
Exhibit 99.2 BROADWAY Topline Results December 10, 2024

Disclaimer This presentation (together with oral statements made in connection herewith, this “Presentation”) is for informational purposes only. This Presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, any securities, nor shall there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful. Forward Looking Statements Certain statements included in this Presentation that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements by NewAmsterdam Pharma Company N.V. (“NewAmsterdam” or the “Company”) regarding the Company's expectations of future MACE benefits, the anticipated results from the Company's ongoing PREVAIL trial, the Company's expectation of the different stakeholder reactions to the BROADWAY top-line data and other statements that are not of historical fact. These statements are based on various assumptions, whether or not identified in this Presentation, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to the approval of NewAmsterdam’s product candidate and the timing of expected regulatory and business milestones; whether topline, initial or preliminary results from a particular clinical trial will be predictive of the final results of that trial and whether results of early clinical trials will be indicative of the results of later clinical trials; , or whether projections regarding clinical outcomes will reflect actual results in future clinical trials or clinical use of our product candidate if approved; ability to negotiate definitive contractual arrangements with potential customers; the impact of competitive product candidates; ability to obtain sufficient supply of materials; global economic and political conditions, including the Russia-Ukraine conflict, and the war in Israel; the effects of competition on NewAmsterdam’s future business; and those factors discussed in the Company's Annual Report on Form 10-K for the year ended December 31, 2023 as supplemented by other documents filed by the Company with the SEC. Additional risks related to NewAmsterdam’s business include, but are not limited to: uncertainty regarding outcomes of the company’s ongoing clinical trials, particularly as they relate to regulatory review and potential approval for its product candidate; risks associated with the Company’s efforts to commercialize a product candidate; the Company’s ability to negotiate and enter into definitive agreements on favorable terms, if at all; the impact of competing product candidates on the Company’s business; intellectual property-related claims; the Company’s ability to attract and retain qualified personnel; and the Company’s ability to continue to source the raw materials for its product candidate, together