT TO THIS AGREEMENT SHALL BE ANY STATE OR FEDERAL COURT IN NEW YORK COUNTY IN THE STATE OF NEW YORK.
6.5 Waiver of Jury Trial. THE PARTIES EACH HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. THE PARTIES EACH HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE AN ORIGINAL COUNTERPART OF A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) EACH SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) EACH SUCH PARTY MAKES THIS WAIVER VOLUNTARILY AND (D) EACH SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 6.5.
6.6 Amendments and Modifications. Upon the written consent of (i) Holdco and (ii) the holders of a majority of the total Registrable Securities at the time in question, compliance with any of the provisions, covenants and conditions set forth in this Agreement may be waived, or any of such provisions, covenants or
G-20
Table of Contents
conditions may be amended or modified; provided, however, that notwithstanding the foregoing, any amendment hereto or waiver hereof shall also require the written consent of the Sponsor so long as the Sponsor and its affiliates hold, in the aggregate, at least one percent (1%) of the outstanding Ordinary Shares; provided, further, that notwithstanding the foregoing, any amendment hereto or waiver hereof shall also require the written consent of each Company Investor so long as such Company Investor and its respective Company Investor Affiliates hold, in the aggregate, at least one percent (1%) of the outstanding Ordinary Shares; and provided, further, that any amendment hereto or waiver hereof that adversely affects one Investor, solely in its capacity as a holder of the shares of capital stock of Holdco, in a manner that is materially different from the other Investors (in such capacity) shall require the consent of the Investor so affected. No course of dealing between any Investor or Holdco and any other party hereto or any failure or delay on the part of an Investor or Holdco in exercising any rights or remedies under this Agreement shall operate as a waiver of any rights or remedies of any Investor or Holdco. No single or partial exercise of any rights or remedies under this Agreement by a party shall operate as a waiver or preclude the exercise of any other rights or remedies hereunder or thereunder by such party.
6.7 Other Registration Rights. Other than the certain Investors and third-party investor shareholders who each have registration rights pursuant to (i) their respective Subscription Agreements and (ii) as provided in the Warrant Agreement, dated as of December 8, 2020, between FLAC and Continental Stock Transfer & Trust Company, as amended by that certain Warrant Assignment, Assumption and Amendment Agreement, dated as of [●], 2022, between FLAC, Holdco and Continental Stock Transfer & Trust Company, FLAC represents and warrants that no person or entity, other than a holder of Registrable Securities pursuant hereto, has any right to require Holdco to register any securities of Holdco for sale or to include such securities of Holdco in any Registration Statement filed by Holdco for the sale of securities for its own account or for the account of any other person or entity. Holdco hereby agrees and covenants that it will not grant rights to register any Ordinary Shares (or securities convertible into or exchangeable for Ordinary Shares) pursuant to the Securities Act that are more favorable, pari passu or senior to those granted to the Investors hereunder without (a) the prior written consent of (i) the Sponsor, for so long as the Sponsor and its affiliates hold, in the aggregate, Registrable Securities representing at least one percent (1%) of the outstanding Ordinary Shares, and (ii) a Company Investor, for so long as such Company Investor and Company Investor Affiliates hold, in the aggregate, Registrable Securities representing at least one percent (1%) of the outstanding Ordinary Shares, or (b) granting economically and legally equivalent rights to the Investors hereunder such that the Investors shall receive the benefit of such more favorable or senior terms and/or conditions. Further, Holdco represents and warrants that this Agreement supersedes any other registration rights agreement or agreement with similar terms and conditions and in the event of a conflict between any such agreement or agreements and this Agreement, the terms of this Agreement shall prevail.
6.8 Term. This Agreement shall terminate with respect to any Investor, on the date that such Investor no longer holds any Registrable Securities. The provisions of Section 3.2, Section 3.5 and Article IV shall survive any termination.
6.9 Investor Information. Each Investor agrees, if requested in writing, to represent to Holdco the total number of Registrable Securities held by such Investor in order for Holdco to make determinations hereunder.
6.10 Additional Investors; Joinder. In addition to persons or entities who may become Investors pursuant to Section 6.2 hereof, subject to the prior written consent of each of the Sponsor (so long as the Sponsor and its affiliates hold, in the aggregate, Registrable Securities representing at least one percent (1%) of the outstanding Ordinary Shares) and each Company Investor (in each case, so long as such Company Investor and Company Investor Affiliates hold, in the aggregate, Registrable Securities representing at least one percent (1%) of the outstanding Ordinary Shares), Holdco may make any person or entity who acquires Ordinary Shares or rights to acquire Ordinary Shares after the date hereof a party to this Agreement (each such person or entity, an “Additional Investor”) by obtaining an executed joinder to this Agreement from such Additional Investor in the form of Exhibit A attached hereto (a “Joinder”). Such Joinder shall specify the rights and obligations of the applicable Additional Investor under this Agreement. Upon the execution and delivery and subject to the terms of
G-21
Table of Contents
a Joinder by such Additional Investor, the Ordinary Shares then owned, or underlying any rights then owned, by such Additional Investor (the “Additional Investor Ordinary Shares”) shall be Registrable Securities to the extent provided herein and therein and such Additional Investor shall be an Investor under this Agreement with respect to such Additional Investor Ordinary Shares.
6.11 Severability. It is the desire and intent of the parties that the provisions of this Agreement be enforced to the fullest extent permissible under the laws and public policies applied in each jurisdiction in which enforcement is sought. Accordingly, if any particular provision of this Agreement shall be adjudicated by a court of competent jurisdiction to be invalid, prohibited or unenforceable for any reason, such provision, as to such jurisdiction, shall be ineffective, without invalidating the remaining provisions of this Agreement or affecting the validity or enforceability of this Agreement or affecting the validity or enforceability of such provision in any other jurisdiction. Notwithstanding the foregoing, if such provision could be more narrowly drawn so as not to be invalid, prohibited or unenforceable in such jurisdiction, it shall, as to such jurisdiction, be so narrowly drawn, without invalidating the remaining provisions of this Agreement or affecting the validity or enforceability of such provision in any other jurisdiction.
6.12 Entire Agreement. This Agreement constitutes the full and entire agreement and understanding between the parties with respect to the subject matter hereof and supersedes all prior agreements and understandings relating to such subject matter. Upon the Closing, the Prior Agreements shall no longer be of any force or effect.
6.13 Adjustments. If, and as often as, there are any changes in the Registrable Securities by way of stock split, stock dividend, combination or reclassification, or through merger, consolidation, reorganization, recapitalization or sale, or by any other means, appropriate adjustment shall be made in the provisions of this Agreement, as may be required, so that the rights, privileges, duties and obligations hereunder shall continue with respect to the Registrable Securities as so changed.
[Signature pages follow]
G-22
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be executed as of the date first written above.
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||
| By: |
| |
| Name: | ||
| Title: | ||
[Signature Page to Investor Rights Agreement]
G-23
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be executed as of the date first written above.
| INVESTORS: | ||
| [ ] | ||
| By: |
| |
| Name: | ||
| Title: | ||
[Signature Page to Investor Rights Agreement]
G-24
Table of Contents
Exhibit A
Form of Joinder Agreement
The undersigned is executing and delivering this joinder agreement (this “Joinder”) pursuant to the Investor Rights Agreement, dated as of [●], 2022 (as the same may hereafter be amended, the “Investor Rights Agreement”), by and among NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”) and the other persons or entities named as parties therein. Capitalized terms used but not otherwise defined herein shall have the meanings provided in the Investor Rights Agreement.
By executing and delivering this Joinder to Holdco, and upon acceptance hereof by Holdco upon the execution of a counterpart hereof, the undersigned hereby agrees to become a party to, to be bound by, and to comply with the Investor Rights Agreement as a holder of Registrable Securities in the same manner as if the undersigned were an original signatory to the Investor Rights Agreement, and the undersigned’s Ordinary Shares shall be included as Registrable Securities under the Investor Rights Agreement to the extent provided therein.
Accordingly, the undersigned has executed and delivered this Joinder as of the day of , 20 .
| ||
| Signature of Stockholder | ||
| ||
| Print Name of Stockholder | ||
| Its: | ||
| Address: |
| |
| ||
| ||
Agreed and Accepted as of
, 20
| NewAmsterdam Pharma Company B.V. | ||
| By: |
| |
| Name: | ||
| Its: | ||
G-25
Table of Contents
FORM OF LOCK-UP AGREEMENT
THIS LOCK-UP AGREEMENT (this “Agreement”), dated as of [●], 2022, is made and entered into by and among NewAmsterdam Pharma Company B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (“Holdco”), and the holders of shares in the capital of the Company listed on Schedule I hereto (collectively, the “Investors” and each, an “Investor”).
RECITALS
WHEREAS, Frazier Lifesciences Acquisition Corporation, a blank check company incorporated as a Cayman Islands exempted company (“FLAC”), Holdco, NewAmsterdam Pharma Holding B.V., a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid) incorporated under the laws of the Netherlands (the “Company”), and NewAmsterdam Pharma Investment Corporation, a Cayman Islands exempted company and a direct wholly-owned subsidiary of Holdco (“Merger Sub”), have entered into that certain Business Combination Agreement, dated as of [●], 2022 (as it may be amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement”), pursuant to which, among other things, (i) each Company Shareholder (as defined in the Business Combination Agreement), including the Investors, of the Company will exchange his, her or its ordinary shares in the share capital of the Company (the “Company Ordinary Shares”) for ordinary shares in the share capital of Holdco (the “Holdco Ordinary Shares”) on the terms and subject to the conditions therein (the “Exchange”) and (ii) Merger Sub will merge with and into FLAC, with FLAC surviving as a wholly-owned subsidiary of Holdco, on the terms and subject to the conditions therein (the “Merger”); and
WHEREAS, as a condition of, and as a material inducement for FLAC to enter into and consummate the transactions contemplated by the Business Combination Agreement, the Investor has agreed to execute and deliver this Agreement.
