
Exhibit 99.2 TANDEM Topline Results November 20, 2024

Disclaimer This presentation (together with oral statements made in connection herewith, this “Presentation”) is for informational purposes only. This Presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, any securities, nor shall there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful. Forward Looking Statements Certain statements included in this Presentation that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements by NewAmsterdam Pharma Company N.V. (“NewAmsterdam” or the “Company”) regarding estimates and forecasts of other financial and performance metrics and projections of market opportunity; the Company's business and strategic plans; expectations and timing related to the success, cost and timing of product development activities, including timing of initiation, completion and data readouts for clinical trials and the potential approval of the Company’s product candidate; the timing for enrolling patients; the timing and forums for announcing data; the size and growth potential of the markets for the Company’s product candidate; the therapeutic and curative potential of the Company’s product candidate; financing and other business milestones; the Company’s expected cash runway; and the Company’s plans for commercialization. These statements are based on various assumptions, whether or not identified in this Presentation, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to the approval of NewAmsterdam’s product candidate and the timing of expected regulatory and business milestones; whether topline, initial or preliminary results from a particular clinical trial will be predictive of the final results of that trial and whether results of early clinical trials will be indicative of the results of later clinical trials; ability to negotiate definitive contractual arrangements with potential customers; the impact of competitive product candidates; ability to obtain sufficient supply of materials; global economic and political conditions, including the Russia-Ukraine conflict, and the war in Israel; the effects of competition on NewAmsterdam’s future business; and those factors discussed in documents filed by the Company with the SEC. Additional risks related to NewAmsterdam’s business include, but are not limited to: uncertainty regarding outcomes of the company’s ongoing clinical trials, particularly as they relate to regulatory review and potential approval for its product candidate; risks associated with the Company’s efforts to commercialize a product candidate; the Company’s ability to negotiate and enter into definitive agreements on favorable terms, if at all; the impact of competing product candidates on the Company’s business; intellectual property-related claims; the Company’s ability to attract and retain qualified personnel; and the Company’s ability to continue to source the raw materials for its product candidate, together with the risks described in the Company’s filings made with the U.S. Securities and Exchange Commission from time to time. If any of these risks materialize or NewAmsterdam’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that are presently unknown by the Company or that NewAmsterdam currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect NewAmsterdam’s expectations, plans, or forecasts of future events and views as of the date of this Presentation and are qualified in their entirety by reference to the cautionary statements herein. NewAmsterdam anticipates that subsequent events and developments will cause the Company’s assessments to change. These forward-looking statements should not be relied upon as representing NewAmsterdam’s assessments as of any date subsequent to the date of this Presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements. Neither NewAmsterdam nor any of its affiliates undertakes any obligation to update these forward-looking statements, except as required by law. Market Data Certain information contained in this Presentation relates to or is based on third-party studies, publications, surveys and NewAmsterdam’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while NewAmsterdam believes its internal research is reliable, such research has not been verified by any independent source and NewAmsterdam cannot guarantee and makes no representation or warranty, express or implied, as to its accuracy and completeness. Trademarks This Presentation contains trademarks, service marks, trade names, and copyrights of NewAmsterdam and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade name or products in this Presentation is not intended to, and does not imply, a relationship with NewAmsterdam or an endorsement or sponsorship by or of NewAmsterdam. Solely for convenience, the trademarks, service marks and trade names referred to in this Presentation may appear with the TM or SM symbols, but such references are not intended to indicate, in any way, that NewAmsterdam will not assert, to the 2 fullest extent permitted under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade names.