with the risks described in the Company’s filings made with the U.S. Securities and Exchange Commission from time to time. If any of these risks materialize or NewAmsterdam’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that are presently unknown by the Company or that NewAmsterdam currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect NewAmsterdam’s expectations, plans, or forecasts of future events and views as of the date of this Presentation and are qualified in their entirety by reference to the cautionary statements herein. NewAmsterdam anticipates that subsequent events and developments will cause the Company’s assessments to change. These forward-looking statements should not be relied upon as representing NewAmsterdam’s assessments as of any date subsequent to the date of this Presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements. Neither NewAmsterdam nor any of its affiliates undertakes any obligation to update these forward-looking statements, except as required by law. Market Data Certain information contained in this Presentation relates to or is based on third-party studies, publications, surveys and NewAmsterdam’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while NewAmsterdam believes its internal research is reliable, such research has not been verified by any independent source and NewAmsterdam cannot guarantee and makes no representation or warranty, express or implied, as to its accuracy and completeness. Trademarks This Presentation contains trademarks, service marks, trade names, and copyrights of NewAmsterdam and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade name or products in this Presentation is not intended to, and does not imply, a relationship with NewAmsterdam or an endorsement or sponsorship by or of NewAmsterdam. Solely for convenience, the trademarks, service marks and trade names referred to in this Presentation may appear with the TM or SM symbols, but such references are not intended to indicate, in any way, that NewAmsterdam will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade names. 2
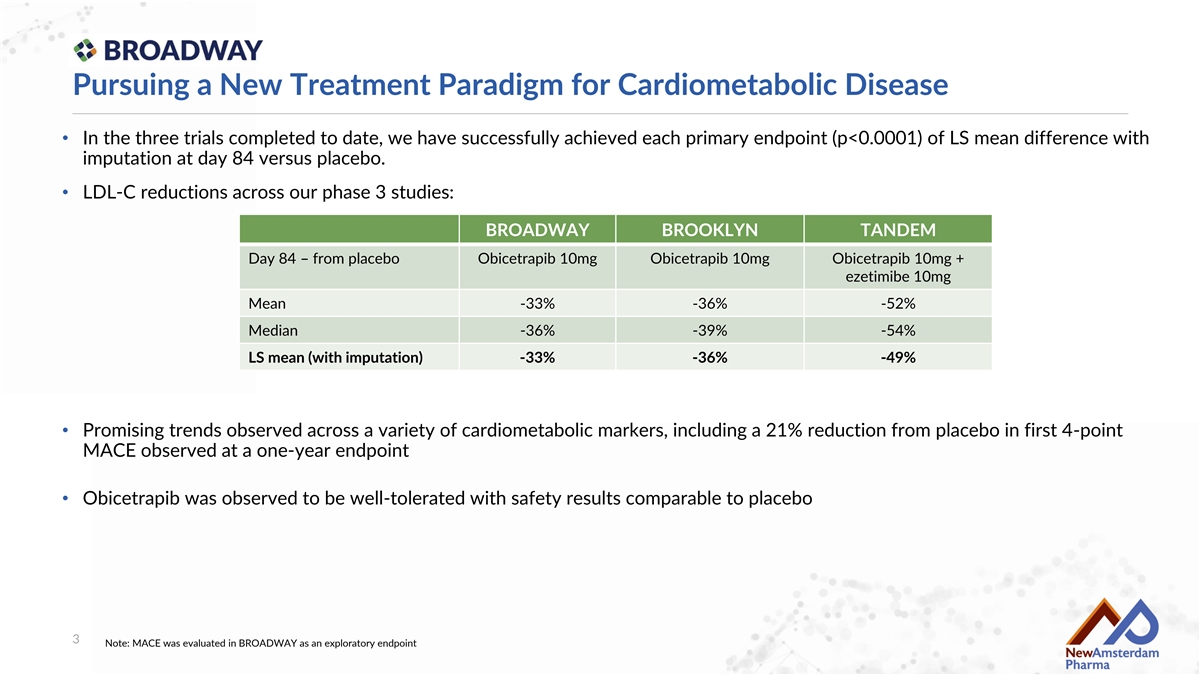
Pursuing a New Treatment Paradigm for Cardiometabolic Disease • In the three trials completed to date, we have successfully achieved each primary endpoint (p<0.0001) of LS mean difference with imputation at day 84 versus placebo. • LDL-C reductions across our phase 3 studies: BROADWAY BROOKLYN TANDEM Day 84 – from placebo Obicetrapib 10mg Obicetrapib 10mg Obicetrapib 10mg + ezetimibe 10mg Mean -33% -36% -52% Median -36% -39% -54% LS mean (with imputation) -33% -36% -49% • Promising trends observed across a variety of cardiometabolic markers, including a 21% reduction from placebo in first 4-point MACE observed at a one-year endpoint • Obicetrapib was observed to be well-tolerated with safety results comparable to placebo 3 Note: MACE was evaluated in BROADWAY as an exploratory endpoint