NOW, THEREFORE, in consideration of the representations, covenants and agreements contained herein, and certain other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE I
DEFINITIONS
1.1 Definitions. The terms defined in this Article I shall, for all purposes of this Agreement, have the respective meanings set forth below:
“Agreement” shall have the meaning given in the Preamble hereto.
“Board” shall mean the Board of Directors of Holdco.
“Business Combination Agreement” shall have the meaning given in the Recitals hereto.
“Closing” shall have the meaning given in the Business Combination Agreement.
“Commission” shall mean the U.S. Securities and Exchange Commission.
H-1
Table of Contents
“Company” shall have the meaning given in the Preamble hereto.
“Company Ordinary Shares” shall have the meaning given in the Preamble hereto.
“Control” means, in relation to any Person, (i) direct, indirect or beneficial ownership of the majority of the voting rights and/or capital interests in such Person, (ii) the power, directly or indirectly, to designate, nominate or remove more than half of the members of the board of directors, management board, supervisory board or similar corporate body of such Person, and/or (iii) the power, directly or indirectly, whether by contract or otherwise, to direct or cause the direction of the management, the affairs, the policies and/or investment decisions of such Person and the terms “Controlled” and “Controlling” have meanings correlative thereto.
“Exchange” shall have the meaning given in the Recitals hereto.
“Exchange Act” shall mean the Securities Exchange Act of 1934, as it may be amended from time to time.
“Final Closing Date” shall have the meaning given in the Business Combination Agreement.
“FLAC” shall have the meaning given in the Preamble hereto.
“Holdco” shall have the meaning given in the Preamble hereto and includes Holdco’s successors by recapitalization, merger, consolidation, spin-off, reorganization or similar transaction.
“Investor Affiliate” means any Person who directly or indirectly, through one or more intermediaries, Controls, is Controlled by, or is under common Control with, the Investor, or in case of an investment fund, its investment manager and/or advisor or an investment fund that is managed and/or advised by an entity that is under common Control with one of the foregoing.
“Investors” shall have the meaning given in the Preamble hereto, for so long as such person or entity holds any Ordinary Shares.
“Lock-up” shall have the meaning given in Section 2.1.
“Lock-up Parties” shall mean, as applicable, the Investors and their respective Permitted Transferees.
“Lock-up Period” shall mean, with respect to each Investor:
(i) with respect to 50% of the Ordinary Shares held by such Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is six (6) months after the Final Closing Date; (b) the date on which the closing price of an Ordinary Share equals or exceeds $12.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any twenty (20) trading days within any thirty (30)-trading day period commencing at least one hundred and fifty (150) days after the Final Closing Date; or (c) the date on which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property; and
(ii) with respect to the remaining 50% of the Ordinary Shares held by such Investor, the period beginning on the Final Closing Date and ending on the earlier of: (a) the date that is six (6) months after the Final Closing Date; or (b) the date on which Holdco completes a liquidation, merger, share exchange, reorganization or other similar transaction that results in all Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property; and
“Lock-up Shares” shall mean the Ordinary Shares and any other equity securities convertible into or exercisable or exchangeable for the Ordinary Shares (including, without limitation, any existing Company
H-2
Table of Contents
Options (as defined in the Business Combination Agreement) and/or awards issued under the Holdco Equity Incentive Plan (as defined in the Business Combination Agreement)) held by the Investors immediately following the Closing (other than the PIPE Shares or Ordinary Shares acquired in the public market).
“Merger” shall have the meaning given in the Recitals hereto.
“Merger Sub” shall have the meaning given in the Recitals hereto.
“Ordinary Shares” shall mean, prior to the Closing, the Company Ordinary Shares and, following the Closing, the Holdco Ordinary Shares.
“Permitted Transferees” shall mean with respect to the Investors and their respective Permitted Transferees, (i) prior to the expiration of the Lock-up Period, any person or entity to whom such Investor is permitted to transfer such Lock-up Shares prior to the expiration of the Lock-up Period pursuant to Section 2.2 and (ii) after the expiration of the Lock-up Period, any person or entity to whom such Investor is permitted to transfer such Lock-up Shares, subject to and in accordance with any applicable agreement between such Investor and/or their respective Permitted Transferees and Holdco and any transferee thereafter.
“Person” means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association, trust, joint venture, investment fund, foundation or other similar entity, whether or not a legal entity.
“PIPE Shares” shall mean the Ordinary Shares acquired by any Investor in connection with such Investor’s participation in the PIPE Financing, as defined in the Business Combination Agreement.
“Securities Act” shall mean the Securities Act of 1933, as amended from time to time.
“Transfer” shall mean the (i) sale or assignment of, offer to sell, contract or agreement to sell, hypothecate, pledge, grant of any option to purchase or otherwise dispose of or agreement to dispose of, directly or indirectly, or establishment or increase of a put equivalent position or liquidation with respect to or decrease of a call equivalent position within the meaning of Section 16 of the Exchange Act, and the rules and regulations of the Commission promulgated thereunder, with respect to, any security, (ii) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (iii) public announcement of any intention to effect any transaction specified in clause (i) or (ii).
ARTICLE II
LOCK-UP
2.1 Lock-Up. Subject to Section 2.2 and Section 2.3, each Investor agrees that it shall not Transfer any Lock-up Shares prior to the end of, in respect of such Investor, the applicable Lock-up Period (the “Lock-up”).
2.2 Permitted Transferees. The foregoing restrictions set forth in Section 2.1 shall not apply to: (i) 10% of the Ordinary Shares held by each of Holdco’s officers and employees or (ii) Transfers made (a) in the case of an individual, pursuant to a bona fide gift to a member of the individual’s immediate family or to a trust, the beneficiary of which is a member of the individual’s immediate family or an affiliate of such person or entity, or to a charitable organization; (b) in the case of an individual, by virtue of laws of descent and distribution upon death of the individual; (c) in the case of an individual, pursuant to a qualified domestic relations order; (d) in the case of a trust, by distribution to one or more of the permissible beneficiaries of such trust; (e) in the case of a corporation, partnership (whether general, limited or otherwise), limited liability company, trust or other business
H-3
Table of Contents
entity, (x) Transfers to another corporation, partnership, limited liability company, trust or other business entity that controls, is controlled by or is under common control or management with an Investor (including, for the avoidance of doubt, where such Investor is a partnership, to its general partner or a successor partnership or fund, or any other funds managed by such partnership), or (y) as part of a distribution, transfer or other disposition of Ordinary Shares to partners, limited liability company members or stockholders of an Investor; (f) in connection with any bona fide mortgage, encumbrance or pledge to a financial institution in connection with any bona fide loan or debt transaction or enforcement thereunder, (g) in connection with a liquidation, merger, stock exchange, reorganization, tender offer approved by the Board or a duly authorized committee thereof or other similar transaction which results in all of Holdco’s shareholders having the right to exchange their Ordinary Shares for cash, securities or other property subsequent to the Final Closing Date or (h) to cover withholding taxes upon the vesting of awards issued pursuant to the Holdco Equity Incentive Plan. The parties acknowledge and agree that any Permitted Transferee of an Investor must enter into a written agreement agreeing to be bound by the terms of this Agreement in form and substance reasonably satisfactory to the Company, including the transfer restrictions set forth in this Article II.
2.3 Notwithstanding the foregoing, except as otherwise agreed to by Holdco, if any Investor is granted a release or waiver from the Lock-up provided in this Article II (such party, a “Triggering Investor”), then each other Investor shall also be granted an early release from its obligations hereunder or under any contractual lock-up agreement with Holdco on the same terms and on a pro-rata basis with respect to such number of Lock-up Shares rounded down to the nearest whole security equal to the product of (i) the total percentage of Lock-up Shares held by the Triggering Investor immediately following the Closing that are being released from the Lock-up agreement multiplied by (ii) the total number of Lock-up Shares held by such other Investor immediately following the Closing.
ARTICLE III
MISCELLANEOUS
3.1 Notices. Any notice or communication under this Agreement must be in writing and given by (i) recorded mail, addressed to the party to be notified, postage prepaid and registered or certified with return receipt requested, (ii) delivery in person or by courier service providing evidence of delivery, or (iii) transmission by hand delivery or electronic mail. Each notice or communication that is mailed, delivered, or transmitted in the manner described above shall be deemed sufficiently given, served, sent, and received, in the case of mailed notices, on the third business day following the date on which it is mailed and, in the case of notices delivered by courier service, hand delivery or electronic mail, at such time as it is delivered to the addressee (with the delivery receipt or the affidavit of messenger) or at such time as delivery is refused by the addressee upon presentation. Any notice or communication under this Agreement must be addressed, if to Holdco, to: NewAmsterdam Pharma Company B.V., c/o NewAmsterdam Pharma Holding B.V., Gooimeer 2-35, 1411 DC Naarden, The Netherlands, Attention: Louise Kooij or by email: , and, if to any Investor, at such Investor’s address, electronic mail address as set forth in Holdco’s books and records. Any party may change its address for notice at any time and from time to time by written notice to the other parties hereto, and such change of address shall become effective thirty (30) days after delivery of such notice as provided in this Section 3.1.
3.2 Assignment; No Third Party Beneficiaries.
3.2.1 This Agreement and the rights, duties and obligations of Holdco hereunder may not be assigned or delegated by Holdco in whole or in part.