Results overview • TANDEM successfully met all co-primary endpoints with statistical significance: − FDC vs. placebo − FDC vs. ezetimibe − FDC vs. obicetrapib monotherapy, and − Obicetrapib vs. placebo • Continued support for potential synergistic benefit of the combination, highlighting potential benefit beyond convenience • Safety results consistent with our prior studies • Growth of ezetimibe and non-statin therapies believed to support significant opportunity for the FDC, if approved • Data supports global regulatory filings of the fixed-dose combination Note: FDC = fixed-dose combination 3

Majority of ASCVD/HeFH Patients have not Demonstrated Achievement of LDL-C Targets Primary prevention HeFH ASCVD patients with an Very high risk ASCVD Despite availability of patients with LDL-C target of LDL<70 or patients with an LDL-C treatments continue to see 2 an LDL-C target <100 mg/dL <55 mg/dL (2017-2018) target <55 mg/dL (2020- minimal uptake, especially 1 3 4 (2011-2017) 2021) adjunct to statins LDL-C < 100 mg/dL LDL-C < 70 mg/dL LDL-C < 55 mg/dL Statin Utilization 65.8M 29.6M 29% 24% 10% PCSK9i Utilization 9.7M <1/3 achieved <1/4 achieved 10% achieved LDL-C <100 mg/dL LDL-C <70 mg/dL LDL-C <55 mg/dL 0.253M ASCVD=atherosclerotic cardiovascular disease; HeFH=heterozygous familial hypercholesterolemia; LDL-C=low-density lipoprotein-cholesterol. 1. Schreuder MM, et al. LDL cholesterol targets rarely achieved in familial hypercholesterolemia patients: A sex and gender-specific analysis. Atherosclerosis. 2023 2. Gao Y, Shah LM, Ding J, Martin SS. US trends in cholesterol screening, lipid levels, and lipid- lowering medication use in US adults, 1999 to 2018. J Am Heart Assoc. 2023;12(3):e028205; 3. Katzmann JL, et al. Simulation study on LDL cholesterol target attainment, treatment costs, and ASCVD events with bempedoic acid in patients at high and very-high cardiovascular risk. PLoS One. 2022;17(10):e0276898; 4. J Am Heart Assoc 2022;11:3026075; doi: 10.1161/JAHA.122.026075 4

(10 of each) Study Design and Baseline Characteristics A Placebo-Controlled, Double-Blind, Randomized, Phase 3 Study to Evaluate the Effect of Obicetrapib 10 mg and Ezetimibe 10 mg Fixed Dose Combination Daily on Top of Maximally Tolerated Lipid-Modifying Therapy in Participants With Heterozygous Familial Hypercholesterolemia (HeFH) and/or Atherosclerotic Cardiovascular Disease (ASCVD) or Multiple ASCVD Risk Factors Study Design Baseline Lipids (total study population) 1º endpoint N = 407 150 160 140 Obicetrapib 10 mg / Ezetimibe 10mg (n=102) 123 120 97 Obicetrapib 10 mg (n=102) 89 100 80 Ezetimibe 10 mg (n=101) 48 60 40 Placebo (n=102) 20 12-weeks 0 Key Inclusion Criteria LDL-C Non-HDL-C HDL-C ApoB TG • ASCVD Demographics • ASCVD risk equivalents • Female 44% • LDL-C≥ 70 mg/dL • White 83% • Maximally tolerated lipid lowering therapy 3 • BMI 32 kg/m Key Exclusion Criteria Baseline Lipid Modifying Therapy • Uncontrolled severe hypertension • High intensity statin: 71% • Diagnosis of homozygous FH Endpoints • Percent change from baseline in LDL-C compared to placebo • Co-Primary Endpoints • FDC vs. Placebo • FDC vs. Eze • FDC vs. Obi • Obi vs. Placebo 5 mg/dL