Majority of ASCVD/HeFH patients do not achieve LDL-C Targets Primary prevention HeFH ASCVD patients with an Very high risk ASCVD patients with LDL-C target of LDL<70 or patients with an LDL-C 2 an LDL-C target <100 mg/dL <55 mg/dL (2017-2018) target <55 mg/dL (2020- 1 3 (2011-2017) 2021) Target: LDL-C < 100 mg/dL Target: LDL-C < 70 mg/dL Target: LDL-C < 55 mg/dL 29% 24% 10% <1/3 achieved <1/4 achieved 10% achieved LDL-C <100 mg/dL LDL-C <70 mg/dL LDL-C <55 mg/dL ASCVD=atherosclerotic cardiovascular disease; HeFH=heterozygous familial hypercholesterolemia; LDL-C=low-density lipoprotein-cholesterol. 1. Schreuder MM, et al. LDL cholesterol targets rarely achieved in familial hypercholesterolemia patients: A sex and gender-specific analysis. Atherosclerosis. 2023 2. Gao Y, Shah LM, Ding J, Martin SS. US trends in cholesterol screening, lipid levels, and lipid- lowering medication use in US adults, 1999 to 2018. J Am Heart Assoc. 2023;12(3):e028205; 3. Katzmann JL, et al. Simulation study on LDL cholesterol target attainment, treatment costs, and ASCVD events with bempedoic acid in patients at high and very-high cardiovascular risk. PLoS One. 2022;17(10):e0276898 4
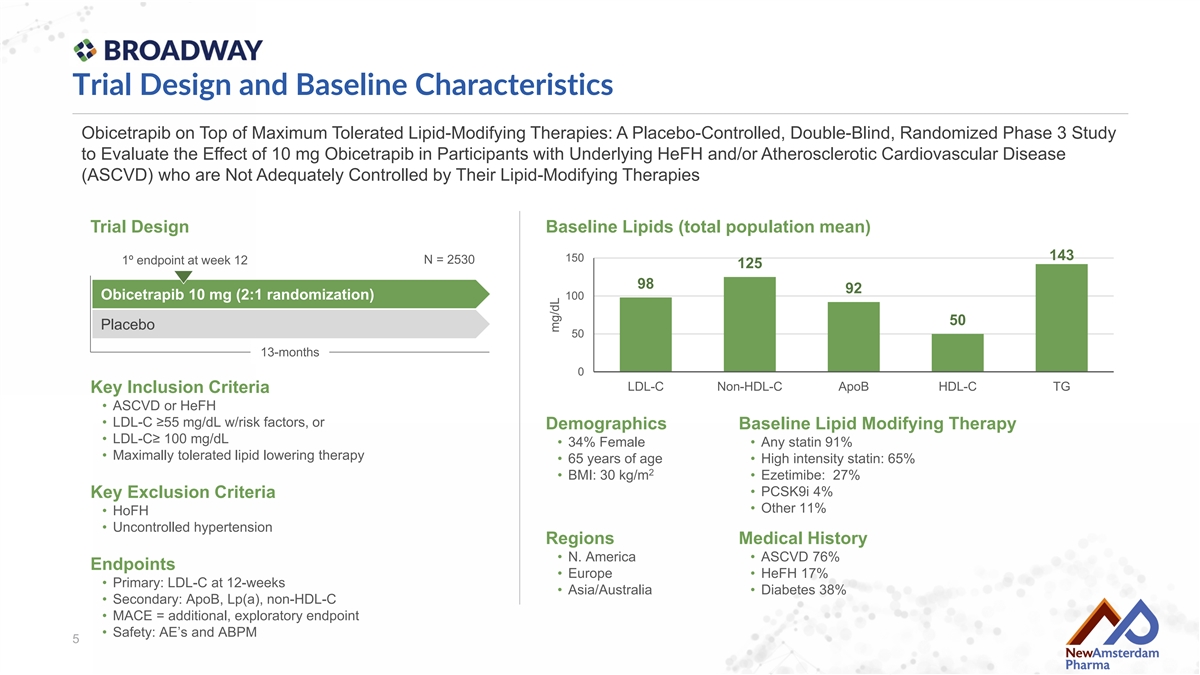
Trial Design and Baseline Characteristics Obicetrapib on Top of Maximum Tolerated Lipid-Modifying Therapies: A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants with Underlying HeFH and/or Atherosclerotic Cardiovascular Disease (ASCVD) who are Not Adequately Controlled by Their Lipid-Modifying Therapies Trial Design Baseline Lipids (total population mean) 143 150 N = 2530 1º endpoint at week 12 125 98 92 Obicetrapib 10 mg (2:1 randomization) 100 50 Placebo 50 13-months 0 LDL-C Non-HDL-C ApoB HDL-C TG Key Inclusion Criteria • ASCVD or HeFH • LDL-C ≥55 mg/dL w/risk factors, or Demographics Baseline Lipid Modifying Therapy • LDL-C≥ 100 mg/dL • 34% Female • Any statin 91% • Maximally tolerated lipid lowering therapy • 65 years of age • High intensity statin: 65% 2 • BMI: 30 kg/m • Ezetimibe: 27% • PCSK9i 4% Key Exclusion Criteria • Other 11% • HoFH • Uncontrolled hypertension Regions Medical History • N. America • ASCVD 76% Endpoints • Europe • HeFH 17% • Primary: LDL-C at 12-weeks • Asia/Australia • Diabetes 38% • Secondary: ApoB, Lp(a), non-HDL-C • MACE = additional, exploratory endpoint • Safety: AE’s and ABPM 5 mg/dL