3.2.2 Subject to Section 3.2.4 and Section 3.2.5, this Agreement and the rights, duties and obligations of an Investor hereunder may be assigned in whole or in part to such Investor’s Permitted Transferees to which it transfers Lock-up Shares; provided that the rights hereunder that are personal to such Investors may not be
H-4
Table of Contents
assigned or delegated in whole or in part, except that the Investors shall be permitted to transfer its rights hereunder as the Investors to one or more Investor Affiliates or any direct or indirect partners, members or equity holders of such Investor (it being understood that no such transfer shall reduce or multiply any rights of such Investor or such transferees).
3.2.3 This Agreement and the provisions hereof shall be binding upon and shall inure to the benefit of each of the parties and its successors and the permitted assigns of the Investors, which shall include Permitted Transferees.
3.2.4 This Agreement shall not confer any rights or benefits on any persons or entities that are not parties hereto, other than as expressly set forth in this Agreement and Section 6.2.
3.2.5 No assignment by any party hereto of such party’s rights, duties and obligations hereunder shall be binding upon or obligate Holdco unless and until Holdco shall have received (i) written notice of such assignment as provided in Section 3.1 hereof and (ii) the written agreement of the assignee, in a form reasonably satisfactory to Holdco, to be bound by the terms and provisions of this Agreement. Any transfer or assignment made other than as provided in this Section 3.2 shall be null and void.
3.3 Counterparts. This Agreement may be executed in multiple counterparts (including PDF counterparts), each of which shall be deemed an original, and all of which together shall constitute the same instrument, but only one of which need be produced.
3.4 Governing Law; Venue. NOTWITHSTANDING THE PLACE WHERE THIS AGREEMENT MAY BE EXECUTED BY ANY OF THE PARTIES HERETO, THE PARTIES EXPRESSLY AGREE THAT (1) THIS AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED UNDER THE LAWS OF THE STATE OF NEW YORK AND (2) THE VENUE FOR ANY ACTION TAKEN WITH RESPECT TO THIS AGREEMENT SHALL BE ANY STATE OR FEDERAL COURT IN NEW YORK COUNTY IN THE STATE OF NEW YORK.
3.5 Waiver of Jury Trial. THE PARTIES EACH HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY LAW, ANY RIGHT TO TRIAL BY JURY OF ANY CLAIM, DEMAND, ACTION OR CAUSE OF ACTION (I) ARISING UNDER THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. THE PARTIES EACH HEREBY AGREES AND CONSENTS THAT ANY SUCH CLAIM, DEMAND, ACTION OR CAUSE OF ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE AN ORIGINAL COUNTERPART OF A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES HERETO TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) EACH SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) EACH SUCH PARTY MAKES THIS WAIVER VOLUNTARILY AND (D) EACH SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 3.5.
3.6 Amendments and Modifications. Upon the written consent of (i) Holdco and (ii) the holders of a majority of the total Lock-up Shares at the time in question, compliance with any of the provisions, covenants and conditions set forth in this Agreement may be waived, or any of such provisions, covenants or conditions
H-5
Table of Contents
may be amended or modified; provided, however, that notwithstanding the foregoing, any amendment hereto or waiver hereof shall also require the written consent of each Investor so long as such Investor and its Investor Affiliates hold, in the aggregate, at least one percent (1%) of the outstanding Ordinary Shares; and provided, further, that any amendment hereto or waiver hereof that adversely affects one Investor, solely in its capacity as a holder of the shares of capital stock of Holdco, in a manner that is materially different from the other Investors (in such capacity) shall require the consent of the Investor so affected. No course of dealing between any Investor or Holdco and any other party hereto or any failure or delay on the part of an Investor or Holdco in exercising any rights or remedies under this Agreement shall operate as a waiver of any rights or remedies of any Investor or Holdco. No single or partial exercise of any rights or remedies under this Agreement by a party shall operate as a waiver or preclude the exercise of any other rights or remedies hereunder or thereunder by such party.
3.7 Term. This Agreement shall terminate with respect to any Investor, on the date that such Investor no longer holds any Lock-up Shares.
3.8 Investor Information. Each Investor agrees, if requested in writing, to represent to Holdco the total number of Lock-up Shares held by such Investor in order for Holdco to make determinations hereunder.
3.9 Severability. It is the desire and intent of the parties that the provisions of this Agreement be enforced to the fullest extent permissible under the laws and public policies applied in each jurisdiction in which enforcement is sought. Accordingly, if any particular provision of this Agreement shall be adjudicated by a court of competent jurisdiction to be invalid, prohibited or unenforceable for any reason, such provision, as to such jurisdiction, shall be ineffective, without invalidating the remaining provisions of this Agreement or affecting the validity or enforceability of this Agreement or affecting the validity or enforceability of such provision in any other jurisdiction. Notwithstanding the foregoing, if such provision could be more narrowly drawn so as not to be invalid, prohibited or unenforceable in such jurisdiction, it shall, as to such jurisdiction, be so narrowly drawn, without invalidating the remaining provisions of this Agreement or affecting the validity or enforceability of such provision in any other jurisdiction.
3.10 Entire Agreement. This Agreement constitutes the full and entire agreement and understanding between the parties with respect to the subject matter hereof and supersedes all prior agreements and understandings relating to such subject matter.
3.11 Adjustments. If, and as often as, there are any changes in the Ordinary Shares by way of stock split, stock dividend, combination or reclassification, or through merger, consolidation, reorganization, recapitalization or sale, or by any other means, appropriate adjustment shall be made in the provisions of this Agreement, as may be required, so that the rights, privileges, duties and obligations hereunder shall continue with respect to the Ordinary Shares as so changed.
[Signature pages follow]
H-6
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be executed as of the date first written above.
| NEWAMSTERDAM PHARMA COMPANY B.V. | ||
| By: |
| |
| Name: | ||
| Title: |
[Signature Page to Lock-Up Agreement]
H-7
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be executed as of the date first written above.
| NEWAMSTERDAM PHARMA HOLDING COMPANY B.V. | ||
| By: |
| |
| Name: | ||
| Title: |
[Signature Page to Lock-Up Agreement]
H-8
Table of Contents
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be executed as of the date first written above.
| INVESTORS: | ||
| [ ] | ||
| By: |
| |
| Name: | ||
| Title: |
[Signature Page to Lock-Up Agreement]
H-9
Table of Contents
Schedule I
Investors
H-10
Table of Contents
This is a translation into English of the official Dutch version of the articles of association of a public company with limited liability under Dutch law. Definitions included in Article 1 below appear in the English alphabetical order, but will appear in the Dutch alphabetical order in the official Dutch version. In the event of a conflict between the English and Dutch texts, the Dutch text shall prevail.
ARTICLES OF ASSOCIATION
NEWAMSTERDAM PHARMA COMPANY N.V.
DEFINITIONS AND INTERPRETATION
Article 1
| 1.1 | In these articles of association the following definitions shall apply: |
| Article | An article of these articles of association. | |
| Board | The Company’s board of directors. | |
| Board Rules | The internal rules applicable to the Board, as drawn up by the Board. | |
| CEO | The Company’s chief executive officer. | |
| Chairperson | The chairperson of the Board. | |
| Company | The company to which these articles of association pertain. | |
| DCC | The Dutch Civil Code. | |
| Director | A member of the Board. | |
| Executive Director | An executive Director. | |
| General Meeting | The Company’s general meeting. | |
| Group Company | An entity or partnership which is organisationally connected with the Company in an economic unit within the meaning of Section 2:24b DCC. | |
| Indemnified Officer | A current or former Director or such other current or former officer or employee of the Company or its Group Companies as designated by the Board. | |
| Meeting Rights | With respect to the Company, the rights attributed by law to the holders of depository receipts issued for shares with a company’s cooperation, including the right to attend and address a General Meeting. | |
| Non-Executive Director | A non-executive Director. | |
| Person with Meeting Rights | A shareholder, a usufructuary or pledgee with voting rights or a holder of depository receipts for ordinary shares issued with the Company’s cooperation. | |
| Record Date | The date of registration for a General Meeting as provided by law. | |
| Simple Majority | More than half of the votes cast. | |
| Subsidiary | A subsidiary of the Company within the meaning of Section 2:24a DCC. | |
| Vice-Chairperson | The vice-chairperson of the Board. | |
| 1.2 | Unless the context requires otherwise, references to “ordinary shares” or “shareholders” are to ordinary shares in the Company’s capital or to the holders thereof, respectively. |
| I-1 |
Table of Contents
| 1.3 | References to statutory provisions are to those provisions as they are in force from time to time. |
| 1.4 | Terms that are defined in the singular have a corresponding meaning in the plural. |
| 1.5 | Words denoting a gender include each other gender. |
| 1.6 | Except as otherwise required by law, the terms “written” and “in writing” include the use of electronic means of communication. |
NAME AND SEAT
Article 2
| 2.1 | The Company’s name is NewAmsterdam Pharma Company N.V. |
| 2.2 | The Company has its corporate seat in Naarden. |
OBJECTS
Article 3
The Company’s objects are:
| a. | to develop, conduct research, produce, commercialize, market and sell medicines in general and innovative medicines for cardiovascular diseases in particular; |
| b. | to incorporate, to participate in, to finance, to hold any other interest in and to conduct the management or supervision of other entities, companies, partnerships and businesses; |
| c. | to provide administrative, technical, financial, economic or other services to other entities, companies, partnerships and businesses; |
| d. | to acquire, to manage, to invest, to exploit, to encumber and to dispose of assets and liabilities; |
| e. | to furnish guarantees, to provide security, to warrant performance in any other way and to assume liability, whether jointly and severally or otherwise, in respect of obligations of group companies or other parties; and |
| f. | to do anything which, in the widest sense, is connected with or may be conducive to the objects described above. |
SHARES - AUTHORISED SHARE CAPITAL AND DEPOSITORY RECEIPTS