Additional baseline details of all randomized patients Placebo (n=102) Ezetimibe (n=101) Obicetrapib (n=102) Obicetrapib and Ezetimibe (n=102) 66.1 67.6 66.7 67.3 Mean Age (years) Sex (F) n (%) 51 (50.0%) 45 (44.6%) 33 (32.4%) 48 (47.1%) Race n (%) White 84 (82.4%) 82 (81.2%) 85 (83.3%) 86 (84.3%) African American 17 (16.7%) 13 (12.9%) 15 (14.7%) 14 (13.7%) Height, cm (mean) 169.9 171.2 169.8 168.8 Weight, kg (mean) 91.7 92.0 92.0 93.6 BMI (mean) 31.7 31.1 31.7 32.8 High intensity statin 75 (73.5%) 71 (70.3%) 66 (64.7%) 75 (73.5%) 6
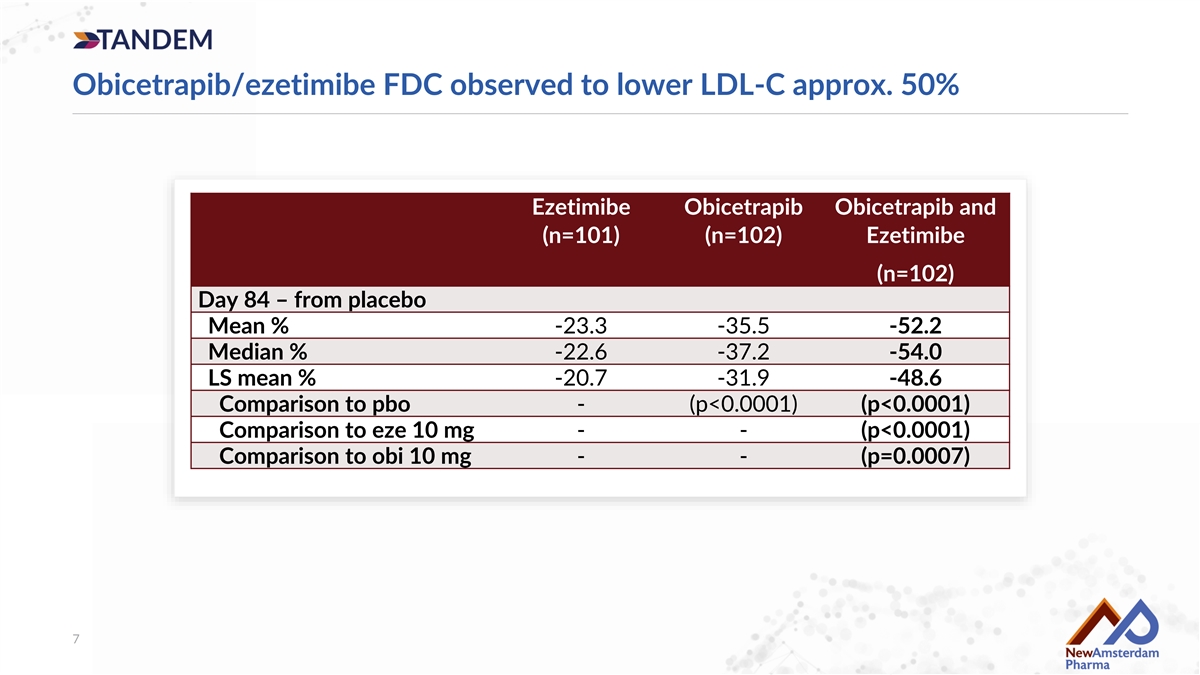
Obicetrapib/ezetimibe FDC observed to lower LDL-C approx. 50% Ezetimibe Obicetrapib Obicetrapib and (n=101) (n=102) Ezetimibe (n=102) Day 84 – from placebo Mean % -23.3 -35.5 -52.2 Median % -22.6 -37.2 -54.0 LS mean % -20.7 -31.9 -48.6 Comparison to pbo - (p<0.0001) (p<0.0001) Comparison to eze 10 mg - - (p<0.0001) Comparison to obi 10 mg - - (p=0.0007) 7