Disposition of All Randomized Participants Placebo Obicetrapib 10 mg 844 1686 Randomized 739 (87.6) 1499 (88.9) Completed treatment 105 (12.4) 187 (11.1) Discontinued treatment 43 (5.1) 68 (4.0) Discontinued due to AE’s 33 (3.9) 57 (3.4) Subject decision 12 (1.4) 30 (1.8) Lost to follow-up 6 (0.7) 7 (0.4) Withdraw of consent 6 (0.7) 8 (0.5) Death 5 (0.6) 17 (1.0) Other 795 (94.2) 1600 (94.9) Completed the trial 49 (5.8) 86 (5.1) Discontinued trial early 844 (100.0) 1686 (100.0) Vital status known at trial completion 6
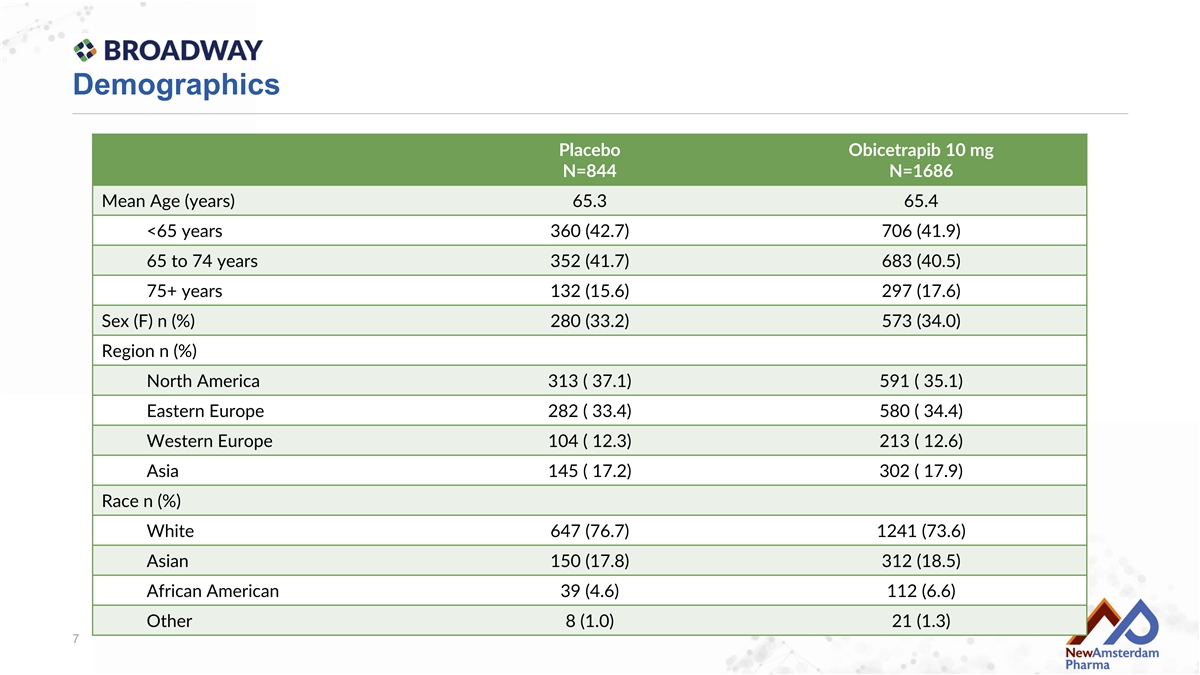
Demographics Placebo Obicetrapib 10 mg N=844 N=1686 Mean Age (years) 65.3 65.4 <65 years 360 (42.7) 706 (41.9) 65 to 74 years 352 (41.7) 683 (40.5) 75+ years 132 (15.6) 297 (17.6) Sex (F) n (%) 280 (33.2) 573 (34.0) Region n (%) North America 313 ( 37.1) 591 ( 35.1) Eastern Europe 282 ( 33.4) 580 ( 34.4) Western Europe 104 ( 12.3) 213 ( 12.6) Asia 145 ( 17.2) 302 ( 17.9) Race n (%) White 647 (76.7) 1241 (73.6) Asian 150 (17.8) 312 (18.5) African American 39 (4.6) 112 (6.6) Other 8 (1.0) 21 (1.3) 7
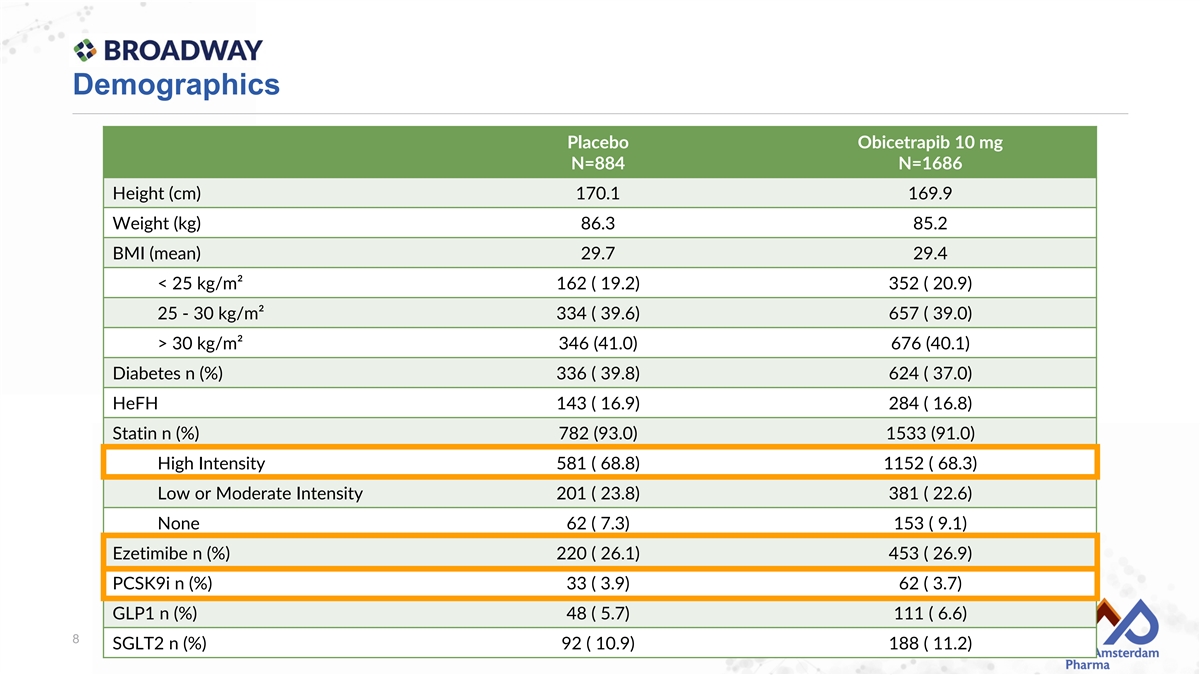
Demographics Placebo Obicetrapib 10 mg N=884 N=1686 Height (cm) 170.1 169.9 Weight (kg) 86.3 85.2 BMI (mean) 29.7 29.4 < 25 kg/m² 162 ( 19.2) 352 ( 20.9) 25 - 30 kg/m² 334 ( 39.6) 657 ( 39.0) > 30 kg/m² 346 (41.0) 676 (40.1) Diabetes n (%) 336 ( 39.8) 624 ( 37.0) HeFH 143 ( 16.9) 284 ( 16.8) Statin n (%) 782 (93.0) 1533 (91.0) High Intensity 581 ( 68.8) 1152 ( 68.3) Low or Moderate Intensity 201 ( 23.8) 381 ( 22.6) None 62 ( 7.3) 153 ( 9.1) Ezetimibe n (%) 220 ( 26.1) 453 ( 26.9) PCSK9i n (%) 33 ( 3.9) 62 ( 3.7) GLP1 n (%) 48 ( 5.7) 111 ( 6.6) 8 SGLT2 n (%) 92 ( 10.9) 188 ( 11.2)
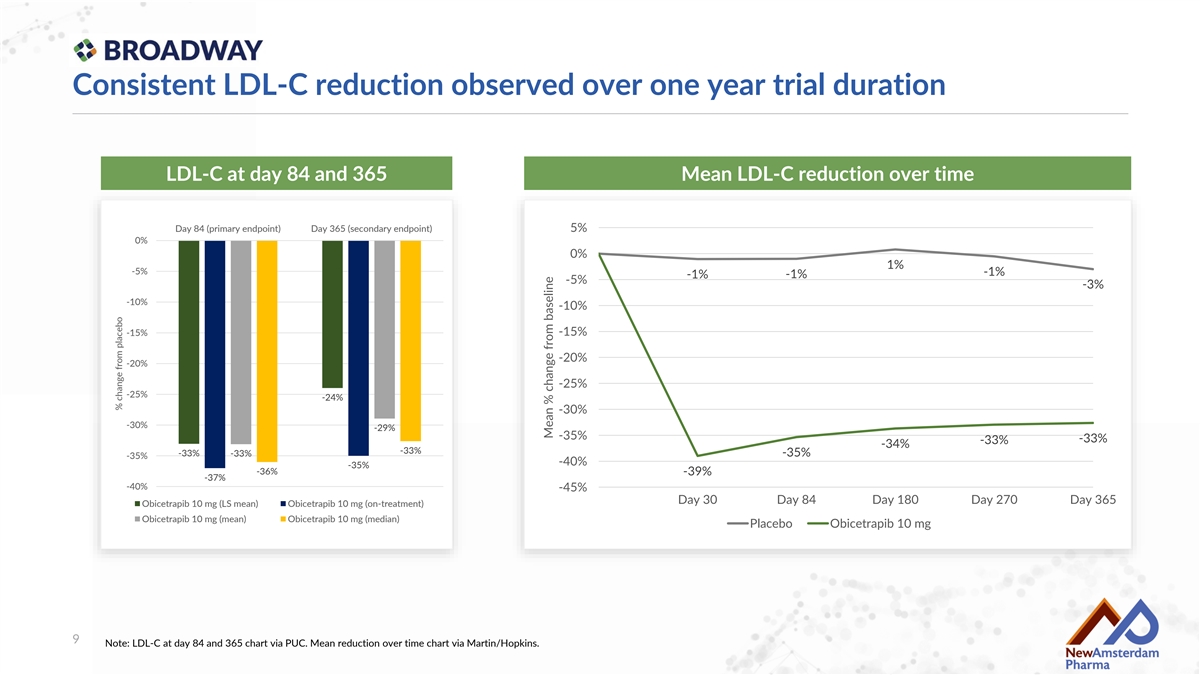
Consistent LDL-C reduction observed over one year trial duration LDL-C at day 84 and 365 Mean LDL-C reduction over time Day 84 (primary endpoint) Day 365 (secondary endpoint) 5% 0% 0% 1% -5% -1% -1% -1% -5% -3% -10% -10% -15% -15% -20% -20% -25% -25% -24% -30% -30% -29% -35% -33% -33% -34% -33% -33% -35% -33% -35% -40% -35% -36% -39% -37% -40% -45% Day 30 Day 84 Day 180 Day 270 Day 365 Obicetrapib 10 mg (LS mean) Obicetrapib 10 mg (on-treatment) Obicetrapib 10 mg (mean) Obicetrapib 10 mg (median) Placebo Obicetrapib 10 mg 9 Note: LDL-C at day 84 and 365 chart via PUC. Mean reduction over time chart via Martin/Hopkins. % change from placebo Mean % change from baseline

Median change from baseline of 40% Obicetrapib 10mg at day 84 100% 80% 60% 40% 20% 0% -20% -40% -60% 33% >50% LDL-C reduction -80% -100% 10 Note: each line represents a single patient in the obicetrapib 10 mg arm, five patients increase more than 100% LDL-C % change versus baseline

Half of the patients on obicetrapib 10 mg achieved less than <55 mg/dL % of patients achieving LDL-C thresholds at: Day 84 Day 365 11
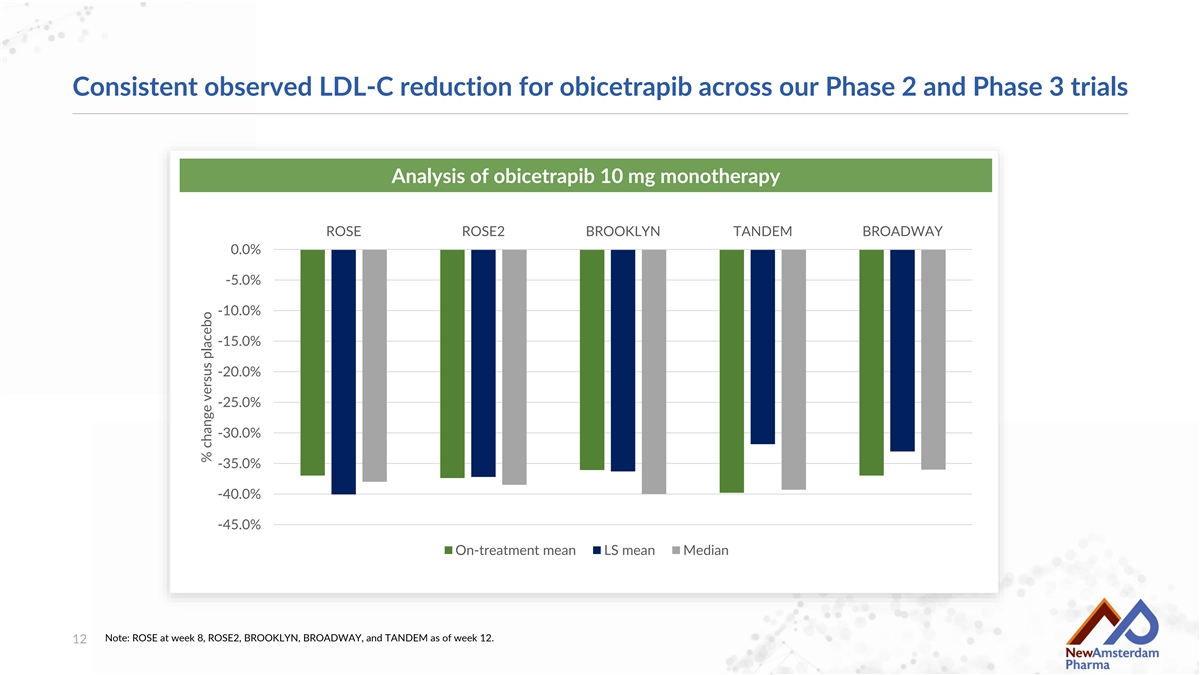