Article 4
| 4.1 | The Company’s authorised share capital amounts to [amount] euro (EUR [amount]). |
| 4.2 | The authorised share capital is divided into [number] ([number]) ordinary shares, each having a nominal value of twelve eurocents (EUR 0.12). |
| 4.3 | The Board may resolve that one or more ordinary shares are divided into such number of fractional ordinary shares as may be determined by the Board. Unless specified differently, the provisions of these articles of association concerning ordinary shares and shareholders apply mutatis mutandis to fractional ordinary shares and the holders thereof, respectively. |
| 4.4 | The Company may cooperate with the issue of depository receipts for ordinary shares in its capital. |
| I-2 |
Table of Contents
SHARES - FORM AND SHARE REGISTER
Article 5
| 5.1 | All ordinary shares are in registered form. The Company may issue share certificates for ordinary shares in registered form as may be approved by the Board. Each Director is authorised to sign any such share certificate on behalf of the Company. |
| 5.2 | Ordinary shares shall be numbered consecutively, starting from 1. |
| 5.3 | The Board shall keep a register setting out the names and addresses of all shareholders and all holders of a usufruct or pledge in respect of ordinary shares. The register shall also set out any other particulars that must be included in the register pursuant to applicable law. Part of the register may be kept outside the Netherlands to comply with applicable local law or pursuant to stock exchange rules. |
| 5.4 | Shareholders, usufructuaries and pledgees shall provide the Board with the necessary particulars in a timely fashion. Any consequences of not, or incorrectly, notifying such particulars shall be borne by the party concerned. |
| 5.5 | All notifications may be sent to shareholders, usufructuaries and pledgees at their respective addresses as set out in the register. |
SHARES - ISSUE
Article 6
| 6.1 | The Company can only issue ordinary shares pursuant to a resolution of the General Meeting or of another body authorised by the General Meeting for this purpose for a specified period not exceeding five years. When granting such authorisation, the number of ordinary shares that may be issued must be specified. The authorisation may be extended, in each case for a period not exceeding five years. Unless stipulated differently when granting the authorisation, the authorisation cannot be revoked. For as long as and to the extent that another body has been authorised to resolve to issue ordinary shares, the General Meeting shall not have this authority. |
| 6.2 | Article 6.1 applies mutatis mutandis to the granting of rights to subscribe for ordinary shares, but does not apply in respect of issuing ordinary shares to a party exercising a previously acquired right to subscribe for ordinary shares. |
| 6.3 | The Company may not subscribe for ordinary shares in its own capital. |
SHARES - PRE-EMPTION RIGHTS
Article 7
| 7.1 | Upon an issue of ordinary shares, each shareholder shall have a pre-emption right in proportion to the aggregate nominal value of his ordinary shares. |
| 7.2 | In deviation of Article 7.1, shareholders do not have pre-emption rights in respect of: |
| a. | ordinary shares issued against non-cash contribution; or |
| b. | ordinary shares issued to employees of the Company or of a Group Company. |
| 7.3 | The Company shall announce an issue with pre-emption rights and the period during which those rights can be exercised in the State Gazette and in a daily newspaper with national distribution, unless the announcement is sent in writing to all shareholders at the addresses submitted by them. |
| 7.4 | Pre-emption rights may be exercised for a period of at least two weeks after the date of announcement in the State Gazette or after the announcement was sent to the shareholders. |
| I-3 |
Table of Contents
| 7.5 | Pre-emption rights may be limited or excluded by a resolution of the General Meeting or of the body authorised as referred to in Article 6.1, if that body was authorised by the General Meeting for this purpose for a specified period not exceeding five years. The authorisation may be extended, in each case for a period not exceeding five years. Unless stipulated differently when granting the authorisation, the authorisation cannot be revoked. For as long as and to the extent that another body has been authorised to resolve to limit or exclude pre-emption rights, the General Meeting shall not have this authority. |
| 7.6 | A resolution of the General Meeting to limit or exclude pre-emption rights, or to grant an authorisation as referred to in Article 7.5, shall require a majority of at least two thirds of the votes cast if less than half of the issued share capital is represented at the General Meeting. |
| 7.7 | The preceding provisions of this Article 7 apply mutatis mutandis to the granting of rights to subscribe for ordinary shares, but do not apply in respect of issuing ordinary shares to a party exercising a previously acquired right to subscribe for ordinary shares. |
SHARES - PAYMENT
Article 8
| 8.1 | Without prejudice to Section 2:80(2) DCC, the nominal value of an ordinary share and, if the ordinary share is subscribed for at a higher price, the difference between these amounts must be paid up upon subscription for that ordinary share. |
| 8.2 | Ordinary shares must be paid up in cash, except to the extent that payment by means of a contribution in another form has been agreed. |
| 8.3 | Payment in a currency other than the euro can only be made with the Company’s consent. Where such a payment is made, the payment obligation is satisfied for the amount in euro for which the paid amount can be freely exchanged. Without prejudice to the last sentence of Section 2:80a(3) DCC, the date of the payment determines the exchange rate. |
SHARES - FINANCIAL ASSISTANCE
Article 9
| 9.1 | The Company may not provide security, give a price guarantee, warrant performance in any other way or commit itself jointly and severally or otherwise with or for others with a view to the subscription for or acquisition of ordinary shares or depository receipts for ordinary shares in its capital by others. This prohibition applies equally to Subsidiaries. |
| 9.2 | The Company and its Subsidiaries may not provide loans with a view to the subscription for or acquisition of ordinary shares or depository receipts for ordinary shares in the Company’s capital by others, unless the Board resolves to do so and Section 2:98c DCC is observed. |
| 9.3 | The preceding provisions of this Article 9 do not apply if ordinary shares or depository receipts for ordinary shares are subscribed for or acquired by or for employees of the Company or of a Group Company. |
SHARES - ACQUISITION OF OWN SHARES
Article 10
| 10.1 | The acquisition by the Company of ordinary shares in its own capital which have not been fully paid up shall be null and void. |
| 10.2 | The Company may only acquire fully paid up ordinary shares in its own capital for no consideration or if and to the extent that the General Meeting has authorised the Board for this purpose and all other relevant statutory requirements of Section 2:98 DCC are observed. |
| I-4 |
Table of Contents
| 10.3 | An authorisation as referred to in Article 10.2 remains valid for no longer than eighteen months. When granting such authorisation, the General Meeting shall determine the number of ordinary shares that may be acquired, how they may be acquired and within which range the acquisition price must be. An authorisation shall not be required for the Company to acquire ordinary shares in its own capital in order to transfer them to employees of the Company or of a Group Company pursuant to an arrangement applicable to them, provided that these ordinary shares are included on the price list of a stock exchange. |
| 10.4 | Without prejudice to Articles 10.1 through 10.3, the Company may acquire ordinary shares in its own capital for cash consideration or for consideration satisfied in the form of assets. In the case of a consideration being satisfied in the form of assets, the value thereof, as determined by the Board, must be within the range stipulated by the General Meeting as referred to in Article 10.3. |
| 10.5 | The previous provisions of this Article 10 do not apply to ordinary shares acquired by the Company under universal title of succession. |
| 10.6 | In this Article 10, references to ordinary shares include depository receipts for ordinary shares. |
SHARES - REDUCTION OF ISSUED SHARE CAPITAL
Article 11
| 11.1 | The General Meeting can resolve to reduce the Company’s issued share capital by cancelling ordinary shares or by reducing the nominal value of ordinary shares by virtue of an amendment to these articles of association. The resolution must designate the ordinary shares to which the resolution relates and it must provide for the implementation of the resolution. |
| 11.2 | A resolution to cancel ordinary shares may only relate to ordinary shares held by the Company itself or in respect of which the Company holds the depository receipts. |
| 11.3 | A resolution of the General Meeting to reduce the Company’s issued share capital shall require a majority of at least two thirds of the votes cast if less than half of the issued share capital is represented at the General Meeting. |
SHARES - ISSUE AND TRANSFER REQUIREMENTS
Article 12
| 12.1 | Except as otherwise provided or allowed by Dutch law, the issue or transfer of an ordinary share shall require a deed to that effect and, in the case of a transfer and unless the Company itself is a party to the transaction, acknowledgement of the transfer by the Company. |
| 12.2 | The acknowledgement shall be set out in the deed or shall be made in such other manner as prescribed by law. |
| 12.3 | For as long as any ordinary shares are admitted to trading on the New York Stock Exchange, the NASDAQ Stock Market or on any other regulated stock exchange operating in the United States of America, the laws of the State of New York shall apply to the property law aspects of the ordinary shares (including the statutory provisions concerning the transfer and ownership of legal title to ordinary shares) reflected in the register administered by the relevant transfer agent, without prejudice to the applicable provisions of Chapters 4 and 5 of Title 10 of Book 10 DCC. |
SHARES - USUFRUCT AND PLEDGE
Article 13
| 13.1 | Ordinary shares can be encumbered with a usufruct or pledge. |
| 13.2 | The voting rights attached to an ordinary share which is subject to a usufruct or pledge vest in the shareholder concerned. |
| I-5 |
Table of Contents
| 13.3 | In deviation of Article 13.2, the holder of a usufruct or pledge on ordinary shares shall have the voting rights attached thereto if this was provided when the usufruct or pledge was created. |
| 13.4 | Usufructuaries and pledgees without voting rights shall not have Meeting Rights. |
BOARD - COMPOSITION
Article 14
| 14.1 | The Company has a Board consisting of: |
| a. | one or more Executive Directors, being primarily charged with the Company’s day-to-day operations; and |
| b. | one or more Non-Executive Directors, being primarily charged with the supervision of the performance of the duties of the Directors. |
The Board shall be composed of individuals.