Over 60% of individuals on the FDC saw a more than 50% decrease in LDL-C Obicetrapib 10mg and ezetimibe 10mg fixed-dose combination 61% >50% LDL-C reduction Note: each line represents a single patient in the fixed-dose combination arm 8
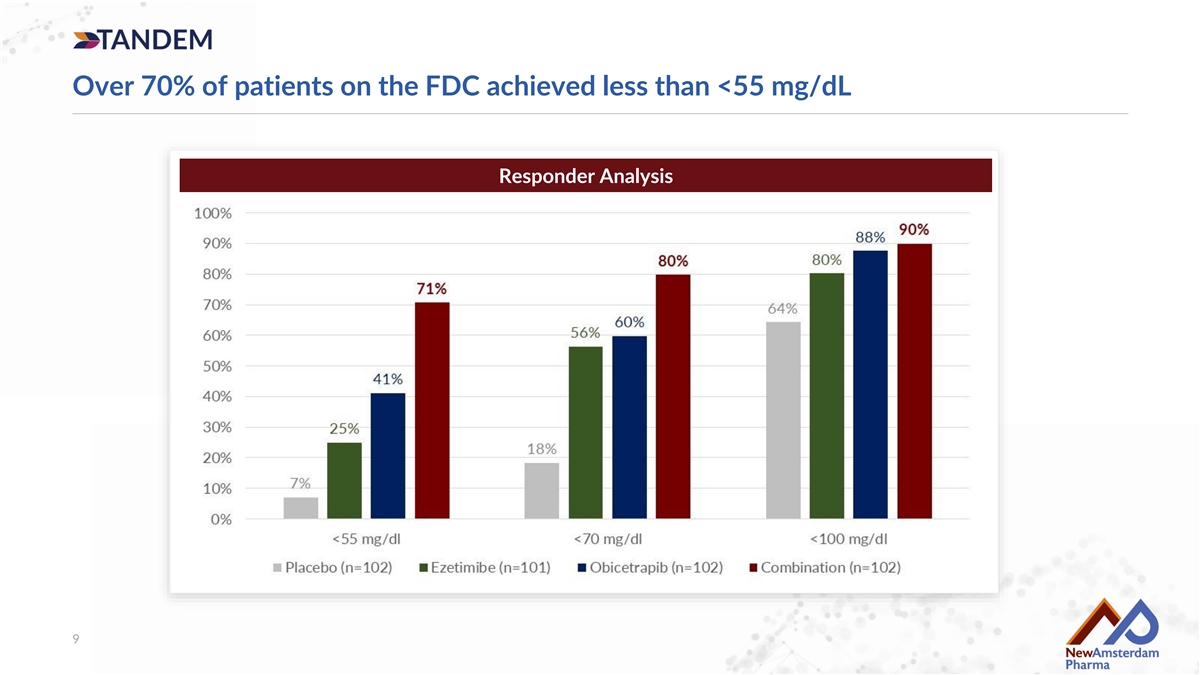
Over 70% of patients on the FDC achieved less than <55 mg/dL Responder Analysis 9

Continued support for potential synergistic effects in combination 18.5% projected greater reduction in LDL-C in the combination than in the ezetimibe monotherapy arm 68 100 31.9% mg/dL mg/dL Mean baseline in Observed LDL-C Post treatment baseline • Ezetimibe monotherapy showed a 20.7% placebo lowering obicetrapib obi mono arm arm 24.5% adjusted reduction in LDL-C drop Observed difference • However, the observed LDL-C reduction between between obi mono and FDC 51 the obicetrapib monotherapy arm and the FDC 100 48.6% mg/dL mg/dL arm was 24.5%, which we believe was driven by Mean baseline in Observed LDL-C Calculated FDC the effect of adding ezetimibe to the FDC obi mono arm lowering FDC arm reduction 18.5% • We therefore project 18.5% greater LDL-C increase reduction by ezetimibe when combined with Observed LDL-C 20.7% obicetrapib in the FDC (24.5% vs 20.7%) than what lowering in eze 10 drop mg arm would be expected with ezetimibe monotherapy alone Note: based on LS mean percent reductions. The calculations above are hypothetical calculations based on the LDL-C reduction observed in the relevant treatment groups in our TANDEM trial. 10