Consistent observed LDL-C reduction for obicetrapib across our Phase 2 and Phase 3 trials Analysis of obicetrapib 10 mg monotherapy ROSE ROSE2 BROOKLYN TANDEM BROADWAY 0.0% -5.0% -10.0% -15.0% -20.0% -25.0% -30.0% -35.0% -40.0% -45.0% On-treatment mean LS mean Median Note: ROSE at week 8, ROSE2, BROOKLYN, BROADWAY, and TANDEM as of week 12. 12 % change versus placebo

Expectation heading into BROADWAY was no difference in MACE (HR=1.0) Separation of curves after one year not seen in prior CETP CVOT trials Month 12 Curves are for the primary efficacy endpoint, which in IMPROVE-IT was defined as the composite of death from cardiovascular disease, a major coronary event (nonfatal myocardial infarction, documented unstable angina requiring hospital admission, or coronary revascularization occurring at least 30 days after randomization), or nonfatal stroke, in ACCELERATE as the composite of death from cardiovascular causes, myocardial infarction, stroke, coronary revascularization, or hospitalization for unstable angina, and in REVEAL as the composite of coronary death, myocardial infarction, or 13 coronary revascularization. Cannon CP, et al. N Engl J Med 2015;372:2387-2397. Lincoff AM, et al. N Engl J Med 2017;376:1933-1942. Bowman L, et al. N Engl J Med 2017;377:1217-1227.

Exploratory endpoint: Major adverse cardiovascular events (MACE) (1) BROADWAY MACE Data Placebo Obicetrapib Hazard Ratio 95% CI N = 844 N= 1686 All-cause mortality – no. (%) 12 (1.4) 19 (1.1) 0.83 (0.40-1.71) Coronary heart death – no. (%) 5 (0.6) 8 (0.5) 0.80 (0.26-2.44) First 4-point MACE – no. (%) 44 (5.2) 70 (4.2) 0.79 (0.54-1.15) (1) BROADWAY + BROOKLYN Pooled MACE Data Placebo Obicetrapib Hazard Ratio 95% CI N = 962 N= 1920 All-cause mortality – no. (%) 14 (1.5) 20 (1.0) 0.78 (0.39-1.58) Coronary heart death – no. (%) 7 (0.7) 9 (0.5) 0.63 (0.24-1.70) First 4-point MACE – no. (%) 49 (5.1) 75 (3.9) 0.75 (0.53-1.08) 4-point MACE: CHD death, Non-fatal myocardial infarction, non-fatal stroke, coronary revascularization 14 1. MACE was evaluated in BROADWAY as an exploratory endpoint. There may be limitations on the interpretation of MACE data derived from both BROOKLYN and BROADWAY studies given they were not designed to assess MACE as the primary and secondary endpoints.
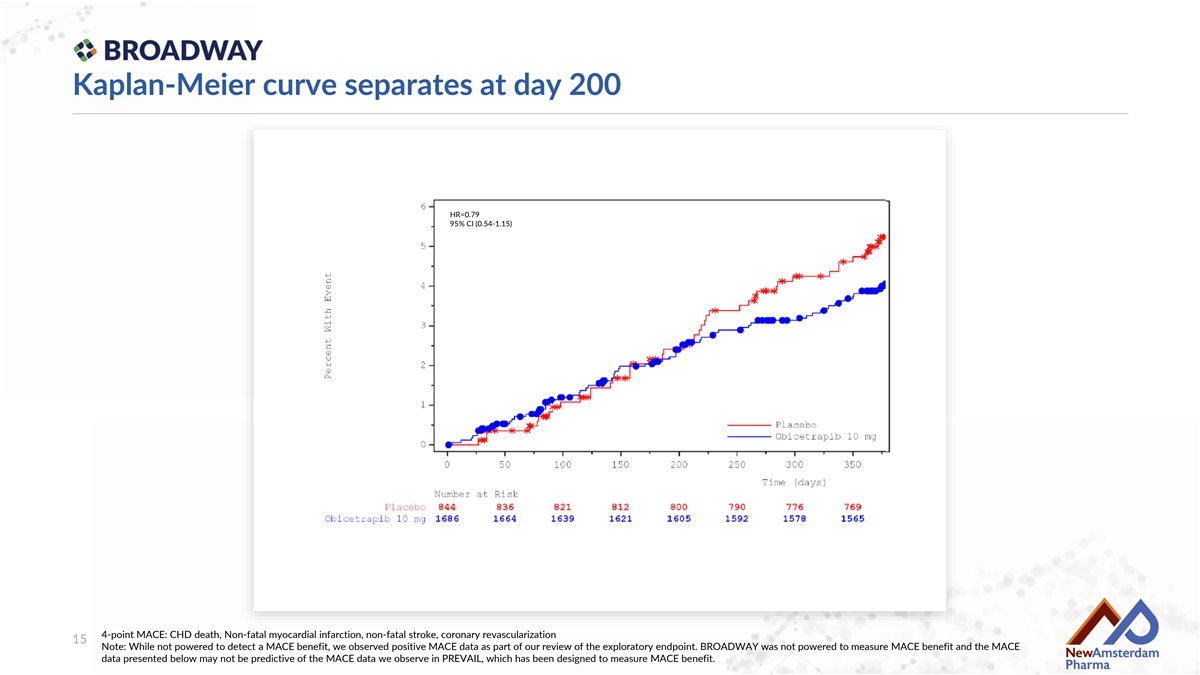
Kaplan-Meier curve separates at day 200 HR=0.79 95% CI (0.54-1.15) 4-point MACE: CHD death, Non-fatal myocardial infarction, non-fatal stroke, coronary revascularization 15 Note: While not powered to detect a MACE benefit, we observed positive MACE data as part of our review of the exploratory endpoint. BROADWAY was not powered to measure MACE benefit and the MACE data presented below may not be predictive of the MACE data we observe in PREVAIL, which has been designed to measure MACE benefit.