| 14.2 | The Board shall determine the number of Executive Directors and the number of Non-Executive Directors. |
| 14.3 | The Board shall elect an Executive Director to be the CEO. The Board may dismiss the CEO, provided that the CEO so dismissed shall subsequently continue his term of office as an Executive Director without having the title of CEO. |
| 14.4 | The Board shall elect a Non-Executive Director to be the Chairperson and another Non-Executive Director to be the Vice-Chairperson. The Board may dismiss the Chairperson or Vice-Chairperson, provided that the Chairperson or Vice-Chairperson so dismissed shall subsequently continue his term of office as a Non-Executive Director without having the title of Chairperson or Vice-Chairperson, respectively. |
| 14.5 | If a Director is absent or unable to act, he may be replaced temporarily by a person whom the Board has designated for that purpose and, until then, the other Director(s) shall be charged with the management of the Company. If all Directors are absent or unable to act, the management of the Company shall be attributed to the person who most recently ceased to hold office as the Chairperson. If such former Chairperson is unwilling or unable to accept that position, the management of the Company shall be attributed to the person who most recently ceased to hold office as the CEO. If such former CEO is also unwilling or unable to accept that position, the management of the Company shall be attributed to one or more persons whom the General Meeting has designated for that purpose. The person(s) charged with the management of the Company in this manner, may designate one or more persons to be charged with the management of the Company instead of, or together with, such person(s). |
| 14.6 | A Director shall be considered to be absent or unable to act, as applicable, within the meaning of Article 14.5: |
| a. | during the existence of a vacancy on the Board, including as a result of: |
| i. | his death; |
| ii. | his dismissal by the General Meeting, other than at the proposal of the Board; |
| iii. | his voluntary resignation before his term of office has expired; or |
| iv. | not being reappointed by the General Meeting, notwithstanding a (binding) nomination to that effect by the Board, |
provided that the Board may always decide to decrease the number of Directors such that a vacancy no longer exists;
| b. | during his suspension; or |
| c. | in a period during which the Company has not been able to contact him (including as a result of illness), provided that such period lasted longer than five consecutive days (or such other period as determined by the Board on the basis of the facts and circumstances at hand). |
| I-6 |
Table of Contents
BOARD - APPOINTMENT, SUSPENSION AND DISMISSAL
Article 15
| 15.1 | The General Meeting shall appoint the Directors and may at any time suspend or dismiss any Director. In addition, the Board may at any time suspend an Executive Director. |
| 15.2 | The General Meeting can only appoint Directors upon a nomination by the Board. The General Meeting may at any time resolve to render such nomination to be non-binding by a majority of at least two thirds of the votes cast representing more than half of the issued share capital. If a nomination is rendered non-binding, a new nomination shall be made by the Board. If the nomination comprises one candidate for a vacancy, a resolution concerning the nomination shall result in the appointment of the candidate, unless the nomination is rendered non-binding. A second meeting as referred to in Section 2:120(3) DCC cannot be convened. |
| 15.3 | At a General Meeting, a resolution to appoint a Director can only be passed in respect of candidates whose names are stated for that purpose in the agenda of that General Meeting or the explanatory notes thereto. |
| 15.4 | Upon the appointment of a person as a Director, the General Meeting shall determine whether that person is appointed as Executive Director or as Non-Executive Director. |
| 15.5 | A resolution of the General Meeting to suspend or dismiss a Director shall require a majority of at least two thirds of the votes cast representing more than half of the issued share capital, unless the resolution is passed at the proposal of the Board. A second meeting as referred to in Section 2:120(3) DCC cannot be convened. |
| 15.6 | If a Director is suspended and the General Meeting does not resolve to dismiss him within three months from the date of such suspension, the suspension shall lapse. |
BOARD - DUTIES AND ORGANISATION
Article 16
| 16.1 | The Board is charged with the management of the Company, subject to the restrictions contained in these articles of association. This includes in any event setting the Company’s policy and strategy. In performing their duties, Directors shall be guided by the interests of the Company and of the business connected with it. |
| 16.2 | The Board shall draw up Board Rules concerning its organisation, decision-making and other internal matters, with due observance of these articles of association. In performing their duties, the Directors shall act in compliance with the Board Rules. |
| 16.3 | The Directors may allocate their duties amongst themselves in or pursuant to the Board Rules or otherwise pursuant to resolutions adopted by the Board, provided that: |
| a. | the Executive Directors shall be charged with the Company’s day-to-day operations; |
| b. | the task of supervising the performance of the duties of the Directors cannot be taken away from the Non-Executive Directors; |
| c. | the Chairperson must be a Non-Executive Director; and |
| d. | the making of proposals for the appointment of a Director and the determination of the compensation of the Executive Directors cannot be allocated to an Executive Director. |
| 16.4 | The Board may determine in writing, in or pursuant to the Board Rules or otherwise pursuant to resolutions adopted by the Board, that one or more Directors can validly pass resolutions in respect of matters which fall under his/their duties. |
| 16.5 | The Board shall establish the committees which the Company is required to have and otherwise such committees as are deemed to be appropriate by the Board. The Board shall draw up (and/or include in the Board Rules) rules concerning the organisation, decision-making and other internal matters of its committees. |
| I-7 |
Table of Contents
| 16.6 | The Board may perform the legal acts referred to in Section 2:94(1) DCC without the prior approval of the General Meeting. |
BOARD - DECISION-MAKING
Article 17
| 17.1 | Without prejudice to Article 17.5, each Director may cast one vote in the decision-making of the Board. |
| 17.2 | A Director can be represented by another Director holding a written proxy for the purpose of the deliberations and the decision-making of the Board. |
| 17.3 | Resolutions of the Board shall be passed, irrespective of whether this occurs at a meeting or otherwise, by Simple Majority unless the Board Rules provide differently. |
| 17.4 | Invalid votes, blank votes and abstentions shall not be counted as votes cast. Directors who casted an invalid or blank vote or who abstained from voting shall be taken into account when determining the number of Directors who are present or represented at a meeting of the Board. |
| 17.5 | Where there is a tie in any vote of the Board, the Chairperson shall have a casting vote, provided that there are at least three Directors in office. Otherwise, the relevant resolution shall not have been passed. |
| 17.6 | The Executive Directors shall not participate in the decision-making concerning: |
| a. | the determination of the compensation of Executive Directors; and |
| b. | the instruction of an auditor to audit the annual accounts if the General Meeting has not granted such instruction. |
| 17.7 | A Director shall not participate in the deliberations and decision-making of the Board on a matter in relation to which he has a direct or indirect personal interest which conflicts with the interests of the Company and of the business connected with it. If, as a result thereof, no resolution can be passed by the Board, the resolution may nevertheless be passed by the Board as if none of the Directors has a conflict of interests as described in the previous sentence. |
| 17.8 | Meetings of the Board can be held through audio-communication facilities, unless a Director objects thereto. |
| 17.9 | Resolutions of the Board may, instead of at a meeting, be passed in writing, provided that all Directors are familiar with the resolution to be passed and none of them objects to this decision-making process. Articles 17.1 through 17.7 apply mutatis mutandis. |
| 17.10 | The approval of the General Meeting is required for resolutions of the Board concerning a material change to the identity or the character of the Company or the business, including in any event: |
| a. | transferring the business or materially all of the business to a third party; |
| b. | entering into or terminating a long-lasting alliance of the Company or of a Subsidiary either with another entity or company, or as a fully liable partner of a limited partnership or general partnership, if this alliance or termination is of significant importance for the Company; and |
| c. | acquiring or disposing of an interest in the capital of a company by the Company or by a Subsidiary with a value of at least one third of the value of the assets, according to the balance sheet with explanatory notes or, if the Company prepares a consolidated balance sheet, according to the consolidated balance sheet with explanatory notes in the Company’s most recently adopted annual accounts. |
| 17.11 | The absence of the approval of the General Meeting of a resolution as referred to in Article 17.10 shall result in the relevant resolution being null and void pursuant to Section 2:14(1) DCC but shall not affect the powers of representation of the Board or of the Directors. |
| I-8 |
Table of Contents
BOARD - COMPENSATION
Article 18
| 18.1 | The General Meeting shall determine the Company’s policy concerning the compensation of the Board with due observance of the relevant statutory requirements. |
| 18.2 | The compensation of Directors shall be determined by the Board with due observance of the policy referred to in Article 18.1. |
| 18.3 | The Board shall submit proposals concerning compensation arrangements for the Board in the form of ordinary shares or rights to subscribe for ordinary shares to the General Meeting for approval. This proposal must at least include the number of ordinary shares or rights to subscribe for ordinary shares that may be awarded to the Board and which criteria apply for such awards or changes thereto. The absence of the approval of the General Meeting shall not affect the powers of representation. |
BOARD - REPRESENTATION
Article 19
| 19.1 | The Board is entitled to represent the Company. |
| 19.2 | The power to represent the Company also vests in the CEO individually, as well as in any other two Executive Directors acting jointly. |
| 19.3 | The Company may also be represented by the holder of a power of attorney to that effect. If the Company grants a power of attorney to an individual, the Board may grant an appropriate title to such person. |
INDEMNITY
Article 20
| 20.1 | The Company shall indemnify and hold harmless each of its Indemnified Officers against: |
| a. | any financial losses or damages incurred by such Indemnified Officer; and |
| b. | any expense reasonably paid or incurred by such Indemnified Officer in connection with any threatened, pending or completed suit, claim, action or legal proceedings of a civil, criminal, administrative or other nature, formal or informal, in which he becomes involved, |
to the extent this relates to his current or former position with the Company and/or a Group Company and in each case to the extent permitted by applicable law.