Observed On-treatment LDL-C reduction for ROSE, ROSE2, BROOKLYN & TANDEM On-Treatment Analysis of obicetrapib 10 mg Note: ROSE at week 8, ROSE2, BROOKLYN, and TANDEM as of week 12. 11

Observed Lp(a) reduction in TANDEM Lp(a) 12

Safety results consistent with our prior studies Placebo (n=102) Ezetimibe Obicetrapib Obicetrapib / (n=101) (n=102) Ezetimibe (n=102) Any study drug-related TEAEs 4 (3.9%) 3 (3.0%) 7 (6.9%) 3 (2.9%) Any study drug-related TEAEs leading to 2 (2.0%) 1 (1.0%) 6 (5.9%) 1 (1.0%) discontinuation of study drug Any study drug related TESAEs 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) Note: treatment emergent adverse events (“TEAEs”) and study drug-related treatment emergent serious adverse events (“TESAEs”) 13

Lipid Lowering Therapy (LLT) Market is a Growing Opportunity 1 2 3 4 5 2 3 4 Patients on Lipid lowering Therapy Patients on Non-statin Treatment Patients on Branded Treatment Branded sales driving market 11.0% 1.3% 0.1% opportunity ($ millions) 56MM 4.0MM 700K 19.6% 31% 4.3% Growth Growth Growth 600K 5.4% Growth 26.3% 51MM 3.5MM 500K Growth 17.8% Growth 400K 87.6% 46MM 3.0MM 638K 3.8MM Generic - Statin Generic - non Statin 54MM 300K 51MM Branded - PCSK9 Branded - ACL Inhibitor 487K 49MM 3.2MM 200K 386K 41MM 2.5MM 2.7MM 100K 256M Prescriptions written 36MM 2.0MM 0K 1 Nov '20 to Nov '21 to Nov '22 to Nov '20 to Nov '21 to Nov '22 to Nov '20 to Nov '21 to Nov '22 to in past 12 months. Oct '21 Oct '22 Oct '23 Oct '21 Oct '22 Oct '23 Oct '21 Oct '22 Oct '23 PCSK9 sales accelerating post Over 250 MM Market growing at over Non-statin market growing Branded market Rx’s annually 4% over the last 2 years at high double digits growing even faster launch miscalculations Recent guideline and label changes driving renewed acceleration 5 2022: ACC updated guidelines to target LDL-C <55 mg/dl in high-risk patients in line with ESC/EAS 2024: FDA highlights need to reduce access restrictions for LLTs. Labels updated from “on top of maximally tolerated statins” to “treatment of primary 6 hyperlipidemia” for some LLTs 1. Source: IQVIA XPT - Data Period – 12 months of TRx from Dec ‘22 to Nov ’23 Source: IQVIA LAAD data from Nov ‘20 to Oct ’23 2. All Lipid Lowering therapies: Statins, Ezetimibe and combinations; PCSK9 and BPA 3. Non-Statins : Ezetimibe and 14 combinations; PCSK9 and BPA 4. Branded: PCSK9 and BPA 5. Lloyd-Jones DM, et al. J Am Coll Cardiol. 2022;80(14):1366-1418 6. Leqvio (inclisiran). Prescribing information. Novartis; 2023.; Nexletol (bempedoic acid). Prescribing information. Esperion Therapeutics Inc; 2023. Note: LTM=last 12 months ending 2Q24

Conclusions • TANDEM successfully hit all co-primary endpoints, observed to lower LDL-C by approximately 50%, including: − A greater than 60% reduction in more that 50% of patients, − More than 70% of patients achieved LDL-C below 55 mg/dL • 18.5% synergy projected with combination, highlighting potential benefit above simple convenience • ~20% ezetimibe and overall market growth believed to support significant market opportunities for obicetrapib and FDC, if approved • Data support global filing of the fixed-dose combination • Other biomarkers consistent with our prior studies • Additional data to be presented at an upcoming medical conference 15