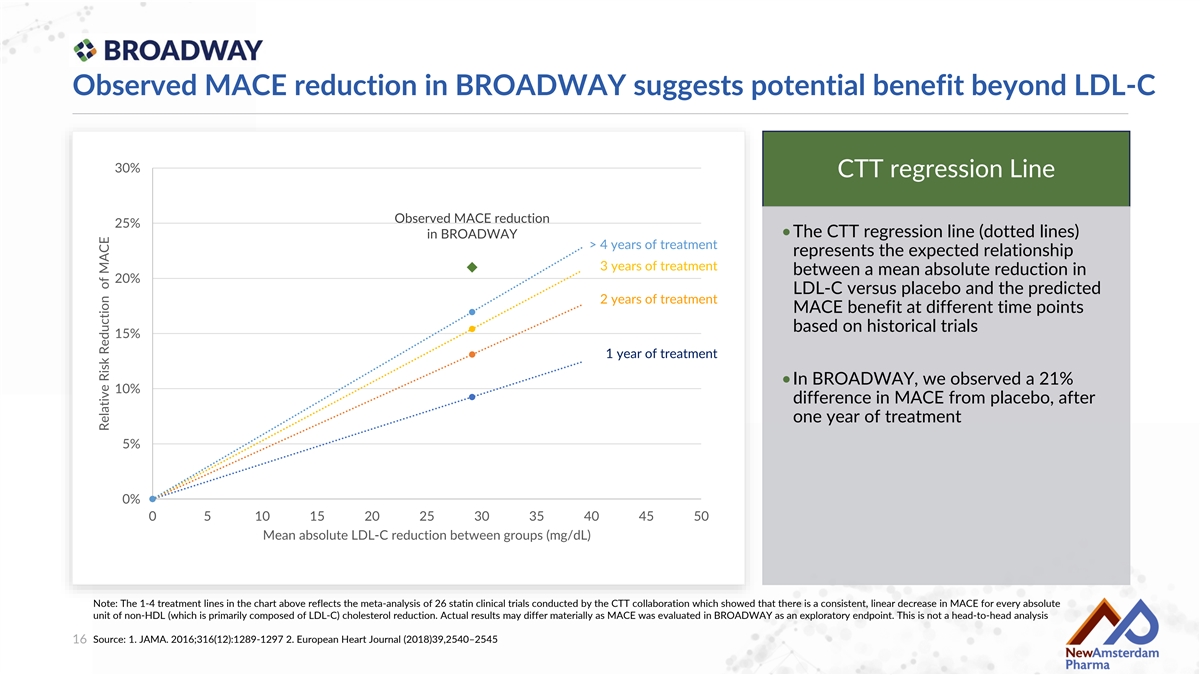
Observed MACE reduction in BROADWAY suggests potential benefit beyond LDL-C 30% CTT regression Line Observed MACE reduction 25% •The CTT regression line (dotted lines) in BROADWAY > 4 years of treatment represents the expected relationship 3 years of treatment between a mean absolute reduction in 20% LDL-C versus placebo and the predicted 2 years of treatment MACE benefit at different time points based on historical trials 15% 1 year of treatment •In BROADWAY, we observed a 21% 10% difference in MACE from placebo, after one year of treatment 5% 0% 0 5 10 15 20 25 30 35 40 45 50 Mean absolute LDL-C reduction between groups (mg/dL) Note: The 1-4 treatment lines in the chart above reflects the meta-analysis of 26 statin clinical trials conducted by the CTT collaboration which showed that there is a consistent, linear decrease in MACE for every absolute unit of non-HDL (which is primarily composed of LDL-C) cholesterol reduction. Actual results may differ materially as MACE was evaluated in BROADWAY as an exploratory endpoint. This is not a head-to-head analysis Source: 1. JAMA. 2016;316(12):1289-1297 2. European Heart Journal (2018)39,2540–2545 16 Relative Risk Reduction of MACE
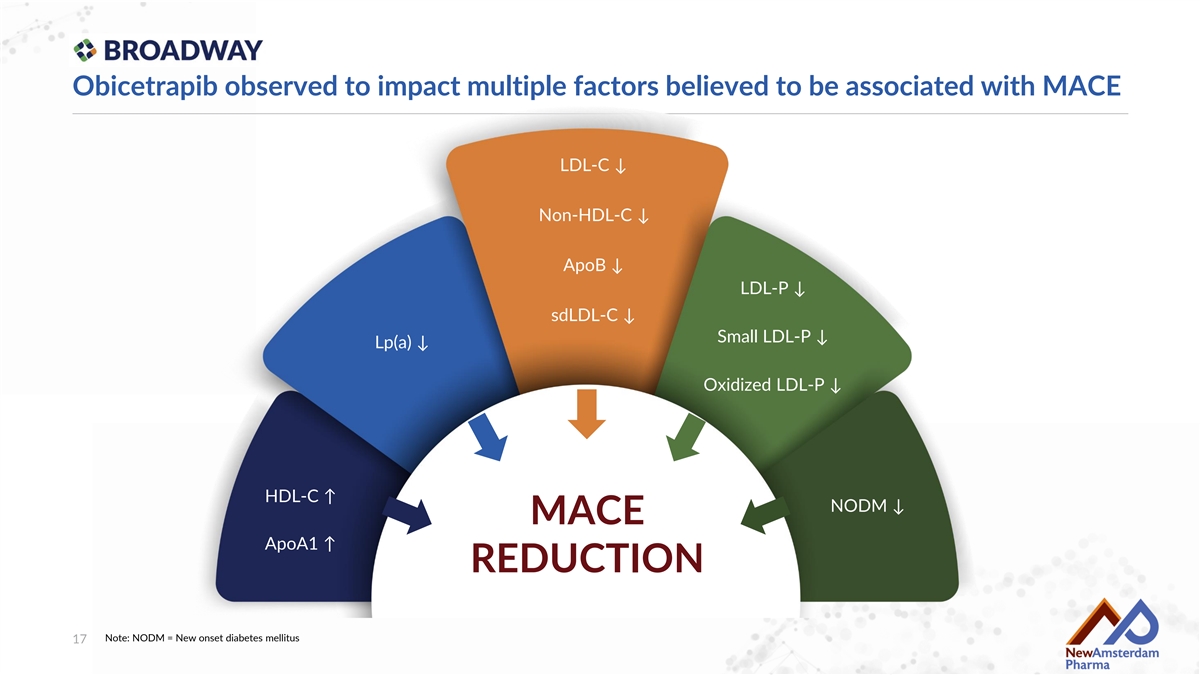
Obicetrapib observed to impact multiple factors believed to be associated with MACE LDL-C ↓ Non-HDL-C ↓ ApoB ↓ LDL-P ↓ sdLDL-C ↓ Small LDL-P ↓ Lp(a) ↓ Oxidized LDL-P ↓ HDL-C ↑ NODM ↓ MACE ApoA1 ↑ REDUCTION Note: NODM = New onset diabetes mellitus 17