| 20.2 | No indemnification shall be given to an Indemnified Officer: |
| a. | if a competent court or arbitral tribunal has established, without having (or no longer having) the possibility for appeal, that the acts or omissions of such Indemnified Officer that led to the financial losses, damages, expenses, suit, claim, action or legal proceedings as described in Article 20.1 are of an unlawful nature (including acts or omissions which are considered to constitute malice, gross negligence, intentional recklessness and/or serious culpability attributable to such Indemnified Officer); |
| b. | to the extent that his financial losses, damages and expenses are covered under insurance and the relevant insurer has settled, or has provided reimbursement for, these financial losses, damages and expenses (or has irrevocably undertaken to do so); |
| c. | in relation to proceedings brought by such Indemnified Officer against the Company, except for proceedings brought to enforce indemnification to which he is entitled pursuant to these articles of association, pursuant to an agreement between such Indemnified Officer and the Company which has been approved by the Board or pursuant to insurance taken out by the Company for the benefit of such Indemnified Officer; or |
| I-9 |
Table of Contents
| d. | for any financial losses, damages or expenses incurred in connection with a settlement of any proceedings effected without the Company’s prior consent. |
| 20.3 | The Board may stipulate additional terms, conditions and restrictions in relation to the indemnification referred to in Article 20.1. |
GENERAL MEETING - CONVENING AND HOLDING MEETINGS
Article 21
| 21.1 | Annually, at least one General Meeting shall be held. This annual General Meeting shall be held within six months after the end of the Company’s financial year. |
| 21.2 | A General Meeting shall also be held: |
| a. | within three months after the Board has considered it to be likely that the Company’s equity has decreased to an amount equal to or lower than half of its paid up and called up capital, in order to discuss the measures to be taken if so required; and |
| b. | whenever the Board so decides. |
| 21.3 | General Meetings must be held in the place where the Company has its corporate seat or in Amsterdam, Arnhem, Assen, The Hague, Haarlem, ‘s-Hertogenbosch, Groningen, Leeuwarden, Lelystad, Maastricht, Middelburg, Rotterdam, Schiphol (municipality of Haarlemmermeer), Utrecht or Zwolle. |
| 21.4 | If the Board has failed to ensure that a General Meeting as referred to in Articles 21.1 or 21.2 paragraph a. is held, each Person with Meeting Rights may be authorised by the court in preliminary relief proceedings to do so. |
| 21.5 | One or more Persons with Meeting Rights who collectively represent at least the part of the Company’s issued share capital prescribed by law for this purpose may request the Board in writing to convene a General Meeting, setting out in detail the matters to be discussed. If the Board has not taken the steps necessary to ensure that the General Meeting could be held within the relevant statutory period after the request, the requesting Person(s) with Meeting Rights may be authorised, at his/their request, by the court in preliminary relief proceedings to convene a General Meeting. |
| 21.6 | Any matter of which the discussion has been requested in writing by one or more Persons with Meeting Rights who, individually or collectively, represent at least the part of the Company’s issued share capital prescribed by law for this purpose shall be included in the convening notice or announced in the same manner, if the Company has received the substantiated request or a proposal for a resolution no later than on the sixtieth day prior to that of the General Meeting. |
| 21.7 | Persons with Meeting Rights who wish to exercise their rights as described in Articles 21.5 and 21.6 must first consult the Board. In that respect, the Board shall have, and Persons with Meeting Rights must observe, the right to invoke any cooling-off period and response period provided under applicable law and/or the Dutch Corporate Governance Code. |
| 21.8 | A General Meeting must be convened with due observance of the relevant statutory minimum convening period. |
| 21.9 | All Persons with Meeting Rights must be convened for the General Meeting in accordance with applicable law. The shareholders may be convened for the General Meeting by means of convening letters sent to the addresses of those shareholders in accordance with Article 5.5. The previous sentence does not prejudice the possibility of sending a convening notice by electronic means in accordance with Section 2:113(4) DCC. |
| I-10 |
Table of Contents
GENERAL MEETING - PROCEDURAL RULES
Article 22
| 22.1 | The General Meeting shall be chaired by one of the following individuals, taking into account the following order of priority: |
| a. | by the Chairperson, if there is a Chairperson and he is present at the General Meeting; |
| b. | by the Vice-Chairperson, if there is a Vice-Chairperson and he is present at the General Meeting; |
| c. | by another Non-Executive Director who is chosen by the Non-Executive Directors present at the General Meeting from their midst; |
| d. | by the CEO, if there is a CEO and he is present at the General Meeting; or |
| e. | by another person appointed by the General Meeting. |
The person who should chair the General Meeting pursuant to paragraphs a. through d. may appoint another person to chair the General Meeting instead of him. |
| 22.2 | The chairperson of the General Meeting shall appoint another person present at the General Meeting to act as secretary and to minute the proceedings at the General Meeting. The minutes of a General Meeting shall be adopted by the chairperson of that General Meeting or by the Board. Where an official report of the proceedings is drawn up by a civil law notary, no minutes need to be prepared. Every Director may instruct a civil law notary to draw up such an official report at the Company’s expense. |
| 22.3 | The chairperson of the General Meeting shall decide on the admittance to the General Meeting of persons other than: |
| a. | the persons who have Meeting Rights at that General Meeting, or their proxyholders; and |
| b. | those who have a statutory right to attend that General Meeting on other grounds. |
| 22.4 | The holder of a written proxy from a Person with Meeting Rights who is entitled to attend a General Meeting shall only be admitted to that General Meeting if the proxy is determined to be acceptable by the chairperson of that General Meeting. |
| 22.5 | The Company may direct that any person, before being admitted to a General Meeting, identify himself by means of a valid passport or driver’s license and/or should be submitted to such security arrangements as the Company may consider to be appropriate under the given circumstances. Persons who do not comply with these requirements may be refused entry to the General Meeting. |
| 22.6 | The chairperson of the General Meeting has the right to eject any person from the General Meeting if he considers that person to disrupt the orderly proceedings at the General Meeting. |
| 22.7 | The General Meeting may be conducted in a language other than the Dutch language, if so determined by the chairperson of the General Meeting. |
| 22.8 | The chairperson of the General Meeting may limit the amount of time that persons present at the General Meeting are allowed to take in addressing the General Meeting and the number of questions they are allowed to raise, with a view to safeguarding the orderly proceedings at the General Meeting. The chairperson of the General Meeting may also adjourn the meeting if he considers that this shall safeguard the orderly proceedings at the General Meeting. |
GENERAL MEETING - EXERCISE OF MEETING AND VOTING RIGHTS
Article 23
| 23.1 | Each Person with Meeting Rights has the right to attend, address and, if applicable, vote at General Meetings, whether in person or represented by the holder of a written proxy. Holders of fractional ordinary shares together constituting the nominal value of an ordinary share shall exercise these rights collectively, whether through one of them or through the holder of a written proxy. |
| I-11 |
Table of Contents
| 23.2 | The Board may decide that each Person with Meeting Rights is entitled, whether in person or represented by the holder of a written proxy, to participate in, address and, if applicable, vote at the General Meeting by electronic means of communication. For the purpose of applying the preceding sentence it must be possible, by electronic means of communication, for the Person with Meeting Rights to be identified, to observe in real time the proceedings at the General Meeting and, if applicable, to vote. The Board may impose conditions on the use of the electronic means of communication, provided that these conditions are reasonable and necessary for the identification of the Person with Meeting Rights and the reliability and security of the communication. Such conditions must be announced in the convening notice. |
| 23.3 | The Board can also decide that votes cast through electronic means of communication or by means of a letter prior to the General Meeting are considered to be votes that are cast during the General Meeting. These votes shall not be cast prior to the Record Date. |
| 23.4 | For the purpose of Articles 23.1 through 23.3, those who have voting rights and/or Meeting Rights on the Record Date and are recorded as such in a register designated by the Board shall be considered to have those rights, irrespective of whoever is entitled to the ordinary shares or depository receipts at the time of the General Meeting. Unless Dutch law requires otherwise, the Board is free to determine, when convening a General Meeting, whether the previous sentence applies. |
| 23.5 | Each Person with Meeting Rights must notify the Company in writing of his identity and his intention to attend the General Meeting. This notice must be received by the Company ultimately on the seventh day prior to the General Meeting, unless indicated otherwise when such General Meeting is convened. Persons with Meeting Rights that have not complied with this requirement may be refused entry to the General Meeting. |
GENERAL MEETING - DECISION-MAKING
Article 24
| 24.1 | Each ordinary share shall give the right to cast one vote at the General Meeting. Fractional ordinary shares, if any, collectively constituting the nominal value of an ordinary share shall be considered to be equivalent to such ordinary share. |
| 24.2 | No vote can be cast at a General Meeting in respect of an ordinary share belonging to the Company or a Subsidiary or in respect of an ordinary share for which any of them holds the depository receipts. Usufructuaries and pledgees of ordinary shares belonging to the Company or its Subsidiaries are not, however, precluded from exercising their voting rights if the usufruct or pledge was created before the relevant ordinary share belonged to the Company or a Subsidiary. Neither the Company nor a Subsidiary can vote ordinary shares in respect of which it holds a usufruct or a pledge. |
| 24.3 | Unless a greater majority is required by law or by these articles of association, all resolutions of the General Meeting shall be passed by Simple Majority. If applicable law requires a greater majority for resolutions of the General Meeting and allows the articles of association to provide for a lower majority, those resolutions shall be passed with the lowest possible majority, except if these articles of association explicitly provide otherwise. |
| 24.4 | Subject to any provision of mandatory Dutch law and any higher quorum requirement stipulated by these articles of association, if the Company is subject to a requirement under applicable securities laws or listing rules that the General Meeting can only pass certain resolutions if a certain part of the Company’s issued share capital is represented at such General Meeting, then such resolutions shall be subject to such quorum as specified by such securities laws or listing rules and a second meeting as referred to in Section 2:120(3) DCC cannot be convened. |
| 24.5 | Invalid votes, blank votes and abstentions shall not be counted as votes cast. Ordinary shares in respect of which an invalid or blank vote has been cast and ordinary shares in respect of which an abstention has been made shall be taken into account when determining the part of the issued share capital that is represented at a General Meeting. |
| I-12 |
Table of Contents
| 24.6 | Where there is a tie in any vote of the General Meeting, the relevant resolution shall not have been passed. |
| 24.7 | The chairperson of the General Meeting shall decide on the method of voting and the voting procedure at the General Meeting. |
| 24.8 | The determination during the General Meeting made by the chairperson of that General Meeting with regard to the results of a vote shall be decisive. If the accuracy of the chairperson’s determination is contested immediately after it has been made, a new vote shall take place if the majority of the General Meeting so requires or, where the original vote did not take place by response to a roll call or in writing, if any party with voting rights who is present so requires. The legal consequences of the original vote shall lapse as a result of the new vote. |
| 24.9 | The Board shall keep a record of the resolutions passed. The record shall be available at the Company’s office for inspection by Persons with Meeting Rights. Each of them shall, upon request, be provided with a copy of or extract from the record, at no more than the cost price. |
| 24.10 | Shareholders may pass resolutions outside a meeting, unless the Company has cooperated with the issuance of depository receipts for ordinary shares in its capital. Such resolutions can only be passed by a unanimous vote of all shareholders with voting rights. The votes shall be cast in writing and may be cast through electronic means. |
| 24.11 | The Directors shall, in that capacity, have an advisory vote at the General Meetings. |
GENERAL MEETING - SPECIAL RESOLUTIONS
Article 25
| 25.1 | The following resolutions can only be passed by the General Meeting at the proposal of the Board: |
| a. | the issue of ordinary shares or the granting of rights to subscribe for ordinary shares; |
| b. | the limitation or exclusion of pre-emption rights; |
| c. | the designation or granting of an authorisation as referred to in Articles 6.1, 7.5 and 10.2, respectively; |
| d. | the disapplication or revocation of a designation or authorisation as referred to in Articles 6.1, 7.5 and 10.2, respectively; |
| e. | the reduction of the Company’s issued share capital; |
| f. | the making of a distribution from the Company’s profits or reserves; |
| g. | the making of a distribution in the form of ordinary shares in the Company’s capital or in the form of assets, instead of in cash; |
| h. | the amendment of these articles of association; |
| i. | the entering into of a merger or demerger; |
| j. | the instruction of the Board to apply for the Company’s bankruptcy; and |
| k. | the Company’s dissolution. |
| 25.2 | A matter which has been included in the convening notice or announced in the same manner by or at the request of one or more Persons with Meeting Rights pursuant to Articles 21.5 and/or 21.6 shall not be considered to have been proposed by the Board for purposes of Article 25.1, unless the Board has expressly indicated that it supports the discussion of such matter in the agenda of the General Meeting concerned or in the explanatory notes thereto. |
| I-13 |
Table of Contents
REPORTING - FINANCIAL YEAR, ANNUAL ACCOUNTS AND MANAGEMENT REPORT
Article 26
| 26.1 | The Company’s financial year shall coincide with the calendar year. |
| 26.2 | Annually, within the relevant statutory period, the Board shall prepare the annual accounts and the management report and deposit them at the Company’s office for inspection by the shareholders. |
| 26.3 | The annual accounts shall be signed by the Directors. If any of their signatures is missing, this shall be mentioned, stating the reasons. |
| 26.4 | The Company shall ensure that the annual accounts, the management report and the particulars to be added pursuant to Section 2:392(1) DCC shall be available at its offices as from the convening of the General Meeting at which they are to be discussed. The Persons with Meeting Rights are entitled to inspect such documents at that location and to obtain a copy at no cost. |
| 26.5 | The annual accounts shall be adopted by the General Meeting. |
REPORTING - AUDIT
Article 27
| 27.1 | The General Meeting shall instruct an external auditor as referred to in Section 2:393 DCC to audit the annual accounts. Where the General Meeting fails to do so, the Board shall be authorised to do so. |
| 27.2 | The instruction may be revoked by the General Meeting and by the body that has granted the instruction. The instruction can only be revoked for well-founded reasons; a difference of opinion regarding the reporting or auditing methods shall not constitute such a reason. |
DISTRIBUTIONS - GENERAL
Article 28
| 28.1 | A distribution can only be made to the extent that the Company’s equity exceeds the amount of the paid up and called up part of its capital plus the reserves which must be maintained by law. |
| 28.2 | The Board may resolve to make interim distributions, provided that it appears from interim accounts to be prepared in accordance with Section 2:105(4) DCC that the requirement referred to in Article 28.1 has been met. |
| 28.3 | Distributions shall be made in proportion to the aggregate nominal value of the ordinary shares. |
| 28.4 | The parties entitled to a distribution shall be the relevant shareholders, usufructuaries and pledgees, as the case may be, at a date to be determined by the Board for that purpose. This date shall not be earlier than the date on which the distribution was announced. |
| 28.5 | The General Meeting may resolve, subject to Article 25, that all or part of a distribution, instead of being made in cash, shall be made in the form of ordinary shares in the Company’s capital or in the form of the Company’s assets. |
| 28.6 | A distribution shall be payable on such date and, if it concerns a distribution in cash, in such currency or currencies as determined by the Board. If it concerns a distribution in the form of the Company’s assets, the Board shall determine the value attributed to such distribution for purposes of recording the distribution in the Company’s accounts with due observance of applicable law (including the applicable accounting principles). |
| 28.7 | A claim for payment of a distribution shall lapse after five years have expired after the distribution became payable. |
| I-14 |
Table of Contents
| 28.8 | For the purpose of calculating the amount or allocation of any distribution, ordinary shares held by the Company in its own capital shall not be taken into account. No distribution shall be made to the Company in respect of ordinary shares held by it in its own capital. |
DISTRIBUTIONS - RESERVES
Article 29
| 29.1 | Subject to Article 25, the General Meeting is authorised to resolve to make a distribution from the Company’s reserves. |
| 29.2 | The Board may resolve to charge amounts to be paid up on ordinary shares against the Company’s reserves, irrespective of whether those ordinary shares are issued to existing shareholders. |
DISTRIBUTIONS - PROFITS
Article 30
| 30.1 | Subject to Article 28.1, the profits shown in the Company’s annual accounts in respect of a financial year shall be appropriated as follows, and in the following order of priority: |
| a. | the Board shall determine which part of the profits shall be added to the Company’s reserves; and |
| b. | subject to Article 25, the remaining profits shall be at the disposal of the General Meeting for distribution on the ordinary shares. |
| 30.2 | Subject to Article 28.1, a distribution of profits shall be made after the adoption of the annual accounts that show that such distribution is allowed. |
DISSOLUTION AND LIQUIDATION
Article 31
| 31.1 | In the event of the Company being dissolved, the liquidation shall be effected by the Board, unless the General Meeting decides otherwise. |
| 31.2 | To the extent possible, these articles of association shall remain in effect during the liquidation. |
| 31.3 | Any assets remaining after payment of all of the Company’s debts shall be distributed to the shareholders. |
| 31.4 | After the Company has ceased to exist, its books, records and other information carriers shall be kept for the period prescribed by law by the person designated for that purpose in the resolution of the General Meeting to dissolve the Company. Where the General Meeting has not designated such a person, the liquidators shall do so. |
FEDERAL FORUM PROVISION
Article 32
Unless the Company consents in writing to the selection of an alternative forum, the sole and exclusive forum for any complaint asserting a cause of action arising under the United States Securities Act of 1933, as amended, or the United States Securities Exchange Act of 1934, as amended, to the fullest extent permitted by applicable law, shall be the United States federal district courts.
| I-15 |
Table of Contents
[TRANSITIONAL PROVISION
Article 33
| 33.1 | Upon the Company’s issued share capital increasing to an amount of at least [amount] euro (EUR [amount]): |
| a. | the Company’s authorised share capital described in Article 4.1 shall immediately and automatically increase to an amount of [amount] euro (EUR [amount]); and |
| b. | the composition of the authorised share capital described in Article 4.2 shall immediately and automatically be adjusted, such that the authorised share capital shall be divided into [number] ([number]) ordinary shares, each having a nominal value of twelve eurocents (EUR 0.12). |
This Article 33.1 shall lapse and shall no longer form part of these articles of association at the moment immediately after the increase of the Company’s issued share capital as described in the first sentence of this Article 33.1 shall have become effective.]
| I-16 |
Table of Contents

July 24, 2022
Board of Directors
Frazier Lifesciences Acquisition Corporation
Two Union Square
601 Union Street, Suite 3200
Seattle, WA 98101
Members of the Board of Directors:
You have requested that Lincoln International LLC (“Lincoln”) render an opinion (the “Opinion”) as to the fairness, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares (as defined below) of the Aggregate Share Consideration (as defined below) to be issued by NewAmsterdam Pharma Company B.V. (“Holdco”) pursuant to the Merger Agreement (as defined below) (the “Transaction”).
Background of the Proposed Transaction
We understand that, in connection with the Transaction, Frazier Lifesciences Acquisition Corporation (“FLAC”) proposes to enter into a Business Combination Agreement (the “Merger Agreement”) with Holdco, NewAmsterdam Pharma Investment Corporation (“Merger Sub”), and NewAmsterdam Pharma Holding B.V. (the “Company”). Pursuant to the Merger Agreement, at the Effective Date (as defined in the Merger Agreement), Merger Sub will be merged with and into FLAC, with FLAC continuing as the surviving entity and as a wholly owned subsidiary of Holdco (the “Merger”). Capitalized terms used but not otherwise defined herein shall have the meanings ascribed to such terms in the Merger Agreement.