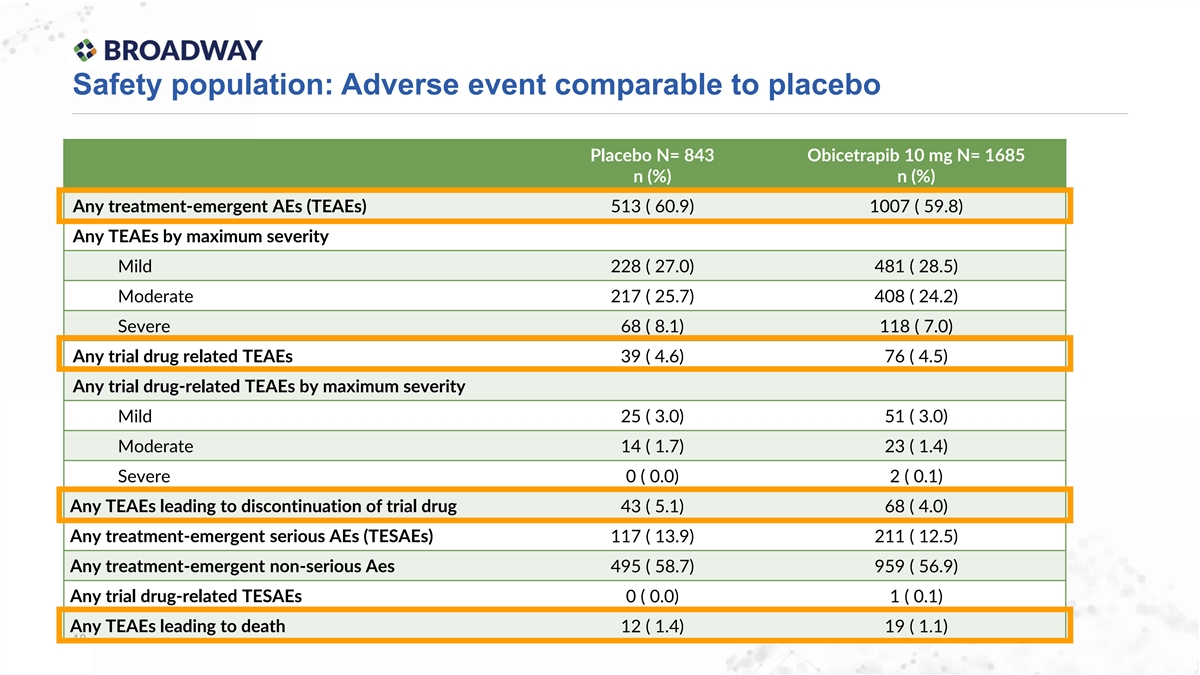
Safety population: Adverse event comparable to placebo Placebo N= 843 Obicetrapib 10 mg N= 1685 n (%) n (%) Any treatment-emergent AEs (TEAEs) 513 ( 60.9) 1007 ( 59.8) Any TEAEs by maximum severity Mild 228 ( 27.0) 481 ( 28.5) Moderate 217 ( 25.7) 408 ( 24.2) Severe 68 ( 8.1) 118 ( 7.0) Any trial drug related TEAEs 39 ( 4.6) 76 ( 4.5) Any trial drug-related TEAEs by maximum severity Mild 25 ( 3.0) 51 ( 3.0) Moderate 14 ( 1.7) 23 ( 1.4) Severe 0 ( 0.0) 2 ( 0.1) Any TEAEs leading to discontinuation of trial drug 43 ( 5.1) 68 ( 4.0) Any treatment-emergent serious AEs (TESAEs) 117 ( 13.9) 211 ( 12.5) Any treatment-emergent non-serious Aes 495 ( 58.7) 959 ( 56.9) Any trial drug-related TESAEs 0 ( 0.0) 1 ( 0.1) Any TEAEs leading to death 12 ( 1.4) 19 ( 1.1) 18
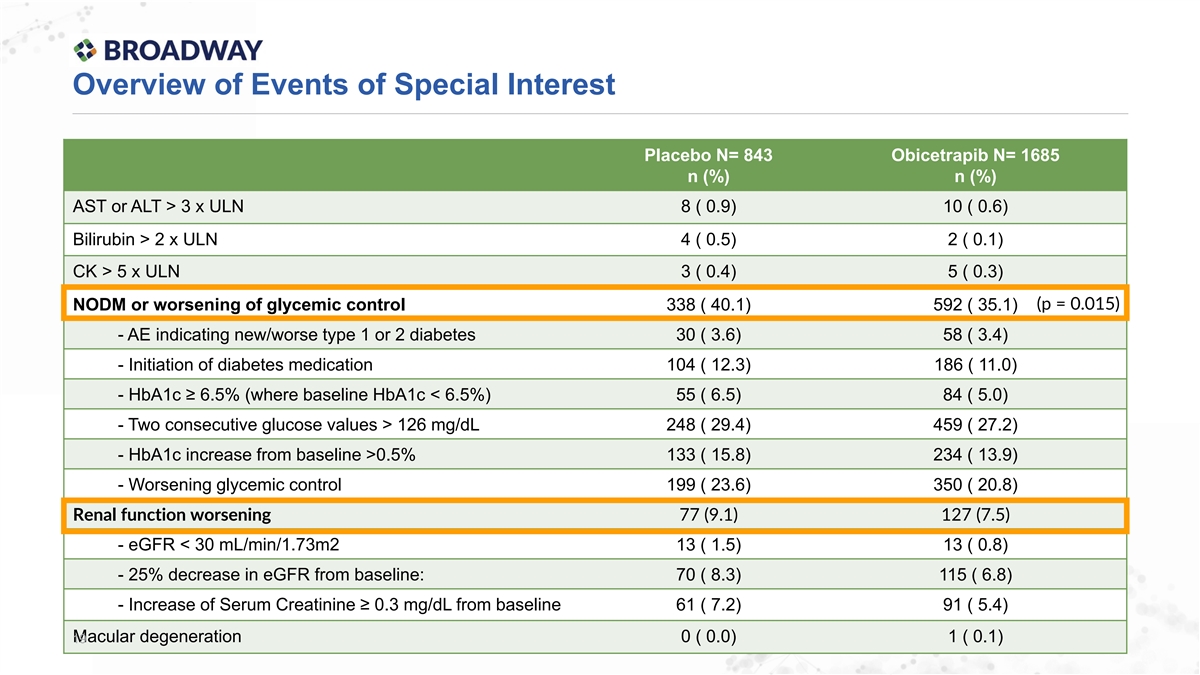
Overview of Events of Special Interest Placebo N= 843 Obicetrapib N= 1685 n (%) n (%) AST or ALT > 3 x ULN 8 ( 0.9) 10 ( 0.6) Bilirubin > 2 x ULN 4 ( 0.5) 2 ( 0.1) CK > 5 x ULN 3 ( 0.4) 5 ( 0.3) (p = 0.015) NODM or worsening of glycemic control 338 ( 40.1) 592 ( 35.1) - AE indicating new/worse type 1 or 2 diabetes 30 ( 3.6) 58 ( 3.4) - Initiation of diabetes medication 104 ( 12.3) 186 ( 11.0) - HbA1c ≥ 6.5% (where baseline HbA1c < 6.5%) 55 ( 6.5) 84 ( 5.0) - Two consecutive glucose