Pursuant to the Merger Agreement, the shareholders of the Company shall contribute and transfer their shares to Holdco for Aggregate Share Consideration consisting of an amount of Holdco Shares equal to the Purchase Price of $491,000,000 divided by the Holdco Per Share Value of $10.00.
By virtue of the Merger, FLAC Shares issued and outstanding immediately prior to the Effective Date (other than any FLAC Shares held by FLAC as treasury shares) and that are held by Pre-Closing FLAC Holders who (x) do not redeem their FLAC Class A Shares for cash pursuant to the FLAC Shareholder Redemption or (y) hold FLAC Class B Shares (collectively, “Relevant FLAC Shares”) shall be automatically cancelled and extinguished in exchange for the corresponding number of Holdco Shares in accordance with the Merger Agreement. For purposes of this opinion, unaffiliated holders do not include officers, directors or affiliates of FLAC or the Sponsor.
In addition, the Merger Agreement sets forth terms and conditions under which up to 1,886,137 additional Holdco Shares may be issued as Earnout Shares.
Lincoln International LLC 110 North Wacker Drive 51st Floor Chicago, Illinois 60606 | www.lincolninternational.com | |||
| J-1 |
| |||
Table of Contents

Scope of Analysis
In connection with this Opinion, Lincoln has, among other things:
| 1) | Reviewed the following documents: |
| a. | Audited income statements for the Company for the fifteen-month period ended December 31, 2020 and balance sheet as of December 31, 2020 provided to us by FLAC; |
| b. | Audited income statements for the Company for the twelve-month period ended December 31, 2021 and balance sheet as of December 31, 2021 provided to us by FLAC; |
| c. | A draft of the Merger Agreement dated as of July 19, 2022; |
| d. | License Agreement between NewAmsterdam Pharma B.V. and A. Menarini International Licensing S.A. dated as of June 23, 2022; and |
| e. | The Company’s PIPE presentation dated as of July 2022; |
| 2) | Discussed the business, financial outlook and prospects of the Company and its addressable market, as well as the terms and circumstances surrounding the Transaction, with management of the Company and FLAC; |
| 3) | Reviewed certain financial and other information for the Company, and compared that data and information with certain financial, stock trading and corresponding data and information for companies with publicly traded securities that we deemed relevant, none of which is directly comparable to the Company; |
| 4) | Reviewed certain financial and other information for the Company and the Transaction, and compared that data and information with certain financial and corresponding data and information for companies that have been subject to change of control M&A transactions that we deemed relevant, none of which is directly comparable to the Company and the Transaction; and |
| 5) | Considered such other information and financial, economic and market criteria and analyses that we deemed relevant. |
Assumptions, Qualifications, and Limiting Conditions
In performing its analyses and rendering this Opinion with respect to the Transaction, Lincoln has, with FLAC management’s consent:
| 1) | Relied upon and assumed the accuracy and completeness of all of the financial, accounting, legal, tax and other information we reviewed, and we have not assumed any responsibility for the independent verification of, nor independently verified, any of such information; |
| 2) | Relied upon the assurances of the management of FLAC that the information provided is complete and correct in all material respects and do not contain any untrue statement of a material fact or omit to state a material fact necessary that would make such information materially incomplete or misleading in the light of the circumstances under which statement are made; |
Lincoln International LLC 110 North Wacker Drive 51st Floor Chicago, Illinois 60606 | www.lincolninternational.com | |||
| J-2 |
| |||
Table of Contents

| 3) | Assumed that the Transaction will be consummated in a timely manner that complies in all respects with all applicable federal and state statutes, rules and regulations; |
| 4) | Assumed that at the consummation of the Transaction, Holdco will be fully funded through the completion of its phase 3 clinical trials of Obicetrapib; |
| 5) | Assumed that in the course of obtaining any necessary regulatory and third-party consents, approvals and agreements for the Transaction, no modification, delay, limitation, restriction, or condition will be imposed that will have an adverse effect on FLAC or the Transaction; |
| 6) | Assumed that the Transaction will be consummated in accordance with the terms outlined by FLAC and other documents made available to Lincoln, without waiver, modification or amendment of any term, condition or agreement therein that is material to Lincoln’s analysis; |
| 7) | Assumed that there has been no material change in the assets, liabilities, business, condition (financial or otherwise), results of operations, or prospects of the Company since the date of the most recent information was made available to Lincoln; |
| 8) | Assumed that the final terms of the Transaction will not vary materially from those set forth in the copies or drafts, as applicable, reviewed by Lincoln; and |
| 9) | Assumed that the final versions of all documents conform in all material respects to the drafts reviewed by Lincoln. |
Lincoln has prepared this Opinion as of the date hereof. This Opinion is necessarily based on financial, economic, market and other conditions as they exist on and the information made available to us as of the date hereof. Although subsequent developments may affect this Opinion, Lincoln does not have any obligation to update, revise or reaffirm this Opinion.
Lincoln did not evaluate the Company’s solvency and was not requested to make, and did not make, an independent evaluation or appraisal of the assets or liabilities (contingent, derivative, off-balance sheet or otherwise) of the Company or any of its subsidiaries, nor was Lincoln furnished with any such evaluations or appraisals. Lincoln was not requested to, nor did Lincoln, participate in the negotiation or structuring of the Transaction. Lincoln was not requested to, nor did Lincoln, seek alternative candidates for the Transaction.
This Opinion (i) does not address the underlying business decision of the Board of Directors of FLAC (the “Board”) or FLAC to proceed with or effect the Transaction or the relative merits of the Transaction as compared to other transaction structures, transactions or business strategies that may be available to FLAC or the effect of any other transaction in which FLAC might engage, and does not address whether the Aggregate Share Consideration to be issued by Holdco pursuant to the Merger Agreement is the best possibly attainable under the circumstances, (ii) does not constitute advice or a recommendation to the Board or any security holder as to how they should act or vote with respect to any matter relating to the Transaction (including as to whether they should redeem any FLAC Shares in the FLAC Shareholder Redemption), and (iii) only addresses the fairness from a financial point of view of the Aggregate Share Consideration to be issued by Holdco pursuant to the Merger Agreement to the unaffiliated holders of Relevant FLAC Shares in the Transaction and does not address any other terms, aspects or implications of the Transaction, or any agreements, arrangements or understandings entered into in connection with the Transaction or otherwise. We express no opinion as to the fairness of any portion or aspect of the Transaction to (i) the holders of any class of securities, creditors or other constituencies
Lincoln International LLC 110 North Wacker Drive 51st Floor Chicago, Illinois 60606 | www.lincolninternational.com | |||
| J-3 |
| |||
Table of Contents

of FLAC, or any other party, except as expressly set forth below, or (ii) any one class or group of FLAC’s security holders, creditors or other constituencies vis-à -vis any other class or group of FLAC’s security holders, creditors or other constituents (including, without limitation, the allocation of any Aggregate Share Consideration among or within such classes or groups of security holders, creditors or other constituents). The decision as to whether to proceed with the Transaction or any related transaction depends on an assessment of various factors, many of which are unrelated to the financial analyses on which this Opinion is based.
Lincoln expresses no opinion as to what the market price or value of the stock of FLAC or Holdco will be after the announcement of the Transaction. This Opinion should not be construed as a valuation opinion, credit rating, solvency opinion, an analysis of FLAC’s or Holdco’s credit worthiness, as tax advice, or as accounting advice. We also express no opinion about the amount or nature of any compensation or equity arrangement to be given to FLAC’s or Holdco’s officers, directors or employees, or class of such persons, in connection with the Transaction relative to the Aggregate Share Consideration in the Transaction.
It is understood that this Opinion is for the use and benefit of the Board in connection with the Transaction. This Opinion may not be used for any other purpose and is not intended to confer any rights or remedies upon any other person. Except as contemplated by the Engagement Letter, dated as of May 31, 2022, among Lincoln and FLAC, neither this Opinion nor any other advice or information provided by Lincoln, whether oral or written, may be disclosed, reproduced, disseminated, summarized, quoted from or referred to, in whole or in part, without our prior written consent.
Disclosure of Relationships
Lincoln will receive a customary fee from FLAC for our services, a portion of which was payable upon our retention, and the balance upon the earlier of termination of the Transaction in accordance with the Merger Agreement or the consummation of the Transaction. In addition, FLAC has agreed to indemnify us and certain related parties against certain liabilities, and to reimburse us for certain expenses, arising in connection with or as a result of our engagement. During the two years preceding the date of this Opinion, we and our affiliates have not had other investment banking relationships with the Company or its affiliates, or any investment banking relationships with Holdco or its affiliates, for which we were paid for our services. We and our affiliates provide a range of investment banking and financial services and, in that regard, we and our affiliates may in the future provide, investment banking and other financial services to FLAC, Holdco and each of their respective affiliates, for which we and our affiliates would expect to receive compensation.
Conclusion
Based on and subject to the foregoing, and in reliance thereon, we are of the opinion that, as of the date hereof the Aggregate Share Consideration to be issued by Holdco pursuant to the Merger Agreement is fair, from a financial point of view, to the unaffiliated holders of Relevant FLAC Shares.
This Opinion has been authorized for issuance by the Opinion Review Committee of Lincoln.
Very truly yours,
LINCOLN INTERNATIONAL LLC
Lincoln International LLC 110 North Wacker Drive 51st Floor Chicago, Illinois 60606 | www.lincolninternational.com | |||
| J-4 |
| |||