values > 126 mg/dL 248 ( 29.4) 459 ( 27.2) - HbA1c increase from baseline >0.5% 133 ( 15.8) 234 ( 13.9) - Worsening glycemic control 199 ( 23.6) 350 ( 20.8) Renal function worsening 77 (9.1) 127 (7.5) - eGFR < 30 mL/min/1.73m2 13 ( 1.5) 13 ( 0.8) - 25% decrease in eGFR from baseline: 70 ( 8.3) 115 ( 6.8) - Increase of Serum Creatinine ≥ 0.3 mg/dL from baseline 61 ( 7.2) 91 ( 5.4) Macular degeneration 0 ( 0.0) 1 ( 0.1) 19
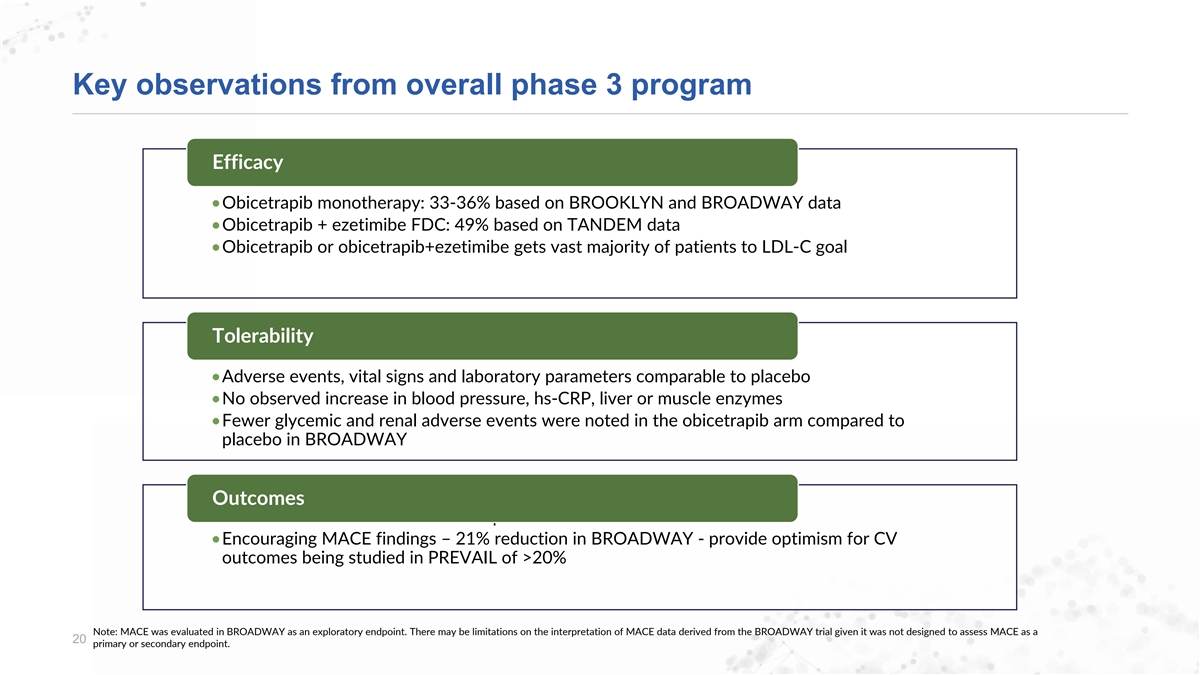
Key observations from overall phase 3 program Efficacy •Obicetrapib monotherapy: 33-36% based on BROOKLYN and BROADWAY data •Obicetrapib + ezetimibe FDC: 49% based on TANDEM data •Obicetrapib or obicetrapib+ezetimibe gets vast majority of patients to LDL-C goal Tolerability •Adverse events, vital signs and laboratory parameters comparable to placebo •No observed increase in blood pressure, hs-CRP, liver or muscle enzymes •Fewer glycemic and renal adverse events were noted in the obicetrapib arm compared to placebo in BROADWAY Outcomes •Confirmation that PREVAIL is set-up for success •Encouraging MACE findings – 21% reduction in BROADWAY - provide optimism for CV outcomes being studied in PREVAIL of >20% Note: MACE was evaluated in BROADWAY as an exploratory endpoint. There may be limitations on the interpretation of MACE data derived from the BROADWAY trial given it was not designed to assess MACE as a 20 primary or secondary endpoint.



















